Attached files
Table of Contents
As filed with the Securities and Exchange Commission on October 5, 2010
Registration No. 333-169031
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
AMENDMENT NO. 1
TO
FORM S-1
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
ORAGENICS, INC.
(Exact name of registrant as specified in its charter)
| Florida | 2836 | 59-3410522 | ||
| (State or other jurisdiction of incorporation or organization) |
(Primary Standard Industrial Classification Code Number) | (I.R.S. Employer Identification No.) |
3000 Bayport Drive, Suite 685
Tampa, Florida 33607
(813) 286-7900
(Address, including zip code, and telephone number, including area code, of principal executive offices)
David Hirsch
Chief Executive Officer and President
Oragenics, Inc.
3000 Bayport Drive, Suite 685
Tampa, Florida 33607
(813) 286-7900
(Name, address, including zip code, and telephone number, including area code, of agent for service)
With copies to:
| Darrell C. Smith, Esq. | Michael D. Maline, Esq. | |
| Mark A. Catchur, Esq. | Thomas S. Levato, Esq. | |
| Shumaker, Loop & Kendrick, LLP | Goodwin Procter LLP | |
| 101 East Kennedy Boulevard | The New York Times Building | |
| Suite 2800 | 620 Eighth Avenue | |
| Tampa, Florida 33602 | New York, New York 10018 | |
| Telephone: (813) 229-7600 | Telephone: (212) 813-8800 | |
| Facsimile: (813) 229-1660 | Facsimile: (212) 355-3333 |
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following box. ¨
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check one):
| Large accelerated filer ¨ | Accelerated filer ¨ | |||
| Non-accelerated filer ¨ | (Do not check if a smaller reporting company) | Smaller reporting company x |
The registrant hereby amends this Registration Statement on such date or dates as may be necessary to delay its effective date until the registrant shall file a further amendment which specifically states that this Registration Statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933, as amended, or until this Registration Statement shall become effective on such date as the Securities and Exchange Commission, acting pursuant to said Section 8(a), may determine.
Table of Contents
Explanatory note
Pursuant to Item 10(f) of Regulation S-K promulgated under the Securities Act of 1933, as amended, we have elected to comply with the scaled disclosure requirements applicable to “smaller reporting companies” throughout this registration statement and prospectus. Except as specifically included in this registration statement and prospectus, items not required by the scaled disclosure requirements have been omitted.
Table of Contents
The information in this prospectus is not complete and may be changed. We may not sell these securities until the registration statement filed with the Securities and Exchange Commission is effective. This prospectus is not an offer to sell these securities and it is not soliciting an offer to buy these securities in any jurisdiction where the offer or sale is not permitted.
| PRELIMINARY PROSPECTUS | SUBJECT TO COMPLETION, DATED OCTOBER 5, 2010 |
|
|
3,000,000 Shares
Common Stock
$ per Share |
We are offering 3,000,000 shares of our common stock.
Our common stock is currently traded on the OTC Bulletin Board under the symbol “ORNID.OB.” Our common stock has been approved for listing on the NASDAQ Capital Market under the proposed symbol “OGEN” subject to notice of issuance. On September 24, 2010 we effected a reverse stock split on a 20-to-1 basis. On September 28, 2010, the last sale price for our common stock as reported on the OTC Bulletin Board was $6.60 per share on an immediate post-split basis.
Investing in our common stock involves a high degree of risk. Please read “Risk Factors” beginning on page 9.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved these securities or determined whether this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
| Per Share | Total | |||||
| Public offering price |
$ | $ | ||||
| Underwriting discounts and commissions(1) |
$ | $ | ||||
| Proceeds, before offering expenses, to us(2) |
$ | $ | ||||
| (1) | Does not include a non-accountable expense allowance equal to 1% of the gross proceeds of this offering (or $ ) payable to the underwriters. |
| (2) | We estimate that the total expenses of this offering will be approximately $1,000,000, consisting of approximately $200,000 for the underwriters’ non-accountable expense allowance (equal to 1% of the gross proceeds of this offering) and approximately $800,000 for legal, accounting, printing costs and various fees associated with the registration and listing of our shares of common stock. |
We have granted the underwriters a 45-day option to purchase up to an additional 450,000 shares of common stock to be offered by us solely to cover over-allotments, if any.
The underwriters expect to deliver our shares of common stock to purchasers in this offering on or about , 2010.
| ThinkEquity LLC | ||
| Caris & Company, Inc. |
The date of this prospectus is , 2010
Table of Contents
Table of Contents
| 1 | ||
| 9 | ||
| 28 | ||
| 29 | ||
| 30 | ||
| 32 | ||
| 33 | ||
| Management’s discussion and analysis of financial condition and results of operations |
35 | |
| 50 | ||
| 74 | ||
| 83 | ||
| 91 | ||
| Security ownership of management and certain beneficial owners |
95 | |
| 97 | ||
| 100 | ||
| 101 | ||
| 104 | ||
| 104 | ||
| 104 | ||
| F-1 |
Unless the context otherwise requires, all references to “Oragenics,” “we,” “us,” “our,” “our company,” or “the Company” in this prospectus refer to Oragenics, Inc., a Florida corporation, and its subsidiaries. You should rely only on the information contained in this prospectus. We have not authorized any other person to provide you with different information. If anyone provides you with different or inconsistent information, you should not rely on it. For further information, please see the section of this prospectus entitled “Where You Can Find More Information.”
This prospectus does not constitute an offer to sell or a solicitation of an offer to buy any common stock in any circumstances in which such offer or solicitation is unlawful. If it is against the law in any state to make an offer to sell these securities, or to solicit an offer from someone to buy these securities, then this prospectus does not apply to any person in that state, and no offer or solicitation is made by this prospectus to any such person.
Neither the delivery of this prospectus nor any sale made in connection with this prospectus shall, under any circumstances, create any implication that there has been no change in our affairs since the date of this prospectus. You should not assume that the information appearing in this prospectus is accurate as of any date other than the date on the front cover of this prospectus, regardless of the time of delivery of this prospectus or any sale of a security. Our business, financial condition, results of operations and prospects may have changed since those dates.
We obtained statistical data, market data and other industry data and forecasts used throughout this prospectus from market research, publicly available information and industry publications. Industry publications generally state that they obtain their information from sources that they believe to be reliable, but they do not guarantee the accuracy and completeness of the information. Similarly, while we believe that the statistical data, industry data and forecasts and market research are reliable, we have not independently verified the data, and we do not make any representation as to the accuracy of the information. We have not sought the consent of the sources to refer to their reports appearing in this prospectus.
We own, have rights to or have applied for the trademarks, trade names, service marks and service names that we use in conjunction with our business, including our logo. EVORAPLUS®, TEDDY’S PRIDE® and PROBIORA3® are registered trademarks of ours. We currently use the following unregistered trademarks: SMaRT Replacement Therapy™, MU1140™, PCMAT™, LPT3-04™ and DPOLT™. Oragenics™ is among our non-registered trademarks. We currently have pending with the U.S. Patent and Trademark Office applications for registration of our principal brands, including the marks for EVORAKIDS™, EVORAPRO™, PROBIORA™, KJ2™, KJ3™ and JH145™. All other trademarks, trade names, service marks and service names appearing in this prospectus are the property of their respective holders.
Table of Contents
This summary highlights information contained elsewhere in this prospectus and does not contain all of the information you should consider in making your investment decision. You should read this summary together with the more detailed information, including our financial statements and the related notes, contained elsewhere in this prospectus. You should carefully consider, among other things, the matters discussed in “Risk Factors.”
Unless otherwise indicated, all historical and pro forma common stock and per share data in this prospectus have been restated to the earliest period presented to account for the 20-to-1 reverse stock split effectuated by the filing of an amendment to our articles of incorporation on September 24, 2010.
Overview
We are a biopharmaceutical company focused primarily on oral health products and novel antibiotics. Within oral health, we are developing our pharmaceutical product candidate, SMaRT Replacement Therapy, and we are also commercializing our oral probiotic blend, ProBiora3. Within antibiotics, we are developing our pharmaceutical product candidate, MU1140-S, and we intend to use our patented, novel organic chemistry platform to create additional antibiotics for therapeutic use.
Our SMaRT Replacement Therapy product candidate is designed to be a painless, one-time, five-minute topical treatment applied to the teeth that has the potential to offer lifelong protection against dental caries, or tooth decay. Dental diseases are the most prevalent chronic infectious diseases in the world, affecting up to 90% of schoolchildren and the vast majority of adults. In 2009, Popular Mechanics magazine named SMaRT Replacement Therapy as the “#1 New Biotech Breakthrough That Will Change Medicine.” In the United States alone, the annual cost to treat tooth decay is estimated to be $40 billion. Our SMaRT Replacement Therapy is based on the creation of a genetically modified strain of bacteria that colonizes in the oral cavity and replaces native decay-causing bacteria. We are commencing a second Phase 1 clinical trial for our SMaRT Replacement Therapy, which we expect to conclude in the first half of 2011.
We have also developed and are commercializing a variety of products that contain our active ingredient ProBiora3, a patent-pending blend of oral probiotics that promote fresher breath, whiter teeth and support overall oral health. The global probiotics market is expected to be $31.2 billion by 2014, representing a compound annual growth rate, or CAGR, of 12.6% from 2009. We have conducted extensive scientific studies on ProBiora3 in order to market our products under self-affirmed Generally Recognized As Safe status, or GRAS. We sell our ProBiora3 products through multiple distribution channels, and our customers include Walgreens, Rite Aid, and Garden of Life, among others.
While developing SMaRT Replacement Therapy, members of our scientific team discovered that the SMaRT bacterial strain produces MU1140, a molecule belonging to the novel class of antibiotics known as lantibiotics. MU1140 has proven active preclinically against Gram positive bacteria responsible for a number of healthcare-associated infections, or HAIs. The direct cost to the U.S. healthcare system from HAIs is estimated to be up to $45 billion annually. We are in the process of scaling up production of our synthetic form of MU1140, or MU1140-S, and expect to commence preclinical testing during the second half of 2010 and to file an Investigational New Drug, or IND, application with the U.S. Food and Drug Administration, or FDA, in mid-2011. The key technology behind the production of MU1140-S is our Differentially Protected Orthogonal Lanthionine Technology platform, or DPOLT, which is a patented, novel organic chemistry platform that we believe will enable the first ever commercial scale, cost-effective production of any of the 50 known lantibiotics. We intend to use DPOLT to create a pipeline of lantibiotics for therapeutic use.
Our core product portfolio is protected by eight issued U.S. patents and eight filed U.S. patent applications. We are the exclusive worldwide licensee to the patents for SMaRT Replacement Therapy and MU1140, which are owned by the University of Florida Research Foundation, Inc. We have retained worldwide commercialization rights to each of these product candidates. Additionally, we believe that our SMaRT Replacement Therapy will qualify for a 12-year exclusivity period in the United States under the recently passed Patient Protection and Affordable Care Act and the Health Care and Education Reconciliation Act.
1
Table of Contents
|
Product/Candidate |
Description |
Application |
Status | |||
| SMaRT Replacement Therapy |
Genetically modified strain of S. mutans that does not produce lactic acid | Tooth decay | Second Phase 1 clinical trial | |||
| ProBiora3 |
Blend of three beneficial oral probiotic bacteria | Oral health, teeth whitening, breath freshening (humans, companion pets) | Commercial (GRAS) | |||
| MU1140-S |
Member of lantibiotic class of antibiotics | Healthcare-associated infections | Preclinical testing | |||
Oragenics was founded in 1996 to commercialize the results of more than 30 years of research in oral biology by our principal founder and Chief Scientific Officer, Dr. Jeffrey Hillman. Dr. Hillman earned a DMD from Harvard School of Dental Medicine and a PhD in Molecular Genetics from Harvard University. He began his research career at the Harvard-affiliated Forsyth Institute in Boston, Massachusetts, where he introduced the concept of replacement therapy to prevent tooth decay by using a genetically modified strain of Streptococcus mutans, or S. mutans, to replace the decay-causing strains of S. mutans that are present on human teeth. He subsequently continued this research, now called SMaRT Replacement Therapy, at the University of Florida College of Dentistry. Under Dr. Hillman’s leadership, our scientific team has also developed other technologies such as ProBiora3, MU1140 and our DPOLT platform. Additionally, we are developing non-core technologies that originated from the discoveries of our scientific team, including LPT3-04, which is a potential weight loss product, and PCMAT, which is a biomarker discovery platform, both of which we believe could provide significant potential opportunities for us.
Since our inception, we have incurred operating losses and generated negative cash flow from operating activities as a result of our product development and commercialization efforts. We expect to incur losses for the foreseeable future as we expand our sales and marketing capabilities for our ProBiora3 products and continue our preclinical testing, clinical trials and research and development activities. Net losses totaled $3,757,421 for the six months ended June 30, 2010 and $5,519,348 and $6,021,742 for the years ended December 31, 2009, and 2008, respectively, and we have an accumulated deficit of $29,269,304 as of June 30, 2010. In light of our recurring losses, accumulated deficit and negative cash flow, the report of our independent registered public accounting firm on our financial statements for the fiscal year ended December 31, 2009 contains an explanatory paragraph raising substantial doubt about our ability to continue as a going concern. However, with the net proceeds from this offering we believe we will have sufficient working capital to avoid such an explanatory paragraph on our financial statements for the fiscal period ending December 31, 2010. The net proceeds from this offering will allow us to strengthen our focus on current and future commercialization of our products and product candidates, which we believe will position us for future profitability and positive cash flows.
Our Business Strategy
Our goal is to develop and commercialize our product portfolio in order to maximize the value of each product or product candidate therein. In order to achieve this goal, we intend to:
| Ø | Develop our SMaRT Replacement Therapy through Phase 1 clinical trials. The SMaRT strain has been extensively and successfully preclinically tested. We concluded our first Phase 1 clinical trial early due to low enrollment, which we believe resulted from the trial’s highly cautious inclusion and exclusion criteria. We are in the process of commencing a second Phase 1 clinical trial, which will examine the safety and genetic stability of an attenuated version of the SMaRT strain during administration to ten healthy adult male subjects over a two-week period. We expect the second Phase 1 clinical trial, including a three-month follow-up examination of subjects, to be concluded in the first half of 2011, and, if successful, we anticipate conducting a third Phase 1 clinical trial using the non-attenuated SMaRT strain shortly thereafter. |
2
Table of Contents
| Ø | Utilize a comprehensive marketing effort to increase sales of our ProBiora3 products across multiple channels. We intend to use a portion of the net proceeds from this offering to drive increased sales of our ProBiora3 products in the mass retail channel by utilizing nationwide marketing efforts. We believe that ProBiora3 is the most comprehensive oral probiotic technology currently available in the oral care market. Our marketing for ProBiora3 includes the cosmetic claims of teeth whitening and breath freshening, along with the general structure-function claim that ProBiora3 supports oral health. We market products containing ProBiora3 under our own house brand names, including EvoraPlus, EvoraKids, Teddy’s Pride and EvoraPro, and have also branded ProBiora3 as an active ingredient for licensing and private labeling. We have selected our distribution channels by focusing on our potential channel impact, as well as the expected potential return on our marketing expenditures. We have experienced early success in our marketing efforts despite limited marketing resources, including nationwide product placements with Walgreens, Rite Aid, GNC and Kroger during the first three quarters of 2010. |
| Ø | Commence the manufacturing and clinical testing of our lantibiotic MU1140-S. We have retained a leading contract manufacturer to refine and scale-up Good Manufacturing Practice, or GMP, production of MU1140-S. We expect to conclude the preclinical testing of MU1140-S, including toxicity testing in rodent and non-rodent animal models, during the first half of 2011. We then intend to file an IND application with the FDA in mid-2011. |
| Ø | Utilize our proprietary novel organic chemistry platform, DPOLT, to synthesize additional lantibiotics of interest. We believe that DPOLT will allow us to synthetically produce any of the 50 known lantibiotics due to the shared chemical structure features of this class of molecule. Analysis of the lantibiotic class suggests that there are possibly six to ten subclasses of lantibiotics as classified by known mechanisms of action, spectra of activity, or structural characteristics. We intend to use DPOLT to create a pipeline of lantibiotics for therapeutic use. |
| Ø | Develop our additional technologies, discovered by our scientific team, including LPT3-04, our weight loss product, and PCMAT, our biomarker discovery platform. Our current product development efforts in LPT3-04 are focused on incorporating the compound into bars, milkshakes, and other food delivery mechanisms. These food products will be used in a blinded placebo-controlled study scheduled to begin in the second half of 2010. Additionally, we intend to use our PCMAT platform to identify protein targets for several widespread disease states. We believe our PCMAT platform rapidly identifies proteins that are expressed when a cell undergoes change, and such proteins may be useful for medical diagnostics and therapeutic strategies. |
| Ø | Selectively establish strategic collaborations to advance and maximize the commercial potential of our product portfolio and potential pipeline. We believe that our product portfolio will be of significant interest to major pharmaceutical and medical diagnostics companies. If SMaRT Replacement Therapy and MU1140-S successfully complete Phase 1 trials, we believe that their respective values will substantially increase, and we would then seek to license these products or partner with one or more major pharmaceutical companies. In addition, if we are able to discover protein targets with sufficient degrees of sensitivity and specificity using our PCMAT biomarker discovery platform, we intend to license these targets to one or more major pharmaceutical or medical diagnostics companies. |
| Ø | Use third-party manufacturers to produce our SMaRT and ProBiora3 products under GMPs. The manufacturing methods for producing the SMaRT strain are commonplace and readily available within the pharmaceutical industry. For our first Phase 1 clinical trial, we engaged a contract manufacturer to produce our SMaRT strain, using a standard operating procedure provided by us that we believe is readily transferable to outside contract manufacturers with fermentation capabilities. We have contracted with multiple manufacturers to produce our active ingredient, ProBiora3, as well as blend, tablet, and package our products. Each of our contract manufacturers for ProBiora3 products has the ability to scale production as needed. We have qualified and used at least two contract manufacturers for each step in our manufacturing process. |
3
Table of Contents
Selected Risk Factors
Our business, our common stock and this offering are subject to numerous risks which are set forth in the section entitled “Risk Factors” beginning on page 9 of this prospectus. Principal risks include the following:
| Ø | Our success will depend on our ability to obtain regulatory approval and achieve successful commercialization of our SMaRT Replacement Therapy and MU1140-S product candidates, as well as our ability to significantly increase sales of our ProBiora3 products, which have only generated modest revenues to date; |
| Ø | Since our inception, while focusing primarily on discovery and development of our commercial products and product candidates, we have experienced significant losses and expect to continue to experience losses for the foreseeable future, and our auditor has expressed substantial doubt about our ability to continue as a going concern; |
| Ø | Our agreements with large national mass retailers with respect to our ProBiora3 products may be delayed, terminated or reduced in scope with little or no notice, and sales of our ProBiora3 products may be adversely affected by fluctuations in and terms and conditions of buying decisions and future returns of our retailers and consolidation among retailers; |
| Ø | We are subject to government regulation of the processing, formulation, packaging, labeling and advertising of our product candidates, and if we are unable to obtain regulatory approval for our product candidates we will be unable to generate revenues; |
| Ø | If we fail to comply with our obligations in the agreements with the University of Florida Research Foundation, Inc., under which we license our rights to SMaRT Replacement Therapy and MU1140, our licenses to these product candidates may be terminated and we will be unable to commercialize these product candidates; |
| Ø | We depend on third-party manufacturers for our ProBiora3 products. The loss of any manufacturer or any interruptions in the supply of our ProBiora3 products, including if our manufacturers in general fail to meet our requirements and the requirements of regulatory authorities, would have a negative impact on our revenues and profitability; |
| Ø | The Koski Family Limited Partnership, or KFLP, together with members of the Koski family, have a controlling interest in our outstanding shares of common stock; and |
| Ø | We have effected a reverse stock split, which may cause the liquidity of our common stock and market capitalization to be materially adversely affected. |
Recent Developments
On September 24, 2010 the amendment to our articles of incorporation that we filed with the Florida Department of State became effective with respect to a 20-to-1 reverse stock split of our authorized and outstanding shares of common stock.
On July 5, 2010, we entered into a common stock purchase agreement (the “July 2010 Financing Transaction”) with the KFLP. At the closing of this financing transaction on July 30, 2010 we issued 250,000 shares of our common stock to the KFLP at a price of $8.00 per share. The $2,000,000 aggregate consideration paid by the KFLP consisted of (i) $1,000,000 cash and (ii) the exchange and cancellation of the $1,000,000 unsecured promissory note (the “May 2010 Note”) issued to the KFLP on May 24, 2010. Accrued interest on the May 2010 Note through closing was waived by the KFLP. Concurrent with the July 2010 Financing Transaction and as part thereof, we entered into an unsecured revolving credit agreement (the “Credit Facility”) with the KFLP. Pursuant to the Credit Facility, we are able to borrow up to $2,000,000 from the KFLP at LIBOR plus 6.0%. The term of the Credit Facility is for 12 months commencing August 1, 2010. As of the date of this prospectus, we have drawn $1,000,000 on this Credit Facility.
4
Table of Contents
We have continued our efforts to broaden the distribution of our ProBiora3 products through the following business development activities:
| Ø | American Dental Association: From October 9-12, 2010, we will exhibit EvoraPro to dental professionals at their Annual Session and World Marketplace Exhibition. |
| Ø | Best Supplies: On September 30, 2010, we announced that Teddy’s Pride will be available in Taiwan through a distribution agreement with The Best Supplies LTD, which has 70 pet supply retail shops across Taiwan. |
| Ø | Rolf C. Hagen, Inc.: On September 23, 2010, we announced the distribution of Teddy’s Pride in Canada through Rolf C. Hagen, Inc., one of the largest privately held pet product manufacturers in the world. |
| Ø | Benelux Cosmetics: On September 21, 2010, we announced that our line of ProBiora3 products will be distributed in Belgium, the Netherlands and Luxembourg through Benelux Cosmetics. |
| Ø | Vetcom: On September 20, 2010, we announced that Teddy’s Pride will be distributed to veterinarians in Korea through Vetcom Korea, Inc. Vetcom Korea is a leading national distributor of products for the veterinarian industry. |
| Ø | Fred Meyer: On September 16, 2010, we announced an initial order of EvoraPlus from Fred Meyer, which operates 129 multi-department stores in four western states. |
| Ø | SuperZoo: From September 14-16, 2010, we introduced Teddy’s Pride to SuperZoo, an annual pet trade show and seminar by the World Pet Association, which was attended by over 9,000 pet professionals from around the world. |
| Ø | Kroger: On September 14, 2010, we announced the expansion of the retail distribution of EvoraPlus through an initial order from Kroger Co. Kroger is the nation’s largest traditional grocery retailer with approximately 2,500 stores in 31 states. We anticipate that EvoraPlus will be available in select Kroger grocery stores beginning in October 2010. |
| Ø | Harris Teeter: On September 10, 2010, we announced an initial order of EvoraPlus and EvoraKids from Harris Teeter. Harris Teeter is a food market chain that operates in eight states with 196 stores. |
| Ø | GNC: On September 7, 2010, we announced that EvoraPlus will be available in corporate-owned GNC stores nationwide and EvoraKids will be available in its concept stores and on GNC.com. GNC is a leading global specialty retailer of nutritional products with over 7,000 locations. |
Corporate History and Structure
We were incorporated in Florida in 1996 and commenced operations in 1999. We amended our articles of incorporation in 2002 in order to change our name from Oragen, Inc. to Oragenics, Inc., and consummated our initial public offering in June 2003. Our executive office is located at 3000 Bayport Drive, Suite 685, Tampa, Florida, 33607 and our research facilities are located at 13700 Progress Boulevard, Alachua, Florida 32615. Our telephone number is (813) 286-7900 and our website is http://www.oragenics.com. Information on, or that can be accessed through, our website is not part of this prospectus and should not be relied on in connection with this offering.
5
Table of Contents
Summary financial information
In the table below we provide you with historical financial data for the six month periods ended June 30, 2010 and 2009 and the years ended December 31, 2009 and 2008, derived from our audited and unaudited financial statements included elsewhere in this prospectus. Historical results are not necessarily indicative of the results that may be expected for any future period. When you read this historical selected financial data, it is important that you read along with it the appropriate historical financial statements and related notes and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” included elsewhere in this prospectus.
Statement of Operations
| Six months ended June 30, |
Years ended December 31, |
|||||||||||||||
| 2010 | 2009 | 2009 | 2008 | |||||||||||||
| (unaudited) |
||||||||||||||||
| Net revenues |
$ | 646,179 | $ | 166,167 | $ | 641,285 | $ | 233,539 | ||||||||
| Cost of goods sold |
326,321 | 35,384 | 221,198 | 14,864 | ||||||||||||
| Operating expenses: |
||||||||||||||||
| Research and development |
909,838 | 979,975 | 1,833,746 | 1,955,488 | ||||||||||||
| Selling, general and administrative |
3,167,626 | 2,718,172 | 4,917,844 | 4,312,246 | ||||||||||||
| Total operating expenses |
4,077,464 | 3,698,147 | 6,751,590 | 6,267,734 | ||||||||||||
| Loss from operations |
(3,757,606 | ) | (3,567,364 | ) | (6,331,503 | ) | (6,049,059 | ) | ||||||||
| Other income (expense): |
||||||||||||||||
| Interest income |
2,535 | 522 | 922 | 32,511 | ||||||||||||
| Interest expense |
(885 | ) | (1,504 | ) | (44,292 | ) | (10,054 | ) | ||||||||
| Gain on sale of property and equipment |
— | 11,274 | 22,743 | 4,860 | ||||||||||||
| Gain on extinguishment of payables |
— | 707,674 | 832,959 | — | ||||||||||||
| Local business tax |
(1,465 | ) | — | (177 | ) | — | ||||||||||
| Total other income, net |
185 | 717,966 | 812,155 | 27,317 | ||||||||||||
| Loss before income taxes |
(3,757,421 | ) | (2,849,398 | ) | (5,519,348 | ) | (6,021,742 | ) | ||||||||
| Net loss |
$ | (3,757,421 | ) | $ | (2,849,398 | ) | $ | (5,519,348 | ) | $ | (6,021,742 | ) | ||||
| Basic and diluted net loss per share |
$ | (0.70 | ) | $ | (1.48 | ) | $ | (1.70 | ) | $ | (3.43 | ) | ||||
| Shares used to compute basic and diluted net loss per share |
5,398,941 | 1,930,207 | 3,244,188 | 1,753,463 | ||||||||||||
6
Table of Contents
Balance Sheet
| June 30, 2010 | |||||||
| Actual | Pro forma as adjusted(1)(2) | ||||||
| (unaudited) | |||||||
| Cash(3) |
$ | 959,434 | $ | 20,375,434 | |||
| Working capital (deficit)(3) |
(140,223 | ) | 19,275,777 | ||||
| Total assets |
1,677,877 | 21,093,877 | |||||
| Total liabilities |
1,759,600 | 1,759,600 | |||||
| Total shareholders’ equity (deficit) |
(81,723 | ) | 19,334,277 | ||||
| (1) | As adjusted to give effect to the sale by us of 3,000,000 shares of common stock at an assumed public offering price of $6.60 per share (the last sale price for our common stock as reported on the OTC Bulletin Board on September 28, 2010 immediately after our reverse stock split) and our receipt of the estimated net proceeds from the offering of $17,416,000 after deducting underwriting discounts and commissions and estimated offering expenses payable by us, including the underwriters’ non-accountable expense allowance. |
| (2) | As adjusted to give effect to the July 2010 Financing Transaction pursuant to which we (i) issued 250,000 shares of common stock through the payment of $2,000,000 in aggregate consideration consisting of (a) $1,000,000 in cash and (b) exchange and cancellation of the May 2010 Note, and (ii) entered into the Credit Facility for $2,000,000, on which we drew down $1,000,000 in September 2010. |
| (3) | Includes $742,682 of cash we have reserved for DPOLT research. |
7
Table of Contents
The offering
Set forth below is a description of the offering. Except as otherwise indicated, all information in this prospectus assumes no exercise by the underwriters of their over-allotment option and no exercise of any outstanding options and warrants; and gives effect to a 20-to-1 reverse split of our common stock.
| Common stock offered by us |
3,000,000 shares |
| Common stock to be outstanding immediately after this offering |
8,663,157 shares |
| Use of proceeds |
We expect the net proceeds from this offering will be approximately $17,416,000 (or $20,178,100 if the underwriters exercise their over-allotment option in full) based upon an assumed public offering price of $6.60 per share (the last sale price of our common stock as reported on the OTC Bulletin Board on September 28, 2010 immediately after our reverse stock split) after deducting underwriting discounts and commissions and estimated offering expenses. We intend to use the net proceeds from this offering primarily for working capital and general corporate purposes, which include, but are not limited to, commercialization of our ProBiora3 products, clinical development of SMaRT Replacement Therapy and MU1140-S, and research and development related to our other products, including our weight loss product and our biomarker discovery platform. See “Use of Proceeds” for more information. |
| Over-allotment option |
We have granted the underwriters an option for a period of 45 days to purchase up to an additional 450,000 shares of common stock to cover over-allotments, if any. |
| OTC Bulletin Board trading symbol |
ORNID.OB |
| Proposed NASDAQ Capital Market trading symbol |
OGEN |
| Risk factors |
The purchase of our common stock involves a high degree of risk. You should carefully review and consider “Risk Factors” beginning on page 9. |
The shares of common stock to be outstanding immediately after this offering as reflected in the table above is based on the actual shares of common stock outstanding as of October 4, 2010, which was 5,663,157 shares, but does not include, as of that date:
| Ø | 306,388 shares of common stock reserved for issuance upon the exercise of outstanding warrants with a weighted average exercise price of $19.20 per share; |
| Ø | 398,112 shares of common stock reserved for issuance upon the exercise of outstanding stock options with a weighted average exercise price of $7.00 per share; and |
| Ø | 223,887 shares of common stock available for future grant under our Amended and Restated 2002 Stock Option and Incentive Plan, or the 2002 Stock Incentive Plan. |
8
Table of Contents
An investment in our common stock involves a high degree of risk. You should carefully consider the risks described below and the other information in this prospectus, including our financial statements and related notes, before deciding to purchase shares of common stock in this offering. The risks described below are not the only ones facing our company. Additional risks not presently known to us or that we currently consider immaterial may also adversely affect our company. If any of the following risks actually occur, our business, financial condition and results of operations could be materially adversely affected. As a result, the trading price of our common stock could decline, and you could lose all or part of your investment.
Risks Related to Our Business
Our success will depend on our ability to obtain regulatory approval of our SMaRT Replacement Therapy and MU1140-S product candidates and their successful commercialization.
Our SMaRT Replacement Therapy and MU1140-S product candidates have not received regulatory approval in any jurisdiction and they may never receive approval or, if approvals are obtained, may never be commercialized successfully. We have incurred and will continue to incur significant costs relating to the preclinical and clinical development of our SMaRT Replacement Therapy and MU1140-S product candidates. We are currently in the process of commencing a second Phase 1 clinical trial to examine the safety and genetic stability of an attenuated version of the SMaRT strain in humans. We do not know whether our planned and current clinical trials for our SMaRT Replacement Therapy product candidate will be completed on schedule, if at all. In addition, we do not know whether any of our clinical trials will be successful or can be completed within our current expected budget. For our MU1140-S product candidate, we have performed extensive preclinical testing using native MU1140 and expect to conclude the preclinical testing of MU1140-S, including toxicity testing in rodent and non-rodent animal models, during the first half of 2011. We intend to file an Investigational New Drug, or IND, application with the FDA in mid-2011. Even if we are able to conduct successful clinical trials or the required regulatory approvals are obtained, we may never be able to generate significant revenues from our SMaRT Replacement Therapy and MU1140-S product candidates. If our SMaRT Replacement Therapy or MU1140-S product candidates are unsuccessful, we may be unable to generate sufficient revenues to sustain and grow our business, and our business, financial condition and results of operations will be materially adversely affected.
Our success will also depend on our ability to significantly increase sales of our ProBiora3 products which have only generated modest revenues to date.
Currently our sole source of product revenues is from sales of our ProBiora3 products, which began in late 2008 and have generated only modest revenues to date. Sales of our ProBiora3 products were $521,115 for the six months ended June 30, 2010 and $366,801 and $8,539 for the years ended December 31, 2009 and 2008, respectively. If we are unable to generate significant revenues from our ProBiora3 products our business, financial condition and results of operations will be materially adversely affected.
We have incurred significant losses since our inception and expect to continue to experience losses for the foreseeable future.
Since our inception, we have incurred operating losses and negative cash flow from operating activities. To achieve and maintain profitability, we must successfully develop, obtain regulatory approval for, manufacture, market and sell, or license, partner or sell the rights to, one or more of the product candidates we either license or own. Furthermore, our cash burn rate and expenses have increased significantly due to our recent commercialization initiatives with our ProBiora3 products. We expect to continue to incur losses for the foreseeable future as we expand our sales and marketing capabilities for our ProBiora3 products and continue our preclinical testing, clinical trials and research and development activities.
9
Table of Contents
Risk factors
Net losses have totaled $3,757,421 for the six months ended June 30, 2010 and $5,519,348 and $6,021,742 for the years ended December 31, 2009 and 2008, respectively. We have experienced losses from operations during the last two years and have an accumulated deficit of $29,269,304 as of June 30, 2010. We have used cash in our operating activities of $3,281,330 for the six months ended June 30, 2010 and $5,799,481 and $3,835,190 for the years ended December 31, 2009 and 2008, respectively. Our accounts payable and accrued expenses have also increased due to operational changes instituted in connection with the launch of our consumer products. We have working capital (deficit) of ($140,223) as of June 30, 2010 ($882,905 deficit when the current cash reserved for DPOLT research is excluded), and $2,564,147 and ($500,672) as of December 31, 2009 and 2008, respectively.
Our auditor has expressed substantial doubt about our ability to continue as a going concern.
In light of our recurring losses, accumulated deficit and negative cash flow, the report of our independent registered public accounting firm on our financial statements for the year ended December 31, 2009 contains an explanatory paragraph raising substantial doubt about our ability to continue as a going concern. Our financial statements do not include any adjustments that may be necessary in the event we are unable to continue as a going concern. If we are unable to establish to the satisfaction of our independent registered public accounting firm that the net proceeds from this offering will be sufficient to allow for the removal of this going concern qualification, we may need to significantly modify our operational plans for us to continue as a going concern.
Our financial results could vary significantly from quarter to quarter and are difficult to predict.
Our revenues and operating results could vary significantly from quarter to quarter due to a variety of factors, many of which are outside of our control. As a result, comparing our operating results on a period-to-period basis may not be meaningful. In addition, we may not be able to predict our future revenues or results of operations. We base our current and future expense levels on our internal operating plans and sales forecasts, and our operating costs are to a large extent fixed. As a result, we may not be able to reduce our costs sufficiently to compensate for an unexpected shortfall in revenues, and even a modest shortfall in revenues could disproportionately and adversely affect financial results for that quarter. Individual products represent meaningful portions of our revenues in any quarter. We may incur significant or unanticipated expenses associated with our research and development efforts of our product candidates under development. In addition to other risk factors discussed in this section, factors that may contribute to the variability of our quarterly results include:
| Ø | the timing of release of new products and services by us and our competitors, particularly those that may represent a significant portion of revenues in any given period; |
| Ø | the popularity of new products, and products released in prior periods; |
| Ø | changes in pricing policies by us or our competitors; |
| Ø | fluctuations in the size and rate of growth of overall consumer and retailer demand for our ProBiora3 products; |
| Ø | our success in entering new geographic markets; |
| Ø | decisions by us to incur additional expenses, such as increases in marketing or research and development; |
| Ø | the level of expenses associated with our clinical trials; |
| Ø | accounting rules governing recognition of revenues; |
| Ø | the amount we reserve against returns and allowances; and |
| Ø | the timing of compensation expense associated with equity compensation grants. |
10
Table of Contents
Risk factors
As a result of these and other factors, our quarterly and annual operating results could be materially adversely affected. Moreover, our operating results may not meet announced guidance or the expectations of research analysts or investors, in which case the price of our common stock could decrease significantly.
Current and future economic and market conditions could materially adversely affect our revenues, expense levels and profitability.
The U.S. economy and the global economy are recovering from a severe recession. Factors such as uncertainties in consumer spending, a sustained regional and/or global economic downturn or slow recovery may reduce the demand for our ProBiora3 products. Furthermore, challenging economic conditions also may impair the ability of our customers to pay for our commercial products. Because consumer spending for our ProBiora3 products can generally be considered a discretionary purchase, we may experience a more negative impact on our business due to these conditions than other companies that do not depend on discretionary spending. If demand for our ProBiora3 products declines or our customers are otherwise unable to pay for our products, we may be required to offer extensive discounts or spend more on marketing than budgeted and our revenues, expense levels, and profitability will be materially adversely affected.
Sales of our ProBiora3 products may be adversely affected by fluctuations in buying decisions of our retailers and consolidation among retailers.
Our ProBiora3 products are sold to national and regional retailers in the United States. Our revenues could be affected by fluctuations in the buying patterns of these customers, which may result from wholesale buying decisions, economic conditions and other factors. In addition, with the growing trend towards retail consolidation, we are increasingly dependent upon a limited number of leading retailers with greater bargaining strength. Such retailers have demanded, and may continue to demand, increased service and order accommodations as well as price and incremental promotional investment concessions. As a result, we may face pressure on our prices and experience increased expenses from promotions to meet these demands, which would reduce our profitability. We also may be negatively affected by changes in the policies of our customers such as inventory destocking, limitations on access to shelf space and other conditions.
Our agreements with large national mass retailers with respect to our ProBiora3 products may be delayed, terminated or reduced in scope with little or no notice, which could adversely impact our profitability.
Our agreements with large national mass retailers with respect to our ProBiora3 products may be terminated or reduced in scope with little or no notice. Cancellations may occur for a variety of reasons, including the failure of our products to satisfy safety requirements, and unexpected health consequences of our products. Agreements with national mass retailers may provide for rights of return that are unfavorable to us and may require us to adjust our future estimates of returns and allowances. We have vendor agreements whereby the vendors reserve the right to cancel a purchase order without penalty by providing notice to us on or before the given cancellation date and at any time if the completion or delivery date is not met.
We are subject to government regulation of the processing, formulation, packaging, labeling and advertising of our consumer products.
Under the Federal Food, Drug, and Cosmetic Act, or FDCA, companies that manufacture and distribute foods, such as our ProBiora3 products, are limited in the claims that they are permitted to make about nutritional support on the product label without FDA approval. Any failure by us to adhere to the labeling requirements could lead to the FDA’s requiring that our products be repackaged and relabeled, which would have a material adverse effect on our business. In addition, companies are responsible for the accuracy and truthfulness of, and must have substantiation for, any such statements. These claims must be truthful and not misleading. Statements must not claim to diagnose, mitigate, treat, cure or prevent a specific disease
11
Table of Contents
Risk factors
or class of disease. We are able to market our ProBiora3 products in reliance on the self-affirmed Generally Recognized As Safe, or GRAS, status of our active ingredient, ProBiora3. No governmental agency or other third party has made a determination as to whether or not ProBiora3 has achieved GRAS status. We make this determination based on independent scientific opinions that ProBiora3 is not harmful under its intended conditions of use. If the FDA, another regulatory authority or other third party denied our self-affirmed GRAS status for ProBiora3, we could face significant penalties or be required to undergo the regulatory approval process in order to market our probiotic products, and our business, financial condition and results of operations will be adversely affected. We cannot guarantee that in such a situation ProBiora3 would be approved.
The FDA’s Good Manufacturing Practices, or GMPs, describe the methods, equipment, facilities, and controls for producing processed food and set the minimum sanitary and processing requirements for producing safe and wholesome food. Those who manufacture, package, or hold human food must comply with the GMPs. If we or our suppliers fail to comply with the GMPs, the FDA may take enforcement action against us or our suppliers.
The processing, formulation, packaging, labeling and advertising of our probiotic products are subject to regulation by one or more federal agencies including the FDA, the Federal Trade Commission and the Environmental Protection Agency. Our activities are also subject to regulation by various agencies of the states and localities in which our probiotic products are sold. Any changes in the current regulatory environment could impose requirements that would limit our ability to market our probiotic products and make bringing new products to market more expensive. In addition, the adoption of new regulations or changes in the interpretation of existing regulations may result in significant compliance costs or discontinuation of product sales and may adversely affect our business, financial condition and results of operations. While our ProBiora3 products are categorized as foods, it is possible that the FDA or a state regulatory agency could classify these products as a cosmetic or a drug. If the products are classified as cosmetics rather than a food, we would be limited to making claims that the products cleanse and beautify, but we could not make structure or function claims. If our probiotic products are classified as drugs, we would not be able to market the ProBoira3 products without going through the drug approval process. Either of these events would limit our ability to effectively market our products and would adversely affect our financial condition and results of operations. If the FDA or a state regulatory agency viewed the products as cosmetics or drugs, they could claim that the products are misbranded and require that we repackage and relabel the products and impose civil and/or criminal penalties. Either or both of these situations could adversely affect our business and operations.
If we undertake product recalls or incur liability claims with respect to our ProBiora3 products, such recalls or claims could increase our costs and adversely affect our reputation, revenues and operating income.
Our ProBiora3 products are designed for human and animal consumption and we may face product recalls or liability claims if the use of our products is alleged to have resulted in injury or death. ProBiora3 is classified as a food ingredient and is not subject to pre-market regulatory approval in the United States. Our ProBiora3 products contain ingredients that do not have long histories of human or animal consumption. Previously unknown adverse reactions resulting from consumption of these ingredients could occur. We may have to undertake various product recalls or be subject to liability claims, including, among others, that our ProBiora3 products include inadequate instructions for use or inadequate warnings concerning possible side effects and interactions with other substances. A product recall or liability claim against us could result in increased costs and could adversely affect our reputation with our customers, which, in turn, could have a material adverse effect on our business, financial condition and results of operations.
Product liability insurance is expensive, is subject to deductibles and coverage limitations, and may not be available in the future. While we currently maintain product liability insurance coverage, we cannot be sure that such coverage will be adequate to cover any incident or all incidents. In addition, we cannot be sure that we will be able to obtain or maintain insurance coverage at acceptable costs or in a sufficient amount, that our insurer will not disclaim coverage as to a future claim or that a product liability claim would not otherwise adversely affect our business, financial condition and results of operations. The cost of any product liability litigation or other proceeding, even if resolved in our favor, could be substantial. Uncertainties resulting from the initiation and continuation of product liability litigation or other proceedings could have a
12
Table of Contents
Risk factors
material adverse effect on our ability to compete in the marketplace. Product liability litigation and other related proceedings may also require significant management attention.
If we fail to comply with our obligations in the agreements with the University of Florida Research Foundation, Inc., under which we license our rights to SMaRT Replacement Therapy and MU1140, our licenses to these product candidates may be terminated and we will be unable to commercialize these products candidates.
We hold our SMaRT Replacement Therapy and MU1140 product candidates under licenses from the University of Florida Research Foundation, Inc., or UFRF. Under the terms of the licenses, we must spend at least $1,000,000 per year on development of those product candidates until the first commercial sale of products derived from those product candidates has occurred. In addition, we must pay $25,000 per quarter as minimum royalties to the UFRF under our license agreements. The UFRF may terminate our licenses to SMaRT Replacement Therapy and MU1140 if we breach our obligations to timely pay these amounts, to submit development reports as required under the license agreements or commit any other breach of any other covenants contained in the license agreements. There is no assurance that we will be able to comply with these conditions in a timely manner or at all. If our license agreements are terminated, we will be unable to commercialize these product candidates.
Until commercial sale of any products developed from these licensed product candidates takes place, we will not earn any revenues from these product candidates and will, therefore, need additional financing to fund our required royalty payments and development expenses, such as through the commercialization and sale of our ProBiora3 products, the sale of our debt or equity securities, or otherwise. There can be no assurance that we will achieve sufficient sales of our ProBiora3 products or be able to raise the capital necessary to meet our obligations under our licenses. If we are unable to meet our obligations under these licenses, we may lose the licenses to these product candidates and our business, financial condition and results of operations will be materially adversely affected.
We depend on third-party manufacturers for our ProBiora3 products. The loss of any manufacturer or any interruptions in the supply of our ProBiora3 products, would have a negative impact on our revenues and profitability.
We currently have no manufacturing facilities and are dependent upon establishing relationships with independent manufacturers to supply our product needs. We have contracted with multiple GMP-certified manufacturers to produce our active ingredient, ProBiora3, under GMPs. We believe our arrangements with our contract manufacturers have the capacity to meet our current and expected future manufacturing needs. Although we have qualified and used at least two contract manufacturers for each step in our manufacturing process, we do not have a long-term supply agreement or commitment with any of our manufacturers. If our manufacturers are unable or unwilling to produce our ProBiora products in sufficient quantities, or at all, at acceptable pricing in accordance with specifications we establish from time to time we would need to find alternative manufacturers that are qualified. If, in such instances, we are unsuccessful in obtaining alternative manufacturers, it could impair our ability to sell our ProBiora3 products and would have a negative impact on our revenues and profitability. In addition, competitors who own their manufacturing facilities may have an advantage over us with respect to pricing, availability of product and in other areas through their control of the manufacturing process.
The risks associated with the numerous factors that could cause interruptions in the supply of our products, including manufacturing capacity limitations, regulatory inspections, changes in our sources for manufacturing, disputes with a manufacturer, our failure to timely locate and obtain replacement manufacturers as needed and conditions affecting the cost and availability of raw materials, are magnified when the suppliers are limited in number. Any interruption in the supply of finished products could hinder our ability to timely distribute our products and satisfy customer demand. If we are unable to obtain adequate product supplies to satisfy our customers’ orders, we may lose those orders, our customers may cancel other orders, and they may choose instead to stock and purchase competing products. This could result in a loss of our market share and negatively affect our revenues and profitability.
13
Table of Contents
Risk factors
If our manufacturers in general fail to meet our requirements and the requirements of regulatory authorities, our revenues and profitability may be materially adversely affected.
We do not have the internal capability to manufacture our ProBiora3 products or our SMaRT Replacement Therapy and MU1140-S product candidates under GMPs, as required by the FDA and other regulatory agencies. In order to continue to develop our product candidates, apply for regulatory approvals for our SMaRT Replacement Therapy and MU1140-S product candidates, and commercialize our ProBiora3 products and other product candidates, we will need to contract with third parties that have, or otherwise develop, the necessary manufacturing capabilities.
There are a limited number of manufacturers that operate under GMPs that are capable of manufacturing our ProBiora3 products and SMaRT Replacement Therapy product candidate. Furthermore, manufacturing MU1140-S on a commercial scale has not yet been achieved, so there are additional technical skills needed for the manufacture of MU1140-S that will further limit the number of potential manufacturers. As such, if we are unable to enter into supply and processing contracts with any of these manufacturers or processors for our ProBiora3 products or our development stage product candidates, we may incur additional costs and delays in development and commercialization.
Problems with these manufacturing processes such as equipment malfunctions, facility contamination, labor problems, raw material shortages or contamination, natural disasters, power outages, terrorist activities, or disruptions in the operations of our suppliers and even minor deviations from normal procedures, could result in product defects or manufacturing failures that result in lot failures, product recalls, product liability claims and insufficient inventory.
If we are required to find an additional or alternative source of supply, there may be additional costs and delays in the development or commercialization of our product candidates. Additionally, the FDA and other regulatory agencies routinely inspect manufacturing facilities before approving a New Drug Application, or NDA, or Biologic License Application, or BLA, for a drug or biologic manufactured at those sites. If any of our manufacturers or processors fails to satisfy regulatory requirements, the approval and eventual commercialization of our commercial products and product candidates may be delayed.
All of our contract manufacturers must comply with the applicable GMPs, which include quality control and quality assurance requirements as well as the corresponding maintenance of records and documentation. If our contract manufacturers do not comply with the applicable GMPs and other FDA regulatory requirements, we may be subject to product liability claims, the availability of marketed products for sale could be reduced, our product commercialization could be delayed or subject to restrictions, we may be unable to meet demand for our products and may lose potential revenues and we could suffer delays in the progress of preclinical testing and clinical trials for products under development. We do not have control over our third-party manufacturers’ compliance with these regulations and standards. Moreover, while we may choose to manufacture ProBiora3 products ourselves in the future, we have no experience in the manufacture of pharmaceutical products for clinical trials or commercial purposes. If we decide to manufacture products, we would be subject to the regulatory requirements described above. In addition, we would require substantial additional capital and would be subject to delays or difficulties encountered in manufacturing pharmaceutical products. Regardless of the manufacturer of our products, we will be subject to continuing obligations regarding the submission of safety reports and other post-market information.
We may be unable to find a method to produce MU1140-S in large-scale commercial quantities. If we cannot, we will be unable to generate revenues from sales of our MU1140-S product candidate.
Our antibiotic product candidate, MU1140-S, is a synthetic form of MU1140 produced by our strain of S. mutans. To date, it has been produced only in laboratory cultures. In March 2005 we successfully developed a methodology for producing MU1140 in quantities sufficient to undertake its preclinical testing. In addition, we developed the DPOLT synthetic chemistry methodology to allow large-scale commercial production of the MU1140-S antibiotic. However, this methodology may not be feasible for cost effective, large scale manufacture. If we are not able to utilize this methodology for large-scale manufacture, we will be unable to generate revenues from this product candidate and our business, financial condition and results of
14
Table of Contents
Risk factors
operations will be materially adversely affected. We have retained Almac Sciences to refine and scale-up GMP production of MU1140-S. The manufacturing of MU1140-S is a highly exacting and complex process. Manufacturing MU1140-S on a commercial scale has not yet been achieved so there are additional risks. Third-party manufacturers must have additional technical skills and must take multiple steps to attempt to control the manufacturing processes.
Our ProBiora3 products and our SMaRT Replacement Therapy and MU1140-S product candidates face various forms of competition from other products in the marketplace.
The pharmaceutical and biotechnology industries are characterized by intense competition, rapid product development and technological change. Most of the competition that our ProBiora3 products and our SMaRT Replacement Therapy and MU1140-S product candidates face comes from companies that are large, well established and have greater financial, marketing, sales and technological resources than we have. Our ProBiora3 products compete with a range of consumer and nutraceutical products. Our commercial success with SMaRT Replacement Therapy and MU1140-S will depend on our ability and the ability of any sub-licensees to compete effectively in marketing and product development areas including, but not limited to, sales and branding, drug safety, efficacy, ease of use, patient or customer compliance, price, marketing and distribution. There can be no assurance that competitors will not succeed in developing and marketing products that are more desirable or effective than our ProBiora3 products or the products developed from our product candidates or that would render our products obsolete and non-competitive.
We anticipate that our SMaRT Replacement Therapy, if approved for the treatment of tooth decay, would compete with other companies attempting to develop technologies in the oral health care market, including vaccines.
We rely on the significant experience and specialized expertise of our senior management and scientific team.
Our performance is substantially dependent on the continued services and on the performance of our senior management and scientific team, who have extensive experience and specialized expertise in our business. Our performance also depends on our ability to retain and motivate our other key employees. The loss of the services of our Chief Executive Officer and President, David Hirsch, our Chief Scientific Officer, Dr. Jeffrey Hillman, and our Chief Financial Officer, Brian Bohunicky, and any members of our scientific team, could harm our ability to develop and commercialize our product candidates. We have no key man life insurance policies. We have employment agreements with Mr. Hirsch, Dr. Hillman and Mr. Bohunicky. The term of each of these employment agreements is for an indefinite period and each employment agreement shall end when the employment relationship is terminated by either party.
