Attached files
Registration No. 333-
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM S-1
REGISTRATION STATEMENT UNDER THE SECURITIES ACT OF 1933
|
Push, Inc.
|
|
(Exact Name of registrant in its charter)
|
|
Nevada
|
8082
|
270 713 568
|
|
(State or jurisdiction of incorporation or organization)
|
(Primary Standard Industrial Classification Code Number)
|
(I.R.S. Employer Identification No.)
|
|
501 Church Street, Suite 317
|
|
Vienna, Virginia 22180
|
|
(703) 938-7500
|
|
(Address and telephone number of principal executive offices)
|
|
Ted Wong
|
|
501 Church Street, Suite 317
Vienna, Virginia 22180
|
|
(703) 938-7500
|
|
(Name, address and telephone number of agent for service)
|
|
Copies to:
|
|
Lynne Bolduc, Esq.
Oswald & Yap LLP
16148 Sand Canyon Avenue
Irvine, CA 92618
|
|
Telephone: (949) 788-8900; fax: (949) 788-8980
|
Approximate date of proposed sale to the public: As soon as practicable after this Registration Statement becomes effective.
If any of the securities being registered are being offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following box x
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the following box and list the Securities Act Registration Statement number of the earlier effective Registration Statement for the same offering. o
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act Registration Statement number of the earlier effective Registration Statement for the same offering. o
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act Registration Statement number of the earlier effective Registration Statement for the same offering. o
Indicate by a check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accredited filer or a smaller reporting company.
| Large accelerated filer | o | Accelerated filer | o | |
| Non-accelerated filer | o | Smaller reporting company | x |
CALCULATION OF REGISTRATION FEE
|
Title of Each Class of Securities to be Registered
|
Amount of Securities to be Registered1
|
Proposed Maximum Offering Price Per Share2
|
Proposed Maximum Aggregate Offering Price
|
Amount of Registration Fee
|
|
Common stock. $0.001 par value per share3
|
2,000,000
|
$0.10
|
$200,000
|
$14
|
|
Common stock, $0.001 par value per share4
|
3,000,000
|
$0.10
|
$300,000
|
$22
|
|
Common stock underlying options exercisable at $0.30 per share5
|
3,000,000
|
$0.306
|
$900,000
|
$64
|
|
Total
|
8,000,000
|
$1,400,000
|
$100
|
The Registrant hereby amends this Registration Statement on such date or dates as may be necessary to delay its effective date until the Registrant shall file a further amendment which specifically states that this Registration Statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933 or until the Registration Statement shall become effective on such date as the Commission, acting pursuant to said Section 8(a), may determine.
1 Pursuant to Rule 416(a) of the Securities Act of 1933, as amended, this registration statement shall be deemed to cover additional securities that may be offered or issued to prevent dilution resulting from stock splits, stock dividends or similar transactions. There is no current market for the securities and the price at which the Shares are being offered has been arbitrarily determined by the Company and used for the purpose of computing the amount of the registration fee in accordance with Rule 457 under the Securities Act of 1933, as amended.
5 Shares of common stock issuable upon exercise of outstanding options being registered for resale by the selling shareholders.
The information in this document is not complete and may be changed. The Company may not sell the securities offered by this document until the registration statement filed with the Securities and Exchange Commission is effective. This prospectus is not an offer to sell these securities, and the Company is not soliciting an offer to buy these securities, in any state or other jurisdiction where the offer or sale is not permitted.
Prospectus
Push, Inc.
8,000,000 Shares of Common Stock
$0.10 per share
We are offering on a best-efforts basis a minimum of 500,000 and a maximum of 2,000,000 shares of our common stock at a price of $0.10 per share. The shares will be sold directly through the efforts of Ted Wong and Howard Sidman, our sole officers and directors. The intended methods of communication include, without limitation, telephone and in-person meetings. See “Plan of Distribution.”
The proceeds from the sale of the shares in this offering will be held in a non-interest bearing escrow account at Security Bank of California pending the achievement of the minimum offering. If the minimum offering is not achieved within 365 days of the date of this prospectus, all subscription funds will be returned to investors promptly without interest thereon or deduction therefrom. See “Plan of Distribution.”
The offering may terminate on the earliest of: (i) the date when the sale of all 2,000,000 shares is completed, (ii) anytime we choose to end the offering, or (iii) 365 days from the effective date of this document, or any extension thereto.
We are also registering 3,000,000 shares of our common stock that are to be sold, from time to time, by one or more of the selling shareholders and 3,000,000 shares of our common stock underlying options currently held by certain selling shareholders. The selling shareholders may only offer and sell, from time to time, common stock using this prospectus in transactions at a fixed offering price of $0.10 per share until a trading market develops in our common stock, at which time the selling shareholders may sell shares at prevailing market prices, which may vary, or at privately negotiated prices. The proceeds from the sale of the shares will go directly to the selling shareholders.
We are a development stage company which currently has limited operations and has not generated any revenue. Therefore, any investment in us involves a high degree of risk. There is currently no public market for our common stock and, there can be no assurance that a meaningful trading market will ever develop.
THIS INVESTMENT INVOLVES A HIGH DEGREE OF RISK. YOU SHOULD PURCHASE ONLY IF YOU CAN AFFORD A COMPLETE LOSS OF YOUR INVESTMENT. SEE THE SECTION ENTITLED “RISK FACTORS” HEREIN BEGINNING ON PAGE 4.
NEITHER THE SECURITIES AND EXCHANGE COMMISSION NOR ANY STATE SECURITIES COMMISSION HAS APPROVED OR DISAPPROVED THESE SECURITIES, OR PASSED UPON THE ADEQUACY OR ACCURACY OF THIS PROSPECTUS. ANY REPRESENTATION TO THE CONTRARY IS A CRIMINAL OFFENSE.
Subject to completion, dated [ _____ ], 2010
TABLE OF CONTENTS
|
SUMMARY INFORMATION
|
1 |
|
RISK FACTORS
|
4 |
|
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
|
15 |
|
USE OF PROCEEDS
|
16 |
|
DETERMINATION OF OFFERING PRICE
|
17 |
|
DILUTION
|
18 |
|
SELLING SHAREHOLDERS
|
19 |
|
PLAN OF DISTRIBUTION
|
20 |
|
DESCRIPTION OF SECURITIES TO BE REGISTERED
|
22 |
|
INTEREST OF NAMED EXPERTS AND COUNSEL
|
23 |
|
DESCRIPTION OF THE BUSINESS
|
24 |
|
DESCRIPTION OF PROPERTY
|
27 |
|
LEGAL PROCEEDINGS
|
27 |
|
MARKET PRICE OF AND DIVIDENDS ON THE ISSUER’S COMMON STOCK
|
28 |
|
MANAGEMENT’S DISCUSSON AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
|
29 |
|
CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
|
30 |
|
DIRECTORS, EXECUTIVE OFFICERS, PROMOTERS AND CONTROL PERSONS
|
30 |
|
EXECUTIVE COMPENSATION
|
31 |
|
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT
|
32 |
|
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS
|
33 |
|
REPORTS TO SECURITY HOLDERS
|
33 |
|
FINANCIAL STATEMENTS
|
34 |
The Company
Push, Inc., a Nevada corporation, is a developmental stage company based in Northern Virginia that is a subsidiary of DKL International, Inc. (“Parent Company”). The Parent Company is a developer of passive human detection technology. The Parent Company’s proprietary technology passively detects the non-uniform electric field that every living human generates. The main source of this field in the human body is the electric activity of the heart. We were formed to apply the Parent Company’s technology to the wellness care market. We hold the worldwide exclusive license to market, distribute, promote, sell, offer for sale and sublicense wellness care products based on the Parent Company’s technology. We have granted to our prior investors certain rights relating to the manufacturing and distribution of our products and, therefore, our rights relating to those activities may be limited. See “Risk Factors—We have granted rights to our prior investors which may restrict our operations and flexibility.”
We are a small, start-up company that has not generated any revenues and that lacks a stable customer base. Since our inception on July 10, 2009 to the present, our activities have been limited to forming our corporate entity, conducting a private placement of our common stock, and acquiring our technology through a license agreement with our Parent Company. We believe that the funds received from our private placement and this current offering if the maximum offering is raised will be sufficient to finance our efforts to become operational and carry us through the next 12 months. We believe that recurring revenues from future operations should be sufficient to support ongoing operations after this 12 month period. Unfortunately, there can be no assurance that the actual expenses incurred over the next 12 months will not materially exceed our current capital base and potential future cash flows from our operations. As a result, our independent auditors have expressed substantial doubt about our ability to continue as a going concern. If we do not produce sufficient cash flow to support our operations over the next 12 months, we may need to raise additional capital by selling additional securities in exchange for cash in order to continue as a going concern. This potential future issuance of securities would dilute the holdings of any purchasers of common stock in this offering or the selling shareholders.1 There are no formal or informal agreements to obtain such financing. We cannot assure you that any financing can be obtained or, if obtained, that it will be on reasonable terms acceptable to us. Without securing additional capital, if needed, it may be unlikely for us to stay in business.
We have filed this prospectus as part of a Registration Statement to register shares of our common stock for sale to raise funding to commence our operations, and to register shares of our common stock for sale by our selling shareholders. We also plan to file a registration statement on Form 8-A to become a reporting company. We then plan to seek a market maker to file an application for our shares of common stock to become quoted on the over-the-counter bulletin board system. There is currently no public market for our common stock and, there can be no assurance that a meaningful trading market will ever develop.
We currently have two officers and directors who are Ted Wong (President, CEO, and Director) and Howard Sidman (Treasurer, Secretary, CFO, and Director). These individuals allocate time and personal resources to us on a part-time basis. As of the date of this prospectus, we have 38,500,000 shares of $0.001 par value common stock issued and outstanding.
Our mailing address is and our principal executive offices are located at 501 Church Street, Suite 317, Vienna, Virginia 22180 with a telephone number of (703) 938-7500.
The Offering
The offering consists of shares offered by the selling shareholders and up to 2,000,000 shares of common stock being offered by us at $0.10 per share. The selling shareholders are offering 3,000,000 shares of our common stock and 3,000,000 shares of common stock underlying options as soon as practicable after this Registration Statement
1 Our Parent Company currently owns 26,000,000 shares of our common stock, and has certain anti-dilution rights which will magnify the effect of any dilutive issuances. See “Risk Factors—Our Parent Company has certain anti-dilution rights which could make it impossible for anyone else to obtain a majority ownership in us, could make our shares less attractive to potential purchasers, and could cause massive dilution to all of our other shareholders.” becomes effective. The selling shareholders will sell at a price of $0.10 per share until the shares are quoted on the OTC Bulletin Board® or in another quotation medium and, thereafter, at prevailing market prices or privately negotiated prices. The options to purchase 3,000,000 shares of common stock are exercisable at $0.30 per share and expire on June 29, 2014.
1
SUMMARY INFORMATION (Continued)
The proceeds of the offering from the sale of the common stock held by the selling shareholders will go directly to the selling shareholders. None of these proceeds will be available to us. We are selling up to 2,000,000 shares of common stock at $0.10 per share, and will receive such proceeds from sale.
We have agreed to pay all costs and expenses relating to the registration of the common stock to be sold in this offering by us and the selling shareholders. The selling shareholders will be responsible for any related commissions; all taxes including, but not limited to, federal and state income taxes; attorney's fees; and related charges in connection with the offer and sale of their shares. The selling shareholders may sell their common stock through one or more broker/dealers, and such broker/dealers may receive compensation in the form of commissions.
The offering price of the common stock has been arbitrarily determined by us and bears no relationship to any objective criterion of value. The price does not bear any relationship to our assets, book value, historical earnings or net worth.
The purchase of our common stock in this offering involves a high degree of risk. The common stock offered in this prospectus is for investment purposes only and currently no market for our common stock exists. See “Risk Factors” and “Dilution.”
Summary of Financial Information
The following table sets forth summary financial data derived from our financial statements. The data should be read in conjunction with our financial statements, related notes and other financial information included in this prospectus.
|
PUSH, INC.
|
||||||||||||
|
(A Development Stage Company)
|
||||||||||||
|
Statements of Operations
|
||||||||||||
|
|
For the
|
From Inception
|
From Inception
|
|||||||||
|
Six Months
|
on July 10,
|
on July 10,
|
||||||||||
|
Ended
|
2009 Through
|
2009 Through
|
||||||||||
|
June 30,
|
December 31,
|
June 30,
|
||||||||||
|
2010
|
2009
|
2010
|
||||||||||
|
(Unaudited)
|
(Audited)
|
(Unaudited)
|
||||||||||
|
REVENUES
|
$ | - | $ | - | $ | - | ||||||
|
OPERATING EXPENSES
|
||||||||||||
|
Consulting
|
- | 114,500 | 114,500 | |||||||||
|
General and administrative
|
7,080 | 6,581 | 13,661 | |||||||||
|
Research
|
18,717 | 14,950 | 33,667 | |||||||||
|
Payroll Expenses
|
69,688 | - | 69,688 | |||||||||
|
Licensing fee
|
- | 90,500 | 90,500 | |||||||||
|
Total Operating Expenses
|
95,485 | 226,531 | 322,016 | |||||||||
|
OTHER INCOME
|
||||||||||||
|
Interest income
|
856 | 104 | 960 | |||||||||
|
NET LOSS
|
$ | (94,629 | ) | $ | (226,427 | ) | $ | (321,056 | ) | |||
|
BASIC AND DILUTED LOSS PER SHARE
|
$ | (0.00 | ) | $ | (0.01 | ) | ||||||
|
WEIGHTED AVERAGE NUMBER
|
||||||||||||
|
OF SHARES OUTSTANDING
|
38,500,000 | 38,091,954 | ||||||||||
2
SUMMARY INFORMATION (Continued)
|
PUSH, INC.
|
||||||||
|
(A Development Stage Company)
|
||||||||
|
Balance Sheets
|
||||||||
|
ASSETS
|
||||||||
|
June 30,
|
December 31,
|
|||||||
|
2010
|
2009
|
|||||||
|
(Unaudited)
|
(Audited)
|
|||||||
|
CURRENT ASSETS
|
||||||||
|
Cash
|
$ | 90,335 | $ | 26,949 | ||||
|
Total Current Assets
|
90,335 | 26,949 | ||||||
|
Accounts receivable - related party
|
- | 146,624 | ||||||
|
TOTAL ASSETS
|
$ | 90,335 | $ | 173,573 | ||||
|
LIABILITIES AND STOCKHOLDERS' EQUITY
|
||||||||
|
CURRENT LIABILITIES
|
||||||||
|
Accounts payable and accrued expenses - related party
|
$ | 6,977 | $ | - | ||||
|
Payroll Liabilities
|
4,414 | - | ||||||
|
Related party payable
|
64,500 | 64,500 | ||||||
|
Total Current Liabilities
|
75,891 | 64,500 | ||||||
|
STOCKHOLDERS' EQUITY
|
||||||||
|
Common stock, 100,000,000 shares authorized
|
||||||||
|
at par value of $0.001; 38,500,000
|
||||||||
|
shares issued and outstanding
|
38,500 | 38,500 | ||||||
|
Additional paid-in capital
|
297,000 | 297,000 | ||||||
|
Deficit accumulated during the development stage
|
(321,056 | ) | (226,427 | ) | ||||
|
Total Stockholders' Equity
|
14,444 | 109,073 | ||||||
|
TOTAL LIABILITIES AND
|
||||||||
|
STOCKHOLDERS' EQUITY
|
$ | 90,335 | $ | 173,573 | ||||
3
RISK FACTORS
Investing in our common stock involves a high degree of risk. You should carefully consider the risks described below and the other information in this prospectus before making a decision to invest in our common stock. If any of these risks, or any other risk not currently known to us or that we currently deem immaterial, should occur, our business, operating results, financial condition or growth prospects could be adversely affected. In those cases, the trading price of our common stock could decline and you may lose all or part of your investment.
Risks Related to Our Business and Industry
We are a development stage detection device company with a limited operating history and a history of losses and negative cash flows from operations. We may never be profitable, and if we incur operating losses and generate negative cash flows from operations for longer than expected, we may be unable to continue operations.
