Attached files
| file | filename |
|---|---|
| EX-32.2 - EXHIBIT 32.2 - IMMUNE PHARMACEUTICALS INC | c08698exv32w2.htm |
| EX-31.2 - EXHIBIT 31.2 - IMMUNE PHARMACEUTICALS INC | c08698exv31w2.htm |
| EX-31.1 - EXHIBIT 31.1 - IMMUNE PHARMACEUTICALS INC | c08698exv31w1.htm |
| EX-32.1 - EXHIBIT 32.1 - IMMUNE PHARMACEUTICALS INC | c08698exv32w1.htm |
Table of Contents
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 10-K/A
(Amendment No. 1)
Annual Report Pursuant to Section 13 or 15(d) of
the Securities Exchange Act of 1934
the Securities Exchange Act of 1934
For the Fiscal Year Ended December 31, 2009
Commission File No. 000-51290
EpiCept Corporation
(Exact name of registrant as specified in its charter)
| Delaware | 52-1841431 | |
| (State or other jurisdiction of | (IRS Employer Identification No.) | |
| incorporation or organization) |
777 Old Saw Mill River Road
Tarrytown, NY 10591
(Address of principal executive offices) (zip code)
Tarrytown, NY 10591
(Address of principal executive offices) (zip code)
Registrant’s telephone number, including area code: (914) 606-3500
Securities registered pursuant to Section 12(b) of the Act:
Common Stock, par value $.0001 per share
(Title of Class)
Common Stock, par value $.0001 per share
(Title of Class)
Securities registered pursuant to Section 12(g) of the Act:
None
(Title of Class)
None
(Title of Class)
Indicate by check mark whether the registrant is a well-known seasoned issuer, as defined in
Rule 405 of the Securities Act.
Yes o No þ.
Indicate by check mark whether the registrant is not required to file reports pursuant to
Section 13 or 15(d) of the Exchange Act. Yes o No þ.
Indicate by check mark whether the registrant (1) has filed all reports required to be filed
by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or
for such shorter period that the registrant was required to file such reports), and (2) has been
subject to such filing requirements for the past 90 days. Yes þ No o.
Indicate by check mark whether the registrant has submitted electronically and posted on its
corporate website, if any, every Interactive Data File required to be submitted and posted pursuant
to Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period that the
registrant was required to submit and post such files).
Yes o No o
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation
S-K is not contained herein and will not be contained, to the best of registrant’s knowledge, in
definitive proxy or information statements incorporated by reference in Part III of this Form 10-K
or any amendments to this Form 10-K. o
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated
filer, a non-accelerated filer or a smaller reporting company. See definitions of “large
accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the
Exchange Act.
| Large accelerated filer o | Accelerated filer þ | Non-accelerated filer o | Smaller reporting company o |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of
the Exchange Act). Yes o No þ.
As of June 30, 2009, the last business day of the registrant’s most recently completed second
fiscal quarter, the aggregate market value of shares of common stock held by non-affiliates was
$101,370,124.
As of March 5, 2010, the registrant had 44,170,984 shares of its common stock, par value
$.0001 per share, outstanding.
Table of Contents
Explanatory Note:
EpiCept Corporation is filing this Amendment No. 1 (this “Form 10-K/A”) to its Annual Report on
Form 10-K for the year ended December 31, 2009 (the “Original Filing”) in response to comments of
the staff of the Securities and Exchange Commission. For purposes of this Annual Report on Form
10-K/A, and in accordance with Rule 12b-15 under the Exchange Act, Items 1 and 11 of our Original
Filing, as amended, have been amended and restated in their entirety.
In addition, as required by Rule 12b-15 under the Securities and Exchange Act of 1934, as amended
(the “Exchange Act”), new certifications by our principal executive officer and financial officer
are filed or furnished, as applicable, as exhibits to this Form 10-K/A under Item 15 of Part IV
hereof.
Except as stated herein, this Form 10-K/A does not reflect events occurring after the filing of the
Original Filing and no attempt has been made in this Form 10-K/A to modify or update other
disclosures as presented in the Original Filing. Accordingly, this Form 10-K/A should be read in
conjunction with our filings with the SEC subsequent to the filing of the Original Filing.
2
Table of Contents
ITEM 1. BUSINESS
We are a specialty pharmaceutical company focused on the clinical development and
commercialization of pharmaceutical products for the treatment of cancer and pain. We focus our
clinical development efforts on innovative cancer therapies and topically delivered analgesics
targeting peripheral nerve receptors. Our lead product is Ceplene®, which when used
concomitantly with low-dose interleukin-2 is intended as remission maintenance therapy in the
treatment of acute myeloid leukemia, or AML, for adult patients who are in their first complete
remission. In October 2008, the European Commission issued a formal marketing authorization for
Ceplene® in the European Union, or EU. In November 2009, Health Canada accepted for
review a New Drug Submission, or NDS, for Ceplene® for the treatment of AML in Canada.
We are continuing our preparation of a New Drug Application, or NDA, filing with the United States
Food and Drug Administration, or FDA for Ceplene® in the same indication. In addition
to Ceplene®, we have two oncology compounds and a pain product candidate for the
treatment of peripheral neuropathies in clinical development. We believe this portfolio of
oncology and pain management product candidates lessens our reliance on the success of any single
product or product candidate.
Our cancer portfolio includes crinobulin, a novel small molecule vascular disruption agent, or
VDA, and apoptosis inducer for the treatment of patients with solid tumors. We have completed our
first Phase I clinical trial for crinobulin. AzixaTM, an apoptosis inducer with VDA
activity licensed by us to Myriad Genetics Inc., or Myriad, as part of an exclusive, worldwide
development and commercialization agreement, is currently in Phase II clinical trials in patients
with primary glioblastoma, melanoma that has metastasized to the brain and non-small-cell lung
cancer that has spread to the brain.
Our late-stage pain product candidate, EpiCeptTM NP-1 cream, which we refer to as
NP-1, is a prescription topical analgesic cream designed to provide effective long-term relief of
pain associated with peripheral neuropathies. In January 2009, we concluded a Phase II clinical
trial of NP-1 in which we studied its safety and efficacy in patients suffering from post-herpetic
neuralgia, or PHN, compared to gabapentin and placebo. This trial met its primary endpoints. In
February 2008, we concluded a Phase II clinical study of NP-1 in patients suffering from diabetic
peripheral neuropathy, or DPN. Both studies support the advancement of NP-1 into a
registration-sized trial. NP-1 utilizes a proprietary formulation to administer FDA approved pain
management therapeutics, or analgesics, directly on the skin’s surface at or near the site of the
pain, targeting pain that is influenced, or mediated, by nerve receptors located just beneath the
skin’s surface.
Product Portfolio
The following chart illustrates the depth of our product pipeline:
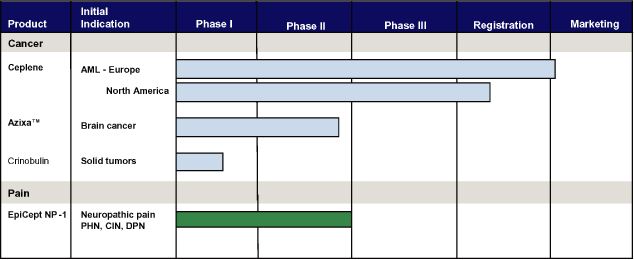
4
Table of Contents
Cancer
Cancer is the second leading cause of death in the United States. Half of all men and one
third of all women in the United States will develop cancer during their lifetimes. Today, millions
of people are living with cancer or have had cancer. Although there are many kinds of cancer, they
are all caused by the out-of-control growth of abnormal cells. Normal body cells grow, divide, and
die in an orderly fashion. During the early years of a person’s life, normal cells divide more
rapidly until the person becomes an adult. After that, cells in most parts of the body divide only
to replace worn-out or dying cells and to repair injuries. Because cancer cells continue to grow
and divide, they are different from normal cells. Instead of dying, they outlive normal cells and
continue to form new abnormal cells.
Cancer usually forms as a tumor. However, some cancers, like leukemia, do not form tumors.
Instead, these cancer cells involve the blood and blood-forming organs and circulate through other
tissues where they grow. Often, cancer cells travel to other parts of the body where they begin to
grow and replace normal tissue. Different types of cancer can behave very differently. For example,
lung cancer and breast cancer are very different diseases. They grow at different rates and respond
to different treatments. That is why people with cancer need treatment that is aimed at their
particular kind of cancer. The risk of developing most types of cancer can be reduced by changes in
a person’s lifestyle, for example, by quitting smoking and following a better diet. The sooner a
cancer is found and treatment begins, the better are the chances for long term survival.
Ceplene®
AML is the most deadly and most common type of acute leukemia in adults. There are
approximately 40,000 AML patients in the EU, with 16,000 new cases occurring each year.
Additionally, there are approximately 13,000 new cases of AML and 9,000 deaths caused by this
cancer each year in the United States. Once diagnosed with AML, patients typically receive
induction and consolidation chemotherapy, with the majority achieving complete remission. However,
about 70-80% of patients who achieve first complete remission will relapse, with the median time in
remission before relapse with current treatments being only 12 months. Less than 15% of relapsed
patients survive long-term.
Ceplene® ( histamine dihydrochloride) is our proprietary product for the remission
maintenance and prevention of relapse in adult patients with AML in first remission. Ceplene®
is to be administered in conjunction with low-dose IL-2 and is designed to protect
lymphocytes responsible for immune-mediated destruction of residual leukemic cells.
Ceplene®reduces the formation of oxygen radicals from phagocytes, inhibiting
nicotinamide adenine dinucleotide phosphate-oxidase, or NADPH oxidase, and protecting
IL-2-activated Natural Killer cells, or NK-cells, and Thymus cells, or T-cells. These two kinds of
cells, NK-cells and T-cells, possess an ability to kill and support the killing of cancer cells and
virally infected cells.
In October 2008, we received a full marketing authorization from the European Commission, or
EU, for Ceplene®. The approval allows Ceplene® to be marketed in the 27
member states of the EU, as well as in Iceland, Liechtenstein and Norway. The approval by the
European Commission is based, in part, on the results of the single pivotal 320-patient Phase III
trial for Ceplene® in conjunction with low dose IL-2. The primary result of this trial
was that treatment with Ceplene/IL-2 significantly reduced the occurrence of relapse among AML
patients in complete remission. The improvement of long-term leukemia-free survival in patients
receiving Ceplene/IL-2 exceeded 50%. Moreover, Ceplene® was well tolerated in this
patient population and conferred an acceptable risk benefit profile for AML patients.
Ceplene was designated as an orphan medicinal product in the EU in April 2005 for the
treatment of AML. As a result of its designation as an Orphan Medical Product, we have been
granted 10 years of market exclusivity in the EU from Ceplene’s approval date. As part of
receiving marketing authorization under Exceptional Circumstances for Ceplene®, we are
required to perform two post-approval clinical studies that have now been combined into a single
clinical study. The first part of the study will seek to further elucidate the clinical
pharmacology of Ceplene® by assessing certain immune biomarkers in AML patients in first
remission. The second part of the study will assess the effect of Ceplene/IL-2 on the development
of minimal residual disease in the same patient population.
In January 2010, we entered into an exclusive commercialization agreement for
Ceplene® with Meda AB, a leading international specialty pharmaceutical company based in
Stockholm, Sweden. Under the terms of the agreement, we granted Meda the right to market
Ceplene® in Europe and several other countries including Japan, China, and Australia.
We received a $3 million fee on signing and will receive an additional $2 million upon the first
commercial launch of Ceplene in a major European market. We will also receive other milestone
payments and an escalating double digit percent royalty on net sales in the covered territories.
Additionally, we will be responsible for Ceplene’s commercial supply.
5
Table of Contents
We have also advanced our efforts to gain approval for Ceplene® as a remission
maintenance treatment for AML patients in North America. In November 2009, Health Canada accepted
for review a New Drug Submission, or NDS, for Ceplene® for the treatment of AML in
Canada. A decision from Health Canada is expected in 2010. We are preparing a NDA filing for
Ceplene® with the FDA for the treatment of AML in the United States later this year.
Crinobulin
Crinobulin is a novel small molecule vascular disruption agent, or VDA, and apoptosis inducer
for the treatment of patients with solid tumors. Crinobulin has shown promising vascular targeting
activity with potent anti-tumor activity in pre-clinical in vitro and in vivo studies. The molecule
has been shown to induce tumor cell apoptosis and selectively inhibit growth of proliferating cell
lines, including multi-drug resistant cell lines. Murine models of human tumor xenografts
demonstrated crinobulin inhibits growth of established tumors of a number of different cancer
types. In preclinical tumored animal models, combination therapy has demonstrated synergistic
activity.
In November 2004, two publications appeared in Molecular Cancer Therapeutics discussing
crinobulin, a journal of the American Association of Cancer Research ( “Discovery and mechanism of
action of a novel series of apoptosis inducers with potential vascular targeting activity”,
Kasibhatla, S., Gourdeau, H., Meerovitch, K., Drewe, J., Reddy, S., Qiu, L., Zhang, H., Bergeron,
F., Bouffard, D., Yang, Q., Herich, J., Lamothe, S., Cai, S. X., Tseng, B., Mol. Cancer Ther. 2004
vol. 3 pp. 1365-1374; and “Antivascular and antitumor evaluation of
2-amino-4-(3-bromo-4,5-dimethoxy-phenyl)-3-cyano-4H-chromenes, a novel series of anticancer
agents”, Henriette Gourdeau, Lorraine Leblond, Bettina Hamelin, Clemence Desputeau, Kelly Dong,
Irenej Kianicka, Dominique Custeau, Chantal Boudreau, Lilianne Geerts, Sui-Xiong Cai, John Drewe,
Denis Labrecque, Shailaja Kasibhatla, and Ben Tseng, Mol. Cancer Ther. 2004 vol. 3 pp.1375-1384).
The manuscripts characterize crinobulin as a potent caspase activator demonstrating vascular
targeting activity and potent antitumor activity in pre-clinical in vitro and in vivo studies.
Crinobulin appeared highly effective in mouse tumor models, producing tumor necrosis at doses that
correspond to only 25% of the maximum tolerated dose, or MTD. Moreover, in combination treatment,
crinobulin significantly enhanced the antitumor activity of cisplatin/taxane, resulting in
tumor-free animals.
In October 2007, we completed a Phase I clinical trial for crinobulin. We successfully
identified the maximum tolerated dose, or MTD, of crinobulin in the Phase I study. The MTD was
below the dose which produced the expected toxicity based on preclinical studies at higher doses.
Crinobulin was administered as a single agent in increasing doses to small cohorts of patients with
advanced solid tumors. A total of seventeen patients were enrolled in the study. The drug was
tested in a variety of cancer types including melanoma, prostate, lung, breast, colon, and
pancreatic cancers. The study, which was initiated in December 2006, was conducted at three cancer
centers in the United States. In addition to determining crinobulin’s MTD, the primary objective
of the study was to determine the pharmacokinetic profile of the drug. In 2008, we enrolled an
additional sixteen patients into the study, lengthening the infusion period of the drug in order to
increase the MTD. The studies helped characterize the pharmacodynamic effects on tumor blood flow
and identified early signs of objective anti-tumor response as measured by computed axial
tomography, or CT scans, magnetic resonance imaging, or MRI, or positron emission tomography, or
PET scan, in advanced cancer patients with well vascularized solid tumors. A Phase Ib study of
crinobulin in combination with other chemotherapeutic agents is planned to commence in 2010.
