Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Vicor Technologies, Inc. | y03817e8vk.htm |
Exhibit 99.1

| Vicor Technologies, Inc. (OTCBB: VCRT) A Medical Diagnostic Company Dedicated to the Commercialization Of Breakthrough Risk Stratification Technology |

| 2 Safe Harbor Statement Forward-looking statements in this presentation are based on current plans and expectations that are subject to uncertainties and risks, which could cause our future results to differ materially. The following factors, among others, could cause our actual results to differ: our ability to generate revenues from the sale of the PD2i Analyzer(tm), our ability to obtain FDA approval of our 510(k) submission to secure a claim for the PD2i CA(tm)(Cardiac Analyzer) for risk stratifying congestive heart failure patients at elevated risk of cardiac mortality and our ability to obtain marketing clearance from the FDA for the PD2i VS(tm) (Vital Sign) for military and civilian applications; our ability to continue to receive financing sufficient to continue operations and complete critical clinical trials; our ability to continue as a going concern; our ability to successfully develop products based on our technologies; our ability to obtain and maintain adequate levels of third-party reimbursement for our products; the impact of competitive products and pricing; our ability to receive regulatory approval for our products; the ability of third-party contract research organizations to perform preclinical testing and clinical trials for our technologies; the ability of third-party manufacturers to manufacture our products; our ability to retain the services of our key personnel; our ability to market and sell our products successfully; our ability to protect our intellectual property; product liability; changes in federal income tax laws and regulations; general market conditions in the medical device and pharmaceutical industries; and other matters that are described in Vicor's Annual Report on Form 10-K for the fiscal year ended December 31, 2009 and subsequent filings with the Securities and Exchange Commission. Forward-looking statements in this presentation speak only as of the date of the presentation and we assume no obligation to update forward-looking statements or the reasons why actual results could differ. |

| 3 Company OverviewMarket Opportunity OverviewPD2i Technology OverviewProduct Overview |

| Company Overview Vicor is a medical diagnostics company, focused on commercializing non-invasive diagnostic technology products based on its patented, proprietary algorithm (the "PD2i(r) Algorithm") and softwareThe PD2i Algorithm and related software facilitates the ability of a physician to accurately risk stratify a specific target population to predict future pathological events. The initial targeted markets are:Diabetics with autonomic nervous system dysfunctionCardiology patients at risk of cardiac events and cardiac mortalityTrauma patients in need of life saving interventionVicor is currently commercializing three proprietary diagnostic medical products based on the PD2i(r) Algorithm and software:PD2i Analyzer(tm): Identifies diabetic patients suffering from autonomic nervous system dysfunctionPD2i Cardiac Analyzer(tm): Identifies patients at an elevated risk of suffering cardiac mortalityPD2i-VS(tm) (Vital Sign): Quickly evaluates the severity of critically-injured soldiers and civilians to determine the need for immediate life saving interventionBased in Boca Raton, FL, Vicor was founded in 2000 and currently employs 12 employees 4 |

| Market Opportunity The total initial domestic target market for diagnostic products utilizing the PD2i Algorithm is 77 million people:23.6 million diabetics (autonomic nervous system testing) - $1.9 billion market15 million immediate at-risk cardiac patients (cardiac mortality) - $1.2 billion market38 million annual trauma events (car accidents, etc) - $0.8 billion market 5 77 Million PotentialPatientPopulation $3.9 Billion Market for PD2i |

