Attached files
| file | filename |
|---|---|
| EX-5.1 - AtheroNova Inc. | ex5-1.htm |
| EX-23.1 - AtheroNova Inc. | ex23-1.htm |
| EX-21.1 - AtheroNova Inc. | ex21-1.htm |
| As filed with the Securities and Exchange Commission on June 29, 2010 | Registration No. ___-____ |
UNITED
STATES
SECURITIES
AND EXCHANGE COMMISSION
Washington,
D.C. 20549
_________________
FORM
S-1
REGISTRATION
STATEMENT
UNDER
THE
SECURITIES
ACT OF 1933
(Amendment
No. __)
_________________
ATHERONOVA
INC.
(Exact
name of registrant as specified in its charter)
|
Delaware
|
2834
|
20-1915083
|
|
(State
or other jurisdiction of
|
(Primary
Standard Industrial
|
(I.R.S.
Employer
|
|
incorporation
or organization)
|
Classification
Code Number)
|
Identification
No.)
|
2301
Dupont Drive, Suite 525
Irvine,
CA 92612
(949)
476-1100
(Address,
including zip code, and telephone number, including area code, of registrant’s
principal executive offices)
Mark
Selawski, Chief Financial Officer
AtheroNova
Inc.
2301
Dupont Drive, Suite 525
Irvine,
CA 92612
(949)
476-1100
(Name,
address, including zip code, and telephone number, including area code, of agent
for service)
Copy
to:
Gregory
Akselrud, Esq.
Stubbs
Alderton & Markiles, LLP
15260
Ventura Boulevard, 20th
Floor
Sherman
Oaks, California 91403
(818)
444-4500
Approximate
date of commencement of proposed sale to the public: From time to
time after the effective date of this Registration Statement.
If any of
the securities being registered on this Form are to be offered on a delayed or
continuous basis pursuant to Rule 415 under the Securities Act of 1933 check the
following box: x
If this
Form is filed to register additional securities for an offering pursuant to Rule
462(b) under the Securities Act, please check the following box and list the
Securities Act registration statement number of the earlier effective
registration statement for the same offering. o
If this
Form is a post-effective amendment filed pursuant to Rule 462(c) under the
Securities Act, check the following box and list the Securities Act registration
statement number of the earlier effective registration statement for the same
offering. o
If this
Form is a post-effective amendment filed pursuant to Rule 462(d) under the
Securities Act, check the following box and list the Securities Act registration
statement number of the earlier effective registration statement for the same
offering. o
Indicate
by check mark whether the registrant is a large accelerated filer, an
accelerated filer, a non-accelerated filer, or a smaller reporting
company. See definitions of “large accelerated filer,” “accelerated
filer” and “smaller reporting company” in Rule 12b-2 of the Exchange
Act.
|
Large
accelerated filer
|
o | Accelerated filer | o |
|
Non-accelerated
filer
|
o (Do not check if
smaller reporting company)
|
Smaller reporting company |
x
|
CALCULATION
OF REGISTRATION FEE
|
Title
of Each Class
of
Securities
to
be Registered
|
Amount
to be
Registered
(1)
|
Proposed
Maximum
Offering
Price
Per
Unit (2)
|
Proposed
Maximum
Aggregate
Offering
Price (2)
|
Amount
of
Registration
Fee
|
|
Common
Stock, par value $0.0001 per share
|
486,722
|
$30.00
|
$14,601,660.00
|
$1,041.10
|
|
Common
Stock, par value $0.0001 per share, issuable upon conversion of
convertible promissory notes
|
3,817,596
|
$30.00
|
$114,527,880.00
|
$8,165.84
|
|
TOTAL
|
4,304,318
|
$30.00
|
$129,129,540.00
|
$9,206.94
|
|
(1)
|
In
the event of a stock split, stock dividend, or other similar transaction
involving the Registrant’s common stock, in order to prevent dilution, the
number of shares registered shall automatically be increased to cover the
additional shares in accordance with Rule 416(a) under the Securities
Act.
|
|
(2)
|
Estimated
solely for the purpose of calculating the registration fee pursuant to
Rule 457(c) under the Securities Act of 1933, using the average of the
high and low prices as reported on the OTC Bulletin Board on June 28, 2010
(accounting for a 1-for-200 reverse stock
split).
|
The
Registrant hereby amends this Registration Statement on such date or dates as
may be necessary to delay its effective date until the Registrant shall file a
further amendment which specifically states that this Registration Statement
shall thereafter become effective in accordance with Section 8(a) of the
Securities Act of 1933 or until the Registration Statement shall become
effective on such date as the Commission, acting pursuant to said Section 8(a),
may determine.
Subject
to Completion, Dated June 29, 2010
ATHERONOVA
INC.
4,304,318 Shares
Common
Stock
_________________
This
prospectus relates to the offer and sale from time to time of up to
4,304,318 shares of
our common stock that are held by the stockholders named in the “Principal and
Selling Stockholders” section of this prospectus. The prices at which
the selling stockholders may sell the shares in this offering will be determined
by the prevailing market price for the shares or in negotiated
transactions. We will not receive any of the proceeds from the sale
of the shares. We will bear all expenses of registration incurred in
connection with this offering. The selling stockholders whose shares
are being registered will bear all selling and other expenses.
Our
common stock is quoted on the OTC Bulletin Board under the symbol
“AHRO.” On June 23, 2010, the last reported sales price of our common
stock on the OTC Bulletin Board was $41.00 per share (accounting for a 1-for-200
reverse stock split).
Investing
in our common stock involves risks. See “Risk Factors” beginning on
page 4.
_________________
Neither
the Securities and Exchange Commission nor any state securities commission has
approved or disapproved of these securities or passed upon the adequacy or
accuracy of this prospectus. Any representation to the contrary is a
criminal offense.
_________________
The date
of this prospectus is ______________
i
TABLE
OF CONTENTS
|
Page
|
Page
|
|||||
|
Prospectus
Summary
|
1
|
Directors,
Executive Officers,
|
||||
|
Risk
Factors
|
4
|
Promoters
and Control Persons
|
35
|
|||
|
Forward-Looking
Statements
|
12
|
Executive
Compensation
|
38
|
|||
|
Use
of Proceeds
|
13
|
Principal
and Selling Stockholders
|
39
|
|||
|
Plan
of Distribution
|
13
|
Certain
Relationships and
|
||||
|
Description
of Registrant’s Securities
|
16
|
Related
Transactions
|
42
|
|||
|
Description
of Business
|
19
|
Legal
Matters
|
44
|
|||
|
Description
of Property
|
26
|
Experts
|
44
|
|||
|
Legal
Proceedings
|
26
|
Where
You Can Find More
|
||||
|
Market
Price of and Dividends on
|
Information
|
44
|
||||
|
the
Registrant’s Common Equity
|
Index
to Annual Financial
|
|||||
|
and
Related Stockholder Matters
|
27
|
Statements
|
45
|
|||
|
Management’s
Discussion and
|
Index
to Quarterly Financial
|
|||||
|
Analysis
of Financial Condition
|
Statements
|
59
|
||||
|
and
Results of
Operations
|
30
|
|||||
You
should rely only on the information contained in this prospectus or any
supplement. We have not authorized anyone to provide information that
is different from that contained in this prospectus. The information
contained in this prospectus is accurate only as of the date of this prospectus,
regardless of the time of delivery of this prospectus or of any sale of our
common stock.
Except as
otherwise indicated, information in this prospectus reflects the reverse merger
(recapitalization) that occurred on May 13, 2010 with AtheroNova Operations,
Inc. (formerly Z&Z Medical Holdings, Inc.), and a 1-for-200 reverse stock
split of our common stock which took effect on and as of June 23,
2010.
ii
PROSPECTUS
SUMMARY
This
summary highlights selected information contained in greater detail elsewhere in
this prospectus. You should read the entire prospectus carefully
before making an investment decision, including “Risk Factors” and the
consolidated financial statements and the related notes. References in this
prospectus to “AtheroNova” and “the Company” refer to AtheroNova Inc. and our
consolidated subsidiary AtheroNova Operations, Inc.
Our
Business
We have
developed intellectual property (“IP”), covered by our pending patent
applications, which uses certain pharmacological compounds uniquely for the
treatment of atherosclerosis, which is the primary cause of various
cardiovascular diseases. Atherosclerosis occurs when cholesterol or
fats are deposited and harden as plaques in the walls of
arteries. This hardening reduces the space within the arteries
through which blood can flow. The plaque can also rupture and greatly
restrict or block altogether blood flow. Through a process called
delipidization, such compounds dissolve the plaques so they can be eliminated
through normal body processes and avoid such rupturing. Such
compounds may be used both to treat and prevent atherosclerosis.
In the
near future, we plan to continue studies and trials to demonstrate the efficacy
our IP. Ultimately, we plan to license our technology to various
licensees throughout the world who may use it in treating or preventing
atherosclerosis and other medical conditions or sublicense the IP to other such
users. Our licensees may also produce, market or distribute products
which utilize or add our compounds and technology in such treatment or
prevention.
Our
Industry
We
compete against well-capitalized pharmacological companies as well as smaller
companies. The market for our products is highly
competitive. The pharmacological sector is evolving and growing
rapidly, and companies are continually introducing new products and
services.
Our
History and Contact Information
We were
incorporated in the State of Delaware on May 13, 1997 under the name Camryn
Information Services, Inc. We operated for a brief period of time
before we ceased operations on February 25, 1999 when we forfeited our charter
for failure to designate a registered agent. We remained dormant
until 2004 when we renewed our operations with the filing of a Certificate of
Renewal and Revival of Charter with the State of Delaware on October 29,
2004. On November 3, 2004, we filed a Certificate of Amendment and
our name was formally changed from Camryn Information Services, Inc. to iStorage
Networks, Inc. Such change became effective on November 8,
2004. On January 26, 2006, we issued 41,000 shares of our common
stock in exchange for all of the membership interests of Landbank, LLC
(“LLC”). We changed our name to Landbank Group, Inc. on January 27,
2006. LLC made bulk acquisitions of parcels of land, primarily
through the real property tax lien foreclosure process. The bulk acquisitions
were then divided into smaller parcels for resale. On December 31,
2007, we transferred all of LLC’s membership interests to Landbank Acquisition,
LLC, ceased business operations, and changed our name to Trist Holdings,
Inc. On May 13, 2010 we changed our name to AtheroNova
Inc.
From
December 31, 2007 through May 13, 2010, we were a public “shell” company with
nominal assets.
1
On March
26, 2010, we entered into an Agreement and Plan of Merger (“Merger Agreement”)
with Z&Z Merger Corporation, a Delaware corporation and our wholly-owned
subsidiary (“MergerCo”), and Z&Z Medical Holdings, Inc., a Delaware
corporation (“Z&Z”). The closing (the “Closing”) of the
transactions contemplated by the Merger Agreement (the “Merger”) occurred on May
13, 2010. At the Closing, (i) MergerCo was merged with and into
Z&Z, whose name was concurrently changed to AtheroNova Operations, Inc.
(“AtheroNova Operations”); (ii) Z&Z, as AtheroNova Operations, became our
wholly-owned subsidiary; (iii) all of AtheroNova Operations’ shares, warrants
and options outstanding prior to the Merger were exchanged (or assumed, in the
case of warrants and options) for comparable securities of our company; and (iv)
approximately 98% of our fully-diluted shares (excluding the shares issuable in
the Capital Raise Transaction (as defined below)) were owned by AtheroNova
Operations’ former stockholders, warrant holders and option
holders. At the Closing, we issued to AtheroNova Operations’ former
stockholders, in exchange for the 9,837,050 shares of AtheroNova Operations’
common stock outstanding prior to the Merger, 88,575,048 shares of our
Super-Voting Common Stock, par value $0.0001 per share (the “Super-Voting Common
Stock”), which, as a result of the approval by the holders a substantial
majority of our outstanding stock entitled to vote and the approval by our board
of directors on May 21, 2010, of amendments to our certificate of incorporation,
as amended, that (i) decreased our authorized number of shares of our common
stock to 100,000,000, (ii) designated 10,000,000 shares of blank check preferred
stock and (iii) adopted a 1-for-200 reverse stock split, on June 23, 2010
converted into 22,143,771 shares of our common stock. As a result of
the Merger we are solely engaged in AtheroNova Operations’ business, AtheroNova
Operations’ officers became our officers and three of AtheroNova Operations’
directors became members of our seven-member board of directors (which currently
has two vacancies).
The
Merger was accounted for as a reverse merger (recapitalization) with AtheroNova
Operations deemed to be the accounting acquirer, and our company deemed to be
the legal acquirer. All financial information in this document is
that of our company and AtheroNova Operations.
On May
13, 2010, we also entered into a Securities Purchase Agreement (the “Securities
Purchase Agreement”) with W-Net Fund I, L.P. (“W-Net”), Europa International,
Inc. (“Europa”) and MKM Opportunity Master Fund, Ltd. (“MKM” and together with
W-Net and Europa, the “Purchasers”), pursuant to which the Purchasers, on May
13, 2010, purchased from us (i) 2.5% Senior Secured Convertible Notes (the
“Notes”) for a cash purchase price of $1,500,000, and (ii) Common Stock Purchase
Warrants pursuant to which the Purchasers may purchase up to 1,908,798 shares of
our common stock at an exercise price of approximately $0.39 per share (the
“Warrants”) (the “Capital Raise Transaction”). The Notes, including
accrued interest through their maturity, are convertible into 4,199,358 shares
of our common stock at a conversion price of approximately $0.39 per
share. We are registering the shares of our common stock issuable
upon the conversion of the Notes.
The
address of our principal executive office is 2301 Dupont Drive, Suite 525,
Irvine, California 92612, and our telephone number is (949)
476-1100.
2
The
Offering
|
Common
stock offered
|
4,304,318 shares
by the selling stockholders
|
|
|
Common
stock outstanding
before
this offering
|
22,680,927
shares
|
|
|
Common
stock to be outstanding after
this offering
|
22,680,927
shares
|
|
|
Use
of
proceeds
|
We
will not receive any of the proceeds from the sale of shares of our common
stock by the selling stockholders. See “Use of
Proceeds.”
|
|
|
OTC
Bulletin Board
symbol
|
“AHRO”
|
|
|
Risk
Factors
|
See
“Risk Factors” beginning on page 4 for a discussion of factors that you
should consider carefully before deciding to purchase our common
stock.
|
In the
table above, the number of shares to be outstanding after this offering is based
on 22,680,927 shares of our common stock outstanding as of June 23,
2010. The number of shares of our common stock to be outstanding
after this offering does not reflect the issuance of the following
shares:
|
·
|
5,497,356
shares of our common stock issuable upon the exercise of common stock
purchase warrants outstanding as of June 23, 2010, with a weighted average
exercise price of approximately $0.28 per
share;
|
|
·
|
549,498
shares of our common stock issuable upon the exercise of stock options
outstanding as of June 23, 2010, with an exercise price of approximately
$0.22 per share;
|
|
·
|
4,199,358
shares of our common stock (including 381,762 shares accounting for
accrued interest through maturity) issuable upon the conversion of
convertible promissory notes outstanding as of June 23, 2010, at a
conversion price of approximately $0.39
and
|
|
·
|
4,362,964
additional shares of common stock reserved for issuance under our 2010
Stock Incentive Plan, as of June 23,
2010.
|
Summary
Financial Data
As of
March 31, 2010, we had an accumulated deficit of $419,531. We
incurred operating losses of $32,633 and $3,163 for the fiscal quarters ended
March 31, 2010 and 2009, respectively. We have not yet achieved
profitability and anticipate that we will continue to incur net losses for at
least the next year. We anticipate that a substantial portion of our
capital resources and efforts will be focused on research and development and
other general corporate purposes. Research and development projects
include the completion of a second animal study at the Cedars-Sinai Division of
Cardiology in conjunction with the University of California Los Angeles to
validate our initial findings and prepare for human trials. We plan
to develop multiple applications for our compounds, to be used in pharmaceutical
grade and over-the-counter grade products, for the treatment of
atherosclerosis. As of March 31, 2010 we had approximately $123,734
in cash and cash equivalents and a working capital deficit of approximately
$90,855 compared to approximately $53,314 in cash and cash equivalents and a
working capital deficit of approximately $62,586 at March 31, 2009.
3
RISK
FACTORS
Investing
in our common stock involves a high degree of risk. You should
carefully consider the following risk factors and all other information
contained in this prospectus before purchasing shares of our common
stock. If any of the following risks occur, our business, financial
condition and/or results of operations could be materially and adversely
affected. In that case, the trading price of our common stock could
decline, and you may lose some or all of your investment.
Risks
Related to Our Business
We
will continue to need additional financing to carry out our business
plan.
The net
proceeds from the Capital Raise Transaction available to fund our business were
reduced by the required payments and reimbursements to stockholders to whom we
were indebted and other transaction costs incurred by AtheroNova
Operations. Although we estimate that the net funds from the Capital
Raise Transaction will be sufficient to fund our planned activities for up to a
year, we will need thereafter or sooner to obtain significant additional funding
successfully to continue our business. Such additional funds may not
be readily available or may not be available on terms acceptable to
us.
We
have a history of operating losses and there can be no assurance that we can
achieve or maintain profitability.
We have a
history of operating losses and may not achieve or sustain
profitability. We cannot guarantee that we will become
profitable. Even if we achieve profitability, given the competitive
and evolving nature of the industry in which we operate, we may not be able to
sustain or increase profitability and our failure to do so would adversely
affect our business, including our ability to raise additional
funds.
We
may not be able to effectively manage our growth.
Our
strategy envisions growing our business. We plan to expand our
technology, sales, administrative and marketing organizations. Any
growth in or expansion of our business is likely to continue to place a strain
on our management and administrative resources, infrastructure and
systems. As with other growing businesses, we expect that we will
need to further refine and expand our business development capabilities, our
systems and processes and our access to financing sources. We also
will need to hire, train, supervise and manage new employees. These
processes are time consuming and expensive, will increase management
responsibilities and will divert management attention. We cannot
assure you that we will be able to:
|
·
|
expand
our systems effectively or efficiently or in a timely
manner;
|
|
·
|
allocate
our human resources optimally;
|
|
·
|
meet
our capital needs;
|
|
·
|
identify
and hire qualified employees or retain valued employees;
or
|
|
·
|
incorporate
effectively the components of any business or product line that we may
acquire in our effort to achieve
growth.
|
4
Our
inability or failure to manage our growth and expansion effectively could harm
our business and materially and adversely affect our operating results and
financial condition.
Technology
changes may make the products we are planning to bring to market
obsolete.
We
believe that the methods for treating and preventing atherosclerosis of the
pharmacological compounds we intend to bring to market enjoy certain competitive
advantages, including superior performance and
cost-effectiveness. Although we are not aware of any other treatments
or methods currently being developed that would compete with the methods we
intend to employ, there can be no assurance that future developments in
technology or pharmacological compounds will not make our technology
non-competitive or obsolete, or significantly reduce our operating margins or
the demand for our offerings, or otherwise negatively impact our
profitability.
We
may not be able to protect our intellectual property.
We and
our licensees may be unable to obtain IP rights to effectively protect our
technology. Patents and other proprietary rights are an important
part of our business plans. The ability to compete effectively may be
affected by the nature and breadth of our IP rights. We intend to
rely on a combination of patents, trade secrets and licensing arrangements to
protect our technology. While we intend to defend against any threats
to our IP rights, there can be no assurance that any of our patents, patent
applications, trade secrets, licenses or other arrangements will adequately
protect our interests.
Although
we have pending patent applications in the United States and under the
international Patent Cooperation Treaty covering uses of our technology, we have
not received, and may never receive, any patent protection for our
technology. We cannot guarantee any particular result or decision by
the U.S. Patent and Trademark Office or a U.S. court of law, or by any patent
office or court of any country in which we have sought patent
protection. If we are unable to secure patent protection for our
technology, our revenue and earnings, financial condition, or results of
operations would be adversely affected. There can also be no
assurance that any patent issued to or licensed by us in the future will not be
challenged or circumvented by competitors, or that any patent issued to or
licensed by us will be found to be valid or be sufficiently broad to protect us
and our technology. A third party could also obtain a patent that may
require us to negotiate a license to conduct our business, and there can be no
assurance that the required license would be available on reasonable terms or at
all.
We do not
warrant any opinion as to patentability or validity of any pending patent
application. We do not warrant any opinion as to non-infringement of
any patent, trademark, or copyright by us or any of our affiliates, providers,
or distributors. Nor do we warrant any opinion as to invalidity of
any third-party patent or unpatentability of any third-party pending patent
application.
We may
also rely on nondisclosure and non-competition agreements to protect portions of
our technology. There can be no assurance that these agreements will
not be breached, that we will have adequate remedies for any breach, that third
parties will not otherwise gain access to our trade secrets or proprietary
knowledge, or that third parties will not independently develop the
technology.
IP
litigation would be costly and could adversely impact our business
operations.
We may
have to take legal action in the future to protect our technology or to assert
our IP rights against others. Any legal action could be costly and
time consuming to us, and no assurances can be made that any action will be
successful. The invalidation of any patent or IP rights that we may
own, or an unsuccessful outcome in lawsuits to protect our technology, could
have a material adverse effect on our business, financial position, or results
of operations.
5
We
operate and compete in an industry that is characterized by extensive IP
litigation. In recent years, it has been common for companies in the
medical product and pharmaceutical businesses to aggressively file
patent-infringement and other intellectual-property litigation in order to
prevent the marketing of new or improved medical products, treatments, or
pharmaceuticals. IP litigation can be expensive, complex, and
protracted. Because of such complexity, and the vagaries of the jury
system, IP litigation may result in significant damage awards and/or injunctions
that could prevent the manufacture, use, distribution, importation, exportation,
and sale of products or require us and/or any of our licensing partners to pay
significant royalties in order to continue to manufacture, use, distribute,
import, export, or sell products. Furthermore, in the event that our
right to license or to market our technology is successfully challenged, and if
we and/or our licensing partners fail to obtain a required license or are unable
to design around a patent held by a third party, our business, financial
condition, or results of operations could be materially adversely
affected. We believe that the patents we have applied for, if
granted, would provide valuable protection for our intellectual property, but
there nevertheless could be no assurances that they would be respected or not
subject to infringement by others.
We
are operating in a highly competitive industry.
We are
involved in a highly competitive industry where we may compete with numerous
other companies who offer alternative methods or approaches, who may have far
greater resources, more experience, and personnel perhaps more qualified than we
do. There can be no assurance that we will be able to successfully
compete against these other entities.
We
and our licensees will be subject to federal and state regulation.
We and
our potential licensing partners are subject to many laws and regulations, and
any adverse regulatory action may affect our ability to exploit our
IP. Developing, manufacturing, and marketing regulated medical
products and pharmaceuticals are subject to extensive and rigorous regulation by
numerous government and regulatory agencies, including the FDA and comparable
foreign agencies. Under the Federal Food, Drug, and Cosmetic Act (the
“FDA Act”), regulated medical devices must receive FDA clearance and approval
before they can be commercially marketed in the U.S. Markets outside the U.S.
require similar clearance and approval before a medical product or
pharmaceutical can be commercially marketed. We cannot guarantee that
we will be able to obtain, directly or through our licensees, marketing
clearance from the FDA and other governing agencies for any new products, or
modifications or enhancements to existing products, which we depend on for
royalty revenues. Furthermore, if FDA clearance is obtained, such
clearance could (a) take a significant amount of time; (b) require the
expenditure of substantial resources; (c) involve rigorous pre-clinical and
clinical testing; (d) require modifications to, or replacements of products;
and/or (e) result in limitations on the proposed uses of products.
Even
after regulated medical products or pharmaceuticals have received marketing
clearance, approvals by the FDA can be withdrawn due to failure to comply with
regulatory standards or the occurrence of unforeseen issues following initial
approval. Failure to comply with regulatory standards or subsequent
discovery of unknown problems with a regulated medical product could result in
fines, suspensions of regulatory approvals, seizures or recalls of devices,
operating restrictions, and/or criminal prosecution. There can be no
assurance that any FDA approval will not be subsequently
withdrawn. Any adverse regulatory action by the FDA or another
regulatory agency may restrict us and our licensees from effectively marketing
and selling our IP applications in medical products. In addition,
foreign laws and regulations have become more stringent and regulated medical
products may become subject to increased regulation by foreign agencies in the
future. Penalties for our licensees for any of their noncompliance
with foreign governmental regulations could be severe, including revocation or
suspension of their business licenses and criminal sanctions. Any
foreign law or regulation imposed on our IP applications may materially affect
our projected operations and revenues, by adverse impact on the distribution and
sale of regulated medical products in foreign jurisdictions through our intended
licensees.
6
Our
licensees may not sustain compliance with regulatory standards and laws
applicable to medical products production, manufacturing, and quality
processes.
Our
licensees, which are manufacturers of medical products or pharmaceuticals, will
be subject to periodic inspection by the FDA for compliance with regulations
that require manufacturers to comply with certain practices and standards,
including testing, quality control and documentation procedures. In
addition, federal medical device reporting regulations require them to provide
information to the FDA whenever there is evidence that reasonably suggests that
a medical product may have caused or contributed to a death or serious injury
or, if a malfunction were to occur, could cause or contribute to a death or
serious injury. Compliance with these requirements is subject to
continual review and is rigorously monitored through periodic FDA
inspections. In foreign markets, our licensing partners are required
to obtain certain certifications in order to sell medical products and must
undergo periodic inspections by regulatory bodies to maintain these
certifications. If our licensees fail to adhere to any laws and
standards applicable to medical product manufacturers, the marketing of products
could be suspended, and such failure could, for our licensees, lead to fines,
withdrawal of regulatory clearances, product recalls, or other consequences, any
of which could in turn adversely affect our projected business operations,
financial condition, or results of operations. Our licensees will
also be subject to certain environmental laws and regulations. Our
licensing partners’ manufacturing operations may involve the use of substances
and materials regulated by various environmental protection agencies and
regulatory bodies. We cannot guarantee that any licensee will sustain
compliance with environmental laws, and that regulations will not have a
material impact on our earnings, financial condition, or business
operations.
