Attached files
| file | filename |
|---|---|
| EX-31.1 - EXHIBIT 31.1 - CHINA JO-JO DRUGSTORES, INC. | a6343246ex311.htm |
| EX-32.1 - EXHIBIT 32.1 - CHINA JO-JO DRUGSTORES, INC. | a6343246ex321.htm |
| EX-99.5 - EXHIBIT 99.5 - CHINA JO-JO DRUGSTORES, INC. | a6343246ex995.htm |
| EX-99.4 - EXHIBIT 99.4 - CHINA JO-JO DRUGSTORES, INC. | a6343246ex994.htm |
| EX-31.2 - EXHIBIT 31.2 - CHINA JO-JO DRUGSTORES, INC. | a6343246ex312.htm |
| EX-32.2 - EXHIBIT 32.2 - CHINA JO-JO DRUGSTORES, INC. | a6343246ex322.htm |
UNITED STATES SECURITIES AND EXCHANGE COMMISSION
Washington, D. C. 20549
FORM 10-K
|
o
|
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
For the fiscal year ended March 31, 2010
|
o
|
TRANSITION REPORT UNDER SECTION 13 OR 15 (d) OF THE EXCHANGE ACT OF 1934
|
For the transition period from _____ to __________
Commission file number 001-34711
CHINA JO-JO DRUGSTORES, INC.
(Exact name of registrant as specified in its charter)
|
Nevada
|
98-0557852
|
|
|
(State or other jurisdiction of incorporation or organization)
|
(IRS Employer Identification No.)
|
Room 507-513, 5th Floor A Building, Meidu Plaza
Gongshu District
Hangzhou, Zhejiang Province
People’s Republic of China
(Address of Principal Executive Offices)
+86 (571) 88077078
(Issuer Telephone Number)
N/A
(Former name or former address, if changed since last report)
|
Title of Each Class
|
Name of Each Exchange on Which Registered
|
|
|
Common Stock $0.001 Par Value
|
NASDAQ Capital Market
|
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes o No þ
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes o No þ
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes þ No o
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes o No o
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (§229.405 of this chapter) is not contained herein, and will not be contained herein, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. o
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act.
|
Large accelerated filer o
|
Accelerated filer o
|
||
|
Non-accelerated filer o
|
Smaller reporting company þ
|
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes o No þ
As of June 25, 2010, the aggregate market value of the voting stock held by non-affiliates of the registrant was approximately $28,480,008 based on a closing price of $4.00 per share of common stock as reported on the NASDAQ Stock Market on such date.
The registrant had a total of 13,500,002 shares of common stock outstanding as of June 25, 2010.
1
TABLE OF CONTENTS
TO ANNUAL REPORT ON FORM 10-K
FOR YEAR ENDED MARCH 31, 2010
|
Page
|
||
| 4 | ||
| 22 | ||
| 42 | ||
| 42 | ||
| 42 | ||
| 42 | ||
| 42 | ||
| 44 | ||
| 44 | ||
| 52 | ||
| 52 | ||
| 52 | ||
| 52 | ||
| 54 | ||
| 54 | ||
| 57 | ||
| 60 | ||
| 61 | ||
| 62 | ||
|
PART IV
|
||
| 62 | ||
| 65 |
2
CAUTION REGARDING FORWARD-LOOKING INFORMATION
This report contains forward-looking statements. All forward-looking statements are inherently uncertain as they are based on current expectations and assumptions concerning future events or future performance of the Company. Readers are cautioned not to place undue reliance on these forward-looking statements, which are only predictions and speak only as of the date hereof. Forward-looking statements usually contain the words “estimate,” “anticipate,” “believe,” “expect,” or similar expressions, and are subject to numerous known and unknown risks and uncertainties. In evaluating such statements, prospective investors should carefully review various risks and uncertainties identified in this Report, including the matters set forth under the captions “Risk Factors” and in the Company’s other SEC filings. These risks and uncertainties could cause the Company’s actual results to differ materially from those indicated in the forward-looking statements. The Company undertakes no obligation to update or publicly announce revisions to any forward-looking statements to reflect future events or developments.
Although forward-looking statements in this annual report on Form 10-K reflect the good faith judgment of our management, such statements can only be based on facts and factors currently known by us. Consequently, forward-looking statements are inherently subject to risks and uncertainties, and actual results and outcomes may differ materially from the results and outcomes discussed in or anticipated by the forward-looking statements. Factors that could cause or contribute to such differences in results and outcomes include, without limitation, those specifically addressed under the heading “Risks Relating to Our Business” below, as well as those discussed elsewhere in this annual report on Form 10-K. Readers are urged not to place undue reliance on these forward-looking statements, which speak only as of the date of this annual report on Form 10-K. We file reports with the Securities and Exchange Commission (“SEC”). You can read and copy any materials we file with the SEC at the SEC’s Public Reference Room, 100 F. Street, NE, Washington, D.C. 20549 on official business days during the hours of 10 a.m. to 3 p.m. You can obtain additional information about the operation of the Public Reference Room by calling the SEC at 1-800-SEC-0330. In addition, the SEC maintains an Internet site (www.sec.gov) that contains reports, proxy and information statements, and other information regarding issuers that file electronically with the SEC, including the Company.
We undertake no obligation to revise or update any forward-looking statements in order to reflect any event or circumstance that may arise after the date of this annual report on Form 10-K. Readers are urged to carefully review and consider the various disclosures made throughout the entirety of this annual report, which attempt to advise interested parties of the risks and factors that may affect our business, financial condition, results of operations and prospects.
3
ITEM 1. BUSINESS
Overview
China Jo-Jo Drugstores, Inc. (“China Jo-Jo” or the “Company”), formerly known as Kerrisdale Mining Corporation, was incorporated in Nevada on December 19, 2006. We own and operate a retail pharmacy chain in the People’s Republic of China (“PRC” or “China”). We currently have 31 stores in Hangzhou, the capital of Zhejiang Province approximately 112 miles south of Shanghai. Our stores provide customers with a wide variety of medicinal products, including prescription and over-the-counter (“OTC”) drugs, nutritional supplements, traditional Chinese medicine (“TCM”) products, personal care products, family care products, medical devices, as well as convenience products including consumable, seasonal and promotional items. Each of our stores typically carries approximately 2,500 to 7,500 different products. In addition to these products, we have licensed doctors of both western medicine and TCM onsite for consultation, examination and treatment of common ailments at scheduled hours. Two of our stores have adjacent medical clinics offering urgent care (to provide treatment for minor ailments such as sprains, minor lacerations and dizziness which can be treated on an outpatient basis), TCM (including acupuncture, therapeutic massage and cupping) and minor outpatient surgical treatments (such as suturing). Our store locations vary in size; however, our 31 stores presently average approximately 3,592 square feet. We attempt to tailor our product offerings, physician access and operating hours based on the community where each individual store is located.
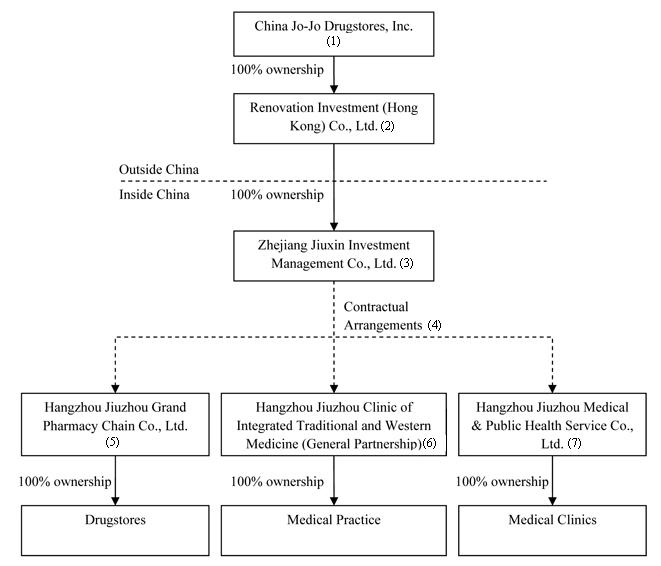
All of our operations are carried out by three PRC companies: (1) Hangzhou Jiuzhou Grand Pharmacy Chain Co., Ltd. (“Jiuzhou Pharmacy”), (2) Hangzhou Jiuzhou Clinic of Integrated Traditional and Western Medicine (General Partnership) (“Jiuzhou Clinic”), and (3) Hangzhou Jiuzhou Medical & Public Health Service Co., Ltd. (“Jiuzhou Service”). Throughout this Form 10-K, we will sometimes refer to Jiuzhou Pharmacy, Jiuzhou Clinic and Jiuzhou Service collectively as “HJ Group.” We do not own any equity interests in any of the three HJ Group companies, but control and receive the economic benefits of their business operations through contractual arrangements. The contractual arrangements are between these companies and their owners, on the one hand, and Zhejiang Jiuxin Investment Management Co., Ltd. (“Jiuxin Management”), the wholly owned subsidiary of Renovation Investment (Hong Kong) Co., Ltd. (“Renovation”), which became our wholly-owned subsidiary subsequent to a share exchange transaction on September 17, 2009.
Such contractual arrangements are necessary to comply with Chinese laws limiting foreign ownership of certain companies. Through these contractual arrangements, we have the ability to substantially influence HJ Group’s daily operations and financial affairs, appoint its senior executives and approve all matters requiring shareholder approval. As a result of these contractual arrangements, which enable us to control HJ Group, we are considered the primary beneficiary of HJ Group.
Our History and Corporate Structure
We were incorporated in Nevada on December 19, 2006, under the name “Kerrisdale Mining Corporation”, with a principal business objective to acquire and develop mineral properties. Although we had acquired certain mining claims, we were not operational.
On July 14, 2008, we amended our Articles of Incorporation to change our authorized capital stock from 75,000,000 shares of common stock, par value $0.001 per share, to 500,000,000 shares of common stock, par value $0.001 per share, and 10,000,000 shares of preferred stock, par value $0.001. The preferred stock is “blank check,” with the right to set its designations, preferences, limitations, privileges, qualifications, dividend, conversion, voting, and other special or relative rights, conferred on our board of directors.
On September 17, 2009 (the “Closing Date”), we executed a share exchange agreement (the “Exchange Agreement”) with Renovation and the holders of 100% of Renovation’s issued and outstanding capital stock (the “Renovation Stockholders”). Pursuant to the Exchange Agreement, on the Closing Date, we issued 7,900,000 shares of our common stock to the Renovation Stockholders in exchange for 100% of the issued and outstanding capital stock of Renovation.
4
The Share Exchange was accounted for as a reverse merger (recapitalization) with Renovation deemed to be the accounting acquirer, and us as the legal acquirer. Accordingly, the historical financial information presented in future financial statements will be that of Renovation as adjusted to give effect to any difference in the par value of ours and Renovation’s capital stock with an offset to capital in excess of par value. The basis of the assets, liabilities and retained earnings of Renovation, the accounting acquirer, have been carried over in the recapitalization. In connection with the Share Exchange, we became a Chinese retail drugstore chain.
On September 24, 2009, we amended our Articles of Incorporation to change our name from “Kerrisdale Ming Corporation” to “China Jo-Jo Drugstores, Inc.” to better reflect our business direction after the Share Exchange.
Renovation
Renovation is a limited liability company incorporated in Hong Kong on September 2, 2008. Renovation was formed by the owners of HJ Group as a special purpose vehicle for purposes of raising capital, in accordance with requirements of the PRC State Administration of Foreign Exchange (“SAFE”). Specifically, on May 31, 2007, SAFE issued the Notice on Relevant Issues Concerning Foreign Exchange Administration for PRC Residents to Engage in Financing and Inbound Investment via Overseas Special Purpose Vehicles (“Circular No. 75,”) on October 21, 2005, and an official notice known as Hi Zhong Fa [2007] No. 106 (“Circular 106”) on May 31, 2007, which requires the owners of any Chinese company to obtain SAFE’s approval before establishing any offshore holding company structure for foreign financing as well as subsequent acquisition matters in China. Accordingly, the owners of HJ Group, namely Lei Liu, Li Qi and Chong’an Jin, submitted their applications to SAFE on July 25, 2008. On August 16, 2008, SAFE approved the application, permitting these Chinese nationals to establish Renovation as an offshore, special purpose vehicle which may have foreign ownership and participate in foreign capital raising activities. After SAFE’s approval, Mr. Liu, Ms. Qi and Dr. Jin became holders of 100% of Renovation’s issued and outstanding capital stock on September 2, 2008.
Jiuxin Management
Jiuxin Management was organized in the PRC on October 14, 2008. Because all of its issued and outstanding capital stock is held by Renovation, a Hong Kong company, Jiuxin Management is deemed a “wholly foreign owned enterprise” (“WFOE”) under PRC laws. The principal purpose of Jiuxin Management is to manage, hold and own rights in and to the businesses and profits of the HJ Group companies through a series of contractual arrangements. We do not own any equity interests in Jiuzhou Pharmacy, Jiuzhou Clinic or Jiuzhou Service, but control and receive the economic benefits of their respective business operations through contractual arrangements. Each of Jiuzhou Pharmacy, Jiuzhou Clinic and Jiuzhou Service has the licenses and approvals necessary to operate its businesses in China. Through Jiuxin Management, we have contractual arrangements with each of them and their owners pursuant to which we provide consulting and other general business operation services. Through these contractual arrangements, we also have the ability to substantially influence their daily operations and financial affairs, since we are able to appoint their senior executives and approve all matters requiring approval of the equity owners. As a result of these contractual arrangements, which enable us to control each constituent HJ Group company and to receive, through Jiuxin Management, all of their profits, we are considered the primary beneficiary of HJ Group. Accordingly, we consolidate its results, assets and liabilities in our financial statements. Other than activities relating to its contractual arrangements with HJ Group, Jiuxin Management has no other separate operations of its own.
HJ Group
Jiuzhou Pharmacy is a PRC limited liability company established in Zhejiang Province on September 9, 2003, with registered capital of RMB 5 million which has been fully paid by its owners. Jiuzhou Pharmacy’s principal offices are in Hangzhou at Room 507-513, 5th Floor, A Building, Meidu Plaza, Gongshu District. The three owners of Jiuzhou Pharmacy are Lei Liu (55%), who is also the executive director of the company, Chong’an Jin (23%) and Li Qi (22%). Jiuzhou Pharmacy operates a chain of pharmacies in Hangzhou that is presently comprised of 31 stores, of which 30 are branch stores with no separate corporate existence and 1 (Kuaileren branch) is a subsidiary of Jiuzhou Pharmacy. Kuaileren was established on May 9, 2006, with registered capital of RMB 100,000. Its sole owner transferred all of his ownership interest to the three owners of HJ Group for no consideration on June 30, 2009, who in turn transferred the ownership interest to Jiuzhou Pharmacy for no consideration on August 28, 2009.
5
Jiuzhou Clinic is a PRC general partnership established in Zhejiang Province on October 10, 2003. Jiuzhou Clinic’s principal offices are in Hangzhou City at No. 12, De Yuan Road, Da Guan Community, Gongshu District. The three partners of Jiuzhou Clinic are Lei Liu (39%), Li Qi (30%) and Chong’an Jin (31%), who is also the managing partner of the partnership. Jiuzhou Clinic is a medical practice currently operating adjacent to Jiuzhou Pharmacy’s Daguan branch, providing primary, urgent, minor surgical and traditional medical care services. Additionally, Jiuzhou Clinic’s physicians consult with, and examine, patients at other Jiuzhou Pharmacy stores.
Jiuzhou Service is a PRC limited liability company established in Zhejiang Province on November 2, 2005, with registered capital of RMB 500,000 which has been fully paid by its owners. Jiuzhou Service’s principal offices are in Hangzhou City at 3rd Floor, No. 2 South Road, Wenjiao Avenue, Xiasha Town. The three owners of Jiuzhou Service are Lei Liu (39%), Li Qi (30%) and Chong’an Jin (31%), who is also the executive director of the company. Jiuzhou Service is licensed as a healthcare management company and currently manages the medical clinic operating adjacent to Jiuzhou Pharmacy’s Wenhua branch that provides services similar to those provided by Jiuzhou Clinic.
Contractual Arrangements with HJ Group and their Owners
Our relationships with the three HJ Group companies and their owners are governed by a series of contractual arrangements that they have entered into with Jiuxin Management.
PRC regulations on foreign investment currently permit foreign companies to establish or invest in WFOEs or joint ventures that engage in wholesale or retail sales of pharmaceuticals in China. For retail sales, however, these regulations restrict the number and size of retail pharmacy stores that a foreign investor may establish. If a foreign investor owns more than 30 stores that sell a variety of branded pharmaceutical products sourced from different suppliers, such foreign investor’s ownership interests in the stores are limited to 49.0%. The contractual arrangements with Jiuzhou Pharmacy enable us to bypass such restrictions, since neither we nor our subsidiaries own equity interests in Jiuzhou Pharmacy, while at the same time, we retain control of the drugstore chain by virtue of the contractual arrangements.
Similarly, PRC regulations place certain restrictions on foreign ownership of medical practice. Foreign investors can acquire ownership interests through a Sino-foreign joint venture only and cannot do so through a WFOE. Since we do not have actual equity interest in Jiuzhou Clinic or Jiuzhou Service, but control these entities through contractual arrangements, the PRC regulations restricting foreign ownership of medical practice are not applicable to us or our structure.
Under PRC laws, Jiuxin Management, Jiuzhou Pharmacy, Jiuzhou Medical and Jiuzhou Clinic are each an independent business entity not exposed to the liabilities incurred by any of the other three entities. The contractual arrangements constitute valid and binding obligations of the parties of such agreements. Each of the contractual arrangements and the rights and obligations of the parties thereto are enforceable and valid in accordance with the laws of the PRC. These contractual arrangements, as amended and in effect, include the following:
Consulting Services Agreement. Pursuant to the exclusive consulting services agreement, Jiuxin Management has the exclusive right to provide to each HJ Group company with general business operation services, including advice and strategic planning, as well as consulting services related to their current and future operations (the “Services”). Additionally, Jiuxin Management owns the intellectual property rights developed or discovered through research and development, in the course of providing the Services, or derived from the provision of the Services. Each HJ Group company pays a quarterly consulting service fees in RMB to Jiuxin Management that is equal to its profits for such quarter. This agreement is in effect unless and until terminated by written notice of either Jiuxin Management or HJ Group in the event that: (a) a party becomes bankrupt, insolvent, is the subject of proceedings or arrangements for liquidation or dissolution, ceases to carry on business, or becomes unable to pay its debts as they become due; (b) Jiuxin Management terminates its operations; or (c) circumstances arise which would materially and adversely affect the performance or the objectives of the agreement. Jiuxin Management may also terminate the agreement if HJ Group breach the terms of the agreement, or without cause.
6
Operating Agreement. Pursuant to the operating agreement, Jiuxin Management agrees to guarantee the HJ Group companies’ contractual performance of their agreements with any third party. In return, the owners of HJ Group (the “Owners”) must appoint designees of Jiuxin Management to HJ Group’s boards of directors and senior management. In addition, each HJ Group company agrees to pledge its accounts receivable and all of its assets to Jiuxin Management. Moreover, the HJ Group companies agree that without the prior consent of Jiuxin Management, they will not engage in any transactions that could materially affect their respective assets, liabilities, rights or operations, including, without limitation, incurrence or assumption of any indebtedness, sale or purchase of any assets or rights, incurrence of any encumbrance on any of their assets or intellectual property rights in favor of a third party or transfer of any agreements relating to their business operation to any third party. The HJ Group companies further agree to abide by corporate policies set by Jiuxin Management with respect to their daily operations, financial management and employment issues. The term of this agreement is from August 1, 2009 until the maximum period of time permitted by law. On the other hand, HJ Group cannot terminate this agreement.