| Results in:High (~100%) sensitivityCorrectly identifying those not at riskHigh (~86%) specificityCorrectly identifying those at riskHigh statistical certainty of results PD2i Technology Overview What is the PD2i Algorithm?A non-invasive, proprietary, patented software and algorithm predictive of future pathological events, including autonomic nervous system dysfunction, cardiac mortality and imminent death from traumaHow does the algorithm work?The PD2i Algorithm measures Heart Rate Variability (HRV)Non-linear, non-stochastic mathematical modelHeart rate variation is controlled by sensory motor loops (temperature, pH level, blood pressure, etc.)If the body is threatened by disease or trauma, the brain tries to marshal the forces in the body to act in concert The PD2i metric measures the degrees-of-freedom of the heart rate variability signalA low PD2i score indicates the body is threatenedWhy is it superior to other HRV and vital sign technologies?More accurate predictive ability (not affected by irregularities in heart rhythm or intrinsic heart disease)Short data collection window: 2-20 minutes depending upon applicationPerformed on a resting patient (less risky)User friendly and non-invasive 6 |

| Product Overview PD2i Analyzer and Cardiac AnalyzerProduct4-lead (12-lead at physician's option) ECG cable with automated blood pressure device paired via USB with a laptopUser-friendly Windows-integrated device that is lighweight and portableProcessLaptop utilizes proprietary collection software, accesses the internet and sends recorded ECG data to Vicor's remote secure server for analysis by the PD2i proprietary algorithm and softwarePhysician instantly receives electronic medical record and a report that the physician interprets to determine whether the patient is at riskSoftware records physician use to enable monthly automated billingPD2i-VSPD2i-VS is a product under developmentECG measurement only (no blood pressure collection)Will be used as a diagnostic/triage tool in trauma patientsWhile the data collection window for the Analyzer and the Cardiac Analyzer is 20 minutes, the PD2i-VS can identify those trauma victims at risk of imminent death and in need of a life saving intervention in under 3 minutes 7 |

| 8 Autonomic Nervous System (ANS) - PD2i Analyzer(tm)Market OpportunityMilestones and Marketing PlanCompetitive Advantages |
| Autonomic Nervous System (ANS) - PD2i Analyzer(tm) Market Opportunity What is the Autonomic Nervous System (ANS)? ANS acts as a control system for the body. It is part of both the central and peripheral nervous system, controlling organs and muscles involuntarily (heart rate, blood pressure, respiration, salivation, etc.)ANS Dysfunction is a complication of diabetes: if left untreated, leads to potentially deadly diabetic autonomic neuropathy (DAN)Cardiovascular autonomic neuropathy (CAN) is a form of DAN (17% of type I and 22% of type II diabetics)Large untapped market23.6 million diabetic patientsGrowing obesity epidemic contributes to increase in the prevalence of diabetes47.2 million tests annually: $1.9 billion market for the PD2i AnalyzerHow is DAN tested?Patient history and physical examinations proved ineffective in the early detection of DANHeart Rate Variability (HRV) measurement is now considered the standard of care for diagnosing DAN/CAN in diabeticsEarly ANS Dysfunction diagnosis:Permits physicians to properly diagnose the cause of many presenting symptoms (dizziness, palpitations, etc.)Permits physicians to adjust/select medications appropriatelyReduces/eliminates effects of ANS dysfunctionANS/DAN testing reimbursable under currently existing CPT codes 9 |
| Autonomic Nervous System (ANS) - PD2i Analyzer(tm) Milestones and Marketing Plan Received 510(k) marketing clearance from the FDA in December 2008Enables Vicor to market and distribute the PD2i Analyzer(tm) for measurement of Heart Rate VariabilityClinical significance of the data determined by physicianPD2i Analyzer(tm) product sales launch in January 2010PD2i currently in use for ANS dysfunction testing in diabetic patientsReimbursement by Medicare, Medicaid and private insurers through existing CPT codes510(k) for Normal Range Study to be submitted in 2H 2010Study will enable Vicor to establish "normal" rangesPhysicians will be able to interpret results as abnormal or normalMarketing PlanSubstantial revenue generation capability with existing 510(k)Initial distribution agreement with VF Medical (25 independent sales reps in NC and SC)In-house sales efforts to initially focus on stockholder physicians and members of its National Cardiac Panel Roll out in Q2 and Q3 2010 in other states (sales personnel, additional distributors) 10 |
| Autonomic Nervous System (ANS) - PD2i Analyzer(tm) Competitive Advantages Heart Rate Variability (HRV) testing is the most common non-invasive method to determine patients at risk of Autonomic Nervous System (ANS) dysfunctionAnsar Group is Vicor's principal competitor to the PD2i AnalyzerAnsar developed the ANX 3.0, a noninvasive, real time digital analyzer of autonomic nervous system functioningANX 3.0 monitor costs ~$40,000 compared to $5,125 for the PD2i Analyzer(tm)Management believes that the PD2i(r) metric is a more sensitive measure of HRV in diabetic patients than the metric used by the ANX 3.0 - the Normal Range Study in conjunction with several studies in diabetics will be used as proofTraditional clinical screening tests (i.e. patient's history, physical exams) are ineffective for early detection of diabetic and cardiac autonomic neuropathy (DAN/CAN) 11 |

