Attached files
| file | filename |
|---|---|
| EX-23.1 - Sinobiopharma, Inc. | sinob_ex23-1.htm |
| EX-32.1 - Sinobiopharma, Inc. | sinob_ex32-1.htm |
| EX-31.1 - Sinobiopharma, Inc. | sinob_ex31-1.htm |
| EX-32.2 - Sinobiopharma, Inc. | sinob_ex32-2.htm |
| EX-31.2 - Sinobiopharma, Inc. | sinob_ex31-2.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
|
[X]
|
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
For the fiscal year ended May 31, 2010
|
or
|
[ ]
|
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
For the transition period from _____________to ______________
|
Commission file number 333-144910
SINOBIOPHARMA, INC.
(Exact name of registrant as specified in its charter)
|
Nevada
|
26-3002371
|
|
State or other jurisdiction of
Incorporation or organization
|
(I.R.S. Employer
Identification No.)
|
8 Zhong Tian Road
Nantong City, Jiangsu Province
People’s Republic of China 226009
(Address of principal executive offices) (Zip Code)
Registrant’s telephone number, including area code: 011 - (86) 51-3853-28336
Securities registered pursuant to Section 12(b) of the Act: None
Securities registered pursuant to section 12(g) of the Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. [ ] Yes [X] No
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. [ ] Yes [X] No
Note – Checking the box above will not relieve any registrant required to file reports pursuant to Section 13 or 15(d) of the Exchange Act from their obligations under those Sections.
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. [X] Yes [ ] No
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). [ ] Yes [ ] No
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (§ 229.405 of this chapter) is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. [ ]
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act.
|
Large accelerated filer [ ]
|
Accelerated filer [ ]
|
|
|
Non-accelerated filer [ ] (Do not check if a smaller reporting company)
|
Smaller reporting company [X]
|
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Ac t). [ ] Yes [X] No
State the aggregate market value of the voting and non-voting common equity held by non-affiliates computed by reference to the price at which the common equity was last sold, or the average bid and asked price of such common equity, as of the last business day of the registrant’s most recently completed second fiscal quarter.
Note. – If a determination as to whether a particular person or entity is an affiliate cannot be made without involving unreasonable effort and expense, the aggregate market value of the common stock held by non-affiliates may be calculated on the basis of assumptions reasonable under the circumstances, provided that the assumptions are set forth in this Form.
The aggregate market value of the voting and non-voting common stock of the issuer held by non-affiliates as of November 30, 2009 was approximately $13,611,600 (56,715,000 shares of common stock held by non-affiliates) based upon a closing price of the common stock of $0.24 as quoted by the OTC Bulletin Board on November 30, 2009.
APPLICABLE ONLY TO REGISTRANTS INVOLVED IN BANKRUPTCY
PROCEEDINGS DURING THE PRECEDING FIVE YEARS:
Indicate by check mark whether the registrant has filed all documents and reports required to be filed by Section 12, 13 or 15(d) of the Securities Exchange Act of 1934 subsequent to the distribution of securities under a plan confirmed by a court. [ ] Yes [ ] No
(APPLICABLE ONLY TO CORPORATE REGISTRANTS)
Indicate the number of shares outstanding of each of the registrant’s classes of common stock, as of the latest practicable date.
As of September 14, 2010, there are presently 117,587,608 shares of common stock, par value $0.0001 issued and outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
List hereunder the following documents if incorporated by reference and the Part of the Form 10-K (e.g., Part I, Part II, etc.) into which the document is incorporated: (1) Any annual report to security holders; (2) Any proxy or information statement; and (3) Any prospectus filed pursuant to Rule 424(b) or (c) under the Securities Act of 1933. The listed documents should be clearly described for identification purposes (e.g., annual report to security holders for fiscal year ended December 24, 1980).
2
| TABLE OF CONTENTS | |
|
Page
|
|
3
FORWARD LOOKING STATEMENTS
In this annual report, references to “Sinobiopharma,” “SNBP,” “the Company,” “we,” “our,” “us,” and the Company’s wholly owned subsidiary, “Dongying BVI,” “Big Global Limited,” and our controlled entity “Dong Ying China” refer to Sinobiopharma, Inc.
This Annual Report on Form 10-K contains forward-looking statements regarding our business, financial condition, results of operations and prospects. Words such as “expects,” “anticipates,” “intends,” “plans,” “believes,” “seeks,” “estimates” and similar expressions or variations of such words are intended to identify forward-looking statements, but are not deemed to represent an all-inclusive means of identifying forward-looking statements as denoted in this Annual Report on Form 10-K. Additionally, statements concerning future matters are forward-looking statements.
Although forward-looking statements in this Annual Report on Form 10-K reflect the good faith judgment of our management, such statements can only be based on facts and factors currently known by us. Consequently, forward-looking statements are inherently subject to risks and uncertainties and actual results and outcomes may differ materially from the results and outcomes discussed in or anticipated by the forward-looking statements. Factors that could cause or contribute to such differences in results and outcomes include, without limitation, those specifically addressed under the headings “Risks Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations.” You are urged not to place undue reliance on these forward-looking statements, which speak only as of the date of this Annual Report on Form 10-K. We file reports with the SEC. The SEC maintains a website (www.sec.gov) that contains reports, proxy and information statements, and other information regarding issuers that file electronically with the SEC, including us. You can also read and copy any materials we file with the SEC at the SEC’s Public Reference Room at 100 F Street, NE, Washington, DC 20549. You can obtain additional information about the operation of the Public Reference Room by calling the SEC at 1-800-SEC-0330.
We undertake no obligation to revise or update any forward-looking statements in order to reflect any event or circumstance that may arise after the date of this Annual Report on Form 10-K, except as required by law. Readers are urged to carefully review and consider the various disclosures made throughout the entirety of this Annual Report, which are designed to advise interested parties of the risks and factors that may affect our business, financial condition, results of operations and prospects.
PART I
Item 1. Business.
Year of Organization and Corporate History
Sinobiopharma, Inc. was incorporated in the State of Nevada under the name of Buzz Media Ltd. on October 26, 2006. On November 8, 2006 the Company acquired all the issued and outstanding shares of Buzz Media Ltd. (“Buzz Nova Scotia”), a corporation incorporated in the province of Nova Scotia, Canada on October 26, 2006. The transaction was treated as an acquisition for accounting purposes. The consideration for the acquisition of Buzz Nova Scotia was 500 shares (on a post-forward stock split basis) of the Company valued at $0.10, the book value of the net assets of Buzz Nova Scotia, since the acquisition was from a related party.
On July 14, 2008 the Company’s President and majority shareholder sold all of her 62.5% interest in the Company to an unrelated individual. On the same day, she resigned from all of her positions as officer and director of the Company and the purchaser was appointed the sole director and officer of the Company.
Effective July 29, 2008, the Company, under its original name of Buzz Media, Ltd., incorporated a subsidiary, “Sinobiopharma, Inc.” with an investment of $0.001 and merged with it for the sole purpose of changing the name of the Company. As a result, the Company changed its name from “Buzz Media Ltd.” to “Sinobiopharma, Inc.”
Effective July 29, 2008, the Company effected a fifty (50) for one (1) stock split of its authorized, issued and outstanding common stock. As a result, the Company’s authorized capital increased from 50,000,000 shares of common stock with a par value of $0.0001 to 2,500,000,000 shares of common stock with a par value of $0.0001. The Company’s issued and outstanding share capital increased as a result of the split from 2,000,010 shares of common stock to 100,000,500 shares of common stock. Share capital figures are presented in these financial statements giving retroactive effect to the stock split and accordingly all share capital figures are presented on a post-split basis as if the split had been affected upon inception.
4
On August 19, 2008, the Company entered into a share exchange agreement (the “Share Exchange Agreement”) with Dongying Pharmaceutical Co, Limited (“Dongying BVI”), a company organized under the laws of the Territory of the British Virgin Islands, and all the shareholders of Dongying BVI, whereby the Company agreed to acquire 100% of the issued and outstanding shares of Dongying BVI through the issuance of approximately 40,000,000 shares of common stock of the Company in aggregate to the shareholders of Dongying BVI on a pro rata basis in accordance with each Dongying BVI shareholder’s percentage of ownership in Dongying BVI. The Share Exchange Agreement closed on September 22, 2008.
Concurrently with the closing of the Share Exchange Agreement, by a letter agreement entered into on September 8, 2008 between Dongying BVI and the Company’s majority shareholder, that shareholder agreed to cancel 60,100,500 shares of the 62,500,500 shares of common stock of the Company registered in his name within ten (10) days of the closing of the Share Exchange Agreement. The 60,100,500 shares were cancelled on September 26, 2008. The share cancellation completed the reverse merger with Dongying BVI as a recapitalization of the Company, such that voting control of the Company was obtained by the former stockholders of Dongying BVI. The net assets of Dongying BVI and the Company have been brought forward at their historical bases. The costs associated with the reverse merger were expensed as incurred.
Dongying BVI was incorporated under the laws of the British Virgin Islands on January 29, 2008. On May 13, 2008 Dongying BVI acquired a 100% interest in Big Global Limited (“Big Global”) from the sole shareholder of Dongying BVI for consideration of $1.00.
Big Global was incorporated under the laws of Hong Kong on November 26, 2007. On December 10, 2007 Big Global acquired a 100% interest in Dong Ying (Jiangsu) Pharmaceuticals Co., Ltd. (“Dong Ying China”) from the sole shareholder of Big Global for consideration of $1.00. The acquisition was approved by the government of the People’s Republic of China (“PRC” or “China”) in May 2008.
Dong Ying China was incorporated under the laws of the PRC in 2003. Dong Ying China’s business is the development, manufacture and sale of pharmaceutical products in China. There are three product lines currently as of May 31, 2010 and several other potential products in various stages of research and development. The product lines currently commercialized are Cisatracurium Besylate, a skeletal muscle relaxant, Clindamycin Hydrochloride, an antibiotic for penicillin-allergic patients and Perindopril, a cardiovascular drug. Dong Ying China’s offices and manufacturing facility are in owned premises located on land used under license in Nantong, China and its research and development is carried out in Nanjing, China.
On August 20, 2008, the Company entered into a share purchase agreement (the “Share Purchase Agreement”) with the Company’s former majority shareholder, effective concurrently with the closing of the Share Exchange Agreement. Pursuant to the Share Purchase Agreement, the former majority shareholder acquired all of the capital of Buzz Media, Ltd. (“Buzz Nova Scotia”), the wholly-owned subsidiary of the Company incorporated in the Province of Nova Scotia, Canada, in exchange for the payment of $10.00.
Our Business
The business of the Company is conducted through its subsidiary, Dongying BVI, which, in turn, conducts its business through its subsidiary, Big Global Limited, which, in turn, conducts its business through Dong Ying China.
The diagram below illustrates the corporate structure of the Company as a result of the completion of the Share Exchange Agreement and the Share Purchase Agreement:

5
Dongying BVI, Big Global Limited and Dong Ying China
Dongying BVI
Dongying BVI was incorporated pursuant to the laws of the British Virgin Islands on January 29, 2008. As a result of the closing of the Share Exchange Agreement, the Company is the sole shareholder of Dongying BVI. Dongying BVI’s sole director is Dr. Lequn Lee Huang. On May 13, 2008, Dongying BVI purchased the sole common share of Big Global Limited from Dr. Lequn Lee Huang in exchange for payment of $1.00HK.
Big Global Limited
Big Global Limited was incorporated pursuant to the laws of Hong Kong on November 26, 2007. Dongying BVI is the sole shareholder of Big Global Limited and Dr. Lequn Lee Huang is the sole director of the Company. Pursuant to an equity transfer contract between Big Global Limited and Cyton International, Inc., a company incorporated pursuant to the laws of the state of Connecticut, USA, dated December 10, 2007, Big Global Limited acquired 100% of the registered capital of Dong Ying China in exchange for the payment of US$1.00 as Cyton International, Inc. was merely a nominee shareholder for Dr. Lequn Huang and there was no change in the beneficial ownership of Dong Ying China.
Dong Ying China
Dong Ying China was incorporated under the name Nantong Dongying Pharmaceutical Co., Ltd. pursuant to the laws of the People’s Republic of China. Effective September 29, 2008, upon the purchase by Cyton International, Inc., of 100% of the issue and outstanding shares of Nantong Dongying Pharmaceutical Co., Ltd., Dong Ying China was reincorporated and approved by the relevant PRC authorities as a wholly-foreign owned enterprise. Effective 11, 2004, Dong Ying China changed its name to Dongying (Jiangsu) Pharmaceutical Co., Ltd. and received the relevant governmental approval for such change. During the last six fiscal years, Dong Ying China has been engaged in the research, development, manufacture and marketing of biopharmaceutical products in China.
Business of Dong Ying China
Dong Ying China is engaged in the research, development, manufacture and marketing of biopharmaceutical products in China. Dong Ying China has developed new methods for synthesis of active pharmaceutical ingredient (“API”) and innovative drug delivery (new formulation) that dramatically reduces the time and cost of drug development. Dong Ying China’s current therapeutic focus is on anesthesia-assisted agents and cardiovascular drugs. Dong Ying China’s R&D focus is new, innovative methods of synthesizing compounds more rapidly at lower cost, and/or improved drug formulation with enhanced usability.
Dong Ying China’s headquarters and 30,000 sq. meter (approximately 322,917.31 sq. foot) Good Manufacturing Practice (“GMP”) certified production facilities are located in the Nantong Economic and Technology Development Zone, is located in Nantong City, Jiangsu, and qualifies Dong Ying China for tax benefits. Dong Ying China’s research and development (“R&D”) labs are located in Nanjing, China. The Nantong Economic and Technology Development Zone is a major R&D center within China with several universities and research institutes. Dong Ying China’s distribution network enables Dong Ying China to market its products in every major city in China.
Dong Ying China’s Strengths
|
*
|
Leadership of CEO, Dr. Lequn Lee Huang (Ph.D., Chemistry, Iowa State University), a former manager, Head of MS lab for drug discovery, at Bayer Co.;
|
|
*
|
Patents applied and granted for technology for fast, low-cost synthesis of API and new drug formulations;
|
|
*
|
Integrated, large capacity GMP-certified manufacturing facilities for freeze-dried powder, injection formula, tablets, hard capsules, granules and pharmaceutical raw materials;
|
|
*
|
10 research specialists and more than 40 of the Company’s 110 employees have college or higher educations.
|
6
Dong Ying China’s Products
Currently the product line of Dong Ying China includes Cisatracurium Besylate (marketed as “Kutai” in China), a skeletal muscle relaxant, Perindopril (Marketed as “Yitai” in China”), a cardiovascular for blood hypertention and Clindamycin Hydrochloride (marketed as “kesu” in China), an antibiotic for penicillin-allergic patients. Dong Ying China has the ability to expand its market through its research cooperation with Nanjing University and Nanjing Suji Pharmaceutical Research Center.
Dong Ying China launched its patented product for new freeze-dried formulation of the pre-surgical skeletal muscle relaxant Cisatracurium Besylate on May 30, 2006. By May 31, 2010, the Company estimated Kutai had captured approximately 65% of the Chinese market and was in use in more than 700 hospitals. Developed using Dong Ying China’s patent granted for method for drug synthesis that can dramatically reduce the time and cost of drug development, Dong Ying China’s Cisatracurium Besylate is the only one can be stored at room temperature whereas competitors’ versions must be kept at between 35.6 to 46.4 degrees Fahrenheit.
Sinobiopharma launched the Perindopril product in early 2010. Perindopril, used alone or in combination with other medications to treat high blood pressure, is the latest generation of a class of medications called ACE inhibitors. It supports circulation by preventing the production of chemicals that occur naturally in the body but constrict blood vessels.
The Company has received the Drug Certificate for Perindopril raw material (Certificate H20073699) from the Chinese State Food and Drug Administration (the “Chinese SFDA”) and obtained GMP Certification as of February 21, 2010. The Company has submitted its application to China's State Intellectual Property Office (SIPO) for a Chinese patent for Perindopril and its new drug compounds and obtained a receiving number (200810242989.1; "New Drug Compounds Containing Perindopril"). Sinobiopharma completed clinical trials for the capsule formulation in September 2007. Sinobiopharma has received approval from the Chinese SFDA to manufacture and market the Perindopril tablet in April 2010 (Certificate H20093504).
As the first-to-market (FTM) Chinese producer of Perindopril, Sinobiopharma will come to market with certain competitive advantages, including higher price approvability by the Chinese Government, exclusivity for penetrating to hospitals, and the ability to export of lower cost active pharmaceutical ingredients (API) to the globe market. Prior to Chinese SFDA approval of Sinobiopharma's version of Perindopril, Servier (France) was the only firm to market imported Perindopril in China.
The following table sets forth information relating to key products that Dong Ying China has launched in the Chinese market:
|
Key Products
|
Dose
|
Approval Number
|
||
|
Cisatracurium Besylate
|
Raw Material (API)
|
H20060926
|
||
|
for injection
|
5 mg
|
H20060927
|
||
|
Clindamycin Hydrochloride
|
||||
|
for injection
|
0.75g/0.9g
|
H20040927
|
||
|
Perindopril
|
Raw Material (API)
|
H20073699
|
||
|
Tablet
|
2 mg
|
H20093504
|
The following table sets forth information relating to a key product that Dong Ying China expects to launch in the Chinese Market in the very near future:
|
Key Products
|
Dose
|
Approval Number
|
||
|
N(2)-L-alanyl-L- glutamine for injection (1)
|
10 g
|
2006B01379
|
7
Dong Ying China has also obtained approved certificates of approval to produce a number of generic drugs in China, which Dong Ying China can produce and market when the market price for such generic products increases and allows production to become profitable, which is currently not the situation.
|
Generic Products
|
Dose
|
Approval Number
|
||
|
Naproxen Tablets
|
0.1g
|
H32021028
|
||
|
Aminophylline tablets
|
0.1g
|
H32021024
|
||
|
Nitrendipine Tablets
|
10mg
|
H32021023
|
||
|
Propafenone tablets
|
50 mg
|
H32021025
|
||
|
Verapamil Tablets
|
40 mg
|
H32021026
|
||
|
Ketotifen Fumarate Tablets
|
1mg
|
H32024391
|
||
|
Oleanolic acid tablets
|
10mg
|
H32024392
|
||
|
Oleanolic acid tablets
|
20mg
|
H32024393
|
||
|
Vitamin C tablets
|
0.1g
|
H32024997
|
||
|
Difenidol tablets
|
25mg
|
H32024914
|
||
|
Pentoxyverine Citrate Tablets
|
25mg
|
H32024999
|
||
|
Atenolol tablets
|
25mg
|
H32024160
|
||
|
Atenolol tablets
|
50mg
|
H32024159
|
||
|
Perphenazine Tablets
|
2mg
|
H32024162
|
||
|
Perphenazine Tablets
|
4mg
|
H32024163
|
||
|
Captopril Tablets
|
25mg
|
H32024167
|
||
|
Clozapine Tablets
|
25mg
|
H32024169
|
||
|
Chlorprothixene Tablets
|
25mg
|
H32024170
|
||
|
Fenfluramine Tablets
|
20mg
|
H32024172
|
||
|
Doxepin Hydrochloride Tablets
|
25mg
|
H32024395
|
||
|
Chlorpromazine Tablets
|
25mg
|
H32024397
|
||
|
Chlorpromazine Tablets
|
50mg
|
H32024396
|
||
|
Breviscapine Tablets
|
20mg
|
Z32021122
|
||
|
Troxerutin Tablets
|
60mg
|
H32025915
|
||
|
Trepibutone Tables
|
40mg
|
H32025533
|
||
|
Tiapride Hydrochloride Tablets
|
0.1g
|
H32025535
|
||
|
Ranitidine Capsules
|
150 mg
|
H32024998
|
||
|
Ciprofloxacin Capsules
|
0.25g
|
H32025000
|
||
|
Ganoderma Capsules
|
0.27g
|
Z32021190
|
Dong Ying China’s Development Pipeline
In addition to those products currently on the market in China, Dong Ying China has product candidates in the development pipeline using its patent applied for method for quickly synthesizing low-cost API and improved delivery formulations. Product candidates currently in the development pipeline include:
|
*
|
Rocuronium bromide (skeletal muscle relaxant): Like its formulation of Cisatracurium Besylate, we expect that Dong Ying China’s next generation Rocuronium Bromide formulation will be the world’s first that can be stored at room temperature, making it more convenient to use; and Rocuronium Bromide is a fast acting muscle relaxant, can be used in cocktail with Cisatracurium Besylate
|
|
*
|
Memantine (treatment of Alzheimer’s): received the Chinese SFDA’s approval for Phase II Clinical Trial. Dong Ying China’s formulation is the latest generation of the drug and Dong Ying China expects to be one of the first companies to launch the drug in the Chinese market.
|
8
The following table sets forth information relating to different product candidates that Dong Ying China has developed or co-developed with Meisu Jining Bio-medicine Research and Development Co., Ltd., which product candidates are in different stages, many of which require additional funds to support clinical trials, pending for launch in the Chinese market:
|
Development Pipeline
|
Dose
|
Approval Number
(only for Pre-clinic)
|
||
|
Rocuronium Bromide injection
|
25mg/50mg
|
|||
|
Memantine Hydrochloride
|
2005L01497
|
|||
|
Memantine Hydrochloride Tablets
|
5mg
|
2005L01498
|
||
|
Azithromycin Granules
(just passed human trials, pending for approval)
|
0.1g
|
2005L03871
|
In 2006, Dong Ying China wrote-off the intangible asset related to the product rights to Azithromycin as it was deemed not realizable through impairment analysis, however, in 2008, Dong Ying China performed clinical trials on Azithromycin, which just passed human trials in August 2008, and which is pending governmental approval.
Dong Ying China’s Marketing Plan
The Industry
With approximately one-fifth of the world’s population and a fast-growing gross domestic product, China represents a significant potential market for the pharmaceutical industry. The Company expects significant growth in the pharmaceutical market in China due to the following factors: robust economic growth; increased pharmaceutical expenditures; an aging population; increased lifestyle-related diseases; government support of the pharmaceutical industry; relatively low research and development and clinical trial costs in China as compared to developed countries; and the increased availability of funding for medical insurance and industry consolidation in China.
Dong Ying China has an established sales and marketing network that distributes its products in 30 provinces and key major cities throughout China over 700 hospitals. The Company intends to expand Dong Ying China’s sales and marketing infrastructure to meet China’s rapidly growing demand for safer, lower-cost, higher-efficacy drugs.
Strategy
Dong Ying China has a clearly defined strategy to drive short, middle and long term growth. All drug development is expected to leverage Dong Ying China’s patent applied for technology for fast, low-cost drug synthesis and new technology for formulation.
In the short term, the Company’s focus is in launching new drugs in the Chinese market. The patented synthesis method allows Dong Ying China to develop new API at lower cost and new formulations that generally offer greater convenience in application and efficacy with fewer side effects.
In the middle term, Dong Ying China intends to focus on exporting API and drug reformulations developed with Dong Ying China’s patent applied for freeze-dried formulation technology for the global market.
In the long term, Dong Ying China intends to focus on applying its technology platform to identifying candidate compounds from traditional Chinese medicines. Dong Ying China intends to accelerate its discovery, synthesis and formulation of pharmaceutical applications.
Distribution Methods
Dong Ying China sells its prescription-based and pharmaceutical products via regional distributors in China. Dong Ying China intends to expand its sales force as well as its coverage with the regional distributors across China. Currently, Dong Ying China has a marketing department and a sales department. The marketing department is responsible for the promotion of new products to the national market and intended global market by attending industry trade shows, conducting educational seminars to physicians, advertising in newspapers and in industry publications, as well as using internet marketing and collaborating with the government.
The sales department has four regional managers with each manager responsible for his or her assigned region in China, with each region having about 7 to 10 provinces. The four regional managers report directly to the VP of Sales. We staff between 1 to 3 agents per province to be in charge of all the cities in such province, which is how Dong Ying China obtains distribution in all major cities in China.
9
During fiscal year 2008, the majority of Dong Ying China’s sales were conducted through a limited number of regional distributors who subsequently sold Dong Ying China’s products to hospitals, clinics, and pharmacies. Since the fiscal year 2008, Dong Ying China has been expanding its distributor base and believes that the number of its regional distributors will increase substantially in the next few years.
In January 2009, we signed distribution agreements for Cisatracurium Besylate with our 32 distribution agents who distribute our products in 30 provinces and key major cities throughout China.
In the first calendar quarter of 2010, we have renewed distribution contracts with 30 distribution agents totaling US$8.4 million for 2010 for distribution of our flagship product Cisatracurium Besylate. These distributors have coverage in more than 30 provinces throughout China and in most major cities.
Our expansion strategy also includes possible targeted acquisitions intended to expand our first-to-market drug pipeline and advancing new development of innovative drugs that is expected to consolidate leadership position in our chosen therapeutic areas.
Dong Ying China’s Competition
The following table sets forth information with respect to competition in China for Dong Ying China’s flagship product of Cisatracurium Besylate:
|
Competitor
|
Percentage of Market
|
||
|
Jiangsu Hengrui Pharmeutical Co., Ltd.
|
30%
|
||
|
GlaxoSmithKline
|
5%
|
Our competitive advantages are discussed in the subsection entitled “Dong Ying China’s Products” above. Although the above-listed competitors occupy a much smaller percentage of market share than Dong Ying China, which is estimated to account for approximately 35% of the market share for Cisatracurium Besylate in China, the main competition for Cisatracurium Besylate comes from these two companies because they are the only companies that currently produce and sell Cisatracurium Besylate in China.
