Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - International Stem Cell CORP | d8k.htm |
 1
(ISCO.OB)
A New Kind of Stem Cell
Company
Superior Technology
Superior Business Model
Superior Execution
Exhibit 99.1 |
 Statements pertaining to anticipated technological developments and
therapeutic applications, the potential benefits of collaborations,
affiliations, and other opportunities for the company and its subsidiaries,
along with other statements about the future expectations, beliefs, goals,
plans, or prospects expressed by management constitute forward-looking
statements. Any statements that are not historical fact (including, but not
limited to statements that contain words such as "will," "should,"
"believes," "plans," "anticipates,"
"expects," "estimates,") should also be considered to be
forward-looking statements. Forward-looking statements involve risks
and uncertainties, including, without limitation, risks inherent in the
development and/or commercialization of potential products, uncertainty in
the results of clinical trials or regulatory approvals, need and ability to
obtain future capital, application of capital resources among competing uses,
and maintenance of intellectual property rights. Actual
results may differ materially from the results anticipated in these
forward- looking
statements
and
as
such
should
be
evaluated
together
with
the
many uncertainties that affect the company's business, particularly those
mentioned in the cautionary statements found in the company's Securities
and Exchange Commission filings. The company disclaims any intent or
obligation to update these forward-looking statements.
2