| 12 Cardiac Mortality - PD2i Cardiac Analyzer(tm)Market OpportunityMilestones and Marketing PlanMUSIC TrialCompetitive Advantages |

| Cardiac Mortality - PD2i Cardiac Analyzer(tm) Market Opportunity 81 million people affected by cardiovascular diseases in the United States:Cardiac Ischemia (heart attack): lack of blood flow and oxygen to the heartCongestive Heart Failure: heart cannot pump enough blood to organs15 million immediate at-risk patient population with cardiac disease:Sudden Cardiac Death (SCD or arrhythmic death): swift and unexpected loss of heart function, largest cause of natural death in United States (>300,000 / year)Post myocardial infarction (MI or heart attack): 8 millionCongestive heart failure (pump failure): 5 millionSyncope (fainting/dizziness) and non-ischemic dilated cardiomyopathy (damaged/enlarged heart): 2 million30 million tests annually: $1.2 billion market for the PD2i Cardiac AnalyzerAccurate identification of at-risk patients needed to implant ICDs or CRT-DsImplantable cardioverter defibrillators (ICDs) monitor heart rate and rhythm and provide a shock to prevent arrhythmic deathICDs enabled with Cardiac Resynchronization Therapy (CRT) provide assistance to the heart's pumping ability as well as a shock to prevent arrhythmic death Conventional diagnostics misidentifiy at-risk patients:Many people who don't need ICDs get implanted - 20 ICDs need to be implanted to save a single life~80% of those who do die from arrhythmic death would not even qualify for implantation under current criteria/methodologyThe PD2i Algorithm is the most accurate and effective easy-to-use technology in predicting the risk of cardiac mortality: it directly addresses the over- and under-implantation of devices 13 |

| Cardiac Mortality - PD2i Cardiac Analyzer(tm) Milestones and Marketing Plan Submitted 510(k) premarket notification for cardiac mortality on July 1, 2010Once approved for cardiac claim, the "PD2i Cardiac Analyzer" can be marketed for the prediction of cardiac mortality in at-risk patients Expect 510(k) clearance in 2H 2010Able to accurately risk stratify patients into those at high or low risk of suffering a fatal cardiac event such as arrhythmic death or pump failure deathResults should be reviewed by qualified medical personnel and be used as an adjunct to clinical history and the results of other testsMarketing PlanBeta tests in several practicesCedars Sinai Medical Group-Los AngelesJackson Memorial Hospital North-MiamiInitially use VF Medical's 25 sales reps in NC and SCThought Leader seminars: 2H 2010Roll out in 2H 2010 of other states 14 |
| Cardiac Mortality - PD2i Cardiac Analyzer(tm) "MUSIC" Trial MUSIC trial data set was sufficient to submit the 510(k) premarket notification for cardiac mortality MERTE SUBITA EN INSUFFICIENCIA CARDIACA (MUSIC) TrialCollaboration with the University of Rochester and the Catalan Institute of Cardiovascular Sciences in BarcelonaThe trial studied the ability of the algorithm to predict cardiac events in congestive heart failure patientsLargest heart failure population ever studied: 651 patients with congestive heart failureThe first trial to document abnormal heart rhythm as a predictor of sudden cardiac deathPatients followed for a mean of 44 months138 total deaths; 52 verified arrhythmic deathsResults show the PD2i algorithm was effective in predicting:Total mortalityCardiac mortalityHeart failure mortalityThe 510(k) will broaden the market for the PD2i Analyzer as a diagnostic tool for cardiologists in their assessment of treatment options for heart failure patients 15 |