Azixaä (MPC6827)
AzixaTM is a compound discovered internally and licensed to Myriad Genetics for
clinical development. AzixaTM demonstrated a broad range of anti-tumor activities
against many tumor types in various animal models as well as activity against different types of
multi-drug resistant cell lines. The Phase I clinical testing was conducted by Myriad, on patients
with solid tumors with a particular focus on brain cancers or brain metastases due to the
pharmacologic properties of AzixaTM in pre-clinical animal studies that indicated higher
drug levels in the brain than in the blood. Myriad reported in the third quarter of 2006 that a MTD
had been reached for AzixaTM and that they had seen evidence of tumor regression at
doses less than the MTD in some patients. In March 2007, Myriad initiated two Phase II
registration-sized clinical trials for AzixaTM in patients with primary brain cancer and
in patients with melanoma that has spread to the brain. The trials are designed to assess the
safety profile of AzixaTM and the extent to which it can improve the overall survival of
these patients. In November 2009 Myriad announced interim results of its Phase II trial of
AzixaTM in melanoma metastases in which ten of the 22 patients treated achieved stable
disease and two patients achieved confirmed partial responses. The median progression-free
survival was 2.8 months. AzixaTM has received orphan drug status in the United States
for the treatment of glioblastoma.
6
Table of Contents
Peripheral Neuropathy
Peripheral neuropathy is a medical condition caused by damage to the nerves in the peripheral
nervous system. The peripheral nervous system includes nerves that run from the brain and spinal
cord to the rest of the body. A 2006 study conducted by Business Insight stated that peripheral
neuropathy affects over 15 million people in the United States and is associated with conditions
that injure peripheral nerves, including herpes zoster, or shingles, diabetes, HIV/AIDS and other
diseases. It can also be caused by trauma or may result from surgical procedures. Peripheral
neuropathy is usually first felt as tingling and numbness in the hands and feet. Symptoms can be
experienced in many ways, including burning, shooting pain, throbbing or aching. Peripheral
neuropathy can cause intense chronic pain that, in many instances, is debilitating.
Post-herpetic neuralgia, or PHN, is one type of peripheral neuropathic pain associated with
herpes zoster, or shingles, which exists after the rash has healed. According to Datamonitor, PHN
affects over 100,000 people in the United States each year. PHN causes pain on and around the area
of skin that was affected by the shingles rash. Most people with PHN describe their pain as “mild”
or “moderate.” However, the pain can be severe in some cases. PHN pain is usually a constant,
burning or gnawing pain but can be an intermittent sharp or stabbing pain. Current treatments for
PHN have limited effectiveness, particularly in severe cases and can cause significant adverse side
effects. One of the initial indications for our NP-1 product candidate is for the treatment of
peripheral neuropathy in PHN patients.
Painful diabetic peripheral neuropathy, or DPN, is common in patients with long-standing Type
1(juvenile) and Type 2 (adult onset) diabetes mellitus. An estimated 18.2 million people have
diabetes mellitus in the United States. The prevalence of neuropathy approaches 50% in those with
diabetes mellitus for greater than 25 years. Specifically, the lifetime incidence of DPN is 11.6%
and 32.1% for Type 1 and 2 diabetes, respectively. Common symptoms of DPN are sharp, stabbing,
burning pain, or allodynia, which is pain to light touch, with numbness and tingling of the feet
and sometimes the hands.
Various drugs are currently used in the treatment of DPN. These include tricyclic
antidepressants, or TCA’s, such as amitriptyline, anticonvulsants such as gabapentin, serotonin and
norepinephrine re-uptake inhibitors (e.g., duloxetine), and opioids (e.g., oxycodone).
Unfortunately, the use of these drugs is often limited by the extent of the pain relief provided
and the occurrence of significant central nervous system, or CNS, side effects such as dizziness,
somnolence, and confusion. Because of its limited systemic absorption into the blood, NP-1 topical
cream potentially fulfills the unmet need for a safe, better tolerated, and effective agent for
painful DPN.
Cancer pain represents a large unmet market. This condition is caused by the cancer tumor
itself as well as the side effects of cancer treatments, such as chemotherapy and radiotherapy.
According to Business Insight’s study, “Pain Market Outlook for 2011”, published in June 2006, over
5 million patients in the United States experience cancer-related pain. This pain can be placed in
three main areas: visceral, somatic and neuropathic. Visceral pain is caused by tissue damage to
organs and may be described as gnawing, cramping, aching or sharp. Somatic pain refers to the skin,
muscle or bone and is described as stabbing, aching, throbbing or pressure. Neuropathic pain is
caused by injury to, or compression of, the structures of the peripheral and central nervous
system. Chemotherapeutic agents, including vinca alkaloids, cisplatin and paclitaxel, are
associated with peripheral neuropathies. Neuropathic pain is often described as sharp, tingling,
burning or shooting.
EpiCeptTM NP-1
EpiCeptTM NP-1, or NP-1, is a prescription topical analgesic cream containing a
patented formulation of two FDA-approved drugs; amitriptyline, which is a widely-used
antidepressant, and ketamine, an NMDA antagonist that is used as an intravenous anesthetic. NP-1 is
designed to provide effective, long-term relief from the pain caused by peripheral neuropathies.
Since each of these ingredients has been shown to have significant analgesic effects and because
NMDA (N-methyl-D-aspartic acid) antagonists, such as ketamine, have demonstrated the ability to
enhance the analgesic effects of amitriptyline, we believe the combination is a good candidate for
the development of a new class of analgesics. We believe that NP-1 can be used in conjunction with
orally delivered analgesics, such as gabapentin. In January 2010, we received orphan drug
protection for NP-1 for the treatment of PHN.
NP-1 is an odorless, white vanishing cream that is applied twice daily and is quickly absorbed
into the applied area. We believe the topical delivery of its patented combination represents a
fundamentally new approach for the treatment of pain associated with peripheral neuropathy. In
addition, we believe that the topical delivery of our product candidate will significantly reduce
the risk of adverse side effects and drug to drug interactions associated with the systemic
delivery of the active ingredients. The results of our clinical trials to date have demonstrated
the safety of the cream for use for up to one year and a potent analgesic effect in subjects with
both post-herpetic neuralgia and other types of peripheral neuropathy, such as those with diabetic,
traumatic and surgical causes.
7
Table of Contents
Current Clinical Initiatives. In January 2009, we completed a Phase IIb, multi-center,
randomized, placebo controlled trial in approximately 360 patients evaluating the analgesic
properties and safety of NP-1 cream in patients with PHN. This trial compared the efficacy and
safety of NP-1 against both gabapentin, the leading drug prescribed for this indication, and
placebo. The first primary endpoint was the change in pain intensity over the four week duration
of the trial. The data demonstrated that NP-1 achieved statistically significant superior efficacy
compared with placebo (p=0.024). An additional primary endpoint, to demonstrate that NP-1 was not
inferior to gabapentin in reducing pain, was also met. A key secondary endpoint measured in the
trial from a responder analysis indicated that 63% of patients in the NP-1 treatment arm achieved a
reduction in pain scores of at least 30%, significantly higher than that of patients in the placebo
arm (p=0.033). Top-line results further indicate that NP-1 achieved a superior safety profile when
compared with gabapentin, especially with regard to dizziness and somnolence, as evaluated by the
reporting of adverse events.
In February 2008, we completed a Phase II clinical trial in 215 patients suffering from DPN.
The results of this double-blind, placebo-controlled study demonstrated that the primary endpoint,
the difference in changes in pain intensity between NP-1 and placebo over the four week duration of
the trial, nearly reached statistical significance (p=0.0715). The analgesic benefits of NP-1
continued to build over time during the course of the study. Key secondary endpoints measured in
the trial from a responder analysis indicate that 60% of patients in the NP-1 treatment arm
achieved a reduction of pain scores of at least 30% compared with 48% of patients in the placebo
arm (p=0.076). In addition, 33% of patients in the NP-1 treatment arm achieved a reduction in pain
scores of at least 50% compared with 21% of patients in the placebo arm (p=0.078). All pain
scores measured trended in favor of the NP-1 treated patients over the placebo group, indicative of
an analgesic effect in this type of peripheral neuropathic pain. We concluded that data derived
from the trial support the continued study of NP-1 in late-stage pivotal clinical trials.
In the third quarter of 2007, the National Cancer Institute, or NCI, initiated a multicenter,
randomized, placebo-controlled clinical trial in approximately 400 patients evaluating the effects
of NP-1 cream in treating patients suffering from chemotherapeutic induced peripheral neuropathy,
also known as CPN. CPN may affect 50% of women undergoing treatment for breast cancer. A common
therapeutic agent for the treatment of advanced breast cancer is paclitaxel, and as many as 80% of
the patients with advanced breast cancer experience some signs and symptoms of CPN, such as
burning, tingling pain associated sometimes with mild muscular weakness, after high dose paclitaxel
administration. The study is being conducted within a network of approximately 25 sites under the
direction of the NCI funded Community Clinical Oncology Program, or CCOP. This trial is expected to
complete in 2010.
Back Pain
In the United States, 80% of the U.S. population will experience significant back pain at some
point. Back pain ranks second only to headaches as the most frequently experienced pain. It is the
leading reason for visits to neurologists and orthopedists and the second most frequent reason for
physician visits overall. Both acute and chronic back pain are typically treated with NSAIDs,
muscle relaxants or opioid analgesics. All of these drugs can subject the patient to systemic
toxicity, significant adverse side effects and drug to drug interactions.
LidoPAIN BP
LidoPAIN BP is a prescription analgesic non-sterile patch designed to provide sustained
topical delivery of lidocaine for the treatment of acute or recurrent lower back pain of moderate
severity of less than three months duration. The LidoPAIN BP patch contains 140 mg of lidocaine in
a 19.0% concentration, is intended to be applied once daily and can be worn for a continuous
24-hour period. The patch’s adhesive is strong enough to permit a patient to move and conduct
normal daily activities but can be removed easily. We licensed LidoPAIN BP and certain other
technology to Endo Pharmaceuticals in December 2003.
Current Clinical Initiatives. We have suspended development of LidoPAIN BP and focused our
resources on our other compounds that we believe present a better risk/reward opportunity. Further
collaboration work with Endo has been postponed pending an affirmation that they have interest in
developing a lidocaine patch for the treatment of back pain.
Our Strategic Alliances
Meda
In January 2010, we entered into an exclusive commercialization agreement for
Ceplene® with Meda AB, a leading international specialty pharmaceutical company based in
Stockholm, Sweden. Under the terms of the agreement, we granted Meda the right to market Ceplene
in Europe and several other countries including Japan, China, and Australia. Pursuant to the terms
of the agreement, we received a $3 million fee and will receive an additional $2 million upon the
first commercial launch of Ceplene® in a major European market. Additional payments
include a $5 million payment upon achievement of a regulatory milestone and up to $30
million in sales-based milestones that commence upon attainment of at least $50 million in
annual sales. We will receive an escalating percentage royalty on net sales in the covered
territories ranging from the low teens to the low twenties and will be responsible for Ceplene’s
commercial supply. The initial term of this agreement is ten years and is subject to automatic two
year extensions at Meda’s option. The agreement can be terminated at any time by Meda upon six
months prior written notice. The agreement can also be terminated by mutual agreement or for
cause.
8
Table of Contents
Myriad
We licensed the MX90745 series of caspase-inducer anti-cancer compounds to Myriad in 2003.
Under the terms of the agreement, we granted to Myriad a research license to develop and
commercialize any drug candidates from the series of compounds with a non-exclusive, worldwide,
royalty-free license, without the right to sublicense the technology. Myriad is responsible for the
worldwide development and commercialization of any drug candidates from the series of compounds. We
also granted to Myriad a worldwide royalty bearing development and commercialization license with
the right to sublicense the technology. The agreement required Myriad to make research payments to
us totaling $3 million which was paid and recognized as revenue prior January 4, 2006. Assuming the
successful commercialization of the compound for the treatment of cancer, we are also eligible to
receive up to $24.0 million upon the achievement of certain milestones and the successful
commercialization of compounds for treatment of cancer as well as an escalating mid to high single
digit percentage royalty on product sales. In March 2007, Myriad initiated Phase II clinical trials
for AzixaTM (MPC6827), a MX90745 series compound. In March 2008, we received a
milestone payment of $1.0 million following dosing of the first patient in these trials.
Durect
In December 2006, we entered into a license agreement with DURECT Corporation (“DURECT”),
pursuant to which we granted DURECT the exclusive worldwide rights to certain of our intellectual
property for a transdermal patch containing bupivacaine for the treatment of back pain. Under the
terms of the agreement, we received a $1.0 million upfront payment. In September 2008, we amended
our license agreement with DURECT. Under the terms of the amended agreement, we granted DURECT
royalty-free, fully paid up, perpetual and irrevocable rights to the intellectual property licensed
as part of the original agreement in exchange for a cash payment of $2.25 million from DURECT.
Endo Pharmaceuticals
In December 2003, we entered into a license agreement with Endo under which we granted Endo
(and its affiliates) the exclusive (including as to us and our affiliates) worldwide right to
commercialize LidoPAIN BP. We also granted Endo worldwide rights to use certain of our patents for
the development of certain other non-sterile, topical lidocaine patches, including Lidoderm, Endo’s
non-sterile topical lidocaine-containing patch for the treatment of chronic lower back pain. Upon
the execution of the Endo agreement, we received a non-refundable payment of $7.5 million. Other
potential compensation under this agreement includes additional milestone payments of up to $82.5
million related to both our LidoPAIN BP product and Lidoderm and a royalty on net sales of LidoPAIN
BP. We do not expect to receive further compensation under this agreement.
The last published clinical study of Lidoderm in back pain occurred in 2005. We believe that
no measurable progress has occurred with Lidoderm for back pain and do not expect further revenue
from this license at this time.
Manufacturing
We currently intend to outsource all of our manufacturing activities. We believe that this
strategy will enable us to direct operational and financial resources to the development of our
product candidates rather than diverting resources to establishing a manufacturing infrastructure.
Under the terms of a supply agreement with Meda, we are responsible for supplying commercial
quantities of Ceplene® based on Meda’s estimate of sales in the licensed territories.
We have entered into arrangements with qualified third parties for the manufacture of
Ceplene® and for the formulation and manufacture of our clinical supplies. We may enter
into additional written supply agreements in the future. We generally purchase our supplies from
current suppliers pursuant to purchase orders. We plan to use a single, separate third party
manufacturer for Ceplene® and each of our product candidates for which we are
responsible for manufacturing. In some cases, the responsibility to manufacture product, or to
identify suitable third party manufacturers, may be assumed by our licensees. We cannot assure you
that our current manufacturers can successfully increase their production to meet full commercial
demand. We believe that in most cases there are several manufacturing sources available to us,
including our current manufacturers, which can meet our commercial supply
requirements on commercially reasonable terms. We will continue to look for and secure the
appropriate manufacturing capabilities and capacity to ensure commercial supply at the appropriate
time.
9
Table of Contents
Sales and Marketing
We hired a senior vice president of sales and marketing in November 2009 in anticipation of
the start of commercial activities for Ceplene® in the United States and Europe. In
order to commercially market Ceplene® or any of our product candidates in the United
States upon receipt of marketing approval, we must either develop an internal sales and marketing
infrastructure or collaborate with third parties with sales and marketing expertise. We have
retained the rights to commercialize NP-1 and crinobulin worldwide and to market Ceplene globally
except for Europe and certain Pacific rim countries. In addition, we have granted Myriad exclusive
worldwide commercialization rights, with rights to sublicense, for AzixaTM. We will
likely market our products in international markets outside of North America through collaborations
with third parties. We intend to make decisions regarding internal sales and marketing of our
product candidates on a product-by-product and country-by-country basis.
Intellectual Property
Our commercial success will depend in part on obtaining and maintaining patent protection and
trade secret protection of our technologies and drug candidates as well as successfully defending
these patents against third-party challenges. We have various compositions of matter and use
patents, which have claims directed to our product candidates or their methods of use. Our patent
policy is to retain and secure patents for the technology, inventions and improvements related to
our core portfolio of product candidates. We also rely on trade secrets, technical know-how and
continuing innovation to develop and maintain our competitive position.
