Attached files
UNITED
STATES
SECURITIES
AND EXCHANGE COMMISSION
Washington,
D.C. 20549
FORM
8-K
CURRENT
REPORT
Pursuant
to Section 13 or 15(d) of
the
Securities Exchange Act of 1934
Date of
Report (Date of earliest event reported): January 25,
2011
|
22nd
CENTURY GROUP, INC.
|
|
(Exact
name of registrant as specified in its
charter)
|
|
Nevada
|
000-54111
|
98-0468420
|
||
|
(State
or other jurisdiction
|
(Commission
File
|
(IRS
Employer
|
||
|
of
incorporation)
|
|
Number)
|
|
Identification
No.)
|
|
8201
Main Street, Suite 6, Williamsville, NY 14221
|
|
(Address
of principal executive offices, including ZIP
code)
|
|
(716)
270-1523
|
|
(Registrant’s
telephone number, including area
code)
|
Check the
appropriate box below if the Form 8-K filing is intended to simultaneously
satisfy the filing obligation of the registrant under any of the following
provisions (see General Instruction A.2. below):
|
¨
|
Written
communications pursuant to Rule 425 under the Securities Act (17 C.F.R.
§230.425)
|
|
¨
|
Soliciting
material pursuant to Rule 14a-12 under the Exchange Act (17 C.F.R.
§230.14a-12)
|
|
¨
|
Pre-commencement
communications pursuant to Rule 14d-2(b) under the Exchange Act (17 C.F.R.
§14d-2(b))
|
|
¨
|
Pre-commencement
communications pursuant to Rule 13e-4(c) under the Exchange Act (17 C.F.R.
§13e-4(c))
|
Cautionary
Note Regarding Forward Looking Statements
This Current Report on Form 8-K and
other written reports and oral statements made from time to time by us may
contain “forward-looking statements,” all of which are subject to risks and
uncertainties. You can identify these forward-looking statements by their use of
words such as “expects,” “plans,” “will,” “estimates,” “forecasts,” “projects”
and other words of similar meaning. You can identify them by the fact that they
do not relate strictly to historical or current facts. These statements are
likely to address our growth strategy, financial results and product and
development programs. You must carefully consider any such statement and should
understand that many factors could cause actual results to differ from these
forward-looking statements. These factors include inaccurate assumptions and a
broad variety of other risks and uncertainties, including some that are known
and some that are not. No forward-looking statement can be guaranteed and actual
future results may vary materially.
Information regarding market and
industry statistics contained in this Current Report on Form 8-K is included
based on information available to us that we believes is accurate. It is
generally based on industry and other publications that are not produced for
purposes of securities offerings or economic analysis. We have not reviewed or
included data from all sources, and cannot assure investors of the accuracy or
completeness of the data included in this Current Report on Form 8-K. Forecasts
and other forward-looking information obtained from these sources are subject to
the same qualifications and the additional uncertainties accompanying any
estimates of future market size, revenue and market acceptance of products and
services. We do not assume the obligation to update any forward-looking
statement. You should carefully evaluate such statements in light of factors
described in our filings with the United States Securities and Exchange
Commission (the “SEC”), especially on Forms 10-K, 10-Q and 8-K. In various
filings, we have identified important factors that could cause actual results to
differ from expected or historic results. We note these factors for investors as
permitted by the Private Securities Litigation Reform Act of 1995. You should
understand that it is not possible to predict or identify all such factors.
Consequently, the reader should not consider any such list to be a complete list
of all potential risks or uncertainties.
Explanatory
Note
This
Current Report on Form 8-K is being filed in connection with a series of
transactions consummated by us that relate to the merger by us with 22nd Century
Limited, LLC, and certain related actions taken by us.
This
Current Report on Form 8-K responds to the following items of Form
8-K:
|
Item
1.01
|
Entry
into a Material Definitive
Agreement.
|
|
Item
2.01
|
Completion
of Acquisition or Disposition of
Assets.
|
|
Item
3.02
|
Unregistered
Sales of Equity Securities.
|
|
Item
4.01
|
Change
in Registrant’s Certifying
Accountants.
|
|
Item
5.01
|
Changes
in Control of Registrant.
|
|
Item
5.02
|
Departure
of Directors or Certain Officers; Election of Directors; Appointment of
Certain Officers; Compensatory Arrangements of Certain
Officers.
|
|
Item
5.03
|
Amendments
to Articles of Incorporation or Bylaws; Change in Fiscal
Year.
|
|
Item
5.06
|
Change
in Shell Company Status.
|
|
Item
9.01
|
Financial
Statements and Exhibits.
|
-2-
As used
in this Current Report on Form 8-K and unless otherwise indicated, the terms the
“Parent,” “we,” “us,” and “our” refer to 22nd Century Group, Inc. after giving
effect to our merger with 22nd Century Limited, LLC, and the related
transactions described below, unless the context requires
otherwise.
|
Item
1.01.
|
Entry into a Material
Definitive Agreement.
|
On January 25, 2011, 22nd Century
Group, Inc., a Nevada corporation (the “Parent”) entered into an Agreement and
Plan of Merger and Reorganization (the “Merger Agreement”) by and among Parent,
22nd Century Limited, LLC, a privately held Delaware limited liability company
(“22nd Century”), and 22nd Century Acquisition Subsidiary, a newly formed,
wholly-owned Delaware limited liability company subsidiary of Parent
(“Acquisition Sub”). Upon the closing of the merger transaction
contemplated under the Merger Agreement (the “Merger”), Acquisition Sub was
merged with and into 22nd Century, and 22nd Century, as the surviving entity,
became a wholly-owned subsidiary of Parent.
The Merger Agreement and the Merger are
described in Item 2.01 below, which disclosure is incorporated herein by
reference.
Prior to the transactions contemplated
by the Merger Agreement with 22nd Century, there were no material relationships
between Parent and 22nd Century, or any of their respective affiliates,
directors or officers, or any associates of their respective officers or
directors.
|
Item
2.01.
|
Completion
of Acquisition or Disposition of
Assets.
|
The
Merger
On January 25, 2011, Parent entered
into the Merger Agreement with 22nd Century and Acquisition Sub. Upon
closing of the Merger on January 25, 2011, Acquisition Sub was merged with and
into 22nd Century, and 22nd Century became a wholly-owned subsidiary of
Parent. Pursuant to the terms and conditions of the Merger
Agreement:
|
·
|
Prior
to the closing of the Merger, Parent (i) obtained forgiveness of all its
outstanding promissory notes in the aggregate principal amount of
$162,327, (ii) cancelled the 386,389 shares of the Parent’s common stock,
$0.00001 par value per share (the “Common Stock”), held by Milestone
Enhanced Fund Ltd. and 10,015,200 shares of Common Stock held by Nanuk
Warman, (iii) entered into contractual agreements with certain
shareholders of Parent pursuant to which an aggregate of 139,800 shares of
Common Stock (the “Contractual Cancellations”) will be cancelled as soon
as practicable following the closing of the Merger (such 139,800 shares of
Common Stock being deemed to be no longer issued and outstanding as of
January 25, 2011) and (iv) effected a 2.782-for-one forward stock split by
way of dividend and subsequent cancellation to ensure that the pre-Merger
shareholders of Parent owned an aggregate of 5,325,200 shares of Common
Stock immediately prior to the closing of the Merger, such 5,325,200
shares of Common Stock representing approximately 19.9% of the issued and
outstanding shares of Common Stock immediately following the closing of
the Merger. In addition, prior to the closing of the Merger,
Parent transferred all of its pre-Merger operating assets and remaining
liabilities to Touchstone Split Corp., a Delaware corporation and
wholly-owned subsidiary of Parent (the “Split-Off Subsidiary”) pursuant to
the terms of that certain Split-Off Agreement dated as of January 25, 2011
by and between Parent, David Rector (the “Buyer”), and the Split-Off
Subsidiary (the “Split-Off Agreement”). Prior to the Merger and
pursuant to the terms of the Split-Off Agreement, Parent transferred and
sold all of the issued and outstanding shares of capital stock of the
Split-Off Subsidiary to Buyer in exchange for $1, such consideration being
deemed to be adequate by Parent’s board of
directors;
|
-3-
|
·
|
Prior
to the closing of the Merger, Parent adopted an equity incentive plan and
reserved 4,250,000 shares of Common Stock for issuance as incentive awards
to officers, directors, employees and other qualified persons in the
future;
|
|
·
|
Prior
to the closing of the Merger, 22nd Century completed a private placement
offering (the “Private Placement Offering”) of 5,434,446 securities (the
“PPO Securities”) at the purchase price of $1.00 per PPO Security (the
“PPO Price”), each such PPO Security consisting of one (1) limited
liability company membership interest unit of the 22nd Century (each, a
“Unit”) and a five year warrant to purchase one half of one (1/2) Unit at
an exercise price of $1.50 per whole
Unit;
|
|
·
|
In
conjunction with the Private Placement Offering, 22nd Century issued to
Rodman & Renshaw, LLC a non-transferrable five-year warrant to
purchase 394,755 Units of 22nd Century at an exercise price of $1.50 per
Unit and issued to Gottbetter Capital Markets, LLC a non-transferrable
five-year warrant to purchase 40,000 Units of 22nd Century at an exercise
price of $1.50 per Unit;
|
|
·
|
At
the closing of Merger, Parent issued to Rodman & Renshaw, LLC a
non-transferrable five-year warrant to purchase 500,000 shares of Common
Stock at an exercise price of $1.50 per share in connection with the
provision of financial advisory services to
Parent;
|
|
·
|
At
the closing of the Merger, each Unit of 22nd Century issued and
outstanding immediately prior to the closing of the Merger was exchanged
for one (1) share of Common Stock, and each warrant to purchase Units of
22nd Century was exchanged for one warrant of like tenor and term to
purchase shares of Common Stock. An aggregate of 21,434,446
shares of Common Stock and warrants to purchase an aggregate of 8,151,980
shares of Common Stock were issued to the holders of Units and warrants,
respectively, of 22nd Century, and immediately following the closing of
the Merger an aggregate of 26,759,646 shares of Common Stock were issued
and outstanding and an aggregate of 10,220,000 shares are Common Stock
were reserved for issuance pursuant to the exercise of warrants to
purchase shares of Common Stock;
|
|
·
|
Upon
the closing of the Merger, the board of directors was expanded and
reconstituted, as described below;
|
|
·
|
Pursuant
to the terms of the Merger Agreement, Parent assumed all of 22nd Century’s
obligations, including those related to 22nd Century’s outstanding
warrants;
|
|
·
|
Each
of Parent, 22nd Century and Acquisition Sub provided customary
representations and warranties, pre-closing covenants and closing
conditions in the Merger Agreement;
and
|
-4-
|
·
|
Following
(i) the closing of the Merger, (ii) the closing of the Private Placement
Offering for $5,434,446, (iii) Parent’s cancellation of 386,389 shares
Common Stock held by Milestone Enhanced Fund Ltd. and 10,015,200 shares of
Common Stock held by Nanuk Warman, (iv) consummation of the Split-Off
Agreement and the transactions contemplated thereby, and (v) taking into
account a 2.782-for-one forward stock split by way of dividend of the
shares of Common Stock that took place on November 29, 2010 (with any
resulting fractional shares being rounded upward to the nearest whole
share) and subsequent cancellation as well as the Contractual
Cancellations, there were 26,759,646 shares of Common Stock issued and
outstanding. Approximately 59.8% of such issued and outstanding
shares were held by individuals and entities that were holders of Units of
22nd Century prior to consummation of the Private Placement Offering,
approximately 20.3% were held by the investors in the Private Placement
Offering and approximately 19.9% were held by the pre-Merger stockholders
of Parent.
|
The foregoing description of the Merger
Agreement does not purport to be complete and is qualified in its entirety by
reference to the complete text of the Merger Agreement, which is filed as
Exhibit 2.1 hereto and incorporated herein by reference.The Merger and related
transactions were approved by the holders of a requisite number of 22nd Century
Units pursuant to written consent dated as of December 15, 2010.
The shares of Common Stock issued to
former holders of 22nd Century Units in connection with the Merger, and 22nd
Century Units and warrants to purchase Units issued in the Private Placement
Offering, were not registered under the Securities Act of 1933, as amended (the
“Securities Act”), and were issued and sold in reliance upon the exemption
from registration provided by Section 4(2) and Section 4(6) of the Securities
Act or pursuant to Regulation D or Regulation S promulgated
thereunder. These securities may not be offered or sold in the United
States absent registration or an applicable exemption from the registration
requirements. Certificates representing these securities contain a legend
stating the same.
The 5,325,200 shares of Common Stock
issued and outstanding immediately prior to the closing of the Merger constitute
the entirety of Parent’s “public float” eligible for resale without further
registration by the holders thereof. Additional shares of Common Stock will be
eligible for resale at such time as a further registration statement is filed
and declared effective pursuant to the Securities Act or at such time as
additional shares of Common Stock are eligible to be resold pursuant to an
exemption from registration under the Securities Act.
Changes
Resulting from the Merger
Parent intends to carry on 22nd
Century’s business as its sole line of business. Parent has relocated
its executive offices to 8201 Main Street, Suite 6, Williamsville, NY 14221 and
its telephone number is (716) 270-1523.
The Parent intends to adopt the fiscal
year of 22nd Century, which ends December 31.
Changes
to the Board of Directors and Officers
In connection with the Merger, the
Parent’s board of directors was expanded to five (5)members. The sole
officer and sole member of the board of directors prior to the closing of the
Merger, David Rector, resigned as an officer but continues to serve as a member
of the board of directors of Parent. Immediately following the
closing of the Merger, Joseph Pandolfino was appointed to serve as a member of
Parent’s board of directors. As of the date ten (10) days following
the filing of a Schedule 14F-1 with the SEC after the closing of the Merger,
David Rector will resign as a member of Parent’s board of directors and will be
replaced by an individual appointed by the pre-Merger stockholders of
Parent. Each of Henry Sicignano III, Joseph Alexander Dunn, Ph.D.,
and James W. Cornell will also be appointed to serve as members of Parent’s
board of directors as of that date. Immediately following the closing
of the Merger, Joseph Pandolfino was appointed as our Chief Executive Officer,
Henry Sicignano III was appointed as our President and Secretary, and C. Anthony
Rider was appointed as our Chief Financial Officer and
Treasurer.
-5-
All directors hold office for one-year
terms until the election and qualification of their
successors. Officers are elected by the board of directors and serve
at the discretion of the board.
Accounting
Treatment
The Merger is being accounted for as a
reverse acquisition and recapitalization of 22nd Century for financial
accounting purposes whereby 22nd Century is deemed to be the acquirer for
accounting and financial reporting purposes. Consequently, the assets and
liabilities and the historical operations that will be reflected in the
financial statements prior to the Merger will be those of 22nd Century and will
be recorded at the historical cost basis of 22nd Century, and the consolidated
financial statements after completion of the Merger will include the assets and
liabilities of Parent and 22nd Century, historical operations of 22nd Century
and operations of Parent beginning on the closing date of the Merger. As a
result, all the historical financial information reported herein is 22nd
Century’s.
Tax
Treatment; Smaller Reporting Company
The transfer of operating assets and
liabilities to the Split-Off Subsidiary, the forgiveness of indebtedness by
certain shareholders of Parent, and the Split-Off of the Split-Off Subsidiary,
will result in taxable income to Parent in an amount equal to the difference
between the fair market value of the assets transferred and Parent’s tax basis
in the assets. Any gain recognized, to the extent not offset by Parent’s net
operating losses carry-forwards, if any, will be subject to federal income tax
at regular corporate income tax rates.
The exchange of Membership Units for
Common Stock in the Merger is expected to qualify for treatment as a tax-free
transfer under section 351 of the United States Internal Revenue Code (“IRC”) as
long as the exchange results in the members of 22nd Century Limited, LLC
immediately prior to the Merger having at least 80% “control” (within the
meaning of IRC §351(a)) of Parent immediately following the Merger and certain
other requirements are met. If the Merger qualifies as a tax-free transfer under
IRC § 351, the shares of Common Stock received in the exchange will have the
same tax basis as the Membership Units for which they were exchanged. A
“significant transferor” (as defined in Treas. Reg. §1.351-3(d)(1)) will be
required to include certain information with his income tax return for the year
of the Merger.
Parent will continue to be a “smaller
reporting company,” as defined in Regulation S-K under the Securities Exchange
Act of 1934, as amended (the “Exchange Act”), following the Merger.
Company
Background
Parent was formed as a Nevada
corporation on September 12, 2005 to engage in the acquisition, exploration and
development of mineral deposits and reserves. Parent has been in a
development stage since its inception and had minimal business operations prior
to the Merger. Immediately prior to the closing of the Merger, the
existing asset and liabilities of Parent were disposed of pursuant to the
cancellation of certain indebtedness owed to shareholders of Parent, the
cancellation of certain shares of Common Stock held by shareholders of Parent,
and the Split-Off.
-6-
22nd Century was formed as a New
York limited liability company on February 20, 1998 as 21st Century
Limited, LLC, which merged with a newly-formed Delaware limited liability
company, 22nd Century Limited, LLC on November 29, 1999. Our offices
are located in Williamsville, New York. Since beginning operations,
we have worked to modify the content of nicotine alkaloids in tobacco plants
through genetic engineering and plant breeding.
After the Merger with Parent, Parent
succeeded to the business of 22nd Century as its sole line of
business.
Company
Overview
Founded
in 1998, we are a plant biotechnology company and a global leader in modifying
the content of nicotinic alkaloids in tobacco plants through genetic engineering
and plant breeding. We own or exclusively control 97 issued patents in 79
countries where at least 75% of the world’s smokers reside. We believe that our
proprietary technology will enable us to capture a significant share of the
global market for approved smoking cessation aids and the emerging market for
modified risk tobacco products.
We plan
to use a substantial portion of the proceeds of the Private Placement Offering
to complete the remaining clinical trials necessary to seek approval from the
U.S. Food and Drug Administration (“FDA”) for X-22, our prescription
smoking cessation aid. X-22 will be a
prescription-only kit containing very low nicotine (“VLN”) cigarettes made from
our proprietary tobacco, which has 95% less nicotine compared to tobacco in
existing “light” cigarettes. The therapy protocol allows the patient to smoke
our VLN cigarettes without restriction over the six-week treatment period to
facilitate the goal of the patient quitting smoking by the end of the treatment
period. We believe this therapy protocol has been successful because VLN
cigarettes made from our proprietary tobacco satisfy smokers’ cravings for
cigarettes while (i) greatly reducing nicotine exposure and nicotine dependence
and (ii) extinguishing the association between the act of smoking and the rapid
delivery of nicotine. We believe X-22 will be more attractive
to smokers than other therapies since it smokes and tastes like a typical
cigarette, involves the same smoking behavior, and does not expose the smoker to
any new drugs or new side effects.
We have
met with the FDA regarding the remaining X-22 clinical trials and,
based on the FDA’s guidance, we plan to conduct a small Phase II-B trial and two
larger and concurrent Phase III trials with the same protocols, all of which
entail measuring the quitting efficacy of the X-22 cigarette against a
typical cigarette with conventional nicotine content that is visually
indistinguishable from X-22. We believe that X-22 will qualify for “Fast
Track” designation by the FDA, and that we will obtain FDA approval for X-22 in the fourth quarter of
2012 at the earliest.
Independent
studies, including two Phase II clinical trials, have demonstrated that VLN
cigarettes made from our proprietary tobacco are at least as effective as
FDA-approved smoking cessation aids. Due to the limited effectiveness and/or
serious side effects of existing FDA-approved smoking cessation products, we
believe that we are well-positioned to capture a significant share of this
market. Since X-22 is
the only smoking cessation product that functions exactly like a regular
cigarette, it will not only take sales and market share from existing smoking
cessation products, but it will also expand the smoking cessation market by
encouraging more smokers to attempt to quit smoking.
We intend
to seek FDA authorization to market BRAND A and BRAND B as Modified Risk
Cigarettes. Compared to other commercial cigarettes, the tobacco in BRAND A has approximately 95%
less nicotine than tobacco in cigarettes marketed as “light” cigarettes and
BRAND B’s smoke
contains the lowest amount of tar per milligram of nicotine. We believe that
BRAND A and BRAND B will achieve
significant market share in the global cigarette market among smokers who will
not quit but are interested in reducing the harmful effects of
smoking.
-7-
The 2009
Family Smoking Prevention and Tobacco Control Act, or Tobacco Control Act,
granted the FDA authority over the regulation of all tobacco products. While it
prohibits the FDA from banning cigarettes outright, it allows the FDA to require
the reduction of nicotine or any other compound in cigarettes. The Tobacco
Control Act also banned all sales in the U.S. of cigarettes with flavored
tobacco (other than menthol). As of June 2010, all cigarette companies were
required to cease the use of the terms “low tar,” “light” and “ultra light” in
describing cigarettes sold in the U.S. We believe this new regulatory
environment represents a paradigm shift for the tobacco industry and will create
opportunities for us in marketing BRAND A and BRAND B and in licensing our
proprietary technology and tobaccos to larger competitors. Within our two
product categories, the Tobacco Control Act offers us the following specific
advantages:
Smoking
Cessation Aids
FDA
approval must be obtained, as has been the case for decades, before a product
can be marketed for quitting smoking or reducing withdrawal symptoms. The
Tobacco Control Act provides that products for smoking cessation, such as X-22, be considered for “Fast
Track” designation by the FDA. The “Fast Track” programs of the FDA are intended
to facilitate development and expedite review of drugs to treat serious and
life-threatening conditions so that an approved product can reach the market
expeditiously. We believe that X-22 will qualify for “Fast
Track” designation by the FDA.
Modified
Risk Cigarettes
For the
first time in history, the FDA will evaluate cigarettes that may pose lower
health risks as compared to conventional cigarettes. The Tobacco Control Act
establishes procedures for the FDA to regulate the labeling and marketing of
Modified Risk Cigarettes and requires the FDA to issue additional guidance
regarding applications that must be submitted to the FDA for approval to market
these Modified Risk Cigarettes. We believe, based in part on the timelines
contained in the Tobacco Control Act, that the FDA will issue such guidance in
2011 and we also believe that BRAND A and BRAND B will qualify as
Modified Risk Cigarettes under these guidelines. In addition, the Tobacco
Control Act allows the FDA to mandate the use of reduced risk technologies in
conventional cigarettes which could create opportunities for us to license our
technology and/or tobaccos.
Tar,
Nicotine, and Smoking Behavior
The
dependence of many smokers on tobacco is largely due to the properties of
nicotine, but the adverse effects of smoking on health are mainly due to other
components present in tobacco smoke, including tar and carbon monoxide. “Tar” is
the common name for the (resinous) total particulate matter minus nicotine and
water produced by the burning of tobacco (or other plant material) during the
act of smoking. Tar and nicotine are commonly measured in milligrams per
cigarette trapped on a Cambridge filter pad under standardized conditions using
smoking machines. These results are referred to as “yields” or, more
specifically, tar yield and nicotine yield.
Individual
smokers generally seek a certain amount of nicotine per cigarette and can easily
adjust how intensely each cigarette is smoked to obtain a satisfactory amount of
nicotine. Smoking of low yield (“light” or “ultra light”) cigarettes compared to
high yield (“full flavor”) cigarettes often results in taking more puffs per
cigarette, larger puffs and/or smoking more cigarettes per day to obtain a
satisfactory amount of nicotine, a phenomenon known as “compensation” or
“compensatory smoking.” A report by the National Cancer Institute in 2001 stated
that due to compensatory smoking, low yield cigarettes are not safer than high
yield cigarettes, which is the reason that the Tobacco Control Act has banned
the use of the terms “low tar,” “light” and “ultra light” in the U.S. market.
Studies have shown that smokers do not compensate when smoking cigarettes made
with our VLN tobacco, and that smoking VLN cigarettes actually assist smokers to
smoke fewer cigarettes per day and reduce their exposure to tar and nicotine.
Other studies have shown that non-commercial cigarettes with low tar-to-nicotine
ratios (tar yield divided by nicotine yield from smoking machines), such as
BRAND B, result in
smokers inhaling less tar and carbon monoxide (CO).
-8-
Market
Cigarettes
and Smoking Cessation Aids
The U.S.
cigarette market consists of approximately 44 million adult smokers who spent
approximately $75 billion in 2009 on 320 billion cigarettes. The World Health
Organization (“WHO”) predicts that the current 1.3 billion smokers worldwide
will increase to 1.7 billion smokers by the year 2025. Worldwide manufacturer
sales in 2009 were 5.91 trillion cigarettes, which has been increasing at
approximately 1.0% per year, resulting in annual retail sales of over $300
billion. Our products address unmet needs of smokers; for those who want to
quit, an innovative smoking cessation aid, and for those who do not quit,
cigarettes that can reduce the level of exposure to nicotine, tar and other
chemicals in cigarettes they smoke.
In 2009,
annual sales of smoking cessation aids in the U.S., all of which must be
approved by the FDA, were approximately $1.0 billion. Outside the United States,
the smoking cessation market is in its infancy. Visiongain estimates the 2008
global smoking cessation market at approximately $3.0 billion. According to
Datamonitor, the prescription smoking cessation market in the United States,
Germany, United Kingdom, France, Italy, Spain and Japan is expected to grow at a
compound annual rate of 16%, reaching approximately $4.6 billion by 2016. This
figure does not consider China, Russia, Brazil, India and other large smoking
markets.
Approximately
50% of U.S. smokers attempt to quit smoking each year, but only 2% to 5%
actually quit smoking in a given year. It takes smokers an average of 8 to 11
“quit attempts” before achieving long-term success. Approximately 95% of
“self-quitters” (i.e., those who attempt to quit smoking without any treatment)
relapse and resume smoking. The Institute of Medicine, the health arm of the
National Academy of Sciences, in a 2007 report concludes: “There is an enormous
opportunity to increase population prevalence of smoking cessation by reaching
and motivating the 57 percent of smokers who currently make no quit attempt per
year.” We believe that our X-22 smoking cessation aid
will be attractive to smokers who have been frustrated in their previous
attempts to quit smoking using other therapies.
Use of
existing smoking cessation aids results in relapse rates that can be as high as
90% in the first year after a smoker initially “quits.” Smokers currently have
only the following limited choices of FDA-approved products to help them quit
smoking:
|
·
|
varenicline
(Chantix®/Champix® outside the U.S.), manufactured by
Pfizer,
|
|
·
|
bupropion
(Zyban®), manufactured by GlaxoSmithKline,
and
|
|
·
|
nicotine
replacement therapy (“NRT”) in several forms — gums, patches,
nasal sprays, inhalers and
lozenges.
|
Chantix®
and Zyban® are pills and are nicotine free. Chantix®, Zyban®, the nicotine nasal
spray and the nicotine inhaler are available by prescription only. Nicotine
gums, nicotine patches, and lozenges are available
over-the-counter.
Chantix®
was introduced in the U.S. market in the fourth quarter 2006. Since 2007,
Chantix® has been the best selling smoking cessation aid in the United States,
with sales of $701 million in 2007, $489 million in 2008 and $386 million in
2009. In July 2009, the FDA required a “Boxed Warning,” the most serious type of
warning in prescription drug labeling, for both Chantix® and Zyban® based on the
potential side effects of these drugs. Despite this warning, sales of Chantix®
in 2009 were approximately $700 million worldwide.
-9-
Other
than Chantix® and Zyban®, the only FDA-approved smoking cessation therapy in the
United States is NRT. These products consist of gums, patches, nasal sprays,
inhalers and lozenges. Nicotine gums and nicotine patches have been sold in the
U.S. for 26 years and 18 years, respectively, and millions of smokers have
already tried NRT products and failed to stop smoking due to the limited
effectiveness of these products. According to Perrigo Company, a pharmaceutical
company that sells NRT products, sales of NRT products in the United States have
averaged approximately $500 million annually from 2007 to 2009.
