Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - REVA Medical, Inc. | a58390e8vk.htm |
Exhibit 99.1

| RevaTvI epical DESIGN FOR LIFE 29th Annual J.P. Morgan Healthcare Conference January 12, 2011 |

| Forward looking Statements The Company cautions you that the statements included in this presentation that are not a description of historical facts are forward-looking statements. These include, but are not limited to, statements regarding: the Company’s interpretation of the results of its prc-clinical trials, proposed regulatory approval and development timelines, future financial requirements and results. Neither the Company, nor its respective directors, officers and advisers or any other person makes any representation, or gives any assurance or guarantee that the occurrence of the events expressed or implied in any forward looking statements in this presentation will occur. Actual results may differ materially from those set forth in this presentation due to the risks and uncertainties inherent in the Company’s business, including, without limitation: the results of any future clinical trials for the ReZolve™ stent may not be favourable and may not support or be consistent with the Company’s interpretation of the results of earlier preclinical trials, the Company may never receive regulatory approval for the ReZolve™ stent; even if approved, the ReZolve™ stent may not achieve market acceptance, and the Company may have underestimated the cost and time required to complete future clinical trials. More detailed information about the Company and the risk factors that may affect the realisation of forward-looking statements is set forth in Section 10 of the Company’s Australian Prospectus and the “Risk Factors” section of the US Prospectus. Given these uncertainties, recipients are cautioned to not place undue reliance on such forward looking statements. Subject to any continuing obligations under applicable law or any relevant listing rules of the ASX, the Company disclaims any obligation or undertaking to disseminate any updates or revisions to any forward looking statements in this presentation to reflect any change in expectations in relation to any forward looking statements or any change in events, conditions