Failure
of our licensees to comply with laws and regulations relating to reimbursement
of health care products may adversely impact our business
operations.
Medical
products are subject to regulation regarding quality and cost by the United
States Department of Health and Human Services, Centers for Medicare &
Medicaid services and comparable state and foreign agencies that are responsible
for payment and reimbursement of healthcare goods and services. In
the U.S., healthcare laws apply to our licensing partners’ business operations
when a reimbursement claim is submitted under a federal government funded
healthcare program. Federal laws and regulations prohibit the filing
of false or improper claims for federal payment and unlawful inducements for the
referral of business reimbursable under federally-funded healthcare programs
(known as the anti-kickback laws). If a governmental agency or
regulatory body were to conclude that our licensees were not in compliance with
applicable laws and regulations regarding payment or reimbursement of medical
products, they could be subject to criminal and civil penalties, including
exclusion from participation as a supplier of products to beneficiaries covered
by government healthcare programs. Such exclusions could negatively affect our
distribution channels, financial condition or results of
operations.
Quality
problems with a licensee’s manufacturing processes could harm our reputation and
affect demand for medical products using our technology.
Ensuring
the quality of products and manufacturing processes is critical for medical
product companies due to the high cost and seriousness of product failures or
malfunctions. If any of our licensees failed to meet adequate quality
standards, its and our reputations could be damaged and our revenues could
decline. In addition, production of medical products which utilize
our technology may depend on our licensees’ abilities to engineer and
manufacture precision components and assemble such components into intricate
medical products and, if they fail to meet these requirements or fail to adapt
to changing requirements, their and our reputations may suffer and demand for
products implementing our technology could decline significantly.
7
Uncertainties
regarding healthcare reimbursements may adversely affect our
business.
Healthcare
cost containment pressures decrease the prices end-users are willing to pay for
medical products, which could have an adverse effect on our royalty
revenue. Products that may implement our technology may be purchased
by hospitals or physicians, which typically bill governmental programs, private
insurance plans and managed care plans for the healthcare devices and services
provided to their patients. The ability of these customers to obtain
reimbursement from private and governmental third-party payors for the products
and services they provide to patients is critical to commercial
success. The availability of reimbursement affects which products
customers purchase and the prices they are willing to
pay. Reimbursement varies from country to country and can
significantly impact the acceptance of new products and
services. Although we and our licensees may have a promising new
product, we and our licensees may find limited demand for the medical product
unless reimbursement approval is obtained from private and governmental
third-party payors. Even if reimbursement approval is obtained from
private and governmental third-party payors, we may still find limited demand
for the product for other reasons. In addition, legislative or
administrative reforms to the U.S., or to international reimbursement systems,
in a manner that significantly reduces reimbursement for products or procedures
using our technology, or denial of coverage for those products or procedures,
could have a material adverse effect on our business, financial condition or
results of operations.
Major
third-party payors for hospital services in the U.S. and abroad continue to work
to contain healthcare costs. The introduction of cost containment
incentives, combined with closer scrutiny of healthcare expenditures by both
private health insurers and employers, has resulted in increased discounts and
contractual adjustments to hospital charges for services performed and has
shifted services between inpatient and outpatient
settings. Initiatives to limit the increase of healthcare costs,
including price regulation, are also ongoing in markets in which our licensees
may do business. Hospitals or physicians may respond to these
cost-containment pressures by insisting that our licensees lower prices, which
may adversely affect our royalties.
In
response to increasing healthcare costs, there has been and may continue to be
proposals by legislators, regulators, and third-party payors to reduce these
costs. If these proposals are approved and passed, limitations and/or
reductions may be placed on the net or allowable price of products implementing
our technology or the amounts of reimbursement available for these products from
customers, governmental bodies, and third-party payors. These
limitations and reductions on prices may have a material adverse effect on our
financial position and results of operations.
We
and our licensees will be required to attract and retain top quality talent to
compete in the marketplace.
We
believe our future growth and success will depend in part on our and our
licensees’ abilities to attract and retain highly skilled managerial, product
development, sales and marketing, and finance personnel. There can be
no assurance of success in attracting and retaining such
personnel. Shortages in qualified personnel could limit our ability
to increase sales of existing products and services and launch new product and
service offerings.
8
Our
forecasts are highly speculative in nature and we cannot predict results in a
development stage company with a high degree of accuracy.
Any
financial projections, especially those based on ventures with minimal operating
history, are inherently subject to a high degree of uncertainty, and their
ultimate achievement depends on the timing and occurrence of a complex series of
future events, both internal and external to the enterprise. There
can be no assurance that potential revenues or expenses we project will, in
fact, be received or incurred.
Our
auditors have expressed going concern opinions on our financial
statements.
Primarily
as a result of our recurring losses and lack of liquidity, the reports of the
independent auditors to both our company and AtheroNova Operations regarding our
respective audited financial statements at December 31, 2009 expressed
substantial uncertainty as to our abilities to continue as going
concerns.
We
will be subject to evolving and expensive corporate governance regulations and
requirements. Our failure to adequately adhere to these requirements
or the failure or circumvention of our controls and procedures could seriously
harm our business.
As a
publicly traded company, we are subject to various federal, state and other
rules and regulations, including applicable requirements of the Sarbanes-Oxley
Act of 2002. Compliance with these evolving regulations is costly and
requires a significant diversion of management time and attention, particularly
with regard to our disclosure controls and procedures and our internal control
over financial reporting. Our internal controls and procedures may
not be able to prevent errors or fraud in the future. Faulty
judgments, simple errors or mistakes, or the failure of our personnel to adhere
to established controls and procedures, may make it difficult for us to ensure
that the objectives of the control system are met. A failure of our
controls and procedures to detect other than inconsequential errors or fraud
could seriously harm our business and results of operations.
Our
limited senior management team size may hamper our ability to effectively manage
a publicly traded company while developing our products and harm our
business.
Our
management team has experience in the management of publicly traded companies
and complying with federal securities laws, including compliance with recently
adopted disclosure requirements on a timely basis. They realize it
will take significant resources to meet these requirements while simultaneously
working on licensing, developing and protecting our IP. Our
management will be required to design and implement appropriate programs and
policies in responding to increased legal, regulatory compliance and reporting
requirements, and any failure to do so could lead to the imposition of fines and
penalties and harm our business.
We
may incur substantial liability associated with registration rights granted to
investors in the Capital Raise Transaction.
Within 60
days following the closing of the Capital Raise Transaction, we are obligated to
file with the Securities and Exchange Commission (“SEC”) a registration
statement covering the resale by investors of the shares represented by the
Notes and Warrants purchased in the Capital Raise Transaction. If we
fail to timely file this registration statement or if the registration statement
does not become effective within 180 days (or 150 days if the SEC does not fully
review the registration statement) following the closing of the Capital Raise
due to our failure to satisfy our obligations, we will be obligated to make
certain payments as liquidated damages to the investors in the Capital Raise
Transaction for each day that elapses after the closing of the Capital Raise
Transaction before the registration statement is filed or becomes effective, as
applicable. There can be no assurance that the registration statement
will be declared effective by the SEC within 180 days following the closing of
the Capital Raise Transaction. Similar penalties may apply if we are
unable to maintain the effectiveness of the registration statement.
9
The
issuance of the Notes in the Capital Raise Transaction has subjected us to
possible remedies of a secured creditor and has limited our financing
alternatives.
Our
obligations under the Notes will be debt obligations, secured by security
interests in all of the assets of our company and its subsidiaries, including
their intellectual property. If we default on our obligations under
the Notes and related agreements, the Note holders will be entitled to all the
remedies of secured creditors including (without limitation) the ability to
accelerate the due date for the entire principal amount, charge default interest
and penalties and foreclose on our assets.
Anti-dilution
adjustments under the Notes and Warrants issued in the Capital Raise Transaction
may dilute the interests of our stockholders.
If we are
forced in the future to issue shares for prices less than the conversion price
of the Notes, that may trigger anti-dilution adjustments that increase the
numbers of shares that are issuable on conversions of the Notes or exercises of
the Warrants issued in the Capital Raise Transaction. Such
adjustments, particularly possible “ratchet” adjustments not weighted by the
relative magnitude of the particular low-price share issuance, may significantly
dilute the holdings of stockholders other than the investors in the Capital
Raise Transaction.
Restrictions
in the Notes and related documents will likely restrict our ability to raise
debt funding or be acquired.
Restrictions
and provisions in the Notes and related documents will restrict our ability to
raise additional debt financing without the Note holders’
consents. Also, financial penalties in the Notes and Warrants may
make it difficult to us to be acquired by a third party.
Our
Chief Executive Officer will not be devoting his full-time efforts to us in the
next stages of operation. His departure could be an event of default under the
Notes.
While it
is believed that Thomas Gardner’s services will be available to us, he currently
has a non-exclusive contractual agreement to perform the services of CEO of
PhyGen LLC, which designs, manufactures and sells instruments and implants for
spine surgery. He is committed to fulfill such contractual
obligations until January 1, 2011. To assist in this transitional
stage, our Chief Financial Officer, Mark Selawski, became a full-time employee
as of April 1, 2010. Mr. Selawski has over 15 years experience in the
healthcare field and has had a previous working relationship with Mr.
Gardner. To supplement this arrangement, we have secured office space
adjacent to Mr. Gardner’s current place of business in order to facilitate a
proximal work environment for him and Mr. Selawski. We feel that the
financial arrangements that we have made for Mr. Gardner, as well as our work
toward a new employment agreement for him, should be sufficient to retain his
services, but there are no assurances these arrangements will be effective and
adequate at this stage in our development. If Mr. Gardner ceases to
be an employee of our company (other than due to a termination without good
cause), that will be an event of default under the Notes unless we obtain a
reasonably acceptable full-time replacement for Mr. Gardner within 90 days after
such termination.
10
Risks
Related to our Common Stock
There
is little current trading of our shares. Our stock price is likely to
be highly volatile.
Although
prices for our shares of common stock are quoted on the OTC Bulletin Board
(“OTCBB”), there is little current trading and no assurance can be given that an
active public trading market will develop or, if developed, that it will be
sustained. The OTCBB is generally regarded as a less efficient and
less prestigious trading market than other national markets. There is
no assurance if or when our common stock will be quoted on another more
prestigious exchange or market. The market price of our stock is
likely to be highly volatile because for some time there will likely be a thin
trading market for the stock, which causes trades of small blocks of stock to
have a significant impact on the stock price.
Because
our common stock is likely to be considered a “penny stock,” our trading will be
subject to regulatory restrictions.
Our
common stock is currently, and in the near future will likely continue to be,
considered a “penny stock.” The SEC has adopted rules that regulate
broker-dealer practices in connection with transactions in “penny
stocks.” Penny stocks generally are equity securities with a price of
less than $5.00 (other than securities registered on certain national securities
exchanges or quoted on the NASDAQ system, provided that current price and volume
information with respect to transactions in such securities is provided by the
exchange or system). The penny stock rules require a broker-dealer,
prior to a transaction in a penny stock not otherwise exempt from those rules,
to deliver a standardized risk disclosure document prepared by the SEC, which
specifies information about penny stocks and the nature and significance of
risks of the penny stock market. The broker-dealer also must provide
the customer with bid and offer quotations for the penny stock, the compensation
of the broker-dealer and any salesperson in the transaction, and monthly account
statements indicating the market value of each penny stock held in the
customer’s account. In addition, the penny stock rules require that,
prior to a transaction in a penny stock not otherwise exempt from those rules,
the broker-dealer must make a special written determination that the penny stock
is a suitable investment for the purchaser and receive the purchaser’s written
agreement to the transaction. These disclosure and other requirements
may adversely affect the trading activity in the secondary market for our common
stock.
Limited
future sales of our common stock in the public market could make it difficult to
generate significant liquidity in our stock.
As noted
above, we will be obligated to file a registration statement with the SEC to
cover resales of shares underlying the Notes and Warrants issued to the
Purchasers. However, upon the effectiveness of this registration
statement, most of the stock covered under the registration may not be
immediately available for trading. Due to a limitation in the number
of shares traded on a regular basis, there may be significant swings in the bid
and ask prices of our stock or there may not be any significant volume of the
stock available to trade.
We
have not paid dividends in the past and do not expect to pay dividends for the
foreseeable future, and any return on investment may be limited to potential
future appreciation on the value of our common stock.
We
currently intend to retain any future earnings to support the development and
expansion of our business and do not anticipate paying cash dividends in the
foreseeable future. Our payment of any future dividends will be at
the discretion of our board of directors after taking into account various
factors, including without limitation, our financial condition, operating
results, cash needs, growth plans and the terms of any credit agreements that we
may be a party to at the time. To the extent we do not pay dividends,
our stock may be less valuable because a return on investment will only occur if
and to the extent our stock price appreciates, which may never
occur. In addition, investors must rely on sales of their common
stock after price appreciation as the only way to realize their investment, and
if the price of our stock does not appreciate, then there will be no return on
investment. Investors seeking cash dividends should not purchase our
common stock.
11
Our
officers, directors and principal stockholders can exert significant influence
over us and may make decisions that are not in the best interests of all
stockholders.
Our
officers, directors and principal stockholders (greater than 5% stockholders)
collectively own approximately 74% of our outstanding common stock, and
approximately 58% of our fully-diluted common stock. As a result of
such ownership and the Voting Agreement that is in place, these stockholders
will be able to affect the outcome of, or exert significant influence over, all
matters requiring stockholder approval, including the election and removal of
directors and any change in control. In particular, this
concentration of ownership of our common stock could have the effect of delaying
or preventing a change of control of us or otherwise discouraging or preventing
a potential acquirer from attempting to obtain control of us. This,
in turn, could have a negative effect on the market price of our common
stock. It could also prevent our stockholders from realizing a
premium over the market prices for their shares of common
stock. Moreover, the interests of this concentration of ownership may
not always coincide with our interests or the interests of other stockholders,
and accordingly, they could cause us to enter into transactions or agreements
that we would not otherwise consider.
Anti-takeover
provisions may limit the ability of another party to acquire us, which could
cause our stock price to decline.
Our
certificate of incorporation, as amended, our bylaws and Delaware law contain
provisions that could discourage, delay or prevent a third party from acquiring
us, even if doing so may be beneficial to our stockholders. In
addition, these provisions could limit the price investors would be willing to
pay in the future for shares of our common stock.
FORWARD-LOOKING
STATEMENTS
This
prospectus, including the sections entitled “Risk Factors,” “Management’s
Discussion and Analysis of Financial Condition and Results of Operations” and
“Business,” contains “forward-looking statements” that include information
relating to future events, future financial performance, strategies,
expectations, competitive environment, regulation and availability of
resources. These forward-looking statements include, without
limitation: statements regarding proposed new services; statements concerning
litigation or other matters; statements concerning projections, predictions,
expectations, estimates or forecasts for our business, financial and operating
results and future economic performance; statements of management’s goals and
objectives; and other similar expressions concerning matters that are not
historical facts. Words such as “may,” “will,” “should,” “could,”
“would,” “predicts,” “potential,” “continue,” “expects,” “anticipates,”
“future,” “intends,” “plans,” “believes” and “estimates,” and similar
expressions, as well as statements in future tense, identify forward-looking
statements.
Forward-looking
statements should not be read as a guarantee of future performance or results,
and will not necessarily be accurate indications of the times at, or by which,
that performance or those results will be achieved. Forward-looking
statements are based on information available at the time they are made and/or
management’s good faith belief as of that time with respect to future events,
and are subject to risks and uncertainties that could cause actual performance
or results to differ materially from those expressed in or suggested by the
forward-looking statements. Important factors that could cause these
differences include, but are not limited to:
12
|
·
|
our
failure to implement our business plan within the time period we
originally planned to accomplish;
and
|
|
·
|
other
factors discussed under the headings “Risk Factors,” “Management’s
Discussion and Analysis of Financial Condition and Results of Operations”
and “Business.”
|
Forward-looking
statements speak only as of the date they are made. You should not put undue
reliance on any forward-looking statements. We assume no obligation
to update forward-looking statements to reflect actual results, changes in
assumptions or changes in other factors affecting forward-looking information,
except to the extent required by applicable securities laws. If we do
update one or more forward-looking statements, no inference should be drawn that
we will make additional updates with respect to those or other forward-looking
statements.
USE
OF PROCEEDS
We will
not receive any proceeds from the sale of shares to be offered by the selling
stockholders. The proceeds from the sale of each selling
stockholder’s common stock will belong to that selling stockholder.
PLAN
OF DISTRIBUTION
We are
registering certain outstanding shares of our common stock and the shares of our
common stock issuable upon conversion of the Notes (including shares of our
common stock issuable upon conversion of accrued interest on the Notes) to
permit the resale of these shares of our common stock by the holders of the
outstanding shares and the Notes from time to time after the date of this
prospectus. We will not receive any of the proceeds from the sale by
the selling stockholders of the shares of our common stock. The
Purchasers will bear all fees and expenses incident to our obligation to
register the shares of our common stock.
The
selling stockholders may sell all or a portion of the shares of our common stock
beneficially owned by them and offered hereby from time to time directly or
through one or more underwriters, broker-dealers or agents. If the
shares of our common stock are sold through underwriters or broker-dealers, the
selling stockholders will be responsible for underwriting discounts or
commissions or agent’s commissions. The shares of our common stock
may be sold in one or more transactions at fixed prices, at prevailing market
prices at the time of the sale, at varying prices determined at the time of
sale, or at negotiated prices. These sales may be effected in
transactions, which may involve crosses or block transactions:
|
·
|
on
any national securities exchange or quotation service on which the
securities may be listed or quoted at the time of
sale;
|
|
·
|
in
the over-the-counter market;
|
13
|
·
|
in
transactions otherwise than on these exchanges or systems or in the
over-the-counter market;
|
|
·
|
through
the writing of options, whether such options are listed on an options
exchange or otherwise;
|
|
·
|
ordinary
brokerage transactions and transactions in which the broker-dealer
solicits purchasers;
|
|
·
|
block
trades in which the broker-dealer will attempt to sell the shares as agent
but may position and resell a portion of the block as principal to
facilitate the transaction;
|
|
·
|
purchases
by a broker-dealer as principal and resale by the broker-dealer for its
account;
|
|
·
|
an
exchange distribution in accordance with the rules of the applicable
exchange;
|
|
·
|
privately
negotiated transactions;
|
|
·
|
short
sales;
|
|
·
|
sales
pursuant to Rule 144;
|
|
·
|
broker-dealers
may agree with the selling stockholders to sell a specified number of such
shares at a stipulated price per
share;
|
|
·
|
a
combination of any such methods of sale;
and
|
|
·
|
any
other method permitted pursuant to applicable
law.
|
If the
selling stockholders effect such transactions by selling shares of our common
stock to or through underwriters, broker-dealers or agents, such underwriters,
broker-dealers or agents may receive commissions in the form of discounts,
concessions or commissions from the selling stockholders or commissions from
purchasers of the shares of our common stock for whom they may act as agent or
to whom they may sell as principal (which discounts, concessions or commissions
as to particular underwriters, broker-dealers or agents may be in excess of
those customary in the types of transactions involved). In connection
with sales of the shares of our common stock or otherwise, the selling
stockholders may enter into hedging transactions with broker-dealers, which may
in turn engage in short sales of the shares of our common stock in the course of
hedging in positions they assume. The selling stockholders may also
sell shares of our common stock short and deliver shares of our common stock
covered by this prospectus to close out short positions and to return borrowed
shares in connection with such short sales. The selling stockholders
may also loan or pledge shares of our common stock to broker-dealers that in
turn may sell such shares.
The
selling stockholders may pledge or grant a security interest in some or all of
the Notes or shares of our common stock owned by them and, if they default in
the performance of their secured obligations, the pledgees or secured parties
may offer and sell the shares of our common stock from time to time pursuant to
this prospectus or any amendment to this prospectus under Rule 424(b)(3) or
other applicable provision of the Securities Act of 1933, as amended, amending,
if necessary, the list of selling stockholders to include the pledgee,
transferee or other successors in interest as selling stockholders under this
prospectus. The selling stockholders also may transfer and donate the
shares of our common stock in other circumstances in which case the transferees,
donees, pledgees or other successors in interest will be the selling beneficial
owners for purposes of this prospectus.
14
The
selling stockholders and any broker-dealer participating in the distribution of
the shares of our common stock may be deemed to be “underwriters” within the
meaning of the Securities Act, and any commission paid, or any discounts or
concessions allowed to, any such broker-dealer may be deemed to be underwriting
commissions or discounts under the Securities Act. At the time a
particular offering of the shares of our common stock is made, a prospectus
supplement, if required, will be distributed which will set forth the aggregate
amount of shares of our common stock being offered and the terms of the
offering, including the name or names of any broker-dealers or agents, any
discounts, commissions and other terms constituting compensation from the
selling stockholders and any discounts, commissions or concessions allowed or
reallowed or paid to broker-dealers.
Under the
securities laws of some states, the shares of our common stock may be sold in
such states only through registered or licensed brokers or
dealers. In addition, in some states the shares of our common stock
may not be sold unless such shares have been registered or qualified for sale in
such state or an exemption from registration or qualification is available and
is complied with.
There can
be no assurance that any selling stockholder will sell any or all of the shares
of our common stock registered pursuant to the registration statement, of which
this prospectus forms a part.
The
selling stockholders and any other person participating in such distribution
will be subject to applicable provisions of the Securities Exchange Act of 1934,
as amended, and the rules and regulations thereunder, including, without
limitation, Regulation M of the Exchange Act, which may limit the timing of
purchases and sales of any of the shares of our common stock by the selling
stockholders and any other participating person. Regulation M may
also restrict the ability of any person engaged in the distribution of the
shares of our common stock to engage in market-making activities with respect to
the shares of our common stock. All of the foregoing may affect the
marketability of the shares of our common stock and the ability of any person or
entity to engage in market-making activities with respect to the shares of our
common stock.
The
Purchasers will pay all expenses of the registration of the shares of our common
stock estimated to be approximately $30,000 in total, including, without
limitation, SEC filing fees and expenses of compliance with state securities or
“blue sky” laws; provided, however, that a selling stockholder will pay all
underwriting discounts and selling commissions, if any. We will
indemnify the selling stockholders against liabilities, including some
liabilities under the Securities Act, or the selling stockholders will be
entitled to contribution. We may be indemnified by the selling
stockholders against civil liabilities, including liabilities under the
Securities Act, that may arise from any written information furnished to us by
the selling stockholder specifically for use in this prospectus, or we may be
entitled to contribution.
Once sold
under the registration statement, of which this prospectus forms a part, the
shares of our common stock will be freely tradable in the hands of persons other
than our affiliates.
15
DESCRIPTION
OF REGISTRANT’S SECURITIES
As of
June 23, 2010, our authorized capital stock consisted of:
|
·
|
100,000,000
shares of common stock, par value $0.0001 per share;
and
|
|
·
|
10,000,000
shares of preferred stock, par value $0.0001 per
share.
|
As of
June 23, 2010, there were outstanding:
|
·
|
22,680,927
shares of common stock held by approximately 20 stockholders of
record;
|
|
·
|
options
to purchase 549,498 shares of common
stock;
|
|
·
|
warrants
to purchase 5,497,396 shares of common
stock;
|
|
·
|
Notes
convertible into 4,199,358 shares of common stock (including 381,762
shares accounting for accrued interest through maturity);
and
|
|
·
|
no
shares preferred stock.
|
Common
Stock
Dividend
Rights
Subject
to preferences that may apply to shares of preferred stock outstanding at the
time, the holders of outstanding shares of our common stock are entitled to
receive dividends out of funds legally available at the times and in the amounts
that our board of directors may determine.
Voting
Rights
Each
holder of common stock is entitled to one vote for each share of common stock
held on all matters submitted to a vote of stockholders. Cumulative
voting for the election of directors is not provided for in our certificate of
incorporation, as amended, which means that the holders of a majority of the
voting shares voted can elect all of the directors then standing for
election.
No
Preemptive or Similar Rights
Holders
of our common stock do not have preemptive rights, and our common stock is not
convertible or redeemable.
Right
to Receive Liquidation Distributions
Upon our
dissolution, liquidation or winding-up, the assets legally available for
distribution to our stockholders are distributable ratably among the holders of
our common stock, subject to the preferential rights and payment of liquidation
preferences, if any, on any outstanding shares of preferred stock.
16
Authorized
but Undesignated Preferred Stock
We are
authorized, subject to limitations prescribed by Delaware law, to issue
preferred stock in one or more series, to establish from time to time the number
of shares to be included in each series, to fix the designation, powers,
preferences and rights of the shares of each series and any of its
qualifications, limitations or restrictions. Our board of directors
can also increase or decrease the number of shares of any series, but not below
the number of shares of that series then outstanding, by the affirmative vote of
the holders of a majority of our capital stock entitled to vote, unless a vote
of any other holders is required by our certificate of incorporation, as
amended, or the Delaware General Corporation Law. Our board of
directors may authorize the issuance of preferred stock with voting or
conversion rights that could adversely affect the voting power or other rights
of the holders of our common stock. The issuance of preferred stock,
while providing flexibility in connection with possible acquisitions and other
corporate purposes, could, among other things, have the effect of delaying,
deferring or preventing a change in control of our company and may adversely
affect the market price of our common stock and the voting and other rights of
the holders of our common stock. We have no current plan to issue any
shares of preferred stock.