Intellectual Property
Patents
As of August 31, 2010, the Company acquired the following patent, “Composition for Lyophilized Powder of Atracurium,” previously jointly owned by Lei Wang and Lequn Huang, the Company’s chief executive officer and director, pursuant to a patent transfer agreement by and among the Company, Dong Ying China, Mr. Wang, and Dr. Huang:
|
Title
|
Application No.
|
Application Date
|
Status
|
Patent No.
|
||||
|
Atracurium combination of freeze-dried formulation
|
200710020588.7
|
2007.3.13
|
Granted
|
ZL200710127756.2
|
New Patents under Application:
Dong Ying China has applied for the following patents:
|
Title
|
Application No.
|
Application Date
|
Status
|
Patent No.
|
||||
|
Method for separating and purifying cisatracurium besylate by column chromatography
|
200910028198.3
|
2009.1.16
|
Pending
|
Waiting for approval
|
||||
|
Pharmaceutical composition comprising perindopril
|
200810242989.1
|
2008.12.31
|
Pending
|
Waiting for approval
|
10
Material Contracts
On March, 29, 2004, Cyton International, Inc., the sole shareholder of Dong Ying China at that time, entered into an agreement (the “Development Agreement”) with the Jiangsu Province Nantong Economic and Technology Development Zone Administration (the “Administration”), for the construction of factories in the Jiangsu Nantong Economic and Technology Development Zone (the “Zone”), a state-level economic and technological development zone qualified for state preferential policies. Pursuant to the Development Agreement, Dong Ying China was provided with 60 acres within the Zone for the construction of factories and is required to pay a land-use fee of 40,000RMB per acre. Dong Ying China ended up paying RMB2,248,000 as there only ended up being 56.2 acres provided to Dong Ying China. All land in China is owned by the State. Individuals and companies are permitted to acquire rights to use land or land use rights for specific purposes. In the case of land used for industrial purposes, the land use rights are granted for a period of 50 years. This period may be renewed at the expiration of the initial and any subsequent terms. Granted land use rights are transferable and may be used as security for borrowings and other obligations. Dong Ying China holds the State-owned Land Use Rights Certificate No.: TONGKAIGUOYONG [2007] No. D0310158, and the RMB40,000 per acre is a one-time payment. In addition, the Administration agreed to provide Dong Ying China with a RMB20,000,000 fund (the “Fund”) to support the construction of factories and the building and purchasing of affiliated equipment. Dong Ying China has three years from the date of each portion of the Fund used to repay such portion. The Administration has the right to convert or exchange the Fund into shares of Dong Ying China, but recently the Administration decided to waive the right to convert or exchange the outstanding Fund into shares of Dong Ying China and agreed to the repayment of the Fund by December 31, 2010. Dong Ying China has repaid RMB12,000,000 as of May 31, 2010, and plans to complete the repayment of the outstanding Fund by the end of 2010. Also in accordance with the Development Agreement, Dong Ying China is required to have a registered capital of USD$5,000,000, which has already been satisfied.
At present, Dong Ying China has used all of the RMB20,000,000 in the Fund and has repaid RMB12,000,000 to date. In addition, Dong Ying China is required to repay the following amounts on the following dates:
|
Amount
|
Due Date
|
|
|
RMB8,000,000
|
December 31, 2010
|
On July 26, 2010, Dong Ying China, entered into a Cooperation Agreement with Jiangsu LianHuan Pharmaceuticals Co., Ltd. (“LianHuan”) for the co-development, manufacture, and marketing of the drug Eplerenone, a therapeutic agent formulated to treat high blood pressure and vascular diseases, and its tablet and capsule forms (the “Eplerenone”). Clinical trial certificates for Eplerenone are currently owned by DongYing. According to the Agreement, LianHuan shall be responsible for all fees and expenses incurred in the clinical trials. Following the completion of clinical trials, LianHuan and DongYing shall jointly complete and submit the drug production application. Once production approval for Eplerenone is obtained, LianHuan and DongYing shall jointly own the new drug production certificate for Eplerenone. LianHuan shall be responsible for the manufacture of Eplerenone and its tablet form, though Dong Ying China retains the option to produce Eplerenone capsules. The net profit from the sales of Eplerenone shall be apportioned as 60 percent to Dong Ying China and 40 percent to LianHuan.
Sources and Availability of Materials
Dong Ying China uses over 120 different medical raw materials and procures its raw materials from various vendors and suppliers in China with no limitation. Dong Ying China always requires an analysis report for quality control purposes to document each and every medicinal raw material received from third parties as its standard operating procedure.
In the fiscal year ended May 31, 2010, 2 suppliers accounted for more than 10% of the Company’s purchases. Purchases of raw material from Lianyungang Youlite Biochemical Science and Technology Co., Ltd. accounted for approximately 55% of total purchases during this period. Purchases of raw material from Nanjing Weier Chemical Co.Ltd. accounted for approximately 15 % of total purchases during this period.
In the fiscal year ended May 31, 2009, 3 suppliers accounted for more than 10% of the Company’s purchases. Purchases of raw material from Lianyungang Youlite Biochemical Science and Technology Co., Ltd. accounted for approximately 47% of total purchases during this period. Purchases of raw material from Suzhou Forth Pharmaceutical Co., Ltd. accounted for approximately 13% of total purchases during this period. Purchases of raw material from Nanjing Weier Chemical Co., Ltd accounted for approximately 12% of total purchases during this period.
Customers
In the fiscal year ended May 31, 2010, Dong Ying China’s five largest customers accounted, in the aggregate, for approximately 61% of sales revenues, as follows:
11
|
*
|
Hebei Dezelong Medicine Co., Ltd. - accounted for approximately 21% of sales
|
|
*
|
Hainan Zhongyu Medicine Co., Ltd. - accounted for approximately 20% of sales
|
|
*
|
Sichuang Ruihai Medicine Co., Ltd. - accounted for approximately 7% of sales
|
|
*
|
Hebei Yongzhengrunsheng Medicine Co., Ltd. - accounted for approximately 7% of sales
|
|
*
|
Zhengzhou Changjin Medicine Co., Ltd - accounted for approximately 6% of sales
|
Research and Development
Research and development expenses were $ 816,262 in the year ended May 31, 2010, compared to $197,286 in the year ended May 31, 2009, which increased as a result of expenses spent on Perindopril, the new product that the Company launched in 2010.
Employees
Dong Ying China has over 110 full time employees, 40 of whom have college or higher education, and 10 research specialists.
Governmental Regulation
The Chinese SFDA regulates pharmaceutical products in China. Dong Ying China takes the following steps to gain approval from the Chinese SFDA to sell its products in China:
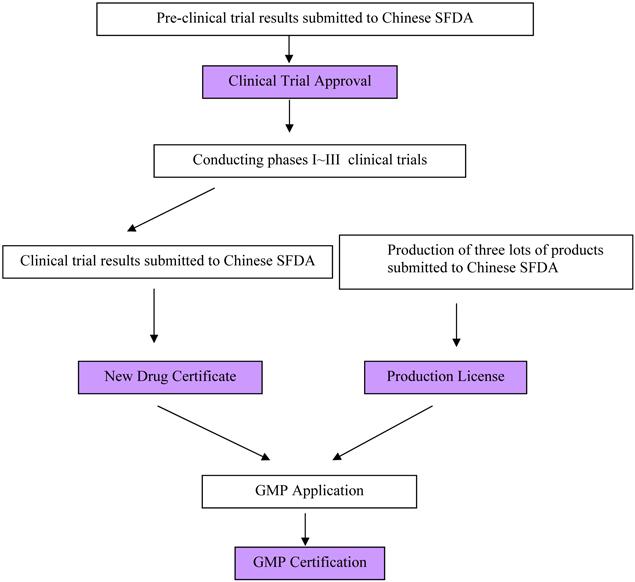
12
Pre-Clinical Study: The pre-clinical studies are divided into two parts: the pharmacy study and the pharmaco-toxicological studies. The pharmacy study includes the construction of the expression strain, the potency & Antigenicity studies, the Batch Production Reports, the data from Quality Control and the preliminary stability test. The pharmaco-toxicological studies include the acute toxic study, long-term toxicity, efficacy study, the pharmacokinetics and the other studies. All these study reports need to be submitted to the Chinese SFDA for review and evaluation. Meanwhile, Dong Ying China is required to produce three continuous lots of bulk and final products and send the samples to the National Institute for the Control of Pharmaceutical and Biological Products (the “NICPBP”) for quality inspection. The NICPBP is directly affiliated with the Chinese SFDA and its responsibility is for inspecting the quality of new products and vaccines.
Clinical Trial Approval and Conduct: During the period of evaluation, the Chinese SFDA may ask Dong Ying China to submit supplementary documents for further evaluation. If the Chinese SFDA is satisfied with everything, then the Chinese SFDA may approve the Phase I trial. After the end of the Phase I trial, Dong Ying China needs to submit the final clinical reports of Phase I trial to the Chinese SFDA for their review and evaluation. If no safety issues are visible or detected in the reports, the approvals for the Phase II trial may be issued. The same process occurs in order to receive the approvals for Phase III.
New Drug Certificate & Production License: Once all three phases of the clinical trial are complete, Dong Ying China must submit all clinical dossiers, including Phase I to Phase III, to the Chinese SFDA. Meanwhile the samples of three lots of bulk and final products manufactured in the Company’s GMP facilities also need to be submitted to the Chinese SFDA for quality inspection. If no serious adverse events are reported, the product shows a good efficacy in the trial, and the bulk and the final products pass quality inspection, the Chinese SFDA may issue the New Drug Certificate & Production License for a new product.
The New Drug Certificate & Production License grants the filing company intellectual property rights to the drug. Chinese administrative protection for the new proprietary drug starts from the date of the issuance of the new drug certificate.
Good Manufacturing Practice Certificate: The GMP certificate is for the monitoring of drug manufacturers and quality management. After receiving a new drug certificate and production license, the company submits an application for GMP certification. The Chinese SFDA will organize a group of specialists to validate the GMP facilities if the facilities satisfy the current GMP requirements. After GMP validation, if qualified, the GMP certificate may be issued. This provides approval for the equipment and control of the manufacturing workshop of the particular drug.
A new drug is only officially approved for sale in the Chinese market once these steps have been completed. The Company believes that the most significant milestone is the new drug certificate.
Environmental Compliance Costs
Our manufacturing operations are subject to numerous laws, regulations, rules and specifications relating to human health and safety and the environment. These laws and regulations address and regulate, among other matters, wastewater discharge, air quality and the generation, handling, storage, treatment, disposal and transportation of solid and hazardous wastes and releases of hazardous substances into the environment. In addition, third parties and governmental agencies in some cases have the power under such laws and regulations to require remediation of environmental conditions and, in the case of governmental agencies, to impose fines and penalties. We make capital expenditures from time to time to stay in compliance with applicable laws and regulations.
We have obtained all permits and approvals and filed all registrations required for the conduct of our business, except where the failure to obtain any permit or approval or file any registration would not have a material adverse effect on our business, financial condition and results of operations. We are in compliance in all material respects with the numerous laws, regulations, rules, specifications and permits, approvals and registrations relating to human health and safety and the environment except where noncompliance would not have a material adverse effect on our business, financial condition and results of operations.
The PRC governmental authorities have not revealed any material environmental liability that would have a material adverse effect on us. We have not been notified by any governmental authority of any continuing noncompliance, liability or other claim in connection with any of our properties or business operations, nor are we aware of any other material environmental condition with respect to any of our properties or arising out of our business operations at any other location.
It is possible that compliance with a new regulatory requirement could impose significant compliance costs on us. Such costs could have a material adverse effect on our business, financial condition and results of operations.
13
Executive Offices
Our executive offices in the PRC are located at 8 Zhong Tian Road, Nantong City, Jiangsu Province, People’s Republic of China. Our telephone is 011 - (86) 51-3853-28336.
Available Information
The Company’s website is www.sinobp.com, where information about the Company may be reviewed and obtained. In addition, the Company’s filings with the Securities and Exchange Commission (“SEC”) may be accessed at the internet address of the SEC, which is http://www.sec.gov. Also, the public may read and copy any materials that the Company files with at the SEC’s Public Reference Room at 100 F Street, N.E., Room 1580 Washington, D.C. 20549. The public may obtain information on the operation of the Public Reference Room by calling the SEC at 1-800-SEC-0330.
Item 1A. Risk Factors.
Risks Relating to our Business
Our operating history may not serve as an adequate basis to judge our future prospects and results of operations.
Dong Ying China commenced its current line of business operations on September 29, 2003 and has received Good Manufacturing Practices (“GMP”) certifications for its production lines that are valid through January 27, 2006 and January 26, 2011, respectively. This certification must be renewed every five years for Dong Ying China to stay in business. Dong Ying China’s operating history may not provide a meaningful basis on which to evaluate its business. We cannot assure you that Dong Ying China will maintain its profitability or that we will not incur net losses in the future. We expect that Dong Ying China’s operating expenses will increase as it expands. Any significant failure to realize anticipated revenue growth could result in significant operating losses. We will continue to encounter risks and difficulties frequently experienced by companies at a similar stage of development, including our potential failure to:
* raise adequate capital for expansion and operations;
* implement our business model and strategy and adapt and modify them as needed;
* increase awareness of our brand name, protect our reputation and develop customer loyalty;
* manage our expanding operations and service offerings, including the integration of any future acquisitions;
* maintain adequate control of our expenses; and
* anticipate and adapt to changing conditions in the medical over the counter, pharmaceutical and nutritional supplement markets in which we operate as well as the impact of any changes in government regulations, mergers and acquisitions involving our competitors, technological developments and other significant competitive and market dynamics.
If we are not successful in addressing any or all of these risks, our business may be materially and adversely affected.
Dong Ying China’s failure to compete effectively may adversely affect our ability to generate revenue.
Dong Ying China competes with other companies, many of whom are developing or can be expected to develop products similar to Dong Ying China. Dong Ying China’s market is a large market with many competitors. Many of its competitors are more established than Dong Ying China is, and have significantly greater financial, technical, marketing and other resources than it presently possess. Some of Dong Ying China’s competitors have greater name recognition and a larger customer base. These competitors may be able to respond more quickly to new or changing opportunities and customer requirements and may be able to undertake more extensive promotional activities, offer more attractive terms to customers, and adopt more aggressive pricing policies. We cannot assure you that Dong Ying China will be able to compete effectively with current or future competitors or that the competitive pressures it faces will not harm it business.
We may not be able to effectively control and manage the growth of Dong Ying China.
If Dong Ying China’s business and markets grow and develop, it will be necessary for us to finance and manage expansion in an orderly fashion. An expansion would increase demands on existing management, workforce and facilities. Failure to satisfy such increased demands could interrupt or adversely affect its operations and cause delay in production and delivery of its pharmaceutical prescription, over the counter and medical nutrient products as well as administrative inefficiencies.
14
We may require additional financing in the future and a failure to obtain such required financing will inhibit Dong Ying China’s ability to grow.
The continued growth of Dong Ying China’s business may require additional funding from time to time, which we expect to raise in private placements of our equity or debt securities with accredited investors or by offering our securities for sale pursuant to an effective registration statement on a market where our common stock is traded. The proceeds of these funding will be forwarded to Dong Ying China and accounted for as a loan to Dong Ying China and eliminated during consolidation. The proceeds would be used for general corporate purposes of Dong Ying China, which could include acquisitions, investments, repayment of debt and capital expenditures among other things. We may also use the proceeds to repurchase our capital stock or for our corporate overhead expenses. If we borrow funds we expect to be the primary obligor on any debt. Obtaining additional funding would be subject to a number of factors including market conditions, operating performance and investor sentiment, many of which are outside of our control. These factors could make the timing, amount, terms and conditions of additional funding unattractive or unavailable to us. Our management believes that we currently have sufficient funds from working capital to meet our current operating costs over the next 12 months.
The terms of any future financing may adversely affect your interest as shareholders.
If we require additional financing in the future, we may be required to incur indebtedness or issue equity securities, the terms of which may adversely affect your interests in us. For example, the issuance of additional indebtedness may be senior in right of payment to your shares upon our liquidation. In addition, indebtedness may be under terms that make the operation of Dong Ying China’s business more difficult because the lender's consent could be required before we take certain actions. Similarly the terms of any equity securities we issue may be senior in right of payment of dividends to your common stock and may contain superior rights and other rights as compared to your common stock. Further, any such issuance of equity securities may dilute your interest in us.
We, through our subsidiaries/affiliated companies Dongying BVI, Big Global Limited or Dong Ying China, may engage in future acquisitions that could dilute the ownership interests of our shareholders, cause us to incur debt and assume contingent liabilities.
We, through our subsidiaries/affiliated companies Dongying BVI, Big Global Limited, or Dong Ying China, may review acquisition and strategic investment prospects that we believe would complement the current product offerings of Dong Ying China, augment its market coverage or enhance its technical capabilities, or otherwise offer growth opportunities. From time to time Dong Ying China reviews investments in new businesses and we, through our subsidiaries/affiliated companies Dongying BVI, Big Global Limited, or Dong Ying China, expect to make investments in, and to acquire, businesses, products, or technologies in the future. We expect that when we raise funds from investors for any of these purposes we will be either the issuer or the primary obligor while the proceeds will be forwarded to Dong Ying China and accounted for as a loan to Dong Ying China and eliminated during consolidation. In the event of any future acquisitions, we could:
|
*
|
issue equity securities which would dilute current shareholders’ percentage ownership;
|
|
*
|
incur substantial debt;
|
|
*
|
assume contingent liabilities; or
|
|
*
|
expend significant cash.
|
These actions could have a material adverse effect on our operating results or the price of our common stock. Moreover, even if through our subsidiary, Big Global Limited or Dong Ying China, we do obtain benefits in the form of increased sales and earnings, there may be a lag between the time when the expenses associated with an acquisition are incurred and the time when we recognize such benefits. Acquisitions and investment activities also entail numerous risks, including:
|
*
|
difficulties in the assimilation of acquired operations, technologies and/or products;
|
|
*
|
unanticipated costs associated with the acquisition or investment transaction;
|
We may not have adequate internal accounting controls. While we have certain internal procedures in our budgeting, forecasting and in the management and allocation of funds, our internal controls may not be adequate.
We are constantly striving to improve our internal accounting controls. We expect to continue to improve our internal accounting control for budgeting, forecasting, managing and allocating our funds and to better account for them as we grow. There is no guarantee that such improvements will be adequate or successful or that such improvements will be carried out on a timely basis. If we do not have adequate internal accounting controls, we may not be able to appropriately budget, forecast and manage our funds, we may also be unable to prepare accurate accounts on a timely basis to meet our continuing financial reporting obligations and we may not be able to satisfy our obligations under US securities laws.
15
Rules adopted by the SEC pursuant to Section 404 of the Sarbanes-Oxley Act of 2002 require annual assessment of our internal control over financial reporting, and attestation of this assessment by our company's independent registered public accountants. The SEC has extended the compliance dates for "non-accelerated filers," as defined by the SEC, for the 2010 fiscal year, and, given the recent passage of the Dodd-Frank Wall Street Reform and Consumer Protection Act, we expect the SEC to permanently exempt smaller reporting companies from the attestation requirement. Notwithstanding the above, this exemption does not affect the requirement that we include a report of management on our internal controls over financial reporting and will not affect the requirement to include the auditor’s attestation if our public float exceeds $75 million and we cease to be a smaller reporting company. Additionally, the standards that must be met for management to assess the internal control over financial reporting as effective are new and complex, and require significant documentation, testing and possible remediation to meet the detailed standards. Though we have evaluated our internal controls over financial reporting in order to allow management to report on our internal controls over financial reporting, as will be required by Section 404 of the Sarbanes-Oxley Act of 2002 and the rules and regulations of the SEC, our lack of familiarity with Section 404 may unduly divert management's time and resources in executing the business plan. If, in the future, management identifies one or more material weaknesses, or our external auditors are unable to express an opinion on the effectiveness of our internal controls, this could result in a loss of investor confidence in our financial reports, have an adverse effect on our stock price and/or subject us to sanctions or investigation by regulatory authorities. So far, our external auditors have not reported to our board of directors any significant weakness on our internal control and provided recommendations accordingly.
We are dependent on certain key personnel and loss of these key personnel could have a material adverse effect on our business, financial condition and results of operations.
Our success is, to a certain extent, attributable to the management, sales and marketing, and pharmaceutical factory operational expertise of key personnel. Dr. Lequn Lee Huang, our President and Chief Executive Officer, and Xinjie Mu, our Chief Financial Officer, perform key functions in the operation of our and Dong Ying China’s business. There can be no assurance that Dong Ying China will be able to retain these officers. The loss of these officers could have a material adverse effect upon our business, financial condition, and results of operations. Dong Ying China must attract, recruit and retain a sizeable workforce of technically competent employees. We do not carry key man life insurance for any of our key personnel or personnel nor do we foresee purchasing such insurance to protect against a loss of key personnel and the key personnel.
We are dependent upon the services of Dr. Huang and Mr. Mu, for the continued growth and operation of our company because of their experience in the industry and their personal and business contacts in the PRC. We have no reason to believe that they will discontinue their services with the Company or Dong Ying China, the interruption or loss of their services would adversely affect our ability to effectively run our business and pursue our business strategy as well as our results of operations.
We may not be able to hire and retain qualified personnel to support its growth and if it is unable to retain or hire these personnel in the future, its ability to improve its products and implement its business objectives could be adversely affected.
Competition for senior management and senior personnel in the PRC is intense, the pool of qualified candidates in the PRC is very limited, and we may not be able to retain the services of our senior executives or senior personnel, or attract and retain high-quality senior executives or senior personnel in the future. This failure could materially and adversely affect our future growth and financial condition. We expect to hire additional sales and plant personnel throughout fiscal year 2010 in order to accommodate our growth.
If we fail to increase our brand recognition, we may face difficulty in obtaining new customers and business partners.
We believe that establishing, maintaining and enhancing our brand in a cost-effective manner is critical to achieving widespread acceptance of our current and future products and services and is an important element in our effort to increase our customer base and obtain new business partners. We believe that the importance of brand recognition will increase as competition in our market develops. Some of our potential competitors already have well-established brands in the pharmaceutical promotion and distribution industry. Successful promotion of our brand will depend largely on our ability to maintain a sizeable and active customer base, our marketing efforts and ability to provide reliable and useful products and services at competitive prices. Brand promotion activities may not yield increased revenue, and even if they do, any increased revenue may not offset the expenses we will incur in building our brand. If we fail to successfully promote and maintain our brand, or if we incur substantial expenses in an unsuccessful attempt to promote and maintain our brand, we may fail to attract enough new customers or retain our existing customers to the extent necessary to realize a sufficient return on our brand-building efforts, in which case our business, operating results and financial condition, would be materially adversely affected.
16
Our operating results may fluctuate as a result of factors beyond our control.
Our operating results may fluctuate significantly in the future as a result of a variety of factors, many of which are beyond our control. These factors include:
|
*
|
the costs of pharmaceutical products and development;
|
|
*
|
the relative speed and success with which we can obtain and maintain customers, merchants and vendors for our products;
|
|
*
|
capital expenditure for equipment;
|
|
*
|
marketing and promotional activities and other costs;
|
|
*
|
changes in our pricing policies, suppliers and competitors;
|
|
*
|
the ability of our suppliers to provide products in a timely manner to their customers;
|
|
*
|
changes in operating expenses;
|
|
*
|
increased competition in the pharmaceutical markets; and
|
|
*
|
other general economic and seasonal factors.
|
We face risks related to product liability claims.
We presently do not maintain product liability insurance. We face the risk of loss because of adverse publicity associated with product liability lawsuits, whether or not such claims are valid. We may not be able to avoid such claims. Although product liability lawsuits in the PRC are rare, and we have not, to date, experienced significant failure of our products, there is no guarantee that we will not face such liability in the future. This liability could be substantial and the occurrence of such loss or liability may have a material adverse effect on our business, financial condition and prospects.
We face marketing risks.
Newly developed drugs and technology may not be compatible with market needs. Because markets for drugs differentiate geographically inside the PRC, we must develop and manufacture our products to accurately target specific markets to ensure product sales. If we fail to invest in extensive market research to understand the health needs of consumers in different geographic areas, we may face limited market acceptance of our products, which could have material adverse effect on our sales and earnings.
We face risks relating to difficulty in defending intellectual property rights from infringement.
Our success depends on protection of our current and future technology and products and our ability to defend our intellectual property rights. Dong Ying China has filed for patent protection for various products that it sells, or plans to sell, in the PRC. However, it is possible for its competitors to develop similar competitive products even though it has taken steps to protect its intellectual property. If we fail to protect Dong Ying China’s intellectual property adequately, competitors may manufacture and market products similar to Dong Ying China. We expect to file patent applications seeking to protect newly developed technology and products in various countries, including the PRC. Some patent applications in the PRC are maintained in secrecy until the patent is issued. Because the publication of discoveries tends to follow their actual discovery by many months, we may not be the first to invent, or file patent applications on any of our discoveries. Patents may not be issued with respect to any of our patent applications and existing or future patents issued to or licensed by us may not provide competitive advantages for our products. Patents that are issued may be challenged, invalidated or circumvented by our competitors. Furthermore, our patent rights may not prevent our competitors from developing, using or commercializing products that are similar or functionally equivalent to our products.
We also rely on trade secrets, non-patented proprietary expertise and continuing technological innovation that we shall seek to protect, in part, by entering into confidentiality agreements with licensees, suppliers, employees and consultants. These agreements may be breached and there may not be adequate remedies in the event of a breach. Disputes may arise concerning the ownership of intellectual property or the applicability of confidentiality agreements. Moreover, our trade secrets and proprietary technology may otherwise become known or be independently developed by our competitors. If patents are not issued with respect to products arising from research, we may not be able to maintain the confidentiality of information relating to these products.
17
We face risks relating to third parties that may claim that we infringe on their proprietary rights and may prevent us from manufacturing and selling certain of our products.
There has been substantial litigation in the pharmaceutical industry with respect to the manufacturing, use and sale of new products. These lawsuits relate to the validity and infringement of patents or proprietary rights of third parties. We may be required to commence or defend against charges relating to the infringement of patents or proprietary rights. Any such litigation could:
|
*
|
require us to incur substantial expense, even if covered by insurance or are successful in the litigation;
|
|
*
|
require us to divert significant time and effort of our technical and management personnel;
|
|
*
|
result in the loss of our rights to develop or make certain products; and
|
|
*
|
require us to pay substantial monetary damages or royalties in order to license proprietary rights from third parties.
|
Although intellectual property disputes within the pharmaceutical industry have often been settled through licensing or similar arrangements, costs associated with these arrangements may be substantial and could include the long-term payment of royalties. These arrangements may be investigated by regulatory agencies and, if improper, may be invalidated. Furthermore, the required licenses may not be made available to us on acceptable terms. Accordingly, an adverse determination in a judicial or administrative proceeding or a failure to obtain necessary licenses could prevent us from manufacturing and selling some of our products or increase our costs to market these products.
In addition, when seeking regulatory approval for some of our products, we may be required to certify to regulatory authorities, including the SFDA, that such products do not infringe upon third party patent rights. Filing a certification against a patent gives the patent holder the right to bring a patent infringement lawsuit against us. Any lawsuit would delay the receipt of regulatory approvals. A claim of infringement and the resulting delay could result in substantial expenses and even prevent us from manufacturing and selling certain of our products.
Our launch of a product prior to a final court decision or the expiration of a patent held by a third party may result in substantial damages to us. If we are found to infringe a patent held by a third party and become subject to such damages, these damages could have a material adverse effect on the results of our operations and financial condition.
We face risks related to research and the ability to develop new drugs.
Our growth and survival depends on our ability to consistently discover, develop and commercialize new products and find new and improve on existing technology and platforms. As such, if we fail to make sufficient investments in research, be attentive to consumer needs or does not focus on the most advanced technology, our current and future products could be surpassed by more effective or advanced products of other companies.
|
|
Risks Related To Doing Business in the PRC
|
Changes in the policies of the PRC government could have a significant impact upon the business we may be able to conduct in the PRC and the profitability of such business.
