As filed with the Securities and Exchange
Commission on October 1, 2010
Registration No. 333-167617
UNITED STATES SECURITIES AND
EXCHANGE COMMISSION
Washington, D.C.
20549
Amendment No. 1
to
Form S-1
REGISTRATION
STATEMENT
UNDER
THE SECURITIES ACT OF
1933
ReSearch Pharmaceutical
Services, Inc.
(Exact Name of Registrant as
Specified in Its Charter)
| |
|
|
|
|
|
Delaware
|
|
8731
|
|
20-4322769
|
(State or Other Jurisdiction
of
Incorporation or Organization)
|
|
(Primary Standard Industrial
Classification Code No.)
|
|
(I.R.S. Employer
Identification Number)
|
520 Virginia Drive
Fort Washington, Pennsylvania 19034
(215) 540-0700
(Address, Including Zip Code,
and Telephone Number, Including Area Code, of Registrant’s
Principal Offices)
Steven Bell
Executive Vice President of Finance and Chief Financial
Officer
520 Virginia Drive
Fort Washington, Pennsylvania 19034
(215) 540-0700
(Name, Address, Including Zip
Code, and Telephone Number, Including Area Code, of Agent for
Service)
Copy to:
| |
|
|
Stephen T. Burdumy, Esq.
Matthew M. McDonald, Esq.
Drinker Biddle & Reath LLP
One Logan Square, Ste. 2000
Philadelphia, Pennsylvania 19103
(215) 988-2700
|
|
Brent B. Siler, Esq.
Cooley LLP
Reston Town Center
11951 Freedom Drive
Reston, Virginia 20190
(703) 456-8000
|
Approximate date of commencement of proposed sale to the
public: As soon as practicable after the
effective date of this Registration Statement.
If any of the securities being registered on this Form are being
offered on a delayed or continuous basis pursuant to
Rule 415 under the Securities Act of 1933, check the
following
box o
If this Form is filed to register additional securities for an
offering pursuant to Rule 462(b) under the Securities Act,
please check the following box and list the Securities Act
registration number of the earlier effective registration
statement for the same
offering. o
If this Form is a post-effective amendment filed pursuant to
Rule 462(c) under the Securities Act, check the following
box and list the Securities Act registration number of the
earlier effective registration statement for the same
offering. o
If this Form is a post-effective amendment filed pursuant to
Rule 462(d) under the Securities Act, check the following
box and list the Securities Act registration statement number of
the earlier effective registration statement for the same
offering. o
Indicate by check mark whether the registrant is a large
accelerated filer, an accelerated filer, a non-accelerated
filer, or a smaller reporting company. See the definitions of
“large accelerated filer,” “accelerated
filer” and “smaller reporting company” in Rule
12b-2 of the Exchange Act. (Check one):
| |
|
|
|
|
|
|
|
Large accelerated filer o
|
|
Accelerated filer o
|
|
Non-accelerated filer þ
(Do not check if a smaller reporting company)
|
|
Smaller reporting company o
|
The Registrant hereby amends this Registration Statement on
such date or dates as may be necessary to delay its effective
date until the Registrant shall file a further amendment which
specifically states that this Registration Statement shall
thereafter become effective in accordance with Section 8(a)
of the Securities Act of 1933 or until the Registration
Statement shall become effective on such date as the Securities
and Exchange Commission, acting pursuant to said
Section 8(a), may determine.
The
information in this preliminary prospectus is not complete and
may be changed. We may not sell these securities until the
registration statement filed with the Securities and Exchange
Commission is effective. This preliminary prospectus is not an
offer to sell these securities and it is not soliciting an offer
to buy these securities in any jurisdiction where the offer or
sale is not permitted.
|
SUBJECT TO COMPLETION, DATED OCTOBER 1, 2010
Preliminary
Prospectus
Shares
Common
Stock
We are
offering shares
of our common stock and the selling stockholders identified in
this prospectus are
offering shares
of our common stock. We will not receive any proceeds from the
sale of shares by the selling stockholders. This is the initial
public offering of our common stock in the United States, and no
public market currently exists for our common stock. We expect
the initial public offering price to be between
$ and
$ per share. We have applied to
list our common stock on The NASDAQ Global Market under the
symbol “RPSE.”
Investing in our common stock involves a high degree of risk.
Please read “Risk Factors” beginning on
page 10.
Neither the Securities and Exchange Commission nor any state
securities commission has approved or disapproved of these
securities or determined if this prospectus is truthful or
complete. Any representation to the contrary is a criminal
offense.
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
PER SHARE
|
|
TOTAL
|
|
|
|
Public Offering Price
|
|
$
|
|
|
|
$
|
|
|
|
Underwriting Discounts and Commissions
|
|
$
|
|
|
|
$
|
|
|
|
Proceeds to ReSearch Pharmaceutical Services, Inc. (Before
Expenses)
|
|
$
|
|
|
|
$
|
|
|
|
Proceeds to Selling Stockholders (Before Expenses)
|
|
$
|
|
|
|
$
|
|
|
|
|
Delivery of the shares of common stock is expected to be made on
or
about ,
2010. We and the selling stockholders have granted the
underwriters an option for a period of 30 days to purchase,
on the same terms and conditions set forth above, up to an
additional shares
of our common stock to cover overallotments. If the underwriters
exercise the option in full, the total underwriting discounts
and commissions payable by us and the selling stockholders will
be $ and the total proceeds to us
and the selling stockholders, before expenses, will be
$ .
Jefferies &
Company
|
|
| William
Blair & Company |
Lazard Capital Markets |
Prospectus
dated ,
2010
Table of
Contents
RPS and the RPS logo are our service marks. All other service
marks, trademarks and trade names referred to in this prospectus
are the property of their respective owners.
We intend to effectuate
a for
reverse stock split of our common stock prior to the
consummation of this offering. As of the date of this
preliminary prospectus, we have not yet effectuated a reverse
stock split.
You should rely only on the information contained in this
prospectus. Neither we, the selling stockholders nor the
underwriters, have authorized anyone to provide you with
additional information or information different from that
contained in this prospectus. We are offering to sell, and
seeking offers to buy, shares of our common stock only in
jurisdictions where offers and sales are permitted. The
information contained in this prospectus is accurate only as of
the date of this prospectus, regardless of the time of delivery
of this prospectus or of any sale of shares of our common stock.
Our business, financial condition, results of operations and
prospects may have changed since that date.
i
Prospectus
Summary
This summary highlights the information appearing elsewhere
in this prospectus. Because it is only a summary, it does not
contain all of the information that may be important to you. For
a more complete understanding of the information that you may
consider important in making your investment decisions, we
encourage you to read the entire prospectus. Among the other
information in this prospectus, you should carefully consider
the information set forth under the heading “Risk
Factors” and our consolidated financial statements and
related notes beginning on
page F-1
of this prospectus. Unless the context requires otherwise, the
words “RPS,” “we,” “our company,”
“us” and “our” refer to ReSearch
Pharmaceutical Services, Inc. and its subsidiaries.
Overview
We are a global, next-generation clinical research organization,
or CRO, providing clinical development services to the
biopharmaceutical industry. Our services support the design,
initiation and management of our clients’ clinical trials
programs that are required to obtain regulatory approval to
market biopharmaceutical products. We provide these services
either as integrated solutions, embedded within the
client’s internal clinical development operations and
supporting the entire breadth of the client’s drug
development pipeline, or on a more traditional
project-by-project
basis. Our innovative, next-generation business model allows us
to leverage our clients’ existing processes to improve
quality, increase the speed of product development and reduce
overall development costs while allowing our clients to maintain
control of their development portfolio. This flexible approach
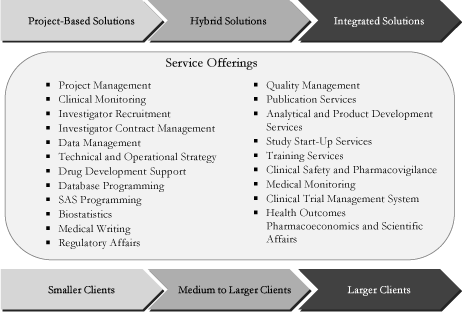
enables us to tailor our service offerings to meet the differing
needs of small, mid-sized and large biopharmaceutical companies.
We offer our services on a global basis, including in North
America, Latin America, Europe and Asia, and we have developed a
diverse client mix including 14 of the 15 largest pharmaceutical
companies in the world. We have grown our service revenue from
$62.8 million in 2005 to $200.5 million in 2009, a
compounded annual growth rate of 33.7%. In the six months ended
June 30, 2010, we generated $122.4 million of service
revenue, a 30.6% increase over service revenue of
$93.7 million for the first six months of 2009.
Traditional CROs are typically engaged to provide various
services on a
project-by-project
basis, such as project planning, clinical investigator and
patient recruitment, clinical trial monitoring, data management,
biostatistical analysis and regulatory reporting for a specific
clinical trial for a drug candidate. Although some of our
clients choose to contract with us for these services on an
individual project basis, we typically provide our services in
the form of integrated, embedded solutions, a business model we
pioneered. In our integrated solutions, we collaborate more
closely with the client, with an ability to work across multiple
clinical trials, product candidates and clinical development
functions. In many of our integrated engagements, we also
provide long-term strategic clinical development planning
services. While our integrated solutions are primarily targeted
at large biopharmaceutical companies, we also offer a wide
spectrum of project-based solutions to small and mid-sized
biopharmaceutical clients, as well as hybrid solutions combining
our integrated and project-based offerings.
We offer a comprehensive suite of outsourced solutions focused
on Phases II through IV of the clinical development
process, which encompasses late-stage and post-marketing
clinical trials. According to Frost and Sullivan, a market
research firm, research and development spending by the global
pharmaceutical industry is expected to grow from
$109 billion in 2009 to $169 billion in 2015, a
compound annual growth rate of 7.7%. Spending in Phase II
through IV in the United States is expected to grow as a
percentage of total research and development spending from 66%
in 2009 to 69% by 2015. Additionally, according to
Frost & Sullivan, CRO revenues, as a percentage of
global research and development spending, is expected to be
19.6% in 2010. Traditional project-based CROs target this 19.6%
of the research and development spending, the outsourced portion
of this market. By contrast, we seek to address a significantly
larger portion of the research and development spending market
by integrating our clinical development solutions into various
levels of our clients’ operations, allowing us to target
both the outsourced and in-house components of our clients’
Phase II through IV clinical expenditures.
We believe that our clinical expertise, coupled with our
personnel resourcing capabilities, represent a competitive
advantage in recruiting and managing the experienced and skilled
professionals required to successfully execute our business
model and differentiate our service offerings from those of
project-based CROs. Our personnel resourcing division is
organized into dedicated teams, each focused on a key functional
area within the clinical development field. We have a highly
experienced professional staff of over 2,300 individuals
averaging over 12 years of
1
experience in the industry, and our proprietary database
includes approximately 188,000 additional clinical trial
professionals around the world. We believe our personnel
resourcing capabilities, which we refer to as resourcing
engines, enable us to proactively allocate human capital by
identifying the expertise needed on each client program and
matching those needs with the availability, capacity and
expertise of the most appropriate professionals. We believe this
ability to rapidly resource, allocate and redeploy, or
“re-resource,” our highly experienced professional
staff is essential in providing integrated solutions that enable
us to achieve improved quality of trial execution, accelerated
clinical development timelines and cost savings for our clients.
We believe our integrated solutions provide clients with the
flexibility to expand their clinical research capabilities while
decreasing permanent headcount, support infrastructure and
overall development expenditures. Our solutions allow our
clients to outsource those portions of the clinical development
process for which the greatest efficiencies and savings can be
realized, while maintaining control over key clinical
development functions. In our integrated engagements, we create
a strategic and interdependent relationship with our clients,
which we believe allows us to better anticipate our
clients’ clinical capacity needs and efficiently deploy our
skilled clinical professionals to meet these requirements. We
believe that our integrated solutions allow our clients to more
effectively utilize their existing resources, processes and
systems, thus minimizing redundancies and avoiding duplicative
costs.
Our expertise allows us to design our solutions in collaboration
with a broad range of clients, from emerging biotechnology
companies to some of the largest biopharmaceutical corporations.
Our project-based solutions are more appropriate for some of our
clients, particularly smaller biopharmaceutical companies that
lack substantial clinical infrastructure. Our integrated
programs typically appeal to larger biopharmaceutical companies.
We aim to leverage our clinical operations and functional
management expertise, along with customized processes and
clinical operating systems, to meet the needs of all of our
clients. We believe our integrated solutions, combined with our
innovative resourcing engines and operational expertise, are
competitive differentiators that have resulted in broad adoption
of our solutions in the biopharmaceutical industry.
Our
Industry
The biopharmaceutical industry is facing a number of pressures,
including rising costs, increasing regulatory complexity, a
struggling global economy and the inherent risks associated with
pre-clinical and clinical biopharmaceutical development. In
order to address these challenges, we believe that
biopharmaceutical companies are increasingly turning to
innovative alternatives to traditional, project-based CRO
outsourcing that better meet their needs, such as our integrated
solutions. The market for CROs originally developed from a need
for additional clinical development capacity, which led to the
outsourcing of basic drug development services and projects. At
first, biopharmaceutical companies tended to work with multiple
CROs simultaneously, resulting in fragmentation, inefficiencies,
lack of coordination and often unreliable performance. Over
time, quality and consistency among CROs became increasingly
important to biopharmaceutical companies, who rationalized their
CRO usage by assigning projects to CROs based on the breadth of
services they offered and their prior experience. Today,
biopharmaceutical companies are seeking an increasingly broad
range of high quality, cost-effective services and more
customized solutions, including, in many cases, the option to
outsource entire clinical development functions. We developed
our business model to meet this emerging demand, providing our
clients with the increased flexibility, quality, commitment and
stability they require to meet their drug development needs
while reducing their costs. Because we are able to provide our
services across our client’s drug development pipeline
rather than limiting our services to a single clinical trial or
project, we believe our business model represents a positive
evolution in strategic outsourcing.
Outsourcing
Drivers
The challenges faced by today’s biopharmaceutical companies
create a number of opportunities to provide outsourced services
at various points throughout the clinical development process.
These drivers include:
|
|
|
| |
•
|
Continued growth in outsourced research and development
spending—Growth in our industry derives both from
growth in the research and development budgets of the
biopharmaceutical industry and from increases in the proportion
of those budgets directed to outsourced service providers.
According to Frost and Sullivan, research and development
spending by the biopharmaceutical industry is expected to grow
at a rate of approximately 7.7% per year from 2009 to 2015. This
growth is expected to be driven by increased competition,
product
|
2
|
|
|
| |
|
innovation, advances in research and clinical analysis, and the
desire to fill the pipelines of many large biopharmaceutical
companies, which are facing patent expirations of a significant
portion of their drug portfolios. As biopharmaceutical companies
have limited internal resources to conduct additional clinical
trials, or seek to more efficiently manage their internal
resources, we believe this results in a growing demand for
outsourced services.
|
|
|
|
| |
•
|
Increasing cost pressures and need for enhanced
efficiencies—With greater pressure on biopharmaceutical
companies to reduce fixed costs and better manage drug
development resources, outsourcing provides a mechanism to shift
from fixed to variable costs, which can improve a
biopharmaceutical company’s cost structure and return on
investment. In addition, biopharmaceutical companies are
increasingly adopting creative solutions that allow them to
reduce costs and enhance the efficiency and quality of clinical
trial execution while maintaining control of the clinical
development process.
|
| |
| |
•
|
Increasing complexity of industry needs driven by tougher
regulatory environment—Global drug regulators,
including the U.S. Food and Drug Administration, or FDA,
are increasingly requiring more clinical trials, longer trial
periods and greater amounts of clinical data before granting
approval to market a drug. According to the Pharmaceutical
Research and Manufacturers of America, a trade group for the
pharmaceutical and biotechnology industry, the average time to
take a new drug from discovery to market in the United States
ranges from approximately 10 to 15 years. This lengthy drug
development timeline has led many companies to increase their
pipelines of drugs in development in order to maximize their
chances of identifying successful products. Outsourcing can
allow biopharmaceutical companies to manage the increased
complexity of a growing drug development pipeline in a
cost-effective manner.
|
| |
| |
•
|
Increased globalization of clinical
trials—Biopharmaceutical companies are increasingly
seeking to access additional patient populations and trial
participants, identify new markets for their products, shorten
development times and reduce costs by conducting trials in
multiple countries. These trends require that biopharmaceutical
companies have access to outsourcing solutions on a global basis.
|
| |
| |
•
|
Priorities of smaller and emerging biopharmaceutical
companies—Smaller and emerging biopharmaceutical
companies commonly elect not to build their own in-house
clinical development capabilities, instead focusing their
resources on early-stage drug discovery or on sales and
marketing. Outsourcing clinical development functions enables
smaller biopharmaceutical companies to focus on developing their
drugs by providing access to necessary clinical development
services, without the high fixed costs of developing such
capabilities internally.
|
Through our innovative, next-generation business model, we
believe we are able to address the needs of the
biopharmaceutical industry by providing flexible outsourcing
solution that includes both integrated and project-based service
offerings. The flexibility of our model benefits our clients by
lowering their costs, improving the quality of their clinical
trial execution, and accelerating the clinical development
timeline for their product candidates.
The RPS
Solution
We have created a next-generation CRO by targeting both the
outsourced and in-house portions of Phase II through
Phase IV clinical development expenditures. Our model
combines innovative, integrated services with traditional
project-based CRO services, enabling us to tailor our solutions
to best meet the differing needs of our clients. The key aspects
of our business model include:
|
|
|
| |
•
|
Integrated, flexible approach and a true partnership with our
clients—Our integrated solutions emphasize close
collaboration with our clients, allowing our clients to realize
efficiencies and cost savings, while permitting them to maintain
control over key medical and regulatory decision-making
processes. We believe that this approach helps to maximize the
effective use of our clients’ existing resources, processes
and systems, while enhancing real-time communication and
coordination between us and our clients and avoiding duplicative
infrastructure costs. Through our integrated solutions, we
create a strategic and interdependent relationship that embeds
our services within our clients’ clinical development
operations. In some cases, we convert our clients’
employees into members of our professional staff.
|
| |
| |
•
|
Greater visibility into our clients’ product pipeline
and needs—We have developed integrated solutions that
|
3
|
|
|
| |
|
have a wider scope and product pipeline coverage than the
project-based services provided by traditional CROs. Our
integrated solutions can potentially cover all aspects of a
client’s clinical development programs. We believe this
deeper and more expansive level of involvement with our clients
allows us to better foresee potential client needs, which can
lead to strategic solutions that directly involve us in our
clients’ clinical development requirements and planning.
|
|
|
|
| |
•
|
Demonstrated ability to identify, recruit, deploy,
re-resource and retain a specialized professional
staff—We believe that our resourcing and re-resourcing
capabilities, combined with our clinical expertise, represent a
significant competitive advantage in attracting and retaining
the high-quality personnel required to successfully execute our
innovative business model and differentiate our service
offerings from those of traditional project-based CROs. We can
rapidly identify and deploy highly experienced professionals to
staff our engagements, which is an essential part of delivering
our integrated solutions. The key driver of this core expertise
has been the development of our resourcing engines, with
dedicated in-house teams that leverage our broad and deep
networks to proactively resource and re-resource experienced
professionals across key functional and therapeutic areas within
the clinical development field. We have built an extensive
database consisting of approximately 188,000 experienced
professionals available to join our professional staff. Through
our resourcing and re-resourcing processes, we can maximize our
professional staff’s expertise, capacity and job
satisfaction, allowing them to more efficiently and effectively
meet our clients’ needs.
|
| |
| |
•
|
Broad range of differentiated, cost-saving services, designed
for large, mid-sized and small biopharmaceutical
companies—We believe we are well-positioned to meet the
differing needs of our broad client base. Our large
biopharmaceutical clients typically prefer our integrated
solutions, which provide the flexibility for our clients to
cost-effectively expand their clinical research capabilities
across their entire pipeline of drug candidates and across
multiple clinical development functions without adding permanent
headcount and additional support infrastructure. The more
tactical needs of our smaller biopharmaceutical clients
typically require project-based solutions. For mid-sized
clients, we are also able to offer the flexibility of a hybrid
approach, combining integrated and project-based solutions
within a single engagement.
|
| |
| |
•
|
High level of flexibility—Our ability to quickly
deploy and redeploy a specialized workforce, combined with a
lack of duplication of infrastructure, allows us to rapidly
scale our programs in accordance with variations in our
clients’ research and development pipeline. With our
integrated solutions, by utilizing a client’s existing
clinical systems and processes, we eliminate the need to build a
separate infrastructure for new projects, thereby reducing costs
and accelerating the drug development process.
|
Investment
Highlights
We believe our next-generation business model has allowed us to
differentiate ourselves from our competitors. Our advantages
include:
|
|
|
| |
•
|
Leader in integrated clinical development
solutions—We built RPS to be the first CRO to approach
the biopharmaceutical industry with integrated solutions. Our
integrated solutions allow us to build strategic working
relationships with our clients, which we believe has helped us
to establish our brand name and develop recognition in the
industry as a leader and an innovator.
|
| |
| |
•
|
Business model validation from a diverse client
base—We believe our next-generation model is validated
by our client base, including many large biopharmaceutical
clients globally. Our client base includes 14 of the 15 largest
biopharmaceutical companies worldwide.
|
| |
| |
•
|
Integrated relationships enhance high customer retention and
revenue visibility—In our integrated engagements, we
create strategic and interdependent relationships with our
clients, which we believe promote long-term customer
relationships with stable revenue streams and enhance our
visibility into future revenue opportunities. We believe the
depth and interconnected nature of our relationships with our
clients helps to maximize customer retention.
|
| |
| |
•
|
Global platform to meet the needs of our
clients—Over the last several years, we have been
building our global infrastructure. We began this process in
2005 with the opening of our Canadian and Latin American
operations and have continued our global expansion with
acquisitions in Europe in 2008 and China in 2009. With our
|
4
|
|
|
| |
|
global infrastructure that is now in place, we believe we are
well positioned to service the growing global needs of our
clients.
|
|
|
|
| |
•
|
Strong financial track record—Our service revenue
grew at a compound annual growth rate of 33.7% from 2005 to
2009. We continue to expand our business through organic growth
supplemented with selective acquisitions. Our growth has
continued in 2010, with service revenue of $122.4 million
for the first six months, representing a 30.6% increase over the
same period in 2009. Our EBITDA has grown from $308,000 in 2005
to $10.0 million in 2009. Our net income has improved from
a loss of $1.7 million in 2005 to income of
$2.6 million in 2009. For a reconciliation of EBITDA to net
income, see the section of this prospectus summary entitled
“Summary Consolidated Financial Data.”
|
| |
| |
•
|
Experienced management—Our senior management team is
composed of highly experienced veterans of public companies and
the CRO, biopharmaceutical, staffing and outsourcing industries.
Daniel M. Perlman, our Chief Executive Officer, has over
20 years of experience in outsourcing services to the
biopharmaceutical industry, and Dr. Harris Koffer, our
President and Chief Operating Officer, has 30 years of
experience in clinical drug development. Steven Bell, our Chief
Financial Officer, has over 30 years of public accounting
and chief financial officer experience.
|
Our Growth
Strategy
We intend to capitalize on increasing customer adoption of our
next-generation model to drive revenue and earnings growth
through 2010 and beyond. Our growth strategy includes the
following initiatives:
|
|
|
| |
•
|
Leverage our global capabilities to enhance and expand our
ability to execute global programs—We have built a
global infrastructure with operations in North America, Latin
America, Europe and Asia. We plan to continue to evaluate
acquisition opportunities, with a particular focus on Eastern
Europe and Asia. We anticipate that increasing our global
capabilities will drive the expansion of existing engagements
and new program awards with global requirements.
|
| |
| |
•
|
Develop further opportunities from our existing integrated
accounts—Our current integrated solutions have allowed
us to grow multiple, long-term engagements by cross-selling and
expanding into other functional areas once we have begun working
with a client. We believe there are opportunities for continued
and accelerated growth by focusing on this strategy. As an
example, we began site management responsibilities for a client
initially and then successfully expanded our responsibilities
into study management, data management, medical writing and
other clinical areas.
|
| |
| |
•
|
Continue to add new accounts—We plan to leverage the
growing demand for outsourced clinical development services to
reach new clients. We see opportunity in the large pipeline of
drugs in development to further drive our growth strategy. We
believe that continued growth in research and development
spending, along with increased demand for outsourced solutions,
represents a significant opportunity for us to add new accounts.
|
| |
| |
•
|
Leverage our infrastructure to enhance
profitability—Our historical investment in our global
expansion provides us with the opportunity to capitalize on our
investment as we grow our revenue. We believe that this
historical investment, along with our ability to leverage our
clients’ existing infrastructure through our integrated
solutions, could mean that with less additional investment in
our own infrastructure, we would be able to expand the services
we provide our clients. By leveraging our innovative model and
current cost structure as our revenues grow, we expect to
enhance our profitability.
|
| |
| |
•
|
Expand suite of services offered—We are expanding
our suite of service offerings to include both non-clinical
services, such as analytical chemistry, and clinical services,
such as Phase IV post-marketing surveillance services. We
believe this will enhance our ability to provide services
tailored to our clients’ needs.
|
Corporate
Information
Our predecessor, ReSearch Pharmaceutical Services, Inc., a
Pennsylvania corporation, or Old RPS, was incorporated in
Pennsylvania in 1994. We were incorporated in Delaware on
January 30, 2006, as Cross Shore Acquisition Corporation,
or Cross Shore. Old RPS merged into a wholly owned subsidiary of
Cross Shore in 2007, and as a result of the merger, Old RPS
became ReSearch Pharmaceutical Services, LLC, a Delaware limited
5
liability company. Cross Shore, which changed its name to
ReSearch Pharmaceutical Services, Inc. in conjunction with the
merger, now conducts all of its operations and business
activities through its wholly owned subsidiary, ReSearch
Pharmaceutical Services, LLC. Our principal executive offices
are located at 520 Virginia Drive, Fort Washington, Pennsylvania
19034. Our telephone number is (215) 540-0700. Our website
address is www.rpsweb.com. Information contained in or that can
be accessed through our website is not incorporated by reference
into this prospectus and should not be considered part of this
prospectus.
6
The
Offering
|
|
|
|
Common stock offered by us |
|
shares |
| |
|
Common stock offered by the selling stockholders
|
|
shares |
| |
|
Common stock to be outstanding immediately after the
offering
|
|
shares |
Use of
proceeds
We estimate that our net proceeds from this offering, after
deducting underwriting discounts and commissions and offering
expenses payable by us, will be
$ million, or
$ million if the underwriters
exercise their overallotment option in full, based upon an
assumed initial public offering price of
$ per share, which is the midpoint
of the range set forth on the cover page of this prospectus. We
will not receive any proceeds from the sale of shares by the
selling stockholders.
We anticipate that we will use the net proceeds we receive from
this offering to repay the outstanding balance of approximately
$10.9 million under our working capital line of credit that
matures in October 2012, with the remainder for general
corporate purposes and working capital, including to promote the
further development and expansion of our service offerings
globally. We may also use a portion of the net proceeds to
acquire complementary businesses or assets, although we
currently have no agreements or commitments with respect to any
acquisitions.
Please read “Use of Proceeds.”
NASDAQ Global
Market listing
We have applied to list our common stock on the NASDAQ Global
Market under the symbol “RPSE.”
Risk
factors
Investing in our common stock involves a high degree of risk.
You should carefully read and consider the information set forth
under the heading “Risk Factors” beginning on
page 10 of this prospectus and all other information set
forth in this prospectus before deciding to invest in our common
stock.
Outstanding
shares
The number of shares of our common stock to be outstanding after
this offering is based on 37,216,052 shares outstanding as
of June 30, 2010, and excludes as of that date:
|
|
|
| |
•
|
2,908,393 shares of common stock issuable upon exercise of
outstanding options with a weighted average exercise price of
$2.07 per share; and
|
| |
| |
•
|
3,788,452 shares of common stock reserved for future grants
under the ReSearch Pharmaceutical Services, Inc. 2007 Equity
Incentive Plan.
|
Except as otherwise indicated, all information contained in this
prospectus assumes no exercise by the underwriters of their
overallotment option and does not give effect to
a -for-
reverse split of our common stock that we plan to complete prior
to the consummation of this offering.
7
Summary
Consolidated Financial Data
The following tables set forth a summary of certain of our
historical consolidated financial data and should be read
together with our consolidated financial statements and related
notes to those statements, as well as “Management’s
Discussion and Analysis of Financial Condition and Results of
Operations,” each included elsewhere in this prospectus. We
have derived the summary consolidated statement of operations
data for each of the years ended December 31, 2007, 2008
and 2009 from our audited consolidated financial statements
included elsewhere in this prospectus. We have derived the
summary consolidated balance sheet data as of June 30, 2010
and the summary consolidated statement of operations data for
each of the six months ended June 30, 2009 and
June 30, 2010 from our unaudited consolidated financial
statements included elsewhere in this prospectus. These interim
financial statements reflect all adjustments, consisting of
normal recurring adjustments, which are necessary in the opinion
of management to present fairly our consolidated financial
position and results of operations for the interim periods. Our
historical financial results are not necessarily indicative of
financial results that may be expected in future periods, and
our financial results for any interim period are not necessarily
indicative of financial results that may be expected for a full
fiscal year.
We have presented the summary balance sheet data as of
June 30, 2010 on an actual basis and on an adjusted basis,
which gives effect to:
|
|
|
| |
•
|
our sale
of shares
of common stock in this offering at an assumed initial public
offering price of $ per share,
which is the midpoint of the range set forth on the cover page
of this prospectus, after deducting estimated underwriting
discounts and commissions and estimated offering expenses
payable by us; and
|
| |
| |
•
|
the repayment in full of the outstanding balance of
approximately $10.9 million under our working capital line
of credit.
|
Each $1.00 increase or decrease in the assumed initial public
offering price of $ per share,
which is the midpoint of the range set forth on the cover page
of this prospectus, would increase or decrease each of cash and
cash equivalents, working capital, total assets and total
stockholders’ equity on an as adjusted basis by
approximately $ , assuming that the
number of shares offered by us, as set forth on the cover page
of this prospectus, remains the same. The as adjusted
information presented in the summary balance sheet data is
illustrative only and will change based on the actual initial
public offering price and other terms of this offering
determined at pricing.
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Six Months
|
|
|
|
Year Ended December 31,
|
|
Ended June 30,
|
|
|
|
2007
|
|
2008
|
|
2009
|
|
2009
|
|
2010
|
|
|
|
|
|
|
|
|
|
(unaudited)
|
|
|
|
(in thousands, except per share data)
|
|
|
|
Consolidated Statement of Operations Data:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Service revenue
|
|
$
|
120,459
|
|
|
$
|
156,967
|
|
|
$
|
200,472
|
|
|
$
|
93,705
|
|
|
$
|
122,409
|
|
|
Reimbursement revenue
|
|
|
13,924
|
|
|
|
18,086
|
|
|
|
23,696
|
|
|
|
10,940
|
|
|
|
15,500
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total revenue
|
|
|
134,383
|
|
|
|
175,052
|
|
|
|
224,168
|
|
|
|
104,646
|
|
|
|
137,909
|
|
|
Direct costs
|
|
|
87,650
|
|
|
|
117,707
|
|
|
|
145,209
|
|
|
|
68,160
|
|
|
|
88,732
|
|
|
Reimbursable
out-of-pocket
costs
|
|
|
13,924
|
|
|
|
18,086
|
|
|
|
23,696
|
|
|
|
10,940
|
|
|
|
15,500
|
|
|
Selling, general, and administrative expenses
|
|
|
26,787
|
|
|
|
31,290
|
|
|
|
44,798
|
|
|
|
21,091
|
|
|
|
25,671
|
|
|
Depreciation and amortization
|
|
|
1,144
|
|
|
|
1,750
|
|
|
|
3,723
|
|
|
|
1,671
|
|
|
|
2,202
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Income from operations
|
|
|
4,879
|
|
|
|
6,219
|
|
|
|
6,742
|
|
|
|
2,784
|
|
|
|
5,804
|
|
|
Interest and other income (expense), net
|
|
|
(5,786
|
)
|
|
|
42
|
|
|
|
(562
|
)
|
|
|
(303
|
)
|
|
|
(396
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Income (loss) before provision for income taxes
|
|
|
(907
|
)
|
|
|
6,261
|
|
|
|
6,180
|
|
|
|
2,481
|
|
|
|
5,409
|
|
|
Provision for income taxes
|
|
|
1,508
|
|
|
|
2,518
|
|
|
|
3,559
|
|
|
|
1,492
|
|
|
|
3,953
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income (loss)
|
|
|
(2,415
|
)
|
|
|
3,743
|
|
|
|
2,620
|
|
|
|
989
|
|
|
|
1,456
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accretion of preferred stock
|
|
|
(321
|
)
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income (loss) applicable to common shares
|
|
$
|
(2,736
|
)
|
|
$
|
3,743
|
|
|
$
|
2,620
|
|
|
$
|
989
|
|
|
$
|
1,456
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income (loss) per common share:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic
|
|
$
|
(0.19
|
)
|
|
$
|
0.11
|
|
|
$
|
0.07
|
|
|
$
|
0.03
|
|
|
$
|
0.04
|
|
|
Diluted
|
|
$
|
(0.19
|
)
|
|
$
|
0.11
|
|
|
$
|
0.07
|
|
|
$
|
0.03
|
|
|
$
|
0.04
|
|
|
Weighted average number of shares outstanding:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic
|
|
|
14,573
|
|
|
|
32,617
|
|
|
|
37,003
|
|
|
|
36,747
|
|
|
|
37,272
|
|
|
Diluted
|
|
|
14,573
|
|
|
|
34,103
|
|
|
|
38,071
|
|
|
|
37,708
|
|
|
|
38,676
|
|
|
|
8
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
As of June 30, 2010
|
|
|
|
Actual
|
|
As Adjusted
|
|
|
|
(unaudited)
|
|
|
|
(in thousands)
|
|
Consolidated Balance Sheet Data:
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents
|
|
$
|
4,252
|
|
|
$
|
|
|
|
Working capital
|
|
|
23,149
|
|
|
|
|
|
|
Total assets
|
|
|
94,558
|
|
|
|
|
|
|
Line of credit
|
|
|
10,861
|
|
|
|
|
|
|
Capital leases
|
|
|
618
|
|
|
|
|
|
|
Stockholders’ equity
|
|
|
43,851
|
|
|
|
|
|
|
|
We use EBITDA, in conjunction with financial measures determined
in accordance with U.S. generally accepted accounting
principles, or GAAP, such as net income and income from
operations, to assess our operating performance. EBITDA is a
non-GAAP financial measure and is defined as net income (loss)
before net interest expense, income taxes, depreciation and
amortization.
We use EBITDA, along with other GAAP measures, to measure our
profitability and to make budgeting decisions relating to
historical performance and future expectations of our business,
and to make performance comparisons of our company compared to
other companies. We believe that, like management, investors
frequently use EBITDA to evaluate our operating performance and
to compare it to that of other companies.
EBITDA should not be considered in isolation or as a substitute
for a measure of our liquidity or operating performance prepared
in accordance with GAAP and is not indicative of net income
(loss) from operations as determined under GAAP. EBITDA and
other non-GAAP financial measures have limitations which should
be considered before using these measures to evaluate our
liquidity or financial performance. EBITDA does not include
interest expense, income tax expense or depreciation and
amortization expense, which may be necessary in evaluating our
operating results and liquidity requirements or those of
businesses we may acquire. Our management compensates for these
limitations by using EBITDA as a supplement to GAAP results to
provide a more comprehensive understanding of the factors and
trends affecting our business or any business we may acquire.
Our computation of EBITDA may not be comparable to other
similarly titled measures provided by other companies, because
not all companies calculate this measure in the same fashion.
The following table reconciles net income (loss) to EBITDA,
which we believe is the most directly comparable GAAP
measurement:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended December 31,
|
|
|
|
2005
|
|
2006
|
|
2007
|
|
2008
|
|
2009
|
|
|
|
(unaudited)
|
|
|
|
(in thousands)
|
|
|
|
Net income (loss)
|
|
$
|
(1,683
|
)
|
|
$
|
1,792
|
|
|
$
|
(2,415
|
)
|
|
$
|
3,743
|
|
|
$
|
2,620
|
|
|
Provision for income taxes
|
|
|
—
|
|
|
|
45
|
|
|
|
1,508
|
|
|
|
2,518
|
|
|
|
3,559
|
|
|
Interest income (expense), net
|
|
|
1,127
|
|
|
|
1,245
|
|
|
|
5,787
|
|
|
|
(66
|
)
|
|
|
110
|
|
|
Depreciation and amortization
|
|
|
864
|
|
|
|
901
|
|
|
|
1,143
|
|
|
|
1,750
|
|
|
|
3,723
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
EBITDA
|
|
$
|
308
|
|
|
$
|
3,983
|
|
|
$
|
6,023
|
|
|
$
|
7,945
|
|
|
$
|
10,012
|
|
|
|
9
Risk
Factors
An investment in our common stock involves a high degree of
risk. You should carefully consider the risks described below,
together with the other information in this prospectus, before
making a decision to purchase shares of our common stock. If any
of the following risks, or the risks described elsewhere in this
prospectus, occur, our business, prospects, results of
operations and financial condition could be materially harmed.
As a result, the market price of our common stock could decline
and you may lose all or part of your investment. The risks and
uncertainties described below are not the only ones we face.
Additional risks and uncertainties not presently known to us or
that we currently believe to be immaterial may also adversely
affect our business.
Risks Related to
Our Business
We depend
on the biopharmaceutical industry for substantially all of our
revenue. Factors or trends affecting that industry could
adversely affect our business.
We provide services and solutions to the biopharmaceutical
industry, and our revenues depend on the outsourcing trends and
research and development expenditures of the biopharmaceutical
industry. Economic factors and industry trends that affect
companies in the biopharmaceutical industry also affect our
business. For example, the practice of many companies in this
industry has been to engage companies like us to manage the
clinical development of their product pipelines. If these
companies reduce their tendency to outsource projects and
development programs in light of current difficult conditions in
credit markets and the economy in general or for any other
reason, our operations, financial condition and growth rate
would be materially and adversely impacted. In the past, factors
such as industry consolidation, product failures and
withdrawals, liability lawsuits and governmental regulation to
control growing healthcare costs have also slowed
decision-making by biopharmaceutical companies and delayed drug
development projects. Any such developments could cause our
clients to reduce their drug discovery and development spending,
which could reduce demand for our services and have an adverse
effect on our business.
Recent consolidation in the biopharmaceutical industry could
lead to a reduction in our revenues.
A number of large biopharmaceutical companies have recently
completed mergers and acquisitions that will consolidate the
research and development expenditures and outsourcing trends of
the biopharmaceutical industry into fewer companies. For
example, Wyeth, our largest customer during 2008 and 2009,
representing 20% and 17%, respectively, of our service revenue
during those years, was acquired by Pfizer, and Schering-Plough,
our second largest customer during 2008 and 2009, representing
12% and 16%, respectively, of our service revenue during those
years, was acquired by Merck. While Pfizer and Merck were also
our clients prior to these acquisitions, we cannot assure you
whether we will continue to generate revenues from these
companies that are consistent with or higher than their historic
levels. As the integration of these acquisitions continues, the
surviving biopharmaceutical companies may decide to use other
CROs, keep clinical research services in-house, or otherwise
diminish the use of our services. We cannot predict the
potential impact of these acquisitions and subsequent
integration, but any resulting decisions related to outsourcing
clinical trial services could reduce our revenues if we are not
engaged to continue providing the same level of services to the
acquiring company. Regardless of the reason, the negative impact
of the loss of business from any large biopharmaceutical
companies may be enhanced due to consolidation in this industry.
Providing
outsourcing services to the biopharmaceutical industry is highly
competitive, and our failure to compete effectively could harm
our business. We also compete with existing in-house personnel
employed by our clients, and the increased use of these
personnel could reduce our revenues.
We compete with a wide range of providers of outsourcing
services to the biopharmaceutical industry, including small,
niche providers and full-service global clinical research
organizations. Outsourcing service providers compete based on a
variety of factors, including reputation for quality,
performance, price, scope of service offerings and geographic
presence. A number of our competitors possess substantially
greater resources and more well-established brand names than we
do, which may hurt our competitive position within the industry.
Additionally, some of our current or potential clients use
in-house personnel to perform the same functions and
10
services that we seek to perform for these clients on an
outsourced basis. The increased use of in-house personnel by
these companies would decrease the likelihood that we could
obtain additional new contracts or extensions of our existing
contracts to participate in our clients’ drug development
process, which could eliminate or substantially reduce our
revenues. In addition, clients and prospective clients may
choose not to utilize our project-based, hybrid or integrated
solutions at any particular period of time, which could lead to
fluctuations and variability in our revenue.
The biopharmaceutical industry generally, and drug discovery and
development more specifically, is also subject to increasingly
rapid technological changes. Our competitors or others might
develop technologies, services or products that are more
effective or more commercially attractive than our current or
future technologies and services, or which render our
technologies and services less competitive or obsolete. If
competitors introduce superior technologies and services or
products and we cannot make enhancements to remain competitive,
our competitive position would be materially and adversely
affected.
Our
contracts may be delayed, terminated or reduced in scope with
little or no notice, which could adversely impact our revenues
and our profitability.
Many of our contracts with our clients may be terminated or
reduced in scope with little or no notice. Cancellations may
occur for a variety of reasons, including the failure of the
client’s product to satisfy safety or efficacy
requirements, unexpected clinical trial results using the
client’s product, regulatory developments, economic issues,
availability of clinical trial material, protocol design matters
or the client’s decision to reduce its research and
development activities. In addition, if we are unable to provide
staff sufficient in number or experience as required for a
project, the contract may be delayed, terminated or reduced in
scope. Any of these developments could lead to an unexpected
reduction in our revenues and an impairment of our profitability
and cash flow.
Our
backlog as of a given date may not be a meaningful predictor of
our future results.
Our backlog, which represents anticipated service revenue from
executed contracts that either have not started but are
anticipated to begin in the near future, or are in process and
have not been completed, can be affected by a number of factors,
such as the size and duration of contracts, many of which are
performed over several years, and changes in labor utilization
over the course of a clinical trial. Also, the scope of a
contract can change significantly during a project, which could
cause our backlog to be adjusted. We cannot assure investors
that we will fully realize our entire backlog, which was
$183.0 million as of June 30, 2010, as service revenue
in the future or at a rate consistent with historic levels.
A
substantial percentage of our revenue is attributable to a
relatively small number of clients. The loss of, or reduction in
services provided to, these clients could significantly reduce
our revenues and profitability.
For the six months ended June 30, 2010, our five largest
clients accounted for 59% of our service revenue and our 20
largest clients accounted for 86% of our service revenue. For
the six months ended June 30, 2010, our largest customer
accounted for 17% of our service revenue, and for the year ended
December 31, 2009, our largest customer accounted for 17%
of our service revenue. The loss of one or more of our largest
clients, or the reduction in scope of a single contract or
several smaller contracts with our largest clients, could
materially reduce our revenues, cash flow and profitability.
If we are
unable to recruit and retain qualified personnel, or to reassign
billable personnel from one project to another as projects are
completed, it will be difficult for us to achieve our financial
and operational goals.
Our success depends to a significant extent upon the efforts of
our senior management team and our ability to hire qualified
management and scientific personnel in the regions in which we
perform services for our clients. There is substantial
competition within the biopharmaceutical and CRO industries for
qualified personnel, and any difficulty that we encounter in
recruiting or retaining qualified personnel would impact our
ability to meet our financial and operational goals.
We rely on our proprietary database of clinical trial
professionals in order to recruit professional staff for our
engagements and to compete with other providers of outsourcing
services to the biopharmaceutical industry. The
11
loss, damage or misappropriation of our database could result in
our inability to meet our contractual obligations with our
clients, a loss of a competitive edge with other outsourcing
service providers or a loss of potential growth opportunities.
Furthermore, our financial and operational success depends to a
significant extent upon our ability to minimize the number of
unassigned billable personnel at any one time by reassigning
billable personnel from one project to another as projects are
completed. Because unassigned personnel remain on our payroll,
we do not earn any revenue, but continue to pay billable
personnel who are unassigned, which increases our expenses and
reduces our profitability.
The fixed
price nature of some of our contracts could result in financial
losses.
Some of our contracts are structured as fixed price contracts.
If we underbid our fixed price contracts or overrun our initial
cost estimates, we may not be able to achieve or maintain
profitability.
Our
business depends on our senior management team and other key
personnel, and the loss of any member of the team could harm our
business.
We believe that our success will depend on the continued
employment of our senior management team, which has substantial
experience in the administration of biopharmaceutical services
businesses. Our growth and profitability could be materially and
adversely affected if the services of our senior management or
other key employees cease to be available. If one or more
members of our senior management team were unable or unwilling
to continue in their present positions, those persons could be
difficult to replace and our business could be harmed. If any of
our key employees were to join a competitor or form a competing
company, some of our clients might choose to use the services of
that competitor or new company instead of our services. While
our senior management employees have entered into
non-competition agreements with us that would restrict their
ability to compete with us after their employment with us
ceases, we cannot assure you that a court would enforce the
non-competition provisions in a manner that would be
advantageous to us. Further, if non-competition provisions were
enforced, they are limited in time and scope and we cannot
assure you that the provisions would adequately protect our
business. In addition, if his employment is terminated,
Mr. Perlman, our chief executive officer, may elect to
forego any severance benefits owed to him in return for the
elimination of the non-compete provisions in his employment
agreement.
Unfavorable
general economic conditions could hurt our business.
Unstable global economic conditions, including the recent
recession in the United States, political and economic unrest
outside of the United States, and the continuing financial
crisis in the banking system and financial markets, could
negatively affect our business. While it is difficult for us to
predict the impact of general economic conditions on our
business, these conditions could reduce client demand for some
of our services or the ability of third parties to provide
services critical to our business, which could cause our revenue
to decline. Also, our clients, particularly smaller
biopharmaceutical companies which are especially reliant on the
credit and capital markets, may not be able to obtain adequate
access to credit or equity funding, which could affect their
ability to make timely payments to us. If that were to occur,
our cash flows could be impaired, and we could be required to
increase our allowance for doubtful accounts, which could impact
our profitability.
We may
not be able to expand through acquisitions
successfully.
From time to time, we evaluate acquisition opportunities
globally and in the United States in order to increase our
market share and our presence in servicing the biopharmaceutical
industry. Our ability to grow successfully through acquisitions
could be affected by, among other things, the following:
|
|
|
| |
•
|
Identification of acquisition targets. We may have
difficulty identifying suitable acquisition opportunities and
successfully consummating proposed transactions.
|
| |
| |
•
|
Competition for acquisitions. Competition in the
acquisition market could limit our ability to grow through
acquisitions or could raise the prices of acquisitions and make
them less accretive or possibly non-accretive.
|
12
|
|
|
| |
•
|
Financing of acquisitions. We may not be able to obtain
necessary financing or may need to incur significant cash
expenditures to consummate desirable strategic acquisitions.
Financing in the form of debt could include covenants
restricting our ability to complete additional acquisitions or
other business activities, while any issuance of new equity
securities could result in the ownership interests of existing
stockholders being significantly diluted.
|
| |
| |
•
|
Expense of acquisitions. The costs and expenses of
acquisitions, including integration expenses and exposure to
unforeseen liabilities, could have a material adverse effect on
our financial condition and results of operations and the
overall effectiveness of our acquisitions.
|
| |
| |
•
|
Accounting charges. We could be required to incur charges
for accounting purposes that could negatively impact our
reported operating results.
|
| |
| |
•
|
Integration of acquisitions. We may experience difficulty
integrating completed acquisitions. The process of integrating
acquired businesses may involve unforeseen difficulties and may
require significant financial and other resources and a
disproportionate amount of management’s attention. We may
not be able to successfully manage and integrate new businesses
or technologies into our existing operations or successfully
maintain the market share attributable to any acquired
businesses. Acquisitions of foreign operations involve the
additional risks of assimilating differences in foreign business
practices, hiring and retaining qualified personnel, and
overcoming language and cultural barriers.
|
To the extent that we are unable to successfully execute our
acquisition strategy, or our recent acquisitions do not prove to
be accretive, it may compromise our ability to expand
domestically and internationally.
Our
international operations are subject to numerous
risks.
We have international operations in Canada, Latin America,
Europe and the Asia-Pacific region, and we intend to develop our
operations globally through organic growth and selective
acquisitions based on client demand. Our current and future
foreign operations are and will be subject to risks inherent in
operating in foreign countries, including government regulations
different from those we face domestically, currency restrictions
and fluctuations, additional taxes and potential political and
civil instability and unrest. Our ability to manage these issues
could be affected by applicable U.S. laws and the need to
protect our assets in those locations. Although we intend to
take steps to mitigate these risks where possible, political,
economic or social instability or other developments could make
less developed countries less suitable for our expansion plans
and may hurt our ability to operate in and contract with persons
in such countries.
Further, our financial statements are denominated in
U.S. dollars. As a result, factors associated with current
and future international operations, including changes in
foreign currency exchange rates, could significantly impair our
results of operations or financial condition. For the year ended
December 31, 2009 and the six months ended June 30,
2010, we generated approximately 17% and 16%, respectively, of
our service revenue from our foreign operations. Exchange rate
fluctuations between local currencies and the U.S. dollar
create risk in several ways, including foreign currency
translation risk related to our revenue and expenses of foreign
operations being generally denominated in local currencies, and
foreign currency transaction risk related to our foreign
contracts that may be denominated in a currency other than the
currency in which we incur expenses related to such contracts.
In addition, as a result of our acquisitions of three CROs in
Europe and one CRO in China during 2008 and 2009, we have
increased our number of paid personnel in foreign countries
significantly, and fluctuations in foreign currency exchange
rates could increase our employee compensation expenses in those
foreign countries accordingly. In the future, we may seek to
limit these risks through exchange rate fluctuation provisions
in our contracts, or by hedging our transaction risk with
foreign currency exchange contracts or options. Despite these
efforts, we may still experience fluctuations in financial
results from our operations outside the United States, and we
cannot assure investors that we will be able to favorably reduce
our currency transaction risk associated with our contracts.
Proposed
and future legislation or regulations may increase the cost of
our business or limit our service offerings.
Federal, state or local authorities might adopt legislation or
regulations that are more burdensome than existing regulations
applicable to our business. For example, recent product safety
concerns and the creation by the FDA of
13
the Drug Safety Oversight Board could change the regulatory
environment for drug products including the process for FDA
product approval and post-approval safety surveillance. These
and other future changes in regulation could increase our
expenses or limit our ability to offer some of our services.
We may be
affected by recently enacted healthcare reform
legislation.
In March 2010, the U.S. Congress enacted the Patient
Protection and Affordable Care Act, which is intended over time
to expand health insurance coverage and impose healthcare cost
containment measures. This legislation may significantly impact
the biopharmaceutical industry. Under this healthcare reform
legislation, medical device manufacturers and biopharmaceutical
companies will be subject to an excise tax in excess of
$2 billion per year that escalates over time and will be
allocated based on market share. In addition, the FDA is
authorized to establish a process to regulate the approval of
generic versions of biologic drugs. The imposition of excise
taxes and a simplified approval process for biologic drugs could
decrease the amount of money our clients can spend on our
services, provide a disincentive to discover new biologic drugs,
or otherwise decrease demand for our services. We are presently
uncertain as to all of the effects the recently enacted
legislation could have on our business and are unable to predict
what legislative proposals will be adopted in the future, if any.
Healthcare reform legislation may also limit the profits that
can be made from the development of new drugs. This could
adversely affect research and development expenditures by the
biopharmaceutical industry, which could in turn decrease the
business opportunities available to us both in the United States
and abroad. In addition, new laws or regulations may create a
risk of liability, increase our costs or limit our service
offerings.
Our
business and our clients’ businesses are subject to
extensive regulation, and our and their results of operations
could be harmed if regulatory standards change significantly or
if we fail to maintain compliance with regulations.
Laws and regulations regarding the development and approval of
drug and biological products have become increasingly stringent
in both the U.S. and foreign jurisdictions, resulting in a
need for more complex and often larger clinical studies.
Pharmaceutical and biologic products and medical devices to be
used in humans are subject to rigorous regulation by the
U.S. government—principally by the FDA, but also by
the Federal Trade Commission and other agencies—and by
foreign governments if products are tested or marketed abroad.
Additional legislation or regulation governing the possession,
use and dissemination of medical record information and other
personal health information might require us to implement new
security measures that require substantial expenditures or limit
our ability to offer certain of our services. Further, a
relaxation of the scope of regulatory requirements, such as the
introduction of simplified marketing applications for
pharmaceuticals and biologics, such as those made by generic
drug manufacturers, could decrease the business opportunities
available to us.
In addition, because we offer services relating to the conduct
of clinical trials and the preparation of marketing
applications, we are required to comply with applicable
regulatory requirements governing, among other things, the
design, conduct, performance, monitoring, auditing, recording,
analysis and reporting of these trials. In the United States,
the FDA governs these activities pursuant to the agency’s
good clinical practice, or GCP, regulations. We have a limited
history of inspection by the FDA. Our failure to maintain
compliance with GCP or other applicable regulations could lead
to a variety of sanctions, including, among other things, and
depending on the nature of the violation and the type of product
involved, the suspension or termination of a clinical study,
civil penalties, criminal prosecution, debarment or prohibition
from assisting in the submission of new drug applications, or
NDAs. Although we recently underwent our first inspection for
GCP compliance and received no observations of noncompliance, we
cannot assure you that future inspections will not identify GCP
or other violations that could subject us to FDA enforcement
actions, including warning letters. In addition, we could be
required to pay monetary damages to our clients in the event we
are found not to have complied with GCP. While we monitor
clinical trials to test for compliance with applicable laws and
regulations in the United States and foreign jurisdictions in
which we operate, and have adopted standard operating procedures
that are designed to satisfy applicable regulatory requirements,
our business spans multiple regulatory jurisdictions with
varying, complex regulatory frameworks, and therefore we cannot
assure investors that our systems will ensure compliance in
every instance in the future. We could be forced to incur
significant costs in complying with new regulations, and we may
incur fines or damage to our reputation as a result of our
failure to comply with any such regulations.
14
Our
clinical research services create a risk of liability, and we
could be required to pay damages or to bear the costs of
defending any claim not covered by contractual
indemnity.
Clinical research services performed by or on behalf of
biopharmaceutical companies involve the testing of new drugs,
biologics, and devices on human volunteers, and, if marketing
approval is received for any of their drug, biologic and device
candidates, their use by patients. This testing creates risks of
liability for personal injury, sickness or death of patients
resulting from their participation in the study. These risks
include, among other things, unforeseen adverse side effects,
improper application or administration of a new drug or device,
and the professional malpractice of medical care providers. Many
volunteer patients are already seriously ill and are at
heightened risk of future illness or death. Clinical trial
agreements between sponsors and investigators do not include us
as a party, which may place us at a disadvantage in the
allocation of risk and regulatory responsibility. Although we do
not believe we are legally accountable for the medical care
rendered by third party investigators, it is possible that, in
the event of the personal injury or death of persons
participating in clinical trials, we could be held liable for
the claims and expenses arising from any professional
malpractice of the investigators. We also could be held liable
for errors or omissions in connection with the services we
perform. While we believe our current insurance coverage is
adequate, our business could be materially harmed if we were
required to pay damages or bear the costs of defending any claim
outside the scope of, or in excess of, the contractual
indemnification provided by our agreements with our clients that
is beyond the level or scope of insurance coverage in effect.
Further, an indemnifying party may not fulfill its
indemnification obligations to us or indemnification agreements
may not enforced by a court in accordance with their terms,
which would compromise our financial position.
We may
not be able to manage our growth effectively.
We have experienced substantial revenue and employee growth over
the last several years, and we believe that sustained growth may
place a strain on our operational, human, and financial
resources. To manage our growth, we must continue to improve
operating and administrative systems and services and attract
and retain qualified management, professional, scientific and
technical operating personnel. We believe that maintaining and
enhancing both our systems and personnel at reasonable costs are
instrumental to our success. The nature and pace of our growth
also introduces risks associated with quality control and client
dissatisfaction due to potential delays in performance or other
problems.
Our
business depends significantly on the continued effectiveness of
our information technology infrastructure, and failures of such
technology could disrupt our operations.
To remain competitive, we must employ information technologies
that capture, manage, and analyze the large streams of data
generated during clinical trials in compliance with applicable
regulatory requirements. In addition, because we provide
services on a global basis, we rely extensively on technology to
allow the concurrent conduct of studies and work sharing around
the world. Any loss of communication services, such as
telephone,
e-mail, or
internet service could compromise our ability to communicate
with our clients and recruit clinical trial professionals. As
with all information technology, our system is vulnerable to
potential damage or interruptions from fires, blackouts,
telecommunications failures, computer-related hardware and
software failures and disruptions and other unexpected events,
as well as to break-ins, sabotage, or intentional acts of
vandalism. Any substantial disruption or resulting loss of data
that is not avoided or corrected by backup measures could
significantly disrupt our operations. While we carry business
interruption insurance policies that we believe to be adequate,
we might suffer losses as a result of business interruptions
that exceed the coverage available under our insurance policies
or for which the policies do not provide coverage.
We may
face significant employment liability risk.
With many of our integrated solutions, we employ and place
people at the physical workplaces of our clients. An inherent
risk of such activity includes possible claims of errors and
omissions, misuse or misappropriation of client proprietary
information, misappropriation of funds, discrimination and
harassment, failure to comply with applicable immigration laws
and regulations, theft of client property, other criminal
activity, and torts or other claims under traditional theories
of employment liability or under co-employment or joint
employment liability. We have policies and guidelines in place
to reduce our exposure to such risks. However, failure of any
employee or
15
personnel to follow these policies and guidelines may result in
negative publicity, loss of client relationships and business,
injunctive relief, or the payment of monetary damages or fines.
Moreover, we could be held responsible for the actions at a
workplace of persons not under our immediate control. To reduce
our exposure, we also maintain insurance covering general
liability, workers compensation claims, errors and omissions and
employee theft. Due to the nature of our assignments, in which
we have access to client information systems and confidential
information, we may not be able to obtain insurance coverage in
amounts adequate to cover any liability on our part on
acceptable terms. In addition, we face various
employment-related risks not covered by insurance, such as
compliance with wage and hour laws and employment and
withholding tax responsibilities, which if not complied with
could result in significant financial penalties.
We are a
holding company and derive substantially all of our cash flow
from our subsidiaries.
We rely upon revenues and distributions from our subsidiaries to
generate the funds necessary to meet our obligations. Our
subsidiaries are separate and independent legal entities and
have no obligation, contingent or otherwise, to make funds
available to us, whether in the form of loans, dividends or
otherwise. The ability of our subsidiaries to pay dividends to
us is also subject to, among other things, the availability of
sufficient funds in such subsidiaries and applicable state or
foreign laws. Claims of creditors of our subsidiaries will
generally have priority as to the assets of such subsidiaries
over our claims and claims of our creditors and stockholders. In
addition, we have pledged the ownership interests in ReSearch
Pharmaceutical Services, LLC, our principal operating
subsidiary, to a bank as security for our line of credit, and
therefore, if we are in default of any of the provisions of our
agreement for the line of credit, our bank could foreclose on
the pledged ownership interests of ReSearch Pharmaceutical
Services, LLC. If the bank were to foreclose on the pledged
ownership interests, we would no longer be entitled to receive
revenues or distributions from our U.S. operating
subsidiaries.
Risks Related to
This Offering
An active
trading market for our common stock may not develop, and our
stockholders may not be able to resell their stock at or above
the current price.
There is currently no public market for shares of our common
stock. Our common stock and warrants were previously listed on
the London Stock Exchange’s AIM, until, at a special
meeting of our stockholders and warrant holders on
August 26, 2009, we received approval from the requisite
percentage of our stockholders to delist our common stock from
AIM. The warrant holders did not approve our proposal to delist
our warrants from AIM. On September 4, 2009, our common
stock was delisted from AIM and our nominated advisor resigned
from its position and trading of our warrants on AIM was
suspended. We did not appoint another nominated advisor within
one month of the suspension of trading of our warrants and the
listing of our warrants was cancelled on October 5, 2009.
All of our unexercised warrants expired by their own terms on
April 28, 2010.
The initial public offering price for our common stock in this
offering will be determined through negotiations between us and
the representative of the underwriters and may bear no
relationship to the price at which our common stock will trade
following the completion of this offering. Although we have
applied to have our common stock listed on The NASDAQ Global
Market, the listing of our common stock may not lead to investor
interest, and an active trading market for our stock may never
develop or may not be sustained following this offering. If an
active market does not develop, it will be difficult or
impossible for you to sell the shares of common stock you
acquire in this offering without depressing the market price of
our common stock or to sell your shares at all.
The
trading price of the shares of our common stock is likely to be
volatile, and purchasers of our common stock could incur
substantial losses.
Our stock price is likely to be volatile. The stock market in
general has experienced extreme volatility that has often been
unrelated to the operating performance of particular companies.
As a result of this volatility, investors may not be able to
sell their common stock at or above the initial public offering
price. The following factors, in
16
addition to the other risks described in this prospectus, may
have a significant impact on the market price of our common
stock:
|
|
|
| |
•
|
variations in our operating results;
|
| |
| |
•
|
announcements of new services, new clients, strategic alliances
or acquisitions by us or by our competitors;
|
| |
| |
•
|
political or regulatory developments, including the
implementation of healthcare reform measures;
|
| |
| |
•
|
economic and market conditions in our industry and the
industries of our clients;
|
| |
| |
•
|
the loss of any of our key personnel;
|
| |
| |
•
|
changes in financial estimates or recommendations by securities
analysts;
|
| |
| |
•
|
sales of large blocks of our common stock; and
|
| |
| |
•
|
sales of our common stock by our executive officers, directors
and significant stockholders.
|
Some or all of these factors may be beyond our control. In
addition, material adverse changes in the market for companies
in the biopharmaceutical and CRO industries could result in the
loss of investor confidence. Fluctuations in our stock price may
be even more pronounced in the trading market for our stock
shortly following this offering. Securities class action
litigation has often been instituted against companies following
periods of volatility in the overall market and in the market
price of a company’s securities. This litigation, if
instituted against us, could result in very substantial costs
and divert our management’s attention and resources.
We incur
increased costs as a public company, which may strain our
resources and adversely affect our operating results and
financial condition.
As a public company since 2008, we incur significant accounting,
legal and other expenses. We will continue to incur costs
associated with our public company reporting requirements, since
we will continue to be subject to the requirements of the
Securities Exchange Act of 1934, the Sarbanes-Oxley Act of 2002
and other rules and regulations, and in addition, we will be
subject to the requirements of NASDAQ after this offering. Our
management and other personnel devote a substantial amount of
time in an effort to comply with these and other public company
requirements. Moreover, these rules and regulations will
increase our legal and financial compliance costs and make some
activities more time-consuming and costly. Further, these laws
and regulations could make it more difficult or more costly for
us to obtain certain types of insurance, including director and
officer liability insurance, and we may be forced to accept
reduced policy limits and coverage or incur substantially higher
costs to obtain the same or similar coverage. The impact of
these requirements could also make it more difficult for us to
attract and retain qualified persons to serve on our Board of
Directors, its committees or as executive officers.
We have been and will be required, pursuant to Section 404 of
the Sarbanes-Oxley Act of 2002, to furnish a report by
management on, among other things, the effectiveness of our
internal control over financial reporting. This assessment will
need to include disclosure of any material weaknesses identified
by our management in our internal control over financial
reporting. We are expending significant resources to develop the
necessary documentation and testing procedures required by
Section 404. We cannot be certain that the actions we are taking
to improve our internal controls over financial reporting will
be sufficient, or that we will be able to implement our planned
processes and procedures in a timely manner.
If we are
not the subject of securities or industry analyst reports, or if
any securities analyst publishes unfavorable research about our
business, our common stock or our industry sector, the price of
our common stock could be negatively affected and trading volume
could decline.
The trading market for our common stock will depend in part on
the research and reports that securities or industry analysts
publish about us or our business. We do not currently have and
may never obtain research coverage by these analysts. There are
many publicly traded companies that provide services to the
biopharmaceutical industry, which may mean it will be less
likely that we receive analysts’ coverage, which in turn
could adversely affect the market price of our common stock. In
addition, if a securities or industry analyst downgrades the
outlook for our stock or one of our competitors’ stocks, or
if analysts cease to cover us or fail to
17
publish regular reports on us, interest in the purchase of our
common stock could decrease, which would cause the trading price
of our common stock and trading volume to be negatively
affected. Securities analysts may publish reports about our
industry containing information that may affect the trading
price of our common stock.
A
substantial number of shares of our common stock will become
eligible for resale by our existing stockholders in the public
market following this offering, and substantial amounts of such
sales may cause the trading price of our common stock to
decline, even if our business is doing well.
Sales of substantial amounts of our common stock could occur at
any time. If our stockholders sell, or the market perceives that
our stockholders intend to sell, substantial amounts of our
common stock in the public market following this offering, this
may depress the market price of our shares and affect the value
of your investment and also make it more difficult for us to
raise capital in the future.
Following completion of this
offering, shares
of our common stock will be outstanding,
and shares
will be issuable upon the exercise of outstanding stock options.
Of
the shares
outstanding upon the completion of this initial public offering,
the shares
sold pursuant to this prospectus will be freely tradable
immediately without restriction, unless such shares are
purchased by our affiliates (as such term is defined in
Rule 144 under the Securities Act).
Our directors, executive officers and certain of our other
existing stockholders have agreed to enter into
lock-up
agreements pursuant to which they have agreed not to sell shares
of our common stock in the public market for a period of
180 days after the date of this prospectus, which
lock-up
period may be extended in certain circumstances. Upon expiration
of the
lock-up
period, shares subject to such
lock-up
agreements will become freely tradable without limitation,
unless held by our affiliates. The representative of the
underwriters may also release the holders from the
lock-up
provisions at any time in its sole discretion, which would allow
for earlier sales of shares in the public market. See
“Shares Eligible for Future
Sale—Lock-Up
Agreements.”
Shares held by our affiliates will become eligible for resale
pursuant to Rule 144 following the expiration of the
lock-up
period, subject to the volume and manner of sale restrictions
set forth in Rule 144. In addition, we have filed a
registration statement on
Form S-8
which permits our current and former employees, including our
directors and officers, to resell shares they have obtained upon
exercise of options.
In addition, at any time after the expiration of the
90-day
period beginning on the date of this prospectus, our existing
stockholders will have the ability to require us to register
shares of common stock for resale, subject to specified
limitations. See “Shares Eligible for Future
Sale—Registration Rights.”
We will
have broad discretion in how we use the proceeds of this
offering and we may not use them effectively, or we may use the
proceeds in ways with which you disagree and in ways that may
not yield a return on your investment.
We will have considerable discretion in applying the net
proceeds of this offering. We anticipate that we will use the
net proceeds we receive from this offering to further develop
and expand of our service offerings globally, including, among
other things, the acquisition and subsequent growth of
businesses similar to ours in Europe and Asia, to repay existing
debt under our revolving line of credit, and for working capital
and general corporate purposes. Because of the number and
variability of factors that determine our use of proceeds from
this offering, our actual uses for the proceeds of this offering
may vary substantially from our currently planned uses.
Stockholders may not deem such uses desirable. In particular, in
order to avoid regulation as an investment company under the
Investment Company Act of 1940, as amended, and pending the use
of the proceeds in the development and expansion of our
business, we will be limited as to the ability to invest a
substantial amount of our proceeds in assets other than cash
items, such as cash, demand deposits with banks, shares of money
market funds, government securities and certain certificates of
deposit. Our short-term use of proceeds in this manner may not
yield a significant return for our stockholders.
18
You will
experience immediate dilution in the book value of the shares of
common stock you purchase in this offering.
We expect the initial public offering price of our common stock
to be substantially higher than the net tangible book value per
share of our common stock. Therefore, after giving effect to the
shares of common stock to be sold in this offering, the price
per share that you pay will be higher than the book value of
that share.
Based on an assumed initial public offering price of
$ per share, which is the midpoint
of the price range set forth on the cover page of this
prospectus, you will experience immediate dilution of
$ per share, representing the
difference between our net tangible book value per share after
giving effect to this offering and the assumed initial public
offering price. In addition, purchasers of common stock in this
offering will have contributed
approximately % of the aggregate
price paid by all purchasers of our common stock but will own
only approximately % of our common
stock outstanding after this offering.
In addition, as of June 30, 2010, we had outstanding stock
options to purchase an aggregate of 2,908,393 shares of
common stock with a weighted average exercise price of $2.07 per
share. To the extent these outstanding options are exercised,
there will be further dilution to investors in this offering.
We do not
intend to pay dividends on shares of our common stock for the
foreseeable future, and capital appreciation, if any, will be
your sole source of gains.
We have never declared or paid any cash dividends on our common
stock. We intend to use our earnings, if any, to fund the
operation, development and growth of our business, and we do not
anticipate paying any cash dividends in the foreseeable future.
In addition, the agreement governing our current line of credit
with a bank contains covenants that provide that we may not pay
any dividends other than stock dividends during the term of the
agreement. As a result, your potential gain from your investment
in our common stock will be solely the capital appreciation, if
any, of our common stock for the foreseeable future.
Our
executive officers, directors and principal stockholders own a
significant percentage of our stock and could exert significant
influence over matters requiring stockholder approval, which may
prevent new investors from influencing significant corporate
decisions.
We anticipate that upon completion of this offering, our
executive officers, directors and beneficial holders of 5% or
more of our common stock will, together with their affiliates,
beneficially own approximately % of
our common stock, or
approximately % if the underwriters
exercise their over-allotment option in full. Accordingly, our
executive officers, directors and principal stockholders and
their affiliated entities will have control over most matters
that require approval by our stockholders, including the
election of directors and approval of significant corporate
transactions. The interests of this group of stockholders may
not coincide with our interests or those of other stockholders.
Corporate action may be taken even if other stockholders,
including those who purchase shares in this offering, oppose
such action. This concentration of ownership might also have the
effect of delaying or preventing a change of control of our
company that other stockholders may view as beneficial.
Provisions
of our corporate charter documents and Delaware law may have the
effect of delaying or preventing attempts by our stockholders to
change our management or an acquisition by a third party, and
the market price of our common stock may be lower as a
result.
Our certificate of incorporation and bylaws contain several
provisions that may make it more difficult for a third party to
acquire control of our company, even if such acquisition would
be beneficial to our stockholders. For example, our certificate
of incorporation authorizes our Board of Directors to issue up
to 1,000,000 shares of “blank check” preferred
stock and to attach special rights, including voting and
dividend rights, to this preferred stock, in each case without
stockholder approval. With these rights, preferred stockholders
could make it more difficult for a third party to acquire us. In
addition, our certificate of incorporation does not provide for
cumulative voting in the election of directors and also provides
for a staggered Board of Directors, whereby directors serve for
three-year terms, with approximately one-third of the directors
coming up for re-election each year. Having a staggered board
makes it more difficult for a third party to obtain control of
our Board of Directors through a proxy contest, which may be a
necessary step in an acquisition of us that is not favored by
our Board of
19
Directors. In addition, our stockholders are not entitled to
remove directors other than by a majority vote and only for
cause.
We are also subject to the anti-takeover provisions of
Section 203 of the Delaware General Corporation Law, which
regulates corporate acquisitions. Under these provisions, if
anyone becomes an “interested stockholder,” we may not
enter into a “business combination” with that person
for three years without special approval, which could discourage
a third party from making a takeover offer or tender offer for
our stock and could delay or prevent a change of control or
changes in our management. For the purposes of Section 203,
“interested stockholder” means, generally, someone
owning 15% or more of our outstanding voting stock or an
affiliate of ours that owned 15% or more of our outstanding
voting stock during the past three years, subject to certain
exceptions as described in Section 203.
20
Cautionary Note
Regarding Forward-Looking Statements and Industry Data
This prospectus contains “forward-looking statements”
within the meaning of the Private Securities Litigation Reform
Act of 1995 that involve risks and uncertainties, many of which
are beyond our control. Our actual results could differ
materially and adversely from those anticipated in such
forward-looking statements as a result of certain factors,
including those set forth in the “Risk Factors”
section of this prospectus. Important factors that may cause
actual results, levels of activity, performance or achievements
to differ from the information expressed or implied by these
forward-looking statements include, but are not limited to:
|
|
|
| |
•
|
adverse economic conditions in general and in the
biopharmaceutical industry in particular;
|
| |
| |
•
|
future demand for our integrated solutions from the
biopharmaceutical industry;
|
| |
| |
•
|
trends in research and development spending;
|
| |
| |
•
|
intense competition in the biopharmaceutical and CRO industries,
including merger and acquisitions activity;
|
| |
| |
•
|
our ability to raise sufficient additional capital to operate
our business;
|
| |
| |
•
|
lower than expected service revenue;
|
| |
| |
•
|
unexpected costs or other liabilities;
|
| |
| |
•
|
changes in laws, rules and regulations affecting our business
and that of our clients;
|
| |
| |
•
|
our ability to predict our revenues, gross margin and operating
income accurately;
|
| |
| |
•
|
expansion of our international operations;
|
| |
| |
•
|
our ability to manage our growth;
|
| |
| |
•
|
adverse results of any legal proceedings; and
|
| |
| |
•
|
our ability to attract or retain qualified personnel, including
management, sales and marketing and scientific personnel.
|
The forward-looking statements are contained principally in the
sections entitled “Prospectus Summary,” “Risk
Factors,” “Management’s Discussion and Analysis
of Financial Condition and Results of Operations” and
“Business,” but are also contained elsewhere in this
prospectus. All statements, other than statements of historical
facts, included in this prospectus regarding our strategy,
future operations, financial position, estimated revenue or
losses, projected costs, prospects, current expectations,
forecasts, and plans and objectives of management are
forward-looking statements, and you should not place undue
reliance on them. When used in this prospectus, the words
“aim,” “will,” “may,”
“believe,” “anticipate,” “intend,”
“estimate,” “expect,” “could,”
“should,” “would,” “project,”
“predict,” “plan,” “objectives,”
“goals,” “potential,” “continue,”
“ongoing” and similar expressions, or negatives of
these words, are intended to identify forward-looking
statements, although not all forward-looking statements contain
such identifying words. All forward-looking statements speak
only as of the date of this prospectus. Although we believe that
our plans, intentions and expectations reflected in or suggested
by the forward-looking statements in this prospectus are
reasonable, we cannot assure you that these plans, intentions or
expectations will be achieved. We do not undertake any
obligation to update any forward-looking statements or other
information contained in this prospectus, whether as a result of
new information, future events or otherwise, except as required
by federal securities laws.
Information regarding market and industry statistics contained
in this prospectus is included based on information available to
us that we believe is accurate as of the date of this
prospectus. Forecasts and other forward-looking information
obtained from these sources are subject to the same
qualifications described above and the additional uncertainties
accompanying any estimates of future market size, revenue and
market acceptance of products and services. We undertake no
obligation to update forward-looking information to reflect
actual results or changes in assumptions or other factors that
could affect those statements. See the “Risk Factors”
section of this prospectus for a more detailed discussion of
uncertainties and risks that may have an impact on our future
results.
21
Use of
Proceeds
We estimate that our net proceeds from the sale of shares of our
common stock in this offering, after deducting underwriting
discounts and commissions and offering expenses payable by us,
will be approximately
$ million, or
$ million if the underwriters
exercise their overallotment option in full, based upon an
assumed initial public offering price of
$ per share, which is the midpoint
of the range set forth on the cover page of this prospectus. We
will not receive any proceeds from the sale of shares by the
selling stockholders.
A $1.00 increase or decrease in the assumed initial public
offering price of $ per share
would increase or decrease, as applicable, our net proceeds by
$ , assuming that the number of
shares offered by us, as set forth on the cover page of this
prospectus, remains the same and after deducting the
underwriting discounts and commissions and our estimated
offering expenses.
A share
increase or decrease in the number of shares of common stock
that we sell in this offering would increase or decrease, as
applicable, our net proceeds by $ .
The primary purposes of this offering are to create a public
market for our common stock, to facilitate future access to the
public equity markets and to obtain additional capital. We
anticipate that we will use the proceeds of this offering as
follows:
|
|
|
| |
•
|
to repay the outstanding principal and interest balance under
our working capital line of credit; and
|
| |
| |
•
|
the remainder for additional working capital and general
corporate purposes, including to promote the further development
and expansion of our service offerings globally.
|
In addition, we may use a portion of the net proceeds to acquire
complementary businesses or assets, although we currently have
no agreements or commitments with respect to any acquisitions.
The anticipated uses of net proceeds from this offering
represent our intentions based upon our current plans and
business conditions. The amounts and timing of our actual
expenditures may vary significantly depending on numerous
factors, including the outstanding principal and interest
balance under our working capital line of credit. Our working
capital line of credit matures on October 31, 2012. It had
a principal and interest balance of approximately
$10.9 million at June 30, 2010 and bears interest at
the Federal funds open rate plus 2%, or 4.75% at June 30,
2010. Our actual expenditures may also vary significantly
depending on the extent to which we acquire or invest in
businesses, assets, and technologies; the global market for our
service offerings; cash flows from operations; the anticipated
growth of our business; and any unforeseen or underestimated
cash needs and other factors. As a result, our management will
retain broad discretion over the allocation of the net proceeds
from this offering.
Pending our use of the net proceeds of this offering, we intend
to invest the net proceeds in short-term, interest-bearing,
investment-grade liquid investments, such as demand deposits
with banks, shares of money market funds, guaranteed obligations
of the U.S. government and certificates of deposit.
