Attached files
| file | filename |
|---|---|
| 8-K - 8-K - AIkido Pharma Inc. | a10-19548_18k.htm |
Exhibit 99.1
|
|
TAGATOSE NON-CONFIDENTIAL OVERVIEW October 18, 2010 |
|
|
2 Forward-Looking Statement This presentation contains forward-looking statements that involve risks and uncertainties, including those described in the Company’s Annual Report for the year ended December 31, 2009 filed on Form 10-K. |
|
|
3 Spherix Highlights • Spherix (NASDAQ: SPEX) has two subsidiaries: – Spherix Consulting: Scientific consulting on food and drug uses for US and overseas clients – Biospherics: Pharmaceutical development division • Biospherics is focused on development of tagatose for the treatment of diabetes and metabolic disorders – Phase 3 trial as monotherapy in ‘mild’ Type 2 Diabetes reported Oct 2010 – Phase 2 dose ranging study in Type 2 Diabetes to complete Dec10 – Phase 2 dose ranging study in Hypertriglyceridemia expected to start in 2011 |
|
|
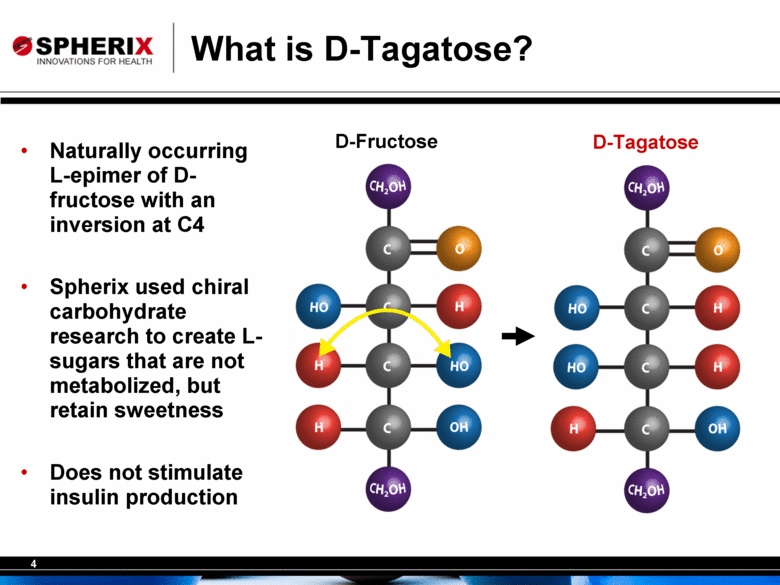
4 What is D-Tagatose? D-Tagatose D-Fructose • Naturally occurring L-epimer of D-fructose with an inversion at C4 • Spherix used chiral carbohydrate research to create L-sugars that are not metabolized, but retain sweetness • Does not stimulate insulin production |
|
|
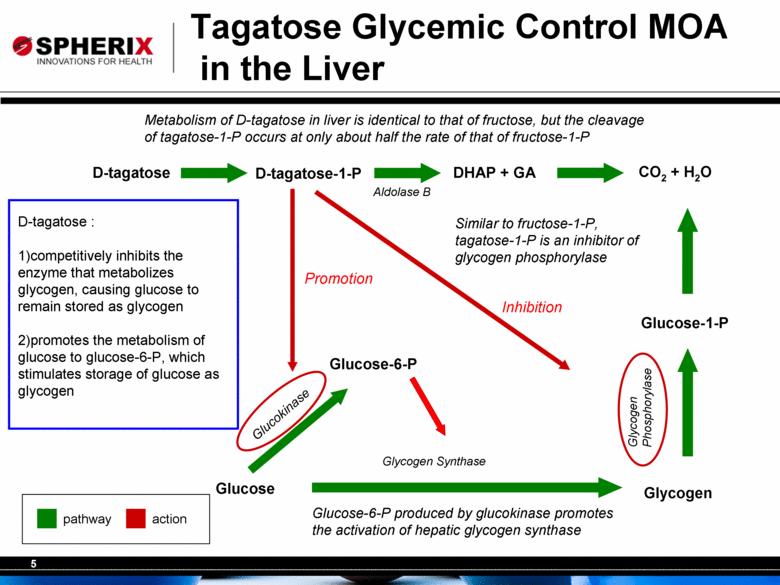
5 Tagatose Glycemic Control MOA in the Liver Metabolism of D-tagatose in liver is identical to that of fructose, but the cleavage of tagatose-1-P occurs at only about half the rate of that of fructose-1-P Similar to fructose-1-P, tagatose-1-P is an inhibitor of glycogen phosphorylase Glucose Glucose-6-P