or circumstances on which any such statement is based. Nothing in this presentation shall under any circumstances create an implication that there has been no change in the affairs of the Company since the date of this presentation. S jkijalijlCW jjfl 1/11/11 |
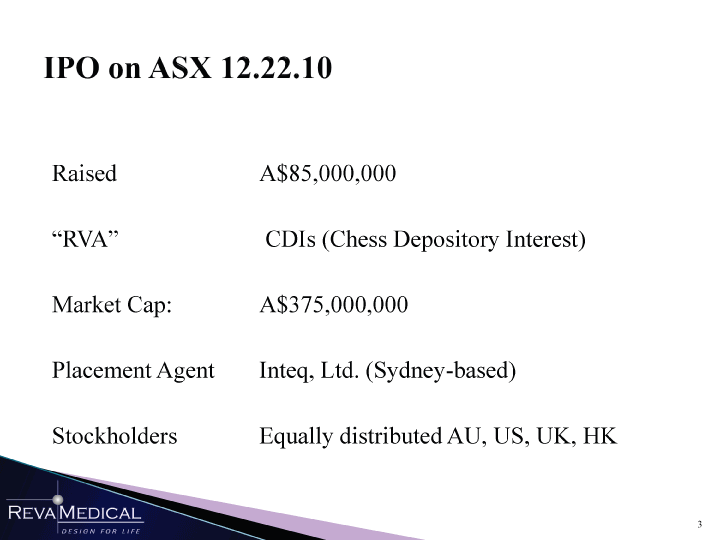
| IPO on ASX 12.22.10 Raised A$85,000,000 “RVA” CDIs (Chess Depository Interest) Market Cap: A$375,000,000 Placement Agent Inteq, Ltd. (Sydney-based) Stockholders Equally distributed AU, US, UK, HK JnniHaB l/ii/n |
| REstore VAscular Function 1/11/11 |

| jpi mam 1/11/11 |

| “Slide & Lock” L ; WÏÏ’ “L/( w%. w SmmmmSmSi 1/11/11 |
| Tyrosine-Derived Polycarbonate Licensed from Rutgers, all vascular applications Has a number of properties: strength, visibility under x-ray, and tunable Breaks down into tyrosine, carbon dioxide, and water MM MM . MMM 1/11/11 |
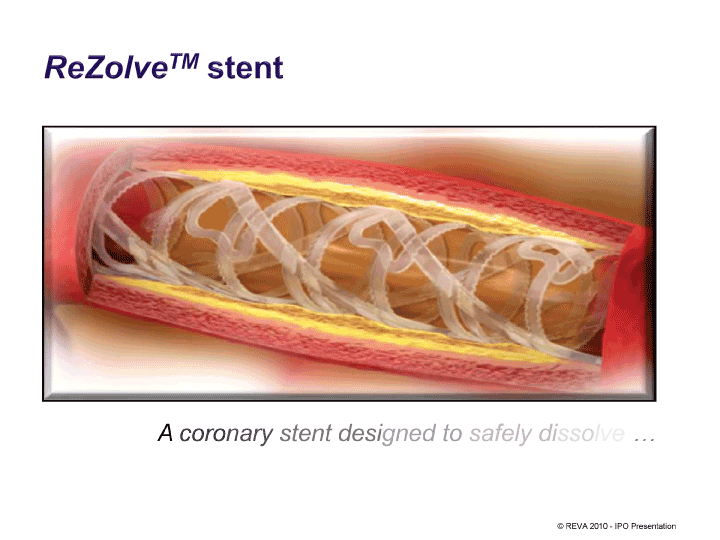
| ReZolve™ stent A coronary stent designed to safely di © REVA 2010 — IPO Presentation |

| Do We Care? Metallic stents work very well thin, deliverable, low MACE rates, low restenosis rates Since 1992: 45,000,000+ people have them So good, improvements are topping out and prices are commoditizing. Has the category matured? |