Warrants,
Options and Convertible Notes
At June
23, 2010, there were outstanding warrants exercisable to purchase shares of our
common stock, as follows:
|
·
|
3,588,558
shares at an exercise price of approximately $0.22 per share, with
expiration dates ranging from February 5, 2013 through March 15, 2015;
and
|
|
·
|
1,908,798
shares at an exercise price of approximately $0.39 per share, which will
expire on May 13, 2014.
|
At June
23, 2010, there were outstanding options exercisable to purchase shares of our
common stock, as follows:
|
·
|
549,498
shares at an exercise price of approximately $0.22 per share, which will
expire on January 7, 2017.
|
At June
23, 2010, there were outstanding Notes convertible (including accrued interest
through maturity) into shares of our common stock, as follows:
|
·
|
4,199,358
shares at a conversion price of approximately $0.39 per share, which will
mature on May 13, 2014.
|
Anti-takeover
Provisions
Certain
provisions of our certificate of incorporation, as amended, and Delaware law may
have the effect of delaying, deferring or discouraging another person from
acquiring control of our company.
17
Charter
and Bylaw Provisions
Our
certificate of incorporation, as amended, allows our board of directors to issue
10,000,000 shares of preferred stock in one or more series and with such rights
and preferences including voting rights, without further stockholder
approval. In the event that our board of directors designates
additional series of preferred stock with rights and preferences, including
super-majority voting rights, and issues such preferred stock, the preferred
stock could make our acquisition by means of a tender offer, a proxy contest or
otherwise, more difficult, and could also make the removal of incumbent officers
and directors more difficult. As a result, these provisions may have
an anti-takeover
effect. The preferred stock authorized in our certificate of
incorporation, as amended, may inhibit changes of control that are not approved
by our board of directors. These provisions could limit the price
that future investors might be willing to pay for our common
stock. This could have the effect of delaying, deferring or
preventing a change in
control. The issuance of preferred stock could also
effectively limit or dilute the voting power of our
stockholders. Accordingly, such provisions of our certificate of
incorporation, as amended, may discourage or prevent an acquisition or
disposition of our business that could otherwise be in the best interest of our
stockholders.
Delaware
Law
In
addition, Delaware has enacted the following legislation that may deter or
frustrate takeovers of Delaware
corporations:
The
Delaware General Corporation Law expressly permits our board of directors, when
evaluating any proposed tender or exchange offer, any merger, consolidation or
sale of substantially all of our assets, or any similar extraordinary
transaction, to consider all relevant factors including, without limitation, the
social, legal, and economic effects on the employees, customers, suppliers, and
other constituencies of our company and its subsidiary, and on the communities
and geographical areas in which they operate. Our board of directors
may also consider the amount of consideration being offered in relation to the
then current market price for our outstanding shares of common stock and our
then current value in a freely negotiated transaction. Our board of
directors believes such provisions are in our long-term best interests and the
long-term best interests of our stockholders.
We are
subject to the Delaware control share acquisitions statute. This
statute is designed to afford stockholders of public corporations in Delaware
protection against acquisitions in which a person, entity or group seeks to gain
voting control. With enumerated exceptions, the statute provides that
shares acquired within certain specific ranges will not possess voting rights in
the election of directors unless the voting rights are approved by a majority
vote of the public corporation’s disinterested
stockholders. Disinterested shares are shares other than those owned
by the acquiring person or by a member of a group with respect to a control
share acquisition, or by any officer of the corporation or any employee of the
corporation who is also a director. The specific acquisition ranges
that trigger the statute are: acquisitions of shares possessing one-fifth or
more but less than one-third of all voting power; acquisitions of shares
possessing one-third or more but less than a majority of all voting power; or
acquisitions of shares possessing a majority or more of all voting
power. Under certain circumstances, the statute permits the acquiring
person to call a special stockholders meeting for the purpose of considering the
grant of voting rights to the holder of the control shares. The
statute also enables a corporation to provide for the redemption of control
shares with no voting rights under certain circumstances.
Transfer
Agent and Registrar
The
transfer agent and registrar for our common stock is Routh Stock
Transfer.
Listing
Our
common stock is quoted on the OTCBB under the trading symbol
“AHRO.” Prior to May 25, 2010, our common stock was quoted on the
OTCBB under the trading symbol “TRHI.”
18
DESCRIPTION
OF BUSINESS
Corporate
History
We were
incorporated in the State of Delaware on May 13, 1997 under the name Camryn
Information Services, Inc. We operated for a brief period of time
before we ceased operations on February 25, 1999 when we forfeited our charter
for failure to designate a registered agent. We remained dormant
until 2004 when we renewed our operations with the filing of a Certificate of
Renewal and Revival of Charter with the State of Delaware on October 29,
2004. On November 3, 2004, we filed a Certificate of Amendment and
our name was formally changed from Camryn Information Services, Inc. to iStorage
Networks, Inc. Such change became effective on November 8,
2004. On January 26, 2006, we issued 41,000 shares of our common
stock in exchange for all of the membership interests of Landbank,
LLC. We changed our name to Landbank Group, Inc. on January 27,
2006. LLC made bulk acquisitions of parcels of land, primarily
through the real property tax lien foreclosure process. The bulk
acquisitions were then divided into smaller parcels for resale. On
December 31, 2007, we transferred all of LLC’s membership interests to Landbank
Acquisition, LLC, ceased business operations, and changed our name to Trist
Holdings, Inc. On May 13, 2010 we changed our name to AtheroNova
Inc. From December 31, 2007 through May 13, 2010, we were a public
“shell” company with nominal assets.
In
December 2006, Z&Z Medical Holdings, Inc. (“Z&Z Nevada”) was formed as a
Nevada corporation with contributed intellectual property from Giorgio Zadini
and Filiberto Zadini. During 2007, Z&Z Nevada sought various
sources of working capital via an offering memorandum first prepared in November
2007, in which up to 1,500,000 shares were offered at $0.50 per share while it
continued to perfect the patent applications previously
filed. Concurrently, Z&Z Nevada was designing its clinical trial
protocol and held discussions with various institutions about conducting a
clinical animal study to test Z&Z Nevada’s conclusions reached in unique in
vitro experiments.
During
2008, Z&Z Nevada signed an agreement with the University of California, Los
Angeles and Dr. Aldons “Jake” Lusis for the initial clinical study in
vivo. The cost of the study was set at $200,000 for all work
associated with the trial. A clinical protocol was established and
the study spanned a period of 30 weeks, with the trial concluding in October,
2009. We expect the publication of the results of the study sometime
in the 3rd
quarter of 2010.
Also
during 2008, Z&Z Nevada raised working capital of $225,000 from sales of
securities pursuant to its offering memorandum from various private sources at
$0.50 per share to start its operations, intellectual property work and clinical
research.
During
2009, Z&Z Nevada continued its research and intellectual property work and
raised an additional $100,000 from various private sources from sales of
securities pursuant to its offering memorandum at $0.50 per
share. From 2007 through 2009 Z&Z Nevada attempted to obtain
larger amounts of working capital without success. Certain potential
investors expressed dissatisfaction with Z&Z Nevada’s status as a Nevada
corporation. As a result Z&Z Nevada’s board of directors chose to
reincorporate in Delaware pursuant to a merger with and into AtheroNova
Operations in 2010.
During
late 2009 Z&Z Nevada was introduced to KOM Capital Management, LLC, a
private equity fund based in New York City (“KOM”), and on November 4, 2009
Z&Z Nevada and KOM signed a Letter of Intent for the company to merge with a
subsidiary of our company whereby a) the holders of Z&Z Nevada’s securities
would obtain approximately 98% of our outstanding shares and b) KOM would
purchase $3,000,000 of our 2.5% Senior Secured Convertible Notes with an
additional $3,000,000 purchasable if certain operating benchmarks were achieved
in the ensuing 24 months, and would receive in connection therewith, warrants to
purchase 100% of the shares of our common stock issuable upon conversion of such
notes.
19
In 2010,
during the due diligence period, Z&Z Nevada raised additional working
capital of $225,000 from the final sales of its securities pursuant to the
offering memorandum at $0.50 per share.
Events
within the capital markets and internal to KOM resulted in the amendment of the
Letter of Intent with KOM to reduce the initial purchase of 2.5% Senior Secured
Convertible Notes to $1,500,000 with 50% warrant coverage, with an additional
$1,500,000 (without warrants) purchasable for up to 14 months following the
initial purchase of such notes. As a result of subsequent
negotiations, AtheroNova Operations consummated the Capital Raise Transaction
with the Purchasers.
On March
26, 2010, we entered into the Merger Agreement with MergerCo and
Z&Z. At the Closing, (i) MergerCo was merged with and into
Z&Z, whose name was concurrently changed to AtheroNova Operations, Inc.;
(ii) Z&Z, as AtheroNova Operations, become our wholly-owned subsidiary;
(iii) all of AtheroNova Operations’ shares, warrants and options outstanding
prior to the Merger were exchanged (or assumed, in the case of warrants and
options) for comparable securities of our company; and (iv) approximately 98% of
our fully-diluted shares (excluding the shares issuable in the Capital Raise
Transaction) were owned by AtheroNova Operations’ former stockholders, warrant
holders and option holders. As a result of the Merger we are solely
engaged in AtheroNova Operations’ business, AtheroNova Operations’ officers
became our officers and three of AtheroNova Operations’ directors became members
of our seven-member board of directors (which currently has two
vacancies).
On May
13, 2010, we entered into the Securities Purchase Agreement with the Purchasers
pursuant to which the Purchasers purchased the Notes for a cash purchase price
of $1,500,000, and the Warrants to purchase up to 1,908,798 shares of our common
stock at an exercise price of approximately $0.39 per share.
The Notes
pay 2.5% interest per annum with a maturity of 4 years after the closing of the
Capital Raise Transaction. No cash interest payments are required,
except that accrued and unconverted interest shall be due on the maturity date
and on each conversion date with respect to the principal amount being
converted, provided that such interest may be added to and included with the
principal amount being converted. If there is an uncured event of
default (as defined in the Notes), the holder of each Note may declare the
entire principal and accrued interest amount immediately due and
payable. Default interest will accrue after an event of default at an
annual rate of 12%. If there is an acceleration, a mandatory default
amount equal to 120% of the unpaid Note principal plus accrued interest may be
payable.
The
Warrants have a term of 4 years and may be exercised on a cashless basis under
which a portion of the shares subject to the exercise are not issued in payment
of the purchase price, based on the then fair market value of the
shares.
On May
13, 2010, we also entered into a Security Agreement and an Intellectual Property
Security Agreement with the Purchasers and AtheroNova Operations, pursuant to
which all of our obligations under the Notes are secured by first priority
security interests in all of our assets and the assets of AtheroNova Operations,
including intellectual property. Upon an event of default under the
Notes or such agreements, the Note holders may be entitled to foreclose on any
of such assets or exercise other rights available to a secured creditor under
California and Delaware law. In addition, under a Subsidiary
Guarantee, AtheroNova Operations will guarantee all of our obligations under the
Notes.
20
The Notes
may not be prepaid, or forced by us to be converted in connection with an
acquisition of our company, except in a limited case more than a year after the
Note issuance where the average of our stock trading price for 30 days on a
national trading market other than the OTCBB is at least three times the
conversion price, in which event, and subject to the satisfaction of certain
other requirements, the Note holders may elect to receive at least double the
unpaid principal amounts in cash and other requirements are
satisfied. In such a limited case acquisition, there could also be a
forced cashless exercise of the Warrants subject to similar requirements and
optional cash payments to the Warrant holders of at least double the exercise
prices of their Warrants.
The Note
conversion price and the Warrant exercise price will be subject to specified
adjustments for certain changes in the numbers of outstanding shares of our
common stock, including conversions or exchanges of such. If
additional shares of our capital stock are issued, except in specified exempt
issuances, for consideration which is less than the then existing Note
conversion or Warrant exercise price, then such conversion or warrant price will
be reduced by anti-dilution adjustments. For the first $400,000 of
such “Dilutive Issuances,” the reduction will be made on a weighted average
basis, taking into account the relative magnitudes of any Dilutive Issuance
relative to the total number of outstanding shares. However, any
further Dilutive Issuance would be subject to a more detrimental “full ratchet”
adjustment that generally reduces the conversion or exercise price to equal the
price in the Dilutive Issuance, regardless of the size of the Dilutive
Issuance.
The Notes
will greatly restrict the ability of our company or AtheroNova Operations to
issue indebtedness or grant liens on our or its respective assets without the
Note holders’ consent. They will also limit and impose financial
costs on our acquisition by any third party.
On May
13, 2010 and in connection with the Capital Raise Transaction, we entered into a
Registration Rights Agreement with the Purchasers (the “Registration Rights
Agreement”) pursuant to which we agreed, at our expense (other than to pay the
initial filing expense which will be paid by the Purchasers), generally to
promptly file, process and keep open a registration statement under the
Securities Act covering all shares that are or may be issued upon conversions of
the Notes or exercises of the Warrants, and to qualify resales of such shares
under certain state securities laws. If the registration statement is
not timely filed, it does not become effective within a specified time or its
effectiveness is not maintained as specified in the agreement, we may owe
liquidated damage amounts to the Purchasers.
Under the
Securities Purchase Agreement, if we meet three specified operating benchmarks
during the first twelve months after the closing of the first Note purchase, an
additional $1,500,000 in Note purchases (without Warrants) can be requested by
us from the Purchasers. The determination of whether we have met the
benchmarks is solely at the discretion of the Purchasers. If the
benchmarks are determined to have been achieved, then we can require the
Purchasers to make the additional $1,500,000 of Note purchases. If
such benchmarks are not attained in the 12-month period, then the Purchasers, in
their discretion, during the next two months may elect to purchase up to
$1,500,000 of Notes (without Warrants) having an initial conversion price which
is 25% higher than the conversion price in the original Notes.
Business
of AtheroNova Inc.
General
Overview
Immediately
prior to the Closing, we were a public “shell” company with nominal
assets. As a result of the Merger, we are solely engaged in
AtheroNova Operations’ business. With respect to this discussion, the
terms “we,” “us,” “our” and “our company” refer to AtheroNova Inc., a Delaware
corporation and its wholly-owned subsidiary AtheroNova Operations, Inc., a
Delaware corporation.
21
We have
developed intellectual property, covered by our pending patent applications,
which uses certain pharmacological compounds uniquely for the treatment of
atherosclerosis, which is the primary cause of various cardiovascular
diseases. Atherosclerosis occurs when cholesterol or fats are
deposited and harden as plaques in the walls of arteries. This
hardening reduces the space within the arteries through which blood can
flow. The plaque can also rupture and greatly restrict or block
altogether blood flow. Through a process called delipidization, such
compounds dissolve the plaques so they can be eliminated through normal body
processes and avoid such rupturing. Such compounds may be used both
to treat and prevent atherosclerosis.
In the
near future, we plan to continue studies and trials to demonstrate the efficacy
our IP. Ultimately, we plan to license our technology to various
licensees throughout the world who may use it in treating or preventing
atherosclerosis and other medical conditions or sublicense the IP to other such
users. Our licensees may also produce, market or distribute products
which utilize or add our compounds and technology in such treatment or
prevention.
Atherosclerosis
Atherosclerosis
(from the Greek words “athero” (gruel or paste) and “sclerosis” (hardness)) is a
common disease of the arteries. It occurs when organic materials,
primarily cholesterol (the waxy, fat-like material found in all parts of the
body) or fats, are deposited and harden in the walls of
arteries. This may occur when such materials accumulate under a
fibrotic cap or at a tear in the inner lining of an artery.
As the
deposits harden, they can restrict and occlude the area through which the blood
can flow through an artery, thereby reducing the amount of blood made available
to organs and other parts of the body. Restricted blood flow in the
arteries, such as to the heart muscle, can lead to symptoms such as chest
pain. It also can cause tissues to receive inadequate oxygen, which
is directly related to a number of circulatory disorders. For
example, arteriosclerosis of the extremities is a disease of the peripheral
blood vessels that is characterized by narrowing and hardening of the arteries
that supply the arms, legs and feet. The narrowing of the arteries
causes a decrease or cessation in blood flow. Symptoms include pain,
numbness, cold tissues and hypoxia resulting in cellular death.
Hardened
plaques can also rupture and dislodge from an artery’s walls, and then greatly
restrict or block altogether blood flow through that or other
arteries. This can lead to heart attacks and other severe
disorders. For example, strokes can be caused when ruptured plaques
in a neck artery impede the flow of blood to parts of the brain and decrease
brain functions.
Cardiovascular
disease is the leading cause of morbidity, disability and mortality in
industrialized countries, and atherosclerosis is its main underlying
pathology.
22
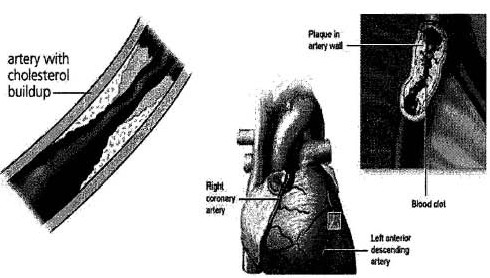
Our
Technology
Our
company has developed technology, covered by our patent applications, which uses
certain pharmacological compounds for the treatment of
atherosclerosis. Through a process called delipidization, such
compounds dissolve plaques in artery walls so they are removed through normal
body processes. The compounds go through the atherosclerotic fibrous
cap and, through delipidization, cause rapid reduction in the size of the
deposits of soft vulnerable plaque in an artery’s walls. We also
believe that the artery walls, once delipidized, will undergo a marked reduction
in inflammation and ultimately undergo a significant restoration of their
integrity. The compounds can be used to reverse the effects of
existing atherosclerosis by widening the area in an artery through which the
blood flows and avoiding the rupturing and dislodging of chunks of the hardened
plaque. The compounds can also be used to prevent significant plaque
buildups in arteries from occurring.
Our
intellectual property for treating atherosclerosis began with the ideas and
research of our two largest stockholders, Dr. Giorgio Zadini and Dr. Filiberto
Zadini, who assigned their related IP rights to Z&Z Medical Holdings, Inc.,
a Nevada corporation and AtheroNova Operations’ predecessor (“Z&Z Nevada”),
in December 2006. That IP was supplemented by subsequent research and
unique in vitro (nonliving) experiments which demonstrated that our chosen
compounds, through delipidization, cause substantial rapid regression of
artherosclerotic plaques. Such demonstration was the confirmation of
a long process of critical reasoning and evaluation of the properties of various
compounds suspected to be effective on the basis of a thorough medical
literature search. Indeed, there is much clinical evidence
accumulated to date in the world medical literature that supports our clinical
premise. Our research experiments are unique in the sense that we
have found no record in medical literature of equivalent experiments
demonstrating substantial regression of atherosclerotic plaques in vitro being
achieved, let alone utilizing a virtually non-toxic compound or class of
compounds such as is used in our technology.
The
compounds used in our technology have a history of approval for use in humans by
regulatory government agencies in a large number of developed countries
throughout the world, including Germany, England, France, and Italy, and have
been used in humans throughout the world, including in the USA, for their
medical indications. The existing human safety record for this class
of compounds, at higher concentrations than we required in our initial research,
is well established for uses other than those which we have claimed in our
IP. Use of the compounds in such other medical configurations, though
approved, is limited to non-competing clinical applications that cannot be
diverted or used off-label for the uses covered by our patent
applications.
23
Our
objective is to conduct animal and clinical trials of our technology at leading
academic research centers under the supervision of recognized researchers and
clinicians. We are currently in the final stages of documenting the
results of our first laboratory study of our atherosclerosis technology, a
proof-of-concept animal study performed at the University of California at Los
Angeles (“UCLA”) in its Atherosclerosis Research Laboratory. We feel
that the results are significant in demonstrating the positive effects
recognized in our prior research and experiments. We shortly plan to
commence two progressive laboratory studies of the technology at Cedars-Sinai
Medical Center in Los Angeles, California and in cooperation with
UCLA. These studies are expected to cost approximately $400,000 and
take 6-8 months to complete, and an additional 3–4 months to determine the final
results.
Over
time, we have approached a number of well known physician/scientist
atherosclerosis researchers regarding our ideas for treating atherosclerosis and
our technology. We entered into non-disclosure agreements with them
and their institutions. Several of these researchers have joined our
Medical Advisory Board.
As noted
above, the delipidization properties of our chosen pharmacological compounds
have been demonstrated in various scientific papers. But we have
filed several applications seeking patents in the United States and under the
international Patent Cooperation Treaty for our uses of such compounds to cause
delipidization of atherosclerotic plaques based on those being “Novel
Uses.” Those applications include known existing delivery methods for
such uses of the compounds as well as several novel delivery
methods. Our applications also extend to potential new synthesized
derivatives of any of such pharmacological compounds. Our
phytochemical (substances appearing naturally in plants) patent applications
apply to, cover and protect “novel use” of an entire class of phytochemical
compounds and new combinations of phytochemical preparations, as well as the
delivery methods included and covered in our IP.
To
protect our IP, we have entered into, and will enter into, confidentiality
agreements with persons to whom we disclose confidential aspects of our
technology who are not required by law to protect such
confidentiality. We further have obtained, and will obtain, covenants
from persons involved in the development of our technology, not only to maintain
its confidentiality, but also to assign or license related IP rights to
us.
Other
Possible Medical Applications
Besides
applications in atherosclerosis, delipidization has significant applications in
other medical fields. The delipidization of subcutaneous fat has been
scientifically demonstrated by researchers at a leading U.S. academic
institution, and was achieved utilizing one of the compounds determined by us to
be an effective delipidizing compound. This has possibly significant
implications for use in the field of clinical cosmesis, for which we have
developed certain IP for delipidization application to unwanted subcutaneous fat
through transdermal delivery.
There are
other promising potential areas of application for our IP that merit further
exploration and testing. We believe that systemic application of our
delipidizing pharmacological compounds may have beneficial effects in the
treatment of obesity and some of the disorders associated with obesity such as
hypertension, diabetes, etc. We also believe that cleansing the lipid
buildup from the small peripheral vessels in the body via delipidization will
have beneficial effects on overall human physiology and well-being.
24
Business
Plan
As noted
above, cardiovascular disease is the leading cause of morbidity, disability and
mortality in industrialized countries, and atherosclerosis is its main
underlying pathology. Atherosclerosis-related disease occurs in all
age and socio-economic groups, with approximate equivalent distribution between
the sexes, but with higher rates of prevalence and severity in minority
populations. Treatments available to date have only barely slowed the
growth rate of this disease. We believe our technology has the
potential to significantly reduce the incidence and severity of atherosclerosis
and that there is a vast potential market for its applications.
We plan
to exploit our IP primarily by entering into various licenses with strategically
selected licensees throughout the world. Such licensees may use our
compounds and technology in treating or preventing atherosclerosis and other
medical conditions or sublicense the IP to other such users. Such
compounds are expected to be suitable for various delivery methods including
parenteral, oral, transdermal and in-loco (through intra-arterial
catherization). Our licensees may also produce, market or distribute
products which utilize or add our compounds and technology in such treatment or
prevention. Also, we anticipate that some of our licensees may couple
our pharmacological compounds within their current commercial offerings to
extend their patent lives with existing statin therapeutics or in-loco drug
cluting technologies. We believe that, through such licensing, we can
achieve significant revenues while maintaining modest staffing and
infrastructure.
Many
clinical uses by licensees of our technology will require regulatory approvals
that will require further animal or clinical trials. However, uses by
our licensees in less regulated over-the-counter markets, such as in
phytochemical or neutraceutical products, may commence sooner.
The
ultimate target audience for applications of our technology will include both
prescribing physicians and other health providers and patient
consumers. Such consumers will include patients who have symptoms of
atherosclerosis as well as persons who do not have such symptoms but have high
risk profiles to develop atherosclerosis. Such consumers may be key
potential influencers and advocates for uses of our technology.
Competition
The
global medical industry presently, through many large and small health care
providers and other vendors of goods and services, generates substantial cash
flows directly related to the treatment of symptomatic atherosclerotic
disease. The clinical applications of our IP are expected to be a
novel class of pharmacological compounds for treating and preventing
atherosclerosis, suitable for parenteral, oral, transdermal and in-loco methods
of delivery. The therapeutic applications of our IP within such a
variety of clinical modalities are likely to be both synergistic and disruptive
to the types of clinical care presently applied within the
atherosclerosis-related markets. The technology or service companies
involved in such markets may be diminished, substantially disrupted, and in some
cases obviated by our technology. We anticipate that the most heavily
affected companies may be prime targets for the licensing of our
IP.
Existing
markets sectors, with their approximate annual cash flows, which may be subject
to obviation or disruption by our technology include Serum Screening ($3
Billion), Imaging ($12 Billion), Diagnostic Catheterizations ($12 Billion),
Statin Drug Therapies ($10 Billion) and Drug Eluting Stents ($6
Billion). In addition, incalculable investment dollars are applied to
emerging therapeutic technologies for cardiovascular diseases.
25
Employees
As of
June 23, 2010, we had 2 full-time employees and no part-time
employees. Since inception, we have never had a work stoppage, and
our employees are not represented by a labor union. We consider our
relationship with our employees to be positive.
