Attached files
| file | filename |
|---|---|
| 8-K - Xtant Medical Holdings, Inc. | v209694_8k.htm |

“Bacterin biologics provides me with a reliable, cost-effective source for allograft tissue.
The quality of their allografts allows my patients to achieve the functional
results my
patients demand.” – Dr. David Mansfield
“OsteoSponge ® has
allowed my patients to heal faster; thus enabling them to get back
to their regular activities and improving their quality of life.”
– Dr. David Yeager
Investor Presentation
February 2011

SAFE HARBOR STATEMENT
Statements in this presentation are forward-looking statements
as that term
is defined in the Private Securities Litigation
Reform Act of 1995. These forward-looking statements are
based on current expectations that could be affected by risks
and uncertainties from time to time described in Bacterin
International Holdings,
Inc’s reports to the Securities and
Exchange Commission, which include Bacterin International
Holdings, Inc’s. Quarterly Reports on Form 10Q and 8k filings.
2
Bacterin International Holdings, Inc.
(BIHI.OB)
600 Cruiser
Lane
Belgrade, MT 59714
www.Bacterin.com

SNAPSHOT: BIHI
HISTORY: 1998 - Company Founded.
1999-2006 - DoD/Grant Funded - $4.1 million.
2007 - First revenue year - $2.3 million.
2008 - Labs/facilities ready for production.
2009 - Initiate marketing and direct sales force.
2010 - OTC BB Listing July 1, 2010.
HEADQUARTERS: Belgrade, MT
SALES: Denver, CO
EMPLOYEE COUNT: 115 (36 prod & dev, 67 Sales & mkt, 12 Admin)
SHARES OUTSTANDING/F.D.: 37.1 million / 49.5 million
OPTIONS / WARRANTS: 4.4 mil (avg. $1.38) / 8.0mil (avg. $2.08)
INSIDE OWNERSHIP: 32% Fully Diluted
As of September 30, 2010
CASH/Acct Rec/Inventory: $ 0.6 million / $2.6 million / $7.0 million
LONG TERM DEBT $ 293,000
SHAREHOLDERS’ EQUITY: $ 1.1 million
Gross Margin: 83%
Recurring Revenues 80+%
3
Latest News
1/20/11 BIHI Secures $5 million Credit Facility with
Bridge Bank.
1/11/11 BIHI Hires Biologics VP of Business
Development with Strong
Orthopedics Background.
11/22/10 BIHI Secures Up to $9 million of Potential
$14 million Financing - up to $8.0 million
in
asset based lending facility , $1.0 million
through exercise of warrants and $5 million
accts receivable credit line proposal.
11/15/10 BIHI Reports Q3 2010; Delivers
Record Revenue with 200% year-
over-year and 31% quarter-over-
quarter growth. BIHI hits EBITDA
breakeven in September.
11/04/10 MT Senator Baucus to award BIHI $237,000
grant for antimicrobial orthopedic surgical
fixation devices.
9/08/10 BIHI to Launch Dermal Scaffold
Product Line
Called hMatrix.
8/10/10 BIHI and RyMed Collaborate and Receive
FDA Consent to Commercialize
Antimicrobial Needleless Connector.
8/05/10 BIHI Closes Private Placement with
Total Raise of $9.2
million.
7/01/10 Bacterin Begins Trading as a Public
Company.
Over 40,000 grafts implanted to date
Bacterin is an accredited tissue bank and medical device
company that designs, processes, manufactures and
markets biological scaffolds for tissue regeneration.
* Assumes no additional non cash warrant derivative liability charges.

BACTERIN
BIOLOGICS DIVISION
4
State-of-the-Art Fully Compliant FDA Registered
- 32,000 sq./ft. production facility
- Four Class 100 Clean Rooms
- Passed 3 FDA Audits (No Warnings or “483’s”)
- Passed 2 AATB Audits ( “A” Rating)
- HCP/T licensures in CA, NY, FL, MD
- Throughput capacity > $ 120 Million/yr. – 2 Shifts

CORE TECHNOLOGY
Human acellular biological scaffolds that can incorporate the patient’s own
stem cells or bioactive agents for accelerated regeneration of bone, skin, and
cartilage.
Accelerates fusion, healing, and repair.
100% human donor derived minimally
processed tissue.
Technology preserves natural growth factors.
The interconnected, porous structure makes
it an ideal scaffold for cellular ingrowth and
proliferation.
Proprietary process with patents pending.
5
Bacterin scaffolds enable surgeons to address regeneration of the damaged
site earlier in the treatment continuum.

