Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - NUPATHE INC. | c10687e8vk.htm |
| EX-99.1 - EXHIBIT 99.1 - NUPATHE INC. | c10687exv99w1.htm |
Exhibit 99.2

| JP Morgan Healthcare Conference January 2011 |

| 2 Forward Looking Statements This presentation contains "forward-looking statements" within the meaning of the Private Securities Litigation Reform Act of 1995. All remarks and information that are not historical facts are hereby identified as forward-looking statements for this purpose and include, among others, remarks and information relating to: receipt and timing of marketing approval for, and launch of, Zelrix; expected PDUFA; scope and duration of IP protection; timing of IND submissions for NP201 and NP202; sufficiency of the Company's capital through approval and into launch of Zelrix; commercialization plans for Zelrix; Zelrix market potential, pricing and reimbursement; and all other remarks and information relating to the Company's projections, expectations, future performance or plans or objectives for future operations (including assumptions underlying or relating to any of the foregoing). Forward-looking statements are based upon the current expectations and beliefs of management. Actual results and events may differ materially from those indicated by the forward-looking statements contained in this presentation as a result of various important factors including, among others: timing and ability to obtain marketing approval for and commercialize Zelrix; the potential benefits of, and market for, Zelrix and the Company's other product candidates; serious adverse events or other safety risks that could require the Company to abandon or delay development of, or preclude or limit approval of, Zelrix or the Company's other product candidates; varying interpretation of clinical and market data; the costs to prepare for and commercialize Zelrix; and the risks, uncertainties and other factors discussed in the Company's Quarterly Report on Form 10-Q for the quarter ended September 30, 2010 under the caption "Risk Factors" and elsewhere in such report, which is