Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - CYPRESS BIOSCIENCE INC | a56678e8vk.htm |
Exhibit 99.1

| Investor Presentation Summer 2010 |

| 2 This presentation may contain certain forward-looking statements concerning the progress and development of the Company and its technology and products, including but not limited to its fibromyalgia program, drug development pipeline, and its personalized medicine services. Actual results could differ materially from those described as a result of a number of factors that may affect the achievement of events, milestones and results in the future. Refer to the most current version of the Company's publicly-available filings with the SEC, including the Company's Annual report on Form 10-K, our most recent quarterly report and any current reports on Form 8-K for a full discussion of such factors and risks prior to making any investment in the Company's securities. The Company undertakes no obligation to revise or update any forward-looking statement to reflect events or circumstances after the date of this presentation. Forward looking statements July, 2010 |

| Overview 3 Cypress has a rich history of CNS expertise and clinical development success While we have managed to build some value in our attempt to transition to the commercial stage, we have come to the conclusion that our commercial business is not likely to provide a source of sustainable competitive advantage or an opportunity for an optimal return on invested capital Therefore, we have decided to return to our source of historical strength - CNS drug development - with a goal of building shareholder value by investing our capital to establish the best CNS pipeline in the industry |

| Experience and expertise Cypress' drug development team has deep expertise in psychiatry, neuropharmacology, and clinical trial methodology Combined 100+ years of clinical development experience Successful approvals and label expansions in: Devices Diagnostics NCEs Peptides Biologics 4 July, 2010 |
| Cypress: History of clinical success 5 PROSORBA column July, 2010 |

| PROSORBA column Approved for Rx of RA Sold to Fresenius 1999 2001 Successfully designed and ran the 1st sham controlled device trial resulting in approval of the Prosorba column for treatment of RA in 1999 Successfully divested Prosorba in 2001 Prosorba(tm): Approval for RA 6 |
| Cypress: History of clinical success 7 PROSORBA column Milnacipran July, 2010 |
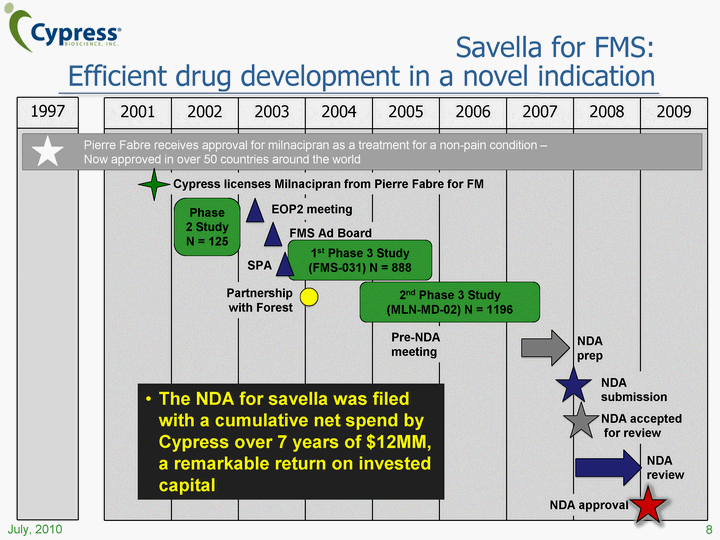
| 2001 2002 2003 2004 2005 2006 2007 2008 2009 1997 Cypress licenses Milnacipran from Pierre Fabre for FM Pierre Fabre receives approval for milnacipran as a treatment for a non-pain condition - Now approved in over 50 countries around the world Phase 2 Study N = 125 2nd Phase 3 Study (MLN-MD-02) N = 1196 NDA accepted for review SPA FMS Ad Board 1st Phase 3 Study (FMS-031) N = 888 EOP2 meeting NDA approval Pre-NDA meeting NDA prep NDA review Partnership with Forest NDA submission Savella for FMS: Efficient drug development in a novel indication The NDA for savella was filed with a cumulative net spend by Cypress over 7 years of $12MM, a remarkable return on invested capital 8 July, 2010 |
| Cypress: History of clinical success 9 PROSORBA column Milnacipran CNS Proof-of-Concept (POC) studies July, 2010 |
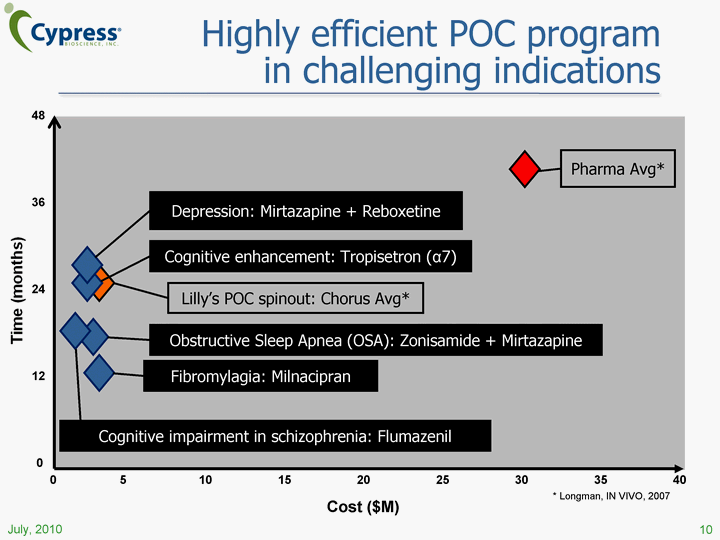
| Highly efficient POC program in challenging indications Time (months) 0 48 12 36 24 Cost ($M) 0 40 35 30 25 20 15 10 5 July, 2010 10 * Longman, IN VIVO, 2007 |