Government
Regulation
Pharmaceutical
companies are subject to extensive regulation by national, state and local
agencies in the countries in which they do business. Of particular
importance is the Food and Drug Administration (“FDA”) in the
U.S. The FDA has jurisdiction over the pharmaceutical business and
administers requirements covering the testing, safety, effectiveness,
manufacturing, labeling, marketing, advertising, and post-marketing surveillance
of pharmaceutical products. In addition, we or our licensees may be
subject to the jurisdiction of various other federal regulatory and enforcement
departments and agencies, such as the Department of Health and Human Services,
the Federal Trade Commission and the Department of
Justice. Individual states, acting through their attorneys general,
have become active as well, seeking to regulate the marketing of prescription
drugs under state consumer protection and false advertising laws. The
FDA and other governing regulatory bodies could enact new regulations or take
actions which are against the industry or our IP applications in the medical and
pharmaceutical industries, or otherwise adversely affect our licensees or our
business.
DESCRIPTION
OF PROPERTY
Our
principal executive offices are located at 2301 Dupont Drive, Suite 525, Irvine,
California 92612. As of May 1, 2010, AtheroNova Operations entered
into a-month-to-month sublease of approximately 1,200 square feet of office
space at that address, for which AtheroNova Operations will pay approximately
$2,100 per month. The sublease is between AtheroNova Operations and
PhyGen LLC, an unrelated medical device company for which Mr. Thomas W. Gardner,
our Chief Executive Officer, also serves as Chief Executive
Officer. Our telephone number is (949) 476-1100.
LEGAL
PROCEEDINGS
We are
not currently party to any legal proceedings, the adverse outcome of which, in
management’s opinion, individually or in the aggregate, would have a material
adverse effect on our results of operations or financial position.
26
MARKET
PRICE OF AND DIVIDENDS ON THE REGISTRANT’S
COMMON
EQUITY AND RELATED STOCKHOLDER MATTERS
Common
Stock
Our
common stock is quoted on the OTC Bulletin Board under the symbol
“AHRO.” The following table sets forth, for the periods indicated,
the high and low bid information for our common stock, as determined from
sporadic quotations on the OTCBB. The information has been adjusted
to reflect a 1-for-200 reverse stock split of our common stock which took effect
on June 23, 2010, after the periods presented. The following
quotations reflect inter-dealer prices, without retail mark-up, mark-down or
commission and may not represent actual transactions.
|
High
|
Low
|
|||
|
Year
Ended December 31, 2008
|
||||
|
First
Quarter
|
$14.00
|
$6.00
|
||
|
Second
Quarter
|
$10.00
|
$6.00
|
||
|
Third
Quarter
|
$10.00
|
$6.00
|
||
|
Fourth
Quarter
|
$ 6.00
|
$4.00
|
||
|
Year
Ended December 31, 2009
|
||||
|
First
Quarter
|
$ 4.00
|
$4.00
|
||
|
Second
Quarter
|
$ 4.00
|
$4.00
|
||
|
Third
Quarter
|
$10.00
|
$4.00
|
||
|
Fourth
Quarter
|
$30.00
|
$4.00
|
||
|
Year
Ended December 31, 2010
|
||||
|
First
Quarter
|
$ 8.00
|
$4.00
|
On June
23, 2010, the closing sales price of our common stock as reported on the OTCBB
was $41.00 per share (accounting for the 1-for-200 reverse stock
split). As of June 23, 2010, there were approximately 20 record
holders of our common stock.
Dividends
We have
never paid dividends on our common stock. We intend to retain our
future earnings to re-invest in our ongoing business.
Equity
Compensation Plan Information
We had no
options outstanding as of December 31, 2009.
2010
Stock Incentive Plan
Our 2010
Stock Incentive Plan (the “Plan”) was adopted and became effective on May 21,
2010. A total of 4,362,964 shares of our common stock remain reserved
for issuance upon exercise of awards granted under the Plan. Any
shares of our common stock subject to an award, which for any reason expires or
terminates unexercised, are again available for issuance under the
Plan.
The Plan
will terminate as to grants of awards after 10 years from the effective date,
unless it is terminated earlier by our board of directors. The Plan
authorizes the award of stock options, stock purchase grants, stock appreciation
rights and stock units.
27
General;
Types of Awards
The Plan
provides for the grant of options to purchase shares of common stock, restricted
stock, stock appreciation rights (“SARs”) and restricted stock units (rights to
receive, in cash or stock, the market value of one share of our commons
stock). Incentive stock options (“ISOs”) may be granted only to
employees. Nonstatutory stock options and other stock-based awards
may be granted to officers, employees, non-employee directors and
consultants.
Administration
The Plan
will be administered by our board of directors or a committee of our board of
directors (the “Administrator”), as provided in the Plan. The
Administrator will have the authority to select the eligible participants to
whom awards will be granted, to determine the types of awards and the number of
shares covered and to set the terms, conditions and provisions of such awards,
to cancel or suspend awards under certain conditions, and to accelerate the
exercisability of awards. The Administrator will be authorized to
interpret the Plan, to establish, amend, and rescind any rules and regulations
relating to the Plan, to determine the terms of agreements entered into with
recipients under the Plan, and to make all other determinations that may be
necessary or advisable for the administration of the Plan. Our board
of directors may at its discretion delegate the responsibility for administering
the Plan to any committee of the board of directors.
Eligibility
Options
and other awards may be granted under the Plan to directors, officers, employees
and consultants of our company and any of our subsidiaries, provided that the
services of such consultants are not in connection with the offer or sale of
securities in a capital-raising transaction and do not directly or indirectly
promote or maintain a market for our securities. At the date of this
prospectus, all of our officers, directors, employees and consultants would have
been eligible to receive awards under the Plan.
Stock
Option and SAR Grants
The
exercise price per share of our common stock purchasable upon exercise of any
stock option or SAR will be determined by the Administrator, but cannot in any
event be less than 100% of the fair market value of our common stock on the date
the option is granted. The Administrator will determine the term of
each stock option or SAR (subject to a maximum term of 10 years) and each option
or SAR will be exercisable pursuant to a vesting schedule determined by the
Administrator. The grants and the terms of ISOs will be restricted to
the extent required for qualification as ISOs by the U.S. Internal Revenue Code
of 1986, as amended. Subject to approval of the Administrator,
options or SARs may be exercised by payment of the exercise price in cash,
shares of common stock or pursuant to a “cashless exercise” through a
broker-dealer under an arrangement approved by the Administrator. The
Administrator may require the grantee to pay to us any applicable withholding
taxes that we are required to withhold with respect to the grant or exercise of
any option. The withholding tax may be paid in cash or, subject to
applicable law, the Administrator may permit the grantee to satisfy these
obligations by the withholding or delivery of shares of our common
stock. We may withhold from any shares of our common stock that may
be issued pursuant to an option or from any cash amounts otherwise due from us
to the recipient of the option an amount equal to such taxes.
28
Restricted
Stock Grants
Restricted
shares may be sold or awarded for consideration determined by the Administrator,
including cash, full-recourse promissory notes, as well as past and future
services. Any award of restricted shares will be subject to a vesting
schedule determined by the Administrator. Any restricted shares that
are not vested will be subject to rights of repurchase, rights of first refusal
or other restrictions as determined by the Administrator. In general,
holders of restricted shares will have the same voting, dividend and other
rights as our other stockholders.
Adjustments
In the
event of any change affecting shares of our common stock by reason of any stock
dividend or split, recapitalization, merger, consolidation, spin-off,
combination or exchange of shares or other similar corporate change, or any
distribution to stockholders other than cash dividends, the Administrator will
make substitutions or adjustments in the aggregate number of shares that may be
distributed under the Plan, and in the number and types of shares subject to,
and the exercise prices under, outstanding awards granted under the Plan, in
accordance with Section 10 and other provisions of the Plan.
Transferability
Unless
otherwise permitted by the Plan and approved by the Administrator as permitted
by the Plan, no award will be assignable or otherwise transferable by the
grantee other than by will or the laws of descent and distribution and, during
the grantee’s lifetime, an award may be exercised only by the
grantee.
Amendment
and Termination
Our board
of directors may amend the Plan in any and all respects without stockholder
approval, except as such stockholder approval may be required under applicable
law or pursuant to the listing requirements of any national market system or
securities exchange on which our equity securities may be listed or quoted, and
except that stockholder approval shall be required for any amendment that
would: (a) increase the maximum number of shares for which awards may
be granted under the Plan; (b) reduce the price at which stock options or SARs
may be granted; (c) extend the term of the Plan; or (d) change the class of
persons eligible to be participants.
Unless
sooner terminated by our board of directors, the Plan will terminate as to
further grants of awards on May 20, 2020.
29
MANAGEMENT’S
DISCUSSION AND ANALYSIS OF
FINANCIAL
CONDITION AND RESULTS OF OPERATIONS
This
discussion summarizes the significant factors affecting the operating results,
financial condition and liquidity and cash flows of AtheroNova Operations for
the fiscal years ended December 31, 2009 and 2008 and three months ended March
31, 2010 and 2009. The discussion and analysis that follows should be
read together with the financial statements of AtheroNova Operations and the
notes to the financial statements included elsewhere in this
prospectus. Except for historical information, the matters discussed
in this Management’s Discussion and Analysis of Financial Condition and Results
of Operations are forward looking statements that involve risks and
uncertainties and are based upon judgments concerning various factors that are
beyond our control.
Overview
We have
developed IP, covered by our pending patent applications, which uses certain
pharmacological compounds uniquely for the treatment of atherosclerosis, which
is the primary cause of various cardiovascular
diseases. Atherosclerosis occurs when cholesterol or fats are
deposited and harden as plaques in the walls of arteries. This
hardening reduces the space within the arteries through which blood can
flow. The plaque can also rupture and greatly restrict or block
altogether blood flow. Through a process called delipidization, such
compounds dissolve the plaques so they can be eliminated through normal body
processes and avoid such rupturing. Such compounds may be used both
to treat and prevent atherosclerosis.
In
December 2006, Z&Z Medical Holdings, Inc. was formed as a Nevada corporation
with the contributed intellectual property from Giorgio Zadini and Filiberto
Zadini. During 2007, Z&Z Nevada sought various sources of working
capital via an offering memorandum first prepared in November 2007, in which up
to 1,500,000 shares were offered at $0.50 per share while it continued to
perfect the patent applications previously filed. Concurrently,
Z&Z Nevada was designing its clinical trial protocol and held discussions
with various institutions about conducting a clinical animal study to test
Z&Z Nevada’s conclusions reached in unique in vitro
experiments.
During
2008, Z&Z Nevada signed an agreement with the University of California, Los
Angeles and Dr. Aldons “Jake” Lusis for the initial clinical study in
vivo. The cost of the study was set at $200,000 for all work
associated with the trial. A clinical protocol was established and
the study spanned a period of 30 weeks, with the trial concluding in October,
2009. We expect the publication of the results of the study sometime
in the 3rd quarter of 2010.
Also
during 2008, Z&Z Nevada raised working capital of $225,000 from sales of
securities pursuant to its offering memorandum from various private sources at
$0.50 per share to start its operations, intellectual property work and clinical
research.
During
2009, Z&Z Nevada continued its research and intellectual property work and
raised an additional $100,000 from various private sources from sales of
securities pursuant to its offering memorandum at $0.50 per
share. From 2007 through 2009 Z&Z Nevada attempted to obtain
larger amounts of working capital without success. Certain potential
investors expressed dissatisfaction with Z&Z Nevada’s status as a Nevada
corporation. As a result Z&Z Nevada’s board of directors chose to
reincorporate in Delaware pursuant to a merger with and into AtheroNova
Operations in 2010.
30
During
late 2009 Z&Z Nevada was introduced to KOM Capital Management, LLC, a
private equity fund based in New York City (“KOM”), and on November 4, 2009
Z&Z Nevada and KOM signed a Letter of Intent for the company to merge with a
subsidiary of our company whereby a) the holders of Z&Z Nevada’s securities
would obtain approximately 98% of our outstanding shares and b) KOM would
purchase $3,000,000 of our 2.5% Senior Secured Convertible Notes with an
additional $3,000,000 purchasable if certain operating benchmarks were achieved
in the ensuing 24 months, and would receive in connection therewith, warrants to
purchase 100% of the shares of our common stock issuable upon conversion of such
notes.
In 2010,
during the due diligence period, Z&Z Nevada raised additional working
capital of $225,000 from the final sales of its securities pursuant to the
offering memorandum at $0.50 per share.
Events
within the capital markets and internal to KOM resulted in the amendment of the
Letter of Intent with KOM to reduce the initial purchase of 2.5% Senior Secured
Convertible Notes to $1,500,000 with 50% warrant coverage, with an additional
$1,500,000 (without warrants) purchasable for up to 14 months following the
initial purchase of such notes. As a result of subsequent
negotiations, AtheroNova Operations consummated the Capital Raise Transaction
with the Purchasers.
General
Operating
expenses consist primarily of payroll and related costs and corporate
infrastructure costs. We expect that our operating expenses will
increase as we finalize clinical testing and continue executing our business
plan, in addition to the added costs of operating as a public
company.
Historically,
we have funded our working capital needs primarily through the sale of shares of
our capital stock.
The
Merger was accounted for as a reverse merger (recapitalization) with AtheroNova
Operations deemed to be the accounting acquirer, and our company deemed to be
the legal acquirer. Accordingly, the following discussing represents
a discussion of the operations of our wholly-owned subsidiary, AtheroNova
Operations for the periods presented.
Results
of Operations
Year
Ended December 31, 2009 Compared with Year Ended December 31,
2008
Revenue
AtheroNova
Operations did not recognize any revenue for the years ended December 31, 2009
and 2008, respectively.
Cost
of Sales
AtheroNova
Operations did not recognize any revenue for the years ended December 31, 2009
and 2008, therefore, did not record any cost of sales in those years,
respectively.
Selling,
General and Administrative Expenses
Selling,
general and administrative expenses were $12,453 and $175,182 for the years
ended December 31, 2009 and 2008, respectively. The decrease in
selling, general and administrative expenses was due to a large progress payment
made in 2008 upon the signing of the clinical trial contract and reversal of
operating expenses in 2009 due to the forgoing of expense debentures originally
recorded in 2008.
31
Interest
Income, Net
Interest
income declined to $130 in 2009 compared to $1,560 in 2008 due to a decline in
cash held in an interest earning saving account.
Three
Months Ended March 31, 2010 Compared with Three Months Ended March 31,
2009
Revenue
AtheroNova
Operations did not recognize any revenue for the three months ended March 31,
2010 and 2009, respectively.
Cost
of Sales
AtheroNova
Operations did not recognize any revenue for the three months ended March 31,
2010 and 2009, therefore, did not record any cost of sales in those three-month
periods, respectively.
Selling,
General and Administrative Expenses
Selling,
general and administrative expenses were $32,633 and $3,163 for the three months
ended March 31, 2010 and 2009, respectively.
Interest
Income, Net
Interest
income declined to $6 in the three months ended March 31, 2010 compared to $108
in the three months ended March 31, 2009 due to a decline in cash held in an
interest earning saving account.
Liquidity
and Capital Resources
Net cash
provided by operating activities was $961 and $24,954 in the years ended
December 31, 2009 and 2008, respectively. The decrease in the amount
of cash provided was largely due to increasing balances in accounts
payables.
Net cash
used in operating activities the three months ended March 31, 2010 was $230,770
due to the operating loss and the cancellation of the Note payable to related
parties, which was a non-cash transaction. Net cash provided in
operations in the three months ended March 31, 2009 was $14,268 due to the
operating loss.
Net cash
used by investing activities was $214,284 and $158,584 in calendar years 2009
and 2008, respectively as the Company continued to use funds to develop and
solidify its intellectual property position.
Net cash
used by investing activities was $1,042 and $52,323 in the three months ended
March 31, 2010 and 2009, respectively as the Company purchased equipment in the
current year and used funds to develop intellectual property in
2009.
Net cash
provided by financing activities was $150,000 and $225,000 in calendar years
2009 and 2008, respectively. Cash provided by financing activities in
both years include funds raised by AtheroNova Operations from working capital
stock sale activity. In 2009, professional services with a value of
$50,000 were provided in exchange for common stock.
32
Net cash
provided by financing activities was $327,500 and $0 in the three months ended
March 31, 2010 and 2009 respectively. Cash provided by financing activities in
2010 include funds raised by AtheroNova Operations from working capital stock
sale activity in the amount of $225,000 and compensation for professional
services in exchange for common stock in the amount of $102,500. No
financing activity occurred in the three months ended March 31,
2009.
AtheroNova
Operations has suffered recurring losses from operations and has an accumulated
deficit of $385,945 and $373,622 in calendar years 2009 and 2008,
respectively. As a development stage company, we are in need of
generating significant cash resources to achieve our future strategic
plan. We consequently consummated the Capital Raise Transaction on
May 13, 2010. Part of the cost of the Capital Raise Transaction
include costs associated with becoming and remaining a public company and filing
and maintaining Registration Statements necessary to comply with our covenants
in the Capital Raise Transaction. Even with the proceeds we generated
from the Capital Raise Transaction, we anticipate that our existing cash and
cash equivalents will not be sufficient to fund our business needs for more than
15 months and expect to have to raise significant additional
capital. Our ability to continue our operations may prove to be more
expensive than we currently anticipate and we may incur significant additional
costs and expenses in connection therewith.
Going
Concern Uncertainties
As of the
date of AtheroNova Operations’ annual report, there is doubt regarding our
ability to continue as a going concern as we have not generated sufficient cash
flow to fund our business operations and loan commitments. Our future
success and viability, therefore, are dependent upon our ability to generate
capital financing. The failure to generate sufficient revenues or
raise additional capital may have a material and adverse effect upon AtheroNova
Operations and our stockholders.
Off-Balance
Sheet Arrangements
AtheroNova
Operations currently does not have any off-balance sheet arrangements or
financing activities with special purpose entities.
Critical
Accounting Policies and Estimates
This
Management’s Discussion and Analysis of Financial Condition and Results of
Operations section discusses AtheroNova Operations’ financial statements, which
have been prepared in accordance with accounting principles generally accepted
in the United States. To prepare these financial statements,
management must make estimates and assumptions that affect the reported amounts
of assets and liabilities. These estimates also affect its reported
revenues and expenses. On an ongoing basis, management evaluates its
estimates and judgment, including those related to revenue recognition, accrued
expenses, financing operations and contingencies and
litigation. Management bases its estimates and judgment on historical
experience and on various other factors that are believed to be reasonable under
the circumstances, the results of which form the basis for making judgments
about the carrying value of assets and liabilities that are not readily apparent
from other sources. Actual results may differ from these estimates
under different assumptions or conditions. The following represents a
summary of AtheroNova Operations’ critical accounting policies, defined as those
policies that it believes are the most important to the portrayal of its
financial condition and results of operations and that require management’s most
difficult, subjective or complex judgments, often as a result of the need to
make estimates about the effects of matters that are inherently
uncertain.
33
Research
and Development Costs
Research
and development costs consist of expenditures for the research and development
of new products and technology. Research and development costs are
expensed as incurred.
Intangible
and Long-Lived Assets
In
accordance with ASC 350-30 (formerly SFAS No. 144, Accounting for the Impairment
or Disposal of Long-Lived Assets), AtheroNova Operations evaluates long-lived
assets for impairment whenever events or changes in circumstances indicate that
their net book value may not be recoverable. When such factors and
circumstances exist, AtheroNova Operations compares the projected undiscounted
future cash flows associated with the related asset or group of assets over
their estimated useful lives against their respective carrying
amount. Impairment, if any, is based on the excess of the carrying
amount over the fair value, based on market value when available, or discounted
expected cash flows, of those assets and is recorded in the period in which the
determination is made. AtheroNova Operations’ management currently
believes there is no impairment of its long-lived assets. There can
be no assurance, however, that market conditions will not change or demand for
its products under development will continue. Either of these could
result in future impairment of long-lived asset.
Common
Stock and Common Stock Warrants
AtheroNova
Operations uses the fair value recognition provision of ASC 718,
“Compensation-Stock Compensation,” which requires AtheroNova Operations to
expense the cost of employee services received in exchange for an award of
equity instruments based on the grant date fair value of such
instruments. AtheroNova Operations uses the Black-Scholes option
pricing model to calculate the fair value of any equity instruments on the grant
date. AtheroNova Operations also uses the provisions of ASC 505-50,
“Equity Based Payments to Non-Employees,” to account for stock-based
compensation awards issued to non-employees for services. Such awards
for services are recorded at either the fair value of the services rendered or
the instruments issued in exchange for such services, whichever is more readily
determinable, using the measurement date guidelines enumerated in ASC
505-50.
Recent
Accounting Pronouncements
Note 2 to
AtheroNova Operations’ financial statements for the years ended December 31,
2009 and 2008 sets forth certain accounting pronouncements that are applicable
to its financial statements.
34
DIRECTORS,
EXECUTIVE OFFICERS, PROMOTERS AND CONTROL PERSONS
Executive
Officers and Directors
The
following table sets forth the names, positions and ages of our current
executive officers and directors. All directors serve until the next
annual meeting of stockholders or until their successors are elected and
qualified. Officers are appointed by the board of directors and their
terms of office are, except to the extent governed by an employment contract, at
the discretion of the board of directors.
|
Name
|
Age
|
Position
|
||
|
Thomas
W. Gardner (1)
|
56
|
Chief
Executive Officer, President and Director
|
||
|
Mark
Selawski (1)
|
54
|
Chief
Financial Officer and Secretary
|
||
|
Filiberto
Zadini, MD (1)
|
65
|
Director
|
||
|
Boris
Ratiner, MD (1)
|
42
|
Director
|
||
|
Chaim
Davis (1)
|
30
|
Director
|
||
|
Gary
Freeman (2)
|
42
|
Director
|
(1) These
persons were appointed to their respective positions effective May 13,
2010.
(2) Mr.
Freeman continued as one of our directors following the Closing.
Thomas W. Gardner, 56, has
been the Chief Executive Officer, the President and a director of AtheroNova
Operations since its formation in December 2009. He held the same
positions with Z&Z Medical Holdings, Inc., a Nevada corporation and the
predecessor in interest to AtheroNova Operations (“Z&Z Nevada”) from
December 2006 until its merger into AtheroNova Operations in March
2010. Since September 2008, he also has been the President of PhyGen
LLC, which designs, manufactures and sells instruments and implants for spine
surgery. He is a senior medical industry executive with twenty-six
years experience in healthcare. He has extensive hands-on experience
with successful start-up ventures, having helped found six healthcare companies,
three of them that were publicly traded. He has served as
President/CEO of Urogen, a San Diego-based Biotech company, President of
Endocare, an Orange County-based urologic products company, President/CEO of
AutoCath, an Orange County based vascular access company, and Executive Vice
President of Medstone International, an Orange County medical products
company.
Mark Selawski, 54, joined
AtheroNova Operations and Z&Z Nevada in January 2010 as Chief Financial
Officer. He became the Secretary of AtheroNova Operations in March
2010. From 2004 to 2009 he served as Chief Financial Officer of
United Polychem, Inc., a privately held petrochemical distribution
company. From 1988 to 2004, he held several positions at Medstone
International, during the last 9 years being the Vice President-Finance, Chief
Financial Officer and Corporate Secretary. Medstone was a
NASDAQ-listed capital medical device manufacturer dedicated to urology
products. Before joining Medstone, he held various financial
positions with a number of manufacturing and high-tech companies in southern
California. He holds a Bachelor of Science in Accounting from Bowling
Green State University.
35
Filiberto Zadini, MD, 65, has
been a director of AtheroNova Operations since December 2009. He was
a director and V.P. of Research & Development for Z&Z Nevada from
December 2006 until March 2010. He is one of the co-inventors of the
core technologies of AtheroNova Operations. He had a past training in
Neurosurgery in Italy and is currently in private general medicine practice in
the San Fernando Valley in southern California.
Boris Ratiner, MD, 42, has
been a director of AtheroNova Operations since December 2009. He was
a director of Z&Z Nevada from December 2006 until March 2010. He
received an Advanced Bachelors degree in Chemistry at Occidental College in Los
Angeles. He then attended Medical School at LSU in New Orleans,
followed by an Internal Medicine Residency and Rheumatology Fellowship at the
University of California San Francisco (UCSF). He is Board Certified
in Internal Medicine and Rheumatology and is in private practice in Tarzana,
California. He is the medical director and founder of Rheumatology
Therapeutics, where he leads a team of 23 staff members that care for patients
with Arthritis and Autoimmune Diseases. He also serves on the board
of the San Fernando Valley Branch of the Arthritis Foundation and is the Program
Director for the Southern CA Rheumatism Society. He is a founder and
active board member of 4Medica, a successful medical informatics company that he
co-founded in 1999. He is also a Clinical Instructor of Medicine at
the David Geffen School of Medicine at the University of California Los Angeles
(UCLA), a teaching attendant with the Cedars-Sinai’s Division of Rheumatology
and an instructor at the Northridge Family Medicine Teaching
Program. He is an active clinical investigator and is actively
involved in trials of new medications for gout, lupus, rheumatoid arthritis,
osteoarthritis, psoriatic arthritis, ankylosing spondylitis and
fibromyalgia. He is published in peer-reviewed papers, abstracts and
textbooks. He is a frequent speaker at local hospitals to physicians
on Rheumatology related diseases. He has authored several book
chapters on osteoarthritis and research papers on Hepatitis C
arthritis.
Chaim Davis, 30, will serve as
a director appointee of the Purchasers under the terms and conditions of the
Voting Agreement entered into in connection with the Merger. He is
currently the Managing Partner of Revach Fund L.P., an investment fund focused
on life science industries. He is also currently serving as a
healthcare industry consultant to KOM (from November 2009) and to Gem Asset
Management (from February 2007). He served as an Account Executive at
Perry Davis & Associates from June 2004 through February 2007, and as a
Healthcare Analyst at The Garnet Group from April 2001 through June
2004. He received his bachelor’s degree from Columbia
University.
