Attached files
| file | filename |
|---|---|
| 8-K - ZOGENIX FORM 8-K - ZOGENIX, INC. | d8k.htm |
 [ 1
] INVESTOR PRESENTATION
January 2011
NASDAQ: ZGNX
Exhibit 99.1 |
 [
2 ]
Forward Looking Statements
Zogenix cautions you that statements included in this presentation that are not a
description of historical facts are forward-looking statements. Words such
as “believes,” “anticipates,” “plans,” “expects,” “indicates,” “will,” “intends,”
“potential,” “suggests,” “assuming,”
“designed” and similar expressions are intended to identify forward looking
statements. These statements are based on Zogenix’s current beliefs and
expectations. These forward-looking statements include statements
regarding: the ability to successfully commercialize Sumavel DosePro and its
continued sales growth; the ability to obtain additional marketing approvals for
Sumavel DosePro in the European Union; the timing of the release of Phase IV
data for Sumavel DosePro; the potential for, and timing of, an NDA submission
for ZX002 and IND submissions for Zogenix’s additional product candidates; the potential for ZX002 to be
the first approved oral, single-entity controlled release formulation of
hydrocodone; the completion of enrollment of one of the Phase 3
clinical trials for ZX002; the timing of results for the Phase 3 clinical trials for ZX002; the potential of
co-promotion discussions for ZX002; the expansion of Zogenix’s existing
sales team; the potential to broaden the application of the DosePro
technology; and the potential market penetration of Sumavel DosePro and ZX002. The
inclusion of forward-looking statements should not be regarded as a
representation by Zogenix that any of its plans will be achieved. Actual
results may differ from those set forth in this presentation due to the risk and uncertainties
inherent in Zogenix’s business, including, without limitation: the market
potential for migraine treatments, and Zogenix’s ability to compete
within that market; inadequate therapeutic efficacy or unexpected adverse side
effects relating to Sumavel DosePro that could delay or prevent commercialization, or
that could result in recalls or product liability claims; Zogenix’s
dependence on its collaboration with Astellas Pharma US, Inc. to promote Sumavel
DosePro; the ability of Zogenix to ensure adequate and continued supply of Sumavel
DosePro to successfully launch commercial sales or meet anticipated market
demand; the progress and timing of Zogenix’s clinical trials; the