The following is a summary of the patent position relating to our three in-house product
candidates:
Ceplene® — The intellectual property protection surrounding our histamine
technology includes 24 U.S. patents issued or allowed, with the term for the latest one expiring in
February 2023. Patents issued or pending in the international markets concern specific therapeutic
areas or manufacturing. Claims include the therapeutic administration of histamine or any H2
receptor agonist in the treatment of cancer, infectious diseases and other diseases, either alone
or in combination therapies, the novel synthetic method for the production of pharmaceutical-grade
histamine dihydrochloride, the mechanism of action including the binding receptor and pathway, and
the rate and route of administration.
Crinobulin — The intellectual property protection regarding this compound is covered by two
issued U.S. patents, with the latest one expiring in May 2022 and one application pending covering
the composition and uses of this compound and structurally related analogs. Additional foreign
patent applications are pending in major pharmaceutical markets outside the United States.
EpiCeptTM NP-1 — We own a U.S. patent with claims directed to a formulation
containing a combination of amitriptyline and ketamine, which can be used as a treatment for the
topical relief of pain, including neuropathic pain, that expires in August 2021. We also have a
license to additional patents, which expire in September 2015 and May 2018, and which have claims
directed to topical uses of tricyclic antidepressants, such as amitriptyline, and NMDA antagonists,
such as ketamine, as treatments for relieving pain, including neuropathic pain.
We may seek to protect our proprietary information by requiring our employees, consultants,
contractors, outside partners and other advisers to execute, as appropriate, nondisclosure and
assignment of invention agreements upon commencement of their employment or engagement. We also
require confidentiality or material transfer agreements from third parties that receive our
confidential data or materials.
We also rely on trade secrets to protect our technology, especially where we do not believe
patent protection is appropriate or obtainable. However, trade secrets are difficult to protect.
While we use reasonable efforts to protect our trade secrets, our employees, consultants,
contractors, partners and other advisors may unintentionally or willfully disclose information to
competitors. Enforcing a claim that a third party illegally obtained and is using trade secrets is
expensive and time consuming, and the outcome is unpredictable. In addition, courts outside the
United States are sometimes less willing to protect trade secrets. Moreover, our competitors may
independently develop equivalent knowledge, methods and know-how.
The pharmaceutical, biotechnology and other life sciences industries are characterized by the
existence of a large number of patents and frequent litigation based upon allegations of patent
infringement. While our drug candidates are in clinical trials, and prior to commercialization, we
believe our current activities fall within the scope of the exemptions provided by 35 U.S.C.
Section 271(e) in
the United States and Section 55.2(1) of the Canadian Patent Act, each of which covers
activities related to developing information for submission to the FDA and its counterpart agency
in Canada. As our drug candidates progress toward commercialization, the possibility of an
infringement claim against us increases. While we attempt to ensure that our drug candidates and
the methods we employ to manufacture them do not infringe other parties’ patents and other
proprietary rights, competitors or other parties may assert that we infringe on their proprietary
rights.
10
Table of Contents
For a discussion of the risks associated with our intellectual property, see Item 1A. “Risk
Factors — Risks Relating to Intellectual Property.”
License Agreements
We have in the past licensed and will continue to license patents from collaborating research
groups and individual inventors.
Epitome/Dalhousie
In August 1999, we entered into a sublicense agreement with Epitome Pharmaceuticals Limited
under which we were granted an exclusive license to certain patents for the topical use of
tricyclic anti-depressants and NMDA antagonists as topical analgesics for neuralgia that were
licensed to Epitome by Dalhousie University. These and other patents cover the combination
treatment consisting of amitriptyline and ketamine in NP-1. This technology has been incorporated
into NP-1. In July 2007, we converted the sublicense agreement previously established with Epitome
Pharmaceuticals Limited, related to NP-1, into a direct license with Dalhousie University. Under
this new arrangement, we gained more favorable terms, including a lower maintenance fee obligation
and reduced royalty rate on future product sales.
We have been granted worldwide rights to make, use, develop, sell and market products
utilizing the licensed technology in connection with passive dermal applications. We are obligated
to make payments to Dalhousie totaling $0.9 million, of which $0.2 million has been paid, upon
achievement of specified milestones and to pay single digit royalties based on annual net sales
derived from the products incorporating the licensed technology. We are obligated to pay Dalhousie
an annual maintenance fee of $0.5 million until the license agreement expires or is terminated, or
an NDA for NP-1 is filed with the FDA, otherwise Dalhousie will have the option to terminate the
contract. The license agreement with Dalhousie terminates upon the expiration of the last to expire
licensed patent. The sublicense agreement with Epitome terminated in July 2007. In each of the
years 2009, 2008 and 2007, we paid Epitome a fee of $0.3 million. During 2009 and 2008, we paid
Dalhousie a maintenance fee of $0.5 million and $0.4 million, respectively. During 2007, we paid
Dalhousie a signing fee of $0.3 million, a maintenance fee of $0.4 million and a milestone payment
of $0.2 million upon the dosing of the first patient in a Phase III clinical trial for NP-1. These
payments were expensed to research and development.
Shire Biochem
In March 2004 and as amended in January 2005, we entered into a license agreement reacquiring
the rights to the MX2105 series of apoptosis inducer anti-cancer compounds from Shire BioChem,
Inc., formerly known as BioChem Pharma, Inc., who had previously announced that oncology would no
longer be a therapeutic focus of the company’s research and development efforts. Under the
agreements, Shire BioChem agreed to assign and/or license to us rights it owned under or shared
under its oncology research program. The agreement requires that we provide Shire BioChem a portion
of any sublicensing payments we receive if we relicense the series of compounds, and make milestone
payments to Shire BioChem totaling up to $26 million, assuming the successful commercialization of
a compound for the treatment of a cancer indication, as well as pay a royalty on product sales. At
December 31, 2009, we accrued a license fee expense of $0.6 million upon the commencement of a
Phase I clinical trial for Crinobulin in 2006.
Hellstrand
In October 1999, we entered into a royalty agreement with Dr. Kristoffer Hellstrand under
which we have an exclusive license to certain patents for Ceplene® configured for the
systemic treatment of cancer, infectious diseases, autoimmune diseases and other medical
conditions. Under this agreement, a predecessor company paid Dr. Hellstrand $1 million in 1999 and
will owe a royalty of 1% of net sales. In November 2009 we expanded our collaboration with Dr.
Hellstrand by concluding a new agreement in which we acquired rights to develop certain new
compounds. As compensation for this agreement and for providing certain ongoing consulting
services, we awarded Dr. Hellstrand options to purchase 50,000 shares of our common stock and
agreed to pay a monthly consulting fee. At December 31, 2009, no royalties have been paid.
11
Table of Contents
Government Regulation
United States
The FDA and comparable state and local regulatory agencies impose substantial requirements
upon the clinical development, manufacture, marketing and distribution of drugs. These agencies and
other federal, state and local entities regulate research and development activities and the
testing, manufacture, quality control, safety, effectiveness, labeling, storage, record keeping,
approval, advertising and promotion of our product candidates. In the United States, the FDA
regulates drugs under the Federal Food, Drug, and Cosmetic Act, and implementing regulations. The
process required by the FDA before our product candidates may be marketed in the United States
generally involves the following:
| • | completion of extensive pre-clinical laboratory tests, pre-clinical animal studies and formulation studies all performed in accordance with the FDA’s good laboratory practice, or GLP, regulations; |
| • | submission to the FDA of an Investigational New Drug, or IND, application that must become effective before clinical trials may begin; |
| • | performance of adequate and well-controlled clinical trials to establish the safety and efficacy of the product candidate for each proposed indication; |
| • | submission of an NDA to the FDA; |
| • | satisfactory completion of an FDA pre-approval inspection of the manufacturing facilities at which the product is produced to assess compliance with current GMP, or cGMP, regulations; and |
| • | FDA review and approval of the NDA prior to any commercial marketing, sale or shipment of the drug. |
The testing and approval process requires substantial time, effort and financial resources,
and we cannot be certain that any approvals for our product candidates will be granted on a timely
basis, if at all.
Pre-clinical Activities. Pre-clinical activities include laboratory evaluation of product
chemistry, formulation and stability, as well as studies to evaluate toxicity in animals. The
results of pre-clinical tests, together with manufacturing information and analytical data, are
submitted as part of an IND application to the FDA. The IND automatically becomes effective 30 days
after receipt by the FDA, unless the FDA, within the 30-day time period, raises concerns or
questions about the conduct of the clinical trial, including concerns that human research subjects
will be exposed to unreasonable health risks. In such a case, the IND sponsor and the FDA must
resolve any outstanding concerns before the clinical trial can begin. Our submission of an IND, or
those of our collaborators, may not result in FDA authorization to commence a clinical trial. A
separate submission to an existing IND must also be made for each successive clinical trial
conducted during product development, and the FDA must grant permission before each clinical trial
can begin. Further, an independent institutional review board, or IRB, for each medical center
proposing to conduct the clinical trial must review and approve the plan for any clinical trial
before it commences at that center, and it must monitor the study until completed. The FDA, the IRB
or the sponsor may suspend a clinical trial at any time on various grounds, including a finding
that the subjects or patients are being exposed to an unacceptable health risk. Clinical testing
also must satisfy extensive Good Clinical Practice, or GCP, regulations and regulations for
informed consent of subjects.
Clinical Trials. For purposes of NDA submission and approval, clinical trials are typically
conducted in the following three sequential phases, which may overlap:
| • | Phase I: Studies are initially conducted in a limited population to test the drug candidate for safety, dose tolerance, absorption, metabolism, distribution and excretion in healthy humans or, on occasion, in subjects. In some cases, a sponsor may decide to run what is referred to as a “Phase Ib” evaluation, which is a second safety-focused Phase I clinical trial typically designed to evaluate the impact of the drug candidate in combination with currently approved drugs. |
| • | Phase II: Studies are generally conducted in a limited patient population to identify possible adverse effects and safety risks, to determine the efficacy of the drug candidate for specific targeted indications and to determine dose tolerance and optimal dosage. Multiple Phase II clinical trials may be conducted by the sponsor to obtain information prior to beginning larger and more expensive Phase III clinical trials. In some instances, a sponsor may decide to run what is referred to as a “Phase IIa” clinical trial, which is designed to provide dose-ranging and additional safety and pharmaceutical data. In other cases, a sponsor may decide to run what is referred to as a “Phase IIb” evaluation, which is a second, confirmatory Phase II clinical trial that could, if positive and accepted by the FDA, serve as a pivotal clinical trial in the approval of a drug candidate. |
12
Table of Contents
| • | Phase III: These are commonly referred to as pivotal studies. When Phase II clinical trials demonstrate that a dose range of the drug candidate is effective and has an acceptable safety profile, Phase III clinical trials are undertaken in large patient populations to further evaluate dosage, to provide substantial evidence of clinical efficacy and to further test for safety in an expanded and diverse patient population at multiple, geographically dispersed clinical trial sites. |
In some cases, the FDA may give conditional approval of an NDA for a drug candidate on the
sponsor’s agreement to conduct additional clinical trials to further assess the drug’s safety and
effectiveness after NDA approval. Such post-approval trials are typically referred to as Phase IV
clinical trials.
New Drug Application. The results of drug candidate development, pre-clinical testing,
chemistry and manufacturing controls and clinical trials are submitted to the FDA as part of an
NDA. The NDA also must contain extensive manufacturing information. Once the submission has been
accepted for filing, by law the FDA has 180 days to review the application and respond to the
applicant. The review process is often significantly extended by FDA requests for additional
information or clarification. The FDA may refer the NDA to an advisory committee for review,
evaluation and recommendation as to whether the application should be approved. The FDA is not
bound by the recommendation of an advisory committee, but it generally follows such
recommendations. The FDA may deny approval of an NDA if the applicable regulatory criteria are not
satisfied, or it may require additional clinical data or an additional pivotal Phase III clinical
trial. Even if such data is submitted, the FDA may ultimately decide that the NDA does not satisfy
the criteria for approval. Data from clinical trials are not always conclusive and the FDA may
interpret data differently than we do. Once issued, the FDA may withdraw drug approval if ongoing
regulatory requirements are not met or if safety problems occur after the drug reaches the market.
In addition, the FDA may require testing, including Phase IV clinical trials, and surveillance
programs to monitor the effect of approved products that have been commercialized, and the FDA has
the power to prevent or limit further marketing of a drug based on the results of these
post-marketing programs. Drugs may be marketed only for the approved indications and in accordance
with the provisions of the approved label. Further, if there are any modifications to the drug,
including changes in indications, labeling or manufacturing processes or facilities, we may be
required to submit and obtain FDA approval of a new NDA or NDA supplement, which may require us to
develop additional data or conduct additional pre-clinical studies and clinical trials.
Satisfaction of FDA regulations and requirements or similar requirements of state, local and
foreign regulatory agencies typically takes several years, and the actual time required may vary
substantially based upon the type, complexity and novelty of the product or disease. Government
regulation may delay or prevent marketing of drug candidates for a considerable period of time and
impose costly procedures upon our activities. The FDA or any other regulatory agency may not grant
approvals for new indications for our drug candidates on a timely basis, if at all. Even if a drug
candidate receives regulatory approval, the approval may be significantly limited to specific
usages, patient populations and dosages. Further, even after regulatory approval is obtained, later
discovery of previously unknown problems with a drug may result in restrictions on the drug or even
complete withdrawal of the drug from the market. Delays in obtaining, or failures to obtain,
regulatory approvals for any of our drug candidates would harm its business. In addition, we cannot
predict what additional governmental regulations may arise from future U.S. governmental action.
Any drugs manufactured or distributed by us or our collaborators pursuant to FDA approvals are
subject to continuing regulation by the FDA, including record keeping requirements and reporting of
adverse experiences associated with the drug. Drug manufacturers and their subcontractors are
required to register their establishments with the FDA and certain state agencies and are subject
to periodic unannounced inspections by the FDA and certain state agencies for compliance with
ongoing regulatory requirements, including cGMPs, which impose certain procedural and documentation
requirements upon us and our third-party manufacturers. Failure to comply with the statutory and
regulatory requirements can subject a manufacturer to potential legal or regulatory action, such as
warning letters, suspension of manufacturing, seizure of product, injunctive action or civil
penalties. We cannot be certain that we or our present or future third-party manufacturers or
suppliers will be able to comply with the cGMP regulations and other ongoing FDA regulatory
requirements. If our present or future third-party manufacturers or suppliers are not able to
comply with these requirements, the FDA may halt our clinical trials, require us to recall a drug
from distribution, or withdraw approval of the NDA for that drug.
The FDA closely regulates the post-approval marketing and promotion of drugs, including
standards and regulations for direct-to-consumer advertising, off-label promotion,
industry-sponsored scientific and educational activities and promotional activities involving the
Internet. A company can make only those claims relating to safety and efficacy that are approved by
the FDA. Failure to comply with these requirements can result in adverse publicity, warning
letters, corrective advertising and potential civil and criminal penalties. Physicians may
prescribe legally available drugs for uses that are not described in the drug’s labeling and that
differ from
those tested by us and approved by the FDA. Such off-label uses are common across medical
specialties. Physicians may believe that such off-label uses are the best treatment for many
patients in varied circumstances. The FDA does not regulate the behavior of physicians in their
choice of treatments. The FDA does, however, impose stringent restrictions on manufacturers’
communications regarding off-label use.
