Attached files
| file | filename |
|---|---|
| 8-K - TENGION, INC. FORM 8-K - TENGION INC | tengion8k.htm |
Exhibit 99.1

1
Tengion Analyst & Investor Meeting
October 27, 2010

2
Forward Looking Statements
Forward Looking Statements
Certain statements in this presentation may constitute forward looking statements within the
meaning of the Private Securities Litigation Reform Act of 1995. Although Tengion believes that
these statements are based upon reasonable assumptions within the bounds of its knowledge of
its business and operations, there are a number of factors that may cause actual results to differ
from these statements.
meaning of the Private Securities Litigation Reform Act of 1995. Although Tengion believes that
these statements are based upon reasonable assumptions within the bounds of its knowledge of
its business and operations, there are a number of factors that may cause actual results to differ
from these statements.
For instance there can be no assurance that: (i) the Company will be able to obtain the capital it
needs to develop its product candidates or continue as a going concern; (ii) the Company will be
able to successfully enroll patients in its clinical trials, including its Phase I clinical trial for the
Neo-Urinary Conduit; (iii) patients enrolled in the Company’s clinical trials will not experience
adverse events related to the Company’s product candidates, which could delay clinical trials or
cause the Company to terminate the development of a product candidate; (iv) the results of the
clinical trial for the Neo-Urinary Conduit will support further development of that product
candidate; (v) data from the Company’s ongoing preclinical studies will continue to be
supportive of advancing its preclinical product candidates; and (vi) the Company will be able to
progress its product candidates that are undergoing preclinical testing, including the Neo-
Kidney Augment, into clinical trials.
needs to develop its product candidates or continue as a going concern; (ii) the Company will be
able to successfully enroll patients in its clinical trials, including its Phase I clinical trial for the
Neo-Urinary Conduit; (iii) patients enrolled in the Company’s clinical trials will not experience
adverse events related to the Company’s product candidates, which could delay clinical trials or
cause the Company to terminate the development of a product candidate; (iv) the results of the
clinical trial for the Neo-Urinary Conduit will support further development of that product
candidate; (v) data from the Company’s ongoing preclinical studies will continue to be
supportive of advancing its preclinical product candidates; and (vi) the Company will be able to
progress its product candidates that are undergoing preclinical testing, including the Neo-
Kidney Augment, into clinical trials.
For additional factors which could cause actual results to differ from expectations, reference is
made to the reports filed by the Company with the Securities and Exchange Commission under
the Securities Exchange Act of 1934, as amended. The forward looking statements in this
presentation are made only as of the date hereof and the Company disclaims any intention or
responsibility for updating predictions or expectations in this presentation.
made to the reports filed by the Company with the Securities and Exchange Commission under
the Securities Exchange Act of 1934, as amended. The forward looking statements in this
presentation are made only as of the date hereof and the Company disclaims any intention or
responsibility for updating predictions or expectations in this presentation.

3
Agenda
Company Overview
Steven Nichtberger, MD, President and CEO
Tengion’s Regenerative Medicine Platform and Product Development Pathway
Tim Bertram, DVM, PhD - Executive Vice President Science & Technology and Chief Scientific Officer
Neo-Urinary Conduit - Clinical
Sunita Sheth, MD, Chief Medical Officer, Vice President Clinical & Regulatory Affairs
Neo-Urinary Conduit - Commercial Opportunity
Mark Stejbach, Vice President & Chief Commercial Officer
- Q&A
Neo-Kidney Augment - Preclinical Research Program
Sharon Presnell, PhD, Senior Vice President Regenerative Medicine & Biology
Neo-Kidney Augment - Research to Clinic
Deepak Jain, PhD, Senior Vice President, Bioprocess Research & Development
Neo-Kidney Augment - Clinical Development Considerations
Sunita Sheth, MD, Chief Medical Officer, Vice President Clinical & Regulatory Affairs
Conclusion
Steven Nichtberger, MD, President and CEO
- Q&A

4
Tengion products harness the body’s natural ability to regenerate
§ Unique and productive research platform
Lead clinical product targets patients with bladder cancer
§ First patient enrolled, biopsied and implanted successfully
– 1 quarter later than anticipated
§ Investigators anticipate more timely enrollment going forward
§ Significant unmet need drives billion dollar market opportunity
Lead preclinical program delays progression of kidney failure
§ Four key animal models of chronic renal failure
– Initial proof of concept data - AJP/RP: Sept 10
– Diabetic renal failure study - ISCT: Sept 10
– Human cells implanted into nude rats with renal failure - TERMIS Dec 10
– Large animal study - American Society of Transplantation Oct 10
§ Strong and growing foundation of evidence for product development
Company Highlights
Company Highlights
5
Catalyzing Regeneration in the Body
INTEGRATED
PLATFORM
Industrialization
Cells
Biomaterials
Implantation
Humans have limited capacity to regenerate
Our platform uniquely catalyzes human tissue regeneration

7
Tengion’s Regenerative Medicine Platform and
Product Development Pathway
Tim Bertram, DVM, PhD

8
Regenerative solutions to address unmet medical needs
safely and efficaciously
Accelerated Product Development Pathway
Translation of Regenerative Medicine Products
Translation of Regenerative Medicine Products
§ Product-directed scientists addressing unmet medical need
§ Optimized product formulation for optimal outcomes
§ Established development pathway to commercialization
§ Scale-up manufacturing to meet market demands

