Attached files
| file | filename |
|---|---|
| 8-K - BIOMIMETIC THERAPEUTICS, INC. | v190026_8k.htm |
| EX-99.2 - BIOMIMETIC THERAPEUTICS, INC. | v190026_ex99-2.htm |
| EX-99.3 - BIOMIMETIC THERAPEUTICS, INC. | v190026_ex99-3.htm |

North American Pivotal Trial
Review
Outcomes of a Randomized, Controlled,
Trial to Evaluate Augment Bone Graft
(rhPDGF-BB/ß-TCP) as a Replacement
for Autograft in Hindfoot and Ankle
Fusion
2010 AOFAS Annual Summer Meeting
Washington, DC
Presented By: Tim Daniels, MD – Toronto, ON

Disclosure
Disclosures available on AOFAS Meeting Book
In the United States, Augment Bone Graft is an
investigational product and is not available for sale
In Canada, Augment Bone Graft is an approved Class IV
medical device and is indicated as a replacement for
autograft in ankle, hindfoot and midfoot fusion surgery

Augment™ Bone Graft ( rhPDGF-BB/ß-TCP)
Developed as a fully synthetic
replacement for autograft
Recombinant PDGF (rhPDGF-BB)
FDA approved for tissue regeneration
periodontal bone defects (GEM 21S®)
diabetic foot ulcers (Regranex®)
Prior Studies in Orthopedics
pilot studies: foot/ankle, wrist

Pivotal Trial Objectives
HYPOTHESIS: Augment Bone Graft (rhPDGF-BB/ß-TCP) is
non-inferior to autograft when used as a healing adjunct during
hindfoot and ankle fusion surgery
PRIMARY ENDPOINT: % patients fused by CT scan at 6
months, defined as ≥50% osseous bridging across the joint
surface (per Coughlin
et al.)
2° ENDPOINTS:
Safety: Adverse Events, Complications