Glycogen Glucokinase Glycogen Synthase Glucose-6-P produced by glucokinase promotes the activation of hepatic glycogen synthase CO2 + H2O Glucose-1-P Glycogen Phosphorylase D-tagatose D-tagatose-1-P DHAP + GA Aldolase B pathway action D-tagatose : 1)competitively inhibits the enzyme that metabolizes glycogen, causing glucose to remain stored as glycogen 2)promotes the metabolism of glucose to glucose-6-P, which stimulates storage of glucose as glycogen Promotion Inhibition |
|
|
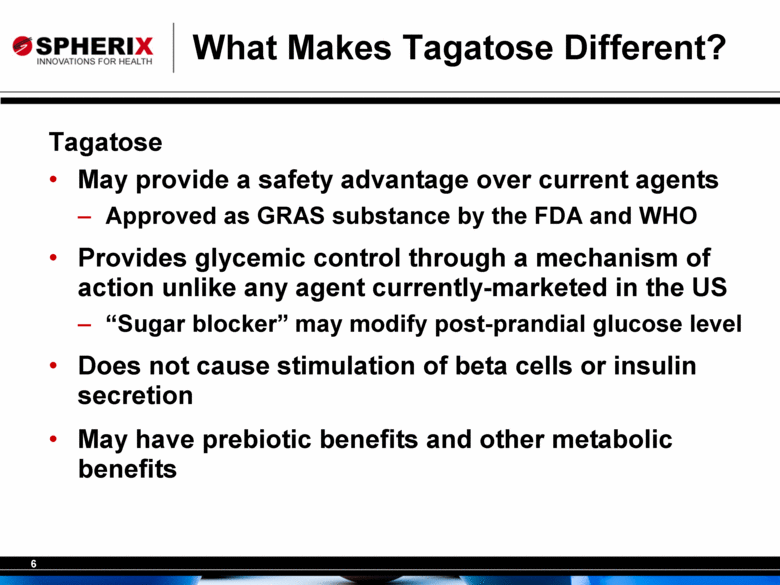
6 What Makes Tagatose Different? Tagatose • May provide a safety advantage over current agents – Approved as GRAS substance by the FDA and WHO • Provides glycemic control through a mechanism of action unlike any agent currently-marketed in the US – “Sugar blocker” may modify post-prandial glucose level • Does not cause stimulation of beta cells or insulin secretion • May have prebiotic benefits and other metabolic benefits |
|
|
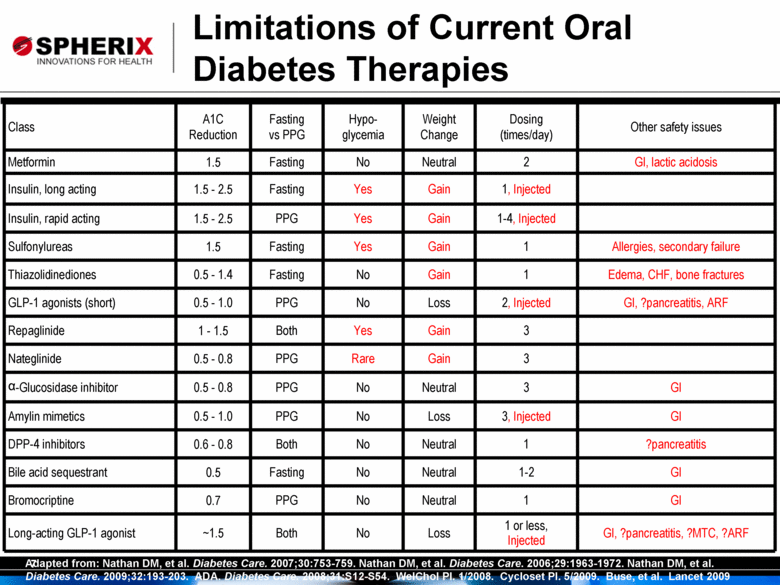
7 Class A1C Reduction Fasting vs PPG Hypoglycemia Weight Change Dosing (times/day) Other safety issues Metformin 1.5 Fasting No Neutral 2 GI, lactic acidosis Insulin, long acting 1.5 - 2.5 Fasting Yes Gain 1, Injected Insulin, rapid acting 1.5 - 2.5 PPG Yes Gain 1-4, Injected Sulfonylureas 1.5 Fasting Yes Gain 1 Allergies, secondary failure Thiazolidinediones 0.5 - 1.4 Fasting No Gain 1 Edema, CHF, bone fractures GLP-1 agonists (short) 0.5 - 1.0 PPG No Loss 2, Injected GI, ?pancreatitis, ARF Repaglinide 1 - 1.5 Both Yes Gain 3 Nateglinide 0.5 - 0.8 PPG Rare Gain 3 a-Glucosidase inhibitor 0.5 - 0.8 PPG No