Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - HUMAN GENOME SCIENCES INC | c10742e8vk.htm |
| EX-99.1 - EXHIBIT 99.1 - HUMAN GENOME SCIENCES INC | c10742exv99w1.htm |
Exhibit 99.2

| BENLYSTA(r) AND BEYOND H. Thomas Watkins President and Chief Executive Officer JP Morgan Healthcare Conference January 10, 2011 |
| NOTE REGARDING FORWARD-LOOKING STATEMENTS 2 JPMorgan Healthcare Conference | January 10, 2011 This presentation contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. The forward-looking statements are based on Human Genome Sciences' current intent, belief and expectations. These statements are not guarantees of future performance and are subject to certain risks and uncertainties that are difficult to predict. Actual results may differ materially from these forward-looking statements because of Human Genome Sciences' unproven business model, its dependence on new technologies, the uncertainty and timing of clinical trials and regulatory approvals, Human Genome Sciences' ability to develop and commercialize products, its dependence on collaborators for services and revenue, its substantial indebtedness and lease obligations, its changing requirements and costs associated with facilities, intense competition, the uncertainty of patent and intellectual property protection, Human Genome Sciences' dependence on key management and key suppliers, the uncertainty of regulation of products, the impact of future alliances or transactions and other risks described in the Company's filings with the SEC. Existing and prospective investors are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. Human Genome Sciences undertakes no obligation to update or revise the information