potential that earlier clinical trials may not be predictive of future results; the
potential for ZX002 to receive regulatory approval on a timely basis or at
all; the potential for adverse safety findings relating to ZX002 to delay or prevent
regulatory approval or commercialization; the ability of Zogenix and its licensors to
obtain, maintain and successfully enforce adequate patent and other
intellectual property protection of its products and product candidates and the
ability to operate its business without infringing the intellectual property rights
of others; and other risks described in Zogenix’s filings with the
Securities and Exchange Commission. You are cautioned not to place undue reliance on
these forward-looking statements, which speak only as of the date hereof, and
Zogenix undertakes no obligation to revise or update this presentation to
reflect events or circumstances after the date hereof. All forward-looking
statements are qualified in their entirety by this cautionary statement. This caution
is made under the safe harbor provisions of Section 21E of the Private
Securities Litigation Reform Act of 1995. |
 [
3 ]
Commercializing and Developing Products for Treatment of
Central Nervous System (CNS) Disorders and Pain
RAMPING SALES
1
Single Use, Needle-Free,
Commercial Product
$3.5B Market
Acute Migraines
and Cluster Headaches
$13B Market
Codeine and Morphine
Based Pain Products
MEDICINE
and
TECHNOLOGY
PHASE 3 DEVELOPMENT
ZX002
1
Oral Single-Entity, Controlled
Release Hydrocodone
st
st
Sources:
Wolters Kluwer Pharma Solutions, Source®
PHAST Institution/Retail (12 months ended June 30, 2010) |
 [
4 ]
Established
Commercial
Infrastructure
•
Sophisticated Manufacturing and Sales Organization in
Place to Support Continued Growth
Positioned for Significant Value Creation
SUMAVEL
®
DosePro
™
:
Differentiated,
Marketed Product
•
Full Commercial Launch Underway, Marketing
Partnership with Astellas, Quarterly Prescription Growth
Phase 3 Ongoing:
ZX002 for
Chronic Pain
•
Potential To Be 1st Approved Oral, Single-Entity,
Controlled Release Formulation of Hydrocodone
DosePro™
Delivery
System:
Proprietary Technology
•
Validated, Innovative Needle-Free Delivery System
•
U.S. FDA approval, initial E.U. marketing authorizations
•
Broad Range of Potential Applications
Experienced
Management Team
•
History of Successful Manufacturing, Development and
Commercialization |
 [
5 ]
1
st
Single Use, Needle-Free, Commercial Product Marketed