BONE SCAFFOLD: OSTEO LINE
OsteoSponge® is on-label for use as a bone graft substitute anywhere
in the human body by all disciplines
of surgeons.
6
OsteoSelect® DBM Putty
Putty-like, can encapsulate bone marrow aspirate and will not leach or migrate
away from site.
OsteoLock® / BacFast® HD
OsteoLock® used to augment spine surgery or as stand alone for
interventional surgery, BacFast HD® is a hyper-demineralized version
with
greater bone generation properties.
Bacterin received the 2009 Spine Technology Award in the Regenerative Technology
Category for the proprietary biologic scaffold OsteoSponge® Demineralized Bone Matrix.
OsteoWrap®
Upon hydration, OsteoWrap ® can be easily bent, folded, or wrapped for
essential
product placement and can be easily sized in the operating room
using sterile scissors or a scalpel and can hold sutures
Total U.S. Bone Repair Addressable Market - $3.5 Billion Annually
OsteoSponge®
OsteoSponge® is on-label for use as a bone void filler anywhere in the human
body by all disciplines of surgeons. It is one-third the cost, statistically proven
clinically equivalent
recombinant protein and far easier for surgeon to handle
and keep in place in the body.

CARTILAGE (EX-US) SCAFFOLD:
OsteoSponge® SC
Osteosponge® SC approved as subchondral bone void filler.
In market today and being used.
Beginning trials for approval to expand claim for primary repair of
osteochondral defect (OCD).
Preliminary results in humans 3-6 months.
Final results expected in 12 months.
Multiple site, prospective, randomized study currently under way.
OCDs of knee, foot and ankle.
7
U.S. ADDRESSABLE MARKET - $1.7 Billion Annually (Growth Market)

Human acellular dermal matrix
Preserves natural growth factors inherent to
scaffold and supports tissue regeneration.
Used in wound care repair and diabetic
ulcers.
Excellent handling characteristics.
Processing and sales synergies within
Bacterin.
Can be extended to non-homologous uses
with 510K application; rotator cuff, tendon
augmentation, etc.
Product Launch Q1 2011.
REGULATED IN THE TISSUE BANKING DOMAIN UNDER HCT/P
U.S. ADDRESSABLE MARKET - $2.6 Billion Annually
DERMAL SCAFFOLD:
hMatrix™
8

BIOLOGICS POTENTIAL
Total U.S. Addressable Market - $8.5 Billion
9
Annual
Ave Product
Total
Number of
Revenue Per
Market
Product
US Procedures
Procedure
Potential
Spine
ALIF
Sponges, Strips, Putty, Structural Allograft
51,124
$4,000
204,496,000
$
PLIF
Sponges, Strips, Putty, Structural Allograft
25,380
$4,000
101,520,000
$
TLIF
Sponges, Strips, Putty, Structural Allograft
53,292
$4,000
213,168,000
$
Cervical
Sponges, Strips, Putty, Structural Allograft
135,795
$1,100
149,374,500
$
Pain Management
Minimally invasive facet stabilization
BacFast
10,000
7,800
78,000,000
$
Neurosurgery
Craniotomy
Cranial plugs
23,000
$4,000
92,000,000
$
Craniomaxillofacial
Strips, Wrap, Putty
10,000
$4,000
40,000,000
$
Foot and Ankle
Hallux Valgas (Bunionectomy)
OsteoSponge SC/Sponge
250,000
$1,200
300,000,000
$
Hallux Rigidis (OCD Big Toe)
OsteoSponge SC/Sponge
775,000
$1,000
775,000,000
$
Ankle Fusions
Sponges/Strips/Wrap/Putty
18,000
$4,000
72,000,000
$
Charcot-Diabetic Related Foot Fusions
Sponges/Strips/Wrap/Putty
100,000
$12,000
1,200,000,000
$
Sports Med
Osteochondral Defects of the Knee
OsteoSponge SC/Disposable MIS Kit
209,079
$3,000
627,237,000
$
ACL Repair
Soft Tissue
125,000
$1,700
212,500,000
$
Hip and Knee Revisions
Sponges/Strips/Wrap/Putty
102,690
$6,000
616,140,000
$
Trauma -non unions are 10% of fractures
Sponges/Strips/Wrap/Putty
620,000
$2,000
1,240,000,000
$
Diabetic Ulcers, wound repair
hMatrix
500,000
$2,000
1,000,000,000
$
Hernia Mesh Repair
hMatrix
200,000
$4,000
800,000,000
$
Breast Reconstruction
hMatrix
200,000
$4,000
800,000,000
$
8,521,435,500
$

