Attached files
| file | filename |
|---|---|
| 8-K - ENDO PHARMACEUTICALS HOLDINGS INC. - ENDO HEALTH SOLUTIONS INC. | d8k.htm |
 ENDO
PHARMACEUTICALS Jefferies 2010 Global SpecPharma
and European Healthcare Conference
October 6, 2010
Exhibit 99.1 |
 grow.
collaborate. innovate. thrive. 2
FORWARD LOOKING STATEMENTS
This presentation contains forward-looking statements regarding, among other things, the
proposed business combination between Endo and Qualitest, Endo’s and
Qualitest’s financial position, results of operations, market position, product development and business strategy, as well as estimates of Endo’s
future total revenues, future expenses, future net income and future earnings per share.
Statements including words such as “believes,” “expects,”
“anticipates,” “intends,” “estimates,” “plan,”
“will,” “may” “intend,” “guidance” or similar expressions are forward-looking statements. Because these
statements reflect our current views, expectations and beliefs concerning future events, these
forward-looking statements involve risks and uncertainties. Investors should note
that many factors could affect the proposed business combination of the companies, future financial results and could cause actual
results to differ materially from those expressed in forward-looking statements contained
in this presentation. These factors include, but are not limited to: the risk that the
acquisition will not close, the risk that Endo’s business and/or Qualitest’s business will be adversely impacted during the pendency of
the acquisition, the risk that the operations of the two companies will not be integrated
successfully, Endo’s ability to successfully develop, commercialize and market new
products; timing and results of pre-clinical or clinical trials on new products; Endo’s ability to obtain regulatory approval of any of Endo’s
pipeline products; competition for the business of Endo’s branded and generic products,
and in connection with its acquisition of rights to intellectual property assets;
market acceptance of our future products; government regulation of the pharmaceutical industry; Endo’s dependence on a small number
of products; Endo’s dependence on outside manufacturers for the manufacture of a majority
of its products; Endo’s dependence on third parties to supply raw materials
and to provide services for certain core aspects of its business; new regulatory action or lawsuits relating to Endo’s use of narcotics in
most of its core products; Endo’s exposure to product liability claims and product
recalls and the possibility that Endo may not be able to adequately insure itself; the