13
Table of Contents
Section 505(b)(2) Drug Applications. Once an FDA-approved new drug is no longer
patent-protected, another company may sponsor a new indication, a new use or put the drug in a new
dosage form. Each new indication from a different company requires an NDA filing. As an alternate
path to FDA approval for new or improved formulations of previously approved products, a company
may file a Section 505(b)(2) NDA. Section 505(b)(2) permits the filing of an NDA where at least
some of the information required for approval comes from studies not conducted by or for the
applicant and for which the applicant has not obtained a right of reference. However, this NDA does
not have to contain all of the information or data that was submitted with the original NDA because
of the FDA’s prior experience with the drug product. An original NDA for an FDA-approved new drug
would have required numerous animal toxicology studies that have been reviewed by the FDA. These
can be referenced in the 505(b)(2) NDA submitted by the new applicant. Many studies in humans that
support the safety of the drug product may be in the published literature. The FDA allows the new
sponsor company to submit these publications to support its 505(b)(2) NDA. By allowing the new
sponsor company to use this information, the time and cost required to obtain approval for a drug
product for the new indication can be greatly reduced. The FDA may also require companies to
perform additional studies or measurements to support the change from the approved product. The FDA
may then approve the new product candidate for all or some of the label indications for which the
referenced product has been approved, as well as for any new indication sought by the Section
505(b)(2) applicant.
To the extent that the Section 505(b)(2) applicant is relying on studies conducted for an
already approved product, the applicant is required to certify to the FDA concerning any patents
listed for the approved product in the FDA’s Orange Book publication. Specifically, the applicant
must certify that: (i) the required patent information has not been filed; (ii) the listed patent
has expired; (iii) the listed patent has not expired, but will expire on a particular date and
approval is sought after patent expiration; or (iv) the listed patent is invalid or will not be
infringed by the new product. If the applicant does not challenge the listed patents, the Section
505(b)(2) application will not be approved until all the listed patents claiming the referenced
product have expired. The Section 505(b)(2) application also will not be approved until any
non-patent exclusivity, such as exclusivity for obtaining approval of a new chemical entity, listed
in the Orange Book for the referenced product has expired.
Foreign Regulation
Whether or not we obtain FDA approval for a product, we must obtain approval of a product by
the comparable regulatory authorities of foreign countries before we can commence clinical trials
or marketing of the product in those countries. The approval process varies from country to
country, and the time may be longer or shorter than that required for FDA approval. The
requirements governing the conduct of clinical trials, product licensing, pricing and reimbursement
also vary greatly from country to country. Although governed by the applicable country, clinical
trials conducted outside of the United States typically are administered with the three-phase
sequential process that is discussed above under “Government Regulation — United States.” However,
the foreign equivalent of an IND is not a prerequisite to performing pilot studies or Phase I
clinical trials.
Under European Union regulatory systems, we may submit marketing authorization applications
either under a centralized or decentralized procedure. The centralized procedure, which is required
for oncology products and is available for medicines produced by biotechnology or which are highly
innovative, provides for the grant of a single marketing authorization that is valid for all member
states. This authorization is a marketing authorization application, or MAA. The decentralized
procedure provides for mutual recognition of national approval decisions. Under this procedure, the
holder of a national marketing authorization may submit an application to the remaining member
states. Within 90 days of receiving the applications and assessment report, each member state must
decide whether to recognize approval. This procedure is referred to as the mutual recognition
procedure.
In addition, regulatory approval of prices is required in most countries other than the United
States. We face the risk that the resulting prices would be insufficient to generate an acceptable
return to us or our collaborators.
Corporate Information
We were incorporated in Delaware in March 1993. We have two wholly-owned subsidiaries, EpiCept
GmbH, based in Munich, Germany, which is engaged in research and development activities on our
behalf and Maxim Pharmaceuticals, Inc. which we acquired in January 2006. Our principal executive
offices are located at 777 Old Saw Mill River Road, Tarrytown, NY, and our telephone number is
(914) 606-3500. Our website address is www.epicept.com. Our website, and the information contained
in our website, is not a part of this annual report.
14
Table of Contents
Employees
As of March 5, 2010, our workforce consists of 17 full-time employees, three of whom hold a
Ph.D. or M.D., and one of whom holds another advanced degree. We have no collective bargaining
agreements with our employees and have not experienced any work stoppages. We believe that our
relations with our employees are good.
Research and Development
Since our inception, we have made substantial investments in research and development. In the
years ended December 31, 2009, 2008 and 2007, we incurred research and development expenses of
$11.6 million, $12.6 million and $15.3 million, respectively.
Availability of SEC Filings
We have filed reports, proxy statements and other information with the SEC. Copies of our
reports, proxy statements and other information may be inspected and copied at the public reference
facilities maintained by the SEC at SEC Headquarters, Public Reference Section, 100 F Street, N.E.,
Washington D.C. 20549. The public may obtain information on the operation of the SEC’s public
reference facilities by calling the SEC at 1-800-SEC-0330. The SEC maintains a website that
contains reports, proxy statements and other information regarding us. The address of the SEC
website is http://www.sec.gov. We will also provide copies of our Forms 8-K, 10-K, 10-Q,
Proxy and Annual Report at no charge available through our website at www.epicept.com as soon as
reasonably practicable after filing electronically such material with the SEC. Copies are also
available, without charge, from EpiCept Corporation, 777 Old Saw Mill River Road, Tarrytown, NY,
10591.
15
Table of Contents
PART III
ITEM 11. EXECUTIVE COMPENSATION
Compensation Discussion and Analysis
The following discussion and analysis of compensation arrangements of our named executive
officers for the year ended December 31, 2009 should be read together with the compensation tables
and related disclosures set forth below. This discussion contains forward looking statements that
are based on our current plans, considerations, expectations and determinations regarding future
compensation programs. Actual compensation programs that we adopt may differ materially from
currently planned programs as summarized in this discussion.
Role of the Compensation Committee
Our executive compensation is administered by the Compensation Committee of the Board of
Directors. The members of this committee are Robert G. Savage (Chairman), A. Collier Smyth and Guy
C. Jackson, each an independent, non-employee director. In 2009, the Compensation Committee met
eight times and all of the members of the Compensation Committee were present during each meeting.
Under the terms of its Charter, the Compensation Committee is responsible for delivering the
type and level of compensation to be granted to our executive officers. In fulfilling its role, the
Compensation Committee reviews and approves for the Chief Executive Officer (CEO) and other
executive officers: (1) the annual base salary, (2) the annual incentive bonus, including the
specific goals and amounts, (3) equity compensation, (4) employment agreements, severance
arrangements and change in control arrangements and (5) any other benefits, compensation,
compensation policies or arrangements.
All new employee equity grants, subsequent grants to existing employees and any grant to
executive officers are approved by the Compensation Committee.
While management may use consultants to assist in the evaluation of the CEO or executive
officer compensation, the Compensation Committee has authority to retain its own compensation
consultant, as it sees fit. The Compensation Committee also has the authority to obtain advice and
assistance from internal or external legal, accounting or other advisors.
During 2009, the Compensation Committee retained Radford, an Aon Consulting Company, or
Radford, to provide compensation information. The Compensation Committee received compensation
recommendations from the CEO, relevant background information on our executive officers and
compensation studies conducted by Radford. The Compensation Committee then reviewed the
compensation recommendation with the CEO for all executives, except for the CEO. The CEO was not
present during the discussion of his compensation. The Compensation Committee then determined the
compensation levels for the executive officers and reported that determination to the Board.
Compensation Objectives Philosophy
The primary objectives of the Compensation Committee with respect to executive compensation
are to attract and retain the most talented and dedicated executives possible, to tie annual cash
bonuses and long-term equity incentives to achievement of performance objectives, and to align
executives’ incentives with stockholder value creation. To achieve these objectives, the
Compensation Committee implements and maintains compensation plans that tie a substantial portion
of executive officer’s overall compensation to (i) operational goals such as meeting operating
plans and budgets, review of organization and staff and the implementation of requisite changes,
(ii) strategic goals such as the establishment and maintenance of key strategic relationships, the
development of our product candidates and the identification and advancement of additional product
candidates and (iii) financial factors, such as success in raising capital and improving our
results of operations. The Compensation Committee evaluates individual executive performance with
the goal of setting compensation at levels the Compensation Committee believes are comparable with
executives in other companies of similar size and stage of development operating in the
biotechnology and specialty pharmaceutical industries while taking into account our relative
performance and our own strategic goals.
16
Table of Contents
Compensation Program
In order to achieve the above goals, our total compensation packages include base salary and
annual bonus, all paid in cash, as well as long-term compensation in the form of stock options,
restricted stock and/or restricted stock units. We believe that appropriately balancing the total
compensation package is necessary in order to provide market-competitive compensation. The costs of
our compensation programs are a significant determinant of our competitiveness. Accordingly, we are
focused on ensuring that the balance of the various components of our compensation program is
optimized to motivate employees to achieve our corporate objectives on a cost-effective basis.
Review of External Data.
The Compensation Committee obtained a survey of the compensation practices of our peers in the
United States in order to assess the competitiveness of our executive compensation. In the fourth
quarter of 2009, the Compensation Committee obtained data from Radford for a number of
biotechnology and specialty pharmaceutical companies with less than $50.0 million in revenue,
comparable numbers of employees, comparable market capitalization and/or similar product offerings
(the general peer group). The peer group consists of Adolor Corporation, Anesiva, Inc., A.P.
Pharma, Inc., Ariad Pharmaceuticals, Inc., BioCryst Pharmaceuticals, Inc., Cell Therapeutics, Inc.,
Depomed, Inc., Durect Corporation, Genta, Inc., Nastech Pharmaceutical Company, Inc., NeoPharm,
Inc., Novacea, Inc., NPS Pharmaceuticals, Inc., Oxigene, Inc., Pain Therapeutics, Inc., Pozen, Inc.
and Titan Pharmaceuticals, Inc. The Compensation Committee asked Radford to conduct assessments in
three areas of compensation for executive positions: 1) total direct compensation (base salary) for
our executive officers; 2) target total cash compensation (salary and bonus); and 3) equity grants.
For executive officers, we targeted the aggregate value of our total cash compensation (base
salary and bonus) near the 50th percentile of the general peer group and long-term equity incentive
compensation near the 75th percentile. The Compensation Committee strongly believes in engaging the
best talent in critical functions, and this may entail negotiations with individual executives who
may have significant retention packages in place with other employers. In order to attract such
individuals to our Company, the Compensation Committee may determine that it is in our best
interests to negotiate packages that deviate from the general principle of benchmarking our
compensation on our general peer group. Similarly, the Compensation Committee may determine to
provide compensation outside of the normal cycle to individuals to address retention issues.
Compensation Elements
Cash Compensation
Base Salary. Base salaries for our executive officers are established based on the scope of
their responsibilities, taking into account competitive market compensation paid by other benchmark
companies for similar positions. Generally, we believe that executive base salaries should be
targeted near the 50th percentile of the range of salaries for executives in similar positions with
similar responsibilities at our peer group companies, in line with our compensation philosophy.
Base salaries are reviewed by the Compensation Committee annually, and adjusted from time to time
to realign salaries with market levels after taking into account individual responsibilities,
performance and experience. This review generally occurs each year in the fourth quarter for
implementation in the first quarter.
Annual Bonus. The Compensation Committee has the authority to award annual bonuses to our
executive officers and other key employees. The Compensation Committee reviews potential annual
cash incentive awards for our named executive officers annually to determine award payments, if
any, for the last completed fiscal year, as well as to establish award opportunities for the
current year. The Compensation Committee intends to utilize annual incentive bonuses to compensate
executive officers for achieving financial and operational goals and for achieving individual
annual performance objectives. These objectives will vary depending on the individual executive
officer, but will relate generally to (i) operational goals such as those related to operating
plans and budgets, review of organization and staff and the implementation of requisite changes,
(ii) strategic goals such as the establishment and maintenance of key strategic relationships, the
development of our product candidates and the identification and advancement of additional product
candidates and (iii) financial factors, such as success in raising capital and our results of
operations. The Compensation Committee evaluates individual executive performance with the goal of
setting compensation at levels the Compensation Committee believes, based on the Radford survey,
are comparable with executive officers in other companies of similar size and stage of development
operating in the biotechnology and specialty pharmaceutical industries while taking into account
our relative performance and our own strategic goals. In 2009, the Compensation Committee awarded
bonuses to certain of our executive officers. The Compensation Committee also has the ability to
grant discretionary bonuses to executive officers. No discretionary bonuses were granted in 2009.
17
Table of Contents
Bonus Determinations For 2009
The target bonus amounts, as a percentage of each named executive officer’s base salary, were
set by the Compensation Committee based on the peer compensation analysis described above. The
individual performance objectives for each of the named executive officers who were awarded bonuses
for 2009, and the analysis of the achievement of those objectives in determining such bonuses, were
as follows:
Jack Talley. The Committee considered Mr. Talley’s performance in the areas of corporate
management, the establishment of the Company’s oncology identity through the commercialization of
Ceplene and other oncology assets and value realization of the company’s analgesic assets.
The Committee established several objectives for consideration of his corporate management
performance, including implementation of the 2009 operating plan/budget, ensuring that actual
results do not exceed authorized budget; and staffing the organization as operational circumstances
dictate to ensure the Company is appropriately staffed and sized with key personnel required to
implement plans. These objectives were met, while an objective to fund the Company’s operations
without tapping the capital markets was not.
2009 Performance Objectives to establish EpiCept as an oncology company included the
completion of a Ceplene marketing agreement(s) for the European Union, providing necessary
scientific, clinical and regulatory support to the successful commercialization of Ceplene in
Europe, and ensuring that all resources required to advance Ceplene towards an NDA filing with FDA
and an NDS submission with Health Canada are implemented. These objectives were partially or
wholly completed. With respect to the advancement of other oncology assets, performance objectives
were set concerning the advancement of crolibulin and Azixa in clinical development.
A 2009 Performance Objective to realize value of the Company’s analgesic assets was primarily
concerned with the licensing of NP-1. Progress was noted in meeting this objective.
The Committee determined, considering the partial achievement of certain of his objectives,
that Mr. Talley had achieved 50% of the objectives set for him, and awarded him a bonus for 2009 of
$119,625, which was 50% of his target bonus.
Robert Cook. The Committee considered Mr. Cook’s performance against performance objectives
in the areas of finance and accounting, investor relations, and corporate administration.
2009 Performance Objectives with respect to finance and accounting included ensuring that
sufficient funds were` available for the Company’s 2009 operating plan, monitoring liquidity and
recommending appropriate action as necessary; monitoring the Company’s performance compared with
the 2009 operating plan/budget, seeking and implementing efficiencies in order to meet or reduce
the G&A expense portion of 2009 operating plan; and ensuring the Company’s accurate and timely
compliance with SEC and OMX reporting requirements.
Performance objectives with respect to investor relations included evaluating the
effectiveness of the Company’s IR program and recommending actions as appropriate to improve IR
effectiveness, and identifying and implementing efficiencies in investor relations in order to meet
or reduce the G&A expense portion of the 2009 operating plan.
The critical corporate administration performance objective was completing the effective
shut-down of the company’s San Diego operations in preparation for a subleasing of the facility to
a third party.
The Committee determined that Mr. Cook had achieved all of the objectives set for him, and
awarded him a bonus for 2009 of $83,120, which was 100% of his target bonus.
Stephane Allard. The Committee established several objectives for consideration of Dr.
Allard’s 2009 performance, all of which were achieved or exceeded. Objectives related to Ceplene
included the initiation of the biomarker study in Europe and related activities required to address
EMEA post-approval commitments, the design and implementation of a NDA filing strategy for Ceplene
in the US, and support of other marketing and regulatory activities. Objectives related to other
company product candidates concerned the completion of various statistical analyses and study
reports, and oversight of ongoing clinical trials. Dr. Allard exceeded his objectives for the
Health Canada NDS filing for Ceplene due to the complexity of the task, the minimal resources
consumed and the time involved. He additionally worked to accelerate the recruitment of the NP-1
CPN study by working with the investigator steering group to modify the protocol.
18
Table of Contents
The Committee determined that Dr. Allard had achieved all of the objectives set for him and,
with respect to his contributions to the Health Canada NDS filing and EpiCept NP-1 CPN study,
exceeded those objectives, and awarded him a bonus for 2009 of $93,150, which was 115% of his
target bonus.
Dileep Bhagwat. The Committee established several objectives for consideration of Dr.