Modified
Risk Tobacco Products
A
substantial number of adult smokers are unable or unwilling to quit smoking. For
example, each year one-half of the adult smokers in the United States do not
attempt to quit. Nevertheless, we believe the majority of these smokers are
interested in reducing the harmful effects of smoking.
In a 2005
analyst report, The Third
Innovation, Potentially Reduced Exposure Cigarettes (PREPs), JP Morgan
examined the effects of FDA regulation of tobacco, including the market for
safer cigarettes. Its proprietary survey of over 600 smokers found that 90% of
smokers are willing to try a safer cigarette. Among JP Morgan’s other
conclusions, it states: “FDA oversight would imbue PREPS [‘potential reduced
exposure products’ equate to modified risk tobacco products] with a regulatory
‘stamp of approval’ and allow for more explicit comparative health claims with
conventional cigarettes. Consumers should trust the FDA more than industry
health claims.” Up until the Tobacco Control Act became law in 2009, no agency
or body had the authority to assess health claims made by tobacco companies or
set standards for what constitutes reduced risk to smokers.
Some
major cigarette manufacturers have developed and marketed alternative cigarette
products. For example, Philip Morris USA developed an alternative cigarette,
called Accord®, in which the tobacco is heated rather than burned. R.J. Reynolds
Tobacco Company has developed and is marketing an alternative cigarette, called
Eclipse®, in which the tobacco is primarily heated, with only a small amount of
tobacco burned. Philip Morris and RJ Reynolds have indicated that their products
may deliver fewer smoke components compared to conventional cigarettes. Vector
Tobacco Inc. has marketed a cigarette offered in three brand styles with reduced
levels of nicotine, called Quest®. Both Accord® and Eclipse®, which are not
conventional cigarettes (e.g., they do not burn down),
have only achieved limited sales. With the exception of Eclipse®, the above
products are no longer being manufactured.
Complete
cessation from all tobacco and medicinal nicotine products is the ultimate goal
of the public health community; however, some public health officials desire to
migrate cigarette smokers en masse to medicinal nicotine (also known as NRT) or
smokeless tobacco products to replace cigarettes. We believe this is
unattainable in the foreseeable future for many reasons including that the
smoking experience is much more complex than simply seeking nicotine. In a 2009
WHO report, statistics demonstrate that approximately 90% of global tobacco
users smoke cigarettes. Worldwide cigarette sales are approximately 20 times
greater than sales of smokeless tobacco products and approximately 100 times
greater than sales of NRT products. Although a small segment of the smoking
population is willing to use NRT or smokeless tobacco products in conjunction
with cigarettes (known as dual users), a large percentage of smokers is not
interested in using NRT or smokeless tobacco products exclusively.
There are
newer forms of smokeless tobacco products that have been introduced in the
market that are less messy to use than chewing tobacco or dry snuff (since
spitting is not involved). These products include Swedish-style snus and
dissolvable tobacco products such as Ariva® and Stonewall® tablets made by Star
Scientific Inc., and Camel® Orbs, Camel® Strips and Camel® Sticks recently
introduced by R.J. Reynolds Tobacco Company. Although use of such products may
be more discreet and convenient than traditional forms of smokeless tobacco,
they have the same route of delivery of nicotine as nicotine gum and nicotine
lozenges, which have been available over-the-counter in the United States for 15
years and 7 years, respectively, and have not significantly replaced
cigarettes.
-10-
Products
X-22
Smoking Cessation Aid
X-22 is a tobacco-based
botanical medical product for use as a smoking cessation therapy. X-22 will be a
prescription-only kit containing very low nicotine (“VLN”) cigarettes made from
our proprietary tobacco, which has 95% less nicotine compared to tobacco in
existing “light” cigarettes. The therapy protocol allows the patient to smoke
our VLN cigarettes without restriction over the six-week treatment period to
facilitate the goal of the patient quitting smoking by the end of the treatment
period. We believe this therapy protocol has been successful because VLN
cigarettes made from our proprietary tobacco satisfy smokers’ cravings for
cigarettes while (i) greatly reducing nicotine exposure and nicotine dependence
and (ii) extinguishing the association between the act of smoking and the rapid
delivery of nicotine. We also believe X-22 will be more attractive
to smokers than other therapies since it smokes and tastes like a typical
cigarette, involves the same smoking behavior, and does not expose the smoker to
any new drugs or new side effects.
We
further believe that X-22 offers the following
advantages over existing smoking cessation products:
|
·
|
X-22 separates the act
of smoking from the rapid delivery of
nicotine;
|
|
·
|
X-22 is more attractive
than other therapies since it smokes, tastes and smells like a typical
cigarette and involves the same smoking
behavior;
|
|
·
|
X-22 does not expose
smokers to any new drugs or new side effects;
and
|
|
·
|
X-22 is more effective
than other smoking cessation aids
because:
|
|
|
·
|
X-22 provides greater
relief from withdrawal symptoms than the FDA-approved nicotine
lozenge;
|
|
|
·
|
X-22 reduces cravings
more than the FDA-approved prescription nicotine inhaler;
and
|
|
|
·
|
X-22 decreases the
likelihood of relapse (in the case of Chantix®, approximately half of
those who quit relapse within 8 weeks after the end of
treatment).
|
We have
met with the FDA regarding the remaining X-22 clinical trials and,
based on the FDA’s guidance, we plan to conduct a small Phase II-B trial and two
larger and concurrent Phase III trials with the same protocols, all of which
entail measuring the quitting efficacy of the X-22 cigarette against a
typical cigarette with conventional nicotine content that is visually
indistinguishable from X-22. As depicted below, we
plan to complete the FDA-approval process for our X-22 smoking cessation aid
and upon such approval launch X-22 in the U.S. market in
the fourth quarter of 2012 at the earliest (as a prescription), and in other top
smoking cessation markets thereafter.
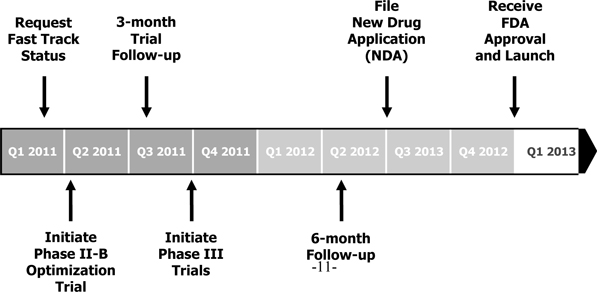
-11-
Our
Modified Risk Cigarettes
We
believe that our BRAND
A and BRAND B
cigarettes will benefit smokers who are unable or unwilling to quit
smoking and who may be attracted to cigarettes which potentially pose a lower
health risk than conventional cigarettes. This includes the approximate one-half
of the 44 million adult smokers in the United States who do not attempt to quit
in a given year. Compared to other commercial cigarettes, the tobacco in BRAND A has approximately 95%
less nicotine than tobacco in cigarettes marketed as “light” cigarettes and
BRAND B’s smoke
contains the lowest amount of tar per milligram of nicotine. We believe that
BRAND A and BRAND B will qualify as
Modified Risk Cigarettes and we intend to seek FDA authorization in 2011 to
market BRAND A and
BRAND B as Modified
Risk Cigarettes. However, the FDA has not yet issued comprehensive guidance
regarding applications that must be submitted to the FDA for Modified Risk
Cigarettes, including the criteria for such authorizations. We believe the FDA
will issue such guidance in 2011.
BRAND
A Cigarettes
Compared
to other commercial tobacco cigarettes, BRAND A has the lowest
nicotine content. The tobacco in BRAND A contains
approximately 95% less nicotine than tobacco in leading “light” cigarette
brands. Clinical studies have demonstrated that smokers who smoke VLN cigarettes
containing our proprietary tobacco smoke fewer cigarettes per day resulting in
significant reductions in smoke exposure, including tar, nicotine and carbon
monoxide. Due to the very low nicotine levels, compensatory smoking does not
occur with VLN cigarettes containing our proprietary tobacco.
In a June
16, 2010 press release, former FDA Commissioner, Dr. David Kessler recommended,
“The FDA should quickly move to reduce nicotine levels in cigarettes to
non-addictive levels. If we reduce the level of the stimulus, we reduce the
craving. It is the ultimate harm reduction strategy.” Shortly thereafter in a
Washington Post
article, Dr. Kessler said that the amount of nicotine in a cigarette should drop
from about 10 milligrams to less than 1 milligram. BRAND A contains
approximately 0.7 milligram of nicotine.
A Phase
II smoking cessation clinical trial at the University of Minnesota Masonic
Comprehensive Cancer Center, which is further described below, also measured
exposure of various smoke compounds in smokers from smoking a VLN cigarette
containing our proprietary tobacco over a 6-week period. Smokers significantly
reduced their smoking as compared to their usual brand of cigarettes. As
depicted below, the number of VLN cigarettes smoked per day on average decreased
from 19 (the baseline number of cigarettes of smokers’ usual brand) to 12 by the
end of the 6-week period, even though participants were instructed to smoke
ad libitum (as many
cigarettes as desired) during treatment. Furthermore, and besides significant
reductions in other biomarkers, carbon monoxide (CO) levels, an indicator of
smoke exposure, significantly decreased from 20 parts per million (baseline) to
15 parts per million. Cotinine, a metabolite and biomarker of nicotine,
significantly decreased from 4.2 micrograms/mL (baseline) to 0.2 micrograms/mL.
All differences were statistically significant (P<0.05).
-12-

We
believe these findings and future exposure studies the FDA may require will
result in a Modified Risk Cigarette claim for BRAND A. We further believe
smokers who desire to smoke fewer cigarettes per day while also satisfying
cravings and reducing exposure to nicotine will find BRAND A beneficial. We intend
that BRAND A will be
available in regular and menthol; with both styles being king size (85 mm)
cigarettes.
BRAND
B Cigarettes
Compared
to other commercial tobacco cigarettes, BRAND B’s smoke contains the
lowest amount of tar per milligram of nicotine. Using a proprietary high
nicotine tobacco blend in conjunction with a unique cigarette design,
BRAND B allows the
smoker to achieve a satisfactory amount of nicotine per cigarette while inhaling
less tar and carbon monoxide. At the same time, we do not expect exposure to
nicotine from BRAND B
to be significantly higher than some full flavor cigarette brands. We believe
smokers who desire to reduce smoke exposure but are less concerned about
nicotine will find BRAND
B beneficial. We intend that BRAND B will be available in
regular and menthol; with both styles being king size (85 mm)
cigarettes.
BRAND B has a tar yield
between typical “light” and “ultra-light” cigarettes, but a nicotine yield of
typical full flavor cigarettes. The graph below compares the tar-to-nicotine
ratios of BRAND B and
BRAND B menthol to
those of the leading cigarette brands. As shown, smokers are expected to inhale
much more tar for every milligram of nicotine from the leading brands than from
BRAND B. For example,
the smoke from BRAND B
has approximately 47% less tar per milligram of nicotine compared to the smoke
from Marlboro Light®.
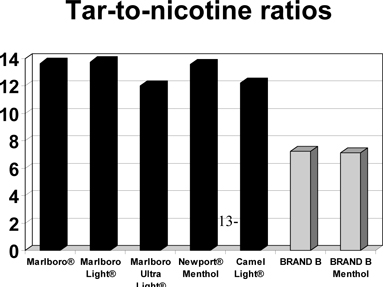
-13-
In a 2001
report, entitled Clearing the
Smoke, Assessing the Science Base for Tobacco Harm Reduction, the
Institute of Medicine notes that a low tar/moderate nicotine cigarette is a
viable strategy for reducing the harm caused by smoking. It states: “Retaining
nicotine at pleasurable or addictive levels while reducing the more toxic
components of tobacco is another general strategy for harm reduction.” We
believe that evaluation of BRAND B in short-term human
exposure studies will confirm that exposure to smoke, including tar and carbon
monoxide, is significantly reduced when smoking BRAND B as compared to
smoking the leading brands of cigarettes. We believe results from these exposure
studies will warrant a Modified Risk Cigarette claim for BRAND B.
Additional
Tobacco Products
We expect
to introduce other cigarettes into the U.S. market in the first quarter of 2011,
particularly to tobacconists, smoke shops and tobacco outlets. The
ban in 2009 by the FDA of all flavored cigarettes (with the exception of
menthol) has resulted in a product void in these tobacco channels. Certain
wholesalers and retailers are now seeking other specialty cigarettes to replace
the banned flavored cigarettes. We believe that certain U.S.
cigarette wholesalers and retailers will purchase these cigarettes to replace
their lost sales of flavored cigarettes as well as lost sales of “light”
cigarettes.
Clinical
Trials with Cigarettes Containing our Very Low Nicotine (“VLN”)
Tobacco
VLN
cigarettes containing our proprietary tobacco have been the subject of various
independent studies, including two Phase II clinical trials for smoking
cessation which were not funded by us. Both of these Phase II clinical trials
were “intent to treat” trials, meaning that any patients who dropped out of the
trials for any reason at any time during treatment or during the follow-up
periods were considered failures (still smoking and not abstinent). Dropout
rates during smoking cessation trials are generally high since patients either
quit smoking or resume smoking their usual brand. In either case, they may
believe there is no reason to continue.
One of
these two Phase II clinical trials compared the quitting efficacy of a VLN
cigarette containing our proprietary tobacco versus a low nicotine cigarette and
an FDA-approved nicotine lozenge (4 mg) in a total of 165 patients treated for 6
weeks (Hatsukami et al. 2010). This clinical trial was led by Dr. Dorothy
Hatsukami, Director of the National Transdisciplinary Tobacco Use Research
Center (TTURC) at the University of Minnesota Masonic Comprehensive Cancer
Center. For reference, Dr. Hatsukami was selected in 2010 as one of the nine
voting members of the 12-person Tobacco Products Scientific Advisory Committee
(“TPSAC”) within the FDA’s Center for Tobacco Products created by the Tobacco
Control Act. TPSAC will make recommendations and issue reports to the FDA
Commissioner on tobacco regulatory matters, including but not limited to, the
impact of the use of menthol in cigarettes, altering levels of nicotine in
tobacco products, and applications submitted to the FDA for modified risk
tobacco products.
Results
from this Phase II trial conclude that patients exclusively using the VLN
cigarette containing our proprietary tobacco achieved a 43% quit rate (confirmed
four-week continuous abstinence) as compared to a quit rate of 35% for the group
exclusively using the nicotine lozenge and a 21% quit rate for the group
exclusively using the low nicotine cigarette. Smoking abstinence at the 6-week
follow-up after the end of treatment was 47% for the VLN cigarette group, 37%
for the nicotine lozenge group and 23% for the low nicotine cigarette group.
Furthermore, the VLN cigarette was also associated with greater relief from
withdrawal symptoms and cravings of usual brand cigarettes than the nicotine
lozenge. Carbon monoxide (CO) levels in patients were tested at each treatment
clinic visit to verify smoking abstinence.
-14-

Unlike
Phase III clinical trials for other FDA-approved smoking cessation aids,
four-week continuous abstinence in the University of Minnesota Phase II trial
was measured after the treatment period, when patients were “off” medication as
shown in the chart below, rather than during the last four weeks of the
treatment period. For example, according to the prescription Chantix® label,
four-week continuous abstinence in the Chantix® Phase III clinical trials (the
44 percent quit rate advertised by Pfizer) was measured during the last four
weeks of the 12-week treatment period, while patients were still taking
Chantix®. In one of these Chantix® Phase III clinical trials, approximately
one-third of those who had been abstinent during the last week of treatment
returned to smoking within four weeks after they stopped taking Chantix®, and
approximately 45% returned to smoking within eight weeks after they stopped
taking Chantix®.

Patients
who used the VLN cigarette containing our proprietary tobacco over the 6-week
treatment period significantly reduced their smoking as compared to their usual
brand of cigarettes. The number of VLN cigarettes smoked per day on average
decreased from 19 (the baseline number of cigarettes of the smoker’s usual
brand) to 12 by the end of the 6-week treatment period, even though participants
in this clinical trial were instructed to smoke ad libitum (as many
cigarettes as desired) during treatment. Carbon monoxide (CO) levels, an
indicator of smoke exposure, significantly decreased from 20 parts per million
(baseline) to 15 parts per million. Cotinine, a metabolite and biomarker of
nicotine, significantly decreased from 4.2 micrograms/mL (baseline) to 0.2
micrograms/mL. All differences in the above three measurements were
statistically significant (P<0.05).
-15-
Additional
biomarkers of smoke exposure were significantly reduced on average from baseline
measurements (taken before the 6-week treatment period) in patients who used the
VLN cigarette containing our proprietary tobacco:
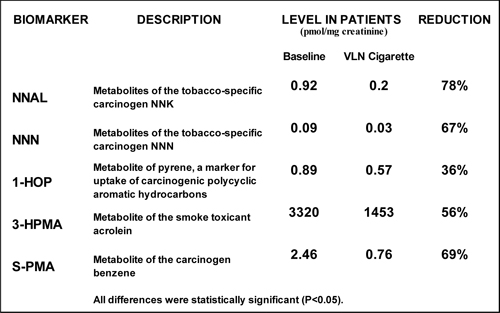
In a
separate Phase II clinical trial funded by Vector Tobacco, our former licensee,
under Investigational New Drug (“IND”) Application 69,185, a randomized
double-blind, active controlled, parallel group, multi-center Phase II smoking
cessation clinical trial was conducted to evaluate the quitting efficacy of
Quest® reduced-nicotine cigarettes as a smoking cessation treatment in 346
patients (Becker et al.
2008). Treatment consisted of smoking three reduced-nicotine cigarette styles
(Quest 1®, Quest 2® and Quest 3®) for 2 weeks each, with nicotine yields per
cigarette of 0.6 mg (a low nicotine cigarette made with a blend of regular
tobacco and our proprietary VLN tobacco), 0.3 mg (an extra low nicotine
cigarette made with a blend of regular tobacco and our proprietary VLN tobacco)
and 0.05 mg (a VLN cigarette made with tobacco only from our proprietary VLN
variety) either in combination with nicotine patch therapy (a nicotine
replacement product) or placebo patches.
In this
three-arm clinical trial in which patients were treated over sixteen weeks, use
of reduced-nicotine cigarettes in combination with nicotine patches was more
effective (the difference was statistically significant) in achieving four-week
continuous abstinence than use of nicotine patches alone (32.8% vs. 21.9%), and
use of reduced-nicotine cigarettes without nicotine patches yielded an
abstinence rate similar (the difference was not statistically significant) to
that of nicotine patches (16.4% vs. 21.9%). No serious adverse events were
attributable to the investigational product.
-16-
The major
difference between the Vector Phase II clinical trial and the University of
Minnesota Phase II clinical trial is that VLN cigarettes in the Vector trial
were smoked by patients for only 2 weeks and either in combination with using a
nicotine patch or placebo patch. In both arms that smoked the VLN cigarette for
2 weeks, patients continued to use nicotine patches or placebo patches for the
subsequent 10 weeks. We believe that the effectiveness of VLN cigarettes for use
in smoking cessation is higher when they are used alone (without another
therapy) for a longer time period, as in the University of Minnesota trial,
rather than with concurrent use of nicotine replacement therapy. We have
therefore decided to have patients use VLN cigarettes alone and for 6 weeks in
our upcoming clinical trials.
A 2008
binding arbitration award, which was completely fulfilled by Vector Tobacco in
2009, provided us with copies of all of Vector’s FDA submissions relating to
Vector’s IND for Quest® and awarded to us a right of reference to Vector’s IND
for Quest®, including all results of Vector’s Phase II clinical trial. This
arbitration award allows us to use all such information in our IND with the FDA
for our VLN cigarette that contains our same proprietary tobacco that Vector
used in its IND submissions to the FDA. This arbitration award has been helpful
to us with the FDA, since analytical reports produced by our former licensee
pertaining to our proprietary tobacco and cigarettes made from our tobacco are
being utilized by us with the FDA.
Another
smoking cessation clinical trial using VLN cigarettes containing our proprietary
tobacco was a randomized controlled trial conducted at Roswell Park Cancer
Institute, Buffalo, New York, to investigate the effect of smoking a very low
nicotine cigarette in combination with a nicotine patch for 2 weeks prior to the
quit date (Rezaishiraz et
al. 2007). Ninety-eight adult smokers were randomized to two treatments:
(i) 2 weeks of a very low nicotine cigarette (Quest 3®) and 21-mg nicotine patch
before the quit date and (ii) a reduced nicotine cigarette (Quest 1®) during the
2 weeks before the quit date. After the quit date, all subjects received
counseling for smoking cessation and nicotine patch therapy for up to 8 weeks (4
weeks of 21-mg patches, 2 weeks of 14-mg patches, and 2 weeks of 7-mg patches).
Group 1, which used very low nicotine cigarettes and a nicotine patch before
quitting, had lower combined craving score during the 2 weeks before and after
the quit date. Self-reported point prevalence of smoking abstinence at the 3-
and 6-month follow-up points was higher in Group 1 (43% vs. 34% and 28% vs.
21%).
A study
at Dalhousie University, Halifax, Nova Scotia (Barrett 2010), compared the
effects of low nicotine cigarettes and an FDA-approved nicotine inhaler on
cravings and smoking behavior of smokers who did not intend to quit. In separate
laboratory sessions, each of twenty-two participants used a VLN cigarette (Quest
3®), a reduced nicotine cigarette (Quest 1®, which contains approximately
two-thirds conventional tobacco and one-third VLN tobacco), a nicotine inhaler
(10 mg; 4 mg deliverable, Pharmacia), or a placebo inhaler (identical in
appearance to the nicotine inhaler, but containing no nicotine). Cravings,
withdrawal and mood descriptors were rated before and after a 20-minute
treatment session during which subjects were instructed to smoke two cigarettes
or to use an inhaler every 10 seconds. The reduction in the rating of intent to
smoke (usual cigarette brand) after using the VLN cigarette (-10.0) was
significantly greater than the reduction with the nicotine inhaler (-1.9). Use
of the VLN cigarette was also associated with significantly increased
satisfaction and relaxation compared to the nicotine inhaler.
Technology
Platform
Our
proprietary technology enables us to decrease or increase the level of nicotine
in tobacco plants by decreasing or increasing the expression of gene(s)
responsible for nicotine production in the tobacco plant using genetic
engineering. The basic techniques are the same as those used in the production
of genetically modified varieties of other crops, which in 2009 were planted on
330 million acres in 25 countries according to the International Service for the
Acquisition of Agri-Biotech Applications (ISAAA). This includes 85% of the corn
and soybeans grown in the United States. The only components of the technology
that are distinct from those in commercialized genetically modified varieties of
major crops are segments of tobacco genes (DNA sequences) that are also present
in all conventional tobacco plants. Genetically modified tobacco that we use in
our products is produced from plants that have been deregulated by the USDA.
Thus, plants may be grown and used in products in the United States without
legal restrictions or labeling requirements related to the genetic modification.
Nevertheless, our proprietary genetically engineered tobacco is grown only by
farmers under contracts that require segregation and prohibit transfer of
material to other parties.
-17-
During
the development of genetically modified varieties, many candidate lines are
evaluated in the field in multiple locations over several years, as in any other
variety development program. This is carried out in order to identify lines that
have not only the specific desired trait, e.g., very low nicotine, but
have overall characteristics that are suitable for commercial production of the
desired product. This allows us to see if there are undesirable effects of the
genetic modification approach or the specific genetic modification event,
regardless of whether the effects are anticipated or unanticipated. For example,
since nicotine is known to be an insecticide effective against a wide range of
insects, reduction of nicotine content in the plants may be expected to affect
susceptibility to insect pests. While there are differences in the
susceptibility of VLN tobacco to some insects, all tobacco is attacked by a
number of insects. The measures taken to control insect pests of conventional
tobacco are adequate to control insect pests in VLN tobacco.
Once a
modified tobacco plant with the desired characteristics is obtained, each plant
can produce hundreds of thousands of seeds. When each seed is germinated, the
resulting tobacco plant has identical characteristics, including nicotine
content, as the parent and sibling plants. Tobacco products with either low or
high nicotine content are easily produced through this method. For example, one
of our proprietary tobacco varieties contains the lowest nicotine content of any
tobacco ever commercialized, with approximately 95% less nicotine than tobacco
in leading “light” cigarette brands. This proprietary tobacco grows with
virtually no nicotine without adversely affecting the other leaf constituents
important to a cigarette’s characteristics, including taste and
aroma.
Intellectual
Property
Our
proprietary technology is covered by 12 patent families consisting of 97 issued
patents in 79 countries, and approximately 44 pending patent applications, which
are either owned by or exclusively licensed to us. A “patent family” is a set of
patents granted in various countries to protect a single invention. Our patent
coverage in the United States, the most valuable smoking cessation market and
cigarette market, consists of 14 issued patents and 6 pending applications. In
China, the world’s largest cigarette market, we exclusively control 5 issued
patents and 3 pending patent applications. We have exclusive worldwide rights to
all uses of the following genes responsible for nicotine content in tobacco
plants: QPT, A622, NBB1, MPO and genes for several
transcription factors. We have exclusive rights to plants with altered nicotine
content produced from modifying expression of these genes and tobacco products
produced from these plants. We also have the exclusive right to license and
sublicense these patent rights. The patents owned by or exclusively licensed to
us are issued in countries where at least 75% of the world’s smokers
reside.
We own
various registered trademarks in the United States. We also have
exclusive rights to plant variety protection (“PVP”) certificates in the United
States (issued by the U.S. Department of Agriculture) and Canada. A PVP
certificate prevents anyone other than the owner/licensee from planting a plant
variety for 20 years in the U.S. or 18 years in Canada. The protections of PVP
are independent of, and in addition to, patent protection.
Sales
and Marketing
X-22
Smoking Cessation Aid
We intend
to enter into arrangements in both the U.S. and international markets with
pharmaceutical companies to market and sell X-22. We will seek marketing
partners with existing pharmaceutical sales forces that already call on medical
and dental offices in their geographic markets.
-18-
There are
approximately 700,000 physicians in the United States, including approximately
80,000 general practitioners, many of whom are aware of new medications, even
before they achieve FDA approval. There are also approximately 170,000 dentists
in the U.S. who can write prescriptions for smoking cessation aids. We plan to
initially concentrate on a “push” strategy to develop demand for X-22 in the United States by
educating physicians and dentists about our X-22 smoking cessation aid.
We intend to advertise in professional journals, use direct mail campaigns to
medical professionals, and attend trade shows and professional conferences. We
also intend to use internet advertising and pharmacy circulars to reach
consumers and to encourage them to ask their physicians and dentists about our
X-22 smoking cessation
aid. We expect to use public relations to increase public awareness about X-22. We will seek to use
federal and state-funded smoking cessation programs and clinics to inform
clinicians and patients about, and encourage the use of, X-22 as a smoking cessation
aid. We will also seek to participate in various government-funded programs
which purchase approved smoking cessation aids and then distribute these to
smokers at no charge or at greatly reduced prices.
BRAND
A and BRAND B
We expect
significant sales in the U.S. of Brand A and Brand B within specialty
tobacco channels such as tobacconists, smoke shops and tobacco outlets. The ban
in 2009 by the FDA of all flavored cigarettes (with the exception of menthol)
has resulted in a product void in these tobacco channels. Certain wholesalers
and retailers are now seeking other specialty cigarettes to replace the banned
flavored cigarettes. We believe that certain U.S. cigarette wholesalers and
retailers will purchase our BRAND A and BRAND B cigarette brands to
replace their lost sales of flavored cigarettes as well as lost sales of “light”
cigarettes.
Government
Research Cigarettes
The
National Institute on Drug Abuse (“NIDA”), a component of the National
Institutes of Health (“NIH”), provides the scientific community with controlled
and uncontrolled research chemicals and drug compounds in its Drug Supply Program. In 2009,
NIDA included an option to develop and produce research cigarettes with ten
different levels of nicotine, including a minimal (placebo) level (“Research
Cigarette Option”) in its request for proposals for a 5-year contract for Preparation and Distribution of
Research and Drug Products. We have agreed, as a subcontractor to RTI
International (“RTI”) in RTI’s contract with NIDA for the Research Cigarette
Option, to supply modified nicotine cigarettes to NIDA. In August 2010, we met
with officials from NIDA, FDA, RTI, the National Cancer Institute and the
Centers for Disease Control and Prevention to finalize certain aspects of the
design of these research cigarettes. These research cigarettes will
be distributed under the mark SPECTRUM.