| Cardiac Mortality - PD2i Cardiac Analyzer(tm) Competitive Advantages Currently existing tools to determine patients at risk of Sudden Cardiac Death (SCD or arrhythmic death) all have significant drawbacksNon-invasive tools: ECG, ambulatory monitoring, echocardiography, cardiac stress testsAre inaccurate because (a) they are insensitive to nonlinearities in the data, (b) they assume random variation in the data (stochastic) and (c) the system generating the data cannot change during the recordingRequires stress testing (risky for heart patients)Invasive diagnostic tools: cardiac catheterization, electrophysiology studySame type of risks that accompany any major surgeryCambridge Heart (competitor to the PD2i Cardiac Analyzer)Requires stress testing (risky for heart patients)More expensive: use costly electrodes (physicians are not separately reimbursed)Procedure cannot be performed on patients with irregular heartbeats or taking beta blockersDevice costs ~$45,000 compared to $5,125 for the PD2i Cardiac Analyzer(tm) 16 |

| 17 Trauma - PD2i-VS(tm) (Vital Sign)Market OpportunityMilestones and Marketing PlanUSAISR Trial |

| Trauma - PD2i-VS(tm) (Vital Sign) Market Opportunity PD2i-VS to be used as a diagnostic toolTo assess the severity of injury and imminent death and to determine the need for immediate life saving interventionMilitary sector opportunity: U.S. and foreign armed forcesCritically injured soldiersCivilian sector opportunity: triage and trauma emergency response38 million annual emergency response calls (e.g. car accidents) in the United StatesTo target hospitals, emergency service providers and critical care personnel$760 million market for the PD2i-VSVS monitoring application would provide critical care personnel with ongoing status updates of the patient's condition 18 |
| Trauma - PD2i-VS(tm) (Vital Sign) Milestones Being developed in collaboration with the United States ArmyResearch and Development Agreement signed in January 2008 Allows for emergency responders to sort victims based on need for medical treatment. US Army has presented the findings at several conferences in the United States and Europe - the PD2i- VS was presented as the most promising diagnostic tool for in-field trauma triageFDA submission for 510(k) marketing clearance expected in 2H 2010If cleared, Vicor can begin marketing the PD2i-VS for a claim of trauma triageAnticipated to be used for civilian triage and trauma emergency response and in intensive care units In addition to the triage application noted above, Vicor completed programming necessary for the PD2i-VS to also operate as a continuous vital sign monitorThe monitor would indicate the ongoing status of the patient's condition and alert critical care personnel if lifesaving intervention was requiredEmergency roomsIntensive care unitsOperating rooms 19 |

| Trauma - PD2i-VS(tm) (Vital Sign) USAISR Trial and Other Studies U.S. Army Institute of Surgical Research (USAISR) TrialSupports a 510(k) submission to the FDA for a marketing claim related to diagnosing patients at imminent risk of death from trauma (trauma triage)Collaborative Research and Development Agreement in January 2008"Prediction of Injury Severity and Outcome in the Critically Ill Using the Point Correlation Dimension Algorithm (PD2i(r))": the trial studied the ability of the algorithm in the prediction of death in trauma patientsStudy of 325 trauma patients to determine which patients would die of their injuries20 deaths: all were correctly identified by low PD2i(r) valueConventional triaging techniques had indentified only 6 of the 20 patients as requiring lifesaving interventionPD2i-VS as a continuous vital sign monitorPlanned and ongoing studies to test the PD2i-VS as a monitoring instrumentMassachusetts General HospitalUniversity of Mississippi Medical CenterFurther studies by the USAISR 20 |