Equity Pledge Agreement. Pursuant to the equity pledge agreement, the Owners pledge all of their equity interests in HJ Group to Jiuxin Management in order to guarantee the three HJ Group companies’ performance of their respective obligations under the consulting services agreement. If HJ Group or the Owners breaches their respective contractual obligations, Jiuxin Management, as pledgee, will be entitled to certain rights, including the right to sell the pledged equity interests. The Owners have also agreed that upon occurrence of any event of default, Jiuxin Management shall be granted an exclusive, irrevocable power of attorney to take actions in the place and stead of the Owners to carry out the security provisions of this agreement and take any action and execute any instrument that Jiuxin Management may deem necessary or advisable to accomplish the purposes of this agreement. The Owners agree not to dispose of the pledged equity interests or take any actions that would prejudice Jiuxin Management’s interests. This agreement will expire two (2) years after HJ Group’s obligations under the consulting services agreements have been fulfilled.
Option Agreement. Pursuant to the option agreement, the Owners irrevocably grant Jiuxin Management or its designee an exclusive option to purchase, to the extent permitted under PRC law, all or part of HJ Group’s equity interests for the cost of the initial contributions to the registered capital or the minimum amount of consideration permitted by applicable PRC law. Jiuxin Management or its designee has sole discretion to decide when to exercise the option, whether in part or in full. The term of this agreement is from August 1, 2009 until the maximum period of time permitted by law.
Proxy Agreement. Pursuant to the proxy agreement, the Owners irrevocably grant a Jiuxin Management designee with the right to exercise their voting and other ownership rights in HJ Group, including the rights to attend any meeting of the Owners (or participate by written consent in lieu of such meeting) in accordance with applicable laws and the HJ Group companies’ incorporating documents, as well as the rights to sell or transfer all or any of the Owners’ equity interests in HJ Group, and to appoint and vote for the directors of HJ Group. The proxy agreement may be terminated by mutual consent of the parties or upon 30-day written notice from Jiuxin Management.
Other than pursuant to the forgoing contractual arrangements, the three HJ Group companies cannot transfer any funds generated from their respective operations. The contractual arrangements were originally entered into on August 1, 2009, and amended on October 27, 2009.
Recent Events
On April 9, 2010, we effected a 1-for-2 reverse stock split of our issued and outstanding shares of common stock and a proportional reduction of our authorized shares of common stock, by filing a Certificate of Change Pursuant to Nevada Revised Statutes 78.209 with the Nevada Secretary of State on April 6, 2010. All share information in this Form 10-K takes into account this reverse stock split.
On April 28, 2010, we completed a registered public offering of 3.5 million shares of common stock at a price of $5.00 per share, resulting in gross proceeds to us, prior to deducting underwriting discounts, commissions and offering expenses, of approximately $17.5 million.
On May 18, 2010, the registration of the Owners’ pledged equity interests in Jiuzhou Pharmacy with the local State Administration of Industry and Commerce was completed.
7
The following diagram illustrates our corporate structure as of the date of this Form 10-K:
Our Industry
We operate in the drugstore industry in China. Below is an overview of our industry’s background, recent environment as well as relevant trends.
Demographics
China has 1.3 billion people, approximately one-fifth of the world’s population. The portion of the Chinese population aged 60 and above has been increasing for the past two decades, both in absolute numbers and as a percentage of the total population, and this trend is likely to continue in the next decade. According to the PRC National Bureau of Statistics, the percentage of the total population that is 65 and older increased from 5.6% in 1990 to 8.5% in 2008. On average, older people spend more on healthcare, particularly because the prevalence of several diseases typically increases with age. For instance, urban residents aged 60 and above in 2000 spent on average five times more than urban residents below the age of 60 in the same year, according to the China National Information Center.
Economic Growth
The key driver of China’s pharmaceutical market over the medium term is the economy, which has grown significantly in recent years. According to the PRC National Bureau of Statistics, China’s gross domestic product, or GDP, increased from RMB 11 trillion in 2001 to RMB 30 trillion in 2008. Over the same period, the average annual household disposable income of urban residents increased from RMB 6,860 to RMB 15,781. Despite the global financial crisis which started in the summer of 2007, the World Bank expects the Chinese economy to grow at 8% in 2009 and expects the growth to be robust in 2010.
8
The significant growth of China’s economy as well as the increase in disposable income among its consumers have led to increased public health awareness and a stronger focus on disease prevention, general wellness and the early diagnosis of medical conditions. This has in turn led to stronger demand for pharmaceutical and other healthcare related products, including nutritional supplements, Traditional Chinese Medicine (“TCM”) and herbal products. In addition, as living standards in China continue to improve, many lifestyle-related illnesses are also growing rapidly, further contributing to the growing demand for pharmaceutical and personal care products.
Healthcare Expenditure
According to World Health Organization, China’s total expenditure on healthcare was RMB 1.5 trillion in 2008 and is forecasted to grow to RMB 2.5 trillion by the end of 2012, representing a compound annual growth rate, or CAGR, of 14.6%. Health expenditure per capita was USD $159.1 in 2008 and is forecasted to increase to USD $303.0 by the end of 2012, representing a CAGR of 17.5%. Health expenditure as a percentage of GDP was 5.1% in 2008 and is forecasted to grow to 6.4% in 2012, representing a CAGR of 6.0%. While China’s expenditure on healthcare as a percentage of GDP and healthcare expenditure per capita have increased at a significant rate, they are both significantly lower when compared to other, especially developed, countries on an absolute basis. The following charts compare China’s healthcare expenditure per capita as well as healthcare expenditure as a percentage of GDP to those of certain other countries in 2006.
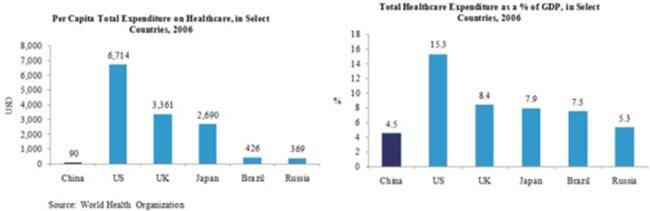
According to Business Monitor International, an independent research firm, China’s expenditure on drugs was RMB 258.3 billion in 2008 and is forecasted to grow to RMB 414.0 billion by the end of 2012, representing a CAGR of 12.5%. Expenditures on drugs per capita was USD $27.6 in 2008 and is forecasted to increase to USD $48.1 by the end of 2012, representing a CAGR of 14.9%. Expenditure on drugs as a percent of GDP was 0.97% in 2008 and is forecasted to increase to 1.13% in 2012.
The chart below provides a breakdown of the Chinese drugs market (excluding TCM) in 2008. The generic drugs market was 74.4% (RMB 192.1 billion) of the total drugs market in 2008 and is forecasted to increase to 76.3% (RMB 316.0 billion) by the end of 2012. The over the counter (OTC) market was 22.6% (RMB 58.4 billion) of the total drugs market in 2008 and is forecasted to decrease to 19.7% (RMB 81.4 billion) by the end of 2012. The patented drugs market was 3.2% (RMB 9.4 billion) of the total drugs market in 2008 and is forecasted to increase to 4.4% (RMB 20.1 billion) by the end of 2012. According to Business Monitor International, sales revenue for TCM in China reached approximately $21.0 billion in 2007 and is forecasted to reach $28.0 billion by 2010.
9
Chinese Drugs Market in 2008, RMB $260 billion
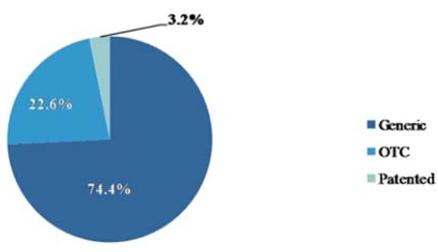
Source: Business Monitor International
Urbanization
The increasing demand for pharmaceutical and other healthcare related products has been especially strong in China’s urban areas, where residents tend to have more spending power and are more health-conscious. China has experienced rapid urbanization over the past eight years. The total urban population in China, according to the PRC National Bureau of Statistics, increased from 502.1 million as of December 31, 2002 to 606.7 million as of December 31, 2008, representing an increase of 21%. Urban population as a percent of total population increased from 39.1% in 2002 to 46.7% in 2008. The United Nations Population Division estimates that by the end of the next decade, China’s urban population will increase to about 756 million, representing approximately 53.2% of the country’s total population. Rapid urbanization is expected to continue to fuel the rapid growth of urban residential communities and presents a significant opportunity for drugstore chains’ expansion in large and fast growing urban markets.
Establishment of a National Essential Drugs List
The draft version of the Healthy China 2020 program, published by China’s National Development and Reform Commission (NDRC) in October 2008, calls for universal insurance, a change in the way hospitals are funded, more subsidies for state-run healthcare facilities and the establishment of a national essential drugs list. Government-controlled pricing of drugs will help poorer Chinese patients, with significant impact on the lives of rural residents. The development of China's National Essential Drug List requiring listed drugs to be sold at government-controlled prices is in line with China’s goal of increasing its population’s access to basic medical infrastructure.
Insurance Coverage
Eligible participants in the national medical insurance program, mainly consisting of urban residents, are entitled to buy medicines when presenting their medical insurance cards in an authorized pharmacy, provided that the medicines they purchase have been included in the national or provincial medical insurance catalogs. The pharmacy in turn obtains reimbursement from the relevant government social security bureaus. The provincial and municipal authorities who are responsible for administering social medical insurance funds to cover such reimbursements have been gradually increasing funding in recent years. The Healthy China 2020 program aims to provide health insurance coverage to China’s entire 1.3 billion population by 2020.
10
Shifting Market Share due to Regulation
In China, retail pharmaceutical and other healthcare related products can be purchased at either hospital pharmacies or non-hospital drugstores, including independent drugstores and drugstore chains. Historically, sales by hospital pharmacies accounted for a larger percent of retail sales of pharmaceutical products in China because patients typically purchase their prescription drugs at hospital pharmacies in accordance with doctors’ prescriptions. However, if a medical condition can be treated with OTC drugs, many choose to purchase OTC drugs from non-hospital drugstores instead of obtaining prescriptions from a hospital due to the lack of accessible physicians. Business Monitor International, an independent research firm, estimates that 15% to 30% of all prescription drugs are currently sold by retail drug stores with the remaining 70% to 85% sold by state run hospitals.
State-run hospitals procure pharmaceutical products in bulk from manufacturers or distributors, and generally decide whether to include a particular medicine on their formularies based upon a number of factors, including doctors’ preference in prescribing the medicine, the cost of the medicine, the perceived efficacy of the medicine and a hospital’s budget. Such decisions may also be affected by corrupt practices, including illegal kickbacks and other benefits offered by pharmaceutical manufacturers and/or distributors, which practices may also influence doctors’ decisions regarding which medicines to prescribe.
In recent years, the PRC government has strengthened its anti-corruption measures and has organized a series of government-sponsored anti-corruption campaigns. In particular, China amended its criminal code in 2006 which, among other changes, has increased the penalties for corrupt business practices. Such enhanced criminal measures against corruption are expected to induce pharmaceutical manufacturers and suppliers to compete more fairly, which in turn is expected to result in more growth opportunities for drugstores that are not affiliated with hospitals. According to Snapshots International Ltd., an independent market research firm, retail sales by China’s non-hospital drugstores is expected to increase from RMB 101.7 billion in 2008 to RMB 185.1 billion in 2013, representing a CAGR of approximately 13.0%, and the number of non-hospital drugstores is expected to increase from 391,500 in 2008 to 569,400 in 2013.
Retail - Chain Stores versus Independent Stores
China’s retail drugstore segment is highly fragmented. According to statistics published by Snapshots International, there were approximately 391,500 non-hospital drugstores in 2008, from 239,800 in 2004. In comparison, the United States had 37,800 traditional drugstore retailing units at the end of 2008, according to the U.S. National Association of Chain Drug Stores. Snapshots International estimates that, in 2008, independent drugstores accounted for 60.7% of China’s retail pharmaceutical market, while the top 10 retail drugstore chains combined for 19.0% of the market. As of December 31, 2008, despite operating 2,709 drug stores, China Nepstar Chain Drugstore Ltd. accounted for only 2.8% of the market, according to Snapshots International.
Non-Pharmaceutical Sales Opportunity at Retail Pharmacies
We believe that non-pharmaceutical merchandise, combined with physician access and prescription and non-prescription drugs, provide customers with a complete wellness solution. Non-pharmaceutical merchandise includes nutrition supplements, beauty, cosmetics and fragrance products, personal care products, as well as consumable, seasonal, promotional and other non-prescription products.
Other Government Initiatives
Pharmaceutical Product Labeling and Prescription Management
The PRC State Food and Drug Administration, or SFDA, promulgated pharmaceutical product labeling regulations in March 2006, requiring labels to list generic ingredients and barring brand name registration for any pharmaceutical product which does not contain active ingredients. In addition, effective May 1, 2007, doctors are not permitted to include brand names in their prescriptions and are required to specify the chemical ingredients of the medicines they prescribe in their prescriptions. These requirements are expected to have the following positive impacts on the business of non-hospital drugstores:
● help curb corrupt practices by pharmaceutical product manufacturers and doctors;
● ensure that patients are given better information on the medicines they purchase; and
● weaken the hospitals’ monopoly on prescriptions and prescription pharmaceutical products.
11
Advertising of Pharmaceutical Products
In 2004, the PRC government began enforcing its regulation prohibiting mass media advertising of prescription drugs. However, advertising of OTC drugs and TCM are not covered by this ban. In March 2007, in order to curb misleading advertising of pharmaceutical products, the PRC government prohibited the advertising of certain pharmaceutical products, and also required that advertising of prescription drugs be limited to authorized medical magazines. In addition, approval of the provincial SFDA must be obtained before a pharmaceutical product may be advertised. Such approval, once obtained, is valid for one year. We believe that Chinese consumers purchase medicines primarily based upon brand name recognition and price, among others factors. Consumers typically become familiar with a medicine through advertising and word-of-mouth recommendations from pharmacy salespeople. With increased restrictions on advertising of pharmaceutical products, drug manufacturers are expected to increasingly rely on retail pharmacies to build brand familiarity among the general public. On the other hand, with continued access to mass media advertising, sales of OTC drugs are expected to grow at a faster pace than those of prescription drugs.
Enhanced Quality Requirements for the Operations of Pharmacies
China has strengthened its enforcement of Good Supply Practice (“GSP”) standards since adopting them at the end of December 2004. As a result, many independent drugstores as well as smaller drugstore chains may have difficulties bringing their operations on par with these enhanced quality requirements. On the other hand, all of our 25 locations are GSP certified, and given our experience with the GSP certification process, we believe that the government’s enhanced enforcement efforts will not be a significant obstacle to our future expansion plans.
Zhejiang Province
We currently have 31 stores in Hangzhou, the capital of Zhejiang Province. We plan to increase our store base in Hangzhou and to expand into other cities within Zhejiang Province. Zhejiang Province has 51.8 million residents, the tenth largest province in terms of population. There are 10 cities in Zhejiang Province with populations over 1 million. The cities with the highest population are, as of the most recent dates available are: Hangzhou (8.1 million), Wenzhou (7.6 million), Taizhou (5.8 million), Ningbo (5.7 million), Shaoxing (4.3 million), Jiaxing (3.4 million), and Yiwu (1.2 million).
The economy of Zhejiang Province has grown significantly in recent years. According to the Zhejiang Bureau of Statistics, Zhejiang Province’s GDP has increased from RMB 614.1 billion in 2000 to RMB 2.28 trillion in 2009. According to the PRC National Bureau of Statistics, from 2000 to 2008, the average per capita annual household disposable income of urban residents in Zhejiang Province increased from RMB 9,279 to RMB 24,611. Zhejiang’s economy was the fourth largest in China among the provinces in 2009.
The total urban population in Zhejiang Province, according to the PRC National Bureau of Statistics, increased from 27.4 million as of December 31, 2005 to 29.5 million as of December 31, 2008, representing an increase of 7.6%. Urban population as a percentage of total population in Zhejiang Province increased from 60.0% in 2005 to 63.0% in 2008. Rapid urbanization is expected to continue to fuel the rapid growth of urban residential communities and presents a significant opportunity for expansion. Also contributing to the growth of the healthcare sector in Zhejiang Province is its aging population: the percentage of people who are 60 and above increased from 13.6% in 2003 to 15.6% in 2008. Urbanization and population aging are both national-level trends that are evident in Zhejiang Province.
Our Products
The products available at our drugstores can be broadly classified into the following categories:
12
Prescription Drugs. We offer approximately 2,198 prescription drugs, of which 353 require a physician’s prescription. Of the 353 drugs that require a physician’s prescription, 90% of these are issued by physicians in our employ. We accept prescriptions only from licensed health care providers. Our in-store pharmacists verify the validity, accuracy and completeness of all prescription drug orders. We ask all prescription drug customers to provide us with information regarding drug allergies, current medical conditions and current medications. All pharmaceutical products in the PRC are subject to price controls, with a recommended price and a price ceiling for each drug that are periodically adjusted by the relevant government authorities in an effort to make healthcare more widely available. The latest such adjustment occurred in October 2009 involving 307 medicines. However, this adjustment only required us to adjust 69 of our 2,198 prescription drug prices for the fiscal year ended March 31, 2010, which did not have a significant effect on our revenues as we generally price our prescription drugs substantially below the price ceilings. Because we have always priced our drugs substantially below price ceilings, price controls have not affected our revenue historically, and we do not expect them to do so in the future. Sales of prescription drugs accounted for approximately 38.5% of our drugstore revenue for fiscal year ended March 31, 2010.
OTC Drugs. We offer approximately 1,500 OTC drugs, including western medicines and traditional Chinese medicines, for the treatment of common diseases. Sales of OTC drugs accounted for approximately 34.5% of our drugstore revenue in fiscal 2010.
Nutritional Supplements. We offer approximately 1,300 nutritional supplements, including a variety of healthcare supplements, vitamin, mineral and dietary products. According to a survey of over 8,000 households across China conducted in 2004 by China Pharmaceutical News, a newspaper sponsored by the State Food and Drug Administration (“SFDA”), a majority of Chinese consumers prefer to buy nutritional supplements from a reputable drugstore as opposed to supermarkets or convenience stores. Sales of nutritional supplements accounted for approximately 9.3% of our drugstore revenue for fiscal year ended March 31, 2010.
TCM Products. Each of our stores maintains a traditional Chinese medicine (“TCM”) counter, staffed by licensed herbalists who put together packages of herbs in a process similar to how our in-store pharmacists fill out prescriptions. Additionally, we offer various types of drinkable herbal remedies and pre-packaged herbal mixtures for making soup, which are used by consumers as health supplements. TCM products typically have higher margins than prescription and OTC drugs. Sales of TCM products accounted for approximately 11.6% of our drugstore revenue for fiscal year ended March 31, 2010.
Sundry Products. Our sundry products include personal care products such as skin care, hair care and beauty products, convenience products such as soft drinks, packaged snacks, and other consumable, cleaning agents, stationeries, and seasonal and promotional items tailored to local consumer demand for convenience and quality. We believe offering these products increases customer visits by increasing the shopping convenience for our customers. Sales of sundry products accounted for approximately 4.1% of our revenue for fiscal year ended March 31, 2010.
Medical Devices. Our medical device offerings include family planning and birth control products, early pregnancy test products, portable electronic diagnostic apparatus, rehabilitation equipment, and surgical tools such as hemostats, needle forceps and surgical scissors. Sales of medical devices accounted for approximately 2.0% of our drugstore revenue for fiscal year ended March 31, 2010.