contained in this presentation whether as a result of new information, future events or circumstances or otherwise. HGS, Human Genome Sciences, and BENLYSTA are trademarks of Human Genome Sciences, Inc. Other trademarks referenced are the property of their respective owners. |
| BROAD POTENTIAL BEYOND INITIAL MARKET ENTRY IN SLE 3 JPMorgan Healthcare Conference | January 10, 2011 Drivers of Near-Term Growth Drivers of Long-Term Growth |
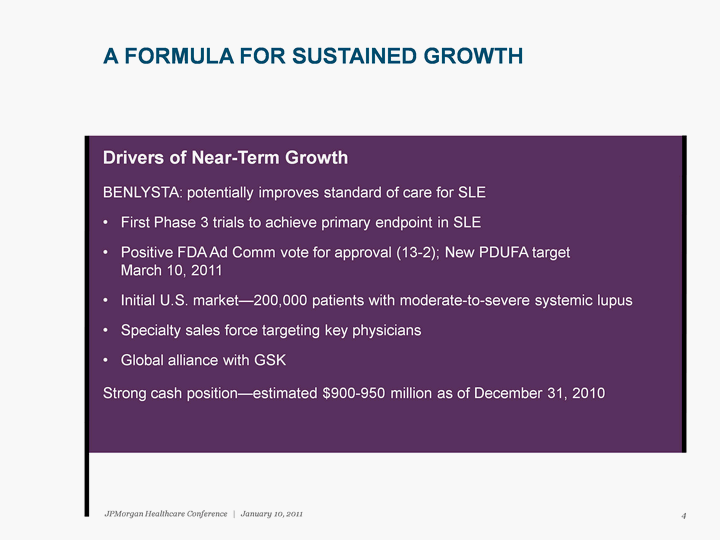
| Drivers of Near-Term Growth BENLYSTA: potentially improves standard of care for SLE First Phase 3 trials to achieve primary endpoint in SLE Positive FDA Ad Comm vote for approval (13-2); New PDUFA target March 10, 2011 Initial U.S. market-200,000 patients with moderate-to-severe systemic lupus Specialty sales force targeting key physicians Global alliance with GSK Strong cash position-estimated $900-950 million as of December 31, 2010 A FORMULA FOR SUSTAINED GROWTH 4 JPMorgan Healthcare Conference | January 10, 2011 |
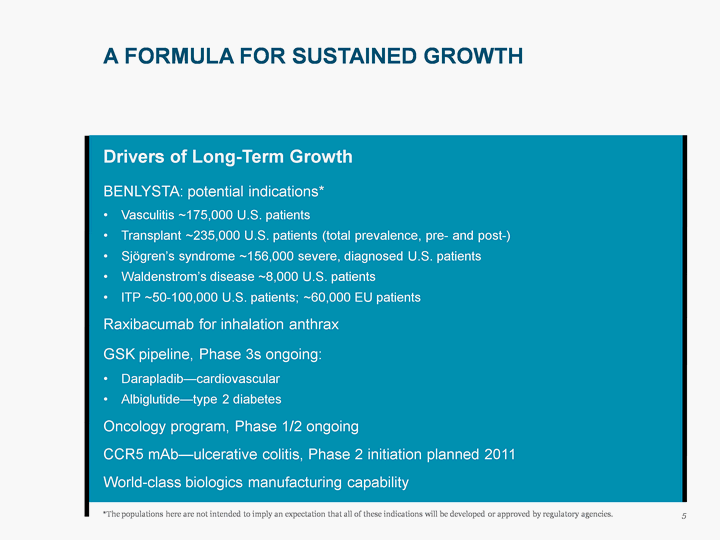
| A FORMULA FOR SUSTAINED GROWTH 5 Drivers of Long-Term Growth BENLYSTA: potential indications* Vasculitis ~175,000 U.S. patients Transplant ~235,000 U.S. patients (total prevalence, pre- and post-) Sjogren's syndrome ~156,000 severe, diagnosed U.S. patients Waldenstrom's disease ~8,000 U.S. patients ITP ~50-100,000 U.S. patients; ~60,000 EU patients Raxibacumab for inhalation anthrax GSK pipeline, Phase 3s ongoing: Darapladib-cardiovascular Albiglutide-type 2 diabetes Oncology program, Phase 1/2 ongoing CCR5 mAb-ulcerative colitis, Phase 2 initiation planned 2011 World-class biologics manufacturing capability *The populations here are not intended to imply an expectation that all of these indications will be developed or approved by regulatory agencies. |