| Overview 11 Cypress has a rich history of CNS expertise and clinical development success While we have managed to build some value in our attempt to transition to the commercial stage, we have come to the conclusion that our commercial business is not likely to provide a source of sustainable competitive advantage or an opportunity for an optimal return on invested capital Therefore, we have decided to return to our source of historical strength - CNS drug development - with a goal of building shareholder value by investing our capital to establish the best CNS pipeline in the industry |

| Cypress: Commercial strategy 12 FRX co-promote* Rheumatology (AviseSM) Dx Net Loss: ~$2M/yr Potential synergy July, 2010 Cypress' Sales Force (N= 100 reps + 15 mgmt) Net Loss: ~$9M/yr * 15% flat royalty off of sales (co-promote notwithstading) + subsidy (~65%) of sales force overhead based on number of Savella details performed |
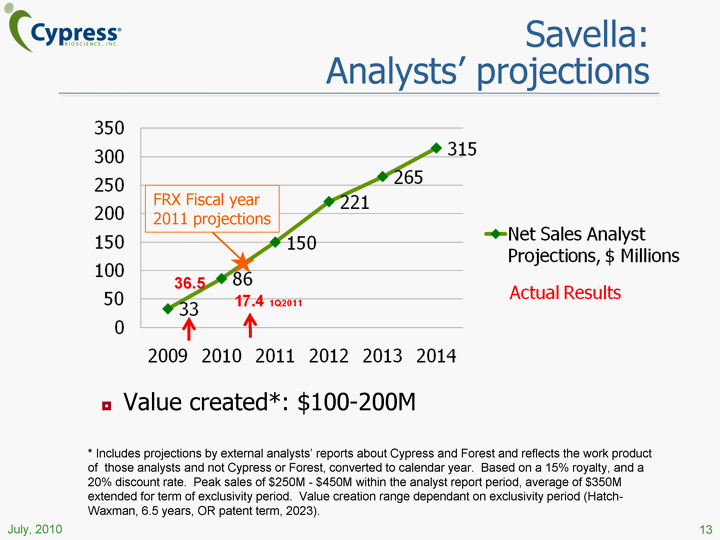
| 13 Value created*: $100-200M * Includes projections by external analysts' reports about Cypress and Forest and reflects the work product of those analysts and not Cypress or Forest, converted to calendar year. Based on a 15% royalty, and a 20% discount rate. Peak sales of $250M - $450M within the analyst report period, average of $350M extended for term of exclusivity period. Value creation range dependant on exclusivity period (Hatch- Waxman, 6.5 years, OR patent term, 2023). (CHART) Savella: Analysts' projections 36.5 FRX Fiscal year 2011 projections |