Bhagwat’s performance, one of which was not achieved. Achieved objectives relating to Ceplene
included securing the appropriate supplies required for a commercial launch in Europe and the
post-approval clinical studies, facilitating the completion of the CMC component and other assigned
sections of the Ceplene NDA filing, and leading efforts to gain regulatory approval in Canada. Dr.
Bhagwat did not meet one performance compensation objective, which resulted in a delay in a US
regulatory filing for Ceplene. Other objectives included ensuring adequate supply of product for
clinical trials of other compounds and internal objectives related to staffing and administration.
The Committee determined that Mr. Bhagwat had achieved 85% of the objectives set for him, and
awarded him a bonus for 2009 of $68,850, which was 85% of his target bonus.
For 2009, annual cash bonus award opportunities for the named executive officers are
summarized below. These awards were determined and paid in 2010.
Annual Cash Bonus Award Opportunity
| Target Performance | ||||||||||||||||
| % of Salary | Amount | Amount Paid | ||||||||||||||
Jack Talley |
FY 2009 | 55 | $ | 239,250 | $ | 119,625 | ||||||||||
Robert Cook |
FY 2009 | 30 | 81,120 | 83,120 | ||||||||||||
Stephane Allard |
FY 2009 | 30 | 81,000 | 93,150 | ||||||||||||
Ben Tseng (1) |
FY 2009 | 30 | 81,000 | — | ||||||||||||
Dileep Bhagwat |
FY 2009 | 30 | 81,000 | 68,850 | ||||||||||||
| (1) | Ben Tseng served as our Chief Scientific Officer from January 2006 to May 2009. |
Long-Term Incentive Program
We believe that long-term performance is achieved through an ownership culture that encourages
such performance by our executive officers through the use of stock and stock-based awards. Our
equity plans have been established to provide our employees, including our executive officers, with
incentives to help align those employees’ interests with the interests of stockholders. The
Compensation Committee believes that the use of stock and stock-based awards offers the best
approach to achieving our compensation goals. We have historically elected to use stock options as
the primary long-term equity incentive vehicle. We believe that the annual aggregate value of these
awards should be set near the 75th percentile of our general peer group. Due to the early stage of
our business, our desire to preserve cash, and the limited nature of our retirement benefit plans,
we expect to provide a greater portion of total compensation to our executives through stock
options, restricted stock units and restricted stock grants than through cash-based compensation.
Stock Options Our stock plans authorize us to grant options to purchase shares of common stock
to our employees, directors and consultants. Our Compensation Committee oversees the administration
of our stock option plan. Stock options may be granted at the commencement of employment, annually,
occasionally following a significant change in job responsibilities or to meet other special
retention objectives.
We expect to continue to use stock options as a long-term incentive vehicle because:
| • | Stock options align the interests of executives with those of the stockholders, support a pay-for-performance culture, foster employee stock ownership, and focus the management team on increasing value for the stockholders. |
| • | Stock options are performance based, in that any value received by the recipient of a stock option is based on the growth of the stock price. |
| • | Stock options help to provide a balance to the overall executive compensation program as base salary and our discretionary annual bonus program focus on short-term compensation, while the vesting of stock options increases stockholders value over the longer term. |
19
Table of Contents
| • | The vesting period of stock options encourages executive retention and the preservation of stockholder value. In determining the number of stock options to be granted to executives, we take into account the individual’s position, scope of responsibility, ability to affect profits and stockholders value and the individual’s historic and recent performance and the value of stock options in relation to other elements of the individual executive’s total compensation. |
The Compensation Committee reviews and approves stock option awards to executive officers
based upon a review of competitive compensation data, its assessment of individual performance, a
review of each executive’s existing long-term incentives, and retention considerations. Periodic
stock option grants are made at the discretion of the Compensation Committee to eligible employees
and, in appropriate circumstances, the Compensation Committee considers the recommendations of our
CEO.
In 2009, certain named executive officers were awarded stock options in the amounts indicated
in the section entitled “Option Grants in Last Fiscal Year (2009).” These grants included grants
made in January 2009 in connection with merit-based grants made by the Board of Directors to
certain of our executive officers, which were intended to encourage an ownership culture among our
executive officers. The January 2009 grants were also made to certain of our executive officers
based on performance of such executive officers and to reward our executive officers for their
service and to encourage continued service with us. Stock options granted by us have an exercise
price equal to the fair market value of our common stock on the day of grant, typically vest
monthly over a four-year period based upon continued employment, and generally expire ten years
after the date of grant.
Stock Appreciation Rights Our 2005 equity incentive plan authorizes us to grant stock
appreciation rights, or SARs. To date, we have not granted any SAR under our 2005 equity incentive
plan. A SAR represents a right to receive the appreciation in value, if any, of our common stock
over the base value of the SAR. The base value of each SAR equals the value of our common stock on
the date the SAR is granted. Upon surrender of each SAR, unless we elect to deliver common stock,
we will pay an amount in cash equal to the value of our common stock on the date of delivery over
the base price of the SAR. SARs typically vest based upon continued employment on a pro-rata basis
over a four-year period, and generally expire ten years after the date of grant. Our Compensation
Committee is the administrator of our stock appreciation rights plan.
Restricted Stock and Restricted Stock Units Our 2005 equity incentive plan authorizes us to
grant restricted stock and restricted stock units. In 2009, certain named non-employee directors
were granted 14,375 restricted stock units with an aggregate fair market value of $31,000. In order
to implement our long-term incentive goals, we may grant restricted stock units in the future in
conjunction with stock options.
Other Compensation
Our executive officers, who are parties to employment agreements, will continue to be parties
to such employment agreements in their current form until such time as the Compensation Committee
determines in its discretion that revisions to such employment agreements are advisable. In
addition, consistent with our compensation philosophy, we intend to continue to maintain our
current benefits for our executive officers, including medical, dental, vision and life insurance
coverage and the ability to contribute to a 401(k) retirement plan; however, the Compensation
Committee in its discretion may revise, amend or add to the officer’s executive benefits if it
deems it advisable. We believe these benefits are currently comparable to the median competitive
levels for comparable companies.
20
Table of Contents
Executive Compensation
The following table sets forth the compensation earned for services rendered to us in all
capacities by our chief executive officer and certain executive officers whose total cash
compensation exceeded $100,000 for the year ended December 31, 2009, collectively referred to in
this annual report as the “named executive officers.”
Summary Compensation Table
| Change in | ||||||||||||||||||||||||||||||||||
| pension | ||||||||||||||||||||||||||||||||||
| value and | ||||||||||||||||||||||||||||||||||
| nonqualified | ||||||||||||||||||||||||||||||||||
| Non-Equity | deferred | |||||||||||||||||||||||||||||||||
| Stock | Option | Incentive Plan | compensation | All Other | ||||||||||||||||||||||||||||||
| Salary | Bonus | Awards | Awards | Compensation | earnings | Compensation | Total | |||||||||||||||||||||||||||
| Name/Principal Position | Year | ($) | ($)(1) | ($)(2) | ($)(3) | ($) | ($) | ($) | ($) | |||||||||||||||||||||||||
John V. Talley |
2009 | 435,000 | 119,625 | — | 345,573 | — | — | 37,175 | (4) | 937,372 | ||||||||||||||||||||||||
President and |
2008 | 435,000 | 358,875 | 117,250 | 329,378 | — | — | 37,776 | (4) | 1,278,279 | ||||||||||||||||||||||||
Chief Executive Officer |
2007 | 400,000 | 200,000 | 99,525 | 278,943 | — | — | 49,685 | (4) | 1,028,154 | ||||||||||||||||||||||||
Robert W. Cook |
2009 | 270,400 | 83,120 | — | 93,039 | — | — | 20,202 | (5) | 466,761 | ||||||||||||||||||||||||
Chief Financial Officer, |
2008 | 270,400 | 81,120 | 42,746 | 114,378 | — | — | 15,891 | (5) | 524,535 | ||||||||||||||||||||||||
Senior Vice President |
2007 | 260,000 | 65,000 | 22,995 | 63,451 | — | — | 24,657 | (5) | 436,103 | ||||||||||||||||||||||||
Finance & Administration |
||||||||||||||||||||||||||||||||||
Stephane Allard |
2009 | 270,000 | 93,150 | — | 106,330 | — | — | 20,788 | (5) | 490,268 | ||||||||||||||||||||||||
Chief Medical Officer |
2008 | 270,000 | 121,500 | 42,746 | 145,816 | — | — | 16,476 | (5) | 596,538 | ||||||||||||||||||||||||
| 2007 | 213,182 | 52,083 | — | 113,797 | — | — | 15,136 | (5) | 394,198 | |||||||||||||||||||||||||
Dileep Bhagwat |
2009 | 270,000 | 68,850 | — | 106,330 | — | — | 26,724 | (5) | 471,904 | ||||||||||||||||||||||||
Senior Vice President, |
2008 | 270,000 | 121,500 | 42,746 | 145,816 | — | — | 26,476 | (5) | 606,538 | ||||||||||||||||||||||||
Pharmaceutical Development |
2007 | 250,000 | 93,750 | 34,482 | 97,612 | — | — | 25,813 | (5) | 501,568 | ||||||||||||||||||||||||
Michael Chen |
2009 | 237,000 | — | — | 39,874 | — | — | 21,816 | (5) | 298,689 | ||||||||||||||||||||||||
Vice President Business |
2008 | 237,000 | 35,550 | 16,750 | 57,105 | — | — | 17,504 | (5) | 363,909 | ||||||||||||||||||||||||
Development |
2007 | 230,000 | 28,750 | 17,476 | 48,222 | — | — | 22,736 | (5) | 347,185 | ||||||||||||||||||||||||
Ben Tseng (6) |
2009 | 276,711 | — | — | 34,898 | — | — | 28,215 | (5) | 333,512 | ||||||||||||||||||||||||
Chief Scientific Officer |
2008 | 270,000 | 81,000 | 42,746 | 145,816 | — | — | 27,978 | (5) | 567,540 | ||||||||||||||||||||||||
| 2007 | 250,000 | 62,500 | 26,525 | 75,086 | — | — | 27,210 | (5) | 441,321 | |||||||||||||||||||||||||
| (1) | Annual cash bonus awards are determined based on the executive’s performance during the year and paid the following year. | |
| (2) | This column represents the grant date fair values for restricted stock granted in 2007 and restricted stock units granted in 2008 to the named executive officers. The 2007 and 2008 stock award values were recalculated from amounts shown in prior annual reports on Form 10-K to reflect their grant date fair values, as required by SEC rules effective for 2010. The grant date fair values have been determined based on the assumptions and methodologies set forth in the Company’s 2009 Annual Report on Form 10-K (Note 11, Share-Based Payments). | |
| (3) | This column represents the grant date fair values of the stock options awarded in 2009, 2008 and 2007, respectively, The 2007 and 2008 option award values were recalculated from the amounts shown in prior annual reports on Form 10-K to reflect the grant date fair value, as required by SEC rules effective for 2010. The grant date fair values have been determined based on the assumptions and methodologies set forth in the Company’s 2009 Annual Report (Note 11, Share-Based Payments). | |
| (4) | Includes premiums for health benefits, life and disability insurance and automobile allowance paid on behalf of Mr. Talley. | |
| (5) | Includes premiums for health benefits and for life and disability insurance paid on behalf of the named executive officer. | |
| (6) | Ben Tseng served as our Chief Scientific Officer from January 2006 to May 2009. During 2009, Dr. Tseng received a salary of $112,500, a lump-sum payment of $29,211 for accrued but unused vacation days and severance payments of $135,000 per his employment agreement. |
21
Table of Contents
Option Grants in Last Fiscal Year (2009)
During 2009, the Company granted approximately 0.7 million stock options and restricted stock
units to employees and directors, of which approximately 0.5 million were to the below named
executive officers.
Grants of Plan-Based Awards
| All | ||||||||||||||||||||||||||||||||||||||||||||
| Other | ||||||||||||||||||||||||||||||||||||||||||||
| Stock | All Other | |||||||||||||||||||||||||||||||||||||||||||
| Awards: | Option | Grant Date | ||||||||||||||||||||||||||||||||||||||||||
| Number | Awards: | Fair Value | ||||||||||||||||||||||||||||||||||||||||||
| of | Number | Exercise | of | |||||||||||||||||||||||||||||||||||||||||
| Estimated Future Payouts Under | Estimated Future Payouts Under | Shares | of Shares | Price of | Stock and | |||||||||||||||||||||||||||||||||||||||
| Non-Equity Incentive Plan Awards | Equity Incentive Plan Awards | of Stock | Underlying | Option | Option | |||||||||||||||||||||||||||||||||||||||
| Name | Grant Date | Threshold | Target | Maximum | Threshold | Target | Maximum | or Units | Options | Awards (1) | Awards (2) | |||||||||||||||||||||||||||||||||
John V. Talley |
01/05/2009 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 108,334 | $ | 1.89 | $ | 151,216 | |||||||||||||||||||||||||||||||
| 02/20/2009 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 140,834 | $ | 1.68 | $ | 194,303 | ||||||||||||||||||||||||||||||||
Robert W. Cook |
01/05/2009 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 29,167 | $ | 1.89 | $ | 40,712 | |||||||||||||||||||||||||||||||
| 02/20/2009 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 37,917 | $ | 1.68 | $ | 52,312 | ||||||||||||||||||||||||||||||||
Stephane Allard |
01/05/2009 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 33,334 | $ | 1.89 | $ | 46,530 | |||||||||||||||||||||||||||||||
| 02/20/2009 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 43,334 | $ | 1.68 | $ | 59,800 | ||||||||||||||||||||||||||||||||
Dileep Bhagwat |
01/05/2009 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 33,334 | $ | 1.89 | $ | 46,530 | |||||||||||||||||||||||||||||||
| 02/20/2009 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 43,334 | $ | 1.68 | $ | 59,800 | ||||||||||||||||||||||||||||||||
Michael Chen |
01/05/2009 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 12,500 | $ | 1.89 | $ | 17,449 | |||||||||||||||||||||||||||||||
| 02/20/2009 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 16,250 | $ | 1.68 | $ | 22,425 | ||||||||||||||||||||||||||||||||
Ben Tseng |
01/05/2009 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 25,000 | $ | 1.89 | $ | 34,898 | |||||||||||||||||||||||||||||||
| (1) | The exercise price of the options was equal to the market value of our common stock on the date of the grant. | |
| (2) | The grant date fair values have been determined based on the assumptions and methodologies set forth in the Company’s 2009 Annual Report (Note 11, Share-Based Payments). |
Aggregate Option Exercises in Last Fiscal Year (2009) and Values at December 31, 2009
None of the named executive officers exercised any options in 2009. The named executive
officers in the “Grants of Plan-Based Awards Table” above received an aggregate of 10,586 shares of
common stock representing the vested portion of their restricted stock grant in 2007.
