Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - HALOZYME THERAPEUTICS, INC. | c10762e8vk.htm |
| EX-10.1 - EXHIBIT 10.1 - HALOZYME THERAPEUTICS, INC. | c10762exv10w1.htm |
Exhibit 99.1

| Gregory I. Frost, Ph.D. President and CEO J.P. Morgan 29th Annual Healthcare Conference January 12, 2011 |

| Safe Harbor All of the statements in this presentation that are not statements of historical facts constitute forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. These forward-looking statements and all references to financial estimates are based upon management's current plans and expectations and are subject to a number of risks and uncertainties which could cause actual results to differ materially from such statements. While the Company believes that the assumptions concerning future events are reasonable, it cautions that there are inherent difficulties in anticipating or predicting certain important factors. A discussion of these factors, including risks and uncertainties, is set forth in the Company's annual and quarterly reports filed from time to time with the Securities and Exchange Commission. The Company disclaims any intention or obligation to revise or update any forward-looking statements, whether as a result of new information, future events, or otherwise. J.P. Morgan 29th Healthcare Conference, 2011 |

| Halozyme Profile and Investment Highlights An Innovative Enzyme Platform A capital efficient biopharmaceutical company developing and commercializing recombinant human enzymes that transiently modify tissue under the skin to facilitate injection of other therapies (biologics, peptides, small molecules and fluids) or correct pathologic tissue structures for clinical benefit (malignancies, edema and aesthetics) Avenues of Value Creation: Royalty bearing partnerships from enzyme delivery technology formulated with high value biologics such as subcutaneous Herceptin(r) (trastuzumab- rHuPH20), subcutaneous MabThera (r) (rituximab-rHuPH20) and HyQ (immune globulin-rHuPH20) Internal programs in endocrinology (analog insulin-rHuPH20), oncology (chemotherapy-PEGPH20) and aesthetics (rHuCATL) Solid Financial Position: Cash of $90 million end of 3Q10 J.P. Morgan 29th Healthcare Conference, 2011 |

| Updates ^ January 2011 Completed enrollment of two insulin analog-PH20 TID studies 110 type 1 and 110 type 2 patients in 24-week crossover study Data available internally 3Q2011 Reacquisition of Hylenex (stand alone 150 U dose rHuPH20) from Baxter Initial product reintroduction planned through a transition services agreement with Baxter Future manufacturing at an alternate fill finish site using second generation API Initiation of insulin pump trials in type 1 patients Studies to evaluate the effects of rHuPH20 on continuous insulin infusions J.P. Morgan 29th Healthcare Conference, 2011 |

| Halozyme's Structure Enables Focus On Core Capabilities in a Capital Efficient Manner BD & Marketing Quality Assurance Research and Preclinical Clinical Development Product Development Manufacturing Regulatory Affairs contracted contracted Finance J.P. Morgan 29th Healthcare Conference, 2011 |

| Core Activities in 2011 Support the Platform Recombinant enzyme manufacturing supports partnerships and pipeline (Roche, Baxter) + (Ultrafast Insulin, PEGPH20, Hylenex) Two cGMP bulk rHuPH20 manufacturing sites established for multi-kg capacity Validation campaign at 300L scale complete 2Q2011 Validation campaign at 2500L scale starting 1Q2011 Indiana Facility 2500L Scale Scalable Manufacturing Processes California Facility 300L Scale J.P. Morgan 29th Healthcare Conference, 2011 |

| 7 Halozyme's Core Technology: Targets Hyaluronan (HA) in "Matrix" matrix create resistance to bulk fluid flow rHuPH20 decreases local resistance to injection of other molecules allowing exposure to capillaries and lymphatics Halozyme's Core Technology: Targets Hyaluronan (HA) in "Matrix" J.P. Morgan 29th Healthcare Conference, 2011 |

| Time Plasma Levels Higher CMAX ^ Higher peak from same dose More Rapid TMAX ^ Fast In/ Fast Out ^CMAX & ^TMAX SC Plus rHuPH20 Standard SC PK Profile CMAX Pharmacokinetic (PK) Implications for Subcutaneous (SC) Peptides and Drugs J.P. Morgan 29th Healthcare Conference, 2011 |