Our business operations may be adversely affected by the current and future political environment in the PRC. The PRC has operated as a socialist state since the mid-1900s and is controlled by the Communist Party of China. The PRC government exerts substantial influence and control over the manner in which we and it must conduct our business activities. The PRC has only permitted provincial and local economic autonomy and private economic activities since 1978. The PRC government has exercised and continues to exercise substantial control over virtually every sector of the PRC economy, including the paper industry, through regulation and state ownership. Our ability to operate in the PRC may be adversely affected by changes in the PRC laws and regulations, including those relating to taxation, import and export tariffs, raw materials, environmental regulations, land use rights, property and other matters. Under current leadership, the PRC government has been pursuing economic reform policies that encourage private economic activity and greater economic decentralization. There is no assurance, however, that the PRC government will continue to pursue these policies, or that it will not significantly alter these policies from time to time without notice.
The PRC’s economy is in a transition from a planned economy to a market oriented economy subject to five-year and annual plans adopted by the government that set national economic development goals. Policies of the PRC government can have significant effects on the economic conditions of the PRC. The PRC government has confirmed that economic development will follow the model of a market economy. Under this direction, we believe that the PRC will continue to strengthen its economic and trading relationships with foreign countries and business development in the PRC will follow market forces. While we believe that this trend will continue, there can be no assurance that this will be the case.
18
A change in policies by the PRC government could adversely affect our interests by, among other factors: changes in laws, regulations or the interpretation thereof, confiscatory taxation, restrictions on currency conversion, imports or sources of supplies, or the expropriation or nationalization of private enterprises. Although the PRC government has been pursuing economic reform policies for more than two decades, there is no assurance that the government will continue to pursue such policies or that such policies may not be significantly altered, especially in the event of a change in leadership, social or political disruption, or other circumstances affecting the PRC’s political, economic and social life.
The PRC laws and regulations governing our current business operations are sometimes vague and uncertain. Any changes in such PRC laws and regulations may harm our business.
The PRC laws and regulations governing our current business operations are sometimes vague and uncertain. The PRC’s legal system is a civil law system based on written statutes, in which system decided legal cases have little value as precedents unlike the common law system prevalent in the United States. There are substantial uncertainties regarding the interpretation and application of PRC laws and regulations, including but not limited to the laws and regulations governing our business, or the enforcement and performance of our arrangements with customers in the event of the imposition of statutory liens, death, bankruptcy and criminal proceedings. The PRC government has been developing a comprehensive system of commercial laws, and considerable progress has been made in introducing laws and regulations dealing with economic matters such as foreign investment, corporate organization and governance, commerce, taxation and trade. However, because these laws and regulations are relatively new, and because of the limited volume of published cases and judicial interpretation and their lack of force as precedents, interpretation and enforcement of these laws and regulations involve significant uncertainties. New laws and regulations that affect existing and proposed future businesses may also be applied retroactively. Our operating entity, Dong Ying China, conduct operations in China, and as a result, we are required to comply with PRC laws and regulations. We cannot assure you that our current ownership and operating structure would not be found in violation of any current or future PRC laws or regulations. Any of these or similar actions could significantly disrupt our business operations or restrict us from conducting a substantial portion of our business operations, which could materially and adversely affect our business, financial condition and results of operations. We cannot predict what effect the interpretation of existing or new PRC laws or regulations may have on our business. If the relevant authorities find that we are in violation of PRC laws or regulations, they would have broad discretion in dealing with such a violation, including, without limitation:
|
*
|
levying fines;
|
|
*
|
revoking Dong Ying China’s business and other licenses;
|
|
*
|
requiring that we restructure our ownership or operations; and
|
|
*
|
requiring that we discontinue any portion or all of our business.
|
Among the material laws that we are subject to are the Price Law of The People’s Republic of China, Measurement Law of The People’s Republic of China, Tax Law, Environmental Protection Law, Contract Law, Patent Law, Accounting Laws, Corporation Laws and Labor Law.
A slowdown, inflation or other adverse developments in the PRC economy may harm our customers and the demand for our services and products.
All of our operations are conducted in the PRC and all of our revenue is generated from sales in the PRC. Although the PRC economy has grown significantly in recent years, we cannot assure you that this growth will continue. A slowdown in overall economic growth, an economic downturn, a recession or other adverse economic developments in the PRC could significantly reduce the demand for our products and harm our business.
While the PRC economy has experienced rapid growth, such growth has been uneven among various sectors of the economy and in different geographical areas of the country. Rapid economic growth could lead to growth in the money supply and rising inflation. If prices for our products rise at a rate that is insufficient to compensate for the rise in the costs of supplies, it may harm our profitability. In order to control inflation in the past, the PRC government has imposed controls on bank credit, limits on loans for fixed assets and restrictions on state bank lending. Such an austere policy can lead to a slowing of economic growth. In January 2010, the People’s Bank of China, the PRC’s central bank, raised interest rates for the first time in nearly five months. Repeated rises in interest rates by the central bank would likely slow economic activity in the PRC which could, in turn, materially increase our costs and also reduce demand for our products.
Any recurrence of severe acute respiratory syndrome, or SARS, or another widespread public health problem, could harm our operations.
A renewed outbreak of SARS or another widespread public health problem (such as bird flu) in the PRC, where all of our revenue is derived, could significantly harm our operations. Our operations may be impacted by a number of health-related factors, including quarantines or closures of some of our offices that would adversely disrupt our operations. Any of the foregoing events or other unforeseen consequences of public health problems could significantly harm our operations.
19
Governmental control of currency conversion may affect the value of your investment.
The PRC government imposes controls on the convertibility of Renminbi into foreign currencies and, in certain cases, the remittance of currency out of the PRC. We receive substantially all of our revenue in Renminbi, which is currently not a freely convertible currency. Shortages in the availability of foreign currency may restrict our ability to remit sufficient foreign currency to pay dividends, or otherwise satisfy foreign currency dominated obligations. Under existing PRC foreign exchange regulations, payments of current account items, including profit distributions, interest payments and expenditures from the transaction, can be made in foreign currencies without prior approval from the PRC State Administration of Foreign Exchange by complying with certain procedural requirements. However, approval from appropriate governmental authorities is required where Renminbi is to be converted into foreign currency and remitted out of the PRC to pay capital expenses such as the repayment of bank loans denominated in foreign currencies.
The PRC government may also in the future restrict access to foreign currencies for current account transactions. If the foreign exchange control system prevents us from obtaining sufficient foreign currency to satisfy our currency demands, we may not be able to pay certain of our expenses as they come due.
The fluctuation of the Renminbi may harm your investment.
The value of the Renminbi against the U.S. dollar and other currencies may fluctuate and is affected by, among other things, changes in the PRC’s political and economic conditions. According to the currency website xe.com, as of May 31, 2010, $1 = 6.83 Yuan (RMB). As we rely entirely on revenues earned in the PRC, any significant revaluation of the Renminbi may materially and adversely affect our cash flows, revenues and financial condition. For example, to the extent that we need to convert U.S. dollars we receive from an offering of our securities into Renminbi for Dong Ying China’s operations, appreciation of the Renminbi against the U.S. dollar would diminish the value of the proceeds of the offering and this could harm our business, financial condition and results of operations because it would reduce the proceeds available to us for capital investment in proportion to the appreciation of the Renminbi. Thus if we raise 1,000,000 dollars and the Renminbi appreciates against the U.S. dollar by 15%, then the proceeds will be worth only RMB 5,940,000 as opposed to RMB 6,830,000 prior to the appreciation. Conversely, if we decide to convert our Renminbi into U.S. dollars for the purpose of making payments for dividends on our common shares or for other business purposes and the U.S. dollar appreciates against the Renminbi; the U.S. dollar equivalent of the Renminbi we convert would be reduced in proportion to the amount the U.S. dollar appreciates. In addition, the depreciation of significant RMB denominated assets could result in a charge to our income statement and a reduction in the dollar value of these assets. Thus if Dong Ying China has RMB1,000,000 in assets and Renminbi is depreciated against the U.S. dollar by 15%, then the assets will be valued at $127,337 as opposed to $146,438 prior to the depreciation.
On July 21, 2005, the PRC government changed its decade-old policy of pegging the value of the Renminbi to the U.S. dollar. Under the new policy, the Renminbi is permitted to fluctuate within a narrow and managed band against a basket of certain foreign currencies. This change in policy has resulted in an approximately 21% appreciation of the Renminbi against the U.S. dollar as of May 31, 2010, based on the exchange rate of the base period at $1 = 8.27 Yuan on July 21, 2005. While the international reaction to the Renminbi revaluation has generally been positive, there remains significant international pressure on the PRC government to adopt an even more flexible currency policy, which could result in a further and more significant appreciation of the Renminbi against the U.S. dollar.
PRC State Administration of Foreign Exchange ("SAFE") regulations regarding offshore financing activities by PRC residents which may increase the administrative burden we face. The failure by our shareholders who are PRC residents to make any required applications and filings pursuant to such regulations may prevent us from being able to distribute profits and could expose us and our PRC resident shareholders to liability under PRC law.
SAFE issued a public notice ("SAFE #75") effective from November 1, 2005, which requires registration with SAFE by the PRC resident shareholders of any foreign holding company of a PRC entity. Without registration, the PRC entity cannot remit any of its profits out of the PRC as dividends or otherwise.
In October 2005, SAFE issued a public notice, the Notice on Relevant Issues in the Foreign Exchange Control over Financing and Return Investment Through Special Purpose Companies by Residents Inside China, or the SAFE notice, which requires PRC residents, including both legal persons and natural persons, to register with the competent local SAFE branch before establishing or controlling any company outside of the PRC, referred to as an "offshore special purpose company," for the purpose of overseas equity financing involving onshore assets or equity interests held by them. In addition, any PRC resident that is the shareholder of an offshore special purpose company is required to amend its SAFE registration with the local SAFE branch with respect to that offshore special purpose company in connection with any increase or decrease of capital, transfer of shares, merger, division, equity investment or creation of any security interest over any assets located in the PRC. Moreover, if the offshore special purpose company was established and owned the onshore assets or equity interests before the implementation date of the SAFE notice, a retroactive SAFE registration is required to have been completed before March 31, 2006. If any PRC shareholder of any offshore special purpose company fails to make the required SAFE registration and amendment, the PRC subsidiaries of that offshore special purpose company may be prohibited from distributing their profits and the proceeds from any reduction in capital, share transfer or liquidation to the offshore special purpose company. Moreover, failure to comply with the SAFE registration and amendment requirements described above could result in liability under PRC laws for evasion of applicable foreign exchange restrictions.
20
It is unclear whether our other PRC resident shareholders must make disclosure to SAFE. While our PRC counsel has advised us that only PRC resident shareholders who receive ownership of the foreign holding company in exchange for ownership in the PRC operating company are subject to SAFE #75, there can be no assurance that SAFE will not require our other PRC resident shareholders to register and make the applicable disclosure. In addition, SAFE #75 requires that any monies remitted to PRC residents outside of the PRC be returned within 180 days; however, there is no indication of what the penalty will be for failure to comply or if shareholder non-compliance will be considered to be a violation of SAFE #75 by us or otherwise affect us.
In the event that the proper procedures are not followed under SAFE #75, Big Global Limited could lose the ability to remit monies outside of the PRC and would therefore be unable to pay dividends or make other distributions. Our PRC resident shareholders could be subject to fines, other sanctions and even criminal liabilities under the PRC Foreign Exchange Administrative Regulations promulgated January 29, 1996, as amended.
The PRC’s legal and judicial system may not adequately protect our business and operations and the rights of foreign investors.
The PRC legal and judicial system may negatively impact foreign investors. In 1982, the National People’s Congress amended the Constitution of China to authorize foreign investment and guarantee the “lawful rights and interests” of foreign investors in the PRC. However, the PRC’s system of laws is not yet comprehensive. The legal and judicial systems in the PRC are still rudimentary, and enforcement of existing laws is inconsistent. Many judges in the PRC lack the depth of legal training and experience that would be expected of a judge in a more developed country. Because the PRC judiciary is relatively inexperienced in enforcing the laws that do exist, anticipation of judicial decision-making is more uncertain than would be expected in a more developed country. It may be impossible to obtain swift and equitable enforcement of laws that do exist, or to obtain enforcement of the judgment of one court by a court of another jurisdiction. The PRC’s legal system is based on the civil law regime, that is, it is based on written statutes; a decision by one judge does not set a legal precedent that is required to be followed by judges in other cases. In addition, the interpretation of the PRC laws may be varied to reflect domestic political changes.
The promulgation of new laws, changes to existing laws and the pre-emption of local regulations by national laws may adversely affect foreign investors. However, the trend of legislation over the last 20 years has significantly enhanced the protection of foreign investment and allowed for more control by foreign parties of their investments in the PRC enterprises. There can be no assurance that a change in leadership, social or political disruption, or unforeseen circumstances affecting the PRC’s political, economic or social life, will not affect the PRC government’s ability to continue to support and pursue these reforms. Such a shift could have a material adverse effect on our business and prospects.
The practical effect of the PRC legal system on our business operations in the PRC can be viewed from two separate but intertwined considerations. First, as a matter of substantive law, the foreign invested enterprise laws provide significant protection from government interference. In addition, these laws guarantee the full enjoyment of the benefits of corporate articles and contracts to foreign invested enterprise participants. These laws, however, do impose standards concerning corporate formation and governance, which are qualitatively different from the general corporation laws of the United States. Similarly, the PRC accounting laws mandate accounting practices, which are not consistent with U.S. generally accepted accounting principles. PRC’s accounting laws require that an annual “statutory audit” be performed in accordance with PRC accounting standards and that the books of account of foreign invested enterprises are maintained in accordance with Chinese accounting laws. Article 14 of the People’s Republic of China Wholly Foreign-Owned Enterprise Law requires a wholly foreign-owned enterprise to submit certain periodic fiscal reports and statements to designated financial and tax authorities, at the risk of business license revocation. While the enforcement of substantive rights may appear less clear than United States procedures, foreign invested enterprises and wholly foreign-owned enterprises are Chinese registered companies, which enjoy the same status as other Chinese registered companies in business-to-business dispute resolution. Any award rendered by an arbitration tribunal is enforceable in accordance with the United Nations Convention on the Recognition and Enforcement of Foreign Arbitral Awards (1958). Therefore, as a practical matter, although no assurances can be given, the PRC legal infrastructure, while different in operation from its United States counterpart, should not present any significant impediment to the operation of foreign invested enterprises.
Because our principal assets are located outside of the United States and most of our directors and officers reside outside of the United States, it may be difficult for you to enforce your rights based on U.S. federal securities laws against us and our officers or to enforce U.S. court judgment against us or them in the PRC.
Most of our directors and officers reside outside of the United States. In addition, our operating company is located in the PRC and substantially all of our assets are located outside of the United States. It may therefore be difficult for investors in the United States to enforce their legal rights based on the civil liability provisions of the U.S. Federal securities laws against us in the courts of either the U.S. or the PRC and, even if civil judgments are obtained in U.S. courts, to enforce such judgments in PRC courts. Further, it is unclear if extradition treaties now in effect between the United States and the PRC would permit effective enforcement against us or our officers and directors of criminal penalties, under the U.S. Federal securities laws or otherwise.
21
Our management is not familiar with the United States securities laws.
Our management is generally unfamiliar with the requirements of the United States securities laws and may not appreciate the need to devote the resources necessary to comply with such laws. A failure to adequately respond to applicable securities laws could lead to investigations by the Securities and Exchange Commission and other regulatory authorities that could be costly, divert management's attention and disrupt our business.
Risks Related to Our Common Stock
Our officers and directors control us through their positions and stock ownership and their interests may differ from other shareholders.
As of September 14, 2010, there were 117,587,608 shares of our common stock issued and outstanding. Our officers and directors own approximately 22.82% of our common stock. As a result, they are able to influence the outcome of shareholder votes on various matters, including the election of directors and extraordinary corporate transactions including business combinations. Yet our officers’ and directors’ interests may differ from those of other shareholders. Furthermore, ownership of 22.82% of our common stock by our officers and directors reduces the public float and liquidity, and may affect the market price, of our common stock when and if our common stock becomes eligible to trade on the OTCBB.
We are not likely to pay cash dividends in the foreseeable future.
We intend to retain any future earnings for use in the operation and expansion of our business. We do not expect to pay any cash dividends in the foreseeable future but will review this policy as circumstances dictate. Should we decide in the future to do so, as a holding company, our ability to pay dividends and meet other obligations depends upon the receipt of dividends or other payments from our operating subsidiaries. In addition, our operating subsidiaries, from time to time, may be subject to restrictions on their ability to make distributions to us, including restrictions on the conversion of local currency into U.S. dollars or other hard currency and other regulatory restrictions.
There is a limited market for our common stock, which may make it difficult for you to sell your stock.
Our common stock trades on the OTCBB under the symbol “SNBP.OB.” There is a limited trading market for our common stock. Accordingly, there can be no assurance as to the liquidity of any markets that may develop for our common stock, the ability of holders of our common stock to sell our common stock, or the prices at which holders may be able to sell our common stock.
Investors may have difficulty liquidating their investment because our common stock is subject to the “penny stock" rules, which require delivery of a schedule explaining the penny stock market and the associated risks before any sale.
Our common stock may be subject to regulations prescribed by the SEC relating to "penny stocks." The SEC has adopted regulations that generally define a penny stock to be any equity security that has a market price (as defined in such regulations) of less than $5 per share, subject to certain exceptions. These regulations impose additional sales practice requirements on broker-dealers who sell penny stocks to persons other than established customers and accredited investors (generally institutions with assets in excess of $5,000,000 and individuals with a net worth in excess of $1,000,000 or annual income exceeding $200,000 (individually) or $300,000 (jointly with their spouse). For transactions covered by these rules, the broker-dealer must make a special suitability determination for the purchase of these securities and have received the purchaser's prior written consent to the transaction. Additionally, for any transaction, other than exempt transactions, involving a penny stock, the rules require the delivery, prior to the transaction, of a risk disclosure document mandated by the SEC relating to the penny stock market. The broker-dealer also must disclose the commissions payable to both the broker-dealer and the registered representative, current quotations for the securities and, if the broker-dealer is the sole market-maker, the broker-dealer must disclose this fact and the broker-dealer's presumed control over the market. Finally, monthly statements must be sent disclosing recent price information for the penny stock held in the account and information on the limited market in penny stocks.
Legal remedies, which may be available to the investor, are as follows:
|
*
|
If penny stocks are sold in violation of the investor's rights listed above, or other federal or state securities laws, the investor may be able to cancel his purchase and get his money back;
|
|
*
|
If the stocks are sold in a fraudulent manner, the investor may be able to sue the persons and firms that caused the fraud for damages;
|
|
*
|
If the investor has signed an arbitration agreement, however, s/he may have to pursue a claim through arbitration.
|
22
If the person purchasing the securities is someone other than an accredited investor or an established customer of the broker-dealer, the broker-dealer must also approve the potential customer's account by obtaining information concerning the customer's financial situation, investment experience and investment objectives. The broker-dealer must also make a determination whether the transaction is suitable for the customer and whether the customer has sufficient knowledge and experience in financial matters to be reasonably expected to be capable of evaluating the risk of transactions in such securities. Accordingly, the SEC's rules may limit the number of potential purchasers of the shares of our common stock and shareholders may have difficulty selling their securities.
A large number of shares may be eligible for future sale and may depress our stock price.
We may be required, under terms of future financing arrangements, to offer a large number of common shares to the public, or to register for sale by future private investors a large number of shares sold in private sales to them.
Sales of substantial amounts of common stock, or a perception that such sales could occur, and the existence of options or warrants to purchase shares of common stock at prices that may be below the then-current market price of our common stock, could adversely affect the market price of our common stock and could impair our ability to raise capital through the sale of our equity securities, either of which would decrease the value of any earlier investment in our common stock.
Item 1B. Unresolved Staff Comments.
Not applicable.
Item 2. Properties.
We maintain our corporate offices at 8 Zhong Tian Road Nantong City, Jiangsu Province, China, 226009.
All land in the PRC is owned by the government and cannot be sold to any individual or entity. Instead, the government grants landholders a "land use right" after a purchase price for such "land use right" is paid to the government. The "land use right" allows the holder the right to use the land for a specified long-term period of time and enjoys all the incidents of ownership of the land. Dong Ying China owns the land use rights with regard to the land that it uses in its business.
Dong Ying China’s headquarters and 30,000 sq. meter (approximately 322,917.31 sq. foot) GMP certified production facilities are located in the Nantong Economic and Technology Development Zone, which is located in Nantong City, Jiangsu, and which qualifies Dong Ying China for tax benefits. Dong Ying China’s R&D labs are located in Nanjing, China.
On March, 29, 2004, Cyton International, Inc., the sole shareholder of Dong Ying China at that time, entered into an agreement (the “Development Agreement”) with the Jiangsu Province Nantong Economic and Technology Development Zone Administration (the “Administration”), for the construction of factories in the Jiangsu Nantong Economic and Technology Development Zone (the “Zone”), a state-level economic and technological development zone qualified for state preferential policies. Pursuant to the Development Agreement, Dong Ying China was provided with 60 acres within the Zone for the construction of factories and is required to pay a land-use fee of 40,000RMB per acre. Dong Ying China ended up paying RMB2,248,000 as there only ended up being 56.2 acres provided to Dong Ying China. All land in China is owned by the State. Individuals and companies are permitted to acquire rights to use land or land use rights for specific purposes. In the case of land used for industrial purposes, the land use rights are granted for a period of 50 years. This period may be renewed at the expiration of the initial and any subsequent terms. Granted land use rights are transferable and may be used as security for borrowings and other obligations. Dong Ying China holds the State-owned Land Use Rights Certificate No.: TONGKAIGUOYONG [2007] No. D0310158, and the RMB40,000 per acre is a one-time payment. In addition, the Administration agreed to provide Dong Ying China with a RMB20,000,000 fund (the “Fund”) to support the construction of factories and the building and purchasing of affiliated equipment. Dong Ying China has three years from the date of each portion of the Fund used to repay such portion. The Administration has the right to convert or exchange the Fund into shares of Dong Ying China, but recently the Administration decided to waive the right to convert or exchange the outstanding Fund into shares of Dong Ying China and agreed to the repayment of the Fund by December 31, 2010. Dong Ying China has repaid RMB12,000,000 as of May 31, 2010, and plans to complete the repayment of the outstanding Fund by the end of 2010. Also in accordance with the Development Agreement, Dong Ying China is required to have a registered capital of USD$5,000,000, which has already been satisfied.
23
At present, Dong Ying China has used all of the RMB20,000,000 in the Fund and has repaid RMB12,000,000 to date. In addition, Dong Ying China is required to repay the following amounts on the following dates:
|
Amount
|
Due Date
|
|
|
RMB8,000,000
|
December 31, 2010
|
Item 3. Legal Proceedings.
We know of no material, active, pending or threatened proceeding against us or our subsidiaries, nor are we, or any subsidiary, involved as a plaintiff or defendant in any material proceeding or pending litigation.
Item 4. (Removed and Reserved).
PART II
Item 5. Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities.
Market Information
Sinobiopharma’s common stock has been quoted on the Over-the-Bulletin Board under the symbol “SNBP” since August 1, 2008. Between August 4, 2008 and September 2, 2008, our common stock traded under the symbol “SNBPD” for the purposes of effecting a 50-for-1 forward split. Prior to August 1, 2008, our common stock bore the symbol “BUZZ” but had no active trading in our common stock.
The range of high and low bid quotations by quarter from June 1, 2008 through May 31, 2010 is listed below. The information for the first fiscal quarter of 2009 commences on August 1, 2008. The quotations are taken from the OTC Bulletin Board. They reflect inter-dealer prices, without retail mark-up, mark-down or commission, and may not necessarily represent actual transactions.
|
Fiscal Quarter
|
High Bid
|
Low Bid
|
|||
|
2009 First Quarter (June 1, 2008 through August 31, 2008)
|
N/A
|
N/A
|
|||
|
2009 Second Quarter (September 1, 2008 through November 30, 2008)
|
1.50
|
1.50
|
|||
|
2009 Third Quarter (December 1, 2008 through February 28, 2009)
|
1.50
|
0.51
|
|||
|
2009 Fourth Quarter (March 1, 2009 through May 31, 2009)
|
1.25
|
0.39
|
|||
|
2010 First Quarter (June 1, 2009 through August 31, 2009)
|
0.50
|
0.26
|
|||
|
2010 Second Quarter (September 1, 2009 through November 30, 2009)
|
0.39
|
0.15
|
|||
|
2010 Third Quarter (December 1, 2009 through February 28, 2010)
|
0.34
|
0.20
|
|||
|
2010 Fourth Quarter (March 1, 2010 through May 31, 2010)
|
0.28
|
0.18
|
|||
As of September 14, 2010, we had approximately 42 shareholders of record of our common stock, excluding the shares held in street name by brokerage firms.
Penny Stock Regulations
The SEC has adopted regulations which generally define "penny stock" to be an equity security that has a market price of less than $5.00 per share. Our common stock, falls within the definition of penny stock and subject to rules that impose additional sales practice requirements on broker-dealers who sell such securities to persons other than established customers and accredited investors (generally those with assets in excess of $1,000,000, or annual incomes exceeding $200,000 or $300,000, together with their spouse).
For transactions covered by these rules, the broker-dealer must make a special suitability determination for the purchase of such securities and have received the purchaser's prior written consent to the transaction. Additionally, for any transaction, other than exempt transactions, involving a penny stock, the rules require the delivery, prior to the transaction, of a risk disclosure document mandated by the SEC relating to the penny stock market. The broker-dealer also must disclose the commissions payable to both the broker-dealer and the registered representative, current quotations for the securities and, if the broker-dealer is the sole market-maker, the broker-dealer must disclose this fact and the broker-dealer's presumed control over the market. Finally, monthly statements must be sent disclosing recent price information for the penny stock held in the account and information on the limited market in penny stocks. Consequently, the "penny stock" rules may restrict the ability of broker-dealers to sell our common stock and may affect the ability of investors to sell their common stock in the secondary market.
24
Dividends
Our Board of Directors has not declared a dividend on our common stock during the last two fiscal years and we do not anticipate the payments of dividends in the near future as we intend to reinvest our profits to grow operations.
Recent Sales of Unregistered Securities
On March 31, 2009 the Company issued 20,000 shares for proceeds of $20,000 in respect of a private placement at $1.00 per share. In connection with this issue the Company agreed to pay a finder’s fee of $2,000.
On June 24, 2009 the Company entered an agreement with Emissary Capital Group LLC to obtain certain research and financing consulting services for one year, for consideration consisting of (i) a fee of $10,000 and 100,000 shares, plus (ii) 500,000 restricted shares of the Company payable upon receiving certain reports and closing a financing of at least $2,500,000, plus (iii) a success fee in cash of 8% of the amount of a successfully completed financing between $250,000 and $5,000,000. The first 100,000 shares were issued pursuant to this agreement on July 10, 2009. We made this determination based on the representations of Emissary Capital Group LLC which included, in pertinent part, that such shareholder was an "accredited investor" within the meaning of Rule 501 of Regulation D promulgated under the Securities Act, and that such shareholder was acquiring our common stock, for investment purposes for its own account and not as a nominees or agent, and not with a view to the resale or distribution thereof, with the understanding that the common stock may not be sold or otherwise disposed of without registration under the Securities Act or an applicable exemption therefrom.
