Attached files
| file | filename |
|---|---|
| EX-23.1 - EX-23.1 - ARTHROCARE CORP | a2201946zex-23_1.htm |
| EX-21.1 - EX-21.1 - ARTHROCARE CORP | a2201946zex-21_1.htm |
| EX-18.1 - EX-18.1 - ARTHROCARE CORP | a2201946zex-18_1.htm |
| EX-31.1 - EX-31.1 - ARTHROCARE CORP | a2201946zex-31_1.htm |
| EX-32.1 - EX-32.1 - ARTHROCARE CORP | a2201946zex-32_1.htm |
| EX-32.2 - EX-32.2 - ARTHROCARE CORP | a2201946zex-32_2.htm |
| EX-99.1 - EX-99.1 - ARTHROCARE CORP | a2201946zex-99_1.htm |
| EX-31.2 - EX-31.2 - ARTHROCARE CORP | a2201946zex-31_2.htm |
| EX-10.42 - EX-10.42 - ARTHROCARE CORP | a2201946zex-10_42.htm |
Use these links to rapidly review the document
TABLE OF CONTENTS
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
ANNUAL REPORT
PURSUANT TO SECTION 13 OR 15(d)
OF THE SECURITIES EXCHANGE ACT OF 1934
For the fiscal year ended December 31, 2010
Commission File Number: 0-27422
ARTHROCARE CORPORATION
(Exact name of registrant as specified in its charter)
| Delaware (State or other jurisdiction of Incorporation or organization) |
94-3180312 (I.R.S. employer Identification number) |
|
7500 Rialto Blvd., Building Two, Suite 100, Austin, TX 78735 (Address of principal executive offices and zip code) |
||
| (512) 391-3900 (Registrant's telephone number, including area code) |
||
Securities registered pursuant to Section 12 (b) of the Act: Common Stock, $0.001 Par Value
Securities registered pursuant to Section 12 (g) of the Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes o No ý
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes o No ý
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the Registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ý No o
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes o No o
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (§229.405 of this chapter) is not contained herein, and will not be contained, to the best of registrant's knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ý
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer or a smaller reporting company. See the definitions of "large accelerated filer," "accelerated filer" and "smaller reporting company" in Rule 12b-2 of the Exchange Act.
| Large accelerated filer ý | Accelerated filer o | Non-accelerated filer o (Do not check if a smaller reporting company) |
Smaller reporting company o |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes o No ý
As of June 30, 2010, the aggregate market value of the voting and non-voting common equity held by non-affiliates of the registrant was approximately $827,736,628 (based upon the closing sales price of such stock as reported by NASDAQ on such date). Shares of Common Stock held by each officer, director and holder of 5 percent or more of the outstanding Common Stock on that date have been excluded in that such persons may be deemed to be affiliates. This determination of affiliate status is not necessarily a conclusive determination for other purposes.
As of February 3, 2011, the number of outstanding shares of the Registrant's Common Stock was 27,228,270.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of our definitive Proxy Statement to be filed pursuant to Regulation 14A under the Exchange Act of 1934, as amended, in connection with our annual meeting of stockholders, scheduled to be held May 12, 2011, that we expect to file with the commission within 120 days after December 31, 2010, are incorporated by reference into Part III of this Annual Report.
ARTHROCARE CORPORATION
FORM 10-K ANNUAL REPORT
TABLE OF CONTENTS
2
"ArthroCare," "we," "us," "our," and "Company" refer to ArthroCare® Corporation, unless the context otherwise requires.
In this Annual Report on Form 10-K, references to and statements regarding North America refer to the United States, or U.S., Canada and Mexico, references to U.S. dollars or USD or $ are to United States Dollars, and references to the euro or € are to the legal currency of the European Union, or EU.
Throughout this Annual Report on Form 10-K we incorporate by reference certain information from other documents filed with the Securities and Exchange Commission, or the SEC. Please refer to such information at www.sec.gov.
This Annual Report on Form 10-K contains forward-looking statements. In "Part I—Item 1A—Risk Factors," we discuss some of the risk factors that could cause our actual results to differ materially from those provided in the forward-looking statements.
Overview
We are a medical device company that develops, manufactures and markets surgical products, many of which are based on our minimally invasive patented Coblation® technology. We have grown beyond our roots in arthroscopy to capitalize on market opportunities across several medical specialties, improving many existing soft-tissue surgical procedures and enabling new minimally invasive procedures. Our innovative technologies improve the lives of individuals as diverse as world class athletes suffering from torn rotator cuffs or anterior cruciate ligaments to senior citizens suffering from herniated discs and children suffering from tonsillitis. We incorporated in California in 1993 and reincorporated in Delaware in 1995.
Our objective is to expand on our position as a leading global provider of minimally invasive surgical products. We seek to differentiate ourselves in this competitive market by providing our patients and user-physicians with improved treatment options. To accomplish this objective, we are focused on a business strategy featuring the following key elements:
- •
- expanding our product offering to address large and rapidly growing domestic and international markets;
- •
- improving selected established medical procedures by replacing current technologies with our technologies;
- •
- continuing to improve sales and cash flow through a combination of direct sales representatives, independent sales agents
and distributors;
- •
- augmenting our current business with complementary and compatible acquisitions that allow us to capitalize on emerging and
existing business opportunities and the strength of our distribution capability; and
- •
- establishing strategic partnerships to further commercialize our technologies and products.
Business Overview
Our business consists of two core product areas: Sports Medicine and Ear Nose and Throat, or ENT. We also manufacture and sell products based on our technology for other procedures, such as certain spine procedures. Our Sports Medicine, ENT and Other products are marketed and sold
3
through two principal geographic distribution channels: Americas (North and South America) and International (all other geographies).
Many of our key products are based on our patented Coblation technology, which uses bipolar radiofrequency energy to generate a precisely focused plasma field to remove and shape soft tissues such as cartilage and tendons. This use of bipolar energy is the primary factor that distinguishes our Coblation technology from traditional monopolar and bipolar radiofrequency surgical tools. Monopolar devices drive electrical energy into the treatment site and requires electrical energy to travel through the patient's body and exit via a grounding pad usually placed underneath the patient. Conversely, traditional bipolar devices include both an active electrode and a return at the tip of the device so a separate grounding pad is not required. To effect treatment by a bipolar device, electrical energy is applied at the surgical site, where it is concentrated at the targeted tissue and exits via the return electrode. These traditional monopolar and bipolar electrosurgical or laser surgery tools use the heat from the electrical current to burn away targeted tissue, often causing thermal damage to tissue surrounding the targeted surgical area.
Our bipolar Coblation devices also include both an active and return electrode at the tip but, in contrast to other bipolar devices, apply electrical energy in a conductive medium at the surgical site. The electrical energy along with electrically conductive fluid, which is found in normal saline solutions, form a highly ionized vapor layer, or plasma field, around the active electrode of the Coblation device. This plasma field enables our Coblation-based products to operate at lower ambient temperatures than traditional devices, minimizing tissue necrosis and damage when compared to traditional bipolar or monopolar instruments.
The use of Coblation allows surgeons to operate with a high level of precision and accuracy to minimize collateral tissue damage. Our Coblation-based systems consist of a controller unit with accessories and an assortment of sterile, single-use disposable devices that are engineered to address specific types of procedures. A single multi-purpose surgical system using Coblation technology can replace the multiple surgical tools traditionally used in soft-tissue surgery procedures.
We have applied Coblation technology to soft-tissue surgery throughout the body, primarily in the areas of arthroscopic sports medicine, ENT and spine applications. We also are exploring the use of Coblation technology in other markets.
Sports Medicine
Overview
Our Sports Medicine product area focuses on the development, production and marketing of surgical products used in the treatment of soft tissue injuries, mostly in the shoulder, knees and hips. Each year, we estimate that more than 600,000 people in the U.S. suffer from instabilities or tendon tears of the shoulder and 1.2 million more people are treated for cartilage, meniscus or tendon injuries in the knee. Sports Medicine is currently our largest product area and accounted for 68.5 percent, 67.9 percent and 67.6 percent of our total product sales during 2010, 2009 and 2008, respectively. We have experienced significant growth in Sports Medicine that we believe has been driven by several factors, including:
- •
- a greater emphasis on physical fitness across all demographics and an aging population accustomed to an active lifestyle,
which results in an increasing incidence of joint and soft tissue injuries;
- •
- increased patient demand for less invasive surgical procedures;
- •
- an increase in the number of arthroscopic procedures to surgically repair soft tissue injuries; and
- •
- an increase in the number of physicians trained in arthroscopic surgical techniques.
4
Sports activities often affect joints that are susceptible to damage from blows, falls or sudden changes in direction, as well as from natural degeneration associated with repeated use and aging. Arthroscopic surgeries are most commonly performed on the knees and shoulders but are also used in the treatment of injuries affecting other joints, such as elbows, wrists, ankles and hips. Prior to the widespread availability of arthroscopic surgery, joint injuries were treated through surgery involving large incisions, and through cutting or dislocating muscles to reach targeted tissues. These open procedures often required a hospital stay and prolonged rehabilitation and recovery. In contrast, arthroscopic surgery is performed through several small incisions, or portals, resulting in lower overall costs, reduced surgical trauma, pain and scarring, and fewer complications. Arthroscopic surgery generally is performed on an outpatient basis with no overnight stay. We believe that the products that we develop, produce, market and sell through Sports Medicine represent a substantial advancement in arthroscopic surgery because our technologies minimize collateral tissue damage to reduce patient pain and speed recovery times.
Our Sports Medicine Products
The ArthroWand® Product Line. Our ArthroWand product line is the principal product line in Sports Medicine. This line features a series of Coblation based specialized disposable radio frequency surgical wands designed for single patient use to treat a number of orthopedic conditions, including injuries of the shoulder, knee, hip, foot, ankle, elbow and wrist. ArthroWands are used with a controller, a software based radio frequency generator that provides electromagnetic energy used to create the localized plasma for tissue removal in arthroscopic surgeries for ablation, resection and coagulation of soft tissue and to seal bleeding vessels.
The Opus® Collection. In 2004, we acquired Opus Medical, Inc., a manufacturer of a proprietary family of specialized implants and instruments, which facilitates a "knotless" arthroscopic repair for shoulder injuries, such as rotator cuff tears. Before Opus, variation in physician skills and training were limitations to wide adoption of completely arthroscopic surgical repairs. Opus technology enabled many surgeons to complete repairs arthroscopically that previously were only possible with open surgical methods. These products may reduce overall procedural costs and surgical time, and expand the number of patients eligible for arthroscopic treatment.
The Atlantech® Collection. In 2002, we acquired two of our European distributors, which allowed us to accelerate the implementation of our direct sales strategy in two key European markets and access to a broad line of hand-held surgical instruments, procedural kits and trays and reusable accessories to facilitate arthroscopic surgery.
ENT
Overview
In ENT, we focus on the development, production and marketing of surgical products used in the treatment of conditions typically treated by ENT healthcare professionals. Our ENT product area accounted for 27.8 percent, 27.8 percent, and 27.3 percent of our total product sales during 2010, 2009 and 2008, respectively. One of the most common ENT procedures which utilize our products is the removal of tonsils and adenoids, and we estimate that there are more than 600,000 tonsillectomies performed in the U.S. each year. The majority of tonsillectomies are performed on pediatric patients. Other commonly performed ENT procedures include endoscopic sinus surgery, turbinate reduction and procedures to remove or stiffen tissue to treat snoring. These procedures are performed by ENT surgeons in hospitals and ambulatory surgery centers. Historically, monopolar electrocautery devices, or bovies, were the standard for removal, or ablation, of soft tissue and cauterization during ENT procedures. Coblation continues to gain widespread market acceptance, particularly for tonsil and adenoid procedures.
5
Our ENT Products
The ENT Wands Product Line. ENT has developed a variety of products for use by ENT surgeons for general head, neck and oral surgical procedures, including sinus surgery, the treatment of snoring, reduction of nasal turbinates, and adenoid and tonsil removal. ENT markets three categories of disposable devices, or wands, to address the ENT Market—suction wands, channeling wands and excision wands, each of which use Coblation technology to provide significant benefits over bovies and similar devices. During a tonsillectomy, suction wands simultaneously ablate and remove tissue providing the surgeon enhanced visibility and shortening anesthesia time. Channeling wands provide controlled ablation with effective hemostasis control to minimize bleeding during turbinate reduction procedures, which relieve nasal obstructions and stiffen the soft palate for the treatment of snoring. Excision wands provide precise dissection of soft tissue with minimal damage to surrounding tissue.
The Rapid Rhino® Product Line. In 2005, we completed the purchase of substantially all of the assets of Applied Therapeutics, Inc., or ATI, a maker of sinus surgery treatment products, that are complementary to our ENT devices. ATI's carboxymethyl-cellulose based packing and tamponade products are used in situations of significant nosebleed, or epistaxis, treatments which are treated in an emergency room setting or surgicenter. Our Rapid Rhino product line includes the dissolvable Stammberger Sinus Dressing and Sinu Knit, which are used after endoscopic sinus surgery to control minor bleeding, facilitate epithelial healing and preserve the spatial integrity of the sinus area. Our Rapid Rhino product line also includes epistaxis balloon tamponade products, which are used primarily in the hospital emergency room environment to control chronic nosebleeds.
Other
Overview
We develop, produce and market surgical products used in the treatment of spine related and other conditions. Other product sales accounted for 3.7 percent, 4.3 percent and 5.1 percent of our product sales during 2010, 2009 and 2008, respectively.
Our Other Products
SpineWand® Product Line. The SpineWand devices use Coblation-based technology to treat soft tissue (intervertebral disc) conditions in the spine. Disc decompression, a common soft tissue minimally-invasive treatment for herniated discs, involves the removal of disc material either through a narrow tube, or cannula, using x-ray technology for guidance or through a minimal surgical incision. Ongoing clinical studies are evaluating minimally invasive disc decompression as an alternative to current forms of standard care for certain indications associated with this condition. Our Plasma Disc Decompression, or PDD, SpineWands are products for the treatment of contained herniated discs. The PDD SpineWands remove portions of these herniated discs to relieve pressure on major spinal nerves and are designed for use in both large and small disc spaces, as well as for treatments requiring the removal of soft tissue in the spine through a much smaller incision rather than through a cannula. Our Cavity SpineWand® is used to reduce the size of malignant lesions within the vertebrae in patients suffering from spinal compression fractures resulting from benign and malignant tumors.
WoundWand® Debridement Device. The WoundWand was Conformité Européenne, or CE, marked in 2010 making it available for distribution in Europe. The WoundWand is intended for wound debridement in acute and chronic wounds and wound cleansing by removal of necrotic tissue in a precise and controlled manner. In 2011, we intend to conduct studies to evaluate the clinical effectiveness of the WoundWand.
6
Reimbursement Assistance Services
Beginning in the fourth quarter of 2005, DiscoCare, Inc., or DiscoCare, an independently owned medical device distributor and reimbursement service provider, purchased SpineWand products from us and sought reimbursement for the wands on a "carve-out" or "stand-alone" basis. DiscoCare provided the products to its customers generally at no charge in exchange for either (i) an assignment of the right to bill private third-party insurance companies and other payors for the product or (ii) a letter of protection or attorney's lien in a personal injury case for separate reimbursement of the product. DiscoCare originally distributed our SpineWand products on a limited geographical basis. DiscoCare approached us about distributing the SpineWands pursuant to this "carve-out" or "stand-alone" model and providing reimbursement services to customers on a national basis, and in the fourth quarter of 2006, our agreement was amended to provide DiscoCare with national distribution and reimbursement services rights and to require us to pay a service fee to DiscoCare. On December 31, 2007, we acquired all of the common stock of DiscoCare from its sole shareholder.
In June 2007, we established a new, wholly-owned subsidiary, Device Reimbursement Services, or DRS, which in the fourth quarter of 2007 began to sell our Sports Medicine products, generally at discounted prices, in exchange for an assignment of the right to bill private third-party insurance companies and other payors for our products upon completion of a procedure using our products.
After the acquisition of DiscoCare, we continued to sell products and provide reimbursement services through our DiscoCare and DRS subsidiaries until the fourth quarter of 2008, when DiscoCare ceased accepting letters of protection in personal injury cases and making any further claims to healthcare carriers. All other claims submission by DiscoCare and DRS ceased in the first quarter of 2009 and the subsidiaries have continued to engage in an orderly wind down of their business operations.
Our Customers and Competition
Our primary customers across all of our product areas are surgeons and other specialized medical professionals who utilize our products to treat their patients. Our primary competitors include Medtronic, Inc., Smith & Nephew, Stryker Corporation, Johnson & Johnson, Olympus (through its subsidiary Gyrus) and Arthrex, Inc. These competitors tend to be large, well-financed companies with diverse product lines.
In the Sports Medicine market, we also compete directly with competitors to whom we have licensed our technology for limited fields of use.
For additional discussion of the risks and considerations concerning our competition, see "Part I—Item 1A—Risk Factors."
Our Distribution Network
Distribution in the Americas
We sell our Sports Medicine products in the Americas through a network of employee sales representatives, independent sales agents and independent distributors. We sell our ENT products in the U.S. exclusively through employee sales representatives and outside of the U.S. we sell to independent distributors. As of December 31, 2010, for these two core business areas our sales organization approximated 480 persons, including sales management. We also sell our other products in the Americas through independent sales agents and distributors. Sales in the Americas accounted for 72.0 percent, 72.9 percent and 73.5 percent of our total product sales during 2010, 2009 and 2008, respectively. We continually evaluate the balance between direct sales representatives, independent agents and distributors in an effort to maximize the coverage and efficiency of our product sales platform. For the year ended December 31, 2010, approximately 52 percent of Americas product sales
7
were from direct sales representatives and the remainder was from independent sales agents or distributors.
Distribution in International
Markets outside of the Americas accounted for 28.0 percent, 27.1 percent and 26.5 percent of our total product sales during 2010, 2009 and 2008, respectively. Our international sales efforts are focused on current key markets where we sell direct to end users of our products, including several countries in Western Europe and Australia, as well as strategic growth markets, including India, China and Russia where we work through independent distributors. If we are successful, we expect that our strategic growth markets will develop into key markets for our products in the future.
For the year ending December 31, 2010, approximately 65 to 70 percent of international sales were derived from direct markets where we manage the relationship with our end customers and the remainder is through distributors.
Geographic Information
For financial reporting purposes, net sales and long-lived assets attributable to significant geographic areas are presented in Note 18, "Segment Information" in the notes to the consolidated financial statements.
Research and Development
We conduct our research and development activities in the U.S. on three campuses in Irvine and Sunnyvale, California and Austin, Texas. Research and development expenses as reported includes experimental research, new product development, process engineering, regulatory affairs and clinical studies and were in aggregate 10.1 percent, 10.9 percent and 10.4 percent of our total net revenues during 2010, 2009 and 2008, respectively. Our efforts are focused on:
- •
- modifying and enhancing the performance of our existing technologies and products;
- •
- designing new surgical devices that incorporate additional functionalities;
- •
- developing new applications of our Coblation and other innovative technologies and
- •
- developing complementary technologies and products for introduction into our product portfolio in order to provide more value to our customers and participate to a greater extent in certain surgical procedures.
We continue to explore and develop the plasma physics underlying our Coblation technology and evaluate the use of our technology in new fields of treatment. We are engaged with a number of doctors, scientists and research institutions to further understand the technology and expand its applications base and technical capability.
To facilitate our research and development efforts, we routinely receive feedback from the physicians who use our products and consult with our Scientific Advisory Boards, which are comprised of respected and experienced physicians and surgeons who advise us on our products, current and prospective, and other areas relating to our medical devices. These medical professionals are valued contributors and advisors to our research and development process and allow us to achieve significant and innovative strides in our product development.
8
Manufacturing and Supply
Our products are primarily manufactured at our facility located in San Jose, Costa Rica. We believe our existing operations will provide adequate capacity for our manufacturing needs at least through 2011.
We have established manufacturing process and quality assurance systems in conformance with the International Organization for Standardization's ISO 13485:2003 standard, the FDA's Quality System Regulation, 21 CFR Part 820 and we are certified under the Canadian Medical Devices Conformity Assessment System. Our facilities are in conformance with domestic and international regulatory requirements that apply to medical device manufacturers and routinely are subject to audits by U.S. Food and Drug Administration, or FDA, Technischer Überwachungs-Verein, or TUV, a Notified Body per the Medical Device Directive for the European Union, and other regulatory agencies representing countries in which our products are sold.
Our products are manufactured from several components, most of which are supplied to us from third parties. Most of the components we use in the manufacture of our products are available from more than one qualified supplier. For some components, however, there are relatively few alternative sources of supply and the establishment of additional or replacement suppliers may not be accomplished quickly. In isolated cases, we rely upon single source suppliers. In such cases, a disruption of our single supply source could materially affect and disrupt our ability to manufacture and distribute certain of our products. Due to regulatory and other requirements regarding the manufacture of our products, we may not be able to quickly establish additional or replacement sources for these certain components or materials. Generally, we have been able to obtain and maintain adequate supplies of such raw materials and components. We also primarily use a single subcontractor located in Costa Rica to sterilize our disposable devices to which we loaned the majority of the funds necessary to construct their Costa Rican sterilization facility. As a part of this agreement we receive guaranteed processing times on their sterilization services. While we have alternate product sterilization service providers, a significant disruption to the primary sterilization provider could temporarily impact our ability to supply products in a timely manner. Disruption of these supply sources and our inability to develop alternative sources for such supply, would result in a material disruption of our ability to sell this line of products for an extended time period and could adversely affect our operations.
DHL Solutions, Inc. is a significant provider of logistic services, including warehousing of finished goods and a variety of transportation services. The company primarily utilizes FedEx Corporation as the provider of transportation services associated with the shipment of finished products to customers and field returns in North America. Shipment of finished products outside of North America and the transportation of raw materials and other production related shipments are handled by a variety of third party common carriers based on a competitive sourcing process. The providers of these services change from time to time and there are alternate sources of these services should any single provider prove unable to supply contracted services. The complex nature of the underlying support technologies utilized to exchange critical product information with these third party transportation providers, such as "Electronic Data Interchange", has, at times, caused disruptions in our ability to deliver products to customers on a timely basis. Failure of these supporting systems could impair our business for the duration of the failure.
Patents and Proprietary Rights
We own over 210 issued U.S. patents and over 170 issued international patents. In addition, we have over 135 U.S. and international pending patent applications. We believe our issued patents are directed at, among other things, the core technology used in our soft-tissue surgery systems, including both multi-electrode and single electrode configurations of our disposable devices, as well as the use of Coblation technology in many different surgical procedures. In addition, our issued patents are directed
9
at many of the core features of our acquired technologies from Opus, Atlantech, Parallax and ATI. Our earliest Coblation-related patents will begin to expire in 2012. The majority of our royalty income is associated with licenses that include core Coblation patent rights which extend through the end of 2014.
In addition to patents, we hold intellectual property that we believe has significant value and provides us with competitive advantages in the form of copyrights, know-how, trademarks and customer relationships.
See "Part I—Item 1A—Risk Factors" and Intangible Asset information outlined in Note 8, "Goodwill and Intangible Assets" in the notes to the consolidated financial statements for additional information.
Government Regulation
U.S.
Our products are considered medical devices and are subject to extensive regulation in the U.S. We must comply with FDA regulations to market our products or obtain pre-market clearance or approval by the FDA for each of our products and indications before they can be commercialized.
FDA regulations are wide-ranging and govern, among other things: product design and development; product testing; product labeling; product storage; premarket clearance or approval; advertising and promotion; and product sales and distribution. Non-compliance with applicable regulatory requirements can result in enforcement action, which may include: warning letters; fines, injunctions and civil penalties against us; recall or seizure of our products; operating restrictions, partial suspension or total shutdown of our production; refusing our requests for premarket clearance or approval of new products; withdrawing product approvals already granted; and criminal prosecution.
Unless an exemption applies, generally, before we can introduce a new medical device or a new use for a medical device into the U.S. market, we must obtain FDA clearance of a 510(k) premarket notification or obtain premarket approval. If we can establish that our device is "substantially equivalent" to an existing device for which the FDA has not called for premarket approvals, we may seek clearance from the FDA to market the device by submitting a 510(k) premarket notification. The 510(k) premarket notification requires the support of appropriate data, including, in some cases, clinical data establishing the claim of substantial equivalence to the satisfaction of the FDA. Where a product is not deemed as substantially equivalent to an existing device, a more rigorous premarket approval process, or a PMA process, is required. This PMA process requires us to independently demonstrate that the new medical device is safe and effective by collecting data regarding design, materials, bench and animal testing, and human clinical data for the medical device. Only if the FDA determines there is reasonable assurance that the medical device is safe and effective, will the FDA authorize the device's commercial release. The premarket approval process is much more detailed, time-consuming and expensive than the 510(k) process.
We have received 510(k) clearance to the extent we believe it is required for all of our products for a variety of indications.
We strive to ensure that our products not requiring clearance meet our manufacturing and quality guidelines and standards to the same extent as if clearance were required.
Our products and the facilities manufacturing our products are subject to continued review and periodic inspections by the FDA. Each U.S. device-manufacturing establishment must be registered with the FDA. Domestic manufacturing establishments are subject to biannual inspections by the FDA, and must comply with the FDA's quality system regulations. Further, we must report adverse events involving our devices to the FDA under regulations issued by the FDA. Failure to maintain compliance with the regulatory requirements of the FDA may result in administrative or judicial actions, such as
10
fines, warning letters, holds on clinical studies, product recalls or seizures, product detention or refusal to permit the import or export of products, refusal to approve pending applications or supplements, restrictions on marketing or manufacturing, injunctions, or civil or criminal penalties.
Federal healthcare laws apply when we or our customers submit claims for items or services that are reimbursed under Medicare, Medicaid or other federally funded healthcare programs. The principal federal laws include: (1) the False Claims Act which prohibits the submission of false or otherwise improper claims for payment to a federally funded healthcare program; (2) the Anti-Kickback Statute which prohibits offers to pay or receive remuneration of any kind for the purpose of inducing or rewarding referrals of items or services reimbursable by a Federal healthcare program; (3) the Stark law which prohibits physicians from referring Medicare or Medicaid patients to a provider that bills these programs for the provision of certain designated health services if the physician (or a member of the physician's immediate family) has a financial relationship with that provider; and (4) healthcare fraud statutes that prohibit false statements and improper claims with any third party payor. Often similar state laws also apply to claims submitted under state Medicaid or state funded healthcare programs. Some states, such as California, Massachusetts and Vermont, mandate implementation of corporate compliance programs to ensure compliance with these laws. In addition, the U.S. Foreign Corrupt Practices Act can be used to prosecute companies in the U.S. for arrangements with physicians, or other parties outside the U.S. if the physician or party is a government official of another country and the arrangement violates the law of that country.
The laws applicable to us are subject to change and to evolving interpretations. To help build more consistent business practices, we have implemented a corporate-wide compliance program that intends to conform business practices throughout our business areas to applicable laws and regulations.
For additional discussion of the risks and considerations surrounding our business in the context of our regulatory environment, see "Part I—Item 1A—Risk Factors."
International
International sales of our products are subject to strict regulatory requirements. The regulatory review process varies from country to country. The member countries of the European Union, or EU, have adopted the European Medical Device Directives, which create a single set of medical device regulations for all EU member countries. These regulations require companies that wish to manufacture and distribute medical devices in EU member countries to obtain the CE Mark, for their products. We have authorization to apply the CE Mark to substantially all of our products. Additionally, we have obtained regulatory clearance to market our key products throughout other countries. In addition, the U.S. Foreign Corrupt Practices Act can be used to prosecute companies in the U.S. for arrangements with physicians, or other parties outside the U.S. if the physician or party is a government official of another country and the arrangement violates the law of that country. Other international laws such as the U.K. Anti-Bribery Act provide further restrictions on interactions with physicians and other potential customers.
General Considerations
The demand for our products is highly dependent on the policies of third-party payors such as Medicare, Medicaid, and private insurance and managed care organizations that reimburse our customers when they use our products. If coverage or payment policies of these third-party payors are revised in light of increased efforts to control healthcare spending or otherwise, the amount we may charge or the demand for our products may decrease. Government programs, including Medicare and Medicaid, private healthcare insurance and managed-care plans have attempted and continue to attempt to control costs by limiting the amount of reimbursement they will pay for particular procedures or treatments. This practice has increased price sensitivity among certain customers for our
11
products. Additionally, some third-party payors require approval of coverage for new or innovative devices or therapies before they will reimburse healthcare providers who use the medical devices or therapies. Even though a new medical device may have been approved for commercial distribution, we may find limited demand for the device until reimbursement approval has been obtained from governmental and private third-party payors. In the U.S., we believe that healthcare reform legislation proposals will most likely remain focused on reducing the cost of healthcare and that efforts by private payors to contain costs through managed care and other methods will continue in the future as efforts to reform the healthcare system continue. We believe we can effectively respond to reasonable changes resulting from the worldwide trend toward cost containment due to our manufacturing efficiencies and cost controls. However, it is difficult for us to predict the potential impact of cost containment trends on future operating results due to uncertainty that remains regarding future legislation.
Impact of Business Outside of the U.S.
Our operations in countries outside the U.S. are accompanied by certain financial and other risks. Relationships with customers and effective terms of sale vary by country, often with longer payment terms on receivables than are typical in the U.S. Currency exchange rate fluctuations can affect net sales from, and profitability of, our operations outside the U.S. See "Part II—Item 7A—Quantitative and Qualitative Disclosures About Market Risk" and Note 18, "Segment Information" in the notes to the consolidated financial statements. In addition, the repatriation of certain earnings of our foreign operations may result in U.S. tax cost of approximately $11.4 million.
Product Liability Risk and Insurance Coverage
The development, manufacture and sale of medical products entail significant risk of product liability claims. We cannot assure you that our current product liability insurance coverage limits are adequate to protect us from any liabilities we might incur in connection with the development, manufacture and sale of our products. In addition, we may require increased product liability coverage as products are successfully commercialized in additional applications. Product liability insurance is expensive and in the future may not be available to us on acceptable terms, if at all. A successful product liability claim or series of claims brought against us in excess of our insurance coverage could have a material adverse effect on our business, financial condition, results of operations and our ability to attract and retain customers for our products.
Employees
At December 31, 2010 we had 1,407 employees. In North America, we had 459 employees, of which 21 were engaged in supply and logistics activities, 110 in research and development, process engineering, and clinical affairs activities, 182 in sales, customer service and marketing activities, 60 in regulatory affairs and quality assurance, and 86 in administration, finance and corporate support functions. In Europe and Asia Pacific, we had 204 employees responsible for our international markets. In Costa Rica, we had 744 employees engaged in manufacturing and plant management. We have no employees covered by collective bargaining agreements, and we believe we maintain good relations with our employees.
Where You Can Find Additional Information
We maintain an internet website at www.ArthroCare.com. On our website, we make available, free of charge, the following filings as soon as reasonably practicable after they are electronically filed with or furnished to the SEC: our annual reports on Form 10-K, our quarterly reports on Form 10-Q, our current reports on Form 8-K and any amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Exchange Act of 1934, as amended, or the Exchange Act. You may also read and copy any materials that we file with the SEC at the SEC's Public Reference Room at 100 F
12
Street, NE, Washington, DC 20549. You may obtain information on the operation of the Public Reference Room by calling the SEC at 1-800-SEC-0330. Our reports, proxy statements and other documents filed electronically with the SEC are available at the website maintained by the SEC at www.sec.gov.
Forward Looking Statements
This Annual Report may include "forward-looking" statements. Forward-looking statements broadly involve our current expectations or forecasts of future results. Our forward-looking statements generally relate to our growth and growth strategies, financial results, product development, regulatory approvals, competitive strengths, intellectual property rights, litigation and tax matters, mergers and acquisitions, market acceptance of our products, accounting estimates, financing activities, ongoing contractual obligations and sales efforts. Such statements can be identified by the use of terminology such as "anticipate," "believe," "could," "estimate," "expect," "forecast," "intend," "may," "plan," "possible," "project," "should," "will" and similar words or expressions.
For more information about the nature of forward-looking statements and risks that could affect our future results and the disclosure provided in this Annual Report, please see "Part I—Item 1A—Risk Factors" and Exhibit 99.1—Forward-Looking Statements, which is incorporated herein by reference.
This section identifies specific risks that should be considered carefully in evaluating our business. Any of these risks could adversely affect our business, results of operations or financial condition. Although we believe that these risks represent the material risks relevant to us, our business and our industry, new material risks may emerge that we are currently unable to predict. As a result, this description of the risks that affect our business and our industry is not exhaustive. The risks discussed below could cause our actual results to differ materially from our historical results or the results contemplated by the forward-looking statements contained in Exhibit 99.1, "Forward-Looking Statements".
The restatement of our previous financial statements, the Audit Committee's review of certain accounting, financial reporting and insurance billing and healthcare compliance practices and the DOJ and other investigations, legal and administrative proceedings have increased our costs and could result in fines, injunctions, orders, and penalties which could materially adversely affect our business, financial condition, results of operations, and liquidity.
As previously reported, during 2009 and 2008, we experienced substantial delays in filing our periodic reports as a result of issues identified during a review of certain accounting and financial reporting matters as well as certain insurance billing and healthcare compliance practices conducted under the direction of our Audit Committee (the "Review"), which began in July 2008. The Review was completed in August 2009 and the results were reported to the Audit Committee and to the Board. In July 2008, we also commenced a separate comprehensive review of our accounting policies and procedures, financial reporting, internal controls and corporate governance. We completed this comprehensive review in November 2009, and as a result of our comprehensive review and the Review, we made extensive organizational and operational changes and improved our internal controls. As a result of errors and possible irregularities identified by the Review and management's review and analysis of certain accounting matters, we restated our financial statements for: the years ended December 31, 2007, 2006, 2005, and 2004; the quarter ended March 31, 2008 and each of the quarters for the years ended December 31, 2007 and 2006 in our Form 10-K for the year ended December 31, 2008 filed on November 18, 2009, or the 2008 Form 10-K.
