Attached files
| file | filename |
|---|---|
| EX-99.1 - EXHIBIT 99.1 - ESPORTS ENTERTAINMENT GROUP, INC. | ex991.htm |
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
____________________________
FORM 8-K/A
Amendment No. 2
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the
Securities Exchange Act of 1934
Date of report (Date of earliest event reported): May 10, 2010
DK SINOPHARMA, INC.
(Exact name of registrant as specified in Charter)
|
Nevada
|
333-156302
|
26-3062752
|
|
(State or other jurisdiction of
incorporation or organization)
|
(Commission File No.)
|
(IRS Employee
Identification No.)
|
|
Dongxing Building, 4th Floor
No.1 Xinke Road,
Xi’an, the People’s Republic of China 710043
|
|
(Address of Principal Executive Offices)
|
86-29-8224-7500
(Issuer’s Telephone Number, Including Area Code
112 North Curry Street, Carson City, NV, 89703
(Former name or former address, if changed since last report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General Instruction A.2. below):
o Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425)
o Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12)
o Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b))
o Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c))
EXPLANATORY NOTE
This Current Report on Form 8-K/A is being filed as Amendment No. 2 to our Current Report on Form 8-K/A which was originally filed on May 10, 2010 with the Securities and Exchange Commission. We are amending and restating our financial statements and notes for the fiscal years ended December 31, 2009 and 2008 as well as our financial statements, notes, and related disclosure for the period ended March 31, 2010, attached as Exhibit 99.1 in Amendment No. 1 to our Current Report on Form 8-K/A filed on May 21, 2010, to provide additional disclosure as relates to our corporate background, organizational structure, andPRC tax treatment, and to clarify certain accounting policies adopted and implemented by the Company.
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
This Form 8-K and other reports filed by us from time to time with the Securities and Exchange Commission (collectively the “Filings”) contain or may contain forward-looking statements and information that are based upon beliefs of, and information currently available to, our management as well as estimates and assumptions made by our management. When used in the filings the words “anticipate”, “believe”, “estimate”, “expect”, “future”, “intend”, “plan” or the negative of these terms and similar expressions as they relate to us or our management identify forward looking statements. Such statements reflect the current view of our management with respect to future events and are subject to risks, uncertainties, assumptions and other factors (including the risks contained in the section of this report entitled “Risk Factors”) as they relate to our industry, our operations and results of operations, and any businesses that we may acquire. Should one or more of the events described in these risk factors materialize, or should our underlying assumptions prove incorrect, actual results may differ significantly from those anticipated, believed, estimated, expected, intended or planned.
Although we believe that the expectations reflected in the forward looking statements are reasonable, we cannot guarantee future results, levels of activity, performance or achievements. Except as required by applicable law, including the U.S. federal securities laws, we do not intend to update any of the forward-looking statements to conform them to actual results. The following discussion should be read in conjunction with our pro forma financial statements and the related notes that will be filed herein.
In this Form 8-K, references to “we,” “our,” “us,” “our company,” “the Company,” “Virtual Closet,” or the “Registrant” refer to Virtual Closet, Inc., a Nevada corporation.
Item 1.01 Entry Into A Material Definitive Agreement.
As more fully described in Item 2.01 below, on May 10, 2010, we consummated a reverse acquisition pursuant to a Share Exchange Agreement (the “Share Exchange Agreement”) entered into by and among Virtual Closet, You Ting Zhu, Ge Lin, and Jia Tan Fang (the “Company Shareholders”), Dong Ke Pharmaceutical, Inc., a Delaware corporation (“Dong Ke”), and the Shareholders of Dong Ke Pharmaceutical, Inc. (the “Dong Ke Shareholders”).
The close of the reverse acquisition (the "Closing") took place on May 10, 2010 (the “Closing Date”). On the Closing Date, pursuant to the terms of the Share Exchange Agreement, we acquired all of the outstanding capital stock and ownership interests of Dong Ke (the “Interests”) from the Dong Ke Shareholders. In exchange for the Interests, we issued to the Dong Ke Shareholders an aggregate of 1,941,818 shares of our common stock. The material terms of the Exchange Agreement are more fully described in Item 2.01 and in Exhibit 2.1 of this report.
As described further below, since January 27, 2010, Dong Ke, through its wholly-owned subsidiary, Yangling Slovan Pharmaceuticals Co., Ltd. (“Slovan”), has been the indirect holding company for Yangling Dongke Maidisen Pharmaceuticals Co., Ltd. ("Maidisen"), a medical and pharmaceutical developer, manufacturer and marketer in the PRC.
Dong Ke was incorporated in Delaware on December 15, 2009. On January 25, 2010, Dongke incorporated Slovan as a “wholly owned foreign enterprise” in the PRC.
Maidisen, our operating entity, was incorporated in the PRC on June 23, 2004 and is in the business of manufacturing, marketing and sales of pharmaceuticals in China.
As described below, on January 27, 2010, an Entrustment Management Agreement was entered into between Slovan and Maidisen to which Dong Ke exercises control over the operations and business of Maidisen through Slovan and receives all net profits and assumes all operational losses of Maidisen through Slovan.
Additionally, on May 10, 2010 pursuant to the Exchange Agreement, You Ting Zhu, our former sole director and officer, Ge Lin and Jia Tan Fang agreed to cancel an aggregate of 10,015,000 shares of our common stock. As of the Closing Date we had 2,181,818 shares issued and outstanding.
1
Item 2.01 Completion of Acquisition or Disposition of Assets.
CLOSING OF EXCHANGE AGREEMENT
As described in Item 1.01 above, on May 10, 2010, pursuant to the Share Exchange Agreement, we acquired all of the outstanding capital stock and ownership interests of Dong Ke from the Dong Ke Shareholders, and in exchange, we issued to the Dong Ke Shareholders 1,941,818 shares, or approximately 89%, of our common stock. As a result of the consummation of the Exchange Agreement, on the Closing Date, Dong Ke became our wholly-owned subsidiary, and Maidisen, the PRC company controlled by Dong Ke via its wholly-owned subsidiary Slovan, became our operating entity.
Pursuant to the Share Exchange Agreement, the Company is also obligated to use its best commercially reasonable efforts to effect a 1 for 13.75 forward share split within 90 days after Closing.
CORPORATE HISTORY AND STRUCTURE
Background of Virtual Closet, Inc. and Corporate Structure
Virtual Closet, Inc. was incorporated on July 22, 2008 under the laws of the State of Nevada. The Company’s U.S. registered office is located at 112 North Curry Street, Carson City, NV 89703-4934.
The Company’s initial business plan was to seek business opportunities in the light manufacturing and household appliances industries specializing in the design, manufacturing and sales of automated household storage systems.
As described above in Item 2.01, on May 10, 2010, the Company entered into an Exchange Agreement with Dong Ke, a Delaware corporation, and as a result the Company adopted the business of Maidisen.
Background of Yangling Dongke Maidisen Pharmaceuticals Co., Ltd.
Maidisen was incorporated in the PRC in on June 23, 2004 and is our operating company and is in the business of manufacturing, marketing and sales of pharmaceuticals in China.
Dong Ke was incorporated in Delaware on December 15, 2009. Slovan is a “wholly foreign-owned enterprise” incorporated in the PRC on January 26, 2010. Slovan is a wholly-owned subsidiary of Dong Ke. Dong Ke, through its wholly owned subsidiary Slovan, and a number of contractual arrangements described below, indirectly controls Maidisen, a medical and pharmaceutical developer, manufacturer and marketer in the PRC.
On January 27, 2010, Slovan, Maidisen and the shareholders of Maidisen entered into a Management Entrustment Agreement, pursuant to which Dong Ke exercises control over the operations and business of Maidisen through Slovan.
Slovan entered into a Management Entrustment Agreement with Maidisen and the shareholders of Maidisen (the “Management Entrustment Agreement”), in which Maidisen and its shareholders agreed to transfer control, or entrust, the operations and management of its business to Slovan. Under the agreement, Slovan manages the operations and assets of Maidisen, controls all of the cash flows of Maidisen through a bank account controlled by Slovan, is entitled to 100% of earnings before tax of Maidisen as a management fee, and is obligated to pay all payables and loan payments of Maidisen. In addition, under the terms of the Management Entrustment Agreement, Slovan has been granted certain rights which include, in part, the right to appoint and terminate members of Maidisen Board of Directors, hire management and administrative personnel and control decisions relating to entering and performing customer contracts and other instruments. We anticipate that Maidisen will continue to be the contracting party under its customer contracts, bank loans and certain other instruments unless Slovan exercises its option. The agreement does not terminate unless the business of Maidisen is terminated or Slovan exercises its option to acquire all of the assets or equity of Maidisen under the terms of the Exclusive Option Agreement as more fully described below.
In exchange for causing Maidisen to enter into the Management Entrustment Agreement, Slovan through its parent company Dong Ke issued an aggregate of 26,700,000 shares of Dong Ke common stock to the shareholders of Maidisen which was allocated based on their respective pro rata ownership of Maidisen.
The Management Entrustment Agreement was utilized instead of a direct acquisition of assets, common stock or a share exchange because we could not pay cash directly or indirectly to acquire Maidisen or its assets. PRC law only permits the purchase of equity interests or assets of a PRC entity by a non PRC entity if the purchase price is paid in cash and the purchase price is based upon the appraised value of the assets or equity. Although we did not conduct a formal appraisal of the value of the assets or equity of Maidisen, based on the book value of the assets at December 31, 2009 $28,532,078 and equity $16,849,032 as shown on the balance sheet, we concluded that we did not have sufficient cash to pay the appraised value of the assets or equity. Therefore, we, through Slovan, entered into the Management Entrustment Agreement in exchange for the right to exercise functional control over Maidisen and allow us to consolidate its financial results for the purposes of GAAP. Slovan also entered into a Voting Proxy Agreement, Option Agreement and Share Pledge Agreement. Biao Dian & Partners, our PRC counsel, has advised us that in their opinion the Management Entrustment Agreement is legal and enforceable under PRC law.
2
In order to give Slovan further control over Maidisen, the Maidisen shareholders and Slovan, a wholly owned subsidiary of Dong Ke in the PRC, entered into a shareholders’ Voting Proxy Agreement (the “Voting Proxy Agreement”) whereby the Maidisen shareholders irrevocably and exclusively appointed the members of the Slovan board of directors, as their proxies to vote on all matters that require Maidisen shareholder approval, including, without limitation, the right to appoint members of the board of directors of Maidisen. The agreement further provides that Slovan will appoint all of the board of directors of Maidisen. As its board of directors and must remove and appoint new members to its board to reflect any changes to the board of Slovan. The agreement terminates upon the exercise of the option by Slovan to purchase the shares of Maidisen, as described below.
In order to enable Maidisen to become an indirectly wholly owned subsidiary of Slovan when permitted under PRC law, Slovan, Maidisen and the Maidisen shareholders entered into an Exclusive Option Agreement (the “Exclusive Option Agreement”) whereby the Maidisen shareholders granted Slovan an irrevocable and exclusive purchase option (the “Option”) to acquire Maidisen equity and/or remaining assets, but only to the extent that the acquisition does not violate limitations imposed by PRC law on such transactions. Current PRC law does not specifically provide for the equity of a non-PRC entity to be used as consideration for the purchase of a PRC entity’s assets or equity unless the value of the shares are equal to or greater than the value of the enterprise acquired. In addition, there is a lengthy appraisal process which must be approved by the provincial PRC government entities. The consideration for the exercise of the Option is to be determined by the parties and memorialized in future definitive agreements setting forth the kind and value of such consideration. Accordingly, we will consider exercising the Option under such circumstances we believe will be in our best interests and our shareholders and the Exclusive Option Agreement has been drafted to give us such flexibility. In considering whether or not we will exercise the Option we may consider such factors as (1) if the exercise price can be lower than the appraised value under current PRC law (2) availability of funds, (3) any relevant tax considerations at the time, (4) any other relevant PRC laws that may exist at the time, (5) the value of our shares that were previously paid to shareholders of Maidisen, and (6) whether or not the exercise of the Option will provide any other additional benefits to us or our shareholders. Upon exercise of the Option, the parties will prepare transfer documents to be submitted for governmental approval and work together to obtain all approvals and permits. The agreement may be terminated by agreement of all parties or by with 30 days notice.
In order to further solidify Slovan’s rights, benefits and control of Maidisen, Slovan and the Maidisen shareholders entered into a hare Pledge Agreement (the “Share Pledge Agreement”) whereby the Maidisen shareholders pledged all of their equity interests in Maidisen, including the proceeds thereof, to guarantee the performance by the shareholders of all of the agreements they entered into with Slovan. Upon breach by any of the shareholders of any of the Management Entrustment Agreement, Voting Proxy Agreement, the Exclusive Option Agreement or the Share Pledge Agreement, Slovan is entitled by operation of law to become the beneficial owner of the shares of Maidisen. Prior to termination of the Share Pledge Agreement, the pledged equity interests of Maidisen cannot be transferred without Slovan’s prior written consent. The provisions of the Share Pledge Agreement and the Voting Proxy Agreement work together to give the board of directors of Slovan control over transfers by the shareholders of Maidisen. The agreement will not terminate until agreed to by all of the parties in writing.
The consolidated financial statements include the accounts of the Company, Slovan and Maidisen, its variable interest entity (“VIE”), for which the Company is the primary beneficiary. All inter-company accounts and transactions have been eliminated in consolidation. The Company has adopted FIN 46R which requires a VIE to be consolidated by a company if that company is subject to a majority of the risk of loss for the VIE or is entitled to receive a majority of the VIE’s residual returns.
3
The Company considered the following indicators in determining the classification of Maidisen as the VIE of Slovan, among others:
|
●
|
Slovan has the full right to control and administrate the financial affairs and daily operation of Maidisen and has the right to manage and control all assets of Maidisen. The equity holders of Maidisen as a group has no right to make any decision about Maidisen activities without the consent of Slovan.
|
|
●
|
Slovan was assigned all voting rights of Maidisen and has the right to appoint all directors and senior management personnel of Maidisen. The equity holders of Maidisen possess no substantive voting rights.
|
|
●
|
Slovan will provide financial support if Maidisen requires additional funds to maintain its operations and to repay its debts.
|
|
●
|
Slovan should be paid a management fee equal to 100% of earnings before tax of Maidisen and should assume all operation risks of Maidisen and bear all losses of Maidisen. Therefore, Slovan is the primary beneficiary of Maidisen.
|
Maidisen is wholly owned by the majority shareholders of the Company. The capital provided to Maidisen by the Company was recorded as interest-free loan to Maidisen. There was no written note to this loan and the loan is not interest bearing and was eliminated during consolidation. Under various contractual agreements, the shareholders of Maidisen are required to transfer their ownership to the Company’s subsidiary in China when permitted by PRC laws and regulations or to designees of the Company at any time when the Company considers it is necessary to acquire Maidisen. In addition, the shareholders of Maidisen have pledged their shares in Maidisen as collateral to secure these contractual arrangements.
In summary, although we have no equity ownership interest in Maidisen, through Slovan, we are entitled to manage the operations of Maidisen, manage and dispose of its assets, nominate officers and directors, make decisions as to the use of funds and manage cash flows and receive a management fee equal to 100% of earnings before tax of Maidisen and we are obligated to pay all of its debts. As a result, we are allowed to consolidate the financial statements of Maidisen under GAAP. When we sell our equity or borrow funds we expect the proceeds will be forwarded to Maidisen and accounted for as a loan to Maidisen and eliminated during consolidation. We may also use the proceeds to repurchase our capital stock or for our corporate overhead expenses. If we borrow funds, we expect to be the primary obligor on any debt.
Thus, by causing our subsidiary Slovan to enter into the Management Entrustment Agreement, Voting Proxy Agreement and Share Pledge Agreement, we are permitted to consolidate the financial results of Maidisen as our VIE.
Reorganization and Revised Structure
The following diagram sets forth the corporate structure of Dong Ke before the reverse acquisition:
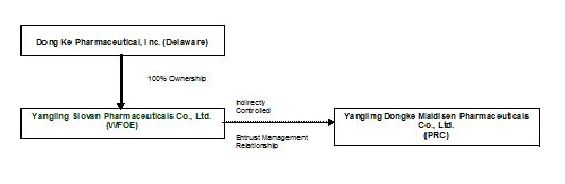
As a result of the consummation of the Exchange Agreement, as of May 10, 2010:
|
●
|
Dong Ke became our wholly-owned subsidiary;
|
|
●
|
In exchange for all of their shares of Dong Ke Pharmaceuticals stock, the Dong Ke Shareholders received 1,941,818 newly issued shares of our common stock.
|
|
●
|
Immediately following the closing of the reverse acquisition, the 1,941,818 shares of the Company’s common stock issued to the Dong Ke Shareholders represent approximately 89% of our issued and outstanding shares on a fully diluted basis.
|
4
The organization and ownership structure of the Company subsequent to the consummation of the reverse acquisition as summarized in the paragraphs above is as follows:
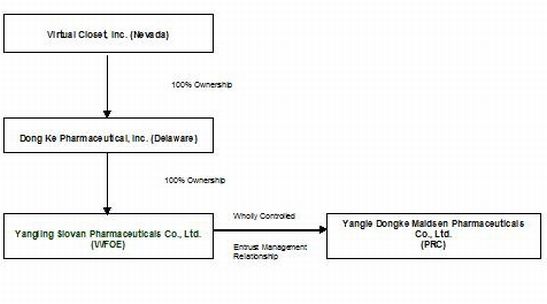
This transaction closed on May 10, 2010.
BUSINESS DESCRIPTION
Overview
As a result of the reverse acquisition, we are a recently organized Nevada corporation that, through our VIE, Maidisen is engaged in the research, development, manufacture, packaging, marketing and distribution of pharmaceutical and medical products in China for human use for a variety of diseases and conditions.
Maidisen, our VIE and operating entity, was incorporated in the PRC on June 23, 2004. All of our operations are conducted in the People’s Republic of China where our one manufacturing facility is located in the City of Yangling in Shaanxi Province. Our current operations involve the manufacture and sale of 36 pharmaceutical products in the form of, capsules, tablets, granules, semisolid ointment, power, ointment, paste ointment. We currently focus on varying diseases relating to respiratory system, digestive system, cardio-cerebral vascular system, antineoplastic, bone diseases-modifying antrheumatic, gynecological, refill nutrition and others. Our products are sold to distributors who distribute our products to the public as well as through representatives that we employ.
Our business focuses on the development and sale of pharmaceutical products and over the counter products based on traditional Chinese medicines (“TCMs”) designed to address varying diseases and conditions. During the past few years 230 representative offices have been found nationwide and the business extended to 26 provinces. Since most of our products are derived from TCMs, our primary raw materials used to manufacture our products are various types of medicinal herbs. We also enter into trading agreements for the supply of many of the materials used to manufacture and package our products including aluminum foil, which we use as the inner packing materials in tablets production. Maidisen has written agreements with all of its suppliers.
Our focus has been on the development and sale of pharmaceutical products based on traditional Chinese medicines (“TCMs”) designed to address numerous diseases and indications, with an emphasis on sales to community hospitals and rural medical institutions.
5
PRINCIPAL PRODUCTS
In the fiscal year ended December 31, 2009, our revenues were principally derived from sales of products listed below. We have SFDA approval for all of the TCMs that we market. The PRC government agency, SFDA, is analogous to the Food and Drug Administration (“FDA”) in the United States. Unlike the FDA, however, the SFDA provides intellectual property and competitive protection to certain classes of approved drugs.
Detailed information of our products are provided in the table below.
Pharmaceutical Products
We market our pharmaceutical products under numerous brand names. The following is a list of some of our approved pharmaceutical products and their intended uses.
|
Product
|
||||
|
No.
|
Product Name
|
Indications
|
||
|
1
|
Huanglong Cough Granules
|
Used for cure acute bronchitis and chronic bronchitis, and bronchial asthma.
|
||
|
2
|
Huanglong Cough & Dyspna Capsules
|
Used for patients with lung-kidney Qi vacuity, cough and panting due to phlegm-heat lying depressed in the lung, acute and chronic bronchitis, bronchial asthma.
|
||
|
3
|
Jinqian Cold Capsule
|
Nasal congestion and runny nose; sore swollen throat; headache and pyrexia.
|
||
|
4
|
Ganhai Stomach Recovery Capsule
|
Used for cure gastric ulcer and duodenal ulcer, chronic gastritis and reflux esophagitis.
|
||
|
5
|
Gynecologic Inflammation Relieving Capsule
|
Used for excessive vaginal discharge, irregular menstruation, and abdominal pain caused by pelvic inflammatory disease, appendicitis, etc.
|
||
|
6
|
Heart Disease Relieving Capsule
|
Used for curing coronary heart disease, angina pectoris, heart disorders, premature beat, and myocarditis.
|
||
|
7
|
Ginseng and Chinese Angelica Blood Nourishing Tablet
|
Used for curing dizziness, palpitation and insomnia, fatigued spirit and lack of strength, shortness of breath, asitia and indigestion.
|
||
|
8
|
Tokay Tablet
|
Used for curing cancers like esophagus cancer, stomach cancer, lung cancer, etc.
|
||
|
9
|
Qinchuan Rheumatism Relieving Tablet
|
Used for curing pain in limbs caused by arthralgia due to wind-cold-dampness, numbness and spasm caused by rheumatoid arthritis and, rheumatoid arthritis, etc.
|
||
|
10
|
Hepatitis B Relieving and Curing Capsule
|
Used for curing CHB and AHB caused by damp-heat stasis obstruction with the symptom of asthenia, hepatalgia, asitia and indigestion, distention and oppression in the stomach duct.
|
||
|
11
|
Compound Cirald Daphne Bark Paining-Relieving Plaster
|
Used for curing lumbar and back pain as well as topoalgia numbness and swelling caused by rheumatism.
|
||
|
12
|
Hepatopathy Relieving Capsule
|
Used for cuing accumulations and gatherings of damp-heat, yellowing of both body and eyes, numbness and ache of both rib- sides, fatigued limbs and laziness to eat, scanty dark urine and loose stools, slimy yellow tongue fur etc as well as acute icteric and non-icteric hepatitis and chronic hepatitis in Western medicine.
|
||
|
13
|
Delicate Plaster
|
Relaxing muscles and tendons, promoting blood circulation, dissipating cold, assuaging pain; used for curing pains in bones and muscles, acute bruising contusion, sprain, rheumatalgia, arthrodynia, hypochondriac pain, muscular soreness, etc.
|
||
|
14
|
Channels-Soothing Capsule
|
Used for ischemic cerebrovascular disease, arteriosclerosis, cerebral thrombosis,cerebral ischemia, coronary heart disease, and angina pectoris.
|
||
|
15
|
Stomach Recovery Capsule
|
Used for fullness and oppression in the chest and abdomen, inappetence, gastric distention and fullness, gastric acid, and gastralgia.
|
|
16
|
Skin Moistening Plaster
|
Used for skin sore and tinea, acne and pimple, rosacea, freckle, sweat macule, leuoderma, noxious dampness, beriberi.
|
||
|
17
|
Musk Bone-Building Plaster
|
Used for rheumatalgia, arthodynia, lumbago, neuralgia, muscular soreness, sprain, bruising.
|
||
|
18
|
Compound Salvia Root Tablet
|
Used for quickening the blood and transforming stasis, regulating the flow of Qi to alleviate pain; used for patients with the symptom of obstruction of Qi in the chest caused by stagnancy of Qi and blood stasis with the symptom of chocking sensation in the chest and precordium prick as well as coronary heart disease and angina pectoris.
|
||
|
19
|
Coughing and Panting Ointment
|
Used for relieving cough and asthma, removing dampness and dispelling phlegm; used for simple chronic tracheitis, breathing chronic tracheitis, asthma (except those caused by heart disease) and other systems.
|
||
|
20
|
Tendon-soothing and Blood Circulation Promoting Powder
|
Used for quickening the blood and transforming stasis, freeing the meridians and collaterals and unfolding the tendons, decreasing swelling and relieving pains; used for pains in articular muscle caused by traumatic injuries, acute parenchyma damages and other chronic tissue damages, lumbar muscle strain, joints bruising, periarthritis of the shoulders, cervical spondylosis and slipped disc.
|
||
|
21
|
Sprain Relieving Plaster
|
Used for rheumatoid arthritis, sore muscle and painful swollen joints.
|
||
|
22
|
Medicinal Moxa Roll
|
Used for promoting the circulation of Qi and blood, dispelling cold dampness; used for arthraglia due to wind-cold-dampness, muscle pain and numbness, pains at joints and limbs, cold pain in the stomach duct and abdomen.
|
||
|
23
|
Stomach-Recovering Capsule
|
Used for removing stagnation and reliving pains; used for stagnation of blood and retardation, gastral pains, postpartum tormina and dysmenorrheal.
|
||
|
24
|
Cold-Resisting and Detoxifying Granules
|
Used for clearing heat and resolving toxin, used for wind-heat common cold.
|
||
|
25
|
VC Honeysuckle-Fructus Forsythiae
|
Used for pungent in flavor and cool in property, relieving exterior syndrome, clearing heat and resolving toxin; used for pyrexia, headache, cough, thirst and throat irritation caused by flu.
|
||
|
26
|
Cow-Bezoar Detoxicating Tablet
|
Used for clearing heat and resolving toxin; used for exuberant internal fire heat, sore swollen throat, painful swollen gums, mouth and tongue sores, sore red swollen eyes.
|
||
|
27
|
Ligusticum Wallichii Tea-mixing Powder
|
Used for dispelling wind and relieving pains; used for headache caused by external contraction of cold or aversion to cold, pyrxia and snuffle.
|
||
|
28
|
Berberine TMP Capsule
|
Used for gastroenteritis, bacillary dysentery caused by sensitive bacteria and other enteric infection.
|
||
|
29
|
Bowels Relaxing Capsule
|
Used for purging away the heat and resolving stagnation, lessening the bowel and relieving constipation, long-term constipation, temporary abdominal distention and constipation, and habitual constipation of the old.
|
6
|
30
|
Blood Circulation Promoting Powder
|
Used for relaxing muscles and tendons and promoting blood circulation, dissipating blood stasis and relieving pains; used for traumatic injuries, ache caused by stagnation of blood, lumber sprain and pain in chest.
|
||
|
31
|
Anti-Bruise Powder
|
Used for eliminating stasis and decreasing swelling, relieving pains and stopping blood; used for traumatic, injuries, blood stasis ache and bleeding due to external injury.
|
||
|
32
|
Poria Five Powder
|
Used for warming yang and promoting Qi, removing dampness and moving water; used for poor Qi promoting in bladders, difficulty in micturition due to water-damp stagnation, dropsy and abdominal distention, vomiting and diarrhea, thirst and heating drinking.
|
||
|
33
|
Rhizoma Corydalis Pain-Alleviating Tablet
|
Used for regulating the flow of Qi,promoting blood circulation, alleviating pain; used for gastropathy due to stagnancy of qi and blood stasis, hypochondriac pains, headache and dysmenorrheal.
|
||
|
34
|
Salicylic Acid Phenol Plaster
|
Used for Helosis.
|
||
|
35
|
Nourishing and Hair-Growing Tablet
|
Used for nourishing liver kidney, invigorating Qi and nourishing construction, activating the collaterals and expediting hair-growing; used for alopecia
|
||
|
36
|
Urine Stagnation Soothing Tablet
|
Used for regulating the flow of Qi and promoting blood circulation, restoring menstrual flow and reducing the stagnation; used for hyperplasia of prostate, urinary retention etc.
|
||
|
37
|
Children Diarrhea Relieving Plaster
|
Used to warm and dissipate cold, alleviate cold and bellyaches, assuage pain and relieve diarrhea
|
||
|
38
|
Hepatopathy Relieving Granules
|
Use to cure accumulations and concentrations of damp heat, jaundice, chest numbness and aches, fatigued limbs and inappetite, infrequent and discolored urination, and acute icteric, non-icteric, and chronic persisting hepatitis
|
Marketing and Sales
We have a trained marketing team and maintain sales offices or agents in approximately 26 provinces throughout China. The sales network covers approximately 230 counties in 26 provinces, municipalities and autonomous regions and is staffed by approximately 427 sales representatives, with each representative averaging a decade of pharmaceuticals sales experience.