| Time (hours to days) Plasma Levels IV Infusion (approved dose and frequency) SC with rHuPH20 SC alone No volume restrictions ^ Capture best dosing frequency Higher Cmax ^ Closer to IV than SC Higher BA ^ Similar to IV for clinical bridging Pharmacokinetic and Dosing Implications for Antibodies and Other Biologics J.P. Morgan 29th Healthcare Conference, 2011 |
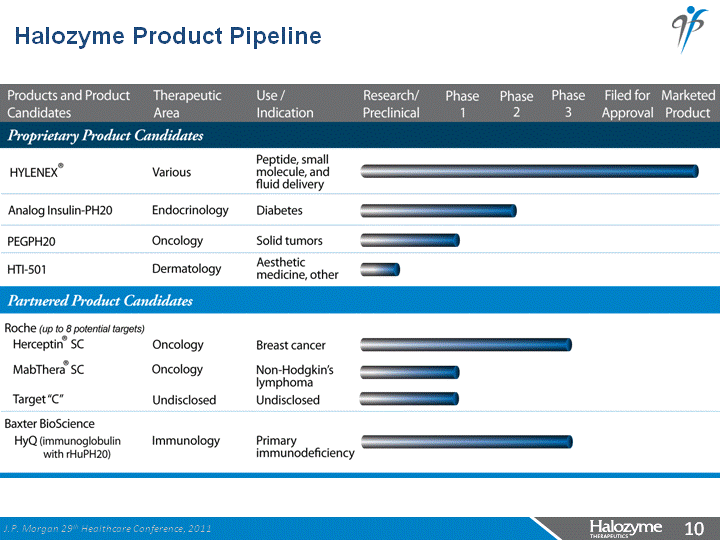
| Halozyme Product Pipeline J.P. Morgan 29th Healthcare Conference, 2011 |

| Alliances Drive Significant Value ~ $108 million of alliance related cash received to date Enhanze technology with Roche ^ up to 8 biologic targets, 5 currently exclusive Alliance worth up to $377M ($20M up-front; $111M milestones for first 3 exclusive targets; $235M up-front & milestones for 5 additional targets; $11M equity), plus royalties; $58M received to date Enhanze technology with Baxter BioScience (HyQ) Alliance worth up to $47M ($10M up-front; $37M milestones), plus royalties; $10M received to date J.P. Morgan 29th Healthcare Conference, 2011 |
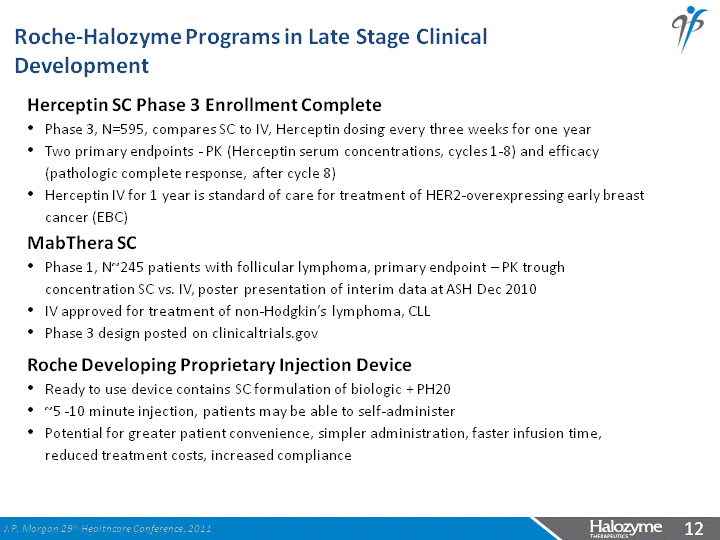
| Herceptin SC Phase 3 Enrollment Complete Phase 3, N=595, compares SC to IV, Herceptin dosing every three weeks for one year Two primary endpoints - PK (Herceptin serum concentrations, cycles 1-8) and efficacy (pathologic complete response, after cycle 8) Herceptin IV for 1 year is standard of care for treatment of HER2-overexpressing early breast cancer (EBC) MabThera SC Phase 1, N~245 patients with follicular lymphoma, primary endpoint - PK trough concentration SC vs. IV, poster presentation of interim data at ASH Dec 2010 IV approved for treatment of non-Hodgkin's lymphoma, CLL Phase 3 design posted on clinicaltrials.gov Roche Developing Proprietary Injection Device Ready to use device contains SC formulation of biologic + PH20 ~5 -10 minute injection, patients may be able to self-administer Potential for greater patient convenience, simpler administration, faster infusion time, reduced treatment costs, increased compliance Roche-Halozyme Programs in Late Stage Clinical Development J.P. Morgan 29th Healthcare Conference, 2011 |