Neutral 3 GI Amylin mimetics 0.5 - 1.0 PPG No Loss 3, Injected GI DPP-4 inhibitors 0.6 - 0.8 Both No Neutral 1 ?pancreatitis Bile acid sequestrant 0.5 Fasting No Neutral 1-2 GI Bromocriptine 0.7 PPG No Neutral 1 GI Long-acting GLP-1 agonist ~1.5 Both No Loss 1 or less, Injected GI, ?pancreatitis, ?MTC, ?ARF Adapted from: Nathan DM, et al. Diabetes Care. 2007;30:753-759. Nathan DM, et al. Diabetes Care. 2006;29:1963-1972. Nathan DM, et al. Diabetes Care. 2009;32:193-203. ADA. Diabetes Care. 2008;31:S12-S54. WelChol PI. 1/2008. Cycloset PI. 5/2009. Buse, et al. Lancet 2009 Limitations of Current Oral Diabetes Therapies |
|
|
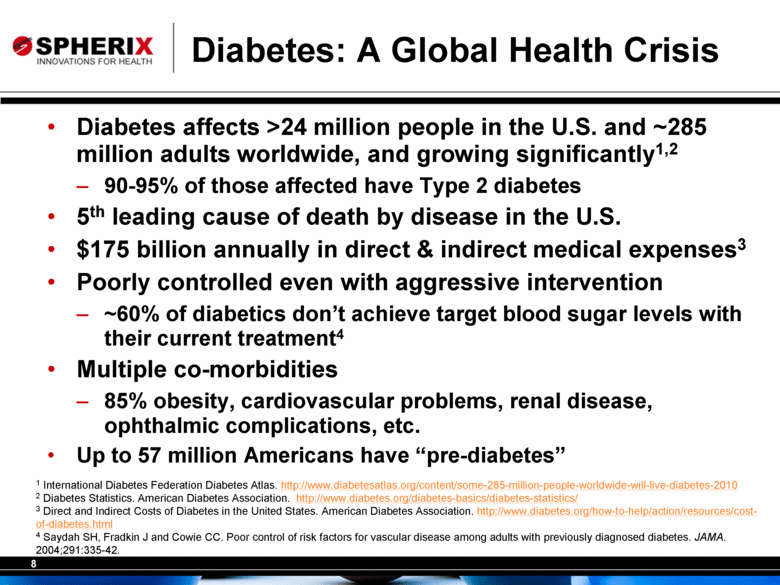
8 Diabetes: A Global Health Crisis • Diabetes affects >24 million people in the U.S. and ~285 million adults worldwide, and growing significantly1,2 – 90-95% of those affected have Type 2 diabetes • 5th leading cause of death by disease in the U.S. • $175 billion annually in direct & indirect medical expenses3 • Poorly controlled even with aggressive intervention – ~60% of diabetics don’t achieve target blood sugar levels with their current treatment4 • Multiple co-morbidities – 85% obesity, cardiovascular problems, renal disease, ophthalmic complications, etc. • Up to 57 million Americans have “pre-diabetes” 1 International Diabetes Federation Diabetes Atlas. http://www.diabetesatlas.org/content/some-285-million-people-worldwide-will-live-diabetes-2010 2 Diabetes Statistics. American Diabetes Association. http://www.diabetes.org/diabetes-basics/diabetes-statistics/ 3 Direct and Indirect Costs of Diabetes in the United States. American