Distribution of our products had already penetrated into more than 1,600 hospitals, and the acesodyre paste has been sold to Korea and Japan through trading companies. The Company has established five sales departments according to different sales regions and product types. Every department works hard to analyze the product market of our competitive counterparts, and the need of the market, which make the marketing efforts more specified and targeted.
In the fiscal year ended December 31, 2009, Maidisen’s five largest customers were Hebei Medicine and Herb Company , Shaanxi Kanghua Medicine Company, Guangdong National Medicine Co., Ltd., Xinjiang Jiuzhoutong Pharmaceutical Medicine Co., Ltd., Zhejiang Haizhou Pharmaceutical Medicine Co., Ltd. They accounted for 31%, 11%, 9%, 7% and 7% of revenues in 2009, respectively.
During the fiscal year ended December 31, 2008, we spent $1,567,784 on marketing. During the year ended December 31, 2009, we spent $1,798,526 on marketing.
7
Production
We manufacture and package our products at one factory located in the City of Yangling in Shaanxi Province. This is in compliance with China’s Good Manufacturing Practice (GMP) standards. All of the production lines at the facility meet international food and drug safety guidelines.
The Yangling factory, built in June 2004, is approximately 27,500 square meters with 17,200 square meters of sterilized area. This production facility has two production lines for high-volume production and manufactures 38 pharmaceutical products with different specifications. This production facility has over 50 machines and supporting parts for pharmaceutical production from domestic and foreign suppliers.
The facility is an independent production base that can process all stages of production, from raw materials to finished goods, including packaging.
We purchase our raw materials from numerous suppliers. We generally utilize more than three suppliers for each raw material.
Raw Materials and Principal Suppliers (API)
The raw materials used to manufacture our products are primarily comprised of Chinese medicinal herbs, which we obtain from numerous qualified suppliers with national qualifications. We also enter into trading agreements for the supply of many of the materials used to manufacture and package our products. Maidisen has written agreements with substantially all of its suppliers.
The five largest suppliers of Maidisen in 2009 are as follows:
|
Supplier
|
Product
|
%
|
|
|
1
|
Ren Yanhuai
|
Raw Material and Supplementary
|
5.0
|
|
2
|
Shaanxi Yungui Subsidiary
|
Raw Material and Supplementary
|
1.0
|
|
3
|
Shanxi Guangsheng Capsule Co., Ltd.
|
Raw Material and Supplementary
|
2.0
|
|
4
|
Xi’an yang Kaiyuan Industrial Co., Ltd.
|
Packaging Materials
|
4.0
|
|
5
|
Xi’an Yinhai Industrial Fabric Co., Ltd.
|
Packaging Materials
|
0.02
|
Quality Control
Our production facilities are designed and maintained with a view towards conforming with good manufacturing practice standards. To comply with GMP operational requirements, we have implemented a quality assurance plan setting forth our quality assurance procedures. Our Quality Control department is responsible for maintaining quality standards throughout the production process. Quality Control executes the following functions:
|
●
|
setting internal controls and regulations for semi-finished and finished products;
|
|
|
●
|
implementing sampling systems and sample files;
|
|
●
|
maintaining quality of equipment, instruments, reagents, test solutions, volumetric solutions, culture media and laboratory animals;
|
|
|
●
|
auditing production records to ensure delivery of quality products;
|
|
●
|
monitoring the number of dust particles and microbes in the clean areas;
|
|
|
●
|
evaluating stability of raw materials, semi-finished products and finished products in order to generate accurate statistics on storage duration and shelf life;
|
|
●
|
articulating the responsibilities of Quality Control staff; and
|
|
|
●
|
on-site evaluation of supplier quality control systems.
|
8
INDUSTRY & COMPETITIVE FACTORS
Competition
The pharmaceutical industry within China is intensely competitive and is characterized by rapid and significant technological progress, and our operating environment is increasingly competitive. Our competitors include large pharmaceutical companies, including Xi’an Jingxi Shuanghe Pharmaceuticals Co., Ltd., that currently engage in or may engage in efforts related to the discovery and development of new pharmaceuticals.
Western pharmaceutical products have more than half of the market share of medications used in China to treat similar medical conditions that our pharmaceutical products treat, with Chinese pharmaceutical products making up the next largest part of the market. Western medicine is all made from chemosynthesis products, while Chinese medicine is made from botanical, animal and mineral components.
There are certain opportunities for growth through the following growth strategies:
Rapidly growing Chinese pharmaceutical market. With approximately one-fifth of the world’s population and a fast-growing gross domestic product, China represents a significant potential market for the pharmaceutical industry. We believe the significant expected growth of the pharmaceutical market in China is due to factors such as robust economic growth and increased pharmaceutical expenditure, aging population and increased lifestyle-related diseases, government support of the pharmaceutical industry, the relatively low research and development and clinical trial costs in China as compared to developed countries, as well as the increased availability of funding for medical insurance and industry consolidation in China.
Currently China has about 3,500 drug companies, falling from more than 5,000 in 2004, according to government figures. The number is expected to drop further due to trends in the industry’s consolidation. The domestic companies compete in the $10 billion market without a dominant leader. Most often cited adverse factors include a lack of protection of intellectual property rights, a lack of visibility for drug approval procedures, a lack of effective governmental incentives, poor corporate support for drug research and differences in the treatment in China accorded to local and foreign firms. The profile of the pharmaceutical industry in China remains very low. China accounts for 20% of the world’s population but only 1.5% of the global drug market. As of 2008, China is the world's eighth largest drug market.
New Product Development. We will continue to evaluate and develop additional product candidates, both through our in-house research and development department and working with our research and development partners, to expand our pipeline where we perceive an unmet need and commercial potential. We will face the risk that in developing new products we may spend substantial sums of money and the new products developed may not effectively meet the perceived need or may not be successfully commercialized.
Focus on brand development. With intense price competition among many similar or identical products in the industry, we believe that building brand equity is the primary means to generate and sustain profitable growth in the future. The company intends to focus its brand development efforts on building its existing brand names with the intent on it becoming a leading medicine brand in China.
Marketing and Sales Function. We intend to grow our internal marketing and sales function and increase our relationships with other distributors to expand the distribution and presence of our pharmaceutical products. In expanding market share of our products, we intend to take advantage of our large manufacturing scale and reasonable cost control mechanisms, and our strong sales network. In addition, our goal is to establish our products as a preferred choice for medicines in local pharmacies. We hope to add other pharmaceutical products into this channel over the next few years. We may face risks in obtaining adequate quantities of raw materials at reasonable prices in order to meet increased demand for our products that result from any growth. In seeking additional employees, sales representatives, and distributors, we will compete with many other established pharmaceutical manufacturers that may have greater resources than we do.
Low cost producer. Our continuing success in optimizing our manufacturing processes and minimizing our production costs provides us with a competitive advantage.
Government sponsored industry consolidation presents opportunities for acquisitions. China’s thousands of domestic companies account for 70 percent of the pharmaceutical market. Anticipating the effects of WTO entry and in an effort to compete with foreign firms, the Chinese government has decided to nurture its own large pharmaceutical companies, by encouraging the consolidation of its government-owned companies which, even though the company is not government-owned, may also present the company with acquisition opportunities. To this end, the Chinese State Economic and Trade Commission (SETC) announced plans to consolidate the industry and support the development of 10 to 15 largest pharmaceutical firms. According to government statistics China currently has about 3,500 drug companies, down from over 5,000 in 2004. The number is expected to drop further. As the industry undergoes further consolidation, the Company may have the opportunity to grow by acquisition.
9
OUR INTELLECTUAL PROPERTY
We regard our service marks, trademarks, trade secrets, patents and similar intellectual property as critical factors to our success. We rely on patent, trademark and trade secret law, as well as confidentiality and license agreements with certain of our employees, customers and others to protect our proprietary rights.
Pursuant to certain PRC laws protecting TCMs, certain ready- made TCM products which have received SFDA approval are automatically afforded intellectual property rights. The administrative protection period covered by SFDA approval is 8 years, after which protection is automatically shifted in form from administrative protection to legal protection to TCM and will cover all dosage types of each product approved for a period of 20 years. The patents listed below have a protection period of 20 years (from 2024 to 2027). Once the SFDA protection period has expired, a company may apply for patent protection.
To a large extent, we rely on such protection regulation to protect our intellectual property rights with respect to such products.
Our Patents
Two types of medicine-related patents exist in PRC: the medicine production technique patent and medicine invention formula patent. In PRC, illegal infringements of production technique patents are widespread. A tiny modification to a filed medicine production technique might be construed as a new one and argued as not violating the patent laws. Invention formula patents are as easily imitated as production technique patents. However, since only one SFDA production certificate can be issued on new branded medicine based on the major pharmaceutical ingredients used, a minor modification to the formula would not warrant a new SFDA production certificate. This means that, even if another manufacturer gets to know a patented formula, it will not be able to produce it in PRC without the proper SFDA production certificate.
Maidisen currently holds eleven patents, which are valid for 20 years from the publication date:
|
Product Description
|
Patent No.
|
Publication date
|
|
A Chinese Traditional Medicine for the treatment of Gynecologic Inflammation
|
ZL2005 10051424.1
|
March 4, 2005
|
|
A Chinese Traditional Medicine for the treatment of Heart Diseases
|
ZL2005 10073342.7
|
June 2, 2005
|
|
A Chinese Traditional Medicine for the treatment of Hepatitis B
|
ZL2005 1008600.3
|
February 23, 2005
|
|
A Chinese Traditional Medicine for the treatment of respiratory disease
|
ZL2004 10026269.3
|
June 24, 2004
|
|
A Chinese Traditional Medicine for the treatment of Children Diarrhea Relieving Plaster
|
ZL2005 10097933.8
|
September 1, 2005
|
|
A Chinese Traditional Medicine for the treatment of Compound Cirald Daphne Bank Paining- Relieving Plaster
|
ZL2005 10097931.9
|
September 1, 2005
|
|
A Chinese Traditional Medicine for the treatment of Stomach Recovery
|
ZL2005 10075090.1
|
June 9, 2005
|
|
A Chinese Traditional Medicine for Blood Nourishing Tablet
|
ZL2005 10097932.3
|
September 1, 2005
|
|
A Chinese Traditional Medicine for the treatment of Cold
|
ZL2005 10041938.9
|
November 14, 2007
|
|
A Chinese Traditional Medicine for the treatment of Rheumatism
|
ZL2005 10071079.8
|
May 24, 2005
|
|
A Chinese Traditional Medicine for the treatment of Qinchuan Tongbi Pill
|
ZL2005 10071079.8
|
December 19, 2007
|
Trademarks
We have two trademarks evidenced by Trademark Registration Certificates for our pharmaceutical products, including all kinds of tablets, capsules, syrups, oral solutions, medicinal liquor and tincture. These trademarks distinguish our pharmaceutical products from those of our competitors. We regard our trademarks, copyrights domain names, trade dress, trade secrets, proprietary technologies and similar intellectual property as important to our success, and we rely on trademark and copyright law, trade-secret protection, and confidentiality and\or license agreements with our employees, customers, partners, suppliers and others to protect or proprietary rights. In an effort to protect our intellectual property, all of our employees are required to follow our confidentiality principles. We have received trademark registration certificates for the following marks: Dongke and Yao Wang MT, which are valid until July 13, 2019 and August 13, 2019, respectively.
RESEARCH AND DEVELOPMENT ACTIVITIES DURING THE PRIOR TWO FISCAL YEARS
Research and Development
We develop new products in-house. During the last two fiscal years, we spent $805,152 for ongoing in-house research and development activities.
10
We also retain outside services to assist our R&D activities. On April 28, 2006, Maidisen entered into a Second Product Development Agency Agreement with Shaanxi Dongke Maidisen Pharmaceutical Technology Co., Ltd. (“DKMP Technology”), a research and development (“R&D) company formerly managed by our chief executive officer, Dongke Zhao. Pursuant to the agreement, DKMP Technology agreed to provide testing facilities and personnel to conduct various R&D activities for 11 of Maidisen’s products according to the quality standards set by Maidisen. Such services include the preparation and compilation of original data and testing results. As consideration for such services, Maidisen is to pay DKMP Technology related testing and development fees of RMB920,000, of which RMB 400,000 shall be used for product-related R&D, RMB300,000 shall be used toward quality improvement R&D, and 220,000 shall be used toward product stability-related R&D.
COMPLIANCE WITH GOVERNMENT REGULATIONS
Environmental Laws
Maidisen operation and facilities are subject to environmental laws and regulations stipulated by the national and the local environment protection bureaus in China. Relevant laws and regulations include provisions governing air emissions, water discharges and the management and disposal of hazardous substances and wastes. The PRC regulatory authorities require pharmaceutical companies to carry out environmental impact studies before engaging in new construction projects to ensure that their production processes meet the required environmental standards.
Maidisen maintains controls at its production facilities to facilitate compliance with environmental rules and regulations. Maidisen is not aware of any investigations, prosecutions, disputes, claims or other proceedings in respect of environmental protection, nor has it been subject to any action made by any environmental administration authorities of the PRC. To management’s knowledge, Maidisen operations meet or exceed the existing requirements of the PRC.
During the last two years, Maidisen spent $3,216 on environmental compliance.
Advertising Laws
Advertisement Law of the People’s Republic of China and Rules of Medicine Advertisements Management from State Admission for Industry and Commerce, Regulations on Control of Advertisements (tentative) from State Council provide guidelines for advertising prescription and OTC drugs and nutrients made by Maidisen. The rules limit where advertisements may be place and govern the claims that may be made by the manufacturer.
Insurance Catalogue
Pursuant to the Decision of the State Council on the Establishment of the State Basic Medical Insurance System for Urban Employees and the Implementation Measures for the Administration of the Scope of Medical Insurance Coverage for Pharmaceuticals for Urban Employees, the Ministry of Labor and Social Security in China established the Insurance Catalogue. The Insurance Catalogue is divided into Parts A and B. The medicines included in Part A are designated by the Chinese governmental authorities for general application. Local governmental authorities may not adjust the content of medicines in Part A. Although the medicines included in Part B are designated by Chinese governmental authorities in the first instance, provincial level authorities may make limited changes to the medicines included in Part B, resulting in some regional variations in the medicines included in Part B from region to region.
Patients purchasing medicines included in Part A are entitled to reimbursement of the costs of such medicines from the social medical fund in accordance with relevant regulations in China. Patients purchasing medicines included in Part B are required to pay a predetermined proportion of the costs of such medicines.
The medicines included in the Insurance Catalogue are selected by the Chinese government authorities based on various factors including treatment requirements, frequency of use, effectiveness and price. Medicines included in the Insurance Catalogue are subject to price control by the Chinese government. The Insurance Catalogue is revised every two years. In connection with each revision, the relevant provincial drug authority collects proposals from relevant enterprises before organizing a comprehensive appraisal. The SFDA then makes the final decision on any revisions based on the preliminary opinion suggested by the provincial drug administration. Approximately 40% of our revenues are derived from pharmaceutical products listed in the Insurance Catalogue so that purchasers can receive reimbursement on these products. Removal of a significant portion of these products from the Insurance Catalogue would adversely affect our total revenue.
11
Price Controls
The prices of approximately 1,500 pharmaceuticals are presently set by the PRC government. These constitute approximately 10% of all distributed drugs. The prices for the other 90%, or approximately 12,000 pharmaceuticals, are established by the market (by the companies themselves). Corporations typically establish these prices based on operating costs, and market supply and demand. The Supervision Department of the PRC government will intervene only if there is a significant fluctuation in prices or a monopoly develops in a particular drug. We have not had any products subject to specific pricing control and production and trading of none of our pharmaceutical products constitutes a monopoly.
Employees
We have approximately 299 full-time, salaried employees who receive labor insurance. These employees are organized into a union under the labor laws of China and can bargain collectively with us. In addition, we employ over 427 sales representatives who are paid on a commission basis. These representatives are not part of the union. We maintain good relations with our employees.
RISK FACTORS
You should carefully consider the risks described below together with all of the other information included in this report before making an investment decision with regard to our securities. The statements contained in or incorporated into this offering that are not historic facts are forward-looking statements that are subject to risks and uncertainties that could cause actual results to differ materially from those set forth in or implied by forward-looking statements. If any of the following risks actually occurs, our business, financial condition or results of operations could be harmed. In that case, the trading price of our common stock could decline, and you may lose all or part of your investment.
Risks Related To Our Business
Our operating history may not serve as an adequate basis to judge our future prospects and results of operations.
Maidisen commenced its current line of business operations on June 23, 2004 and has received Good Manufacturing Practices (“GMP”) certifications for its two production lines that are valid through July 2011 and May 2014, respectively. This certification must be renewed every five years for Maidisen to stay in business. Maidisen’s operating history may not provide a meaningful basis on which to evaluate its business. We cannot assure you that Maidisen will maintain its profitability or that we will not incur net losses in the future. We expect that Maidisen’s operating expenses will increase as it expands. Any significant failure to realize anticipated revenue growth could result in significant operating losses. We will continue to encounter risks and difficulties frequently experienced by companies at a similar stage of development, including our potential failure to:
|
· raise adequate capital for expansion and operations;
· implement our business model and strategy and adapt and modify them as needed;
· increase awareness of our brand name, protect our reputation and develop customer loyalty;
· manage our expanding operations and service offerings, including the integration of any future acquisitions;
· maintain adequate control of our expenses;
· anticipate and adapt to changing conditions in the medical over the counter, pharmaceutical and nutritional supplement markets in which we operate as well as the impact of any changes in government regulations, mergers and acquisitions involving our competitors, technological developments and other significant competitive and market dynamics
|
If we are not successful in addressing any or all of these risks, our business may be materially and adversely affected.
12
The loss of Maidisen as our operating business would have a material adverse effect on our business and the price of our common stock.
We have no equity ownership interest in Maidisen. Our ability to control Maidisen and consolidate its financial results is through a series of contractual agreements between Maidisen and our wholly-owned subsidiary Slovan. The management of Maidisen is an affiliate of the Company and of Slovan and the stockholders of Maidisen are also our shareholders. Thus the Management Entrustment Agreement was not entered into as a result of arms’ length negotiations because the parties to the agreement are under common control. Dongke Zhao, our President, CEO and Chairman of the Board, holds approximately 40.4% of the shares of Maidisen and approximately 36% of our common stock. The Management Entrustment Agreement may be terminated upon the termination of the business of Maidisen or upon the date upon which Slovan completes the acquisition of Maidisen. Any other termination would be a breach of the agreement. While the Company has been advised by its PRC counsel that the Management Entrustment Agreement is legal and enforceable under PRC law, these affiliates control the parties to the Management Entrustment Agreement and it could be possible for them to cause Maidisen to breach the Management Entrustment Agreement and our unaffiliated investors would have little or no recourse because of the inherent difficulties in enforcing their rights since all our assets are located in the PRC. (See, Risk Factor “The PRC laws and regulations governing our current business operations are sometimes vague and uncertain. Any changes in such PRC laws and regulations may harm its business.”) In the event that management of Maidisen decides to breach the Management Entrustment Agreement, the risk of loss of the affiliated shareholders of Maidisen could be lower than unaffiliated investors and the interests of the management and shareholders of Maidisen would be in conflict with the interest of our other stockholders.
Maidisen’sfailure to compete effectively may adversely affect our ability to generate revenue.
Maidisen competes with other companies, many of whom are developing or can be expected to develop products similar to Maidisen. Maidisen’s market is a large market with many competitors. Many of its competitors are more established than Maidisen is, and have significantly greater financial, technical, marketing and other resources than it presently possess. Some of Maidisen’s competitors have greater name recognition and a larger customer base. These competitors may be able to respond more quickly to new or changing opportunities and customer requirements and may be able to undertake more extensive promotional activities, offer more attractive terms to customers, and adopt more aggressive pricing policies. We cannot assure you that Maidisen will be able to compete effectively with current or future competitors or that the competitive pressures it faces will not harm it business.
We may not be able to effectively control and manage the growth of Maidisen.
If Maidisen’s business and markets grow and develop, it will be necessary for us to finance and manage expansion in an orderly fashion. An expansion would increase demands on existing management, workforce and facilities. Failure to satisfy such increased demands could interrupt or adversely affect its operations and cause delay in production and delivery of its pharmaceutical prescription, over the counter and medical nutrient products as well as administrative inefficiencies.
We may require additional financing in the future and a failure to obtain such required financing will inhibit Maidisen’ sability to grow.
The continued growth of Maidisen’s business may require additional funding from time to time, which we expect to raise in private placements of our equity or debt securities with accredited investors or by offering our securities for sale pursuant to an effective registration statement on a market where our common stock is traded. The proceeds of these funding will be forwarded to Maidisen and accounted for as a loan to Maidisen and eliminated during consolidation. The proceeds would be used for general corporate purposes of Maidisen, which could include acquisitions, investments, repayment of debt and capital expenditures among other things. We may also use the proceeds to repurchase our capital stock or for our corporate overhead expenses. If we borrow funds we expect to be the primary obligor on any debt. Obtaining additional funding would be subject to a number of factors including market conditions, operating performance and investor sentiment, many of which are outside of our control. These factors could make the timing, amount, terms and conditions of additional funding unattractive or unavailable to us. Our management believes that we currently have sufficient funds from working capital to meet our current operating costs over the next 12 months.
13
The terms of any future financing may adversely affect your interest as stockholders.
If we require additional financing in the future, we may be required to incur indebtedness or issue equity securities, the terms of which may adversely affect your interests in us. For example, the issuance of additional indebtedness may be senior in right of payment to your shares upon our liquidation. In addition, indebtedness may be under terms that make the operation of Maidisen’s business more difficult because the lender's consent could be required before we take certain actions. Similarly the terms of any equity securities we issue may be senior in right of payment of dividends to your common stock and may contain superior rights and other rights as compared to your common stock. Further, any such issuance of equity securities may dilute your interest in us.
We, through our subsidiaries/affiliated companies Dong Ke, Slovan or Maidisen, may engage in future acquisitions that could dilute the ownership interests of our stockholders, cause us to incur debt and assume contingent liabilities.
We, through our subsidiaries/affiliated companies Dong Ke, Slovan, or Maidisen, may review acquisition and strategic investment prospects that we believe would complement the current product offerings of Maidisen, augment its market coverage or enhance its technical capabilities, or otherwise offer growth opportunities. From time to time Maidisen reviews investments in new businesses and we, through our subsidiaries/affiliated companies Dong Ke, Slovan, or Maidisen, expect to make investments in, and to acquire, businesses, products, or technologies in the future. We expect that when we raise funds from investors for any of these purposes we will be either the issuer or the primary obligor while the proceeds will be forwarded to Maidisen and accounted for as a loan to Maidisen and eliminated during consolidation. In the event of any future acquisitions, we could:
|
·
|
issue equity securities which would dilute current stockholders’ percentage ownership;
|
|
·
|
incur substantial debt;
|
|
·
|
assume contingent liabilities; or
|
|
·
|
expend significant cash.
|
These actions could have a material adverse effect on our operating results or the price of our common stock. Moreover, even if through our subsidiary, Slovan or Maidisen, we do obtain benefits in the form of increased sales and earnings, there may be a lag between the time when the expenses associated with an acquisition are incurred and the time when we recognize such benefits. Acquisitions and investment activities also entail numerous risks, including:
|
·
|
difficulties in the assimilation of acquired operations, technologies and/or products;
|
|
·
|
unanticipated costs associated with the acquisition or investment transaction;
|
We may not have adequate internal accounting controls. While we have certain internal procedures in our budgeting, forecasting and in the management and allocation of funds, our internal controls may not be adequate.
We are constantly striving to improve our internal accounting controls. We expect to continue to improve our internal accounting control for budgeting, forecasting, managing and allocating our funds and to better account for them as we grow. There is no guarantee that such improvements will be adequate or successful or that such improvements will be carried out on a timely basis. If we do not have adequate internal accounting controls, we may not be able to appropriately budget, forecast and manage our funds, we may also be unable to prepare accurate accounts on a timely basis to meet our continuing financial reporting obligations and we may not be able to satisfy our obligations under US securities laws.
Rules adopted by the SEC pursuant to Section 404 of the Sarbanes-Oxley Act of 2002 require annual assessment of our internal control over financial reporting, and attestation of this assessment by our company's independent registered public accountants. The SEC extended the compliance dates for "non-accelerated filers," as defined by the SEC. Accordingly, we believe that the annual assessment of our internal controls requirement first applied to our annual report for the 2007 fiscal year and the attestation requirement of management's assessment by our independent registered public accountants will first apply to our annual report for the 2010 fiscal year. The standards that must be met for management to assess the internal control over financial reporting as effective are new and complex, and require significant documentation, testing and possible remediation to meet the detailed standards. We have not yet evaluated our internal controls over financial reporting in order to allow management to report on, and our independent auditors to attest to, our internal controls over financial reporting, as will be required by Section 404 of the Sarbanes-Oxley Act of 2002 and the rules and regulations of the SEC. We have never performed the system and process evaluation and testing required in an effort to comply with the management assessment and auditor certification requirements of Section 404, which will initially apply to us as of December 31, 2007 and December 31, 2010 respectively. Our lack of familiarity with Section 404 may unduly divert management's time and resources in executing the business plan. If, in the future, management identifies one or more material weaknesses, or our external auditors are unable to attest that our management's report is fairly stated or to express an opinion on the effectiveness of our internal controls, this could result in a loss of investor confidence in our financial reports, have an adverse effect on our stock price and/or subject us to sanctions or investigation by regulatory authorities. So far, our external auditors have not reported to our board of directors any significant weakness on our internal control and provided recommendations accordingly.