| Cypress: Commercial strategy 14 FRX co-promote* Rheumatology (AviseSM) Dx Net Loss: ~$2M/yr Potential synergy July, 2010 Cypress' Sales Force (N= 100 reps + 15 mgmt) Net Loss: ~$9M/yr * 15% flat royalty off of sales (co-promote notwithstading) + subsidy (~65%) of sales force overhead based on number of Savella details performed |
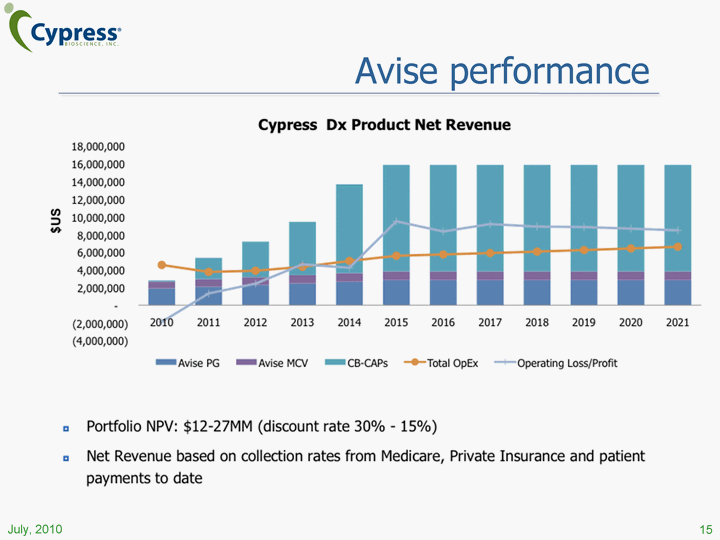
| Avise performance 15 |
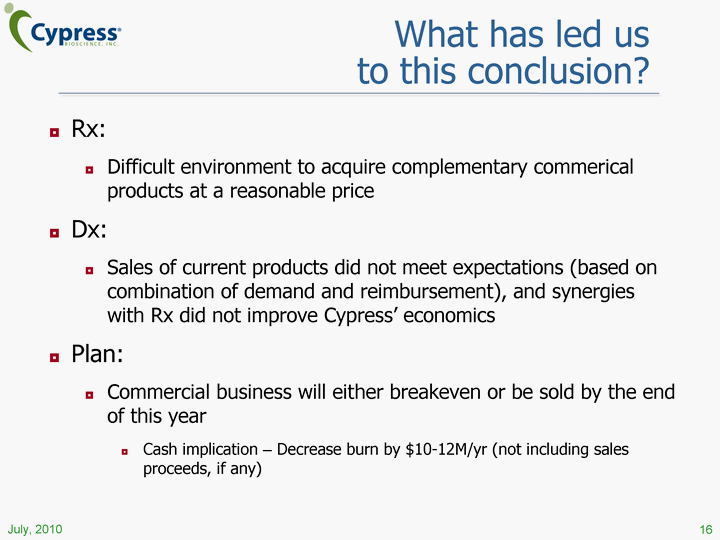
| What has led us to this conclusion? 16 Rx: Difficult environment to acquire complementary commerical products at a reasonable price Dx: Sales of current products did not meet expectations (based on combination of demand and reimbursement), and synergies with Rx did not improve Cypress' economics Plan: Commercial business will either breakeven or be sold by the end of this year Cash implication - Decrease burn by $10-12M/yr (not including sales proceeds, if any) |

| Overview 17 Cypress has a rich history of CNS expertise and clinical development success While we have managed to build some value in our attempt to transition to the commercial stage, we have come to the conclusion that our commercial business is not likely to provide a source of sustainable competitive advantage or an opportunity for an optimal return on invested capital Therefore, we have decided to return to our source of historical strength - CNS drug development - with a goal of building shareholder value by investing our capital to establish the best CNS pipeline in the industry |
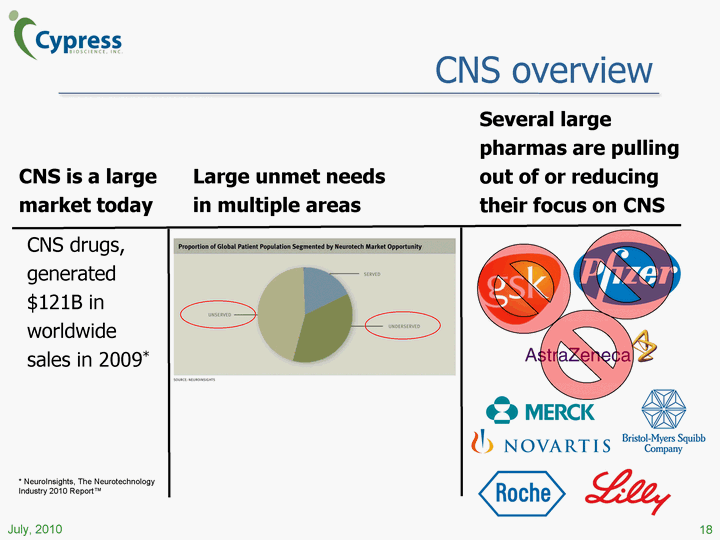
| CNS overview CNS is a large market today CNS drugs, generated $121B in worldwide sales in 2009* Large unmet needs in multiple areas 18 July, 2010 Several large pharmas are pulling out of or reducing their focus on CNS * NeuroInsights, The Neurotechnology Industry 2010 Report(tm) |