On December 18, 2009, Dong Ying China entered into three intellectual property transfer agreements with Meisu Jining Science and Technology (Nanjing) Limited Company for transfer of the approval certificates to engage in clinical trials for the drug Epleronone and its tablet and capsule forms in China. As consideration for the intellectual property transfer to Dong Ying China, the Company issued an aggregate of 9,500,000 restricted shares of the Company's common stock with a fair value of $1,776,500 to Meisu Jining Science and Technology (Nanjing) Limited Company.
On December 22, 2009, Dong Ying China entered into a patent transfer agreement with Lei Wang, a third party, and Lequn Huang, the Company’s chief executive officer and director, for the transfer of a patent, “Composition for Lyophilized Powder of Atracurium,” jointly owned by Mr. Wang and Mr. Huang. Mr. Wang was issued 8,000,000 restricted shares of common stock as consideration for his respective ownership in the patent. On April 2, 2010, pursuant to the terms of the patent transfer agreement, the Company issued 1,000,000 shares of the Series A Preferred Stock to Mr. Lequn Huang as consideration for his respective ownership in the patent.
On January 11, 2010, the Company settled certain outstanding debt owed to its chief executive and financial officers by issuing an aggregate of 5,067,608 shares of common stock of the Company. The Company’s chief executive officer, Lequn Huang, was issued 4,234,275 shares of common stock for the outstanding $508,113 in loans owed to Mr. Huang, while the Company’s chief financial officer, Xinjie Mu, was issued 833,333 shares of common stock for the outstanding $100,000 in loans due to Mr. Mu. Both debt were settled by shares at a price of $0.12 per share. At the conversion date, the stock market trading price of the Company’s stock was $0.22 per share. The difference of $423,427 and $83,333 was treated as compensation to Mr. Huang and Mr. Mu.
On January 15, 2010, the Company entered into a Subscription Agreement with certain accredited investors to sell to them an aggregate of 15,000,000 shares of common stock, par value $0.0001 of the Company, for an aggregate purchase price of $1,500,000.
With the exception of issuances to Emissary Capital Group LLC, the above issuances of the common stock were exempt from registration under Section 4(2) of the Securities Act based upon our compliance with Regulation S as promulgated by the SEC under the Securities Act of 1933, as amended (the “Securities Act”). Each of the above investors acquired the Company’s common stock for investment purposes for their own respective accounts and not as a nominee or agent, and not with a view to the resale or distribution thereof, with the understanding that the common stock may not be sold or otherwise disposed of without registration under the Securities Act or an applicable exemption therefrom. Transfers of such shares were restricted by the Company in accordance with the requirements of the Securities Act. Each of the investors was provided with access to our Securities and Exchange Commission filings.
25
Equity Compensation Plan Information
|
Number of securities to be
issued upon exercise of
outstanding options,
warrants and rights
|
Weighted average exercise
price of outstanding options,
warrants and rights
|
Number of securities
remaining available for
future issuance under equity
compensation plans
(excluding securities
reflected in column (a))
|
||||||||||
|
Plan Category
|
(a)
|
(b)
|
(c)
|
|||||||||
|
Equity compensation plans approved by security holders
|
0
|
$
|
0
|
0
|
||||||||
|
Equity compensation plans not approved by security holders
|
2,525,000
|
$
|
$1.80
|
7,475,000
|
||||||||
|
Total
|
2,525,000
|
$
|
$1.80
|
7,475,000
|
||||||||
Purchases of Equity Securities by the Issuer and Affiliated Purchasers
None.
Item 6. Selected Financial Data.
Not Applicable.
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations
Cautionary Notice Regarding Forward-Looking Statements
The following discussion of the financial condition and results of operation of the Company for the years ended May 31, 2010 and 2009 should be read in conjunction with the selected financial data, the financial statements, and the notes to those statements that are included elsewhere in this Annual Report.
Some of the information contained in this discussion and analysis or set forth elsewhere in this Report, including information with respect to our plans and strategy for our business and related financing, includes forward-looking statements that involve risks and uncertainties. You should review the “Risk Factors” section of this Report for a discussion of important factors that could cause actual results to differ materially from the results described in or implied by the forward-looking statements contained in the following discussion and analysis.
Results of Operations
Comparison of the Years Ended May 31, 2010 and 2009
Overview
The Company, through its operating subsidiary Dong Ying China, is involved in the Chinese biopharmaceutical industry. We are engaged in R&D, manufacturing, marketing and distribution of biopharmaceutical products, with a primary focus on the muscle relaxant and heart disease areas. By using its innovative methods of active pharmaceutical ingredient (“API”) synthesis and drug delivery, the company intends to introduce products that enhance usability, reduce drug development timelines and costs. Sinobiopharma has successfully brought four drugs to the Chinese market, including its flagship product, KuTai (cisatracurium), a leading muscle relaxant and its recently launched product, YiTai (perindopril), a first-to-market cardiovascular ACE inhibitor. The company has six additional drug candidates in the pipeline. Sinobiopharma’s production facilities are GMP-certified by the China State Food and Drug Administration (SFDA) and the company has an established distribution network in China.
Results of Operations
The Company had revenue of $7,731,039 for the year ended May 31, 2010, as compared to revenue of $3,850,278 for the year ended May 31, 2009, and realized a net income of $2,863,572 for the year ended May 31, 2010, as compared to a net loss of $2,154,137 for the year ended May 31, 2009.
26
Revenue and cost of goods sold
Revenue increased 101% to $7,731,039 for the year ended May 31, 2010, from $3,850,278 for the year ended May 31, 2009. Gross margin increased 142% to $6,151,430 (80% of sales) for the year ended May 31, 2010, from $2,538,441 (66% of sales) for the year ended May 31, 2009.
The increase in revenue was due to the continuing growth in sales of Cisatracurium Besylate. Sales of this product increased to $7,516,436 for the year ended May 31, 2010, from $3,592,406 for the year ended May 31, 2009, representing 97% of sales and 94% of sales for the year ended May 31, 2010 and 2009, respectively. The improvement in gross margin is attributable to the fact that, as volume increased, the unit production cost of Cisatracurium Besylate decreased because the allocation of the unit indirect cost decreased. The improvement in gross margin is also attributable to an increase in the price of Cisatracurium Bestylate for the sales to certain distributors, because these distributors only provide potential customer contact information to us, while all the marketing effort, quantity and delivery term negotiation were conducted by ourselves. Since this distributor is GSP-licensed, the sales have to go through it first to get to the final customer.
Operating Expenses
The operating expenses for the year ended May 31, 2010 were $3,218,398 representing a 29% decrease as compared to $4,541,699 for the year ended May 31, 2009.
The decrease is primarily attributable to the decrease of $2,086,240 in the stock-based compensation of $830,885 for the year ended May 31, 2010, compared to $2,917,125 for the year ended May 31, 2009. The stock options have been fully vested and expensed as of August 31, 2009.
On January 11, 2010, the Company settled certain outstanding debt owed to its chief executive and financial officers by issuing an aggregate of 5,067,608 unregistered shares of common stock of the Company. The Company's chief executive officer, Lequn Huang, was issued 4,234,275 shares of common stock for the outstanding $508,113 in loans owed to Mr. Huang, while the Company's chief financial officer, Xinjie Mu, was issued 833,333 unregistered shares of common stock for the outstanding $100,000 in loans due to Mr. Mu. The shares were issued at the price of $0.12 per share. At the conversion date, the stock market trading price of the Company’s stock was $0.22 per share. The difference of $423,427 and $83,333 was treated as compensation to Mr. Huang and Mr. Mu, which increased general and administration expense by $506,760.
Research and development expenses increased $618,976 from $197,286 for the year ended May 31, 2009 to $816,262 for the year ended May 31, 2010. The increase was due to the spending on Perindopril, our new product that the Company launched in 2010.
Other income was $114,085 for the year ended May 31, 2010 as compare to other expenses of $150,879 for the year ended May 31, 2009. The change was due to the gain of $222,227 on the transfer of technology and the decrease in the balance of the shareholder loans, bank loans and government loans, which resulted in a decrease in interest expense of $17,426.
Net income increased $5,017,709 from net loss of $2,154,137 for the year ended May 31, 2009 to net income of $2,863,572 for the year ended May 31, 2010. The increase in net income was due to the increase in sales of Cisatracurium Besylate and decrease of stock-based compensation expense.
Liquidity and Capital Resources
On January 15, 2010, the Company raised $1,500,000 capital through a private placement by selling to the investors an aggregate of 15,000,000 shares of common stock, par value $0.0001 of the Company, for an aggregate purchase price of $1,500,000. As of May 31, 2010, the Company has paid off the bank loan.
The operations of Dong Ying China have generated profits for the year ended May 31, 2010. The Company had $1,331,959 in cash. The profit generated from operation is sufficient to enable the Company to support its current operations and pay current debt due for repayment. However, the Company plans to raise more capital through equity finance to provide cash to expand the business development, fund further drug product development and to launch new products. The Company is also working in developing markets to increase sales and generate positive cash flow of the existing products.
Net cash provided by the operating activities for the year ended May 31, 2010 was $2,527,766 compared to the net cash provided in the operating activities of $607,295 for the year ended May 31, 2009, an increase of $1,920,471. The increase is primarily attributable to the increase in net income of $5,017,709 which was offset by decrease in stock based compensation of $2,086,240, an increase in the change in notes receivables and accounts receivables for the amount of $598,261 due to the increase in sales for the year ended May 31, 2010 compared to the year ended May 31, 2009, and a decrease of the change in other payables of $440,242.
27
Net cash used in the investment activities for the year ended May 31, 2010 and 2009 was $1,579,081 and $147,576, respectively. The increase is primarily attributable to the deposit for the purchase of new technologies in the amount of $733,200. The Company has purchased a financial product from the bank with the fair market value of $293,280 as of May 31, 2010. The Company has purchased $552,601 more fixed assets in the year ended May 31, 2010 than in the same period of last year.
Net cash used in the financing activities for the year ended May 31, 2010 was $525,286 and net cash provided by the financing activities for the year ended May 31, 2009 was $169,481. During the year ended May 31, 2010, the Company reduced loans from banks and government in the net amount of $1,466,400. The company has also reduced shareholder loans $643,886 during the year ended May 31, 2010. The Company has raised $1,500,000 through a private placement and borrowed $100,000 from Xinjie Mu, the CFO of the Company, which was later exchanged for 833,333 common stock shares during the year ended May 31, 2010.
Short-Term Loans
On June 10, 2009 the Company received a loan in the amount of RMB10,000,000 (approximately $1,464,000 at the exchange rate applicable at May 31, 2010) from the Agricultural Bank of China. The loan bears interest at the rate of 5.31% per annum paid monthly. The loan is due for repayment in half on May 12, 2010 and June 9, 2010, respectively. The Company paid RMB5,000,000 (approximately $732,000 at the exchange rate applicable at May 31, 2010) on November 13, 2009. On December 3, 2009, the Company borrowed RMB5,000,000 ($732,000 at the exchange rate applicable at May 31, 2010) from the Agricultural Bank of China. The loan bears interest at the rate of 5.31% per annum paid monthly. The loan is due for repayment on November 25, 2010. On January 29, 2010, the Company paid RMB8,500,000 ($1,244,400 at the exchange rate applicable at May 31, 2010) to the Agricultural Bank of China for the loan balance. On April 13, 2010, the Company paid off the remaining balance of RMB1,500,000 ($219,600 at the exchange rate applicable at May 31, 2010). The loan balance as of May 31, 2010 is nil.
As of May 31, 2009, the balance RMB5,000,000 ($732,000 at the exchange rate applicable at May 31, 2009) represents loan from Bank of Communications and it has been repaid in full at June 8, 2009. The loan bore interest at the rate of 6.37% per annum paid monthly.
The interest expense from bank loans for the year ended May 31, 2010 and 2009 was $ 65,349 and $29,232, respectively.
Loans from Government
The Company has a loan from the Nantong Economic and Technology Development Zone Administration. The loan bears no interest. The original principal amount of the loan was RMB20 million ($2,928,000 at the exchange rate applicable at May 31, 2010) and was due for repayment in full in March 2007. During 2007 the Company repaid RMB3,000,000 ($439,200 at the exchange rate applicable at May 31, 2010). In December 2007 the Company was granted an extension of the due date to December 31, 2008. During the year ended December 31, 2008 the Company repaid RMB2,000,000 ($292,800 at the exchange rate applicable at May 31, 2010). On February 1, 2009 the Company repaid another RMB2,000,000 ($292,800 at the exchange rate applicable at May 31, 2010) and the Company was granted an extension to December 31, 2009 over the remainder of the loan RMB13,000,000 ($1,903,200 at the exchange rate applicable at May 31, 2010). The Company repaid RMB3,000,000 ($439,200 at the exchange rate applicable at May 31, 2010) in December 2009, and was granted an extension to December 31, 2010 over the remaining of the loan RMB10,000,000 ($1,464,000 at the exchange rate applicable at May 31, 2010). On April 7, 2010, the Company repaid RMB1,000,000 ($146,400 at the exchange rate applicable at May 31, 2010). On May 6, 2010, the Company paid RMB1,000,000 ($146,400 at the exchange rate applicable at May 31, 2010) leaving a remaining balance of RMB8,000,000 ($1,171,200 at the exchange rate applicable at May 31, 2010).
Since the loan bears no interest, the obligation is carried at its net present value using interest rates equal to the prevailing Bank of China one-year rate at the time the loan was received (5.6% in 2004, applied in respect of the year 2006) or the date the extension was effective (6.4% in March 2007, applied in respect of the years 2007 and 2008, 5.6% in February, 2009, applied in respect of the year 2009 and 5.31% in February 2010, applied in respect of the year 2010). Imputed loan interest expense included in the accounts for the year ended as of May 31, 2010 and 2009 was $77,491 and $124,486 which is determined as the amortization on the interest method basis of the discount over the remaining period to maturity of the loan. Gain from discount of no-interest loans was $43,903 and $96,515 for the year ended as of May 31, 2010 and 2009, respectively.
The present value of the total government loan was $1,145,726 as of May 31, 2010 and $1,844,193 as of May 31, 2009.
Shareholder Loans
Shareholder loans carry the value of $0 and $1,169,032 at May 31, 2010 and May 31, 2009.
The loans of $1,169,032 at May 31, 2009 consisted of $102,521 due to Peter Chen, a former shareholder of the company, and $1,066,511 due to Mr. Lequan Huang, the majority shareholder of the Company. The loans do not bear interest and have no stated repayment terms.
28
On January 11, 2010, the Company settled outstanding debt owed to its chief executive and financial officers by issuing an aggregate of 5,067,608 shares of common stock of the Company. The shareholder loans had the balance of $608,113 as of January 11, 2010. The amount includes $508,113 owed to Mr. Lequn Huang from prior borrowing and $100,000 owed to the chief financial officer, Xinjie Mu. Mr. Mu had transferred in $50,000 on December 14, 2009 and $50,000 on December 24, 2009 to the Company for the Company’s needs to pay off certain expense in US currency. The Company's chief executive officer, Lequn Huang, was issued 4,234,275 shares of common stock for the outstanding $508,113 in loans owed to Mr. Huang, while the Company's chief financial officer, Xinjie Mu, was issued 833,333 shares of common stock for the outstanding $100,000 in loans due to Mr. Mu. Both debts were settled through the issuance of shares at a price of $0.12 per share. At the conversion date, the stock market trading price of the Company’s stock was $0.22 per share. The difference of $423,427 and $83,333 was treated as compensation to Mr. Huang and Mr. Mu, respectively.
Off-Balance Sheet Arrangements
None.
Critical Accounting Policies and Estimates
The Company’s financial statements are prepared in accordance with accounting principles generally accepted in the United States, which require us to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting periods. Management makes these estimates using the best information available at the time the estimates are made. However, actual results could differ materially from those estimates (See Note 2 in the Notes to Financial Statements).
Item 7A. Quantitative and Qualitative Disclosures About Market Risk.
Not Applicable.
Item 8. Financial Statements and Supplementary Data.
Our audited financial statements for the fiscal years ended May 31, 2010 and 2009, together with the report of the independent certified public accounting firm thereon and the notes thereto, are presented beginning at page F-1.
|
Page
|
|
29
Report of Independent Registered Public Accounting Firm
To the Board of Directors and Stockholders of
Sinobiopharma, Inc.
We have audited the accompanying consolidated balance sheet of Sinobiopharma, Inc. and its subsidiaries (“the Company”) as of May 31, 2010, and the related consolidated statements of operations and comprehensive income / (loss), changes in stockholders’ equity and cash flows for the year then ended. The Company’s management is responsible for these financial statements. Our responsibility is to express an opinion on these financial statements based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audit included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company's internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audit provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of the Company as of May 31, 2010, and the results of its operations and its cash flows for the year then ended in conformity with accounting principles generally accepted in the United States of America.
/s/ Bernstein & Pinchuk LLP
New York, New York
September 14, 2010
F-1
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
Board of Directors
Sinbiopharma
We have audited the accompanying consolidated balance sheet of Sinobiopharma as of May 31, 2009, and the related consolidated statements of operations, stockholders’ (deficit), and cash flows for the year ended May 31, 2009. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audit included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audit provides a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of Sinobiopharma as of May 31, 2009, and the results of its operations, and its cash flows for the year ended May31, 2009, in conformity with accounting principles generally accepted in the United States of America.
/s/ Schumacher & Associates, Inc.
Schumacher & Associates, Inc.
Certified Public Accountants
2525 Fifteenth Street, Suite 3H
Denver, Colorado 80211
September 14, 2009
F-2
|
SINOBIOPHARMA, INC. AND SUBSIDIARIES
|
|||||||||
|
CONSOLIDATED BALANCE SHEETS
|
|||||||||
|
May 31,
|
May 31,
|
||||||||
|
2010
|
2009
|
||||||||
| ASSETS | |||||||||
|
CURRENT ASSETS
|
|||||||||
|
Cash and cash equivalents
|
$ | 1,331,959 | $ | 891,132 | |||||
|
Accounts receivable, net
|
670,662 | 208,673 | |||||||
|
Notes receivable
|
274,148 | 5,505 | |||||||
|
Inventories, net
|
758,090 | 547,317 | |||||||
|
Advance payments
|
67,971 | - | |||||||
|
Other receivables
|
321,493 | 39,825 | |||||||
|
Available for Sale (AFS) Securities
|
293,064 | - | |||||||
|
Total Current Assets
|
3,717,387 | 1,692,452 | |||||||
|
Advance payment to shareholder for intangible assets
|
985,760 | 253,760 | |||||||
|
Fixed assets, net
|
2,912,983 | 2,691,258 | |||||||
|
Intangible assets, net
|
4,336,832 | 1,318,973 | |||||||
| $ | 11,952,962 | $ | 5,956,443 | ||||||
|
LIABILITIES AND STOCKHOLDERS’ EQUITY
|
|||||||||
|
CURRENT LIABILITIES
|
|||||||||
|
Accounts payable
|
$ | 419,707 | $ | 655,064 | |||||
|
Short-term bank loans
|
- | 732,000 | |||||||
|
Loans from government
|
1,145,726 | 1,844,193 | |||||||
|
Due to shareholders
|
- | 1,169,032 | |||||||
|
Advance from customers
|
135,521 | 136,755 | |||||||
|
Due to related parties
|
- - | 256,200 | |||||||
|
Income tax payable
|
155,397 | - | |||||||
|
Other payables
|
343,780 | 386,368 | |||||||
|
Total Current Liabilities
|
2,200,131 | 5,179,612 | |||||||
|
COMMITMENTS AND CONTINGENCIES
|
|||||||||
|
STOCKHOLDERS' EQUITY
|
|||||||||
|
Preferred stock, $0.0001 par value;10,000,000 shares authorized;
|
|||||||||
|
1,000,000 shares issued and outstanding at May 31, 2010 and 0 shares at May 31, 2009
|
100 | - | |||||||
|
Common stock; $0.0001 par value; 2,490,000,000 shares authorized; 117,587,608 shares issued and outstanding at May 31, 2010 and 79,920,000 shares at May 31, 2009
|
11,759 | 7,992 | |||||||
|
Additional paid-in capital
|
14,360,487 | 8,254,991 | |||||||
|
Accumulated deficit
|
(4,847,707 | ) | (7,711,278 | ) | |||||
|
Accumulated other comprehensive income
|
228,192 | 225,126 | |||||||
|
Total Stockholders' Equity
|
9,752,831 | 776,831 | |||||||
| $ | 11,952,962 | $ | 5,956,443 | ||||||
See notes to the consolidated financial statements
F-3
|
SINOBIOPHARMA, INC. AND SUBSIDIARIES
|
||||||||
|
CONSOLIDATED STATEMENTS OF OPERATIONS AND COMPREHENSIVE INCOME/(LOSS)
|
||||||||
|
Year ended May 31,
|
||||||||
|
2010
|
2009
|
|||||||
|
REVENUE
|
$ | 7,731,039 | $ | 3,850,278 | ||||
|
COST OF GOODS SOLD
|
1,579,609 | 1,311,837 | ||||||
|
GROSS Margin
|
6,151,430 | 2,538,441 | ||||||
|
OPERATING EXPENSES
|
||||||||
|
Selling expenses
|
490,850 | 327,405 | ||||||
|
Research and development
|
816,262 | 197,286 | ||||||
|
Depreciation and amortization
|
210,835 | 182,130 | ||||||
|
General and administrative expenses
|
1,700,451 | 3,834,878 | ||||||
|
TOTAL OPERATING EXPENSES
|
3,218,398 | 4,541,699 | ||||||
|
INCOME /(LOSS) FROM OPERATIONS
|
2,933,032 | (2,003,258 | ) | |||||
|
OTHER INCOME / (EXPENSES)
|
||||||||
|
Interest income
|
6,709 | 1,049 | ||||||
|
Interest expenses
|
(114,851 | ) | (132,277 | ) | ||||
|
Other income/(expenses)
|
222,227 | (19,651 | ) | |||||
|
TOTAL OTHER INCOME/ (EXPENSES)
|
114,085 | (150,879 | ) | |||||
|
INCOME/(LOSS) BEFORE INCOME TAX EXPENSE
|
3,047,117 | (2,154,137 | ) | |||||
|
INCOME TAX EXPENSE
|
183,545 | - | ||||||
|
NET INCOME/(LOSS)
|
2,863,572 | (2,154,137 | ) | |||||
|
OTHER COMPREHENSIVE INCOME:
|
||||||||
|
Foreign Currency Translation Adjustment
|
2,802 | 13,900 | ||||||
|
Unrealized gain from available for sale securities
|
264 | - | ||||||
|
COMPREHENSIVE INCOME/(LOSS)
|
$ | 2,866,638 | $ | (2,140,237 | ) | |||
|
Earnings/(losses) per share:
|
||||||||
|
Basic and Diluted
|
$ | 0.03 | $ | (0.03 | ) | |||
|
Weighted average number of common shares outstanding – basic and diluted
|
95,420,364 | 79,903,342 | ||||||
See notes to the consolidated financial statements
F-4
|
SINOBIOPHARMA, INC. AND SUBSIDIARIES
|
||||||||||||||||||||||||||||||||
|
CONSOLIDATED STATEMENTS OF CHANGES IN STOCKHOLDERS’ EQUITY
|
||||||||||||||||||||||||||||||||
|
Common stock issued
|
Preferred stock issued
|
|||||||||||||||||||||||||||||||
|
Shares of Common stock
|
Amount
|
Shares of Preferred stock
|
Amount
|
Additional paid-in capital
|
Accumulated deficit
|
Accumulated other Comprehensive Income
|
Total
|
|||||||||||||||||||||||||
|
Balance as of May 31, 2008
|
40,000,000 | $ | 4,000 | - | $ | - | $ | 5,246,422 | $ | (5,557,141 | ) | $ | 211,226 | $ | (95,493 | ) | ||||||||||||||||
|
Capital changes due to reverse acquisition
|
39,900,000 | 3,990 | - | - | (3,990 | ) | - | - | - | |||||||||||||||||||||||
|
Stock-based compensation
|
- | - | - | - | 2,917,125 | - | - | 2,917,127 | ||||||||||||||||||||||||
|
Shares issued March 31, 2009 pursuant to private placement at $1.00 per share
|
20,000 | 2 | - | - | 19,998 | - | - | 19,998 | ||||||||||||||||||||||||
|
Finder's fees payable related to private placement of shares
|
- - | - | - | - | (2,000 | ) | - | - | (2,000 | ) | ||||||||||||||||||||||
|
Imputed interest on shareholders' loans
|
- - | - | - | - | 77,436 | - | - | 77,436 | ||||||||||||||||||||||||
|
Net income (loss) for the year
|
- | - | - | - | (2,154,137 | ) | 13,900 | (2,140,237 | ) | |||||||||||||||||||||||
|
Balance as of May 31, 2009
|
79,920,000 | $ | 7,992 | - | $ | - | $ | 8,254,991 | $ | (7,711,278 | ) | $ | 225,126 | $ | 776,831 | |||||||||||||||||
|
Stock-based compensation
|
- | - | - | - | 324,125 | - | - | 324,125 | ||||||||||||||||||||||||
|
Preferred stock issued during the year
|
1,000,000 | 100 | (100 | ) | - | |||||||||||||||||||||||||||
|
Common stock issued during the year
|
37,667,608 | 3,767 | - | - | 5,765,607 | - | - | 5,769,374 | ||||||||||||||||||||||||
|
Imputed interest on shareholders' loans
|
- | - | - | - | 15,864 | - | - | 15,864 | ||||||||||||||||||||||||
|
Net income for the year
|
- | - | - | - | - | 2,863,571 | 3,066 | 2,866,637 | ||||||||||||||||||||||||
|
Balance as of May 31, 2010
|
117,587,608 | $ | 11,759 | 1,000,000 | $ | 100 | $ | 14,360,487 | $ | (4,847,707 | ) | $ | 228,192 | $ | 9,752,831 | |||||||||||||||||
See notes to the consolidated financial statements
F-5
|
SINOBIOPHARMA, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CASH FLOWS
(Expressed in US Dollars)
|
||||||||
|
Years ended May 31,
|
||||||||
|
2010
|
2009
|
|||||||
|
CASH FLOWS FROM OPERATING ACTIVITIES
|
||||||||
|
Net income/(loss)
|
$ | 2,863,572 | $ | (2,154,137 | ) | |||
|
Adjustments to reconcile net income/(loss) to net cash
|
||||||||
|
provided by operating activities
|
||||||||
|
Depreciation and amortization
|
442,923 | 304,398 | ||||||
|
Write-down of inventory
|
- | 22,000 | ||||||
|
Stock-based compensation
|
830,885 | 2,917,125 | ||||||
|
Imputed interest expense on shareholders' loans
|
15,448 | 77,436 | ||||||
|
Amortization of discount in interest expense
|
77,490 | 128,540 | ||||||
|
Consulting fee by issuing stock
|
33,000 | - | ||||||
|
Gain from discount of non-interest government loan
|
(43,903 | ) | (96,645 | ) | ||||
|
Changes in assets and liabilities:
|
||||||||
|
Notes receivable
|
(274,597 | ) | - | |||||
|
Accounts receivable, net
|
(457,233 | ) | (133,569 | ) | ||||
|
Inventories
|
(211,082 | ) | (126,359 | ) | ||||
|
Advance payments
|
(68,082 | ) | - | |||||
|
Other receivables
|
(282,106 | ) | (204,713 | ) | ||||
|
Accounts payable
|
(235,344 | ) | 56,114 | |||||
|
Advance from customers
|
(1,237 | ) | (305,517 | ) | ||||
|
Income tax payable
|
155,652 | - | ||||||
|
Other payables
|
(317,620 | ) | 122,622 | |||||
|
Net Cash Provided by Operating Activities
|
2,527,766 | 607,295 | ||||||
|
CASH FLOWS FROM INVESTING ACTIVITIES
|
||||||||
|
Payment in acquiring available for sales securities
|
(293,280 | ) | - | |||||
|
Acquisition of property and equipment
|
(552,601 | ) | (147,576 | ) | ||||
|
Advance payment for purchase of intangible assets
|
(733,200 | ) | - | |||||
|
Net Cash Used in Investing Activities
|
(1,579,081 | ) | (147,576 | ) | ||||
|
CASH FLOWS FROM FINANCING ACTIVITIES
|
||||||||
|
Subscription received for issuing stock
|
1,600,000 | 18,000 | ||||||
|
Proceeds from shareholder loans
|
- | 18,698 | ||||||
|
Repayment from shareholder loans
|
(643,886 | ) | (27,177 | ) | ||||
|
Proceeds from bank loan and government loan
|
2,199,600 | 731,000 | ||||||
|
Repayment of bank loan and government loan
|
(3,666,000 | ) | (576,800 | ) | ||||
|
Repayments of loans by related parties
|
- | 5,760 | ||||||
|
Payment for common stock issuance costs
|
(15,000 | ) | - | |||||
|
Net Cash (Used in)/Provided by Financing Activities
|
(525,286 | ) | 169,481 | |||||
|
EFFECT OF FOREIGN CURRENCY FLUCTUATIONON CASH
|
17,428 | (1,036 | ) | |||||
|
NET INCREASE IN CASH AND CASH EQUIVALENTS
|
440,827 | 628,164 | ||||||
|
CASH AND CASH EQUIVALENTS - BEGINNING OF YEAR
|
$ | 891,132 | $ | 262,968 | ||||
|
CASH AND CASH EQUIVALENTS - ENDING OF YEAR
|
$ | 1,331,959 | $ | 891,132 | ||||
|
Supplemental cash flow information:
|
||||||||
|
Cash paid for income taxes
|
$ | 27,893 | $ | - | ||||
|
Cash paid for interest expense
|
$ | 65,349 | $ | 29,232 | ||||
|
Non-cash investing and financing activities:
|
||||||||
|
100,000 common shares issued in exchange for consulting services received
|
$ | 33,000 | $ | - | ||||
|
17,500,000 common shares issued in exchange for intangible assets
|
$ | 3,136,500 | $ | - | ||||
|
4,234,275 common shares issued to settle debt
|
$ | 508,113 | $ | - | ||||
|
1,000,000 Preferred stock issued in exchange for intangible assets
|
$ | 100 | $ | - | ||||
See notes to the consolidated financial statements
F-6
SINOBIOPHARMA, INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements
May 31, 2010 and 2009
1. ORGANIZATION AND DESCRIPTION OF BUSINESS
Sinobiopharma, Inc. (the “Company”) is a fully integrated and highly innovative specialty biopharmaceutical company engaged in the research and development, manufacture and marketing of biopharmaceutical products in China. The Company's current therapeutic focus is on anesthesia-assisted agents and cardiovascular drugs. Sinobiopharma has patented new methods for synthesizing active pharmaceutical ingredients (API) at a lower cost and owns drug delivery formulations that improve usability.