| 6 JPMorgan Healthcare Conference | January 10, 2011 POTENTIALLY IMPROVES STANDARD OF CARE FOR SLE |
| Potential to be the first drug approved for SLE in 50 years High unmet need Global alliance with GSK Targeted commercial strategy BENLYSTA(r) NEAR TERM OPPORTUNITY SLE THE MEANINGFUL DRIVER 7 JPMorgan Healthcare Conference | January 10, 2011 |

| SLE IS A SYSTEMIC DISEASE AFFECTING MULTIPLE ORGANS 8 JPMorgan Healthcare Conference | January 10, 2011 A chronic, life-threatening autoimmune disease resulting from excess autoantibody production Multiple organ systems often affected simultaneously Skin, joint and immunologic most common Symptoms include severe pain, extreme fatigue, swollen joints, skin rash and kidney problems Mortality rate 2-5x normal Eyes and Mucous Membranes Heart and Lungs Skin Gastrointestinal System Reproductive System Musculoskeletal System Joints Kidneys Central Nervous System Blood |
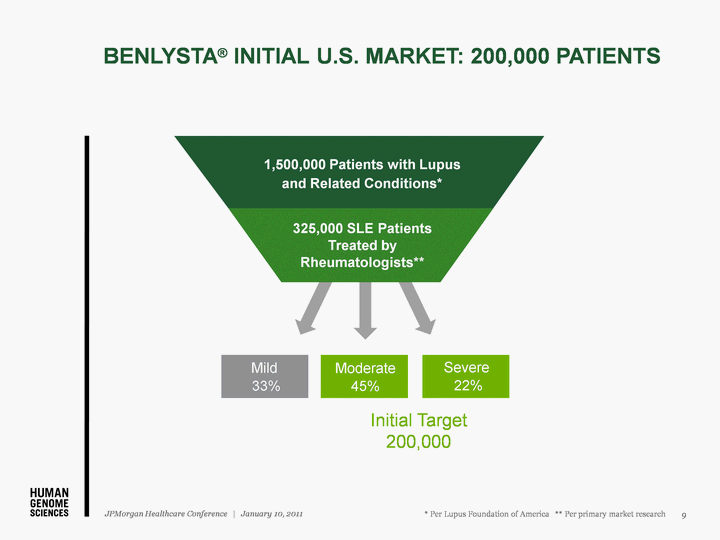
| Mild 33% Severe 22% Moderate 45% Initial Target 200,000 1,500,000 Patients with Lupus and Related Conditions* 325,000 SLE Patients Treated by Rheumatologists** * Per Lupus Foundation of America ** Per primary market research BENLYSTA(r) INITIAL U.S. MARKET: 200,000 PATIENTS 9 JPMorgan Healthcare Conference | January 10, 2011 |
| Met primary endpoint in two Phase 3 trials Reduction of disease activity over and above standard therapy Signals of other potential benefits Reduced use of steroids and risk of severe SLE flare rates Improved quality of life Normalized serological activity Nearly 2,000 patients studied for SLE Up to 6 years