13
We are involved in ongoing material legal proceedings and the actions currently pending result primarily from matters relating to our restatement and Review. See Note 11, "Litigation and Contingencies," in the notes to the consolidated financial statements. These proceedings and other legal actions may harm our business or liquidity in the future.
We have incurred substantial cost and expenses for legal and accounting services due to the Review and resulting Department of Justice, or DOJ, investigations. These matters will likely continue to divert our management's time and attention periodically and cause us to continue to incur substantial costs and expenses. Such investigations can also lead to fines or injunctions or orders with respect to future activities. We could incur substantial additional costs to defend and resolve litigation, investigations or proceedings arising out of or related to these matters. In addition, we could be exposed to enforcement or other actions with respect to these matters by the DOJ or other federal or state agencies. At this point, we are unable to predict the duration, scope or result of these investigations or whether any of these agencies will commence any legal action. Any such investigations may result in us being subject to criminal and civil penalties and other remedial measures, which could have an adverse impact on our business, results of operations, financial condition and liquidity.
On October 18, 2010, we, our directors' and officers' insurance carriers, and the plaintiffs in the Federal Court and State Court derivative actions agreed in principal to a settlement. Under the terms of the proposed settlement, the director and officer insurers will collectively pay us $8.0 million, on behalf of the individuals named as defendants in the Federal Court and State Court derivative actions, to settle the State and Federal Derivative actions. The lawyers for the plaintiffs will be entitled to seek an award of legal fees and costs from the Federal Court in an amount not to exceed $2.25 million. The agreement also provides that the directors' and officers' insurers will pay the Company an additional $2.0 million for a broad mutual release by us and the insurers of any further rights or obligations under the directors' and officers' liability insurance policies. The settlement calls for certain mutually acceptable governance changes and is subject to approval of the former officers and directors who are parties to the derivative actions, an agreement by those former officers and directors to release our directors' and officers' liability insurance carriers of any further rights or obligations under the applicable directors' and officers' liability insurance policies, the execution of a full and final settlement agreement, and the approval of the settlement of the derivative actions by the U.S. District Court, Western District of Texas. Upon approval of this settlement agreement, any further litigation cost or damages we incur that is based on the restatement or related events, including the ongoing DOJ investigation, the consolidated securities Class Action case described in Note 11, "Litigation and Contingencies" in the notes to the consolidated financial statements, and any ongoing indemnity obligations related to these matters will be funded without the benefit of further insurance coverage.
Many of our pending legal proceedings are in the early stages and we cannot predict their outcomes. However, irrespective of the outcomes, we may incur substantial costs, including defense and indemnity costs, and these matters may divert the attention of our technical and management personnel, which could materially harm our business. We may continue to incur ongoing indemnity obligations with respect to certain former officers of ours who are still subject to potential legal action against them. Moreover, if we do not prevail in any of the actions still pending against us, we could be required to pay substantial damages or settlement costs. Adverse outcomes or other developments during the course of litigation or other proceedings may harm our business, financial condition, results of operations or liquidity as well as investors' perception of our business, any of which could harm our stock price.
Our failure to comply with extensive government regulations could subject us to penalties and materially adversely affect our business, results of operations and financial condition.
Our medical device products and operations are subject to extensive regulation by the FDA and various other federal, state and foreign governmental authorities. Government regulations and foreign
14
requirements specific to medical devices are wide ranging and govern, among other things: design, development and manufacturing; testing, labeling and storage; clinical trials; product safety; marketing, sales and distribution; pre-market clearance and approval; record keeping procedures; advertising and promotions; recalls and field corrective actions; post-market surveillance, including reporting of deaths or serious injuries and malfunctions that, if they were to recur, could lead to death or serious injury; and product import and export.
We must also comply with the U.S. anti-kickback statute and the foreign corrupt practices act and similar foreign anti-bribery statutes in the sales and distribution of our products.
The FDA, DOJ and other governmental authorities have broad enforcement powers, and our failure to comply with these regulatory requirements could result in governmental agencies or a court taking action, including any of the following, which would adversely affect our business: issuing public warning letters to us; imposing fines and penalties on us; obtaining an injunction preventing us from manufacturing or selling our products; bringing civil or criminal charges against us; delaying the introduction of our products into the market; delaying pending requests for clearance or approval of new uses or modifications to our existing products; recalling, detaining or seizing our products; or withdrawing or denying approvals or clearances for our products.
If we fail to comply with complex and rapidly evolving laws and regulations, we could suffer civil and criminal penalties, be required to pay substantial damages and make significant changes to our products and operations.
During the past several years, the U.S. health care industry has been subject to an increase in governmental regulation at both the federal and state levels. We are subject to numerous federal and state regulations. As part of our internal compliance program, we review our sales and marketing materials, contracts and programs with counsel, and require employees and marketing representatives to participate in regular training. We also have adopted and train our personnel on the code of conduct for Interactions with Health Care Professionals promulgated by the Advanced Medical Technology Association, or AdvaMed, a leading trade association representing medical device manufacturers. Most recently, we amended our Code of Business Conduct and Ethics, the "Code of Ethics," in August 2009. Originally adopted in 2004 to qualify as a "code of ethics" within the meaning of Section 406 of the Sarbanes-Oxley Act of 2002, the Code of Ethics was amended to qualify as well as a "code of conduct" under the current Federal Sentencing Guidelines and "Compliance Program Guidance for Pharmaceutical Manufacturers" 68 FR 23731 (May 5, 2003) applicable to medical device companies as published by the Office of Inspector General for the Department of Human Services. The Code of Ethics applies to all of our directors, officers, employees and agents. It also serves as an essential element of our Corporate Compliance Program, as announced in August 2009. However, we can give no assurances that our compliance program will ensure that the Company is in compliance with existing or future applicable laws and regulations. If our compliance program fails to meet its objectives, or if we otherwise fail to comply with existing or future applicable laws and regulations, we could suffer civil or criminal penalties. We devote significant operational and managerial resources to comply with these laws and regulations. Different interpretations and enforcement policies of these laws and regulations could subject our current practices to allegations of impropriety or illegality or could require us to make significant changes to our products and operations. In addition, we cannot predict the impact of future legislation and regulatory changes on our business or assure you that we will be able to obtain or maintain the regulatory approvals required to operate our business.
15
Our accounting policies and methods require management to make judgments and estimates about matters that are inherently uncertain, which may result in our actual results differing significantly from those generated by our judgments and estimates.
We are required to exercise judgment in the application of our accounting policies and methods to comply with generally accepted accounting principles in the U.S., or GAAP, and to reflect management's judgment of the most appropriate manner to report our financial condition and results of operations. See Note 3, "Summary of Significant Accounting Policies" in the notes to the consolidated financial statements for a description of our significant accounting policies.
The accounting policies we have identified as critical to the presentation of our financial condition and results of operations are described in "Part II—Item 7—Management's Discussion and Analysis of Financial Condition and Results of Operations—Critical Accounting Policies and Estimates". We believe these policies are critical because they require management to make particularly subjective or complex judgments about matters that are inherently uncertain at the end of a period and because of the likelihood that materially different amounts would be reported under different conditions or using different assumptions. Due to the complexity of these critical accounting policies, our accounting methods relating to these policies involve substantial use of estimates. Estimates are inherently imperfect predictors of actual results because they are based on assumptions, including assumptions about future events. Our estimates may not include assumptions that reflect very positive or very negative market conditions and, accordingly, our actual results could differ significantly from those generated by our estimates. As a result, the estimates that we use to prepare our financial statements, as well as our estimates of our future results of operations, may be significantly inaccurate.
We may be the subject of federal, state and/or foreign government civil and criminal enforcement efforts against health care companies, which could result in civil and/or criminal penalties which could have a material negative impact on our operations and financial condition.
In addition to uncertainties surrounding coverage policies, both federal and state government agencies have heightened civil and criminal enforcement efforts against health care companies, as well as their executives and managers. These efforts generally relate to a wide variety of matters, including referral and billing practices, and implicate a variety of state and federal laws, including state insurance fraud provisions, common law fraud theories, as well as both state and federal fraud-and-abuse, anti-kickback, false claims statutes, and the foreign corrupt practices act and similar international anti-bribery laws and regulations. Although we no longer directly bill any payor, some of our prior activities could become the subject of additional governmental investigations or inquiries. In addition, even though we no longer directly bill any payor, we are still subject to health care fraud and abuse regulation and enforcement by both the federal government and the states in which we conduct our business.
The risk of our being found in violation of these laws is increased by the fact that many of them have not been fully interpreted by the regulatory authorities or the courts, and their provisions are open to a variety of interpretations. Moreover, recent health care reform legislation has strengthened these laws. For example, the Patient Protection and Affordable Care Act (PPACA) which was signed into law in March 2010, among other things, amends the intent requirement of the federal anti-kickback and criminal health care fraud statutes; a person or entity no longer needs to have actual knowledge of this statute or specific intent to violate it. In addition, PPACA provides that the government may assert that a claim including items or services resulting from a violation of the federal anti-kickback statute constitutes a false or fraudulent claim for purposes of the false claims statutes. Moreover, we expect there will continue to be federal and state laws and/or regulations, proposed and implemented, that could impact our operations and business. The extent to which future legislation or regulations if any relating to health care fraud abuse laws and/or enforcement may be enacted or what effect such legislation or regulation would have on our business remains uncertain. If our operations
16
are found to be in violation of any of the laws described above or any other governmental regulations that apply to us, we may be subject to penalties, including civil and criminal penalties, damages, fines, exclusion from governmental health care programs, and the curtailment or restructuring of our operations, any of which could adversely affect our ability to operate our business and our financial results.
We may in the future be subject to intellectual property claims, which are costly to defend, could require us to pay damages and could limit our ability to use certain technologies in the future.
The medical device industry has been characterized by extensive litigation regarding patents and other intellectual property rights, and companies in the medical device industry have employed intellectual property litigation to gain a competitive advantage.
We cannot assure you that we will not become subject to additional patent infringement claims or other litigation, including interference proceedings declared by the U.S. Patent and Trademark Office, or USPTO, to determine the priority of inventions. The defense and prosecution of intellectual property lawsuits, USPTO reexamination and interference proceedings and related legal and administrative proceedings, generally are costly and time-consuming. If other parties violate our proprietary rights, further litigation may be necessary to enforce our patents, to protect trade secrets or know-how we own or to determine the enforceability, scope and validity of the proprietary rights of others. Any litigation or reexamination or interference proceedings will be costly and cause significant diversion of effort by our technical and management personnel.
An adverse determination in existing claims, additional litigation or reexamination or interference proceedings to which we may become a party could subject us to significant liabilities to third parties, require disputed rights to be licensed from third parties or require us to cease using certain technology. Furthermore, we cannot assure you that we could obtain necessary licenses on satisfactory terms, if at all. Adverse determinations in judicial or administrative proceedings or failure to obtain necessary licenses could prevent us from manufacturing and selling our products, which would have a material adverse effect on our business, financial condition, results of operations, and future growth prospects.
Our intellectual property rights are valuable, and any inability to protect them could reduce the value of our products, business, financial condition, results of operations and future growth prospects.
Our ability to compete effectively depends in part on developing and maintaining the proprietary aspects of our Coblation technology and our acquired technologies. We believe that our issued patents are directed at the core technology used in our soft-tissue surgery systems, including both multi-electrode and single electrode configurations of our disposable devices, as well as the use of Coblation technology in specific surgical procedures. In addition, we believe that our issued patents are directed at many of the core features of our acquired technologies from Opus, Parallax, Atlantech and Applied Therapeutics, Inc. ("ATI"). Our earliest Coblation-related patents will begin to expire in 2012. The majority of our royalty income is associated with licenses that include core Coblation patent rights which extend through the end of 2014.
There is no assurance that the patents we have obtained, or any patents we may obtain as a result of our pending U.S. or international patent applications, will provide any competitive advantages for our products. We also cannot assure investors that those patents will not be successfully challenged, invalidated or circumvented prior to their expiration. In addition, we cannot provide assurance that competitors, many of which have substantial resources and have made substantial investments in competing technologies, have not already applied for or obtained, or will not seek to apply for and obtain, patents that will prevent, limit or interfere with our ability to make, use and sell our products either in the U.S. or in international markets. In Sports Medicine, many of our competitors have licensed our technology for limited fields of use. Patent applications are maintained in secrecy for a
17
period after filing. We may not be aware of all of the patents and patent applications potentially adverse to our interests.
A number of medical device and other companies, universities and research institutions have filed patent applications or have issued patents relating to monopolar and/or bipolar electrosurgical methods and apparatus. We have received, and we may receive in the future, notifications of potential conflicts of existing patents, pending patent applications and challenges to the validity of existing patents. In addition, we have become aware of, and may become aware of in the future, patent applications and issued patents that relate to our products and/or the surgical applications and issued patents and, in some cases, have obtained internal and/or external opinions of counsel regarding the relevance of certain issued patents to our products. We do not believe that our products currently infringe any valid and enforceable claims of the issued patents that we have reviewed. However, if third-party patents or patent applications contain claims infringed by our technology and such claims are ultimately determined to be valid, we may not be able to obtain licenses to those patents at a reasonable cost, if at all, or be able to develop or obtain alternative technology. Our inability to do either would have a material adverse effect on our business, financial condition, results of operations and future growth prospects. We cannot assure investors that we will not have to defend ourselves in court against allegations of infringement of third-party patents, or that such defense would be successful.
In addition to patents, we rely on trade secrets and proprietary know-how, which we seek to protect, in part, through confidentiality and proprietary information agreements. We require our key employees and consultants to execute confidentiality agreements upon the commencement of an employment or consulting relationship with us. These agreements generally provide that all confidential information, developed or made known to the individual during the course of the individual's relationship with us, is to be kept confidential and not disclosed to third parties. These agreements also generally provide that inventions conceived by the individual in the course of rendering services to us shall be our exclusive property. We cannot assure investors that employees will not breach such agreements, that we would have adequate remedies for any breach or that our trade secrets will not otherwise become known to or be independently developed by competitors.
We may not be able to keep pace with technological change or successfully develop new products with wide market acceptance, which could cause us to lose business to competitors.
We compete in a market characterized by rapidly changing technology. We may not be able to keep pace with technology or to develop viable new products. Our future financial performance will depend in part on our ability to develop and manufacture new products in a cost-effective manner, to introduce these products to the market on a timely basis, and to achieve market acceptance of these products. Factors which may result in delays of new product introductions or cancellation of our plans to manufacture and market new products include capital constraints, research and development delays, and delays in acquiring regulatory approvals. Our new products and new product introductions may fail to achieve expected levels of market acceptance. Factors impacting the level of market acceptance include our ability to successfully implement new technologies, the timeliness of our product introductions, our product pricing strategies, our available financial and technological resources for product promotion and development, our ability to show clinical benefit from our products, and the availability of coverage and reimbursement for our products as discussed in the risk factor titled "Changes in coverage and reimbursement for procedures using our products could affect physicians" below.
18
The markets for our products are intensely competitive, which may result in our competitors developing technologies and products that are more effective than ours or that make our technologies and products obsolete. Many of our competitors have significantly greater resources and market power than we do.
The markets for our current products in our core businesses are intensely competitive. We cannot assure you that other companies will not succeed in developing technologies and products that are more effective than ours, or that would render our technologies or products obsolete or uncompetitive in these markets.
Our primary competitors across all of our operating units consist of Medtronic, Inc., Smith & Nephew, Stryker Corporation, Johnson & Johnson, Olympus (through their subsidiary Gyrus), and Arthrex, Inc.. These competitors tend to be large, well-financed companies with diverse product lines who may have significantly greater financial, manufacturing, marketing, distribution and technical resources than we do. Some of these companies offer broad product lines that they may offer as a single package and frequently offer significant price discounts as a competitive tactic. Furthermore, some of our competitors utilize purchasing contracts that link discounts on the purchase of one product to purchases of other products in their broad product lines. Many of the hospitals in the U.S. have purchasing contracts with our competitors. Accordingly, customers may be dissuaded from purchasing our products instead of our competitors' products to the extent the purchase would cause them to lose discounts on our competitors' products. In addition, we are aware of several small companies offering alternative treatments for back pain and other ailments or indications that may indirectly compete with our products.
If we were to be unable to continue to compete for any of the above reasons, it could have a material adverse effect on our business, financial condition, results of operations and future growth prospects.
Until we resolve our contingencies, we may not have access to a cost effective credit facility and we will be required to fund a significant amount of cash to fund our working capital needs and planned expenditure with cash on hands. Our ability to generate cash depends on many factors beyond our control.
In the absence of a credit facility as a possible source of liquidity, our ability to meet our working capital needs and to fund capital expenditures will depend on our ability to generate cash from operations and effectively manage our cash balances. This is subject to general economic, financial, competitive and other factors that may be beyond our control. We are unable to predict the outcome of ongoing litigation and investigations to which we are a party and these matters could have a material adverse effect on our liquidity and cash flows. See Note 11, "Litigation and Contingencies," in the notes to the condensed consolidated financial statements for further discussion on legal proceedings. We expect that our cash flows from operations together with cash on hand will be sufficient to satisfy our short-term and, excluding the uncertainty related to the ongoing litigation and investigations to which we are a party, long-term normal operating liquidity requirements; however, we cannot assure you that our business will generate sufficient cash flow from operations in an amount sufficient to enable us to fund our other liquidity needs.
As a result, from time to time, we may be required to seek financing from alternative sources. We cannot assure you that such alternative funding will be available to us on terms and conditions acceptable to us, or at all.
In addition, we maintain deposit accounts with numerous financial institutions around the world in amounts that exceed applicable governmental deposit insurance levels. While we actively monitor our deposit relationships, the unanticipated failure of a financial institution in which we maintain deposits could cause us to suffer losses that could materially harm our results of operations and financial condition.
19
Any control deficiencies relating to our internal control over financial reporting could result in errors in our reported results and could have a material adverse effect on our operations, investor confidence in our business and the trading prices of our securities.
Internal control over financial reporting cannot provide absolute assurance of achieving financial reporting objectives because of its inherent limitations. Internal control over financial reporting is a process that involves human diligence and compliance and is subject to lapses in judgment and breakdowns resulting from human failures. Internal control over financial reporting can also be circumvented by collusion or improper management override. Because of such limitations, there is a risk that material misstatements will not be prevented or detected on a timely basis by internal control over financial reporting.
A failure to remain current in our filings may have material impacts on our business and liquidity.
If we are not able to remain current in our filings with the SEC, we will face several adverse consequences and restrictions. We will not be able to have a registration statement under the Securities Act of 1933, as amended (the "Securities Act"), covering a public offering of securities, declared effective by the SEC, or make offerings pursuant to existing registration statements; we will not be able to make an offering to any purchasers not qualifying as "accredited investors" under certain "private placement" rules of the SEC under Regulation D; we will not be eligible to use a "short form" registration statement on Form S-3 for a period of at least 12 months after the time we become current in our periodic and current reports under the Securities Exchange Act of 1934, as amended (the "Exchange Act"); we will not be able to deliver the requisite annual report and proxy statement to our stockholders to hold our annual stockholders meeting; our employees cannot be granted stock options, nor will they be able to exercise stock options registered on Form S-8, because Form S-8 would not be available to us; and our Common Stock may be delisted from the NASDAQ Stock Market. These restrictions may impair our ability to raise funds in the public markets, should we desire to do so, and to attract and retain employees.
In addition, pursuant to the Registration Rights Agreement between us and the holders of our Series A Preferred Stock, if our registration statement ceases to be available to the holders of our Series A Preferred Stock, a Registration Default occurs. If we are not able to cure this Registration Default in a timely manner we are obligated to pay the holders of the Series A Preferred Stock 2.00 percent per annum of the original liquidation preference of the Series A Preferred Stock, accruing from the date of the Registration Default until the Registration Default is cured.
Medical device reprocessors that reprocess our single-use devices and then provide them to customers at a lower cost than a new device may adversely affect our business.
Certain medical device reprocessors routinely collect used or opened but unused single-use devices from customers and reprocess and repackage these products to customers at a price lower than the price of a new device. These reprocessed devices may be confused with our authorized products, reduce our product sales and harm our reputation with key physicians who may not have knowledge that the devices have been reprocessed. In addition, this may increase time and expense spent investigating and addressing performance issues, including product liability issues, related to reprocessed devices. We may be required to incur additional expense to protect ourselves against performance claims associated with reprocessed devices and to enforce infringements by reprocessors of our proprietary and contractual rights.
20
Failure to obtain FDA clearance or approval for our products could materially adversely affect our business, results of operations, financial condition, and future growth prospects.
In the U.S., before we can market a new medical device, or a new use of, or claim for, or significant modification to, an existing product, we must first receive either premarket clearance under Section 510(k) of the U.S. Federal Food, Drug, and Cosmetic Act, or FDCA, or approval of a PMA submission from the FDA, unless an exemption applies. In the 510(k) clearance process, the FDA must determine that a proposed device is "substantially equivalent" to a device legally on the market, known as a "predicate" device, with respect to intended use, technology and safety and effectiveness, in order to clear the proposed device for marketing. Clinical data is sometimes required to support substantial equivalence. However, as was the case in our recent submission for 510(k) clearance of Coblator IQ ENT Plasma Wands, the FDA has a high degree of latitude when evaluating submissions and may determine that a proposed device submitted for 510(k) clearance is not substantially equivalent, or NSE, to a predicate device.
Recent trends in FDA's review of 501(k) submissions suggest that FDA is often requiring manufacturers to provide new, more expansive or different information regarding a particular device than what the manufacturer anticipated. This has resulted in increasing uncertainty and delay in the premarket notification review process. In January 2011, the FDA announced twenty-five specific action items it intends to take with respect to the 510(k) process to add greater transparency and certainty to the review process. These reforms, when fully implemented, could impose additional regulatory requirements upon us which could delay our ability to obtain new clearances, increase the costs of compliance, or restrict our ability to maintain current clearances.
An FDA NSE determination means that the device is automatically reclassified into Class III and we cannot market the device unless it is either approved through the PMA process or reclassified into Class I or Class II based on further submissions with supporting data. A new submission may require clinical data. The PMA approval pathway requires an applicant to demonstrate the safety and effectiveness of the device based, in part, on data obtained in clinical trials. Both of these processes can be expensive and lengthy and entail significant user fees, unless exempt. The FDA's 510(k) clearance process usually takes from three to twelve months, but it can last longer. The process of obtaining PMA approval is much more costly and uncertain than the 510(k) clearance process. It generally takes from one to three years, or even longer, from the time the PMA application is submitted to the FDA until an approval is obtained. There is no assurance that we will be able to obtain FDA clearance or approval for any of our new products on a timely basis, or at all. Failure to obtain FDA clearance or approval for our new products could materially adversely affect our business, results of operations, financial condition, and future growth prospects.
Modifications to our currently FDA-cleared products may require new regulatory clearance or approvals or require us to recall or cease marketing our current products until clearances or approvals are obtained.
After a device receives 510(k) premarket notification clearance from the FDA, any modification that could significantly affect its safety or effectiveness, or that would constitute a major change in the intended use of the device, technology, materials, packaging, and certain manufacturing processes may require a new 510(k) clearance or Premarket Approval, or PMA. The FDA requires every manufacturer to make the determination regarding the need for a new 510(k) clearance or PMA approval in the first instance, but the FDA may review any manufacturer's decision and may retroactively require the manufacturer to submit a premarket notification requesting 510(k) clearance or an application for PMA approval. The FDA also can require the manufacturer to cease marketing and/or recall the modified device until 510(k) clearance or approval is obtained. FDA guidance documents define when to submit premarket notifications for new or modified devices. These guidance documents also define modifications for which a new 510(k) is not required. We have modified some of our marketed devices, and have determined that in certain instances new 510(k) clearances or PMA
21
approvals are not required. No assurance can be made that the FDA would agree with any of our decisions not to seek 510(k) clearance or PMA approval. If the FDA requires us to cease marketing and/or recall the modified device until we obtain a new 510(k) clearance or PMA approval, our business, financial condition, results of operations and future growth prospects could be materially adversely affected.
We may be subject to fines, penalties or injunctions if we are determined to be promoting the use of our products for unapproved or "off-label" uses, which would adversely affect our financial condition.
Our promotional materials and training methods must comply with FDA regulations and other applicable laws and regulations, including the prohibition of the promotion of a medical device for a use that has not been cleared or approved by the FDA. Use of a device outside its cleared or approved indications is known as "off-label" use. Physicians may use our products off-label, as the FDA does not restrict or regulate a physician's choice of treatment within the practice of medicine. However, if the FDA determines that our promotional materials or training constitutes the promotion of an off-label use, it could subject us to certain sanctions ranging from a request that we modify our training or promotional materials to further regulatory or enforcement actions, including the issuance of an untitled letter, a warning letter, injunction, seizure, civil fine and/or criminal penalties against us or our officers or employees. It is also possible that other federal, state or foreign enforcement authorities might take action if they consider our promotional or training materials to constitute promotion of an unapproved use, which could result in significant fines or penalties under other statutory authorities, such as laws prohibiting false claims for reimbursement. In that event, our reputation could be damaged and adoption of the products would be impaired. Although our policy is to refrain from statements that could be considered off-label promotion of our products, the FDA or another regulatory agency could disagree and conclude that we have engaged in off-label promotion. In addition, the off-label use of our products may increase the risk of injury to patients, and, in turn, the risk of product liability claims. Product liability claims are expensive to defend and could divert our management's attention and result in substantial damage awards against us.
Our products may in the future be subject to product recalls that could harm our reputation, business operations and financial results, and if our products cause or contribute to a death or serious injury, we will be subject to medical device reporting regulations, which can result in voluntary corrective actions or FDA enforcement actions.
The FDA and similar foreign governmental authorities have the authority to require the recall of commercialized products in the event of material deficiencies or defects in design or manufacture or in the event that a product poses an unacceptable risk to health. Manufacturers may, under their own initiative, recall a product if any material deficiency in a device is found. A government mandated or voluntary recall by us or one of our distributors could occur as a result of an unacceptable risk to health, component failures, manufacturing errors, design or labeling defects or other deficiencies and issues. Recalls of any of our products would divert managerial and financial resources and have an adverse effect on our financial condition and results of operations. Any of these sanctions could impair our ability to produce our products in a cost-effective and timely manner in order to meet our customers' demands. We may also be required to bear other costs or take other actions that may have a negative impact on our future sales and our ability to generate profits.
Further, under the FDA medical device reporting regulations, or MDR, we are required to report to the FDA any incident in which our product may have caused or contributed to a death or serious injury or in which our product malfunctioned and, if the malfunction were to recur, would likely cause or contribute to death or serious injury. Any adverse event involving our products could result in future voluntary corrective actions, such as recalls or customer notifications, or agency action, such as inspection, mandatory recall or other enforcement action. Any corrective action, whether voluntary or
22
involuntary, as well as defending ourselves in a lawsuit, will require the dedication of our time and capital, distract management from operating our business, and may harm our reputation and financial results.
If we or our third-party suppliers fail to comply with the FDA's Quality System Regulations, our manufacturing operations could be interrupted and our sales and our ability to generate profits could suffer.
We and certain of our third-party suppliers are required to comply with the FDA's Quality System Regulation, or QSR, which covers the methods and documentation of the design, testing, production, control, quality assurance, labeling, packaging, sterilization, storage and shipping of our products. We and our suppliers are also subject to the regulations of foreign jurisdictions regarding the manufacturing process if we market our products in these jurisdictions. The FDA enforces the QSR through periodic and unannounced inspections of manufacturing facilities. If our facilities or those of our suppliers fail to take satisfactory corrective action in response to an adverse QSR inspection, the FDA could take enforcement action, including any of the following sanctions: untitled letters; warning letters, fines, injunctions, consent decrees and civil penalties; customer notifications or repair, replacement, refunds, recall, detention or seizure of our products; operating restrictions or partial suspension or total shutdown of production; refusing or delaying our requests for 510(k) clearance or premarket approval of new products or modified products; withdrawing 510(k) clearances on PMA approvals that have already been granted; refusal to grant export approval for our products; or criminal prosecution.
Any of these sanctions could impair our ability to produce our products in a cost-effective and timely manner in order to meet our customers' demands. We may also be required to bear other costs or take other actions that may have a negative impact on our future sales and our ability to generate profits.
We require foreign regulatory approvals to market and sell our products in other countries. Failure to obtain and maintain these regulatory approvals could harm our ability to generate revenue.
To be able to market and sell our products in other countries, we must obtain regulatory approvals and comply with the regulations of those countries. These regulations, including the requirements for approvals and the time required for regulatory review, vary from country to country. Obtaining and maintaining foreign regulatory approvals is expensive, and we cannot be certain that we will receive regulatory approvals in any foreign country in which we plan to market our products. If we fail to obtain or maintain regulatory approval in any foreign country in which we plan to market our products, our ability to generate revenue could be harmed.
Changes in coverage and reimbursement for procedures using our products could affect use of our devices and our future revenue.
The demand for our products is highly dependent on the policies of third-party payors such as Medicare, Medicaid, private insurance, and managed care organizations that reimburse our customers when they use our products. Failure by physicians, hospitals and other users of our products to obtain sufficient coverage and reimbursement from healthcare payors for procedures in which our products are used, or adverse changes in environmental and private third-party payors' policies toward coverage and reimbursement for such procedures would have a material adverse effect on our business, financial condition, results of operations and future growth prospects.
23
In the U.S., third-party payors continue to implement initiatives that restrict the use of certain technologies to those that meet certain clinical evidentiary requirements. We are aware of several third-party payors in the U.S., including governmental payors such as Medicare and Medicaid and private health insurance companies, who consider Coblation technology used in certain procedures to treat certain clinical conditions to be experimental or investigational. These payors have developed policies that deny coverage and therefore make no reimbursement for such procedures using our devices. Procedures using our devices that are not covered by some payors include such procedures as plasma disc decompression, or Coblation nucleoplasty, as well as Coblation or radiofrequency volumetric tissue reduction for (1) removing soft tissue during arthroscopic surgery, (2) hypertrophied nasal turbinates for the treatment of chronic nasal obstruction or obstructive sleep apnea and (3) soft palate and tongue for the treatment of obstructive sleep apnea. However, some payors in the U.S. provide coverage and reimbursement for Coblation tonsillectomy for certain clinical indications and percutaneous vertebroplasty for patients that meet specified criteria. In addition, some payors are moving toward a managed care system and control their health care costs by limiting authorizations for surgical procedures, including elective procedures using our devices. Failure to obtain favorable payor policies could have a material adverse effect on our business and operations.
In addition to uncertainties surrounding coverage policies, third-party payors from time to time update reimbursement amounts and also revise the methodologies used to determine reimbursement amounts. This includes annual updates to payments to ambulatory surgical centers and physicians for procedures using our products. Since reimbursement for our products generally is included as part of the payments for procedures, these updates directly impact the amounts recognized for the costs of our products. An example of payment updates is the Medicare program updates to physician payments, which is done on an annual basis using a prescribed statutory formula. In the past, when the application of the formula resulted in lower payment, Congress has passed interim legislation to prevent the reductions. For 2010, CMS projected a rate reduction of 21.2% under the statutory formula and a number of legislative measures were passed to prevent this reduction. For the second half of 2010, the update factor was increased by 2.2 percent. For 2011, the Medicare and Medicaid Extenders Act of 2010 which was signed into law on December 15, 2010, froze the 2010 update through 2011. Because CMS was required to make its other changes to the Medicare Physician Fee Schedule (including adjustments made to certain codes identified to be misvalued) budget neutral, CMS made a downward adjustment to what is known as the "conversion factor," which translates values into dollar amounts. Whereas the conversion factor for the end of 2010 was $36.8729, it is $33.9764 for 2011. At this time, is it uncertain how the change would affect our business. If Congress fails to intervene to prevent the negative update factor in the future, the resulting decrease in payment may adversely affect our revenues and results of operations.
New laws, regulations and judicial decisions, or new interpretations of existing laws, regulations and decisions that relate to healthcare availability, methods of delivery or payment for products and services, or sales, marketing or pricing may cause our revenue to decline. In addition, we may need to revise our research and development plans if a program or programs no longer are commercially viable. Such changes could cause our stock price to decline or experience periods of volatility.
The PPACA legislation, among other things, imposes significant new taxes on medical device makers which will result in a significant increase in the tax burden on our industry, and could have a material, negative impact on our results of operations and our cash flows. Other elements of this legislation such as comparative effectiveness research, an independent payment advisory board, payment system reforms including shared savings pilots and other provisions could significantly change the way healthcare is developed and delivered, and may materially impact many aspects of our business.
If we obtain the necessary international regulatory approvals, market acceptance of our products in international markets would be dependent, in part, upon the availability of coverage and
24
reimbursement within prevailing health care payment systems. Reimbursement and health care payment systems in international markets vary significantly by country and include both government sponsored health care and private insurance. We intend to seek international reimbursement approvals, although we cannot assure investors that any such approvals will be obtained in a timely manner, if at all. We are also unable to predict at this time the impact on our business of any future changes, if any, that are made to coverage and reimbursement policies by government action in key international markets.
Product liability claims could adversely impact our financial condition and impair our reputation.
Our business exposes us to potential product liability risks which are inherent in the design, manufacture and marketing of medical devices. In addition, many of our products are designed to be implanted in the human body for long periods of time. Component failures, manufacturing flaws, design defects or inadequate disclosure of product related risks or product related information with respect to these or other products we manufacture or sell could result in an unsafe condition or injury to a patient. The occurrence of such a problem could result in product liability claims or a recall of one or more of our products, which could ultimately result in the removal from the body of such products and claims regarding associated costs and damages, which could materially adversely impact our business, financial condition, results of operations, our ability to attract and retain customers for our products, and future growth prospects.
We cannot assure you that our current product liability insurance coverage limits are adequate to protect us from any liabilities we might incur in connection with the development, manufacture and sale of our products. In addition, we may require increased product liability coverage as products are successfully commercialized in additional applications. Product liability insurance is expensive and in the future may not be available to us on acceptable terms, if at all. A successful product liability claim or series of claims brought against us in excess of our insurance coverage could have a material adverse effect on our business, financial condition, results of operations, our ability to attract and retain customers for our products, and future growth prospects.