We need to hire and retain additional qualified scientists and other highly skilled personnel to maintain and grow our business.
Our future success depends on our ability to identify, attract, hire, train, retain and motivate highly skilled technical, managerial and research personnel in all areas within our organization. We plan to continue to grow our business and will need to hire additional personnel to support this growth. We believe that there are only a limited number of individuals with the requisite skills to serve in many of our key positions, and we compete for key personnel with other biotechnology companies, as well as universities and research institutions. It is often difficult to hire and retain these persons, and we may be unable to replace key persons if they leave or be unable to fill new positions requiring key persons with appropriate experience. If we fail to attract, integrate and retain the necessary personnel, our ability to maintain and grow our business could suffer significantly.
If our SMaRT Replacement Therapy and MU1140-S product candidates are shown to be ineffective or harmful in humans, we will be unable to generate revenues from these product candidates.
Before obtaining regulatory approvals for the commercial sale of our SMaRT Replacement Therapy or MU1140-S product candidates, we must demonstrate through preclinical testing and clinical trials that our products are safe and effective for use
15
Table of Contents
Risk factors
in humans. To date, the testing of our SMaRT Replacement Therapy product candidate has been undertaken solely in animals and a limited number of humans. Studies have proven our genetically altered strain of S. mutans to be effective in preventing tooth decay in animals. It is possible that our strain of S. mutans will be shown to be less effective in preventing tooth decay in humans in clinical trials. If our SMaRT Replacement Therapy product candidate is shown to be ineffective in preventing tooth decay in humans, we will be unable to commercialize and generate revenues from this product candidate. To date the testing of the antibiotic substance, MU1140, has been undertaken solely in the laboratory and in animals. We have not yet conducted human studies of MU1140-S. It is possible that when these studies are conducted, they will show that MU1140-S is ineffective or harmful in humans. If MU1140-S is shown to be ineffective or harmful in humans, we will be unable to commercialize and generate revenues from sales of this compound. If we are unable to generate revenues from our SMaRT Replacement Therapy and MU1140-S product candidates, our business, financial condition and results of operations will be materially adversely affected.
We intend to rely on third parties to pay the majority of the costs associated with obtaining regulatory approval for, and manufacturing and marketing of, our MU1140-S and SMaRT Replacement Therapy product candidates. If we are unable to obtain agreements with third parties to fund these costs, we will have to fund such costs ourselves or we may be unable to continue our operations.
Assuming the successful completion of Phase 1 trials for our MU1140-S and SMaRT Replacement Therapy product candidates, we intend to either license these product candidates to, or partner with, one or more major pharmaceutical companies prior to commercialization. If we do so, we intend for these licensees or partners to pay the costs associated with our remaining clinical trials and the manufacturing and marketing of our product candidates. If we are unable to license our product candidates or otherwise partner with third parties, we will have to fund the costs of our Phase 2 and Phase 3 trials ourselves. We will also have to establish our own manufacturing facilities and find our own distribution channels. This would greatly increase our future capital requirements and we cannot assure you that we would be able to obtain the necessary financing to pay these costs or that we will be generating significant revenues from any of our products, such as ProBiora3, sufficient to cover the associated costs. If we do not generate sufficient revenues to cover the associated costs or we cannot obtain financing on acceptable terms or at all, our business, financial condition and results of operations will be materially adversely affected.
Our dependence on collaborative arrangements with third parties subjects us to a number of risks. These collaborative arrangements may not be on terms favorable to us. Agreements with collaborative partners typically allow partners significant discretion in electing whether or not to pursue any of the planned activities. We cannot control the amount and timing of resources our collaborative partners may devote to products based on the collaboration, and our partners may choose to pursue alternative products. Our partners may not perform their obligations as expected. Business combinations or significant changes in a collaborative partner’s business strategy may adversely affect a partner’s willingness or ability to complete its obligations under the arrangement. Moreover, we could become involved in disputes with our partners, which could lead to delays or termination of the collaborations and time-consuming and expensive litigation or arbitration. Even if we fulfill our obligations under a collaborative agreement, our partner may be able to terminate the agreement under certain circumstances. If any collaborative partner were to terminate or breach our agreement with it, or otherwise fail to complete its obligations in a timely manner, our chances of successfully commercializing our product candidates would be materially and adversely affected.
We may require additional financing to complete the development of and to commercialize our SMaRT Replacement Therapy and MU1140-S product candidates and we do not know if additional financing will be available to us when and if needed, or, if available, on terms that we find acceptable, particularly given the current and potential future strain in the financial and credit markets.
Our operations have required substantial capital funding since inception and we expect to continue to spend substantial amounts to develop and commercialize our SMaRT Replacement Therapy and MU1140-S product candidates. We may need
16
Table of Contents
Risk factors
substantial additional funding and may be unable to raise capital when needed or on attractive terms, which would force us to significantly delay, scale back or discontinue the development or commercialization of our product candidates. Changing circumstances may cause us to use capital significantly faster than we currently anticipate, and we may incur higher expenses than currently expected because of circumstances beyond our control. If we are not able to raise additional capital and we are not generating positive cash flow from our ProBiora3 products and are unable to commercialize our product candidates, we may be unable to pursue further development of our product candidates, be forced to divest our product candidates prior to maximizing their potential value, be unable to maintain the licenses for our SMaRT Replacement Therapy and MU1140-S product candidates, or be forced to significantly scale back or cease our operations.
Other than our $2,000,000 Credit Facility with the KFLP, we have no other committed sources of capital and do not know whether additional financing will be available to us when and if needed, or, if available, that the terms will be acceptable to us, particularly if the financial and credit markets continue to be constrained.
We may seek additional financing through public or private equity offerings or through arrangements with strategic third parties. If we raise additional financing by issuing equity securities, further dilution to existing stockholders may result. In addition, as a condition to providing additional financing to us, future investors may demand, and may be granted, rights superior to those of existing stockholders. If we raise additional financing through arrangements with strategic third parties, we may be required to relinquish rights to or sell certain of our product candidates or products that we would not otherwise relinquish or sell.
We may seek additional financing through long-term debt and lines of credit or through the issuance of debt securities. If we raise additional financing through borrowing or the issuance of debt securities, our debt service obligations may be significant. If we are unable to generate sufficient cash to meet these debt service obligations, we will need to use existing cash or liquidate investments in order to fund these obligations and to repay our debt, which could force us to delay or terminate our research, development and commercialization efforts.
We are subject to risks of doing business internationally as we attempt to expand our sales through international distributor relationships.
We recently entered into a number of international distributor agreements for our ProBiora3 products. As a result, we expect to increase our revenues from international sales. A number of factors can slow or prevent international sales, or substantially increase the cost of international sales, and we may encounter certain risks of doing business internationally including the following:
| Ø | reduced protection and enforcement for our intellectual property rights; |
| Ø | unexpected changes in, or impositions of, legislative or regulatory requirements that may limit our ability to sell our products and repatriate funds to the United States; |
| Ø | political and economic instability; |
| Ø | fluctuations in foreign currency exchange rates; |
| Ø | difficulties in developing and maintaining distributor relationships in foreign countries; |
| Ø | difficulties in negotiating acceptable contractual terms and enforcing contractual obligations; |
| Ø | exposure to liabilities under the U.S. Foreign Corrupt Practices Act; |
| Ø | potential trade restrictions and exchange controls; |
17
Table of Contents
Risk factors
| Ø | creditworthiness of foreign distributors, customer uncertainty and difficulty in foreign accounts receivable collection; and |
| Ø | the burden of complying with foreign laws. |
As we attempt to expand our sales internationally, our exposure to these risks could result in our inability to attain the anticipated benefits and our business could be adversely impacted. Our success will depend, in large part, on our ability to anticipate and effectively manage these and other risks associated with our international operations. However, any of these factors could adversely affect our international operations and, consequently, our operating results.
If our intellectual property rights do not adequately protect our products or product candidates, or if third parties claim we are infringing their intellectual property rights, others could compete against us more directly or we could be subject to significant litigation. Such results could prevent us from marketing our products or product candidates and hurt our profitability.
Our product portfolio is protected by eight issued U.S. patents and eight filed U.S. patent applications, including patents exclusively licensed from the University of Florida Research Foundation, Inc., or the UFRF. We are the exclusive worldwide licensee to the patents for SMaRT Replacement Therapy and MU1140, which are owned by the UFRF. Our success depends in part on our ability to obtain patents or rights to patents, protect trade secrets, operate without infringing upon the proprietary rights of others, and prevent others from infringing on our patents, trademarks and other intellectual property rights. We will be able to protect our intellectual property from unauthorized use by third parties only to the extent that it is covered by valid and enforceable patents, trademarks and licenses. Patent protection generally involves complex legal and factual questions and, therefore, enforceability of patent rights cannot be predicted with certainty. Patents, if issued, may be challenged, invalidated or circumvented. Thus, any patents that we own or license from others may not provide adequate protection against competitors. In addition, any future patent applications may fail to result in patents being issued. Also, those patents that are issued may not provide us with adequate proprietary protection or competitive advantages against competitors with similar product candidates. Moreover, the laws of certain foreign countries do not protect intellectual property rights to the same extent as do the laws of the United States.
In addition to patents and trademarks, we rely on trade secrets and proprietary know-how. We seek protection of these rights, in part, through confidentiality and proprietary information agreements. These agreements may not provide meaningful protection or adequate remedies for violation of our rights in the event of unauthorized use or disclosure of confidential and proprietary information. Failure to protect our proprietary rights could seriously impair our competitive position.
In the event of an infringement or violation, we may face litigation and may be prevented from pursuing product development or commercialization. We may receive in the future, notice of claims of infringement of other parties’ proprietary rights. Infringement or other claims could be asserted or prosecuted against us in the future and it is possible that past or future assertions or prosecutions could harm our business.
The FDA instituted numerous clinical holds with respect to our Phase 1 clinical trial program for our SMaRT Replacement Therapy product candidate. Although the clinical holds were lifted with respect to the attenuated version of the SMaRT strain, a clinical hold remains in effect for our IND for the non-attenuated SMaRT strain. If the FDA does not lift the clinical hold on this IND, we will be unable to conduct the clinical trials necessary to obtain marketing approval of our product candidate. The FDA’s concerns that resulted in these clinical holds may lead to the imposition of a new clinical hold or holds or the failure of our product candidates to obtain marketing approval.
In December 1998, the FDA imposed a clinical hold on the IND for our SMaRT strain. The FDA expressed concerns about the potential for DNA transfer into or from the SMaRT strain; the possible creation of an organism with increased ability to
18
Table of Contents
Risk factors
colonize and produce dental caries; and the potential for the transfer of the strain between humans. We met with the FDA to discuss proposed trials of an attenuated SMaRT strain to address the FDA’s concerns, and the FDA informed us that such trials required a separate IND. We submitted an IND for the attenuated strain in March 2003.
The FDA then imposed multiple clinical holds with respect to the Phase 1 clinical trials of the attenuated strain. Specifically, we received clinical hold letters in May 2003, February 2004, June 2007 and August 2007. In connection with the clinical hold letter in May 2003, the FDA requested that we provide preclinical data on eradication of the attenuated version of the SMaRT strain once it had been implanted and a plan for the eradication of the attenuated strain in the trial subjects and their spouse or partners. In an April 2004 meeting, the FDA suggested that we conduct two eradication trials: one in subjects without teeth, and, if successful, another trial in subjects with teeth. We submitted revised protocols in accordance with FDA’s suggestions, and in November 2004 the FDA lifted the clinical hold for the attenuated strain.
We initiated our first Phase 1 clinical trial in April 2005, but we found it difficult to find subjects who fit the FDA’s highly cautious inclusion and exclusion criteria, particularly with respect to the subjects’ lack of dentition. We concluded this trial early after enrolling only two of the 15 planned subjects. The FDA then recommended that we revise the protocol for the evaluation of ten healthy male subjects, ranging from 18 to 30 years old and with normal dentition, in an institutionalized setting. After we submitted additional information, the FDA issued another clinical hold letter in June 2007 for the proposed trial with the attenuated strain, citing the need for a plan with respect to serious adverse effects; a plan for the eradication of the attenuated strain in trial subjects’ offspring; and a required pregnancy test for female partners of subjects. We submitted additional protocols in response to the FDA’s concerns. In August 2007, the FDA issued another clinical hold letter with required revisions to the protocol for offspring of subjects. We submitted a response to the clinical hold letter in September 2007, and the FDA removed the clinical hold for our Phase 1 trial in the attenuated strain in October 2007.
We plan to discuss with the FDA whether the clinical hold for the non-attenuated SMaRT strain can be lifted after the completion of our second Phase 1 clinical trial using the attenuated strain, because we believe the results from the trial may address the FDA’s concerns with the non-attenuated SMaRT strain. However, there is no guarantee that our clinical trials for the attenuated strain will be successful, or that the FDA will ever lift the clinical hold on the non-attenuated SMaRT strain. If the FDA does not lift the clinical hold on the non-attenuated SMaRT strain, we cannot commence our anticipated third Phase 1 trial and we may not be able to conduct the clinical trials necessary to obtain marketing approval of the SMaRT strain.
The FDA may impose additional requirements that may significantly increase the time and expense of obtaining FDA approval. Although the FDA has removed the clinical holds with respect to our Phase 1 trials for the attenuated strain, if the FDA has further concerns regarding the safety or risks associated with our trials, the FDA could implement another clinical hold on our ongoing trial or future trials or raise concerns as a part of a future NDA review process for our SMaRT Replacement Therapy product candidate, which could delay or prevent marketing.
Our SMaRT Replacement Therapy and MU1140-S product candidates are subject to substantial government regulation, including the regulation of preclinical testing and clinical trials. If we are unable to obtain regulatory approval for our product candidates we will be unable to generate revenues.
The production and marketing of products which may be developed from our SMaRT Replacement Therapy and MU1140-S product candidates and our ongoing research and development, preclinical testing and clinical trial activities are subject to extensive regulation and review by numerous governmental authorities. Most of the product candidates we are developing must undergo rigorous preclinical testing and clinical trials and an extensive regulatory approval process before they can be marketed in the United States or internationally.
The FDA accepted our protocols to conduct Phase 1 human clinical trials of our SMaRT Replacement Therapy product candidate. We expect to file an IND application with the FDA in mid-2011 for our MU1140-S product candidate. If we fail to maintain regulatory approval for the clinical trials of our SMaRT Replacement Therapy, if the FDA fails to lift the clinical hold
19
Table of Contents
Risk factors
on our IND for the non-attenuated version of the SMaRT strain, or if we fail to obtain regulatory approval for our MU1140-S product candidate, we may have to cease further development. Clinical trials on our SMaRT Replacement Therapy and MU1140-S product candidates are expected to take several years to fully complete. The commencement or completion of preclinical studies or clinical trials can be delayed or prevented for a number of reasons, including:
| Ø | our belief that SMaRT Replacement Therapy is one of the first genetically modified bacterial strains for use in humans, which may cause the FDA to proceed with additional caution; |
| Ø | findings in preclinical studies; |
| Ø | difficulties obtaining regulatory approval to commence a clinical trial or complying with conditions imposed by a regulatory authority regarding the scope or term of a clinical trial; |
| Ø | delays in reaching or failing to reach agreement on acceptable terms with prospective contract research organizations, or CROs, and trial sites, the terms of which can be subject to extensive negotiation and may vary significantly among different CROs and trial sites; |
| Ø | insufficient or inadequate supply or quality of a product candidate or other materials necessary to conduct our clinical trials; |
| Ø | difficulties obtaining institutional review board, or IRB, approval to conduct a clinical trial at a prospective site; |
| Ø | challenges recruiting and enrolling patients to participate in clinical trials for a variety of reasons, including the size and nature of patient population, proximity of patients to clinical sites, eligibility criteria for the trial, nature of the trial protocol, the availability of approved effective treatments for the relevant condition and competition from other clinical trial programs for similar indications; |
| Ø | severe or unexpected drug or biologic-related side effects experienced by patients in a clinical trial; and |
| Ø | difficulties retaining patients who have enrolled in a clinical trial but may be prone to withdraw due to rigors of the trial, lack of efficacy, side effects, or personal issues, or who are lost to further follow up. |
Clinical trials also may be delayed or terminated as a result of ambiguous or negative interim results. In addition, a clinical trial may be suspended or terminated by us, the FDA, the IRBs at the sites where the IRBs are overseeing a trial, or a data safety monitoring board, or DSMB, overseeing the clinical trial at issue, or other regulatory authorities due to a number of factors, including:
| Ø | failure to conduct the clinical trial in accordance with regulatory requirements or our clinical protocols; |
| Ø | inspection of the clinical trial operations or trial sites by the FDA or other regulatory authorities; |
| Ø | unforeseen safety issues or lack of effectiveness; and |
| Ø | lack of adequate funding to continue the clinical trial. |
We cannot assure you that clinical trials will demonstrate the safety or effectiveness of our SMaRT Replacement Therapy or MU1140-S product candidates, or will otherwise satisfy regulatory requirements. Our preclinical studies or clinical trials may produce negative or inconclusive results, there may be inconsistencies between early clinical trial results and results obtained in later clinical trials. and we may decide, or regulators may require us, to conduct additional preclinical studies or clinical
20
Table of Contents
Risk factors
trials. Moreover, preclinical and clinical data are often susceptible to varying interpretations and analyses, and many companies that have believed their product candidates performed satisfactorily in preclinical studies and clinical trials have nonetheless failed to obtain FDA approval for their products. If we are unable to resolve the FDA’s concerns, we will not be able to obtain regulatory approval for these product candidates.
The pre-marketing approval process can be particularly expensive, uncertain and lengthy, and a number of products for which FDA or other governmental regulatory approval has been sought by other companies have never been approved for marketing. In addition to testing and approval procedures, extensive regulations also govern marketing, manufacturing, distribution, labeling, and record-keeping procedures. If we do not comply with applicable regulatory requirements, such violations could result in warning letters, non-approval, suspensions of regulatory approvals or ongoing clinical trials, civil penalties and criminal fines, product seizures and recalls, operating restrictions, injunctions, and criminal prosecution.
We may encounter such delays and rejection of our product candidates by the FDA or other regulatory authority may also adversely affect our business. Such delays or rejection may be encountered due to, among other reasons, government or regulatory delays, lack of efficacy during clinical trials, unforeseen safety issues, or changes in regulatory policy during the period of product development. More stringent regulatory approval processes in product clearance and enforcement activities could result in our experiencing longer approval cycles, more uncertainty, greater risk, and higher expenses. Even if regulatory approval of a product is granted, this approval may entail limitations on uses for which the product may be labeled and promoted. It is possible, for example, that we may not receive FDA approval to market products based on our licensed, patented product candidates for different indications or to market updated products that represent extensions of our basic product candidates. In addition, we may not receive FDA approval to export our products based on our licensed, patented product candidates in the future, and countries to which products are to be exported may not approve them for import.
From time to time, legislative or regulatory proposals are introduced that could alter the review and approval process relating to our product candidates. It is possible that the applicable regulatory authority will issue additional regulations further restricting the sale of our product candidates. Any change in legislation or regulations that govern the review and approval process relating to our future product candidates could make it more difficult and costly to obtain approval for new products based on our product candidates, or to produce, market, and distribute such products if approved.
We cannot assure you that the market and consumers will accept our product candidates. If they do not, we will be unable to generate significant revenues from our product candidates and our business, financial condition and results of operations will be materially adversely affected.
The commercial success of our MU1140-S and SMaRT Replacement Therapy, ProBiora3, LPT3-04 and other product candidates will depend in part on market acceptance by consumers. Biopharmaceutical companies have received substantial support from the scientific community, regulatory agencies and many governmental officials in the United States and internationally. Future scientific developments, media coverage and political events may diminish such support. Public attitudes may be influenced by claims that health products are unsafe for consumption or pose unknown risks to the environment or to traditional social or economic practices. Securing governmental approvals for, and consumer confidence in, such products poses numerous challenges, particularly outside the United States. The market success of product candidates developed by biopharmaceutical companies could be delayed or impaired in certain geographical areas as a result. Products based on our product candidates may compete with a number of traditional dental therapies and drugs manufactured and marketed by major pharmaceutical companies and biotechnology companies. Market acceptance of products based on our product candidates will depend on a number of factors including potential advantage over alternative treatment methods. We can offer you no assurance that dentists, physicians, patients or the medical and dental communities in general will accept and utilize products developed from our product candidates. If they do not, we may be unable to generate significant revenues from our product candidates and our business, financial condition and results of operations will be materially adversely affected.
21
Table of Contents
Risk factors
If our products do not receive favorable third-party reimbursement, or if new restrictive legislation is adopted, market acceptance of our products may be limited and we may not generate significant revenues.
Our ability to commercialize our products will depend in part on the extent to which appropriate reimbursement levels for the cost of our proposed formulations and products and related treatments are obtained by governmental authorities, private health insurers and other organizations, such as Health Maintenance Organizations, or HMOs. Reimbursement from third parties depends greatly on our ability to present data which demonstrate positive outcomes and reduced utilization of other products or services as well as cost data which show that treatment costs using the new product are equal to or less than what is currently covered for other products. If our products do not receive favorable third-party reimbursement and patients are unwilling or unable to pay for our products out-of-pocket, it could limit our revenues and harm our business.
The continuing efforts of government and insurance companies, health maintenance organizations and other payers of healthcare costs to contain or reduce costs of health care may affect our future revenues and profitability, and the future revenues and profitability of our potential customers, suppliers and collaborative partners and the availability of capital. For example, in certain foreign markets, pricing or profitability of prescription pharmaceuticals is subject to government control. In the United States, recent federal and state government initiatives have been directed at lowering the total cost of health care. In March 2010, President Obama signed into law the Patient Protection and Affordable Care Act, a sweeping law intended to broaden access to health insurance, reduce or constrain the growth of healthcare spending, enhance remedies against fraud and abuse, add new transparency requirements for healthcare and health insurance industries, impose new taxes and fees on the health industry and impose additional health policy reforms. Federal and state legislatures will likely continue to focus on health care reform, controlling the cost of prescription pharmaceuticals and on the reform of the Medicare and Medicaid systems. While we cannot predict whether any such legislative or regulatory proposals will be adopted, the announcement or adoption of such proposals could materially harm our business, financial condition and results of operations.
Our ProBiora3 products are sold subject to a right of return to mass retailers. If our estimates for returned products are incorrect, there could be a materially adverse impact on our net revenues and results of operations.
Our ProBiora3 products may be sold to mass retailers with a right of return, which is a common practice in the mass retail channel. For example, a right of return may be granted when the shelf life has reached its expiration or the product has remained unsold for a period of time. We are required to estimate the amount of product that will ultimately be returned pursuant to our return policy and to record a related reserve at the time of sale. These amounts are deducted from our gross revenues to determine our net revenues. In order to reasonably estimate future returns, we will analyze both quantitative and qualitative information including, but not limited to, actual return rates by product, the level of product in the distribution channel, expected shelf life of the product, product demand, the introduction of competitive or generic products that may erode current demand, our new product launches and general economic and industry wide indicators. There are inherent limitations in estimating future product returns due to the time lapse between sale and actual return of the product. If we over- or under-estimate the amount of product that will ultimately be returned, there could be a material impact to our results of operations. We are just beginning our roll out of ProBiora3 products on a larger scale to mass retailers and we have limited data to determine the expected returns in future periods in situations when we may grant rights of return.
If we fail to maintain an effective system of internal controls, we may not be able to accurately report our financial results or prevent fraud which could subject us to regulatory sanctions, harm our business and operating results and cause the trading price of our stock to decline.
Effective internal controls required under Section 404 of the Sarbanes-Oxley Act of 2002, or Sarbanes-Oxley Act, are necessary for us to provide reliable financial reports and effectively prevent fraud. If we cannot provide reliable financial reports or prevent fraud, our business, reputation and operating results could be harmed. We have discovered, and may in the future discover, areas of our internal controls that need improvement. For example, “material weaknesses” were identified in our year ended December 31, 2009 which means that there was “a significant deficiency, or a combination of significant
22
Table of Contents
Risk factors
deficiencies, that results in more than a remote likelihood that a material misstatement of the annual or interim financial statements will not be prevented or detected.” During the year ended December 31, 2009, we were under significant operational stress due to a lack of capital resources, and much of our staff was terminated. Until we can complete our remediation efforts including the re-staffing and training of our accounting personnel, we have a higher risk of deficiencies in our financial reporting. We cannot be certain that the measures we have taken or intend to take will ensure that we maintain adequate controls over our financial processes and reporting in the future. Any failure to implement required new or improved controls or difficulties encountered in their implementation could subject us to regulatory sanctions, harm our business and operating results or cause us to fail to meet our reporting obligations. Inferior internal controls could also harm our reputation and cause investors to lose confidence in our reported financial information, which could have a negative impact on the trading price of our stock.
Risks Related to Our Common Stock
The Koski Family Limited Partnership, or KFLP, together with members of the Koski family, have a controlling interest in our outstanding shares of common stock.
The KFLP, together with members of the Koski family, own approximately 56.6% of our outstanding shares of common stock. Following the sale of the shares offered under this prospectus, the KFLP, together with members of the Koski family, will own approximately 37.0% of our outstanding shares of common stock. In addition, two members of the Koski family serve on our Board of Directors. As a result, the Koski family will be able to affect the outcome of, or exert significant influence over, all matters requiring shareholder approval, including the election and removal of directors and any change in control. In particular, this concentration of ownership of our common stock could have the effect of delaying or preventing a change of control of us or otherwise discouraging or preventing a potential acquirer from attempting to obtain control of us. This, in turn, could have a negative effect on the market price of our common stock. It could also prevent our shareholders from realizing a premium over the market price for their shares of common stock. Moreover, the interests of this concentration of ownership may not always coincide with our interests or the interests of other shareholders, and, accordingly, the Koski family could cause us to enter into transactions or agreements that we would not otherwise consider.
Certain provisions of our articles of incorporation, bylaws, executive employment agreements and stock option plan may prevent a change of control of our company that a shareholder may consider favorable.
Provisions of our articles of incorporation, bylaws, executive employment agreements and stock option plan may discourage, delay or prevent a change of control of our company that a shareholder may consider favorable. These provisions include:
| Ø | authorization of the issuance of “blank check” preferred stock that could be issued by our Board of Directors without shareholder approval and that may be substantially dilutive or contain preferences or rights objectionable to an acquiror; |
| Ø | the ability of our Board of Directors to amend the bylaws without shareholder approval; |
| Ø | vacancies on our Board may only be filled by the remaining directors and not our shareholders; |
| Ø | requirements that only our Board, our President or holders of more than 10% of our shares can call a special meeting of shareholders; |
| Ø | obligations to make certain payments under executive employment agreements in the event of a change of control and termination of employment; and |
| Ø | immediate vesting of all stock options. |
23
Table of Contents
Risk factors
As a result, these provisions could discourage bids for our common stock at a premium and limit the price that investors are willing to pay in the future for shares of our common stock. However, in our articles of incorporation we expressly elected not to be governed by Sections 607.0901 and 607.0902 of the Florida Business Corporation Act, which are statutory anti-takeover provisions relating to affiliated transactions and control share acquisitions, respectively. As such, we do not have the protection of these statutes in connection with any unwanted takeover attempts which could have the effect of encouraging an attempted change of control without first negotiating with our Board of Directors.
Our stock price has historically been volatile and the trading volume of our stock has been low.
Since our initial public offering in June 2003 and through June 30, 2010 our stock price has fluctuated from $100.00 to $1.00 per share. The trading price of our common stock has been, and may be, subject to wide fluctuations in response to a number of factors, many of which are beyond our control. These factors include:
| Ø | quarter-to-quarter variations in our operating results; |
| Ø | our perceived funding needs; |
| Ø | the results of testing, technological innovations, or new commercial products by us or our competitors; |
| Ø | governmental regulations, rules, and orders; |
| Ø | our level of, and expected future use of, working capital; |
| Ø | general conditions in the healthcare, dentistry, or biopharmaceutical industries; |
| Ø | comments, research reports and/or earnings estimates by securities analysts; |
| Ø | developments concerning patents or other intellectual property rights; |
| Ø | litigation or public concern about the safety of our products; |
| Ø | announcements by us or our competitors of significant acquisitions, strategic partnerships, joint ventures or capital commitments; |
| Ø | additions or departures of directors, officers and key personnel; |
| Ø | release of transfer restrictions on our outstanding shares of common stock or sales of additional shares of common stock; |
| Ø | potential litigation initiated against us; and |
| Ø | the additional sale of common stock by us in capital raising transactions. |
Historically, the daily trading volume of our common stock has been relatively low and on some days our stock does not trade at all. We cannot guarantee that an active public market for our common stock will be sustained or that the average trading volume will increase. In addition, the stock market in general has experienced significant price and volume fluctuations. Volatility in the market price for particular companies has often been unrelated or disproportionate to the operating performance of those companies. Broad market factors may seriously harm the market price of our common stock, regardless of our operating performance. In addition, securities class action litigation has often been initiated following periods of volatility
24
Table of Contents
Risk factors
in the market price of a company’s securities. A securities class action suit against us could result in substantial costs, potential liabilities, and the diversion of management’s attention and resources. To the extent our stock price fluctuates or remains low, it could impair our ability to raise capital through the offering of additional equity securities.
The issuance of additional equity securities by us in the future could result in dilution to our existing shareholders.
Any issuance of additional equity securities by us in the future could result in dilution to our existing shareholders. Such issuances could be made at a price that reflects a discount or a premium to the then-current trading price of our common stock. In addition, our business strategy may include expansion through internal growth by acquiring complementary businesses, acquiring or licensing additional products or brands, or establishing strategic relationships with targeted customers and suppliers. In order to do so, or to finance the cost of our other activities, we may issue additional equity securities that could result in further dilution to our existing shareholders.
Future sales of our common stock may depress our stock price.
The market price of our common stock could decline as a result of sales of substantial amounts of our common stock in the public market, or the perception that these sales could occur. In addition, these factors could make it more difficult for us to raise funds through future offerings of common stock. As of October 4, 2010, there were 5,663,157 shares of our common stock outstanding, with another 306,388 shares of common stock issuable upon exercise of warrants to investors, 398,112 shares issuable upon exercise of options outstanding and an additional 223,887 shares available for option grants under our stock option plans. The issuance of shares of our common stock under our 2002 Stock Incentive Plan is covered by Form S-8 registration statements we filed with the Securities and Exchange Commission, or SEC, and upon exercise of the options, such shares may be resold into the market. We have also issued shares of common stock and warrants in connection with private placements. Such shares are available for resale as well as the shares of common stock issuable upon exercise of the warrants. We issued an aggregate of 1,050,812 shares of our common stock in the December 2009 Private Placement and July 2010 Financing Transaction which are deemed to be “restricted securities,” as that term is defined in Rule 144 promulgated under the Securities Act of 1933, as amended, or Securities Act, and may be resold pursuant to the provisions of Rule 144. The resale of shares acquired from us in private transactions could cause our stock price to decline significantly.
In addition, from time to time, certain of our shareholders may be eligible to sell all or some of their restricted shares of common stock by means of ordinary brokerage transactions in the open market pursuant to Rule 144, subject to certain limitations. In general, pursuant to Rule 144, after satisfying a six-month holding period: (i) affiliated shareholders, or shareholders whose shares are aggregated, may, under certain circumstances, sell within any three-month period a number of securities which does not exceed the greater of 1% of the then-outstanding shares of common stock or the average weekly trading volume of the class during the four calendar weeks prior to such sale and (ii) non-affiliated shareholders may sell without such limitations, in each case provided we are current in our public reporting obligations. Rule 144 also permits the sale of securities by non-affiliates that have satisfied a one-year holding period without any limitation or restriction.
The requirements of being a stock exchange listed public company may strain our resources, divert management’s attention and affect our ability to attract and retain qualified members for our Board of Directors.
As a public company, we are already subject to the reporting requirements of the Securities Exchange Act of 1934, as amended, or the Exchange Act, and the Sarbanes-Oxley Act. However, we will also be subject to additional NASDAQ requirements if our common stock is approved for listing on the NASDAQ Capital Market. The requirements of these rules and regulations may increase our legal, accounting and financial compliance costs; may make some activities more difficult, time-consuming and costly; and may also place undue strain on our personnel, systems and resources. For example, NASDAQ listing requirements require that listed companies satisfy certain corporate governance requirements relating to
25
Table of Contents
Risk factors
independent directors, audit committees, distribution of annual and interim reports, shareholder meetings, shareholder approvals, solicitation of proxies, conflicts of interest, shareholder voting rights and codes of business conduct. Our management and other personnel will need to devote a substantial amount of time to comply with these requirements. Moreover, these rules and regulations will increase our legal and financial compliance costs and will make some activities more time-consuming and costly. These rules and regulations could also make it more difficult for us to attract and retain qualified persons to serve on our Board of Directors and Board committees or as executive officers.
As a public company we are also required to assess our internal control over financial reporting under Section 404 of the Sarbanes-Oxley Act and file periodic reports with the SEC. If we are unable to comply with these requirements in a timely manner, or if material weaknesses or significant deficiencies persist, the market price of our stock could decline and we could be subject to sanctions or regulatory investigations, which could harm our business. We must perform an assessment of our internal control over financial reporting to allow management to report on the effectiveness of our internal control over financial reporting, as required by Section 404 of the Sarbanes-Oxley Act. Our compliance with Section 404 of the Sarbanes-Oxley Act requires that we incur substantial accounting expense and expend significant management efforts. If we are unable to comply with the requirements of Section 404 of the Sarbanes-Oxley Act or the SEC reporting requirements in a timely manner, or if we continue to note or identify deficiencies in our internal control over financial reporting that are deemed to be material weaknesses, the market price of our stock could decline and we could be subject to sanctions or investigations by the stock exchange upon which our common stock is listed, the SEC or other regulatory authorities, which would require additional financial and management resources. As a smaller reporting company, we do not have to have our independent registered public accounting firm report on the effectiveness of our internal control over financial reporting. If in the future we no longer meet the requirements of a smaller reporting company, our independent registered public accounting firm will have to report on our internal controls over financial reporting. There can be no assurance that our independent registered public accounting firm will not identify material weaknesses which could have an adverse effect on our stock price.
Risks Related to this Offering
We will have broad discretion as to the use of the proceeds from this offering, and we may not use the proceeds effectively.
We will have broad discretion in the application of the net proceeds from this offering and could allocate the net proceeds in ways that do not improve our results of operations or enhance the value of our common stock. Our failure to apply these funds effectively could have a material adverse effect on our business, delay the development of our product candidates and cause the price of our common stock to decline.
Purchasers in this offering will experience immediate and substantial dilution.
The public offering price of our common stock pursuant to this prospectus will be substantially higher than the pro forma net tangible book value per share of our common stock immediately after this offering. Therefore, if you purchase our common stock in this offering, you will incur an immediate dilution of $4.37 per share, which represents the difference between our net tangible book value per share after this offering and the price you paid, based upon the assumed public offering price of $6.60 per share (the last sale price of our common stock as reported on the OTC Bulletin Board on September 28, 2010 immediately after our reverse stock split) and the pro forma as adjusted net tangible book value and shares outstanding as of June 30, 2010. The exercise of outstanding options and warrants, or the exercise by the underwriters of their over-allotment option, will result in further dilution in your investment. In addition, if we issue additional equity securities in the future, the newly issued securities may further dilute your ownership interest.
26
Table of Contents
Risk factors
We have effected a reverse stock split, which may cause the liquidity of our common stock and market capitalization to be materially adversely affected.
At our 2010 Annual Meeting of Shareholders held on August 25, 2010, our shareholders approved an amendment to our articles of incorporation which gave our Board of Directors the authority to effect a reverse stock split of our authorized and issued and outstanding common stock at a ratio to be determined by the Board of Directors, between 2-to-1 and 20-to-1, without further approval of our shareholders, upon a determination by the Board of Directors that such a reverse stock split is in our best interests. On September 24, 2010, we effectuated a reverse stock split of 20-to-1 in order to increase the stock price to a level that will enable our common stock to qualify for listing on the NASDAQ Capital Market.
A reverse stock split is often viewed negatively by the market and, consequently, can lead to a decrease in our overall market capitalization. If the per-share market price does not increase proportionately as a result of the reverse split, then the value of our company as measured by our market capitalization will be reduced, perhaps significantly. In addition, because the reverse split significantly reduced the number of shares of our common stock that are outstanding, the liquidity of our common stock could be materially and adversely affected and you may find it more difficult to purchase or sell shares of our common stock.
If we cannot continue to satisfy the NASDAQ Capital Market’s listing requirements, our common stock may be delisted, which could negatively impact the price of our common stock and your ability to sell our common stock after this offering.
Our common stock has been approved for listing on the NASDAQ Capital Market, subject to notice of issuance. We cannot assure you that we will be able to satisfy the NASDAQ Capital Market criteria for maintaining our listing. If we are unable to maintain the listing criteria of the NASDAQ Capital Market our common stock could be subject to delisting. To qualify for continued listing on the NASDAQ Capital Market, we must meet the NASDAQ Capital Market continued listing requirements, which include maintaining a minimum stock price of $1.00 and satisfying standards relative to minimum shareholders’ equity, minimum market value of publicly held shares and various additional requirements. If we fail to comply with all listing standards applicable to issuers listed on the NASDAQ Capital Market, our common stock may be delisted. If our common stock is delisted, our shares will likely be subject to the “penny stock” rules for the foreseeable future and our shareholders will, in all likelihood, find it difficult to sell their securities. Furthermore, the “penny stock” rules could also hamper our ability to raise funds in the future. These additional sales practice and disclosure rules could impede the sale of our securities. We have a limited active public market for our common stock. We cannot assure you that a more active public market will develop. Consequently, you may not be able to readily liquidate your investment. Delisting from the NASDAQ Capital Market could also result in other negative consequences, including the potential loss of confidence by customers and employees, the loss of institutional investor interest and fewer business development opportunities.
27
Table of Contents
Special note regarding forward-looking statements
This prospectus contains, in addition to historical information, forward-looking statements within the meaning of the “safe harbor” provisions of the Private Securities Litigation Reform Act of 1995. These statements relate to future events or our future financial performance and can be identified by the use of forward-looking terminology such as “project,” “may,” “could,” “expect,” “anticipate,” “estimate,” “continue” or other similar words. These forward-looking statements are based on management’s current expectations and are subject to a number of factors and uncertainties which could cause actual results to differ materially from those described in these statements. The following are some of the important factors that could cause our actual performance to differ materially from those discussed in the forward-looking statements:
| Ø | We have incurred significant operating losses since our inception and cannot assure you that we will generate revenues or achieve profitability. |
| Ø | As a result of our lack of financial liquidity, our auditors have indicated there is substantial doubt about our ability to continue as a going concern. |
| Ø | If we fail to achieve positive cash flows from our operations and we fail to raise additional capital to cover any future capital needs, we may need to significantly curtail operations. |
| Ø | We are subject to extensive and costly regulation by the FDA, which must approve our product candidates in development and could restrict or delay the future commercialization of these product candidates. |
| Ø | We may be unable to achieve commercial viability and acceptance of our ProBiora3 products and proposed product candidates or increase sales of our ProBiora3 products. |
| Ø | Orders we receive for our consumer products may be subject to terms and conditions that could result in their cancellation or the return of products to us. |
| Ø | We may become dependent on a few large retail customers for sales of our consumer products. |
| Ø | We may be unable to successfully operate internationally. |
| Ø | We may be unable to improve upon, protect and/or enforce our intellectual property. |
| Ø | We may be unable to enter into strategic collaborations or partnerships for the development, commercialization, manufacturing and distribution of our proposed product candidates or maintain strategic collaborations or partnerships. |
| Ø | We may be adversely impacted by a continuing or worsening worldwide financial crises and its impact on consumers, retailers and equity and debt markets as well as our ability to obtain required additional funding to conduct our business. |
| Ø | We are subject to significant competition. |
| Ø | As a public company, we must implement additional and expensive finance and accounting systems, procedures and controls as we grow our business and organization to satisfy new reporting requirements, which will increase our costs and require additional management resources. |
| Ø | Success, timing and expenses of our expected clinical trials. |
We caution you that actual results or business conditions may differ materially from those projected or suggested in forward-looking statements as a result of various factors including, but not limited to, those described above and in the “Risk Factors” section of this prospectus. We cannot assure you that we have identified all the factors that create uncertainties. Moreover, new risks emerge from time to time and it is not possible for us to predict all risks, nor can we assess the impact of all risks on our business or the extent to which any risk, or combination of risks, may cause actual results to differ from those contained in any forward-looking statements. You should not place undue reliance on forward-looking statements. Except as required by applicable law, including the securities laws of the United States, we undertake no obligation to publicly release the result of any revision of these forward-looking statements to reflect events or circumstances after the date they are made or to reflect the occurrence of unanticipated events.
28
Table of Contents
We estimate that the net proceeds to us from the sale of shares of common stock that we are offering will be approximately $17,416,000 (or approximately $20,178,100 if the underwriters exercise their over-allotment option in full) based on an assumed public offering price of $6.60 per share (the last sale price for our common stock as reported on the OTC Bulletin Board on September 28, 2010 immediately after our reverse stock split), after deducting underwriting discounts and commissions and estimated offering expenses that we must pay, including the underwriters’ non-accountable expense allowance.
We intend to use the net proceeds from this offering for working capital and general corporate purposes, which include, but are not limited to, commercialization of our ProBiora3 products, clinical development of our SMaRT Replacement Therapy and MU1140-S product candidates, and research and development related to our other products, including our weight loss product and our biomarker discovery platform.
If we receive additional net proceeds from the exercise of the underwriters’ over-allotment option, we anticipate that we would use the additional funds for working capital and general corporate purposes as described above.
Pending any use, as described above, we plan to invest the net proceeds in investment-grade, short-term, interest-bearing securities.
29
Table of Contents
Market for common equity and related shareholder matters
Market Information
Our common stock is currently traded on the OTC Bulletin Board under the symbol “ORNID.OB.” There can be no assurances that an active public market for our common stock will develop or be sustained. The public market for our common stock has often been sporadic, volatile and limited. The closing sales price of our common stock as reported on the OTC Bulletin Board on September 28, 2010 was $6.60 per share on an immediate post-split basis.
Our common stock has been approved for listing on the NASDAQ Capital Market under the proposed symbol “OGEN” subject to notice of issuance.
The following table sets forth, for the periods indicated, the high and low sales prices for our common stock. The prices reflect inter-dealer quotations, without retail mark-up, markdowns or commissions, and may not represent actual transactions.
| 2010 |
High | Low | ||||
| 1st Quarter |
$ | 20.40 | $ | 6.00 | ||
| 2nd Quarter |
14.60 | 6.80 | ||||
| 3rd Quarter (through September 28, 2010) |
8.00 | 5.80 | ||||
| 2009 |
||||||
| 1st Quarter |
$ | 9.00 | $ | 4.40 | ||
| 2nd Quarter |
8.20 | 1.00 | ||||
| 3rd Quarter |
10.20 | 4.40 | ||||
| 4th Quarter |
6.40 | 4.00 | ||||
| 2008 |
||||||
| 1st Quarter |
$ | 12.00 | $ | 7.20 | ||
| 2nd Quarter |
15.60 | 7.60 | ||||
| 3rd Quarter |
17.60 | 7.20 | ||||
| 4th Quarter |
17.60 | 3.00 | ||||
Number of Shareholders
As of October 4, 2010, there were approximately 97 holders of record of our common stock. There were also approximately 1,584 beneficial owners of our common stock who hold their stock in the names of various dealers, clearing agencies, banks, brokers and other fiduciaries.
Dividend Policy
Historically, we have not paid any dividends to the holders of our common stock and we do not expect to pay any such dividends in the foreseeable future as we expect to retain our future earnings for use in the operation and expansion of our business.
30
Table of Contents
Market for common equity and related shareholder matters
Securities Authorized For Issuance Under Equity Compensation Plan
We currently maintain an equity compensation plan known as the Amended and Restated 2002 Stock Option and Incentive Plan, or the 2002 Stock Incentive Plan. Our Compensation Committee is responsible for making, reviewing and recommending grants of options or awards of restricted stock under this plan. The 2002 Stock Incentive Plan was approved by our Board of Directors and shareholders in September 2002. The 2002 Stock Incentive Plan allows for the grant of options to purchase, or the issuance of restricted shares of up to 625,000 shares of our common stock. The 2002 Stock Incentive Plan provides for the granting of options to purchase shares of our common stock at prices not less than the fair market value of the stock at the date of grant and, subject to vesting and early termination, generally expire ten years after the date of grant. The stock options are subject to vesting requirements, generally three or four years or as determined by our Compensation Committee and in certain instances are subject to early termination if vesting does not occur by designated time periods or events. The 2002 Stock Incentive Plan also provides for the granting of restricted shares of common stock subject to requirements established by our Compensation Committee. As of October 4, 2010, a total of 223,887 shares of common stock remain available for future awards under the 2002 Stock Incentive Plan.
31
Table of Contents
The following table sets forth our cash and cash equivalents, and capitalization as of June 30, 2010 on an actual and on a pro forma as adjusted basis to give effect to (i) the sale of 3,000,000 shares of common stock by us in this offering at an assumed public offering price of $6.60 per share (the last sale price for our common stock as reported on the OTC Bulletin Board on September 28, 2010 immediately after our reverse stock split), after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us; (ii) the July 2010 Financing Transaction which included the repayment of the May 2010 Note with shares of our common stock prior to this offering; (iii) the draw down of $1,000,000 on our Credit Facility in September 2010, and (iv) the application of the estimated net proceeds of this offering as described under “Use of Proceeds.”
This table should be read in conjunction with our financial statements and related notes and the sections entitled “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” “Use of Proceeds,” and “Description of Capital Stock” appearing elsewhere in this prospectus.
| June 30, 2010 | ||||||||
| Actual | As adjusted(1)(2) |
|||||||
| (unaudited) |
||||||||
| Cash and cash equivalents(3) |
$ | 959,434 | $ | 20,375,434 | ||||
| Current note payable |
1,000,000 | — | ||||||
| Credit Facility note payable |
— | 1,000,000 | ||||||
| Shareholders’ equity: |
||||||||
| Common stock, $0.001 par value: 15,000,000 shares authorized; 5,410,157 issued and outstanding, actual; and 8,663,157 shares issued and outstanding, as adjusted |
$ | 5,410 | $ | 8,663 | ||||
| Preferred stock, no par value: 20,000,000 shares authorized and no shares issued and outstanding, actual; and no shares issued and outstanding, as adjusted |
— | |||||||
| Additional paid-in capital |
$ | 29,182,171 | $ | 48,594,918 | ||||
| Accumulated deficit |
$ | (29,269,304 | ) | $ | (29,269,304 | ) | ||
| Total shareholders’ equity (deficit) |
$ | (81,723 | ) | $ | 19,334,277 | |||
| Total capitalization |
$ | 918,277 | $ | 20,334,277 | ||||
| (1) | The above table excludes the following: |
| Ø | 306,388 shares of common stock reserved for issuance upon the exercise of outstanding warrants with a weighted average exercise price of $19.20 per share; |
| Ø | 401,602 shares of common stock reserved for issuance upon the exercise of outstanding stock options with a weighted average exercise price of $7.00 per share; |
| Ø | 223,398 shares of common stock available for future grant under our 2002 Stock Incentive Plan; and |
| Ø | 450,000 shares of common stock that may be issued upon exercise of the underwriters’ over-allotment option. |
| (2) | Includes the effect of the July 2010 Financing Transaction pursuant to which we (i) issued 250,000 shares of common stock through the payment of $2,000,000 in aggregate consideration consisting of (a) $1,000,000 in cash and (b) exchange and cancellation of the May 2010 Note, and (ii) entered into the Credit Facility for $2,000,000, on which we drew down $1,000,000 in September 2010. See “Management’s Discussion and Analysis of Financial Condition and Results of Operations.” |
| (3) | Includes $742,682 of cash we have reserved for DPOLT research. |
32
Table of Contents
If you invest in our common stock, your interest will be diluted to the extent of the difference between the public offering price per share you pay and the as adjusted net tangible book value per share of our common stock after this offering. Our net tangible book value as of June 30, 2010 was ($81,723), or ($0.02) per share of common stock. We calculate net tangible book value per share by calculating the difference between the total assets less goodwill and other intangible assets and total liabilities, and dividing the result by the number of shares of common stock outstanding.
Net tangible book value dilution per share represents the difference between the amount per share paid by new investors who purchase shares in this offering and the pro forma net tangible book value per share of common stock immediately after completion of this offering as of June 30, 2010, after giving effect to:
| Ø | the sale by us of 3,000,000 shares of common stock at an assumed public offering price of $6.60 per share (the last sale price of our common stock as reported on the OTC Bulletin Board on September 28, 2010 immediately after our reverse stock split) and the application of the estimated net proceeds in this offering as described under “Use of Proceeds”; |
| Ø | the sale by us of 250,000 shares in the July 2010 Financing Transaction as described under “Management’s Discussion and Analysis of Financial Condition and Results of Operations”; |
| Ø | the borrowing of $1,000,000 under our Credit Facility; and |
| Ø | the estimated underwriting discounts and commissions and offering expenses payable by us. |
Our pro forma as adjusted net tangible book value would have been $19,334,277, or $2.23 per share. The assumed public offering price of $6.60 per share exceeds $2.23 per share, which is the per share pro forma value of total tangible assets less total liabilities after this offering. This represents an immediate increase in net tangible book value of $2.25 per share to our existing shareholders, and an immediate dilution in net tangible book value of $4.37 per share to new investors purchasing shares in this offering.
The following table illustrates this dilution to new investors on a per share basis.
| Assumed public offering price per share |
$ | 6.60 | ||||
| Net tangible book value as of June 30, 2010 |
(0.02 | ) | ||||
| Increase per share attributable to this offering |
2.25 | |||||
| Adjusted net tangible book value per share after this offering |
2.23 | |||||
| Dilution in net tangible book value per share to new investors |
$ | 4.37 | ||||
If the underwriters exercise their over-allotment option to purchase additional shares of common stock in full, the pro forma net tangible book value after this offering will increase to approximately $2.42 per share, representing an increase to existing shareholders of approximately $2.44 per share, and there will be an immediate dilution of approximately $4.18 per share to new investors.