in Phase 2 continuation studies Administered with wide range of standard therapies SAFETY: Rates of overall adverse events, serious and/or severe adverse events, all infections, serious and/or severe infections, and discontinuations due to adverse events were comparable in Phase Three trials between BENLYSTA and placebo BENLYSTA(r) STUDY PROGRAM FIRST SUCCESSFUL PHASE 3 TRIALS FOR SLE 10 |
| BENLYSTA(r) STUDY PROGRAM STUDY POPULATION REPRESENTATIVE OF MAJORITY OF SLE PATIENTS 11 JPMorgan Healthcare Conference | January 10, 2011 Patients included in the trials had active SLE Autoantibody positive (ANA or anti-dsDNA) SELENA SLEDAI score ^6 Most on 2 or more stable therapies Patients excluded from the trials At risk for acute intervention with highly aggressive therapy Severe active lupus kidney disease Severe active CNS disease (including seizures, psychosis) |
| Bringing BENLYSTA(r) to Patients 12 JPMorgan Healthcare Conference | January 10, 2011 |
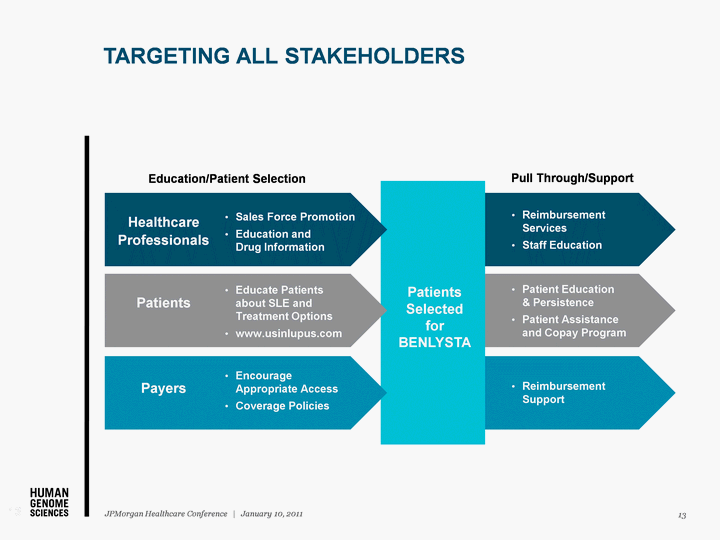
| Patients Selected for BENLYSTA TARGETING ALL STAKEHOLDERS 13 JPMorgan Healthcare Conference | January 10, 2011 13 Education/Patient Selection Pull Through/Support Healthcare Professionals Sales Force Promotion Education and Drug Information Reimbursement Services Staff Education Patient Education & Persistence Patient Assistance and Copay Program Reimbursement Support Patients Educate Patients about SLE and Treatment Options www.usinlupus.com Payers Encourage Appropriate Access Coverage Policies |
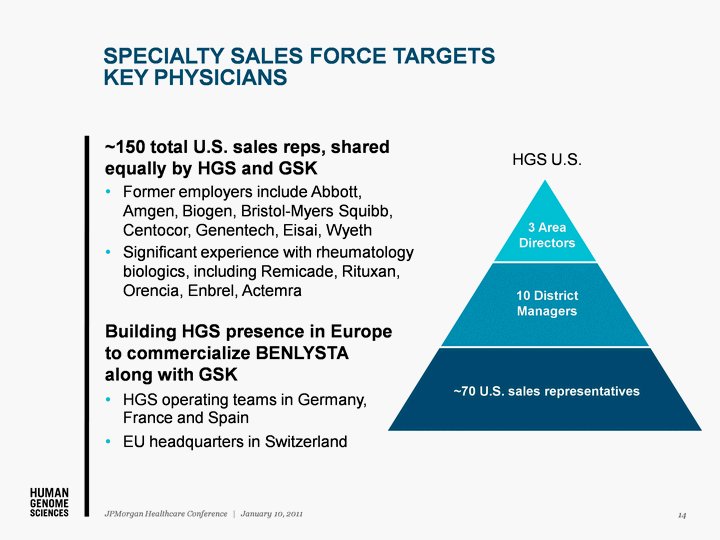