Gary Freeman, 42, has served
as one of our directors since July 2007. He will serve as a director
appointee of the Purchasers under the terms and conditions of the Voting
Agreement entered into in connection with the Merger. He is currently
a Partner in Beach, Freeman, Lim & Cleland’s Audit and Accounting services
division. In conjunction with various consulting engagements, he has
assumed interim senior level management roles at numerous public and private
companies during his career, including Co-President and Chief Financial Officer
of Trestle Holdings, Inc., Chief Financial Officer of Silvergraph International
and Chief Financial Officer of Galorath Incorporated. He served as a
member of the Board of Directors of Blue Holdings, Inc., Trestle Holdings, Inc.
and GVI Security Solutions, Inc. His previous experience includes ten
years with BDO Seidman, LLP, including two years as an Audit
Partner.
On May
13, 2010, Filiberto Zadini, Giorgio Zadini, Thomas W. Gardner, Boris Ratiner,
W-Net, Europa and MKM entered into the Voting Agreement pursuant to which such
parties became obligated, for four years, to vote for the directors determined
as described below. The authorized number of directors will be
seven. Those initially include three persons who before the Closing
were members of AtheroNova Operations’ board of directors—Thomas W. Gardner,
Boris Ratiner and Filiberto Zadini—whose replacements will be determined under
the terms of the Voting Agreement by the holders of a majority of the shares
held by the Z&Z Shareholders (Filiberto Zadini, Giorgio Zadini, Boris
Ratiner and Thomas W. Gardner). Two other directors will be Gary
Freeman, who is presently a member of our board of directors, and Chaim Davis,
and their replacements will be determined under the Voting Agreement by the
holders of a majority of the shares held by the Purchasers. The final
two directors, and their replacements, will be determined jointly by the holders
of a majority of the shares held by the Z&Z Shareholders and the holders of
a majority of the shares held by the Purchasers.
36
None of
the newly appointed officers or directors, nor any of their affiliates,
beneficially owned any of our equity securities or rights to acquire any of our
securities prior to the Closing, and no such persons have been involved in any
transaction with us or any of our directors, executive officers or affiliates
that is required to be disclosed pursuant to the rules and regulations of the
SEC, other than with respect to the transactions that have been described
herein. None of the newly appointed officers and directors have been
convicted in a criminal proceeding, excluding traffic violations or similar
misdemeanors, nor have they been a party to any judicial or administrative
proceeding during the past ten years, except for matters that were dismissed
without sanction or settlement, that resulted in a judgment, decree or final
order enjoining the person from future violations of, or prohibiting activities
subject to, federal or state securities laws, or a finding of any violation of
federal or state securities laws.
Director
Independence
Our Audit
Committee currently consists of Messrs. Davis and Freeman. Our Audit
Committee is responsible for selecting and engaging our independent accountant,
establishing procedures for the confidential, anonymous submission by our
employees of, and receipt, retention and treatment of concerns regarding
accounting, internal controls and auditing matters, reviewing the scope of the
audit to be conducted by our independent public accountants, and periodically
meeting with our independent public accountants and our chief financial officer
to review matters relating to our financial statements, our accounting
principles and our system of internal accounting controls. Our Audit
Committee reports its recommendations as to the approval of our financial
statements to our board of directors. The role and responsibilities
of our Audit Committee are more fully set forth in an amended and restated
written charter adopted by our board of directors on June 17,
2010. Our Audit Committee reviews and reassesses the Audit Committee
Charter annually and recommends any changes to our board of directors for
approval. We are not a “listed company” under SEC rules and are
therefore not required to have an audit committee comprised of independent
directors. We have, however, determined that Messrs. Davis and
Freeman are “independent” as that term is defined in Section 5605 of the
Marketplace Rules as required by the NASDAQ Stock Market.
Our
Compensation Committee currently consists of Messrs. Davis and
Freeman. Generally, our Compensation Committee is responsible for
considering and making recommendations to our board of directors regarding
executive compensation and for administering the Plan. The role and
responsibilities of our Compensation Committee are more fully set forth in a
written charter adopted by our board of directors on June 17,
2010. Our Compensation Committee reviews and reassesses the
Compensation Committee Charter annually and recommends any changes to our board
of directors for approval. We are not a “listed company” under SEC
rules and are therefore not required to have a compensation committee comprised
of independent directors. We have, however, determined that Messrs.
Davis and Freeman are “independent” as that term is defined in Section 5605 of
the Marketplace Rules as required by the NASDAQ Stock Market.
We do not
have a nominating committee or nominating committee charter for persons to be
proposed as directors for election to our board of directors. The
duties and functions performed by such committee are performed by the full board
of directors. We do not have any restrictions on stockholder
nominations under our certificate of incorporation, as amended, or
bylaws. The only restrictions are those applicable generally under
the Delaware General Corporation Law and the federal proxy
rules. Currently, our entire board of directors decides on nominees,
on the recommendation of one or more members of our board of
directors. We are not a “listed company” under SEC rules and are
therefore not required to have a nominating committee comprised of independent
directors. We have, however, determined that Messrs. Davis and
Freeman are “independent” as that term is defined in Section 5605 of the
Marketplace Rules as required by the NASDAQ Stock Market.
37
EXECUTIVE
COMPENSATION
Summary
Compensation Table
The following table and related footnotes
show the compensation paid during the fiscal years ended December 31, 2009 and
2008, to our named executive officers. No other executive officers
received salary and bonus in excess of $100,000 for the prior two fiscal
years.
Summary
Compensation Table
| Option |
All
Other
|
||||||||||||||||||||
|
Name
and Principal
|
Year
|
Salary
|
Bonus
|
Awards
|
Compensation
|
Total
|
|||||||||||||||
|
Position
|
($)
|
($)
|
($)
|
($)
|
($)
|
||||||||||||||||
|
Eric
Stoppenhagen (1)
|
2009
|
$
|
48,000
|
--
|
--
|
--
|
$
|
48,000
|
|||||||||||||
|
Interim
President & Secretary
|
2008
|
$
|
48,000
|
--
|
--
|
--
|
$
|
48,000
|
|||||||||||||
|
|
(1)
|
Mr.
Stoppenhagen served as our Interim President & Secretary from
September 2007 through May 13, 2010. Represents consulting fees
paid to Mr. Stoppenhagen’s company, Venor Consulting,
Inc.
|
On
September 27, 2007, we entered into a Consulting Agreement with Venor
Consulting, Inc. (“Venor”), a company owned by Mr.
Stoppenhagen. Under the terms of the Consulting Agreement, Venor
performed certain consulting services for us with respect to, among other
things, the provision of executive services (including, without limitation, the
services of Mr. Stoppenhagen) for a period of nine months. We paid
Venor a monthly fee for certain of the services to be provided, with additional
services to be billed at an hourly rate. We terminated this agreement
at the Closing.
AtheroNova
Operations paid no compensation to its officers during 2009 or
2008.
On
October 15, 2007, Z&Z Nevada entered into an employment agreement with Mr.
Gardner to assure his continued service to the company, which we assumed
pursuant to the Merger. The agreement runs for a term of 3 years,
expiring on October 15, 2010. The agreement provides for a base
salary of not less than $150,000 per year in the first year following the
receipt by the company of $2,000,000 in operating funds, $170,000 per year in
the second year following such event and $190,000 per year in the third year
following such event. The agreement provides for payment of 50% of
each aforementioned amount in the event of his inability to serve in his
position due to mental or physical incapacitation. The agreement
cannot be terminated for any other reason than fraud, misappropriation,
embezzlement or willful misconduct or because of any violation of the agreement
regarding confidentiality and protection of company secrets.
38
We are in
current negotiations with Mr. Gardner regarding a new contract in anticipation
of his prior commitment to serve as CEO of PhyGen LLC until the end of calendar
2010. It is anticipated that he will share executive management
duties for our company and AtheroNova Operations with Mr. Selawski during that
time.
On
January 7, 2010, Z&Z Nevada and AtheroNova Operations entered into two
agreements with Mr. Selawski. The first was a consulting contract
with Z&Z Nevada, which we assumed pursuant to the Merger, under which he was
to be paid $5,000 per month until such time as the company raises $1,000,000 in
operating funds. Concurrent with this agreement, Mr. Selawski was
awarded a stock grant of 5,000 shares of AtheroNova Operations’ common stock
which were converted into 11,215 shares of our common stock in connection with
the Merger. Upon closing of the Capital Raise Transaction, the second
agreement commenced under which Mr. Selawski will receive $144,000 per year,
subject to annual review by our board of directors. He is also
eligible to receive up to 10% of his annual compensation as a
bonus. Under this agreement Mr. Selawski will be an “at will”
employee and can be terminated with or without cause with 30 days
notice. On March 6, 2010, Mr. Selawski was granted stock options to
purchase up to 245,000 shares of AtheroNova Operations’ common stock at a
purchase price of $0.50 per share. We assumed these options in the
Merger which now entitle Mr. Selawski to purchase 549,498 shares of our common
stock at a purchase price of approximately $0.22 per share. Such
options vest 25% on January 6, 2011 and 75% evenly on a monthly basis over the
next three years thereafter and expire January 7, 2017.
We are in
current negotiations with Mr. Selawski regarding a new contract in anticipation
of his assumption of additional duties with us and AtheroNova Operations due to
Mr. Gardner’s prior commitment to serve as full-time President of PhyGen LLC
until the end of calendar 2010.
Outstanding
Equity Awards at Fiscal Year-End
We did
not grant options to our executive officers during 2009.
AtheroNova
Operations did not grant options to its executive officers during
2009.
Compensation
of Directors
We did
not pay compensation to our directors during 2009. Members of our
board of directors may be paid their expenses, if any, of attendance at a
meeting of our board of directors, and may be paid a fixed sum for attendance at
each meeting of our board of directors or a stated salary as a
director. No such payment shall preclude any director from serving us
in any other capacity and receiving compensation therefor except as otherwise
provided under applicable law.
AtheroNova
Operations did not pay compensation to its directors during 2009.
PRINCIPAL
AND SELLING STOCKHOLDERS
The
following table presents information regarding the beneficial ownership of our
common stock by the following persons both as of June 23, 2010 and as adjusted
to reflect the sale of the common stock in this offering by the selling
stockholders: (i) each executive officer and director, (ii) all executive
officers and directors as a group, (iii) each stockholder known to be the
beneficial owner of more than 5% of our outstanding common stock and (iv) each
selling stockholder.
39
Beneficial
ownership is determined in accordance with the rules of the SEC and generally
includes voting or investment power with respect to
securities. Unless otherwise indicated below, to our knowledge, the
persons and entities named in the table have sole voting and sole investment
power with respect to all shares beneficially owned, subject to community
property laws where applicable. Shares of our common stock subject to
options or warrants that are currently exercisable or exercisable within 60 days
of June 23, 2010 are deemed to be outstanding and to be beneficially owned by
the person holding the options for the purpose of computing the percentage
ownership of that person but are not treated as outstanding for the purpose of
computing the percentage ownership of any other person.
W-Net
Fund I, L.P., Europa International, Inc. and MKM Opportunity Master Fund, Ltd.,
the three selling stockholders, acquired the Notes convertible into, and
Warrants exercisable for, shares of our common stock on May 13, 2010, pursuant
to the Capital Raise Transaction, as further described in the Corporate History
subsection of the Business section of this registration statement.
The
information presented in this table is based on 22,680,927 shares of our common
stock outstanding on June 23, 2010. Unless otherwise indicated, the
address of each of the executive officers and directors and 5% or more
stockholders named below is c/o AtheroNova Inc., 2301 Dupont Drive, Suite 525,
Irvine, CA 92612.
|
Number
of Shares
Beneficially
Owned
Prior
to Offering
|
Number
of Shares
Beneficially
Owned
After
Offering
|
|||||||||||||||||||
|
Name
of Beneficial Owner
|
Number
|
Percentage
of
Shares
Outstanding
|
Number
of
Shares
Being
Offered
|
Number
|
Percentage
of
Shares
Outstanding
|
|||||||||||||||
|
Executive
Officers and Directors:
|
||||||||||||||||||||
|
Thomas
W. Gardner
|
3,158,044 | 13.9 | % | -- | 3,158,044 | 13.9 | % | |||||||||||||
|
Mark
Selawski
|
11,215 | * | -- | 11,215 | * | |||||||||||||||
|
Filiberto
Zadini, M.D.
|
6,078,122 | 26.8 | % | -- | 6,078,122 | 26.8 | % | |||||||||||||
|
Boris
Ratiner, M.D. (1)
|
2,915,704 | 12.1 | % | -- | 2,915,704 | 12.1 | % | |||||||||||||
|
Chaim
Davis
|
-- | -- | -- | -- | -- | |||||||||||||||
|
Gary
Freeman
|
-- | -- | -- | -- | -- | |||||||||||||||
|
All
directors and executive
officers as a group (2)
|
12,163,085 | 50.4 | % | -- | 12,163,085 | 50.4 | % | |||||||||||||
|
5%
Stockholders:
|
||||||||||||||||||||
|
Giorgio
Zadini
2237
Hilltop Lane
Camarillo,
CA 93012
|
6,078,122 | 26.8 | % | -- | 6,078,122 | 26.8 | % | |||||||||||||
|
Selling
Stockholders:
|
||||||||||||||||||||
|
W-Net
Fund I, L.P. (3)
12400
Ventura Blvd., Ste. 327
Studio
City, CA 91604
|
2,152,159 | 8.8 | % | 1,515,893 | 636,266 | 2.6 | % | |||||||||||||
|
Europa
International, Inc. (4)
1114
Avenue of the Americas
45th
Floor
New
York, NY 10036
|
2,152,159 | 8.8 | % | 1,515,893 | 636,266 | 2.6 | % | |||||||||||||
|
MKM
Opportunity Master Fund, Ltd. (5)
c/o
MKM Capital Advisors
1515
Broadway, 11th
Fl.
New
York, NY 10036
|
1,908,798 | 7.8 | % | 1,272,532 | 636,266 | 2.6 | % | |||||||||||||
|
TOTAL:
|
24,454,323 | 81.9 | % | 4,304,318 | 20,150,005 | 83.5 | % | |||||||||||||
40
|
*
|
Less
than 1%
|
|
(1)
|
Includes
1,457,852 shares of our common stock that may be acquired pursuant to the
exercise of warrants within 60 days of June 23,
2010.
|
|
(2)
|
Includes
1,457,852 shares of our common stock that may be acquired pursuant to the
exercise of warrants within 60 days of June 23,
2010.
|
|
(3)
|
Includes
1,272,532 shares of our common stock that may be acquired pursuant to the
conversion of a Note, and 636,266 shares of our common stock that may be
acquired pursuant to the exercise of a Warrant, within 60 days of June 23,
2010. The Note and the Warrant prohibit W-Net from converting
the Note or exercising the Warrant if after such conversion and/or
exercise W-Net would own more than 4.9% of our outstanding common
stock. W-Net’s beneficial ownership is therefore limited to
4.9% of our outstanding common stock until such time as the shares
issuable under the Note and the Warrant, along with shares of our common
stock held by W-Net, constitute 4.9% or less of our outstanding common
stock, or W-Net elects to remove such restriction. David
Weiner, as the manager of W-Net Fund GP I, LLC, the general partner of
W-Net, exercises voting and dispositive power over the shares held by
W-Net, and may be deemed to beneficially own such shares. Mr.
Weiner disclaims any beneficial interest in the shares of our common stock
owned by W-Net except to the extent of his pecuniary interest
therein.
|
|
(4)
|
Includes
1,272,532 shares of our common stock that may be acquired pursuant to the
conversion of a Note, and 636,266 shares of our common stock that may be
acquired pursuant to the exercise of a Warrant, within 60 days of June 23,
2010. The Note and the Warrant prohibit Europa from converting
the Note or exercising the Warrant if after such conversion and/or
exercise Europa would own more than 4.9% of our outstanding common
stock. Europa’s beneficial ownership is therefore limited to
4.9% of our outstanding common stock until such time as the shares
issuable under the Note and the Warrant, along with shares of our common
stock held by Europa, constitute 4.9% or less of our outstanding common
stock, or Europa elects to remove such restriction. Fred Knoll,
the principal of Knoll Capital Management, L.P., the investment manager
for Europa, exercises voting and dispositive power over the shares held by
Europa, but disclaims any beneficial interest in the shares of our common
stock owned by Europa except to the extent of his pecuniary interest
therein.
|
|
(5)
|
Consists
of 1,272,532 shares of our common stock that may be acquired pursuant to
the conversion of a Note, and 636,266 shares of our common stock that may
be acquired pursuant to the exercise of a Warrant, within 60 days of June
23, 2010. The Note and the Warrant prohibit MKM from converting
the Note or exercising the Warrant if after such conversion and/or
exercise MKM would own more than 4.9% of our outstanding common
stock. MKM’s beneficial ownership is therefore limited to 4.9%
of our outstanding common stock until such time as the shares issuable
under the Note and the Warrant, along with shares of our common stock held
by MKM, constitute 4.9% or less of our outstanding common stock, or MKM
elects to remove such restriction. David Skriloff, the
portfolio manager of MKM Capital Advisors, LLC, the managing entity of
MKM, exercises voting and dispositive power over the shares held by MKM,
but disclaims any beneficial interest in the shares of our common stock
owned by MKM except to the extent of his pecuniary interest
therein.
|
Changes
in Control.
There are
currently no arrangements which may result in a change of control of our
company.
41
CERTAIN
RELATIONSHIPS AND RELATED TRANSACTIONS
Other
than the transactions described below, since January 1, 2009 there has not been,
nor is there currently proposed, any transaction or series of similar
transactions to which we were or will be a party:
|
·
|
in
which the amount involved exceeds $120,000;
and
|
|
·
|
in
which any director, executive officer, selling stockholder named in this
prospectus, other stockholder of more than 5% of our common stock or any
member of their immediate family had or will have a direct or indirect
material interest.
|
AtheroNova
On
December 31, 2007, we executed a Demand Promissory Note (the “Demand Note”)
payable to Landbank Acquisition LLC (“Landbank”), in the principal amount of
$500,000 with simple interest on the unpaid principal from the date of the note
at the rate of eight percent (8%) per annum. Landbank was related to
us through common major stockholders. The Note was due on
demand.
On
October 19, 2009, we entered into a Revolving Promissory Note (the “Revolving
Note”) with Landbank. Under the terms of the Revolving Note, Landbank
agreed to advance to us, from time to time and at our request, amounts up to an
aggregate of $500,000 until October 19, 2010. All advances had to be
paid on or before October 19, 2010 and interest accrued from the date of any
advances on any principal amount withdrawn, and on accrued and unpaid interest
thereon, at the rate of eight percent (8%) per annum, compounded
annually. Our obligations under the Revolving Note would accelerate
upon our bankruptcy, any default by us of our payment obligations under the
Revolving Note or our breach of any provision of any material agreement between
us and the noteholder.
In
connection with Landbank’s sale to each of Europa and Woodman Management
Corporation, the predecessor in interest to W-Net (“Woodman”), of 198,278 shares
of our common stock, the Note was assigned to Woodman and Europa in equal
parts. The Revolving Note was cancelled, and new notes (the
“Replacement Notes”) were issued by us to Woodman and Europa on October 19,
2009. The Replacement Notes contained identical terms and conditions
to the Note, except that each Replacement Note provided that the noteholder
would advance up to $250,000. At the Closing, we converted all
outstanding indebtedness under the Note and the Replacement Notes, other than an
aggregate amount of $250,000, into 90,166 shares of our common
stock. We repaid the remaining $250,000 from the proceeds of the
Capital Raise Transaction.
AtheroNova
Operations
On
October 15, 2007, Z&Z Nevada entered into an employment agreement with Mr.
Gardner to assure his continued service to the company, which we assumed
pursuant to the Merger. The agreement runs for a term of 3 years,
expiring on October 15, 2010. The agreement provides for a base
salary of not less than $150,000 per year in the first year following the
receipt by the company of $2,000,000 in operating funds, $170,000 per year in
the second year following such event and $190,000 per year in the third year
following such event. The agreement provides for payment of 50% of
each aforementioned amount in the event of his inability to serve in his
position due to mental or physical incapacitation. The agreement
cannot be terminated for any other reason than fraud, misappropriation,
embezzlement or willful misconduct or because of any violation of the agreement
regarding confidentiality and protection of company secrets.
42
We are in
current negotiations with Mr. Gardner regarding a new contract in anticipation
of his prior commitment to serve as CEO of PhyGen LLC until the end of calendar
2010. It is anticipated that he will share executive management
duties for our company and AtheroNova Operations with Mr. Selawski during that
time.
On
January 7, 2010, Z&Z Nevada and AtheroNova Operations entered into two
agreements with Mr. Selawski. The first was a consulting contract
with Z&Z Nevada, which we assumed pursuant to the Merger, under which he was
to be paid $5,000 per month until such time as the company raises $1,000,000 in
operating funds. Concurrent with this agreement, Mr. Selawski was
awarded a stock grant of 5,000 shares of AtheroNova Operations’ common stock
which converted into 11,215 shares of our common stock in connection with the
Merger. Upon the closing of the Capital Raise Transaction, the second
agreement commenced under which Mr. Selawski will receive $144,000 per year,
subject to annual review by our board of directors. He is also
eligible to receive up to 10% of his annual compensation as a
bonus. Under this agreement Mr. Selawski will be an “at will”
employee and can be terminated with or without cause with 30 days
notice. On March 6, 2010, Mr. Selawski was granted stock options to
purchase up to 245,000 shares of AtheroNova Operations’ common stock at a
purchase price of $0.50 per share. We assumed these options in the
Merger which now entitle Mr. Selawski to purchase 549,498 shares of our common
stock at a purchase price of approximately $0.22 per share. Such
options vest 25% on January 6, 2011 and 75% evenly on a monthly basis over the
next three years thereafter and expire January 7, 2017.
We are in
current negotiations with Mr. Selawski regarding a new contract in anticipation
of his assumption of additional duties with us and AtheroNova Operations due to
Mr. Gardner’s prior commitment to serve as full-time President of PhyGen LLC
until the end of calendar 2010.
On March
6, 2010, AtheroNova Operations issued warrants to Boris Ratiner to purchase
650,000 shares of AtheroNova Operations’ common stock. The warrants
had a term of 5 years and were exercisable at a purchase price of
$0.50. We assumed these warrants in the Merger, which now entitle Mr.
Ratiner to purchase 1,457,852 shares of our common stock for a term of 5 years
at a per share price of approximately $0.22.
Transactions
with Selling Stockholders
On May
13, 2010, we entered into the Securities Purchase Agreement with the Purchasers
pursuant to which we sold to the Purchasers Notes having an aggregate purchase
price of $1,500,000 and Warrants to purchase 1,908,798 shares of our common
stock at a per share price of approximately $0.39.
We also
entered into the Registration Rights Agreement pursuant to which, among other
things, we agreed to register the resale of the shares issuable upon conversion
of the Notes and exercise of the Warrants by the Purchasers and to keep the
registration statement continuously effective until the earlier of the date on
which (a) all such shares have been publicly sold by the Purchasers or (b) the
date that all of such shares have been registered for resale pursuant to a
registration statement on Form S-3 (the “Effectiveness Period”). The
Registration Rights Agreement provides that if (i) we do not file a registration
statement on or before July 12, 2010, (ii) a registration statement is not
declared effective on or prior to October 10, 2010 if not subject to a full
review or November 9, 2010 if subject to a full review, or the date on which our
management and the SEC resolve any applicable comments or inquiries, or (iii)
after its effective date, such registration statement ceases to remain
continuously effective and available to the holders of such shares at any time
prior to the expiration of the Effectiveness Period for an aggregate of more
than 5 consecutive trading days or for more than an aggregate of 20 trading days
in any 12-month period (which need not be consecutive), then we must pay each
Purchaser on the date of such event, and for each month thereafter that such
event continues, an amount in cash as partial relief for damages equal to 1% of
the aggregate Purchase Price paid by such Purchaser pursuant to the Securities
Purchase Agreement for any securities then held by such
Purchaser. Pursuant to the Registration Rights Agreement, we filed
the registration statement of which this prospectus is a part with the SEC to
register for resale the shares of common stock identified in this prospectus and
owned by the selling stockholders.
43
LEGAL
MATTERS
Stubbs
Alderton & Markiles, LLP will pass upon the validity of the common stock
offered by this prospectus for us.
EXPERTS
The
audited financial statements of AtheroNova Operations for the years ended
December 31, 2009 and 2008, included in this prospectus have been so included in
reliance on the report of Anton & Chia, LLP, independent registered public
accountants, given on the authority of said firm as experts in auditing and
accounting. We acquired AtheroNova Operations as our subsidiary in
the Merger. Immediately prior to the Merger, we had no material
operations, assets, or liabilities. Accordingly, for all meaningful
purposes the audited and unaudited financial statements for AtheroNova
Operations which are included in this prospectus comprise our pro forma
financials as well.
WHERE
YOU CAN FIND MORE INFORMATION
We file
annual, quarterly and current reports, proxy statements and other information
with the SEC. We have also filed with the SEC under the Securities
Act a registration statement on Form S-1 with respect to the common stock
offered by this prospectus. This prospectus, which constitutes part
of the registration statement, does not contain all the information set forth in
the registration statement or the exhibits and schedules which are part of the
registration statement, portions of which are omitted as permitted by the rules
and regulations of the SEC. Statements made in this prospectus
regarding the contents of any contract or other document are summaries of the
material terms of the contract or document. With respect to each
contract or document filed as an exhibit to the registration statement,
reference is made to the corresponding exhibit. For further
information pertaining to us and the common stock offered by this prospectus,
reference is made to the registration statement, including the exhibits and
schedules thereto, copies of which may be inspected without charge at the public
reference facilities of the SEC at 100 F Street, N.E., Washington, D.C.