The following table summarizes as of June 30, 2010 on a pro forma basis to reflect the same adjustments described above, the number of shares of common stock purchased from us, the total consideration paid and the average price per share paid by:
| Ø | The existing common shareholders; and |
| Ø | The new investors in this offering, assuming the sale of 3,000,000 shares offered hereby at an assumed public offering price of $6.60 per share (the last sale price of our common stock as reported on the OTC Bulletin Board on September 28, 2010 immediately after our reverse stock split). |
33
Table of Contents
Dilution
The calculations are based upon total consideration given by new and existing shareholders, before any deduction of estimated underwriting discounts and commissions and offering expenses.
| Shares of common stock purchased |
Total consideration | Average
price per share | ||||||||||||
| Number | Percent | Amount | Percent | |||||||||||
| Existing shareholders |
5,663,157 | 65.4 | % | $ | 29,187,581 | 59.6 | % | $ | 5.15 | |||||
| New investors |
3,000,000 | 34.6 | 19,800,000 | 40.4 | 6.60 | |||||||||
| Total |
8,663,157 | 100 | % | $ | 48,987,581 | 100 | % | |||||||
The number of shares of common stock to be outstanding immediately prior to and after this offering as reflected in the table above is based on the actual number of shares of common stock outstanding as of June 30, 2010, which was 5,410,157 shares, but does not include, as of that date:
| Ø | 306,388 shares of common stock reserved for issuance upon the exercise of outstanding warrants with a weighted average exercise price of $19.20 per share; |
| Ø | 401,602 shares of common stock reserved for issuance upon the exercise of outstanding stock options with a weighted average exercise price of $7.00 per share; |
| Ø | 223,398 shares of common stock available for future grant under our 2002 Stock Incentive Plan; and |
| Ø | 450,000 shares of common stock that may be issued upon exercise of the underwriters’ over-allotment option. |
To the extent that options and warrants outstanding as of June 30, 2010 have been or may be exercised or other shares have been or will be issued, there will be further dilution to new investors.
In addition, we may choose to raise additional capital due to market conditions or strategic considerations even if we believe we have sufficient funds for our current or future operating plans. To the extent that additional capital is raised through the sale of equity or convertible debt securities, the issuance of these securities could result in further dilution to our shareholders.
34
Table of Contents
Management’s discussion and analysis of financial condition and results of operations
The following discussion and analysis of our financial condition and results of operations should be read in conjunction with our financial statements and related notes that appear elsewhere in this prospectus. In addition to historical financial information, the following discussion contains forward-looking statements that reflect our plans, estimates and beliefs. Our actual results could differ materially from those discussed in the forward-looking statements. Factors that could cause or contribute to these differences include those discussed below and elsewhere in this prospectus, particularly in “Risk Factors.”
Overview
We are a biopharmaceutical company focused primarily on oral health products and novel antibiotics. Within oral health, we are developing our pharmaceutical product candidate, SMaRT Replacement Therapy, and we are also commercializing our oral probiotic blend, ProBiora3. Within antibiotics, we are developing our pharmaceutical product candidate, MU1140-S, and we intend to use our patented, novel organic chemistry platform to create additional antibiotics for therapeutic use.
Our SMaRT Replacement Therapy product candidate is designed to be a painless, one-time, five-minute topical treatment applied to the teeth that has the potential to offer lifelong protection against dental caries, or tooth decay. Our SMaRT Replacement Therapy is based on the creation of a genetically modified strain of bacteria that colonizes in the oral cavity and replaces native bacteria that cause tooth decay. We are commencing a second Phase 1 clinical trial for SMaRT Replacement Therapy which we expect to conclude in the first half of 2011. We have also developed and are commercializing a variety of products that contain the active ingredient ProBiora3, a patent pending blend of oral probiotics that promote fresher breath, whiter teeth and support overall oral health. We have conducted extensive scientific studies on ProBiora3 in order to market our products under self-affirmed Generally Recognized As Safe status, or GRAS. We sell our ProBiora3 products through multiple distribution channels and our customers include Walgreens, Rite Aid, and Garden of Life, among others.
While developing SMaRT Replacement Therapy, members of our scientific team discovered that the SMaRT bacterial strain produces MU1140, a molecule belonging to the novel class of antibiotics known as lantibiotics. MU1140 has proven active preclinically against Gram positive bacteria responsible for a number of HAIs. We are in the process of scaling up production of our synthetic form of MU1140, or MU1140-S, and expect to commence preclinical testing during the second half of 2010 and to file an Investigational New Drug, or IND, application with the FDA in mid-2011. The key technology behind the production of MU1140-S is our Differentially Protected Orthogonal Lanthionine Technology platform, or DPOLT, which is a patented, novel organic chemistry platform that we believe will enable the first ever commercial scale, cost-effective production of any of the 50 known lantibiotics. We intend to use DPOLT to create a pipeline of lantibiotics for therapeutic use. Additionally, we are developing non-core technologies that originated from the discoveries of our scientific team, including LPT3-04, which is a weight loss product, and PCMAT, which is a biomarker discovery platform, both of which we believe could provide significant potential opportunities for us.
We were incorporated in November 1996 and commenced operations in 1999. We consummated our initial public offering in June 2003. We have devoted substantially all of our resources to the commercialization of our ProBiora3 products as well as our discovery efforts comprising research and development, clinical trials for our product candidates, protection of our intellectual property and the general and administrative support of these operations. We have generated limited revenues from grants and ProBiora3 product sales through June 30, 2010, and have principally funded our operations through the sale of debt and equity securities, including the exercise of warrants issued in connection with these financing transactions. Prior to 2008 our revenues were derived solely from research grants. Since 2008, our revenues have also included sales of our ProBiora3 products, which we initiated in late 2008. Our net revenues were $646,179 for the six months ended June 30, 2010 and $641,285 and $233,539 for the years ended December 31, 2009 and 2008, respectively.
35
Table of Contents
Management’s discussion and analysis of financial condition and results of operations
We have never been profitable and, as of June 30, 2010, we had an accumulated deficit of $29,269,304. We incurred net losses of $3,757,421 for the six months ended June 30, 2010 and $5,519,348 and $6,021,742 for the years ended December 31, 2009 and 2008, respectively. We expect to incur significant and increasing operating losses for the foreseeable future as we advance our product candidates through preclinical testing and clinical trials to seek regulatory approval and eventual commercialization. The report of our independent registered public accounting firm with respect to our financial statements appearing at the end of this prospectus contains an explanatory paragraph stating that our operating losses and negative cash flows from operations since inception, and our need to raise additional financing and/or financial support prior to December 31, 2010 in order to continue to fund our operations, raise substantial doubt about our ability to continue as a going concern. Adequate additional funding may not be available to us on acceptable terms, or at all. We expect that research and development expenses will increase along with general and administrative costs, as we grow and operate our business. The proceeds from this offering will allow us to strengthen our focus on current and future commercialization of our products and product candidates, which we believe will position us for future profitability and positive cash flows.
Recent Developments
On September 24, 2010 the amendment to our articles of incorporation that we filed with the Florida Department of State became effective with respect to a 20-to-1 reverse stock split of our authorized and outstanding shares of common stock.
On July 5, 2010, we entered into a common stock purchase agreement (the “July 2010 Financing Transaction”) with the Koski Family Limited Partnership, or KFLP. At the closing of this financing transaction on July 30, 2010 we issued 250,000 shares of our common stock to the KFLP at a price of $8.00 per share. The $2,000,000 aggregate consideration paid by the KFLP consisted of (i) $1,000,000 cash and (ii) the exchange and cancellation of the outstanding May 2010 Note issued to the KFLP on May 28, 2010. Accrued interest on the May 2010 Note through closing was waived by the KFLP. Concurrent with the July 2010 Financing Transaction and as part thereof, we entered into an unsecured revolving credit agreement (the “Credit Facility”) with the KFLP. Pursuant to the Credit Facility, we are able to borrow up to $2,000,000 from the KFLP at LIBOR plus 6.0%. The term of the Credit Facility is for 12 months commencing August 1, 2010. As of the date of this prospectus, we have drawn $1,000,000 on this Credit Facility.
We have continued our efforts to broaden the distribution of our ProBiora3 products through the following business development activities:
| Ø | American Dental Association: From October 9-12, 2010, we will exhibit EvoraPro to dental professionals at their Annual Session and World Marketplace Exhibition. |
| Ø | Best Supplies: On September 30, 2010, we announced that Teddy’s Pride will be available in Taiwan through a distribution agreement with The Best Supplies LTD, which has 70 pet supply retail shops across Taiwan. |
| Ø | Rolf C. Hagen, Inc.: On September 23, 2010, we announced the distribution of Teddy’s Pride in Canada through Rolf C. Hagen, one of the largest privately held pet product manufacturers in the world. |
| Ø | Benelux Cosmetics: On September 21, 2010, we announced that our line of ProBiora3 products will be distributed in Belgium, the Netherlands and Luxembourg through Benelux Cosmetics. |
| Ø | Vetcom: On September 20, 2010, we announced that Teddy’s Pride will be distributed to veterinarians in Korea through Vetcom Korea, Inc. Vetcom Korea is a leading national distributor of products for the veterinarian industry. |
| Ø | Fred Meyer: On September 16, 2010, we announced an initial order of EvoraPlus from Fred Meyer, which operates 129 multi-department stores in four western states. |
| Ø | SuperZoo: From September 14-16, 2010, we introduced Teddy’s Pride to SuperZoo, an annual pet trade show and seminar by the World Pet Association, which was attended by over 9,000 pet professionals from around the world. |
36
Table of Contents
Management’s discussion and analysis of financial condition and results of operations
| Ø | Kroger: On September 14, 2010, we announced the expansion of the retail distribution of EvoraPlus through an initial order from Kroger Co. Kroger is the nation’s largest traditional grocery retailer with approximately 2,500 stores in 31 states. We anticipate that EvoraPlus will be available in select Kroger grocery stores beginning in October 2010. |
| Ø | Harris Teeter: On September 10, 2010, we announced an initial order of EvoraPlus and EvoraKids from Harris Teeter. Harris Teeter is a food market chain that operates in eight states with 196 stores. |
| Ø | GNC: On September 7, 2010, we announced that EvoraPlus will be available in corporate-owned GNC stores nationwide and EvoraKids will be available in its concept stores and on GNC.com. GNC is a leading global specialty retailer of nutritional products with over 7,000 locations. |
Financial Overview
Net Revenues
Our revenues prior to 2008 consisted exclusively of grant funding from government agencies under the National Science Foundation’s, or NSF, and National Institutes of Health’s, or NIH, Small Business Innovation Research, or SBIR, grants. Since the initial launch of our ProBiora3 products in late 2008, our net revenues for the year ended December 31, 2008 also included sales of our ProBiora3 products. Sales of our ProBiora3 products were $521,115, for the six months ended June 30, 2010 and $366,801 and $8,539 for the years ended December 31, 2009 and 2008, respectively. Because of our efforts to increase the distribution of our ProBiora3 products, we expect net revenues to continue to increase in the near future. However, our success will depend on a number of factors, including our marketing efforts related to our ProBiora3 products.
We expect that our revenues will fluctuate from quarter to quarter as a result of the volume of sales of our products and the amount of license fees, research and development reimbursements, milestone and other payments we may receive upon any license or strategic partnerships we may enter into in the future.
Cost of Goods Sold
Our cost of goods sold includes the production and manufacture of our ProBiora3 products, as well as shipping and processing expenses and scrap expense. Scrap expense represents product rework charges, inventory adjustments, and damaged inventory. We expect our costs of goods sold to increase as we expand our distribution and sales efforts for our ProBiora3 products.
Research and Development Expenses
Research and development consists of expenses incurred in connection with the discovery and development of our product candidates. These expenses consist primarily of employee-related expenses, which include salaries and benefits and attending science conferences; expenses incurred under agreements with contract research organizations, investigative sites and consultants that conduct our clinical trials and a substantial portion of our preclinical studies; the cost of acquiring and manufacturing clinical trial materials; facilities, depreciation and other allocated expenses, which include direct and allocated expenses for rent and maintenance of facilities and equipment, and depreciation of fixed assets; license fees for and milestone payments related to in-licensed products and technology; stock-based compensation expense; and costs associated with non-clinical activities and regulatory approvals. We expense research and development costs as incurred.
We plan to increase our research and development expenses for the foreseeable future as we seek to advance the development of our SMaRT Replacement Therapy and MU1140-S product candidates, and to further advance our earlier stage research and development projects, such as LPT3-04, our potential weight loss product, and PCMAT, our biomarker discovery platform.
37
Table of Contents
Management’s discussion and analysis of financial condition and results of operations
Prior to January 1, 2009, we did not track our internal research and development costs or our personnel and personnel-related costs on a project-by-project basis, instead, our research and development resources were allocated among all of our programs. Since January 1, 2009, we have tracked development expenses and personnel expense on a project-by-project basis and have allocated common expenses, such as scientific consultants and lab supplies, to each program based on the personnel resources allocated to each program.
Our research and development expenses were $909,838 for the six months ended June 30, 2010 and $1,833,746 and $1,955,488 for the years ended December 31, 2009 and 2008, respectively. Our research and development expenses can be divided into (i) clinical research and development, and (ii) preclinical research and development activities. Clinical research and development costs consist of clinical trials, manufacturing services, regulatory activities and related personnel costs, and other costs such as rent, utilities, depreciation and stock-based compensation. Preclinical research and development costs consist of our research activities, preclinical studies, related personnel costs and laboratory supplies, and other costs such as rent, utilities, depreciation and stock-based compensation. While we are currently focused on advancing each of our product development programs, our future research and development expenses will depend on the clinical success of each product candidate, as well as ongoing assessments of each product candidate’s commercial potential. In addition, we cannot forecast with any degree of certainty which product candidates may be subject to future collaborations, when such arrangements will be secured, if at all, and to what degree such arrangements would affect our development plans and capital requirements.
We expect our research and development expenses to increase in the future as we continue the advancement of our clinical trials and preclinical product development programs. The lengthy process of completing clinical trials and seeking regulatory approval for our product candidates requires expenditure of substantial resources. Any failure or delay in completing clinical trials, or in obtaining regulatory approvals, could cause a delay in generating product revenues and cause our research and development expenses to increase and, in turn, have a material adverse effect on our operations. Our current product development candidates are not expected to be commercially available before 2011.
Selling, General and Administrative Expenses
Selling, general and administrative expenses consist principally of salaries and related costs for personnel in executive, finance, business development, marketing, information technology, legal and human resources functions. Other general and administrative expenses include facility costs not otherwise included in research and development expenses, patent filing, prosecution and defense costs and professional fees for legal, consulting, auditing and tax services.
We anticipate that our general and administrative expenses will increase for, among others, the following reasons:
| Ø | the sales and marketing of our ProBiora3 products; |
| Ø | to support our research and development activities, which we expect to expand as we continue the development of our product candidates; and |
| Ø | the increased payroll, expanded infrastructure and higher consulting, legal, accounting and investor relations costs associated with being a public company. |
Other Income and (Expense)
Other income and expense includes gain or loss on sale of assets, local business taxes and extinguishment of payables as well as interest income and expense. Interest income consists of interest earned on our cash and cash equivalents and marketable securities. The primary objective of our investment policy is capital preservation. Interest expense consists primarily of interest and costs associated with our loans payable.
38
Table of Contents
Management’s discussion and analysis of financial condition and results of operations
Income Taxes
As of December 31, 2009, we had federal and state net operating loss carryforwards and research and development tax credit carryforwards of approximately $23,125,665 and $384,276, respectively. Our net operating loss and research and development tax credit carryforwards will expire, if not used, between 2010 and 2029. Our ability to utilize our net operating loss and tax credit carryforwards may be limited in the event a change in ownership, as defined in Section 382 of the Internal Revenue Code of 1986, as amended, or the Code, has occurred or may occur in the future. The private placement transaction with the KFLP in June 2009 (the “June 2009 Private Placement”) constituted such an event and our historical loss carryfowards were limited. See “Tax Loss Carryforwards.” In each period since our inception, we have recorded a 100% valuation allowance for the full amount of our deferred tax asset, as the realization of the deferred tax asset is uncertain. As a result, we have not recorded any federal tax benefit in our statements of operations.
Critical Accounting Estimates and Policies
Our discussion and analysis of our financial condition and results of operations are based upon our financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States, or U.S. GAAP. The preparation of financial statements in accordance with U.S. GAAP requires us to make estimates and assumptions that affect reported amounts and related disclosures. We consider an accounting estimate to be critical if it requires assumptions to be made that were uncertain at the time the estimate was made; and changes in the estimate or different estimates that could have been made could have a material impact on our results of operations or financial condition. The principal areas of estimation reflected in the financial statements are stock-based compensation, valuation of warrants, sales returns and allowances and allowance for doubtful accounts.
Revenue Recognition
We recognize revenues from the sales of product when title and risk of loss pass to the customer, which is generally when the product is shipped. Grant revenues are recognized as the reimbursable expenses are incurred over the life of the related grant. Grant revenues are deferred when reimbursable expenses have not been incurred.
We record allowances for discounts and product returns at the time of sales as a reduction of revenues as such allowances can be reliably estimated based on historical experience or known trends. Product returns are limited to specific mass retail customers for expiration of shelf life or unsold product over a period of time. We maintain a return policy that allows our customers to return product within a specified period of time prior to and subsequent to the expiration date of the product. Our estimate of the provision for returns is analyzed quarterly and is based upon many factors, including industry data of product return rates, historical experience of actual returns, analysis of the level of inventory in the distribution channel, if any, and reorder rates. If the history or our product returns changes, the reserve will be adjusted. While we believe that the reserves we have established are reasonable and appropriate based upon current facts and circumstances, applying different judgments to the same facts and circumstances would result in the estimated amounts for sales returns and chargebacks to vary. Because our ProBiora3 products have only recently been introduced, we could experience different circumstances in the future and these differences could be material.
Accounts Receivable
Accounts receivable are recorded at their net realizable value and consist of trade receivables from the sale of product to customers. We analyze accounts receivable on a monthly basis and determine the collectability based on the facts and circumstances relating to each customer. We do not have a history of accounts receivable or write offs, therefore, we estimate our allowance for doubtful accounts based on sales trends and specific review of the creditworthiness of each customer.
39
Table of Contents
Management’s discussion and analysis of financial condition and results of operations
Inventory
Inventories are stated at the lower of cost or market. Cost, which includes material, labor and overhead, is determined on a first-in, first-out basis. On a quarterly basis, we analyze our inventory levels and write-down inventory that is expected to expire prior to being sold, inventory that has a cost basis in excess of its expected net realizable value, inventory in excess of expected sales requirements, or inventory that fails to meet commercial sale specifications through a charge to cost of goods sold. Expired inventory is disposed of and the related costs are written off to cost of goods sold. Charges for inventory write-downs are not reversed if we later determine that the product is saleable. Therefore, any such written-down inventory would be sold at significantly higher margin. If actual conditions are less favorable than those projected by management, additional inventory write-downs may be required.
Stock-Based Compensation
U.S. GAAP requires all share-based payments to employees, including grants of employee stock options, to be recognized in the financial statements based on their fair values as of the grant dates. Stock-based compensation expense is recorded over the requisite service period in which the grantee provides services to us, to the extent the options or warrants do not vest at the grant date and are not subject to forfeiture.
Income Taxes
Income taxes are accounted for under the asset and liability method. Deferred tax assets and liabilities are recognized for future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases and operating loss and tax credit carryforwards.
Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rate is recognized in operations in the period that includes the enactment date. Deferred tax assets are reduced to estimated amounts expected to be realized by the use of a valuation allowance. Based on our historical operating losses, a valuation allowance has been recognized for all deferred tax assets.
New Accounting Pronouncements
In August 2009, the FASB issued ASU 2009-05, “Fair Value Measurements and Disclosures (ASC Topic 820)—Measuring Liabilities at Fair Value” (“Update 2009-05”). Update 2009-05 provides clarification regarding valuation techniques when a quoted price in an active market for an identical liability is not available in addition to treatment of the existence of restrictions that prevent the transfer of a liability. Update 2009-05 also clarifies that both a quoted price in an active market for an identical liability at the measurement date and the quoted price for an identical liability when traded as an asset in an active market (when no adjustments to the quoted price of the asset are required) are Level 1 fair value measurements. This standard is effective for the first reporting period, including interim periods, beginning after issuance. Adoption of Update 2009-05 did not have a material effect on our financial statements.
In October 2009, the FASB issued ASC Update No. 2009-13, Revenue Recognition (Topic 605): Multiple-Deliverable Revenue Arrangements (“Update No. 2009-13”). The consensus in Update No. 2009-13 supersedes certain guidance in Topic 605 (formerly EITF Issue No. 00-21, Multiple-Element Arrangements) and requires an entity to allocate arrangement consideration at the inception of an arrangement to all of its deliverables based on their relative selling prices. The consensus eliminates the use of the residual method of allocation and requires the use of the relative-selling-price method in all circumstances in which an entity recognizes revenues for an arrangement with multiple deliverables subject to ASC 605-25. We are required to adopt Update No. 2009-13 as of January 1, 2011 and are in the process of determining the impact, if any. However, we do not believe that the adoption of Update No. 2009-13 will have a material effect on our financial statements.
40
Table of Contents
Management’s discussion and analysis of financial condition and results of operations
Results of Operations
| Six months ended June 30, |
Years ended December 31, |
|||||||||||||||
| 2010 | 2009 | 2009 | 2008 | |||||||||||||
| (unaudited) |
||||||||||||||||
| Net revenues |
$ | 646,179 | $ | 166,167 | $ | 641,285 | $ | 233,539 | ||||||||
| Cost of goods sold |
326,321 | 35,384 | 221,198 | 14,864 | ||||||||||||
| Operating expenses: |
||||||||||||||||
| Research and development |
909,838 | 979,975 | 1,833,746 | 1,955,488 | ||||||||||||
| Selling, general and administrative |
3,167,626 | 2,718,172 | 4,917,844 | 4,312,246 | ||||||||||||
| Total operating expenses |
4,077,464 | 3,698,147 | 6,751,590 | 6,267,734 | ||||||||||||
| Loss from operations |
(3,757,606 | ) | (3,567,364 | ) | (6,331,503 | ) | (6,049,059 | ) | ||||||||
| Other income (expense): |
||||||||||||||||
| Interest income |
2,535 | 522 | 922 | 32,511 | ||||||||||||
| Interest expense |
(885 | ) | (1,504 | ) | (44,292 | ) | (10,054 | ) | ||||||||
| Gain on sale of property and equipment |
— | 11,274 | 22,743 | 4,860 | ||||||||||||
| Gain on extinguishment of payables |
— | 707,674 | 832,959 | — | ||||||||||||
| Local business tax |
(1,465 | ) | — | (177 | ) | — | ||||||||||
| Total other income, net |
185 | 717,966 | 812,155 | 27,317 | ||||||||||||
| Loss before income taxes |
(3,757,421 | ) | (2,849,398 | ) | (5,519,348 | ) | (6,021,742 | ) | ||||||||
| Net loss |
$ | (3,757,421 | ) | $ | (2,849,398 | ) | $ | (5,519,348 | ) | $ | (6,021,742 | ) | ||||
For the Six Months Ended June 30, 2010 and 2009
Net Revenues. We generated net revenues of $646,179 for the six months ended June 30, 2010 compared to $166,167 in the same period in 2009, an increase of $480,012. The increase was primarily attributable to an increase in ProBiora3 product sales, offset by increases in returns, allowances, and discounts of $21,073. The increase in net revenues also included a $25,064 increase in grant revenues attributable to an NSF SBIR Phase II grant for the small peptide antibiotic synthesis program using our proprietary DPOLT.
Cost of Goods Sold. Cost of goods sold was $326,321 for the six months ended June 30, 2010 compared to $35,384 in the same period in 2009, an increase of $290,937. The increase was attributable to increased sales of our ProBiora3 products. Cost of goods sold includes the production and manufacturing costs of our ProBiora3 products sold of $145,850, shipping and processing expenses of $72,086, and scrap expense of $108,385. Scrap expenses represent product rework charges, inventory adjustments, and damaged inventory.
Research and Development. Research and development expenses were $909,838 for the six months ended June 30, 2010 compared to $979,975 in the same period in 2009, a decrease of $70,137, or 7.2%. This decrease in research and development expenses was primarily due to depreciation expense savings stemming from the full depreciation of certain lab equipment.
Selling, General and Administrative. Selling, general and administrative expenses were $3,167,626 for the six months ended June 30, 2010 compared to $2,718,172 in the same period in 2009, an increase of $449,454, or 16.5%. This increase was due to increases in stock-based compensation expense of $219,880, salary and fringe costs of $227,052 as a result of
41
Table of Contents
Management’s discussion and analysis of financial condition and results of operations
additional staff and increases in compensation of existing personnel, advertising and marketing expenses of $656,560 and non-employee director stock-based compensation expense of $61,879. The increase in selling, general and administrative expense was offset by reductions in other expenses, including reductions in travel and convention expenses of $55,110 as a result of reduced global travel, legal and professional support service fee savings of $409,786, and consultant expenses of $259,227 associated primarily with reduced investor relations consulting spending.
Other Income (Expense). Other income and expense was $185 for the six months ended June 30, 2010 compared to $717,966 in the same period in 2009, a decrease of $717,781. The decrease was primarily attributable to a $707,674 gain we recognized in 2009 associated with the extinguishment of payables in connection with the June 2009 Private Placement, and a $11,274 gain we realized in 2009 on the sale of assets. In addition, the decrease in other income and expense for the period was impacted by a decrease in interest expense of $619, an increase in interest income of $2,013, as well as local business tax expenses we incurred during the period of $1,465.
For the Years Ended December 31, 2009 and 2008
Net Revenues. We generated net revenues of $641,285 for the year ended December 31, 2009 compared to $233,539 in the same period in 2008, an increase of $407,746. The increase in net revenues was primarily attributable to increased ProBiora3 product sales. The increase in net revenues also included a $49,484 increase in grant revenues.
Cost of Goods Sold. Cost of goods sold was $221,198 for the year ended December 31, 2009 compared to $14,864 in the same period in 2008, an increase of $206,334. This increase was attributable to increased sales of our ProBiora3 products. Cost of goods sold also includes shipping and warehouse processing expenses of $67,864 and scrap expense of $24,737.
Research and Development. Research and development expenses were $1,833,746 for the year ended December 31, 2009 compared to $1,955,488 in the same period in 2008, a decrease of $121,742, or 6.2%. The decrease was primarily attributable to a reduction in stock based compensation expense of $153,191.
Selling, General and Administrative. Selling, general and administrative expenses were $4,917,844 for the year ended December 31, 2009 compared to $4,312,246 in the same period in 2008, an increase of $605,598, or 14.0%. The increase was primarily attributable to increases in selling and marketing salaries and benefits and advertising expenses of $983,349, consulting expenses of $280,604, officer/staff salaries and benefits of $117,174, travel related expenses of $48,768, stock based compensation expense of $31,709 and non-employee director fees of $26,347. The increases in selling, general and administrative expense was offset by decreases in legal fees of $440,463, filing/registration fees of $360,263, relocation expenses of $44,300 and bank financing fees of $22,572.
Other Income (Expense). Other income and expense was $812,155 for the year ended December 31, 2009 compared to $27,317 in the same period in 2008, an increase of $784,838. The increase was primarily attributable to a $832,959 gain we recognized on the extinguishment of payables due to the reduction in expenses associated with the June 2009 Private Placement. Interest expense was $44,292 for the year ended December 31, 2009 compared to $10,054 in the same period in 2008, an increase of $34,238. This increase was primarily attributable to interest paid on a short term note with an accredited investor and on a long term note we entered into in connection with the June 2009 Private Placement. Interest income decreased by $31,589 for the year ended December 31, 2009 compared to the same period in 2008. This decrease was primarily due to the nominal money market interest rates available during 2009.
42
Table of Contents
Management’s discussion and analysis of financial condition and results of operations
Liquidity and Capital Resources
Since our inception, we have funded our operations primarily through the sale of equity securities in our initial public offering, the sale of equity securities and warrants in private placements, debt financing and grants. The following table sets forth the primary sources and uses of cash for each of the periods indicated:
| Six months ended June 30, |
Years ended December 31, |
|||||||||||
| 2010 | 2009 | 2008 | ||||||||||
| (unaudited) | ||||||||||||
| Net cash used in operating activities |
$ | (3,281,330 | ) | $ | (5,799,481 | ) | $ | (3,835,190 | ) | |||
| Net cash provided by (used in) investing activities |
(6,198 | ) | 30,927 | (13,072 | ) | |||||||
| Net cash provided by financing activities |
3,202,688 | 4,904,213 | 4,538,687 | |||||||||
| Net increase (decrease) in cash and cash equivalents |
$ | (84,840 | ) | $ | (864,341 | ) | $ | 690,425 | ||||
During the six months ended June 30, 2010 and the years ended December 31, 2009 and 2008, our operating activities used cash of $3,281,330, $5,799,481 and $3,835,190, respectively. The use of cash in all periods primarily resulted from our net losses adjusted for non-cash items and changes in operating assets and liabilities. We had negative working capital of $140,223 as of June 30, 2010 compared to positive working capital of $2,564,147 as of December 31, 2009 and negative working capital of $500,672 as of December 31, 2008.
During the six months ended June 30, 2010 and the years ended December 31, 2009 and 2008, our investing activities provided (used) cash of ($6,198), $30,927 and ($13,072), respectively. The cash provided and used in connection with investing activities primarily related to purchases and sales of equipment.
During the six months ended June 30, 2010 and the years ended December 31, 2009 and 2008, our financing activities provided cash of $3,202,688, $4,904,213 and $4,538,687, respectively. The cash provided by financing activities in the year ended December 31, 2008 was primarily due to sales of our common stock in private placement transactions. The cash provided by financing activities in the year ended December 31, 2009 was primarily due to our debt and equity financings including the June 2009 Private Placement and the December 2009 Private Placement. The cash provided by investing activities in the six months ended June 30, 2010 was primarily due to the release of restrictions on cash, borrowings under a note payable from a shareholder, offset by reductions in long term notes payable and reductions in proceeds from issuances of common stock.
August 2007 Private Placement
In August 2007, we issued a total of 230,000 shares of restricted common stock and warrants to acquire 230,000 shares of common stock (the “August 2007 Warrants”) in a private placement to accredited investors, for total proceeds of $1,171,591. The shares were sold at a price per share of $5.00, except that per requirements of our former listing exchange applicable at the time, our former chief executive officer participating in the purchase was required to acquire shares at $8.80 per share, which was the closing share price on August 7, 2007. The August 2007 Warrants were exercisable at $11.60 per share. The August 2007 Warrants expired on August 8, 2008. During the first quarter of 2008 we amended the August 2007 Warrants to reduce the exercise price to $8.80, which was the fair market value on the date of the amendment. The amended exercise price was applicable from January 28, 2008 to February 29, 2008. In February 2008, we issued 226,818 shares of common stock upon exercise of the August 2007 Warrants at the amended exercise price resulting in additional working capital proceeds to us of $1,996,000. The remaining unexercised August 2007 Warrants expired on August 8, 2008.
43
Table of Contents
Management’s discussion and analysis of financial condition and results of operations
June 2008 Private Placement
In June 2008, we issued a total of 288,888 shares of restricted common stock and warrants to acquire 288,888 shares of common stock (the “June 2008 Warrants”) in a private placement to accredited investors, for net proceeds of $2,515,000 (the “June 2008 Private Placement”). The shares were sold at price per share of $9.00. The June 2008 Warrants were exercisable at $26.00 per share. The June 2008 Warrants expire May 30, 2013. Of the June 2008 Warrants, 161,000 were subsequently amended to reduce the exercise price in connection with the June 2009 Private Placement discussed below.
June 2009 Private Placement
On June 29, 2009, we issued a total of 2,500,000 shares of restricted common stock and warrants to acquire 50,000 shares of common stock in a private placement to the Koski Family Limited Partnership, or KFLP, for total proceeds of $4,000,000 (the “June 2009 Private Placement”). The shares were sold at $1.60 per share. The warrants to purchase 50,000 shares of our common stock were exercisable at $2.00 per share and had a five year term. The consideration paid by the KFLP for the shares of common stock consisted of $4,000,000 as follows: $1,500,000 in cash at closing and $2,500,000 pursuant to a non-interest bearing promissory note providing for five consecutive monthly installment payments of $500,000 commencing July 31, 2009. In addition, pursuant to the securities purchase agreement (the “June 2009 Purchase Agreement”) with the KFLP, the KFLP also provided a secured loan of $1,000,000 to us. The loan was secured by substantially all of our assets, excluding receivables, and paid interest at the rate of prime plus 4.0% which was payable quarterly. This loan was subsequently repaid by us in connection with the December 2009 Private Placement described below. The principal of the loan was due in five years. As a result of the June 2009 Private Placement the Board of Directors believes there was a change of control, with the KFLP acquiring a controlling interest in our outstanding voting common stock. We also agreed to provide the KFLP with certain registration rights in connection with any underwritten or other offering by us over the next five years. Specifically, we are obligated to register on behalf of the KFLP shares of common stock held by the KFLP equal to 15% of the total number of shares of common stock to be sold by us in a public offering subject to the discretion of the managing underwriter on the inclusion of shares in the offering to be sold by selling shareholders.
In addition to the above, as a further condition to the consummation of the transaction contemplated by the June 2009 Purchase Agreement, we were required to obtain satisfactory arrangements with three main creditors for reductions in the amounts payable by us to these creditors. As of June 30, 2009, these reductions amounted to $707,674 in the aggregate and were conditioned upon prompt payment of the remaining balances owed to such creditors after taking into account the agreed upon reductions. As of December 31, 2009, the amount of reductions arranged with our creditors totaled $832,959. These agreed upon reductions in payables have been fully reflected in our financial statements for the periods and reported under other income.
In connection with, and as a closing condition to the June 2009 Private Placement, the purchasers in the June 2008 Private Placement (including George Hawes, our largest shareholder prior to the June 2009 Private Placement), entered into a consent, waiver and mutual release agreement with us on June 25, 2009. In addition, the purchasers in the June 2008 Private Placement waived and relinquished any special rights they possessed pursuant to the agreements with us as part of the June 2008 Private Placement, including, but not limited to, (i) rights of first refusal, (ii) antidilution regarding future equity sales and (iii) covenants regarding secured lending by us. In connection with such consents, waivers and mutual releases, warrants to acquire 161,000 shares that were previously issued in connection with the June 2008 Private Placement were subject to the right of exchange for new replacement warrants to acquire the same number of shares under the same terms except for a change in the exercise price from $26.00 to $15.00. In addition, to the extent of any future underwritten registered offerings of our common stock, or the filing of any resale registration statement by us, in each case occurring within five years from the date of the consent, waiver and mutual release, the purchasers shall have the right to include an aggregate of up to 5.0% of the shares being registered in such offering or registration statement, subject to the discretion, in any underwritten primary offerings by us, of the underwriter on the inclusion of shares in the offering to be sold by selling shareholders.
44
Table of Contents
Management’s discussion and analysis of financial condition and results of operations
December 2009 Private Placement
On December 30, 2009, we issued a total of 500,812 shares of restricted common stock in the initial closing of a private placement to accredited investors including the KFLP, our largest shareholder (the “December 2009 Private Placement”), for initial proceeds of $2,504,062. The shares were sold at $5.00 per share. The initial closing proceeds of $2,504,062 included the cancellation at closing of $54,062 in outstanding obligations we owed to Dr. Jeffrey Hillman, our Chief Scientific Officer, for compensation that had been deferred. Approximately half of the total investment, or $1,250,000, was made by the KFLP. In conjunction with, and as a condition to the initial closing of the December 2009 Private Placement, we also issued 200,000 shares of our common stock to the KFLP at $5.00 per share, which was the same price per share paid by the participating accredited investors, in exchange for the cancellation of the KFLP’s $1,000,000 secured promissory note we previously issued to the KFLP in connection with the June 2009 Private Placement.
Approximately $1,000,000 of the total proceeds from the December 2009 Private Placement were committed to further our development of the DPOLT synthetic chemistry platform, essential to the production of our lead antibiotic, MU1140, subject to the goals set forth by the two-year NSF SBIR Phase II grant that we received on February 15, 2008. Such allocation enabled us to be eligible to receive up to an additional $500,000 matching grant from the NSF, which grant was subsequently awarded in June 2010.
Contemporaneously with the initial closing of the December 2009 Private Placement, the KFLP also elected to exercise warrants it received as part of the June 2009 Private Placement to purchase 50,000 shares of our common stock. The warrants were exercised through the payment by the KFLP of the warrant exercise price of $2.00 per share. Additionally, Christine Koski and Robert Koski, as directors, each exercised previously issued options to purchase 5,000 shares of our common stock at the option exercise price of $2.00 per share. These options were granted to Christine Koski and Robert Koski when they became non-employee directors on June 30, 2009 in connection with our non-employee director compensation program.
On January 13, 2010, we completed the $3,004,062 private placement contemplated by the December 2009 Private Placement and issued another 100,000 shares of common stock at a price per share of $5.00 to the accredited investors for $500,000. Of this amount, the KFLP again participated in half of the remainder of the aggregate investment by acquiring 50,000 shares for $250,000.
May 2010 Note Financing
On May 28, 2010, we entered into an unsecured promissory note with a conversion provision (the “May 2010 Note”) to the KFLP pursuant to which we borrowed $1,000,000 from the KFLP. Interest on the May 2010 Note accrued at the rate of LIBOR plus 6.0% and the principal of the May 2010 Note, together with all accrued interest thereon, was due and payable the earlier of: (i) the closing date of a registered public offering of newly issued equity securities by us resulting in cash proceeds to us, other than in connection with employee option plans, or (ii) the May 24, 2011 maturity date; provided, however, that in the event we completed a subsequent private offering of equity securities prior to the May 24, 2011 maturity date, we could elect to convert the principal of the May 2010 Note into the same equity securities being sold in the private offering at the same price and terms to the KFLP.
July 2010 Financing Transaction
On July 5, 2010, we entered into a common stock purchase agreement (the “July 2010 Financing Transaction”) with the Koski Family Limited Partnership, or KFLP. At the closing of this financing transaction on July 30, 2010 we issued 250,000 shares of our common stock to the KFLP at a price of $8.00 per share. The $2,000,000 aggregate consideration paid by the KFLP consisted of (i) $1,000,000 cash and (ii) the exchange and cancellation of the outstanding May 2010 Note issued to the KFLP on May 28, 2010. Accrued interest on the May 2010 Note through closing was waived by the KFLP. Concurrent with the July 2010 Financing Transaction and as part thereof, we entered into an unsecured revolving credit agreement (the “Credit Facility”) with the KFLP. Pursuant to the Credit Facility, we are able to borrow up to $2,000,000 from the KFLP at LIBOR plus
45
Table of Contents
Management’s discussion and analysis of financial condition and results of operations
6.0%. The term of the Credit Facility is for 12 months commencing August 1, 2010. As of the date of this prospectus, we have drawn $1,000,000 on this Credit Facility. Our continued ability to draw on the Credit Facility is subject to (i) the receipt by the KFLP of a certificate of no adverse change from us in form and substance acceptable to the KFLP, (ii) the receipt by the KFLP of a revolving unsecured promissory note from us in the principal drawn down in the form attached to the Credit Facility and (iii) our compliance with the terms of the Credit Facility.
Other Financings
In June 2008, we entered into a short-term note payable for $67,426 bearing interest at the rate of 5.75% to finance directors’ and officers’ and employment-related practices liability insurance. This note required principal and interest payments to be made evenly over a ten month period and was repaid in full on April 30, 2009 in accordance with its terms.
On March 17, 2009, we entered into a short-term note payable for $53,087 with an interest rate of 5.75% to finance product liability insurance. This note required principal and interest payments to be made evenly over a ten-month period and was repaid in full at December 31, 2009.
On April 15, 2009 we entered into a loan agreement with an accredited investor for a short-term note in the amount of $100,000. The note included an interest rate of 15% per annum and its maturity date was April 15, 2011. On August 21, 2009 we repaid this short-term note and outstanding accrued interest in full. In connection with this borrowing we also issued warrants to acquire 5,000 shares of our common stock at an exercise price of $10.00 per share to the investor and such warrants are exercisable for five years.
On May 4, 2009 and June 10, 2009, we borrowed $32,556 and $13,100, respectively, from Dr. Jeffery Hillman, our founder, Chief Scientific Officer and Director. These borrowings were to be repaid upon demand by Dr. Hillman, were unsecured and did not bear interest. The proceeds from these borrowings were used to purchase inventory for our Consumer Healthcare products division. On June 29, 2009 the aggregate amount of these obligations of $45,656 were repaid by us in full through the issuance of 22,828 shares of our common stock at a price of $2.00 per share, which was the closing price of our common stock on June 29, 2009.
On August 6, 2009 we entered into a short-term note payable for $70,025 with an interest rate of 5.75% to finance directors’ and officers’ liability insurance. This note required principal and interest payments to be made evenly over a ten-month period and was repaid in full on May 24, 2010 in accordance with its terms.
On March 17, 2010, we entered into a short-term note payable for $50,637 with an interest rate of 5.75% to finance product liability insurance. Payments on this note are made evenly based on a straight line amortization over a ten-month period with the final payment due on January 10, 2011. At June 30, 2010 the outstanding balance due was $30,382.
On July 9, 2010, we entered into a non-interest bearing short-term note payable for $22,188 to finance a portion of our new enterprise resource planning system. Payments on this note began July 9, 2010 and are made quarterly with the final payment due on April 1, 2011.
On July 20, 2010 we entered into a short-term note payable for $65,529 with an interest rate of 5.75% to finance directors’ and officers’ liability insurance. Payments on this note begin on August 24, 2010 and are made evenly based upon a straight line amortization over a ten-month period with the final payment due on July 24, 2011.
On July 31, 2010, we entered into a short-term note payable for $85,185 bearing interest at 7.5% to finance a portion our new enterprise resource planning system. Principal and interest payments on this note begin August 31, 2010 and are made evenly based on a straight line amortization over a 17-month period with the final payment due on December 31, 2011.
46
Table of Contents
Management’s discussion and analysis of financial condition and results of operations
Grants
On February 15, 2008, we were awarded a two-year NSF SBIR Phase II grant to advance development of DPOLT. This federal grant supports studies focused on the synthesis and testing of our lead antibiotic, MU1140. While the grant will total $500,000, to date we have received $425,000 of these restricted funds.
On September 1, 2009 we received a grant funding from the University of Florida under the prime grant with the Florida Citrus Production Advisory Council in the amount of $124,570. The purpose of the University of Florida grant is to identify disease-specific proteins expressed during citrus greening using our proprietary PCMAT biomarker technology.
On June 14, 2010 we were awarded the matching $500,000 grant from the NSF to support the previously awarded SBIR Phase II grant for further development of our DPOLT platform. On June 17, 2010, we received $125,000 of a $500,000 NSF awarded SBIR II Phase II grant for the company’s DPOLT platform. Proceeds from the financing are to be allocated to further the development of our DPOLT platform, essential to the production of our lead antibiotic, MU1140, subject to the goals set forth by the NSF SBIR Phase II grant received by us. The remainder of these grant funds are expected to be provided to us in $125,000 increments over the next 18 to 24 months.
Future Capital Requirements
Our capital requirements for the remainder of 2010 will depend on numerous factors, including the success of our commercialization efforts and of our research and development, the resources we devote to develop and support our technologies and the success of pursuing strategic licensing and funded product development relationships with external partners. Subject to our ability to generate revenues and cash flow from our ProBiora3 products and our ability to raise additional capital including through possible joint ventures and/or partnerships, we expect to incur substantial expenditures to further commercialize or develop each of our technologies including continued increases in costs related to research, preclinical testing and clinical studies, as well as significant costs associated with being a public company. We will require substantial funds to conduct research and development and preclinical and Phase 1 clinical testing of our licensed, patented technologies and to develop sublicensing relationships for the Phase 2 and 3 clinical testing and manufacture and marketing of any products that are approved for commercial sale. Our plans include seeking both equity and non-dilutive financing, alliances or other partnership agreements with entities interested in our technologies, or other business transactions that would generate sufficient resources to ensure continuation of our operations and research and development programs as well as seeking equity financing.
In addition, the report of our independent registered public accounting firm with respect to our financial statements appearing at the end of this prospectus contains an explanatory paragraph stating that our operating losses and negative cash flows from operations since inception, and our need to raise additional financing and/or financial support prior to December 31, 2010 in order to continue to fund our operations, raise substantial doubt about our ability to continue as a going concern. We believe that the successful completion of this offering will eliminate this doubt and enable us to continue as a going concern; however, if we are unable to raise sufficient capital in this offering, we will need to obtain alternative financing or significantly modify our operational plans for us to continue as a going concern. Further, even if we successfully complete and receive the net proceeds from this offering, given our planned expenditures for the next several years, including, without limitation, expenditures in connection with our contemplated clinical trials of SMaRT Replacement Therapy and MU1140-S, it is possible that our independent registered public accounting firm may conclude, in connection with their attestation of our financial statements for fiscal years after 2010, that there is substantial doubt regarding our ability to continue as a going concern.
We believe that the net proceeds from this offering, together with our existing cash and cash equivalents, and committed research and development funding, will allow us to fund our operating plan through at least the next 12 months. If our available cash and cash equivalents are insufficient to satisfy our liquidity requirements, or if we develop additional opportunities to do so, we may seek to sell additional equity or debt securities or obtain a credit facility. The sale of additional equity and debt securities may result in additional dilution to our shareholders. If we raise additional funds through the
47
Table of Contents
Management’s discussion and analysis of financial condition and results of operations
issuance of debt securities or preferred stock, these securities could have rights senior to those of our common stock and could contain covenants that would restrict our operations. We may require additional capital beyond our currently forecasted amounts, such as, for example, if we determine to proceed independently with a Phase 3 clinical trial for our SMaRT Replacement Therapy. Any such required additional capital may not be available on reasonable terms, if at all. If we were unable to obtain additional financing, we may be required to reduce the scope of, delay or eliminate some or all of our planned research, development and commercialization activities, which could harm our business.
Because of the numerous risks and uncertainties associated with sales of our ProBiora3 products as well as research, development and commercialization of pharmaceutical products, we are unable to estimate the exact amounts of our working capital requirements. Our future funding requirements will depend on many factors, including, but not limited to:
| Ø | the cash flow generated from our ProBiora3 product sales; |
| Ø | the number and characteristics of the product candidates we pursue; |
| Ø | the scope, progress, results and costs of researching and developing our product candidates, and conducting preclinical and clinical trials; |
| Ø | the timing of, and the costs involved in, obtaining regulatory approvals for our product candidates; |
| Ø | the cost of commercialization activities for our ProBiora3 products and, if any of our product candidates are approved for sale, including marketing, sales and distribution costs; |
| Ø | the cost of manufacturing our ProBiora3 products and product candidates and any products we successfully commercialize; |
| Ø | our ability to establish strategic partnerships, licensing or other arrangements and the financial terms of such agreements; |
| Ø | the costs involved in preparing, filing, prosecuting, maintaining, defending and enforcing patent claims, including litigation costs and the outcome of such litigation; and |
| Ø | the timing, receipt and amount of sales of, or royalties on, our products and future products, if any. |
Off-Balance Sheet Arrangements
We have no off-balance sheet arrangements.
Tax Loss Carryforwards
As of December 31, 2009, we have net operating loss carryforwards of approximately $23,000,000 to offset future federal and state income taxes. We also have research and development and investment tax credit carryforwards of approximately $400,000 to offset future federal and state income taxes. Any greater than 50% change in ownership under Section 382 of the Internal Revenue Code, or the Code, places significant annual limitations on the use of such net operating loss carryforwards and we exceeded the 50% threshold when we consummated the June 2009 Private Placement transaction with the KFLP. As a result, our historical loss carryforwards through June 2009 will be limited to $172,000 per year over the next 20 years, or limited to an aggregate amount of up to $3,440,000 of such historical loss carryforwards over such period of time, and the remaining balance of our historical loss carryforwards prior to June 2009 will expire unused. Provided that there are no future
48
Table of Contents
Management’s discussion and analysis of financial condition and results of operations
ownership changes that would trigger the limitations on loss carryforwards provided under the Code, the operating losses we experience after the June 2009 Private Placement transaction are expected to add to our loss carryforwards and to be fully available to us.
At December 31, 2009, we recorded a 100% valuation allowance against our deferred tax assets of approximately $8,700,000, as our management believes it is uncertain that they will be fully realized. If we determine in the future that we will be able to realize all or a portion of our net operating loss carryforwards, an adjustment to our net operating loss carryforwards would increase net income in the period in which we make such a determination.
Inflation
Inflation affects the cost of raw materials, goods and services that we use. In recent years, inflation has been modest. However, high energy costs and fluctuations in commodity prices can affect the cost of all raw materials and components. The competitive environment somewhat limits our ability to recover higher costs resulting from inflation by raising prices. Although we cannot precisely determine the effects of inflation on our business, it is our belief that the effects on revenues and operating results will not be significant. We do not believe that inflation has had a material impact on our results of operations for the periods presented, except with respect to payroll-related costs and other costs arising from or related to government imposed regulations.
49
Table of Contents
Overview
We are a biopharmaceutical company focused primarily on oral health products and novel antibiotics. Within oral health, we are developing our pharmaceutical product candidate, SMaRT Replacement Therapy, and we are also commercializing our oral probiotic blend, ProBiora3. Within antibiotics, we are developing our pharmaceutical product candidate, MU1140-S, and we intend to use our patented, novel organic chemistry platform to create additional antibiotics for therapeutic use.
Our SMaRT Replacement Therapy is designed to be a painless, one-time, five-minute topical treatment applied to the teeth that has the potential to offer lifelong protection against dental caries, or tooth decay. Dental diseases are the most prevalent chronic infectious diseases in the world, affecting up to 90% of schoolchildren and the vast majority of adults. In 2009, Popular Mechanics magazine named SMaRT Replacement Therapy as the “#1 New Biotech Breakthrough That Will Change Medicine.” In the United States alone, the annual cost to treat tooth decay is estimated to be $40 billion. SMaRT is based on the creation of a genetically modified strain of bacteria that colonizes in the oral cavity and replaces native decay-causing bacteria. We are commencing a second Phase 1 clinical trial for our SMaRT Replacement Therapy, which we expect to conclude in the first half of 2011.
We have also developed and are commercializing a variety of products that contain the active ingredient ProBiora3, a patent-pending blend of oral probiotics that promote fresher breath, whiter teeth and support overall oral health. The global probiotics market is expected to be $31.2 billion by 2014, representing a compound annual growth rate, or CAGR, of 12.6% from 2009. We have conducted extensive scientific studies on ProBiora3, in order to market our products under self-affirmed Generally Recognized As Safe status, or GRAS. We sell our ProBiora3 products through multiple distribution channels, and our customers include Walgreens, Rite Aid, and Garden of Life, among others.
While developing SMaRT Replacement Therapy, members of our scientific team discovered that the SMaRT bacterial strain produces MU1140, a molecule belonging to the novel class of antibiotics known as lantibiotics. MU1140 has proven active preclinically against Gram positive bacteria responsible for a number of HAIs. The direct cost to the U.S. healthcare system from HAIs is estimated to be up to $45 billion annually. We are in the process of scaling up production of our synthetic form of MU1140, or MU1140-S, and expect to commence preclinical testing during the second half of 2010 and to file an Investigational New Drug, or IND, application with the FDA in mid-2011. The key technology behind the production of MU1140-S is our Differentially Protected Orthogonal Lanthionine Technology platform, or DPOLT, which is a patented, novel organic chemistry platform that we believe will enable the first ever commercial scale, cost-effective production any of the 50 known lantibiotics. We intend to use DPOLT to create a pipeline of lantibiotics for therapeutic use.