Dividend
Policy
We have never declared or paid any cash dividends on our common
stock. We currently intend to retain any future earnings to
finance the operation, development and expansion of our business
and we do not anticipate paying any cash dividends in the
foreseeable future. In addition, the agreement governing our
line of credit provides that we may not pay any dividends other
than stock dividends during the term of the agreement, which
expires on October 31, 2012.
22
Capitalization
The following table sets forth our cash and cash equivalents,
short-term debt and capitalization at June 30, 2010:
|
|
|
| |
•
|
on an actual basis; and
|
| |
| |
•
|
on an as adjusted basis to give effect to our sale
of shares
of common stock in this offering at an assumed initial public
offering price of $ per share,
which is the midpoint of the range set forth on the cover page
of this prospectus, and our receipt of the estimated net
proceeds after deducting underwriting discounts and commissions
and offering expenses payable by us, and our use of a portion of
the net proceeds to repay in full the outstanding balance under
our working capital line of credit, which was $10.9 million
as of June 30, 2010.
|
The as adjusted information below is illustrative only, and our
capitalization following the completion of this offering will be
adjusted based on the actual initial public offering price and
other terms of this offering determined at pricing. You should
read this table in conjunction with “Use of Proceeds,”
“Selected Consolidated Financial Data,”
“Management’s Discussion and Analysis of Financial
Condition and Results of Operations,” “Description of
Capital Stock” and our financial statements and related
notes included elsewhere in this prospectus.
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
As of June 30, 2010
|
|
|
|
Actual
|
|
As Adjusted
|
|
|
|
(unaudited)
|
|
|
|
(in thousands, except per share data)
|
|
|
|
Cash and cash equivalents
|
|
$
|
4,252
|
|
|
$
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Line of credit
|
|
$
|
10,861
|
|
|
$
|
|
|
|
Stockholders’ equity:
|
|
|
|
|
|
|
|
|
|
Common stock, $0.0001 par value: Authorized
shares—150,000,000, issued and outstanding
shares—37,216,052
actual; as
adjusted
|
|
|
4
|
|
|
|
|
|
|
Additional paid-in capital
|
|
|
45,896
|
|
|
|
|
|
|
Accumulated other comprehensive loss
|
|
|
(4,165
|
)
|
|
|
|
|
|
Retained earnings
|
|
|
2,116
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total stockholders’ equity
|
|
|
43,851
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total capitalization
|
|
$
|
54,712
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Each $1.00 increase or decrease in the assumed initial public
offering price of $ per share,
which is the midpoint of the range set forth on the cover of
this prospectus, would increase or decrease, as applicable, our
as adjusted cash and cash equivalents, additional paid-in
capital, total stockholders’ equity and total
capitalization by $ , assuming that
the number of shares offered by us, as set forth on the cover of
this prospectus, remains the same. Assuming this initial public
offering price per share,
a share
increase or decrease in the number of shares of common stock
that we sell in this offering would increase or decrease, as
applicable, our as adjusted cash and cash equivalents,
additional paid-in capital, total stockholders’ equity and
total capitalization by $ .
The number of shares of our common stock outstanding in the
table above does not include:
|
|
|
| |
•
|
2,908,393 shares of common stock issuable upon the exercise
of outstanding options as of June 30, 2010, with a weighted
average exercise price of $2.07 per share; and
|
| |
| |
•
|
3,788,452 shares of common stock reserved for future
issuance under our 2007 Equity Incentive Plan.
|
23
Dilution
If you invest in our common stock, your interest will be diluted
to the extent of the difference between the initial public
offering price per share of our common stock and the net
tangible book value per share of our common stock immediately
after this offering. Net tangible book value per share
represents the amount of our total tangible assets less total
liabilities, divided by the number of shares of common stock
outstanding.
Investors participating in this offering will incur immediate
and substantial dilution. Our net tangible book value was
$27.8 million, or approximately $0.75 per share of common
stock, at June 30, 2010. After giving effect to the sale by
us of
the shares
of common stock offered by us in this offering at an assumed
initial public offering price of $
per share, which is the midpoint of the range set forth on the
cover of this prospectus, and after deducting estimated
underwriting discounts and commissions and estimated offering
expenses, our as adjusted net tangible book value at
June 30, 2010, would have been
$ million, or approximately
$ per share of common stock. This
represents an immediate increase in net tangible book value of
$ per share of common stock to our
existing stockholders and an immediate dilution in net tangible
book value of $ per share to the
new investors purchasing shares in this offering at the assumed
initial public offering price. We determine dilution to new
investors participating in this offering by subtracting the net
tangible book value per share after this offering from the
amount of cash that a new investor paid for a share of common
stock in this offering.
The following table illustrates this per share dilution:
| |
|
|
|
|
|
|
|
|
|
Assumed initial public offering price per share
|
|
|
|
|
|
$
|
|
|
|
Net tangible book value per share at June 30, 2010
|
|
$
|
0.75
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net increase in tangible book value per share attributable to
this offering
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net tangible book value per share after this offering
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Dilution per share to new investors
|
|
|
|
|
|
$
|
|
|
|
|
|
|
|
|
|
|
|
|
The dilution information discussed above is illustrative only
and will change based on the actual initial public offering
price and other terms of this offering determined at pricing.
The information in the preceding table has been calculated using
an assumed initial public offering price of
$ per share, which is the midpoint
of the estimated price range set forth on the cover page of this
prospectus. A $1.00 increase or decrease in the assumed initial
public offering price per share would decrease or increase, as
applicable, the net tangible book value per share of common
stock after this offering by $ per
share and increase or decrease, as applicable, the dilution per
share of common stock to new investors in this offering by
$ per share, in each case
calculated as described above and assuming that the number of
shares offered by us, as set forth on the cover page of this
prospectus, remains the same. Likewise, the information in the
preceding table has been calculated assuming that we issue a
number of shares of common stock in this offering equal to the
number of shares appearing on the cover of this prospectus.
A share
increase or decrease in the number of shares of common stock
that we issue in this offering would decrease or increase, as
applicable, the net tangible book value per share of common
stock after this offering by $ per
share and increase or decrease, as applicable, the dilution per
share of common stock to new investors in this offering by
$ per share, in each case
calculated as described above and assuming an initial public
offering price of $ per share.
24
The following table sets forth on the basis described above, as
of June 30, 2010, the number of shares of common stock
purchased or to be purchased from us, the total consideration
paid or to be paid and the weighted average price per share paid
or to be paid by existing holders of common stock, and by the
new investors in this offering, at an assumed initial public
offering price of $ per share,
which is the midpoint of the estimated price range set forth on
the cover page of this prospectus, and before deducting
estimated underwriting discounts and commissions and estimated
offering expenses payable by us.
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Shares Purchased
|
|
Total Consideration
|
|
Weighted Average
|
|
|
|
Number
|
|
Percent
|
|
Amount
|
|
Percent
|
|
Price per Share
|
|
|
|
Existing Stockholders
|
|
|
|
|
|
|
|
%
|
|
$
|
|
|
|
|
|
%
|
|
$
|
|
|
|
New Investors in this Offering
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total
|
|
|
|
|
|
|
100
|
%
|
|
$
|
|
|
|
|
100
|
%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The discussion and tables above assume no exercise of the
underwriters’ overallotment option. If the
underwriters’ overallotment option is exercised in full:
|
|
|
| |
•
|
The number of shares of common stock held by existing
stockholders will represent % of
the total number of shares of common stock to be outstanding
after this offering and the number of shares of common stock
held by new investors participating in this offering will
represent % of the total number of
shares of common stock to be outstanding after this
offering; and
|
|
|
|
| |
•
|
Our net tangible book value at June 30, 2010 would have
been $ million, or
$ per share of common stock,
representing an immediate increase in net tangible book value of
$ per share of common stock to our
existing stockholders and an immediate dilution of
$ per share to new investors
participating in this offering.
|
As of June 30, 2010, there were options outstanding to
purchase 2,908,393 shares of our common stock, with
exercise prices ranging from $0.37 to $5.05 per share and a
weighted average exercise price of $2.07 per share. The tables
and calculations above assume that those options have not been
exercised. To the extent outstanding options are exercised, you
would experience further dilution if the exercise price is less
than our net tangible book value per share. If all of the
outstanding options as of June 30, 2010 had been exercised,
our net tangible book value would have been
$ per share of common stock, net
tangible book value after this offering would be
$ per share of common stock and
dilution in net tangible book value to new investors in this
offering would be $ per share of
common stock. In addition, if all of the outstanding options as
of June 30, 2010 were so exercised as of such date, before
deducting underwriting discounts and commissions and our
estimated offering expenses, (i) existing stockholders
would have
purchased shares
representing % of the total shares
for $ , or
approximately % of the total
consideration paid, with an average price per share of
$ and (ii) using an assumed
public offering price of $ per
share, shares purchased by new stockholders in this offering
would represent approximately % of
the total shares for approximately
$ , or
approximately % of the total
consideration paid.
If we grant options, warrants, preferred stock, or other
convertible securities or rights to purchase our common stock in
the future with exercise prices below the initial public
offering price, new investors will incur additional dilution
upon exercise of such securities or rights.
25
Price Range of
Our Common Stock
Our common stock is not currently traded on any established
public trading market. Our common stock, along with warrants to
purchase common stock that have since expired, were traded on
the Alternative Investment Market of the London Stock Exchange,
or AIM, under the symbols “RPSE” for our common stock
and “RPSW” for our warrants until September 4,
2009 and October 4, 2009, respectively. At a special
meeting of stockholders and warrant holders on August 26,
2009, we received approval from the requisite percentage of our
stockholders to delist our common stock from AIM. The warrant
holders did not approve our proposal to delist our warrants from
trading on AIM. Upon the date of delisting of our common stock
from AIM, our nominated advisor resigned from its position, and
the trading of our warrants on AIM was suspended. We did not
appoint another nominated advisor within one month after the
suspension of our warrants from trading on AIM, and as a result
the listing of our warrants on AIM was cancelled on
October 4, 2009. Due to the limited quotations of our
common stock and warrants on AIM while listed, AIM was not
considered an established public trading market under
U.S. securities laws.
As of September 30, 2010, there were approximately 101
record holders of our common stock. Because some of our common
stock may be held by brokers and other institutions on behalf of
stockholders, we are unable to estimate the total number of
beneficial holders represented by these record holders.
The initial public offering price for the common stock being
offered by this prospectus will be determined by negotiation
between us and the underwriters based on a number of factors
which are described in “Underwriting.”
26
Selected
Consolidated Financial Data
The following tables set forth certain of our selected
historical consolidated financial data and should be read
together with our consolidated financial statements and related
notes to those statements, as well as “Management’s
Discussion and Analysis of Financial Condition and Results of
Operations,” each included elsewhere in this prospectus. We
have derived the consolidated statement of operations data for
each of the years ended December 31, 2007, 2008 and 2009
and the consolidated balance sheet data as of December 31,
2008 and 2009 from our audited consolidated financial statements
included elsewhere in this prospectus. We have derived the
consolidated statement of operations data for each of the years
ended December 31, 2005 and 2006 and the balance sheet data
as of December 31, 2005, 2006 and 2007 from our audited
consolidated financial statements not included in this
prospectus. We have derived the consolidated statement of
operations data for the six months ended June 30, 2009 and
June 30, 2010 and the consolidated balance sheet data as of
June 30, 2010 from our unaudited consolidated financial
statements included in this prospectus. These interim financial
statements reflect all adjustments, consisting of normal
recurring adjustments, which are necessary in the opinion of
management to present fairly our consolidated financial position
and results of operations for the interim periods. Our
historical financial results are not necessarily indicative of
financial results that may be expected in future periods, and
our financial results for any interim period are not necessarily
indicative of financial results that may be expected for a full
fiscal year.
A description of the merger between Old RPS and Cross Shore in
August 2007 and the accounting treatment thereof is further
described in Note 2 to our consolidated financial
statements, beginning on
page F-8
of this prospectus.
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Six Months
|
|
|
|
Year Ended December 31,
|
|
Ended June 30,
|
|
|
|
2005
|
|
2006
|
|
2007
|
|
2008
|
|
2009
|
|
2009
|
|
2010
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(unaudited)
|
|
|
|
(in thousands, except per share data)
|
|
|
|
Consolidated Statement of Operations Data:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Service revenue
|
|
$
|
62,799
|
|
|
$
|
84,418
|
|
|
$
|
120,459
|
|
|
$
|
156,967
|
|
|
$
|
200,472
|
|
|
$
|
93,705
|
|
|
$
|
122,409
|
|
|
Reimbursement revenue
|
|
|
8,074
|
|
|
|
10,273
|
|
|
|
13,924
|
|
|
|
18,086
|
|
|
|
23,696
|
|
|
|
10,940
|
|
|
|
15,500
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total revenue
|
|
|
70,873
|
|
|
|
94,961
|
|
|
|
134,383
|
|
|
|
175,052
|
|
|
|
224,168
|
|
|
|
104,646
|
|
|
|
137,909
|
|
|
Direct costs
|
|
|
45,744
|
|
|
|
61,365
|
|
|
|
87,650
|
|
|
|
117,707
|
|
|
|
145,209
|
|
|
|
68,160
|
|
|
|
88,732
|
|
|
Reimbursable
out-of-pocket
costs
|
|
|
8,074
|
|
|
|
10,273
|
|
|
|
13,924
|
|
|
|
18,086
|
|
|
|
23,696
|
|
|
|
10,940
|
|
|
|
15,500
|
|
|
Selling, general, and administrative expenses
|
|
|
16,747
|
|
|
|
19,070
|
|
|
|
26,787
|
|
|
|
31,290
|
|
|
|
44,798
|
|
|
|
21,091
|
|
|
|
25,671
|
|
|
Depreciation and amortization
|
|
|
864
|
|
|
|
901
|
|
|
|
1,144
|
|
|
|
1,750
|
|
|
|
3,723
|
|
|
|
1,671
|
|
|
|
2,202
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Income (loss) from operations
|
|
|
(556
|
)
|
|
|
3,082
|
|
|
|
4,879
|
|
|
|
6,219
|
|
|
|
6,742
|
|
|
|
2,784
|
|
|
|
5,804
|
|
|
Interest and other income (expense), net
|
|
|
(1,127
|
)
|
|
|
(1,245
|
)
|
|
|
(5,786
|
)
|
|
|
42
|
|
|
|
(562
|
)
|
|
|
(303
|
)
|
|
|
(396
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Income (loss) before provision for income taxes
|
|
|
(1,683
|
)
|
|
|
1,837
|
|
|
|
(907
|
)
|
|
|
6,261
|
|
|
|
6,180
|
|
|
|
2,481
|
|
|
|
5,409
|
|
|
Provision for income taxes
|
|
|
—
|
|
|
|
45
|
|
|
|
1,508
|
|
|
|
2,518
|
|
|
|
3,559
|
|
|
|
1,492
|
|
|
|
3,953
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income (loss)
|
|
|
(1,683
|
)
|
|
|
1,792
|
|
|
|
(2,415
|
)
|
|
|
3,743
|
|
|
|
2,620
|
|
|
|
989
|
|
|
|
1,456
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accretion of preferred stock
|
|
|
(485
|
)
|
|
|
(485
|
)
|
|
|
(321
|
)
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income (loss) applicable to common shares
|
|
$
|
(2,168
|
)
|
|
$
|
1,307
|
|
|
$
|
(2,736
|
)
|
|
$
|
3,743
|
|
|
$
|
2,620
|
|
|
$
|
989
|
|
|
$
|
1,456
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income (loss) per common share:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic
|
|
$
|
(0.39
|
)
|
|
$
|
0.24
|
|
|
$
|
(0.19
|
)
|
|
$
|
0.11
|
|
|
$
|
0.07
|
|
|
$
|
0.03
|
|
|
$
|
0.04
|
|
|
Diluted
|
|
$
|
(0.39
|
)
|
|
$
|
0.08
|
|
|
$
|
(0.19
|
)
|
|
$
|
0.11
|
|
|
$
|
0.07
|
|
|
$
|
0.03
|
|
|
$
|
0.04
|
|
|
Weighted average number of shares outstanding
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic
|
|
|
5,506
|
|
|
|
5,502
|
|
|
|
14,573
|
|
|
|
32,617
|
|
|
|
37,003
|
|
|
|
36,747
|
|
|
|
37,272
|
|
|
Diluted
|
|
|
5,506
|
|
|
|
15,484
|
|
|
|
14,573
|
|
|
|
34,103
|
|
|
|
38,071
|
|
|
|
37,708
|
|
|
|
38,676
|
|
|
|
|
|
27
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
As of
|
|
|
|
As of December 31,
|
|
June 30,
|
|
|
|
2005
|
|
2006
|
|
2007
|
|
2008
|
|
2009
|
|
2010
|
|
|
|
|
|
|
|
(in thousands)
|
|
|
|
(unaudited)
|
|
|
|
Consolidated Balance Sheet Data:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents
|
|
$
|
540
|
|
|
$
|
197
|
|
|
$
|
11,060
|
|
|
$
|
6,565
|
|
|
$
|
3,468
|
|
|
$
|
4,252
|
|
|
Working capital
|
|
|
4,351
|
|
|
|
6,603
|
|
|
|
31,355
|
|
|
|
24,608
|
|
|
|
21,601
|
|
|
|
23,149
|
|
|
Total assets
|
|
|
17,983
|
|
|
|
26,124
|
|
|
|
50,419
|
|
|
|
87,089
|
|
|
|
96,261
|
|
|
|
94,558
|
|
|
Line of credit
|
|
|
4,840
|
|
|
|
8,992
|
|
|
|
—
|
|
|
|
7,500
|
|
|
|
9,566
|
|
|
|
10,861
|
|
|
Capital lease obligations
|
|
|
73
|
|
|
|
21
|
|
|
|
950
|
|
|
|
1,555
|
|
|
|
804
|
|
|
|
618
|
|
|
Long-term debt
|
|
|
3,980
|
|
|
|
4,165
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
Redeemable convertible preferred stock
|
|
|
7,517
|
|
|
|
8,002
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
Total stockholders’ equity (deficit)
|
|
|
(5,788
|
)
|
|
|
(4,350
|
)
|
|
|
30,429
|
|
|
|
42,282
|
|
|
|
46,306
|
|
|
|
43,851
|
|
|
|
|
|
28
Management’s
Discussion and Analysis of
Financial Condition and Results of Operations
You should read the following discussion and analysis of our
financial condition and results of operations together with the
“Selected Consolidated Financial Data” section of this
prospectus and our audited and unaudited consolidated financial
statements and the related notes to those statements that appear
elsewhere in this prospectus. In addition to historical
information, this discussion contains forward-looking statements
reflecting our current plans, estimates, beliefs and
expectations that involve risks and uncertainties. Actual
results may differ materially from those discussed or
anticipated in these forward-looking statements due to a number
of important factors, including those set forth in the sections
entitled “Risk Factors” and “Cautionary Note
Regarding Forward-Looking Statements and Industry Data” and
elsewhere in this prospectus.
Introduction
Management’s discussion and analysis of financial
condition, changes in financial condition and results of
operations is provided as a supplement to the accompanying
consolidated financial statements and notes to help provide an
understanding of our financial condition and results of
operations. This discussion is organized as follows:
|
|
|
| |
•
|
Overview. This section provides a general description of
our business, the components of our operating results and
anticipated trends that we expect to affect our financial
condition and results of operations.
|
| |
| |
•
|
Critical Accounting Policies and Estimates. This section
discusses accounting policies that we consider to be important
to our financial condition and results of operations, require
significant judgment or require estimates on our part in
applying them. Our significant accounting policies, including
those we consider to be critical accounting policies, are
discussed in Note 2 to the accompanying consolidated
financial statements.
|
| |
| |
•
|
Results of Operations. This section provides an analysis
of our results of operations for the six months ended
June 30, 2010 and 2009 and for the three years in the
period ended December 31, 2009.
|
| |
| |
•
|
Liquidity and Capital Resources. This section provides an
analysis of our cash flows for the six months ended
June 30, 2010 and 2009 and the three years in the period
ended December 31, 2009, as well as a discussion of our
capital requirements and the resources available to us to meet
those requirements.
|
Overview
We are a global, next-generation CRO providing a broad range of
clinical development solutions and services to biopharmaceutical
companies. Our services support the design, initiation and
management of our clients’ clinical trials programs that
are required to obtain regulatory approval to market
biopharmaceutical products.
We offer a comprehensive suite of outsourced solutions focused
on Phases II through IV of the clinical development
process, which encompasses late-stage and post-marketing
clinical trials. We provide our services both as integrated
solutions within a client’s internal clinical development
operations, with an ability to work across multiple clinical
trials, product candidates and clinical development functions,
and on a more traditional project basis. We also offer hybrid
solutions combining our integrated and project-based offerings.
Our flexible model enables us to tailor our services to the
differing needs of small, mid-sized, and large biopharmaceutical
companies. We believe that the combination of our clinical
expertise and our extensive personnel resourcing capabilities
enables us to achieve cost savings, improvements in the quality
of clinical trial execution and accelerated clinical timelines
on behalf of our clients.
International
Expansion and Acquisitions
We began our investment in global expansion in 2005 through 2007
with the opening of international offices in Canada, Mexico,
Argentina, Brazil, Colombia and Chile. In December 2008, we
completed the acquisition of three CROs located in France,
Germany, and Spain, and in July 2009, we completed the
acquisition of Paramax International Inc., a CRO located in
China. We have accounted for each of these acquisitions as a
purchase, and, accordingly, the results of operations of the
acquired company have been included in our consolidated
financial
29
statements commencing on the dates of the respective
acquisitions. We believe that these acquisitions provide us with
further expansion opportunities in Europe and the Asia-Pacific
region and complement our operations in North America and Latin
America. We expect that our international expansion will enable
us to meet the growing global needs of our clients in the
rapidly expanding market for integrated clinical research
services.
Merger
with Cross Shore and Basis of Presentation
Our predecessor company, Old RPS, merged with and into a wholly
owned subsidiary of Cross Shore. As a result of the merger, Old
RPS became a limited liability company organized under the laws
of Delaware under the name Research Pharmaceutical Services, LLC
and Cross Shore changed its name to ReSearch Pharmaceutical
Services, Inc. We are now a holding company for, and conduct all
of our operations through, our wholly owned subsidiary, Research
Pharmaceutical Services, LLC. When we discuss our business and
our financial results throughout this prospectus, we are
referring to Old RPS for periods prior to August 30, 2007
and to our combined company for periods after that date.
The merger between Old RPS and Cross Shore was accounted for
under the purchase method of accounting as a reverse acquisition
in accordance with GAAP. Under this method of accounting, Cross
Shore was treated as the “acquired” company for
financial reporting purposes. Accordingly, for accounting
purposes, the merger was treated as the equivalent of Old RPS
issuing stock for the net assets of Cross Shore, which amounted
to $50.6 million and consisted of cash and investments of
$51.3 million, $600,000 of other assets, and
$1.3 million of accrued transaction fees. The purchase
price was allocated to the assets acquired and liabilities
assumed based upon their respective fair values as of the date
of the merger. All operating results shown in our financial
statements for periods prior to August 30, 2007, the date
of the merger, reflect solely the operations of Old RPS.
Components
of Operating Results
|
|
|
| |
•
|
Service Revenue. We derive our service revenue from
contracts with biopharmaceutical companies under which we
provide clinical development services. Many of our contracts
with our clients are based on fixed hourly or monthly fees per
professional, plus reimbursable expenses. Some of our contracts
are for a flat fee, subject to fixed or formulaic inflation
adjustments. In addition, certain of our contracts are
units-based contracts, whereby revenues are recognized based on
the units completed multiplied by the applicable contract
per-units
price. Some of our fees are contractually capped. In some cases,
our contracts contain provisions providing for increased
discounts as the fees increase. In cases where the contracts are
set at a fixed price, we generally bear the cost of overruns,
but we benefit if the costs are lower than we anticipated.
Contracts may range in duration from a few months to several
years or longer depending on the nature of the services we
provide. In some cases, a portion of the contract fee is paid at
the time the study or trial is started, with the balance of the
contract fee payable over the course of the study.
|
Many of our contracts may be terminated by the client either
immediately or upon short notice, typically 30 to 120 days.
In the case of early termination, these contracts typically
require payment to us of expenses to wind down a program and
payment of our fees earned to date.
Our backlog consists of anticipated service revenue from
executed contracts that either have not started but are
anticipated to begin in the near future, or are in process and
have not been completed. Amounts included in backlog represent
anticipated future service revenue, excluding revenues that have
been recognized previously, and have been adjusted for foreign
currency fluctuations. Once contracted work begins, service
revenue is recognized over the life of the contract. We do not
include potential reimbursement revenue or investigator fees in
our backlog. We cannot assure investors that we will fully
realize our entire backlog in the future or at a rate consistent
with historic levels.
For the years ended December 31, 2007, 2008 and 2009, our
service revenue was $120.5 million, $157.0 million and
$200.5 million, respectively. We expect that our service
revenue will increase as we continue to expand our operations.
|
|
|
| |
•
|
Reimbursement Revenue and Reimbursable
Out-of-Pocket
Costs. Under our service contracts, we receive
reimbursements for our
out-of-pocket
expenses from the client. We account for expense reimbursements
as revenue in the statement of operations. We also record an
equal amount of offsetting expense in the
|
30
|
|
|
| |
|
statement of operations, characterized as reimbursable
out-of-pocket
costs, in each period. We exclude fees paid to clinical
investigators from our reimbursement revenue and our
reimbursable
out-of-pocket
expenses because these fees are funded from our clients’
restricted cash and are recorded on a “pass-through
basis” without risk or reward to us.
|
|
|
|
| |
•
|
Direct Costs. Direct costs consist of amounts necessary
to carry out our revenue-generating activities, including direct
labor and related benefits charges. Direct cost levels are
correlated with changes in our service revenue levels. As our
revenues increase, we expect that our direct costs will also
increase, although we aim to reduce direct costs as a percentage
of our service revenue as we increase operational efficiencies.
|
| |
| |
•
|
Selling, General and Administrative Expense. Selling,
general and administrative expense, or SG&A, consists
primarily of administrative payroll and related benefit charges,
stock-based compensation expense, sales, advertising and
promotional expenses, recruiting and relocation expenses and
overhead costs such as information technology and rent expense.
We expect that SG&A will continue to increase in absolute
dollars, although we expect that such expenses will decline as a
percentage of our service revenue as we leverage our revenue
growth and existing infrastructure.
|
| |
| |
•
|
Depreciation and Amortization. Depreciation represents
the depreciation charged on our fixed assets. We record
depreciation using the straight-line method, based on estimated
useful lives of one to seven years. Amortization expense
consists of amortization costs recorded on identified
finite-lived intangible assets, acquired in our international
acquisitions, on a straight-line method over their estimated
useful lives. We expect that our depreciation and amortization
expense will increase to the extent that we continue to make
additional investments in our global expansion.
|
| |
| |
•
|
Interest Income (Expense). Interest income represents
interest earned on our cash and cash equivalents. Interest
expense consists primarily of the interest accrued on
outstanding borrowings under our lines of credit with commercial
banks.
|
| |
| |
•
|
Income Tax Expense. Income tax expense consists of
U.S. federal, state and foreign income taxes. We are
required to pay income taxes in jurisdictions in which we have
operations and in certain foreign jurisdictions.
|
Critical
Accounting Policies and Estimates
Our consolidated financial statements are prepared in accordance
with GAAP, which requires our management to make estimates and
assumptions about future events that affect the amounts reported
in the financial statements and the accompanying notes. Actual
results could differ from these estimates. Our significant
accounting policies are also discussed in Note 2 to the
audited financial statements beginning on
page F-1
of this prospectus. The following discussion highlights what we
believe to be the critical accounting policies and judgments
made in the preparation of these consolidated financial
statements.
Revenue
and Cost Recognition
Our service revenue is derived from
fee-for-service
contracts, some of which are fixed price contracts. We recognize
revenues and the related direct costs of
fee-for-service
contracts in the period in which services are performed. We
recognize fixed price contract revenue on a proportional
performance basis based on the rate that costs incurred to date
bear to estimated total costs at completion. We also recognize
revenue under units-based contracts by multiplying units
completed by the applicable contract
per-unit
price. We recognize revenue related to contract modifications
when realization is assured and the amounts are reasonably
determinable. We make adjustments to contract estimates in the
periods in which the facts that require the revisions become
known. When the revised estimate indicates a loss, the loss is
provided for in the financial statements during that period.
Deferred revenue represents amounts billed to clients in excess
of revenues recognized.
Income
Taxes
We account for income taxes using the asset and liability
approach, which requires that deferred tax assets and
liabilities be recognized using enacted tax rates for the effect
of temporary differences between the book and tax bases of
recorded assets and liabilities. This approach also requires
that deferred tax assets be reduced by a
31
valuation allowance if it is more likely than not that some
portion or the entire deferred tax asset will not be realized.
We evaluate whether our deferred tax assets are realizable on an
ongoing basis by assessing the valuation allowance and by
adjusting the amount of such allowance, if necessary. The
factors used to assess the likelihood of realization include our
forecast of future taxable income and available tax planning
strategies that could be implemented to realize the net deferred
tax assets. Failure to achieve forecasted taxable income might
affect the ultimate realization of the net deferred tax assets.
Effective January 1, 2007, we adopted Financial Accounting
Standards Board, or FASB, guidance related to accounting for
uncertainty in income taxes. This authoritative interpretation
clarified and standardized the manner by which companies are
required to account for uncertain income tax positions. Under
this guidance, we may recognize the tax benefit from an
uncertain tax position only if it is more likely than not to be
sustained upon examination based on the technical merits of the
position. The amount of the accrual for which an exposure exists
is measured as the largest amount of benefit determined on a
cumulative probability basis that we believe is more likely than
not to be realized upon ultimate settlement of the position.
Our provision for income taxes and the determination of the
resulting deferred tax assets and liabilities involve a
significant amount of our management’s judgment.
Management’s judgments, assumptions and estimates relative
to the current provision for income tax take into account
current tax laws, our interpretation of current tax laws and
possible outcomes of current and future audits conducted by
foreign and domestic tax authorities. We operate within federal,
state and international taxing jurisdictions and we are subject
to audit in those jurisdictions. These audits can involve
complex issues, which may require an extended period of time to
resolve.
Stock-Based
Compensation
FASB guidance requires all stock-based payments to employees,
including grants of employee stock options, to be recognized in
the financial statements based on their fair values as of the
date of grant. This guidance requires that an entity measure the
cost of equity-based service awards based on the grant-date fair
value of the award and then recognize the cost of the award over
the vesting period during which the employee is required to
provide services to earn the award.
We estimated the weighted-average fair value of the options
granted during the year ended December 31, 2007, 2008 and
2009 to be $1.70, $1.96 and $0.87 per share, respectively, and
the weighted-average fair value of options granted during the
six months ended June 30, 2009 and 2010 to be $0.86 and
$1.78 per share, respectively, using the Black-Scholes
option-pricing model with the following assumptions, which are
based upon our history or industry comparative information:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Six Months Ended
|
|
|
|
Year Ended December 31,
|
|
June 30,
|
|
|
|
2007
|
|
2008
|
|
2009
|
|
2009
|
|
2010
|
|
|
|
Expected dividend yield
|
|
|
0.00
|
%
|
|
|
0.00
|
%
|
|
|
0.00
|
%
|
|
|
0.00
|
%
|
|
|
0.00
|
%
|
|
Expected volatility
|
|
|
52
|
%
|
|
|
50
|
%
|
|
|
50
|
%
|
|
|
50
|
%
|
|
|
50
|
%
|
|
Risk-free interest rate
|
|
|
3.34
|
%
|
|
|
3.01
|
%
|
|
|
2.19
|
%
|
|
|
1.92
|
%
|
|
|
2.21
|
%
|
|
Expected life
|
|
|
6 years
|
|
|
|
6 years
|
|
|
|
6 years
|
|
|
|
6 years
|
|
|
|
6 years
|
|
|
|
Prior to August 30, 2007, our common stock was not publicly
traded, and the expected volatility was calculated as of each
grant date based on an alternative method, or calculated value.
As of August 30, 2007, our common stock and warrants to
purchase our common stock were listed on the Alternative
Investment Market of the London Stock Exchange, or AIM, although
we continued to utilize the calculated value for expected
volatility because a sufficient level of history as a publicly
traded company was not available.
In September and October 2009, in anticipation of a potential
listing in the United States, we delisted our common stock and
warrants from AIM, respectively, and our common stock and
warrants are no longer publicly traded. Accordingly, we will
continue to use the calculated value in connection with our
stock-based awards as long as our stock is not listed on a
national securities exchange. We have identified similar public
entities for which share price information is available, and we
have considered the historical volatility of these
entities’ share prices in determining our estimated
expected volatility. We used the average volatility of these
guideline companies over a
32
six-year period, consistent with the expected term calculated
pursuant to FASB guidance. From August 30, 2007 through the
September 2009 AIM delisting date, we utilized our quoted stock
price on the AIM as the sole determinant of the fair value of
our common stock. Subsequent to the AIM delisting date, we
estimate the fair value of our common stock using the market and
income valuation approaches, with the assistance of a valuation
consultant.
For the years ended December 31, 2007, 2008 and 2009 and
the six months ended June 30, 2009 and 2010, stock-based
compensation expense amounted to approximately $212,000,
$569,000, $597,000, $309,000 and $294,000, respectively. We
recognize the compensation expense of such stock-based service
awards on a straight-line basis. Total compensation cost of
options granted but not yet vested as of June 30, 2010 was
$327,000, net of estimated forfeitures, which is expected to be
recognized over the weighted average remaining vesting period of
1.7 years.
Valuation
of Long-lived Assets
Intangible assets consist primarily of non-compete agreements,
customer contracts and lists, brand names and goodwill. The
majority of the intangible asset balances consist of intangible
assets acquired from our European acquisitions in 2008 and the
Paramax acquisition in 2009. We amortize finite-lived intangible
assets on a straight-line basis over the following periods:
Customer lists—three to five years, brand names—two
years, and non-compete agreements—three to six years.
Goodwill represents the excess of the cost over the fair value
of net assets acquired in a business combination. If we
determine that the carrying value of definite lived long-lived
assets may not be recoverable based upon the existence of one or
more indicators of impairment, we perform an undiscounted cash
flow analysis to determine if impairment exists. If impairment
exists, we measure the impairment based on the difference
between the asset’s carrying amount and its fair value.
Goodwill is tested for impairment on an annual basis as of
October 1 of each year and more frequently if an event occurs or
circumstances change that would more likely than not reduce our
fair value below the carrying value. If our fair value is less
than the carrying value, goodwill may be impaired, in which case
we write it down to the estimated fair market value, if
necessary.
Results of
Operations
Six
Months Ended June 30, 2010 Compared to the Six Months Ended
June 30, 2009
Revenues. Service revenue increased by
$28.7 million, or 30.6%, to $122.4 million for the six
months ended June 30, 2010 from $93.7 million for the
six months ended June 30, 2009 as we generated additional
business from existing and new clients. The majority of the
increase is related to the continued build from existing
contracts with several biopharmaceutical companies that use our
integrated programs. Service revenue from integrated programs
for the six months ended June 30, 2010 grew 38.0% over the
comparable prior period, and accounted for 65.0% of our total
service revenue and accounted for approximately 76.0% of our
revenue growth for the six months ended June 30, 2010 over
the prior year period. The remaining 24.0% of our revenue growth
for the six months ended June 30, 2010 over the prior year
period was the result of new project awards and growth in our
hybrid and traditional project-based offerings from our clients
worldwide.
Reimbursement revenue and offsetting reimbursable out-of-pocket
costs fluctuate from period to period due primarily to the level
of pass-through expenses in a particular period. Reimbursement
revenue and reimbursable out-of-pocket costs increased by
$4.6 million, or 41.7%, to $15.5 million during the
six months ended June 30, 2010 from $10.9 million
during the six months ended June 30, 2009. The increase is
due primarily to an increase in the number of programs for which
we provide our various services.
Direct Costs. Direct costs increased by
$20.6 million, or 30.2%, to $88.7 million for the six
months ended June 30, 2010 as compared to
$68.2 million for the six months ended June 30, 2009.
As a percentage of service revenue, direct costs decreased from
72.7% to 72.5% between periods. Although the increase in direct
costs is directly correlated with the increase in revenues as
described above, the improvement in direct costs as a percentage
of service revenue was the result of increased labor
efficiencies that we have continued to implement.
Selling, general and administrative
expenses. SG&A increased by $4.6 million, or
21.7%, to $25.7 million for the six months ended
June 30, 2010, from $21.1 million for the six months
ended June 30, 2009. As a percentage of
33
service revenue, however, SG&A decreased from 22.5% to
21.0% between periods. The overall increase in SG&A was
primarily the result of our investment in infrastructure related
to our European acquisitions and the Paramax acquisition and an
increase in the number of corporate personnel to support our
expanded operations. Employee-related costs, including new
salaries, health benefits and payroll taxes, increased to
$15.6 million for the six months ended June 30, 2010
as compared to $12.3 million for the six months ended
June 30, 2009. We also incurred an increase of
$1.0 million in professional fees, office expenses and
license fees compared to the six months ended June 30,
2009, and an increase in rent and travel expense to
$3.1 million for the six months ended June 30, 2010 as
compared to $2.7 million for the six months ended
June 30, 2009, as a result of our expanded global
operations.
Depreciation and amortization expense. Depreciation
and amortization expense increased by $531,000, or 31.8%, to
$2.2 million for the six months ended June 30, 2010,
as compared to $1.7 million for the six months ended
June 30, 2009. The increase was due primarily to an
increase in our depreciable asset base and the amortization of
intangible assets related to the Paramax acquisition.
Income from operations. As a result of the revenue
and expense increases described above and our leverage of fixed
costs across our larger revenue base, our income from operations
increased by $3.0 million, or 108.5%, to $5.8 million
for the six months ended June 30, 2010 as compared to
$2.8 million for the six months ended June 30, 2009.
Interest expense. Interest expense increased by
$135,000, or 44.3%, to $438,000 for the six months ended
June 30, 2010, as compared to $304,000 for the six months
ended June 30, 2009. The increase was due to an increase in
borrowings on our line of credit.
Interest income. Interest income from our
interest-bearing cash balances decreased by $163,000, or 96.9%,
to $5,000 for the six months ended June 30, 2010, as
compared to interest income of $168,000 during the six months
ended June 30, 2009. The decrease in interest income during
the six months ended June 30, 2010 was due to both
fluctuations in and a decrease in the level of investable cash
on hand throughout the period.
Other income. We recorded other income of $37,000
during the six months ended June 30, 2010 as a result of
favorable foreign currency fluctuations impacting our Latin
American operations, as compared to other expense of $167,000
from such currency fluctuations during the six months ended
June 30, 2009.
Provision for income taxes. The provision for income
taxes for the six months ended June 30, 2010 increased by
$2.5 million, to $4.0 million, as compared to a
provision of $1.5 million for the six months ended
June 30, 2009. The increase in the provision was due to an
increase in taxable income in the United States during the
period, as well as an increase in our overall effective tax
rate. Our effective tax rate increased as we are not recording a
tax benefit for certain net operating losses generated in our
foreign subsidiaries, as we may not realize the tax benefit of
those operating losses.
Net income. As a result of the factors discussed
above, we reported net income of $1.5 million, or $0.04 per
basic and diluted share, for the six months ended June 30,
2010, as compared to net income of $989,000, or $0.03 per basic
and diluted share, for the six months ended June 30, 2009.
Year
Ended December 31, 2009 Compared to the Year Ended
December 31, 2008
Revenues. Service revenue increased by
$43.5 million, or 27.7%, to $200.5 million for 2009
from $157.0 million for 2008 as we generated additional
business from existing and new clients. The $43.5 million
increase in revenue was related to significant new contracts and
the continued growth of existing contracts, including growth in
the revenue attributable to our integrated solutions of
$22.1 million or 50.8% of the increase in service revenue,
and $21.4 million, or 49.2% of the increase in service
revenue, from the European acquisitions and the Paramax
acquisition. Service revenue from our integrated solutions for
the year ended December 31, 2009 grew 24.7% over the
comparable prior period, and accounted for $123.6 million,
or 61.6%, of our total service revenue for the year ended
December 31, 2009, as compared to growth in revenue from
our integrated solutions of 16.1% for the period ended
December 31, 2008 over the comparable prior period, which
accounted for $99.1 million, or 63.2%, of total service
revenue for the year ended December 31, 2008.
34
Reimbursement revenue and reimbursable
out-of-pocket
costs increased by $5.6 million, or 31.0%, to
$23.7 million in 2009 from $18.1 million in 2008. The
increase was due primarily to an increase in the number of
programs for which we provide our various services.
Direct Costs. Direct costs increased by
$27.5 million, or 23.4%, to $145.2 million for 2009,
as compared to $117.7 million for 2008. As a percentage of
service revenue, direct costs decreased from 75.0% to 72.4%
between years. Although the increase in direct costs was
directly correlated with the increase in revenues, the
improvement in direct costs as a percentage of service revenue
was the result of increased labor efficiencies.
Selling, general and administrative
expenses. SG&A increased by $13.5 million, or
43.2%, to $44.8 million from $31.3 million for 2008.
As a percentage of service revenue, SG&A increased from
19.9% to 22.3% between years. Growth in SG&A expenditures
outpaced revenue growth primarily as a result of our investment
in infrastructure related to our European acquisitions and the
Paramax acquisition, which included an increase in the number of
corporate personnel to support our expanded operations.
Employee-related costs, such as new salaries, health benefits
and payroll taxes, increased to $25.9 million for the year
ended December 31, 2009 as compared to $18.8 million
for the year ended December 31, 2008. We also incurred a
$1.9 million increase in rent and travel expense to
$5.4 million for the year ended December 31, 2009, as
compared to $3.5 million for the year ended
December 31, 2008, as a result of expanded international
operations.
Depreciation and amortization expense. Depreciation
and amortization expense increased by $2.0 million, or
112.7%, to $3.7 million for 2009, as compared to
$1.8 million for 2008. The increase was due primarily to
the amortization of intangibles related to the European
acquisitions and the Paramax acquisition.
Income from operations. As a result of the revenue
and expense changes described above and our additional
leveraging of fixed costs across a larger revenue base, our
income from operations increased by $523,000, or 8.4%, to
$6.7 million for 2009, as compared to $6.2 million for
2008.
Interest expense. Interest expense increased by
$422,000, or 186.4%, to $650,000 for 2009, as compared to
$227,000 for 2008. The increase was due to the interest expense
incurred on the higher average outstanding balance on our line
of credit.
Interest income. Interest income increased by
$246,000, or 84%, to $539,000 during the year ended
December 31, 2009 from $293,000 during 2008. The increase
was due to an increase in the level of our interest-bearing cash
balances during 2009 as compared to 2008.
Other expense. We recorded other expense of $452,000
during the year ended December 31, 2009 due to adverse
foreign currency fluctuations impacting our Latin American
operations in Brazil and Colombia.
Provision for income taxes. The provision for income
taxes for 2009 increased by $1.1 million to
$3.6 million, as compared to $2.5 million for 2008.
The increase in the provision was due to both an increase in
taxable income in the United States during the year, as well as
an increase in the overall effective tax rate. The effective tax
rate increased as we are not recording a tax benefit for net
operating losses generated in certain foreign subsidiaries,
since we may not realize the tax benefit of these operating
losses.
Net income. As a result of the factors discussed
above, our net income for 2009 decreased to $2.6 million,
or $0.07 per basic and diluted share, from net income of
$3.7 million, or $0.11 per basic and diluted share, for
2008.
Year
Ended December 31, 2008 Compared to the Year Ended
December 31, 2007
Revenues. Service revenue increased by
$36.5 million, or 30.3%, to $157.0 million for 2008
from $120.5 million for 2007 as we generated additional
business from existing and new clients. The majority of the
increase was related to significant new contracts and the
continued growth of existing contracts with several
pharmaceutical companies for which we provide our integrated
solutions. Service revenue from providing our integrated
solutions for the year ended December 31, 2008 grew 56.3%
over the comparable prior period, and accounted for
$99.1 million or 63.2% of our total service revenue for the
year ended December 31, 2008, as compared to growth in
revenue from our integrated solutions of 181.1% for the period
ended December 31, 2007 over the comparable prior period,
which accounted for $63.4 million or 53.2% of total service
revenue for the year ended December 31, 2007.
35
Reimbursement revenue and reimbursable
out-of-pocket
costs increased by $4.2 million, or 29.9%, to
$18.1 million in 2008 from $13.9 million in 2007. The
increase was due primarily to an increase in the number of
programs for which we provided our various services.
Direct Costs. Direct costs increased by
$30.0 million, or 34.3%, to $117.7 million for 2008,
as compared to $87.7 million for 2007. As a percentage of
service revenue, direct costs increased from 72.8% to 75.0%
between years. The increase in direct costs was directly
correlated with the increase in revenues, and the increase in
direct costs as a percentage of service revenue was the result
of providing service offerings that tend to increase direct
costs as a percentage of revenue.
Selling, general and administrative
expenses. SG&A increased by $4.5 million, or
16.8%, to $31.3 million for 2008, from $26.8 million
for 2007 to support the increase in revenues. As a percentage of
service revenue, SG&A decreased from 22.2% to 19.9% between
years. The decrease in SG&A as a percentage of revenue was
attributable to our ability to leverage fixed infrastructure
costs and contain semi-variable overhead costs at a slower rate
of growth than revenues. The primary reason for the increase in
overall SG&A costs was an increase in the number of
corporate personnel, which resulted in increases in
employee-related costs, such as new salaries, as well as
increases in salaries for existing employees, bonuses,
commissions, health benefits and payroll taxes, to
$18.8 million for the year ended December 31, 2008 as
compared to $16.2 million for the year ended
December 31, 2007. We also incurred a $877,000 increase in
rent and travel expense to $3.5 million for the year ended
December 31, 2008, as compared to $2.6 million for the
year ended December 31, 2007, as a result of increased
international operations. Our expenditures for insurance
premiums, licenses and professional fees decreased to
$3.1 million for the year ended December 31, 2008,
from $3.4 million for the year ended December 31, 2007.
Depreciation and amortization expense. Depreciation
and amortization expense increased by $607,000, or 53.0%, to
$1.8 million for 2008, as compared to $1.1 million for
2007. The increase was due primarily to an increase in our
depreciable asset base.
Income from operations. As a result of growth in
revenues in excess of the corresponding growth in direct costs
and SG&A, our income from operations increased by
$1.3 million, or 27.5%, to $6.2 million for 2008, as
compared to $4.9 million for 2007.
Interest expense. Interest expense decreased by
$5.8 million, or 96.2%, to $227,000 for 2008, as compared
to $6.0 million for 2007. During the year ended
December 31, 2007, we recorded a non-cash charge of
$4.7 million to adjust our put warrant liability to its
market value during the period. The put warrants were exchanged
for a combination of common stock and cash in connection with
our merger with Cross Shore on August 30, 2007, and
therefore there was no corresponding expense for the put
warrants during the year ended December 31, 2008.
Provision for income taxes. The provision for income
taxes for 2008 increased by $1.0 million to
$2.5 million, as compared to $1.5 million for 2007.
The increased provision is reflective of our increased income
before provision for income taxes. Our effective tax rate for
2007 was significantly higher than in prior year, as the
$4.7 million non-cash interest charge recorded related to
the put warrant liability discussed above was non-deductible for
income tax purposes.
Net income. As a result of the factors discussed
above, net income for 2008 increased to $3.7 million, or
$0.11 per basic and diluted share, from a net loss of
$2.4 million, or $0.19 per basic and diluted share, for
2007.
Liquidity and
Capital Resources
Cash
Flows
Operating
Activities
During the six months ended June 30, 2009 and 2010, we used
cash of $8.8 million and generated cash of $893,000,
respectively, in our operating activities. We generated net
income of $1.0 million and $1.5 million for the six
months ended June 30, 2009 and 2010, respectively.
Increases in our accounts receivable resulted in net cash
outflows of $7.6 million and $3.4 million during the
six months ended June 30, 2009 and 2010, respectively. In
addition, changes in our prepaid expenses, accounts payable,
accrued expenses, other liabilities, customer
36
deposits and deferred revenue resulted in net cash outflows of
$3.9 million during the six months ended June 30, 2009
and net cash inflows of $373,000 during the six months ended
June 30, 2010. The net income for the periods also includes
non-cash charges for depreciation, amortization, stock-based
compensation and the deferred tax provision totaling
$1.7 million and $2.5 million during the six months
ended June 30, 2009 and 2010, respectively.
During the year ended December 31, 2007, we generated
$1.6 million in cash from operating activities. During the
years ended December 31, 2008 and 2009, we used cash of
$2.8 million and $744,000, respectively, in our operating
activities. For the year ended December 31, 2007, we had a
net loss of $2.4 million and we generated net income of
$3.7 million and $2.6 million for the years ended
December 31, 2008 and 2009, respectively. Increases in our
accounts receivable resulted in net cash outflows of
$10.0 million, $4.9 million and $10.9 million
during the years ended December 31, 2007, 2008 and 2009,
respectively. In addition, changes in our prepaid expenses,
accounts payable, accrued expenses, other liabilities, customer
deposits and deferred revenue resulted in net cash inflows of
$8.0 million for the year ended December 31, 2007, net
cash outflows of $3.4 million for the year ended
December 31, 2008 and net cash inflows of $3.7 million
for the year ended December 31, 2009. The net income (loss)
for the periods also includes non-cash charges for depreciation,
amortization, stock-based compensation and the deferred tax
provision totaling $1.3 million, $1.8 million and
$3.9 million during the years ended December 31, 2007,
2008 and 2009, respectively. During the year ended
December 31, 2007, our net loss also included the
$4.7 million non-cash interest charge related to our put
warrant liability.
Investing
Activities
During the six months ended June 30, 2009, we used cash of
$1.2 million in our investing activities, consisting of
$1.6 million used in connection with the European
acquisitions and $1.3 million used for the purchase of
equipment, offset by a change of $1.6 million in our
restricted cash balances. For the six months ended June 30,
2010, we used cash of $1.0 million in our investing
activities, consisting of $1.1 million used for the
purchase of equipment, offset by a change of $53,000 in our
restricted cash balances.
During the years ended December 31, 2007, 2008 and 2009, we
used cash of $2.0 million, $8.7 million and
$3.6 million, respectively, in our investing activities.
During the years ended December 31, 2008 and 2009, we used
an aggregate of $7.9 million and $3.0 million,
respectively, for the European and Paramax acquisitions. For the
years ended December 31, 2007, 2008 and 2009, we used
$2.2 million, $1.3 million and $2.7 million,
respectively, for the purchase of equipment. These uses were
offset by changes in our restricted cash balances of $146,000,
$420,000 and $2.1 million, respectively, during these years.
Financing
Activities
During the six months ended June 30, 2009 and 2010, we
generated cash of $4.5 million and $1.0 million,
respectively, from our financing activities. During these
periods, we had net borrowings of $4.9 million and
$1.3 million, respectively, under our line of credit, we
made principal payments on our capital lease obligations of
$350,000 and $187,000, respectively, and we made $105,000 in
payments on deferred equity financing costs during the six
months ended June 30, 2010 in connection with the
contemplated public offering of our common stock.
During the years ended December 31, 2007, 2008 and 2009, we
generated cash of $11.3 million, $6.9 million and
$1.3 million, respectively, from our financing activities.
During the year ended December 31, 2007, we received cash
proceeds of $51.4 million, net of fees, from the merger
with Old RPS. In connection with the merger, we also paid
$20.0 million in cash distributions to our stockholders and
$2.6 million to former holders of preferred stock for
accrued dividends, and we used $3.8 million to repurchase
shares of common stock from former stockholders of Cross Shore.
We also used proceeds from the merger with Old RPS to repay an
aggregate of $13.6 million of indebtedness, including on
our line of credit, during the year ended December 31,
2007. During the years ended December 31, 2008 and 2009, we
had net borrowings of $7.5 million and $2.1 million,
respectively, under our line of credit. During the years ended
December 31, 2007, 2008 and 2009, we also repaid $194,000,
$605,000 and $750,000, respectively, in principal on our capital
lease obligations.
37
Sources
of Liquidity
We manage our liquidity primarily through cash flows from
operations and borrowings under our lines of credit. We monitor
our accounts receivable balances to ensure sufficient operating
cash flow. In the United States, we manage our cash function
using collection and cash management accounts. Daily collections
of our accounts receivable are swept into our operating account,
with excess funds invested in high-quality money market funds of
short duration. Disbursements presented for payment are funded
daily out of the money market accounts. Outside of the United
States, our cash balances are maintained at levels necessary to
support operating activities. As in the United States, cash
balances for our foreign subsidiaries are generally maintained
in the functional currency of the applicable subsidiary.
We maintain a working capital line of credit with a bank, with a
maximum potential borrowing capacity of $30.0 million,
depending on our borrowing base of eligible accounts receivable.
At June 30, 2010 and December 31, 2009, we had
$10.9 million and $9.6 million, respectively, in
outstanding borrowings under this facility and
$18.7 million and $13.5 million, respectively, in
available borrowings under this facility. Interest on
outstanding borrowings under our facility accrues at an annual
rate equal to the Federal Funds open rate plus 2%, which was
4.75% at June 30, 2010. Our credit facility contains
various financial and other covenants, including a prohibition
on paying dividends or distributions other than dividends or
distributions payable in our stock. At June 30, 2010 and
December 31, 2009, we were in compliance with these
covenants. Our credit facility is secured by all of our assets.
At June 30, 2010, we had available cash and cash equivalent
balances of $4.3 million and working capital of
$23.1 million, which we believe will provide sufficient
liquidity for at least the next twelve months.
Contractual
Obligations
Our industry is generally not capital-intensive. Our principal
operating cash needs are for payment of salaries, office rents,
and travel expenditures. From time to time we may also make
capital expenditures for facilities, information system
enhancements and potential acquisitions to support our expansion.
Set forth below is information concerning our known contractual
obligations as of December 31, 2009, consisting only of
obligations under operating and capital leases:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Payments Due by Period
|
|
|
|
|
|
Less than
|
|
|
|
|
|
More than
|
|
|
|
Total
|
|
1 year
|
|
1-3 Years
|
|
3-5 Years
|
|
5 Years
|
|
|
|
Contractual Obligations
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Capital leases
|
|
$
|
861,875
|
|
|
$
|
601,683
|
|
|
$
|
260,192
|
|
|
$
|
—
|
|
|
$
|
—
|
|
|
Operating leases
|
|
|
17,422,366
|
|
|
|
3,634,646
|
|
|
|
6,168,276
|
|
|
|
4,782,630
|
|
|
|
2,836,814
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total
|
|
$
|
18,284,241
|
|
|
$
|
4,236,329
|
|
|
$
|
6,428,468
|
|
|
$
|
4,782,630
|
|
|
$
|
2,836,814
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Off-Balance Sheet
Arrangements
We are not a party to any off-balance sheet arrangements as
defined by Regulation S-K Item 303(a)(4)(ii).
Inflation
A portion of our revenues are earned under long-term contracts
having terms in excess of one year, which generally include an
inflation or cost of living adjustment for the portion of
services to be performed more than one year from the contract
date. As a result, we believe that the effects of inflation
generally do not have a material effect on our operations or
financial condition.
Recently Issued
Accounting Standards
We adopted new accounting guidance on fair value measurements
effective January 1, 2008, for our financial assets and
liabilities. In addition, effective January 1, 2009, we
adopted this guidance as it relates to nonfinancial assets
38
and liabilities that are not recognized or disclosed at fair
value in the financial statements on at least an annual basis.
This guidance defines fair value as the price that would be
received to sell an asset or paid to transfer a liability,
referred to as the exit price, in an orderly transaction between
market participants at the measurement date. The standard
outlines a valuation framework and creates a fair value
hierarchy in order to increase the consistency and comparability
of fair value measurements and the related disclosures. In
determining fair value of financial assets, we primarily use
prices and other relevant information generated by market
transactions involving identical or comparable assets, called
the market approach. As of June 30, 2010 and
December 31, 2009, the fair value of all of our financial
assets are based on level one observable inputs. The provisions
of this guidance will be applied at such time a fair value
measurement of a nonfinancial asset or liability is required,
which may result in a fair value that is materially different
than would have been calculated prior to the adoption of this
guidance. The implementation of this fair value guidance has not
had an impact on our consolidated financial statements.
In December 2007, the FASB issued new guidance related to
business combinations. This guidance retains the fundamental
requirements of existing guidance that the acquisition method of
accounting be used for all business combinations and for an
acquirer to be identified for each business combination. This
guidance defines the acquirer as the entity that obtains control
of one or more businesses in the business combination and
establishes the acquisition date as the date the acquirer
achieves control. This guidance was effective beginning
January 1, 2009 and was followed in connection with the
Paramax acquisition. The impact of this guidance will depend
upon the nature and terms of business combinations that we may
consummate in the future.
In June 2008, the FASB issued new guidance related to assessing
whether an equity-linked financial instrument (or embedded
feature) is indexed to an entity’s own stock for the
purposes of determining whether such equity-linked financial
instrument (or embedded feature) is subject to derivative
accounting. We adopted this new guidance effective
January 1, 2009. The adoption of this guidance did not have
a material impact on our consolidated financial statements.
In May 2009, the FASB issued new guidance on subsequent events.
The standard provides guidance on management’s assessment
of subsequent events and incorporates this guidance into
accounting literature. We adopted this guidance commencing with
our June 30, 2009 consolidated financial statements. The
implementation of this standard did not have a material impact
on our consolidated financial statements.
In April 2009, the FASB issued a staff position requiring fair
value disclosures in both interim as well as annual financial
statements in order to provide more timely information about the
effects of current market conditions on financial instruments.
We adopted this guidance commencing with our June 30, 2009
consolidated financial statements. The implementation of this
standard did not have a material impact on our consolidated
financial statements.
In June 2009, FASB Accounting Standards Codification, or
Codification, was issued, effective for financial statements
issued for interim and annual periods ending after
September 15, 2009. The Codification supersedes literature
of the FASB, Emerging Issues Task Force and other sources. The
Codification did not change GAAP. The implementation of this
standard did not have a material impact on our consolidated
financial statements.
Quantitative and
Qualitative Disclosures about Market Risk
Foreign currency risks. Since we operate in
countries other than the United States, we are exposed to
various foreign currency risks. The majority of the services we
provide to our clients result in revenues that are denominated
in U.S. dollars. However, at times, a portion of the work
is performed by one of our foreign subsidiaries under a contract
specifying that costs are to be incurred in the local
denomination of that subsidiary. When expenses are incurred in a
denomination other than U.S. dollars, our net earnings can
be affected by fluctuations in exchange rates. In addition, any
fluctuation in the exchange rates of the net assets of our
foreign subsidiaries denominated in local currencies would be
reflected in translation gains or losses, which are accounted
for in other comprehensive income in our statements of
redeemable convertible preferred stock and stockholders’
equity. We do not believe that a change of 10% in the applicable
foreign currency exchange rates as of and during the six months
ended June 30, 2009 and 2010 or as of and for the years
ended December 31, 2007, 2008 or 2009 would have had a
material impact on our financial position or results of
operations as of those dates and during those periods.
39
Approximately 16% of our service revenue for the six months
ended June 30, 2010 and 2009, and approximately 5%, 6% and
17% of our service revenue for the years ended December 31,
2007, 2008 and 2009, respectively, were derived from our
operations outside of the United States. We currently do not
engage in derivative or hedging activities related to our
potential foreign exchange exposures. However, as we contemplate
future anticipated foreign currency working capital
requirements, capital asset acquisitions of our foreign
operations, and our continued international expansion, we will
consider maintaining a portion of our cash and cash equivalents
denominated in foreign currencies sufficient to satisfy these
possible future requirements. We will also evaluate the need and
cost of financial instruments to hedge currency exposures on an
ongoing basis and may hedge against exchange rate exposure in
the future.
Interest rate risk. The primary objective of our
investment activity is to preserve principal, provide liquidity
and maximize income without increasing risk. Our investments
have limited exposure to market risk. To minimize this risk, we
maintain our portfolio of cash and cash equivalents in a variety
of investments, consisting primarily of bank deposits and money
market funds. The interest rates are variable and fluctuate with
current market conditions. The risk associated with fluctuating
interest rates is limited to this investment portfolio and the
variable interest rate under our line of credit, and we do not
believe that a 100 basis point change in interest rates would
have had a material impact on our interest income during the
years ended December 31, 2007, 2008 and 2009 or the six
months ended June 30, 2009 and 2010. We do not believe that
a 100 basis point change in the variable interest rate on our
line of credit would have had a material impact on our interest
expense during the years ended December 31, 2007, 2008 and
2009 or the six months ended June 30, 2009 and 2010.
40
Business
Business
Overview
We are a global, next-generation clinical research organization,
or CRO, providing a broad range of clinical development
solutions and services to biotechnology and pharmaceutical
companies. Our services, which we provide across a broad range
of therapeutic areas, include clinical trial project management,
site identification, management and monitoring, patient
enrollment, data collection and management, statistical analysis
and report writing, quality assurance, regulatory and medical
affairs, and pharmacovigilance services. These services support
the design, initiation and management of our clients’
clinical trials programs that are required to obtain regulatory
approval to market biopharmaceutical products.
We offer a comprehensive suite of outsourced solutions covering
the entire range of our clients’ biopharmaceutical clinical
development activity. Our innovative business model allows us to
use the existing processes and systems of our clients to improve
quality, increase the speed of product development and reduce
overall development costs, while allowing the client to maintain
control of their development portfolio. We provide our services
both as integrated solutions embedded within a client’s
internal clinical development operations, with an ability to
work across multiple clinical trials, product candidates and
clinical development functions, and on a more traditional
project basis. We also offer hybrid solutions combining our
integrated and project-based offerings. Our flexible model
enables us to tailor our services to meet the differing needs of
small, medium and large biopharmaceutical companies.
We believe that our integrated solutions address shortcomings of
the traditional CRO model by providing clients with more control
of the strategic aspects of their clinical trials, greater
integration of in-house capabilities with outsourced resources,
reduced costs and improved productivity and efficiency. By
focusing on creating strategic relationships with our clients
for their entire pipeline of drugs in development, we attempt to
create effective partnerships with our clients that enhance
long-term revenue opportunities and minimize dependence on
individual trials.
Our business model focuses on integrating our clinical
development solutions within the internal clinical development
operations of our clients, and in doing so, we address both the
outsourced and in-house components of our clients’
Phase II through Phase IV research and development
expenditures. We believe that the combination of our clinical
expertise and our extensive personnel resourcing capabilities
enables us to achieve cost savings, improvements in the quality
of clinical trial execution and accelerated clinical timelines
on behalf of our clients. We have a professional staff of over
2,300 individuals averaging over 12 years of experience in the
biotechnology and pharmaceutical industries, and we maintain a
proprietary human resources database that includes approximately
188,000 additional clinical professionals around the world.
We offer our services on a global basis, with international
operations in North America, Latin America, Europe and Asia. Our
diverse client mix includes 14 of the 15 largest pharmaceutical
companies in the world as ranked by 2009 global revenues, and
our annual service revenue has increased from $62.8 million
in 2005 to $200.5 million in 2009, a compounded annual
growth rate of 33.7%. In the six months ended June 30,
2010, we generated $122.4 million of service revenue, a
30.6% increase over service revenue of $93.7 million for
the first six months of 2009.
Industry
Background and Outsourcing Drivers
Discovering and developing new drugs is an extremely expensive,
complex, high-risk and time-consuming process. According to
Frost and Sullivan, a market research firm, the average
development cost of a new drug in the United States from
conception stage to marketing approval from the U.S. Food
and Drug Administration, or FDA, increased from
$138 million in 1975 to approximately $800 million by
2000 and was estimated to be over $1.3 billion by the end
of 2008. The Pharmaceutical Research and Manufacturers of
America estimates that it typically takes between 10 and
15 years to develop and obtain approval to market a new
prescription drug in the United States.
The rising cost of drug development and stringent regulatory
guidelines have been major drivers for outsourcing of drug
development by biopharmaceutical companies. These companies
outsource portions of their drug
41
development needs to CROs in order to manage the product
development process more efficiently and cost effectively and to
accelerate time to market. CROs typically provide a variety of
clinical drug development services, including protocol design
and management of Phase I through Phase IV clinical trials,
data management, laboratory testing, medical and safety reviews
and statistical analysis. These services are intended to
generate high-quality and timely data in support of applications
for regulatory approval of new drugs and reformulations of
existing drugs, as well as to support new and existing marketing
claims. By outsourcing drug development activities,
biopharmaceutical companies can reduce their fixed costs and
their investments in clinical infrastructure and focus their
resources on their core competencies, such as sales and
marketing and drug discovery.
We and other CROs derive revenues from the research and
development expenditures of biopharmaceutical companies, which
have increased substantially in recent years. We target
Phase II through Phase IV clinical development
programs, which encompass late-stage and post-marketing clinical
trials of biopharmaceutical products. According to Frost and
Sullivan, research and development spending by the global
pharmaceutical industry is expected to grow from
$109 billion in 2009 to $169 billion in 2015, a
compound annual growth rate of 7.7%. Spending in Phase II
through IV in the United States is expected to grow as a
percentage of total research and development spending from 66%
in 2009 to 69% by 2015. Additionally, according to
Frost & Sullivan, CRO revenues, as a percentage of
global research and development spending, is expected to be
19.6% in 2010.
Traditional project-based CROs target this 19.6% of the research
and development spending, the outsourced portion of this market.
By contrast, we seek to address a significantly larger portion
of the research and development spending market because we
integrate our clinical development solutions into various levels
of our clients’ operations, allowing us to target both the
outsourced and in-house components of our clients’
Phase II through IV clinical expenditures.
The growth in total research and development spending is
expected to be driven by increased competition, product
innovation, advances in research and clinical analysis and the
desire to fill the pipelines of many large biopharmaceutical
companies, which are facing patent expirations of a significant
portion of their drug portfolios. Continued investment in
research and development has resulted in growing pipelines of
drugs in development, leading to an increase in demand for
clinical trials.
In addition to the overall increase in research and development
spending, we believe that there are a number of trends and
challenges facing biopharmaceutical companies that will increase
their reliance on outsourced services, including:
|
|
|
| |
•
|
Increasing cost pressures and need for enhanced efficiencies.
Market forces and governmental initiatives place significant
pressures on biopharmaceutical companies to reduce drug prices.
In addition, increased competition as a result of patent
expiration, market acceptance of generic drugs and governmental
and privately managed care organization efforts to reduce
healthcare costs have added to drug pricing pressures. With
greater pressure on biopharmaceutical companies to better manage
their drug development resources, outsourcing offers a way to
convert fixed costs to variable costs, thereby improving a
company’s cost structure and return on investment. In
addition, biopharmaceutical companies are increasingly seeking
to enhance the efficiency of their clinical development
processes while maintaining control of the strategic aspects of
their development programs.
|
| |
| |
•
|
Increasing complexity of industry needs driven by tougher
regulatory environment. Global regulatory agencies including
the FDA are increasingly requiring more clinical trials, longer
trial periods and greater amounts of clinical data before
granting approval to market a drug. Lengthy drug development
timelines along with the inherent risks involved have led many
companies to increase their pipeline of drugs in development in
order to maximize their chances of identifying successful
products. Outsourcing can allow biopharmaceutical companies to
manage the increased complexity of a growing drug development
pipeline in a cost-effective manner.
|
| |
| |
•
|
Increased globalization of clinical trials.
Biopharmaceutical companies are increasingly seeking to
access additional patient populations and trial participants,
identify new markets for their products, shorten development
times and reduce costs by conducting clinical trials in multiple
countries. This trend requires that biopharmaceutical companies
have access to outsourcing solutions on a global scale.
|
| |
| |
•
|
Priorities of smaller and emerging biopharmaceutical
companies. Smaller and emerging biopharmaceutical
|
42
|
|
|
| |
|
companies commonly elect not to build their own in-house
clinical development capabilities, instead focusing their
resources on early-stage drug discovery or on sales and
marketing. Outsourcing clinical development functions enables
smaller biopharmaceutical companies to focus on developing their
drugs by providing access to necessary clinical development
services, without the high fixed costs of developing such
capabilities internally.
|
In order to address these challenges and to better meet their
needs, we believe that biopharmaceutical companies are
increasingly turning to innovative solutions, such as our
integrated service offerings, as an alternative to the exclusive
use of traditional, project-based CRO outsourcing.
Our
Solution
We have created a next-generation CRO that addresses both the
outsourced and in-house components for Phase II through
Phase IV clinical development activities. Our model
combines innovative integrated services with traditional,
project-based CRO services, as well as hybrid solutions. This
flexible approach enables us to tailor our service offerings to
meet the differing needs of small, mid-sized and large
biopharmaceutical companies.
We believe that our approach, combining innovative integrated
services with traditional project-based CRO services, results in
a number of advantages that differentiate us within our
industry, including:
|
|
|
| |
•
|
An integrated, flexible approach and a true partnership with
our clients. Our integrated solutions emphasize close
collaboration with our clients, allowing efficiencies and cost
savings to be realized, while permitting clients to maintain
control over their key medical and regulatory decision-making
processes. We believe that this approach helps to maximize the
effective use of our clients’ existing resources, processes
and systems, while enhancing real-time communication and
coordination between us and our clients and avoiding duplicative
infrastructure costs. Through our integrated solutions, we
create a strategic and interdependent relationship that embeds
our services within our clients’ clinical development
operations. In some cases, our clients’ employees may
become members of our professional staff in connection with an
engagement.
|
| |
| |
•
|
Greater visibility into our clients’ product pipeline
and needs. Our integrated solutions can potentially cover
all aspects of a client’s clinical development programs
across therapeutic and functional areas. We believe this deeper
and more expansive level of involvement with our clients allows
us to better foresee potential client needs than in a
traditional project-based context. These integrated engagements
can also lead to strategic solutions that directly involve us in
our clients’ clinical development planning. In our
integrated engagements, we create strategic and interdependent
relationships with our clients, which we believe
|
43
|
|
|
| |
|
promote long-term customer relationships with stable revenue
streams, enhance our visibility into future revenue
opportunities and reduce our dependence on individual clinical
trials. We believe the depth and interconnected nature of the
relationship between us and our clients also maximizes customer
retention.
|
|
|
|
| |
•
|
Demonstrated ability to identify, recruit, deploy,
re-resource and retain a specialized professional staff. We
believe that our recruiting and deployment capabilities,
combined with our clinical expertise, represent a significant
competitive advantage in attracting and retaining the
high-quality personnel required to successfully execute our
innovative business model and to differentiate our service
offerings from those of traditional CROs. We can rapidly
identify and deploy highly experienced professionals to staff
our engagements, which is an essential part of delivering our
integrated solutions. The key driver of this core expertise has
been the development of what we call our resourcing engines,
with dedicated in-house teams that leverage our broad and deep
personnel networks to proactively resource and re-resource
experienced professionals across key functional and therapeutic
areas within the clinical development field. We have built an
extensive database of experienced clinical professionals
available to join our professional staff. We believe that our
resourcing engines enable us to proactively allocate human
capital depending on the stage of our clients’ development
programs by identifying the expertise needed on each client
program and matching those needs with the availability, capacity
and expertise of the most appropriate professionals. We believe
that rapidly resourcing and re-resourcing our professional staff
is essential to our ability to provide integrated solutions,
enabling us to achieve cost savings, improvements in clinical
trial execution quality and accelerated clinical timelines for
our clients.
|
| |
| |
•
|
Broad range of differentiated, cost-saving services designed
for large, medium and small biopharmaceutical companies. We
tailor our solutions to meet the differing needs of our broad
client base. Our large biopharmaceutical clients typically
prefer our integrated solutions, which provide the flexibility
for our clients to cost-effectively expand their clinical
research capabilities across their entire pipeline of drug
candidates and across multiple clinical development functions
without adding permanent headcount and additional support
infrastructure. The more tactical needs of our smaller
biopharmaceutical clients typically require project-based
solutions. For mid-sized clients, we are also able to offer the
flexibility of a hybrid approach, combining integrated and
project-based solutions within a single engagement.
|
| |
| |
•
|
High level of flexibility. Our ability to quickly deploy
and redeploy a specialized workforce, combined with the lack of
duplication of our clients’ clinical development
infrastructure, allows us to rapidly expand the scope of our
engagements in accordance with variations in our clients’
research and development pipelines. With our integrated
solutions, by utilizing a client’s existing clinical
systems and processes, we eliminate the need to build a separate
infrastructure for new projects, thereby reducing costs and
accelerating the drug development process.
|
Our Growth
Strategy
Our corporate mission is to help our clients maximize the return
on their research and development investments and to accelerate
the delivery of safe and effective therapeutics to patients. The
key parts of our growth strategy that enable this mission and
expand our business include the following initiatives:
|
|
|
| |
•
|
Leveraging our international capabilities to enhance and
expand our ability to execute clinical programs globally. We
currently maintain operations in North America, Latin America,
Europe and Asia. We intend to further expand globally when we
deem it appropriate to meet our existing and prospective
clients’ demands, as demonstrated by our recent
acquisitions of Paramax International, a CRO in China, and three
CROs located in Spain, France and Germany. We evaluate
acquisition opportunities as they arise, with a current focus on
Eastern Europe and Asia. We anticipate that increasing our
global capabilities will drive the expansion of existing
engagements and new program awards with global requirements.
|
| |
| |
•
|
Developing further opportunities from our existing integrated
accounts. Our current integrated solutions have allowed us
to grow multiple, long-term engagements by cross-selling and
expanding into other functional areas once we have begun working
with a client. We believe there is an excellent opportunity for
continued growth by focusing on this strategy. As an example, we
began site management responsibilities for a large
pharmaceutical client initially and then successfully expanded
our responsibilities into study management, data management,
medical writing and other clinical areas.
|
44
|
|
|
| |
•
|
Continuing to add new accounts. We plan to leverage the
growing demand for outsourced clinical development services to
reach new clients. We believe the large pipeline of drugs in
development and the continued growth in research and development
spending, along with increased demand for outsourced solutions,
represents a significant market opportunity.
|
| |
| |
•
|
Leveraging our investments in global infrastructure to
enhance our profitability. Over the last several years, we
have been investing in building our global infrastructure. We
began this process in 2005 with the opening of our Latin America
operations and have continued our global expansion with our
acquisitions in Europe in 2008 and China in 2009. With this
global infrastructure now in place, we believe we are
well-positioned to leverage these investments by continuing to
grow our revenue with the potential for improved profitability.
|
| |
| |
•
|
Expanding our suite of services offered. We are expanding
our suite of service offerings to include both non-clinical
services, such as analytical chemistry, and clinical services,
such as Phase IV post-marketing surveillance services. We
believe this will enhance our ability to provide services
tailored to our clients’ needs.
|
The Drug
Development Process
Before a new prescription drug or biologic product reaches the
commercialization stage, it must undergo extensive clinical
testing and, eventually, regulatory review for verification that
the product is safe and efficacious for its intended use.
Regulatory requirements are a significant factor in the time and
cost of drug development and contribute to the small number of
approved products that reach the market. The Pharmaceutical
Research and Manufacturers of America estimates that for every
5,000 to 10,000 potential new molecular compounds researched,
only five will be evaluated through clinical trials and only one
will be approved for use in humans.
The drug development process consists of two
stages: research (comprising pre-discovery, discovery and
pre-clinical) and clinical development. In the research stage,
targets are identified, and candidate drugs or biologics are
developed and tested in a test tube and in animals generally to
assess and optimize potential use in humans. The research stage
generally occurs over a three- to six-year period. After
successful preclinical testing, the new drug can be advanced to
the clinical development stage, which involves testing in
humans. The FDA and equivalent agencies outside the United
States regulate both the research and clinical phases of the
drug development cycle.
Prior to commencing human clinical trials in the United States,
a biopharmaceutical company must file with the FDA an
investigational new drug application, or IND, containing details
for at least one study protocol and outlines of other planned
studies. The biopharmaceutical company must provide available
manufacturing data, pre-clinical data, information about any use
of the drug or biologic in humans for other purposes, and a
detailed plan for the proposed clinical trials. The design of
these clinical trials, also referred to as the study protocol,
is essential to the success of the drug development effort. The
protocols must correctly anticipate the nature of the data to be
generated and results that the FDA will require before approving
the product. If the FDA does not comment on an IND within
30 days after filing, human clinical trials may begin.