We are a development stage detection device company and have a limited operating history, limited capital, and no current sources of revenue. We have incurred losses since inception. Our operations to date have consisted of organizing our company, obtaining funds from private investors, developing and engaging in trials of our product candidates (i.e., a device that passively monitors the movement of humans, and a separate device that passively monitors certain human vital signs), expanding and strengthening our intellectual property position by obtaining an exclusive license from our Parent Company, and recruiting management personnel. Consequently, you may have difficulty in predicting our future success or viability due to our lack of operating history. As of June 30, 2010, we have accumulated a deficit during our development stage of $321,056. None of our product candidates have been developed yet and we have not received regulatory approval for the sale of such product(s). Accordingly, we have not generated any material revenues. We anticipate that we will incur significant and increasing net losses and negative cash flows from operations for the foreseeable future as we:
|
·
|
continue research and development and undertake clinical trials, where necessary, with respect to our product candidate(s);
|
|
·
|
apply for regulatory approvals, where necessary;
|
|
·
|
make capital expenditures to increase our research and development;
|
|
·
|
add operational, financial and management information systems and personnel, and develop and protect our intellectual property;
|
|
·
|
make payments pursuant to our license agreement with the Parent Company;
|
|
·
|
purchase products from those entities that we have granted manufacturing rights;
|
|
·
|
establish sales and marketing capabilities to commercialize products for which we obtain regulatory approval, if any; and
|
|
·
|
provide our prior investors with a share of any licensing fees or royalty payments paid to us.
|
Our lack of experience in conducting and managing preclinical development activities, clinical trials and the application process necessary to obtain regulatory approvals might prevent us from successfully designing or implementing a preclinical study or clinical trial. If we do not succeed in conducting and managing our preclinical development activities or clinical trials, or in obtaining regulatory approvals, we might not be able to commercialize certain of our product candidates, or might be significantly delayed in doing so, which will materially harm our business.
We anticipate that we will need to transition from a company with a research and development focus to a company capable of supporting commercial activities, and we may not succeed in such a transition.
Because of the numerous risks and uncertainties associated with our product development and commercialization efforts, we are unable to predict the extent of our future losses or when or if we will become profitable. Any failure to successfully commercialize our product candidate(s) or to become and remain profitable could depress the market price of our common stock and impair our ability to raise capital, expand our business, and continue our operations.
4
RISK FACTORS (Continued)
We have granted rights to our prior investors which may restrict our operations and flexibility.
Prior to commencing this offering, we raised funds through a private placement of our common stock. These private investors were granted rights of refusal on exclusive manufacturing rights, exclusive distribution rights in Taiwan and China, and a right to receive 15% of our initial licensing fees and royalty payments. If any of these investors exercises its right to manufacture our products, then we will lose a significant amount of control over the commercialization of our products unless we purchase the products manufactured by the investor at a price equal to cost plus a 25% premium. Even if we purchase such products, we will not have the right to distribute such products in Taiwan or China.
Our Parent Company has certain anti-dilution rights which could make it impossible for anyone else to obtain a majority ownership in us, could make our shares less attractive to potential purchasers, and could cause massive dilution to all of our other shareholders.
The license agreement through which we obtained intellectual property rights from our Parent Company contains “full-ratchet” anti-dilution provisions which protect the Parent Company’s ownership interest in us. (See Exhibit 10.7, Amendment to License Agreement with DKL International, Inc.) These provisions ensure that the Parent Company will have at least 51% of our outstanding common stock, so long as it does not sell or otherwise dispose of more than 5,345,000 of the 26,000,000 shares it currently holds. If we issue any shares which dilute the Parent Company’s common stock holdings below 51% of all then-outstanding shares of common stock, we will automatically issue a number of shares to the Parent Company to restore its 51% interest. The Parent Company need not provide any additional consideration to receive such shares.
These rights make it impossible for anyone else to obtain a majority ownership interest in us, unless the Parent Company sells more than 5,345,000 shares of our common stock, at which time its anti-dilution rights will be proportionately reduced. Potential investors in our common stock may be discouraged from purchasing our common stock due to these anti-dilution rights.
Furthermore, anti-dilution rights have the effect of magnifying the dilution experienced by all other shareholders in any dilutive issuance. Once the anti-dilution rights are triggered, we anticipate issuing one or more shares of common stock to the Parent Company for each share we offer and sell to other persons. Thus, we expect that our shareholders will experience dilution far worse than would otherwise occur if these anti-dilution rights did not exist.
We may need substantial additional funding and may be unable to raise capital when needed. An inability to obtain additional financing on acceptable terms could adversely affect our business, financial condition, results of operations, and could even prevent us from continuing our business.
Based upon an offering price of $0.10 per share, we expect to raise net proceeds of up to $200,000 in this offering. Even if we obtain this entire amount with this offering, our demand for capital may be significantly higher than anticipated. We may require substantial future capital in order to continue the research and development, preclinical and clinical programs, and regulatory activities necessary to obtain regulatory approval of our product candidates. In addition, subject to obtaining regulatory approval for any of our product candidates, we expect to incur significant commercialization expenses for product sales, marketing and manufacturing. The extent of our need for additional capital will depend on numerous factors, including, but not limited to:
|
·
|
the scope, rate of scientific progress, results and cost of our clinical trials and other research and development activities;
|
|
·
|
our ability to obtain sufficient third-party insurance coverage or reimbursement for our product candidates;
|
|
·
|
the effectiveness of commercialization activities (including the volume and profitability of any sales achieved);
|
|
·
|
our ability to establish additional strategic, collaborative and licensing relationships with third parties with respect to the sales, marketing and distribution of our products, research and development and other matters, and the economic and other terms and timing of any such relationships;
|
|
·
|
the costs involved in any potential litigation that may occur;
|
|
·
|
decisions by us to pursue the development of new product candidates or technologies or to make acquisitions or investments; and
|
|
·
|
the effect of competing products, technologies and market developments.
|
5
RISK FACTORS (Continued)
We have no commitments or arrangements from third parties for any additional financing to fund the research, development and commercialization of any of our product candidates. We may need to seek substantial additional financing through public or private financing, which may include equity or debt financings, and through other arrangements, including collaborative arrangements. However, financing may not be available when we need it, or may not be available on acceptable terms. Our ability to obtain additional debt financing may be limited by the amount of, terms and restrictions of our then current debt. Debt financing, if available, may involve restrictive covenants that limit or further limit our operating and financial flexibility and prohibit us from making distributions, if any, to shareholders. If we raise additional funds by issuing equity, equity-related or convertible securities, the economic, voting and other rights of our existing shareholders, including investors who purchase shares in this offering, may be diluted, and those securities may have rights superior to those of our common stock. If we obtain additional capital through collaborative arrangements, we may be required to relinquish greater rights to our technologies or product candidates than we might otherwise have or become subject to restrictive covenants that may affect our business. If we are unable to raise additional funds when we need them, we may be required to delay, scale back or eliminate expenditures for our development programs, curtail efforts to commercialize our product candidates or reduce the scale of our operations, any of which could adversely affect our business, financial condition, results of operations, and could even prevent us from continuing our business at all.
We are a small company in a highly competitive industry.
Competition from other detection and medical device companies, diversified healthcare companies and research and academic institutions is intense and expected to increase. Many companies engaged in the medical device sector have substantially greater financial and other resources and development capabilities than we do, and have substantially greater experience in testing of products, obtaining regulatory approvals and manufacturing and marketing medical devices. Therefore, our competitors may succeed in obtaining approval for products more rapidly than we can. Other companies may succeed in developing and commercializing products earlier than we do. In addition to competing with universities and other research institutions in the development of products, technologies and processes, we may compete with other companies in acquiring rights to products or technologies from universities. Also, the medical device market is experiencing increasing customer concentration, due to the emergence of large purchasing groups. We cannot assure you that we will develop products that are more effective or achieve greater market acceptance than competitive products, or that our competitors will not succeed in developing products and technologies that are more effective than those being developed by us or that would render our products and technologies less competitive or obsolete. Moreover, there can be no assurance that we will be able to successfully sell to large purchasing groups, which are increasingly looking to suppliers that can provide a broader range of products than we currently offer.
We devote substantial resources to the development of a single type of product.
We expect to devote a significant amount of resources to continue the development of a device that passively monitors human specific movement. Thereafter, we plan to enhance this technology to measure human vital signs. Both of these product candidates rely on the same technology. We believe that substantial resources are required to prepare this novel technology for commercialization. Such investments include further research and development, including significant expenditures for equipment, clinical studies, marketing expenditures and general working capital requirements. There can be no assurance that we will be successful in these endeavors. Because we devote substantially all of our resources to the development of this single product candidate, our failure to develop and commercialize this product candidate would critically impair our ability to continue operations.
Our approach of monitoring patient vital signs without the direct bodily contact of electrodes is unproven.
To the best of our knowledge, no company has yet been successful in obtaining regulatory approval in the United States of a cardiac and respiratory activity monitoring device that does not require the direct contact of wearable electrodes. Such passive monitoring devices, in general, may be susceptible to inaccurate readings due to interference or other issues. These complications may prevent or limit their approval by regulators for commercial use. Although a related passive monitoring device developed by our Parent Company has been tested and quantified by the Parent Company’s internal testing department, these tests were not conducted on a product intended for use in the wellness care market and did not involve the measurement of vital signs. If we cannot sufficiently advance the technology or adapt it for use by patients at home, our business and results of operation would suffer.
6
RISK FACTORS (Continued)
The sale of products we may develop could result in significant product liability exposure.
As a potential manufacturer of medical diagnostic equipment, we may face product liability claims for any products that we ultimately develop. The monitors we seek to develop are electrical instruments that can fail due to variety of electrical, mechanical or design problems. We plan to obtain product liability insurance, but cannot assure you that any insurance coverage we obtain will be adequate to cover any product liability claims that occur in the future or that product liability insurance will be available at reasonable prices. Any product liability judgments or settlements in excess of insurance coverage could have a material adverse effect on our business and results of operations.
We are subject to significant government regulation.
Our business is subject to varying degrees of governmental regulation. We expect that our proposed human vital sign monitoring product will be more rigorously regulated than our product candidate which only detects human movement. Products which measure vital signs are generally subject to regulation as a medical device by the Food and Drug Administration (“FDA”), and by other federal and state agencies. These regulations pertain to the manufacturing, labeling, development and testing of any device we create as well as to the maintenance of required records. An FDA regulation also requires prompt reporting by all medical device manufacturers of an event or malfunction involving a medical device where the device caused or contributed to death or serious injury or is likely to do so.
Federal law provides for several routes by which the FDA reviews medical devices before their entry into the marketplace. Medical products such as our proposed vital sign monitoring device are subject to regulation under the Food, Drug and Cosmetic Act (the “FD&C Act”) and numerous acts and amendments such as the Quality System Regulations which replaced the regulations formerly called Good Manufacturing Practices. In addition, depending upon product type, we must also comply with those regulations governing the Conduct of Human Investigations, Pre-Market Regulations and other requirements, as promulgated by the FDA. The FDA is authorized to inspect a device, its labeling and advertising, and the facilities in which it is manufactured in order to ensure that the device is not manufactured or labeled in a manner which could cause it to be injurious to health.
The FDA has adopted regulations which classify medical devices based upon the degree of regulation believed necessary to assure safety and efficacy. A device is classified as a Class I, II, or III device. Class I devices are subject only to general controls. Class II devices, in addition to general controls, are or will be subject to “performance standards.” Most devices are also subject to the 510(k) pre-market notification provision. In addition, some Class III devices require FDA pre-market approval before they may be marketed commercially because their safety and effectiveness cannot be assured by the general controls and performance standards of Class I or II devices. We believe that a device which passively monitors human cardiac and respiratory rates will be categorized as a Class I or Class II device and may be subject to FDA notification requirement under Section 510(k) of the FD&C Act.
Satisfaction of clearance or approval requirements may take up to several years or more and may vary substantially based upon the type, complexity and novelty of the product. The effect of government regulation may be to delay marketing of new products for a considerable or indefinite period of time, to impose costly procedures upon our activities and to furnish a competitive advantage to larger companies that compete with us. We cannot assure you that FDA or other regulatory clearance or approval for any products we develop will be granted on a timely basis, if at all, or, once granted, that clearances or approvals will not be withdrawn or other regulatory action taken which might limit our ability to market our proposed products. Any delay in obtaining or failure to obtain these clearances or approvals would adversely affect the manufacturing and marketing of our products and the ability to generate additional product revenue.
Federal regulatory reforms may adversely affect our ability to successfully market the products we may create.
Recent efforts to reform the U.S. health care industry led by President Obama and certain members of Congress have resulted in legislation and other measures which will effect changes in healthcare delivery and coverage, and public and private reimbursements for healthcare products. Federal initiatives may also affect state programs. Legislative changes may affect market expenditures for medical devices, the type and volume of procedures performed, and the demand for new and innovative products. These changes could be significant and may adversely affect the demand for any products we create, our results of operations, cash flows and our overall financial condition.
7
RISK FACTORS (Continued)
There is substantial uncertainty as to the insurance coverage that may be available and the reimbursement rates that may be established for our product candidates. Any failure to obtain third party coverage or an adequate level of reimbursement for our product candidates will likely have a material adverse effect on our business.
If we successfully develop, and obtain necessary regulatory approvals for, our proposed passive monitoring devices, we intend to sell them in the United States and abroad. We have not yet submitted our product candidate to the Center for Medicare and Medicaid Services, or CMS, or any private or governmental third party payer in the United States to determine whether or not our proposed monitoring devices will be covered under private or public health insurance plans or, if they will be covered, what coverage or reimbursement rates may be available. We cannot assure you that reimbursement will be adequate or available at all.
We believe that our revenues will depend in part upon the coverage and reimbursement rates and policies established for our product candidates by third party payers, including governmental authorities, managed-care providers, public health insurers, private health insurers and other organizations. These third party payers are increasingly attempting to contain healthcare costs by limiting both coverage and the level of reimbursement for new healthcare products approved for marketing by the FDA or regulatory agencies in other countries. As a result, significant uncertainty exists as to whether detection devices or newly approved medical products will be eligible for coverage by third party payers or, if eligible for coverage, what the reimbursement rates will be for those products. Furthermore, devices based on novel technologies may be more expensive than similar devices based on existing technology, due to, among other things, the higher cost and complexity associated with the research, development and production of these devices. This, in turn, may make it more difficult for us to obtain adequate reimbursement from third party payers, particularly if we cannot demonstrate a favorable cost-benefit relationship. Third party payers may also deny coverage or offer inadequate levels of reimbursement for our potential products if they determine that the product has not received appropriate clearances from the FDA or other government regulators or is experimental, unnecessary or inappropriate. Accordingly, we cannot assure you that adequate third party coverage or reimbursement will be available to allow us to successfully commercialize any products we develop.
Coverage and reimbursement rates for our product candidates may be subject to increased restrictions both in the United States and in other countries in the future. Coverage policies and reimbursement rates are subject to change and we cannot guarantee that current coverage policies and reimbursement rates will be applicable to our product candidates in the future. U.S. federal, state and foreign agencies and legislatures from time to time may seek to impose restrictions on coverage, pricing, and reimbursement level of drugs, devices and healthcare services in order to contain healthcare costs.
The products we may create could become rapidly obsolete.
Our product candidates involve rapidly developing technology. Others may develop products that might cause products being developed, distributed or licensed by us to become obsolete or uneconomical or result in products superior to our products.
We depend highly on certain key management personnel.
Our success depends on our continued ability to attract, retain and motivate highly qualified management, clinical and scientific personnel and on our ability to develop and maintain important relationships with academic institutions, clinicians and scientists. We believe that our future success will depend to a significant extent on the efforts and abilities of our senior management, in particular Ted Wong, our President, Chief Executive Officer and director, and Howard Sidman, our Chief Financial Officer, Treasurer and Secretary and director. We are highly dependent upon our senior scientific staff, many of whom have developed very specialized expertise in their position. The loss of services of one or more members of our senior scientific staff could significantly delay or prevent the successful development of our product candidates. The employment of each of these employees can be terminated by us or the employee with or without cause upon 30 days’ prior written notice to the other party. We do not have a succession plan in place for any of our officers and key employees. Although we are seeking to secure “key person” insurance on Mr. Wong and Mr. Sidman, we do not carry insurance on any of our other key employees and, accordingly, their death or disability may have a material adverse effect on our business.
8
RISK FACTORS (Continued)
The competition for qualified personnel in our industry is intense. We will need to hire additional personnel, including regulatory and sales personnel, as we continue to expand our development activities. We may not be able to attract and retain quality personnel on acceptable terms given our geographic location and the competition for such personnel among life sciences, biotechnology, and other companies. If we are unable to attract new employees and retain existing employees, we may be unable to continue our development and commercialization activities and our business may be harmed.
Our success will depend in part on establishing and maintaining effective strategic partnerships, collaborations and licensing agreements.