Customers
For fiscal year ended March 31, 2010, our stores served an average of approximately 8,229 customers per day. We periodically conduct qualitative customer surveys, helping us to build a stronger understanding of our market position and our customers’ purchasing habits.
Our customers pay by cash, debit or credit cards, or medical insurance cards under municipal and provincial medical insurance programs. During our fiscal year ended March 31, 2010, approximately 82% of our revenue came from cash sales, 12% from Hangzhou’s medical insurance cards and 6% come from debit, credit, provincial medical insurance and other charge card sources. We obtain payments from the relevant government social security bureaus, for sales made to eligible participants in the national medical insurance program on a monthly basis. See “Regulation — Reimbursement under the National Medical Insurance Program.” It takes approximately one year from the opening date for a store to be licensed to accept Hangzhou’s medical insurance cards. Of our 31 stores, 16 are licensed to accept Hangzhou’s medical insurance cards while 15 are awaiting approval as of the date of this Form 10-K. Our stores accepting Hangzhou’s medical insurance are designated as such on our outer signage.
13
Our Stores
Prior to opening a store, we carefully evaluate sites to maximize consumer traffic, store visibility and convenience for our customers. All of our stores are located in well-established residential communities and prime retail locations where consumer purchasing power is relatively concentrated. Depending on its size, each drugstore has between two to twelve pharmacists on staff, all of whom are properly licensed. As of the date of this Form 10-K, we operate a chain of 31 drugstores.
After opening, a location may take up to one hundred twenty days to achieve our projected revenue goals for that particular location. Various factors influence individual store revenue including, but not limited to: location, nearby competition, local population demographics, and square footage. To date, we have not closed or targeted for closure any stores due to underperformance.
Employees
We had 295 employees as of March 31, 2010, including 282 fulltime and 13 part-time employees. The following table sets forth the number of our employees for each of our areas of operations and as a percentage of our total workforce as of March 31, 2010:
|
|
As of March 31, 2010
|
|||||||
|
|
Employees
|
Percentage
|
||||||
|
|
||||||||
|
Non-pharmacist store staff
|
116
|
39
|
%
|
|||||
|
Pharmacists
|
37
|
13
|
%
|
|||||
|
Management- non-pharmacists
|
48
|
16
|
%
|
|||||
|
Management- non-pharmacists
|
64
|
22
|
%
|
|||||
|
Physicians
|
14
|
5
|
%
|
|||||
|
Non-physician clinic staff
|
16
|
5
|
%
|
|||||
|
Total
|
295
|
100
|
%
|
|||||
We place strong emphasis on the quality of our employees at all levels, including in-store pharmacists and store staff who directly interact with our customers. We provide extensive training for newly recruited employees in the first three months of their employment. The training is designed to encompass a number of areas, such as knowledge about our products and how best to interact with our customers. In addition, we regularly carry out training programs on medicine information, nutritional information, selling skills for our store staff and in-store pharmacists. We believe these programs have played an important role in strengthening the capabilities of our management team.
In addition to our employees, there are 275 sales personnel provided to our drugstores by various manufacturers, which pay us a fee for their presence in our stores. These manufacturers also compensate us to train these salespersons in our stores’ policies and procedures.
Marketing and Promotion
Our marketing and promotion strategy is to build brand recognition, increase customer traffic to our stores, attract new customers, build strong customer loyalty, maximize repeat customer visits and develop incremental revenue opportunities.
Our marketing department designs our chain-wide marketing efforts while each store designs local promotions based on local demographics and market conditions. We also launch single store promotional campaigns and community activities in connection with the openings of new stores. Our store managers and staff are also encouraged to propose their own advertising and promotion plans, including holiday promotions, posters and billboards. In addition, we offer special discounts and gift promotions for selected merchandise periodically in conjunction with our suppliers’ marketing programs. We also provide ancillary services such as providing free blood pressure measurements in our stores.
14
Many of our promotion programs are designed to encourage manufacturers to invest resources to market their brands within our stores. We charge manufacturers promotional fees in exchange for granting them the right to promote and display their products in our stores during promotional periods. We also allow manufacturers and distributors to station salespeople at our drugstore locations to promote their products, for which we receive a fee. We believe that manufacturer promotions improve our customers’ shopping experience because manufacturers provide purchasing incentives and information to help customers to make informed purchase decisions. We work to maintain strong inventory positions for merchandise featured in our promotions, as we believe this increases the effectiveness of our spending on promotion activities.
As part of our marketing campaign, we offer our customers the Jiuzhou Drugstore Rewards Card (the “Rewards Card”). Certain discount pricing is only available to our customers who have a Rewards Card. After a customer signs up for the Rewards Card, we communicate via the customer’s preferred method: e-mail, traditional mail or text messages. Approximately 35% of total customers use the Rewards Card when making purchases. We intend to further extend this program to enhance customer acquisition and retention.
We run advertisements periodically in selected newspapers to promote our brand and the products carried in our stores. Under our agreements with certain newspapers, we run one-page weekly or monthly advertisements in these newspapers, and the newspapers publish healthcare-related feature articles relating to the products we advertise near the dates of our advertisements. We also promote our brand and products using billboards and radio and television commercials. Advertising expenses are borne either by the manufacturers of the products being advertised or us, or are shared, depending on our agreement with the particular manufacturer. Our advertisements are designed to promote our brand, our corporate image and the prices of products available for sale in our stores.
Distribution Methods of Our Products or Services
We currently outsource all of the typical operations of a distribution center, including inventory, delivery and distribution, to Zhejiang Yingte Logistics Co., Ltd. (“Yingte Logistics”), one of the largest logistics companies in Zhejiang Province. Yingte Logistics is certified by Zhejiang Province to distribute prescription medication and our other products. The outsourcing of our distribution center functions to Yingte Logistics is designed to reduce our costs of operations and to provide us with the ability and flexibility for rapid expansion into other cities in Zhejiang Province.
Pursuant to our annual contract with Yingte Logistics, which was most recently renewed on January 1, 2010, in addition to providing delivery and distribution services, Yingte Logistics provides us with a 5,000 square meter capacity warehouse for our exclusive use, sufficient to support up to 50 stores. Inventory and inventory management is controlled through our centralized management system that tracks inventory status retrieval, and is linked to all of our drugstores to track sales volume by product. Based on such information, we can instruct Yingte Logistics to make deliveries to each drugstore as necessary.
Suppliers
We currently source our merchandise from approximately 276 suppliers, including 175 wholesalers and 101 direct manufacturers. For the year ended March 31, 2010, two vendors accounted for 22.72% of our total purchases: Zhejiang Yingte Pharmaceutical Co., Ltd. And Hunan Yiyang Pharmaceutical Co., Ltd. We believe that competitive sources are readily available for substantially all of the merchandise we carry in our stores. We believe that as we grow in size, our greater sourcing capability will make us a more attractive distribution channel for many drug manufacturers who can reduce their marketing expense while increasing their sales volume by selling directly to us, thereby reducing our cost of purchase.
15
Cash Control
For the fiscal year ended March 31, 2010, approximately 82% of our sales were made in cash. Therefore, we have adopted strict cash control procedures in all of our stores. Specifically, the details of each sales event are recorded in our integrated information management system, and the cash generated at our stores is collected and deposited frequently in designated bank accounts, which are controlled by our headquarters. Depending on store’s activity, cash will be either deposited daily or several times per week. Our accounting department also carries out a daily reconciliation of sales data collected on our information management system with cash receipts as confirmed by the banks.
Quality Control
We place strong emphasis on quality control for both merchandise sourcing and in-store services. Our quality control starts with procurement. We select products based on the manufacturers and wholesalers’ GMP and GSP compliance status and their product quality, manufacturing facilities and technology, packaging, transportation and storage capabilities as well as market acceptance and cost competitiveness of the products. Additionally, we conduct random quality inspections of each batch of products we procure. We replace our suppliers if they fail to pass our quality inspections. Since there is a significant manufacturing capability surplus within the Chinese pharmaceutical industry, it is possible for us to change suppliers without a material interruption to our business.
All of our employees participate in a mandatory 36-hour training program regarding quality control annually, and we regularly dispatch quality inspectors to our stores to monitor the service quality of our staff.
Competition
The drugstore industry in China is intensely competitive, rapidly evolving and highly fragmented. We primarily compete with other retail drugstore chains or drugstores, but also increasingly face competition from discount stores, convenience stores and supermarkets as we increase our offering of non-drug convenience products and services. We compete for customers primarily on the basis of store location, merchandise selection, prices, the unique combination of pharmacy and medical care services that we offer and brand name recognition. We believe that continued consolidation of the drugstore industry and new store openings by chain store operators will further increase competitive pressures.
We believe the primary competitive factors include: (i) the ability to negotiate favorable discounts from drug manufacturers; (ii) responsiveness to customers’ needs; (iii) the ability to identify and apply effective cost management programs utilizing clinical strategies; (iv) the commitment to provide flexible, clinically-oriented services to customers; and (v) the quality, scope and costs of products and services offered to our customers. We compete with a number of large, national drugstore chains that may have more financial resources and stronger brand strength and management expertise than us, including China Nepstar Chain Drugstore Ltd. (“Nepstar”), LBX Pharmacy (“LBX”) and Tian Tian Hao Grand Pharmacy (“Tian Tian”). In Hangzhou, as of December 31, 2009, Nepstar operated approximately 195 stores while both Tian Tian and LBX Pharmacy operated approximately 30 stores. We additionally compete with local and independent drugstores and government-operated pharmacies. On average, the square footage of Tian Tian and Nepstar stores are significantly smaller than our average store size and do not have the breadth of product offerings or categories. Moreover, none of our competitors provide the medical consultations that we offer at our drugstores.
Intellectual Property
We do not currently own any patents or trademarks, nor do we have any pending patent or trademark applications, and we are not a beneficiary of any licenses, franchises, concessions or royalty agreements. All our employees are required to enter into written employment agreements with us, pursuant to which they are subject to confidentiality obligations.
16
Relevant PRC Regulations
SAFE Registration
In October 2005, China’s State Administration of Foreign Exchange (“SAFE”) issued the Notice on Relevant Issues Concerning Foreign Exchange Administration for PRC Residents Engaging in Financing and Roundtrip Investments via Overseas Special Purpose Vehicles, or Circular 75. Circular 75 regulates foreign exchange matters in relation to the use of a “special purpose vehicle”, or SPV, by PRC residents to seek offshore equity financing and conduct “round trip investment” in China. Under Circular 75, a SPV is an offshore entity established or controlled, directly or indirectly, by PRC residents or PRC entities for the purpose of seeking offshore equity financing using assets or interests owned by such PRC residents or PRC entities in onshore companies, while round trip investment refers to direct investment in China by the PRC residents through the SPV, including without limitation establishing foreign invested enterprises and using such foreign invested enterprises to purchase or control (by way of contractual arrangements) onshore assets. Circular 75 requires that, before establishing or controlling a SPV, PRC residents and PRC entities are required to complete foreign exchange registration with the local offices of SAFE for their overseas investments. In addition, any PRC resident that is the shareholder of SPV is required to amend his or her SAFE registration with the SAFE or its competent local branch, with respect to that SPV in connection with any of its increase or decrease of capital, transfer of shares, merger, division, equity investment or creation of any security interest over any assets located in China.
To further clarify the implementation of Circular 75, on May 31, 2007, SAFE issued an official notice known as “Circular 106”. Under the Circular 106, PRC subsidiaries of an offshore company governed by Circular 75 are required to coordinate and supervise the filing of SAFE registrations in a timely manner by such company’s shareholders who are PRC residents. If these shareholders fail to comply, the PRC subsidiaries are required to report to the local SAFE authorities.
Dividend Distribution
The principal laws, rules and regulations governing dividends paid by our PRC affiliated entities include the Company Law of the PRC (1993), as amended in 2005 and effective in 2006, Wholly Foreign Owned Enterprise Law (1986), as amended in 2000, and Wholly Foreign Owned Enterprise Law Implementation Rules (1990), as amended in 2001. Under these laws and regulations, each of our consolidated PRC entities, including wholly foreign owned enterprises, or WFOEs, and domestic companies in China may pay dividends only out of their accumulated profits, if any, determined in accordance with PRC accounting standards and regulations. In addition, each of our consolidated PRC entities, including WFOEs and domestic companies, is required to set aside at least 10% of its after-tax profit based on PRC accounting standards each year to its statutory surplus reserve fund until the accumulative amount of such reserve reaches 50% of its respective registered capital. These reserves are not distributable as cash dividends. As of March 31, 2010, the accumulated balance of our statutory reserve funds reserves amounted to RMB 9.5 million (US$1.3 million) and the accumulated profits of our consolidated PRC entities that were available for dividend distribution amounted to RMB 101.6 million (US $15.5 million).
Taxation
Under the EIT Law, enterprises are classified as resident enterprises and non-resident enterprises. An enterprise established outside of China with its “de facto management bodies” located within China is considered a “resident enterprise,” meaning that it can be treated in a manner similar to a Chinese enterprise for enterprise income tax purposes. The implementing rules of the EIT Law define “de facto management bodies” as a managing body that in practice exercises “substantial and overall management and control over the production and operations, personnel, accounting, and properties” of the enterprise; however, it remains unclear whether the PRC tax authorities would deem our managing body as being located within China. Due to the short history of the EIT Law and lack of applicable legal precedents, the PRC tax authorities determine the PRC tax resident treatment of entities organized under the laws of foreign jurisdictions on a case-by-case basis.
If the PRC tax authorities determine that we and/or Renovation and/or us is a “resident enterprise” for PRC enterprise income tax purposes, a number of PRC tax consequences could follow. First, Renovation and/or us may be subject to enterprise income tax at a rate of 25% on our respective worldwide taxable income, as well as PRC enterprise income tax reporting obligations. Second, although the EIT Law provides that “dividends, bonuses and other equity investment proceeds between qualified resident enterprises” is exempted income, and the implementing rules of the EIT Law refers “dividends, bonuses and other equity investment proceeds between qualified resident enterprises” as the investment proceeds obtained by a resident enterprise from its direct investment in another resident enterprise, it is still unclear whether the dividends we receives indirectly from Jiuxin Management constitute “dividend between qualified resident enterprises” and consequently be qualified for tax exemption.
17
If Renovation is treated as a PRC “non-resident enterprise” under the EIT Law, then dividends that Renovation receives from Jiuxin Management (assuming such dividends were considered sourced within the PRC) (i) may be subject to a 5% PRC withholding tax, provided that Renovation owns more than 25% of the registered capital of Jiuxin Management incessantly within 12 months immediately prior to obtaining dividend from Jiuxin Management, and if the Arrangement between the Mainland of China and the Hong Kong Special Administrative Region for the Avoidance of Double Taxation and the Prevention of Fiscal Evasion with Respect to Taxes on Income is applicable, or (ii) if such treaty does not apply (i.e., because the PRC tax authorities may deem Renovation to be a conduit not entitled to treaty benefits), may be subject to a 10% PRC withholding tax. Similarly, if we are treated as a PRC “non-resident enterprise” under the EIT Law, and Renovation were treated as a PRC “resident enterprise” under the EIT Law, then dividends that we receives from Renovation (assuming such dividends were considered sourced within the PRC) may be subject to a 10% PRC withholding tax. Any such taxes on dividends could materially reduce the amount of dividends, if any, Jo-Jo Drugstores could pay to Jo-Jo Drugstores’ shareholders.
Finally, the new “resident enterprise” classification could result in a situation in which a 10% PRC tax is imposed on dividends we pay to our investors that are non-resident enterprises so long as such non-resident enterprise investors do not have an establishment or place of business in China or, despite the existence of such establishment of place of business in China, the relevant income is not effectively connected with such establishment or place of business in China, to the extent that such dividends have their sources within the PRC. Similarly, any gain realized on the transfer of our shares by such investors is also subject to a 10% PRC income tax if such gain is regarded as income derived from sources within China. In such event, we may be required to withhold a 10% PRC tax on any dividends paid to its investors that are non-resident enterprises. Our investors that are non-resident enterprises also may be responsible for paying PRC tax at a rate of 10% on any gain realized from the sale or transfer of its ordinary shares in certain circumstances. We would not, however, has an obligation to withhold PRC tax with respect to such gain.
Moreover, the State Administration of Taxation issued a circular, or Circular 698, on December 10, 2009, that reinforces taxation on transfer of non-listed shares by non-resident enterprises through overseas holding vehicles. Circular 698 applies retroactively and was deemed to be effective as of January 2008. Pursuant to Circular 698, where (i) a foreign investor who indirectly holds equity interest in a PRC resident enterprise through an offshore holding company indirectly transfers equity interests in a PRC resident enterprise by selling the shares of the offshore holding company, and (ii) the offshore holding company is located in a jurisdiction where the effective tax rate is lower than 12.5% or where the offshore income of its residents is not taxable, the foreign investor is required to provide the tax authority in charge of that PRC resident enterprise with certain relevant information within 30 days of the transfer. The tax authorities in charge will evaluate the offshore transaction for tax purposes. In the event that the tax authorities determine that such transfer is abusing forms of business organization and there is no reasonable commercial purpose other than avoidance of PRC enterprise income tax, the tax authorities will have the power to conduct a substance-over-form re-assessment of the nature of the equity transfer. A reasonable commercial purpose may be established when the overall offshore structure is set up to comply with the requirements of supervising authorities of international capital markets. If the State Administration of Taxation’s challenge of a transfer is successful, they will deny the existence of the offshore holding company that is used for tax planning purposes. Since Circular 698 has a short history, there is uncertainty as to its application.
General PRC Government Approval
As a distributor and retailer of pharmaceutical products, we are subject to regulation and oversight by different levels of the food and drug administration in China, in particular, the SFDA. The Law of the PRC on the Administration of Pharmaceutical Products, as amended, provides the basic legal framework for the administration of the production and sale of pharmaceutical products in China and governs the manufacturing, distributing, packaging, pricing and advertising of pharmaceutical products in China. The corresponding implementation regulations set out detailed rules with respect to the administration of pharmaceuticals in China. We are also subject to other PRC laws and regulations that are applicable to business operators, retailers and foreign-invested companies.
18
Distribution of Pharmaceutical Products
A distributor of pharmaceutical products must obtain a distribution permit from the relevant provincial- or designated municipal- or county-level food and drug administration. The grant of such permit is subject to an inspection of the distributor’s facilities, warehouses, hygienic environment, quality control systems, personnel and equipment. The distribution permit is valid for five years, and the holder must apply for renewal of the permit within six months prior to its expiration. In addition, a pharmaceutical product distributor needs to obtain a business license from the relevant administration for industry and commerce prior to commencing its business. All of our consolidated entities that engage in retail pharmaceutical business have obtained necessary pharmaceutical distribution permits, and we do not expect any difficulties for us to renew these permits and/or certifications.
In addition, under the Supervision and Administration Rules on Pharmaceutical Product Distribution promulgated by the SFDA on January 31, 2007, and effective May 1, 2007, a pharmaceutical product distributor is responsible for its procurement and sales activities and is liable for the actions of its employees or agents in connection with their conduct of distribution on behalf of the distributor. A retail distributor of pharmaceutical products is not allowed to sell prescription pharmaceutical products, or Tier A OTC pharmaceutical products, listed in the national or provincial medical insurance catalogs without the presence of a certified in-store pharmacist. See “Reimbursement under the National Medical Insurance Program.”
Restrictions on Foreign Ownership of Wholesale or Retail Pharmaceutical Business in China
PRC regulations on foreign investment currently permit foreign companies to establish or invest in wholly foreign-owned enterprises or joint ventures that engage in wholesale or retail sales of pharmaceuticals in China. For retail sales, these regulations restrict the number and size of retail pharmacy stores that a foreign investor may establish. If a foreign investor owns more than 30 stores that sell a variety of branded pharmaceutical products sourced from different suppliers, the foreign investor’s ownership interests in the stores are limited to 49.0%.