Oragenics was founded in 1996 to commercialize the results of more than 30 years of research in oral biology by our principal founder and Chief Scientific Officer, Dr. Jeffrey Hillman. Dr. Hillman earned a DMD from Harvard School of Dental Medicine and a PhD in Molecular Genetics from Harvard University. He began his research career at the Harvard-affiliated Forsyth Institute in Boston, Massachusetts, where he introduced the concept of replacement therapy to prevent tooth decay by using a genetically modified strain of Streptococcus mutans, or S. mutans, to replace the decay-causing strains of S. mutans that are present on human teeth. He subsequently continued this research, now called SMaRT Replacement Therapy, at the University of Florida College of Dentistry. Under Dr. Hillman’s leadership, our scientific team has also developed other technologies such as ProBiora3, MU1140 and our DPOLT platform. Additionally, we are developing non-core technologies that originated from the discoveries of our scientific team, including LPT3-04, which is a potential weight loss product, and PCMAT, which is a biomarker discovery platform, both of which we believe could provide significant potential opportunities for us.
50
Table of Contents
Business
Our Product Portfolio
We are currently developing or commercializing three primary products or product candidates, including SMaRT Replacement Therapy, ProBiora3, and MU1140-S. Our product portfolio is protected by eight issued U.S. patents and eight filed U.S. patent applications, including patents exclusively licensed from the University of Florida Research Foundation, Inc., or UFRF. We are the exclusive worldwide licensee to the patents for SMaRT Replacement Therapy and MU1140, which are owned by the UFRF. We have retained worldwide commercialization rights to each of these products. Additionally, we believe that our SMaRT Replacement Therapy will qualify for a 12-year exclusivity period in the United States under the recently passed Patient Protection and Affordable Care Act and the Health Care and Education Reconciliation Act.
| Product/Candidate |
Description |
Application |
Status | |||
| SMaRT Replacement Therapy | Genetically modified strain of S. mutans that does not produce lactic acid | Tooth decay | Second Phase 1 clinical trial | |||
| ProBiora3 | Blend of three beneficial oral probiotic bacteria | Oral health, teeth whitening, breath freshening (humans, companion pets) | Commercial (GRAS) | |||
| MU1140-S | Member of lantibiotic class of antibiotics | Healthcare-associated infections | Preclinical testing | |||
SMaRT Replacement Therapy
SMaRT Replacement Therapy is designed to be a painless, one-time, five-minute topical treatment applied to the teeth that has the potential to offer lifelong protection against tooth decay caused by S. mutans, the principal cause of this disease. We have extensively and successfully tested the SMaRT strain for safety and efficacy in laboratory and animal models, and we are in the process of commencing a second Phase 1 clinical trial with an attenuated version of our SMaRT Replacement Therapy.
Market Opportunity
Dental diseases are the most prevalent chronic infectious diseases in the world, affecting up to 90% of schoolchildren and the vast majority of adults. Annual expenditures on the treatment of dental caries in the U.S. are estimated to be $40 billion a year according to the Dental, Oral and Craniofacial Data Resource Center. Tooth decay is characterized by the demineralization of enamel and dentin, eventually resulting in the destruction of the teeth. Dietary sugar is often misperceived as the cause of tooth decay; however, the immediate cause of tooth decay is lactic acid produced by microorganisms that metabolize sugar on the surface of the teeth. Studies suggest that of the approximately 700 oral microorganisms, S. mutans, a bacterium found in virtually all humans, is the principal causative agent in the development of tooth decay. Residing within dental plaque on the surface of teeth, S. mutans derives energy from carbohydrate metabolism as it converts dietary sugar to lactic acid which, in turn, promotes demineralization in enamel and dentin, eventually resulting in a cavity. The rate at which mineral is lost depends on several factors, most importantly the frequency and amount of sugar that is consumed.
Fluoride is used to reduce the effect of lactic acid-based demineralization of enamel and dentin. Despite the widespread use of fluoride in public water systems, toothpastes, dental treatments and sealants, and antiseptic mouth rinses, over 50% of 5-to-9-year-olds and almost 80% of 17-year-olds in the United States have at least one cavity or filling, according to the U.S. Surgeon General. In addition to non-compliance with the behavioral guidelines of the American Dental Association such as routine brushing and flossing, there are several factors that are likely to increase the incidence and frequency of tooth decay,
51
Table of Contents
Business
including increasing consumption of both dietary sugar and bottled water. Bottled water generally does not contain fluoride, and thus does not impart any of the protective effects of fluoridated water from public systems. In 2008, U.S. consumers drank more bottled water than any other alcoholic or non-alcoholic beverage, with the exception of carbonated soft drinks, according to the Beverage Marketing Corporation.
Our Solution
Our replacement therapy technology is based on the creation of a genetically altered strain of S. mutans, called SMaRT, which does not produce lactic acid. Our SMaRT strain is engineered to have a selective colonization advantage over native S. mutans strains in that SMaRT produces minute amounts of a lantibiotic that kills off the native strains but leaves the SMaRT strain unharmed. Thus SMaRT Replacement Therapy can permanently replace native lactic acid-producing strains of S. mutans in the oral cavity, thereby potentially providing lifelong protection against the primary cause of tooth decay. The SMaRT strain has been extensively and successfully tested for safety and efficacy in laboratory and animal models.
SMaRT Replacement Therapy is designed to be applied topically to the teeth by a dentist, pediatrician or primary care physician during a routine office visit. A suspension of the SMaRT strain is administered using a cotton-tipped swab during a single five-minute, pain-free treatment. Following treatment, the SMaRT strain should displace the native, decay-causing S. mutans strains over a six to twelve month period and permanently occupy the niche on the tooth surfaces normally occupied by native S. mutans.
Tooth decay is a largely preventable disease through implementation of an appropriate oral care hygiene program including brushing, flossing, irrigation, sealants and antiseptic mouth rinses. Nevertheless, tooth decay remains the most common chronic infectious disease in the world, which indicates that the lack of patient compliance with an overall oral care regimen remains a critical issue in tooth decay prevention. We believe that SMaRT Replacement Therapy addresses the issue of patient compliance by requiring only a one-time, five-minute treatment for the potential lifelong prevention of tooth decay.
Regulatory Status
We initiated our first Phase 1 clinical trial in April 2005, but we found it difficult to find subjects who fit the trials’s highly cautious inclusion and exclusion criteria, particularly with respect to the subjects’ lack of dentition. We concluded this trial early after enrolling only two of the 15 planned subjects. The FDA then recommended that we revise the protocol for the evaluation of ten healthy male subjects, ranging from 18 to 30 years old and with normal dentition, in an institutionalized setting. After we submitted additional information, the FDA issued a clinical hold letter in June 2007 for the proposed trial with the attenuated strain, citing the need for a plan with respect to serious adverse effects; a plan for the eradication of the attenuated strain in trial subjects’ offspring; and a required pregnancy test for female partners of subjects. We submitted additional protocols in response to the FDA’s concerns. In August 2007, the FDA issued a clinical hold letter with required revisions to the protocol for offspring of subjects. We submitted a response to the clinical hold letter in September 2007, and the FDA removed the clinical hold for our Phase 1 trial in the attenuated strain in October 2007.
We are in the process of commencing a second Phase 1 clinical trial of an attenuated version of our SMaRT Replacement Therapy, which will examine the safety and genetic stability of the SMaRT strain during administration to ten healthy adult male subjects over a two-week period. As a precautionary measure, this trial will use an attenuated version of the SMaRT strain that is dependent on D-alanine, which is a specific amino acid not normally found in the human diet. D-Alanine will be administered though a mouthwash provided to the patient group, and must be administered daily or the attenuated strain will perish in the oral cavity. We expect the second Phase 1 clinical trial of the attenuated strain, including a three-month follow-up examination of subjects, to be concluded in the first half of 2011. If the second Phase 1 trial of the attenuated strain is successful and if the FDA lifts the clinical hold on the IND for the non-attenuated version of the SMaRT strain, we anticipate that we would conduct a third Phase 1 trial using the non-attenuated SMaRT strain instead of the attenuated version.
52
Table of Contents
Business
The SMaRT strain has been extensively and successfully tested in the laboratory as well as in animal models , and has demonstrated the following:
| Ø | No lactic acid creation under any cultivation conditions tested; |
| Ø | Dramatically reduced ability to cause tooth decay; |
| Ø | Genetic stability as demonstrated in mixed culture and biofilm studies and in rodent model studies; |
| Ø | Production of a level of MU1140 that is comparable to its wild-type parent strain, which was previously shown to readily and persistently colonize the human oral cavity; and |
| Ø | Aggressive displacement of native, decay-causing strains of S. mutans and preemptive colonization of its niche on the teeth of laboratory rats. |
In addition, during preclinical and early-stage clinical testing of our SMaRT Replacement Therapy, we observed the following:
| Ø | No adverse side effects in either acute or chronic testing in rodent models; |
| Ø | Colonization of the treated subjects following a five-minute application of SMaRT Replacement Therapy in our first Phase 1 study using the attenuated strain; and |
| Ø | No adverse side effects during our first Phase 1 study. |
We conducted a preclinical study in which one group of rats was treated with the native S. mutans strain, and a second group of rats was treated with the SMaRT strain. Both groups of rats were subsequently fed a high-sugar diet for eight weeks. The group of rats treated with the SMaRT strain showed dramatically increased protection from tooth decay as compared to the group of rats treated with native S. mutans.
Rodent Teeth Treated with Native S. mutans (left) and SMaRT Strain (right)
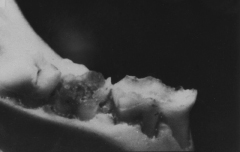
|
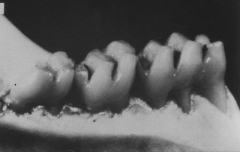
|
Our Strategy
Our strategy is to develop our SMaRT Replacement Therapy through Phase 1 clinical trials. Assuming the successful completion of Phase 1 trials, we intend to license our SMaRT Replacement Therapy to, or partner with, a major pharmaceutical company. We believe that the completion of Phase 1 trials would definitively establish clinical safety and therefore would represent a significant milestone in the development of SMaRT Replacement Therapy, which we anticipate would result in a substantial increase in the value of this technology. If we are unable to negotiate acceptable terms with a
53
Table of Contents
Business
licensee or partner after Phase 1 trials have been completed, and assuming we are not required to undertake Phase 2 trials, we may consider pursuing Phase 3 clinical trials independently. However, we anticipate that we would partner with a major pharmaceutical company prior to marketing the product if our SMaRT Replacement Therapy ultimately achieves FDA approval.
Manufacturing
The manufacturing methods for producing the SMaRT strain of S. mutans are standard Good Manufacturing Practice, or GMP, fermentation techniques. These techniques involve culturing bacteria in large vessels and harvesting them at saturation by centrifugation or filtration. The cells are then freeze dried or suspended in a pharmaceutical medium appropriate for application in the human oral cavity. These manufacturing methods are commonplace and readily available within the pharmaceutical industry. A single dose of our SMaRT Replacement Therapy contains approximately 10 billion S. mutans cells, which represents approximately 10 milliliters of fermentation product. The SMaRT strain grows readily in a variety of cultivation media and under a variety of common growth conditions including both aerobic and anaerobic incubations. The SMaRT strain can also utilize various carbon and nitrogen sources and is highly acid tolerant. There is no significant limitation to the manufacturing scale of our SMaRT strain other than the size of the containment vessel. For our first Phase 1 clinical trial, we engaged a contract manufacturer to produce an attenuated version our SMaRT strain, using a standard operating procedure provided by us that we believe is readily transferable to outside contract manufacturers with fermentation capabilities.
ProBiora3
ProBiora3 is a proprietary blend of three naturally occurring strains of beneficial bacteria, including Streptococcus oralis, Streptococcus uberis, and Streptococcus rattus, which promote fresher breath, whiter teeth, and support overall oral health. We believe that ProBiora3 is the most comprehensive oral probiotic technology currently available in the oral care market. The scientific basis for the oral health and cosmetic benefits provided by these three strains of bacteria has been documented in numerous peer-reviewed publications over the last 30 years. We promote ProBiora3 as the active ingredient in our over-the-counter consumer branded products, including EvoraPlus, EvoraKids, Teddy’s Pride and EvoraPro. EvoraPlus and EvoraKids are flavored probiotic tablets intended for twice-daily use by adults and children, respectively, after brushing their teeth. Teddy’s Pride is intended for companion pets such as cats and dogs, and comes in powder form, which is odorless and tasteless. The powder is intended to be sprinkled on a pet’s food once per day. EvoraPro is a professional strength product designed for the dental office channel. In addition to our house-branded products, we also market ProBiora3 as an active ingredient for private label products, as well as in bulk for licensing applications.
Market Opportunity
Probiotics are live microorganisms that confer a health benefit to their host when administered in sufficient amounts. The beneficial bacteria in a probiotic formulation help to maintain a healthy balance with bacteria in the body. Examples of common probiotic applications are yogurt containing live cultures, acidophilus capsules to improve digestion, and products for improved immune system and vaginal and urinary tract health. According to MarketsandMarkets, the global probiotics market is expected to reach $31.2 billion by 2014, representing a CAGR of 12.6% from 2009 to 2014. Probiotics products are relatively more common in Asia and Europe, with Europe accounting for nearly 42% and Asia accounting for 30% of the global market. The probiotics market in the United States, however, is emerging, and products that address gastrointestinal problems and other uses are rapidly becoming available, especially as dietary supplements and cultured foods and beverages. The Probiotic Foods & Beverages category currently represents over 75% of the overall probiotics market in the United States.
| Ø | Oral Care: The oral care market in the United States was $9.1 billion in 2008 and is expected to reach $10.9 billion by 2014, according to Packaged Facts. Packaged Facts segments this market into three comprehensive |
54
Table of Contents
Business
| product categories: (i) Dental Preparations, which include toothpastes, tooth cleaners/whiteners, and denture products; (ii) Implements/Appliances, including toothbrushes, dental floss and irrigators; and (iii) Gum/Mouthwash/Breath Fresheners, which represented $2.4 billion of the market in 2009. |
| Ø | Companion Pets: In 2009, approximately 62% of U.S. households owned a pet, with an estimated 38.2 million and 45.6 million households owning cats and dogs, respectively, according to the American Pet Products Association, or APPA. The APPA also estimates that total 2009 U.S. pet industry expenditures were $45.5 billion, representing an increase of 5.3% from 2008. Within this market, approximately $10.4 billion was spent on Supplies/OTC Medicine, representing a 4.0% increase over 2008. |
Our Solution
ProBiora3 is a blend of three naturally occurring strains of bacteria for use in the promotion of oral health, including Streptococcus oralis strain KJ3SM, or S. oralis; Streptococcus uberis strain KJ2SM, or S. uberis; and Streptococcus rattus strain JH145SM, or S. rattus. In a healthy human oral cavity, S. oralis and S. uberis are commonly found in significant amounts, and conversely, the levels of bacteria associated with periodontal disease are usually quite low. The opposite situation prevails in periodontal disease sites, at which the beneficial bacteria S. oralis and S. uberis are usually undetectable. Our scientists have demonstrated that S. oralis and S. uberis produce hydrogen peroxide, which interferes with the growth of certain potentially harmful periodontal bacteria, and also gently and naturally whitens teeth. The third bacterial strain in our ProBiora3 blend, S. rattus, is able to establish and maintain a healthy balance of bacteria on the tooth surfaces by competing with certain other potentially harmful bacteria associated with tooth decay.
ProBiora3 has been extensively tested for safety and efficacy in the laboratory and in animal and human trials. In our pilot human study, a twice-daily administration of ProBiora3 was well tolerated by subjects and no safety issues were observed. ProBiora3 produced substantial decreases in the numbers of key potentially pathogenic bacteria associated with tooth decay and periodontal disease in young healthy adults.
We market products containing ProBiora3 under our own house brand names, and have branded ProBiora3 as an active ingredient for licensing and private labeling. Our house brand products contain different ratios, or blends, of the three natural strains contained in ProBiora3, which vary depending on the intended use of the product. Our ProBiora3 products are designed for repetitive use in order to achieve the intended benefits, which we believe provides us with the potential for recurring revenues as consumers who continue to seek the benefits of our products will continue to make purchases. Our ProBiora3 products include:
| Ø | EvoraPlus: a product with equal weight of all three strains that is optimally designed for the general consumer market. EvoraPlus is a mint-flavored probiotic tablet packaged in a 60-unit box with four 15-dose blister packs, representing a one-month supply. The intended use for EvoraPlus is for consumers to take one tablet twice per day after brushing their teeth. EvoraPlus was initially launched in December 2008, but distribution was limited to sales through our own website. In March 2010, we obtained national distribution for EvoraPlus in the domestic mass retail channel with the addition of Walgreens as a vendor. We have continued to expand our distribution in the domestic mass retail channel, and we believe that EvoraPlus is currently available for stocking at over 17,000 retail stores. The manufacturer’s suggested retail price, or MSRP, for EvoraPlus is $19.95 per box. |
| Ø | EvoraKids: a product that has higher levels of S. rattus, which addresses dental health, but reduced levels of S. oralis and S. uberis since periodontal disease is not typically a pediatric concern. EvoraKids is a fruit-flavored probiotic tablet packaged in a 60-unit box with four 15-dose blister packs, representing a one-month supply. The intended use for EvoraKids is for consumers to take one tablet twice per day after brushing their teeth. We launched distribution of EvoraKids in January 2010 with an MSRP of $19.95 per box. |
55
Table of Contents
Business
| Ø | Teddy’s Pride: a product that has higher levels of S. oralis and S. uberis, which address tooth staining and breath problems common to dogs and cats, but reduced levels of S. rattus since tooth decay is not typically a concern in companion pets. Teddy’s Pride comes in powder form, which is odorless and tasteless. The powder is intended to be sprinkled on a pet’s food once per day. It is sold in a pail containing a measuring scoop that provides the recommended dosage per application, representing a two-month supply. We launched Teddy’s Pride in October 2009 with an MSRP of $24.95 per pail. |
| Ø | EvoraPro: a professional strength version of EvoraPlus that is designed for the dental office channel. EvoraPro is packaged as a ten-dose blister pack and is shrink-wrapped with one box of EvoraPlus. The intended use for EvoraPro is to take one tablet per day for ten days after a routine dental cleaning. EvoraPro can only be purchased from a professional dental office. EvoraPro was launched in early August 2010 with an MSRP of $29.95 per pack. |
Our Regulatory Strategy
We market ProBiora3 as a food ingredient utilizing self-affirmed Generally Recognized as Safe, or GRAS, status. GRAS is available for food products that are generally recognized as being safe for human use and do not claim to treat, prevent, or cure a disease. Furthermore, food products that make only cosmetic or structure-function claims are typically able to enter the market through what is known as self-affirmed GRAS status, which designates that we have performed all necessary research, including the formation of an expert panel to review safety concerns, and are prepared to use these findings to defend ProBiora3’s self-affirmed GRAS status. In 2008, we convened a panel, the members of which we believed to be qualified as experts by their scientific training and professional experience, to analyze and evaluate the safety data for ProBiora3. After review, the panel concluded that the safety data of ProBiora3 was sufficient to support our claim to self-affirmed GRAS status for human consumption. The same data dossier could be applied to support the safety for companion pet consumption of ProBiora3.
Our marketing for ProBiora3 includes the cosmetic claims of teeth whitening and breath freshening, along with the general structure-function claim that ProBiora3 supports oral health. Regulations vary in markets outside the United States and it may be possible to assert other benefits including health and disease prevention claims associated with probiotic use, especially after independent clinical studies have been completed and appropriate regulatory fillings are approved. At present, we are aware of several independent academic studies that have been initiated on a variety of potential health and cosmetic benefits associated with ProBiora3 probiotic use by humans.
Sales, Marketing and Distribution
All of our house-branded ProBiora3 products have been launched and are available through various distribution channels. We have selected our distribution channels by focusing on our potential channel impact, as well as potential return on marketing expenditures.
| Ø | Mass Retail: The mass retail channel encompasses several sub-channels including large national retail stores, mass drugstore chains, independent drugstores, and grocery stores. In order to develop and manage this channel, we retained a team of independent manufacturers’ representatives with industry expertise and strong relationships with the buyers for many of the large national mass retailers. We worked with this team to identify the mass drugstore channel as the lead sub-channel in the overall mass retail channel. Mass drugstores are typically the first to adopt a new wellness-related product or technology, and once mass drugstores adopt such a product, other sub-channels typically follow. In March 2010 we received an initial order from Walgreens for EvoraPlus, and in April 2010, we received an initial order from Rite Aid for EvoraPlus. Both Walgreens and Rite Aid received delivery for initial stocking of EvoraPlus during the second quarter of 2010. In addition to these mass drugstore chains, we have received orders for EvoraPlus from a number of |
56
Table of Contents
Business
| larger national and regional mass retailers, including GNC, Kroger, A&P Supermarkets, Pathmark, Albertsons Supermarkets, Sweetbay Supermarkets, and Hannaford Supermarkets, among others. |
| Ø | Direct-to-Consumer: The direct-to-consumer channel encompasses four sub-channels, including (i) Internet sales through our own websites; (ii) direct mail; (iii) direct-response television, or DRTV, which is usually initiated through an infomercial; and (iv) electronic-response television, or ERTV, which entails marketing through television shopping networks such as Home Shopping Network and QVC. |
| i. | Internet sales: We currently operate two websites through which we market our products directly, and we have incorporated an ‘Oragenics Store’ into our corporate website. We also intend to develop an affiliate marketing program whereby we will pay external website operators click-through revenues when a customer visits our websites via an affiliate site and subsequently makes a purchase. |
| ii. | Direct mail: We are currently in discussions with one of the largest direct mailers of nutraceutical products in the United States, although we may also initiate our own direct mail campaign. |
| iii. | DRTV: We have developed a two-minute spot infomercial that we expect to test on select networks and in select markets. The infomercial has been designed to promote a direct response from viewers, as well as to drive traffic to the mass retail channel. If tests prove successful and we are able to forecast a positive return on our marketing spend, we will expand the geographic area and broadcasting frequency of the infomercial. We have also been in discussions with a number of companies that specialize in DRTV advertising. |
| iv. | ERTV: We have been in discussions with both of the major domestic ERTV operators as well as companies that have established brands on their respective channels. We anticipate consummating one or more ERTV marketing opportunities by the end of 2010. |
| Ø | Professional Offices: The professional offices channel encompasses several sub-channels, including (i) the dental professional channel, which includes dentists, orthodontists and dental hygienists; (ii) the veterinarian professional channel; and (iii) the alternative medicine professional channel, which includes chiropractors, massage therapists and occupational therapists, among others. In August 2010, we launched EvoraPro, which is a product exclusively for the dental professional sub-channel. EvoraPro is an extra-strength, probiotic mint that comes in a 10-unit box designed to be taken after dental cleaning or treatment. It is coupled with a box of EvoraPlus to be used once the EvoraPro has been exhausted. We have also established an affiliate marketing program through which dental professionals can earn recurring revenues from their patients’ subsequent purchases of EvoraPlus. If successful, we intend on following a similar plan to penetrate the other sub-channels in the professional offices channel. We would look to initiate a campaign in the veterinarian channel by the end of 2010. |
| Ø | Private Label: The private label channel encompasses arrangements whereby we or third-party manufacturers market our products for resale under a third-party’s brand name. We typically establish private labeling arrangements in order to leverage an existing company’s brand equity and distribution channels. The first major private labeling agreement we consummated was with Garden of Life, which is a leading U.S. nutritional supplement products brand. Garden of Life has contracted to sell our EvoraPlus product under the brand name Probiotic Smile. Garden of Life sells exclusively in the health food channel, which includes many stores geographically disbursed around the United States. Another notable private labeling sub-channel is the multi-level marketing, or MLM, channel. We have been in discussions with a number of large MLM companies regarding private labeling opportunities for our products. |
57
Table of Contents
Business
| Ø | International: Europe and Asia account for over 70% of the global probiotic market. Since the launch of our first product, EvoraPlus, we have received substantial interest from the international community, and we have therefore initiated relationships with partners to distribute our products worldwide. To date, we have executed a number of distributorship agreements, including agreements with RicciPharma and Celgen. The international distributorship agreements we have entered into to date typically require that the distributors provide upfront payment to us either by irrevocable letters of credit or wire transfers prior to our initiating production and as a result, we believe that we do not bear any credit risk with such agreements. We also require distributors to take possession of product at our manufacturing facility, which substantially reduces our inventory risk. |
| Ø | Licensing/Bulk: The licensing/bulk channel encompasses the incorporation of ProBiora3 as an active ingredient into existing branded products. We have been in discussions with a number of companies regarding the licensing or bulk sale of ProBiora3. |
Manufacturing
When produced, ProBiora3 comes in powder form. ProBiora3 is manufactured by separate fermentation of each of the three strains. The cells are recovered by centrifugation or filtration and freeze dried. Experimentally determined amounts of the resulting powders are blended with natural bulking agents to deliver the proper number of viable cells of each strain per dose. ProBiora3 for human use may be incorporated into various delivery vehicles; for example, in the case of EvoraPlus and EvoraKids, flavoring agents are added and the powder is pressed into tablets, which are sealed in blister packs. In the case of Teddy’s Pride, the powder is not flavored and is simply added in bulk to a plastic container. Freeze-dried cells in ProBiora3-containing products are stable for up to 18 months after manufacture when kept in cool, dry conditions. The cells are revitalized when they come in contact with moisture, for example the saliva present in the oral cavity.
We have contracted with multiple manufacturers to: (i) produce our active ingredient, ProBiora3, (ii) blend and tablet EvoraPlus, EvoraKids, Teddy’s Pride and EvoraPro, and (iii) package our products. Each of our contract manufacturers has the ability to scale production as needed. With each manufacturer, we place orders for components or finished product to be produced for a fixed fee which we are expected to pay upon completion of the manufacturing process. Packaged probiotics products are shipped to us or to a destination specified by us, which is a central distribution center in the case of a mass retail customer or a private label distributor. We currently maintain an inventory of our products for Internet sales and other sales to distributors. We believe our arrangements with our contract manufacturers are satisfactory to meet our current and expected future needs. We have qualified and used at least two contract manufacturers for each step in our manufacturing process, although we do not have a long-term supply agreement or commitment with any of our manufacturers.
MU1140 and Other Lantibiotics
Our lantibiotic, MU1140, was discovered by Dr. Hillman in the course of developing SMaRT Replacement Therapy. MU1140 is a potent antibiotic that is naturally produced by the parent of the SMaRT strain, and we have produced a synthetic version of MU1140 known as MU1140-S. MU1140 is active against all Gram positive bacteria against which it has been tested, including those responsible for a variety of healthcare-associated infections, or HAIs. The key technology that enables our production of MU1140-S is our Differentially Protected Orthogonal Lanthionine Technology, or DPOLT, which is a patented, novel organic chemistry synthesis platform developed by our scientific team. We reported the successful, analytical scale synthesis of MU1140-S using DPOLT in October 2008, and thus achieved what we believe will lead to the first-ever synthetic route to commercial-scale production of lantibiotics.
Market Opportunity
The most common HAIs are caused by drug-resistant bacteria, including methicillin-resistant Staphylococcus aureus, or MRSA; vancomycin-resistant Enterococcus faecalis, or VRE; and Clostridium difficile, or C. diff. According to the Centers for
58
Table of Contents
Business
Disease Control and Prevention, or CDC, HAIs are estimated to occur in approximately 5% of all acute-care hospitalizations, based on the 35 million patients admitted to 7,000 acute-care institutions in the United States, with an annual incidence of approximately 1.7 million cases, which result in 99,000 deaths. The CDC also estimates that the total direct medical cost to the U.S. healthcare system from HAIs is between $35.7 billion to $45 billion annually. HAIs are estimated to more than double the mortality and morbidity risks of any admitted patient in a U.S. hospital, which is the equivalent of 350,000 years of life lost annually. The critical care market for antibiotics is approximately $7 billion in the United States alone. Cubicin, a Gram positive lipopeptide antibiotic which was recently introduced by the biotechnology company Cubist, had 2009 sales of $524 million in the United States.
The need for novel antibiotics is increasing as a result of the growing resistance of target pathogens. In 2002, the CDC estimated that pathogenic bacteria resistant to known antibiotics cause between 6.3% and 89.1% of HAIs, and individual hospitals have resistance rates as high as 70% for many Gram positive infections. HAIs are not exclusively a problem in the United States as the rest of the world has also seen a dramatic rise in HAIs during the last decade. Vancomycin, which was introduced in 1956, has served as the last line of defense against certain life-threatening infections, and, more recently, Cubicin has also served in this capacity, but bacterial resistance to these drugs has been growing at an increasing rate. Novel antibiotics have become increasingly scarce as major pharmaceutical companies have focused more research and development resources on lifestyle drugs and fewer resources on specialty pharmaceuticals such as antibiotics. Between 1983 and 1987, 16 new antibiotics were approved by the FDA. Twenty years later, from 2003 to 2007, only five new antibiotics were approved, of which only two possessed a novel mechanism of action.
Lantibiotics such as MU1140 are highly modified peptide antibiotics made by a small group of Gram positive bacterial species. Approximately 50 lantibiotics have been discovered since 1927 when the first lantibiotic, nisin, was discovered. Lantibiotics are known to be potent antibiotic agents, however, all attempts to investigate their usefulness have met with uniform failure due to the inability to produce sufficient pure amounts of any of these molecules to be able to test them as a therapeutic agent for the treatment of infectious diseases. Standard fermentation methods, such as those used to make a variety of other antibiotics, typically result in production of only minute amounts of the lantibiotic. In cases where large amounts of a lantibiotic are made, such as with nisin, the unique chemical structure of lantibiotics has prevented the necessary purification needed for clinical testing.
Our Solution
MU1140 has demonstrated activity against a wide variety of disease-causing Gram positive bacteria, including MRSA, VRE, C. diff., Mycobacterium tuberculosis, or M. tuberculosis, and anthrax. We have performed extensive preclinical testing on MU1140, which has demonstrated the molecule’s novel mechanism of action. In order to produce sufficient quantities for our clinical trials and commercialization efforts, we intend to use a synthetic version of MU1140, known as MU1140-S.
We created MU1140-S using our patented, novel organic chemistry synthesis platform known as DPOLT. We believe that DPOLT will enable large-scale, cost-effective production of clinical grade MU1140-S. We reported the successful, analytical scale synthesis of MU1140-S using DPOLT in October 2008, which we believe will lead to the first-ever synthetic route to commercial-scale production of a lantibiotic. In addition, we believe that DPOLT will allow us to synthetically produce any of the 50 known lantibiotics due to the shared chemical structure features of this class of molecule. We intend to use DPOLT to create a pipeline of lantibiotics for therapeutic use.
Regulatory Status
We have performed extensive preclinical testing using native MU1140, which demonstrated the following features:
| Ø | Bactericidal activity against Gram positive strains and against both replicating and non-replicating M. tuberculosis; |
59
Table of Contents
Business
| Ø | Unusual chemical structure, which makes it extremely stable; |
| Ø | No immune response in a variety of animal models, even with the use of strong adjuvants and carriers; |
| Ø | Negligible toxicity when supra-therapeutic doses were tested in yeast, and fibroblast and kidney cell lines; |
| Ø | In vivo efficacy in mouse and rat models, in which animals were infected intraperitoneally with MRSA (60xLD50) and MU1140 was administered intravenously at doses well below its maximum tolerated dose; |
| Ø | Novel mechanism of action that involves binding to and abducting Lipid II, which is required for cell wall biosynthesis; |
| Ø | No spontaneous, genetically stable resistant mutants to MU1140; |
| Ø | Synergy with an aminoglycoside; and |
| Ø | Good pharmaceutical properties. |
We expect to conclude the preclinical testing of MU1140-S, including toxicity testing in rodent and non-rodent animal models, during the first half of 2011. We then intend to file an Investigational New Drug, or IND, application with the FDA in mid-2011. We estimate that, once commenced, the regulatory process will require at least four years of clinical testing and the application and FDA approval of a New Drug Application, or NDA, before MU1140-S would be commercially available. We have engaged Celerion (formerly known as MDS Pharma Services) on a fee-for-service basis to represent us in regulatory meetings with the FDA, and to perform the first-in-human trials with MU1140-S.
Our Strategy
We intend to develop MU1140-S through Phase 1 clinical trials. If MU1140-S successfully completes Phase 1 trials, we believe that its value will substantially increase, and we would then seek to license MU1140-S to or partner with a major pharmaceutical company. If we are unable to consummate an acceptable licensing or partnership arrangement, we may pursue Phase 2 clinical trials independently.
Analysis of the 50 known lantibiotics suggests that there are possibly six to ten subclasses of lantibiotics as classified by known mechanisms of action, spectra of activity, or structural characteristics. In addition to MU1140-S, we intend to utilize DPOLT to synthesize additional lantibiotics of interest in the future.
Manufacturing
We have retained Almac Sciences, a leading contract manufacturer, to refine and scale-up GMP production of MU1140-S. We expect to have sufficient amounts of MU1140-S by second half of 2010, which will enable preliminary testing to demonstrate equivalence between the synthetic and native molecule.
Additional Areas of Development
As part of our past research efforts, we have identified and filed patent applications covering two technologies that we may seek to further develop internally or monetize through a sale, license, or partnership in the future. These areas include LPT3-04, our weight loss product, and PCMAT, our biomarker discovery platform.
60
Table of Contents
Business
Weight Loss Product (LPT3-04)
LPT3-04 is a naturally occurring compound, which is normally consumed in the human diet in small amounts. In the course of our SMaRT Replacement Therapy research, we discovered program that consumption of significantly larger amounts of LPT3-04 resulted in dose-dependent weight loss in experimental animal models. The mechanism of action appears to be induction of apoptosis, or programmed cell death, specifically in white fat cells. LPT3-04 consumption in the required amounts has been shown to be safe in humans. Anecdotally, weight loss has been observed in human volunteers. Due to the natural sweetness of LPT3-04 and the relatively large amounts of it that need to be consumed on a daily basis to achieve the desired weight loss effect, current product development efforts are focused on incorporating the compound into bars, milkshakes, and other food products. These food products will be used in a blinded placebo-controlled study scheduled to begin in the third quarter of 2010. We have submitted a patent application for the use of LPT3-04 for weight regulation with the United States Patent and Trademark Office, or U.S. PTO.
Biomarker Discovery Platform (PCMAT)
Our biomarker discovery platform is based on our Proteomics-based Change Mediated Antigen Technology, or PCMAT, and was discovered by members of our scientific team while searching for protein targets associated with the diagnosis of periodontal disease. This technology rapidly identifies proteins that are expressed when a cell undergoes any sort of change. Such proteins are excellent targets for medical diagnostics and therapeutic strategies. PCMAT is able to identify proteins shed from diseased tissues into bodily fluids such as blood, saliva and urine. We believe that PCMAT is faster, more cost-efficient and significantly more sensitive than competing technologies such as differential proteomics and microarrays. In addition, our technology uses the actual diseased host rather than an animal model, so that biomarkers that we discover are more likely to be of high clinical value. We have identified several widespread disease states that we intend to pursue. If we are able to discover protein targets with sufficient degrees of sensitivity and specificity, we intend to license these targets to major pharmaceutical or medical diagnostics companies.
Our In-Licensed Technology Agreements
SMaRT Replacement Therapy
We have exclusively licensed the intellectual property for our replacement therapy technology from the University of Florida Research Foundation, Inc., or the UFRF. The original license agreement was dated August 4, 1998 and was subsequently amended on September 15, 2000, July 10, 2002, September 25, 2002 and March 17, 2003. The amended license agreement provides us with an exclusive worldwide license to make, use and sell products and processes covered by Patent No. 5,607,672, entitled “Replacement Therapy for Dental Caries”, which was filed in the U.S. PTO on June 7, 1995 and made effective on March 4, 1997. The patent will expire on June 7, 2015. Our license is for the period of the patent, subject to the performance of terms and conditions contained therein. The patent covers the genetically altered strain of S. mutans which does not produce lactic acid, a pharmaceutical composition for administering the genetically altered strain and the method of preventing tooth decay by administering the strain. See “Our Intellectual Property.”
We issued 29,997 shares of our common stock to the UFRF as partial consideration for the initial license.
MU1140
We have exclusively licensed the intellectual property for our MU1140 lantibiotic technology from the UFRF. The original license agreement was dated June 22, 2000 and was subsequently amended on September 15, 2000, July 10, 2002, September 25, 2002 and March 17, 2003. The amended license agreement provides us with an exclusive worldwide license to make, use and sell products and processes covered by Patent No. 5,932,469 entitled “Antimicrobial Polypeptide, Nucleic Acid and Methods of Use.” Our license is for the period of the patent, subject to the performance of terms and conditions contained therein. See “Our Intellectual Property.”
61
Table of Contents
Business
Additional Terms of License Agreements
In the amended license agreements for SMaRT Replacement Therapy and MU1140 the UFRF has reserved the right to use and sell products and services for research purposes only. The amended license agreements also provide the UFRF with a license, for research purposes only, to any improvements that we make to the products and processes covered by the patents.
We are obligated to pay 5% of the selling price of any products developed from the licensed technologies that we may sell as royalty to the UFRF. In addition, if we sublicense any rights granted by the amended license agreements, we are obligated to pay the UFRF 20% of all revenues received from the sublicenses, excluding monies received solely for development costs.
We are also obligated to make minimum annual royalty payments to the UFRF for the term of the amended license agreement in the amount of $50,000 by the end of each year for each license agreement. The minimum royalty payments are required to be paid in advance on a quarterly basis. For the SMaRT Replacement Therapy and MU1140 minimum royalty payments, we must pay the UFRF an aggregate of $100,000 which is required to be paid in equal quarterly installments of $25,000.
Under the terms of the amended license agreements, in each calendar year and in addition to the royalty payment obligations, we are obligated to spend, or cause to be spent, an aggregate of $1,000,000 on the research, development, and regulatory prosecution of our SMaRT Replacement Therapy and MU1140 technologies combined, until a product which is covered wholly or partially by the claims of the patent, or is manufactured using a process which is covered wholly or partially by the claims of the patent, is sold commercially. If we fail to make these minimum research and development expenditures, the UFRF may terminate our license agreement.
We must also pay all patent costs and expenses incurred by the UFRF for the preparation, filing, prosecution, issuance and maintenance of the patent.
We have agreed to indemnify and hold the UFRF harmless from any damages caused as a result of the production, manufacture, sale, use, lease, consumption or advertisement of the product.
We are required to maintain liability insurance coverage appropriate to the risk involved in marketing our products. Our liability insurance has been renewed through March 2011, however, there is no assurance that we can obtain continued coverage on reasonable terms.
The amended license agreements further provide that the U.S. government funded research grant No. RO1 DE04529 during the course of or under which the licensed inventions covered by the patent were conceived. As such the U.S. government is entitled, as a right, to a nonexclusive, nontransferable, irrevocable, paid-up license to practice or have practiced the inventions of such patents for governmental purposes.
In order to protect our license rights and their patents, we or the UFRF may have to file lawsuits and obtain injunctions. If we do that, we will have to spend large sums of money for attorney fees in order to obtain the injunctions. Even if we do obtain the injunctions, there is no assurance that those infringing on our licenses or the UFRF’s patents will comply with the injunctions. Further, we may not have adequate funds available to prosecute actions to protect or to defend the licenses and patents, in which case those infringing on the licenses and patents could continue to do so in the future.
Government Regulations
The formulation, manufacturing, processing, packaging, labeling, advertising, distribution and sale of our products are subject to regulation by federal agencies, including, but not limited to the Food and Drug Administration, or FDA, and the Federal
62
Table of Contents
Business
Trade Commission, or FTC. These activities also are regulated by various agencies of the states, localities and foreign countries in which our products are sold. In particular, the FDA, under the Federal Food, Drug, and Cosmetic Act, or FDCA, regulates the safety, manufacturing, labeling and distribution of drugs, medical devices, food, and dietary supplements. In addition, the FTC has primary jurisdiction to regulate the advertising of drugs, medical devices, food and dietary supplements.
In foreign countries these same activities may be regulated by Ministries of Health, or other local regulatory agencies. The manner in which products sold in foreign countries are registered, how they are formulated, or what claims may be permitted may differ from similar products and practices in the United States.
FDA Regulation—Food
Under the FDCA, the FDA is responsible for ensuring that foods are safe, wholesome, and correctly labeled. The FDA enforces statutory prohibitions against misbranded and adulterated foods, and establishes safety standards for food processing and ingredients, manufacturing procedures for processed foods, and labeling standards for food products.
All facilities engaged in manufacturing, processing, packing or holding food for consumption in the United States must be registered with FDA before such activities begin. Those who manufacture, package, or hold food must comply with the Good Manufacturing Practices, or GMPs, for foods. The GMPs describe the methods, equipment, facilities, and controls for producing processed food, including requirements for personnel such as education, training and cleanliness requirements; proper maintenance and sanitization of buildings, facilities, and equipment; and processes and controls.
Acceptable claims for foods fall into three categories: health claims, structure/function claims and nutrient content claims. Health claims describe a relationship between a food, food component, or dietary ingredient and reducing the risk of a disease or health-related condition. The FDA authorizes these types of health claims based on an extensive review of the scientific literature, generally as a result of the submission of a health claim petition. Manufacturers also may make certain health claims based on “authoritative statements” from a scientific body of the U.S. Government or the National Academy of Sciences. Structure/function claims describe the role of a nutrient or dietary ingredient intended to affect or maintain normal structure or function of the body, and may characterize the means by which a nutrient or dietary ingredient acts to maintain such structure or function. Nutrient content claims expressly or by implication characterize the level of a nutrient in a food, by using terms such as “free,” “high” or “low.” The FDA’s regulations define the nutrient content claims that may be used and the requirements for making such claims.
Labels for food must not be false or misleading. Required information for labels includes the name of the food, the net quantity, the name and address of the manufacturer, packer or distributor, the ingredient list, and a Nutrition Facts label. In addition to the information required to be in a Nutrition Facts label, other nutrients must be included in the Nutrition Facts label if the nutrients are added as a nutrient supplement to the food, if the label makes a nutrition claim about them, or if advertising or product literature connects the nutrients to the food. The FDA considers information that is required or permitted in the Nutrition Facts label, on the front label or elsewhere on the package to be a nutrition content claim. In such cases, the package label must comply with the regulations for nutrient content claims.
Under the FDCA, any substance that is intentionally added to food is a food ingredient, which is subject to premarket review and approval by the FDA, unless the substance is Generally Recognized As Safe, or GRAS, which means that the substance is generally recognized, among qualified experts, as having been adequately shown to be safe under the conditions of its intended use, or unless the use of the substance is otherwise excluded from the definition of a food ingredient. Under FDA’s regulations, the use of a food substance may be GRAS either through scientific procedures that may be voluntarily submitted to the FDA, or, for a substance used in food before 1958, through experience based on common use in food. General recognition of safety through scientific procedures requires the same quantity and quality of scientific evidence as required to obtain approval of the substance as a food ingredient and ordinarily is based upon published studies, which may be corroborated by unpublished studies and other data and information. General recognition of safety through experience based
63
Table of Contents
Business
on common use in foods requires a substantial history of consumption for food use by a significant number of consumers. To be considered “safe” for its intended use, there must be a reasonable certainty in the minds of competent scientists that the substance is not harmful under its intended conditions of use. The specific data and information that demonstrate safety depend on the characteristics of the substance, the estimated dietary intake, and the population that will consume the substance.
Registered food facilities that manufacture, process, pack, or hold food for human or animal consumption in the United States are required to submit a report to the FDA’s Reportable Food Registry, or RFR, when there is a reasonable probability that the use of, or exposure to, an article of food will cause serious adverse health consequences or death. The RFR covers all foods regulated by FDA except infant formula and dietary supplements. Registered facilities must report as soon as practicable, but in no case later than 24 hours after it is determined that an article of food is a reportable food.
FDA Regulation—Dietary Supplements
The Dietary Supplement Health and Education Act of 1994, or DSHEA, amended the FDCA by establishing regulatory standards with respect to dietary supplements, and defining dietary supplements as a new category of food. Dietary supplements include vitamins, minerals, amino acids, nutritional supplements, herbs and botanicals intended for ingestion that are labeled as dietary supplements and are not represented for use as a conventional food or as a sole item of a meal or the diet. Under DSHEA, a firm that manufactures or distributes dietary supplements must determine that such products are safe and that any representations or claims made about the products are substantiated by adequate evidence to show that the claims are not false or misleading.
DSHEA does not require manufacturers or distributors to seek approval from the FDA before producing or selling a dietary supplement unless the supplement contains one or more ingredients that are considered to be a “new dietary ingredient.” A “new dietary ingredient” is one that was not marketed in the United States before October 15, 1994. The manufacturer or distributor of a dietary supplement that contains a “new dietary ingredient” must provide the FDA with information, including any citations to published articles, demonstrating why the ingredient is reasonably expected to be safe for use in a dietary supplement at least 75 days before the dietary supplement is introduced or delivered for introduction into interstate commerce. This requirement does not apply if the ingredient has been recognized as a food substance and is present in the food supply.
Because dietary supplements are foods, manufacturers of dietary supplements must register the facilities where the supplements are manufactured, processed, packed or held with the FDA before such activities begin. Those who manufacture, package or hold dietary supplements also must comply with GMPs for dietary supplements. According to the GMPs, dietary supplements must be prepared, packaged, labeled and held in compliance with specific requirements, including detailed quality control requirements, such as those for maintaining and cleaning facilities and instruments, hiring and training personnel and ensuring the appropriate manufacturing environment, testing requirements, recordkeeping requirements and handling of customer complaints. Anyone who manufactures, packages, labels or holds dietary supplements must evaluate and ensure the identity, purity, strength and composition of the products. FDA regulations also require that certain information appear on dietary supplement labels, including the name of the dietary supplement, the amount of the dietary supplement, nutrition labeling, a complete list of ingredients and the name and place of business of the manufacturer, packer or distributor. Manufacturers must ensure, and have substantiation showing, that claims made about dietary supplements are truthful and not misleading. Acceptable claims for dietary supplements are the same as those for conventional foods: health claims, structure/function claims and nutrient content claims. However, additional requirements apply to manufacturers of dietary supplements who make structure/function claims. Manufacturers of dietary supplements must notify the FDA of any structure/function claims made for a dietary supplement within 30 days of first marketing the product with the identified claims. A dietary supplement that includes a structure/function claim on its labeling is also required to bear a prescribed disclaimer: “This statement has not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure or prevent any disease.”
64
Table of Contents
Business
The manufacturer, packer, or distributor of a dietary supplement must submit to the FDA any report it receives of a serious adverse event associated with the dietary supplement when used in the United States, accompanied by a copy of the label of the dietary supplement, no later than 15 business days after the report is received. A “serious adverse event” is an adverse event that results in death, a life-threatening experience, inpatient hospitalization, a persistent or significant disability or incapacity, a congenital anomaly or birth defect, or requires, based on a reasonable medical judgment, medical or surgical intervention to prevent such outcomes.
The FDA may take action to restrict use of a dietary supplement or to remove it from the marketplace if the agency believes the supplement presents a significant or unreasonable risk of illness or injury under conditions of use suggested in the labeling or under ordinary conditions of use. Under DSHEA, the FDA bears the burden of proof to show that a dietary supplement presents a significant or unreasonable risk of illness or injury. The FDA also may take enforcement action against a dietary supplement manufacturer or distributor for unlawful promotion of a dietary supplement, such as making claims that a supplement treats, prevents or cures a specific disease or condition. These claims would subject the dietary supplement to regulation as a drug product. If dietary supplements do not meet applicable requirements, the manufacturer may need to undertake a voluntary recall.
FDA Regulation—Biological Products and New Drug Products
Under the FDCA all new drugs and biological products are subject to pre-market approval by the FDA. In contrast to chemically synthesized small molecular weight drugs, which have a well-defined structure and can be thoroughly characterized, biological products are generally derived from living material—human, animal, or microorganism—are complex in structure, and thus are usually not fully characterized. Biological products include blood-derived products, vaccines, in vivo diagnostic allergenic products, immunoglobulin products, products containing cells or microorganisms, and most protein products. Biological products subject to the Public Health Service, or PHS, Act also meet the definition of drugs under the FDCA, therefore both biological products and drugs are regulated under provisions of the FDCA. However, only biological products are licensed under the PHS Act. The overall development process for biological products is similar to that for drugs. The steps ordinarily required before a biological product or new drug may be marketed in the United States include:
| Ø | completion of preclinical studies according to Good Laboratory Practice, or GLP, regulations; |
| Ø | the submission of an IND application to the FDA, which must become effective before human clinical trials may commence; |
| Ø | performance of adequate and well-controlled human clinical trials according to Good Clinical Practices to establish the safety and efficacy of the proposed biological product or new drug for its intended use; |
| Ø | satisfactory completion of an FDA pre-approval inspection of the manufacturing facility or facilities at which the product is manufactured, processed, packaged or held to assess compliance with GMPs; and |
| Ø | the submission to, and review and approval by, the FDA of a biologics license application, or BLA, or new drug application, or NDA, that includes satisfactory results of preclinical testing and clinical trials. |
Preclinical tests include laboratory evaluation of the product candidate, its formulation and stability, as well as animal studies. The FDA requires that preclinical tests be conducted in compliance with GLP regulations. The results of preclinical testing are submitted as part of an IND application to the FDA together with manufacturing information for the clinical supply, analytical data, the protocol for the initial clinical trials and any available clinical data or literature. The IND automatically becomes effective 30 days after receipt by the FDA, unless the FDA, within the 30-day time period, places the clinical trial on a clinical hold. In such a case, the IND sponsor and the FDA must resolve any outstanding concerns before the clinical trial can begin. FDA may also impose clinical holds at any time before or during studies due to safety concerns or non-compliance.
65
Table of Contents
Business
Clinical trials to support BLAs and NDAs involve the administration of the investigational product to human subjects under the supervision of qualified investigators. Clinical trials are conducted under protocols detailing, among other things, the objectives of the study, the parameters to be used in monitoring safety and the efficacy criteria to be evaluated. Clinical trials are typically conducted in three sequential phases, but the phases may overlap.
In Phase 1 clinical trials, the biological or new drug product candidate is initially introduced into human subjects or patients and assessed for safety, dosage tolerance, absorption, metabolism, distribution and excretion, including any side effects associated with increasing doses.
Phase 2 clinical trials usually involve studies in a limited patient population to identify possible adverse effects and safety risks; preliminarily assess the efficacy of the product candidate in specific, targeted indications; and assess dosage tolerance and optimal dosage.
If a product candidate is found to be potentially effective and to have an acceptable safety profile in Phase 2 evaluations, Phase 3 trials are undertaken within an expanded patient population at multiple study sites to further demonstrate clinical efficacy and safety, further evaluate dosage and establish the risk-benefit ratio of the product and an adequate basis for product labeling.
Phase 4, or post-marketing, trials may be mandated by the FDA or may be conducted voluntarily. Phase 4 trials are typically initiated to monitor the safety and efficacy of a biological product or new drug in its approved population and indication over a longer period of time, so that rare or long-term adverse effects can be detected over a much larger patient population and time than was possible during prior clinical trials. Alternatively, Phase 4 trials may be used to test a new method of product administration, or to investigate a product’s use in other indications. Adverse effects detected by Phase 4 trials may result in the withdrawal or restriction of a product.