14
We are dependent on certain key personnel and loss of these key personnel could have a material adverse effect on our business, financial condition and results of operations.
Our success is, to a certain extent, attributable to the management, sales and marketing, and pharmaceutical factory operational expertise of key personnel. Dongke Zhao, our President, Chief Executive Officer and Chairman of the Board, and Yanhong Ren, our Chief Financial Officer, perform key functions in the operation of our and Maidisen’s business. There can be no assurance that Maidisen will be able to retain these officers after the term of their employment contracts expire. The loss of these officers could have a material adverse effect upon our business, financial condition, and results of operations. Maidisen must attract, recruit and retain a sizeable workforce of technically competent employees. We do not carry key man life insurance for any of our key personnel or personnel nor do we foresee purchasing such insurance to protect against a loss of key personnel and the key personnel.
We are dependent upon the services of Mr. Zhao and Ms. Ren, for the continued growth and operation of our company because of their experience in the industry and his personal and business contacts in the PRC. Although we have entered into two-year employment agreements with Mr. Zhao and Ms. Ren and we have no reason to believe that they will discontinue their services with the Company or Maidisen, the interruption or loss of his services would adversely affect our ability to effectively run our business and pursue our business strategy as well as our results of operations.
We may not be able to hire and retain qualified personnel to support its growth and if it is unable to retain or hire these personnel in the future, its ability to improve its products and implement its business objectives could be adversely affected.
Competition for senior management and senior personnel in the PRC is intense, the pool of qualified candidates in the PRC is very limited, and we may not be able to retain the services of our senior executives or senior personnel, or attract and retain high-quality senior executives or senior personnel in the future. This failure could materially and adversely affect our future growth and financial condition. We expect to hire additional sales and plant personnel throughout fiscal year 2010 in order to accommodate its growth.
If we fail to increase our brand recognition, we may face difficulty in obtaining new customers and business partners.
We believe that establishing, maintaining and enhancing our brand in a cost-effective manner is critical to achieving widespread acceptance of our current and future products and services and is an important element in our effort to increase our customer base and obtain new business partners. We believe that the importance of brand recognition will increase as competition in our market develops. Some of our potential competitors already have well-established brands in the pharmaceutical promotion and distribution industry. Successful promotion of our brand will depend largely on our ability to maintain a sizeable and active customer base, our marketing efforts and ability to provide reliable and useful products and services at competitive prices. Brand promotion activities may not yield increased revenue, and even if they do, any increased revenue may not offset the expenses we will incur in building our brand. If we fail to successfully promote and maintain our brand, or if we incur substantial expenses in an unsuccessful attempt to promote and maintain our brand, we may fail to attract enough new customers or retain our existing customers to the extent necessary to realize a sufficient return on our brand-building efforts, in which case our business, operating results and financial condition, would be materially adversely affected.
Our operating results may fluctuate as a result of factors beyond our control.
Our operating results may fluctuate significantly in the future as a result of a variety of factors, many of which are beyond our control. These factors include:
|
·
|
the costs of pharmaceutical products and development;
|
|
·
|
the relative speed and success with which we can obtain and maintain customers, merchants and vendors for our products;
|
|
·
|
capital expenditure for equipment;
|
|
·
|
marketing and promotional activities and other costs;
|
|
·
|
changes in our pricing policies, suppliers and competitors;
|
|
·
|
the ability of our suppliers to provide products in a timely manner to their customers;
|
|
·
|
changes in operating expenses;
|
|
·
|
increased competition in the pharmaceutical markets; and
|
|
·
|
other general economic and seasonal factors.
|
15
We face risks related to product liability claims.
We presently do not maintain product liability insurance. We face the risk of loss because of adverse publicity associated with product liability lawsuits, whether or not such claims are valid. We may not be able to avoid such claims. Although product liability lawsuits in the PRC are rare, and we have not, to date, experienced significant failure of our products, there is no guarantee that we will not face such liability in the future. This liability could be substantial and the occurrence of such loss or liability may have a material adverse effect on our business, financial condition and prospects.
We face marketing risks.
Newly developed drugs and technology may not be compatible with market needs. Because markets for drugs differentiate geographically inside the PRC, we must develop and manufacture our products to accurately target specific markets to ensure product sales. If we fail to invest in extensive market research to understand the health needs of consumers in different geographic areas, we may face limited market acceptance of our products, which could have material adverse effect on our sales and earnings.
We face risks relating to difficulty in defending intellectual property rights from infringement.
Our success depends on protection of our current and future technology and products and our ability to defend our intellectual property rights. Maidisen has filed for trademark protection for the various names and brands of its products sold in the PRC. However, it is possible for its competitors to develop similar competitive products even thought it has taken steps to protect its intellectual property. If we fail to protect Maidisen’s intellectual property adequately, competitors may manufacture and market products similar to Maidisen. We expect to file patent applications seeking to protect newly developed technology and products in various countries, including the PRC. Some patent applications in the PRC are maintained in secrecy until the patent is issued. Because the publication of discoveries tends to follow their actual discovery by many months, we may not be the first to invent, or file patent applications on any of our discoveries. Patents may not be issued with respect to any of our patent applications and existing or future patents issued to or licensed by us may not provide competitive advantages for our products. Patents that are issued may be challenged, invalidated or circumvented by our competitors. Furthermore, our patent rights may not prevent our competitors from developing, using or commercializing products that are similar or functionally equivalent to our products.
We also rely on trade secrets, non-patented proprietary expertise and continuing technological innovation that we shall seek to protect, in part, by entering into confidentiality agreements with licensees, suppliers, employees and consultants. These agreements may be breached and there may not be adequate remedies in the event of a breach. Disputes may arise concerning the ownership of intellectual property or the applicability of confidentiality agreements. Moreover, our trade secrets and proprietary technology may otherwise become known or be independently developed by our competitors. If patents are not issued with respect to products arising from research, we may not be able to maintain the confidentiality of information relating to these products.
16
We face risks relating to third parties that may claim that we infringe on their proprietary rights and may prevent us from manufacturing and selling certain of our products.
There has been substantial litigation in the pharmaceutical industry with respect to the manufacturing, use and sale of new products. These lawsuits relate to the validity and infringement of patents or proprietary rights of third parties. We may be required to commence or defend against charges relating to the infringement of patents or proprietary rights. Any such litigation could:
|
·
|
require us to incur substantial expense, even if covered by insurance or are successful in the litigation;
|
|
·
|
require us to divert significant time and effort of our technical and management personnel;
|
|
·
|
result in the loss of our rights to develop or make certain products; and
|
|
·
|
require us to pay substantial monetary damages or royalties in order to license proprietary rights from third parties.
|
Although intellectual property disputes within the pharmaceutical industry have often been settled through licensing or similar arrangements, costs associated with these arrangements may be substantial and could include the long-term payment of royalties. These arrangements may be investigated by regulatory agencies and, if improper, may be invalidated. Furthermore, the required licenses may not be made available to us on acceptable terms. Accordingly, an adverse determination in a judicial or administrative proceeding or a failure to obtain necessary licenses could prevent us from manufacturing and selling some of our products or increase our costs to market these products.
In addition, when seeking regulatory approval for some of our products, we may be required to certify to regulatory authorities, including the SFDA, that such products do not infringe upon third party patent rights. Filing a certification against a patent gives the patent holder the right to bring a patent infringement lawsuit against us. Any lawsuit would delay the receipt of regulatory approvals. A claim of infringement and the resulting delay could result in substantial expenses and even prevent us from manufacturing and selling certain of our products.
Our launch of a product prior to a final court decision or the expiration of a patent held by a third party may result in substantial damages to us. If we are found to infringe a patent held by a third party and become subject to such damages, these damages could have a material adverse effect on the results of our operations and financial condition.
We face risks related to research and the ability to develop new drugs.
Our growth and survival depends on our ability to consistently discover, develop and commercialize new products and find new and improve on existing technology and platforms. As such, if we fail to make sufficient investments in research, be attentive to consumer needs or does not focus on the most advanced technology, our current and future products could be surpassed by more effective or advanced products of other companies.
Risk Related To the PharmaceuticalIndustry
Our certificates, permits, and licenses related to our pharmaceutical operations are subject to governmental control and renewal and failure to obtain renewal will cause all or part of our operations to be terminated.
Maidisen is subject to various PRC laws and regulations pertaining to the pharmaceutical industry. Maidisen has attained certificates, permits, and licenses required for the operation of a pharmaceutical enterprise and the manufacturing of pharmaceutical products in the PRC.
In 1998, the SFDA introduced the Good Manufacturing Practice (GMP) Certificate in order to promote quality and safety of pharmaceutical production. The Good Manufacturing Practices were revised in July and October, 2004. We and our competitors are required to meet GMP standards in order to continue manufacturing pharmaceutical products and health foods. For each new product, Maidisen prepares documentation of pharmacological, toxicity, pharmacokinetics and drug metabolism studies in addition to providing samples of the drug. The documentation and samples are then submitted to provincial food and drug administration. This process typically takes approximately three months. After the documentation and samples have been approved by the provincial food and drug administration, the provincial administration submits the approved documentation and samples to the SFDA. The SFDA examines the documentation and tests the samples and presents the findings to the New Drug Examination Committee for approval. If the application is approved by the SFDA, the SFDA will issue a clinical trial license to the applicant for clinical trials. This clinical trial license approval typically takes one year, followed by approximately two years of trials, depending on the category and class of the new drug. The SFDA then examines the documentation from the trial and, if approved, issues the new drug license to the applicant. This process usually takes eight months. The entire process takes anywhere from three to four years.
17
Maidisen initially obtained pharmaceutical products and health food production permits by submitting its manufacturing processes and product tests to the SFDA who verified that its production processes and products met the standards by onsite inspections, review of test results and a determination that the market was not saturated by its products. The production permits are permanent once issued as long as they are renewed by the expiration date.
The GMP certificate is valid for a term of five years, the pharmaceutical products production permits are subject to renewal every five years, and each must be renewed before its expiration, if applicable. We manufacture and package our products at one factory located in Yangling City, Shaanxi Province. This facility is in compliance with Good Manufacturing Practice (GMP) standards and has two GMP certificates for its 38 products, dated July 27, 2006 (Certificate No. Shaan H0202) and May 21, 2009 (Certificate No. Shaan K0331). The certificates remain valid until July 26, 2011 and May 20, 2014, respectively. All of the complete production lines at each facility meet international food and drug safety guidelines. If the GMP certificates expire without renewal, Maidisen will not be able to continue medicine production, which will cause its operations to be terminated. Maidisen intends to apply for renewed GMP certificates for its two production facilities before its current certificates expire.
According to Drug Administration Law of the PRC and its implementing rules, the SFDA approvals, including Pharmaceutical Manufacturing Permit and Drug Approval Numbers, may be suspended or revoked prior to the expiration date under circumstances that include:
|
·
|
producing counterfeit medicine;
|
|
·
|
producing inferior quality products;
|
|
·
|
failing to meet the drug GMP standards;
|
|
·
|
purchasing medical ingredients used in the production of products sources that do not have Pharmaceutical Manufacturing Permit or Pharmaceutical Trade Permit;
|
|
·
|
fraudulent reporting of results or product samples in application process;
|
|
·
|
failing to meet drug labeling and direction standards;
|
|
·
|
bribing doctors or hospital personnel to entice them to use products,
|
|
·
|
producing pharmaceuticals for use or resale by companies that are not approved by the SFDA, or
|
|
·
|
the approved drug has a serious side effect.
|
If our pharmaceutical products fail to receive regulatory approval or are severely limited in these products' scope of use, we may be unable to recoup considerable research and development expenditures.
Our research and development of pharmaceutical products is subject to the regulatory approval of the SFDA in the PRC. The regulatory approval procedure for pharmaceuticals can be quite lengthy, costly, and uncertain. Depending upon the discretion of the SFDA, the approval process may be significantly delayed by additional clinical testing and require the expenditure of resources not currently available; in such an event, it may be necessary for us to abandon our application. Even where approval of the product is granted, it may contain significant limitations in the form of narrow indications, warnings, precautions, or contra-indications with respect to conditions of use. If approval of our product is denied, abandoned, or severely limited in terms of the scope of products use, it may result in the inability to recoup considerable research and development expenditures. If we do not receive timely approval for any of our drugs, then production will be delayed and sales of the products cannot be planned for.
Price control regulations may decrease our profitability.
The laws of the PRC provide for the government to fix and adjust prices. The prices of certain medicines we distribute, including those listed in the Chinese government's catalogue of medications that are reimbursable under the PRC’s social insurance program, or the Insurance Catalogue, are subject to control by the relevant state or provincial price administration authorities. The PRC establishes price levels for products based on market conditions, average industry cost, supply and demand and social responsibility. In practice, price control with respect to these medicines sets a ceiling on their retail price. The actual price of such medicines set by manufacturers, wholesalers and retailers cannot historically exceed the price ceiling imposed by applicable government price control regulations. Although, as a general matter, government price control regulations have resulted in drug prices tending to decline over time, there has been no predictable pattern for such decreases.
18
For the year ended December 31, 2009 and December 31, 2008, we have not had any products subject to specific pricing control and production and trading of none of our pharmaceutical products constitutes a monopoly. However, it is possible that our products may be subject to price control in the future. To the extent that our products are subject to price control, our revenue, gross profit, gross margin and net income will be affected since the revenue we derive from our sales will be limited and we may face no limitation on our costs. Further, if price controls affect both our revenue and costs, our ability to be profitable and the extent of our profitability will be effectively subject to determination by the applicable regulatory authorities in the PRC.
If the medicines we produce are replaced by other medicines or are removed from the PRC's insurance catalogue in the future, our revenue may suffer.
Under PRC regulations, patients purchasing medicine listed by the PRC's state and/or provincial governments in the Insurance Catalogue may be reimbursed, in part or in whole, by a social medicine fund. Accordingly, pharmaceutical distributors prefer to engage in the distribution of medicine listed in the Insurance Catalogue. Currently, our main prescription products are listed in the Insurance Catalogue. The content of the Insurance Catalogue is subject to change by the PRC Ministry of Labor and Social Security, and new medicine may be added to the Insurance Catalogue by provincial level authorities as part of their limited ability to change certain medicines listed in the Insurance Catalogue. If the medicine we produce are replaced by other medicines or removed from the Insurance Catalogue in the future, our revenue may suffer.
Adverse publicity associated with our products, ingredients or network marketing program, or those of similar companies, could harm our financial condition and operating results.
The results of our operations may be significantly affected by the public's perception of our product and similar companies. This perception is dependent upon opinions concerning:
|
·
|
the safety and quality of our products and ingredients;
|
|
·
|
the safety and quality of similar products and ingredients distributed by other companies; and
|
|
·
|
our sales force.
|
Adverse publicity concerning any actual or purported failure to comply with applicable laws and regulations regarding product claims and advertising, good manufacturing practices, or other aspects of our business, whether or not resulting in enforcement actions or the imposition of penalties, could have an adverse affect on our goodwill and could negatively affect our sales and ability to generate revenue.
In addition, our consumers' perception of the safety and quality of products and ingredients as well as similar products and ingredients distributed by other companies can be significantly influenced by media attention, publicized scientific research or findings, widespread product liability claims and other publicity concerning our products or ingredients or similar products and ingredients distributed by other companies. Adverse publicity, whether or not accurate or resulting from consumers' use or misuse of our products, that associates consumption of our products or ingredients or any similar products or ingredients with illness or other adverse effects, questions the benefits of our or similar products or claims that any such products are ineffective, inappropriately labeled or have inaccurate instructions as to their use, could negatively impact our reputation or the market demand for our products.
If we fail to develop new products with high profit margins, and our high profit margin products are substituted by competitor's products, our gross and net profit margins will be adversely affected.
There is no assurance that we will be able to sustain our profit margins in the future. The pharmaceutical industry is very competitive, and there may be pressure to reduce sale prices of products without a corresponding decrease in the price of raw materials. In addition, the medical industry in the PRC is highly competitive and new products are constantly being introduced to the market. In order to increase our sales and expand our market share, we may be forced to reduce prices in the future, leading to a decrease in gross profit margin. The research and development of new products and technology is costly and time consuming, and there are no assurances that our research and development of new products will either be successful or completed within the anticipated timeframe, if ever at all. There is no assurance that our competitors' new products, technology, and processes will not render our existing products obsolete or non-competitive. To the extent that we fail to develop new products with high profit margins and our high profit margin products are substituted by competitors' products, our gross profit margins will be adversely affected.
19
The commercial success of our products depends upon the degree of market acceptance among the medical community and failure to attain market acceptance among the medical community may have an adverse impact on our operations and profitability.
The commercial success of our products depends upon the degree of market acceptance among the medical community, such as hospitals and physicians. Even if our products are approved by the SFDA, there is no assurance that physicians will prescribe or recommend our products to patients. Furthermore, a product's prevalence and use at hospitals may be contingent upon its relationship with the medical community, particularly products that are only available by medical prescription. The acceptance of our products among the medical community may depend upon several factors, including but not limited to, the product's acceptance by physicians and patients as a safe and effective treatment, cost effectiveness, potential advantages over alternative treatments, and the prevalence and severity of side effects. Failure to attain market acceptance among the medical community may have an adverse impact on our operations and profitability.
We enjoy certain preferential tax concessions and loss of these preferential tax concessions will cause its tax liabilities to increase and its profitability to decline.
Maidisen enjoys preferential tax concessions in the PRC as a high-tech enterprise because of the research and development methodologies it employs to develop new products. Pursuant to the State Council's Regulations on Encouraging Investment in and Development, Maidisen was granted a reduction in its income tax rate under which it paid no income taxes from January 1, 2005 to December 31, 2006 and had had an income tax rate of 16.5% since January 1, 2007 which is a 50% reduction on the current effective income tax rate. This favorable 50% tax exemption treatment expired on December 31, 2009. There is no assurance that the preferential tax treatment in the PRC will remain unchanged and effective. Its tax liabilities will increase and its profits may accordingly decline if its reduced income tax rate is no longer applicable and/or the tax relief on investment in PRC is no longer available.
Additionally, as a company organized in the PRC, Maidisen is subject to the PRC Enterprise Income Tax Law (the "EIT Law"), which was enacted on March 16, 2007. Under the EIT Law, effective January 1, 2008, the PRC adopted a uniform tax rate of 25.0% for all enterprises (including foreign-invested enterprises) and cancelled several tax incentives enjoyed by foreign-invested enterprises. However, for foreign-invested enterprises established before the promulgation of the EIT Law, a five-year transition period is provided during which reduced rates will apply but gradually be phased out. As a high-tech enterprise and under the EIT Law, Maidisen enjoyed a two-year tax exemption for 2006 through 2007, and since 2008, it has received the statutory rate of 15%. Since the PRC government has not announced implementation measures for the transitional policy with regards to such preferential tax rates, we cannot reasonably estimate the financial impact of the new tax law to Maidisen at this time. Further, any future increase in the enterprise income tax rate applicable to it or other adverse tax treatments, such as the discontinuation of preferential tax treatments for high and new technology enterprises, would have a material adverse effect on its results of operations and financial condition.
Risks Related to Doing Business in the PRC
Changes in the policies of the PRC government could have a significant impact upon the business we may be able to conduct in the PRC and the profitability of such business.
Our business operations may be adversely affected by the current and future political environment in the PRC. The PRC has operated as a socialist state since the mid-1900s and is controlled by the PRC’s Communist Party. The Chinese government exerts substantial influence and control over the manner in which we and it must conduct our business activities. The PRC has only permitted provincial and local economic autonomy and private economic activities since 1988. The government of the PRC has exercised and continues to exercise substantial control over virtually every sector of the Chinese economy, particularly the pharmaceutical industry, through regulation and state ownership. Our ability to operate in the PRC may be adversely affected by changes in Chinese laws and regulations, including those relating to taxation, import and export tariffs, raw materials, environmental regulations, land use rights, property and other matters. Under current leadership, the government of the PRC has been pursuing economic reform policies that encourage private economic activity and greater economic decentralization. There is no assurance, however, that the government of the PRC will continue to pursue these policies, or that it will not significantly alter these policies from time to time without notice.
20
The PRC's economy is in a transition from a planned economy to a market oriented economy subject to five-year and annual plans adopted by the government that set national economic development goals. Policies of the PRC government can have significant effects on the economic conditions of the PRC. The PRC government has confirmed that economic development will follow the model of a market economy. Under this direction, we believe that the PRC will continue to strengthen its economic and trading relationships with foreign countries and business development in the PRC will follow market forces. While we believe that this trend will continue, there can be no assurance that this will be the case.
A change in policies by the PRC government could adversely affect our interests by, among other factors: changes in laws, regulations or the interpretation thereof, confiscatory taxation, restrictions on currency conversion, imports or sources of supplies, or the expropriation or nationalization of private enterprises. Although the PRC government has been pursuing economic reform policies for more than two decades, there is no assurance that the government will continue to pursue such policies or that such policies may not be significantly altered, especially in the event of a change in leadership, social or political disruption, or other circumstances affecting the PRC's political, economic and social life.
The PRC laws and regulations governing our current business operations are sometimes vague and uncertain. Any changes in such PRC laws and regulations may harm its business.
The PRC laws and regulations governing our current business operations are sometimes vague and uncertain. The PRC’s legal system is a civil law system based on written statutes, in which system decided legal cases have little value as precedents unlike the common law system prevalent in the United States. There are substantial uncertainties regarding the interpretation and application of PRC laws and regulations, including but not limited to the laws and regulations governing our business, or the enforcement and performance of our arrangements with customers in the event of the imposition of statutory liens, death, bankruptcy and criminal proceedings. The Chinese government has been developing a comprehensive system of commercial laws, and considerable progress has been made in introducing laws and regulations dealing with economic matters such as foreign investment, corporate organization and governance, commerce, taxation and trade. However, because these laws and regulations are relatively new, and because of the limited volume of published cases and judicial interpretation and their lack of force as precedents, interpretation and enforcement of these laws and regulations involve significant uncertainties. New laws and regulations that affect existing and proposed future businesses may also be applied retroactively. We are considered a foreign persons or foreign funded enterprises under PRC laws, and as a result, we are required to comply with PRC laws and regulations. We cannot predict what effect the interpretation of existing or new PRC laws or regulations may have on its businesses. If the relevant authorities find that we are in violation of PRC laws or regulations, they would have broad discretion in dealing with such a violation, including, without limitation:
|
·
|
levying fines;
|
|
·
|
revoking Maidisen’s business and other licenses;
|
|
·
|
requiring that we restructure our ownership or operations; and
|
|
·
|
requiring that we discontinue any portion or all of our business.
|
Among the material laws that we are subject to are the Medicine Management Law, governing the management of pharmaceutical companies, medicine production procedure, packaging, prices, Advertisement Law of the People’s Republic of China and Rules of Medicine Advertisements Management from State Admission for Industry and Commerce, Regulations on Control of Advertisements from State Council, governing rules on advertising, Standardization of the Management on the Quality of Medicine Production issued by SFDA, providing standards for staff, plants, equipment, materials, environment, production management, products laws, and the Price Law of The People’s Republic of China, Measurement Law of The People’s Republic of China, Tax Law, Environmental Protection Law, Contract Law, Patent Law, Accounting Laws and Labor Law.
21
A slowdown, inflation or other adverse developments in the PRC economy may harm our customers and the demand for our services and products.
All of our operations are conducted in the PRC and all of our revenue is generated from sales in the PRC. Although the PRC economy has grown significantly in recent years, we cannot assure you that this growth will continue. A slowdown in overall economic growth, an economic downturn, a recession or other adverse economic developments in the PRC could significantly reduce the demand for our products and harm our business.
While the PRC economy has experienced rapid growth, such growth has been uneven among various sectors of the economy and in different geographical areas of the country. Rapid economic growth could lead to growth in the money supply and rising inflation. If prices for our products rise at a rate that is insufficient to compensate for the rise in the costs of supplies, it may harm our profitability. In order to control inflation in the past, the PRC government has imposed controls on bank credit, limits on loans for fixed assets and restrictions on state bank lending. Such an austere policy can lead to a slowing of economic growth. In October 2004, the People's Bank of China, the PRC's central bank, raised interest rates for the first time in nearly a decade and indicated in a statement that the measure was prompted by inflationary concerns in the Chinese economy. Repeated rises in interest rates by the central bank would likely slow economic activity in the PRC which could, in turn, materially increase its costs and also reduce demand for its products.
Governmental control of currency conversion may affect the value of your investment.
The PRC government imposes controls on the convertibility of Renminbi into foreign currencies and, in certain cases, the remittance of currency out of the PRC. We receive substantially all of our revenue in Renminbi, which is currently not a freely convertible currency. Shortages in the availability of foreign currency may restrict our ability to remit sufficient foreign currency to pay dividends, or otherwise satisfy foreign currency dominated obligations. Under existing PRC foreign exchange regulations, payments of current account items, including profit distributions, interest payments and expenditures from the transaction, can be made in foreign currencies without prior approval from the PRC State Administration of Foreign Exchange by complying with certain procedural requirements. However, approval from appropriate governmental authorities is required where Renminbi is to be converted into foreign currency and remitted out of the PRC to pay capital expenses such as the repayment of bank loans denominated in foreign currencies.
The PRC government may also in the future restrict access to foreign currencies for current account transactions. If the foreign exchange control system prevents us from obtaining sufficient foreign currency to satisfy our currency demands, we may not be able to pay certain of our expenses as they come due.
The fluctuation of the Renminbi may harm your investment.
The value of the Renminbi against the U.S. dollar and other currencies may fluctuate and is affected by, among other things, changes in the PRC's political and economic conditions. According to the currency website xe.com, as of December 31, 2009, $1 = 6.828 Renminbi. As we rely entirely on revenue earned in the PRC, any significant revaluation of the Renminbi may materially and adversely affect our cash flows, revenue and financial condition. For example, to the extent that we need to convert U.S. dollars we receive from an offering of our securities into Renminbi for Maidisen’s operations, appreciation of the Renminbi against the U.S. dollar would diminish the value of the proceeds of the offering and this could harm Maidisen’s business, financial condition and results of operations because it would reduce the proceeds available to us for capital investment in proportion to the appreciation of the Renminbi. Thus if we raise 1,000,000 dollars and the Renminbi appreciates against the U.S. dollar by 15%, then the proceeds will be worth only RMB 5,803,800 as opposed to RMB 6,828,000 prior to the appreciation. Conversely, if we decide to convert our Renminbi into U.S. dollars for the purpose of making payments for dividends on our common shares or for other business purposes and the U.S. dollar appreciates against the Renminbi; the U.S. dollar equivalent of the Renminbi we convert would be reduced in proportion to the amount the U.S. dollar appreciates. In addition, the depreciation of significant RMB denominated assets could result in a charge to our income statement and a reduction in the dollar value of these assets. Thus if Maidisen has RMB 1,000,000 in assets and Renminbi is depreciated against the U.S. dollar by 15%, then the assets will be valued at $124,487 as opposed to $146,456 prior to the depreciation.