In 2010,
we received our first purchase order of $152,660 for 1.15 million research
cigarettes which included a design phase fee of $40,604. We expect to receive
two more purchase orders for an additional 8.275 million research cigarettes
over the next three months. We estimate the revenue from this contract,
including other direct orders from researchers, will be approximately $700,000
in 2011 and $3 million over the next 5 years.
Healthcare
Reimbursement
Government
and private sector initiatives to limit the growth of healthcare costs,
including price regulation, competitive pricing, coverage and payment policies,
and managed-care arrangements, are continuing in many countries where we intend
to sell our X-22
smoking cessation aid, including the United States. These changes are causing
the marketplace to put increased emphasis on the delivery of more cost-effective
medical products.
-19-
Government
healthcare programs in the United States, including Medicare and Medicaid,
private healthcare insurance and managed-care plans have attempted to control
costs by limiting the amount of reimbursement for which they will pay for
particular procedures or treatments. This may create price sensitivity among
potential customers for our X-22 smoking cessation aid,
even if we obtain FDA approval for it. Some third-party payers must also approve
coverage for new or innovative devices or therapies before they will reimburse
healthcare providers who use the medical devices or therapies. Even though a new
medical product may have been cleared for commercial distribution, we may find
limited demand for X-22
until reimbursement approval has been obtained from governmental and private
third-party payers.
Approximately
160 million Americans have private health insurance with prescription coverage
and the majority, and an increasing number of these plans, cover pharmacologic
treatments for smoking cessation. Healthcare payers, including governmental
bodies, are increasingly willing to fund smoking cessation treatments due to the
expected savings from reducing the incidence of smoking-related illnesses.
Approximately 46 million Americans were covered by Medicare in 2009. Medicare
provides insurance coverage for up to two smoking cessation attempts per year
and each attempt may include four counseling sessions.
Approximately
47 million Americans were covered by state Medicaid programs in 2009.
Approximately 30% of Medicaid recipients are smokers. Medicaid programs in 42
states and the District of Columbia cover at least one form of pharmacologic
treatment for smoking cessation (Chantix®, Zyban® or NRT). The new healthcare
legislation is expanding Medicaid coverage to all 50 states. The current retail
price of the 12-week prescription of Chantix® is over $450, which should give us
great latitude in pricing X-22. We expect X-22 to be price competitive
with any FDA-approved smoking cessation aid, especially Chantix®, which will not
only encourage governmental and private third-party payers to cover X-22, but will encourage
smokers to attempt to quit with X-22 since they will not have
to purchase their usual brand of cigarettes over the 6-week treatment period.
This equates to approximately $239 in out-of-pocket savings to the consumer if
their insurance plan covers X-22.
Manufacturing
We are in
the process of entering into agreements with several cigarette manufacturing
companies to manufacture X-22 for us for sale in the
United States and foreign markets. We are also in the process of
entering into agreements with several cigarette manufacturing companies to
manufacture BRAND A and
BRAND B for us for sale
in the Unites States and foreign markets, subject to FDA approval to market
BRAND A and BRAND B as Modified Risk
Cigarettes.
Competition
In the
market for FDA-approved smoking cessation aids, our principal competitors
include Pfizer Inc., GlaxoSmithKline PLC, Novartis International AG, and
Niconovum AB, a subsidiary of Reynolds American Inc. The industry consists of
major domestic and international companies, most of which have existing
relationships in the markets into which we plan to sell, as well as financial,
technical, marketing, sales, manufacturing, scaling capacity, distribution and
other resources and name recognition substantially greater than
ours.
Cigarette
companies compete primarily on the basis of product quality, brand recognition,
brand loyalty, taste, innovation, packaging, service, marketing, advertising,
retail shelf space and price. Cigarette sales can be significantly influenced by
weak economic conditions, erosion of consumer confidence, competitors’
introduction of low-price products or innovative products, higher cigarette
taxes, higher absolute prices and larger gaps between price categories, and
product regulation that diminishes the ability to differentiate tobacco
products. Domestic competitors include Philip Morris USA, Reynolds American
Inc., Lorillard Inc., Commonwealth Brands, Inc., Liggett Group LCC, Vector
Tobacco Inc., and Star Scientific Inc. International competitors include Philip
Morris International, British American Tobacco, Japan Tobacco Inc. and regional
and local tobacco companies; and, in some instances, government-owned tobacco
enterprises, principally in China, Egypt, Thailand, Taiwan, Vietnam and
Algeria.
-20-
Potential
Smoking Cessation Aids
Nicotine
Vaccines
Nicotine
vaccines are under development in clinical trials; however they have not yet
achieved the efficacy of other FDA-approved smoking cessation therapies.
Nicotine itself is not recognized by the body as a foreign compound since the
molecule is too small. In order to stimulate the production of antibodies,
nicotine must be attached to a carrier to make the vaccine work. Different
vaccine development programs use different carriers. Four companies, Cytos
Biotechnology AG, Celtic Pharmaceuticals Holdings, Nabi Biopharmaceuticals, L.P.
and Independent Pharmaceutica AB have or have had vaccine candidates in clinical
trials. Cytos exclusively licensed its nicotine vaccine candidate to Novartis in
2007 for 35 million Swiss Francs ($30 million) and up to 565 million Swiss
Francs ($492 million) in milestone payments and royalties. In October 2009, it
was announced that Cytos’ nicotine vaccine candidate failed to show efficacy in
a Phase II trial.
GlaxoSmithKline
Biologicals SA exclusively licensed Nabi’s nicotine vaccine candidate,
NicVAX®, in a
deal which was approved by Nabi’s shareholders in March 2010. Together with an
upfront non-refundable fee of $40 million paid by GlaxoSmithKline, Nabi is
eligible to receive over $500 million in option fees and milestones, not
including potential royalties on global sales. Phase III NicVAX® clinical
trials are commenced in 2010.
These
vaccine treatments entail six to seven consecutive monthly injections. Increases
in abstinence rates have been reported but only among a minority of trial
subjects with the highest levels of anti-nicotine antibodies. To date, all
subjects do not develop sufficient antibody levels despite receiving multiple
injections. Even in those who do develop sufficient antibody levels, cravings
for cigarettes are not addressed by this treatment, although the pharmacological
reward of nicotine is suppressed. Expectations are that the treatment, if
approved, would need to be repeated every 12 to 18 months to assist in
preventing relapse. Dr. Michael C. Fiore, lead chairperson and author of the
2008 U.S. government report on clinical practice guidelines for treating tobacco
use and co-principal Investigator of the Transdisciplinary Tobacco Use Research
Center at the University of Wisconsin, Madison, estimated in 2009 that any
approval of a nicotine vaccine may be 5 to 10 years away.
Electronic
or E-cigarettes
Although
the FDA has not evaluated electronic cigarettes, or e-cigarettes, for quitting
smoking, and we are not aware of any published result of a controlled clinical
trial of e-cigarettes as a smoking cessation aid, e-cigarettes are included here
since there have been unconfirmed claims that these products facilitate
cessation. E-cigarettes have been the subject of much controversy for this and
various other reasons, including the fact that these products are actually not
cigarettes or tobacco products at all but are battery-operated devices filled
with nicotine, flavor and other chemicals. They turn nicotine and other
chemicals into a vapor that is inhaled. E-cigarettes have very similar nicotine
delivery as nicotine inhalers, a prescription NRT product already approved by
the FDA, which is the reason we believe that using e-cigarettes to quit smoking
is not likely to be any more effective than other nicotine replacement
products.
In a
September 9, 2010 press release, the FDA issued warning letters to five
e-cigarette distributors for various violations of the Federal Food, Drug, and
Cosmetic Act, including unsubstantiated claims and poor manufacturing practices.
The FDA said these e-cigarette companies are illegally marketing their products
as tools to help people quit using cigarettes. The FDA believes e-cigarettes,
“Meet the definition of a combination drug-device product under the Federal
Food, Drug and Cosmetic Act.” In a letter to the Electronic Cigarette
Association of the same date, the FDA said the agency intends to regulate
electronic cigarette and related products in a manner consistent with its
mission of protecting the public health.
-21-
The FDA
has also been confiscating imports of e-cigarettes and has been in litigation
with importers of these products. A federal appeals court ruled on December 7,
2010 that the FDA can regulate electronic cigarettes as tobacco products rather
than a drug-delivery device. The FDA is appealing this decision,
however, the U.S. Court of Appeals for the District of Columbia Circuit on
January 2011 rejected the FDA’s request to have the entire court review the
December 7, 2010 decision that went against the agency. The FDA,
which has always contended that e-cigarettes should be regulated as
drug-delivery devices not tobacco products, now has the option of asking
the U.S. Supreme Court to take up the case. An FDA spokesman said that the
agency is evaluating the latest court ruling “and considering its legal and
regulatory options.” Many countries have already banned e-cigarettes
as has the state of Oregon and other states are in the process of banning
them.
Government
Regulation
Smoking
Cessation Aids
Government
authorities in the United States and foreign countries extensively regulate the
research, development, testing, manufacture, labeling, promotion, advertising,
distribution, sampling, marketing and import and export of pharmaceutical
products. FDA approval must be obtained, as has been the case for decades,
before a product can be marketed for quitting smoking or reducing withdrawal
symptoms. In addition, as with all FDA-approved prescription drugs, the FDA must
approve the brand name of our X-22 smoking cessation aid.
The FDA approval process for smoking cessation aids is similar to that required
by the FDA for new drug approvals, although the cost to complete clinical trials
for a smoking cessation aid such as X-22 are generally far less
than clinical trials for drugs. The primary endpoint of the clinical trial for
smoking cessation aids is smoking abstinence, which is generally confirmed by
inexpensive, noninvasive biomarker tests. Since potential quitters are already
smokers, X-22 will not
expose participants in the clinical trials to any new compounds, unlike a new
chemical entity, such as Chantix®.
The
process of obtaining governmental approvals and complying with ongoing
regulatory requirements requires the expenditure of substantial time and
financial resources. In addition, statutes, rules, regulations and policies may
change and new legislation or regulations may be issued that could delay such
approvals. If we fail to comply with applicable regulatory requirements at any
time during the product development process, approval process, or after
approval, we may become subject to administrative or judicial sanctions. These
sanctions could include the FDA’s refusal to approve pending applications,
withdrawals of approvals, clinical holds, warning letters, product recalls,
product seizures, total or partial suspension of our operations, injunctions,
fines, civil penalties or criminal prosecution. Any agency enforcement action
could have a material adverse effect on us.
The U.S.
regulatory scheme for the development and commercialization of new drugs can be
divided into three distinct phases: an investigational phase including both
preclinical and clinical investigations leading up to the submission of a New
Drug Application (“NDA”); a period of FDA review culminating in the approval or
refusal to approve the NDA; and the post-marketing period.
-22-
Preclinical
Phase
The
preclinical phase involves the characterization, product formulation and animal
testing necessary to prepare an IND Application for submission to the FDA. The
IND must be reviewed and authorized by the FDA before the drug can be tested in
humans. Once a new drug agent has been identified and selected for further
development, preclinical testing is conducted to confirm pharmacological
activity, to generate safety data, to evaluate prototype dosage forms for
appropriate release and activity characteristics, and to confirm the integrity
and quality of the material to be used in clinical trials. A bulk supply of the
active ingredient to support the necessary dosing in initial clinical trials
must be secured. Data from the preclinical investigations and detailed
information on proposed clinical investigations are compiled in an IND
submission and submitted to the FDA before human clinical trials may begin. If
the FDA does not formally communicate an objection to the IND within 30 days,
the specific clinical trials outlined in the IND may go forward.
Clinical
Phase
The
clinical phase of drug development follows an IND submission and involves the
activities necessary to demonstrate the safety, tolerability, efficacy, and
dosage of the substance in humans, as well as the ability to produce the
substance in accordance with the FDA’s cGMP requirements. Data from these
activities are compiled in an NDA requesting approval to market the drug for a
given use, or indication. Clinical trials must be conducted under the
supervision of qualified investigators in accordance with good clinical
practice, and according to IND-approved protocols detailing, among other things,
the study objectives and the parameters, or endpoints, to be used in assessing
safety and efficacy. Each trial must be reviewed, approved and conducted under
the auspices of an independent Institutional Review Board, or IRB, and each
trial, with limited exceptions, must include all subjects’ informed consent. The
clinical evaluation phase typically involves the following sequential
process:
Phase I
clinical trials are conducted in a limited number of healthy subjects to
determine the drug’s safety, tolerability, and biological performance. The total
number of subjects in Phase I clinical trials varies, but is generally in the
range of 20 to 80 people (or less in some cases, such as drugs with significant
human experience).
Phase II
clinical trials involve administering the drug to subjects suffering from the
target disease or condition to evaluate the drug’s potential efficacy and
appropriate dose. The number of subjects in Phase II trials is typically several
hundred subjects or less.
Phase III
clinical trials are performed after preliminary evidence suggesting
effectiveness has been obtained and safety, tolerability, and appropriate dosing
have been established. Phase III clinical trials are intended to gather
additional data needed to evaluate the overall benefit-risk relationship of the
drug and to provide adequate instructions for its use. Phase III trials usually
include several hundred to several thousand subjects.
Throughout
the clinical testing phase, samples of the product made in different batches are
tested for stability to establish shelf life constraints. In addition,
increasingly large-scale production protocols and written standard operating
procedures must be developed for each aspect of commercial manufacturing and
testing.
The
clinical trial phase is both costly and time-consuming, and may not be completed
successfully within any specified time period, if at all. The FDA closely
monitors the progress of each of the three phases of clinical trials that are
conducted under an IND and may, at its discretion, reevaluate, alter, suspend,
or terminate the testing at any time for various reasons, including a finding
that the subjects or patients are being exposed to an unacceptable health risk.
The FDA can also request additional clinical testing as a condition to product
approval. Additionally, new government requirements may be established that
could delay or prevent regulatory approval of our products under development.
Furthermore, institutional review boards, which are independent entities
constituted to protect human subjects in the institutions in which clinical
trials are being conducted, have the authority to suspend clinical trials in
their respective institutions at any time for a variety of reasons, including
safety issues.
-23-
New
Drug Application and Review
After the
completion of Phase III clinical trials, the sponsor of the new drug submits an
NDA to the FDA requesting approval to market the product for one or more
indications. An NDA is a comprehensive, multi-volume application that includes,
among other things, the results of all preclinical and clinical studies,
information about the drug’s composition, and the sponsor’s plans for producing,
packaging, and labeling the drug. In most cases, the NDA must be accompanied by
a substantial user fee. FDA has 60 days after submission to review the
completeness and organization of the application, and may refuse to accept it
for continued review, or refuse to file, if the application is found deficient.
After filing, the FDA reviews an NDA to determine, among other things, whether a
product is safe and effective for its intended use. Drugs that successfully
complete NDA review may be marketed in the United States, subject to all
conditions imposed by the FDA.
Prior to
granting approval, the FDA generally conducts an inspection of the facilities,
including outsourced facilities that will be involved in the manufacture,
production, packaging, testing and control of the drug for cGMP compliance. The
FDA will not approve the application unless cGMP compliance is satisfactory. If
the FDA determines that the marketing application, manufacturing process, or
manufacturing facilities are not acceptable, it will outline the deficiencies in
the submission and will often request additional testing or information.
Notwithstanding the submission of any requested additional information, the FDA
ultimately may decide that the marketing application does not satisfy the
regulatory criteria for approval and refuse to approve the application by
issuing a “not approvable” letter.
The
length of the FDA’s review can range from a few months to several years or more.
Once an NDA is in effect, significant changes such as the addition of one or
more new indications for use generally require prior approval of a supplemental
NDA including additional clinical trials or other data required to demonstrate
that the product as modified remains safe and effective.
Fast
Track Development
The Food
and Drug Administration Modernization Act of 1997, or the Modernization Act,
establishes a statutory program for relatively streamlined approval of “Fast
Track” products, which are defined under the Modernization Act as new drugs or
biologics intended for the treatment of a serious or life-threatening condition
that demonstrates the potential to address unmet medical needs for this
condition. Fast Track status requires an official designation by the FDA. The
2009 Family Smoking Prevention and Tobacco Control Act (“Tobacco Control Act”)
provides that products for smoking cessation, such as X-22, be considered for “Fast
Track” designation by the FDA.
We intend
to submit a request to the FDA for Fast Track designation in the fourth quarter
2010 and, although there can be no assurance, we believe that our X-22 smoking cessation aid
will be granted Fast Track designation by the FDA. A product that receives Fast
Track designation is eligible for (i) more frequent meetings with the FDA to
discuss the drug’s development plan and ensure collection of appropriate data
needed to support drug approval, and (ii) more frequent written correspondence
from the FDA about such things as the design of the proposed clinical trials. A
Fast Track product is also eligible for Rolling Review, in which sections of the
NDA can be submitted for review by the FDA before the entire application is
completed. A Fast Track product would ordinarily meet FDA criteria for Priority
Review. The FDA goal for reviewing a drug with Priority Review status is six
months from the filing of the NDA.
Post-Approval
Phase
Once the
FDA has approved a new drug for marketing, the product becomes available for
physicians to prescribe in the United States. After approval, we must comply
with post-approval requirements, including ongoing compliance with cGMP
regulations, delivering periodic reports to the FDA, submitting descriptions of
any adverse reactions reported, and complying with drug sampling and
distribution requirements. We are required to maintain and provide updated
safety and efficacy information to the FDA. We must also comply with
requirements concerning advertising, product promotions, and
labeling.
-24-
X-22
Clinical Trials
We have
met with the FDA regarding the remaining X-22 clinical trials and, based on the
FDA’s guidance, we plan to conduct a small Phase II-B trial and two larger and
concurrent Phase III trials with the same protocols that entail measuring the
quitting efficacy of the X-22 cigarette against a
typical cigarette with conventional nicotine content that is visually
indistinguishable from X-22
(the “active control”). The Phase II-B optimization trial will consist of
approximately 200 participants over a 6-week treatment period, and the Phase III
trials will use the same protocol with larger groups of participants. In all of
the remaining clinical trials, half of the participants will smoke X-22 for 6 weeks and half of
the participants will smoke the active control for 6 weeks, with all
participants instructed to quit on the last day of the 6-week treatment
period.
Smokers
who do not smoke over the four-week period immediately following the conclusion
of the 6-week treatment period (weeks 7 through 10) are considered abstinent.
The abstinence (quit) rates of the X-22 group and the active
control group will then be compared for statistical significance. With adequate
funding, we will be able to conduct our two concurrent Phase III clinical trials
with the same protocols in order to expedite the FDA approval process. We have
submitted our Pre-IND (PIND 103,589) to the FDA and, subject to closing this
Offering, we expect to initiate our Phase II-B clinical trial in the first
quarter of 2011 after we file our IND. Our IND will contain all of the
information and data of our PIND 103,589 plus standard tobacco industry smoke
analyses of the X-22
clinical trial cigarette and the active control. Before Phase III trials, some
additional information and testing of X-22 and its tobacco are
required by the FDA, some of which we already have from our former licensee’s
IND 69,185. All analyses that FDA requires are efficiently outsourced to Arista
Laboratories which is the industry leader in tobacco and tobacco smoke analyses
that we have been contracting with for years. We intend to initiate our Phase
III clinical trials in the third quarter of 2011 and to file our NDA with the
FDA for X-22 by the
first quarter of 2011. We expect the FDA to Fast Track the approval of X-22 and that we should
receive FDA approval to commence the marketing and sales of X-22 in the U.S. as early as
the fourth quarter of 2012.
Following
FDA approval, we intend to register X-22 as a Medicinal Product
(pharmacological) for smoking cessation with the European Medicines Agency and
other international FDA-equivalent agencies in targeted countries. Regulatory
approval for X-22 as a
smoking cessation aid is not required in some international markets since,
unlike the FDA, some foreign drug regulatory agencies do not require approval to
market a product as a smoking cessation aid if the product is allowed to be sold
for other purposes.
Modified
Risk Cigarettes
The
Tobacco Control Act, which became law in June 2009, prohibits the FDA from
banning cigarettes outright or mandating that nicotine levels be reduced to
zero. However, among other things, it allows the FDA to require the reduction of
nicotine or any other compound in cigarettes. In 2009, the Tobacco Control Act
banned all sales in the United States of cigarettes with flavored tobacco (other
than menthol). As of June 2010, all cigarette companies were required to cease
using the terms “low tar,” “light” and “ultra light” in describing cigarettes
sold in the United States. We believe this new regulatory environment represents
a paradigm shift for the tobacco industry and will create opportunities for us
in marketing BRAND A
and BRAND B and
in licensing our proprietary technology and/or tobaccos to larger
competitors.
-25-
For the
first time in history, the FDA will evaluate cigarettes that may pose lower
health risks as compared to conventional cigarettes. The Tobacco Control Act
established procedures for the FDA to regulate the labeling and marketing of
Modified Risk Cigarettes and requires the FDA to issue additional guidance
regarding applications that must be submitted to the FDA for Modified Risk
Cigarettes. We believe the FDA will issue such guidance in 2011. We also believe
that BRAND A and BRAND B will qualify as
Modified Risk Cigarettes. In addition, the Tobacco Control Act allows the FDA to
mandate the use of reduced risk technologies in conventional cigarettes (e.g., Marlboro®) which could
create opportunities for us to license our technology or tobaccos.
We have
begun to supply our cigarettes to the National Transdisciplinary Tobacco Use
Research Centers in the United States so they can conduct studies to obtain
additional information on our products, including results from exposure studies
(for BRAND A and BRAND B) and smoking clinical
trials (for X-22). We
expect this information will assist us, along with our own funded studies, in
obtaining the necessary FDA authorizations and approvals to market BRAND A and BRAND B as Modified Risk
Cigarettes and for X-22
as a prescription smoking cessation aid.
Biomass
Products
We have
funded extensive biomass field trials conducted by North Carolina State
University (“NCSU”) and work on feedstock digestibility and bioconversion at the
National Renewable Energy Lab. The results have been included in a comprehensive
feasibility study relating to our nicotine-free tobacco biomass crop (Verfola) to produce a variety
of bioproducts. First, protein and other plant fractions are extracted, and then
biofuels and other products are produced from the remaining cellulosic residue.
In 2008, we put our biomass development projects on hold so that our management
could focus its attention and resources on X-22, BRAND A and BRAND B. We plan to move
forward in our biomass business activities when we have sufficient resources to
do so. We plan to form a separate subsidiary in the future which will be
dedicated to our biomass business model.
Tobacco
has a number of advantages as a starting point for development of novel
bioproduct crop systems. Because tobacco is a widely cultivated crop, grown in
over 100 countries throughout the world, tobacco agronomy is highly understood.
For decades tobacco has been used as a model system for plant biology, and
recently the tobacco genome has been mapped. Tobacco plants rapidly sprout back
after each harvest and produce large amounts of leaf and total biomass. Tobacco
grown for cigarettes yields about 3,000 pounds of cured leaf per acre (~20%
moisture) per year from 7,500 tobacco plants. In our field trials in North
Carolina, nicotine-free tobacco grown for biomass yields about 100,000 pounds of
fresh weight per acre (which equals 10,000 pounds of dry weight) per year with
multiple machine harvests from about 80,000 tobacco plants.
About
2,000 pounds (20%) of the per-acre dry weight biomass consists of extractable
protein fractions. Of this protein, about 500 pounds (25%) is a protein known as
Rubisco (RibUlose
BISphosphate
Carboxylase-Oxygenase)
which is involved in photosynthesis. All green leaf plants contain Rubisco.
However, it is most easily extracted from tobacco by a proven and simple
two-step process. We believe that Rubisco has many valuable uses. Additional
high-quality protein fractions can be extracted along with other plant fractions
such as sugars, starches, cellulose and other components can be utilized
directly, or for production of biofuels, including ethanol and butanol, by
fermentation.
Rubisco
is a crystalline (greater than 99 pure) pharmaceutical grade protein that is
tasteless, odorless, and colorless when mixed with water. It is not perishable
and can be stored for years. As a plant-based protein source, it is useful as a
food additive or supplement. Rubisco includes all the essential amino acids in
quantities that equal or exceed the Food and Agriculture Organization (“FAO”)
Provisional Pattern and compares favorably to soybeans in essential amino acid
content (measured in grams of each essential amino acid per 100 grams of
protein). Rubisco has a low lysine-to-arginine (“L/A”) ratio (0.95) compared to
L/A ratios in protein from animal sources (2.4 for milk protein, 1.9 for casein,
and 1.4 for fish meal). A low L/A ratio is reportedly correlated with low serum
cholesterol and atherosclerotic incidence in animals. Rubisco can be added to
fortify almost any food or beverage with a high quality protein without
affecting the aroma or taste.
-26-
We
believe Rubisco is a superior substitute for casein, an animal-based protein
source derived from milk. The United States currently imports about 70,000
metric tons of casein per year. The market price fluctuates like other
commodities but is currently $4.10 per pound. Besides human nutrition, Rubisco
will also favorably compete in the following markets: personal care products,
nutraceuticals, and pharmaceutical grade protein (e.g., for dialysis patients).
Additional protein concentrates from Verfola will compete
favorably in animal feed, in particular aquaculture.
We
believe Verfola
provides significant advantages over any other green leaf crop, including
conventional tobacco. If tobacco with conventional nicotine levels was utilized
for biomass, for every acre grown, hundreds of pounds of toxic alkaloids would
have to be extracted, stored and disposed.
Research
and Development
Most
research and development (R&D) since 22nd Century’s inception have been
outsourced to highly qualified groups in their respective
fields. Since 1998, 22nd Century has had multiple R&D agreements
with North Carolina State University (“NCSU”) resulting in exclusive
worldwide licenses to various patented technologies. We have utilized
the model of many public-sector research organizations which entails obtaining
an exclusive option or license agreement to any invention arising out of the
funded research. In all cases, we fund and exclusively control all
patent filings as the exclusive licensee. This model of contracting
with public-sector researchers has enabled 22nd Century to control R&D costs
while achieving our desired results, including obtaining exclusive intellectual
property rights relating to all of our funded R&D.
Other
R&D partners with the same arrangement have included the National Research
Counsel of Canada, Plant Biotechnology Institute in Saskatoon, Canada (“NRC”)
and the Nara Institute of Science and Technology in Nara, Japan
(“NAIST”). Our R&D agreements with NCSU, NRC and NAIST have
expired in 2009 and the majority these agreements have involved the
biosynthesis of nicotine in plants. During the years ended
December 31, 2009 and 2008, we incurred research and development expenses of
approximately $540,000 and $654,000, respectively. In 2010,
NAIST assigned all of their worldwide patents to 22nd Century which were a
result of our R&D at NAIST and that were previously licensed to 22nd
Century on a exclusive basis.
Other
than our planned clinical trials for X-22 and exposure studies for
our Modified Risk Cigarette candidates, we have no other third-party R&D
commitments requiring funding in 2011. However, we do plan to carry
out a minimal amount of other R&D in 2011 not to exceed $250,000 per year,
including the execution of more field trials from the inventory of hundreds of
seed lots that resulted from our R&D at NCSU, NRC and
NAIST.
Employees
We
currently employ six people, none of whom are represented by a union, and we
consider our employee relations to be good.
Description
of Property
Our
principal administrative offices are located in Williamsville, New
York. We currently lease such facilities and the lease
expires on October 31, 2011, subject to automatic renewal for an additional
one-year term absent notice of non-renewal from either party.
-27-
Legal
Proceedings
From time
to time we may be involved in claims arising in the ordinary course of
business. To our knowledge, no legal proceedings, governmental
actions, investigations or claims are currently pending against us or involve us
that, in the opinion of management, could reasonably be expected to have a
material adverse effect on our business and financial condition.