successful efforts of manufacturers of branded pharmaceuticals to use litigation and legislative and regulatory efforts to limit the use of
generics and certain other products; Endo’s ability to successfully implement its
acquisition and in-licensing strategy; regulatory or other limits on the
availability of controlled substances that constitute the active ingredients of some of its
products and products in development; the availability of third- party reimbursement
for Endo’s products; the outcome of any pending or future litigation or claims by third parties or the government, and the
performance of indemnitors with respect to claims for which Endo has been indemnified;
Endo’s dependence on sales to a limited number of large pharmacy chains and
wholesale drug distributors for a large portion of its total revenues; a determination by a regulatory agency that Endo is engaging or
has engaged in inappropriate sales or marketing activities, including promoting the
“off-label” use of its products, the risk that demand for and acceptance
of Endo’s products or services may be reduced; the risk of changes in governmental
regulations; the impact of economic conditions; the impact of competition and pricing
and other risks and uncertainties, including those detailed from time to time in the companies’ periodic reports filed with the
Securities and Exchange Commission, including current reports on Form 8-K, quarterly
reports on Form 10-Q and annual reports on Form 10-K, particularly the
discussion under the caption “RISK FACTORS" in their annual reports on Form 10-K for the year ended December 31, 2009, which were filed with the
Securities and Exchange Commission. The forward-looking statements in this presentation
are qualified by these risk factors. These are factors that, individually or in the
aggregate, could cause our actual results to differ materially from expected and historical results. The companies’ assume no
obligation to publicly update any forward-looking statements, whether as a result of new
information, future developments or otherwise. |
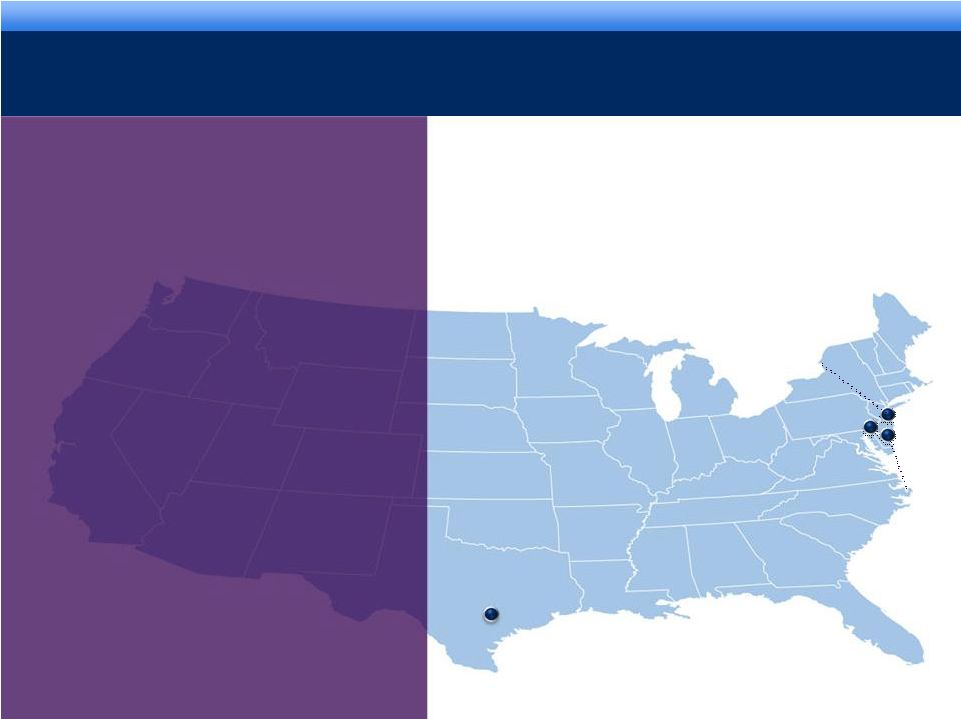   grow. collaborate.
innovate. thrive. COMPANY OVERVIEW
Chadds
Ford, PA
Corporate Headquarters
Cranbury, NJ
Manufacturing
Facilities
Westbury, NY
R&D Facilities
Austin, TX
HealthTronics
Headquarters
3
Endo is a specialty healthcare
solutions company focused on
high-value branded products
and specialty generics as well
as devices and services. Our
portfolio contains products in
pain, urology, endocrinology
and oncology. We are a
customer-centric organization
that considers the needs of
patients, healthcare providers
and shareholders, as well as
the development of
employees, in all actions. |
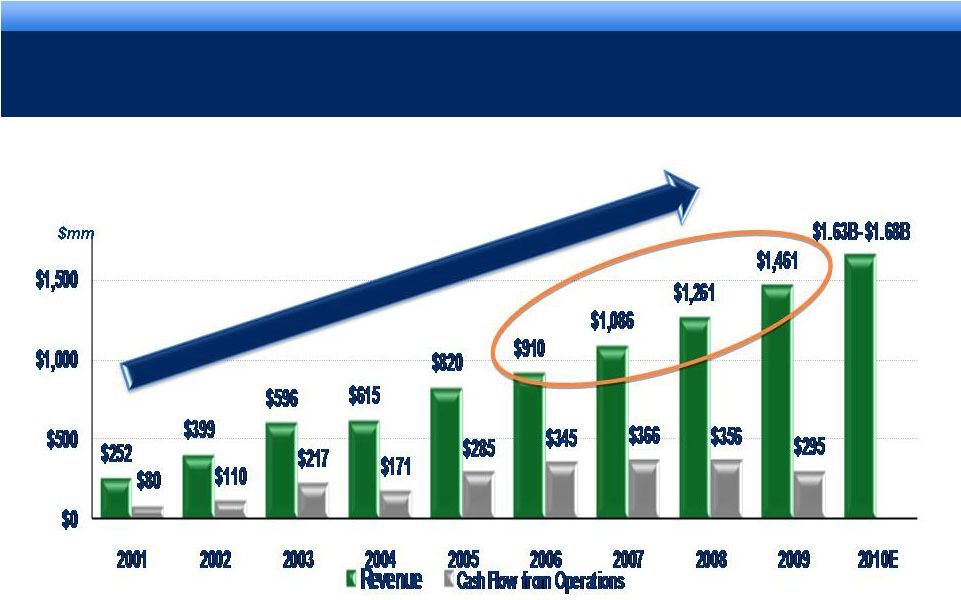 grow.