We are dependent on key suppliers, and supply disruptions could materially adversely affect our business, financial condition, results of operations and future growth prospects.
Some of the key components of our products are purchased from single vendors. If the supply of materials from a single source supplier were interrupted, replacement or alternative sources might not be readily obtainable due to the regulatory and other requirements applicable to our manufacturing operations or the availability of certain product drawings and/or specifications. A new or supplemental filing with applicable regulatory authorities may require us to obtain clearance prior to our marketing a product containing new material. This clearance process may take a substantial period of time and we cannot assure investors that we would be able to obtain the necessary regulatory approval for a new material to be used in our products on a timely basis, if at all. This could create supply disruptions that would materially adversely affect our business, financial condition, results of operations and future growth prospects.
In addition, we primarily use a subcontractor located in Costa Rica to sterilize our disposable devices. We have the ability to use alternative sterilization service providers for most of our products. If our Costa Rica sterilization service were to be disrupted for any reason, we would be required to use alternative sources with longer processing and logistics cycles, which could lead to a disruption in our ability to supply products for a period of time.
We are dependent on warehouses in the U.S. and Belgium owned and operated by Deutsche Post DHL, Inc. If our ability to use these warehouses is disrupted, we may be unable to supply products to our customers on a timely basis, which could materially adversely affect our business.
25
The complex nature of the underlying support technologies utilized to exchange critical product information with third party transportation and logistics providers, such as Electronic Data Interchange, has, at times, caused disruptions in our ability to deliver products to customers on a timely basis. Failure of these supporting systems could impair our business for the duration of the failure.
Our business is susceptible to risks associated with international operations.
International operations are generally subject to a number of risks, including: protectionist laws, business practices, licenses, tariffs and other trade barriers that favor local competition; multiple, conflicting and changing governmental laws and regulations, such as tax laws regulating intercompany transactions; difficulties in managing foreign operations, including staffing, seasonality of operations, dependence on local vendors, and collecting accounts receivable; loss of proprietary information due to piracy, misappropriation or weaker laws regarding intellectual property protection; foreign currency exchange rate fluctuations; and political and economic instability.
We derived 28.0 percent, 27.1 percent and 26.5 percent of our total product sales for the year ended December 31, 2010, 2009 and 2008, respectively, from customers located outside of the Americas. We expect international revenue to remain a significant percentage of total revenue and we believe that we must continue to expand our international sales activities to be successful. Historically, a majority of our international revenues and costs have been denominated in foreign currencies and we expect future international revenues and costs will be denominated in foreign currencies. Our international sales growth will be limited if we are unable to establish appropriate foreign operations, expand international sales channel management and support organizations, hire additional personnel, develop relationships with international sales representatives, and establish relationships with additional distributors. In that case, our business, operating results and financial condition could be materially adversely affected. Even if we are able to successfully expand international operations, we cannot be certain that we will be able to maintain or increase international market demand for our products.
Disruptions or other adverse developments at our Costa Rica facility could materially adversely affect our business.
Our high-volume disposable devices and controllers are manufactured at our Company owned facility in an industrial park in San Jose, Costa Rica. If our Costa Rica facility is not able to produce sufficient quantities of our controllers and products with adequate quality, or if our Costa Rica operations are disrupted for any reason, then we may be forced to locate alternative manufacturing facilities, including facilities operated by third parties. Disruptions may include, but are not limited to: changes in the legal and regulatory environment in Costa Rica; slowdowns or work stoppages within the Costa Rican customs authorities; acts of God (including but not limited to potential disruptive effects from an active volcano near the facility or earthquakes, hurricanes and other natural disasters); and other issues associated with significant operations that are remote from our headquarters and operations centers. Additionally, continued growth in product sales could outpace the ability of our Costa Rican operation to supply ordered products on a timely basis or cause us to take actions within our supply and manufacturing operations which increase costs, complexity and timing. Locating alternative facilities would be time-consuming, would disrupt our production and cause shipment delays and could result in damage to our reputation and profitability. Additionally, we cannot assure you that alternative manufacturing facilities would offer the same cost structure as our Costa Rica facility.
Future changes in technology or market conditions could result in adjustments to our recorded asset balance for intangible assets, including goodwill, resulting in additional non-cash charges that could significantly impact our operating results.
Our balance sheet includes significant intangible assets, including goodwill and other acquired intangible assets. The determination of related estimated useful lives and whether these assets are
26
impaired involves significant judgments. Our ability to accurately predict future cash flows related to these intangible assets may be adversely affected by unforeseen and uncontrollable events. In the highly competitive medical device industry, new technologies could impair the value of our intangible assets if they create market conditions that adversely affect the competitiveness of our products. We test our goodwill for impairment in the fourth quarter of each year, but we also test goodwill and other intangible assets for impairment at any time when there is a change in circumstances that indicates that the carrying value of these assets may be impaired. Any future determination that these assets are carried at greater than their fair value could result in substantial non-cash impairment charges, which could significantly impact our reported operating results.
Delaware law and provisions in our charter could make the acquisition of our company by another company more difficult, which could adversely affect our stock price.
Certain provisions of our certificate of incorporation and bylaws may have the effect of making it more difficult for a third party to acquire, or of discouraging a third party from attempting to acquire, control of our company. This could limit the price that certain investors might be willing to pay in the future for shares of our Common Stock. Certain provisions of our certificate of incorporation and bylaws allow us to issue preferred stock without any vote or further action by the stockholders, to eliminate the right of stockholders to act by written consent without a meeting, to specify advance notice and other procedures for director nominations by stockholders and submission of other proposals for consideration at stockholder meetings, and to eliminate cumulative voting in the election of directors. Certain provisions of Delaware law applicable to us could also delay or make more difficult a merger, tender offer or proxy contest involving us, including Section 203, which prohibits a Delaware corporation from engaging in any business combination with any interested stockholder for a period of three years unless certain conditions are met. The procedures required for director nominations and stockholder proposals and Delaware law could have the effect of delaying, deferring or preventing a change in control of the company, including without limitation, discouraging a proxy contest or making more difficult the acquisition of a substantial block of our Common Stock. These provisions of Delaware law, our certificate of incorporation, and our bylaws may have the effect of delaying or preventing changes in control or management of the Company, which could have an adverse effect on the market price of our stock.
One Equity Partners, a private equity firm, may have influence on our major corporate decisions.
As previously reported, on September 1, 2009, an affiliate of One Equity Partners, or OEP, a private equity firm, acquired our Series A Preferred Stock. See "Part II—Item 7—Management's Discussion and Analysis of Financial Condition and Results of Operations—Liquidity and Capital Resources" in our 2009 Form 10-K for a description of this transaction. As a result of this transaction, OEP became the largest beneficial owner of our stock. At this time, assuming conversion of the Series A Preferred Stock to our Common Stock, including make-whole amounts as defined in the Certificate of Designation for the Series A Preferred Stock but excluding outstanding options and warrants held by other parties, OEP would represent an ownership interest of approximately 17.6 percent of our voting stock. In connection with this transaction, we expanded the Board of Directors to eight members and granted OEP the right to nominate two directors. Effective September 1, 2009, Messrs. Gregory Belinfanti and Christian Ahrens, both partners of OEP, were appointed to our Board of Directors.
Consequently, OEP may have the ability to influence our Board of Directors and matters requiring stockholder approval, subject to the restrictions placed on OEP by the Securities Purchase Agreement, including without limitation OEP's agreement to vote for any director nominated by the Board and to comply with the terms of the standstill.
27
Since September 1, 2010, OEP is free to convert the Series A Preferred Stock into our Common Stock and, subject to the registration requirements of the federal securities laws, dispose of such stock, which could result in a decrease of the market price of our Common Stock, particularly if such dispositions are made in the open market and are substantial.
We are dependent upon key management, technical personnel and advisors, and loss of our key personnel and advisors could have a material adverse effect on our business and results of operations.
The loss of the services of one or more key employees or consultants could have a material adverse effect on us. Our success also depends on our ability to attract and retain additional highly qualified management and technical personnel. We face intense competition for qualified personnel, any of whom often receive competing employment offers. We cannot assure you that we will continue to be able to attract and retain such personnel. Loss of key personnel would materially impact our ability to meet our financial and operational objectives and could have a material adverse effect on our business and our results of operations.
Circumstances associated with our integration of acquisitions may adversely affect our operating results.
An element of our growth strategy in the future may be the pursuit of acquisitions of other businesses that expand or complement our existing products and distribution channels. Integrating businesses, however, involves a number of special risks, including: the possibility that management attention may be diverted from regular business concerns by the need to integrate operations; unforeseen costs, difficulties and liabilities in integrating our and the acquired company's employees, operations and systems; accounting, regulatory, or compliance issues that could arise in connection with, or as a result of, the acquisition of the acquired company; challenges in retaining our customers or the customers of the acquired company following the acquisition; the difficulty of incorporating acquired technology and rights into our products and services; and impairment charges if our acquisitions are not successful due to these risks.
In addition, we may finance future acquisitions by issuing equity securities, which may dilute the holdings of our current stockholders. If we are unable to successfully complete and integrate strategic acquisitions in a timely manner, our business, financial condition or operating results may be adversely affected.
Our operating results may fluctuate.
In our experience, our results of operations may fluctuate significantly from period to period. Adverse fluctuations due to these factors may adversely affect our level of revenues and profitability, results of operations, financial condition, and future growth prospects in the short and long term: the introduction of new product lines and increased penetration in existing applications; achievement of research and development milestones; the amount and timing of expenditures and the receipt and recognition of license fees; and timing of the receipt of orders and product shipments, absence of a backlog of orders, and the rate of product returns.
Factors may make the market price of our stock highly volatile.
The market price of our Common Stock could fluctuate substantially in the future. Investors may be unable to resell our Common Stock at or above their purchase price. This volatility may subject our stock price to material fluctuations due to the factors discussed in this "Risk Factors" section, and other factors including market reaction to acquisitions and trends in sales, marketing, and research and development; rumors or dissemination of false information; changes in coverage or earnings estimates by analysts; our ability to meet analysts' or market expectations; and sales of Common Stock by existing stockholders.
28
As previously reported, as of September 1, 2009, OEP owns all of our outstanding Series A Preferred Stock. Since September 1, 2010, OEP may convert the Series A Preferred Stock into our Common Stock and, subject to the registration requirements of the federal securities laws, dispose of such stock, which could result in a decrease of the market price of our Common Stock, particularly if such dispositions are made in the open market and are substantial.
ITEM 1B. UNRESOLVED STAFF COMMENTS
None.
We lease a facility in Austin, Texas, which serves as our corporate headquarters. The Austin, Texas facility is used for general and administrative purposes as well as for research and development activities. Our lease for this building will expire in June 2016. We lease a facility in Irvine, California, which is used for product and prototype development. This lease expires in March 2016. In Sunnyvale, California we lease a facility for research and development, general and administrative purposes and prototype manufacturing. Our lease for this building will expire in February 2014. We also lease, on a month-to-month basis, a warehouse in a neighboring building in Sunnyvale, California for warehousing and distribution. Internationally, we lease a facility in Stockholm, Sweden, which serves as our coordination center for international sales and marketing activities, which expires in 2014. We have access to a facility in Opglabbeek, Belgium that is used for our European repair center. In Costa Rica, we own a building located in a tax-advantaged business park. This facility serves as our principal manufacturing location for disposable devices and implants.
We believe these facilities, in addition to multiple less significant facilities leased in various countries around the world for sales, marketing and administrative purposes are in good condition, well maintained, and are suitable and adequate to carry on our business.
In addition to the matters specifically described below, we are involved in other legal and regulatory proceedings that arise in the ordinary course of business that do not have a material impact on our business. Litigation claims and proceedings of all types are subject to many factors that generally cannot be predicted accurately.
We record reserves for claims and lawsuits when they are probable and reasonably estimable. Except as otherwise specifically noted, we currently cannot determine the ultimate resolution of the matters described below. For matters where the likelihood or extent of a loss is not probable or cannot be reasonably estimated, we have not recognized in our consolidated financial statements the potential liability that may result from these matters. If one or more of these matters is determined against us, it could have a material adverse effect on our earnings, liquidity and financial condition.
We continue to gather additional facts and information related to insurance billing and healthcare compliance issues and marketing and promotional practices in connection with these legal and administrative proceedings with the assistance of legal counsel.
SEC Investigation
The Fort Worth Regional Office of the Securities and Exchange Commission's ("SEC") Division of Enforcement conducted a formal investigation into accounting matters related to the restatement of financial results as announced by us on July 21, 2008 and completed on November 18, 2009, when we filed our Annual Report on Form 10-K for the fiscal year ended December 31, 2008, in which we provided a detailed description of these matters with respect to the Review and the restatement. We
29
have entered a settlement with the SEC that fully resolves the SEC investigation against the Company. Under the settlement, we have consented to the entry by the SEC of an administrative order (the "Order"), on February 9, 2011, directing us to cease and desist from committing or causing violations of the reporting, books and records and internal control provisions of the federal securities laws in Sections 13(a), 13(b)(2)(A) and 13(b)(2)(B) of the Securities Exchange Act of 1934 and under Rules 12b-20, 13a-1 and 13a-13 promulgated under the Exchange Act. We consented to the entry of the Order without admitting or denying the Order's assertions of factual findings. No monetary penalty or fine was imposed on us, and none of our current officers or employees were charged.
DOJ Investigation
The DOJ is investigating certain activities including past sales, accounting, and billing procedures primarily in relation to the operation of our Spine product sales. The DOJ is also reviewing our relationship with our DiscoCare subsidiary. We are cooperating with this investigation. At this stage of the investigation, we cannot predict the ultimate outcome and we are unable to estimate any potential liability we may incur.
Private Securities Class Action and Shareholder Derivative Lawsuits
Federal Court Actions
On April 4, 2008, a putative securities class action was filed in Federal court in the Southern District of Florida against us and certain of our former executive officers, alleging violations of Sections 10(b) and 20(a) of the Exchange Act and Rule 10b-5 promulgated thereunder. Plaintiffs allege that the defendants violated federal securities laws by issuing false and misleading financial statements and making material misrepresentations regarding our internal controls, business, and financial results. On October 28, 2008, the court granted our motion to transfer this case to the U.S. District Court, Western District of Texas. (McIlvaine v. ArthroCare, et al).
On July 25, 2008, a putative securities class action was filed in Federal court in the Western District of Texas against us and certain of our current and former executive officers, alleging violations of Sections 10(b) and 20(a) of the Exchange Act and Rule 10b-5 promulgated thereunder. Plaintiffs allege that the defendants violated federal securities laws by issuing false and misleading financial statements and making material misrepresentations regarding our internal controls, business, and financial results. (Strong v. ArthroCare, et al).
On August 7, 2008, a derivative action was filed in Federal court in the Southern District of Florida against us and our then-current directors alleging breach of fiduciary duty based on our alleged improper revenue recognition, improper reporting of such revenue in SEC filings and press releases, failure to maintain adequate internal controls, and failure to supervise management. On October 14, 2008, the court granted our motion to transfer this case to the U.S. District Court, Western District of Texas. (Weil v. Baker, et al).
On March 4, 2009, a derivative action was filed in Federal court in the Western District of Texas against our then current directors, a former director, certain of our current and former executive officers and other employees and PricewaterhouseCoopers LLP alleging (i) disgorgement under Section 304 of the Sarbanes-Oxley Act; (ii) violations of Section 10(b) of the Exchange Act and Rule 10b-5; (iii) breach of fiduciary duty; (iv) abuse of control; (v) gross mismanagement of the Company; (vi) waste of corporate assets; (vii) insider trading; and (viii) unjust enrichment. (King v. Baker, et al).
On April 29, 2009, a derivative action was filed in Federal court in the Western District of Texas against our then current directors and a former director alleging breach of fiduciary duty based on improper revenue recognition, improper reporting of such revenue in SEC filings and press releases,
30
failure to maintain adequate internal controls, and failure to supervise management. (Barron v. Baker, et al).
On October 28, 2008, and thereafter, the two putative securities class actions and the shareholder derivative actions were consolidated and designated: In Re ArthroCare Corporation Securities Litigation, Case No. 1:08-cv-00574-SS (consolidated) in the U.S. District Court, Western District of Texas. On December 10, 2008, Lead Plaintiffs and Lead Plaintiffs' counsel were appointed in the putative consolidated securities class action. The Lead Plaintiff filed an Amended Consolidated Class Action Complaint on December 18, 2009, seeking unspecified monetary damages and interest. ArthroCare filed a Motion to Dismiss the Amended Consolidated Class Action Complaint on February 16, 2010. On July 20, 2010, the federal court issued a ruling granting in part and denying in part ArthroCare's Motion to Dismiss, permitting certain claims related to statements after December 11, 2007 to continue.
State Court Actions
On September 23, 2008, a derivative action was filed in Texas State District Court against us, and our then current directors and certain of our current and former officers. (Wieser v. Baker). In this action, one of our shareholders alleged derivative claims on behalf of us, that our directors and officers breached their fiduciary duties to shareholders by allowing improper financial reporting, failing to maintain adequate financial controls over revenue recognition, disseminating false financial statements, abuse of control, gross mismanagement, waste of corporate assets, and engaging in insider trading.
On October 20, 2008, a derivative action was filed in Texas State District Court against us, our then directors and certain of our current and former executive officers. (Bocklet v. Baker). In this action, one of the Company's shareholders alleged derivative claims on behalf of us, that our directors and officers breached their fiduciary duties to shareholders by failing to maintain adequate financial controls over revenue recognition, allowing improper financial reporting, disseminating false financial statements, and engaging in insider trading.
On October 27, 2008, a derivative action was filed in Texas State District Court against us, our then directors and certain of our current and former executive officers. (Guthrie v. Baker). In this action, one of our shareholders alleged derivative claims on behalf of us that our directors and officers breached their fiduciary duties to shareholders by failing to maintain adequate financial controls over revenue recognition, allowing improper financial reporting, disseminating false financial statements, and engaging in insider trading.
On March 18, 2009, these three shareholder derivative actions were consolidated and designated: In Re ArthroCare Corporation Derivative Litigation, Case No. D-1-GN-08-3484 (consolidated), Travis County District Court. The consolidated action seeks financial compensation, certain governance changes and removal of certain directors. On our motion, the state shareholder derivative actions were stayed pending resolution of all motions to dismiss the federal Amended Consolidated Class Action Complaint and class certification, if any, of a class in the federal Amended Consolidated Class Action Complaint.
Possible Settlement of Shareholder Derivative Actions
On October 18, 2010, we, our directors' and officers' insurance carriers, and the plaintiffs in the Federal Court and State Court derivative actions agreed in principal to a settlement. Under the terms of the proposed settlement, the director and officer insurers will collectively pay $8 million to us, on behalf of the individuals named as defendants in the Federal Court and State Court derivative actions, to settle the State and Federal Derivative actions. The lawyers for the plaintiffs will be entitled to seek an award of legal fees and costs from the Federal Court in an amount not to exceed $2.25 million. The agreement also provides that the directors' and officers' insurers will pay us an additional $2.0 million
31
for a broad mutual release by us and the insurers of any further rights or obligations under the directors' and officers' liability insurance policies. The settlement calls for certain mutually acceptable governance changes and is subject to approval of the former officers and directors who are parties to the derivative actions, an agreement by those former officers and directors to release our directors' and officers' liability insurance carriers of any further rights or obligations under the applicable directors' and officers' liability insurance policies, the execution of a full and final settlement agreement, and the approval of the settlement of the derivative actions by the U.S. District Court, Western District of Texas. Upon approval of this settlement agreement, we will incur any further litigation cost or damages allegedly resulting from or based on the restatement or related events, including the ongoing DOJ investigation, the consolidated securities Class Action case described in Note 11, "Litigation and Contingencies" and any ongoing indemnity obligations without the benefit of further insurance coverage.
ITEM 5. MARKET FOR REGISTRANT'S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
On January 19, 2010, Nasdaq accepted our application for listing on the Nasdaq Global Select Market and our shares began trading on the Nasdaq Global Select Market on January 22, 2010, under the symbol "ARTC". From January 16, 2009 until January 22, 2010 our securities were traded on an electronic quotation service maintained by Pink OTC Markets, Inc., or Pink Sheets, under the symbol "ARTC.PK". Prior to January 16, 2009 our common stock was publicly traded on The Nasdaq Stock Market LLC, or Nasdaq, under the symbol "ARTC." The following table sets forth, for the periods indicated, the quarterly high and low closing sales prices of our Common stock.
| |
December 31, 2010 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Quarter 1 | Quarter 2 | Quarter 3 | Quarter 4 | |||||||||
High |
$ | 30.92 | $ | 32.18 | $ | 29.94 | $ | 31.77 | |||||
Low |
23.30 | 26.31 | 25.21 | 26.79 | |||||||||
| |
December 31, 2009 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Quarter 1 | Quarter 2 | Quarter 3 | Quarter 4 | |||||||||
High |
$ | 8.42 | $ | 11.50 | $ | 20.55 | $ | 23.70 | |||||
Low |
2.95 | 4.90 | 10.99 | 18.00 | |||||||||
As of February 3, 2011, there were 27,228,270 shares of our common stock outstanding, and the closing price of our common stock was $28.83 per share.
As of December 31, 2010, there were 75,000 outstanding shares of preferred stock and 198 holders of record of 27,104,984 shares of outstanding common stock. We have not paid any cash dividends since our inception and do not anticipate paying cash dividends on our common stock or preferred stock in the foreseeable future.
On September 1, 2009, we sold 75,000 shares of Series A Preferred Stock to OEP AC Holdings LLC, an affiliate of OEP, for gross proceeds of $75.0 million. The sale of the Series A Preferred Stock was to an accredited investor and is exempt from registration under Rule 506 Regulation D of the Securities Act of 1933.
The holders of the Series A Preferred Stock may convert their shares at any time, in whole or in part, at a rate of 66.667 shares of the Company's Common Stock per $1,000 of Liquidation Preference of the Series A Preferred Stock, subject to customary anti-dilution adjustments (the "Conversion Rate"), representing an initial conversion price of $15.00 per share of Common Stock. If a conversion occurs prior to the expiration of the Dividend Duration Period, the number of shares of Common
32
Stock received shall be increased for a make-whole adjustment equal to the number of additional shares of Series A Preferred Stock the holder would have otherwise been paid during the Dividend Duration Period, multiplied by the Conversion Rate (the "Make-Whole Adjustment").
The Company may, at any time, cause an automatic conversion of all outstanding shares of Series A Preferred Stock upon no less than 10 days prior notice if the closing sales price of the Common Stock equals or exceeds $35.00 per share (subject to adjustment in the event of a stock split, stock dividend, combination or other similar recapitalization) for the prior 20 consecutive trading days, the Company is current on its reporting requirements with the SEC, the Company has an effective resale registration statement and related prospectus that permits the Common Stock issued upon such automatic conversion to be immediately resold thereunder, and the current investigation of the Company by the DOJ has been terminated, settled or finally adjudicated.
No conversion of Series A Preferred Stock will be permitted to the extent that any holder of Series A Preferred Stock would individually hold in excess of 19.99 percent of the Company's voting power after the proposed conversion, or to the extent that the holders of Series A Preferred Stock would hold in excess of 17.8 percent of the Company's voting power in the aggregate, in each case solely attributable to their holdings of Series A Preferred Stock and any Common Stock received upon conversion thereof (such limitations collectively, the "Conversion Cap"). Shares of Series A Preferred Stock not convertible as a result of the Conversion Cap shall remain outstanding and shall become convertible to the extent the Conversion Cap no longer applies.
We describe our equity compensation plans in our definitive Proxy Statement to be filed pursuant to Regulation 14A under the Exchange Act in connection with our annual meeting of stockholders scheduled to be held May 12, 2011, which is hereby incorporated herein by reference.
33
This performance graph shall not be deemed "filed" for purposes of Section 18 of the Exchange Act, or incorporated by reference into any filing of ArthroCare under the Securities Act or the Exchange Act, except as shall be expressly set forth by specific reference in such filing.
The following graph shows a comparison of total stockholder return for holders of our common stock for the five years ended December 31, 2010 compared with the Nasdaq Stock Exchange, U.S. Index and the Nasdaq Health Services Index. This graph is presented pursuant to SEC rules. The information below is based on $100 invested on December 31, 2005, including reinvestment of dividends with fiscal years ending December 31. The stock prices of medical device companies like ours are subject to a number of factors, such as those discussed in "Part I—Item 1A—Risk Factors," above.
COMPARISON OF 5 YEAR CUMULATIVE TOTAL RETURN*
Among ArthroCare Corporation, the NASDAQ Composite Index
and the NASDAQ Health Services Index
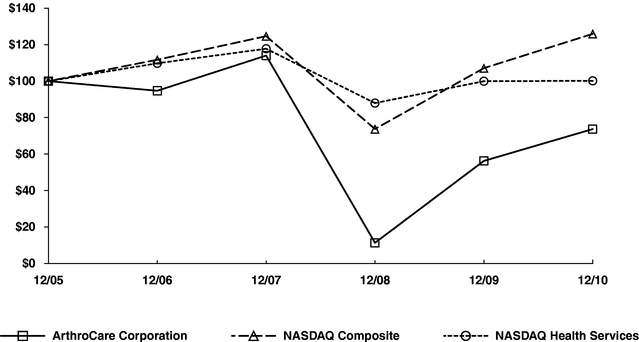
*$100 invested on 12/31/05 in stock or index, including reinvestment of dividends. Fiscal year ended December 31.
| |
Cumulative Total Return | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
12/2005 | 12/2006 | 12/2007 | 12/2008 | 12/2009 | 12/2010 | |||||||||||||
ArthroCare Corporation |
100.00 | 94.73 | 114.02 | 11.32 | 56.24 | 73.71 | |||||||||||||
NASDAQ Composite |
100.00 | 111.74 | 124.67 | 73.77 | 107.12 | 125.93 | |||||||||||||
NASDAQ Health Services |
100.00 | 109.80 | 117.78 | 87.97 | 99.96 | 100.19 | |||||||||||||
34
ITEM 6. SELECTED CONSOLIDATED FINANCIAL DATA
The following selected financial data (in thousands, except per share data) should be read in conjunction with "Part II—Item 7—Management's Discussion and Analysis of Financial Condition and Results of Operations" and the consolidated financial statements and notes thereto included in this Annual Report on Form 10-K. The statements of operations data for the years ended December 31, 2010, 2009 and 2008 and the balance sheet data as of December 31, 2010 and 2009 have been derived from audited consolidated financial statements included elsewhere in this report. The historical results are not necessarily indicative of the results of operations to be expected in the future.
| |
Year Ended December 31,(1) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2010 | 2009 | 2008 | 2007 | 2006 | ||||||||||||
Statements of Operations Data: |
|||||||||||||||||
Product sales(2) |
$ | 338,757 | $ | 308,493 | $ | 295,118 | $ | 261,632 | $ | 226,026 | |||||||
Royalties, fees and other |
16,622 | 15,624 | 12,165 | 11,099 | 10,015 | ||||||||||||
Total revenues |
355,379 | 324,117 | 307,283 | 272,731 | 236,041 | ||||||||||||
Gross profit |
244,628 | 233,177 | 214,638 | 188,234 | 168,055 | ||||||||||||
Operating expenses(3) |
190,358 | 233,124 | 238,509 | 192,957 | 134,115 | ||||||||||||
Net income (loss) from continuing operations |
37,518 | (5,296 | ) | (24,582 | ) | 455 | 19,342 | ||||||||||
Net income (loss) |
37,084 | (5,777 | ) | (35,025 | ) | 491 | 27,673 | ||||||||||
Net income (loss) applicable to common stockholders(4) |
33,820 | (33,822 | ) | (35,025 | ) | 491 | 27,673 | ||||||||||
Net earnings (loss) per share from continuing operations applicable to common stockholders |
|||||||||||||||||
Basic |
$ | 1.04 | $ | (1.24 | ) | $ | (0.92 | ) | $ | 0.02 | $ | 0.74 | |||||
Diluted |
$ | 1.03 | $ | (1.24 | ) | $ | (0.92 | ) | $ | 0.02 | $ | 0.69 | |||||
Net earnings (loss) per share applicable to common stockholders |
|||||||||||||||||
Basic |
$ | 1.03 | $ | (1.26 | ) | $ | (1.32 | ) | $ | 0.02 | $ | 1.06 | |||||
Diluted |
$ | 1.02 | $ | (1.26 | ) | $ | (1.32 | ) | $ | 0.02 | $ | 0.99 | |||||
| |
December 31, | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2010 | 2009 | 2008 | 2007 | 2006 | |||||||||||
Balance Sheet Data: |
||||||||||||||||
Cash, cash equivalents, restricted cash equivalents and investments |
$ | 132,536 | $ | 57,386 | $ | 37,980 | $ | 43,176 | $ | 30,756 | ||||||
Working capital |
191,939 | 106,267 | 43,406 | 121,991 | 97,395 | |||||||||||
Total assets |
439,195 | 391,128 | 389,485 | 397,947 | 365,791 | |||||||||||
Long-term liabilities |
13,979 | 5,147 | 10,058 | 66,919 | 4,196 | |||||||||||
Series A 3% Redeemable Convertible Preferred Stock(5) |
73,768 | 70,504 | — | — | — | |||||||||||
Total stockholders' equity |
295,728 | 250,395 | 246,491 | 281,053 | 309,923 | |||||||||||
- (1)
- In
the fourth quarter of 2010, our non-Coblation spine products, which include the Parallax and Contour product lines, met the criteria to be
classified as discontinued operations. As a result, the Selected Consolidated Financial Data for all prior periods have been adjusted to remove the product sales, expenses and net assets associated
with these product lines from products sales, total revenues, gross profit, operating expenses, net income (loss) from continuing operations, net earnings (loss) per share from continuing operations
applicable to common stockholders, and working capital. See Note 4—"Discontinued Operations" in the notes to the consolidated financial statements for more information.
- (2)
- Product
sales in 2010 include recognition of $6.6 million of previously deferred product sales and a $13.2 million increase in contract
manufacturing when compared to the same period in 2009.
- (3)
- Operating
expenses in 2009 include $31.7 million of investigation and restatement related costs. Operating expenses in 2008 include
$17.0 million of investigation and restatement related costs, $15.1 million charge related to the arbitration matter involving Gyrus and Ethicon and a $6.3 million impairment of
goodwill. In 2007, $34.1 million of costs related to reimbursement service activities were incurred, including $25.0 million for settlement of the DiscoCare pre-acquisition
agreement.
- (4)
- The 2010 net income applicable to common stockholders include a charge of $3.3 million related to the accretion of preferred stock discount and accrued dividends on the Series A Preferred Stock. The 2009 net loss applicable to common stockholders included a charge of $28.0 million related to the accretion of the beneficial conversion feature, accretion of preferred stock discount, and accrued dividends on the Series A Preferred Stock.
35
- (5)
- In 2009 we sold 75,000 shares of Series A Preferred Stock for gross proceeds of $75.0 million. See Note 12—"Mezzanine Equity" in the notes to the consolidated financial statements for more information.
ITEM 7. MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following discussion should be read in conjunction with the consolidated financial statements and notes thereto appearing elsewhere in this report. Readers should also review carefully "Part I—Item 1A—Risk Factors" and Exhibit 99.1—"Forward-Looking Statements," which provides information about the forward-looking statements in this report and a discussion of the factors that might cause our actual results to differ, perhaps materially, from these forward-looking statements.
Overview
We are a medical device company that develops, manufactures and markets surgical products, many of which are based on our minimally invasive patented Coblation technology. Our products are used across several medical specialties, improving many existing soft-tissue surgical procedures and enabling new minimally invasive surgical procedures. Our business is divided into two core product areas: Sports Medicine and ENT. We also manufacture and sell products based on our technology for other procedures, such as certain spine procedures. Each of the Sports Medicine, ENT and Other product areas market and sell their products in two principal geographic markets: Americas (North and South America) and International (all other geographies).
As previously reported, in 2009 and 2008, we experienced substantial delays in filing our periodic reports as a result of issues identified during the Review, which was completed in August 2009, and the restatement of prior financial statements, which was completed in November 2009 with the filing of our delinquent periodic reports. During the year ended December 31, 2009, our results of operations, financial position and cash flows were adversely affected by the effect that the Review and the restatement had on our management and employees and the significant costs of the Review and restatement.
First Quarter 2010 Accounting Corrections
Our results for the year ended December 31, 2010 include reductions of stock compensation expense to correct forfeiture calculation errors in prior periods. The impact of the error was an overstatement of operating expenses of $0.7 million in the year ended December 31, 2009 and $0.4 million in the year ended December 31, 2008. To correct this error, we recorded reductions of $0.8 million to general and administrative expense and $0.3 million to sales and marketing expense in the quarter ended March 31, 2010. We have determined that the impact of this uncorrected error is immaterial to all prior periods and the year ended December 31, 2010.
Key Financial Items, Trends and Uncertainties Affecting Our Business
Our management reviews and analyzes several metrics and ratios in order to manage our business and assess the quality of and potential variability of our operating performance. The most important of these financial metrics and ratios include:
Product Sales Growth
Our principal source of revenue is from sales of our products, which primarily include disposable surgical devices and implants. Product sales growth in 2010, 2009, and 2008 was 9.8 percent, 4.5 percent and 12.8 percent, respectively. We also contract manufacture disposable surgical devices for use in Sports Medicine procedures for another medical devices company which is included in product sales. During the year ended December 31, 2010, product sales from contract manufacturing was
36
$25.6 million compared to $12.4 million in 2009. Contract manufactured products sold in 2010 may not be indicative of contract manufactured products in the future. Product sales growth in 2010, 2009, and 2008 related to our proprietary products which are sold through our direct sales employees, independent sales agents and independent distributors was 5.1 percent, 2.8 percent and 13.1 percent, respectively.
We anticipate that our proprietary disposable device sales and implants will remain the key component of our product sales for the foreseeable future. We also generate revenue from royalties and fees from licensing of our products and technology to other companies and earn other revenues from shipping and handling costs billed to customers.