| 21 Business Model / EconomicsVicorPhysician |
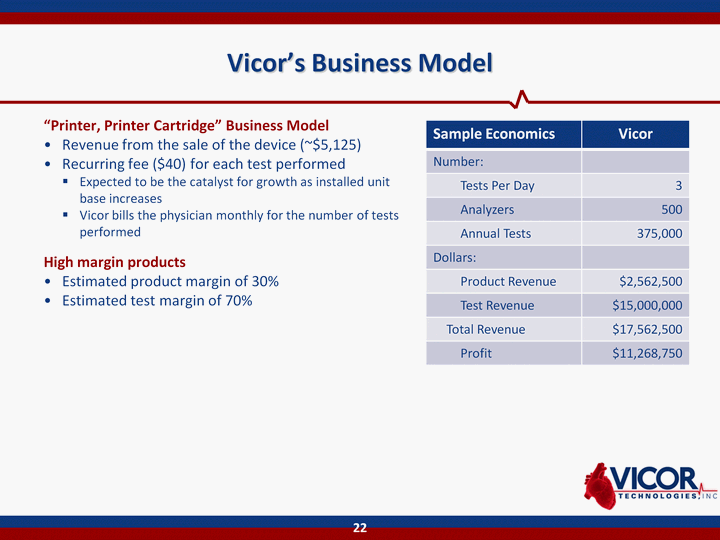
| Vicor's Business Model "Printer, Printer Cartridge" Business ModelRevenue from the sale of the device (~$5,125)Recurring fee ($40) for each test performedExpected to be the catalyst for growth as installed unit base increasesVicor bills the physician monthly for the number of tests performedHigh margin productsEstimated product margin of 30%Estimated test margin of 70% 22 Sample Economics Vicor Number: Tests Per Day 3 Analyzers 500 Annual Tests 375,000 Dollars: Product Revenue $2,562,500 Test Revenue $15,000,000 Total Revenue $17,562,500 Profit $11,268,750 |

| Physician's Economics Reimbursement to healthcare providers by third party insurersThere are currently 60 ICD-9 diagnosis codes available for reimbursement by Medicare, Medicaid and private insurersReimbursement to physicians will likely be ~$160 per testIncentive for Physician UseLow up-front investmentPer test revenue (Vicor and Physician)Physician break-even in less than a month (after 51 tests performed) 23 Sample Economics Physician Number: Tests Per Day 3 Analyzers 1 Annual Tests 750 Dollars: Up-front investment $5,125 Revenue $160 / test Gross Profit $100 / test Total Annual Gross Profit $75,000 (1) Estimated gross profit per test after payment to Vicor, technician's time and disposables. |
| 24 ManagementScientific Advisory BoardInvestment Highlights |

| Management Highly experienced management team has track record with public and high-growth companies as well as discovery and commercialization of products.David Fater - President and CEOOver 13 years experience as a senior executive with 3 public healthcare companiesSpent 24 years as a senior international partner with Ernst & YoungDr. Jerry Anchin, Ph.D. - VP of Product Development25 year's accumulated experience in the biotechnology diagnostic and medical device sectorActively involved in immunology, molecular biology, and drug discoveryHolds patents for 14 immunoassay and drug discovery platforms and an additional 16 patents pendingDr. Richard M. Cohen, Ph.D. - Chief Operating Officer30 year's experience in worldwide operations and sourcing for manufacturing companiesCOO of four health care companies, including two medical device companies 25 |