available on the Company's website at www.nupathe.com in the "Investor Relations - SEC Filings" section. As a result, you are cautioned not to place undue reliance on any forward-looking statements. Additionally, the forward-looking statements contained in this presentation represent management views as of the date of this presentation. While the Company may update certain forward-looking statements from time to time, it specifically disclaims any obligation to do so, whether as a result of new information, future developments or otherwise. (c) 2011 NuPathe Inc. All rights reserved |
| 3 NuPathe Investment Highlights ZelrixTM - the first and only migraine patch 505(b)(2) NDA filed with FDA on October 29, 2010 PDUFA in 3Q11 expected Launch planned in 1H12 Migraine remains a large, but underserved, market Capital sufficient through approval and into launch Zelrix IP protection through 2023 CNS pipeline provides long-term growth |
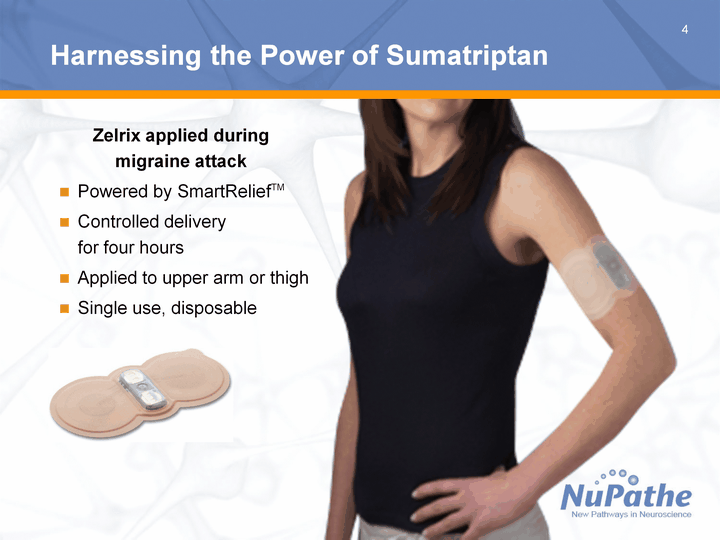
| 4 Harnessing the Power of Sumatriptan Zelrix applied during migraine attack Powered by SmartReliefTM Controlled delivery for four hours Applied to upper arm or thigh Single use, disposable |
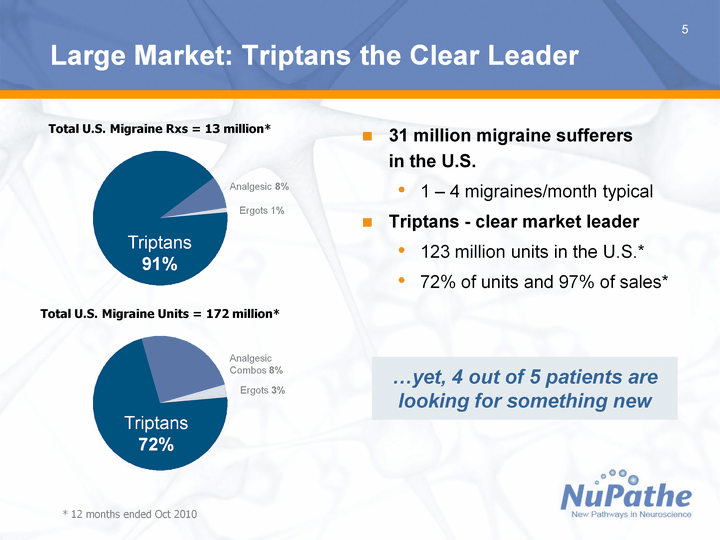
| 5 Large Market: Triptans the Clear Leader 31 million migraine sufferers in the U.S. 1 - 4 migraines/month typical Triptans - clear market leader 123 million units in the U.S.* 72% of units and 97% of sales* * 12 months ended Oct 2010 ...yet, 4 out of 5 patients are looking for something new Total U.S. Migraine Rxs = 13 million* Total U.S. Migraine Units = 172 million* Triptans Analgesic Ergots Total 91 8 1 Analgesic 8% Ergots 1% Triptans 91% Triptans Analgesic Combos Ergots Total 72 25 3 Triptans 72% Ergots 3% Analgesic Combos 8% |

| 6 Why a Patch for Migraine? * Overlap amongst patient types |
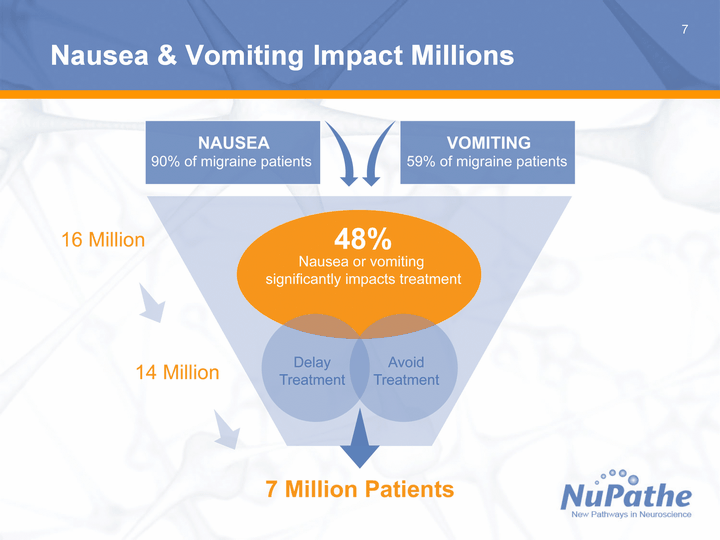
| 7 Nausea & Vomiting Impact Millions |

| 8 Therapeutic Level Zelrix: Designed to Address Shortcomings of Current Therapies Control provided by SmartRelief allows Zelrix to deliver efficacy with minimal triptan AEs |
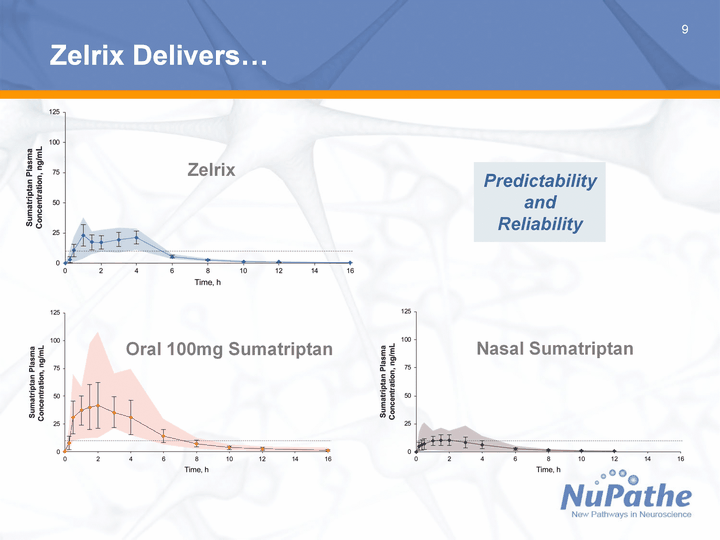