Forward-looking statements |
 The
Stem Cell Industry Model 3 |
 The
ISCO Model—Multiple Chances to Succeed IP
Revenue
4 |
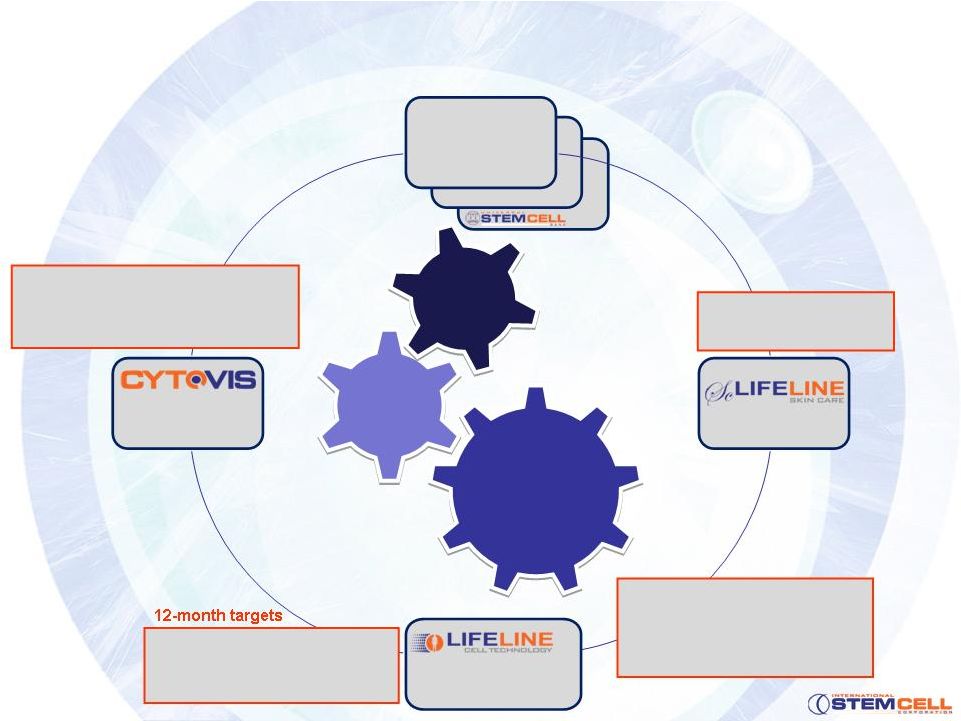 Hepatocytes
The Corporate Structure
Parthenogenic
stem cells
Parthenogenic
stem cells
Human
cell
culture
Human
cell
culture
Int’l
partners
Int’l
partners
Therapeutics
where cell therapy
is proven yet cells
in limited supply
Cosmeceuticals
•Research
products •
Toxicity Testing
Cellular
ophthalmology
•
Expanded Asian distribution
•
Asian manufacturing base
•
First quarterly profitability
12-month targets
•
First commercial
sale
•
Full commercial plan
12-month targets
•
Immunogenetics
review/data
•
Functional differentiation
•
Industry-first, homozygous
stem cell lines under cGMP
12-month targets
•
CytoCor
funded development
•
CytoCor
chemical testing partner
•
CytoRet
functionality data
12-month targets
5 |
 6
Growth Stages to Success
•
Equity Capital
•
@$35M to Date
•
Partners & Licensing
•
Product Sales
•
Reduced Equity Need
•
Partners & Licensing
•
Product Sales
•
Therapy Products
•
No Further Equity Need |
 7
Categories of Human Stem Cells
ISCO
parthenotes
Embryonic
stem cells
iPS
cells
Adult stem
cells
Pluripotent
Yes
Yes
Possibly
No
Proliferation
Expansion to clinical volume
Strong
Strong
Varies
Weak
Ethical concerns
Embryo use or destruction
None
Significant
None
None
Gene manipulation
None
None
Substantial
None
Immune matching
No Immune
Rejection
Immune
Rejection
(Unless
Autologous)
Immune
Rejection
(Unless
Autologous)
Immune
Rejection
(Unless
Autologous) |
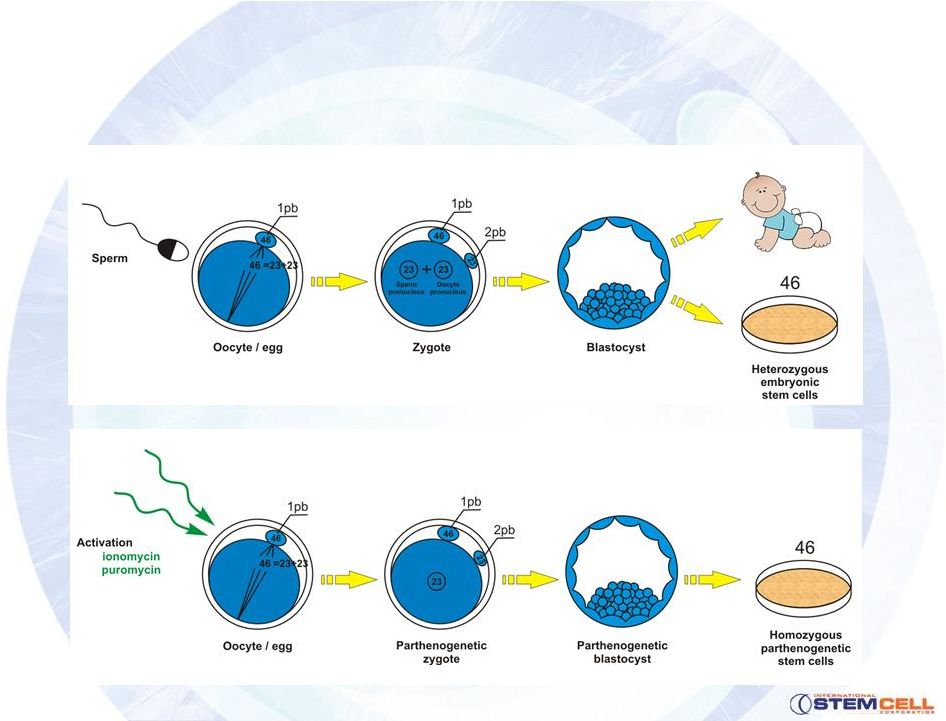 Parthenogenesis –
Solving Ethical and Immune
Matching Problems
8