9
Platform - Cells
Platform - Cells
Defined regenerative cells taken from the patient
Defined regenerative cells taken from the patient
§ Autologous cells
– Identify, characterize and expand selected regenerative cells
– Indifferent as to tissue source or cell type - focus is optimizing regeneration
– Avoids risks associated with allogeneic and other sources
Fat biopsy
Selected Regenerative
Cells (SMC)
Cells (SMC)
GMP shipping conditions
INTEGRATED
PLATFORM
Industrialization
Biomaterials
Cells
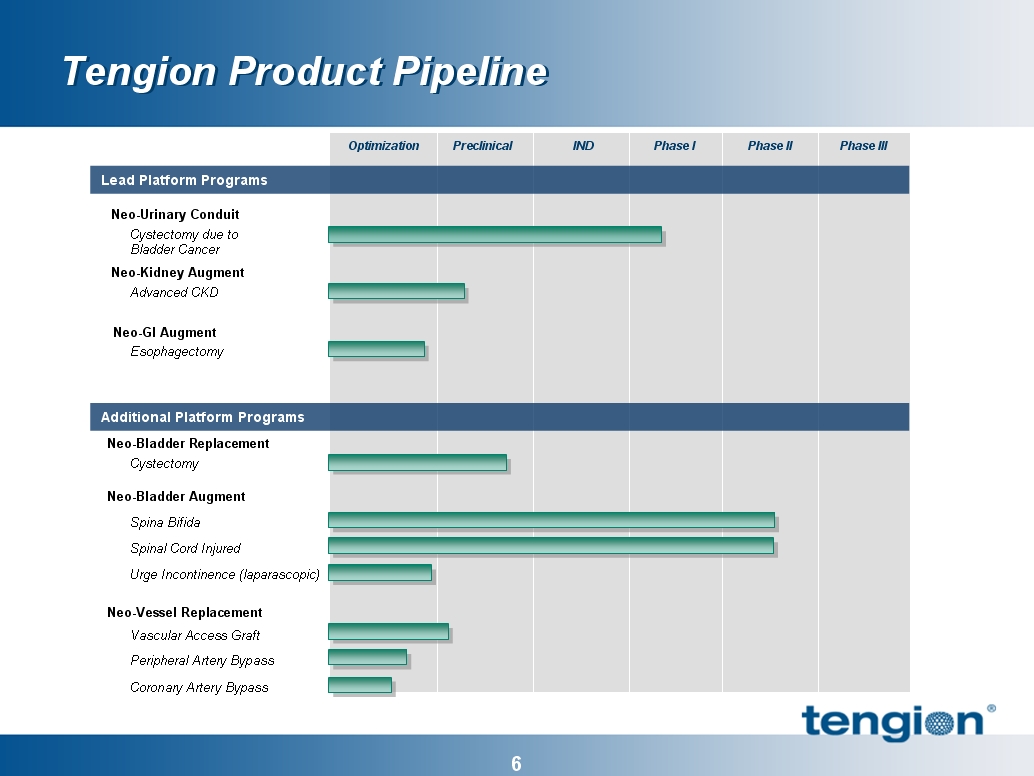
Kidney Augment: Chronic Kidney Disease
Conduit: Bladder cancer patients requiring bladder removal

10
Platform - Biomaterials
Platform - Biomaterials
Biomaterials for enhancing formulation and outcomes
Biomaterials for enhancing formulation and outcomes
Optimized formulation
Cellular control
Biodegradable scaffold
Cellular alignment
Functional regeneration
§ Focus on materials with clinical precedent to support regeneration
§ Optimize formulation for maximal product stability
Industrialization
Biomaterials
Cells
Kidney Augment: Chronic Kidney Disease
INTEGRATED
PLATFORM
Conduit: Bladder cancer patients requiring bladder removal
* Regeneration demonstrated in preclinical models

11
Platform - Industrialization
Platform - Industrialization
GMP compliant scale-up and focus on cost of goods
GMP compliant scale-up and focus on cost of goods
§ Integrated, GMP-compliant processing, bio-analytical methods and delivery devices to
produce scalable, cost-efficient products
produce scalable, cost-efficient products
Expansion
Production bioreactor
Production facilities
Expansion and isolation
Production facilities
Production bioreactor
INTEGRATED
PLATFORM
Industrialization
Biomaterials
Cells
Kidney Augment: Chronic Kidney Disease
Conduit: Bladder cancer patients requiring bladder removal
12
Platform - Implantation
Platform - Implantation
3-Dimensional tissue and organ regeneration
3-Dimensional tissue and organ regeneration
Delivery/Transport
system
Regenerative outcome
Neo-Urinary
Conduit
Conduit
Delivery system
Minimally invasive
Regenerative outcome
NX
NX + NKA
Healthy
§ Key opinion leaders have performed preclinical surgeries to inform product design
§ Implant procedure being standardized for consistent outcomes
INTEGRATED
PLATFORM
Industrialization
Biomaterials
Cells
Conduit: Bladder cancer patients requiring bladder removal *
Kidney Augment: Chronic Kidney Disease
* Actively evaluating in clinical program

13
§ BLA with CBER in the lead and CDRH collaborating
§ IND accepted in 30 days
§ Neo-Bladder Augment experience in US and Europe is instructive for
conduit
conduit
§ FDA interactions planned 2011
§ Combination product development pathway expected
§ Previous development experience instructive for optimal plan
Regulatory Pathway - Combination Products
Regulatory Pathway - Combination Products
CBER lead with CDRH collaboration
CBER lead with CDRH collaboration
Conduit: Bladder cancer patients requiring bladder removal
Kidney Augment: Chronic kidney disease
14
IP and Barriers to Entry
Issued patent protection to 2021, plus recent applications pending
§ 37 US and 130 international patents and patent applications
§ Core patents cover composition, design and methods of manufacture*
Plus know-how, trade secrets and integrated capabilities associated with
the discovery, development and manufacturing of our product candidates
the discovery, development and manufacturing of our product candidates
CELLS
DEVICE /
MATERIAL
PROCESS
THX USE
Urologic Franchise
= Patent Applications
= Issued Patents
Neo-Kidney Augment
CELLS
DEVICE /
MATERIAL
PROCESS
THX USE
* Individual patents may cover multiple elements

15
Neo-Urinary Conduit
Neo-Urinary Conduit
Clinical Update
Clinical Update
Sunita Sheth, MD
Sunita Sheth, MD
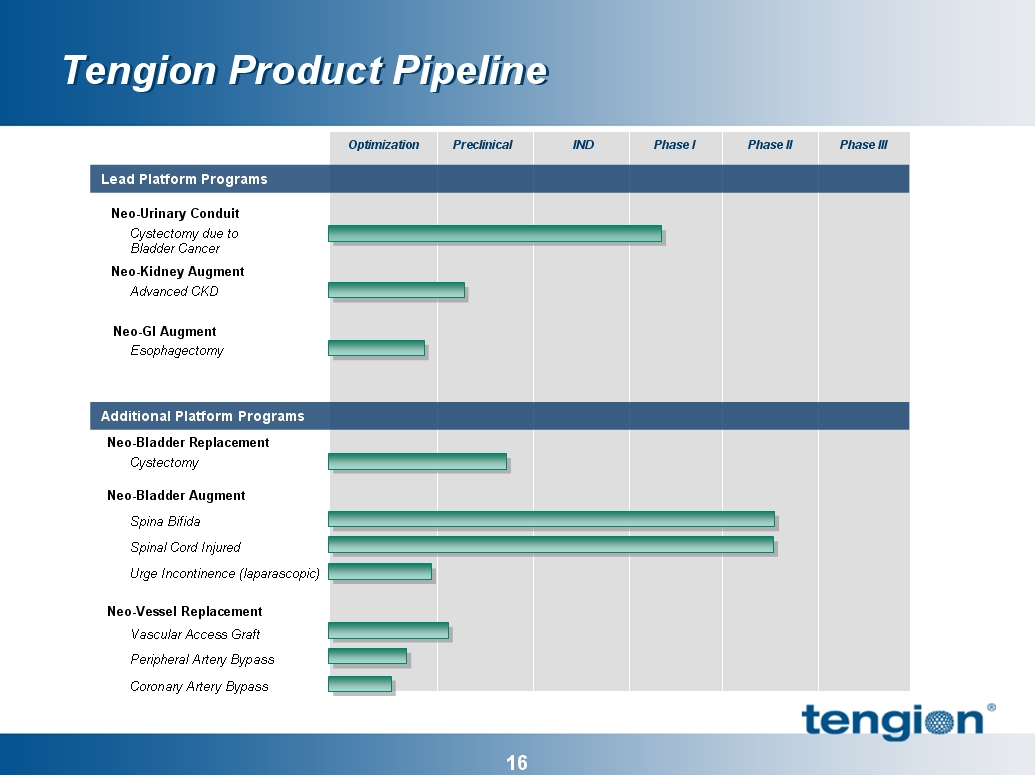