| Cypress' revised mission and plan Mission: Develop innovative drugs that target large unmet clinical needs for patients suffering from a variety of disorders of the central nervous system Plan: Establish a leading portfolio of CNS assets, all of which will meet the following criteria: Address a large unmet clinical need and market opportunity Must be well-characterized, with primary residual risk being clinical/regulatory Must be innovative, potential to be first-in-class Able to reach a significant value-creating milestone within ~2 years 19 July, 2010 |
| In-licensing of CYP-1020* Unmet need - Cognitive deficits main predictor of long-term outcome, at least ~80% of patients with schizophrenia are affected Well-characterized - Based on known molecule (perphenazine) with robust P2b data Innovative - Potential to be 1st antipsychotic with pro-cognitive effect 2-years to milestone - Forthcoming trial, if successful, will significantly de-risk P3 program 20 July, 2010 |
| Cognitive impairment in schizophrenia Cognitive Impairment (CI) is a core deficit in schizophrenia: If defined as a failure to meet one's expected level of cognitive functioning, nearly all patients with schizophrenia would qualify as cognitively impaired (Keefe, 2004) Major determinant of poor functional outcome Joint FDA-NIMH-Academia-Industry effort: Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) Broad concern among psychiatrists - modern second generation antipsychotics have been over-hyped, and do not improve cognition 21 |
| In-licensing of CYP-1020 Unmet need - Cognitive deficits main predictor of long-term outcome, at least ~80% of patients with schizophrenia are affected Well-characterized - Based on known molecule (perphenazine) with robust P2b data Innovative - Potential to be 1st antipsychotic with pro-cognitive effect 2-years to milestone - Forthcoming trial, if successful, will significantly de-risk P3 program 22 July, 2010 |
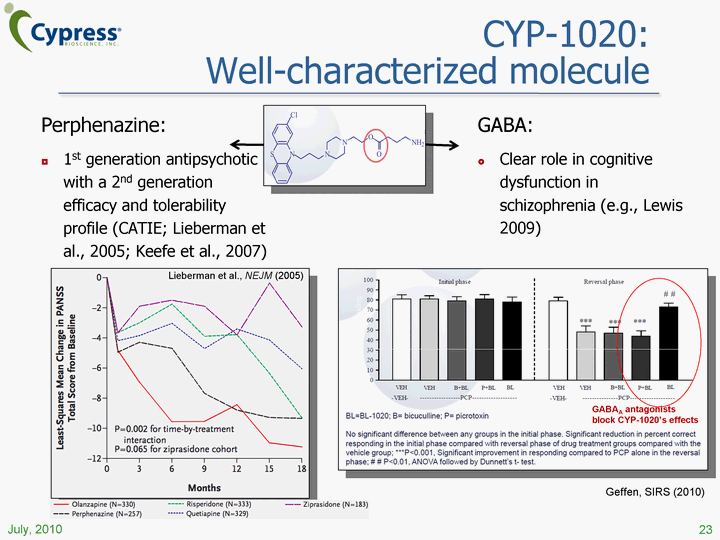
| CYP-1020: Well-characterized molecule Perphenazine: 1st generation antipsychotic with a 2nd generation efficacy and tolerability profile (CATIE; Lieberman et al., 2005; Keefe et al., 2007) 23 July, 2010 GABA: Clear role in cognitive dysfunction in schizophrenia (e.g., Lewis 2009) GABAA antagonists block CYP-1020's effects Lieberman et al., NEJM (2005) Geffen, SIRS (2010) |
| In-licensing of CYP-1020 Unmet need - Cognitive deficits main predictor of long-term outcome, at least ~80% of patients with schizophrenia are affected Well-characterized - Based on known molecule (perphenazine) with robust P2b data Innovative - Potential to be 1st antipsychotic with pro-cognitive effect 2-years to milestone - Forthcoming trial, if successful, will significantly de-risk P3 program 24 July, 2010 |
| CYP-1020: Innovative, first-in-class potential To date, no pharmacological therapy has succeeded in producing clinically relevant improvements in cognition in a regulatory context, either as a primary or adjunctive agent Multiple drugs in development, most focused on ?7 nicotinic agonism (e.g., TC-5619, MEM3454), H3 antagonism (e.g., GSK-239512), 5-HT6 antagonism (e.g., SAM-531), glutamate modulation (e.g., AZD-8529), or D2/5-HT2A antagonist (e.g., lurisidone) In contrast, the P2b data on CYP-1020 suggests a robust, clinically meaningful effect 25 July, 2010 |
| EAGLE P2b trial overview Double blind, multi-center, 6-week study Designed to determine the efficacy, safety and tolerability of BL-1020 in hospitalized patients with acute exacerbation of schizophrenia Full GCP trial conducted under U.S. FDA IND at 40 sites in the US, Europe and India 363 patients randomized as follows: BL-1020, 10mg/d BL-1020, 20-30mg/d Risperidone, 2-8mg/d Placebo 26 July, 2010 |