Our strategy for the commercialization of our product candidates relies on establishing and maintaining numerous collaborations with various sales and marketing personnel. We plan to license any product we develop to companies which provide a first alert service to the senior sector of the population. The process of establishing and maintaining such partnerships and licensing agreements on acceptable terms is difficult. Any collaboration that we enter into may not be successful. The success of our collaboration arrangements, if any, will depend heavily on the efforts and activities of our collaborators. Possible future collaborations have risks, including the following:
|
·
|
our collaboration agreements are likely to be for fixed terms and subject to termination by our collaborators in the event of a material breach or lack of scientific progress by us;
|
|
·
|
our collaborators are likely to have the first right to maintain or defend our intellectual property rights and, although we would likely seek to secure the right to assume the maintenance and defense of our intellectual property rights if our collaborators do not, our ability to do so may be compromised by our collaborators’ acts or omissions;
|
|
·
|
our collaborators may utilize our intellectual property rights in such a way as to invite litigation that could jeopardize or invalidate our intellectual property rights or expose us to potential liability;
|
|
·
|
our collaborators may underfund or not commit sufficient resources to the testing, marketing, distribution or development of our product candidates; and
|
|
·
|
our collaborators may develop alternative products either on their own or in collaboration with others, or encounter conflicts of interest or changes in business strategy or other business issues, which could adversely affect their willingness or ability to fulfill their obligations to us.
|
These arrangements also may require us to grant certain rights to third parties, including exclusive marketing rights to one or more products, or may have other terms that are burdensome to us, and may involve the issuance of our securities. If any of our partners terminates its relationship with us or fails to perform its obligations in a timely manner, the development or commercialization of our technology and product candidates may be substantially delayed. Further, disputes may arise with our collaborators about inventorship and corresponding rights in know-how and inventions resulting from the joint creation or use of intellectual property by us and our collaborators.
If we are unable to sustain profitable operations by licensing our technology to third-parties willing to commercialize our product candidate, we may need to establish our own sales and marketing capabilities.
We primary plan is to license any product we develop to companies which provide a first alert service to the senior sector of the population. However, if we cannot sustain profitable operations by licensing our technology to third parties, we will need to either develop an internal direct sales and marketing force or enter into agreements with third parties to have these services performed. We do not currently have a sales and marketing force and related infrastructure, and have no experience in the sales, marketing and distribution of our product candidate. Furthermore, to sell products outside of the U.S., we may need to obtain an export license which we currently do not possess. The development of our own sales and marketing force will result in us incurring significant costs before the time that we may generate revenues. We may not be able to attract, hire, train and retain qualified sales and marketing personnel to build a significant or effective marketing and sales force for sales of our product candidate.
9
RISK FACTORS (Continued)
Risks Related to Our Intellectual Property
Our license agreement with the Parent Company may limit our ability to perform research and development activities, and creates doubt as to whether we will own the intellectual property rights derived from our research.
Our license agreement with the Parent Company requires us to contract with the Parent Company for all research and development activities relating to new or improved products or processes using the Parent Company’s technology. Furthermore, it requires that we fund all such research and development activities. Therefore, if we are unable to reach an agreement on the terms upon which research and development activities will be conducted, we may not be able to expand our operations. Moreover, it is uncertain whether we will own the intellectual property rights arising from such research and development activities, even if they are funded by us.
Our business could be adversely affected if we cannot protect our proprietary technology or if we infringe on the proprietary technology of others.
Our proprietary technology aids our ability to compete effectively with other companies in certain markets in which we compete. We license the rights to certain proprietary technology from our Parent Company, and we seek to expand our proprietary rights by further developing this technology and applying it toward the wellness care market. Although our Parent Company has filed applications for, and has been awarded, patents relating to the covert surveillance of vital signs, these patents may not fully protect our competitive position or use of this technology. Our commercial success depends to a significant degree on our ability to:
|
·
|
obtain and/or maintain protection for our product candidate under the patent laws of the United States and other countries;
|
|
·
|
defend and enforce our patents once obtained;
|
|
·
|
obtain and/or maintain appropriate licenses to patents, patent applications or other proprietary rights held by others with respect to our technology, both in the United States and other countries;
|
|
·
|
maintain trade secrets and other intellectual property rights relating to our product candidates; and
|
|
·
|
operate without infringing upon the patents and proprietary rights of third parties.
|
The degree of intellectual property protection for our technology is uncertain, and only limited intellectual property protection may be available for our product candidate, which may prevent us from gaining or keeping any competitive advantage against our competitors. Although we believe the patents and patent applications that have been licensed to us generally provide us a competitive advantage, the patent positions of medical device companies are generally highly uncertain, involve complex legal and factual questions and have been the subject of much litigation. Neither the U.S. Patent and Trademark Office nor the courts have a consistent policy regarding the breadth of claims allowed or the degree of protection afforded under many biotechnology patents. Even if issued, patents may be challenged, narrowed, invalidated or circumvented, which could limit our ability to stop competitors from marketing similar products or limit the length of term of patent protection we may have for our products. Further, a court or other government agency could interpret our patents in a way such that the patents do not adequately cover our current or future product candidates. Changes in either patent laws or in interpretations of patent laws in the United States and other countries may diminish the value of our intellectual property or narrow the scope of our patent protection.
In particular, we cannot assure you that:
|
·
|
we or the owners or other inventors of the patents that we own or that have been licensed to us or that may be issued or licensed to us in the future were the first to file patent applications or to invent the subject matter claimed in patent applications relating to the technologies upon which we rely;
|
|
·
|
others will not independently develop similar or alternative technologies or duplicate any of our technologies;
|
|
·
|
any patent applications that we file will result in issued patents;
|
|
·
|
the patents and the patent applications that have been licensed to us or that may be issued or licensed to us in the future will provide a basis for commercially viable products or will provide us with any competitive advantages, or will not be challenged by third parties;
|
|
·
|
the patents and the patent applications that have been licensed to us are valid and enforceable;
|
|
·
|
we will develop additional proprietary technologies that are patentable;
|
|
·
|
we will be successful in enforcing the patents that we own or that have been licensed to us and any patents that may be issued or licensed to us in the future against third parties; or
|
|
·
|
the patents of third parties will not have an adverse effect on our ability to do business.
|
10
RISK FACTORS (Continued)
Litigation may be necessary to enforce patents issued to the Parent Company or us, to defend ourselves against claimed infringement of the rights of others or to determine the scope and validity of the proprietary rights of others. The defense and prosecution of patent suits are both costly and time-consuming, even if the outcome is favorable to us. Such proceedings can be extremely expensive and their outcome very unpredictable. An adverse outcome in the defense of a patent suit could cause us to lose proprietary rights, subject us to significant liabilities to third parties or require us to license rights from third parties or to cease selling our products.
If we are not able to protect and control unpatented trade secrets, know-how and other technological innovation, we may suffer competitive harm.
In addition to patented intellectual property, we also rely on unpatented technology, trade secrets, confidential information and proprietary know-how to protect our technology and maintain our competitive position, especially when we do not believe that patent protection is appropriate or can be obtained. Trade secrets are difficult to protect. In order to protect proprietary technology and processes, we rely in part on confidentiality and intellectual property assignment agreements with our employees, consultants and others. These agreements generally provide that the individual must keep confidential and not disclose to other parties any confidential information developed or learned by the individual during the course of the individual’s relationship with us except in limited circumstances. These agreements generally also provide that we shall own all inventions conceived by the individual in the course of rendering services to us. These agreements may not effectively prevent disclosure of confidential information or result in the effective assignment to us of intellectual property, and may not provide an adequate remedy in the event of unauthorized disclosure of confidential information or other breaches of the agreements. In addition, others may independently discover trade secrets and proprietary information that have been licensed to us or that we own, and in such case we could not assert any trade secret rights against such party. Enforcing a claim that a party illegally obtained and is using trade secrets that have been licensed to us or that we own is difficult, expensive and time-consuming, and the outcome is unpredictable. In addition, courts outside the United States may be less willing to protect trade secrets. Costly and time-consuming litigation could be necessary to seek to enforce and determine the scope of our proprietary rights, and failure to obtain or maintain trade secret protection could have a material adverse effect on our business. If we cannot maintain the confidentiality of our technologies and other confidential information in connection with our collaborations, our ability to protect our proprietary information or obtain patent protection in the future may be impaired, which could have a material adverse effect on our business.
We have licensed and therefore do not own the intellectual property that is critical to our business. Any events or circumstances that result in the termination or limitation of our rights under our agreement between us and the licensor of our intellectual property could have a material adverse effect on our business.
The intellectual property that is critical to our business has been licensed to us by our Parent Company. If we do not meet our obligations under the license agreement, we may lose our license. Under our patent license agreement, we are subject to payment, commercialization and other obligations and, if we fail to comply with any of these requirements or otherwise breach our agreement, our Parent Company may terminate the license in whole or in part, terminate the exclusive nature of the license to the extent such license is exclusive or otherwise limit our rights thereunder. For instance, we are obligated to pay all research and development fees and also to make royalty payments to the Parent Company (subject to annual minimum payment amounts). Any termination or limitation of, or loss of exclusivity under, our exclusive or conditionally exclusive license agreements would have a material adverse effect on us and could delay or completely terminate our product development efforts.
We may not have the right under our license agreement to control the protection of the patents licensed thereunder and, as a result, our licensor may take actions and make decisions that could materially adversely affect our business.
11
RISK FACTORS (Continued)
We generally have the responsibility of initiating law suits against suspected infringers of the rights licensed by us.
However, the Parent Company retains certain rights to take control of such lawsuits. We may not be able control whether or how zealously the Parent Company enforces its and our rights against others. Under our material license agreements, our licensors generally have the right to control the filing, prosecution, maintenance and defense of all licensed patents and patent applications and, if a third party infringes on any of those licensed patents, to control any legal or other proceedings instituted against that third party for infringement. As a result, our licensors may take actions or make decisions relating to these matters with which we do not agree or which could have a material adverse effect on our business. Likewise, our licensors may in the future grant licenses to third parties to use the patents and other intellectual property to the extent that we do not have the exclusive or conditionally exclusive right to such intellectual property. Should our licensors elect not to pursue the filing, prosecution or maintenance of a licensed patent application or patent or institute legal or other proceedings against a third party for infringements of those patents, then we may be required to undertake these proceedings alone or jointly with others, who may have interests that are different from ours. Under certain of our license agreements where we are the licensor, we may have no right to undertake these proceedings even if our licensees refuse to do so. As a result, we may have no control or only limited control over the prosecution, maintenance, defense and enforcement of patent applications and patents that are critical to our business. In that regard, certain of our license agreements require that we contribute to the costs of filing, prosecuting, maintaining, defending and enforcing the licensed patent applications, patents and other intellectual property, whether or not we agree with those actions. Further, such actions typically require the expenditure of considerable time and money.
Patent protection for our technology outside of the United States is limited and we may not be able to effectively enforce our intellectual property rights in certain countries, which could have a material adverse effect on our business.
The technology upon which our product candidate is based is not protected by patents in every country in which we plan to operate, which means that competitors will be free to sell products that incorporate the same technologies that are used in our product candidate in those countries. In addition, the laws and practices in some of those countries, or others in which we may seek to market our other product candidates in the future, may not protect intellectual property rights to the same extent as in the United States. We or our licensors may not be able to effectively obtain, maintain or enforce rights with respect to the intellectual property relating to our product candidates in those countries. Our lack or shortage of patent protection in one or more countries, or the inability to obtain, maintain or enforce intellectual property rights in one or more countries, could adversely affect our ability to commercialize our products in those countries and otherwise have a material adverse effect on our business.
Risks Related to This Offering
There is no public market for our stock. If a market develops, our stock price may be volatile and you may be unable to sell your shares at or above the offering price.
There is no public market for our common stock. The offering price for our shares was arbitrarily determined by us and does not purport to be indicative of prices that will prevail in any trading market that may develop. The market price of our common stock could be subject to wide fluctuations in response to many risk factors described in this section and other matters, including:
|
·
|
publications of our business developments;
|
|
·
|
fluctuations in the valuation of companies perceived by investors to be comparable to us;
|
|
·
|
retention and departures of key personnel;
|
|
·
|
strategic decisions by us or our competitors, such as acquisitions, divestitures, spin-offs, joint ventures, strategic investments or changes in business strategy;
|
|
·
|
the passage of legislation or other regulatory developments affecting us or our industry;
|
|
·
|
speculation in the press or investment community; and
|
|
·
|
natural disasters, terrorist acts, acts of war or periods of widespread civil unrest.
|
12
RISK FACTORS (Continued)
Furthermore, the stock markets have experienced extreme price and volume fluctuations that have affected and continue to affect the market prices of equity securities of many companies, especially life sciences and medical device companies. These fluctuations often have been unrelated or disproportionate to the operating performance of those companies. These broad market and industry fluctuations, as well as general economic, political and market conditions, may negatively affect the market price of our common stock. As a result, the market price of our common stock is likely to be similarly volatile and investors in our common stock may experience a decrease, which could be substantial, in the value of their stock. In the past, many companies that have experienced volatility in the market price of their stock have been subject to securities class action litigation. We may be the target of this type of litigation in the future. Securities litigation against us could result in substantial costs and divert our management’s attention from other business concerns, which could have a material adverse effect on our business.
Our existing shareholders have significant control of our management and affairs, which they could exercise against your best interests.
Through our Parent Company and H & W Associates 99, Inc., Ted Wong and Howard Sidman (our sole officers and directors) have significant control over our operations. These individuals will continue to control us following the completion of this offering, and will continue to have a significant influence over the outcome of all corporate actions requiring shareholder approval. Consequently, this concentration of ownership may have the effect of delaying or preventing a change of control, including a merger, consolidation or other business combination involving us, or discouraging a potential acquirer from making a tender offer or otherwise attempting to obtain control, even if such a change of control would benefit our other shareholders. The interests of these shareholders may not coincide with our interests or the interests of other shareholders.
An active trading market for our common stock may never develop.
This is our initial public offering of common stock, and prior to this offering there has been no public market for our common stock. Although we plan to seek a market maker to file an application for our shares of common stock to become quoted on the over-the-counter bulletin board system, there can be no assurance that a meaningful trading market will ever develop or be sustained following this offering. If an active market for our common stock does not develop, it may be impossible for you to sell shares you purchase in this offering.
The Penny Stock Rules could restrict the ability of broker-dealers to sell our shares having a negative effect on our offering.
The Securities and Exchange Commission (“SEC”) has adopted penny stock regulations which apply to securities traded over-the-counter. These regulations generally define penny stock to be any equity security that has a market price of less than $5.00 per share or an equity security of an issuer with net tangible assets of less than $5,000,000 as indicated in audited financial statements, if the corporation has been in continuous operations for less than three years. Subject to certain limited exceptions, the rules for any transaction involving a penny stock require the delivery, prior to the transaction, of a risk disclosure document prepared by the SEC that contains certain information describing the nature and level of risk associated with investments in the penny stock market. The broker-dealer also must disclose the commissions payable to both the broker-dealer and the registered representative and current quotations for the securities. Monthly account statements must be sent by the broker-dealer disclosing the estimated market value of each penny stock held in the account or indicating that the estimated market value cannot be determined because of the unavailability of firm quotes. In addition, the rules impose additional sales practice requirements on broker-dealers who sell such securities to persons other than established customers and institutional accredited investors (generally institutions with assets in excess of $5,000,000). These practices require that, prior to the purchase, the broker-dealer determined that transactions in penny stocks were suitable for the purchaser and obtained the purchaser’s written consent to the transaction. If a market for our common stock does develop and our shares trade below $5.00 per share, it will be a penny stock. Consequently, the penny stock rules will likely restrict the ability of broker-dealers to sell our shares and will likely affect the ability of purchasers in the offering to sell our shares in the secondary market. If trading in our common stock develops, it will be subject to the “penny stock” rules. Due to the thinly traded market of these shares investors are at a much higher risk to lose all or part of their investment. Not only are these shares thinly traded but they are subject to higher fluctuations in price due to the instability of earnings of these smaller companies. As a result of the lack of a highly traded market in our shares investors are at risk of a lack of brokers who may be willing to trade in these shares.
Because we have operated as a private company, we have no experience complying with public company obligations. Compliance with these requirements will increase our costs and require additional management resources, and we may still fail to comply.
13
RISK FACTORS (Continued)
We will incur significant additional legal, accounting, insurance and other expenses as a result of being a public company that we have not incurred as a private company. For example, laws and regulations affecting public companies, including the provisions of the Sarbanes-Oxley Act of 2002, and rules related to corporate governance and other matters subsequently adopted by the SEC, will result in substantially increased costs to us, including legal and accounting costs, and may divert our management’s attention from other matters that are important to our business. These rules and any related regulations that may be proposed in the future will likely make it more difficult or more costly for us to obtain certain types of insurance, including directors’ and officers’ liability insurance, and we may be forced to accept reduced policy limits and coverage or incur substantially higher costs to obtain the same or similar coverage. The impact of these laws, rules and regulations could also make it more difficult for us to attract and retain qualified persons to serve on our board of directors, our board committees or as executive officers. We cannot predict or estimate the amount of the additional costs we may incur, but we expect our operating results will be adversely affected by the costs of operating as a public company.
Failure to achieve and maintain effective internal control over financial reporting in accordance with Section 404 of the Sarbanes-Oxley Act could have a material adverse effect on our business.