On July 21, 2005, the PRC government changed its decade-old policy of pegging the value of the Renminbi to the U.S. dollar. Under the new policy, the Renminbi is permitted to fluctuate within a narrow and managed band against a basket of certain foreign currencies. This change in policy has resulted in an approximately 17.5% appreciation of the Renminbi against the U.S. dollar as of December 31, 2009. While the international reaction to the Renminbi revaluation has generally been positive, there remains significant international pressure on the PRC government to adopt an even more flexible currency policy, which could result in a further and more significant appreciation of the Renminbi against the U.S. dollar.
22
PRC State Administration of Foreign Exchange ("SAFE") regulations regarding offshore financing activities by PRC residents which may increase the administrative burden we face. The failure by our shareholders who are PRC residents to make any required applications and filings pursuant to such regulations may prevent us from being able to distribute profits and could expose us and our PRC resident shareholders to liability under PRC law.
SAFE issued a public notice ("SAFE #75") effective from November 1, 2005, which requires registration with SAFE by the PRC resident shareholders of any foreign holding company of a PRC entity. Without registration, the PRC entity cannot remit any of its profits out of the PRC as dividends or otherwise.
In October 2005, SAFE issued a public notice, the Notice on Relevant Issues in the Foreign Exchange Control over Financing and Return Investment Through Special Purpose Companies by Residents Inside China, or the SAFE notice, which requires PRC residents, including both legal persons and natural persons, to register with the competent local SAFE branch before establishing or controlling any company outside of the PRC, referred to as an "offshore special purpose company," for the purpose of overseas equity financing involving onshore assets or equity interests held by them. In addition, any PRC resident that is the shareholder of an offshore special purpose company is required to amend its SAFE registration with the local SAFE branch with respect to that offshore special purpose company in connection with any increase or decrease of capital, transfer of shares, merger, division, equity investment or creation of any security interest over any assets located in the PRC. Moreover, if the offshore special purpose company was established and owned the onshore assets or equity interests before the implementation date of the SAFE notice, a retroactive SAFE registration is required to have been completed before March 31, 2006. If any PRC shareholder of any offshore special purpose company fails to make the required SAFE registration and amendment, the PRC subsidiaries of that offshore special purpose company may be prohibited from distributing their profits and the proceeds from any reduction in capital, share transfer or liquidation to the offshore special purpose company. Moreover, failure to comply with the SAFE registration and amendment requirements described above could result in liability under PRC laws for evasion of applicable foreign exchange restrictions.
It is unclear whether our other PRC resident shareholders must make disclosure to SAFE. While our PRC counsel has advised us that only PRC resident shareholders who receive ownership of the foreign holding company in exchange for ownership in the PRC operating company are subject to SAFE #75, there can be no assurance that SAFE will not require our other PRC resident shareholders to register and make the applicable disclosure. In addition, SAFE #75 requires that any monies remitted to PRC residents outside of the PRC be returned within 180 days; however, there is no indication of what the penalty will be for failure to comply or if shareholder non-compliance will be considered to be a violation of SAFE #75 by us or otherwise affect us.
In the event that the proper procedures are not followed under SAFE #75, Slovan could lose the ability to remit monies outside of the PRC and would therefore be unable to pay dividends or make other distributions. Our PRC resident shareholders could be subject to fines, other sanctions and even criminal liabilities under the PRC Foreign Exchange Administrative Regulations promulgated January 29, 1996, as amended.
The PRC's legal and judicial system may not adequately protect our business and operations and the rights of foreign investors.
The PRC legal and judicial system may negatively impact foreign investors. In 1982, the National People's Congress amended the Constitution of the PRC to authorize foreign investment and guarantee the "lawful rights and interests" of foreign investors in the PRC. However, the PRC's system of laws is not yet comprehensive. The legal and judicial systems in the PRC are still rudimentary, and enforcement of existing laws is inconsistent. Many judges in the PRC lack the depth of legal training and experience that would be expected of a judge in a more developed country. Because the PRC judiciary is relatively inexperienced in enforcing the laws that do exist, anticipation of judicial decision-making is more uncertain than would be expected in a more developed country. It may be impossible to obtain swift and equitable enforcement of laws that do exist, or to obtain enforcement of the judgment of one court by a court of another jurisdiction. The PRC's legal system is based on the civil law regime, that is, it is based on written statutes; a decision by one judge does not set a legal precedent that is required to be followed by judges in other cases. In addition, the interpretation of Chinese laws may be varied to reflect domestic political changes.
23
The promulgation of new laws, changes to existing laws and the pre-emption of local regulations by national laws may adversely affect foreign investors. However, the trend of legislation over the last 20 years has significantly enhanced the protection of foreign investment and allowed for more control by foreign parties of their investments in Chinese enterprises. There can be no assurance that a change in leadership, social or political disruption, or unforeseen circumstances affecting the PRC's political, economic or social life, will not affect the PRC government's ability to continue to support and pursue these reforms. Such a shift could have a material adverse effect on our business and prospects.
The practical effect of the PRC legal system on our business operations in the PRC can be viewed from two separate but intertwined considerations. First, as a matter of substantive law, the Foreign Invested Enterprise laws provide significant protection from government interference. In addition, these laws guarantee the full enjoyment of the benefits of corporate Articles and contracts to Foreign Invested Enterprise participants. These laws, however, do impose standards concerning corporate formation and governance, which are qualitatively different from the general corporation laws of the United States. Similarly, the PRC accounting laws mandate accounting practices, which are not consistent with U.S. generally accepted accounting principles. PRC's accounting laws require that an annual "statutory audit" be performed in accordance with PRC accounting standards and that the books of account of Foreign Invested Enterprises are maintained in accordance with Chinese accounting laws. Article 14 of the People's Republic of China Wholly Foreign-Owned Enterprise Law requires a wholly foreign-owned enterprise to submit certain periodic fiscal reports and statements to designated financial and tax authorities, at the risk of business license revocation. While the enforcement of substantive rights may appear less clear than United States procedures, the Foreign Invested Enterprises and Wholly Foreign-Owned Enterprises are Chinese registered companies, which enjoy the same status as other Chinese registered companies in business-to-business dispute resolution. Any award rendered by an arbitration tribunal is enforceable in accordance with the United Nations Convention on the Recognition and Enforcement of Foreign Arbitral Awards (1958). Therefore, as a practical matter, although no assurances can be given, the Chinese legal infrastructure, while different in operation from its United States counterpart, should not present any significant impediment to the operation of Foreign Invested Enterprises
Any recurrence of severe acute respiratory syndrome, or SARS, or another widespread public health problem, could harm our operations.
A renewed outbreak of SARS or another widespread public health problem (such as bird flu) in the PRC, where all of our revenue is derived, could significantly harm our operations. Our operations may be impacted by a number of health-related factors, including quarantines or closures of some of our offices that would adversely disrupt our operations. Any of the foregoing events or other unforeseen consequences of public health problems could significantly harm our operations.
Because our principal assets are located outside of the United States and most of our directors and all of our officers reside outside of the United States, it may be difficult for you to enforce your rights based on U.S. Federal Securities Laws against us and our officers or to enforce U.S. Court Judgments against us or them in the PRC
Most of our directors and all of our officers reside outside of the United States. In addition, our operating company is located in the PRC and substantially all of our assets are located outside of the United States. It may therefore be difficult for investors in the United States to enforce their legal rights based on the civil liability provisions of the U.S. Federal securities laws against us in the courts of either the U.S. or the PRC and, even if civil judgments are obtained in U.S. courts, to enforce such judgments in PRC courts. Further, it is unclear if extradition treaties now in effect between the United States and the PRC would permit effective enforcement against us or our officers and directors of criminal penalties, under the U.S. Federal securities laws or otherwise.
The relative lack of public company experience of our management team may put us at a competitive disadvantage.
Our management team lacks public company experience, which could impair our ability to comply with legal and regulatory requirements such as those imposed by Sarbanes-Oxley Act of 2002. The individuals who now constitute our senior management have never had responsibility for managing a publicly traded company. Such responsibilities include complying with federal securities laws and making required disclosures on a timely basis. Our senior management may not be able to implement programs and policies in an effective and timely manner that adequately responds to such increased legal, regulatory compliance and reporting requirements. Our failure to comply with all applicable requirements could lead to the imposition of fines and penalties and distract our management from attending to the growth of our business.
24
Risks Related to our Common Stock
Our officers and directors control us through their positions and stock ownership and their interests may differ from other stockholders.
As of May 10, 2010, there were 2,181,818 shares of our common stock issued and outstanding. Our officers and directors own approximately 36.9% of our common stock. As a result, they are able to influence the outcome of stockholder votes on various matters, including the election of directors and extraordinary corporate transactions including business combinations. Yet our officers’ and directors’ interests may differ from those of other stockholders. Furthermore, ownership of 36.9% of our common stock by our officers and directors reduces the public float and liquidity, and may affect the market price, of our common stock when and if our common stock becomes eligible to trade on the OTCBB.
We are not likely to pay cash dividends in the foreseeable future.
We intend to retain any future earnings for use in the operation and expansion of our business. We do not expect to pay any cash dividends in the foreseeable future but will review this policy as circumstances dictate. Should we decide in the future to do so, as a holding company, our ability to pay dividends and meet other obligations depends upon the receipt of dividends or other payments from our operating subsidiaries. In addition, our operating subsidiaries, from time to time, may be subject to restrictions on their ability to make distributions to us, including restrictions on the conversion of local currency into U.S. dollars or other hard currency and other regulatory restrictions.
There is currently no trading market for our common stock.
Our common stock is not quoted on any exchange or inter-dealer quotation system. There is no trading market for our common stock and our common stock may never be included for trading on any stock exchange or through any quotation system (including, without limitation, the NASDAQ Stock Market and the FINRA over-the-counter Bulletin Board). You may not be able to sell your shares due to the absence of a trading market.
Our common stock may be also subject to the "penny stock" rules to the extent that its price is below $5.00, which rules require delivery of a schedule explaining the penny stock market and the associated risks before any sale. These requirements may further limit your ability to sell your shares.
Our common stock is illiquid and subject to price volatility unrelated to our operations.
If a market for our common stock does develop, its market price could fluctuate substantially due to a variety of factors, including market perception of our ability to achieve our planned growth, quarterly operating results of other companies in the same industry, trading volume in our common stock, changes in general conditions in the economy and the financial markets or other developments affecting us or our competitors. In addition, the stock market itself is subject to extreme price and volume fluctuations. This volatility has had a significant effect on the market price of securities issued by many companies for reasons unrelated to their operating performance and could have the same effect on our common stock.
Investors may have difficulty liquidating their investment because our common stock is subject to the “penny stock" rules, which require delivery of a schedule explaining the penny stock market and the associated risks before any sale.
Our common stock may be subject to regulations prescribed by the SEC relating to "penny stocks." The SEC has adopted regulations that generally define a penny stock to be any equity security that has a market price (as defined in such regulations) of less than $5 per share, subject to certain exceptions. These regulations impose additional sales practice requirements on broker-dealers who sell penny stocks to persons other than established customers and accredited investors (generally institutions with assets in excess of $5,000,000 and individuals with a net worth in excess of $1,000,000 or annual income exceeding $200,000 (individually) or $300,000 (jointly with their spouse). For transactions covered by these rules, the broker-dealer must make a special suitability determination for the purchase of these securities and have received the purchaser's prior written consent to the transaction. Additionally, for any transaction, other than exempt transactions, involving a penny stock, the rules require the delivery, prior to the transaction, of a risk disclosure document mandated by the SEC relating to the penny stock market. The broker-dealer also must disclose the commissions payable to both the broker-dealer and the registered representative, current quotations for the securities and, if the broker-dealer is the sole market-maker, the broker-dealer must disclose this fact and the broker-dealer's presumed control over the market. Finally, monthly statements must be sent disclosing recent price information for the penny stock held in the account and information on the limited market in penny stocks.
25
Legal remedies, which may be available to the investor, are as follows:
|
·
|
If penny stocks are sold in violation of the investor's rights listed above, or other federal or state securities laws, the investor may be able to cancel his purchase and get his money back;
|
|
·
|
If the stocks are sold in a fraudulent manner, the investor may be able to sue the persons and firms that caused the fraud for damages;
|
|
·
|
If the investor has signed an arbitration agreement, however, s/he may have to pursue a claim through arbitration.
|
If the person purchasing the securities is someone other than an accredited investor or an established customer of the broker-dealer, the broker-dealer must also approve the potential customer's account by obtaining information concerning the customer's financial situation, investment experience and investment objectives. The broker-dealer must also make a determination whether the transaction is suitable for the customer and whether the customer has sufficient knowledge and experience in financial matters to be reasonably expected to be capable of evaluating the risk of transactions in such securities. Accordingly, the SEC's rules may limit the number of potential purchasers of the shares of our common stock and stockholders may have difficulty selling their securities.
A large number of shares may be eligible for future sale and may depress our stock price.
We may be required, under terms of future financing arrangements, to offer a large number of common shares to the public, or to register for sale by future private investors a large number of shares sold in private sales to them.
Sales of substantial amounts of common stock, or a perception that such sales could occur, and the existence of options or warrants to purchase shares of common stock at prices that may be below the then-current market price of our common stock, could adversely affect the market price of our common stock and could impair our ability to raise capital through the sale of our equity securities, either of which would decrease the value of any earlier investment in our common stock.
MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following Management’s Discussion and Analysis of Financial Condition and Results of Operations (“MD&A”) is intended to help the reader understand our results of operations and financial condition.
Our discussion and analysis of our financial condition and results of operations are based upon Dong Ke audited consolidated financial statements for the years’ ended December 31, 2009 and 2008 which have been prepared in accordance with accounting principles generally accepted in the United States. Through various contractual arrangements completed on January 27, 2010, Slovan has a controlling interest in Maidisen and therefore we are required to consolidate Slovan and Maidisen's financial statements and ultimately consolidate them with the financial statements of Dong Ke. The preparation of financial statements in conformity with accounting principles generally accepted in the United States requires us to make estimates and assumptions that affect the reported amounts of assets and liabilities, disclosure of any contingent liabilities at the financial statement date and reported amounts of revenue and expenses during the reporting period. On an on-going basis we review our estimates and assumptions. Our estimates are based on our historical experience and other assumptions that we believe to be reasonable under the circumstances. Actual results are likely to differ from those estimates under different assumptions or conditions. Our critical accounting policies, the policies we believe are most important to the presentation of our financial statements and require the most difficult, subjective and complex judgments are outlined below in “Critical Accounting Policies.”
26
FORWARD-LOOKING STATEMENTS
Certain statements made in this report may constitute “forward-looking statements on our current expectations and projections about future events.” These forward-looking statements involve known or unknown risks, uncertainties and other factors that may cause our actual results, performance, or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements. In some cases you can identify forward-looking statements by terminology such as “may,” “should,” “potential,” “continue,” “expects,” “anticipates,” “intends,” “plans,” “believes,” “estimates,” and similar expressions. These statements are based on our current beliefs, expectations, and assumptions and are subject to a number of risks and uncertainties. Although we believe that the expectations reflected in the forward-looking statements are reasonable, we cannot guarantee future results, levels of activity, performance or achievements. These forward-looking statements are made as of the date of this report, and we assume no obligation to update these forward-looking statements whether as a result of new information, future events, or otherwise, other than as required by law. In light of these assumptions, risks, and uncertainties, the forward-looking events discussed in this report might not occur and actual results and events may vary significantly from those discussed in the forward-looking statements.
Overview
We are a recently formed Delaware corporation that through our wholly owned subsidiary and our variable interest entity (“VIE”) Maidisen is primarily engaged in the research, development, manufacture, packaging, marketing and distribution of pharmaceutical and medical products in China for human use for a variety of diseases and conditions. All of our operations are conducted in the People’s Republic of China where our manufacturing facility is located. Through various contractual arrangements completed on January 27, 2010, Slovan has a controlling interest in Maidisen and therefore we are required to consolidate Slovan's and Maidisen’s financial statements and ultimately consolidate with the financial statements of Dong Ke.
Our current operations involve the manufacture and sale of 36 pharmaceutical products in the form of, capsules, tablets, granules, semisolid ointment, power, ointment, paste ointment. We currently focus on varying diseases relating to respiratory system, digestive system, cardio-cerebral vascular system, antineoplastic, bone disease-modifying antirheumatic, gynecological, refill nutrition and others. Our products are sold to distributors who distribute our products to the public as well as through representatives that we employ. Our focus is on the development and sale of pharmaceutical products and over the counter products based on traditional Chinese medicines designed to address varying diseases and conditions. During the past few years 230 representative offices have been found nationwide and the business extended to 26 provinces.
Critical Accounting Policies
Management believes that the critical accounting policies and estimates discussed below involve the most complex management judgments due to the sensitivity of the methods and assumptions necessary in determining the related asset, liability, revenue and expense amounts. Specific risks associated with these critical accounting policies are discussed throughout this MD&A, where such policies have a material effect on reported and expected financial results. For a detailed discussion of the application of these and other accounting policies, refer to the individual Notes to the Financial Statements for the years ended December 31, 2008 and 2009.
Principles of Consolidation
The consolidated financial statements include the accounts of the Company, Dong Ke, Slovan and Maidisen, its VIE for which the Company is the primary beneficiary. All inter-company accounts and transactions have been eliminated in consolidation. The Company has adopted FIN 46R which requires a VIE to be consolidated by a company if that company is subject to a majority of the risk of loss for the VIE or is entitled to receive a majority of the VIE’s residual returns.
In determining Maidisen is the VIE of Slovan, the Company considered the following indicators, among others:
|
●
|
Slovan has the full right to control and administrate the financial affairs and daily operation of Maidisen and has the right to manage and control all assets of Maidisen. The equity holders of Maidisen as a group have no right to make any decision about Maidisen activities without the consent of Slovan.
|
|
●
|
Slovan was assigned all voting rights of Maidisen and has the right to appoint all directors and senior management personnel of Maidisen. The equity holders of Maidisen possess no substantive voting rights.
|
|
●
|
Slovan should be paid a management fee equal to 100% of the earnings before tax of Maidisen and should assume all operation risks of Maidisen and bear all losses of Maidisen. Therefore, Slovan is the primary beneficiary of Maidisen.
|
27
Maidisen is wholly owned by the majority shareholders of the Company. Under various contractual agreements, the shareholders of Maidisen are required to transfer their ownership to the Company’s subsidiary in China when permitted by PRC laws and regulations or to designees of the Company at any time when the Company considers it is necessary to acquire Maidisen. In addition, the shareholders of Maidisen have pledged their shares in Maidisen as collateral to secure these contractual arrangements.
Foreign Currency
The Company’s reporting currency is the U.S. dollar. The Company’s operation in China uses Chinese Renminbi (CNY) as its functional currency. The financial statements of the subsidiary are translated into U.S. Dollars (USD) in accordance with Statement of Financial Accounting Standards (SFAS) No. 52, “Foreign Currency Translation.” According to the Statement, all assets and liabilities were translated at the current exchange rate, stockholders’ equity are translated at the historical rates and income statement items are translated at the average exchange rate for the period. The resulting translation adjustments are reported under other comprehensive income in accordance with SFAS No. 130, “Reporting Comprehensive Income,” as a component of shareholders’ equity. Foreign exchange transaction gains and losses are reflected in the income statement. For the year ended December 31, 2009 and 2008, the foreign currency translation adjustments to the Company’s comprehensive income were $11,067 and ($47,178), respectively.
Accounts Receivable
The Company maintains reserves for potential credit losses on accounts receivable. Management reviews the composition of accounts receivable and analyzes historical bad debts, customer concentrations, customer credit worthiness, current economic trends and changes in customer payment patterns to evaluate the adequacy of these reserves. Reserve balances at March 31, 2010 and December 31, 2009 were $0.
Allowance for Doubtful Accounts
We do not currently maintain allowances for doubtful accounts for estimated losses resulting from the inability of our customers to make required payments. The determination of whether to have an allowance for doubtful accounts is based on management’s best estimate of the likelihood of potential loss, taking into account such factors as the financial condition and payment history of major customers. Management evaluates the collectability of the receivables at least quarterly and has determined that at December 31, 2008 and 2009 that all accounts receivable were collectible. If the financial condition of the customers were to deteriorate, resulting in an impairment of their ability to make payments an allowance may be required. The differences could be material and could significantly impact cash flows from operating activities.
The following table sets out the aging of our accounts receivable for each balance sheet period presented.
|
Accounts Receivable Aging
|
Total
|
1-30 days
|
31-60 days
|
61-90 days
|
91-180 days
|
181-365 days
|
> 365 days
|
|||||||||||||||||||||
|
As of December 31, 2009
|
$
|
3,460,649
|
$
|
902,455
|
$
|
549,471
|
$
|
478,124
|
$
|
767,180
|
$ |
763,419
|
––
|
|||||||||||||||
|
As of December 31, 2008
|
$
|
4,322,013
|
$
|
970,040
|
$
|
1,059,987
|
$
|
474,987
|
$
|
1,030,573
|
$
|
697,973
|
$
|
88,453
|
||||||||||||||
The following presents the days sales outstanding calculated based on sales and accounts receivables in RMB term for each income statement periods presented.
|
Year Ended
December 31,
|
||||||||
|
2009
|
2008
|
|||||||
|
Days sales outstanding
|
66
|
130
|
||||||
The number of days that sales were outstanding decreased to 66 days for the year ended December 31, 2009 from 130 days for the same period last year. This decrease was due to better enforcement of the policy of debt collection from customers within 90 days by the sale department.
28
Use of Estimates
The preparation of financial statements in conformity with generally accepted accounting principles requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates.
Recently Issued Accounting Standards
For a discussion of the adoption and potential impact of recently issued accounting standards refer to the Recent Accounting Pronouncements section of Note 2 “Summary of Significant Accounting Policies,” in the Notes to Interim and Annual Financial Statements.
Results of Operation for the years ended December 31, 2009 and 2008
Revenue
Revenue for the year ended December 31, 2009 was $18,943,917, an increase of 6,884,480 or approximately 57% as compared to revenue of $12,059,437 for year ended December 31, 2008. The increase was primarily the result of an increase in sales volume of our new products and the exploration of new sales markets, such as new hospitals. Distribution of these products had already penetrated into more than 1,600 hospitals, and the acesodyre paste has been sold to Korea and Japan through trading companies. The Company has established five sales departments according to different sales regions and product types. Every department works hard to analyze the product market of our competitive counterparts and the needs of the market, which make our marketing more specified and targeted.
Cost of Sales
Cost of sales as a percentage of net sales was approximately 56% for the year ended December 31, 2009 as compared to 56% for the year ended December 31, 2008. The cost of sales as a percentage of net sales remains at the same level for both years.
Selling, General and Administrative Expenses
Selling, general and administrative expenses increased approximately 15% from $3,092,660 for the year ended December 31, 2008 to $3,568,670 for the year ended December 31, 2009. This increase was mainly due to the increase in payroll expenses from approximately $0.54 million in 2008 to $0.73 million in 2009, an increase in entertainment expenses from approximately $0.09 million in 2008 to approximately $0.15 million in 2009, an increase in development expenses from approximately $0.01 million in 2008 to approximately $0.06 million in 2009, and an increase in business travelling expenses from approximately $0.05 million in 2008 to approximately $0.10 million in 2009, all resulting from on-going marketing expansion.
Other Expenses
Total other expense decreased approximately 69% from $41,989 for the year ended December 31, 2008 to $12,967 for the year ended December 31, 2009. The decrease was mainly due to the decrease in donations of $28,061. As a result of a catastrophic earthquake in Szechwan in May 2008, donation of $28,061 was made in 2008.
Net Income
For the year ended December 31, 2009 as compared to the year ended December 31, 2008 net income increased approximately 98% from $2,437,927 to $4,827,608. This increase was primarily a result of the increase in net sales.
29
Liquidity and Capital Resources
Year Ended December 31, 2009
As of December 31, 2009, we had cash and cash equivalents of approximately $1,058,157. We believe our existing cash and cash equivalents will be sufficient to maintain our operations at present level for at least the next twelve months. We plan to review acquisition opportunities as a strategy for further growth.
Maidisen’s primary sources and uses of cash for the year ended December 31, 2009 included income from continuing operations, adjusted for non-cash items of income and expense, borrowings and repayment of short term bank loans and a purchase of equipment. Net cash provided by operating activities from continuing operations was $2,386,785 for the year ended December 31, 2009.
Net cash used in operating activities for the year ended December 31, 2008 was $827,773. This was primarily due to the net income of $2,437,927, adjusted by non-cash related expenses including depreciation and amortization of $1,069,735, offset by a net decrease in working capital items reflected in the statement of cash flows. The net decrease in working capital items was mainly due to increase in accounts receivable, inventories, advance to suppliers and prepaid expenses and other receivable and decrease in customer and other deposits. The net decrease in working capital items was partially offset by the increase in accounts payable, accrued expenses and other payable.
Net cash used in investing activities for the year ended December 31, 2009, was $1,383,847, an increase of $1,240,407 or about 865% from the comparable period in 2008. This increase was primarily attributable to the additional purchases of property and equipment in 2009.
Net cash used in financing activities for the year ended December 31, 2009, was $439,264 representing amounts use to make certain short-term bank loan repayments and interest paid on short-term borrowings.
Net cash provided by financing activities for the year ended December 31, 2008, was $1,273,255 representing borrowing from short-term loans.