| 9 Zelrix Delivers... Oral 100mg Sumatriptan Nasal Sumatriptan Zelrix Predictability and Reliability |
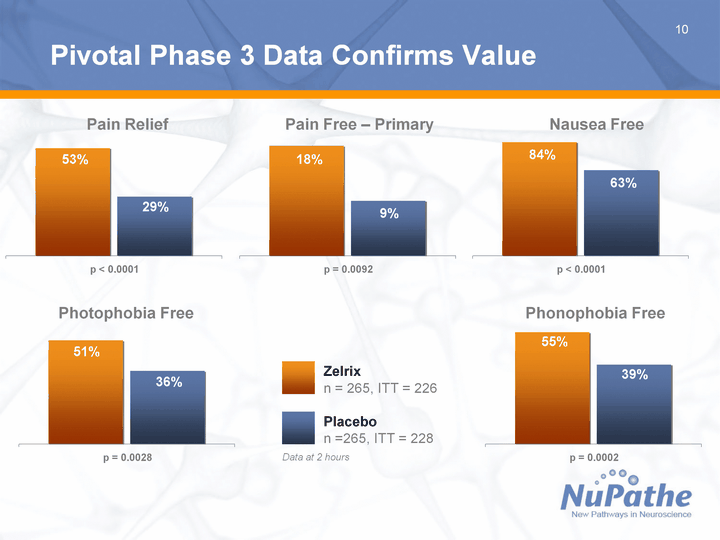
| 10 p < 0.0001 East 0.53 West 0.29 Pivotal Phase 3 Data Confirms Value Photophobia Free Phonophobia Free Pain Free - Primary Nausea Free Pain Relief p = 0.0092 East 0.18 West 0.09 p < 0.0001 East 0.84 West 0.63 p = 0.0028 East 0.51 West 0.36 p = 0.0002 East 0.55 West 0.39 Data at 2 hours Zelrix n = 265, ITT = 226 Placebo n =265, ITT = 228 |

| 11 Rapid and Sustained Pain Relief Rapid pain relief 38% more Zelrix patients than placebo at 30 minutes^ Significant and comparable to oral and nasal triptans at one hour Sustained pain relief 34% of Zelrix patients compared to 21% of placebo (p=0.0015) from 2 to 24 hours ^ Not statistically significant |
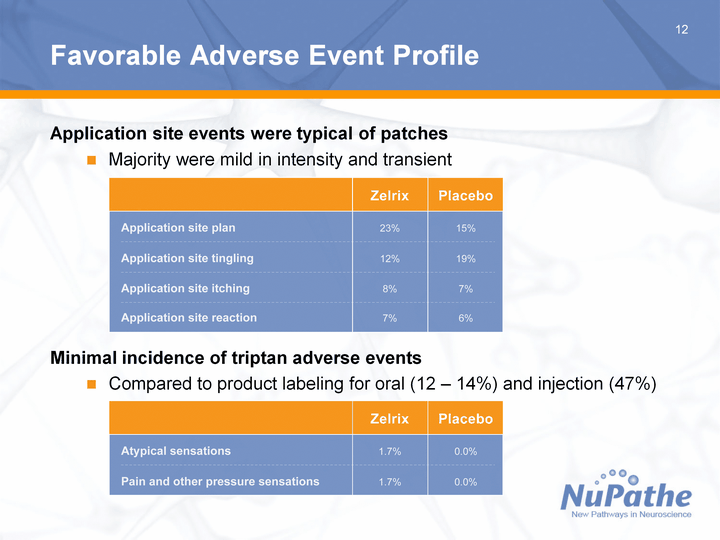
| 12 Favorable Adverse Event Profile Application site events were typical of patches Majority were mild in intensity and transient Minimal incidence of triptan adverse events Compared to product labeling for oral (12 - 14%) and injection (47%) |
| 13 Positive Long-Term Data for Zelrix Two 12 month trials (NP101-008, NP101-009) NP101-008: top-line efficacy and safety results Strong, consistent efficacy within two hours Headache pain relief for 58% of migraines treated Headache pain free for 24% of migraines treated Nausea free for 79% of migraines treated Well tolerated Only three patients (1.6%) reported a triptan AE The most common AEs were related to the application site Itching (21.9%), pain (21.3%) and hypersensitivity (6.0%) No increase in skin irritation with cumulative usage |
| 14 Setting the Stage for Commercialization Commercial infrastructure Gradual build - hire core commercial team prior to FDA approval Sales force of ~100 to be hired after approval US partnership NDA filing - important milestone To expand product reach and revenue Manufacturing scale-up Commercial equipment and process on track for launch in first half of 2012 |
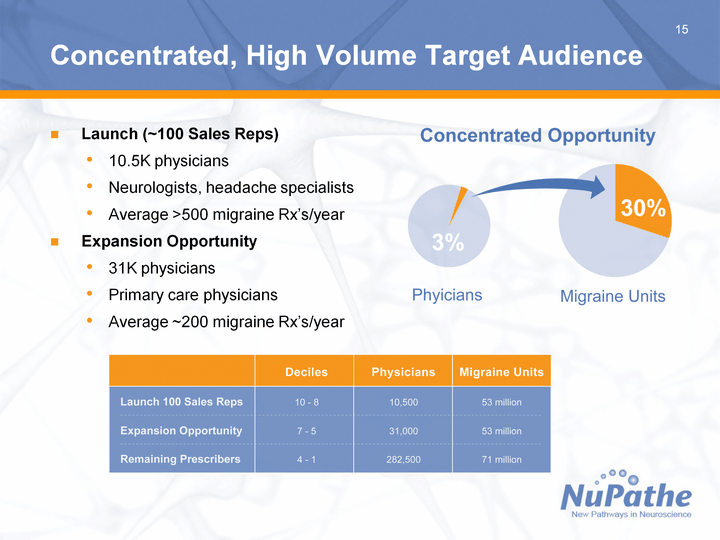