| SPECIALTY SALES FORCE TARGETS KEY PHYSICIANS ~150 total U.S. sales reps, shared equally by HGS and GSK Former employers include Abbott, Amgen, Biogen, Bristol-Myers Squibb, Centocor, Genentech, Eisai, Wyeth Significant experience with rheumatology biologics, including Remicade, Rituxan, Orencia, Enbrel, Actemra Building HGS presence in Europe to commercialize BENLYSTA along with GSK HGS operating teams in Germany, France and Spain EU headquarters in Switzerland 3 Area Directors 10 District Managers ~70 U.S. sales representatives HGS U.S. 14 JPMorgan Healthcare Conference | January 10, 2011 |
| OPTIMIZING ACCESS TO BENLYSTA(r) A KEY PRIORITY BENLYSTA will primarily be covered under the Medical Benefit Access broader in scope than Pharmacy Benefit Payers establish policies for product access Prior authorization requirements GSK Payer Account Managers support coverage policy determination SLE education and product information on BENLYSTA Proactive in defining appropriate patient types Encourage appropriate prior authorization requirements 15 JPMorgan Healthcare Conference | January 10, 2011 |
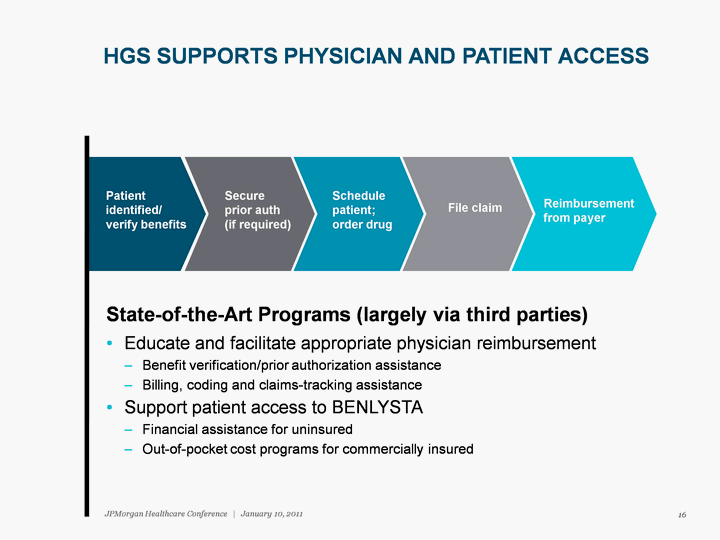
| Patient identified/ verify benefits Secure prior auth (if required) Schedule patient; order drug File claim Reimbursement from payer HGS SUPPORTS PHYSICIAN AND PATIENT ACCESS State-of-the-Art Programs (largely via third parties) Educate and facilitate appropriate physician reimbursement Benefit verification/prior authorization assistance Billing, coding and claims-tracking assistance Support patient access to BENLYSTA Financial assistance for uninsured Out-of-pocket cost programs for commercially insured 16 JPMorgan Healthcare Conference | January 10, 2011 |
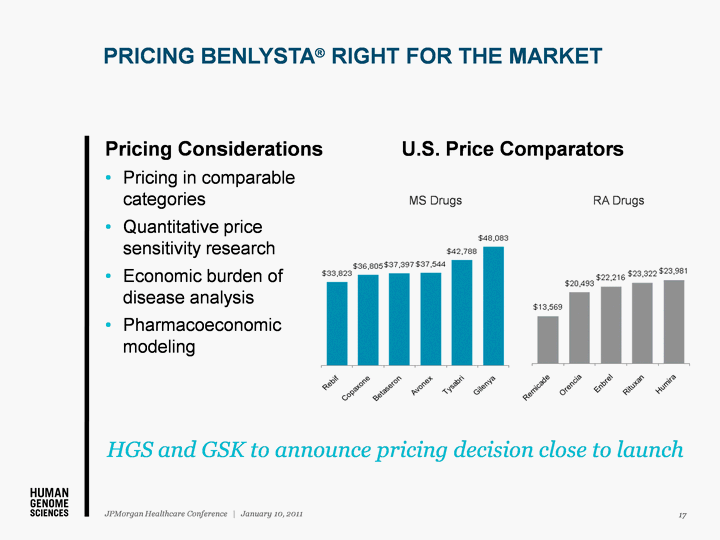