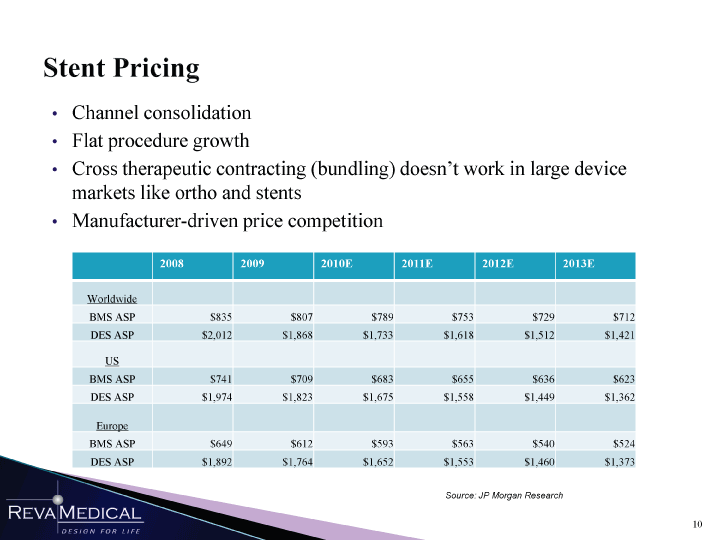
| Stent Pricing Channel consolidation Flat procedure growth Cross therapeutic contracting (bundling) doesn’t work in large device markets like ortho and stents Manufacturer-driven price competition Worldwide BMS ASP $835 $807 $789 $753 $729 $712 DES ASP $2,012 $1,868 $1,733 $1,618 $1,512 $1,421 US BMS ASP $741 $709 $683 $655 $636 $623 DES ASP $1,974 $1,823 $1,675 $1,558 $1,449 $1,362 Europe BMS ASP $649 $612 $593 $563 $540 $524 DES ASP $1,892 $1,764 $1,652 $1,553 $1,460 $1,373 Source: JP Morgan Research W mmSSmSSSm 1/11/11 |
| Stent’s Purpose: Allow artery to “remodel” , . MMn 1/11/11 |

| Our Band Aid Sff BiBWi iB 4W * m m TWP MBIHBH BBB . BBB 1/11/11 |
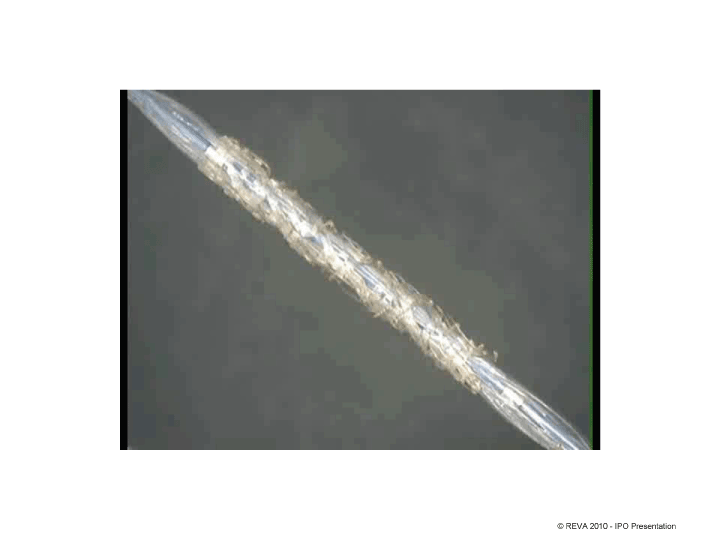
| © REVA 2010 — IPO Presentation |
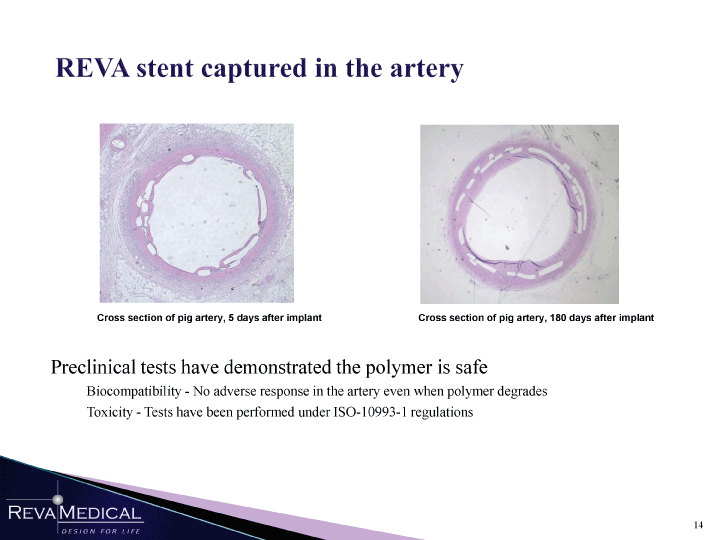
| REVA stent captured in the artery Cross section of pig artery, 5 days after implant Cross section of pig artery, 180 days after implant Preclinical tests have demonstrated the polymer is safe Biocompatibility — No adverse response in the artery even when polymer degrades Toxicity — Tests have been performed under ISO-10993-1 regulations |