| 15 15 Concentrated, High Volume Target Audience Launch (~100 Sales Reps) 10.5K physicians Neurologists, headache specialists Average >500 migraine Rx's/year Expansion Opportunity 31K physicians Primary care physicians Average ~200 migraine Rx's/year |
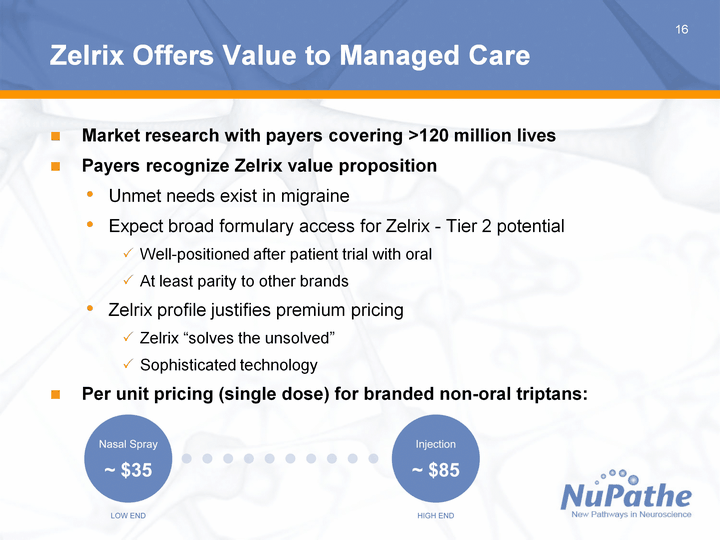
| 16 Zelrix Offers Value to Managed Care Market research with payers covering >120 million lives Payers recognize Zelrix value proposition Unmet needs exist in migraine Expect broad formulary access for Zelrix - Tier 2 potential Well-positioned after patient trial with oral At least parity to other brands Zelrix profile justifies premium pricing Zelrix "solves the unsolved" Sophisticated technology Per unit pricing (single dose) for branded non-oral triptans: |

| 17 Pipeline Addresses Unmet Needs in CNS Pipeline powered by proprietary technology platforms SmartRelief - Zelrix LAD(tm) - NP201 and NP202 1. 505(b)(2) NDA submitted October 29, 2010 2. 505(b)(2) |
| 18 LAD: Improved Treatment Through Continuous Delivery NP201: Long term delivery of ropinirole to treat the signs and symptoms of Parkinson's disease Designed to address tolerability and need for consistent long-term efficacy Current status: Preclinical POC study complete - return to normal function Pre-IND FDA Meeting - 1Q10 IND expected in 1H11 NP202: Long term delivery of an atypical antipsychotic to treat schizophrenia and bipolar disorder Designed to address significant compliance issues Current status: Preclinical POC studies complete Prototype development ongoing IND expected in 2012 |
| 19 Financial Strength to Execute Strong cash position $47 million at September 30, 2010 Cash sufficient through approval and into launch of Zelrix Efficient use of resources Near-term revenues with launch of Zelrix in 2012 |
| 20 Demonstrated Execution Post-IPO Completion of PK study of Zelrix in young & elderly Completion of PK study of Zelrix to evaluate bioavailability Completion of study of Zelrix to evaluate cumulative skin irritation Zelrix NDA filing Completion of long-term safety study Zelrix NDA acceptance NP201 IND Completion of second long-term safety study Zelrix NDA Approval Zelrix Partnership NP202 IND |

