Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - CLINICAL DATA INC | b84305e8vk.htm |
Exhibit 99.1

| J. P. Morgan Healthcare Conference January 12, 2011 1 |

| Forward-Looking Statement This presentation contains forward-looking information and statements that are intended to be covered by the safe harbor for "forward looking" statements provided by the Private Securities Litigation Reform Act of 1995. Forward-looking statements are statements that are not historical facts. Words such as "expect(s)", "feel(s)", "believe(s)", "will", "may", "anticipate(s)" and similar expressions are intended to identify forward-looking statements. These statements include, but are not limited to, statements about our ability to obtain regulatory approval for, and successfully introduce, vilazodone, Stedivaze and our other drug candidates; our ability to expand our long-term business opportunities; and all other statements regarding future performance. All such information and statements are subject to certain risks and uncertainties, the effects of which are difficult to predict and generally beyond the control of the Company, that could cause actual results to differ materially from those expressed in, or implied or projected by, the forward-looking information and statements contained in this presentation. These risks and uncertainties include, but are not limited to, whether vilazodone, Stedivaze or any of our other therapeutic products will advance further in the clinical trials process and whether and when, if at all, vilazodone, Stedivaze or any of our other therapeutic products will receive final approval from the U.S. Food and Drug Administration and equivalent foreign regulatory agencies and for which indications; whether vilazodone, Stedivaze or any of our other therapeutic products will be successfully marketed if approved; the strength of our intellectual property rights; competition from pharmaceutical and biotechnology companies; general economic conditions; and those risks identified and discussed by us in our filings with the U.S. Securities and Exchange Commission. Our audience is cautioned not to place undue reliance on these forward looking statements that speak only as of the date hereof. We do not undertake any obligation to publish revised forward-looking statements to reflect events or circumstances after the date of this presentation, or to reflect the occurrence of unanticipated events. Our audience is also urged to carefully review and consider the various disclosures in our periodic and interim reports filed with the SEC, including but not limited to our Annual Report on Form 10-K for the fiscal year ended March 31, 2010, Quarterly Report on Form 10-Q for the fiscal quarter ended September 30, 2010, and Current Reports on Form 8-K filed from time to time by the company. 2 |
| Clinical Data, Inc. at a Glance Completed transformation into a pharmaceutical company with two late-stage drug candidates that address large markets Vilazodone: a serotonin 1A receptor partial agonist and reuptake inhibitor in development for the treatment of depression Stedivaze: potential best-in-class coronary vasodilator for cardiac stress testing in Phase III trial Growing therapeutics pipeline, including Phase II compound with Orphan Drug Designation for pulmonary arterial hypertension Multiple partnership and licensing opportunities 3 PDUFA date January 22, 2011 |

| Depression Market Remains Large and Unsatisfied $12 billion U.S. market and growing1 Majority of patients do not achieve success with first therapy2 Side effects are common, e.g., gastrointestinal, sexual function, CNS disorders, and weight gain Co-morbid conditions often require additional medications Patient compliance is poor Despite billions spent on R&D for drugs with unique mechanisms, nothing truly novel developed to treat depression since the introduction of SNRIs in 1993 4 1 IMS Health's National Prescription Audit and National Sales Perspective, September 2009 2 STAR*D Study, January, 2006 American Journal of Psychiatry |

| Vilazodone: A Serotonin 1A Receptor Partial Agonist and Reuptake Inhibitor 5 Blocks serotonin reuptake Binds with high affinity to 5-HT1A auto- and hetero-receptors, Down-regulates 5-HT1A auto-receptors Richardson-Jones JW et al. Neuron. 2010;65:40-52 |

| 2nd Phase III Vilazodone N = 231 Placebo N = 231 MADRS (primary) -13.3 -10.8 -2.5 0.009 HAMD17 -10.7 -9.1 -1.6 0.026 HAMA -7.0 -5.7 -1.2 0.037 Vilazodone Demonstrated Superiority to Placebo in Two Phase III Trials1 Endpoints Least Squares Mean Change at Week 8 LS Mean Treatment Difference p-value 1st Phase III Vilazodone N = 198 Placebo N = 199 MADRS (primary) -12.9 -9.6 -3.2 0.001 HAMD17 -10.4 -8.6 -1.7 0.022 HAMA -6.5 -5.2 -1.4 0.031 1 Rickels et al. J Clin Psychiatry, 2009 and data on file with Clinical Data, Inc. All p-values are from ITT/LOCF analyses 6 |