The Company was incorporated in the State of Nevada under the name of Buzz Media Ltd. on October 26, 2006. On November 8, 2006 the Company acquired all the issued and outstanding shares of Buzz Media Ltd. (“Buzz Nova Scotia”), a corporation incorporated in the province of Nova Scotia, Canada on October 26, 2006. The transaction was treated as an acquisition for accounting purposes. The consideration for the acquisition of Buzz Nova Scotia was 500 shares (on a post-forward stock split basis) of the Company valued at $0.10, the book value of the net assets of Buzz Nova Scotia, since the acquisition was from a related party.
On July 14, 2008 the Company’s President and majority shareholder sold all of her 62.5% interest in the Company, to an unrelated individual. On the same day, she resigned from all of her positions as officer and director of the Company and the purchaser was appointed the sole director and officer of the Company.
Effective July 29, 2008, the Company under its original name of Buzz Media, Ltd. incorporated a subsidiary, “Sinobiopharma, Inc.” with an investment of $0.001 and merged with it for the sole purpose of changing the name of the Company. As a result, the Company changed its name from “Buzz Media Ltd.” to “Sinobiopharma, Inc.”.
Effective July 29, 2008, the Company effected a fifty (50) for one (1) stock split of its authorized, issued and outstanding common stock. As a result, the Company’s authorized capital increased from 50,000,000 shares of common stock with a par value of $0.0001 to 2,500,000,000 shares of common stock with a par value of $0.0001. The Company’s issued and outstanding share capital increased as a result of the split from 2,000,010 shares of common stock to 100,000,500 shares of common stock. Share capital figures are presented in these financial statements giving retroactive effect to the stock split and accordingly all share capital figures are presented on a post-split basis as if the split had been affected upon inception.
On August 19, 2008, the Company entered into a share exchange agreement (the “Share Exchange Agreement”) with Dongying Pharmaceutical Co, Limited (“Dongying BVI”), a company organized under the laws of the Territory of the British Virgin Islands, and all the shareholders of Dongying BVI, whereby the Company agreed to acquire 100% of the issued and outstanding shares of Dongying BVI through the issuance of approximately 40,000,000 shares of common stock of the Company in aggregate to the shareholders of Dongying BVI on a pro rata basis in accordance with each Dongying BVI shareholder’s percentage of ownership in Dongying BVI. The Share Exchange Agreement closed on September 22, 2008.
Concurrently with the closing of the Share Exchange Agreement, by a letter agreement entered into on September 8, 2008 between Dongying BVI and the Company’s majority shareholder, that shareholder agreed to cancel 60,100,500 shares of the 62,500,500 shares of common stock of the Company registered in his name within ten (10) days of the closing of the Share Exchange Agreement. The 60,100,500 shares were cancelled on September 26, 2008. After the cancellation, the shareholders of Sinobiopharma, Inc. owned 39,900,000 shares. The share cancellation completed the reverse merger with Dongying BVI as a recapitalization of the Company such that voting control of the Company was obtained by the former stockholders of Dongying BVI. The net assets of Dongying BVI and the Company have been brought forward at their historical bases. The costs associated with the reverse merger were expensed as incurred.
Dongying BVI was incorporated under the laws of the British Virgin Islands on January 29, 2008. On May 13, 2008 Dongying BVI acquired a 100% interest in Big Global Limited (“Big Global”) from the sole shareholder of Dongying BVI for consideration of $1.00. The purpose of the transaction was the change of domicile to the British Virgin Islands.
Big Global was incorporated under the laws of Hong Kong on November 26, 2007. On December 10, 2007 Big Global acquired a 100% interest in Dong Ying (Jiangsu) Pharmaceuticals Co., Ltd. (“Dong Ying China”) from the sole shareholder of Big Global for consideration of $1.00. The purpose of the transaction was the change of domicile to Hong Kong. The acquisition was approved by the Chinese government in May 2008.
F-7
Dong Ying China was incorporated under the laws of the People’s Republic of China in 2003. Dong Ying China’s business is the development, manufacture and sale of pharmaceutical products in China. There are three product lines currently as of May 31, 2010 and several other potential products in various stages of research and development. The product lines currently commercialized are Cisatracurium Besylate, a skeletal muscle relaxant, Clindamycin Hydrochloride, an antibiotic for penicillin-allergic patients and Perindopril, a cardiovascular drug. Dong Ying China’s offices and manufacturing facility are in owned premises located on land used under license in Nantong, China and its research and development is carried out in Nanjing, China.
On August 20, 2008, the Company entered into a share purchase agreement (the “Share Purchase Agreement”) with the Company’s former majority shareholder, effective concurrently with the closing of the Share Exchange Agreement. Pursuant to the Share Purchase Agreement, the former majority shareholder acquired all of the capital of Buzz Media, Ltd. (“Buzz Nova Scotia”), the wholly-owned subsidiary of the Company incorporated in the Province of Nova Scotia, Canada, in exchange for the payment of $10.00.
2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Change of Reporting Entity
As a result of the Share Exchange closed on September 22, 2008, the former Dongying BVI shareholders owned a majority of the common stock of the Company. The transaction was regarded as a reverse merger whereby Dongying BVI was considered to be the accounting acquirer as its shareholders retained control of the Company after the Share Exchange, although the Company is the legal parent company. As such, Dongying BVI (and its historical financial statements) is the continuing entity for financial reporting purpose. Financial Statements have been prepared as if Dongying BVI had always been the reporting company and then on the share exchange date, had changed its name and reorganized its capital stock.
Principles of Consolidation and Basis of Presentation
The accompanying consolidated financial statements of the Company have been prepared in accordance with accounting principles generally accepted in the United States of America (“U.S. GAAP”) and include the financial statements of the Company and its subsidiaries. The accompanying consolidated balance sheets as of May 31, 2010 and May 31, 2009, consolidated statements of operations and comprehensive income and consolidated statements of cash flows for the years ended May 31, 2010 and May 31, 2009 include Sinobiopharma, Inc. and its directly owned subsidiaries, Dongying BVI, Big Global and Dong Ying China.
The accompanying consolidated financial statements as of May 31, 2010 and for the years ended May 31, 2010 and 2009 have been prepared in accordance with generally accepted accounting principles. All significant intercompany transactions and balances are eliminated on consolidation.
Use of Estimates
The preparation of the Company’s consolidated financial statements in conformity with generally accepted accounting principles of the United States of America requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Management makes its best estimate of the ultimate outcome for these items based on historical trends and other information available when the financial statements are prepared. Actual results could differ from those estimates. Significant items subject to such estimates and assumptions include the useful lives of fixed assets; the allowance for doubtful accounts; the fair value determination of financial and equity instruments, the realizability of deferred tax assets and inventories; the recoverability of intangible assets, land use right and property, plants and equipment; and accruals for income tax uncertainties and other contingencies. The current economic environment has increased the degree of uncertainty inherent in those estimates and assumptions.
Cash and cash equivalents
The Company’s cash and cash equivalents consist of all cash deposited in large financial institution in the PRC with acceptable credit rating. For purposes of the statements of cash flows the Company considers all highly liquid investments with an original maturity of three months or less to be cash and cash equivalents.
F-8
Accounts receivable and allowance for doubtful accounts
Accounts receivable consist of receivables for sales of product on credit. Management allows credit sales with six months terms only to customers with a long time business relationship and high transaction value. All the other customers are required to make prepayments for purchasing our products. The carrying amount of accounts receivable is reduced by an allowance that reflects management’s best estimate of the amounts that will not be collected. The Company reviews delinquent accounts over six months old, management considers many factors in estimating its allowance, including historical data, experience, credit worthiness, and economic trends. From time to time, management may adjust its assumptions for anticipated changes in any of those or other factors expected to affect collectibility. Account balances are charged against the allowance after all collection efforts have been exhausted and the potential for recovery is considered remote.
Property, plant and equipment
Property, plant and equipment are stated at cost less accumulated depreciation and amortization.
The cost of an asset comprises its purchase price and any directly attributable costs of bringing that asset to its present working condition and location for its intended use. Expenditures incurred after the assets have been put into operation, such as repairs and maintenance, overhaul and minor renewals and betterments, are normally charged to operating expenditures. Expenditures that expect to enhance the life of the assets are capitalized.
Depreciation is computed using the straight line method over the estimated useful life from the date on which they become fully operational and after taking into account their estimated residual values as follows:
|
Mould
|
Useful lives
|
|
Buildings
|
10-20 years
|
|
Land use rights
|
40 years
|
|
Manufacturing equipment
|
5-10 years
|
|
Office furniture and equipment
|
5 years
|
|
Road and grounds
|
20 years
|
|
Vehicles
|
8 years
|
|
Leasehold improvements
|
5 years
|
|
Software
|
5years
|
Intangible Assets
Intangible assets are carried at cost less a provision for amortization on a straight-line basis over their estimated useful lives of 20 years.
Revenue recognition
Revenues are recognized in accordance with ASC topic 605 (formely SEC Staff Accounting Bulletin (SAB) No. 104, "Revenue Recognition."). Under ASC No 605, product revenues (or service revenues) are recognized when persuasive evidence of an arrangement exists, delivery has occurred (or service has been performed), the sales price is fixed and determinable and collectibility is reasonably assured. Sales revenue represents the invoiced value of goods, net of value-added tax. All sales are final. Therefore, we do not estimate deductions or allowances for sales returns.
Research and development
Research and development costs are expensed as incurred. These costs consist primarily of cost of materials used and salaries paid for the development of the Company’s products. Research and development costs are $816,262 and $197,286 for the years ended May 31, 2010 and 2009, respectively.
F-9
Income taxes
The Company records deferred taxes in accordance with ASC topic 740 (formerly Statement of Financial Accounting Standards (SFAS) No. 109, "Accounting for Income Taxes.") The statement requires recognition of deferred tax assets and liabilities for temporary differences between the tax bases of assets and liabilities and the amounts at which they are carried in the financial statements, based upon the enacted tax rates in effect for the year in which the differences are expected to reverse. A valuation allowance is established when necessary to reduce deferred tax assets to the amount expected to be realized.
Fair value measurements
The Company applies the provisions of ASC Subtopic 820-10, Fair Value Measurements, for fair value measurements of financial assets and financial liabilities and for fair value measurements of nonfinancial items that are recognized or disclosed at fair value in the financial statements. ASC Subtopic 820-10 also establishes a framework for measuring fair value and expands disclosures about fair value measurements.
ASC Subtopic 820-10 defines fair value as the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. When determining the fair value measurements for assets and liabilities required or permitted to be recorded at fair value, the Company considers the principal or most advantageous market in which it would transact and it considers assumptions that market participants would use when pricing the asset or liability.
ASC Subtopic 820-10 establishes a fair value hierarchy that requires an entity to maximize the use of observable inputs and minimize the use of unobservable inputs when measuring fair value. ASC Subtopic 820-10 establishes three levels of inputs that may be used to measure fair value. The hierarchy gives the highest priority to unadjusted quoted prices in active markets for identical assets or liabilities (Level 1 measurements) and the lowest priority to measurements involving significant unobservable inputs (Level 3 measurements). The three levels of the fair value hierarchy are as follows:
|
·
|
Level 1 inputs are quoted prices (unadjusted) in active markets for identical assets or liabilities that the Company has the ability to access at the measurement date.
|
|
·
|
Level 2 inputs are inputs other than quoted prices included within Level 1 that are observable for the asset or liability, either directly or indirectly.
|
|
·
|
Level 3 inputs are unobservable inputs for the asset or liability.
|
The level in the fair value hierarchy within which a fair value measurement in its entirety falls is based on the lowest level input that is significant to the fair value measurement in its entirety.
The Company’s financial instruments consist of cash, accounts receivable, notes receivable, securities available for sale, accounts payable and loans payable. Accounts receivable and notes receivable are carried at estimated net realizable values net of provisions for uncollectible amounts. Uncollectible accounts are charged off when determined to be unrecoverable. The loan payable is carried at discounted present value. Securities available for sale are carried at market value with any unrealized gain or loss is recorded in comprehensive income or loss. The carrying values of the remaining financial instruments reflected in these financial statements approximate their fair values due to the short-term maturity of the instruments.
Foreign currency translation
The reporting currency of the Company is the U.S. dollar. The Company uses the Chinese Renminbi (“RMB”) as its functional currency. In accordance with ASC topic 830 (formerly Statement of Financial Accounting Standards (“SFAS”) No. 52, “Foreign Currency Translation,”) Results of operations and cash flows are translated at average exchange rates during the period, and assets and liabilities are translated at the unified exchange rates at the balance sheet dates, and equity is translated at the historical exchange rates. As a result, amounts related to assets and liabilities reported on the statements of cash flows will not necessarily agree with changes in the corresponding accounts on the balance sheets. Translation adjustments resulting from this process are included in accumulated other comprehensive income in the statements of shareholders’ equity. Translation gains and losses that arise from exchange rate fluctuations on transactions denominated in a currency other than the functional currency are included in the results of operations as incurred.
F-10
Assets and liabilities were translated at RMB6.8306 and RMB6.8306 to $1.00 at May 31, 2010 and 2009, respectively. The average translation rates applied to consolidated statements of operations and comprehensive income / (loss) and cash flows for the years ended May 31, 2010 and 2009 was RMB6.8194 and RMB6.9156 to $1.00, respectively.
Earning/(Loss Per Share
Basic earnings (loss) per share of common stock are computed by dividing the net income (loss) by the weighted average number of (post-split) common shares outstanding during the year. Diluted earnings (loss) per share are equal to the basic earnings (loss) per share for the years ended May 31, 2010 and 2009 because the common stock equivalents outstanding are anti-dilutive due to the fact that the weighted average exercise price per share of the stock option is higher than the weighted average market price per share of the common stock during the year ended May 31, 2010.
Stock-based compensation
ASC 718-10 requires the Company to measure the cost of employee services received in exchange for an award of equity instruments based on the grant-date fair value of the award. The Company records the cost as expense over the offering period and vesting term in connection with compensation expense for stock-based employee compensation plans.
Comprehensive Income / (Loss)
The Company has adopted ASC topic 220 (formerly Statement of Financial Accounting Standards (SFAS) No. 130, “Reporting Comprehensive Income”.) Comprehensive income / (Loss) include net income (loss) and all changes in equity during the period that arise from non-owner sources, such as foreign currency items and unrealized gains and losses on certain investments in equity securities.
RECENTLY ISSUED ACCOUNTING PRONOUNCEMENTS
The FASB issued ASU 2010-13, Compensation—Stock Compensation (ACS Topic 718): Effect of Denominating the Exercise Price of a Share-Based Payment Award in the Currency of the Market in Which the Underlying Equity Security Trades. The ASU codifies the consensus reached in Emerging Issues Task Force (EITF) Issue No. 09-J. The amendments to the Codification clarify that an employee share-based payment award with an exercise price denominated in the currency of a market in which a substantial portion of the entity’s equity shares trades should not be considered to contain a condition that is not a market, performance, or service condition. Therefore, an entity would not classify such an award as a liability if it otherwise qualifies as equity.
The amendments in the ASU are effective for fiscal years, and interim periods within those fiscal years, beginning on or after December 15, 2010. Earlier adoption is permitted. The amendments are to be applied by recording a cumulative-effect adjustment to beginning retained earnings. We are currently evaluating the impact of adopting this update on our consolidated financial statements.
In February 2010, FASB issued ASU No. 2010-09 “Subsequent Events” (“ASC Topic 855”) which removes the requirement for an SEC filer to disclose a date in both issued and revised financial statements. This amendment shall be applied prospectively for interim or annual financial periods ending after June 15, 2010. The Company does not believe the adoption will have a material effect on the Company’s consolidated financial statements.
The FASB has issued ASU 2010-06, Fair Value Measurements and Disclosures (ACS Topic 820): Improving Disclosures about Fair Value Measurements. This ASU requires some new disclosures and clarifies some existing disclosure requirements about fair value measurement as set forth in Codification Subtopic 820-10. The FASB’s objective is to improve these disclosures and, thus, increase the transparency in financial reporting. Specifically, ASU 2010-06 amends Codification Subtopic 820-10 to now require:
|
|
·
|
A reporting entity to disclose separately the amounts of significant transfers in and out of Level 1 and Level 2 fair value measurements and describe the reasons for the transfers; and
|
|
|
·
|
In the reconciliation for fair value measurements using significant unobservable inputs, a reporting entity should present separately information about purchases, sales, issuances, and settlements.
|
F-11
In addition, ASU 2010-06 clarifies the requirements of the following existing disclosures:
|
|
·
|
For purposes of reporting fair value measurement for each class of assets and liabilities, a reporting entity needs to use judgment in determining the appropriate classes of assets and liabilities; and
|
|
|
·
|
A reporting entity should provide disclosures about the valuation techniques and inputs used to measure fair value for both recurring and nonrecurring fair value measurements.
|
ASU 2010-06 is effective for interim and annual reporting periods beginning after December 15, 2009, except for the disclosures about purchases, sales, issuances, and settlements in the roll forward of activity in Level 3 fair value measurements. Those disclosures are effective for fiscal years beginning after December 15, 2010, and for interim periods within those fiscal years. Early adoption is permitted. This guidance was effective for our third quarter ended February 28, 2010.
Since the filing of 2009 Form 10-K, the FASB issued ASU No. 2010-1 through No. 2010-21. These ASUs entail technical corrections to existing guidance or affect guidance related to specialized industries or entities and therefore have minimal, if any, impact on the Company.
3. NOTES RECEIVALBE
Notes receivables of $274,148 and $5,505 as of May 31, 2010 and 2009, repectively, represent bank acceptance notes the Company received from customers for sales of products. The notes have maturity durations of 6 months, and are accepted by banks.
4. ACCOUNTS RECEIVABLE, NET
Accounts receivable consist of the following at May 31:
|
2010
|
2009
|
|||||||
|
Accounts receivables
|
$ | 670,662 | $ | 241,010 | ||||
|
Allowance for doubtful accounts
|
- | (32,337 | ) | |||||
| $ | 670,662 | $ | 208,673 | |||||
Allowance for doubtful accounts movement for year ended May 31, 2010:
|
May 31, 2009
|
Provision
|
Reverse
|
Write-off
|
May 31, 2010
|
||||||||||||||
|
Allowance for doubtful accounts
|
$
|
(32,337
|
)
|
$
|
-
|
$
|
16,814
|
$
|
15,523
|
$
|
-
|
|||||||
5. SECURITIES AVAILABLE FOR SALE
On May 17, 2010, the Company purchased a low risk short term financial product from China Agricultural Bank. The maturity term of this product is three months with no guarantee from the bank of repayment of the principle. The principle is $292,800, the fair value as May 31, 2010 is $293,064, the unrealized gain is $264 which is booked into comprehensive income or loss.
F-12
6. INVENTORIES, NET
Inventories consist of the following at May 31:
|
2010
|
2009
|
|||||||
|
Raw materials
|
$ | 127,883 | $ | 356,187 | ||||
|
Goods in process
|
553,450 | - | ||||||
|
Finished goods
|
76,757 | 213,130 | ||||||
|
Provision
|
- | (22,000 | ) | |||||
| $ | 758,090 | $ | 547,317 | |||||
7. ADVANCE PAYMENTS
Advance payments of $67,971 and $0 as of May 31, 2010 and May 31, 2009 represent prepayments to suppliers for the purchase of raw materials.
8. ADVANCE PAYMENTS TO A SHAREHOLDER FOR INTANGIBLE ASSETS
Advance payment to a shareholder for intangible assets of $985,760 and $253,760 represent prepayments to the Meisu Jining Science and Technology (Nanjing) Limited Company for the purchase of two technologies for new product manufacturing (“intellectual property”). As of May 31, 2010, the Company has not obtained the technologies. If these technologies cannot be delivered by the due date stipulated in the contracts, the prepayment will be returned to the Company.
Meisu Jining Science and Technology (Nanjing) Limited Company was a related party prior February 22, 2010. The Company’s CEO and shareholder Mr. Lequn Huang owned 25%. On February 22, 2010, Mr. Lequn Huang sold his interest in Meisu Jining Science and Technology (Nanjing) Limited Company to a third party which was approved by Chinese Government. Meisu Jining Science and Technology (Nanjing) Limited Company then became a shareholder of the Company with 8% interest through another Intellectual Property purchase transaction (as decribed in Note 11) which occurred in December 2009.
9.OTHER RECEIVABLES
The $321,493 balance of other receivables as of May 31, 2010 includes the payment of $289,840 to a non related party for purchasing research lab property. The intended purchase of the property was subsequently cancelled and paid back in July 2010. The balance of the account consists of advances to employees for business expenses.
F-13
10. PROPERTY, PLANT AND EQUIPMENT, NET
Property, plant and equipment consist of the following:
|
May 31, 2010
|
May 31, 2009
|
|||||||
|
Buildings
|
$ | 1,990,207 | $ | 1,990,207 | ||||
|
Land use rights
|
405,507 | 405,507 | ||||||
|
Manufacturing equipment
|
999,665 | 769,317 | ||||||
|
Office furniture and equipment
|
102,263 | 79,391 | ||||||
|
Road and grounds
|
212,791 | 212,791 | ||||||
|
Vehicles
|
146,171 | 10,011 | ||||||
|
Leasehold improvements
|
31,138 | - | ||||||
|
Software
|
2,127 | - | ||||||
|
Less: Accumulated depreciation
|
1,105,938 | 775,966 | ||||||
| 2,783,931 | 2,691,258 | |||||||
|
In-progress tangible assets
|
129,052 | - | ||||||
| $ | 2,912,983 | $ | 2,691,258 | |||||
Depreciation expense for the years ended May 31, 2010 and 2009 was $ 330,513 and $250,764, respectively.
11. INTANGIBLE ASSETS, NET
Intangible assets consist of the cost of a patent, certificates for clinical trials, and technology and licensed rights to manufacture the company’s products.
The patent and certificates for clinical trial were acquired intangible assets during fiscal year ending 2010.
The patent was purchased from Lei Wang and Lequn Huang by issuing 8,000,000 unregistered shares common stock at the price of $0.17 per share and preferred stock, respectively.
The certificates for clinical trial were purchased from Meisu Jining Science and Technology (Nanjing) Limited Company by issuing 9,500,000 unregistered shares of common stock at a price of $0.187 per share. The acquiring clinical trial certificates were approved by the government for the drug Eplerenone, the Company does not intend to continue the R&D process of this drug internally since obtaining these certificates, but want to use them to exchange for other “ready for production” drugs or profit sharing with other companys who complete the next development stage of this drug.
The unit prices per share of common stock issued with respect to the patent and certificates represents the estimated fair values of the Company’s unregistered common shares which were determined on the purchase dates, December 18, 2009 and December 20, 2009, using the Black-Scholes Model with level 2 inputs (See Note 18.)
No cost was recorded for the consideration of preferred stock which has been issued on April 2, 2010. According to ASC 845-10-S99, transfers of nonmonetary assets to a company by its promoters or shareholders in exchange for stock prior to or at the time of the Company's initial public offering normally should be recorded at the transferors' historical cost basis determined under GAAP. The patent was internally developed by the shareholder, Lequn Huang, therefore, it has a basis of zero.