Normal fertilization
Parthenogenesis |
    9
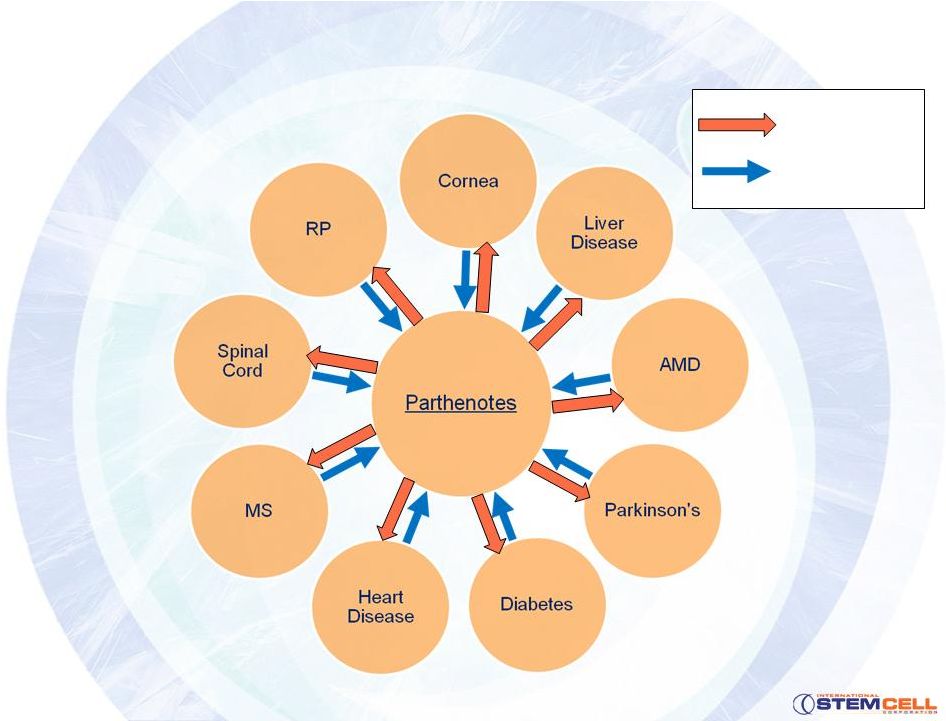
Therapeutic Targets -
The Low Hanging Fruit
Cornea transplant market
•
10M corneally
blind
•
Untouched Asian/Indian markets
•
Can use either hpSCs
or hESs
Toxicity testing
Retinal
conditions
-
cell therapy is proven
•
Age-related macular degeneration (AMD)
•
Retinitis pigmentosa
(RP)-
100,000 Americans and no therapy
ISCO RPE technology
•
Demonstrated morphology, markers and pigmentation
•
In vivo functionality testing underway
Liver diseases (acute and chronic)
•
30-40M with fatty liver and app. 30,000 deaths annually in the US
•
15,750 on liver waiting list ($200-$300k surgery, $12-20k annually)
Neurodegenerative diseases
•
Cell therapy proven
•
Large unmet medical need
•
Increasing with the aging of the population
ISCO objectives
•
Demonstrate parthenote
neuronal differentiation
•
Contribute to parthenote
validation
ISCO hepatocyte
technology
•
Demonstrated markers and glycogen storage
•
In vivo functionality testing underway |
 Products
•
70+ growth factors, media and human cell cultures
•
More new products in 2009 than the market-leader
Applications
•
Across therapeutic areas
•
Regenerative medicine
•
Safety and toxicology
•
Basic cell biology
Commercialization
•
Direct sales from in-house staff
•
Distributors (ATCC, Millipore, Life Technologies,
Cell Systems, Veritas)
•
Asian expansion opportunity
•
2010 first quarterly profitability for unit
10 |
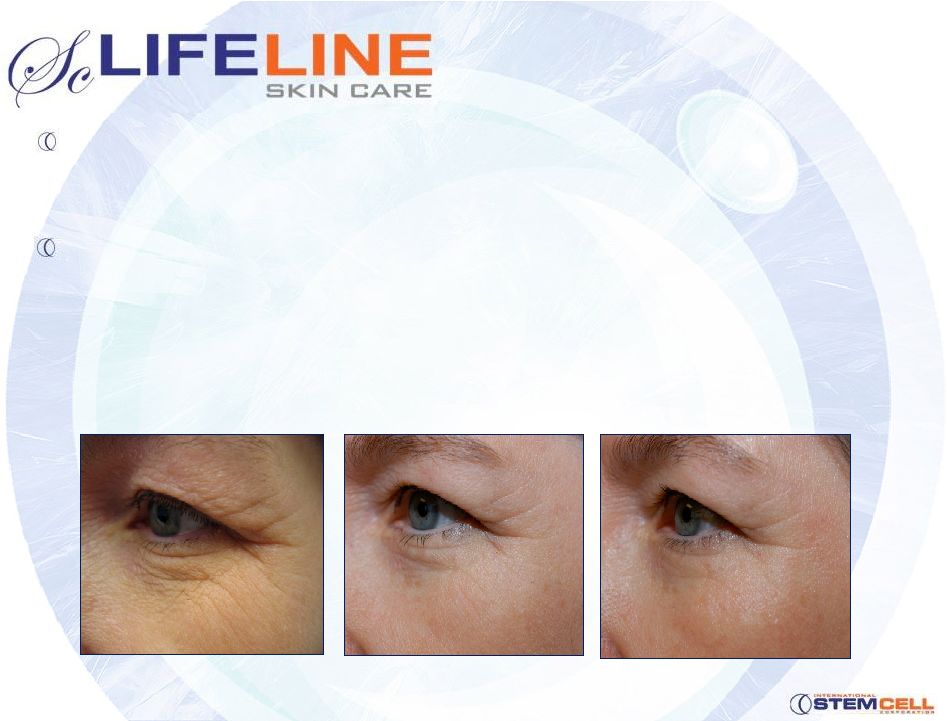 Strong technical
foundation
•
Proven Lifeline skin cell growth products
•
Human safety established
Commercialization
•
Market-differentiating stem cell technology
•