| Management Dr. Daniel N. Weiss, M.D.,F.A.C.C. - Chief Medical OfficerExtensive experience as a practicing cardiologist and electrophysiologistPartner of Florida Arrhythmia Consultants. Director of the Jim Moran Heart and Vascular Center since 1994Consultant to Medtronics, St. Jude Medical, and GuidantDr. James Skinner, Ph.D. - VP of Research and ScienceExtensive experience as a scientist and manager of large research and development projectsProfessor of Medicine at Baylor College of Medicine. Recipient of many research grantsPrincipal investigator of a program that operated 5 laboratories and 3 core facilitiesVicor is in the process of searching for an SVP of Sales & Marketing. Candidates in consideration are currently heading up sales efforts at major medical device companies. 26 |

| Scientific Advisory Board Vicor has assembled a Scientific Advisory Board consisting of medical doctors and other professionals with experience in relevant scientific and medical fields. The advisory board provides thought-leader guidance to management regarding science, research and product development.Mark E. Josephson, M.D.Chief of Cardiology at Beth Israel Deaconness Medical CenterAuthor of "Clinical Cardiac Electrophysiology"Scientific Advisor to over 20 companiesHein J. J. Wellens, M.D.Professor & Chairman of Department of Cardiology Academisch Ziekenhuis MaastrichtDirector of Interuniversity Cardiology Institute of the NetherlandsRichard M. Luceri, M.D., F.A.C.C. Director Interventional Arrhythmia Center, Holy Cross HospitalClinical investigator in MADIT II TrialClinical investigator & Principal Author SCD-HeFT TrialJules Mitchel, Ph.D., MBAFounder and President/CEO Target Health Inc. NYC 27 |

| Scientific Advisory Board Robert G. Hauser, M.D., F.A.C.C., FHRSSenior Consulting Cardiologist - Minneapolis Heart InstituteChairman Cardiovascular Services Division at Abbott Northwestern HospitalChief Executive Officer of Cardiac Pacemakers, Inc -acquired by Guidant Jonathan Kaplan, M.D., M.P.H.Medical Director of Fidelis Care in New YorkFormer Medical Director for Excellus Blue Cross Blue ShieldDavid ChazanovitzFormer CEO of Cambridge Heart, Inc.Edward F. Lundy, M.D., Ph.D. Chief of Cardiothoracic Surgery at Good Samaritan HospitalPh.D. in Physiology with focus on altered-state physiologies (hibernation) 28 |
| Investment Highlights Life Saving TechnologySubstantial Unmet Medical Need in Risk StratificationIn the Process of Entering Three Large MarketsHigh Margin, High Operating Profit ProductsAttractive Business Model: "Printer, Printer Cartridge" Model with Recurring Test RevenueHighly Experienced Management TeamWorld-Class Scientific Advisory Board 29 |

| Thank You. Vicor Technologies, Inc. 2300 NW Corporate Blvd.,Suite 123 Boca Raton, FL 33431 Phone: (877) 528-PD2i (7324) Fax: (561) 995-2449 www.vicortech.com |

| 31 AppendixAvailable ICD-9 Diagnostic CodesICD-9 Coding ExampleClinical Trials SummaryUSAISR Trial DetailsIntellectual Property |