Efficacy: Clinical Healing, additional
radiographic endpoints (XR,CT), delayed/non
union rate
QOL: Pain, function indices

Study Protocol
2:1 randomization, Augment™ (rhPDGF-BB/ß-TCP): Autograft
Autograft from iliac crest, Gerdy’s, distal tibia, calcaneus
Std joint preparation, ORIF using ≤ 3 screws/joint
Post-op immobilization for 12 wks, NWB first 6 wk
CT evaluation: 9, 16, 24, 36 wks
XR evaluation: 1-3, 6, 9, 12, 16, 24, 36 and 52 wks
Clinical assessment: pain, QOL, functional outcomes
Serum screening for antibody formation (pre/post-op)
CT/XR analysis by independent radiologist
Non-inferiority statistical analysis
Statistically powered sample size
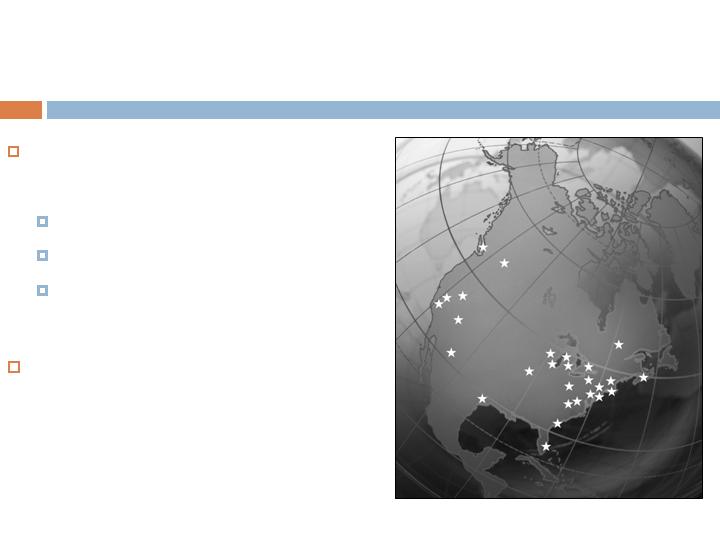
Largest clinical study ever
performed in foot and ankle
434 patients
37 clinical centers (USA/CAN)
72 investigators
First of its kind with a
recombinant protein
Study Scope
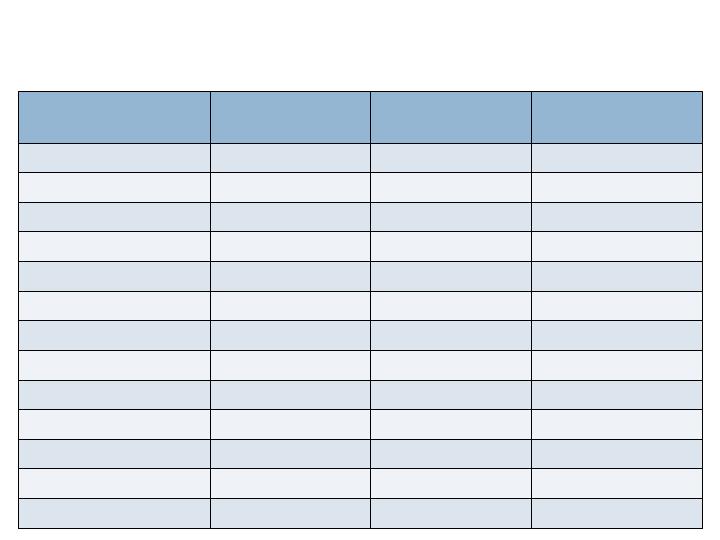
Study Demographics
All Patients
n= 434
Augment™
n= 285
Autograft
n=149
Sex (M/F)
49.8% / 49.1%
46.3% / 52.3%
56.4% / 43.0%
Age (Mean)
56.6 years
56.2 years
57.5 years
BMI (Mean)
30.8
30.7
31.1
Diagnosis
Post Traumatic OA
48.2%
48.8%
47.0%
Primary Arthritis
34.3%
32.6%
37.6%
Rheumatoid
6.7%
8.4%
3.4%
Other
9.7%
8.8%
11.4%
Risk Factors
Obesity (BMI>30)
48.4%
46.3%
52.3%
Smoking History
24.2%
24.9%
22.8%
Prior Surgery
23.3%
22.8%
24.2%
Diabetes
12.0%
11.2%
13.4%
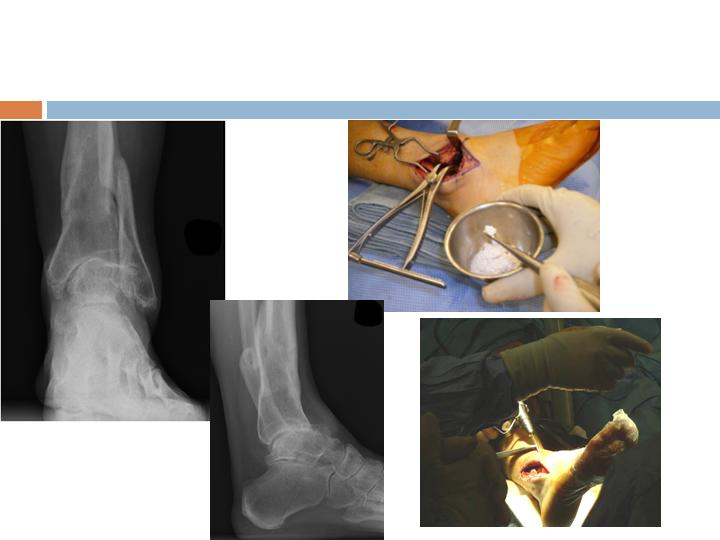
Clinical Example, Ankle
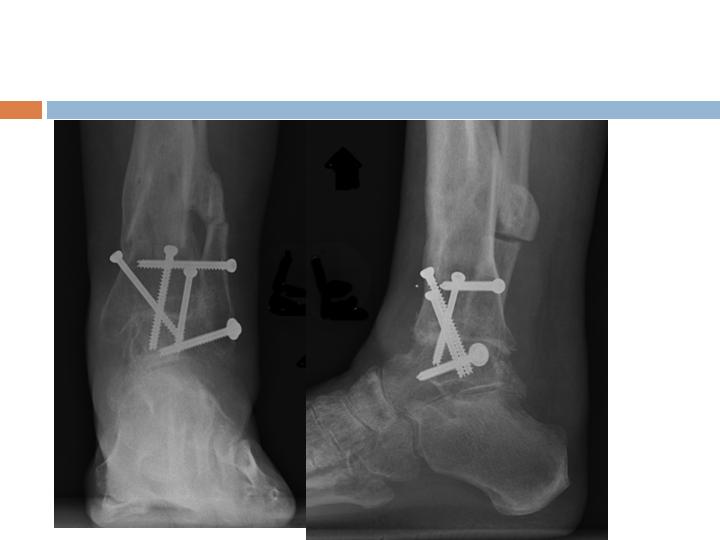