As a reporting company, we will be required to document and test our internal financial control procedures in order to satisfy the requirements of Section 404 of the Sarbanes-Oxley Act, which will require annual management assessments of the effectiveness of our internal control over financial reporting. During the course of our testing, we may identify deficiencies which we may not be able to remediate in time to meet our deadline for compliance with Section 404, and we may also identify inaccuracies or deficiencies in our financial reporting that could require revisions to or restatement of prior period results. Testing and maintaining internal control over financial reporting also will involve significant costs and can divert our management’s attention from other matters that are important to our business. We may not be able to conclude on an ongoing basis that our internal control over financial reporting is effective in accordance with Section 404. Failure to achieve and maintain an effective internal control environment could harm our operating results and could cause us to fail to meet our reporting obligations and could require that we restate our financial statements for prior periods, any of which could cause investors to lose confidence in our reported financial information and cause a decline, which could be material, in the trading price of our common stock.
Our management has broad discretion in the use of the net proceeds from this offering and may not use them effectively.
As of the date of this prospectus, we cannot specify with certainty the amount of net proceeds from this offering that we will spend on particular uses. Although our management currently intends to use the net proceeds in the manner described in “Use of Proceeds,” it will have broad discretion in the application of the net proceeds. The failure by our management to apply these funds effectively could adversely affect our ability to continue to maintain and expand our business.
We do not intend to pay cash dividends on our common stock in the foreseeable future and, accordingly, capital appreciation of our common stock, if any, will be a shareholder’s sole source of gain from an investment in our common stock.
Our policy is to retain earnings to provide funds for the operation and expansion of our business and, accordingly, we have never declared or paid any cash dividends on our common stock or other securities and do not currently anticipate paying any cash dividends in the foreseeable future. Consequently, shareholders will need to sell shares of our common stock to realize a return on their investments, if any and this capital appreciation, if any, will be a shareholder’s sole source of gain from an investment in the common stock. The declaration and payment of dividends by us are subject to the discretion of our Board of Directors and the restrictions specified in our articles of incorporation, in any agreements we enter into containing negative covenants, and by applicable law. Any future determination to pay cash dividends will depend on our results of operations, financial condition, capital requirements, contractual restrictions and other factors deemed relevant by our Board of Directors.
14
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus contains forward-looking statements about our business, financial condition and prospects that reflect our management’s assumptions and beliefs based on information currently available. We can give no assurance that the expectations indicated by such forward-looking statements will be realized. If any of our assumptions should prove incorrect, or if any of the risks and uncertainties underlying such expectations should materialize, the actual results may differ materially from those indicated by the forward-looking statements.
The key factors that are not within our control and that may have a direct bearing on operating results include, but are not limited to, public acceptance of the proposed merchandise and services that we expect to market, our ability to establish a sufficient customer base, management’s ability to raise capital in the future (if required), the retention of key employees, changes in the regulation of the industry in which we function, and changes under the laws, rules and regulations of governmental entities.
There may be other risks and circumstances that management may be unable to predict.
When used in this prospectus, words such as, “believes,” “expects,” “intends,” “plans,” “anticipates,” “estimates” and similar expressions are intended to identify and qualify forward-looking statements, although there may be certain forward-looking statements not accompanied by such expressions.
15
USE OF PROCEEDS
We will realize gross proceeds from the Offering of $50,000 if only the minimum offering is achieved and $200,000 if the maximum offering is achieved. We anticipate the proceeds will generally be used as detailed below. In the case that the Offering does not reach the maximum and the total proceeds are less than those indicated in the table, we will have the discretion to apply the available net proceeds to various indicated uses within the dollar limits established in the table below.
We intend to use the proceeds from this offering as follows:
|
Minimum
|
50% of Maximum
|
Maximum
|
||||||||||||||||||||||
|
Application Of Proceeds
|
Dollar Amount
|
% of Gross Proceeds
|
Dollar Amount
|
% of Gross Proceeds
|
Dollar Amount
|
% of Gross Proceeds
|
||||||||||||||||||
|
Total Offering Proceeds
|
50,000 | 100.00 | 100,000 | 100.00 | 200,000 | 100.00 | ||||||||||||||||||
|
Offering Expenses
|
||||||||||||||||||||||||
|
Legal and Professional Fees
|
20,000 | 40.00 | 20,000 | 20.00 | 20,000 | 10.00 | ||||||||||||||||||
|
Accounting Fees
|
5,000 | 10.00 | 5,000 | 5.00 | 5,000 | 2.50 | ||||||||||||||||||
|
Filing Fees
|
115 | * | 115 | * | 115 | * | ||||||||||||||||||
|
Escrow Fees
|
1,500 | 3.00 | 1,500 | 1.50 | 1,500 | * | ||||||||||||||||||
|
Total Offering Expenses
|
26,615 | 53.23 | 26,615 | 26.62 | 26,615 | 13.31 | ||||||||||||||||||
|
Net Proceeds from Offering
|
23,385 | 46.77 | 73,385 | 73.39 | 173,385 | 86.69 | ||||||||||||||||||
|
Use of Net Proceeds
|
||||||||||||||||||||||||
|
Regulatory Approval
|
- | * | 20,385 | 20.39 | 55,385 | 27.69 | ||||||||||||||||||
|
Marketing Expenses
|
8,000 | 16.00 | 10,000 | 10.00 | 20,000 | 10.00 | ||||||||||||||||||
|
Product Development
|
5,000 | 10.00 | 10,000 | 10.00 | 48,500 | 24.25 | ||||||||||||||||||
|
Web Site Preparation
|
2,885 | 5.77 | 5,000 | 5.00 | 5,000 | 2.50 | ||||||||||||||||||
|
Legal and Professional Fees
|
7,500 | 15.00 | 7,500 | 7.50 | 15,000 | 7.50 | ||||||||||||||||||
|
Travel Expense
|
- | * | 6,250 | 6.25 | 10,000 | 5.00 | ||||||||||||||||||
|
Working Capital
|
- | * | 14,250 | 14.25 | 19,500 | 9.75 | ||||||||||||||||||
|
Total Use of Net Proceeds
|
23,385 | 46.77 | 73,385 | 73.39 | 173,385 | 86.69 | ||||||||||||||||||
|
Total Use of Proceeds
|
50,000 | 100.00 | 100,000 | 100.00 | 200,000 | 100.00 | ||||||||||||||||||
* Less than 1% of gross proceeds
16
DETERMINATION OF OFFERING PRICE
The offering price of the common stock has been arbitrarily determined by us and bears no relationship to any objective criterion of value. The price does not bear any relationship to our assets, book value, historical earnings or net worth. In determining the offering price, our management considered such factors as the prospects, if any, for similar companies, anticipated results of operations, present financial resources and the likelihood of success of this offering.
17
DILUTION
If you invest in our common stock, your interest will be diluted to the extent of the difference between the offering price per share of our common stock and the pro forma net tangible book value per share of our common stock immediately after completion of the offering. “Net tangible book value per share” is the amount that results from subtracting our total liabilities from our total tangible assets, divided by the total number of shares outstanding. In this offering, the level of dilution is increased as a result of the relatively low tangible book value of our issued and outstanding common stock.
Our historical net tangible book value of our common stock as of June 30, 2010 was $14,444, or $0.000375 per share. After giving effect to our issuance of 2,000,000 shares at a public offering price of $0.10 per share, our pro forma net tangible book value as adjusted as of June 30, 2010 would have been $214,444, or $0.005295 per share of common stock. This represents an immediate increase in pro forma net tangible book value of $0.004920 per share to our existing shareholders and immediate dilution of $0.094705 per share to the new investors who purchase shares in this offering. The following table illustrates the dilution to the purchasers of the common stock in this offering:
|
Offering price per share
|
$ | 0.100000 | ||||||
|
Pro forma net tangible book value per share before this offering
|
$ | 0.000375 | ||||||
|
Increase in pro forma net tangible book value per share attributable to investors in this offering
|
$ | 0.004920 | ||||||
|
Pro forma net tangible book value per share, as adjusted to give effect to this offering
|
$ | 0.005295 | ||||||
|
Dilution per share to investors in this offering
|
$ | 0.094705 | ||||||
The following table summarizes as of June 30, 2010, on a pro forma as adjusted basis described above, the differences between the existing shareholders and the new investors purchasing our common stock in this offering with respect to the number of shares purchased, the total consideration paid and the average price per share paid, assuming the maximum offering of 2,000,000 shares is sold:
|
Shares Purchased
|
Total Consideration
|
|||||||||||||||||||
|
Number
|
Percent
|
Amount
|
Percent
|
Average Price
Per Share
|
||||||||||||||||
|
Existing Shareholder(s)
|
38,500,000 | 95.1 | % | $ | 300,000 | 60.0 | % | $ | 0.0078 | |||||||||||
|
New Investors
|
2,000,000 | 4.9 | % | $ | 200,000 | 40.0 | % | $ | 0.1000 | |||||||||||
|
Total:
|
40,500,000 | 100 | % | $ | 500,000 | 100 | % | |||||||||||||
The above discussion and tables do not include shares sold in this offering by our selling shareholders (which do not affect our net tangible book value) or shares underlying options to purchase common stock (which will be issued only upon exercise of the option at a price of $0.30 per share).
18
SELLING SHAREHOLDERS
The following table sets forth (i) the number of outstanding shares beneficially owned by the selling shareholders prior to the offering; (ii) the aggregate number of shares offered by each such stockholder pursuant to this prospectus; and (iii) the number of outstanding shares and the percentage of the class to be beneficially owned by such shareholder after the offering is complete.
|
Name of Selling Shareholder
|
Number of Shares Owned
Before the Offering
|
Number of Shares Offered by
Selling Shareholder
|
Number of Shares
Owned
After the Offering
|
Percentage of Shares Owned
After the Offering
|
|
Shih Chung Chen
|
2,000,0001
|
2,000,000
|
0
|
0.00%
|
|
Tsung Wen Chang
|
2,000,0001
|
2,000,000
|
0
|
0.00%
|
|
John Phillips
|
2,000,0001
|
2,000,000
|
0
|
0.00%
|
|
Total (Three Shareholders)
|
6,000,000
|
6,000,000
|
0
|
0.00%
|
|
|
1
|
Includes an option to purchase up to 1,000,000 shares of common stock at $0.30 per share, which underlying shares of common stock are being registered in this offering. The option expires on June 29, 2014.
|
19
PLAN OF DISTRIBUTION
There is no public market for our common stock. Our common stock is currently held only by three non-affiliated shareholders and four affiliated shareholders. Therefore, the current and potential market for our common stock is limited and the liquidity of our shares may be severely limited. To date, we have made no effort to obtain listing or quotation of our securities on a national stock exchange or electronic quotation system. We have not identified or approached any broker/dealers with regard to assisting us in applying for such listing or quotation. We are unable to estimate when we expect to undertake this endeavor. In the absence of being listed or quoted, no market is available for investors in our common stock to sell their shares. We cannot guarantee that a meaningful trading market will ever develop.
If a trading market develops, the trading price of our common stock could be subject to wide fluctuations in response to various events or factors, many of which are beyond our control. As a result, investors may be unable to sell their shares at or greater than the price at which they are being offered, and investors may be unable to sell their shares at all.
Common Stock Offered by the Company
This offering will be conducted on a best-efforts basis utilizing the efforts of Ted Wong and Howard Sidman, our officers and directors. Potential investors include, but are not limited to, family, friends and acquaintances of the officers and directors and their associates. The intended methods of communication include, without limitation, telephone and personal contact. In their endeavors to sell this offering, they will not use any mass advertising methods such as the internet or print media.
Funds received in connection with sales of our securities will be transmitted immediately into an escrow account until the minimum sales threshold is reached. There can be no assurance that all, or any, of the shares will be sold.
Ted Wong and Howard Sidman will not receive commissions for any sales originated on our behalf. We believe that Ted Wong and Howard Sidman are exempt from registration as a broker under the provisions of Rule 3a4-1 promulgated under the Securities Exchange Act of 1934. In particular, as to Ted Wong or Howard Sidman, they individually:
1. are not subject to a statutory disqualification, as that term is defined in Section 3(a)(39) of the Securities Exchange Act of 1934, as amended, at the time of his participation;
2. are not to be compensated in connection with his participation by the payment of commissions or other remuneration based either directly or indirectly on transactions in securities (they are paid salaries as stated in their employment agreements);
3. are not an associated person of a broker or dealer; and
4. meet the following conditions:
a. Primarily performs, or is intended primarily to perform at the end of the offering, substantial duties for or on behalf of the issuer otherwise than in connection with transactions in securities;
b. Was not a broker or dealer, or associated person of a broker or dealer, within the preceding 12 months; and
c. Did not participate in selling an offering of securities for any issuer more than once every 12 months other than in reliance on paragraphs within this section, except that for securities issued pursuant to rule 415 under the Securities Act of 1933, the 12 months shall begin with the last sale of any security included within rule 415 registration.
None of our officers or directors may purchase any securities in this offering.
20
PLAN OF DISTRIBUTION (Continued)
As of this date, we have not entered into any agreements or arrangements for the sale of the shares with any broker/dealer or sales agent. However, if we were to enter into such arrangements, we will file a post effective amendment to disclose those arrangements because any broker/dealer participating in the offering would be acting as an underwriter and would have to be so named herein.
In order to comply with the applicable securities laws of certain states, the securities may not be offered or sold unless they have been registered or qualified for sale in such states or an exemption from such registration or qualification requirement is available and with which we have complied. The purchasers in this offering and in any subsequent trading market must be residents of such states where the shares have been registered or qualified for sale or an exemption from such registration or qualification requirement is available. As of this date, we have not identified the specific states where the offering will be sold.
The proceeds from the sale of the shares in this offering will be held in a non-interest bearing escrow account at Security Bank of California (“Escrow Account”) until the minimum offering of $50,000 is raised. Failure to reach the minimum offering will result in checks being returned to the investor(s) who submitted the check(s). No interest will be paid to any investor or us. All subscription agreements and checks are irrevocable, unless otherwise required by law. All subscription funds will be held in the Escrow Account pending achievement of the minimum offering and no funds shall be released to us until such a time as the minimum proceeds are raised. The escrow agent will continue to receive funds and perform additional disbursements until (i) the maximum offering of $200,000 is achieved, (ii) anytime we choose to end the offering, or (iii) a period of 365 days from the effective date of this document elapses, whichever event occurs first. Thereafter, this escrow arrangement and the offering shall terminate. If the minimum offering is not achieved within 365 days of the date of this prospectus, all subscription funds will be returned to investors promptly without interest thereon or deduction therefrom. The fee of the escrow agent is $1,500.
Investors can purchase common stock in this offering by completing a Subscription Agreement and sending it together with payment in full. All payments must be made in United States currency either by personal check, bank draft, or cashier’s check. There is no minimum subscription requirement. All subscription agreements and checks are irrevocable. We expressly reserve the right to either accept or reject any subscription. Any subscription rejected will be returned to the subscriber within ten business days of the rejection date. Furthermore, once a subscription agreement is accepted, it will be executed without reconfirmation to or from the subscriber. Once we accept a subscription, the subscriber cannot withdraw it.
Selling Shareholders
The selling shareholders may offer their shares at various times in one or more of the following transactions:
|
|
·
|
In any over-the-counter market if the shares are quoted there;
|
|
|
·
|
On any exchange which the shares may hereafter be listed;
|
|
|
·
|
In negotiated transactions other than on an over-the-counter market or exchange;
|
|
|
·
|
By pledge to secure debts and other obligations;
|
|
|
·
|
In connection with the writing of non-traded and exchange-traded call options, in hedge transactions, in covering previously established short positions and in settlement of other transactions in standardized or over-the-counter options; or
|
|
|
·
|
In a combination of any of the above transactions.
|
The selling shareholders may only offer and sell, from time to time, common stock using this prospectus in transactions at a fixed offering price of $0.10 per share until a trading market develops in our common stock, at which time the selling shareholders may sell shares at market prices, which may vary, or at negotiated prices. The selling shareholders may use broker/dealers to sell their shares. The broker/dealers may receive commissions for sales of shares.
In order to comply with the applicable securities laws of certain states, the securities may not be offered or sold unless they have been registered or qualified for sale in such states or an exemption from such registration or qualification requirement is available and with which we and the selling shareholders have complied. The purchasers in this offering and in any subsequent trading market must be residents of such states where the shares have been registered or qualified for sale or an exemption from such registration or qualification requirement is available.
21
PLAN OF DISTRIBUTION (Continued)
Some of the selling shareholders may be eligible and may elect to sell some or all of their shares pursuant to additional exemptions to the registration requirements of the Securities Act, including but not limited to, Rule 144 promulgated under the Securities Act, rather than pursuant to this Registration Statement.