| GAMMAGARD Liquid - plasma derived immunoglobulin (IgG) indicated for primary immunodeficiency, approved for IV infusion $1B+ product franchise for Baxter, ~ $6B worldwide market Current SC IgG limited by low bioavailability (~70%) and small injection site volumes - requires weekly dosing with multiple needles Phase 1/2a trial demonstrated that once monthly SC administration with PH20 resulted in bioavailability equal to 92% of IV administration Potential for convenient self-administration once monthly in a single SC injection site; unique SC product for Baxter franchise Phase 3 completed with ~ 89 patients; presented interim data Oct 2010 at ESID; BLA filing in 2011 and launch in 2012* HyQ (immunoglobulin) - SC Monthly Dosing at Single Site with Halozyme's rHuPH20 * Baxter projections as stated on investor conference calls J.P. Morgan 29th Healthcare Conference, 2011 |

| Halozyme Reacquires Hylenex Distribution Rights from Baxter J.P. Morgan 29th Healthcare Conference, 2011 HyQ relationship with Baxter Bioscience unchanged Hylenex indicated for the dispersion and absorption of other injected drugs; for subcutaneous fluid hydration Composition of matter IP to recombinant protein until 2027 FDA feedback provides path to reintroduction 2H2011 through existing site with new process at future location Initiated activities to establish alternate manufacturing site using second generation API Additional Phase 4 studies in areas of Halozyme's strategic interests (insulin pumps, aesthetics) planned for 2011 Opportunities for strategic partnerships in emerging geographic markets |

| Proprietary Product Development Candidates Ultrafast Insulin - Analog insulins formulated with rHuPH20 for diabetes PEGPH20 - PEGylated rHuPH20 targets HA expressing tumors HTI-501 - Novel Matrix remodeling enzyme targets collagen degradation for aesthetics J.P. Morgan 29th Healthcare Conference, 2011 |

| Goal - To develop best-in-class rapid-acting insulin products that surpass current standards of care 8 clinical studies completed or ongoing with insulin products to optimize product configuration, safety profile and clinical attributes Proprietary insulin formulations of novel rHuPH20 permeation enhancing excipient with rapid-acting insulin analogs Humalog(r), NovoLog(r), Apidra(r) ? "Analog-PH20" Currently in Phase 2, targeting commercialization by 2014, depending on most attractive product candidate or partnership Halozyme's Ultrafast Injectable Insulin Program J.P. Morgan 29th Healthcare Conference, 2011 |
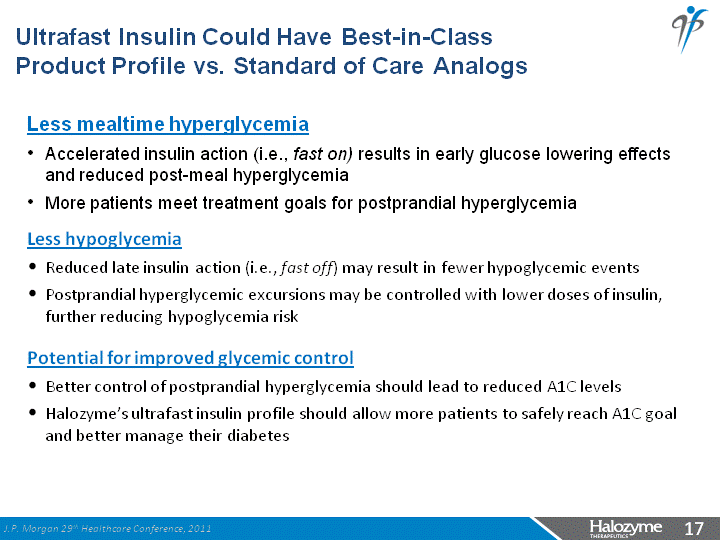
| Less mealtime hyperglycemia Accelerated insulin action (i.e., fast on) results in early glucose lowering effects and reduced post-meal hyperglycemia More patients meet treatment goals for postprandial hyperglycemia Ultrafast Insulin Could Have Best-in-Class Product Profile vs. Standard of Care Analogs Less hypoglycemia Reduced late insulin action (i.e., fast off) may result in fewer hypoglycemic events Postprandial hyperglycemic excursions may be controlled with lower doses of insulin, further reducing hypoglycemia risk Potential for improved glycemic control Better control of postprandial hyperglycemia should lead to reduced A1C levels Halozyme's ultrafast insulin profile should allow more patients to safely reach A1C goal and better manage their diabetes J.P. Morgan 29th Healthcare Conference, 2011 |