Gross Product Margin
Gross product margin as a percentage of product sales in 2010, 2009, and 2008 was 67.3 percent, 70.5 percent and 68.6 percent, respectively. Cost of product sales consists of all product manufacturing costs (including material costs, labor costs, manufacturing overhead, warranty and other direct product costs), adjustments to the carrying value of inventory for excess or obsolete items, certain stock based compensation costs associated with manufacturing and operations personnel and costs of product shipped to our customers. Cost of product sales also includes the amortization of controller units that have been placed at customer locations to enable the use of our disposable surgical products. We maintain ownership of all placed controllers and the cost of controller units are capitalized and amortized into cost of product sales over the estimated useful life of the controller unit. Most of our manufactured products are produced at our Costa Rica facility which has experienced stable operations. Raw materials used to produce our products are generally not subject to substantial commodity price volatility.
The comparability of gross product margin between periods will be impacted by several items, including the mix between proprietary and contract manufactured product sales; the stability of our average sales price on proprietary products; changes in the estimated amount of research and engineering activities related to manufacturing process design or improvement; and, changes in our product emphasis which could result in obsolescence charges being included in the cost of product sales in a particular period.
Operating Margin
Operating margin is our income (loss) from operations as a percentage of total revenues. Our key operating expenses include expenses incurred in connection with research and development, sales and marketing, general and administrative and amortization of intangible assets. During 2009 and 2008 our operating expenses were significantly impacted by the substantial expense incurred in connection with the Review and the restatement of our financial statements and goodwill impairment. Operating margin in 2010, 2009, and 2008 was positive 15.3 percent, 0.0 percent, and negative 7.8 percent, respectively.
We expect to continue to spend approximately $9 to $10 million each quarter in 2011 in research, engineering, new product development, regulatory affairs, and clinical studies. We anticipate that sales and marketing spending could temporarily increase as a percentage of total revenue if we undertake initiatives to expand our direct sales and distribution capabilities. Over the long-term, we expect sales and marketing expense as a percentage of total revenue to remain approximately the same as our current experience.
Net Earnings
Net earnings represent the net income or loss in the period. Net earnings will be affected by the same trends that impact our revenues, gross product margin and operating margin. In addition, net earnings will also be affected by other income and expenses, such as interest expense, foreign currency
37
gains and losses and income taxes. Interest expense and bank fees were lower in the year ended December 31, 2010 as we repaid all outstanding bank borrowings on September 1, 2009 and we anticipate this trend to continue until we enter into credit facilities in the future. We expect that we will report foreign currency gains or losses each period due primarily to changes in the value of the euro, British pound and Australian dollar versus the U.S. dollar.
Our effective income tax rate is less than the U.S. statutory rate as many of the jurisdictions in which we operate have lower corporate statutory tax rates, including Costa Rica, where we have a tax holiday that extends through December 2015. In years of loss, our effective tax rate may exceed the U.S. statutory rate depending on the apportionment of income or loss between jurisdictions in which we operate. We expect to be able to fully utilize our deferred tax assets.
38
Results of Operations
Overview of our Results of Operations
Results of operations for the three years ending December 31, 2010, 2009 and 2008 (in thousands, except percentages and per-share data) were as follows:
| |
Year Ended December 31,(1) | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2010 | 2009 | 2008 | |||||||||||||||||
| |
Dollars | % Total Revenue |
Dollars | % Total Revenue |
Dollars | % Total Revenue |
||||||||||||||
Revenues: |
||||||||||||||||||||
Product sales |
$ | 338,757 | 95.3 | % | $ | 308,493 | 95.2 | % | $ | 295,118 | 96.0 | % | ||||||||
Royalties, fees and other |
16,622 | 4.7 | % | 15,624 | 4.8 | % | 12,165 | 4.0 | % | |||||||||||
Total revenues |
355,379 | 100.0 | % | 324,117 | 100.0 | % | 307,283 | 100.0 | % | |||||||||||
Cost of product sales |
110,751 | 31.2 | % | 90,940 | 28.1 | % | 92,645 | 30.1 | % | |||||||||||
Gross profit |
244,628 | 68.8 | % | 233,177 | 71.9 | % | 214,638 | 69.9 | % | |||||||||||
Operating expenses: |
||||||||||||||||||||
Research and development |
35,846 | 10.1 | % | 35,203 | 10.9 | % | 31,930 | 10.4 | % | |||||||||||
Sales and marketing |
107,852 | 30.3 | % | 114,539 | 35.4 | % | 125,502 | 40.9 | % | |||||||||||
General and administrative |
38,491 | 10.8 | % | 46,034 | 14.2 | % | 50,359 | 16.4 | % | |||||||||||
Amortization of intangible assets |
5,237 | 1.5 | % | 5,309 | 1.6 | % | 5,479 | 1.8 | % | |||||||||||
Reimbursement services |
92 | 0.0 | % | 353 | 0.1 | % | 1,966 | 0.6 | % | |||||||||||
Impairment of goodwill |
— | 0.0 | % | — | 0.0 | % | 6,252 | 2.0 | % | |||||||||||
Investigation and restatement—related costs |
2,840 | 0.9 | % | 31,686 | 9.7 | % | 17,021 | 5.5 | % | |||||||||||
Total operating expenses |
190,358 | 53.6 | % | 233,124 | 71.9 | % | 238,509 | 77.6 | % | |||||||||||
Income (loss) from operations |
54,270 | 15.3 | % | 53 | 0.0 | % | (23,871 | ) | (7.8 | )% | ||||||||||
Interest and other expense, net |
(3,864 |
) |
(2,212 |
) |
(8,886 |
) |
||||||||||||||
Income (loss) from continuing operations before income taxes |
50,406 | (2,159 | ) | (32,757 | ) | |||||||||||||||
Income tax provision (benefit) |
12,888 |
3,137 |
(8,175 |
) |
||||||||||||||||
Net income (loss) from continuing operations |
37,518 | (5,296 | ) | (24,582 | ) | |||||||||||||||
Loss from discontinued operations, net of taxes |
(434 |
) |
(481 |
) |
(10,443 |
) |
||||||||||||||
Net income (loss) |
37,084 | (5,777 | ) | (35,025 | ) | |||||||||||||||
Accrued dividend, beneficial conversion feature and accretion charges on Series A 3% Convertible Preferred Stock |
(3,264 | ) | (28,045 | ) | — | |||||||||||||||
Net income (loss) applicable to common stockholders |
$ | 33,820 | $ | (33,822 | ) | $ | (35,025 | ) | ||||||||||||
Weighted-average shares outstanding: |
||||||||||||||||||||
Basic |
27,006 | 26,803 | 26,610 | |||||||||||||||||
Diluted |
27,348 | 26,803 | 26,610 | |||||||||||||||||
Earnings (loss) per share from continuing operations applicable to common stockholders: |
||||||||||||||||||||
Basic |
$ | 1.04 | $ | (1.24 | ) | $ | (0.92 | ) | ||||||||||||
Diluted |
$ | 1.03 | $ | (1.24 | ) | $ | (0.92 | ) | ||||||||||||
Earnings (loss) per share applicable to common stockholders: stockholders: |
||||||||||||||||||||
Basic |
$ | 1.03 | $ | (1.26 | ) | $ | (1.32 | ) | ||||||||||||
Diluted |
$ | 1.02 | $ | (1.26 | ) | $ | (1.32 | ) | ||||||||||||
- (1)
- In the fourth quarter of 2010, we determined that our non-Coblation spine products met the criteria under generally accepted accounting principles to be reported as discontinued operations. Our non-Coblation spine products consist of our Parallax and Contour product lines. As a result of this determination, reported product sales for the years ended December 31, 2010, 2009, and 2008 have been reduced by $5.9 million, $7.5 million, and $10.5 million, respectively and the impact from these discontinued product lines on our current and previously reported net income (loss) in all periods is separately reported as "Loss from discontinued operations, net of taxes" in our Consolidated Statements of Operations.
39
2010 Comparison with 2009
Product Sales
Product sales consist principally of sales of disposable surgical devices and implants. Product sales by each of our product groups and geographic markets for the years ended December 31, 2010 and 2009 are as follows (in thousands, except percentages):
| |
Year Ended December 31, 2010 | Year Ended December 31, 2009 | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Americas | International | Total Product Sales |
% Net Product Sales |
Americas | International | Total Product Sales |
% Net Product Sales |
|||||||||||||||||
Sports Medicine |
$ | 160,859 | $ | 71,120 | $ | 231,979 | 68.5 | % | $ | 147,169 | $ | 62,533 | $ | 209,702 | 67.9 | % | |||||||||
ENT |
79,019 | 15,270 | 94,289 | 27.8 | % | 72,375 | 13,299 | 85,674 | 27.8 | % | |||||||||||||||
Other |
4,122 | 8,367 | 12,489 | 3.7 | % | 5,231 | 7,886 | 13,117 | 4.3 | % | |||||||||||||||
Total Product Sales |
$ | 244,000 | $ | 94,757 | $ | 338,757 | 100.0 | % | $ | 224,775 | $ | 83,718 | $ | 308,493 | 100.0 | % | |||||||||
% Net Product Sales |
72.0 | % | 28.0 | % | 100.0 | % | 72.9 | % | 27.1 | % | 100.0 | % | |||||||||||||
Sports Medicine product sales increased 10.6 percent, or $22.3 million, in 2010 compared to 2009. Product sales in the Americas increased by $13.7 million, due largely to a $13.2 million increase in contract manufacturing volume pursuant to an existing supply and distribution agreement with Smith & Nephew and included $6.6 million for the recognition of product sales from prior periods that were deferred pending resolution of certain contract issues with customers which occurred during the first quarter of 2010. Excluding the recognition of deferred revenue, proprietary product sales in the Americas decreased $6.1 million, or 4.2 percent, in 2010. Approximately half of this decline we believe is attributable to lower sales volume due to an overall slowdown in the underlying Sports Medicine procedure utilization in the United States and half of the decline is related to lower average sales prices. International product sales increased $8.6 million, due primarily to volume increases in our direct markets.
ENT product sales increased 10.1 percent, or $8.6 million, in 2010 compared to 2009. Product sales increased in the Americas as a result of increased Coblation product sales volumes, increased Rapid Rhino product sales volumes and higher average sales price. International product sales increased primarily due to higher sales to distributors, particularly in the Asia Pacific region.
Other product sales decreased $0.6 million, or 4.8 percent, in 2010 compared to 2009. In the Americas, PDD SpineWand sales continue to be negatively affected by the Centers for Medicare & Medicaid Services, or CMS, negative reimbursement determination in September 2008. International product sales increased primarily as a result of increased sales volumes in our direct international markets.
In total, International product sales across all product groups increased $11.0 million or 13.2 percent during 2010 when compared to 2009. Product sales increased by approximately $8.7 million in 2010 in our direct International markets, while sales to distributors increased by approximately $2.3 million. For the year ended December 31, 2010, changes in foreign currency rates had a negligible effect on product sales when compared to 2009.
Royalties, Fees and Other Revenues
Royalties, fees, and other revenues consist mainly of revenue from the licensing of our products and technology along with shipping and handling costs billed to customers. Such revenues increased $1.0 million in 2010 compared to 2009. The increase was primarily a result of increased royalties from third parties that use our radio frequency, or RF, technology pursuant to a license.
40
Cost of Product Sales
Cost of product sales for the years ended December 31, 2010 and 2009 were as follows (in thousands, except percentages):
| |
Year Ended December 31, | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2010 | 2009 | |||||||||||
| |
Dollars | % Net Product Sales |
Dollars | % Net Product Sales |
|||||||||
Product cost |
$ | 91,931 | 27.1 | % | $ | 74,046 | 24.0 | % | |||||
Controller amortization |
10,019 | 3.0 | % | 9,108 | 3.0 | % | |||||||
Other |
8,801 | 2.6 | % | 7,786 | 2.5 | % | |||||||
Total Cost of Product Sales |
$ | 110,751 | 32.7 | % | $ | 90,940 | 29.5 | % | |||||
Gross product margin as a percentage of product sales for 2010 was 67.3 percent in 2010 compared to 70.5 percent in 2009. Gross product margin was lower in 2010 due to increased revenue from contract manufactured product which is sold at a lower average sales price, but for which we do not incur sales commission or marketing expense. Additionally, charges for excess and obsolete inventory increased $5.8 million in 2010 compared to 2009 as we chose to de-emphasize certain handhelds and instrumentation sets associated with our Sports Medicine Atlantech product line.
Operating Expenses
Operating expenses for the periods shown were as follows (in thousands, except percentages):
| |
Year Ended December 31, | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2010 | 2009 | ||||||||||||
| |
Dollars | % Total Revenue |
Dollars | % Total Revenue |
||||||||||
Research and development |
$ | 35,846 | 10.1 | % | $ | 35,203 | 10.9 | % | ||||||
Sales and marketing |
107,852 | 30.3 | % | 114,539 | 35.3 | % | ||||||||
General and administrative |
38,491 | 10.8 | % | 46,034 | 14.2 | % | ||||||||
Amortization of intangible assets |
5,237 | 1.5 | % | 5,309 | 1.6 | % | ||||||||
Reimbursement services |
92 | 0.0 | % | 353 | 0.1 | % | ||||||||
Investigation and restatement- |
||||||||||||||
related costs |
2,840 | 0.9 | % | 31,686 | 9.8 | % | ||||||||
Total operating expenses |
$ | 190,358 | 53.6 | % | $ | 233,124 | 71.9 | % | ||||||
Sales and marketing expense decreased $6.7 million, to 30.3 percent of total revenues in 2010 compared to 35.3 percent of total revenues in 2009. Sales and marketing expense as a percentage of total revenues decreased as a result of deferred revenue that was recognized in the first quarter of 2010 and an increase in contract manufacturing product revenues, neither of which incurred selling expenses such as commissions. Sales and marketing expenses decreased in 2010 as well due to a result of lower product demonstration costs and a decrease in bad debt allowance requirements due to improved cash collection of accounts receivable. Additionally, sales and marketing expense in the Americas decreased in 2010 compared to 2009 as we restructured our distribution channel for our spine products from primarily a direct sales organization to a network of independent agents.
General and administrative expense decreased $7.5 million, to 10.8 percent of total revenues in 2010 compared to 14.2 percent of total revenues in 2009. The decrease in expenses was primarily a result of a decrease in legal fees related to certain patent matters that were settled in December 2009, partially offset by an increase in accounting and compliance personnel.
41
Investigation and restatement expense was $2.8 million in 2010 compared to $31.7 million in 2009. The decrease is a result of completing the Review and restatement in November of 2009. We expect to continue to incur legal defense costs in connection with the DOJ and shareholder class action and shareholder derivative matters arising from the Review and restatement and the defense costs will continue to be classified as investigation and restatement-related expense in future periods as incurred.
Interest and Other Expense, Net
Interest and other expense for the periods shown were as follows (in thousands, except percentages):
| |
Year Ended December 31, | ||||||
|---|---|---|---|---|---|---|---|
| |
2010 | 2009 | |||||
Interest and other income, net |
$ | 216 | $ | 312 | |||
Interest expense |
(769 | ) | (3,757 | ) | |||
Foreign exchange gain (loss), net |
(3,311 | ) | 1,233 | ||||
Interest and other expense, net |
$ | (3,864 | ) | $ | (2,212 | ) | |
Interest expense decreased $3.0 million in 2010 from $3.8 million in 2009, primarily as a result of repaying outstanding borrowings under our credit agreement in September 2009. We expect that we will report foreign currency gains or losses each period due primarily to changes in the value of the euro, British pound and Australian dollar versus the U.S. dollar.
Discontinued Operations
In the fourth quarter of 2010, we determined that our non-Coblation spine products met the criteria under generally accepted accounting principles to be reported as discontinued operations. Our non-Coblation spine products consist of our Parallax and Contour product lines. As a result of this determination, reported product sales for the years ended December 31, 2010, 2009, and 2008 have been reduced by $5.9 million, $7.5 million, and $10.5 million, respectively and the impact from these discontinued product lines on our current and previously reported net income (loss) in all periods is separately reported as "Loss from discontinued operations, net of taxes" in our Consolidated Statements of Operations. Net assets of $3.1 million identifiable with these discontinued product lines is reported in our December 31, 2010 Consolidated Balance Sheet as "Assets held for sale".
Income Tax Provision (Benefit)
Our effective tax rate in 2010 was 25.6 percent. Lower foreign tax rates reduced income tax expense in 2010 by $6.6 million when compared to the U.S. corporate tax rate, and R&D credits reduced income tax expense by $1.3 million. In January 2009, the U.S. Treasury issued temporary cost-sharing regulations and as a result, we decided to adjust our intercompany payments for all open tax years. As a result of these adjustments to our intercompany payments, we utilized approximately $1.9 million of our reserves for uncertain tax positions and recorded additional income tax expense of approximately $3.6 million in 2009. Adjusted for the effect of this change in regulations, our effective tax rate for 2009 was 21.5 percent.
Accrued Dividends, Beneficial Conversion Feature and Accretion Charges on Series A 3% Preferred Stock
Accrued dividends, beneficial conversion feature and accretion charges on Series A 3% Convertible Preferred Stock decreased to $3.3 million in 2010 compared to $28.0 million in 2009. We accrued $3.3 million of dividends and accretion charges for the full year in 2010 compared to $1.0 million in 2009 for the period from September 1, 2009 (the date of issuance) to the end of the year. In 2009 we
42
also recognized a beneficial conversion feature of $27.0 million on the sale of the Series A Preferred Stock that was immediately charged against net loss applicable to common stockholders.
2009 Comparison with 2008
Product Sales
Product sales consist principally of sales of disposable surgical devices and implants. Product sales by each of our product groups and geographic markets for the years ended December 31, 2009 and 2008 are as follows (in thousands, except percentages):
| |
Year Ended December 31, 2009 | Year Ended December 31, 2008 | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Americas | International | Total Product Sales |
% Net Product Sales |
Americas | International | Total Product Sales |
% Net Product Sales |
|||||||||||||||||
Sports Medicine |
$ | 147,169 | $ | 62,533 | $ | 209,702 | 67.9 | % | $ | 142,856 | $ | 56,676 | $ | 199,532 | 67.6 | % | |||||||||
ENT |
72,375 | 13,299 | 85,674 | 27.8 | % | 68,412 | 12,072 | 80,484 | 27.3 | % | |||||||||||||||
Other |
5,231 | 7,886 | 13,117 | 4.3 | % | 5,772 | 9,330 | 15,102 | 5.1 | % | |||||||||||||||
Total Product Sales |
$ | 224,775 | $ | 83,718 | $ | 308,493 | 100.0 | % | $ | 217,040 | $ | 78,078 | $ | 295,118 | 100.0 | % | |||||||||
% Net Product Sales |
72.9 | % | 27.1 | % | 100.0 | % | 73.5 | % | 26.5 | % | 100.0 | % | |||||||||||||
Sports Medicine product sales increased 5.1 percent primarily as a result of International market sales growth of 10.3 percent and higher contract manufacturing volume. Sports Medicine product sales increased 3.0 percent in the Americas market. Approximately half of the increase was from higher contract manufacturing volume pursuant to a supply and distribution agreement with Smith & Nephew. In the U.S., which is the largest component of our Americas market, Sports Medicine product sales were also influenced by slowed procedure volume growth in the first half of 2009 caused by weaker general economic conditions.
ENT product sales increased by 6.4 percent as a result of higher Coblation sales both in the Americas and International markets. The increase in Coblation sales is attributed to greater market share in tonsillectomy and adenoidectomy procedures.
Other product sales decreased $2.0 million in 2009 compared to 2008. In the Americas, PDD SpineWand sales were negatively affected by a Centers for Medicare & Medicaid Services, or CMS, negative reimbursement determination in September 2008 as well as management's decision in the fourth quarter of 2008 to cease our reimbursement assistance service activities which had previously been a significant distribution focus for Spine in the Americas. International Spine sales were negatively affected by reduced private insurance reimbursement and weaker general economic conditions in Europe.
Across all product areas, the U.S. dollar reported value of International product sales increased 7.2 percent in 2009. The translation effect of changes in foreign currency rates reduced the 2009 U.S. dollar reported value of local currency product sales by $5.4 million. In 2009, product sales increased in existing direct International markets, we expanded our direct market presence in certain European countries including Switzerland and Scandinavian countries, and product sales increased to distributors in the Asia Pacific region.
Royalties, Fees and Other Revenues
Royalties, fees, and other revenues consist mainly of revenue from the licensing of our products and technology, plus shipping and handling costs billed to customers. Royalties, fees, and other
43
revenues increased to $15.6 million in 2009 compared to $12.2 million in 2008. 2009 includes a lump sum royalty settlement received in the fourth quarter of $2.5 million. Adjusted for this lump sum settlement, royalties, fees, and other revenues increased 7.4 percent primarily as a result of increased sales by third parties of products that use our RF technology pursuant to a license.
Cost of Product Sales
Cost of product sales for the periods shown were as follows (in thousands, except percentages):
| |
Year Ended December 31, | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2009 | 2008 | |||||||||||
| |
Dollars | % Net Product Sales |
Dollars | % Net Product Sales |
|||||||||
Product cost |
$ | 74,046 | 24.0 | % | $ | 76,183 | 25.8 | % | |||||
Controller amortization |
9,108 | 3.0 | % | 8,856 | 3.0 | % | |||||||
Other |
7,786 | 2.5 | % | 7,606 | 2.6 | % | |||||||
Total Cost of Product Sales |
$ | 90,940 | 29.5 | % | $ | 92,645 | 31.4 | % | |||||
Product margin as a percentage of net product sales increased to 70.5 percent in 2009 from 68.6 percent in 2008. The increase in 2009 product margin is a result of higher excess and obsolescence reserves recorded in 2008 that reduced the carrying value of inventory in 2008, partially offset by higher royalties costs incurred in 2009 due to the arbitration matter involving Gyrus and Ethicon.
Operating Expenses
Operating expenses for the periods shown were as follows (in thousands, except percentages):
| |
Year Ended December 31, | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2009 | 2008 | |||||||||||
| |
Dollars | % Total Revenue |
Dollars | % Total Revenue |
|||||||||
Research and development |
$ | 35,203 | 10.9 | % | $ | 31,930 | 10.4 | % | |||||
Sales and marketing |
114,539 | 35.3 | % | 125,502 | 40.9 | % | |||||||
General and administrative |
46,034 | 14.2 | % | 50,359 | 16.4 | % | |||||||
Amortization of intangible assets |
5,309 | 1.6 | % | 5,479 | 1.8 | % | |||||||
Reimbursement services |
353 | 0.1 | % | 1,966 | 0.6 | % | |||||||
Impairment of goodwill |
— | 0.0 | % | 6,252 | 2.0 | % | |||||||
Investigation and restatement-related costs |
31,686 | 9.8 | % | 17,021 | 5.5 | % | |||||||
Total operating expenses |
$ | 233,124 | 71.9 | % | $ | 238,509 | 77.6 | % | |||||
Research and development expense increased $3.3 million to 10.9 percent of total revenues in 2009 from 10.4 percent in 2008. The increase in research and development expenses in 2009 compared to 2008 is primarily attributable to an increase in personnel cost as a result of incentive compensation in 2009 and increased headcount in quality control personnel.
Sales and marketing expense decreased $11.0 million, to 35.3 percent of total revenues in 2009 compared to 40.9 percent of total revenues in 2008 primarily as a result of sales and marketing cost reduction efforts in the Americas. ENT sales and marketing expenses for the Americas were reduced by approximately 20 percent in 2009 as a result of terminating various marketing initiatives from 2008. Other product area sales and marketing expenses were reduced by approximately one-third as we restructured the Spine distribution channel from a fixed cost direct sales force to an independent agent structure.
44
General and administrative expense decreased $4.3 million in 2009 and was 14.2 percent of total revenues compared to 16.4 percent of total revenues in 2008. In 2008, we accrued $15.1 million for the arbitration panel's award decision announced in June 2009 in the arbitration matter involving Gyrus and Ethicon originally brought against us in May 2008. We also incurred legal costs in 2008 associated with our strategic options process that was terminated in July 2008 and support costs related to our March 2008 NASDAQ hearing. These additional costs in 2008 were partially offset by $8.3 million of increased legal defense costs related to the arbitration matter involving Gyrus and Ethicon and the Delaware proceedings against Gyrus and increased accounting and compliance personnel in response to internal control remediation needs in 2009.
Reimbursement services expense declined in 2009 as we continued the orderly wind-down of our reimbursement assistance services subsidiaries during 2009.
Investigation and restatement expenses are costs incurred in 2009 and 2008 as a result of the Review and associated restatement of previously reported financial statements contained in our 2008 Form 10-K. These expenses include legal fees, forensic accounting fees and incremental audit fees.
Interest and Other Expense, Net
Interest and other expense for the periods shown were as follows (in thousands, except percentages):
| |
Year Ended December 31, | ||||||
|---|---|---|---|---|---|---|---|
| |
2009 | 2008 | |||||
Interest and other income, net |
$ | 312 | $ | 563 | |||
Interest expense |
(3,757 | ) | (3,792 | ) | |||
Foreign exchange gain (loss), net |
1,233 | (5,657 | ) | ||||
Interest and other expense, net |
$ | (2,212 | ) | $ | (8,886 | ) | |
The $6.7 million decrease in net interest and other expense in 2009 is primarily attributable to the U.S. dollar weakening against the material foreign currencies in which we operate, partially offset by a decrease in interest earned on investments as a result of declining investment balances.
Income Tax Provision (Benefit)
In January 2009, the U.S. Treasury Department issued temporary cost-sharing regulations and as a result, we decided to adjust our intercompany payments for all open tax years. As a result of these adjustments to our intercompany payments, we utilized approximately $1.9 million of our reserves for uncertain tax positions and recorded additional income tax expense of approximately $3.6 million in 2009. Adjusted for the effect of this change in regulations, our effective tax rate for 2009 was 21.5 percent. Our effective tax rate in 2008 was 25.0 percent.
Accrued Dividends, Beneficial Conversion Feature and Accretion Charges on Series A 3% Preferred Stock
We recognized a beneficial conversion feature of $27.0 million on the sale of the Series A Preferred Stock that was immediately charged against net loss applicable to common stockholders. We also accrued $1.0 million for additional dividend and accretion charges on the Series A Preferred Stock in 2009. For additional information relating to our Series A Preferred Stock, see Note 12, "Mezzanine Equity" in the notes to the consolidated financial statements.
45
Liquidity and Capital Resources
Historically, our liquidity needs have been to finance the costs of operations, to acquire complementary businesses or assets and to make repurchases of our common stock. Our operating cash flow has historically been affected by the overall profitability of the sales of our products, our ability to invoice and collect from customers in a timely manner, and our ability to efficiently implement our acquisition strategy and manage costs. We expect that our cash flows from operations together with cash on hand will be sufficient to satisfy our short-term requirements and, excluding the uncertainty related to the ongoing litigation and investigations to which we are a party, long-term normal operating liquidity requirements. As a result of repaying and terminating our Credit Agreement on September 1, 2009, we do not currently have access to a credit facility as a possible source of liquidity.
Cash Flows and Working Capital for the Year Ended December 31, 2010 compared to the year ended December 31, 2009
As of December 31, 2010, we had $191.9 million in working capital compared to $106.3 million at December 31, 2009. The change in working capital is primarily a result of cash received from operating activities.
Cash provided by operating activities in 2010 was $82.6 million, compared to $19.5 million in 2009, and differed from our net income of $37.1 million as a result of non-cash expenses such as depreciation and amortization expense of $24.1 million, deferred taxes of $2.9 million, stock-based compensation of $8.0 million, changes in inventory and warranty reserves of $8.1 million, and changes in operating assets and liabilities of $1.2 million. Cash provided by operating activities included cash provided by discontinued operations of $0.5 million and $0.7 million for the years ended December 31, 2010 and 2009, respectively.
Cash used in investing activities was $12.2 million in 2010 consisting primarily of property and equipment additions. Cash used in investing activities was $12.4 million in 2009 and consisted primarily of property and equipment purchases offset by the removal of cash restrictions due to the termination of the Credit Agreement.
Cash provided by financing activities was $3.6 million in 2010 and consisted primarily of proceeds from the exercise of stock options and tax benefit resulting from the exercise of employee stock options. Cash provided by financing activities was $15.9 million in 2009 as we sold our Series A Preferred Stock for an aggregate purchase price of $75.0 million less direct issuance cost of $5.6 million and we repaid our outstanding principal balance under our Credit Agreement of $55.0 million.
Cash Flows and Working Capital for the Year Ended December 31, 2009 compared to the year ended December 31, 2008
As of December 31, 2009, we had $106.3 million in working capital compared to $43.4 million at December 31, 2008. At December 31, 2008 we had $55 million of borrowings outstanding under our Credit Agreement that was classified as short-term debt and which reduced our working capital. The change in working capital is primarily a result of the sale of our Series A Preferred Stock for $75.0 million as discussed above which was used to repay the outstanding borrowings under our Credit Agreement. As of December 31, 2009, our principal sources of liquidity consisted of $57.4 million in cash and cash equivalents.
Cash provided by operating activities in 2009 was $19.5 million, compared to $32.0 million in 2008, and differed from our net loss of $5.8 million as a result of non-cash expenses such as depreciation and amortization expense of $23.9 million and stock-based compensation of $6.6 million. Accrued liabilities were reduced by $17.4 million, primarily as a result of final settlement and payment of the Gyrus arbitration matter. Cash provided by operating activities included cash provided by discontinued
46
operations of $0.7 million and $3.3 million for the years ended December 31, 2009 and 2008, respectively.
Cash used in investing activities was $12.4 million in 2009 consisting primarily of property and equipment additions offset by the removal of cash restrictions due to the termination of the Credit Agreement. Cash used in investing activities was $24.7 million in 2008 consisting primarily of property and equipment additions.
Cash provided by financing activities was $15.9 million in 2009 as we sold our Series A Preferred Stock for an aggregate purchase price of $75.0 million less direct issuance cost of $5.6 million and we repaid our outstanding principal balance under our Credit Agreement of $55.0 million. Cash used in financing activities was $11.5 million in 2008 as we made common stock repurchases of $11.8 million.
Contractual Obligations and Commercial Commitments
The following table aggregates all material contractual obligations and commercial commitments that affected our financial condition and liquidity as of December 31, 2010 (in thousands):
| |
Payments Due by Period (in thousands) |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Total(1) | Less than 1 Year |
1-3 Years |
3-5 Years |
More than 5 Years |
|||||||||||
Operating lease obligations |
$ | 13,125 | $ | 3,297 | $ | 5,327 | $ | 2,980 | $ | 1,521 | ||||||
Educational grants(2) |
150 | 50 | 100 | — | — | |||||||||||
Purchase commitments with suppliers(3) |
9,769 | 7,111 | 2,658 | — | — | |||||||||||
Total |
$ | 23,044 | $ | 10,458 | $ | 8,085 | $ | 2,980 | $ | 1,521 | ||||||
- (1)
- The
Company has entered into indemnification agreements with several former executives and employees, and will likely continue to incur legal costs
associated with the ongoing litigation and investigations discussed in Note 11 "Litigation and Contingencies". While the potential for future payments exists, the amounts are not estimable.
- (2)
- Represents
contributions to foundations that promote and support education and research initiatives.
- (3)
- Represents agreements to purchase materials and products that are enforceable, legally binding and specify terms, including: fixed or minimum quantities to be purchased and the approximate timing of the payments.
The Company has not included liabilities for uncertain tax positions in the above table of contractual obligations and commercial commitments because the Company is unable to estimate the anticipated timing of payment.
Critical Accounting Policies and Estimates
The preparation of financial statements and related disclosures in conformity with accounting principles generally accepted in the U.S., or GAAP, requires our management to make judgments, assumptions and estimates that affect the amounts of revenue, expenses, income, assets and liabilities, reported in our consolidated financial statements and accompanying notes. Understanding our accounting policies and the extent to which our management uses judgment, assumptions and estimates in applying these policies is integral to understanding our financial statements. We describe our most significant accounting policies in Note 3, "Summary of Significant Accounting Policies" in the notes to the consolidated financial statements. We have identified the following accounting policies as those that require significant judgments, assumptions and estimates and that have a significant impact on our
47
financial condition and results of operations. These policies are considered critical because they may result in fluctuations in our reported results from period to period due to the significant judgments, estimates and assumptions about highly complex and inherently uncertain matters and because the use of different judgments, assumptions or estimates could have a material impact on our financial condition or results of operations. We evaluate our critical accounting estimates and judgments required by our policies on an ongoing basis and update them as appropriate based on changing conditions.
Revenue Recognition
Our principal sources of revenue are from sales of our products through our direct sales professionals, independent sales agents and independent distributors. We recognize these revenues when there is persuasive evidence of an arrangement providing for the sale of a product, delivery of the product has occurred, the sale price of the product is fixed or determinable and collectability is reasonably assured.
For product sales made by our direct sales professionals or independent agents, our criteria for revenue recognition is complete when our product is delivered to end customers, such as surgical centers and hospitals. These sales occur either through a purchase order submitted by the customer or through trunk sales. For purchase orders, we ship product to and invoice the end customer directly. For trunk sales, product is hand-delivered at the end customer's premises from trunk stock maintained by the direct sales representative or independent agent and a bill-only order is received notifying us of the product's delivery at which time we invoice the end customer.
Sales to distributors occur either as stocking and replenishment orders or as drop shipments to end customers. In the majority of cases involving sales to distributors, our requirements for revenue recognition are met when we deliver product to a particular distributor. In the case of certain distributors, prior to March 2010, we had agreed to pricing reductions based on the distributors' ultimate reselling price and in those cases we recognized revenue on a "sell-through" basis only after the distributor had sold our products to an end user. In order to determine the amount of sell-through revenue that should be recognized during a given reporting period, we relied on reports from the distributors that provided us with information about sales to end customers during the reporting period as well as the amount and types of our products in the distributor's inventory at the end of a reporting period. Our management was required to exercise a significant level of judgment to determine the accuracy and reliability of the reported information. We used a first-in-first-out accounting method to account for products sold by these distributors where we applied the sell-through method.