for Treating Acute Migraine and Cluster Headaches
RAMPING SALES
Launched
Mid-January 2010 |
 [
6 ]
75%
of
Patients
Are Women
The
9th
Leading Cause
of Disability
in Women Globally
7th
Most Costly Disease
to US Employers
30 Million People in the U.S. Suffer Migraines**
Symptoms and Nature of Episodes Vary Widely
Disabling Headache Pain, 4-72 Hours in Duration
$25 B Annual Estimated Cost to Employers
63% Suffer
1 Attack per
Month
25% Have
1 Attack per Week
48% Upon Morning Wakening (between 4 -9 am)
29% Reported Vomiting as a Symptom of Migraine Attacks
Treatment Depends on Type of Episode
Migraine Frequency, Severity, Speed of Onset, and Previous
Response to Medication Determine Treatment
25% Have
2 Active Prescriptions
Oral Triptans
Often Prescribed as 1
st
Line Therapy
Sources:
NHF(2010),
World
Health
Organization
(2000),
Goetzel
et
all,
JOEM
(2004),
International
Headache
Society
(www.ihs-headache.org), Thomson
Medstat
(2006),
Lipton
et
al,
Neurology
(2002),
Fox
and
Davis,
Headache
(1998),
Lipton
et
al,
Neurology
(2001),
Boston
Healthcare
Associates,
Inc.
(2007) |
 [
7 ]
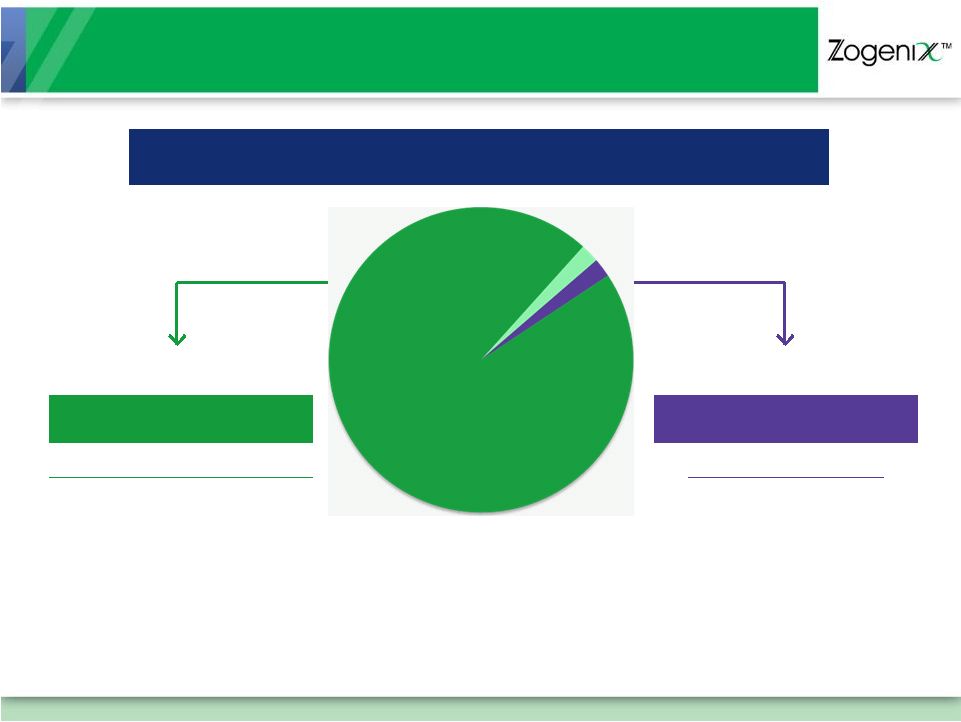
$3.5 Billion Migraine Market
TARGET AUDIENCE: DISSATISFIED ORAL USERS
2% Nasal
2% Injectable*
Oral and Nasal
128 Million Doses
CONVENIENCE
But…
Limited Efficacy for
Many Attacks
Injectable
3.2 Million Doses
EFFECTIVE
But…
50% of Patients Refuse
Needle-based Injection
Due to Anxiety, Fear,
and Complexity
96%
Tablets and
Melt Tablets
*Sumatriptan Is the Only Injectable Triptan
Sources: Palace Healthcare Group, Inc. (2006), Wolters Kluwer Pharma Solutions,
Source® PHAST Institution/Retail (12 months ended June 2010) |
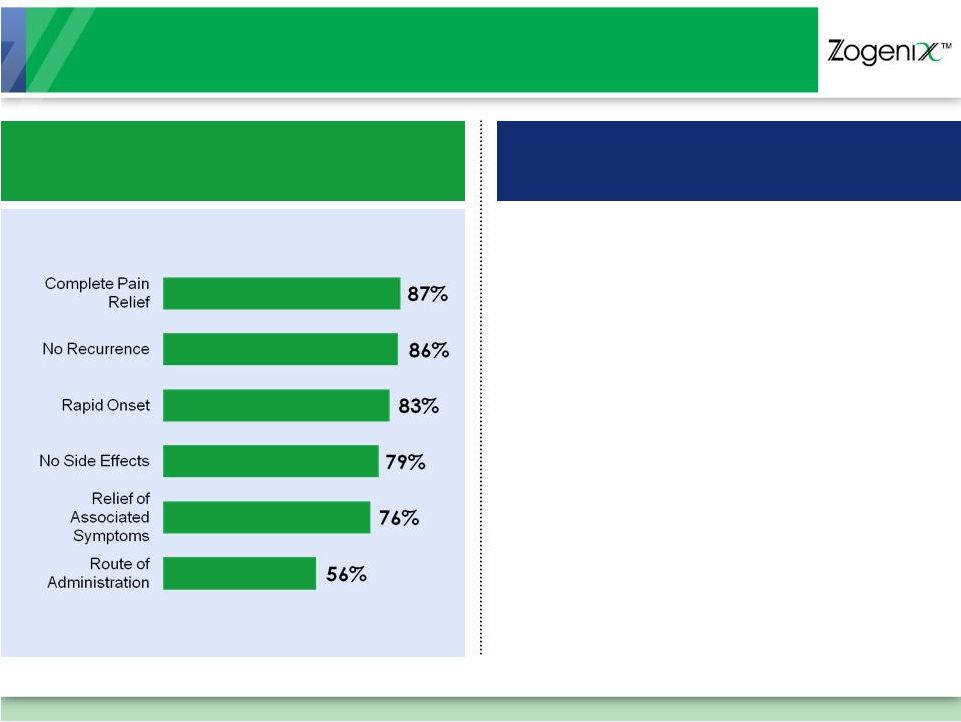 Significant Unmet Needs
Patients Want Fast,
Complete Pain Relief…
…
But Most Patients Are Dissatisfied
50%
Unsatisfied with Their Current Therapy
75%
Said Medication Didn’t
Work Fast Enough
Majority
Said Prescription Oral Medication
Not Useful for Every Migraine Attack
30%
Fail to Respond to Oral or Nasal Triptans
[ 8
]
Sources: Lipton et al, Headache (1999), NHF Survey of 500 Migraine Sufferers (June
2010), Gerson Lehrman Group (2006) |
 SUMAVEL
DosePro: A Differentiated Migraine Therapy
COMPLETE
Pain Free
EFFECTIVE
Pain Relief
EASY TO USE
98%
of
Patients
Correctly
Used SUMAVEL DosePro
During Migraine Attacks
on Their First Try At Home
FAST
Pain Relief in as Little as
10 Minutes
for Some Patients
Sources: SUMAVEL DosePro
Prescribing Information
[ 9
] |
 [
10 ]
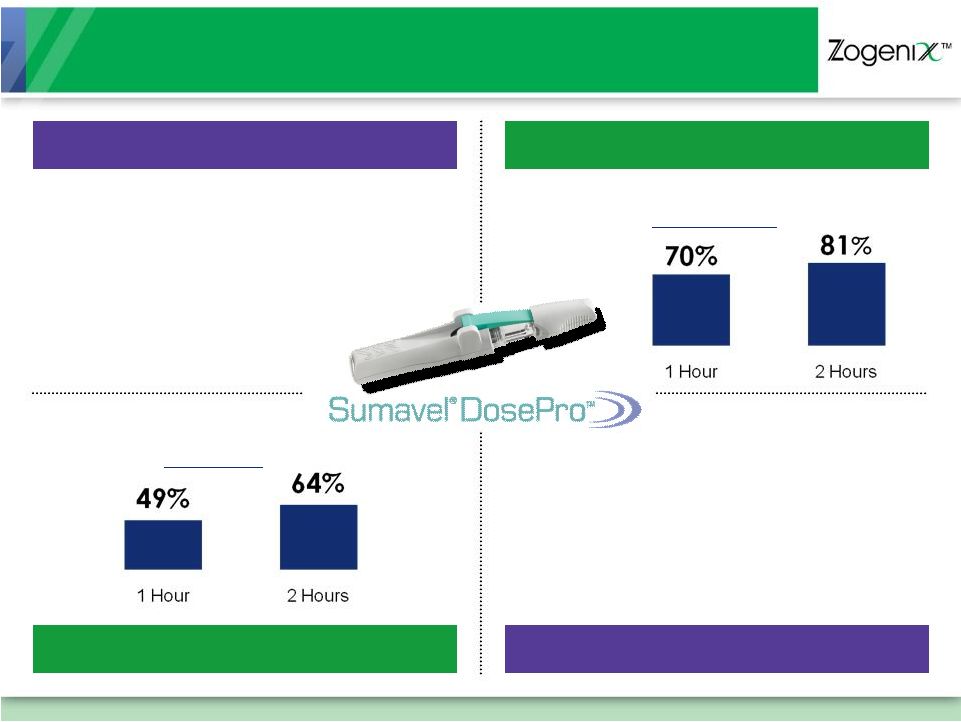