Diabetes Association. http://www.diabetes.org/how-to-help/action/resources/cost-of- diabetes.html 4 Saydah SH, Fradkin J and Cowie CC. Poor control of risk factors for vascular disease among adults with previously diagnosed diabetes. JAMA. 2004;291:335-42. |
|
|
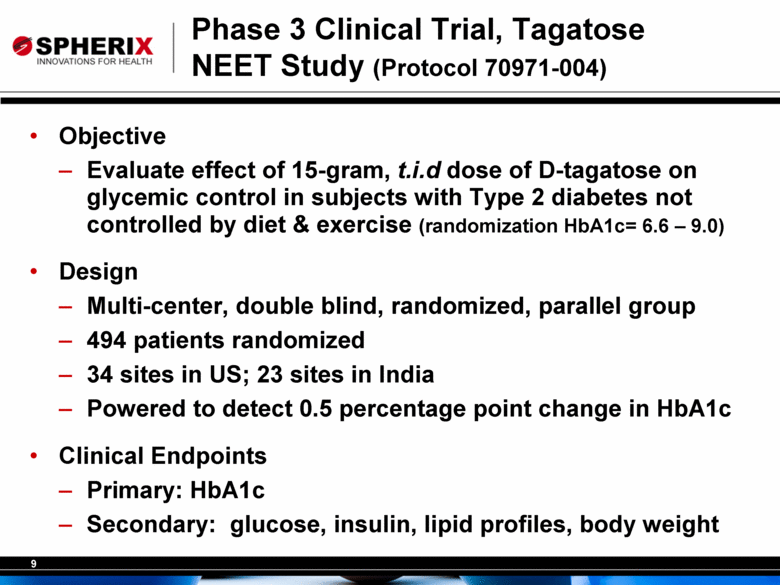
9 Phase 3 Clinical Trial, Tagatose NEET Study (Protocol 70971-004) • Objective – Evaluate effect of 15-gram, t.i.d dose of D-tagatose on glycemic control in subjects with Type 2 diabetes not controlled by diet & exercise (randomization HbA1c= 6.6 – 9.0) • Design – Multi-center, double blind, randomized, parallel group – 494 patients randomized – 34 sites in US; 23 sites in India – Powered to detect 0.5 percentage point change in HbA1c • Clinical Endpoints – Primary: HbA1c – Secondary: glucose, insulin, lipid profiles, body weight |
|
|
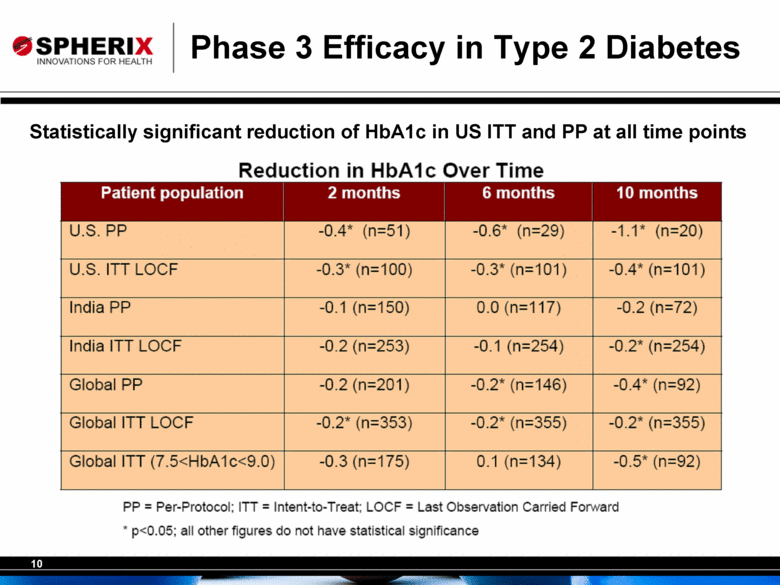
10 Phase 3 Efficacy in Type 2 Diabetes Statistically significant reduction of HbA1c in US ITT and PP at all time points Reduction in HbA1c Over Time Patient population 2 months 6 months 10 months U.S. PP -0.4* (n=51) -0.6* (n=29) -1.1* (n=20) U.S. ITT LOCF -0.3* (n=100) -0.3* (n=101) -0.4* (n=101) India PP -0.1 (n=150) 0.0 (n=117) -0.2 (n=72) India ITT LOCF -0.2 (n=253) -0.1 (n=254) -0.2* (n=254) Global PP -0.2 (n=201) -0.2* (n=146) -0.4* (n=92) Global ITT LOCF -0.2* (n=353) -0.2* (n=355) -0.2* (n=355) Global ITT (7.5<HbA1c<9.0) -0.3 (n=175) 0.1 (n=134) -0.5* (n=92) PP = Per-Protocol; ITT = Intent-to-Treat; LOCF = Last Observation Carried Forward * p<0.05; all other figures do not have statistical significance. |
|