If the required Phase 1, 2 and 3 clinical testing is completed successfully, the results of product development, preclinical studies and clinical trials, descriptions of the manufacturing process and other relevant information concerning the safety and effectiveness of the biological product or new drug candidate are submitted to the FDA in the form of a BLA or NDA. In most cases, the BLA or NDA must be accompanied by a substantial user fee. The FDA may deny a BLA or NDA if all applicable regulatory criteria are not satisfied or may require additional data, including clinical, toxicology, safety or manufacturing data. It can take several years for the FDA to approve a BLA or NDA once it is submitted, if at all, and the actual time required for any product candidate may vary substantially, depending upon the nature, complexity and novelty of the product candidate.
Before approving an application, the FDA will inspect the facility or facilities where the product is manufactured. The FDA will not approve a BLA or NDA unless it determines that the manufacturing processes and facilities are in compliance with GMP requirements.
If the FDA evaluations of the BLA or NDA and the manufacturing facilities are favorable, the FDA will issue an approval letter. If the FDA determines that it will not approve an NDA or BLA in its present form for one or more reasons, the FDA will issue a complete response letter. The complete response letter usually contains a number of conditions that must be met before FDA will approve the BLA or NDA. If the BLA or NDA does not meet the criteria for approval, the FDA may deny the application.
The required testing, data collection, analysis and compilation of an IND and a BLA or NDA are labor intensive and costly and may take a great deal of time. Tests may have to be redone or new tests performed in order to comply with FDA requirements. It can take considerable time (e.g. 5-10 years) and resources to achieve enrollment sufficient to commence such trials and complete Phase 2 or 3 clinical trials. Moreover, there is no guarantee a product will be approved.
66
Table of Contents
Business
FDA Regulation—Medical Devices
Medical devices also are subject to extensive regulation by the FDA. To be commercially distributed in the United States, devices that are not exempt from FDA’s premarket notification, or 510(k) procedures, or are pre-amendment devices, meaning they were on the market prior to May 28, 1976, must receive either 510(k) clearance or pre-market approval, or PMA, from the FDA prior to marketing. Devices are assigned to one of three classes depending on the controls the FDA deems necessary to ensure the safety and effectiveness of the devices. Devices deemed to pose the least risk are placed in Class I. A Class I device is 510(k) exempt unless the device is intended for a use which is of substantial importance in preventing impairment of human health, or the device presents a potential unreasonable risk of illness or injury. Class II devices require the manufacturer to submit a pre-market notification to FDA unless they are 510(k) exempt. Devices deemed by the FDA to pose the greatest risk, such as life-sustaining, life-supporting or implantable devices, devices deemed not substantially equivalent to a previously 510(k) cleared device and certain other devices are placed in Class III. Most Class III devices require approved PMAs before marketing, although some Class III devices can get to market through the 510(k) process.
To obtain 510(k) clearance, a manufacturer must submit a pre-market notification demonstrating that the proposed device is “substantially equivalent” to a “predicate device,” which is a previously 510(k) cleared Class I or Class II device, a pre-amendment Class III device for which the FDA has not yet called for PMA applications or a device that was in commercial distribution before May 28, 1976. To demonstrate substantial equivalence, the applicant must show that the device has the same intended use and the same technological characteristics as the predicate, or if the device has different technological characteristics than the predicate, the device does not raise new questions of safety and effectiveness, and is at least as safe and effective as the predicate. The FDA’s 510(k) clearance pathway usually takes from four to twelve months, but it can last longer. After a device receives 510(k) clearance, any modification that could significantly affect its safety or effectiveness, or that would constitute a major change in its intended use, requires a new 510(k) clearance or could require a PMA approval.
A product not eligible for 510(k) clearance must follow the PMA pathway, which requires proof that there is a reasonable assurance of a device’s safety and efficacy to the FDA’s satisfaction. The PMA pathway is much more costly and lengthy than the 510(k) pathway. A PMA application typically must provide extensive preclinical and clinical trial data and also information about the device and its components including, among other things, device design, manufacturing and labeling. As part of the PMA review, the FDA will typically inspect the manufacturer’s facilities for compliance with quality system regulation requirements, which impose elaborate testing, control, documentation and other quality assurance procedures. Upon acceptance by the FDA of what it considers a completed filing, the FDA commences an in-depth review of the PMA application, which typically takes from one to two years, but may last longer. Even after approval of a PMA, a new PMA or PMA supplement is required in the event of a modification affecting the safety or effectiveness of the device.
FDA Regulation—Post-Market Requirements
Even if regulatory clearances or approvals for our product candidates are obtained, our products and the facilities manufacturing our products, including foods, will be subject to continued review and periodic inspections by the FDA. The FDA may perform these inspections at any time without advanced notice. For example, as a condition of approval of an NDA, the FDA may require us to engage in post-marketing testing and surveillance and to monitor the safety and efficacy of our products. Holders of an approved NDA, BLA, or PMA, or 510(k) clearance are subject to several post-market requirements, including the reporting of certain adverse events involving their products to the FDA, provision of updated safety and efficacy information, and compliance with requirements concerning the advertising and promotion of their products.
The FDA will inspect manufacturing facilities to confirm that the facilities comply with GMP requirements. To comply with GMP requirements, manufacturers must expend money, time and effort in the area of production and quality control to ensure full compliance. For example, manufacturers of biologic products must establish validated systems to ensure that products meet high standards of sterility, safety, purity, potency and identity, and must report to the FDA any deviations from GMP or any
67
Table of Contents
Business
unexpected or unforeseeable event that may affect a product’s safety, purity, or potency. The regulations also impose documentation requirements and require manufacturers of drugs, biologics or devices to investigate and correct any unexplained discrepancy or the failure of a lot or unit to meet any of its specifications.
Failure to comply with the applicable FDA requirements may subject manufacturers and distributors to administrative or judicial sanctions. These sanctions could include, among other things, warning letters, product seizures, total or partial suspension of production or distribution, injunctions, civil money penalties, fines, restitution, disgorgement, or civil or criminal penalties. Further, the FDA has authority to issue mandatory recalls for medical devices and biologics, and we may need to undertake a voluntary recall for any of our products.
In addition to regulations enforced by the FDA, we are also subject to regulation under the Occupational Safety and Health Act, the Environmental Protection Act, the Toxic Substances Control Act, the Resource Conservation and Recovery Act and other federal, state and local regulations. Our research and development activities involve the controlled use of hazardous materials, chemicals, biological materials and radioactive compounds.
FTC Regulation
The advertising of our products is subject to regulation by the FTC under the Federal Trade Commission Act, in addition to state and local regulation. The Federal Trade Commission Act prohibits unfair methods of competition and unfair or deceptive acts or practices in or affecting commerce. The Federal Trade Commission Act also provides that the dissemination or the causing to be disseminated of any false advertisement pertaining to drugs or foods, which would include dietary supplements, is an unfair or deceptive act or practice. Under the FTC’s Substantiation Doctrine, an advertiser is required to have a “reasonable basis” for all objective product claims before the claims are made. Failure to adequately substantiate claims may be considered either deceptive or unfair practices. Pursuant to this FTC requirement we are required to have adequate substantiation for all advertising claims made for our products.
In recent years the FTC has initiated numerous investigations of dietary supplement and weight loss products and companies. We may be the subject of investigation in the future, and the FTC may impose limitations on our advertising of products. The FTC has a variety of processes and remedies available to it for enforcement, both administratively and judicially, including compulsory processes, cease and desist orders, and injunctions. FTC enforcement can result in orders requiring, among other things, limits on advertising, corrective advertising, consumer redress, divestiture of assets, rescission of contracts and such other relief as may be deemed necessary.
International Regulation
Our product candidates are subject to regulation in every country where they will be tested or used. Whether or not we obtain FDA approval for a product candidate, we must obtain the necessary approvals from the comparable regulatory authorities of foreign countries before we can commence testing or marketing of a product candidate in those countries. The requirements governing the conduct of clinical trials and the approval processes vary from country to country and the time required may be longer or shorter than that associated with FDA approval. As in the United States, post-approval regulatory requirements, such as those regarding product manufacture, marketing, or distribution, would apply to any product that is approved outside the United States.
Future Legislation and Regulations
In the future we may be subject to additional laws or regulations by the FDA or other federal, state or foreign regulatory authorities, the repeal of laws or regulations, or more stringent interpretations of current laws or regulations. We are unable to predict the nature of such future laws, regulations, or interpretations, nor can we predict what effect additional governmental regulations or administrative orders, when and if promulgated, would have on our business in the future. For example, for
68
Table of Contents
Business
dietary supplements, the FDA or other governmental regulatory bodies could require the reformulation of certain products to meet new standards, the recall or discontinuance of certain products not able to be reformulated, imposition of additional record keeping requirements, expanded documentation of the properties of certain products, expanded or different labeling and scientific substantiation. Any or all of such requirements could have a materially adverse effect on our business, financial condition, results of operations and cash flows.
Competition
Our industry is subject to rapid and intense technological change. Competition is intense among manufacturers of nutritional, non-prescription, and prescription pharmaceuticals. We face, and will continue to face, competition from nutraceutical, pharmaceutical, biopharmaceutical, medical device and biotechnology companies developing similar products and technologies both in the United States and abroad, as well as numerous academic and research institutions, governmental agencies and private organizations engaged in drug funding or research and discovery activities both in the United States and abroad. Academic institutions, government agencies and other public and private research organizations may also conduct research, seek patent protection and establish collaborative arrangements for discovery, research and clinical development of technologies and products similar to ours. We also face competition from entities and healthcare providers using more traditional methods. We believe there are a substantial number of products under development by numerous nutraceutical, pharmaceutical, biopharmaceutical, medical device and biotechnology companies, and it is likely that other competitors will emerge.
Many of our existing and potential competitors are large, well established pharmaceutical, chemical or healthcare companies with considerably greater research and product development capabilities and financial, scientific, marketing and human resources than we have. As a result, these competitors may succeed in developing competing products earlier than we do; obtain patents that block or otherwise inhibit our ability to further develop and commercialize our product candidates; obtain approvals from the FDA or other regulatory agencies for products more rapidly than we do; or develop treatments or cures that are safer or more effective than those we propose to develop. These competitors may also devote greater resources to marketing or selling their products and may be better able to withstand price competition. In addition, these competitors may introduce or adapt more quickly to new technologies or scientific advances, which could render our technologies obsolete, and may introduce products or technologies that make the continued development, production, or marketing of our product candidates uneconomical. These competitors may also be more successful in negotiating third-party licensing or collaborative arrangements and may be able to take advantage of acquisitions or other strategic opportunities more readily than we can. These actions by competitors or potential competitors could materially affect our business, financial condition and results of operations. We cannot assure you that we will be able to compete successfully.
We have a limited ability to predict how competitive our products, technology platforms and replacement therapy will be in the market place. The competition we believe currently exists with respect to each of our products is as follows:
SMaRT Replacement Therapy
We are not aware of any direct competitors with respect to our licensed, patented replacement therapy technology. However, there may be several ways to disable or eradicate S. mutans. We know that certain companies and several academic and research institutions, such as the Forsyth Institute, the University of Alabama, and Guy’s Hospital of London, are developing and testing caries vaccines aimed at eradicating S. mutans. An alternative approach involves topical application of adhesion- blocking synthetic peptides that prevent S. mutans from attaching to the tooth surface. Products that result in the elimination of S. mutans from the natural ecosystem would require major studies to determine whether such eradication of naturally occurring bacteria might not create serious, unintended consequences. The problem with eradicating S. mutans is that it disrupts the natural ecosystem leaving a void for another pathogen potentially more harmful than S. mutans to dominate. We are not aware that any other company has filed an IND with the FDA to test their technology to address the matter.
69
Table of Contents
Business
Any product based on our replacement therapy technology will compete against traditional oral care products used to combat tooth decay. These products include fluoride-based toothpastes as well as fluoride treatments and tooth sealants administered by dentists. These competitors could include, among others, Colgate, Procter & Gamble, Unilever, GlaxoSmithKline, and Dentsply.
ProBiora3
Many companies sell probiotics that are principally designed for digestive health, vaginal and urinary tract health, and immune system support. Our product will not compete directly with the products of these companies. Recently, researchers at the University of Hiroshima in Japan have published studies indicating that Lactobacillus reuteri, or L. reuteri, a bacterial species isolated from the gastrointestinal tract, can reduce the levels of S. mutans in the mouth and may aid in the prevention of tooth decay. L. reuteri is widely used as a probiotic for other indications and recently has been promoted for dental health. We are aware of a probiotic product from BioGaia AB/Sunstar, containing a strain of L. reuteri, which is on the market today as GUM® PerioBalance® and is targeted to maintain oral health. Another probiotic bacteria for oral care, known as BLIS K12 probiotic, is commercially available from Frutarom, an Israeli company. BLIS K12 is promoted as a probiotic for bad breath and contains the bacterium, Streptococcus salivarius K12. This bacterium principally colonizes the cheek and tongue surfaces in the oral cavity, and as such is promoted only for its oral care activity as an aid for halitosis. As compared to all of these competitors, ProBiora3, with its unique blend of three proprietary probiotic strains, potentially has greater beneficial actions for maintaining oral health.
MU1140-S and Other Lantibiotics
MU1140-S will likely compete directly with antibiotic drugs such as vancomycin and newer drugs, including Cubicin (daptomycin) and Zyvox (linezolid). Given the growing resistance of target pathogens to even new antibiotics, we believe that there is ample room in the marketplace for additional antibiotics. We are aware of another mutacin peptide patented in the United States by the University of Laval in Quebec which patent expired on April 17, 2009 for failure to pay required maintenance fees. Management believes that the Laval peptide, if developed would infringe on the MU1140 patent.
Many of our competitors are taking approaches to drug development differing from our approach, including using traditional screening of natural products; genomics to identify new targets, and combinatorial chemistry to generate new chemical structures. Competition in the pharmaceutical industry is based on drug safety, efficacy, ease of use, patient compliance, price, marketing, and distribution. Commercial success of MU1140-S technology will depend on our ability and/or the ability of our licensees and partners to compete effectively in all of these areas, against other companies with existing and pipeline antibiotics to be commercialized in the future.
Producers of antibiotic products include many large, global pharmaceutical companies, who have much greater financial and technical resources than us.
Our Intellectual Property
We rely upon a combination of licenses, patents, trade secrets, know-how, and licensing opportunities to develop our business. Our future prospects depend on our ability to protect our intellectual property, particularly our patents. We also need to operate without infringing the proprietary rights of third parties.
License Agreements
We have exclusively licensed the intellectual property for our SMaRT Replacement Therapy and MU1140 technologies from the UFRF. The related patents to which our exclusive license applies are U.S. Patent No. 5,607,672, “Replacement Therapy for Dental Caries,” and U.S. Patent No. 5,932,469, “Antimicrobial Polypeptide, Nucleic Acid and Methods of Use” (including derivative patents: 6,391,285, 6,475,771, 6,964,760 and 7,067,125). See “Our In-licensed Technology Agreements.”
70
Table of Contents
Business
Patents
We attempt to protect our technology and products through patents and patent applications. We have built a portfolio of patents and applications covering certain of our technologies. We have rights to eight issued U.S. patents and we have eight U.S. patent applications on file with the U.S. PTO directed toward our products and technologies, including patents exclusively licensed from the UFRF. Our pending applications cover a range of technologies, including specific embodiments and applications for treatment of various medical indications, improved application methods and adjunctive utilization with other therapeutic modalities. We reassess the value of each patent at the time maintenance fees are due, and in cases where maintaining the patent is judged to be of no significant strategic value we decline to pay the fee. The patents and patent applications we have with respect to our products and technologies are set forth below:
| Ø | Consumer Products. We filed two U.S. patent applications on our probiotic technology (U.S. patent application serial number 10/567592, filed June 30, 2006; U.S. patent application serial number 12/482,881, filed June 11, 2009). These applications were internationally filed as PCT/US04/025899 on August 10, 2004, and PCT/US09/047040. We also filed a U.S. patent application entitled “Methods for Regulating Weight and Size of Animals” (U.S. patent application serial number 11/265, 414, filed November 2, 2005). This application was internationally filed as PCT/US05/39657 on November 2, 2005. |
| Ø | Biomarker Discovery. In our Biomarker Discovery division we acquired the rights to our platform technology in November 2006 in connection with our acquisition of IviGene Corporation. We own patents and applications directed toward the identification and isolation of polynucleotides expressed during the process of infection: In Vivo Induced Antigen Technology-U.S. Patent No. 7,033,748, filed March 6, 2002, and U.S. patent application serial number 09/980,845; filed August 4, 2000 (internationally filed as PCT/US00/21340 on August 4, 2000); and 12/327,056, filed December 3, 2008; In Vivo Induced Genes of Mycobacterium Tuberculosis, U.S. patent application serial number 12/293,497 having an effective U.S. filing date of March 13, 2007 (internationally filed as PCT/US07/63850, on March 13, 2007); Compositions for Detection and Treatment of Colorectal Cancer, PCT/US09/050938, filed July 17, 2009. |
| Ø | Antibiotics. In our Antibiotics division we have filed a patent application directed at the intellectual property surrounding the DPOLT solid/liquid phase peptide synthesis platform technology, as well as associated areas of lantibiotics technology, in the U.S. (Pat. No. 7,521,529 filed August 11, 2006; U.S. Pat. Appl. 12/413,551, filed march 28, 2009) and internationally (PCT/US06/31510 filed August 11, 2006; PCT/US10/028620 filed March 25, 2010). In addition, we have the exclusive license for our MU1140 lantibiotic technology from the UFRF. |
| Ø | Biologics. We have licensed our SMaRT Replacement Therapy technology, and the use of recombinant Streptococcus strains to combat dental caries, from the UFRF. |
We also have applications pending and/or allowed in Australia, Canada, China, Hong Kong, Israel, Japan, Mexico, New Zealand, South Africa, South Korea, as well as in the European Patent Office. Because of the differences in patent laws and laws concerning proprietary rights, the extent of protection provided by U.S. patents or proprietary rights owned by us may differ from that of their foreign counterparts.
The recently passed Patient Protection and Affordable Care Act and the Health Care and Education Reconciliation Act provide a 12-year market exclusivity period for new biologics. We believe that our SMaRT Replacement Therapy technology would qualify for this exclusivity.
71
Table of Contents
Business
Trademarks
Our trademarks are of material importance to our business. We have developed many brand names and trademarks for our products. Accordingly, our future success may depend in part upon the goodwill associated with our brand names. We currently use the following unregistered trademarks: SMaRT Replacement Therapy™, MU1140™, IVIAT™ and CMAT™, LPT3-04™ and DPOLT™. Oragenics™ is among our non-registered trademarks. We currently have pending with the U.S. PTO, applications for registration of our principal brands, including the marks for EVORAKIDS™, EVORAPRO™ PROBIORA™, KJ2™, KJ3™ and JH145™ . We also hold U.S. trademark registrations for EVORAPLUS®, TEDDY’S PRIDE® and PROBIORA3®. Finally, we hold a European Community trademark registration for PROBIORA3®.
We also have rights to use other names essential to our business. Federally registered trademarks have a perpetual life, as long as they are maintained and renewed on a timely basis and used properly as trademarks, subject to the rights of third parties to seek cancellation of the trademarks if they claim priority or confusion of usage. We regard our trademarks and other proprietary rights as valuable assets and believe they have significant value in marketing our products.
Protection of Trade Secrets
We attempt to protect our trade secrets, including the processes, concepts, ideas and documentation associated with our technologies, through the use of confidentiality agreements and non-competition agreements with our current employees and with other parties to whom we have divulged such trade secrets. If our employees or other parties breach our confidentiality agreements and non-competition agreements or if these agreements are not sufficient to protect our technology or are found to be unenforceable, our competitors could acquire and use information that we consider to be our trade secrets and we may not be able to compete effectively. Most of our competitors have substantially greater financial, marketing, technical and manufacturing resources than we have and we may not be profitable if our competitors are also able to take advantage of our trade secrets.
Government Grants
We have applied for and have received funding from government agencies under the National Science Foundation’s and National Institute of Health’s Small Business Innovation Research, or SBIR, grants. Eligibility of public companies to receive such grants is based on size and ownership criteria which are under review by the Small Business Administration, or SBA. As a result, our eligibility may change in the future and additional funding from this source may not be available. In addition, although we seek to protect the competitive benefits we derive from our patents, proprietary information, and other intellectual property, we may not have the right to prohibit the U.S. government from using certain technologies developed or acquired by us due to federal research grants or to prohibit third-party companies, including our competitors, from using those technologies in providing products and services to the U.S. government. The U.S. government could have the right to royalty-free use of technologies that we may develop under such grants. We may commercially exploit those government-funded technologies and may assert our intellectual property rights against other non-government users of technology developed by us, but we may not be successful in our efforts to do so.
Research and Development Costs
We have spent $909,838 on research and development of our technologies in the six months ended June 30, 2010 and $1,833,746 and $1,955,488 in the years ended December 31, 2009 and 2008, respectively.
Employees
We have 19 full-time and no part-time employees. We have three employees in research and development, nine employees in general and administrative and seven employees in sales, marketing and business development. We enjoy good relations
72
Table of Contents
Business
with our employees. None of our employees is a member of any labor union, and we are not a party to any collective bargaining agreement.
Properties
In October 2009, we began leasing the office space located at 3000 Bayport Drive, Suite 685, Tampa, Florida 33607. This new location has become our principal executive office and is also being used for sales and marketing and some administrative matters. The office space is approximately 3,150 square feet and the annual lease cost is $63,317 which includes insurance, utilities and taxes. The lease term expires January 2013. Lease payments are capped during the term with the exception of taxes and insurance exceeding 3%. In addition to our Tampa location we continue to lease our research facility located at 13700 Progress Boulevard, Alachua, Florida 32615. This lease was renewed for a two-year period beginning December 2009 and expires November 2011. The facility is approximately 5,300 square feet of which approximately 60% is laboratory space and the remainder is office space and common areas. The 12-month lease costs for the year ended December 31, 2009 was approximately $97,187, of which 11 months were net of insurance, taxes and utilities that are paid by us and the new lease cost beginning December 2009 includes these amounts. Lease payments are capped during the term. We expect the location in Alachua, Florida to continue to be used primarily as our research and laboratory space as we seek to migrate more of the administrative and accounting functions to our Tampa, Florida office location. There were no leasehold improvements in 2009 and 2008. The Company terminated two lease agreements in August and October 2009 for office space located in Alachua and St. Petersburg, Florida, respectively.
Legal Proceedings
We are not a party to any material legal proceedings and there are no material legal proceedings pending with respect to our property. We are not aware of any legal proceedings contemplated by any governmental authorities involving either us or our property. None of our Directors, officers or affiliates is an adverse party in any legal proceedings involving us, or has an interest in any proceeding which is adverse to us.
73
Table of Contents
Our Board of Directors, director nominee, executive officers and key employees are as follows:
| Name |
Age | Position | ||
| Christine L. Koski |
52 | Chairperson and Director | ||
| David B. Hirsch |
41 | Chief Executive Officer, President and Director | ||
| Dr. Jeffrey D. Hillman |
61 | Chief Scientific Officer and Director | ||
| Robert C. Koski |
51 | Director | ||
| Dr. Frederick W. Telling |
58 | Director | ||
| Charles L. Pope |
58 | Director | ||
| Dr. Alan W. Dunton |
56 | Nominee Director | ||
| Brian J. Bohunicky |
56 | Chief Financial Officer, Secretary and Treasurer | ||
| Gerard “Gerry” V. David |
58 | Executive Vice President of Sales and Marketing | ||
| Dr. Martin Handfield |
40 | Director of Research and Development | ||
Directors of the Company
Christine L. Koski. Ms. Koski has served as a Director and the Chairperson of our Board of Directors since June 2009. Ms. Koski also serves as head of strategic marketing of nMetrics, LLC, a provider of web-based manufacturing system software. Prior to joining nMetrics in September 2006, Ms. Koski founded Koski Consulting Group, Inc. in June 2001 to advise start-up companies in the areas of business strategy and marketing. In addition to her positions at nMetrics and Oragenics, Ms. Koski serves as a director at Sun Hydraulics Corporation (NASDAQ: SNHY), a manufacturer of high performance hydraulic valves and solutions, and Cheltec, Inc., a specialty chemical company. Ms. Koski is a managing partner of the Koski Family Limited Partnership, which beneficially owns a controlling interest in the Company. Ms. Koski is a member of the nonprofit National Association of Corporate Directors. Ms. Koski holds an Executive MBA degree from Southern Methodist University’s Cox School of Business and a B.S. degree in Chemistry from St. Lawrence University. Ms. Koski is the sister of our Director, Robert Koski.
Ms. Koski brings to the Board over a decade of experience as an executive officer and as a director of other privately held and public technology-based companies. Through her extensive executive management and board experience, Ms. Koski has developed the leadership, business judgment and consensus-building skills necessary to effectively lead our Board as a non-executive Chairperson. Her strong expertise and background in management and marketing and track record as an accomplished executive have provided her with the business acumen and skills necessary to serve as our Chairperson.
David B. Hirsch. Mr. Hirsch has served as a Director and our Chief Executive Officer and President since June 2009. Mr. Hirsch joined the Company as a consultant in April 2008. Mr. Hirsch was appointed as our Chief Operating Officer effective June 2008 and assumed the role of Chief Financial Officer in July 2008. Mr. Hirsch was appointed as our acting President and Chief Executive Officer in March 2009 and relinquished his positions as Chief Operating Officer and Chief Financial Officer shortly thereafter. Prior to joining the Company, Mr. Hirsch operated a boutique legal and consulting practice since January 2002 with a focus on financing and advising emerging technology companies. Prior to starting his own firm, Mr. Hirsch worked at Deloitte and Touche, LLP in San Francisco, California as a manager in its restructuring group. Mr. Hirsch also served as a registered investment advisor at Mutual Ascent and as an associate at The Cottonwood Group, a venture capital firm in San Mateo, California. He holds a MSIA (MBA) degree from the Carnegie Mellon University Tepper School of Business, a JD degree from Drake University Law School and a B.A. degree in Economics from Indiana University, Bloomington. Mr. Hirsch is also a licensed attorney in the states of Florida and Indiana.
74
Table of Contents
Management
As our Chief Executive Officer, President and a member of our Board, Mr. Hirsch draws upon over a decade of experience in venture capital, restructuring and corporate finance. In addition to his industry experience, Mr. Hirsch brings to the Board the critical expertise gained over his career in corporate and business strategy and in law.
Dr. Jeffrey D. Hillman. Dr. Hillman has served as our Chief Scientific Officer and as a Director since November 1996, and as Chairman of our Board of Directors from November 1996 to December 2004. From 1992 through July 2008, Dr. Hillman was a Professor at the University of Florida College of Dentistry. Dr. Hillman received undergraduate training at the University of Chicago (Phi Beta Kappa), and holds a DMD degree (cum laude) from the Harvard School of Dental Medicine and a PhD from Harvard University Medical School. He is the inventor or co-inventor of various Oragenics’ technologies.
Dr. Hillman, our founder and longest serving Board member, brings to our Board an extensive background spanning nearly 30 years in biotechnology research and development and a deep knowledge and understanding of Oragenics’ business, operation and employees.
Robert C. Koski. Mr. Koski has served as a Director since June 2009. Mr. Koski has practiced as an attorney with the Koski Firm, a sole proprietorship located in Atlanta, Georgia since 1992, where his practice includes litigation and tax law. Mr. Koski has also served as a partner in the Koski Family Limited Partnership, which beneficially owns a controlling interest in the Company, and as a director of the Koski Family Foundation since December 1996. Mr. Koski holds a B.A. degree in Philosophy and English from Colgate University, a JD from Emory School of Law and an LLM degree in Taxation and Litigation from Emory University. He is the brother of our Director and Chairperson, Christine Koski.
Mr. Koski brings to our Board over two decades of experience in the legal field as a practicing attorney. In addition to his legal experience, Mr. Koski’s educational background provides a foundation for leadership and consensus-building.
Dr. Frederick W. Telling. Dr. Telling has served as a Director since June 2010. Dr. Telling retired from Pfizer Inc. in June 2007 after 30 years of service. At Pfizer Dr. Telling served as its Corporate Vice President and Vice President of Corporate Strategic Planning and Policy since October 1994. Dr. Telling also serves as a director and member of the Compensation Committee and Audit Committee at Cell Therapeutics Inc. (NASDAQ: CTIC), a public company based in Seattle, Washington. Dr. Telling also serves on the boards of various civic and non-profit organizations. Dr. Telling holds a B.A. degree in History and Economics from Hamilton College and a MA degree in Industrial and Labor Relations and a PhD in Economics and Public Policy from Cornell University.
Dr. Telling brings to our Board an extensive array of business and industry experience as well as experience as a director of public companies.
Charles L. Pope. Mr. Pope has served as a Director since June 2010. Mr. Pope currently serves as the Chief Financial Officer of Palm Bancorp, Inc. since June 2009. From September 2007 through June 2009, Mr. Pope served as the Chief Financial Officer of Aerosonic Inc., a manufacturer of aviation products. Mr. Pope served as the Chief Financial Officer of Reptron Inc., a manufacturer of electronic products, from March 2005 through June 2007. From March 2002 to February 2005, Mr. Pope served as Chief Financial Officer of SRI/Surgical Express, Inc. From February 2001 to March 2002 Mr. Pope served as Chief Financial Officer of Innovaro, Inc. (formerly UTEK Corporation). Prior to this time, Mr. Pope served as a Partner in the Audit and Financial Advisory Consulting Divisions and was a Partner in the Accounting and SEC Directorate at PricewaterhouseCoopers LLP. Mr. Pope serves on the board of directors of Inuvo, Inc. in Clearwater, Florida and Innovaro Inc. in Tampa, Florida, each of which are public companies. Mr. Pope holds a B.S. degree in Economics and Accounting from Auburn University and is a Certified Public Accountant in Florida.
Mr. Pope brings to our Board over three decades of experience in the finance and accounting fields. In addition, Mr. Pope also has experience serving as a director of public companies.
75
Table of Contents
Management
Nominee Director
Dr. Alan W. Dunton. Dr. Dunton is expected to become a member of our Board of Directors immediately after completion of the offering. Dr. Dunton is the principal owner of Danerius, LLC, a biotechnology consulting company which he founded in 2006. From January 2007 until March 2009, Dr. Dunton served as President and Chief Executive Officer of Panacos Pharmaceuticals, Inc. Since 2005, Dr. Dunton has served as the Non-Executive Chairman of the board of directors of ActivBiotics, Inc., a privately held biopharmaceutical company. Previously, he was the President and Chief Executive Officer of Metaphore Pharmaceuticals, Inc. from 2003 until 2006, when it merged with ActivBiotics. From 2004 until 2005, Dr. Dunton served as a member of the board of directors of Vicuron Pharmaceuticals until it was acquired by Pfizer, Inc. In 2002, Dr. Dunton served as President, Chief Operating Officer and a director of Emisphere Technologies, Inc., a biopharmaceutical company. From 1994 to 2001, Dr. Dunton was a senior executive in various capacities in the Pharmaceuticals Group of Johnson & Johnson. From 1999 to 2001, Dr. Dunton was President and Managing Director of The Janssen Research Foundation, a Johnson & Johnson company. From 1998 to 1999, he served as Group Vice President of Global Clinical Research and Development of Janssen. Prior to joining Janssen, Dr. Dunton was Vice President of Global Clinical Research and Development at the R.W. Johnson Pharmaceutical Research Institute, also a Johnson & Johnson company. Prior to joining Johnson & Johnson, Dr. Dunton held positions in clinical research and development at Syntex Corporation, CIBA-GEIGY Corporation and Hoffmann La Roche Inc. Dr. Dunton is currently serving as a member of the board of directors of Targacept, Inc. (NASDAQ: TRGT) and MediciNova, Inc. (NASDAQ: MNOV), positions which he has held since 2006. Dr. Dunton holds a MD degree from New York University School of Medicine, where he completed his residency in internal medicine. He also was a Fellow in Clinical Pharmacology at the New York Hospital/Cornell University Medical Center.
Dr. Dunton was selected to serve as one of our directors due to his extensive business and industry experience as well as his experience as a director of public companies.
Executive Management
David B. Hirsch. The biography of Mr. Hirsch is included above under the section “Directors of the Company.”
Dr. Jeffrey D. Hillman. The biography of Dr. Hillman is included above under the section “Directors of the Company.”
Brian J. Bohunicky. Mr. Bohunicky has served as our Chief Financial Officer since June 2009 and as Secretary and Treasurer since August 2009. Mr. Bohunicky joined the Company in January 2009 as the Company Controller. Prior to joining the Company, Mr. Bohunicky was the Vice President and Controller of Idex Corporation’s (NYSE: IEX) Fire & Safety Segment from October 2002 to November 2008. Mr. Bohunicky holds a B.A. degree in Economics from Moravian College.
Key Employees
Gerard “Gerry” V. David. Mr. David has served as our Executive Vice President of Sales and Marketing since September 2008. Prior to that time, he provided consulting services to Oragenics through his company, Certified Nutrition for Less, LLC. Mr. David served as President and Chief Operating Officer of Växa International in Tampa, Florida from March 2007 to July 2008. From August 2006 to February 2007 he served as Chief Operating Officer of Cyberwize in Sarasota, Florida. From March 2003 to July 2006, he served as President and Chief Operating Officer of Vitarich Laboratories, Inc. in Naples, Florida. Prior to his service at Vitarich Laboratories, Mr. David served as Chief Operating Officer of Oxyfresh. Mr. David attended Western Michigan University.
Dr. Martin Handfield. Dr. Handfield has served as our Director of Research and Development since January 2009. Prior to joining our Company, Dr. Handfield held a position as Tenured Associate Professor at the Center for Molecular Microbiology and the Department of Oral Biology at the University of Florida College of Dentistry, where he co-invented IVIAT and co-founded iviGene Corp. and Epicure Corp. to commercialize this and related technologies. Dr. Handfield holds a
76
Table of Contents
Management
B.S. degree in Biochemistry, and a MS degree and PhD in Microbiology and Immunology from the Université Laval College of Medicine in Canada, and did postdoctoral training at the University of Florida under the mentorship of Dr. Hillman.
Corporate Governance Principles
Our Board has adopted the Board of Directors Corporate Governance Policy, which sets forth the principles that guide the Board’s responsibility to oversee corporate governance, maintain its independence, evaluate its own performance and the performance of our executive officers, and set corporate strategy. Our Corporate Governance Policy states that different individuals must fill the roles of Chairman and Chief Executive Officer. Our Board first adopted the Corporate Governance Policy in December 2009 and may refine them from time to time. Our Corporate Governance Policy can be accessed on our website at http://www.oragenics.com/investors/governance.
Code of Ethics/Standards of Business Conduct
It is our policy to conduct our operations in compliance with all applicable laws and regulations and to operate our business under the fundamental principles of honesty, integrity and ethical behavior. This policy can be found in our Company Operating Principles, which is applicable to all of our Directors, officers and employees, and which complies with the SEC’s requirements. In connection with this offering and our application for listing on the NASDAQ Capital Market, we expect to adopt and be in compliance with the NASDAQ Capital Market listing standards upon consummation of the offering. Such NASDAQ Capital Market listing standards include standards relating to our Company Operating Principles, director independence and other corporate governance requirements which we believe we meet.
Our Company Operating Principles are designed to promote honest and ethical conduct and compliance with all applicable laws, rules and regulations and to deter wrongdoing. Our Company Operating Principles are also aimed at ensuring that information we provide to the public, including our filings with and submissions to the SEC, is accurate, complete, fair, relevant, timely and understandable. Our Company Operating Principles can be accessed on our web site at http://www.oragenics.com/investors/governance. We intend to disclose amendments to certain provisions of our Company Operating Principles, or waivers of such provisions granted to directors and executive officers, on our website in accordance with applicable SEC and NASDAQ Capital Markets requirements.
Board Leadership Structure
We currently separate the positions of Chief Executive Officer and Chairperson of the Board. Since June 2009, Christine Koski, one of our independent directors, has served as our non-executive Chairperson of the Board. The responsibilities of the Chairperson of the Board include: setting the agenda for each Board meeting, in consultation with the Chief Executive Officer; presiding at executive sessions; facilitating and conducting, with the Nominating Committee, the annual self-assessments by the Board and each standing committee of the Board, including periodic performance reviews of individual directors; and conducting, with the Compensation Committee, a formal evaluation of the Chief Executive Officer and other executive officers in the context of the annual compensation review.
Separating the positions of Chief Executive Officer and Chairperson of the Board allows our Chief Executive Officer to focus on our day-to-day business, while allowing the Chairperson of the Board to lead the Board in its fundamental role of providing advice to and independent oversight of management. The Board believes that having an independent director serve as Chairperson of the Board is the appropriate leadership structure for the Company at this time and demonstrates our commitment to good corporate governance.
In addition, as described in more detail below, our Board has three standing committees, each chairperson and each member of which is an independent director. Our Board delegates substantial responsibility to each Board committee, which reports their activities and actions back to the Board. We believe that our independent Board committees and their chairpersons are an important aspect of our Board leadership structure.
77
Table of Contents
Management
Board of Directors and Committees
Board of Directors
Our property, affairs and business are under the general management of our Board of Directors as provided by the laws of the State of Florida and our Bylaws.
On June 29, 2009, we consummated a private placement of equity and debt financing pursuant to a securities purchase agreement (the “June 2009 Private Placement”) with the KFLP. Pursuant to the terms of the June 2009 Private Placement we issued 2,500,000 shares of our common stock to the KFLP in exchange for $4,000,000. As a result of the June 2009 Private Placement there was a change of control of the Company with the KFLP acquiring a controlling interest in our outstanding voting common stock. See “Certain Relationships and Related Transactions.”
Effective upon the closing of the June 2009 Private Placement former independent directors Richard Welch, Derek Hennecke and Kevin Sills resigned from our Board of Directors and our President and Chief Executive Officer, David Hirsch, as well as Christine Koski and Robert Koski, were appointed to fill the vacancies on our Board of Directors created by the aforementioned resignations. Ms. Koski was elected as Chairperson to succeed Mr. Welch. Ms. Koski and Mr. Koski are principals of the KFLP. Following the closing of the June 2009 Private Placement, David Hirsch became our Chief Executive Officer and President and our Controller, Brian Bohunicky, was appointed to be our Chief Financial Officer.
On June 4, 2010 our Board of Directors expanded the size of our Board by two additional seats in order to accommodate its appointment of Charles Pope and Dr. Frederick Telling to serve as additional non-employee, independent directors. As such, the Board currently consists of six members. The Board periodically reviews the size of the Board and recommends any changes it determines to be appropriate given our needs. Under our Bylaws, the number of members on the Board may be increased or decreased by resolution of the Board.
The Board of Directors conducts its business through meetings of the full Board and through committees of the Board. The Board of Directors has appointed standing Audit, Compensation and Nominating Committees of the Board of Directors. The Board has no formal policy regarding board member attendance at our annual meeting of shareholders. All of our Directors attended the prior year’s annual meeting and are expected to attend the 2010 annual meeting. The Board of Directors met or unanimously consented to resolutions 18 times during the year ending December 31, 2009, or Fiscal 2009. Our Directors attended at least 75% of the aggregate number of meetings of the Board of Directors and Committees during Fiscal 2009. In conjunction with regularly scheduled meetings, our independent Directors met in separate executive sessions.
Director Independence
Since our securities are not listed on a national securities exchange or in an inter-dealer quotation system, we are not currently required to comply with director independence requirements. Notwithstanding the foregoing, historically we have determined director independence in accordance with the rules of a designated exchange. Our common stock has been approved for listing on the NASDAQ Capital Market subject to notice of issuance. Accordingly, in determining whether our Directors are independent, we intend to comply with the rules of the NASDAQ Capital Market following completion of the offering. We also expect to continue to comply with securities and other laws and regulations regarding the independence of directors, including those adopted under Section 301 of the Sarbanes-Oxley Act and Rule 10A-3 under the Securities and Exchange Act of 1934 with respect to the independence of Audit Committee members. The NASDAQ Capital Market listing standards define an “independent director” generally as a person, other than an officer of a company, who does not, in the view of the company’s Board of Directors, have a relationship with the company that would interfere with the director’s
78
Table of Contents
Management
exercise of independent judgment. The Board has determined that each of the following directors and nominee director, constituting a majority of the Board, is independent within the meaning of the NASDAQ Capital Market listing standards:
Christine L. Koski
Robert C. Koski
Frederick W. Telling
Charles L. Pope
Alan W. Dunton (nominee director)
Such independence definition includes a series of objective tests, including that the director is not an employee of the company and has not engaged in various types of business dealings with the company. In addition, as further required by the NASDAQ listing standards, the Board has made a subjective determination as to each independent director that no relationships exist which, in the opinion of the Board, would interfere with the exercise of independent judgment in carrying out the responsibilities of a director. Commensurate with this offering we expect to add Dr. Dunton as an independent director and as a member of our Audit Committee in order for us to be in compliance with the listing requirements of the NASDAQ Capital Market.
Audit Committee
The Audit Committee members currently consist of Charles Pope and Dr. Frederick Telling (and following the offering will also include Dr. Dunton) and the Board has determined that each such person met the requirements of independence, with it also being determined that Mr. Pope met the requirements of a financial expert. The Audit Committee members during Fiscal 2009 consisted of former directors Mr. Hennecke and Mr. Welch until their resignations and the Board determined that each such person met the requirements of independence, with it also being determined that Mr. Welch met the requirement as a financial expert. As a result of, and following, the June 2009 Private Placement described above, we did not have any Directors meeting the requirements for “independence” under the Sarbanes–Oxley Act or the NASDAQ Capital Market rules for service on an Audit Committee because our two outside Directors, Christine Koski and Robert Koski, are affiliates due to their relationship to the KFLP and the June 2009 Private Placement. Accordingly, our full Board functioned as our Audit Committee between the date of the June 2009 Private Placement and the recent appointment of Mr. Pope and Dr. Telling as Directors on June 4, 2010. In March 2004, the Audit Committee adopted a written charter which was modified in April 2007 and on December 29, 2009. We believe that our Audit Committee charter complies with the requirements related to the Sarbanes-Oxley Act and a current copy of the Audit Committee charter is available on our website at http://www.oragenics.com.
The Audit Committee has the sole authority to engage and discharge, review the independence, qualifications, activities and compensation of our independent registered certified public accountants. The Audit Committee reports to the Board the appointment of the independent registered certified public accountants. The Audit Committee must assure regular rotation of the lead and concurring audit partners. The Audit Committee is responsible for the oversight of our financial policies, control procedures, accounting staff, and reviews and approves our financial statements. The Audit Committee is responsible for the review of transactions between us and any of our officers, Directors or entity in which one of our officers or Directors has a material interest. The Audit Committee must develop and maintain procedures for the submission of complaints and concerns about accounting and auditing matters. The Audit Committee must assure that CEO and CFO certifications meet their obligations by performing a review and evaluation of our disclosure controls and procedures. The Audit Committee has the authority to engage the services of an outside advisor when required. The Audit Committee must receive reports from the independent registered certified public accountants on critical accounting policies, significant accounting judgments and estimates, off-balance sheet transactions and non-U.S. GAAP financial measures.
79
Table of Contents
Management
Compensation Committee
From the date of the June 2009 Private Placement through June 3, 2010, the members of the Compensation Committee consisted of Christine Koski and Robert Koski. Currently, and following the appointment to the Board of Dr. Telling and Charles Pope, the Compensation Committee consists of Dr. Telling, Mr. Pope, Ms. Koski and Mr. Koski with Dr. Telling serving as Chairman. The Board has determined that each current member of the Compensation Committee meets the requirements for independence. Prior to the June 2009 Private Placement, the Compensation Committee members Mr. Welch and Mr. Hennecke and the Board determined that each such person met the requirements of independence. None of the Compensation Committee members has ever been an officer or employee of the Company. The Compensation Committee is responsible for establishing the compensation of our Directors, Chief Executive Officer and all other executive officers, including salaries, bonuses, severance arrangements, and other executive officer benefits. The Compensation Committee also administers the Company’s various incentive and stock option plans and designates both the persons receiving awards and the amounts and terms of the awards. The Compensation Committee adopted a charter in March 2004 to outline its compensation, benefits and management development philosophy and to communicate to shareholders our compensation policies and the reasoning behind such policies as required by the SEC. The Compensation Committee charter was modified on April 24, 2007 and again on December 29, 2009. A current copy of the Compensation Committee charter is available on our website at http://www.oragenics.com.
Nominating Committee
The Board of Directors did not historically have a separate Nominating Committee and as such the entire Board functioned as our Nominating Committee. On December 29, 2009, however, the Board formed a Nominating Committee, consisting of Christine Koski and Robert Koski, with Mr. Koksi serving as Chairman. In conjunction therewith, the Board adopted a Nominating Committee charter. In addition to recommending candidates to the Board for election at the annual shareholder meeting, the Nominating Committee oversees the evaluation of the Board as a whole and its committees, as well as individual evaluations of those Directors who are being considered for possible re-nomination to our Board. The evaluation process occurs annually. The Nominating Committee has not established specific minimum age, education, and years of business experience or specific types of skills for potential Director candidates, but, in general, expects qualified candidates will have ample experience and a proven record of business success and leadership. The Nominating Committee also believes it is appropriate for certain key members of our management to participate as members of the Board of Directors. The Nominating Committee will only consider as candidates for Director those individuals who possess a high level of ethics, integrity and values, and who are committed to representing the long-term interests of our shareholders. Such candidates must be able to make a significant contribution to the governance of our Company by virtue of their business and financial expertise, educational and professional background. The business discipline that may be sought at any given time will vary depending on the needs and strategic direction of our Company, and the disciplines represented by incumbent directors. In evaluating candidates for nomination as a director, the Nominating Committee will also consider other criteria, including geographical representation, independence, practical wisdom, mature judgment and having sufficient time to devote to our affairs in order to carry out the responsibilities of a Director. One or more of our Directors is required to possess the education or experience required to qualify as an Audit Committee financial expert as defined in the applicable rules of the Securities and Exchange Commission. The Nominating Committee does not have a formal policy with respect to diversity; however, the Board of Directors and the Nominating Committee believe that it is essential that the members of the Board of Directors represent diverse viewpoints and a diverse mix of the specific criteria above. The entire Board of Directors is polled for suggestions as to individuals meeting the aforementioned criteria. Research may also be performed to identify qualified individuals. To date we have not engaged third parties to identify or evaluate or assist in identifying potential nominees.
Compensation of Directors
Due primarily to our limited operating capital, our Director compensation program during the fiscal year ended December 31, 2009 consisted of a one-time option grant to acquire 5,000 shares of common stock in lieu of the payment of any meeting
80
Table of Contents
Management
fees. Outside non-employee Directors are reimbursed for their expenses associated with travel to and from Board meetings and meetings with management. Certain fees previously earned by former non-employee Directors for attending Board and Committee meetings in the amount of $34,000 have been deferred instead of being paid.
On June 4, 2010, commensurate with the appointment of two new independent Directors, the Board approved changes to the standard Board compensation to be paid to non-employee Directors. Such changes primarily relate to the reinstating of a cash fee component to the Director compensation program for non-employee directors. The Director compensation program consists of the following:
Cash Compensation
The Director compensation program changes provide that all non-employee Directors will receive an annual base fee for service on the Board of $24,000. In addition, the Chairperson of the Board and of our Audit Committee, Compensation Committee and Nominating Committee will also receive annual fees of $25,000, $20,000, $15,000 and $10,000, respectively. All non-employee Directors serving on committees (other than as the Chairperson) shall receive an annual fee of $5,000 in connection with such committee service. All fees for Board service are to be paid quarterly in arrears.
Equity Compensation
Equity compensation is to be issued to Directors upon joining our Board. Non-employee Directors will receive a stock option for the purchase of 5,000 shares of our common stock at an exercise price per share equal to the fair market value per share on date they became a Director, which will immediately vest and be exercisable. As part of the Director compensation program, the Board may also make discretionary equity based awards from time to time under the Company’s existing Amended and Restated 2002 Stock Option and Incentive Plan, or 2002 Stock Incentive Plan.
Reimbursement of Expenses
Non-employee Directors are also reimbursed for expenses incurred in connection with their attendance at Board or committee meetings and reasonable out-of-pocket business expenses associated with their Board service.
Employee Directors
Consistent with past practice, the Director compensation program provides that employee Directors receive no additional compensation in connection with their board service.
The following table sets forth the compensation of our non-employee Directors in 2009.
| Name |
Fees earned or paid in cash(1) |
Option awards (2) |
All other compensation (3) |
Total | |||||||
| Christine L. Koski |
— | $ | 6,000 | — | $ | 6,000 | |||||
| Robert C. Koski |
— | $ | 6,000 | — | $ | 6,000 | |||||
| *Richard T. Welch |
$ | 30,000 | — | — | $ | 30,000 | |||||
| *Derek G. Hennecke |
$ | 6,000 | — | — | $ | 6,000 | |||||
| *Kevin H. Sills |
$ | 6,000 | — | — | $ | 6,000 | |||||
| *Marc K. Siegel |
$ | 3,000 | — | — | $ | 3,000 | |||||
| * | Former directors. |
| (1) | Amounts represent cash compensation paid to these former Directors during 2009 in connection with their service on a special committee tasked with exploring strategic alternatives on behalf of the Company. This cash compensation was |
81
Table of Contents
Management
| paid following the successful completion of the investment of capital by the Koski Family Limited Partnership in June 2009. Commensurate with such transaction, Messrs. Welch, Hennecke and Sills resigned from our Board of Directors and Christine Koski and Robert Koski were appointed to our Board. Mr. Siegel resigned as a Director on May 9, 2009. |
| (2) | The compensation amount reflected with respect to these awards represents the 2009 compensation expense associated with outstanding option grants to our non-employee directors. Upon joining our Board of Directors in June 2009, Ms. Koski and Mr. Koski as non-employee Directors were each granted options to acquire 5,000 shares of our common stock at $2.00 per share in accordance with our Director compensation program. On December 30, 2009 Ms. Koski and Mr. Koski each exercised these options in full. The amounts reflected in the table with respect to these awards represent the 2009 compensation expense associated with such grants. The Company uses a Black-Scholes option pricing model to estimate the fair value of the stock option grant. The use of a valuation model requires the Company to make certain assumptions with respect to selected model inputs. The average expected life is based on the contractual term of the option and on the simplified approach provided by SAB 107. The risk-free interest rate is based on the U.S. Treasury zero-coupon issues equal to the expected life assumed at the date of the grant. As non-employee Directors, the options previously awarded to Messrs. Welch, Hennecke, Sills and Siegel in connection with their Board service were not exercised following their departure from our Board of Directors and as such the shares covered by such options reverted back to the pool of available shares covered by our 2002 Stock Incentive Plan. |
| (3) | No other compensation was paid to the non-employee Directors except for reimbursement for travel expenses to Board meetings, which did not exceed $10,000 individually or in the aggregate for our non-employee Directors. |
Indemnification of Directors and Officers
Florida law permits us to indemnify our officers and Directors in connection with certain actions, suits and proceedings brought against them if they acted in good faith and believed their conduct to be in our best interests and, in the case of criminal actions, had no reasonable cause to believe that their conduct was unlawful. Florida law requires such indemnification when a Director entirely prevails in the defense of any proceeding to which he was a party because he is or was a Director of our company, and further provides that we may make any further indemnity and additional provision for advances and reimbursement of expenses, if authorized by our articles of incorporation or shareholder-adopted bylaws, except an indemnity against willful misconduct or a knowing violation of the criminal law.