Before a new product is ready for submission for approval by
regulatory authorities, it must undergo a rigorous clinical
trial process. The clinical trial process must be conducted in
accordance with regulations promulgated by the FDA or the
appropriate foreign regulatory body, which require the product
to be tested and studied in a setting analogous to real-world
use of the drug. Human clinical trials seek to establish the
safety and efficacy of the product in the proposed class of
patients. The clinical trial process generally consists of the
following interrelated phases, which may overlap:
|
|
|
| |
•
|
Phase I. Phase I trials are conducted in healthy
individuals and usually involve 20 to 80 subjects and typically
range from six to 12 months. These trials are designed to
establish the basic safety, dose tolerance, and metabolism of
the clinical product candidate. When the trial establishes basic
safety and metabolism of the clinical product candidate,
Phase II trials can begin.
|
| |
| |
•
|
Phase II. Phase II trials are conducted in patients
who have the disorder the drug is designed to treat. These
trials typically test 100 to 300 patients and last on
average 12 to 24 months. Phase II trials are typically
designed to identify possible adverse effects and safety risks,
to determine the efficacy of the drug, and to determine dose
tolerance. If the drug appears safe and effective,
Phase III trials can begin.
|
| |
| |
•
|
Phase III. Phase III trials involve significantly
larger and more diverse populations than Phase I and II
trials
|
45
|
|
|
| |
|
and are conducted at multiple sites. On average, this phase
lasts from one to three years. During this phase, the
drug’s safety and efficacy are further examined and
evaluated.
|
After the completion of all clinical phases, a biopharmaceutical
company may submit to the FDA a new drug application, or NDA, or
a biologic license application, or BLA. Equivalent regulatory
agencies outside of the United States follow a similar procedure
by requiring an equivalent application for a new drug or
biologic. The biopharmaceutical company submits an NDA, BLA or
equivalent application for a drug or a biologic, requesting that
the product be approved for marketing. The NDA, BLA or
equivalent application includes, among other things, the
clinical trial data generated and analyzed during the clinical
development process.
|
|
|
| |
•
|
Post-Approval/Phase IV. During the course of the review
process, regulatory authorities may approve a product for
marketing and sale on the condition that additional clinical
trials be conducted. Usually referred to as post-approval, or
Phase IV, trials, these trials may either be for submission of
additional data to regulatory authorities or for
non-registration purposes, such as additional marketing
information. These trials are intended to investigate a new
indication for an existing drug, monitor the drug’s
long-term risks and benefits, analyze different dosage levels,
evaluate different safety and efficacy parameters in target
populations, or to substantiate marketing claims. Phase IV
trials typically enroll thousands of patients and last from six
to 24 months.
|
The FDA’s regulatory requirements have served as the model
for much of the regulation of new drug development worldwide,
and regulatory requirements similar to those of the FDA exist in
the other countries in which we operate. Over the past two
decades, the FDA and corresponding regulatory agencies of the
European Union and Japan have developed harmonized standards for
pre-clinical and clinical studies and the format and content of
applications for new drug approvals. Data from multinational
studies adhering to international standards of clinical practice
are now generally acceptable to the FDA and Canadian, European
and Japanese regulators, and a common format drug and biologic
marketing authorization application is mandatory in Europe and
Japan and highly recommended by the FDA and by Canadian
regulatory authorities.
Our Clinical
Development Services
We provide our clients with a range of clinical development
services, primarily from Phase II to Phase IV in the
drug development cycle. Our employees have drug development
experience across a number of therapeutic areas, including
cardiovascular, gastroenterology, hematology, immunology,
infectious diseases, metabolic disorders, neurology, oncology,
ophthalmology, orthopedics, pulmonology, rheumatology and
women’s health.
We provide the following core Phase II to Phase IV
clinical trial management services to our clients:
|
|
|
| |
•
|
Study Protocol and Case Report Form Design. We
assist our clients in designing clinical trial protocols and
preparing case report forms. The protocol defines the medical
conditions to be examined and the statistical methods that will
be used. The protocol specifies, for example, the frequency and
type of laboratory and clinical measures to be tracked and
analyzed, the number of patients required to produce
statistically valid results, the period of time over which they
must be tracked, and the frequency and dosage of drug
administration. Once the study protocol has been finalized, we
assist in the preparation of electronic or paper case report
forms, which are used by investigative sites to record the
necessary clinical data dictated by the study protocol.
|
| |
| |
•
|
Site and Investigator Recruitment. During clinical
trials, independent physicians, referred to as investigators,
administer the drug candidate being investigated to patients at
hospitals, clinics or other locations, referred to as sites. A
significant portion of a trial’s success depends on the
successful identification and recruitment of experienced
investigators with an adequate base of patients who satisfy the
requirements of the trial protocol. We recruit investigators to
participate in clinical trials and have a database of thousands
of investigators who conduct clinical trials worldwide.
|
| |
| |
•
|
Patient Recruitment and Retention. We assist our clients
in the recruitment and retention of patients participating in
clinical trials through existing investigator relationships and
potential new relationships through our investigator recruitment
division and project teams. After performing protocol-specific
feasibility, our investigator recruitment division and project
team collaborate on the identification of the most suitable
patient recruitment or retention plan that can be tailored to a
specific program or site based
|
46
|
|
|
| |
|
on a variety of characteristics, including past site experience,
geography, patient population and demographics.
|
|
|
|
| |
•
|
Study Monitoring and Data Collection. We provide study
monitoring services, which include pre-study qualification
visits to ensure the quality and appropriateness of site
selection, investigational site initiation, interim monitoring
visits to ensure the quality and completeness of data and other
critical study and site information, study close-out visits and
patient enrollment assistance. Specially trained persons known
as monitors visit sites regularly to ensure that case report
forms are being completed correctly and to verify that the trial
is being conducted in accordance with good clinical practice, or
GCP. Our study monitoring and data collection services are
designed to comply with the safety reporting guidelines of the
FDA and other relevant regulatory agencies.
|
| |
| |
•
|
Data Management and Biostatistical Analysis. We have
extensive experience in the development and statistical analysis
of scientific databases for the clinical drug development
process. These databases are designed to comply with established
industry standards and government regulations. We prepare
statistical planning and analysis summaries for interim and
final analyses, data safety monitoring committees and regulatory
submissions, including NDAs, BLAs and equivalent regulatory
filings.
|
| |
| |
•
|
Report Writing. A description of the study conducted,
along with the statistical analysis of data collected during the
trial and other clinical data, are presented and summarized in a
final report generated for inclusion in a regulatory document.
We assist clients with writing reports for inclusion in these
documents.
|
| |
| |
•
|
Medical and Drug Safety Services. Throughout the course
of a development program, our physicians provide a wide range of
medical research and consulting services to improve the
efficiency and quality of clinical research, including medical
supervision of clinical trials, medical monitoring of patient
safety, review and reporting of adverse events, medical writing
and strategy and product development. Our medical services
professionals also provide lifecycle drug safety services
combining operational pharmacovigilance and pharmacovigilance
consulting. Operational pharmacovigilance capabilities cover all
phases of clinical development and drug safety for marketed
products.
|
| |
| |
•
|
Project Management. Throughout the entire spectrum of
activities described above, we provide project management
services to our client’s project team, including project
planning, managing progress against study goals and
deliverables, budget management and issue resolution.
|
Although some of our clients choose to contract with us for
these services on an individual project basis, we also provide
these services in the form of our integrated solutions, a
business model we pioneered. In our integrated solutions, we
collaborate more closely with the client, across multiple
clinical trials and product candidates and often across multiple
clinical development functions. While our integrated solutions
are primarily targeted at large biopharmaceutical companies, we
also offer a wide spectrum of project-based solutions to small
and mid-sized biopharmaceutical clients, as well as hybrid
solutions combining our integrated and project-based offerings.
Our Personnel
Resourcing Capabilities
In addition to our clinical expertise, we have extensive
personnel resourcing capabilities that enable us to rapidly
identify and deploy highly experienced professionals to staff
our clinical engagements. We have developed and maintain a
proprietary database, currently consisting of approximately
188,000 clinical trial professionals throughout the United
States, Latin America, Europe and Asia, that we use to recruit
clinical development professionals in support of our
engagements. This database contains not only general contact
information for candidates, but also includes specific candidate
job requirements such as travel preferences and salary
requirements, work history and current clinical trial
experience, as well as specialty and
sub-specialty
information. To ensure security of the data, our database system
has both physical and application security restrictions, as well
as role-based access restrictions.
Our personnel resourcing division is organized into dedicated
teams, each focused on a key functional area within the clinical
development field. We are organized into seven specialty
recruiting teams: clinical research; biometrics; regulatory
affairs, safety and medical writing; physicians; scientific and
analytical chemistry; and health outcomes and pharmacoeconomics.
Each team is staffed by a marketing recruiter, a project leader,
senior recruiters, recruiters and researchers. We refer to our
teams, coupled with our proprietary database and our processes,
as “resourcing
47
engines.” Our resourcing engines provide us with the
resourcing capabilities to proactively allocate human capital by
identifying the expertise needed on each client program and
matching those needs with the availability, capacity and
expertise of the most appropriate professionals. Our resourcing
engines give us the ability to rapidly resource, allocate and
redeploy, or re-resource, our highly experienced professional
staff, which is essential in providing integrated solutions that
enable us to achieve improved quality of trial execution,
accelerated clinical development timelines and cost reductions
for our clients.
Our
Clients
We provide development services to the biopharmaceutical
industry and view our operations and manage our business as one
operating segment. Our clients range from the world’s
largest pharmaceutical companies and biotechnology companies to
small and
start-up
organizations. Our diverse client mix includes 14 of the 15
largest pharmaceutical companies in the world as ranked by 2009
global revenues.
Wyeth, which is now a subsidiary of Pfizer Inc., accounted for
$28.4 million, $31.1 million, and $34.2 million,
or 23%, 20% and 17%, of our service revenue during the years
ended December 31, 2007, 2008 and 2009, respectively, and
Pfizer and Wyeth collectively accounted for $19.3 million,
or 16%, of our service revenue for the six months ended
June 30, 2010. Aggregate service revenue from Pfizer and
Wyeth was $7.6 million in the first quarter of 2009, when
the acquisition of Wyeth by Pfizer was announced. In the second
quarter of 2010, following completion of the acquisition of
Wyeth, combined revenue from Pfizer and Wyeth accounted for
$9.8 million in service revenue, an increase of 28%.
Schering-Plough, which is now a subsidiary of Merck &
Co., accounted for $11.1 million, $18.5 million, and
$31.0 million, or 9%, 12% and 16%, of our service revenue,
respectively, during these years, and Merck and Schering Plough
collectively accounted for $17.8 million, or 15%, of our
service revenue for the six months ended June 30, 2010.
Aggregate service revenue from Merck and Schering-Plough was for
$6.5 million in the first quarter of 2009, when the
acquisition of Schering-Plough by Merck was announced. In the
second quarter of 2010, following completion of the acquisition
of Schering-Plough, combined revenue from Merck and
Schering-Plough accounted for $8.5 million in service
revenue, an increase of 31.4%. Additionally, Johnson &
Johnson accounted for $20.0 million, or 17%, of our service
revenue during the six months ended June 30, 2010. We
provide Pfizer, Merck and Johnson & Johnson with
integrated solutions.
For the years ended December 31, 2007, 2008 and 2009,
approximately 95%, 94% and 83%, respectively, of our service
revenue was derived from work performed in the United States. As
a result of our recent international acquisitions during 2008
and 2009, we expect that the percentage of our service revenue
derived from foreign countries will increase. As of
December 31, 2007, 2008 and 2009, approximately 97%, 76%
and 65% of our consolidated tangible assets were located in the
United States.
Backlog
Our backlog consists of anticipated service revenue from
executed contracts that either have not started but are
anticipated to begin in the near future, or are in process and
have not been completed. Amounts included in our backlog
represent anticipated future service revenue, excluding revenues
that have been recognized previously and have been adjusted for
foreign currency fluctuations. Once contracted work begins,
service revenue is recognized over the life of the contract. We
do not include potential reimbursement revenue in our backlog.
Our backlog was $183.0 million in anticipated service
revenue at June 30, 2010, compared to $231.6 million
at December 31, 2009 and $187.2 million at
December 31, 2008. Of our backlog as of June 30, 2010,
we expect that approximately $61.9 million will be
recognized after December 31, 2010. While traditional CROs
may use book-to-bill metrics that compare new project awards
booked to revenue reported during a particular period as a
future indicator of revenue performance, we do not currently
believe such metrics to be relevant to our business, based on
the contractual structure of our integrated programs.
Our backlog as of any date is not necessarily a meaningful
predictor of future results because our backlog can be affected
by a number of factors, including the size and duration of
contracts, many of which are performed over several years.
Additionally, contracts are subject to early termination by the
client. Clinical trials can be delayed or canceled for many
reasons, including unexpected test results, safety concerns,
regulatory developments, economic issues, availability of
clinical trial material and protocol design matters, all of
which could lead to early termination of a contract. Also, the
scope of a contract can change significantly during the course
of a study. If the scope of a
48
contract is revised, the adjustment to our backlog occurs when
the revised scope is approved by the client. For these and other
reasons, we might not fully realize our entire backlog as
revenue.
Sales and
Marketing
We use corporate marketing to support the efforts of our
centralized business development staff, calling on
biopharmaceutical companies. Our sales teams focus on client
segments and service areas. In addition, while service area
representatives call on particular functional groups within a
given client, our key account directors are responsible for
managing the relationship and book of business with clients
across our portfolio of services.
Our business development personnel consult with potential
pharmaceutical and biotechnology clients early in the project
consideration stage in order to determine their requirements.
Along with the appropriate operational, technical or scientific
personnel, our business development representatives invest
significant time to determine the optimal means to design and
execute the potential client’s program requirements. As an
example, recommendations we make to a potential client with
respect to a drug development study design and implementation
are an integral part of our bid proposal process and an
important aspect of the integrated services we offer. We believe
that our preliminary efforts relating to the evaluation of a
proposed clinical protocol and implementation plan enhance the
opportunity for accelerated initiation and overall success of
the trial.
Our global marketing initiatives include integrated,
multi-channel campaigns designed to help differentiate and
promote our expertise and services. Through trade events, online
and print advertising in trade publications, direct
communication, newsletters and our website, we provide our
perspective on current industry challenges or developments to
create an ongoing dialogue with our clients and to promote our
industry expertise, quality, technology and innovation. We
reinforce key messages and selling points through client
presentations, corporate materials, participation in trade
events and speaking engagements at industry conferences.
We encourage and sponsor the participation of our scientific and
technical personnel in a variety of professional endeavors,
including speaking and the presentation of papers at national
and international professional, scientific and trade meetings
and the publication of scientific articles in medical and
pharmaceutical journals. Through these presentations and
publications, we seek to further our reputation for professional
excellence.
Competition
The CRO industry is highly competitive and fragmented,
consisting of hundreds of smaller, limited-service providers and
a number of full-service global companies. In the past, the
industry experienced some consolidation and a group of large,
full-service competitors emerged. Consolidations and
acquisitions have continued. These consolidations and other
transactions could potentially increase competition in our
industry for clients, experienced clinical personnel, geographic
markets and acquisition candidates. Additional business
combinations by competitors or clients are possible and could
have a significant impact on the competitive landscape of the
CRO industry.
Our significant CRO competitors include Covance Inc.,
Pharmaceutical Product Development, Inc., Quintiles
Transnational Corp., PAREXEL International Corporation, Kendle
International Inc., ICON plc, PRA International, i3 Research and
Pharmanet Development Group Inc. These and other potential
competitors have financial and other resources substantially
greater than ours.
In addition to competing with a number of other global,
full-service companies, services similar to ours are also
provided by medium-sized companies, in-house research and
development departments of biopharmaceutical companies,
universities and teaching hospitals. Newer, smaller entities
with specialty focuses, such as those focused on a specific
disease or therapeutic area, compete aggressively against larger
companies for clients. Increased competition might lead to price
and other forms of competition that could adversely affect our
operating results.
Providers of outsourced drug development services compete on the
basis of a number of factors. These factors include:
|
|
|
| |
•
|
the reputation for on-time quality performance;
|
| |
| |
•
|
expertise and experience in specific therapeutic areas;
|
| |
| |
•
|
scope of service offerings;
|
| |
| |
•
|
staff expertise and qualifications;
|
49
|
|
|
| |
•
|
price;
|
| |
| |
•
|
strengths in various geographic markets;
|
| |
| |
•
|
technological expertise and systems;
|
| |
| |
•
|
data management capabilities;
|
| |
| |
•
|
ability to acquire, process, analyze and report data in a
time-saving, accurate manner; and
|
| |
| |
•
|
ability to manage large-scale clinical trials both domestically
and internationally.
|
Although there can be no assurance that we will continue to do
so, we believe we compete favorably in these areas.
Despite recent consolidation, our industry remains highly
fragmented, with several hundred smaller, limited-service
providers and a small number of full-service companies with
global capabilities. Although there are few barriers to entry
for smaller, limited-service providers, there are significant
barriers to becoming a global provider offering a broad range of
services. These barriers include:
|
|
|
| |
•
|
the cost and experience necessary to develop broad therapeutic
expertise;
|
| |
| |
•
|
the ability to manage large, global, complex clinical trials;
|
| |
| |
•
|
the ability to deliver high-quality services consistently for
large drug development projects;
|
| |
| |
•
|
the cost of building or acquiring the infrastructure to manage
global clinical programs;
|
| |
| |
•
|
the experience to prepare regulatory submissions throughout the
world; and
|
| |
| |
•
|
the infrastructure and knowledge to respond to the global needs
of clients.
|
Intellectual
Property
We have developed a number of technically derived processes and
procedures and other intellectual property, including our
proprietary resourcing database, that are intended to maximize
the quality, efficiency and effectiveness of our services.
Although our intellectual property rights are valuable to our
success, we believe that the technical expertise, proprietary
know-how, ability and experience of our professional staff are
more important and that, overall, these capabilities provide
significant benefits to our clients. Where we consider it to be
appropriate, we take steps to protect trade secrets and know-how
through confidentiality agreements with employees and
consultants. If these arrangements are not honored, we might not
have adequate remedies for breach. We have no patents,
trademarks, licenses or franchises that are material and upon
which any of our service offerings are dependent.
Government
Regulation
In the United States, the FDA governs the conduct of clinical
trials of drug products in human subjects, the form and content
of regulatory applications, including, but not limited to, INDs
for human clinical testing and the development, approval,
manufacture, safety, labeling, storage, recordkeeping and
marketing of drug products. The FDA has similar authority and
similar requirements with respect to the clinical testing of
biological products. Outside the United States, the European
Medicines Agency and other regulatory agencies require that test
results submitted to such authorities be based on studies
conducted in accordance with the FDA’s GCP regulations.
Governmental regulation directly affects our business. Increased
regulation leads to more complex clinical trials and an increase
in potential business for us. Conversely, a relaxation in the
scope of regulatory requirements, such as the introduction of
simplified marketing applications for pharmaceutical and
biological products, could decrease the business opportunities
available to us. Changing levels of business opportunities and
government regulation will result in a corresponding change in
our direct and indirect costs incurred in providing services.
For example, additional legislation or regulation governing the
possession, use and dissemination of medical record information
and other personal health information might require us to
implement new security measures that require substantial
expenditures or limit our ability to offer some of our services
and products. These regulations might also increase costs by
creating new privacy procedures and requirements.
In the United States, we must perform our clinical drug and
biologic services in compliance with applicable laws, rules and
regulations, including GCP regulations, which govern, among
other things, the design, conduct,
50
performance, monitoring, auditing, recording, analysis, and
reporting of clinical trials. Before a human clinical trial may
begin, the manufacturer or sponsor of the clinical product
candidate must file an IND with the FDA, which contains, among
other things, the results of preclinical tests, manufacturer
information and other analytical data. A separate submission
relating to an existing IND must also be made for each
successive clinical trial conducted during product development.
Each clinical trial must be conducted pursuant to, and in
accordance with, an effective IND.
In addition, under the GCP regulations, each human clinical
trial is subject to the oversight of an institutional review
board, which is an independent committee that has the authority
to review, approve, monitor and suspend a clinical trial for
which the institutional review board has responsibility. The
FDA, an institutional review board or a biopharmaceutical
company may suspend or terminate a clinical trial at any time on
various grounds, including a finding that the study subjects are
being exposed to an unacceptable health risk.
In order to comply with the GCP and other regulations, either we
or our clients must, among other things:
|
|
|
| |
•
|
comply with specific requirements governing the selection of
qualified investigators;
|
| |
| |
•
|
obtain specific written commitments from the investigators;
|
| |
| |
•
|
obtain institutional review board approval of the clinical trial;
|
| |
| |
•
|
verify that appropriate patient informed consent is obtained
before the patient participates in a clinical trial;
|
| |
| |
•
|
ensure adverse drug reactions resulting from the administration
of a drug or use of a device during a clinical trial are
medically evaluated and reported in a timely manner;
|
| |
| |
•
|
monitor the validity and accuracy of data;
|
| |
| |
•
|
verify drug or device accountability;
|
| |
| |
•
|
instruct investigators and study staff to maintain records and
reports; and
|
| |
| |
•
|
permit appropriate governmental authorities access to data for
review.
|
We must also maintain reports and other related information and
documents in compliance with applicable regulatory requirements
for each study. These reports, other information and documents
may be audited by our clients, the FDA or similar regulatory
authorities.
A failure to comply with applicable regulations relating to the
conduct of clinical trials or the preparation of marketing
applications could lead to a variety of sanctions. For example,
violations of the GCP regulations could result, depending on the
nature of the violation and the type of product involved, in the
issuance of a warning letter, suspension or termination of a
clinical study, refusal by the FDA to approve clinical trial or
marketing applications or withdrawal of such applications,
injunction, seizure of investigational products, civil
penalties, criminal prosecutions or debarment from assisting in
the submission of new drug applications.
We monitor our clients’ clinical trials to test for
compliance with applicable laws and regulations in the United
States and the foreign jurisdictions in which we operate. We
have adopted standard operating procedures that are designed to
satisfy regulatory requirements and serve as a mechanism for
controlling and enhancing the quality of those clinical trials.
In the United States, our procedures were developed to ensure
compliance with the GCP regulations and associated guidelines.
The Standards for Privacy of Individually Identifiable Health
Information, or Privacy Rule, issued under the Health Insurance
Portability and Accountability Act of 1996, or HIPAA, restrict
the use and disclosure of certain protected health information.
Under the Privacy Rule, specified entities may not use or
disclose protected health information without the authorization
of the individual whose information is protected, unless the use
or disclosure of the information is specifically permitted by
regulation or law.
We are not a covered entity under the HIPAA Privacy Rule.
However, in connection with our clinical development activities,
we do receive protected health information from covered entities
subject to HIPAA. In order for those covered entities to
disclose protected health information to us, the covered entity
must obtain an authorization meeting Privacy Rule requirements
from the research subject, or make a disclosure under an
exception to the Privacy Rule’s authorization requirement.
As part of our research activities, we require covered entities
that perform research activities on our behalf to comply with
HIPAA, including the Privacy Rule’s authorization
requirement.
51
Outside of the United States, many countries have enacted laws
to safeguard the privacy and security of personal information,
including individually identifiable health information. The
member states of the European Union have adopted a rigorous
system of data protection regulations, based upon a framework
imposed by the 1995 European Commission Directive on Data
Protection, or the Directive. The Directive provides broad
protections for personal information, including, among other
things, notice requirements, limits on the scope and duration
that personal information may be maintained and processed,
restrictions on disclosures of personal information, standards
for providing individuals with control over the manner in which
personal information is processed, and restrictions on transfers
of such data to countries that the European Union finds to lack
adequate data protection laws of their own. The Directive
applies standards for the protection of all personal data, not
just health information, in the European Union and requires its
member states to enact national laws implementing the Directive.
Our operations in Europe are subject to the Directive and to any
variations in the Directive as enacted by individual member
states in which we operate.
Professional
Staff
As of August 31, 2010, we had approximately 2,365
professional staff located throughout North America, Latin
America, Europe and the Asia-Pacific regions. Of these,
approximately 2,236 are full-time employees, and the remainder
are part-time employees or independent contractors.
Approximately 24% of our professional staff are located outside
of the United States. None of our employees are subject to a
collective bargaining agreement. Employees in certain of our
non-U.S. locations
are represented by works councils as required by local laws. We
believe that our relations with our employees are good.
Legal
Proceedings
We are party to lawsuits and administrative proceedings
incidental to the normal course of our business. We do not
believe that any liabilities related to any current lawsuits or
proceedings will have a material adverse effect on our financial
condition, results of operations or cash flows.
Properties
Our headquarters are located on our campus in
Fort Washington, Pennsylvania, where we lease approximately
96,000 square feet of space under leases with terms that
are scheduled to expire between April 2014 and June 2017. This
facility accommodates our executive offices, recruiting and
management operations. We lease additional office space in
Montreal, Canada; Buenos Aires, Argentina; Mexico City, Mexico;
Bogota, Colombia; Sao Paolo, Brazil; Santiago, Chile; Lima,
Peru; Paris and Caen, France; Nuremburg, Germany; Barcelona and
Madrid, Spain; Beijing, China; and Seoul, South Korea.
Corporate
Information
We were incorporated in Delaware on January 30, 2006 as
Cross Shore Acquisition Corporation, a blank check company
formed for the sole purpose of acquiring an operating business
engaged in the delivery of business services to consumers and
companies in the United States. On April 24, 2006, we
consummated an initial public offering on the Alternative
Investment Market of the London Stock Exchange, or AIM, and on
August 30, 2007, a wholly owned subsidiary of our company
completed a merger with ReSearch Pharmaceutical Services, Inc.,
a Pennsylvania corporation providing services to the
biopharmaceutical industry since 1994, which we refer to in this
prospectus as Old RPS. Upon the completion of the merger, we
changed our corporate name to ReSearch Pharmaceutical Services,
Inc. and Old RPS, which survives as our wholly owned subsidiary,
was converted into a Delaware limited liability company with the
name ReSearch Pharmaceutical Services, LLC. We are now a holding
company for, and conduct all of our operations through, ReSearch
Pharmaceutical Services, LLC.
On September 4, 2009, we delisted our common stock from AIM
following approval of the delisting by the requisite number of
our stockholders. Trading of our warrants on AIM was suspended
simultaneously, and our warrants were delisted from AIM on
October 5, 2009. All of our unexercised warrants expired on
April 28, 2010.
In December 2008, we completed the acquisition of three CROs
located in France, Germany, and Spain, and in July 2009 we
completed the acquisition of a CRO located in China.
52
Management
The following table sets forth certain information about our
executive officers and directors:
| |
|
|
|
|
|
|
|
|
|
Name
|
|
Age
|
|
Position
|
|
|
|
Daniel M. Perlman
|
|
|
54
|
|
|
Chief Executive Officer and Chairman of the Board of Directors
|
|
Harris Koffer
|
|
|
57
|
|
|
Chief Operating Officer, President, and Director
|
|
Steven Bell
|
|
|
52
|
|
|
Chief Financial Officer and Executive Vice President of Finance
|
|
Janet L. Brennan
|
|
|
50
|
|
|
Chief International Affairs Officer and Executive Vice President
|
|
Samir Shah
|
|
|
35
|
|
|
Executive Vice President, Strategic Development
|
|
Thomas R. Armstrong
|
|
|
66
|
|
|
Director
|
|
Jack H. Dean
|
|
|
68
|
|
|
Director
|
|
James R. Macdonald
|
|
|
53
|
|
|
Director
|
|
Warren W. Myers
|
|
|
48
|
|
|
Director
|
|
Daniel Raynor
|
|
|
50
|
|
|
Director
|
|
Stephen E. Stonefield
|
|
|
62
|
|
|
Director
|
|
Peter M. Yu
|
|
|
48
|
|
|
Director
|
|
|
|
|
|
|
|
|
|
|
Daniel M. Perlman joined Old RPS in 1998 as President and
became its Chief Executive Officer and Chairman of the Board of
Directors in 2001. Mr. Perlman continued as our Chief
Executive Officer and Chairman of the Board of Directors
following the merger between Cross Shore and Old RPS. From 1993
until joining Old RPS, Mr. Perlman served as Vice
President—Operating Specialties at Kforce Inc., a
professional staffing company, where he started the contract
staffing divisions in the pharmaceutical, healthcare,
engineering, legal and scientific industries. From 1990 to 1993,
Mr. Perlman served as Managing Director of a local division
of CDI Corporation, a professional staffing company, where he
specialized in pharmaceutical outsourcing. Prior to that,
Mr. Perlman worked at a private label division of Goodyear
where he last served as Vice President - Sales and Marketing,
Private Label Division. From 1985 until 1990, Mr. Perlman
was President of TKA, a tire company in eastern Pennsylvania. He
graduated from The Haverford School and The Wharton School,
University of Pennsylvania.
The Board of Directors believes that Mr. Perlman’s
extensive experience leading both our Board of Directors and our
company, his intimate familiarity with our business, and his
20 years of management experience in the pharmaceutical
outsourcing and staffing industry give him the expertise,
skills, and judgment to serve as Chairman. Under
Mr. Perlman’s guidance, our Board of Directors has
expanded our global footprint and increased our growth in a
manner that we believe has positioned us well for the future.
Harris Koffer joined Old RPS in July 2006 as President
and Chief Operating Officer and a member of its board of
directors, and has continued as our President and Chief
Operating Officer and as a director of our company following the
merger between Cross Shore and Old RPS. Prior to joining Old
RPS, from December 2005 to June 2006, Dr. Koffer served as
Corporate Executive Vice President and President, Cardiac Safety
Services, for Medifacts International, a cardiac safety service
provider. Dr. Koffer resigned from all positions he held at
Medifacts International in June 2006. On January 28, 2007,
Medifacts International filed for Chapter 11 bankruptcy
protection. Dr. Koffer also served as Vice President,
Clinical Trials and Pharmaceutical Business Development, for
Quest Diagnostics from 2000 to November 2005, and served in
various positions at Covance Inc., a global CRO, and its
predecessor companies from 1981 to 2000, including as Vice
President and General Manager of Covance Clinical Services from
1995 to 1998, and as President of Covance Periapproval Services
from 1992 to 1995. In addition, Dr. Koffer served on the
board of directors for BioImaging Technologies from 1995 to
1998. Dr. Koffer has served as Adjunct Assistant Professor
of Pharmacy in Medicine at the University of Pennsylvania School
of Medicine and as Clinical Associate Professor of Pharmacy at
the Philadelphia College of Pharmacy and Science.
Dr. Koffer has published and presented numerous papers in
the fields of cardiovascular clinical pharmacology and
pharmacoeconomics. He earned both a B.S. degree in pharmacy and
a PharmD. degree
53
from the Philadelphia College of Pharmacy and Science and
completed a Fellowship in Clinical Pharmacology at Thomas
Jefferson University Hospital in Philadelphia.
The Board of Directors believes that Dr. Koffer’s
service as a director since 2006 and his more than 30 years
of experience in biopharmaceutical research and development
provides him with a substantial knowledge and understanding of
our company, the biopharmaceutical industry, and the contract
service organization marketplace that enable him to be a
valuable member of our Board of Directors.
Dr. Koffer’s experience in the academic and private
sector, his doctorate in pharmacy, and his expertise in
pharmacoeconomics, clinical pharmacology, pharmacoepidemiology,
and drug development in general, as well as his experience as an
executive at several clinical research and related organizations
give him particular professional expertise and management
experience relevant to his qualifications as a director.
Steven Bell joined Old RPS in 2003 as Executive Vice
President, Finance and Chief Financial Officer, and has
continued in those positions with our company following the
merger between Cross Shore and Old RPS. Prior to joining Old
RPS, Mr. Bell served as Chief Financial Officer for
CareScience, Inc., a publicly traded healthcare technology
company located in Philadelphia. Before that, Mr. Bell
spent four years at The MRC Group, Inc., a national medical
transcription company, where he served as Senior Vice President
of Finance. In addition to his executive experience,
Mr. Bell’s career includes 13 years in public
accounting, first at Price Waterhouse, and then as a partner in
the firm Zelenkofske, Axelrod and Co. Mr. Bell is a
certified public accountant and received his B.S. degree in
Business Administration from Temple University in Philadelphia.
Janet L. Brennan joined Old RPS in 1999 as its Vice
President of Clinical Operations and was promoted to Chief
Operating Officer in 2001, and then promoted to the position of
Chief Clinical Officer and Executive Vice President of Global
Operations in 2006, and remained in that position with our
company following the merger between Cross Shore and Old RPS.
Ms. Brennan was promoted to the position of Chief
International Affairs Officer and Executive Vice President in
2009. She has been instrumental in the expansion of our company
into global markets and is currently responsible for directing
and managing the business and clinical research operations for
the Asia-Pacific, Europe and Latin America regions.
Ms. Brennan has over 19 years of experience in
clinical trial operational and strategic development activities
in the contract resourcing arena of the biopharmaceutical
industry. Prior to joining our company, Ms. Brennan had
managerial responsibilities as a director of several large
project management and clinical monitoring departments and began
her career in the industry in the pharmacovigilance area.
Ms. Brennan’s clinical trial experience includes
Phase I, Phase II, Phase III, Phase IV, post-marketing
surveillance and treatment IND applications. Ms. Brennan is
a registered nurse and holds a B.S.N degree from Thomas
Jefferson University in Philadelphia.
Samir Shah joined Old RPS in 2000 and served as its Vice
President, Strategic Development and continued in that position
with our company following the merger of Cross Shore and Old
RPS. Mr. Shah was promoted to the position of Executive
Vice President, Strategic Development of our company in June
2010. Mr. Shah oversees business development and has
responsibilities for corporate expansion. From 1992 until 2000,
Mr. Shah worked in the pharmaceutical, biotech and clinical
research organization industries in various roles in both
clinical research and business operations at Parexel
International, US Bioscience, Zeneca Pharmaceuticals and IBAH
(Bio-Pharm) Clinical Services. Mr. Shah began his career in
the Department of Psychiatry/Pharmacology at the University of
Pennsylvania Medical Center where he was a Research Scientist.
Mr. Shah received a B.S. degree in Bio-Psychology from
Saint Joseph’s University in Philadelphia.
Thomas R. Armstrong has served as a director of our
company since 2008. He co-founded Cartesian Capital Group, LLC,
a global private equity firm with more than $1 billion in
commitments under management. Mr. Armstrong served as
Senior Advisor to AIG Capital Partners, or AIGCP, from 1999 to
2005, playing an active role in a number of the firm’s
investments. Mr. Armstrong previously co-founded Advent
International, a global private equity investment firm, in 1984,
where he served as Executive Vice President and Chief Operating
Officer from its inception to 1998. During that period, he
served on Advent’s investment committee and assisted in the
formation and operation of over 20 affiliated private equity
firms around the world. Mr. Armstrong has also served as
Vice President, International, of The Allen Group, a publicly
listed manufacturer of capital equipment, automotive parts, and
consumer products. Mr. Armstrong also previously co-founded
and served as Chief Operating Officer of Thrasos, Inc., a
pharmaceutical development firm using combinatorial and
computational chemistry technology to validate early stage
biological targets and accelerate the development of new
therapeutic agents. Mr. Armstrong
54
holds engineering degrees from Princeton University and Cornell
University, and an MBA from Harvard Business School.
Mr. Armstrong is a director of SCM Private Equity GP Ltd.
and SCM Private Equity II GP Ltd. Mr. Armstrong was
previously a director of Lexent Technologies.
The Board of Directors believes that Mr. Armstrong’s
background in drug development as a founder and former chief
operating officer of Thrasos, and his financial and investment
expertise developed from investments in more than
200 companies in over a dozen different industries, gives
him an experienced perspective on both the technical and the
economic aspects of our business, and provides the Board of
Directors with experienced views on the matters that come before
the Board of Directors. In addition, Mr. Armstrong’s
experience in international investments and transactions has
been valuable in developing international operations as we
expand our global footprint.
Jack H. Dean has served as a director of our company
since 2008. Dr. Dean retired in January 2006 as the
President of U.S. Science and Medical Affairs for
Sanofi-Aventis, a global pharmaceutical company, and as the
Global Director of Preclinical Development for Sanofi-Aventis,
SA. Dr. Dean is currently a director of Drug Development
Advisors, LLC, his drug development and drug safety consulting
company, and a research professor in the departments of
Pharmacology and Toxicology at the College of Medicine at the
University of Arizona. Prior to retiring after his
18-year
tenure with Sanofi-Aventis and legacy companies, Dr. Dean
was the Executive Vice President, Development and Director of
the Department of Toxicology and Vice President, Drug Safety
Assessment, for Sterling Winthrop, a global pharmaceutical
company, as well as the Director of the Sterling Winthrop
Pharmaceuticals Research Center. From 1982 to 1988,
Dr. Dean was the head of the Department of Cellular and
Molecular Toxicology at the Chemical Industry Institute of
Toxicology, and was the head of the Immunotoxicology Section of
National Institute of Environmental Health Services and National
Toxicology Program at the National Institutes of Health.
Dr. Dean holds a B.S. degree in microbiology and a M.S.
degree in medical microbiology from California State University,
Long Beach, and a Ph.D. in molecular biology, with a minor in
biochemistry, from the University of Arizona Health Sciences
Center in Tucson, Arizona. Dr. Dean is a Chevalier in the
Ordre national de la Légion d’honneur for his
contribution to medical and pharmaceutical research.
The Board of Directors believes that Dr. Dean’s more
than 20 years of experience in the drug development
industry and management experience in government, academic, and
the private sector gives him extensive knowledge of our industry
and business. Dr. Dean’s drug development and drug
safety expertise, his advanced degrees in relevant scientific
fields, and his position as a professor of pharmacology and
toxicology give him particular technical expertise related to
our business that is relevant to his qualifications as a
director. In addition, the Board of Directors believes that
Mr. Dean’s consulting experience gives him a broad
perspective and insight on effectively managing and advising a
business.
James R. Macdonald began serving as a director of Old RPS
in 2001 and has continued to serve as a director of our company
following the merger between Cross Shore and Old RPS.
Mr. Macdonald is a Managing Director of First Analysis
Corporation, an investment research and private equity
management company he joined in 1997. Prior to that, he was
employed by Nalco Chemical Company from 1983 to 1997.
Mr. Macdonald is on the boards of several other private
companies as part of his investment role with First Analysis.
Mr. Macdonald graduated with a B.S. degree in civil
engineering from Cornell University and an MBA degree from
Harvard Business School.
The Board of Directors believes that Mr. Macdonald’s
service as a director of Old RPS and our company for more than
nine years gives him significant knowledge of our company, its
history, and its businesses. Additionally, Mr. Macdonald is
a director of three private companies in the healthcare and
outsourced services field, a registered research analyst
covering multiple public healthcare and outsourcing companies,
and the managing partner of private equity funds responsible for
investments in more than 10 companies in the healthcare and
outsourced services industries, giving him specialized insight
into both our business and the business of our clients.
Warren W. Myers began serving as a director of our
company in 2008. Mr. Myers presently serves as a consultant
to the biopharmaceutical industry, and most recently was the
Executive Director, Strategic Sourcing and Procurement at Amgen
Inc., serving Amgen’s Research and Development
organization. Mr. Myers joined Amgen in 1997 and left to
start his consulting business in late 2007. Prior to his time
with Amgen, Mr. Myers was Associate Director, Medical
Research with Bayer Pharmaceuticals. Mr. Myers joined Bayer
in 1991. Mr. Myers
55
holds a B.A. degree in biology from the University of
California, Santa Barbara, and an M.S. degree in technology
management from Pepperdine University.
The Board of Directors believes that Mr. Myers’ more
than 10 years of experience in sourcing and procurement for
the biopharmaceutical industry, his advanced degrees in relevant
fields, and his medical research and development experience
provide Mr. Myers with extensive knowledge of our business
and our industry, as well as the sourcing and procurement needs
of our clients. In addition, Mr. Myers’ consulting
experience gives him a broad perspective and insight on
effectively managing and advising a business.
Daniel Raynor began serving as a director of Old RPS in
2001 and has continued to serve as a director of our company
following the merger between Cross Shore and Old RPS. He is a
managing partner of The Argentum Group, a private equity firm, a
position he has held since co-founding the firm in 1987. In this
capacity, Mr. Raynor also serves as a director of several
privately held companies engaged in outsourced, healthcare and
technology-enabled services in which Argentum’s managed
funds have an equity interest. Mr. Raynor also serves as a
director of Comforce, Inc., a publicly traded New York-based
provider of staffing, consulting and outsourcing solutions.
Previously, Mr. Raynor served as a director and
compensation committee member of NuCo2, Inc., a provider of
carbon dioxide to the hospitality industry, from February 1998
until it was acquired in May 2008 and ceased being a publicly
traded company. He received a B.S. degree in economics from The
Wharton School, University of Pennsylvania.
The Board of Directors believes that Mr. Raynor’s
service as a director of Old RPS and our company for more than
nine years gives him extensive knowledge of our history and
business, and his position as a current or former director of
several private companies in the outsourcing, consulting and
technology-enabled services fields provides him with significant
knowledge of our industry. Additionally, Mr. Raynor
currently serves as a director of a publicly traded company, and
has served as a director of another leading clinical research
organization, and is the managing partner of private equity
funds responsible for investments in more than 50 companies
in the healthcare and outsourcing fields, which provides the
Board of Directors with significant guidance relating to our
business and our industry.
Stephen E. Stonefield became a director of Cross Shore in
2006 and has continued to serve as a director of our company
following the merger between Cross Shore and Old RPS.
Mr. Stonefield is a senior advisor and global strategy
officer for Sabrient Systems LLC, a quantitative equity research
publishing and advisory company. Mr. Stonefield has also
served as Director of Precise Asset Management Pte. Ltd. since
2004, and is also serving in senior advisory roles to two
privately held companies in Asia. In 2003, Mr. Stonefield
retired after three decades of senior positions in investment
banking, largely in Asia, most recently as Chairman, Pacific
Region, of Credit Suisse First Boston, or CSFB, and former
Vice-Chairman and member of the Executive Board of CSFB. Prior
to joining CSFB, Mr. Stonefield was a Managing Director at
Smith Barney in New York, where he was head of Equity Capital
Markets and Financing Services and a member of the firm’s
Steering Committee. Prior to that, he was a Managing Director at
Morgan Stanley in Tokyo and New York. He began his career in
finance at Continental Illinois Ltd. Mr. Stonefield has
also served as a member of the Economic Review Committee for
financial services in Singapore, the Securities Industry Council
of Singapore, and as a member of the International Advisory
Board Kuala Lumpur Stock Exchange in Malaysia.
Mr. Stonefield graduated summa cum laude from
Dartmouth College with a B.A. degree and has an M.A. degree from
Harvard University.
The Board of Directors believes that Mr. Stonefield’s
service as a director of our company since 2006 and his
extensive career in global investment banking enhance his
qualifications to serve on the Board of Directors, especially
with our recent expansion into the Asia-Pacific market. His
service as a director and executive of a number of global
investment banks allows him to provide a broad perspective on
techniques for effectively running a business, while his
particular expertise in finance and investment, especially as it
relates to international investments and management of those
investments, has made Mr. Stonefield particularly valuable
in the growth of our international footprint.
Peter M. Yu began serving as a director of our company in
2008. Mr. Yu founded Cartesian Capital Group, LLC, a global
private equity firm with more than $1 billion in
commitments under management and responsible for more than 12
investments in a variety of fields and industries. Prior to
founding Cartesian, Mr. Yu founded and served as President
and Chief Executive Officer of AIGCP. Under his leadership,
AIGCP became a leading international private equity firm, with
more than $4.5 billion in committed capital. Prior to
founding AIGCP in
56
1996, Mr. Yu served President Clinton as Director to the
National Economic Council, the White House office responsible
for developing and coordinating economic policy. A graduate of
Harvard Law School, Mr. Yu served as President of the
Harvard Law Review and as a law clerk on the U.S. Supreme
Court. Mr. Yu received a B.A. degree from Princeton
University’s Woodrow Wilson School. Mr. Yu is a
director of Banco Daycoval, S.A., a publicly traded bank
headquartered in Brazil. Mr. Yu is also a director of a
number of private entities partly or wholly owned by funds
sponsored by Cartesian Capital Group.
The Board of Directors believes that Mr. Yu’s
background in business management and financial and investment
expertise gives him an experienced perspective on the economic
side of our business and provides our Board of Directors with
access to different and current views on the issues facing our
company and our business. In addition, Mr. Yu’s
extensive experience in international management and
transactions has been instrumental in our recent global
acquisitions and the integration of those acquisitions into our
operations, which has made Mr. Yu also particularly
valuable in the growth of our international footprint.
Board
Composition, Committees, and Board Independence
Our business is managed under the direction of our Board of
Directors, in accordance with the General Corporation Law of the
State of Delaware and our bylaws. Members of the Board of
Directors are kept informed of developments in our business
through discussions with the Chairman and Chief Executive
Officer and other officers, by reviewing materials provided to
them, and by participating in regular and special meetings of
the Board of Directors and its committees.
Our amended and restated certificate of incorporation provides
that the size of our Board of Directors shall consist of not
less than one nor more than eleven directors. Our Board of
Directors currently consists of nine directors, with three
classes of directors, each consisting of three directors. The
directors serve for staggered three-year terms:
|
|
|
| |
•
|
Messrs. Macdonald, Armstrong, and Stonefield are each
Class I Directors whose terms will expire at our 2011
annual meeting of stockholders;
|
| |
| |
•
|
Messrs. Raynor and Myers and Dr. Dean are each
Class II Directors whose terms will expire at our 2012
annual meeting of stockholders; and
|
| |
| |
•
|
Messrs. Perlman and Yu and Dr. Koffer are each
Class III Directors whose terms will expire at our 2013
annual meeting of stockholders.
|
Pursuant to an agreement between us, certain of our
stockholders, and Pangaea One Acquisition Holdings I, LLC,
or Pangaea, an affiliate of Cartesian Capital Group, LLC, or
Cartesian, Pangaea has the right to nominate and have elected up
to two designees to our Board of Directors as long as Pangaea
owns at least 20% of our outstanding common stock, and one
designee as long as Pangaea owns at least 10% of our outstanding
common stock. Pangaea currently owns approximately 25% of our
common stock, and Messrs. Yu and Armstrong were
Pangaea’s nominees and have been elected to our Board of
Directors. The agreement expired on August 30, 2010.
Board of
Directors
Our Board of Directors has a long-standing commitment to sound
and effective corporate governance practices. The foundation for
our corporate governance is the Board of Directors’ policy
that a majority of its members should be independent. We have
applied to list our stock on the NASDAQ Global Market. Under the
applicable standards promulgated by NASDAQ, our Board of
Directors has determined that five, and therefore a majority, of
our current directors, Messrs. Raynor, Macdonald, Meyers
and Stonefield and Dr. Dean, have no relationship which, in
the opinion of the Board of Directors, would interfere with the
exercise of independent judgment in carrying out the
responsibilities of a director, and that each meets the
objective requirement of “independence,” as defined by
the applicable listing rules of NASDAQ.
Mr. Perlman serves as both our Chief Executive Officer and
as Chairman of the Board of Directors. The Board of Directors
believes that it is appropriate for Mr. Perlman to serve as
both Chief Executive Officer and Chairman because the Board of
Directors believes that his role in transforming our company
from a pharmaceutical staffing company into a next generation
CRO uniquely qualifies Mr. Perlman as the individual who
generally sets the agenda for, and leads discussions of,
strategic issues for our company at the Board of Directors
level, and
57
implements those strategic decisions while managing our company
as its Chief Executive Officer. In addition, the Board of
Directors believes that combining Mr. Perlman’s roles
of Chief Executive Officer and Chairman of the Board of
Directors provides an effective bridge between management and
the Board of Directors, which facilitates a better flow of
information in both directions and more efficient execution of
the strategic goals developed by the Board of Directors
throughout our company. Further, the Board of Directors believes
that our corporate governance structure provides the appropriate
balance between the need for consistent strategic direction and
the need for the objectivity and independence of the
non-management directors. The Board of Directors believes that
there are a number of effective oversight mechanisms currently
in place, including that a majority of the Board of Directors is
independent and that our Audit Committee, Nominating and
Corporate Governance Committee and Compensation Committee are
composed entirely of independent directors and perform
significant oversight functions, as detailed below under the
heading “Board Committees.” The Board of Directors
believes that separating the roles of Chairman and Chief
Executive Officer would result in a less efficient
implementation of our strategies without providing any added
benefit beyond that already achieved by our existing governance
structure.
The Board of Directors has considered designating a “lead
independent director” but has elected not to do so at this
time because, as described above, the Board of Directors
believes that the current structure under which Mr. Perlman
serves as Chairman and Chief Executive Officer is the most
efficient and effective leadership structure for the Board of
Directors and for our company. The Board of Directors has
determined that each independent director represents expertise
in the technical, financial, business, and international facets
of our operations, and together provide comprehensive guidance
in developing strategies for our growth. Further, the
composition of our Audit Committee and Compensation Committee,
each of which consists solely of independent directors and is
chaired by a different director, provides our independent
directors with leadership opportunities and promotes the
potential for differing perspectives in these key areas of
governance. Because of these factors, the Board of Directors has
determined that each independent director plays an equally
important role in the overall function of the Board of Directors
and that designating one as the “lead independent
director” would serve no additional benefit beyond that
already achieved by our existing governance structure. However,
with a view towards our commitment to sound and effective
corporate governance practices, the Board of Directors,
depending on circumstances, will consider other leadership
models, including designating a “lead independent
director,” if it becomes appropriate in the future.
Board
Committees
Our Board of Directors has established an Audit Committee,
Nominating and Corporate Governance Committee and a Compensation
Committee, all of which operate pursuant to written charters.
Audit
Committee
Our Audit Committee assists our Board of Directors in the
oversight of the integrity of our consolidated financial
statements, as well as the qualifications, independence and
performance of our independent registered public accounting
firm. Our Audit Committee currently consists of
Mr. Macdonald as the chairman and Messrs. Raynor and
Stonefield, each of whom our Board of Directors has determined
to be independent under SEC and NASDAQ rules. The Audit
Committee currently operates under a written charter that has
been approved by our Board of Directors. The Audit Committee
Charter is reviewed annually by the Audit Committee with any
recommended changes approved by the Board of Directors.
The Audit Committee’s primary responsibility is to assist
the Board of Directors in fulfilling its oversight
responsibilities to our stockholders and other constituencies by
reviewing our financial statements and other financial
information we provide, and reviewing our system of internal
controls, including accounting, auditing and financial reporting
practices. In furtherance of those oversight responsibilities,
the Audit Committee’s primary duties are to:
(1) appoint (and terminate, as appropriate), compensate,
retain and oversee the work of the independent accountants,
including the audit plan, scope and procedures; (2) approve
or pre-approve, as applicable, the engagement of our independent
accountants, the terms of such engagements, and all audit
services and permissible non-audit services provided by the
independent accountants to us; (3) confirm and take action
to assure the independence of the independent accountants by
reviewing and discussing the formal written statement and other
periodic written reports received from the independent
accountants regarding their objectivity and
58
independence, including statements concerning other
relationships and services that may affect their independence;
(4) set clear hiring policies for employees and former
employees of the independent accountants; (5) consider and
review, with the independent accountants, the manager of our
internal accounting controls, and management, the adequacy and
effectiveness of our internal controls, including processes for
identifying significant risks or exposures, and elicit
recommendations for the improvement of such internal control
procedures where desirable; (6) review with the independent
accountants and management (i) our financial reporting
(including financial statements and related footnotes),
(ii) any significant changes required in the independent
accountants’ audit plan, (iii) any material
difficulties or disputes with management encountered during the
course of the audit, (iv) other matters related to the
conduct of the audit, (v) any material written
communications provided by the independent accountants to
management, and (vi) any legal and regulatory matters that
may have a material impact on the financial statements;
(7) review the appointment, replacement, reassignment or
dismissal of the manager of our internal accounting controls;
(8) review the processes of our management for performing
its required certifications under the Sarbanes-Oxley Act;
(9) review and discuss all earnings press releases and
guidance; (10) review and approve all related party
transactions; (11) establish procedures for (i) the
receipt, retention and treatment of complaints received by us
regarding accounting, internal accounting controls, or auditing
matters, and (ii) the confidential, anonymous submission by
our employees of concerns regarding these issues;
(12) report Audit Committee actions to the Board of
Directors with such recommendations as the Audit Committee may
deem appropriate; (13) prepare the audit committee report
required to be filed with the SEC; (14) review and reassess
the adequacy of the Audit Committee’s Charter annually and
submit recommended amendments to the Board of Directors for
approval; (15) investigate any matter brought to the
attention of the Audit Committee within the scope of the Audit
Committee’s duties, with the power to retain and determine
the appropriate compensation for independent legal, accounting,
financial and other advisors as the Audit Committee may deem
necessary or appropriate to carry out its duties, at our
expense; and (16) enforce our Code of Business Conduct and
Ethics. The Audit Committee has been established in accordance
with Section 3(a)(58)(A) of the Securities Exchange Act of
1934, as amended.
Messrs. Macdonald, Raynor and Stonefield all have
significant past employment experience in finance, and our Board
of Directors has designated each of them as audit committee
financial experts under SEC rules.
In addition to the Audit Committee’s responsibilities set
forth above, the Audit Committee has, pursuant to its current
charter, primary responsibility for the oversight of risks that
could affect us. The entire Board of Directors is actively
involved in, and has ultimate responsibility for, the oversight
of risks facing us and management of that risk, but the Audit
Committee conducts preliminary evaluations of risk and addresses
risk prior to review by the Board of Directors. The Audit
Committee considers and reviews, in conjunction with our
internal control processes, independent public accounting firm
and management, the adequacy of our internal controls, including
the processes for identifying significant risks or exposures,
and elicits recommendations for the improvements of such
procedures where desirable. In addition to the Audit
Committee’s role, the full Board of Directors is involved
in oversight and administration of risk and risk management
practices by overseeing members of senior management in their
risk management capacities, and regularly reviewing and
analyzing the investment of our available cash and accompanying
risk levels. Members of our senior management have
day-to-day
responsibility for risk management and establishing risk
management practices, and members of management are expected to
report matters relating to financial or accounting risk to the
Audit Committee, and to report all other matters directly to the
Board of Directors as a whole. Members of our senior management
have an open line of communication to the Board of Directors and
have the discretion to raise issues from time to time in any
manner they deem appropriate, and management’s reporting on
issues relating to risk management typically occurs through
direct communication with directors or committee members as
matters requiring attention arise.
Compensation
Committee
The Compensation Committee operates under a written charter that
has been approved by the Board of Directors. The Compensation
Committee Charter is reviewed annually by the Compensation
Committee with any recommended changes approved by the Board of
Directors. The Compensation Committee is responsible for
formulating, evaluating, and approving the compensation of our
executive officers, overseeing all compensation programs
involving the issuance of our common stock and other equity
securities, and preparing the annual report on executive
compensation for inclusion in our annual proxy statement. The
Compensation Committee:
59
(1) annually reviews, determines, and approves the
compensation of our Chief Executive Officer and all other
executive officers, including the use of cash incentives and
deferred compensation plans; (2) determines our policy with
respect to the application of Section 162(m) of the
Internal Revenue Code; (3) approves compensation programs
and grants involving the use of our common stock and other
equity securities; (4) reviews, approves, and when
appropriate, recommends to our Board of Directors for approval,
any employment agreements or any severance arrangements for our
executive officers; (5) reviews and discusses with
management our analysis and discussion of compensation for
inclusion in our annual proxy statement; and (6) reviews
our incentive compensation programs to determine whether they
encourage excessive risk-taking and assesses, at least annually,
the relationship between risk management policies and practices
and compensation. To perform its duties, the Compensation
Committee has the power to retain, at our expense, experts and
other professionals, including compensation consultants.
Our Compensation Committee consists of Mr. Raynor as the
chairman and Mr. Stonefield, each of whom our Board of
Directors has determined to be independent under NASDAQ listing
rules.
The section of this prospectus entitled “Compensation
Discussion and Analysis” includes additional information
about the processes and procedures of the Board of Directors and
the Compensation Committee for considering and determining
executive officer compensation.
In addition to the Audit Committee, the Compensation Committee
considers the risks that may be implicated by our executive
compensation practices, as described under the heading
“Executive Compensation—Compensation Discussion and
Analysis—Performance Bonuses.”
Nominating
and Corporate Governance Committee
The Nominating and Corporate Governance Committee, or Nominating
Committee, operates under a written charter that has been
approved by our Board of Directors. The Nominating Committee
Charter is reviewed annually by the Nominating Committee with
any recommended changes approved by the Board of Directors. The
Nominating Committee is responsible for establishing criteria
for selecting new directors, identifying, screening, and
recruiting new directors, recommending director nominees to the
full Board of Directors, and recommending to the Board of
Directors a set of corporate governance principles applicable to
our company. The Nominating Committee: (1) establishes and
reviews as necessary the criteria for selecting new directors,
including the review and approval of any written policies
relating to the submission of candidates for director by
stockholders; (2) assesses the contributions of the current
directors to the Board of Directors in connection with their
re-nomination and makes recommendations to the Board of
Directors for director nominees at our annual meeting of
stockholders and to fill vacancies; (3) recommends
standards for determining director independence to the Board of
Directors and periodically reviews director independence;
(4) recommends and reviews corporate governance guidelines
to the Board of Directors, including guidelines in our bylaws
and committee charters; (5) oversees the evaluation of the
effectiveness of the Board of Directors; (6) recommends the
assignment of members of committees to the Board of Directors;
and (7) periodically reviews all elements of non-employee
director compensation and makes recommendations to the Board of
Directors regarding changes. To perform its duties, the
Nominating Committee has the power to retain, at our expense,
experts and other professionals, including director search firms.
Our Nominating Committee consists of Dr. Dean as the
chairman and Mr. Myers, each of whom our Board of Directors
has determined to be independent under NASDAQ listing rules.
Compensation
Committee Interlocks and Insider Participation
Daniel Raynor and Stephen Stonefield were the only members of
our Compensation Committee during the last fiscal year, neither
of whom has ever been an employee or officer of our company.
None of our executive officers serves as a member of the Board
of Directors or compensation committee, or other committee
serving an equivalent function, of any entity that has one or
more executive officers who serve as members of our Board of
Directors or our Compensation Committee.
60
Director
Compensation
Until August 30, 2007, the Board of Directors of Cross
Shore consisted of Edward V. Yang (chair), Dennis M. Smith,
Stephen E. Stonefield and Jon A. Burgman. The directors were not
paid compensation of any kind until August 30, 2007, with
the exception of reimbursement for
out-of-pocket
expenses incurred by or on behalf of the director in identifying
and performing due diligence on potential acquisition targets.
Effective August 29, 2007, Messrs. Smith and Yang each
entered into service agreements with us. Messrs. Smith and
Yang resigned as directors effective December 6, 2007, but
their respective service agreements remained in place.
Mr. Burgman resigned as a director on August 30, 2007,
and Mr. Stonefield remained as a director of our company.
Pursuant to the service agreements, Messrs. Smith and Yang
provided consulting services, were entitled to receive their
respective annual base salaries ($60,000 each), and were
eligible to participate in all of our benefit plans and equity
incentive plans, and to receive an annual bonus at the sole
discretion of our Board of Directors for a period of two years.
Under the terms of this arrangement, Messrs. Smith and Yang
received $60,000 each for the year ended December 31, 2008
and additional compensation of $15,168 and $10,417,
respectively, related to our cost of medical, dental and other
insurance premiums covered under our benefit plans.
Messrs. Smith and Yang received $40,000 each for the year
ended December 31, 2009 and additional compensation of
$9,152 and $6,391, respectively, related to the cost of medical,
dental and other insurance premiums covered under our benefit
plans. The service agreements were not renewed and expired on
their terms on August 29, 2009.
In conjunction with the merger with Old RPS, Daniel Perlman,
Harris Koffer, Daniel Raynor and James Macdonald were appointed
as directors. Thomas Armstrong, Peter Yu, Warren Myers, and Jack
Dean were appointed as directors on May 12, 2008.
Dr. Dean and Messrs. Stonefield and Myers are each
entitled to receive $6,250 per quarter for their service on our
Board of Directors, totaling $25,000 annually. In addition,
Dr. Dean and Mr. Myers were each granted options to
purchase 5,000 shares of our common stock at an exercise
price of $3.70 per share on the day they were appointed to the
Board of Directors in May 2008, and Mr. Stonefield was
granted an option to purchase 5,000 shares of our common
stock at an exercise price of $1.75 per share on May 20,
2009. One-third of the stock options granted to Dr. Dean
and Messrs. Myers and Stonefield vest on each anniversary
of the grant date over three years. The directors of our
predecessor, Old RPS, were not compensated for their services.
Mr. Perlman and Dr. Koffer receive no additional
compensation for their service as directors of RPS. Compensation
for Mr. Perlman and Dr. Koffer is set forth in the
Summary Compensation Table below. Messrs. Yu, Armstrong,
Raynor and Macdonald receive no compensation for their service
as directors.
The following table provides compensation information for the
year ended December 31, 2009 for each member of our Board
of Directors serving during 2009.
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Fees Earned or
|
|
Option
|
|
|
|
Name(1)(3)
|
|
Paid in Cash
|
|
Awards(2)
|
|
Total
|
|
|
|
Stephen E. Stonefield
|
|
$
|
25,000
|
|
|
$
|
4,350
|
|
|
$
|
29,350
|
|
|
Jack H. Dean
|
|
$
|
25,000
|
|
|
|
—
|
|
|
$
|
25,000
|
|
|
Warren W. Myers
|
|
$
|
25,000
|
|
|
|
—
|
|
|
$
|
25,000
|
|
|
Peter M. Yu
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
Thomas R. Armstrong
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
Daniel Perlman
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
Harris Koffer
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
Daniel Raynor
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
James Macdonald
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
|
|
(1) |
|
In 2009, no director received any stock awards, non-equity
incentive plan compensation, or other compensation, nor were
there any pensions or nonqualified deferred compensation
available to the directors solely as compensation for their
services as directors. Therefore, the columns with the headings
“Stock Awards,” “Non-Equity Incentive Plan
Compensation,” “Changes in Pension Value and
Nonqualified Deferred Compensation Earnings,” and “All
Other Compensation” have been omitted from this table. |
61
|
|
|
|
(2) |
|
Amount reflects the grant date fair value of options granted in
2009 computed in accordance with Codification Topic 718
regarding stock compensation, excluding the effect of estimated
forfeitures. The exercise price of the options granted to
Mr. Myers and Dr. Dean on May 12, 2008 was $3.70
per share with an aggregate fair value of $9,450 each. The
exercise price of the options granted to Mr. Stonefield on
May 20, 2009 was $1.75 per share with an aggregate fair
value of $4,350. The fair value was calculated using a
Black-Scholes option-pricing model for those grants issued to
directors and executive officers. For a discussion of the
assumptions utilized in the Black-Scholes option-pricing model,
please see Note 2 to our consolidated financial statements
for the fiscal year ended December 31, 2009, which are
included in this prospectus. As of December 31, 2009, each
of Messrs. Stonefield and Myers and Dr. Dean held
stock options to purchase an aggregate of 5,000 shares. As
of December 31, 2009, Mr. Perlman and Dr. Koffer
held stock options to purchase 450,000 and 989,279 shares,
respectively. |
| |
|
(3) |
|
Messrs. Yang and Smith did not serve as directors during
the 2009 fiscal year, and are therefore not included in this
table. Compensation arrangements with Messrs. Smith and
Yang are described above under the heading entitled
“Director Compensation.” |
62
Executive
Compensation
Compensation
Discussion and Analysis
Our Compensation Committee, currently comprised of
Mr. Raynor as chairman and Mr. Stonefield, has overall
responsibility for reviewing and approving the recommendations
of management with respect to the appropriate compensation
policies, programs and levels, and for continually monitoring
adherence to our compensation philosophy. The Compensation
Committee is responsible for ensuring that the total
compensation paid to our executive officers is fair, reasonable
and competitive and approves all changes to the compensation
packages for our Chief Executive Officer and our other executive
officers.
Objectives
of Our Compensation Program
The primary objective of our compensation program is to ensure
that members of our executive management team are provided with
appropriate incentives to encourage enhanced performance and
are, in a fair and responsible manner, rewarded for their
individual contributions to our success. The Compensation
Committee reviews and approves our compensation program to
provide sufficient compensation opportunities for executives in
order to attract, retain and motivate the best possible
management team to lead us in the achievement of both our short-
and long-term performance goals. The Compensation Committee
believes that the first step in attracting and retaining
executives is to ensure that our compensation program is
competitive in the marketplace. Furthering this goal, the
compensation packages for each of our named executive officers,
including the Chief Executive Officer, consist of a base salary,
opportunities for annual cash compensation in the form of
performance bonuses, and long-term compensation in the form of
equity ownership.
Each of our named executive officers has a written employment
agreement setting forth the material terms of his or her
employment. The material terms of the named executive
officers’ employment agreements currently in effect are
described below under “Named Executive Officer Employment
Agreements.”
On an ongoing basis, the Compensation Committee determines
adjustments to base salary, the amount and timing of performance
bonuses, the performance targets required to be achieved for the
payment of performance bonuses, and the appropriate level and
targets for other compensation, if any, to be paid to our named
executive officers. The Compensation Committee, annually and as
it otherwise deems appropriate, meets with the Board of
Directors to obtain recommendations with respect to our
compensation programs for executives and other employees. The
Board of Directors may make recommendations to the Compensation
Committee on base salary, performance targets and other terms,
which the Compensation Committee may consider. No director or
executive is involved in any decisions as to his or her own
compensation.
Short-Term
versus Long-Term Compensation
Short-term compensation paid to our named executive officers
includes:
|
|
|
| |
•
|
base salaries, which are paid in regular installments in
accordance with our general payroll practices and are subject to
customary withholding;
|
| |
| |
•
|
cash performance bonuses at the sole discretion of the Board of
Directors or based on achieving business and financial goals
determined by the Board of Directors or the Compensation
Committee and as approved by the Compensation Committee; and
|
| |
| |
•
|
perquisites and personal benefits, which are paid consistent
with our policies in appropriate circumstances.
|
Our long-term compensation currently consists of grants of stock
options. Our executives may also participate in our 401(k) plan,
which is open to all employees who have completed at least three
months of service and are at least 21 years of age.
The Compensation Committee seeks to balance the need of our
named executive officers for current income with the need to
create longer-term incentives that are directly tied to
achievement of our long-term targets and the enhancement of
stockholder value. Our allocation between cash and non-cash and
between short-term and long-term incentive compensation is set
by the terms of the individual employment agreement and the
terms of our equity incentive plan. Income from elements of
incentive compensation is realized as a result of the
performance of
63
our company or the executive, depending on the type of award,
compared to goals proposed and approved by the Compensation
Committee on an annual basis.
Benchmarking
and Use of Compensation Consultants
The Compensation Committee does not engage in formal
benchmarking when setting compensation for our named executive
officers, including Mr. Perlman, although the Compensation
Committee has in the past and would expect in the future to
consider information regarding compensation of executive
officers of other similar companies in revising our compensation
plan. Neither the Board of Directors nor the Compensation
Committee has engaged compensation consultants for the purposes
of determining executive or director compensation. We have
engaged compensation consultants for the purposes of general
human resources and benefits advisory services that are
applicable and available to all salaried employees, and the fees
for such services did not exceed $120,000 in 2009.
Compensation
Components
The principal compensation components for the named executive
officers consists of the following:
|
|
|
| |
•
|
Base salary: fixed pay that takes into account an
individual’s role and responsibilities, experience,
expertise, and individual performance.
|
| |
| |
•
|
Performance cash bonuses: paid to reward attainment of annual
business and financial performance targets that the Board of
Directors set and the Compensation Committee approved.
|
| |
| |
•
|
Long-term incentives: issued to reward increases in stockholder
value over longer terms and align the interests of our
executives with the interests of our stockholders.
|
Historically, the largest portion of our executives’
compensation packages have been in the form of annual base
salary.
Base
Salary
The Compensation Committee adjusts base salaries of our named
executive officers based on a number of factors, with the
primary factor being the base salary agreed upon in each named
executive officer’s employment agreement, but also
including an individual’s role and responsibilities,
experience, expertise, individual job performance relative to
his or her responsibilities, impact on development and
achievement of our business strategy, and competitive market
factors for comparable talent. The Compensation Committee may
also adjust base salaries from time to time if a change in scope
of the officer’s responsibilities justifies an adjustment
or, in limited circumstances, to maintain salary competitiveness.
In 2009, the Compensation Committee increased the base salaries
of Messrs. Perlman and Bell and Dr. Koffer by
approximately 1%, and increased Ms. Brennan’s base
salary by approximately 2%, when compared to the base salaries
earned by those named executive officers in 2008, while
Mr. Shah’s base salary remained the same.
Base salary for our named executive officers in fiscal years
2007 through 2009 is shown in the Summary Compensation Table
below, under the heading “Salary.”
Annual
Performance Bonuses
The Compensation Committee believes that some portion of overall
cash compensation for executive officers should be contingent on
the successful achievement of business and financial targets
determined by the Board of Directors on an annual basis. To that
end, and depending on our financial and operating performance,
cash compensation is augmented in appropriate circumstances by
the payment of performance bonuses. These performance-based
bonuses more closely align an individual’s overall
compensation with his or her performance and our financial
performance. The Compensation Committee believes that this bonus
arrangement focuses our executives on strategic goals
established for the fiscal year and aligns management’s
interests with those of our stockholders. The Compensation
Committee historically established a bonus pool to be
distributed to our executive officers upon the achievement of
specified objectives.
64
In prior years, the Board of Directors had the discretion to
increase the amount available in the aggregate bonus pool based
on whether we exceed an established earnings before interest,
taxes, depreciation, and amortization, or EBITDA, target. For
example, if EBITDA exceeded the established target, a percentage
ranging from 15% to 100% of such excess could be added to the
aggregate amount available for bonuses. Exceeding the EBITDA
performance target could result in performance bonuses exceeding
the amounts initially reserved in the aggregate bonus pool, and
falling short of the EBITDA performance target could result in
performance bonuses less than the amount reserved for the
aggregate bonus pool. The Compensation Committee has sole
discretion whether the excess over targeted EBITDA will be added
to the aggregate bonus pool, the percentage over targeted EBITDA
that can be contributed to the aggregate bonus pool, and the
percentage of the aggregate bonus pool to be paid to individual
executive officers.
In determining the amounts to be paid to individual executive
officers out of the established bonus pool, the Board of
Directors considered factors including the performance of the
individual executive and our performance as a whole, including
performance as measured by EBITDA. In fiscal year 2008, the
Board of Directors reviewed and approved an annual budget that
included a provision for awarding performance bonuses to the
executive officers based upon achieving performance targets
established by the Board of Directors for 2008. Depending on
whether we achieved, exceeded or fell short of the financial
target established by the Board of Directors, the Compensation
Committee determined, in its sole discretion, whether an amount
equal to or greater or less than the budgeted amount was paid in
performance bonuses. The targets established by the Board of
Directors serve as general guidelines for determining bonuses,
but the ultimate determination regarding the performance bonus
amount awarded to individual executive officers is at the
discretion of the Compensation Committee, taking into account
any contractual provisions in an executive’s employment
agreement. During 2008, progress towards meeting the financial
target was evaluated on a quarterly basis, and each executive
officer was awarded 50% of the bonus that the Board of Directors
determined that executive officer was entitled to receive for
the relevant quarter, and the remaining bonus amounts were paid
at the end of the fiscal year.
In 2009, the Board of Directors did not establish a performance
bonus target using the same criteria used in 2008, and instead
elected to award bonuses at its discretion based on overall
financial performance, including EBITDA. Based upon a review of
2009 financial performance, the Compensation Committee
established a discretionary bonus pool to be distributed to the
executive officers. The performance bonus amounts were
determined in the fourth quarter of 2009 and approved by the
Compensation Committee and paid in January 2010.
The performance target for 2008 and the aggregate bonus pool
available to our named executive officers for 2008 and 2009 are
summarized below:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Performance
|
|
|
|
|
|
|
|
|
|
Target
|
|
Available Aggregate
|
|
|
|
|
|
Fiscal Year
|
|
(EBITDA)
|
|
Bonus Pool
|
|
|
|
|
|
|
|
2008
|
|
$13,000,000
|
|
$720,000
|
|
|
|
|
|
2009
|
|
N/A
|
|
$350,000
|
|
|
|
|
The Board of Directors, in connection with its assessment of
performance criteria for 2009, concluded that while the
consideration of the performance of both the individual and our
company as a whole in determining performance bonuses may
promote prudent risk-taking in support of our objectives,
balancing our financial performance against an individual’s
personal performance and our overall ability to reach our
objectives did not encourage or promote inappropriate
risk-taking by the participants.
Comparing performance bonuses as a percentage of base salary
earned by the named executive officers in 2008 to 2009, the
performance bonuses for all of our executive officers increased
between the two years. As a percentage of base salary,
Mr. Perlman’s performance bonus increased 12%,
Dr. Koffer’s increased by 10%, Mr. Bell’s
increased by 9%, Ms. Brennan’s increased by 5%, and
Mr. Shah’s increased by 8%.
Performance bonuses for our named executive officers earned in
fiscal 2007, 2008 and 2009 are shown in the Summary Compensation
Table below, under the heading “Non-Equity Incentive Plan
Compensation.”
65
Long-Term
Incentives
The Compensation Committee considers incentives that vest over
time to be an essential component of executive compensation so
that a proper balance exists between short- and long-term
considerations and enhancing stockholder value. The Compensation
Committee believes that management ownership of stock and
equity-based performance compensation arrangements are useful
tools to align the interests of management with those of our
stockholders. Certain of our named executive officers previously
participated in the equity incentive plan maintained by Old RPS.
These named executive officers received option grants under the
Old RPS plan that vested over a period of three years from the
date of grant. After the merger between Cross Shore and Old RPS,
we adopted the 2007 Equity Incentive Plan, which we refer to in
this prospectus as the 2007 Plan, and terminated the plan
maintained by Old RPS. All prior awards made under the Old RPS
plan were terminated unless exercised in connection with the
merger. Options to purchase common stock of Old RPS were
replaced with options to purchase our common stock, based upon
the exchange ratio established in the merger agreement. As a
result, Mr. Bell, Dr. Koffer, Ms. Brennan and
Mr. Shah received replacement options to purchase
72,560 shares, 899,279 shares, 83,445 shares and
130,610 shares, respectively.
In December 2007, following the merger between Cross Shore and
Old RPS, we granted options to Mr. Perlman, Mr. Bell
and Dr. Koffer to purchase 450,000 shares,
180,000 shares and 120,000 shares, respectively, as an
additional incentive for them to remain with our company and to
align their interests with those of our public company
stockholders. These options vest over a three-year period
through December 6, 2010 and have an exercise price of
$5.05 per share, which was the closing price of our common stock
on the AIM on the date of grant. The aggregate grant date fair
value of stock option awards in 2007 for Messrs. Perlman
and Bell and Dr. Koffer was $760,500, $304,200, and
$202,800 respectively. Ms. Brennan and Mr. Shah were
not granted any stock options in 2007.
Because retention of these executives following the merger
between Cross Shore and Old RPS was one factor in the decision
to make stock option awards following the merger, and those
executives have remained with us since the merger, the
Compensation Committee’s goal of retaining these executive
officers in the years immediately following the merger was
largely completed and therefore no additional equity
compensation was awarded in 2008 or 2009. The Compensation
Committee believes that the possible forfeiture of options upon
the departure of our executives provides an additional retention
incentive, and the vesting of options over time aligns the
interests of our executives with our stockholders. To that end,
our Compensation Committee authorized the grant of an option to
purchase 100,000 shares of common stock and issued
40,000 shares of restricted stock to Mr. Bell in
August 2010 as a retention incentive and in recognition of his
continued service to our company.
Other
Compensation and Benefits
All of our named executive officers are eligible to participate
in certain benefit plans and arrangements offered to employees
generally, including health, dental, life and disability
insurance, our 401(k) plan, and our Section 125 cafeteria
plan. With the exception of the perquisites described below, we
do not generally differentiate between the benefits we offer our
named executives and the benefits we offer our other employees.
We do not maintain any executive retirement programs such as
executive pension plans, deferred compensation plan, or other
executive retirement benefits. We intend to continue to maintain
our current benefits for our executive officers, although the
Compensation Committee in its discretion may reduce, revise,
amend or add to any executive’s benefits or perquisites as
it deems advisable.
We provide our executive officers with limited perquisites and
other personal benefits that are not otherwise available to all
of our employees. These perquisites consist of
automobile-related reimbursements and premiums paid on behalf of
the executive for health, dental, life and disability insurance
coverage that is not made generally available to our other
employees. Perquisites and personal benefits are taken into
account in evaluating the total compensation package for our
named executive officers. We believe the few perquisites and
other personal benefits that we make available to our executive
officers are reasonable and consistent with our overall
compensation program, and better enable us to attract and retain
superior employees for key positions. Certain perquisites may be
subject to the approval of the Compensation Committee, depending
on the amount and type. Additional details are provided in the
“All Other Compensation Table” below.
66
Tax
Considerations
Section 162(m) of the Internal Revenue Code, which is
referred to in this prospectus as the Code, generally disallows
a tax deduction for compensation in excess of $1.0 million
paid to our Chief Executive Officer and the four other most
highly paid executive officers. Qualifying performance-based
compensation is not subject to the deduction limitation if
specified requirements are met. The Compensation Committee
generally intends to structure the performance-based portion of
our executive compensation, when feasible, to comply with the
exemptions provided in Section 162(m) so that the
compensation remains tax deductible to us. However, the Board of
Directors may, in its judgment, authorize compensation payments
that do not comply with the exemptions in Section 162(m)
when it believes that such payments are appropriate to attract
and retain executive talent.
Summary
Compensation Table
The table below summarizes for the fiscal years ended
December 31, 2009, 2008, and 2007 the total compensation
earned by our Chief Executive Officer, Chief Financial Officer
and our three other most highly compensated executive officers
as of December 31, 2009. These persons are referred to in
this prospectus as the named executive officers.