Highly Effective Treatment for Migraine Pain
Fastest Time to Maximum Drug
Concentration in Blood (Hours)
Most Effective Patient
Pain Relief at 1 Hour
70%
38-43%
28%
20-30%
12 Minutes
1.6 -
2.5
Hours
1 Hour
1.5 Hours
ORAL
TABLETS
ORAL
MELT
ORAL TABLETS
ORAL MELT
Sources: Products’
Prescribing Information
(sumatriptan injection)
(sumatriptan
injection) |
 [
11 ]
Rapid, Growing
Market Adoption Since January Launch
Weekly Prescriptions: Rolling 4-Week Average
(Through week ending October 22, 2010)
SUMAVEL DosePro
Prescriptions: Over 25,000 TRx
To Date
$1.9M
Net Sales
$4.2M
Net Sales
$5.7M
Net Sales
Refill Prescriptions
New Prescriptions
Total
Prescriptions
New
Prescriptions
Sources: Wolters
Kluwer
Pharma
Solutions (Source®
LaunchTrac, (w/e
January 15, 2010 –
w/e
October 22, 2010), Source®
PHAST Retail (Jan 2010 –
Sep 2010)
Refill
Prescriptions
at
31%
of
Total
(September)
Over 6,100 Prescribing Physicians |
 [
12 ]
Market Experience To Date …
New Physicians
32%
of Prescribing Physicians Had Not
Written a Prescription for Needle-
Based
Sumatriptan
Injections
in
prior
12 months
We’re Changing the Way Migraines Are Treated
Sources:
Wolters
Kluwer
Pharma
Solutions
:
Source®
LaunchTrac
(w/e
January
15,
2010
–
w/e
July
30,
2010),
Source®
Lx
PTA
(Jan
2010
–
Aug
2010);
Infomedics, based on 1,071 patient respondents (through September 11, 2010)
Patients Switching
77%
of Patients Switching Dosage Forms
through August were from Oral
Triptans
Higher Patient Satisfaction
Average Patient Satisfaction Score
vs. 5.5 (9 point scale) for Prior
Migraine Medication;
Confirmed by Phase 4 Study Results
7.1
New Patients
34%
of Patients Were New to the
Triptan
Market since launch |
 [
13 ]
SUMAVEL DosePro:
CONNECT 2010 Most Innovative Product (MIP) Winner
2010
Winner
in
Life
Sciences
–
Medical
Products
category
Recognizes emerging technologies and cutting-edge
products that drive San Diego’s innovation economy
DosePro
Technology…as easy as 1-2-3 |
 [
14 ]
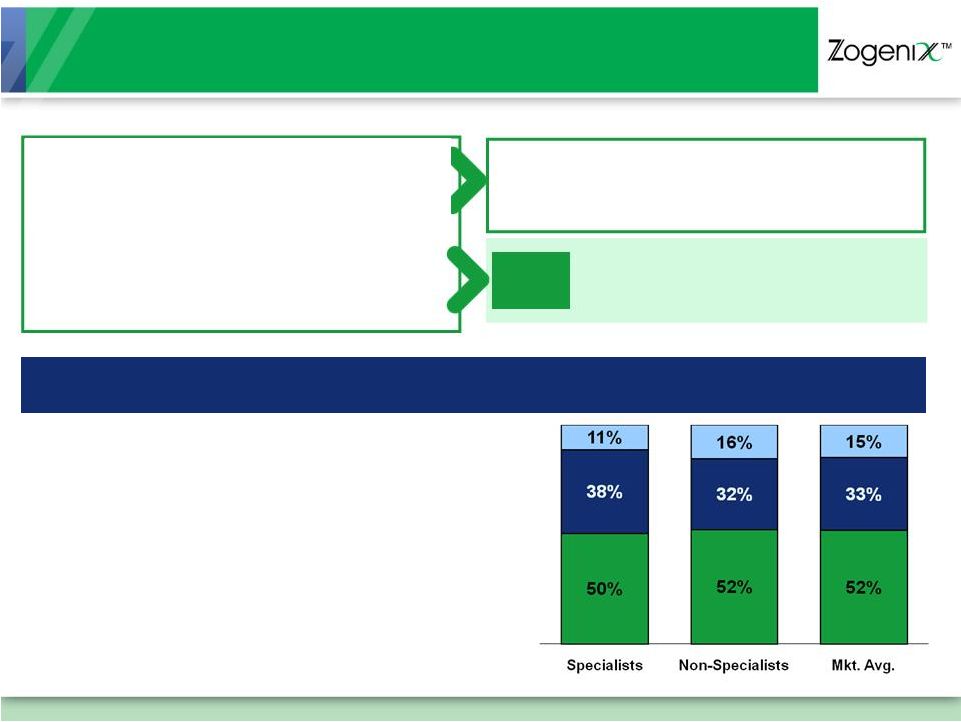
Successfully Executing with
Well-Established Sales and Marketing Organization
~ 80 Field Sales Reps, Most Have
Neurology and/or Migraine
Experience
Targeting Neurologists, Headache
Clinics and Specialists, Select PCPs
12+ Years Average Experience per
Sales Rep
~ 400 Field Sales Reps
Targeting PCPs, OB/GYNs, EM
Physicians and Urologists
Multi-Year Co-Marketing and
Co-Promotion Agreement
$20M Upfront and Milestone Payments
Pay-for-Performance Sales Results
Positioning
SUMAVEL
DosePro
as
a
First-Line