Our bylaws provide that an officer or Director or former officer or Director shall be indemnified to the full extent permitted by Florida law as currently in effect or as hereafter amended in connection with any action, suit or proceeding brought by or in the right of our company or brought by or on behalf of our shareholders. Our articles of incorporation further provide for the elimination of the liability of our officer or Director or former officer or director for monetary damages to us or our shareholders in any action, suit or proceeding, to the full extent permitted by Florida law as currently in effect or as hereafter amended. In addition, we carry insurance on behalf of Directors and officers.
Insofar as indemnification by us for liabilities arising under the Securities Act may be permitted to our Directors, officers and controlling persons pursuant to the provisions referenced in Item 17 of the registration statement to which this prospectus forms a part, or otherwise, we have been advised that in the opinion of the SEC, such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable. If a claim for indemnification against such liabilities (other than the payment by us of expenses incurred or paid by one of our Directors, officers, or controlling persons in the successful defense of any action, suit or proceeding) is asserted by a Director, officer or controlling person in connection with the securities being registered hereunder, we will, unless in the opinion of our counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by us is against public policy as expressed in the Securities Act, and will be governed by the final adjudication of such issue.
82
Table of Contents
Summary Compensation Table
The following table sets forth the aggregate compensation in 2008 and 2009 for services in all capacities paid or accrued by the Company to our Principal Executive Officer and our next most highly compensated officers who earned more than $100,000 in total salary and bonus during the fiscal year ended December 31, 2009 (the “Named Executive Officers”).
| Name and principal position |
Year | Salary | Bonus | Option awards (5) |
All other compensation (6) |
Total | |||||||||||
| David B. Hirsch |
2009 | $ | 214,583 | $ | 100,000 | $ | 413,211 | $ | 9,417 | $ | 737,211 | ||||||
| Chief Executive Officer, President and Principal Executive Officer(1) | 2008 | $ | 94,903 | $ | 50,000 | $ | 16,348 | $ | 23,744 | $ | 184,995 | ||||||
| Dr. Jeffrey D. Hillman |
2009 | $ | 182,278 | — | $ | 318,205 | $ | 86,650 | $ | 587,133 | |||||||
| Chief Scientific Officer(2) |
2008 | $ | 180,000 | — | $ | 34,069 | $ | 5,400 | $ | 219,469 | |||||||
| Brian J. Bohunicky |
2009 | $ | 156,832 | — | $ | 195,750 | $ | 3,840 | $ | 356,422 | |||||||
| Chief Financial Officer and Principal Financial Officer(3) | 2008 | — | — | — | — | — | |||||||||||
| Gerard “Gerry” V. David |
2009 | $ | 161,250 | — | $ | 207,706 | — | $ | 368,956 | ||||||||
| Executive Vice President Sales and Marketing |
2008 | $ | 43,750 | — | $ | 2,344 | — | $ | 46,094 | ||||||||
| Dr. Martin Handfield |
2009 | $ | 158,808 | — | $ | 136,281 | — | $ | 295,089 | ||||||||
| Director of Research and Development |
2008 | $ | 6,875 | — | $ | 11,391 | — | $ | 18,266 | ||||||||
| Former officer |
|||||||||||||||||
| Stanley Stein |
2009 | $ | 39,824 | — | — | $ | 120,000 | $ | 159,824 | ||||||||
| Former President, CEO and PEO(4) |
2008 | $ | 145,833 | $ | 75,000 | $ | 54,050 | $ | 40,000 | $ | 314,833 | ||||||
| (1) | Mr. Hirsch joined our Company as an executive on May 14, 2008 and was subsequently appointed to Chief Operating Officer and entered into an employment agreement with us. On July 15, 2008, Mr. Hirsch also assumed the role of our Chief Financial Officer and Principal Financial Officer. On March 18, 2009, Mr. Hirsch relinquished his position as Chief Operating Officer and assumed the positions of acting President, Chief Executive Officer and Principal Executive Officer. In connection with his employment, Mr. Hirsch was awarded a bonus of $50,000 during 2008 of which $33,333 was deferred and subsequently paid during 2009. In June 2009 Mr. Hirsch was awarded a bonus of $100,000 payable in 50,000 shares of our common stock at a price per share of $2.00. This bonus was paid to Mr. Hirsch in recognition of his efforts in guiding us through a significant adverse capital resources crisis. On August 13, 2009, the Compensation Committee also approved an increase in Mr. Hirsch’s annual base salary from $150,000 to $225,000. |
| (2) | Effective December 1, 2009 Dr. Hillman’s annual salary was increased from $180,000 to $200,000. In addition, an amount of $81,250 in the other column reflects payments to Dr. Hillman in December 2009 for compensation and consulting fees that had previously been deferred. This amount net of applicable fees was paid through the issuance of restricted common stock to Dr. Hillman as part of our December 2009 Private Placement . See “Certain Relationships and Related Transactions.” |
| (3) | Mr. Bohunicky joined our Company in January 2009 and became our Chief Financial Officer and Principal Financial Officer on June 29, 2009. Following the 2009 Private Placement Mr. Bohunicky’s annual compensation was increased by the Compensation Committee to $200,000. Included in Mr. Bohunicky’s salary for 2009 is $25,000 in compensation that had been deferred during a portion of the year which was paid to Mr. Bohunicky immediately following the 2009 Private Placement in 12,500 shares of our common stock at a price per share of $2.00. |
83
Table of Contents
Executive compensation
| (4) | On March 18, 2009, Mr. Stein resigned as our President, Chief Executive Officer and Principal Executive Officer and was succeeded by Mr. Hirsch as our acting President, Chief Executive Officer and Principal Executive Officer. Pursuant to our separation agreement with Mr. Stein he was to be paid a severance and was to provide consulting services to us. Following a period in which no payments were made to Mr. Stein under his separation agreement and the June 2009 Private Placement, we entered into a settlement and release agreement with Mr. Stein on August 31, 2009, pursuant to which we paid him $120,000 and terminated the consulting agreement and Mr. Stein’s outstanding options. Amounts paid to Mr. Stein as severance or in connection with a settlement agreement are included under “All Other Compensation.” |
| (5) | The amounts included in this column do not reflect compensation actually received by the named executive officers. Instead the amounts in this column represent the aggregate grant date fair value computed in accordance with Financial Accounting Standards Board Accounting Standards Codification, Topic 718, Compensation-Stock Compensation (ASC 718). Under SEC rules relating to executive compensation disclosure, the amounts shown exclude the impact of estimated forfeitures related to service-based vesting conditions. Fair values relating to share grants have been determined under ASC 718 and were calculated using the common stock closing price on the date of grant and multiplying that price by the number of shares subject to the share grant. The equity-based compensation expense relating to the stock grants is recognized over the requisite service period of the grant. For option awards, we utilize the Black-Scholes option pricing model to determine the fair value on the date of the grant multiplied by the number of options subject to the option grants in accordance with ASC 718. The stock-based compensation expense relating to the stock option grants is recognized over the requisite service period of the grant. For information on the assumptions used to calculate the fair value of stock option grants, refer to Footnote 1, “Organization and Significant Accounting Policies” in our financial statements for the year ended December 31, 2009 included elsewhere in this prospectus. These amounts reflect our accounting expense for these awards, and do not necessarily correspond to the actual value that will be recognized by the executive officers. Other than our former Chief Executive Officer, Mr. Stanley Stein, no stock option awards received by our named executives above were forfeited or cancelled during 2009. Amounts in this column include $52,086, $41,455, $51,156 and $32,196 for Mr. Hirsch, Dr. Hillman, Mr. David and Dr. Handfield, respectively, which reflect the impact of the acceleration of the vesting of previously outstanding stock options by our Compensation Committee on August 13, 2009. The number of shares covered by the accelerated vesting were 21,666, 25,000, 15,166 and 13,000 for Mr. Hirsch, Dr. Hillman, Mr. David, and Dr. Handfield, respectively. All other terms of the prior option awards, including the share amounts covered by the options and exercise price remained the same. |
| (6) | Our Simple IRA retirement plan requires us to match employee contributions up to the first 3% of compensation earned and amounts presented also include our matching contribution and the amounts in this column represent such contributions. This column excludes certain payments for personal benefits for Mr. Hirsch and Dr. Hillman that do not exceed $10,000 individually or in the aggregate. |
84
Table of Contents
Executive compensation
Option Awards
The Compensation Committee believes that our future success depends, in large part, upon our ability to maintain a competitive position in attracting, retaining and motivating key personnel. Accordingly, on December 1, 2009, options to purchase a total of 281,590 shares of our common stock which are subject to time vesting and performance vesting were awarded to our executive officers and employees. These option awards each have exercise prices of $5.40 per share, which was the closing price on the date the Compensation Committee granted the options. These option awards were made pursuant to individual award agreements substantially similar to the form of stock option agreement attached as an exhibit to our 2002 Stock Incentive Plan which has been previously filed with the SEC. Included in these option grants were the following awards to executive officers:
| Name |
Time vested(1) |
Performance vested(2) |
Performance vested(3) |
Performance vested(4) |
Total | |||||
| David B. Hirsch |
50,000 | 5,625 | 6,250 | 5,000 | 66,875 | |||||
| Dr. Jeffrey D. Hillman |
35,000 | 5,000 | 6,250 | 5,000 | 51,250 | |||||
| Brian J. Bohunicky |
25,000 | 5,000 | 6,250 | — | 36,250 | |||||
| Gerard “Gerry” V. David |
17,500 | 4,500 | 6,250 | — | 28,250 | |||||
| Dr. Martin Handfield |
12,500 | 4,275 | — | 2,500 | 19,275 |
| (1) | Represents awards that are time vested with each award vesting evenly on an annual basis over three years, subject to earlier vesting upon a change in control as defined in the award agreements. |
| (2) | Represents awards that vest upon the first calendar quarter in which we report a net profit in a Form 10-Q Report or Form 10-K Report. These awards expire on the earlier of (i) December 1, 2019 or (ii) such date we cease to be required to file quarterly or annual reports with the Securities and Exchange Commission. |
| (3) | Represents awards that vest upon our achieving certain performance goals tied to the shipment and invoicing of our ProBiora3 products with a third of these options expiring if we have not achieved the vesting performance targets by September 1, 2010, and another third expiring if we have not achieved the vesting performance targets by December 1, 2010, and the remaining third expiring if we have not achieved the vesting performance targets by March 1, 2011. To the extent any of these option become vested and exercisable, they shall expire December 1, 2019. |
| (4) | Represents awards that are subject to vesting based on certain scientific performance milestones being achieved. These options expire and are void unless they become vested and exercisable on or before December 31, 2011. To the extent these options become vested and exercisable, they shall expire December 1, 2019. |
85
Table of Contents
Executive compensation
Outstanding Equity Awards
The following table provides information concerning unexercised options, stock that has not vested, and equity incentive plan awards outstanding as of December 31, 2009:
| Name |
Number of securities underlying unexercised options (#) exercisable |
Number of securities underlying unexercised options (#) unexercisable |
Equity incentive plan awards: number of securities underlying unexercised unearned options (#) |
Option exercise price ($) |
Option expiration date | ||||||
| David B. Hirsch |
25,000 | — | $ | 9.80 | 5/30/2018 | ||||||
| 50,000 | 5.40 | 12/01/2019 | |||||||||
| 5,625 | 5.40 | 12/01/2019 | |||||||||
| 6,250 | 5.40 | 12/01/2019 | |||||||||
| 5,000 | 5.40 | 12/01/2019 | |||||||||
| Dr. Jeffrey D. Hillman |
3,750 | — | 14.80 | 09/08/2011 | |||||||
| 35,000 | — | 17.00 | 5/21/2018 | ||||||||
| 35,000 | 5.40 | 12/01/2019 | |||||||||
| 5,000 | 5.40 | 12/01/2019 | |||||||||
| 6,250 | 5.40 | 12/01/2019 | |||||||||
| 5,000 | 5.40 | 12/01/2019 | |||||||||
| Brian J. Bohunicky |
25,000 | 5.40 | 12/01/2019 | ||||||||
| 5,000 | 5.40 | 12/01/2019 | |||||||||
| 6,250 | 5.40 | 12/01/2019 | |||||||||
| Gerard “Gerry” V. David |
17,500 | 5.60 | 12/17/2018 | ||||||||
| 17,500 | 6,250 | 5.40 | 12/01/2019 | ||||||||
| 4,500 | 5.40 | 12/01/2019 | |||||||||
| Dr. Martin Handfield |
15,000 | 10.40 | 8/18/2018 | ||||||||
| 12,500 | 5.40 | 12/01/2019 | |||||||||
| 4,275 | 5.40 | 12/01/2019 | |||||||||
| Former Officer |
|||||||||||
| Stanley Stein(1) |
— | — | — | — | — | ||||||
| — | — | — | — | — | |||||||
| (1) | Mr. Stein was originally granted 3,250 shares upon becoming a Director which vested immediately. These shares expired on June 18, 2009 following Mr. Stein’s resignation as a Director on March 18, 2009. Mr. Stein’s other option grant of 37,500 shares consisted of 5,000 of the option shares that became exercisable on April 9, 2008 and the remaining 32,500 option shares become exercisable upon the Company’s stock reaching certain share prices as follows: 7,500 option shares at $20.00 per share, 7,500 option shares at $40.00 per share, 7,500 option shares at $60.00 per share and 10,000 option shares at $100.00 per share. This option award was amended to continue in connection with Mr. Stein’s consulting agreement with us. Pursuant to a subsequent agreement with Mr. Stein on August 31, 2009 his consulting arrangement with us and his options were terminated. |
There were no stock options exercised by the named executive officers during the year ending December 31, 2009. No stock awards were made during 2009. We do not have any long-term incentive plans that provide compensation intended to serve as incentives for performance other than options granted pursuant to our 2002 Stock Incentive Plan.
86
Table of Contents
Executive compensation
Employment Contracts and Change in Control Arrangements
David Hirsch, Chief Executive Officer and President
David Hirsch, our Chief Executive Officer and President, began working for us as a consultant in April 2008 and became a full time employee in May 2008. In connection with Mr. Hirsch’s appointment, effective June 27, 2008, as our Chief Operating Officer, Mr. Hirsch entered into an employment agreement with us which was amended on July 15, 2008 when he also became our Chief Financial Officer upon the retirement of our former chief financial officer. Mr. Hirsch’s initial employment agreement was for one year, and was automatically extended for successive one-year renewal terms. Pursuant to his initial employment agreement, Mr. Hirsch initially received an annual salary of not less than $150,000 and was eligible to receive bonuses at the discretion of the Compensation Committee of the Board of Directors. Mr. Hirsch was granted stock options to acquire 25,000 shares of common stock under our Amended and Restated 2002 Stock Option and Incentive Plan, or 2002 Stock Incentive Plan. These options were scheduled to vest as follows: 3,333 shares vest immediately, 5,000 shares on the dates which the Company’s stock price equals or exceeds $20.00 per share, $40.00 per share and $60.00 per share respectively, and 6,666 shares on the date which the Company’s stock price equals or exceeds $100.00 per share.
Under the terms of his initial employment agreement, if Mr. Hirsch was involuntarily terminated he would receive his base salary accrued through the date of termination, and any nonforfeitable benefits earned and payable to him under the terms of the deferred compensation, incentive or other benefit plan, payable in accordance with the terms of the applicable plan. In addition, if Mr. Hirsch’s separation from employment was not voluntary, for cause or due to death or disability, the Company would be obligated to pay Mr. Hirsch a series of nine equal monthly payments equal to one-twelfth of his annual base salary in effect on the date of such termination as severance and any unvested options shall vest. If he was terminated for cause, he would be entitled to receive his base salary accrued through the date of termination and any nonforfeitable benefits already earned and payable to Mr. Hirsch under the terms of the deferred compensation or incentive plans maintained by the Company. If Mr. Hirsch voluntarily resigned, he would be entitled to this base salary accrued through termination and any nonforfeitable benefits already earned and payable to Mr. Hirsch under the terms of the deferred compensation or incentive plans maintained by the Company. In the event of a change in corporate control, the vesting of any stock options or other awards under the terms of the 2002 Stock Incentive Plan would become immediately vested in full and in the case of stock options, exercisable in full. If Mr. Hirsch is terminated within six months of a change in control, as such term is defined in his employment agreement, Mr. Hirsch would be entitled to receive, in lieu of the foregoing severance payment described above, a series of 24 equal monthly payments equal to one-twelfth of Mr. Hirsch’s annual base salary in effect at the time of a change in control. The initial employment agreement also included non-disclosure and non-compete provisions as well as a lump sum payment equal to the sum of the executive’s accrued base salary, unpaid amounts of any bonuses earned with respect to the fiscal year of the Company most recently ended and the death benefits payable under any retirement, deferred compensation or other employee benefit plan maintained by the Company in the event of Mr. Hirsch’s death during the term of the agreement.
Mr. Hirsch became our acting President and Chief Executive Officer effective March 18, 2009. He also continued in his role as our Chief Financial Officer. Mr. Hirsch did not receive any adjustment in his compensation upon assuming the role of our acting President and Chief Executive Officer. On June 29, 2009, immediately following the June 2009 Private Placement, Mr. Hirsch became our President and Chief Executive Officer and relinquished his position as Chief Financial Officer to Mr. Bohunicky. On August 13, 2009, the Compensation Committee approved acceleration of the vesting of the unvested, unexerciseable options awarded to Mr. Hirsch and approved an increase in his annual base salary to $225,000.
On March 11, 2010 our Compensation Committee approved a new employment agreement for Mr. Hirsch. See “New 2010 Employment Agreements—Hirsch, Hillman and Bohunicky.”
87
Table of Contents
Executive compensation
Dr. Jeffrey Hillman, Chief Scientific Officer
We also had an employment agreement with Dr. Jeffrey Hillman, our Chief Scientific Officer. His three-year agreement commenced on January 1, 2004 and provides for automatic one-year extensions after December 31, 2007. Under the terms of our employment agreement with Dr. Hillman, we were obligated to pay Dr. Hillman compensation of $180,000. He is also eligible for participation in incentive stock compensation plans. The employment agreement also provided for other benefits including the right to participate in fringe benefit plans, life and disability insurance plans, expense reimbursement and 20 days accumulating vacation/sick leave annually. If Dr. Hillman was terminated by the Company without cause (as defined in the agreement) or within 12 months following a change of control (as defined in the agreement), or if he left for good reason (as defined in the agreement), he would have been entitled to severance payments, at his then annual base salary and all stock options granted to the executive and any benefits under any benefit plans would have become immediately vested and exercisable. If Dr. Hillman voluntarily resigned he would have received no further compensation after the effective date of such resignation. The employment agreement also included non-disclosure and non-compete provisions, as well as salary payments for a three month period in the event of Dr. Hillman’s death or disability during the term of the agreement. Dr. Hillman was awarded options to acquire 35,000 shares of common stock under the 2002 Stock Incentive Plan on May 21, 2008. These options vested as follows: 10,000 shares immediately and the remaining 25,000 shares were scheduled to vest when the Company’s stock price reached certain levels as follows: 7,500 shares vest at $20.00 per share, 7,500 shares vest at $40.00 per share and 10,000 shares vest at $60.00 per share. On August 13, 2009 the Compensation Committee approved the accelerated vesting of the unvested, unexerciseable options.
On March 11, 2010 our Compensation Committee approved a new employment agreement for Dr. Hillman. See “New 2010 Employment Agreements—Hirsch, Hillman and Bohunicky.”
Brian Bohunicky, Chief Financial Officer, Secretary and Treasurer
Mr. Bohunicky joined the Company in January 2009 and became our Chief Financial Officer and Principal Financial Officer on June 29, 2009 following the June 2009 Private Placement. Also at this time, Mr. Bohunicky’s annual compensation was increased by the Compensation Committee to $200,000. Included in Mr. Bohunicky’s salary for 2009 is $25,000 in compensation that had been deferred during a portion of the year which was paid to Mr. Bohunicky immediately following the June 2009 Private Placement, in 12,500 shares of our common stock at a price per share of $2.00. During 2009, Mr. Bohunicky’s employment with us was at will and was not subject to the terms of an employment agreement.
On March 11, 2010 our Compensation Committee approved a new employment agreement for Mr. Bohunicky. See “New 2010 Employment Agreements—Hirsch, Hillman and Bohunicky.”
Stanley Stein, our former Chief Executive Officer
On April 8, 2008, we entered into an employment agreement with our former Chief Executive Officer and President, Mr. Stanley Stein. Mr. Stein’s initial compensation arrangement was pursuant to an offer letter that provided for an annual rate of compensation of $175,000 and relocation expenses of $10,000. Mr. Stein also was compensated in the amount of $30,000 in connection with his initial services and was expected to receive an award of stock options under our 2002 Stock Incentive Plan. The initial term of the employment agreement was for one year and was subject to automatically being extended for successive one year renewal terms. Pursuant to the employment agreement, Mr. Stein received an annual salary of not less than $175,000 and was eligible to receive bonuses at the discretion of the Compensation Committee of the Board of Directors. Mr. Stein was granted stock options to acquire 37,500 shares of common stock under our 2002 Stock Incentive Plan. The options were subject to vesting as follows: 5,000 shares on April 9, 2008; 7,500 shares on the dates which the Company’s stock price equals or exceeds $20.00 per share, $40.00 per share and $60.00 per share respectively, and 10,000 shares on the date which the Company’s stock price equals or exceeds $100.00 per share. Mr. Stein resigned as President, Chief Executive Officer and Director effective March 18, 2009 and his employment agreement with us was terminated. In
88
Table of Contents
Executive compensation
connection with Mr. Stein’s separation from employment he was to be paid his accrued compensation earned through the date of termination, which included an accrued bonus payment of $50,000 upon the occurrence of certain specified events. In addition, Mr. Stein was to be paid $1,500 for nine months to cover post-separation expenses. After separation from employment with us, Mr. Stein also became a consultant to the Company with his previously granted options continuing so long as Mr. Stein served as a consultant to the Company. On August 31, 2009, pursuant to a subsequent agreement with Mr. Stein, all continuing obligations and payments to Mr. Stein including his consulting agreement and options were terminated in exchange for a one time payment of $120,000. As a result of Mr. Stein’s resignation in March 2009, Mr. Hirsch was appointed to serve as our acting President and Chief Executive Officer.
New 2010 Employment Agreements—Hirsch, Hillman and Bohunicky
On March 11, 2010 our Compensation Committee met, approved and authorized new employment agreements with Mr. David Hirsch, our Chief Executive Officer and President, Dr. Hillman, our Chief Scientific Officer and Mr. Brian Bohunicky, our Chief Financial Officer, Secretary and Treasurer. Each of the new employment agreements have substantially similar terms other than with respect to their annual compensation and title (the “2010 Employment Agreements”). As to Mr. Hirsch and Dr. Hillman, the 2010 Employment Agreements replaced the existing employment agreements discussed above and as to Mr. Bohunicky, the 2010 Employment Agreement constitutes a new employment agreement between us and Mr. Bohunicky. The annual base salaries provided in the 2010 Employment Agreements are $225,000, $200,000 and $200,000 for Mr. Hirsch, Dr. Hillman and Mr. Bohunicky, respectively, and are payable in installments consistent with our normal payroll practices. The entering into of these 2010 Employment Agreements did not result in any change to any of the executive officers existing and previously disclosed annual base compensation. The executive officers are also eligible under the 2010 Employment Agreements to receive bonuses during the term at the discretion of the Compensation Committee and the Board of Directors.
The 2010 Employment Agreements are terminable at any time by either party and if the executive officer is involuntarily terminated by us he shall receive his base salary and vacation pay each accrued through the date of termination, and any nonforfeitable benefits earned and payable to him under the terms of the employee handbook (which applies to all employees) and benefits available under any applicable incentive plan in which employee participates. In addition, if the executive officer’s separation from employment is not voluntary and without cause, we would be obligated to pay the executive officer six months of his annual base salary as severance and the executive shall be entitled to out placement service benefits. If the executive officer is terminated for cause, he shall be entitled to receive his base salary and accrued vacation due through the date of termination and any nonforfeitable benefits already earned and payable to the executive under the terms of the employee handbook or other applicable incentive plans maintained by us. Cause is defined in the 2010 Employment Agreements as any action that is illegal, immoral, or improper that reflects on the Company, the employee, or the ability of either to function optimally. If the executive officer voluntarily resigns, he shall be entitled to this base salary and accrued vacation due through the date of termination (including any mutually agreed upon notice period) and any nonforfeitable benefits already earned and payable to the executive officer under the terms of the employee handbook or other incentive plans maintained by us.
If the executive officer dies during the term of employment with us, the estate of the employee shall be paid the salary of the employee as it would have accrued over a period of thirty days after the executive officer’s death. We shall also extend the executive officer’s right to exercise vested stock options for six months provided such extension is permitted under the 2002 Stock Incentive Plan. In the event the executive officer becomes disabled (as defined in the then applicable short and long-term disability insurance policies) we shall pay to the executive officer his salary as it would have accrued over a period of 30 days after the executive became so disabled and we shall extend the executive officer’s right to exercise vested stock options for six months provided such extension is permitted under the 2002 Stock Incentive Plan.
The 2010 Employment Agreements also each include non-disclosure and Company ownership of development provisions, as well as a provision providing for the Company to defend and indemnify the executive if the executive is named as a defendant in any lawsuit regarding any action taken within the scope of employment.
89
Table of Contents
Executive compensation
In the event of a change in control, any stock options or other awards granted (other than performance awards) under our 2002 Stock Incentive Plan shall become immediately vested in full and in the case of stock options exercisable in full. If the change in control results in an involuntary separation from employment of the executive officer within 180 days following a change in control, the executive officer would be entitled to (i) receive six months of salary and the extension of his benefits (excluding vacation time and paid time off) and (ii) exercise vested options for six months from the date of separation, provided said extension period is allowed under the 2002 Stock Incentive Plan. Under the 2010 Employment Agreements, “involuntary separation of employment” means (i) termination without cause, (ii) any reduction in responsibilities of office altering the status of the executive officer as an employee, or (iii) the duplication of the executive officers position by an equivalent executive in an acquiring entity and “change in control” means the sale of the entire company, or substantially all of its assets, or the sale of the business unit employing an individual which results in the termination of employment or subsequent transfer of the employment relationship to another legal entity, or entity, or single party acquiring more shares than are owned by the Koski Family Limited Partnership, including its members and their immediate families, including spouses and their children.
90
Table of Contents
Certain relationships and related transactions
The Audit Committee of the Board of Directors (or, to the extent applicable, our disinterested directors) is responsible for reviewing all transactions between us and any of our officers or Directors or any entity in which an officer or Director has a material interest. Any such transactions must be on terms no less favorable than those that could be obtained on an arms-length basis from independent third parties.
Consulting Fees
We paid Dr. Hillman, our Chief Scientific Officer, (i) $55,000 for consulting services he provided to us in 2001 and 2002, and (ii) $26,250 for salary deferred prior to 2008. Together these amounts, net of applicable taxes, totaled $54,063 and were paid by us through the issuance of 10,812 shares of restricted common stock at a price per share of $5.00 in accordance with Dr. Hillman’s participation in the December 2009 Private Placement discussed below.
Our former director, Dr. Marc Siegel, entered into a consulting agreement with us to provide certain media relations services to us. In connection with Dr. Siegel’s services as a consultant he was paid $9,600 in 2008. Mr. Siegel resigned as a Director on May 9, 2009.
Financing Transactions
August 2007 Private Placement
In August 2007, we issued a total of 230,000 shares of restricted common stock and warrants to acquire 230,000 shares of common stock (the “August 2007 Warrants”) in a private placement to accredited investors, for total proceeds of $1,171,591. The shares were sold at a price per share of $5.00, except that per our former exchange listing requirements, our former Director and chief executive officer who participated in the purchase was required to acquire his shares at $8.80 per share, which was the closing share price on August 7, 2007. The August 2007 Warrants were exercisable at $11.60 per share. The August 2007 Warrants expired on August 8, 2008. One of our largest shareholders at the time, George Hawes, participated in this private placement and acquired 55,000 shares and warrants to acquire 55,000 shares of our common stock. During the first quarter of 2008 we amended the August 2007 Warrants to reduce the exercise price to $8.80, which was the fair market value on the date of the amendment. The amended exercise price was applicable from January 28, 2008 to February 29, 2008. In February 2008, we issued 226,818 shares of common stock upon exercise of the August 2007 Warrants at the amended exercise price. The August 2007 Warrants that were not exercised at the amended exercise price expired unexercised on August 8, 2008. Mr. Hawes acquired 25,000 shares of common stock upon exercise of his August 2007 Warrants.
June 2008 Private Placement
In June 2008, we issued a total of 288,888 shares of restricted common stock and warrants to acquire 288,888 shares of common stock (the “June 2008 Warrants”) in a private placement to two accredited investors including our largest shareholder and affiliate at the time, George Hawes, for total proceeds of $2,600,000 (the “June 2008 Private Placement”). The shares were sold at price per share of $9.00. The June 2008 Warrants were exercisable at $26.00 per share. The June 2008 Warrants expire May 30, 2013. Pursuant to the June 2008 Private Placement, the purchasers acquired certain rights from us. However, these rights were the subject of a subsequent consent, waiver and mutual release agreement that we received from the purchasers in connection with, and as condition precedent to, the June 2009 Private Placement discussed below, and such rights are no longer of any force or effect. In addition, as part of the consent, waiver and mutual release agreement, warrants to acquire 161,000 shares of our common stock issued to the purchasers in the June 2008 Private Placement were subsequently amended in connection with the June 2009 Private Placement discussed below to reduce the exercise price from $26.00 per share to $15.00 per share, with all other terms of the warrants remaining the same.
91
Table of Contents
Certain relationships and related transactions
June 2009 Private Placement
On June 29, 2009, we issued a total of 2,500,000 shares of restricted common stock and warrants to acquire 50,000 shares of common stock in a private placement to the Koski Family Limited Partnership, or KFLP, for total proceeds of $4,000,000 (the “June 2009 Private Placement”). The shares were sold at $1.60 per share. The warrants to purchase 50,000 shares of our common stock were exercisable at $2.00 per share and had a five-year term. The consideration paid by the KFLP for the shares of common stock consisted of $4,000,000 as follows: (i) $1,500,000 in cash at closing, and (ii) $2,500,000 pursuant to a non-interest bearing promissory note providing for five consecutive monthly installment payments of $500,000 commencing July 31, 2009.
In addition, as a closing condition to the securities purchase agreement (the “June 2009 Purchase Agreement”) with the KFLP, the KFLP also provided a secured loan of $1,000,000 to us. The loan was secured by substantially all of our assets, excluding receivables, and paid interest at the rate of prime plus 4.0%, compounded quarterly. The principal of the loan, together with all accrued interest, was due in five years or upon a change of control of the Company. This loan was subsequently repaid by us in connection with the December 2009 Private Placement described below.
As a condition to the June 2009 Private Placement, we also agreed to provide the KFLP with certain registration rights in connection with any public offering consummated by us within five years from the date of closing. Specifically, we have agreed to register shares of common stock beneficially owned by the KFLP in an amount not to exceed 15% of the total number of shares publicly offered, subject to the discretion of the managing underwriter in the event of an underwritten public offering. Effective upon the closing of the June 2009 Private Placement, three of our then existing directors resigned from our Board of Directors and our President and Chief Executive Officer, Mr. David Hirsch, as well as two principals from the KFLP, Ms. Christine Koski and Mr. Robert Koski, were appointed to fill the vacancies created by such resignations, with Ms. Koski being elected as Chairperson of our Board of Directors. Ms. Koski and Mr. Koski are siblings and partners in the KFLP. As a result of the June 2009 Private Placement, a change of control occurred at the Company with the KFLP acquiring a controlling interest of greater than 50% our outstanding voting common stock.
In addition to the above, as a further condition to the consummation of the transaction contemplated by the June 2009 Purchase Agreement, we were required to obtain satisfactory arrangements with three main creditors for reductions in the amounts payable by us to these creditors. As of June 30, 2009, these reductions amounted to $707,674 in the aggregate and were conditioned upon payment of the remaining balances owed to such creditors after taking into account the agreed upon reductions. As of December 31, 2009, the amount of reductions arranged with our creditors totaled $832,959. These agreed upon reductions in payables have been fully reflected in our financial statements for the periods and reported under other income.
In connection with, and as a closing condition to, the June 2009 Private Placement, the purchasers in the June 2008 Private Placement (including George Hawes, our largest shareholder prior to the June 2009 Private Placement), entered into a consent, waiver and mutual release agreement with us on June 25, 2009 in which the purchasers waived and relinquished any special rights they obtained in connection with the June 2008 Private Placement, including, but not limited to, (i) rights of first refusal, (ii) preemptive rights and (iii) consent rights regarding future financings or secured lending. In connection with such consent, waiver and mutual release agreement, 161,000 of the June 2008 Warrants that were previously issued in connection with the June 2008 Private Placement became subject to the right of exchange for new replacement warrants to acquire the same number of shares under the same terms except for a change in the exercise price from $26.00 to $15.00. In addition, to the extent of any future underwritten registered offerings of our common stock, or the filing of any resale registration statement by us, in each case occurring within five years from the date of the consent, waiver and mutual release, the purchasers shall have the right to include an aggregate of up to 5% of the shares being registered in such offering or registration statement, subject to the discretion, in any underwritten primary offerings by us, of the underwriter on the inclusion of shares in the offering to be sold by selling shareholders.
92
Table of Contents
Certain relationships and related transactions
Financing Transactions with Dr. Hillman
On May 4, 2009 and June 10, 2009, we borrowed $32,556 and $13,100, respectively, from Dr. Jeffery Hillman, our Chief Scientific Officer. These borrowings were to be repaid upon demand by Dr. Hillman, were unsecured and did not bear interest. The proceeds from these borrowings were used to facilitate the manufacturing of ProBiora3 products for inventory. On June 29, 2009 the aggregate amount of these obligations of $45,656 was repaid by us in full through the issuance of 22,828 shares of our common stock at a price of $2.00 per share, which was the closing price of our common stock on June 29, 2009.
December 2009 Private Placement
On December 30, 2009, we issued a total of 500,812 shares of restricted common stock in a private placement to accredited investors, including the KFLP, our largest shareholder, for total proceeds of $2,504,062 (the “December 2009 Private Placement”). The shares were sold at $5.00 per share. The proceeds of $2,504,062 included the cancellation at closing of $54,062 in outstanding obligations we owed to Dr. Jeffrey Hillman, our Chief Scientific Officer and director, for compensation that had been deferred. Approximately half of the total investment, or $1,250,000, was made by the KFLP. In conjunction with, and as a condition to the initial closing of the December 2009 Private Placement, we also issued 200,000 shares of our common stock to the KFLP at $5.00 per share which was the same price per share paid by the participating accredited investors in exchange for the cancellation of the $1,000,000 secured promissory note we previously issued to the KFLP in connection with the June 2009 Private Placement.
Approximately $1,000,000 of the total proceeds from the December 2009 Private Placement were committed to further our development of the DPOLT synthetic chemistry platform, essential to the production of our lead antibiotic, MU1140, subject to the goals set forth by the two-year NSF SBIR Phase II grant received by us on February 15, 2008. Such allocation enabled us to be eligible to receive up to an additional $500,000 matching grant from the NSF, which was subsequently awarded on June 14, 2010.
Contemporaneously with the initial closing of the December 2009 Private Placement, the KFLP also elected to exercise warrants it received as part of the June 2009 Private Placement to purchase 50,000 shares of our common stock. The warrants were exercised through the payment by the KFLP of the warrant exercise price of $2.00 per share. Additionally, Christine Koski and Robert Koski, as Directors, each exercised previously issued options to purchase 100,000 shares of our common stock at the option exercise price of $2.00 per share. These options were granted to Ms. Koski and Mr. Koski when they became non-employee directors on June 30, 2009 in connection with our non-employee director compensation program.
On January 13, 2010, we completed the $3,004,062 December 2009 Private Placement and issued another 100,000 shares of common stock at a price per share of $5.00 to the accredited investors for $500,000. Of this amount, the KFLP again participated in half of the remainder of the aggregate investment by acquiring 50,000 shares for $250,000.
May 2010 Note Financing
On May 28, 2010, we entered into a $1,000,000 unsecured promissory note with conversion provisions (the “May 2010 Note”) with the KFLP. Interest on the May 2010 Note accrued at the rate of LIBOR plus 6.0%, and the principal, together with all accrued interest thereon, was due and payable the earlier of: (i) the closing date of a registered public offering of newly issued equity securities by us resulting in cash proceeds to us, other than in connection with employee option plans, or (ii) the May 24, 2011 maturity date; provided, however, that in the event we completed a subsequent private offering of equity securities prior to the May 24, 2011 maturity date, we could elect to convert the principal of the May 2010 Note into the same equity securities being sold in the private offering at the same price and terms to the KFLP. The May 2010 Note was cancelled in connection with the July 2010 Financing Transaction, described below.
93
Table of Contents
Certain relationships and related transactions
July 2010 Financing Transaction
On July 5, 2010, we entered into a common stock purchase agreement (the “July 2010 Financing Transaction”) with the KFLP. At the closing at this financing transaction on July 30, 2010, we issued 250,000 shares of our common stock to the KFLP at a price of $8.00 per share. The $2,000,000 aggregate consideration paid by the KFLP consisted of (i) $1,000,000 cash and (ii) the exchange and cancellation of the outstanding May 2010 Note. Accrued interest on the May 2010 Note through closing was waived by the KFLP. Concurrent with the July 2010 Financing Transaction and a condition thereof, we entered into an unsecured revolving credit agreement (the “Credit Facility”) with the KFLP. Pursuant to the Credit Facility, we are able to borrow up to $2,000,000 from the KFLP at LIBOR plus 6.0%. The term of the Credit Facility is for 12 months commencing August 1, 2010. As of the date of this prospectus, we have drawn $1,000,000 on this Credit Facility.
Other Relationships
During 2008, we utilized the services of the spouse of our former chief executive officer and current Vice President of Product Development, Dr. Robert Zahradnik who provided administrative services to us as an independent contractor on an as-needed basis at an hourly rate and was paid an aggregate of $5,925 during fiscal 2008. As of February 15, 2008, Ms. Zahradnik was no longer providing these services to us.
During 2008 and 2009 we paid $201,665 and $150,406, respectively, to a law firm that employs the daughter-in-law of our Chief Scientific Officer, Dr. Jeffrey Hillman, and for which we received intellectual property related legal services.
94
Table of Contents
Security ownership of management and certain beneficial owners
The following table sets forth, as of October 4, 2010, certain information concerning the beneficial ownership of each class of our voting securities by: (i) each person known by us to own beneficially 5% or more of the outstanding shares of our common stock, (ii) each of our Directors and named executive officers, and (iii) all executive officers and Directors as a group.
The number of shares beneficially owned by each 5% shareholder, Director or named executive officer is determined under rules of the SEC and the information is not necessarily indicative of beneficial ownership for any other purpose. Under those rules, beneficial ownership includes any shares to which the individual has sole or shared voting power or investment power and also any shares that the individual has the right to acquire within 60 days after October 4, 2010 through the exercise of any stock option, warrant or other right, or conversion of any security. Unless otherwise indicated, each person has sole investment and voting power (or shares such power with his or her spouse) with respect to the shares set forth in the following table. The inclusion in the table below of any shares deemed beneficially owned does not constitute an admission of beneficial ownership of those shares.
| Name and address(1) |
Number of shares beneficially owned |
Percentage of ownership(2) |
|||
| 5% shareholders |
|||||
| Koski Family Limited Partnership(3) |
3,206,998 | 56.6 | % | ||
| George T. Hawes(4) |
669,499 | 11.8 | % | ||
| Directors and officers |
|||||
| David B. Hirsch(5) |
75,000 | 1.3 | % | ||
| Dr. Jeffrey D. Hillman(6) |
271,697 | 4.7 | % | ||
| Christine L. Koski(3)(7) |
3,042,666 | 53.7 | % | ||
| Robert C. Koski(3)(8) |
3,090,666 | 54.6 | % | ||
| Charles L. Pope(9) |
5,000 | * | |||
| Dr. Frederick W. Telling(9) |
5,000 | * | |||
| Brian J. Bohunicky(10) |
12,500 | * | |||
| Dr. Alan W. Dunton (Nominee Director)(11) |
— | — | |||
| All Directors and officers as a group (7 persons) |
3,434,531 | 59.9 | % | ||
| * | less than one percent |
| (1) | Except as indicated, the address of the person named in the table is c/o Oragenics, Inc., 3000 Bayport Drive, Suite 685, Tampa, Florida 33607. |
| (2) | For each person and group included in this table, percentage ownership is calculated by dividing the number of shares beneficially owned by such person or group by the sum of 5,663,157 shares of common stock outstanding as of October 4, 2010, plus the number of shares of common stock that such person has the right to acquire within 60 days. |
| (3) | Based upon information provided by the Koski Family Limited Partnership, or KFLP, in the amendment to its Schedule 13D filing with the SEC on August 6, 2010, includes (i) 2,998,000 shares held directly by the KFLP, and (ii) 44,666 shares held by KFLP partner Christine Koski, 22,666 shares held by KFLP partner Robert Koski, 2,000 shares held by KFLP partner Koski Management, Inc. (solely owned by Beverly Koski), 69,666 shares held by KFLP partner, Thomas Koski and 70,000 shares held in trusts which Robert Koski serves as sole trustee. (See Note 8 below) The address for the KFLP is 3525 Turtle Creek Boulevard, Unit 19-B, Dallas, Texas 75219. |
| (4) | Based upon information provided by Mr. Hawes in his Form 5, Form 4s and Schedule 13D filings with the SEC. The number of shares includes 541,611 shares owned directly, (as reflected on Form 4 filed August 26, 2010) and 127,888 shares issuable pursuant to currently exercisable warrants, and excludes 5,000 shares of common stock and warrants to purchase 5,250 shares of common stock owned by Mr. Hawes’ wife for which he disclaimed beneficial ownership. Mr. Hawes’ address as reflected in Schedule 13D/A is 390 Plandome Road, Suite 222, Manhasset, New York 11030. |
95
Table of Contents
Security ownership of management and certain beneficial owners
| (5) | Includes 25,000 shares of common stock from currently exercisable options awarded to Mr. Hirsch in connection with his employment with us, and excludes an aggregate of 66,875 shares able to be acquired pursuant to stock options which have not vested. |
| (6) | Includes 202,845 shares held by the 2002 Jeffrey Hillman Trust, 39,283 shares held directly by Dr. Hillman and 38,750 shares pursuant to currently exercisable outstanding options, and excludes an aggregate of 51,250 shares able to be acquired pursuant to stock options which have not vested. Dr. Hillman disclaims beneficial ownership of 1,000 shares gifted to his minor grandchild. |
| (7) | In addition to the shares reflected as being directly owned by the KFLP in Note 3, the share amounts include 44,666 shares owned directly by Ms. Koski (which includes 5,000 shares of our common stock acquired during 2009 upon exercise of Director options). |
| (8) | In addition to the shares reflected as directly owned by the KFLP in Note 3, the share amounts include: (i) 22,666 shares owned directly by Mr. Koski (which includes 5,000 shares of our common stock acquired during 2009 upon exercise of Director options) and (ii) 70,000 shares owned by trusts which Mr. Koski serves as sole trustee as follows: the Robert Clayton Koski Trust for the benefit of Anthony James Hunter (10,000 shares); The Robert Clayton Koski Trust for the benefit of Hunter Buchanan Koski (25,000 shares); The Robert Clayton Koski Trust for the benefit of Clayton Ward Bennett (25,000 shares); and The Robert Clayton Koski Trust for the benefit of Robert Edward Koski (10,000 shares). |
| (9) | Includes 5,000 shares able to be acquired upon the exercise of currently exercisable stock options granted pursuant to our Director compensation program upon initially becoming Directors. |
| (10) | Excludes an aggregate of 36,250 shares able to be acquired pursuant to stock options which have not vested. |
| (11) | Upon becoming a non-employee independent director in connection with this offering, Dr. Dunton is expected to be granted options to acquire 5,000 shares of our common stock pursuant to our director compensation program, which, when granted, would be immediately exercisable. |
96
Table of Contents
The following descriptions are summaries of the material terms that are included in our amended and restated articles of incorporation and our bylaws. This summary is qualified in its entirety by the specific terms and provisions contained in our articles of incorporation and our bylaws, copies of forms of which we have filed as exhibits to the registration statement of which this prospectus is a part, and by the provisions of applicable law. We encourage you to read our articles of incorporation and our bylaws.
Overview
Authorized Capital Stock
Our authorized capital stock consists of 15,000,000 shares of common stock, par value $0.001, and 20,000,000 shares of preferred stock, without par value. As of October 4, 2010 there were 5,663,157 shares of our common stock issued and outstanding and no shares of our preferred stock issued and outstanding.
Authorized but Unissued Capital Stock
Florida law does not require shareholder approval for any issuance of authorized shares other than in connection with certain mergers to which we may be a party. However, the NASDAQ Capital Market rules will require shareholder approval of certain issuances of common stock or securities convertible into or exchangeable for common stock equal to or exceeding 20% of the then outstanding voting power or then outstanding number of shares of our common stock. These additional shares may be used for a variety of corporate purposes, including future public offerings to raise additional capital or to facilitate corporate acquisitions.
Common Stock
Voting
The holders of our common stock are entitled to one vote for each share held of record on all matters submitted to a vote of the shareholders. Approval of an amendment of our articles of incorporation, a merger, a share exchange, a sale of all our property or a dissolution must be approved by a majority of all votes entitled to be cast. Such votes may be cast in person or by proxy as provided in Article I Section 8 of our bylaws.
Distributions
Our Board of Directors, subject to any restrictions contained in (i) the Florida Business Corporation Act, or FBCA; or (ii) our amended and restated articles of incorporation, as amended, or Articles of Incorporation, may make distributions upon our securities. Distributions may be paid in cash, in property, or in our securities.
We have not declared or paid any distributions on our common stock. We presently intend to retain our future earnings, if any, to fund the development and growth of our business and, therefore, do not have plans to pay any dividends in the foreseeable future.
Other Rights
Upon our liquidation, dissolution or winding-up, after payment in full of our liabilities and the amounts required to be paid to holders of any outstanding shares of preferred stock, if any, all holders of our common stock will be entitled to receive a pro rata distribution of all of our assets and funds legally available for distribution.
97
Table of Contents
Description of capital stock
No shares of our common stock are subject to redemption or have preemptive rights to purchase additional shares of our common stock or any of our other securities.
Listing of Common Stock
Our common stock is currently traded on the OTC Bulletin Board under the trading symbol “ORNID.OB.” Our common stock has been approved for listing on the NASDAQ Capital Market under the proposed symbol “OGEN” subject to notice of issuance.
Transfer Agent and Registrar
The transfer agent and registrar of our common stock is Continental Stock Transfer & Trust Company, 17 Battery Place, New York, New York 10004, telephone: (212) 509-4000.
Preferred Stock
Our Board of Directors has the authority, without action by our shareholders, to designate and issue up to 20,000,000 shares of preferred stock in one or more series or classes and to designate the rights, preferences and privileges of each series or class, which may be greater than the rights of our common stock. As of October 4, 2010, we did not have any shares of preferred stock either designated or outstanding. It is not possible to state the actual effect of the issuance of any shares of preferred stock upon the rights of holders of our common stock until our Board of Directors determines the specific rights of the holders of the preferred stock. However, the effects might include:
| Ø | restricting dividends on our common stock; |
| Ø | diluting the voting power of our common stock; |
| Ø | impairing liquidation rights of our common stock; or |
| Ø | delaying or preventing a change in control of us without further action by our shareholders. |
The Board of Directors’ authority to issue preferred stock without shareholder approval could make it more difficult for a third-party to acquire control of our company, and could discourage such attempt. We have no present plans to issue any shares of preferred stock.
Options and Warrants
As of October 4, 2010, 398,112 options to acquire shares of our common stock were outstanding at exercises prices between $5.40 and $17.00 under the 2002 Stock Incentive Plan and 223,887 shares were available for future grants under our 2002 Stock Incentive Plan. As of such date, we also have warrants outstanding to acquire an aggregate of 306,388 shares of our common stock at an exercise price of between $6.00 to $26.00. Holders of options and warrants do not have any of the rights or privileges of our shareholders, including voting rights, prior to exercise of the options and warrants. The number of shares of common stock for which these options and warrants are exercisable and the exercise price of these options and warrants are subject to proportional adjustment for stock splits and similar changes affecting our common stock. We have reserved sufficient shares of authorized common stock to cover the issuance of common stock subject to the options and warrants.
98
Table of Contents
Description of capital stock
Registration Rights
We agreed with George Hawes and William Matlack that to the extent of any future underwritten registered offerings of our common stock, or the filing of any resale registration statement, in each case occurring within five years from the date of their consent, waiver and mutual release on June 25, 2009, they shall have the right to include an aggregate of up to 5% of the shares being registered in such offering or registration statement, subject to the discretion, in any underwritten primary offerings, of the underwriters in such offering.
Pursuant to the June 2009 Private Placement with the KFLP, we also agreed to provide the KFLP with certain registration rights in connection with any underwritten or other offering by us within five years of such agreement. We are required under the June 2009 Private Placement to register on behalf of KFLP 15% of the total number of shares being offered, except that in an underwritten public offering the inclusion of shares is subject to the discretion of the managing underwriter.
Certain Anti-Takeover Provisions
Florida Law
We are not subject to the statutory anti-takeover provisions under Florida law because in our articles of incorporation we have specifically elected to opt out of both the “control-share acquisitions” (F.S. 607.0902) and the “affiliated transactions” (F.S. 607.0901) statutes. Since these anti-takeover statutes do not apply to a corporation that has specifically elected to opt out of such provisions we would not be able to invoke the protection of such statutes in the event of a hostile takeover attempt.
Articles of Incorporation and Bylaw Provisions
Our articles of incorporation and bylaws contain provisions that could have an anti-takeover effect. These provisions include
| Ø | authorization of the issuance of “blank check” preferred stock that could be issued by our Board of Directors without shareholder approval and that may be substantially dilutive or contain preferences or rights objectionable to an acquiror; |
| Ø | the ability of the Board of Directors to amend the bylaws without shareholder approval; |
| Ø | vacancies on our board may only be filled by the remaining Directors and not our shareholders; and |
| Ø | requirements that only our Board, our President or holders of more than 10% of our shares can call a special meeting of shareholders. |
These provisions in our articles of incorporation and bylaws could delay or discourage transactions involving an actual or potential change in control of us, including transactions in which shareholders might otherwise receive a premium for their shares over their current prices. Such provisions could also limit the ability of shareholders to approve transactions that shareholders may deem to be in their best interests and could adversely affect the price of our common stock.
99
Table of Contents
Shares eligible for future sales
As of October 4, 2010 we had outstanding 5,663,157 shares of our common stock, of which 2,002,376 shares are freely tradable. The remaining shares of our common stock outstanding are “restricted securities” as defined in Rule 144 and are held by our “affiliates” (as that term is defined in Rule 144 under the Securities Act). These restricted securities may be sold in the future pursuant to registration statements filed with the SEC or without registration under the Securities Act to the extent permitted by Rule 144 or other exemptions under the Securities Act.