BACTERIN
MEDICAL DEVICE
AND COATINGS DIVISION
10
State-of-the-Art Fully Compliant FDA & ISO Registered
- 12,000 sq./ft. production facility
- Two Class 100 Clean Rooms
- ISO Class 7 Environmentally Controlled Areas

CORE TECHNOLOGY
Patented technology applies a micron-thin coating to medical
devices creating an anti-microbial barrier against infection.
Infection post-surgery is common for implanted medical devices.
Institute of Medicine (Washington,D.C.) reports 44,000-98,000
deaths annually due to hospital-originated infections.
Hospitals within 28 States are now required to publish infection
rates. This practice is expected to be applied nationwide.
October 2008, hospitals are no longer reimbursed by Medicare and
Medicaid for the treatment of staph infection -- approximate cost
per incident is $30,000.
Bacterin’s medical devices division develops medical devices
intended for use in several diverse clinical areas including
orthopedic, plastic and cardiovascular surgery.
11

August 2010 FDA approval for
needleless connectors. Product launch
Q1 2011.
Needleless connector market –
approximately $2 Billion annually.
Bacterin’s anti-infective coating
addressable market approximately
$240 million.
Joint development project with RyMed.
Bacterin Technology –
chlorhexidine/silver ion engineering.
PRODUCTS
Elutia® Coated Wound Drains
The Elutia® family of wound drains
feature a micron-thin coating that is
intended
to inhibit microbial
contamination of the drain.
Bacterin’s first FDA approved product.
In 2010, Brook Army Medical Institute.
InVision-Plus® CS™ Connector
12

BACTERIN
LEADERSHIP – FINANCIALS
GROWTH STRATEGY
13

MANAGEMENT TEAM
Guy Cook (45), Founder, CEO – 20 years managerial experience in industry, considered international expert in
biofilm science; previous president
of Delta Resources focused on customized image analysis solutions;
confocal microscopist for the Center for Biofilm Engineering (Montana State University). Board member of West
Coast Tissue Services and American Donor Services.
John Gandolfo (50), CFO – 25 years of senior management experience, CFO of public and private companies
with a primary focus in
the life sciences and medical device areas; previously CFO of Progenitor Cell Therapy,
Power Medical, Bioject, Impath and Medical Resources, Inc.
Jesus Hernandez (52), VP of Biologics – 30 years of organ/tissue bank experience; previously Director of the
Organ and Tissue Bank at the
University of California Irvine Medical Center; served as CEO and COO for two
national tissue banks.
Darrel Holmes (55), VP of Devices – 25 years technical operations experience in device/diagnostics industry;
previously Director of Operations
for American Qualex, HYCOR Biomedical and Stratagene (now Agilient
Technologies).
Robert Taggart (48), National Sales Manager – 25 years of sales management
experience; previously principle
of RAF Financial, where he grew revenue from $5 million to $70 million in 2 years. Mr. Taggart provided the
initial professional capital for Bacterin and joined the company full time in November 2009.
Steven M. Scott, M.D.,M.B.A.
, Chairman of Bacterin Scientific Advisory Board - Salt Lake City orthopedic
practice, member of the American Academy
of Orthopedic Surgeons, the Musculoskeletal Tumor Society and
the Pediatric Society Orthopedic of North America, authored many scientific publications. University of Colorado
Medical School, orthopedic training at the University of Utah and
Mayo Clinic.
Middle management team includes 3 PhDs and have all been with the company
through the development stage.
14