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Non-Equity
|
|
Option
|
|
All Other
|
|
|
|
|
|
Fiscal
|
|
|
|
Incentive Plan
|
|
Awards(4)
|
|
Compensation(2)
|
|
|
|
Name and Principal Position(1)(3)
|
|
Year
|
|
Salary ($)
|
|
Compensation ($)
|
|
($)
|
|
($)
|
|
Total ($)
|
|
|
|
Daniel M. Perlman
|
|
|
2007
|
|
|
|
350,000
|
|
|
|
171,205
|
|
|
|
760,500
|
|
|
|
327,741
|
|
|
|
1,609,446
|
|
|
Chief Executive Officer
|
|
|
2008
|
|
|
|
350,000
|
|
|
|
45,662
|
|
|
|
—
|
|
|
|
75,934
|
|
|
|
471,596
|
|
|
|
|
|
2009
|
|
|
|
353,750
|
|
|
|
88,114
|
|
|
|
—
|
|
|
|
70,888
|
|
|
|
512,752
|
|
|
Steven Bell
|
|
|
2007
|
|
|
|
280,000
|
|
|
|
91,309
|
|
|
|
304,200
|
|
|
|
273,863
|
|
|
|
949,372
|
|
|
Chief Financial Officer
|
|
|
2008
|
|
|
|
280,000
|
|
|
|
24,353
|
|
|
|
—
|
|
|
|
25,128
|
|
|
|
329,481
|
|
|
|
|
|
2009
|
|
|
|
283,750
|
|
|
|
46,994
|
|
|
|
—
|
|
|
|
27,986
|
|
|
|
358,730
|
|
|
Harris Koffer
|
|
|
2007
|
|
|
|
300,000
|
|
|
|
122,289
|
|
|
|
202,800
|
|
|
|
78,780
|
|
|
|
703,869
|
|
|
President and Chief
|
|
|
2008
|
|
|
|
300,000
|
|
|
|
32,616
|
|
|
|
—
|
|
|
|
30,048
|
|
|
|
362,664
|
|
|
Operating Officer
|
|
|
2009
|
|
|
|
303,750
|
|
|
|
62,938
|
|
|
|
—
|
|
|
|
30,568
|
|
|
|
397,256
|
|
|
Janet Brennan
|
|
|
2007
|
|
|
|
260,000
|
|
|
|
52,992
|
|
|
|
—
|
|
|
|
19,159
|
|
|
|
332,151
|
|
|
Chief International Affairs
|
|
|
2008
|
|
|
|
260,000
|
|
|
|
14,134
|
|
|
|
—
|
|
|
|
19,907
|
|
|
|
294,041
|
|
|
Officer and Executive Vice President
|
|
|
2009
|
|
|
|
265,833
|
|
|
|
27,273
|
|
|
|
—
|
|
|
|
21,145
|
|
|
|
314,251
|
|
|
Samir Shah
|
|
|
2007
|
|
|
|
250,000
|
|
|
|
81,526
|
|
|
|
—
|
|
|
|
26,101
|
|
|
|
357,627
|
|
|
Executive Vice President,
|
|
|
2008
|
|
|
|
250,000
|
|
|
|
21,744
|
|
|
|
—
|
|
|
|
26,970
|
|
|
|
298,714
|
|
|
Strategic Development
|
|
|
2009
|
|
|
|
250,000
|
|
|
|
41,959
|
|
|
|
—
|
|
|
|
29,247
|
|
|
|
321,206
|
|
|
|
|
|
|
|
(1) |
|
Neither the Chief Executive Officer, Chief Financial Officer nor
any of our other three most highly compensated executive
officers as of December 31, 2009 received any compensation
in the form of stock awards, bonuses, or a change in pension
value and nonqualified deferred compensation earnings in 2007,
2008 or 2009. Accordingly, the corresponding columns have been
omitted. |
| |
|
(2) |
|
Additional details regarding the amounts included in the
“All Other Compensation” column are set forth in the
table immediately following this Summary Compensation Table. |
| |
|
(3) |
|
Mr. Dennis M. Smith was the Chief Executive Officer of
Cross Shore until completion of the merger, when
Mr. Perlman replaced him as Chief Executive Officer.
Mr. Smith did not receive any compensation for services
rendered as Chief Executive Officer of Cross Shore during 2006
and until August 29, 2007, except reimbursement for
out-of-pocket
expenses incurred in identifying and performing due diligence on
a target for a qualified business combination. Our compensation
arrangements with Mr. Smith are set forth under the heading
“Director Compensation” in this prospectus. |
| |
|
(4) |
|
The amounts reported in the “Option Awards” column
represent the fair value of options granted in the indicated
year, excluding the effect of estimated forfeitures. |
67
All Other
Compensation Table
The table below summarizes the amounts included in the “All
Other Compensation” column of the Summary Compensation
Table earned by our named executive officers in 2007, 2008 and
2009.
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
401(k) Matches,
|
|
|
|
|
|
|
|
|
|
|
|
Medical, Dental and
|
|
Automobile-Related
|
|
Bonus upon
|
|
|
|
|
|
Fiscal
|
|
other Insurance
|
|
Compensation(1)
|
|
Completion of the
|
|
|
|
Name
|
|
Year
|
|
Premiums ($)
|
|
($)
|
|
Merger ($)
|
|
Total ($)
|
|
|
|
Daniel M. Perlman
|
|
|
2007
|
|
|
|
29,229
|
|
|
|
48,512
|
|
|
|
250,000
|
|
|
|
327,741
|
|
|
Chief Executive Officer
|
|
|
2008
|
|
|
|
30,521
|
|
|
|
45,413
|
|
|
|
—
|
|
|
|
75,934
|
|
|
|
|
|
2009
|
|
|
|
31,053
|
|
|
|
39,835
|
|
|
|
—
|
|
|
|
70,888
|
|
|
Steven Bell
|
|
|
2007
|
|
|
|
14,863
|
|
|
|
9,000
|
|
|
|
250,000
|
|
|
|
273,863
|
|
|
Chief Financial Officer
|
|
|
2008
|
|
|
|
16,128
|
|
|
|
9,000
|
|
|
|
—
|
|
|
|
25,128
|
|
|
|
|
|
2009
|
|
|
|
16,671
|
|
|
|
11,315
|
|
|
|
—
|
|
|
|
27,986
|
|
|
Harris Koffer
|
|
|
2007
|
|
|
|
16,780
|
|
|
|
12,000
|
|
|
|
50,000
|
|
|
|
78,780
|
|
|
President and Chief Operating
|
|
|
2008
|
|
|
|
18,048
|
|
|
|
12,000
|
|
|
|
—
|
|
|
|
30,048
|
|
|
Officer
|
|
|
2009
|
|
|
|
18,568
|
|
|
|
12,000
|
|
|
|
—
|
|
|
|
30,568
|
|
|
Janet Brennan
|
|
|
2007
|
|
|
|
6,111
|
|
|
|
13,048
|
|
|
|
—
|
|
|
|
19,159
|
|
|
Chief International Affairs Officer
|
|
|
2008
|
|
|
|
6,539
|
|
|
|
13,368
|
|
|
|
—
|
|
|
|
19,907
|
|
|
and Executive Vice President
|
|
|
2009
|
|
|
|
6,211
|
|
|
|
14,934
|
|
|
|
—
|
|
|
|
21,145
|
|
|
Samir Shah
|
|
|
2007
|
|
|
|
14,684
|
|
|
|
11,417
|
|
|
|
—
|
|
|
|
26,101
|
|
|
Executive Vice President, Strategic
|
|
|
2008
|
|
|
|
15,963
|
|
|
|
11,007
|
|
|
|
—
|
|
|
|
26,970
|
|
|
Development
|
|
|
2009
|
|
|
|
16,598
|
|
|
|
12,649
|
|
|
|
—
|
|
|
|
29,247
|
|
|
|
|
|
|
|
(1) |
|
Automobile-related compensation includes monthly automobile
lease payments, payment or reimbursement of automobile insurance
premiums, automobile maintenance, repairs, and gasoline, or a
flat automobile allowance, as applicable. |
Named
Executive Officer Employment Agreements
Each of our named executive officers has a written employment
agreement setting forth the material terms of his or her
employment as summarized below. Messrs. Perlman and Bell
and Dr. Koffer entered into new employment agreements in
conjunction with the merger between Cross Shore and Old RPS. We
entered into an employment agreement with Mr. Shah on
December 6, 2007, following the merger. These employment
agreements were reviewed by the Compensation Committee and
approved by the respective boards of directors of Old RPS and
Cross Shore. Janet Brennan’s employment agreement entered
into before the merger remained in effect after the merger.
Under these employment agreements, these executives receive
annual base salaries at rates not less than the amounts reported
in the Summary Compensation Table for 2009, which may be
adjusted from time to time.
Daniel M.
Perlman
We entered into an employment agreement with Mr. Perlman on
April 26, 2007 to serve as our Chairman and Chief Executive
Officer, and the employment agreement became effective on
August 29, 2007, upon completion of the merger with Old
RPS. The employment agreement has an initial term of three
years, and will be automatically renewed for successive one-year
periods after the initial term, unless terminated by either us
or Mr. Perlman within a specified period prior to the end
of the initial term or any renewal term.
Mr. Perlman is entitled to receive a base salary of
$400,000 per year, which includes automobile payments, expenses,
insurance, and maintenance, or such higher rate as the Board of
Directors may designate from time to time, payable in accordance
with our normal payroll practices. Mr. Perlman is eligible
to receive an annual target bonus equal to 60% of his base
salary, with the actual amount of any bonus based on achieving
our business and financial objectives. In addition,
Mr. Perlman is entitled to participate in the 2007 Plan,
with any stock options granted to be incentive stock options to
the maximum extent possible, and any other compensation programs
available to executive officers and all benefit plans, including
medical, dental, retirement, short- and long-term disability and
other such plans as we may establish from time to time for our
executives or employees generally.
68
We have agreed under the employment agreement to obtain and
maintain a life insurance policy covering the life of
Mr. Perlman with death benefits in an aggregate amount of
not less than $4,000,000, with the beneficiaries of such policy
to be selected by Mr. Perlman. Mr. Perlman is also
entitled under the employment agreement to receive the benefits
under a disability insurance policy maintained by us that would
pay Mr. Perlman at least 60% of his then current annual
base salary. Mr. Perlman is also entitled to receive
severance payments and benefits in the event his employment is
terminated by us or he voluntarily resigns his employment, as
well as acceleration of his equity awards in connection with a
change of control, in each case as described below under
“Potential Payments Upon Termination or Change in
Control.”
Additionally, Mr. Perlman has made customary agreements and
representations regarding confidentiality, assignment of
inventions, and non-competition, and we have made agreements and
representations regarding indemnification under our certificate
of incorporation and bylaws.
Harris
Koffer
We entered into an employment agreement with Dr. Koffer on
April 26, 2007, to serve as our President and Chief
Operating Officer and as a director of our company. The
employment agreement became effective on August 29, 2007,
upon completion of the merger between Cross Shore and Old RPS.
The employment agreement can be terminated by either us or
Dr. Koffer at any time for any reason, and Dr. Koffer
will be entitled to receive severance payments and benefits in
the event his employment is terminated by us or he voluntarily
resigns his employment, as described below under “Potential
Payments Upon Termination or Change in Control.”
Dr. Koffer is entitled to receive a base salary of
$300,000, as may be adjusted by the Board of Directors from time
to time, payable in accordance with our normal payroll
practices. Dr. Koffer is also eligible to receive an annual
target bonus equal to 50% of his base salary for achieving our
business and financial objectives. In addition, Dr. Koffer
is entitled to participate in the 2007 Plan and all other
benefit plans, including medical, dental, retirement, flexible
spending account, Section 125 plan, Section 401(k)
plan, short- and long-term disability, life insurance, in an
amount equal to three times his base salary, and accident and
disability insurance, and other such plans as we may establish
from time to time for our executives or employees generally.
Dr. Koffer has entered into a separate agreement that contains
customary agreements and representations by Dr. Koffer
relating to confidentiality, assignment of inventions, and
non-competition.
Steven
Bell
We entered into an employment agreement with Mr. Bell on
April 26, 2007 to serve as our Executive Vice President of
Finance and Chief Financial Officer. The employment agreement
became effective on August 29, 2007, upon completion of the
merger between Cross Shore and Old RPS. The employment agreement
had an initial term of one year, and has been automatically
renewed for successive one-year periods unless terminated by
either us or Mr. Bell within a specified period prior to
the end of any renewal term. Mr. Bell is entitled to
receive severance payments and benefits in the event his
employment is terminated by us, as well as acceleration of
vesting upon a change of control, in each case as described
below under “Potential Payments Upon Termination or Change
in Control.”
Mr. Bell is entitled to receive a base salary of $310,000
per year, or such other higher rate as the Chief Executive
Officer may designate from time to time, payable in accordance
with our normal payroll practices. Mr. Bell is also
eligible to receive an annual bonus in such amount as determined
by the Board of Directors in its sole discretion. In addition,
Mr. Bell is entitled to participate in all benefit plans,
including medical, dental, retirement, short- and long-term
disability, the premiums and fees for which will be fully paid
by us, and stock incentive and other such plans as we may
establish from time to time for our executives or employees
generally. Any stock options granted to Mr. Bell are to be
treated as incentive stock options to the maximum extent
possible.
The employment agreement contains customary agreements and
representations by Mr. Bell regarding confidentiality,
assignment of inventions, and non-competition.
69
Janet
Brennan
Old RPS entered into an employment agreement with
Ms. Brennan on April 28, 2001, which was assumed by
our company following the merger between Cross Shore and Old
RPS. Under Ms. Brennan’s employment agreement,
Ms. Brennan currently serves as our Chief International
Affairs Officer and Executive Vice President. The initial term
of the agreement was one year, and the agreement has been
automatically renewed for successive one-year periods unless
terminated by either us or Ms. Brennan within a specified
period prior to the end of any renewal term.
Ms. Brennan’s base salary under the agreement was
increased to $280,000 per year in 2009, and may be further
increased by the Board of Directors from time to time, payable
in accordance with our normal payroll practices.
Ms. Brennan is eligible to receive an annual performance
bonus in such amount as determined in the sole discretion of the
Board of Directors. In addition, Ms. Brennan is eligible to
participate in our benefit plans, including medical, dental,
retirement, short- and long-term disability and other such plans
as we may establish from time to time for our executives or
employees generally.
Ms. Brennan is also entitled to receive severance payments
and benefits from us in the event we terminate her employment or
she voluntarily resigns her employment, as described below under
“Potential Payments Upon Termination or Change in
Control.”
The employment agreement contains customary agreements and
representations by Ms. Brennan regarding confidentiality,
assignment of inventions, and non-competition.
Samir
Shah
We entered into an employment agreement with Mr. Shah on
December 6, 2007. Under Mr. Shah’s employment
agreement, Mr. Shah currently serves as our Executive Vice
President, Strategic Development. The initial term was one year
and the agreement has been automatically renewed for successive
one-year periods unless terminated by either us or Mr. Shah
within a specified period prior to the end of any renewal term.
Mr. Shah is entitled to receive a base salary of $250,000
per year, or such higher rate as the Board of Directors may
designate from time to time, payable in accordance with our
general payroll practices. Mr. Shah is eligible to receive
an annual performance bonus in such amount as determined in the
sole discretion of the Board of Directors. In addition,
Mr. Shah is eligible to participate in our benefit plans,
including medical, dental, retirement, short- and long-term
disability and other such plans as we may establish from time to
time for our executives or employees generally.
Mr. Shah is also entitled to receive severance payments and
benefits from us in the event that we terminate his employment
or he voluntarily resigns his employment, as described below
under “Potential Payments Upon Termination or Change in
Control.”
The employment agreement contains customary agreements and
representations by Mr. Shah regarding confidentiality,
assignment of inventions, and non-competition.
70
Grants of
Plan-Based Awards
The table below sets forth information regarding all plan-based
awards granted to our named executive officers during 2009.
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Estimated Future
|
|
|
|
|
|
|
|
Payouts Under
|
|
|
|
|
|
|
|
Non-Equity
|
|
|
|
|
|
|
|
Incentive Plan
|
|
Name
|
|
Year
|
|
Grant Date(1)
|
|
Awards(2)(3)
|
|
|
|
Daniel M. Perlman
|
|
|
2009
|
|
|
|
12/31/2009
|
|
|
$
|
88,114
|
|
|
Chief Executive Officer
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Steven Bell
|
|
|
2009
|
|
|
|
12/31/2009
|
|
|
$
|
46,994
|
|
|
Chief Financial Officer
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Harris Koffer
|
|
|
2009
|
|
|
|
12/31/2009
|
|
|
$
|
62,938
|
|
|
President and Chief Operating Officer
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Janet Brennan
|
|
|
2009
|
|
|
|
12/31/2009
|
|
|
$
|
27,273
|
|
|
Chief International Affairs Officer and
Executive Vice President
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Samir Shah
|
|
|
2009
|
|
|
|
12/31/2009
|
|
|
$
|
41,959
|
|
|
Executive Vice President,
Strategic Development
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(1) |
|
Each executive officer was awarded 100% of his or her respective
non-equity incentive plan awards in January 2010, as detailed
under the heading “Performance Bonuses” in the
“Compensation Discussion and Analysis” section above. |
| |
|
(2) |
|
Our non-equity incentive plan does not provide for threshold,
target or maximum amounts of bonuses to be awarded upon
satisfaction of conditions under the plan. The performance
bonuses awarded to executive officers under the non-equity
incentive plan are determined by the Board of Directors as
described in the “Compensation Discussion and
Analysis” section above under the heading “Performance
Bonuses.” |
| |
|
(3) |
|
The columns with the headings “All Other Stock Awards:
Number of Shares of Stock or Units,” “All Other Option
Awards: Number of Securities Underlying Options,”
“Estimated Future Payouts Under Equity Incentive Plan
Awards,” “Exercise or Base Price of Option
Awards,” and “Grant Date Fair Value of Stock and
Option Awards” have been omitted from the table because we
did not issue any shares of stock, units, or stock options to
named executive officers during 2009. |
71
Outstanding
Equity Awards at Fiscal Year End
The table below sets forth information regarding our named
executive officers’ outstanding equity awards at
December 31, 2009, all of which are stock options.
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Option Awards(1)(2)(3)
|
|
|
|
Number of Securities
|
|
Number of Securities
|
|
|
|
|
|
|
|
Underlying
|
|
Underlying
|
|
Option
|
|
Option
|
|
|
|
Unexercised Options
|
|
Unexercised Options
|
|
Exercise
|
|
Expiration
|
|
Name
|
|
Exercisable (#)
|
|
Unexercisable (#)
|
|
Price ($/Sh)
|
|
Date
|
|
|
|
Daniel M. Perlman
|
|
|
300,000
|
|
|
|
150,000
|
(4)
|
|
|
5.05
|
|
|
|
12/6/2017
|
|
|
Chief Executive Officer
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Steven Bell
|
|
|
36,280
|
|
|
|
—
|
|
|
|
0.83
|
|
|
|
6/1/2014
|
|
|
Chief Financial Officer
|
|
|
36,280
|
|
|
|
—
|
|
|
|
0.83
|
|
|
|
5/23/2016
|
|
|
|
|
|
135,000
|
|
|
|
45,000
|
(4)
|
|
|
5.05
|
|
|
|
12/6/2017
|
|
|
Harris Koffer
|
|
|
899,279
|
|
|
|
—
|
|
|
|
0.83
|
|
|
|
7/10/2016
|
|
|
President and Chief Operating Officer
|
|
|
90,000
|
|
|
|
30,000
|
(4)
|
|
|
5.05
|
|
|
|
12/6/2017
|
|
|
Janet Brennan
|
|
|
5,442
|
|
|
|
—
|
|
|
|
0.37
|
|
|
|
12/31/2011
|
|
|
Chief International Affairs Officer
|
|
|
5,442
|
|
|
|
—
|
|
|
|
0.37
|
|
|
|
12/31/2012
|
|
|
And Executive Vice President
|
|
|
72,561
|
|
|
|
—
|
|
|
|
0.83
|
|
|
|
6/1/2014
|
|
|
Samir Shah
|
|
|
1,814
|
|
|
|
—
|
|
|
|
0.37
|
|
|
|
12/31/2011
|
|
|
Executive Vice President, Strategic
|
|
|
1,814
|
|
|
|
—
|
|
|
|
0.37
|
|
|
|
12/31/2012
|
|
|
Development
|
|
|
36,280
|
|
|
|
—
|
|
|
|
0.83
|
|
|
|
6/1/2014
|
|
|
|
|
|
90,702
|
|
|
|
—
|
|
|
|
0.83
|
|
|
|
5/23/2016
|
|
|
|
|
|
|
|
(1) |
|
None of the securities underlying any of the named executive
officers’ options are unearned, and therefore the column
with the heading “Equity Incentive Plan Awards: Number of
Securities Underlying Unexercised Unearned Options” has
been omitted from this table. |
| |
|
(2) |
|
The four columns under the heading “Stock Awards” have
been omitted from this table because no unvested awards of stock
were held by our named executive officers at December 31,
2009. |
| |
|
(3) |
|
With the exception of the 450,000 options granted to
Mr. Perlman and the 899,279 options granted to
Dr. Koffer upon commencing employment with Old RPS on
July 10, 2006, stock options vest on the following
schedule:
331/3%
of options vested on the first anniversary of the date of grant,
and
81/3%
of options vested every 90 days until fully vested. The
options become fully vested three years after grant, provided
that the employee has remained continuously employed by us
during those three years. One-third of Mr. Perlman’s
options vest each year over a three-year period. The option
becomes fully vested three years after the date of grant.
Dr. Koffer’s 899,279 options vested on the following
schedule:
331/3%
vested on July 10, 2007, and
27/9%
vested every month thereafter until the option fully vested on
July 10, 2009. |
| |
|
(4) |
|
These options will become fully vested on December 6, 2010. |
ReSearch
Pharmaceutical Services, Inc. 2007 Equity Incentive
Plan
Our Board of Directors adopted the 2007 Plan in conjunction with
the merger between Cross Shore and Old RPS in August 2007 and
our stockholders approved the 2007 Plan at our 2008 annual
meeting of stockholders. The purpose of the 2007 Plan is to
attract and retain the best possible individuals to promote our
success and to better align the interests of our executive
officers and employees with the interests of our stockholders.
Upon the closing of the merger, the equity incentive plan
maintained by Old RPS was terminated, and any options granted
under the Old RPS plan that were not terminated or exercised
prior to the merger were cancelled and replaced with options
issued under the 2007 Plan.
Set forth below is a summary of the principal provisions of the
2007 Plan. This summary is qualified in its entirety by
reference to the 2007 Plan, which is filed as an exhibit to our
registration statement of which this prospectus is a part.
All employees of and consultants and advisors to our company and
our affiliated entities, and all of our directors,
72
are eligible to receive awards under the 2007 Plan. The 2007
Plan permits awards of options to purchase our common stock as
well as grants of restricted stock. We have reserved
6,792,271 shares of common stock for issuance under the
2007 Plan. In addition, on the first day of each fiscal year,
the aggregate number of shares reserved for issuance under the
2007 Plan will be increased, but not decreased, automatically by
a number of shares, if needed, such that the total number of
shares reserved for issuance under the 2007 Plan equals 15% of
the number of shares outstanding, calculated on a fully diluted
basis, on that date. As of the date of this prospectus, no
adjustment to the number of shares reserved for issuance under
the 2007 Plan in accordance with the previous sentence has been
necessary. Following the completion of this offering, we expect
that the number of shares to be reserved for issuance under the
2007 Plan will be adjusted in accordance with this provision
beginning on January 1, 2011. Any shares underlying options
that expire or are not fully exercised, and restricted stock
that is cancelled, repurchased, or forfeited, will once again
become available for grant under the 2007 Plan.
If there is a change in the number of our outstanding shares of
common stock by reason of a stock split, reverse stock split,
stock dividend, reclassification, recapitalization, merger,
consolidation, exchange of shares, or a similar change affecting
our common stock, the number of shares which may be issued and
the number of shares subject to outstanding awards under the
2007 Plan will be adjusted proportionately.
The 2007 Plan is administered by our Board of Directors.
Pursuant to the 2007 Plan, the Board of Directors has delegated
its authority to administer the 2007 Plan to our Compensation
Committee. The Board of Directors or Compensation Committee has
sole discretion to determine when options are exercisable and
when they expire, provided that the term cannot exceed
10 years from the date of grant. The exercise price of any
option must be at least equal to the fair market value of the
stock on the date of the grant. No participant may receive
options relating to more than 1,000,000 shares of our
common stock in any calendar year.
The 2007 Plan also permits grants of restricted stock to
eligible participants. Restricted stock is subject to vesting
restrictions and to restrictions on sale or other transfer by
the participant. The Board of Directors or the Compensation
Committee determines eligible participants, the time and number
of shares of restricted stock granted, the price, if any, to be
paid by the recipient, the time when the restricted stock will
be subject to forfeiture, when the restrictions will terminate,
and all other terms and conditions of the grants. Vesting
conditions may include continued employment or service, or
attaining specified individual or corporate performance goals.
Unless otherwise set forth in an award agreement, grants of
restricted stock include the right to be credited with dividends
and the right to vote the shares. As of the date of this
prospectus, no shares of restricted stock have been granted
under the 2007 Plan.
If a Change of Control, as defined in the 2007 Plan, occurs or
is anticipated, our Board of Directors or Compensation Committee
may prohibit the exercise of any option until either the Change
of Control is no longer anticipated or has occurred. Contingent
upon a Change of Control, our Board of Directors or Compensation
Committee, in its sole and absolute discretion and without the
consent of the participant, may (a) cause outstanding
options to become fully vested and immediately exercisable, and
outstanding restricted stock to become non-forfeitable;
(b) cancel any option or restricted stock in exchange for
an option to purchase common stock of any successor corporation
or for restricted shares of the common stock of any successor
corporation, as applicable; or (c) redeem any restricted
stock or cancel any option in exchange for cash or other
substitute consideration.
The Board of Directors or the Compensation Committee may
suspend, terminate, discontinue or amend the 2007 Plan, or
modify, extend or renew any award, provided that no amendment or
modification of an award may adversely affect the rights of any
participant without the participant’s consent. If not
terminated earlier by the Board of Directors or Compensation
Committee, the 2007 Plan will terminate in 2017.
Option
Exercises and Stock Vested
None of our named executive officers exercised any options
awarded by either us or Old RPS during 2009 and no stock awards
to the named executive officers vested in 2009. Therefore, the
Option Exercises and Stock Vested table is not included in this
prospectus.
73
Pension
Benefits and Nonqualified Deferred Compensation
We do not offer any pension benefit plans to our named executive
officers, and none of our named executive officers participated
in any nonqualified deferred compensation arrangements, and
therefore the Pension Benefits and the Nonqualified Deferred
Compensation tables are not included in this prospectus.
Potential
Payments Upon Termination or Change in Control
The employment agreements with our named executive officers each
provide for severance payments upon a termination of the
executive’s employment in specified circumstances. For our
named executive officers other than Mr. Perlman:
|
|
|
| |
•
|
“cause” generally means:
|
|
|
|
| |
•
|
conviction of a felony or the commission of any other act or
omission involving dishonesty or fraud;
|
| |
| |
•
|
failure of the executive to perform his or her duties as
directed by our Board of Directors, provided those duties are
reasonable and consistent with the duties generally performed by
an executive with the same title;
|
| |
| |
•
|
gross negligence or willful misconduct; or
|
| |
| |
•
|
material breach of the employment agreement; and
|
|
|
|
| |
•
|
“good reason,” except in the case of
Mr. Bell’s employment agreement, in which case it is
not used or defined, generally means:
|
|
|
|
| |
•
|
a material alteration or reduction in the executive’s
duties;
|
| |
| |
•
|
a reduction in the executive’s compensation package; or
|
| |
| |
•
|
a requirement that the executive be based at a location in
excess of 40 miles from the employee’s current
residence.
|
In the case of Mr. Perlman:
|
|
|
| |
•
|
conviction of a felony;
|
| |
| |
•
|
indictment for a felony involving dishonesty or fraud or the
commission of an act or omission involving dishonesty or fraud;
or
|
| |
| |
•
|
gross negligence or willful misconduct; and
|
|
|
|
| |
•
|
a material breach of our obligations to Mr. Perlman under
the employment agreement that is not remedied within a specified
amount of time;
|
| |
| |
•
|
his relocation outside the metropolitan Philadelphia area;
|
| |
| |
•
|
a material change in his job description, office title, or
responsibilities, excluding promotions or increased
responsibility;
|
| |
| |
•
|
his removal from our Board of Directors without cause; or
|
| |
| |
•
|
our failure to nominate him as a candidate for election to our
Board of Directors.
|
Daniel M.
Perlman
If we terminate Mr. Perlman’s employment without cause
or if Mr. Perlman voluntarily resigns for good reason,
there are two severance options depending on whether or not he
chooses to be bound by the non-competition and non-solicitation
covenants contained in the employment agreement:
|
|
|
| |
•
|
Option one permits Mr. Perlman to choose to be bound by the
employment agreement’s non-competition and non-solicitation
covenants for a period of 18 months following such
termination or resignation and entitles him to receive a lump
sum amount equal to 2.99 times his then current annual base
salary, plus the
|
74
|
|
|
| |
|
pro rata portion of any bonus to which he is entitled for the
year in which his employment is terminated, plus payment of his
premiums under the Consolidated Omnibus Budget Reconciliation
Act of 1986, as amended, or COBRA, for a period of
18 months following termination if Mr. Perlman elects
to continue COBRA coverage.
|
|
|
|
| |
•
|
Option two permits Mr. Perlman to choose not to be bound by
the agreement’s non-competition and non-solicitation
covenants and entitles him to receive a lump sum amount equal to
his then current annual base salary, plus the pro rata portion
of any bonus to which he is entitled for the year in which his
employment is terminated, plus payment of his premiums under
COBRA for a period of 18 months following termination if
Mr. Perlman elects to continue COBRA coverage.
|
If we terminate Mr. Perlman with cause, he will have two
severance options depending on whether or not he chooses to be
bound by the non-competition and non-solicitation covenants
contained in the employment agreement:
|
|
|
| |
•
|
Option one permits Mr. Perlman to choose to be bound by the
employment agreement’s non-competition and non-solicitation
covenants for a period of one year following termination and
entitles him to receive a lump sum amount equal to his then
current annual base salary, plus the pro rata portion of any
bonus to which he is entitled for the year in which his
employment is terminated, plus payment of his premiums under
COBRA for a period of 12 months following termination if
Mr. Perlman elects to continue COBRA coverage.
|
| |
| |
•
|
Option two permits Mr. Perlman to choose not to be bound by
the employment agreement’s non-competition and
non-solicitation covenants but does not entitle him to receive
any severance payments or benefits from us.
|
If Mr. Perlman voluntarily resigns his employment without
good reason, and a change of control has not occurred prior to
his resignation, we will pay Mr. Perlman all compensation
accrued through the date of resignation, and Mr. Perlman
will be bound by the employment agreement’s non-competition
and non-solicitation covenants for one year following the date
of resignation.
If we terminate Mr. Perlman’s employment due to his
suffering a permanent disability as defined in his employment
agreement, he is entitled to receive a lump sum payment equal to
two times his then current annual base salary, plus the pro rata
portion of any bonus to which he is entitled for the year in
which his employment is terminated. We will also pay
Mr. Perlman’s premiums under COBRA for a period of
18 months following termination if he elects to continue
COBRA coverage.
If Mr. Perlman dies during the term of the employment
agreement, we will pay his estate all compensation and
reimbursements accrued for Mr. Perlman through the date of
his death.
In addition to the termination benefits described above, if we
terminate Mr. Perlman’s employment for any reason
other than death, disability, or cause within six months
preceding or 12 months after a change of control, or if he
resigns for any reason during this period, he has two severance
options depending on whether or not he chooses to be bound by
the non-competition and non-solicitation covenants contained in
the employment agreement.
|
|
|
| |
•
|
Option one permits Mr. Perlman to choose to be bound by the
employment agreement’s non-competition and non-solicitation
covenants for a period of 18 months following his
termination or resignation and entitles him to receive any
amounts earned but not yet paid under the employment agreement,
plus a lump sum payment equal to 2.99 times the sum of his then
current annual base salary plus his bonus for the previous year.
Mr. Perlman will also be entitled to receive, for a period
of three years following the later of the change of control,
termination or resignation, medical benefits for him, his spouse
and any dependents to the same extent he was so entitled prior
to such termination or resignation, at our expense if and to the
extent we were paying for such benefits at the time of such
termination or resignation. If our medical benefits plans do not
allow for such payment, we will pay Mr. Perlman a lump sum
equal to the amount we would have paid for such coverage over
the three-year period had such coverage been permitted.
Mr. Perlman, his spouse, and any dependents would also be
entitled to such rights as he or they may have to continue
coverage at his sole expense under COBRA for the COBRA coverage
period following the expiration of the period during which he,
his spouse and any dependents continue to receive such medical
benefits coverage.
|
75
|
|
|
| |
•
|
Option two permits Mr. Perlman to choose not to be bound by
the employment agreement’s non-competition and
non-solicitation covenants and entitles him to receive any
amounts earned but not yet paid under the employment agreement
plus a lump sum payment equal to the sum of his then current
annual base salary plus his bonus for the previous year.
Mr. Perlman would also receive the same continuation of
medical benefits, or lump sum payment if continued coverage is
not permitted, described above, except that such medical
benefits would extend only for a period of one year.
|
Upon a change of control, whether or not Mr. Perlman’s
employment is thereafter terminated, all of
Mr. Perlman’s outstanding stock grants shall become
fully vested immediately before the change of control, all stock
options previously granted shall become immediately vested and
exercisable without regard to continued employment or
performance-based vesting standards, and each non-qualified
stock option shall remain exercisable until 180 days after
the change of control, unless a later period following the
change of control is set forth in the relevant stock option
agreement or, if earlier, the scheduled expiration date of the
non-qualified stock option. The exercise period of any incentive
stock options granted to Mr. Perlman will continue to be
governed by the relevant stock option agreement.
Harris
Koffer
If we terminate Dr. Koffer’s employment without cause
or Dr. Koffer terminates his employment for good reason, he
will be entitled to receive a lump sum payment equal to his then
current annual base salary.
If we terminate Dr. Koffer’s employment for cause or
Dr. Koffer terminates his employment without good reason,
he will be entitled to receive his then current base salary
through the date of termination. If Dr. Koffer’s
employment is terminated as a result of his death or disability,
there will be no further payments of his base salary under the
employment agreement.
Steven
Bell
If we terminate Mr. Bell’s employment without cause,
he will be entitled to receive his then current base salary and
benefits for a period of 18 months following the date of
termination and any earned but unpaid bonuses, determined based
on the partial year in which the termination occurs. If we
terminate Mr. Bell’s employment without cause at any
time after the date which is three months before a change of
control or at any time thereafter, he will be entitled to
receive his then current base salary and benefits for a period
of 24 months following the date of termination and any
earned but unpaid bonuses, determined based on the partial year
in which the termination occurs. In each case, one-half of the
amount owed to Mr. Bell is to be paid on the date that is
six months following termination, with the remainder payable on
the date that is nine months following the termination date.
If we terminate Mr. Bell’s employment for cause, or
due to his death or disability, or if Mr. Bell resigns for
any reason, he will be entitled to receive his then current base
salary through the date of termination or resignation.
Upon a change of control, all of Mr. Bell’s stock
options will immediately vest in full.
Janet
Brennan
Ms. Brennan’s employment agreement provides that if
she is terminated without cause, she is entitled to receive a
continuation of her base salary and benefits for one year
following termination. If Ms. Brennan is terminated with
cause, she terminates the employment agreement, or the
employment is terminated due to her death or disability,
Ms. Brennan is entitled to receive her base salary through
the date of termination.
Samir
Shah
Mr. Shah’s employment agreement provides that if he is
terminated without cause, he is entitled to receive, as
severance, a continuation of his base salary and benefits for
one year following termination, and any earned but unpaid
bonuses. If Mr. Shah is terminated with cause, he
terminates his employment agreement, or his employment is
terminated due to death or disability, Mr. Shah is entitled
to receive his base salary through the date of termination.
76
Quantification
of Potential Payments upon Termination or Change in
Control
The following table summarizes the estimated termination
payments that we would have been required to make to our named
executive officers for the termination scenarios listed and
assuming the triggering events for the scenarios occurred on
December 31, 2009:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Termination
|
|
Termination
|
|
|
|
Name
|
|
without cause(1)
|
|
with cause(2)
|
|
Change in Control
|
|
|
|
Daniel M. Perlman
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash and Benefits
|
|
$
|
1,332,280
|
(4)
|
|
$
|
518,114
|
(6)
|
|
$
|
1,466,973
|
(9)
|
|
|
|
|
|
|
|
|
—
|
(7)
|
|
|
|
|
|
|
|
$
|
518,114
|
(5)
|
|
$
|
948,114
|
(8)
|
|
$
|
549,167
|
(10)
|
|
Equity Acceleration(3)
|
|
|
—
|
|
|
|
—
|
|
|
$
|
757,500
|
(11)(12)
|
|
Harris Koffer
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash and Benefits
|
|
$
|
330,000
|
|
|
|
—
|
|
|
|
—
|
|
|
Equity Acceleration(3)
|
|
|
—
|
|
|
|
—
|
|
|
$
|
202,000
|
(11)
|
|
Steven Bell
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash and Benefits
|
|
$
|
553,973
|
|
|
|
—
|
|
|
$
|
720,424
|
(13)
|
|
Equity Acceleration(3)
|
|
|
—
|
|
|
|
—
|
|
|
$
|
303,000
|
(11)(14)
|
|
Janet Brennan
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash and Benefits
|
|
$
|
301,145
|
|
|
|
—
|
|
|
|
—
|
|
|
Equity Acceleration(3)
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
Samir Shah
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash and Benefits
|
|
$
|
279,247
|
|
|
|
—
|
|
|
|
—
|
|
|
Equity Acceleration(3)
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
(1) |
|
Termination without cause also includes resignation with good
reason, if applicable under the terms of the named executive
officer’s employment agreement. |
| |
|
(2) |
|
Unless otherwise noted, each of the named executive officers
would receive his or her base salary through the date of
termination if termination is made with cause. Termination with
cause also includes resignation without good reason, where
applicable. No additional payments would be made if the named
executive officer was terminated on December 31, 2009. All
compensation amounts in this table assume payment of the 2009
performance bonus as set forth in the Summary Compensation Table. |
| |
|
(3) |
|
Pursuant to the 2007 Plan, no option awards would accelerate in
the event of the named executive officer’s termination on
December 31, 2009. Options exercisable on the date of
termination would remain exercisable for a period of three
months from the date of termination. |
| |
|
(4) |
|
Assumes Mr. Perlman agrees to be bound by the
non-competition and non-solicitation provisions of his
employment agreement for a period of 18 months.
Mr. Perlman may also elect coverage under COBRA for
18 months, which we would pay if so elected. |
| |
|
(5) |
|
Assumes Mr. Perlman does not agree to be bound by the
non-competition and non-solicitation provisions of his
employment agreement. Mr. Perlman may also elect coverage
under COBRA for 18 months, which we would pay if so elected. |
| |
|
(6) |
|
Assumes Mr. Perlman resigns without good reason, resulting
in Mr. Perlman being bound by the non-competition and
non-solicitation provisions of his employment agreement for a
period of 12 months or Mr. Perlman is terminated with
cause and agrees to be bound by the non-competition and
non-solicitation provisions of his employment agreement for one
year. Mr. Perlman may also elect coverage under COBRA for
12 months, which we would pay if so elected. |
| |
|
(7) |
|
Assumes Mr. Perlman does not agree to be bound by the
non-competition and non-solicitation provisions of his
employment agreement. |
| |
|
(8) |
|
Assumes Mr. Perlman is permanently disabled and has
received payment from his disability insurance for one year.
Mr. Perlman is entitled to also receive at least 60% of his
then base salary at the time of permanent |
77
|
|
|
|
|
|
disability from a disability insurance policy maintained by us.
Mr. Perlman may also elect coverage under COBRA for
18 months, which we would pay if so elected. |
| |
|
(9) |
|
Assumes Mr. Perlman is terminated without cause or resigns
within six months before or 12 months after a change of
control and agrees to be bound by the non-competition and
non-solicitation provisions of his employment agreement for a
period of 18 months and the cost of Mr. Perlman’s
benefits remains the same over three years. Mr. Perlman may
also elect coverage under COBRA for the maximum period under
which COBRA coverage is offered, which we would pay if so
elected. |
| |
|
(10) |
|
Assumes Mr. Perlman is terminated without cause or resigns
within six months before or 12 months after a change of
control and does not agree to be bound by the non-competition
and non-solicitation provisions of his employment agreement and
the cost of Mr. Perlman’s benefits remain the same
over the next year. |
| |
|
(11) |
|
Pursuant to the 2007 Plan, upon a change in control the Board of
Directors may, but is not obligated to, cause all outstanding
options to become fully vested and immediately exercisable. The
figures in the table represent the value of the options if they
became fully vested and exercisable on December 31, 2009. |
| |
|
(12) |
|
Under the terms of Mr. Perlman’s employment agreement,
all of his stock options fully and immediately vest upon a
change of control. This amount assumes a change of control and
vesting of all unvested stock options on December 31, 2009. |
| |
|
(13) |
|
This figure assumes Mr. Bell was terminated without cause
and within three months prior to a change of control occurring
on December 31, 2009, or any time thereafter. |
| |
|
(14) |
|
Under the terms of Mr. Bell’s employment agreement,
all of his stock options fully and immediately vest upon a
change of control. This amount assumes a change of control and
vesting of all unvested stock options on December 31, 2009. |
78
Certain
Relationships and Related Transactions
In addition to the director and executive compensation
arrangements discussed above in “Management” and
“Executive Compensation,” we have been a party to the
following transactions since January 1, 2007, in which any
director, executive officer or holder of more than 5% of any
class of our voting stock, or any member of the immediate family
of or entities affiliated with any of them, had or will have a
material interest and the amount involved exceeds $120,000.
We repurchased 750,000 shares of our common stock from
Pangaea One Acquisition Holdings I, or Pangaea, at a price
of $4.85 per share, the quoted price on AIM on the repurchase
date, for a total repurchase price of $3,637,500 pursuant to a
Share Repurchase Agreement with Pangaea dated October 4,
2007. In addition, pursuant to an agreement between us, certain
of our stockholders, and Pangaea, Pangaea has the right to
nominate and have elected to our Board of Directors up to two
designees as long as Pangaea owns at least 20% of our
outstanding common stock, and one designee as long as Pangaea
owns at least 10% of our outstanding common stock. Pangaea
currently owns approximately 25% of our common stock, and
Messrs. Yu and Armstrong were Pangaea’s nominees and
have been elected to our Board of Directors. The agreement
expired on August 30, 2010.
On November 16, 2007, we entered into a consulting
agreement with Cartesian Capital Group LLC, or Cartesian, for
consulting and advisory services relating to
non-U.S. acquisitions.
Messrs. Yu and Armstrong are principals of Cartesian.
Cartesian received advisory service fees in the amount of
$600,000 in January 2008 following expiration of the consulting
agreement on December 31, 2007.
Review, Approval
or Ratification of Transactions with Related Persons
Our former written Code on Dealing in Securities, adopted prior
to the merger between Cross Shore and Old RPS, required the
Chairman of the Board of Directors and the Chief Executive
Officer to notify and receive approval from the Board of
Directors when the acquisition or disposition of our securities
was proposed, and the party proposing to sell or buy those
securities owned more than 3% of our outstanding securities. No
such acquisition or disposition was permitted without approval
of the Board of Directors, and the Board of Directors was
required to determine that the proposed transaction was fair and
reasonable to our stockholders. The Board of Directors had up to
five days to review the proposed transaction, written approval
or disapproval was prepared, and the transaction must have taken
place within two days after the date of the approval, if
approval was granted. Our Code on Dealing in Securities
terminated upon the delisting of our common stock and warrants
from AIM.
The repurchase of 750,000 shares of our common stock from
Pangaea was reviewed and unanimously approved by the Board of
Directors pursuant to the procedures of the Code on Dealing in
Securities. In 2007, at the time we entered into the
transactions with Pangaea and Cartesian, we did not have a
specific policy for related party transactions not involving our
securities. Our past practice was that the Board of Directors
must review and grant approval for transactions involving
related parties or significant expenditures, such as the
consulting agreement with Cartesian. In accordance with this
practice, the Board of Directors reviewed and unanimously
approved entry into the consulting agreement.
On March 3, 2009, we adopted our written Code of Business
Conduct and Ethics, which requires that our officers and
directors must disclose to the Chief Executive Officer any
material transaction or relationship that reasonably could be
expected to give rise to a conflict of interest, including an
interest in a transaction involving our company, and the Chief
Executive Officer shall notify the Board of Directors of any
such disclosure. Our Audit Committee is primarily responsible
for enforcing the Code of Business Conduct and Ethics.
79
Principal and
Selling Stockholders
The following table sets forth certain information with respect
to the beneficial ownership of our common stock, as of
September 30, 2010, as adjusted to reflect the sale of
common stock offered by us and the selling stockholders in this
offering, for:
|
|
|
| |
•
|
each person or group of affiliated persons who is known by us to
beneficially own more than 5% of our common stock;
|
| |
| |
•
|
each of our directors;
|
| |
| |
•
|
each of our named executive officers;
|
| |
| |
•
|
all of our directors and executive officers as a group; and
|
| |
| |
•
|
each of the selling stockholders.
|
The percentages of shares owned before the offering shown in the
following table is based on 37,216,052 shares of common
stock outstanding as of September 30, 2010 and the numbers
and percentages of shares owned after the offering gives effect
to the issuance
of shares
of common stock by us
and shares
of common stock by the selling stockholders in this offering.
Each individual or entity shown in the table has furnished
information to us with respect to the holder’s beneficial
ownership. Except as otherwise indicated below, the address of
each officer and director listed below is
c/o ReSearch
Pharmaceutical Services, Inc., 520 Virginia Drive,
Fort Washington, Pennsylvania 19034.
We have determined beneficial ownership in accordance with the
rules of the Securities and Exchange Commission and generally
includes any shares over which a person exercises sole or shared
voting or investment power. Under these rules, beneficial
ownership also includes any shares which the individual has the
right to acquire currently or within 60 days after
September 30, 2010 through the exercise of any option or
other right to purchase shares of common stock. Such shares are
deemed outstanding for computing the percentage ownership of the
person holding such options or rights, but are not deemed
outstanding for computing the percentage ownership of any other
person. Unless otherwise indicated, the persons or entities
identified in the table below have sole voting and investment
power with respect to all shares shown as beneficially owned by
them, subject to applicable community property laws.
The information contained in the following table is not
necessarily indicative of beneficial ownership for any other
purpose and the inclusion of any shares in the table does not
constitute an admission of beneficial ownership of those shares.
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Number of
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Shares to be
|
|
Shares Beneficially
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Sold if
|
|
Owned
|
|
|
|
|
|
|
|
|
|
Beneficial
|
|
Underwriters’
|
|
After this Offering if
|
|
|
|
Beneficial Ownership
|
|
|
|
Ownership
|
|
Option is
|
|
Underwriters’ Option is
|
|
|
|
Before Offering
|
|
Shares
|
|
After Offering
|
|
Exercised in
|
|
Exercised in Full
|
|
Name and Address of Beneficial Holder
|
|
Shares
|
|
Percentage
|
|
Offered
|
|
Shares
|
|
Percentage
|
|
Full
|
|
Shares
|
|
Percentage
|
|
|
|
Named Executive Officers and Directors
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Daniel M. Perlman(1)
|
|
|
2,851,613
|
|
|
|
7.6
|
%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Harris Koffer(2)
|
|
|
989,279
|
|
|
|
2.7
|
%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Steven Bell(3)
|
|
|
423,265
|
|
|
|
1.1
|
%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Janet L. Brennan(4)
|
|
|
569,730
|
|
|
|
1.5
|
%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Samir Shah(5)
|
|
|
280,042
|
|
|
|
*
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Thomas R. Armstrong(6)
|
|
|
9,237,673
|
|
|
|
24.8
|
%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Jack H. Dean(2)
|
|
|
3,334
|
|
|
|
*
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
James R. Macdonald(7)
|
|
|
3,454,127
|
|
|
|
9.3
|
%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Warren M. Myers(2)
|
|
|
3,334
|
|
|
|
*
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Daniel Raynor(8)
|
|
|
5,766,604
|
|
|
|
15.5
|
%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Stephen E. Stonefield(9)
|
|
|
4,617
|
|
|
|
*
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Peter M. Yu(6)
|
|
|
9,237,673
|
|
|
|
24.8
|
%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
All directors and executive officers as a group (consists of
12 persons)(10)
|
|
|
23,583,618
|
|
|
|
63.3
|
%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
80
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Number of
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Shares to be
|
|
Shares Beneficially
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Sold if
|
|
Owned
|
|
|
|
|
|
|
|
|
|
Beneficial
|
|
Underwriters’
|
|
After this Offering if
|
|
|
|
Beneficial Ownership
|
|
|
|
Ownership
|
|
Option is
|
|
Underwriters’ Option is
|
|
|
|
Before Offering
|
|
Shares
|
|
After Offering
|
|
Exercised in
|
|
Exercised in Full
|
|
Name and Address of Beneficial Holder
|
|
Shares
|
|
Percentage
|
|
Offered
|
|
Shares
|
|
Percentage
|
|
Full
|
|
Shares
|
|
Percentage
|
|
|
|
5% Stockholders
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Pangaea One Acquisition Holdings(6)
|
|
|
9,237,673
|
|
|
|
24.8
|
%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The Argentum Group(8)
|
|
|
5,719,441
|
|
|
|
15.3
|
%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The Productivity Fund IV(7)
|
|
|
3,454,127
|
|
|
|
9.3
|
%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Lehman Brothers International (Europe)(11)
|
|
|
2,142,736
|
|
|
|
5.7
|
%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Other Selling Stockholders
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
* |
|
Represents beneficial ownership of less than one percent. |
|
|
|
|
(1) |
|
Includes 300,000 shares issuable upon the exercise of
options that are exercisable within 60 days of
September 30, 2010. |
|
|
|
|
(2) |
|
Consists solely of shares issuable upon the exercise of options
that are exercisable within 60 days of September 30,
2010. |
|
|
|
|
(3) |
|
Includes 207,560 shares issuable upon the exercise of
options that are exercisable within 60 days of
September 30, 2010, and an additional 40,000 shares of
restricted stock that are subject to repurchase by us.
Mr. Bell has voting power with respect to the restricted
shares but does not have investment power with respect to such
shares until our repurchase option lapses. |
|
|
|
|
(4) |
|
Includes 83,445 shares issuable upon the exercise of
options that are exercisable within 60 days of
September 30, 2010. |
|
|
|
|
(5) |
|
Includes 130,610 shares issuable upon the exercise of
options that are exercisable within 60 days of
September 30, 2010. |
|
|
|
|
(6) |
|
Consists of 7,862,010 shares of common stock held by
Pangaea One Acquisition Holdings I, LLC, or Pangaea, and
1,452,324 shares of common stock held by Pangaea One
Acquisition Holdings II, LLC, or Pangaea II, which is a
wholly-owned subsidiary of Pangaea. Pangaea One GP2 (Cayman),
Co. is the general partner of Pangaea One GP2 (Cayman), L.P.,
which is the general partner of Pangaea One Parallel Fund, L.P.,
referred to collectively as Pangaea One GP2. Pangaea One GP
(Cayman), Co. is the general partner of Pangaea One GP (Cayman),
L.P. , which is the general partner of Pangaea One (Cayman),
L.P., referred to collectively as Pangaea One GP. Pangaea One
GP, LLC is the general partner of both Pangaea One, L.P.,
referred to as Pangaea One DE GP, and Pangaea One Parallel Fund
(B), L.P., referred to as Pangaea One Parallel GP. Pangaea One
GP2, Pangaea One GP, Pangaea One DE GP, and Pangaea One Parallel
GP each own a minority percentage of the membership interests of
Pangaea and together own all of the outstanding membership
interests of Pangaea. Mr. Armstrong and Mr. Yu,
through the investment committee of Pangaea One GP2, Pangaea One
GP, Pangaea One DE GP, and Pangaea One Parallel GP, have shared
voting and investment power over the shares held by Pangaea and
Pangaea II. Mr. Armstrong and Mr. Yu have disclaimed
such beneficial ownership. Pursuant to a previous agreement we
entered with Pangaea, Pangaea had the right to nominate and have
elected up to two directors to our Board of Directors as long as
it owns at least 20% of our outstanding common stock, and one
director as long as it owns at least 10% of our outstanding
common stock. Pangaea nominated, our Board of Directors
appointed, and our stockholders elected Mr. Armstrong and
Mr. Yu as directors at our 2008 and 2010 annual meetings of
stockholders, respectively. The address for
Messrs. Armstrong and Yu is
c/o Cartesian
Capital Group, LLC, 505 Fifth Avenue, 15th Floor New York,
NY 10017. |
81
|
|
|
|
(7) |
|
Consists of shares of common stock owned of record by The
Productivity Fund IV, L.P., which beneficially owns
3,326,213 shares, and The Productivity Fund IV
Advisors Fund, L.P., which beneficially owns
127,914 shares, which are referred to collectively as the
Productivity Funds. Mr. Macdonald is a managing director of
First Analysis Corporation, which is the manager of First
Analysis Venture Operations and Research, L.L.C., which is the
managing member of First Analysis Management Company IV, L.L.C.,
which is the general partner of the Productivity Funds.
Mr. Macdonald may be deemed to have beneficial ownership
over the shares held by the Productivity Funds.
Mr. Macdonald disclaims such beneficial ownership. The
address for Mr. Macdonald is
c/o First
Analysis Corporation, One South Wacker Drive, Suite 3900
(39th floor), Chicago, Illinois 60606. |
| |
|
(8) |
|
Consists of shares of common stock owned of record by Argentum
Capital Partners, L.P, or ACP, which beneficially owns
905,632 shares, Argentum Capital Partners II, L.P., or ACP
II, which beneficially owns 4,813,809 shares, and
47,163 shares owned of record by CGM IRA Custodian for the
benefit of Daniel Raynor. Mr. Raynor is the managing member
of Argentum Investments, LLC, which is the managing member of
Argentum Partners II, L.P., which is the general partner of ACP
II. Mr. Raynor is also the chairman of B.R. Associates,
Inc., which is the general partner of ACP. Mr. Raynor may
be deemed to have beneficial ownership over the shares held by
these entities. Mr. Raynor disclaims such beneficial
ownership. The address for Mr. Raynor is
c/o The
Argentum Group, 60 Madison Avenue, Suite 701, New York,
NY 10010. |
|
|
|
|
(9) |
|
Includes 1,667 shares issuable upon the exercise of options
that are exercisable within 60 days of September 30,
2010. |
|
|
|
|
(10) |
|
Includes 1,719,229 shares issuable upon the exercise of
options that are exercisable within 60 days of
September 30, 2010 and the shares identified in footnotes
(6), (7) and (8). Also includes 40,000 shares of
restricted stock that are subject to repurchase by us. |
|
|
|
|
(11) |
|
The address for Lehman Brothers International (Europe) is
c/o PricewaterhouseCoopers
LLP, 1 Embankment Place, London WC2N 6RH25, United Kingdom. |
82
Description of
Capital Stock
The following description of our capital stock and provisions of
our certificate of incorporation and by-laws are summaries and
are qualified by reference to our Second Restated Certificate of
Incorporation, as amended, and our Amended and Restated By-laws.
We have filed copies of these documents with the SEC as exhibits
to our registration statement of which this prospectus forms a
part.
Common
Stock
We are authorized to issue 150,000,000 shares of common
stock, par value $0.0001 per share. As of June 30, 2010,
there were 37,216,052 shares of common stock outstanding,
held of record by approximately 101 stockholders. The number of
shares of common stock outstanding as of June 30, 2010 does
not include 2,908,393 shares of common stock issuable upon
the exercise of outstanding options to purchase common stock
with a weighted average exercise price of $2.07 per share.
Holders of our common stock are entitled to one vote for each
share held of record on all matters submitted to a vote of
stockholders and do not have cumulative voting rights. Subject
to the rights of any preferred stock that may be issued, holders
of our common stock are entitled to receive such dividends on a
pro rata basis, if any, as may be declared by the Board of
Directors out of any funds legally available for that purpose.
Upon our dissolution, liquidation or
winding-up,
holders of our common stock, subject to the rights of any
preferred stock that may be issued, are entitled to share
ratably in our net assets legally available for distribution to
our stockholders after the payment of all of our debts and other
liabilities. Authorized but unissued shares of common stock may
generally be issued upon the approval of the Board of Directors
without obtaining the approval of the stockholders, unless the
issuance relates to a transaction that requires stockholder
consent. Holders of common stock have no preemptive, conversion,
subscription or other rights, and there are no redemption or
sinking fund provisions applicable to the common stock. The
rights, preferences and privileges of the holders of common
stock are subject to and may be adversely affected by, the
rights of the holders of shares of any series of preferred stock
that we may designate in the future. All of our outstanding
shares of common stock are, and the shares of common stock to be
issued in this offering will be, fully paid and non-assessable.
Preferred
Stock
Our Second Restated Certificate of Incorporation, as amended,
authorizes us to issue up to 1,000,000 shares of preferred
stock, par value $0.0001 per share, with such designations,
rights and preferences as may be determined from time to time by
the Board of Directors. Accordingly, the Board of Directors is
empowered, without stockholder approval, to issue preferred
stock with dividend, liquidation, conversion, voting or other
rights which could adversely affect the voting power or other
rights of the holders of common stock. The issuance of preferred
stock, while providing flexibility in connection with possible
acquisitions and other corporate purposes, could, among other
things, have the effect of delaying, deferring or preventing a
change in control of our company and may adversely affect the
market price of the common stock and the voting and other rights
of the holders of common stock. No preferred stock has been
issued.
Certain
Provisions of Our Second Restated Certificate of Incorporation,
as amended and Amended and Restated By-laws and Delaware
Anti-Takeover Law
Second
Restated and Amended Certificate of Incorporation
Pursuant to our Second Restated Certificate of Incorporation, as
amended, we have a classified board of directors divided into
three classes with staggered three-year terms. Only one class of
directors may be elected each year, while the directors in the
other classes continue to hold office for the remainder of their
three-year terms. Each class of the Board of Directors is
required to have approximately the same number of directors. The
Board of Directors may, on its own, determine the size of the
exact number of directors on the Board of Directors between one
and eleven directors and may fill vacancies on the Board of
Directors by vote of a majority of the directors then in office,
even if less than a quorum, or by a sole remaining director. The
procedure for electing and removing directors on a classified
board of directors generally makes it more difficult for
stockholders to change
83
control of a company by replacing a majority of the classified
board of directors at any one time, and our classified structure
may discourage a third party tender offer or other attempt to
gain control of our company and may maintain the incumbency of
directors. In addition, under our Second Restated Certificate of
Incorporation, as amended, our directors may only be removed
from office for cause and by a vote of the majority of the
shares then outstanding and eligible to vote.
Amended
and Restated By-Laws
Under our amended and restated by-laws, special meetings of
stockholders may be called at the request, in writing to our
corporate secretary, of stockholders holding of record at least
50% of the issued and outstanding stock entitled to vote.
Delaware
Anti-Takeover Law
We are subject to the provisions of Section 203 of the
Delaware General Corporation Law regulating corporate takeovers.
In general, Section 203 prohibits a publicly held Delaware
corporation from engaging, under certain circumstances, in a
business combination with an interested stockholder for a period
of three years following the date the person became an
interested stockholder, unless:
|
|
|
| |
•
|
prior to the date of the transaction, the board of directors of
the corporation approved either the business combination or the
transaction which resulted in the stockholder becoming an
interested stockholder;
|
| |
| |
•
|
upon completion of the transaction that resulted in the
stockholder becoming an interested stockholder, the stockholder
owned at least 85% of the voting stock of the corporation
outstanding at the time the transaction commenced, excluding for
purposes of determining the number of shares outstanding
(1) shares owned by persons who are directors and also
officers and (2) shares owned by employee stock plans in
which employee participants do not have the right to determine
confidentially whether shares held subject to the plan will be
tendered in a tender or exchange offer; or
|
| |
| |
•
|
on or subsequent to the date of the transaction, the business
combination is approved by the board and authorized at an annual
or special meeting of stockholders, and not by written consent,
by the affirmative vote of at least
662/3%
of the outstanding voting stock which is not owned by the
interested stockholder.
|
Generally, a business combination includes a merger, asset or
stock sale, or other transaction resulting in a financial
benefit to the interested stockholder. An interested stockholder
is a person who, together with affiliates and associates, owns
or, within three years prior to the determination of interested
stockholder status, did own 15% or more of a corporation’s
outstanding voting securities. We expect the existence of this
provision to have an anti-takeover effect with respect to
transactions our Board of Directors does not approve in advance.
Section 203 may also discourage attempts that might result
in a premium over the market price for the shares of common
stock held by stockholders.
Transfer Agent
and Registrar
The transfer agent and registrar for our common stock is
StockTrans, Inc. The transfer agent and registrar’s address
is 44 West Lancaster Avenue, Ardmore, Pennsylvania 19003.
NASDAQ Global
Market Listing
We have applied for listing of our common stock on The NASDAQ
Global Market under the trading symbol “RPSE.”
84
Shares Eligible
for Future Sale
Prior to this offering, there was no public market in the United
States for our securities. Our common stock and warrants to
purchase common stock traded only on AIM, prior to their
delisting in September and October 2009, respectively, or in
private transactions. All unexercised warrants expired on
April 28, 2010. Future sales of substantial amounts of
common stock in the public market could adversely affect the
market price of our common stock. After this offering is
completed, the number of shares eligible for future sale into
the public markets is subject to legal and contractual
restrictions, some of which are described below. The expiration
of these restrictions will permit sales of substantial amounts
of our common stock in the public market or could create the
perception that these sales could occur, which could adversely
affect the market price for our common stock. These factors
could also make it more difficult for us to raise funds through
future offerings of common stock.
Based on the number of shares of common stock outstanding as
of ,
2010, upon completion of this
offering, shares of common stock
will be outstanding, assuming no exercise of the
underwriters’ over-allotment option and no exercise of
stock options prior to the completion of this offering. All of
the shares sold in this offering will be freely tradable without
restrictions or further registration under the Securities Act,
unless purchased by our affiliates as that term is defined under
Rule 144 under the Securities Act, in which case sales of
the shares are subject to specified restrictions under
Rule 144 described below.
The
remaining shares
of common stock outstanding upon the closing of this offering
will be freely tradable without restrictions or further
registration under the Securities Act, subject to:
|
|
|
| |
•
|
lock-up
agreements described below between the holders of some of the
shares and the underwriters of this offering;
|
| |
| |
•
|
arrangements under which some of the shares issued in our
previous acquisitions are held in escrow; and
|
| |
| |
•
|
in the case of shares held by affiliates, restrictions as to
volume, manner of sale and other limitations under Rule 144.
|
Additionally, of the 2,908,393 shares of common stock
issuable upon exercise of options outstanding as of
June 30, 2010, substantially all are subject to the lockup
agreements with the underwriters, but upon expiration of those
lockup agreements, they will be eligible for sale without
restriction under the Securities Act, unless held by affiliates,
in which case they will be subject to the restrictions of
Rule 144 described below.
Lock-up
Agreements
Our executive officers and directors, and certain holders of our
outstanding common stock, have agreed, subject to specified
exceptions, not to directly or indirectly sell, offer, contract
or grant any option to sell (including without limitation any
short sale), pledge, transfer, establish an open “put
equivalent position” within the meaning of
Rule 16a-1(h)
under the Securities Exchange Act of 1934, as amended, or the
Exchange Act, or otherwise dispose of any shares of our common
stock, options or warrants to acquire shares of our common
stock, or securities exchangeable or exercisable for or
convertible into shares of our common stock currently or
hereafter owned either of record or beneficially (as defined in
Rule 13d-3
under the Exchange Act) by such person, or publicly announce an
intention to do any of the foregoing. We have also agreed,
subject to specified exceptions, not to directly or indirectly
sell (including, without limitation, any short sale), offer,
contract or grant any option to sell, pledge, transfer or
establish an open “put equivalent position” within the
meaning of
Rule 16a-1(h)
under the Exchange Act, or otherwise dispose of or transfer, or
announce the offering of, or file any registration statement
under the Securities Act in respect of, any shares of our common
stock, options, rights or warrants to acquire shares of our
common stock, or securities exchangeable or exercisable for or
convertible into shares of common stock, or publicly announce
the intention to do any of the foregoing.
These restrictions terminate as to us and our executive
officers, directors and stockholders after the close of trading
of our common stock on the 180th day after the date of this
prospectus. Jefferies & Company, Inc. may, in its sole
discretion and at any time or from time to time before the
termination of the
180-day
period, without notice, release all or any portion of the
securities subject to the
lock-up
agreements. However, subject to specified exceptions, if
(i) during the last 17 days of the
180-day
period, we issue an earnings release or material news or a
material event relating to our company occurs or (ii) prior
to the expiration of the
180-day
period, we announce
85
that we will release earnings results during the
16-day
period beginning on the last day of the
180-day
period, then the
180-day
period will be extended until the expiration of the
18-day
period beginning on the date of the issuance of the earnings
release or the occurrence of the material news or material
event, as applicable, unless Jefferies & Company, Inc.
waives, in writing, such extension.
Shares in
Escrow
In connection with our acquisition of Imerem on
December 22, 2008, we issued 1,296,165 shares of
common stock to Imerem’s sole shareholder. Pursuant to an
escrow agreement between Imerem’s sole shareholder and our
acquiring subsidiary, ReSearch Pharmaceutical Services
Netherlands B.V., or RPS Dutch BV, one-half, or 648,083, of the
shares of common stock were placed in escrow, to be released in
equal portions on the first, second and third anniversaries of
the acquisition date, subject to there being no indemnity claims
outstanding. The shares held in escrow may not be sold until
they are released from escrow. As of the date of this
prospectus, 216,028 shares have been released from escrow
and 432,055 shares remain subject to escrow, of which
one-half will be released in December 2010 and one-half will be
released in December 2011.
In connection with our acquisition of Infociencia on
December 22, 2008, we issued 1,404,856 shares of
common stock to Infociencia’s shareholders. Pursuant to an
escrow agreement between Infociencia’s shareholders and our
acquiring subsidiary, RPS Spain S.L., or RPS Spain, one-half, or
702,428, of the shares of common stock were placed in escrow, to
be released in equal portions on the first, second and third
anniversaries of the acquisition date, subject to there being no
indemnity claims outstanding. The shares held in escrow may not
be sold until they are released from escrow. As of the date of
this prospectus, 234,143 shares have been released from
escrow and 468,285 shares remain subject to escrow, of
which one-half will be released in December 2010 and one-half
will be released in December 2011.
In connection with our acquisition of Therapharm on
December 23, 2008, we issued 1,497,864 shares of
common stock to Therapharm’s shareholder. Pursuant to an
escrow agreement between Therapharm’s shareholder and our
acquiring subsidiary, RPS Dutch BV, one-half, or 748,932, of the
shares of common stock were placed in escrow, to be released in
equal portions on the first, second and third anniversaries of
the acquisition date, subject to there being no indemnity claims
outstanding. The shares held in escrow may not be sold until
they are released from escrow. As of the date of this
prospectus, 187,344 shares have been released from escrow,
62,300 shares were returned to us pursuant to a working
capital adjustment escrow claim, which shares we subsequently
cancelled, and 499,288 shares remain subject to escrow, of
which one-half will be released in December 2010 and one-half
will be released in December 2011.
In connection with our acquisition of Paramax on July 10,
2009, we issued 530,973 shares of common stock to
Paramax’s shareholder. Pursuant to an escrow agreement
between Paramax’s shareholder and our acquiring subsidiary,
RPS Dutch BV, all of the consideration shares were placed in
escrow, and were, or will be released in three equal portions on
October 7, 2009, July 7, 2010 and January 31,
2011, subject to there being no indemnity claims outstanding.
The shares held in escrow may not be sold until they are
released. As of the date of this prospectus, 353,982 shares
have been released from escrow, and 176,991 shares remain
subject to escrow.
Rule 144
In general, under Rule 144 under the Securities Act, as in
effect on the date of this prospectus, our affiliates who have
beneficially owned shares of our common stock for at least six
months are entitled to sell within any three-month period a
number of shares that does not exceed the greater of:
|
|
|
| |
•
|
one percent of the number of shares of our common stock then
outstanding, which will equal
approximately shares
immediately after this offering; and
|
| |
| |
•
|
the average weekly trading volume of our common stock on The
NASDAQ Global Market during the four calendar weeks preceding
the filing of a notice on Form 144 with respect to the sale.
|
Sales of shares under Rule 144 by our affiliates are also
subject to requirements regarding the manner of sale, notice and
the availability of current public information about our company.
86
Registration
Rights
Most of our stockholders prior to this offering have rights to
require us to register their shares under the Securities Act
pursuant to three separate registration rights agreements.
Investor
Rights Agreement
We entered into an Investor Rights Agreement, dated
April 24, 2006, in connection with the initial public
offering of Cross Shore’s common stock. The Investor Rights
Agreement covers an aggregate of 16,783,891 shares of our
outstanding common stock.
Under the Investor Rights Agreement, we are also required to
file a shelf registration statement on
Form S-3
within 90 days after becoming eligible to do so. In
addition, the holders of our common stock that are party to the
Investor Rights Agreement are entitled to no more than three
demand registrations, covering in each case a minimum of 15% of
the shares then outstanding, and piggyback registration rights.
If we file a shelf registration statement for the resale of
shares, demand and piggyback registration rights will be
suspended except for underwritten offerings. Registration rights
are generally available only for stock that is subject to
restrictions on transfer under the U.S. securities laws.
Registration
Rights Agreement
We entered into a Registration Rights Agreement, dated
August 30, 2007, or the Registration Rights Agreement, with
the holders of shares of common stock of Old RPS who were issued
shares of our common stock in the merger between Cross Shore and
Old RPS. The Registration Rights Agreement covers an aggregate
of 15,758,497 shares of our outstanding common stock.
Under the Registration Rights Agreement, we have granted the
stockholders that previously held shares in Old RPS the rights
to include shares on any registration statement we file pursuant
to the Securities Act in connection with a public offering of
stock, whether such offering is being made for our own account
or for the account of stockholders other than the stockholders
that previously held shares in Old RPS. These registration
rights are applicable to any registration of stock that is made
pursuant to a demand from the existing stockholders pursuant to
the Investor Rights Agreement. The number of shares that the
existing stockholders may include in an underwritten public
offering by exercising their registration rights under the
Registration Rights Agreement is subject to reduction in the
event the managing underwriters of such offering advise us that
the number of shares to be included in such offering exceeds the
amount of stock that can be sold without adversely affecting the
offering.
The Registration Rights Agreement also provides similar shelf
registration rights as are set forth in the Investor Rights
Agreement described above.
Founders’
Shares Agreement
We entered into a Registration Rights Agreement, dated
April 24, 2006, with the founders of Cross Shore who
acquired shares of common stock prior to Cross Shore’s
initial public offering. We refer to this agreement as the
Founders’ Shares Agreement. The Founders’
Shares Agreement covers an aggregate of
1,806,772 shares of our outstanding common stock.
Under the Founders’ Shares Agreement, we are required
to file a shelf registration statement on
Form S-3
upon request of the stockholders who are parties to the
Founders’ Shares Agreement, as long as the holders
propose to sell registrable securities and such other
securities, if any, at an aggregate price to the public of at
least $2,000,000. In addition, the stockholders who are parties
to the Founders’ Shares Agreement are entitled to no
more than two demand registrations, covering in each case as
many shares as the requesting stockholders propose to sell,
subject to certain restrictions imposed by an underwriter, and
piggyback registration rights. If we file a shelf registration
statement for the resale of shares, demand and piggyback
registration rights will be suspended except for underwritten
offerings. Registration rights are generally available only for
stock that is subject to restrictions on transfer under the
U.S. securities laws.
We are required to bear all expenses incident to our compliance
with the terms of the Registration Rights Agreement, the
Investor Rights Agreement and the Founders’
Shares Agreement. The Registration Rights
87
Agreement and Founders’ Shares Agreement also contain
customary indemnification obligations from us to the applicable
stockholders with respect to untrue statements or material
omissions in any registration statement that includes the
applicable shares.
If we do not effect a registration required under the Investor
Rights Agreement or the Registration Rights Agreement, the
stockholders who are party to those agreements may be entitled
to receive liquidated damages in the form of additional shares
in an amount per month equal to 1% of all or a portion of such
holder’s registrable securities for up to two months, in
the case of the Registration Rights Agreement, or four months,
in the case of the Investor Rights Agreement.
88
Certain U.S.
Federal Tax Considerations Applicable To
Non-U.S.
Holders
The following is a summary of certain U.S. federal income
and estate tax considerations related to the purchase, ownership
and disposition of our common stock that are applicable to a
“non-U.S. holder,”
as defined below. This section does not address tax
considerations applicable to other investors, such as
U.S. persons, as defined below. They are urged to consult
their own tax advisors to determine the specific tax
consequences and risks to them of purchasing, holding and
disposing of the common stock.
This summary:
|
|
|
| |
•
|
is based on the Code, U.S. federal tax regulations
promulgated or proposed under it, referred to in this prospectus
as the Treasury Regulations, judicial authority, and published
rulings and administrative pronouncements of the
U.S. Internal Revenue Service, or the IRS, each as of the
date of this prospectus and each of which are subject to change
at any time, possibly with retroactive effect;
|
| |
| |
•
|
is applicable only to
non-U.S. holders
who hold the shares as “capital assets” within the
meaning of section 1221 of the Code;
|
| |
| |
•
|
does not discuss the applicability of any U.S. state or
local taxes,
non-U.S. taxes
or any other U.S. federal tax except for U.S. federal
income tax and estate tax; and
|
| |
| |
•
|
does not address all aspects of U.S. federal income
taxation that may be relevant to holders in light of their
particular circumstances or who are subject to special treatment
under U.S. federal income tax laws, including but not
limited to:
|
|
|
|
| |
•
|
certain former citizens and long-term residents of the United
States;
|
| |
| |
•
|
banks, financial institutions, or “financial services
entities”;
|
| |
| |
•
|
insurance companies;
|
| |
| |
•
|
tax-exempt organizations;
|
| |
| |
•
|
dealers in securities;
|
| |
| |
•
|
investors holding the common stock as part of a
“straddle,” “hedge,” “conversion
transaction,” or other risk-reduction transaction; and
|
| |
| |
•
|
“controlled foreign corporations” and “passive
foreign investment companies,” as defined in the Code.
|
This summary constitutes neither tax nor legal advice.
Prospective investors are urged to consult their own tax
advisors to determine the specific tax consequences and risks to
them of purchasing, holding and disposing of our common stock,
including the application to their particular situations of any
U.S. federal, state, local, and
non-U.S. tax
laws and of any applicable income tax treaty.
Non-U.S.
Holder Defined
For purposes of this discussion, a
non-U.S. holder
is a beneficial owner of common stock that is neither a
“U.S. person” nor a partnership or entity or
arrangement treated as a partnership for U.S. federal
income tax purposes. A “U.S. person” is:
|
|
|
| |
•
|
an individual citizen or resident of the United States;
|
| |
| |
•
|
a corporation, or other entity taxable as a corporation for
U.S. federal income tax purposes, that is created or
organized in or under the laws of the United States, any state
thereof or the District of Columbia;
|
| |
| |
•
|
an estate the income of which is subject to U.S. federal
income taxation regardless of its source; or
|
| |
| |
•
|
a trust if it (1) is subject to the primary supervision of
a court within the United States and one or more
U.S. persons have the authority to control all substantial
decisions of the trust or (2) has a valid election in
effect under applicable Treasury Regulations to be treated as a
U.S. person.
|
If a partnership (or an entity or arrangement treated as a
partnership for U.S. federal income tax purposes) owns our
common stock, then the U.S. federal income tax treatment of
a partner in that partnership generally will depend on the
status of the partner and the partnership’s activities.
Partners and partnerships should consult their own tax advisors
with regard to the U.S. federal income tax treatment of an
investment in our common stock.
89
Distributions to
Non-U.S.
Holders
Distributions of cash or property, if any, paid to a
non-U.S. holder
of the common stock will constitute “dividends” for
U.S. federal income tax purposes to the extent paid out of
our current or accumulated earnings and profits, as determined
for U.S. federal income tax purposes. If the amount of a
distribution exceeds both our current and accumulated earnings
and profits, such excess will first constitute a nontaxable
return of capital, which will reduce the holder’s tax basis
in the common stock, but not below zero, and thereafter will be
treated as gain from the sale of the common stock. See
“—Sale or Taxable Disposition of Common Stock by
Non-U.S. Holders”
below.
Subject to the following paragraphs, dividends on the common
stock generally will be subject to U.S. federal withholding
tax at a 30% gross rate, subject to any exemption or lower rate
as may be specified by an applicable income tax treaty. We may
withhold up to 30% of either (1) the gross amount of the
entire distribution, even if the amount of the distribution is
greater than the amount constituting a dividend, as described
above, or (2) the amount of the distribution we project
will be a dividend, based upon a reasonable estimate of both our
current and our accumulated earnings and profits for the taxable
year in which the distribution is made. If tax is withheld on
the amount of a distribution in excess of the amount
constituting a dividend, then you may obtain a refund of that
excess amount by timely filing a claim for refund with the IRS.
To claim the benefit of a reduced rate of or an exemption from
U.S. federal withholding tax under an applicable income tax
treaty, a
non-U.S. holder
will be required (1) to satisfy certain certification
requirements, which may be made by providing us or our agent
with a properly executed and completed IRS
Form W-8BEN
(or other applicable form) certifying, under penalty of perjury,
that the holder qualifies for treaty benefits and is not a
U.S. person or (2) if the common stock is held through
certain
non-U.S. intermediaries,
to satisfy the relevant certification requirements of the
applicable Treasury Regulations. Special certification and other
requirements apply to certain
non-U.S. holders
that are pass-through entities.
Dividends that are effectively connected with the conduct of a
trade or business by the
non-U.S. holder
within the United States (and, if required by an applicable
income tax treaty, are attributable to a permanent
establishment, or a fixed base in the case of an individual
non-U.S. holder,
that is maintained by the
non-U.S. holder
in the United States) (“effectively connected
dividends”) are not subject to the U.S. federal
withholding tax, provided that the
non-U.S. holder
certifies, under penalty of perjury, that the dividends paid to
such holder are effectively connected dividends on a properly
executed and completed IRS
Form W-8ECI
(or other applicable form). Instead, any such dividends will be
subject to U.S. federal income tax on a net income basis in
a manner similar to that which would apply if the
non-U.S. holder
were a U.S. person.