| Consistent Ultrafast PK Profile from Study to Study Proof of Concept Study in HV PH20 Dose Selection Study, Stage 4 Variability Study Comprehensive Analog Study Type 1 Meal Study Type 2 Meal Study J.P. Morgan 29th Healthcare Conference, 2011 |

| Ultrafast Analog+PH20 Profile Improves Glucose Control in Both T1DM and T2DM Patients T1DM Meal Study (Identical dose with and without PH20) T2DM Meal Study (Dose optimized for each test article separately) T1DM Meal T2DM Meal % to Glycemic Goal (ADA) 36% More (91% v. 55%) 23% More (71% v. 48%) Hyperglcyemic Excursions (>140 mg/dL) 79% Less 44% Less Hypoglycemic Events No Change 25 to 30% Fewer J.P. Morgan 29th Healthcare Conference, 2011 |

| Two Ongoing Phase 2 Studies - Analogs +- PH20, Part of Intensive TID Insulin Treatment Regime HALO-205, HALO-206, N=110 for each study, 3x/day, 12 weeks x-over Designed to demonstrate meaningful improvement of lispro-PH20 and/or aspart-PH20 vs. Humalog in patients with T1DM or T2DM Enrollment completed Jan 2011 Results available to Halozyme 3Q11 for presentation in 2012 Primary Endpoint Compare Lispro-PH20 or Aspart-PH20 to insulin lispro alone with respect to glycemic control - change in A1C from baseline Secondary Endpoints Blood glucose profiles over 3 days, SMBG and CGM Postprandial glucose changes from pre-meal baseline Proportion of patients achieving A1C <7.0%, and A1C ^6.5% Overall rate of hypoglycemia Composite endpoint of proportion of patients achieving A1C goals without unacceptable hypoglycemia J.P. Morgan 29th Healthcare Conference, 2011 |

| 0 Insulin Pump Market Represents an Additional Large Opportunity 54.0892193308612 89.5787139689681 45.9107806691502 10.4212860310433 MDI* Pump Patients with T2 Diabetes Patients with T2 Diabetes 90% 10% Patients with T1 Diabetes Patients with T1 Diabetes 54% 46% Primary Route of Prandial Insulin Delivery in U.S.1 ~400,000 patients with diabetes in the U.S. use insulin pumps *Multiple daily injections 1: Roper Diabetes Group, U.S. only 2: JPM Securities Number of pump patients expected to grow rapidly 389.000000000044 538.000000000061 2015E +38% 2010E Population of Pump Users in U.S.2 J.P. Morgan 29th Healthcare Conference, 2011 |