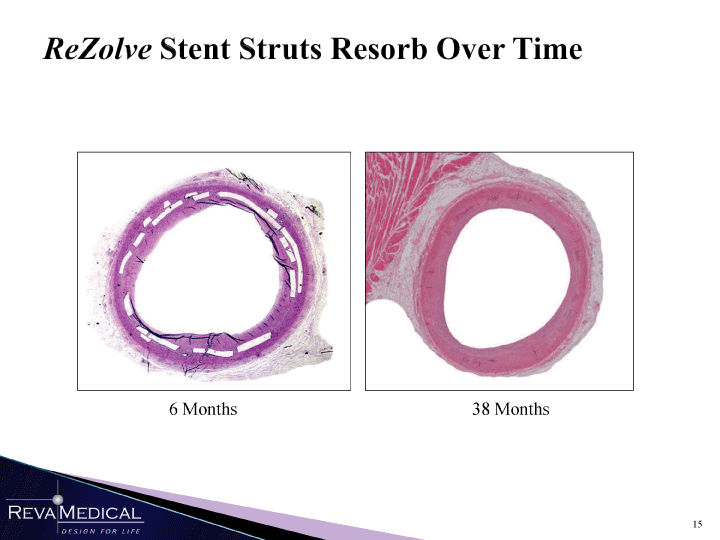
| ReZolve Stent Struts Resorb Over Time g fm 1 6 Months 38 Months MMM 1/11/11 |

| ReZolve Stent Struts Resorb Over Time 3 Mos * 52 Mos K HI HHÎ l/ii/ii |
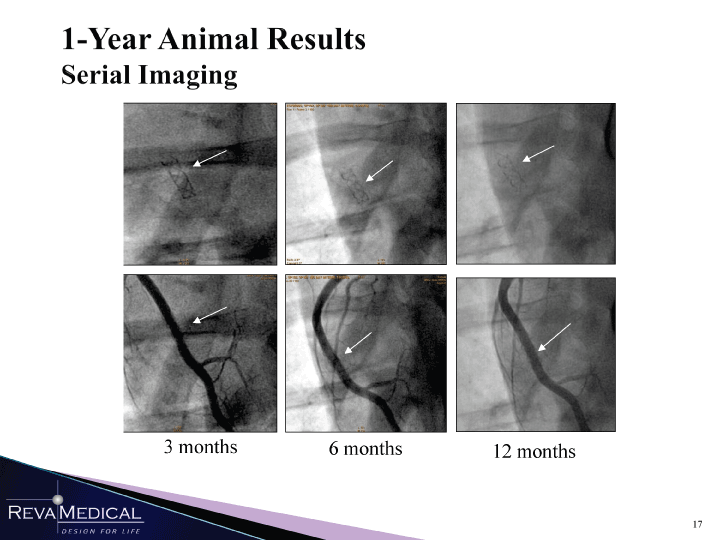
| 1-Year Animal Results Serial Imaging I F 1 3 months 6 months 12 months HI RBI 1/11/11 |
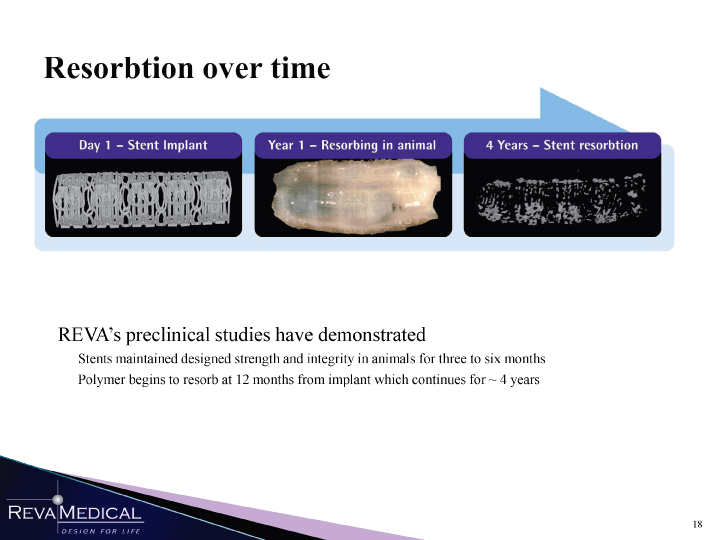
| Resorbtion over time R K W K r ff B m F W S S M K K W S REVA’s preclinical studies have demonstrated Stents maintained designed strength and integrity in animals for three to six months Polymer begins to resorb at 12 months from implant which continues for ~ 4 years MMM 1/11/11 |
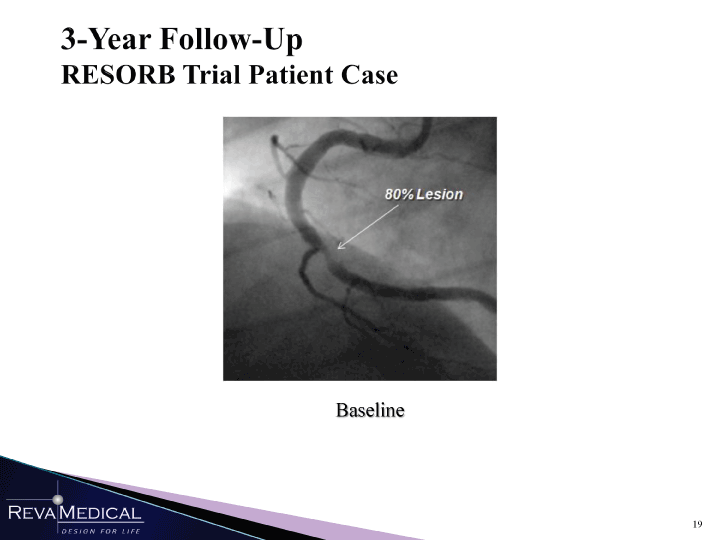
| 3-Year Follow-Up RESORB Trial Patient Case Baseline MMn 1/11/11 |
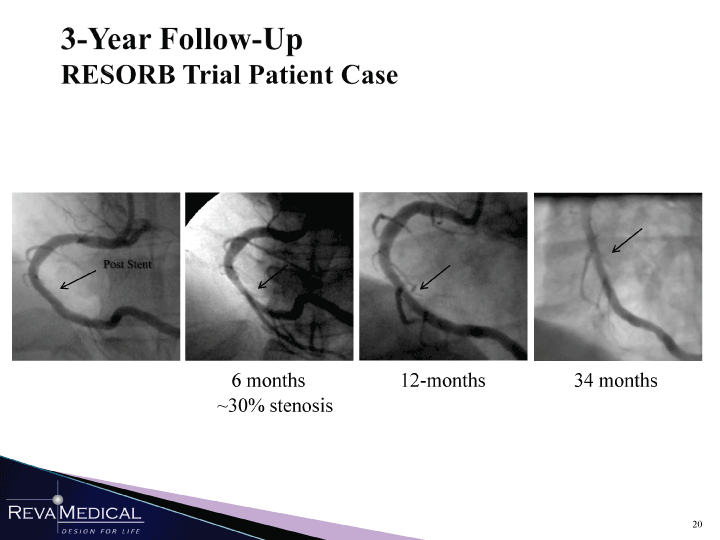
| 3-Year Follow-Up RESORB Trial Patient Case 6 months 12-months 34 months -30% stenosis MMn l/ii/ii |
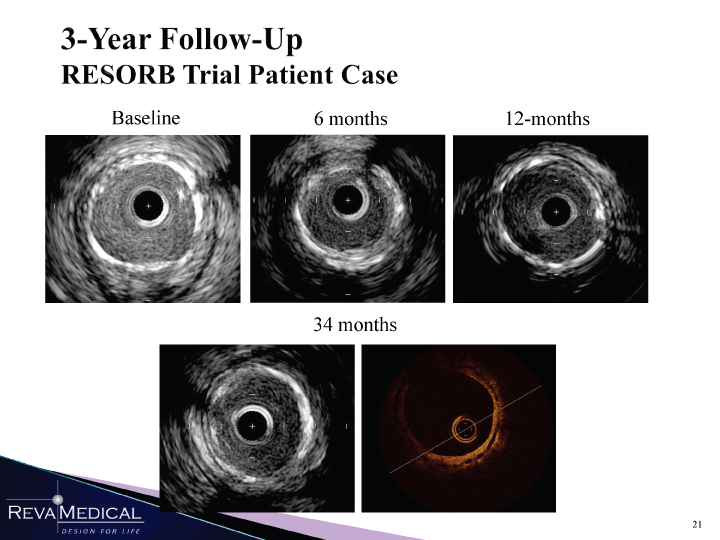