Contractual Obligations
To date, our business has been dependent upon short term bank loans. At December 31, 2009 we had total outstanding bank loans of $4,107,258, of which $1,320,190 represented short term debt. As of December 31, 2009 we had $1,320,190 outstanding under our loan from the China Merchant's Bank and $2,787,068 outstanding under our loans from the Xi'an City Commercial Bank. The following table sets forth Dong Ke’s bank loan obligations as of December 31, 2009:
|
12/31/2009
|
||||
|
China Merchant's Bank
|
||||
|
Terms of this loan call for interest 7% per annum,
with principal due November 23, 2010. Loan is secured by personal guarantee of Dongke Zhao and Jinkun Wei.
|
$
|
1,320,190
|
||
|
InXi'an City Commercial Bank, Zhonglou Branch
Term of this loan called for interest from 5.94% per annum,
with principal due July 22, 2011. Loan is secured by the land and building of the Yangling factory.
|
2,787,068
|
|||
|
Total
|
$
|
4,107,258
|
||
In April 2009, we obtained a loan from China Bank, Yangling Agriculture Zone in the amount of approximately $1,464,558 (RMB 10,000,000). As of March 25, 2010, this loan has been paid off and is no longer outstanding.
Our anticipated needs for the future are to be negotiated in accordance with manufacturing and operation needs, and market conditions of next year.
Valuation of Intangibles
From time to time, we acquire intangible assets that are beneficial to our product development processes. Management periodically evaluates the carrying value of intangibles, including the related amortization periods. In evaluating acquired intangible assets, management determines whether there has been impairment by comparing the anticipated undiscounted cash flows from the operation and eventual disposition of the product line with its carrying value. If the undiscounted cash flows are less than the carrying value, the amount of the impairment, if any, will be determined by comparing the carrying value of each intangible asset with its fair value. Fair value is generally based on either a discounted cash flows analysis or market analysis. Future operating income is based on various assumptions, including regulatory approvals, patents being granted, and the type and nature of competing products. If regulatory approvals or patents are not obtained or are substantially delayed, or other competing technologies are developed and obtain general market acceptance or market conditions otherwise change, our intangibles may have a substantially reduced value, which could be material.
30
In April 2008, the FASB issued FASB Staff Position (FSP) FAS 142-3, “Determination of the Useful Life of Intangible Assets,” which amends the factors that should be considered in developing renewal or extension assumptions used to determine the useful life of a recognized intangible asset under FASB Statement No. 142, “Goodwill and Other Intangible Assets.” This Staff Position is effective for financial statements issued for fiscal years beginning after December 15, 2008, and interim periods within those fiscal years. Early adoption is prohibited. The adoption of this new FSP did not have a material impact on the Company’s financial position, results of operations or cash flows.
The Fair Value Option for Financial Assets and Financial Liabilities
In February, 2007, FASB issued SFAS 159, ‘The Fair Value Option for Financial Assets and Financial Liabilities – Including an Amendment of FASB Statement No. 115.” This Statement permits entities to choose to measure many financial instruments and certain other items at fair value. This Statement is expected to expand the use of fair value measurement, which is consistent with the Board’s long-term measurement objectives for accounting for financial instruments. This Statement is effective as of the beginning of an entity’s first fiscal year that begins after November 15, 2007. The adoption of this new standard did not have a material impact on the Company’s financial position, results of operations or cash flows.
Income Taxes
The Company utilizes SFAS No. 109, "Accounting for Income Taxes," which requires the recognition of deferred tax assets and liabilities for the expected future tax consequences of events that have been included in the financial statements or tax returns. Under this method, deferred income taxes are recognized for the tax consequences in future years of differences between the tax bases of assets and liabilities and their financial reporting amounts at each period end based on enacted tax laws and statutory tax rates applicable to the periods in which the differences are expected to affect taxable income. Valuation allowances are established, when necessary, to reduce deferred tax assets to the amount expected to be realized.
In July 2006, the FASB issued Interpretation No. 48 (FIN 48), "Accounting for uncertainty in Income Taxes." FIN 48 clarifies the accounting for Income Taxes by prescribing the minimum recognition threshold a tax position is required to meet before being recognized in the financial statements. It also provides guidance on de-recognition, measurement, classification, interest and penalties, accounting in interim periods, disclosure and transition and clearly scopes income taxes out of SFAS 5, "Accounting for Contingencies." FIN 48 is effective for fiscal years beginning after December 15, 2006. As a result of implementing FIN 48, there have been no adjustments to the Company's financial statements.
Statutory Reserve
In accordance with the laws and regulations of the PRC, a wholly-owned Foreign Invested Enterprises income, after the payment of the PRC income taxes, shall be allocated to the statutory surplus reserves and statutory public welfare fund. Prior to January 1, 2006 the proportion of allocation for reserve was 10 percent of the profit after tax to the surplus reserve fund and additional 5-10 percent to the public affair fund. The statutory public welfare fund reserve is limited to an amount equals to 50 percent of the registered capital. Effective January 1, 2006, there is now only one fund requirement. The reserve is 10 percent of income after tax, not to exceed 50 percent of registered capital.
Statutory Reserve funds are restricted for set off against losses, expansion of production and operation or increase in register capital of the respective company. Statutory public welfare fund is restricted to the capital expenditures for the collective welfare of employees. These reserves are not transferable to the Company in the form of cash dividends, loans or advances. These reserves are therefore not available for distribution except in liquidation. As of December 31, 2009 and December 31, 2008, the Company had allocated $2,448,456 and $275,233, respectively, to these non-distributable reserve funds.
31
Inflation
Inflation in recent years has affected the business results of the Company. First of all, on the global economic expansion, supply has been restricted and coupled with the fact that USA has adopted a relaxed currency policy etc., these increase inflation risks. Secondly, GNP increases in China also elevates consumption ability and production cost, prices increase as a natural tendency. Finally, as a result of the macro economic trend, prices increase, the Company’s procurement prices are also affected resulting in increase in cost of sales.
The Company operates in China and as such, the Company’s business activities, financial position and operational results will be affected by PRC politics, economic and legal environments and also affected by the overall economic situation of China. The business of the Company may be affected by the relevant laws, regulations, anti-inflation measures, currency conversion and overseas remittance and exchange rates issues etc that are related to China politics.
Off-Balance Sheet Commitments and Arrangements
We do not have any financial undertaking or any other guarantee responsible for third party obligations and commitments. We have not entered into any derivative products contracts having the Company’s equity linked or that have not been reflected in the consolidated Pro Forma financial statements. Furthermore, in passing our assets to those providing credits, clearing or providing market risks support Bodies, we have not reserved any rights or undefined rights on the assets passing. The Company does not have any benefits from Bodies providing finance, clearing or market risks or credit support or with Bodies we engage in leasing, hedging or research and development services.
Recent Accounting Pronouncements
In May 2009, the FASB issued accounting guidance for “Subsequent Events”, which is included in ASC Topic 855, Subsequent Events. ASC Topic 855 established principles and requirements for evaluating and reporting subsequent events and distinguishes which subsequent events should be recognized in the financial statements versus which subsequent events should be disclosed in the financial statements. ASC Topic 855 also required disclosure of the date through which subsequent events are evaluated by management. ASC Topic 855 was effective for interim periods ending after June 15, 2009 and applies prospectively. Because ASC Topic 855 impacted the disclosure requirements, and not the accounting treatment for subsequent events, the adoption of ASC Topic 855 did not impact our results of operations or financial condition.
Effective July 1, 2009, the Company adopted the Financial Accounting Standards Board (“FASB”) Accounting Standards Codification (“ASC”) 105-10, Generally Accepted Accounting Principles – Overall (“ASC 105-10”). ASC 105-10 establishes the FASB Accounting Standards Codification (the “Codification”) as the source of authoritative accounting principles recognized by the FASB to be applied by nongovernmental entities in the preparation of financial statements in conformity with U.S. GAAP. Rules and interpretive releases of the SEC under authority of federal securities laws are also sources of authoritative U.S. GAAP for SEC registrants. All guidance contained in the Codification carries an equal level of authority. The Codification superseded all existing non-SEC accounting and reporting standards. All other non-grandfathered, non-SEC accounting literature not included in the Codification is non-authoritative. The FASB will not issue new standards in the form of Statements, FASB Staff Positions or Emerging Issues Task Force Abstracts. Instead, it will issue Accounting Standards Updates (“ASUs”). The
FASB will not consider ASUs as authoritative in their own right. ASUs will serve only to update the Codification, provide background information about the guidance and provide the bases for conclusions on the change(s) in the Codification. References made to FASB guidance throughout this document have been updated for the Codification.
In August 2009, the FASB issued, Measuring Liabilities at Fair Value, which provides additional guidance on how companies should measure liabilities at fair value under ASC 820. The ASU clarifies that the quoted price for an identical liability should be used. However, if such information is not available, an entity may use, the quoted price of an identical liability when traded as an asset, quoted prices for similar liabilities or similar liabilities traded as assets, or another valuation technique (such as the market or income approach). The ASU also indicates that the fair value of a liability is not adjusted to reflect the impact of contractual restrictions that prevent its transfer and indicates circumstances in which quoted prices for an identical liability or quoted price for an identical liability traded as an asset may be considered level 1 fair value measurements. This ASU is effective October 1, 2009. The Company is currently evaluating the impact of this standard, but would not expect it to have an impact on the Company’s consolidated results of operations or financial condition.
32
In September 2009, the FASB issued, Investments in Certain Entities That Calculate Net Asset Value per Share (or Its Equivalent), that amends ASC 820 to provide guidance on measuring the fair value of certain
alternative investments such as hedge funds, private equity funds and venture capital funds. The ASU indicates that, under certain circumstance, the fair value of such investments may be determined using net asset value (NAV) as a practical expedient, unless it is probable the investment will be sold at something other than NAV. In those situations, the practical expedient cannot be used and disclosure of the remaining actions necessary to complete the sale is required. The ASU also requires additional disclosures of the attributes of all investments within the scope of the new guidance, regardless of whether an entity used the practical expedient to measure the fair value of any of its investments. This ASU is effective October 1,
2009. The Company is currently evaluating the impact of this standard, but would not expect it to have an impact on the Company’s consolidated results of operations or financial condition.
In October 2009, the FASB issued, Multiple-Deliverable Revenue Arrangements—a consensus of the FASB Emerging Issues Task Force, that provides amendments to the criteria for separating consideration in multiple-deliverable arrangements. As a result of these amendments, multiple-deliverable revenue arrangements will be separated in more circumstances than under existing U.S. GAAP. The ASU does this by establishing a selling price hierarchy for determining the selling price of a deliverable. The selling price used for each deliverable will be based on vendor-specific objective evidence if available, third-party evidence if vendor-specific objective evidence is not available, or estimated selling price if neither vendor-specific objective evidence nor third-party evidence is available. A vendor will be required to determine its best estimate of selling price in a manner that is consistent with that used to determine the price to sell the deliverable on a standalone basis. This ASU also eliminates the residual method of allocation and will require that arrangement consideration be allocated at the inception of the arrangement to all deliverables using the relative selling price method, which allocates any discount in the overall arrangement proportionally to each deliverable based on its relative selling price. Expanded disclosures of qualitative and quantitative information regarding application of the multiple-deliverable revenue arrangement guidance are also required under the ASU. The ASU does not apply to arrangements for which industry specific allocation and measurement guidance exists, such as long-term construction contracts and software transactions. This is effective beginning January 1, 2011. The Company is currently evaluating the impact of this standard on its consolidated results of operations and financial condition.
In October 2009, the FASB issued, Certain Revenue Arrangements That Include Software Elements—a consensus of the FASB Emerging Issues Task Force, that reduces the types of transactions that fall within the current scope of software revenue recognition guidance. Existing software revenue recognition guidance requires that its provisions be applied to an entire arrangement when the sale of any products or services containing or utilizing software when the software is considered more than incidental to the product or service. As a result of the amendments, many tangible products and services that rely on software will be accounted for under the multiple-element arrangements revenue recognition guidance rather than under the software revenue recognition guidance. Under the amendments, the following components would be excluded from the scope of software revenue recognition guidance: the tangible element of the product, software products bundled with tangible products where the software components and non-software components function together to deliver the product’s essential functionality, and undelivered components that relate to software that is essential to the tangible product’s functionality. The ASU also provides guidance on how to allocate transaction consideration when an arrangement contains both deliverables within the scope of software revenue guidance (software deliverables) and deliverables not within the scope of that guidance (non-software deliverables). This amendment is effective beginning January 1, 2011. The Company is currently evaluating the impact of this standard on its consolidated results of operations and financial condition.
Reports to Security Holders
We are subject to the reporting and other requirements of the Exchange Act and we intend to furnish our shareholders with annual reports containing financial statements audited by our independent auditors and to make available quarterly reports containing unaudited financial statements for each of the first three quarters of each year.
The public may read and copy any materials that we file with the SEC at the SEC's Public Reference Room at 100 F Street, NE, Washington, D.C. 20549. The public may obtain information on the operation of the Public Reference Room by calling the SEC at 1-800-SEC-0330. The SEC maintains an Internet site that contains reports, proxy and information statements, and other information regarding issuers that file electronically with the SEC. The address of that site is www.sec.gov.
33
PROPERTIES
Headquarters and Administration Offices
Our principal executive offices are located at Dongxing Building, 4th Floor, Xincheng Technology Zone, No.1 Xinke Road, Xi’an, the People’s Republic of China 710043. Our headquarters, which house our sales and management departments, occupies approximately 1600 square meters of space. It is leased free of charge from our Chief Executive Officer, Dongke Zhao for a period of five years, expiring December 31, 2011.
Production Plant
We manufacture and package our products at one factory, located in the City of Yangling in Shaanxi Province. Our facility is in compliance with Good Manufacturing Practices 2 (“GMP”) standards. All of the complete production lines at the facility meet international food and drug safety guidelines.
All land in the PRC is owned by the government and cannot be sold to any individual or entity. Instead, the government grants landholders a "land use right" after a purchase price for such "land use right" is paid to the government. The "land use right" allows the holder the right to use the land for a specified long-term period of time and enjoys all the incidents of ownership of the land. The following are the details regarding Maidisen’s land use rights with regard to the land that it uses in its business.
We have land use rights to the parcel of land on which our production base is located. The Yangling production base covers an area of 25,737 square meters, including a building area of 15,601 square meters. The land use rights for the parcel in Yangling expires in February 2049. The production facility located on this premises has two production lines for high-volume production and manufactures 38 pharmaceutical products with different specifications. This production facility has more than 50 machines and supporting parts for pharmaceutical production from domestic and foreign suppliers.
MANAGEMENT
In connection with the Share Exchange Agreement, we appointed five new directors to our board and hired two new officers. Furthermore, concurrent with the closing of the Exchange Agreement, You Ting Zhu, our former Chief Executive Officer and Chief Financial Officer resigned from his positions.
The following table sets forth the names, ages, and positions of our current executive officers and directors as of the Closing Date. Executive officers are elected annually by our Board of Directors. Each executive officer holds his office until he resigns, is removed by the Board, or his successor is elected and qualified. Directors are elected annually by our stockholders at the annual meeting. Each director holds his office until his successor is elected and qualified or his earlier resignation or removal.
The directors, officers and key employees of the Company are as follows:
|
Name
|
Age
|
Position
|
||
|
Dongke Zhao
|
56
|
Chief Executive Officer, President and Chairman
|
||
|
Yanhong Ren
|
35
|
Chief Financial Officer and
Director
|
||
|
Gengchang Wang
|
42
|
Director
|
||
|
Jinhuan Chang
|
34
|
Director
|
||
|
Michael Segal
|
67
|
Director
|
||
34
The business experience, principal occupations and employment of each of the above persons during at least the last five years are set forth below.
DONGKE ZHAO – Chairman, Chief Executive Officer and President. Mr. Zhao was appointed Chairman, Chief Executive Officer and President of Virtual Closet on May 10, 2010. Mr. Zhao has been serving as Chief Executive Officer, President and Chairman of Maidisen since 2004. Prior to Mr. Zhao’s employment with Maidisen, Mr. Zhao served as Director of Shaanxi Dongke Medical Technology Co., Ltd. from November 2002 to October 2006. From January 1999 to October 2002, Mr. Zhao served as the Director of Shaanxi Dongke Chinese Medicine Institution and from December 1994 to December 1998 Mr. Zhao served as a Director of Xianyang Health Products Institute. Mr. Zhao was employed as an Associate Professor of Pharmacology, Xi’an Medical University, Xi’an, Shaanxi from 1979 through 1988 and served as a Professor of Pharmacology, Xi’an Medical University, Xi’an, Shaanxi from 1988 through 2004. At the time of his appointment in 1988 he was only 33 years old and became the youngest Professor of Pharmacology academia in China. Mr. Zhao graduated from Xi’an Medical University with a Bachelor Degree of Pharmacology and a Masters Degree in Pharmacology.
YANHONG REN – Chief Financial Officer, Director. Ms. Ren was appointed Chief Financial Officer of Virtual Closet on May 10, 2010. Ms. Ren has been serving as Chief Financial Officer and Financial Manager of Maidisen since 2004. Prior to her appointment as CFO of Maidisen, Ms. Ren was Cashier, Accountant and Accounting Supervisor, Shaanxi Xi’an Gyang Health Products Institute, Xi’an, Shaanxi from May 1995 to December 2000, and served as Financial Manager and Audit Manager, Shaanxi Medical Technology Development Co., Ltd., Xi’an, Shaanxi from January 2001 to April 2004. Ms. Ren graduated from Shaanxi Finance and Economics University with a Bachelor of Accounting and a Master Degree of Business Administration, Northwestern University in Xi’an, Shaanxi.
GENGCHANG WANG – Director. Mr. Wang has been serving as Manager of the Audit Department of Xi’an Jingwei Accounting Firm, Xi’an, Shaanxi since January 2008. Prior to his employment at Xi’an Jingwei Accounting Firm, Mr. Wang served as a Chief Accountant, Shaanxi Jiuzhou Pharmaceutical Co., Ltd., Xi’an, Shaanxi from February 2004 to December 2007. Prior to working as a Chief Accountant, Mr. Wang has previously served as Manager of the Audit Department, Xi’an Yongming Accounting Firm, Xi’an, Shaanxi and as Accountant and Accounting Manager, at Xi’an Building Materials Factory, Xi’an, Shaanxi. Mr. Wang graduated from the Shaanxi Institute of Finance in Xi’an with a Bachelor of Accounting. Mr. Wang has also held the National Certified Public Accountant professional designation since 2002.
JINHUAN CHANG – Director. Mr. Chang has been serving as the Chief Financial Officer and Vice General Manager, Xi’an Ounaide Biotechnology Co., Ltd., Xi’an, Shaanxi since January 2009. Mr. Chang was previously employed as the Manager of the Financial Department, Shaanxi Shuangyu Constructure Company, Xi’an, Shaanxi from May 2003 through December 2008. Mr. Chang was employed also at Accountant and the Accounting Manager, Shaanxi Shuangyu Constructure Company, Xi’an, Shaanxi from January 1998 through May 2003. Mr. Chang has a Bachelor of Financial Management, Chinese PLA Second Artillery Engineering College.
MICHAEL SEGAL – Director. Since 2001, Mr. Segal has been President of Segal Cirone Services Inc., a financial consulting company that advises institutions, banks and high net worth individuals. He has been an Officer, General Securities Principal, Options Compliance Principal and an Investment Banking Representative of B & B Securities, Inc., a member of the New York Stock Exchange and Financial Industry Regulatory Authority (FINRA) since January 2010. From 2006 to 2009 and 2003 to 2005, Mr. Segal was a Principal, Option Compliance Principal and Branch Manager of Whitaker Securities LLC. Mr. Segal had served as President of Alexander Westcott & Co., Inc., a Broker/Dealer registered with NASD and Secretary of the board of directors of its parent company, the Financial Commerce Network Inc.(FCNI.OB), a public company. He is also individually registered as a Commodity Trading Advisor with the Commodity Futures Trading Commission (CFTC) and a founding member of the Managed Funds Association. Mr. Segal received a B.B.A. in marketing and economics from the University of Miami in Florida. Mr. Segal sits on the board of directors of China Agri Business Inc. (CHBU.OB) and China Power Equipment Inc. (CPQQ.OB) public traded companies on the US OTC Bulletin Board. Additionally Mr. Segal sits on the board of directors of privately held companies SunGame Inc., International American Capital Inc. and Asia Carbon Industries Inc.
The Board believes that each of the Company’s directors is highly qualified to serve as a member of the Board. Each of the directors has contributed to the mix of skills, core competencies and qualifications of the Board. When evaluating candidates for election to the Board, the Board seeks candidates with certain qualities that it believes are important, including integrity, an objective perspective, good judgment, leadership skills. The Company’s directors are highly educated and have diverse backgrounds and talents and extensive track records of success in what the Company believes are highly relevant positions. Some of the Company’s directors have served in its new operating entity, Dongke Maidisen, for many years and benefit from an intimate knowledge of our operations and corporate philosophy.
35
Committees of the Board of Directors
The Company presently does not have an audit committee, compensation committee or nominating committee or committee performing similar functions, as the management of the Company believes that until this point it has been premature at the early stage of the Company’s management and business development to form an audit, compensation or nominating committee. As of April 1, 2010, we have adopted our charters to the following committees of the Board of Directors, with membership to be announced upon the appointment of independent Directors.
Audit Committee
Our audit committee will consist of our independent directors upon appointment. The Committee will oversee our accounting and financial reporting processes and the audits of the financial statements of our company. The audit committee is responsible for, among other things:
|
●
|
selecting our independent auditors and pre-approving all auditing and non-auditing services permitted to be performed by our independent auditors;
|
|
●
|
reviewing with our independent auditors any audit problems or difficulties and management’s response;
|
|
●
|
reviewing and approving all proposed related-party transactions, as defined in Item 404 of Regulation S-K under the Securities Act;
|
|
●
|
discussing the annual audited financial statements with management and our independent auditors;
|
|
●
|
reviewing major issues as to the adequacy of our internal controls and any special audit steps adopted in light of material control deficiencies;
|
|
●
|
annually reviewing and reassessing the adequacy of our audit committee charter;
|
|
●
|
such other matters that are specifically delegated to our audit committee by our board of directors from time to time;
|
|
●
|
meeting separately and periodically with management and our internal and independent auditors; and
|
|
●
|
reporting regularly to the full board of directors.
|
Compensation Committee
Our compensation committee will consist of our independent directors upon appointment. Our compensation committee assists the board in reviewing and approving the compensation structure of our directors and executive officers, including all forms of compensation to be provided to our directors and executive officers. Members of the compensation committee are not prohibited from direct involvement in determining their own compensation. Our chief executive officer may not be present at any committee meeting during which his compensation is deliberated. The compensation committee is responsible for, among other things:
|
●
|
approving and overseeing the compensation package for our executive officers;
|
|
●
|
reviewing and making recommendations to the board with respect to the compensation of our directors;
|
|
●
|
reviewing and approving corporate goals and objectives relevant to the compensation of our chief executive officer, evaluating the performance of our chief executive officer in light of those goals and objectives, and setting the compensation level of our chief executive officer based on this evaluation; and
|
|
●
|
reviewing periodically and making recommendations to the board regarding any long-term incentive compensation or equity plans, programs or similar arrangements, annual bonuses, employee pension and welfare benefit plans.
|
36
Corporate Governance and Nominating Committee
Our corporate governance and nominating committee will consist of our independent directors upon appointment. The corporate governance and nominating committee assists the board of directors in identifying individuals qualified to become our directors and in determining the composition of the board and its committees. The corporate governance and nominating committee is responsible for, among other things:
|
●
|
identifying and recommending to the board nominees for election or re-election to the board, or for appointment to fill any vacancy;
|
|
●
|
reviewing annually with the board the current composition of the board in light of the characteristics of independence, age, skills, experience and availability of service to us;
|
|
●
|
identifying and recommending to the board the directors to serve as members of the board’s committees;
|
|
●
|
advising the board periodically with respect to significant developments in the law and practice of corporate governance as well as our compliance with applicable laws and regulations, and making recommendations to the board on all matters of corporate governance and on any corrective action to be taken; and
|
|
●
|
monitoring compliance with our code of business conduct and ethics, including reviewing the adequacy and effectiveness of our procedures to ensure proper compliance.
|
To the best of our knowledge, none of the following ever occurred to any of our directors and officers:
|
1.
|
A petition under the Federal bankruptcy laws or any state insolvency law was filed by or against, or a receiver, fiscal agent or similar officer was appointed by a court for the business or property of such person, or any partnership in which he was a general partner at or within two years before the time of such filing, or any corporation or business association of which he was an executive officer at or within two years before the time of such filing;
|
|
2.
|
|
3.
|
|
iii.
|
|
5.
|
37
|
7.
|
Such person was the subject of, or a party to, any Federal or State judicial or administrative order, judgment, decree, or finding, not subsequently reversed, suspended or vacated, relating to an alleged violation of:
|
|
ii.
|
|
iii.
|
Board Leadership and Risk Oversight
Our Chief Executive Officer also serves as Chairman of the Board. The Board believes that the Company’s Chief Executive Officer is best situated to serve as Chairman of the Board because he is the director most familiar with our business and industry and the director most capable of identifying strategic priorities and executing our business strategy. In addition, having one person serve as both Chairman and Chief Executive Officer eliminates potential for confusion and provides clear leadership for the Company, with a single person setting the tone and managing our operations. The Board oversees specific risks, including, but not limited to:
|
·
|
appointing, retaining and overseeing the work of the independent auditors, including resolving disagreements between the management and the independent auditors relating to financial reporting;
|
|
|
·
|
approving all auditing and non-auditing services permitted to be performed by the independent auditors;
|
|
|
·
|
reviewing annually the independence and quality control procedures of the independent auditors;
|
|
|
·
|
reviewing, approving, and overseeing risks arising from proposed related party transactions;
|
|
|
·
|
discussing the annual audited financial statements with the management;
|
|
|
·
|
meeting separately with the independent auditors to discuss critical accounting policies, management letters, recommendations on internal controls, the auditor’s engagement letter and independence letter and other material written communications between the independent auditors and the management; and
|
|
|
·
|
monitoring the risks associated with management resources, structure, succession planning, development and selection processes, including evaluating the effect the compensation structure may have on risk decisions.
|
Code of Ethics
We currently have a code of ethics that applies to our officers, employees and directors, including our Chief Executive Officer and senior executives.
Conflicts of Interest
Certain potential conflicts of interest are inherent in the relationships between our officers and directors, and us.
38
From time to time, one or more of our affiliates may form or hold an ownership interest in and/or manage other businesses both related and unrelated to the type of business that we own and operate. These persons expect to continue to form, hold an ownership interest in and/or manage additional other businesses which may compete with ours with respect to operations, including financing and marketing, management time and services and potential customers. These activities may give rise to conflicts between or among the interests of us and other businesses with which our affiliates are associated. Our affiliates are in no way prohibited from undertaking such activities, and neither we nor our shareholders will have any right to require participation in such other activities.
Further, because we intend to transact business with some of our officers, directors and affiliates, as well as with firms in which some of our officers, directors or affiliates have a material interest, potential conflicts may arise between the respective interests of us and these related persons or entities. We believe that such transactions will be effected on terms at least as favorable to us as those available from unrelated third parties.