We may register additional shares in the future in connection with acquisitions, compensation or otherwise. We have not entered into any agreements or understanding regarding any future acquisitions and cannot ensure that we will be able to identify or complete any acquisition in the future. Sales of shares of common stock in the public markets or through Rule 144 may have an adverse effect on prevailing market prices for our common stock.
Rule 144 governs resale of “restricted securities” for the account of any person (other than an issuer), and restricted and unrestricted securities for the account of an “affiliate” of the issuer. Restricted securities generally include any securities acquired directly or indirectly from an issuer or its affiliates which were not issued or sold in connection with a public offering registered under the Securities Act. An affiliate of the issuer is any person who directly or indirectly controls, is controlled by, or is under common control with, the issuer. Affiliates of ours may include our Directors, executive officers, and persons directly or indirectly owning 10% or more of our outstanding common stock. Under new rules adopted by the SEC, unregistered resales of restricted securities of reporting companies are able to be made by non-affiliates and affiliates after such securities have been held for six months (assuming the issuer remains current in its SEC periodic reporting obligations for an additional six months, and subject to any affiliates complying with certain volume limitations and other resale requirements as set forth in Rule 144), and after one year by affiliates and non-affiliates of non-reporting companies, subject to certain requirements under Rule 144, as it has been amended (including that there is current public information regarding the issuer for sales by affiliates and that other volume limitations are complied with for sales of affiliates, as described in greater detail in Rule 144).
Options and Warrants
As of October 4, 2010, there were an aggregate of 306,388 shares of our common stock issuable upon exercise of outstanding stock options and an aggregate of 398,112 shares of stock issuable upon exercise of outstanding warrants. We have an effective registration statement on Form S-8 under the Securities Act covering shares of our common stock issued or reserved for issuance under our 2002 Stock Incentive Plan. Accordingly, shares of our common stock registered under such registration statement will be available for sale in the open market upon exercise by the holders, subject to vesting restrictions with us, contractual lock-up restrictions and/or market stand-off provisions applicable to each option agreement that prohibit the sale or other disposition of the shares of common stock underlying the options for a period of 180 days after the date of this prospectus, subject to a possible extension under certain circumstances, without the prior written consent from us or ThinkEquity LLC, as representative of the underwriters, as the case may be.
Lock-Up Agreements
Our Directors, executive officers, and certain of our significant shareholders have signed lock-up agreements under which they have agreed not to sell, transfer or dispose of, directly or indirectly, any shares of our common stock or any securities into or exercisable or exchangeable for shares of our common stock without the prior written consent of ThinkEquity LLC, as representative of the underwriters, for a period of 180 days, subject to a possible extension under certain circumstances, after the date of this prospectus. The holders of 3,370,781 shares of common stock, or 59.5% of our outstanding shares of common stock, have executed lock-up agreements. These agreements are described below under “Underwriting.” ThinkEquity LLC may, in its sole discretion, at any time and without prior notice, release all or any portion of the shares from the restrictions contained in these lock-up agreements.
100
Table of Contents
ThinkEquity LLC is acting as sole bookrunning manager of the offering and as representative of the underwriters named below. Subject to the terms and conditions stated in the underwriting agreement dated as of the date of this prospectus, each underwriter named below has agreed to purchase, and we have agreed to sell to that underwriter, the number of the shares set forth opposite the underwriter’s name.
| Underwriter |
Number of shares | |
| ThinkEquity LLC |
||
| Caris & Company, Inc. |
||
| Total |
||
The underwriting agreement provides that the obligations of the underwriters to purchase shares included in this offering are subject to approval of legal matters by counsel and to other conditions. The underwriters are obligated to purchase all the shares, other than those covered by the over-allotment option described below, if they purchase any of the shares. The obligation of the underwriters to purchase the shares is several and not joint meaning that, subject to the terms of the underwriting agreement, each underwriter is obligated to purchase only the number of shares set forth opposite its name.
The underwriters have advised us that they propose to offer the shares initially to the public at $ per share. The underwriters propose to offer the shares to certain dealers at the same price less a concession of not more than $ per share. If all the shares are not sold at the initial offering price, the representative may change the offering price and other selling terms.
We have granted to the underwriters an over-allotment option to purchase up to an additional 450,000 shares of our common stock from us at the same price as to the public, and with the same underwriting discount, as set forth on the front cover of this prospectus. The underwriters may exercise this option any time during the 45-day period after the date of this prospectus, but only to cover over-allotments, if any. To the extent the underwriters exercise the option, each underwriter will become obligated, subject to certain conditions, to purchase approximately the same percentage of the additional shares as it was obligated to purchase under the underwriting agreement.
We and each of our officers and directors and certain of our significant shareholders have agreed not to offer to sell, sell, pledge or otherwise dispose of (or enter into any transaction or device that is designed to, or could be expected to, result in the disposition by any person at any time in the future of), directly or indirectly, any shares of our common stock or any securities convertible into or exchangeable for our common stock, or enter into any swap or other derivatives transaction that transfers to another, in whole or in part, any of the economic consequences of ownership of our common stock, whether any transaction is to be settled by delivery of common stock or other securities, in cash or otherwise, without the prior written consent of ThinkEquity LLC for a period of 180 days after the date of this prospectus. Notwithstanding the foregoing, if (i) during the last 17 days of the initial 180-day lock-up period, we release earnings results or material news or a material event relating to us occurs or (ii) prior to the expiration of the initial 180-day lock-up period, we announce that we will release earnings results during the 16-day period beginning on the last day of the initial 180-day lock-up period, then in each case the initial 180-day lock-up period will be extended until the expiration of the 18-day period beginning on the date of release of the earnings results or the occurrence of the material news or material event, as applicable.
Our common stock is currently quoted on the OTC Bulletin Board under the symbol “ORNID.OB”. Our common stock has been approved for listing on the NASDAQ Capital Market under the proposed symbol “OGEN” subject to notice of issuance.
101
Table of Contents
Underwriting
The following table summarizes the compensation to be paid to the underwriters by us. We have agreed to pay the underwriters a non-accountable expense reimbursement equal to 1% of the gross proceeds received by us from the sale of the shares subject to this offering. Non-accountable expenses refer to the expense of the underwriters for which independent documentation is not required. The information assumes either no exercise or full exercise by the underwriters of the over-allotment option.
| Per share | Total | |||||||
| Without over-allotment |
With over-allotment |
Without over-allotment |
With over-allotment | |||||
| Underwriting discount paid by us(1) |
||||||||
| Non-accountable expense allowance paid by us(2) |
||||||||
| (1) | Does not include a non-accountable expense allowance equal to 1% of the gross proceeds of this offering (or $ ) payable to the underwriters. |
| (2) | The non-accountable expense allowance of 1% is not payable with respect to the shares sold upon exercise of the underwriters’ over-allotment option. |
Upon successful completion of this offering, ThinkEquity LLC will be granted a right of first refusal to manage any public underwriting or private placement of equity or equity-linked securities by us or to act as financial advisor to us with respect to certain business combination transactions entered into by us through July 13, 2011.
We estimate that the total expenses of this offering, including registration, filing and listing fees and legal and accounting expenses, but excluding the underwriting discounts and commissions and non-accountable expense allowance, payable by us in connection with the sale and distribution of the shares being registered will be approximately $800,000.
In connection with the offering, the underwriters may purchase and sell shares of our common stock in the open market. These transactions may include short sales, syndicate covering transactions and stabilizing transactions. Short sales involve syndicate sales of shares of our common stock in excess of the number of shares to be purchased by the underwriters in this offering, which creates a syndicate short position. “Covered” short sales are sales of shares made in an amount up to the number of shares represented by the underwriters’ over-allotment option. In determining the source of shares to close out the covered syndicate short position, the underwriters will consider, among other things, the price of shares available for purchase in the open market as compared to the price at which they may purchase shares through the over-allotment option. Transactions to close out the covered syndicate short positions involve either purchases of the common stock in the open market after the distribution has been completed or the exercise of the over-allotment option. The underwriters may also make “naked” short sales of shares in excess of the over-allotment option. The underwriters must close out any naked short position by purchasing shares of our common stock in the open market. A naked short position is more likely to be created if the underwriters are concerned that there may be downward pressure on the price of the shares in the open market after pricing that could adversely affect investors who purchase in this offering. Stabilizing transactions consist of bids for or purchases of shares in the open market while the offering is in progress.
The underwriters also may impose a penalty bid. Penalty bids permit the underwriters to reclaim a selling concession from a syndicate member when the underwriters repurchase shares originally sold by that syndicate member in order to cover syndicate short positions or make stabilizing purchases.
Any of these activities may have the effect of preventing or retarding a decline in the market price of the common stock. They may also cause the price of the common stock to be higher than the price that would otherwise exist in the open market in the absence of these transactions. The underwriters may conduct these transactions on the NASDAQ Capital Market, the OTC Bulletin Board or on any other applicable trading market. If the underwriters commence any of these transactions, they may discontinue them at any time.
102
Table of Contents
Underwriting
A prospectus in electronic format may be made available by one or more of the underwriters on a website maintained by a third-party vendor or by one or more of the underwriters. The representative of the underwriters may agree to allocate a number of shares to underwriters for sale to their online brokerage account holders. The representative will allocate shares to underwriters that may make Internet distributions on the same basis as other allocations. In addition, shares may be sold by the underwriters to securities dealers who resell shares to online brokerage account holders. Other than the prospectus in electronic format, the information on such website is not part of the prospectus. We have agreed to indemnify the underwriters against certain liabilities, including liabilities under the Securities Act, or to contribute to payments the underwriters may be required to make because of any of those liabilities.
103
Table of Contents
The legality of the shares of common stock being offered by this prospectus will be passed upon for us by Shumaker, Loop & Kendrick, LLP, Tampa, Florida. Goodwin Procter LLP, New York, New York, has acted as counsel to the underwriters in connection with this offering.
The financial statements included in this prospectus as of December 31, 2009 and 2008, and for each of the two years ended December 31, 2009 and 2008, have been audited by Kirkland Russ Murphy & Tapp, P.A., an independent registered public accounting firm, as stated in their report appearing herein (which report expresses an unqualified opinion on the financial statements and includes an explanatory paragraph relating to the substantial doubt about our ability to continue as a going concern). Such financial statements have been so included in reliance upon the report of such firm given upon their authority as experts in accounting and auditing.
Where you can find more information
We have filed with the SEC a registration statement on Form S-1 under the Securities Act with respect to the common stock offered by this prospectus. This prospectus, which is part of the registration statement, omits certain information, exhibits, schedules and undertakings set forth in the registration statement. For further information pertaining to us or our common stock, reference is made to the registration statement and the exhibits and schedules to the registration statement. Statements contained in this prospectus as to the contents or provisions of any documents referred to in this prospectus are not necessarily complete, and in each instance where a copy of the document has been filed as an exhibit to the registration statement, reference is made to the exhibit for a more complete description of the matters involved.
We are a reporting company and file annual, quarterly and current reports, proxy statements and other information with the SEC. You may read and copy all or any portion of the registration statement without charge at the Public Reference Room, Room 1580, of the SEC at 100 F Street, N.E., Washington, D.C. 20549, on official business days during the hours of 10:00 a.m. to 3:00 p.m. Copies of the registration statement may be obtained from the SEC at prescribed rates from the public reference room of the SEC at such address. You may also obtain information on the operations of the Public Reference Room by calling the SEC at (800) SEC-0330. In addition, registration statements and certain other filings made with the SEC electronically are publicly available through the SEC’s website at http://www.sec.gov. The registration statement, including all exhibits and amendments to the registration statement, have been filed electronically with the SEC.
Our website is http://www.oragenics.com. Information on, or that can be accessed through, our website is not part of this prospectus and should not be relied on in connection with this offering.
104
Table of Contents
Oragenics, Inc.
| F-1 | ||
| Report of Kirkland Russ Murphy & Tapp, P.A., independent registered public accounting firm |
F-2 | |
| Audited financial statements |
||
| F-3 | ||
| F-4 | ||
| F-5 | ||
| F-6 | ||
| F-7 to F-20 | ||
| Supplemental financial information |
||
| Balance sheets as of June 30, 2010 (unaudited) and December 31, 2009 |
F-21 | |
| Statements of operations for the six months ended June 30, 2010 and 2009 (unaudited) |
F-22 | |
| Statements of cash flows for the six months ended June 30, 2010 and 2009 (unaudited) |
F-23 | |
| Notes to financial statements (unaudited) |
F-24 to F-27 | |
F-1
Table of Contents
Report of independent registered public accounting firm
To the Board of Directors and
Shareholders of Oragenics, Inc.
We have audited the accompanying balance sheets of Oragenics, Inc. (the Company) as of December 31, 2009 and 2008, and the related statements of operations, shareholders’ equity (deficit), and cash flows for the years ended December 31, 2009 and 2008. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audit included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion of the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of Oragenics, Inc. as of December 31, 2009 and 2008, and the results of its operations and its cash flows for the years ended December 31, 2009 and 2008 in conformity with accounting principles generally accepted in the United States of America.
The accompanying financial statements have been prepared assuming Oragenics, Inc. will continue as a going concern. As more fully described in Note 1, the Company has incurred recurring operating losses, negative operating cash flows and has an accumulated deficit. These conditions raise substantial doubt about the Company’s ability to continue as a going concern. Management’s plans in regard to these matters are also described in Note 1. The financial statements do not include any adjustments to reflect the possible future effects on the recoverability and classification of assets or the amounts and classification of liabilities that may result from the outcome of this uncertainty.
| March 29, 2010 | /s/ Kirkland Russ Murphy & Tapp, P.A. | |
| Clearwater, Florida | Certified Public Accountants |
F-2
Table of Contents
Balance sheets
December 31, 2009 and 2008
| 2009 | 2008 | ||||||
| Assets |
| ||||||
| Current assets: |
|||||||
| Cash and cash equivalents |
$ | 301,592 | 1,165,933 | ||||
| Restricted cash |
2,450,000 | — | |||||
| Accounts receivable, net |
162,813 | 6,286 | |||||
| Inventory |
132,112 | 11,814 | |||||
| Prepaid expenses and other current assets |
80,839 | 86,666 | |||||
| Total current assets |
3,127,356 | 1,270,699 | |||||
| Property and equipment, net |
75,480 | 323,424 | |||||
| Total assets |
$ | 3,202,836 | 1,594,123 | ||||
| Liabilities and Shareholders’ Equity (Deficit) |
| ||||||
| Current liabilities: |
|||||||
| Accounts payable and accrued expenses |
$ | 478,111 | 1,743,684 | ||||
| Short term note payable |
35,012 | 27,687 | |||||
| Deferred grant revenues |
50,086 | — | |||||
| Total current liabilities |
563,209 | 1,771,371 | |||||
| Shareholders’ equity (deficit): |
|||||||
| Preferred stock, no par value; 20,000,000 shares authorized; none issued and outstanding |
— | — | |||||
| Common stock, $0.001 par value; 15,000,000 and 5,000,000 shares authorized at December 31, 2009 and 2008, respectively; 5,304,157 and 1,915,829 shares issued and outstanding at December 31, 2009 and December 31, 2008, respectively. |
|
5,304 |
|
1,916 |
| ||
| Additional paid-in capital |
28,146,206 | 19,813,371 | |||||
| Accumulated deficit |
(25,511,883 | ) | (19,992,535 | ) | |||
| Total shareholders’ equity (deficit) |
2,639,627 | (177,248 | ) | ||||
| Total liabilities and shareholders’ equity (deficit) |
$ | 3,202,836 | 1,594,123 | ||||
See accompanying Report of Independent Registered Public Accounting Firm and notes to the financial statements.
F-3
Table of Contents
Statements of operations
For the years ended December 31, 2009 and 2008
| Years ended December 31, | |||||||
| 2009 | 2008 | ||||||
| Net revenues |
$ | 641,285 | 233,539 | ||||
| Cost of goods sold |
221,198 | 14,864 | |||||
| Operating expenses: |
|||||||
| Research and development |
1,833,746 | 1,955,488 | |||||
| Selling, general and administration |
4,917,844 | 4,312,246 | |||||
| Total operating expenses |
6,751,590 | 6,267,734 | |||||
| Loss from operations |
(6,331,503 | ) | (6,049,059 | ) | |||
| Other income (expense): |
|||||||
| Interest income |
922 | 32,511 | |||||
| Interest expense |
(44,292 | ) | (10,054 | ) | |||
| Gain on sale of property and equipment |
22,743 | 4,860 | |||||
| Gain on extinguishment of payables |
832,959 | — | |||||
| Local business tax |
(177 | ) | — | ||||
| Total other income, net |
812,155 | 27,317 | |||||
| Loss before income taxes |
(5,519,348 | ) | (6,021,742 | ) | |||
| Net loss |
$ | (5,519,348 | ) | (6,021,742 | ) | ||
| Basic and diluted net loss per share |
$ | (1.70 | ) | (3.43 | ) | ||
| Shares used to compute basic and diluted net loss per share |
3,244,188 | 1,753,463 | |||||
See accompanying Report of Independent Registered Public Accounting Firm and notes to the financial statements.
F-4
Table of Contents
Statements of changes in shareholders’ equity (deficit)
For the years ended December 31, 2009 and 2008
| Common stock | Additional paid In capital |
Accumulated deficit |
Total shareholders’ equity (deficit) |
|||||||||||||
| Shares | Amount | |||||||||||||||
| Balances at December 31, 2007 |
1,400,122 | $ | 1,400 | $ | 14,789,276 | $ | (13,970,793 | ) | $ | 819,883 | ||||||
| Exercise of common stock warrants |
226,818 | 227 | 1,995,773 | — | 1,996,000 | |||||||||||
| Issuance of common stock and warrants |
288,889 | 289 | 2,514,711 | — | 2,515,000 | |||||||||||
| Compensation expense relating to option issuances |
— | — | 513,611 | — | 513,611 | |||||||||||
| Net loss |
— | — | — | (6,021,742 | ) | (6,021,742 | ) | |||||||||
| Balances at December 31, 2008 |
1,915,829 | $ | 1,916 | $ | 19,813,371 | $ | (19,992,535 | ) | $ | (177,248 | ) | |||||
| Exercise of common stock options and warrants |
60,000 | 60 | 119,940 | — | 120,000 | |||||||||||
| Issuance of common stock and warrants, net of expenses |
3,328,328 | 3,328 | 7,820,766 | — | 7,824,094 | |||||||||||
| Compensation expense relating to option issuances |
— | — | 392,129 | — | 392,129 | |||||||||||
| Net loss |
— | — | — | (5,519,348 | ) | (5,519,348 | ) | |||||||||
| Balances at December 31, 2009 |
5,304,157 | $ | 5,304 | $ | 28,146,206 | $ | (25,511,883 | ) | $ | 2,639,627 | ||||||
See accompanying Report of Independent Registered Public Accounting Firm and notes to the financial statements.
F-5
Table of Contents
Statements of cash flows
For the years ended December 31, 2009 and 2008
| Year ended December 31, | |||||||
| 2009 | 2008 | ||||||
| Cash flows from operating activities: |
|||||||
| Net loss |
$ | (5,519,348 | ) | (6,021,742 | ) | ||
| Adjustments to reconcile net loss to net cash used in operating activities: |
|||||||
| Non-cash bonus paid in common stock |
100,000 | — | |||||
| Non-cash services paid in common stock |
115,000 | — | |||||
| Non-cash settlement of amounts owed to employees |
113,439 | — | |||||
| Depreciation and amortization |
239,760 | 253,857 | |||||
| Stock-based compensation expense |
392,129 | 513,611 | |||||
| Gain on extinguishment of payables |
(832,959 | ) | — | ||||
| Gain on sale of property and equipment |
(22,743 | ) | (4,860 | ) | |||
| Changes in operating assets and liabilities: |
|||||||
| Accounts receivable, net |
(156,527 | ) | (6,286 | ) | |||
| Inventory |
(120,298 | ) | (11,814 | ) | |||
| Prepaid expenses and other current assets |
128,939 | 29,854 | |||||
| Accounts payable and accrued expenses |
(286,959 | ) | 1,412,190 | ||||
| Deferred grant revenues |
50,086 | — | |||||
| Net cash used in operating activities |
(5,799,481 | ) | (3,835,190 | ) | |||
| Cash flows from investing activities: |
|||||||
| Purchase of property and equipment, net |
(9,073 | ) | (55,322 | ) | |||
| Proceeds from sale of property and equipment, net |
40,000 | 42,250 | |||||
| Net cash provided by (used in) investing activities |
30,927 | (13,072 | ) | ||||
| Cash flows from financing activities: |
|||||||
| Borrowings under short term note payable |
100,000 | 79,518 | |||||
| Borrowings under long term note payable |
1,000,000 | — | |||||
| Payments on short term note payable |
(215,787 | ) | (51,831 | ) | |||
| Net proceeds from issuance of common stock |
6,470,000 | 4,511,000 | |||||
| Restricted cash from common stock issuance proceeds |
(2,450,000 | ) | — | ||||
| Net cash provided by financing activities |
4,904,213 | 4,538,687 | |||||
| Net (decrease) increase in cash and cash equivalents |
(864,341 | ) | 690,425 | ||||
| Cash and cash equivalents at beginning of year |
1,165,933 | 475,508 | |||||
| Cash and cash equivalents at end of year |
$ | 301,592 | 1,165,933 | ||||
| Interest paid |
$ | 25,915 | 10,054 | ||||
| Non-cash investing and financing activities: |
|||||||
| Issuance of common stock to employees as settlement of amounts owed |
$ | 32,556 | — | ||||
| Borrowings under short term notes payable for prepaid expense |
$ | 123,112 | — | ||||
| Long-term note payable converted into common stock |
$ | 1,000,000 | — | ||||
See accompanying Report of Independent Registered Public Accounting Firm and notes to the financial statements.
F-6
Table of Contents
Notes to financial statements
December 31, 2009 and 2008
1. Organization and Significant Accounting Policies
Oragenics, Inc. (formerly known as Oragen, Inc.) (the Company) was incorporated in November, 1996; however, operating activity did not commence until 1999. The Company is focused on the discovery, development and commercialization of a variety of technologies associated with oral health, broad spectrum antibiotics and other general health benefits.
Basis of Presentation
The accompanying financial statements of the Company have been prepared in accordance with accounting principles generally accepted in the United States (GAAP) including the assumption of a going concern basis which contemplates the realization of assets and the settlement of liabilities and commitments in the normal course of business. The Company incurred a net loss of $5,519,348 for the year ended December 31, 2009 and as of that date had an accumulated deficit of $25,511,883. Cash used in operations for the year ended December 31, 2009 was $5,799,481 and cash flow from operations was negative throughout 2009. The Company expects to incur substantial expenditures to further develop each of its technologies. The Company believes the working capital at December 31, 2009 will be sufficient to meet the business objectives as presently structured through June 2010. Management recognizes that the Company must generate additional capital resources or consider modifications to its technology development plans to enable it to continue as a going concern. Management’s plans include seeking financing, alliances or other partnership agreements with entities interested in the Company’s technologies, or other business transactions that would generate sufficient resources to assure continuation of the Company’s operations and research and development programs.
The Company intends to seek additional funding through sublicensing arrangements, joint venturing or partnering, sales of rights to technology, government grants and public or private financings. The Company’s future success depends on its ability to raise capital and ultimately generate revenues and attain profitability. The Company cannot be certain that additional capital, whether through selling additional debt or equity securities or obtaining a line of credit or other loan, will be available to it or, if available, will be on terms acceptable to the Company. If the Company issues additional securities to raise funds, these securities may have rights, preferences, or privileges senior to those of its common stock, and the Company’s current shareholders may experience dilution. If the Company is unable to obtain funds when needed or on acceptable terms, the Company may be required to curtail their current development programs, cut operating costs and forego future development and other opportunities. Without sufficient capital to fund their operations, the Company will be unable to continue as a going concern. The accompanying financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Recently Adopted Accounting Pronouncements
In August 2009, the FASB issued ASU 2009-05, “Fair Value Measurements and Disclosures (ASC Topic 820)—Measuring Liabilities at Fair Value” (“Update 2009-05”). Update 2009-05 provides clarification regarding valuation techniques when a quoted price in an active market for an identical liability is not available in addition to treatment of the existence of restrictions that prevent the transfer of a liability. Update 2009-05 also clarifies that both a quoted price in an active market for an identical liability at the measurement date and the quoted price for an identical liability when traded as an asset in an active market (when no adjustments to the quoted price of the asset are required) are Level 1 fair value measurements. This standard is effective for the first reporting period, including interim periods, beginning after issuance. Adoption of Update 2009-05 did not have a material effect on Company’s financial statements.
F-7
Table of Contents
Oragenics, Inc.
Notes to financial statements
December 31, 2009 and 2008
On July 1, 2009 the Financial Accounting Standards Board (“FASB”) Accounting Standards CodificationTM (“ASC”) became the authoritative source of accounting principles to be applied to financial statements prepared in accordance with U.S. Generally Accepted Accounting Principles (“GAAP”). In accordance with the ASC, citations to accounting literature in this report are to the relevant topic of the ASC or are presented in plain English. This standard is effective for financial statements issued for interim and annual periods ending after September 15, 2009. The Company adopted this standard at its effective date.
New Accounting Pronouncements
In June 2003, the SEC adopted final rules under Section 404 of the Sarbanes-Oxley Act of 2002 (“Section 404”), as amended, by SEC Release No. 33-8760 on December 15, 2006. Commencing with the Company’s annual report for the year ended December 31, 2007, the Company is required to include a report of management on the Company’s internal control over financial reporting. The internal control report must include a statement of management’s responsibility for establishing and maintaining adequate internal control over financial reporting for the Company; of management’s assessment of the effectiveness of the Company’s internal control over financial reporting as of year-end; and of the framework used by management to evaluate the effectiveness of the Company’s internal control over financial reporting. Furthermore, beginning with the Company’s annual report for fiscal year 2010, the Company is required to file the auditor’s attestation report separately on the Company’s internal control over financial reporting on whether it believes that the Company has maintained, in all material respects, effective internal control over financial reporting.
In October 2009, the FASB issued ASU No. 2009-13, “Revenue Recognition (Topic 605)—Multiple-Deliverable Revenue Arrangements.” ASU No. 2009-13 addresses the accounting for multiple-deliverable arrangements to enable vendors to account for products or services (deliverables) separately rather than as a combined unit. This guidance establishes a selling price hierarchy for determining the selling price of a deliverable, which is based on: (a) vendor-specific objective evidence; (b) third-party evidence; or (c) estimates. This guidance also eliminates the residual method of allocation and requires that arrangement consideration be allocated at the inception of the arrangement to all deliverables using the relative selling price method. In addition, this guidance significantly expands required disclosures related to a vendor’s multiple-deliverable revenue arrangements. ASU No. 2009-13 is effective prospectively for revenue arrangements entered into or materially modified in fiscal years beginning on or after June 15, 2010 and early adoption is permitted. A company may elect, but will not be required, to adopt the amendments in ASU No. 2009-13 retrospectively for all prior periods. Management is currently evaluating the requirements of ASU No. 2009-13 and has not yet determined the impact on the Company’s consolidated financial statements.
Use of Estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America (GAAP) requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements, as well as the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates. The principal areas of estimation reflected in the financial statements are stock based compensation, valuation of warrants, sales returns and allowances and allowance for doubtful accounts.
Fair Value of Financial Instruments
The fair value of the Company’s cash and cash equivalents, accounts payable and accrued expenses approximate their carrying values due to their short-term nature.
Cash and Cash Equivalents
Cash and cash equivalents consist of all cash balances and highly liquid investments with an original maturity of three months or less. The Company’s cash and cash equivalents are deposited in a financial institution and consist of demand deposits and overnight repurchase agreement investments and at times deposits are in excess of federally insured limits.
F-8
Table of Contents
Oragenics, Inc.
Notes to financial statements
December 31, 2009 and 2008
Restricted Cash
As of December 31, 2009, the Company had $2,450,000 of cash that was restricted and held in escrow for three days pursuant to the Common Stock Purchase Agreement dated December 30, 2009. On January 2, 2010, $1,450,000 was released from restrictions. In accordance with this agreement, the Company shall reserve and allocate $1,000,000 of the proceeds from this common stock sale solely to the expenses incurred in the further development of the Company’s DPOLT synthetic chemistry platform.
Accounts Receivable
Accounts receivable are recorded at their net realizable value and consist of trade receivables from the sale of product to customers. Management analyzes accounts receivable on a regular basis and determines the collectability based on the facts and circumstances relating to each customer. The Company does not have a history of accounts receivable or write offs, therefore, the Company estimated their allowance for doubtful accounts based on sales trend. As of December 31, 2009 and 2008, the Company has recorded an allowance for doubtful accounts of $5,410 and $0, respectively.
Inventory
Inventories are stated at the lower of cost or market. Cost, which includes material, labor and overhead, is determined on a first-in, first-out basis.
Property and Equipment
Property and equipment is stated at cost less accumulated depreciation and amortization. Depreciation is provided on the straight-line method over the estimated useful lives of the assets (three to seven years). Leasehold improvements are amortized over the shorter of the estimated useful life or the lease term of the related asset (five years).
Business Segments
In accordance with GAAP, the Company is required to report segment information. As the Company only operates principally in one business segment, no additional reporting is required.
Stock-Based Compensation
GAAP requires all share-based payments to employees, including grants of employee stock options, to be recognized in the financial statements based on their grant date fair values. Stock-based compensation expense is recorded over the requisite service period in which the employee or non-employee provides services to Oragenics, to the extent the options or warrants do not vest at the grant date and are not subject to forfeiture.
Net Loss Per Share
During all periods presented, the Company had securities outstanding that could potentially dilute basic earnings per share in the future, but were excluded from the computation of diluted net loss per share, as their effect would have been antidilutive. Because the Company reported a net loss for all periods presented, shares associated with the stock options and warrants are not included because they are antidilutive. Basic and diluted net loss per share amounts are the same for the periods presented. Net loss per share is computed using the weighted average number of shares of common stock outstanding.
Revenue Recognition
The Company recognizes revenues from the sales of product when title and risk of loss pass to the customer, which is generally when the product is shipped. Grant revenues are recognized as the reimbursable expenses are incurred over the life of the related grant. Grant revenues are deferred when reimbursable expenses have not been incurred.
F-9
Table of Contents
Oragenics, Inc.
Notes to financial statements
December 31, 2009 and 2008
The Company records allowances for discounts and product returns at the time of sales as a reduction of revenues as such allowances can be reliably estimated based on historical experience or known trends. Product returns are limited to specific mass retail customers for expiration of shelf life or unsold product over a period of time.
Impairment of Long-Lived Assets
The Company periodically reviews their long-lived assets for impairment and reduces the carrying value to fair value whenever events or changes in circumstances indicate that the carrying value may not be recoverable. There were no impairment losses recorded during the years ended December 31, 2009 and 2008.
Advertising Expenses
The Company’s policy is to expense advertising and marketing costs as incurred. For the years ended December 31, 2009 and 2008, advertising and marketing expense was $421,038 and $45,308, respectively.
Research and Development Expenses
Expenditures for research and development are expensed as incurred.
Income Taxes
Income taxes are accounted for under the asset and liability method. Deferred tax assets and liabilities are recognized for future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases and operating loss and tax credit carryforwards.
Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rate is recognized in operations in the period that includes the enactment date. Deferred tax assets are reduced to estimated amounts expected to be realized by the use of a valuation allowance.
Concentrations
The Company is dependent on three key suppliers to provide probiotics, blending and packaging of its EvoraPlus, EvoraPlus Kids and Teddy’s Pride products.
2. Inventory
Inventory consists of the following as of December 31, 2009 and 2008:
| 2009 | 2008 | |||||
| Finished goods |
$ | 77,826 | $ | 11,814 | ||
| Work-in-process |
27,286 | — | ||||
| Raw materials |
27,000 | — | ||||
| Total inventory |
$ | 132,112 | $ | 11,814 | ||
F-10
Table of Contents
Oragenics, Inc.
Notes to financial statements
December 31, 2009 and 2008
3. Property and Equipment, net
Property and equipment, net consists of the following as of December 31, 2009 and 2008:
| 2009 | 2008 | |||||||
| Furniture and fixtures |
$ | 17,109 | $ | 8,035 | ||||
| Laboratory equipment |
804,279 | 825,193 | ||||||
| Leasehold improvements |
476,777 | 481,606 | ||||||
| Office and computer equipment |
33,908 | 82,915 | ||||||
| 1,332,073 | 1,397,749 | |||||||
| Accumulated depreciation and amortization |
(1,256,593 | ) | (1,074,325 | ) | ||||
| Property and equipment, net |
$ | 75,480 | $ | 323,424 | ||||
Depreciation and amortization expense for the years ending December 31, 2009 and 2008 were $239,760 and $253,857, respectively.
4. Related Party Transactions
At December 31, 2009 deferred payments totaling $34,000 were owed to former directors in connection with their service on our Board and are included in the accompanying balance sheet in accrued expenses as of December 31, 2009. These meeting fees were deferred until such time as management determines that we have sufficient funding to pay them to the former directors. The deferrals of payments to our former directors, do not reduce our expenses, but serve to preserve our limited cash resources to the extent necessary to maintain our operations. These amounts are non-interest bearing.
At December 31, 2008 deferred payments totaling $143,583 were owed to Jeffrey D. Hillman, David Hirsch, Stanley Stein and $34,000 to the former directors and are included in the accompanying balance sheet in accounts payable and accrued expenses as of December 31, 2008. Amounts were repaid through stock issuances or cash payments during 2009, excluding $34,000 due to former directors which remain outstanding as of December 31, 2009.
5. Accounts Payable and Accrued Expenses
| 2009 | 2008 | |||||
| Accounts payable trade |
$ | 194,025 | $ | 493,599 | ||
| Legal fees |
107,656 | 909,881 | ||||
| Vacation |
88,473 | 65,907 | ||||
| Deferred compensation |
34,000 | 143,583 | ||||
| Royalties payable |
25,000 | 50,000 | ||||
| Interest |
18,377 | — | ||||
| Other |
6,291 | 10,714 | ||||
| Consulting fees |
4,289 | 70,000 | ||||
| Total accounts payable and accrued expenses |
$ | 478,111 | $ | 1,743,684 | ||
Accounts payable and accrued expenses as of December 31, 2009 and 2008 were $478,111 and $1,743,684, respectively. Excluding accounts payable trade, legal fees represent the most significant expense totaling $107,656 as of December 31, 2009 and $909,881 as of December 31, 2008. In 2009, accrued legal fees are primarily for general counsel and patent work. The fees incurred during 2008 supported legal activities related to the delisting from the NYSE Alternext US (formerly known
F-11
Table of Contents
Oragenics, Inc.
Notes to financial statements
December 31, 2009 and 2008
as the American Stock Exchange), listing on the Alternext – Paris exchange, requirements for trading on the Over-the-Counter (OTC) Bulletin Board and global expansion activities in Mexico and France. During 2009, the Company recorded $832,959 of extinguished accounts payable due to the reduction in payments owed to several creditors following the June 29, 2009 financing transaction.
6. Short Term Note Payable
On August 6, 2009 the Company entered into a short term note payable for $70,025 with an interest rate of 5.75% to finance directors and officers liability insurance. This note matures on May 24, 2010. At December 31, 2009 the balance due was $35,012.
On April 15, 2009 we entered into a loan agreement with an accredited investor for a short term note in the amount, of $100,000. On August 21, 2009 we paid the short term note and outstanding accrued interest in full. The note included an interest rate of 15% per annum and its maturity date was April 15, 2011. In connection with this borrowing we also issued warrants to acquire 5,000 shares of our common stock at an exercise price of $10.00 per share and such warrants are exercisable for five years. The fair value of the warrants was determined to be immaterial by the Company.
In March 2009, the Company entered into a short term note payable for $53,087 with an interest rate of 5.75% to finance product liability insurance. This note matures on January 10, 2010. At December 31, 2009 the balance was paid off.
The Company entered into a short term note payable in June of 2008 with an interest rate of 5.75% to finance D&O and employment related practices liability insurance at December 31, 2008, the balance due was $27,687. The amount was paid off as of April 30, 2009.
7. Line of Credit
There were no lines of credit established by the Company during 2009.
The Company opened a Line of Credit with Signature Bank, NY during 2008. The line of credit was established for short term loans for working capital purposes, provided the aggregate principal amount of loans at any time outstanding would not exceed $1,000,000. Signature Bank was entitled to receive interest at a fluctuating rate per annum equal to the Prime Rate and the interest rate was subject to change as the Prime Rate changes. The Company agreed to pay the Bank an additional compensation facility fee in the amount of $10,000 which was payable upon the Company’s acceptance. The Line of Credit was fully secured by the Company’s Signature Bank Fidelity Prime Fund money market. As of December 31, 2008 there were no amounts outstanding on the line of credit. The Company canceled the line of credit on January 24, 2009 without ever drawing upon it.
8. Shareholders’ Equity
Common Stock
On June 12, 2008, we issued an aggregate of 288,889 shares of common stock accredited to investors, including an affiliate, George T. Hawes at a price of $9.00 per share pursuant to a private offering of the Company’s stock. Net proceeds of $2,515,000 were received from this private offering.
In November, 2008, the Company began the process of listing on the NYSE Euronext Alternext Paris exchange. We were sponsored by Bryan Garnier, a European investment banking firm. We were approved in early December, and on Monday, December 15, 2008, trading of the Company’s shares on Alternext Paris commenced.
F-12
Table of Contents
Oragenics, Inc.
Notes to financial statements
December 31, 2009 and 2008
On December 10, 2008, the Company received notice from NYSE Alternext US LLC (formerly known as the American Stock Exchange* hereinafter the “Exchange” or “Alternext US”) that the Listings Qualifications Panel of the Exchange’s Committee on Securities (the “Panel”), denied the Company’s appeal and affirmed the Staff’s previous decision to delist the Company’s common stock. The notice from the Exchange indicated that the Panel agreed with the Staffs determination that the Company did not meet the continued listing standards under the Alternext US Company Guide: Section 1003(a)(ii) in that the Company’s shareholders’ equity is less than $4 million and it has sustained losses in three of its four most recent fiscal years. Accordingly, the delisting became effective at the close of market on December 19, 2008. On Monday, December 22, 2008, quotations for the Company’s shares became available on the Over-the-Counter (OTC) Bulletin Board under the ticker symbol ORNI. Quotes became available, among other places, on the OTCBB website at http://www.otcbb.com.
On May 4, 2009 and June 10, 2009, we borrowed $32,556 and $13,100, respectively, from Dr. Jeffery Hillman, our founder, Chief Science Officer and director. These borrowings were to be repaid upon demand by Dr. Hillman, were unsecured and did not bear interest. The proceeds from these borrowings were used to purchase inventory for our Consumer Healthcare products division. On June 29, 2009 the aggregate amount of these obligations of $45,656 were repaid by us in full through the issuance of 22,828 shares of our common stock at a price of $2.00 per share, which was the closing price of our common stock on June 29, 2009.
On June 29, 2009, we successfully entered into and consummated a private placement of equity and debt financing pursuant to a Securities Purchase Agreement with an accredited investor (the “June 2009 Private Placement”). Pursuant to the terms of the Securities Purchase Agreement the Company issued 2,500,000 shares of its Common Stock to the Koski Family Limited Partnership (“KFLP”) and issued warrants to the KFLP to acquire 50,000 shares of Company common stock at an exercise price of $2.00 per share in exchange for $4,000,000, the payment of which consisted of the following: $1,500,000 in cash at closing and $2,500,000 pursuant to a non-interest bearing promissory note providing for five consecutive monthly installment payments of $500,000 commencing July 31, 2009 and the KFLP provided a secured convertible loan of $1,000,000 to the Company. The loan is secured by substantially all of the Company’s assets (excluding receivables) and bears interest at the rate of Prime plus 4.0% which is payable quarterly. The principal of the loan is due in five years. The warrants expire in five years and are immediately exercisable. Immediately following the closing of the aforementioned June 2009 Private Placement, our Chief Executive Officer Mr. Hirsch was awarded a bonus of $100,000 which was paid in 50,000 shares of our common stock at a price per share of $2.00. We issued 12,500 shares of our common stock to our newly appointed Chief Financial Officer for deferred compensation we owed to him and we issued 17,187 shares of our common stock to another employee for deferred compensation we owed to him.
As a result of the transaction the board of directors believes there was a change of control of the Company with the KFLP acquiring a controlling interest of approximately 56.6% of our outstanding voting common stock. Two Koski family members, Robert C. Koski and Christine L. Koski were appointed to our Board of Directors. In addition, following the transaction, the KFLP also has the ability to consent to the selection and appointment of two outside directors.
The KFLP was also granted registration rights in connection with any offerings by the Company of its shares. Such registration rights require the Company to include a certain amount of the KFLP shares in a Company offering determined based upon 15% of the shares to be publicly offered.
In connection with, and as a condition to the Securities Purchase Agreement, the purchasers, including George Hawes our largest shareholder prior to this transaction, under that certain securities purchase agreement dated June 12, 2008, (the “Hawes Agreement”) entered into waiver and release agreements with us. In addition, such individuals waived and relinquished any special rights they possessed pursuant to agreements with the Company, including, but not limited to, (i) rights of first refusal (ii) antidilution regarding future equity sales and (iii) covenants regarding secured lending. In connection with such waivers and releases, warrants to acquire 161,000 shares of our common stock at an exercise price of $26.00 per share that were previously issued under the Hawes Agreement were subject to the right of exchange for new replacement warrants to acquire the same number of shares under the same terms except for a change in the exercise price from $26.00 to $15.00.
F-13
Table of Contents
Oragenics, Inc.
Notes to financial statements
December 31, 2009 and 2008
In addition to the above, as a further condition to the consummation of the transaction contemplated by the Securities Purchase Agreement the Company was required to obtain satisfactory arrangements with three main creditors for reductions in the amounts payable by the Company to such creditors. As of June 29, 2009 the agreed upon reductions in accounts payable with such creditors amounted to $707,674 in aggregate and the reductions were conditioned upon prompt payment of the remaining balances owed to such creditors after taking into account the reductions agreed to by such creditors. Further reductions to amounts owed to creditors were agreed to during the three months ending December 31, 2009 in the amount of $79,017. The total amount of reductions for the year ending December 31, 2009 was $832,959 which was recorded as a gain on extinguishment of payables and reported as Other Income.
In September 2009, the Company issued 25,000 shares of restricted common stock to Media4Equity LLC (“M4E”) pursuant to an agreement with M4E effective September 3, 2009 whereby M4E will provide consulting services to us with respect to national media exposure of placements of print and radio features. The agreement also requires us to pay a monthly fee to M4E of $10,000 during the three year term of the agreement, subject to certain termination rights. The shares of common stock have a fair market value of $115,000 based on a price of $4.60 per share. This amount is included in selling, general and administrative expenses in the accompanying 2009 statement of operations.
On October 28, 2009 at our annual shareholder meeting our proposal to amend the Company’s articles of incorporation to increase the authorized shares of common stock from 500,000 to 15,000,000 was approved by shareholders and the amendment to our articles of incorporation was filed with the Florida Department of State. In addition, at our annual meeting our shareholders also approved a second amendment to our Amended and Restated 2002 Stock Option and Incentive Plan to increase the shares available for grant thereunder from 250,000 to 625,000.
On December 30, 2009, we completed the initial closing of a private placement of equity pursuant to a Common Stock Purchase Agreement with accredited investors (the “December 2009 Private Placement”). The Company issued 500,812 shares of its Common Stock at a price of $5.00 per share to the investors for $2,504,062, the payment of which consisted of the following: $2,450,000 in cash at closing and $54,062 pursuant to the cancellation of the same dollar amount of outstanding deferred compensation obligation owed by the Company to Dr. Jeffrey Hillman. Approximately half of the total investment, or $1,250,000, was made by the Koski Family Limited Partnership (the “KFLP”). In conjunction with, and as a condition to the initial closing of the December 2009 Private Placement, the KFLP was issued 200,000 shares of the Company’s Common Stock at $5.00 per share, which was the same price per share paid by the investors, in exchange for the cancellation of its $1,000,000 secured note. The loan originally had been secured by substantially all of the Company’s assets (excluding receivables) and required interest payments at the rate of Prime plus 4.0% which were payable quarterly. The transaction was consummated pursuant to, and in reliance upon, an exemption from registration set forth under Section 4(2) of the Securities Act of 1933 as amended, as this transaction did not involve a public offering.
Contemporaneously with the December 2009 Private Placement contemplated by the Common Stock Purchase Agreement, the KFLP also elected to exercise previously issued warrants (issued on June 30, 2009) to purchase 50,000 shares of Company Common Stock. The warrants were exercised through the payment by the KFLP of the warrant exercise price of $2.00 per share. Additionally, Christine L. Koski and Robert C. Koski, as Directors of the Company, each exercised previously issued options to purchase 5,000 shares of the Company’s Common Stock at the option exercise price of $2.00 per share. These options were automatically granted to both Christine and Robert Koski when they became non-employee directors of the Company on June 30, 2009.
Warrants
On January 11, 2008 the Company approved an amendment to the outstanding warrants that were originally issued in connection with the Company’s private placement on March 6, 2006. The warrants were to expire on February 8, 2008 and the Board of Directors determined it would be in the best interest of the Company to amend the exercise price from $12.00 to
F-14
Table of Contents
Oragenics, Inc.
Notes to financial statements
December 31, 2009 and 2008
$8.80 for the balance of the remaining term. The outstanding warrants totaled 75,000 shares of common stock. On February 8, 2008, we issued an aggregate of 57,500 shares of common stock to warrant holders in connection with their exercise of the warrants at a reduced price of $8.80. The warrants were originally issued to accredited investors in connection with our March 6, 2006 private placement. The 17,500 remaining unexercised warrants expired as of February 8, 2008 in accordance with the terms of the warrants. Proceeds of $506,000 were received by us from the exercise of the warrants. As holders of these outstanding warrants, Jeffery Hillman, our Chief Science Officer; Robert Zahradnik, our former Chief Operating Officer; and an affiliate, George Hawes; acquired 3,125 shares, 3,125 shares and 36,875 shares, respectively.
On January 29, 2008 the Company approved an amendment to the outstanding warrants that were originally issue in connection with the Company’s private placement on August 7, 2007. The original warrants that totaled 230,000 shares of common stock and expire on August 7, 2008, were amended prior to expiration by the Board of Directors from the original $11.60 to $8.80. This amended price was only exercisable during the period from January 28, 2008 to February 29, 2008. On February 29, 2008, we issued an aggregate of 169,318 shares of common stock to warrant holders in connection with their exercise of the warrants at a reduced exercised price of $8.80. The warrants were originally issued to accredited investors in connection with our August 7, 2007 private placement. The remaining 60,682 outstanding warrants associated with this original private placement expired August 8, 2008 at an exercise price of $11.60. Proceeds of $1,490,000 were received from the exercise of warrants. As part of the warrant exercises, George T. Hawes, an affiliate, acquired 25,000 shares of the Company.
Coupled with the private offering on June 12, 2008 investors also received warrants to purchase 288,889 shares common stock at a price of $26.00 per share. These warrants expire five years from their date of issuance. A portion of these warrants to acquire 161,000 shares of our commons stock were amended in connection with the June 2009 Private Placement to reduce the exercise price from $26.00 to $15.00.
In connection with the June 2009 Private Placement the Company issued warrants to the KFLP to acquire 50,000 shares of Company common stock at an exercise price of $2.00 per share. The warrants expired in five years and were immediately exercisable. In connection with the December 2009 Private Placement, the KFLP elected to exercise its warrants to purchase 50,000 shares of Company Common Stock through the payment by the KFLP of the warrant exercise price of $2.00 per share. Additionally, Christine L. Koski and Robert C. Koski, as Directors of the Company, each exercised previously issued options to purchase 5,000 shares of the Company’s Common Stock at the option exercise price of $2.00 per share. These options were automatically granted to both Christine and Robert Koski when they became non-employee directors of the Company on June 30, 2009.
On September 14, 2009 the Company issued 12,500 warrants to Strategic Growth International to purchase common stock at an exercise price of $6.00 per share. These shares were issued in connection with a contract to provide investor relations services.
A summary of the status of the Company’s outstanding and exercisable warrants as of December 31, 2009 is presented below:
| Shares underlying warrant outstanding |
Exercise price |
Expiration date | |||
| 127,888 | $ | 26.00 | 5/30/2013 | ||
| 161,000 | 15.00 | 5/30/2013 | |||
| 5,000 | 10.00 | 4/15/2014 | |||
| 12,500 | 6.00 | 9/14/2012 | |||
| 306,388 | |||||
F-15
Table of Contents
Oragenics, Inc.
Notes to financial statements
December 31, 2009 and 2008
A summary of the status of the Company’s outstanding and exercisable warrants as of December 31, 2008 is presented below:
| Shares underlying warrant outstanding |
Exercise price |
Expiration date | |||
| 288,888 | $ | 26.00 | 5/30/2013 | ||
Stock Compensation Plan
The Company originally adopted the Oragenics, Inc. 2002 Stock Option and Incentive Plan on September 17, 2002. An Amended and Restated 2002 Stock Option and Incentive Plan was subsequently adopted by our Board and approved by our shareholders in May 2006 (the “Plan”). The First Amendment to the Plan, which increased the number of shares from 150,000 to 250,000 was approved by our shareholders in April 2008. Our stockholders approved the Second Amendment to the Plan to increase the shares available for issuance under the Plan from 250,000 to 625,000 shares in October 2009. The purpose of the Plan is to advance the interests of the Company by affording certain employees and directors of the Company and key consultants and advisors an opportunity to acquire or increase their proprietary interests in the Company. The Plan authorizes the grant of stock options (incentive and non-statutory), stock appreciation rights and restricted stock. As of December 31, 2009 and 2008, the Company had not awarded any stock appreciation rights or restricted stock under the Plan. The Company has reserved an aggregate of 625,000 and 250,000 shares of common stock for grants under the Plan at December 31, 2009 and 2008, respectively, of which 239,035 and 21,500 shares, respectively, are available for future grants as of December 31, 2009 and 2008. The exercise price of each option shall be determined by the Board and an option’s maximum term is ten years.
During the years ended December 31, 2009 and 2008, the Company recognized stock compensation expense of $392,129 and $513,611, respectively, in accordance with GAAP.
During 2009, there were 132,250 options forfeited due to lack of exercise of employees and directors. On August 13, 2009, the compensation committee approved the acceleration of the vesting of certain outstanding option awards, the vesting of which was tied to our share price reaching certain levels in the future. Option awards previously made to Mr. David Hirsch, our President and Chief Executive Officer, Dr. Jeffrey Hillman, our Chief Science Officer and certain other Company employees were impacted by the accelerated vesting of these options (21,666 shares for Mr. Hirsch, 25,000 shares for Dr. Hillman, 28,166 for other Company employees). Following the acceleration of vesting by the compensation committee, Mr. Hirsch’s grant of options to acquire 25,000 shares of our common stock at $9.80 per share is now fully vested and exercisable (including the 21,666 shares impacted by the acceleration of vesting), Dr. Hillman’s grant of options to acquire 35,000 shares of our common stock at $17.00 per share is now fully vested and exercisable (including the 25,000 shares impacted by the acceleration of vesting). The options previously had a performance condition that was not probable. They are vested without any condition and a compensation expense of $177,800 was recognized at the modification date, no compensation expense was previously recognized. All other terms of the prior option awards, including the share amounts covered by the options and exercise prices remained the same.