| AVAILABLE DIAGNOSTIC CODES (ICD-9 for test authorization) 32 INTERNAL MEDICINE278.01 Morbid Obesity279.3 Unspecified Immunity Deficiency296.80 Bipolar Disease311 Depression300.00 Anxiety307.4** Sleep Disorders530.11 GERD536.3 Gastroparesis564.1 Irritable Bowel Syndrome729.1 Fibromyalgia309.81 Post-Traumatic Stress Syndrome782.3 Edema CARDIOLOGY401.0 - 405.99 Hypertension412 Post - MI413.9 Angina440.9 Atherosclerosis424.0 Mitral Valve Prolapse Syndrome425.4 Cardiomyopathy427.9 Cardiac Dysrhythmias428.0 Congestive Heart Failure ENDOCRINOLOGY244.8 Acquired Hypothyroidism246.9 Thyroid Disorders256.31 Premature Menopausal Symptoms627.2 Menopausal Syndromes NEPHROLOGY402.90 Hypertensive Renal Disease586 Renal Failure DIABETES 250 - 250.8** NEUROLOGY307.01 Tension Headache314.01 ADD/ADHD332.0 Parkinsonism333.0 Shy-Drager Syndrom (Orthostatic Hypotension with Multisystem Degeneration)337.00 Idiopathic Peripheral Autonomic Neuropathy 358.1 Myasthenic Syndrome (Eaton-Lambert)337.2 Chronic Regional Pain Syndrome or RSD 458.0 Orthostatic Hypotension340 Multiple Sclerosis 458.1 Chronic Hypotension346.0 - 346.9** Migraine 742.8 Other Specified Congenital Anomalies of the Nervous System356.4 Idiopathic Progressive Polyneuropathy 780.2 Syncope and Collapse356.8 Other Specified Idiopathic Peripheral Neuropathy 780.4 Dizziness, Light-headedness and Vertigo356.9 Unspecified Peripheral Idiopathic Neuropathy 780.71 Chronic Fatigue Syndrome357.2 Polyneuropathy in Diabetes 780.0 Headache357.4 Polyneuropathy in Other Disease Classified Elsewhere 785.0 Tachycardia (Postural) PULMONOLOGY780.5 - 780.59 Sleep Disturbances493.9 - 493.92** Asthma493.2** COPD (Asthma with Chronic Obstructive Pulmonary Disease) This example was put together to help aid you on proper reimbursement procedures. If you have any questions regarding reimbursement, please feel free to contact David Fater toll-free at 800-998-9964 |

| ATTN: BILLING DEPARTMENT ICD-9 CODING FOR VICOR MONITORING 33 CPT CODING EXAMPLE:STEP 1: Use proper diagnosis code(s) for patient. See reverse side for ICD-9 Coding list.*** (i.e., 250.6 Diabetes with neurological manifestations)STEP 2: CPT CODING for VICOR TESTING (95921 & 95922) 95921 & 95922 are separate and distinct procedures. On occasion, certain Coding Software requires the -59 Modifier be used to unbundle claims. Please contact David Fater with any questions or concerns. CPT CODE DESCRIPTION 2010 NATIONAL REIMBURSEMENT AVERAGE SUBMITTAL REQUEST 95921 Parasympathetic Nervous System Testing $75.59 $150 95922 Sympathetic Nervous System Testing $91.81 $150 PT CODING for 3-Lead Rhythm ECG: During the Vicor test a 3-lead ECG is also performed. You may bill for this procedure in addition to Sympathetic and Parasympathetic testing, but you may not be paid separately if it's included in Vicor's testing.** This code is not specific to our technology. It is not reimbursed by every insurance provider; those that are reimbursing may require a 59Modifier. (Please refer to your states policies.) CPT CODE DESCRIPTION 2010 NATIONAL REIMBURSEMENT AVERAGE SUBMITTAL REQUEST 93000** Routine ECG with at least 12 leads; with interpretation and report $20.28 $25 |

| Ongoing Clinical Trials & Market Potential Ongoing Clinical Trials & Market Potential 34 Current trials underway at various institutions (some of which are sponsored by Vicor) include the following: |

| PD2i VS(tm) 35 USAISR Study #1USE OF HEART-RATE COMPLEXITY TO DETERMINE THE NEED FOR LIFE SAVING INTERVENTIONS IN COMBAT CASUALTIES Abnormal HRC values exhibited in all patients Those requiring LSI's had Min PD2i values < 1 Findings suggest utility of PD2i for diagnosis of need for LSI |

| Intellectual Property Intellectual Property 36 Patents issued related to the PD2i technology includes 4 U.S. patents (listed below) and 3 foreign patents. Pending patent applications include numerous U.S. and foreign patents. |