Alternative
for
Oral
Triptan
Non-Responders
and
Dissatisfied
Patients
Collaboration With Over
475 Sales Representatives |
 [
15 ]
Comprehensive Marketing and Patient Support
Programs |
 Broad
Reimbursement Coverage Source:
Wolters
Kluwer
Pharma
Solutions
Source®
Dynamic
Claims
(January
2010
–
November
2010)
of Reimbursement Claims
Submitted Are Approved
79%
Tier 3 Reimbursement Strategy
Meeting Expectations
Broad Coverage with the Largest
Commercial Healthcare Plans
Range of Physician and Patient
Reimbursement and
Support Programs ($15 Co-Pay)
Non-Retail Initiatives Underway
$87 WAC per Unit
$522 Sold in 6-Packs
$15 Cost to Patient
[ 16
] |
 [
17 ]
High Quality Partnerships Support
Domestic and European Commercialization
Manufactured and Released Over 1,000,000
SUMAVEL DosePro
Units to Date
Manufacturing
Contract Manufacturers and Component Suppliers
Distribution and Marketing
Europe |
 [
18 ]
ZX002
1
st
Oral, Single-Entity, Controlled Release Hydrocodone
for Treating Chronic Pain
PHASE 3 DEVELOPMENT |
 ZX002:
Filling A Significant Treatment Gap for Chronic Pain Sources:
Wolters
Kluwer
Pharma
Solutions,
Source®
PHAST
Retail
(12
months
ended
June
30,
2010),
bioStrategies
Group
(2007)
NO
Controlled Release Hydrocodone
Single Entity Hydrocodone
$13 Billion Market (202M TRx)
Codeine and Morphine
Based Pain Products
Hydrocodone
Rx Share By Pain Type: ~50% for Chronic Pain
Acute
Chronic
–
Other
Chronic
Back/
OA
June 2009 FDA Joint Advisory
Committee Meeting
Highlighted public health problem of
liver injury related to use of
acetaminophen in OTC and Rx
products
$3.1 Billion Annual Sales (126M TRx)
Generic, Immediate Release
Hydrocodone
Combination Products
Oral ER Formulations Accepted at
Significant Premiums to Generics/IR
[ 19
] |
    [
20 ]
ZX002: Phase 3 Clinical Development Program
Study 801
Study 802
Open Label Safety:
Enrollment Completed 4Q10
Initiated May 2010, ~55 Sites
~450 patients on therapy
Open Label Safety Study to Provide
Safety Database for 505(b)(2) NDA:
300 Subjects at 6 Months and 100
Subjects at 1 Year Exposure
Broad Spectrum of Chronic Pain
Pivotal Phase 3 Efficacy Trial:
Enrollment Completion 1Q11
Initiated March 2010, ~60 Sites
~340 patients to be randomized
Standard Opioid Trial Design with
12-Weeks Active Treatment Following
Dose Titration and Randomization
Chronic Lower Back Pain Patients
Top Line Data Expected 2H 2011
Intend to Submit NDA by Early 2012 |
 ZX002
Single Entity Hydrocodone, Multiple Strengths (10-50 mg)
Uses Elan’s