We
anticipate that we will expend significant financial and management resources to
the defense of our intellectual property rights in the future if we believe that
our rights have been infringed. We also anticipate that we will
expend significant financial and management resources to defend against claims
that our products and services infringe upon the intellectual property rights of
third parties.
Risk
Factors
There are numerous and varied risks,
known and unknown, that may prevent the Company from achieving its goals. The
risks described below are not the only ones the Company will face. If any of
these risks actually occurs, our business, financial condition or results of
operation may be materially adversely affected. In such case, the trading price
of our Common Stock could decline and investors in our Common Stock could lose
all or part of their investment.
Risks
Related to Our Business and Operations
We
may not be able to continue as a going concern.
Recurring
losses from operations, our negative working capital of $3.6 million as of
September 30, 2010 ($3.2 million at December 31, 2009), members’ deficit of $1.8
million as of September 30, 2010 ($1.8 million at December 31, 2009) and the
uncertainty of obtaining additional financing on a timely basis, raise doubt
about our ability to continue as a going concern. The report of our independent
registered public accounting firm on our financial statements for the year ended
December 31, 2009 (dated June 1, 2010 and restated on October 15, 2010),
includes an emphasis of a matter paragraph expressing substantial doubt whether
we can continue as a going concern. Even in light of the proceeds of the Private
Placement Offering, we cannot guarantee our ability to continue as a going
concern.
We
have had a history of losses, and we may be unable to achieve or sustain
profitability.
We
experienced net losses of approximately $1.0 million during the nine month
period ended September 30, 2010 and $1.2 million and $0.74 million in the years
2009 and 2008, respectively. We expect to continue to incur net losses and
negative operating cash flows in the foreseeable future and cannot be certain
that we will ever achieve profitability. Since 2007, we have received only
limited licensing revenue from a former licensee and have achieved limited
revenue of product sales from test marketing. We will need to spend significant
capital to fulfill planned operating goals and conduct clinical studies, achieve
regulatory approvals and, subject to such approvals, successfully produce
products for commercialization. In addition, as a public company, we
will incur significant legal, accounting and other expenses that we did not
incur as a private company.
We
have a history of negative cash flow, and our ability to generate positive cash
flow is uncertain.
We had
negative cash flow before financing activities of approximately $739,000 during
the nine months ended September 30, 2010 and $172,000 and $762,000 in the years
2009 and 2008, respectively. We anticipate that we will continue to have
negative cash flow for the foreseeable future as we will continue to incur
increased expenses from seeking regulatory approvals, including clinical trials
and exposure studies, sales and marketing, and general and administrative
expenses, as well as to purchase inventory. Our business will also require
significant amounts of working capital to support our growth. Therefore, we may
need to raise additional investment capital to achieve growth, and we may not
achieve sufficient revenue growth to generate positive future cash flow. An
inability to generate positive cash flow for the foreseeable future or raise
additional capital on reasonable terms may decrease our long-term
viability.
-28-
Our
limited operating history makes it difficult to evaluate our current business
and future prospects.
We have
been in existence since 1998, but our activities have been limited primarily to
licensing and funding research and development activities. Our limited operating
history may make it difficult to evaluate our current business and our future
prospects. We have encountered and will continue to encounter risks and
difficulties frequently experienced by growing companies in rapidly changing
industries, including increasing expenses as we continue to grow our business.
If we do not manage these risks successfully, our business will be
harmed.
We
have no experience in managing growth. If we fail to manage our growth
effectively, we may be unable to execute our business plan or address
competitive challenges adequately.
We
currently have six employees. Any growth in our business will place a
significant strain on our managerial, administrative, operational, financial,
information technology and other resources. We intend to further expand our
overall business, customer base, employees and operations, which will require
substantial management effort and significant additional investment in our
infrastructure. We will be required to continue to improve our operational,
financial and management controls and our reporting procedures and we may not be
able to do so effectively. As such, we may be unable to manage our growth
effectively.
Our
working capital requirements involve estimates based on demand expectations and
may decrease or increase beyond those currently anticipated, which could harm
our operating results and financial condition.
We have
no experience in selling smoking cessation products or Modified Risk Cigarettes
on a commercial basis. As a result, we intend to base our funding and inventory
decisions on estimates of future demand. If demand for our products does not
increase as quickly as we have estimated or drops off sharply, our inventory and
expenses could rise, and our business and operating results could suffer.
Alternatively, if we experience sales in excess of our estimates, our working
capital needs may be higher than those currently anticipated. Our ability to
meet any demand for our products may depend on our ability to arrange for
additional financing for any ongoing working capital shortages, since it is
likely that cash flow from sales will lag behind our investment
requirements.
The
net proceeds of the Private Placement Offering will not be sufficient to enable
us to complete the FDA approval process for our X-22 smoking cessation product
and the FDA authorization process for our Modified Risk Cigarettes.
We will
require additional capital in the future beyond the net proceeds of the Private
Placement Offering to complete the FDA approval process for our X-22 smoking cessation
product and the FDA authorization process for our Modified Risk Cigarettes, and
we may not be able to obtain additional debt or equity financing on favorable
terms, if at all. If we raise additional funds through the issuance of equity
securities, our stockholders may experience substantial dilution, or the equity
securities may have rights, preferences or privileges senior to those of
existing stockholders. If we raise additional funds through debt financings,
these financings may involve significant cash payment obligations and covenants
that restrict our ability to operate our business and make distributions to our
stockholders. We also could elect to seek funds through arrangements with
collaborators. To the extent that we raise additional funds through
collaboration and licensing arrangements, it may be necessary to relinquish some
rights to our technologies or our potential products or grant licenses on terms
that are not favorable to us.
-29-
Due to
market conditions and the status of our product development activities,
additional funding may not be available to us on acceptable terms, or at all.
Having insufficient funds may require us to delay, scale back or eliminate some
or all of our clinical programs or to relinquish greater rights to potential
products at an earlier stage of development or on less favorable terms than we
would otherwise choose. Our failure to raise additional financing would
adversely affect our ability to maintain, develop, enhance or grow our business,
take advantage of future opportunities or respond to competitive pressures. If
we cannot raise additional capital on acceptable terms, we may not be able to,
among other things:
|
·
|
continue
or complete clinical trials of our X-22 smoking cessation
aid;
|
|
·
|
continue
or complete the steps necessary to seek FDA authorization of our Modified
Risk Cigarettes;
|
|
·
|
develop
or enhance our potential products or introduce new
products;
|
|
·
|
expand
our development, sales and marketing and general and administrative
activities;
|
|
·
|
attract
tobacco growers, customers or manufacturing and distribution
partners;
|
|
·
|
acquire
complementary technologies, products or
businesses;
|
|
·
|
expand
our operations in the United States or
internationally;
|
|
·
|
hire,
train and retain employees; or
|
|
·
|
respond
to competitive pressures or unanticipated working capital
requirements.
|
Continued
instability in the credit and financial market conditions may negatively impact
our business, results of operations, and financial condition.
Financial
markets in the United States, Canada, Europe and Asia continue to experience
disruption, including, among other things, significant volatility in security
prices, declining valuations of certain investments, severely diminished
liquidity and credit availability. Business activity across a wide range of
industries and regions continues to be greatly reduced and local governments and
many businesses are still in serious difficulty due to the lack of consumer
spending and the lack of liquidity in the credit markets. As a clinical-stage
biotechnology company, we rely on third parties for several important aspects of
our business, including the supply of tobacco, manufacturing and distribution of
our products, development of our potential products, and conduct of our clinical
trials. Such third parties may be unable to satisfy their commitments to us due
to tightening of global credit from time to time, which would adversely affect
our business. The continued instability in the credit and financial market
conditions may also negatively impact our ability to access capital and credit
markets and our ability to manage our cash balance. While we are unable to
predict the continued duration and severity of the adverse conditions in the
United States and other countries, any of the circumstances mentioned above
could adversely affect our business, financial condition, operating results and
cash flow or cash position.
We
will depend on the success of our X-22 smoking cessation aid and our Modified
Risk Cigarettes and we may not be able to successfully commercialize these
potential products.
Our goal
is to develop products whose potential for risk reduction can be substantiated
and that meet adult smokers’ taste expectations. We may not succeed in these
efforts. If we do not succeed, but one or more of our competitors do, we may be
at a competitive disadvantage. The success of our business depends in part on
our ability to obtain FDA approval for our X-22 smoking cessation aid
and FDA authorization under the Tobacco Control Act to market our BRAND A and BRAND B cigarettes as
Modified Risk Cigarettes. We have not obtained approval to market X-22 in any jurisdiction, nor
have we obtained authorization to market our BRAND A or BRAND B cigarettes as
Modified Risk Cigarettes, and we cannot predict whether we will be able to
obtain such approval or authorizations, or if regulators will permit the
marketing of tobacco products with claims of reduced risk to consumers. Any
failure to obtain such approval or authorizations would significantly undermine
the commercial viability of the applicable product. If we fail to successfully
commercialize or continue to sell these products, we may be unable to generate
sufficient revenue to sustain and grow our business, and our business, financial
condition, results of operations and cash flows will be adversely
affected.
-30-
We
will depend on third parties to manufacture our potential products.
We
currently do not intend to manufacture any of our products and depend on
contract manufacturers to produce our products according to our specifications,
in sufficient quantities, on time, in compliance with appropriate regulatory
standards and at competitive prices. We do not currently have an arrangement
with any contract manufacturer to produce our final version of X-22 smoking cessation aid
once it is approved by the FDA.
Manufacturers
supplying our potential products must comply with FDA regulations which require,
among other things, compliance with the FDA’s evolving regulations on Current
Good Manufacturing Practices (“cGMP(s)”), which are enforced by the FDA through
its facilities inspection program. The manufacture of products at any facility
will be subject to strict quality control, testing and record keeping
requirements, and continuing obligations regarding the submission of safety
reports and other post-market information. We cannot guarantee that any facility
utilized by third-party manufacturers which we engage will pass FDA and/or
similar inspections in foreign countries to produce the final version of our
X-22 smoking cessation
aid, or that future changes to cGMP manufacturing standards will not also affect
the manufactures of our products. Therefore, we may have to build our
own manufacturing facility which would require additional capital.
We
will mainly depend on third parties to market, sell and distribute our products,
and we currently have no commercial arrangements for the marketing, sale or
distribution of our X-22 smoking cessation aid.
We expect
to mainly depend on third parties to market, sell and distribute our products
and we currently have no arrangements with third parties in place to provide
such services for our X-22
smoking cessation aid. We cannot be sure that we will be able to enter
into such arrangements on acceptable terms, or at all.
If we are
unable to enter into marketing, sales and distribution arrangements with third
parties for our X-22
smoking cessation aid, we would need to incur significant sales,
marketing and distribution expenses in connection with the commercialization of
our X-22 and any future
potential products. We do not currently have a dedicated sales force, and we
have no experience in the sales, marketing and distribution of pharmaceutical
products. Developing a sales force is expensive and time-consuming, and we may
not be able to develop this capacity. If we are unable to establish adequate
sales, marketing and distribution capabilities, independently or with others, we
may not be able to generate significant revenue and may not become
profitable.
If
our X-22 smoking cessation aid does not gain market acceptance among physicians,
patients, third-party payers and the medical community, we may be unable to
generate significant revenue.
Our X-22 smoking cessation aid
may not achieve market acceptance among physicians, patients, third-party payers
and others in the medical community. If we receive the regulatory approvals
necessary for commercialization of our X-22 smoking cessation aid in
the U.S., the degree of market acceptance could depend upon a number of factors,
including:
|
·
|
continue
limited indications of regulatory
approvals;
|
|
·
|
the
establishment and demonstration in the medical community of the clinical
efficacy and safety of our potential products and their potential
advantages over existing products;
|
|
·
|
the
prevalence and severity of any side
effects;
|
|
·
|
the
strength of marketing and distribution support;
and/or
|
|
·
|
sufficient
third-party coverage or
reimbursement.
|
-31-
The
market may not accept our X-22
smoking cessation aid, based on any number of the above factors. Even if
the FDA approves the marketing of X-22 as a smoking cessation
aid, there are other FDA-approved products available and there will also be
future competitive products which directly compete with X-22. The market may choose
to continue utilizing such existing or future competitive products for any
number of reasons, including familiarity with or pricing of such products. The
failure of any of our potential products to gain market acceptance could impair
our ability to generate revenue, which could have a material adverse effect on
our future business, financial condition, results of operations and cash
flows.
Our
principal competitors in the smoking cessation market have, and any future
competitors may have, greater financial and marketing resources than we do, and
they may therefore develop products or other technologies similar or superior to
ours or otherwise compete more successfully than we do.
We have
no experience in selling smoking cessation products. Competition in the smoking
cessation aid products industry is intense, and we may not be able to
successfully compete in the market. In the market for FDA-approved smoking
cessation aids, our principal competitors include Pfizer Inc., GlaxoSmithKline
PLC, Perrigo Company, Novartis International AG, and Niconovum AB, a subsidiary
of Reynolds American Inc. The industry consists of major domestic and
international companies, most of which have existing relationships in the
markets into which we plan to sell, as well as financial, technical, marketing,
sales, manufacturing, scaling capacity, distribution and other resources and
name recognition substantially greater than ours. In addition, we expect new
competitors will enter the markets for our products in the future. Potential
customers may choose to do business with our more established competitors,
because of their perception that our competitors are more stable, are more
likely to complete various projects, can scale operations more quickly, have
greater manufacturing capacity, are more likely to continue as a going concern
and lend greater credibility to any joint venture. If we are unable to compete
successfully against manufacturers of other smoking cessation products, our
business could suffer, and we could lose or be unable to obtain market
share.
We
face intense competition in the market for our BRAND A and BRAND B cigarettes,
and our failure to compete effectively could have a material adverse effect on
our profitability and results of operations.
Cigarette
companies compete primarily on the basis of product quality, brand recognition,
brand loyalty, taste, innovation, packaging, service, marketing, advertising,
retail shelf space and price. We are subject to highly competitive conditions in
all aspects of our business and we may not be able to effectively market and
sell our BRAND A
and BRAND B
cigarettes or other cigarettes we may introduce to the market, even if we are
able to market our BRAND A
and BRAND B
cigarettes as Modified Risk Cigarettes. The competitive environment and our
competitive position can be significantly influenced by weak economic
conditions, erosion of consumer confidence, competitors’ introduction of
low-price products or innovative products, higher cigarette taxes, higher
absolute prices and larger gaps between price categories, and product regulation
that diminishes the ability to differentiate tobacco products. Domestic
competitors include Philip Morris USA, Reynolds American Inc., Lorillard Inc.,
Commonwealth Brands, Inc., Liggett Group LLC, Vector Tobacco Inc. and Star
Scientific Inc. International competitors include Philip Morris International,
British American Tobacco, Japan Tobacco Inc. and regional and local tobacco
companies; and, in some instances, government-owned tobacco enterprises,
principally in China, Egypt, Thailand, Taiwan, Vietnam and Algeria.
Our
competitors may develop products that are less expensive, safer or more
effective, which may diminish or eliminate the commercial success of any
potential products that we may commercialize.
If our
competitors market products that are less expensive, safer or more effective
than our potential products, or that reach the market before our potential
products, we may not achieve commercial success. The market may choose to
continue utilizing existing products for any number of reasons, including
familiarity with or pricing of these existing products. The failure of our X-22 smoking cessation aid or
BRAND A and BRAND B cigarettes to compete
with products marketed by our competitors would impair our ability to generate
revenue, which would have a material adverse effect on our future business,
financial condition, results of operations and cash flows. Our
competitors may:
-32-
|
·
|
develop
and market products that are less expensive or more effective than our
proposed products;
|
|
·
|
commercialize
competing products before we or our partners can launch our proposed
products;
|
|
·
|
operate
larger research and development programs or have substantially greater
financial resources than we do;
|
|
·
|
initiate
or withstand substantial price competition more successfully than we
can;
|
|
·
|
have
greater success in recruiting skilled technical and scientific workers
from the limited pool of available
talent;
|
|
·
|
more
effectively negotiate third-party licenses and strategic relationships;
and
|
|
·
|
take
advantage of acquisition or other opportunities more readily than we
can.
|
In
addition, if we fail to stay at the forefront of technological change, we may be
unable to compete effectively. Our competitors may render our technologies
obsolete by advances in existing technological approaches or the development of
new or different approaches, potentially eliminating the advantages that we
believe we derive from our research approach and proprietary
technologies.
Government
mandated prices, production control programs, shifts in crops driven by economic
conditions and adverse weather patterns may increase the cost or reduce the
quality of the tobacco and other agricultural products used to manufacture our
potential products.
We depend
upon independent tobacco producers to grow our specialty proprietary tobaccos
with specific nicotine contents for our potential products. As with other
agricultural commodities, the price of tobacco leaf can be influenced by
imbalances in supply and demand, and crop quality can be influenced by
variations in weather patterns, diseases and pests. We must also compete with
other tobacco companies for contract production with independent tobacco
growers. Tobacco production in certain countries is subject to a variety of
controls, including government mandated prices and production control programs.
Changes in the patterns of demand for agricultural products could cause farmers
to plant less tobacco. Any significant change in tobacco leaf prices, quality
and quantity could affect our profitability and our business.
We
may not be able to successfully recruit and retain skilled employees,
particularly scientific, technical and management professionals.
We
believe that our future success will depend in large part on our ability to
attract and retain highly skilled technical, managerial and marketing personnel.
There is currently intense competition for skilled executives and employees with
relevant scientific and technical expertise, and this competition is likely to
continue. The inability to retain sufficient scientific, technical and
managerial personnel or quickly recruit and attract qualified replacements could
limit or delay our product development efforts, which could adversely affect the
development and commercialization of our potential products and growth of our
business. This competition will intensify if the smoking cessation market
continues to grow, and if a market for Modified Risk Cigarettes develops. We
compete in the market for personnel against numerous companies, including
larger, more established competitors who have significantly greater financial
resources than we do and may be in a better financial position to offer higher
compensation packages to attract and retain human capital. We cannot be certain
that we will be successful in attracting and retaining the skilled personnel
necessary to operate our business effectively in the future.
Our
future success depends on our ability to retain key personnel.
Our
success will depend to a significant extent on the continued services of our
senior management team, and in particular Joseph Pandolfino, our Chief Executive
Officer, Henry Sicignano III, our President, and Michael Moynihan, 22nd Century
Limited, LLC’s Vice President of R&D. The loss or unavailability of any of
these individuals may significantly delay or prevent the development of our
potential products and other business objectives by diverting management’s
attention to transition matters. Identification of suitable
management replacements, if any, and could have a material adverse effect on our
business, operating results, cash flows and financial condition. While each of
these individuals is party to employment agreements with us, they could
terminate their relationships with us at any time, and we may be unable to
enforce any applicable employment or non-compete agreements.
-33-
We also
rely on consultants and advisors to assist us in formulating our research and
development, manufacturing, distribution, marketing and sales strategies. All of
our consultants and advisors are either self-employed or employed by other
organizations, and they may have conflicts of interest or other commitments,
such as consulting or advisory contracts with other organizations, that may
affect their ability to contribute to us.
Product
liability claims, product recalls or other claims could cause us to incur losses
or damage our reputation.
The risk
of product liability claims or product recalls, and associated adverse
publicity, is inherent in the development, manufacturing, marketing and sale of
cigarettes and smoking cessation products. We do not currently have product
liability insurance for our potential products and do not expect to be
able to obtain product liability insurance at reasonable commercial rates for
our potential products. Any product recall or lawsuit seeking significant
monetary damages may have a material adverse affect on our business and
financial condition. A successful product liability claim against us could
require us to pay a substantial monetary award. We cannot assure you that such
claims will not be made in the future.
We
may be unable to complete or integrate acquisitions effectively, which may
adversely affect our growth, profitability and results of
operations.
We may
pursue acquisitions as part of our business strategy. However, we cannot be
certain that we will be able to identify attractive acquisition targets, obtain
financing for acquisitions on satisfactory terms or successfully acquire
identified targets. Additionally, we may not be successful in integrating
acquired businesses into our existing operations and achieving projected
synergies. Competition for acquisition opportunities in the industries in which
we operate may rise, thereby increasing our costs of making acquisitions or
causing us to refrain from making further acquisitions. These and other
acquisition-related factors could negatively and adversely impact our growth,
profitability and results of operations.
Risks
Related to Regulatory Approvals and Insurance Reimbursement
If
we fail to obtain FDA and foreign regulatory approvals of X-22 as a smoking
cessation aid and FDA authorization to market BRAND A and BRAND B as Modified
Risk Cigarettes, we will be unable to commercialize these potential products in
and outside the U.S., other than the sale of our BRAND A and BRAND B cigarettes
as conventional cigarettes.
There can
be no assurance that our X-22
smoking cessation aid will be approved by the FDA, EMEA or any other
governmental body. In addition, there can be no assurance that all necessary
approvals will be granted for our potential products or that review or actions
will not involve delays caused by requests for additional information or testing
that could adversely affect the time to market for and sale of our potential
products. Even if X-22
is approved by the FDA, the FDA may require the product to only be prescribed to
patients who have already failed to quit smoking with another approved therapy.
Further, failure to comply with applicable regulatory requirements can, among
other things, result in the suspension of regulatory approval as well as
possible civil and criminal sanctions.
-34-
The
development, testing, manufacturing and marketing of our potential products are
subject to extensive regulation by governmental authorities in the United States
and throughout the world. In particular, the process of obtaining approvals by
the FDA, European Medicines Agency (“EMEA”) and other international
FDA-equivalent agencies in targeted countries is costly and time consuming, and
the time required for such approval is uncertain. Our X-22 smoking cessation aid
must undergo rigorous clinical testing and an extensive regulatory approval
process mandated by the FDA or EMEA. Such regulatory review includes the
determination of manufacturing capability and product performance. Generally,
only a small percentage of pharmaceutical products are ultimately approved for
commercial sale.
The scope
of review, including product testing and exposure studies, to be required by the
FDA under the Tobacco Control Act in order for cigarettes such as BRAND A and BRAND B to be marketed as
Modified Risk Cigarettes has not yet been fully established. We may be
unsuccessful in establishing that BRAND A or BRAND B are Modified Risk
Cigarettes, and we may fail to demonstrate that either BRAND A or BRAND B significantly reduces
tar exposure for smokers. Even if we are able to demonstrate reduced nicotine or
tar exposure, the FDA may decide that allowing a reduced risk claim is not in
the best interest of the public health, and the FDA may not allow us to market
our BRAND A and/or
BRAND B cigarettes as
Modified Risk Cigarettes. The FDA may prevent us from selling BRAND A or BRAND B or both products in
the U.S. market before the FDA makes a determination of whether to authorize us
to market our BRAND A
or BRAND B
cigarettes as Modified Risk Cigarettes. Furthermore, the FDA
could force us to remove other tobacco products that we may
commercialize.
If
we fail to comply with extensive regulations enforced by the FDA and other
agencies, the commercialization of our potential products could be prevented,
delayed or halted.
Clinical
trials, manufacturing and marketing of X-22, BRAND A and BRAND B are subject to
extensive regulation by various government authorities. We have not received
marketing approval for our X-22 smoking cessation aid,
nor have we applied for or received FDA authorization to market BRAND A or BRAND B cigarettes as
Modified Risk Cigarettes. The process of obtaining FDA and other required
regulatory approvals and authorizations is lengthy and expensive, and the time
required for such approvals and authorizations is uncertain. The
processes are affected by such factors as:
|
·
|
the
severity of the disease involved;
|
|
·
|
the
quality of submissions relating to the potential
product;
|
|
·
|
the
potential product’s clinical efficacy and
safety;
|
|
·
|
the
strength of the chemistry and manufacturing control of the
process;
|
|
·
|
the
manufacturing facility’s
compliance;
|
|
·
|
the
availability of alternative
treatments;
|
|
·
|
the
risks and benefits demonstrated in clinical trials;
and
|
|
·
|
the
patent status and marketing exclusivity rights of certain innovative
products.
|
Any
regulatory approvals or authorizations that we receive for our potential
products may also be subject to limitations on the indicated uses for which the
product may be marketed or contain requirements for potentially costly
post-marketing follow-up studies. The subsequent discovery of previously unknown
problems with the product, including adverse events of unanticipated severity or
frequency, may result in restrictions on the marketing of the product and/or
withdrawal of the product from the market.
Manufacturing,
labeling, storage and distribution activities in the United States also are
subject to strict regulation and licensing by the FDA. The manufacturing
facilities for biopharmaceutical products are subject to periodic inspection by
the FDA and other regulatory authorities and from time to time, these agencies
may send notice of deficiencies as a result of such inspections. Our failure or
the failure of our contractors’ manufacturing facilities to continue to meet
regulatory standards or to remedy any deficiencies could result in corrective
action by the FDA or these other authorities, including the interruption or
prevention of marketing, closure of our contractors’ manufacturing facilities,
and fines or penalties.
-35-
Regulatory
authorities also could require post-marketing surveillance to monitor and report
to the FDA potential adverse effects of our potential products. The U.S.
Congress or the FDA in specific situations can modify the regulatory process. If
approved, any of our potential products’ subsequent failure to comply with
applicable regulatory requirements could, among other things, result in warning
letters, fines, suspension or revocation of regulatory approvals, product
recalls or seizures, operating restrictions, injunctions and criminal
prosecutions.
The FDA’s
policies may change and additional government regulations may be enacted that
could prevent or delay regulatory approval of our potential products. We cannot
predict the likelihood, nature or extent of adverse government regulation that
may arise from future legislation or administrative action. If we are not able
to maintain regulatory compliance, we might not be permitted to market our
potential products and our business could suffer.
In
the future, we intend to distribute and sell our potential products outside of
the United States, which will subject us to further regulatory
risk.
In
addition to seeking approval from the FDA for our X-22 smoking cessation aid in
the United States, we intend to seek governmental approvals required to market
X-22 and our other
potential products in other countries. Marketing of our X-22 smoking cessation aid is
not permitted in certain countries until we have obtained required approvals or
exemptions in the individual country. The regulatory review process varies from
country to country, and approval by foreign government authorities is
unpredictable, uncertain and generally expensive. Our ability to market our
potential products could be substantially limited due to delays in receipt of,
or failure to receive, the necessary approvals or clearances. We anticipate
commencing the applications required in some or all of these countries following
approval by the FDA; however, we may decide to file applications in advance of
the FDA approval if we determine such filings to be both time and cost
effective. If we export any of our potential products that have not yet been
cleared for commercial distribution in the United States, such products may be
subject to FDA export restrictions. Failure to obtain necessary regulatory
approvals could impair our ability to generate revenue from international
sources.
Market
acceptance of our X-22 smoking cessation aid could be limited if users are
unable to obtain adequate reimbursement from third-party payers.
Government
health administration authorities, private health insurers and other
organizations generally provide reimbursement for FDA-approved smoking cessation
products, and our commercial success could depend in part on these third-party
payers agreeing to reimburse patients for the costs of our X-22 smoking cessation aid.
Even if we succeed in bringing our X-22 smoking cessation aid to
market, there is no assurance that third-party payers will consider X-22 cost effective or
provide reimbursement in whole or in part for its use.
Significant
uncertainty exists as to the reimbursement status of newly approved health care
products. Our X-22
smoking cessation aid is intended to replace or alter existing therapies or
procedures. These third-party payers may conclude that our X-22 smoking cessation aid
is less safe, effective or cost-effective than these existing therapies or
procedures. Therefore, third-party payers may not approve X-22 for
reimbursement.
If
third-party payers do not approve our potential products for reimbursement or
fail to reimburse for them adequately, sales could suffer as some physicians or
their patients could opt for a competing product that is approved for
reimbursement or is adequately reimbursed. Even if third-party payers make
reimbursement available, these payers’ reimbursement policies may adversely
affect our ability and the ability of our potential collaborators to sell our
potential products on a profitable basis.