Our WFOE, Jiuxin Management, has entered into contractual arrangements with Jiuzhou Pharmacy and its owners.
Good Supply Practice Standards
GSP standards regulate wholesale and retail pharmaceutical product distributors to ensure the quality of distribution of pharmaceutical products in China. The current applicable GSP standards require pharmaceutical product distributors to implement strict controls on the distribution of medicine products, including standards regarding staff qualifications, distribution premises, warehouses, inspection equipment and facilities, management and quality control. The GSP certificate is usually valid for five years. Prior to opening, each of our stores must go through GSP certification. All 31 of our locations are GSP certified.
Prescription Administration
Under the Rules on Administration of Prescriptions promulgated by the SFDA, effective May 1, 2007, doctors are required to include the chemical ingredients of the medicine they prescribe in their prescription and are not allowed to include brand names in their prescription. This regulation is designed to provide consumers with choices among different pharmaceutical products that contain the same chemical ingredients.
Advertisement of Pharmaceutical Products
In order to prevent misleading advertising of pharmaceutical products, the State Administration for Industry and Commerce (“SAIC”) and the SFDA jointly promulgated the Standards for Examination and Publication of Advertisements of Pharmaceutical Products and Rules for Examination of Advertisement of Pharmaceutical Products in March 2007. Under these regulations, there are prohibitions on the advertising of certain pharmaceutical products, and advertisement of prescription pharmaceutical products may only be made in authorized medical magazines. In addition, an approval must be obtained from the provincial level of food and drug administration before a pharmaceutical product may be advertised. Such approval, once obtained, is valid for one year.
19
Product Liability and Consumers Protection
Product liability claims may arise if the products sold have any harmful effect on the consumers. The injured party may make a claim for damages or compensation. The General Principles of the Civil Law of the PRC, which became effective in January 1987, state that manufacturers and sellers of defective products causing property damage or injury shall incur civil liabilities for such damage or injuries.
The Product Quality Law of the PRC was enacted in 1993 and amended in 2000 to strengthen the quality control of products and protect consumers’ rights and interests. Under this law, manufacturers and distributors who produce or sell defective products may be subject to confiscation of earnings from such sales, revocation of business licenses and imposition of fines, and in severe circumstances, may be subject to criminal liability.
The Law of the PRC on the Protection of the Rights and Interests of Consumers was promulgated on October 31, 1993 and became effective on January 1, 1994 to protect consumers’ rights when they purchase or use goods or services. All business operators must comply with this law when they manufacture or sell goods and/or provide services to customers. In extreme situations, pharmaceutical product manufacturers and distributors may be subject to criminal liability if their goods or services lead to the death or injuries of customers or other third parties.
The Tort Law of the PRC was promulgated on December 26, 2009 and will come into force on July 1, 2010. The Tort Law provides that manufacturers and distributors who produce or sell defective products shall be responsible for the damage caused by the defective products.
Price Controls
The retail prices of some pharmaceutical products sold in China, primarily those included in the national and provincial medical insurance catalogs and those pharmaceutical products whose production or distribution are deemed to constitute monopolies, are subject to price controls in the form of fixed prices (for non-profit medical institutions) or price ceilings. Manufacturers or distributors cannot freely set or change the retail price for any price-controlled product above the applicable price ceiling or deviate from the applicable fixed price imposed by the PRC government. The prices of medicines that are not subject to price controls are determined freely at the discretion of the respective pharmaceutical companies, subject to notification to the provincial pricing authorities.
The retail prices of medicines that are subject to price controls are administered by the Price Control Office of the National Development and Reform Commission (“NDRC”), and provincial and regional price control authorities. The retail price, once set, also effectively determines the wholesale price of that medicine. From time to time, the NDRC publishes and updates a list of medicines that are subject to price control. Fixed prices and price ceilings on medicine are determined based on profit margins that the relevant government authorities deem reasonable, the type and quality of the medicine, its production costs, the prices of substitute medicine and the extent of the manufacturer’s compliance with the applicable Good Manufacturing Practice (“GMP”) standards. The NDRC directly regulates the pricing of a portion of the medicine on the list, and delegates to provincial and regional price control authorities the authority to regulate the pricing of the rest of the medicine on the list. Provincial and regional price control authorities have discretion to authorize price adjustments based on the local conditions and the level of local economic development. There are approximately 14,784 OTC and prescription drug products that are subject to price controls. The price controls of all of those pharmaceutical products are administered by the NDRC.
Only the manufacturer of a medicine may apply for an increase in the retail price of the medicine, and it must either apply to the provincial price control authorities in the province where it is incorporated, if the medicine is provincially regulated, or to the NDRC, if the medicine is NDRC regulated. For a provincially regulated medicine, in cases where provincial price control authorities approve an application, manufacturers must file the newly approved price with the NDRC for record and thereafter the newly approved price will become binding and enforceable across China.
Since May 1998, the PRC government has been ordering reductions in the retail prices of various pharmaceutical products. The latest price reduction occurred in October 2009 and it affected 307 different pharmaceutical products, which required us to make minimal price adjustments to 71 OTC drugs and 69 prescription drugs. Currently, of the total number of pharmaceutical products and OTC drugs we offered, 1,092 are subject to price controls. Price controls, however, have had no significant impact on our operations as our price points have historically been substantially below such government-imposed ceilings.
20
The NDRC may grant premium pricing status to certain pharmaceutical products that are under price control. The NDRC may set the retail prices of pharmaceutical products that have obtained premium pricing status at a level that is significantly higher than comparable products.
Reimbursement under the National Medical Insurance Program
Eligible participants in the national medical insurance program, mainly consisting of urban residents, are entitled to purchase medicine when presenting their medical insurance cards in an authorized pharmacy, provided that the medicine they purchase have been included in the national or provincial medical insurance catalogs. Depending on relevant local regulations, authorized pharmacies either sell medicine on credit and obtain reimbursement from relevant government social security bureaus on a monthly basis, or receive payments from the participants at the time of their purchases, and the participants in turn obtain reimbursement from relevant government social security bureaus.
Medicine included in the national and provincial medical insurance catalogs is divided into two tiers. Purchases of Tier A pharmaceutical products are generally fully reimbursable, except that certain Tier A pharmaceutical products are only reimbursable to the extent the medicine are used for specifically stated purposes in the medical insurance catalogs. Purchasers of Tier B pharmaceutical products, which are generally more expensive than Tier A pharmaceutical products, are required to make a certain percentage of co-payments, with the remaining amount being reimbursable. The percentage of reimbursement for Tier B OTC pharmaceutical products varies in different regions in the PRC. Factors that affect the inclusion of medicine in the medical insurance catalogs include whether the medicine is consumed in large volumes and commonly prescribed for clinical use in China and whether it is considered to be important in meeting the basic healthcare needs of the general public.
The PRC Ministry of Labor and Social Security, together with other government authorities, has the power to determine every two years which medicine are included in the national medical insurance catalog, under which of the two tiers the included medicine falls, and whether an included medicine should be removed from the catalog. Provincial governments are required to include all Tier A medicines listed on the national Medical Insurance Catalog in their provincial medical insurance catalogs. For Tier B medicines listed in the national medical insurance catalog, provincial governments have the discretion to adjust upwards or downwards by no more than 15% from the number of Tier B medicine listed in the national medical insurance catalog that is to be included in the provincial medical insurance catalogs. The amount in a participant’s individual account under the program varies, depending on the amount of contributions from the participant and his or her employer. Generally, participants under the national medical insurance program who are from relatively wealthier parts of China and metropolitan centers have greater amounts in their individual accounts than those from other parts of the country. Different regions in China have different requirements regarding the caps of reimbursements in excess of the amounts in the individual accounts.
Sales of Nutritional Supplements and other Food Products
According to the PRC Food Hygiene Law and Rules on Food Hygiene Certification, a distributor of nutritional supplements and other food products must obtain a food hygiene certificate from relevant provincial or local health regulatory authorities. The grant of such certificate is subject to an inspection of the distributor’s facilities, warehouses, hygienic environment, quality control systems, personnel and equipment. The food hygiene certificate is valid for four years, and the holder must apply for renewal of the certificate within six months prior to its expiration.
Medical Practice
Healthcare providers in China are required to comply with many laws and regulations at the national and local government levels. The laws and regulations applicable to our medical practice include the following:
21
|
●
|
We must register with and maintain an operating license from the local public health authority for each clinic that we operate, and is subject to annual review by the public health authority;
|
|
●
|
The Licensed Physician Act requires that we only hire PRC licensed physicians;
|
|
●
|
All waste material from our clinics must be properly collected, sterilized, deposited, transported and disposed of, and we are required to keep records of the origin, type and amount of all waste materials that we generate;
|
|
●
|
We must have at least 3 physicians, 5 nurses and 1 technician on staff at each clinic; and
|
|
●
|
We must establish and follow protocols to prevent medical malpractice, which require us to: (i) insure that patients are adequately informed before they consent to medical operations or procedures; (ii) maintain complete medical records which are available for review by the patient, physicians and the courts; (iii) voluntarily report any event of malpractice to a local government agency; and (iv) support and justify the medical services we provide in any administrative investigation or litigation. If we fail to comply with applicable laws and regulations, we could suffer penalties, including the loss of our license to operate.
|
Interim Regulations on Administration of Sino-Foreign Joint Venture and Cooperative Medical Institutions
As per China’s WTO commitments, “Foreign service suppliers are permitted to establish joint venture hospitals or clinics with local Chinese partners with quantitative limitations in line with China’s needs. Foreign majority ownership is permitted.” In accordance with the “Interim Regulations on Administration of Sino-Foreign Joint Venture and Cooperative Medical Institutions” jointly issued by the Ministry of Health (“MOH”) and MOFCOM in 2000, the Chinese party of Sino-foreign joint ventures and cooperative medical institutions shall hold no less than 30% of shares and legal rights or interest, which also mean foreign investors are allowed to hold a maximum stake of 70%. Such regulations also specify that the establishment of Sino-foreign joint venture and cooperative medical institutions should be approved respectively by MOH and MOFCOM. In other words, foreigners are allowed to run hospitals or clinics in the form of equity or co-operative joint ventures with an equity interest of up to 70% and a duration for co-operation of up to 20 years.
Environmental Matters
Our drugstore operations do not involve any activities subject to specific PRC environmental regulations. Our medical clinics are in compliance with applicable regulations regarding the administration of medical wastes, including collections, temperate storage, and packaging and labeling of medical wastes. Pursuant to such regulations, we contract with Dadi Weikang Medical Wastes Disposal Center to dispose of all medical wastes generated by our clinics.
ITEM 1A. RISK FACTORS
RISK FACTORS
You should carefully consider the risks described below together with all of the other information included in this report before making an investment decision with regard to our securities. The statements contained in or incorporated into this report that are not historic facts are forward-looking statements that are subject to risks and uncertainties that could cause actual results to differ materially from those set forth in or implied by forward-looking statements. If any of the following risks actually occurs, our business, financial condition or results of operations could be harmed. In that case, the trading price of our common stock could decline, and you may lose all or part of your investment.
22
Risks Relating to Our Business
Our relatively limited operating history makes it difficult to evaluate our future prospects and results of operations.
We have a limited operating history. Jiuzhou Pharmacy opened its first drugstore in March 2004, Jiuzhou Clinic began its first clinic in October 2003, and Jiuzhou Service commenced operation in November 2005. Accordingly, you should consider our future prospects in light of the risks and uncertainties experienced by early stage companies in evolving industries such as the pharmaceutical industry in China. Some of these risks and uncertainties relate to our ability to:
|
●
|
maintain our market position;
|
|
●
|
attract additional customers and increase spending per customer;
|
|
●
|
respond to competitive market conditions;
|
|
●
|
increase awareness of our brand and continue to develop customer loyalty;
|
|
●
|
respond to changes in our regulatory environment;
|
|
●
|
maintain effective control of our costs and expenses;
|
|
●
|
raise sufficient capital to sustain and expand our business;
|
|
●
|
attract, retain and motivate qualified personnel; and
|
|
●
|
ability to find and open new locations.
|
If we are unsuccessful in addressing any of these risks and uncertainties, our business may be materially and adversely affected.
We depend substantially on the continuing efforts of our executive officers, and our business and prospects may be severely disrupted if we lose their services.
Our future success is dependent on the continued services of the key members of our management team. In particular, we depend on the services of the three co-founders of HJ Group, Mr. Lei Lu, who is also our chief executive officer and the chairman of our board of directors, and Ms. Li Qi and Mr. Chong’an Jin, who are also members of our board of directors. The implementation of our business strategy and our future success depend in large part on our continued ability to attract and retain highly qualified management personnel. We face competition for personnel from other drugstore chains, retail chains, supermarkets, convenience stores, pharmaceutical companies and other organizations. Competition for these individuals could cause us to offer higher compensation and other benefits in order to attract and retain them, which could materially and adversely affect our financial condition and results of operations. We may be unable to attract or retain the personnel required to achieve our business objectives and failure to do so could severely disrupt our business and prospects. The process of hiring suitably qualified personnel is also often lengthy. In the past, we have had two major challenges to our recruiting efforts: (1) unqualified candidates who represent themselves as being qualified, and (2) talented and competent candidates who do not match our job requirements. If our recruitment and retention efforts are unsuccessful in the future, it may be more difficult for us to execute our business strategy.
We do not maintain key-man insurance for members of our management team. If we lose the services of any senior management, we may not be able to locate suitable or qualified replacements, and may incur additional expenses to recruit and train new personnel, which could severely disrupt our business and prospects. Furthermore, as we expect to continue to expand our operations, we will need to continue attracting and retaining experienced management. Each of our three founders has entered into a confidentiality and non-competition agreement with us regarding these agreements. However, if any disputes arise between our founders and us, we cannot assure you, in light of uncertainties associated with the PRC legal system, that any of these agreements could be enforced in China, where the three founders reside and hold some of their assets. See “Risks Related to Doing Business in China — Uncertainties with respect to the PRC legal system could limit the protections available to you and us.”
23
We may need additional capital, and the sale of additional shares or other equity securities could result in dilution to our stockholders.
We believe that our current cash and cash equivalents and anticipated cash flow from operations will be sufficient to meet our anticipated cash needs for the near future. We may, however, require additional cash resources due to changed business conditions or other future developments, including any investments or acquisitions we may decide to pursue. If our resources are insufficient to satisfy our cash requirements, we may seek to sell additional equity or debt securities or obtain credit facility. The sale of additional equity securities could result in dilution to our stockholders. The incurrence of additional indebtedness would result in increased debt service obligations and could result in further operating and financing covenants that would further restrict our freedom to operate our business, such as conditions that:
|
●
|
limit our ability to pay dividends or require us to seek consent for the payment of dividends;
|
|
●
|
increase our vulnerability to general adverse economic and industry conditions;
|
|
●
|
require us to dedicate a portion of our cash flow from operations to payments on our debt, thereby reducing the availability of our cash flow to fund capital expenditures, working capital and other general corporate purposes; and
|
|
●
|
limit our flexibility in planning for, or reacting to, changes in our business and our industry.
|
We cannot guarantee that we will be able to obtain any additional financing on terms that are acceptable to us, or at all.
We have significant cash deposits with our suppliers and landlords in order to obtain and maintain our inventory and maintain and establish store locations, which we may not be able to recover in the event of bankruptcy by our suppliers or landlords or other events beyond our control.
Our ability to obtain products and maintain inventory at our existing and new locations, and to maintain and establish leases for our existing and new locations, is dependent upon our ability to post and maintain significant cash deposits with our suppliers and landlords. In China many vendors are unwilling to extend credit terms for product sales which require cash deposits to be made, and landlords may require 12 months or longer of cash deposit as security from tenants. At March 31, 2010, we had approximately $6,850,240 on deposit with suppliers and approximately $3,745,305 as deposits with landlords for our retail locations. Were we unable or unwilling to establish such advances and deposits our ability to generate sales and expand our business would be adversely affected. In general, we expect the amounts required for advances and deposits to increase as we undertake our expansion plans, complete store openings and expand our business through acquisitions or otherwise. We do not generally receive interest on any of our supplier or landlord deposits and such deposits are subject to loss as a result of the creditworthiness or bankruptcy of the party who holds our funds, as well as the risk from illegal acts such as conversion, fraud, theft or dishonesty associated with the third party. If these circumstances were to arise, we would find it difficult or impossible, due to the unpredictability of legal proceedings in China, to recover all or a portion of the amount on deposit with our vendors or landlords.
Risks Relating to Our Pharmacy Operations
Our operating results are difficult to predict, and we may experience significant fluctuations in our operating results.
Our operating results may fluctuate significantly. As a result, you may not be able to rely on period to period comparisons of our operating results as an indication of our future performance. Factors causing these fluctuations include, among others:
24
|
●
|
our ability to maintain and increase sales to existing customers, attract new customers and satisfy our customers’ demands;
|
|
●
|
the frequency of customer visits to our drugstores and the quantity and mix of products our customers purchase;
|
|
●
|
the price we charge for our products or changes in our pricing strategies or the pricing strategies of our competitors;
|
|
●
|
timing and costs of marketing and promotional programs organized by us and/or our suppliers, including the extent to which we or our suppliers offer promotional discounts to our customers;
|
|
●
|
our ability to acquire merchandise, manage inventory and fulfill orders;
|
|
●
|
technical difficulties, system downtime or interruptions that may affect our product selection, procurement, pricing, distribution and retail management processes;
|
|
●
|
the introduction by our competitors of new products or services;
|
|
●
|
the effects of strategic alliances, potential acquisitions and other business combinations, and our ability to successfully and timely integrate them into our business;
|
|
●
|
changes in government regulations with respect to pharmaceutical and retail industries; and
|
|
●
|
current economic and geopolitical conditions in China and elsewhere.
|
In addition, a significant percentage of our operating expenses are fixed in the short term. As a result, a delay in generating revenue for any reason could result in substantial operating losses.
Moreover, our business is subject to seasonal variations in demand. In particular, traditional retail seasonality affects the sales of certain pharmaceuticals and other non-pharmaceutical products. Sales of our pharmaceutical products benefit in our fiscal third quarter (October 1st through December 31st) from the winter cold and flu season, and are lower in our fiscal fourth quarter (January 1st through March 31st ) because Chinese New Year falls into that quarter each year and our customers generally pay fewer visits to drugstores during this period. In addition, sales of some health and beauty products are driven, to some extent, by seasonal purchasing patterns and seasonal product changes. Failure to manage the increased sales effectively in the high sale season, and increases in inventory in anticipation of sales increase could have a material adverse effect on our financial condition, results of operations and cash flow.
Many of the factors discussed above are beyond our control, making our quarterly results difficult to predict, which could cause the trading price of our securities to decline below investor expectations. You should not rely on our operating results for prior periods as an indication of our future results.
We may not be able to timely identify or otherwise effectively respond to changing customer preferences, and we may fail to optimize our product offering and inventory position.
The drugstore industry in China is rapidly evolving and is subject to rapidly changing customer preferences that are difficult to predict. Our success depends on our ability to anticipate and identify customer preferences and adapt our product selection to these preferences. In particular, we must optimize our product selection and inventory positions based on sales trends. We cannot assure you that our product selection, especially our selections of nutritional supplements and food products, will accurately reflect customer preferences at any given time. If we fail to anticipate accurately either the market for our products or customers’ purchasing habits or fail to respond to customers’ changing preferences promptly and effectively, we may not be able to adapt our product selection to customer preferences or make appropriate adjustments to our inventory positions, which could significantly reduce our revenue and have a material adverse effect on our business, financial condition and results of operations.
25
Our success depends on our ability to establish effective advertising, marketing and promotional programs.