Radiographic Assessment
3 months
Post-op
Radiographs and clinical
evaluation useful but not
always reliable
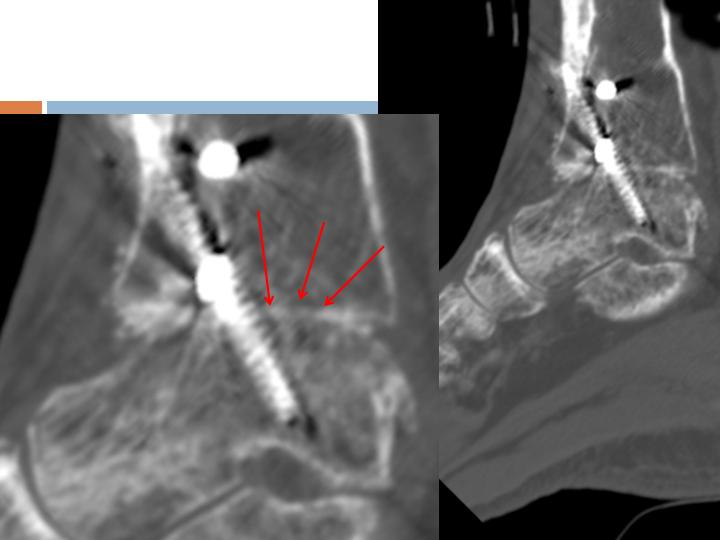
CT Assessment
6 weeks
Post-op
Multiple areas of
Osseous-bridging
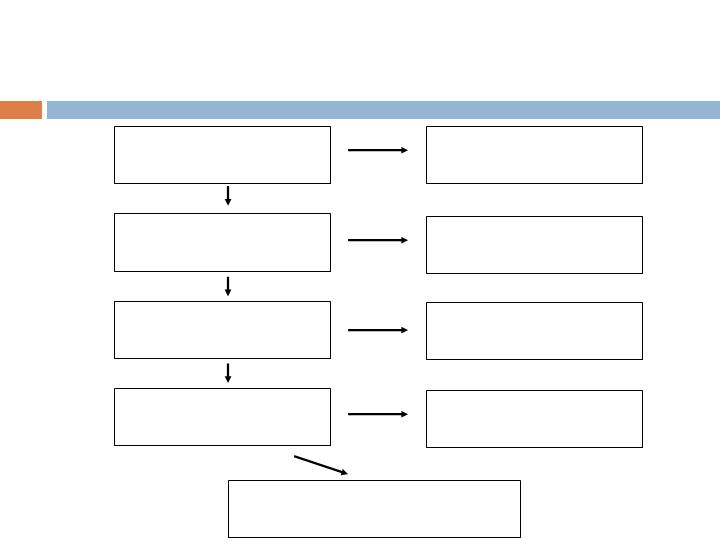
Patient Accountability
Screened Population
n=457 (456*)
*Less one subject screened twice
Randomized Population
n=435
ITT Population
n=434
Augment (285)/Autograft (149)
mITT Population
n=397
Augment (260)/Autograft (137)
Not randomized
n=21
Not randomized
prior to treatment
n=1
Excluded from mITT
n=17
Augment (12)/Autograft (5)
Safety Population
(Treated), n=414
Augment (272)/Autograft (142)
Not treated
n=20
Augment (13)/Autograft (7)
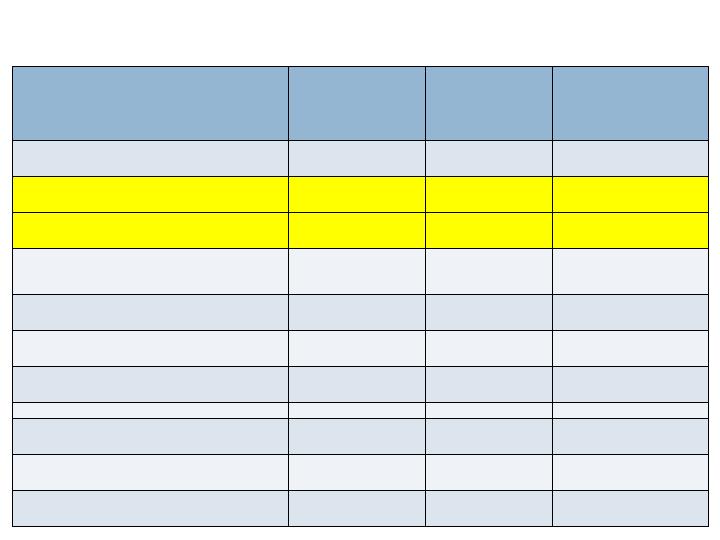
Radiographic Endpoints -36/52 wks
Augment
N=260
Joints=394
Autograft
N=137
Joints=203
Non-
Inferiority
(p-value)
CT Fusion - 36 wks
Full Complement of Joints
63.5%
69.3%
No (0.202)
All Joints
68.8%
73.9%
No (0.103)
XR Fusion (3 views) - 52 wks
Full Complement of Joints
36.9%
36.5%
Yes (0.020)
All Joints
48.5%
44.3%
Yes (<0.001)
XR Fusion (2 views) - 52 wks
Full Complement of Joints
70.8%
75.2%
No (0.115)
All joints
77.2%
77.8%
Yes (0.050)
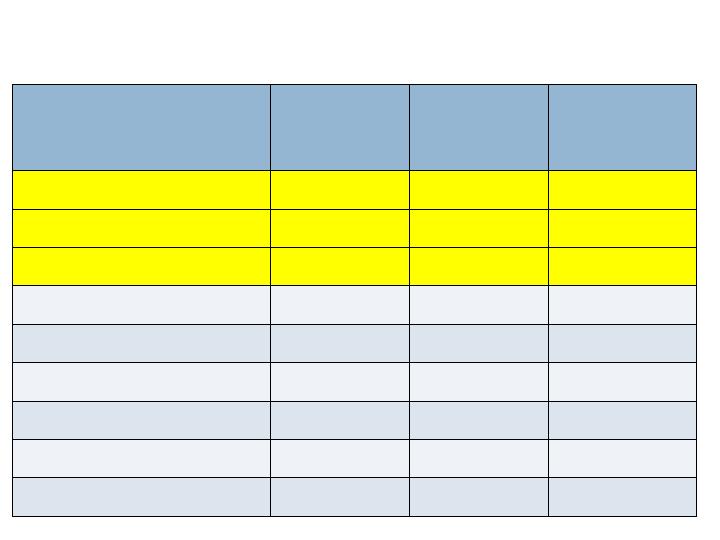
Radiographic Endpoints – 24 wks
Augment
N=260
Joints=394
Autograft
N=137
Joints=203
Non-
Inferiority
(p-value)
CT Fusion Rate
Full complement of joints
61.2%
62.0%
Yes (0.038)
All joints
66.5%
62.6%
Yes (<0.001)
XR Fusion (3 views)
Full complement of joints
30.8%
32.8%
No (0.054)
All joints