-36-
The trend
toward managed healthcare in the United States, the growth of organizations such
as health maintenance organizations and legislative proposals to reform
healthcare and government insurance programs could significantly influence the
purchase of healthcare services and products, resulting in lower prices and
reduced demand for our potential products which could adversely affect our
business, financial condition, results of operations and cash
flows.
In
addition, legislation and regulations affecting the pricing of our potential
products may change in ways adverse to us before or after the FDA or other
regulatory agencies approve any of our potential products for marketing. While
we cannot predict the likelihood of any of these legislative or regulatory
proposals, if any government or regulatory agency adopts these proposals, they
could materially adversely affect our business, financial condition, results of
operations and cash flows.
We
could be negatively impacted by the application or enforcement of federal and
state fraud and abuse laws, including anti-kickback laws and other federal and
state anti-referral laws.
We will
need to establish a program to assure compliance with all potentially applicable
laws in connection with the development, manufacturing, marketing and sales of
our potential products. For example, all product marketing efforts must be
strictly scrutinized to assure that they are not associated with improper
remunerations to referral sources in violation of the federal Anti-Kickback
Statute and similar state statutes. Remunerations may include potential future
activities for our potential products, including discounts, rebates and bundled
sales, which must be appropriately structured to take advantage of statutory and
regulatory “safe harbors.” From time to time, we may engage physicians in
consulting activities. In addition, we may decide to sponsor continuing medical
education activities for physicians or other medical personnel. We also may
award or sponsor study grants to physicians from time to time. All relationships
with physicians, including consulting arrangements, continuing medical education
and study grants, must be similarly reviewed for compliance with the
Anti-Kickback Statute to assure that remuneration is not provided in return for
referrals. Patient inducements may also be unlawful. Inaccurate reports of
product pricing, or a failure to provide product at an appropriate price to
various governmental entities, could also serve as a basis for an enforcement
action under various theories.
Claims
which are “tainted” by virtue of kickbacks or a violation of self-referral rules
may be alleged as false claims if other elements of a violation are established.
The federal False Claims Act, which includes a provision allowing whistleblowers
to bring actions on behalf of the federal government and receive a portion of
the recovery, applies to those who submit a false claim and those who cause a
false claim to be submitted. Because our potential customers may seek payments
from the federal healthcare programs for our potential products, even during the
clinical trial stages, we must assure that we take no actions which could result
in the submission of false claims. For example, free product samples which are
knowingly or with reckless disregard billed to the federal healthcare programs
could constitute false claims. If the practice was facilitated or fostered by
us, we could be liable. Similarly, inadequate accounting for or a misuse of any
federal grant funds used for product research and development could be alleged
as a violation of the False Claims Act or other relevant statutes.
The risk
of our being found in violation of these laws is increased by the fact that many
of them have not been fully interpreted by the regulatory authorities or the
courts, and their provisions are open to a variety of interpretations, and
additional legal or regulatory change.
Delays
in clinical testing could result in increased costs to us and delay our ability
to generate revenue.
Significant
delays in clinical testing could materially increase our product development
costs. Clinical trials can be delayed for a variety of reasons, including delays
in obtaining regulatory approval to commence and continue a study, delays in
reaching agreement on acceptable clinical study terms with prospective sites,
delays in obtaining institutional review board approval to conduct a study at a
prospective site and delays in recruiting patients to participate in a
study.
-37-
In
addition, we plan to rely on third-party clinical investigators to conduct our
clinical trials and other third-party organizations to oversee the operations of
these clinical trials and to perform data collection and analysis. As a result,
we may face additional delays outside of our control if these parties do not
perform their obligations in a timely fashion. Significant delays in testing or
regulatory approvals or authorizations for any of our current or future
potential products, including our X-22 smoking cessation aid or
our BRAND A and BRAND B cigarettes as
Modified Risk Cigarettes, could prevent or cause delays in the commercialization
of such potential products, reduce potential revenues from the sale of such
potential products and cause our costs to increase.
Our
clinical trials for any of our potential products may produce negative or
inconclusive results and we may decide, or regulators may require us, to conduct
additional clinical and/or preclinical testing for these potential products or
cease our trials.
We do not
know whether clinical trials of our potential products will demonstrate safety
and efficacy sufficiently to result in marketable products. Because our clinical
trials for our X-22
smoking cessation aid and any other potential products may produce negative or
inconclusive results, we may decide, or regulators may require us, to conduct
additional clinical and/or preclinical testing for these potential products or
cease our clinical trials. If this occurs, we may not be able to obtain approval
for these potential products or our anticipated time of bringing these potential
products to the market may be substantially delayed and we may also experience
significant additional development costs. We may also be required to undertake
additional clinical testing if we change or expand the indications for our
potential products.
The
use of hazardous materials in our operations may subject us to environmental
claims or liabilities.
Our
research and development activities involve the use of hazardous materials.
Injury or contamination from these materials may occur and we could be held
liable for any damages, which could exceed our available financial resources.
This liability could materially adversely affect our business, financial
condition, results of operations and cash flows.
We are
subject to federal, state and local laws and regulations governing the use,
manufacture, storage, handling and disposal of hazardous materials and waste
products. We may be required to incur significant costs to comply with
environmental laws and regulations in the future that could materially adversely
affect our business, financial condition, results of operations and cash
flows.
The
degree of public acceptance or perceived public acceptance of our genetically
modified tobacco may affect our sales and operations.
Some
opponents of genetically modified crops have actively raised public concern
about the potential adverse effects these crops, and the products made from
them, may have on human and animal health, other plants, and the environment.
Public concern may affect the timing of, and whether we are able to obtain,
government approvals. Even after approvals are granted, public concern may lead
to increased regulation or legislation, which could affect our sales and
profitability, and may adversely affect sales of our products, due to concerns
about products derived from biotechnology. In addition, opponents of
agricultural biotechnology have attacked farmers’ fields and facilities used by
agricultural biotechnology companies, and may launch future attacks against
farmers’ fields and our research, production or other facilities, which could
affect our sales and our costs.
-38-
Risks
Related to the Tobacco Industry
Our
business faces significant governmental action aimed at increasing regulatory
requirements with the goal of preventing the use of tobacco
products.
Cigarette
companies face significant governmental action, especially in the United States
pursuant to the Tobacco Control Act, including efforts aimed at reducing the
incidence of tobacco use, restricting marketing and advertising, imposing
regulations on packaging, warnings and disclosure of flavors or other
ingredients, prohibiting the sale of tobacco products with certain
characterizing flavors or other characteristics, limiting or prohibiting the
sale of tobacco products by certain retail establishments and the sale of
tobacco products in certain packaging sizes, and seeking to hold them
responsible for the adverse health effects associated with both smoking and
exposure to environmental tobacco smoke. Governmental actions, combined with the
diminishing social acceptance of smoking and private actions to restrict
smoking, have resulted in reduced industry volume in the United States and other
countries, and we expect that these factors will continue to reduce consumption
levels in these countries.
Certain
of such actions may have a favorable impact on our X-22 smoking cessation aid,
or on our BRAND A and
BRAND B cigarettes if
we are able to market them as Modified Risk Cigarettes. However, there is no
assurance of such favorable impact, and such actions may have a negative impact
on our ability to market our BRAND A and BRAND B cigarettes as
conventional cigarettes.
Significant
regulatory developments will take place over the next few years in many markets,
driven principally by the World Health Organization’s Framework Convention on
Tobacco Control (“FCTC”). The FCTC is the first international public health
treaty on tobacco, and its objective is to establish a global agenda for tobacco
regulation with the purpose of reducing initiation of tobacco use and
encouraging cessation. In addition, the FCTC has led to increased efforts by
tobacco control advocates and public health organizations to reduce the
palatability and appeal of tobacco products. Partly because of some or a
combination of these efforts, unit sales of tobacco products in certain markets,
principally Western Europe and Japan, have been in general decline and we expect
this trend to continue. Our operating results could be significantly affected by
any significant decrease in demand for cigarettes, any significant increase in
the cost of complying with new regulatory requirements and requirements that
lead to a commoditization of tobacco products.
We
may become subject to litigation related to cigarette smoking and exposure to
environmental tobacco smoke (“ETS”), which could severely impair our results of
operations and liquidity.
Although
we are not currently subject to legal proceedings, we may become subject to
litigation related to the sale of our BRAND A and BRAND B cigarettes. Legal
proceedings covering a wide range of matters related to tobacco use are pending
or threatened in various U.S. and foreign jurisdictions. Various types of claims
are raised in these proceedings, including product liability, consumer
protection, antitrust, tax, contraband shipments, patent infringement,
employment matters, claims for contribution and claims of competitors and
distributors.
Litigation
is subject to uncertainty and it is possible that there could be adverse
developments in pending cases. An unfavorable outcome or settlement of pending
tobacco related litigation could encourage the commencement of additional
litigation. The variability in pleadings, together with the actual experience of
management in litigating claims, demonstrates that the monetary relief that may
be specified in a lawsuit bears little relevance to the ultimate
outcome.
Damages
claimed in some tobacco-related litigation are significant and, in certain cases
range into the billions of dollars. We anticipate that new cases will continue
to be filed. The FCTC encourages litigation against tobacco product
manufacturers. It is possible that our results of operations, cash flows or
financial position could be materially affected by an unfavorable outcome or
settlement of litigation, whether or not we are a party to such
litigation.
Cigarettes
are subject to substantial taxes. Significant increases in cigarette-related
taxes have been proposed or enacted and are likely to continue to be proposed or
enacted in numerous jurisdictions. These tax increases may affect our sales and
profitability and make us less competitive versus certain of our
competitors.
-39-
Tax
regimes, including excise taxes, sales taxes and import duties, can
disproportionately affect the retail price of manufactured cigarettes versus
other tobacco products, or disproportionately affect the relative retail price
of our BRAND A and
BRAND B cigarettes
versus lower-priced cigarette brands manufactured by our competitors. Increases
in cigarette taxes are expected to continue to have an adverse impact on sales
of cigarettes resulting in (i) lower consumption levels, (ii) a shift in sales
from manufactured cigarettes to other tobacco products or to lower-price
cigarette categories, (iii) a shift from local sales to legal cross-border
purchases of lower price products, and (iv) illicit products such as contraband
and counterfeit.
We
may become subject to governmental investigations on a range of
matters.
Cigarette
companies are often subject to investigations, including allegations of
contraband shipments of cigarettes, allegations of unlawful pricing activities
within certain markets, allegations of underpayment of custom duties and/or
excise taxes, and allegations of false and misleading usage of descriptors such
as “lights” and “ultra lights.” We cannot predict the outcome of any to which we
may become subject, and we may be materially affected by an unfavorable outcome
of any future investigations.
Risks
Related to Intellectual Property
Our
proprietary rights may not adequately protect our intellectual property and
potential products, and if we cannot obtain adequate protection of our
intellectual property and potential products, we may not be able to successfully
market our potential products.
Our
commercial success will depend in part on obtaining and maintaining intellectual
property protection for our technologies and potential products. We will only be
able to protect our technologies and potential products from unauthorized use by
third parties to the extent that valid and enforceable patents cover them, or
other market exclusionary rights apply.
The
patent positions of life sciences companies, like ours, can be highly uncertain
and involve complex legal and factual questions for which important legal
principles remain unresolved. No consistent policy regarding the breadth of
claims allowed in such companies’ patents has emerged to date in the United
States. The general patent environment outside the United States also involves
significant uncertainty. Accordingly, we cannot predict the breadth of claims
that may be allowed or that the scope of these patent rights could provide a
sufficient degree of future protection that could permit us to gain or keep our
competitive advantage with respect to these products and technology.
Additionally, life science companies like ours are often dependent on creating a
pipeline of products. We may not be able to develop additional potential
products, or proprietary technologies that produce commercially viable products
or that are themselves patentable.
Our
issued patents may be subject to challenge and possibly invalidated by third
parties. Changes in either the patent laws or in the interpretations of patent
laws in the United States or other countries may diminish the value of our
intellectual property.
In
addition, others may independently develop similar or alternative products and
technologies that may be outside the scope of our intellectual property. Should
third parties obtain patent rights to similar products or technology, this may
have an adverse effect on our business.
We also
rely on trade secrets to protect our technology, especially where we do not
believe patent protection is appropriate or obtainable. Trade secrets, however,
are difficult to protect. While we believe that we use reasonable efforts to
protect our trade secrets, our own or our strategic partners’ employees,
consultants, contractors or advisors may unintentionally or willfully disclose
our information to competitors. We seek to protect this information, in part,
through the use of non-disclosure and confidentiality agreements with employees,
consultants, advisors and others. These agreements may be breached, and we may
not have adequate remedies for a breach. In addition, we cannot ensure that
those agreements will provide adequate protection for our trade secrets,
know-how or other proprietary information or prevent their unauthorized use or
disclosure.
-40-
To the
extent that consultants or key employees apply technological information
independently developed by them or by others to our potential products, disputes
may arise as to the proprietary rights of the information, which may not be
resolved in our favor. Consultants and key employees that work with our
confidential and proprietary technologies are required to assign all
intellectual property rights in their discoveries to us. However, these
consultants or key employees may terminate their relationship with us, and we
cannot preclude them indefinitely from dealing with our competitors. If our
trade secrets become known to competitors with greater experience and financial
resources, the competitors may copy or use our trade secrets and other
proprietary information in the advancement of their products, methods or
technologies. If we were to prosecute a claim that a third party had illegally
obtained and was using our trade secrets, it could be expensive and time
consuming and the outcome could be unpredictable. In addition, courts outside
the United States are sometimes less willing to protect trade secrets than
courts in the United States. Moreover, if our competitors independently develop
equivalent knowledge, we would lack any contractual claim to this information,
and our business could be harmed.
Our
ability to commercialize our potential products will depend on our ability to
sell such products without infringing the patent or proprietary rights of third
parties. If we are sued for infringing intellectual property rights of third
parties, such litigation could be costly and time consuming and an unfavorable
outcome could have a significant adverse effect on our business.
Our
ability to commercialize our potential products will depend on our ability to
sell such products without infringing the patents or other proprietary rights of
third parties. Third-party intellectual property rights in our field are
complicated, and third-party intellectual property rights in these fields are
continuously evolving. We have not performed searches for third-party
intellectual property rights that may raise freedom-to-operate issues, and we
have not obtained legal opinions regarding commercialization of our potential
products. As such, there may be existing patents that may affect our ability to
commercialize our potential products.
In
addition, because patent applications are published up to 18 months after their
filing, and because applications can take several years to issue, there may be
currently pending third-party patent applications that are unknown to us, which
may later result in issued patents.
If a
third-party claims that we infringe on its patents or other proprietary rights,
we could face a number of issues that could seriously harm our competitive
position, including:
|
·
|
infringement
claims that, with or without merit, can be costly and time consuming to
litigate, can delay the regulatory approval process and can divert
management’s attention from our core business
strategy;
|
|
·
|
substantial
damages for past infringement which we may have to pay if a court
determines that our products or technologies infringe upon a competitor’s
patent or other proprietary rights;
|
|
·
|
a
court order prohibiting us from commercializing our potential products or
technologies unless the holder licenses the patent or other proprietary
rights to us, which such holder is not required to
do;
|
|
·
|
if
a license is available from a holder, we may have to pay substantial
royalties or grant cross licenses to our patents or other proprietary
rights; and
|
|
·
|
redesigning
our process so that it does not infringe the third-party intellectual
property, which may not be possible, or which may require substantial time
and expense including delays in bringing our potential products to
market.
|
-41-
Such
actions could harm our competitive position and our ability to generate revenue
and could result in increased costs.
Our
patent applications may not result in issued patents, which may have a material
adverse effect on our ability to prevent others from commercially exploiting
products similar to ours.
We own or
exclusively control 97 issued patents in 79 countries. In addition, we also have
approximately 44 pending patent applications. We cannot assure you these patent
applications will issue, in whole or in part, as patents. Patent applications in
the United States are maintained in secrecy until the patents are published or
are issued. Since publication of discoveries in the scientific or patent
literature tends to lag behind actual discoveries by several months, we cannot
be certain that we are the first creator of inventions covered by pending patent
applications or the first to file patent applications on these inventions. We
also cannot be certain that our pending patent applications will result in
issued patents or that any of our issued patents will afford protection against
a competitor. In addition, patent applications filed in foreign countries are
subject to laws, rules and procedures that differ from those of the United
States, and thus we cannot be certain that foreign patent applications related
to U.S. patents will be issued. Furthermore, if these patent applications issue,
some foreign countries provide significantly less effective patent enforcement
than in the United States.
The
status of patents involves complex legal and factual questions and the breadth
of claims allowed is uncertain. Accordingly, we cannot be certain that the
patent applications that we file will result in patents being issued, or that
our patents and any patents that may be issued to us in the near future will
afford protection against competitors with similar technology. In addition,
patents issued to us may be infringed upon or designed around by others and
others may obtain patents that we need to license or design around, either of
which would increase costs and may adversely affect our operations.
We
license certain patent rights from third-party owners. If such owners do not
properly maintain or enforce the patents underlying such licenses, our
competitive position and business prospects could be harmed.
We
license rights to third-party intellectual property that is necessary or useful
for our business, and we may enter into additional licensing agreements in the
future. Our success could depend in part on the ability of some of our licensors
to obtain, maintain and enforce patent protection for their intellectual
property, in particular, those patents to which we have secured exclusive
rights. Our licensors may not successfully prosecute the patent applications to
which we are licensed. Even if patents are issued with respect to these patent
applications, our licensors may fail to maintain these patents, may determine
not to pursue litigation against other companies that are infringing these
patents, or may pursue such litigation less aggressively than we could. In
addition, our licensors may terminate their agreements with us in the event we
breach the applicable license agreement and fail to cure the breach within a
specified period of time. Without protection for the intellectual property we
license, other companies might be able to offer substantially identical products
for sale, which could adversely affect our competitive business position and
harm our business prospects.
We
are currently in default pursuant to the terms of an intellectual property
license to which we are a party.
We are
currently in payment default pursuant to the terms of that certain License
Agreement dated as of March 6, 2009 by and between us and North Carolina
State University. To date, we have not received any notice of
termination from North Carolina State University. We plan to use a
portion of the net proceeds from the Offering to cure the payment default.
The intellectual property licensed to us pursuant to the License Agreement is
crucial to our business and, if North Carolina State University chooses to
invoke its right to terminate the License Agreement and we are unable to cure
the default, our business would be materially and adversely
affected.
-42-
Risks
Related to Ownership of our Common Stock
The
Securities issued in the Merger are “restricted securities” and, as such, may
not be sold except in limited circumstances.
None of
the shares of Common Stock or warrants issued in the Merger or the shares of
Common Stock issuable upon exercise of such warrants (collectively, the
“Securities”) have been registered under the Securities Act, or registered or
qualified under any state securities laws. The Securities were sold and/or
issued pursuant to exemptions contained in and under those laws. Accordingly,
the Securities are “restricted securities” as defined in Rule 144 under the
Securities Act and must, therefore, be held indefinitely unless registered under
applicable federal and state securities laws, or an exemption from the
registration requirements of those laws is available. The securities purchase
agreements, warrants and certificates representing the Securities will contain
legends reflecting their restricted status.
Although
we are required to register the shares of Common Stock issued to the investors
in the Private Placement Offering in exchange for the Membership Units included
in the Units purchased by such investors in the Private Placement Offering, we
cannot assure that the SEC will declare the registration statement effective,
thereby enabling the shares of Common Stock to be freely tradable. Rule 144
under the Securities Act, which permits the resale, subject to various terms and
conditions, of limited amounts of restricted securities after they have been
held for six months will not immediately apply to our Common Stock because we
were at one time designated as a “shell company” under SEC regulations. Pursuant
to Rule 144(i), securities issued by a current or former shell company that
otherwise meet the holding period and other requirements of Rule 144
nevertheless cannot be sold in reliance on Rule 144 until one year after the
date on which the issuer filed current “Form 10 information” (as defined in Rule
144(i)) with the SEC reflecting that it ceased being a shell company, and
provided that at the time of a proposed sale pursuant to Rule 144, the issuer
has satisfied certain reporting requirements under the Exchange Act. Because, as
a former shell company, the reporting requirements of Rule 144(i) will apply
regardless of holding period, the restrictive legends on certificates for the
shares of Common Stock issued to the investors in the Private Placement Offering
in exchange for the Membership Units included in the Units sold in the Private
Placement Offering or issued upon exercise of the warrants cannot be removed
except in connection with an actual sale that is subject to an effective
registration statement under, or an applicable exemption from the registration
requirements of, the Securities Act.
Because
the Merger was a reverse merger, the registration statement we file with respect
to the shares of Common Stock received by investors in the Private Placement
Offering as a result of the Merger might be subject to heightened scrutiny by
the SEC, and we may not be able to attract the attention of major brokerage
firms if we seek to raise additional capital in the future.
Additional
risks may exist since the Merger was a “reverse merger.” Certain SEC rules are
more restrictive when applied to reverse merger companies, such as the ability
of stockholders to resell their shares of Common Stock pursuant to Rule 144. In
addition, securities analysts of major brokerage firms may not provide coverage
of our Common Stock following the Merger since there may be little incentive for
brokerage firms to recommend the purchase of our Common Stock. We cannot assure
you that brokerage firms will want to conduct any secondary offerings on our
behalf if we seek to raise additional capital in the future.
-43-
If
we are unable to register in a timely manner the shares of Common Stock issued
to investors in the Private Placement Offering as a result of the Merger, then
the ability to resell shares of our Common Stock so issued will be
delayed.
We have
agreed, at our expense, to prepare a registration statement, and to cause our
company to file a registration statement with the SEC within seventy-five (75)
days after the effective date of the Merger. We shall use our best efforts to
cause such registration statement to be declared effective by the SEC within one
hundred eighty (180) calendar days of filing with the SEC (or 240 days if the
SEC reviews such registration statement). The registration statement will cover
the resale of the shares of Common Stock issued to investors in the Private
Placement Offering in exchange for the Membership Units purchased in the Private
Placement Offering. There are many reasons, including some over which we have
little or no control, which could delay our filing of the registration statement
beyond seventy-five (75) days after the effective date of the Merger or which
could keep the registration statement from being declared effective by the SEC,
including delays resulting from the SEC review process and comments raised by
the SEC during that process. Accordingly, in the event that the registration
statement is not filed or declared effective within these timeframes, the shares
of Common Stock proposed to be covered by such registration statement will not
be eligible for resale until the registration statement is effective or an
exemption from registration, such as Rule 144, becomes available.
We
will incur increased costs and demands upon management as a result of complying
with the laws and regulations affecting public companies, which could harm our
operating results.
As a
public company, we will incur significant legal, accounting and other expenses,
including costs associated with public company reporting requirements. We will
also incur substantial expenses in connection with the preparation and filing of
the registration statement and responding to SEC comments in connection with its
review of the registration statement. We also incur costs associated with
current corporate governance requirements, including requirements under Section
404 and other provisions of the Sarbanes-Oxley Act, as well as rules implemented
by the SEC and the OTC Bulletin Board or any stock exchange on which our Common
Stock may be listed in the future. The expenses incurred by public companies for
reporting and corporate governance purposes have increased dramatically in
recent years. We expect these rules and regulations to substantially increase
our legal and financial compliance costs and to make some activities more
time-consuming and costly. We are unable to currently estimate these costs with
any degree of certainty. We also expect these new rules and regulations may make
it difficult and expensive for us to obtain director and officer liability
insurance, and if we are able to obtain such insurance, we may be required to
accept reduced policy limits and coverage or incur substantially higher costs to
obtain the same or similar coverage available to privately-held companies. As a
result, it may be more difficult for us to attract and retain qualified
individuals to serve on our board of directors or as our executive
officers.
If
we fail to maintain proper and effective internal controls, our ability to
produce accurate and timely financial statements could be impaired, which could
harm our operating results, our ability to operate our business and investors’
views of us.
Ensuring
that we have adequate internal financial and accounting controls and procedures
in place so that we can produce accurate financial statements on a timely basis
is a costly and time-consuming effort that will need to be evaluated frequently.
Section 404 of the Sarbanes-Oxley Act requires public companies to conduct an
annual review and evaluation of their internal controls and attestations of the
effectiveness of internal controls by independent auditors. Our failure to
maintain the effectiveness of our internal controls in accordance with the
requirements of the Sarbanes-Oxley Act could have a material adverse effect on
our business. We could lose investor confidence in the accuracy and completeness
of our financial reports, which could have an adverse effect on the price of our
Common Stock. In addition, if our efforts to comply with new or changed laws,
regulations, and standards differ from the activities intended by regulatory or
governing bodies due to ambiguities related to practice, regulatory authorities
may initiate legal proceedings against us and our business may be
harmed.
-44-
An
active trading market for our Common Stock may not develop or be sustained, and
you may not be able to resell your shares at or above the price at which you
purchased them.
An active
trading market for our shares may never develop or be sustained. In the absence
of an active trading market for the Common Stock, shares of Common Stock may not
be able to be resold at or above the purchase price of such shares. Although
there can be no assurances, we expect that our Common Stock will continue to be
quoted on the OTC Bulletin Board, an over-the-counter quotation system, on which
the shares of our Common Stock are currently quoted. However, even if our Common
Stock continues to be quoted on the OTC Bulletin Board, it is unlikely that an
active market for our Common Stock will develop in the foreseeable future. It
may be more difficult to dispose of shares or obtain accurate quotations as to
the market value of our Common Stock compared to securities of companies whose
shares are traded on the NASDAQ or another stock exchange.
Our
stock price may be highly volatile and our Common Stock could decline in
value.
The
number of shares of Common Stock and warrants issued as a result of the Merger
bears no relationship to our assets, book value or historical results of
operations or any other established criterion of value on a stand alone or pro
forma combined basis with Parent, or the trading price of the shares of Common
Stock prior to the Merger, and may bear no relationship to the trading price of
our Common Stock after the Merger.
The
market prices for securities in general have been highly volatile and may
continue to be highly volatile in the future. The following factors, in addition
to other risk factors described in this section, may have a significant impact
on the market price of our Common Stock:
|
·
|
results
from and any delays in any clinical trials
programs;
|
|
·
|
failure
or delays in entering potential products into clinical
trials;
|
|
·
|
failure
or discontinuation of any of our research
programs;
|
|
·
|
delays
in establishing new strategic
relationships;
|
|
·
|
delays
in the development of our potential products and commercialization of our
potential products;
|
|
·
|
market
conditions in our sector and issuance of new or changed securities
analysts’ reports or
recommendations;
|
|
·
|
general
economic conditions, including recent adverse changes in the global
financial markets;
|
|
·
|
actual
and anticipated fluctuations in our quarterly financial and operating
results;
|
|
·
|
developments
or disputes concerning our intellectual property or other proprietary
rights;
|
|
·
|
introduction
of technological innovations or new commercial products by us or our
competitors;
|
|
·
|
issues
in manufacturing or distributing our potential
products;
|
|
·
|
market
acceptance of our potential
products;
|
|
·
|
third-party
healthcare reimbursement policies;
|
|
·
|
FDA
or other United States or foreign regulatory actions affecting us or our
industry;
|
|
·
|
litigation
or public concern about the safety of our potential products or
products;
|
|
·
|
additions
or departures of key personnel;
|
|
·
|
third-party
sales of large blocks of our Common
Stock;
|
|
·
|
sales
of the Common Stock by our executive officers, directors or significant
stockholders; and
|
|
·
|
equity
sales by us of the Common Stock to or securities convertible into Common
Stock to fund our operations.
|
-45-
These and
other external factors may cause the market price and demand for our Common
Stock to fluctuate substantially, which may limit or prevent investors from
readily selling their shares of Common Stock and may otherwise negatively affect
the liquidity of our Common Stock. In addition, in the past, when the market
price of a stock has been volatile, holders of that stock have instituted
securities class action litigation against the company that issued the stock. If
any of our stockholders brought a lawsuit against us, we could incur substantial
costs defending the lawsuit. Such a lawsuit could also divert the time and
attention of our management.