| PANSS total scores: Change from baseline 27 (CHART) July, 2010 |

| Brief Assessment of Cognition in Schizophrenia (BACS)* Includes 6 tests that measure 6 cognitive domains Approximately 30 min to administer Composite score has high test-retest reliability in patients with schizophrenia 28 Cognitive Domain Test Motor speed Token Motor Task Attention and processing speed Symbol Coding Working memory Digit Sequencing Task Verbal memory List Learning Verbal fluency Tests of Category Instances and Controlled Oral Word Association Test Reasoning and problem solving Tower of London July, 2010 *Keefe et al., Schiz Res (2004) |
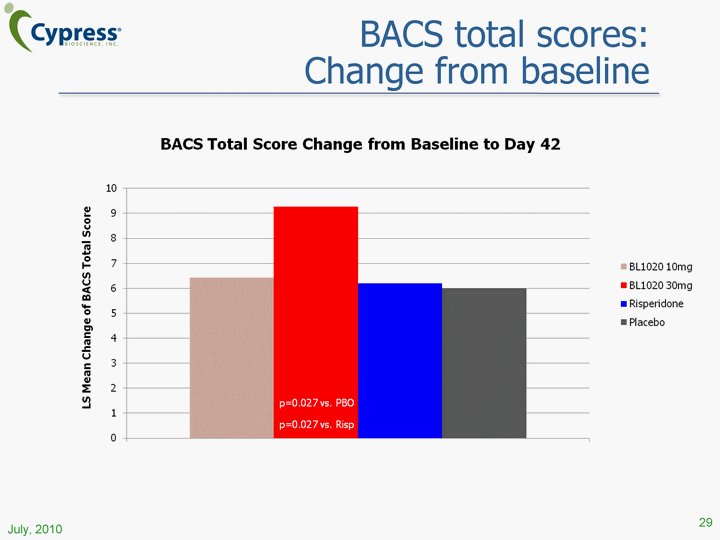
| BACS total scores: Change from baseline 29 (CHART) July, 2010 |
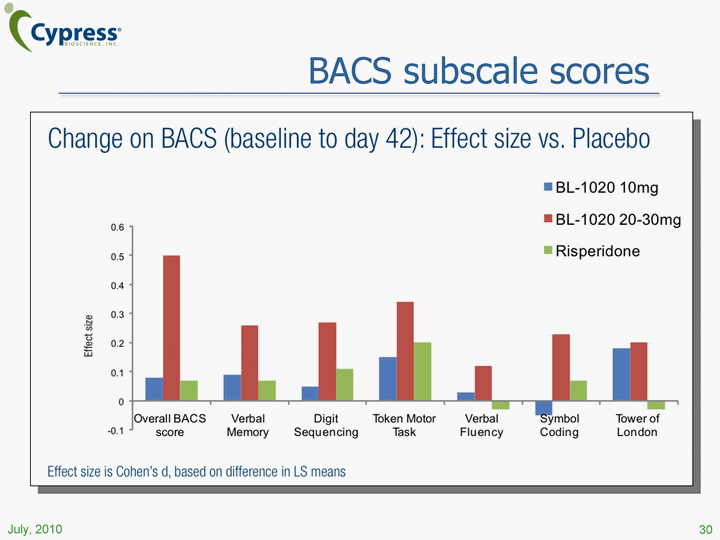
| BACS subscale scores 30 July, 2010 |