38.3%
37.9%
Yes (0.007)
XR Fusion (2 views)
Full complement of joints
60.8%
66.4%
No (0.194)
All joints
67.5%
70.9%
Yes (0.049)
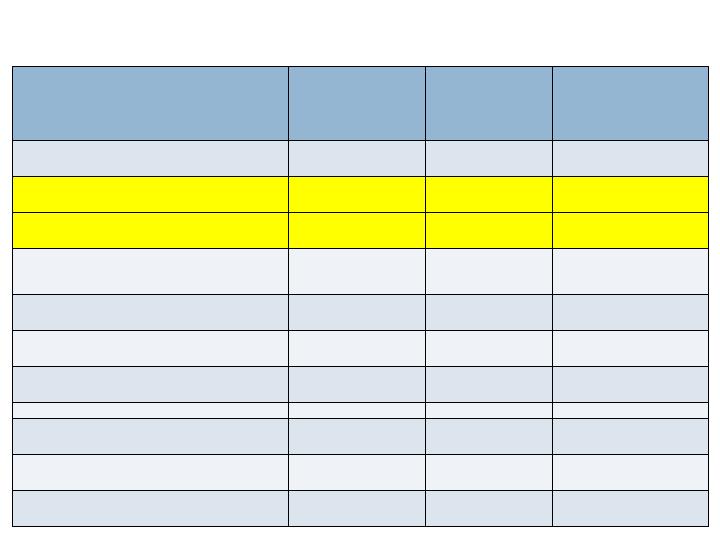
Radiographic Endpoints - 36/52 wks
Augment
N=260
Joints=394
Autograft
N=137
Joints=203
Non-
Inferiority
(p-value)
CT Fusion - 36 wks
Full Complement of Joints
63.5%
69.3%
No (0.202)
All Joints
68.8%
73.9%
No (0.103)
XR Fusion (3 views) - 52 wks
Full Complement of Joints
36.9%
36.5%
Yes (0.020)
All Joints
48.5%
44.3%
Yes (<0.001)
XR Fusion (2 views) - 52 wks
Full Complement of Joints
70.8%
75.2%
No (0.115)
All joints
77.2%
77.8%
Yes (0.050)
Successful fusion but
Missed 36 wk CT-scan
N = 8
N = 1
Clinical Endpoints – 52 wks
Augment
N=260
Joints=394
Autograft
N=137
Joints=203
Non-Inferiority
(p-value)
Clinical Healing
(Investigator Assessed)
Patient Level
87.7%
88.3%
Yes (0.003)
Full complement of joints
86.2%
87.6%
Yes (0.008)
All joints
88.3%
87.2%
Yes (<0.001)
Nonunion
4.2%
5.8%
Yes (<0.001)
Clinical Success
76.9%
78.1%
Yes (0.022)
Therapeutic Failure
7.3%
8.0%
Yes (<0.001)
Functional Endpoints – 52 wks
Augment
N=260
Autograft
N=137
Non-
Inferiority
(p-value)
SF-12 (Mean PCS)
42.4
45.0
Yes (<0.001)
Foot Fusion Index
(Mean Total)
20.1
17.5
Yes (0.012)
AOFAS Ankle-Hindfoot
Scale
77.8
78.2
Yes (<0.001)
VAS Pain Scores
Fusion Site
13.2
12.9
Yes (<0.001)
Weight Bearing
15.6
15.8
Yes (<0.001)
Graft Site Pain (any)
(% of pts)
0
44%
Yes (<0.001)

Safety Endpoints – 52 wks
Augment
N=272
Autograft
N=142
P-value