A
significant portion of the total outstanding shares of Common Stock may be sold
into the public market in the near future, which could cause the market price of
our Common Stock to drop significantly, even if our business is doing
well.
Sales of
a substantial number of shares of our Common Stock in the public market could
occur at any time. These sales, or the perception in the market that the holders
of a large number of shares intend to sell shares, could reduce the market price
of our Common Stock.
We will
file the registration statement covering the resale of the shares of Common
Stock issued to investors in the Private Placement Offering as a result of the
Merger. Once these shares are registered, they can be freely sold in the public
market.
We
currently have outstanding 26,759,646 shares of Common Stock, of which 5,434,446
will be included in the registration statement. Of the 26,759,646 shares of
Common Stock outstanding, unless they are registered for resale pursuant to the
registration statement, or included in a subsequent registration statement
declared effective by the SEC, 21,434,446 shares are restricted shares owned by
“affiliates” and by “non-affiliates” who have held such shares for less than one
year (including investors in the Private Placement Offering), which could be
sold after meeting certain requirements of Rule 144 of the Securities Act.
Shares of Common Stock held by “non-affiliates” (including investors in the
Private Placement Offering) may be resold after such shares have been held for
longer than one year, without meeting such requirements. In addition,
14,924,903 of such restricted shares held by our directors, executive officers,
and beneficial owners of 10% of more of our issued and outstanding Common Stock
are subject to lock-up agreements preventing the re-sale of such shares for 18
months following the date of the closing of the Merger, except to another
individual or entity that is subject to a similar lock-up
agreement. These lock-up agreements do not apply to the 422,544
shares of Common Stock or warrants to purchase 211,272 shares of Common Stock
issued to Clearwater Partners, LLC and Angelo Tomasello upon consummation of the
Merger in exchange for the securities contained in the PPO Securities purchased
by Clearwater Partners, LLC and Angelo Tomasello in the Private Placement
Offering nor to any shares of Common Stock issued to Clearwater Partners, LLC or
Angelo Tomasello upon exercise of such warrants.
We also
intend to register all shares of Common Stock that we may issue under our
company’s equity incentive plan, including 4,250,000 shares reserved for future
issuance under such plan. Once we register and issue these shares, they can be
freely sold in the public market upon issuance.
Our
Common Stock will likely be considered a “penny stock,” which is likely to limit
its liquidity.
The
market price of our Common Stock is, and will likely remain for the foreseeable
future, less than $5.00 per share, and therefore will be a “penny stock”
according to SEC rules, unless our Common Stock is listed on a national
securities exchange. The OTC Bulletin Board is not a national securities
exchange. Designation as a “penny stock” requires any broker or dealer selling
these securities to disclose certain information concerning the transaction,
obtain a written agreement from the purchaser and determine that the purchaser
is reasonably suitable to purchase the securities. These rules may restrict the
ability of brokers or dealers to sell our Common Stock and may affect the
ability of current holders of our Common Stock to sell their shares. Such rules
may also deter broker-dealers from recommending or selling the Common Stock,
which may further limit its liquidity. This may also make it more difficult for
us to raise additional capital in the future. Accordingly, although we will
undertake to register under the Securities Act the resale of the shares of
Common Stock issued in the Merger, these shares will be highly illiquid. Because
of such expected illiquidity, it will likely be difficult to re-sell shares of
our Common Stock as desired.
-46-
If
securities or industry analysts do not publish or cease publishing research or
reports about us, our business or our market, or if they change their
recommendations regarding our stock adversely, our stock price and trading
volume could decline.
No
securities or industry analysts currently publish research or reports about us.
The trading market for our Common Stock will be influenced by the research and
reports that industry or securities analysts may publish about us, our business,
our market or our competitors. If any of the analysts who may cover us in the
future change their recommendation regarding our stock adversely, or provide
more favorable relative recommendations about our competitors, our stock price
would likely decline. If any analyst who may cover us were to cease coverage of
us or fail to regularly publish reports on us, we could lose visibility in the
financial markets, which in turn could cause our stock price or trading volume
to decline.
We
are controlled by our current officers, directors and principal
stockholders.
Our
directors and executive officers beneficially own approximately 37% of the
outstanding shares of the Common Stock. Accordingly, our directors and executive
officers will have substantial influence over, and may have the ability to
control, the election of our board of directors and the outcome of issues
submitted to a vote of our stockholders.
We
do not expect to declare any dividends in the foreseeable future.
We have
not paid cash dividends to date. We currently intend to retain our future
earnings, if any, to fund the development and growth of our business, and we do
not anticipate paying any cash dividends on our capital stock for the
foreseeable future. In addition, the terms of any future debt facilities may
preclude us from paying dividends on the Common Stock. As a result, capital
appreciation, if any, of the Common Stock could be the sole source of gain for
the foreseeable future.
Anti-takeover
provisions contained in our articles of incorporation and bylaws, as well as
provisions of Nevada law, could impair a takeover attempt.
Our
amended and restated articles of incorporation and bylaws currently contain
provisions that, together with Nevada law, could have the effect of rendering
more difficult or discouraging an acquisition deemed undesirable by our board of
directors. Our corporate governance documents presently include the following
provisions:
|
·
|
authorizing
blank check preferred stock, which could be issued with voting,
liquidation, dividend and other rights superior to our common stock;
and
|
|
·
|
limiting
the liability of, and providing indemnification to, our directors and
officers.
|
These
provisions, alone or together, could delay hostile takeovers and changes in
control of us or changes in our management.
As a
Nevada corporation, we also may become subject to the provisions Nevada Revised
Statutes Sections 78.378 through 78.3793, which prohibit an acquirer, under
certain circumstances, from voting shares of a corporation’s stock after
crossing specific threshold ownership percentages, unless the acquirer obtains
the approval of the stockholders of the issuer corporation. The first such
threshold is the acquisition of at least one-fifth, but less than one-third of
the outstanding voting power of the issuer. We may become subject to the above
referenced Statutes if we have 200 or more stockholders of record, at least 100
of whom are residents of the State of Nevada, and do business in the State of
Nevada directly or through an affiliated corporation.
-47-
As a
Nevada corporation, we are subject to the provisions of Nevada Revised Statutes
Sections 78.411 through 78.444, which prohibit an “interested stockholder”
from entering into a combination with the corporation, unless certain conditions
are met. An “interested stockholder” is a person who, together with affiliates
and associates, beneficially owns (or within the prior three years did own) 10
percent or more the corporation’s voting stock.
Any
provision of our amended and restated articles of incorporation, our bylaws or
Nevada law that has the effect of delaying or deterring a change in control
could limit the opportunity for our stockholders to receive a premium for their
shares of our Common Stock, and could also affect the price that some investors
are willing to pay for our Common Stock.
Management’s
Discussion and Analysis or Plan of Operation
This discussion should be read in
conjunction with the other sections of this Report, including “Risk Factors,”
“Company Overview” and the Financial Statements attached as Exhibits 99.1 and
99.1 to this Current Report on Form 8-K. The various sections of this discussion
contain a number of forward-looking statements, all of which are based on our
current expectations and could be affected by the uncertainties and risk factors
described throughout this Current Report on Form 8-K. See “Forward-Looking
Statements.” Our actual results may differ materially.
Overview
We have
operated at a loss since 2006, when we increased our research and development
expenditures. Our license agreement with our former licensee was discontinued in
2007. In 2008, we realized royalty income of $201,635 from the final
payment of royalties due under an agreement with a former licensee, and in 2009
we realized sales of $27,612 from limited test marketing of our cigarettes. Our
operating losses in 2008 and 2009 were also partially attributable to funding
four research projects with third parties, which were completed by the end of
2009. We also transitioned over this period from solely developing proprietary
technology and tobacco to developing and commercializing our own
products. Other than our planned clinical trials for X-22 and exposure studies for
our modified risk cigarette candidates, we have no third-party R&D commitments requiring
funding in 2011. We do however plan to carry out a minimal amount of
R&D in terms of field trials from the large inventory of seed lots resulting
from our R&D at NCSU, NRC and NAIST.
Our
prospects depend on our ability to generate and sustain revenues from our X-22 smoking cessation aid,
our BRAND A and BRAND B cigarettes and other
cigarettes we may introduce to the market. Our ability to generate meaningful
revenue from X-22,
especially in the United States, depends in large part on FDA approval, and our
ability to generate meaningful revenue from BRAND A and BRAND B depends on obtaining
FDA authorization to market these brands as Modified Risk Cigarettes and the
successful marketing, distribution and consumer acceptance of these brands. We
do not expect FDA approval of X-22 until the fourth quarter
of 2012 at the earliest. We believe the FDA will issue regulations for modified
risk tobacco products in 2011, and we therefore expect to submit applications to
the FDA to authorize the marketing and labeling of BRAND A and BRAND B as Modified Risk
Cigarettes in 2011. This process is likely to take at least one year.
Accordingly, our cash flow from product sales will be limited and may require
additional equity or debt financing to continue funding our business and
operations.
In
connection with our FDA activities we will incur substantial costs related to
clinical trials and smoke exposure studies related to our modified risk product
candidates. In December 2010, we entered into two contracts for our Phase II- B
clinical trial and
made a deposit of approximately $200,000. The financial commitment
under these contracts during 2011 is approximately $650,000, not including
various other expenses of our Phase II- B clinical trial.
-48-
At
September 30, 2010, we had current assets of approximately $383,000 and current
liabilities of approximately $3,954,000 and total assets of
$2,212,471 and total liabilities of $4,018,497.
Critical
Accounting Policies and Estimates
Accounting
principles generally accepted in the United States of America, or U.S. GAAP,
require estimates and assumptions to be made that affect the reported amounts in
our consolidated financial statements and accompanying notes. Some of these
estimates require difficult, subjective and/or complex judgments about matters
that are inherently uncertain and, as a result, actual results could differ from
those estimates. Due to the estimation processes involved, the following
summarized accounting policies and their application are considered to be
critical to understanding our business operations, financial condition and
results of operations.
Revenue
Recognition
Revenue
is recognized when tobacco products are shipped to customers and title passes.
We also record appropriate provisions for rebates and discounts and credits for
returns. These amounts are estimated based on information and historical
experience.
Impairment
of Long-Lived Assets
We review
the carrying value of amortizing long-lived assets whenever events or changes in
circumstances indicate that the historical cost-carrying value of an asset may
no longer be appropriate. We also assess recoverability of the asset by
estimating the future undiscounted net cash flows expected to result from the
asset, including eventual disposition. If the estimated future undiscounted net
cash flows are less than the carrying value of the asset, an impairment loss is
recorded equal to the difference between the asset’s carrying value and its fair
value. Non-amortizing intangibles (trademarks) are reviewed annually for
impairment. We have not recognized any impairment losses during the two years
ended December 31, 2009 or in the interim period ended September 30,
2010.
Amortization
Estimates
We
generally determine amortization based on the estimated useful lives of the
assets and record amortization expense on a straight-line method over such
lives. The remaining life of a patent is generally used to determine the
estimated useful life of the related patent costs.
Valuation
of our Equity Securities
We have
issued Units to satisfy obligations to vendors or employees that were due in
cash. These securities have been valued based on the cash value of the
obligation satisfied by their issuance. We have also issued warrants in
connection with the issuance of debt obligations. These warrants have been
valued based on the value ascribed to the underlying Units issued in cash
transactions or in settlement of cash obligations.
Income
taxes
Prior to
the closing of the merger, 22nd Century was organized as a limited liability
company and treated as a partnership for income tax purposes;
accordingly, 22nd Century was not directly responsible for income taxes
(income and loses passed through to its LLC members) and did not have to account
for them. As of the merger, our results of operations will be subject
to income taxes and accounting for income taxes will likely be a critical
accounting policy. In addition to accounting for taxes on our current
taxable income, we will need to account for deferred tax assets and liabilities,
including the evaluation of the recoverability of deferred tax
assets.
-49-
Derivative
Financial Instruments
The
warrants that were issued in connection with the Merger will be treated as
derivative instruments for accounting purposes. Accordingly, these
instruments will be treated as liabilities rather than equity upon
issuance. As a result, this accounting policy is expected to be
considered critical in future periods. We do not use derivative
instruments to hedge exposures to cash flow, market or foreign currency risks.
We evaluate all of our financial instruments to determine if such instruments
are derivatives or contain features that qualify as embedded derivatives. For
derivative financial instruments that are accounted for as liabilities, the
derivative instrument is initially recorded at its fair market value and then is
revalued at each reporting date, with changes in fair value reported in the
consolidated statement of operations. The methodology for valuing our
outstanding warrants classified as derivative instruments will use a lattice
model approach which includes probability weighted estimates of future events
including volatility of our Common Stock. The classification of derivative
instruments, including whether such instruments should be recorded as
liabilities or equity, is evaluated at the end of each reporting period.
Derivative instrument liabilities are classified in the balance sheet as current
or non-current based on whether or not net-cash settlement of the derivative
instrument could be required within twelve months of the balance sheet
date.
Results
of Operations
Quarterly
Period Ended September 30, 2010 Compared to Quarterly Period Ended September 30,
2009
Revenues
We had
$20,302 in revenue from the sale of research cigarettes in the third quarter of
2010 compared to no revenue in the third quarter of 2009.
Costs
of Goods Sold
Costs of
goods sold in the third quarter of 2010 was $5,302 and equal to 26.1% of
revenue. There were no product sales in the third quarter of 2009 so costs of
goods sold was zero. This increase of $5,302 was due to the increase in revenues
we received from sales of research cigarettes in the third quarter of 2010
versus not selling any such research cigarettes in the third quarter of
2009.
Amortization
Expense — Patent Costs and Trademarks
Amortization
expense increased by 6.5% in the third quarter of 2010 to $40,803 from $38,313
in the third quarter of 2009. This increase of $2,490 was due to increased
investment in patent costs and trademarks during 2009 and the first nine months
of 2010.
General
and Administrative Expense
General
and administrative expense was $138,911 in the third quarter of 2010, an
increase of $80,129, or 136%, from $58,782 during the third quarter of 2009. The
increase was primarily was due to increases in payroll, accounting and legal
fees.
Research
and Development Expense
Research
and development expense was $67,528 in the third quarter of 2010, a decrease of
$49,493, or 42.3%, from $117,021 during the third quarter of 2009. The decrease
was the result of reduced compensation and license fees.
-50-
Interest
Expense and Debt Expense
Interest
expense and debt expense, which includes interest amortization of debt discount
and debt issuance costs, decreased in the third quarter of 2010 to $68,642 from
$70,035 in the same quarter in 2009. This decrease of $1,393 or 2.0%, was a
result of reduced amortization of debt discount offset by an increase in
outstanding borrowing during the third quarter of 2010 compared to
2009.
Net
Loss
We had a
net loss in the third quarter of 2010 of $300,884 as compared to a net loss of
$284,151 in the same period in 2009. The increase in the net loss of $16,733, or
5.9%, was a result of increased general and administrative expense partially
offset by reduced research and development expense, revenues and lower interest
and debt expense.
Nine
Month Period Ended September 30, 2010 Compared to Nine Month Period Ended
September 30, 2009
Revenues
We had
$22,102 in revenue from the sale of research cigarettes in first nine months of
2010 compared to no revenue in the first nine months of 2009.
Cost
of Goods Sold
Cost of
goods sold in the first nine months of 2010 was $6,302 and equal to 28.5% of
revenue. There were no product sales in the first none months of 2009
so the cost of goods sold was zero. This increase of $6,302 was due
to the increase in revenues we received from sales of research cigarettes in the
first nine months of 2010 versus not selling any such research cigarettes in the
first nine months of 2009.
Amortization
Expense — Patent Costs and Trademarks
Amortization
expense increased by 12% in the first nine months of 2010 to $121,735 from
$108,691 in the first nine months of 2009. This increase of $13,044 was due to
increased investment in patent costs and trademarks during 2009 and the first
nine months of 2010.
General
and Administrative Expense
General
and administrative expense was $383,576 in the first nine months of 2010, an
increase of $123,845 or 47.7%, from $259,731 during the first nine months of
2009. The increase was primarily was due to increases in payroll, accounting and
audit fees.
Research
and Development Expense
Research
and development expense was $282,971 in the first nine months of 2010, a
decrease of $128,733, or 31.3%, from $411,704 during the first nine months of
2009. The decrease was the result of reduced compensation and license
fees.
Interest
Expense and Debt Expense
Interest
expense and debt expense, which includes interest amortization of debt discount
and debt issuance costs, increased in the first nine months of 2010 to $218,519
from $202,525 in the same period in 2009. This increase of $15,994, or 7.9%, was
a result of increased outstanding borrowings during the first nine months of
2010 compared to 2009.
Net
Loss
We had a
net loss in the first nine months of 2010 of $991,001 as compared to a net loss
of $982,651 in the same period in 2009. The increase in the net loss of $8,350,
or 0.8%, was a result of the increases in general and administrative expense and
interest and debt expense partially offset by revenue and reduced research and
development expense.
-51-
Year
Ended December 31, 2009 Compared to Year Ended December 31, 2008
Revenues
Revenue
of $27,612 in 2009 resulted solely from the sale of tobacco products. We test
marketed certain of our cigarettes during the third and fourth quarters of
2009. There were no tobacco product sales in 2008. There were no
royalty revenues in 2009 as compared to 2008 during which we recorded $201,635
as the final payment under a license agreement with a former licensee. As a
result, there was a net decrease in total revenue in 2009 of $174,023 as
compared to 2008.
Gross
Profit
In 2009,
gross profit (sales less costs of goods sold including federal excise taxes) was
$7,500 or approximately 27% of sales.
Amortization
Expense — Patent Costs and Trademarks
Amortization
expense increased 45% in 2009 to $144,792 from $99,970 in 2008. This increase of
$44,822 is due to our investment in patent costs and trademarks in 2009 and 2008
of $227,942 and $737,518, respectively.
Selling,
General and Administrative Expense
Selling
general and administrative expense was $280,709 in 2009, an increase of
$132,839, or 90%, from $147,870 in 2008. This increase was primarily was due to
increased costs associated with product development of BRAND A and BRAND B and professional
fees.
Research
and Development Expense
Research
and Development expense was $540,300 in 2009, a decrease of $114,197, or 17%,
from $654,497 in 2008. This decrease was the result of successfully completing
certain research projects during the two year period ending December 31, 2009.
As these projects were completed, we reduced these expenses.
Interest
Expense and Debt Expense
Interest
expense and debt expense, which includes interest amortization of debt discount
and debt issuance costs, increased in 2009 to $268,503 from $70,563 in 2008.
This increase of $197,940 or 281% was directly a result of additional borrowings
in late 2008 and 2009. In order to fund our research commitments, payments under
our license agreements, patent costs and working capital, we borrowed additional
amounts from private sources at higher average total costs. Interest and debt
expense as a percentage of the average net carrying amount of the related
obligations was 30% in 2009 as compared to 11% in 2008.
Interest
Income
We had no
interest income in 2009 as compared to $34,886 in 2008. The interest income in
2008 was a result of an arbitration award that we obtained which granted
interest on amounts owed to us by a former licensee from 2006.
Net
Loss
We had a
net loss in 2009 of $1,226,804 as compared to a net loss of $736,379 in 2008.
The increase in the net loss of $490,425, or 67%, was a result of having no
royalty or interest income in 2009 as compared to 2008 and higher total expenses
of $261,404 in 2009 as compared to 2008 offset by the gross margin on product
sales of $7,500.
-52-
Liquidity
and Capital Resources
Summary
of Balances and Recent Sources and Uses
As of
September 30, 2010, we had negative working capital of approximately $3.6
million, consisting of approximately $2.8 million in accounts payable and
accrued liabilities and approximately $1.2 million of notes, advances and loans
payable offset by $0.4 million in current assets.
As of
December 31, 2009, we had negative working capital of approximately $3.2
million, consisting of approximately $2.1 million in accounts payable and
approximately $1.0 million of notes, advances and loans payable, compared to as
of December 31, 2008, negative working capital of approximately $2.3 million,
consisting of approximately $1.7 million of accounts payable, and approximately
$0.6 million of notes, advances and loans payable.
Cash
Demands on Operations
In the
first nine months of 2010, we had a net loss of approximately
$991,000. In 2009, we had a net loss of approximately $1.2
million.
In 2009,
we continued to incur material expenditures for research and in connection with
the development and protection of our intellectual property portfolio. Although
we are in a position to reduce our expenditures on research, we will have to
increase our expenditures on product and market development. We also recognize
that we will need to continue to spend money maintaining and protecting our
patent portfolio and for expenditures related to the FDA approval processes for
our smoking cessation aid and our Modified Risk Cigarettes.
Net
Cash Used in Operating Activities
In the
first nine months of 2010, we used $650,516 in cash compared $105,057 in cash
for the first nine months of 2009. This increase use of cash of $545,459 was due
to the net change in components of working capital of which $290,766 relate to
increases in accounts receivable and inventory and $328,783 relate to lower
increases in accounts payable and accrued expenses in 2010 as compared to the
2009 period.
In 2009,
approximately $165,000 of cash was used in operating activities compared to
approximately $494,000 of cash used in operating activities in 2008. The
decrease is due primarily to increases in accounts payable, accrued expenses and
non-cash expenses.
Net
Cash Used in Investing Activities
In the
first nine months of 2010, we used $88,382 in cash for patents compared to no
cash for the first nine months of 2009. During the first nine months of 2009
payment for all amounts incurred for patent costs and trademarks were
deferred.
During
2009, we used $7,000 of cash from the net activity related to third party costs
incurred for patents and trademarks as compared to $268,000 used in
2008.
Net
Cash From Financing Activities
During
the first nine months of 2010, we generated $740,028 from our financing
activities through the issuance of units, warrants and notes with total proceeds
of $904,470 offset by approximately $94,000 in repayments of advances from a
related party, payment of private placement costs of approximately $58,970 and
repayment of debts of $11,772. In the first nine months of 2009 we issued notes
for $45,000 and received approximately $48,000 in net advances from one of our
LLC members and a related party, which accounted for most of our financing
activity during this period.
During
2009, we generated net cash of approximately $159,000 from financing activities.
Approximately $55,000 was generated by the issuance of notes and related
warrants. We also received cash advances from our LLC members and a related
party of $105,000 and repaid $1,000 of bank demand loans. In 2008, approximately
$776,000 was generated from financing activities. Of that amount, approximately
$656,000 was generated by the issuance of notes and related warrants. We also
received cash advances from LLC members and a related party, net of repayments,
of approximately $142,000 and repaid $22,000 of bank demand
loans.
-53-
Based on
our current operating plans, we believe that the net proceeds from the Private
Placement Offering will be sufficient to finance our planned operations through
the completion of our next clinical trial – a small Phase
II-B. However, we expect to require additional funds at approximately
year-end 2011 to completely satisfy past due amounts, complete the FDA clinical
trials for X-22, the
FDA requirements for our Modified Risk Cigarettes, including exposure studies,
and launch X-22. Our future
capital requirements will depend on many factors, including the progress made in
our X-22 clinical
trials.
We may
also need additional funds for possible future strategic acquisitions of
businesses, products or technologies complementary to our
business. If additional funds are required, we may raise such funds
from time to time through public or private sales of equity or debt securities.
Financing may not be available on acceptable terms, or at all, and our failure
to raise capital when needed could materially adversely impact our growth plans
and its financial condition and results of operations. Additional equity
financing may be dilutive to holders of the Common Stock, and debt financing, if
available, may involve significant cash payment obligations and covenants that
restrict our ability to operate our business.
Off-Balance
Sheet Arrangements
We do not
have any off-balance sheet arrangements as defined by Item 303(a)(4) of
Regulation S-K.
Accounting
and Reporting Developments
In
June 2009, the FASB issued Statement No. 168, The FASB Accounting Standards
Codification TM and the Hierarchy of Generally Accepted Accounting Principles—a
replacement of FASB Statement No. 162 (FAS 168). The Codification
became the source of authoritative GAAP recognized by the FASB to be applied by
non-governmental entities. Rules and interpretive releases of the SEC under
authority of federal securities laws are also sources of authoritative GAAP for
SEC registrants. On the effective date of FAS 168, the Codification superseded
all then-existing non-SEC accounting and reporting standards. All other
non-grandfathered non-SEC accounting literature not included in the Codification
became non-authoritative. FAS 168 was effective for financial statements issued
for interim and annual periods ending after September 15, 2009. The
adoption of FAS 168 did not affect our consolidated financial position, results
of operations, or cash flows.
Effective
June 1, 2009, the Company adopted new guidance on subsequent events. The
objective of this guidance is to establish general standards of accounting for
and disclosures of events that occur after the consolidated balance sheet date
but before the consolidated financial statements are issued or are available to
be issued. We has evaluates and discloses any material subsequent events through
the date of issuance of its financial statements; such date is disclosed in the
notes to the financial statements. This adoption did not have any impact on our
results of operations or financial condition.
In
December 2007, the FASB issued SFAS 160, Non-controlling Interests in
Consolidated Financial Statements, an amendment of ARB 51 (subsequently
incorporated into the FASB Accounting Standards Codification). This Statement
amends U.S. GAAP to establish accounting and reporting standards for the
non-controlling interest in a subsidiary and for the deconsolidation of a
subsidiary. It also clarifies that a non-controlling interest in a subsidiary is
an ownership interest in the consolidated entity that should be reported as
equity in the consolidated financial statements. It requires consolidated net
income or loss to be reported at amounts that include the amounts attributable
to both the parent and the non-controlling interest. Additionally, this
Statement establishes a single method of accounting for changes in a parent’s
ownership interest in a subsidiary that does not result in a change in control.
This Statement is effective for the first annual reporting period beginning on
or after December 31, 2008. We adopted the FASB’s guidance on Non-controlling Interests in
Consolidated Financial Statements on January 1, 2009 and disclosed the
balance of the non-controlling interest in our subsidiary, Xodus LLC, on our
balance sheet.
-54-
Securities
Ownership of Certain Beneficial Owners and Management
The following tables set forth certain
information regarding the beneficial ownership of our Common Stock taking into
account the consummation of the Merger, the closing of the Private Placement
Offering and the consummation of the Split-Off, by (i) each person who, to our
knowledge, owns more than 5% of our Common Stock, (ii) each of our directors and
executive officers, and (iii) all of our executive officers and directors as a
group. Unless otherwise indicated in the footnotes to the following table, each
person named in the table has sole voting and investment power and that person’s
address is c/o 22nd Century Group, Inc., 8201 Main Street, Suite 6,
Williamsville, NY 14221. Shares of our Common Stock subject to options,
warrants, or other rights currently exercisable or exercisable within 60 days of
January 25, 2011, are deemed to be beneficially owned and outstanding for
computing the share ownership and percentage of the person holding such options,
warrants or other rights, but are not deemed outstanding for computing the
percentage of any other person. Except as otherwise noted below, the
address for each person or entity listed in the table below is c/o 22nd Century
Group, Inc., 8201 Main Street, Suite 6, Williamsville, NY 14221.
|
Name of Beneficial Owner
|
Number of Shares
Beneficially Owned
|
Percentage Beneficially Owned
(1)
|
||||||
|
Management
and Directors
|
||||||||
|
Joseph
Pandolfino(2)
|
6,010,396 | 21.3 | % | |||||
|
Henry
Sicignano, III(3)
|
3,634,927 | 13.2 | % | |||||
|
Michael
R. Moynihan, Ph.D.(4)
|
1,017,645 | 3.8 | % | |||||
|
C.
Anthony Rider(5)
|
243,473 | * | ||||||
|
David
Rector
|
0 | * | ||||||
|
All
directors and executive officers as a group (5
persons) (2)-(5)
|
10,906,441 | 37.1 | % | |||||
|
Other
5% Owners
|
||||||||
|
Clearwater
Partners, LLC(6)
|
5,144,279 | 18.4 | % | |||||
|
Angelo
Tomasello(7)
|
4,193,881 | 15.1 | % | |||||
|
Henry
Sicignano III Group, LLC(8)
|
3,342,760 | 12.1 | % | |||||
* Less
than 1%
(1) Based
on 26,759,646 shares of Common Stock issued and outstanding, plus Common Stock
subject to options, warrants, or other rights currently exercisable or
exercisable within 60 days of January 25, 2011, held by the beneficial owner to
whom the disclosure pertains.