EXECUTIVE COMPENSATION
The following Executive Compensation Chart highlights the compensation for our executive officers. No other executive officers received salary and bonus in excess of $100,000 for the prior two fiscal years.
Compensation of Directors and Executive Members
|
Long Term Compensation
|
||||||||||||||||
|
Annual Compensation
|
Awards
|
Payouts
|
||||||||||||||
|
Nameand
PrincipalPosition
|
Year
|
Salary
($)
|
Bonus ($)
|
Other
Annual Compensation ($)
|
RestrictedStockAward(s)($)
|
Securities
Underlying Options/
SARs (#) (#)
|
LTIP
Payouts ($)
|
All Other
Compensation
($)
|
||||||||
|
You Ting Zhou (former CEO)(1)
|
2009
2008
|
$0
$0
|
$0
$0
|
$0
$0
|
N/A
N/A
|
N/A
N/A
|
N/A
N/A
|
N/A
N/A
|
||||||||
|
Dongke Zhao
Chairman, President and CEO (2)
|
2009
2008
|
$17,750
$17,750
|
$0
$0
|
$0
$0
|
N/A
N/A
|
N/A
N/A
|
N/A
N/A
|
N/A
N/A
|
||||||||
|
Yanhong Ren
CFO(2)
|
2009
2008
|
$4,620
$4,620
|
$0
$0
|
$0
$0
|
N/A
N/A
|
N/A
N/A
|
N/A
N/A
|
N/A
N/A
|
||||||||
|
(1)
|
You Ting Zhou resigned as our Chief Executive Officer, Chief Financial Officer, Secretary, Treasurer and Director, on April 29, 2010.
|
|
(2)
|
These amounts represent compensation paid by Dong Ke Pharmaceutical, Inc. and its subsidiaries. As of the Closing of the Share Exchange Agreement, we had executed employment agreements with our CEO/President and CFO. The employment agreements will provide for annual salaries and annual bonuses in amounts not less than the amounts set forth in the table above.
|
Family Relationships
There are no family relationships between any of our directors or executive officers and any other directors or executive officers.
Option Grants
We do not maintain any equity incentive or stock option plan. Accordingly, we did not grant options to purchase any equity interests to any employees or officers, and no stock options are issued or outstanding to any officers.
Employment Agreements
On March 1, 2010, Dong Ke entered into a two year Employment Agreement with Dongke Zhao to serve as our Chief Executive Officer. The agreement provides for an annual salary of USD$17,750 and an annual bonus of up to 50% of the executive’s annual salary. A copy of this agreement is included in this Current Report as an Exhibit.
39
On March 1, 2010, Dong Ke entered into a two year Employment Agreement with Yanhong Ren to serve as our Chief Financial Officer. The agreement provides for an annual salary of USD$4,620 and an annual bonus of up to 50% of the executive’s annual salary. A copy of this agreement is included in this Current Report as an Exhibit.
Outstanding Equity Awards
None.
Bonuses and Deferred Compensation
The Company does not have any bonus, deferred compensation or retirement plan. Decisions regarding compensation will be determined by our compensation committee once established; our compensation committee will consist of our three independent directors effective until the expiration of the ten day period following the mailing of the Schedule 14f-1 to the Company’s shareholders.
No stock options were granted to the executive officers during the fiscal year ended December 31, 2009. No stock options were exercised during the fiscal year ended December 31, 2009.
As of December 31, 2009, there are no other arrangements, standard or otherwise, with any director or officer wherein the director or officer is compensated other than as stated above.
Effective May 10, 2010, the Company entered into an agreement with Michael Segal pursuant to which he is to receive $24,000 per yearfor his services as director, beginning May 10, 2010, payable on a quarterly basis, with the first installment to be received on August 10, 2010. Additionally he is to receive 19,636 shares of common stock on May 10, 2010 and annual stock payments of the equivalent of $24,000, based upon the closing price of the Company’s common stock on December 31 of each year that Mr. Segal remains in service as a director.
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT
Pre-Closing
The following table sets forth certain information regarding our common stock beneficially owned on May 10, 2010, prior to the closing, for (i) each shareholder known to be the beneficial owner of 5% or more of our outstanding common stock, (ii) each of our officers and directors, and (iii) all executive officers and directors as a group. In general, a person is deemed to be a "beneficial owner" of a security if that person has or shares the power to vote or direct the voting of such security, or the power to dispose or to direct the disposition of such security. A person is also deemed to be a beneficial owner of any securities of which the person has the right to acquire beneficial ownership within 60 days. To the best of our knowledge, all persons named have sole voting and investment power with respect to such shares, except as otherwise noted. Except as set forth in this Information Statement, there are not any pending or anticipated arrangements that may cause a change in control. At May 10, 2010, 10,255,000 shares of our common stock were outstanding immediately prior to the Closing Date.
|
Name
|
Number of Shares
Beneficially Owned
|
Percent of Shares
|
||||||
|
You Ting Zhou
|
10,000,000 | 97.51 | % | |||||
|
All Executive Officers and Directors as a group
|
10,000,000 | 97.51 | % | |||||
40
Post-Closing
Upon the consummation of the Share Exchange Agreement on May 10, 2010, the following table sets forth certain information that would exist as of May 10, 2010 with respect to the beneficial ownership of the Company’s outstanding common stock by (i) any holder of more than five (5%) percent; (ii) each of the named executive officers, directors, and director nominees; and (iii) our directors, director nominees and named executive officers as a group. Except as otherwise indicated, each of the stockholders listed below has sole voting and investment power over the shares beneficially owned.
|
Name of Beneficial Owner (1)
|
Common Stock
Beneficially Owned
|
Percentage of
Common Stock (2)
|
||
|
Dongke Zhao
Chairman, President, and Chief Executive Officer
|
785,454
|
36.0%
|
||
|
Yanhong Ren
Director and Chief Financial Officer
|
0
|
--
|
||
|
Gengchang Wang
Independent Director
No.148, Zhongcun Villiage, Hongmiaopo, Lianhu District
Xi’an, People’s Republic of China 710016
|
0
|
--
|
||
|
Jinhuan Chang
Independent Director
No.1 Floor 2, Building 1, No.10 North Road of Erfuzhuang, Weiyang District
Xi’an, People’s Republic of China 710016
|
0
|
--
|
||
|
Michael Segal
Independent Director
11 East 86th Street, Suite 19B
New York, NY 10028
|
19,636
|
*
|
||
|
All officers and directors as a group (6 persons)
|
805,090
|
36.9%
|
*Less than 1% of the Company’s issued and outstanding common stock.
|
(1)
|
Except as otherwise indicated, the address of each beneficial owner is c/o Virtual Closet, Inc., Dongxing Building, 4th Floor, Xincheng Technology Zone, No.1 Xinke Road, Xi’an, the People’s Republic of China 710043.
|
|
(2)
|
Applicable percentage ownership is based on an assumption of 2,181,818 shares of common stock issued and outstanding as of May 10, 2010. The number of shares beneficially owned by a person includes shares of common stock underlying options or warrants held by that person that are currently exercisable or exercisable within 60 days of May 10, 2010. Beneficial ownership is determined in accordance with the rules of the Securities and Exchange Commission and generally includes voting or investment power with respect to securities. Shares of common stock that are currently obtainable or obtainable within 60 days of May 10, 2010 by exercise or conversion of other securities are deemed to be beneficially owned by the person holding such securities for the purpose of computing the percentage of ownership of such person, but are not treated as outstanding for the purpose of computing the percentage ownership of any other person.
|
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS
Pursuant to the Share Exchange Agreement, the Company acquired 100% of the capital stock of Dong Ke in exchange for 1,941,818 newly-issued shares of the Company’s common stock, and You Ting Zhou, the Virtual Closet controlling stockholder and former sole director and officer, as well as Ge Lin and Jia Tan Fang agreed to cancel an aggregate of 10,015,000 shares of the Company’s common stock.
As a result of the consummation of the Share Exchange Agreement of May 10, 2010, we acquired control of Dong Ke, a Delaware corporation and the parent company of Slovan, a wholly foreign-owned enterprise organized under the laws of the People’s Republic of China by issuing to the Dong Ke shareholders shares our of Common Stock as consideration for all of the outstanding capital stock of Dong Ke.
41
The table below sets forth our significant stockholders, officers and directors; the significant stockholders, officers, and directors of Dong Ke and Slovan and their relationships with Maidisen.
|
Name
|
Position/Interrelationship
|
|
|
Dongke Zhao
|
Chairman, Chief Executive Officer and President of Virtual Closet, Inc., Dong Ke, Slovan and Maidisen, stockholder of Virtual Closet, Inc. with 36.0% of our common stock; Director of Slovan with right to vote Maidisen shares under the terms of the Voting Proxy Agreement.
|
|
|
Yanhong Ren
|
Chief Financial Officer of Virtual Closet, Inc., Dong Ke, Slovan and Maidisen.
|
The following transactions relate to the activities of Dong Ke and our new operating entity, Maidisen:
Pursuant to a Property Borrowing Agreement dated December 26, 2006, we lease our headquarters, which house our sales and management departments and occupies approximately 1600 square meters of space, from our Chief Executive Officer, Dongke Zhao. The agreement is valid for a period of five years, expiring December 31, 2011.
On April 28, 2006, Maidisen entered into a Second Product Development Agency Agreement with Shaanxi Dongke Maidisen Pharmaceutical Technology Co., Ltd. (“DKMP Technology”), a research and development (“R&D) company formerly managed by our chief executive officer, Dongke Zhao. Pursuant to the agreement, DKMP Technology agreed to provide testing facilities and personnel to conduct various R&D activities for 11 of Maidisen’s products according to the quality standards set by Maidisen. Such services include the preparation and compilation of original data and testing results. As consideration for such services, Maidisen is to pay DKMP Technology related testing and development fees of RMB920,000, of which RMB 400,000 shall be used for product-related R&D, RMB300,000 shall be used toward quality improvement R&D, and 220,000 shall be used toward product stability-related R&D. Mr. Zhao resigned from his position as chairman of DKMP Technology in December 2006. Since that time he has had no affiliated relationship with DKMP Technology.
In February 2007, Mr. Zhao was authorized by Maidisen and advanced RMB260,000 to act as agent for Maidisen to apply for the Maidisen’s access to the state medical insurance program, which enables the sale of some of Maidisen’s products to be covered by government insurance, . The processing of such application is expected to be completed by June 30, 2010. As such, as of December 31, 2009, $172,655 in receivables was outstanding from Mr. Zhao. The Company expects such receivables to be paid in full before the end of 2010.
In November 2009, Mr. Zhao and Jinkun Wei, a former director of Maidisen and shareholder of the Company, agreed to serve as personal guarantors to a loan in the amount of RMB 9,000,000 (approximately $1,320,190) from China Merchant’s Bank. The loan bears interest at a rate of 7% per annum and becomes due on November 23, 2010.
On March 9, 2010, Dong Ke entered into an agreement with NSD Consulting Services, Inc. Dong Ke requires assistance in the public company sector including advising on a merger/acquisition transaction, regulatory filings and other consulting services support as requested. As consideration for the Consulting Services to be performed by Consultant under this agreement Dong Ke, agreed to issue NSD Consulting Services, Inc. warrants to acquire 72,727 common shares
of the Company’s stock, adjusted for any forward or reverse splits, with registration rights, at a $13.75 exercise price and expiration date of three years after the closing of the acquisition or merger for time spent on consulting services.
On April 2, 2010, Dong Ke entered into an investor relations agreement with Avisair IR Services, Inc., pursuant to which the Company required assistance in the public company sector including advising on and with respect to investor relations services. The term of the contract expires April 30, 2011. As consideration for certain investor relations services to be performed by Avisair IR Services, Inc., Dong Ke agreed to issue to Avisair IR Services, Inc. warrants to acquire 72,727 common shares of the Company’s stock, adjusted for any forward or reverse splits, with registration rights, at a $13.75 exercise price and expiration date of April 26, 2013, for time spent on consulting services.
42
Other than as disclosed above, there have been no other transactions since the beginning of our last fiscal year, or any currently proposed transactions in which we are, or plan to be, a participant and the amount involved exceeds $120,000 or one percent of the average of our total assets at year end for the last two completed fiscal years, and in which any related person had or will have a direct or indirect material interest.
Except with respect to the Share Exchange Agreement, none of the Company’s directors or officers, nor any incoming director, nor any person who beneficially owns, directly or indirectly, shares carrying more than 10% of the voting rights attached to the Company’s outstanding shares, nor any of the Company’s promoters, nor any relative or spouse of any of the foregoing persons has or will have any material interest, direct or indirect, in any transaction for the past two years or in any presently proposed transaction to which the Company was or is to be party. None of the Company’s directors or officers, nor any incoming director is indebted to the Company.
Procedures for Approval of Related Party Transactions
Our Board of Directors is charged with reviewing and approving all potential related party transactions. All such related party transactions must then be reported under applicable SEC rules. We have not adopted other procedures for review, or standards for approval, of such transactions, but instead review them on a case-by-case basis.
Director Independence
Gengchang Wang, Jinhuan Chang, and Michael Segal are independent directors of the Company. The determination of independence of directors has been made using the definition of “independent director” contained under Rule 4200(a)(15) of the Rules of National Association of Securities Dealers.
DESCRIPTION OF SECURITIES
As of May 10, 2010 our authorized capital stock consists of 75,000,000 shares of common stock, par value $0.001 per share. As of May 10, 2010, an aggregate of 2,181,818 shares of Common Stock were outstanding.
There are no shares of preferred stock authorized or outstanding.
Common Stock
Subject to preferences that may apply to shares of preferred stock outstanding at the time, the holders of outstanding shares of Common Stock are entitled to receive dividends out of assets legally available therefore at times and in amounts as our board of directors may determine. Each stockholder is entitled to one vote for each share of Common Stock held on all matters submitted to a vote of the stockholders. Cumulative voting is not provided for in our amended articles of incorporation, which means that the majority of the shares voted can elect all of the directors then standing for election. The Common Stock is not entitled to pre-emptive rights and is not subject to conversion or redemption. Upon the occurrence of a liquidation, dissolution or winding-up, the holders of shares of Common Stock are entitled to share rateably in all assets remaining after payment of liabilities and satisfaction of preferential rights of any outstanding preferred stock. There are no sinking fund provisions applicable to the Common Stock. The outstanding shares of Common Stock are, and the shares of Common Stock to be issued upon conversion of the Warrants will be, fully paid and non-assessable.
Voting Rights
Holders of our common stock have the right to cast one vote for each share of stock in their name on the books of our company, whether represented in person or by proxy, on all matters submitted to a vote of holders of common stock, including election of directors. There is no right to cumulative voting in the election of directors. Except where a greater requirement is provided by statute or by the articles of incorporation, or in the by-laws, the presence, in person or by proxy duly authorized, of the one or more holders of a majority of the outstanding shares of our common stock constitutes a quorum for the transaction of business. The vote by the holders of a majority of outstanding shares is required to effect certain fundamental corporate changes such as liquidation, merger, or amendment of our articles of incorporation.
43
Dividends
There are no restrictions in our articles of incorporation or by-laws that prevent us from declaring dividends. The Nevada Revised Statutes, however, do prohibit us from declaring dividends where, after giving effect to the distribution of the dividend (1) we would not be able to pay our debts as they become due in the usual course of business or (2) our total assets would be less than the sum of our total liabilities plus the amount that would be needed to satisfy the rights of stockholders who have preferential rights superior to those receiving the distribution.
We have not declared any dividends, and we do not plan to declare any dividends in the foreseeable future.
Preemptive Rights
Holders of our common stock are not entitled to preemptive rights, and no redemption or sinking fund provisions are applicable to our common stock. All outstanding shares of our common stock are, and the shares of common stock sold in the offering will when issued be, fully paid and non-assessable.
Warrants
For a more detailed description of our Warrants, please see the discussion in Item 3.02 of this report.
MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER
PURCHASES OF EQUITY SECURITIES
Our common stock, having $0.001 par value per share, is traded on the Over-The-Counter Bulletin Board ("OTCBB") Following the Share Exchange, the combined Company will continue to be traded on the ("OTCBB"), under the symbol “VIRZ”.
Our common stock is listed but not quoted or traded on OTC Bulletin Board at this time under the symbol "VIRZ". Should a market develop for our shares, the trading price of the common stock is likely to be highly volatile and could be subject to wide fluctuations in response to factors such as actual or anticipated variations in quarterly operating results, announcements of technological innovations, new sales formats, or new services by us or our competitors, changes in financial estimates by securities analysts, conditions or trends in Internet or traditional retail markets, changes in the market valuations of other equipment and furniture leasing service providers or accounting related business services, announcements by us or our competitors of significant acquisitions, strategic partnerships, joint ventures, capital commitments, additions or departures of key personnel, sales of common stock and other events or factors, many of which are beyond our control. In addition, the stock market in general, and the market for instant messaging business services in particular, has experienced extreme price and volume fluctuations that have often been unrelated or disproportionate to the operating performance of such companies. These broad market and industry factors may materially adversely affect the market price of the common stock, regardless of our operating performance.
Consequently, future announcements concerning us or our competitors, litigation, or public concerns as to the commercial value of one or more of our products or services may cause the market price of our common stock to fluctuate substantially for reasons which may be unrelated to operating results. These fluctuations, as well as general economic, political and market conditions, may have a material adverse effect on the market price of our common stock.
As of May 10, 2010, we had approximately 45 shareholders of record of our common stock.
Transfer Agent and Registrar
Pacific Stock Transfer Company, 4045 South Spencer Street, Suite 403, Las Vegas, Nevada, 89119.
Dividend Policy
Any future determination as to the declaration and payment of dividends on shares of our Common Stock will be made at the discretion of our board of directors out of funds legally available for such purpose. We are under no contractual obligations or restrictions to declare or pay dividends on our shares of Common Stock. In addition, we currently have no plans to pay such dividends. However, even if we wish to pay dividends, because our cash flow is dependent on dividend distributions from our affiliated entities in PRC, we may be restricted from distributing dividends to our holders of shares of our common stock in the future if at the time we are unable to obtain sufficient dividend distributions from Dong Ke, Slovan and Maidisen. Our board of directors currently intends to retain all earnings for use in the business for the foreseeable future. See “Risk Factors.”
44
Securities Authorized for Issuance under Equity Compensation Plans
As of the date of this Current Report, we do not have any securities authorized for issuance under any equity compensation plans and we do not have any equity compensation plans.
Penny Stock Regulations
Our shares of common stock are subject to the "penny stock" rules of the Securities Exchange Act of 1934 and various rules under this Act. In general terms, "penny stock" is defined as any equity security that has a market price less than $5.00 per share, subject to certain exceptions. The rules provide that any equity security is considered to be a penny stock unless that security is registered and traded on a national securities exchange meeting specified criteria set by the SEC, issued by a registered investment company, and excluded from the definition on the basis of price (at least $5.00 per share), or based on the issuer's net tangible assets or revenues. In the last case, the issuer's net tangible assets must exceed $3,000,000 if in continuous operation for at least three years or $5,000,000 if in operation for less than three years, or the issuer's average revenues for each of the past three years must exceed $6,000,000.
Trading in shares of penny stock is subject to additional sales practice requirements for broker-dealers who sell penny stocks to persons other than established customers and accredited investors. Accredited investors, in general, include individuals with assets in excess of $1,000,000 or annual income exceeding $200,000 (or $300,000 together with their spouse), and certain institutional investors. For transactions covered by these rules, broker-dealers must make a special suitability determination for the purchase of the security and must have received the purchaser's written consent to the transaction prior to the purchase. Additionally, for any transaction involving a penny stock, the rules require the delivery, prior to the first transaction, of a risk disclosure document relating to the penny stock. A broker-dealer also must disclose the commissions payable to both the broker-dealer and the registered representative, and current quotations for the security. Finally, monthly statements must be sent disclosing recent price information for the penny stocks. These rules may restrict the ability of broker-dealers to trade or maintain a market in our common stock, to the extent it is penny stock, and may affect the ability of shareholders to sell their shares.
LEGAL PROCEEDINGS
We know of no material, active, pending or threatened proceeding against us or our subsidiaries, nor are we, or any subsidiary, involved as a plaintiff or defendant in any material proceeding or pending litigation.
RECENT SALES OF UNREGISTERED SECURITIES
Reference is made to Item 3.02 of this Current Report on Form 8-K for a description of recent sales of unregistered securities, which is hereby incorporated by reference.
INDEMNIFICATION OF OFFICERS AND DIRECTORS
Nevada Revised Statutes
Section 78.138 of the NRS provides for immunity of directors from monetary liability, except in certain enumerated circumstances, as follows:
“Except as otherwise provided in NRS 35.230, 90.660, 91.250, 452.200, 452.270, 668.045 and 694A.030, or unless the Articles of Incorporation or an amendment thereto, in each case filed on or after October 1, 2003, provide for greater individual liability, a director or officer is not individually liable to the corporation or its stockholders or creditors for any damages as a result of any act or failure to act in his capacity as a director or officer unless it is proven that:
|
(a)
|
his act or failure to act constituted a breach of his fiduciary duties as a director or officer; and
|
|
(b)
|
his breach of those duties involved intentional misconduct, fraud or a knowing violation of law.”
|
45
Section 78.5702 of the NRS provides as follows:
|
1.
|
A corporation may indemnify any person who was or is a party or is threatened to be made a party to any threatened, pending or completed action, suit or proceeding, whether civil, criminal, administrative or investigative, except an action by or in the right of the corporation, by reason of the fact that he is or was a director, officer, employee or agent of the corporation, or is or was serving at the request of the corporation as a director, officer, employee or agent of another corporation, partnership, joint venture, trust or other enterprise, against expenses, including attorneys’ fees, judgments, fines and amounts paid in settlement actually and reasonably incurred by him in connection with the action, suit or proceeding if he:
|
|
(a)
|
is not liable pursuant to NRS 78.138; or
|
|
(b)
|
acted in good faith and in a manner which he reasonably believed to be in or not opposed to the best interests of the corporation, and, with respect to any criminal action or proceeding, had no reasonable cause to believe his conduct was unlawful.
|
|
2.
|
A corporation may indemnify any person who was or is a party or is threatened to be made a party to any threatened, pending or completed action or suit by or in the right of the corporation to procure a judgment in its favor by reason of the fact that he is or was a director, officer, employee or agent of the corporation, or is or was serving at the request of the corporation as a director, officer, employee or agent of another corporation, partnership, joint venture, trust or other enterprise against expenses, including amounts paid in settlement and attorneys’ fees actually and reasonably incurred by him in connection with the defense or settlement of the action or suit if he:
|
|
(a)
|
is not liable pursuant to NRS 78.138; or
|
|
(b)
|
acted in good faith and in a manner which he reasonably believed to be in or not opposed to the best interests of the corporation.
|
To the extent that a director, officer, employee or agent of a corporation has been successful on the merits or otherwise in defense of any action, suit or proceeding referred to in subsections 1 and 2, or in defense of any claim, issue or matter therein, the corporation shall indemnify him against expenses, including attorneys’ fees, actually and reasonably incurred by him in connection with the defense.
Our by-laws provide that we shall indemnify our officers and directors in any action, suit or proceeding unless such officer or director shall be adjudged to be derelict in his or her duties.
Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to our directors, officers or person controlling us, we have been informed that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the act and is therefore unenforceable.
CHANGES AND DISAGREEMENTS WITH ACCOUNTANTS
Please see Item 4.01 of this report for details.
Item 3.02 Unregistered Sales of Equity Securities.
Pursuant to the Share Exchange Agreement, on May 10, 2010 we issued 1,941,818 shares of our Common Stock to the shareholders Dong Ke in exchange for 100% of the outstanding shares of Dong Ke. The issuance of these shares was exempt from registration, in part pursuant to Regulation S and Regulation D under the Securities Act of 1933 and in part pursuant to Section 4(2) of the Securities Act of 1933. We made this determination based on the representations of Dong Ke which included, in pertinent part, that such shareholders were either (a) "accredited investors" within the meaning of Rule 501 of Regulation D promulgated under the Securities Act, or (b) not a "U.S. person" as that term is defined in Rule 902(k) of Regulation S under the Act, and that such shareholders were acquiring our common stock, for investment purposes for their own respective accounts and not as nominees or agents, and not with a view to the resale or distribution thereof, and that Dong Ke shareholders understood that the shares of our common stock may not be sold or otherwise disposed of without registration under the Securities Act or an applicable exemption therefrom.
46
Concurrent with the Share Exchange Agreement on May 10, 2010, we issued to certain consultants three year warrants each to purchase 72,727 shares of our common stock at an exercise price of $13.75 per share (the “Warrants”). Such securities were not registered under the Securities Act of 1933. The issuance of these securities was exempt from registration under Section 4(2) of the Securities Act. We made this determination based on the representations of the consultants, which included, in pertinent part, that such consultants were an "accredited investors" within the meaning of Rule 501 of Regulation D promulgated under the Securities Act and that the consultants was acquiring our common stock for investment purposes for their own respective accounts and not as nominees or agents, and not with a view to the resale or distribution thereof, and that the consultants understood that the shares of our common stock may not be sold or otherwise disposed of without registration under the Securities Act or an applicable exemption therefrom.
The Warrants were issued pursuant to the following consulting agreements:
On January 7, 2010, Dong Ke entered into an agreement with Jinhao Zhang to provide for public shell referral services. As consideration for the referral services to be performed by Jinhao Zhang, Dong Ke agreed to issue to Jinhao Zhang warrants to acquire 72,727 common shares of the Company’s common stock, subject to any forward or reverse splits, with registration rights, at a $13.75 exercise price and expiration date of three years after acquiring control of or merging with said public shell for time spent on referral services, at the time of acquiring control or merging with said public shell. The agreement had no fixed term of expiry.
On March 9, 2010, Dong Ke entered into an agreement with NSD Consulting Services, Inc. Dong Ke requires assistance in the public company sector including advising on a merger/acquisition transaction, regulatory filings and other consulting services support as requested. As consideration for the Consulting Services to be performed by Consultant under this agreement Dong Ke, agreed to issue NSD Consulting Services, Inc. warrants to acquire 72,727 common shares of the Company’s stock, adjusted for any forward or reverse splits, with registration rights, at a $ 13.75 exercise price and expiration date of three years after the closing of the acquisition or merger for time spent on consulting services.
On April 2, 2010, Dong Ke entered into an investor relations agreement with Avisair IR Services, Inc., pursuant to which the Company required assistance in the public company sector including advising on and with respect to investor relations services. The term of the contract expires April 30, 2011. As consideration for certain investor relations services to be performed by Avisair IR Services, Inc., Dong Ke agreed to issue to Avisair IR Services, Inc. warrants to acquire 72,727 common shares of the Company’s stock, adjusted for any forward or reverse splits, with registration rights, at a $13.75 exercise price and expiration date of April 26, 2013, for time spent on consulting services.