| Findings from Long-term Safety Study (LTSS) Consistent with Phase III, Placebo-Controlled Trials Improvement Weeks Receiving Treatment 1Observed cases analysis (scores for 254 patients who remained in the study). N = 596 in effectiveness population. Data on file with Clinical Data, Inc. MADRS scores improved over 20 weeks and was sustained over 52-weeks1 Subject retention was twice that seen in historical evaluation of other antidepressants 7 |

| Vilazodone Was Generally Well Tolerated in Phase III Trials and LTSS Most common adverse events in Phase III trials were diarrhea, nausea, and insomnia1 Most were mild or moderate in intensity and did not require treatment No single adverse event led to discontinuation in >1% of patients No effects on vital signs, ECG, lab tests (including liver function tests), or body weight, when compared to placebo Sexual function was similar to placebo, as measured by validated scales. Low incidence of patient reported adverse events related to sexual function. LTSS results were consistent with Phase III studies 1 ¡A 5% and twice placebo according to results of Phase III trials 8 |

| Extended Intellectual Property Protection for Vilazodone U.S. Composition of Matter patent expires in 2014 5-year extension anticipated via Hatch-Waxman providing protection into 2019 Newly U.S. issued patent for most commercially viable polymorphic form extends protection for ~3 more years to 2022 European patent also granted, related applications granted in other territories and applications pending in many other countries Process to secure additional patents is well underway and anticipate further claims in the future 9 |
| Market Dynamics in Treatment of Depression Are Changing Only one currently branded depression drug may be on the market beyond 2014 Brand promotion is decreasing in historically promotionally sensitive category Targeted approaches and smaller sales forces are becoming more common Market is awaiting new treatment options and has historically embraced drugs with new mechanisms 10 |

| Brands Continue to Thrive 11 Source: IMS HEALTH'S National Sales Perspective |
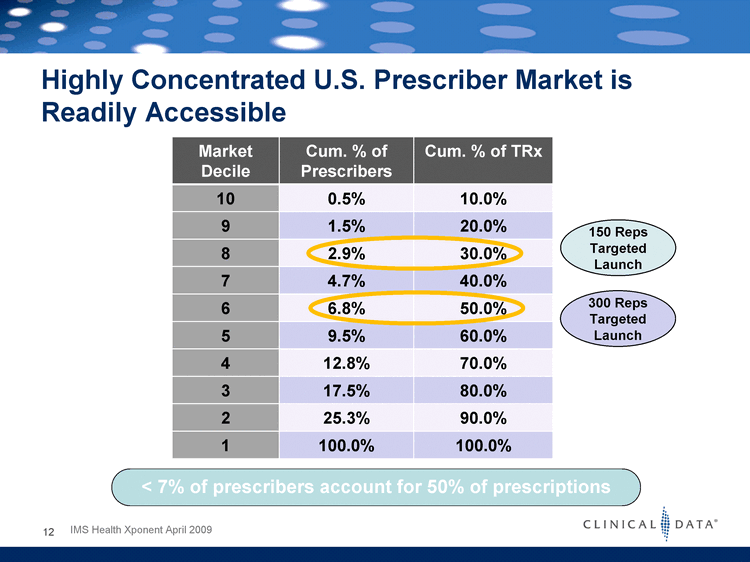
| Market Decile Cum. % of Prescribers Cum. % of TRx 10 0.5% 10.0% 9 1.5% 20.0% 8 2.9% 30.0% 7 4.7% 40.0% 6 6.8% 50.0% 5 9.5% 60.0% 4 12.8% 70.0% 3 17.5% 80.0% 2 25.3% 90.0% 1 100.0% 100.0% 9 8 7 6 5 4 3 2 1 Highly Concentrated U.S. Prescriber Market is Readily Accessible < 7% of prescribers account for 50% of prescriptions 12 IMS Health Xponent April 2009 150 Reps Targeted Launch 300 Reps Targeted Launch |

| Revenue Opportunity Remains Large in Depression Market Market Decile Cum. % of Prescribers 5% Share of TRx (000's) 10% Share of TRx (000's) 10 0.5% $ 158,250 $ 316,500 9 1.5% $ 316,500 $ 633,000 8 2.9% $ 474,750 $ 949,500 7 4.7% $ 633,000 $1,266,000 6 6.8% $ 791,250 $1,582,500 5 9.5% $ 949,500 $1,899,000 4 12.8% $1,107,750 $2,215,500 150 Reps Targeted Launch 300 Reps Targeted Launch IMS Health Xponent April 2009. Assumes 211M annual prescriptions at $150/scrip 13 |