The patent has a useful life of 20 years from the date of application. There was 16.5 years of useful life remaining when the Company purchased patent. The certificates have a useful life of 20 years but, because they are not intentionally used in production, no amortization is recorded. The technology and rights have an estimated useful life of 20 years. The amounts related to the particular products are as follows:
F-14
|
May 31, 2010
|
May 31, 2009
|
|||||||||||||||||||||||||||||||
|
Gross Carrying Amount
|
Accumulated Amortization
|
Net Carrying Amount
|
Weight Average Useful Life
|
Gross Carrying Amount
|
Accumulated Amortization
|
Net Carrying Amount
|
Weight Average Useful Life
|
|||||||||||||||||||||||||
|
(in years)
|
(in years)
|
|||||||||||||||||||||||||||||||
|
KuTai Patent
|
$ | 1,357,219 | $ | (41,128 | ) | $ | 1,316,091 | 16.5 | $ | - | $ | - | $ | - | ||||||||||||||||||
|
Clinical Trial Certificates for Eplerenone
|
1,772,867 | - | 1,772,867 | - | - | - | ||||||||||||||||||||||||||
|
Cisatracurium Besylate Technology
|
732,000 | (108,063 | ) | 623,937 | 20 | 732,000 | (72,514 | ) | 659,486 | 20 | ||||||||||||||||||||||
|
Perindopril Technology
|
732,000 | (108,063 | ) | 623,937 | 20 | 732,000 | (72,513 | ) | 659,487 | 20 | ||||||||||||||||||||||
|
Total intangible assets
|
$ | 4,594,086 | $ | (257,254 | ) | $ | 4,336,832 | $ | 1,464,000 | $ | (145,027 | ) | $ | 1,318,973 | ||||||||||||||||||
As of May 31, 2009, the Company reviewed newly acquired intangible assets; KuTai Patent and Clinical Trial Certificates for Eplerenone for impairment. An independent appraiser was engaged to appraise the fair value of the above mentioned intangible assets, who determined that there is no impairment as of May 31, 2010.
Amortization for the year ended May 31, 2010 and 2009 of $112,410 and $60,803, respectively is included in depreciation and amortization expense.
12. SHORT TERM BANK LOANS
On June 10, 2009 the Company received a loan in the amount of RMB 10,000,000 (approximately $1,464,000 at the exchange rate applicable at May 31, 2010) from the Agricultural Bank of China. The loan bears interest at the rate of 5.31% per annum paid monthly and due for repayment in half on May 12, 2010 and the balance on June 9, 2010, respectively. The Company paid RMB5,000,000 (approximately $732,000 at the exchange rate applicable at May 31, 2010) on November 13, 2009.
On December 3, 2009, the Company borrowed RMB5,000,000 ($732,000 at the exchange rate applicable at May 31, 2010) from the Agricultural Bank of China. This loan bears interest at the rate of 5.31% per annum paid monthly. and is due on November 25, 2010. On January 29, 2010, the Company paid RMB8,500,000 ($1,244,400 at the exchange rate applicable at May 31, 2010) to the Agricultural Bank of China for the outstanding loan balance. On April 13, 2010, the Company paid the remaining balance of RMB1,500,000 ($219,600 at the exchange rate applicable at May 31, 2010).
As of May 31, 2009, the balance of RMB5,000,000 ($732,000 at the exchange rate applicable at May 31, 2009) represents loan from Bank of Communications which was repaid in full at June 8, 2009. This loan beared interest at the rate of 6.37% per annum paid monthly.
The interest expense from bank loans for the year ended May 31, 2010 and 2009 was $ 65,349 and $29,232.
13. LOANS FROM GOVERNMENT
The Company has a loan from the Nantong Economic and Technology Development Zone Administration. The loan bears no interest. The original principal amount of the loan was RMB20million ($2,928,000 at the exchange rate applicable at May 31, 2010) and was due for repayment in full in March 2007. During 2007 the Company repaid RMB3,000,000($439,200 at the exchange rate applicable at May 31, 2010). In December 2007 the Company was granted an extension to December 31, 2008. During the year ended December 31, 2008 the Company repaid RMB2,000,000 ($292,800 at the exchange rate applicable at May 31, 2010). On February 1, 2009 the Company repaid another RMB2,000,000 ($292,800 at the exchange rate applicable at May 31, 2010) and the Company was granted an extension to December 31, 2009 for the remainder of the loan of RMB13,000,000 ($1,903,200 at the exchange rate applicable at May 31, 2010). The Company repaid RMB3,000,000 ($439,200 at the exchange rate applicable at May 31, 2010) in December 2009, and was granted another extension to December 31, 2010 for the remaining loan balance of RMB10,000,000 ($1,464,000 at the exchange rate applicable at May 31, 2010). On April 7, 2010, the Company repaid RMB1,000,000 ($146,400 at the exchange rate applicable at May 31, 2010). On May 6, 2010, the Company paid RMB1,000,000 ($146,400 at the exchange rate applicable at May 31, 2010) leaving a remaining balance of RMB8,000,000 ($1,171,200 at the exchange rate applicable at May 31, 2010).
F-15
Since the loan bore no interest, the obligation is carried at its net present value using interest rates equal to the prevailing Bank of China one-year rate at the time the loan was received (5.6% in 2004, applied in respect of the 2006 year) or the date the extension was effective (6.4% in March 2007, applied in respect of the 2007 and 2008 years, 5.6% in February, 2009, applied in respect of the 2009 year and 5.31% in February2010, applied in respect of the 2010 year). Imputed loan interest expense included in the accounts for the year ended as of May 31, 2010 and 2009 was $77,490 and $124,486 which is determined as the amortization on the interest method basis of the discount over the remaining period to maturity of the loan. Gain from discount of non-interest loans was $43,903 and $96,515 for the year ended as of May 31, 2010 and 2009, respectively.
The present value of the total government loan was $1,145,726 as of May 31, 2010 and $1,844,193 as of May 31, 2009.
14. AMOUNTS DUE TO SHAREHOLDERS
Shareholder loans are $0 and $1,169,032 at May 31, 2010 and 2009, respectively.
The balance of $1,169,032 at May 31, 2009 consisted of $102,521 due to Peter Chen, a former shareholder of the Company, and $1,066,511 due to Mr. Lequan Huang, the majority shareholder of the Company. The loans did not bear interest and had no stated repayment terms.
On January 11, 2010, the Company settled outstanding debt of $608,113 owed to its chief executive and financial officers by issuing an aggregate of 5,067,608 shares of the Company’s common stock. The amount included $508,113 owed to Mr. Lequn Huang from prior borrowings and $100,000 owed to the chief financial officer , Xinjie Mu. Mr.Mu loaned the Company $50,000 on December 14, 2009 and $50,000 on December 24, 2009. The Company's chief executive officer, Lequn Huang, was issued 4,234,275 shares of common stock for the outstanding $508,113 in loans owed to Mr. Huang, while the Company's chief financial officer, Xinjie Mu, was issued 833,333 shares of common stock for the outstanding $100,000 loan to Mr. Mu. All shares were issued at a market price of $0.12 per share At the conversion date, the stock market trading price of the Company’s stock was $0.22 per share. The difference of $423,427 and $83,333 was treated as compensation to Mr. Huang and Mr. Mu and booked as general and administration expenses on the income statement.
15. AMOUNTS DUE TO RELATED PARTY
$256,200 due to related party as of May 31, 2009, represents the payables to Meisu Jining Science and Technology (Nanjing) Limited Company (the “Meisu Jining”) for the Company technology purchased in October 2007. The amount was reclassed to due to a shareholder and was paid off as of May 31, 2010, since Meisu Jining became a shareholder with 8% interests of the Company through the transaction of the intellectual property transfer (Note 11) at December 18, 2009.
16. OTHER PAYABLES
$343,780 and $386,368 other payables as of May 31, 2010 and 2009, respectively, represent deposits received from customers, payroll payables, accrued professional fees and VAT payables.
17. INCOME TAXES
Taxes payable balance of $155,397 represents the income tax accrual of Dong Ying China for the year ended May 31, 2010. Income tax was accrued startingfrom January 1, 2010 to May 31, 2010 since Dong Ying China was exempt from income tax for the first 7 months in the fiscal year of 2010.
The Company’s operating subsidiary Dong Ying China is entitled to tax preferential policies of no enterprise income taxes for two years and half income tax for three years starting January 1, 2008 due to the Company’s wholly-owned foreign enterprises status. Starting January 1, 2010, the Company’s Chinese operations will be subject to enterprise income tax with half of tax rate for next three years. The Company’s applicable tax rate will be 11% for 2010, 12% for 2011 and 12.5% for 2012 as approved by the Nantong Economic and Technical Development Zone Office of State Administration of Taxation.
F-16
Income tax expense of $183,545 for the year ended May 31, 2010 represents PRC current income taxes payable.
A reconciliation of income tax expense to the amount computed at the statutory rates is as follows:
|
2010
|
2009
|
|||||||
|
Income (loss) for the year ended May 31
|
3,047,117 | (2,154,137 | ) | |||||
|
Average statutory tax rate
|
25 | % | 25 | % | ||||
|
Expected income tax provision
|
761,779 | (538,534 | ) | |||||
|
Exemption due to tax holiday
|
(960,092 | ) | (322,704 | ) | ||||
|
Non-deductible expense
|
300,442 | 791,221 | ||||||
|
Non-taxable income
|
(6,124 | ) | (24,129 | ) | ||||
|
Unrecognized tax losses
|
87,540 | 94,146 | ||||||
|
Income tax expense
|
183,545 | - | ||||||
The Company is subject to United States income tax to the extent of its operations in the United States. The Company had no U.S. income tax expense in 2009 and 2010 due to net losses in the US operations.
The Company has a deferred tax asset on net operating losses of approximately $ 246,951as of May 31, 2010. The ultimate realization of deferred tax assets depends on the generation of future taxable income during the periods in which those net operating losses are available. The Company considers projected future taxable income and tax planning strategies in making its assessment. At present, the Company does not have a sufficient operations in the United States to conclude that it is more-likely-than-not that the Company will be able to realize all of its tax benefits in the near future and therefore a valuation allowance of $246,951 was established for the full value of the deferred tax asset.
A valuation allowance will be maintained until sufficient positive evidence exists to support the reversal of any portion or all of the valuation allowance. Should the Company start operations in the United States in future periods with supportable trend, the valuation allowance will be reversed accordingly.
18. COMMON STOCK
On July 10, 2009 the Company issued 100,000 common shares to Emissary Capital Group LLC in exchange of financial advisory and independent equity research consultation. The common stock par value is $0.0001 and the market price on July 10, 2009 as fair value is $0.33. No cash proceed will be paid to the Company for this issuance.
On December 18, 2009, the Company authorized its wholly-owned subsidiary Dong Ying China entered into three intellectual property transfer agreements with Meisu Jining Science and Technology (Nanjing) Limited Company for transfer of the approval certificates to engage in clinical trials for the drug Eplerenone and its tablet and capsule forms in China (‘Intellectual Property”). As consideration for Intellectual Property to Dong Ying China, the Company issued an aggregate of 9,500,000 unregistered shares of the Company's common stock which at a price of $0.1870 per share and $1,776,500 was recorded to Meisu Jining Science and Technology (Nanjing) Limited Company on the same day. As a result of this transaction, Meisu Jining Science and Technology (Nanjing) Limited Company became the shareholder of the company with 8% interests. (See Note 11).
On December 22, 2009, the Company's wholly-owned subsidiary Dong Ying China entered into a patent transfer agreement with Lei Wang the employee of Dong Ying China and Lequn Huang, the Company's chief executive officer and director, for the transfer of a patent, "Composition for Lyophilized Powder of Atracurium," jointly owned by Mr. Wang and Mr. Huang. As consideration for the patent, the Company issued 8,000,000 unregistered shares of the Company's common stock to Mr. Wang, of which the value was determined at the market price as of the same date, and also agreed to issue the preferred stock to Mr. Huang, but prior to the issuance of the preferred stock, the Company shall: (i) undertake its commercially reasonable best efforts to seek shareholder, board, and relevant governmental approval to authorize a class of blank check preferred stock, and (ii) following the effectiveness of the authorization of blank check preferred stock, designate and issue such number of shares of preferred stock as to give Mr. Huang approximately 51% of the voting rights and 0% of the equity rights of the Company. Should the Company fail to obtain sufficient approval to authorize any preferred stock after having made commercially reasonable best efforts to do so, Mr. Huang waived his right to redress under the dispute settlement terms of the agreement. The preferred stocks were issued on April 2, 2010, please refer to Note 19. The fair value of the consideration of issuance of 8,000,000 common stock on December 22, 2009 was $1,360,000.
F-17
The Company measures the fair value of the unregistered common shares and pure voting right preferred stock based on the assumptions that market participants would use in pricing the asset or liability and establishes a fair value hierarchy that ranks the inputs used to measure fair value by their reliability. The three levels of the fair value hierarchy are as follows:
Level 1 - quoted prices (unadjusted) in active markets for identical assets or liabilities that a company has the ability to access at the measurement date.
Level 2 - inputs other than quoted prices included within Level 1 that are observable for similar assets or liabilities, either directly or indirectly.
Level 3 - unobservable inputs for the asset or liability. Unobservable inputs are used to measure fair value to the extent that observable inputs are not available, thereby allowing for situations in which there is little, if any, market activity for the asset or liability at the measurement date.
The estimated fair values of the Company’s unregistered common shares and pure voting right preferred stock were determined at December 18, 2009 and December 22, 2009 using the Black-Scholes Model with level 2 inputs.
The following table sets forth, by level within the fair value hierarchy, the Company’s unregistered common shares and pure voting right preferred stock that were measured on a non-recurring basis at fair value as of December 18, 2009 and December 22, 2009.
|
Fair Value Measurements Using:
|
||||
|
Clinical Trial Certificates for Eplerenone
|
Quoted Prices in Active Markets for
Identical Financial Assets and Liabilities
|
Significant Other
Observable Inputs
|
Significant
Unobservable Inputs
|
|
|
December 18, 2009
|
Total
|
Level 1
|
Level 2
|
Level 3
|
|
Equity at fair value:
|
||||
|
Unregistered common shares
|
$1,776,500
|
-
|
$1,776,500
|
-
|
|
Fair Value Measurements Using:
|
||||
|
KuTai Patent
|
Quoted Prices in Active Markets for
Identical Financial Assets and Liabilities
|
Significant Other
Observable Inputs
|
Significant
Unobservable Inputs
|
|
|
December 22, 2009
|
Total
|
Level 1
|
Level 2
|
Level 3
|
|
Equity at fair value:
|
||||
|
Unregistered common shares and pure voting right preferred stock
|
$1,360,000
|
-
|
$1,360,000
|
-
|
|
Shares issued
|
Market price per share
|
Fair value price per unregistered share
|
Total amount of estimated fair value
|
|||||||||||||
|
KuTai Patent
|
8,000,000
|
$
|
0.2000
|
$
|
0.1700
|
1,360,000
|
||||||||||
|
Clinical Trial Certificates for Eplerenone
|
9,500,000
|
$
|
0.2200
|
$
|
0.1870
|
1,776,500
|
||||||||||
The fair values of the unregistered common shares as of December 18, 2009 and December 22, 2009 was determined based on the Black-Scholes option pricing model Because shares issued to Meisu Jining Science and Technology (Nanjing) Limited Company for this transaction are non registered restricted shares, their value was considered lower than the market share price with a discount of 15%. Key assumptions to value the stock were as follows:
|
Unregistered common shares
|
Unregistered common shares
|
|||||||
|
December 18, 2009
|
December 22, 2009
|
|||||||
|
Volatility:
|
56.00
|
%
|
56.00
|
%
|
||||
|
Risk-free interest rate:
|
1.17
|
%
|
1.17
|
%
|
||||
|
Dividend yield:
|
-
|
-
|
||||||
|
Expected lives (years):
|
0.50
|
0.50
|
||||||
On January 15, 2010, Sinobiopharma, Inc. entered into a Subscription Agreement with certain accredited investors (collectively, the "Buyers") to sell to the Buyers an aggregate of 15,000,000 unregistered shares of the Company’s common stock, par value $0.0001, for an aggregate purchase price of $1,500,000. On January 15, 2010, the Company issued an aggregate of 15,000,000 unregistered shares to the Buyers in the private placement.
On January 11, 2010, the Company settled certain outstanding debt owed to its chief executive and financial officers by issuing an aggregate of 5,067,608 unregistered shares of common stock of the Company. The Company's chief executive officer, Lequn Huang, was issued 4,234,275 shares of common stock for the outstanding $508,113 in loans owed to Mr. Huang, while the Company's chief financial officer, Xinjie Mu, was issued 833,333 unregistered shares of common stock for the outstanding $100,000 in loans due to Mr. Mu. The shares were issued at the price of $0.12 per share. At the conversion date, the stock market trading price of the Company’s stock was $0.22 per share. The difference of $423,427 and $83,333 was treated as compensation to Mr. Huang and Mr. Mu.
19. PREFERRED STOCK
Effective March 29, 2010, the Company filed Amended and Restated Articles of Incorporation, pursuant to which the Company changed its authorized capital stock to consist of 2,500,000,000 shares, consisting of 2,490,000,000 shares of common stock, $0.0001 par value and 10,000,000 shares of preferred stock, $0.0001 par value. On the same day, the Company filed a Certificate of Designation with the Secretary of State of Nevada whereby it designated 1,000,000 shares of preferred stock as Series A Preferred Stock, par value $0.0001 per share. Each holder of the Series A Preferred Stock shall be entitled to vote on all matters submitted to shareholders of the Company and shall be entitled to 60 votes for each share of Series A Preferred Stock owned at the record date for the determination of shareholders entitled to vote on such matter or, if no such record date is established, at the date such vote is taken or any written consent of shareholders is solicited. Except as otherwise required by law, the holders of shares of Series A Preferred Stock shall vote together with the holders of common stock on all matters and shall not vote as a separate class.
After majority shareholders’ approval process, on April 2, 2010, pursuant to the terms of the patent transfer agreement for "Composition for Lyophilized Powder of Atracurium” signed on December 22, 2009 (as mentioned in note 18), the Company issued 1,000,000 shares of the Series A Preferred Stock (the "Preferred Stock") to Mr. Lequn Huang as consideration for the patent. The issuance of the Preferred Stock to Mr. Huang was exempt from registration under Section 4(2) of the Securities Act based upon our compliance with Regulation S as promulgated by the SEC under the Securities Act of 1933, as amended.
F-18
20. STOCK-BASED COMPENSATION
On September 29, 2008, the Company adopted a stock option and incentive plan (the “2008 Stock Option and Incentive Plan”). The 2008 Stock Option and Incentive Plan provides authorization to the Board of Directors to grant Stock Options and Incentives to a total number of shares of the Company’s common stock, not to exceed ten million (10,000,000) (post forward stock split) shares. The following option awards are part of this plan.
On September 29, 2008, the Company granted to certain directors, officers and consultants of the Company in aggregate 1,800,000 stock options having an exercise price of $1.80 per share and an expiry date of five years from the date of grant. These stock options have vesting provisions of 10% on the date of grant and 10% on the last day of each month thereafter beginning on October 31, 2008. The total vesting period is 9 months.
On October 2, 2008, the Company granted to certain mid-level managers of DongYing China an aggregate of 500,000 stock options with an exercise price of $1.80 per share and an expiration date of five years from the date of grant. These stock options have vesting provisions of 10% on the date of grant and 10% on the last day of each month thereafter beginning on October 31, 2008. The total vesting period is 9 months.
On October 22, 2008, the Company granted to a scientific consultant and advisory board member of the Company 225,000 stock options having an exercise price of $1.80 per share and an expiration date of five years from the date of grant. These stock options have vesting provisions of 10% on the date of grant and 10% on the last day of each month thereafter beginning on October 31, 2008. The total vesting period is 9 months.
A summary of the Company’s stock option activities is presented below:
|
Number of options
|
Weighted average
exercise price per share
|
Weighted average grant
date fair value Per Share
|
|||
|
|
|||||
|
|
|||||
|
|
|||||
|
Options Outstanding,
|
|||||
|
June 1, 2009- May 31, 2010
|
2,525,000
|
$ 1.8
|
$ 1.28
|
Compensation cost related to options was recognized as the related options vest. The vesting period was the requisite service period for each option holder. The stock-based compensation cost was $324,125 and $ 2,917,125 for the year ended May 31, 2010 and 2009. As of May 31, 2010, all the outstanding options have vested as follows:
Vested options are as follows:
|
Number
outstanding
|
Total fair
value
|
Weighted average grant-
date fair value
per share
|
||||||||||
|
Options vested at ,
|
||||||||||||
|
June 1, 2009
|
2,272,500 | $ | 2,917,125 | $ | 1.28 | |||||||
|
Vested during the fiscal year of 2010
|
252,500 | 324,125 | 1.28 | |||||||||
|
May 31, 2010
|
2,525,000 | $ | 3,241,250 | $ | 1.28 | |||||||
F-19
If not previously exercised or canceled, options outstanding at May 31, 2010 will expire as follows:
|
Range of
Exercise Prices
|
Number
|
Weighted average
|
||||||||||||||
|
Expiry Date
|
High
|
Low
|
of Shares
|
exercise price
|
||||||||||||
|
September 29, 2013
|
$
|
1.8
|
$
|
1.8
|
1,800,000
|
$
|
1.8
|
|||||||||
|
October 2, 2013
|
$
|
1.8
|
$
|
1.8
|
500,000
|
$
|
1.8
|
|||||||||
|
October 22, 2013
|
$
|
1.8
|
$
|
1.8
|
225,000
|
$
|
1.8
|
|||||||||
The fair values of the options granted September 29, October 2 and October 22, 2008 were estimated at values of $1.25 per share, $1.42 per share and $1.25, respectively, using the Black-Scholes Option Pricing Model with the following weighted average assumptions:
|
Volatility:
|
88.3
|
%
|
||
|
Risk-free interest rate:
|
2.30
|
%
|
||
|
Dividend yield:
|
—
|
|||
|
Expected lives (years):
|
5
|
Option-pricing models require the use of highly subjective estimates and assumptions including the expected stock price volatility. Changes in the underlying assumptions can materially affect the fair value estimates and therefore, in management’s opinion, existing models do not necessarily provide reliable measure of the fair value of the Company’s stock options.
On January 11, 2010, the Company settled outstanding debt of $608,113 owed to its chief executive and financial officers by issuing an aggregate of 5,067,608 shares of the Company’s common stock. The amount included $508,113 owed to Mr. Lequn Huang from prior borrowings and $100,000 owed to the chief financial officer , Xinjie Mu. Mr.Mu loaned the Company $50,000 on December 14, 2009 and $50,000 on December 24, 2009. The Company's chief executive officer, Lequn Huang, was issued 4,234,275 shares of common stock for the outstanding $508,113 in loans owed to Mr. Huang, while the Company's chief financial officer, Xinjie Mu, was issued 833,333 shares of common stock for the outstanding $100,000 loan to Mr. Mu. All shares were issued at the price of $0.12 per share At the conversion date, the stock market trading price of the Company’s stock was $0.22 per share. The difference of $423,427 and $83,333 was treated as compensation to Mr. Huang and Mr. Mu and booked as general and administration expenses on the income statement.
21. EARNINGS/(LOSS) PER SHARE
ASC 260 “Earnings Per Share,” requires dual presentation of basic and diluted earnings per share (“EPS”) with a reconciliation of the numerator and denominator of the basic EPS computation to the numerator and denominator of the diluted EPS computation. Basic EPS excludes dilution. Diluted EPS reflects the potential dilution that could occur if securities or other contracts to issue common stock were exercised or converted into common stock or resulted in the issuance of common stock that then shared in the earnings of the entity.
Basic net income per share is computed by dividing net income available to common shareholders by the weighted average number of shares of common stock outstanding during the period. Diluted income per share is computed by dividing net income by the weighted average number of shares of common stock, common stock equivalents and potentially dilutive securities outstanding during each period. Potentially dilutive common shares consist of common shares issuable upon the conversion of common stock options (using the treasury stock method). The following table presents a reconciliation of basic and diluted net income per share:
F-20
|
|
The years ended
|
|||||||
|
|
May 31, 2010
|
May 31, 2009
|
||||||
|
|
|
|
||||||
|
Numerator used in basic net income per share:
|
||||||||
|
Net income/(loss)
|
$
|
2,863,572
|
$
|
(2,154,137
|
)
|
|||
|
Shares (denominator):
|
||||||||
|
Weighted average common shares outstanding
|
95,420,364
|
79,903,342
|
||||||
|
Weighted average common shares outstanding used in computing diluted earnings/(loss) per
|
||||||||
|
ordinary share
|
95,420,364
|
79,903,342
|
||||||
|
Earnings/(loss) per common share-basic
|
$
|
0.03
|
$
|
(0.03
|
)
|
|||
|
Earnings/(loss) per common share-diluted
|
$
|
0.03
|
$
|
(0.03
|
)
|
|||
As of May 31, 2010, the Company had 2,525,000 common share equivalents outstanding that could potentially dilute basic income per share in the future, but which were excluded in the computation of diluted income per share in the periods presented, as their effect would have been anti-dilutive due to the fact that the weighted average exercise price per share of the stock option is higher than the weighted average market price per share of the common stock during the year ended May 31, 2010.
22. RELATED PARTY TRANSACTIONS AND BALANCES
The Company purchased two intangible assets in prior year from Meisu Jining Science and Technology (Nanjing) Limited Company, previously 25% owned by the Company’s CEO and shareholder Mr. Lequn Huang; and Bio-Medical Research and Development Limited Company, previously 30% owned by Mr. Lequn Huang at prices to be negotiated at the time. As of May 31, 2010, the cost of these two intangible assets is $732,000 for each. (See Note 11).
Addition of intangible assets
The patent and certificates for clinical trial are additional intangible assets. The patent was purchased from Lei Wang and Lequn Huang by issuing 8,000,000 unregistered shares common stock. The consideration is $1,360,000.The certificates for clinical trial were purchased from Meisu Jining Science and Technology (Nanjing) Limited Company by issuing 9,500,000 unregistered shares common stock. The consideration is $1,776,500. (See Note 11 and 18)
On February 22, 2010, Mr. Lequn Huang sold his interest in Meisu Jining Science and Technology (Nanjing) Limited Company to a third party. The transaction was approved by Chinese Government Agency and legally became effective on February 22, 2010. On January 12, 2010, Chinese Government Agency approved the application of transferring the legal representative of SUJI Bio-Medical Research and Development Limited Company from Mr. Lequn Huang to a third party. After these transactions, neither Mr. Lequn Huang nor any other person in the Company has any interest in Meisu Jining Science and Technology (Nanjing) Limited Company and SUJI Bio-Medical Research and Development Limited.
Meisu Jining became a shareholder of the Company through the above mentioned transaction of the intellectual property transfer at December 18, 2009.