22
Table of Contents
Outstanding Equity Awards at December 31, 2009
| Option Awards | Stock Awards | |||||||||||||||||||||||||||||||||||
| Equity | Equity Incentive | Equity Incentive | ||||||||||||||||||||||||||||||||||
| Incentive Plan | Plan | Plan Awards: | ||||||||||||||||||||||||||||||||||
| Awards: | Market | Awards: | Market or | |||||||||||||||||||||||||||||||||
| Number of | Number of | Value of | Number of | Payout Value of | ||||||||||||||||||||||||||||||||
| Number of Securities | Securities | Shares or | Shares or | Unearned | Unearned | |||||||||||||||||||||||||||||||
| Underlying | Underlying | Units of | Units of | Shares, Units or | Shares, Units or | |||||||||||||||||||||||||||||||
| Unexercised Options | Unexercised | Option | Option | Stock | Stock | Other Rights | Other Rights | |||||||||||||||||||||||||||||
| Number | Number | Unearned | Exercise | Expiration | That have | That have | That have | That have | ||||||||||||||||||||||||||||
| Name | Exercisable | Unexercisable | Options | Price | Date | Not Vested | Not Vested | Not Vested | Not Vested | |||||||||||||||||||||||||||
John V. Talley |
27,695 | — | — | $ | 3.60 | 11/1/2011 | — | — | — | — | ||||||||||||||||||||||||||
| 695 | — | — | $ | 3.60 | 1/1/2012 | — | — | — | — | |||||||||||||||||||||||||||
| 27,778 | — | — | $ | 3.60 | 1/1/2012 | — | — | — | — | |||||||||||||||||||||||||||
| 414,219 | — | — | $ | 17.52 | 1/4/2016 | — | — | — | — | |||||||||||||||||||||||||||
| 68,416 | 22,806 | — | $ | 4.38 | 1/8/2017 | 5,680 | $ | 9,883 | — | — | ||||||||||||||||||||||||||
| 58,332 | 58,335 | — | $ | 4.02 | 1/7/2018 | 29,167 | $ | 50,750 | — | — | ||||||||||||||||||||||||||
| 25,000 | — | — | $ | 1.89 | 9/8/2018 | — | — | — | — | |||||||||||||||||||||||||||
| 27,081 | 81,253 | — | $ | 1.89 | 1/5/2019 | — | — | — | — | |||||||||||||||||||||||||||
| — | 140,834 | — | $ | 1.68 | 2/20/2019 | — | — | — | — | |||||||||||||||||||||||||||
Robert Cook |
70,523 | — | — | $ | 17.52 | 1/4/2016 | — | — | — | — | ||||||||||||||||||||||||||
| 15,563 | 5,187 | — | $ | 4.38 | 1/8/2017 | 1,312 | $ | 2,283 | — | — | ||||||||||||||||||||||||||
| 21,283 | 21,284 | — | $ | 4.02 | 1/7/2018 | 10,634 | $ | 18,500 | — | — | ||||||||||||||||||||||||||
| 5,000 | — | — | $ | 1.89 | 9/8/2018 | — | — | — | — | |||||||||||||||||||||||||||
| 7,291 | 21,876 | — | $ | 1.89 | 1/5/2019 | — | — | — | — | |||||||||||||||||||||||||||
| — | 37,917 | — | $ | 1.68 | 2/20/2019 | — | — | — | — | |||||||||||||||||||||||||||
Stephane Allard |
23,611 | 9,723 | — | $ | 4.89 | 3/23/2017 | — | — | — | — | ||||||||||||||||||||||||||
| 21,283 | 21,284 | — | $ | 4.02 | 1/7/2018 | 10,634 | $ | 18,500 | — | — | ||||||||||||||||||||||||||
| 16,667 | — | — | $ | 1.89 | 9/8/2018 | — | — | — | — | |||||||||||||||||||||||||||
| 8,333 | 25,001 | — | $ | 1.89 | 1/5/2019 | — | — | — | — | |||||||||||||||||||||||||||
| — | 43,334 | — | $ | 1.68 | 2/20/2019 | — | — | — | — | |||||||||||||||||||||||||||
Dileep Bhagwat |
37,500 | — | — | $ | 17.52 | 1/4/2016 | — | — | — | — | ||||||||||||||||||||||||||
| 23,941 | 7,981 | — | $ | 4.38 | 1/8/2017 | 1,968 | $ | 3,424 | — | — | ||||||||||||||||||||||||||
| 21,283 | 21,284 | — | $ | 4.02 | 1/7/2018 | 10,634 | $ | 18,500 | — | — | ||||||||||||||||||||||||||
| 16,667 | — | — | $ | 1.89 | 9/8/2018 | — | — | — | — | |||||||||||||||||||||||||||
| 8,333 | 25,001 | — | $ | 1.89 | 1/5/2019 | — | — | — | — | |||||||||||||||||||||||||||
| — | 43,334 | — | $ | 1.68 | 2/20/2019 | — | — | — | — | |||||||||||||||||||||||||||
Ben Tseng |
— | — | — | — | — | — | — | — | — | |||||||||||||||||||||||||||
Michael Chen |
24,445 | 2,222 | — | $ | 8.10 | 5/26/2016 | — | — | — | — | ||||||||||||||||||||||||||
| 11,828 | 3,942 | — | $ | 4.38 | 1/8/2017 | 996 | $ | 1,733 | — | — | ||||||||||||||||||||||||||
| 8,333 | 8,334 | — | $ | 4.02 | 1/7/2018 | 4,167 | $ | 7,251 | — | — | ||||||||||||||||||||||||||
| 3,125 | 9,375 | — | $ | 1.89 | 1/5/2019 | — | — | — | — | |||||||||||||||||||||||||||
| — | 16,250 | — | $ | 1.68 | 2/20/2019 | — | — | — | — | |||||||||||||||||||||||||||
Employment Agreements and Potential Payments Upon Termination or Change-in-Control
We believe that reasonable and appropriate severance and change-in-control benefits are
appropriate to protect our named executives against circumstances over which they have no control.
Furthermore, we believe change-in-control severance payments align the named executives’ interests
with our own by enabling the named executive to evaluate a transaction in the best interests of our
shareholders and our other constituents without undue concern over whether the transaction may
jeopardize the named executive’s own employment.
23
Table of Contents
We have entered into employment agreements with Messrs. John V. Talley and Robert W. Cook,
each dated as of October 28, 2004. Effective January 4, 2006, pursuant to their employment
agreements, Messrs. Talley and Cook received base salaries of $350,000 and $250,000, respectively.
For 2010, Messrs. Talley and Cook will receive a base salary of $435,000, and $278,512,
respectively. Each employment agreement also provides for discretionary bonuses and stock option
awards and reimbursement of reasonable expenses incurred in connection with services performed
under each officer’s respective employment agreement. The
discretionary bonuses and stock options are based on performance standards determined by our Board.
Individual performance is determined based on quantitative and qualitative objectives, including
our operating performance relative to budget and the achievement of certain milestones largely
related to the clinical development of our products and licensing activities. The future objectives
will be established by our Board. In addition, Mr. Talley’s employment agreement provides for
automobile benefits and term life and long term disability insurance coverage. Both employment
agreements expired on December 31, 2007 but are automatically extended for unlimited additional
one-year periods.
The information below describes and quantifies certain compensation that would become payable
under each of Messrs. Talley and Cook’s respective employment agreements if, as of December 31,
2009, his employment had been terminated or there was a change in control. Due to the number of
factors that affect the nature and amount of any benefits provided upon the events discussed below,
any actual amounts paid or distributed may be different. Factors that could affect these amounts
include the timing during the year of any
such event. We do not have employment agreements with any other of our named executive
officers. If Messrs. Allard, and Bhagwat are terminated without cause, each is entitled to
severance payments of $139,050.
Termination for any Reason. Upon termination for any reason and in addition to any other
payments disbursed in connection with termination, Mr. Talley and Mr. Cook are entitled to:
| • | receive payment of his applicable base salary through the termination date; |
| • | the balance of any annual, long-term or incentive award earned in any period prior to the termination date; and |
| • | a lump-sum payment for any accrued but unused vacation days. |
Termination due to Death or Disability. If termination occurs due to death or disability, Mr.
Talley is or his estate is entitled to:
| • | a lump-sum payment equal to one-third of his base salary times a fraction, the numerator being the number of days he was employed in the calendar year of termination and the denominator being the number of days in that year; |
| • | have 50% of outstanding stock options that are not then vested or exercisable become vested and exercisable as of the termination date; |
| • | have the remaining outstanding stock options that are not then vested or exercisable become vested and exercisable ratably and quarterly for two years following the termination date; and |
| • | have each outstanding stock option remain exercisable for all securities for the later of (i) the 90th day following the date that the option becomes fully vested and exercisable and (ii) the first anniversary of the termination date. |
If termination occurs due to death or disability, Mr. Cook or his estate is entitled to
receive the same benefits as Mr. Talley, except the equation for his lump-sum payment is based on
one-fourth of his base salary.
Termination Without Cause. If Mr. Talley is terminated without cause or the term of his
agreement is not extended pursuant to the employment agreement, he is entitled to receive payments
equal to:
| • | the payments he would receive if he were terminated due to death or disability; and |
| • | a lump-sum payment equal to one and one-third times his base salary times the number of whole and partial months remaining in the term of the agreement (but no more than 12 and no less than 6) divided by 12. |
If Mr. Cook is terminated without cause or the term of his agreement is not extended pursuant
to the employment agreement, he is entitled to the same benefits as Mr. Talley, but the equation
for his lump-sum payment is based on one and one-fourth times his base salary.
Change-in-Control Arrangements. If Mr. Talley is terminated in anticipation of, or within one
year following, a change of control, he is entitled to:
| • | a lump-sum payment equal to one and one third times his base salary times the number of whole and partial months remaining in the term of the agreement (but not less than 24) divided by 12; |
| • | have 50% of outstanding stock options that are not then vested or exercisable become vested and exercisable as of the termination date; |
| • | the remaining outstanding stock options that are not then vested or exercisable become vested and exercisable ratably and monthly for the first year following the termination date; and |
| • | have each outstanding stock option remain exercisable for all securities for the later of (i) the 90th day following the date that the option becomes fully vested and exercisable and (ii) the first anniversary of the termination date. |
24
Table of Contents
If Mr. Cook is terminated in anticipation of, or within one year following, a change of
control, he is entitled to the same benefits as Mr. Talley, except his lump sum is equal to one and
one-fourth times his base salary times the number of whole and partial months remaining in the term
of the agreement (but no more than 18 and no less than 12) divided by 12.
The following table summarizes the potential payments to our named executive officers with
whom we have entered into employment agreements, assuming that such events occurred as of December
31, 2009.
| Accelerated | ||||||||||||||||||||||||
| Vested | Vesting of | |||||||||||||||||||||||
| Severance | Benefit | Incentive | Incentive | |||||||||||||||||||||
| Amounts | Benefits | Continuation | Units | Units | Total | |||||||||||||||||||
| ($) | ($) | ($) | ($) | ($) | ($) | |||||||||||||||||||
John V. Talley |
||||||||||||||||||||||||
Termination for any reason (other than without
cause or for good reason) |
$ | 54,375 | $ | — | $ | — | $ | — | $ | — | $ | 54,375 | ||||||||||||
Termination without cause or for good reason |
$ | 435,000 | $ | — | $ | — | $ | — | $ | — | $ | 435,000 | ||||||||||||
Change in control |
1,160,000 | $ | — | $ | — | $ | — | $ | — | $ | 1,160,000 | |||||||||||||
Robert W. Cook |
||||||||||||||||||||||||
Termination for any reason (other than without
cause or for good reason) |
$ | 34,814 | $ | — | $ | — | $ | — | $ | — | $ | 34,814 | ||||||||||||
Termination without cause or for good reason |
$ | 243,698 | $ | — | $ | — | $ | — | $ | — | $ | 243,698 | ||||||||||||
Change in control |
$ | 348,140 | $ | — | $ | — | $ | — | $ | — | $ | 348,140 | ||||||||||||
Stock Option Plans
2005 Equity Incentive Plan
The 2005 Equity Incentive Plan, as amended, was adopted on September 1, 2005 and approved by
stockholders on September 5, 2005, and subsequently amended and approved by stockholders on May 23,
2007 and June 2, 2009. The 2005 Equity Incentive Plan provides for the grant of incentive stock
options, within the meaning of Section 422 of the Internal Revenue Code, to our employees and our
parent and subsidiary corporations’ employees, and for the grant of nonstatutory stock options,
restricted stock, restricted stock units, performance-based awards and cash awards to our
employees, directors and consultants and our parent and subsidiary corporations’ employees and
consultants.
A total of 13,000,000 shares of our common stock are reserved for issuance pursuant to the
2005 Equity Incentive Plan. As of December 31, 2009, 2.4 million shares are outstanding. No
optionee may be granted an option to purchase more than 1,500,000 shares in any fiscal year.
Our board of directors or a committee of its board administers the 2005 Equity Incentive Plan.
In the case of options intended to qualify as “performance-based compensation” within the meaning
of Section 162(m) of the Internal Revenue Code, the committee will consist of two or more “outside
directors” within the meaning of Section 162(m) of the Code. The administrator has the power to
determine the terms of the awards, including the exercise price, the number of shares subject to
each such award, the exercisability of the awards and the form of consideration, if any, payable
upon exercise. The administrator also has the authority to institute an exchange program by which
outstanding awards may be surrendered in exchange for awards with a lower exercise price.
The administrator will determine the exercise price of options granted under the 2005 Equity
Incentive Plan, but with respect to nonstatutory stock options intended to qualify as
“performance-based compensation” within the meaning of Section 162(m) of the Code and all incentive
stock options, the exercise price must at least be equal to the fair market value of our common
stock on the date of grant. The term of an incentive stock option may not exceed ten years, except
that with respect to any participant who owns 10% of the voting power of all classes of our
outstanding stock, the term must not exceed five years and the exercise price must equal at least
110% of the fair market value on the grant date. The administrator determines the term of all other
options.
25
Table of Contents
Restricted stock may be granted under the 2005 Equity Incentive Plan. Restricted stock awards
are shares of our common stock that vest in accordance with terms and conditions established by the
administrator. The administrator will determine the number of shares of restricted stock granted to
any employee. The administrator may impose whatever conditions to vesting it determines to be
appropriate. For example, the administrator may set restrictions based on the achievement of
specific performance goals. Shares of restricted stock that do not vest are subject to our right of
repurchase or forfeiture.
Performance-based awards may be granted under the 2005 Equity Incentive Plan.
Performance-based awards are awards that will result in a payment to a participant only if
performance goals established by the administrator are achieved or the awards otherwise vest. The
administrator will establish organizational or individual performance goals in its discretion,
which, depending on the extent to which they are met, will determine the number and/or the value of
performance units and performance shares to be paid out to participants. The 2005 Equity Incentive
Plan generally does not allow for the transfer of awards and only the recipient of an award may
exercise an award during his or her lifetime.
The 2005 Equity Incentive Plan provides that if we experience a Change of Control (as defined
in the Plan), the administrator may provide at any time prior to the Change of Control that all
then outstanding stock options and unvested cash awards shall immediately vest and become
exercisable and any restrictions on restricted stock awards shall immediately lapse. In addition,
the administrator may provide that all awards held by participants who are at the time of the
Change of Control in our service or the service of one of our subsidiaries or affiliates shall
remain exercisable for the remainder of their terms notwithstanding any subsequent termination of a
participant’s service. All awards will be subject to the terms of any agreement effecting the
Change of Control, which agreement may provide, without limitation, that in lieu of continuing the
awards, each outstanding stock option shall terminate within a specified
number of days after notice to the holder, and that such holder shall receive, with respect to
each share of common stock subject to such stock option, an amount equal to the excess of the fair
market value of such shares of common stock immediately prior to the occurrence of such Change of
Control over the exercise price (or base price) per share underlying such stock option with such
amount payable in cash, in one or more kinds of property (including the property, if any, payable
in the transaction) or in a combination thereof, as the administrator, in its discretion, shall
determine. A provision like the one contained in the preceding sentence shall be inapplicable to a
stock option granted within six months before the occurrence of a Change of Control if the holder
of such stock option is subject to the reporting requirements of Section 16(a) of the Exchange Act
and no exception from liability under Section 16(b) of the Exchange Act is otherwise available to
such holder.
The 2005 Equity Incentive Plan will automatically terminate ten years from the effective date,
unless it is terminated sooner. In addition, our board of directors has the authority to amend,
suspend or terminate the 2005 Equity Incentive Plan provided such action does not impair the rights
of any participant.
1995 Stock Option Plan
The 1995 Stock Option Plan, as amended, was approved by our board of directors in November
1995, and subsequently amended in April 1997, March 1999, February 2002 and June 2002. A total of
797,080 shares of our common stock were authorized for issuance under the 1995 Stock Option Plan.
As of December 31, 2009 and 2008, 83,981 shares were available for issuance under the 1995 Stock
Option Plan. We do not plan to grant any further options from this plan.