Proprietary SODAS Delivery System to Enhance
Hydrocodone
Release Profile
Planning expansion of existing sales team to 250+
representatives to support ZX002 launch
ZX002: A Late Stage Product Candidate
with Numerous Benefits Over Existing Opioids
Effective12-Hour Pain Relief
Enables Dose
Optimization and
Switching from
Other Opioids
Improves
Adherence and
Patient
Convenience
Provides Consistent,
Around-the-Clock
Relief
Eliminates
Safety Risks
Associated with
Acetaminophen
[ 21
] |
 DosePro: Proprietary Drug Delivery System
Validated by FDA Approval of SUMAVEL DosePro
10+ Years
Development
and Validation
US and Foreign IP
61 Issued Patents
31 Patent
Applications Pending
Proven Ability
to Deliver Biologics
and Highly
Viscous Formulations
[ 22
] |
 DosePro: Broad Range of Potential Applications
Attractive Delivery Option for Additional Medications,
Including Biologics and Small Molecules
DosePro
Internal Development
Injectable
CNS Drug
Product Candidates
(2 Pre-Clinical)
Second Generation
Technology
(1mL device)
Technology Out Licensing
Enhance, Differentiate
or Extend Injectable
Product Lifecycles
Prototypes of Second Generation DosePro
[ 23
] |
 [
24 ]
TRx
Market
1.26 Million TRx
$244 Million
48% of Episodes Upon Morning Wakening
(between 4 -9 am)
29% of Sufferers Reported Vomiting as a
Symptom of Migraine Attacks
30% of Patients Fail to Respond to Oral or
Nasal Triptans
Net Revenue/Dose: $70/Unit*
7 Units/TRx
Average TRx
Value: $490
12.1 Million TRx
per Year
1% Triptan
Share
ZX002
126M Hydrocodone
(~50% for Chronic Use)
76M Other ER and IR Opioids
Net Revenue/Day: $7.75**
Average TRx: 25 Days
Average TRx
Value: $194
202 Million TRx
per Year
1% Hydrocodone
Share
Large Commercial Opportunities
Patient/ Market
Segments
Prescription
Values
*Includes Co-Pay Assistance ** Based on current pricing of
branded ER opioids Sources:
Wolters
Kluwer
Pharma
Solutions
,
Source®
PHAST
Retail
(12
months
ended
June
30,
2010)
Illustrative
Market
Penetration
121,000 TRx
$59 Million |
 Financial Summary
9 months ended September 30, 2010
P&L
$ Millions
Financial Highlights
$ Millions
Revenue
$
14.6
Operating Expense
$
66.2
Loss From Operations
$
51.6
Other (Income)/Loss
$ 19.9
Net Loss
$
71.5
Gross Invoiced Sales
$
16.4
Invested Capital
$ 164.0
Cash Balance
$
11.7
Debt Facility
$25M Term Loan
$10M Working Capital Line
•
Balance $2.7M
Cash Flow Used In
Operations
$ 58.5
[ 25
] |
 2010
Achievements SUMAVEL DosePro
Full U.S. Commercial Launch by
Zogenix
with co-promote