17
When bladder removal is needed, a urinary diversion procedure is performed…
§ Native bladder removed
§ Section of bowel isolated, with blood supply maintained
§ Bowel continuity re-established without the removed segment
§ Ureters connected to the bowel segment, which is connected to
abdominal wall for ostomy bag drainage
abdominal wall for ostomy bag drainage
Non-continent Urinary Diversion Conduit (20,000 annually in the US & EU)
§ Native bladder removed
§ Section of bowel isolated, with blood supply maintained
§ Bowel continuity re-established without the removed segment
§ Isolated bowel segment fashioned into a pouch
§ Ureters connected to the bowel segment, which is connected to
urethra
urethra
Orthotopic Neo-bladder (1,600 annually in the US & EU)
Bladder Cancer Management
Urinary diversion procedures
Urinary diversion procedures
Bladder Cancer Management
Urinary diversion procedures
Urinary diversion procedures

18
Current Standard of Care - Common Side Effects
Current Standard of Care - Common Side Effects
Absorption Issues
Electrolyte and metabolic imbalances,
drug re-absorption issues
drug re-absorption issues
Infection
Persistent infections in
~23% of conduit patients
~23% of conduit patients
Cancer
Malignancy may result from
use of bowel tissue
use of bowel tissue
Mucus Secretion
Bowel tissue secretes
mucus into urine
mucus into urine
Bowel Complications
Leaks, fistulas, obstructions, anemia
and neurological abnormalities
and neurological abnormalities
Stone Formation
Recurrent stones in ~10%
of conduit patients, due to
mucus secretion
of conduit patients, due to
mucus secretion

19
Neo-Urinary Conduit
Expanding an established regeneration technology platform
Expanding an established regeneration technology platform
SMC from Adipose
Scaffold
Construct
Surgical Implantation
In vivo Regeneration
Urinary diversion
Neo-Urinary Conduit

20
Open label study to define surgical procedure and safety profile
§ 5 patients with primary bladder cancer requiring bladder removal
– U of Chicago and Johns Hopkins screening
§ Selective enrollment criteria
– Bladder cancer, stage T1/T2 and N0, M0 only
– Not obese
– No history of pelvic radiation
– No uncontrolled diabetes or hypertension
§ Primary efficacy assessment
– Conduit integrity and patency at 1 year
§ Quarterly interim assessments
– Weekly visits in post-operative period
Neo-Urinary Conduit
Neo-Urinary Conduit
Clinical trial
Clinical trial

21
Neo-Urinary Conduit Trial
Incorporating years of clinical development experience
Incorporating years of clinical development experience
Selected entry criteria for initial clinical study
§ Enroll a less complicated and more homogeneous population
– Minimize risk of unrelated events
Standardize surgical approach and post-op stoma care
§ Same 2 surgeons at each surgery for the first 3 patients
Sequential patient enrollment first 3 patients
§ Minimum of 8 weeks between implants for first 3 patients
§ Permits optimization of surgical approach prior to the next patient

22
First patient has been implanted at U of Chicago
§ Biopsied, manufactured product to specifications, shipped, implanted
§ 1 quarter longer than anticipated to identify first patient
Investigators expect more timely enrollment going forward
§ First patient with Neo-Urinary Conduit now implanted
§ Two sites now actively screening rather than one
Actively evaluating
§ Broadening of patient selection criteria
§ Addition of more sites (minimal incremental cost per site)
Neo-Urinary Conduit
Neo-Urinary Conduit
Enrollment
Enrollment

23
Neo-Urinary Conduit
Commercial Opportunity
Mark Stejbach
Commercial Opportunity
Mark Stejbach

24
Conduit Commercial Opportunity
Efficacy without the morbidity of using bowel
Efficacy without the morbidity of using bowel
Compelling potential advantages across stakeholders
§ Eliminates need to harvest bowel from the patient
§ May reduce / eliminate many acute and chronic complications
§ Value proposition driven by targeted safety profile
Sources: US: NIS, average 2003-5; EU: National health records for UK,
France, Germany, (2004-6), population-based estimates for others
France, Germany, (2004-6), population-based estimates for others
11,000
Procedures*
Procedures*
13,000
Procedures*
Procedures*
*Annual procedures for all indications

25
Today, these patients get either a conduit or a bladder replacement
created from their own gastrointestinal tissue
Other conditions include:
radiation cystitis
pelvic trauma
diabetic neurogenic bladder
~15% of Conduits are placed for conditions
other than bladder cancer
other than bladder cancer
Cystectomy Procedures (IMS 2005-6)
Provides a sound basis for current market assumptions
Provides a sound basis for current market assumptions
ICD-9 Code Description Procedures
57.71 Radical Cystectomy 12,778

26
BASE: Total physicians performing cystectomies, n=204)
PPQ1. What advantages, if any, do these products have over existing options?
PPQ1. What advantages, if any, do these products have over existing options?
BioVid survey, Jul 08
Most Important Importance Rankings Least Important
“If this works,
why would I ever
do the traditional
procedure?”
why would I ever
do the traditional
procedure?”