Our success depends on our ability to establish effective advertising, marketing and promotional programs, including pricing strategies implemented in response to competitive pressures and/or to drive demand for our products. Our advertisements are designed to promote our brand, our corporate image and the prices of products available for sale in our stores. Our pricing strategies and value proposition must be appropriate for our target customers. If we are not able to maintain and increase the awareness of our pharmacy brand, products and services, we may not be able to attract and retain customers and our reputation may also suffer. We expect to incur substantial expenses in our marketing and promotional efforts to both attract and retain customers. However, our marketing and promotional activities may be less successful than we anticipate, and may not be effective at building our brand awareness and customer base. We also cannot assure you that our current and planned spending on marketing activities will be adequate to support our future growth. Failure to successfully execute our advertising, marketing and promotional programs may result in material decreases in our revenue and profitability.
If we are unable to optimize management of our distribution activities, we may be unable to meet customer demand.
We currently outsource our distribution and inventory functions to Yingte Logistics. Our ability to meet customer demand may be significantly limited if we do not successfully and efficiently conduct our distribution activities, or if Yingte Logistics’ facilities are destroyed or shut down for any reason, including as the result of a natural disaster. Any disruption in the operation of our distribution could result in higher costs or longer lead times associated with distributing our products. In addition, as it is difficult to predict accurate sales volume in our industry, we may be unable to optimize our distribution activities, which may result in excess or insufficient inventory, warehousing, fulfillment or distribution capacity. Furthermore, failure to effectively control product damage during distribution process could decrease our operating margins and reduce our profitability.
Failure to maintain optimal inventory levels could increase our inventory holding costs or cause us to lose sales, either of which could have a material adverse effect on our business, financial condition and results of operations.
We need to maintain sufficient inventory levels to operate our business successfully as well as meet our customers’ expectations. However, we must also guard against the risk of accumulating excess inventory. We are exposed to inventory risks as a result of our increased offering of private label products, rapid changes in product life cycles, changing consumer preferences, uncertainty of success of product launches, seasonality, and manufacturer backorders and other vendor-related problems. We cannot assure you that we can accurately predict these trends and events and avoid over-stocking or under-stocking products. In addition, demand for products could change significantly between the time product inventory is ordered and the time it is available for sale.
When we begin selling a new product, it is particularly difficult to forecast product demand accurately. The purchase of certain types of inventory may require significant lead-time. As we carry a broad selection of products and maintain significant inventory levels for a substantial portion of our merchandise, we may be unable to sell such inventory in sufficient quantities or during the relevant selling seasons. Carrying too much inventory would increase our inventory holding costs, and failure to have inventory in stock when a customer orders or purchases it could cause us to lose that order or lose that customer, either of which could have a material adverse effect on our business, financial condition and results of operations.
The centralization of procurement may not help us achieve anticipated savings and may place additional burdens on the management of our supply chain.
All of the product procurement for our drugstore chain is handled through our corporate headquarters. Such centralization of merchandise procurement and replenishment operations is intended to reduce cost of goods sold as a result of volume purchase benefits. However, we may be less successful than anticipated in achieving these volume purchase benefits. In addition, the centralization of merchandise procurement is expected to increase the complexity of tracking inventory, create additional inventory handling and transportation costs and place additional burdens on the management of our supply chain. Furthermore, we may not be successful in achieving the cost savings expected from the renegotiation of certain supplier contracts due to the nature of the products covered by those contracts and the market position of the related suppliers. If we cannot successfully reduce our costs through centralizing procurement, our profitability and prospects would be materially and adversely affected.
26
Our brand name, trade secrets and other intellectual property are valuable assets. If we are unable to protect them from infringement, our business and prospects may be harmed.
We consider our pharmacy brand name to be a valuable asset. We may be unable to prevent third parties from using our brand name without authorization. Unauthorized use of our brand name by third parties may adversely affect our business and reputation, including the perceived quality and reliability of our products and services. We plan to apply for trademark protection of our logo in China, although we have not done so nor have we registered our brand name as of the date of this Form 10-K.
We also rely on trade secrets to protect our know-how and other proprietary information, including pricing, purchasing, promotional strategies, customer lists and/or suppliers lists. However, trade secrets are difficult to protect. While we use reasonable efforts to protect our trade secrets, our employees, consultants, contractors or advisors may unintentionally or willfully disclose our information to competitors. In addition, confidentiality agreements, if any, executed by the foregoing persons may not be enforceable or provide meaningful protection for our trade secrets or other proprietary information in the event of unauthorized use or disclosure. Our employees are required to sign an employment agreement as a condition of employment, which contains a confidentiality provision.
If we were to enforce a claim that a third party had illegally obtained and was using our trade secrets, our enforcement efforts could be expensive and time-consuming, and the outcome is unpredictable. In addition, if our competitors independently develop information that is equivalent to our trade secrets or other proprietary information, it will be even more difficult for us to enforce our rights and our business and prospects could be harmed.
Litigation may be necessary in the future to enforce our intellectual property rights or to determine the validity and scope of the intellectual property rights of others. However, because the validity, enforceability and scope of protection of intellectual property rights in the PRC are uncertain and still evolving, we may not be successful in prosecuting these cases. In addition, any litigation or proceeding or other efforts to protect our intellectual property rights could result in substantial costs and diversion of our resources and could seriously harm our business and operating results. Furthermore, the degree of future protection of our proprietary rights is uncertain and may not adequately protect our rights or permit us to gain or keep our competitive advantage. If we are unable to protect our trade names, trade secrets and other propriety information from infringement, our business, financial condition and results of operations may be materially and adversely affected.
We may be exposed to intellectual property infringement and other claims by third parties which, if successful, could disrupt our business and have a material adverse effect on our financial condition and results of operations.
Our success depends, in large part, on our ability to use our proprietary information and know-how without infringing third party intellectual property rights. As litigation becomes more common in China, we face a higher risk of being the subject of claims for intellectual property infringement, invalidity or indemnification relating to other parties’ proprietary rights. Our current or potential competitors, many of which have substantial resources, may have or may obtain intellectual property protection that will prevent, limit or interfere with our ability to conduct our business in China. Moreover, the defense of intellectual property suits, including trademark infringement suits, and related legal and administrative proceedings can be both costly and time consuming and may significantly divert the efforts and resources of our management personnel. Furthermore, an adverse determination in any such litigation or proceedings to which we may become a party could cause us to:
|
●
|
pay damage awards;
|
|
●
|
seek licenses from third parties;
|
|
●
|
pay ongoing royalties;
|
|
●
|
redesign our product offerings; or
|
|
●
|
be restricted by injunctions,
|
each of which could effectively prevent us from pursuing some or all of our business and result in our customers or potential customers deferring or limiting their purchase from our stores, which could have a material adverse effect on our financial condition and results of operations.
27
We rely on computer software and hardware systems in managing our operations, the capacity of which may restrict our growth and the failure of which could adversely affect our business, financial condition and results of operations.
We are dependent upon our integrated information management system to monitor daily operations of our drugstores and to maintain accurate and up-to-date operating and financial data for compilation of management information. In addition, we rely on our computer hardware and network for the storage, delivery and transmission of the data of our retail system. Any system failure which causes interruptions to the input, retrieval and transmission of data or increase in the service time could disrupt our normal operation. Although we believe that our disaster recovery plan is adequate in handling the failure of our computer software and hardware systems, we cannot assure you that we can effectively carry out this disaster recovery plan and that we will be able to restore our operation within a sufficiently short time frame to avoid our business being disrupted. Any failure in our computer software and/or hardware systems could have a material adverse effect on our business, financial condition and results of operations. In addition, if the capacity of our computer software and hardware systems fails to meet the increasing needs of our expanding operations, our ability to grow may be constrained.
As a retailer of pharmaceutical and other healthcare products, we are exposed to inherent risks relating to product liability and personal injury claims.
Pharmacies are exposed to risks inherent in the packaging and distribution of pharmaceutical and other healthcare products, such as with respect to improper filling of prescriptions, labeling of prescriptions, adequacy of warnings, unintentional distribution of counterfeit drugs. Furthermore, the applicable laws, rules and regulations require our in-store pharmacists to offer counseling, without additional charge, to our customers about medication, dosage, delivery systems, common side effects and other information the in-store pharmacists deem significant. Our in-store pharmacists may also have a duty to warn customers regarding any potential negative effects of a prescription drug if the warning could reduce or negate these effects and we may be liable for claims arising from advices given by our in-store pharmacists. In addition, product liability claims may be asserted against us with respect to any of the products we sell and as a retailer, we are required to pay for damages for any successful product liability claim against us, although we may have the right under applicable PRC laws, rules and regulations to recover from the relevant manufacturer for compensation we paid to our customers in connection with a product liability claim. We may also be obligated to recall affected products. If we are found liable for product liability claims, we could be required to pay substantial monetary damages. Furthermore, even if we successfully defend ourselves against this type of claim, we could be required to spend significant management, financial and other resources, which could disrupt our business, and our reputation as well as our brand name may also suffer. We, like many other similar companies in China, do not carry product liability insurance. As a result, any imposition of product liability could materially harm our business, financial condition and results of operations. In addition, we do not have any business interruption insurance due to the limited coverage of any business interruption insurance in China, and as a result, any business disruption or natural disaster could severely disrupt our business and operations and significantly decrease our revenue and profitability.
Future acquisitions are expected to be a part of our growth strategy, and could expose us to significant business risks.
One of our strategies is to grow our business through acquisition. However, we cannot assure you that we will be able to identify and secure suitable acquisition opportunities. Our ability to consummate and integrate effectively any future acquisitions on terms that are favorable to us may be limited by the number of attractive acquisition targets, internal demands on our resources and, to the extent necessary, our ability to obtain financing on satisfactory terms for larger acquisitions, if at all. Moreover, if an acquisition target is identified, the third parties with whom we seek to cooperate may not select us as a potential partner or we may not be able to enter into arrangements on commercially reasonable terms or at all. The negotiation and completion of potential acquisitions, whether or not ultimately consummated, could also require significant diversion of management’s time and resources and potential disruption of our existing business. Furthermore, we cannot assure you that the expected synergies from future acquisitions will actually materialize. In addition, future acquisitions could result in the incurrence of additional indebtedness, costs, and contingent liabilities. Future acquisitions may also expose us to potential risks, including risks associated with:
28
|
●
|
the integration of new operations, services and personnel;
|
|
●
|
unforeseen or hidden liabilities;
|
|
●
|
the diversion of financial or other resources from our existing businesses;
|
|
●
|
our inability to generate sufficient revenue to recover costs and expenses of the acquisitions; and
|
|
●
|
potential loss of, or harm to, relationships with employees or customers.
|
Any of the above could significantly disrupt our ability to manage our business and materially and adversely affect our business, financial condition and results of operations.
We may not be able to manage our expansion of operations effectively and failure to do so could strain our management, operational and other resources, which could materially and adversely affect our business and growth potential.
We anticipate continued expansion of our business to address growth in demand for our products and services, as well as to capture new market opportunities. The continued growth of our business has resulted in, and will continue to result in, substantial demands on our management, operational and other resources. In particular, the management of our growth will require, among other things:
|
●
|
our ability to continue to identify and lease new store locations at acceptable prices;
|
|
●
|
our ability to optimize product offerings and increase sales of private label products;
|
|
●
|
our ability to control procurement cost and optimize product pricing;
|
|
●
|
our ability to control operating expenses and achieve a high level of efficiency, including, in particular, our ability to manage the amount of time required to open new stores and for stores to become profitable, to maintain sufficient inventory levels and to manage warehousing, buying and distribution costs;
|
|
●
|
information technology system enhancement;
|
|
●
|
strengthening of financial and management controls;
|
|
●
|
increased marketing, sales and sales support activities; and
|
|
●
|
hiring and training of new personnel.
|
If we are not able to manage our growth successfully, our business and prospects would be materially and adversely affected.
We depend on the continued service of, and on the ability to attract, motivate and retain a sufficient number of qualified and skilled staff, especially in-store pharmacists, for our stores.
29
Our ability to continue expanding our retail drugstore chain and deliver high quality products and customer service depends on our ability to attract and retain qualified and skilled staff, especially in-store pharmacists. In particular, the applicable PRC regulations require at least one qualified pharmacist to be stationed in every drugstore to instruct or advise customers on prescription drugs. Over the years, a significant shortage of pharmacists has developed due to increasing demand within the drugstore industry as well as demand from other businesses in the healthcare industry. We cannot assure you that we will be able to attract, hire and retain sufficient numbers of skilled personnel and in-store pharmacists necessary to continue to develop and grow our business. In the past, our major recruiting challenges included unqualified candidates who represent themselves as being qualified, and talented and competent candidates who do not match our job requirements. The inability to attract and retain a sufficient number of skilled personnel and in-store pharmacists could limit our ability to open additional stores, increase revenue or deliver high quality customer service. In addition, competition for these individuals could cause us to offer higher compensation and other benefits in order to attract and retain them, which could materially and adversely affect our financial condition and results of operations.
We face significant competition, and if we do not compete successfully against existing and new competitors, our revenue and profitability would be materially and adversely affected.
The drugstore industry in China is highly competitive, and we expect competition to intensify in the future. Our primary competitors include other drugstore chains and independent drugstores. We also increasingly face competition from discount stores, convenience stores and supermarkets as we increase our offering of non-drug convenience products and services. We compete for customers and revenue primarily on the basis of store location, merchandise selection, price, services that we offer and our brand name. We believe that the continued consolidation of the drugstore industry and continued new store openings by chain store operators will further increase competitive pressures in the industry. In addition, we may be subject to additional competition from new entrants to the drugstore industry in China. If the PRC government removes the barriers for the foreign companies to operate majority-owned retail drugstore business in China, we could face increased competition from foreign companies. Some of our larger competitors may enjoy competitive advantages, such as:
|
●
|
greater financial and other resources;
|
|
●
|
larger variety of products;
|
|
●
|
more extensive and advanced supply chain management systems;
|
|
●
|
greater pricing flexibility;
|
|
●
|
larger economies of scale and purchasing power;
|
|
●
|
more extensive advertising and marketing efforts;
|
|
●
|
greater knowledge of local market conditions;
|
|
●
|
stronger brand recognition; and
|
|
●
|
larger sales and distribution networks.
|
As a result, we may be unable to offer products similar to, or more desirable than, those offered by our competitors, market our products as effectively as our competitors or otherwise respond successfully to competitive pressures. In addition, our competitors may be able to offer larger discounts on competing products, and we may not be able to profitably match those discounts. Furthermore, our competitors may offer products that are more attractive to our customers or that render our products uncompetitive. In addition, the timing of the introduction of competing products into the market could affect the market acceptance and market share of our products. Our failure to compete successfully could materially and adversely affect our business, financial condition, results of operation and prospects.
30
Changes in economic conditions and consumer confidence in China may influence the retail industry, consumer preferences and spending patterns.
Our business and revenue growth primarily depend on the size of the retail market of pharmaceutical products in China. As a result, our revenue and profitability may be negatively affected by changes in national, regional or local economic conditions and consumer confidence in China. In particular, as we focus our expansion of retail stores in metropolitan markets, where living standards and consumer purchasing power are relatively high, we are especially susceptible to changes in economic conditions, consumer confidence and customer preferences of the urban Chinese population. External factors beyond our control that affect consumer confidence include unemployment rates, levels of personal disposable income, national, regional or local economic conditions and acts of war or terrorism. Changes in economic conditions and consumer confidence could adversely affect consumer preferences, purchasing power and spending patterns. In addition, acts of war or terrorism may cause damage to our facilities, disrupt the supply of the products and services we offer in our stores or adversely impact consumer demand. Any of these factors could have a material adverse effect on our business, financial condition and results of operations.
The retail prices of some of our products are subject to control, including periodic downward adjustment, by PRC governmental authorities.
An increasing percentage of our pharmaceutical products, primarily those included in the national and provincial medical insurance catalogs, are subject to price controls in the form of fixed retail prices or retail price ceilings. See “Relevant PRC Regulations — Price Controls” below. In addition, the retail prices of these products are also subject to periodic downward adjustments as the PRC governmental authorities seek to make pharmaceutical products more affordable to the general public. Since May 1998, the relevant PRC governmental authorities have ordered price reductions of thousands of pharmaceutical products. The latest price reduction occurred in October 2009 and affected 307 different pharmaceutical products. Currently, 1,092 of the prescription and OTC drugs that we offer are subject to price controls. As we generally price our product substantially below the price ceilings, only 69 of our prescription and 71 of our OTC drug prices required adjustment for the fiscal year ended March 31, 2010, the impact of which was negligible. Any future price controls or government mandated price reductions, however, may have a material adverse affect on our financial condition and results of operations, including significantly reducing our revenue and profitability.
Our retail operations require a number of permits and licenses in order to carry on their business.
Drugstores in China are required to obtain certain permits and licenses from various PRC governmental authorities, including Drug Distribution Permit and GSP certification. We are also required to obtain food hygiene certificates for the distribution of nutritional supplements and food products. We cannot assure you that we can maintain all required licenses, permits and certifications to carry on our business at all times, and from time to time we may have not been in compliance with all such required licenses, permits and certifications. Moreover, these licenses, permits and certifications are subject to periodic renewal and/or reassessment by the relevant PRC governmental authorities and the standards of such renewal or reassessment may change from time to time. We intend to apply for the renewal of these licenses, permits and certifications when required by applicable laws and regulations. Any failure by us to obtain and maintain all licenses, permits and certifications necessary to carry on our business at any time could have a material adverse effect on our business, financial condition and results of operations. In addition, any inability to renew these licenses, permits and certifications could severely disrupt our business, and prevent us from continuing to carry on our business. Any changes in the standards used by governmental authorities in considering whether to renew or reassess our business licenses, permits and certifications, as well as any enactment of new regulations that may restrict the conduct of our business, may also decrease our revenue and/or increase our costs and materially reduce our profitability and prospects. Furthermore, if the interpretation or implementation of existing laws and regulations changes or if new regulations come into effect requiring us to obtain any additional licenses, permits or certifications that were previously not required to operate our existing businesses, we cannot assure you that we may successfully obtain such licenses, permits or certifications.
31
The continued penetration of counterfeit products into the retail market in China may damage our brand and reputation and have a material adverse effect on our business, financial condition, results of operations and prospects.
There has been continued penetration of counterfeit products into the pharmaceutical retail market in China. Counterfeit products are generally sold at lower prices than the authentic products due to their low production costs, and in some cases are very similar in appearance to the authentic products. Counterfeit pharmaceuticals may or may not have the same chemical content as their authentic counterparts, and are typically manufactured without proper licenses or approvals as well as fraudulently mislabeled with respect to their content and/or manufacturer. Although the PRC government has been increasingly active in combating counterfeit pharmaceutical and other products, there is not yet an effective counterfeit pharmaceutical product regulation control and enforcement system in China. Although we have implemented a series of quality control procedures in our procurement process, we cannot assure you that we would not be selling counterfeit pharmaceutical products inadvertently. Any unintentional sale of counterfeit products may subject us to negative publicity, fines and other administrative penalties or even result in litigation against us. Moreover, the continued proliferation of counterfeit products and other products in recent years may reinforce the negative image of retailers among consumers in China. The continued proliferation of counterfeit products in China could have a material adverse effect on our business, financial condition, and results of operation.
We may be subject to fines and penalties if we fail to comply with the applicable PRC laws and regulations governing sales of medicines under the PRC National Medical Insurance Program.
Eligible participants in the PRC national medical insurance program, mainly consisting of urban residents in China, are entitled to buy medicines using their medical insurance cards in an authorized pharmacy, provided that the medicines they purchase have been included in the national or provincial medical insurance catalogs. The pharmacy, in turn, obtains reimbursement from the relevant government social security bureaus. Moreover, the applicable PRC laws, rules and regulations prohibit pharmacies from selling goods other than pre-approved medicines when purchases are made with medical insurance cards. We have established procedures to prohibit our drugstores from selling unauthorized goods to customers who make purchases with medical insurance cards. However, we cannot assure you that those procedures will be strictly followed by all of our employees in all of our stores.