| 3-Year Follow-Up RESORB Trial Patient Case Baseline 6 months 12-months 34 months kJi ->_ 49» HI HHÎ 1/11/11 |
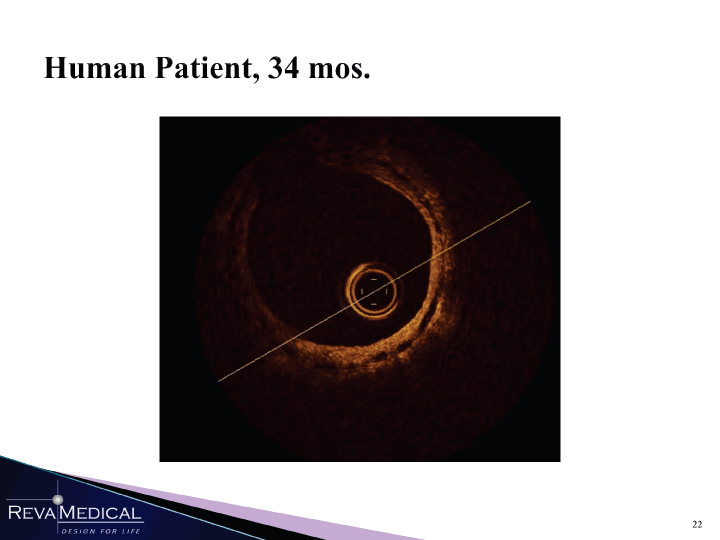
| Human Patient, 34 mos. V It 1 1 , H HI HHÎ 1/11/11 |

| Do We Care? MMn 1/11/11 |

| Do We Care? Industry sought a “temporary stent” when stents were conceived—25 years ago Late Stent Thrombosis affects .5% of the general population, and 3.5% of African Americans Can you have an “LST” if you don’t have a stent? Does the restoration of an artery’s contractility matter? Will DAPT be curtailed? wO ËmMmmmmmmmm 1/11/11 |

| We think so!! \ \ iData Research Nov 29, 2010 07:45 ET Survey Reveals Cardiologist Preference for Bioabsorbable Stents Will Fuel Intervention a I Cardiology Market by 2013 MM MM . MMM 1/11/11 |
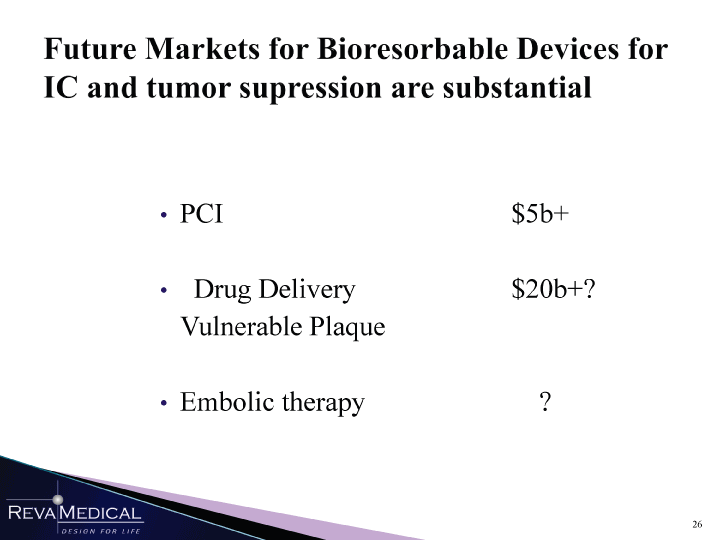
| Future Markets for Bioresorbable Devices for IC and tumor supression are substantial PCI $5b+ Drug Delivery $20b+? Vulnerable Plaque Embolic therapy ? MMH 1/11/11 |

| Can REVA deliver? HI HHÎ 1/11/11 |
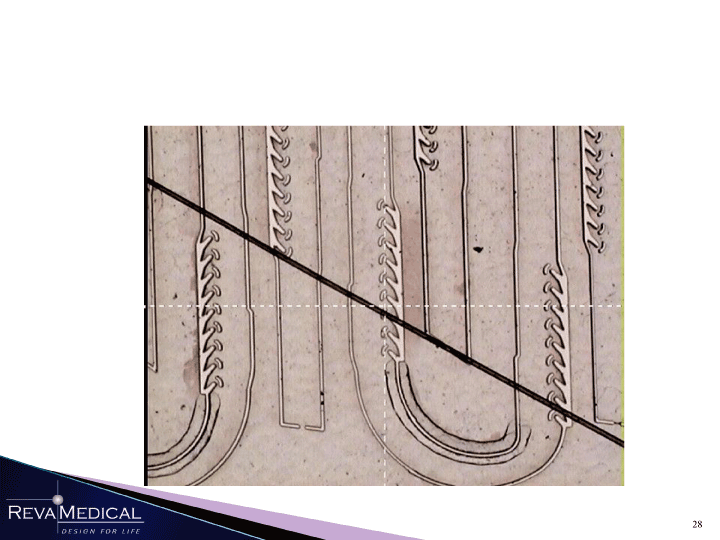
| jpi mam 1/11/11 |

| jpi mam 1/11/11 |
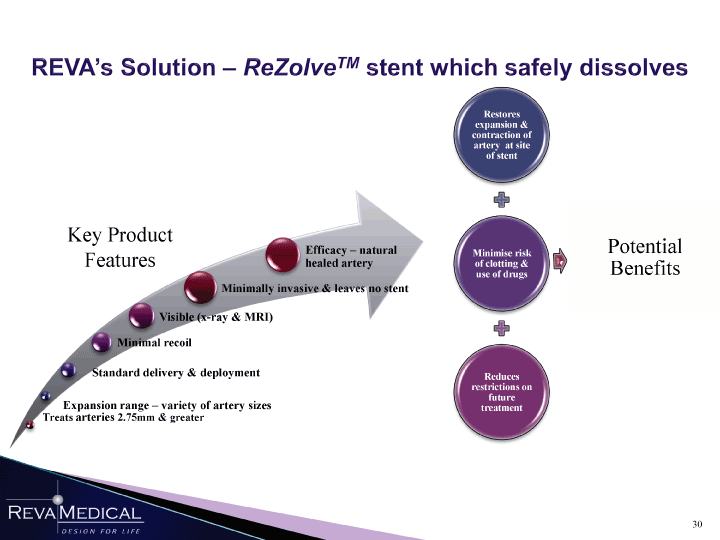