27
Market Research Reinforces Rapid Uptake Expectations
Market Research Reinforces Rapid Uptake Expectations
Based on the product profile, urologists were asked
to rate the Neo-Urinary Conduit compared to the
standard of care
to rate the Neo-Urinary Conduit compared to the
standard of care
BASE: MDs (n=30) performing 3-25 ileal diversions per year.
Q19. How advantageous would this procedure be as opposed to the current
standard of care?
standard of care?
Very advantageous
Advantageous
No difference
Disadvantageous
Very Disadvantageous
0%
20%
40%
60%
80%
100%
52%
45%
3%
GLG Survey, Dec 08
Percent of respondents

28
Direct Sales Force
§ Sequenced product launches through centers of excellence
§ >65% of market covered by a specialty sales force of ~ 20 reps
Commercial Model
§ Certified urologist requests biopsy kit
§ Reimbursement confirmation by Tengion
§ Upon pre-clearance, biopsy kit sent
§ Biopsy received at Tengion, cells isolated, conduit grown
§ Neo-Urinary Conduit sent to hospital for implant
US Commercial Strategy
Fully accessible market internally or through partnering
Fully accessible market internally or through partnering

29
Conduit Commercial Assessment
Summary
Summary
Most of the 24,000 US / EU annual conduit procedures are for bladder cancer
Market uptake driven by elimination of bowel surgery
§ Market research with urologists is unambiguous
§ Potential for acute and chronic benefits
§ Potential for new standard of care by eliminating the use of bowel tissue
Intend to model offsets based on clinical trial experience including:
§ Length of OR procedure and hospital stay
§ Peri-op, post-op and long-term complications
§ Impact on QoL and activities of daily living
Payer preference for new products with narrow populations and well-defined
needs, strong clinical support and some direct costs offset
needs, strong clinical support and some direct costs offset
Targeted and consultative hospital call point

30
Neo-Kidney Augment
Preclinical Research
Sharon Presnell, PhD
Preclinical Research
Sharon Presnell, PhD

31
100,000 new dialysis patients each year in the US
§ 350,000 currently on dialysis
§ 20% annual mortality
§ $60,000 1st year cost per patient
§ $22 billion in direct US costs annually for end stage kidney disease
Neo-Kidney Augment (NKA) Overview
Neo-Kidney Augment (NKA) Overview
Intended to delay the need for dialysis or transplantation
Intended to delay the need for dialysis or transplantation
***Kelley et al, Am J Physiol Renal Physiol 2010
(Published online 9/6/2010)
*Selected Regenerative Cells used in the NKA
** In development

32
*Kelley et al, Am J Physiol Renal Physiol 2010 (In Press)
**Presnell et al., presented at ISCT (San Francisco) 9/28/2010
Neo-Kidney Augment Preclinical Research
Summary
Summary
Proof-of-concept established for regenerative renal cells in three
independent small animal models of chronic kidney disease (CKD)
independent small animal models of chronic kidney disease (CKD)
§ 5/6 Nephrectomy model of terminal renal insufficiency*
§ ZSF1 model of metabolic syndrome and diabetic nephropathy**
§ Human cells in a nude rat model of I-R/G, chronic after acute renal failure
Early observations from ongoing large animal studies are consistent with
early small animal results
early small animal results
§ Isolation and delivery of renal cells from canines with renal insufficiency
§ Early results indicate in vivo function of regenerative cellular components
Isolation of renal cells from human kidneys with CKD
§ Reproducible isolation from core needle biopsies
§ Supports autologous sourcing strategy from target patient population

33
*Kelley et al, Am J Physiol Renal Physiol 2010 (In Press)
**Presnell et al., presented at ISCT (San Francisco) 9/28/2010
Neo-Kidney Augment Preclinical Research
Summary
Summary
Neo-Kidney Augment Preclinical Research
Summary
Summary
Proof-of-concept established for regenerative renal cells in three
independent small animal models of chronic kidney disease (CKD)
independent small animal models of chronic kidney disease (CKD)
§ 5/6 Nephrectomy model of terminal renal insufficiency*
§ ZSF1 model of metabolic syndrome and diabetic nephropathy**
§ Human cells in a nude rat model of I-R/G, chronic after acute renal failure
Early observations from ongoing large animal studies are consistent with
early small animal results
early small animal results
§ Isolation and delivery of renal cells from canines with renal insufficiency
§ Early results indicate in vivo function of regenerative cellular components
Isolation of renal cells established from human kidneys with CKD
§ Reproducible isolation from core needle biopsies
§ Supports autologous sourcing strategy from target patient population

34
Rodent 5/6 Nx-induced
model of renal failure
5/6 Nephrectomy Model of CKD
Renal disease secondary to reduced kidney mass
Renal disease secondary to reduced kidney mass
Terminal progressive renal failure
§ 0% survival at 6M post-nephrectomy
§ Progressive decline in GFR
§ Uremic
§ Anemic
§ Proteinuria
§ Hypertensive
§ Weight loss
§ Progressive glomerular sclerosis
§ Progressive tubulointerstitial fibrosis
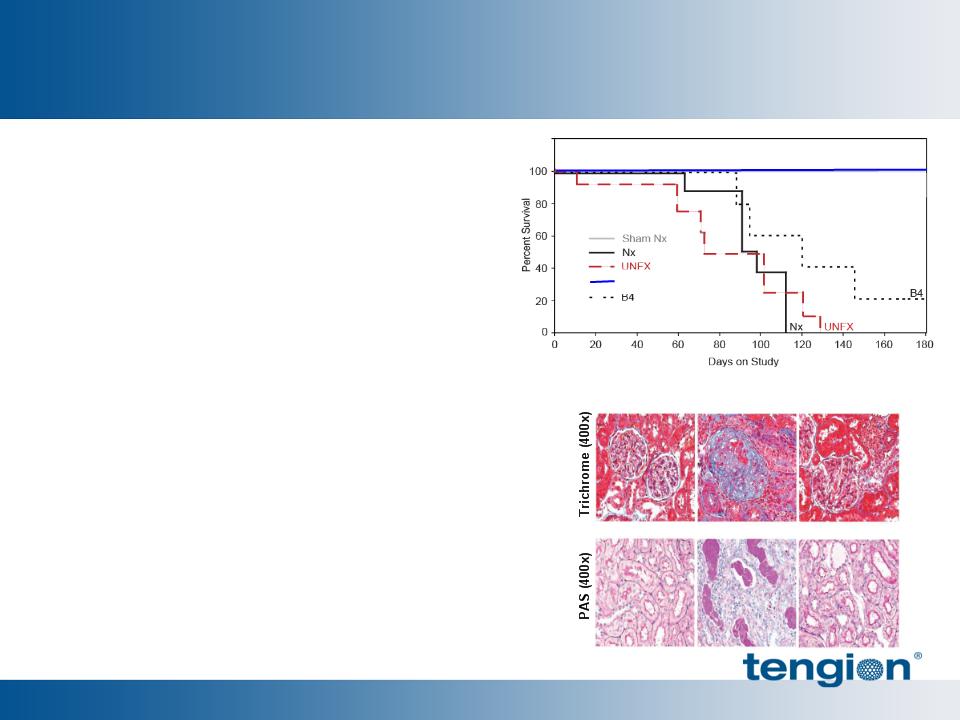
35
Renal cells delivered after chronic
disease state established
disease state established
§ sCREAT sustained at >200%
§ BUN sustained at >150%
Selected regenerative renal cells
(NKA) outperform unfractionated
mixture (UNFX) and improve multiple
physiologic parameters
(NKA) outperform unfractionated
mixture (UNFX) and improve multiple
physiologic parameters
§ Enhanced survival
– (100% (NKA) vs. 0% (Nx and UNFX)
§ Stabilized filtration (sCreatinine)
§ Improved protein retention
§ Reduced phosphatemia
Kelley et al, Am J Physiol Renal Physiol 2010
(Published online 9/6/2010)
NKA
NKA
SHAM NX
HEALTHY NX NX + NKA
Renal Cell function Validated in vivo
In rodent 5/6 nephrectomy model of chronic renal failure
In rodent 5/6 nephrectomy model of chronic renal failure