Risks Relating to Our Medical Services
If we do not attract and retain qualified physicians and other medical personnel, our ability to provide medical services would be adversely affected.
The success of our medical services will be, in part, dependent upon the number and quality of doctors, nurses and other medical support personnel that we employ and our ability to maintain good relations with them. Our medical staff may terminate their employment with us at any time. If we are unable to successfully maintain good relationships with them, our ability to provide medical services may be adversely affected.
The provision of medical services is heavily regulated in the PRC and failure to comply with those regulations could result in penalties, loss of licensure, additional compliance costs or other adverse consequences.
Healthcare providers in China, as in most other populous countries, are required to comply with many laws and regulations at the national and local government levels. These laws and regulations relate to: licensing; the conduct of operations; the ownership of facilities; the addition of facilities and services; advertising; confidentiality, maintenance and security issues associated with medical records; billing for services; and prices for services. If we fail to comply with applicable laws and regulations, we could suffer penalties, including the loss of our licenses to operate. In addition, further healthcare legislative reform is likely, and could materially adversely affect our business and results of operations in the event we do not comply or if the cost of compliance is expensive. The above list of certain regulated areas is not exhaustive and it is not possible to anticipate the exact nature of future healthcare legislative reform in China. Depending on the priorities determined by the Chinese Ministry of Health, the political climate at any given time, the continued development of the Chinese healthcare system and many other factors, future legislative reforms may be highly diverse, including stringent infection control policies, improved rural healthcare facilities, increased regulation of the distribution of pharmaceuticals and numerous other policy matters. Consequently, the implications of these future reforms could result in penalties, loss of licensure, additional compliance costs or other adverse consequences.
32
As a provider of medical services, we are exposed to inherent risks relating to malpractice claims.
As a provider of medical services, any misdiagnosis or improper treatment may result in adverse publicity regarding us, which would harm our reputation. If we are found liable for malpractice claims, we could be required to pay substantial monetary damages. Furthermore, even if we successfully defend ourselves against this type of claim, we could be required to spend significant management, financial and other resources, which could disrupt our business, and our reputation as well as our brand name may also suffer. Because malpractice claims are not common in China, we do not carry malpractice insurance. As a result, any imposition of malpractice liability could materially harm our business, financial condition and results of operations.
We face competition that could adversely affect our results of operations.
Our clinics compete with a large number and variety of healthcare facilities in their respective markets. There are numerous government-run and private hospitals and clinics available to the general populace. There can be no assurance that these or other clinics, hospitals or other facilities will not commence or expand such operations, which would increase their competitive position. Further, there can be no assurance that a healthcare organization, having greater resources in the provision or management of healthcare services, will not decide to engage in operations similar to those being conducted by us in Hangzhou.
Risks Related to Our Corporate Structure
Chinese regulations limit foreign ownership of Chinese pharmacy chain operating 30 or more stores and limit foreign ownership of Chinese medical clinics to Sino-foreign joint venture. We conduct our drugstore business through Jiuzhou Pharmacy and our clinics through Jiuzhou Clinic and Jiuzhou Service by means of contractual arrangements. None of our businesses are owned and the validity of our contractual arrangements is uncertain. If the Chinese government determines that these contractual arrangements do not comply with applicable regulations, our business could be adversely affected.If the PRC regulatory bodies determine that the agreements that establish the structure for operating our business in China do not comply with PRC regulatory restrictions on foreign investment in drugstore and medical practice or in our business generally, we could be subject to severe penalties.In addition, changes in such Chinese laws and regulations may materially and adversely affect our business.
Current PRC regulations limit any foreign investor’s ownership of drugstores to 49.0% if the investor owns interests in more than 30 drugstores in China that sell a variety of branded pharmaceutical products sourced from different suppliers. Since we do not own any equity interests in Jiuzhou Pharmacy, but controls the drugstore chain through contractual arrangements with our wholly foreign owned enterprise (“WFOE”), Jiuxin Management, we have been advised by our PRC counsel, Allbright Law Offices (“Allbright”), that the regulations on foreign ownership of drugstores do not apply to us even if our drugstore chain expands beyond 30 stores. Similarly, foreign ownership of medical practice in China is limited to means of Sino-foreign joint venture. Since we do not have actual equity interest in Jiuzhou Clinic or Jiuzhou Service, but control these entities through contractual arrangements, Allbright has advised us that the PRC regulations restricting foreign ownership of medical practice are not applicable to us or our structure.
There are, however, uncertainties regarding the interpretation and application of PRC laws, rules and regulations, including but not limited to the laws, rules and regulations governing the validity and enforcement of our contractual arrangements. Although we have been advised by Allbright, that based on their understanding of the current PRC laws, rules and regulations, the structure for operating our business in China (including our corporate structure and contractual arrangements with Jiuzhou Pharmacy, Jiuzhou Clinic, Jiuzhou Service and their respective owners) comply with all applicable PRC laws, rules and regulations, and do not violate, breach, contravene or otherwise conflict with any applicable PRC laws, rules or regulations, we cannot assure you that the PRC regulatory authorities will not determine that our corporate structure and contractual arrangements violate PRC laws, rules or regulations. If the PRC regulatory authorities determine that our contractual arrangements are in violation of applicable PRC laws, rules or regulations, our contractual arrangements will become invalid or unenforceable, and we may not be able to consolidate the operations of the HJ Group with our results of operations. In addition, new PRC laws, rules and regulations may be introduced from time to time to impose additional requirements that may be applicable to our contractual arrangements. For example, pursuant to the PRC Property Rights Law that became effective on October 1, 2007, the pledge of any equity interests of a PRC private entity shall become effective once it is duly registered with the local branches of the State Administration for Industry and Commerce (“SAIC”). Following the promulgation of the Property Law, SAIC further issued the Administrative Measures for Registrations of Share Pledge on September 1, 2008, which provided detailed procedural guidance for the local SAIC offices to handle the registrations of share pledge. The Equity Pledge Agreements entered by Jiuxin Management with each HJ Group company as part of the contractual arrangements have created a legally binding obligation on the parties upon the execution date; however, the pledge established under these agreements does not become effective until due registration with local SAIC office. On May 18, 2010, registration of the pledged equity interests in Jiuzhou Pharmacy was completed.
33
The Chinese government has broad discretion in dealing with violations of laws and regulations, including levying fines, revoking business and other licenses and requiring actions necessary for compliance. In particular, licenses and permits issued or granted to us by relevant governmental bodies may be revoked at a later time by higher regulatory bodies. We cannot predict the effect of the interpretation of existing or new Chinese laws or regulations on our businesses. We cannot assure you that our current ownership and operating structure would not be found in violation of any current or future Chinese laws or regulations. As a result, we may be subject to sanctions, including fines, and could be required to restructure our operations or cease to provide certain services. Any of these or similar actions could significantly disrupt our business operations or restrict us from conducting a substantial portion of our business operations, which could materially and adversely affect our business, financial condition and results of operations.
If we are determined to be in violation of any existing or future PRC laws, rules or regulations or fail to obtain or maintain any of the required governmental permits or approvals, the relevant PRC regulatory authorities would have broad discretion in dealing with such violations, including:
|
●
|
revoking the business and operating licenses of our PRC consolidated entities;
|
|
●
|
discontinuing or restricting the operations of our PRC consolidated entities;
|
|
●
|
imposing conditions or requirements with which we or our PRC consolidated entities may not be able to comply;
|
|
●
|
requiring us or our PRC consolidated entities to restructure the relevant ownership structure or operations;
|
|
●
|
restricting or prohibiting our use of the proceeds from our initial public offering to finance our business and operations in China; or
|
|
●
|
imposing fines.
|
The imposition of any of these penalties would severely disrupt our ability to conduct business and have a material adverse effect on our financial condition, results of operations and prospects.
We may be adversely affected by complexity, uncertainties and changes in Chinese regulation of drugstores and the practice of medicine.
The Chinese government regulates drugstores and the practice of medicine including foreign ownership, and the licensing and permit requirements. These laws and regulations are relatively new and evolving, and their interpretation and enforcement involve significant uncertainty. As a result, in certain circumstances it may be difficult to determine what actions or omissions may be deemed to be a violation of applicable laws and regulations. Issues, risks and uncertainties relating to Chinese government regulation of the industry include the following:
|
●
|
we only have contractual control over Jiuzhou Pharmacy, Jiuzhou Clinic and Jiuzhou Service. We do not own them due to the restriction of foreign investment in pharmacy chains with 30 or more drugstores and foreign ownership of medical practice; and
|
|
●
|
uncertainties relating to the regulation of drugstores and medical practice in China, including evolving licensing practices, means that permits, licenses or operations at our company may be subject to challenge. This may disrupt our business, or subject us to sanctions, requirements to increase capital or other conditions or enforcement, or compromise enforceability of related contractual arrangements, or have other harmful effects on us.
|
34
The interpretation and application of existing Chinese laws, regulations and policies and possible new laws, regulations or policies have created substantial uncertainties regarding the legality of existing and future foreign investments in, and the businesses and activities of, pharmaceutical businesses in China, including our business
Our contractual arrangements with Jiuzhou Pharmacy, Jiuzhou Clinic, Jiuzhou Service and their respective owners may not be as effective in providing control over these entities as direct ownership.
We have no equity ownership interest in Jiuzhou Pharmacy, Jiuzhou Clinic or Jiuzhou Service, and rely on contractual arrangements to control and operate these companies and their businesses. These contractual arrangements may not be as effective in providing control over these companies as direct ownership. For example, Jiuzhou Pharmacy, Jiuzhou Clinic or Jiuzhou Service could fail to take actions required for our business despite its contractual obligation to do so. If Jiuzhou Pharmacy, Jiuzhou Clinic or Jiuzhou Service fails to perform under their agreements with us, we may have to rely on legal remedies under Chinese law, which may not be effective. In addition, we cannot assure you that the respective owners of Jiuzhou Pharmacy, Jiuzhou Clinic or Jiuzhou Service will act in our best interests.
Because we rely on contractual arrangements to control HJ Group and for our revenue, the termination of such agreements will severely and detrimentally affect our continuing business viability under our current corporate structure.
We are a holding company and do not have any assets or conduct any business operations other than the contractual arrangements between Jiuxin Management, our WFOE, and each of Jiuzhou Pharmacy, Jiuzhou Clinic and Jiuzhou Service. All of our business operations are conducted by, and we derive all of our revenues from, the three HJ Group companies. Because neither we nor our WFOE own equity interests of Jiuzhou Pharmacy, Jiuzhou Clinic and Jiuzhou Service, the termination of the contractual arrangements would sever our ability to continue receiving payments from these companies under our current holding company structure.
As we do not have any equity interests in any of the HJ Group companies, in the event the contractual arrangements terminate, we will lose our control over them and their business operations, as well as our sole source of revenues. Should this occur, we may seek to acquire control of the HJ Group companies through other means, although we cannot guarantee that we will do so, nor can we guarantee that we will be successful if we do.
We cannot assure you that there will not be any event or reason that may cause the contractual arrangements to terminate. In the event that the contractual arrangements are terminated for any reason, this may have a severe and detrimental effect on our continuing business viability under our current corporate structure, which in turn may affect the value of your investment.
We rely principally on dividends paid by our consolidated operating entities to fund any cash and financing requirements we may have, and any limitation on the ability of our consolidated PRC entities to pay dividends to us could have a material adverse effect on our ability to conduct our business.
We are a holding company, and rely principally on dividends paid by our consolidated PRC operating entities for cash requirements, including the funds necessary to service any debt we may incur. In particular, we rely on earnings generated by each of Jiuzhou Pharmacy, Jiuzhou Clinic and Jiuzhou Service, which are passed on to us through Jiuxin Management. If any of the consolidated operating entities incurs debt in its own name in the future, the instruments governing the debt may restrict dividends or other distributions on its equity interest to us. In addition, the PRC tax authorities may require us to adjust our taxable income under the contractual arrangements in a manner that would materially and adversely affect our ability to pay dividends and other distributions on our equity interest.
35
Furthermore, applicable PRC laws, rules and regulations permit payment of dividends by our consolidated PRC entities only out of their retained earnings, if any, determined in accordance with PRC accounting standards. Under PRC laws, rules and regulations, our consolidated PRC entities are required to set aside at least 10.0% of their after-tax profit based on PRC accounting standards each year to their statutory surplus reserve fund until the accumulative amount of such reserves reach 50.0% of their respective registered capital. As a result, our consolidated PRC entities are restricted in their ability to transfer a portion of their net income to us whether in the form of dividends, loans or advances. As of March 31, 2010, our restricted reserves totaled RMB 9,460,695 ($1,309,109) and we had unrestricted retained earnings of RMB 101,555,429 ($15,513,507). Our restricted reserves are not distributable as cash dividends. Any limitation on the ability of our consolidated operating entities to pay dividends to us could materially and adversely limit our ability to grow, make investments or acquisitions that could be beneficial to our businesses, pay dividends or otherwise fund and conduct our business.
Management members of Jiuzhou Pharmacy, Jiuzhou Clinic and Jiuzhou Service have potential conflicts of interest with us, which may adversely affect our business and your ability for recourse.
Mr. Lei Liu, chairman of the board of directors and our chief executive officer, is also the executive director of Jiuzhou Pharmacy, a general partner of Jiuzhou Clinic, and the supervising director of Jiuzhou Service. Mr. Chong’an Jin, one of our directors, is the supervising director of Jiuzhou Pharmacy, the managing general partner of Jiuzhou Clinic, and the executive director of Jiuzhou Service. Ms. Li Qi, corporate secretary and also a member of the board of directors, is the general manager of each of Jiuzhou Pharmacy, Jiuzhou Clinic and Jiuzhou Service and a general partner of Jiuzhou Clinic. Conflicts of interests between their respective duties to our company and HJ Group may arise. As our directors and executive officer (in the case of Mr. Liu), they have a duty of loyalty and care to us under U.S. and Hong Kong law when there are any potential conflicts of interests between our company and HJ Group. We cannot assure you, however, that when conflicts of interest arise, every one of them will act completely in our interests or that conflicts of interests will be resolved in our favor. For example, they may determine that it is in HJ Group’s interests to sever the contractual arrangements with Jiuxin Management, irrespective of the effect such action may have on us. In addition, any one of them could violate his or her legal duties by diverting business opportunities from us to others, thereby affecting the amount of payment that HJ Group is obligated to remit to us under the consulting services agreement.
In the event that you believe that your rights have been infringed under the securities laws or otherwise as a result of any one of the circumstances described above, it may be difficult or impossible for you to bring an action against HJ Group or our officers or directors who are members of HJ Group’s management, all of whom reside within China. Even if you are successful in bringing an action, the laws of China may render you unable to enforce a judgment against the assets of Jiuzhou Pharmacy, Jiuzhou Clinic and Jiuzhou Service and their respective management, all of which are located in China.
Risks Related to Doing Business in China
The three HJ Group companies are subject to restrictions on making payments to us.
We are a holding company incorporated in Nevada and do not have any assets or conduct any business operations other than our indirect investments in the three HJ Group companies. As a result of our holding company structure, we rely entirely on payments from these companies under their contractual arrangements with our WFOE, Jiuxin Management. The Chinese government also imposes controls on the conversion of RMB into foreign currencies and the remittance of currencies out of China. We may experience difficulties in completing the administrative procedures necessary to obtain and remit foreign currency. See “Government control of currency conversion may affect the value of your investment.” Furthermore, if our affiliated entity in China incurs debt on their own in the future, the instruments governing the debt may restrict their ability to make payments. If we are unable to receive all of the revenues from our operations through these contractual arrangements, we may be unable to pay dividends on our ordinary shares.
36
Uncertainties with respect to the Chinese legal system could adversely affect us.
We conduct our business primarily through the three HJ Group companies, all of which are PRC entities. Our operations in China are governed by Chinese laws and regulations. We are generally subject to laws and regulations applicable to foreign investments in China and, in particular, laws applicable to wholly foreign-owned enterprises. The Chinese legal system is based on written statutes. Prior court decisions may be cited for reference but have limited precedential value.
Since 1979, Chinese legislation and regulations have significantly enhanced the protections afforded to various forms of foreign investments in China. However, China has not developed a fully integrated legal system and recently enacted laws and regulations may not sufficiently cover all aspects of economic activities in China. In particular, because these laws and regulations are relatively new, and because of the limited volume of published decisions and their nonbinding nature, the interpretation and enforcement of these laws and regulations involve uncertainties. In addition, the Chinese legal system is based in part on government policies and internal rules (some of which are not published on a timely basis or at all) that may have a retroactive effect. As a result, we may not be aware of our violation of these policies and rules until some time after the violation. In addition, any litigation in China may be protracted and result in substantial costs and diversion of resources and management attention.
You may experience difficulties in effecting service of legal process, enforcing foreign judgments or bringing original actions in China based on United States or other foreign laws against us or our management.
We are a holding company and do not have any assets or conduct any business operations other than the contractual arrangements between Jiuxin Management and the three HJ Group companies. In addition, all of HJ Group’s assets are located in, and all of our other senior executive officers (excepting our chief financial officer) reside within, China. As a result, it may not be possible to effect service of process within the United States or elsewhere outside China upon our senior executive officers and directors not residing in the United States, including with respect to matters arising under U.S. federal securities laws or applicable state securities laws. Moreover, our Chinese counsel has advised us that China does not have treaties with the United States or many other countries providing for the reciprocal recognition and enforcement of judgment of courts. As a result, our public shareholders may have substantial difficulty in protecting their interests through actions against our management or directors than would shareholders of a corporation with assets and management members located in the United States.
We may need to obtain additional governmental approvals to open new drugstores. Our inability to obtain such approvals will have a material adverse effect on our business and growth.
According to the Measures on the Administration of Foreign Investment in the Commercial Sector promulgated by the PRC Ministry of Commerce (the “Measures”), which became effective on June 1, 2004, a company that is directly owned by a foreign invested enterprise needs to obtain relevant governmental approvals before it opens new retail stores. However, there are no specific laws, rules or regulations with respect to whether it is necessary for a company contractually controlled by a foreign invested enterprise to obtain approvals to open new retail stores. In addition, the Measures state that PRC Ministry of Commerce will promulgate a detailed implementation regulation to govern foreign invested enterprises engaging in drug sale. However, such implementation regulation has not yet been promulgated. Therefore we cannot assure you that the PRC Ministry of Commerce will not require that such approvals to be obtained. If additional governmental approval is deemed to be necessary and we are not able to obtain such approvals on a timely basis or at all, our business, financial condition, results of operations and prospects, as well as the trading price of our common stock, will be materially and adversely affected.
The advent of recent healthcare reform directives from the PRC government may increase both competition and our cost of doing business.
Under the auspices of the Healthy China 2020 program (the “Program”), published by China’s National Development and Reform Commission in October 2008, the PRC government has set in motion a series of policies in fairly rapid successions aimed to improve China’s healthcare system. Such policies include (1) discouraging hospitals from both prescribing and dispensing medication, (2) the unveiling of formal healthcare reform guidelines in April 2009, aimed to improve the availability of and subsidies for “essential” drugs, and (3) the announcement of China’s National Essential Drugs List (“NEDL”) in August 2009, listing approximately 300 medicines that are expected to be sold at government-controlled prices. While an underlying goal of these policies is to make drugs more accessible to China’s poorer populations, such policy as discouraging hospitals from both prescribing and dispensing medication also serve to create opportunities that in turn will intensify business competition in the Chinese retail drugstore industry, as well as competition for skilled labor and retail spaces. Additionally, we expect the NEDL to lead to a rise in the number of government-subsidized community healthcare service centers, which will erode the convenience and price advantage that our drugstores traditionally enjoy against hospitals.