Warrants
Each Warrant entitles the holder to purchase one share of our Common Stock. The Warrants will be exercisable, in whole or in part, at an exercise price equal to $13.75 per share (“Exercise Price”). The Warrants may be exercised at any time upon the election of the warrant holder beginning on the date of issuance and ending of the third anniversary of the closing of the Share Exchange Agreement.
The exercise price and number of shares of Common Stock to be received upon the exercise of Warrants are subject to adjustment upon the occurrence of certain events, such as stock splits, stock dividends or our recapitalization. In the event of our liquidation, dissolution or winding up, the holders of Warrants will not be entitled to participate in the distribution of our assets.
If we shall make any dividend or other distribution of assets to holders of its common stock (a “Distribution”), then the Exercise Price shall be reduced by multiplying such Exercise Price by a fraction of which (i) the numerator shall be the closing bid price of the shares of common stock on the trading day immediately preceding such record date minus the value of the Distribution applicable to one share of common stock, and (ii) the denominator shall be the closing bid price of the shares of common stock on the trading day immediately preceding such record date. Additionally, the number of shares underlying the Warrant shall be increased to a number of shares of common stock obtainable immediately prior to the close of business on the record date fixed for determining holders of shares entitled to receive the Distribution multiplied by the reciprocal of the fraction set forth above. Holders of Warrants will have no voting, pre-emptive, subscription or other rights of shareholders in respect of the Warrants, nor shall the Holders be entitled to receive dividends.
We have agreed to register an amount of stock equal to 100% of the shares of common stock issuable in connection with the Warrants, on a registration statement to be filed by us. We shall pay the usual costs of such registration.
47
Item 4.01 Change in Registrant’s Certifying Accountant.
On May 10, 2010, the Company dismissed Chang G. Park, CPA, PhD. the independent registered principal accountants of Virtual Closet, Inc.
During the company’s two most recent fiscal years and subsequent interim period preceding the termination of Chang G. Park, Park, CPA, PhD, there were no disagreements with Chang G. Park, CPA, PhD. which were not resolved on any matter concerning accounting principles or practices, financial statement disclosure, or auditing scope or procedure, which disagreements, if not resolved to the satisfaction of Chang G. Park, CPA, PhD. would have caused to make reference to the subject matter of the disagreements in connection with its reports. Chang G. Park, CPA, PhD as the Company’s principal independent accountant, did not provide an adverse opinion or disclaimer of opinion to the Company’s financial statements, nor modify its opinion as to uncertainty, audit scope or accounting principles, except that the reports of Chang G. Park, CPA, PhD for the fiscal years ended September 30, 2008, and 2009 indicated conditions which raised substantial doubt about the Company’s ability to continue as a going concern.
The Company provided Chang G. Park, CPA, PhD with a copy of this disclosure before its filing with the SEC. The Company requested that Chang G. Park, CPA, PhD provide us with a letter addressed to the SEC stating whether or not it agrees with the above statements. The Company will file an amendment to this report to file as an exhibit a copy of the requested letter from Chang G. Park, CPA, PhD. upon receipt.
On May 10, 2010 the board of directors of the Company approved and authorized the engagement of Acquavella, Chiarelli, Shuster, Berkower & Co., LLP Certified Public Accountants and Advisors, 518 Route One, Suite 4103, Iselin, NJ, 08830 as the Company's independent public accountants for the Company for the fiscal year ending December 31, 2010.
Prior to engaging Acquavella, Chiarelli, Shuster, Berkower & Co., LLP, Acquavella, Chiarelli, Shuster, Berkower & Co., LLP did not provide our company with either written or oral advice that was an important factor considered by our company in reaching a decision to change our independent registered public accounting firm from Chang G. Park, CPA, PhD. to Acquavella, Chiarelli, Shuster, Berkower & Co., LLP.
Item 5.01 Changes in Control of Registrant.
As explained more fully in Item 2.01, in connection with the Share Exchange Agreement, on May 10, 2010, we issued 1,941,818 shares of our Common Stock to the Dong Ke shareholders in exchange for the transfer of 100% of the outstanding shares of Dong Ke capital stock by the Dong Ke shareholders to us and cancelled 10,015,000 shares of our common stock held by 3 former shareholders. As such Dong Ke has, immediately following the Exchange and Financing transactions, the Dong Ke shareholders held approximately 89% of the total combined voting power of all classes of our outstanding stock entitled to vote. Reference is made to the disclosures set forth under Item 2.01 of this Current Report on Form 8-K, which disclosure is incorporated herein by reference.
In connection with the Closing of the Share Exchange Agreement, and as explained more fully in Item 2.01 above under the section titled “Management” and in Item 5.02 of this Current Report dated May 10, 2010, You Ting Zhou resigned as a Chief Executive Officer Chief Financial Officer, Treasurer, Secretary and Director. Further, effective May 10, 2010, Dongke Zhao, Yanhong Ren, Gengchang Wang, Jinhuan Chang, and Michael Segal were appointed as members of our board of directors. Finally, effective May 10, 2010 our Directors appointed Dongke Zhao as our Chief Executive Officer and President and Yanhong Ren as our Chief Financial Officer.
Item 5.02 Departure of Directors or Principal Officers; Election of Directors; Appointment of Principal Officers.
Please see Executive Compensation on page 36.
Item 5.03 Amendments to Articles of Incorporation or Bylaws; Forward Share Split and Change in Fiscal Year.
As of May 10, 2010, the Company changed its fiscal year-end from September 30 to December 31. Please see Item 1.01 of this report for information with regard to the Share Exchange Agreement.
Item 5.06 Change In Shell Company Status.
As explained more fully in Item 2.01 above, we have been a "shell company" (as such term is defined in Rule 12b-2 under the Securities Exchange Act of 1934, as amended) immediately before the Closing of the Share Exchange Agreement. As a result of the Share Exchange Agreement, Dong Ke became our wholly owned subsidiary, and Dong Ke’s indirectly controlled entity, Maidisen, became our primary operating entity. Consequently, we believe that the Exchange Agreement has caused us to cease to be a shell company if we were one prior to the share exchange. For information about the Share Exchange, please see the information set forth above under Item 2.01 of this Current Report on Form 8-K which information is incorporated herein by reference.
48
Item 9.01 Financial Statement and Exhibits.
(a) FINANCIAL STATEMENTS OF BUSINESS ACQUIRED.
The Audited Consolidated Financial Statements of Dong Ke, as of December 31, 2009 and 2008 are filed below to this current report and are incorporated herein by reference.
DONGKE PHARMACEUTICALS INC.
CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2009
49
TABLE OF CONTENTS
|
Report of Independent Registered Public Accounting Firm
|
F-1
|
|
Consolidated Balance Sheets
|
F-2
|
|
Consolidated Statements of Income
|
F-3
|
|
Consolidated Statements of Cash Flows
|
F-4
|
|
Consolidated Statements of Stockholders’ Equity
|
F-5
|
|
Notes to Consolidated Financial Statements
|
F-6 - F-17
|
50
ACSBAcquavella, Chiarelli, Shuster, Berkower & Co., LLP
|
517 Route one
Iselin, New Jersey, 08830
732.855.9600
|
1 Penn Plaza
36the Floor
New York, NY 10119
|
Report of Independent Registered Public Accounting Firm
Board of Directors and Stockholders of
Dongke Pharmaceuticals Inc.
We have audited the accompanying consolidated balance sheets of Dongke Pharmaceuticals Inc. as of December 31, 2009 and 2008, and the related consolidated statements of income, stockholders’ equity and comprehensive income, and cash flows for each of the years in the two-year period ended December 31, 2009. Dongke Pharmaceutical Inc.’s management is responsible for these financial statements. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. The company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audit included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that out audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the consolidated financial position of Dongke Pharmaceuticals, Inc. as of December 31, 2009 and 2008, and the consolidated results of its operations and its cash flows for each of the years in the two-year period ended December 31, 2009 in conformity with accounting principles generally accepted in the United States of America.
Acquavella, Chiarelli, Shuster, Berkower & Co., LLP
Certified Public Accountants
New York, N.Y.
March 29, 2010
F-1
DONGKE PHARMACEUTICALS, INC
CONSOLIDATED BALANCE SHEETS
|
December 31,
|
December 31,
|
|||||||
|
2009
|
2008
|
|||||||
|
ASSETS
|
||||||||
|
Current Assets
|
||||||||
|
Cash and cash equivalents
|
$ | 1,058,157 | $ | 491,626 | ||||
|
Accounts receivable
|
3,460,649 | 4,322,013 | ||||||
|
Other receivables
|
762,158 | 70,941 | ||||||
|
Advances to suppliers
|
537,578 | 753,109 | ||||||
|
Prepaid expenses
|
326,783 | 125,826 | ||||||
|
Inventories
|
10,509,797 | 4,354,008 | ||||||
|
Due from related parties
|
172,655 | 144,528 | ||||||
|
Total Current Assets
|
16,827,777 | 10,262,051 | ||||||
|
Property and equipment, net
|
5,067,401 | 4,165,264 | ||||||
|
Intangible assets, net
|
6,636,900 | 7,358,332 | ||||||
|
Total Assets
|
$ | 28,532,078 | $ | 21,785,647 | ||||
|
LIABILITIES AND STOCKHOLDERS' EQUITY
|
||||||||
|
Current Liabilities
|
||||||||
|
Accounts payable
|
$ | 2,812.073 | $ | 2,296,000 | ||||
|
Accrued expenses and other payable
|
4,379,631 | 2,127,451 | ||||||
|
Customer and other deposits
|
384,084 | 820,204 | ||||||
|
Short-term bank loan
|
1,320,190 | 4,092,371 | ||||||
|
Total Current Liabilities
|
8,895,978 | 9,336,026 | ||||||
|
Long-term bank loan
|
2,787,068 | - | ||||||
|
Total Liabilities
|
11,683,046 | 9,336,026 | ||||||
|
Stockholders' Equity
|
||||||||
|
Common stock, $.001 par value, 100,000,000 shares authorized,
|
||||||||
|
26,700,000 shares issued and outstanding at December 31, 2009
and 2008
|
26,700 | 26,700 | ||||||
|
Subscription receivable
|
(26,700 | (26,700 | ) | |||||
|
Additional paid-in capital
|
10,339,590 | 10,339,590 | ||||||
|
Statutory reserves
|
2,448,456 | 275,233 | ||||||
|
Accumulated other comprehensive loss
|
(127,703 | (138,770 | ) | |||||
|
Retained earnings
|
4,188,689 | 1,973,568 | ||||||
|
Total Stockholders' Equity
|
16,849,032 | 12,449,621 | ||||||
|
Total Liabilities and Stockholders' Equity
|
$ | 28,532,078 | $ | 21,785,647 | ||||
The accompanying notes are an integral part of these consolidated financial statements.
F-2
|
DONGKE PHARMACEUTICALS, INC
|
||||||||
|
CONSOLIDATED STATEMENTS OF INCOME
|
||||||||
|
FOR THE YEARS ENDED DECEMBER 31, 2009 AND 2008
|
||||||||
|
2009
|
2008
|
|||||||
|
Sales, net
|
$ | 18,943,917 | $ | 12,059,437 | ||||
|
Cost of sales
|
10,591,759 | 6,792,216 | ||||||
|
Gross profit
|
8,352,158 | 5,267,221 | ||||||
|
Selling, general and administrative expenses
|
3,568,670 | 3,092,660 | ||||||
|
Income from operations
|
4,783,488 | 2,174,561 | ||||||
|
Other Income (Expense)
|
||||||||
|
Interest income
|
3,304 | 6,729 | ||||||
|
Interest expense
|
(327,141 | ) | (319,472 | ) | ||||
|
Government grant
|
- | 205,135 | ||||||
|
Other income
|
380,924 | 412,963 | ||||||
|
Other expense
|
(12,967 | ) | (41,989 | ) | ||||
|
Total other Income (Expense)
|
44,120 | 263,366 | ||||||
|
Income before income taxes
|
4,827,608 | 2,437,927 | ||||||
|
Provision for income taxes
|
- | - | ||||||
|
Net income
|
$ | 4,827,608 | $ | 2,437,927 | ||||
|
Net income per common share
|
||||||||
|
Basic
|
26,700,000 | 26,700,000 | ||||||
|
Diluted
|
26,700,000 | 26,700,000 | ||||||
|
Weighted average common shares outstanding
|
||||||||
|
Basic
|
$ | .18 | $ | 0.09 | ||||
|
Diluted
|
$ | .18 | $ | 0.09 | ||||
|
Net income
|
$ | 4,827,608 | $ | 2,437,927 | ||||
|
Other comprehensive income (loss)
|
11,067 | (47,178 | ) | |||||
|
Comprehensive income (loss)
|
$ | 4,838,675 | $ | 2,390,749 | ||||
The accompanying notes are an integral part of these consolidated financial statements.
F-3
|
DONGKE PHARMACEUTICALS, INC
|
||||||||
|
CONSOLIDATED STATEMENTS OF CASH FLOWS
|
||||||||
|
FOR THE YEARS ENDED DECEMBER 31, 2009 AND 2008
|
||||||||
|
2009
|
2008
|
|||||||
|
CASH FLOWS FROM OPERATING ACTIVITIES
|
||||||||
|
Net income
|
$ | 4,827,608 | $ | 2,437,927 | ||||
|
Adjustments to reconcile net income to net cash
|
||||||||
|
provided by (used in) operating activities:
|
||||||||
|
Depreciation and amortization
|
1,203,142 | 1,069,735 | ||||||
|
Provision for doubtful accounts
|
175,865 | 154,075 | ||||||
|
Loss on assets retirement
|
- | 7,285 | ||||||
|
Changes in operating assets and liabilities:
|
||||||||
|
Accounts receivable
|
875,495 | (3,203,345 | ) | |||||
|
Inventories
|
(6,128,801 | ) | (2,245,275 | ) | ||||
|
Advances to suppliers
|
42,010 | (367,239 | ) | |||||
|
Prepaid expenses and other receivable
|
(889,840 | (44,219 | ) | |||||
|
Due from related parties
|
(27,551 | ) | (2,213 | ) | ||||
|
Accounts payable
|
506,798 | 205,132 | ||||||
|
Accrued expense and other payable
|
1,418,673 | 1,163,547 | ||||||
|
Customer and other deposits
|
383,386 | (3,183 | ) | |||||
|
Net cash provided by (used in) operating activities
|
2,386,785 | (827,773 | ) | |||||
|
CASH FLOWS FROM INVESTING ACTIVITIES
|
||||||||
|
Purchase of property and equipment
|
(1,383,847 | ) | (138,873 | ) | ||||
|
Purchase of intangible assets
|
- | (4,567 | ) | |||||
|
Net cash provided by (used in) Investing activities
|
(1,383,847 | ) | (143,440 | ) | ||||
|
CASH FLOWS FROM FINANCING ACTIVITIES
|
||||||||
|
Payment from short-term loans
|
(4,099,800 | ) | - | |||||
|
Borrowings from bank loans
|
4,099,800 | 1,273,255 | ||||||
|
Dividends paid
|
(439,264 | ) | - | |||||
|
Net cash provided by (used in) financing activities
|
(439,264 | ) | 1,273,255 | |||||
|
Effect of exchange rate changes on cash and cash equivalents
|
2,857 | (71,970 | ) | |||||
|
Net Increase (Decrease) in cash and cash equivalents
|
566,531 | 230,072 | ||||||
|
Cash and cash equivalents, beginning balance
|
491,626 | 261,554 | ||||||
|
Cash and cash equivalents, ending balance
|
$ | 1,058,157 | $ | 491,626 | ||||
|
SUPPLEMENTAL DISCLOSURES:
|
||||||||
|
Interest payments
|
$ | 327,141 | $ | 319,472 | ||||
The accompanying notes are an integral part of these consolidated financial statements.
F-4
|
DONGKE PHARMACEUTICALS INC.
FOR THE YEARS ENDED DECEMBER 31, 2009 AND 2008
|
|
Additional
|
Other
|
Total
|
||||||||||||||||||||||||||
|
Capital Stock
|
Subscription
|
Paid-in
|
Comprehensive
|
Statutory
|
Retained
|
Stockholders
|
||||||||||||||||||||||
|
Amount
|
Receivables
|
Capital
|
Income
|
Reserves
|
Earnings
|
Equity
|
||||||||||||||||||||||
|
Balance December 31, 2007
|
$ | 26,700 | $ | (26,700 | ) | $ | 10,339,590 | $ | (91,592 | ) | $ | 31,440 | $ | (220,566 | ) | $ | 10,058,872 | |||||||||||
| - | ||||||||||||||||||||||||||||
|
Foreign currency translation adjustments
|
(47,178 | ) | (47,178 | ) | ||||||||||||||||||||||||
|
Transferred to Statutory reserve
|
243,793 | (243,793 | ) | - | ||||||||||||||||||||||||
|
Income for the year ended December 31, 2008
|
2,437,927 | 2,437,927 | ||||||||||||||||||||||||||
|
Balance December 31, 2008
|
26,700 | (26,700 | ) | 10,339,590 | (138,770 | ) | 275,233 | 1,973,568 | 12,449,621 | |||||||||||||||||||
|
Foreign currency translation adjustments
|
11,067 | 11,067 | ||||||||||||||||||||||||||
|
Transferred to Statutory reserve
|
2,173,223 | (2,173,223 | ) | - | ||||||||||||||||||||||||
|
Dividend paid
|
(439,264 | ) | (439,264 | ) | ||||||||||||||||||||||||
|
Income for the year ended December 31, 2009
|
4,827,608 | 4,827,608 | ||||||||||||||||||||||||||
|
Balance December 31, 2009
|
$ | 26,700 | $ | (26,700 | ) | $ | 10,339,590 | $ | (127,703 | ) | $ | 2,448,456 | $ | 4,188,689 | $ | 16,849,032 | ||||||||||||
The accompanying notes are an integral part of these consolidated financial statements
F-5
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2009
Note 1 - ORGANIZATION
In January 2010, WFOE has entered into an Agreement on Entrustment for Operation and Management, Exclusive Option Agreement, Shareholders’ Voting Proxy Agreement and Agreement on share pledge Agreement (collectively, the “Control Agreements”) with Maidisen through which the Company operates the restricted businesses. Under accounting principles generally accepted in the United States of America, or US GAAP, Maidisen is considered a Variable Interest Entity (“VIE”) and the related accounts are recorded at carrying value. US GAAP requires the Company to consolidate entities controlled by contract as a VIE in financial statements because the control agreements related to those entities provide the Company with the risks and rewards associated with equity ownership. Although the Company does not currently own any of the outstanding equity interests in Maidisen, it has: (1) exclusive decision making authority over all business decisions, (2) a significant financial interest in the entity giving it the right to receive income as well as the right to unilaterally sell or transfer these rights to a 3rd party based upon the exclusive option agreement and (3) a long-term commitment expected to be in excess of ten years. Accordingly, all of the income since the inception of Maidisen is included in the consolidated financial statements
The following are brief descriptions of contracts entered between WFOE and Maidisen:
(1)Agreement on Entrustment for Operation and Management. The domestic companies, Maidisen and WFOE, have entered into an Entrusted Management Agreement, which provides that WFOE will be fully and exclusively responsible for the management of Maidisen. As consideration for such services, Maidisen has agreed to pay WFOE the management fee during the term of this agreement and the management fee shall equal to Maidisen’s the earnings before tax. Also, WFOE will assume all operating risks related to this entrusted management service to Maidisen and bear all losses of Maidisen.
(2) Exclusive Option Agreement. All the shareholders of Maidisen as well as Maidisen have entered into an Exclusive Option Agreement with WFOE, which provides that WFOE will be entitled to acquire such shares from the current shareholders upon certain terms and conditions. Meanwhile, WFOE will be entitled an irrevocable exclusive purchase option to purchase all or part of the assets and business of Maidisen, if such a purchase is or becomes allowable under PRC laws and regulations. The Exclusive Option Agreement also prohibits the current shareholders of Maidisen as well as Maidisen from transferring any portion of their equity interests, business or assets to anyone other than WFOE. WFOE has not yet taken any corporate action to exercise this right of purchase, and there is no guarantee that it will do so or will be permitted to do so by applicable law at such times as it may wish to do so.
(3) Shareholders’ Voting Proxy Agreement. All the shareholders of Maidisen have executed a Shareholders’ Voting Proxy Agreement to irrevocably appoint the persons designated by WFOE with the exclusive right to exercise, on their behalf, all of their Voting Rights in accordance with the laws and Maidisen’s Articles of Association, including but not limited to the rights to sell or transfer all or any of their equity interests of Maidisen, and to appoint and elect the directors and Chairman as the authorized legal representative of Maidisen. This agreement can only be terminated prior to the completion of acquisition of all of the equity interests in, or all assets or business of Maidisen.
(4) Share Pledge Agreement. WFOE and the shareholders of Maidisen have entered into a Share Pledge Agreement, pursuant to which all shareholders pledge all of their shares (100%) of Maidisen, as appropriate, to WFOE. If Maidisen or any of its respective shareholders breaches its respective contractual obligations in the “Entrusted Management Agreement”, “Exclusive Option Agreement” and “Shareholders’ Voting Proxy Agreement”, WFOE as Pledge, will be entitled to certain rights to foreclose on the pledged equity interests. Such Maidisen shareholders cannot dispose of the pledged equity interests or take any actions that would prejudice WFOE’s interest
Except for the disclosed above, there are no arrangements that could require WFOE to provide financial support to the variable interest entity, including events or circumstances that could expose WFOE to a loss. As stated in the disclosure of various agreements between WFOE and its VIE, WFOE has rights to acquire any portion of the equity interests of the VIE. Also, WFOE may allocate its available funds to its VIE for business purposes. There are no fixed terms of such arrangements.
The Company, through its subsidiary, and exclusive contractual arrangement with Maidisen, is engaged in the business of manufacturing and marketing over-the-counter (“OTC”) and pharmaceutical products based on traditional Chinese medicines designed to address for a variety of diseases and conditions.
These consolidated financial statements present the Company and its subsidiaries on a historical proforma basis.
Note 2 - SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Basis of Presentation
The accompanying consolidated financial statements have been prepared in conformity with accounting principles generally accepted in the United States of America and represent the pro forma historical results of the consolidated group. The Company adopted the new accounting guidance (“Codification”) on July 1, 2009. For the year ended December 31, 2009, all reference for periods subsequent to July 1, 2009 are based on the codification. The Company's functional currency is the Chinese Renminbi; however the accompanying consolidated financial statements have been translated and presented in the United States Dollars.
Principles of Consolidation
The consolidated financial statements include the accounts of the Company, its subsidiary and variable interest entity (“VIE”) for which the Company is the primary beneficiary. All inter-company accounts and transactions have been eliminated in consolidation. The Company has adopted FIN 46R which requires a VIE to be consolidated by a company if that company is subject to a majority of the risk of loss for the VIE or is entitled to receive a majority of the VIE’s residual returns.
F-6
DONGKE PHARMACEUTICALS INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2009
Note 2 - SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (CONTINUED)
Translation Adjustment
As of December 31, 2009 and December 31, 2008, the accounts of the Company were maintained, and its financial statements were expressed, in Chinese Yuan Renminbi (“CNY”). Such financial statements were translated into U.S. Dollars (“USD”) in accordance with the Foreign Currency Matters Topic of the Codification, with the CNY as the functional currency. According to the Codification, all assets and liabilities were translated at the current exchange rate, stockholders’ equity are translated at the historical rates and income statement items are translated at the average exchange rate for the period. The resulting translation adjustments are reported under other comprehensive income in accordance with the Comprehensive Income Topic of the Codification, as a component of shareholders’ equity. Transaction gains and losses are reflected in the income statement.
Use of Estimates
The preparation of financial statements in conformity with generally accepted accounting principles requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the
reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates.
Comprehensive Income
The Company follows the Comprehensive Income Topic of the Codification. Comprehensive income is a more inclusive financial reporting methodology that includes disclosure of certain financial information that historically has not been recognized in the calculation of net income.
Risks and Uncertainties
The Company’s operations are carried out in the PRC. Accordingly, the Company’s business, financial condition and results of operations may be influenced by the political, economic and legal environments in the PRC, and by the general state of the PRC’s economy. The Company’s business may be influenced by changes in governmental policies with respect to laws and regulations, anti-inflationary measures, currency conversion and remittance abroad, and rates and methods of taxation, among other things.
The Company is subject to substantial risks from, among other things, intense competition associated with the industry in general, other risks associated with financing, liquidity requirements, rapidly changing customer requirements, limited operating history, foreign currency exchange rates and the volatility of public markets.
Contingencies
Certain conditions may exist as of the date the financial statements are issued, which may result in a loss to the Company but which will only be resolved when one or more future events occur or fail to occur. The Company’s management and legal counsel assess such contingent liabilities, and such assessment inherently involves an exercise of judgment. In assessing loss contingencies related to legal proceedings that are pending against the Company or unasserted claims that may result in such proceedings, the Company’s legal counsel evaluates the perceived merits of any legal proceedings or unasserted claims as well as the perceived merits of the amount of relief sought or expected to be sought. There were no contingencies of this type as of December 31, 2009 and 2008.
F-7
DONGKE PHARMACEUTICALS INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2009
Note 2 - SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (CONTINUED)
If the assessment of a contingency indicates that it is probable that a material loss has been incurred and the amount of the liability can be estimated, then the estimated liability would be accrued in the Company’s financial statements. If the assessment indicates that a potential material loss contingency is not probable but is reasonably possible, or is probable but cannot be estimated, then the nature of the contingent liability, together with an estimate of the range of possible loss if determinable and material would be disclosed. There were no contingencies of this type as of December 31, 2009 and 2008.
Loss contingencies considered to be remote by management are generally not disclosed unless they involve guarantees, in which case the guarantee would be disclosed.
Cash and Cash Equivalents
Cash and cash equivalents include cash in hand and cash in time deposits, certificates of deposit and all highly liquid debt instruments with original maturities of three months or less.
Accounts Receivable
The Company maintains reserves for potential credit losses on accounts receivable. Management reviews the composition of accounts receivable and analyzes historical bad debts, customer concentrations, customer credit worthiness, current economic trends and changes in customer payment patterns to evaluate the adequacy of these reserves. Reserve balances at December 31, 2009 and 2008 were $0.