The purpose of the 1995 Stock Option Plan was to provide us and our stockholders the benefits
arising out of capital stock ownership by our employees, officers, directors, consultants and
advisors and any of our subsidiaries, who are expected to contribute to our future growth and
success. The 1995 Stock Option Plan provides for the grant of non-statutory stock options to our
(and our majority-owned subsidiaries’) employees, officers, directors, consultants or advisors, and
for the grant of incentive stock options meeting the requirements of Section 422 of the Internal
Revenue Code to our employees and employees of our majority-controlled subsidiaries.
A committee duly appointed by our board of directors administered the 1995 Stock Option Plan.
The committee has the authority to (a) construe the respective option agreements and the terms of
the plan; (b) prescribe, amend and rescind rules and regulations relating to the plan; (c)
determine the terms and provisions of the respective option agreements, which need not be
identical; (d) make all other determinations in the judgment of the committee necessary or
desirable for the administration of the plan. From and after the registration of our common stock
under the Securities Exchange Act of 1934, the selection of a director or an officer who is a
“reporting person” under Section 16(a) of the Exchange Act as a recipient of an option, the timing
of the option grant, the exercise price of the option and the number of shares subject to the
option shall be determined by (a) the committee of the Board, each of which members shall be an
outside director or (b) by a committee consisting of two or more directors having full authority to
act in the matter, each of whom shall be an outside director.
26
Table of Contents
The committee shall determine the exercise price of stock options granted under the 1995 Stock
Option Plan, but with respect to all incentive stock options, the exercise price must be at least
equal to the fair market value of our common stock on the date of the grant or, in the case of
grants of incentive stock options to holders of more than 10% of the total combined voting power of
all classes of our stock (“10% owners”), at least equal to 110% of the fair market value of our
common stock on the date of the grant.
The committee shall determine the term of stock options granted under the 1995 Stock Option
Plan, but such date shall not be later than 10 years after the date of the grant, except in the
case of incentive stock options granted to 10% owners in which case such date shall not be later
than five years after the date of the grant.
Each option granted under the 1995 Stock Option Plan is exercisable in full or in installments
at such time or times and during such period as is set forth in the option agreement evidencing
such option, but no option granted to a “reporting person” shall be exercisable during the first
six months after the grant.
No optionee may be granted an option to purchase more than 350,000 shares in any fiscal year.
In addition, no incentive stock option may be exercisable for the first time in any one calendar
year for shares of common stock with an aggregate fair market value (as of the date of the grant)
of more than $100,000.
The 1995 Stock Option Plan generally does not allow for the transfer of options and only the
optionee may exercise an option during his or her lifetime.
An optionee may exercise an option at any time within three months following the termination
of the optionee’s employment or other relationship with us or within one year if such termination
was due to the death or disability of the optionee, but except in the case of the optionee’s death,
in no event later than the expiration date of the option. If the termination of the optionee’s
employment is for cause, the option expires immediately upon termination.
The 1995 Stock Option Plan automatically terminated on November 14, 2005.
2005 Employee Stock Purchase Plan
The 2005 Employee Stock Purchase Plan was adopted on September 1, 2005 and approved by the
stockholders on September 5, 2005. The Employee Stock Purchase Plan became effective at the
effective time of the merger with Maxim and a total of 500,000 shares of our common stock have been
reserved for sale.
Our board of directors or a committee of the board will administer the 2005 Employee Stock
Purchase Plan. Our board of directors or the committee will have full and exclusive authority to
interpret the terms of the 2005 Employee Stock Purchase Plan and determine eligibility.
All of our employees are eligible to participate if they are customarily employed by us or any
participating subsidiary for at least 20 hours per week and more than five months in any calendar
year. However, an employee may not be granted an option to purchase stock if such employee:
| • | immediately after the grant owns stock possessing 5% or more of the total combined voting power or value of all classes of our capital stock, or |
| • | whose rights to purchase stock under all of our employee stock purchase plans accrues at a rate that exceeds $25,000 worth of stock for each calendar year. |
The 2005 Employee Stock Purchase Plan is intended to qualify under Section 423 of the Internal
Revenue Code and generally provides for six-month offering periods beginning on January 1 and July
1 of each calendar year, commencing on January 1, 2006 or such other date as may be determined by
the committee appointed by us to administer the 2005 Employee Stock Purchase Plan. The plan
commenced on November 16, 2007.
The 2005 Employee Stock Purchase Plan permits participants to purchase common stock through
payroll deductions from their eligible compensation, which includes a participant’s base salary,
wages, overtime pay, shift premium and recurring commissions, but does not include payments for
incentive compensation or bonuses.
27
Table of Contents
Amounts deducted and accumulated by the participant are used to purchase shares of our common
stock at the end of each six-month purchase period. The price is 85% of the lower of the fair
market value of our common stock at the beginning of an offering period or end of an offering
period. Participants may end their participation at any time during an offering period, and will be
paid their payroll deductions to date. Participation ends automatically upon termination of
employment with us.
A participant may not transfer rights granted under the 2005 Employee Stock Purchase Plan
other than by will, the laws of descent and distribution or as otherwise provided under the 2005
Employee Stock Purchase Plan.
Our board of directors has the authority to amend or terminate the 2005 Employee Stock
Purchase Plan, except that, subject to certain exceptions described in the 2005 Employee Stock
Purchase Plan, no such action may adversely affect any outstanding rights to purchase stock under
the 2005 Employee Stock Purchase Plan.
2009 Employee Stock Purchase Plan
The 2009 Employee Stock Purchase Plan was adopted by the board of directors on December 4,
2008. The 2009 Employee Stock Purchase Plan became effective on January 1, 2009 and a total of
1,000,000 shares of our common stock have been reserved for sale, pending stockholder approval.
A committee appointed by the board of directors will administer the 2009 Employee Stock
Purchase Plan, and the committee will have full and exclusive authority to interpret its terms and
determine eligibility.
All of our employees are eligible to participate, however, an employee may not purchase stock
if such employee:
| • | is designated by the committee as being ineligible to participate in the 2009 Employee Stock Purchase Plan as permitted by Section 423(b)(4)(D) of the Internal Revenue Code; |
| • | has not been employed by us or any participating subsidiary for at least two years; |
| • | is not customarily employed by us or any participating subsidiary for at least 20 hours per week and more than five months in any calendar year; |
| • | immediately after the grant owns stock possessing 5% or more of the total combined voting power or value of all classes of our capital stock; or |
| • | whose rights to purchase stock under all of our employee stock purchase plans accrues at a rate that exceeds $25,000 worth of stock for each calendar year. |
The 2009 Employee Stock Purchase Plan is intended to qualify under Section 423 of the Internal
Revenue Code and generally provides for successive six-month offering periods beginning on January
1 and July 1 of each calendar year, commencing on January 1, 2009.
The 2009 Employee Stock Purchase Plan permits participants to purchase common stock through
payroll deductions from their eligible compensation, which includes a participant’s base salary and
any other compensation components determined by the committee.
Amounts deducted and accumulated by the participant are used to purchase shares of our common
stock at the end of each six-month offering period. The maximum number of shares purchasable by a
participant in any offering period is 100,000 shares. The price is 85% of the lower of the fair
market value of our common stock at the beginning of an offering period or end of an offering
period. Participants may end their participation at any time during an offering period, and will be
paid their payroll deductions to date. Participation ends automatically upon termination of
employment with us.
A participant may not transfer rights granted under the 2009 Employee Stock Purchase Plan
other than by will, the laws of descent and distribution or as otherwise provided under the 2009
Employee Stock Purchase Plan.
Our board of directors has the authority to amend or terminate the 2009 Employee Stock
Purchase Plan, except that, subject to certain exceptions described in the 2009 Employee Stock
Purchase Plan, no such action may adversely diminish any outstanding rights under the 2009 Employee
Stock Purchase Plan at the time of termination.
28
Table of Contents
The following table sets forth the participation in our employee stock purchase plans by our
named executive officers and all other employees as a group since inception:
| Shares Purchased | ||||
| As of December 31, 2009 | ||||
John V. Talley |
45,698 | |||
Robert W. Cook |
23,351 | |||
Stephane Allard |
44,822 | |||
Dileep Bhagwat |
13,213 | |||
Michael Chen |
— | |||
Ben Tseng |
17,300 | |||
All other employees |
67,730 | |||
Total |
212,114 | |||
401(k) Plan
In January 2007, we adopted a new Retirement Savings and Investment Plan, the 401(k) Plan,
whereby the two previously existing plans were terminated. The 401(k) Plan provides for matching
contributions by us in an amount equal to the lesser of 50% of the employee’s deferral or 3% of the
employee’s qualifying compensation. The 401(k) Plan is intended to qualify under Section 401(k) of
the Internal Revenue Code, so that contributions to the 401(k) Plan by employees or by us, and the
investment earnings thereon, are not taxable to the employees until withdrawn. If the 401(k) Plan
qualifies under Section 401(k) of the Internal Revenue Code, the contributions will be tax
deductible by us when made. Our employees may elect to reduce their current compensation by up to
the statutorily prescribed annual limit of $16,500 if under 50 years old and $22,000 if over 50
years old in 2009 and to have those funds contributed to the 401(k) Plan.
In 1998, we adopted a Retirement Savings and Investment Plan, the old EpiCept 401(k) Plan,
covering its full-time employees located in the United States. The old EpiCept 401(k) Plan was
intended to qualify under Section 401(k) of the Internal Revenue Code, so that contributions to the
401(k) Plan by employees or by us, were the investment earnings thereon, are not taxable to the
employees until withdrawn. The old EpiCept 401(k) Plan was terminated in January 2007.
Upon the completion of the merger with Maxim on January 4, 2006, we adopted and continued the
existing 401(k) retirement plan, the Maxim 401(k) Plan, under which employees of our San Diego
office who met the eligibility requirements may participate and contribute to the 401(k) Plan. The
Maxim 401(k) Plan was terminated in January 2007.
Director Compensation
We compensate our non-employee directors in cash, stock options and restricted stock units.
We also reimburse our non-employee directors for their expenses incurred in connection with
attending board and committee meetings.
For 2009, the compensation committee retained Radford to analyze the Company’s non-executive
director and chairman compensation. The committee determined that cash compensation should be
benchmarked to the 50th percentile and that equity-based compensation should be benchmarked to the
75th percentile for comparable companies in the biotechnology and specialty pharmaceutical
industries. As a result of that analysis, the board approved a 2009 annual equity grant for each
named director and for the chairman of 12,500 shares and 21,875 shares, respectively. Eighty
percent of the annual director equity grant was in the form of stock options vesting monthly over
two years and the remainder was comprised of restricted stock units cliff vesting after two years.
29
Table of Contents
The following table set forth all material Director compensation information during the year
ended December 31, 2009:
Director Compensation Table
| Change in pension | ||||||||||||||||||||||||||||
| value and | ||||||||||||||||||||||||||||
| nonqualified | ||||||||||||||||||||||||||||
| Fees Earned | Non-equity | deferred | ||||||||||||||||||||||||||
| or Paid in | Stock | Option | Incentive plan | compensation | All Other | |||||||||||||||||||||||
| Cash (1) | Awards ($)(2) | Awards ($) (3) | compensation($) | earnings($) | Compensation | Total | ||||||||||||||||||||||
Robert G. Savage |
$ | 64,750 | $ | 9,568 | $ | 30,996 | $ | — | $ | — | $ | — | $ | 105,314 | ||||||||||||||
Guy C. Jackson |
53,500 | 5,468 | 17,712 | — | — | — | 76,680 | |||||||||||||||||||||
Gerhard Waldheim |
39,000 | 5,468 | 17,712 | — | — | — | 62,180 | |||||||||||||||||||||
A. Collier Smyth |
27,750 | 5,468 | 32,328 | — | — | — | 65,546 | |||||||||||||||||||||
Wayne P. Yetter |
48,000 | 5,468 | 17,712 | — | — | — | 71,180 | |||||||||||||||||||||
| (1) | This column reports the amount of cash compensation earned in 2009 for Board and committee service. | |
| (2) | This column represents the grant date fair values for restricted stock units granted in to the named directors. The grant date fair values have been determined based on the assumptions and methodologies set forth in the Company’s 2009 Annual Report on Form 10-K (Note 11, Share-Based Payments). | |
| (3) | This column represents the grant date fair values of the stock options awarded to the named directors in 2009, The grant date fair values have been determined based on the assumptions and methodologies set forth in the Company’s 2009 Annual Report (Note 11, Share-Based Payments). |
In 2009, the chair person of the board received an annual retainer of $40,000, while each
other non-employee director received an annual retainer of $25,000. In addition, the chairperson of
the audit committee received an annual retainer of $10,000 and the chairperson of each of the other
committees received an annual retainer of $7,500. Each non-employee director also received $1,500
for their attendance at each board meeting, $750 for their participation in each telephonic board
meeting, $750 for their attendance at each committee meeting and $500 for their participation in a
telephonic committee meeting. We have in the past granted non-employee directors restricted stock
units and options to purchase our common stock pursuant to the terms of our 2005 Equity Incentive
Plan upon joining the board and annually thereafter as determined by the Compensation Committee
after considering market data. In 2009, Dr. Smyth, our only new director, received an initial
grant of 11,667 options vesting monthly over three years. Typically annual equity compensation
vests monthly over two years. The option and restricted stock unit awards to the directors in 2009
represent awards to Messrs. Savage, Jackson, Waldheim, Smyth and Yetter. The value of the options
and restricted stock units granted to non-employee directors set forth in the Director Compensation
Table above reflect grants of options and restricted stock units to compensate for their service
and were issued at the market value of our common stock at the date of grant.
Tax Implications of Executive Compensation
We do not believe that Section 162(m) of the Internal Revenue Code, which limits deductions
for executive compensation paid in excess of $1.0 million, is applicable to us, and accordingly,
our Compensation Committee did not consider its impact in determining compensation levels for our
named executive officers in 2008.
Accounting Implications of Executive Compensation
Effective January 1, 2006, we were required to recognize compensation expense of all
stock-based awards pursuant to the principles set forth in in accordance with ASC 718-10,
“Compensation – Stock Compensation” (“ASC 718-10”). The Summary Compensation and Director
Compensation Tables used the principles set forth in ASC 718-10 to recognize expense for new awards
granted after January 1, 2006 and for unvested awards as of January 1, 2006. The non-cash stock
compensation expense for stock-based awards that we grant is generally recognized ratably over the
requisite vesting period. We continue to believe that stock options, restricted stock, restricted
stock units and other forms of equity compensation are an essential component of our compensation
strategy, and we intend to continue to offer these awards in the future.
30
Table of Contents
Compensation Committee Interlocks and Insider Participation
All members of the Compensation Committee of the Board of Directors during the fiscal year
ended December 31, 2009 were independent directors and none of them were our employees or our
former employees. During the fiscal year ended December 31,
2009, none of our executive officers served on the Compensation Committee (or equivalent), or
the board of directors, of another entity whose executive officers served on the Compensation
Committee of our board of directors.
Compensation Committee Report
The Compensation Committee of the Board has reviewed and discussed with management the
Compensation Discussion and Analysis above, and based on such discussions, the Compensation
Committee recommended to the Board that the Compensation Discussion and Analysis be included in our
annual report on Form 10-K.