| PRICING BENLYSTA(r) RIGHT FOR THE MARKET Pricing Considerations Pricing in comparable categories Quantitative price sensitivity research Economic burden of disease analysis Pharmacoeconomic modeling U.S. Price Comparators HGS and GSK to announce pricing decision close to launch MS Drugs RA Drugs 17 JPMorgan Healthcare Conference | January 10, 2011 |
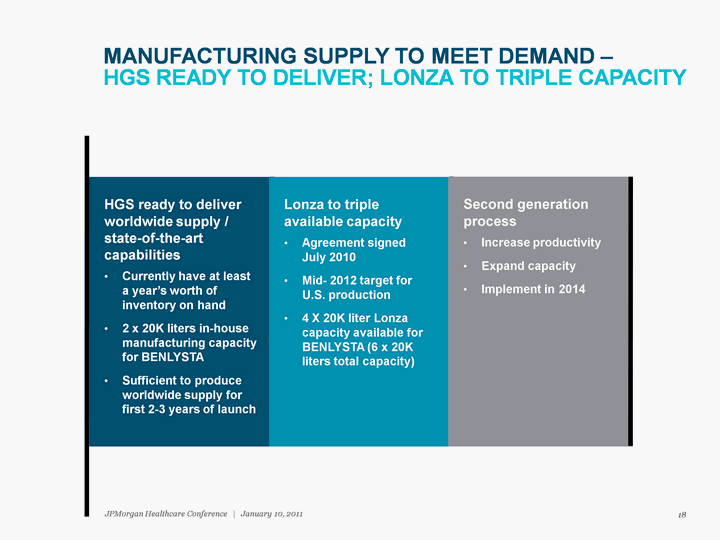
| MANUFACTURING SUPPLY TO MEET DEMAND - HGS READY TO DELIVER; LONZA TO TRIPLE CAPACITY 18 JPMorgan Healthcare Conference | January 10, 2011 |
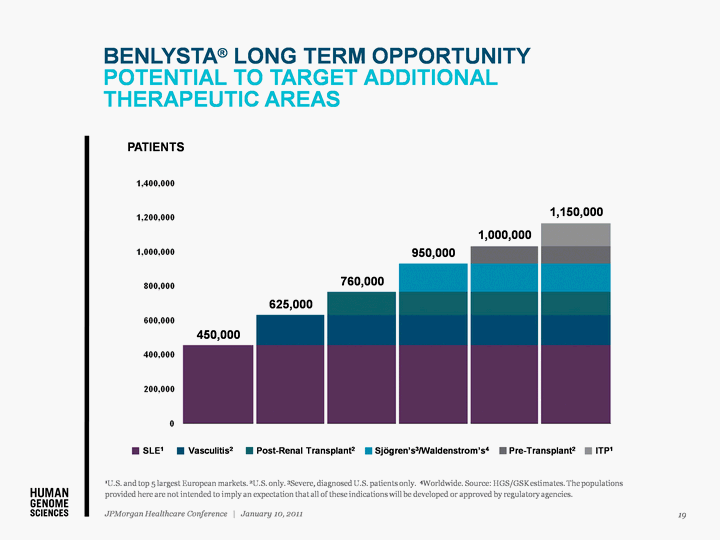
| BENLYSTA(r) LONG TERM OPPORTUNITY POTENTIAL TO TARGET ADDITIONAL THERAPEUTIC AREAS 19 JPMorgan Healthcare Conference | January 10, 2011 1U.S. and top 5 largest European markets. 2U.S. only. 3Severe, diagnosed U.S. patients only. 4Worldwide. Source: HGS/GSK estimates. The populations provided here are not intended to imply an expectation that all of these indications will be developed or approved by regulatory agencies. SLE1 Vasculitis2 Post-Renal Transplant2 Sjogren's3/Waldenstrom's4 Pre-Transplant2 ITP1 450,000 625,000 760,000 950,000 1,000,000 1,150,000 PATIENTS |
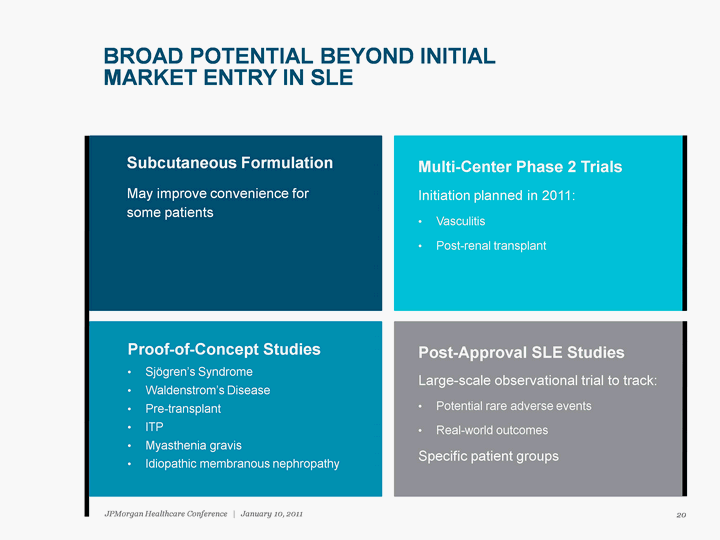
| Proof-of-Concept Studies Sjogren's Syndrome Waldenstrom's Disease Pre-transplant ITP Myasthenia gravis Idiopathic membranous nephropathy BROAD POTENTIAL BEYOND INITIAL MARKET ENTRY IN SLE Subcutaneous Formulation May improve convenience for some patients Multi-Center Phase 2 Trials Initiation planned in 2011: Vasculitis Post-renal transplant Post-Approval SLE Studies Large-scale observational trial to track: Potential rare adverse events Real-world outcomes Specific patient groups 20 JPMorgan Healthcare Conference | January 10, 2011 |

| Beyond 21 JPMorgan Healthcare Conference | January 10, 2011 |
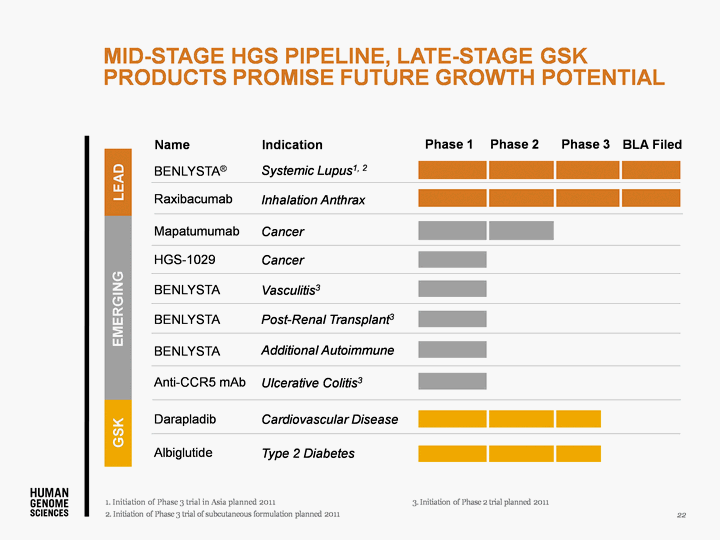