(2)
Includes 1,441,761 share of Common Stock issuable upon exercise of
warrants.
(3)
Consists of 222,603 shares of Common Stock held by Mr. Sicignano, 2,543,347
shares of Common Stock held by Henry Sicignano III Group, LLC, 69,564 shares of
Common Stock issuable to Mr. Sicignano upon exercise of warrants, and 800,413
shares of Common Stock issuable to Henry Sicignano III Group, LLC upon exercise
of warrants.
-55-
(4)
Includes 243,711 share of Common Stock issuable upon exercise of
warrants. Mr. Moynihan is Vice-President, Research and Development of
22nd Century Limited, LLC.
(5)
Includes 57,970 share of Common Stock issuable upon exercise of
warrants.
(6)
Includes 1,238,763 share of Common Stock issuable upon exercise of
warrants.
(7)
Includes 1,044,972 share of Common Stock issuable upon exercise of
warrants.
(8)
Includes 800,413 share of Common Stock issuable upon exercise of
warrants.
Executive
Officers and Directors
The following persons became our
executive officers and directors upon effectiveness of the Merger and hold the
positions set forth opposite their respective names:
|
Name
|
Age
|
Position
|
||
|
Joseph
Pandolfino
|
42
|
Chief
Executive Officer and Director
|
||
|
Henry
Sicignano, III
|
43
|
President
|
||
|
C.
Anthony Rider
|
59
|
Chief
Financial Officer
|
||
|
David
Rector
|
|
63
|
|
Director
|
Biographies
Joseph
Pandolfino, MBA, Chief Executive Officer and Director
Mr.
Pandolfino has served as our Chief Executive Officer and as a Director since the
closing of the Merger. He founded 22nd Century in 1998 and has over
15 years experience in all aspects of the tobacco industry, including 12 years
with genetically-engineered tobacco. He served as President of 22nd Century from
its inception until April 2010 and as Chief Executive Officer of 22nd Century
since April 2010. Mr. Pandolfino oversees our operations, strategy and product
development. Mr. Pandolfino holds a Bachelor of Science Degree in Business
Administration from Medaille College and a Master of Business Administration
Degree from the State University of New York at Buffalo. Mr.
Pandolfino’s significant experience in all aspect of the tobacco industry as
well as his experience leading 22nd Century led to our conclusion that Mr.
Pandolfino should serve as a Director of our Company.
Henry
Sicignano, III, MBA, President
Mr.
Sicignano has served as our President and Secretary since the closing of the
Merger and served as President of 22nd Century since April, 2010. From August
2005 to April 2009, Mr. Sicignano served as a General Manager and as the
Director of Corporate Marketing for NOCO Energy Corp., a petroleum products
company; and from March 2003 to July 2005, as Vice President of Kittinger
Furniture Company, Inc., a fine furniture manufacturer. From February 1997
through July 2002, he served as Vice President and Marketing Director of Santa
Fe Natural Tobacco Company, a specialty tobacco company, prior to the sale of
that company to R.J. Reynolds Tobacco Company in 2002. Mr. Sicignano holds a
Bachelors of Arts Degree in Government from Harvard College and a Master of
Business Administration Degree from Harvard University.
C.
Anthony Rider, CPA, Chief Financial Officer
Mr. Rider
has served as our Chief Financial Officer and Treasurer since the closing of the
Merger and served as the Chief Financial Officer of 22nd Century on a part-time
basis since 2007. He has also served, since 2007, as Chief Financial Officer of
Locke Acquisition Group LLC, which is unrelated to us. Mr. Rider served as the
Chief Financial Officer of Astronics Corporation, a public company, from 2000 to
2005, and as the Chief Financial Officer of IIMAK, a private-equity sponsored
international manufacturing company, from 2005 to 2007. Mr. Rider holds a
Bachelor of Science Degree from Canisius College. Mr. Rider is a member of the
AICPA and the New York State Society of CPAs. From 1973 to 2000, Mr. Rider was
employed by Ernst & Young, where he specialized in working with
entrepreneurial growth companies, and was the partner responsible for developing
and administering Ernst & Young’s entrepreneurial services practice for
Upstate New York and Western Pennsylvania.
-56-
David
Rector, Director
Mr.
Rector has served as a Director since November 22, 2010. He served as
our Chief Executive Officer, Chief Financial Officer, President, Secretary and
Treasurer from November 22, 2010 until the effective date of the
Merger. Mr. Rector served as the Chief Executive Officer, President,
Principal Accounting Officer, Secretary, Treasurer and a Director of Universal
Gold Mining Corp. from September 30, 2008 through November 17,
2010. Mr. Rector previously served as the Chief Executive Officer,
Chief Financial Officer, President, Secretary, Treasurer, and Director of Nevada
Gold Holdings, Inc. (formerly known as Nano Holdings International, Inc.) from
April 19, 2004 through December 31, 2008. He has served as the Chief
Executive Officer, Chief Financial Officer, President, Secretary, Treasurer, and
Director of Standard Drilling, Inc. since November 2007. Mr. Rector
has served as President, Treasurer, Secretary and a Director of Li3 Energy, Inc.
since June 6, 2008, was also the Chief Executive Officer and Chief Financial
Officer of the same company from June 6, 2008 until October 19, 2009 and January
13, 2010, respectively.
Mr.
Rector previously served as President, Chief Executive Officer and Chief
Operating Officer of Nanoscience from June 2004 to December 2006, when he
resigned as an officer and Director of Nanoscience. Mr. Rector also
served as President, Chief Executive Officer, Chief Financial Officer and
Treasurer of California Gold Corp. (f/k/a US Uranium, Inc.) from June 15, 2007
to July 11, 2007 and again from August 8, 2007 to November 12,
2007. Since June 1985, Mr. Rector has been the principal of the David
Stephen Group, which provides enterprise consulting services to emerging and
developing companies in a variety of industries. From January 1995
until June 1995, Mr. Rector served as the General Manager of the Consumer
Products Division of Bemis-Jason Corporation. Mr. Rector was employed by Sunset
Designs Inc., a manufacturer and marketer of consumer product craft kits from
June 1980 until June 1985. From June 1983 until June 1985, Mr. Rector served as
President and General Manager of Sunset, from August 1981 until May 1985, Mr.
Rector served as an Administrative and International Director of Sunset, and
from June 1980 until August 1981, Mr. Rector served as Group Product Manager for
Sunset. Mr. Rector’s significant experience as a director and officer
of publicly traded companies led to our conclusion that he should serve as a
Director of our Company.
Executive
Compensation
Summary
Compensation Table
The
following summary compensation table sets forth the compensation paid during the
two years ended December 31, 2009 to our Chief Executive Officer and the two
most highly compensated executive officer other than our CEO.
-57-
|
Name and Principal Position
|
Year
|
Salary/
guaranteed
payment
|
Bonus
|
Other
Annual
Compensation(1)
|
Securities
Underlying
Options
|
Annual
Unfunded
Accrued
Pension
|
||||||||||||||||
|
Joseph
Pandolfino,
|
2009
|
$ | 150,000 | 0 | 0 | N/A | 0 | |||||||||||||||
|
Chief
Executive Officer
|
2008
|
150,000 | 0 | 0 | N/A | 0 | ||||||||||||||||
|
Michael
R. Moynihan, Ph.D.
|
||||||||||||||||||||||
|
Vice
President of R&D of 22nd
|
2009
|
92,000 | 0 | $ | 258,660 | N/A | 0 | |||||||||||||||
|
Century
Limited, LLC
|
2008
|
80,000 | 0 | 0 | N/A | 0 | ||||||||||||||||
|
Deborah
Aguglia
|
2009
|
$ | 36,000 | 0 | 0 | N/A | 0 | |||||||||||||||
|
(1)
|
Value
of equity grants.
|
Outstanding
Equity Awards at Fiscal Year-End
As of
December 31, 2010, there were no outstanding equity awards held by executive
officers of either 22nd Century or Parent.
Agreements
with Executive Officers
We have
entered into employment agreements with each of Messrs. Pandolfino, Sicignano
and Rider and, that provide for annual compensation of $150,000, $150,000, and
$72,000, respectively, subject to increases as contained in such employment
agreements and/or as decided by our board of directors. These employment
agreements also contain non-compete covenants and change of control
provisions.
The
employment agreement of each such executive officer provides that during the
executive officer’s employment by us and for a period of two (2) years after the
executive officer ceases to be employed by us, the following non-compete
covenants will apply: (i) the executive officer will not (except on behalf of
us) provide or offer to provide any goods or services to any entity engaged in
the United States in the making, offering, marketing, distributing and/or
selling of products made from the tobacco (Nicotiana) plant, and/or providing or
offering to provide the same or substantially similar services to any customer
or prospective customer, (ii) the executive officer will not interfere with our
relationships with any customer, prospective customer, supplier, distributer,
farmer and/or manufacturer, and (iii) the executive will not induce or attempt
to induce any persons employed by us to leave their employment with us, nor hire
or employ, or attempt to hire or employ, any persons employed by us, nor assist
or facilitate in any way any other person or entity in the hiring of any persons
employed by us.
The
employment agreement of Mr. Rider provides that in the event of a change of
control (as defined in the employment agreement) of our Company, Mr. Rider may
resign his employment with the Company (or, if involuntarily terminated, give
notice of his intention to collect benefits) and shall be entitled to receive
the base salary which remains unpaid for the remainder of the initial term of
the employment agreement as set forth on Addendum A thereto.
-58-
The
employment agreements of Messers. Pandolfino and Sicignano provide that in the
event of a change in control (as defined in the employment agreements) of our
Company, then during the three- (3) year period following such change in control
if certain triggering events occur as defined in such employment agreements,
such as if the executive is terminated other than for cause (as defined in the
agreements), death or disability, or if the executive officer’s responsibilities
are diminished after the change in control as compared to the executive
officer’s responsibilities prior to the change in control, or if the executive
officer’s base salary or benefits are reduced, or the executive is required to
relocate more than twenty-five (25) miles from his current place of employment,
then in any such events the executive officer will have the option, exercisable
within ninety (90) days of the occurrence of such an event, to resign his
employment with us, in which case the executive officer will be entitled to
receive (A) the greater of either his base salary for the then remaining portion
of the initial 5-year term of the agreement or his base salary for three (3)
years thereafter, (B) reimbursement for eighteen (18) months of his reasonable
costs for medical, dental, life, disability and other benefits and insurance
coverage that the executive officer received during his employment, (C)
outplacement services for two (2) years, and (D) the immediate vesting of all
options and/or restricted stock grants previously granted or to be granted to
the executive officer.
We also
provide each of these individuals with health insurance and vacation
benefits.
Director
Compensation
We
currently do not have a set compensation package for members of our board of
directors for acting as such, but we expect to establish these arrangements in
the near future.
Board
of Directors and Corporate Governance
Upon the
closing of the Merger, Parent’s board of directors was expanded to consist of
five (5) members. The sole officer and sole member of the board of
directors prior to the closing of the Merger, David Rector, resigned as an
officer effective as of the closing of the Merger but continues to serve as a
member of our board of directors. Immediately following the closing
of the Merger, Joseph Pandolfino was appointed to serve as a member of our board
of directors. As of the date ten (10) days following the filing of a
Schedule 14F-1 with the United States Securities and Exchange Commission after
the closing of the Merger, David Rector will resign as a member of our board of
directors and will be replaced by an individual appointed by the pre-Merger
stockholders of Parent. Each of Henry Sicignano III, Joseph Alexander
Dunn, and James W. Cornell will also be appointed to serve as members of our
board of directors as of that date.
Code
of Ethics
In 2006,
we adopted a Code of Ethics that applies to all of our employees. A
copy of our Code of Ethics will be provided to any person requesting same
without charge. To request a copy of our Code of Ethics, please make
written request to our Chief Executive Officer c/o 22nd Century Group, Inc.,
8201 Main Street, Suite 6, Williamsville, NY 14221.
Board
Committees
We intend
to appoint such persons to the board of directors and committees of the board of
directors as are expected to be required to meet the corporate governance
requirements imposed by a national securities exchange, although we are not
required to comply with such requirements until we elect to seek listing on a
securities exchange. We intend that a majority of our directors will be
independent directors, of which at least one director will qualify as an “audit
committee financial expert,” within the meaning of Item 407(d)(5) of Regulation
S-K, as promulgated by the SEC. Additionally, the board of directors is expected
to appoint an audit committee, nominating committee and compensation committee,
and to adopt charters relative to each such committee, in the near future. We do
not currently have an “audit committee financial expert” since we currently do
not have an audit committee in place.
-59-
Equity
Incentive Plans
On October 21, 2010, we established an
equity incentive compensation plan for directors, officers and employees,
consisting of 4,250,000 shares of Common Stock, which will represent
approximately 10.7% of the total number of outstanding shares of Common Stock,
on a fully diluted basis. This equity incentive compensation plan
will have a term of ten (10) years and will be administered by a committee to be
established by our board of directors, with such committee to determine the
various types of incentive awards that may be granted to recipients under this
plan, such as stock options, stock appreciation rights, performance share
awards, restricted stock and restricted stock units, and the number of shares of
Common Stock to underlie each such award under this plan. This plan also
contains a provision which restricts the plan to granting awards relating to no
more than 1,600,000 shares of Common Stock during the first twelve (12) months
following the effective date of the plan.
Certain
Relationships and Related Transactions
Transactions
with Parent Directors, Executive Officers, and holders of 5% or more of our
issued and outstanding Common Stock
Immediately prior to the closing of the
Merger, pursuant to the terms of the Split-Off Agreement, we transferred all of
our pre-Merger operating assets and liabilities to Split-Off
Subsidiary. We then transferred all of the outstanding capital stock
of Split-Off Subsidiary to David Rector, our sole director and executive officer
prior to the Merger, in exchange for $1, such consideration being deemed to be
adequate by our board of directors prior to the Merger. Prior to the
closing of the Merger, we paid Mr. Rector $1,500 in consideration for his
service as our sole director and executive officer.
Prior to the closing of the Merger, we
utilized office space located at 11923 SW 37 Terrace, Miami, Florida 33175 that
was provided to us on a rent-free basis by Nanuk Warman, our former director and
executive officer. Also, prior to the closing of the merger, we
cancelled 10,015,200 shares of our Common Stock held by Mr. Warman and entered
into a mutual release agreement with Mr. Waraman regarding such
cancellation. In each of fiscal years 2009 and 2010, we paid Mr.
Warman aggregate compensation of $8,000 in consideration for his service as our
sole director and executive officer during those periods. We also
paid Mr. Warman aggregate of $1,500 in consideration for his accounting services
in preparation of our most recent Form 10-K and Form 10-Q.
Transactions
with 22nd Century Founders, Executive Officers, and holders 5% of more of our
issued and outstanding Common Stock
The
following discussion pertaining to the number of shares of our Common Stock
involved in the related party transactions. At the time of these
transactions, the parties thereto received Units of 22nd Century, which were
converted into shares of our Common Stock in the Merger.
We have
had numerous transactions with Alternative Cigarettes, Inc. (“AC”). AC is 95%
owned by three holders of our Common Stock, including Joseph Pandolfino, our
Chief Executive Officer, and Angelo Tomasello, who currently owns approximately
11.8% of our issued and outstanding Common Stock. We share office space and
employee services with AC. AC reimburses us from time to time for the value of
these activities. AC paid us $32,387 during fiscal year 2009 and $57,667 during
fiscal year 2008 for these services. AC has also advanced funds to us from time
to time. Since January 1, 2009, the largest net amount due from us to AC was
approximately $127,000. No interest has been accrued or paid on these amounts
due to AC and there are no repayment terms between the parties.
-60-
In
January 2008, we issued convertible promissory notes due and payable on January
15, 2011 to Messrs. Pandolfino and Tomasello in the principal amounts of $77,435
and $100,315, respectively, with 7% interest per annum accruing thereon. In
December 2009, Mr. Pandolfino converted the principal balance and accrued
interest under his note ($88,172) into 151,760 shares of our Common
Stock. In June 2010, Mr. Tomasello agreed to amend his note to
eliminate his right to convert the balance into shares of our Common
Stock.
In
November 2008, we issued a promissory note due and payable on November 11, 2010
to Mr. Tomasello in the principal amount of $325,000 with 10% interest per annum
accruing thereon and a warrant to purchase 371,006 shares of our Common Stock,
which have since been exercised at a price of $.0001 per share. The note is
guaranteed by Virgil Properties, LLC, which is jointly owned by Messrs.
Pandolfino and Tomasello. Mr. Tomasello continued to make funds available to us
in the form of cash advances. The largest net amount outstanding since January
1, 2009 was approximately $166,000. No interest was accrued or paid on such
advances and there were no repayment terms between the parties. In December
2009, Mr. Tomasello was issued 504,553 shares of our Common Stock in lieu of
repayment of $135,996 of such advances, and we issued him a promissory note that
was exchanged for 204,639 shares of our Common Stock in June
2010. Effective December 1, 2010 the original $325,000 promissory
note was amended to extend the maturity date until January 10, 2012 and to
increase the interest rate to 15% during this extension period. On
January 25, 2011, Mr. Tomasello converted the principal amount of this
promissory note into 325,000 shares of Common Stock through an investment in the
Private Placement Offering and was issued a new promissory note in the principal
amount of $79,401.06 with 10% interest per annum, which represents the accrued
interest on the original $325,000 promissory note that was not
converted.
Mr.
Pandolfino continues to make funds available to us in the form of cash advances
and deferred guaranteed payments due to him by us as consideration for his
services as our Chief Executive Officer. The largest net amount of such advances
and deferred guaranteed payments outstanding since January 1, 2009 was
approximately $137,000. No interest was accrued or paid on such advances or
deferrals and there are no repayment terms between the parties. In December
2009, Mr. Pandolfino was issued 504,553 shares of our Common Stock in lieu of
repayment of $135,996 of such advances. During the period between
January 1 and October 5, 2010, we issued Mr. Pandolfino 455,331 shares of our
Common Stock in lieu of $103,573 due and payable to him for his
services. On October 5, 2010, we issued Mr. Pandolfino a promissory
note, which was assigned to Mr. Sicignano, due and payable on January 31, 2011
in the principal amount of $58,873 with 15% interest per annum accruing
thereon.
In
September 2010, Henry Sicignano III, our President, loaned us $35,000, which
amount is due and payable in November 2010 with 15% interest per annum accruing
thereon. On December 16, 2010, Mr. Sicignano agreed to extend the
maturity date of this loan until January 25, 2011. Mr. Sicignano is
also the managing member of Henry Sicignano III Group, LLC. On
October 5, 2010, Henry Sicignano III Group, LLC purchased 112,396 shares of our
Common Stock for $30,295 and we issued Henry Sicignano III Group, LLC a
promissory note due and payable on January 31, 2011 in the principal amount of
$30,295 with 15% interest per annum accruing thereon.
On
December 29, 2010, we issued a promissory note to Henry Sicignano, III in the
amount of $100,000 due and payable on January 20, 2011 with 15% interest per
annum accruing thereon. On January 25, 2011, Henry Sicignano III Group, LLC
converted the principal amount of this promissory and the accrued interest
thereon into 31,626 shares of Common Stock through an investment in the Private
Placement Offering.
-61-
Michael
R. Moynihan, Ph.D., Vice President of Research and Development of 22nd Century
Limited, LLC, has deferred guaranteed payments due and payable to him as
consideration for his services to us. The largest net balance of such amounts
outstanding since January 1, 2009 was approximately $79,000. No interest was
accrued or paid on such amounts owed and there were no repayment terms between
the parties. In December 2009, Dr. Moynihan was issued 74,201 shares
of our Common Stock and 4 membership interests in our subsidiary (100 units
outstanding), Xodus LLC, in lieu of $54,000 of such amount due and payable to
him. During the period between January 1 and October 5, 2010, we
issued Dr. Moynihan 109,584 shares of our Common Stock in lieu of $23,538 of
such amount due and payable to him for his services.
On
September 15 and October 15, 2009, we issued notes payable to Clearwater
Partners, LLC in the amounts of $15,000 and $10,000, respectively. In
conjunction with the $15,000 note, a warrant to purchase 185,503 membership
units at less than $.0001 per unit was issued, and in conjunction with the
$10,000 note, a warrant to purchase 92,751 Membership Units at less than $.0001
per unit was issued. The notes bear interest at a rate of 10%. The notes had
original maturity dates September 15, 2010 and October 15, 2010, respectively,
and as of May 27, 2010, the maturity dates of these notes were extended to
January 31, 2012.
On March
1, 2010, we issued a four-year warrant to purchase 1,706,626 shares of our
Common Stock to Clearwater Partners, LLC that was exercised in full on May 27,
2010 at a price per share of $0.0001. On May 27, 2010, we further issued
to Clearwater Partners, LLC a four-year warrant to purchase 1,409,821 shares of
our Common Stock, which was immediately exercised in full at a price per share
of $0.0001, and a promissory note due and payable on January 31, 2012 in the
principal amount of $45,000 with 10% interest per annum accruing thereto.
These warrants and this promissory note were issued to Clearwater Partners, LLC
in lieu of repayment of $450,000 in funds previously advanced by Clearwater
Partners, LLC.
On
October 5, 2010, we issued Clearwater Partners, LLC a promissory note due and
payable on January 31, 2011 in the principal amount of $47,535 with 15% interest
per annum accruing thereon.
Board
Independence
We currently have no “independent”
directors, as that term is defined in the applicable listing standards of The
NASDAQ Stock Market and SEC rules; however, we plan to appoint three (3)
independent directors ten (10) days following the filing of a Schedule 14F-1
with the SEC that will occur after consummation of the Merger.
|
Item
3.02
|
Unregistered
Sale of Securities
|
Sales
by 22nd Century
The
following discussion pertaining to the number of shares of our Common Stock
involved in certain sales of unregistered securities by 22nd
Century. At the time of these transactions, the parties thereto
received Units of 22nd Century, warrants to purchase Units of 22nd Century, or
debt securities convertible into Units of 22nd Century, which were converted
into shares of our Common Stock and warrants to purchase shares of our Common
Stock in the Merger.
All of the sales of unregistered
securities set forth herein were made solely to “accredited investors” as that
term is defined in Rule 501 of Regulation D under the Securities Act and to
individuals and entities that are not “U.S. Persons” as that term is defined in
Regulation S under the Securities Act. The securities discussed
herein were not registered under the Securities Act, or the securities laws of
any state, and were offered and sold in reliance on the exemption from
registration provided by Section 4(2) and/or Section 4(6) of the Securities Act
or pursuant to Regulation D or Regulation S and corresponding provisions of
state securities laws.
-62-
On
January 1, 2008 we issued convertible promissory notes due and payable on
January 15, 2011 to Joseph Pandolfino and Angelo Tomasello in the principal
amounts of $77,435 and $100,013, respectively with 7% interest per annum
accruing thereon. On December 30, 2009, Mr. Pandolfino converted the
principal balance and accrued interest under his promissory note ($88,172) into
151,760 shares of our Common Stock. On June 10, 2010, Mr. Tomasello’s
promissory note was amended to eliminate Mr. Tomasello’s right to convert the
principal balance and accrued interest under his promissory note into shares of
our Common Stock.
On
October 28, 2008, we issued a promissory note due and payable on October 28,
2010 to Joseph M. Anderson in the principal amount of $325,000 with 10% interest
per annum accruing thereon together with a five-year warrant to purchase 371,006
shares of our Common Stock, which was exercised in full July 1, 2010 at a price
per share of $0.0001. On May 20, 2009, we issued a subsequent
promissory note to Mr. Anderson due and payable on May 19, 2010 with 10%
interest per annum accruing thereon together with a four-year warrant to
purchase 185,503 shares of our Common Stock, which was exercised in full on July
1, 2010 at price per share of $0.0001. In connection with the Private
Placement Offering, Mr. Anderson partially converted the principal balance and
accrued interest under both of these promissory notes ($150,000). He was issued
a new promissory note due and payable on June 30, 2013 in the principal amount
of $140,000 with 12% interest per annum accruing thereon.
On
November 11, 2008, we issued a promissory note due and payable on November 11,
2010 to Mr. Tomasello in the principal amount of $325,000 with 10% interest per
annum accruing thereon together with a five-year warrant to purchase 371,006
shares of our Common Stock, which was exercised on May 1, 2010 at a price per
shares of $0.0001. On December 1, 2010 the maturity date of this
promissory note was extended until January 10, 2012. In connection
with the Private Placement Offering, Mr. Tomasello converted the principal
balance under this promissory notes into 325,000 shares of our Common Stock and
was issued a new promissory note due and payable on January 28, 2011 in the
principal amount of $79,401.06 with 10% interest per annum accruing thereon,
representing the accrued interest on the original promissory note.
On
December March 1, 2009 we issued a three year warrant to purchase 37,624 shares
of our Common Stock at an exercise price of $0.0001 to The Kane Firm, PC in
consideration of $21,154 of professional services rendered.
On March
1, 2009 we issued a three-year warrant to purchase 37,100 shares of our Common
Stock at an exercise price of $0.0001 to Lee R. Guterman in consideration of
$21,154 of professional services rendered.
On
September 15, 2009 we issued a promissory note due and payable on September 15,
2010 to Clearwater Partners, LLC in the principal amount of $15,000 with 10%
interest per annum accruing thereon together with a four-year warrant to
purchase 278,254 shares of our Common Stock, which was exercised in full on May
27, 2010 at price per share of $0.0001. On October 15, 2009 we issued
a subsequent promissory note due and payable on October 15, 2010 to Clearwater
Partners, LLC in the principal amount of $10,000 with 10% interest per annum
accruing thereon. On May 27, 2010, the maturity date of both of these
promissory notes was extended until January 31, 2012.
-63-
On
December 1, 2009 we issued 74,201 shares of our Common Stock to The Kane Firm,
PC in consideration of $18,333 of professional services rendered.
On
December 30, 2009, we issued 504,553 shares of our Common Stock to Mr. Tomasello
in lieu of repayment of $135,996 in funds previously advanced by Mr.
Tomasello. On that same date, we further issued to Mr. Tomasello
a promissory note due and payable on June 30, 2011 in the principal
amount of $30,054 with 10% interest per annum accruing thereon, which was
exchanged for 204,639 shares of our Common Stock on June 10, 2010.
On
December 30, 2009 we issued 504,533 shares of our Common Stock to Mr. Pandolfino
in lieu of repayment of $135,996 in funds previously advanced by Mr.
Pandolfino. On December 1, 2009, we further issued Mr. Pandolfino
506,508 shares of our Common Stock in lieu of repayment of $137,500 due and
payable to Mr. Pandolfino in compensation for his services. On June
7, 2010, we further issued Mr. Pandolfino 236,909 shares of our Common Stock in
lieu of repayment of $44,700 due and payable to Mr. Pandolfino in compensation
for his services.
On
December 1, 2009, we issued 74,201 shares of our Common Stock and 4 membership
interest units in our subsidiary, Xodus, LLC, to Michael Moynihan in lieu of
repayment $54,000 due and payable to Dr. Moynihan in compensation for his
services.
On March
1, 2010, we issued a four-year warrant to purchase 1,706,626 shares of our
Common Stock to Clearwater Partners, LLC was exercised in full on May 27, 2010
at a price per share of $0.0001. On May 27, 2010, we further issued
to Clearwater Partners, LLC a four- year warrant to purchase 1,409,821 shares of
our Common Stock, which was immediately exercised in full at a price per share
of $0.0001, and a promissory note due and payable on January 31, 2012 in the
principal amount of $45,000 with 10% interest per annum accruing
thereto. These warrants and this promissory note were issued to
Clearwater Partners, LLC in lieu of repayment of $450,000 in funds previously
advanced by Clearwater Partners, LLC.