|
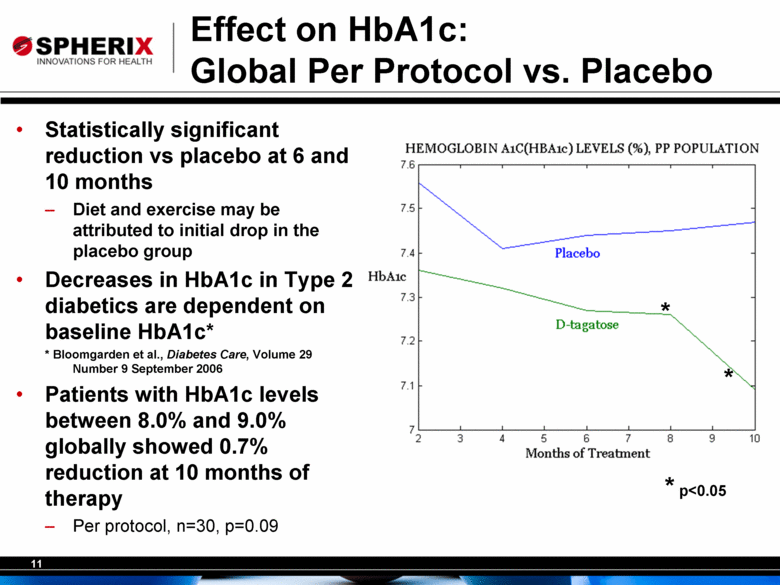
11 Effect on HbA1c: Global Per Protocol vs. Placebo • Statistically significant reduction vs placebo at 6 and 10 months – Diet and exercise may be attributed to initial drop in the placebo group • Decreases in HbA1c in Type 2 diabetics are dependent on baseline HbA1c* * Bloomgarden et al., Diabetes Care, Volume 29 Number 9 September 2006 • Patients with HbA1c levels between 8.0% and 9.0% globally showed 0.7% reduction at 10 months of therapy – Per protocol, n=30, p=0.09 * * * p<0.05 |
|
|
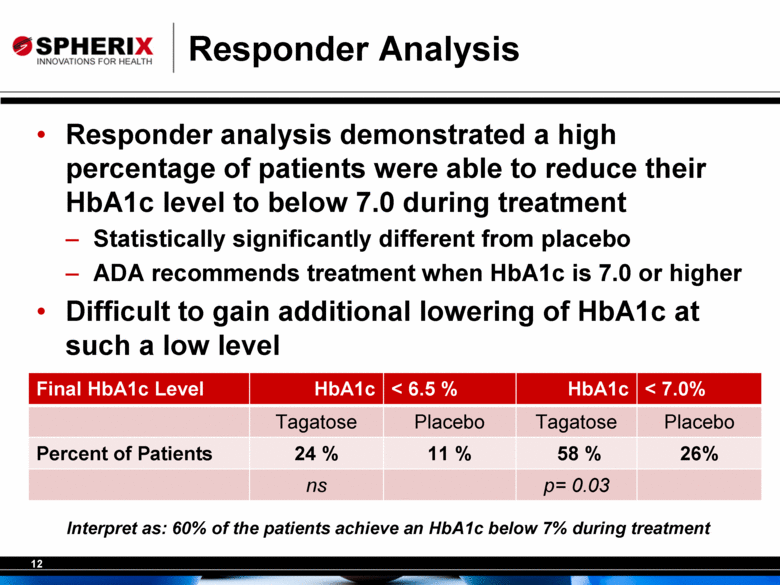
12 Responder Analysis • Responder analysis demonstrated a high percentage of patients were able to reduce their HbA1c level to below 7.0 during treatment – Statistically significantly different from placebo – ADA recommends treatment when HbA1c is 7.0 or higher • Difficult to gain additional lowering of HbA1c at such a low level Final HbA1c Level HbA1c < 6.5 % HbA1c < 7.0% Tagatose Placebo Tagatose Placebo Percent of Patients 24 % 11 % 58 % 26% ns p= 0.03 Interpret as: 60% of the patients achieve an HbA1c below 7% during treatment |
|
|
13 Other Diabetes Clinical Findings • Patients with >1 treatment-emergent adverse events in the active group (163) was comparable to the placebo group (166) – No serious adverse event deemed treatment related – No episodes of hypoglycemia or pancreatitis were reported among any trial subjects • Limited size of patient population with abnormal triglycerides and BMI were not powered for significance in secondary endpoints |
|
|
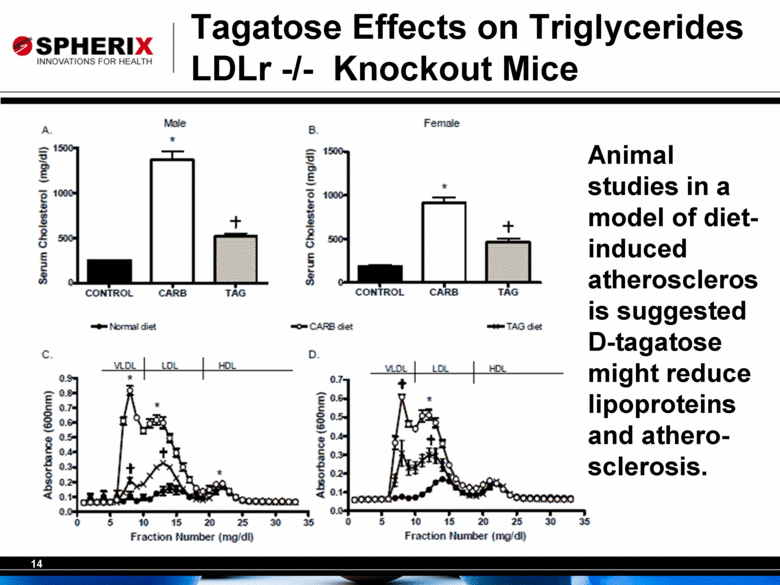