Corporate
non-U.S. holders
who receive effectively connected dividends may also be subject
to an additional “branch profits tax” at a gross rate
of 30% on their earnings and profits for the taxable year that
are effectively connected with the holder’s conduct of a
trade or business within the United States, subject to any
exemption or reduction provided by an applicable income tax
treaty.
Sale or Taxable
Disposition of Common Stock by
Non-U.S.
Holders
Any gain realized on the sale, exchange or other taxable
disposition of the common stock generally will not be subject to
U.S. federal income tax unless:
|
|
|
| |
•
|
the gain is effectively connected with the conduct of a trade or
business by the
non-U.S. holder
within the United States (and, if required by an applicable
income tax treaty, is attributable to a permanent establishment,
or fixed base in the case of an individual
non-U.S. holder,
that is maintained by the
non-U.S. holder
in the United States);
|
| |
| |
•
|
the
non-U.S. holder
is an individual who is present in the United States for
183 days or more in the taxable year of that disposition,
and certain other conditions are met; or
|
| |
| |
•
|
we are or have been a “United States real property holding
corporation” for U.S. federal income tax purposes at
any time during the shorter of the five-year period ending on
the date of such disposition and the
non-U.S. holder’s
holding period in the common stock.
|
A
non-U.S. holder
described in the first bullet point above generally will be
subject to U.S. federal income tax on
90
the net gain derived from the sale or disposition under regular
graduated U.S. federal income tax rates as if the holder
were a U.S. person. If the
non-U.S. holder
is a corporation, then the gain may also, under certain
circumstances, be subject to the “branch profits tax,”
which was discussed above.
An individual
non-U.S. holder
described in the second bullet point above will be subject to a
tax at a 30% gross rate, subject to any reduction or reduced
rate under an applicable income tax treaty, on the net gain
derived from the sale, which may be offset by
U.S.-source
capital losses, even though the individual is not considered a
resident of the United States for U.S. federal income tax
purposes.
We believe we are not, have not been and will not become a
“United States real property holding corporation” for
U.S. federal income tax purposes. In the event that we are
or become a United States real property holding corporation at
any time during the applicable period described in the third
bullet point above, any gain recognized on a sale or other
taxable disposition of the common stock may be subject to
U.S. federal income tax, including any applicable
withholding tax, if (1) the
non-U.S. holder
beneficially owns, or has owned, more than 5% of our common
stock at any time during the applicable period, or (2) our
common stock ceases to be traded on an “established
securities market” within the meaning of the Code.
Non-U.S. holders
who intend to acquire more than 5% of our common stock are
encouraged to consult their tax advisors with respect to the
U.S. tax consequences of a disposition of the common stock.
Information
Reporting and Backup Withholding
We must report annually to the IRS and to each
non-U.S. holder
the amount of dividends and other distributions paid to the
holder and the tax withheld, if any, from those payments. These
reporting requirements apply regardless of whether withholding
was reduced or eliminated by any applicable income tax treaty.
Copies of the information returns reporting such dividends and
the tax withheld may also be made available to the tax
authorities in the country in which the
non-U.S. holder
resides under the provisions of an applicable income tax treaty.
A
non-U.S. holder
that is not a corporation will generally be subject to backup
withholding, currently at a 28% rate, for dividends paid to the
holder unless the holder certifies under penalty of perjury that
it is not a U.S. person or the holder otherwise establishes
an exemption (provided that the payor does not have actual
knowledge or reason to know that such holder is a
U.S. person or that the conditions of any other exemptions
are not in fact satisfied).
Information reporting and, depending on the circumstances,
backup withholding will apply to the proceeds of a sale of the
common stock by a
non-U.S. holder
within the United States or conducted through certain
U.S.-related
financial intermediaries, unless the holder certifies under
penalty of perjury that it is not a U.S. person or the
holder otherwise establishes an exemption (provided that neither
the broker nor intermediary has actual knowledge or reason to
know that such holder is a U.S. person or that the
conditions of any other exemptions are not in fact satisfied).
Backup withholding is not an additional tax. Any amounts
withheld under the backup withholding rules may be allowed as a
refund or a credit against a
non-U.S. holder’s
U.S. federal income tax liability, if any, provided the
required information is timely furnished to the IRS.
Federal Estate
Tax
Individual
non-U.S. holders
and entities the property of which is potentially includible in
such an individual’s gross estate for U.S. federal
estate tax purposes (for example, a trust funded by such an
individual and with respect to which the individual has retained
certain interests or powers) should note that, absent an
applicable treaty benefit, the common stock will be treated as
U.S.-situs
property subject to U.S. federal estate tax.
Recent
Legislative Developments
The recently enacted Hiring Incentives to Restore Employment Act
has, among other things, added new sections 1471 to 1474 of
the Code, which will, effective January 1, 2013, impose new
information reporting and withholding tax requirements for
dividends and sales proceeds paid to certain
non-U.S. entities
that hold shares in
91
U.S. corporations. In general, to avoid a 30% withholding
tax under these provisions, (1) foreign financial
institutions that hold shares in U.S. corporations will be
required to identify for the IRS each U.S. account owner
who is a beneficial owner of such shares and to provide certain
information regarding the account, and also to agree to comply
with certain other requirements, and (2) other foreign
entities (aside from public companies) that are beneficial
owners of shares will be required to identify U.S. persons
who own a 10% or greater interest in such foreign entity.
Foreign entities, and other foreign persons who plan to have
their shares of our common stock held through a foreign
financial institution, should consider the potential
applicability of these new provisions.
92
Underwriting
Under the terms and subject to the conditions contained in an
underwriting agreement dated ,
2010, by and among us and the underwriters named below, for whom
Jefferies & Company, Inc. is acting as representative,
the underwriters have agreed to purchase, and we have agreed to
sell to them, the number of shares of common stock indicated in
the table below:
| |
|
|
|
|
|
|
|
Number
|
|
Name
|
|
of Shares
|
|
|
|
Jefferies & Company, Inc.
|
|
|
|
|
|
William Blair & Company, L.L.C.
|
|
|
|
|
|
Lazard Capital Markets LLC
|
|
|
|
|
|
|
|
|
|
|
|
Total
|
|
|
|
|
|
|
|
|
|
|
The underwriters are offering the common stock subject to their
acceptance of the shares from us and subject to prior sale. The
underwriting agreement provides that the obligations of the
underwriters to pay for and accept delivery of the common stock
offered by this prospectus are subject to the approval of
certain legal matters by their counsel and to certain other
conditions. The underwriting agreement provides that the
underwriters are obligated to take and pay for all of the common
stock if any such shares are purchased, other than those shares
covered by the overallotment option described below.
Commissions and
Expenses
The underwriters have advised us that they propose to offer the
shares to the public at the public offering price per share set
forth on the cover page of this prospectus and to certain
dealers at that price less a concession not in excess of
$ per share. After the offering,
the public offering price and concession to dealers may be
reduced by the underwriters. No such reduction shall change the
amount of proceeds to be received by us as set forth on the
cover page of this prospectus. The shares are offered by the
underwriters as stated herein, subject to receipt and acceptance
by them and subject to their right to reject any order in whole
or in part.
The following table shows the public offering price, the
underwriting discounts and commissions payable to the
underwriters by us and the proceeds, before expenses, to us.
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total with
|
|
|
|
|
|
|
|
Full Exercise of
|
|
|
|
|
|
|
|
Overallotment
|
|
|
|
Per Share
|
|
Total
|
|
Option
|
|
|
|
Public offering price
|
|
$
|
|
|
|
$
|
|
|
|
$
|
|
|
|
Underwriting discounts and commissions
|
|
$
|
|
|
|
$
|
|
|
|
$
|
|
|
|
Proceeds to ReSearch Pharmaceutical Services, Inc. (before
expenses)
|
|
$
|
|
|
|
$
|
|
|
|
$
|
|
|
We estimate expenses payable by us in connection with the
offering of common stock, other than the underwriting discounts
and commissions referred to above, will be approximately
$ .
Option to
Purchase Additional Shares
We have granted to the underwriters an option, exercisable for
30 days from the date of this prospectus, to purchase up to
an aggregate
of
additional shares at the same price they are paying for the
shares shown in the table above. The underwriters may exercise
this option at any time and from time to time, in whole or in
part, within 30 days after the date of this prospectus.
Indemnification
We have agreed to indemnify the underwriters against certain
liabilities, including liabilities under the Securities
93
Act. We have also agreed to contribute to payments that the
underwriters may be required to make in respect of those
liabilities.
Lock-up
Agreements
Our executive officers and directors and the holders of
substantially all of our outstanding common stock have agreed,
subject to specified exceptions, not to directly or indirectly
sell, offer, contract or grant any option to sell (including
without limitation any short sale), pledge, transfer, establish
an open “put equivalent position” within the meaning
of
Rule 16a-1(h)
under the Exchange Act, or otherwise dispose of any shares of
our common stock, options or warrants to acquire shares of our
common stock, or securities exchangeable or exercisable for or
convertible into shares of our common stock currently or
hereafter owned either of record or beneficially (as defined in
Rule 13d-3
under the Exchange Act) by such person, or publicly announce an
intention to do any of the foregoing. We have also agreed,
subject to specified exceptions, not to directly or indirectly
sell (including, without limitation, any short sale), offer,
contract or grant any option to sell, pledge, transfer or
establish an open “put equivalent position” within the
meaning of
Rule 16a-1(h)
under the Exchange Act, or otherwise dispose of or transfer, or
announce the offering of, or file any registration statement
under the Securities Act in respect of, any shares of our common
stock, options, rights or warrants to acquire shares of our
common stock, or securities exchangeable or exercisable for or
convertible into shares of common stock, or publicly announce
the intention to do any of the foregoing.
These restrictions terminate as to us and our executive
officers, directors and stockholders after the close of trading
of our common stock on the 180th day after the date of this
prospectus. Jefferies & Company, Inc. may, in its sole
discretion and at any time or from time to time before the
termination of the
180-day
period, without notice, release all or any portion of the
securities subject to the
lock-up
agreements. However, subject to specified exceptions, if
(i) during the last 17 days of the
180-day
period, we issue an earnings release or material news or a
material event relating to our company occurs or (ii) prior
to the expiration of the
180-day
period, we announce that we will release earnings results during
the 16-day
period beginning on the last day of the
180-day
period, then the
180-day
period will be extended until the expiration of the
18-day
period beginning on the date of the issuance of the earnings
release or the occurrence of the material news or material
event, as applicable, unless Jefferies & Company, Inc.
waives, in writing, such extension.
Electronic
Distribution
This prospectus in electronic format may be made available on
websites or through other online services maintained by the
underwriters of the offering, or by their affiliates. Other than
the prospectus in electronic format, the information on the
underwriters’ websites and any information contained in any
other website maintained by the underwriters is not part of the
prospectus or the registration statement of which this
prospectus forms a part, has not been approved or endorsed by us
or the underwriters in their capacity as underwriters and should
not be relied upon by investors.
Upon receipt of a request by an investor or its representative
who has received an electronic prospectus from an underwriter
within the period during which there is an obligation to deliver
a prospectus, we will promptly transmit, or cause to be
transmitted, without charge, a paper copy of the prospectus.
No Public
Market
We have applied to list our common stock on The NASDAQ Global
Market under the symbol “RPSE,” but there has been no
public market for our common stock since its delisting from AIM
in September 2009. The initial public offering price for the
shares being offered hereby will be determined by us and the
representative, based on the following factors:
|
|
|
| |
•
|
the history and prospects for the industry in which we compete;
|
| |
| |
•
|
our past and present operations;
|
| |
| |
•
|
our historical results of operations;
|
| |
| |
•
|
our prospects for future business and earning potential;
|
94
|
|
|
| |
•
|
our management;
|
| |
| |
•
|
the general condition of the securities markets at the time of
this offering;
|
| |
| |
•
|
the recent market prices of securities of generally comparable
companies;
|
| |
| |
•
|
the market capitalization and stages of development of other
companies which we and the representative believe to be
comparable to us; and
|
| |
| |
•
|
other factors deemed to be relevant.
|
We cannot assure you that the initial public offering price will
correspond to the price of which our common stock will trade in
the public market after this offering or that an active trading
market for the common stock will develop and continue after this
offering.
Price
Stabilization, Short Positions and Penalty Bids
Until the distribution of the shares of common stock is
completed, SEC rules may limit the underwriters from bidding for
and purchasing shares of our common stock.
In connection with this offering, the underwriters may engage in
transactions that stabilize, maintain or make short sales of our
common stock and may purchase our common stock on the open
market to cover positions created by short sales. Short sales
involve the sale by the underwriters of a greater number of
shares than they are required to purchase in this offering.
Establishing short sales positions may involve either
“covered” short sales or “naked” short
sales. Covered short sales are short sales made in an amount not
greater than the underwriters’ over-allotment option
described above. The underwriters may close out any covered
short position either by exercising their over-allotment option
or by purchasing shares in the open market. To determine how
they will close the covered short position, the underwriters
will consider, among other things, the price of shares available
for purchase in the open market, as compared to the price at
which they may purchase shares through the over-allotment
option. Naked short sales are short sales in excess of the
over-allotment option. The underwriters must close out any naked
short position by purchasing shares in the open market. A naked
short position is more likely to be created if the underwriters
are concerned that, in the open market after the pricing of this
offering, there may be downward pressure on the price of the
shares that could adversely affect investors who purchase shares
in this offering.
A “stabilizing bid” is a bid for or the purchase of
common stock on behalf of the underwriters in the open market
prior to the completion of this offering for the purpose of
fixing or maintaining the price of the shares of common stock. A
“syndicate covering transaction” is the bid for or
purchase of common stock on behalf of the underwriters to reduce
a short position incurred by the underwriters in connection with
the offering.
Similar to other purchase transactions, the underwriters’
purchases to cover the syndicate short sales may have the effect
of raising or maintaining the market price of our shares or
preventing or retarding a decline in the market price of our
shares. As a result, the price of our shares may be higher than
the price that might otherwise exist in the open market.
In connection with this offering, the underwriters may also
engage in passive market making transactions in our common stock
on The NASDAQ Global Market in accordance with Rule 103 of
Regulation M during a period before the commencement of
offers or sales of shares of our common stock in this offering
and extending through the completion of distribution. A passive
market maker must display its bid at a price not in excess of
the highest independent bid of that security. However, if all
independent bids are lowered below the passive market
maker’s bid, that bid must then be lowered when specified
purchase limits are exceeded.
Neither we nor the underwriters make any representation or
prediction as to the direction or magnitude of any effect that
the transactions described above may have on the price of our
common stock. In addition, neither we nor the underwriters make
any representation that the underwriters will engage in these
transactions or that any transaction, if commenced, will not be
discontinued without notice.
Affiliations
In the future, the underwriters and their affiliates may provide
various investment banking, commercial banking, financial
advisory and other services to us and our affiliates for which
services they have received, and may in the
95
future receive, customary fees. In the course of their
businesses, the underwriters and their affiliates may actively
trade our securities or loans for their own accounts or for the
accounts of customers, and, accordingly, the underwriters and
their affiliates may at any time hold long or short positions in
such securities or loans.
Lazard Frères & Co. LLC referred this transaction
to Lazard Capital Markets LLC and will receive a referral fee
from Lazard Capital Markets LLC in connection therewith.
European Economic
Area
In relation to each Member State of the European Economic Area
which has implemented the Prospectus Directive (as defined
below) (each, a Relevant Member State), with effect from and
including the date on which the Prospectus Directive is
implemented in that Relevant Member State, or the Relevant
Implementation Date, an offer of our common stock to the public
may not be made in that Relevant Member State prior to the
publication of a prospectus in relation to our common stock
which has been approved by the competent authority in that
Relevant Member State or, where appropriate, approved in another
Relevant Member State and notified to the competent authority in
that Relevant Member State, all in accordance with the
Prospectus Directive, except that an offer to the public in that
Relevant Member State of any shares of our common stock may be
made at any time under the following exemptions under the
Prospectus Directive if they have been implemented in the
Relevant Member State:
(a) to legal entities which are authorized or regulated to
operate in the financial markets or, if not so authorized or
regulated, whose corporate purpose is solely to invest in
securities;
(b) to any legal entity which has two or more of
(1) an average of at least 250 employees during the
last financial year; (2) a total balance sheet of more than
€43,000,000 and (3) an annual net turnover of more
than €50,000,000, as shown in its last annual or
consolidated accounts;
(c) to fewer than 100 natural or legal persons per Relevant
Member State (other than qualified investors as defined in the
Prospectus Directive); or
(d) in any other circumstances falling within
Article 3(2) of the Prospectus Directive,
provided that no such offer of our common stock shall result in
a requirement for the publication by us or any underwriter of a
prospectus pursuant to Article 3 of the Prospectus
Directive.
For the purposes of this provision, the expression an
“offer of our common stock to the public” in relation
to any shares of our common stock in any Relevant Member State
means the communication in any form and by any means of
sufficient information on the terms of the offer and our common
stock to be offered so as to enable an investor to decide to
purchase or subscribe our common stock, as the same may be
varied in that Member State by any measure implementing the
Prospectus Directive in that Member State and the expression
“Prospectus Directive” means Directive 2003/71/EC and
includes any relevant implementing measure in each Relevant
Member State.
United
Kingdom
Shares of our common stock may not be offered or sold and will
not be offered or sold to any persons in the United Kingdom
other than to persons whose ordinary activities involve them in
acquiring, holding, managing or disposing of investments (as
principal or as agent) for the purposes of their businesses or
otherwise in circumstances which have not resulted or will not
result in an offer to the public in the United Kingdom within
the meaning of the Financial Services and Markets Act 2000, or
the FSMA.
In addition, any invitation or inducement to engage in
investment activity (within the meaning of section 21 of
the FSMA) in connection with the issue or sale of shares of our
common stock may only be communicated or caused to be
communicated in circumstances in which Section 21(1) of the
FSMA does not apply to us. Without limitation to the other
restrictions referred to herein, this prospectus is directed
only at (1) persons outside the United Kingdom or
(2) persons who:
(a) are qualified investors as defined in
section 86(7) of FSMA, being persons falling within the
meaning of article 2.1(e)(i), (ii) or (iii) of
the Prospectus Directive; and
96
(b) are either persons who fall within article 19(1)
of the Financial Services and Markets Act 2000 (Financial
Promotion) Order 2005, as amended, or Order, or are persons who
fall within article 49(2)(a) to (d) (“high net worth
companies, unincorporated associations, etc.”) of the
Order; or
(c) to whom it may otherwise lawfully be communicated in
circumstances in which Section 21(1) of the FSMA does not
apply.
Without limitation to the other restrictions referred to herein,
any investment or investment activity to which this offering
circular relates is available only to, and will be engaged in
only with, such persons, and persons within the United Kingdom
who receive this communication (other than persons who fall
within (2) above) should not rely or act upon this
communication.
Germany
Any offer or solicitation of securities within Germany must be
in full compliance with the German Securities Prospectus Act
(Wertpapierprospektgesetz—WpPG). The offer and solicitation
of securities to the public in Germany requires the publication
of a prospectus that has to be filed with and approved by the
German Federal Financial Services Supervisory Authority
(Bundesanstalt für
Finanzdienstleistungsaufsicht—BaFin). This prospectus has
not been and will not be submitted for filing and approval to
the BaFin and, consequently, will not be published. Therefore,
this prospectus does not constitute a public offer under the
German Securities Prospectus Act (Wertpapierprospektgesetz).
This prospectus and any other document relating to our common
stock, as well as any information contained therein, must
therefore not be supplied to the public in Germany or used in
connection with any offer for subscription of our common stock
to the public in Germany, any public marketing of our common
stock or any public solicitation for offers to subscribe for or
otherwise acquire our common stock. This prospectus and other
offering materials relating to the offer of our common stock are
strictly confidential and may not be distributed to any person
or entity other than the designated recipients hereof.
France
This prospectus has not been prepared in the context of a public
offering of financial securities in France within the meaning of
Article L.411-1
of the French Code Monétaire et Financier and Title I
of Book II of the Règlement Général of the
Autorité des marchés financiers, or AMF, and therefore
has not been and will not be filed with the AMF for prior
approval or submitted for clearance to the AMF. Consequently,
the shares of our common stock may not be, directly or
indirectly, offered or sold to the public in France and offers
and sales of the shares of our common stock may only be made in
France to qualified investors (investisseurs qualifiés)
acting for their own, as defined in and in accordance with
Articles L.411-2
and D.411-1 to D.411-4, D.734-1, D.744-1, D.754-1 and D.764-1 of
the French Code Monétaire et Financier. Neither this
prospectus nor any other offering material may be released,
issued or distributed to the public in France or used in
connection with any offer for subscription on sale of the shares
of our common stock to the public in France. The subsequent
direct or indirect retransfer of the shares of our common stock
to the public in France may only be made in compliance with
Articles L.411-1,
L.411-2, L.412-1 and L.621-8 through L.621-8-3 of the French
Code Monétaire et Financier.
Sweden
This is not a prospectus under, and has not been prepared in
accordance with the prospectus requirements provided for in, the
Swedish Financial Instruments Trading Act [lagen (1991:980) om
handel med finasiella instrument] nor any other Swedish
enactment. Neither the Swedish Financial Supervisory Authority
nor any other Swedish public body has examined, approved, or
registered this document.
97
Legal
Matters
The validity of the shares of our common stock offered by this
prospectus will be passed upon for us by Drinker
Biddle & Reath LLP, Philadelphia, Pennsylvania. The
underwriters are represented by Cooley LLP, Reston, Virginia.
Experts
The consolidated financial statements of ReSearch Pharmaceutical
Services, Inc. and Subsidiaries at December 31, 2009 and
2008, and for each of the three years in the period ended
December 31, 2009, appearing in this prospectus and the
related registration statement have been audited by
Ernst & Young LLP, independent registered public
accounting firm, as set forth in their report thereon appearing
elsewhere herein, and are included in reliance upon such report
given on the authority of such firm as experts in accounting and
auditing.
The consolidated financial statements of each of Imerem,
Infociencia and Therapharm for the year ended and as of
December 31, 2007, included in this prospectus and
registration statement have been audited by
McGladrey & Pullen, LLP, independent registered public
accounting firm, as set forth in their report thereon appearing
elsewhere herein and are included in reliance on such report
given on the authority of such firm as experts in accounting and
auditing.
Where You Can
Find More Information
We have filed with the SEC a registration statement on
Form S-1
under the Securities Act relating to the shares of our common
stock being offered by this prospectus. This prospectus, which
constitutes part of that registration statement, does not
contain all of the information set forth in the registration
statement or the exhibits and schedules which are part of the
registration statement. For further information about us and the
common stock offered, we refer you to the registration statement
and the exhibits and schedules thereto. Statements contained in
this prospectus regarding the contents of any contract or any
other document to which reference is made are not necessarily
complete, and, in each instance where a copy of a contract or
other document has been filed as an exhibit to the registration
statement, reference is made to the copy so filed, each of those
statements being qualified in all respects by the reference.
A copy of the registration statement, the exhibits and schedules
thereto and any other document we file may be inspected without
charge at the public reference facilities maintained by the SEC
at 100 F Street, N.E., Washington, D.C. 20549,
and copies of all or any part of the registration statement may
be obtained from this office upon the payment of the fees
prescribed by the SEC. The public may obtain information on the
operation of the public reference facilities in
Washington, D.C. by calling the SEC at
1-800-732-0330.
Our filings with the SEC are available to the public from the
SEC’s website at www.sec.gov.
We are subject to the information and periodic reporting
requirements of the Exchange Act and, accordingly, have filed
annual reports containing our financial statements audited by an
independent public accounting firm, quarterly reports containing
our unaudited financial statements, current reports, proxy
statements and other information with the SEC. You may read and
copy this information at the SEC’s public reference room
and the website of the SEC referred to above. In addition, we
also make our filings with the SEC available on our website at
www.rpsweb.com. Information presented on, or accessible through,
our website is not a part of, and is not incorporated into, this
prospectus. We will also provide copies of our filings with the
SEC free of charge to our stockholders upon request.
98
Index to
Consolidated Financial Statements
| |
|
|
|
|
|
|
|
Page
|
|
|
|
ReSearch Pharmaceutical Services, Inc. and Subsidiaries
|
|
|
|
|
|
|
|
|
F-3
|
|
|
|
|
|
F-4
|
|
|
|
|
|
F-5
|
|
|
|
|
|
F-6
|
|
|
|
|
|
F-7
|
|
|
|
|
|
F-8
|
|
|
ReSearch Pharmaceutical Services, Inc. and Subsidiaries Interim
Financial Statements
|
|
|
|
|
|
|
|
|
F-31
|
|
|
|
|
|
F-32
|
|
|
|
|
|
F-33
|
|
|
|
|
|
F-34
|
|
|
IMEREM Institute for Medical Research Management and
Biometrics—Institute für medizinisches
Forschungsmanagement und Biometrie—Ein unabhaengiges
Forschungsunternehmen GmbH
|
|
|
|
|
|
|
|
|
F-44
|
|
|
|
|
|
F-45
|
|
|
|
|
|
F-46
|
|
|
|
|
|
F-47
|
|
|
|
|
|
F-48
|
|
|
|
|
|
F-49
|
|
|
IMEREM Institute for Medical Research Management and
Biometrics—Institute für medizinisches
Forschungsmanagement und Biometrie—Ein unabhaengiges
Forschungsunternehmen GmbH Interim Financial Statements
|
|
|
|
|
|
|
|
|
F-56
|
|
|
|
|
|
F-57
|
|
|
|
|
|
F-58
|
|
|
|
|
|
F-59
|
|
|
Infociencia S.L. and Infociencia Clinical Research S.L.
|
|
|
|
|
|
|
|
|
F-63
|
|
|
|
|
|
F-64
|
|
|
|
|
|
F-65
|
|
|
|
|
|
F-66
|
|
|
|
|
|
F-67
|
|
|
|
|
|
F-68
|
|
|
Infociencia S.L. and Infociencia Clinical Research S.L. Interim
Financial Statements
|
|
|
|
|
|
|
|
|
F-74
|
|
F-1
| |
|
|
|
|
|
|
|
Page
|
|
|
|
|
|
|
F-75
|
|
|
|
|
|
F-76
|
|
|
|
|
|
F-77
|
|
|
Therapharm Recherches Th. R.
|
|
|
|
|
|
|
|
|
F-80
|
|
|
|
|
|
F-81
|
|
|
|
|
|
F-82
|
|
|
|
|
|
F-83
|
|
|
|
|
|
F-84
|
|
|
|
|
|
F-85
|
|
|
Therapharm Recherches Th. R. Interim Financial Statements
|
|
|
|
|
|
|
|
|
F-90
|
|
|
|
|
|
F-91
|
|
|
|
|
|
F-92
|
|
|
|
|
|
F-93
|
|
|
|
|
|
|
|
|
|
F-2
Report of
Independent Registered Public Accounting Firm
The Board of Directors and Shareholders
Research Pharmaceutical Services, Inc. and Subsidiaries
We have audited the accompanying consolidated balance sheets of
Research Pharmaceutical Services, Inc. and Subsidiaries as of
December 31, 2009 and 2008 and the related consolidated
statements of operations, redeemable convertible preferred stock
and stockholders’ equity, and cash flows for each of the
three years in the period ended December 31, 2009. These
financial statements are the responsibility of the
Company’s management. Our responsibility is to express an
opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the
Public Company Accounting Oversight Board (United States). Those
standards require that we plan and perform the audit to obtain
reasonable assurance about whether the financial statements are
free of material misstatement. We were not engaged to perform an
audit of the Company’s internal control over financial
reporting. Our audits included consideration of internal control
over financial reporting as a basis for designing audit
procedures that are appropriate in the circumstances, but not
for the purpose of expressing an opinion on the effectiveness of
the Company’s internal control over financial reporting.
Accordingly, we express no such opinion. An audit also includes
examining, on a test basis, evidence supporting the amounts and
disclosures in the financial statements, assessing the
accounting principles used and significant estimates made by
management, and evaluating the overall financial statement
presentation. We believe that our audits provide a reasonable
basis for our opinion.
In our opinion, the financial statements referred to above
present fairly, in all material respects, the consolidated
financial position of Research Pharmaceutical Services, Inc. and
Subsidiaries as of December 31, 2009 and 2008, and the
consolidated results of their operations and their cash flows
for each of the three years in the period ended
December 31, 2009, in conformity with U.S. generally
accepted accounting principles.
Philadelphia, Pennsylvania
March 24, 2010
F-3
ReSearch
Pharmaceutical Services, Inc. and Subsidiaries
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
December 31,
|
|
|
|
2009
|
|
2008
|
|
|
|
Assets
|
|
|
|
|
|
|
|
|
|
Current assets:
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents
|
|
$
|
3,468,104
|
|
|
$
|
6,565,003
|
|
|
Restricted cash
|
|
|
5,195,841
|
|
|
|
7,247,532
|
|
|
Accounts receivable, less allowance for doubtful accounts of
$398,000 at December 31, 2009 and $654,000 at
December 31, 2008, respectively
|
|
|
54,516,875
|
|
|
|
43,225,016
|
|
|
Current deferred tax asset
|
|
|
473,940
|
|
|
|
970,797
|
|
|
Prepaid expenses and other current assets
|
|
|
4,795,030
|
|
|
|
2,377,838
|
|
|
|
|
|
|
|
|
|
|
|
|
Total current assets
|
|
|
68,449,790
|
|
|
|
60,386,186
|
|
|
Property and equipment, net
|
|
|
6,404,747
|
|
|
|
5,993,387
|
|
|
Other assets
|
|
|
1,627,453
|
|
|
|
1,179,018
|
|
|
Intangible assets subject to amortization, net
|
|
|
2,792,481
|
|
|
|
3,880,000
|
|
|
Goodwill
|
|
|
16,742,614
|
|
|
|
15,145,585
|
|
|
Deferred tax asset
|
|
|
243,593
|
|
|
|
504,366
|
|
|
|
|
|
|
|
|
|
|
|
|
Total assets
|
|
$
|
96,260,678
|
|
|
$
|
87,088,542
|
|
|
|
|
|
|
|
|
|
|
|
|
Liabilities and stockholders’ equity
|
|
|
|
|
|
|
|
|
|
Current liabilities:
|
|
|
|
|
|
|
|
|
|
Accounts payable
|
|
$
|
3,526,931
|
|
|
$
|
3,496,309
|
|
|
Accrued expenses
|
|
|
14,551,527
|
|
|
|
12,069,957
|
|
|
Customer deposits
|
|
|
9,695,841
|
|
|
|
7,247,532
|
|
|
Deferred revenue
|
|
|
8,910,551
|
|
|
|
4,781,935
|
|
|
Line of credit
|
|
|
9,565,808
|
|
|
|
7,500,000
|
|
|
Current deferred tax liability
|
|
|
44,267
|
|
|
|
—
|
|
|
Current portion of capital lease obligations
|
|
|
553,689
|
|
|
|
682,695
|
|
|
|
|
|
|
|
|
|
|
|
|
Total current liabilities
|
|
|
46,848,614
|
|
|
|
35,778,428
|
|
|
Customer deposits
|
|
|
—
|
|
|
|
4,500,000
|
|
|
Deferred tax liability
|
|
|
345,121
|
|
|
|
1,331,955
|
|
|
Other liabilities
|
|
|
2,510,351
|
|
|
|
2,323,794
|
|
|
Capital lease obligations, less current portion
|
|
|
250,576
|
|
|
|
871,963
|
|
|
|
|
|
|
|
|
|
|
|
|
Total liabilities
|
|
|
49,954,662
|
|
|
|
44,806,140
|
|
|
Stockholders’ equity:
|
|
|
|
|
|
|
|
|
|
Common stock, $.0001 par value:
|
|
|
|
|
|
|
|
|
|
Authorized shares—150,000,000; issued and outstanding
shares—37,277,808 and 36,746,291 at December 31, 2009
and December 31, 2008, respectively.
|
|
|
3,728
|
|
|
|
3,675
|
|
|
Additional paid-in capital
|
|
|
45,601,325
|
|
|
|
44,083,184
|
|
|
Accumulated other comprehensive income
|
|
|
40,507
|
|
|
|
155,535
|
|
|
Retained earnings (accumulated deficit)
|
|
|
660,456
|
|
|
|
(1,959,992
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Total stockholders’ equity
|
|
|
46,306,016
|
|
|
|
42,282,402
|
|
|
|
|
|
|
|
|
|
|
|
|
Total liabilities and stockholders’ equity
|
|
$
|
96,260,678
|
|
|
$
|
87,088,542
|
|
|
|
|
|
|
|
|
|
|
|
|
|
See accompanying notes.
F-4
ReSearch
Pharmaceutical Services, Inc. and Subsidiaries
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended December 31,
|
|
|
|
2009
|
|
2008
|
|
2007
|
|
|
|
Service revenue
|
|
$
|
200,471,816
|
|
|
$
|
156,966,558
|
|
|
$
|
120,459,459
|
|
|
Reimbursement revenue
|
|
|
23,696,162
|
|
|
|
18,085,514
|
|
|
|
13,923,784
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total revenue
|
|
|
224,167,978
|
|
|
|
175,052,072
|
|
|
|
134,383,243
|
|
|
Direct costs
|
|
|
145,208,645
|
|
|
|
117,707,287
|
|
|
|
87,650,346
|
|
|
Reimbursable
out-of-pocket
costs
|
|
|
23,696,162
|
|
|
|
18,085,514
|
|
|
|
13,923,784
|
|
|
Selling, general, and administrative expenses
|
|
|
44,797,903
|
|
|
|
31,289,566
|
|
|
|
26,786,748
|
|
|
Depreciation and amortization
|
|
|
3,722,907
|
|
|
|
1,750,252
|
|
|
|
1,143,734
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Income from operations
|
|
|
6,742,361
|
|
|
|
6,219,453
|
|
|
|
4,878,631
|
|
|
Interest expense
|
|
|
(649,878
|
)
|
|
|
(226,911
|
)
|
|
|
(6,025,467
|
)
|
|
Interest income
|
|
|
539,424
|
|
|
|
293,056
|
|
|
|
238,178
|
|
|
Other income (expense)
|
|
|
(452,138
|
)
|
|
|
(24,435
|
)
|
|
|
1,404
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income (loss) before provision for income taxes
|
|
|
6,179,769
|
|
|
|
6,261,163
|
|
|
|
(907,254
|
)
|
|
Provision for income taxes
|
|
|
3,559,321
|
|
|
|
2,518,379
|
|
|
|
1,508,087
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income (loss)
|
|
|
2,620,448
|
|
|
|
3,742,784
|
|
|
|
(2,415,341
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accretion of preferred stock
|
|
|
—
|
|
|
|
—
|
|
|
|
(320,819
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income (loss) applicable to common shares:
|
|
$
|
2,620,448
|
|
|
$
|
3,742,784
|
|
|
$
|
(2,736,160
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income (loss) per common share:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic
|
|
$
|
0.07
|
|
|
$
|
0.11
|
|
|
$
|
(0.19
|
)
|
|
Diluted
|
|
$
|
0.07
|
|
|
$
|
0.11
|
|
|
$
|
(0.19
|
)
|
|
Weighted average number of common shares outstanding:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic
|
|
|
37,002,773
|
|
|
|
32,616,846
|
|
|
|
14,572,881
|
|
|
Diluted
|
|
|
38,071,113
|
|
|
|
34,103,258
|
|
|
|
14,572,881
|
|
|
|
See accompanying notes.
F-5
Research
Pharmaceutical Services, Inc. and Subsidiaries
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Redeemable Convertible Preferred Stock
|
|
Stockholders’ equity
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accumulated
|
|
Retained
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Additional
|
|
Other
|
|
Earnings/
|
|
|
|
|
|
Series A
|
|
Series B
|
|
Common Stock
|
|
Treasury Shares
|
|
Paid-In
|
|
Comprehensive
|
|
(Accumulated
|
|
|
|
|
|
Shares
|
|
Amount
|
|
Shares
|
|
Amount
|
|
Shares
|
|
Amount
|
|
Shares
|
|
Amount
|
|
Capital
|
|
Income
|
|
Deficit)
|
|
Total
|
|
|
|
Balance at December 31, 2006
|
|
|
7,593,198
|
|
|
$
|
5,489,688
|
|
|
|
1,271,694
|
|
|
$
|
2,512,345
|
|
|
|
12,404,751
|
|
|
$
|
1,240
|
|
|
$
|
6,903,077
|
|
|
$
|
(1,187,650
|
)
|
|
$
|
117,388
|
|
|
$
|
6,297
|
|
|
$
|
(3,287,435
|
)
|
|
$
|
(4,350,160
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accretion of dividends on Series A and Series B
Convertible Preferred Stock
|
|
|
—
|
|
|
|
214,747
|
|
|
|
—
|
|
|
|
106,072
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(320,819
|
)
|
|
|
—
|
|
|
|
—
|
|
|
|
(320,819
|
)
|
|
Stock-based compensation
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
211,817
|
|
|
|
—
|
|
|
|
—
|
|
|
|
211,817
|
|
|
Exercise of common stock options
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
6,324
|
|
|
|
1
|
|
|
|
—
|
|
|
|
—
|
|
|
|
6,747
|
|
|
|
—
|
|
|
|
—
|
|
|
|
6,748
|
|
|
Repurchase of shares from stockholders
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
24,592
|
|
|
|
(172,909
|
)
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(172,909
|
)
|
|
Payment of preferred stock dividends
|
|
|
—
|
|
|
|
(2,039,334
|
)
|
|
|
—
|
|
|
|
(588,000
|
)
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
Conversion of preferred stock and put warrants to common stock
|
|
|
(7,593,198
|
)
|
|
|
(3,665,101
|
)
|
|
|
(1,271,694
|
)
|
|
|
(2,030,417
|
)
|
|
|
10,287,698
|
|
|
|
1,029
|
|
|
|
—
|
|
|
|
—
|
|
|
|
10,906,580
|
|
|
|
—
|
|
|
|
—
|
|
|
|
10,907,609
|
|
|
Retirement of treasury shares
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(6,927,669
|
)
|
|
|
1,360,559
|
|
|
|
(1,360,559
|
)
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
Issuance of common stock and proceeds received in connection
with reverse acquisition of Cross Shore, net of distribution to
stockholders and fees
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
10,250,450
|
|
|
|
1,025
|
|
|
|
—
|
|
|
|
—
|
|
|
|
30,154,871
|
|
|
|
—
|
|
|
|
—
|
|
|
|
30,155,896
|
|
|
Repurchase and retirement of shares from stockholder
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(750,000
|
)
|
|
|
(75
|
)
|
|
|
—
|
|
|
|
—
|
|
|
|
(3,637,425
|
)
|
|
|
—
|
|
|
|
—
|
|
|
|
(3,637,500
|
)
|
|
Comprehensive loss:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(2,415,341
|
)
|
|
|
(2,415,341
|
)
|
|
Other comprehensive income—foreign currency translation
adjustment
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
44,008
|
|
|
|
—
|
|
|
|
44,008
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total comprehensive loss
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(2,371,333
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance at December 31, 2007
|
|
|
—
|
|
|
$
|
—
|
|
|
|
—
|
|
|
$
|
—
|
|
|
|
32,199,223
|
|
|
$
|
3,220
|
|
|
$
|
—
|
|
|
$
|
—
|
|
|
$
|
36,078,600
|
|
|
$
|
50,305
|
|
|
$
|
(5,702,776
|
)
|
|
$
|
30,429,349
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Exercise of common stock options
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
12,183
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
8,952
|
|
|
|
—
|
|
|
|
|
|
|
|
8,952
|
|
|
Issuance of common stock and proceeds received in connection
with reverse acquisition of Cross Shore, net of distribution to
stockholders and fees
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
336,000
|
|
|
|
35
|
|
|
|
—
|
|
|
|
—
|
|
|
|
356,309
|
|
|
|
—
|
|
|
|
—
|
|
|
|
356,344
|
|
|
Issuance of common stock in connection with acquisitions of
Imerem, Infociencia and Therapharm
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
4,198,885
|
|
|
|
420
|
|
|
|
—
|
|
|
|
—
|
|
|
|
7,070,502
|
|
|
|
—
|
|
|
|
—
|
|
|
|
7,070,922
|
|
|
Stock-based compensation
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
568,821
|
|
|
|
—
|
|
|
|
—
|
|
|
|
568,821
|
|
|
Comprehensive income:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
3,742,784
|
|
|
|
3,742,784
|
|
|
Other comprehensive income—foreign currency translation
adjustment
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
105,230
|
|
|
|
—
|
|
|
|
105,230
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total comprehensive income
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
3,848,014
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance at December 31, 2008
|
|
|
—
|
|
|
$
|
—
|
|
|
|
—
|
|
|
$
|
—
|
|
|
|
36,746,291
|
|
|
$
|
3,675
|
|
|
$
|
—
|
|
|
$
|
—
|
|
|
$
|
44,083,184
|
|
|
$
|
155,535
|
|
|
$
|
(1,959,992
|
)
|
|
$
|
42,282,402
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Exercise of common stock options
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
544
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
201
|
|
|
|
—
|
|
|
|
—
|
|
|
|
201
|
|
|
Issuance of common stock in connection with acquisitions of
Paramax
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
530,973
|
|
|
|
53
|
|
|
|
—
|
|
|
|
—
|
|
|
|
920,655
|
|
|
|
—
|
|
|
|
—
|
|
|
|
920,708
|
|
|
Stock-based compensation
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
597,285
|
|
|
|
—
|
|
|
|
—
|
|
|
|
597,285
|
|
|
Comprehensive income:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
2,620,448
|
|
|
|
2,620,448
|
|
|
Other comprehensive loss—foreign currency translation
adjustment
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(115,028
|
)
|
|
|
—
|
|
|
|
(115,028
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total comprehensive income
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
2,505,420
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance at December 31, 2009
|
|
|
—
|
|
|
$
|
—
|
|
|
|
—
|
|
|
$
|
—
|
|
|
|
37,277,808
|
|
|
$
|
3,728
|
|
|
$
|
—
|
|
|
$
|
—
|
|
|
$
|
45,601,325
|
|
|
$
|
40,507
|
|
|
$
|
660,456
|
|
|
$
|
46,306,016
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
See accompanying notes.
F-6
Research
Pharmaceutical Services, Inc. and Subsidiaries
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended December 31,
|
|
|
|
2009
|
|
2008
|
|
2007
|
|
|
|
Operating activities
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income (loss)
|
|
$
|
2,620,448
|
|
|
$
|
3,742,784
|
|
|
$
|
(2,415,341
|
)
|
|
Adjustments to reconcile net income (loss) to net cash (used in)
provided by operating activities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Depreciation and amortization
|
|
|
3,722,907
|
|
|
|
1,750,252
|
|
|
|
1,478,337
|
|
|
Interest charge related to put warrant liability
|
|
|
—
|
|
|
|
—
|
|
|
|
4,723,451
|
|
|
Stock-based compensation
|
|
|
597,285
|
|
|
|
568,821
|
|
|
|
211,817
|
|
|
Deferred tax benefit
|
|
|
(441,982
|
)
|
|
|
(552,680
|
)
|
|
|
(409,460
|
)
|
|
Changes in operating assets and liabilities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accounts receivable
|
|
|
(10,946,527
|
)
|
|
|
(4,896,994
|
)
|
|
|
(10,004,080
|
)
|
|
Prepaid expenses and other assets
|
|
|
(2,340,453
|
)
|
|
|
(371,262
|
)
|
|
|
(686,109
|
)
|
|
Accounts payable
|
|
|
(12,838
|
)
|
|
|
(270,086
|
)
|
|
|
67,861
|
|
|
Accrued expenses and other liabilities
|
|
|
4,106,552
|
|
|
|
(315,104
|
)
|
|
|
2,303,212
|
|
|
Customer deposits
|
|
|
(2,109,179
|
)
|
|
|
(419,544
|
)
|
|
|
4,354,112
|
|
|
Deferred revenue
|
|
|
4,060,238
|
|
|
|
(2,001,238
|
)
|
|
|
1,988,877
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net cash (used in) provided by operating activities
|
|
|
(743,549
|
)
|
|
|
(2,765,051
|
)
|
|
|
1,612,677
|
|
|
Investing activities
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Change in restricted cash
|
|
|
2,109,179
|
|
|
|
419,544
|
|
|
|
145,888
|
|
|
Business combinations, net of cash acquired
|
|
|
(3,044,244
|
)
|
|
|
(7,867,466
|
)
|
|
|
—
|
|
|
Purchase of property and equipment
|
|
|
(2,680,376
|
)
|
|
|
(1,269,245
|
)
|
|
|
(2,198,108
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net cash used in investing activities
|
|
|
(3,615,441
|
)
|
|
|
(8,717,167
|
)
|
|
|
(2,052,220
|
)
|
|
Financing activities
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net borrowings (repayments) on line of credit
|
|
|
2,060,834
|
|
|
|
7,500,000
|
|
|
|
(8,991,544
|
)
|
|
Principal payments on capital lease obligations
|
|
|
(750,393
|
)
|
|
|
(604,550
|
)
|
|
|
(194,355
|
)
|
|
Repurchase of shares from stockholders in connection with
reverse acquisition of Cross Shore
|
|
|
—
|
|
|
|
—
|
|
|
|
(3,810,409
|
)
|
|
Cross Shore merger consideration, net of fees paid
|
|
|
—
|
|
|
|
(22,667
|
)
|
|
|
51,375,660
|
|
|
Distribution to stockholders
|
|
|
—
|
|
|
|
—
|
|
|
|
(20,000,000
|
)
|
|
Payment of preferred stock dividends
|
|
|
—
|
|
|
|
—
|
|
|
|
(2,627,334
|
)
|
|
Proceeds from exercise of options
|
|
|
201
|
|
|
|
8,952
|
|
|
|
6,748
|
|
|
Payment of note payable
|
|
|
—
|
|
|
|
—
|
|
|
|
(4,500,000
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net cash provided by financing activities
|
|
|
1,310,642
|
|
|
|
6,881,735
|
|
|
|
11,258,766
|
|
|
Effect of exchange rates on cash and cash equivalents
|
|
|
(48,551
|
)
|
|
|
105,231
|
|
|
|
44,008
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net change in cash and cash equivalents
|
|
|
(3,096,899
|
)
|
|
|
(4,495,252
|
)
|
|
|
10,863,231
|
|
|
Cash and cash equivalents, beginning of year
|
|
|
6,565,003
|
|
|
|
11,060,255
|
|
|
|
197,024
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents, end of year
|
|
$
|
3,468,104
|
|
|
$
|
6,565,003
|
|
|
$
|
11,060,255
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Supplemental disclosures of cash flow information
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash paid during the year for:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Interest
|
|
$
|
618,571
|
|
|
$
|
251,346
|
|
|
$
|
921,000
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Income taxes
|
|
$
|
5,168,208
|
|
|
$
|
2,924,777
|
|
|
$
|
1,459,000
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Supplemental disclosures of noncash financing activities
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Issuance of shares in connection with business combinations
|
|
$
|
918,583
|
|
|
$
|
7,070,922
|
|
|
$
|
320,819
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accretion of preferred stock dividends
|
|
$
|
—
|
|
|
$
|
—
|
|
|
$
|
320,819
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Acquisition of fixed assets under capital leases
|
|
$
|
—
|
|
|
$
|
1,388,843
|
|
|
$
|
1,123,097
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
See accompanying notes.
F-7
Research
Pharmaceutical Services, Inc. and Subsidiaries
1.
Business
ReSearch Pharmaceutical Services, Inc. and Subsidiaries (the
“Company” or “RPS”) is a next generation CRO
(clinical research organization) serving biotechnology and
pharmaceutical companies, which the Company refers to
collectively as the biopharmaceutical industry. The RPS business
model combines the expertise of a traditional CRO with the
ability to provide flexible outsourcing solutions that are fully
integrated within the Company’s clients’ clinical
infrastructure. The Company is able to leverage its clinical
expertise, industry knowledge and specialization to reduce the
expense and time frame of clinical development that meets the
varied needs of small, medium and large biopharmaceutical
companies. The Company’s revenues are generated principally
from customers located in the United States.
The Company has wholly owned subsidiaries in over 40 countries
around the world with its core operations located in North
America, Latin America, Europe and Asia.
2. Significant
Accounting Policies
2007
Merger and Accounting Treatment
Cross Shore Acquisition Corporation (“Cross Shore”)
was incorporated in Delaware on January 30, 2006 as a blank
check company, the objective of which was to acquire one or more
operating companies engaged in the delivery of business services
to companies and consumers in the United States. On
April 28, 2006, Cross Shore completed an initial public
offering (the “Offering”) on the Alternative
Investment Market (“AIM”) of the London Stock Exchange
and raised proceeds of $112 million before offering
expenses. Of the net proceeds from the Offering,
$102.7 million was placed in trust to be held until the
earlier of (i) consummation of Cross Shore’s first
business combination or (ii) liquidation of Cross Shore.
On August 30, 2007, the Company’s predecessor company
(“Old RPS”) merged with and into a wholly-owned
subsidiary of Cross Shore. As a result of the merger, Old RPS
became a limited liability company organized under the laws of
Delaware under the name ReSearch Pharmaceutical Services, LLC,
and Cross Shore changed its name to ReSearch Pharmaceutical
Services, Inc.. RPS is now a holding company for, and conducts
substantially all of its operations through its wholly-owned
subsidiary, ReSearch Pharmaceutical Services, LLC. Results of
operations prior to the August 30, 2007 merger with Cross
Shore reflect the operations of Old RPS.
The merger was accounted for under the purchase method of
accounting as a reverse acquisition in accordance with
U.S. generally accepted accounting principles for
accounting and financial reporting purposes. Under this method
of accounting, Cross Shore was treated as the
“acquired” company for financial reporting purposes.
Accordingly, for accounting purposes, the merger was treated as
the equivalent of Old RPS issuing stock for the net assets of
Cross Shore which amounted to $50.6 million and consisted
of cash and investments of $51.3 million, other assets of
$0.6 million and $1.3 million of accrued transaction
fees. The purchase price was allocated to the assets acquired
and liabilities assumed based on their fair value at the date of
the merger. Stockholders’ equity has been retroactively
adjusted for all periods prior to the merger to reflect the
number of shares of common stock received by holders of common
stock of Old RPS in connection with the merger based upon the
exchange ratio of approximately 1.4 shares of Cross Shore
common stock for each share of Old RPS common stock as per the
merger agreement. Stockholders’ equity has not been
retroactively adjusted for periods prior to the merger for the
10,250,499 shares of Cross Shore issued to Old RPS holders
of preferred stock and common stock warrants.
The shares of preferred stock, common stock, and common stock
warrants held by Old RPS stockholders prior to the merger were
converted into a total of 15,758,497 shares of Cross Shore
common stock, or 47.34% of the subsequently outstanding common
stock of the combined company. Upon consummation of the merger,
$49.9 million, net of $1.4 million of accrued
transaction fees, was released from trust and became available
to the Company. Of this amount, existing holders of shares of
preferred stock, common stock and common stock
F-8
warrants of Old RPS received a total cash distribution of
$20 million as merger consideration pursuant to the terms
of the merger agreement.
The remaining cash of $29.9 million is available for use by
the Company to fund business operations. Total direct and
incremental fees incurred by the Company in connection with the
merger are reflected as a reduction of additional paid in
capital. The senior management team of Old RPS prior to the
merger continued as senior management of the Company after the
merger, and Old RPS controlled the majority of the Board of
Directors of the Company.
Use of
Estimates
The preparation of financial statements in conformity with
accounting principles generally accepted in the United States
requires management to make estimates and assumptions that
affect the amounts reported in the financial statements and
accompanying notes. Actual results could differ from those
estimates.
Cash and
Cash Equivalents
Cash and cash equivalents include highly liquid investments with
an original maturity of three months or less from date of
purchase.
Restricted
Cash
The Company receives cash in advance from certain customers
specifically for the payment of investigator fees relating to
specific projects. Such amounts are recorded in restricted cash
and short term customer deposits in the accompanying
consolidated balance sheets.
Concentration
of Credit Risk
Financial instruments, which potentially subject the Company to
credit risk, consist principally of cash and accounts
receivable. The Company performs periodic evaluations of the
financial institutions in which its cash is invested. The
majority of the Company’s revenues and accounts receivable
are derived from pharmaceutical companies located in the United
States. The Company’s two largest customers accounted for
approximately 17% and 16%, respectively, of service revenue
during the year ended December 31, 2009, and the
Company’s three largest customers accounted for
approximately 20%, 12% and 12%, respectively, of service revenue
during the year ended December 31, 2008, and the
Company’s largest customer represented approximately 23% of
service revenue during the year ended December 30, 2007.
The two largest customers represented approximately 16% and 17%,
respectively of the accounts receivable balance at
December 31, 2009, while the three largest customers
represented approximately 14%, 13% and 8%, respectively, of the
accounts receivable balance at December 31, 2008. No other
customers represented more than 10% of net service revenue or
accounts receivable during those periods or at those times. The
Company provides an allowance for doubtful accounts based on
experience and specifically identified risks. Accounts
receivable are carried at fair value and charged off against the
allowance for doubtful accounts when management determines that
recovery is unlikely and the Company ceases collection efforts.
The following table summarizes the changes in the Company’s
allowance for doubtful accounts for the periods indicated.
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended December 31,
|
|
|
|
2009
|
|
2008
|
|
2008
|
|
|
|
Balance at the beginning of the period
|
|
$
|
654,000
|
|
|
$
|
547,000
|
|
|
$
|
200,000
|
|
|
Amounts charged to expense
|
|
|
213,000
|
|
|
|
107,000
|
|
|
|
347,000
|
|
|
Accounts written off
|
|
|
(469,000
|
)
|
|
|
—
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance at the end of the period
|
|
$
|
398,000
|
|
|
$
|
654,000
|
|
|
$
|
547,000
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
F-9
Consolidation
The consolidated financial statements include the accounts of
the Company and its wholly owned subsidiaries. All significant
intercompany balances and transactions have been eliminated in
consolidation.
Fair
Value of Financial Instruments
The carrying value of financial instruments including cash,
accounts receivable, accounts payable, and lines of credit
approximates their fair value based on the short-term nature of
these instruments.
Property
and Equipment
Property and equipment are recorded at cost. Expenditures for
repairs and maintenance which do not extend the useful life of
the related assets are charged to expense as incurred.
Depreciation is computed using the straight-line method over the
estimated useful lives of the assets ranging from 1 to
5 years.
Intangible
Assets
Intangible assets consist primarily of noncompete agreements,
customer contracts and lists, brand names, and goodwill mainly
related to the European Acquisitions (Note 3). Finite-lived
intangible assets are amortized on a straight line basis over
the following periods: Customer lists—three and five years,
brand names—two years and non-compete agreements—three
and six years. Goodwill represents the excess of the cost over
the fair value of net assets acquired in a business combination.
The Company accounts for goodwill, noncompete agreements, brand
names and customer lists in accordance with the Financial
Accounting Standards Board (“FASB”) guidance for
intangible assets. Goodwill is tested for impairment on an
annual basis (as of October 1 of each year) and more frequently
if an event occurs or circumstances change that would more
likely than not reduce the fair value of the Company below its
carrying value. If the fair value of the Company is less than
the carrying value, goodwill may be impaired, and will be
written down to its estimated fair market value, if necessary.
Revenue
and Cost Recognition
The majority of the Company’s service revenue is derived
from
fee-for-service
contracts, some of which are fixed price contracts. Revenues and
the related costs of
fee-for-service
contracts are recognized in the period in which services are
performed. Fixed price contract revenue is calculated on a
proportional performance basis based on the ratio that costs
incurred to date bear on the estimated total costs at
completion. The Company also recognizes revenue under
units-based contracts by multiplying units completed by the
applicable contract
per-units
price. Revenue related to contract modifications is recognized
when realization is assured and the amounts are reasonably
determinable. Adjustments to contract estimates are made in the
periods in which the facts that require the revisions become
known. When the revised estimate indicates a loss, such loss is
provided for in the financial statements during that period.
Deferred revenue represents amounts billed to customers in
excess of revenues recognized.
FASB guidance requires reimbursable
out-of-pocket
expenses to be characterized as revenue in the statements of
operations. Reimbursements for
out-of-pocket
expenses included in total revenue in the Company’s
consolidated statements of operations were $23,696,162,
$18,085,514 and $13,923,784 for the years ended
December 31, 2009, 2008 and 2007 respectively.
The Company excludes investigator fees from its
out-of-pocket
expenses because these fees are funded from the customer’s
restricted cash and are recorded on a “pass-through
basis” without risk or reward to the Company. Investigator
fees paid on behalf of clients for the years ended
December 31, 2009, 2008 and 2007 were approximately
$7,019,000, $4,708,000 and $6,666,000 respectively.
F-10
Income
Taxes
The Company accounts for income taxes using an asset and
liability approach which requires the recognition of deferred
tax assets and liabilities for the expected future tax
consequences of temporary differences between the carrying
amount of assets and liabilities for financial reporting
purposes and amounts reportable for income tax purposes. On
January 1, 2007, the Company adopted the FASB guidance
related to accounting for uncertainty in income taxes. This
guidance creates a single model to address uncertainty in tax
positions and clarifies the accounting for income taxes by
prescribing the minimum recognition threshold a tax position is
required to meet before it is recognized in the financial
statements.
Foreign
Currency Translation
The financial statements of the Company’s foreign
subsidiaries have been translated into U.S. dollars in
accordance with the FASB guidance on foreign currency
translation. All balance sheet accounts have been translated
using the exchange rates in effect at the balance sheet dates.
Income statement amounts have been translated using average
exchange rates in effect for the relevant periods. The gains and
losses resulting from the changes in exchange rates during the
year have been reported separately in other comprehensive income
in the consolidated financial statements.
Stock-Based
Compensation
FASB guidance requires all share-based payments to employees,
including grants of employee stock options, to be recognized in
the financial statements based on their fair values. This
guidance requires that an entity measure the cost of
equity-based service awards based on the grant-date fair value
of the award and recognize the cost of such award over the
period during which the employee is required to provide service
in exchange for the award (vesting period).
The per-share weighted average fair value of the options granted
during the years ended December 31, 2009, 2008 and 2007 was
estimated at $0.87, $1.96 and $1.70 on the date of grant,
respectively, using the Black-Scholes option-pricing model with
the following weighted average assumptions which are based upon
the Company’s history or industry comparative information:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended December 31,
|
|
|
|
2009
|
|
2008
|
|
2007
|
|
|
|
Expected dividend yield
|
|
|
0.00
|
%
|
|
|
0.00
|
%
|
|
|
0.00
|
%
|
|
Expected volatility
|
|
|
50
|
%
|
|
|
50
|
%
|
|
|
52
|
%
|
|
Risk-free interest rate
|
|
|
2.19
|
%
|
|
|
3.01
|
%
|
|
|
3.34
|
%
|
|
Expected life
|
|
|
6 years
|
|
|
|
6 years
|
|
|
|
6 years
|
|
Prior to August 30, 2007, the Company’s common stock
was not publicly traded, and the expected volatility was
calculated for each date of grant based on an alternative method
(defined as “calculated value”). Subsequent to
August 30, 2007, as a public company listed on AIM, the
Company continued to utilize the calculated value for expected
volatility as a sufficient level of history was not available as
a publicly traded company. In September and October 2009, the
Company delisted its common stock and warrants from AIM,
respectively, and its common stock and warrants are no longer
publicly traded. As such, the Company will continue to use the
calculated value. The Company identified similar public entities
for which share price information is available and has
considered the historical volatility of these entities’
share prices in determining its estimated expected volatility.
The Company used the average volatility of these guideline
companies over a six-year period, consistent with the expected
term of the options. From August 30, 2007 through the
September 2009 AIM delisting date, the Company utilized the
quoted stock price on the AIM as a determinant of fair value of
the Company’s common stock. Subsequent to the AIM delisting
date, the Company estimates the fair value of its common stock
using the market and income valuation approaches. Stock based
compensation expense for the years ended December 31, 2009,
2008 and 2007 related to share-based service awards was
$597,000, $569,000 and $212,000, respectively and is included in
selling, general, and administrative expenses in the
accompanying consolidated statements of operations. The
F-11
Company recognizes the compensation expense of such share-based
service awards on a straight-line basis. Total compensation cost
of options granted but not yet vested as of December 31,
2009 was $506,000 net of estimated forfeitures, which is
expected to be recognized over the weighted average period of
2.2 years.
Segment
Information
Operating segments are identified as components of an enterprise
about which separate financial information is available for
evaluation by the chief operating decision-maker, or
decision-making group, in making decisions on how to allocate
resources and assess performance. The Company views its
operations and manages its business as one operating segment.
The Company’s foreign operations accounted for
approximately 17%, 6% and 5% of service revenue during the year
ended December 31, 2009, 2008 and 2007 respectively. As a
result of the European Acquisitions and the Paramax Acquisition
(see Note 3) the Company expects the percentage of
service revenue resulting from foreign operations to increase
going forward. In addition, approximately 35%, 34% and 3% of the
Company’s consolidated tangible assets are located in
foreign locations at December 31, 2009, 2008 and 2007,
respectively.
Service revenue from external customers and long-lived assets
for each significant geographic location for the years ended
December 31, 2009, 2008 and 2007 are as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Americas
|
|
Europe
|
|
Asia-Pacific
|
|
Total
|
|
|
|
Service revenue from customers(1)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2009
|
|
$
|
179,134,695
|
|
|
$
|
20,485,290
|
|
|
$
|
851,832
|
|
|
$
|
200,471,817
|
|
|
2008
|
|
|
156,966,558
|
|
|
|
—
|
|
|
|
—
|
|
|
|
156,966,558
|
|
|
2007
|
|
|
120,459,459
|
|
|
|
—
|
|
|
|
—
|
|
|
|
120,459,459
|
|
|
Long-lived assets(2)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2009
|
|
|
4,228,432
|
|
|
|
1,982,932
|
|
|
|
193,383
|
|
|
|
6,404,747
|
|
|
2008
|
|
|
4,251,207
|
|
|
|
1,742,179
|
|
|
|
—
|
|
|
|
5,993,386
|
|
|
2007
|
|
|
3,343,371
|
|
|
|
—
|
|
|
|
—
|
|
|
|
3,343,371
|
|
|
|
|
|
(1) |
|
Service revenue is attributable to geographic locations based on
the physical location where the services are performed. |
| |
|
(2) |
|
Long-lived assets represents the net book value of property and
equipment. |
Recent
Accounting Pronouncements
The Company adopted new accounting guidance on fair value
measurements effective January 1, 2008, for financial
assets and liabilities. In addition, effective January 1,
2009, the Company adopted this guidance as it relates to
nonfinancial assets and liabilities that are not recognized or
disclosed at fair value in the financial statements on at least
an annual basis. This guidance defines fair value as the price
that would be received to sell an asset or paid to transfer a
liability, referred to as the exit price, in an orderly
transaction between market participants at the measurement date.
The standard outlines a valuation framework and creates a fair
value hierarchy in order to increase the consistency and
comparability of fair value measurements and the related
disclosures. In determining fair value of financial assets, the
Company primarily uses prices and other relevant information
generated by market transactions involving identical or
comparable assets, called the market approach. As of
December 31, 2009 and December 31, 2008, the fair
value of all of the Company’s financial assets are based on
level one observable inputs. The implementation of this guidance
for nonfinancial assets and liabilities did not have an impact
on the Company’s consolidated financial statements as of
December 31, 2009. The provisions of this guidance will be
applied at such time a fair value measurement of a nonfinancial
asset or liability is required, which may result in a fair value
that is materially different than would have been calculated
prior to the adoption of this guidance.
F-12
In December 2007, the FASB issued new guidance related to
business combinations. This guidance retains the fundamental
requirements of existing guidance that the acquisition method of
accounting be used for all business combinations and for an
acquirer to be identified for each business combination. This
guidance defines the acquirer as the entity that obtains control
of one or more businesses in the business combination and
establishes the acquisition date as the date the acquirer
achieves control. This guidance was effective for the Company
beginning January 1, 2009 and the impact of the adoption of
this guidance depends upon the nature and terms of business
combinations that the Company consummates on or after
January 1, 2009.
In June 2008, the FASB issued new guidance related to assessing
whether an equity-linked financial instrument (or embedded
feature) is indexed to an entity’s own stock for the
purposes of determining whether such equity-linked financial
instrument (or embedded feature) is subject to derivative
accounting The Company adopted this new guidance effective
January 1, 2009. The adoption of this guidance did not have
a material impact on the Company’s consolidated financial
statements.
In May 2009, the FASB issued new guidance on subsequent events.
The standard provides guidance on management’s assessment
of subsequent events and incorporates this guidance into
accounting literature. The standard is effective prospectively
for interim and annual periods ending after June 15, 2009
and the Company adopted this guidance commencing with its
June 30, 2009 consolidated financial statements. The
implementation of this standard did not have a material impact
on the Company’s consolidated financial position and
results of operations.
In April 2009, the FASB issued a staff position requiring fair
value disclosures in both interim as well as annual financial
statements in order to provide more timely information about the
effects of current market conditions on financial instruments.
The guidance is effective for interim and annual periods ending
after June 15, 2009, and the Company adopted this guidance
commencing with its June 30, 2009 consolidated financial
statements. The implementation of this standard did not have a
material impact on the Company’s consolidated financial
statements.
In June 2009, FASB Accounting Standards Codification
(Codification) was issued, effective for financials statements
issued for interim and annual periods ending after
September 15, 2009. The Codification supersedes literature
of the FASB, Emerging Issues Task Force and other sources. The
Codification did not change U.S. generally accepted
accounting principles. The implementation of this standard did
not have a material impact on the Company’s consolidated
financial statements.
Net
Income (Loss) Per Share
Basic net income (loss) per share is computed by dividing net
income (loss) applicable to common stock by the weighted average
number of shares of common stock outstanding during the periods
presented. Diluted net income (loss) per share is computed by
dividing net income (loss) applicable to common stock by the
weighted average number of shares of common stock outstanding
during the periods plus the dilution that would occur upon the
exercise or conversion of stock options or common stock warrants.
The following table is a reconciliation of the numerator and
denominator of the computation of basic and diluted net income
(loss) per share.
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended December 31,
|
|
|
|
2009
|
|
2008
|
|
2007
|
|
|
|
Net income (loss) applicable to common stock
|
|
$
|
2,620,448
|
|
|
$
|
3,742,784
|
|
|
$
|
(2,736,160
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted average common shares outstanding—basic
|
|
|
37,002,773
|
|
|
|
32,616,846
|
|
|
|
14,572,881
|
|
|
Dilutive effect of stock options and warrants
|
|
|
1,068,340
|
|
|
|
1,486,412
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted average common shares outstanding—diluted
|
|
|
38,071,113
|
|
|
|
34,103,258
|
|
|
|
14,572,881
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Options to purchase 968,911 and 967,304 shares,
respectively, of the Company’s common stock were excluded
from the computation of diluted weighted average shares
outstanding for the years ended December 31, 2009 and
F-13
2008 because their effect would have been anti-dilutive. Since
the Company reported a net loss applicable to common shares for
the year ended December 31, 2007, all of the outstanding
stock options, warrants and shares of preferred stock were
excluded from the calculation of diluted weighted average shares
outstanding as their effect would have been anti-dilutive. The
outstanding stock options and warrants could potentially dilute
earnings per share in the future.
Reclassification
Certain prior year amounts in the consolidated financial
statements and notes thereto have been reclassified to conform
to the current year’s presentation.
3.
Acquisitions
2009
Acquisition
Paramax
International Inc. (“Paramax”)
On July 7, 2009, a subsidiary of RPS acquired the
outstanding shares of Paramax for consideration of
$1.0 million in cash and 530,973 shares of RPS common
stock (the “Paramax Shares”) issued to Paramax’s
sole shareholder (the “Paramax Acquisition”). Paramax,
which is active in the same fields as RPS, provides the Company
with opportunities in the Asia-Pacific market and complements
its ongoing operations in the Americas and Europe. In addition,
the acquisition provides RPS with greater scale to meet the
growing needs of its customers in the rapidly expanding market
for globally integrated clinical research services. The Paramax
Shares were valued by management utilizing the assistance of a
valuation specialist at $1.73 per share, which resulted in total
acquisition consideration of approximately $1.9 million.
The shareholder of Paramax has entered into a share escrow
agreement whereby all of the Paramax Shares are held in escrow,
to be released in equal portions on October 7, 2009,
July 7, 2010 and January 31, 2011, subject to there
being no indemnity claims outstanding (as defined within the
acquisition agreement). As of December 31, 2009, there were
no indemnity claims outstanding. In addition, the shareholder of
Paramax has agreed to a 24 month
lock-up on
all Paramax Shares commencing on the date of closing of the
Paramax Acquisition. Paramax, founded in 2007, is located in
Beijing, China. Paramax operates throughout China and the
Asia-Pacific market, providing clinical research services to the
biopharmaceutical industry.
The acquisition has been accounted for as a purchase.
Accordingly, the results of operations of Paramax have been
included in the consolidated financial statements commencing
July 7, 2009. A preliminary allocation of the purchase
price is outlined below:
| |
|
|
|
|
|
Purchase Price:
|
|
|
|
|
|
Cash paid
|
|
$
|
1,000,000
|
|
|
Value of RPS Shares
|
|
|
918,583
|
|
|
|
|
|
|
|
|
Total purchase price
|
|
$
|
1,918,583
|
|
|
|
|
|
|
|
F-14
| |
|
|
|
|
|
Allocation of Purchase Price:
|
|
|
|
|
|
Cash
|
|
$
|
163,692
|
|
|
Accounts receivable
|
|
|
87,367
|
|
|
Property and equipment
|
|
|
31,780
|
|
|
Other assets
|
|
|
9,130
|
|
|
Goodwill
|
|
|
1,504,355
|
|
|
Customer lists
|
|
|
18,000
|
|
|
Non-compete agreement
|
|
|
117,000
|
|
|
Current liabilities
|
|
|
(12,741
|
)
|
|
|
|
|
|
|
|
|
|
$
|
1,918,583
|
|
|
|
|
|
|
|
The allocation of the purchase price to the estimated fair
values of the assets acquired and liabilities assumed as
reflected in the consolidated financial statements is
preliminary and subject to change based on finalization of the
Company’s valuation of the assets acquired and liabilities
assumed. The Company is currently assessing the fair value of
the identifiable tangible and intangible assets acquired and
liabilities assumed. It is expected that the assets and
liabilities assumed will approximate the values assigned as of
the date of the acquisition. A valuation study is presently
being conducted to establish the fair market value of the
identifiable intangibles acquired. The intangible assets
acquired consist primarily of customer lists and a non-compete
agreement. The final purchase price allocation to reflect the
fair values of the assets acquired and liabilities assumed will
be based on the outcome of the Company’s valuation study.
The final valuation will be completed in 2010.
2008
Acquisitions
In December 2008, the Company completed the acquisitions of
three European companies located in Spain, France and Germany
(“the European Acquisitions”). The European
Acquisitions, which involved companies that are active in the
same fields as RPS, provide the Company with opportunities in
the European market and complement its ongoing operations in the
Americas. In addition, the European Acquisitions provide RPS
with greater scale to meet the growing needs of its customers in
the rapidly expanding market for globally integrated clinical
research services.
IMEREM Institute for Medical Research Management and
Biometrics—Institut für medizinisches
Forschungsmanagement und Biometrie—Ein unabhaengiges
Forschungsunternehmen GmbH (“Imerem”)
On December 22, 2008, a subsidiary of RPS acquired the
outstanding shares of Imerem for a consideration of
€2.7 million ($3.9 million) in cash and issuance
of 1,296,165 shares of RPS common stock (the “Imerem
Shares”) issued to Imerem’s sole shareholder. The
Imerem Shares were valued at $1.68 per share, which, along with
transaction costs of approximately $1.0 million that were
paid by the Company, resulted in total acquisition consideration
of approximately $7.1 million. The sole shareholder of
Imerem has entered into a share escrow agreement whereby
50 percent of the Imerem Shares are held in escrow, to be
released in equal portions on the first, second and third
anniversaries of the acquisition date, subject to there being no
indemnity claims outstanding (as defined within the acquisition
agreement). As of December 31, 2009 there were no indemnity
claims outstanding. In addition, the shareholder of Imerem has
agreed to a 12 month
lock-up on
all Imerem Shares commencing on the date of closing of the
acquisition. Imerem, founded in 1990, is located in
Nürnberg, Germany. Imerem operates throughout Eastern and
Western Europe and Scandinavia, providing clinical research
services to the biopharmaceutical industry and academic
institutions.
F-15
The acquisition has been accounted for as a purchase.
Accordingly, the results of operations of Imerem have been
included in the consolidated financial statements commencing
December 22, 2008. The allocation of the purchase price is
outlined below:
| |
|
|
|
|
|
Purchase Price:
|
|
|
|
|
|
Cash paid
|
|
$
|
3,924,089
|
|
|
Value of RPS Shares
|
|
|
2,182,742
|
|
|
Transaction costs
|
|
|
1,041,325
|
|
|
|
|
|
|
|
|
Total purchase price
|
|
$
|
7,148,156
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
Allocation of Purchase Price:
|
|
|
|
|
|
Cash
|
|
$
|
1,499,696
|
|
|
Restricted cash
|
|
|
1,079,203
|
|
|
Accounts receivable
|
|
|
886,369
|
|
|
Prepaid expense and other current assets
|
|
|
68,708
|
|
|
Property and equipment
|
|
|
101,179
|
|
|
Goodwill
|
|
|
4,519,008
|
|
|
Customer lists
|
|
|
800,000
|
|
|
Brand name
|
|
|
330,000
|
|
|
Non-compete agreements
|
|
|
350,000
|
|
|
Accrued expenses
|
|
|
(378,583
|
)
|
|
Customer deposits
|
|
|
(1,079,203
|
)
|
|
Accounts payable
|
|
|
(562,465
|
)
|
|
Deferred tax liability
|
|
|
(465,756
|
)
|
|
|
|
|
|
|
|
|
|
$
|
7,148,156
|
|
|
|
|
|
|
|
Infociencia,
S.L. and Infociencia Clinical Research S.L.
(“Infociencia”)
On December 22, 2008, a subsidiary of RPS acquired the
outstanding shares of Infociencia for consideration of
€2.5 million ($3.6 million) in cash and issuance
of 1,404,856 shares of RPS common stock (the
“Infociencia Shares”) to Infociencia’s
shareholders. The Infociencia Shares were valued at $1.68 per
share which, along with transaction costs of approximately
$1.0 million that were paid by the Company, resulted in
total acquisition consideration of $7.0 million. The
shareholders of Infociencia entered into share escrow agreements
whereby 50 percent of the Infociencia Shares are held in
escrow, to be released in equal portions on the first, second
and third anniversaries of the acquisition date, subject to
there being no indemnity claims outstanding (as defined within
the acquisition agreement). As of December 31, 2009 there
were no indemnity claims outstanding. In addition, the
shareholders of Infociencia have agreed to a 12 month
lock-up on
all of the Infociencia Shares commencing on the date of closing
of the acquisition. Infociencia founded in 1998, has offices in
Barcelona and Madrid, Spain and operates throughout Western
Europe providing clinical research services to the
biopharmaceutical industry and academic and government
institutions.
F-16
The acquisition has been accounted for as a purchase.
Accordingly, the results of operations of Infociencia have been
included in the consolidated financial statements commencing
December 22, 2008. The allocation of the purchase price is
outlined below:
| |
|
|
|
|
|
Purchase Price:
|
|
|
|
|
|
Cash paid
|
|
$
|
3,563,536
|
|
|
Value of RPS Shares
|
|
|
2,365,778
|
|
|
Transaction costs
|
|
|
1,034,929
|
|
|
|
|
|
|
|
|
Total purchase price
|
|
$
|
6,964,243
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
Allocation of Purchase Price:
|
|
|
|
|
|
Cash
|
|
$
|
446,939
|
|
|
Restricted cash
|
|
|
4,702,100
|
|
|
Accounts receivable
|
|
|
3,612,585
|
|
|
Prepaid expense and other current assets
|
|
|
493,413
|
|
|
Property and equipment
|
|
|
1,496,736
|
|
|
Goodwill
|
|
|
4,872,958
|
|
|
Customer lists
|
|
|
280,000
|
|
|
Brand name
|
|
|
640,000
|
|
|
Non-compete agreements
|
|
|
550,000
|
|
|
Other liabilities
|
|
|
(1,141,933
|
)
|
|
Customer deposits
|
|
|
(4,702,100
|
)
|
|
Accounts payable
|
|
|
(876,983
|
)
|
|
Accrued expenses
|
|
|
(2,863,472
|
)
|
|
Deferred tax liability
|
|
|
(546,000
|
)
|
|
|
|
|
|
|
|
|
|
$
|
6,964,243
|
|
|
|
|
|
|
|
Therapharm
Recherches Th.R. (“Therapharm”)
On December 23, 2008, a subsidiary of RPS acquired the
outstanding shares of Therapharm for consideration of
€2.6 million ($3.8 million) in cash and issuance
of 1,497,864 shares of RPS common stock (the
“Therapharm Shares,” and along with the Paramax
Shares, Imerem Shares and the Infociencia Shares, the
“Shares”) to Therapharm’s shareholder. The
Therapharm Shares were valued at $1.68 per share which, along
with transaction costs of approximately $1.1 million that
were paid by the Company, resulted in total acquisition
consideration of $7.4 million. The shareholder of
Therapharm entered into a share escrow agreement whereby
50 percent of the Therapharm Shares are held in escrow, to
be released in equal portions on the first, second and third
anniversaries of the acquisition date, subject to there being no
indemnity claims outstanding (as defined within the acquisition
agreement). In the fourth quarter of 2009, the Company made one
working capital adjustment pursuant to the acquisition agreement
that decreased the purchase price by $104,913 and resulted in
the release of 62,300 Therapharm Shares from the escrow to the
Company, which the Company subsequently cancelled. In addition,
the shareholder of Therapharm has agreed to a 12 month
lock-up on
all of the Therapharm Shares commencing on the date of closing
of the acquisition.