36
*Kelley et al, Am J Physiol Renal Physiol 2010 (In Press)
**Presnell et al., presented at ISCT (San Francisco) 9/28/2010
Neo-Kidney Augment Preclinical Research
Summary
Summary
Proof-of-concept established for regenerative renal cells in three
independent small animal models of chronic kidney disease (CKD)
independent small animal models of chronic kidney disease (CKD)
§ 5/6 Nephrectomy model of terminal renal insufficiency*
§ ZSF1 model of metabolic syndrome and diabetic nephropathy**
§ Human cells in a nude rat model of I-R/G, chronic after acute renal failure
Early observations from ongoing large animal studies are consistent with
early small animal results
early small animal results
§ Isolation and delivery of renal cells from canines with renal insufficiency
§ Early results indicate in vivo function of regenerative cellular components
Isolation of renal cells established from human kidneys with CKD
§ Reproducible isolation from core needle biopsies
§ Supports autologous sourcing strategy from target patient population

37
Tofovic et al., Ren. Fail. 22:387 (2000)
ZSF1 rodent model of
diabetic nephropathy
Obese ZSF1 Rats Model Progressive Nephropathy
Renal disease secondary to diabetes mellitus and hypertension
Renal disease secondary to diabetes mellitus and hypertension
Aggressive Metabolic Syndrome
§ Morbid obesity (leptin-receptor deficient)
§ ~50% mortality at ~1yr
§ Multiple co-morbid conditions
– Hyperglycemia
– Vasculopathy
– Hypertension
§ Progressive disease occurs throughout
the nephron
the nephron
– Renal hypertrophy
– Progressive glomerular sclerosis
– Progressive decline in GFR
– Tubular / interstitial fibrosis
– Severe proteinuria
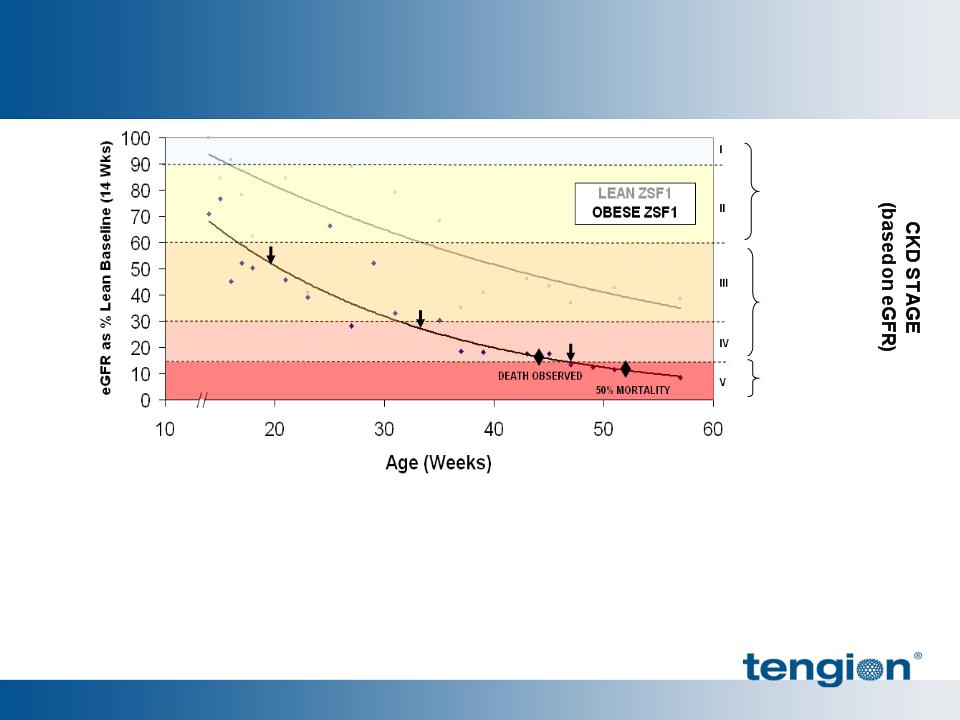
38
STABLE
DECLINE
FAILURE
CKD 3
CKD 4
CKD 5
Validating Renal Cells in Chronic Disease
Renal failure secondary to obesity and Type 2 diabetes (ZSF1)
Renal failure secondary to obesity and Type 2 diabetes (ZSF1)
Intervention windows:
§ Stage 3 CKD with no control of hyperglycemia
§ Stage 4 CKD with moderate control of hyperglycemia
§ Stage 5 CKD with moderate control of hyperglycemia
§ Lean ZSF1 = positive control
Intervention strategies:
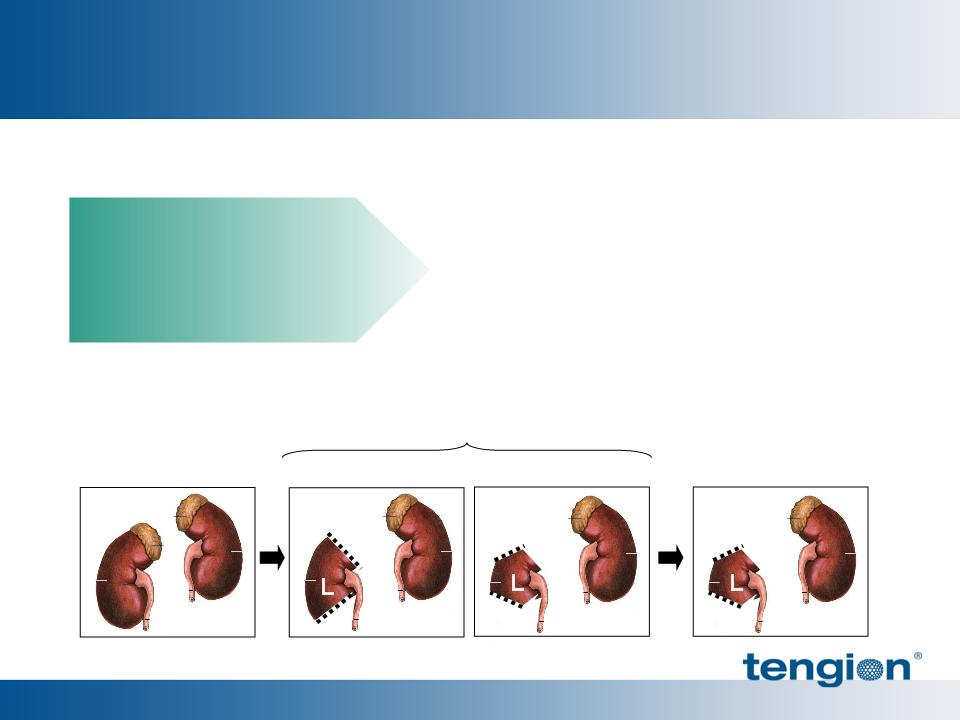
§ Syngeneic diseased donors
§ Treated (1) or both kidney(s)