partner Astellas
Enhanced Formulary Coverage
and Patient Support
Phase IV Study Completed
Consistent Monthly Rx Growth
Since Launch
1 Million Commercial Units
Produced
Initial EU Approvals by Desitin
Named a 2010 “Most Innovative
Product”
Winner by CONNECT
ZX002
Initiated Phase 3 Safety Study
(Study 802)
Initiated Phase 3 Efficacy Study
(Study 801)
Completed Enrollment in
Phase 3 Safety Study
Corporate
Appointed Ann D. Rhoads to
Serve as CFO
Established Term Loan and
Revolving Line of Credit
Completed Initial Public
Offering
[ 26
] |
 [
27 ]
2011 Goals and Milestones
SUMAVEL DosePro
Continue US Sales Growth
Phase IV Data Publication
Additional EU Approvals
New Direct-to-Patient
Initiatives
ZX002
Pivotal Study 801 Efficacy Top–
Line Results
Open Label Study 802 Top-Line
Safety Results
Initiate Co-Promotion Discussions
Pre-NDA Meeting with FDA
Prepare to File NDA by early
2012
DosePro
Technology
File IND for Next Zogenix
CNS
Product
Seek First Licensee for Biologic
Building on Track Record of
Successful Execution |
 [
28 ]
Experienced Management Team with a History
of Successful Development & Commercialization
*Co-Founders
Aradigm, Invacare
19
John Turanin*
VP/GM, Zogenix
Technologies
GlaxoSmithKline, Elan, Skin Medica, InterMune
30
Mark Thompson
VP, Sales & Managed Markets
Nektar, Connetics
24
Edward Smith, Ph.D., RAC
VP, Regulatory Affairs
Avera, Windamere
17
Bret Megargel*
VP, Corporate Dev
Elan, Allergan, Valient
12
Stephen Jenner
VP, Marketing
Elan, InterMune
19
Cynthia Robinson, Ph.D.
CDO
Premier, Sprout, DLJ, Bain & Co, Merrill Lynch
18
Ann Rhoads
CFO
Aradigm, Cardiff University
20
Stephen Farr, Ph.D.*
President & COO
GlaxoSmithKline, Elan, InterMune
23
Roger Hawley*
CEO
Years
Experience |
 [
29 ]
Established
Commercial
Infrastructure
•
Sophisticated Manufacturing and Sales Organization in
Place to Support Continued Growth
Positioned for Significant Value Creation
SUMAVEL
®
DosePro
™
:
Differentiated,
Marketed Product
•
Full Commercial Launch Underway, Marketing
Partnership with Astellas, Quarterly Prescription Growth
Phase 3 Ongoing:
ZX002 for
Chronic Pain
•
Potential To Be 1st Approved Oral, Single-Entity,
Controlled Release Formulation of Hydrocodone
DosePro™
Delivery
System:
Proprietary Technology
•
Validated, Innovative Needle-Free Delivery System
•
U.S. FDA approval, initial E.U. marketing authorizations
•
Broad Range of Potential Applications
Experienced
Management Team
•
History of Successful Manufacturing, Development and
Commercialization |