Therapharm, founded in 1980, is located in Boulogne Billancourt,
France. Therapharm provides clinical research services to the
biopharmaceutical industry and operates throughout Western
Europe, focusing its efforts on France, Belgium and Switzerland.
F-17
The acquisition has been accounted for as a purchase.
Accordingly, the results of operations of Therapharm have been
included in the consolidated financial statements commencing
December 23, 2008. The allocation of the purchase price is
outlined below:
| |
|
|
|
|
|
Purchase Price:
|
|
|
|
|
|
Cash paid
|
|
$
|
3,799,459
|
|
|
Value of RPS Shares
|
|
|
2,417,490
|
|
|
Transaction costs
|
|
|
1,103,445
|
|
|
|
|
|
|
|
|
Total purchase price
|
|
$
|
7,320,394
|
|
|
|
|
|
|
|
Allocation of Purchase Price:
| |
|
|
|
|
|
Cash
|
|
$
|
2,356,206
|
|
|
Restricted cash
|
|
|
563,896
|
|
|
Accounts receivable
|
|
|
3,430,837
|
|
|
Prepaid expense and other current assets
|
|
|
632,878
|
|
|
Property and equipment
|
|
|
144,265
|
|
|
Goodwill
|
|
|
5,838,873
|
|
|
Customer lists
|
|
|
280,000
|
|
|
Brand name
|
|
|
440,000
|
|
|
Non-compete agreements
|
|
|
210,000
|
|
|
Customer deposits
|
|
|
(563,896
|
)
|
|
Accounts payable
|
|
|
(884,066
|
)
|
|
Accrued expenses
|
|
|
(3,097,466
|
)
|
|
Deferred revenue
|
|
|
(1,710,934
|
)
|
|
Deferred tax liability
|
|
|
(320,199
|
)
|
|
|
|
|
|
|
|
|
|
$
|
7,320,394
|
|
|
|
|
|
|
|
The unaudited pro forma information below presents combined
results of operations as if the Paramax Acquisition and the
European Acquisitions had occurred as of the beginning of the
applicable reporting periods instead of in July 2009 and
December 2008, respectively. The pro forma information is based
on historical results and is not necessarily indicative of the
results of operations of the combined entity had the acquisition
occurred at the beginning of the periods presented, nor is it
necessarily indicative of future results.
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended December 31,
|
|
|
|
2009
|
|
2008
|
|
2007
|
|
|
|
(unaudited)
|
|
|
|
Service revenue
|
|
$
|
200,682,702
|
|
|
$
|
181,247,167
|
|
|
$
|
142,380,066
|
|
|
Reimbursement revenue
|
|
|
23,696,162
|
|
|
|
25,856,370
|
|
|
|
27,044,389
|
|
|
Total revenue
|
|
|
224,378,864
|
|
|
|
207,103,537
|
|
|
|
169,424,455
|
|
|
Net income
|
|
|
2,317,141
|
|
|
|
6,719,188
|
|
|
|
540,583
|
|
|
Net income per common share:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic
|
|
$
|
0.06
|
|
|
$
|
0.18
|
|
|
$
|
0.03
|
|
|
Diluted
|
|
$
|
0.06
|
|
|
$
|
0.17
|
|
|
$
|
0.03
|
|
|
Weighted average number of common shares outstanding:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic
|
|
|
37,002,773
|
|
|
|
37,346,704
|
|
|
|
18,771,766
|
|
|
Diluted
|
|
|
38,071,113
|
|
|
|
38,833,116
|
|
|
|
20,415,640
|
|
F-18
The Shares issued in connection with the consummation of the
Paramax Acquisition and the European Acquisitions were valued
utilizing the assistance of an independent third party valuation
specialist, which resulted in fair values of $1.73 and $1.68 per
share, respectively.
4. Intangible
Assets
The following table summarizes the changes in the carrying
amount of the Company’s goodwill for the years ended
December 31, 2009 and 2008, respectively:
| |
|
|
|
|
|
Balance as of December 31, 2007
|
|
$
|
275,536
|
|
|
Goodwill as a result of European Acquisitions
|
|
|
14,870,049
|
|
|
|
|
|
|
|
|
Balance as of December 31, 2008
|
|
|
15,145,585
|
|
|
Purchase accounting adjustments
|
|
|
360,790
|
|
|
Goodwill acquired (Paramax)
|
|
|
1,504,355
|
|
|
Currency exchange
|
|
|
(268,116
|
)
|
|
|
|
|
|
|
|
Balance as of December 31, 2009
|
|
$
|
16,742,614
|
|
|
|
|
|
|
|
None of the goodwill acquired in connection with the European
Acquisitions in December 2008 or with the Paramax Acquisition in
July 2009 is expected to be tax deductible.
The following tables summarize intangible assets and their
amortization as of:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
December 31, 2009
|
|
Intangible assets subject to amortization:
|
|
Gross
|
|
Accumulated Amortization
|
|
Net
|
|
|
|
Customer contracts and lists
|
|
$
|
3,291,114
|
|
|
$
|
(2,242,057
|
)
|
|
$
|
1,049,057
|
|
|
Brand name
|
|
|
1,403,059
|
|
|
|
(696,360
|
)
|
|
|
706,699
|
|
|
Non-compete agreements
|
|
|
1,569,459
|
|
|
|
(532,734
|
)
|
|
|
1,036,725
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total
|
|
$
|
6,263,632
|
|
|
$
|
(3,471,151
|
)
|
|
$
|
2,792,481
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
December 31, 2008
|
|
Intangible assets subject to amortization:
|
|
Gross
|
|
Accumulated Amortization
|
|
Net
|
|
|
|
Customer contracts and lists
|
|
$
|
3,280,128
|
|
|
$
|
(1,920,128
|
)
|
|
$
|
1,360,000
|
|
|
Brand name
|
|
|
1,410,000
|
|
|
|
—
|
|
|
|
1,410,000
|
|
|
Non-compete agreements
|
|
|
1,460,000
|
|
|
|
(350,000
|
)
|
|
|
1,110,000
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total
|
|
$
|
6,150,128
|
|
|
$
|
(2,270,128
|
)
|
|
$
|
3,880,000
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The estimated amortization expense for each of the five years
ending December 31, 2014 is as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year ending December 31,
|
|
2010
|
|
2011
|
|
2012
|
|
2013
|
|
2014
|
|
|
|
$
|
1,185,000
|
|
|
$
|
500,000
|
|
|
$
|
477,000
|
|
|
$
|
450,000
|
|
|
$
|
180,000
|
|
F-19
5. Property and
Equipment
Property and equipment consist of the following:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
December 31,
|
|
|
|
Useful life
|
|
2009
|
|
2008
|
|
|
|
Computers, software and other equipment
|
|
2 to 3 years
|
|
$
|
5,052,139
|
|
|
$
|
4,951,507
|
|
|
Automobiles
|
|
1 to 3 years
|
|
|
1,615,000
|
|
|
|
2,163,123
|
|
|
Leasehold improvements
|
|
7 years
|
|
|
379,645
|
|
|
|
314,882
|
|
|
Software
|
|
2 to 3 years
|
|
|
498,683
|
|
|
|
350,000
|
|
|
Furniture and fixtures
|
|
5 years
|
|
|
2,980,807
|
|
|
|
2,000,212
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10,523,274
|
|
|
|
9,779,724
|
|
|
Less accumulated depreciation
|
|
|
|
|
(4,121,527
|
)
|
|
|
(3,786,337
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$
|
6,404,747
|
|
|
$
|
5,993,387
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Automobiles, computers, software and other equipment include
assets acquired under capital lease obligations
(Note 14).Total depreciation expense was approximately
$2,434,000, $1,750,000 and $1,144,000 for the years ended
December 31, 2009, 2008 and 2007, respectively.
6. Accrued
Expenses
Accrued expenses consist of the following:
| |
|
|
|
|
|
|
|
|
|
|
|
December 31,
|
|
|
|
2009
|
|
2008
|
|
|
|
Accrued compensation
|
|
$
|
5,824,601
|
|
|
$
|
4,280,576
|
|
|
Accrued professional fees
|
|
|
1,953,424
|
|
|
|
1,755,192
|
|
|
Volume rebate accrual
|
|
|
1,507,603
|
|
|
|
1,049,534
|
|
|
Accrued taxes
|
|
|
1,975,566
|
|
|
|
1,583,950
|
|
|
Accrued transaction costs
|
|
|
—
|
|
|
|
1,573,752
|
|
|
Other
|
|
|
3,290,333
|
|
|
|
1,826,953
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$
|
14,551,527
|
|
|
$
|
12,069,957
|
|
|
|
|
|
|
|
|
|
|
|
7. Lines of
Credit
In November 2006, the Company entered into a bank line of credit
agreement (the “Credit Agreement”), expiring
October 31, 2009. The Credit Agreement provided for
$15,000,000 of available borrowings, and was subject to certain
borrowing base restrictions. Borrowings under the Credit
Agreement required interest at the Federal Funds open rate, as
defined, plus 1%. The Credit Agreement was secured by all
corporate assets and also contained financial and nonfinancial
covenants, including restrictions on the payment of dividends,
restrictions on acquisitions and restrictions on the repurchase,
redemption, or retirement of outstanding equity. At
December 31, 2008, there were $7.5 million in
outstanding borrowings under this line of credit.
In July 2009, the Credit Agreement was amended (the
“Amended Credit Agreement”) to extend the termination
date to October 31, 2012. The Amended Credit Agreement
provides for an increase to $30,000,000 of available borrowings,
and is subject to certain borrowing base restrictions.
Borrowings under the Amended Credit Agreement require interest
at the Federal Funds open rate, as defined, plus 2% (4.75% at
December 31, 2009). The Amended Credit Agreement remains
secured by all corporate assets and continues the financial and
nonfinancial covenants, including restrictions on the payment of
dividends, restrictions on acquisitions and
F-20
restrictions on the repurchase, redemption, or retirement of
outstanding equity present under the Credit Agreement. At
December 31, 2009, there was $9.4 million in
outstanding borrowings under this line of credit.
In addition to the line of credit for the Company’s
U.S.-based operations, the Company maintains various local lines
of credit for its operations based around the world. At
December 31, 2009, there was $0.2 million in
outstanding borrowings under these lines of credit.
8. Note Payable
and Other Long-Term Liabilities
On December 29, 2003, Old RPS raised $4,500,000 in the form
of a senior subordinated note payable (the “Senior
Subordinated Note”). The note required the payment of
interest at 12% per annum, due and payable in arrears monthly.
No principal payments were due on the note until maturity on
December 31, 2008. Interest expense on the note amounted to
$405,000 for the year ended December 31, 2007.
In connection with the original issuance of the note payable,
the lenders received warrants (the “2003 Warrants”) to
purchase 956,839 shares of Old RPS’ common stock at
$.007 per share. Such warrants were set to expire in 2013. In
addition, in connection with the execution and delivery of an
amendment in March 2005, the lenders received additional
warrants (the “2005 Warrants”) to purchase 35,141
shares of Old RPS’ common stock at $.007 per share.
The 2003 Warrants and 2005 Warrants contained put features which
enabled the holder to require Old RPS to redeem the warrants for
cash at any time subsequent to the fifth anniversary of the
issuance date, subject to certain exceptions. The redemption
price was equal to the greater of the estimated fair value of
common stock as determined by a formula, or the estimated fair
value of common stock as determined by an independent appraisal.
The fair value of the 2003 Warrants was determined to be
$442,465 upon issuance, and such amount was recorded as debt
discount and put warrant liability in 2003. The fair value of
the 2005 Warrants was determined to be $16,250 upon issuance,
and was recorded as put warrant liability in 2005. The debt
discount was amortized to interest expense through December
2008. Changes in the estimated value of the put warrant
liability were recorded as charges to interest expense during
the period of the change.
In 2007, the Company recorded a charge of approximately
$4.7 million related to the increase in the estimated fair
value of the put warrants. Such amount was included in interest
expense in the consolidated statement of operations.
In connection with the merger with Cross Shore on
August 30, 2007 (Note 2), all of the outstanding 2003
Warrants and 2005 Warrants were exchanged for a combination of
cash and shares of RPS common stock.
In addition, the Company repaid the Senior Subordinated Note and
the remaining accrued interest thereon upon the closing of the
merger with Cross Shore.
In connection with the acquisition of Infociencia, the Company
assumed outstanding grant loans from government institutions in
Spain. These loans have a two-year grace period for payments.
The aggregate amount of remaining payments required on all
long-term grant debt at December 31, 2009 was $1,339,224
and is included with other liabilities on the consolidated
balance sheet. Certain amounts of these loans may be forgiven
should the Company submit qualifying expenses pursuant to the
terms of the loan agreements.
9. Retirement
Plan
The Company maintains a defined contribution retirement plan
under Section 401(k) of the Internal Revenue Code (the
“Plan”), which covers all eligible employees as
defined in the Plan. Employees who are at least 21 years of
age and have completed three months of service are eligible for
the Plan. Under the Plan, participating employees may defer up
to 15% of their pretax salary but not more than statutory
limits. Employee contributions vest immediately. The Company
accrued and paid out a discretionary match of $180,678 and
$119,322 on employee contributions for the years ended
December 31, 2008 and 2007, respectively. The Company did
not match any employee contributions for the year ended
December 31, 2009.
F-21
10. Income
Taxes
Net income (loss) before provision for income taxes consists of
the following components:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended December 31,
|
|
|
|
2009
|
|
2008
|
|
2007
|
|
|
|
Domestic
|
|
$
|
10,654,095
|
|
|
$
|
5,915,363
|
|
|
$
|
(822,162
|
)
|
|
Foreign
|
|
|
(4,474,326
|
)
|
|
|
345,800
|
|
|
|
(85,092
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total net income (loss) before provision for income taxes
|
|
$
|
6,179,769
|
|
|
$
|
6,261,163
|
|
|
$
|
(907,254
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The provision for income taxes is as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended December 31,
|
|
|
|
2009
|
|
2008
|
|
2007
|
|
Current:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Federal
|
|
$
|
3,096,672
|
|
|
$
|
2,426,985
|
|
|
$
|
1,654,022
|
|
|
State
|
|
|
647,631
|
|
|
|
535,165
|
|
|
|
258,708
|
|
|
Foreign
|
|
|
257,000
|
|
|
|
108,909
|
|
|
|
4,818
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
4,001,303
|
|
|
|
3,071,059
|
|
|
|
1,917,548
|
|
|
Deferred:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Federal
|
|
|
424,826
|
|
|
|
(395,480
|
)
|
|
|
(316,730
|
)
|
|
State
|
|
|
64,490
|
|
|
|
(105,866
|
)
|
|
|
(77,630
|
)
|
|
Foreign
|
|
|
(931,298
|
)
|
|
|
(51,334
|
)
|
|
|
(15,101
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(441,982
|
)
|
|
|
(552,680
|
)
|
|
|
(409,461
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total
|
|
$
|
3,559,321
|
|
|
$
|
2,518,379
|
|
|
$
|
1,508,087
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Deferred income taxes reflect the net tax effects of temporary
differences between the carrying amounts of assets and
liabilities for financial reporting purposes and the amounts
used for income tax purposes. Significant components of the
Company’s deferred tax assets and liabilities are as
follows:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended December 31,
|
|
|
|
2009
|
|
2008
|
|
2007
|
|
|
|
Deferred tax assets:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net operating loss carryforwards and tax credits
|
|
$
|
1,361,623
|
|
|
$
|
318,867
|
|
|
$
|
—
|
|
|
Property, plant and equipment
|
|
|
—
|
|
|
|
130,796
|
|
|
|
41,037
|
|
|
Start-up
costs
|
|
|
230,620
|
|
|
|
241,654
|
|
|
|
267,620
|
|
|
Stock-based compensation
|
|
|
426,731
|
|
|
|
195,745
|
|
|
|
57,188
|
|
|
Allowance for bad debts
|
|
|
351,014
|
|
|
|
257,181
|
|
|
|
221,999
|
|
|
Other reserves
|
|
|
212,895
|
|
|
|
330,920
|
|
|
|
121,862
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total deferred tax assets
|
|
|
2,582,883
|
|
|
|
1,475,163
|
|
|
|
709,706
|
|
|
Deferred tax liabilities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Property, plant and equipment
|
|
|
(448,491
|
)
|
|
|
—
|
|
|
|
—
|
|
|
Intangibles
|
|
|
(928,943
|
)
|
|
|
(1,331,955
|
)
|
|
|
—
|
|
|
Other
|
|
|
(105,790
|
)
|
|
|
—
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total deferred tax liabilities
|
|
|
(1,483,224
|
)
|
|
|
(1,331,955
|
)
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Valuation allowance for deferred tax assets
|
|
|
(771,514
|
)
|
|
|
—
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net deferred tax assets
|
|
$
|
328,145
|
|
|
$
|
143,208
|
|
|
$
|
709,706
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
F-22
In December 2008, the Company completed the European
Acquisitions and acquired amortizable intangibles for which the
Company recorded a deferred tax liability.
A reconciliation of income taxes computed at the
U.S. federal statutory rate to the provision for income
taxes is as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended December 31,
|
|
|
|
2009
|
|
2008
|
|
2007
|
|
|
|
Federal statutory income tax expense (benefit)
|
|
$
|
2,101,122
|
|
|
$
|
2,128,795
|
|
|
$
|
(317,540
|
)
|
|
State taxes, net of federal benefit
|
|
|
469,999
|
|
|
|
358,059
|
|
|
|
224,558
|
|
|
Impact of foreign taxes
|
|
|
95,530
|
|
|
|
(70,032
|
)
|
|
|
36,847
|
|
|
Increase (decrease) in valuation allowance
|
|
|
771,514
|
|
|
|
—
|
|
|
|
(156,979
|
)
|
|
Other permanent differences
|
|
|
121,156
|
|
|
|
101,557
|
|
|
|
1,721,201
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Provision for income taxes
|
|
$
|
3,559,321
|
|
|
$
|
2,518,379
|
|
|
$
|
1,508,087
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The effective tax rate for 2007 was significantly higher than
the federal statutory rate primarily as a result of the
significant interest charge for the put warrants discussed in
Note 8, for which the Company did not receive a tax
deduction. The tax effected amount of the interest charge for
the put warrants was included in other permanent differences in
the rate reconciliation for the year ended December 31,
2007. The effective tax rate for 2009 is higher than the federal
statutory rate, as the Company is not recording a tax benefit
for net operating losses generated in certain of its foreign
subsidiaries as it may not realize the tax benefit of these net
operating losses.
The Company has foreign net operating loss carryforwards
generated mostly among European affiliates at December 31,
2009 aggregating $3.8 million, which expire through 2019.
Effective January 1, 2007, the Company adopted FASB
ASC 740-10-50,
formerly known as Financial Accounting Standards Board
Interpretation No. 48, Accounting for Uncertainty in Income
Taxes, an interpretation of FASB ASC 740, formerly known as
FASB Statement No. 109 (the Interpretation). This
Interpretation requires that the company recognizes, in its
financial statements, the impact of a tax position taken, or
expected to be taken, in tax returns if that position is more
likely than not of being sustained on audit, based on the
technical merits of the position. Under FASB
ASC 740-10-50,
tax positions are evaluated for recognition using a
more-likely-than-not threshold, and those tax positions
requiring recognition are measured as the largest amount of tax
benefit that is greater than 50 percent likely of being
realized upon ultimate settlement with a taxing authority that
has full knowledge of all relevant information.
The Company records accrued interest and penalties related to
unrecognized tax benefits in the income tax provision. There
have been no material changes to unrecognized tax benefits or
accrued interest and penalties during 2007, 2008 or 2009. The
Company does not expect a significant increase or decrease in
unrecognized tax benefits over the next twelve months.
The Company files U.S. federal income tax returns as well
as income tax returns in various states and for foreign
jurisdictions. The Company may be subject to examination by the
various taxing authorities generally for calendar years 2005
through 2009. Additionally, any net operating losses and other
tax attribute carryovers that were generated in prior years and
utilized in these years may also be subject to examination. The
Company cannot predict with certainty how theses audits will be
resolved and whether the Company will be required to make
additional tax payments, which may or may not include penalties
and interest. The Company is currently under audit by the
Internal Revenue Service for the tax year of 2007. For most
states where the Company conducts business, the Company is
subject to examination for the preceding three to six years. In
certain states, the period could be longer.
Management believes the Company has provided sufficient tax
provisions for tax periods that are within the statutory period
of limitation not previously audited and that are potentially
open for examination by the taxing authorities. Potential
liabilities associated with these years will be resolved when an
event occurs to warrant closure,
F-23
primarily through the completion of audits by the taxing
jurisdictions. To the extent audits or other events result in a
material adjustment to the accrued estimates, the effect would
be recognized during the period of the event. There can be no
assurance, however, that the ultimate outcome of audits will not
have a material adverse impact on the Company’s financial
position, results of operations or cash flows.
11.
Stockholders’ Equity
Prior to
the Merger with Cross Shore
Old RPS was authorized to issue up to 25,301,475 shares of
common stock with no par value. Of the shares authorized,
2,108,456 shares of common stock were reserved for issuance
pursuant to Old RPS’ 2002 Equity Incentive Plan
(Note 13).
Old RPS issued warrants to purchase 393,579 shares of
common stock to certain investors in 2003 in connection with a
bridge loan (Note 8). The warrants were exercisable at
$0.4695 per share at any time through 2013. In connection with
the merger, the warrants were exchanged for a combination of
cash and RPS common shares.
Subsequent
to the Merger with Cross Shore
Subsequent to the merger with Cross Shore on August 30,
2007, the Company is authorized to issue up to
1,000,000 shares of preferred stock, $0.0001 par
value, and 150,000,000 shares of common stock,
$0.0001 par value. Of the shares authorized,
6,792,271 shares of common stock have been reserved for
issuance pursuant to the Company’s 2007 equity incentive
plan (Note 13).
The Company’s stockholders have been granted certain rights
to register their shares under the securities laws of the United
States pursuant to three separate registration rights
agreements. The Registration Rights Agreement (as defined below)
pertains to those holding shares in Old RPS prior to the merger
with Cross Shore. The Investor Rights Agreement (as defined
below) pertains to those acquiring shares and warrants in Cross
Shore’s initial public offering in April of 2006. The
Founders’ Shares Agreement (as defined below) pertains
to those acquiring shares prior to Cross Shore’s initial
public offering in April 2006.
The Company entered into the Investor Rights Agreement, dated
April 24, 2006 (the “Investor Rights Agreement”),
in connection with the initial public offering of Cross
Shore’s common stock.
Under the Investor Rights Agreement, the Company is required to
file a shelf registration statement on
Form S-3
within 90 days after becoming eligible to do so. In
addition, the holders of the Company’s common stock that
are party to the Investor Rights Agreement are entitled to no
more than three demand registrations, covering in each case a
minimum of 15% of the shares then outstanding, and piggyback
registration rights. If the Company files a shelf registration
statement for the resale of shares, demand and piggyback
registration rights will be suspended except for underwritten
offerings. Registration rights are generally available only for
stock that is subject to restrictions on transfer under the
U.S. securities laws.
The Company entered into a Registration Rights Agreement, dated
August 30, 2007 (the “Registration Rights
Agreement”), with the holders of shares of common stock of
Old RPS who were issued shares of the Company’s common
stock in the merger between Cross Shore and Old RPS.
Under the Registration Rights Agreement, the Company granted the
stockholders that previously held shares in Old RPS the rights
to include shares on any registration statement the Company
files pursuant to the Securities Act in connection with a public
offering of stock, whether such offering is being made for the
Company’s own account or for the account of stockholders
other than the stockholders that previously held shares in Old
RPS. These registration rights are applicable to any
registration of stock that is made pursuant to a demand from the
existing stockholders pursuant to the Investor Rights Agreement.
The number of shares that the existing stockholders may include
in an underwritten public offering by exercising their
registration rights under the Registration Rights Agreement is
subject to reduction in the event the managing underwriters of
such offering advise us that the number of shares to be included
in such offering exceeds the amount of stock that can be sold
without adversely affecting the offering.
F-24
The Registration Rights Agreement also provides similar shelf
registration rights as are set forth in the Investor Rights
Agreement described above.
The Company entered into a Registration Rights Agreement, dated
April 24, 2006 (the “Founders’
Shares Agreement”), with the founders of Cross Shore
who acquired shares of common stock prior to Cross Shore’s
initial public offering.
Under the Founders’ Shares Agreement, the Company is
required to file a shelf registration statement on
Form S-3
upon request of the stockholders who are parties to the
Founders’ Shares Agreement, as long as the holders
propose to sell registrable securities and such other
securities, if any, at an aggregate price to the public of at
least $2,000,000. In addition, the stockholders who are parties
to the Founders’ Shares Agreement are entitled to no
more than two demand registrations, covering in each case as
many shares as the requesting stockholders propose to sell,
subject to certain restrictions imposed by an underwriter, and
piggyback registration rights. If the Company files a shelf
registration statement for the resale of shares, demand and
piggyback registration rights will be suspended except for
underwritten offerings. Registration rights are generally
available only for stock that is subject to restrictions on
transfer under the U.S. securities laws.
The Company is required to bear all expenses incident to its
compliance with the terms of the Registration Rights Agreement,
the Investor Rights Agreement and the Founders’
Shares Agreement. The Registration Rights Agreement and
Founders’ Shares Agreement also contain customary
indemnification obligations from the Company to the applicable
stockholders with respect to untrue statements or material
omissions in any registration statement that includes the
applicable shares.
If the Company does not effect a registration required under the
Investor Rights Agreement or the Registration Rights Agreement,
the stockholders who are party to those agreements may be
entitled to receive liquidated damages in the form of additional
shares in an amount per month equal to 1% of all or a portion of
such holder’s registrable securities for up to two months,
in the case of the Registration Rights Agreement, or four
months, in the case of the Investor Rights Agreement
Subsequent to the date of the merger with Cross Shore, the
Company also had a total of 1,357,179 common stock warrants (the
“IPO Warrants”) outstanding. The IPO Warrants were
immediately exercisable at any time through April 28, 2010
at $5.00 per. The IPO Warrants were issued to investors in
connection with the initial public offering of Cross Shore in
April 2006 (Note 2) and were delisted from AIM on
October 5, 2009.
The IPO Warrants were redeemable at the Company’s option at
a price of $.0001 per warrant only in the event that the last
sale price of the Company’s common stock is at least $8.50
per share for any 20 trading days within a 30 trading day period
ending on the third day prior to the date on which notice of
redemption is given and the weekly trading volume of the
Company’s common shares has been at least
550,000 shares for each of the two calendar weeks before
the Company sends the notice of redemption.
In addition, a total of 186,667 options were outstanding from
the date of the Cross Shore initial public offering in April
2006 (Note 2). These options (the “Underwriter
Purchase Options”) were issued to representatives of the
underwriters of the Cross Shore initial public offering. The
options entitled the holder to one share of common stock and two
common stock warrants in exchange for an exercise price of $6.60
per share. Should the options be exercised, the warrants
received will be fully vested with exercise prices of $5.00 per
share at any time through April 28, 2010. Such warrants are
subject to the same provisions as the IPO Warrants discussed
above.
In January 2008, the Company issued 336,000 shares of
common stock to certain investors pursuant to the provisions of
Underwriter Purchase Options that were tendered by such
investors in connection with the merger with Cross Shore.
In December 2008, the Company issued a total of
4,198,885 shares of common stock in connection with the
European Acquisitions. The shareholders of Therapharm,
Infociencia and Imerem have entered into share escrow agreements
whereby 50 percent of the shares are held in escrow, to be
released in equal portions on the first, second and third
anniversaries of the acquisition date, subject to there being no
claims outstanding against each corporation being acquired (as
defined within the respective acquisition agreements). As of
December 31, 2009, the Company made one working capital
adjustment pursuant to the acquisition agreement with
Therapharm, resulting in the release of 62,300 shares,
which the Company has subsequently cancelled. In addition, the
F-25
shareholders of Therapharm, Infociencia and Imerem have agreed
to a 12 month
lock-up on
all of the Shares, all of which commenced on the closing date of
the respective acquisitions and had expired as of
December 31, 2009.
In July 2009, the Company issued a total of 530,973 shares
of common stock in connection with the Paramax Acquisition. The
shareholder of Paramax has entered into a share escrow agreement
whereby the Paramax Shares are being held in escrow, to be
released in equal portions on the three and twelve month
anniversaries of the acquisition date and on January 31,
2011, subject to there being no claims outstanding against
Paramax (as defined within the acquisition agreement). As of
December 31, 2009 there were no indemnity claims
outstanding. In addition, the shareholder of Paramax has agreed
to a 24 month
lock-up on
all of the Paramax Shares, which commenced on the closing date
of the acquisition.
12. Redeemable
Convertible Preferred Stock
Prior to
the Merger with Cross Shore
Old RPS authorized the issuance of up to 7,593,198 shares
of Series A 8% Convertible Preferred Stock (the
“Series A Preferred Stock”) and
1,279,130 shares of Series B 8% Convertible
Preferred Stock (the “Series B Preferred Stock”
and collectively with the Series A Preferred Stock, the
“Preferred Stock”). The rights and preferences of the
Preferred Stock were as follows:
Dividends
The holders of shares of Preferred Stock were entitled to
receive an annual cash dividend at a rate of 8% (or $0.0424 per
share of Series A Preferred Stock and $0.1273 per share of
Series B Preferred Stock). Such dividends were cumulative
and were payable, whether or not declared by the board of
directors of Old RPS, upon conversion, redemption, liquidation,
or disposition of the preferred shares subject to full payment
of the Senior Subordinated Note (Note 8). Old RPS recorded
Preferred Stock accretion for the preferred dividends in the
amount of $320,819 in 2007 through the August 30, 2007
merger with Cross Shore.
Liquidation
In the event of a liquidation of Old RPS, the holders of
Preferred Stock were entitled to receive the accrued but unpaid
dividends to the date of liquidation plus an amount equal to the
greater of $0.5301 per outstanding share for the Series A
Preferred Stock and $1.5914 per outstanding share for the
Series B Preferred Stock or such additional amount as would
have been received if the holders of the Preferred Stock
converted their securities into common stock immediately prior
to liquidation and participated in the liquidation on a pro rata
basis in relation to the stock held by the common stockholders.
Redemption
At any time on or after the fifth anniversary of the
Series B Preferred Stock issuance date (December 2008), Old
RPS would, upon written notice of holders of not less than a
majority of the then-outstanding shares of Preferred Stock,
redeem all or a portion of the outstanding shares at a price
equal to $0.5301 per share for the Series A Preferred Stock
and $1.5914 per share for the Series B Preferred Stock plus
all accrued but unpaid preferred dividends through the
redemption date.
Conversion
Each share of Preferred Stock was convertible at the election of
the holder into such number of shares of common stock as
determined by dividing $0.5301 for the Series A Preferred
Stock and $1.5914 for the Series B Preferred Stock by the
applicable conversion price in effect at the time of conversion.
Upon conversion, the holders of Preferred Stock were entitled to
receive, in cash, an amount equal to all unpaid dividends
accreted through the date of conversion.
Old RPS was required to reserve, out of its authorized but
unissued common stock, the full number of shares of common stock
deliverable upon the conversion of the outstanding shares of the
Preferred Stock. The conversion
F-26
price was subject to adjustment in the event that Old RPS issued
additional stock at a price per share that is less than the
Preferred Stock conversion price in effect immediately prior to
the issuance of such stock. In such an event, the Preferred
Stock conversion price would be reduced to an amount equal to
such lower purchase price or $0.0071 if there was no
consideration. The conversion price was also subject to
adjustment for events of dilution including, but not limited to,
stock dividends and stock splits. Shares of the Preferred Stock
would automatically be converted into shares of common stock, at
the then-effective conversion rate, immediately prior to the
closing of an underwritten public offering of common stock with
gross proceeds of at least $20 million and an offering
price equal to at least 300% of the Series A Preferred
Stock conversion price and 100% of the Series B Preferred
Stock conversion price then in effect.
Voting
Rights
The holders of the Preferred Stock were entitled to elect two
directors to the board of directors of Old RPS and vote on all
matters on which holders of common stock were entitled to vote,
casting such number of votes equal to the number of shares of
common stock into which the Preferred Stock was then
convertible. In addition, Old RPS would not, without the
approval of the holders of the Preferred Stock (i) amend
the articles of incorporation in a manner adverse to the rights
of the preferred stockholders, (ii) authorize any class or
series of capital stock ranking senior to the Preferred Stock,
(iii) increase the number of authorized shares of Preferred
Stock, (iv) change the rights of the Preferred Stock,
(v) repurchase or declare a dividend on any shares of
common stock other than as provided in agreements in existence
on the Preferred Stock issuance date, or (vi) authorize a
merger or consolidation of Old RPS.
Subsequent
to the Merger with Cross Shore
Subsequent to the merger with Cross Shore on August 30,
2007, all of the outstanding shares of Preferred Stock were
converted into shares of common stock of the Company. In
addition, all accumulated dividends of the Preferred Stock
accrued through the date of the merger, totaling
$2.63 million, were paid to the investors.
13. Stock Option
Plan
In June 2002, Old RPS adopted the 2002 Equity Incentive Plan
(the “2002 Plan”) which permitted the granting of
incentive stock options, nonqualified stock options and
restricted stock. The 2002 Plan authorized the issuance of up to
2,108,456 shares of common stock to satisfy grants under
the 2002 Plan. Stock options issued generally vested over a
three-year period. The exercise period was determined by Old
RPS’ board of directors, but could not exceed ten years
from the date of grant. Each option entitled the holder to
purchase one share of common stock at the indicated exercise
price.
In connection with the merger with Cross Shore, the Company
adopted the 2007 Equity Incentive Plan (the “2007 Incentive
Plan”) on August 30, 2007 and terminated the 2002
Plan. The 2007 Incentive Plan permits awards of options and
restricted stock. At December 31, 2009, the total number of
shares reserved under the 2007 Incentive Plan was
6,792,271 shares. On an annual basis, this amount would be
automatically adjusted to increase to an amount equal to 15% of
the number of shares outstanding should that number of shares
exceed the amount of shares reserved under the 2007 Incentive
Plan (calculated on a fully diluted basis). Stock options issued
generally vest over a three year period. The exercise period is
determined by the Board of Directors, but may not exceed
10 years from the date of grant.
F-27
The following table summarizes activity under the 2002 Plan and
2007 Incentive Plan:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Options
|
|
Number of
|
|
Weighted
|
|
|
|
Available For
|
|
Options
|
|
Average Exercise
|
|
|
|
Grant
|
|
Outstanding
|
|
Price
|
|
|
|
Balance, December 31, 2006
|
|
|
693,069
|
|
|
|
2,022,594
|
|
|
$
|
0.75
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Authorized
|
|
|
4,076,608
|
|
|
|
—
|
|
|
|
—
|
|
|
Granted
|
|
|
(775,107
|
)
|
|
|
775,107
|
|
|
|
4.98
|
|
|
Exercised
|
|
|
—
|
|
|
|
(52,773
|
)
|
|
|
0.58
|
|
|
Forfeited/cancelled
|
|
|
(14,963
|
)
|
|
|
(14,963
|
)
|
|
|
0.91
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance, December 31, 2007
|
|
|
3,979,607
|
|
|
|
2,729,965
|
|
|
|
1.94
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Authorized
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
Granted
|
|
|
(217,304
|
)
|
|
|
217,304
|
|
|
|
3.86
|
|
|
Exercised
|
|
|
—
|
|
|
|
(11,639
|
)
|
|
|
0.77
|
|
|
Forfeited/cancelled
|
|
|
15,181
|
|
|
|
(15,181
|
)
|
|
|
1.04
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance, December 31, 2008
|
|
|
3,777,484
|
|
|
|
2,920,449
|
|
|
|
2.09
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Authorized
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
Granted
|
|
|
(45,155
|
)
|
|
|
45,155
|
|
|
|
1.75
|
|
|
Exercised
|
|
|
—
|
|
|
|
(544
|
)
|
|
|
0.37
|
|
|
Forfeited/cancelled
|
|
|
49,648
|
|
|
|
(49,648
|
)
|
|
|
3.44
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance, December 31, 2009
|
|
|
3,781,977
|
|
|
|
2,915,412
|
|
|
|
2.06
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The weighted average grant date fair value of options granted
was $0.87, $1.96 and $1.70 per share during the years ended
December 31, 2009, 2008 and 2007, respectively. The total
intrinsic value of options exercised during the years ended
December 31, 2009, 2008 and 2007 was $952, $38,000 and
$177,000 respectively.
At December 31, 2009, options to purchase
2,595,485 shares were exercisable at a weighted average
exercise price of $1.79 per share. The weighted average
remaining contractual life of the outstanding options at
December 31, 2009 was 6.0 years. The weighted average
remaining contractual life of the fully vested options at
December 31, 2009 was 6.0 years. The aggregate
intrinsic value of options outstanding and fully vested at
December 31, 2009 was $1.9 million.
14. Commitments
and Contingencies
The Company occupies its corporate headquarters and other
offices and uses certain equipment under various leases. The
Company’s current lease for its corporate headquarters
expires in June 2017. Rent expense under such arrangements,
including for rent obligations around the world, was
approximately $3,334,000, $1,987,000 and $1,578,000 during the
years ended December 31, 2009, 2008 and 2007, respectively.
The Company is the lessee of approximately $1,615,000 of
automobiles and equipment under capital leases expiring through
2012. The equipment is recorded at the present value of minimum
lease payments and is amortized over its estimated useful life.
Amortization of the assets under capital lease agreements of
approximately $531,000, $650,000 and $180,000 is included in
depreciation expense for the years ended December 31, 2009,
2008 and 2007 respectively.
F-28
Future minimum lease payments subsequent to December 31,
2009 under capital and non-cancelable operating leases are as
follows:
| |
|
|
|
|
|
|
|
|
|
|
|
Capital
|
|
Operating
|
|
|
|
Leases
|
|
Leases
|
|
|
|
2010
|
|
$
|
601,683
|
|
|
$
|
3,634,646
|
|
|
2011
|
|
|
255,602
|
|
|
|
3,153,276
|
|
|
2012
|
|
|
4,590
|
|
|
|
3,015,000
|
|
|
2013
|
|
|
—
|
|
|
|
2,656,260
|
|
|
2014
|
|
|
—
|
|
|
|
2,126,370
|
|
|
Thereafter
|
|
|
—
|
|
|
|
2,836,814
|
|
|
|
|
|
|
|
|
|
|
|
|
Total minimum lease payments
|
|
|
861,875
|
|
|
|
17,422,366
|
|
|
Less amount representing interest
|
|
|
(57,610
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Present value of net minimum lease payments
|
|
$
|
804,265
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The Company is involved in various claims incidental to the
conduct of its business. Management does not believe that any
such claims to which the Company is a party, both individually
and in the aggregate, will have a material adverse effect on the
Company’s financial position or results of operations.
15. Related Party
Transactions
In November 2007, the Company entered into a consulting
agreement with a shareholder to assist the Company in
identifying potential acquisition candidates. The consulting
agreement expired in December 2007 and required payment to the
shareholder totaling $600,000 for such services. Such amount was
recognized as selling, general and administrative expense during
2007 and was included in accrued expenses at December 31,
2007. The amount was paid in the first quarter of 2008.
The Company is the lessee of office space for its German
subsidiary, RPS ReSearch Germany GmbH. The lessor of the office
space is a shareholder and an employee of the Company and former
shareholder of Imerem. The Company pays rent for the facility in
the amount of $14,500 on a
month-to-month
basis.
F-29
16. Interim
Consolidated Financial Information (unaudited)
The following table sets forth certain unaudited quarterly
consolidated financial data for each quarter in the
Company’s two last completed fiscal years. In the opinion
of the Company’s management, this unaudited information has
been prepared on the same basis as the audited consolidated
financial statements contained herein and includes all
adjustments, consisting only of normal recurring adjustments,
necessary to present fairly the information set forth therein
when read in conjunction with the consolidated financial
statements and notes thereto. The operating results for any
quarter are not necessarily indicative of results for any future
period.
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Three Months Ended
|
|
|
|
March 31, 2009
|
|
June 30, 2009
|
|
September 30, 2009
|
|
December 31, 2009
|
|
|
|
Service revenue
|
|
$
|
45,258,874
|
|
|
$
|
48,446,362
|
|
|
$
|
51,669,235
|
|
|
$
|
55,097,345
|
|
|
Reimbursement revenue
|
|
|
5,034,975
|
|
|
|
5,905,352
|
|
|
|
5,588,148
|
|
|
|
7,167,687
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total revenue
|
|
|
50,293,849
|
|
|
|
54,351,714
|
|
|
|
57,257,383
|
|
|
|
62,265,032
|
|
|
Direct costs
|
|
|
33,219,359
|
|
|
|
34,940,337
|
|
|
|
37,167,441
|
|
|
|
39,881,508
|
|
|
Reimbursable
out-of-pocket
costs
|
|
|
5,034,975
|
|
|
|
5,905,352
|
|
|
|
5,588,148
|
|
|
|
7,167,687
|
|
|
Selling, general and administrative expenses
|
|
|
10,045,270
|
|
|
|
11,045,742
|
|
|
|
11,279,779
|
|
|
|
12,427,112
|
|
|
Depreciation and amortization
|
|
|
796,422
|
|
|
|
874,207
|
|
|
|
884,177
|
|
|
|
1,168,101
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Income from operations
|
|
|
1,197,823
|
|
|
|
1,586,076
|
|
|
|
2,337,838
|
|
|
|
1,620,624
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income
|
|
$
|
502,952
|
|
|
$
|
485,777
|
|
|
$
|
994,033
|
|
|
$
|
637,686
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income per share:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic
|
|
$
|
0.01
|
|
|
$
|
0.01
|
|
|
$
|
0.03
|
|
|
$
|
0.02
|
|
|
Diluted
|
|
$
|
0.01
|
|
|
$
|
0.01
|
|
|
$
|
0.03
|
|
|
$
|
0.02
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Three Months Ended
|
|
|
|
March 31, 2008
|
|
June 30, 2008
|
|
September 30, 2008
|
|
December 31, 2008
|
|
|
|
Service revenue
|
|
$
|
38,047,853
|
|
|
$
|
40,286,342
|
|
|
$
|
39,113,267
|
|
|
$
|
39,519,096
|
|
|
Reimbursement revenue
|
|
|
3,794,541
|
|
|
|
4,554,955
|
|
|
|
4,900,378
|
|
|
|
4,835,640
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total revenue
|
|
|
41,842,394
|
|
|
|
44,841,297
|
|
|
|
44,013,645
|
|
|
|
44,354,736
|
|
|
Direct costs
|
|
|
28,316,024
|
|
|
|
30,076,813
|
|
|
|
29,555,433
|
|
|
|
29,759,017
|
|
|
Reimbursable
out-of-pocket
costs
|
|
|
3,794,541
|
|
|
|
4,554,955
|
|
|
|
4,900,378
|
|
|
|
4,835,640
|
|
|
Selling, general and administrative expenses
|
|
|
7,120,510
|
|
|
|
7,759,741
|
|
|
|
7,845,537
|
|
|
|
8,563,778
|
|
|
Depreciation and amortization
|
|
|
365,295
|
|
|
|
418,969
|
|
|
|
449,187
|
|
|
|
516,801
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Income from operations
|
|
$
|
2,246,024
|
|
|
$
|
2,030,819
|
|
|
$
|
1,263,110
|
|
|
$
|
679,500
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income
|
|
$
|
1,323,049
|
|
|
$
|
1,153,084
|
|
|
$
|
748,239
|
|
|
$
|
518,412
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income per share:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic
|
|
$
|
0.04
|
|
|
$
|
0.04
|
|
|
$
|
0.02
|
|
|
$
|
0.02
|
|
|
Diluted
|
|
$
|
0.04
|
|
|
$
|
0.03
|
|
|
$
|
0.02
|
|
|
$
|
0.02
|
|
F-30
ReSearch
Pharmaceutical Services, Inc. and Subsidiaries
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
June 30,
|
|
December 31,
|
|
|
|
2010
|
|
2009
|
|
|
|
(unaudited)
|
|
|
|
|
|
Assets
|
|
|
|
|
|
|
|
|
|
Current assets:
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents
|
|
$
|
4,252,240
|
|
|
$
|
3,468,104
|
|
|
Restricted cash
|
|
|
5,161,878
|
|
|
|
5,195,841
|
|
|
Accounts receivable, less allowance for doubtful accounts of
$535,000 at June 30, 2010 and $398,000 at December 31,
2009, respectively
|
|
|
56,747,607
|
|
|
|
54,516,875
|
|
|
Current deferred tax asset
|
|
|
500,900
|
|
|
|
473,940
|
|
|
Prepaid expenses and other current assets
|
|
|
4,442,707
|
|
|
|
4,795,030
|
|
|
|
|
|
|
|
|
|
|
|
|
Total current assets
|
|
|
71,105,332
|
|
|
|
68,449,790
|
|
|
Property and equipment, net
|
|
|
5,608,563
|
|
|
|
6,404,747
|
|
|
Other assets
|
|
|
1,564,898
|
|
|
|
1,627,453
|
|
|
Intangible assets subject to amortization, net
|
|
|
1,867,216
|
|
|
|
2,792,481
|
|
|
Goodwill
|
|
|
14,199,770
|
|
|
|
16,742,614
|
|
|
Deferred tax asset
|
|
|
212,332
|
|
|
|
243,593
|
|
|
|
|
|
|
|
|
|
|
|
|
Total assets
|
|
$
|
94,558,111
|
|
|
$
|
96,260,678
|
|
|
|
|
|
|
|
|
|
|
|
|
Liabilities and stockholders’ equity
|
|
|
|
|
|
|
|
|
|
Current liabilities:
|
|
|
|
|
|
|
|
|
|
Accounts payable
|
|
$
|
5,409,160
|
|
|
$
|
3,526,931
|
|
|
Accrued expenses
|
|
|
10,992,273
|
|
|
|
14,551,527
|
|
|
Customer deposits
|
|
|
9,661,878
|
|
|
|
9,695,841
|
|
|
Deferred revenue
|
|
|
10,493,214
|
|
|
|
8,910,551
|
|
|
Line of credit
|
|
|
10,860,648
|
|
|
|
9,565,808
|
|
|
Current deferred tax liability
|
|
|
44,267
|
|
|
|
44,267
|
|
|
Current portion of capital lease obligations
|
|
|
494,937
|
|
|
|
553,689
|
|
|
|
|
|
|
|
|
|
|
|
|
Total current liabilities
|
|
|
47,956,377
|
|
|
|
46,848,614
|
|
|
Deferred tax liability
|
|
|
314,421
|
|
|
|
345,121
|
|
|
Other liabilities
|
|
|
2,313,028
|
|
|
|
2,510,351
|
|
|
Capital lease obligations, less current portion
|
|
|
122,823
|
|
|
|
250,576
|
|
|
|
|
|
|
|
|
|
|
|
|
Total liabilities
|
|
|
50,706,649
|
|
|
|
49,954,662
|
|
|
Stockholders’ equity:
|
|
|
|
|
|
|
|
|
|
Common stock, $.0001 par value:
|
|
|
|
|
|
|
|
|
|
Authorized shares—150,000,000 at June 30, 2010 and
December 31, 2009, issued and outstanding
shares—37,216,052 and 37,277,808 at June 30, 2010 and
December 31, 2009, respectively
|
|
|
3,728
|
|
|
|
3,728
|
|
|
Additional paid-in capital
|
|
|
45,895,863
|
|
|
|
45,601,325
|
|
|
Accumulated other comprehensive (loss) income
|
|
|
(4,164,581
|
)
|
|
|
40,507
|
|
|
Retained earnings
|
|
|
2,116,452
|
|
|
|
660,456
|
|
|
|
|
|
|
|
|
|
|
|
|
Total stockholders’ equity
|
|
|
43,851,462
|
|
|
|
46,306,016
|
|
|
|
|
|
|
|
|
|
|
|
|
Total liabilities and stockholders’ equity
|
|
$
|
94,558,111
|
|
|
$
|
96,260,678
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Please see accompanying notes.
F-31
ReSearch
Pharmaceutical Services, Inc. and Subsidiaries
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Six Months Ended June 30,
|
|
|
|
2010
|
|
2009
|
|
|
|
(unaudited)
|
|
|
|
Service revenue
|
|
$
|
122,409,197
|
|
|
$
|
93,705,236
|
|
|
Reimbursement revenue
|
|
|
15,499,981
|
|
|
|
10,940,328
|
|
|
|
|
|
|
|
|
|
|
|
|
Total revenue
|
|
|
137,909,178
|
|
|
|
104,645,564
|
|
|
Direct costs
|
|
|
88,731,922
|
|
|
|
68,159,696
|
|
|
Reimbursable
out-of-pocket
costs
|
|
|
15,499,981
|
|
|
|
10,940,328
|
|
|
Selling, general, and administrative expenses
|
|
|
25,671,180
|
|
|
|
21,091,012
|
|
|
Depreciation and amortization
|
|
|
2,201,768
|
|
|
|
1,670,629
|
|
|
|
|
|
|
|
|
|
|
|
|
Income from operations
|
|
|
5,804,327
|
|
|
|
2,783,899
|
|
|
Interest expense
|
|
|
(438,372
|
)
|
|
|
(303,766
|
)
|
|
Interest income
|
|
|
5,186
|
|
|
|
167,829
|
|
|
Other income (expense)
|
|
|
37,464
|
|
|
|
(167,102
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Income before provision for income taxes
|
|
|
5,408,605
|
|
|
|
2,480,860
|
|
|
Provision for income taxes
|
|
|
3,952,609
|
|
|
|
1,492,131
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income
|
|
$
|
1,455,996
|
|
|
$
|
988,729
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income per common share:
|
|
|
|
|
|
|
|
|
|
Basic
|
|
$
|
0.04
|
|
|
$
|
0.03
|
|
|
Diluted
|
|
$
|
0.04
|
|
|
$
|
0.03
|
|
|
Weighted average number of common shares outstanding:
|
|
|
|
|
|
|
|
|
|
Basic
|
|
|
37,271,665
|
|
|
|
36,746,648
|
|
|
Diluted
|
|
|
38,676,380
|
|
|
|
37,707,889
|
|
|
|
|
|
Please see accompanying notes.
F-32
ReSearch
Pharmaceutical Services, Inc. and Subsidiaries
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Six Months Ended June 30,
|
|
|
|
2010
|
|
2009
|
|
|
|
(unaudited)
|
|
|
|
Operating activities
|
|
|
|
|
|
|
|
|
|
Net income
|
|
$
|
1,455,996
|
|
|
$
|
988,729
|
|
|
Adjustments to reconcile net income to net cash provided by
(used in) operating activities:
|
|
|
|
|
|
|
|
|
|
Depreciation and amortization
|
|
|
2,201,768
|
|
|
|
1,670,629
|
|
|
Stock-based compensation
|
|
|
294,337
|
|
|
|
308,675
|
|
|
Deferred tax benefit
|
|
|
(26,339
|
)
|
|
|
(229,418
|
)
|
|
Changes in operating assets and liabilities:
|
|
|
|
|
|
|
|
|
|
Accounts receivable
|
|
|
(3,406,062
|
)
|
|
|
(7,620,488
|
)
|
|
Prepaid expenses and other assets
|
|
|
437,265
|
|
|
|
(1,717,888
|
)
|
|
Accounts payable
|
|
|
2,016,987
|
|
|
|
(1,496,494
|
)
|
|
Accrued expenses and other liabilities
|
|
|
(3,005,662
|
)
|
|
|
504,627
|
|
|
Customer deposits
|
|
|
33,757
|
|
|
|
(1,645,565
|
)
|
|
Deferred revenue
|
|
|
890,603
|
|
|
|
446,898
|
|
|
|
|
|
|
|
|
|
|
|
|
Net cash provided by (used in) operating activities
|
|
|
892,650
|
|
|
|
(8,790,295
|
)
|
|
Investing activities
|
|
|
|
|
|
|
|
|
|
Change in restricted cash
|
|
|
52,641
|
|
|
|
1,645,565
|
|
|
Business combinations, net of cash acquired
|
|
|
—
|
|
|
|
(1,573,752
|
)
|
|
Purchase of property and equipment
|
|
|
(1,051,447
|
)
|
|
|
(1,254,484
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Net cash used in investing activities
|
|
|
(998,806
|
)
|
|
|
(1,182,671
|
)
|
|
Financing activities
|
|
|
|
|
|
|
|
|
|
Net borrowings on line of credit
|
|
|
1,294,840
|
|
|
|
4,873,471
|
|
|
Principal payments on capital lease obligations
|
|
|
(186,505
|
)
|
|
|
(349,647
|
)
|
|
Payments on deferred equity financing costs
|
|
|
(105,000
|
)
|
|
|
—
|
|
|
Proceeds from exercise of options
|
|
|
201
|
|
|
|
201
|
|
|
|
|
|
|
|
|
|
|
|
|
Net cash provided by financing activities
|
|
|
1,003,536
|
|
|
|
4,524,025
|
|
|
Effect of exchange rates on cash and cash equivalents
|
|
|
(113,244
|
)
|
|
|
38,927
|
|
|
|
|
|
|
|
|
|
|
|
|
Net change in cash and cash equivalents
|
|
|
784,136
|
|
|
|
(5,410,014
|
)
|
|
Cash and cash equivalents, beginning of period
|
|
|
3,468,104
|
|
|
|
6,565,003
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents, end of period
|
|
$
|
4,252,240
|
|
|
$
|
1,154,989
|
|
|
|
|
|
|
|
|
|
|
|
|
Supplemental disclosures of cash flow information
|
|
|
|
|
|
|
|
|
|
Cash paid during the period for:
|
|
|
|
|
|
|
|
|
|
Interest
|
|
$
|
425,387
|
|
|
$
|
470,868
|
|
|
|
|
|
|
|
|
|
|
|
|
Income taxes
|
|
$
|
3,618,401
|
|
|
$
|
4,033,194
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Please see accompanying notes.
F-33
ReSearch
Pharmaceutical Services, Inc. and Subsidiaries
June 30,
2010 (unaudited)
1.
Business
ReSearch Pharmaceutical Services, Inc. and Subsidiaries (the
“Company” or “RPS”) is a global next
generation CRO (clinical research organization) serving
biotechnology and pharmaceutical companies, which the Company
refers to collectively as the biopharmaceutical industry. The
RPS business model combines the expertise of a traditional CRO
with the ability to provide flexible outsourcing solutions that
are fully integrated within the Company’s clients’
clinical infrastructure. The Company is able to leverage its
high degree of clinical expertise, industry knowledge and
specialization to reduce the expense and time frame of clinical
development that meets the varied needs of small, medium and
large biopharmaceutical companies.
On August 30, 2007, the Company’s predecessor company
(“Old RPS”) merged with and into a wholly-owned
subsidiary of Cross Shore Acquisition Corporation (“Cross
Shore”), a blank check company incorporated in Delaware in
2006 as a vehicle to acquire one or more operating companies in
the United States. Prior to the merger, Cross Shore completed an
initial public offering on the Alternative Investment Market
(“AIM”) of the London Stock Exchange to raise proceeds
to fund such an acquisition. As a result of the merger, Cross
Shore changed its name to ReSearch Pharmaceutical Services,
Inc., and RPS is now a holding company for, and conducts
substantially all of its operations through its wholly-owned
subsidiary, ReSearch Pharmaceutical Services, LLC.
On September 4, 2009, RPS delisted its common stock from
AIM following approval of the delisting by the requisite number
of shareholders. Trading in RPS’ warrants to purchase
common stock, also listed on AIM, was suspended following the
delisting of the common stock, and the warrants were delisted on
October 5, 2009. RPS common stock and warrants are no
longer traded on AIM, but remain transferable as described in
the proxy statement which was mailed to shareholders and warrant
holders on July 24, 2009, and subject to applicable
securities laws.
The Company has wholly-owned subsidiaries in 47 countries around
the world with its core operations located in North America,
Latin America, Europe and Asia.
2. Significant
Accounting Policies
Basis of
Presentation
The accompanying unaudited consolidated financial statements
have been prepared in accordance with accounting principles
generally accepted in the United States for interim financial
information and with the instructions to
Form 10-Q
and Article 10 of
Regulation S-X.
The consolidated balance sheet as of June 30, 2010, the
consolidated statements of operations for the six months ended
June 30, 2010 and 2009 and the consolidated statements of
cash flows for the six months ended June 30, 2010 and 2009
are unaudited, but include all adjustments, consisting only of
normal recurring adjustments, which the Company considers
necessary for a fair presentation of its financial position,
operating results and cash flows for the periods presented. The
consolidated balance sheet at December 31, 2009 has been
derived from audited financial statements.
Although the Company believes that the disclosures in these
financial statements are adequate to make the information
presented not misleading, certain information and footnote
information normally included in financial statements prepared
in accordance with accounting principles generally accepted in
the United States have been condensed or omitted as permitted by
the rules and regulations of the Securities and Exchange
Commission.
Results for any interim period are not necessarily indicative of
results for any future interim period or for the entire year.
The accompanying financial statements should be read in
conjunction with the financial statements and notes thereto
included in the Company’s Annual Report on
Form 10-K
for the year ended December 31, 2009.
F-34
Concentration
of Credit Risk
Financial instruments that potentially subject the Company to
credit risk consist principally of cash and accounts receivable.
The Company performs periodic evaluations of the financial
institutions in which its cash is invested. The majority of the
Company’s revenues and accounts receivable are derived from
pharmaceutical companies located in the United States. The
Company’s three largest customers accounted for
approximately 17%, 16% and 15% of service revenue during the six
months ended June 30, 2010, respectively. The
Company’s two largest customers for the six months ended
June 30, 2009 represented approximately 17% and 11% of
service revenue, respectively.
The Company’s largest customer accounted for approximately
16% of the accounts receivable balance at June 30, 2010 and
at December 31, 2009. The Company’s second largest
customer accounted for approximately 14% of the accounts
receivable balance at June 30, 2010, and approximately 17%
of the accounts receivable balance at December 31, 2009. No
other customers represented more than 10% of net service revenue
or accounts receivable during those periods or at those times.
The Company provides an allowance for doubtful accounts based on
experience and specifically identified risks. Accounts
receivable are carried at fair value and charged off against the
allowance for doubtful accounts when management determines that
recovery is unlikely and the Company ceases collection efforts.
Revenue
and Cost Recognition
The majority of the Company’s service revenue is derived
from
fee-for-service
contracts, some of which are fixed-price contracts. Revenues and
the related costs of
fee-for-service
contracts are recognized in the period in which services are
performed. Fixed-price contract revenue is calculated on a
proportional performance basis based on the ratio that costs
incurred to date bear on the estimated total costs at
completion. The Company also recognizes revenue under
units-based contracts by multiplying units completed by the
applicable contract
per-unit
price. Revenue related to contract modifications is recognized
when realization is assured and the amounts are reasonably
determinable. Adjustments to contract estimates are recorded in
the periods in which the facts that require the revisions become
known. When the revised estimate indicates a loss, such loss is
recorded in the financial statements for that period. No such
losses were recognized in the six months ended June 30,
2010 or 2009. Deferred revenue represents amounts billed to
customers in excess of revenue recognized.
Financial Accounting Standards Board (“FASB”) guidance
requires reimbursable
out-of-pocket
expenses to be classified as revenue in the statements of
operations. Reimbursements for
out-of-pocket
expenses, included in total revenue in the Company’s
consolidated statements of operations were $15,449,981 and
$10,940,328 for the six months ended June 30, 2010 and
2009, respectively.
The Company excludes investigator fees from its
out-of-pocket
expenses because these fees are funded from the customer’s
restricted cash and are recorded on a “pass-through
basis” without risk or reward to the Company. Investigator
fees paid on behalf of clients were approximately $3,996,000 and
$1,803,000 for the six months ended June 30, 2010 and 2009,
respectively.
Income
Taxes
The Company accounts for income taxes using an asset and
liability approach which requires the recognition of deferred
tax assets and liabilities for the expected future tax
consequences of temporary differences between the carrying
amount of assets and liabilities for financial reporting
purposes and amounts reportable for income tax purposes. On
January 1, 2007, the Company adopted the FASB guidance
related to accounting for uncertainty in income taxes. This
guidance creates a single model to address uncertainty in tax
positions and clarifies the accounting for income taxes by
prescribing the minimum recognition threshold a tax position is
required to meet before it is recognized in the financial
statements.
The effective tax rate for the six months ended June 30,
2010 and 2009 was higher than the federal statutory rate, as the
Company did not record a tax benefit for net operating losses
generated in certain of its foreign subsidiaries as it may not
realize the tax benefit of these net operating losses.
F-35
Foreign
Currency Translation
The financial statements of the Company’s foreign
subsidiaries have been translated into U.S. dollars in
accordance with the FASB guidance on foreign currency
translation. All balance sheet accounts have been translated
using the exchange rates in effect at the respective balance
sheet dates. Income statement amounts have been translated using
average exchange rates in effect for the relevant periods. The
gains and losses resulting from the changes in exchange rates
during the year have been reported separately in other
comprehensive income in the consolidated financial statements.
Stock-Based
Compensation
The per-share weighted average fair value of the options granted
during the six months ended June 30, 2010 and 2009 was
estimated at $1.78 and $0.86, respectively, using the
Black-Scholes option-pricing model with the following weighted
average assumptions, which are based upon Company history or
industry comparative information:
| |
|
|
|
|
|
|
|
|
|
|
|
Six Months Ended June 30,
|
|
|
|
2010
|
|
2009
|
|
|
|
Expected dividend yield
|
|
|
0.00
|
%
|
|
|
0.00
|
%
|
|
Expected volatility
|
|
|
50
|
%
|
|
|
50
|
%
|
|
Risk-free interest rate
|
|
|
2.21
|
%
|
|
|
1.92
|
%
|
|
Expected life
|
|
|
6 years
|
|
|
|
6 years
|
|
Prior to August 30, 2007, the Company’s common stock
was not publicly traded, and the expected volatility was
calculated for each date of grant based on an alternative method
(defined as the “calculated value”). Subsequent to
August 30, 2007, as a public company on the AIM, the
Company continued to utilize the calculated value for expected
volatility as a sufficient level of history was not available as
a publicly traded company. In September and October 2009, the
Company delisted its common stock and warrants from AIM,
respectively, and its common stock and warrants are no longer
publicly traded. As such, the Company will continue to use the
calculated value to estimate fair value. The Company identified
similar public entities for which share price information is
available and has considered the historical volatility of these
entities’ share prices in determining its estimated
expected volatility. The Company used the average volatility of
these guideline companies over a six-year period, consistent
with the expected term calculated pursuant to FASB guidance.
From August 30, 2007 through the September 2009 AIM
delisting date, the Company utilized the quoted stock price on
the AIM as a determinant of fair value of the Company’s
common stock. Subsequent to the AIM delisting date, the Company
estimates the fair value of its common stock using the market
and income valuation approaches, with the assistance of a
valuation consultant. Stock-based compensation expense for the
six months ended June 30, 2010 and 2009 related to share
based service awards was approximately $294,000 and $309,000,
respectively, and is included in selling, general, and
administrative expenses in the accompanying consolidated
statements of operations. The Company recognizes the
compensation expense of such stock-based service awards on a
straight-line basis. Total compensation cost of options granted
but not yet vested as of June 30, 2010 was $327,000, net of
estimated forfeitures, which is expected to be recognized over
the weighted average period of 1.7 years.
Segment
Information
Operating segments are identified as components of an enterprise
about which separate financial information is available for
evaluation by the chief operating decision-maker, or
decision-making group, in making decisions on how to allocate
resources and assess performance. The Company views its
operations and manages its business as one operating segment.
The Company’s foreign operations accounted for
approximately 16% of service revenue during the six months
F-36
ended June 30, 2010 and 2009, respectively. In addition,
approximately 29% and 35% of the Company’s consolidated
tangible assets are located in foreign locations at
June 30, 2010 and December 31, 2009, respectively.
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Americas
|
|
Europe
|
|
Asia-Pacific
|
|
Total
|
|
|
|
Service revenue from customers (1)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Six months ended June 30, 2010
|
|
$
|
110,867,474
|
|
|
$
|
10,252,641
|
|
|
$
|
1,289,082
|
|
|
$
|
122,409,197
|
|
|
Six months ended June 30, 2009
|
|
|
84,114,209
|
|
|
|
9,591,027
|
|
|
|
—
|
|
|
|
93,705,236
|
|
|
Long-lived assets (2)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
As of June 30, 2010
|
|
|
3,967,809
|
|
|
|
1,454,706
|
|
|
|
186,046
|
|
|
|
5,608,561
|
|
|
As of December 31, 2009
|
|
|
4,228,432
|
|
|
|
1,982,932
|
|
|
|
193,383
|
|
|
|
6,404,747
|
|
|
|
|
|
|
|
|
|
(1) |
|
Service revenue is attributable to geographic locations based on
the physical location where the services are performed. |
| |
|
(2) |
|
Long-lived assets represents the net book value of property and
equipment. |
Net
Income Per Share
Basic net income per share is computed by dividing net income by
the weighted average number of shares of common stock
outstanding during the periods presented. Diluted net income per
share is computed by dividing net income by the weighted average
number of shares of common stock outstanding during the period
plus the dilution that would occur upon the exercise or
conversion of stock options or common stock warrants.
The following table is a reconciliation of the numerator and
denominator of the computation of basic and diluted net income
per share.
| |
|
|
|
|
|
|
|
|
|
|
|
Six Months Ended June 30,
|
|
|
|
2010
|
|
2009
|
|
|
|
Net income
|
|
$
|
1,455,996
|
|
|
$
|
988,729
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted average common shares outstanding—basic
|
|
|
37,271,665
|
|
|
|
36,746,648
|
|
|
Dilutive effect of stock options and warrants
|
|
|
1,404,715
|
|
|
|
961,241
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted average common shares outstanding—diluted
|
|
|
38,676,380
|
|
|
|
37,707,889
|
|
|
|
|
|
|
|
|
|
|
|
Options to purchase 856,224 shares of the Company’s
common stock and warrants outstanding to purchase
1.4 million shares of the Company’s common stock
were excluded from the computation of diluted weighted average
shares outstanding for the six months ended June 30, 2010
because their effect would have been anti-dilutive. Warrants
outstanding to purchase a total of 1.4 million shares
of the Company’s common stock, and options to purchase
997,349 shares of the Company’s common stock were
excluded from the computation of diluted weighted average shares
outstanding for the six months ended June 30, 2009 because
their effect would have been anti-dilutive. Outstanding stock
options and warrants could potentially dilute earnings per share
in the future.
Comprehensive
Income (Loss)
The Company’s comprehensive income (loss) was as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
Six Months Ended June 30,
|
|
|
|
2010
|
|
2009
|
|
|
|
Net income as reported
|
|
$
|
1,455,996
|
|
|
$
|
988,729
|
|
|
Other comprehensive income (loss):
|
|
|
|
|
|
|
|
|
|
Foreign currency translation adjustment
|
|
|
(4,205,088
|
)
|
|
|
(384,175
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Comprehensive income (loss)
|
|
$
|
(2,749,092
|
)
|
|
$
|
604,554
|
|
|
|
|
|
|
|
|
|
|
|
F-37
3.
Acquisitions
2009
Acquisition
Paramax
International Inc. (“Paramax”)
On July 7, 2009, RPS acquired the outstanding shares of
Paramax for consideration of $1.0 million in cash and
530,973 shares of common stock (the “Paramax
Shares”) issued to Paramax’s sole shareholder (the
“Paramax Acquisition”). Paramax, which is active in
the same fields as RPS, provides the Company with opportunities
in the Asia-Pacific market and complements its current
operations in the Americas and Europe. In addition, the Paramax
Acquisition provides RPS with greater scale to meet the growing
needs of its customers in the market for globally integrated
clinical research services. The Paramax Shares were valued by
management utilizing the assistance of a valuation specialist at
$1.73 per share, which resulted in total acquisition
consideration of approximately $1.9 million. The
shareholder of Paramax has entered into a share escrow agreement
whereby all of the Paramax Shares are held in escrow, and were,
or will be, released in equal portions on October 7, 2009,
July 7, 2010 and January 31, 2011, subject to there
being no indemnity claims outstanding (as defined within the
acquisition agreement). As of June 30, 2010, there were no
indemnity claims outstanding. In addition, the shareholder of
Paramax has agreed to a
lock-up on
all Paramax Shares to expire 24 months following
consummation of the Paramax Acquisition. Paramax, founded in
2007, is located in Beijing, China. Paramax operates throughout
China and the Asia-Pacific market, providing clinical research
services to the biopharmaceutical industry.
The acquisition has been accounted for as a purchase.
Accordingly, the results of operations of Paramax have been
included in the Company’s consolidated financial statements
commencing July 7, 2009. The allocation of the purchase
price is outlined below:
| |
|
|
|
|
|
Purchase Price:
|
|
|
|
|
|
Cash paid
|
|
$
|
1,000,000
|
|
|
Value of RPS Shares
|
|
|
918,583
|
|
|
|
|
|
|
|
|
Total purchase price
|
|
$
|
1,918,583
|
|
|
|
|
|
|
|
Allocation of Purchase Price:
| |
|
|
|
|
|
Cash
|
|
$
|
163,692
|
|
|
Accounts receivable
|
|
|
87,367
|
|
|
Fixed assets
|
|
|
31,780
|
|
|
Other assets
|
|
|
9,130
|
|
|
Goodwill
|
|
|
1,504,355
|
|
|
Customer lists
|
|
|
18,000
|
|
|
Non compete agreements
|
|
|
117,000
|
|
|
Current liabilities
|
|
|
(12,742
|
)
|
|
|
|
|
|
|
|
|
|
$
|
1,918,583
|
|
|
|
|
|
|
|
The unaudited pro forma information below presents combined
results of operations as if the Paramax Acquisition had occurred
as of the beginning of the applicable reporting period instead
of in July 2009. The pro forma information is based on
historical results and is not necessarily indicative of the
results of operations of the combined entity had the Paramax
Acquisition occurred at the beginning of the periods presented,
nor is it necessarily indicative of future results.
F-38
| |
|
|
|
|
|
|
|
Six months ended
|
|
|
|
June 30, 2009
|
|
|
|
(unaudited)
|
|
|
|
Service revenue
|
|
$
|
93,811,112
|
|
|
Reimbursement revenue
|
|
|
10,940,328
|
|
|
|
|
|
|
|
|
Total revenue
|
|
|
104,751,440
|
|
|
|
|
|
|
|
|
Net income
|
|
$
|
836,453
|
|
|
Net income per common share:
|
|
|
|
|
|
Basic
|
|
$
|
0.02
|
|
|
Diluted
|
|
$
|
0.02
|
|
|
Weighted average number of common shares outstanding:
|
|
|
|
|
|
Basic
|
|
|
37,277,621
|
|
|
Diluted
|
|
|
38,238,862
|
|
The shares issued in connection with the consummation of the
Paramax Acquisition were valued by management utilizing the
assistance of a valuation specialist, which resulted in a fair
value of $1.73 per share. This value is consistent with the
trading price of the Company’s common stock on AIM at the
time of the Paramax Acquisition, discounted to reflect the
escrow and lock up arrangements underlying certain of the shares
issued as discussed above.
4. Intangible
Assets
The following table summarizes the changes in the carrying
amount of the Company’s goodwill for the six months ended
June 30, 2010:
| |
|
|
|
|
|
Balance as of December 31, 2009
|
|
$
|
16,742,614
|
|
|
Currency exchange
|
|
|
(2,542,844
|
)
|
|
|
|
|
|
|
|
Balance as of June 30, 2010
|
|
$
|
14,199,770
|
|
|
|
|
|
|
|
The following tables summarize intangible assets and their
amortization as of:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
June 30, 2010
|
|
|
|
|
|
Accumulated
|
|
|
|
|
|
Gross
|
|
Amortization
|
|
Net
|
|
|
|
Intangible assets subject to amortization:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Customer contracts and lists
|
|
$
|
3,362,958
|
|
|
$
|
(2,550,693
|
)
|
|
$
|
812,265
|
|
|
Brand name
|
|
|
1,195,042
|
|
|
|
(909,378
|
)
|
|
|
285,664
|
|
|
Non-compete agreements
|
|
|
1,338,663
|
|
|
|
(619,376
|
)
|
|
|
769,287
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total
|
|
$
|
5,946,663
|
|
|
$
|
(4,079,447
|
)
|
|
$
|
1,867,216
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
F-39
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
December 31, 2009
|
|
|
|
|
|
Accumulated
|
|
|
|
|
|
Gross
|
|
Amortization
|
|
Net
|
|
|
|
Intangible assets subject to amortization:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Customer contracts and lists
|
|
$
|
3,291,114
|
|
|
$
|
(2,242,057
|
)
|
|
$
|
1,049,057
|
|
|
Brand name
|
|
|
1,403,059
|
|
|
|
(696,360
|
)
|
|
|
706,669
|
|
|
Non-compete agreements
|
|
|
1,569,459
|
|
|
|
(532,734
|
)
|
|
|
1,036,725
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total
|
|
$
|
6,263,632
|
|
|
$
|
(3,471,151
|
)
|
|
$
|
2,792,481
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The estimated amortization expense for the six months ending
December 31, 2010 and the four years ending
December 31, 2014 is as follows:
| |
|
|
|
|
|
|
|
|
Six Months
|
|
|
|
|
|
|
|
|
Ending
|
|
|
|
|
|
|
|
|
December 31,
|
|
Year ending December 31,
|
|
2010
|
|
2011
|
|
2012
|
|
2013
|
|
2014
|
|
|
|
$501,000
|
|
$425,000
|
|
$406,000
|
|
$382,500
|
|
$152,500
|
5. Property and
Equipment
Property and equipment consist of the following:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
June 30,
|
|
December 31,
|
|
|
|
Useful life
|
|
2010
|
|
2009
|
|
|
|
Computers, software and other equipment
|
|
2 to 3 years
|
|
$
|
4,853,971
|
|
|
$
|
5,052,139
|
|
|
Automobiles
|
|
1 to 3 years
|
|
|
1,615,071
|
|
|
|
1,615,000
|
|
|
Leasehold improvements
|
|
7 years
|
|
|
711,237
|
|
|
|
379,645
|
|
|
Software
|
|
2 to 3 years
|
|
|
1,146,186
|
|
|
|
498,683
|
|
|
Furniture and fixtures
|
|
5 years
|
|
|
2,679,339
|
|
|
|
2,980,807
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
11,005,804
|
|
|
|
10,526,274
|
|
|
Less accumulated depreciation
|
|
|
|
|
(5,397,241
|
)
|
|
|
(4,121,527
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$
|
5,608,563
|
|
|
$
|
6,404,747
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Automobiles, computers, software and other equipment include
assets acquired under capital lease obligations (Note 10).
Depreciation expense was approximately $1,579,000 and $1,050,000
for the six months ended June 30, 2010 and 2009,
respectively.
6. Accrued
Expenses
Accrued expenses consist of the following:
| |
|
|
|
|
|
|
|
|
|
|
|
June 30,
|
|
December 31,
|
|
|
|
2010
|
|
2009
|
|
|
|
Accrued compensation
|
|
$
|
4,732,849
|
|
|
$
|
5,824,601
|
|
|
Accrued professional fees
|
|
|
1,867,422
|
|
|
|
1,953,424
|
|
|
Volume rebate accrual
|
|
|
781,922
|
|
|
|
1,507,603
|
|
|
Accrued taxes
|
|
|
734,967
|
|
|
|
1,975,566
|
|
|
Other
|
|
|
2,875,113
|
|
|
|
3,290,333
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$
|
10,992,273
|
|
|
$
|
14,551,527
|
|
|
|
|
|
|
|
|
|
|
|
F-40
7. Lines of
Credit
In November 2006, the Company entered into a bank line of credit
agreement (the “Credit Agreement”), expiring
October 31, 2009. The Credit Agreement provided for
$15,000,000 of available borrowings, and was subject to certain
borrowing base restrictions. Borrowings under the Credit
Agreement required interest at the Federal Funds open rate, as
defined, plus 1%. The Credit Agreement was secured by all
corporate assets and also contained financial and nonfinancial
covenants, including restrictions on the payment of dividends,
restrictions on acquisitions and restrictions on the repurchase,
redemption, or retirement of outstanding equity.
In July 2009, the Credit Agreement was amended (the
“Amended Credit Agreement”) to extend the termination
date to October 31, 2012. The Amended Credit Agreement also
provides for an increase to $30,000,000 of available borrowings,
and is subject to certain borrowing base restrictions.
Borrowings under the Amended Credit Agreement require interest
at the Federal Funds open rate, as defined, plus 2% (4.75% at
June 30, 2010). The Amended Credit Agreement remains
secured by all corporate assets and continues the financial and
nonfinancial covenants, including restrictions on the payment of
dividends, restrictions on acquisitions and restrictions on the
repurchase, redemption, or retirement of outstanding equity
present under the Credit Agreement. At June 30, 2010 there
were $10.9 million in outstanding borrowings under this
line of credit.
In addition to the Company’s line of credit for its
U.S.-based operations, the Company maintains various local lines
of credit for its operations based around the world. At
June 30, 2010, there were no outstanding borrowings under
these lines of credit.
8.
Stockholders’ Equity
The Company is authorized to issue up to 1,000,000 shares
of preferred stock, $0.0001 par value, and
150,000,000 shares of common stock, $.0001 par value.
