Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Assertio Therapeutics, Inc | a10-17848_18k.htm |
Exhibit 99.1
|
|
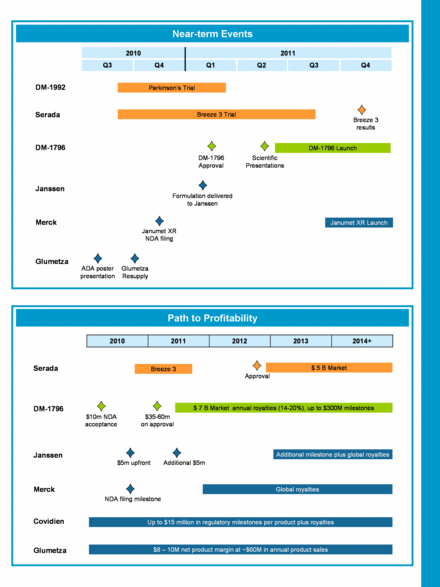
Investor Newsletter A message from our CEO, Carl A. Pelzel Please see note regarding forward-looking statements and risk factors on last page September 13, 2010 Dear Shareholders of Depomed, We have progressed well on our corporate objectives this year, advanced our late-stage pipeline, received a $10 million cash payment from Abbott Products, and entered into a technology license deal with Janssen Pharmaceutica. The acceptance of the New Drug Application of DM-1796 by the FDA triggered a $10 million milestone payment by Abbott and sets us up for another milestone payment of $35 - $60 million upon approval of DM-1796, which we expect in the first quarter of 2011. The path to approval was further cleared from uncertainty regarding potential litigation after we received confirmation that Pfizer would not impose a 30-month stay of approval on the NDA. With the approval of DM-1796, we will look forward to a potential royalty stream to us which could reach $40 million or more annually if DM-1796 captures even a modest percentage market share of the 40 million prescriptions that make up the combined gabapentin/ Lyrica market. In addition, we may receive up to $300 million in sales milestones. We are pleased that we reached an SPA (Special Protocol Assessment) agreement with the FDA on our Breeze 3 Phase 3 trial assessing Serada® in August. We immediately initiated Breeze 3 as all clinical sites were on line and more than 200 patients were pre-screened. We believe that we have taken appropriate measures to keep the placebo response within the 45% to 55% range observed in other published hot flash trials. We have a two week run-in period to reduce regression to the mean, significantly reduced patient interactions with clinical centers to limit the “TLC” aspects of the study, and reduced boredom and mechanical diary entry by having patients record hot flashes only every fourth week during the last 12 weeks of the study. Furthermore, there is only one active treatment arm, so Breeze 3 needs to reach a statistical p value of 0.05. Breeze 3 is expected to be completed by the end of the third quarter of 2011, with results to be reported in the fourth quarter. In August, we also closed our third licensing deal with a Big Pharma partner in the past 2 years. We further monetized the value of our Acuform® extended-release metformin technology through a worldwide license to Janssen Pharmaceutica for the development of fixed-dose combination formulations of canagliflozin, a Sodium Glucose Transport 2 (SGLT2) inhibitor, and extended-release metformin. Janssen also has a right of reference to our Glumetza® NDA. We received a $5 million upfront license fee. The deal provides for reimbursements for our formulation work and an additional $5 million license fee following completion of the formulation activities. In addition, we are eligible for a development milestone and will receive royalties on net sales. This deal adds another potential technology-derived royalty stream to our Covidien and Merck collaborations, and supports our path to profitability. We are very pleased that our Glumetza promotion partner, Santarus, has licensed the FDA-approved novel type 2 diabetes drug Cycloset ® (bromocriptine mesylate). With an enhanced focus on diabetes with two complementary products, we believe that Santarus will be able to further grow Glumetza. Santarus’ efforts on Glumetza will be assisted by the results of the Glumetza observational study presented to the ADA in June which suggests that patients previously intolerant of metformin may be able to tolerate higher doses of metformin when treated with Glumetza. The study enrolled patients previously unable to tolerate more than 1000mg of metformin per day. After switching to Glumetza, 95% of patients tolerated a once-daily dose greater than 1000mg, 57% tolerated a once-daily dose greater than 1500mg, and 43% tolerated a once-daily dose of 2000mg or more. We hope to conclude our investigation into the Glumetza 500mg supply chain and resume product shipments in mid-to-late fourth quarter 2010, depending on the results of our investigation. We anticipate the remainder of 2010 will be productive on multiple fronts. We expect to advance our Parkinson’s program with a Phase 1 trial, Merck to file an NDA for Janumet XR by the end of this year, and progression of Serada’s Breeze 3 trial. Our business development team will focus their efforts on out licensing our early-stage GERD and Parkinson’s Disease programs, technology deals, and an ex-US transaction for DM-1796. Thank you again for your continued support and interest in Depomed. Sincerely, Carl A. Pelzel President and CEO |