15
Guy Cook, Chairman, CEO, President and Chief Scientific Officer
Expert in biofilm science and its application. Product specialist in the Image Analysis Department
for Laboratory
Equipment Company in Chicago; President of Delta Resources in Crystal Lake, Illinois(specialized in developing
customized image analysis solutions for the academic community); Confocal Microscopist for the Center for Biofilm
Engineering at the Montana State University (developed proprietary med device testing models).
Mitchell T. Godfrey, Director, Secretary and Treasurer, and Compensation Committee Chairman
25 years consulting and participation in firms in the manufacturing, medical devices, nuclear,
service and
animal health industries; Lieutenant in the U.S. Navy for a period of four years in the 1960s; Director of the Utah
Vietnam Agent Orange Program; Chairman of the Montana based Crow Creek Falls Conservation Group.
Michael Lopach, Director and Audit Committee Chairman
Served 27 years with Galusha, Higgens, Galusha & Co., the largest
privately held accounting firm in Montana
and northern Idaho, a firm establish in 1919 that employs 60 accounting professionals in six offices, where he served
as president and CEO. In 1999, founded Lopach & Carparelli PC, an accounting
firm that focuses on medical
practitioners. Certified public account ; University of Notre Dame MBA.
Kent Swanson, Director
Senior Partner of Accenture (retired after 32 years); Board Chair of ALN Medical Management;
Board Chair for
Boys Hope Girls Hope of Colorado; Board member, Audit Committee member and Compensation Committee Chair for
MPC Computers(2002 to 2009); University of Chicago MBA (1969).
Jon M. Wickwire, Director and Chairman of Corporate Governance and Nominating Committee
Attorney and founding shareholder of Wickwire Gavin, P.C., a national construction
law firm, which merged
with Akerman Senterfitt, one of the top 100 law firms in the U.S. Served as lead counsel on major infrastructure
litigation and alternative dispute resolutions throughout his 30 year career. Graduate of the
University of Maryland
and Georgetown University Law Center.
BOARD OF DIRECTORS
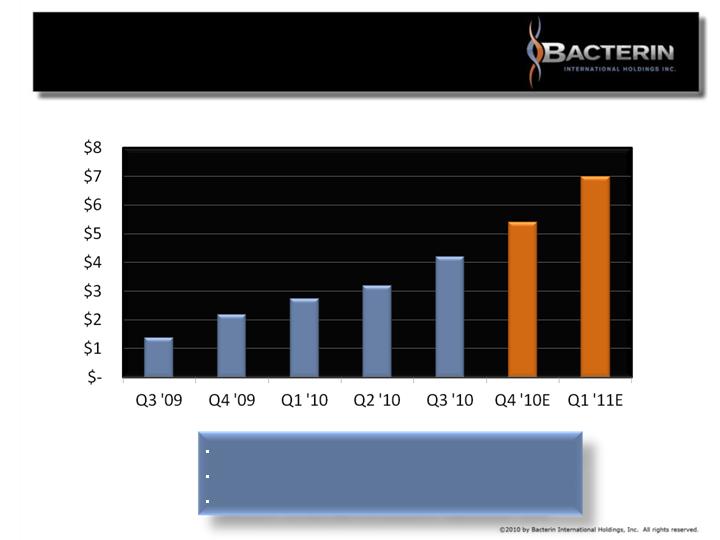
BACTERIN QUARTERLY
REVENUE GROWTH
Revenues in US$ millions
16
Bacterin consistently generates gross margin of 80%+
Bacterin generates 80%+ recurring revenue
Bacterin turned EBITDA positive September 2010
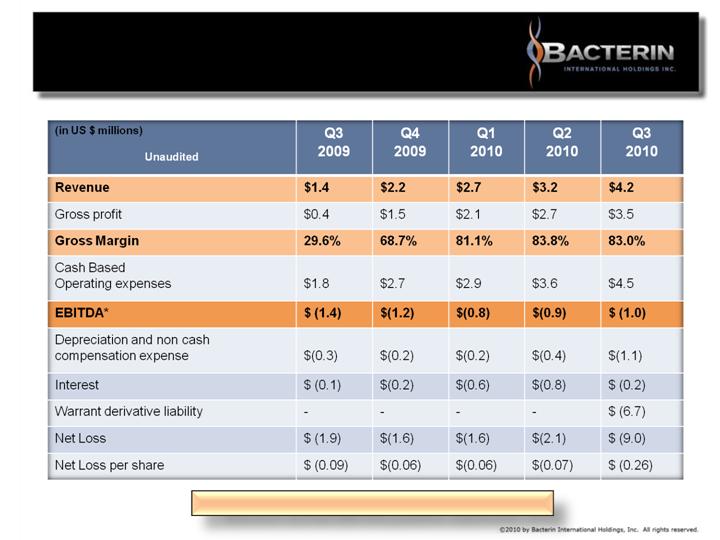
ABBREVIATED QUARTERLY
INCOME STATEMENT
17
* Bacterin turned EBITDA positive September 2010