Of the shares authorized, 6,792,271 shares of common stock
have been reserved for issuance pursuant to the Company’s
2007 equity incentive plan (Note 9).
The Company’s stockholders have been granted certain rights
to register their shares under the securities laws of the United
States pursuant to three separate registration rights
agreements. The Registration Rights Agreement (as defined below)
pertains to those holding shares in RPS prior to the merger with
Cross Shore. The Investor Rights Agreement (as defined below)
pertains to those acquiring shares and warrants in Cross
Shore’s initial public offering in April of 2006. The
Founders’ Shares Agreement (as defined below) pertains
to those that acquired shares prior to Cross Shore’s
initial public offering in April 2006.
Under the Investor Rights Agreement dated April 24, 2006
(the “Investor Rights Agreement”), the Company is
required to file a shelf registration statement on
Form S-3
within 90 days after becoming eligible to do so. In
addition the holders of the Company’s stock are entitled to
no more than three demand registrations (covering in each case a
minimum of 15% of the shares then outstanding) and piggyback
registration rights. If the Company files a shelf registration
for resale of shares, demand and piggyback registration rights
will be suspended except for underwritten offerings.
Registration rights are generally available only for stock that
is subject to restrictions on transfer under the
U.S. securities laws.
Under the terms of the Registration Rights Agreement dated
August 30, 2007 (the “Registration Rights
Agreement”), the Company will grant the existing
stockholders the rights to include shares on any registration
statement filed by the Company pursuant to the Securities Act of
1933, as amended (the “Securities Act”) in connection
with a public offering of stock, whether such offering is being
made for the Company’s own account or for the account of
stockholders other than the existing stockholders. These
registration rights are applicable to any registration of stock
that is made pursuant to a demand from the existing stockholders
pursuant to the Investor Rights Agreement. The number of shares
that the existing stockholders may include in an underwritten
public offering by exercising their registration rights under
the Registration Rights Agreement is subject to reduction in the
event the managing underwriters of such offering advise the
Company that the number of shares to be included in such
offering exceeds the amount of stock that can be sold without
adversely affecting the offering. The Registration Rights
Agreement also provides the Old RPS stockholders similar shelf
registration rights as those in the Investor Rights Agreement.
Registration rights are generally available only for stock that
is subject to restrictions on transfer under the U.S. securities
laws. If the Company fails to make filings under the Securities
Act or the Securities Exchange Act of 1934, as amended (the
“Exchange Act”) that are required to be made pursuant
F-41
to its contractual arrangements with the existing stockholders,
the Registration Rights Agreement entitles the holders of shares
to receive liquidated damages in the form of additional shares
in an amount per month equal to 1% of all or a portion of such
holder’s registrable securities for up to two months, or up
to four months under the Investor Rights Agreement.
Under the Registration Rights Agreement dated April 24,
2006 (the “Founders’ Shares Agreement”), the
Company is required to file a shelf registration statement on
Form S-3
upon request of the founding stockholders after becoming
eligible to do so. In addition, the holders of the
Company’s founding stock are entitled to no more than two
demand registrations (covering in each case as many shares as
the founding stockholders propose to sell, subject to certain
restrictions imposed by an underwriter) and piggyback
registration rights. If the Company files a shelf registration
statement for resale of shares, demand and piggyback
registration rights will be suspended except for underwritten
offerings. Registration rights are generally available only
until, in the opinion of the Company’s counsel, the
founding stock is no longer subject to restrictions on transfer
under the U.S. securities laws.
The Company is required to bear all expenses incident to its
compliance with the terms of the Registration Rights Agreement,
the Investor Rights Agreement, and the Founders’
Shares Agreement. The Registration Rights Agreement and
Founders’ Shares Agreement also contain customary
indemnification obligations from the Company to the applicable
stockholders with respect to untrue statements or material
omissions in any registration statement that includes the
applicable shares.
The Company also had a total of 1,357,179 common stock warrants
(the “IPO Warrants”). The unexercised IPO Warrants
expired on April 28, 2010. The IPO Warrants were issued to
investors in connection with the initial public offering of
Cross Shore in April 2006 and were delisted from AIM on
October 5, 2009. The IPO Warrants were exercisable at $5.00
per share.
In addition, a total of 186,667 options (“Underwriter
Purchase Options”) remained outstanding from the date of
the date of the Cross Shore initial public offering in April
2006 until the unexercised options expired on April 27,
2010. These Underwriter Purchase Options were issued to
representatives of the underwriters of the Cross Shore initial
public offering. The options entitled the holder to one share of
common stock and two common stock warrants in exchange for an
exercise price of $6.60 per share. If the options were
exercised, the warrants received would have been fully vested
with an exercise price of $5.00 per share at any time through
April 28, 2010. Such warrants were subject to the same
provisions as the IPO Warrants discussed above.
9. Stock Option
Plans
In June 2002, the Company adopted the 2002 Equity Incentive Plan
(the “2002 Plan”) which permitted the granting of
incentive stock options, nonqualified stock options and
restricted stock. The Company authorized the issuance of up to
2,108,456 shares of common stock to satisfy grants under
the 2002 Plan. Stock options issued generally vested over a
three-year period. The exercise period was determined by the
Company’s Board of Directors, but could not exceed ten
years from the date of grant. Each option entitled the holder to
purchase one share of common stock at the indicated exercise
price.
The Company adopted the 2007 Equity Incentive Plan (the
“2007 Incentive Plan”) on August 30, 2007 and
terminated the 2002 Plan. The 2007 Incentive Plan permits awards
of options and restricted stock. At June 30, 2010, the
total number of shares reserved under the 2007 Incentive Plan
was 6,792,271 shares. On an annual basis, this amount is
automatically increased to an amount equal to 15% of the number
of shares outstanding (calculated on a fully diluted basis).
Stock options issued under the 2007 Incentive Plan generally
vest over a three year period. The exercise period is determined
by the Board of Directors, but may not exceed 10 years from
the date of grant.
F-42
The following table summarizes activity under the 2002 Plan and
the 2007 Incentive Plan:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Options Available
|
|
Number of Options
|
|
Weighted Average
|
|
|
|
For Grant
|
|
Outstanding
|
|
Exercise Price
|
|
|
|
Balance, December 31, 2009
|
|
|
3,781,977
|
|
|
|
2,915,412
|
|
|
$
|
2.06
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Granted
|
|
|
(71,940
|
)
|
|
|
71,940
|
|
|
|
3.23
|
|
|
Exercised
|
|
|
—
|
|
|
|
(544
|
)
|
|
|
0.37
|
|
|
Forfeited/Cancelled
|
|
|
78,415
|
|
|
|
(78,415
|
)
|
|
|
1.27
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance, June 30, 2010
|
|
|
3,788,452
|
|
|
|
2,908,393
|
|
|
|
2.07
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The weighted average grant date fair value of options granted
was $1.78 per share during the six months ended June 30,
2010.
At June 30, 2010, options to purchase 2,683,289 shares were
exercisable at a weighted average exercise price of $1.98 per
share. The weighted average remaining contractual life of the
fully vested options at June 30, 2010 was 5.6 years.
The aggregate intrinsic value of options outstanding and fully
vested at June 30, 2010 was $5.2 million.
10. Commitments
and Contingencies
The Company occupies its corporate headquarters and other
offices and uses certain equipment under various operating
leases. The Company’s current lease for its corporate
headquarters expires in June 2017. Rent expense under such lease
arrangements was approximately $1,962,000 and $1,523,000 during
the six months ended June, 2010 and 2009, respectively. The
Company is the lessee of approximately $1,615,000 of automobiles
and equipment under capital leases expiring through 2012. The
equipment is recorded at the present value of minimum lease
payments and is amortized over its estimated useful life.
Amortization of the assets under capital lease agreements of
approximately $230,000 and $289,000 for the six months ended
June 30, 2010 and 2009, respectively, and is included in
depreciation expense.
Future minimum lease payments subsequent to June 30, 2010
under capital and non-cancelable operating leases are as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
Capital
|
|
Operating
|
|
|
|
Leases
|
|
Leases
|
|
|
|
2010
|
|
$
|
385,060
|
|
|
$
|
1,819,201
|
|
|
2011
|
|
|
255,601
|
|
|
|
3,262,305
|
|
|
2012
|
|
|
4,590
|
|
|
|
3,148,147
|
|
|
2013
|
|
|
—
|
|
|
|
2,630,569
|
|
|
2014
|
|
|
—
|
|
|
|
2,059,714
|
|
|
Thereafter
|
|
|
—
|
|
|
|
2,836,814
|
|
|
|
|
|
|
|
|
|
|
|
|
Total minimum lease payments
|
|
|
645,251
|
|
|
$
|
15,756,750
|
|
|
Less amount representing interest
|
|
|
27,491
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Present value of net minimum lease payments
|
|
$
|
617,760
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The Company is involved in various claims incidental to the
conduct of its business. Management does not believe that any
such claims to which the Company is a party, both individually
and in the aggregate, will have a material adverse effect on the
Company’s financial position, results of operations or cash
flows.
F-43
Independent
Auditor’s Report
The Board of Directors and Stockholders
IMEREM Institute for Medical Research Management and
Biometrics—Institute für medizinisches
Forschungsmanagement und Biometrie—Ein unabhaengiges
Forschungsunternehmen GmbH
We have audited the accompanying balance sheet of IMEREM
Institute for Medical Research Management and
Biometrics—Institute für medizinisches
Forschungsmanagement und Biometrie—Ein unabhaengiges
Forschungsunternehmen GmbH as of December 31, 2007, and the
related statements of income, stockholder’s equity and cash
flows for the year then ended. These financial statements are
the responsibility of the Company’s management. Our
responsibility is to express an opinion on these financial
statements based on our audit.
We conducted our audit in accordance with auditing standards
generally accepted in the United States of America. Those
standards require that we plan and perform the audit to obtain
reasonable assurance about whether the financial statements are
free of material misstatement. An audit includes examining, on a
test basis, evidence supporting the amounts and disclosures in
the financial statements. An audit also includes assessing the
accounting principles used and significant estimates made by
management, as well as evaluating the overall financial
statement presentation. We believe that our audit provides a
reasonable basis for our opinion.
In our opinion, the financial statements referred to above
present fairly, in all material respects, the financial position
of IMEREM Institute for Medical Research Management and
Biometrics—Institute für medizinisches
Forschungsmanagement und Biometrie—Ein unabhaengiges
Forschungsunternehmen GmbH as of December 31, 2007, and the
results of its operations and its cash flows for the year then
ended, in conformity with accounting principles generally
accepted in the United States.
/s/ McGladrey & Pullen, LLP
Chicago, Illinois
March 9, 2009
F-44
IMEREM Institute
for Medical Research Management and Biometrics—
Institute für medizinisches Forschungsmanagement und
Biometrie—Ein unabhaengiges Forschungsunternehmen GmbH
Balance Sheet
In Euros
| |
|
|
|
|
|
|
|
|
|
December 31,
|
|
|
|
2007
|
|
|
|
Assets
|
|
|
|
|
|
Current assets:
|
|
|
|
|
|
Cash and cash equivalents
|
|
€
|
1,657,468
|
|
|
Restricted cash
|
|
|
687,849
|
|
|
Available-for-sale
securities
|
|
|
1,095,529
|
|
|
Unbilled Revenues
|
|
|
1,361,824
|
|
|
Accounts receivable, less allowance for doubtful accounts
|
|
|
1,355,308
|
|
|
Prepaid expenses and other current assets
|
|
|
127,826
|
|
|
|
|
|
|
|
|
Total current assets
|
|
|
6,285,804
|
|
|
Intangible assets, net
|
|
|
38,446
|
|
|
Property and equipment, net
|
|
|
659,433
|
|
|
Cash surrender value of life insurance
|
|
|
519,293
|
|
|
Deferred income taxes
|
|
|
9,000
|
|
|
|
|
|
|
|
|
Total assets
|
|
€
|
7,511,976
|
|
|
|
|
|
|
|
|
Liabilities and stockholders’ equity
|
|
|
|
|
|
Current liabilities:
|
|
|
|
|
|
Accounts payable
|
|
€
|
15,235
|
|
|
Accrued expenses
|
|
|
305,453
|
|
|
Customer deposits
|
|
|
687,849
|
|
|
Deferred revenue
|
|
|
659,854
|
|
|
Due to stockholder
|
|
|
786,840
|
|
|
Other current liabilities
|
|
|
623,149
|
|
|
Deferred income taxes
|
|
|
—
|
|
|
|
|
|
|
|
|
Total current liabilities
|
|
|
3,078,380
|
|
|
Deferred compensation
|
|
|
612,078
|
|
|
Deferred income taxes—long term
|
|
|
193,000
|
|
|
Long term debt
|
|
|
135,986
|
|
|
|
|
|
|
|
|
Total liabilities
|
|
|
4,019,444
|
|
|
Stockholders’ equity:
|
|
|
|
|
|
Common stock; no par value, 1 share authorized and issued
|
|
|
25,565
|
|
|
Accumulated other comprehensive loss
|
|
|
(27,471
|
)
|
|
Retained earnings
|
|
|
3,494,438
|
|
|
|
|
|
|
|
|
Total stockholders’ equity
|
|
|
3,492,532
|
|
|
|
|
|
|
|
|
Total liabilities and stockholders’ equity
|
|
€
|
7,511,976
|
|
|
|
|
|
|
|
|
|
The accompanying notes are an integral part of the financial
statements.
F-45
IMEREM Institute
for Medical Research Management and Biometrics—
Institute für medizinisches Forschungsmanagement und
Biometrie—Ein unabhaengiges Forschungsunternehmen GmbH
Statement of Income
In Euros
| |
|
|
|
|
|
|
|
|
|
12 Months
|
|
|
|
December 31, 2007
|
|
|
|
Service revenue
|
|
€
|
4,030,386
|
|
|
Other income
|
|
|
158,924
|
|
|
|
|
|
|
|
|
Total revenue
|
|
|
4,189,310
|
|
|
Direct costs
|
|
|
2,277,925
|
|
|
Selling, general, and administrative expenses
|
|
|
718,925
|
|
|
Depreciation and amortization
|
|
|
78,441
|
|
|
|
|
|
|
|
|
Income from operations
|
|
|
1,114,019
|
|
|
Interest expense
|
|
|
(45,071
|
)
|
|
Interest income
|
|
|
64,145
|
|
|
|
|
|
|
|
|
Income before provision for income taxes
|
|
|
1,133,093
|
|
|
Provision for income taxes
|
|
|
369,601
|
|
|
|
|
|
|
|
|
Net income
|
|
€
|
763,492
|
|
|
|
|
|
|
|
|
|
The accompanying notes are an integral part of the financial
statements.
F-46
IMEREM Institute
for Medical Research Management and Biometrics—
Institute für medizinisches Forschungsmanagement und
Biometrie—Ein unabhaengiges Forschungsunternehmen GmbH
Statement of Stockholder’s
Equity
In Euros
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accumulated
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Other
|
|
|
|
Total
|
|
|
|
|
|
Comprehensive
|
|
|
|
Comprehensive
|
|
Retained
|
|
Stockholder’s
|
|
|
|
Share
|
|
Income
|
|
Amount
|
|
loss
|
|
Earnings
|
|
Equity
|
|
|
|
Balance at January 1, 2007
|
|
|
1
|
|
|
€
|
—
|
|
|
€
|
25,565
|
|
|
€
|
(20,877
|
)
|
|
€
|
2,800,946
|
|
|
€
|
2,805,634
|
|
|
|
|
|
|
Dividends to stockholder
|
|
|
—
|
|
|
|
763,492
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(70,000
|
)
|
|
|
(70,000
|
)
|
|
Comprehensive income:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income
|
|
|
—
|
|
|
|
|
|
|
|
—
|
|
|
|
—
|
|
|
|
763,492
|
|
|
|
763,492
|
|
|
Unrealized loss on
available-for-sale
securities, net of tax
|
|
|
—
|
|
|
|
|
|
|
|
—
|
|
|
|
(6,594
|
)
|
|
|
—
|
|
|
|
(6,594
|
)
|
|
Total comprehensive income
|
|
|
—
|
|
|
|
(6,594
|
)
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance at December 31, 2007
|
|
|
1
|
|
|
|
756,898
|
|
|
€
|
25,565
|
|
|
€
|
(27,471
|
)
|
|
€
|
3,494,438
|
|
|
€
|
3,492,532
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The accompanying notes are an integral part of the financial
statements.
F-47
IMEREM Institute
for Medical Research Management and Biometrics—
Institute für medizinisches Forschungsmanagement und
Biometrie—Ein unabhaengiges Forschungsunternehmen GmbH
Statement of Cash Flows
In Euros
| |
|
|
|
|
|
|
|
|
|
12 Months Ended
|
|
|
|
December 31, 2007
|
|
|
|
Operating activities
|
|
|
|
|
|
Net income
|
|
€
|
763,486
|
|
|
Adjustments to reconcile net income to net cash provided by
operating activities:
|
|
|
|
|
|
Depreciation and amortization
|
|
|
78,441
|
|
|
Deferred tax provision (benefit)
|
|
|
(269,000
|
)
|
|
Increase in cash value of life insurance
|
|
|
(120,153
|
)
|
|
Changes in operating assets and liabilities:
|
|
|
|
|
|
Accounts receivable
|
|
|
(551,859
|
)
|
|
Unbilled revenue
|
|
|
319,923
|
|
|
Prepaid expenses and other current assets
|
|
|
172,084
|
|
|
Accounts payable
|
|
|
8,226
|
|
|
Accrued expenses
|
|
|
188,354
|
|
|
Customer deposits
|
|
|
276,338
|
|
|
Deferred revenue
|
|
|
157,067
|
|
|
Deferred compensation
|
|
|
56,599
|
|
|
Other liabilities
|
|
|
57,732
|
|
|
|
|
|
|
|
|
Net cash provided by operating activities
|
|
|
1,137,244
|
|
|
Investing activities
|
|
|
|
|
|
Change in restricted cash
|
|
|
(276,338
|
)
|
|
Proceeds from sale of investments
|
|
|
476,930
|
|
|
Purchase of intangible assets
|
|
|
(37,504
|
)
|
|
Purchase of property and equipment
|
|
|
(30,424
|
)
|
|
|
|
|
|
|
|
Net cash provided by investing activities
|
|
|
132,664
|
|
|
Financing activities
|
|
|
|
|
|
Principal payment on long-term debt
|
|
|
(119,522
|
)
|
|
Dividends
|
|
|
(70,000
|
)
|
|
|
|
|
|
|
|
Net cash used in financing activities
|
|
|
(189,522
|
)
|
|
Net change in cash
|
|
|
1,080,386
|
|
|
Cash and cash equivalents, beginning of period
|
|
|
577,082
|
|
|
|
|
|
|
|
|
Cash and cash equivalents, end of period
|
|
€
|
1,657,468
|
|
|
|
|
|
|
|
|
|
The accompanying notes are an integral part of the financial
statements.
F-48
IMEREM Institute
for Medical Research Management and Biometrics—
Institute für medizinisches Forschungsmanagement und
Biometrie—
Ein unabhaengiges Forschungsunternehmen GmbH
Notes to Financial Statements
December 31, 2007
1. Nature of
Business
IMEREM Institute for Medical Research Management and
Biometrics—Institute für medizinisches
Forschungsmanagement und Biometrie—Ein unabhaengiges
Forschungsunternehmen GmbH (the “Company” or
“Imerem”) is a Clinical Resource Organization (CRO),
providing high-quality, efficient and flexible clinical
development solutions to the pharmaceutical industry. The
Company is able to leverage its high degree of clinical
expertise, industry knowledge and specialization to reduce the
expense and time frame of clinical development. The
Company’s revenues are generated principally from customers
located in Germany, while it does support clients and perform
services in several European countries.
2. Significant
Accounting Policies
Use of
Estimates
The preparation of financial statements in conformity with
accounting principles generally accepted in the United States
requires management to make estimates and assumptions that
affect the amounts reported in the financial statements and
accompanying notes. Actual results could differ from those
estimates.
Cash and
Cash Equivalents
Cash and cash equivalents include highly liquid investments with
an original maturity of three months or less from date of
purchase.
Restricted
Cash
The Company receives cash in advance from certain customers
specifically for the payment of investigator fees relating to
specific projects. Such amounts are recorded in restricted cash
and short term customer deposits in the accompanying
consolidated balance sheets.
Concentration
of Credit Risk
Financial instruments, which potentially subject the Company to
credit risk, consist principally of cash and accounts
receivable. The Company performs periodic evaluations of the
financial institutions in which its cash is invested. The
majority of the Company’s revenues and accounts receivable
are derived from pharmaceutical companies located in Germany.
The Company’s four largest customers accounted for
approximately 29%, 29%, 20% and 11% of service revenue during
the year ended December 31, 2007.
The four largest customers represented approximately 35%, 12%,
20% and 8% of the accounts receivable balance at
December 31, 2007. No other customers represented more than
10% of net service revenue or accounts receivable during the
period. The Company provides an allowance for doubtful accounts
based on experience and specifically identified risks. Accounts
receivable are carried at amounts due from customers and charged
off against the allowance for doubtful accounts when management
determines that recovery is unlikely and the Company ceases
collection efforts. At December 31, 2007, the allowance for
doubtful accounts totaled approximately €11,000.
Marketable
Securities
The Company determines the appropriate classification of its
investments in equity securities at the time of purchase and
reevaluates such determinations at each balance sheet date.
Marketable equity securities are classified
F-49
as available for sale, and are carried at fair market value,
with the unrealized gains and losses, net of tax, included in
the determination of comprehensive income and reported in
stockholder’s equity.
Property
and Equipment
Property and equipment are recorded at cost. Expenditures for
repairs and maintenance which do not extend the useful life of
the related assets are charged to expense as incurred.
Depreciation is computed using the straight-line method over the
estimated useful lives of the assets ranging from 1 to
36 years.
Revenue
and Cost Recognition
The majority of the Company’s service revenue is derived
from
fee-for-service
contracts, some of which are fixed-price contracts. Revenues and
the related costs of
fee-for-service
contracts are recognized in the period in which services are
performed. Fixed-price contract revenue is recognized as
services are performed. The Company measures progress for fixed
price contracts using the concept of proportional performance
based upon a unit-based output method. Under the unit-based
output method, output units are pre-defined in the contract and
revenue is recognized based upon completion of such output
units. Revenue related to contract modifications is recognized
when realization is assured and the amounts are reasonably
determinable. Adjustments to contract estimates are made in the
periods in which the facts that require the revisions become
known. When the revised estimate indicates a loss, such loss is
provided for in the financial statements during that period. No
such losses were recognized in 2007. Deferred revenue represents
amounts billed to customers in excess of revenue recognized.
Value
Added Taxes
The Company accounts for value added taxes as a net component of
selling, general, and administrative expenses in accordance with
EITF 06-03,
“How Taxes Collected from Customers and Remitted to
Governmental Authorities Should be Presented in the Income
Statement.”
Comprehensive
Income
The Company’s comprehensive income was as follows (in
Euros):
| |
|
|
|
|
|
|
|
Year
|
|
|
|
Ended
|
|
|
|
December 31,
|
|
|
|
2007
|
|
|
|
Net income as reported
|
|
€
|
763,492
|
|
|
Other comprehensive income (loss):
|
|
|
|
|
|
Unrealized loss on
available-for-sale
securities, net of tax
|
|
|
(6,594
|
)
|
|
|
|
|
|
|
|
Comprehensive income
|
|
€
|
756,898
|
|
|
|
|
|
|
|
Income
Taxes
Deferred taxes are provided on a liability method whereby
deferred tax assets are recognized for deductible temporary
differences and operating loss and tax credit carryforwards and
deferred tax liabilities are recognized for taxable temporary
differences. Temporary differences are the differences between
the reported amounts of assets and liabilities and their tax
bases. Deferred tax assets are reduced by a valuation allowance
when, in the opinion of management, it is more likely than not
that some portion or all of the deferred tax assets will not be
realized. Deferred tax assets and liabilities are adjusted for
the effects of changes in tax laws and rates on the date of
enactment.
In July 2006, the Financial Accounting Standards Board
(“FASB”) issued Interpretation No. 48, Accounting
for Uncertainty in Income Taxes—an interpretation of FASB
Statement 109 (FIN 48). FIN 48 clarifies the
accounting for uncertainty in income taxes recognized in an
enterprise’s financial statements in accordance with
Statement No. 109, Accounting for Income Taxes. FIN 48
prescribes a recognition threshold and measurement attribute for
F-50
the financial statement recognition and measurement of a tax
position taken or expected to be taken in a tax return.
FIN 48 also provides guidance on derecognition of tax
benefits, classification on the balance sheet, interest and
penalties, accounting in interim periods, disclosure, and
transition.
In December 2008, the FASB provided for a deferral of the
effective date of FIN 48 for certain nonpublic enterprises
to annual financial statements for fiscal years beginning after
December 15, 2008. The Company has elected this deferral
and accordingly will be required to adopt FIN 48 in its
2009 annual financial statements. Prior to adoption of
FIN 48, the Company will continue to evaluate its uncertain
tax positions and related income tax contingencies under
Statement No. 5, Accounting for Contingencies.
SFAS No. 5 requires the Company to accrue for losses
it believes are probable and can be reasonably estimated. The
Company does not expect the adoption of FIN 48 to have a
material impact on its financial condition or results of
operations.
Recently
Issued Accounting Pronouncements
In September 2006 the FASB issued Statement of Financial
Accounting Standards No. 157, “Fair Value Measurements
(SFAS No. 157).” SFAS No. 157 defines
fair value, establishes a framework for measuring fair value,
and expands disclosures about fair value measurement.
SFAS No. 157 also emphasizes that fair value is a
market-based measurement, not an entity-specific measurement,
and sets out a fair value hierarchy with the highest priority
being quoted prices in active markets. Under
SFAS No. 157, fair value measurements are disclosed by
level within that hierarchy. In February 2008, the FASB issued
FASB Staff Position
No. 157-2,
“Effective Date of FASB Statement No. 157,” which
permits a one-year deferral for the implementation of
SFAS No. 157 with regard to nonfinancial assets and
liabilities that are not recognized or disclosed at fair value
in the financial statements on a recurring basis. The Company
adopted SFAS No. 157 for the fiscal year beginning
January 1, 2008, except for nonfinancial assets and
nonfinancial liabilities that are recognized or disclosed at
fair value in the financial statements on a nonrecurring basis
for which delayed application is permitted until our fiscal year
beginning January 1, 2009. The Company is currently
assessing the potential effect of the adoption of the remaining
provisions of SFAS No. 157 on its financial position,
results of operations and cash flows.
In February 2007, the FASB issued SFAS No. 159,
“The Fair Value Option for Financial Assets and Financial
Liabilities—Including an amendment of FASB Statement
No. 115,” or SFAS No. 159. This standard
permits, but does not require, all entities to choose to measure
eligible items at fair value at specified election dates. For
items for which the fair value option has been elected, an
entity would report unrealized gains and losses in earnings at
each subsequent reporting date. SFAS No. 159 is
effective as of the beginning of an entity’s first fiscal
year beginning after November 15, 2007. The Company is not
electing to adopt the provisions permitting the measurement of
eligible financial assets and liabilities at January 1,
2008 using the fair value option.
In June 2007, the FASB reached a consensus on EITF Issue
No. 07-03,
“Accounting for Nonrefundable Advance Payments for Goods or
Services to Be Used in Future Research and Development
Activities.”
EITF 07-03
requires companies to defer and capitalize, until the goods have
been delivered or the related services have been rendered,
non-refundable advance payments for goods that will be used or
services that will be performed in future research and
development activities.
EITF 07-03
is effective for fiscal years beginning after December 15,
2007. The Company does not expect
EITF 07-03
will have a material impact on its financial condition or
results of operations.
In December 2007, the FASB reached a consensus on EITF Issue
No. 07-01,
“Accounting for Collaborative Arrangements.”
EITF 07-01
defines collaborative arrangements and establishes reporting
requirements for transactions between participants in a
collaborative arrangement and between participants in the
arrangement and third parties.
EITF 07-01
also establishes the appropriate income statement presentation
and classification for joint operating activities and payments
between participants, as well as the required disclosures
related to these arrangements.
EITF 07-01
is effective for fiscal years beginning after December 15,
2008. The Company does not expect
EITF 07-01
will have a material impact on its financial condition or
results of operations.
In December 2007, the FASB issued SFAS No. 141
(revised 2007), “Business Combinations,” or
SFAS 141(R). FAS 141(R) expands the definition of a
business and a business combination, requires that: the purchase
price of an acquisition, including the issuance of equity
securities to be determined on the acquisition date, be recorded
at fair value at the acquisition date; all assets, liabilities,
contingent consideration, contingencies and in-process
F-51
research and development costs of an acquired business be
recorded at fair value at the acquisition date; acquisition
costs generally be expensed as incurred; restructuring costs
generally be expensed in periods subsequent to the acquisition
date; and changes be made in accounting for deferred tax asset
valuation allowances and acquired income tax uncertainties after
the measurement period to impact income tax expense.
SFAS 141(R) applies prospectively to business combinations
for which the acquisition date is on or after the beginning of
the first annual reporting period beginning on or after
December 15, 2008. The Company does not expect
SFAS 141(R) will have a material impact on its financial
condition or results of operations.
In December 2007, the FASB issued SFAS No. 160,
“Noncontrolling Interests in Consolidated Financial
Statements—an amendment of ARB No. 51,” or
SFAS 160. SFAS 160 amends ARB 51 to establish
accounting and reporting standards for the noncontrolling
interest in a subsidiary and for the deconsolidation of a
subsidiary. An ownership interest in subsidiaries held by
parties other than the parent should be presented in the
consolidated statement of financial position within equity, but
separate from the parent’s equity. SFAS 160 requires
that changes in a parent’s ownership interest while the
parent retains its controlling financial interest in its
subsidiary should be accounted for similarly to equity
transactions. When a subsidiary is deconsolidated, any retained
noncontrolling equity investment should be initially measured at
fair value, with any gain or loss recognized in earnings.
SFAS 160 requires consolidated net income to be reported at
amounts that include the amounts attributable to both the parent
and the noncontrolling interest. It also requires disclosure, on
the face of the consolidated income statement, of the amounts of
consolidated net income attributable to the parent and to the
noncontrolling interests. SFAS 160 is effective for fiscal
years, including interim periods within those fiscal years,
beginning on or after December 15, 2008. The Company does
not expect SFAS 160 will have a material impact on its
financial condition or results of operations.
3. Property
and Equipment
Property and equipment consist of the following (in Euros):
| |
|
|
|
|
|
|
|
|
|
|
|
December 31,
|
|
|
|
Useful life
|
|
2007
|
|
|
|
Buildings
|
|
36 years
|
|
€
|
586,009
|
|
|
Furniture and fixtures
|
|
1-15 years
|
|
|
114,567
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
700,576
|
|
|
Less accumulated depreciation
|
|
|
|
|
(41,143
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
€
|
659,433
|
|
|
|
|
|
|
|
|
|
4. Marketable
Securities
Available-for-sale
securities consist primarily of debt securities that are due
within one to three years. Net unrealized holding losses on
available-for-sale
securities in the amount of €9,594, net of tax of
€3,000 for the year ended December 31, 2007 have been
included in accumulated other comprehensive income. Proceeds
from the sale of
available-for-sale
securities totaled €476,930 for the year ended
December 31, 2007. The specific identification method is
used to measure the cost of securities sold. There were no
realized gains or losses on those sales.
The following is a summary of investments in
available-for-sale
debt securities (in Euros):
| |
|
|
|
|
|
|
|
Year Ended
|
|
|
|
December 31,
|
|
|
|
2007
|
|
|
|
Amortized Cost
|
|
€
|
1,136,000
|
|
|
Gross unrealized losses
|
|
|
(40,471
|
)
|
|
|
|
|
|
|
|
Fair value
|
|
€
|
1,095,529
|
|
|
|
|
|
|
|
F-52
5. Long Term
Debt
The Company has a term note payable to a bank expiring
March 31, 2012. The note is collateralized by a building,
is guaranteed by the sole shareholder of the Company and bears
interest at 5.25%. The balance of the note was paid in full in
July 2007.
The Company also has a term note payable to a bank expiring
January 31, 2014. The note is collateralized by a building,
is guaranteed by the sole shareholder of the Company and bears
interest at 5.20% At December 31, 2007 the outstanding
balance on this note totaled €157,340.
Aggregate maturities of long-term debt for the years ending
December 31, 2008 through 2013 and beyond are as follows
(in Euros):
| |
|
|
|
|
|
|
|
2008
|
|
€
|
21,354
|
|
|
2009
|
|
|
23,405
|
|
|
2010
|
|
|
24,651
|
|
|
2011
|
|
|
25,964
|
|
|
2012
|
|
|
27,364
|
|
|
2013 and beyond
|
|
|
34,602
|
|
|
|
|
|
|
|
|
|
|
€
|
157,340
|
|
|
Current maturities
|
|
|
21,354
|
|
|
|
|
|
|
|
|
|
|
€
|
135,986
|
|
|
|
|
|
|
|
6. Retirement
Plan
The Company maintains a defined benefit retirement plan (Plan),
which covers only the Company’s Managing Director and sole
shareholder as defined in the Plan. Under the Plan, actuarial
calculations are performed annually by Bayern—Versicherung
to calculate the amount required to fund the Plan in order to
meet the terms of the guaranteed payout over the expected life
of the participant. This provision has been endowed with a life
insurance policy on the Company’s sole shareholder for the
full amount in accordance with all applicable local tax laws and
in accordance with the terms of the Plan as of December 31,
2007. The Company’s sole shareholder has a lien against the
life insurance policy in the event of non-payment of the amount
due under the retirement plan.
7. Income
Taxes
Income before income taxes consists of the following components
(in Euros):
| |
|
|
|
|
|
|
|
Year Ended
|
|
|
|
December 31,
|
|
|
|
2007
|
|
|
|
Income before income taxes
|
|
€
|
1,133,093
|
|
|
|
|
|
|
|
The provision for income taxes is as follows:
| |
|
|
|
|
|
|
|
Year Ended
|
|
|
|
December 31,
|
|
|
|
2007
|
|
|
|
Current:
|
|
€
|
638,601
|
|
|
Deferred:
|
|
|
(269,000
|
)
|
|
|
|
|
|
|
|
Total
|
|
€
|
369,601
|
|
|
|
|
|
|
|
Deferred income taxes reflect the net tax effects of temporary
differences between the carrying amounts of assets
F-53
and liabilities for financial reporting purposes and the amounts
used for income tax purposes. Significant components of the
Company’s deferred tax assets and liabilities are as
follows:
| |
|
|
|
|
|
|
|
Year Ended
|
|
|
|
December 31,
|
|
|
|
2007
|
|
|
|
Current deferred tax assets:
|
|
|
|
|
|
Customer deposits
|
|
€
|
115,000
|
|
|
Other
|
|
|
23,000
|
|
|
|
|
|
|
|
|
|
|
|
138,000
|
|
|
Gross deferred tax liabilities:
|
|
|
|
|
|
Unbilled/deferred revenues
|
|
|
279,000
|
|
|
Accrued expenses
|
|
|
35,000
|
|
|
Other
|
|
|
8,000
|
|
|
|
|
|
|
|
|
|
|
|
322,000
|
|
|
|
|
|
|
|
|
Net deferred tax liabilities
|
|
€
|
(184,000
|
)
|
|
|
|
|
|
|
|
Presented in the accompanying balance sheet as follows:
|
|
|
|
|
|
Long-term deferred tax assets
|
|
€
|
9,000
|
|
|
Current deferred tax liabilities
|
|
|
(193,000
|
)
|
|
Long-term deferred tax liabilities
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
€
|
(184,000
|
)
|
|
|
|
|
|
|
8. Commitments
The Company uses certain equipment under various operating
leases.
Future minimum lease payments subsequent to December 31,
2007 under non-cancelable operating leases are as follows (in
Euros):
| |
|
|
|
|
|
|
|
Operating
|
|
|
|
Leases
|
|
|
|
2008
|
|
€
|
42,462
|
|
|
2009
|
|
|
26,146
|
|
|
2010
|
|
|
9,382
|
|
|
|
|
|
|
|
|
Total minimum lease payments
|
|
€
|
77,990
|
|
|
|
|
|
|
|
Lease expense is included in selling, general, and
administrative expenses in the accompanying statements of income
and totaled approximately €49,500 for the year ended
December 31, 2007.
9. Related
Party Transactions
During 2007, the Company extended several interest free loans to
other companies, where the sole stockholder of the Company was
also a stockholder. The loans totaled approximately €70,000
and have been recorded as dividends in the accompanying
financial statements.
In September 2005, the sole stockholder of the Company loaned
the Company €350,000. During 2007, the sole stockholder of
the Company made various advances to the Company which have been
added to the balance due to stockholder. Interest accrues on the
entire balance due to stockholder at a rate of 4% and has been
added to the balance due to stockholder. Interest expense
related to the loans and advances included in the accompanying
F-54
statement of income totaled €22,908 for the year ended
December 31, 2007. The loan and advances are due on demand.
10. Cash
Flows Information
Supplemental information relative to the statements of cash
flows for the year ended December 31, 2007 is as follows
(in Euros):
| |
|
|
|
|
|
|
|
Year Ended
|
|
|
|
December 31,
|
|
|
|
2007
|
|
|
|
Supplemental disclosures of cash flows information:
|
|
|
|
|
|
Cash payments for:
|
|
|
|
|
|
Interest
|
|
€
|
11,437
|
|
|
|
|
|
|
|
|
Taxes
|
|
€
|
321,129
|
|
|
|
|
|
|
|
11. Subsequent
Events
On December 22, 2008 the stockholder of Imerem sold 100% of
his interest in the Company to an unrelated party.
F-55
IMEREM Institute
for Medical Research Management and Biometrics—
Institute für medizinisches Forschungsmanagement und
Biometrie—Ein unabhaengiges Forschungsunternehmen GmbH
Unaudited Condensed Balance
Sheet
In Euros
| |
|
|
|
|
|
|
|
|
|
September 30, 2008
|
|
|
|
(unaudited)
|
|
|
|
Assets
|
|
|
|
|
|
Current assets:
|
|
|
|
|
|
Cash and cash equivalents
|
|
€
|
1,480,833
|
|
|
Restricted cash
|
|
|
251,842
|
|
|
Unbilled revenues
|
|
|
766,682
|
|
|
Accounts receivable, less allowance for doubtful accounts
|
|
|
1,224,583
|
|
|
Prepaid expenses and other current assets
|
|
|
130,679
|
|
|
|
|
|
|
|
|
Total current assets
|
|
|
3,854,619
|
|
|
Intangible assets, net
|
|
|
56,004
|
|
|
Property and equipment, net
|
|
|
640,041
|
|
|
Cash surrender value of life insurance
|
|
|
725,720
|
|
|
|
|
|
|
|
|
Total assets
|
|
€
|
5,276,384
|
|
|
|
|
|
|
|
|
Liabilities and stockholders’ equity
|
|
|
|
|
|
Current liabilities:
|
|
|
|
|
|
Accounts payable
|
|
€
|
171,610
|
|
|
Accrued expenses
|
|
|
248,746
|
|
|
Customer deposits
|
|
|
251,842
|
|
|
Deferred revenue
|
|
|
537,429
|
|
|
Due to stockholder
|
|
|
454,183
|
|
|
Total current liabilities
|
|
|
1,663,810
|
|
|
Deferred compensation
|
|
|
612,078
|
|
|
Deferred income taxes—long term
|
|
|
188,386
|
|
|
Long term debt
|
|
|
135,986
|
|
|
|
|
|
|
|
|
Total liabilities
|
|
|
2,600,260
|
|
|
Stockholders’ equity:
|
|
|
|
|
|
Common stock
|
|
|
25,565
|
|
|
Comprehensive income
|
|
|
(27,471
|
)
|
|
Retained earnings
|
|
|
2,678,030
|
|
|
|
|
|
|
|
|
Total stockholders’ equity
|
|
|
2,676,124
|
|
|
|
|
|
|
|
|
Total liabilities and stockholders’ equity
|
|
€
|
5,276,384
|
|
|
|
|
|
|
|
|
|
The accompanying notes are an integral part of the financial
statements.
F-56
IMEREM Institute
for Medical Research Management and Biometrics—
Institute für medizinisches Forschungsmanagement und
Biometrie—Ein unabhaengiges Forschungsunternehmen GmbH
Unaudited Condensed Statements of
Income
In Euros
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Nine Months Ended September 30,
|
|
|
|
2008
|
|
2007
|
|
|
|
(unaudited)
|
|
|
|
Service revenue
|
|
€
|
2,551,850
|
|
|
€
|
3,022,790
|
|
|
Other Income
|
|
|
36,788
|
|
|
|
119,193
|
|
|
|
|
|
|
|
|
|
|
|
|
Total revenue
|
|
|
2,588,638
|
|
|
|
3,141,983
|
|
|
Direct costs
|
|
|
1,527,711
|
|
|
|
1,708,444
|
|
|
Selling, general, and administrative expenses
|
|
|
528,430
|
|
|
|
539,198
|
|
|
Depreciation and amortization
|
|
|
53,449
|
|
|
|
58,831
|
|
|
|
|
|
|
|
|
|
|
|
|
Income from operations
|
|
|
479,048
|
|
|
|
835,510
|
|
|
Interest expense
|
|
|
(32,000
|
)
|
|
|
(33,803
|
)
|
|
Interest income
|
|
|
99,820
|
|
|
|
44,647
|
|
|
Gain due to financial investments
|
|
|
—
|
|
|
|
3,462
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income before provision for income taxes
|
|
|
546,868
|
|
|
|
849,815
|
|
|
Provision for income taxes
|
|
|
207,810
|
|
|
|
277,201
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income
|
|
€
|
339,058
|
|
|
€
|
572,615
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The accompanying notes are an integral part of the financial
statements.
F-57
IMEREM Institute
for Medical Research Management and Biometrics—
Institute für medizinisches Forschungsmanagement und
Biometrie—Ein unabhaengiges Forschungsunternehmen GmbH
Unaudited Condensed Statements of
Cash Flows
In Euros
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Nine Months Ended September 30,
|
|
|
|
2008
|
|
2007
|
|
|
|
(unaudited)
|
|
|
|
Operating activities
|
|
|
|
|
|
|
|
|
|
Net income
|
|
€
|
339,058
|
|
|
€
|
572,615
|
|
|
Adjustments to reconcile net income to net cash used in
operating activities:
|
|
|
|
|
|
|
|
|
|
Depreciation and amortization
|
|
|
53,449
|
|
|
|
56,306
|
|
|
Deferred tax expense (benefit)
|
|
|
4,386
|
|
|
|
(272,000
|
)
|
|
Changes in operating assets and liabilities:
|
|
|
|
|
|
|
|
|
|
Accounts receivable
|
|
|
130,725
|
|
|
|
(551,859
|
)
|
|
Unbilled revenue
|
|
|
595,142
|
|
|
|
19,923
|
|
|
Increase in cash value of life insurance
|
|
|
—
|
|
|
|
56,599
|
|
|
Prepaid expenses and other current assets
|
|
|
(2,853
|
)
|
|
|
122,084
|
|
|
Other assets
|
|
|
(131,424
|
)
|
|
|
(21,029
|
)
|
|
Accounts payable
|
|
|
156,374
|
|
|
|
8,230
|
|
|
Accrued expenses
|
|
|
(56,707
|
)
|
|
|
(190,308
|
)
|
|
Customer deposits
|
|
|
(436,006
|
)
|
|
|
99,999
|
|
|
Deferred revenue
|
|
|
(122,425
|
)
|
|
|
57,067
|
|
|
Other liabilities
|
|
|
(623,149
|
)
|
|
|
53,838
|
|
|
|
|
|
|
|
|
|
|
|
|
Net cash used in operating activities
|
|
|
(93,430
|
)
|
|
|
(45,134
|
)
|
|
Investing activities
|
|
|
|
|
|
|
|
|
|
Change in restricted cash
|
|
|
436,007
|
|
|
|
(100,000
|
)
|
|
Change in short term investments
|
|
|
1,165,529
|
|
|
|
167,401
|
|
|
Change in intangible assets
|
|
|
(17,558
|
)
|
|
|
(207
|
)
|
|
Purchase of property and equipment
|
|
|
(34,057
|
)
|
|
|
(42,729
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Net cash provided by investing activities
|
|
|
1,549,921
|
|
|
|
24,465
|
|
|
Financing activities
|
|
|
|
|
|
|
|
|
|
Net repayments on lines of credit
|
|
|
—
|
|
|
|
(115,628
|
)
|
|
Dividends
|
|
|
(1,300,468
|
)
|
|
|
—
|
|
|
Shareholder loan
|
|
|
(332,658
|
)
|
|
|
22,908
|
|
|
|
|
|
|
|
|
|
|
|
|
Net cash used in financing activities
|
|
|
(1,633,126
|
)
|
|
|
(36,121
|
)
|
|
Net change in cash
|
|
|
(176,635
|
)
|
|
|
(63,384
|
)
|
|
Cash and cash equivalents, beginning of period
|
|
|
1,657,468
|
|
|
|
577,082
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents, end of period
|
|
€
|
1,480,833
|
|
|
€
|
513,698
|
|
|
|
|
|
|
|
|
|
|
|
|
Supplemental disclosures of cash flow information
|
|
|
|
|
|
|
|
|
|
Cash paid during the period for:
|
|
|
|
|
|
|
|
|
|
Income taxes
|
|
€
|
510,181
|
|
|
€
|
701,396
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The accompanying notes are an integral part of the financial
statements.
F-58
1. Nature of
Business
IMEREM Institute for Medical Research Management and
Biometrics—Institute für medizinisches
Forschungsmanagement und Biometrie—Ein unabhaengiges
Forschungsunternehmen GmbH (the “Company” or
“Imerem”) is a Clinical Resource Organization (CRO),
providing high-quality, efficient and flexible clinical
development solutions to the pharmaceutical industry. The
Company is able to leverage its high degree of clinical
expertise, industry knowledge and specialization to reduce the
expense and time frame of clinical development. The
Company’s revenues are generated principally from customers
located in Germany, while it does support clients and perform
services in several European countries.
2. Significant
Accounting Policies
Use of
Estimates
The preparation of financial statements in conformity with
accounting principles generally accepted in the
United States requires management to make estimates and
assumptions that affect the amounts reported in the financial
statements and accompanying notes. Actual results could differ
from those estimates.
Cash and
Cash Equivalents
Cash and cash equivalents include highly liquid investments with
an original maturity of three months or less from date of
purchase.
Restricted
Cash
The Company receives cash in advance from certain customers
specifically for the payment of investigator fees relating to
specific projects. Such amounts are recorded in restricted cash
and short term customer deposits in the accompanying
consolidated balance sheets.
Concentration
of Credit Risk
Financial instruments, which potentially subject the Company to
credit risk, consist principally of cash and accounts
receivable. The Company performs periodic evaluations of the
financial institutions in which its cash is invested. The
majority of the Company’s revenues and accounts receivable
are derived from pharmaceutical companies located in Germany.
The Company’s four largest customers accounted for
approximately 26%, 22%, 19% and 19% of service revenue during
the nine months ended September 30, 2008, the
Company’s four largest customers accounted for
approximately 29%, 29%, 20% and 11% of service revenue during
the nine months ended September 30, 2007.
The four largest customers represented approximately 29%, 20%,
10% and 9% of the accounts receivable balance at
September 30, 2008. No other customers represented more
than 10% of net service revenue or accounts receivable during
those periods or at those times. The Company provides an
allowance for doubtful accounts based on experience and
specifically identified risks. Accounts receivable are carried
at amounts due from customers and charged off against the
allowance for doubtful accounts when management determines that
recovery is unlikely and the Company ceases collection efforts.
During the periods contained within, the Company had not
identified any specific risks, and therefore no allowance for
doubtful accounts was booked.
F-59
Marketable
Securities
The Company determines the appropriate classification of its
investments in equity securities at the time of purchase and
reevaluates such determinations at each balance-sheet date
marketable equity securities are classified as available for
sale, and are carried at fair market value, with the unrealized
gains and losses, net of tax, included in the determination of
comprehensive income and reported in shareholders’ equity.
Property
and Equipment
Property and equipment are recorded at cost. Expenditures for
repairs and maintenance which do not extend the useful life of
the related assets are charged to expense as incurred.
Depreciation is computed using the straight-line method over the
estimated useful lives of the assets ranging from 1 to
36 years.
Revenue
and Cost Recognition
The majority of the Company’s service revenue is derived
from
fee-for-service
contracts, some of which are fixed-price contracts. Revenues and
the related costs of
fee-for-service
contracts are recognized in the period in which services are
performed. Fixed-price contract revenue is recognized as
services are performed. The Company measures progress for fixed
price contracts using the concept of proportional performance
based upon a unit-based output method. Under the unit-based
output method, output units are pre-defined in the contract and
revenue is recognized based upon completion of such output
units. Revenue related to contract modifications is recognized
when realization is assured and the amounts are reasonably
determinable. Adjustments to contract estimates are made in the
periods in which the facts that require the revisions become
known. When the revised estimate indicates a loss, such loss is
provided for in the financial statements during that period. No
such losses were recognized in 2008 or 2007. Deferred revenue
represents amounts billed to customers in excess of revenue
recognized. Accounts receivable from customers, which represent
deposits to be applied to customer invoices in future years or
returned to the customer upon expiration of the contract are
recorded in long term customer deposits.
Comprehensive
Income
The Company’s comprehensive income was as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
Nine Months Ended September 30,
|
|
|
|
2008
|
|
2007
|
|
|
|
Net income as reported
|
|
€
|
339,058
|
|
|
€
|
572,615
|
|
|
Other comprehensive loss:
|
|
|
|
|
|
|
|
|
|
Unrealized loss on
available-for-sale
securities, net of tax
|
|
|
—
|
|
|
|
(6,594
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Comprehensive income
|
|
€
|
339,058
|
|
|
€
|
566,021
|
|
|
|
|
|
|
|
|
|
|
|
Recently
Issued Accounting Pronouncements
See Note 2 to the audited financial statements for the
fiscal year ended December 31, 2007.
F-60
3. Property
and Equipment
Property and equipment consist of the following:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
September 30,
|
|
|
|
Useful life
|
|
2008
|
|
Buildings
|
|
|
36 years
|
|
|
€
|
586,009
|
|
|
Furniture and fixtures
|
|
|
1-15 years
|
|
|
|
109,437
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
695,446
|
|
|
Less accumulated depreciation
|
|
|
|
|
|
|
(55,405
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
€
|
640,041
|
|
|
|
|
|
|
|
|
|
|
|
4. Long Term
Debt
The Company has a term note payable to a bank expiring
March 31, 2012. The note is secured by a building, is
guaranteed by the sole shareholder of the Company and bears
interest at 5.25%. The balance of the note was paid in full in
July 2007.
The Company also has a term note payable to a bank expiring
January 31, 2014. The note is collateralized by a building,
is guaranteed by a the sole shareholder of the Company and bears
interest at 5.20% At December 31, 2007, the outstanding
balance on this note totaled €157,340.
Aggregate maturities of long-term debt for the years ending
December 31, 2008 through 2013 and beyond are as follows
(in Euros):
| |
|
|
|
|
|
|
|
2008
|
|
€
|
21,354
|
|
|
2009
|
|
|
23,405
|
|
|
2010
|
|
|
24,651
|
|
|
2011
|
|
|
25,964
|
|
|
2012
|
|
|
27,364
|
|
|
2013 and beyond
|
|
|
34,602
|
|
|
|
|
|
|
|
|
|
|
€
|
157,340
|
|
|
Current maturities
|
|
|
21,354
|
|
|
|
|
|
|
|
|
|
|
€
|
135,986
|
|
|
|
|
|
|
|
5. Retirement
Plan
The Company maintains a defined benefit retirement plan (Plan),
which covers only the Company’s Managing Director and sole
shareholder as defined in the Plan. Under the Plan, actuarial
calculations are performed annually by Bayern—Versicherung
to calculate the amount required to fund the Plan in order to
meet the terms of the guaranteed payout over the expected life
of the participant. This provision has been endowed with a life
insurance policy on the Company’s sole shareholder for the
full amount in accordance with all applicable local tax laws and
in accordance with the terms of the Plan as of December 31,
2007. The Company’s sole shareholder has a lien against the
life insurance policy in the event of non-payment of the amount
due under the retirement plan.
F-61
6. Commitments
and Contingencies
The Company uses certain equipment under various operating
leases.
Future minimum lease payments subsequent to December 31,
2007 under non-cancelable operating leases are as follows:
| |
|
|
|
|
|
|
|
Operating
|
|
|
|
Leases
|
|
|
|
2008
|
|
€
|
10,661
|
|
|
2009
|
|
|
26,146
|
|
|
2010
|
|
|
9,382
|
|
|
|
|
|
|
|
|
Total minimum lease payments
|
|
€
|
46,189
|
|
|
|
|
|
|
|
7. Subsequent
Events
On December 22, 2008 the shareholder of Imerem sold 100% of
his interest in the Company to an unrelated party.
F-62
Independent
Auditor’s Report
The Board of Directors and Stockholders
Infociencia S.L. and Infociencia Clinical Research S.L.
We have audited the accompanying combined balance sheet of
Infociencia S.L. and Infociencia Clinical Research S.L. as of
December 31, 2007, and the related combined statements of
operations, stockholders equity and cash flows for the year then
ended. These financial statements are the responsibility of the
Company’s management. Our responsibility is to express an
opinion on these financial statements based on our audit.
We conducted our audit in accordance with auditing standards
generally accepted in the United States of America. Those
standards require that we plan and perform the audit to obtain
reasonable assurance about whether the financial statements are
free of material misstatement. An audit includes examining, on a
test basis, evidence supporting the amounts and disclosures in
the financial statements. An audit also includes assessing the
accounting principles used and significant estimates made by
management, as well as evaluating the overall financial
statement presentation. We believe that our audit provides a
reasonable basis for our opinion.
In our opinion, the financial statements referred to above
present fairly, in all material respects, the combined financial
position of Infociencia S.L. and Infociencia Clinical Research
S.L. as of December 31, 2007, and the combined results of
their operations and their cash flows for the year then ended,,
in conformity with accounting principles generally accepted in
the United States.
/s/ McGladrey & Pullen, LLP
Chicago, Illinois
March 9, 2009
F-63
Infociencia S.L.
and Infociencia Clinical Research S.L.
In
Euros
| |
|
|
|
|
|
|
|
|
|
December 31,
|
|
|
|
2007
|
|
|
|
Assets
|
|
|
|
|
|
Current assets:
|
|
|
|
|
|
Cash and cash equivalents
|
|
€
|
352,501
|
|
|
Restricted cash
|
|
|
4,586,862
|
|
|
Available for sale securities
|
|
|
256,772
|
|
|
Accounts receivable
|
|
|
3,100,389
|
|
|
Grant receivable
|
|
|
1,559,135
|
|
|
Income tax receivable
|
|
|
32,866
|
|
|
Due from stockholders
|
|
|
300,000
|
|
|
Prepaid expenses and other current assets
|
|
|
18,074
|
|
|
|
|
|
|
|
|
Total current assets
|
|
|
10,206,599
|
|
|
Intangible assets
|
|
|
11,839
|
|
|
Equipment, net
|
|
|
184,442
|
|
|
Other current assets
|
|
|
20,204
|
|
|
|
|
|
|
|
|
Total assets
|
|
€
|
10,423,084
|
|
|
|
|
|
|
|
|
Liabilities and stockholders’ equity
|
|
|
|
|
|
Current liabilities:
|
|
|
|
|
|
Accounts payable
|
|
|
2,242,577
|
|
|
Customer deposits
|
|
|
4,025,516
|
|
|
Accrued expenses
|
|
|
769,827
|
|
|
Deferred revenue
|
|
|
138,873
|
|
|
Other current liabilities
|
|
|
58,110
|
|
|
Total current liabilities
|
|
|
7,234,903
|
|
|
Grant debt
|
|
|
809,009
|
|
|
|
|
|
|
|
|
Total liabilities
|
|
|
8,043,912
|
|
|
Stockholders’ equity:
|
|
|
|
|
|
Common stock; €1 par value; 416,278 shares
authorized, issued and outstanding
|
|
|
416,278
|
|
|
Additional paid in capital
|
|
|
106,605
|
|
|
Retained earnings
|
|
|
1,856,289
|
|
|
|
|
|
|
|
|
Total stockholders’ equity
|
|
|
2,379,172
|
|
|
|
|
|
|
|
|
Total liabilities and stockholders’ equity
|
|
€
|
10,423,084
|
|
|
|
|
|
|
|
|
|
The accompanying notes are an integral part of the financial
statements.
F-64
Infociencia S.L.
and Infociencia Clinical Research S.L.
In
Euros
| |
|
|
|
|
|
|
|
|
|
12 Months Ended
|
|
|
|
December 31, 2007
|
|
|
|
Service revenue
|
|
€
|
6,176,375
|
|
|
Reimbursement revenue
|
|
|
6,817,885
|
|
|
Other revenue
|
|
|
654,666
|
|
|
|
|
|
|
|
|
Total revenue
|
|
|
13,648,926
|
|
|
Direct costs
|
|
|
3,054,183
|
|
|
Reimbursable
out-of-pocket
costs
|
|
|
6,817,885
|
|
|
Selling, general, and administrative expenses
|
|
|
2,522,329
|
|
|
Depreciation and amortization
|
|
|
70,587
|
|
|
|
|
|
|
|
|
Income from operations
|
|
|
1,263,942
|
|
|
Interest expense
|
|
|
1,412
|
|
|
Loss on disposal of assets
|
|
|
462
|
|
|
Realized loss on available for sale securities
|
|
|
107,797
|
|
|
Other income
|
|
|
156,262
|
|
|
|
|
|
|
|
|
Income before provision for income taxes
|
|
|
1,230,533
|
|
|
Provision for income taxes
|
|
|
288,597
|
|
|
|
|
|
|
|
|
Net income
|
|
€
|
941,936
|
|
|
|
|
|
|
|
|
|
The accompanying notes are an integral part of the financial
statements.
F-65
Infociencia S.L.
and Infociencia Clinical Research S.L.
In
Euros
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accumulated
|
|
|
|
|
|
|
|
|
|
|
|
Additional
|
|
Other
|
|
|
|
Total
|
|
|
|
Comprehensive
|
|
Common
|
|
Paid In
|
|
Comprehensive
|
|
Retained
|
|
Stockholders’
|
|
|
|
Income
|
|
Stock
|
|
Capital
|
|
Income (loss)
|
|
Earnings
|
|
Equity
|
|
|
|
Balance at January 1, 2007
|
|
€
|
—
|
|
|
€
|
416,278
|
|
|
€
|
106,605
|
|
|
€
|
9,470
|
|
|
€
|
1,103,592
|
|
|
€
|
1,635,945
|
|
|
|
|
|
|
|
|
|
|
Dividends
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(189,239
|
)
|
|
|
(189,239
|
)
|
|
Comprehensive income:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income
|
|
|
941,936
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
941,936
|
|
|
|
941,936
|
|
|
Other comprehensive income:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Unrealized gain on
available-for-sale
securities, net tax
|
|
|
(9,470
|
)
|
|
|
—
|
|
|
|
—
|
|
|
|
(9,470
|
)
|
|
|
—
|
|
|
|
(9,470
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Comprehensive income
|
|
|
932,466
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance at December 31, 2007
|
|
|
|
|
|
€
|
416,278
|
|
|
€
|
106,605
|
|
|
€
|
—
|
|
|
€
|
1,856,289
|
|
|
€
|
2,379,172
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The accompanying notes are an integral part of the financial
statements.
F-66
Infociencia S.L.
and Infociencia Clinical Research S.L.
In
Euros
| |
|
|
|
|
|
|
|
12 Months Ended
|
|
|
|
December 31, 2007
|
|
|
|
Operating activities
|
|
|
|
|
|
Net income
|
|
€
|
941,936
|
|
|
Adjustments to reconcile net income to net cash provided by
operating activities:
|
|
|
|
|
|
Depreciation and amortization
|
|
|
70,587
|
|
|
Realized loss on available for sale securities
|
|
|
107,797
|
|
|
Changes in operating assets and liabilities:
|
|
|
|
|
|
Accounts receivable
|
|
|
(547,135
|
)
|
|
Income taxes receivable
|
|
|
68,247
|
|
|
Grants receivable
|
|
|
(1,187,892
|
)
|
|
Prepaid expenses and other current assets
|
|
|
36,596
|
|
|
Other assets
|
|
|
(4,116
|
)
|
|
Accounts payable
|
|
|
213,426
|
|
|
Customer deposits
|
|
|
913,958
|
|
|
Accrued expenses
|
|
|
242,795
|
|
|
Deferred revenue
|
|
|
(3,599
|
)
|
|
Other current liabilities
|
|
|
12,229
|
|
|
Other liabilities
|
|
|
250,942
|
|
|
|
|
|
|
|
|
Net cash provided by operating activities
|
|
|
1,115,771
|
|
|
Investing activities
|
|
|
|
|
|
Restricted cash
|
|
|
(915,238
|
)
|
|
Intangible assets
|
|
|
(1,697
|
)
|
|
Due from stockholders
|
|
|
(300,000
|
)
|
|
Purchase of equipment
|
|
|
(105,000
|
)
|
|
|
|
|
|
|
|
Net cash used in investing activities
|
|
|
(1,321,935
|
)
|
|
Financing activities
|
|
|
|
|
|
Dividends
|
|
|
(189,239
|
)
|
|
|
|
|
|
|
|
Net cash used in financing activities
|
|
|
(189,239
|
)
|
|
Net change in cash
|
|
|
(395,403
|
)
|
|
|
|
|
|
|
|
Cash and cash equivalents, beginning of period
|
|
€
|
747,904
|
|
|
|
|
|
|
|
|
Cash and cash equivalents, end of period
|
|
€
|
352,501
|
|
|
|
|
|
|
|
|
Supplemental disclosures of cash flow information
|
|
|
|
|
|
Cash paid during the period for:
|
|
|
|
|
|
Income taxes
|
|
€
|
163,930
|
|
|
|
|
|
|
|
|
|
F-67
Infociencia S.L.
and Infociencia Clinical Research S.L.
December 31,
2007
1. Nature of
Business
Infociencia S.L. and Infociencia Clinical Research S.L.
(referred to collectively as the “Company” or
“Infociencia”) are Clinical Resource
Organization’s (CRO’s), providing high-quality,
efficient and flexible clinical development solutions to the
pharmaceutical industry. The Company is able to leverage its
high degree of clinical expertise, industry knowledge and
specialization to reduce the expense and time frame of clinical
development. The Company’s revenues are generated
principally from customers located in Spain, while it does
support clients and perform services in several European
countries.
2. Significant
Accounting Policies
Use of
Estimates
The preparation of financial statements in conformity with
accounting principles generally accepted in the United States
requires management to make estimates and assumptions that
affect the amounts reported in the financial statements and
accompanying notes. Actual results could differ from those
estimates.
Cash and
Cash Equivalents
Cash and cash equivalents include highly liquid investments with
an original maturity of three months or less from date of
purchase.
Restricted
Cash
The Company receives cash in advance from certain customers
specifically for the payment of investigator fees relating to
specific projects. Such amounts are recorded in restricted cash
and short term customer deposits in the accompanying
consolidated balance sheets.
Concentration
of Credit Risk
Financial instruments, which potentially subject the Company to
credit risk, consist principally of cash and accounts
receivable. The Company performs periodic evaluations of the
financial institutions in which its cash is invested. The
majority of the Company’s revenues and accounts receivable
are derived from pharmaceutical companies located in Spain. The
Company’s two largest customers accounted for approximately
17% and 14% of service revenue during the year ended
December 31, 2007.
The two largest customers represented approximately 7% and 0% of
the accounts receivable balance at December 31, 2007 while
three other customers represented approximately 47%, 16% and 15%
of the accounts receivable balance at December 31, 2007. No
other customers represented more than 10% of net service revenue
or accounts receivable during the period. The Company provides
an allowance for doubtful accounts based on experience and
specifically identified risks. Accounts receivable are carried
at amounts due from customers and charged off against the
allowance for doubtful accounts when management determines that
recovery is unlikely and the Company ceases collection efforts.
During the period contained within, the Company had not
identified any specific risks, and therefore no allowance for
doubtful accounts has been recorded.
Grant
Receivable
Grant receivables mainly include receivables from government
bodies related to research and development grants. When the
grants are approved, the Company records a receivable for the
total amount of the grant. Income is recognized upon receipt of
official communication from the public entity granting the
subsidy. The projects are
F-68
typically completed in the year the grants are received. When a
project overlaps a year-end, a reasonable average for the split
of work load is determined.
Principles
of Combination
The combined financial statements include the accounts of
Infociencia S.L and Infociencia Clinical Research S.L., which
are under common ownership. All significant intercompany
balances and transactions have been eliminated in combination.
Marketable
Securities
The Company determines the appropriate classification of its
investments in debt securities at the time of purchase and
reevaluates such determination at each-balance sheet date
Marketable debt securities are classified as available for sale,
and are carried at fair market value, with the unrealized gains
and losses, net of tax, included in the determination of
comprehensive income and reported in stockholders equity.
Equipment
Equipment is recorded at cost. Expenditures for repairs and
maintenance which do not extend the useful life of the related
assets are charged to expense as incurred. Depreciation is
computed using the straight-line method over the estimated
useful lives of the assets ranging from 1 to 10 years.
Revenue
and Cost Recognition
The majority of the Company’s service revenue is derived
from
fee-for-service
contracts, some of which are fixed-price contracts. Revenues and
the related costs of
fee-for-service
contracts are recognized in the period in which services are
performed. Fixed-price contract revenue is recognized as
services are performed. The Company measures progress for fixed
price contracts using the concept of proportional performance
based upon a unit-based output method. Under the unit-based
output method, output units are pre-defined in the contract and
revenue is recognized based upon completion of such output
units. Revenue related to contract modifications is recognized
when realization is assured and the amounts are reasonably
determinable. Adjustments to contract estimates are made in the
periods in which the facts that require the revisions become
known. When the revised estimate indicates a loss, such loss is
provided for in the financial statements during that period. No
such losses were recognized in 2007. Deferred revenue represents
amounts billed to customers in excess of revenue recognized.
Accounts receivable from customers, which represent deposits to
be applied to customer invoices in future years or returned to
the customer upon expiration of the contract are recorded in
long term customer deposits.
In connection with the management of clinical trials, the
Company pays, on behalf of its clients, fees to investigators
and test subjects as well as other
out-of-pocket
costs for items such as travel, printing, meetings and couriers.
The Company’s clients reimburse the Company for these
costs. As required by
EITF 01-14,
amounts paid by the Company as a principal for
out-of-pocket
costs are included in direct costs as reimbursable
out-of-pocket
expenses and the reimbursements the Company receives as a
principal are reported as reimbursed
out-of-pocket
revenue. In the statements of operations, the Company combines
amounts paid by the Company as an agent for
out-of-pocket
costs with the corresponding reimbursements, or revenue, the
Company receives as an agent. During the year ended
December 31, 2007 fees paid to investigators and other fees
the Company paid as an agent and the associated reimbursements
were approximately €6,818,000.
Reporting
Currency
The reporting currency of the Company is the Euro.
Income
Taxes
The Company accounts for income taxes using an asset and
liability approach which requires the recognition of deferred
tax assets and liabilities for the expected future tax
consequences of temporary differences between the carrying
amount of assets and liabilities for financial reporting
purposes and amounts reportable for income tax
F-69
purposes. The Company recognizes deferred tax assets on
deductible temporary differences and deferred tax liabilities on
taxable temporary differences. Temporary differences are the
differences between the reported amounts of assets and
liabilities and their tax bases. As those differences reverse,
they will enter into the determination of future taxable income
included in the consolidated tax returns. Deferred tax assets
are reduced by a valuation allowance when, in the opinion of
management, it is more likely than not that some portion or all
of the deferred tax assets will not be realized. Deferred tax
assets and liabilities are adjusted for the effects of changes
in tax laws and rates on the date of enactment.
In July 2006, the Financial Accounting Standards Board
(“FASB”) issued Interpretation No. 48, Accounting
for Uncertainty in Income Taxes—an interpretation of FASB
Statement 109 (FIN 48). FIN 48 clarifies the
accounting for uncertainty in income taxes recognized in an
enterprise’s financial statements in accordance with
Statement No. 109, Accounting for Income Taxes. FIN 48
prescribes a recognition threshold and measurement attribute for
the financial statement recognition and measurement of a tax
position taken or expected to be taken in a tax return.
FIN 48 also provides guidance on derecognition of tax
benefits, classification on the balance sheet, interest and
penalties, accounting in interim periods, disclosure, and
transition.
In December 2008, the FASB provided for a deferral of the
effective date of FIN 48 for certain nonpublic enterprises
to annual financial statements for fiscal years beginning after
December 15, 2008. The Company has elected this deferral
and accordingly will be required to adopt FIN 48 in its
2009 annual financial statements. Prior to adoption of
FIN 48, the Company will continue to evaluate its uncertain
tax positions and related income tax contingencies under
Statement No. 5, Accounting for Contingencies.
SFAS No. 5 requires the Company to accrue for losses
it believes are probable and can be reasonably estimated. The
Company does not expect the adoption of FIN 48 to have a
material impact on its financial condition or results of
operations.
Recently
Issued Accounting Pronouncements
In September 2006 the FASB issued Statement of Financial
Accounting Standards No. 157, “Fair Value Measurements
(SFAS No. 157).” SFAS No. 157 defines
fair value, establishes a framework for measuring fair value,
and expands disclosures about fair value measurement.
SFAS No. 157 also emphasizes that fair value is a
market-based measurement, not an entity-specific measurement,
and sets out a fair value hierarchy with the highest priority
being quoted prices in active markets. Under
SFAS No. 157, fair value measurements are disclosed by
level within that hierarchy. In February 2008, the FASB issued
FASB Staff Position
No. 157-2,
“Effective Date of FASB Statement No. 157,” which
permits a one-year deferral for the implementation of
SFAS No. 157 with regard to nonfinancial assets and
liabilities that are not recognized or disclosed at fair value
in the financial statements on a recurring basis. The Company
adopted SFAS No. 157 for the fiscal year beginning
January 1, 2008, except for nonfinancial assets and
nonfinancial liabilities that are recognized or disclosed at
fair value in the financial statements on a nonrecurring basis
for which delayed application is permitted until our fiscal year
beginning January 1, 2009. The Company is currently
assessing the potential effect of the adoption of the remaining
provisions of SFAS No. 157 on its financial position,
results of operations and cash flows.
In February 2007, the FASB issued SFAS No. 159,
“The Fair Value Option for Financial Assets and Financial
Liabilities—Including an amendment of FASB Statement
No. 115,” or SFAS No. 159. This standard
permits, but does not require, all entities to choose to measure
eligible items at fair value at specified election dates. For
items for which the fair value option has been elected, an
entity would report unrealized gains and losses in earnings at
each subsequent reporting date. SFAS No. 159 is
effective as of the beginning of an entity’s first fiscal
year beginning after November 15, 2007. The Company is not
electing to adopt the provisions permitting the measurement of
eligible financial assets and liabilities at January 1,
2008 using the fair value option.
In June 2007, the FASB reached a consensus on EITF Issue
No. 07-03,
“Accounting for Nonrefundable Advance Payments for Goods or
Services to Be Used in Future Research and Development
Activities.”
EITF 07-03
requires companies to defer and capitalize, until the goods have
been delivered or the related services have been rendered,
non-refundable advance payments for goods that will be used or
services that will be performed in future research and
development activities.
EITF 07-03
is effective for fiscal years beginning after December 15,
2007. The Company does not expect
EITF 07-03
will have a material impact on its financial condition or
results of operations.
In December 2007, the FASB reached a consensus on EITF Issue
No. 07-01,
“Accounting for Collaborative
F-70
Arrangements.”
EITF 07-01
defines collaborative arrangements and establishes reporting
requirements for transactions between participants in a
collaborative arrangement and between participants in the
arrangement and third parties.
EITF 07-01
also establishes the appropriate income statement presentation
and classification for joint operating activities and payments
between participants, as well as the required disclosures
related to these arrangements.
EITF 07-01
is effective for fiscal years beginning after December 15,
2008. The Company does not expect
EITF 07-01
will have a material impact on its financial condition or
results of operations.
In December 2007, the FASB issued SFAS No. 141
(revised 2007), “Business Combinations,” or
SFAS 141(R). FAS 141(R) expands the definition of a
business and a business combination, requires that: the purchase
price of an acquisition, including the issuance of equity
securities to be determined on the acquisition date, be recorded
at fair value at the acquisition date; all assets, liabilities,
contingent consideration, contingencies and in-process research
and development costs of an acquired business be recorded at
fair value at the acquisition date; acquisition costs generally
be expensed as incurred; restructuring costs generally be
expensed in periods subsequent to the acquisition date; and
changes be made in accounting for deferred tax asset valuation
allowances and acquired income tax uncertainties after the
measurement period to impact income tax expense.
SFAS 141(R) applies prospectively to business combinations
for which the acquisition date is on or after the beginning of
the first annual reporting period beginning on or after
December 15, 2008. The Company does not expect
SFAS 141(R) will have a material impact on its financial
condition or results of operations.
In December 2007, the FASB issued SFAS No. 160,
“Noncontrolling Interests in Consolidated Financial
Statements—an amendment of ARB No. 51,” or
SFAS 160. SFAS 160 amends ARB 51 to establish
accounting and reporting standards for the noncontrolling
interest in a subsidiary and for the deconsolidation of a
subsidiary. An ownership interest in subsidiaries held by
parties other than the parent should be presented in the
consolidated statement of financial position within equity, but
separate from the parent’s equity. SFAS 160 requires
that changes in a parent’s ownership interest while the
parent retains its controlling financial interest in its
subsidiary should be accounted for similarly to equity
transactions. When a subsidiary is deconsolidated, any retained
noncontrolling equity investment should be initially measured at
fair value, with any gain or loss recognized in earnings.
SFAS 160 requires consolidated net income to be reported at
amounts that include the amounts attributable to both the parent
and the noncontrolling interest. It also requires disclosure, on
the face of the consolidated income statement, of the amounts of
consolidated net income attributable to the parent and to the
noncontrolling interests. SFAS 160 is effective for fiscal
years, including interim periods within those fiscal years,
beginning on or after December 15, 2008. The Company does
not expect SFAS 160 will have a material impact on its
financial condition or results of operations.
3.
Equipment
Equipment consists of the following (in Euros):
| |
|
|
|
|
|
|
|
|
|
Useful life
|
|
December 31, 2007
|
|
|
|
Furniture and fixtures
|
|
1 to 10 years
|
|
€
|
314,697
|
|
|
|
|
|
|
|
|
|
|
Less accumulated depreciation
|
|
|
|
|
(130,255
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
€
|
184,442
|
|
|
|
|
|
|
|
|
|
4. Marketable
Securities
Available-for-sale
securities consist primarily of debt securities that are due
within three to ten years. During 2007, the Company recognized
€ 107,797 of
other-than-temporary
impairment losses on
available-for-sale
securities. The impairment charges for the Marketable securities
were recognized in light of significant subsequent loss on sale
of securities in 2008. This created a new adjusted cost basis of
€256,772.
The following is a summary of investments in
available-for-sale
debt securities (in Euros):
| |
|
|
|
|
|
|
|
Year Ended
|
|
|
|
December 31, 2007
|
|
|
|
Amortized Cost
|
|
€
|
256,772
|
|
|
Fair value
|
|
€
|
256,772
|
|
F-71
5. Grant
Debt
The Company has numerous outstanding grant loans from government
institutions. These loans bear zero interest and have a two-year
grace period for payments. The Company is not required to pay
back the government as long as they submit qualifying expenses.
The aggregate amounts of remaining payments required on all
long-term grant debt at December 31, 2007, are as follows
(in Euros):
| |
|
|
|
|
|
2009
|
|
€
|
94,299
|
|
|
2010
|
|
|
94,299
|
|
|
2011
|
|
|
137,344
|
|
|
2012
|
|
|
137,344
|
|
|
2013
|
|
|
137,344
|
|
|
Thereafter
|
|
|
208,379
|
|
|
|
|
|
|
|
|
|
|
€
|
809,009
|
|
|
|
|
|
|
|
6.
Stockholder’s Equity
Components of stockholder’s equity at December 31,
2007 is presented below.
| |
|
|
|
|
|
|
|
Year Ended
|
|
|
|
December 31,
|
|
|
|
2007
|
|
|
|
Common stock, Infociencia SL:
|
|
|
|
|
|
€1 par value; authorized 398,278 shares;
|
|
€
|
398,278
|
|
|
issued and outstanding 398,278 shares
|
|
|
|
|
|
Common stock, Infociencia Clinical Research S.L.:
|
|
|
|
|
|
€1 par value; authorized 18,000 shares;
|
|
|
|
|
|
issued and outstanding 398,278 shares
|
|
|
18,000
|
|
|
|
|
|
|
|
|
|
|
€
|
416,278
|
|
|
|
|
|
|
|
|
Additional paid-in capital, Infociencia SL
|
|
€
|
106,605
|
|
|
|
|
|
|
|
|
Accumulated other comprehensive income, Infociencia SL
|
|
€
|
—
|
|
|
|
|
|
|
|
|
Retained Earnings:
|
|
€
|
1,856,289
|
|
|
|
|
|
|
|
7. Income
Taxes
Income before income taxes consists of the following component
(in Euros):
| |
|
|
|
|
|
|
|
Year Ended
|
|
|
|
December 31,
|
|
|
|
2007
|
|
|
|
Income before income taxes
|
|
€
|
1,230,533
|
|
|
|
|
|
|
|
The provision for income taxes is as follows (in Euros):
| |
|
|
|
|
|
|
|
Year Ended
|
|
|
|
December 31,
|
|
|
|
2007
|
|
|
|
Provision for income taxes
|
|
€
|
288,597
|
|
|
|
|
|
|
|
F-72
The income tax provision differs from the amount of income tax
determined by applying statutory federal income tax rate to
pretax income from continuing operations for the year ended
December 31, 2007, due to the following (in Euros):
| |
|
|
|
|
|
|
|
2007
|
|
|
|
Computed tax
|
|
€
|
430,687
|
|
|
Decrease in income taxes from tax credits
|
|
|
(142,090
|
)
|
|
|
|
|
|
|
|
Total
|
|
€
|
288,597
|
|
|
|
|
|
|
|
8.