| In-licensing of CYP-1020 Unmet need - Cognitive deficits main predictor of long-term outcome, at least ~80% of patients with schizophrenia are affected Well-characterized - Based on known molecule (perphenazine) with robust P2b data Innovative - Potential to be 1st antipsychotic with pro-cognitive effect 2-years to milestone - Forthcoming trial, if successful, will significantly de-risk P3 program 31 July, 2010 |

| CYP-1020: Milestones 32 July, 2010 2010 2010 2010 2011 2011 2011 2011 2012 2012 2012 2012 2013 2013 2013 2013 2014 2014 2014 2014 2015 2015 2015 2015 q2 q3 q4 q1 q2 q3 q4 q1 q2 q3 q4 q1 q2 q3 q4 q1 q2 q3 q4 q1 q2 q3 q4 P2b ~$15M ~$15M ~$15M ~$15M ~$15M ~$15M ~$15M P3-1 P3-2 P3-OL NDA NDA submission NDA submission NDA submission NDA submission Topline |
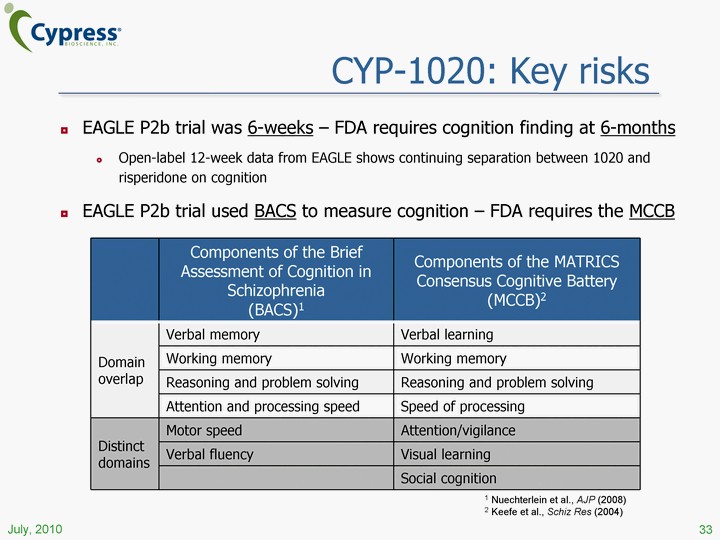
| CYP-1020: Key risks EAGLE P2b trial was 6-weeks - FDA requires cognition finding at 6-months Open-label 12-week data from EAGLE shows continuing separation between 1020 and risperidone on cognition EAGLE P2b trial used BACS to measure cognition - FDA requires the MCCB 33 July, 2010 Components of the Brief Assessment of Cognition in Schizophrenia (BACS)1 Components of the MATRICS Consensus Cognitive Battery (MCCB)2 Domain overlap Verbal memory Verbal learning Domain overlap Working memory Working memory Domain overlap Reasoning and problem solving Reasoning and problem solving Domain overlap Attention and processing speed Speed of processing Distinct domains Motor speed Attention/vigilance Distinct domains Verbal fluency Visual learning Distinct domains Social cognition 1 Nuechterlein et al., AJP (2008) 2 Keefe et al., Schiz Res (2004) |

| CYP-1020 deal terms* $30M upfront Anticipated R&D commitment for next trial of $15M over ~2 years Total clinical and regulatory milestones of up to $160M through to approval The majority of which are dependent on success in improving cognition Commercial milestones of up to $85M Additional $90M for approval in additional indications or other countries Cypress will fund all development activities and pay BioLineRx royalties on net sales 34 July, 2010 * Subject to approval from Office of Chief Scientist of the Ministry of Industry, Trade and Labor of the State of Israel |

| 35 We have financial wherewithal to execute on our strategy We expect to add more programs this year to achieve our stated goal of building the best CNS pipeline in industry We expect that all programs will meet the objectives outlined above (i.e., unmet need, well-characterized, innovative, milestone within 2 years) We have the financial wherewithal to execute our strategy Cash and short-term investment balance as of 31 March 2010: ~$137M Ongoing cash flow from Savella(r) Royalty on total net sales of the product - Forest Fiscal 2011* guidance for total net sales = $100M Savella milestones - $165M related to development and commercialization: $71 million paid to date (including $25 million upfront) and $45M related to additional indications July, 2010 * Forest fiscal year April 1, 2010 - March 31, 2011 |

| 36 We have financial wherewithal to execute on our strategy Expect to end 2010 with ~$95 million Shares outstanding: 38M basic, 46M fully diluted No debt July, 2010 |