20549. Copies of all or any portion of the registration statement may
be obtained from the SEC at prescribed rates. Information on the
public reference facilities may be obtained by calling the SEC at
1-800-SEC-0330. In addition, the SEC maintains a web site that
contains reports, proxy and information statements and other information that is
filed through the SEC’s EDGAR System. The web site can be accessed at http://www.sec.gov.
44
INDEX
TO ANNUAL FINANCIAL STATEMENTS
Z&Z
Medical Holdings, Inc.
(A
Developmental Stage Company)
Index
to Financial Statements
|
Report
of Independent Registered Public Accounting Firm
|
46
|
|
Balance
Sheets as of December 31, 2009 and 2008
|
47
|
|
Statements
of Operations for the Years Ended December 31, 2009 and 2008, and for the
period December 13, 2006 (Inception) through December 31,
2009
|
48
|
|
Statements
of Stockholders’ Equity for the Years Ended December 31, 2009 and 2008,
and for the period December 13, 2006 (Inception) through
December 31, 2009
|
49
|
|
Statements
of Cash Flows for the Years Ended December 31, 2009 and 2008, and for the
period December 13, 2006 (Inception) through December 31,
2009
|
50
|
|
Notes
to Financial Statements
|
51
|
45
REPORT
OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
The Board
of Directors and Stockholders
Z&Z
Medical Holdings, Inc:
We have
audited the accompanying balance sheets of Z&Z Medical Holdings, Inc. (the
"Company"), a development stage company, as of December 31, 2009 and 2008, and
the related statements of operations, stockholders' equity and cash flows for
each of the years ended December 31, 2009 and 2008 and for the period December
13, 2006 (Inception) through to December 31, 2009. These financial statements
are the responsibility of the Company's management. Our responsibility is to
express an opinion on these financial statements based on our
audits.
We
conducted our audits in accordance with the standards of the Public Company
Accounting Oversight Board (United States). Those standards require that we plan
and perform the audits to obtain reasonable assurance about whether the
financial statements are free of material misstatement. The Company is not
required to have, nor were we engaged to perform, an audit of its internal
control over financial reporting. Our audits include consideration of internal
control over financial reporting as a basis for designing audit procedures that
are appropriate in the circumstances, but not for the purpose of expressing an
opinion on the effectiveness of the Company's internal control over financial
reporting. Accordingly, we express no such opinion. An audit includes examining,
on a test basis, evidence supporting the amounts and disclosures in the
financial statements. An audit also includes assessing the accounting principles
used and significant estimates made by management, as well as evaluating the
overall financial statement presentation. We believe that our audits provide a
reasonable basis for our opinion.
In our
opinion, the financial statements referred to above present fairly, in all
material respects, the financial position of Z&Z Medical Holdings, Inc. as
of December 31, 2009 and 2008, and the results of its operations and its cash
flows for each of the years ended December 31, 2009 and 2008 and for the period
December 13, 2006 (Inception) through to December 31, 2009 then ended, in
conformity with accounting principles generally accepted in the United States of
America.
The
accompanying financial statements have been prepared assuming that the Company
will continue as a going concern. The Company has recurring losses from
operations and had a deficit accumulated during the development stage of
$385,945 at December 31, 2009. As discussed in Note 3 to the financial
statements, a significant amount of additional capital will be necessary to
advance operations to the point at which the Company is profitable. These
conditions, among others, raise substantial doubt about the Company's ability to
continue as a going concern. Management's plans regarding these matters are also
described in Note 3. The accompanying financial statements do not include any
adjustments that might result from the outcome of this uncertainty.
/s/
Anton & Chia, LLP
Newport
Beach, California
May 17,
2010
46
Z&Z
Medical Holdings, Inc.
(A
Development Stage Company)
Balance
Sheets
As
of December 31, 2009 and 2008
|
ASSETS
|
||||||||
|
2009
|
2008
|
|||||||
|
Current
Assets:
|
||||||||
|
Cash
|
$
|
28,047
|
$
|
91,370
|
||||
|
Total
current assets
|
28,047
|
91,370
|
||||||
|
Intellectual
property rights
|
572,867
|
358,584
|
||||||
|
Total
Assets
|
$
|
600,914
|
$
|
449,954
|
||||
|
Current
Liabilities:
|
||||||||
|
Accounts
payable and accrued expenses
|
$
|
211,859
|
$
|
98,576
|
||||
|
Notes
payable related parties, current
|
-
|
100,000
|
||||||
|
Total
current liabilities
|
211,859
|
198,576
|
||||||
|
Notes
payable related parties, net of current portion
|
200,000
|
200,000
|
||||||
|
Total
liabilities
|
411,859
|
398,576
|
||||||
|
Commitments and
Contingencies
|
||||||||
|
Stockholders'
Equity:
|
||||||||
|
Common
stock, par value $0.001 per share, 20,000,000 shares
authorized;
9,218,050 and 8,918,050 shares issued and
outstanding
at 2009 and 2008, respectively
|
9,218
|
8,918
|
||||||
|
Additional
paid-in capital
|
565,782
|
416,082
|
||||||
|
Deficit
accumulated during the development stage
|
(385,945)
|
(373,622)
|
||||||
|
Total
stockholders' equity
|
189,055
|
51,378
|
||||||
|
Total
Liabilities and Stockholders' Equity
|
$
|
600,914
|
$
|
449,954
|
||||
See
accompanying notes, which are an integral part of the financial
statements.
47
Z&Z
Medical Holdings, Inc.
(A
Development Stage Company)
Statements
of Operations
For
the Years Ended December 31, 2009 and 2008
And
for the period from December 13, 2006 (Inception) through December 31,
2009
|
Cumulative
|
||||||||||||
|
From
|
||||||||||||
|
2009
|
2008
|
Inception
|
||||||||||
|
Revenues:
|
$
|
-
|
$
|
-
|
$
|
-
|
||||||
|
Expenses:
|
||||||||||||
|
General
and administrative
|
12,453
|
175,182
|
487,635
|
|||||||||
|
Loss
from operations:
|
(12,453)
|
(175,182)
|
(487,635)
|
|||||||||
|
Interest
income
|
130
|
1,560
|
1,690
|
|||||||||
|
Provision
for income taxes:
|
-
|
-
|
-
|
|||||||||
|
Net
loss:
|
$
|
(12,323)
|
$
|
(173,622)
|
$
|
(485,945)
|
||||||
|
Net
loss per share attributable to commons shares – Basic and
Diluted:
|
$
|
(0.00)
|
$
|
(0.02)
|
-
|
|||||||
|
Weighted
average number of shares outstanding:
|
9,097,217
|
8,980,550
|
-
|
|||||||||
See
accompanying notes, which are an integral part of the financial
statements.
48
Z&Z
Medical Holdings, Inc.
(A
Development Stage Company)
Statements
of Stockholders’ Equity
For
the Year Ended December 31, 2009 and 2008
and
for the period from December 13, 2006 (Inception) through December 31,
2009
|
Deficit
|
||||||||||||||||||||
|
Accumulated
|
||||||||||||||||||||
|
Additional
|
During
the
|
|||||||||||||||||||
|
Common
stock
|
Paid-in
|
Development
|
||||||||||||||||||
|
Description
|
Shares
|
Amount
|
Capital
|
Stage
|
Total
|
|||||||||||||||
|
Balance
inception – December 13, 2006
|
-
|
$
|
-
|
$
|
-
|
$
|
-
|
$
|
-
|
|||||||||||
|
Net
loss
|
-
|
-
|
||||||||||||||||||
|
Balance,
December 31, 2006
|
-
|
$
|
-
|
$
|
-
|
$
|
-
|
$
|
-
|
|||||||||||
|
|
||||||||||||||||||||
|
Net
loss
|
-
|
-
|
-
|
-
|
-
|
|||||||||||||||
|
Issuance
of common stock to founders
|
8,468,050
|
8,468
|
191,532
|
(200,000)
|
-
|
|||||||||||||||
|
|
||||||||||||||||||||
|
Balance
- December 31, 2007
|
8,468,050
|
$
|
8,468
|
$
|
191,532
|
$
|
(200,000)
|
$
|
-
|
|
||||||||||
|
|
||||||||||||||||||||
|
Issuance
of common stock for cash
|
450,000
|
450
|
224,550
|
-
|
225,000
|
|||||||||||||||
|
Net
loss
|
-
|
-
|
-
|
(173,622)
|
(173,622)
|
|||||||||||||||
|
Balance
- December 31, 2008
|
8,918,050
|
$
|
8,918
|
$
|
416,082
|
$
|
(373,622)
|
$
|
51,378
|
|||||||||||
|
Issuance
of common stock for cash
|
300,000
|
300
|
149,700
|
-
|
150,000
|
|||||||||||||||
|
Net
loss
|
-
|
-
|
-
|
(12,323)
|
(12,323)
|
|||||||||||||||
|
Balance
- December 31, 2009
|
9,218,050
|
$
|
9,218
|
$
|
565,782
|
$
|
(385,945)
|
$
|
189,055
|
|
||||||||||
See
accompanying notes to the financial statements.
49
Z&Z
Medical Holdings, Inc.
(A
Development Stage Company)
Statements
of Cash Flows
For
the Years Ended December 31, 2009 and 2008
and
for the period December 13, 2006 from (Inception) through December 31,
2009
|
Cumulative
|
||||||||||||
|
From
|
||||||||||||
|
2009
|
2008
|
Inception
|
||||||||||
|
Operating
Activities:
|
||||||||||||
|
Net
loss
|
$
|
(12,323)
|
$
|
(173,622)
|
$
|
(485,945)
|
||||||
|
Changes
in operating assets and liabilities
|
||||||||||||
|
Accounts
payable and accrued expenses
|
13,284
|
198,576
|
311,860
|
|||||||||
|
Net
Cash Provided (Used) by Operating Activities
|
961
|
24,954
|
(174,805)
|
|||||||||
|
Investing
Activities:
|
||||||||||||
|
Acquisition
of intellectual property
|
(214,284)
|
(158,584)
|
(372,868)
|
|||||||||
|
Net
Cash Used in Investing Activities
|
(214,284)
|
(158,584)
|
(372,868)
|
|||||||||
|
Financing
Activities:
|
||||||||||||
|
Proceeds
from issuance of common stock
|
150,000
|
225,000
|
575,000
|
|||||||||
|
Net
Cash Provided by Financing Activities
|
150,000
|
225,000
|
575,000
|
|||||||||
|
Net
Increase (Decrease) in Cash
|
(63,323)
|
91,370
|
28,047
|
|||||||||
|
Cash
- Beginning of Period
|
91,370
|
-
|
-
|
|||||||||
|
Cash
- End of Period
|
$
|
28,047
|
$
|
91,370
|
$
|
28,047
|
||||||
|
Supplemental
Disclosures of Non Cash Investing and Financing
Transactions:
|
||||||||||||
|
Common
stock issued to founders
|
$
|
-
|
-
|
200,000
|
||||||||
|
Notes
payable related parties issued in exchange for intellectual
property
|
$
|
-
|
$
|
200,000
|
$
|
200,000
|
||||||
|
Notes
payable related parties issued in lieu of expense reports
|
$
|
-
|
$
|
100,000
|
$
|
100,000
|
||||||
See
accompanying notes to the financial statements.
50
Z&Z
Medical Holdings, Inc.
(A
Development Stage Company)
Notes
to Financial Statements
(1) Description
of Business
Z&Z
Medical Holdings, Inc. (the “Company”) was incorporated in the State of Nevada
on December 13, 2006. On March 3, 2010, the Company reincorporated in
Delaware.
The
Company owns certain intellectual property (“IP”) consisting of pharmacological
compounds and delivery systems for the treatment of cardiovascular disease. The
Company plans to develop commercial relationships with third parties for the
development, marketing and sale of products based on the IP and to derive
revenue through the licensing of the IP to such third parties. The Company plans
to further establish the curative aspects of and the licensing value of the
Company’s IP reflective of the global market size and impact that its
patents-pending pharmacological compounds and delivery systems will have on the
world’s largest healthcare market, the cardiovascular diseases
market.
(2) Summary
of Significant Accounting Policies
Basis
of Presentation
The
accompanying financial statements have been prepared in accordance with
generally accepted accounting principles (“GAAP”) as promulgated in the United
States of America.
In July
2009, the Financial Accounting Standards Board (“FASB”) issued Accounting
Standards Codification (“ASC”) 105-10, formerly Statement of Financial
Accounting Standards ("SFAS") No. 168, The FASB Accounting Standards
Codification and Hierarchy of Generally Accepted Accounting Principles, which
became the single source of authoritative GAAP recognized by the
FASB. ASC 105-10 does not change current U.S. GAAP, but on the
effective date, the FASB ASC superseded all then existing non-SEC accounting and
reporting standards. The ASC is effective for interim and annual
reporting periods ending after September 15, 2009. The Company
adopted ASC 105-10 during the year ended December 31, 2009 and revised its
referencing of GAAP accounting standards in these financial statements to
reflect the new standards.
Use
of Estimates
Financial
statements prepared in accordance with accounting principles generally accepted
in the United States require management to make estimates and assumptions that
affect the reported amounts of assets and liabilities at the date of the
financial statements and the reported amounts of revenues and expenses during
the reporting period. Among other things, management has estimated
the useful lives of patents, the valuation of long lived assets, and the
variables used to calculate the valuation of warrants using the Black-Scholes
option valuation model. Actual results could differ from those
estimates.
Cash
and Cash Equivalents
The
Company considers all highly liquid temporary cash investments with an original
maturity of three months or less to be cash equivalents. At December
31, 2009 and 2008, respectively, the Company had no cash
equivalents.
Concentration
of Credit Risk
Financial
instruments that potentially subject the Company to concentrations of credit
risk consist principally of cash deposits. Accounts at each institution are
insured by the Federal Deposit Insurance Corporation (FDIC) up to $250,000. At
December 31, 2009, the Company had no amounts in excess of FDIC insured limit.
While the Company periodically evaluates the credit quality of the financial
institutions in which it holds deposits, it cannot reasonably alleviate the risk
associated with the sudden possible failures of such institutions.
51
Z&Z
Medical Holdings, Inc.
(A
Development Stage Company)
Notes
to Financial Statements
Revenue
Recognition
The
Company is in the development stage and has yet to realize revenues from planned
operations. The Company will recognize revenue on arrangements in
accordance with FASB ASC No. 605, “Revenue Recognition”. In all
cases, revenue is recognized only when the price is fixed and determinable,
persuasive evidence of an arrangement exists, the service is performed and
collectability of the resulting receivable is reasonably assured. The Company
plans to expand the application and development of clinical modalities for the
Company’s IP through licensing agreements with select licensing partners to
administer therapeutics. The Company will generate revenues primarily
from IP licensing royalties. For licensing activities, revenue from such
agreements will be realized over the term and under the conditions of each
specific license once all contract conditions have been met. Payments for
licensing fees are generally received at the time the license agreements are
executed, unless other terms for delayed payment are documented and agreed to
between the parties.
Research
and Development Costs
Research
and development costs consist of expenditures for the research and development
of new products and technology. Research and development costs are expensed as
incurred.
Intellectual
Property
The
Company obtained certain intellectual property from two Directors who are also
stockholders. The intellectual property obtained was by assignment
effective on December 1, 2006, the date of the initial meeting of the Board of
Directors. Under Staff Accounting Bulletin Topic 5G, “Transfers of
Nonmonetary Assets by Promoters and Shareholders,” the Company recorded the
transaction as an obligation payable to the Directors and stockholders’ at the
historical cost basis in the amount of $200,000. The Company
accounts for its intellectual property and patent applications in accordance
with ASC 350-30 and ASC 360 (formerly SFAS No. 142, Goodwill and Other
Intangible Assets). The Company amortizes the capitalized intellectual property
and patent costs on a straight line basis over a period of 19.5 years,
management’s estimated legal life of the patents, which approximates their
estimated useful life. No amortization expense relating to these assets was
recognized for the years ended December 31, 2009 and 2008, respectively, as the
Company is still in the development stage.
Intangible
and Long-Lived Assets
In
accordance with ASC 350-30 (formerly SFAS No. 144, Accounting for the Impairment
or Disposal of Long-Lived Assets), the Company evaluates long-lived assets for
impairment whenever events or changes in circumstances indicate that their net
book value may not be recoverable. When such factors and circumstances exist,
the Company compares the projected undiscounted future cash flows associated
with the related asset or group of assets over their estimated useful lives
against their respective carrying amount. Impairment, if any, is based on the
excess of the carrying amount over the fair value, based on market value when
available, or discounted expected cash flows, of those assets and is recorded in
the period in which the determination is made. The Company’s management
currently believes there is no impairment of its long-lived assets. There can be
no assurance, however, that market conditions will not change or demand for the
Company’s products under development will continue. Either of these could result
in future impairment of long-lived asset.
52
Z&Z
Medical Holdings, Inc.
(A
Development Stage Company)
Notes
to Financial Statements
Income
Taxes
The
Company accounts for income taxes under FASB Codification Topic 740-10-25 (“ASC
740-10-25”). Under ASC 740-10-25, deferred tax assets and liabilities
are recognized for the future tax consequences attributable to differences
between the financial statement carrying amounts of existing assets and
liabilities and their respective tax bases. Deferred tax assets and
liabilities are measured using enacted tax rates expected to apply to taxable
income in the years in which those temporary differences are expected to be
recovered or settled. Under ASC 740-10-25, the effect on deferred tax
assets and liabilities of a change in tax rates is recognized in income in the
period that includes the enactment date.
The
Company maintains a valuation allowance with respect to deferred tax
assets. The Company establishes a valuation allowance based upon the
potential likelihood of realizing the deferred tax asset and taking into
consideration the Company’s financial position and results of operations for the
current period. Future realization of the deferred tax benefit
depends on the existence of sufficient taxable income within the carryforward
period under the Federal tax laws.
Changes
in circumstances, such as the Company generating taxable income, could cause a
change in judgment about the realizability of the related deferred tax
asset. Any change in the valuation allowance will be included in
income in the year of the change in estimate.
Common
Stock and Common Stock Warrants
The
Company uses the fair value recognition provision of ASC 718, “Stock
Compensation,” which requires the Company to expense the cost of employee
services received in exchange for an award of equity instruments based on the
grant date fair value of such instruments. The Company uses the Black-Scholes
option pricing model to calculate the fair value of any equity instruments on
the grant date. The Company also uses the provisions of ASC 505-50, “Equity
Based Payments to Non-Employees,” to account for stock-based compensation awards
issued to non-employees for services. Such awards for services are recorded at
either the fair value of the services rendered or the instruments issued in
exchange for such services, whichever is more readily determinable, using the
measurement date guidelines enumerated in ASC 505-50.
Fair
value of Financial Instruments
The
Company adopted ASC topic 820, “Fair Value Measurements and Disclosures” (ASC
820), formerly SFAS No. 157 “Fair Value Measurements,” effective January 1,
2009. ASC 820 defines “fair value” as the price that would be received for an
asset or paid to transfer a liability (an exit price) in the principal or most
advantageous market for the asset or liability in an orderly transaction between
market participants on the measurement date. There was no impact relating to the
adoption of ASC 820 to the Company’s financial statements.
Financial
instruments consist principally of cash, accounts payable and accrued
liabilities, and notes payable. The carrying amounts of such financial
instruments in the accompanying balance sheets approximate their fair values due
to their relatively short-term nature. It is management’s opinion that the
Company is not exposed to any significant currency or credit risks arising from
these financial instruments.
53
Z&Z
Medical Holdings, Inc.
(A
Development Stage Company)
Notes
to Financial Statements
Loss
Per Share
Basic
loss per share is calculated using the weighted-average number of common shares
outstanding during each reporting period. Diluted loss per share includes
potentially dilutive securities such as outstanding options and warrants, using
various methods such as the treasury stock or modified treasury stock method in
the determination of dilutive shares outstanding during each reporting period.
Common equivalent shares are excluded from the computation of net loss per share
since their effect is anti-dilutive.
For the
years ended December 31, 2009 and 2008, we excluded any effect of the 650,000
and 450,000 outstanding warrants, respectively, as their effect would be
anti-dilutive.
Recent
Accounting Pronouncements
In June
2009, the FASB issued changes to the consolidation guidance applicable to a
variable interest entity (VIE). FASB ASC Topic 810, "Consolidation," amends the
guidance governing the determination of whether an enterprise is the primary
beneficiary of a VIE, and is, therefore, required to consolidate an entity, by
requiring a qualitative analysis rather than a quantitative analysis. The
qualitative analysis will include, among other things, consideration of who has
the power to direct the activities of the entity that most significantly impact
the entity's economic performance and who has the obligation to absorb losses or
the right to receive benefits of the VIE that could potentially be significant
to the VIE. This standard also requires continuous reassessments of whether an
enterprise is the primary beneficiary of a VIE. FASB ASC 810 also requires
enhanced disclosures about an enterprise's involvement with a VIE. Topic 810 is
effective as of the beginning of interim and annual reporting periods that begin
after November 15, 2009. This will not have an impact on the Company’s financial
position, results of operations or cash flows.
FASB ASC
No. 860 is effective as of the beginning of each reporting entity's first annual
reporting period that begins after November 15, 2009, for interim periods within
that first annual reporting period and for interim and annual reporting periods
thereafter. This will not have an impact on the Company’s financial position,
results of operations or cash flows.
(3) Development
Stage Activities and Going Concern
The
Company is currently in the development stage, and its business plan is to
develop commercial relationships with third parties for the development,
marketing and sale of products based on the IP and to derive revenue through the
licensing of the IP to such third parties.
While
management of the Company believes that the Company will be successful in its
planned operating activities, there can be no assurance that the Company will be
successful in the development of its intellectual property, or services that
will generate sufficient revenues to sustain the operations of the
Company. The Company also intends to conduct additional capital
formation activities through the issuance of its common stock and to commence
operations.
The
accompanying financial statements have been prepared in conformity with
accounting principles generally accepted in the United States of America, which
contemplate continuation of the Company as a going concern. The
Company has incurred an operating loss since inception, had negative working
capital as of December 31, 2009, and 2008, and the cash resources of the Company
were insufficient to meet its planned business objectives. These and
other factors raise substantial doubt about the Company’s ability to continue as
a going concern. The accompanying financial statements do not include
any adjustments to reflect the possible future effects on the recoverability and
classification of assets or the amounts and classification of liabilities that
may result from the possible inability of the Company to continue as a going
concern.
54
Z&Z
Medical Holdings, Inc.
(A
Development Stage Company)
Notes
to Financial Statements
(4) Common
Stock and Common Stock Warrants
On
December 1, 2007, the Company issued 8,468,050 shares of common stock to its
founders at approximately $0.02 per share based on the fair value of the shares
on the grant date. During the years ended December 31, 2009 and 2008,
the Company issued 300,000 and 450,000 shares, respectively, of common
stock. These subsequent issuances of common stock were issued for
cash at a per share amount of $0.50. The Company recognized proceeds
from these issuances during the years ended December 31, 2009 and 2008 of
$150,000 and $225,000, respectively.
The
Company also issued warrants exercisable into common stock of the
Company. Certain common stock subscribers also received a warrant to
purchase one share of common stock for every subscription share
purchased. The exercise price of these warrants are equal to the
price of shares of securities of the Company issued in a subsequent equity
financing with an aggregate gross proceeds to the Company of at least
$2,500,000. However, in the event the Company’s valuation immediately prior to
the financing is less than $20,000,000, the exercise price of the warrant shall
be 50% of the purchase price per share of the Equity Securities offered. As of
December 31, 2009 there are warrants to purchase 650,000 shares of the Company’s
common stock outstanding with expiration dates ranging from February 2013
through September 2014.
| Shares underlying warrants issued: | 2009 | 2008 | ||||||
|
Beginning
balance
|
450,000 | - | ||||||
|
Shares
granted
|
200,000 | 450,000 | ||||||
|
Ending
balance
|
650,000 | 450,000 | ||||||
(5) Income
Taxes
The
provision (benefit) for income taxes for the periods ended December 31, 2009,
and 2008, was as follows (using a 42.8 percent effective Federal and state
income tax rate):
|
2009
|
2008
|
|||||||
|
Current
Tax Provision:
|
||||||||
|
Federal
and state-
|
||||||||
|
Taxable
income
|
$
|
-
|
$
|
-
|
||||
|
Total
current tax provision
|
$
|
-
|
$
|
-
|
||||
|
Deferred
Tax Provision:
|
||||||||
|
Federal
and state-
|
||||||||
|
Loss
carryforwards
|
$
|
(26,620
|
)
|
$
|
(41,148
|
)
|
||
|
Valuation
allowance
|
26,620
|
41,148
|
||||||
|
Total
deferred tax provision
|
$
|
-
|
$
|
-
|
||||
55
Z&Z
Medical Holdings, Inc.
(A
Development Stage Company)
Notes
to Financial Statements
The
Company had deferred income tax assets as of December 31, 2009, and 2008, as
follows:
|
2009
|
2008
|
|||||||
|
Loss
carryforwards
|
$
|
(67,768
|
)
|
$
|
(41,148
|
)
|
||
|
Less
- valuation allowance
|
67,768
|
41,148
|
||||||
|
Total
net deferred tax assets
|
$
|
-
|
$
|
-
|
||||
As of
December 31, 2009 and 2008, respectively, the Company had net operating loss
carryforwards for income tax reporting purposes of approximately $67,768 and
$41,148 that may be offset against future taxable income. Current
tax laws limit the amount of loss available to be offset against future taxable
income when a substantial change in ownership occurs or a change in the nature
of the business. Therefore, the amount available to offset future
taxable income may be limited.