| MID-STAGE HGS PIPELINE, LATE-STAGE GSK PRODUCTS PROMISE FUTURE GROWTH POTENTIAL 22 BENLYSTA(r) Anti-CCR5 mAb HGS-1029 BENLYSTA BENLYSTA BENLYSTA Raxibacumab Mapatumumab Darapladib Systemic Lupus1, 2 Ulcerative Colitis3 Cancer Additional Autoimmune Vasculitis3 Post-Renal Transplant3 Inhalation Anthrax Cancer Type 2 Diabetes Cardiovascular Disease BLA Filed Albiglutide |
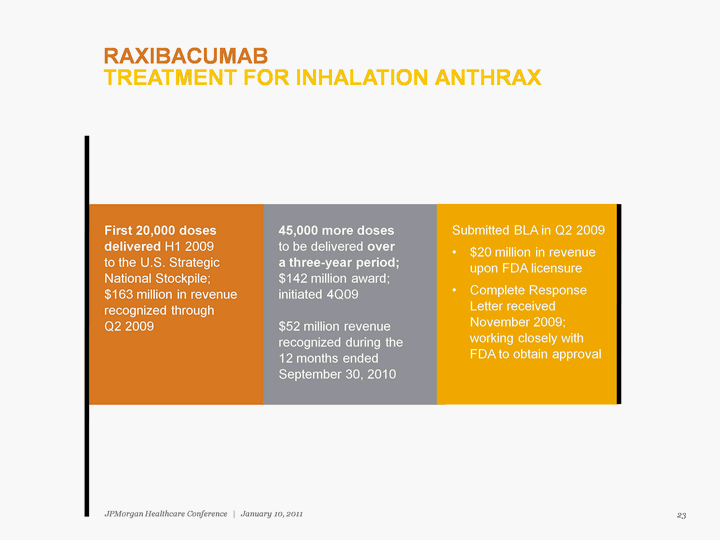
| First 20,000 doses delivered H1 2009 to the U.S. Strategic National Stockpile; $163 million in revenue recognized through Q2 2009 45,000 more doses to be delivered over a three-year period; $142 million award; initiated 4Q09 $52 million revenue recognized during the 12 months ended September 30, 2010 RAXIBACUMAB TREATMENT FOR INHALATION ANTHRAX 23 JPMorgan Healthcare Conference | January 10, 2011 |
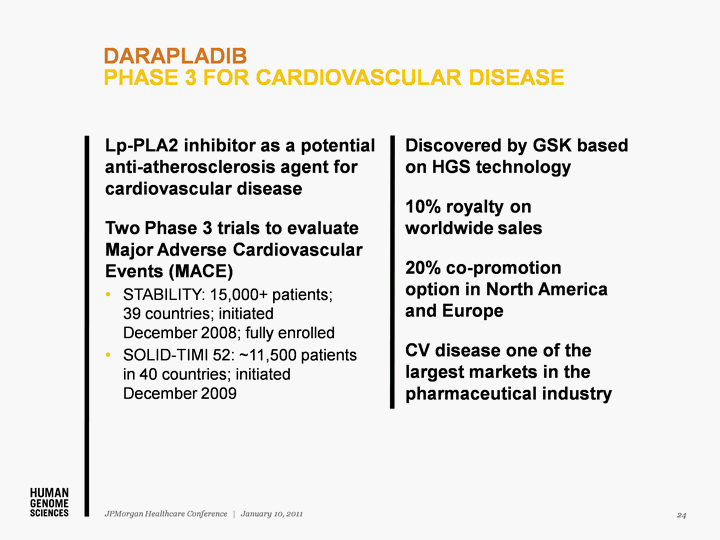
| Discovered by GSK based on HGS technology 10% royalty on worldwide sales 20% co-promotion option in North America and Europe CV disease one of the largest markets in the pharmaceutical industry DARAPLADIB PHASE 3 FOR CARDIOVASCULAR DISEASE 24 JPMorgan Healthcare Conference | January 10, 2011 Lp-PLA2 inhibitor as a potential anti-atherosclerosis agent for cardiovascular disease Two Phase 3 trials to evaluate Major Adverse Cardiovascular Events (MACE) STABILITY: 15,000+ patients; 39 countries; initiated December 2008; fully enrolled SOLID-TIMI 52: ~11,500 patients in 40 countries; initiated December 2009 |