| Positioned for Commercialization Upon Approval 14 |

| Medical Education Activities Ongoing Advisory Board meetings and interactions with KOLs continues Implementing fully integrated publication plan: 27 abstracts published to date 19 manuscripts to date 16 posters presented at major medical/HCP meetings in 2010 ~20 abstracts/posters projected in 2011; two abstracts have already been accepted for APA in May 20+ manuscripts ongoing/proposed 15 |

| Managed Care Markets Communications Underway Conducted payer landscape assessment and segmentation Developed and validated managed markets messages Producing vilazodone AMCP formulary dossier Began market shaping campaign with first of a series of advertorials introduced 16 |

| Sales Force Development and Projected Launch Top level sales and marketing management team on board Key sales force candidates identified, interviewed and contingent offer letters ready upon approval 150 sales representatives targeting high prescribers accounting for approximately 30% of U.S. MDD prescriptions Additional hires as physician education and payer adoption expands Sales training to begin early spring Official product launch, upon approval, within 3-4 months 17 |

| Stedivaze for Cardiac Stress Testing Potential best-in-class coronary vasodilator for myocardial perfusion imaging (MPI) Adenosine 2A receptor agonist in Phase III development Positive attributes position Stedivaze to be potential best-in-class Superior adenosine 2A receptor binding selectivity Potential for improved tolerability with reduced incidence of most common adverse events (e.g. shortness of breath, flushing, chest pain, headache, nausea, and dizziness) Optimal pharmacokinetics for SPECT MPI Convenient, single, fixed-dose bolus IV injection Potential for no contraindication in patients with COPD/asthma Superior tolerability compared to Adenoscan in Phase II trials 18 |
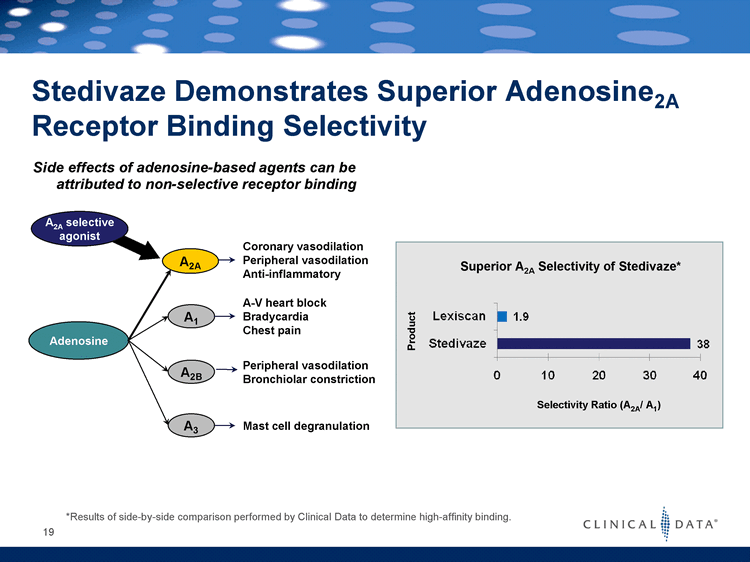
| Stedivaze Demonstrates Superior Adenosine2A Receptor Binding Selectivity Adenosine A1 A2B A3 A2A selective agonist A2A Coronary vasodilation Peripheral vasodilation Anti-inflammatory A-V heart block Bradycardia Chest pain Peripheral vasodilation Bronchiolar constriction Mast cell degranulation *Results of side-by-side comparison performed by Clinical Data to determine high-affinity binding. 19 Side effects of adenosine-based agents can be attributed to non-selective receptor binding Superior A2A Selectivity of Stedivaze* Product Selectivity Ratio (A2A/ A1) |

| Stedivaze Phase III ASPECT 1 First of two Phase III trials: Apadenoson Single Photo Emission Computed Tomography (ASPECT) Objective is to establish comparable efficacy and superior tolerability compared to adenosine N=750 patients over 18-24 months Patients undergo SPECT MPI, then are randomized to receive a second SPECT MPI using either Stedivaze or adenosine ASPECT 2 trial to begin in 1H 2011 20 |

| 21 Pharmacologic SPECT-MPI Market Continues to Grow U.S. Market Projected to be $800M in 20111 Sources: 1996-2008 data provided by Clinical Data to Third Party . 2008 data scaled from 2H 2009 data, and 2009 data scaled from 1H 2009 data, AMR. The Myocardial Perfusion Study Market Guide, Jul - Dec 2008 & Jan - Jun 2009, USA. Produced by AMRI Inc. Malvern PA. 2008-Lexiscan approved 1995-Adenoscan approved 1 AMR Monthly Monitor |