The selling shareholders and any broker/dealers that participate in the distribution are deemed to be “underwriters” within the meaning of the Securities Act. Any commissions received by such broker/dealers and any profits realized on the resale of shares by them may be considered underwriting discounts and commissions under the Securities Act. The selling shareholders may agree to indemnify such broker/dealers against certain liabilities, including liabilities under the Securities Act.
The selling shareholders will also be subject to applicable provisions of the Exchange Act and regulations under the Exchange Act, which may limit the timing of purchases and sales of the shares by the selling shareholders.
Furthermore, under Regulation M under the Exchange Act, any person engaged in the distribution or the resale of shares may not simultaneously engage in market making activities with respect to our common stock for a period of two business days prior to the commencement of such distribution. All of the above may affect the marketability of the securities and the availability of any person or entity to engage in market-making activities with respect to our common stock.
The selling shareholders will pay all commissions, transfer fees, and other expenses associated with the sale of securities by them. The shares offered hereby are being registered by us, and we have paid the expenses of the preparation of this prospectus. We have not made any underwriting arrangements with respect to the sale of shares offered hereby.
We do not intend to engage in any distribution efforts on behalf of any of the holders of our common stock other than providing for registration of the securities registered for sale with the SEC.
Each of the selling shareholders is acting independently of us in making decisions with respect to the timing, manner and size of each with the distribution of the shares. The selling shareholders may only offer and sell common stock using this prospectus in transactions at a fixed offering price of $0.10 per share until a trading market develops in our common stock, at which time the selling shareholders may sell shares at market prices, which may vary, or at negotiated prices. There is no assurance, therefore, that the selling shareholders will sell any or all of the shares. In connection with the offer and sale of the shares, we have agreed to make available to the selling shareholders copies of this prospectus and any applicable prospectus supplement and have informed the selling shareholders of the need to deliver copies of this prospectus and any applicable prospectus supplement to purchasers at or prior to the time of any sale of the shares offered hereby.
DESCRIPTION OF SECURITIES TO BE REGISTERED
Common Stock
We are authorized to issue 100,000,000 shares of $0.001 par value common stock. As of the date of this prospectus, we have 38,500,000 shares of common stock issued and outstanding, which are held by seven shareholders of record. We also have issued and outstanding options to purchase up to 3,000,000 shares of our common stock, which have been granted to three investors. The options expire on June 29, 2014 and may be exercised at the price of $0.30 per share.
Our shares of common stock are entitled to one vote per share on each matter submitted for a vote at any meeting of shareholders. Our shares of common stock do not carry cumulative voting rights.
22
DESCRIPTION OF SECURITIES TO BE REGISTERED (Continued)
Our shares have no pre-emptive rights to acquire additional shares or any other securities. Our shares are not subject to redemption and carry no subscription or conversion rights. In the event of liquidation, our shares are entitled to share equally in corporate assets after satisfaction of all liabilities.
Shareholders are entitled to receive such dividends as the Board of Directors may from time to time declares out of funds legally available for the payment of dividends. However, we are currently seeking growth and expansion of our business through the reinvestment of profits, if any, and we do not anticipate that we will pay cash dividends in the foreseeable future.
INTEREST OF NAMED EXPERTS AND COUNSEL
No expert or counsel named in this prospectus as having prepared or certified any part of this prospectus or having given an opinion upon the validity of the securities being registered or upon other legal matters in connection with the registration or offering of the common stock was employed on a contingency basis, or had, or is to be connected with us as a promoter, managing or principal underwriter, voting trustee, director, officer, or employee.
M&K CPAS PLLC has audited our Financial Statements for the period ended December 31, 2009 and to the extent set forth in its reports, which are included herein in reliance upon the authority of said person as an expert in accounting and auditing.
23
DESCRIPTION OF THE BUSINESS
Overview
We are a developmental stage detection device company formed as a Nevada corporation on July 10, 2009 and based in Northern Virginia. We are a subsidiary of DKL International, Inc. (“Parent Company”), a provider of passive human detection technology. The Parent Company’s proprietary technology passively detects the non-uniform electric field that every living human generates. The main source of this field in the human body is the electric activity of the heart. We were formed to apply the Parent Company’s technology to the wellness care market. We hold the exclusive license to market, distribute, promote, sell, offer for sale and sublicense wellness care products based on the Parent Company’s technology. We have granted to our prior investors certain rights relating to the manufacturing and distribution of our products and, therefore, our rights relating to those activities may be limited. See “Risk Factors—We have granted rights to our prior investors which may restrict our operations and flexibility.”
Business
We intend to create an intelligent environment in which people who are confined to their homes can collaborate with their healthcare providers. With aging baby boomers making up a substantial portion of the U.S. population, the cost of public health entitlement is expected to increase in the future. Additionally, we believe that a significant number of all mortalities that occur within hospitals are due to human errors. Both of these factors highlight the need for a high tech safety-net in the home. Such a home based system may be able to extend the hospital prime care into individual households and provide wellness monitoring for patients at home alone. The home based system will allow the establishment of an over-sampled “wellness baseline profile” and “individual diagnostic aids.”
Principal Products / Services
We plan to adapt the military covert persistent surveillance technology and methodology developed by our Parent Company for use by patients at home in need of wellness care. We believe this can be accomplished through a set of affordable and effective, non-contact, passive smart monitoring devices. The monitoring devices currently available in the market are often expensive and require the use of wearable electrodes or are triggered by contact with the person. Our technology requires no or very minimum action by the monitored person to enable the passive, invisible, non-intrusive monitoring of people. This noninvasive detection of electric fields and electric field variations generated by people can be accomplished from a distance. Our proposed wellness care application can easily be integrated into the existing environment, with detectors embedded within everyday objects that are near the patient, such as furniture, bedding, walls, floors and ceilings, for enhanced signal detection that results in a high signal to noise ratio. The main task in accomplishing this objective is measuring the pumping of the heart and blood, whose dielectric oscillation generates low frequency electromagnetic waves. Based on results from our sensors, we are able to differentiate a human’s heartbeat from a pet’s heartbeat. Our Parent Company’s internal studies confirm the sensor’s ability to passively detect human movement. After we develop a product which can reliably measure human movement, we plan to develop a medical device which can measure the respiratory rate and heart rate of people.
Our focus is to create a home-care and alert system to ensure continuity of a person’s care. This includes the development of a new concept of tele-medicine as “care at the point of need in co-operative environments.” The project will employ innovative sensors, intelligent agents and wireless communications technologies to develop a system that is user-friendly, reliable and respects the privacy of patients. As shown below, with as few as five sensors, this system will be able to monitor and collect critical information for caregivers and medical personnel.
24
DESCRIPTION OF THE BUSINESS (Continued)
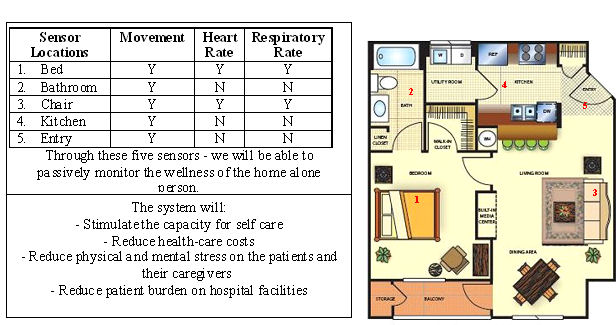
It is our intention to develop an intelligent and unobtrusive monitoring environment for people who need constant or frequent surveillance, including the disabled, the aging, and those who require care and health services. The system will improve the efficiency, quality, and acceptability of health and social-care services, reduce the need for long-term hospitalization, and enable people to remain in their own homes.
Competition
Most companies which provide the first-alert type of service require the patient to press and activate a device to notify an answering service that the patient needs help.
There are companies which offer tele-medicine services which can provide the patient’s heart rate and respiratory rate information to the medical service provider. With those companies, the patient must wear a device around his waist or on his body to acquire the necessary information.
We have numerous competitors which have greater financial and other resources than we do. For instance, Boston Scientific Corporation has developed a patient monitoring system called Latitude that provides health care practitioners with the ability to remotely follow their patients. Similarly, BioHeart, Inc. markets a monitoring system that enables remotely located medical professionals to measure their patients’ vital signs. In 2005, TeleVital Inc. received FDA clearance for its VitalWare VMS remote real-time bedside monitoring solution. We compete with these companies by developing product candidates that can measure vital signs from greater distances without intruding upon the patient.
Technology / Research and Development
On July 12, 2009, we entered into a license agreement with our Parent Company. Pursuant to this agreement, we were granted an exclusive worldwide license to distribute, market, promote, sell, offer for sale, sublicense and otherwise exploit any and all products embodying the Parent Company’s dielectrokinetic techniques within the wellness care market. This technique generally involves the passive detection of the non-uniform electric field generated by living human beings. The term of the license is the longer of 15 years or the expiration date of the last applicable patent, but may be canceled by the Parent Company if we fail to comply with our obligations under the agreement.
The license agreement requires us to put the Parent Company’s technology into commercial use within three years, or to be engaged within a marketing or sublicensing program. We must contract with the Parent Company for all research and development activities that improve the Parent Company’s technology, and we must pay fund all approved research and development plans. Additionally, we must make annual royalty payments to the Parent
Company.
25
DESCRIPTION OF THE BUSINESS (Continued)
Our licensed electronic sensor is capable of detecting the changes caused by low frequency electric fields. It can discriminate the signature of the human electric field from electric fields generated by other animals and materials. The device in its simplest form consists of the sensor circuit with an attached collector. The operating mode of the sensor circuit is a null detector (i.e., it samples the background of its environment and establishes a zero point). It then reacts to any change from that zero point. A conductive material (i.e., collector) connected to the sensor circuit is placed in contact with the local environment materials to accumulate the changing voltage gradients and transfer them to the circuit.
Humans generate a large unique ultra-low-frequency (“ULF”) electric field. The major source of this detectable electric field is the rapid depolarization and repolarization of the heart as seen in an electrocardiogram. Additionally, because humans are highly polarized with high dielectric constants, it is possible to uniquely differentiate the humans from other animals or objects in the environment.
The passive unattended sensor specifically detects the change in “volts per meter” in the ULF 0.2 to 10 Hz range. The sensor constantly adjusts to the slow changes in the background voltage gradients. When a person walks into the detection area, the person’s large ULF electric field causes a major change to the charge pattern on the materials in the detection area. When the “volts per meter” pattern shift, the circuit detects the shift, identifies the source as human or non-human and sends an alert as necessary. At close ranges, the sensor can monitor cardiac and respiratory rates. At greater distances, it is still able to detect movement.
Patents
The Parent Company has eight registered U.S. utility patents. Internationally, the Parent Company has 15 registered patents and four pending patents. Additionally, the Parent Company has three registered U.S. trademarks and four registered international trademarks. The Parent Company’s business is built on intellectual property protection. A considerable share of the Parent Company's paid in capital has been spent on patent protection. As with every technology-based company, it is imperative for the Parent Company to further develop its technological patent portfolio to continue to enhance its value to licensees such as us.
In addition to protecting its product technology in as many markets as possible, the Parent Company seeks to protect its scientific and engineering breakthroughs in the commercial application of dielectrokinetics. The Parent Company achieves this by patenting both broad and specific applications of its new technology to the greatest extent possible. The patents that have been issued to date give the Parent Company the right to exclude others from making, using or selling several types apparatus that locate entities using the principles of dielectrokinesis. This broad protection could be extended with the additional, currently pending international patent applications covering new technological developments.
Marketing / Distribution
We plan to license any products we create to companies which provide first alert services to the senior sector of the U.S. population. Several of these companies have already built the infrastructure necessary for providing such services and as such, we believe that our product candidates could be implemented efficiently. We believe this licensing approach is the quickest and most cost-effective method for generating royalties and entering the wellness care market, which traditionally operates with high volume transactions.
We have granted rights of first refusal on exclusive manufacturing rights and we have already granted certain distribution rights. These rights were granted to our initial investors in order to attract their infusion of capital. Specifically, once the manufacturing of the sensor is conducted off-shore, three investors have successive rights of refusal to manufacture the sensor. We have retained the right to purchase such sensors for a price equal to cost plus a 25% premium. The specific terms of the right of first refusal and the manufacturing rights are yet to be determined. See “Exhibits 10.4, 10.5 and 10.6—Deal Points Agreements.” Additionally, we have granted to an affiliated company exclusive distribution rights for our products in Taiwan and China. See “Exhibits 10.4, 10.5 and 10.6—Deal Points Agreements.” The prior investors described above own 75% of this affiliated entity, and we own 25%. We have also granted these investors 15% of our initial licensing fees and royalty payments.
26
DESCRIPTION OF THE BUSINESS (Continued)
Government Regulation
We anticipate that we will need to obtain approval from the U.S. Food and Drug Administration to manufacture and sell any products in the United States that monitor human vital signs. However, we believe that FDA approval is not required for our product candidate which only detects movement. Therefore, we believe obtaining the necessary approvals will delay our ability to bring certain of our product candidates to market and will increase the costs of constructing compliant manufacturing facilities.
Employees
We are currently in the development stage. During this development period, we plan to rely exclusively on the services of our two officers to establish business operations and perform or supervise the minimal services required at this time. Our officers provide services to us on a part-time basis. We believe that our operations are currently manageable by these individuals, and plan to hire independent contractors on an as-needed basis. There are no other full or part-time employees.
DESCRIPTION OF PROPERTY
We sublease approximately 120 square feet of office space from our Parent Company, DKL International, Inc., located at 501 Church Street, Vienna, Virginia 22180, for rent of $205 per month. We do not have a written sublease with our Parent Company. Our Parent Company’s lease is on a month to month basis. We do not currently manufacture any products and do not maintain a manufacturing facility.
LEGAL PROCEEDINGS
There are no legal proceedings pending or threatened against us.
27
MARKET PRICE OF AND DIVIDENDS ON THE ISSUER’S COMMON STOCK
Market Information
As of the date of this prospectus, there is no public market for our common stock and there can be no assurance that any trading market will ever develop.
As of the date of this prospectus,
1. There are outstanding options to purchase 3,000,000 shares of common stock at an exercise price of $0.30 per share, with an expiration date of June 29, 2014;
2. 3,000,000 shares of common stock currently outstanding are eligible to be sold pursuant to Rule 144 under the Securities Act, and the shares included in this Registration Statement are the only shares that we have agreed to register under the Securities Act for sale by security holders; and
3. Other than the shares registered under this Registration Statement, there is no other common equity that has been proposed to be publicly offered which could have a material effect on the market price of our common equity.
Holders
As of the date of this prospectus, we have 38,500,000 shares of $0.001 par value common stock issued and outstanding held by seven shareholders of record.
Dividends
We have neither declared nor paid any cash dividends on our common stock. For the foreseeable future, we intend to retain any earnings to finance the development and expansion of our business, and do not anticipate paying any cash dividends. Any future determination to pay dividends will be at the discretion of the Board of Directors and will be dependent upon then existing conditions, including our financial condition, results of operations, capital requirements, contractual restrictions, business prospects, and other factors that the Board of Directors considers relevant.
Equity Compensation Plans
As of the date of this prospectus, we have not adopted an equity compensation plan and have no plans to adopt any equity compensation plans in the foreseeable future.
28
MANAGEMENT’S DISCUSSON AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
This section must be read in conjunction with the Financial Statements included in this prospectus.
Liquidity / Capital Resources
Since our inception, we have financed our operations primarily through a private placement of our common stock which was conducted pursuant to Regulation D under the Securities Act (hereinafter referred to as the “Private Placement”), whereby we raised $300,000 from three accredited investors. This Private Placement was closed on September 30, 2009.
We used part of our paid-in capital to acquire and partially pay for the license agreement whereby we acquired our technology which makes up our business plan. This License Agreement was executed on July 12, 2009 with our Parent Company, DKL International, Inc., whereby we received the right to distribute, market, promote, sell, offer for sale, sublicense and otherwise exploit any and all products embodying the Parent Company’s dielectrokinetic techniques within the wellness care market. See “Description of the Business—Technology / Research and Development.”
To date, we have not implemented our planned principal operations. Our ability to commence operations will be entirely dependent upon the proceeds to be raised in this offering. We anticipate that the funds from this offering, if the maximum amount is raised, will be sufficient to finance our efforts to become operational and carry us through the next 12 months. If we do not raise at least the minimum offering amount, we will be unable to establish a base of operations, without which we will be unable to begin to generate any revenues. The realization of revenues in the next 12 months is important in the execution of our plan of operation. We believe that recurring revenues from future operations should be sufficient to support ongoing operations after this 12 month period. However, we cannot guarantee that we will generate such growth. If we do not produce sufficient cash flow to support our operations over the next 12 months, we may need to raise additional capital by issuing capital stock in exchange for cash in order to continue as a going concern. There are no formal or informal agreements to attain such financing. We cannot provide any assurance that, if needed, sufficient financing can be obtained or, if obtained, that it will be on reasonable terms. Without receipt of capital from this offering and, if necessary, future public or private offerings, it is unlikely that our operations will continue.