Our customers generally have the right to return or exchange products purchased from us for up to 60 days from the date of product shipment. At each period end, we determine the extent to which our revenues need to be reduced to account for returns and exchanges and we record a reserve against revenue recognized during that period for product returns and exchanges that our management estimates are likely to occur after the end of that period. We base these estimates on our historical experience with our customers and on direct customer feedback. We had an agreement with one customer that provided for an extended right of exchange. Our management concluded that the duration of the exchange agreement with this customer was inconsistent with our revenue recognition criteria and as a result revenue related to product sales to this customer was deferred until the related right of exchange period expired during the first quarter of 2010. This customer no longer has this extended right of return.
Our revenue recognition is also impacted by management's estimate of our customers' ability to pay us pursuant to the terms of our customer agreements. If after we have recognized revenue, collectability of an account receivable becomes doubtful, we will establish an allowance for doubtful accounts with respect to the previously recognized revenue that remains uncollected. Where our
48
management forms a judgment that a particular customer has not established a sufficient credit history but decides to deliver products to the customer, we will defer recognizing revenues on product sales to that customer until collectability is reasonably assured, which typically coincides with the collection of cash. Once the customer establishes a reliable payment history we generally return to normal revenue recognition based on our criteria. We regularly review the creditworthiness of our customers considering such factors as historical collection experience, a customer's current credit standing, the age of accounts receivable balances, and general economic conditions that may affect a customer's ability to pay (both individually and in the aggregate).
Prior to ceasing activities in our DiscoCare and DRS subsidiaries in early 2009, products were billed to and cash collected from third-party payors for which we have determined that we are unable to conclude as to whether the exchange represented persuasive evidence of an arrangement providing for the sale of product or whether amounts billed, including those collected, would be subject to future disputes with the payor due to insurance reimbursement practices. Accordingly, product sales revenues have been reversed until the Company is able to conclude on the above, including revenue for product sales where the Company has collected cash. Cash received from third-party payors related to DiscoCare and DRS has been recorded on the consolidated balance sheet as an accrued liability pending the resolution of any possible dispute. With the cessation of the DiscoCare and DRS businesses, we are no longer engaged in product sales activities where we bill third-party payors.
Although the terms of our agreements with many of our customers provide that risk of loss passes to the customer upon shipment, we have historically accepted the risk of loss until product delivery. Accordingly, we only recognize revenues on a product sale if, in management's judgment, it has been delivered during the relevant reporting period. The application of this policy requires our management to make estimates as to the timing of deliveries. We base these estimates on our historical experience with product shipments to particular markets that we serve. We regularly compare these estimates to actual results based on delivery confirmations to determine whether a change of estimate is required.
We generally recognize license fees and other revenue over the term of the associated agreement. Royalties represent the largest component of non-product sales revenue and are recognized as earned, based on our estimates using current and historical trends adjusted for market factors, and are classified as royalties, fees and other revenues in the accompanying statements of operations.
When we make payments to our customers, we recognize those payments as a reduction of revenue unless we can determine that we received products or services that have a fair value equal to the amount of the payment to the customer. In order to determine the fair value of the products or services received and whether alternative sources of supply exist, management is required to use judgment and make estimates to determine the amount that should be classified as a reduction of revenue and the amount to be classified as an expense.
Inventory Allowance
We value our inventory based on cost. We adjust the value of our inventory to the extent our management determines that our cost cannot be recovered due to obsolescence or other factors. In order to make these determinations, our management uses estimates of future demand and sales prices for each product to determine appropriate inventory reserves and to make corresponding reductions in inventory values to reflect the lower of cost or market value. In the event of a sudden significant decrease in demand for our products, or a higher incidence of inventory obsolescence, we could be required to increase our inventory reserve, which would increase our cost of product sales and decrease our gross profit.
49
Long-lived Assets
Our goodwill balance is not amortized to expense; instead it is tested for impairment at least annually. During 2010, we changed our annual goodwill impairment test date from December 31 to October 31, which represents a change in accounting principle. Management concluded that this change was preferable as it facilitates the timely reporting of our financial results If events or indicators of impairment occur between annual impairment analyses, we perform an impairment analysis of goodwill at that date. These events or circumstances could include a significant change in the business climate, legal factors, operating performance indicators, competition, or sale or disposition of a significant asset. In testing for a potential impairment of goodwill, we: (1) verify there are no changes to our reporting units with goodwill balances; (2) allocate goodwill to our various reporting units to which the acquired goodwill relates; (3) determine the carrying value, or book value, of our reporting units, as some of the assets and liabilities related to each reporting unit are held by a corporate function; (4) estimate the fair value of each reporting unit using a discounted cash flow model; (5) reconcile the fair value of our reporting units in total to our market capitalization adjusted for a subjectively estimated control premium and other identifiable factors; (6) compare the fair value of each reporting unit to its carrying value; and (7) if the estimated fair value of a reporting unit is less than the carrying value, we must estimate the fair value of all identifiable assets and liabilities of that reporting unit, in a manner similar to a purchase price allocation for an acquired business to calculate the implied fair value of the reporting unit's goodwill and recognize an impairment charge if the implied fair value of the reporting unit's goodwill is less than the carrying value.
The process of evaluating the potential impairment of goodwill is subjective and requires significant judgment at many points during the analysis, including the identification of our reporting units, identification and allocation of the assets and liabilities to each of our reporting units and determination of fair value. In estimating the fair value of a reporting unit for the purposes of our annual or periodic impairment analyses, we make estimates and significant judgments about the future cash flows of that reporting unit. Our cash flow forecasts are based on assumptions that represent the highest and best use for our reporting units. Changes in judgment on these assumptions and estimates could result in further goodwill impairment charges. We believe that the assumptions and estimates utilized are appropriate based on the information available to management.
On October 31, 2010, we performed our required annual goodwill impairment test. Our goodwill has been allocated to our Sports Medicine—Americas, ENT—Americas and International reporting units. The test requires us to compare the fair value of each reporting unit to its carrying value. If the fair value is less than the carrying value, we are required to compare the carrying value of the reporting unit's goodwill to its implied fair value. The fair values of our Sports Medicine—Americas, ENT—Americas and International reporting units, with goodwill balances of $80.7 million, $11.1 million and $27.2 million, respectively, were determined to be in excess of the carrying values of the reporting units and therefore no impairment charges were recognized related to these reporting units. The discounted cash flow analyses utilized a discount rate of 13.3 percent for each of the reporting units. The average growth rates utilized in our discounted cash flow models for the Sports Medicine—Americas, ENT—Americas and International reporting units approximated the average growth rate between 2008 and 2010. We believe these growth rates are reasonable estimates of future performance. We noted that a 25 percent reduction in the growth rates or a 25 percent increase in the discount rate for the Sports Medicine—Americas, ENT—Americas and International reporting units would not result in an impairment charge. The enterprise fair values implied by the discounted cash flow analysis were below our total market capitalization, which reflects the conservative nature of our forecasts. As the fair value of the reporting units was significantly above the carrying value using these conservative estimates, we concluded that the second step of the goodwill impairment test was not necessary.
50
Intangible assets with finite lives and property, plant and equipment are amortized or depreciated over their estimated useful life on a straight line basis. We monitor conditions related to these assets to determine whether events and circumstances warrant a revision to the remaining amortization or depreciation period. We test these assets for potential impairment whenever our management concludes events or changes in circumstances indicate that the carrying amount may not be recoverable. The original estimate of an asset's useful life and the impact of an event or circumstance on either an asset's useful life or carrying value involve significant judgment regarding estimates of the future cash flows associated with each asset.
In addition to testing goodwill impairment, we tested our $2.1 million of intangible assets associated with our Other products and discontinued operations for impairment. We determined that the future net cash flows associated with each asset were in excess of the carrying value of each asset and therefore, no impairment charge was recorded.
Legal and Other Contingencies
The outcomes of legal proceedings and claims brought against us and other loss contingencies are subject to significant uncertainty. We accrue a charge against income when our management determines that it is probable that an asset has been impaired or a liability has been incurred and the amount of loss can be reasonably estimated. In addition, we accrue for the authoritative judgments or assertions made against us by government agencies at the time of their rendering regardless of our intent to appeal. In determining the appropriate accounting for loss contingencies, we consider the likelihood of loss or impairment of an asset or the incurrence of a liability, as well as our ability to reasonably estimate the amount of loss. We regularly evaluate current information available to us to determine whether an accrual should be established or adjusted. Estimating the probability that a loss will occur and estimating the amount of a loss or a range of loss involves significant judgment.
Income Taxes
We account for income taxes under the liability method, whereby deferred tax asset or liability account balances are determined based on the difference between the financial statement and the tax bases of assets and liabilities using current tax laws and rates in effect for the year in which the differences are expected to affect taxable income. Valuation allowances are established, when necessary, to reduce deferred tax assets when we expect the amount of tax benefit to be realized is less than the carrying value of the deferred tax asset.
We make an evaluation at the end of each reporting period as to whether or not some or all of the undistributed earnings of our foreign subsidiaries are permanently reinvested. While we may have concluded in the past that some of such undistributed earnings are permanently reinvested, facts and circumstances may change in the future. Changes in facts and circumstances may include a change in the estimated capital needs of our foreign subsidiaries, or a change in our corporate liquidity requirements. Such changes could result in our management determining that some or all of such undistributed earnings are no longer permanently reinvested. In that event, we would be required to recognize income tax liabilities on the assumption that our foreign undistributed earnings will be distributed to the U.S.
Accounting for income taxes involves uncertainty and judgment on how to interpret and apply tax laws and regulations within our annual tax filings. Such uncertainties from time to time may result in a tax position that may be challenged and overturned by a tax authority in the future which could result in additional tax liability, interest charges and possibly penalties. An income tax benefit from an uncertain tax position is recognized only if it is more likely than not that the tax position will be sustained upon examination by the relevant taxing authorities, based on the technical merits of the position. The liability for uncertain tax positions, including related interest and penalties, is recorded as
51
other non-current liabilities or offsetting deferred tax asset, as applicable. Interest on unpaid taxes and penalties when incurred are classified as a component of income tax expense.
Series A 3.00% Convertible Preferred Stock
Our Series A Preferred Stock is classified as mezzanine equity and is shown on our balance sheet net of issuance costs. The difference between the carrying value of the Series A Preferred Stock and its redemption value is accreted over 5 years using the effective interest method. As the Series A Preferred Stock is convertible at the option of the holder, we immediately amortized the entire amount of the beneficial conversion feature, as determined on the date of issuance, as a dividend. Additional dividends are accrued at the stated rate each period so that the mezzanine equity carrying value will equal its redemption value on the fifth anniversary of the issuance when any remaining unconverted Series A Preferred Stock could be redeemable at the option of the holder. Dividends declared on the Series A Preferred Stock shares, the accretion of issuance costs and amortization of the beneficial conversion feature reduce the amount of net earnings that are available to common stockholders and are presented together as a separate amount on the consolidated statements of operations.
Stock-Based Compensation
We account for stock-based compensation by measuring and recognizing as compensation expense the fair value of all share-based payment awards made to employees, including employee stock options and restricted stock awards. The determination of fair value involves a number of significant estimates. We use the Black-Scholes option pricing model to estimate the value of employee stock options which requires a number of assumptions to determine the model inputs. These include the expected volatility of our stock and employee exercise behavior which are based on historical data as well as expectations of future developments over the term of the option. As stock-based compensation expense is based on awards ultimately expected to vest it has been reduced for estimated forfeitures. Forfeitures are estimated at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differ from those estimates. Management's estimate of forfeitures is based on historical experience but actual forfeitures could differ materially as a result of voluntary employee actions and involuntary actions which would result in a significant change in our stock-based compensation expense amounts in the future.
Recently Issued Accounting Pronouncements
New accounting pronouncements or changes in existing accounting pronouncements may have a significant effect on our results of operations, our financial condition, our net worth or our business operations.
In October 2009, the Financial Accounting Standards Board ("FASB") issued amended revenue recognition guidance for arrangements with multiple deliverables. This guidance is effective for all new or materially modified arrangements entered into on or after January 1, 2011 with earlier application permitted as of the beginning of a fiscal year. We do not expect the implementation of this guidance to have a material impact on the consolidated financial statements.
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
We are exposed to certain market risks as part of our ongoing business operations, primarily risks from changing interest rates and foreign currency exchange rates that may impact, adversely or otherwise, our financial condition, results of operations, or cash flows.
Our interest income is dependent on changes in the general level of U.S. interest rates. Our cash and cash equivalents consist of money market funds and various deposit accounts.
52
The table below presents principal amounts and related weighted average interest rates as of December 31, 2010 for our cash and cash equivalents (in thousands, except percentages):
Cash and cash equivalents |
$ | 132,536 | ||
Average interest rate earned on cash and cash equivalents |
0.24 | % |
Foreign Currency Risk
A significant portion of our international sales and operating expenses are denominated in currencies other than the U.S. dollar. To the extent that the exchange rates for these currencies fluctuate against the U.S. dollar, we will experience variations in our results of operations and financial condition.
Our cash and cash equivalents at December 31, 2010 are denominated primarily in U.S. dollars; however, we also maintain balances in Euros, British pounds, Swedish kroner, Swiss francs, Australian dollars and Costa Rican colones in support of local subsidiary operations. A 10 percent change in the December 31, 2010 exchange rates for these currencies would have an impact on pre-tax income of approximately $1.2 million.
We have not used derivative financial instruments to hedge against foreign currency exchange risk and do not anticipate doing so in the future. Our objective to minimize foreign currency gains and losses has been managed by maintaining only enough cash necessary for immediate working capital requirements in accounts denominated in currencies other than the U.S. dollar and holding the majority of our cash and cash equivalents in U.S. dollar accounts.
53
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
The following financial statements of the Company and the Report of Independent Registered Public Accounting Firm are included in this Report on the pages indicated.
All other schedules are omitted because they are not applicable.
54
Report of Independent Registered Public Accounting Firm
To Board of Directors and Stockholders of ArthroCare Corporation:
In our opinion, the consolidated financial statements listed in the accompanying index present fairly, in all material respects, the financial position of ArthroCare Corporation and its subsidiaries at December 31, 2010 and 2009, and the results of their operations and their cash flows for each of the three years in the period ended December 31, 2010 in conformity with accounting principles generally accepted in the United States of America. In addition, in our opinion, the financial statement schedule listed in the accompanying index presents fairly, in all material respects, the information set forth therein when read in conjunction with the related consolidated financial statements. Also in our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of December 31, 2010, based on criteria established in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). The Company's management is responsible for these financial statements and financial statement schedule, for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in Management's Report on Internal Control over Financial Reporting appearing under Item 9A. Our responsibility is to express opinions on these financial statements, on the financial statement schedule, and on the Company's internal control over financial reporting based on our integrated audits. We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the financial statements are free of material misstatement and whether effective internal control over financial reporting was maintained in all material respects. Our audits of the financial statements included examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. Our audit of internal control over financial reporting included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audits also included performing such other procedures as we considered necessary in the circumstances. We believe that our audits provide a reasonable basis for our opinions.
A company's internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company's internal control over financial reporting includes those policies and procedures that (i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company's assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
/s/
PricewaterhouseCoopers LLP
Austin, Texas
February 14, 2011
55
ARTHROCARE CORPORATION
CONSOLIDATED BALANCE SHEETS
(in thousands, except par value data)
| |
December 31, | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
2010 | 2009 | ||||||||
ASSETS |
||||||||||
Current assets: |
||||||||||
Cash and cash equivalents |
$ | 132,536 | $ | 57,386 | ||||||
Accounts receivable, net of allowances of $2,445 and $4,069 at 2010 and 2009, respectively |
48,870 | 45,789 | ||||||||
Inventories, net |
34,087 | 48,628 | ||||||||
Deferred tax assets |
24,661 | 12,983 | ||||||||
Prepaid expenses and other current assets |
4,424 | 6,563 | ||||||||
Assets held for sale |
3,081 | — | ||||||||
Total current assets |
247,659 | 171,349 | ||||||||
Property and equipment, net |
41,582 |
47,386 |
||||||||
Intangible assets, net |
10,733 | 17,975 | ||||||||
Goodwill |
119,020 | 119,076 | ||||||||
Deferred tax assets |
16,019 | 30,526 | ||||||||
Other assets |
4,182 | 4,816 | ||||||||
Total assets |
$ | 439,195 | $ | 391,128 | ||||||
LIABILITIES, REEDEMABLE CONVERTIBLE PREFERRED AND STOCKHOLDERS' EQUITY |
||||||||||
Current liabilities: |
||||||||||
Accounts payable |
$ | 13,819 | $ | 14,299 | ||||||
Accrued liabilities |
40,197 | 46,077 | ||||||||
Deferred tax liabilities |
149 | 3 | ||||||||
Deferred revenue |
— | 4,508 | ||||||||
Income tax payable |
1,555 | 195 | ||||||||
Total current liabilities |
55,720 | 65,082 | ||||||||
Deferred tax liabilities |
213 | 303 | ||||||||
Other non-current liabilities |
13,766 | 4,844 | ||||||||
Total liabilities |
69,699 | 70,229 | ||||||||
Commitments and contingencies (Notes 10 and 11) |
||||||||||
Series A 3% Redeemable Convertible Preferred Stock, par value $0.001; Authorized: 100 shares; Issued and outstanding: 75 shares at 2010 and 2009 |
73,768 |
70,504 |
||||||||
Stockholders' equity: |
||||||||||
Preferred stock, par value $0.001; Authorized: 4,900 shares; Issued and outstanding: none |
— | — | ||||||||
Common stock, par value, $0.001: Authorized: 75,000 shares; Issued: 31,102 and 30,905 shares; Outstanding: 27,112 and 26,886 shares, at December 31, 2010 and 2009, respectively |
27 | 27 | ||||||||
Treasury stock: 3,990 and 4,019 shares at December 31, 2010 and 2009, respectively |
(107,899 | ) | (108,724 | ) | ||||||
Additional paid-in capital |
386,395 | 379,921 | ||||||||
Accumulated other comprehensive income |
4,246 | 1,645 | ||||||||
Retained earnings (accumulated deficit) |
12,959 | (22,474 | ) | |||||||
Total stockholders' equity |
295,728 | 250,395 | ||||||||
Total liabilities, redeemable convertible preferred stock and stockholders' equity |
$ | 439,195 | $ | 391,128 | ||||||
The accompanying notes are an integral part of these consolidated financial statements.
56
ARTHROCARE CORPORATION
CONSOLIDATED STATEMENTS OF OPERATIONS
(in thousands, except per-share data)
| |
Years Ended December 31, | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2010 | 2009 | 2008 | |||||||||
Revenues: |
||||||||||||
Product sales |
$ | 338,757 | $ | 308,493 | $ | 295,118 | ||||||
Royalties, fees and other |
16,622 | 15,624 | 12,165 | |||||||||
Total revenues |
355,379 | 324,117 | 307,283 | |||||||||
Cost of product sales |
110,751 |
90,940 |
92,645 |
|||||||||
Gross profit |
244,628 | 233,177 | 214,638 | |||||||||
Operating expenses: |
||||||||||||
Research and development |
35,846 | 35,203 | 31,930 | |||||||||
Sales and marketing |
107,852 | 114,539 | 125,502 | |||||||||
General and administrative |
38,491 | 46,034 | 50,359 | |||||||||
Amortization of intangible assets |
5,237 | 5,309 | 5,479 | |||||||||
Impairment of goodwill |
— | — | 6,252 | |||||||||
Reimbursement services |
92 | 353 | 1,966 | |||||||||
Investigation and restatement-related costs |
2,840 | 31,686 | 17,021 | |||||||||
Total operating expenses |
190,358 | 233,124 | 238,509 | |||||||||
Income (loss) from operations |
54,270 | 53 | (23,871 | ) | ||||||||
Other income, net |
216 | 312 | 563 | |||||||||
Interest expense and bank fees |
(769 | ) | (3,757 | ) | (3,792 | ) | ||||||
Foreign exchange gain (loss), net |
(3,311 | ) | 1,233 | (5,657 | ) | |||||||
Interest and other expense, net |
(3,864 | ) | (2,212 | ) | (8,886 | ) | ||||||
Income (loss) from continuing operations before income taxes |
50,406 | (2,159 | ) | (32,757 | ) | |||||||
Income tax provision (benefit) |
12,888 |
3,137 |
(8,175 |
) |
||||||||
Net income (loss) from continuing operations |
37,518 | (5,296 | ) | (24,582 | ) | |||||||
Loss from discontinued operations, net of taxes |
(434 |
) |
(481 |
) |
(10,443 |
) |
||||||
Net income (loss) |
37,084 | (5,777 | ) | (35,025 | ) | |||||||
Accrued dividend, beneficial conversion feature and accretion charges on Series A 3% Convertible Preferred Stock |
(3,264 | ) | (28,045 | ) | — | |||||||
Net income (loss) applicable to common stockholders |
$ | 33,820 | $ | (33,822 | ) | $ | (35,025 | ) | ||||
Weighted-average shares outstanding: |
||||||||||||
Basic |
27,006 | 26,803 | 26,610 | |||||||||
Diluted |
27,348 | 26,803 | 26,610 | |||||||||
Earnings (loss) per share from continuing operations applicable to common stockholders: |
||||||||||||
Basic |
$ | 1.04 | $ | (1.24 | ) | $ | (0.92 | ) | ||||
Diluted |
$ | 1.03 | $ | (1.24 | ) | $ | (0.92 | ) | ||||
Earnings (loss) per share applicable to common stockholders: |
||||||||||||
Basic |
$ | 1.03 | $ | (1.26 | ) | $ | (1.32 | ) | ||||
Diluted |
$ | 1.02 | $ | (1.26 | ) | $ | (1.32 | ) | ||||
The accompanying notes are an integral part of these consolidated financial statements.
57
ARTHROCARE CORPORATION
CONSOLIDATED STATEMENTS OF STOCKHOLDERS' EQUITY
(in thousands)
| |
Common Stock Shares |
Common Stock Amount |
Treasury Stock |
Additional Paid-In Capital |
Accumulated Other Comprehensive Income |
Retained Earnings (Accumulated Deficit) |
Total Stockholders' Equity |
Comprehensive Income (Loss) |
||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Balance, December 31, 2007 |
26,808 | $ | 27 | $ | (101,321 | ) | $ | 358,065 | $ | 3,970 | $ | 20,312 | $ | 281,053 | ||||||||||||
Issuance of common stock through: |
||||||||||||||||||||||||||
Exercise of options and restricted stock |
97 | — | — | 1,221 | — | — | 1,221 | |||||||||||||||||||
Employee stock purchase plan |
15 | — | — | 391 | — | — | 391 | |||||||||||||||||||
Contribution to employee benefit plan |
79 | — | 2,154 | (639 | ) | — | (655 | ) | 860 | |||||||||||||||||
Stock compensation |
— | — | — | 9,797 | — | — | 9,797 | |||||||||||||||||||
Income tax effect of exercise of stock options |
— | — | — | 3,816 | — | — | 3,816 | |||||||||||||||||||
Purchase of common stock |
(244 | ) | — | (11,778 | ) | — | — | — | (11,778 | ) | ||||||||||||||||
Currency translation adjustment |
— | — | — | — | (3,844 | ) | — | (3,844 | ) | $ | (3,844 | ) | ||||||||||||||
Net loss |
— | — | — | — | — | (35,025 | ) | (35,025 | ) | (35,025 | ) | |||||||||||||||
Balance, December 31, 2008 |
26,755 | 27 | (110,945 | ) | 372,651 | 126 | (15,368 | ) | 246,491 | (38,869 | ) | |||||||||||||||
Issuance of common stock through: |
||||||||||||||||||||||||||
Exercise of options and restricted stock |
50 | — | — | 359 | — | — | 359 | |||||||||||||||||||
Contribution to employee benefit plan |
81 | — | 2,221 | — | — | (1,329 | ) | 892 | ||||||||||||||||||
Stock compensation |
— | — | — | 6,557 | — | — | 6,557 | |||||||||||||||||||
Income tax effect of exercise of stock options |
— | — | — | 1,412 | — | — | 1,412 | |||||||||||||||||||
Accrued dividends and accretion charges on Series A 3% Convertible Preferred Stock |
— | — | — | (1,058 | ) | — | — | (1,058 | ) | |||||||||||||||||
Currency translation adjustment |
— | — | — | — | 1,519 | — | 1,519 | 1,519 | ||||||||||||||||||
Net loss |
— | — | — | — | — | (5,777 | ) | (5,777 | ) | (5,777 | ) | |||||||||||||||
Balance, December 31, 2009 |
26,886 | 27 | (108,724 | ) | 379,921 | 1,645 | (22,474 | ) | 250,395 | (4,258 | ) | |||||||||||||||
Issuance of common stock through: Exercise of options and restricted stock |
190 |
— |
— |
1,868 |
— |
— |
1,868 |
|||||||||||||||||||
Employee stock purchase plan |
5 | — | — | 128 | — | — | 128 | |||||||||||||||||||
Contribution to employee benefit plan |
31 | — | 825 | 80 | — | — | 905 | |||||||||||||||||||
Stock compensation |
— | — | — | 8,001 | — | — | 8,001 | |||||||||||||||||||
Income tax effect of exercise of stock options |
— | — | — | (1,990 | ) | — | — | (1,990 | ) | |||||||||||||||||
Accrued dividends and accretion charges on Series A 3% Redeemable Convertible Preferred Stock |
— | — | — | (1,613 | ) | — | (1,651 | ) | (3,264 | ) | ||||||||||||||||
Currency translation adjustment |
— | — | — | — | 2,601 | — | 2,601 | 2,601 | ||||||||||||||||||
Net income |
— | — | — | — | — | 37,084 | 37,084 | 37,084 | ||||||||||||||||||
Balance, December 31, 2010 |
27,112 | $ | 27 | $ | (107,899 | ) | $ | 386,395 | $ | 4,246 | $ | 12,959 | $ | 295,728 | $ | 39,685 | ||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
58
ARTHROCARE CORPORATION
CONSOLIDATED STATEMENTS OF CASH FLOWS
(in thousands)
| |
Year Ended December 31, | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2010 | 2009 | 2008 | ||||||||||
Cash flows from operating activities: |
|||||||||||||
Net income (loss) |
$ | 37,084 | $ | (5,777 | ) | $ | (35,025 | ) | |||||
Adjustments to reconcile net income (loss) to net cash provided by by operating activities: |
|||||||||||||
Depreciation and amortization |
24,130 | 23,904 | 22,994 | ||||||||||
Impairment of goodwill and intangible assets |
— | — | 18,945 | ||||||||||
Provision for doubtful accounts receivable and product returns |
(336 | ) | 3,147 | 7,258 | |||||||||
Provision for inventory and warranty reserves |
8,149 | 2,778 | 7,987 | ||||||||||
Non-cash stock compensation expense |
8,001 | 6,557 | 9,878 | ||||||||||
Deferred taxes |
2,885 | 1,339 | (10,516 | ) | |||||||||
Income tax benefits relating to employee stock options |
(1,608 | ) | (1,099 | ) | (3,746 | ) | |||||||
Unrealized foreign exchange (gain) loss |
4,594 | (1,939 | ) | (1,505 | ) | ||||||||
Other |
1,027 | 1,224 | 1,990 | ||||||||||
Changes in operating assets and liabilities |
|||||||||||||
Accounts receivable |
(2,057 | ) | (2,800 | ) | (9,227 | ) | |||||||
Inventories |
5,990 | 6,936 | (7,792 | ) | |||||||||
Prepaid expenses and other current assets |
2,615 | 3,136 | (2,558 | ) | |||||||||
Accounts payable |
(698 | ) | (3,375 | ) | 2,405 | ||||||||
Accrued liabilities |
(2,001 | ) | (17,391 | ) | 29,548 | ||||||||
Deferred revenue |
(4,508 | ) | 1,298 | 1,445 | |||||||||
Income taxes payable |
(631 | ) | 1,530 | (48 | ) | ||||||||
Net cash provided by operating activities |
82,636 | 19,468 | 32,033 | ||||||||||
Cash flows from investing activities: |
|||||||||||||
Purchases of property and equipment |
(12,231 | ) | (16,140 | ) | (20,890 | ) | |||||||
Earnout payment for purchase of ATI, net |
— | — | (483 | ) | |||||||||
Payments for business combinations, including earnouts, and purchases of intangible assets |
(149 | ) | (439 | ) | (1,375 | ) | |||||||
Proceeds from principle payments of loan receivable |
168 | — | — | ||||||||||
Disbursement of loan receivable |
— | (180 | ) | (2,320 | ) | ||||||||
Net change in restricted cash equivalents and investments |
— | 4,362 | 396 | ||||||||||
Proceeds from maturities of available-for-sale securities |
38 | 33 | — | ||||||||||
Net cash used in investing activities |
(12,174 | ) | (12,364 | ) | (24,672 | ) | |||||||
Cash flows from financing activities: |
|||||||||||||
Proceeds from issuance of preferred stock, net of issuance cost |
— | 69,446 | — | ||||||||||
Payments for purchases of treasury stock |
— | — | (11,778 | ) | |||||||||
Repayments on revolving credit agreement |
— | (55,000 | ) | (5,000 | ) | ||||||||
Payment for deferred debt cost |
— | — | (114 | ) | |||||||||
Proceeds from issuance of common stock, net of issuance costs |
128 | — | 391 | ||||||||||
Proceeds from exercise of options to purchase common stock, net of issuance costs |
1,868 | 359 | 1,221 | ||||||||||
Income tax benefit relating to employee stock options |
1,608 | 1,099 | 3,746 | ||||||||||
Net cash provided by (used in) financing activities |
3,604 | 15,904 | (11,534 | ) | |||||||||
Effect of exchange rate changes on cash and cash equivalents |
1,084 | 872 | (570 | ) | |||||||||
Net increase (decrease) in cash and cash equivalents |
75,150 | 23,880 | (4,743 | ) | |||||||||
Cash and cash equivalents, beginning of the period |
57,386 | 33,506 | 38,249 | ||||||||||
Cash and cash equivalents, end of the period |
$ | 132,536 | $ | 57,386 | $ | 33,506 | |||||||
The accompanying notes are an integral part of these consolidated financial statements.
59
ARTHROCARE CORPORATION
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
NOTE 1—FORMATION AND BUSINESS OF THE COMPANY
ArthroCare Corporation ("ArthroCare" or the "Company") is a medical device company incorporated on April 29, 1993, that develops, manufactures and markets surgical products, many of which are based on the Company's minimally invasive patented Coblation® technology. The Company's products are used across several medical specialties, improving many existing soft-tissue surgical procedures and enabling new minimally invasive surgical procedures. The Company's business consists of two core product areas: Sports Medicine and ENT. The Company's products are sold through two geographic distribution channels: Americas (North and South America) and International (all other geographies).
NOTE 2—FIRST QUARTER 2010 ACCOUNTING CORRECTIONS
Our results for the period ended December 31, 2010 include reductions of stock compensation expense to correct forfeiture calculation errors in prior periods. The impact of the error was an overstatement of operating expenses of $0.4 million in the year ended December 31, 2008 and $0.7 million in the year ended December 31, 2009. To correct this error, we recorded reductions of $0.8 million to general and administrative expense and $0.3 million to sales and marketing expense in the quarter ended March 31, 2010. We have determined that the impact of this uncorrected error is immaterial to all prior periods, and the correction is immaterial to the year ended December 31, 2010.
NOTE 3—SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Basis of Presentation. The consolidated financial statements include the accounts of the Company and all of its wholly owned subsidiaries. All significant intercompany transactions and accounts have been eliminated. The Company uses the calendar year as its fiscal year.
Use of Estimates. The preparation of financial statements in conformity with accounting principles generally accepted in the U.S. requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent liabilities in the consolidated financial statements and the reported amount of revenue and expenses during the reporting period. Actual results could differ from those estimates.
Cash and Cash Equivalents. The Company considers all highly liquid investments purchased with original maturities of ninety days or less to be cash equivalents. Cash and cash equivalents include money market funds and various deposit accounts.
Inventories and Inventory Allowances. The Company's inventories are stated at standard cost, which include material, labor and overhead costs and approximates actual cost determined on a first-in, first-out basis. The Company adjusts the value of its inventory to the extent management determines that the cost cannot be recovered due to obsolescence or other factors. In order to make these determinations, management uses estimates of future demand and sales prices for each product to determine appropriate inventory reserves and to make corresponding reductions in the carrying value of inventory to reflect the lower of cost or market value. In the event of a sudden significant decrease in demand for the Company's products, or a higher incidence of inventory obsolescence, the Company could be required to increase its inventory reserve, which would increase cost of product sales and decrease gross profit.
60
ARTHROCARE CORPORATION
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
NOTE 3—SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
Property and Equipment. Property and equipment is stated at cost and is depreciated on a straight-line basis over the asset's estimated useful life. The Company places the majority of its manufactured controller units with customers in order to facilitate the sale of disposable devices. Controller units placed with customers are capitalized at cost and amortized to cost of product sales over a four-year period. Leasehold improvements are amortized over the shorter of five years or the lease term. Furniture, fixtures, machinery and equipment are amortized over a five year period, while computer equipment and software are amortized over three to five years. Buildings are depreciated over a thirty year life. Maintenance and repair costs are charged to operations as incurred. Upon retirement or sale, the cost of disposed assets and their accumulated depreciation are removed from the balance sheet and any gain or loss is recognized in current operations. The Company tests its property, plant and equipment for potential impairment whenever management concludes events or changes in circumstances indicate that the carrying amount may not be recoverable. The original estimate of an asset's useful life and the impact of an event or circumstance on either an asset's useful life or carrying value involve significant judgment.
Revenue Recognition. The Company's principal sources of revenue are from sales of its products through direct sales professionals, independent sales agents and independent distributors. These revenues are recognized when there is persuasive evidence of an arrangement providing for the sale of a product, delivery of the product has occurred, the sale price of the product is fixed or determinable and collectability is reasonably assured. Revenues include shipping and handling costs billed to customers. Shipping and handling costs are presented as a part of cost of product sales, when related to revenue producing activities.