| 0 "Faster-In", "Faster-Out" Profile of Halozyme's Ultrafast Insulin Could Deliver Key Benefits to Pump Users Benefits of "Faster-in" Less waiting between delivery of bolus and meal consumption Accelerated insulin action and reduced post-meal hyperglycemia More patients meet treatment goals for postprandial hyperglycemia Benefits of "Faster-out" Ability to bolus more frequently due to reduced 'bolus stacking' Faster recovery from hypoglycemia and less hypoglycemia overall due to reduced late insulin action Benefits could allow pump patients to better manage their diabetes and safely reach A1C goals J.P. Morgan 29th Healthcare Conference, 2011 |

| Halozyme Continuous Subcutaneous Insulin Infusion (CSII) Study Design Primary Objective: Compare early insulin exposure (first 60 minutes following bolus infusion) for rapid-acting analog insulins (NovoLog, Apidra) with and without rHuPH20 during euglycemic glucose clamp procedures in patients with type 1 diabetes Secondary Objectives: Pharmacokinetic and glucodynamic parameters between treatments during euglycemic clamp procedures and, separately, following standardized test meals Assess the "off" and "on" rates for pharmacokinetic and serum glucose responses following suspension of CSII and after compensatory bolus treatment Compare infusion set performance as assessed by serial pharmacokinetic and glucodynamic measurements over 72 hours Evaluate safety and tolerability of subcutaneous infusion of insulin analogs with rHuPH20 Study population: Approximately 18 generally healthy CSII patients with type 1 diabetes recruited into each of two study stages * J.P. Morgan 29th Healthcare Conference, 2011 |

| Kultti et al., J Biol Chem, 2006 PEGPH20 (PEGylated Recombinant Human PH20) Many tumors create a complex matrix environment 20-30% of all solid tumors produce a matrix comprising hyaluronan (HA) and proteoglycans Matrix-rich environment can generate resistance to therapies poor perfusion, high interstitial fluid pressure, resistance to angiogenesis inhibitors and chemotherapeutics First-in-class PEGylated enzyme, unique mechanism of action eliminates protective HA-containing coats produced by tumor cells normalizes tumor perfusion and pressure, shrinks tumors, and slows expansion Animal models show that enzymatically degrading the HA rich matrix of tumors with systemic PEGPH20 slows tumor growth when used alone or in combination with other anticancer therapies chemotherapy, radiation, monoclonal antibodies, immunotherapies Thompson et al, Mol. Cancer Ther. 2010; 9(11):3052-64. Shepard et al, EORTC, 2010 Our goal is to turn these challenging cancers into a manageable disease, through this completely unique mechanism of action, complimentary to nearly all current solid tumor therapies J.P. Morgan 29th Healthcare Conference, 2011 |

| PEGPH20 Addresses a Large, Unmet Medical Need PEGPH20 therapy could provide broad access to multiple HA(+) tumor markets with large potential sales ($1B+) Cancers with the highest proportion of HA(+) patients include: Currently conducting two Phase 1 multicenter, open-label, escalating, repeat dose safety and tolerability trials with PEGPH20 in patients with advanced solid tumors to identify dose/frequency for Phase 2 clinical studies Study 101 dosed every 21 days Study 102 dosed twice weekly (first cycle, 28 days) and weekly (each subsequent cycle) with pre- and post-dose dexamethasone Preparing to initiate a Phase 2 study in metastatic pancreatic adenocarcinoma with gemcitabine +/- PEGPH20 in 2H2011 J.P. Morgan 29th Healthcare Conference, 2011 Pancreas (87%) Breast (64%) NSCLC (42%) Prostate (22%) |
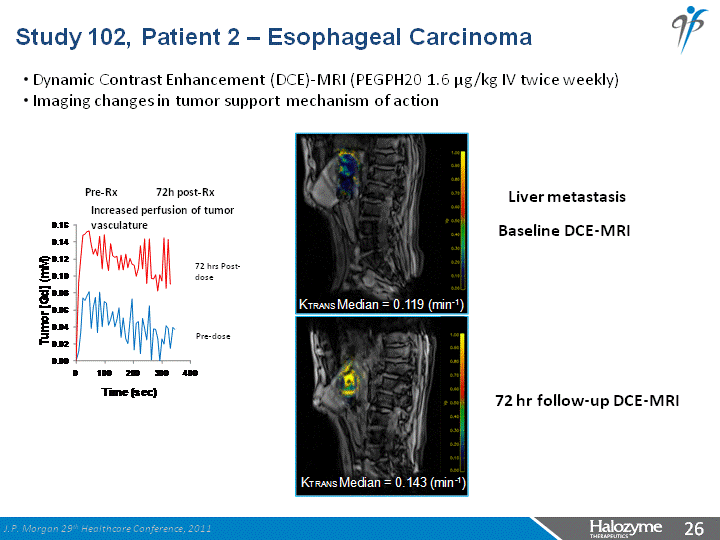
| Study 102, Patient 2 - Esophageal Carcinoma Baseline DCE-MRI 72 hr follow-up DCE-MRI Pre-Rx 72h post-Rx Dynamic Contrast Enhancement (DCE)-MRI (PEGPH20 1.6 µg/kg IV twice weekly) Imaging changes in tumor support mechanism of action Increased perfusion of tumor vasculature 72 hrs Post-dose Pre-dose Liver metastasis J.P. Morgan 29th Healthcare Conference, 2011 |