As of the date of this prospectus, we had $33,018.57 cash on hand.
Results of Operations
To date, we have generated no revenues, but have incurred substantial expenses. This has resulted in a net loss of $321,056 since inception, which is primarily attributable to salary payments, consulting, general and administrative expenses and licensing fees.
We do not own any significant plant or equipment, nor do we intend to purchase any significant plant or equipment in the near future. We plan to outsource our initial manufacturing requirements. We have granted rights of refusal on exclusive manufacturing rights to the three investors in our Private Placement. See “Description of the Business—Marketing / Distribution.”
Our management does not anticipate any significant changes in the number of employees in the next 12 months. Currently, we believe the services provided by our two officers and directors are sufficient at this time.
We have not paid for expenses on behalf of any director. Additionally, we believe that this practice will not materially change in the foreseeable future.
Our independent auditor has expressed substantial doubt about our ability to continue as a going concern and believes that our ability to do so is dependent on our ability to implement our business plan, raise capital and generate revenues.
29
MANAGEMENT’S DISCUSSON AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS (Continued)
Off-Balance Sheet Arrangements
We currently have no off-balance sheet arrangements that will have or are reasonably likely to have a current or future effect on our financial condition.
Inflation
The effect of inflation on our operating results has not been significant.
CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
Since inception until the present time, our principal independent accounting firm has neither resigned (nor declined to stand for reelection) nor been dismissed. Our independent accountant is M&K CPAS, PLLC, 13831 Northwest Freeway, Suite 575, Houston, Texas 77040.
DIRECTORS, EXECUTIVE OFFICERS, PROMOTERS AND CONTROL PERSONS
Directors are elected by our shareholders to a term of one year and serve until a successor is elected and qualified. Officers are appointed by the Board of Directors to a term of one year and serve until a successor is duly appointed and qualified, or until removed from office. The Board of Directors has no nominating, auditing or compensation committees.
The following table sets forth certain information regarding our directors, executive officers, promoters and control persons as of the date of this prospectus:
|
Name
|
Age
|
Position
|
Period of Service
|
|
Ted Wong
|
70
|
President, CEO, and Director
|
Since Inception
|
|
Howard Sidman
|
60
|
Treasurer, Secretary, CFO, and Director
|
Since Inception
|
The present officers and directors have obligations to entities other than us. We expect them to spend approximately 20 hours per week on our business affairs. At the date of this prospectus, we are not engaged in any transactions, either directly or indirectly, with any persons or organizations considered promoters.
Backgrounds of Executive Officers, Promoters, and Control Persons
Ted Wong – President, CEO, and Director – Dr. Wong served as CEO and President of NanoSensors, Inc. from September 2003 to August 2007, a publicly traded company he co-founded to develop and market biosensors to detect E-Coli and Salmonella based on technologies licensed from a major university in the U.S. Dr. Wong has served as President of H & W Associates 99, Inc., one of our founding shareholders, since September 2007. Dr. Wong holds a Bachelor and Ph.D. degrees in Chemical Engineering from the University of Utah.
Howard Sidman – Treasurer, Secretary, CFO, and Director – Mr. Sidman has served as President of DKL International, Inc., our Parent Company, since November 1998. Mr. Sidman graduated from the United States Naval Academy in 1973 and earned a Masters Degree in International Affairs from Catholic University in 1989.
Directors
Our Board of Directors presently consists of two members. Our Board of Directors may appoint a Compensation Committee to review all employee and consultant compensation, including payroll expenditures, salaries, stock options, stock incentives and bonuses, but has not appointed any committees yet. Our Bylaws generally provide for majority approval of disinterested directors in order to adopt resolutions, including any borrowings by us or the issuance of any additional securities. Although we anticipate appointing additional directors in the future, we have not identified any such additional directors at this time.
30
EXECUTIVE COMPENSATION
|
Summary Compensation Table
|
||||||||
|
Annual Compensation
|
Long-Term Compensation
|
|||||||
|
Name and
Principal Position
|
Year
|
Salary ($)
|
Bonus ($)
|
Other Annual Compensation ($)
|
Restricted Stock Awards ($)
|
Securities Underlying Options (#)
|
LTIP Payouts ($)
|
All Other Compensation ($)
|
|
Ted Wong
|
2009
|
35,000
|
-
|
-
|
-
|
-
|
-
|
-
|
|
Officer and Director
|
2010
|
60,000
|
-
|
-
|
-
|
-
|
-
|
-
|
|
Howard Sidman
|
2009
|
35,000
|
-
|
-
|
-
|
-
|
-
|
-
|
|
Officer and Director
|
2010
|
60,000
|
-
|
-
|
-
|
-
|
-
|
-
|
Director’s Compensation
Directors are not entitled to receive compensation for services rendered to us or for each meeting attended except for reimbursement of out-of-pocket expenses. There are no formal or informal arrangements or agreements to compensate directors for services provided as a director.
Employment Contracts and Officers’ Compensation
We have part-time employment agreements with each of our named executive officers. We entered into employment agreements with Messrs. Wong and Sidman on February 1, 2010. The primary purpose of the agreements is to establish the terms of employment and to protect both us and the executive. We are provided with reasonable protections that the executive will perform at acceptable levels and will not compete with or recruit employees from us during or after the termination of employment. The executive is provided financial protection in the event of certain reasons for termination of employment in recognition of the executive’s professional career and a forgoing of present and future career options.
Each agreement provides for a rolling 30 month term of employment, with each agreement renewing only by written agreement. Each agreement may be terminated by us or the employee with or without cause upon 30 days’ prior written notice. We may terminate the agreement immediately for cause.
The employment agreements provide for severance payments in the event that we terminate such agreements without cause. If we do so, the employee shall be paid the lesser of (i) an amount equal to six months’ base salary, or (ii) the base salary for the remainder of the employment term.
The salaries of Messrs. Wong and Sidman are initially established pursuant to their respective employment agreements. The base salary for each of these individuals is $60,000 per year, but may be increased by our Board of Directors after the first 180 days after employment. The employment agreements also provide that each executive is entitled to participate in any benefits plan comparable to our other executives.
Non-Disclosure, Non-Competition and Non-Solicitation
Pursuant to their respective employment agreements, each of Messrs. Wong and Sidman are subject to non-disclosure, non-competition and non-solicitation clauses. Each executive has agreed not to disclose confidential information and, if we exercise a non-competition option by continuing to pay salary after termination of their employment, not to engage or participate in, directly or indirectly, any business that offers products or provides related services and products or engages in any other business that we are engaged in or have taken steps to engage in, within our competitive area, for a period of one year. Additionally, during the term of their employment agreement and a period of one year thereafter, Messrs. Wong and Sidman have agreed to not solicit or attempt to directly solicit any of our officers, directors, consultants or executives (other than immediate family members) to
leave.
31
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT
The following table sets forth certain information as of the date of this prospectus with respect to the beneficial ownership of our common stock by all persons known by us to be beneficial owners of more than 5% of any such outstanding classes, and by each director and executive officer, and by all officers and directors as a group. Unless otherwise specified, the named beneficial owner has, to our knowledge, either sole or majority voting and investment power.
|
Title Of Class
|
Name, Title and Address of Beneficial Owner of Shares1
|
Amount of Beneficial Ownership
|
Percent of Class
|
|
|
Before Offering
|
After Offering
|
|||
|
Common
|
Ted Wong, President, CEO, and Director
|
8,850,000 2
|
22.99%
|
21.85%
|
|
Common
|
Howard Sidman, Treasurer, Secretary, CFO, and Director
|
- 0 -
|
-
|
-
|
|
All Directors and Officers as a group (two persons)
|
8,850,000
|
22.99%
|
21.85%
|
|
|
Common
|
Shih Chung Chen, 5% or greater shareholder
#21-30 Ta-Pu, Wu-Chuan Chung, Ta-Yuan Hsaing
Tao-Yuan, Taiwan, R.O.C.
|
2,000,000 3
|
5.06%
|
0.00%
|
|
Common
|
Tsung Wen Chang, 5% or greater shareholder
#86-1 Sheng Lin Road, Sheng Kang Hsaing
Taichung County, Taiwan, ROC, Postal Code 501 429
|
2,000,000 3
|
5.06%
|
0.00%
|
|
Common
|
John Phillips, 5% or greater shareholder
726 E State Highway 121
Lewisville, TX 75057
|
2,000,000 3
|
5.06%
|
0.00%
|
|
Common
|
DKL International, Inc.
|
26,000,000 4
|
67.53%
|
64.20%
|
1 As used in this table, “beneficial ownership” means the sole or shared power to vote, or to direct the voting of, a security, or the sole or share investment power with respect to a security (i.e., the power to dispose of, or to direct the disposition of a security). Two or more persons might count as beneficial owners of the same security. Unless otherwise noted, the address of the following persons listed below is c/o Push, Inc., 501 Church Street, Suite 317, Vienna, Virginia 22180.
2 Consists of 8,850,000 shares of our common stock held by H & W Associates 99, Inc., of which Dr. Wong exercises voting power and control.
3 Includes 1,000,000 shares of common stock underlying options exercisable at the price of $0.30 per share, expiring on June 29, 2014.
4 Howard Sidman is the President, a director and a 3.76% shareholder of DKL International, Inc. DKL LLC and DKL Europe NVeach own 40.31% of the outstanding capital stock of DKL International, Inc. DKL LLC is controlled by Howard Sidman and DKL Europe NV is controlled by Jaap Alting.
32
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS
Conflict of Interest
Our officers and directors are involved in other business activities and may, in the future, become involved in other business opportunities. If a specific business opportunity becomes available, such persons may face a conflict in selecting between our business and their other business interests. Our policy is that any personal business or corporate opportunity proposed by an officer or director must be disclosed to and examined by the Board and approved by the Board before an officer or director can engage or take advantage of a business opportunity which could result in a conflict of interest.
Related Transactions
During the period ended December 31, 2009, we paid a $90,500 non-refundable technology license fee to a related party (DKL International, Inc., our Parent Company). $26,000 of this amount was paid in shares of our common stock issued at $0.001 per share. We owe $64,500 in cash for the balance of the purchase which is classified as a related party payable. The related party payable is due no later than January 12, 2011, is unsecured and non-interest bearing.
REPORTS TO SECURITY HOLDERS
1. After this offering, we plan to file periodic and current reports with the SEC as required to maintain fully reporting status.
2. The public may read and copy any materials we file with the SEC at the SEC's Public Reference Room at 450 Fifth Street, N.W., Washington, D.C. 20549. The public may obtain information on the operation of the Public Reference Room by calling the SEC at 1-800-SEC-0330. Our SEC filings will also be available on the SEC’s Internet site. The address of that site is: http://www.sec.gov.
The SEC’s Policy on Indemnification
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers, and controlling persons of the company pursuant to any provisions contained in its Articles of Incorporation, Bylaws, or otherwise, we have been advised that, in the opinion of the SEC, such indemnification is against public policy as expressed in the Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by us of expenses incurred or paid by a director, officer or controlling person in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, we will, unless in the opinion of our legal counsel, the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether indemnification is against public policy as expressed in the Act and will be governed by the final adjudication of such issue.
33
FINANCIAL STATEMENTS
The following financial statements and schedules are filed as a part of this report.
|
Report of Independent Registered Public Accounting Firm
|
35 | |
|
Balance Sheets at June 30, 2010 (unaudited) and December 31, 2009
|
36 | |
|
Statements of Operations for the Six Months Ended June 30, 2010 (unaudited), from Inception (July 10, 2009) to the Fiscal Year Ended December 31, 2009, and from Inception (July 10, 2009) to the Six Months Ended June 30, 2010 (unaudited)
|
37 | |
|
Statements of Owners’ Equity for the Period from Inception (July 10, 2009) to the Fiscal Year Ended December 31, 2009 and for the Six Months Ended June 30, 2010 (unaudited)
|
38 | |
|
Statements of Cash Flows for the Period for the Six Months Ended June 30, 2010, from Inception (July 10, 2009) to the Fiscal Year Ended December 31, 2009, and from Inception (July 10, 2009) to the Six Months Ended June 30, 2010 (unaudited)
|
39 | |
|
Notes to Financial Statements
|
40 |
34
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors
Push, Inc.
(A Development Stage Company)
We have audited the accompanying balance sheet of Push, Inc. (A Development Stage Company) as of December 31, 2009, and the related statement of operations, stockholders’ equity and cash flows for the period from inception on July 10, 2009 through December 31, 2009. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audit.
We conducted our audit in accordance with standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. The Company was not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audit included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audit provides a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of Push, Inc. (A Development Stage Company) as of December 31, 2009, and the related statement of operations, stockholders' equity and cash flows for the period from inception on July 10, 2009 through December 31, 2009, in conformity with accounting principles generally accepted in the United States of America.
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 4 to the financial statements, the Company has an accumulated deficit of $321,056, which raises substantial doubt about its ability to continue as a going concern. Management’s plans concerning these matters are also described in Note 4. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
/s/ M & K CPAS, PLLC
www.mkacpas.com
Houston, Texas
October 13, 2010
35
|
PUSH, INC.
|
||||||||
|
(A Development Stage Company)
|
||||||||
|
Balance Sheets
|
||||||||
|
ASSETS
|
||||||||
|
June 30,
|
December 31,
|
|||||||
|
2010
|
2009
|
|||||||
|
(Unaudited)
|
(Audited)
|
|||||||
|
CURRENT ASSETS
|
||||||||
|
Cash
|
$ | 90,335 | $ | 26,949 | ||||
|
Total Current Assets
|
90,335 | 26,949 | ||||||
|
Accounts receivable - related party
|
- | 146,624 | ||||||
|
TOTAL ASSETS
|
$ | 90,335 | $ | 173,573 | ||||
|
LIABILITIES AND STOCKHOLDERS' EQUITY
|
||||||||
|
CURRENT LIABILITIES
|
||||||||
|
Accounts payable and accrued expenses - related party
|
$ | 6,977 | $ | - | ||||
|
Payroll Liabilities
|
4,414 | - | ||||||
|
Related party payable
|
64,500 | 64,500 | ||||||
|
Total Current Liabilities
|
75,891 | 64,500 | ||||||
|
STOCKHOLDERS' EQUITY
|
||||||||
|
Common stock, 100,000,000 shares authorized
|
||||||||
|
at par value of $0.001; 38,500,000
|
||||||||
|
shares issued and outstanding
|
38,500 | 38,500 | ||||||
|
Additional paid-in capital
|
297,000 | 297,000 | ||||||
|
Deficit accumulated during the development stage
|
(321,056 | ) | (226,427 | ) | ||||
|
Total Stockholders' Equity
|
14,444 | 109,073 | ||||||
|
TOTAL LIABILITIES AND
|
||||||||
|
STOCKHOLDERS' EQUITY
|
$ | 90,335 | $ | 173,573 | ||||
|
The accompanying notes are an integral part of these financial statements.
|
||||||||
36
|
PUSH, INC.
|
||||||||||||
|
(A Development Stage Company)
|
||||||||||||
|
Statements of Operations
|
||||||||||||
|
|
For the
|
From Inception
|
From Inception
|
|||||||||
|
Six Months
|
on July 10,
|
on July 10,
|
||||||||||
|
Ended
|
2009 Through
|
2009 Through
|
||||||||||
|
June 30,
|
December 31,
|
June 30,
|
||||||||||
|
2010
|
2009
|
2010
|
||||||||||
|
(Unaudited)
|
(Audited)
|
(Unaudited)
|
||||||||||
|
REVENUES
|
$ | - | $ | - | $ | - | ||||||
|
OPERATING EXPENSES
|
||||||||||||
|
Consulting
|
- | 114,500 | 114,500 | |||||||||
|
General and administrative
|
7,080 | 6,581 | 13,661 | |||||||||
|
Research
|
18,717 | 14,950 | 33,667 | |||||||||
|
Payroll Expenses
|
69,688 | - | 69,688 | |||||||||
|
Licensing fee
|
- | 90,500 | 90,500 | |||||||||
|
Total Operating Expenses
|
95,485 | 226,531 | 322,016 | |||||||||
|
OTHER INCOME
|
||||||||||||
|
Interest income
|
856 | 104 | 960 | |||||||||
|
NET LOSS
|
$ | (94,629 | ) | $ | (226,427 | ) | $ | (321,056 | ) | |||
|
BASIC AND DILUTED LOSS PER SHARE
|
$ | (0.00 | ) | $ | (0.01 | ) | ||||||
|
WEIGHTED AVERAGE NUMBER
|
||||||||||||
|
OF SHARES OUTSTANDING
|
38,500,000 | 38,091,954 | ||||||||||
|
The accompanying notes are an integral part of these financial statements
|
||||||||||||
37
|
PUSH, INC.