(Fisher Exact Test)
Serious
Treatment
Emergent AE’s
10.3%
14.8%
No difference
(0.201)
Complications
(Surgically
Related)
23.9%
30.3%
No difference
(0.195)
Complications
(Serious)
5.1%
6.3%
No difference
(0.654)
Infections
8.5%
11.3%
No difference
(0.378)

Pivotal Trial Data Summary
Augment Bone Graft met the primary endpoint of the study –
statistically significant non-inferiority to autograft per CT fusion
rate at 24 wks
52 wk non-union rate (4.2% augment
vs 5.8% autograft) is
consistent with the published literature, despite high
percentage of patients (75%) with risk factors for
poor healing
Radiographic, clinical and functional assessments at 24 and 52
weeks demonstrate comparability to autograft, the standard
adjunct for bone healing in reconstructive orthopedic surgery
52 week data support reliability of fusion for both Augment and
autograft groups (no patients regressed between 24&52 weeks)

Discussion/Conclusions
Harvesting autograft is a time-consuming procedure; which is
associated with potential complications and prolonged post-
operative patient pain
Augment Bone Graft treated patients experienced fewer serious
adverse events and were spared the pain and morbidity of bone
graft harvest
There are few materials that have been shown to be equivalent
to autograft in large RCT studies – none indicated for use in
foot and ankle surgery
The results from this study establish Augment Bone Graft as a
safe an effective alternative to autograft in hindfoot and ankle
surgery