| REVA’s Solution — ReZolve™ stent which safely dissolves Efficacy — natural / \ jy Potential Features healed artery nimise r S Benefits A (l Minimally invasive & leaves no stent \ / fc Visible (x-ray & MRI) n A Minimal recoil « / Standard delivery & deployment à \ T Expansion range -variety of artery sizes \ treatmen / TTreats arteries 2.75mm & greater \ / |
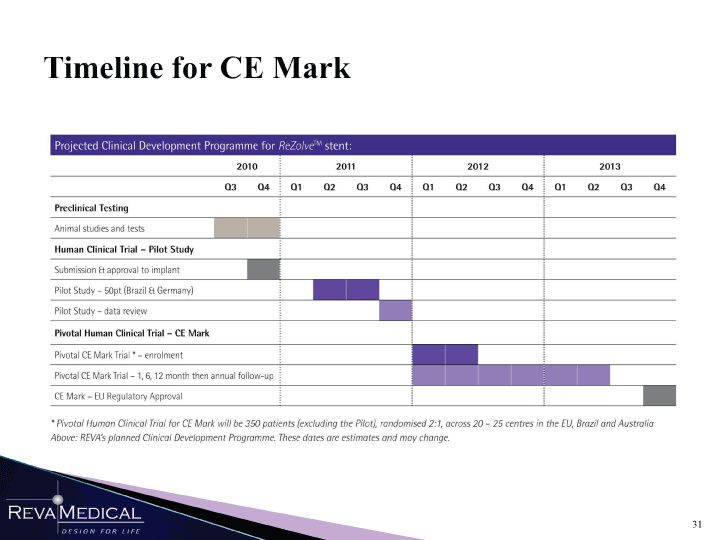
| Timeline for CE Mark 2010 2011 2012 2013 Q3 Q4 j Q1 Q2 Q3 Q4 ! Q1 Q2 Q3 Q4 ! Ql Q2 Q3 Q4 Preclinical Testing Animal studies and tests j Human Clinical Trial — Pilot Study Submission Et approval to implant Pilot Study — 50pt (Brazil Et Germany) j Pilot Study — data review B Pivotal Human Clinical Trial — CE Mark Pivotal CE Mark Trial’-enrolment j j Pivotal CE Mark Trial — 1, 6, 12 month then annual follow-up j CE Mark-EU Regulatory Approval j * Pivotal Human Clinical Trial for CE Mark will be 350 patients (excluding the Pilot), randomised 2:1, across 20 — 25 centres in the EU, Brazil and Australia Above: REVA’s planned Clinical Development Programme. These dates are estimates and may change. w mmSSmSSSm 1/11/11 |
| Thank you! MMM 1/11/11 |