CONDENSED BALANCE SHEET
Cash
$ 571,844
Receivables
2,560,692
Inventory
6,971,792
Notes receivable - trade
518,905
Prepaid and other current assets
221,567
Property and Equipment
3,117,439
Intangibles and other
639,257
Total Assets
$ 14,601,496
Accounts Payable
$ 1,749,938
Accrued liability and other
1,367,972
Warrant derivative liability
7,429,968
Notes Payable
1,119,375
Convertible Notes Payable
393,834
Current Portion of Long Term debt and other
1,133,305
Long Term liabilities
292,800
Total Liabilities
13,487,192
Total Stockholders’ Equity
1,114,304
Total Liabilities and Stockholders’ Equity
$ 14,601,496
September 30, 2010
18
11/22/10 BIHI Secures Up to $9 million of Potential $14 million Financing - up to $8.0 million in asset based lending
facility , $1.0 million through
exercise of warrants and $5 million accts receivable credit line (effective 1/20/11).

CAPITALIZATION TABLE
Directors and
Executive Officers
Common
Shares
Warrants
Avg.
Exercise
Price
Options
Avg.
Exercise
Price
Fully
Diluted
Shares
%
Ownership
Guy Cook, CEO
13,207,000
121,000
25,000
13,353,000
26.9%
Members of the Board
1,321,000
270,000
150,000
1,741,000
3.5%
John Gandolfo, CFO
-
-
250,000
250,000
0.5%
Jesus Hernandez, EVP
-
-
558,000
558,000
1.1%
Darrel Holmes, EVP
10,000
-
150,000
160,000
0.3%
Sub-Total
14,538,000
391,000
1,133,000
16,062,000
32.3%
Employees
-
-
3,316,000
3,316,000
6.7%
Other (Retail)
22,733,000
7,606,000
-
30,339,000
61.0%
Shares Outstanding
37,271,000
7,997,000
$2.08
4,449,000
$1.38
49,717,000
100.0%
19
As of December 9, 2010

POTENTIAL 6 MONTH MILESTONES
Bacterin has successfully established a direct sales force to supplement a multiple
call
point sales initiative. Immediate results - 5 quarters of sequential revenue growth
with future growth expected to accelerate.
Direct Sales: 7 RVP - 21 reps (12/31/09) versus 1 NSM - 3 EVPs – 13 RVPs – 52 reps (12/31/10)
Independent Distributors: ~40 (12/31/09) versus 148 (12/31/10)
Broadlane Distribution Agreement : (June 2009) – “open door” to market product into 6,000 medical facilities.
Continued Balance Sheet improvement.
Second half 2010 EBITDA breakeven and 2011 profitability.
hMatrix launch expected Q1 2011.
OsteoSpongeSC Talar Dome Repair Study results / OsteoSponge 2 year follow up data
published.
Additional distribution agreements and/or joint ventures.
Expansion of core technology into additional product.
Additional hires – senior biologics and financial support personnel.
Increased participation at industry conventions and analyst presentations.
20

SUMMARY
Strong opportunities for revenue high growth.
Product line expansion.
Increased use by existing and new surgeons.
Strategic relationships and distribution partners.
Geographic expansion into International markets.
Business model is capable of generating superior margins (80%+ gross
margins), and recurring revenues (80%+).
High Barriers to entry (regulatory and product development expenses/patents)
with limited competition and first to market advantage in several markets.
Strong technical and scientific management team.
Continued industry consolidation.
21

THANK YOU
Bacterin Contacts:
Guy Cook, CEO 406-388-0480 gcook@bacterin.com
John Gandolfo, CFO 973-768-6784 jgandolfo@bacterin.com
Yvonne Zappulla, IR 212-681-4108 yvonne@grannusfinancial.com
22