collaborate. innovate. thrive. STRONG OPERATING PERFORMANCE
17% 3-YEAR CAGR FOR REVENUE*
* Revenue CAGR 2006-2009.
Sustaining our Growth
4 |
 grow.
collaborate. innovate. thrive. 5
Through Organic Growth
•
Diversified business lines to maximize growth
•
Enhanced commercial model driving growth via
strategic resource deployment
•
Invested in R&D portfolio yielding a diversified
pipeline of products with two branded products
pending FDA approval
•
Bolstered management team by adding
expertise and experience in managing and
growing a larger enterprise
5
ENDO’S TRANSFORMATION
Through Strategic Growth
•
With Indevus, we secured a position in urology
•
HealthTronics gave us an established presence
in Devices & Services and critical mass in
urology
•
Penwest strengthened our pain business
enhancing profitability & flexibility in the opioid
franchise
•
Qualitest brings critical mass to our generics
business & strengthens our pain portfolio |
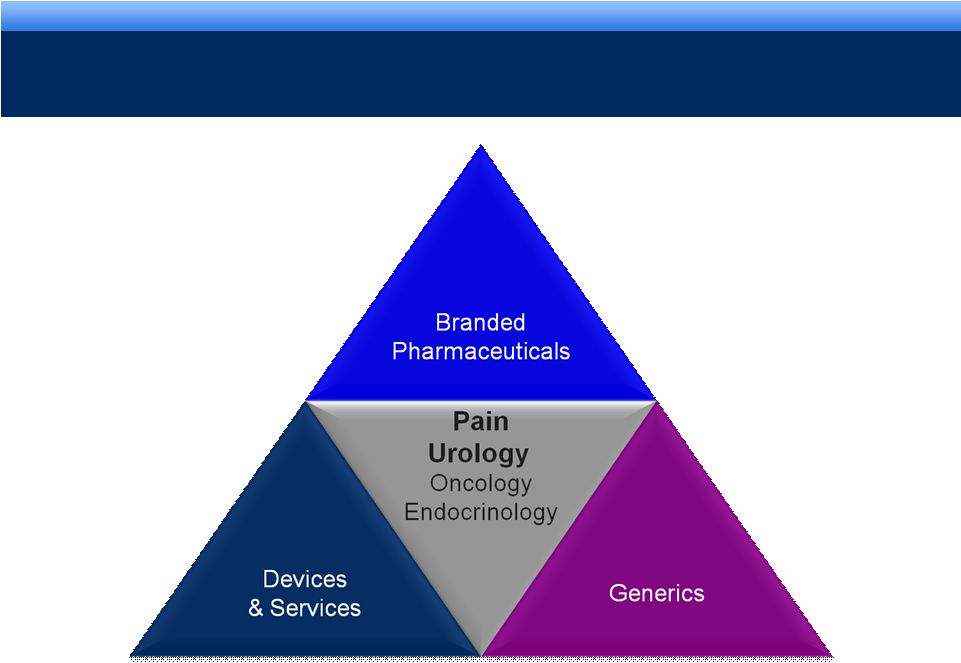 grow.
collaborate. innovate. thrive. 6
ENDO’S INTEGRATED BUSINESS MODEL |
 grow.