For product sales made by direct sales professionals or independent agents, the Company's criteria for revenue recognition is generally complete when the product is delivered to end customers, such as surgical centers and hospitals. These sales occur either through a purchase order submitted by the customer or through trunk sales. For purchase orders, the Company ships product to and invoices the end customer directly. For trunk sales, product is hand-delivered at the end customer's premises from trunk stock maintained by the direct sales representative or independent agent and a bill-only order is received notifying the Company of the product's delivery at which time the end customer is invoiced.
Sales to distributors occur either as stocking and replenishment orders or as drop shipments to end customers. In the majority of cases involving sales to distributors, the requirements for revenue recognition are met when the product is delivered to a particular distributor. Prior to March of 2010, the Company agreed to pricing concessions for certain distributors based on the distributors' ultimate reselling price and in these cases revenue was recognized on a "sell-through" basis only after the distributor sold the products to an end user. In order to determine the amount of sell-through revenue that should be recognized during a given reporting period, the Company relied on reports from the distributors that provided information about sales to end customers during the reporting period as well as the amount and types of products in the distributor's inventory at the end of a reporting period. The Company's management was required to make estimates and exercise a significant level of judgment to determine the accuracy and reliability of the reported information. A first-in-first-out accounting method was used to account for products sold by these distributors where the sell-through method had been applied.
Customers generally have the right to return or exchange products purchased from the Company for up to 60 days from the date of product shipment. At each period end, the Company determines the
61
ARTHROCARE CORPORATION
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
NOTE 3—SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
extent to which its revenues need to be reduced to account for expected returns and exchanges and a reserve is recorded against revenue recognized. These estimates are based on historical experience with customers and on direct customer feedback. The Company had an agreement with one customer that provided for an extended right of exchange. Management determined that for this customer, it was not possible to reasonably estimate future return levels and as a result, revenue related to product sales to this customer was deferred until the related right of exchange period expired in the first quarter of 2010. This customer no longer has this extended right of return.
Revenue recognition is also impacted by management's estimate of customers' ability to pay pursuant to the terms of customer agreements. If after the Company has recognized revenue, collectability of an account receivable becomes doubtful, an allowance for doubtful accounts will be established with respect to the previously recognized revenue that remains uncollected. When management forms a judgment that a particular customer has not established a sufficient credit history but decides to deliver products to the customer, revenue recognition will be deferred. Once the customer establishes a reliable payment history the Company generally returns to normal revenue recognition based on the Company's criteria. The creditworthiness of customers is periodically reviewed considering such factors as historical collection experience, a customer's current credit standing, the age of accounts receivable balances, and general economic conditions that may affect a customer's ability to pay.
Prior to early 2009, certain products were billed to and cash collected from third-party payors that the Company may judge to be subject to possible disputes or re-pricing by the payors. Because of the Company's limited history with such disputes and re-pricings, management has concluded that the criteria for revenue recognition are not satisfied with respect to these product sales and revenue recognition was deferred for these sales until the risk of disputes or claims was judged by management to be remote. Since early 2009 the Company is no longer engaged in product sales activities where it bills third-party payors.
Although the terms of the agreements with many of the Company's customers provide that title and risk of loss passes to the customer upon shipment, the Company has historically accepted the risk of loss until product delivery. Accordingly, revenue is recognized on a product sale if in management's judgment it has been delivered during the relevant reporting period. The application of this policy requires management to make estimates as to the timing of deliveries. These estimates are based on historical experience with product shipments to particular markets that are served. These estimates are regularly compared to actual results based on delivery confirmations to determine whether a change of estimate is required.
The Company generally recognizes license fees and other revenues over the term of the associated agreement. Royalties represent the largest component of non-product sales revenue and are recognized as earned, based on management's estimates using current and historical trends adjusted for market factors, and are classified as royalties, fees and other revenues in the accompanying consolidated statements of operations.
When payments are made to customers, the Company recognizes those payments as a reduction of revenue unless it can be determined that the Company received products or services that are separable from the customer purchasing its product that have a fair value equal to the amount of the payment to the customer. When the fair value of the products or services received is less than the consideration
62
ARTHROCARE CORPORATION
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
NOTE 3—SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
paid, only the portion that meets the above criteria is recognized as an expense and the excess is recorded as a reduction in revenue.
Discontinued Operation. In the fourth quarter of 2010, management determined that the Company's non-Coblation spine products met the criteria under generally accepted accounting principles to be reported as discontinued operations. The Company's non-Coblation spine products consist of its Parallax and Contour product lines. As a result of this determination, the reported impact from these discontinued product lines on current and previously reported net income (loss) in all periods is separately reported as "Loss from discontinued operations, net of taxes" in the Consolidated Statements of Operations. Assets identifiable with these discontinued product lines is reported in the December 31, 2010 Consolidated Balance Sheet as "Assets held for sale".
Business Combinations. Assets acquired and liabilities assumed as part of a business acquisition are generally recorded at their fair value at the date of acquisition. The excess of purchase price over the fair value of assets acquired and liabilities assumed is recorded as goodwill. Determining fair value of identifiable assets, particularly intangibles, and liabilities acquired also requires management to make estimates, which are based on all available information and in some cases assumptions with respect to the timing and amount of future revenues and expenses associated with an asset. Accounting for business acquisitions requires management to make judgments as to whether a purchase transaction is a multiple element contract, meaning that it includes other transaction components such as a settlement of a preexisting relationship. This judgment and determination affects the amount of consideration paid that is allocable to assets and liabilities acquired in the business purchase transaction.
Goodwill. The Company's goodwill balance is not amortized to expense; instead it is tested for impairment at least annually. The Company performs its annual goodwill impairment analysis in the fourth quarter. During 2010, the Company changed its annual goodwill impairment test date from December 31 to October 31, which represents a change in accounting principle. Management concluded that this change was preferable in order to facilitate the timely reporting of our financial results. This change did not have an impact on the consolidated balance sheet or the consolidated statement of operations. If events or indicators of impairment occur between annual impairment analyses, the Company performs an impairment analysis of goodwill at that date. These events or circumstances could include a significant change in the business climate, legal factors, operating performance indicators, competition, or sale or disposition of a significant asset. In testing for a potential impairment of goodwill, the Company: (1) verifies there are no changes to its reporting units with goodwill balances; (2) allocates goodwill to its various reporting units to which the acquired goodwill relates; (3) determines the carrying value, or book value, of its reporting units, as some of the assets and liabilities related to each reporting unit are held by a corporate function; (4) estimates the fair value of each reporting unit using a discounted cash flow model; (5) reconciles the fair value of its reporting units in total to the Company's market capitalization adjusted for a subjectively estimated control premium and other identifiable factors; (6) compares the fair value of each reporting unit to its carrying value; and (7) if the estimated fair value of a reporting unit is less than the carrying value, the Company must estimate the fair value of all identifiable assets and liabilities of that reporting unit, in a manner similar to a purchase price allocation for an acquired business to calculate the implied fair value of the reporting unit's goodwill and recognize an impairment charge if the implied fair value of the reporting unit's goodwill is less than the carrying value.
63
ARTHROCARE CORPORATION
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
NOTE 3—SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
The process of evaluating the potential impairment of goodwill is subjective and requires significant judgment at many points during the analysis, including the identification of the Company's reporting units, identification and allocation of the assets and liabilities to each of its reporting units and determination of fair value. In estimating the fair value of a reporting unit for the purposes of the annual or periodic impairment analyses, the Company makes estimates and significant judgments about the future cash flows of that reporting unit. The cash flow forecasts are based on assumptions that represent the highest and best use for the Company's reporting units. Changes in judgment on these assumptions and estimates could result in further goodwill impairment charges. The Company believes that the assumptions and estimates utilized are appropriate based on the information available to management.
Intangible assets with finite lives are amortized over their estimated useful life. The Company monitors conditions related to these assets to determine whether events and circumstances warrant a revision to the remaining amortization period. The Company tests its intangible assets with finite lives for potential impairment whenever management concludes events or changes in circumstances indicate that the carrying amount may not be recoverable. The original estimate of an asset's useful life and the impact of an event or circumstance on either an asset's useful life or carrying value involve significant judgment.
Series A 3% Redeemable Convertible Preferred Stock. The Company's Series A Preferred Stock is classified as mezzanine equity and is shown net of issuance costs. The difference in carrying value and redemption value resulting from offering costs and return of capital to the investor is accreted over the redemption period using the effective interest method. As the preferred stock is convertible at the option of the holder, the Company elected to immediately amortize the entire amount of the beneficial conversion feature as a dividend on the date of issuance. As the Company had an accumulated deficit, the election to immediately amortize the beneficial conversion feature had no impact on the accompanying consolidated balance sheets. Additional dividends are accrued at the stated rate each period so that the mezzanine equity carrying value will equal its redemption value at the date the equity is redeemable and are recorded on the declaration date at fair market value. Dividends on the preferred stock shares, including declared dividends, accrued cumulative dividends, deemed dividends on the accretion of issuance costs and amortization of the beneficial conversion feature, reduce the net income available to common stockholders and are presented as a separate amount on the accompanying consolidated statements of operations.
Earnings Per Share. The Company calculates earnings per share ("EPS") using the two class method for all periods ending after September 1, 2009, the date of issuance of the Series A Preferred Stock. Under the two-class method, distributed and undistributed earnings are allocated to common stockholders based on their participation rights in those earnings.
Treasury Stock. Treasury stock is accounted for under the cost method and is included as a component of stockholders' equity. When treasury stock is sold, the treasury stock balance is relieved using the weighted average cost.
Research and Development. Research and development costs are charged to operations as incurred.
64
ARTHROCARE CORPORATION
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
NOTE 3—SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
Advertising Expense. Advertising expenses are charged to operations as sales and marketing expenses as incurred. Advertising expense was $1.1 million, $1.5 million and $3.0 million in 2010, 2009 and 2008, respectively.
Reimbursement Services. Reimbursement services represent the costs the Company incurred related to DiscoCare and DRS activities and to affect their subsequent cessation of activities.
Stock-Based Compensation. The Company accounts for stock-based compensation by measuring and recognizing as compensation expense the grant date fair value of all share-based payment awards made to employees, including employee stock options and restricted stock awards. The determination of fair value involves a number of significant estimates. The Company uses the Black-Scholes option pricing model to estimate the value of employee stock options which requires a number of assumptions to determine the model inputs. These include the expected volatility of stock and employee exercise behavior which are based on historical data as well as expectations of future developments over the term of the option. As stock-based compensation expense is based on awards ultimately expected to vest it has been reduced for estimated forfeitures. Forfeitures are estimated at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differ from those estimates. Management's estimate of forfeitures is based on historical experience but actual forfeitures could differ materially as a result of voluntary employee actions and involuntary actions which would result in a significant change in future stock-based compensation expense.
The Company presents excess tax benefits from the exercise of stock options, if any, as financing cash flows rather than operating cash flows.
Legal and Other Contingencies. The outcomes of legal proceedings and claims brought against the Company and other loss contingencies are subject to significant uncertainty. The Company accrues a charge against income when management determines that it is probable that an asset has been impaired or a liability has been incurred and the amount of loss can be reasonably estimated. In determining the appropriate accounting for loss contingencies, the Company will consider the likelihood of loss or impairment of an asset or the incurrence of a liability, as well as management's ability to reasonably estimate the amount of loss. Current information available to the Company is regularly evaluated to determine whether an accrual should be established or adjusted. Estimating the probability that a loss will occur and estimating the amount of a loss or a range of loss involves significant judgment.
Foreign Currency Translation. Assets and liabilities of foreign subsidiaries that operate primarily in a currency other than the U.S. dollar are translated into U.S. dollars using the current exchange rate in effect at the balance sheet date, and revenues and expenses are translated using the average exchange rate in effect during the period. The gains and losses from foreign currency translation of these subsidiaries' financial statements are recorded directly as a separate component of stockholders' equity and represent essentially all of the balance under the caption "accumulated other comprehensive income."
For the Company's foreign subsidiaries that operate primarily in the U.S. dollar, all foreign currency denominated monetary assets and liabilities are remeasured into U.S. dollars at exchange rates in effect at the balance sheet date, and non-monetary assets and related elements of expense are remeasured using historical rates of exchange. Income and expense elements are remeasured into U.S.
65
ARTHROCARE CORPORATION
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
NOTE 3—SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
dollars using average exchange rates in effect during the period. Remeasurement gains and losses are recorded each period in the statement of operations.
Concentration of Risks and Uncertainties. A majority of the Company's cash and cash equivalents are maintained at financial institutions in the U.S. Deposits at these institutions may exceed federally insured limits on such deposits. The Company has not experienced any losses on deposits of cash and cash equivalents.
Sales to both international and domestic customers are generally made on open credit terms. Management performs ongoing credit evaluations of the Company's customers and maintains an allowance for potential credit losses when needed. No individual customer represented more than 10 percent of product sales during the years ended December 31, 2010, 2009 and 2008 or the accounts receivable balance as of December 31, 2010 and 2009.
Income Taxes. The Company accounts for income taxes under the liability method, whereby deferred tax asset or liability account balances are determined based on the difference between the financial statement and the tax bases of assets and liabilities using current tax laws and rates in effect for the year in which the differences are expected to affect taxable income. Valuation allowances are established, when necessary, to reduce deferred tax assets when the amount of expected tax benefit to be realized is less than the carrying value of the deferred tax asset.
An evaluation is made at the end of each reporting period as to whether or not some or all of the undistributed earnings of foreign subsidiaries are permanently reinvested. While the Company may have concluded in the past that earnings of its foreign subsidiaries are permanently reinvested, facts and circumstances may change in the future based on the estimated capital needs of foreign subsidiaries, or a change in corporate liquidity requirements. Such changes could result in management determining that some or all of such undistributed earnings are no longer permanently reinvested. In that event, the Company would be required to recognize income tax liabilities on the assumption that foreign undistributed earnings will be distributed to the U.S.
Accounting for income taxes involves uncertainty and judgment on how to interpret and apply tax laws and regulations within the Company's annual tax filings. Such uncertainties from time to time may result in a tax position that may be challenged and overturned by a tax authority in the future which could result in additional tax liability, interest charges and possibly penalties. An income tax benefit from an uncertain tax position is recognized only if it is more likely than not that the tax position will be sustained upon examination by the relevant taxing authorities, based on the technical merits of the position. The liability for unrecognized tax benefits, including related interest and penalties, is recorded as other non-current liabilities or offsetting deferred tax asset, as applicable. Interest and penalties are classified as a component of income tax expense.
Investigation and Restatement Related Costs. Investigation and restatement related costs are expenses incurred as a result of the SEC and DOJ investigations described in Note 11 "Litigation and Contingencies" and costs associated with the Review and restatement of previously reported financial statements contained in the 2008 Form 10-K filed on November 18, 2009. These costs include legal expenses, forensic accountant fees and incremental external audit costs.
66
ARTHROCARE CORPORATION
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
NOTE 3—SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
Recently Issued Accounting Pronouncements
In October 2009, the Financial Accounting Standards Board ("FASB") issued amended revenue recognition guidance for arrangements with multiple deliverables. For the Company, this guidance is effective for all new or materially modified arrangements entered into on or after January 1, 2011. We do not expect it to have a material impact on the consolidated financial statements.
NOTE 4—DISCONTINUED OPERATIONS
In the fourth quarter of 2010, we determined that our non-Coblation spine products met the criteria under generally accepted accounting principles to be reported as discontinued operations. Our non-Coblation spine products consist of our Parallax and Contour product lines. As a result of this determination, reported product sales for the years ended December 31, 2010, 2009, and 2008 have been reduced by $5.9 million, $7.5 million and $10.5 million, respectively and the impact from these discontinued product lines on our current and previously reported net income (loss) in all periods is separately reported as "Loss from discontinued operations, net of taxes" in our Consolidated Statements of Operations. Net assets of $3.1 million identifiable with these discontinued product lines is reported in our December 31, 2010 Consolidated Balance Sheet as "Assets held for sale".
The following table presents the assets held for sale by major class (in thousands):
| |
December 31, 2010 |
||||
|---|---|---|---|---|---|
Inventory |
$ | 1,719 | |||
Equipment, net of acumulated depreciation of $349 |
63 | ||||
Intangible assets, net of accumulated amortization of $6,800 |
1,299 | ||||
Assets held for sale |
$ | 3,081 | |||
The results of non-Coblation spine products, net of tax, are reported as discontinued operations for the years ended December 31, 2010, 2009 and 2008. Revenues and income from discontinued operations and income taxes related to discontinued operations for the years ended December 31, 2010, 2009 and 2008 were as follows (in thousands):
| |
Year Ended December 31, | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
2010 | 2009 | 2008 | |||||||
Revenue |
$ | 5,851 | $ | 7,491 | $ | 10,454 | ||||
Loss from discontinued operations before income taxes(1) |
(616 | ) | (739 | ) | (10,123 | ) | ||||
Income tax benefit |
(182 | ) | (258 | ) | 320 | |||||
Loss from discontinued operations |
$ | (434 | ) | $ | (481 | ) | $ | (10,443 | ) | |
- (1)
- The 2008 loss from discontinued operations before income taxes includes an allocated goodwill impairment charge of $12.7 million.
67
ARTHROCARE CORPORATION
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
NOTE 5—COMPUTATION OF EARNINGS (LOSS) PER SHARE
The Company's Series A Preferred Stock has participation rights in dividends issued to common stockholders. As a result, the Company calculates earnings per share using the two class method for periods ending after September 1, 2009, the date on which the Company issued the Series A Preferred Stock. The following is a reconciliation of net income (loss) applicable to common stockholders and the number of shares used in the calculation of basic and diluted earnings (loss) per share applicable to common stockholders (in thousands, except per-share data):
| |
Year Ended December 31, | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2010 | 2009 | 2008 | |||||||||
Income (loss) from continuing operations allocated to common stockholders (net of $6,061 attributable to Series A Preferred Stock for 2010) |
$ | 28,193 | $ | (33,341 | ) | $ | (24,582 | ) | ||||
Loss from discontinued operations (net of $77 attributable to Series A Preferred Stock for 2010) |
(357 | ) | (481 | ) | (10,443 | ) | ||||||
Net income (loss) allocated to common stockholders (net of $5,984 attributable to Series A Preferred Stock for 2010) |
27,836 | (33,822 | ) | (35,025 | ) | |||||||
Basic: |
||||||||||||
Weighted-average common shares outstanding |
27,006 | 26,803 | 26,610 | |||||||||
Earnings (loss) from continuing operations per share allocated to common stockholders |
$ | 1.04 | $ | (1.24 | ) | $ | (0.92 | ) | ||||
Loss from discontinued operations per share allocated to common stockholders |
(0.01 | ) | (0.02 | ) | (0.40 | ) | ||||||
Earnings (loss) per share allocated to common stockholders |
$ | 1.03 | $ | (1.26 | ) | $ | (1.32 | ) | ||||
Diluted: |
||||||||||||
Weighted-average shares outstanding used in |
||||||||||||
basic calculation |
27,006 | 26,803 | 26,610 | |||||||||
Dilutive effect of options |
266 | — | — | |||||||||
Dilutive effect of unvested restricted stock |
76 | — | — | |||||||||
Weighted-average common stock and common stock equivalents |
27,348 | 26,803 | 26,610 | |||||||||
Earnings (loss) from continuing operations per share allocated to common stockholders |
$ | 1.03 | $ | (1.24 | ) | $ | (0.92 | ) | ||||
Loss from discontinued operations per share allocated to common stockholders |
(0.01 | ) | (0.02 | ) | (0.40 | ) | ||||||
Earnings (loss) per share allocated to common stockholders |
$ | 1.02 | $ | (1.26 | ) | $ | (1.32 | ) | ||||
Stock issuable upon conversion of the Series A Preferred Stock |
5,806 | 5,806 | — | |||||||||
Stock awards excluded from calculation as their effect would be anti-dilutive |
1,977 | 3,221 | 3,137 | |||||||||
68
ARTHROCARE CORPORATION
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
NOTE 6—INVENTORY
The following summarizes the Company's inventories (in thousands):
| |
December 31, | ||||||
|---|---|---|---|---|---|---|---|
| |
2010 | 2009 | |||||
Raw materials |
$ | 9,387 | $ | 8,937 | |||
Work-in-process |
7,687 | 6,990 | |||||
Finished goods |
27,372 | 39,081 | |||||
|
44,446 | 55,008 | |||||
Inventory valuation reserves |
(10,359 | ) | (6,380 | ) | |||
Inventories, net |
$ | 34,087 | $ | 48,628 | |||
NOTE 7—PROPERTY AND EQUIPMENT
The following summarizes the Company's property and equipment (in thousands):
| |
December 31, | ||||||
|---|---|---|---|---|---|---|---|
| |
2010 | 2009 | |||||
Controller placements |
$ | 40,438 | $ | 40,117 | |||
Computer equipment and software |
28,938 | 28,652 | |||||
Machinery and equipment |
20,271 | 19,288 | |||||
Furniture, fixtures and leasehold improvements |
12,074 | 11,656 | |||||
Building and improvements |
7,178 | 6,668 | |||||
Land |
745 | 745 | |||||
Construction in process |
208 | 1,639 | |||||
|
109,852 | 108,765 | |||||
Less: accumulated depreciation |
(68,270 | ) | (61,379 | ) | |||
Total property and equipment, net |
$ | 41,582 | $ | 47,386 | |||
Depreciation expense related to the Company's property and equipment was $18.1 million, $17.3 million and $16.5 million for the years ended December 31, 2010, 2009 and 2008, respectively.
69
ARTHROCARE CORPORATION
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
NOTE 8—GOODWILL AND INTANGIBLE ASSETS
Goodwill
The changes in the carrying amount of goodwill are as follows (in thousands):
| |
December 31, | ||||||
|---|---|---|---|---|---|---|---|
| |
2010 | 2009 | |||||
Goodwill as of January 1, 2010 and 2009 net of accumulated impairment losses of $18,945 |
$ | 119,076 | $ | 118,054 | |||
Acquisitions, earnouts, and purchase accounting adjustments |
83 | (328 | ) | ||||
Translation adjustments |
(139 | ) | 1,350 | ||||
Goodwill as of December 31, 2010 and 2009 net of accumulated impairment losses of $18,945 |
$ | 119,020 | $ | 119,076 | |||
Changes to goodwill in 2010 and 2009 were related to purchase accounting adjustments, which were immaterial both individually and in the aggregate, and foreign translation adjustments related to goodwill.
Intangible assets with finite lives
Intangible assets are originally recorded at historical cost, in the case of separately purchased intangible assets, or at estimated fair value, in the case of assets acquired in the purchase of a business, and all of the Company's intangible assets are subject to amortization ratably over their estimated useful lives. Intangible assets consist of the following (in thousands, except weighted average useful life):
| |
December 31, | |
||||||
|---|---|---|---|---|---|---|---|---|
| |
Weighted- Average Useful Life |
|||||||
| |
2010 | 2009 | ||||||
Intellectual property rights |
$ | 21,399 | $ | 26,901 | 8.0 years | |||
Patents |
9,766 | 11,700 | 8.0 years | |||||
Distribution/customer relationships |
5,797 | 6,459 | 6.5 years | |||||
Trade name/trademarks |
4,228 | 4,828 | 7.8 years | |||||
Licensing, employment and non-competition agreements |
426 | 1,118 | 3.0 years | |||||
|
41,616 | 51,006 | 7.7 years | |||||
Less: accumulated amortization |
(30,883 | ) | (33,031 | ) | ||||
Total intangible assets, net |
$ | 10,733 | $ | 17,975 | ||||
Total amortization expense for the years ended December 31, 2010, 2009 and 2008 was approximately $5.3 million, $5.3 million and $5.5 million, respectively.
70
ARTHROCARE CORPORATION
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
NOTE 8—GOODWILL AND INTANGIBLE ASSETS (Continued)
Estimated future amortization expense is as follows (in thousands):
2011 |
$ | 5,332 | ||
2012 |
4,692 | |||
2013 |
626 | |||
2014 |
42 | |||
2015 |
6 | |||
Thereafter |
35 | |||
|
$ | 10,733 | ||
NOTE 9—ACCRUED LIABILITIES
The following summarizes the Company's accrued liabilities (in thousands):
| |
December 31, | ||||||
|---|---|---|---|---|---|---|---|
| |
2010 | 2009 | |||||
Compensation |
$ | 19,236 | $ | 14,865 | |||
Insurance dispute reserve |
9,830 | 15,268 | |||||
Legal fees and arbitration accrual |
2,041 | 2,862 | |||||
Agent commissions |
1,837 | 2,107 | |||||
Royalties and discounts |
1,660 | 2,946 | |||||
Accounting and reporting costs |
863 | 2,750 | |||||
Other |
4,730 | 5,279 | |||||
|
$ | 40,197 | $ | 46,077 | |||
The Company entered into settlement and release agreements in the year ended December 31, 2010 with certain private insurers related to cases in which letters of protection were held by its DiscoCare subsidiary. The Company agreed to pay these insurers an aggregate of $6.0 million, which reduced the Company's insurance dispute reserve when paid in 2010.
NOTE 10—COMMITMENTS
The Company leases certain facilities and equipment under operating leases. The Company recognizes rent expense on a straight-line basis over the lease term. Rent expense for 2010, 2009 and 2008 was $6.4 million, $6.1 million and $6.0 million, respectively.
The Company provides contributions to foundations that promote and support education and research initiatives.
In the ordinary course of business, the Company enters into agreements to purchase materials in the future that are enforceable, legally binding and specify terms with fixed or minimum quantities to be purchased and the approximate timing of payments.
The Company advances legal fees as required pursuant to indemnification agreements that were entered into with certain former executives and employees while they were employed with the Company. During the year ended December 31, 2010, the Company advanced $3.8 million and has
71
ARTHROCARE CORPORATION
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
NOTE 10—COMMITMENTS (Continued)
recorded additional liabilities for $1.1 million at December 31, 2010 under these indemnity agreements. We expect to continue to advance payments pursuant to these agreements in future periods; however, management is unable to estimate the amount or timing of future payments under such agreements.
At December 31, 2010, total future minimum commitments were as follows (in thousands):
| |
Minimum Lease Payments |
Educational Grants |
Purchase Commitments |
Total | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
2011 |
$ | 3,297 | $ | 50 | $ | 7,111 | $ | 10,458 | |||||
2012 |
2,796 | 50 | 2,658 | 5,504 | |||||||||
2013 |
2,531 | 50 | — | 2,581 | |||||||||
2014 |
1,705 | — | — | 1,705 | |||||||||
2015 |
1,275 | — | — | 1,275 | |||||||||
Thereafter |
1,521 | — | — | 1,521 | |||||||||
|
$ | 13,125 | $ | 150 | $ | 9,769 | $ | 23,044 | |||||
Warranties
Disposable medical devices are guaranteed for materials, function and workmanship for a single usage. The Company's warranty obligation is affected by product failure rates, material usage and service delivery costs incurred in correcting a product failure. The Company periodically evaluates and adjusts the warranty reserve to the extent actual warranty expense varies from the original estimates.
NOTE 11—LITIGATION AND CONTINGENCIES
In addition to the matters specifically described below, the Company is involved in other legal and regulatory proceedings that arise in the ordinary course of business that do not have a material impact on the Company's business. Litigation claims and proceedings of all types are subject to many factors that generally cannot be predicted accurately.
The Company records reserves for claims and lawsuits when they are probable and reasonably estimable. Except as otherwise specifically noted, the Company currently cannot determine the ultimate resolution of or the amount of monetary damages sought as relief in the matters described below. For matters where the likelihood or extent of a loss is not probable or cannot be reasonably estimated, the Company has not recognized in its consolidated financial statements the potential liability that may result from these matters. If one or more of these matters is determined against the Company, it could have a material adverse effect on the Company's earnings, liquidity and financial condition.
The Company continues to gather additional facts and information related to insurance billing and healthcare compliance issues and marketing and promotional practices in connection with these legal and administrative proceedings with the assistance of legal counsel.
SEC Investigation
The Fort Worth Regional Office of the Securities and Exchange Commission's ("SEC") Division of Enforcementconducted a formal investigation into accounting matters related to the restatement of financial results as announced by the Company on July 21, 2008 and completed on November 18, 2009,
72
ARTHROCARE CORPORATION
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
NOTE 11—LITIGATION AND CONTINGENCIES (Continued)
when the Company filed its Annual Report on Form 10-K for the fiscal year ended December 31, 2008, in which it provided a detailed description of these matters with respect to the Review and the restatement. The Company has entered a settlement with the SEC that fully resolves the SEC investigation against the Company. Under the settlement, the Company has consented to the entry by the SEC of an administrative order (the "Order"), on February 9, 2011, directing the Company to cease and desist from committing or causing violations of the reporting, books and records and internal control provisions of the federal securities laws in Sections 13(a), 13(b)(2)(A) and 13(b)(2)(B) of the Securities Exchange Act of 1934 and under Rules 12b-20, 13a-1 and 13a-13 promulgated under the Exchange Act. The Company consented to the entry of the Order without admitting or denying the Order's assertions of factual findings. No monetary penalty or fine was imposed on the Company, and none of the Company's current officers or employees were charged.
DOJ Investigation
The Department of Justice ("DOJ") is investigating certain of the Company's activities including past sales, accounting, and billing procedures primarily in relation to the operation of the Company's Spine product sales. The DOJ is also reviewing the Company's relationship with its DiscoCare subsidiary. The Company is cooperating with this investigation. At this stage of the investigation, the Company cannot predict the ultimate outcome and is unable to estimate any potential liability the Company may incur.
Private Securities Class Action and Shareholder Derivative Lawsuits
Federal Court Actions
On April 4, 2008, a putative securities class action was filed in Federal court in the Southern District of Florida against the Company and certain of its former executive officers, alleging violations of Sections 10(b) and 20(a) of the Exchange Act and Rule 10b-5 promulgated thereunder. Plaintiffs allege that the defendants violated federal securities laws by issuing false and misleading financial statements and making material misrepresentations regarding the Company's internal controls, business, and financial results. On October 28, 2008, the court granted the Company's motion to transfer this case to the U.S. District Court, Western District of Texas. (McIlvaine v. ArthroCare, et al).
On July 25, 2008, a putative securities class action was filed in Federal court in the Western District of Texas against the Company, and certain of its current and former executive officers, alleging violations of Sections 10(b) and 20(a) of the Exchange Act and Rule 10b-5 promulgated thereunder. Plaintiffs allege that the defendants violated federal securities laws by issuing false and misleading financial statements and making material misrepresentations regarding the Company's internal controls, business, and financial results. (Strong v. ArthroCare, et al).
On August 7, 2008, a derivative action was filed in Federal court in the Southern District of Florida against the Company and its then-current directors alleging breach of fiduciary duty based on the Company's alleged improper revenue recognition, improper reporting of such revenue in SEC filings and press releases, failure to maintain adequate internal controls, and failure to supervise management. On October 14, 2008, the court granted the Company's motion to transfer this case to the U.S. District Court, Western District of Texas. (Weil v. Baker, et al).
73
ARTHROCARE CORPORATION
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
NOTE 11—LITIGATION AND CONTINGENCIES (Continued)
On March 4, 2009, a derivative action was filed in Federal court in the Western District of Texas against the Company's current directors, a former director, certain of its current and former executive officers and other employees and PricewaterhouseCoopers LLP alleging (i) disgorgement under Section 304 of the Sarbanes-Oxley Act; (ii) violations of Section 10(b) of the Exchange Act and Rule 10b-5; (iii) breach of fiduciary duty; (iv) abuse of control; (v) gross mismanagement of the Company; (vi) waste of corporate assets; (vii) insider trading; and (viii) unjust enrichment. (King v. Baker, et al).
On April 29, 2009, a derivative action was filed in Federal court in the Western District of Texas against the Company's current directors and a former director alleging breach of fiduciary duty based on the Company's improper revenue recognition, improper reporting of such revenue in SEC filings and press releases, failure to maintain adequate internal controls, and failure to supervise management. (Barron v. Baker, et al).
On October 28, 2008, and thereafter, the two putative securities class actions and the shareholder derivative actions were consolidated and designated: In Re ArthroCare Corporation Securities Litigation, Case No. 1:08-cv-00574-SS (consolidated) in the U.S. District Court, Western District of Texas. On December 10, 2008, Lead Plaintiffs and Lead Plaintiffs' counsel were appointed in the putative consolidated securities class action. The Lead Plaintiff filed an Amended Consolidated Class Action Complaint on December 18, 2009, seeking unspecified monetary damages and interest. ArthroCare filed a Motion to Dismiss the Amended Consolidated Class Action Complaint on February 16, 2010. On July 20, 2010, the federal court issued a ruling granting in part and denying in part ArthroCare's Motion to Dismiss, permitting certain claims related to statements after December 11, 2007 to continue.
State Court Actions
On September 23, 2008, a derivative action was filed in Texas State District Court against the Company, and its then current directors and certain of its current and former officers. (Wieser v. Baker). In this action, one of the Company's shareholders alleged derivative claims on behalf of the Company that its directors and officers breached their fiduciary duties to shareholders by allowing improper financial reporting, failing to maintain adequate financial controls over revenue recognition, disseminating false financial statements, abuse of control, gross mismanagement, waste of corporate assets, and engaging in insider trading.
On October 20, 2008, a derivative action was filed in Texas State District Court against the Company, its then directors and certain of its current and former executive officers. (Bocklet v. Baker). In this action, one of the Company's shareholders alleged derivative claims on behalf of the Company that its directors and officers breached their fiduciary duties to shareholders by failing to maintain adequate financial controls over revenue recognition, allowing improper financial reporting, disseminating false financial statements, and engaging in insider trading.
On October 27, 2008, a derivative action was filed in Texas State District Court against the Company, its then directors and certain of its current and former executive officers. (Guthrie v. Baker). In this action, one of the Company's shareholders alleged derivative claims on behalf of the Company that its directors and officers breached their fiduciary duties to shareholders by failing to maintain adequate financial controls over revenue recognition, allowing improper financial reporting, disseminating false financial statements, and engaging in insider trading.