14 Tagatose Effects on Triglycerides LDLr -/- Knockout Mice Animal studies in a model of dietinduced atheroscleros is suggested D-tagatose might reduce lipoproteins and atherosclerosis. |
|
|
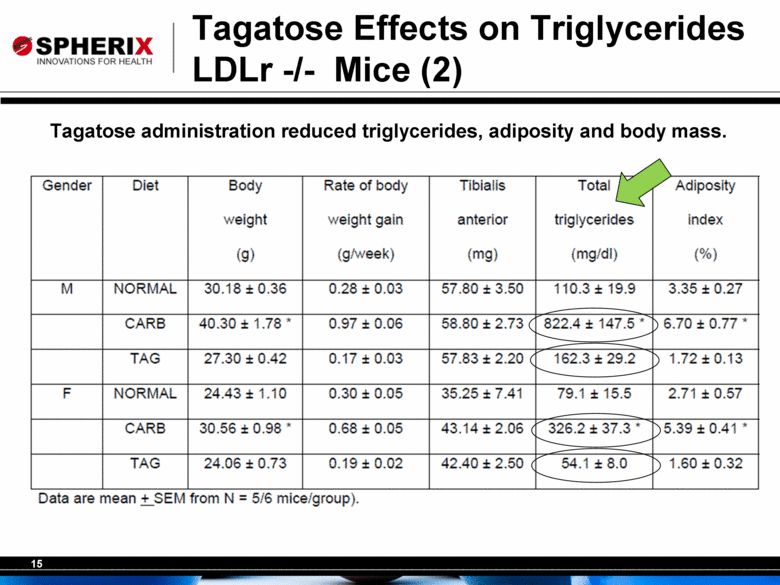
15 Tagatose Effects on Triglycerides LDLr -/- Mice (2) Tagatose administration reduced triglycerides, adiposity and body mass. |
|
|
16 Near-Term Milestones • Report Phase 2 dose-finding data on diabetes, hypertriglyceridemia, body mass index 4Q10 • Engage partner for D-tagatose in diabetes to continue development Ongoing • Perform additional animal studies on hypertriglyceridemia mechanism of action 1Q11 • Begin Phase 2 study with D-Tagatose in hypertriglyceridemia 1Q11 |
|
|
17 Tagatose Summary • Tagatose medical use focused on large and growing Type 2 diabetes and dyslipidemic markets • Proof of concept demonstrated in human trials (diabetes) and animal models (triglycerides) • Market position based on expected safety advantages, good tolerability and modest efficacy • Interested in out-licensing commercial rights, outright sale of asset or entering a development agreement |
|
|
18 Robert Lodder, Ph.D. Leisa Dennehy President Commercial and Corporate rlodder@spherix.com Development Advisor Phone: 301-476-0705 ldennehy@spherix.com Phone: 301-897-0617 Robert Clayton Claire Kruger, Ph.D. Chief Financial Officer Chief Executive Officer rclayton@spherix.com ckruger@spherix.com Phone: 301-897-0616 Phone: 301-897-0611 For More Information, Please Contact: SPHERIX 6430 Rockledge Drive #503 Bethesda, MD 20817 301-897-2540 • Fax: 301-897-2567 Toll Free: 1-866-SPHERIX http://www.spherix.com |