Respectfully Submitted by:
MEMBERS OF THE COMPENSATION COMMITTEE
Robert G. Savage
Guy C. Jackson
A. Collier Smyth
Robert G. Savage
Guy C. Jackson
A. Collier Smyth
31
Table of Contents
Table of Contents
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934,
Registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto
duly authorized.
| EPICEPT CORPORATION |
||||
| By: | /s/ John V. Talley | |||
| John V. Talley | ||||
| President and Chief Executive Officer November 19, 2010 |
||||
33
Table of Contents
Exhibit Index
| Exhibit | ||||
| Number | Description of Exhibits | |||
| 2.1 | Agreement and Plan of Merger, dated as of September 6, 2005, among EpiCept Corporation,
Magazine Acquisition Corp. and Maxim Pharmaceuticals, Inc. (incorporated by reference to
Exhibit 2.1 to Maxim Pharmaceuticals Inc.’s Current Report on Form 8-K filed September 6,
2005). |
|||
| 3.1 | Third Amended and Restated Certificate of Incorporation of EpiCept Corporation (incorporated
by reference to Exhibit 3.1 to EpiCept Corporation’s Current Report on Form 8-K filed May 21,
2008). |
|||
| 3.2 | Amendment to the Third Amended and Restated Certificate of Incorporation (incorporated by
reference to Exhibit 3.1 to EpiCept Corporation’s Current Report on Form 8-K filed July 9,
2009). |
|||
| 3.3 | Certificate of Amendment to the Third Amended and Restated Certificate of Incorporation,
filed with the Secretary of State of the State of Delaware on January 14, 2010 (incorporated
by reference to Exhibit 3.1 to EpiCept Corporation’s Current Report on Form 8-K filed
January 14, 2010). |
|||
| 3.4 | Amended and Restated Bylaws of EpiCept Corporation (incorporated by reference to Exhibit 3.1
to EpiCept Corporation’s Current Report on Form 8-K filed February 18, 2010). |
|||
| 4.1 | Specimen Common Stock Certificate (incorporated by reference to Exhibit 4.1 to the EpiCept’s
Corporation’s Registration Statement on Form S-1 (File No. 333-121938) (the “EpiCept
Form S-1”)). |
|||
| 4.2 | Convertible Debenture due April 10, 2009 (incorporated by reference to Exhibit 4.1 to EpiCept
Corporation’s Current Report on Form 8-K, filed December 9, 2008). |
|||
| 4.3 | Indenture between EpiCept Corporation and Bank of New York Mellon, as Trustee, dated February
9, 2009 (incorporated by reference to Exhibit 4.1 to Epicept Corporation’s Current Report on
Form 8-K, filed February 10, 2009). |
|||
| 10.1 | Form of Indemnification Agreement between EpiCept Corporation and each of its directors and
executive officers (incorporated by reference to Exhibit 10.1 to the EpiCept Form S-1). |
|||
| †10.2 | 1995 Stock Option Plan (incorporated by reference to Exhibit 10.2 to the EpiCept Form S-1). |
|||
| †10.3 | 2005 Equity Incentive Plan (Amended and Restated May 23, 2007) (incorporated by reference to
Exhibit 10.1 to EpiCept’s Current Report on Form 8-K filed May 30, 2007). |
|||
| †10.4 | 2005 Employee Stock Purchase Plan (Incorporated by reference to Exhibit 10.4 of the Form S-4). |
|||
| †10.5 | Employment Agreement, dated as of October 28, 2004, between EpiCept Corporation and John V.
Talley (incorporated by reference to Exhibit 10.5 to the EpiCept Form S-1). |
|||
| †10.6 | Employment Agreement, dated as of October 28, 2004, between EpiCept Corporation and Robert
Cook (incorporated by reference to Exhibit 10.6 to the EpiCept Form S-1). |
|||
| 10.7 | License Agreement, dated as of December 18, 2003, between Endo Pharmaceuticals Inc. and
EpiCept Corporation (incorporated by reference to Exhibit 10.9 to the EpiCept Form S-1). |
|||
| 10.8 | Royalty Agreement, dated as of July 16, 2003, between EpiCept Corporation and R. Douglas
Cassel, M.D. (incorporated by reference to Exhibit 10.10 to the EpiCept Form S-1). |
|||
| 10.9 | Cooperation Agreement between APL American Pharmed Labs, Inc. and
Technologie-Beteiligungs-Gesellschaft mbH, dated August 1997 (incorporated by reference to
Exhibit 10.13 to the EpiCept Form S-1). |
|||
34
Table of Contents
| Exhibit | ||||
| Number | Description of Exhibits | |||
| 10.10 | Investment Agreement between Pharmed Labs GmbH and Technologie-Beteiligungs-Gesellschaft mbH,
dated August 1997 (incorporated by reference to Exhibit 10.14 to the EpiCept Form S-1). |
|||
| 10.11 | Investment Agreement among Pharmed Labs GmbH, American Pharmed Labs, Inc. and
Technologie-Beteiligungs-Gesellschaft mbH, dated February 17, 1998 (incorporated by reference
to Exhibit 10.15 to the EpiCept Form S-1). |
|||
| 10.12 | Lease Agreement between BMR-Landmark at Eastview LLC, as Landlord, and EpiCept Corporation,
as Tenant, dated August 28, 2006 (incorporated by reference to Exhibit 10.12 to EpiCept
Corporation’s Annual Report on Form 10-K filed March 18, 2008). |
|||
| 10.13 | First Exchange Option Agreement, dated as of December 31, 1997, by and between American
Pharmed Labs, Inc. and tbg Technologie-Beteiligungs-Gesellschaft mbg der Deutschen
Ausgleichsbank (incorporated by reference to Exhibit 10.22 to the EpiCept Form S-1). |
|||
| 10.14 | Second Exchange Option Agreement, dated as of February 17, 1998, by and between American
Pharmed Labs, Inc. and tbg Technologie-Beteiligungs-Gesellschaft mbh der Deutschen
Ausgleichsbank (incorporated by reference to Exhibit 10.23 to the EpiCept Form S-1). |
|||
| 10.15 | Amendment to Second Exchange Option Agreement, dated as of August 26, 2005, by and between
EpiCept Corporation and tbg Technologie-Beteiligungs-Gesellschaft mbh der Deutschen
Ausgleichsbank (incorporated by reference to Exhibit 10.28 to the EpiCept Form S-4). |
|||
| †10.16 | Amended and Restated Note Purchase Agreement (the “Note Purchase Agreement”), dated as of
March 3, 2005, by and among EpiCept Corporation and the Purchasers indentified therein
(incorporated by reference to Exhibit 10.18 to the EpiCept Form S-1). |
|||
| 10.17 | Amendment No. 1 to License Agreement between EpiCept Corporation and DURECT Corporation,
dated as of September 12, 2008 (incorporated by reference to Exhibit 10.1 to EpiCept
Corporation’s Current Report on Form 8-K filed September 17, 2008). |
|||
| 10.18 | Loan and Security Agreement with Hercules Technology Growth Capital, Inc., dated August 30,
2008 (incorporated by reference to Exhibit 10.1 to EpiCept Corporation’s Current Report on
Form 10-Q filed November 9, 2006). |
|||
| 10.19 | First Amendment to the Loan and Security Agreement with Hercules Technology Growth Capital,
Inc., dated May 7, 2008 (incorporated by reference to Exhibit 10.1 to EpiCept Corporation’s
Current Report on Form 8-K filed May 9, 2008). |
|||
| 10.20 | Second Amendment to Loan and Security Agreement, by and among EpiCept Corporation, Maxim
Pharmaceuticals Inc. and Hercules Technology Growth Capital, Inc., dated as of June 23, 2008
(incorporated by reference to Exhibit 10.4 to EpiCept Corporation’s Current Report on Form
8-K filed June 23, 2008). |
|||
| 10.21 | Warrant Agreement with Hercules Technology Growth Capital, Inc., dated August 30, 2008
(incorporated by reference to Exhibit 10.2 to EpiCept Corporation’s Current Report on Form
10-Q filed November 9, 2006). |
|||
| 10.22 | Second Amendment to the Warrant Agreement with Hercules Technology Growth Capital, Inc.,
dated May 7, 2008 (incorporated by reference to Exhibit 10.2 to EpiCept Corporation’s Current
Report on Form 8-K filed May 9, 2008). |
|||
| 10.23 | First Amendment to the Deposit Account Control Agreement with Hercules Technology Growth
Capital, Inc., dated May 7, 2008 (incorporated by reference to Exhibit 10.3 to EpiCept
Corporation’s Current Report on Form 8-K filed May 9, 2008). |
|||
| 10.24 | Common Stock Warrant, by and between EpiCept Corporation and Hercules Technology Growth
Capital, Inc., dated as of June 23, 2008 (incorporated by reference to Exhibit 10.6 to
EpiCept Corporation’s Current Report on Form 8-K filed June 23, 2008). |
|||
| 10.25 | Amendment to the Repayment Agreement by and between EpiCept Corporation and
Technologie-Beteiligungs Gesellschaft mbH der Deutschen Ausgleichsbank, dated May 23, 2008
(incorporated by reference to Exhibit 10.1 to EpiCept Corporation’s Current Report on Form
8-K filed May 28, 2008). |
|||
35
Table of Contents
| Exhibit | ||||
| Number | Description of Exhibits | |||
| 10.26 | Securities Purchase Agreement, dated June 28, 2007 (incorporated by reference to Exhibit 10.1
to EpiCept’s Current Report on Form 8-K filed June 29, 2007). |
|||
| 10.27 | Form of Warrant, dated as of June 28, 2007 (incorporated by reference to Exhibit 10.3 to
EpiCept Corporation’s Current Report on Form 8-K filed June 29, 2007). |
|||
| 10.28 | Securities Purchase Agreement, dated October 10, 2007 (incorporated by reference to Exhibit
10.1 to EpiCept Corporation’s Current Report on Form 8-K filed October 11, 2007). |
|||
| 10.29 | Form of Warrant, dated as of October 10, 2007 (incorporated by reference to Exhibit 10.2 to
EpiCept Corporation’s Current Report on Form 8-K filed October 11, 2007). |
|||
| 10.30 | Securities Purchase Agreement, dated December 4, 2007 (incorporated by reference to Exhibit
10.1 to EpiCept Corporation’s Current Report on Form 8-K filed December 5, 2007). |
|||
| 10.31 | Form of Common Stock Purchase Warrant, dated as of December 4, 2007 (incorporated by
reference to Exhibit 10.2 to EpiCept Corporation’s Current Report on Form 8-K filed December
5, 2007). |
|||
| 10.32 | Securities Purchase Agreement, dated March 6, 2008 (incorporated by reference to Exhibit 10.1
to EpiCept Corporation’s Current Report on Form 8-K dated March 6, 2008). |
|||
| 10.33 | Securities Purchase Agreement, dated as of June 23, 2008 (incorporated by reference to
Exhibit 10.1 to EpiCept Corporation’s Current Report on Form 8-K filed June 25, 2008). |
|||
| 10.34 | Form of Warrant, dated as of June 23, 2008 (incorporated by reference to Exhibit 10.6 to
EpiCept Corporation’s Current Report on Form 8-K filed June 23, 2008). |
|||
| 10.35 | Securities Purchase Agreement, dated July 15, 2008 (incorporated by reference to Exhibit 10.1
to EpiCept Corporation’s Current Report on Form 8-K filed July 16, 2008). |
|||
| 10.36 | Form of Warrant, dated as of July 15, 2008 (incorporated by reference to Exhibit 10.2 to
EpiCept Corporation’s Current Report on Form 8-K filed July 16, 2008). |
|||
| 10.37 | Securities Purchase Agreement, dated August 1, 2008 (incorporated by reference to Exhibit
10.1 to EpiCept Corporation’s Current Report on Form 8-K filed August 4, 2008). |
|||
| 10.38 | Form of Warrant, dated as of August 1, 2008 (incorporated by reference to Exhibit 10.2 to
EpiCept Corporation’s Current Report on Form 8-K filed August 4, 2008). |
|||
| 10.39 | Securities Purchase Agreement, dated August 11, 2008 (incorporated by reference to Exhibit
10.1 to EpiCept Corporation’s Current Report on Form 8-K filed August 12, 2008). |
|||
| 10.40 | Form of Warrant, dated as of August 11, 2008 (incorporated by reference to Exhibit 10.2 to
EpiCept Corporation’s Current Report on Form 8-K filed August 12, 2008). |
|||
| 10.41 | Securities Purchase Agreement, dated as of December 8, 2008 (incorporated by reference to
Exhibit 10.2 to EpiCept Corporation’s Current Report on Form 8-K filed December 9, 2008). |
|||
| 10.42 | 2009 Employee Stock Purchase Plan (incorporated by reference to Exhibit 10.1 to EpiCept
Corporation’s Registration Statement on Form S-8 filed December 23, 2008). |
|||
| 10.43 | Second Amendment to the Repayment Agreement by and between EpiCept Corporation and
Technologie-Beteiligungs Gesellschaft mbH der Deutschen Ausgleichsbank, dated November 26,
2008 (incorporated by reference to Exhibit 10.1 to EpiCept Corporation’s Current Report on
form 8-K filed December 3, 2008). |
|||
36
Table of Contents
| Exhibit | ||||
| Number | Description of Exhibits | |||
| 10.44 | Placement Agent Agreement (incorporated by reference to Exhibit 10.1 to EpiCept Corporation’s
Current Report on form 8-K filed December 9, 2008) |
|||
| 10.45 | Securities Purchase Agreement, dated as of February 4, 2009 (incorporated by reference to
Exhibit 10.1 to EpiCept Corporation’s Current Report on Form 8-K filed February 10, 2009). |
|||
| 10.46 | Form of Warrant (incorporated by reference to Exhibit 10.2 to EpiCept Corporation’s Current
Report on Form 8-K filed February 10, 2009). |
|||
| 10.47 | Placement Agent Agreement, dated February 4, 2009 (incorporated by reference to Exhibit 10.3
to EpiCept Corporation’s Current Report on Form 8-K filed February 10, 2009). |
|||
| 10.48 | Securities Purchase Agreement, dated as of June 18, 2009 (incorporated by reference to
Exhibit 10.1 to EpiCept Corporation’s Current Report on Form 8-K filed June 19, 2009). |
|||
| 10.49 | Form of Warrant (incorporated by reference to Exhibit 10.2 to EpiCept Corporation’s Current
Report on Form 8-K filed June 19, 2009). |
|||
| 10.50 | Placement Agent Agreement, dated June 18, 2009 (incorporated by reference to Exhibit 10.3 to
EpiCept Corporation’s Current Report on Form 8-K filed June 19, 2009). |
|||
| 10.51 | Amendment to the Repayment Agreement, dated June 25, 2009 (English translation from the
original German) (incorporated by reference to Exhibit 10.1 to EpiCept Corporation’s Current
Report on Form 8-K filed June 30, 2009). |
|||
| 10.52 | License and Collaboration Agreement, dated as of November 19, 2003, between Maxim
Pharmaceuticals, Inc. and Cytovia, Inc. and Myriad Genetics, Inc. (incorporated by reference
to Exhibit 10.7 to the Maxim Pharmaceuticals, Inc. Current Report on Form 10-Q filed February
10, 2004). |
|||
| 11.1 | Statement Regarding Computation of Per Share Earnings (incorporated by reference to the Notes
to Consolidated Financial Statements included in Part II of this Report). |
|||
| 21.1 | * | List of Subsidiaries of EpiCept Corporation. |
||
| 23.1 | * | Consent of Deloitte & Touche LLP, Independent Registered Public Accounting Firm. |
||
| 31.1 | Certification of Chief Executive Officer, pursuant to Exchange Act Rules 13a-14(a) and 15(d)-14(a), adopted pursuant to Section 302 of the
Sarbanes-Oxley Act of 2002. |
|||
| 31.2 | Certification of Chief Financial Officer, pursuant to Exchange Act Rules 13a-14(a) and 15(d)-14(a), adopted pursuant to Section 302 of the
Sarbanes-Oxley Act of 2002. |
|||
| 32.1 | Certification of Chief Executive Officer, pursuant to 18 U.S.C. 1350, adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. |
|||
| 32.2 | Certification of Chief Financial Officer, pursuant to pursuant to 18 U.S.C. 1350, adopted Section 906 of the Sarbanes-Oxley Act of 2002. |
|||
| * | Previously filed. | |
| † | Management contract or compensatory plan or arrangement |
| (c) | Financial Statements Schedules. |
None.
37