No tax
benefit has been reported in the financial statements for the realization of
loss carryforwards, as the Company believes there is high probability that the
carryforwards will not be utilized in the foreseeable
future. Accordingly, the potential tax benefits of the loss
carryforwards are offset by a valuation allowance of the same
amount.
The
Company is primarily subject to U.S. federal and state income tax. As
a result of the implementation of certain provisions of ASC 740, Income Taxes,
(formerly FIN 48, Accounting for Uncertainty in Income Taxes – An Interpretation
of FASB Statement No. 109), the Company performed an analysis of its previous
tax filings and determined that there were no positions taken that it considered
uncertain. Therefore, there were no unrecognized tax benefits as of December 31,
2009 and 2008.
Future
changes in the unrecognized tax benefit are not expected to have an impact on
the effective tax rate due to the existence of the valuation allowance. The
Company estimates that the unrecognized tax benefit will not change within the
next twelve months. The Company will continue to classify income tax penalties
and interest, if any, as part of interest and other expenses in its statements
of operations. The Company has incurred no interest or penalties as of December
31, 2009 and 2008.
6) Related
Party Transactions
On
December 1, 2006, the Company received certain intellectual property through an
intellectual property assignment agreement from two Directors of the Company who
are also stockholders. In exchange for the intellectual property, the
Company issued two $100,000 non-interest bearing notes payable. These
notes payable are recorded in the accompanying financial statements in notes
payable related parties, net of current portion.
During
the year ended December 31, 2008, the Company issued four $25,000 non-interest
bearing notes payable to certain Directors and employees of the Company in lieu
of expense reports. These notes payable are recorded in the
accompanying financial statements in notes payable related parties, current. The
Directors and employees forgave the notes payable on December 31,
2009.
56
Z&Z
Medical Holdings, Inc.
(A
Development Stage Company)
Notes
to Financial Statements
(7) Commitments
and Contingencies
The
Company has executed three year employment contracts with Thomas Gardener,
Filiberto Zadini, Giorgio Zadini and Aaron Sandoval, each with a salary and
salary increases at the discretion of the Board of Directors not to exceed 10%
per annum or the previous calendar year's percentage increase in the Consumer
Price Index, whichever is greater. The contracts are not in effect until the
Company raises the equity financing with an aggregate gross proceeds to the
Company of at least $2,500,000.
The
Company has executed a contract in May 2008 with The University of California
(the “University”), on behalf of its Los Angeles Campus under which the
University shall conduct a laboratory study to demonstrate the efficacy of the
Company’s biocompatible emulsifiers on atherosclerotic lesions. The
contract calls for progress payments upon the completion of various stages of
the study and the total obligation to the University for completion of the study
totals $200,600. To date, a total of $90,150 has been paid on the
contract.
(8) Subsequent
Events
In
preparing these financial statements, the Company has evaluated events and
transactions for potential recognition or disclosure through May 17, 2010, the
date the financial statements were issued.
On March
6, 2010, the Company issued options to purchase 245,000 shares of common stock
of the Company at $0.50 per share as part of an employment agreement with our
Chief Financial Officer. The option vests 25% on January 6, 2011 and 75%
evenly on a monthly basis over the next three years thereafter and expire
January 7, 2017.
On
January 8, 2010, the Company issued 5,000 shares of its common stock for cash at
a price of $0.50 per share. The Company recorded proceeds for this
issuance of $2,500.
During
the first quarter, the Company sold an additional common stock subscription unit
consisting of 450,000 shares and a warrant to purchase an additional 450,000
shares. The subscription unit was sold at a price of $0.50 per share,
with a net proceeds to the company of $225,000.
The
Company has also issued warrants to purchase 500,000 shares of common stock at
$0.50 per share to a director of the Company as compensation for services
rendered in connection with the reverse merger and financing consented to by a
majority of the stockholders of the Company.
On May
13, 2010, the Company completed its reverse merger transaction with Trist
Holdings, Inc. (“Trist”). Effective as of the closing, Z&Z became a
wholly-owned subsidiary of Trist and changed its name to AtheroNova Operations,
Inc. As a result of the closing, the business operations of Z&Z will
comprise Trist's principal business operations going forward. Immediately after
the closing of the merger, Trist closed a capital raise transaction through the
placement of $1.5 million in 2.5% Senior Secured Convertible Notes which mature
4 years after issuance. Interest on the Convertible Notes, which will accrue at
the rate of 2.5% per year, is not payable until maturity or conversion, and is
payable in cash or common stock. Also, Trist has changed its name to AtheroNova
Inc. to more accurately reflect the company's emphasis in the healthcare market
and anticipates that the company will begin trading under the symbol AHRO on the
OTC Bulletin Board as of May 25, 2011.
57
Z&Z
Medical Holdings, Inc.
(A
Development Stage Company)
Notes
to Financial Statements
At the
closing, pursuant to the terms of the Merger Agreement dated March 26, 2010, by
and among Trist, Z&Z and Z&Z Merger Corp., all of the outstanding
shares, warrants and options of Z&Z were exchanged for shares of, and
warrants and options to purchase, Trist's Super-Voting Common Stock. Each share
of Trist's Super-Voting Common Stock will automatically convert into 50 shares
(on a pre-reverse split basis) of Trist's Common Stock upon the consummation of
a 200-for-1 reverse split of Trist's Common Stock. As a result of the merger,
Z&Z stockholders own 88,575,048 shares of Trist's Super-Voting Common Stock
(22,143,771 shares of Trist's Common Stock on a post-reverse split basis), or
approximately 97.6% of the total shares outstanding.
The
Convertible Notes are convertible into 3,817,596 shares of common stock (on a
post-reverse split basis), excluding accrued interest, which may also be paid in
stock. If held to maturity and all accrued interest is paid in common stock an
additional 381,762 shares would be issued. The purchasers of the Convertible
Notes also received Warrants to purchase another 1,908,798 shares of common
stock (on a post-reverse split basis) at an exercise price of $0.39 per share
(on a post-reverse split basis). The shares of Trist's Common Stock issuable
upon conversion (excluding the conversion of accrued interest) and exercise of
the Notes and Warrants represent approximately 17.6% of the Company's
outstanding capital stock as of the closing of the capital raise transaction on
a fully-diluted basis.
As a
result of the merger and the capital raise transaction, Z&Z stockholders own
80.8% of Trist's fully-diluted Common Stock, Trist stockholders immediately
prior to the merger own 1.6% of Trist's fully-diluted Common Stock and the
holders of Convertible Notes and Warrants issued in the capital raise
transaction own 17.6% of Trist's fully-diluted Common Stock.
58
INDEX
TO QUARTERLY FINANCIAL STATEMENTS
Z&Z
Medical Holdings, Inc.
(A
Developmental Stage Company)
Index
to Financial Statements
|
Balance
Sheets as of March 31, 2010 and December 31, 2009
|
60
|
|
Statements
of Operations for the three Months ended March 31, 2010 and 2009, and for
the period December 13, 2006 (Inception) through
March 31, 2010
|
61
|
|
Statements
of Cash Flows for the three months ended March 31, 3010 and 2009, and for
the period December 13, 2006 (Inception) through March 31,
2010
|
62
|
|
Notes
to Financial Statements
|
63
|
59
Z&Z
Medical Holdings, Inc.
(A
Development Stage Company)
Balance
Sheets
|
March
31,
2010
|
December
31,
2009
|
|||||||
|
(Unaudited)
|
||||||||
| ASSETS | ||||||||
| Current Assets: | ||||||||
|
Cash
|
$ | 123,734 | $ | 28,047 | ||||
|
Total
current assets
|
123,734 | 28,047 | ||||||
|
Equipment,
net
|
955 | - | ||||||
|
Intellectual
property rights
|
572,867 | 572,867 | ||||||
|
Total
Assets
|
697,557 | 600,914 | ||||||
|
LIABILITIES
AND STOCKHOLDERS' EQUITY
|
||||||||
|
Current
Liabilities:
|
||||||||
|
Accounts
payable and accrued expenses
|
$ | 214,589 | $ | 211,859 | ||||
|
Total
current liabilities
|
214,589 | 211,859 | ||||||
|
Commitments
and Contingencies
|
||||||||
|
Stockholders'
Equity:
|
||||||||
|
Common
stock, par value $0.001 per share, 20,000,000 shares authorized;
9,873,050 and 9,218,050 shares issued and outstanding
at
March 31, 2010 and December 31, 2009, respectively
|
9,873 | 9,218 | ||||||
|
Additional
paid-in capital
|
892,626 | 565,782 | ||||||
|
Deficit
accumulated during the development stage
|
(419,531 | ) | (385,945 | ) | ||||
|
Total
stockholders' equity
|
482,968 | 189,055 | ||||||
|
Total
Liabilities and Stockholders' Equity
|
$ | 697,557 | $ | 600,914 | ||||
See
accompanying notes, which are an integral part of the financial
statements.
60
Z&Z
Medical Holdings, Inc.
(A
Development Stage Company)
Statements
of Operations
For
the three Months Ended March 31, 2010 and 2009
And
for the period from December 13, 2006 (Inception) through March 31,
2010
|
Three
Months
ended
|
Three
Months
ended
|
December
13,
2006
(Inception)
|
||||||||||
|
March
31,
|
March
31,
|
through
March
|
||||||||||
|
2010
|
2009
|
31,
2010
|
||||||||||
|
Revenues:
|
$
|
-
|
$
|
-
|
$
|
-
|
||||||
|
Expenses:
|
||||||||||||
|
General
and administrative
|
32,633
|
3,163
|
420,268
|
|||||||||
|
Loss
from operations:
|
(32,633)
|
(3,163)
|
(420,268)
|
|||||||||
|
Interest
income
|
6
|
108
|
1,696
|
|||||||||
|
Provision
for income taxes
|
959
|
-
|
959
|
|||||||||
|
Net
loss:
|
$
|
(33,586)
|
$
|
(3,055)
|
$
|
(419,531)
|
||||||
|
Net
loss per share attributable to commons shares – Basic and
Diluted:
|
$
|
(0.00)
|
$
|
(0.00)
|
-
|
|||||||
|
Weighted
average number of shares outstanding:
|
9,583,050
|
8,918,050
|
-
|
|||||||||
See
accompanying notes, which are an integral part of the financial
statements.
61
Z&Z
Medical Holdings, Inc.
(A
Development Stage Company)
Statements
of Cash Flows
For
the Three Months Ended March 31, 2010 and 2009
and
for the period December 13, 2006 from (Inception) through March 31,
2010
| For the three months ended March 31, |
December
13,
2006
(Inception)
through
March
|
|||||||||||
| 2010 | 2009 |
31, 2010
|
||||||||||
| Operating Activities: | ||||||||||||
| Net loss | $ | (33,586 | ) | $ | (3,055 | ) | $ | (419,531 | ) | |||
| Adjustments to reconcile net income | ||||||||||||
| to net cash (used) provided by operating activities: | ||||||||||||
|
Depreciation and
amortization
|
87 | - | 87 | |||||||||
|
Changes
in operating assets and liabilities
|
||||||||||||
|
Accounts payable and accrued expenses
|
(197,271 | ) | 17,323 | 14,589 | ||||||||
|
Net
Cash (Used) Provided by Operating Activities
|
(230,770 | ) | 14,268 | (404,855 | ) | |||||||
|
Investing
Activities:
|
||||||||||||
| Equipment | (1,042 | ) | - | (1,042 | ) | |||||||
| Acquisition of intellectual property | - | (52,323 | ) | (372,868 | ) | |||||||
|
Net
Cash Used in Investing Activities
|
(1,042 | ) | (52,323 | ) | (373,910 | ) | ||||||
|
Financing
Activities:
|
||||||||||||
|
Proceeds
from issuance of common stock
|
327,500 | - | 902,500 | |||||||||
|
Net
Cash Provided by Financing Activities
|
327,500 | - | 902,500 | |||||||||
|
Net
Increase in Cash
|
95,688 | (38,056 | ) | 123,734 | ||||||||
|
Cash
- Beginning of Period
|
28,047 | 91,370 | - | |||||||||
|
Cash
- End of Period
|
$ | 123,734 | $ | 52,314 | $ | 123,734 | ||||||
| Supplemental Cash Flow Disclosures: | ||||||||||||
|
Cash
paid during the period for income taxes
|
$ | 959 | $ | - | $ | 959 | ||||||
| Supplemental Disclosures of Non Cash Investing and Financing Transactions: | ||||||||||||
|
Common
stock issued in lieu of compensation
|
$ | 102,500 | $ | - | $ | 102,500 | ||||||
|
Common
stock issued to founders
|
$ | - | $ | - | $ | 200,000 | ||||||
|
Notes
payable related parties issued in exchange for intellectual
property
|
$ | (200,000 | ) | - | $ | - | ||||||
|
Notes
payable related parties issued in lieu of expense reports
|
$ | - | $ | - | $ | 100,000 | ||||||
See
accompanying notes, which are an integral part of the financial
statements.
62
Z&Z
Medical Holdings, Inc.
(A
Development Stage Company)
Notes
to Financial Statements
(1) Description
of Business
Z&Z
Medical Holdings, Inc. (the “Company” and/or “Z&Z”) was incorporated in the
State of Nevada on December 13, 2006. On March 3, 2010, the Company
reincorporated in Delaware.
The
Company owns certain intellectual property (“IP”) consisting of pharmacological
compounds and delivery systems for the treatment of cardiovascular disease. The
Company plans to develop commercial relationships with third parties for the
development, marketing and sale of products based on the IP and to derive
revenue through the licensing of the IP to such third parties. The Company plans
to further establish the curative aspects of and the licensing value of the
Company’s IP reflective of the global market size and impact that its
patents-pending pharmacological compounds and delivery systems will have on the
world’s largest healthcare market, the cardiovascular diseases
market.
On March
26, 2010, we signed an agreement of merger with Trist Holding, Inc. an OTCBB
“shell” company traded under the symbol TRHI. Under the terms of the
agreement, a reverse merger will take place whereby the operations of our
Company will become the operations of the merged company. The
stockholders of Z & Z will receive approximately 98% of the total
outstanding shares of the merger company. The officers of Z & Z
will become the officers of the merged company and three members of our Board of
Directors will become directors of the merged company. Once merged,
Trist will change its name to AtheroNova, Inc. to more accurately reflect the
healthcare emphasis of the Company.
Concurrent
with the merger, a senior convertible debt placement of $1.5 million will be
closed with KOM Capital Management. The debt carries an interest rate
of 2.5% per annum and is not due and payable until maturity or upon conversion
of the underlying debt, and is payable in cash or common stock. The
debt matures 4 years from the date of issuance if not converted into common
stock prior to that date. The debt is convertible at $0.39 per share,
with the total debt representing 3,817,596 shares of the company’s common stock.
If the full debt is outstanding for the full term and is paid in common stock,
there will be an additional 381,762 shares issued. As part of the
debt placement, warrants to purchase common stock are also issued to the debt
holders. The warrants are exercisable at $0.39 per share and allow
for purchase of 1,908,798 shares of our common stock and have an automatic
cashless conversion feature if not exercised prior to expiration, which is 4
years after closure of the debt placement.
Both
transactions closed on May 13, 2010, please see Subsequent Events Footnote
9.
(2) Summary
of Significant Accounting Policies
Basis
of Presentation
The
accompanying financial statements have been prepared in accordance with
generally accepted accounting principles (“GAAP”) as promulgated in the United
States of America.
Use
of Estimates
Financial
statements prepared in accordance with accounting principles generally accepted
in the United States require management to make estimates and assumptions that
affect the reported amounts of assets and liabilities at the date of the
financial statements and the reported amounts of revenues and expenses during
the reporting period. Among other things, management has estimated
the useful lives of equipment and patents, along with the variables used to
calculate the valuation of stock options and warrants using the Black-Scholes
option valuation model. Actual results could differ from those
estimates.
63
Z&Z
Medical Holdings, Inc.
(A
Development Stage Company)
Notes
to Financial Statements
Cash
and Cash Equivalents
The
Company considers all highly liquid temporary cash investments with an original
maturity of three months or less to be cash equivalents. At March 31,
2010 and 2009, respectively, the Company had no cash equivalents.
Concentration
of Credit Risk
Financial
instruments that potentially subject the Company to concentrations of credit
risk consist principally of cash deposits. Accounts at each institution are
insured by the Federal Deposit Insurance Corporation (FDIC) up to $250,000. At
March 31, 2010, the Company had no amounts in excess of FDIC insured limit.
While the Company periodically evaluates the credit quality of the financial
institutions in which it holds deposits, it cannot reasonably alleviate the risk
associated with the sudden possible failures of such institutions.
Revenue
Recognition
The
Company is in the development stage and has yet to realize revenues from planned
operations. The Company will recognize revenue on arrangements in
accordance with FASB ASC No. 605, “Revenue Recognition”. The Company
plans to expand the application and development of clinical modalities for the
Company’s IP through licensing agreements with select licensing partners to
administer therapeutics. The Company will generate revenues primarily
from IP licensing royalties. For licensing activities, revenue from such
agreements will be realized over the term and under the conditions of each
specific license once all contract conditions have been met. Payments for
licensing fees are generally received at the time the license agreements are
executed, unless other terms for delayed payment are documented and agreed to
between the parties.
Research
and Development Costs
Research
and development costs consist of expenditures for the research and development
of new products and technology. Research and development costs are expensed as
incurred.
Intellectual
Property
The
Company obtained certain intellectual property from two Directors who are also
stockholders. The intellectual property obtained was by assignment
effective on December 1, 2006, the date of the initial meeting of the Board of
Directors. Under Staff Accounting Bulletin Topic 5G, “Transfers of
Nonmonetary Assets by Promoters and Shareholders,” the Company recorded the
transaction as an obligation payable to the Directors and stockholders’ at the
historical cost basis in the amount of $200,000. The Company
accounts for its intellectual property and patent applications in accordance
with ASC 350-30 and ASC 360 (formerly SFAS No. 142, Goodwill and Other
Intangible Assets). The Company amortizes the capitalized intellectual property
and patent costs on a straight line basis over a period of 19.5 years,
management’s estimated legal life of the patents, which approximates their
estimated useful life. No amortization expense relating to these assets was
recognized for the three months ended March 31, 2010 and 2009, respectively, as
the Company is still in the development stage.
64
Z&Z
Medical Holdings, Inc.
(A
Development Stage Company)
Notes
to Financial Statements
Intangible
and Long-Lived Assets
In
accordance with ASC 350-30 (formerly SFAS No. 144, Accounting for the Impairment
or Disposal of Long-Lived Assets), the Company evaluates long-lived assets for
impairment whenever events or changes in circumstances indicate that their net
book value may not be recoverable. When such factors and circumstances exist,
the Company compares the projected undiscounted future cash flows associated
with the related asset or group of assets over their estimated useful lives
against their respective carrying amount.
Impairment,
if any, is based on the excess of the carrying amount over the fair value, based
on market value when available, or discounted expected cash flows, of those
assets and is recorded in the period in which the determination is made. The
Company’s management currently believes there is no impairment of its long-lived
assets. There can be no assurance, however, that market conditions will not
change or demand for the Company’s products under development will continue.
Either of these could result in future impairment of the Company’s long-lived
assets.
Income
Taxes
The
Company accounts for income taxes under FASB Codification Topic 740-10-25 (“ASC
740-10-25”). Under ASC 740-10-25, deferred tax assets and liabilities
are recognized for the future tax consequences attributable to differences
between the financial statement carrying amounts of existing assets and
liabilities and their respective tax bases. Deferred tax assets and
liabilities are measured using enacted tax rates expected to apply to taxable
income in the years in which those temporary differences are expected to be
recovered or settled. Under ASC 740-10-25, the effect on deferred tax
assets and liabilities of a change in tax rates is recognized in income in the
period that includes the enactment date.
The
Company maintains a valuation allowance with respect to deferred tax
assets. The Company establishes a valuation allowance based upon the
potential likelihood of realizing the deferred tax asset and taking into
consideration the Company’s financial position and results of operations for the
current period. Future realization of the deferred tax benefit
depends on the existence of sufficient taxable income within the carryforward
period under the Federal tax laws.
Changes
in circumstances, such as the Company generating taxable income, could cause a
change in judgment about the realizability of the related deferred tax
asset. Any change in the valuation allowance will be included in
income in the year of the change in estimate.
Stock
Based Compensation
The
Company uses the fair value recognition provision of ASC 718, “Stock
Compensation,” which requires the Company to expense the cost of employee
services received in exchange for an award of equity instruments based on the
grant date fair value of such instruments. The Company uses the Black-Scholes
option pricing model to calculate the fair value of any equity instruments on
the grant date. The Company also uses the provisions of ASC 505-50, “Equity
Based Payments to Non-Employees,” to account for stock-based compensation awards
issued to non-employees for services. Such awards for services are recorded at
either the fair value of the services rendered or the instruments issued in
exchange for such services, whichever is more readily determinable, using the
measurement date guidelines enumerated in ASC 505-50.
65
Z&Z
Medical Holdings, Inc.
(A
Development Stage Company)
Notes
to Financial Statements
Fair
Value of Financial Instruments
The
Company adopted ASC topic 820, “Fair Value Measurements and Disclosures” (ASC
820), formerly SFAS No. 157 “Fair Value Measurements,” effective January 1,
2009. ASC 820 defines “fair value” as the price that would be received for an
asset or paid to transfer a liability (an exit price) in the principal or most
advantageous market for the asset or liability in an orderly transaction between
market participants on the measurement date.
There was
no impact relating to the adoption of ASC 820 to the Company’s financial
statements. The carrying amounts of such financial instruments in the
accompanying balance sheets approximate their fair values due to their
relatively short-term nature. It is management’s opinion that the Company is not
exposed to any significant currency or credit risks arising from these financial
instruments.
Loss
Per Share
Basic
loss per share is calculated using the weighted-average number of common shares
outstanding during each reporting period. Diluted loss per share includes
potentially dilutive securities such as outstanding options and warrants, using
various methods such as the treasury stock or modified treasury stock method in
the determination of dilutive shares outstanding during each reporting
period.
For the
three months ended March 31, 2010 and 2009, we excluded any effect of the
1,845,000 and 450,000 outstanding options and warrants, respectively, as their
effect would be anti-dilutive.
Recent
Accounting Pronouncements
In June
2009, the FASB issued changes to the consolidation guidance applicable to a
variable interest entity (VIE). FASB ASC Topic 810, "Consolidation," amends the
guidance governing the determination of whether an enterprise is the primary
beneficiary of a VIE, and is, therefore, required to consolidate an entity, by
requiring a qualitative analysis rather than a quantitative analysis. The
qualitative analysis will include, among other things, consideration of who has
the power to direct the activities of the entity that most significantly impact
the entity's economic performance and who has the obligation to absorb losses or
the right to receive benefits of the VIE that could potentially be significant
to the VIE. This standard also requires continuous reassessments of whether an
enterprise is the primary beneficiary of a VIE. FASB ASC 810 also requires
enhanced disclosures about an enterprise's involvement with a VIE. Topic 810 is
effective as of the beginning of interim and annual reporting periods that begin
after November 15, 2009. This will not have an impact on the Company’s financial
position, results of operations or cash flows.
FASB ASC
No. 860 is effective as of the beginning of each reporting entity's first annual
reporting period that begins after November 15, 2009, for interim periods within
that first annual reporting period and for interim and annual reporting periods
thereafter. This will not have an impact on the Company’s financial position,
results of operations or cash flows.
66
Z&Z
Medical Holdings, Inc.
(A
Development Stage Company)
Notes
to Financial Statements
(3) Development
Stage Activities and Going Concern
The
Company is currently in the development stage, and its business plan is to
develop commercial relationships with third parties for the development,
marketing and sale of products based on the IP and to derive revenue through the
licensing of the IP to such third parties.
While
management of the Company believes that the Company will be successful in its
planned operating activities, there can be no assurance that the Company will be
successful in the development of its intellectual property, or services that
will generate sufficient revenues to sustain the operations of the
Company. The Company also intends to conduct additional capital
formation activities through the issuance of its common stock and to commence
operations.
The
accompanying financial statements have been prepared in conformity with
accounting principles generally accepted in the United States of America, which
contemplate continuation of the Company as a going concern. The
Company has incurred an operating loss since inception, had negative working
capital as of March 31, 2010, and 2009, and the cash resources of the Company
were insufficient to meet its planned business objectives. These and
other factors raise substantial doubt about the Company’s ability to continue as
a going concern. The accompanying financial statements do not include
any adjustments to reflect the possible future effects on the recoverability and
classification of assets or the amounts and classification of liabilities that
may result from the possible inability of the Company to continue as a going
concern.
(4) Common
Stock and Common Stock Warrants
On
December 1, 2007, the Company issued 8,468,050 shares of common stock to its
founders at approximately $0.02 per share based on the fair value of the shares
on the grant date. During the three months ended March 31, 2010 and
2009, the Company issued 450,000 and 0 shares, respectively, of common
stock. These subsequent issuances of common stock were issued for
cash at a per share amount of $0.50. The Company recognized proceeds
from these issuances during the three months ended March 31, 2010 and 2009 of
$225,000 and $0, respectively.