On April
9, 2010 we issued and sold 2,124,387 shares of our Common Stock to Henry
Sicignano III Group, LLC for an aggregate purchase price of
$330,000.
On May
28, 2010 we issued and sold 185,503 shares of our Common Stock to Mr. Tomasello
for an aggregate purchase price of $30,000.
In June
and July 2010, we issued an aggregate of 583,009 shares of our Common Stock to
five of our then existing shareholders for an aggregate purchase price of
$110,000. Such aggregate number of shares and aggregate purchase
price includes the issuance 236,909 and 74,201 shares of our Common Stock to Mr.
Pandolfino and Dr. Moynihan, respectively, in lieu of repayment of $44,700 and
$14,000 due and payable to Mr. Pandolfino and Dr. Moynihan, respectively, in
compensation for their services.
On
September 1, 2010 we issued a promissory note due and payable on October 1, 2010
to Henry Sicignano III in the principal amount of $35,000 with 10% interest per
annum accruing thereon. On December 16, 2010, the maturity date of
this promissory note was extended until January 25, 2010.
In
October 2010, we issued an aggregate of 556,508 shares of our Common Stock and
promissory notes in the aggregate principal amount of $150,000 with 15% interest
per annum accruing thereon to eight of our then existing shareholders for an
aggregate purchase price of $300,000. Such aggregate purchase price
includes the issuance of shares of our Common Stock to Dr. Moynihan and The Kane
Firm PC in lieu of repayment of $19,046 and $4,384, respectively, in
compensation for their services.
-64-
On
January 1, 2010, we issued a promissory note due and payable on September 30,
2010 to Isidore Redstone and Elizabeth Redstone in the principal amount of
$30,629 with 7% interest per annum accruing thereon.
On October 25, 2010, a resolution was passed to issue five-year
warrants to purchase an aggregate of
5,000,000 shares of our Common Stock to our then existing
shareholders at an exercise price per share of $3.00, contingent on the
closing of the Private Placement Offering .
On
January 25, 20111 in the Private Placement Offering, we issued and sold an
aggregate of 5,434,446 shares of our Common Stock and five-year warrants to
purchase an aggregate of 2,717,223 shares of our Common Stock at an exercise price of 1.50 per
share.
Net
proceeds received from the Private Placement Offering are expected to be used
for the FDA-approval process for our X-22 smoking cessation aid
and exposure studies for our modified risk cigarettes, including Phase II-B
trial and Phase III trial with the same protocol, reduction of currently
liabilities, protection and defense of our intellectual property, our investors
relations program, and our future working capital needs.
Rodman & Renshaw, LLC (the
“Placement Agent”) acted as placement agent in the Private Placement Offering
and Gottbetter Capital Markets, LLC (the “Sub-Act”) acted as sub-placement agent
in the Private Placement Offering. In connection with the closing of
the Private Placement Offering on January 25, 2011, 22nd Century was obligated
to compensate the Placement Agent with (i) a cash fee of equal to 8% of the
aggregate purchase price paid by purchasers of PPO Securities in the Private
Placement Offering (the “Cash Fee”); (ii) five-year warrants to purchase such
number of our Common Stock equal to eight percent (8%) of the total number of
PPO Securities sold in the Private Placement Offering at an exercise price of
$1.50 per share, and (iii) reimbursement for all reasonable out of pocket
expenses incurred in connection with the engagement, including, but not limited
to reasonable expense of counsel. On January 24, 2011, pursuant to
the terms of that certain Conversion Agreement by and between 22nd Century and
the Placement Agent, the Placement Agent elected to convert the Cash Fee due and
payable to it into shares of our Common Stock. Upon the closing of
the Merger, (i) the Sub-Agent was paid $40,000, (ii) the Sub-Agent was issued a
five-year warrant to purchase 40,000 shares of our Common Stock at an exercise
price of $1.50 per share, and (iii) the Placement Agent was issued a warrant to
purchase 394,755 share of our Common Stock at an exercise price of $1.50 per
share.
Sales
by Parent
Pre-Merger
On February 6, 2008 Parent sold 138,889
shares of Common Stock to one person at a price of $0.36 per share, or an
aggregate sale price of $50,000. The sale was made pursuant to the
exception provided by Section 4(2) of the Securities Act since the issuance did
not involve a public offering, the recipient had access to information that
would be included in a registration statement, the recipient took the shares for
investment and not resale, and Parent took appropriate measures to restrict
resale.
In September 2007, Parent issued
3,000,000 shares of restricted Common Stock to Douglas Scheving, its then-sole
officer and director, in exchange for the forgiveness of $34,502 in indebtedness
that was owed to him by Parent. The shares were issued pursuant to the exemption
from registration provided by Section 4(2) of the Securities Act.
-65-
Merger
See Item 2.01 for a description of the
Parent securities issued in connection with the Merger. The issuance
of securities in the Merger was not registered under the Securities Act, or the
securities laws of any state, and were offered and sold in relation on the
exemption from registrations provided by Section 4(2) and/or Section 4(6) of the
Securities Act or pursuant to Regulation D or Regulation S and corresponding
provisions of state securities laws.
Description
of Capital Stock
General
Our
authorized capital stock consists of 300,000,000 shares of Common Stock and
10,000,000 shares of preferred stock, of which 26,759,646 shares of Common Stock
are issued and outstanding. No shares of preferred stock are issued
and outstanding. We will also have reserved 14,470,000 shares of
Common Stock for (i) issuance upon the exercise of the warrants issued in
connection with the Merger, (ii) issuance upon the exercise of the warrants that
were issued to our financial advisor upon the closing of the Merger, and (iii)
the shares of Common Stock underlying our equity incentive plan.
The
following summary of certain provisions of our capital stock does not purport to
be complete and is subject to and is qualified in its entirety by our articles
of incorporation and by-laws following the Merger and by the provisions of
applicable law.
Common
Stock
Holders
of the Common Stock are entitled to one vote per share with respect to each
matter presented to our shareholders on which holders of Common Stock are
entitled to vote. The Common Stock does not have cumulative voting rights. No
share of Common Stock affords any preemptive rights or is convertible,
redeemable, assessable or entitled to the benefits of any sinking or repurchase
fund.
Subject
to the prior rights of holders of preferred stock, if any, holders of Common
Stock are entitled to receive dividends as may be lawfully declared from time to
time by our board of directors. Upon our liquidation, dissolution or winding up,
whether voluntary or involuntary, holders of Common Stock will be entitled to
receive such assets as are available for distribution to our shareholders after
there shall have been paid, or set aside for payment, the full amounts necessary
to satisfy any preferential or participating rights to which the holders of each
outstanding series of preferred stock are entitled by the express terms of the
series.
The
shares of Common Stock outstanding as of the closing of the Merger will be fully
paid and non-assessable. The Common Stock is quoted on the OTC Bulletin Board
under the symbol “XXII.OB.”
Preferred
Stock
Our board
of directors is authorized, without action by our stockholders, to designate and
issue up to an aggregate of 10,000,000 shares of preferred stock in one or more
series. Our board of directors has the discretion to determine the rights,
preferences, privileges and restrictions, including voting rights, dividend
rights, conversion rights, redemption privileges and liquidation preferences, of
each series of preferred stock.
-66-
No shares
of preferred stock are currently outstanding, and we have no current plans to
issue preferred stock. The issuance of shares of preferred stock, or the
issuance of rights to purchase preferred stock, could be used to discourage an
unsolicited acquisition proposal. For example, a business combination could be
impeded by the issuance of a series of preferred stock containing class voting
rights that would enable the holder or holders of such series to block any such
transaction. Alternatively, a business combination could be facilitated by the
issuance of a series of preferred stock having sufficient voting rights to
provide a required percentage vote of our shareholders. In addition, under some
circumstances, the issuance of preferred stock could adversely affect the voting
power and other rights of the holders of our Common Stock. Although prior to
issuing any series of preferred stock our board is required to make a
determination as to whether the issuance is in the best interests of our
shareholders, our board could act in a manner that would discourage an
acquisition attempt or other transaction that some, or a majority, of our
shareholders might believe to be in their best interests or in which our
shareholders might receive a premium for their stock over prevailing market
prices of such stock. Our board of directors does not presently intend to seek
shareholder approval prior to any issuance of currently authorized preferred
stock, unless otherwise required by law or applicable stock exchange
requirements.
Warrants
We issued
five-year warrants to purchase 2,717,223 shares of our Common Stock, at an
exercise price of $1.50 per share, in exchange for the warrants contained in the
PPO Securities purchased by investors in the Private Placement Offering (the
“Investor Warrants”). These warrants contain among other things, a
cashless exercise provision to become operative upon the later of: (A) one (1)
year following the date hereof, if a registration statement pursuant to the
Securities Act with regard to the shares of Common Stock issuable upon exercise
of these warrants has not been filed within one (1) year following the date
hereof and (B) thirty (30) days following the date on which the earlier filed
registration statement with regard to the shares of Common Stock received by the
investors in the Private Placement Offering as a result of the Merger has been
declared effective by the SEC, if a registration statement pursuant to the
Securities Act with regard to the shares of Common Stock issuable upon exercise
of these warrants has not been filed prior to the expiry of such thirty- (30)
day period. A cashless exercise means that in lieu of paying the
aggregate purchase price for the shares being purchased upon exercise of the
warrants in cash, the holder will forfeit a number of shares underlying the
warrants with a “fair market value” equal to the aggregate exercise
price. We will not receive additional proceeds to the extent that
warrants are exercised on a cashless basis. The exercise price and
number of shares of our Common Stock issuable upon exercise of the warrants may
be adjusted in certain circumstances, including in the event of a stock
dividend, or our recapitalization, reorganization, merger or
consolidation. These warrants also provide holders with
weighted-average anti-dilution price protection. No fractional shares
will be issued upon exercise of these warrants. If, upon exercise of
these warrants, a holder would be entitled to receive a fractional interest in a
share, we may, in our discretion, upon exercise, round up to the nearest whole
number of shares of our Common Stock to be issued to the warrant holder or
otherwise equitably adjust the exercise and exercise price per
share.
We issued
five-year warrants to purchase 5,000,000 shares of our Common Stock, at an
exercise price of $3.00 per share, in exchange for the warrants held by the LLC
members of 22nd Century prior to the consummation of the Private Placement
Offering (the “Century Warrants”). These warrants contain among other
things, a cashless exercise provision to become operative upon the later of: (A)
one (1) year following the date hereof, if a registration statement pursuant to
the Securities Act with regard to the shares of Common Stock issuable upon
exercise of these warrants has not been filed within one (1) year following the
date hereof and (B) thirty (30) days following the date on which the earlier
filed registration statement with regard to the share of Common Stock received
by the investors in the Private Placement Offering as a result of the Merger has
been declared effective by the SEC, if a registration statement pursuant to the
Securities Act with regard to the shares of Common Stock issuable upon exercise
of the these warrants has not been filed prior to the expiry of such thirty-
(30) day period. A cashless exercise means that in lieu paying the
aggregate purchase price for the shares being purchased upon exercise of the
warrants in cash, the holder will forfeit a number of shares underlying the
warrants with a “fair market value” equal to the aggregate exercise
price. We will not receive additional proceeds to the extent that
warrants are exercised on a cashless basis. The exercise price
and number of shares of our Common Stock issuable upon exercise of the warrants
may be adjusted in certain circumstances, including in the event of a stock
dividend, or our recapitalization, reorganization, merger or
consolidation. These warrants also provide holders with
weighted-average anti-dilution price protection. No fractional shares
will be issued upon exercise of these warrants. If, upon exercise of
these warrants, a holder would be entitled to receive a fractional interest in a
share, we may, in our discretion, upon exercise, round up to the nearest whole
number of shares of our Common Stock to be issued to the warrant holder or
otherwise equitably adjust the exercise and exercise price per
share.
-67-
We issued
five-year warrants to purchase an aggregate of 434,755 shares of our Common
Stock, at an exercise price of $1.50 per share, in exchange for the warrants
issued to the Placement Agent and the Sub-Agent (the “Placement Agent Conversion
Warrants”). These warrants contain among other things, a cashless
exercise provision. A cashless exercise means that in lieu of paying
the aggregate purchase price for the shares being purchased upon exercise of the
warrants in cash, the holder will forfeit a number of shares underlying the
warrants with a “fair market value” equal to the aggregate exercise
price. We will not receive additional proceeds to the extent that
warrants are exercised on a cashless basis. The exercise price and
number of shares of our Common Stock issuable upon exercise of the warrants may
be adjusted in certain circumstances, including in the event of a stock
dividend, or our recapitalization, reorganization, merger or
consolidation. These warrants also provide holders with
weighted-average anti-dilution price protection. No fractional shares
will be issued upon exercise of these warrants. If, upon exercise of
these warrants, a holder would be entitled to receive a fractional interest in a
share, we may, in our discretion, upon exercise, round up to the nearest whole
number of shares of our Common Stock to be issued to the warrant holder or
otherwise equitably adjust the exercise and exercise price per
share.
We issued
five year warrants to purchase 500,000 shares of our Common Stock, at an
exercise price of $1.50 per share, to the Placement Agent (the “Advisor
Warrants”). These warrants contain among other things, a cashless
exercise provision. A cashless exercise means that in lieu of paying
the aggregate purchase price for the shares being purchased upon exercise of the
warrants in cash, the holder will forfeit a number of shares underlying the
warrants with a “fair market value” equal to the aggregate exercise
price. We will not receive additional proceeds to the extent that
warrants are exercised on a cashless basis. The exercise price and
number of shares of our Common Stock issuable upon exercise of the warrants may
be adjusted in certain circumstances, including in the event of a stock
dividend, or our recapitalization, reorganization, merger or
consolidation. These warrants also provide holders with
weighted-average anti-dilution price protection. No fractional shares
will be issued upon exercise of these warrants. If, upon exercise of
these warrants, a holder would be entitled to receive a fractional interest in a
share, we may, in our discretion, upon exercise, round up to the nearest whole
number of shares of our Common Stock to be issued to the warrant holder or
otherwise equitably adjust the exercise and exercise price per
share.
Registration
Rights
We agreed
to a covenant in conjunction with the Private Placement Offering to use our best
efforts to file, within 75 days following the effective date of the Merger, with
the SEC a registration statement, which will cover the resale of the Common
Stock issued to the investors in the Private Placement as a result of the Merger
in exchange for the Membership Units contained in the PPO Securities. We will
use our best efforts to cause this registration statement to be declared
effective by the SEC within one hundred eighty (180) calendar days of filing
with the SEC (240 days if the SEC reviews such registration statement). If we
are late in filing this registration statement or if this registration statement
is not declared effective within the prescribed time periods, then the holders
of registrable Common Stock shall be entitled to monetary penalties payable by
us at a rate equal to one-half percent (0.50%) of the offering price per Unit in
the Private Placement Offering for each full month that (i) we are late in
filing this registration statement or (ii) this registration statement is late
in being declared effective by the SEC; provided, however, that in no event
shall the aggregate of any such penalties exceed five percent (5%) of the
offering price per Unit in the Private Placement Offering. Notwithstanding the
foregoing, no penalties shall accrue with respect to any shares of Common Stock
removed from the registration statement in response to a comment from the staff
of the SEC limiting the number of shares of Common Stock which may be included
in this registration statement (a “Cutback Comment”) or which may be resold by
the holders of registrable Common Stock in accordance with Rule 144 under the
Securities Act. We shall keep this registration statement effective and up to
date for two (2) years from the date it is declared effective by the SEC or
until Rule 144 is available to the investors in the Private Placement Offering
with respect to all of their shares of registrable Common Stock, whichever is
earlier.
-68-
The
holders of the Investor Warrants and the Placement Agent Conversion Warrants, as
well as the holders of any shares of Common Stock removed from the registration
statement described above as a result of a Cutback Comment (but not the
Restricted Holders (as defined below)), shall have “piggyback” registration
rights for the shares of Common Stock underlying such warrants with respect to
any registration statement filed by us following the effectiveness of the
registration statement described above, which would permit the inclusion of such
underlying shares.
All
officers, directors, stockholders holding ten percent (10%) or more of our
Common Stock after giving effect to the Merger, the Split-Off and the Private
Placement Offering and our key employees (each a “Restricted Holder,” and
collectively, the “Restricted Holders”), have entered into lock-up agreements
with us for a term of eighteen (18) months following the date of the closing of
the Merger during which time no Restricted Holder will offer or sell any shares
of Common Stock owned by such Restricted Holder, except to another Restricted
Holder. The lock-up agreements entered into by Clearwater Partners,
LLC and Angelo Tomasello do not apply to any shares our Common Stock or any
Investor Warrant issued to Clearwater Partners, LLC or Mr. Tomasello upon
consummation of the Merger in exchange for the Units and warrant of 22nd Century
contained in the PPO Securities purchased by Clearwater Partners, LLC or Mr.
Tomasello in the Private Placement Offering nor to any shares of our Common
Stock issued to Clearwater Partners, LLC or Mr. Tomasello upon exercise of any
Investor Warrant.
In
addition, for a period of eighteen (18) months following the closing of the
Merger, we will not register or take any action to facilitate registration under
the Securities Act of the shares of Common Stock issued pursuant to the Merger
to the Restricted Holders.
Liability
and Indemnification of Directors and Officers
Nevada Revised Statutes Sections
78.7502 and 78.751 provide us with the power to indemnify any of our directors
and officers. The director and officer must have conducted himself or
herself in good faith and reasonably believe that his or her conduct was in, or
not opposed to, out best interests. In a criminal action, the
director, officer, employee, or agent must not have had reasonable cause to
believe that his or her conduct was unlawful.
Under Nevada Revised Statutes Section
78.751, advances for expenses may be made by agreement if the director or
officer affirms in writing that he or she believes that he or she has met the
statutory standards and will personally repay the expenses if it is determined
that such officer or director did not meet the statutory standards.
Our amended and restated articles of
incorporation allow for indemnification of directors and officers to the maximum
extent permitted by the Nevada Revised Statutes.
-69-
Insofar as indemnification for
liability under the Securities Act may be permitted for our directors, officers
and controlling persons pursuant to the foregoing provisions, or otherwise, we
have been advised that in the opinion of the SEC such indemnification is against
public policy as expressed in the Securities Act and is, therefore,
unenforceable.
Future
Stock Issuances
We intend to engage one or more
third-parties to provide investors’ relations services to the
Company. In addition to other consideration, such third-parties may
be compensated with warrants to purchase up to 250,000 shares of Common
Stock.
Except as expressly set forth herein or
pursuant to our equity incentive plan, we have no current plans to issue any
additional shares of our capital stock.
Trading
Information
The
Common Stock is quoted on the OTC Bulletin Board under the symbol
“XXII.OB.”
The
transfer agent and registrar for the Common Stock is Continental Stock Transfer
& Trust Company, 17 Battery Place, 8th Floor,
New York, NY 10004. We will serve as warrant agent for the
outstanding warrants.
|
Item
4.01
|
Changes
in Registrant’s Certifying
Accountant
|
On January 27, 2011, our board of
directors approved the dismissal of Child, Van Wagoner & Bradshaw, PLLC
(“Child”) as our independent registered public accounting firm and engaged Freed
Maxick & Battaglia, PC (“Freed”) as our independent registered public
accounting firm, both effective as of January 27, 2011. Freed was the
independent registered public accounting firm of 22nd Century prior to the
Merger and, given that the business of 22nd Century is now our sole line of
business, our board of directors concluded that Freed should serve as our
independent registered public accounting firm.
Child’s report on our financial
statements for each of the past two fiscal years ended September 30, 2010 and
2009 did not contain an adverse opinion or disclaimer of opinion, nor was it
qualified or modified as to uncertainty, audit scope or accounting principles,
except that the report was qualified as to our ability to continue as a going
concern.
During the fiscal years ended September
30, 2010 and 2009 and the subsequent interim period through January 27, 2011,
there were no: (i) disagreements with Child on any matter of accounting
principles or practices, financial statement disclosure, or auditing scope of
procedure which, if not resolved to the satisfaction of Child, would have caused
Child to make reference to the matter in their report, or (ii) reportable events
as defined in Item 304(a)(1)(v) of Regulation S-K.
During the fiscal years ended September
30, 2010 and 2009 and the subsequent interim period through January 27, 2011,
neither 22nd Century Group, Inc. nor anyone acting on its behalf
consulted Freed regarding either: (i) the application of accounting principles
to a specific transaction, either completed or proposed, or the type of audit
opinion that might be rendered on our financial statements; or (ii) any matter
that was either the subject of a disagreement (as defined in Item 304(a)(1)(iv)
of Regulation S-K) or a reportable event (as described in Item 304(a)(1)(v) of
Regulation S-K).
|
Item
5.01
|
Changes
in Control of Registrant
|
Reference is made to the disclosure set
forth under Item 2.01 of this Current Report on Form 8-K, which disclosure is
incorporated herein by reference.
-70-
|
Item
5.02
|
Departure
of Directors or Certain Officers; Appointment of Certain Officers;
Compensatory Arrangements of Certain
Officers
|
Reference is made to the disclosure set
forth under Item 2.01 of this Current Report on Form 8-K, which disclosure is
incorporated herein by reference.
|
Item
5.03
|
Amendments
to Articles of Incorporation or Bylaws; Change in Fiscal
Year
|
On November 21, 2010, our board of
directors approved an amendment to our articles of incorporation, recommending
(i) a change of our name from “Touchstone Mining Limited” to “22nd Century
Group, Inc.”, (ii) increasing our authorized capitalization from 100,000,000
shares, consisting of 100,000,000 shares of common stock, $0.00001 par value per
share, to 310,000,000 shares, consisting of 300,000,000 shares of common stock,
$0.00001 par value per share and 10,000,000 shares of preferred stock, $0.00001
par value per share, and (iii) limiting the liability of the Company’s officers
and directors to the Company, its stockholders and creditors to the fullest
extent permitted by Nevada law. On November 21, 2010, stockholders
representing the requisite number of votes necessary to approve the amendment to
our articles of incorporation took action via written consent, approving the
above listed actions. On November 23, 2010, we filed our Amended and
Restated Articles of Incorporation with the Secretary of State of the State of
Nevada.
|
Item
5.06
|
Change
in Shell Company Status
|
As a result of the consummation of the
Merger described in Item 1.01 and Item 2.01 of this Current Report on Form 8-K,
we ceased to be a shell corporation, as that term is defined in Rule 405 of the
Securities Act and Rule 12b-2 of the Exchange Act, as of the closing date of the
Merger.
|
Item
9.01
|
Financial
Statements and Exhibits
|
(a) Financial
Statements of the Businesses Acquired
In accordance with Item 9.01(a), (i)
22nd Century’s audited financial statements for the fiscal year ended December
31, 2009 and 2008 are filed in this Current Report on Form 8-K as Exhibit 99.1
and (ii) 22nd Century’s unaudited financial statements for the three and nine
month interim periods ended September 30, 2010 and 2009 are filed in this
Current Report on Form 8-K as Exhibit 99.2.
(b) Pro
Forma Financial Information
In accordance with Item 9.01(b), our
pro forma financial statements are filed in this Current Report on Form 8-K as
Exhibit 99.3.
(d) Exhibits
The exhibits listed in the following
Exhibit Index are filed as part of this Current Report on Form 8-K.
|
Exhibit No.
|
Description
|
|
|
2.1
|
Agreement
and Plan of Merger and Reorganization dated as of January 25, 2011 by and
among Parent, 22nd Century, and Acquisition Sub.
|
|
|
2.2
|
Certificate
of Merger dated as of January 25, 2011 Acquisition Sub. with and into 22nd
Century
|
|
|
3.1(1)
|
Certificate
of Incorporation of Parent
|
|
|
3.2(2)
|
Amended
and Restate Certificate of Incorporation of Parent
|
|
|
3.3(1)
|
Bylaws
of Parent
|
|
|
10.1(3)
|
Parent
2010 Equity Incentive
Plan
|
-71-
|
10.2
|
Form
of Securities Purchase Agreement dated as of January 25, 2011 by and among
22nd Century, the purchaser(s) identified on the signature pages thereto
and Parent, solely for the purposes of Section E and Section G thereof, as
amended.
|
|
|
10.3
|
Form
of Conversion Agreement
|
|
|
10.4
|
Form
of Warrant dated as of January 25, 2011 issued to LLC members of 22nd
Century prior to the consummation of the Private Placement Offering upon
consummation of the Merger
|
|
|
10.5
|
Form
of Warrant dated as of January 25, 2011 issued to investors in the Private
Placement Offering upon consummation of the Merger
|
|
|
10.6
|
Form
of Warrant dated as of January 25, 2011 issued to the Placement Agent and
Sub-Agent upon consummation of the Merger
|
|
|
10.7
|
Advisor
Warrant dated as of January 25, 2011 issued to the Placement Agent in
connection with that certain Advisory Agreement dated as of January 25,
2011 by and between Parent and the Placement Agent
|
|
|
10.8
|
Advisory
Agreement dated as of January 25, 2011 by and between Parent and the
Placement Agent
|
|
|
10.9
|
Placement
Agency Agreement dated as of December 1, 2010 by and between 22nd Century
and the Placement Agent
|
|
|
10.10
|
Escrow
Agreement dated as of December 2, 2010 by and among 22nd
Century, the Placement Agent and Bank of America, National
Association
|
|
|
10.11
|
Split-Off
Agreement dated as of January 25, 2010 by and among Parent, Touchstone
Split. Corp and David
Rector
|
|
10.12
|
Letter
from Paramount Strategy Corp dated as of December 21, 2010 regarding loan
forgiveness
|
|
|
10.13
|
Letter
from Milestone Enhanced Fund Ltd. dated as of December 28, 2010 regarding
loan forgiveness
|
|
|
10.14
|
Letter
from Mark Tompkins dated as of January 25, 2011 regarding loan
forgiveness
|
|
|
10.15
|
Employment
Agreement dated as of January 25, 2011 by and between Parent and Joseph
Pandolfino
|
|
|
10.16
|
Employment
Agreement dated as of January 25, 2011 by and between Parent and Henry
Sicignano III
|
|
|
10.17
|
Employment
Agreement dated as of January 25, 2011 by and between Parent and C.
Anthony Rider
|
|
|
10.18
|
Form
of Lock-Up Agreement
|
|
|
14.1(4)
|
Code
of Ethics
|
|
|
16.1
|
Letter
from Child, Van Wagoner & Bradshaw, PLLC regarding change in
independent registered public accountants.
|
|
|
17.1
|
Letter
from David Rector dated as of January 25, 2011 resigning as a director and
officer of
Parent
|
-72-
|
99.1
|
22nd
Century financial statements for the fiscal years ended December 31, 2009
and 2008
|
|
|
99.2
|
22nd
Century financial statements for the three and nine months ended September
30, 2010 and 2009 (unaudited)
|
|
|
99.3
|
Pro
forma unaudited consolidated financial statement as of September 30, 2010
for the nine months ended September 30, 2010 and the year ended December
31,
2009
|
(1)
Incorporated herein by reference to Exhibit 3.1 of the Company’s Registration
Statement on Form SB-2 filed with the Commission on December 27,
2005.
(2)
Incorporated herein by reference to Exhibit 3.2 of the Company’s Annual Report
on Form 10-K for the year ended September 30, 2010 filed with the Commission on
November 2, 2010.
(3)
Incorporated herein by reference to Appendix B of the Company’s Definitive
Information Statement on Schedule 14C filed with the Commission on November 2,
2010.
(4)
Incorporated herein by reference to Exhibit 14.1 of the Company’s Annual Report
on Form 10-KSB for the year ended September 30, 2006 filed with the Commission
on December 20, 2006.
-73-
SIGNATURES
Pursuant
to the requirements of the Securities Exchange Act of 1934, the Registrant has
duly caused this Current Report to be signed on its behalf by the undersigned,
hereunto duly authorized, on the 31st day
of January, 2011.
| 22nd CENTURY GROUP, INC. | ||
|
By:
|
 |
|
|
Name:
Joseph Pandolfino
|
||
|
Title:
Chief Executive
Officer
|
||
-74-