On December 30, 2009, we completed the initial closing of a private placement of equity pursuant to a Common Stock Purchase Agreement with accredited investors. Contemporaneously with the financing transaction, Christine L. Koski and Robert C. Koski, as Directors of the Company, each exercised previously issued options to purchase 5,000 shares of the Company’s Common Stock at the option exercise price of $2.00 per share. These options were automatically granted to both Christine and Robert Koski when they became non-employee directors of the Company on June 30, 2009.
As of the date of this filing there are approximately 306,388 warrants outstanding and there are approximately 394,090 stock options have been granted that have not been forfeited. The total number of outstanding warrants and unexercised stock options is 700,478. If all warrants and stock options were exercised, the total number of outstanding shares would be approximately 6,110,636.
F-16
Table of Contents
Oragenics, Inc.
Notes to financial statements
December 31, 2009 and 2008
A summary of the status of the Company’s outstanding stock options as of December 31, 2009 and 2008 and changes during the periods ending on those dates is presented below:
| Options | Option price per share |
Weighted average exercise price | |||||||
| Outstanding at December 31, 2007 |
67,250 | $ | 6.40 - 85.00 | $ | 25.00 | ||||
| Forfeited |
(46,500 | ) | 6.40 - 85.00 | 27.40 | |||||
| Granted |
207,750 | 5.60 - 40.00 | 11.20 | ||||||
| Outstanding at December 31, 2008 |
228,500 | $ | 5.60 - 85.00 | $ | 12.60 | ||||
| Forfeited |
(132,250 | ) | 5.60 - 80.00 | 12.00 | |||||
| Granted |
299,715 | 2.00 - 6.00 | 5.40 | ||||||
| Exercised |
(10,000 | ) | 2.00 - 2.00 | 2.00 | |||||
| Outstanding at December 31, 2009 |
385,965 | $ | 5.40 - 17.00 | $ | 7.00 | ||||
| Exercisable at the end of the year |
281,106 | $ | 5.60 - 17.00 | $ | 12.40 | ||||
The range of exercise price for outstanding options at December 31, 2009 is $5.40 to $17.00 per share. The weighted-average per option fair value of options granted during 2009 and 2008 was $5.40 and $11.20, respectively, and the weighted average remaining contractual life of those options is 9.5 years. Options vest over a period of two to three years from respective grant dates and the options expire 10 years after the date of grant. The fair value of these options was estimated at the date of grant using the Black-Scholes option pricing model with the following weighted-average assumptions: weighted average risk-free interest rate of 0.04%; dividend yields of 0%; weighted-average volatility factors of the expected market price of the Company’s common stock of 146.0%; and an expected life of the option of ten years. Future compensation expense related to the outstanding options as of December 31, 2009 is $1,421,982.
9. Licenses
The Company has two license agreements with the University of Florida Research Foundation, Inc. (“the UFRF”) for their technologies. The Company issued 29,997 shares of common stock as partial consideration in 1998. The license agreements provide for, among other things, the Company to make minimum annual research expenditures of $1,000,000 and to adhere to specific milestones. Beginning in 2005, the Company was required to pay minimum royalties on product sales of $50,000 annually per agreement. If the Company fails to perform certain of its obligations, the UFRF may terminate the license agreements. The Company’s milestones are in compliance with the UFRF and the Company had $25,000 and $50,000 of royalties payable to the UFRF recorded in the accompanying balance sheets in accounts payable and accrued expenses at December 31, 2009 and 2008, respectively.
10. Retirement Plan
In January 2004, the Company established a defined contribution Simple Individual Retirement Arrangement (IRA) plan, replacing the previous plan that had been established in 2001. The new plan covers all employees and provides for a Company match of up to 3% of all employee contributions to the plan. Total matching contributions made by the Company in 2009 and 2008 were $24,718 and $17,644, respectively.
F-17
Table of Contents
Oragenics, Inc.
Notes to financial statements
December 31, 2009 and 2008
11. Income Taxes
At December 31, 2009 and 2008, the Company had temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and their respective income tax bases, as measured by enacted state and federal tax rates, as follows:
| 2009 | 2008 | |||||||
| Deferred tax assets: |
||||||||
| Net operating loss carryforward |
$ | 8,702,188 | $ | 7,075,638 | ||||
| Compensation to Directors & Officers and consulting services |
14,486 | 38,601 | ||||||
| Total deferred tax assets |
8,716,674 | 7,114,239 | ||||||
| Less valuation allowance |
(8,716,674 | ) | (7,114,239 | ) | ||||
| Total net deferred taxes |
$ | — | $ | — | ||||
The following is a reconciliation of tax computed at the statutory federal rate to the income tax benefit in the statements of operations for the years ended December 31, 2009 and 2008:
| Years ended December 31, | ||||||||
| 2009 | 2008 | |||||||
| Income tax benefit computed at statutory federal rate of 34% |
$ | (1,876,579 | ) | $ | (2,047,392 | ) | ||
| State income tax benefits, net of federal expense/benefit |
(200,352 | ) | (218,589 | ) | ||||
| Change in valuation allowance |
1,602,435 | 2,066,955 | ||||||
| Non-deductible expenses |
474,496 | 199,026 | ||||||
| Other |
— | — | ||||||
| Total |
$ | — | $ | — | ||||
In assessing the realizability of deferred tax assets, management considers whether it is more likely than not that some portion or all of the deferred tax assets will not be realized. The ultimate realization of deferred tax assets is dependent upon the generation of future taxable income during the periods in which those temporary differences become deductible. Management considers the scheduled reversal of deferred tax liabilities, projected future taxable income, and tax planning strategies in making this assessment. Based upon the levels of historical taxable income and projections of future taxable income over which the deferred tax assets are deductible, the Company believes that it is more likely than not that it will not be able to realize the benefits of some of these deductible differences.
Accordingly, a valuation allowance of $8,716,674 and $7,114,239 has been provided in the accompanying financial statements as of December 31, 2009 and 2008, respectively. The 2009 net change in valuation allowance related to deferred tax assets was an increase of $1,602,435 primarily relating to net operating loss carryforwards.
At December 31, 2009, the Company has federal and state tax net operating loss carryforwards of approximately $23,125,665. The federal and state tax loss carryforward will expire through 2029, unless previously utilized. The Company also has federal research and development tax credit carryforwards of approximately $384,276. The federal tax credit carryforward will expire through 2029, unless previously utilized.
Pursuant to Internal Revenue Service Code Sections 382 and 383, use of the Company’s net operating losses and credit carryforwards are limited due to a cumulative change in ownership of more than 50% that occurred in 2009. As a result of the 50% change in ownership, the annual amount of pre-change net operating losses that may be used in periods subsequent to the change in ownership is approximately $172,000. The impact of this limitation is factored into management’s valuation allowance placed against the Company’s deferred tax assets.
F-18
Table of Contents
Oragenics, Inc.
Notes to financial statements
December 31, 2009 and 2008
In July 2006, the FASB issued guidance which clarifies accounting for uncertainty in income taxes recognized in an entity’s financial statements in accordance with GAAP and prescribes a recognition threshold and measurement attributes for financial statement disclosure of tax positions taken or expected to be taken on a tax return. Under GAAP, the impact of an uncertain income tax position on the income tax return must be recognized at the largest amount that is more-likely-than-not to be sustained upon audit by the relevant taxing authority. An uncertain income tax position will not be recognized if it has less than a 50% likelihood of being sustained. Additionally, GAAP provides guidance on derecognition, classification, interest and penalties, accounting for interim periods, disclosure and transition.
For the years ended December 31, 2009 and 2008, the Company incurred $43,057 and $50,890, respectively, of additional unrecognized tax benefits that resulted in a decrease to the deferred tax asset valuation allowance, related to research and development credits. The entire amount of this unrecognized tax benefit, if recognized, would result in an increase to the deferred tax asset valuation allowance, and would not have an impact on the effective tax rate.
The Company files its income tax returns in the U.S. federal jurisdiction and in Florida. With few exceptions, the Company is no longer subject to federal or state income tax examinations by tax authorities for years before 2006.
A reconciliation of the beginning and ending amount of unrecognized tax benefits is as follows:
| Balance as of December 31, 2007 |
$ | 290,329 | |
| Additions based on tax positions related to the current year |
50,890 | ||
| Additions for the tax positions of prior years |
— | ||
| Reductions for the tax positions of prior years |
— | ||
| Balance as of December 31, 2008 |
$ | 341,219 | |
| Additions based on tax positions related to the current year |
43,057 | ||
| Additions for the tax positions of prior years |
— | ||
| Reductions for the tax positions of prior years |
— | ||
| Balance as of December 31, 2009 |
$ | 384,276 |
Included in the balance at December 31, 2009 and 2008, are $384,276 and $341,219, respectively, of tax positions for which there is uncertainty about the validity of certain credits. The disallowance of the credits would impact the amount of gross deferred tax assets reflected in the accompanying footnotes.
During the years 2009 and 2008 the Company did not recognize any interest and penalties. Due to the potential offset of the Company’s operating loss carryforward for any future activity, the amount attributed to interest and penalties would be immaterial.
12. Commitments and Contingencies
The Company’s Alachua facility is being leased from a real estate developer for a term of two years renewed in December 2009. Lease payments are capped during the term with the exception of taxes and insurance exceeding 3%. This operating lease agreement required the Company to pay a deposit of $6,400 and provides for monthly lease payments of $8,993, inclusive of utilities, insurance, sales taxes and real estate taxes. Rent expense under this lease was $97,187 and $89,753 for the years ended December 31, 2009 and 2008, respectively. On October 1, 2009 the Company leased office space for Corporate, Sales and Marketing personnel located in Tampa, FL. The lease is for approximately 3,150 square feet and is occupied by seven employees. The lease period for the office space is forty months in the amount of $5,276 per month inclusive of insurance, taxes and utilities. The lease expires on January 31, 2013. Rent expense under this lease was $15,828 for the year ended December 31, 2009.
F-19
Table of Contents
Oragenics, Inc.
Notes to financial statements
December 31, 2009 and 2008
The Company terminated two lease agreements in August and October 2009 for office spaces which were located in Alachua and St. Petersburg, Florida, respectively.
Future annual minimum payments under all non-cancelable operating leases are approximately as follows as of December 31, 2009:
| Year ended: |
|||
| 2010 |
171,229 | ||
| 2011 |
162,236 | ||
| 2012 |
63,317 | ||
| 2013 |
5,276 | ||
| $ | 402,058 | ||
13. Unaudited Quarterly Financial Information
The quarterly interim financial information shown below has been prepared by the Company’s management and is unaudited. It should be read in conjunction with the audited financial statements appearing herein.
| 2009 | ||||||||||||||||
| First | Second | Third | Fourth | |||||||||||||
| Net revenues |
$ | 124,272 | $ | 41,895 | $ | 199,675 | $ | 275,444 | ||||||||
| Total operating expenses |
2,092,819 | 1,605,328 | 1,593,353 | 1,460,091 | ||||||||||||
| Net loss |
(1,980,350 | ) | (869,048 | ) | (1,437,184 | ) | (1,232,766 | ) | ||||||||
| Loss per share: |
||||||||||||||||
| Basic and diluted |
$ | (1.20 | ) | $ | (0.40 | ) | $ | (0.40 | ) | $ | (0.20 | ) | ||||
| 2008 | ||||||||||||||||
| First | Second | Third | Fourth | |||||||||||||
| Net revenues |
$ | 125,000 | $ | — | $ | 100,000 | $ | 8,539 | ||||||||
| Total operating expenses |
926,095 | 1,111,553 | 1,253,200 | 2,983,596 | ||||||||||||
| Net loss |
(791,636 | ) | (1,095,112 | ) | (1,138,117 | ) | (2,996,877 | ) | ||||||||
| Loss per share: |
||||||||||||||||
| Basic and diluted |
$ | (0.60 | ) | $ | (0.60 | ) | $ | (0.60 | ) | $ | (1.60 | ) | ||||
14. Subsequent Events
On January 13, 2010 the Company completed the $3,004,062 private placement contemplated by the Common Stock Purchase Agreement and December 2009 Private Placement and issued another 100,000 shares of common stock at a price of $5.00 per share to accredited investors for $500,000. Of this amount the KFLP again participated in one half of the remainder of the aggregate investment by acquiring 50,000 of the shares for $250,000.
F-20
Table of Contents
Balance sheets
June 30, 2010 (unaudited) and December 31, 2009
| June
30, 2010 |
December 31, 2009 |
|||||||
| (unaudited) | ||||||||
| Assets |
| |||||||
| Current assets: |
||||||||
| Cash and cash equivalents |
$ | 216,752 | $ | 301,592 | ||||
| Restricted cash |
742,682 | 2,450,000 | ||||||
| Accounts receivables, net |
173,540 | 162,813 | ||||||
| Inventory |
416,474 | 132,112 | ||||||
| Prepaid expenses and other current assets |
69,929 | 80,839 | ||||||
| Total current assets |
1,619,377 | 3,127,356 | ||||||
| Property and equipment, net |
58,500 | 75,480 | ||||||
| Total assets |
$ | 1,677,877 | $ | 3,202,836 | ||||
| Liabilities and Shareholders’ Equity (Deficit) |
| |||||||
| Current liabilities: |
||||||||
| Accounts payable and accrued expenses |
$ | 678,636 | $ | 478,111 | ||||
| Short term notes payable |
30,382 | 35,012 | ||||||
| Note payable to shareholder |
1,000,000 | — | ||||||
| Deferred revenues |
50,582 | 50,086 | ||||||
| Total current liabilities |
1,759,600 | 563,209 | ||||||
| Shareholders’ equity (deficit): |
||||||||
| Preferred stock, no par value; 20,000,000 shares authorized; none issued and outstanding |
— | — | ||||||
| Common stock, $0.001 par value; 15,000,000 shares authorized; 5,410,157 and 5,304,157 shares issued and outstanding at June 30, 2010 and December 31, 2009, respectively. |
5,410 | 5,304 | ||||||
| Additional paid-in capital |
29,182,171 | 28,146,206 | ||||||
| Accumulated deficit |
(29,269,304 | ) | (25,511,883 | ) | ||||
| Total shareholders’ equity (deficit) |
(81,723 | ) | 2,639,627 | |||||
| Total liabilities and shareholders’ equity (deficit) |
$ | 1,677,877 | $ | 3,202,836 | ||||
See accompanying notes.
F-21
Table of Contents
Statements of operations
For the six months ended June 30, 2010 and 2009 (unaudited)
| Six months
ended June 30, |
||||||||
| 2010 | 2009 | |||||||
| (unaudited) | ||||||||
| Net revenues |
$ | 646,179 | $ | 166,167 | ||||
| Cost of goods sold |
326,321 | 35,384 | ||||||
| Operating expenses: |
||||||||
| Research and development |
909,838 | 979,975 | ||||||
| Selling, general and administrative |
3,167,626 | 2,718,172 | ||||||
| Total operating expenses |
4,077,464 | 3,698,147 | ||||||
| Loss from operations |
(3,757,606 | ) | (3,567,364 | ) | ||||
| Other income (expense): |
||||||||
| Interest income |
2,535 | 522 | ||||||
| Interest expense |
(885 | ) | (1,504 | ) | ||||
| Gain on sale of property and equipment |
— | 11,274 | ||||||
| Gain on extinguishment of payables |
— | 707,674 | ||||||
| Local business tax |
(1,465 | ) | — | |||||
| Total other income (expense), net |
185 | 717,966 | ||||||
| Loss before income taxes |
(3,757,421 | ) | (2,849,398 | ) | ||||
| Net loss |
$ | (3,757,421 | ) | $ | (2,849,398 | ) | ||
| Basic and diluted net loss per share |
$ | (0.70 | ) | $ | (1.48 | ) | ||
| Shares used to compute basic and diluted net loss per share |
5,398,941 | 1,930,207 | ||||||
See accompanying notes.
F-22
Table of Contents
Statements of cash flows
For the six months ended June 30, 2010 and 2009 (unaudited)
| Six months
ended June 30, |
||||||||
| 2010 | 2009 | |||||||
| (unaudited) | ||||||||
| Cash flows from operating activities: |
||||||||
| Net loss |
$ | (3,757,421 | ) | $ | (2,849,398 | ) | ||
| Adjustments to reconcile net loss to net cash used in operating activities: |
||||||||
| Non-cash bonus paid in common stock |
— | 100,000 | ||||||
| Depreciation and amortization |
23,178 | 141,168 | ||||||
| Stock-based compensation expense |
461,071 | 33,943 | ||||||
| Non-cash services paid in common stock |
75,000 | — | ||||||
| Gain on extinguishment of payables |
— | (707,674 | ) | |||||
| Gain on sale of property and equipment |
— | (11,274 | ) | |||||
| Changes in operating assets and liabilities: |
||||||||
| Accounts receivable, net |
(10,727 | ) | (13,962 | ) | ||||
| Inventory |
(284,362 | ) | (30,991 | ) | ||||
| Prepaid expenses and other current assets |
10,910 | 16,935 | ||||||
| Accounts payable and accrued expenses |
200,525 | 1,107,632 | ||||||
| Deferred compensation |
496 | 211,727 | ||||||
| Net cash used in operating activities |
(3,281,330 | ) | (2,001,894 | ) | ||||
| Cash flows from investing activities: |
||||||||
| Purchase of property and equipment, net |
(6,198 | ) | (9,074 | ) | ||||
| Proceeds from sale of property and equipment, net |
— | 28,000 | ||||||
| Net cash (used in) provided by investing activities |
(6,198 | ) | 18,926 | |||||
| Cash flows from financing activities: |
||||||||
| Borrowings under short term notes payable |
50,637 | 198,742 | ||||||
| Borrowings under long term notes payable |
— | 1,000,000 | ||||||
| Borrowings under note payable from shareholder |
1,000,000 | — | ||||||
| Payments on short term notes payable |
(55,267 | ) | (62,021 | ) | ||||
| Net proceeds from issuance of common stock |
500,000 | 1,500,000 | ||||||
| Restricted cash released from common stock proceeds |
1,707,318 | — | ||||||
| Net cash provided by financing activities |
3,202,688 | 2,636,721 | ||||||
| Net (decrease) increase in cash and cash equivalents |
(84,840 | ) | 653,753 | |||||
| Cash and cash equivalents at beginning of the period |
301,592 | 1,165,933 | ||||||
| Cash and cash equivalents at end of the period |
$ | 216,752 | $ | 1,819,686 | ||||
| Supplemental disclosure of cash flow information |
||||||||
| Interest paid |
$ | 18,377 | $ | 1,503 | ||||
| Non-cash investing and financing activities: |
||||||||
| Stock subscription receivable |
$ | — | $ | 2,500,000 | ||||
| Issuance of common stock to employees as settlement of amounts owed |
$ | — | $ | 205,032 | ||||
See accompanying notes.
F-23
Table of Contents
Supplemental financial information
Notes to financial statements
(unaudited)
1. Organization and Significant Accounting Policies
Oragenics, Inc. (the “Company”) was incorporated in November 1996; however, operating activity did not commence until 1999. The Company is dedicated to developing technologies associated with oral health, broad spectrum antibiotics and general health benefits.
Basis of Presentation
The accompanying unaudited condensed financial statements as of June 30, 2010 and for the six months ended June 30, 2010 and 2009 have been prepared in accordance with accounting principles generally accepted in the United States of America (GAAP) for interim financial information and with the instructions to Form 10-Q and Article 10 of Regulation S-X. Accordingly, they do not include all of the information and footnotes required by GAAP for complete financial statements. In the opinion of management, the accompanying financial statements include all adjustments, consisting of normal recurring accruals, necessary for a fair presentation of the financial condition, results of operations and cash flows for the periods presented. The results of operations for the interim period June 30, 2010 are not necessarily indicative of the results that may be expected for the year ended December 31, 2010 or any future period. The balance sheet as of December 31, 2009 has been audited.
These financial statements should be read in conjunction with the audited financial statements and notes thereto for the year ended December 31, 2009, which are included in our Annual Report on Form 10-K filed with the Securities and Exchange Commission on March 31, 2010. In that report the Company disclosed that it expects to incur substantial expenditures to further develop each of its technologies and that it believes its working capital will be insufficient to meet the business objectives as presently structured and that without sufficient capital to fund its operations, the Company will be unable to continue as a going concern. The accompanying financial statements do not include any adjustments that might result from the outcome of this uncertainty. Although the Company currently believes that it will have sufficient resources to commercialize selective products, it intends to seek additional funding to further develop and commercialize other products.
Adoption of New Accounting Standards
In August 2009, the FASB issued ASU 2009-05, “Fair Value Measurements and Disclosures (ASC Topic 820)—Measuring Liabilities at Fair Value” (“Update 2009-05”). Update 2009-05 provides clarification regarding valuation techniques when a quoted price in an active market for an identical liability is not available in addition to treatment of the existence of restrictions that prevent the transfer of a liability. Update 2009-05 also clarifies that both a quoted price in an active market for an identical liability at the measurement date and the quoted price for an identical liability when traded as an asset in an active market (when no adjustments to the quoted price of the asset are required) are Level 1 fair value measurements. This standard is effective for the first reporting period, including interim periods, beginning after issuance. Adoption of Update 2009-05 did not have a material effect on Company’s financial statements.
Revenue Recognition
The Company recognizes revenues from the sales of product when title and risk of loss pass to the customer, which is generally when the product is shipped. Grant revenues are recognized as the reimbursable expenses are incurred over the life of the related grant. Grant revenues are deferred when reimbursable expenses have not been incurred.
F-24
Table of Contents
Oragenics, Inc.
Supplemental financial information
Notes to financial statements
(unaudited)
The Company records allowances for discounts and product returns at the time of sales as a reduction of revenues as such allowances can be reasonably estimated based on historical experience or known trends. Product returns are limited to specific mass retail customers for expiration of shelf life or unsold product over a period of time.
Inventory
Inventories are stated at the lower of cost or market. Cost, which includes material, labor and overhead, is determined on a first-in, first-out basis. As of June 30, 2010, we had $67,858 in consignment inventory with a mass retailer which will be reduced as shipments are made.
2. Net Loss Per Share
During all periods presented, the Company had securities outstanding that could potentially dilute basic earnings per share in the future, but were excluded from the computation of diluted net loss per share, as their effect would have been antidilutive. Because the Company reported a net loss for all periods presented, shares associated with the stock options and warrants are not included because they are antidilutive. Basic and diluted net loss per share amounts are the same for the periods presented. Net loss per share is computed using the weighted average number of shares of common stock outstanding.
3. Income Taxes
Income taxes are accounted for under the asset and liability method. Deferred tax assets and liabilities are recognized for future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases and operating loss and tax credit carryforwards.
Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rate is recognized in operations in the period that includes the enactment date. Deferred tax assets are reduced to estimated amounts expected to be realized by the use of a valuation allowance.
In July 2006, the FASB issued guidance which clarifies accounting for uncertainty in income taxes recognized in an entity’s financial statements in accordance with GAAP and prescribes a recognition threshold and measurement attributes for financial statement disclosure of tax positions taken or expected to be taken on a tax return. Under GAAP, the impact of an uncertain income tax position on the income tax return must be recognized at the largest amount that is more-likely-than-not to be sustained upon audit by the relevant taxing authority. An uncertain income tax position will not be recognized if it has less than a 50% likelihood of being sustained. Additionally, GAAP provides guidance on derecognition, classification, interest and penalties, accounting for interim periods, disclosure and transition.
The Company files its income tax returns in the U.S. federal jurisdiction and in Florida. With few exceptions, the Company is no longer subject to federal or state income tax examinations by tax authorities for years before 2006.
4. Fair Value of Financial Instruments
ASC 820, Fair Value Measurements and Disclosures, defines fair value, provides guidance for measuring fair value and requires certain disclosures. This standard discusses valuation techniques, such as the market approach (comparable market prices), the income approach (present value of future income or cash flow), and the cost approach (cost to replace the service capacity of an asset or replacement cost). The standard utilizes a fair value hierarchy that prioritizes the inputs to valuation techniques used to measure fair value into three broad levels. The following is a brief description of those three levels:
| Ø | Level 1. Observable inputs such as quoted prices in active markets; |
| Ø | Level 2. Inputs, other than the quoted prices in active markets, that are observable either directly or indirectly; and |
F-25
Table of Contents
Oragenics, Inc.
Supplemental financial information
Notes to financial statements
(unaudited)
| Ø | Level 3. Unobservable inputs in which there is little or no market data, which require the reporting entity to develop its own assumptions. |
The Company does not have any assets or liabilities measured at fair value on a recurring basis at June 30, 2010. The Company did not have any fair value adjustments for assets and liabilities measured at fair value on a nonrecurring basis during the six months ended June 30, 2010.
5. Stock Options Expense During the six months ended June 30, 2010
During the six months ended June 30, 2010, the Company issued 24,325 stock options of which 10,000 vested immediately. From January 1, 2010 to the date of this filing, 38,000 stock options previously granted have vested and 8,687 have been forfeited. Stock option compensation expense of $461,071 was recorded for the six months ended June 30, 2010 and is a non-cash expense. This amount is included in research and development and selling, general and administrative expenses in the accompanying statement of operations.
6. Common Stock Issued During the six months ended June 30, 2010
In January 2010, we completed the closing of a $3,000,000 million private placement of common stock pursuant to a Common Stock Purchase Agreement with accredited investors. The Company issued an additional 100,000 shares of its Common Stock at a price of $5.00 per share to the investors for $500,000, the payment of which consisted of $500,000 in cash at closing. Half of the total investment, or $250,000, was made by the Koski Family Limited Partnership.
In June 2010, we issued 6,000 shares to Athorn Clark Partners (“Athorn”) at a price per share of $12.50 (based on the value of the services required to be provided by Athorn) in connection with an agreement for Athorn to provide media related services to us.
7. Short Term Notes Payable
Insurance Premium Financing. In March 2010, we entered into a short term note payable for $50,637 with an interest rate of 5.75% to finance our product liability insurance. This note matures January 10, 2011. At June 30, 2010 the balance due was $30,382. The payment terms are calculated on a straight line amortization over a ten month period.
8. Note Payable to Shareholder
May 2010 Note Financing. On May 28, 2010, the Company entered into an unsecured promissory note with conversion provisions (the “May 2010 Note”) with the Koski Family Limited Partnership (the “KFLP”), our largest shareholder. Pursuant to the May 2010 Note the Company borrowed $1,000,000 from the KFLP. Interest on the May 2010 Note accrued at the rate of LIBOR plus 6% (6.54% at June 30, 2010) and the principal of the May 2010 Note, together with all accrued interest thereon,
F-26
Table of Contents
Oragenics, Inc.
Supplemental financial information
Notes to financial statements
(unaudited)
was due and payable on such date that is the earlier of: (a) the closing date of a registered public offering of newly issued equity securities by the Company resulting in cash proceeds to the Company (other than in connection with employee option plans) or (b) May 24, 2011 (the “Due Date”); provided, however, that in the event the Company completes a private offering of equity securities prior to such Due Date (a “Private Placement”), the Company may at its option, upon five (5) days written notice to the KFLP, elect to convert the principal of the May 2010 Note, together with all accrued interest thereon, into the same equity securities being sold in the Private Placement at the same price and terms (the “Conversion Securities”) and issue the Conversion Securities to the KFLP. Company directors Christine L. Koski and Robert C. Koski are partners in the KFLP. The issuance of the May 2010 Note was approved by the disinterested members of the Company’s Board of Directors. See Note 10, Subsequent Events below.
9. Outstanding Warrants and Stock Options
As of June 30, 2010 there were 306,388 warrants outstanding and there are approximately 398,112 outstanding stock options that have been granted that have not been forfeited. The total number of outstanding warrants and unexercised stock options was 707,990. If all warrants and stock options were exercised, the total number of outstanding shares would be approximately 6,100,000.
10. Subsequent Events
On July 5, 2010, we entered into a Common Stock Purchase Agreement (the “Agreement”) with the KFLP. At the closing thereof on July 30, 2010 we issued 250,000 shares of our common stock to the KFLP at a price of $8.00 per share. The $2,000,000 aggregate consideration paid by the KFLP consisted of (i) $1,000,000 cash and (ii) the exchange and cancellation of the outstanding May 2010 financing described above in Note 8, “Note Payable to Shareholder.” Accrued interest on the May 2010 Note through closing was waived by the KFLP. Simultaneously with this purchase of common stock by the KFLP (including note conversion) and as part thereof, we entered into an unsecured revolving credit agreement (the “Credit Facility”) with the KFLP. Pursuant to the Credit Facility, we are able to borrow up to $2,000,000 from the KFLP at LIBOR plus 6.0%. The term of the Credit Facility is for twelve months commencing August 1, 2010. As of the date hereof, we have not drawn on this Credit Facility. Our ability to draw on the Credit Facility is subject to (i) the receipt by the KFLP of a certificate of no adverse change from us in form and substance acceptable to the KFLP, (ii) the receipt by the KFLP of a revolving unsecured promissory note from us in the principal drawn down in the form attached to the Credit Facility and (iii) our compliance with the terms of the Credit Facility.
F-27
Table of Contents

Table of Contents

Table of Contents
Part II. Information not required in prospectus
Item 13. Other Expenses of Issuance and Distribution.
The following table sets forth the expenses payable by us in connection with this offering of securities described in this registration statement. All amounts shown are estimates, except for the SEC and FINRA filing fees. The Registrant will bear all expenses shown below.
| SEC filing fee |
$ | 1,640 | |
| FINRA filing fee |
2,800 | ||
| NASDAQ Capital Market listing fee |
50,000 | ||
| Accounting fees and expenses |
85,000 | ||
| Legal fees and expenses |
450,000 | ||
| Printing and related expenses |
125,000 | ||
| Other |
85,560 | ||
| Total |
$ | 800,000 | |
Item 14. Indemnification of Directors and Officers.
As provided in our bylaws and under Florida law, our Directors shall not be personally liable to our company or any other person for monetary damages for breach of duty of care or any other duty owed to our company as a Director, unless the breach of or failure to perform those duties constitutes:
| Ø | a violation of criminal law, unless the Director had reasonable cause to believe his conduct was lawful, or had no reasonable cause to believe his conduct was unlawful; |
| Ø | a transaction from which the Director received an improper personal benefit, directly or indirectly; |
| Ø | in a proceeding by or in the right of our company or a shareholder, an act or omission which involves a conscious disregard for the best interests of our company or which involves willful misconduct; |
| Ø | in a proceeding by or in the right of someone other than our company or a shareholder, an act of recklessness or an act or omission which was committed in bad faith or with malicious purpose or in a manner exhibiting wanton and willful disregard of human rights, safety, or property; or |
| Ø | a distribution made in violation of Florida law. |
Our bylaws provide that we are required to indemnify any Director, officer, employee or agent made a party to a proceeding because he is or was our Director, officer, employee or agent against liability incurred in the proceeding if he acted in good faith and in a manner the Director reasonably believed to be in or not opposed to our best interests and, in the case of any criminal proceeding, he had no reasonable cause to believe his conduct was unlawful.
Our bylaws and Florida law also provide that we shall indemnify a Director, officer, employee or agent who has been successful on the merits or otherwise in the defense of any proceeding to which he was a party, or in defense of any claim, issue or matter therein, because he is or was a Director, officer, employee or agent of our company against expenses actually and reasonably incurred by him in connection with such defense.
II-1
Table of Contents
Item 15. Recent Sales of Unregistered Securities.
The following information relates to all securities issued or sold by the Registrant within the past three years and not registered under the Securities Act. Each of the transactions described below was conducted in reliance upon the exemptions from registration provided in Section 4(2) of the Securities Act and the rules and regulations promulgated thereunder. There were no underwriters employed in connection with any of the transactions set forth in this Item 15.
On July 30, 2010, we issued 250,000 shares of common stock to the Koski Family Limited Partnership, or KFLP, an accredited investor and our largest shareholder at a price of $8.00 per share. The $2,000,000 aggregate consideration consisted of (i) $1,000,000 cash and (ii) the exchange and cancellation of the outstanding May 2010 Note issued to the KFLP on May 28, 2010. Simultaneously with such purchase (including note conversion) and as part thereof, we entered into an unsecured revolving credit agreement (the “Credit Facility”) which enables us to borrow up to $2,000,000 from the KFLP at LIBOR plus 6.0% after August 1, 2010 for a period of 12 months.
In June 2010, we issued 6,000 shares to Athorn Clark Partners, or Athorn, at a price per share of $12.50, based on the value of the services required to be provided by Athorn, in connection with an agreement for Athorn to provide media-related services to us.
On May 28, 2010, we entered into an unsecured promissory note with conversion provisions (the “May 2010 Note”) with the KFLP pursuant to which we borrowed $1,000,000 from the KFLP. Interest on the May 2010 Note accrues at the rate of LIBOR plus 6.0% and the principal of the May 2010 Note, together with all accrued interest thereon, was due and payable on such date that is the earlier of: (i) the closing date of a registered public offering of newly issued equity securities by us resulting in cash proceeds to us (other than in connection with employee option plans) or (ii) the May 24, 2011 maturity date; provided, however, that in the event we complete a private offering of equity securities prior to such May 24, 2011 maturity date, we may elect to convert the principal of the May 2010 Note into the same equity securities being sold in the private placement at the same price and terms.
On December 30, 2009, we completed the initial closing of a private placement (the “December 2009 Private Placement”) of common stock pursuant to a common stock purchase agreement (the “December 2009 Securities Purchase Agreement”) with accredited investors, including the KFLP. We issued 500,812 shares of our common stock at a price of $5.00 per share to the investors for $2,504,062, the payment of which consisted of the following: $2,450,000 in cash at closing and $54,063 pursuant to the cancellation of the same dollar amount of outstanding deferred compensation obligation owed by the Company to Dr. Jeffrey Hillman, our Chief Scientific Officer and director. Approximately half of the total investment, or $1,250,000, was made by the KFLP. In conjunction with, and as a condition to closing of this financing, the KFLP was also issued 200,000 shares of our common stock at $5.00 per share, (which was the same price per share paid by the investors), in exchange for the cancellation of its $1,000,000 secured note. The loan was entered into in June 2009 and originally had been secured by substantially all of our assets (excluding receivables) and required interest payments at the rate of prime plus 4.0% which were payable quarterly. The accredited investors were: Carol E. Martin, Kelly H. Leaird, Mark Bailey, Kris A. Persinger, First Clearing, LLC C/F Roth IRA FBO Kris Persinger, Richard Dresden, John Diana and Michael Wells, Dr. Hillman, and the KFLP.
On January 13, 2010, we completed the $3,004,062 private placement contemplated by the December 2009 Securities Purchase Agreement and December 2009 Private Placement and issued another 100,000 shares of common stock at a price of $5.00 per share to the accredited investors for $500,000. Of this amount the KFLP again participated in half of the remainder of the aggregate investment by acquiring 50,000 shares for $250,000.
Contemporaneously with the December 2009 Private Placement, the KFLP also elected to exercise previously issued warrants (issued on June 30, 2009 as part of the June 2009 Private Placement) to purchase 50,000 shares of Company common stock. The warrants were exercised through the payment by the KFLP of the warrant exercise price of $2.00 per share.
II-2
Table of Contents
On September 16, 2009 we issued 25,000 shares of our common stock to Media4Equity LLC, or M4E. The restricted shares were issued to M4E pursuant to the terms of an agreement we entered into with M4E effective September 3, 2009, whereby M4E will provide national media exposure consulting services to us relating to the placement of print and radio features. The shares have a fair market value of $115,000 based on a price of $4.60 per share. This amount is included in selling, general and administrative expenses in the accompanying statements of operations. In addition to the issuance of common stock the agreement with M4E requires us to make monthly payments to M4E of $10,000 over the three-year term of the agreement, subject to certain termination rights.
On September 14, 2009 we issued a warrant to Strategic Growth International, Inc., or SGI, to acquire 12,500 shares of our common stock at $6.00 per share. The warrant was issued in connection with an agreement with SGI to provide investor relations services to us.
On June 29, 2009, we entered into a private placement of equity and debt financing (the “June 2009 Private Placement”) pursuant to a securities purchase agreement (the “June 2009 Purchase Agreement”) with the KFLP. Pursuant to the terms of the June 2009 Purchase Agreement, the Company issued 2,500,000 shares of its common stock to the KFLP and issued warrants to the KFLP to acquire 50,000 shares of Company common stock at an exercise price of $2.00 per share in exchange for $4,000,000, the payment of which consisted of the following: (i) $1,500,000 in cash at closing and (ii) $2,500,000 pursuant to a non-interest bearing promissory note providing for five consecutive monthly installment payments of $500,000 commencing July 31, 2009, and the KFLP provided a secured loan of $1,000,000 to the Company. The loan was secured by substantially all of our assets, excluding receivables, and bore interest at the rate of prime plus 4.0% payable quarterly. The principal of the loan was due in five years. The warrants expired in five years and were immediately exercisable. As a result of this transaction the Board of Directors believes there was a change of control of the Company with the KFLP acquiring a controlling interest of our then outstanding voting common stock.
In connection with, and as a closing condition to the June 2009 Private Placement, the purchasers, (George Hawes, our largest shareholder prior to the June 2009 Private Placement, and William Matlack, an accredited investor), under that certain securities purchase agreement dated June 12, 2008, (the “June 2008 Purchase Agreement”) entered into consent, waiver and mutual release agreements with us on June 25, 2009. In addition, such individuals waived and relinquished any special rights they possessed pursuant to agreements with the Company, including, but not limited to, (i) rights of first refusal, (ii) antidilution regarding future equity sales and (iii) covenants regarding secured lending contained in the June 2008 Purchase Agreement. In connection with such consents, waivers and releases, warrants to acquire 161,000 shares of our common stock at an exercise price of $26.00 per share that were previously issued under the June 2008 Purchase Agreement were subject to the right of exchange for new replacement warrants to acquire the same number of shares under the same terms except for a change in the exercise price from $26.00 to $15.00.
On April 15, 2009, we issued a warrant to acquire 5,000 shares at $10.00 per share to Kelly Leaird, an accredited investor, as part of the short-term loan agreement we entered into with Mr. Leaird.
On September 1, 2008, we were obligated to issue 94 shares of common stock to our consultant, Certified Nutrition for Less, LLC. The obligation to issue the shares was incurred in accordance with the consulting agreement entered into between the Company and the consultant. The price per share was $10.60.
On June 12, 2008, we issued an aggregate of 288,888 shares of common stock to accredited investors at a price of $9.00 per share pursuant to a private offering of the Company’s common stock (the “June 2008 Private Placement”). Mr. Hawes, a significant shareholder and affiliate at the time, acquired 288,888 shares in the June 2008 Private Placement and Mr. Matlack, acquired the remaining shares. The aforementioned June 2008 Private Placement investors also received a proportionate number of warrants to purchase 288,888 shares of common stock at a price of $26.00 per share. These warrants expire five years from their vesting date on June 12, 2013.
II-3
Table of Contents
On February 8, 2008, we issued an aggregate of 57,500 shares of common stock to warrant holders in connection with their exercise of the warrants at the amended price of $8.80. The remaining 17,500 unexercised warrants, expired as of February 8, 2008 in accordance with the terms of the warrants. Proceeds of $506,000 were received by us from the exercise of the warrants. Our Chief Scientific Officer, Dr. Hillman, acquired 3,125 shares upon exercising of his warrants, our former president, Robert T. Zahradnik, acquired 3,125 shares upon exercising of his warrants, and Mr. Hawes acquired 36,875 shares upon the exercise of his warrants each at the amended price.
On January 17, 2008 we amended the outstanding warrants that were originally issued to accredited investors in connection with our March 2006 private placement. Pursuant to the amendment, the warrant exercise price was reduced from $12.00 to $8.80 per share, which was the fair market value per share on the date of the amendment.
On August 7, 2007, we closed on $1,171,591 in equity based financing. We issued a total of 230,000 shares of restricted common stock and warrants to acquire 230,000 shares of common stock in a private placement to accredited investors. The shares were sold to accredited investors at $5.00 per share, except that per applicable trading market requirements, a former chief executive officer and director acquired his shares at $8.80 per share, which was the closing share price on August 7, 2007. Each warrant to purchase shares of common stock was exerciseable at the price of $11.60 per share. The warrants expired on August 8, 2008 (the “August 2007 Warrants”). On January 31, 2008 we amended the August 2007 Warrants to reduce the exercise price to $8.80, which was the fair market value on the date of the amendment, from January 28, 2008 to February 29, 2008 following which the exercise price reverted back to $11.60. Prior to the expiration of the August 2007 Warrants and during the amendment period on February 29, 2008, 169,318 shares of common stock were issued to the warrant holders upon exercise at the amended exercise price resulting in additional working capital proceeds to us of $1,490,000. Mr. Hawes acquired 25,000 shares upon the exercise of his warrants.
Item 16. Exhibits and Financial Statements Schedules.
(a) Exhibits.
See “Exhibit Index” on the page immediately following the signature page for a list of exhibits filed as part of this registration statement, which is incorporated herein by reference.
(b) Financial Statement Schedules.
The financial statement schedules required by Item 16 have been omitted because of the absence of conditions under which they are required or because the required information is included in the financial statements or notes thereto provided with this registration statement.
Item 17. Undertakings.
The undersigned registrant hereby undertakes that:
(1) For the purpose of determining liability under the Securities Act of 1933, the information omitted from the form of prospectus filed as part of this registration statement in reliance on Rule 430A and contained in a form of prospectus filed by the registrant pursuant to Rule 424(b)(1) or (4) or 497(h) under the Securities Act shall be deemed to be a part of this registration statement as of the time it was declared effective.
(2) For the purpose of determining any liability under the Securities Act, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
II-4
Table of Contents
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers and controlling persons of the Registrant pursuant to the foregoing provisions, or otherwise, the Registrant has been advised that in the opinion of the Commission such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the Registrant of expenses incurred or paid by a director, officer or controlling person of the Registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
II-5
Table of Contents
Signatures
Pursuant to the requirements of the Securities Act of 1933, as amended, the registrant has duly caused this Amendment No. 1 to our Registration Statement on Form S-1 to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Tampa, State of Florida on October 5, 2010.
| ORAGENICS, INC. | ||
| By: | /S/ DAVID B. HIRSCH | |
| Name: David B. Hirsch | ||
| Title: Chief Executive Officer, President, Principal Executive Officer | ||
Pursuant to the requirements of the Securities Act of 1933, this Amendment No. 1 to our Registration Statement has been signed by the following persons in the capacities and on the dates indicated below.
| Signature |
Title |
Date | ||
| /s/ David B. Hirsch David B. Hirsch |
Chief Executive Officer, President, Principal Executive Officer and Director | October 5, 2010 | ||
| /s/ * Brian J. Bohunicky |
Chief Financial Officer (Principal Accounting Officer, Secretary and Treasurer) | October 5, 2010 | ||
| /s/ * Jeffrey D. Hillman |
Chief Scientific Officer and Director | October 5, 2010 | ||
| /s/ * Christine L. Koski |
Chairperson of the Board | October 5, 2010 | ||
| /s/ * Robert C. Koski |
Director | October 5, 2010 | ||
| /s/ * Charles L. Pope |
Director | October 5, 2010 | ||
| /s/ * Frederick W. Telling |
Director | October 5, 2010 | ||
| */s/ David B. Hirsch David B. Hirsch, Attorney in-fact |
October 5, 2010 | |||
S-1
Table of Contents
Exhibit index
| Incorporated by reference to | ||||||||||||
| Exhibit number |
Exhibit description |
Form | File no. | Exhibit | Filing date |
Filed herewith | ||||||
| 1.1 | Form of Underwriting Agreement | X | ||||||||||
| 3.1 | Amended and Restated Articles of Incorporation | SB-2 | 333-100568 | 3.3 | 10/16/02 | |||||||
| 3.2 | Articles of Amendment to Amended and Restated Articles of Incorporation | 8-K | 001-32188 | 10.2 | 10/30/09 | |||||||
| 3.3 | Articles of Amendment to Amended and Restated Articles of Incorporation | 8-K | 001-32188 | 3.1 | 9/27/10 | |||||||
| 3.4 | Bylaws | SB-2 | 333-100568 | 3.2 | 10/16/02 | |||||||
| 3.5 | First Amendment to Bylaws | 8-K | 001-32188 | 3.1 | 6/9/10 | |||||||
| 3.6 | Second Amendment to Bylaws | 8-K | 001-32188 | 3.1 | 8/24/10 | |||||||
| 4.1 | Specimen Stock Certificate | X | ||||||||||
| 4.2 | Securities Purchase Agreement between George Hawes, William Matlack and Oragenics, Inc. dated June 12, 2008 (including form of June 2008 Warrant) | 8-K | 001-32188 | 10.1 | 6/16/08 | |||||||
| 4.3 | Securities Purchase Agreement dated June 29, 2009 by and between the Company and the Koski Family Limited Partnership (including the Form of the Promissory Note and Form of the Warrant) | 8-K | 001-32188 | 10.1 | 7/6/09 | |||||||
| 4.4 | Secured Promissory Note issued to the Koski Family Limited Partnership | 8-K | 001-32188 | 10.2 | 7/6/09 | |||||||
| 4.5 | Security Agreement between the Company and the Koski Family Limited Partnership | 8-K | 001-32188 | 10.3 | 7/6/09 | |||||||
| 5.1 | Form of Opinion of Shumaker, Loop & Kendrick, LLP (including the consent of such firm) regarding the legality of the securities being offered. | X | ||||||||||
| 10.1 | Exclusive License Agreement between the Company and the University of Florida Research Foundation, Inc. effective August 4, 1998 for Replacement Therapy for Dental Caries (the “Replacement Therapy License Agreement”) | SB-2 | 333-100568 | 10.1 | 10/16/02 | |||||||
| 10.2 | First Amendment to Replacement Therapy License Agreement dated September 15, 2000 | SB-2 | 333-100568 | 10.2 | 10/16/02 | |||||||
| 10.3 | Second Amendment to Replacement Therapy License Agreement dated June 2002 | SB-2 | 333-100568 | 10.3 | 10/16/02 | |||||||
| 10.4 | Third Amendment to Replacement Therapy License Agreement dated September 25, 2002 | SB-2 | 333-100568 | 10.4 | 10/16/02 | |||||||
| 10.5 | Fourth Amendment to Replacement Therapy License Agreement dated March 2003 | SB-2/A-3 | 333-100568 | 10.36 | 4/9/03 | |||||||
Table of Contents
| Exhibit description |
Incorporated by reference to | |||||||||||
| Exhibit number |
Form | File no. | Exhibit | Filing date |
Filed herewith | |||||||
| 10.6 | Standard Exclusive License Agreement with Sublicensing Terms between the Company and the University of Florida Research Foundation, Inc. effective June 22, 2000 (the “Antimicrobial Polypeptide License Agreement”) | SB-2 | 333-100568 | 10.5 | 10/16/02 | |||||||
| 10.7 | First Amendment to the Antimicrobial Polypeptide License Agreement dated September 15, 2000 | SB-2 | 333-100568 | 10.6 | 10/16/02 | |||||||
| 10.8 | Second Amendment to the Antimicrobial Polypeptide License Agreement dated June 10, 2002 | SB-2 | 333-100568 | 10.7 | 10/16/02 | |||||||
| 10.9 | Third Amendment to the Antimicrobial Polypeptide License Agreement dated September 25, 2002 | SB-2 | 333-100568 | 10.4 | 10/16/02 | |||||||
| 10.10 | Fourth Amendment to the Antimicrobial Polypeptide License Agreement dated March 2003 | SB-2/A-3 | 333-100568 | 10.36 | 4/9/03 | |||||||
| 10.11 | Amended and Restated 2002 Stock Option and Incentive Plan (including Form of Stock Option Agreement) | 10-QSB/A | 001-32188 | 10.1 | 9/29/06 | |||||||
| 10.12 | First Amendment to Amended and Restated 2002 Stock Option and Incentive Plan | 8-K | 001-32188 | 4.2 | 4/14/08 | |||||||
| 10.13 | Second Amendment to Amended and Restated 2002 Stock Option and Incentive Plan | 8-K | 001-32188 | 10.1 | 10/30/09 | |||||||
| 10.14 | Proprietary Information and Invention Agreement between the Company and Jeffrey D. Hillman | SB-2 | 333-100568 | 99.4 | 10/16/02 | |||||||
| 10.15 | Lease Agreement between the Company and Hawley-Wiggins LLC dated January 28, 2004; Subordination Agreement dated April 14, 2004; and First Amendment dated November 15, 2004 | 10-KSB | 001-32188 | 10.46 | 3/14/05 | |||||||
| 10.16 | Sublease Agreement between the Company and Astrazenca LP dated October 12, 2009 (3000 Bayport Drive, Suite 685, Tampa, FL 33607) | 10-K | 001-32188 | 10.17 | 3/31/10 | |||||||
| 10.17 | Lease Agreement between the Company and Hawley-Wiggins LLC dated October 23, 2009 (13700 Progress Blvd, Alachua, FL 32615) | 10-K | 001-32188 | 10.18 | 3/31/10 | |||||||
| 10.18 | Common Stock Purchase Agreement dated December 30, 2009 | 10-K | 001-32188 | 10.19 | 3/3/10 | |||||||
| 10.19† | Executive Employment Agreement between the Company and David B. Hirsch, dated May 11, 2010 | 10-Q | 001-32188 | 10.1 | 5/14/10 | |||||||
Table of Contents
| Exhibit description |
Incorporated by reference to | |||||||||||
| Exhibit number |
Form | File no. | Exhibit | Filing date |
Filed herewith | |||||||
| 10.20† | Executive Employment Agreement between the Company and Jeffrey D. Hillman, dated May 11, 2010 | 10-Q | 001-32188 | 10.2 | 5/14/10 | |||||||
| 10.21† | Executive Employment Agreement between the Company and Brian J. Bohunicky, dated May 10, 2010 | 10-Q | 001-32188 | 10.3 | 5/14/10 | |||||||
| 10.22 | Unsecured Promissory Note with Conversion Provisions issued to the Koski Family Limited Partnership, dated May 28, 2010 | 8-K | 001-32188 | 1.01 | 5/28/10 | |||||||
| 10.23 | Common Stock Purchase Agreement dated July 5, 2010, by and between Oragenics, Inc. and the Koski Family Limited Partnership | 8-K | 001-32188 | 10.1 | 7/7/10 | |||||||
| 10.24 | Revolving Credit Agreement dated July 30, 2010, by and between Oragenics, Inc. and the Koski Family Limited Partnership (including form of revolving unsecured promissory note). | 8-K | 001-32188 | 10.2 | 8/2/10 | |||||||
| 10.25 | Revolving Unsecured Promissory Note dated September 13, 2010 | 8-K | 001-32188 | 10.2 | 9/16/10 | |||||||
| 23.1 | Consent of Kirkland Russ Murphy & Tapp, PA, an independent public accounting firm | X | ||||||||||
| 23.2 | Consent of Shumaker, Loop & Kendrick, LLP (included as part of Exhibit 5.1 hereto) | |||||||||||
| 24.1 | Powers of Attorney (incorporated by reference to that Registration Statement on Form S-1 filed with the Securities and Exchange Commission on August 25, 2010). | |||||||||||
| 99.1 | Consent of Nominee Director – Dr. Alan W. Dunton | X | ||||||||||
| † | Management compensation agreement. |