Advance payment for intangible assets to shareholder
Advance payments for intangible assets of $985,760 and $253,760 to a shareholder as of May 31, 2010 and 2009 represent the prepayments to Meisu Jining Science and Technology (Nanjing) Limited Company.
The company has signed two contracts for purchase of intellectual property with Meisu Jining Science and Technology (Nanjing) Limited Company. One was signed in July 2008, for an amount of $292,800. As of May 31, 2010 the company prepaid $253,760. The other contract was signed in July 2009, for a contract amount of $732,000 and as of May 31, 2010, the company prepaid $732,000.(See Note 8)
F-21
Amounts due to shareholders
Shareholder loans carry the value of $0 and $1,169,032 at May 31, 2010 and 2009. For detail refer to Note 14.
Issuance of Preferred stock
On April 2, 2010, pursuant to the terms of the patent transfer agreement for "Composition for Lyophilized Powder of Atracurium” signed on December 22, 2009, the Company issued 1,000,000 shares of the Series A Preferred Stock (the "Preferred Stock") to Mr. Lequn Huang, the Company’s chairman, president and chief executive officer as consideration for the patent. For detail refer to Note 19.
23. COMMITMENTS AND CONTINGENCIES
Commitments
In August 2009, the Company signed a technical support contract of Perindopril and Rocuronium with SUJI Bio-Medical Research and Development Limited Company for the total price of RMB8,000,000($1,171,200). As of May 31, 2010, the Company has paid RMB1,800,000 ($263,520) which had been expensed and is committed to pay the remaining of RMB6, 200,000 ($907,680) before Jan 2015.
On May 30, 2010, the Company’s board resolved that the company would issue Ms. Luk 300,000 shares of the Company's common stock within three months from May 30, 2010 as compensation for serving as an independent director. Ms. Luk was elected as a director of the company on May 30, 2010.
Contingencies
Contingencies through August 30, 2010 have been considered by the Company and none were noted which were required to be disclosed.
24. CONCENTRATIONS AND CREDIT RISK
At May 31, 2010 and 2009, the Company had a credit risk exposure of cash in banks of $1,331,959 and $891,132 respectively that is uninsured by the government authority. To limit exposure to credit risk relating to deposits, the Company primarily places cash deposits only with large financial institution in the PRC with acceptable credit rating.
The Company’s operations are carried out in the PRC. Accordingly, the Company’s business, financial condition and results of operations may be influenced by the political, economic and legal environments in the PRC as well as by the general state of the PRC’s economy. The business may be influenced by changes in governmental policies with respect to laws and regulations, anti-inflationary measures, currency conversion and remittance abroad, and rates and methods of taxation, among other things.
(1) Our main products include Clindamycin Hydrochloride and Cisatracurium Besylate which accounted for 100% of the Company’s total sales.
|
Year ended May 31,
|
|||||||||||||||||||
|
2010
|
2009
|
||||||||||||||||||
|
Amount of Sales
|
% of Total Sales
|
Amount of Sales
|
% of Total Sales
|
||||||||||||||||
|
Amount of Sales
|
% of Total Sales
|
Amount of Sales
|
% of Total Sales
|
||||||||||||||||
|
Clindamycin Hydrochloride 0.75g
|
$ | 51,632 | 1 |
%
|
$ | 77,122 | 2 | % | |||||||||||
|
Clindamycin Hydrochloride 0.90g
|
144,234 | 2 |
%
|
180,750 | 5 | % | |||||||||||||
|
Cisatracurium Besylate 5mg
|
7,516,436 | 97 |
%
|
3,592,406 | 93 | % | |||||||||||||
|
Perindopril troche
|
18,737 | 0 |
%
|
- | - | % | |||||||||||||
|
Total
|
$ | 7,731,039 | 100 |
%
|
$ | 3,850,278 | 100 | % | |||||||||||
F-22
(2) Customers accounted for over 10% of the Company’s total sales are as follows:
|
For the year ended May 31,
|
||||||||||||||
|
2010
|
2009
|
|||||||||||||
|
Amount of sales
|
% of Total Sales
|
Amount of sales
|
% of Total Sales
|
|||||||||||
|
Customer A
|
$
|
1,625,736
|
21
|
%
|
$
|
170,850
|
4
|
%
|
||||||
|
Customer B
|
1,513,375
|
20
|
%
|
842,028
|
22
|
%
|
||||||||
|
Total
|
$
|
3,139,111
|
41
|
%
|
$
|
1,012,878
|
26
|
%
|
||||||
(3) Suppliers accounted for over 10% of the Company’s total purchases are as follows:
|
For the year ended May 31,
|
||||||||||||||||
|
2010
|
2009
|
|||||||||||||||
|
Amount of sales
|
% of Total Sales
|
Amount of sales
|
% of Total Sales
|
|||||||||||||
|
Supplier A
|
$
|
1,019,323
|
55
|
%
|
$
|
529,419
|
47
|
%
|
||||||||
|
Supplier B
|
287,378
|
15
|
%
|
147,781
|
13
|
%
|
||||||||||
|
Supplier C
|
137,518
|
12
|
%
|
|||||||||||||
|
Total
|
$
|
1,306,701
|
70
|
%
|
$
|
814,718
|
72
|
%
|
||||||||
25. RECLASSIFICATION
The financial statements for periods prior to May 31, 2010 have been reclassified to conform to the headings and classifications used in the May 31, 2010 financial statements which are mainly reflected in the account of advance payment for intangible assets and amounts due to related party.
26. SUBSEQUENT EVENTS
As of September 14, 2010, which is the financial statement issuance date, management identified the following subsequent events:
On July 26, 2010, Dong Ying China, entered into a Cooperation Agreement with Jiangsu LianHuan Pharmaceuticals Co., Ltd. (“LianHuan”) for the co-development, manufacture, and marketing of the drug Eplerenone, a therapeutic agent formulated to treat high blood pressure and vascular diseases, and its tablet and capsule forms (the “Eplerenone”). Clinical trial certificates for Eplerenone are currently owned by DongYing. According to the Agreement, LianHuan shall be responsible for all fees and expenses incurred in the clinical trials. Following the completion of clinical trials, LianHuan and DongYing shall jointly complete and submit the drug production application. Once production approval for Eplerenone is obtained, LianHuan and DongYing shall jointly own the new drug production certificate for Eplerenone. LianHuan shall be responsible for the manufacture of Eplerenone and its tablet form, though Dong Ying China retains the option to produce Eplerenone capsules. The net profit from the sales of Eplerenone shall be apportioned as 60 percent to Dong Ying China and 40 percent to LianHuan.
F-23
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure.
None.
Item 9A. Controls and Procedures.
Evaluation of Disclosure Controls and Procedures
The Company maintains a set of disclosure controls and procedures designed to ensure that information required to be disclosed by the Company in the reports filed under the Securities Exchange Act, is recorded, processed, summarized and reported within the time periods specified by the SEC's rules and forms. Disclosure controls are also designed with the objective of ensuring that this information is accumulated and communicated to the Company's management, including the Company's chief executive officer and chief financial officer, as appropriate, to allow timely decisions regarding required disclosure.
Based upon their evaluation as of the end of the period covered by this report, the Company's chief executive officer and chief financial officer concluded that, the Company's disclosure controls and procedures are effective to ensure that information required to be included in the Company's periodic SEC filings is recorded, processed, summarized, and reported within the time periods specified in the SEC rules and forms. Our chief executive officer and chief financial officer also concluded that our disclosure controls and procedures are effective to ensure that information required to be disclosed in the reports required to be filed or submitted under the Exchange Act is accumulated and communicated to the our management, including our chief executive officer and chief financial officer, to allow timely decisions regarding required disclosure.
Management’s Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over our financial reporting as defined in Rules 13a-15(f) and 15d-15(f) under the Securities Exchange Act. Our management is also required to assess and report on the effectiveness of our internal control over financial reporting in accordance with Section 404 of the Sarbanes-Oxley Act of 2002 (“Section 404”). Internal control over financial reporting is a process to provide reasonable assurance regarding the reliability of our financial reporting for external purposes in accordance with generally accepted accounting principles. Internal control over financial reporting includes policies and procedures that: (i) pertain to maintaining records that in reasonable detail accurately and fairly reflect our transactions; (ii) provide reasonable assurance that transactions are recorded as necessary for preparation of our financial statements and that receipts and expenditures of company assets are made in accordance with management authorization; and (iii) provide reasonable assurance that unauthorized acquisition, use or disposition of company assets that could have a material effect on our financial statements would be prevented or detected on a timely basis.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies and procedures may deteriorate.
For the fiscal year ended May 31, 2010, we carried out an evaluation of the effectiveness of the design and operation of its disclosure controls and procedures pursuant to Exchange Act Rule 13a-15(b). In making this assessment, we used the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission (COSO) in Internal Control – Integrated Framework. This evaluation was conducted by Dr. Lequn Huang, our chief executive officer, and Xinjie Mu, our chief financial officer. Based upon this evaluation, the chief executive officer and the chief financial officer concluded that our internal control over financial reporting is effective based on those criteria.
This Annual Report does not include an attestation report of our registered public accounting firm regarding internal control over financial reporting. Management’s report was not subject to attestation by our registered public accounting firm pursuant to temporary rules of the Securities and Exchange Commission that permit us to provide only management’s report in this Annual Report.
Changes in Internal Controls over Financial Reporting
Other than as disclosed above, no changes in the Company's internal control over financial reporting have come to management's attention during the Company's last fiscal quarter that have materially affected, or are likely to materially affect, the Company's internal control over financial reporting.
53
Limitations on Controls
Management does not expect that our disclosure controls and procedures or our internal control over financial reporting will prevent or detect all error and fraud. Any control system, no matter how well designed and operated, is based upon certain assumptions and can provide only reasonable, not absolute, assurance that its objectives will be met. Further, no evaluation of controls can provide absolute assurance that misstatements due to error or fraud will not occur or that all control issues and instances of fraud, if any, within the Company have been detected. Our disclosure controls and procedures are designed to provide reasonable assurance of achieving their objectives and our chief executive officer and chief financial officer have concluded that our disclosure controls and procedures are effective at that reasonable assurance level.
Item 9B. Other Information.
None.
PART III
Item 10. Directors, Executive Officers and Corporate Governance
Set forth below is certain information regarding our directors and executive officers. Our Board of Directors is comprised of five directors. There are no family relationships between any of our directors or executive officers. Each of our directors is elected to serve until the next annual meeting of our shareholders and until his successor is elected and qualified or until such director’s earlier death, removal or termination.
The following table sets forth certain information with respect to our directors and executive officers:
|
Name
|
Age
|
Positions and Offices Held
|
||
|
Lequn Lee Huang(1)
|
55
|
President, CEO and Director
|
||
|
Xinjie Mu(2)
|
40
|
CFO and Director
|
||
|
Eric Shi(3)
|
50
|
Director
|
||
|
Xuejun Chen(4)
|
34
|
Senior Vice President and Director
|
||
|
Yuanwei Chen(5)
|
47
|
Director
|
||
|
Du Wang(6)
|
48
|
Director
|
||
|
Hoki Luk(7)
|
34
|
Director
|
Notes:
|
(1)
|
Mr. Lequn Lee Huang was appointed the President, CEO CFO and a director of our Company on September 22, 2008. On September 25, 2009, Mr. Huang resigned as CFO of the Company but retains all other positions.
|
|
(2)
|
Mr. Xinjie Mu was appointed the CFO and a director of our Company on September 25, 2009.
|
|
(3)
|
Mr. Eric Shi was appointed a director of our Company on May 30, 2010.
|
|
(4)
|
Mr. Xuejun Chen was appointed the Senior Vice President and a director of our Company on September 22, 2008.
|
|
(5)
|
Mr. Yuanwei Chen was appointed a director of our Company on September 22, 2008.
|
|
(6)
|
Mr. Du Wang was appointed a director of our Company on May 30, 2010.
|
|
(7)
|
Ms. Hoki Luk was appointed a director of our Company on May 30, 2010.
|
Our directors hold office until the next annual meeting of our shareholders and until their successors have been qualified after being elected or appointed. Our officers serve at the discretion of our Board of Directors.
Set forth below is biographical information about our current directors and executive officers:
Dr. Lequn Lee Huang, age 55, is the President, CEO and a director of the Company. Dr. Huang was one of the founders of Dong Ying China and has been the Chairman, CEO and a director of Dong Ying China since 2004. Since 2004, Dr. Huang has been a professor at the Medical School at Nanjing University. From 1991 to 2004, Dr. Huang worked at the Bayer Research Center in New Haven, Conn., U.S., for Bayer Co. where he became the Principal Research Scientist and Head of the MS Lab. At the Bayer Research Center, Dr. Huang’s group was responsible for analysis of drug discovery. In 1981, Dr. Huang received his B.S. in Organic Chemistry from Nanjing University in China and, in 1987, he received his Ph.D. in Analytical Chemistry from Iowa State University. Dr. Huang is not an officer or director of any other reporting issuer.
54
Mr. Xinjie Mu, age 40, is the CFO and a director of the Company. Mr. Mu has been an independent consultant from January 2008 to present and since March 9, 2008, Mr. Mu has been a director of China Infrastructure Investment Corporation (NASDAQ: CIIC). From April 2007 to December 2007, Mr. Mu was the Chief Financial Officer of Jingwei International Limited (OTCBB: JNGW.OB). From January 2006 to April 2007, Mr. Mu served as the Senior Accountant for Geller and Company. From September 1999 to March 2005, Mr. Mu served as the Senior Accountant of Flightsafety International, Inc., a wholly-owned subsidiary of Berkshire Hathaway. Mr. Mu earned his B.S. at Hebei University of Science and Technology (Chemical Engineering) in 1992 and his MBA at Baruch College of the City University of New York in 1997. Mr. Mu received his CPA designation from the State of Maryland in 1997.
Dr. Eric Shi, age 50, is a director of the Company. Mr. Shi is the founder of DiereTech Bio-Pharma, Inc. since 2009. DiereTech Bio-Pharma focuses on developing new drugs for the China market. Prior to founding DiereTech Bio-Pharma, in 2004, Dr. Shi incorporated DiereTech Investment Limited, Inc., a Hong Kong company engaged in investing new drug projects in China and in European countries. In 2005, Dr. Shi incorporated DierePharma, Inc. in New York. DierePharma conducts deep research and development on drugs with IP obtained by DiereTech Investment Limited. In 2006, Mr. Shi incorporated Beijing Unimedex Science & Technology Development Co., Ltd., a company located in Beijing and currently conducting research on medical apparatus and instruments for urine control. Dr. Shi was the Chief Professor at the Long Island Jewish Medical Center Department of Radiation since 2004. Prior to that, from 1994 to 2003, Dr. Shi was Associate Professor at Albert Einstein College of Medicine. In 1993, Dr. Shi received his post-doctorate training from Georgetown University Cancer Research Center and earned his PhD in Biochemistry from Dartmouth Medical School in 1989. He received his Bachelor’s degree from Beijing University in 1983.
Mr. Xuejun Chen, age 34, is the Senior Vice President and a director of the Company. Since 2006, Mr. Chen has been the Executive Vice President of Sales and a director of DongYing China where he is responsible for sales. From 2005 to 2006, Mr. Chen was the Vice President of Sales for Nanjing Langkun Medicine Co., Ltd., a company in the business of pharmaceuticals where he was responsible for sales. From 2004 to 2005, Mr. Chen was the Marketing Director for the Province of Jiangsu for Medicine Co., Ltd., a company in the business of pharmaceuticals, where he was responsible for sales. From 2003 to 2004, Mr. Chen was the Sales director for the Province of Jiangsu for Xiamen Beidazhilu Biotech Co., Ltd., a company in the business of pharmaceuticals, where he was responsible for regional sales. In 1998, Mr. Chen received his B.S. from Lanzhou University in China and, in 2007, he received his M.B.A from Nanjing University. Mr. Chen is not an officer or director of any other reporting issuer.
Mr. Yuanwei Chen, age 47, is a director of the Company. Since March, 2008, Mr. Chen has been the General Manager of China Gateway Pharma Products (Chengdu) Co. Ltd., a company in the business of pharmaceutical research, and the Vice President of Medicinal Chemistry of Shanghai ChemPartner Co., a company in the business of pharmaceutical research. As the Vice President of Medicinal Chemistry of Shanghai ChemPartner Co., Mr. Chen is responsible for managing the operations of his department, allocating resources, managing projects and recruiting employees. From February, 2005, to March, 2008, Mr. Chen was the Chief Scientific Officer of Egret Pharma (Shanghai) Ltd. in Shanghai, China where he was responsible for establishing the company’s drug discovery platform, the company’s research strategy, managing finances and recruiting employees. From December, 1998, to February, 2005, Mr. Chen worked with Bayer Healthcare in West Haven, Connecticut, U.S. At Bayer Healthcare, Mr. Chen worked in the Pharmaceutical Division as a Senior Research Scientist I, Senior Research Scientist II and as a Senior lab head. As a Senior lab head, Mr. Chen was responsible for proposing and implementing research and development strategies for various projects and supervising and training research staff. In 1983, Mr. Chen received his B.S. in Organic Chemistry and, in 1986, he received his M.S. in Organic Chemistry from Sichuan University in China. In 1993, Mr. Chen received his Ph.D. in Organic Chemistry from the University Lausanne in Switzerland and, in 1995, he completed his Postdoctoral Fellowship with the Scripps Research Institute in La Jolla, California, U.S. Mr. Chen is not an officer or director of any other reporting issuer.
Dr. Du Wang, age 48, is the director of the Company. Dr. Wang has been a private investor since 2000. His major accomplishments include founding the first non-performing loan (NPL) investment fund in China. From 1998 until 2000 he was president of JP Morgan Chase Hong Kong. Prior, beginning in 1997 until 1998 he managed Smith Investments, a distressed asset investment fund based in New York. Dr. Du Wang was also a fund manager at Princeton Capital Management, a New York security investment fund, from 1991 to 1997. He received his Ph.D. in Economics from the State University of New York (SUNY) at Stony Brook and holds a Masters in Management from Stevens Institute of Technology in New Jersey. He also has received a Bachelor Degree in Business Administration from Tianjin University in China.
Ms. Hoki Luk, age 34, is a director of the Company. Ms. Luk has been the founder and managing partner of Western Bridge LLC based in Stamford Connecticut since June 2009. Western Bridge is an investment relations and business development company facilitating relationship between U.S. and Chinese companies. Prior to serving at Western Bridge LLC, from November 2007 until May 2009 she was an Analyst for Citigroup, Hong Kong where she concentrated on the Chinese healthcare market. From December 2005 to October 2007, Ms. Luk served as associate and junior analyst at Sivic Global Healthcare in New York, where her primary responsibilities were to identify new investments and make investment recommendations for U.S. and Chinese companies. Ms. Luk also previously served as an associate analyst in New York working in the U.S. biotechnology and China healthcare markets with UBS from May 2002 to August 2004, Credit Suisse First Boston from February 2001 to May 2002, and Salomon Smith Barney from June 1999 to February 2001. Mr. Hoki Luk graduated from the University of California at Berkeley with a bachelor’s degree in Business Administration.
55
The Board believes that each of the Company’s director-nominees is highly qualified to serve as a member of the Board. Each of the director-nominees has contributed to the mix of skills, core competencies and qualifications of the Board. When evaluating candidates for election to the Board, the Nominating Committee seeks candidates with certain qualities that it believes are important, including integrity, an objective perspective, good judgment, leadership skills. Our director-nominees are highly educated and have diverse backgrounds and talents and extensive track records of success in what we believe are highly relevant positions. Some of our director-nominees have served in our operating entity, Dong Ying China, for many years and benefit from an intimate knowledge of our operations and corporate philosophy.
Save as otherwise reported above, none of our directors held directorships in other reporting companies and registered investment companies at any time during the past five years.
Significant Employees
The following persons are directors, officers of significant employees of Dong Ying China as of May 31, 2010, whom are not directors or executive officers of the Company:
Ms. Qiu Zhang, age 49, has been the General Manager of Production for Dong Ying China since January, 2008. Ms. Zhang is a licensed pharmacist in China. From October, 2003, to December, 2007, Ms. Zhang was the Deputy General Manager of Nanjing Hicin Pharmaceutical Co. Ltd. where she was responsible for production. From October, 2001, to October, 2003, Ms. Zhang was the Deputy General Manager for the Jilin Weiwei Group (Joint Venture) where she was responsible for production. In 1983, Ms. Zhang earned her B.S. in Pharmacy from Shenyang Pharmaceutical University in China and, in 1981, she earned her Bachelor of Engineering from the Jilin Institute of Chemical Technology in China.
Family Relationships
There are no family relationships between any of the Company’s directors or executive officers.
Involvement in Certain Legal Proceedings
We are not aware of any material legal proceedings that have occurred within the past ten years concerning any director, director nominee, or control person which involved a criminal conviction, a pending criminal or bankruptcy proceeding, a pending or concluded administrative or civil proceeding limiting one’s participation in the securities or banking industries, or a finding of securities or commodities law violations.
Section 16(a) Beneficial Ownership Reporting Compliance
Our officers, directors and shareholders owning greater than ten percent of our shares are not required to comply with Section 16(a) of the Securities Exchange Act of 1934 because we do not have a class of securities registered under Section 12 of the Securities Exchange Act of 1934.
Committees
Our business, property and affairs are managed by or under the direction of the board of directors. Members of the board are kept informed of our business through discussion with the chief executive and financial officers and other officers, by reviewing materials provided to them and by participating at meetings of the board and its committees.
Our board of directors has three committees - the audit committee, the compensation committee and the corporate governance/nominating committee. The audit committee is comprised of Du Wang, Hoki Luk, and Yuanwei Chen, with Mr. Du Wang serving as chairman. The compensation committee is comprised of Yuanwei Chen, Eric Shi, and Hoki Luk, with Mr. Yuanwei Chen as chairman. The nominating committee is comprised of Yuanwei Chen, Du Wang, and Eric Shi, with Mr. Yuanwei Chen as chairman.
Our audit committee is involved in discussions with our independent auditor with respect to the scope and results of our year-end audit, our quarterly results of operations, our internal accounting controls and the professional services furnished by the independent auditor. Each member of our Audit Committee is independent as that term is defined under the NYSE Corporate Governance Rules. The Board has determined that Du Wang qualifies as an Audit Committee “Financial Expert” under applicable rules of the Securities and Exchange Commission. Our board of directors has adopted a written charter for the audit committee which the audit committee reviews and reassesses for adequacy on an annual basis. A copy of the Audit Committee’s current charter is available on our website at www.sinobp.com.
56
The compensation committee oversees the compensation of our chief executive officer and our other executive officers and reviews our overall compensation policies for employees generally. If so authorized by the board of directors, the committee may also serve as the granting and administrative committee under any option or other equity-based compensation plans which we may adopt. The compensation committee does not delegate its authority to fix compensation; however, as to officers who report to the chief executive officer, the compensation committee consults with the chief executive officer, who may make recommendations to the compensation committee. Any recommendations by the chief executive officer are accompanied by an analysis of the basis for the recommendations. The committee will also discuss compensation policies for employees who are not officers with the chief executive officer and other responsible officers. A copy of the Compensation Committee’s current charter is available on our website at www.sinobp.com .
The nominating committee is involved in evaluating the desirability of and recommending to the board any changes in the size and composition of the board, evaluation of and successor planning for the chief executive officer and other executive officers. The qualifications of any candidate for director will be subject to the same extensive general and specific criteria applicable to director candidates generally. A copy of the Nominating Committee’s current charter is available on our website at www.sinobp.com.
When considering potential director-nominees, the Nominating Committee also will consider the current composition of our Board and our evolving needs, including expertise, diversity and balance of inside, outside and independent directors. Although we do not have a formal policy for the consideration of diversity in identifying director-nominees, the Nominating Committee recognizes the benefits associated with a diverse board, and strives to create diversity in perspective, background and experience in the Board as a whole when identifying and selecting director-nominees. On an annual basis, as part of the Board’s self-evaluation, the Board assesses whether the mix of Board members is appropriate for our Company.
http://www.ir-site.com/images/library/orientalpaper/Nominating%20Committee%20Charter.pdf
Code of Ethics
As of July 1, 2010, we have adopted a code of ethics to apply to our principal executive officer, principal financial officer, principal accounting officer and controller, or persons performing similar functions. A copy of the code of ethics is available on our website at www.sinobp.com .
The board and its committees held the following number of meetings during the fiscal year ended May 31, 2010:
|
Board of Directors
|
10
|
|
Audit Committee
|
N/A (not formed until July 1, 2010)
|
|
Compensation Committee
|
N/A (not formed until July 1, 2010)
|
|
Nominating Committee
|
N/A (not formed until July 1, 2010)
|
The meetings include meetings that were held by means of a conference telephone call, but do not include actions taken by unanimous written consent.
Each director attended at least 75% of the total number of meetings of the board and those committees on which he served during the year.
Our non-management directors did not meet in executive session during the fiscal year ended May 31, 2010.
To our knowledge, during the last ten years, none of our directors and executive officers (including those of our subsidiaries) has:
|
*
|
Had a bankruptcy petition filed by or against any business of which such person was a general partner or executive officer either at the time of the bankruptcy or within two years prior to that time.
|
|
|
*
|
Been convicted in a criminal proceeding or been subject to a pending criminal proceeding, excluding traffic violations and other minor offenses.
|
|
|
*
|
Been subject to any order, judgment or decree, not subsequently reversed, suspended or vacated, of any court of competent jurisdiction, permanently or temporarily enjoining, barring, suspending or otherwise limiting his involvement in any type of business, securities or banking activities.
|
|
|
*
|
Been found by a court of competent jurisdiction (in a civil action), the SEC, or the Commodities Futures Trading Commission to have violated a federal or state securities or commodities law, and the judgment has not been reversed, suspended or vacated.
|
|
|
*
|
Been the subject to, or a party to, any sanction or order, not subsequently reverse, suspended or vacated, of any self-regulatory organization, any registered entity, or any equivalent exchange, association, entity or organization that has disciplinary authority over its members or persons associated with a member.
|
|
57
Board Leadership Structure and Role in Risk Oversight
Lequn Huang is our chairman, president and chief executive officer. At the advice of other members of the management or the Board, Mr. Huang calls meetings of Board of Directors when necessary. We have three independent directors. We do not have a lead independent director. Our Board has three standing committees, each of which is comprised solely of independent directors with a committee chair. The Board believes that the Company’s chief executive officer is best situated to serve as chairman of the Board because he is the director most familiar with our business and industry and the director most capable of identifying strategic priorities and executing our business strategy. In addition, having a single leader eliminates the potential for confusion and provides clear leadership for the Company. We believe that this leadership structure has served the Company well.