| HTI-501 (conditionally active recombinant human cathepsin) A first-in-class injectable recombinant human enzyme shown preclinically to locally break down the collagen fibrils that can cause disfiguring scars, contractures and cellulite Disfiguring scars (keloids, hypertrophic scars) and aesthetic applications, such as cellulite, have few viable treatment options rHuCATL - Temporal-spatially controlled recombinant human enzyme, can tightly control the duration and extent of collagen degradation Proof of mechanism study in cellulite has clear safety and pharmacology endpoints CTA filing on track for ex-U.S. trial to begin in 3Q2011 J.P. Morgan 29th Healthcare Conference, 2011 |

| Potential Milestones for 2011 Top line Phase 3 HyQ results by Baxter, 1H11 Filing of HyQ BLA by Baxter, 2011 Initiate randomized PEGPH20 Phase 2 clinical trial in pancreatic cancer, 2H11 Presentation of data from analog insulin pump studies with rHuPH20 3Q11 Initiate dosing of HTI-501 in ex-U.S. proof of concept study in aesthetic medicine, 3Q11 Presentation of data from PEGPH20 Phase 1 clinical trial, 2H11 Completion of two insulin Analog-PH20 Phase 2, 3x/day treatment studies, 3Q11 Potential for additional clinical progress from the Roche partnership J.P. Morgan 29th Healthcare Conference, 2011 |

| Halozyme's Late Stage Programs Target Over $20 Billion in Product Opportunities Roche's Herceptin SC in Phase 3 pivotal trial, $5.1B WW product, MabThera SC in Phase 1, $5.9B WW product Baxter BioScience's HyQ with rHuPH20 completed pivotal Phase 3, $6B WW market Interim manufacturing resolution in 2H2011, with multiple strategic opportunities in U.S. and abroad Ultrafast Insulin in Phase 2, targeting $3.8B rapid- acting analog insulin market with potential best-in- class profile Partnered Programs Proprietary Programs J.P. Morgan 29th Healthcare Conference, 2011 |

| Halozyme Therapeutics - Supplementary Slides Investor Presentation J.P. Morgan 29th Healthcare Conference, 2011 |