37
Governmental control of currency conversion may affect the value of your investment.
The Chinese government imposes controls on the convertibility of RMB into foreign currencies and, in certain cases, the remittance of currency out of China. We receive substantially all of our revenues in RMB. Under our current structure, our income is primarily derived from payments from the three HJ Group companies. Shortages in the availability of foreign currency may restrict the ability of our subsidiaries and our PRC affiliated entities to remit sufficient foreign currency to pay dividends or other payments to us, or otherwise satisfy their foreign currency denominated obligations. Under existing Chinese foreign exchange regulations, payments of current account items, including profit distributions, interest payments and expenditures from trade-related transactions, can be made in foreign currencies without prior approval from China State Administration of Foreign Exchange by complying with certain procedural requirements. However, approval from appropriate government authorities is required where RMB is to be converted into foreign currency and remitted out of China to pay capital expenses such as the repayment of bank loans denominated in foreign currencies. The Chinese government may also at its discretion restrict access in the future to foreign currencies for current account transactions. If the foreign exchange control system prevents us from obtaining sufficient foreign currency to satisfy our currency demands, we may not be able to pay dividends in foreign currencies to our stockholders.
Fluctuation in the value of RMB may have a material adverse effect on your investment.
The value of RMB against the U.S. dollar and other currencies may fluctuate and is affected by, among other things, changes in political and economic conditions. Our revenues, costs, and financial assets are mostly denominated in RMB while our reporting currency is the U.S. dollar. Accordingly, this may result in gains or losses from currency translation on our financial statements. We rely entirely on fees paid to us by our affiliated entities in China. Therefore, any significant fluctuation in the value of RMB may materially and adversely affect our cash flows, revenues, earnings and financial position, and the value of, and any dividends payable on, our stock in U.S. dollars. For example, an appreciation of RMB against the U.S. dollar would make any new RMB denominated investments or expenditures more costly to us, to the extent that we need to convert U.S. dollars into RMB for such purposes. An appreciation of RMB against the U.S. dollar would also result in foreign currency translation losses for financial reporting purposes when we translate our U.S. dollar denominated financial assets into RMB, as RMB is our reporting currency.
Dividends we receive from our subsidiary located in the PRC may be subject to PRC withholding tax.
The recently enacted PRC Enterprise Income Tax Law, or the EIT Law, and the implementation regulations for the EIT Law issued by the PRC State Council, became effective as of January 1, 2008. The EIT Law provides that a maximum income tax rate of 20% is applicable to dividends payable to non-PRC investors that are “non-resident enterprises,” to the extent such dividends are derived from sources within the PRC, and the State Council has reduced such rate to 10% through the implementation regulations. We are a Nevada holding company and substantially all of our income is derived from the operations of the three HJ Group companies located in the PRC, who are contractually obligated to pay their quarterly profits to our WFOE. Therefore, dividends paid to us by our WFOE in China may be subject to the 10% income tax if we are considered as a “non-resident enterprise” under the EIT Law. If we are required under the EIT Law and its implementation regulations to pay income tax for any dividends we receive from our WFOE, it may have a material and adverse effect on our net income and materially reduce the amount of dividends, if any, we may pay to our shareholders.
We face risks related to health epidemics and other outbreaks.
Our business could be adversely affected by the effects of an epidemic outbreak, such as the SARS epidemic in April 2004. Any prolonged recurrence of such adverse public health developments in China may have a material adverse effect on our business operations. For instance, health or other government regulations adopted in response may require temporary closure of our stores or offices. Such closures would severely disrupt our business operations and adversely affect our results of operations. We have not adopted any written preventive measures or contingency plans to combat any future outbreak of SARS or any other epidemic.
38
If relations between the United States and China worsen, investors may be unwilling to hold or buy our stock and our stock price may decrease.
At various times during recent years, the United States and China have had significant disagreements over political and economic issues. Controversies may arise in the future between these two countries. Any political or trade controversies between the United States and China, whether or not directly related to our business, could reduce the price of our common stock.
Risks Related to an Investment in Our Securities
To date, we have not paid any cash dividends and no cash dividends will be paid in the foreseeable future.
We do not anticipate paying cash dividends on our common stock in the foreseeable future and we may not have sufficient funds legally available to pay dividends. Even if the funds are legally available for distribution, we may nevertheless decide not to pay any dividends. We intend to retain all earnings for our operations.
The NASDAQ Capital Market may delist our common stock from trading on its exchange, which could limit investors’ ability to effect transactions in our common stock and subject us to additional trading restrictions.
Our common stock is listed on the NASDAQ Capital Market. We cannot assure you that our common stock will continue to be listed on the NASDAQ Capital Market in the future. If the NASDAQ Capital Market delists our common stock from trading on its exchange, we could face significant material adverse consequences including:
|
|
●
|
a limited availability of market quotations for our common stock;
|
|
|
●
|
a limited amount of news and analyst coverage for our company; and
|
|
|
●
|
a decreased ability to issue additional securities or obtain additional financing in the future.
|
Although publicly traded, the trading market in our common stock may be substantially less liquid than the average stock quoted on the NASDAQ Capital Market, and such low trading volume may adversely affect the price of our common stock.
Although our common stock has been listed on the NASDAQ Capital Market since April 22, 2010, the historical trading volume of our common stock has generally been very low. Reported average daily trading volume in our common stock from October 1, 2009 through June 25, 2010 was approximately 11,762 shares. Limited trading volume will subject our shares of common stock to greater price volatility and may make it difficult for you to sell your shares of common stock at a price that is attractive to you.
Our officers and directors own a substantial portion of our outstanding common stock, which will enable them to influence many significant corporate actions and in certain circumstances may prevent a change in control that would otherwise be beneficial to our shareholders.
Our directors and executive officers collectively control approximately 47.3% of our outstanding shares of stock that are entitled to vote on all corporate actions. These stockholders, acting together, could have a substantial impact on matters requiring the vote of the shareholders, including the election of our directors and most of our corporate actions. This control could delay, defer or prevent others from initiating a potential merger, takeover or other change in our control, even if these actions would benefit our shareholders and us. This control could adversely affect the voting and other rights of our other shareholders and could depress the market price of our common stock.
39
The elimination of monetary liability against our directors, officers and employees under Nevada law and the existence of indemnification rights to our directors, officers and employees may result in substantial expenditures by us and may discourage lawsuits against our directors, officers and employees.
Our bylaws contain specific provisions that eliminate the liability of our directors for monetary damages to our company and shareholders, and we are prepared to give such indemnification to our directors and officers to the extent provided by Nevada law. We may also have contractual indemnification obligations under our employment agreements with our officers. The foregoing indemnification obligations could result in our company incurring substantial expenditures to cover the cost of settlement or damage awards against directors and officers, which we may be unable to recoup. These provisions and resultant costs may also discourage our company from bringing a lawsuit against directors and officers for breaches of their fiduciary duties, and may similarly discourage the filing of derivative litigation by our shareholders against our directors and officers even though such actions, if successful, might otherwise benefit our company and shareholders.
Legislative actions, higher insurance costs and potential new accounting pronouncements may impact our future financial position and results of operations.
There have been regulatory changes, including the Sarbanes-Oxley Act of 2002, and there may potentially be new accounting pronouncements or additional regulatory rulings that will have an impact on our future financial position and results of operations. The Sarbanes-Oxley Act of 2002 and other rule changes as well as proposed legislative initiatives following the Enron bankruptcy are likely to increase general and administrative costs and expenses. In addition, insurers are likely to increase premiums as a result of high claims rates over the past several years, which we expect will increase our premiums for insurance policies. Further, there could be changes in certain accounting rules. These and other potential changes could materially increase the expenses we report under generally accepted accounting principles, and adversely affect our operating results
The market price for our stock may be volatile.
The market price for our stock may be volatile and subject to wide fluctuations in response to factors including the following:
|
●
|
actual or anticipated fluctuations in our quarterly operating results;
|
|
●
|
changes in financial estimates by securities research analysts;
|
|
●
|
conditions in the retail pharmacy markets;
|
|
●
|
changes in the economic performance or market valuations of other retail pharmacy operators;
|
|
●
|
announcements by us or our competitors of new products, acquisitions, strategic partnerships, joint ventures or capital commitments;
|
|
●
|
addition or departure of key personnel;
|
|
●
|
fluctuations of exchange rates between RMB and the U.S. dollar;
|
|
●
|
intellectual property litigation; and
|
|
●
|
general economic or political conditions in China.
|
In addition, the securities market has from time to time experienced significant price and volume fluctuations that are not related to the operating performance of particular companies. These market fluctuations may also materially and adversely affect the market price of our stock.
40
Volatility in our common share price may subject us to securities litigation
The market for our common stock is characterized by significant price volatility when compared to seasoned issuers, and we expect that our share price will continue to be more volatile than a seasoned issuer for the indefinite future. As an illustration of such volatility, the closing price of our common stock during the 52 weeks preceding the date of this Form 10-K ranged from a low of $0.10 to a high of $10.00. In the past, plaintiffs have often initiated securities class action litigation against a company following periods of volatility in the market price of its securities. We may, in the future, be the target of similar litigation. Securities litigation could result in substantial costs and liabilities and could divert management's attention and resources.
If we fail to maintain an effective system of internal controls, we may not be able to accurately report our financial results or prevent fraud.
Since HJ Group operated as a private enterprise without public reporting obligations prior to the Share Exchange, we have committed limited personnel and resources to the development of the external reporting and compliance obligations that would be required of a public company. Recently, we have taken measures to address and improve our financial reporting and compliance capabilities and we are in the process of instituting changes to satisfy our obligations in connection with joining a public company, when and as such requirements become applicable to us. Prior to taking these measures, we did not believe we had the resources and capabilities to do so. We plan to obtain additional financial and accounting resources to support and enhance our ability to meet the requirements of being a public company. We will need to continue to improve our financial reporting systems and procedures, and documentation thereof. If our financial reporting systems or procedures fail, we may not be able to provide accurate financial statements on a timely basis or comply with the Sarbanes-Oxley Act of 2002 as it applies to us. Any failure of our ability to provide accurate financial statements could cause the trading price of our common stock to decrease substantially.
We will incur increased costs as a result of being a public company.
As a public company, we will incur significant legal, accounting and other expenses that we did not incur as a private company prior to the Share Exchange. We will incur costs associated with our public company reporting requirements. We also anticipate that we will incur costs associated with recently adopted corporate governance requirements, including certain requirements under the Sarbanes-Oxley Act of 2002, as well as new rules implemented by the SEC and the Financial Industry Regulatory Authority (“FINRA”). We expect these rules and regulations, in particular Section 404 of the Sarbanes-Oxley Act of 2002, to significantly increase our legal and financial compliance costs and to make some activities more time-consuming and costly. Like many smaller public companies, we face a significant impact from required compliance with Section 404 of the Sarbanes-Oxley Act of 2002. Section 404 requires management of public companies to evaluate the effectiveness of internal control over financial reporting and the independent auditors to attest to the effectiveness of such internal controls and the evaluation performed by management. The SEC has adopted rules implementing Section 404 for public companies as well as disclosure requirements. The Public Company Accounting Oversight Board, or PCAOB, has adopted documentation and attestation standards that the independent auditors must follow in conducting its attestation under Section 404. We are currently preparing for compliance with Section 404; however, there can be no assurance that we will be able to effectively meet all of the requirements of Section 404 as currently known to us in the currently mandated timeframe. Any failure to implement effectively new or improved internal controls, or to resolve difficulties encountered in their implementation, could harm our operating results, cause us to fail to meet reporting obligations or result in management being required to give a qualified assessment of our internal controls over financial reporting or our independent auditors providing an adverse opinion regarding management’s assessment. Any such result could cause investors to lose confidence in our reported financial information, which could have a material adverse effect on our stock price.
We also expect these new rules and regulations may make it more difficult and more expensive for us to obtain director and officer liability insurance and we may be required to accept reduced policy limits and coverage or incur substantially higher costs to obtain the same or similar coverage. As a result, it may be more difficult for us to attract and retain qualified individuals to serve on our board of directors or as executive officers. We are currently evaluating and monitoring developments with respect to these new rules, and we cannot predict or estimate the amount of additional costs we may incur or the timing of such costs.
41
Shares eligible for future sale may adversely affect the market.
From time to time, certain of our stockholders may be eligible to sell all or some of their shares of common stock by means of ordinary brokerage transactions in the open market pursuant to Rule 144, promulgated under the Securities Act, subject to certain limitations. In general, pursuant to amended Rule 144, non-affiliate stockholders may sell freely after six months subject only to the current public information requirement (which disappears after one year). Affiliates may sell after six months subject to the Rule 144 volume, manner of sale (for equity securities), current public information and notice requirements. Of the 13,500,002 shares of our common stock outstanding as of June 25, 2010, approximately 4,500,000 million shares are, or will be, freely tradable without restriction, unless held by our "affiliates", as of such date. Any substantial sale of our common stock pursuant to Rule 144 or pursuant to any resale prospectus may have a material adverse effect on the market price of our common stock. Under Rule 144, we estimate that our founders and service consultants who received shares in September 2009 as a result of the Share Exchange will be unable to sell these shares until September 2010. If our founders and service consultants who received shares were to sell their shares, they would be subject to volume and/or other restrictions imposed by Rule 144.
ITEM 1B. UNRESOLVED STAFF COMMENTS
Not applicable.
ITEM 2. PROPERTIES
All of our current business operations, including our corporate headquarters and branch stores, are located in Hangzhou, and all of the space for our operations is leased from third parties. As of June 25, 2010, we have entered into 33 operating leases for our corporate headquarters and retail spaces with various terms, periods and other rental provisions. Our leases do not contain any escalating lease payments or contingent rental payment terms. See Note 7, "Long Term Deposits," and Note 17, “Commitments and Contingencies” of the footnotes to our audited financial statements for the years ended March 31, 2010 and 2009, included elsewhere in this Form 10-K, which sections are incorporated here by reference
We must negotiate with the landlords for an extension of the old leases or enter into new leases upon their termination, and our landlords may request a rent increase. Under applicable PRC law, we have priority over other potential lessees with respect to the leased store space on the same terms. We also do not expect any significant difficulties in renewal of existing leases upon their expiration, where desired. Our community stores are normally relatively small in size and the facilities inside the store are easily movable. As a result, we do not expect our drugstore operations to be materially and adversely affected by any failure to renew or enter into new leases.
We are also leasing two lands from The People's Government of Qianhong Village in Lin'an, Zhejiang Province, and have entered into 30-year lease in connection therewith in February 2010. One piece of land is approximately 4.6 acres and is zoned for industrial use; the other is approximately 48.6 acres of forestry land. We have not utilized these lands since entering into the lease, although we have prepaid the lease amount in full as of March 31, 2010. See Note 8, "Prepaid Rent - Non Current," and Note 17, "Commitments and Contingencies," of the footnotes to our audited financial statements for the years ended March 31, 2010 and 2009, included elsewhere in this Form 10-K, which sections are incorporated here by reference.
ITEM 3. LEGAL PROCEEDINGS
Except as described below, we know of no material, existing or pending legal proceedings against us, nor are we involved as a plaintiff in any material proceeding or pending litigation. There are no proceedings in which any of our directors, officers or affiliates, or any registered or beneficial stockholder, is an adverse party or has a material interest adverse to our company.
On December 8, 2009, Jiuzhou Pharmacy filed suit against The Ventana Group, LLC and Michael Hom in the California Superior Court for the County of San Mateo (Case Number CV490272), alleging breach of contract of an agreement entered into with the defendants in 2008 and seeking damages of $25,000. The suit was subsequently amended to remove Mr. Hom as a defendant. In May 2010, Jiuzhou Pharmacy sought for default judgment against the remaining defendant, which remains pending.
ITEM 4. RESERVED
ITEM 5. MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASE OF EQUITY SECURITIES
Market Information
Our common stock has been trading on the Nasdaq Capital Market under the symbol “CJJD” since April 22, 2010. Our common stock was previously quoted on the Over-The-Counter Bulletin Board, or OTCBB, under the trading symbol “CJJD.OB” until April 21, 2010.
42
The following table sets forth the high and low bid information for our common stock on the OTCBB through April 21, 2010 and on the Nasdaq Capital Market since April 22, 2010 for the periods indicated. The bid prices reflect inter-dealer quotations, do not include retail markups, markdowns, or commissions, and do not necessarily reflect actual transactions.
|
The OTCBB
Bid
Price per Share (1)
|
The Nasdaq
Capital Market
Price per Share (2)
|
|||||||||||||||
| High | Low | High | Low | |||||||||||||
|
Quarter ended
|
||||||||||||||||
|
June 30, 2010 (3)
|
$
|
9.50
|
$
|
3.48
|
$
|
5.49
|
$
|
3.80
|
||||||||
|
March 31, 2010
|
$
|
10.00
|
$
|
3.60
|
$
|
N/A
|
$
|
N/A
|
||||||||
|
December 31, 2009
|
$
|
5.30
|
$
|
0.10
|
$
|
N/A
|
$
|
N/A
|
||||||||
|
September 30, 2009
|
$
|
0.00
|
$
|
0.00
|
$
|
N/A
|
$
|
N/A
|
||||||||
|
June 30, 2009
|
$
|
0.00
|
$
|
0.00
|
$
|
N/A
|
$
|
N/A
|
||||||||
|
March 31, 2009
|
$
|
0.00
|
$
|
0.00
|
$
|
N/A
|
$
|
N/A
|
||||||||
|
December 31, 2008
|
$
|
1.10
|
$
|
0.30
|
$
|
N/A
|
$
|
N/A
|
||||||||
|
September 30, 2008
|
$
|
2.24
|
$
|
0.50
|
$
|
N/A
|
$
|
N/A
|
||||||||
|
June 30, 2008 (4)
|
$
|
2.22
|
$
|
0.00
|
$
|
N/A
|
$
|
N/A
|
||||||||
(1) Through April 21, 2010.
(2) From April 22, 2010 forward.
(3) Through June 25, 2010.
(4) Reflect prices beginning May 1, 2008.
As of June 25, 2010, we had approximately 13,500,002 shares of common stock issued and outstanding.
As of March 31, 2010, one of our directors is owed 1,725 share of common stock pursuant to the terms of his agreement with us.
Assuming the issuance of the shares owed to our director, we would have approximately 13,501,727 shares of common stock outstanding as of June 25, 2010.
Holders
As of March 31, 2010, there were 17 stockholders of record of our common stock (not including beneficial owners who hold shares at broker/dealers in “street name”).
Dividends
While there are no restrictions that limit our ability to pay dividends, we have not paid, and do not currently intend to pay cash dividends on our common stock in the foreseeable future. Our policy is to retain all earnings, if any, to provide funds for operation and expansion of our business. The declaration of dividends, if any, will be subject to the discretion of our Board of Directors, which may consider such factors as our results of operations, financial condition, capital needs and acquisition strategy, among others.
43
Securities Authorized for Issuance under Equity Compensation Plans
Please see the discussion in Item 12 titled “Equity Compensation Plan Information” below.
Recent Sales of Unregistered Securities
None during the three months ended March 31, 2010.
ITEM 6. SELECTED FINANCIAL DATA
Not applicable.