collaborate. innovate. thrive. STRONG CORE BUSINESS SUPPORTING GROWTH
7 |
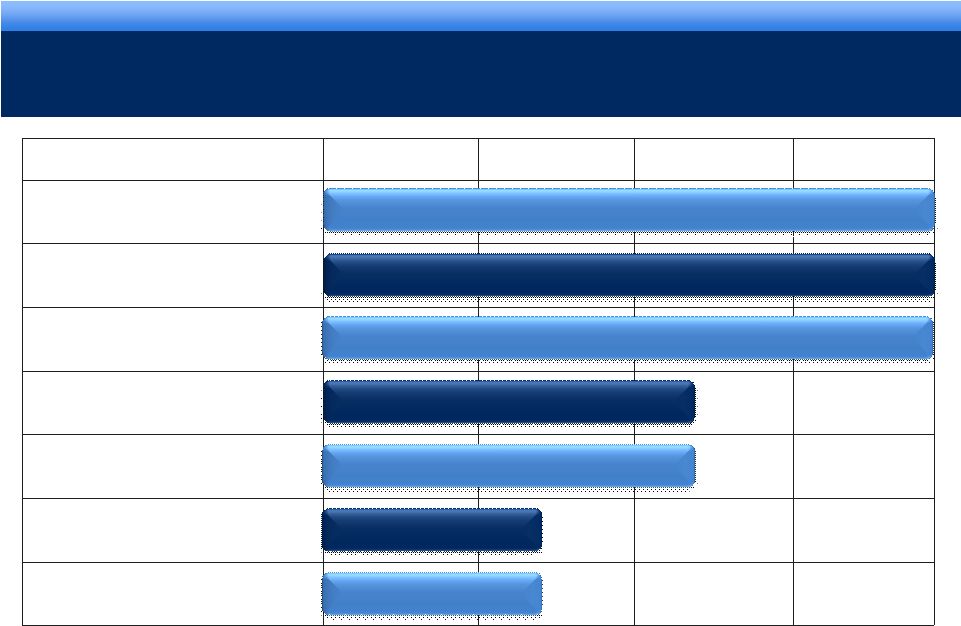 grow.
collaborate. innovate. thrive. DEVELOPMENT PIPELINE
Phase I
Phase II
Phase III
NDA
Oxymorphone
Formulation designed to be crush-resistant
FORTESTA™
2% Testosterone Gel
AVEED™
Long Acting Injectable
Testosterone
Urocidin™
Bladder Cancer
Octreotide
Implant
Acromegaly*
Axomadol
Moderate to moderately severe chronic pain
Octreotide
Implant
Carcinoid
Syndrome
Pending
Pending
* Granted orphan drug designation
8
Update Pending |
 grow.
collaborate. innovate. thrive. Leading provider of urology services
Leader in lithotripsy, BPH laser and cryosurgery
Emerging urologic businesses in:
Anatomic pathology
Radiation therapy
Unique business relationship with 1/3 of urologists in U.S.
Total solution for the urology marketplace
Improve patient care
Enhance practice economics
9
HEALTHTRONICS OVERVIEW |
 grow.
collaborate. innovate. thrive. 10
QUALITEST PHARMACEUTICALS –
A PERFECT STRATEGIC FIT
Furthers strategy to respond to the changing
economics that drive the U.S. healthcare
environment
•
Healthcare reform puts a premium on providing cost-
effective health solutions
•
Evolving from a product-driven company to a
healthcare solutions provider
•
Integrated business model
Strengthens Endo’s core pain franchise
•
Over 40% of Qualitest’s
sales come from pain
products
•
In the $15BN pain market, more than 90% of all pain
scripts and just less than 50% of sales are being
filled by generic drugs
•
Qualitest
strengthens Endo’s ability to provide
products at multiple value points
Brings critical mass to generics business
•
Combined
generics
business
will
be
the
6
th
largest
U.S. generics manufacturer by prescriptions filled
•
Focused in categories that have high barriers to
entry
•
Brings a robust ANDA pipeline, seasoned
management team & solid assets
By acquiring a high-growth asset, will
diversify and boost revenue and earnings’
streams
•
Advances growth on several metrics
•
Further diversifies Endo across Branded
Pharmaceuticals, Generic and Devices & Services
segments
•
Combined company has robust number of ANDAs
on
file and near-term pipeline projects |
 grow.