|
||||||||||||||||||||
|
(A Development Stage Company)
|
||||||||||||||||||||
|
Statements of Stockholders' Equity
|
||||||||||||||||||||
|
Deficit
|
||||||||||||||||||||
|
Accumulated
|
||||||||||||||||||||
|
Additional
|
During the
|
Total
|
||||||||||||||||||
|
Common Stock
|
Paid-in
|
Development
|
Stockholders'
|
|||||||||||||||||
|
Shares
|
Amount
|
Capital
|
Stage
|
Equity (Deficit)
|
||||||||||||||||
|
Balance, July 9, 2009
|
- | $ | - | $ | - | $ | - | $ | - | |||||||||||
|
Shares issued to founder
|
||||||||||||||||||||
|
at $0.001 per share
|
9,500,000 | 9,500 | - | 9,500 | ||||||||||||||||
|
Shares issued to founder
|
||||||||||||||||||||
|
at $0.001 per share
|
26,000,000 | 26,000 | - | - | 26,000 | |||||||||||||||
|
Shares issued for cash
|
||||||||||||||||||||
|
$0.10 per share
|
3,000,000 | 3,000 | 297,000 | - | 300,000 | |||||||||||||||
|
Net loss from inception to
|
||||||||||||||||||||
|
December 31, 2009
|
- | - | - | (226,427 | ) | (226,427 | ) | |||||||||||||
|
Balance, December 31, 2009 (Audited)
|
38,500,000 | 38,500 | 297,000 | (226,427 | ) | 109,073 | ||||||||||||||
|
Net loss for the six months
|
||||||||||||||||||||
|
ended June 30, 2010
|
- | - | - | (94,629 | ) | (94,629 | ) | |||||||||||||
|
Balance, June 30, 2010 (Unaudited)
|
38,500,000 | $ | 38,500 | $ | 297,000 | $ | (321,056 | ) | $ | 14,444 | ||||||||||
|
The accompanying notes are an integral part of these financial statements.
|
||||||||||||||||||||
38
|
PUSH, INC.
|
||||||||||||
|
(A Development Stage Company)
|
||||||||||||
|
Statements of Cash Flows
|
||||||||||||
|
For the
|
From Inception
|
From Inception
|
||||||||||
|
Six Months
|
on July 10,
|
on July 10,
|
||||||||||
|
Ended
|
2009 Through
|
2009 Through
|
||||||||||
|
June 30,
|
December 31,
|
June 30,
|
||||||||||
|
2010
|
2009
|
2010
|
||||||||||
|
(Unaudited)
|
(Audited)
|
(Unaudited)
|
||||||||||
|
CASH FLOWS FROM OPERATING ACTIVITIES
|
||||||||||||
|
Net loss
|
$ | (94,629 | ) | $ | (226,427 | ) | $ | (321,056 | ) | |||
|
Adjustments to reconcile net (loss) to
|
||||||||||||
|
net cash provided by operating activities:
|
||||||||||||
|
Stock issued for Licensing fee
|
- | 26,000 | 26,000 | |||||||||
|
Stock issued for Services
|
- | 9,500 | 9,500 | |||||||||
|
Changes to operating assets and liabilities:
|
||||||||||||
|
Increase (decrease)
|
||||||||||||
|
Accounts Receivable - related party
|
146,624 | (146,624 | ) | - | ||||||||
|
Accounts Payable - related party
|
6,977 | 64,500 | 71,477 | |||||||||
|
Accured Expenses
|
4,414 | - | 4,414 | |||||||||
|
Net Cash Provided by Operating Activities
|
63,386 | (273,051 | ) | (209,665 | ) | |||||||
|
CASH FLOWS FROM FINANCING ACTIVITIES
|
||||||||||||
|
Common stock issued for cash
|
- | 300,000 | 300,000 | |||||||||
|
|
||||||||||||
|
Net Cash Provided by Financing Activities
|
- | 300,000 | 300,000 | |||||||||
|
NET INCREASE IN CASH
|
63,386 | 26,949 | 90,335 | |||||||||
|
CASH AT BEGINNING OF PERIOD
|
26,949 | - | - | |||||||||
|
CASH AT END OF PERIOD
|
$ | 90,335 | $ | 26,949 | $ | 90,335 | ||||||
|
SUPPLEMENTAL DISCLOSURES OF
|
||||||||||||
|
CASH FLOW INFORMATION
|
||||||||||||
|
CASH PAID FOR:
|
||||||||||||
|
Interest
|
$ | - | $ | - | $ | - | ||||||
|
Income Taxes
|
$ | - | $ | - | $ | - | ||||||
|
NON CASH FINANCING ACTIVITIES:
|
||||||||||||
| $ | - | $ | - | $ | - | |||||||
|
The accompanying notes are an integral part of these financial statements.
|
||||||||||||
39
PUSH, INC.
(A Development Stage Company)
Notes to Financial Statements
June 30, 2010 (Unaudited) and December 31, 2009
NOTE 1. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Nature of Business
Push, Inc, (the Company) was incorporated under the laws of the State of Nevada on July 10, 2009. The Company is currently developing technology for and engineering a circuit board for human detection technology for wellness care application.
Use of Estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities at the date of the financial statements and the reported amounts of revenue and expenses during the reporting period. Actual results could differ from those estimates.
Development Stage Company
The Company is currently considered a development stage company. As a development stage enterprise, the Company discloses the deficit accumulated during the development stage and the cumulative statements of operations and cash flows from inception to the current balance sheet date. An entity remains in the development stage until such time as, among other factors, revenues have been realized. To date, the development stage of the Company’s operations consists of developing the business model and marketing concepts.
Unaudited Interim Financial Information
The accompanying balance sheet as of June 30, 2010, statement of operations for the six months ended June 30, 2010, statement of stockholders’ equity for the six months ended June 30, 2010 and statement of cash flows for the six months ended June 30, 2010 are unaudited. These unaudited interim financial statements have been prepared in accordance with accounting principles generally accepted in the United States of America (“GAAP”). In the opinion of the Company’s management, the unaudited interim financial statements have been prepared on the same basis as the audited financial statements and include all adjustments necessary for the fair presentation of the Company’s statement of financial position at June 30, 2010, its results of operations and its cash flows for the six months ended June 30, 2010. The results for the six months ended June 30, 2010 are not necessarily indicative of the results to be expected for the fiscal year ending December 31, 2010.
Earnings per Common Share
Basic earnings per share are calculated by dividing the Company’s net income applicable to common shareholders by the weighted average number of common shares during the period. Diluted earnings per share is calculated by dividing the Company’s net income available to common shareholders by the diluted weighted average number of shares outstanding during the year. The diluted weighted average number of shares outstanding is the basic weighted number of shares adjusted for any potentially dilutive debt or equity. There are no such common stock equivalents outstanding as of June 30, 2010 and December 31, 2009.
Revenue Recognition
The Company's revenue recognition policy is consistent with the criteria set forth in Staff Accounting Bulletin (SAB) 104, "Revenue Recognition in Financial Statements," (codified in FASB ASC Topic 605) for determining when revenue is realized or realizable and earned. In accordance with professional guidance, the Company recognizes revenue when (1) persuasive evidence of an arrangement exists, (2) delivery has occurred, (3) the seller's price is fixed or determinable, and (4) collectability is reasonably assured.
Advertising Costs
The Company’s policy regarding advertising is to expense advertising costs when incurred. The Company has not incurred any advertising expenses as of June 30, 2010 and December 31, 2009.
Cash and Cash Equivalents
Cash equivalents are purchased, short-term, highly liquid investments, readily convertible to cash with original maturities of no more than three months. There are no cash balances in federally insured banks that exceed the maximum insured amounts by the by the FDIC.
Fair Value of Financial Instruments
Financial instruments consist of cash and cash equivalents, accounts payable, accrued expenses and short-term and long-term convertible debt obligations. Including promissory notes, and related party liabilities, the fair value of these financial instruments approximates their carrying amount as of June 30, 2010 and December 31, 2009 due to the nature of or the short maturity of these instruments.
40
PUSH, INC.
(A Development Stage Company)
Notes to Financial Statements
June 30, 2010 (Unaudited) and December 31, 2009
NOTE 1. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
Accounting Basis
These financial statements and related notes are presented in accordance with accounting principles generally accepted in the United States, and are expressed in U.S. dollars. The Company’s fiscal year end is December 31.
Share-based compensation
On July 2009, the Company adopted ASC 718, Share Based Payment, which establishes standards for the accounting of transactions in which an entity exchanges its equity instruments for goods or services, primarily focusing on accounting for transactions where an entity obtains services in share based payment transactions. ASC 718 requires entities to measure the cost of services received in exchange for an award of equity instruments, including stock options and warrants, based on the grant date fair value of the award and to recognize it as compensation expense over the period required to provide service in exchange for the award, usually the vesting period.
Financial Instruments
Pursuant to ASC 820, Fair Value Measurements and Disclosures and ASC 825, Financial Instruments, an entity is required to maximize the use of observable inputs and minimize the use of unobservable inputs when measuring fair value. ASC 820 and 825 establishes a fair value hierarchy based on the level of independent, objective evidence surrounding the inputs used to measure fair value. A financial instrument’s categorization within the fair value hierarchy is based upon the lowest level of input that is significant to the fair value measurement. ASC 820 and 825 prioritizes the inputs into three levels that may be used to measure fair value:
Level 1
Level 1 applies to assets or liabilities for which there are quoted prices in active markets for identical assets or liabilities.
Level 2
Level 2 applies to assets or liabilities for which there are inputs other than quoted prices that are observable for the asset or liability such as quoted prices for similar assets or liabilities in active markets; quoted prices for identical assets or liabilities in markets with insufficient volume or infrequent transactions (less active markets); or model-derived valuations in which significant inputs are observable or can be derived principally from, or corroborated by, observable market data.
Level 3
Level 3 applies to assets or liabilities for which there are unobservable inputs to the valuation methodology that are significant to the measurement of the fair value of the assets or liabilities.
The Company’s financial instruments consist principally of cash, and amounts due to related parties. Pursuant to ASC 820 and 825, the fair value of our cash is determined based on “Level 1” inputs, which consist of quoted prices in active markets for identical assets. We believe that the recorded values of all of our other financial instruments approximate their current fair values because of their nature and respective maturity dates or durations.
Income Taxes
The Company uses the asset and liability approach to account for income taxes. Under this method, deferred tax assets and liabilities are recognized for the expected future tax consequences of differences between the carrying amounts of assets and liabilities and their respective tax bases using tax rates in effect for the year in which the differences are expected to reverse. A valuation allowance is provided when it is more likely than not that some portion or all of the deferred tax assets will not be realized. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period when the change is enacted. Since the company has a history of operating losses, the company has fully reserved any deferred tax asset.
On January 1, 2007, the Company adopted an accounting standard which clarifies the accounting for uncertainty in income taxes recognized in financial statements. This standard provides guidance on recognizing, measuring, presenting and disclosing in the financial statements uncertain tax positions that a company has taken or expects to take on a tax return. See Note 2 for further information related to the Company’s accounting for income taxes.
41
PUSH, INC.
(A Development Stage Company)
Notes to Financial Statements
June 30, 2010 (Unaudited) and December 31, 2009
NOTE 1. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
Recent Accounting Pronouncements
In January 2010, the FASB issued Accounting Standards Update 2010-02, Consolidation (Topic 810): Accounting and Reporting for Decreases in Ownership of a Subsidiary. This amendment to Topic 810 clarifies, but does not change, the scope of current US GAAP. It clarifies the decrease in ownership provisions of Subtopic 810-10 and removes the potential conflict between guidance in that Subtopic and asset derecognition and gain or loss recognition guidance that may exist in other US GAAP. An entity will be required to follow the amended guidance beginning in the period that it first adopts FAS 160 (now included in Subtopic 810-10). For those entities that have already adopted FAS 160, the amendments are effective at the beginning of the first interim or annual reporting period ending on or after December 15, 2009. The amendments should be applied retrospectively to the first period that an entity adopted FAS 160. The Company does not expect the provisions of ASU 2010-02 to have a material effect on the financial position, results of operations or cash flows of the Company.
In January 2010, the FASB issued Accounting Standards Update 2010-01, Equity (Topic 505): Accounting for Distributions to Shareholders with Components of Stock and Cash (A Consensus of the FASB Emerging Issues Task Force). This amendment to Topic 505 clarifies the stock portion of a distribution to shareholders that allows them to elect to receive cash or stock with a limit on the amount of cash that will be distributed is not a stock dividend for purposes of applying Topics 505 and 260. Effective for interim and annual periods ending on or after December 15, 2009, and would be applied on a retrospective basis. The Company does not expect the provisions of ASU 2010-01 to have a material effect on the financial position, results of operations or cash flows of the Company.
In December 2009, the FASB issued Accounting Standards Update 2009-17, Consolidations (Topic 810): Improvements to Financial Reporting by Enterprises Involved with Variable Interest Entities. This Accounting Standards Update amends the FASB Accounting Standards Codification for Statement 167. (See FAS 167 effective date below.)
In December 2009, the FASB issued Accounting Standards Update 2009-16, Transfers and Servicing (Topic 860): Accounting for Transfers of Financial Assets. This Accounting Standards Update amends the FASB Accounting Standards Codification for Statement 166. (See FAS 166 effective date below)
In October 2009, the FASB issued Accounting Standards Update 2009-15, Accounting for Own-Share Lending Arrangements in Contemplation of Convertible Debt Issuance or Other Financing. This Accounting Standards Update amends the FASB Accounting Standard Codification for EITF 09-
1. (See EITF 09-1 effective date below)
In October 2009, the FASB issued Accounting Standards Update 2009-14, Software (Topic 985): Certain Revenue Arrangements That Include Software Elements. This update changed the accounting model for revenue arrangements that include both tangible products and software elements. Effective prospectively for revenue arrangements entered into or materially modified in fiscal years beginning on or after June 15, 2010.
Early adoption is permitted. The Company does not expect the provisions of ASU 2009-14 to have a material effect on the financial position, results of operations or cash flows of the Company.
In October 2009, the FASB issued Accounting Standards Update 2009-13, Revenue Recognition (Topic 605): Multiple-Deliverable Revenue Arrangements. This update addressed the accounting for multiple-deliverable arrangements to enable vendors to account for products or services (deliverables) separately rather than a combined unit and will be separated in more circumstances that under existing US GAAP. This amendment has eliminated that residual method of allocation. Effective prospectively for revenue arrangements entered into or materially modified in fiscal years beginning on or after June 15, 2010. Early adoption is permitted. The Company does not expect the provisions of ASU 2009-13 to have a material effect on the financial position, results of operations or cash flows of the Company.
42
PUSH, INC.
(A Development Stage Company)
Notes to Financial Statements
June 30, 2010 (Unaudited) and December 31, 2009
NOTE 1. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
Recent Accounting Pronouncements (Continued)
In September 2009, the FASB issued Accounting Standards Update 2009-12, Fair Value Measurements and Disclosures (Topic 820): Investments in Certain Entities That Calculate Net Asset Value per Share (or Its Equivalent). This update provides amendments to Topic 820 for the fair value measurement of investments in certain entities that calculate net asset value per share (or its equivalent). It is effective for interim and annual periods ending after December 15, 2009. Early application is permitted in financial statements for earlier interim and annual periods that have not been issued. The Company does not expect the provisions of ASU 2009-12 to have a material effect on the financial position, results of operations or cash flows of the Company.
In February 2010, the FASB issued ASU No. 2010-09 “Subsequent Events (ASC Topic 855) “Amendments to Certain Recognition and Disclosure Requirements” (“ASU No. 2010-09”). ASU No. 2010-09 requires an entity that is an SEC filer to evaluate subsequent events through the date that the financial statements are issued and removes the requirement for an SEC filer to disclose a date, in both issued and revised financial statements, through which the filer had evaluated subsequent events. The adoption did not have an impact on the Company’s financial position and results of operations.
In January 2010, the FASB issued Accounting Standards Update (“ASU”) No. 2010-06, “Improving Disclosures about Fair Value Measurements.” ASU No. 2010-06 amends FASB Accounting Standards Codification (“ASC”) 820 and clarifies and provides additional disclosure requirements related to recurring and non-recurring fair value measurements and employers’ disclosures about postretirement benefit plan assets. This ASU is effective for interim and annual reporting periods beginning after December 15, 2009. The adoption of ASU 2010-06 did not have a material impact on the Company’s financial statements.
In January 2010, the FASB issued an amendment to ASC 505, Equity, where entities that declare dividends to shareholders that may be paid in cash or shares at the election of the shareholders are considered to be a share issuance that is reflected prospectively in EPS, and is not accounted for as a stock dividend. This standard is effective for interim and annual periods ending on or after December 15, 2009 and is to be applied on a retrospective basis. The adoption of this standard is not expected to have a significant impact on the Company’s consolidated financial statements.