Our Board has overall responsibility for risk oversight. The Board has delegated responsibility for the oversight of specific risks to Board committees as follows:
|
*
|
The Audit Committee oversees the Company’s risk policies and processes relating to the financial statements and financial reporting processes, as well as key credit risks, liquidity risks, market risks and compliance, and the guidelines, policies and processes for monitoring and mitigating those risks.
|
|
*
|
The Nominating Committee oversees risks related to the company’s governance structure and processes.
|
Our Board is responsible to approve all related party transactions according to our Code of Ethics. We have not adopted written policies and procedures specifically for related person transactions.
Item 11. Executive Compensation.
The following summary compensation table indicates the cash and non-cash compensation earned during the years ended May 31, 2010 and 2009 by each person who served as principal executive officer, principal financial officer, and secretary during the fiscal year ended May 31, 2010. No officer received compensation of $100,000 or more during the fiscal year ended May 31, 2010.
|
Name and
principal
position
|
Year
|
Salary
($)
|
Bonus
($)
|
Stock
awards
($)
|
Option
awards
($)
|
Non-equity
incentive
plan
compensation
($)
|
Nonqualified
deferred
compensation
earnings
($)
|
All other
compensation
($)
|
Total
($)
|
||||||||||
|
Dr. Lequn Huang(1)
|
2010
|
45,000
|
Nil
|
423,427
|
Nil
|
Nil
|
Nil
|
Nil
|
468,427
|
||||||||||
|
President, CEO & Director
|
2009
|
Nil
|
Nil
|
Nil
|
562,500
|
Nil
|
Nil
|
Nil
|
562,500
|
||||||||||
|
Xinjie Mu(2)
|
2010
|
Nil
|
Nil
|
83,333
|
Nil
|
Nil
|
Nil
|
Nil
|
83,333
|
||||||||||
|
CFO & Director
|
2009
|
Nil
|
Nil
|
Nil
|
Nil
|
Nil
|
Nil
|
Nil
|
Nil
|
Notes:
|
(1)
|
Mr. Lequn Lee Huang was appointed the President, CEO, CFO, Treasurer and a director of our Company on September 22, 2008. On September 25, 2009, Mr. Huang resigned as the CFO of the Company.
|
|
(2)
|
Mr. Xinjie Mu was appointed the CFO and a director of our Company on September 25, 2009.
|
Narrative Disclosure to the Summary Compensation Table
On September 29, 2008, the Board of Directors adopted the Stock Option and Incentive Plan and granted 500,000 stock options to Dr. Lequn Lee Huang –President and CEO. The stock options have an exercise price of $1.80 per share and an expiry date of five years from the date of grant. These stock options have vesting provisions of 10% on the date of grant and 10% on the last day of each month thereafter beginning on October 31, 2008.
58
On January 11, 2010, the Company settled outstanding debt of $608,113 owed to its chief executive and financial officers by issuing an aggregate of 5,067,608 shares of the Company’s common stock. The amount included $508,113 owed to Mr. Lequn Huang from prior borrowings and $100,000 owed to the chief financial officer , Xinjie Mu. Mr.Mu loaned the Company $50,000 on December 14, 2009 and $50,000 on December 24, 2009. The Company's chief executive officer, Lequn Huang, was issued 4,234,275 shares of common stock for the outstanding $508,113 in loans owed to Mr. Huang, while the Company's chief financial officer, Xinjie Mu, was issued 833,333 shares of common stock for the outstanding $100,000 loan to Mr. Mu. All shares were issued at the price of $0.12 per share. At the conversion date, the stock market trading price of the Company’s stock was $0.22 per share. The difference of $423,427 and $83,333 was treated as compensation to Mr.Huang and Mr. Mu..
Other than the foregoing and the issuance of stock options pursuant to our 2008 Stock Option and Incentive Plan, we do not pay any compensation to our Named Executive Officers at this time. However, we reserve the right to compensate our Named Executive Officers in the future with cash, stock, options, or some combination of the foregoing. See “Item 5. Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities – Securities Authorized for Issuance Under Equity Compensation Plans” for details of the Company’s 2008 Stock Option and Incentive Plan.
Employment Agreements
No employment agreements.
Compensation Discussion and Analysis
We strive to provide our named executive officers (as defined in Item 402 of Regulation S-K) with a competitive base salary that is in line with their roles and responsibilities when compared to peer companies of comparable size in similar locations.
We plan to implement a more comprehensive compensation program, which takes into account other elements of compensation, including, without limitation, short and long term compensation, cash and non-cash, and other equity-based compensation such as stock options. We expect that this compensation program will be comparable to the programs of our peer companies and aimed to retain and attract talented individuals.
Our compensation committee oversees the compensation of our named executive officers.
Compensation of Directors
The following table discloses the compensation of the directors of the Company for the Company’s fiscal year ended May 31, 2010 (unless already disclosed above):
|
Name
|
Fees
earned or
paid in
cash
($)
|
Stock
awards
($)
|
Option
awards
($)
|
Non-equity
incentive plan
compensation
($)
|
Nonqualified
deferred
compensation
earnings
($)
|
All other
compensation
($)
|
Total
($)
|
|||||||
|
(a)
|
(b)
|
(c)
|
(d)
|
(e)
|
(f)
|
(g)
|
(h)
|
|||||||
|
Lequn Lee Huang(1)
|
See Above.
|
See Above.
|
See Above.
|
See Above.
|
See Above.
|
See Above.
|
See Above.
|
|||||||
|
Xinjie Mu(2)
|
See Above.
|
|
See Above.
|
|
See Above.
|
|
See Above.
|
|
See Above.
|
|
See Above.
|
|
See Above.
|
|
|
Eric Shi(3)
|
nil
|
nil
|
nil
|
nil
|
nil
|
nil
|
nil
|
|||||||
|
Xuejun Chen(4)
|
nil
|
nil
|
nil
|
nil
|
nil
|
nil
|
nil
|
|||||||
|
Yuanwei Chen(5)
|
nil
|
nil
|
nil
|
nil
|
nil
|
nil
|
nil
|
|||||||
|
Du Wang(6)
|
|
nil
|
nil
|
nil
|
nil
|
nil
|
nil
|
nil
|
||||||
|
Hoki Luk(7)
|
nil
|
nil
|
nil
|
nil
|
nil
|
nil
|
nil
|
Notes:
|
(1)
|
Mr. Lequn Lee Huang was appointed the President, CEO, CFO, Treasurer and a director of our Company on September 22, 2008. On September 25, 2009, Mr. Huang resigned as the CFO of the Company.
|
|
(2)
|
Mr. Xinjie Mu was appointed the CFO and a director of our Company on September 25, 2009.
|
|
(3)
|
Mr. Eric Shi was appointed a director of our Company on May 30, 2010.
|
|
(4)
|
Mr. Xuejun Chen was appointed the Senior Vice President and a director of our Company on September 22, 2008.
|
|
(5)
|
Mr. Yuanwei Chen was appointed a director of our Company on September 22, 2008
|
|
(6)
|
Mr. Du Wang was appointed a director of our Company on May 30, 2010.
|
|
(7)
|
Ms. Hoki Luk was appointed a director of our Company on May 30, 2010.
|
59
Narrative Disclosure to the Director Compensation Table
Other than the issuance of stock options pursuant to our 2008 Stock Option and Incentive Plan, we do not pay any compensation to our directors at this time. However, we reserve the right to compensate our directors in the future with cash, stock, options, or some combination of the foregoing.
Other than as described above, there are no understandings or arrangements between Mr. Huang, Mr. Mu, Mr. Shi, Mr. Xuejun Chen, Mr. Yuanwei Chen, Mr. Wang, or Ms. Luk and any other person pursuant to which Mr. Huang, Mr. Mu, Mr. Shi, Mr. Xuejun Chen, Mr. Yuanwei Chen, Mr. Wang, or Ms. Luk was appointed as a director. None of Mr. Huang, Mr. Mu, Mr. Shi, Mr. Xuejun Chen, Mr. Yuanwei Chen, Mr. Wang, or Ms. Luk has any family relationship with any director, executive officer or person nominated or chosen by us to become a director or executive officer.
Outstanding Equity Awards at Fiscal Year-End
None.
Pension and Retirement Plans
Currently, except for contributions to the PRC government-mandated social security retirement endowment fund for those employees who have not waived their coverage, we do not offer any annuity, pension or retirement benefits to be paid to any of our officers, directors or employees. There are also no compensatory plans or arrangements with respect to any individual named above which results or will result from the resignation, retirement or any other termination of employment with our company, or from a change in our control.
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters.
The following table sets forth certain information with respect to the beneficial ownership of our voting securities by (i) any person or group owning more than 5% of any class of voting securities, (ii) each director, (iii) our Chief Executive Officer and President and (iv) all executive officers and directors as a group as of September 14, 2010.
Amount and Nature of Beneficial Ownership
|
Name and Address
of Beneficial Owner
|
Amount and Nature of Beneficial Ownership (1)
|
Percentage of
Common
Stock
|
||||
|
Shareholders Holding 5% or More of Class
|
||||||
|
More Big Group Limited (2)
1225 Prince's Building
10 Charter Road
Central, Hong Kong
|
10,800,000
|
9.18
|
%
|
|||
|
Sino Run International Limited (3)
1225 Prince's Building
10 Charter Road
Central, Hong Kong
|
7,500,000
|
6.38
|
%
|
|||
|
Sino Stature Ltd. (6)
1225 Prince's Building
10 Charter Road
Central, Hong Kong
|
8,000,000
|
6.80
|
%
|
|||
|
Meisu Jining Science and Technology (Nanjing) Limited Company
Hubin New Village, Jianging Econ.
Development Area, Nanjing City
Jiangsu Province, China
|
9,500,000
|
8.08
|
%
|
|||
60
|
Directors and Executive Officers
|
||||||
|
Lequn Huang
CEO and Director
|
15,534,275
|
(2)
|
13.15
|
%
|
||
|
Xinjie Mu
CFO
|
933,333
|
*
|
||||
|
Xuejun Chen
Director (3)
|
7,750,000
|
6.58
|
%
|
|||
|
Yuanwei Chen
Director (4)
|
250,000
|
*
|
||||
|
Du Wang
Director
|
0
|
0
|
||||
|
Eric Shi
Director
|
0
|
0
|
||||
|
Hoki Luk
Director
|
0
|
0
|
||||
|
Jianguo Wang
Former Director (5)
|
2,650,000
|
2.25%
|
||||
|
All Directors and Executive Officers as a Group (8 persons)
|
27,117,608
|
22.82
|
%
|
*less than 1% of the Company’s issued and outstanding common shares.
Notes:
(*) Indicates less than 1%.
|
(1)
|
Beneficial ownership of Common Stock has been determined for this purpose in accordance with Rule 13d-3 under the Exchange Act, under which a person is deemed to be the beneficial owner of securities if such person has or shares voting power or investment power with respect to such securities, has the right to acquire beneficial ownership within 60 days or acquires such securities with the purpose or effect of changing or influencing the control of the Company.
|
|
(2)
|
This figure includes 10,800,000 shares indirectly owned by Dr. Lequn Lee Huang through More Big Group Limited, of which Dr. Huang has sole voting power and sole dispositive power over the shares held by More Big Group Limited, and 500,000 shares underlying stock options which have vested.
This figure does not include 1,000,000 shares of Series A Preferred Stock issued to Dr. Huang on April 2, 2010, pursuant to the terms of a patent transfer agreement dated December 22, 2009. The Series A Preferred Stock does not bear any conversion rights but entitles Mr. Huang to vote on all matters submitted to shareholders of the Company and shall be entitled to 60 votes for each share of Series A Preferred Stock owned.
|
|
(3)
|
This figure includes 7,500,000 shares indirectly owned by Mr. Xuejun Chen through Sino Run International Limited, which Mr. Chen has sole voting power and sole dispositive power over the shares held by Sino Run International Limited and 250,000 shares underlying stock options which have vested.
|
|
(4)
|
This figure represents 250,000 shares underlying stock options which have vested.
|
|
(5)
|
This figure includes 2,400,000 shares held directly by Jianguo Wang and 250,000 shares underlying stock options which have vested.
|
|
(6)
|
Liu Tianpei has sole voting power and sole dispositive power over the shares held by Sino Stature Ltd.
|
61
Item 13. Certain Relationships and Related Transactions, and Director Independence.
On December 18, 2009, the Company authorized its wholly-owned subsidiary, Dong Ying China, to enter into three intellectual property transfer agreements with Meisu Jining Science and Technology (Nanjing) Limited Company for transfer of the approval certificates to engage in clinical trials for the drug Eplerenone and its tablet and capsule forms in China (the ‘Intellectual Property”). As consideration for Intellectual Property to Dong Ying China, the Company issued an aggregate of 9,500,000 unregistered shares of the Company's common stock which at a price of $0.1870 per share and $1,776,500 was recorded to Meisu Jining Science and Technology (Nanjing) Limited Company on the same day. As a result of this transaction, Meisu Jining Science and Technology (Nanjing) Limited Company became a shareholder owning approximately 8% of the issued and outstanding shares of the Company’s common stock
On December 22, 2009, Dong Ying China entered into a patent transfer agreement with Lei Wang the employee of Dong Ying China and Lequn Huang, the Company's chief executive officer and director, for the transfer of a patent, "Composition for Lyophilized Powder of Atracurium," jointly owned by Mr. Wang and Dr. Huang. As consideration for the patent, the Company issued 8,000,000 unregistered shares of the Company's common stock, of which the value is determined at the market price of the date, to Mr. Wang and agreed prior to issuance of the preferred stock to Dr. Huang, the Company shall: (i) undertake its commercially reasonable best efforts to seek shareholder, board, and relevant governmental approval to authorize a class of blank check preferred stock, and (ii) following the effectiveness of the authorization of blank check preferred stock, designate and issue such number of shares of preferred stock as to give Dr. Huang approximately 51% of the voting rights and 0% of the equity rights of the Company. Should the Company fail to obtain sufficient approval to authorize any preferred stock after having made commercially reasonable best efforts to do so, Dr. Huang waived his right to redress under the dispute settlement terms of the agreement. The fair value of the consideration on December 22, 2009 was $1,360,000. On April 2, 2010, pursuant to the terms of the patent transfer agreement, the Company issued 1,000,000 shares of the Series A Preferred Stock to Dr. Lequn Huang as consideration for the patent.
On January 11, 2010, the Company settled certain outstanding debt owed to its chief executive and financial officers into an aggregate of 5,067,608 shares of common stock of the Company. The Company’s chief executive officer, Lequn Huang, was issued 4,234,275 shares of common stock for the outstanding $508,113 in loans owed to Mr. Huang, while the Company’s chief financial officer, Xinjie Mu, was issued 833,333 shares of common stock for the outstanding $100,000 in loans due to Mr. Mu. Both debts were settled by shares at a price of $0.12 per share. At the conversion date, the stock market trading price of the Company’s stock was $0.22 per share. The difference of $423,427 and $83,333 was treated as compensation to Mr.Huang and Mr. Mu.
Other than as disclosed above, there are no transactions in the last two fiscal years, or any currently proposed transaction, in which the Company was or is to be a participant and the amount involved exceeds $120,000 or one percent of the average of the Company’s total assets at year end for the last two fiscal years, and in which any related person had or will have a direct or indirect material interest.
Procedures for Approval of Related Party Transactions
Our Board of Directors is charged with reviewing and approving all potential related party transactions. All such related party transactions must then be reported under applicable SEC rules. We have not adopted other procedures for review, or standards for approval, of such transactions, but instead review them on a case-by-case basis.
Director Independence
The Company currently has four independent directors, Yuanwei Chen, Du Wang, Eric Shi, and Hoki Luk, as that term is defined under the National Association of Securities Dealers Automated Quotation system.
Item 14. Principal Accounting Fees and Services
The following table discloses the fees billed by our auditor in connection with the audit of our annual financial statements for the years ended May 31, 2010 and 2009.
62
|
Financial Statements for Year Ended May 31
|
Audit Fees(1)
|
Audit Related
Fees(2)
|
Tax Fees(3)
|
All Other Fees(4)
|
||||||||||||
|
2010
|
$ | 82,000 | $ | - | $ | - | $ | - | ||||||||
|
2009
|
$ | 9,600 | $ | - | $ | - | $ | - | ||||||||
Notes:
|
(1)
|
The aggregate fees billed for the fiscal year for professional services rendered by the principal accountant for the audit of the Company’s annual financial statements and review of financial statements included in the Company’s Form 10-Qs or services that are normally provided by the accountant in connection with statutory and regulatory engagements for that fiscal years.
|
|
(2)
|
Represents aggregate fees billed in the fiscal year for assurance and related services by the principal accountants that are reasonably related to the performance of the audit or review of the Company’s financial statements and are not reported in Note 1.
|
|
(3)
|
Represents aggregate fees billed in the fiscal year for professional services rendered by the principal accountant for tax compliance, tax advice, and tax planning.
|
|
(4)
|
Represents aggregate fees billed in the fiscal year for the products and services provided by the principal accountant, other than the services reported in Notes (1), (2) and (3).
|
Audit Committee’s Pre-Approval Practice
Section 10A(i) of the Securities Exchange Act of 1934, as amended, prohibits our auditors from performing audit services for us as well as any services not considered to be audit services unless such services are pre-approved by our audit committee or, in cases where no such committee exists, by our board of directors (in lieu of an audit committee) or unless the services meet certain deminimis standards. In the fiscal year ended May 31, 2010, our auditors did not provide any non-audit-related services.
PART IV
Item 15. Exhibits, Financial Statements Schedules
|
Exhibit No.
|
Description of Exhibit
|
|
|
2.1(1)
|
Share Exchange Agreement, dated as of August 19, 2008, among the Company, Dongying BVI and all the shareholders of Dongying BVI.
|
|
|
3.1(2)
|
Articles of Incorporation.
|
|
|
3.2(2)
|
Amendment to Articles of Incorporation.
|
|
|
3.3(3)
|
Certificate of Amendment to Articles of Incorporation.
|
|
|
3.3(2)
|
Corporate Bylaws.
|
|
|
3.4(4)
|
Articles of Merger.
|
|
|
3.5(4)
|
Certificate of Change.
|
|
|
3.6(13)
|
Amended and Restated Articles of Incorporation.
|
|
|
3.7(13)
|
Certificate of Designation of Preferences, Rights and Limitations of Series A Preferred Stock.
|
|
|
3.8
|
Certificates of Correction.
|
|
|
10.1(2)
|
Share Purchase Agreement between Tiffany Walsh and Buzz Media, Ltd. for Buzz Media Nova Scotia.
|
|
|
10.2(2)
|
Artists Agreements for student contributors program
|
|
|
10.3(2)
|
Office Space Rental Agreement
|
|
|
10.4(5)
|
Stock Purchase Agreement, dated as of July 2, 2008, by and between Ms. Tiffany Walsh and Mr. Jianguo Wang.
|
|
|
10.5(6)
|
Share Exchange Agreement, dated as of August 19, 2008, among the Company, Dongying BVI and all the shareholders of Dongying BVI.
|
|
|
10.6(6)
|
Letter Agreement among Mr. Jianguo Wang and Dongying BVI, dated effective August 19, 2008.
|
|
|
10.7(6)
|
Share Purchase Agreement, dated as of August 20, 2008, among the Company and Ms. Tiffany Walsh.
|
|
|
10.8(7)
|
Extension Agreement, dated August 29, 2008, among the Company, Dongying BVI and all the shareholders of Dongying BVI.
|
|
|
10.9(1)
|
Amended Letter Agreement between Dongying BVI and Jianguo Wang, dated September 8, 2008.
|
|
|
10.10(1)
|
Technology Transfer Contract for Prulifloxacin, dated November 30, 2003, between Nantong Cyton Pharmaceutical Co., Ltd. and Nanjing Suji Bio-medicine Research and Development Co., Ltd.
|
|
|
10.11(1)
|
Technology Transfer Contract for Clarithromycin, Olmesartan and Eplerenone, dated November 30, 2003, between Nantong Cyton Pharmaceutical Co., Ltd. and Meisu Jining Bio-medicine Research and Development Co., Ltd.
|
|
|
10.12(1)
|
Technology Transfer Contract for Cisatracurium Besylate, dated January 20, 2004 between Nantong Cyton Phramaceutical Co., Ltd. and Nanjing Suji Bio-medicine Research and Development Co., Ltd.
|
|
|
10.13(1)
|
Technology Transfer Contract for Lansoprazole, Azitromvcin, Ganciclovir and Clindamycin Hydrochloride, dated February 6, 2004, between Nantong Cyton Pharmaceutical Co., Ltd. and Nanjing Suji Bio-medicine Research and Development Co., Ltd.
|
63
|
10.14(1)
|
Development and Loan Agreement between Nantong Economic and Technology Development Zone and Cyton International, Inc., dated March 29, 2004.
|
|
|
10.15(1)
|
Technology Transfer Contract for Alanyl Glutamine and Perindopril, dated June 1, 2006, between Nantong Cyton Pharmaceutical Co., Ltd. and Meisu Jining Bio-medicine Research and Development Co., Ltd.
|
|
|
10.16(1)
|
Loan Extension Approval from Nantong Economic and Technology Development Zone, dated December 4, 2007.
|
|
|
10.17(1)
|
Waiver of rights to convert to shares of Dong Ying China provided by Nantong Economic and Technology Development Zone, dated August 21, 2008.
|
|
|
10.18(9)
|
Maximum Amount Loan Agreement between Dong Ying China and the Bank of Communications, Nantong Branch, dated Jan. 19, 2009
|
|
|
10.19(9)
|
Maximum Mortgage Contract between Dong Ying China and the Community Bank, Nantong Branch, dated Jan. 19, 2009.
|
|
|
10.20(10)
|
Purchase Agreement by and between Dong Ying China and Meisu Jining Science and Technology (Nanjing) Limited Company, dated December 18, 2009.
|
|
|
10.21(10)
|
Purchase Agreement by and between Dong Ying China and Meisu Jining Science and Technology (Nanjing) Limited Company, dated December 18, 2009.
|
|
|
10.22(10)
|
Purchase Agreement by and between Dong Ying China and Meisu Jining Science and Technology (Nanjing) Limited Company, dated December 18, 2009.
|
|
|
10.23(11)
|
Patent Transfer Agreement, dated December 22, 2009 by and among DongYing (Jiangsu) Pharmaceutical Limited Company, Lei Wang, Lequn Huang, and the Company.
|
|
|
10.24(12)
|
Form of Subscription Agreement by and between Sinobiopharma and certain accredited investors, dated January 15, 2010.
|
|
|
10.25(14)
|
Cooperation Agreement, dated July 26, 2010, by and between Dong Ying China and Jiangsu LianHuan Pharmaceuticals Co., Ltd.
|
|
|
21.1(9)
|
List of Subsidiaries.
|
|
|
31.1
|
Certificate pursuant to Rule 13a-14(a).
|
|
|
31.2
|
Certificate pursuant to Rule 13a-14(a).
|
|
|
32.1
|
Certificate pursuant to 18 U.S.C. Section 1350.
|
|
|
32.2
|
Certificate pursuant to 18 U.S.C. Section 1350.
|
|
|
99.1(1)
|
Property Rights Certificate of PRC issued to Dong Ying China, dated September 11, 2007.
|
|
|
99.2(1)
|
Abstract for the Patent Application for Atracurium freeze-dried formulation.
|
|
|
99.3(1)
|
Description of verbal right of first refusal arrangement between Dong Ying China, Nanjing Suji and Meisu Jining relating to any new products developed by Nanjing Suji or Meisu Jining.
|
|
|
99.4(1)
|
Description of terms of shareholder loan by Dr. Lequn Huang.
|
|
|
99.7(1)
|
Trust Declaration executed by the inventors and applicant of the Patent Application titled “Atracurium freezing-dried composition” being held in trust for Dong Ying China, dated September 23, 2008.
|
|
|
99.8(8)
|
Consulting Agreement between Sinobiopharma, Inc. and Michael Tan, dated September 25, 2008.
|
|
|
99.9(8)
|
2008 Stock Option and Incentive Plan.
|
|
|
99.10(8)
|
Sample Stock Option Agreement.
|
|
|
99.11(9)
|
Certificate of Patent for Atracurium freeze-dried composition issued by the State Intellectual Property Office of the People’s Republic of China on August 26, 2009.
|
|
(1)
|
Incorporated by reference to the exhibit to our current report on Form 8-K/A-2 filed with the SEC on September 26, 2008.
|
|
(2)
|
Incorporated by reference to the exhibit to our registration statement on Form SB-2 filed with the SEC on July 21, 2007.
|
|
(3)
|
Incorporated by reference to the exhibit to our amended annual report on form 10-KSB filed with the SEC on September 15, 2008.
|
|
(4)
|
Incorporated by reference to the exhibit to our current report on form 8-K filed with the SEC on August 7, 2008.
|
|
(5)
|
Incorporated by reference to the exhibit to our current report on form 8-K filed with the SEC on July 3, 2008.
|
|
(6)
|
Incorporated by reference to the exhibit to our current report on form 8-K filed with the SEC on August 22, 2008.
|
|
(7)
|
Incorporated by reference to the exhibit to our current report on form 8-K filed with the SEC on September 5, 2008.
|
|
(8)
|
Incorporated by reference to the exhibit to our current report on form 8-K filed with the SEC on October 15, 2008.
|
|
(9)
|
Incorporated by reference to the exhibit to our annual report on form 10-K filed with the SEC on September 15, 2009.
|
|
(10)
|
Incorporated by reference to the exhibit to our current report on Form 8-K filed with the SEC on December 23, 2009.
|
|
(11)
|
Incorporated by reference to the exhibit to our current report on form 8-K filed with the SEC on December 29, 2009.
|
|
(12)
|
Incorporated by reference to the exhibit to our current report on form 8-K filed with the SEC on January 15, 2010.
|
|
(13)
|
Incorporated by reference to the exhibit to our current report on form 8-K filed with the SEC on April 2, 2010.
|
|
(14)
|
Incorporated by reference to the exhibit to our current report on form 8-K filed with the SEC on August 10, 2010.
|
64
In accordance with Section 13 or 15(d) of the Exchange Act of 1934, the registrant caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
Date: September 14, 2010
|
|
SINOBIOPHARMA, INC.
|
|
|
|
||
|
By:
|
/s/ Lequn Huang
|
|
|
Lequn Huang
|
||
|
Chief Executive Officer
|
||
In accordance with the Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
|
Name
|
Title
|
Date
|
||
|
/s/ Lequn Huang
|
Chief Executive Officer and Director (principal executive officer)
|
September 14, 2010
|
||
|
Lequn Huang
|
||||
|
/s/ Xinjie Mu
|
Chief Financial Officer (principal financial and accounting officer)
|
September 14, 2010
|
||
|
Xinjie Mu
|
||||
|
/s/ Yuanwei Chen
|
Director
|
September 14, 2010
|
||
|
Yuanwei Chen
|
||||
|
/s/ Du Wang
|
Director
|
September 14, 2010
|
||
|
Du Wang
|
||||
|
/s/ Eric Shi
|
Director
|
September 14, 2010
|
||
|
Eric Shi
|
||||
|
/s/ Hoki Luk
|
Director
|
September 14, 2010
|
||
|
Hoki Luk
|
65