collaborate. innovate. thrive. 11
11
KEY TRANSACTION TERMS
Purchase Price
•
$1.2 billion
Cash/Share Mix
•
All cash
Funding Mix
•
Combination of cash and fully-committed term loans &
revolver borrowings
Time to Accretion
•
Immediately accretive by $0.40 to adjusted diluted EPS in
first full year after close
•
Dilutive by $0.51 to GAAP EPS
Closing Conditions
•
Subject to customary closing conditions and regulatory
approval
Transaction Close
•
Expected in late 4Q10 / early 1Q11 |
 grow.
collaborate. innovate. thrive. 12
Sixth Largest U.S. Generics Company
•
A cost competitive producer of products in categories with high barriers to entry
(controlled substances, liquids)
•
175 product families covering multiple therapeutic areas
Strong History of Growth Supported by Robust Future Pipeline
•
2010E Sales of approximately $350MM, which has grown at a three-year CAGR of 21%
•
Poised for strong growth with 30 ANDAs
under active review, a robust pipeline of ANDAs
expected to be filed and new product launches
Seasoned Management Team and High Quality Assets
•
Team has significant experience running a growing generics business
•
Four
manufacturing/distribution
facilities
–
three
in
Huntsville,
AL
and
one
in
Charlotte,
NC
12
QUALITEST COMPANY PROFILE |
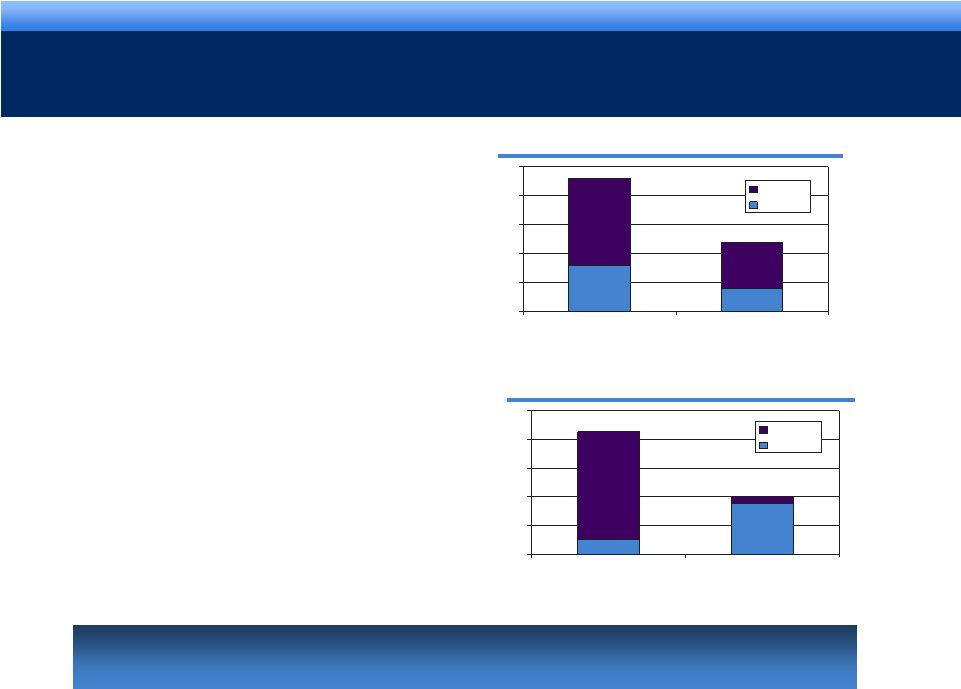 grow.
collaborate. innovate. thrive. 13
A STRONGER GENERICS BUSINESS WITH ROBUST
PIPELINE
Qualitest is a High-Growth Asset
•
Expect revenue to be approx. $350MM in
2010
•
30 ANDAs under active review by FDA
•
Expects to file 16 ANDAs in 2011-12
•
16 products launches in 2011-12
Enhances Endo’s Generics Business
•
16 ANDAs under active review by FDA
•
Expects to file 8 ANDAs in 2011-12
•
9 products launches in 2011-12
16
8
30
16
0
10
20
30
40
50
Under Active Review
Expected Filings in 2011-12
Qualitest
Endo
Pipeline
2
7
15
1
0
4
8
12
16
20
2011
2012
Qualitest
Endo
Combined Business has 25 projected
launches in 2011-2012
Combined Endo/Qualitest portfolio will grow 15% over 2010-12 period
driven by 25 product launches over 2011-12 |
 grow.