Commitments
The Company uses certain equipment under various operating
leases.
Future minimum lease payments subsequent to December 31,
2007 under non-cancelable operating leases are as follows (in
Euros):
| |
|
|
|
|
|
|
|
Operating
|
|
|
|
Leases
|
|
|
|
2008
|
|
€
|
7,944
|
|
|
2009
|
|
|
9,468
|
|
|
2010
|
|
|
9,468
|
|
|
2011
|
|
|
9,468
|
|
|
2012
|
|
|
6,258
|
|
|
Thereafter
|
|
|
3,045
|
|
|
|
|
|
|
|
|
Total minimum lease payments
|
|
€
|
37,707
|
|
Rent expense under the non-cancelable operating leases for the
year ended December 31, 2007 was € 9,292.
In addition to the operating leases listed above, the Company
leases office space in two locations. The office space is leased
on a month to month agreement, and can be cancelled without
penalty at any time. Rent expense under such arrangements was
approximately € 99,715 during the year ended
December 31, 2007.
The Company is involved in various claims incidental to the
conduct of its business. Management does not believe that any
such claims to which the Company is a party, both individually
and in the aggregate, will have a material adverse effect on the
Company’s financial position or results of operations.
9. Related Party
Transactions (in Euros)
During November 2007, the Company extended three interest
bearing loans to the major stockholders of Infociencia. There
was a €100,000 loan made to each major shareholder at 5%
interest rate. The Company had earned interest income of
€2,500 in 2007. The loans are due in full in March 2008.
10. Subsequent
Events (in Euros)
During 2008, the Company sold 100% of its
available-for-sale
securities. The original costs of the securities were
€350,000, the adjusted cost at December 31, 2007 was
€256,772 and were sold for €214,200. The sale of the
securities resulted in a realized loss of €42,475 that will
be recognized in 2008.
In 2008, the €300,000 in stockholders loans were repaid to
the Company along with accrued interest.
On December 22, 2008 the stockholders of Infociencia S.L.
and Infociencia Clinical Research S.L. sold 100% of their
interest in the Company to an unrelated party.
F-73
Infociencia S.L.
and Infociencia Clinical Research S.L.
In
Euros
| |
|
|
|
|
|
|
|
|
|
September 30, 2008
|
|
|
|
(unaudited)
|
|
|
|
Assets
|
|
|
|
|
|
Current assets:
|
|
|
|
|
|
Cash and cash equivalents
|
|
€
|
1,266,585
|
|
|
Restricted cash
|
|
|
2,761,668
|
|
|
Accounts receivable, less allowance for doubtful accounts
|
|
|
1,899,314
|
|
|
Income tax receivable
|
|
|
606,835
|
|
|
Prepaid expenses and other current assets
|
|
|
16,969
|
|
|
|
|
|
|
|
|
Total current assets
|
|
|
6,551,372
|
|
|
Intangible assets
|
|
|
7,309
|
|
|
Equipment, net
|
|
|
656,178
|
|
|
Other non current assets
|
|
|
350,005
|
|
|
|
|
|
|
|
|
Total assets
|
|
€
|
7,564,865
|
|
|
|
|
|
|
|
|
Liabilities and stockholders’ equity
|
|
|
|
|
|
Current liabilities:
|
|
|
|
|
|
Accounts payable
|
|
|
3,105,269
|
|
|
Deferred revenue
|
|
|
2,761,668
|
|
|
|
|
|
|
|
|
Total current liabilities
|
|
|
5,866,937
|
|
|
Grant debt
|
|
|
812,621
|
|
|
|
|
|
|
|
|
Total liabilities
|
|
|
6,679,558
|
|
|
Stockholders’ equity:
|
|
|
|
|
|
Additional paid in capital
|
|
|
416,278
|
|
|
Accumulated other comprehensive loss
|
|
|
(65,988
|
)
|
|
Retained earnings
|
|
|
535,015
|
|
|
|
|
|
|
|
|
Total stockholders’ equity
|
|
|
885,305
|
|
|
|
|
|
|
|
|
Total liabilities and stockholders’ equity
|
|
€
|
7,564,863
|
|
|
|
|
|
|
|
The accompanying notes are an integral part of the financial
statements.
F-74
Infociencia S.L.
and Infociencia Clinical Research S.L.
In
Euros
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Nine Months Ended September 30,
|
|
|
|
2008
|
|
2007
|
|
|
|
(unaudited)
|
|
|
|
Service revenue
|
|
€
|
3,684,080
|
|
|
€
|
3,363,172
|
|
|
Reimbursement revenue
|
|
|
2,522,588
|
|
|
|
6,385,898
|
|
|
Other revenue
|
|
|
153,362
|
|
|
|
212,686
|
|
|
|
|
|
|
|
|
|
|
|
|
Total revenue
|
|
|
6,360,030
|
|
|
|
9,961,756
|
|
|
Direct costs
|
|
|
2,211,596
|
|
|
|
1,423,734
|
|
|
Reimbursable
out-of-pocket
costs
|
|
|
2,522,588
|
|
|
|
6,385,898
|
|
|
Selling, general, and administrative expenses
|
|
|
1,049,559
|
|
|
|
1,352,178
|
|
|
Depreciation and amortization
|
|
|
337,955
|
|
|
|
63,220
|
|
|
|
|
|
|
|
|
|
|
|
|
Income from operations
|
|
|
238,332
|
|
|
|
736,728
|
|
|
Other income
|
|
|
107,125
|
|
|
|
87,612
|
|
|
|
|
|
|
|
|
|
|
|
|
Income before provision for income taxes
|
|
|
345,457
|
|
|
|
824,340
|
|
|
Provision for income taxes
|
|
|
90,027
|
|
|
|
228,265
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income
|
|
€
|
255,430
|
|
|
€
|
596,075
|
|
|
|
|
|
|
|
|
|
|
|
The accompanying notes are an integral part of the financial
statements.
F-75
Infociencia S.L.
and Infociencia Clinical Research S.L.
In
Euros
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Nine Months Ended September 30,
|
|
|
|
2008
|
|
2007
|
|
|
|
(unaudited)
|
|
|
|
Net income
|
|
€
|
255,430
|
|
|
€
|
596,075
|
|
|
Adjustments to reconcile net income to net cash provided by
operating activities:
|
|
|
|
|
|
|
|
|
|
Depreciation and amortization
|
|
|
337,955
|
|
|
|
63,220
|
|
|
Changes in operating assets and liabilities:
|
|
|
|
|
|
|
|
|
|
Accounts receivable
|
|
|
1,201,075
|
|
|
|
1,562,239
|
|
|
Income taxes payable (receivable)
|
|
|
1,017,505
|
|
|
|
(1,143,261
|
)
|
|
Notes receivable
|
|
|
40,000
|
|
|
|
—
|
|
|
Prepaid expenses and other current assets
|
|
|
301,104
|
|
|
|
36,601
|
|
|
Other assets
|
|
|
(73,029
|
)
|
|
|
(4,446
|
)
|
|
Accounts payable
|
|
|
(1,219,623
|
)
|
|
|
137,548
|
|
|
Deferred revenue
|
|
|
(138,873
|
)
|
|
|
—
|
|
|
Other liabilities
|
|
|
3,615
|
|
|
|
294,229
|
|
|
|
|
|
|
|
|
|
|
|
|
Net cash provided by operating activities
|
|
|
1,725,159
|
|
|
|
1,542,205
|
|
|
Investing activities
|
|
|
|
|
|
|
|
|
|
Change in restricted cash
|
|
|
1,825,194
|
|
|
|
(250,080
|
)
|
|
Change in intangible assets
|
|
|
4,530
|
|
|
|
(11,636
|
)
|
|
Purchase of property and equipment
|
|
|
(809,691
|
)
|
|
|
(463,442
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Net cash provided by (used in) investing activities
|
|
|
1,020,033
|
|
|
|
(725,158
|
)
|
|
Financing activities
|
|
|
|
|
|
|
|
|
|
Dividends
|
|
|
(1,831,108
|
)
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
Net cash used in financing activities
|
|
|
(1,831,108
|
)
|
|
|
—
|
|
|
Net change in cash
|
|
|
914,084
|
|
|
|
817,047
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents, beginning of period
|
|
|
352,501
|
|
|
|
747,904
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents, end of period
|
|
€
|
1,266,585
|
|
|
€
|
1,564,951
|
|
|
|
|
|
|
|
|
|
|
|
|
Supplemental disclosures of cash flow information
|
|
|
|
|
|
|
|
|
|
Cash paid during the period for:
|
|
|
|
|
|
|
|
|
|
Income taxes
|
|
€
|
471,336
|
|
|
€
|
163,930
|
|
|
|
|
|
|
|
|
|
|
|
The accompanying notes are an integral part of the financial
statements.
F-76
Infociencia S.L.
and Infociencia Clinical Research S.L.
September 30,
2008
1. Nature of
Business
Infociencia S.L. and Infociencia Clinical Research S.L.
(referred to collectively as the “Company” or
“Infociencia”) are Clinical Resource
Organization’s (CROs), providing high-quality, efficient
and flexible clinical development solutions to the
pharmaceutical industry. The Company is able to leverage its
high degree of clinical expertise, industry knowledge and
specialization to reduce the expense and time frame of clinical
development. The Company’s revenues are generated
principally from customers located in Spain, while it does
support clients and perform services in several European
countries.
2. Significant
Accounting Policies
Use of
Estimates
The preparation of financial statements in conformity with
accounting principles generally accepted in the United States
requires management to make estimates and assumptions that
affect the amounts reported in the financial statements and
accompanying notes. Actual results could differ from those
estimates.
Cash and
Cash Equivalents
Cash and cash equivalents include highly liquid investments with
an original maturity of three months or less from date of
purchase.
Restricted
Cash
The Company receives cash in advance from certain customers
specifically for the payment of investigator fees relating to
specific projects. Such amounts are recorded in restricted cash
and short term customer deposits in the accompanying combined
balance sheets.
Concentration
of Credit Risk
Financial instruments, which potentially subject the Company to
credit risk, consist principally of cash and accounts
receivable. The Company performs periodic evaluations of the
financial institutions in which its cash is invested. The
majority of the Company’s revenues and accounts receivable
are derived from pharmaceutical companies located in France. The
Company’s two largest customers accounted for approximately
34% and 14% of service revenue during the nine months ended
September 30, 2008, the Company’s two largest
customers accounted for approximately 17% and 14% of service
revenue during the nine months ended September 30, 2007.
The two largest customers represented approximately 35% and 12%
of the accounts receivable balance at September 30, 2008.
No other customers represented more than 10% of net service
revenue or accounts receivable during those periods or at those
times. The Company provides an allowance for doubtful accounts
based on experience and specifically identified risks. Accounts
receivable are carried at fair value and charged off against the
allowance for doubtful accounts when management determines that
recovery is unlikely and the Company ceases collection efforts.
During the periods contained within, the Company had not
identified any specific risks, and therefore no allowance for
doubtful accounts was booked.
Principles
of Combination
The combined financial statements include the accounts of
Infociencia S.L. and Infociencia Clinical Research S.L. All
significant intercompany balances and transactions have been
eliminated in combination.
F-77
Equipment
Equipment is recorded at cost. Expenditures for repairs and
maintenance which do not extend the useful life of the related
assets are charged to expense as incurred. Depreciation is
computed using the straight-line method over the estimated
useful lives of the assets ranging from 1 to 10 years.
Revenue
and Cost Recognition
The majority of the Company’s service revenue is derived
from
fee-for-service
contracts, some of which are fixed-price contracts. Revenues and
the related costs of
fee-for-service
contracts are recognized in the period in which services are
performed. Fixed-price contract revenue is recognized as
services are performed. We measure progress for fixed price
contracts using the concept of proportional performance based
upon a unit-based output method. Under the unit-based output
method, output units are pre-defined in the contract and revenue
is recognized based upon completion of such output units.
Revenue related to contract modifications is recognized when
realization is assured and the amounts are reasonably
determinable. Adjustments to contract estimates are made in the
periods in which the facts that require the revisions become
known. When the revised estimate indicates a loss, such loss is
provided for in the financial statements during that period. No
such losses were recognized in 2008 or 2007. Deferred revenue
represents amounts billed to customers in excess of revenue
recognized. Accounts receivable from customers, which represent
deposits to be applied to customer invoices in future years or
returned to the customer upon expiration of the contract are
recorded in long term customer deposits.
In connection with the management of clinical trials, the
Company pays, on behalf of its clients, fees to investigators
and test subjects as well as other
out-of-pocket
costs for items such as travel, printing, meetings and couriers.
The Company’s clients reimburse the Company for these
costs. As required by
EITF 01-14,
amounts paid by the Company as a principal for
out-of-pocket
costs are included in direct costs as reimbursable
out-of-pocket
expenses and the reimbursements the Company receives as a
principal are reported as reimbursed
out-
of-pocket
revenue. In the statements of operations, the Company combines
amounts paid by the Company as an agent for
out-of-pocket
costs with the corresponding reimbursements, or revenue, the
Company receives as an agent. During the years ended
September 30, 2008 and 2007 fees paid to investigators and
other fees the Company paid as an agent and the associated
reimbursements were approximately €2,523,000 and
€6,386,000 respectively.
Income
Taxes
The Company accounts for income taxes using an asset and
liability approach which requires the recognition of deferred
tax assets and liabilities for the expected future tax
consequences of temporary differences between the carrying
amount of assets and liabilities for financial reporting
purposes and amounts reportable for income tax purposes.
Recently
Issued Accounting Pronouncements
See Note 2 to the audited financial statements for the
fiscal years ended December 31, 2007.
3.
Equipment
Equipment consist of the following:
| |
|
|
|
|
|
|
|
|
|
|
|
September 30,
|
|
|
|
Useful life
|
|
2008
|
|
|
|
Furniture and fixtures
|
|
1 to 10 years
|
|
€
|
792,081
|
|
|
|
|
|
|
|
|
|
|
Less accumulated depreciation
|
|
|
|
|
(135,903
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
€
|
656,178
|
|
|
|
|
|
|
|
|
|
F-78
4. Commitments
and Contingencies
The Company uses certain equipment under various operating
leases.
Future minimum lease payments subsequent to September 30,
2008 under non-cancelable operating leases are as follows:
| |
|
|
|
|
|
|
|
Operating Leases
|
|
|
|
2008
|
|
€
|
1,986
|
|
|
2009
|
|
|
9,468
|
|
|
2010
|
|
|
9,468
|
|
|
2011
|
|
|
6,258
|
|
|
2012
|
|
|
3,045
|
|
|
Thereafter
|
|
|
1,524
|
|
|
|
|
|
|
|
|
Total minimum lease payments
|
|
€
|
31,749
|
|
In addition to the operating leases listed above, the Company
leases office space in two locations. The office space is leased
on a month to month agreement, and can be cancelled without
penalty at any time. Rent expense under such arrangements was
€76,850 and €74,785 during the nine months ended
September 30, 2008 and 2007, respectively.
5. Subsequent
Events
On December 22, 2008 the shareholders of Infociencia sold
100% of their interest in the Company to an unrelated party.
F-79
Independent
Auditor’s Report
The Board of Directors and Stockholders
Therapharm Recherches Th. R.
We have audited the accompanying balance sheet of Therapharm
Recherches Th. R. as of December 31, 2007, and the related
statements of operations, stockholders’ equity and cash
flows for the year then ended. These financial statements are
the responsibility of the Company’s management. Our
responsibility is to express an opinion on these financial
statements based on our audit.
We conducted our audit in accordance with auditing standards
generally accepted in the United States of America. Those
standards require that we plan and perform the audit to obtain
reasonable assurance about whether the financial statements are
free of material misstatement. An audit includes examining, on a
test basis, evidence supporting the amounts and disclosures in
the financial statements. An audit also includes assessing the
accounting principles used and significant estimates made by
management, as well as evaluating the overall financial
statement presentation. We believe that our audit provides a
reasonable basis for our opinion.
In our opinion, the financial statements referred to above
present fairly, in all material respects, the financial position
of Therapharm Recherches Th. R. as of December 31, 2007,
and the results of its operations and its cash flows for the
year then ended, in conformity with accounting principles
generally accepted in the United States.
/s/ McGladrey & Pullen, LLP
Chicago, Illinois
March 9, 2009
F-80
Therapharm
Recherches Th. R.
In
Euros
| |
|
|
|
|
|
|
|
|
|
December 31, 2007
|
|
|
|
Assets
|
|
|
|
|
|
Current assets:
|
|
|
|
|
|
Cash and cash equivalents
|
|
€
|
764,746
|
|
|
Restricted cash
|
|
|
423,534
|
|
|
Accounts receivable
|
|
|
1,913,224
|
|
|
Unbilled revenue
|
|
|
648,949
|
|
|
Income tax receivable
|
|
|
182,335
|
|
|
Prepaid expenses and other current assets
|
|
|
182,145
|
|
|
|
|
|
|
|
|
Total current assets
|
|
|
4,114,933
|
|
|
Leasehold improvements and equipment, net
|
|
|
117,035
|
|
|
|
|
|
|
|
|
Total assets
|
|
€
|
4,231,968
|
|
|
|
|
|
|
|
|
Liabilities and stockholders’ equity
|
|
|
|
|
|
Current liabilities:
|
|
|
|
|
|
Accounts payable
|
|
€
|
631,789
|
|
|
Accrued expenses
|
|
|
994,024
|
|
|
Customer deposits
|
|
|
400,000
|
|
|
Deferred revenue
|
|
|
1,572,759
|
|
|
Other current liabilities
|
|
|
81,971
|
|
|
|
|
|
|
|
|
Total current liabilities
|
|
|
3,680,543
|
|
|
Other liabilities
|
|
|
87,755
|
|
|
|
|
|
|
|
|
Total liabilities
|
|
|
3,768,298
|
|
|
Stockholders’ equity:
|
|
|
|
|
|
Stock at par value, 1,409 shares authorized, issued and
outstanding
|
|
|
38,043
|
|
|
Additional paid in capital
|
|
|
370,746
|
|
|
Accumulated retained earnings
|
|
|
54,881
|
|
|
|
|
|
|
|
|
Total stockholders’ equity
|
|
|
463,670
|
|
|
|
|
|
|
|
|
Total liabilities and stockholders’ equity
|
|
€
|
4,231,968
|
|
|
|
|
|
|
|
The accompanying notes are an integral part of the financial
statements.
F-81
Therapharm
Recherches Th. R.
In
Euros
| |
|
|
|
|
|
|
|
|
|
12 Months Ended
|
|
|
|
December 31, 2007
|
|
|
|
Service revenue
|
|
€
|
5,785,044
|
|
|
Reimbursable revenue
|
|
|
1,940,439
|
|
|
|
|
|
|
|
|
Total revenue
|
|
|
7,725,483
|
|
|
Direct costs
|
|
|
3,745,187
|
|
|
Reimbursable expense
|
|
|
1,940,439
|
|
|
Selling, general, and administrative expenses
|
|
|
1,269,295
|
|
|
Depreciation and amortization
|
|
|
83,369
|
|
|
|
|
|
|
|
|
Income from operations
|
|
|
687,193
|
|
|
Other expense
|
|
|
(2,760
|
)
|
|
Other income
|
|
|
11,966
|
|
|
|
|
|
|
|
|
Income before provision for income taxes
|
|
|
696,399
|
|
|
Provision for income taxes
|
|
|
245,383
|
|
|
|
|
|
|
|
|
Net income
|
|
€
|
451,016
|
|
|
|
|
|
|
|
|
|
The accompanying notes are an integral part of the financial
statements.
F-82
Therapharm
Recherches Th. R.
In
Euros
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accumulated
|
|
|
|
|
|
|
|
Additional
|
|
Retained
|
|
Total
|
|
|
|
Common
|
|
Paid In
|
|
Earnings
|
|
Stockholder’s
|
|
|
|
Stock
|
|
Capital
|
|
(Deficit)
|
|
Equity
|
|
|
|
Balance at January 1, 2007
|
|
€
|
38,043
|
|
|
€
|
370,746
|
|
|
€
|
(396,135
|
)
|
|
€
|
12,654
|
|
|
Net income
|
|
|
—
|
|
|
|
—
|
|
|
|
451,016
|
|
|
|
451,016
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance at December 31, 2007
|
|
€
|
38,043
|
|
|
€
|
370,746
|
|
|
€
|
54,881
|
|
|
€
|
463,670
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The accompanying notes are an integral part of the financial
statements.
F-83
Therapharm
Recherches Th. R.
In
Euros
| |
|
|
|
|
|
|
|
|
|
12 Months Ended
|
|
|
|
December 31, 2007
|
|
|
|
Operating activities
|
|
|
|
|
|
Net income
|
|
€
|
451,016
|
|
|
Adjustments to reconcile net income to net cash provided by
operating activities:
|
|
|
|
|
|
Depreciation and amortization
|
|
|
83,369
|
|
|
Changes in operating assets and liabilities:
|
|
|
|
|
|
Unbilled revenue
|
|
|
(163,751
|
)
|
|
Accounts receivable
|
|
|
(593,959
|
)
|
|
Prepaid expenses and other current assets
|
|
|
(61,047
|
)
|
|
Accounts payable
|
|
|
61,120
|
|
|
Accrued expenses
|
|
|
223,329
|
|
|
Customer deposits
|
|
|
400,000
|
|
|
Deferred revenue
|
|
|
474,204
|
|
|
Other current liabilities
|
|
|
81,971
|
|
|
Other liabilities
|
|
|
(16,100
|
)
|
|
|
|
|
|
|
|
Net cash provided by operating activities
|
|
|
940,152
|
|
|
Investing activities
|
|
|
|
|
|
Change in restricted cash
|
|
|
(400,000
|
)
|
|
Purchase of leasehold improvements and equipment
|
|
|
(23,339
|
)
|
|
|
|
|
|
|
|
Net cash used in investing activities
|
|
|
(423,339
|
)
|
|
Net change in cash
|
|
|
516,813
|
|
|
|
|
|
|
|
|
Cash and cash equivalents, beginning of period
|
|
€
|
247,933
|
|
|
|
|
|
|
|
|
Cash and cash equivalents, end of period
|
|
€
|
764,746
|
|
|
|
|
|
|
|
|
|
The accompanying notes are an integral part of the financial
statements.
F-84
Therapharm
Recherches Th. R.
December 31,
2007
1. Nature of
Business
Therapharm Recherches Th. R. (the “Company” or
“Therapharm”) is a Clinical Resource Organization
(CRO), providing high-quality, efficient and flexible clinical
development solutions to the pharmaceutical industry. The
Company is able to leverage its high degree of clinical
expertise, industry knowledge and specialization to reduce the
expense and time frame of clinical development. The
Company’s revenues are generated principally from customers
located in France, while it does support clients and perform
services in several European countries.
2. Significant
Accounting Policies
Use of
Estimates
The preparation of financial statements in conformity with
accounting principles generally accepted in the United States of
America requires management to make estimates and assumptions
that affect the amounts reported in the financial statements and
accompanying notes. Actual results could differ from those
estimates.
Cash and
Cash Equivalents
Cash and cash equivalents include highly liquid investments with
an original maturity of three months or less from date of
purchase.
Restricted
Cash
The Company receives cash in advance from certain customers
specifically for the payment of investigator fees relating to
specific projects. Such amounts are recorded in restricted cash
and short term customer deposits in the accompanying
consolidated balance sheets.
Concentration
of Credit Risk
Financial instruments, which potentially subject the Company to
credit risk, consist principally of cash and accounts
receivable. The Company performs periodic evaluations of the
financial institutions in which its cash is invested. The
majority of the Company’s revenues and accounts receivable
are derived from pharmaceutical companies located in France. The
Company’s largest customer accounted for approximately 11%
of service revenue during the year ended December 31, 2007.
The four largest customers represented approximately 16%, 14%,
13% and 11% of the accounts receivable balance at
December 31, 2007. No other customers represented more than
10% of net service revenue or accounts receivable during those
periods or at those times. The Company provides an allowance for
doubtful accounts based on experience and specifically
identified risks. Accounts receivable are carried at the amounts
due from customers and charged off against the allowance for
doubtful accounts when management determines that recovery is
unlikely and the Company ceases collection efforts. During the
period contained within, the Company had not identified any
specific risks, and therefore no allowance for doubtful accounts
was booked.
Leasehold
Improvements and Equipment
Leasehold improvements and equipment are recorded at cost.
Expenditures for repairs and maintenance which do not extend the
useful life of the related assets are charged to expense as
incurred. Depreciation and amortization expense is computed
using the straight-line method over the estimated useful lives
of the assets ranging from 1 to 10 years.
F-85
Revenue
and Cost Recognition
The majority of the Company’s service revenue is derived
from
fee-for-service
contracts, some of which are fixed-price contracts. Revenues and
the related costs of
fee-for-service
contracts are recognized in the period in which services are
performed. Fixed-price contract revenue is recognized as
services are performed. The Company measures progress for fixed
price contracts using the concept of proportional performance
based upon a unit-based output method. Under the unit-based
output method, output units are pre-defined in the contract and
revenue is recognized based upon completion of such output
units. Revenue related to contract modifications is recognized
when realization is assured and the amounts are reasonably
determinable. Adjustments to contract estimates are made in the
periods in which the facts that require the revisions become
known. When the revised estimate indicates a loss, such loss is
provided for in the financial statements during that period. No
such losses were recognized in 2007. Deferred revenue represents
amounts billed to customers in excess of revenue recognized.
Unbilled revenue represents revenue recognized in excess of
amounts billed.
In connection with the management of clinical trials, the
Company pays, on behalf of its clients, fees to investigators
and test subjects as well as other
out-of-pocket
costs for items such as travel, printing, meetings and couriers.
The Company’s clients reimburse the Company for these
costs. As required by
EITF 01-14,
amounts paid by the Company as a principal for
out-of-pocket
costs are included in direct costs as reimbursable
out-of-pocket
expenses and the reimbursements the Company receives as a
principal are reported as reimbursed
out-of-pocket
revenue. During the year ended December 31, 2007, fees paid
to investigators and other fees the Company paid as an agent and
the associated reimbursements were approximately €1,940,000
and are included in the statements of operations as reimbursable
revenue and expense.
Foreign
Currency
The reporting currency of the Company is the Euro.
Value
Added Taxes
The Company accounts for value added taxes as a net component of
selling, general, and administrative expenses in accordance with
EITF 06-03,
“How Taxes Collected from Customers and Remitted to
Governmental Authorities Should be Presented in the Income
Statement.”
Income
Taxes
The Company is a member of a group that files a consolidated tax
return in France. Accordingly, income taxes payable to
(refundable from) the tax authority is recognized on the
financial statements of the parent company who is the taxpayer
for income tax purposes. The members of the consolidated group
allocate payments to any member of the group for the income tax
reduction resulting from the member’s inclusion in the
consolidated return, or the member makes payments to the parent
company for its allocated share of the consolidated income tax
liability. This allocation approximates the amounts that would
be reported if the Company was separately filing its tax return.
The Company also recognizes deferred tax assets on deductible
temporary differences and deferred tax liabilities on taxable
temporary differences. Temporary differences are the differences
between the reported amounts of assets and liabilities and their
tax bases. As those differences reverse, they will enter into
the determination of future taxable income included in the
consolidated tax returns. Deferred tax assets are reduced by a
valuation allowance when, in the opinion of management, it is
more likely than not that some portion or all of the deferred
tax assets will not be realized. Deferred tax assets and
liabilities are adjusted for the effects of changes in tax laws
and rates on the date of enactment.
In July 2006, the Financial Accounting Standards Board
(“FASB”) issued Interpretation No. 48, Accounting
for Uncertainty in Income Taxes—an interpretation of FASB
Statement 109 (FIN 48). FIN 48 clarifies the
accounting for uncertainty in income taxes recognized in an
enterprise’s financial statements in accordance with
Statement No. 109, Accounting for Income Taxes. FIN 48
prescribes a recognition threshold and measurement attribute for
the financial statement recognition and measurement of a tax
position taken or expected to be taken in a tax
F-86
return. FIN 48 also provides guidance on derecognition of
tax benefits, classification on the balance sheet, interest and
penalties, accounting in interim periods, disclosure, and
transition.
In December 2008, the FASB provided for a deferral of the
effective date of FIN 48 for certain nonpublic enterprises
to annual financial statements for fiscal years beginning after
December 15, 2008. The Company has elected this deferral
and accordingly will be required to adopt FIN 48 in its
2009 annual financial statements. Prior to adoption of
FIN 48, the Company will continue to evaluate its uncertain
tax positions and related income tax contingencies under
Statement No. 5, Accounting for Contingencies.
SFAS No. 5 requires the Company to accrue for losses
it believes are probable and can be reasonably estimated.
Recently
Issued Accounting Pronouncements
In September 2006 the FASB issued Statement of Financial
Accounting Standards No. 157, “Fair Value Measurements
(SFAS No. 157).” SFAS No. 157 defines
fair value, establishes a framework for measuring fair value,
and expands disclosures about fair value measurement.
SFAS No. 157 also emphasizes that fair value is a
market-based measurement, not an entity-specific measurement,
and sets out a fair value hierarchy with the highest priority
being quoted prices in active markets. Under
SFAS No. 157, fair value measurements are disclosed by
level within that hierarchy. In February 2008, the FASB issued
FASB Staff Position
No. 157-2,
“Effective Date of FASB Statement No. 157,” which
permits a one-year deferral for the implementation of
SFAS No. 157 with regard to nonfinancial assets and
liabilities that are not recognized or disclosed at fair value
in the financial statements on a recurring basis. The Company
adopted SFAS No. 157 for the fiscal year beginning
January 1, 2008, except for nonfinancial assets and
nonfinancial liabilities that are recognized or disclosed at
fair value in the financial statements on a nonrecurring basis
for which delayed application is permitted until our fiscal year
beginning January 1, 2009. The Company is currently
assessing the potential effect of the adoption of the remaining
provisions of SFAS No. 157 on its financial position,
results of operations and cash flows.
In February 2007, the FASB issued SFAS No. 159,
“The Fair Value Option for Financial Assets and Financial
Liabilities—Including an amendment of FASB Statement
No. 115,” or SFAS No. 159. This standard
permits, but does not require, all entities to choose to measure
eligible items at fair value at specified election dates. For
items for which the fair value option has been elected, an
entity would report unrealized gains and losses in earnings at
each subsequent reporting date. SFAS No. 159 is
effective as of the beginning of an entity’s first fiscal
year beginning after November 15, 2007. The Company is not
electing to adopt the provisions permitting the measurement of
eligible financial assets and liabilities at January 1,
2008 using the fair value option.
In June 2007, the FASB reached a consensus on EITF Issue
No. 07-03,
“Accounting for Nonrefundable Advance Payments for Goods or
Services to Be Used in Future Research and Development
Activities.”
EITF 07-03
requires companies to defer and capitalize, until the goods have
been delivered or the related services have been rendered,
non-refundable advance payments for goods that will be used or
services that will be performed in future research and
development activities.
EITF 07-03
is effective for fiscal years beginning after December 15,
2007. The Company does not expect
EITF 07-03
will have a material impact on its financial condition or
results of operations.
In December 2007, the FASB reached a consensus on EITF Issue
No. 07-01,
“Accounting for Collaborative Arrangements.”
EITF 07-01
defines collaborative arrangements and establishes reporting
requirements for transactions between participants in a
collaborative arrangement and between participants in the
arrangement and third parties.
EITF 07-01
also establishes the appropriate income statement presentation
and classification for joint operating activities and payments
between participants, as well as the required disclosures
related to these arrangements.
EITF 07-01
is effective for fiscal years beginning after December 15,
2008. The Company does not expect
EITF 07-01
will have a material impact on its financial condition or
results of operations.
In December 2007, the FASB issued SFAS No. 141
(revised 2007), “Business Combinations,” or
SFAS 141(R). FAS 141(R) expands the definition of a
business and a business combination, requires that: the purchase
price of an acquisition, including the issuance of equity
securities to be determined on the acquisition date, be recorded
at fair value at the acquisition date; all assets, liabilities,
contingent consideration, contingencies and in-process research
and development costs of an acquired business be recorded at
fair value at the acquisition date; acquisition costs generally
be expensed as incurred; restructuring costs generally be
expensed in periods subsequent to the
F-87
acquisition date; and changes be made in accounting for deferred
tax asset valuation allowances and acquired income tax
uncertainties after the measurement period to impact income tax
expense. SFAS 141(R) applies prospectively to business
combinations for which the acquisition date is on or after the
beginning of the first annual reporting period beginning on or
after December 15, 2008. The Company does not expect
SFAS 141(R) will have a material impact on its financial
condition or results of operations.
In December 2007, the FASB issued SFAS No. 160,
“Noncontrolling Interests in Consolidated Financial
Statements—an amendment of ARB No. 51,” or
SFAS 160. SFAS 160 amends ARB 51 to establish
accounting and reporting standards for the noncontrolling
interest in a subsidiary and for the deconsolidation of a
subsidiary. An ownership interest in subsidiaries held by
parties other than the parent should be presented in the
consolidated statement of financial position within equity, but
separate from the parent’s equity. SFAS 160 requires
that changes in a parent’s ownership interest while the
parent retains its controlling financial interest in its
subsidiary should be accounted for similarly to equity
transactions. When a subsidiary is deconsolidated, any retained
noncontrolling equity investment should be initially measured at
fair value, with any gain or loss recognized in earnings.
SFAS 160 requires consolidated net income to be reported at
amounts that include the amounts attributable to both the parent
and the noncontrolling interest. It also requires disclosure, on
the face of the consolidated income statement, of the amounts of
consolidated net income attributable to the parent and to the
noncontrolling interests. SFAS 160 is effective for fiscal
years, including interim periods within those fiscal years,
beginning on or after December 15, 2008. The Company does
not expect SFAS 160 will have a material impact on its
financial condition or results of operations.
3. Leasehold
Improvements and Equipment
Furniture and equipment consist of the following (in Euros):
| |
|
|
|
|
|
|
|
|
|
|
|
December 31,
|
|
|
|
Useful life
|
|
2007
|
|
|
|
Leasehold improvements
|
|
10 years
|
|
€
|
134,494
|
|
|
Technical facilities, materials and tools
|
|
2 to 3 years
|
|
|
242,287
|
|
|
Computer equipment
|
|
1 to 3 years
|
|
|
404,385
|
|
|
Furniture and fixtures
|
|
2 to 3 years
|
|
|
301,547
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
1,082,713
|
|
|
Less accumulated depreciation
|
|
|
|
|
(965,678
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
€
|
117,035
|
|
|
|
|
|
|
|
|
|
4. Income
Taxes
Income before income taxes consists of the following components
(in Euros):
| |
|
|
|
|
|
|
|
Year Ended
|
|
|
|
December 31,
|
|
|
|
2007
|
|
|
|
Income before income taxes
|
|
€
|
696,399
|
|
|
|
|
|
|
|
The provision for income taxes is as follows:
| |
|
|
|
|
|
|
|
Year Ended
|
|
|
|
December 31,
|
|
|
|
2007
|
|
|
|
Current:
|
|
€
|
245,383
|
|
|
Total
|
|
€
|
245,383
|
|
|
|
|
|
|
|
F-88
5. Commitments
and Contingencies
The Company occupies its corporate headquarters and other
offices and uses certain equipment under various operating
leases. The Company’s current lease for its corporate
headquarters expires in December 2015 and its Caen location
lease expires in December 2010. Rent expense under such office
and equipment arrangements was approximately €418,000
during the years ended December 31, 2007.
Future minimum lease payments subsequent to December 31,
2007 under capital and noncancelable operating leases are
approximately as follows (in Euros):
| |
|
|
|
|
|
|
|
Operating Leases
|
|
|
|
2008
|
|
€
|
556,000
|
|
|
2009
|
|
|
529,000
|
|
|
2010
|
|
|
464,000
|
|
|
2011
|
|
|
396,000
|
|
|
2012
|
|
|
379,000
|
|
|
Thereafter
|
|
|
1,107,000
|
|
|
|
|
|
|
|
|
Total minimum lease payments
|
|
€
|
3,431,000
|
|
The Company is involved in various claims incidental to the
conduct of its business. Management does not believe that any
such claims to which the Company is a party, both individually
and in the aggregate, will have a material adverse effect on the
Company’s financial position results of operations, or cash
flows.
6. Related Party
Transactions
During 2007, the Company was the subject of a lawsuit by one of
its clients. The lawsuit was subsequently settled out of court
by Therapharm’s parent company APA Research S.A.S.
(“APA”). Although the settlement was between APA and
the client, Therapharm guaranteed the payment to the client. The
settlement was for €740,000 to be paid back over a period
of ten years, or in full upon a change in control of
Therapharm’s ownership structure.
7. Subsequent
Events
On December 23, 2008 the stockholder of APA sold 100% of
its interest in the Company to an unrelated party. Commensurate
with the sale, the settlement amount (Note 6) was paid in
full and Therapharm was not required to perform on its guarantee.
F-89
Therapharm
Recherches Th. R.
In
Euros
| |
|
|
|
|
|
|
|
|
|
September 30, 2008
|
|
|
|
(unaudited)
|
|
|
|
Assets
|
|
|
|
|
|
Current assets:
|
|
|
|
|
|
Cash and cash equivalents
|
|
€
|
623,013
|
|
|
Restricted cash
|
|
|
423,534
|
|
|
Accounts receivable, less allowance for doubtful accounts
|
|
|
2,179,962
|
|
|
Unbilled revenue
|
|
|
474,336
|
|
|
Income tax receivable
|
|
|
221,156
|
|
|
Prepaid expenses and other current assets
|
|
|
133,135
|
|
|
|
|
|
|
|
|
Total current assets
|
|
|
4,055,136
|
|
|
Leasehold improvements and equipment, net
|
|
|
173,233
|
|
|
|
|
|
|
|
|
Total assets
|
|
€
|
4,228,369
|
|
|
|
|
|
|
|
|
Liabilities and stockholders’ equity
|
|
|
|
|
|
Current liabilities:
|
|
|
|
|
|
Accounts payable
|
|
€
|
662,138
|
|
|
Accrued expenses
|
|
|
1,040,630
|
|
|
Customer deposits
|
|
|
417,879
|
|
|
Deferred revenue
|
|
|
1,303,770
|
|
|
Other current liabilities
|
|
|
3,720
|
|
|
|
|
|
|
|
|
Total current liabilities
|
|
|
3,428,137
|
|
|
Other liabilities
|
|
|
24,670
|
|
|
|
|
|
|
|
|
Total liabilities
|
|
|
3,452,807
|
|
|
Stockholders’ equity:
|
|
|
|
|
|
Stock at par value
|
|
|
38,043
|
|
|
Additional paid in capital
|
|
|
370,746
|
|
|
|
|
|
|
|
|
Accumulated deficit
|
|
|
|
|
|
Stock at par value, 1,409 shares authorized, issued, and
outstanding
|
|
|
38,043
|
|
|
Additional paid in capital
|
|
|
370,746
|
|
|
Accumulated retained earnings
|
|
|
366,773
|
|
|
|
|
|
|
|
|
Total stockholders’ equity
|
|
|
775,562
|
|
|
|
|
|
|
|
|
Total liabilities and stockholders’ equity
|
|
€
|
4,228,369
|
|
|
|
|
|
|
|
|
|
The accompanying notes are an integral part of the financial
statements.
F-90
Therapharm
Recherches Th. R.
In
Euros
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Nine Months Ended
|
|
|
|
September 30,
|
|
|
|
2008
|
|
2007
|
|
|
|
(unaudited)
|
|
|
|
Service revenue
|
|
€
|
4,467,093
|
|
|
€
|
4,338,783
|
|
|
Reimbursable revenue
|
|
|
1,498,367
|
|
|
|
1,455,329
|
|
|
|
|
|
|
|
|
|
|
|
|
Total revenue
|
|
|
5,965,460
|
|
|
|
5,794,112
|
|
|
Direct costs
|
|
|
1,936,322
|
|
|
|
2,808,890
|
|
|
Reimbursable expense
|
|
|
1,498,367
|
|
|
|
1,455,329
|
|
|
Selling, general, and administrative expenses
|
|
|
1,793,019
|
|
|
|
941,359
|
|
|
Depreciation and amortization
|
|
|
45,979
|
|
|
|
62,527
|
|
|
|
|
|
|
|
|
|
|
|
|
Income from operations
|
|
|
691,773
|
|
|
|
526,007
|
|
|
Other expense
|
|
|
(2,700
|
)
|
|
|
(2,070
|
)
|
|
Other income
|
|
|
5,676
|
|
|
|
8,975
|
|
|
|
|
|
|
|
|
|
|
|
|
Income before provision for income taxes
|
|
|
694,749
|
|
|
|
532,912
|
|
|
Provision for income taxes
|
|
|
(229,267
|
)
|
|
|
(197,212
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Net income
|
|
€
|
465,482
|
|
|
€
|
335,700
|
|
|
|
|
|
|
|
|
|
|
|
The accompanying notes are an integral part of the financial
statements.
F-91
Therapharm
Recherches Th. R.
In
Euros
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Nine Months Ended
|
|
|
|
September 30,
|
|
|
|
2008
|
|
2007
|
|
|
|
(unaudited)
|
|
|
|
Operating activities
|
|
|
|
|
|
|
|
|
|
Net income
|
|
€
|
465,482
|
|
|
€
|
335,700
|
|
|
Adjustments to reconcile net cash provided by operating
activities:
|
|
|
|
|
|
|
|
|
|
Depreciation and amortization
|
|
|
45,979
|
|
|
|
62,527
|
|
|
Changes in operating assets and liabilities:
|
|
|
|
|
|
|
|
|
|
Work in progress
|
|
|
174,613
|
|
|
|
(163,751
|
)
|
|
Accounts receivable
|
|
|
(266,738
|
)
|
|
|
(893,959
|
)
|
|
Income taxes receivable
|
|
|
(38,821
|
)
|
|
|
—
|
|
|
Prepaid expenses and other current assets
|
|
|
49,010
|
|
|
|
(11,047
|
)
|
|
Accounts payable
|
|
|
30,349
|
|
|
|
91,120
|
|
|
Accrued expenses
|
|
|
46,606
|
|
|
|
313,885
|
|
|
Customer deposits
|
|
|
17,879
|
|
|
|
400,000
|
|
|
Deferred revenue
|
|
|
(268,989
|
)
|
|
|
474,204
|
|
|
Other current liabilities
|
|
|
(78,251
|
)
|
|
|
88,975
|
|
|
Other liabilities
|
|
|
(63,085
|
)
|
|
|
(35,570
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Net cash provided by operating activities
|
|
|
114,043
|
|
|
|
662,084
|
|
|
Investing activities
|
|
|
|
|
|
|
|
|
|
Change in restricted cash
|
|
|
—
|
|
|
|
(400,000
|
)
|
|
Purchase of leasehold improvements and equipment
|
|
|
(102,177
|
)
|
|
|
(2,497
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Net cash used in investing activities
|
|
|
(102,177
|
)
|
|
|
(402,497
|
)
|
|
Financing activities
|
|
|
|
|
|
|
|
|
|
Dividends
|
|
|
(153,590
|
)
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
Net cash used in financing activities
|
|
|
(153,590
|
)
|
|
|
—
|
|
|
Net change in cash
|
|
|
(141,733
|
)
|
|
|
259,587
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents, beginning of period
|
|
|
764,746
|
|
|
|
247,933
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents, end of period
|
|
€
|
623,013
|
|
|
€
|
507,520
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The accompanying notes are an integral part of the financial
statements.
F-92
Therapharm
Recherches Th. R.
September 30,
2008
1. Nature of
Business
Therapharm Recherches Th. R. (the “Company” or
“Therapharm”) is a Clinical Resource Organization
(CRO), providing high-quality, efficient and flexible clinical
development solutions to the pharmaceutical industry. The
Company is able to leverage its high degree of clinical
expertise, industry knowledge and specialization to reduce the
expense and time frame of clinical development. The
Company’s revenues are generated principally from customers
located in France, while it does support clients and perform
services in several European countries.
2. Significant
Accounting Policies
Use of
Estimates
The preparation of financial statements in conformity with
accounting principles generally accepted in the United States of
America requires management to make estimates and assumptions
that affect the amounts reported in the financial statements and
accompanying notes. Actual results could differ from those
estimates.
Cash and
Cash Equivalents
Cash and cash equivalents include highly liquid investments with
an original maturity of three months or less from date of
purchase.
Restricted
Cash
The Company receives cash in advance from certain customers
specifically for the payment of investigator fees relating to
specific projects. Such amounts are recorded in restricted cash
and short term customer deposits in the accompanying
consolidated balance sheets.
Concentration
of Credit Risk
Financial instruments, which potentially subject the Company to
credit risk, consist principally of cash and accounts
receivable. The Company performs periodic evaluations of the
financial institutions in which its cash is invested. The
majority of the Company’s revenues and accounts receivable
are derived from pharmaceutical companies located in France. The
Company’s two largest customers accounted for approximately
15% and 10% of service revenue during the nine months ended
September 30, 2008, the Company’s largest customer
accounted for approximately 11% of service revenue during the
nine months ended September 30, 2007.
The two largest customers represented approximately 28% and 13%
of the accounts receivable balance at September 30, 2008.
No other customers represented more than 10% of net service
revenue or accounts receivable during those periods or at those
times. The Company provides an allowance for doubtful accounts
based on experience and specifically identified risks. Accounts
receivable are carried at fair value and charged off against the
allowance for doubtful accounts when management determines that
recovery is unlikely and the Company ceases collection efforts.
During the periods contained within, the company had not
identified any specific risks, and therefore no allowance for
doubtful accounts was booked.
Property
and Equipment
Property and equipment are recorded at cost. Expenditures for
repairs and maintenance which do not extend the useful life of
the related assets are charged to expense as incurred.
Depreciation and amortization expense is computed using the
straight-line method over the estimated useful lives of the
assets ranging from 1 to 10 years.
F-93
Revenue
and Cost Recognition
The majority of the Company’s service revenue is derived
from
fee-for-service
contracts, some of which are fixed-price contracts. Revenues and
the related costs of
fee-for-service
contracts are recognized in the period in which services are
performed. Fixed-price contract revenue is recognized as
services are performed. The Company measures progress for fixed
price contracts using the concept of proportional performance
based upon a unit-based output method. Under the unit-based
output method, output units are pre-defined in the contract and
revenue is recognized based upon completion of such output
units. Revenue related to contract modifications is recognized
when realization is assured and the amounts are reasonably
determinable. Adjustments to contract estimates are made in the
periods in which the facts that require the revisions become
known. When the revised estimate indicates a loss, such loss is
provided for in the financial statements during that period. No
such losses were recognized in 2008 or 2007. Deferred revenue
represents amounts billed to customers in excess of revenue
recognized. Unbilled revenue represents revenue recognized in
excess of amounts billed.
In connection with the management of clinical trials, the
Company pays, on behalf of its clients, fees to investigators
and test subjects as well as other
out-of-pocket
costs for items such as travel, printing, meetings and couriers.
The Company’s clients reimburse the Company for these
costs. As required by
EITF 01-14,
amounts paid by the Company as a principal for
out-of-pocket
costs are included in direct costs as reimbursable
out-of-pocket
expenses and the reimbursements the Company receives as a
principal are reported as reimbursed
out-of-pocket
revenue. In the statements of operations, the Company combines
amounts paid by the Company as an agent for
out-of-pocket
costs with the corresponding reimbursements, or revenue, the
Company receives as an agent. During the years ended
September 30, 2008 and 2007 fees paid to investigators and
other fees the Company paid as an agent and the associated
reimbursements were approximately €1,455,000 and
€1,498,000 respectively.
Income
Taxes
The Company accounts for income taxes using an asset and
liability approach which requires the recognition of deferred
tax assets and liabilities for the expected future tax
consequences of temporary differences between the carrying
amount of assets and liabilities for financial reporting
purposes and amounts reportable for income tax purposes.
Recently
Issued Accounting Pronouncements
See Note 2 to the audited financial statements for the
fiscal year ended December 31, 2007.
3. Leasehold
Improvements and Equipment
Leasehold improvements and equipment consist of the following:
| |
|
|
|
|
|
|
|
|
|
Useful life
|
|
September 30, 2008
|
|
|
|
|
|
(Unaudited)
|
|
|
|
Leasehold improvements
|
|
10 years
|
|
€
|
67,457
|
|
|
Technical facilities, materials and tools
|
|
2 to 3 years
|
|
|
242,287
|
|
|
Computer equipment
|
|
1 to 3 years
|
|
|
272,348
|
|
|
Furniture and fixtures
|
|
2 to 3 years
|
|
|
560,113
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
1,142,205
|
|
|
Less accumulated depreciation
|
|
|
|
|
(968,973
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
€
|
173,232
|
|
|
|
|
|
|
|
|
|
F-94
4. Commitments
and Contingencies
The Company occupies its corporate headquarters and other
offices and uses certain equipment under various operating
leases. The Company’s current lease for its corporate
headquarters expires in March 2015. Rent expense under such
arrangements was approximately € 327,465 and € 277,500
during the nine months ended September 30, 2008 and 2007,
respectively.
Future minimum lease payments subsequent to December 31,
2007 under capital and noncancelable operating leases are as
follows:
| |
|
|
|
|
|
|
|
Operating Leases
|
|
|
|
2008
|
|
€
|
139,007
|
|
|
2009
|
|
|
528,999
|
|
|
2010
|
|
|
464,096
|
|
|
2011
|
|
|
395,923
|
|
|
2012
|
|
|
379,421
|
|
|
Thereafter
|
|
|
1,107,359
|
|
|
|
|
|
|
|
|
Total minimum lease payments
|
|
€
|
3,014,805
|
|
The Company is involved in various claims incidental to the
conduct of its business. Management does not believe that any
such claims to which the Company is a party, both individually
and in the aggregate, will have a material adverse effect on the
Company’s financial position or results of operations.
5. Related Party
Transactions
During 2006 and into 2007, the Company was the subject of a
lawsuit by one of its clients. The lawsuit was subsequently
settled out of court by Therapharm’s parent company APA
Research S.A.S. (“APA”). Although the settlement was
between APA and the client, Therapharm guaranteed the payment to
the client. The settlement was for €740,000 to be paid back
over a period of ten years, or in full upon a change in control
of Therapharm’s ownership structure.
6. Subsequent
Events
On December 23, 2008 the shareholders of Therapharm, APA,
sold 100% of their interest in the Company to an unrelated
party. Commensurate with the sale, the litigation settlement
amount was paid in full and Therapharm was not required to
perform on its guarantee.
F-95
Shares
Common
Stock
Prospectus
Jefferies &
Company
William
Blair &
Company
Lazard Capital Markets
,
2010
Until ,
2010 (25 days after the date of this prospectus), all
dealers that buy, sell or trade in our common stock, whether or
not participating in this offering, may be required to deliver a
prospectus. This is in addition to the dealers’ obligation
to deliver a prospectus when acting as underwriters and with
respect to their unsold allotments or subscriptions.
PART II
Information Not
Required in Prospectus
|
|
|
Item 13.
|
Other
Expenses of Issuance and Distribution.
|
The following table sets forth the various expenses, other than
underwriting discounts and commissions, payable by us in
connection with the sale of the common stock being registered.
All of the amounts shown are estimated except the SEC
registration fee, the NASDAQ initial listing fee, and the FINRA
filing fee.
| |
|
|
|
|
|
Securities and Exchange Commission registration fee
|
|
$
|
7,130
|
|
|
NASDAQ Global Market initial listing fee
|
|
|
125,000
|
|
|
FINRA filing fee
|
|
|
10,500
|
|
|
Printing expenses
|
|
|
*
|
|
|
Accounting fees and expenses
|
|
|
*
|
|
|
Legal fees and expenses
|
|
|
*
|
|
|
Transfer agent fees and expenses
|
|
|
*
|
|
|
Miscellaneous
|
|
|
*
|
|
|
|
|
|
|
|
|
Total
|
|
|
*
|
|
|
|
|
|
* |
|
To be supplied by amendment. |
|
|
|
Item 14.
|
Indemnification
of Directors and Officers.
|
Section 145 of the Delaware General Corporation Law
(“DGCL”) provides, in general, that a corporation
incorporated under the laws of the State of Delaware, such as
us, may indemnify any person who was or is a party or is
threatened to be made a party to any threatened, pending or
completed action, suit or proceeding (other than a derivative
action by or in the right of the corporation) by reason of the
fact that such person is or was a director, officer, employee or
agent of the corporation, or is or was serving at the request of
the corporation as a director, officer, employee or agent of
another enterprise, against expenses (including attorneys’
fees), judgments, fines and amounts paid in settlement actually
and reasonably incurred by such person in connection with such
action, suit or proceeding if such person acted in good faith
and in a manner such person reasonably believed to be in or not
opposed to the best interests of the corporation, and, with
respect to any criminal action or proceeding, had no reasonable
cause to believe such person’s conduct was unlawful. In the
case of a derivative action, a Delaware corporation may
indemnify any such person against expenses (including
attorneys’ fees) actually and reasonably incurred by such
person in connection with the defense or settlement of such
action or suit if such person acted in good faith and in a
manner such person reasonably believed to be in or not opposed
to the best interests of the corporation, except that no
indemnification will be made in respect of any claim, issue or
matter as to which such person will have been adjudged to be
liable to the corporation unless and only to the extent that the
Court of Chancery of the State of Delaware or any other court in
which such action was brought determines such person is fairly
and reasonably entitled to indemnity for such expenses.
Our Second Restated Certificate of Incorporation, as amended,
and our Amended and Restated By-laws provide that we will
indemnify any person who was or is a party or is threatened to
be made a party to any threatened, pending or completed action,
suit or proceeding, whether civil, criminal, administrative or
investigative (other than an action by or in the right of the
corporation) by reason of the fact that he is or was a director,
officer, employee, or agent of the corporation, or is or was
serving at the request of the corporation as a director,
officer, employee, or agent of another corporation, partnership,
joint venture, trust or other enterprise, against expenses
(including attorneys’ fees), judgments, fines, and amounts
paid in settlement actually and reasonably incurred by him in
connection with such action, suit, or proceeding if he acted in
good faith and in a manner he reasonably believed to be in or
not opposed to the best interests of the corporation, and, with
respect to any criminal action or proceeding, had no reasonable
cause to believe his conduct was unlawful.
II-1
Our Second Restated Certificate of Incorporation, as amended,
and our Amended and Restated By-laws provide that we will
indemnify any person who was or is a party or is threatened to
be made a party to any threatened, pending or completed action
or suit by or in the right of the corporation to procure a
judgment in its favor by reason of the fact that he is or was a
director, officer, employee or agent of the corporation, or is
or was serving at the request of the corporation as a director,
officer, employee or agent of another corporation, partnership,
joint venture, trust or other enterprise against expenses
(including attorneys’ fees) actually and reasonably
incurred by him in connection with the defense of settlement of
such action or suit if he acted in good faith and in a manner he
reasonably believed to be in or not opposed to the best
interests of the corporation, provided that no indemnification
shall be made in respect of any claim, issue or matter as to
which such person shall have been adjudged to be liable to the
corporation unless and only to the extent that the Court of
Chancery of the State of Delaware or the court in which such
action or suit was brought shall determine upon application
that, despite the adjudication of liability but in view of all
the circumstances of the case, such person is fairly and
reasonably entitled to indemnity for such expenses which the
Court of Chancery of such other court shall deem proper.
We are also permitted to apply for insurance on behalf of any
director, officer, employee or agent for liability arising out
of his actions, whether or not the corporation’s provisions
for indemnification would permit indemnification.
|
|
|
Item 15.
|
Recent
Sales of Unregistered Securities.
|
On August 30, 2007 we issued 15,758,497 shares of our
common stock to the stockholders of Old RPS in connection with
the merger of Longxia Acquisition, Inc. with and into Old RPS
with Old RPS being the surviving corporation and the subsequent
merger of Old RPS with and into ReSearch Pharmaceutical
Services, LLC, our wholly-owned subsidiary. The issuance of the
shares to the former shareholders of Old RPS was exempt from
registration under Section 4(2) of the Securities Act
because shares were issued to a limited number of existing
shareholders of Old RPS and did not involve a public offering.
On December 6, 2007 we granted an option to purchase
450,000 shares of our common stock at an exercise price of
$5.05 to Daniel Perlman, which is subject to vesting. The
issuance of the option was exempt from registration under
Section 4(2) of the Securities Act and Rule 506 of
Regulation D as a transaction not involving a public
offering.
On December 6, 2007 we granted an option to purchase
120,000 shares of our common stock at an exercise price of
$5.05 to Harris Koffer, which is subject to vesting. The
issuance of the option was exempt from registration under
Section 4(2) of the Securities Act and Rule 506 of
Regulation D as a transaction not involving a public
offering.
On December 6, 2007 we granted an option to purchase
180,000 shares of our common stock at an exercise price of
$5.05 to Steven Bell, which is subject to vesting. The issuance
of the option was exempt from registration under
Section 4(2) of the Securities Act and Rule 506 of
Regulation D as a transaction not involving a public
offering.
On December 17, 2008 we issued 2,794,029 shares of our
common stock to our wholly owned Netherlands-based subsidiary,
RPS Dutch BV, upon payment of aggregate consideration of
€5,457,088 ($7,818,241 at the exchange rate on
December 17, 2008) and 1,404,856 shares of our
common stock to our wholly owned Spain-based subsidiary, RPS
Spain, upon payment of aggregate consideration of
€2,743,859 ($3,928,479 at the exchange rate on
December 17, 2008) as part of the acquisitions
described below. The shares were issued to our subsidiaries in
reliance upon the exemption from registration under
Section 4(2) of the Securities Act as transactions not
involving a public offering.
On December 22, 2008, RPS Dutch BV entered into and
consummated a definitive stock purchase agreement to purchase
all of the issued and outstanding shares of Imerem, a
Germany-based clinical research organization, from the sole
shareholder of Imerem. In addition, RPS Spain entered into and
consummated a definitive stock purchase agreement to purchase
all of the issued and outstanding shares of Infociencia, a
Spain-based clinical research organization, from
Infociencia’s shareholders.
II-2
On December 23, 2008, RPS Dutch BV entered into and
consummated a definitive stock purchase agreement to purchase
all of the issued and outstanding shares of Therapharm, a
French-based clinical research organization, from
Therapharm’s sole shareholder.
RPS Dutch BV transferred 1,296,165 shares of our common
stock with an aggregate value of $2,275,000 to the shareholder
of Imerem. RPS Dutch BV transferred 1,497,864 shares of our
common stock with an aggregate value of $2,625,000 to the
shareholder of Therapharm. RPS Spain transferred
1,404,856 shares of our common stock with an aggregate
value of $2,450,000 to the shareholders of Infociencia. The
shares of common stock transferred by RPS Dutch BV to the
shareholders of Imerem and Therapharm, and the shares of common
stock transferred by RPS Spain to the shareholders of
Infociencia were transferred in reliance upon exemptions from
registration for transactions which constitute “offshore
transactions” as defined in Regulation S under the
Securities Act.
On July 10, 2009, in connection with the consummation of
the purchase of the issued and outstanding shares of Paramax, a
British Virgin Islands-based company operating a clinical
research organization subsidiary (Paramax International
(Beijing) Inc.) in Beijing and Shanghai, China, pursuant to a
definitive share purchase agreement between RPS Dutch BV and
Paramax dated March 30, 2009, we issued 530,973 shares
of our common stock to RPS Dutch BV upon payment of aggregate
consideration of $1,061,946. The shares were issued to RPS Dutch
BV in reliance upon the exemption from registration under
Section 4(2) of the Securities Act as a transaction not
involving a public offering.
On July 10, 2009, RPS Dutch BV transferred
530,973 shares of our common stock to the shareholder of
Paramax in consideration for all of the issued and outstanding
shares of Paramax. The shares were transferred to the
shareholder of Paramax in reliance upon exemptions from
registration for transactions which constitute “offshore
transactions” as defined in Regulation S under the
Securities Act.
|
|
|
Item 16.
|
Exhibits
and Financial Statement Schedules.
|
(a) Exhibits.
| |
|
|
|
|
|
|
|
Exhibit No.
|
|
Description
|
|
|
|
|
1
|
.1*
|
|
Form of Underwriting Agreement
|
|
|
2
|
.1**
|
|
Agreement and Plan of Merger dated as of April 26, 2007
among Cross Shore Acquisition Corporation, Longxia Acquisition,
Inc., Research Pharmaceutical Services, Inc., The RPS
Securityholders and Daniel M. Perlman and Daniel Raynor, as the
RPS Securityholders Committee
|
|
|
2
|
.2**
|
|
First Amendment to Agreement and Plan of Merger dated as of
June 5, 2007 among Cross Shore Acquisition Corporation,
Longxia Acquisition, Inc., Research Pharmaceutical Services,
Inc., and Daniel M. Perlman and Daniel Raynor, as the RPS
Securityholders Committee
|
|
|
2
|
.3**
|
|
Second Amendment to Agreement and Plan of Merger dated as of
July 6, 2007 among Cross Shore Acquisition Corporation,
Longxia Acquisition, Inc., Research Pharmaceutical Services,
Inc., and Daniel M. Perlman and Daniel Raynor, as the RPS
Securityholders Committee
|
|
|
2
|
.4**
|
|
Agreement for the Sale and Purchase of the Share Capital in
IMEREM Institute for Medical Research Management and
Biometrics—Institut für medizinisches
Forschungsmanagement und Biometrie—Ein unabhaengiges
Forschungsunternehmen GmbH dated December 22, 2008
|
|
|
2
|
.5**
|
|
Agreement for the Sale and Purchase of the Share Capital in
Infociencia, S.L. dated December 22, 2008
|
|
|
2
|
.6**
|
|
Agreement for the Sale and Purchase of the Share Capital in
Therapharm Recherches Th.R. dated December 23, 2008
|
|
|
2
|
.7**
|
|
Share Purchase Agreement Relating to Paramax International Inc.
dated March 30, 2009
|
|
|
3
|
.1**
|
|
Second Restated Certificate of Incorporation of Cross Shore
Acquisition Corporation, as amended
|
|
|
3
|
.2†
|
|
Amended and Restated By-laws of ReSearch Pharmaceutical
Services, Inc.
|
|
|
4
|
.1**
|
|
Registration Rights Agreement dated as of August 30, 2007
between Cross Shore Acquisition Corporation and Daniel M.
Perlman and Daniel Raynor as the RPS Securityholders Committee
|
|
|
4
|
.2**
|
|
Investor Rights Agreement dated as of April 24, 2006 among
Cross Shore Acquisition Corporation, Sunrise Securities Corp.
and Collins Stewart Limited
|
II-3
| |
|
|
|
|
|
|
|
Exhibit No.
|
|
Description
|
|
|
|
|
4
|
.3**
|
|
Registration Rights Agreement dated as of April 24, 2006 by
and among Cross Shore Acquisition Corporation, Stephen
Stonefield, Jon Burgman, CSA I, LLC, CSA II, LLC, CSA III,
LLC, and Sunrise Securities Corp.
|
|
|
4
|
.4**
|
|
Specimen Certificate of Common Stock
|
|
|
4
|
.5**
|
|
ReSearch Pharmaceutical Services, Inc. 2007 Equity Incentive Plan
|
|
|
5
|
.1*
|
|
Opinion of Drinker Biddle & Reath LLP
|
|
|
10
|
.1**
|
|
Pennsylvania Full Service Lease between Brandywine Operating
Partnership, L.P. and ReSearch Pharmaceutical Services, Inc. for
520 Virginia Drive, Fort Washington, Pennsylvania, dated as
of August 7, 2006
|
|
|
10
|
.2**
|
|
Revolving Credit and Security Agreement by and among ReSearch
Pharmaceutical Services, Inc. and PNC Bank, N.A. dated
November 1, 2006
|
|
|
10
|
.3**
|
|
First Amendment and Waiver by and among ReSearch Pharmaceutical
Services, Inc. and PNC Bank, N.A. dated August 29, 2007
|
|
|
10
|
.4**
|
|
Third Amendment to Revolving Credit and Security Agreement by
and among ReSearch Pharmaceutical Services, Inc and PNC Bank,
N.A. dated July 9, 2009
|
|
|
10
|
.5**
|
|
Employment Agreement dated April 26, 2007 between Cross
Shore Acquisition Corporation and Daniel Perlman
|
|
|
10
|
.6**
|
|
Employment Agreement dated April 26, 2007 between Cross
Shore Acquisition Corporation and Harris Koffer
|
|
|
10
|
.7**
|
|
Employment Agreement dated April 26, 2007 between Cross
Shore Acquisition Corporation and Steven Bell
|
|
|
10
|
.8**
|
|
Employment Agreement dated December 6, 2007 between
ReSearch Pharmaceutical Services, LLC and Samir Shah
|
|
|
10
|
.9**
|
|
Employment Agreement dated April 28, 2001 between ReSearch
Pharmaceutical Services, Inc. and Janet Brennan
|
|
|
10
|
.10**
|
|
Standard form of Non-Qualified Stock Option Award Agreement
|
|
|
10
|
.11**
|
|
Standard form of Replacement Incentive Stock Option Award
Agreement
|
|
|
10
|
.12**
|
|
Agreement Concerning Board of Directors dated August 20,
2007 between ReSearch Pharmaceutical Services, Inc. and Pangaea
One Acquisition Holdings I, LLC
|
|
|
10
|
.13**
|
|
Share Repurchase Agreement dated October 4, 2007 between
ReSearch Pharmaceutical Services, Inc. and Pangaea One
Acquisition Holdings I, LLC
|
|
|
10
|
.14**
|
|
Consulting Agreement dated November 16, 2007 between
ReSearch Pharmaceutical Services, Inc. and Cartesian Capital
Management, LLC
|
|
|
10
|
.15**
|
|
Employment Agreement Relating to Business Information, Trade
Secrets and Non-Competition dated May 28, 2006 between
ReSearch Pharmaceutical Services, Inc. and Harris Koffer
|
|
|
21
|
.1**
|
|
List of subsidiaries of ReSearch Pharmaceutical Services, Inc.
|
|
|
23
|
.1*
|
|
Consent of Drinker Biddle & Reath LLP (included in
Exhibit 5.1)
|
|
|
23
|
.2†
|
|
Consent of Ernst & Young LLP
|
|
|
23
|
.3†
|
|
Consent of McGladrey & Pullen, LLP
|
|
|
24
|
.1
|
|
Power of Attorney (included in signature pages of
Form S-1
filed on June 18, 2010)
|
|
|
|
|
|
|
* |
|
To be filed by amendment. |
|
|
|
|
(b) |
|
Financial Statement Schedules. |
All financial statement schedules have been omitted as they are
not required, not applicable, or the required information is
otherwise included in the notes to the consolidated financial
statements.
II-4
The undersigned registrant hereby undertakes that:
1. For purposes of determining any liability under the
Securities Act of 1933, the information omitted from the form of
prospectus filed as part of this registration statement in
reliance upon Rule 430A and contained in a form of
prospectus filed by the registrant pursuant to Rule 424(b)
(1) or (4) or 497(h) under the Securities Act shall be
deemed to be part of this registration statement as of the time
it was declared effective.
2. For the purpose of determining any liability under the
Securities Act of 1933, each post-effective amendment that
contains a form of prospectus shall be deemed to be a new
registration statement relating to the securities offered
therein, and the offering of such securities at that time shall
be deemed to be the initial bona fide offering thereof.
Insofar as indemnification for liabilities arising under the
Securities Act may be permitted to directors, officers and
controlling persons of the registrant pursuant to the foregoing
provisions, or otherwise, the registrant has been advised that
in the opinion of the SEC, such indemnification is against
public policy as expressed in the Securities Act, and is,
therefore, unenforceable. In the event that a claim for
indemnification against such liabilities (other than the payment
by the registrant of expenses incurred or paid by a director,
officer or controlling person of the registrant in the
successful defense of any action, suit or proceeding) is
asserted by such director, officer or controlling person in
connection with the securities being registered, the registrant
will, unless in the opinion of its counsel the matter has been
settled by controlling precedent, submit to a court of
appropriate jurisdiction the question whether such
indemnification by it is against public policy as expressed in
the Securities Act and will be governed by the final
adjudication of such issue.
II-5
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the
registrant has duly caused this registration statement to be
signed on its behalf by the undersigned, thereunto duly
authorized in the City of Fort Washington, Commonwealth of
Pennsylvania, on October 1, 2010.
ReSearch Pharmaceutical Services, Inc.
|
|
|
| |
By:
|
Daniel M. Perlman
|
| |
Title:
|
Chief Executive Officer and Chairman
|
of the Board of Directors
Pursuant to the requirements of the Securities Act of 1933, this
Amendment No. 1 to the Registration Statement has been
signed by the following persons in the capacities and on the
dates indicated.
| |
|
|
|
|
|
|
|
Name
|
|
Title
|
|
Date
|
|
|
|
|
|
|
|
|
|
/s/ Daniel
Perlman
Daniel
M. Perlman
|
|
Chief Executive Officer
(principal executive officer)
and Chairman of the Board of Directors
|
|
October 1, 2010
|
|
|
|
|
|
|
|
/s/ Steven
Bell
Steven
Bell
|
|
Executive Vice President of Finance, Chief Financial Officer
(principal financial officer and principal accounting officer),
Secretary, and Treasurer
|
|
October 1, 2010
|
|
|
|
|
|
|
|
*
Harris
Koffer
|
|
President, Chief Operating Officer and Director
|
|
October 1, 2010
|
|
|
|
|
|
|
|
*
Thomas
R. Armstrong
|
|
Director
|
|
October 1, 2010
|
|
|
|
|
|
|
|
*
Jack
H. Dean
|
|
Director
|
|
October 1, 2010
|
|
|
|
|
|
|
|
*
James
R. Macdonald
|
|
Director
|
|
October 1, 2010
|
|
|
|
|
|
|
|
*
Warren
W. Myers
|
|
Director
|
|
October 1, 2010
|
|
|
|
|
|
|
|
*
Daniel
Raynor
|
|
Director
|
|
October 1, 2010
|
II-6
| |
|
|
|
|
|
|
|
Name
|
|
Title
|
|
Date
|
|
|
|
|
|
|
|
|
|
*
Stephen
E. Stonefield
|
|
Director
|
|
October 1, 2010
|
|
|
|
|
|
|
|
*
Peter
M. Yu
|
|
Director
|
|
October 1, 2010
|
|
|
|
|
|
|
|
|
|
*
|
|
Daniel Perlman, by signing his name hereto, does hereby sign
this document on behalf of each of the above-named directors of
the registrant pursuant to powers of attorney duly executed by
such persons.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
By:
|
|
/s/ Daniel
Perlman
Daniel
PerlmanAttorney-in-fact
|
|
|
|
|
II-7
EXHIBIT LIST
| |
|
|
|
|
|
|
|
Exhibit No.
|
|
Description
|
|
|
|
|
1
|
.1*
|
|
Form of Underwriting Agreement
|
|
|
2
|
.1**
|
|
Agreement and Plan of Merger dated as of April 26, 2007 among
Cross Shore Acquisition Corporation, Longxia Acquisition, Inc.,
Research Pharmaceutical Services, Inc., The RPS Securityholders
and Daniel M. Perlman and Daniel Raynor, as the RPS
Securityholders Committee
|
|
|
2
|
.2**
|
|
First Amendment to Agreement and Plan of Merger dated as of June
5, 2007 among Cross Shore Acquisition Corporation, Longxia
Acquisition, Inc., Research Pharmaceutical Services, Inc., and
Daniel M. Perlman and Daniel Raynor, as the RPS Securityholders
Committee
|
|
|
2
|
.3**
|
|
Second Amendment to Agreement and Plan of Merger dated as of
July 6, 2007 among Cross Shore Acquisition Corporation, Longxia
Acquisition, Inc., Research Pharmaceutical Services, Inc., and
Daniel M. Perlman and Daniel Raynor, as the RPS Securityholders
Committee
|
|
|
2
|
.4**
|
|
Agreement for the Sale and Purchase of the Share Capital in
IMEREM Institute for Medical Research Management and
Biometrics—Institut für medizinisches
Forschungsmanagement und Biometrie—Ein unabhaengiges
Forschungsunternehmen GmbH dated December 22, 2008
|
|
|
2
|
.5**
|
|
Agreement for the Sale and Purchase of the Share Capital in
Infociencia, S.L. dated December 22, 2008
|
|
|
2
|
.6**
|
|
Agreement for the Sale and Purchase of the Share Capital in
Therapharm Recherches Th.R. dated December 23, 2008
|
|
|
2
|
.7**
|
|
Share Purchase Agreement Relating to Paramax International Inc.
dated March 30, 2009
|
|
|
3
|
.1**
|
|
Second Restated Certificate of Incorporation of Cross Shore
Acquisition Corporation, as amended
|
|
|
3
|
.2†
|
|
Amended and Restated By-laws of ReSearch Pharmaceutical
Services, Inc.
|
|
|
4
|
.1**
|
|
Registration Rights Agreement dated as of August 30, 2007
between Cross Shore Acquisition Corporation and Daniel M.
Perlman and Daniel Raynor as the RPS Securityholders Committee
|
|
|
4
|
.2**
|
|
Investor Rights Agreement dated as of April 24, 2006 among Cross
Shore Acquisition Corporation, Sunrise Securities Corp. and
Collins Stewart Limited
|
|
|
4
|
.3**
|
|
Registration Rights Agreement dated as of April 24, 2006 by and
among Cross Shore Acquisition Corporation, Stephen Stonefield,
Jon Burgman, CSA I, LLC, CSA II, LLC, CSA III, LLC, and
Sunrise Securities Corp.
|
|
|
4
|
.4**
|
|
Specimen Certificate of Common Stock
|
|
|
4
|
.5**
|
|
ReSearch Pharmaceutical Services, Inc. 2007 Equity Incentive Plan
|
|
|
5
|
.1*
|
|
Opinion of Drinker Biddle & Reath LLP
|
|
|
10
|
.1**
|
|
Pennsylvania Full Service Lease between Brandywine Operating
Partnership, L.P. and ReSearch Pharmaceutical Services, Inc. for
520 Virginia Drive, Fort Washington, Pennsylvania, dated as
of August 7, 2006
|
|
|
10
|
.2**
|
|
Revolving Credit and Security Agreement by and among ReSearch
Pharmaceutical Services, Inc and PNC Bank, N.A. dated November
1, 2006
|
|
|
10
|
.3**
|
|
First Amendment and Waiver by and among ReSearch Pharmaceutical
Services, Inc. and PNC Bank, N.A. dated August 29, 2007
|
|
|
10
|
.4**
|
|
Third Amendment to Revolving Credit and Security Agreement by
and among ReSearch Pharmaceutical Services, Inc. and PNC Bank,
N.A. dated July 9, 2009
|
|
|
10
|
.5**
|
|
Employment Agreement dated April 26, 2007 between Cross Shore
Acquisition Corporation and Daniel Perlman
|
|
|
10
|
.6**
|
|
Employment Agreement dated April 26, 2007 between Cross Shore
Acquisition Corporation and Harris Koffer
|
|
|
10
|
.7**
|
|
Employment Agreement dated April 26, 2007 between Cross Shore
Acquisition Corporation and Steven Bell
|
|
|
10
|
.8**
|
|
Employment Agreement dated December 6, 2007 between ReSearch
Pharmaceutical Services, LLC and Samir Shah
|
|
|
10
|
.9**
|
|
Employment Agreement dated April 28, 2001 between ReSearch
Pharmaceutical Services, Inc. and Janet Brennan
|
|
|
10
|
.10**
|
|
Standard form of Non-Qualified Stock Option Award Agreement
|
| |
|
|
|
|
|
|
|
Exhibit No.
|
|
Description
|
|
|
|
|
10
|
.11**
|
|
Standard form of Replacement Incentive Stock Option Award
Agreement
|
|
|
10
|
.12**
|
|
Agreement Concerning Board of Directors dated August 20, 2007
between ReSearch Pharmaceutical Services, Inc. and Pangaea One
Acquisition Holdings I, LLC
|
|
|
10
|
.13**
|
|
Share Repurchase Agreement dated October 4, 2007 between
ReSearch Pharmaceutical Services, Inc. and Pangaea One
Acquisition Holdings I, LLC
|
|
|
10
|
.14**
|
|
Consulting Agreement dated November 16, 2007 between
ReSearch Pharmaceutical Services, Inc. and Cartesian Capital
Management, LLC
|
|
|
10
|
.15**
|
|
Employment Agreement Relating to Business Information, Trade
Secrets and Non-Competition dated May 28, 2006 between
ReSearch Pharmaceutical Services, Inc. and Harris Koffer
|
|
|
21
|
.1**
|
|
List of subsidiaries of ReSearch Pharmaceutical Services, Inc.
|
|
|
23
|
.1*
|
|
Consent of Drinker Biddle & Reath LLP (included in Exhibit
5.1)
|
|
|
23
|
.2†
|
|
Consent of Ernst & Young LLP
|
|
|
23
|
.3†
|
|
Consent of McGladrey & Pullen, LLP
|
|
|
24
|
.1
|
|
Power of Attorney (included in signature pages of
Form S-1
filed on June 18, 2010)
|
|
|
|
|
|
|
* |
|
To be filed by amendment. |