|
|
Near-term Events Path to Profitability 2010 Q3 Q4 2011 Q4 Q3 Q2 Q1 DM - 1796 Approval Breeze 3 Trial Breeze 3 results Parkinson ’ s Trial DM - 1796 Launch Scientific Presentations Glumetza Resupply ADA poster presentation Janumet XR NDA filing Janumet XR Launch Formulation delivered to Janssen Serada DM - 1796 Glumetza Janssen Merck DM - 1992 2013 2010 2011 2014+ 2012 Breeze 3 Serada DM - 1796 $ 7 B Market annual royalties (14 - 20%), up to $300M milestones $8 – 10M net product margin at ~$60M in annual product sales Glumetza Janssen $5m upfront Additional $5m Merck NDA filing milestone Global royalties Covidien Up to $15 million in regulatory milestones per product plus royalties Additional milestone plus global royalties $35 - 60m on approval $10m NDA acceptance Approval $ 5 B Market |
|
|
NOTE REGARDING FORWARD-LOOKING STATEMENTS AND RISK FACTORS. Statements in this newsletter that are not historical facts are forward-looking statements that involve risks and uncertainties. The inclusion of forward-looking statements, including those related to expectations regarding the commercialization of Serada® potential business transactions, financial matters, clinical development programs, and potential benefits of our products and product candidates, should not be regarded as a representation that any of our plans will be achieved. Actual results may differ materially from those set forth in this newsletter due to the risks and uncertainties inherent in our business, including, without limitation, risks and uncertainties related to: our research and development efforts, including pre-clinical and clinical testing; regulation by the FDA and other government agencies; the timing of regulatory applications and product launches; our ability to successfully commercialize our products; the success of our collaborative arrangements with development and commercialization partners; and other risks detailed in our filings with the Securities and Exchange Commission filings, including our most recent Annual Report on Form 10-K and our most recent Quarterly Report on Form 10-Q. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. We undertake no obligation to revise or update this newsletter to reflect events or circumstances that occur after the date of this newsletter. Investment in our common stock involves a high degree of risk. This newsletter is not intended to be, and is not, a prospectus. You should review our most recent Annual Report on Form 10-K and any subsequently filed Quarterly Report on Form 10-Q or Current Report on Form 8-K before making any investment decision related to our securities. You should pay particular attention to the “Risk Factors” sections in the Form 10-K and Form 10-Q. Each of the risks described in those documents could adversely affect our business, financial condition, results of operations and prospects, and could result in a complete loss of any investment you may have in our securities. Breeze 3 Trial currently enrolling § Special Protocol Assessment (SPA) approved and Breeze 3 Trial initiated in Q3 2010 § Key Aspects of SPA – Reductions in the mean frequency of moderate-to-severe hot flashes, and the average severity of hot flashes, measured after 4, 12 (primary endpoint) and 24 (secondary endpoint) weeks of stable treatment – Single active arm (1800mg BID) with required p-value of .05 – Two-week run in period prior to randomization limits regression to the mean – Non-parametric analysis limits impact of significant outliers – Responder analysis to assess the clinical meaningfulness of any reduction in the frequency of hot flashes in the active arm relative to the placebo arm Breeze 3 Placebo 1800mg 4 wks 12 wks 24 wks |