collaborate. innovate. thrive. 14
QUALITEST –
HIGHLY COMPLEMENTARY,
ATTRACTIVE ASSET
Provides cost competitive manufacturing capabilities
•
Commercial-scale dosage form manufacturing
•
Capabilities in range of dosage forms including liquids, suspensions, creams, ointments,
suppositories, tablets and capsules
Deep institutional knowledge in controlled substances
Experienced management team and employee base
Extensive commercial portfolio
•
175 product families in multiple therapeutic areas including pain, CNS, hypertension and
women’s health |
 grow.
collaborate. innovate. thrive. 15
Ensures that Endo remains at the forefront of providing pain solutions
•
In the $15BN pain market, more than 90% of all pain prescriptions are filled by generic drugs
which accounts for just under 50% of sales
•
Combined portfolio will have products that cover the vast majority of all pain prescriptions
written
–
25 product offerings with 16% share of pain product sales
•
Provides critical mass enabling Endo to provide a breadth of products at multiple value
points allowing for market share gains
Aligned with existing strategy of targeting high barriers to entry
•
Endo’s existing generics business focuses on niche products that are difficult to
formulate •
Approximately 40% of Qualitest’s product portfolio is comprised of controlled
substances, which cannot be imported into the U.S.
•
In addition, roughly 17% of Qualitest’s product portfolio is made up of liquids, which
are less profitable to ship into the U.S.
15
ENHANCES ENDO’S CORE PAIN FRANCHISE |
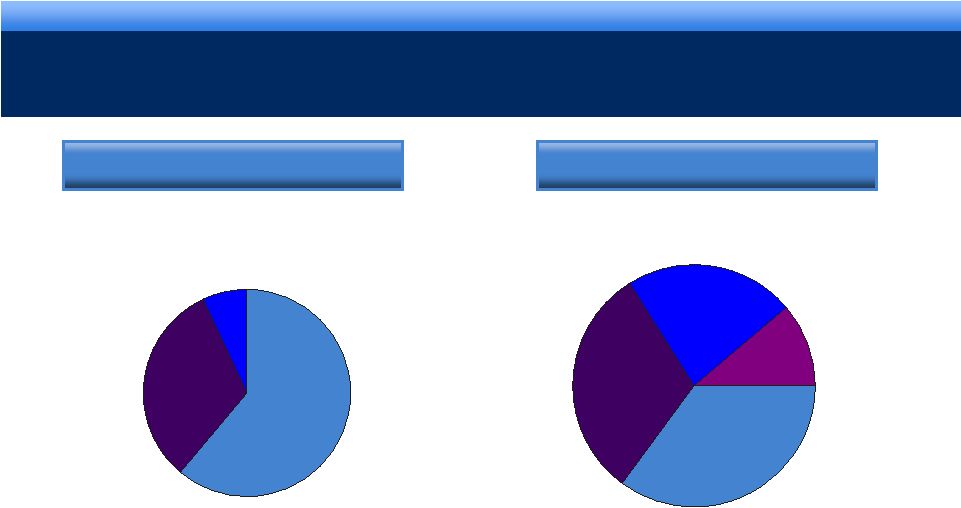 grow.
collaborate. innovate. thrive. 16
DIVERSIFICATION OF BUSINESS
Lidoderm
Generics
Other
Brands
2008
2008
Pro-forma 2011
Pro-forma 2011
Lidoderm currently contributes 50% of revenue to the business and following closing,
Lidoderm will now represent less than 40%
Lidoderm
Generics
Devices &
Services
Other Brands |
 grow.