| Herceptin(r) - For Treatment of HER2-Overexpressing Breast Cancer Breast Cancer Most common cancer among women worldwide More than 1M new cases of breast cancer (BC) diagnosed annually worldwide 20-25% of women with breast cancer are HER2-positive Herceptin Humanized monoclonal antibody that targets and blocks the HER2 receptor Approved for adjuvant treatment of HER2-overexpressing BC and metastatic BC Herceptin for 1 year is standard of care in HER2-positive early BC, HERA 2 year results expected in 2012 Herceptin until progression is standard of care in HER2-positive metastatic breast cancer Improves response rates, disease-free survival and overall survival as monotherapy or in combination with chemotherapy Has been used to treat > 650,000 women with HER2-positive BC worldwide Sources: www.herceptin.com, World Health Organization, http://www.who.int/cancer/detection/breastcancer/en/, Ferlay J, et al., GLOBOCAN 2002. Cancer Incidence, Mortality and Prevalence Worldwide. IARC CancerBase No.5, Version 2.0. IARCPress, Lyon, 2004. J.P. Morgan 29th Healthcare Conference, 2011 |
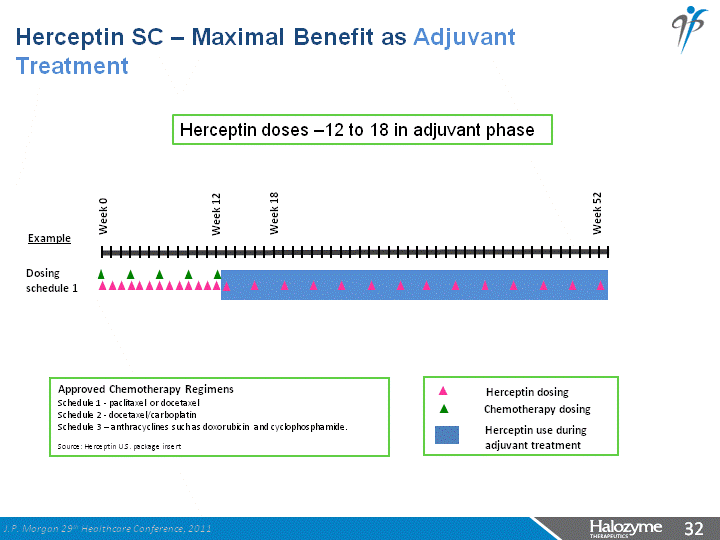
| Herceptin dosing Approved Chemotherapy Regimens Schedule 1 - paclitaxel or docetaxel Schedule 2 - docetaxel/carboplatin Schedule 3 - anthracyclines such as doxorubicin and cyclophosphamide. Source: Herceptin U.S. package insert Chemotherapy dosing Herceptin use during adjuvant treatment Dosing schedule 1 Week 0 Week 18 Week 52 Week 12 Herceptin doses -12 to 18 in adjuvant phase Example Herceptin SC - Maximal Benefit as Adjuvant Treatment J.P. Morgan 29th Healthcare Conference, 2011 |

| Herceptin SC - Phase 3 Clinical Trial Design Registration trial began Oct 2009, enrollment completed Dec 2010, N = 595, women with HER2-positive operable or locally advanced breast cancer, HANNAH Pre-operative treatment with 8 cycles of chemotherapy (docetaxel followed by 5-FU/epirubicin/cyclophosphamide) concurrent with IV or SC Herceptin, followed by 10 cycles of Herceptin SC or IV for 10 cycles after surgery, q3 weeks Surgery between cycles 8 and 9 Follow up for 24 months after last dose of Herceptin Two primary endpoints Herceptin serum concentration, comparing SC vs. IV cycles 1-8 Pathologic complete response post-surgery European filing targeted in 2012 (as stated by Roche, March 2010, NY analyst meeting) J.P. Morgan 29th Healthcare Conference, 2011 |

| MabThera(r) SC - Phase 1 Trial Underway Phase 1 began 9/09, N=245 patients with follicular lymphoma, maintenance treatment after response to IV induction therapy Two stages, involves randomization to SC or IV, Stage 1 data presented at ASH, Dec 2010 Primary endpoint - PK trough concentration for SC vs. IV measured at intervals up to day 84 Secondary endpoints: PK measures including AUC, Cmax, Tmax, T1/2 up to day 84 Adverse events, laboratory parameters measured at each clinic visit J.P. Morgan 29th Healthcare Conference, 2011 |

| % Herceptin and MabThera/Rituxan - A Large and Growing ~$11 Billion Market Opportunity *2005-2009 year end exchange rates from oanda.com Source: Roche annual reports, 2005-2009 (CHART) ~54% of sales in EU/Int'l. in 2009 J.P. Morgan 29th Healthcare Conference, 2011 |