The
Company also issued warrants exercisable into common stock of the
Company. Certain common stock subscribers also received a warrant to
purchase one share of common stock for every subscription share
purchased. The exercise price of these warrants are equal to the
price of shares of securities of the Company issued in a subsequent equity
financing with an aggregate gross proceeds to the Company of at least
$2,500,000. However, in the event the Company’s valuation immediately prior to
the financing is less than $20,000,000, the exercise price of the warrant shall
be 50% of the purchase price per share of the Equity Securities
offered. Warrants issued in connection with the subscription
agreements were 450,000 and 0 in the three months ended March 31, 2010 and 2009,
respectively.
The
Company has also issued warrants to purchase 500,000 shares of common stock at
$0.50 per share to a director of the Company as compensation for services
rendered in connection with the reverse merger and financing consented to by a
majority of the stockholders of the Company.
67
Z&Z
Medical Holdings, Inc.
(A
Development Stage Company)
Notes
to Financial Statements
As of
March 31, 2010, there are warrants to purchase 1,600,000 shares of the Company’s
common stock outstanding with expiration dates ranging from February 2013
through March 2015.
|
Shares
underlying warrants issued:
|
March
31, 2010 |
March
31, 2009 |
|||
|
Beginning
balance
|
650,000 | 450,000 | |||
|
Shares
granted
|
950,000 | - | |||
|
Ending
balance
|
1,600,000 | 450,000 | |||
(5) Stock-Based
Compensation
The
Company’s 2010 Equity Incentive Plan (the “Plan”), which is not yet received
stockholder-approval, permits the grant of share options and shares to its
employees and affiliates for up to 4,362,964 shares of common stock. The Company
believes that such awards better align the interests of its employees and
affiliates with those of its stockholders. Option awards are generally granted
with an exercise price that approximates the market price of the Company’s stock
at the date of grant.
The
Company has granted and issued the stock options in the financial statements as
stockholder approval is nothing more than an administrative matter, consistent
with ASC 718, “Stock Compensation.”
On March
6, 2010, the Company issued options to purchase 245,000 shares of common stock
of the Company at $0.50 per share as part of an employment agreement with our
Chief Financial Officer. The option vests 25% on January 6, 2011 and
75% evenly on a monthly basis over the next three years thereafter and expire
January 7, 2017.
The fair
value of each option award is estimated on the date of grant using the
Black-Scholes option valuation model that uses the assumptions noted in the
following table. Because the Black-Scholes option valuation model incorporate
ranges of assumptions for inputs, those ranges are disclosed. Expected
volatilities are based on implied volatilities from traded options on the
companies in the industry that are very similar to Z&Z, and other factors.
The Company uses historical data of reference companies to estimate option
exercise and employee termination within the valuation model. The expected term
of options granted is derived from estimates and represents the period of time
that options granted are expected to be outstanding. The risk-free rate for
periods within the contractual life of the option is based on the U.S. Treasury
yield curve in effect at the time of grant. Assumptions used to calculate the
fair value of the options issued was as follows:
|
Three
months ended March
31, 2010 |
|||
|
Expected
life in years
|
3-7 | ||
|
Stock
price volatility
|
100%
to 263%
|
||
|
Risk
free interest rate
|
1.62 | ||
|
Expected
dividends
|
None
|
||
|
Forfeiture
rate
|
0 | % | |
68
Z&Z
Medical Holdings, Inc.
(A
Development Stage Company)
Notes
to Financial Statements
A summary
of option activity as of March 31, 2010 and changes during the three months
ended presented below:
|
Shares
|
Weighted
Exercise
Price
|
Remaining
Contractual
Term
|
|||||||||
|
Outstanding
- January 1, 2010
|
- | $ | - | - | |||||||
|
Shares
granted
|
245,000 | 0.50 | 7 | ||||||||
|
Exercised
|
- | - | - | ||||||||
|
Forfeited
|
- | - | - | ||||||||
|
Outstanding
– March 31, 2010
|
245,000 | $ | 0.50 | 7 | |||||||
|
Exercisable
– March 31, 2010
|
- | - | - | ||||||||
There
were no options exercised during the three months ended March 31, 2010 and 2009.
There is approximately $1,880 of unvested compensation at March 31,
2010.
(6) Income
Taxes
The
provision for income taxes for the periods ended March 31, 2010, and 2009, was
as follows (using a 42.8 percent effective Federal and state income tax
rate):
|
2010
|
2009
|
|||||||
|
Current
Tax Provision:
|
||||||||
|
Federal
and state-
|
||||||||
|
Taxable
income
|
$
|
-
|
$
|
-
|
||||
|
Total
current tax provision
|
$
|
-
|
$
|
-
|
||||
|
Deferred
Tax Provision:
|
||||||||
|
Federal
and state-
|
||||||||
|
Loss
carryforwards
|
$
|
(14,375
|
)
|
$
|
(1,308
|
)
|
||
|
Valuation
allowance
|
14,375
|
1,308
|
||||||
|
Total
deferred tax provision
|
$
|
-
|
$
|
-
|
||||
The
Company had deferred income tax assets as of March 31, 2010, and 2009, as
follows:
|
2010
|
2009
|
|||||||
|
Loss
carryforwards
|
$
|
(82,143
|
)
|
$
|
(42,456
|
)
|
||
|
Less
- valuation allowance
|
82,143
|
42,456
|
||||||
|
Total
net deferred tax assets
|
$
|
-
|
$
|
-
|
||||
As of
March 31, 2010 and 2009, respectively, the Company had net operating loss
carryforwards for income tax reporting purposes of approximately $82,143 and
$42,456 that may be offset against future taxable income. Current
tax laws limit the amount of loss available to be offset against future taxable
income when a substantial change in ownership occurs or a change in the nature
of the business. Therefore, the amount available to offset future
taxable income may be limited.
69
Z&Z
Medical Holdings, Inc.
(A
Development Stage Company)
Notes
to Financial Statements
No tax
benefit has been reported in the financial statements for the realization of
loss carryforwards, as the Company believes there is high probability that the
carryforwards will not be utilized in the foreseeable
future. Accordingly, the potential tax benefits of the loss
carryforwards are offset by a valuation allowance of the same
amount.
The
Company is primarily subject to U.S. federal and state income tax. As
a result of the implementation of certain provisions of ASC 740, Income Taxes,
(formerly FIN 48, Accounting for Uncertainty in Income Taxes – An Interpretation
of FASB Statement No. 109), the Company performed an analysis of its previous
tax filings and determined that there were no positions taken that it considered
uncertain. Therefore, there were no unrecognized tax benefits as of March 31,
2010 and 2009.
Future
changes in the unrecognized tax benefit are not expected to have an impact on
the effective tax rate due to the existence of the valuation allowance. The
Company estimates that the unrecognized tax benefit will not change within the
next twelve months. The Company will continue to classify income tax penalties
and interest, if any, as part of interest and other expenses in its statements
of operations. The Company has incurred no interest or penalties as of March 31,
2010.
(7) Commitments
and Contingencies
The
Company has executed three year employment contracts with Thomas Gardener,
Filiberto Zadini, Giorgio Zadini and Aaron Sandoval, each with a salary and
salary increases at the discretion of the Board of Directors not to exceed 10%
per annum or the previous calendar year's percentage increase in the Consumer
Price Index, whichever is greater. The contracts are not in effect until the
Company raises the equity financing with an aggregate gross proceeds to the
Company of at least $2,500,000. Effective March 22, 2010, Mr.
Sandoval resigned all positions with the company, effective
immediately.
Mr.
Gardner has signed an amended contract that will pay him $144,000 per year
effective upon closure of the Trist merger agreement. Mr. Selawski,
our Chief Financial Officer, has also executed an amended contract that will pay
him $144,000 per year upon closure of the Trist merger agreement.
The
Company has executed a contract in May 2008 with The University of California
(the “University”), on behalf of its Los Angeles Campus under which the
University shall conduct a laboratory study to demonstrate the efficacy of the
Company’s biocompatible emulsifiers on atherosclerotic lesions. The
contract calls for progress payments upon the completion of various stages of
the study and the total obligation to the University for completion of the study
totals $200,600. To date, a total of $190,150 has been paid on the
contract.
(8) Related
Party Transactions
The
Company had previously executed Notes Payable totaling $200,000 to two directors
of the Company for contribution of certain intellectual property. On
March 21, 2010, the Company and the note holders agreed to cancellation of the
obligation without recourse. The disinterested members of the Board
of Directors ratified this action.
70
Z&Z
Medical Holdings, Inc.
(A
Development Stage Company)
Notes
to Financial Statements
(9) Subsequent
Events
In
preparing these financial statements, the Company has evaluated events and
transactions for potential recognition or disclosure through May 17, 2010, the
date the financial statements were issued.
On April
23, 2010, the Board approved an amendment to all subscription warrants setting
the pre-merger price at $0.50 per share and making them immediately
exercisable. The respective expiration dates remain
unchanged.
May 13,
2010, the Company completed its reverse merger transactions with Trist Holdings,
Inc. (“Trist”), Immediately after the closing of the merger, Trist closed a
capital raise transaction through the placement of $1.5 million in 2.5% Senior
Secured Convertible Notes which mature 4 years after issuance. Interest on
the Convertible Notes, which will accrue at the rate of 2.5% per year, is not
payable until maturity or conversion, and is payable in cash or common stock.
Also, Trist has changed its name to AtheroNova Inc. to more accurately reflect
the Company's emphasis in the healthcare market and anticipates that the Company
will begin trading under a new symbol on the OTC Bulletin Board in the coming
days.
At the
closing, pursuant to the terms of the Merger Agreement dated March 26, 2010, by
and among Trist, Z&Z and Z&Z Merger Corp., all of the outstanding
shares, warrants and options of Z&Z were exchanged for shares of, and
warrants and options to purchase, Trist's Super-Voting Common Stock. Each
share of Trist's Super-Voting Common Stock will automatically convert into 50
shares (on a pre-reverse split basis) of Trist's Common Stock upon the
consummation of a 200-for-1 reverse split of Trist's Common Stock. As a
result of the merger, Z&Z stockholders own 88,575,048 shares of Trist's
Super-Voting Common Stock (22,143,771 shares of Trist's Common Stock on a
post-reverse split basis), or approximately 97.6% of the total shares
outstanding.
The
Convertible Notes are convertible into 3,817,596 shares of common stock (on a
post-reverse split basis), excluding accrued interest, which may also be paid in
stock. If held to maturity and all accrued interest is paid in common stock
an additional 381,762 shares would be issued.
The
purchasers of the Convertible Notes also received Warrants to purchase another
1,908,798 shares of common stock (on a post-reverse split basis) at an exercise
price of $0.39 per share (on a post-reverse split basis). The shares of
Trist's Common Stock issuable upon conversion (excluding the conversion of
accrued interest) and exercise of the Notes and Warrants represent approximately
17.6% of the Company's outstanding capital stock as of the closing of the
capital raise transaction on a fully-diluted basis.
As a
result of the merger and the capital raise transaction, Z&Z stockholders own
80.8% of Trist's fully-diluted Common Stock, Trist stockholders immediately
prior to the merger own 1.6% of Trist's fully-diluted Common Stock and the
holders of Convertible Notes and Warrants issued in the capital raise
transaction own 17.6% of Trist's fully-diluted Common Stock.
71
PART
II
INFORMATION
NOT REQUIRED IN PROSPECTUS
ITEM
13. Other Expenses of Issuance and Distribution.
The
Purchasers will bear all expenses of registration incurred in connection with
this offering. The selling stockholders whose shares are being
registered will bear all selling and other expenses. The following
table itemizes the expenses in connection with the offering. All the
amounts shown are estimates except the SEC registration fee.
|
Amount
|
||||
|
Registration
fee – SEC
|
$ | 12,275.91 | ||
|
Legal
fees and expenses
|
$ | 11,000.00 | ||
|
Accounting
fees and expenses
|
$ | 1,187.50 | ||
|
Miscellaneous
expenses
|
$ | 5,536.59 | ||
|
Total
|
$ | 30,000.00 | ||
ITEM
14. Indemnification of Directors and Officers.
The
Delaware General Corporation Law and certain provisions of our certificate of
incorporation, as amended, and bylaws under certain circumstances provide for
indemnification of our officers, directors and controlling persons against
liabilities which they may incur in such capacities.
In
general, any officer, director, employee or agent may be indemnified against
expenses, fines, settlements or judgments arising in connection with a legal
proceeding to which such person is a party, if that person’s actions were in
good faith, were believed to be in our best interest, and were not
unlawful. Unless such person is successful upon the merits in such an
action, indemnification may be awarded only after a determination by independent
decision of our board of directors, by legal counsel, or by a vote of the
stockholders, that the applicable standard of conduct was met by the person to
be indemnified.
The
circumstances under which indemnification is granted in connection with an
action brought on our behalf is generally the same as those set forth above;
however, with respect to such actions, indemnification is granted only with
respect to expenses actually incurred in connection with the defense or
settlement of the action. In such actions, the person to be
indemnified must have acted in good faith and in a manner believed to have been
in our best interest, and have not been adjudged liable for negligence or
misconduct.
Indemnification
may also be granted pursuant to the terms of agreements which may be entered in
the future or pursuant to a vote of stockholders or directors. The
provision cited above also grants us the power to purchase and maintain
insurance which protects our officers and directors against any liabilities
incurred in connection with their service in such a position, and such a policy
may be obtained by us.
We do not
have any indemnification agreements with any of our directors or executive
officers.
A
stockholder’s investment may be adversely affected to the extent we pay the
costs of settlement and damage awards against directors and officers as required
by these indemnification provisions. At present, there is no pending
litigation or proceeding involving any of our directors, officers or employees
regarding which indemnification by us is sought, nor are we aware of any
threatened litigation that may result in claims for
indemnification.
II-1
Insofar
as indemnification for liabilities arising under the Securities Act of 1933 may
be permitted to directors, officers or persons controlling the registrant
pursuant to the foregoing provisions, the registrant has been informed that in
the opinion of the Securities and Exchange Commission such indemnification is
against public policy as expressed in the Act and is therefore
unenforceable.
ITEM
15. Recent Sales of Unregistered Securities.
AtheroNova
On May
13, 2010, in connection with the Merger we converted $540,984 of outstanding
indebtedness into 90,166 shares (18,032,810 shares on a pre-reverse stock split
basis) of our common stock, issued 88,575,048 shares of Super-Voting Common
Stock to AtheroNova Operations’ former stockholders (which converted into
22,143,771 shares of our common stock on June 23, 2010) and assumed options and
warrants which were exercisable to purchase an aggregate of 1,845,000 shares of
AtheroNova Operations’ common stock at a per share price of $0.50 and are now
exercisable to purchase 4,138,056 shares of our common stock at a per share
price of approximately $0.22, and, in connection with the Capital Raise
Transaction, issued the Notes and Warrants.
In
connection with the above security issuances, we did not pay any underwriting
discounts or commissions. None of the sales of securities described
or referred to above was registered under the Securities Act. In
making the sales without registration under the Securities Act, we relied upon
one or more of the exemptions from registration contained in Sections 4(2) of
the Securities Act, and in Regulation D promulgated under the Securities
Act. No general solicitation or advertising was used in connection
with the sales.
AtheroNova
Operations
On March
6, 2010, AtheroNova Operations issued options to Mark Selawski to purchase
245,000 shares of AtheroNova Operations’ common stock at a purchase price of
$0.50 per share. We assumed these options in the Merger which now
entitle Mr. Selawski to purchase 549,498 shares of our common stock at a
purchase price of approximately $0.22 per share. Such options vest
25% on January 6, 2011 and 75% evenly on a monthly basis over the next three
years thereafter and expire on January 7, 2017.
On March
6, 2010, AtheroNova Operations issued warrants to Boris Ratiner to purchase
650,000 shares of AtheroNova Operations’ common stock at an exercise price of
$0.50 per share. We assumed these warrants in the Merger, which now
entitle Mr. Ratiner to purchase 1,457,852 shares of our common at a per share
price of approximately $0.22. Such warrants are fully vested and
expire on January 7, 2015.
In
connection with the above security issuances, AtheroNova Operations did not pay
any underwriting discounts or commissions. None of the grants of
securities described above was registered under the Securities Act in reliance
upon Rule 701 thereunder.
II-2
ITEM
16. Exhibits.
See
attached Exhibit Index.
ITEM
17. Undertakings.
The
undersigned registrant hereby undertakes to:
(1) File,
during any period in which offers or sells are being made, a post-effective
amendment to this registration statement:
(i) To
include any prospectus required by section 10(a)(3) of the Securities Act of
1933;
(ii) To
reflect in the prospectus any facts or events arising after the effective date
of the registration statement (or the most recent post-effective amendment
thereof) which, individually or in the aggregate, represent a fundamental change
in the information set forth in the registration
statement. Notwithstanding the foregoing, any increase or decrease in
volume of securities offered (if the total dollar value of securities offered
would not exceed that which was registered) and any deviation from the low or
high end of the estimated maximum offering range may be reflected in the form of
prospectus filed with the Commission pursuant to Rule 424(b) if, in the
aggregate, the changes in volume and price represent no more than 20% change in
the maximum aggregate offering price set forth in the "Calculation of
Registration Fee" table in the effective registration statement.
(iii) To
include material information with respect to the plan of distribution not
previously disclosed in the registration statement or any material change to
such information in the registration statement;
(2) That,
for the purpose of determining any liability under the Securities Act of 1933,
each such post-effective amendment shall be deemed to be a new registration
statement relating to the securities offered therein, and the offering of such
securities at that time shall be deemed to be the initial bona fide offering
thereof.
(3) To
remove from registration by means of a post-effective amendment any of the
securities being registered which remain unsold at the termination of the
offering.
(4) That,
for the purpose of determining liability under the Securities Act of 1933 to any
purchaser, each prospectus filed pursuant to Rule 424(b) as part of a
registration statement relating to an offering, other than registration
statements relying on Rule 430B or other than prospectuses filed in reliance on
Rule 430A, shall be deemed to be part of and included in the registration
statement as of the date it is first used after
effectiveness. Provided, however, that no statement made in a
registration statement or prospectus that is part of the registration statement
or made in a document incorporated or deemed incorporated by reference into the
registration statement or prospectus that is part of the registration statement
will, as to a purchaser with a time of contract of sale prior to such first use,
supersede or modify any statement that was made in the registration statement or
prospectus that was part of the registration statement or made in any such
document immediately prior to such date of first use.
II-3
Insofar
as indemnification for liabilities arising under the Securities Act of 1933 may
be permitted to directors, officers and controlling persons of the registrant
pursuant to the foregoing provisions, or otherwise, the registrant has been
advised that in the opinion of the Securities and Exchange Commission such
indemnification is against public policy as expressed in the Act and is,
therefore, unenforceable. In the event that a claim for
indemnification against such liabilities (other than the payment by the
registrant of expenses incurred or paid by a director, officer or controlling
person of the registrant in the successful defense of any action, suit or
proceeding) is asserted by such director, officer or controlling person in
connection with the securities being registered, the registrant will, unless in
the opinion of its counsel the matter has been settled by controlling precedent,
submit to a court of appropriate jurisdiction the question whether such
indemnification by it is against public policy as expressed in the Act and will
be governed by the final adjudication of such issue.
II-4
SIGNATURES
Pursuant
to the requirements of the Securities Act of 1933, as amended, the registrant
has duly caused this registration statement to be signed on its behalf by the
undersigned, thereunto duly authorized in the City of Irvine, State of
California, on June 29, 2010.
|
ATHERONOVA
INC.
(Registrant)
|
|||
|
|
By:
|
/s/ Mark Selawski | |
|
Mark
Selawski
Chief
Financial Officer & Secretary
|
|||
POWER
OF ATTORNEY
Each
person whose signature appears below constitutes and appoints each of Thomas W.
Gardner and Mark Selawski as his true and lawful attorney-in-fact and agent with
full power of substitution and resubstitution, for him and his name, place and
stead, in any and all capacities, to sign any or all amendments (including
post-effective amendments) to this Registration Statement and to file a new
registration statement under Rule 461, and to file the same, with all exhibits
thereto, and other documents in connection therewith, with the Securities and
Exchange Commission, granting unto said attorneys-in-fact and agents, and each
of them, full power and authority to do and perform each and every act and thing
requisite and necessary to be done in and about the foregoing, as fully to all
intents and purposes as he might or could do in person, hereby ratifying and
confirming all that said attorneys-in-fact and agents, or either of them, or
their substitutes, may lawfully do or cause to be done by virtue
hereof.
Pursuant
to the requirements of the Securities Act of 1933, as amended, this registration
statement has been signed by the following persons in the capacities and on the
dates indicated.
|
Signature
|
Title
|
Date
|
||
|
/s/
Thomas
W. Gardner
|
Chief
Executive Officer and President
|
June
29, 2010
|
||
|
Thomas
W. Gardner
|
||||
|
/s/
Mark
Selawski
|
Chief
Financial Officer and Secretary
|
June
29, 2010
|
||
|
Mark
Selawski
|
||||
|
/s/
Filiberto
Zadini, M.D.
|
Director
|
June
29, 2010
|
||
|
Filiberto
Zadini, M.D.
|
|
/s/
Boris
Ratiner, M.D.
|
Director
|
June
29, 2010
|
||
|
Boris
Ratiner, M.D.
|
||||
|
/s/
Chaim
Davis
|
Director
|
June
29, 2010
|
||
|
Chaim
Davis
|
||||
|
/s/
Gary
Freeman
|
Director
|
June
29, 2010
|
||
|
Gary
Freeman
|
EXHIBIT
INDEX
|
Exhibit
Number
|
Description of Exhibit
|
|
|
2.1
|
Merger
Agreement by and between Trist Holdings, Inc., Z&Z Merger Corporation
and Z&Z Medical Holdings, Inc., dated March 26,
2010. Incorporated by reference to Exhibit 2.1 to the Current
Report on Form 8-K (File No. 000-52315) filed with the Securities and
Exchange Commission on April 1, 2010.
|
|
|
3.1
|
Amended
and Restated Certificate of Incorporation. Incorporated by
reference to Exhibit 3.1 to the Current Report on Form 8-K (File No.
000-52315) filed with the Securities and Exchange Commission on June 25,
2010.
|
|
|
3.2
|
Amended
and Restated Bylaws. Incorporated by reference to Exhibit 3.2
to the Current Report on Form 8-K (File No. 000-52315) filed with the
Securities and Exchange Commission on June 23, 2010.
|
|
|
4.1
|
Amended
and Restated Certificate of Incorporation. Incorporated by
reference to Exhibit 3.1.
|
|
|
4.2
|
Amended
and Restated Bylaws. Incorporated by reference to Exhibit
3.2.
|
|
|
4.3
|
2010
Stock Incentive Plan. Incorporated by reference to Exhibit B to
the Definitive Information Statement on Schedule 14C (File No. 000-52315)
filed with the Securities and Exchange Commission on June 3,
2010.
|
|
|
5.1
|
Opinion
re legality.
|
|
|
10.1
|
Employment
Agreement of Thomas W. Gardner. Incorporated by reference to
Exhibit 10.1 to the Current Report on Form 8-K (File No. 000-52315) filed
with the Securities and Exchange Commission on May 20,
2010.
|
|
|
10.2
|
Employment
Agreement of Mark Selawski. Incorporated by reference to
Exhibit 10.2 to the Current Report on Form 8-K (File No. 000-52315) filed
with the Securities and Exchange Commission on May 20,
2010.
|
|
|
10.3
|
Securities
Purchase Agreement dated May 13, 2010, among AtheroNova Inc., W-Net Fund
I, L.P., Europa International, Inc. and MKM Opportunity Master Fund,
Ltd. Incorporated by reference to Exhibit 10.3 to the Current
Report on Form 8-K (File No. 000-52315) filed with the Securities and
Exchange Commission on May 20, 2010.
|
|
|
10.4
|
Registration
Rights Agreement dated May 13, 2010, among AtheroNova Inc., W-Net Fund I,
L.P., Europa International, Inc. and MKM Opportunity Master Fund,
Ltd. Incorporated by reference to Exhibit 10.4 to the Current
Report on Form 8-K (File No. 000-52315) filed with the Securities and
Exchange Commission on May 20, 2010.
|
|
|
10.5
|
Security
Agreement dated May 13, 2010, among AtheroNova Inc., W-Net Fund I, L.P.,
Europa International, Inc. and MKM Opportunity Master Fund,
Ltd. Incorporated by reference to Exhibit 10.5 to the Current
Report on Form 8-K (File No. 000-52315) filed with the Securities and
Exchange Commission on May 20, 2010.
|
|
|
10.6
|
IP
Security Agreement dated May 13, 2010, among AtheroNova Inc., W-Net Fund
I, L.P., Europa International, Inc. and MKM Opportunity Master Fund,
Ltd. Incorporated by reference to Exhibit 10.1 to the Current
Report on Form 8-K (File No. 000-52315) filed with the Securities and
Exchange Commission on May 20, 2010.
|
|
|
10.7
|
Form
of Promissory Note. Incorporated by reference to Exhibit 10.7
to the Current Report on Form 8-K (File No. 000-52315) filed with the
Securities and Exchange Commission on May 20, 2010.
|
|
|
10.8
|
Form
of Warrant. Incorporated by reference to Exhibit 10.8 to the
Current Report on Form 8-K (File No. 000-52315) filed with the Securities
and Exchange Commission on May 20, 2010.
|
|
|
21.1
|
Subsidiaries
of the Registrant.
|
|
|
23.1
|
Consent
of Independent Registered Public Accounting Firm.
|
|
|
23.2
|
Consent
of Legal Counsel. Incorporated by reference to Exhibit
5.1.
|
|
|
24.1
|
Power
of Attorney. Incorporated by reference to the signature page to
this Registration Statement on Form
S-1.
|