74
ARTHROCARE CORPORATION
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
NOTE 11—LITIGATION AND CONTINGENCIES (Continued)
On March 18, 2009, these three shareholder derivative actions were consolidated and designated: In Re ArthroCare Corporation Derivative Litigation, Case No. D-1-GN-08-3484 (consolidated), Travis County District Court. The consolidated action seeks financial compensation, certain governance changes and removal of certain directors. On the Company's motion, the state shareholder derivative actions were stayed pending resolution of all motions to dismiss the federal Amended Consolidated Class Action Complaint and class certification, if any, of a class in the federal Amended Consolidated Class Action Complaint.
Proposed Settlement of Shareholder Derivative Actions
On October 18, 2010, the Company, its directors' and officers' insurance carriers, and the plaintiffs in the Federal Court and State Court derivative actions agreed in principal to a settlement. Under the terms of the proposed settlement, the director and officer insurers will collectively pay $8 million to the Company, on behalf of the individuals named as defendants in the Federal Court and State Court derivative actions, to settle the State and Federal Derivative actions. The lawyers for the plaintiffs will be entitled to seek an award of legal fees and costs from the Federal Court in an amount not to exceed $2.25 million. The agreement also provides that the directors' and officers' insurers will pay the Company an additional $2 million for a broad mutual release by the Company and the insurers of any further rights or obligations under the directors' and officers' liability insurance policies. The settlement calls for certain mutually acceptable governance changes and is subject to approval of the former officers and directors who are parties to the derivative actions, an agreement by those former officers and directors to release the Company's directors' and officers' liability insurance carriers of any further rights or obligations under the applicable directors' and officers' liability insurance policies, the execution of a full and final settlement agreement, and the approval of the settlement of the derivative actions by the U.S. District Court, Western District of Texas. Upon approval of this settlement agreement, we will incur any further litigation cost or damages allegedly resulting from or based on the restatement or related events, including the ongoing DOJ investigation, the consolidated securities Class Action case and any ongoing indemnification obligations without the benefit of further insurance coverage.
NOTE 12—MEZZANINE EQUITY
Series A 3.00% Redeemable Convertible Preferred Stock
On September 1, 2009, the Company issued and sold 75,000 shares of the Company's Series A 3 Percent Redeemable Convertible Preferred Stock, par value $0.001 per share (the "Series A Preferred Stock"), for an aggregate purchase price of $75.0 million (the "Equity Financing") pursuant to the Securities Purchase Agreement dated August 14, 2009, by and between the Company and OEP AC Holdings, LLC ("OEP").
The Company used approximately $39 million of the proceeds to repay all outstanding indebtedness under a 5-year revolving credit agreement dated January 13, 2006 (the "Credit Agreement") and to cash collateralize a letter of credit. After the repayment of the Credit Agreement and the payment of fees and expenses related thereto and to the issuance of the Preferred Stock to OEP, the Company had approximately $35 million of net proceeds, which the Company has used together with cash from operations to fund its general business requirements.
75
ARTHROCARE CORPORATION
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
NOTE 12—MEZZANINE EQUITY (Continued)
Cumulative dividends on the Series A Preferred Stock are payable-in-kind on a quarterly basis at the rate per annum of 3 percent of the liquidation preference of $1,000 per share (the "Liquidation Preference") until October 1, 2014, (the "Dividend Duration Period"). As of December 31, 2010 and 2009, dividends of $3.1 million and $0.8 million were accrued, respectively.
The holders of the Series A Preferred Stock may convert their shares at any time, in whole or in part, at a rate of 66.667 shares of the Company's Common Stock per $1,000 of Liquidation Preference of the Series A Preferred Stock, subject to customary anti-dilution adjustments (the "Conversion Rate"), representing an initial conversion price of $15.00 per share of Common Stock. If a conversion occurs prior to the expiration of the Dividend Duration Period, the number of shares of Common Stock received shall be increased for a make-whole adjustment equal to the number of additional shares of Series A Preferred Stock the holder would have otherwise been paid during the Dividend Duration Period, multiplied by the Conversion Rate (the "Make-Whole Adjustment"). As of December 31, 2010, none of the shares of Series A Preferred Stock have been converted into Common Stock.
The Company may, at any time after September 1, 2010, cause an automatic conversion of all outstanding shares of Series A Preferred Stock upon no less than 10 days prior notice if the closing sales price of the Common Stock equals or exceeds $35.00 per share (subject to adjustment in the event of a stock split, stock dividend, combination or other similar recapitalization) for the prior 20 consecutive trading days, the Company is current on its reporting requirements with the SEC, the Company has an effective resale registration statement and related prospectus that permits the Common Stock issued upon such automatic conversion to be immediately resold thereunder, and the current DOJ investigations of the Company by the U.S. Attorney's offices in Florida and North Carolina have been terminated, settled or finally adjudicated.
No conversion of Series A Preferred Stock will be permitted to the extent that any holder of Series A Preferred Stock would individually hold in excess of 19.99 percent of the Company's voting power after the proposed conversion, or to the extent that the holders of Series A Preferred Stock would hold in excess of 17.8 percent of the Company's voting power in the aggregate, in each case solely attributable to their holdings of Series A Preferred Stock and any Common Stock received upon conversion thereof (such limitations collectively, the "Conversion Cap"). Shares of Series A Preferred Stock not convertible as a result of the Conversion Cap shall remain outstanding and shall become convertible to the extent the Conversion Cap no longer applies.
At any time after September 1, 2014, or in connection with a change in control of the Company, holders of the Series A Preferred Stock may require the Company to redeem any or all outstanding shares of Series A Preferred Stock at the Liquidation Preference amount plus any applicable Make-Whole Adjustment. The Series A Preferred Stock has a maximum redemption value of $87.1 million.
The Series A Preferred Stock will rank subordinate and junior in right of payments to all indebtedness of the Company. Holders of the Series A Preferred Stock will vote with the Common Stock on an as-converted basis, including any applicable Make-Whole Adjustment. However, no holder of the Series A Preferred Stock shall be permitted to vote more than an equivalent of 19.99 percent of the Company's outstanding voting securities solely attributable to its ownership of the Series A Preferred Stock and any Common Stock received upon conversion thereof. Holders of a majority of the Series A Preferred Stock then outstanding, voting as a separate class, must approve any amendment to
76
ARTHROCARE CORPORATION
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
NOTE 12—MEZZANINE EQUITY (Continued)
the Company's articles of incorporation or bylaws that would adversely affect the rights of the holders of the Series A Preferred Stock.
The Series A Preferred Stock can be converted into a maximum of 5,805,921 shares of the Company's common stock (the "Conversion Shares") representing a conversion price after the Make-Whole Adjustment of $12.92 (the "Conversion Price"). The closing value of the Company's common stock on the date of issuance was $17.45 (the "Closing Price") resulting in an intrinsic value of $4.65 per Conversion Share calculated as the Closing Price of $17.45 less the Conversion Price of $12.92 less approximately $0.7 million in proceeds which were used to pay expenses of the investor. This resulted in a beneficial conversion feature of $27.0 million on the sale of the Series A Preferred Stock based on the intrinsic value of each Conversion Share multiplied by the number of Conversion Shares. The beneficial conversion feature was immediately charged against net loss applicable to common stockholders as the Series A Preferred stock can be converted into common stock at the option of the holder.
Gross proceeds from the sale of the Series A Preferred Stock were reduced by direct issuance cost of $5.6 million, including the amount used to pay the expenses of the investor, and will be accreted over a five year period using the effective interest rate method. Total accretion charges for the periods ended December 31, 2010 and 2009 were $1.0 million and $0.3 million and were recorded as a dividend.
Pursuant to the Registration Rights Agreement between us and the holders of our Series A Preferred Stock, the Company was required to file a registration statement on Form S-1 prior to September 1, 2010, to register the resale of the Common Stock underlying the Series A Preferred Stock. If the registration statement was not filed or became effective September 1, 2010, then the Company would be required to pay the holders of the Series A Preferred Stock an amount equal to 2.00 percent per annum of the liquidation preference of the Series A Preferred Stock until the registration default was cured. The Company filed a registration statement on Form S-1 on May 13, 2010, a notice of effectiveness on August 8, 2010, and a Post Effective Amendment on December 6, 2010, to convert the registration statement on Form S-1 to a registration statement on Form S-3. Accordingly, as of December 31, 2010, the Company was in compliance with its registration statement obligations contained in the Registration Rights Agreement.
NOTE 13—STOCKHOLDERS EQUITY
Treasury Stock
In 2010, 2009 and 2008, the Company issued 30,500 shares from treasury valued at $0.9 million, 81,524 shares valued at $0.9 million, 78,669 shares valued at $0.9 million, respectively, in order to fulfill the Company's obligations to match employees' 401(k) contributions with shares of Company stock.
Employee Stock Purchase Plan
The Company has an Employee Stock Purchase Plan, or ESPP. Under the ESPP plan, regular full-time employees (subject to certain exceptions) may contribute up to 10 percent of base compensation to the semi-annual purchase of shares of the Company's common stock at 95 percent of the fair market value at certain plan-defined dates. The Company reserved a total of 450,000 shares of
77
ARTHROCARE CORPORATION
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
NOTE 13—STOCKHOLDERS EQUITY (Continued)
common stock for issuance under the ESPP and as of December 31, 2010, 52,239 shares remained available for future purchases under the ESPP.
Stock Option Plans
In August 1999, the Company adopted the Non-statutory Option Plan ("1999 Plan") which expired in 2009. Awards that were granted prior to the expiration of the 1999 Plan will continue to operate in accordance with the terms of the 1999 Plan.
In May 2003, the Company adopted the 2003 Incentive Stock Plan ("2003 Plan") under which the Board of Directors is authorized to grant incentive and non-statutory stock option awards and restricted stock awards to employees and consultants. The 2003 Plan has been amended with approval of the Company's stockholders to increase the number of shares available for issuance to a total of 5,900,000 shares. As of December 31, 2010, the Company had 3,247,439 shares available for future grant under the 2003 Plan.
Options granted under the 1999 Plan and the 2003 Plan generally become exercisable over a 36-month period for the current CEO, 48-month period for all other employees and restricted stock awards generally become exercisable over a three Board of Directors and five year period for all other employees. All options and awards granted under each of the Company's plans have a legal life of seven years, effective February 2005. Prior to that, options granted had a legal life of 10 years from the grant date. The Company has historically issued new shares upon exercises of stock options and vesting of restricted stock.
The 1999 Plan and the 2003 Plan authorize the Company to issue Stock Appreciation Rights ("SARs") to directors, employees and consultants of the Company. SARs granted under the plans have an expiration term of seven years and generally become exercisable over a 48-month period.
78
ARTHROCARE CORPORATION
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
NOTE 13—STOCKHOLDERS EQUITY (Continued)
Activity for all stock based compensation plans for the years ended December 31, 2010, 2009 and 2008 is as follows:
| |
Stock Options Outstanding | Restricted Stock Awards Outstanding | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Number of Shares |
Weighted- Average Exercise Price |
Number of Shares |
Weighted- Average Grant Date Fair Value |
||||||||||
Balance as of December 31, 2007 |
2,995,245 | $ | 28.78 | 358,870 | $ | 35.41 | ||||||||
Granted |
81,686 | $ | 42.85 | 128,507 | $ | 43.21 | ||||||||
Exercised/vested |
(74,590 | ) | $ | 21.89 | (133,979 | ) | $ | 20.71 | ||||||
Canceled/forfeited |
(164,656 | ) | $ | 36.76 | (54,006 | ) | $ | 42.28 | ||||||
Balance as of December 31, 2008 |
2,837,685 | $ | 28.90 | 299,392 | $ | 44.10 | ||||||||
Granted |
1,153,462 | $ | 23.50 | 177,619 | $ | 23.50 | ||||||||
Exercised/vested |
(22,234 | ) | $ | 20.38 | (52,677 | ) | $ | 39.88 | ||||||
Canceled/forfeited |
(1,110,135 | ) | $ | 28.04 | (62,301 | ) | $ | 44.43 | ||||||
Balance as of December 31, 2009 |
2,858,778 | $ | 27.12 | 362,033 | $ | 34.55 | ||||||||
Granted |
102,100 | $ | 27.38 | 79,708 | $ | 28.24 | ||||||||
Exercised/vested |
(115,254 | ) | $ | 17.96 | (87,670 | ) | $ | 32.35 | ||||||
Canceled/forfeited |
(245,192 | ) | $ | 30.30 | (27,596 | ) | $ | 44.78 | ||||||
Balance as of December 31, 2010 |
2,600,432 | $ | 27.24 | 326,475 | $ | 32.74 | ||||||||
Net cash proceeds from the exercise of stock option awards were $1.9 million, $0.4 million, and $1.2 million for the years ended December 31, 2010, 2009 and 2008, respectively.
As of December 31, 2010, there was $8.4 million of unrecognized compensation expense related to unvested stock option awards granted under the Company's stock plans. That unrecognized cost is expected to be recognized over a weighted average period of 2.6 years. At December 31, 2010, the aggregate intrinsic value associated with these options was $16.2 million.
During the year ended December 31, 2010, the Company issued 79,708 shares of restricted common stock, which were valued at $2.1 million when reduced by estimated forfeitures. At December 31, 2010, there was total unrecognized compensation expense of $5.8 million, net of estimated forfeitures, related to unvested restricted stock awards and units. That unrecognized cost is expected to be recognized over a weighted average period of 2.7 years. At December 31, 2010, the aggregate intrinsic value associated with the unvested restricted stock awards was $10.1 million.
79
ARTHROCARE CORPORATION
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
NOTE 13—STOCKHOLDERS EQUITY (Continued)
Stock option awards outstanding and currently exercisable by exercise price for all plans at December 31, 2010 were as follows:
| |
Options Outstanding | Options Exercisable | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Range of Exercise Prices
|
Shares Outstanding |
Weighted-Average Remaining Contractual Life |
Weighted- Average Exercise Price |
Options Exercisable |
Weighted- Average Exercise Price |
||||||||||||
$ 9.84 - $16.10 |
301,898 | 1.85 | $ | 13.49 | 301,898 | $ | 13.49 | ||||||||||
$17.30 - $22.77 |
205,851 | 0.93 | $ | 21.94 | 205,851 | $ | 21.94 | ||||||||||
$23.50 - $23.50 |
1,026,218 | 5.85 | $ | 23.50 | 379,262 | $ | 23.50 | ||||||||||
$24.38 - $28.42 |
292,378 | 3.49 | $ | 26.20 | 210,465 | $ | 26.05 | ||||||||||
$28.70 - $36.08 |
324,544 | 2.18 | $ | 33.90 | 297,267 | $ | 33.99 | ||||||||||
$37.24 - $44.04 |
287,633 | 3.30 | $ | 41.23 | 263,757 | $ | 41.19 | ||||||||||
$44.50 - $48.05 |
142,038 | 2.05 | $ | 46.41 | 138,140 | $ | 46.46 | ||||||||||
$50.62 - $54.15 |
19,872 | 3.59 | $ | 51.09 | 16,137 | $ | 51.09 | ||||||||||
Total |
2,600,432 | 3.77 | $ | 27.24 | 1,812,777 | $ | 28.24 | ||||||||||
Valuation and Expense Information
The following table summarizes the reporting of total stock based compensation expense resulting from employee stock options, including stock appreciation rights, and restricted stock awards for the years ended December 31, 2010, 2009 and 2008 (in thousands):
| |
Year Ended December 31, | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2010 | 2009 | 2008 | ||||||||
Cost of product sales |
$ | 1,401 | $ | 1,079 | $ | 1,309 | |||||
Research and development |
849 | 671 | 1,208 | ||||||||
Sales and marketing |
2,282 | 2,403 | 3,170 | ||||||||
General and administrative |
3,469 | 2,404 | 4,191 | ||||||||
Stock based compensation expense before income taxes |
8,001 | 6,557 | 9,878 | ||||||||
Income tax benefit |
2,360 | 2,227 | 3,431 | ||||||||
Total stock-based compensation expense, net of income taxes |
$ | 5,641 | $ | 4,330 | $ | 6,447 | |||||
The weighted average fair value of each option granted to employees during the years ended December 31, 2010, 2009 and 2008 were estimated at $12.76, $10.73 and $20.63, respectively. The fair value of each stock option was estimated on the grant date using the Black Scholes option pricing
80
ARTHROCARE CORPORATION
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
NOTE 13—STOCKHOLDERS EQUITY (Continued)
model with the following weighted average assumptions for the years ended December 31, 2010, 2009 and 2008:
| |
2010 | 2009 | 2008 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
Expected term (in years) |
4.4 | 4.2 | 3.8 | |||||||
Expected volatility |
56 | % | 56 | % | 66 | % | ||||
Risk-free interest rate |
1.7 | % | 1.9 | % | 2.6 | % | ||||
Expected dividends |
— | — | — | |||||||
Stock-based compensation expense recognized has been reduced for estimated forfeitures at a rate of 5.7 percent based on the Company's historical experience. Forfeitures are estimated at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differ from those estimates.
NOTE 14—LOAN RECEIVABLE
The Company loaned $2.5 million to a sterilization subcontractor with proceeds used to construct the subcontractor's Costa Rican sterilization facility. The loan, which is recorded in other assets in the accompanying consolidated balance sheet, bears interest at a fixed rate of 7.0 percent and calls for repayment during the period of 2010 through 2015. The loan is secured by all of the subcontractor's assets in Costa Rica. The balance of the loan receivable, including accrued interest, at December 31, 2010 and 2009 is $2.5 million and $2.7 million respectively. The Company has not recorded an allowance for collectability on the loan, and there are no amounts past due.
NOTE 15—INCOME TAXES
The components of income (loss) before income taxes were as follows (in thousands):
| |
Year Ended December 31, | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2010 | 2009 | 2008 | ||||||||
Domestic |
$ | 29,433 | $ | (2,949 | ) | $ | (30,961 | ) | |||
Foreign |
20,973 | 790 | (1,796 | ) | |||||||
Total |
$ | 50,406 | $ | (2,159 | ) | $ | (32,757 | ) | |||
The amounts identified above exclude loss from discontinued operations before income taxes of $0.6 million, $0.7 million and $10.1 million for 2010, 2009 and 2008, respectively.
81
ARTHROCARE CORPORATION
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
NOTE 15—INCOME TAXES (Continued)
The income tax provision (benefit) consisted of the following (in thousands):
| |
Year Ended December 31, | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2010 | 2009 | 2008 | |||||||||
Current: |
||||||||||||
Federal |
$ | 7,801 | $ | 845 | $ | 579 | ||||||
State |
1,221 | 205 | 903 | |||||||||
Foreign |
981 | 748 | 859 | |||||||||
Total current |
10,003 | 1,798 | 2,341 | |||||||||
Deferred: |
||||||||||||
Federal |
3,088 | 2,033 | (8,133 | ) | ||||||||
State |
(8 | ) | (485 | ) | (2,196 | ) | ||||||
Foreign |
(195 | ) | (209 | ) | (187 | ) | ||||||
Total deferred |
2,885 | 1,339 | (10,516 | ) | ||||||||
Total income tax provision (benefit) |
$ | 12,888 | $ | 3,137 | $ | (8,175 | ) | |||||
The amounts identified above exclude the provision (benefit) for income tax from discontinued operations. These amounts are ($0.2 million), ($0.3 million), and $0.3 million for 2010, 2009 and 2008, respectively.
The income tax provision (benefit) differed from a provision computed at the U.S. statutory tax rate as follows (in thousands):
| |
Year Ended December 31, | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2010 | 2009 | 2008 | ||||||||
Tax at federal statutory rate |
$ | 17,642 | $ | (756 | ) | $ | (11,465 | ) | |||
State income taxes |
890 | (70 | ) | (306 | ) | ||||||
Differences in foreign tax rates |
(6,555 | ) | 289 | 1,060 | |||||||
Research and development credits |
(1,276 | ) | (940 | ) | (1,282 | ) | |||||
Stock-based compensation |
693 | 330 | 419 | ||||||||
Nondeductible expenses |
324 | 391 | 470 | ||||||||
Goodwill impairment |
— | — | 2,188 | ||||||||
Accruals for intercompany payments |
1,814 | 3,867 | 312 | ||||||||
Other |
(644 | ) | 26 | 429 | |||||||
Total income tax provision (benefit) |
$ | 12,888 | $ | 3,137 | $ | (8,175 | ) | ||||
The Company's tax expense for the period 2008 through 2010 was significantly reduced due to a tax holiday for its Costa Rica manufacturing operations. Under its holiday, the Company will not be subject to Costa Rican income tax until January 2016. At that time, the Company will become subject to the normal Costa Rican corporate taxes, which are currently 30 percent. Without the Costa Rican tax holiday, the increase in income tax and the impact on earnings (loss) per share would have been
82
ARTHROCARE CORPORATION
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
NOTE 15—INCOME TAXES (Continued)
$2.7 million and $0.10, $2.7 million and $0.10 and $2.4 million and $0.09, respectively, for 2010, 2009 and 2008, respectively.
| |
December 31, | |||||||
|---|---|---|---|---|---|---|---|---|
| |
2010 | 2009 | ||||||
Federal loss carryover |
$ | 10,284 | $ | 18,914 | ||||
Portion related to unrecognized stock-based compensation deductions |
— | 401 | ||||||
State loss carryover |
— | 6,550 | ||||||
Portion related to unrecognized stock-based compensation deductions |
— | 401 | ||||||
Federal research and development credit carryover |
7,111 | 6,988 | ||||||
State research and development credit carryover |
8,972 | 8,505 | ||||||
The Company's federal net operating loss carryforwards will expire during the period 2020 through 2024 if not utilized. The Company's federal research and development credit carryforwards will expire during the period 2012 through 2030. The state research and development credits are not subject to expiration.
Under U.S. tax law, certain changes in the Company's ownership could result in an annual limitation on the amount of net operating loss carryforwards and income tax credits that could be utilized to offset future tax liabilities. At December 31, 2010, all of the Company's federal net operating loss carryforwards of approximately $10.3 million are subject to an annual utilization limit of approximately $0.9 million pursuant to IRC Section 382.
83
ARTHROCARE CORPORATION
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
NOTE 15—INCOME TAXES (Continued)
The Company's deferred tax assets and liabilities consist of the following (in thousands):
| |
December 31, | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
2010 | 2009 | ||||||||
Deferred tax assets: |
||||||||||
Net operating loss carryovers, net of reserves |
$ | 3,600 | $ | 7,555 | ||||||
R&D and other tax credits, net of reserves |
12,943 | 12,610 | ||||||||
Allowances and reserves |
8,794 | 7,183 | ||||||||
Deferred revenue |
14,130 | 17,116 | ||||||||
Alternative Minimum Tax credit |
822 | 371 | ||||||||
Stock-based compensation |
6,022 | 7,303 | ||||||||
Other |
454 | 10 | ||||||||
Gross deferred tax assets |
46,765 | 52,148 | ||||||||
Valuation allowance |
— | (292 | ) | |||||||
Subtotal |
46,765 | 51,856 | ||||||||
Deferred tax liabilities: |
||||||||||
Property and equipment |
(3,107 | ) | (3,692 | ) | ||||||
Non-goodwill intangibles |
(3,191 | ) | (4,948 | ) | ||||||
Other |
(149 | ) | (13 | ) | ||||||
Gross deferred tax liabilities |
(6,447 | ) | (8,653 | ) | ||||||
Net deferred tax assets |
$ | 40,318 | $ | 43,203 | ||||||
During 2010, the Company utilized $0.3 million of deferred tax assets which had a full valuation allowance as of the beginning of the year.
Income tax benefits resulting from employee stock compensation of $1.2 million, $1.4 million, and $3.8 million, were credited to additional paid-in capital for the years ended December 31, 2010, 2009 and 2008, respectively. Additionally, in 2010, the Company made an adjustment to reduce additional paid-in capital by $3.2 million to reflect the impact of the amendment and filing of prior year returns for 2006 through 2009.
Deferred U.S. income taxes and foreign withholding taxes are not provided on the undistributed cumulative earnings of foreign subsidiaries because those earnings are considered to be permanently reinvested in those operations. The permanently reinvested undistributed earnings were approximately $30.1 million as of December 31, 2010. The tax impact resulting from a distribution of these earnings would be approximately $11.4 million.
84
ARTHROCARE CORPORATION
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
NOTE 15—INCOME TAXES (Continued)
The Company's change in uncertain tax benefit reserves during 2010, 2009 and 2008 were as follows (in thousands):
| |
2010 | 2009 | 2008 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
Balance at January 1 |
$ | 7,078 | $ | 8,490 | $ | 8,451 | |||||
Additions for tax positions of current period |
2,252 | 652 | 632 | ||||||||
Additions for tax positions of prior years |
7,384 | — | — | ||||||||
Decreases for tax positions of prior years |
(236 | ) | (2,064 | ) | (593 | ) | |||||
Balance at December 31 |
$ | 16,478 | $ | 7,078 | $ | 8,490 | |||||
As of December 31, 2010, total uncertain tax positions amounted to $16.5 million. Should the tax positions prove successful, the Company's tax expense would be reduced by $15.2 million (net of federal benefit). $11.6 million of the uncertain tax position reserves is included in noncurrent liabilities, while the remaining $3.6 million of uncertain tax positions reduce the research and development carry-forward deferred tax assets. No interest or penalty provisions have been recorded as of December 31, 2010 as the Company had net operating loss carryovers available.
In February 2008, the Company submitted an application with the Internal Revenue Service (the "IRS") for an Advanced Pricing Agreement, ("APA"), relating to the transfer pricing policies between the Company and its foreign subsidiaries. The Company has subsequently withdrawn from the process.
In 2010, the Company was notified by the IRS of its intention to examine the 2006 federal income tax return. No adjustments have yet been proposed; however, it is expected that the examination will not be completed until 2012.
The Company is subject to audit by the IRS and California Franchise Tax Board for the years 1994 through 2010 as a result of its net operating loss and credit carryover positions, and by the Texas State Comptroller for the years 2006 through 2010. As the Company has operations in most other U.S. states, other state tax authorities may assess deficiencies related to prior year activities; however, the years open to assessment vary with each state. The Company also files income tax returns for non-U.S. jurisdictions; the most significant of which are the UK, Sweden, and Germany. The years open to adjustment for the UK and Germany are 2006 through 2010. The years open to adjustment for Sweden are 2008 and 2010.
In 2009, the Company had adjusted its accrued inter-company payments for all open years as a result of the issuance of new U.S. cost sharing regulations. As a result of the realization of changes to the payment amounts, approximately $1.9 million of the income tax reserve was reversed in 2009. The impact of this change resulted in approximately $3.5 million of the additional income tax expense during first quarter of 2009.
In 2010, the Company amended or filed its U.S. income tax returns for its 2006, 2007, 2008 and 2009 tax years. The Company's tax filings were based on its historical intercompany cost sharing and transfer pricing policies whereas the tax liability accrued in prior years was based on certain possible modifications to these policies. The excess income tax payable over the amount reported in the Company's income tax returns of approximately $7.4 million has been recorded as an addition to uncertain tax positions of prior years during 2010.
85
ARTHROCARE CORPORATION
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
NOTE 16—EMPLOYEE BENEFIT PLAN
The Company maintains a Retirement Savings and Investment Plan ("401(k) Plan"), which covers all U.S.-based employees. Eligible employees may defer salary (before tax) up to a federally specified maximum. Management, at its discretion, may make matching contributions in stock on behalf of the participants in the 401(k) Plan. The Company matched up to $2,500 in Company stock annually in 2010, 2009 and 2008. The Company matched approximately $0.9 million of employee contributions to the 401(k) Plan in each of the years ended December 31, 2010, 2009 and 2008.
NOTE 17—FAIR VALUE MEASUREMENTS
At December 31, 2010, the assets measured at fair value on a recurring basis consisted of short-term investments of $0.1 million, which were fair valued based on quoted prices from active markets for identical assets. The Company's short-term investments consist primarily of corporate bonds. The Company had no assets or liabilities that were measured at fair value on a non-recurring basis. The estimated fair value of the Company's notes payable and loan receivable approximate the carrying value presented in its consolidated balance sheet based on discounting the expected future cash flows using current market rates as of December 31, 2010.
NOTE 18—SEGMENT INFORMATION
The Company has organized its marketing and sales efforts based on four operating segments which are aggregated into one reportable segment—the development, manufacture and marketing of disposable devices for less invasive surgical procedures and implants. Each of the Company's operating segments has similar economic characteristics, technology, manufacturing processes, customers, distribution and marketing strategies, regulatory environments, and shared infrastructures. These operating segments are Sports Medicine—Americas, ENT—Americas, Other-Americas and International.
Product sales by operating segments and product area for the periods shown were as follows (in thousands):
| |
Year Ended December 31, 2010 | Year Ended December 31, 2009 | Year Ended December 31, 2008 | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Americas | International | Total Product Sales |
Americas | International | Total Product Sales |
Americas | International | Total Product Sales |
|||||||||||||||||||
Sports Medicine |
$ | 160,859 | $ | 71,120 | $ | 231,979 | $ | 147,169 | $ | 62,533 | $ | 209,702 | $ | 142,856 | $ | 56,676 | $ | 199,532 | ||||||||||
ENT |
79,019 | 15,270 | 94,289 | 72,375 | 13,299 | 85,674 | 68,412 | 12,072 | 80,484 | |||||||||||||||||||
Other |
4,122 | 8,367 | 12,489 | 5,231 | 7,886 | 13,117 | 5,772 | 9,330 | 15,102 | |||||||||||||||||||
Total Product Sales |
$ | 244,000 | $ | 94,757 | $ | 338,757 | $ | 224,775 | $ | 83,718 | $ | 308,493 | $ | 217,040 | $ | 78,078 | $ | 295,118 | ||||||||||
Internationally, the Company markets and supports its products primarily through its subsidiaries and various distributors. Revenues attributed to geographic areas are based on the country or regional
86
ARTHROCARE CORPORATION
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
NOTE 18—SEGMENT INFORMATION (Continued)
area where the Company's customer is domiciled. Product sales by geography for the periods shown were as follows (in thousands):
| |
Year Ended December 31, | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2010 | 2009 | 2008 | ||||||||
United States |
$ | 226,383 | $ | 212,766 | $ | 208,554 | |||||
Non-United States(1) |
112,374 | 95,727 | 86,564 | ||||||||
Total product sales |
$ | 338,757 | $ | 308,493 | $ | 295,118 | |||||
- (1)
- No additional locations are individually significant.