collaborate. innovate. thrive. 17
GROWTH PROSPECTS
Qualitest Business Growth Prospects
•
Qualitest stand-alone sales expected to grow at a double-digit CAGR over
2010-2012 –
16 product launches expected in 2011-12
•
Qualitest stand-alone revenue is estimated to be roughly $400MM in 2011
Combined Company Growth Prospects
•
Pro forma generics sales CAGR of at least 15% over 2010-2012
–
25 product launches expected in 2011-12
•
Generics revenue is forecast to be more than $500MM for 2011
•
Consolidated pro forma gross margin of approximately 70%
•
Qualitest EPS accretion is approximately $0.40 for first full-year after
close 17 |
 grow.
collaborate. innovate. thrive. 18
Transaction is Primarily About Growth
•
Given the scale of Endo’s existing generics business, there is minimal operating
overlap, but synergies were identified
•
Deal was driven by an enhancement to our revenue and earnings growth
Cost Synergies Achieving a $30MM Annualized Run-Rate in 2013
•
Savings expected in procurement, supply chain and manufacturing efficiencies
Expect Revenue Synergies with Strategic Benefits of the Acquisition Post
Integration Including
•
Broader portfolio and scale in generics marketplace
•
Greater contracting opportunity
•
Opens door to multiple distribution channels
•
Enhanced preferred buying arrangements with key customers
18
SYNERGIES |
 grow.
collaborate. innovate. thrive. 19
2010 ENDO GUIDANCE AFFIRMED
Guidance
Revenue range
$1.63BN -
$1.68BN
Adjusted diluted EPS range
$3.30 -
$3.35
Reported (GAAP) diluted EPS range
$1.88 -
$1.96
Expect
to
generate
more
than
$400M
in
annual
Operating
Cash
Flow
this
year |
 grow.
collaborate. innovate. thrive. 20
A PERFECT STRATEGIC FIT
Furthers our stated strategy to build a healthcare company better able to
respond
to
the
changing
economics
that
drive
the
U.S.
healthcare
environment
Enhances our core pain franchise
Adds critical mass to our current generics business, alongside Branded
Pharmaceuticals and Devices & Services
As a high-growth asset, will significantly diversify and accelerate the growth of
Endo’s revenue and earnings’
streams |
 ENDO
PHARMACEUTICALS |
 grow.
collaborate. innovate. thrive. 22
22
RECONCILIATION OF NON-GAAP MEASURES
22
For an explanation of Endo’s reasons for using non-GAAP measures, see Endo’s
Current Report on Form 8-K filed today with the Securities and Exchange Commission
Reconciliation of Projected GAAP Diluted Earnings Per Share to Adjusted Diluted Earnings Per
Share Guidance for the Year Ending December 31, 2010 Lower End of Range
Upper End of Range
Projected GAAP diluted income per common share
$1.88
$1.96
Upfront and milestone-related payments to partners
$0.38
$0.33
Amortization of commercial intangible assets
$0.59
$0.59
Costs incurred in connection with continued efforts to enhance the
cost structure of the Company
$0.08
$0.08
Indevus
related costs and change in fair value of contingent
consideration
$0.01
$0.01
Impairment of indefinite-lived intangibles
$0.11
$0.11
Costs related to the acquisition of HealthTronics, Inc.
$0.30
$0.30
Costs related to the acquisition of Penwest
Pharmaceuticals Co.
$0.22
$0.22
Costs related to the acquisition of Qualitest
Pharmaceuticals
$0.02
$0.02
Interest expense adjustment for ASC 470-20
and the amortization of
the premium on debt acquired from Indevus
$0.15
$0.15
Tax effect of pre-tax adjustments at the applicable tax rates
and
certain other expected cash tax savings as a result of the Indevus,
HealthTronics, Penwest
and Qualitest
acquisitions
($0.44)
($0.42)
Diluted adjusted income per common share guidance
$3.30
$3.35
The company's guidance is being issued based on certain assumptions including:
•Certain
of
the
above
amounts
are
based
on
estimates
and
there
can
be
no
assurance
that
Endo
will
achieve
these
results
•Includes
all
completed
business
development
transactions
as
of
September
28,
2010
and
the
acquisitions
of
Penwest
Pharmaceuticals
Co.
and
Qualitest
Pharmaceuticals. |
 ENDO
PHARMACEUTICALS Jefferies 2010 Global SpecPharma
and European Healthcare Conference
October 6, 2010 |