Inventories
Inventories are valued at the lower of cost (determined on a weighted average basis) or market. The Management compares the cost of inventories with the market value and allowance is made for writing down their inventories to market value, if lower. As of December 31, 2009 and December 31, 2008, inventories consist of the following:
|
2009
|
2008
|
|||||||
|
Raw materials
|
$ | 7,090,312 | $ | 2,621,106 | ||||
|
Work in process
|
701,317 | 521,825 | ||||||
|
Finished goods
|
2,718,168 | 1,211,077 | ||||||
|
Total
|
$ | 10,509,797 | $ | 4,354,008 | ||||
F-8
DONGKE PHARMACEUTICALS INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2009
Note 2 - SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (CONTINUED)
Property, Plant & Equipment
Property and equipment are stated at cost. Expenditures for maintenance and repairs are charged to earnings as incurred; additions, renewals and betterments are capitalized. When property and equipment are retired or otherwise disposed of, the related cost and accumulated depreciation are removed from the respective accounts, and any gain or loss is included in operations. Depreciation of property and equipment is provided using the straight-line method for substantially all assets with estimated lives of:
|
Buildings
|
10- 30 years
|
|
Leasehold improvement
|
6-10 years
|
|
Transportation equipment
|
5-10 years
|
|
Fixture and furniture
|
5-10 years
|
|
Machinery and equipment
|
5 -12 years
|
As of December 31, 2009 and 2008 Property, Plant & Equipment consist of the following:
|
2009
|
2008
|
|||||||
|
Buildings
|
$ | 4,389,792 | $ | 4,123,292 | ||||
|
Leasehold improvements
|
41,715 | 41,715 | ||||||
|
Transportation equipment
|
99,604 | 47,284 | ||||||
|
Fixture and furniture
|
117,922 | 116,604 | ||||||
|
Machinery and equipment
|
2,389,875 | 1,326,165 | ||||||
|
Total
|
$ | 7,038,908 | $ | 5,655,060 | ||||
|
Accumulated depreciation
|
(1,971,507 | ) | (1,489,796 | ) | ||||
| $ | 5,067,401 | $ | 4,165,264 | |||||
Depreciation expense for the years ending December 31, 2009 and 2008 was $481,711 and $373,243, respectively.
F-9
DONGKE PHARMACEUTICALS INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2009
Note 2 - SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (CONTINUED)
Intangible Assets
Intangible assets are amortized using the straight-line method over their estimated period of benefit, ranging from six to fifty years. Management evaluate the recoverability of intangible assets periodically and take into account events or circumstances that warrant revised estimates of useful lives or that indicate that impairment exists. No impairments of intangible assets have been identified during any of the periods presented. The land use rights will expire in 2056 and 2058. The proprietary technologies were contributed by four shareholders of the Company and relate to the production of the Company's five state approved drugs. All of the Company’s intangible assets are subject to amortization with estimated lives of:
|
Land use right
|
50 years
|
|
Software
|
6 years
|
|
Proprietary technologies
|
10-20 years
|
The components of finite-lived intangible assets are as follows:
|
December 31,
|
December 31,
|
|||||||
|
2009
|
2008
|
|||||||
|
Land use right
|
$ | 1,691,077 | $ | 1,691,077 | ||||
|
Software
|
4,567 | 4,567 | ||||||
|
Proprietary technologies
|
7,138,063 | 7,138,063 | ||||||
| 8,833,707 | 8,833,707 | |||||||
|
Less: Accumulated amortization
|
(2,196,807 | ) | (1,475,375 | ) | ||||
| $ | 6,636,899 | $ | 7,358,332 | |||||
Amortization expense for the years ending December 31, 2009 and 2008 was $721,432 and $696,492 respectively.
The estimated future amortization expenses related to intangible asset as of December 31, 2008 are as follows:
|
Years Ending December 31,
|
||||
|
2010
|
$ | 721,432 | ||
|
2011
|
721,432 | |||
|
2012
|
721,432 | |||
|
2013
|
721,432 | |||
|
2014
|
721,432 | |||
|
Thereafter
|
$ | 3,029,739 | ||
Long-Lived Assets
Effective January 1, 2002, the Company adopted the Property, Plant and Equipment Topic of the Codification, which addresses financial accounting and reporting for the impairment or disposal of long-lived assets and supersedes previous accounting guidance, “Accounting for the Impairment of Long-Lived Assets and for Long-Lived Assets to be Disposed Of,” and “Reporting the Results of Operations for a Disposal of a Segment of a Business.” The Company periodically evaluates the carrying value of long-lived assets to be held and used, which requires impairment losses to be recorded on long-lived assets used in operations when indicators of impairment are present and the undiscounted cash flows estimated to be
F-10
DONGKE PHARMACEUTICALS INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2009
Note 2 - SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (CONTINUED)
Long-Lived Assets (Continued)
generated by those assets are less than the assets carrying amounts. In that event, a loss is recognized based on the amount by which the carrying amount exceeds the fair market value of the long-lived assets. Loss on long-lived assets to be disposed of is determined in a similar manner, except that fair market values are reduced for the cost of disposal. Based on its review, the Company believes that, as of September 30, 2009, there were no impairments of its long-lived assets.
Fair Value of Financial Instruments
The Financial Instrument Topic of the Codification requires that the Company disclose estimated fair values of financial instruments. The carrying amounts reported in the statements of financial position for current assets and current liabilities qualifying as financial instruments are a reasonable estimate of fair value.
Revenue Recognition
The Company records revenues for goods shipped at the time of shipment. For goods delivered by the Company to a customer’s site, revenues are recognized at time of delivery.
Advertising
Advertising expenses consist primarily of costs of promotion for corporate image and product marketing and costs of direct advertising. The Company expenses all advertising costs as incurred.
Income Taxes
The Company utilizes the accounting guidance, “Accounting for Income Taxes,” which requires the recognition of deferred tax assets and liabilities for the expected future tax consequences of events that have been included in the financial statements or tax returns. Under this method, deferred income taxes are recognized for the tax consequences in future years of differences between the tax bases of assets and liabilities and their financial reporting amounts at each period end based on enacted tax laws and statutory tax rates applicable to the periods in which the differences are expected to affect taxable income. Valuation allowances are established, when necessary, to reduce deferred tax assets to the amount expected to be realized.
It is the Company’s intention to permanently reinvest earnings from activity with china. And thereby indefinitely postpone repatriation of these funds to the US. Accordingly, no domestic deferred income tax provision has been made fro US income tax which could result from paying dividend to the Company.
There were no deferred tax difference in 2009 and 2008
F-11
DONGKE PHARMACEUTICALS INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2009
Note 2 - SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (CONTINUED)
Statement of Cash Flows
In accordance with Statement of Cash Flows of the Codification, cash flows from the Company’s operations are based upon the local currencies. As a result, amounts related to assets and liabilities reported on the statement of cash flows will not necessarily agree with changes in the corresponding balances on the balance sheet.
Concentration of Credit Risk
Financial instruments that potentially subject the Company to concentrations of credit risk are cash, accounts receivable and other receivables arising from its normal business activities. The Company places its cash in what it believes to be credit-worthy financial institutions. The Company has a diversified customer base, most of which are in China. The Company controls credit risk related to accounts receivable through credit approvals, credit limits and monitoring procedures. The Company routinely assesses the financial strength of its customers and, based upon factors surrounding the credit risk, establishes an allowance, if required, for uncollectible accounts and, as a consequence, believes that its accounts receivable credit risk exposure beyond such allowance is limited.
Recent Accounting Pronouncements
In May 2009, the FASB issued accounting guidance for “Subsequent Events”, which is included in ASC Topic 855, Subsequent Events. ASC Topic 855 established principles and requirements for evaluating and reporting subsequent events and distinguishes which subsequent events should be recognized in the financial statements versus which subsequent events should be disclosed in the financial statements. ASC Topic 855 also required disclosure of the date through which subsequent events are evaluated by management. ASC Topic 855 was effective for interim periods ending after June 15, 2009 and applies prospectively. Because ASC Topic 855 impacted the disclosure requirements, and not the accounting treatment for subsequent events, the adoption of ASC Topic 855 did not impact our results of operations or financial condition.
Effective July 1, 2009, the Company adopted the Financial Accounting Standards Board (“FASB”) Accounting Standards Codification (“ASC”) 105-10, Generally Accepted Accounting Principles – Overall (“ASC 105-10”). ASC 105-10 establishes the FASB Accounting Standards Codification (the “Codification”) as the source of authoritative accounting principles recognized by the FASB to be applied by nongovernmental entities in the preparation of financial statements in conformity with U.S. GAAP. Rules and interpretive releases of the SEC under authority of federal securities laws are also sources of authoritative U.S. GAAP for SEC registrants. All guidance contained in the Codification carries an equal level of authority. The Codification superseded all existing non-SEC accounting and reporting standards. All other non-grandfathered, non-SEC accounting literature not included in the Codification is non-authoritative. The FASB will not issue new standards in the form of Statements, FASB Staff Positions or Emerging Issues Task Force Abstracts. Instead, it will issue Accounting Standards Updates (“ASUs”). The
FASB will not consider ASUs as authoritative in their own right. ASUs will serve only to update the Codification, provide background information about the guidance and provide the bases for conclusions on the change(s) in the Codification. References made to FASB guidance throughout this document have been updated for the Codification.
In August 2009, the FASB issued, Measuring Liabilities at Fair Value, which provides additional guidance on how companies should measure liabilities at fair value under ASC 820. The ASU clarifies that the quoted price for an identical liability should be used. However, if such information is not available, an
F-12
DONGKE PHARMACEUTICALS INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2009
Note 2 - SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (CONTINUED)
entity may use, the quoted price of an identical liability when traded as an asset, quoted prices for similar liabilities or similar liabilities traded as assets, or another valuation technique (such as the market or income approach). The ASU also indicates that the fair value of a liability is not adjusted to reflect the impact of contractual restrictions that prevent its transfer and indicates circumstances in which quoted prices for an identical liability or quoted price for an identical liability traded as an asset may be considered level 1 fair value measurements. This ASU is effective October 1, 2009. The Company is currently evaluating the impact of this standard, but would not expect it to have an impact on the Company’s consolidated results of operations or financial condition.
In September 2009, the FASB issued, Investments in Certain Entities That Calculate Net Asset Value per Share (or Its Equivalent), that amends ASC 820 to provide guidance on measuring the fair value of certain
alternative investments such as hedge funds, private equity funds and venture capital funds. The ASU indicates that, under certain circumstance, the fair value of such investments may be determined using net asset value (NAV) as a practical expedient, unless it is probable the investment will be sold at something other than NAV. In those situations, the practical expedient cannot be used and disclosure of the remaining actions necessary to complete the sale is required. The ASU also requires additional disclosures of the attributes of all investments within the scope of the new guidance, regardless of whether an entity used the practical expedient to measure the fair value of any of its investments. This ASU is effective October 1,
2009. The Company is currently evaluating the impact of this standard, but would not expect it to have an impact on the Company’s consolidated results of operations or financial condition.
In October 2009, the FASB issued, Multiple-Deliverable Revenue Arrangements—a consensus of the FASB Emerging Issues Task Force, that provides amendments to the criteria for separating consideration in multiple-deliverable arrangements. As a result of these amendments, multiple-deliverable revenue arrangements will be separated in more circumstances than under existing U.S. GAAP. The ASU does this by establishing a selling price hierarchy for determining the selling price of a deliverable. The selling price used for each deliverable will be based on vendor-specific objective evidence if available, third-party evidence if vendor-specific objective evidence is not available, or estimated selling price if neither vendor-specific objective evidence nor third-party evidence is available. A vendor will be required to determine its best estimate of selling price in a manner that is consistent with that used to determine the price to sell the deliverable on a standalone basis. This ASU also eliminates the residual method of allocation and will require that arrangement consideration be allocated at the inception of the arrangement to all deliverables using the relative selling price method, which allocates any discount in the overall arrangement proportionally to each deliverable based on its relative selling price. Expanded disclosures of qualitative and quantitative information regarding application of the multiple-deliverable revenue arrangement guidance are also required under the ASU. The ASU does not apply to arrangements for which industry specific allocation and measurement guidance exists, such as long-term construction contracts and software transactions. This is effective beginning January 1, 2011. The Company is currently evaluating the impact of this standard on its consolidated results of operations and financial condition.
In October 2009, the FASB issued, Certain Revenue Arrangements That Include Software Elements—a consensus of the FASB Emerging Issues Task Force, that reduces the types of transactions that fall within the current scope of software revenue recognition guidance. Existing software revenue recognition guidance requires that its provisions be applied to an entire arrangement when the sale of any products or services containing or utilizing software when the software is considered more than incidental to the product or service. As a result of the amendments, many tangible products and services that rely on software will be accounted for under the multiple-element arrangements revenue recognition guidance rather than under the software revenue recognition guidance. Under the amendments, the following components would be excluded from the scope of software revenue recognition guidance: the tangible element of the product, software products bundled with tangible products where the software components and non-software components function together to deliver the product’s essential functionality, and
F-13
DONGKE PHARMACEUTICALS INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2009
Note 2 - SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (CONTINUED)
undelivered components that relate to software that is essential to the tangible product’s functionality. The ASU also provides guidance on how to allocate transaction consideration when an arrangement contains both deliverables within the scope of software revenue guidance (software deliverables) and deliverables not within the scope of that guidance (non-software deliverables). This amendment is effective beginning January 1, 2011. The Company is currently evaluating the impact of this standard on its consolidated results of operations and financial condition.
Note 3 –OTHER RECEIVABLES
Other receivables mainly consist of cash advances to employees. As of December 31, 2009 and December 31, 2008, the other receivables were $762,158 and $70,941, respectively. December 31, 2009 amounts also include $234,700 of R&D income receivable.
Note 4 – DUE FROM RELATED PARTY
The Company has a receivable due from related parties. As of December 31, 2009 and December 31, 2008, due from relate party were $ 172,655and $144,528, respectively.
Note 5 – COMPENSATED ABSENCES
Regulation 45 of the local labor law of the People’s Republic of China (“PRC”) entitles employees to annual vacation leave after 1 year of service. In general, all leave must be utilized annually, with proper notification. Any unutilized leave is cancelled.
Note 6 – DEBT
As of December 31, 2009 and 2008, the Company had debt as follows:
|
12/31/2009
|
12/31/2008
|
||||||||
|
China Merchant Bank
|
$ | 1,320,190 |
China Merchant Bank
|
$ | 1,315,405 | ||||
|
Terms of the loan call for interest 7.00% per annum, with principal due in November 2010
|
Terms of the loan call for interest 7.47% per annum, with principal due in 2009
|
||||||||
|
Commercial Bank of China –Zhonglou Branch
|
2,787,068 |
Commercial Bank of China –Zhonglou Branch
|
2,776,966 | ||||||
|
Term of the loan called for interest 5.94% per annum, with principal due in July 2011
|
Term of these loans called for interest 4.5% per annum, with principal due in 2009
|
||||||||
|
Total
|
$ | 4,107,258 | $ | 4,092,371 | |||||
|
Less current portion
|
1,320,190 | 4,092,371 | |||||||
|
Non current portion
|
$ | 2,787,068 | $ | 0 | |||||
The Company is using these loans for working capital purposes and secured by land of the Company and a PRC stated-owned company.
F-14
DONGKE PHARMACEUTICALS INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2009
Note 7 - INCOME TAXES
Pursuant to the PRC Income Tax Laws, the Enterprise Income Tax (“EIT”) through December 31, 2007 is at a statutory rate of 33%, which is comprised of 30% national income tax and 3% local income tax. As of January 1, 2008, the EIT is at a statutory rate of 25%. The Company is a high tech enterprise and under PRC Income Tax Laws, it is entitled to a five-year exemption for 2007 through 2011. If the Company were subject to the standard 25% enterprise income tax rate, it would have incurred $1,206,902 and $609,482 of income tax expense for the years ended December 31, 2009 and 2008, respectively. The standard income tax rate would have caused the Company to incur an additional expense of $0.05 and $0.02 per share for the years ended December 31, 2009 and 2008, respectively.
ASC 740, Accounting for Income Taxes (“ASC 740”), requires that deferred tax assets be evaluated for future realization and reduced by a valuation allowance to the extent that the Company believes a portion, more likely than not, will not be realized. The Company considers many factors when assessing the likelihood of future realization of our deferred tax assets, including our recent cumulative earnings experience and expectations of future taxable income by taxing jurisdiction, the carry-forward periods available for tax reporting purposes, and other relevant factors.
The Company through its VIE adopted the provisions of FASB Accounting Standards Codification Topic 740, Accounting for Uncertainty in Income Taxes. ASC 740 clarifies the accounting for uncertainty in income taxes recognized in an enterprise’s financial statements. The standard prescribes a recognition and measurement method for the financial statement recognition measurement of a tax position taken or expected to be taken in a tax return. The standard also provides guidance on recognition, classification, interest and penalties, accounting interim periods, disclosure and transition. The Company considers many factors when evaluating and estimating tax positions and tax benefits, which may require periodic adjustments and which may not accurately forecast actual outcomes.
Based on a review of tax positions, the Company was not required to record a liability for unrecognized tax benefits as a result of adopting ASC 740. Further, there has been no change during the years ended December 31, 2009 and 2008. Accordingly, no interest and penalties were accrued through December 31, 2009 and 2008.
A reconciliation of the PRC Statutory income tax rate to the effective income tax rate is as follows:
|
December 31, 2009
|
December 31, 2008
|
|||||||
|
PRC Statutory income tax rate
|
25 | % | 25 | % | ||||
|
Preferential allowance as permitted
|
-25 | % | -25 | % | ||||
|
Other adjustments
|
0 | % | 0 | % | ||||
|
Total
|
0 | % | 0 | % | ||||
Note 8 – STATUTORY RESERVE
In accordance with the laws and regulations of the PRC, a wholly-owned Foreign Invested Enterprises income, after the payment of the PRC income taxes, shall be allocated to the statutory surplus reserves and statutory public welfare fund. Prior to January 1, 2006 the proportion of allocation for reserve was 10 percent of the profit after tax to the surplus reserve fund and additional 5-10 percent to the public affair fund. The public welfare fund reserve was limited to 50 percent of the registered capital. Effective January 1, 2006, there is now only one fund requirement. The reserve is 10 percent of income after tax, not to exceed 50 percent of registered capital. Statutory Reserve funds are restricted for set off against losses, expansion of production and operation or increase in register capital of the respective company. Statutory public welfare fund is restricted to the capital expenditures for the collective welfare of employees. These
reserves are not transferable to the Company in the form of cash dividends, loans or advances. These reserves are therefore not available for distribution except in liquidation. As of December 31, 2009 and December 31, 2008, the Company had allocated $2,448,456 and $275,233, respectively, to these non-distributable reserve funds.
Note 9 - OTHER COMPREHENSIVE INCOME
Balances of related after-tax components comprising accumulated other comprehensive income, included in stockholders’ equity, at December 31, 2009 and 2008, are as follows:
|
Foreign Currency Translation Adjustment
|
Accumulated Other Comprehensive Income
|
|||||||
|
Balance at December 31, 2007
|
$ | (91,592 | ) | $ | (91,592 | ) | ||
|
Change for 2008
|
(47,178 | ) | (47,178 | ) | ||||
|
Balance at December 31, 2008
|
(138,770 | ) | (138,770 | ) | ||||
|
Change for 2009
|
11,067 | 11,067 | ||||||
|
Balance at December 31, 2009
|
$ | (127,703 | ) | $ | (127,703 | ) | ||
Note 10- CURRENT VULNERABILITY DUE TO CERTAIN RISK FACTORS
The Company’s operations are carried out in the PRC. Accordingly, the Company’s business, financial condition and results of operations may be influenced by the political, economic and legal environments in the PRC, by the general state of the PRC's economy. The Company’s business may be influenced by changes in governmental policies with respect to laws and regulations, anti-inflationary measures, currency conversion and remittance abroad, and rates and methods of taxation, among other things.
F-15
DONGKE PHARMACEUTICALS INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2009
Note 11 – MAJOR CUSTOMERS AND CREDIT RISK
Four customers accounted for more than 10% of accounts receivable at December 31, 2009, totaling 52%; at December 31, 2008, one customer accounted for more than 10% of accounts receivable, totaling 22%. One vendor accounted for 17% of accounts payable at December 31, 2009; at December 31, 2008, one vendor accounted for 13% of accounts payable. One customer accounted for greater than 10% of sales at year end December 31, 2009, totaling 26%; at December 31, 2008, two customers accounted for greater than 10% of sales, totaling 38%. Four vendors supplied 56% of purchases at December 31, 2009. For December 31, 2008, two vendors accounted for 36% of purchases.
Note 12 – SUBSEQUENT EVENTS
For the year ended December 31, 2009, the Company has evaluated subsequent events for potential recognition and disclosure through March 29, 2010, the date of the financial statement issuance.
F - 16
(b) SHELL COMPANY TRANSACTIONS
Reference is made to Items 9.01(a) and 9.01(b) and the exhibits referred to therein, which are incorporated herein by reference.
(c) EXHIBITS
|
Exhibit Number
|
Description
|
|
|
2.1
|
Share Exchange Agreement dated May 10, 2010, by and among Dong Ke Pharmaceutical Inc., the shareholders of Dong Ke Pharmaceutical Inc, Virtual Closet, Inc. and You Ting Zhou, Ge Lin and Si Yu Min, shareholders of Virtual Closet, Inc.*
|
|
|
3.1
|
Articles of Incorporation of Virtual Closet, Inc., a Nevada corporation. (1)
|
|
|
3.2
|
Bylaws of Virtual Closet, Inc. (1)
|
|
|
10.1
|
Property Lease Agreement, Dated December 26, 2006, by and between Dongke Zhao and Maidisen.
|
|
|
10.2
|
Referral Agreement, dated January 7, 2010, by and between Jinhao Zhang and Dong Ke
|
|
|
10.3
|
Consulting Services Agreement, dated March 9, 2010, by and between NSD Consulting Services, INC. and Dong Ke
|
|
|
10.4
|
Investor Relations Agreement, dated April 2, 2010, by and between Avisa IR Services, Inc. and Dong Ke
|
|
|
10.5
|
Warrant, dated May 10, 2010, by and between Virtual Closet, Inc. and Jinhao Zhang
|
|
|
10.6
|
Warrant, dated May 10, 2010, by and between Virtual Closet, Inc. and NSD Consulting Services, Inc.
|
|
|
10.7
|
Warrant, dated May 10, 2010, by and between Virtual Closet, Inc. and Avisa IR Services, Inc.
|
|
|
10.8
|
Employment Agreement, dated March 1, 2010, by and between Dongke Zhao and Dong Ke
|
|
|
10.9
|
Employment Agreement , dated March 1, 2010, by and between Yanhong Ren and Dong Ke
|
|
|
10.10
|
Agreement on Entrustment for Operation and Management, dated January 27, 2010, by and between Maidisen and Slovan
|
|
|
10.11
|
Agreement on Share Pledge, dated January 27, 2010, by and between the shareholders of Maidisen and Slovan
|
|
|
10.12
|
Exclusive Option Agreement, dated January 27, 2010, by and between Slovan, Maidisen and the shareholders of Maidisen
|
|
|
10.13
|
Shareholders' Voting Proxy Agreement, dated January 27, 2010, by and between Slovan and shareholders of Slovan
|
|
|
10.14
|
Regional Sales Agency Agreement, Guangdong Province, dated January 1, 2009, by and between Maidisen, Guangdong National Medicine Co. Ltd and Dong Tiemin
|
|
|
10.15
|
Regional Sales Agency Agreement, Hebei Province, dated January 1, 2009, by and between Maidisen, Heibei Medicine and Herb Company and Zhang Jiansheng
|
|
|
10.16
|
Regional Sales Agency Agreement, Shaanxi Province, dated January 1, 2009, by and between Maidisen and Shaanxi Kanghua Medicine Company
|
|
|
10.17
|
Regional Sales Agency Agreement, Xingjiang Uygur Autonomous Region, dated January 1, 2009, by and between Maidisen, Xinjiang Jiuzhoutong Pharmaceutical Medicine Co., Ltd and Chu Jingchao
|
|
|
10.18
|
Regional Sales Agency Agreement, Zhejiang Province, dated January 1, 2009, by and between Maidisen and Zhejiang Haizhou Pharmaceutical Medicine Co., Ltd.
|
|
|
10.19
|
Supply Agreement, dated January 10, 2009, by and between Ren Yanhuai and Maidisen
|
|
|
10.20
|
Supply Agreement, dated November 1, 2009, by and between Maidisen, Shaanxi Yungui Subsidiary Foodstuff Corporation Ltd
|
|
|
10.21
|
Supply Agreement, dated September 1, 2009, by and between Maidisen and Xi'an Yinhai Industrial Fabrics Co., Ltd
|
|
|
10.22
|
Supply Agreement, dated February 10, 2009, by and between Maidisen and Xianyang Kaiyuan Industrial Co., Ltd.
|
|
|
10.23
|
Purchase and Sale Agreement, dated July 1, 2008, by and between Maidisen and Shanxi Guangsheng Capsule Co., Ltd.
|
|
|
10.24
|
Loan Agreement, dated December 2009, for a loan of RMB 9,000,000, by and between China Merchant Bank and Maidisen
|
|
|
10.25
|
Loan Agreement, date July 17, 2009, for a loan of RMB 19,000,000, by and between Xi’an City Commercial Bank and Maidisen
|
|
|
10.26
|
Patent Agency Agreement, dated February 16, 2005, by and between Maidisen and Dongke Zhao
|
|
|
10.27
|
Patent Transfer Agreement, dated January 1, 2007, by and between Maidisen and Dongke Zhao
|
|
|
10.28
|
Second Product Development Agreement, dated April 28, 2006, by and between Maidisen and Dong Ke
|
|
|
10.29
|
Letter of Appointment of Director, dated May 10, 2010, by and between Dongke Zhao and Michael Segal
|
|
|
16.1
|
Letter from the Company to Chang G. Park, CPA, dated May 10, 2010
|
|
|
99.1
|
Consolidated Financial Statements of Dong Ke Pharmaceutical, Inc. for the period ended March 31, 2010
|
|
(1)
|
Incorporated by reference to the exhibit to our registration statement on Form S-1 filed with the SEC on December 19, 2008.
|
* The schedules to this document are not being filed herewith. Virtual Closet, Inc. agrees to furnish supplementally a copy of any such schedule to the Securities and Exchange Commission upon request.
51
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the Registrant has duly caused this Report on Form 8-K /A to be signed on its behalf by the undersigned hereunto duly authorized.
|
VIRTUAL CLOSET, INC.
|
|||
|
Date: November 3, 2010
|
By:
|
/s/ Dongke Zhao
|
|
|
Dongke Zhao
|
|||
|
Chief Executive Officer (Chief Principal Officer)
|
|||
52