Long-lived assets by geography at the balance sheet dates shown were as follows (in thousands):
| |
December 31, | |||||||
|---|---|---|---|---|---|---|---|---|
| |
2010 | 2009 | ||||||
United States |
$ | 27,285 | $ | 31,791 | ||||
Costa Rica |
12,750 | 13,693 | ||||||
Other |
5,729 | 6,719 | ||||||
Total long-lived assets |
$ | 45,764 | $ | 52,203 | ||||
NOTE 19—QUARTERLY FINANCIAL INFORMATION (unaudited)
The following tables present certain unaudited condensed consolidated quarterly financial information for each quarter in the years ended December 31, 2010 and 2009. In the Company's opinion, this unaudited quarterly information has been prepared on the same basis as the consolidated
87
ARTHROCARE CORPORATION
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
NOTE 19—QUARTERLY FINANCIAL INFORMATION (unaudited) (Continued)
financial statements and includes all adjustments necessary to state fairly the information for the periods presented (in thousands, except per share data).
| |
Year Ended December 31, 2010 | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
First Quarter | Second Quarter | Third Quarter | Fourth Quarter | |||||||||||
Revenues: |
|||||||||||||||
Product sales |
$ | 85,165 | $ | 83,066 | $ | 82,917 | $ | 87,609 | |||||||
Royalties, fees and other |
3,949 | 4,113 | 3,536 | 5,024 | |||||||||||
Total revenues |
89,114 | 87,179 | 86,453 | 92,633 | |||||||||||
Cost of product sales |
26,579 | 28,358 | 28,632 | 27,182 | |||||||||||
Gross profit |
62,535 | 58,821 | 57,821 | 65,451 | |||||||||||
Operating expenses: |
|||||||||||||||
Research and development |
8,879 | 8,414 | 9,244 | 9,309 | |||||||||||
Sales and marketing |
26,855 | 26,887 | 26,156 | 27,954 | |||||||||||
General and administrative |
8,970 | 9,083 | 9,283 | 11,155 | |||||||||||
Amortization of intangible assets |
1,315 | 1,302 | 1,315 | 1,305 | |||||||||||
Reimbursement services |
21 | 24 | 25 | 22 | |||||||||||
Investigation and restatement-related costs |
1,043 | 528 | 317 | 952 | |||||||||||
Total operating expenses |
47,083 | 46,238 | 46,340 | 50,697 | |||||||||||
Income from operations |
15,452 | 12,583 | 11,481 | 14,754 | |||||||||||
Interest and other income (expense), net |
(3,061 | ) | (1,164 | ) | 1,227 | (866 | ) | ||||||||
Income from continuing operations |
|||||||||||||||
before income taxes |
12,391 | 11,419 | 12,708 | 13,888 | |||||||||||
Income tax provision |
3,314 | 3,134 | 3,536 | 2,904 | |||||||||||
Net income from continuing operations |
9,077 | 8,285 | 9,172 | 10,984 | |||||||||||
Income (loss) from discontinued operations, net of taxes |
(250 | ) | (203 | ) | 85 | (66 | ) | ||||||||
Net income |
8,827 | 8,082 | 9,257 | 10,918 | |||||||||||
Accrued dividend, beneficial conversion |
|||||||||||||||
feature and accretion charges on Series A |
|||||||||||||||
3% Redeemable Convertible Preferred Stock |
(802 | ) | (812 | ) | (820 | ) | (830 | ) | |||||||
Net income attributable to common stockholders |
$ | 8,025 | $ | 7,270 | $ | 8,437 | $ | 10,088 | |||||||
Weighted-average shares outstanding: |
|||||||||||||||
Basic |
26,922 | 26,976 | 27,017 | 27,059 | |||||||||||
Diluted |
27,221 | 27,352 | 27,323 | 27,446 | |||||||||||
Earnings per share from continuing operations applicable to common stockholders: |
|||||||||||||||
Basic |
$ | 0.25 | $ | 0.23 | $ | 0.25 | $ | 0.31 | |||||||
Diluted |
$ | 0.25 | $ | 0.22 | $ | 0.25 | $ | 0.30 | |||||||
Earnings per share applicable to common stockholders: |
|||||||||||||||
Basic |
$ | 0.25 | $ | 0.22 | $ | 0.26 | $ | 0.31 | |||||||
Diluted |
$ | 0.24 | $ | 0.22 | $ | 0.25 | $ | 0.30 | |||||||
88
ARTHROCARE CORPORATION
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
NOTE 19—QUARTERLY FINANCIAL INFORMATION (unaudited) (Continued)
| |
Year Ended December 31, 2009 | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
First Quarter | Second Quarter | Third Quarter | Fourth Quarter | |||||||||||
Revenues: |
|||||||||||||||
Product sales |
$ | 73,575 | $ | 75,443 | $ | 74,716 | $ | 84,759 | |||||||
Royalties, fees and other |
3,159 | 3,230 | 3,066 | 6,169 | |||||||||||
Total revenues |
76,734 | 78,673 | 77,782 | 90,928 | |||||||||||
Cost of product sales |
21,288 |
21,555 |
23,684 |
24,413 |
|||||||||||
Gross profit |
55,446 | 57,118 | 54,098 | 66,515 | |||||||||||
Operating expenses: |
|||||||||||||||
Research and development |
8,136 | 9,262 | 9,019 | 8,786 | |||||||||||
Sales and marketing |
28,475 | 29,224 | 27,517 | 29,323 | |||||||||||
General and administrative |
11,932 | 12,157 | 11,967 | 9,978 | |||||||||||
Amortization of intangible assets |
1,334 | 1,337 | 1,330 | 1,308 | |||||||||||
Reimbursement services |
168 | 98 | 46 | 41 | |||||||||||
Investigation and restatement-related costs |
8,724 | 7,306 | 9,293 | 6,363 | |||||||||||
Total operating expenses |
58,769 | 59,384 | 59,172 | 55,799 | |||||||||||
Income (loss) from operations |
(3,323 | ) | (2,266 | ) | (5,074 | ) | 10,716 | ||||||||
Interest and other income (expense), net |
(1,338 | ) | (1,929 | ) | (1,653 | ) | 2,708 | ||||||||
Income (loss) from continuing operations before income taxes before income taxes |
(4,661 | ) | (4,195 | ) | (6,727 | ) | 13,424 | ||||||||
Income tax provision (benefit) |
1,757 |
(1,597 |
) |
(2,573 |
) |
5,550 |
|||||||||
Net income (loss) from continuing operations |
(6,418 | ) | (2,598 | ) | (4,154 | ) | 7,874 | ||||||||
Loss from discontinued operations, net of taxes |
(89 |
) |
(91 |
) |
(273 |
) |
(28 |
) |
|||||||
Net income (loss) |
(6,507 | ) | (2,689 | ) | (4,427 | ) | 7,846 | ||||||||
Accrued dividend, beneficial conversion feature and accretion charges on Series A 3% Redeemable Convertible Preferred Stock |
— |
— |
(27,252 |
) |
(793 |
) |
|||||||||
Net income (loss) attributable to common stockholders |
$ | (6,507 | ) | $ | (2,689 | ) | $ | (31,679 | ) | $ | 7,053 | ||||
Weighted-average shares outstanding: |
|||||||||||||||
Basic |
26,746 | 26,810 | 26,838 | 26,857 | |||||||||||
Diluted |
26,746 | 26,810 | 26,838 | 27,024 | |||||||||||
Earnings (loss) per share from continuing operations applicable to common stockholders: |
|||||||||||||||
Basic |
$ | (0.24 | ) | $ | (0.10 | ) | $ | (1.17 | ) | $ | 0.22 | ||||
Diluted |
$ | (0.24 | ) | $ | (0.10 | ) | $ | (1.17 | ) | $ | 0.22 | ||||
Earnings (loss) per share applicable to common stockholders: |
|||||||||||||||
Basic |
$ | (0.24 | ) | $ | (0.10 | ) | $ | (1.18 | ) | $ | 0.22 | ||||
Diluted |
$ | (0.24 | ) | $ | (0.10 | ) | $ | (1.18 | ) | $ | 0.21 | ||||
89
ARTHROCARE CORPORATION
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
NOTE 20—SUPPLEMENTAL CASH FLOW INFORMATION
Information below is in thousands:
| |
Year Ended December 31, | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
2010 | 2009 | 2008 | |||||||
Cash paid for interest |
$ | — | $ | 2,312 | $ | 2,991 | ||||
Cash paid for income taxes |
1,802 | — | 4,553 | |||||||
Non cash Series A Preferred Stock beneficial conversion feature and accretion charges |
3,264 | 28,045 | — | |||||||
90
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
None.
ITEM 9A. CONTROLS AND PROCEDURES
EVALUATION OF DISCLOSURE CONTROLS AND PROCEDURES
As required by Rule 13a-15 under the Securities Exchange Act of 1934, management has evaluated, with the participation of our Chief Executive Officer and Chief Financial Officer, the effectiveness of our disclosure controls and procedures as of the end of the period covered by this report. Disclosure controls and procedures refer to controls and other procedures designed to ensure that information required to be disclosed in the reports we file or submit under the Exchange Act is recorded, processed, summarized and reported within the time periods specified in the rules and forms of the SEC. Disclosure controls and procedures include, without limitation, controls and procedures designed to ensure that information required to be disclosed by us in the reports that we file or submit under the Exchange Act is accumulated and communicated to management, including our CEO and CFO, as appropriate, to allow timely decisions regarding our required disclosure. In designing and evaluating our disclosure controls and procedures, management recognizes that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving the desired control objectives, and management was required to apply its judgment in evaluating and implementing possible controls and procedures.
Pursuant to this evaluation, our CEO and CFO concluded that, as of December 31, 2010, the end of the period covered by this report, our disclosure controls and procedures were effective at the reasonable assurance level.
MANAGEMENT'S REPORT ON INTERNAL CONTROL OVER FINANCIAL REPORTING
Management, under the supervision of the CEO and CFO, is responsible for establishing and maintaining adequate internal control over financial reporting. Internal control over financial reporting, as defined in Rules 13a-15(f) under the Exchange Act, refers to a process designed by, or under the supervision of, our CEO and CFO, and effected by our board of directors, management and other personnel, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external reporting purposes in accordance with GAAP and includes those policies and procedures that:
- •
- pertain to the maintenance of records that in reasonable detail accurately and fairly reflect the transactions and
dispositions of our assets;
- •
- provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in
accordance with GAAP and that our receipts and expenditures are being made only in accordance with authorizations of our management and members of our board of directors; and
- •
- provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of our assets that could have a material effect on our financial statements.
Internal control over financial reporting cannot provide absolute assurance of achieving financial reporting objectives because of its inherent limitations. Internal control over financial reporting is a process that involves human diligence and compliance and is subject to lapses in judgment and breakdowns resulting from human failures. Internal control over financial reporting can also be circumvented by collusion or improper management override. Because of such limitations, there is a
91
risk that material misstatements will not be prevented or detected on a timely basis by internal control over financial reporting. However, these inherent limitations are known features of the financial reporting process, and it is possible to design into the process safeguards to reduce, though not eliminate, the risk.
Management assessed the effectiveness of our internal control over financial reporting as of December 31, 2010, using the framework set forth in the report of the Treadway Commission's Committee of Sponsoring Organizations (COSO), "Internal Control—Integrated Framework." As a result of that assessment, management has concluded that our internal control over financial reporting was effective as of December 31, 2010.
PricewaterhouseCoopers LLP, the independent registered public accounting firm that audited the consolidated financial statements included in this Report on Form 10-K for the year ended December 31, 2010, issued an audit report on the effectiveness of our internal control over financial reporting as of December 31, 2010, which report is set forth on page 45 of this annual report.
CHANGES IN INTERNAL CONTROL OVER FINANCIAL REPORTING
Management has evaluated, with the participation of our CEO and CFO, whether any changes in our internal control over financial reporting that occurred during the quarter ended December 31, 2010 have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting. Based on that evaluation, management concluded that there have been no changes in ArthroCare's internal control over financial reporting during the quarter ended December 31, 2010 that has materially affected, or is reasonably likely to materially affect, ArthroCare's internal control over financial reporting.
REMEDIATION OF PREVIOUSLY EXISTING MATERIAL WEAKNESSES
Management previously concluded that we did not maintain an effective control environment with the sufficient complement of personnel, procedures for gathering and disseminating information pertinent to our financial reporting or activities (information and communication) that effectively monitor the performance of controls. Management previously concluded that we did not maintain effective controls related to our period-end financial reporting process to provide reasonable assurance that (a) account reconciliations were performed properly and that reconciliations and journal entries were consistently reviewed for completeness and accuracy; (b) foreign currency transactions and balances were valued accurately; (c) stock-based compensation was accurately recorded; and (d) operating expenses were recognized in the proper period which also affected the accuracy and valuation of certain intangible assets, prepaid expenses, and accrued liabilities. Management also previously concluded that we did not maintain effective controls related to our accounting for business acquisitions.
Remediation measures have been implemented to address the above-described material weaknesses throughout the year. These measures included adding personnel to the accounting function with knowledge, experience and training suitable to our financial reporting requirements and changing the accounting organization to better align personnel to specific accounting activities and responsibilities; strengthening the communication flow between business and accounting personnel to provide a sufficient level of assurance that essential data required to properly apply GAAP to our transactions is completely and accurately communicated to our accounting personnel in a timely manner; enhancing our financial reporting policies, procedures and processes involving account reconciliations, journal entries and corresponding supporting documentation; and changing monitoring practices concerning the review of key accounting controls and processes to ensure consistent compliance to established procedures and policies. Throughout 2010, management has continued to monitor, evaluate and improve upon these measures as previously reported under "Remediation Activities" in Item 4 in our
92
Form 10-Q for each of the quarters ended September 30, 2010, June 30, 2010, and March 31, 2010. As of the date of this filing, management concluded that the previously disclosed remediation activities have been effectively functioning in place for a sufficient period of time, including the fourth quarter, to demonstrate reliance in their design and implementation. Accordingly, management believes that the aforementioned material weaknesses relating to the control environment, information and communication, monitoring the performance of controls, the period-end financial reporting process, and acquisition accounting have been remediated as of December 31, 2010.
None.
93
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
Effective August 14, 2009, our Board of Directors adopted amendments to our Code of Business Conduct and Ethics, or the Code. These amendments provide that our employees may not provide non-employee health care professionals with gifts, entertainment or recreational items, regardless of value. Limited exceptions to this general rule are made for modest meals incidental to a scientific presentation and educational items of modest value that serve a genuine educational function and benefit patients. The amendments also set forth the roles of our new General Counsel and new Compliance Officer in certain governance matters.
Originally adopted in 2004 to qualify as a "code of ethics" within the meaning of Section 406 of the Sarbanes-Oxley Act of 2002 and the rules promulgated thereunder, the Code was amended to qualify as well as a "code of conduct" under the current Federal Sentencing Guidelines and "Compliance Program Guidance for Pharmaceutical Manufacturers" 68 FR 23731 (May 5, 2003) (applicable to medical device companies according to end note 5) published by the Office of Inspector General for the Department of Human Services. The Code applies to all of our directors, officers, employees and agents. It also serves as an essential element of our Corporate Compliance Program, as announced in August 2009. A copy of the Code is available on our website at www.ArthroCare.com. We intend to post any amendments to, or waivers from, if any, our Code on our website.
All additional information required by this item is included in our definitive Proxy Statement to be filed pursuant to Regulation 14A under the Exchange Act in connection with our annual meeting of stockholders scheduled to be held May 12, 2011 and is incorporated herein by reference.
ITEM 11. EXECUTIVE COMPENSATION
All information required by this item is included in our definitive Proxy Statement to be filed pursuant to Regulation 14A under the Exchange Act of 1934 in connection with our annual meeting of stockholders scheduled to be held May 12, 2011 and is incorporated herein by reference.
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
Except as set forth below regarding securities authorized for issuance under equity compensation plans, the information required by this item is included in our definitive Proxy Statement to be filed pursuant to Regulation 14A under the Exchange Act in connection with our annual meeting of stockholders scheduled to be held May 12, 2011 and is incorporated herein by reference.
94
EQUITY COMPENSATION PLAN INFORMATION
The following table provides information as of December 31, 2010 regarding the common stock that may be issued upon the exercise of options, warrants and rights under our existing equity compensation plans.
Plan category
|
Number of securities to be issued upon exercise of outstanding options, warrants and rights |
Weighted average exercise price of outstanding options, warrants and rights |
Number of securities remaining available for future issuance under equity compensation plans (excluding securities reflected in the first column) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
Equity compensation plans approved by security holders(1) |
2,979,146 | 28.74 | 3,247,439 | |||||||
Equity compensation plans not approved by security holders(2) |
601,001 | 27.34 | — | |||||||
Total |
3,580,147 | 28.50 | 3,247,439 | |||||||
- (1)
- Includes
52,239 shares remaining available for future issuance under the Company's Employee Stock Purchase Plan.
- (2)
- Consists of shares issuable under our Amended and Restated Non-statutory Option Plan, which does not require the approval of, and has not been approved by, our stockholders.
ITEM 13. CERTAIN RELATIONSHIPS, RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
Under its charter, the Audit Committee is responsible for reviewing and approving all related party transactions, regardless of the dollar amount involved, that, if entered into, would be required to be disclosed under the rules and regulations of the SEC. No member of the Audit Committee having an interest in a related party transaction may participate in any decision regarding that transaction.
All additional information required by this item is included in our definitive Proxy Statement to be filed pursuant to Regulation 14A under the Exchange Act in connection with our annual meeting of stockholders scheduled to be held May 12, 2011 and is incorporated herein by reference.
DIRECTOR INDEPENDENCE
Our Board of Directors, with the assistance of the Nominating and Corporate Governance Committee, has reviewed the independence of all current Board members under the NASDAQ listing standards and our Corporate Governance Guidelines. The Board of Directors has determined that all current Board members, other than David Fitzgerald, meet the general requirements to be independent directors under the NASDAQ listing standards and our Corporate Governance Guidelines. Based on its review, the Board of Directors has also determined that Terrence E. Geremski, James G. Foster and Tord B. Lendau meet the specific independence requirements to serve on the Audit Committee under the Nasdaq listing standards and the Securities Exchange Act.
ITEM 14. PRINCIPAL ACCOUNTANT FEES AND SERVICES
All information required by this item is included in our definitive Proxy Statement to be filed pursuant to Regulation 14A under the Exchange Act, in connection with our annual meeting of stockholders scheduled to be held May 12, 2011 and is incorporated herein by reference.
95
ITEM 15. EXHIBITS AND FINANCIAL STATEMENT SCHEDULES
(a)(1). Documents filed as part of this annual report.
- •
- Consolidated Balance Sheets at December 31, 2010 and 2009
- •
- Consolidated Statements of Income for the years ended December 31, 2010, 2009 and 2008
- •
- Consolidated Statements of Shareholders' Equity for the years ended December 31, 2010, 2009 and 2008
- •
- Consolidated Statements of Cash Flows for the years ended December 31, 2010, 2009 and 2008
- •
- Notes to Consolidated Financial Statements
- •
- Report of Independent Registered Public Accounting Firm
(a)(2). Financial statement schedule
- •
- Valuation and Qualifying Accounts.
The following report and schedule are included in this Part IV, and are found in this report:
All other schedules are omitted, as the required information inapplicable or the information is presented in the consolidated financial statements or related notes.
(a)(3). Exhibits
| 2.1 | Agreement and Plan of Merger, dated as of September 3, 2004, by and among ArthroCare Corporation, Opus Medical, Inc., OC Merger Sub Corporation, OC Acquisition Sub LLC, and James W. Hart and Steven L. Gex, as the Shareholders' Agents (Incorporated herein by reference to Exhibit 2.1 to the registrant's Current Report on Form 8-K filed on November 18, 2004). | |
2.2 |
Amendment No. 1, dated as of October 6, 2004, to the Agreement and Plan of Merger, dated as of September 3, 2004, by and among ArthroCare Corporation, Opus Medical, Inc., OC Merger Sub Corporation, OC Acquisition Sub LLC, and James W. Hart and Steven L. Gex, as the Shareholders' Agents (Incorporated herein by reference to Exhibit 2.2 to the registrant's Current Report on Form 8-K filed on November 18, 2004). |
|
2.3 |
Asset Purchase Agreement, dated as of August 16, 2005, by and among ArthroCare Corporation, a Delaware corporation, ArthroCare (Deutschland) GmbH, a corporation organized under the laws of Germany and a wholly-owned subsidiary of ArthroCare, ArthroCare UK, Ltd., a corporation registered in England & Wales and a wholly-owned subsidiary of ArthroCare, Applied Therapeutics, Inc., a Florida corporation, Applied Therapeutics, Ltd., a corporation registered in England & Wales, Applied Therapeutics GmbH, a corporation organized under the laws of Germany and BHK Holding, a corporation organized under the laws of the Cayman Islands. (Incorporated herein by reference to Exhibit 2.1 to the Company's Current Report on Form 8-K filed on August 22, 2005). |
|
3.1 |
Restated Certificate of Incorporation of the Company. (Incorporated herein by reference to Exhibit 3.1 filed previously with the Company's Annual Report on Form 10-K for the period ended December 30, 2000). |
96
| 3.2 | Amended and Restated Bylaws of the Company. (Incorporated herein by reference to Exhibit 3.1 filed previously with the Company's Current Report on Form 8-K filed on August 17, 2009). | |
4.1 |
Specimen Common Stock Certificate. (Incorporated herein by reference to Exhibit 4.1 filed previously with the Company's Registration Statement on Form 8-A (Registration No. 000-27422)). |
|
4.2 |
Certificate of Designations of Series A 3.00 percent Convertible Preferred Stock (Par Value $0.001) (Incorporated herein by reference to Exhibit 99.2 filed previously with the Company's Current Report on Form 8-K filed on August 17, 2009). |
|
10.1* |
Form of Indemnification Agreement between the registrant and each of its directors and officers. (Incorporated herein by reference to Exhibit 10.1 filed previously with the Company's Registration Statement on Form S-1 (Registration No. 33-80453)). |
|
10.2* |
Incentive Stock Plan and form of Stock Option Agreement there under. (Incorporated herein by reference to Exhibit 10.2 filed previously with the Company's Registration Statement on Form S-1 (Registration No. 33-80453)). |
|
10.3* |
Director Option Plan and form of Director Stock Option Agreement there under. (Incorporated herein by reference to Exhibit 10.3 filed previously with the Company's Registration Statement on Form S-1 (Registration No. 33-80453)). |
|
10.4* |
Employee Stock Purchase Plan and forms of agreements there under. (Incorporated herein by reference to Exhibit 10.4 filed previously with the Company's Registration Statement on Form S-1 (Registration No. 33-80453)). |
|
10.5 |
Form of Exclusive Distribution Agreement. (Incorporated herein by reference to Exhibit 10.5 filed previously with the Company's Registration Statement on Form S-1 (Registration No. 33-80453)). |
|
10.6† |
Litigation Settlement Agreement, between the Company and ETHICON Inc. dated June 24, 1999 (Incorporated herein by reference to Exhibit 10.33 previously filed with the Company's Quarterly Report on Form 10-Q for the period ended July 3, 1999). |
|
10.7† |
License Agreement between ArthroCare Corporation and Stryker Corporation, dated June 28, 2000. (Incorporated herein by reference to Exhibit 10.36 filed previously with this the Company's Quarterly Report on Form 10-Q for the period ended July 1, 2000). |
|
10.8* |
Amendment to the 1995 Director Option Plan (Incorporated herein by reference to Exhibit 4.3 to the Company's Statement on Form S-8 filed on August 8, 2000). |
|
10.9* |
Amended and Restated 2003 Incentive Stock Plan (Incorporated herein by reference to the Company's Proxy Statement on Schedule 14A filed with the Securities and Exchange Commission on March 23, 2010. |
|
10.10* |
Second Amendment to the 1995 Director Option Plan (Incorporated herein by reference to Exhibit 4.3 to the Company's Registration Statement on Form S-8 filed with the Securities and Exchange Commission on May 8, 2003). |
|
10.11 |
Agreement and Plan of Merger, dated as of October 23, 2003, as amended by Amendment No. 1 to Agreement and Plan of Merger, dated as of January 5, 2004, by and among ArthroCare Corporation, Alpha Merger Sub Corporation and Medical Device Alliance Inc. (Incorporated herein by reference to Exhibit 2.1 to the Company's Current Report on Form 8-K filed on February 11, 2004). |
97
| 10.12 | Contingent Value Rights Agreement, dated as of January 28, 2004, by and among ArthroCare Corporation, Alpha Merger Sub Corporation, Medical Device Alliance Inc., Wells Fargo Bank, N.A. and Frank Bumstead (Incorporated herein by reference to Exhibit 2.2 to the Company's Current Report on Form 8-K filed on February 11, 2004). | |
10.13 |
Form of Stockholder Waiver Agreement, dated as of October 23, 2003, by each of Vegas Ventures, LLC, Jeffrey Barber and Howard Preissman (Incorporated herein by reference to Exhibit 99.3 to the Company's Current Report on Form 8-K filed on October 31, 2003). |
|
10.14 |
Form of Option Agreement under the Amended and Restated 2003 Incentive Plan (Incorporated herein by reference to Exhibit 10.48 to the Company's Current Report on Form 8-K filed on February 22, 2005). |
|
10.15 |
Form of Restricted Stock Bonus Agreement under the Amended and Restated Director Option Plan (Incorporated herein by reference to Exhibit 10.49 to the Company's Current Report on Form 8-K filed on February 22, 2005). |
|
10.16 |
Form of Restricted Stock Bonus Agreement under the Amended and Restated 2003 Incentive Stock Plan (Incorporated herein by reference to Exhibit 10.50 to the Company's Current Report on Form 8-K filed on February 22, 2005). |
|
10.17† |
Settlement and License Agreement between the Company, ArthroCare Corporation Cayman Islands, a corporation organized under the laws of the Cayman Islands and a wholly-owned subsidiary of the Company and Smith & Nephew, Inc., dated September 2, 2005 (Incorporated herein by reference to Exhibit 10.52 to the Company's Quarterly Report on Form 10-Q filed November 3, 2005). |
|
10.18† |
Supply and Distribution Agreement between the Company, ArthroCare Corporation Cayman Islands, a corporation organized under the laws of the Cayman Islands and a wholly-owned subsidiary of the Company and Smith & Nephew, Inc., dated September 2, 2005 (Incorporated herein by reference to Exhibit 10.53 to the Company's Quarterly Report on Form 10-Q filed November 3, 2005). |
|
10.19 |
Lease Agreement between Lantana Office Properties I, L.P. and the Company (Incorporated herein by reference to Exhibit 10.57 to the Company's Quarterly Report on 10-Q filed on June 30, 2006). |
|
10.20† |
Settlement and License Agreement between the Company, ArthroCare Corporation Cayman Islands and MarcTec, LLC effective January 3, 2007 (Incorporated herein by reference to the Company's Annual Report on Form 10-K filed February 27, 2007). |
|
10.21* |
Form of "Amended VP Continuity Agreement" between ArthroCare Corporation and its Vice Presidents (Incorporated herein by reference to Exhibit 10.61 to the Company's Current Report on Form 8-K filed on April 5, 2007). |
|
10.22* |
Form of "Amended Senior VP Continuity Agreement" between ArthroCare Corporation and its Senior Vice Presidents (Incorporated herein by reference to Exhibit 10.62 to the Company's Current Report on Form 8-K filed on April 5, 2007). |
|
10.23 |
Amendment dated September 20, 2007 to Supply and Distribution Agreement between the Company ArthroCare Corporation Cayman Islands and Smith & Nephew, Inc. (Incorporated herein by reference to Exhibit 10.31 to the Company's Annual Report on Form 10-K filed on November 18, 2009). |
|
10.24† |
Stock Purchase Agreement, dated as of December 31, 2007, by and between ArthroCare Corporation and DiscoCare, Inc. (Incorporated herein by reference to Exhibit 10.65 to the Company's Annual Report on Form 10-K filed on February 29, 2008). |
98
| 10.25 | 2008 Executive Officer Bonus Plan (Incorporated herein by reference to Exhibit 10.66 to the Company's Current Report on Form 8-K filed on February 28, 2008). | |
10.26† |
Pricing and Delivery Amendment dated June 16, 2008 to Supply and Distribution Agreement between the Company, ArthroCare Corporation Cayman Islands and Smith & Nephew, Inc. (Incorporated herein by reference to Exhibit 10.1 to the Company's Quarterly Report on Form 10-Q for the period ended June 30, 2008 filed on November 18, 2009). |
|
10.27† |
Amendment dated June 16, 2008 for Development and Supply of S&N Branded Probes and Controllers to Supply and Distribution Agreement between the Company, ArthroCare Corporation Cayman Islands and Smith & Nephew, Inc. (Incorporated herein by reference to Exhibit 10.2 to the Company's Quarterly Report on Form 10-Q for the period ended June 30, 2008 filed on November 18, 2009). |
|
10.28 |
Resignation Letter of Michael A. Baker dated February 18, 2009 (Incorporated herein by reference to Exhibit 99.2 to the Company's Current Report on Form 8-K filed on February 19. 2009). |
|
10.29 |
General Release and Separation Agreement between John Raffle and the Company, dated February 21, 2009 (Incorporated herein by reference to Exhibit 10.71 to the Company's Current Report on Form 8-K filed on February 26, 2009). |
|
10.30* |
Employment Agreement between ArthroCare Corporation and David Fitzgerald, effective March 30, 2009 (Incorporated herein by reference to Exhibit 10.4 to the Company's Current Report on Form 8-K filed on April 3, 2009). |
|
10.31* |
Employment Agreement between ArthroCare Corporation and Todd Newton, effective April 2, 2009 (Incorporated herein by reference to Exhibit 10.75 to the Company's Current Report on Form 8-K filed on April 3, 2009). |
|
10.32* |
Restatement Bonus Plan adopted March 30, 2009 (Incorporated herein by reference to Exhibit 10.76 to the Company's Current Report on Form 8-K filed on April 3, 2009). |
|
10.33* |
Indemnification Agreement dated April 2, 2009 between ArthroCare Corporation and Todd Newton (Incorporated herein by reference to Exhibit 10.77 to the Company's Current Report on Form 8-K filed on April 3, 2009). |
|
10.34† |
Securities Purchase Agreement, dated as of August 14, 2009, by and between ArthroCare Corporation and OEP AC Holdings, LLC. (Incorporated herein by reference to Exhibit 10.50 to the Company's Annual Report on 10-K filed on November 18, 2009). |
|
10.35† |
Disclosure Letter to Securities Purchase Agreement, dated as of August 14, 2009, by and between ArthroCare Corporation and OEP AC Holdings, LLC. (Incorporated herein by reference to Exhibit 10.51 to the Company's Annual Report on 10-K filed on November 18, 2009). |
|
10.36 |
Registration Rights Agreement, dated as of August 14, 2009, by and between ArthroCare Corporation and OEP AC Holdings, LLC. (Incorporated herein by reference to Exhibit 10.52 to the Company's Annual Report on 10-K filed on November 18, 2009). |
|
10.37 |
Letter Agreement dated September 1, 2009 between ArthroCare Corporation and OEP AC Holdings, LLC with regard to director seats for OEP representatives. (Incorporated herein by reference to Exhibit 10.53 to the Company's Annual Report on 10-K filed on November 18, 2009). |
99
| 10.38 | Third Amendment to Credit Agreement and Forbearance Agreement with respect to Credit Agreement between the Company, Bank of America, N.A., Wells Fargo Bank National Association, and certain other lenders dated January 13, 2006. (Incorporated herein by reference to Exhibit 10.54 to the Company's Annual Report on 10-K filed on November 18, 2009). | |
10.39* |
2009 Executive Officer Bonus Plan dated December 9, 2009 (Incorporated herein by reference to Exhibit 10.1 to the Company's Current Report on 8-K filed on December 14, 2009). |
|
10.40* |
2010 Executive Officer Bonus Plan dated January 7, 2010 (Incorporated herein by reference to Exhibit 10.1 to the Company's Current Report on 8-K filed on January 11, 2010). |
|
10.41* |
Correction to Certificate of Designations of Series A 3.00% Convertible Preferred Stock (Incorporated herein by reference to Exhibit 99.2 to the Company's current report on 8-K filed on August 2, 2010.) |
|
10.42** |
General Release and Separation Agreement between Rich Christensen and the Company dated December 31, 2010. |
|
18.1** |
Preferability letter from Independent Registered Public Accounting firm indicating change to preferable method of accounting. |
|
21.1** |
Subsidiaries of the Company. |
|
23.1** |
Consent of Independent Registered Public Accounting Firm |
|
31.1** |
Certification of the Chief Executive Officer pursuant to Rule 13a-14(a) of the Exchange Act, as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. |
|
31.2** |
Certification of the Chief Financial Officer pursuant to Rule 13a-14(a) of the Exchange Act, as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. |
|
32.1** |
Certification of the Chief Executive Officer pursuant to Rule 13a-14(b) of the Exchange Act and 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. |
|
32.2** |
Certification of the Chief Financial Officer pursuant to Rule 13a-14(b) of the Exchange Act and 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. |
|
99.1** |
Forward-Looking Statements. |
- †
- Confidential
treatment has been granted as to portions of this exhibit.
- *
- Management
contract or compensatory plan or arrangement.
- **
- Filed herewith.
100
The following financial statement schedule is filed as a part of this report under Schedule II immediately preceding the signature page: Schedule II—Valuation and Qualifying Accounts for the three fiscal years ended December 31, 2010, 2009 and 2008. All other schedules called for by Form 10-K are omitted because they are not applicable or the required information is shown in the consolidated financial statements, or notes thereto, included herein.
Schedule II
ARTHROCARE CORPORATION
VALUATION AND QUALIFYING ACCOUNTS
(in thousands)
| |
Balance at Beginning of Period |
Additional Charges to Costs and Expenses |
Deductions | Balance at End of Period |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Year ended December 31, 2010 |
||||||||||||||
Deducted from asset accounts: |
||||||||||||||
Allowance for doubtful accounts |
$ | 3,637 | $ | (508 | ) | $ | (956 | ) | $ | 2,173 | ||||
Product returns |
432 | 172 | (332 | ) | 272 | |||||||||
Inventory reserves |
6,380 | 6,894 | (2,915 | ) | 10,359 | |||||||||
Warranty reserves |
477 | 1,255 | (1,203 | ) | 529 | |||||||||
Year ended December 31, 2009 |
||||||||||||||
Deducted from asset accounts: |
||||||||||||||
Allowance for doubtful accounts |
$ | 2,973 | $ | 924 | $ | (260 | ) | $ | 3,637 | |||||
Product returns |
1,028 | 2,223 | (2,819 | ) | 432 | |||||||||
Inventory reserves |
7,769 | 888 | (2,277 | ) | 6,380 | |||||||||
Warranty reserves |
429 | 1,890 | (1,842 | ) | 477 | |||||||||
Year ended December 31, 2008 |
||||||||||||||
Deducted from asset accounts: |
||||||||||||||
Allowance for doubtful accounts |
$ | 2,508 | $ | 2,069 | $ | (1,604 | ) | $ | 2,973 | |||||
Product returns |
1,801 | 5,189 | (5,962 | ) | 1,028 | |||||||||
Inventory reserves |
3,401 | 6,569 | (2,201 | ) | 7,769 | |||||||||
Warranty reserves |
441 | 1,418 | (1,430 | ) | 429 | |||||||||
101
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized:
| ARTHROCARE CORPORATION a Delaware Corporation |
||||
By: |
/s/ DAVID FITZGERALD David Fitzgerald President and Chief Executive Officer |
|||
Date: February 14, 2011
Pursuant to the requirements of the Securities Exchange Act of 1934, the following persons on behalf of the registrant and in the capacities and on the dates indicated have signed this Report below:
Signature
|
Title
|
Date
|
||
|---|---|---|---|---|
| /s/ DAVID FITZGERALD David Fitzgerald |
President, Chief Executive Officer and Director (Principal Executive Officer) |
February 14, 2011 | ||
/s/ TODD NEWTON Todd Newton |
Senior Vice President Finance and Chief Financial Officer (Principal Financial and Accounting Officer) |
February 14, 2011 |
||
/s/ JAMES G. FOSTER James G. Foster |
Director |
February 14, 2011 |
||
/s/ PETER L. WILSON Peter L. Wilson |
Director |
February 14, 2011 |
||
/s/ TORD B. LENDAU Tord B. Lendau |
Director |
February 14, 2011 |
||
/s/ BARBARA D. BOYAN Barbara D. Boyan |
Director |
February 14, 2011 |
||
/s/ TERRENCE E. GEREMSKI Terrence E. Geremski |
Director |
February 14, 2011 |
||
/s/ GREGORY A. BELINFANTI Gregory A. Belinfanti |
Director |
February 14, 2011 |
||
/s/ CHRISTIAN P. AHRENS Christian P. Ahrens |
Director |
February 14, 2011 |
102
| Exhibit Number |
Exhibit Name | ||
|---|---|---|---|
| 10.42 | General Release and Separation between Rich Christensen and the Company dated December 31, 2010. | ||
18.1 |
Preferability letter from Independent Registered Public Accounting firm indicating change to preferable method of accounting. |
||
21.1 |
Subsidiaries of the Registrant. |
||
23.1 |
Consent of Independent Registered Public Accounting Firm. |
||
31.1 |
Certification of the Chief Executive Officer pursuant to Rule 13a-14(a) of the Exchange Act, as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. |
||
31.2 |
Certification of the Chief Financial Officer pursuant to Rule 13a-14(a) of the Exchange Act, as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. |
||
32.1 |
Certification of the Chief Executive Officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. |
||
32.2 |
Certification of the Chief Financial Officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. |
||
99.1 |
Forward-Looking Statements. |
||
- *
- Only exhibits actually filed are listed. Exhibits incorporated by reference are set forth in the exhibit listing included in Item 15 of the Report on Form 10-K.
103