39
Polynomial fit w/ 95% C.I.
Deaths in
untreated
p = 0.033
*
Untreated
OB ZSF1
+ NKA
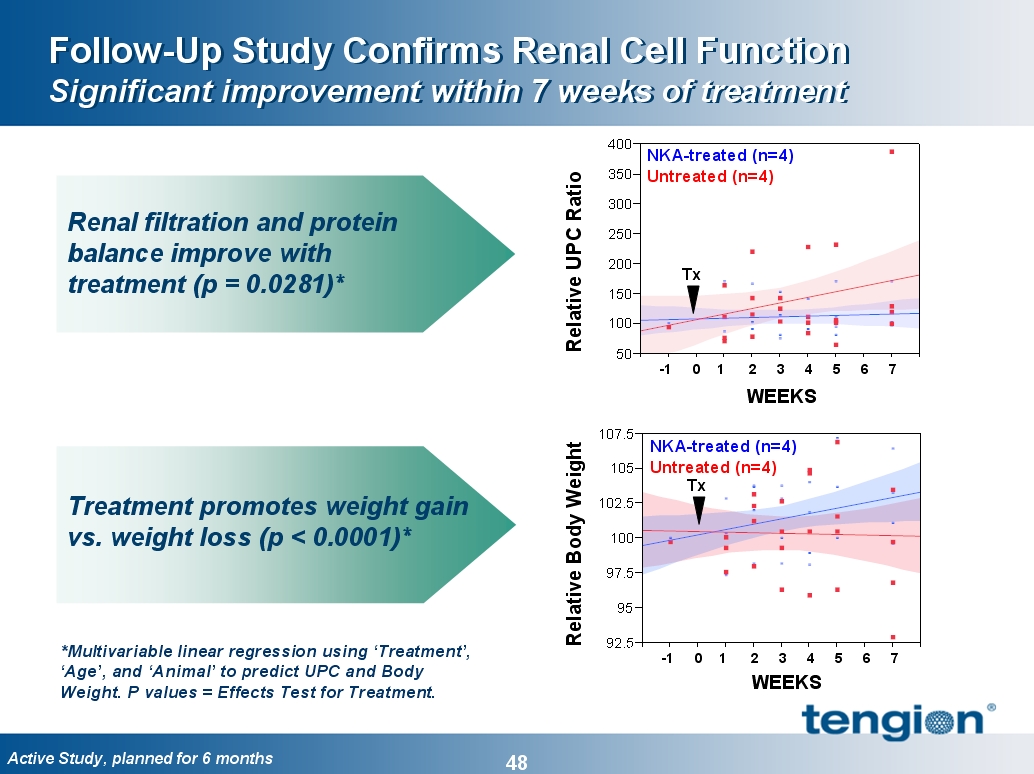
Renal Cells Improved Function Throughout the Nephron
Glomeruli, tubules and collecting ducts
Glomeruli, tubules and collecting ducts
At > 1 year of age:
§ 35% improvement in eGFR
(Filtration)
§ 15% reduction in phosphatemia
(Tubular Function)
§ 10% improvement in uOSM
(Concentration)
40
|
Treatment
Group |
63-week
Survival |
|
Untreated
OB ZSF1
|
20% (1/5)
|
|
OB ZSF1
+ NKA |
100%
(5/5) |
p = 0.042
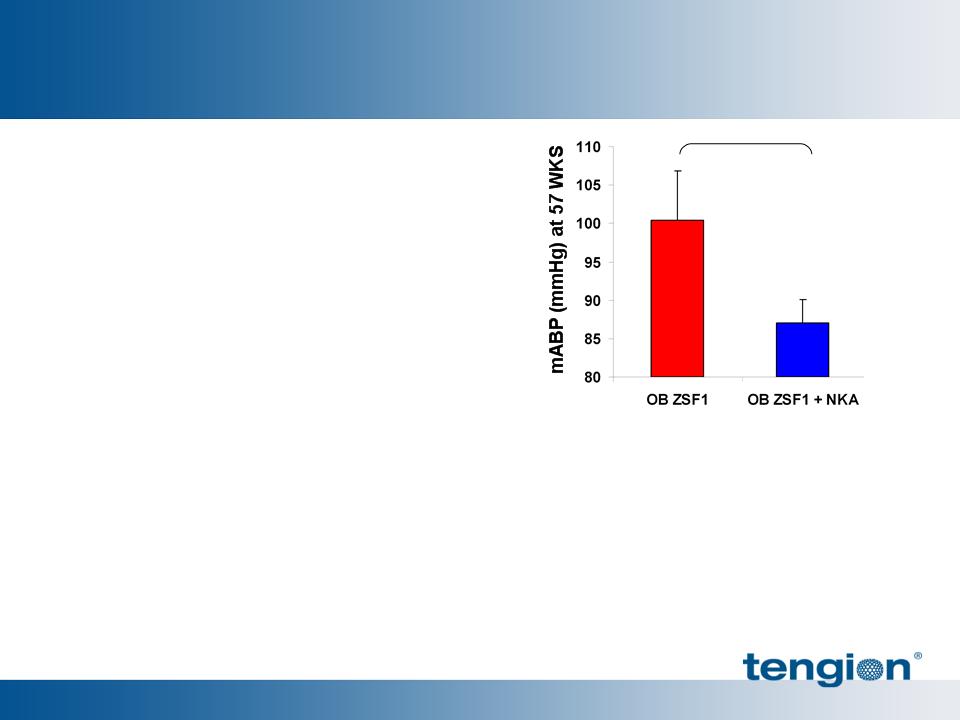
Renal Cells Reduced Hypertension and Improved Survival
ZSF1 rats at >1 year of age
ZSF1 rats at >1 year of age
§ Renal Cells reduced mean
arterial blood pressure (MABP)
significantly
arterial blood pressure (MABP)
significantly
§ Renal Cells support survival
beyond 50% mortality time
point for OB ZSF1
beyond 50% mortality time
point for OB ZSF1