| Intellectual Property Protection for Stedivaze Anticipated Through 2024 U.S. Composition of Matter and Method of Use patents expire in 2019 Anticipate 5-year extension through Hatch-Waxman providing protection into 2024 Activities to secure additional patents is well underway with several patents pending 22 |

| PRX-8066 in Phase II Development for PAH Selective serotonin 2B (5-HT2B) receptor antagonist with potential for disease modification 5-HT2B receptors regulate abnormal cell growth and proliferation in the pulmonary vasculature of PAH patients Granted Orphan Drug Designation from FDA for Pulmonary Arterial Hypertension (PAH) November 2010 Phase II study in pulmonary hypertension associated with COPD demonstrated that it is generally well tolerated and has capacity to reduce elevated pulmonary pressures Developing clinical program for PRX-8066 23 |

| 24 Vilazodone: Depression Pain Oncology: B-cell Cancers 1222: Acute Inflammation 8066: Pulmonary Arterial Hypertension (PAH) 844: Diabetes Asthma 313: Glaucoma Stedivaze: Cardiac Stress Testing Novartis Option Santen, IND filed 12/10 Zalicus* Granted Orphan Drug Status Nov 2010 PDUFA Date of January 22, 2011 Phase III Initiated Nov 2009 Lead Discovery Preclinical Phase I Phase II Phase III NDA Marketed Clinical Data Drugs in Development *Formerly CombinatoRx |
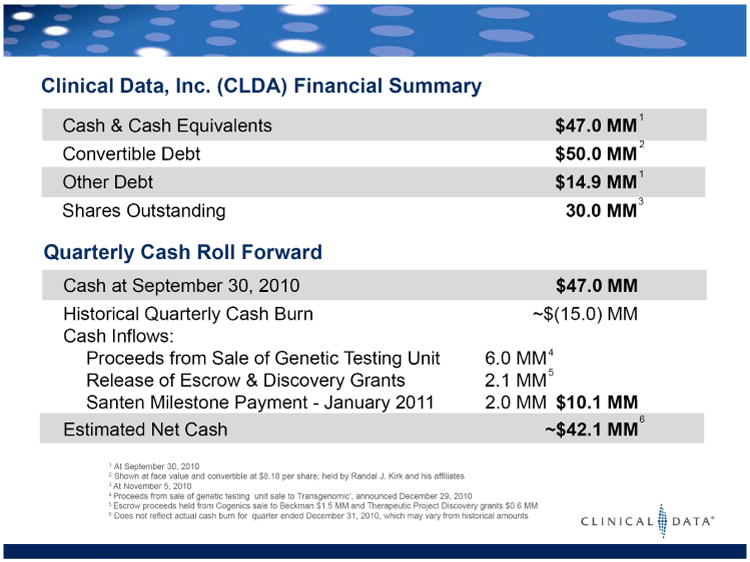
| Clinical Data, Inc. (CLDA) Financial Summary 1 At September 30, 2010 2 Shown at face value and convertible at $8.18 per share; held by Randal J. Kirk and his affiliates 3 At November 5, 2010 4 Proceeds from sale of genetic testing unit sale to Transgenomic’, announced December 29, 2010 5 Escrow proceeds held from Cogenics sale to Beckman $1.5 MM and Therapeutic Project Discovery grants $0.6 MM 6 Does not reflect actual cash burn for quarter ended December 31, 2010, which may vary from historical amounts Cash & Cash Equivalents $47.0 MM Convertible Debt $50.0 MM Other Debt $14.9 MM Shares Outstanding 30.0 MM 2 1 3 Cash at September 30, 2010 $47.0 MM Historical Quarterly Cash Burn ~$(15.0) MM Cash Inflows: Proceeds from Sale of Genetic Testing Unit 6.0 MM Release of Escrow & Discovery Grants 2.1 MM Santen Milestone Payment - January 2011 2.0 MM $10.1 MM Estimated Net Cash ~$42.1 MM 1 4 5 Quarterly Cash Roll Forward 6 |

| Investment Highlights Two late-stage products target large and growing markets: vilazodone and Stedivaze Near-term events Potential approval of vilazodone: PDUFA date January 22, 2011 2nd Phase III Stedivaze trial to begin in 1H 2011 IND on at least one of several pipeline programs Multiple partnership/licensing opportunities Follow-on pipeline of promising drug candidates 26 |

| Thank You |