| Licensed to GSK Fees and milestone payments up to $183 million; up to $150 million still to be received Single-digit net royalties on worldwide sales Approximately 17.9 million diagnosed type 2 diabetes patients in the U.S.1 ALBIGLUTIDE PHASE 3 FOR TYPE 2 DIABETES 25 JPMorgan Healthcare Conference | January 10, 2011 Once-a-week GLP-1 agonist Eight Phase 3 studies ongoing to evaluate efficacy and safety as mono- and add-on therapy in patients with type 2 diabetes First Phase 3 clinical trial initiated 1Q09; expected study duration of up to 3 years 1National Diabetes Fact Sheet, 2007, Centers for Disease Control and Prevention |
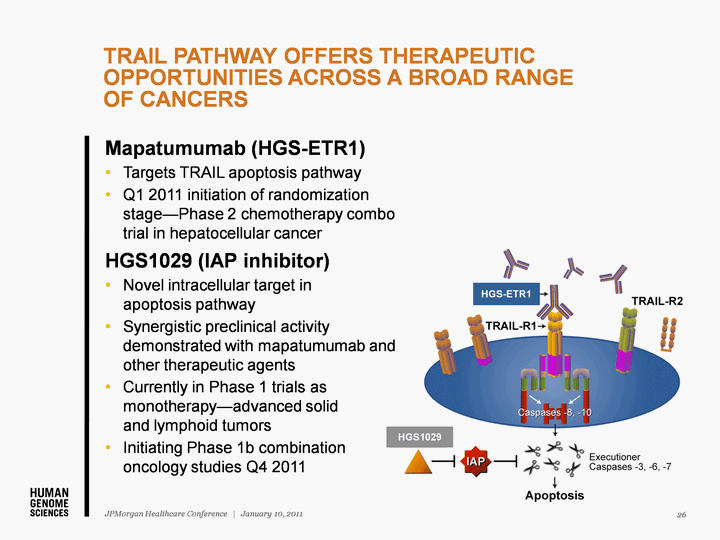
| TRAIL PATHWAY OFFERS THERAPEUTIC OPPORTUNITIES ACROSS A BROAD RANGE OF CANCERS 26 JPMorgan Healthcare Conference | January 10, 2011 Mapatumumab (HGS-ETR1) Targets TRAIL apoptosis pathway Q1 2011 initiation of randomization stage-Phase 2 chemotherapy combo trial in hepatocellular cancer HGS1029 (IAP inhibitor) Novel intracellular target in apoptosis pathway Synergistic preclinical activity demonstrated with mapatumumab and other therapeutic agents Currently in Phase 1 trials as monotherapy-advanced solid and lymphoid tumors Initiating Phase 1b combination oncology studies Q4 2011 HGS1029 HGS-ETR1 |
| Cash and investments at Sept 30, 2010: $1.0 billion Expected net cash burn 2010: $300-$350 million Expected cash and investments at year-end 2010: $900-950 million Products Nearing Commercialization High-Potential Pipeline STRONG FINANCIAL POSITION TO FUEL PRODUCT LAUNCHES, PIPELINE ADVANCEMENT 27 JPMorgan Healthcare Conference | January 10, 2011 Strong Cash Position |
| Potential Blockbuster Product Pipeline to Fuel Future Growth Manufacturing Excellence Commercial Readiness World-Class Partner Seasoned Leadership A FORMULA FOR SUSTAINABLE GROWTH 28 JPMorgan Healthcare Conference | January 10, 2011 Building Shareholder Value |

| The End 29 |