Formulation and packaging
•
Direct sales and global marketing partnering
11
Baseline
6 weeks
16 weeks |
 Management
Kenneth Aldrich, Executive Chairman
•
>30 years investment in and management of public and private companies
Andrey
Semechkin, CEO
•
Member of Russian Academy of Sciences
•
Successful founder and manager of international companies
Brian Lundstrom, President
•
Novo Nordisk, SangStat/Genzyme
and ACADIA
•
>24 years clinical-commercial development and transactional experience
Jeffrey Janus, ISCO SVP and Lifeline CEO
•
>24 years experience with FDA-compliant biological products
•
Founder of Clonetics
(now research product market leader Lonza)
Ruslan
Semechkin, CEO Lifeline Skin Care
•
MD, PhD, Medical Genetics and Parkinson’s
Simon Craw, VP UniStemCell
•
Merck, AstraZeneca and Novartis
•
>21 years scientific operational experience
12 |
 12-month milestones
13
Lifeline
•
Expanded Asian distribution
•
Asian manufacturing base
•
First quarterly profitability
Lifeline Skin Care
•
First commercial sale
•
Large-scale commercial plan
Cytovis
•
CytoCor
funded development for therapeutic use
•
CytoCor
IND development path
•
CytoCor
chemical testing partnership(s)
•
CytoRet
functionality (animal data)
Core technology and UniStemCell
•
Immunogenetics
review and experimental data
•
Functional differentiation
•
Industry-first, homozygous stem cell lines under cGMP
|
 Financial overview
14
Shares outstanding
72 million
Stock price (9/1/2010)
$1.08
Market capitalization
$78 million
Product revenue (2009)
$1.1 million |
 15
Contact information
www.internationalstemcell.com
Kenneth Aldrich, Chairman
760-940-6383
kaldrich@intlstemcell.com |