41
*Kelley et al, Am J Physiol Renal Physiol 2010 (In Press)
**Presnell et al., presented at ISCT (San Francisco) 9/28/2010
Neo-Kidney Augment Preclinical Research
Summary
Summary
Proof-of-concept established for regenerative renal cells in three
independent small animal models of chronic kidney disease (CKD)
independent small animal models of chronic kidney disease (CKD)
§ 5/6 Nephrectomy model of terminal renal insufficiency*
§ ZSF1 model of metabolic syndrome and diabetic nephropathy**
§ Human cells in a nude rat model of I-R/G, chronic after acute renal failure
Early observations from ongoing large animal studies are consistent with
early small animal results
early small animal results
§ Isolation and delivery of renal cells from canines with renal insufficiency
§ Early results indicate in vivo function of regenerative cellular components
Isolation of renal cells established from human kidneys with CKD
§ Reproducible isolation from core needle biopsies
§ Supports autologous sourcing strategy from target patient population

42
Nude Rat Model of CKD
Nude Rat Model of CKD
Enables functional assessment of bioactive human cells
Enables functional assessment of bioactive human cells
Rodent model of CKD
after acute injury
after acute injury
Chronic renal insufficiency
§ Renal failure due to Ischemia-
Reperfusion + Gentamicin (I-R/G)
Reperfusion + Gentamicin (I-R/G)
§ Rats that fail to recover 3M post-injury
are utilized (chronic after acute)
are utilized (chronic after acute)
§ Model characterized by stable
insufficiency with partial recovery
insufficiency with partial recovery
§ Ischemia-Reperfusion + Gentamicin
injury in nude rats enables functional
testing of human cellular components
injury in nude rats enables functional
testing of human cellular components

43
Human-derived cells
prevent renal failure in
CKD Nude Rats for 3 mo.
prevent renal failure in
CKD Nude Rats for 3 mo.
Human Kidney Tissue Regeneration in Nude Rats
Human Kidney Tissue Regeneration in Nude Rats
Delayed progression of CKD and stabilized renal function
Delayed progression of CKD and stabilized renal function
Human-derived cells
improve CKD Nude Rat
nephron function
improve CKD Nude Rat
nephron function
I-R/G Injury +
Human Renal Cells
Human Renal Cells
I-R/G Injury
Treatment
1 4 12
weeks post-treatment
*
*
* p<0.05
Human
Renal Cells
NoTx

44
*Kelley et al, Am J Physiol Renal Physiol 2010 (In Press)
**Presnell et al., presented at ISCT (San Francisco) 9/28/2010
Neo-Kidney Augment Preclinical Research
Summary
Summary
Proof-of-concept established for regenerative renal cells in three
independent small animal models of chronic kidney disease (CKD)
independent small animal models of chronic kidney disease (CKD)
§ 5/6 Nephrectomy model of terminal renal insufficiency*
§ ZSF1 model of metabolic syndrome and diabetic nephropathy**
§ Human cells in a nude rat model of I-R/G, chronic after acute renal failure
Early observations from ongoing large animal studies are consistent with
early small animal results
early small animal results
§ Isolation and delivery of renal cells from canines with renal insufficiency
§ Early results indicate in vivo function of regenerative cellular components
Isolation of renal cells established from human kidneys with CKD
§ Reproducible isolation from core needle biopsies
§ Supports autologous sourcing strategy from target patient population
45
Canine 5/6 Nx-induced
model of renal failure
model of renal failure
INTACT
R
L
POLE REMOVAL A
POLE REMOVAL B
RIGHT KIDNEY REMOVED
R
R
R
x
STEP 1
STEP 2
Modified 5/6 Nephrectomy Canine Model of CKD
Renal disease secondary to reduced kidney mass
Renal disease secondary to reduced kidney mass
Chronic renal insufficiency
§ >50% reduction in GFR
§ Mild hypertension
§ Uremia
§ Mild anemia
§ Progressive proteinuria
§ Gradual weight loss

46
Caudal Renal
Pole Exposed
Caudal Renal
Pole Injected
NKA Product
R-Kidney
(Control), 40x
(Control), 40x
L-Kidney
(NKA), 40x
(NKA), 40x
L-Kidney
(NKA), 40x
(NKA), 40x
L-Kidney
(NKA), 600x
(NKA), 600x
Compensation
Focus of tubular regeneration
Renal Cell Delivery Verified
In dogs with single kidney (n=2)
In dogs with single kidney (n=2)
No adverse events
No systemic evidence
of renal injury
of renal injury
§ Serum chemistry
Normal histology (1M
post-implant)
post-implant)
§ Normal post-
nephrectomy
compensation
nephrectomy
compensation
§ Foci of tubular
regeneration at
delivery site
regeneration at
delivery site


49
*Kelley et al, Am J Physiol Renal Physiol 2010 (In Press)
**Presnell et al., presented at ISCT (San Francisco) 9/28/2010
Neo-Kidney Augment Preclinical Research
Summary
Summary
Proof-of-concept established for regenerative renal cells in three
independent small animal models of chronic kidney disease (CKD)
independent small animal models of chronic kidney disease (CKD)
§ 5/6 Nephrectomy model of terminal renal insufficiency*
§ ZSF1 model of metabolic syndrome and diabetic nephropathy**
§ Human cells in a nude rat model of I-R/G, chronic after acute renal failure
Early observations from ongoing large animal studies are consistent with
early small animal results
early small animal results
§ Isolation and delivery of renal cells from canines with renal insufficiency
§ Early results indicate in vivo function of regenerative cellular components
Isolation of renal cells established from human kidneys with CKD
§ Reproducible isolation from core needle biopsies
§ Supports autologous sourcing strategy from target patient population