The Company has implemented all new accounting pronouncements that are in effect. These pronouncements did not have any material impact on the financial statements unless otherwise disclosed, and the Company does not believe that there are any other new accounting pronouncements that have been issued that might have a material impact on its financial position or results of operations.
In July 2009, the FASB ratified the consensus reached by EITF (Emerging Issues Task Force) issued EITF No. 09-1, (ASC Topic 470) "Accounting for Own-Share Lending Arrangements in Contemplation of Convertible Debt Issuance" ("EITF 09-1"). The provisions of EITF 09-1, clarifies the accounting treatment and disclosure of share-lending arrangements that are classified as equity in the financial statements of the share lender. An example of a share-lending arrangement is an agreement between the Company (share lender) and an investment bank (share borrower) which allows the investment bank to use the loaned shares to enter into equity derivative contracts with investors. EITF 09-1 is effective for fiscal years that beginning on or after December 15, 2009 and requires retrospective application for all arrangements outstanding as of the beginning of fiscal years beginning on or after December 15, 2009. Share-lending arrangements that have been terminated as a result of counterparty default prior to December 15, 2009, but for which the entity has not reached a final settlement as of December 15, 2009 are within the scope. Effective for share-lending arrangements entered into on or after the beginning of the first reporting period that begins on or after June 15, 2009. The Company does not expect the provisions of EITF 09-1 to have a material effect on the financial position, results of operations or cash flows of the Company.
43
PUSH, INC.
(A Development Stage Company)
Notes to Financial Statements
June 30, 2010 (Unaudited) and December 31, 2009
|
|
NOTE 2. INCOME TAXES
|
The Company uses the liability method where deferred tax assets and liabilities are determined based on the future tax consequences of temporary differences between the carrying amounts of assets and liabilities for financial and income tax reporting purposes. During the period from inception to June 30, 2010, The Company incurred net losses and therefore has no tax liability accrued in the accompanying financial statements. The net deferred tax asset generated by the loss carry forward has been fully reserved. The net operating loss carry forward is approximately $224,427 at December 31, 2009 and $94,629 as of June 30, 2010 for a total of $321,056, and will expire beginning in the year 2025.
At June 30, 2010 and December 31, 2009, deferred tax assets consisted of the following:
|
June 30, 2010
(Unaudited)
|
December 31, 2009
|
|||||||
|
Deferred tax asset
|
$ | 109,260 | $ | 76,985 | ||||
|
Valuation allowance
|
(109,260 | ) | (76,985 | ) | ||||
|
Net deferred tax asset
|
$ | - | $ | - | ||||
NOTE 3. EQUITY TRANSACTIONS
On July 10, 2009, the Company issued at par 9,500,000 shares of our common stock to H&W Associates 99, Inc. (Control Person, Ted Wong, Officer and Director) as founder’s shares, in exchange for services related to the Company formation valued at $9,500.
On July 12, 2009, the Company entered into a licensing agreement with DKL International, Inc. and agreed to pay DKL a non-refundable license fee of $90,500. As consideration for the licensing agreement, the Company issued 26,000,000 common shares at a par value of $0.001 per share as founder’s shares and agreed to pay the remaining balance of $64,500 in cash within the next 18 months.
On July 15, 2009, the Company issued 3,000,000 common shares for cash at $0.10 per share along with option agreements for the issuance of 3,000,000 additional shares upon exercise of the options at an exercise price of $.30 per share. The attached options were valued using the Black-Scholes stock option valuation mode. The relative fair value of the common stock was $160,806 and relative fair value of the options were $139,194 based on each instrument’s percentage of the total value applied to the consideration given.
There were no equity issuances from December 31, 2009 through June 30, 2010.
NOTE 4. GOING CONCERN
The accompanying financial statements have been prepared in conformity with generally accepted accounting principle, which contemplate continuation of the Company as a going concern. The Company currently has limited liquidity, and has not completed its efforts to establish a stabilized source of revenues sufficient to cover operating costs over an extended period of time.
Management anticipates that the Company will be dependent, for the near future, on additional investment capital to fund operating expenses The Company intends to position itself so that it may be able to raise additional funds through the capital markets. In light of management’s efforts, there are no assurances that the Company will be successful in this or any of its endeavors or become financially viable and continue as a going concern.
NOTE 5. RELATED PARTY TRANSACTIONS
During the period ended December 31, 2009, the Company issued 9,500,000 shares of our common stock to H&W Associates 99, Inc. (Control Person, Ted Wong, Officer and Director) in exchange for services related to our formation valued at $9,500.
During the period ended December 31, 2009, the Company purchased a non- refundable technology license from a related party for shares valued at $90,500 of which $26,000 was paid in shares of the Company’s common stock. The Company owes $64,500 in cash for the balance of the purchase which is classified as a related party payable. The related party payable is due no later than January 12, 2011, is unsecured and non interest bearing.
The Company’s parent corporation, DKL International, Inc., maintains a bank account on behalf of Push. Certain expenses and cash receipts are recorded by Push when they occur and the net balance of this account has be recorded as a related party payable or receivable for the periods ended. The balance in this account was net receivable of $146,624 for the period ended December 31, 2009 and net payable of $6,977 as of June 30, 2010.
44
PUSH, INC.
(A Development Stage Company)
Notes to Financial Statements
June 30, 2010 (Unaudited) and December 31, 2009
NOTE 6. COMMITMENTS AND CONTINGENCIES
Under the terms of the license agreement the Company is obligated to “use reasonable efforts” to market the licensed products. If the Company does not generate minimum adjusted gross annual sales of $25,000 over the next three years the licensor may terminate the license.
Beginning in 2011, the Company is also obligated to make minimum annual royalty payments as follows:
| 2011 | $ 100,000 |
| 2012-2015 | $ 250,000 |
| 2016-2019 | $ 500,000 |
| 2020-termination | $ 1,000,000 |
NOTE 7. SUBSEQUENT EVENTS
In accordance with SFAS 165 (ASC 855-10) Company management reviewed all material events through October 13, 2010, and there are no material subsequent events to report.
45
PART II: INFORMATION NOT REQUIRED IN PROSPECTUS
OTHER EXPENSES OF ISSUANCE AND DISTRIBUTION
The following table sets forth the costs and expenses payable by us in connection with the sale of the common stock being registered. We have agreed to pay all costs and expenses in connection with this offering of common stock. The estimated expenses of issuance and distribution, assuming the maximum proceeds are raised, are set forth below.
|
Legal and Professional Fees
|
$ | 20,000 | ||
|
Accounting Fees
|
$ | 5,000 | ||
|
Filing Fees
|
$ | 115 | ||
|
Escrow Fees
|
$ | 1,500 | ||
|
Total
|
$ | 26,615 | ||
INDEMNIFICATION OF DIRECTORS AND OFFICERS
Our Articles of Incorporation and Bylaws provide for the indemnification of a present or former director or officer. We indemnify any director, officer, employee or agent who is successful on the merits or otherwise in defense on any action or suit. Such indemnification shall include, but not necessarily be limited to, expenses, including attorney's fees actually or reasonably incurred by him. Nevada law also provides for discretionary indemnification for each person who serves as or at our request as an officer or director. We may indemnify such individual against all costs, expenses and liabilities incurred in a threatened, pending or completed action, suit or proceeding brought because such individual is a director or officer. Such individual must have conducted himself in good faith and reasonably believed that his conduct was in, or not opposed to, our best interests. In a criminal action, he must not have had a reasonable cause to believe his conduct was unlawful.
Nevada Law
Pursuant to the provisions of Nevada Revised Statutes 78.751, we shall indemnify any director, officer and employee as follows: every director, officer, or employee of ours shall be indemnified by us against all expenses and liabilities, including counsel fees, reasonably incurred by or imposed upon him/her in connection with any proceeding to which he/she may be made a party, or in which he/she may become involved, by reason of being or having been a director, officer, employee or agent of us or is or was serving at our request as a director, officer, employee or agent of us, partnership, joint venture, trust or enterprise, or any settlement thereof, whether or not he/she is a director, officer, employee or agent at the time such expenses are incurred, except in such cases wherein the director, officer, employee or agent is adjudged guilty of willful misfeasance or malfeasance in the performance of his/her duties; provided that in the event of a settlement the indemnification herein shall apply only when the Board of Directors approves such settlement and reimbursement as being for our best interests. We shall provide to any person who is or was a director, officer, employee or agent of us or is or was serving at our request as a director, officer, employee or agent of the corporation, partnership, joint venture, trust or enterprise, the indemnity against expenses of a suit, litigation or other proceedings which is specifically permissible under applicable law.
RECENT SALES OF UNREGISTERED SECURITIES
Since inception, we issued the following unregistered securities in private transactions under the Securities Act:
On July 10, 2009, we issued 9,500,000 shares of our common stock to H&W Associates 99, Inc. (Control Person: Ted Wong, Officer and Director) in exchange for services related to our formation valued at $9,500. This issuance of stock qualifies for the exemption from registration contained in Section 4(2) of the Securities Act. H & W Associates 99, Inc. has sold 400,000 shares of our common stock to Jennifer Lee and 250,000 shares of our common stock to Anne Wong at a price of $0.001 per share.
46
RECENT SALES OF UNREGISTERED SECURITIES (Continued)
On July 12, 2009, we entered into a licensing agreement with DKL International, Inc. (“DKL”) (Control Person:
Howard Sidman, Officer and Director) and agreed to pay DKL a non-refundable license fee of $90,500. In partial satisfaction of the $90,500 obligation, we issued 26,000,000 common shares at a value of $0.001 per share for a total value of $26,000 and agreed to pay the remaining balance of $64,500 in cash within the next 18 months. At the time of the issuance, DKL International, Inc. was in possession of all available material information about us, as it is now our parent company. This issuance of stock qualifies for the exemption from registration contained in Section 4(2) of the Securities Act.
On or about July 15, 2009, Shih Chung Chen purchased 1,000,000 shares of our common stock for $100,000 ($0.10 per share) in a private placement offering exempt from the registration provision of the Securities Act, pursuant to Regulation D, Rule 506. Mr. Chen is an accredited investor as defined by Rule 501 of Regulation D. Pursuant to this purchase, Mr. Chen also has the right to purchase an additional 1,000,000 shares of our common stock for $0.30 per share pursuant to an option, expiring on June 29, 2014.
On or about July 15, 2009, Tsung Wen Chang purchased 1,000,000 shares of our common stock for $100,000 ($0.10 per share) in a private placement offering exempt from the registration provision of the Securities Act, pursuant to Regulation D, Rule 506. Mr. Chang is an accredited investor as defined by Rule 501 of Regulation D. Pursuant to this purchase, Mr. Chang also has the right to purchase an additional 1,000,000 shares of our common stock for $0.30 per share pursuant to an option, expiring on June 29, 2014.
On or about July 15, 2009, John Phillips purchased 1,000,000 shares of our common stock for $100,000 ($0.10 per share) in a private placement offering exempt from the registration provision of the Securities Act, pursuant to Regulation D, Rule 506. Mr. Phillips is an accredited investor as defined by Rule 501 of Regulation D. Pursuant to this purchase, Mr. Phillips also has the right to purchase an additional 1,000,000 shares of our common stock for $0.30 per share pursuant to an option, expiring on June 29, 2014.
As of the date of this prospectus, there were 38,500,000 shares of common stock issued and outstanding being held by seven shareholders of record.
47
EXHIBITS AND FINANCIAL STATEMENT SCHEDULES
| Exhibit No. | Name/Identification of Exhibit |
| 3.1 | Articles of Incorporation, dated July 10, 2009 |
| 3.2 | Certificate of Amendment to Articles of Incorporation, dated August 19, 2009 |
| 3.3 | Bylaws of Push, Inc., dated July 10, 2009 |
| 4.1 | Subscription Agreement1 |
| 4.2 | Escrow Agreement with Security Bank of California, dated [_____]1 |
| 5 | Opinion regarding legality (includes consent)1 |
| 10.1 | Employment Agreement with Ted Wong, dated February 1, 2010 |
| 10.2 | Employment Agreement with Howard Sidman, dated February 1, 2010 |
| 10.3 | License Agreement with DKL International, Inc., dated July 12, 2009 |
| 10.4 | Deal Points Agreement with Shih Chung Chen, dated June 29, 2009 |
| 10.5 | Deal Points Agreement with Tsung Wen Chang, dated June 29, 2009 |
| 10.6 | Deal Points Agreement with John Phillips, dated June 29, 2009 |
| 10.7 | Amendment to License Agreement with DKL International, Inc., dated October 11, 2009 |
| 14 | Push, Inc. Code of Ethics, dated September 30, 2009 |
| 23 | Consent of M&K CPAS, PLLC |
1 To be filed by amendment.
48
UNDERTAKINGS
The registrant hereby undertakes:
(1) To file, during any period in which it offers or sells securities, a post-effective amendment to this registration statement to:
(i) Include any prospectus required by Section 10(a)(3) of the Securities Act;
(ii) Reflect in the prospectus any facts or events which, individually or together, represent a fundamental change in the information in the registration statement; Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the Commission pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than a 20% change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective registration statement; and
(iii) Include any additional or changed material information on the plan of distribution.
(2) For determining liability under the Act, to treat each post-effective amendment as a new registration statement of the securities offered, and the offering of the securities at that time to be the initial bona fide offering.
(3) To file a post-effective amendment to remove from registration any of the securities which remain unsold at the end of the offering.
(4) For determining liability under the Act, to any purchaser in the initial distribution of securities, the undersigned small business issuer undertakes that in a primary offering of securities of the undersigned small business issuer pursuant to this registration statement, regardless of the underwriting method used to sell the securities to the purchaser, if the securities are offered or sold to such purchaser by means of any of the following communications, the undersigned small business issuer will be a seller to the purchaser and will be considered to offer or sell such securities to such purchaser:
(i) Any preliminary prospectus or prospectus of the undersigned small business issuer relating to the offering required to be filed pursuant to Rule 424 (230.424 of this chapter);
(ii) Any free writing prospectus relating to the offering prepared by or on behalf of the undersigned small business issuer or used or referred to by the undersigned small business issuer;
(iii) The portion of any other free writing prospectus relating to the offering containing material information about the undersigned small business issuer or its securities provided by or on behalf of the undersigned small business issuer; and
(iv) Any other communication that is an offer in the offering made by the undersigned small business issuer to the purchaser.
(5) Insofar as indemnification for liabilities arising under the Act may be permitted to directors, officers and controlling persons of the small business issuer pursuant to the foregoing provisions, or otherwise, the small business issuer has been advised that in the opinion of the SEC such indemnification is against public policy as expressed in the Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the small business issuer of expenses incurred or paid by a Director, officer or controlling person of the small business issuer in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the small business issuer will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Act and will be governed by the final adjudication of such issue.
(6) For the purpose of determining liability under the Securities Act to any purchaser, each prospectus filed pursuant to Rule 424(b) as a part of a registration statement relating to an offering, other than registration statements relying on Rule 430B or other than prospectuses filed in reliance on Rule 430A, shall be deemed to be part of and included in the registration statement as of the date it is first used after effectiveness.
Provided, however, that no statement made in the registration statement or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such first use, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such document immediately prior to such date of first use.
49
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the Registrant has duly caused this amended registration statement to be signed on its behalf by the undersigned, thereunto authorized in the City of Vienna, State of Virginia on or about October 15, 2010.
|
Push, Inc.
|
|||
| (Registrant) | |||
|
|
By:
|
/s/ Ted Wong | |
| Ted Wong | |||
| President and Director | |||
POWER OF ATTORNEY
KNOW ALL MEN BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints and hereby authorizes Ted Wong such person’s true and lawful attorneys-in-fact, with full power of substitution or resubstitution, for such person and in such person’s name, place and stead, in any and all capacities, to sign on such person’s behalf, individually and in each capacity stated below, any and all amendments, including post-effective amendments to this registration statement, and to file the same, with all exhibits thereto, and other documents in connection therewith, with the SEC, granting unto said attorneys-in-fact, full power and authority to do and perform each and every act and thing requisite or necessary to be done in and about the premises, as fully to all intents and purposes as such person might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact, or their substitute or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Act of 1933, this registration statement has been signed by the following persons in the capacities and on the dates indicated.
|
Signature
|
Title
|
Date
|
|
/s/ Ted Wong
|
President and Director
(Principal Executive Officer)
|
October 15, 2010
|
|
Ted Wong
|
||
|
/s/ Howard Sidman
|
Secretary, Treasurer and Director
(Principal Financial Officer and Principal Accounting Officer)
|
October 15, 2010
|
|
Howard Sidman
|
||
50