50
Human Translation of Cellular Components
Isolation, characterization and expansion from diseased human kidney
Isolation, characterization and expansion from diseased human kidney
§ Standard core needle biopsy procedure
(0.02g tissue)
(0.02g tissue)
§ Cells can be expanded and
cryopreserved
cryopreserved
§ Salient phenotypic attributes are
preserved
preserved
§ Supports autologous sourcing strategy
Presnell et al., Tissue Engineering Part C, 2010
(Published online 9/20/2010)

51
*Kelley et al, Am J Physiol Renal Physiol 2010 (In Press)
**Presnell et al., presented at ISCT (San Francisco) 9/28/2010
Validation of Renal Cell Function in Four Models
Increases probability of success through development
Increases probability of success through development
1. Renal cells demonstrated durable function in 5/6 Nx rodent model of CKD due to renal
mass insufficiency*
mass insufficiency*
§ Robust therapeutic effect (animals followed 6M post-implant)
§ Reproducible across independent studies
2. Renal cells function in a model of CKD secondary to obesity and Type 2 Diabetes
(Active)**
(Active)**
§ Evaluation of intervention at CKD Stages 3-4 and followed to >1 year of age
§ Renal cells derived from diseased donors
3. Will human-derived renal cells function in vivo? (Active)
§ Ischemia-Reperfusion-Gentamicin (I-R/G) model of kidney damage (chronic after acute)
§ Feasibility established in I-R/G model with human renal cells
4. Will the renal cells function in a large animal model of CKD? (Active)
§ Isolation and delivery of renal cells from canines with renal insufficiency
§ Early results indicate in vivo function of regenerative cellular components

52
Neo-Kidney Augment
Research to Clinic
Deepak Jain, PhD
Research to Clinic
Deepak Jain, PhD
53
Neo-Kidney Augment
Research to clinic
Research to clinic
INTEGRATED
PLATFORM
Industrialization
Cells
Biomaterials
Implantation
Manufacturing/Product Release
Product Delivery
Selected Regenerative Cells
Product Formulation
INTEGRATED
PLATFORM
Industrialization
Biomaterials
Cells

54
Cell Factory (10 stack)
INTEGRATED
PLATFORM
Industrialization
Biomaterials
Cells
COBE
Tissue Culture Flasks
Neo-Kidney Augment
Cells: selected regenerative cell sourcing
Cells: selected regenerative cell sourcing
Cell Sourcing
§ Kidney needle biopsy
Cell Isolation
§ Enzyme digestion of biopsy
§ Cell washing and plating
Cell Expansion
§ By serial passage
§ Separated by density gradient
centrifugation
centrifugation

55
Cell-biomaterial formulations optimized in
combinatorial screening platform
combinatorial screening platform
Renal Cell - Biomaterial Formulations
INTEGRATED
PLATFORM
Industrialization
Biomaterials
Cells
Neo-Kidney Augment
Biomaterials: product formulations
Biomaterials: product formulations
Neo-Kidney Augment Program
Selected Regenerative Renal Cell Population
§ Demonstrated efficacy
Biomaterial Formulation Provides
§ Cell stability and durability for transport
§ Predictable and persistent delivery of cells
§ Structure - architecture for cell interactions
§ Space - displacement of fibrous tissue

56
Product Characterization and
Quality Release Criteria:
Quality Release Criteria:
X
Cell Function
X
Product Integrity
X
X
X
X
X
X
Final Construct
X
Cell Phenotype
X
Cell Viability
X
X
X
X
X
Total Viable Cells
X
Cell Morphology
Product Release Criteria
Quality Attribute
INTEGRATED
PLATFORM
Industrialization
Biomaterials
Cells
Neo-Kidney Augment
Industrialization: manufacturing
Industrialization: manufacturing
Manufacturing:
§ Closed system
– aseptic, scalable manufacturing
§ Maintain integrity during transport
§ User-friendly handling in the OR
57
INTEGRATED
PLATFORM
Industrialization
Biomaterials
Cells
Neo-Kidney Augment
Implantation: Product Delivery
Implantation: Product Delivery
Delivery of Injectable Product:
§ Syringe and needle
§ Catheter
Product Implantation:
§ Effective delivery

58
Neo-Kidney Augment
Neo-Kidney Augment
Clinical Development Considerations
Clinical Development Considerations
Sunita Sheth, MD
Sunita Sheth, MD

59
Sources: NHANES (2004) and US Renal Data System (2008)
CKD Prevalence by Stage
(000s)
Primary Diagnosis at ESRD
44%
28%
7%
2%
1%
13%
4%
1%
Diabetes
Hypertension
Glomerulonephritis
Cystic Kidney
Disease
Urologic Disease
Other known
Unknown
Missing data
Chronic Kidney Disease Affects Over 20 million in US
Expected to double by 2020, driven by diabetes
Expected to double by 2020, driven by diabetes

60
Clinical Development Pathway
Informed by preclinical studies and regulatory history
Informed by preclinical studies and regulatory history
Clinical Benefit
§ Improve kidney function in patients at risk of requiring dialysis
§ Delay progression of kidney failure or time to onset of dialysis
Target Population
§ Stage of kidney failure
– Stage 3 and 4
§ Underlying disease
– Diabetes, hypertension, other
Efficacy Measures
§ Functional changes
– Improvement in GFR or change in rate of decline in GFR
– Time to doubling of the serum creatinine
– Reduction in proteinuria
§ Event reduction
– End-stage renal disease
• Defined as initiation dialysis or transplant
– Mortality

61
Neo-Urinary Conduit Trial in Bladder Cancer Patients
§ Successful first implant
– Biopsy, manufacturing, quality release and implant procedure
§ Investigators optimistic regarding future enrollment timelines
– First patient successfully implanted
– Two sites actively screening
§ Anticipate update on clinical progress by next implant
Neo-Kidney Augment to Delay Progression or Eliminate Need for Dialysis
§ Strong and growing foundation of evidence for product development
– Optimizing product formulation
– Continuing follow-up from ongoing studies
§ Anticipate discussions with FDA in 2H11 regarding path to clinical trials
Conclusions
Conclusions

62