ITEM 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following discussion and analysis of our results of operations and financial condition for fiscal years ended March 31, 2010 and 2009 should be read in conjunction with our financial statements and the notes to those financial statements that are included elsewhere in this Form 10-K. Our discussion includes forward-looking statements based upon current expectations that involve risks and uncertainties, such as our plans, objectives, expectations and intentions. Actual results and the timing of events could differ materially from those anticipated in these forward-looking statements as a result of a number of factors, including those set forth under the “Risk Factors”, “Cautionary Notice Regarding Forward-Looking Statements” and “Description of Business” sections and elsewhere in this Form 10-K. We use words such as “anticipate,” “estimate,” “plan,” “project,” “continuing,” “ongoing,” “expect,” “believe,” “intend,” “may,” “will,” “should,” “could,” “predict,” and similar expressions to identify forward-looking statements. Although we believe the expectations expressed in these forward-looking statements are based on reasonable assumptions within the bound of our knowledge of our business, our actual results could differ materially from those discussed in these statements. Factors that could contribute to such differences include, but are not limited to, those discussed in the “Risk Factors” section of this Form 10-K. We undertake no obligation to update publicly any forward-looking statements for any reason even if new information becomes available or other events occur in the future.
Our financial statements are prepared in US$ and in accordance with accounting principles generally accepted in the United States. See “Exchange Rates” below for information concerning the exchanges rates at which Renminbi (“RMB”) were translated into US$ at various pertinent dates and for pertinent periods.
Overview
The Company was incorporated in Nevada on December 19, 2006, originally under the name Kerrisdale Mining Corporation. On September 24, 2009, The Company changed its name to “China Jo-Jo Drugstores, Inc.” in connection with the share exchange transaction described below.
On September 17, 2009, the Company completed a share exchange transaction with Renovation, a Hong Kong company, and Renovation became a wholly-owned subsidiary of the Company. On the closing date, the Company issued 7,900,000 of its common stock to the Renovation Stockholders in exchange for 100% of the capital stock of Renovation. Prior to the share exchange transaction, the Company had 2,100,000 shares of common stock issued and outstanding. After the share exchange transaction, the Company had 10,000,000 shares of common stock outstanding and the Renovation Stockholders owned 79% of the issued and outstanding shares. The management members of Renovation became the directors and officers of the Company. The share exchange transaction was accounted for as a reverse acquisition and recapitalization and, as a result, the consolidated financial statements of the Company (the legal acquirer) is, in substance, those of Renovation (the accounting acquirer), with the assets and liabilities, and revenues and expenses, of the Company being included effective from the date of the share exchange transaction.
Renovation is a holding company that, through its wholly-owned PRC subsidiary, Jiuxin Management, controls the three HJ Group companies in the PRC, namely Jiuzhou Pharmacy, Jiuzhou Clinic, and Jiuzhou Service by a series of contractual arrangements. All of our business operations are carried out by HJ Group.
44
Critical Accounting Policies and Estimates
In preparing our consolidated financial statements in accordance with accounting principles generally accepted in the United States, we are required to make judgments, estimates and assumptions that affect: (i) the reported amounts of our assets and liabilities; (ii) the disclosure of our contingent assets and liabilities at the end of each reporting period; and (iii) the reported amounts of revenue and expenses during each reporting period. We continually evaluate these estimates based on our own historical experience, knowledge and assessment of current business and other conditions, our expectations regarding the future based on available information and reasonable assumptions, which together form our basis for making judgments about matters that are not readily apparent from other sources. Since the use of estimates is an integral component of the financial reporting process, our actual results could differ from those estimates.
We believe that any reasonable deviation from those judgments and estimates would not have a material impact on our financial condition or results of operations. To the extent that the estimates used differ from actual results, however, adjustments to the statement of operations and corresponding balance sheet accounts would be necessary. These adjustments would be made in future financial statements.
When reading our financial statements, you should consider: (i) our critical accounting policies; (ii) the judgment and other uncertainties affecting the application of such policies; and (iii) the sensitivity of reported results to changes in conditions and assumptions. We believe the following accounting policies involve the most significant judgment and estimates used in the preparation of our financial statements. We have not made any material changes in the methodology used in these accounting policies during the past eighteen months.
Revenue recognition
Revenue from sales of prescription medicine at the drugstores is recognized when the prescription is filled and the customer picks up and pays for the prescription.
Revenue from sales of other merchandise at the drugstores is recognized at the point of sale, which is when the customer pays for and receives the merchandise.
Revenue from medical services (which is nominal) is recognized after the service has been rendered to the customer.
Revenue from sales of merchandise to non-retail customers is recognized when the following conditions are met: 1) persuasive evidence of an arrangement exists (sales agreements and customer purchase orders are used to determine the existence of an arrangement); 2) delivery of goods has occurred and risks and benefits of ownership have been transferred, which is when the goods are received by the customer at its designated location in accordance with the sales terms; 3) the sales price is fixed or determinable; and 4) collectability is probable. Historically, sales returns have been immaterial.
Our revenue is net of value added tax (“VAT”) collected on behalf of tax authorities in respect of the sale of merchandise. VAT collected from customers, net of VAT paid for purchases, is recorded as a liability in the balance sheet until it is paid to the tax authorities.
Vendor allowances
The Company accounts for vendor allowances according the accounting standards, Accounting by a Customer (Including a Reseller) for Certain Consideration Received from a Vendor, and by Reseller to Sales Incentives Offered to Consumers by Manufacturers. Vendor allowances reduce the carrying value of inventories and subsequently transferred to cost of goods sold when the inventories are sold, unless those allowances are specifically identified as reimbursements for advertising, promotion and other services, in which case they are recognized as a reduction of the related advertising and promotion costs.
45
Slotting allowances are a major portion of total allowances. With slotting allowances, the vendors reimburse the Company for the cost of placing new product on the shelf. The Company has no obligation or commitment to keep the product on the shelf for a minimum period.
A small portion of vendor allowance also includes advertising and promotion allowances for the promotion of vendors' products in stores. The promotion may be any combination of a temporary price reduction or a feature in print ads.
Depreciation and Amortization
Our non-current assets include property and equipment, including leasehold improvements, long term deposits and long term advances to suppliers. We depreciate our equipment assets using the straight-line method over the estimated useful lives of the assets. We make estimates of the useful lives of the equipment (including the salvage values), in order to determine the amount of depreciation expense to be recorded during any reporting period. We amortize leasehold improvements of our retail drugstores and other business premises over the shorter of five years or lease term. A majority of our leases have a five-year term. We estimate the useful lives of our other property and equipment at the time we acquire the assets based on our historical experience with similar assets as well as anticipated technological and other changes. If technological changes were to occur more rapidly than anticipated or in a different form than anticipated, we may shorten the useful lives assigned to these assets as appropriate, which will result in the recognition of increased depreciation and amortization expense in future periods. There has been no change to the estimated useful lives and salvage values during the years ended March 31, 2010 and 2009.
Impairment of Long-Lived Assets
We evaluate our long lived tangible and intangible assets for impairment, at least annually, but more often whenever events or changes in circumstances indicate that the carrying value may not be recoverable from its estimated future cash flows. Recoverability is measured by comparing the asset’s net book value to the related projected undiscounted cash flows from these assets, considering a number of factors including past operating results, budgets, economic projections, market trends and product development cycles. If the net book value of the asset exceeds the related undiscounted cash flows, the asset is considered impaired, and a second test is performed to measure the amount of impairment loss. Based on its review, we believe that, as of March 31, 2010, there was no impairment.
Inventories
We state our inventory at the lower of cost or market. Cost is determined using the weighted average cost method. Market is the lower of replacement cost or net realizable value. We carry out physical inventory counts on a monthly basis at each store and distribution location to ensure that the amounts reflected in the consolidated financial statements at each reporting period are properly stated and valued. We record write-downs to inventory for shrinkage losses and damaged merchandise that are identified during the inventory counts. The inventory write downs for the years ended March 31, 2010 and 2009 have been immaterial.
Recent Accounting Pronouncements
46
In December 2009, FASB issued ASU No. 2009-16, Accounting for Transfers of Financial Assets. This Accounting Standards Update amends the FASB Accounting Standards Codification for the issuance of FASB Statement No. 166, Accounting for Transfers of Financial Assets—an amendment of FASB Statement No. 140.The amendments in this Accounting Standards Update improve financial reporting by eliminating the exceptions for qualifying special-purpose entities from the consolidation guidance and the exception that permitted sale accounting for certain mortgage securitizations when a transferor has not surrendered control over the transferred financial assets. In addition, the amendments require enhanced disclosures about the risks that a transferor continues to be exposed to because of its continuing involvement in transferred financial assets. Comparability and consistency in accounting for transferred financial assets will also be improved through clarifications of the requirements for isolation and limitations on portions of financial assets that are eligible for sale accounting. The adoption of this ASU on January 1, 2010 did not have a material impact on the Company’s financial statements.
In December, 2009, FASB issued ASU No. 2009-17, Improvements to Financial Reporting by Enterprises Involved with Variable Interest Entities. This Accounting Standards Update amends the FASB Accounting Standards Codification for the issuance of FASB Statement No. 167, Amendments to FASB Interpretation No. 46(R). The amendments in this Accounting Standards Update replace the quantitative-based risks and rewards calculation for determining which reporting entity, if any, has a controlling financial interest in a variable interest entity with an approach focused on identifying which reporting entity has the power to direct the activities of a variable interest entity that most significantly impact the entity’s economic performance and (1) the obligation to absorb losses of the entity or (2) the right to receive benefits from the entity. An approach that is expected to be primarily qualitative will be more effective for identifying which reporting entity has a controlling financial interest in a variable interest entity. The amendments in this Update also require additional disclosures about a reporting entity’s involvement in variable interest entities, which will enhance the information provided to users of financial statements. The adoption of this ASU on January 1, 2010 did not have a material impact on the Company’s financial statements.
47
In January 2010, FASB issued ASU No. 2010-01- Accounting for Distributions to Shareholders with Components of Stock and Cash. The amendments in this Update clarify that the stock portion of a distribution to shareholders that allows them to elect to receive cash or stock with a potential limitation on the total amount of cash that all shareholders can elect to receive in the aggregate is considered a share issuance that is reflected in EPS prospectively and is not a stock dividend for purposes of applying Topics 505 and 260 (Equity and Earnings Per Share). The amendments in this update are effective for interim and annual periods ending on or after December 15, 2009, and should be applied on a retrospective basis. The Company does not expect the adoption of this ASU to have a material impact on its consolidated financial statements.
In January 2010, FASB issued ASU No. 2010-02 – Accounting and Reporting for Decreases in Ownership of a Subsidiary – a Scope Clarification. The amendments in this Update affect accounting and reporting by an entity that experiences a decrease in ownership in a subsidiary that is a business or nonprofit activity. The amendments also affect accounting and reporting by an entity that exchanges a group of assets that constitutes a business or nonprofit activity for an equity interest in another entity. The amendments in this update are effective beginning in the period that an entity adopts SFAS No. 160, “Non-controlling Interests in Consolidated Financial Statements – An Amendment of ARB No. 51.” If an entity has previously adopted SFAS No. 160 as of the date the amendments in this update are included in the Accounting Standards Codification, the amendments in this update are effective beginning in the first interim or annual reporting period ending on or after December 15, 2009. The amendments in this update should be applied retrospectively to the first period that an entity adopted SFAS No. 160. The adoption of this ASU did not have a material impact on its consolidated financial statements.
In January 2010, FASB issued ASU No. 2010-06 – Improving Disclosures about Fair Value Measurements. This update provides amendments to Subtopic 820-10 that requires new disclosure as follows: 1) Transfers in and out of Levels 1 and 2. A reporting entity should disclose separately the amounts of significant transfers in and out of Level 1 and Level 2 fair value measurements and describe the reasons for the transfers. 2) Activity in Level 3 fair value measurements. In the reconciliation for fair value measurements using significant unobservable inputs (Level 3), a reporting entity should present separately information about purchases, sales, issuances, and settlements (that is, on a gross basis rather than as one net number).This update provides amendments to Subtopic 820-10 that clarify existing disclosures as follows: 1) Level of disaggregation. A reporting entity should provide fair value measurement disclosures for each class of assets and liabilities. A class is often a subset of assets or liabilities within a line item in the statement of financial position. A reporting entity needs to use judgment in determining the appropriate classes of assets and liabilities. 2) Disclosures about inputs and valuation techniques. A reporting entity should provide disclosures about the valuation techniques and inputs used to measure fair value for both recurring and nonrecurring fair value measurements. Those disclosures are required for fair value measurements that fall in either Level 2 or Level 3.The new disclosures and clarifications of existing disclosures are effective for interim and annual reporting periods beginning after December 15, 2009, except for the disclosures about purchases, sales, issuances, and settlements in the roll forward of activity in Level 3 fair value measurements. Those disclosures are effective for fiscal years beginning after December 15, 2010, and for interim periods within those fiscal years. The Company is currently evaluating the impact of this ASU, however, the Company does not expect the adoption of this ASU to have a material impact on its consolidated financial statements.
48
In February 2010, the FASB issued ASU 2010-09, “Subsequent Events (Topic 855): Amendments to Certain Recognition and Disclosure Requirements,” or ASU 2010-09. ASU 2010-09 primarily rescinds the requirement that, for listed companies, financial statements clearly disclose the date through which subsequent events have been evaluated. Subsequent events must still be evaluated through the date of financial statement issuance; however, the disclosure requirement has been removed to avoid conflicts with other SEC guidelines. ASU 2010-09 was effective immediately upon issuance and was adopted in February 2010.
In March 2010, FASB issued ASU No. 2010-10 –Amendments for Certain Investment Funds. This update defers the effective date of the amendments to the consolidation requirements made by FASB Statement 167 to a reporting entity’s interest in certain types of entities. The deferral will mainly impact the evaluation of reporting enterprises’ interests in mutual funds, private equity funds, hedge funds, real estate investment entities that measure their investment at fair value, real estate investment trusts, and venture capital funds. The ASU also clarifies guidance in Statement 167 that addresses whether fee arrangements represent a variable interest for all service providers and decision makers. The ASU is effective for interim and annual reporting periods in fiscal year beginning after November 15, 2009. The Company is currently evaluating the impact of this ASU, however, the Company does not expect the adoption of this ASU to have a material impact on its consolidated financial statements.
Results of Operations
The following table summarizes our results of operations for the years ended March 31, 2010 and 2009.
|
Years Ended March 31,
|
||||||||||||||||
| 2010 | 2009 | |||||||||||||||
| Percentage of | Percentage of | |||||||||||||||
| Amount | total revenue | Amount | total revenue | |||||||||||||
|
Revenues
|
$ | 55,174,929 | 100.0 | % | $ | 44,776,652 | 100.0 | % | ||||||||
|
Gross Profit
|
$ | 16,898,040 | 30.6 | % | $ | 12,168,911 | 27.2 | % | ||||||||
|
Selling Expenses
|
$ | 2,630,332 | 4.8 | % | $ | 1,712,474 | 3.8 | % | ||||||||
|
General and Administrative Expenses
|
$ | 1,510,154 | 2.7 | % | $ | 1,399,305 | 3.1 | % | ||||||||
|
Income from Operations
|
$ | 12,757,554 | 23.1 | % | $ | 9,057,132 | 20.2 | % | ||||||||
|
Other Income (Expense)
|
$ | (55,520 | ) | (0.1 | %) | $ | 17,369 | 0.0 | % | |||||||
|
Income Tax Expenses
|
$ | 2,880,293 | 5.2 | % | $ | 2,260,985 | 5.0 | % | ||||||||
|
Net Income
|
$ | 9,821,741 | 17.8 | % | $ | 6,813,516 | 15.2 | % | ||||||||
Revenue. Our revenue increased by $10,398,277 or 23.2% to $55,174,929 for the year ended March 31, 2010 from $44,776,652 for the year ended March 31, 2009 due to operating additional store locations. Of this increase, $3,214,016 was attributable to new stores or 31% of the increase, with the remainder primarily attributable to our maturing stores that we opened during the year ended March 31, 2010. We operated 25 stores as of March 31, 2010, as compared to 16 stores as of March 31, 2009. We anticipate that our overall revenue will continue to increase as we open additional stores.
Gross Profit. Our gross profit increased by $4,729,129 or 38.9% to $16,898,040 for the year ended March 31, 2010 from $12,168,911 for the year ended March 31, 2009. Our gross margin slightly increased from 27.2% for the year ended March 31, 2009 to 30.6% for the year ended March 31, 2010 as a result of selling additional traditional Chinese medicines and sundry products, which typically have a higher margin contribution. We anticipate that our overall gross profit will continue to increase as our sales increase. Additionally, we anticipate that our gross margin will increase as we will be able to obtain better pricing terms from our suppliers and achieve further economies of scale as a result of purchasing larger quantities of products. However, in order to increase sales volume we may lower our prices and therefore may maintain our margins. We presently do not privately label any of our products and are constantly adjusting our product mix to meet customer demand and to maximize our gross margin.
Sales and Marketing Expenses. Our sales and marketing expenses increased by $917,858 or 53.6% to $2,630,332 for the year ended March 31, 2010 from $1,712,474 for the year ended March 31, 2009. The increase in sales and marketing expense was primarily a result of the continued expansion of our drugstore chain from 18 stores as of March 31, 2009 to 25 locations as of March 31, 2010. Sales and marketing expenses as a percentage of our revenue increased slightly to 4.8% for the year ended March 31, 2010 from 3.8% for the year ended March 31, 2009. We expect that our sales and marketing expenses will increase as we continue to expand our store network within the city of Hangzhou as well as into other cities within Zhejiang Province.
49
General and Administrative Expenses. Our general and administrative expenses increased by $110,849 or 7.9% to $1,510,154 for the year ended March 31, 2010 from $1,399,305 for the year ended March 31, 2009. General and administrative expenses as a percentage of our revenue remained relatively consistent at 2.7% for the year ended March 31, 2010 as compared to 3.1% for the year ended March 31, 2009. However, as we continue to open drugstores in Hangzhou and begin to expand into other cities within Zhejiang Province, further develop our infrastructure, and incur expenses related to being a U.S. public company, we anticipate that our general and administrative expenses will increase in absolute dollars as well as a percentage of total revenues.
Income from Operations. As a result of the increased gross margin, our income from operations increased by $3,700,422 or 40.9% to $12,757,554 for the year ended March 31, 2010 from $9,057,132 for the year ended March 31, 2009. Our operating margin for the years ended March 31, 2010 and 2009 was 23.1% and 20.2%, respectively.
Income Taxes. Our income tax expense increased to $2,880,293 for the year ended March 31, 2010 from $2,260,985 for the year ended March 31, 2009 as a result of increased operating income. Our effective tax rate decreased from 25% for the year ended March 31, 2009 to 23% for the year ended March 31, 2010.
Net Income. As a result of the foregoing, our net income increased to $9,821,741 for the year ended March 31, 2010 from $6,813,516 for the year ended March 31, 2009.
Liquidity
Year Ended March 31, 2010
For the year ended March 31, 2010, we generated cash from operating activities of $807,309, as compared to cash used in operating activities of $230,990 for the year ended March 31, 2009. The increase is primarily attributable to an increase of $3,008,225 in net income, and a $5,986,506 net liquidation in advances paid to both related and unrelated supplier. The increase to operating cash flow was offset by a $1,100,565 increase in inventory, a $582,606 increase in other accounts receivables, and an increase of $3,452,646 in prepaid rent.
We used $602,225 in investing activities during the year March 31, 2010 as compared to $474,072 during the year March 31, 2009 as a result of purchasing leasehold improvement.
Cash used in financing activities was $400,503 for the year ended March 31, 2010 as compared to cash provided by financing activities of $805,193 for the year ended March 31, 2009. We borrowed $1,465,600 and partially repaid loans totaling $512,400 during the year ended March 31, 2009. During the year ended March 31, 2010, we repaid loans totaling $2,932,400, while borrowing $935,000 from a shareholder and director and received proceeds from short term loans of $2,343,600. Additionally, for the year ended March 31, 2010 our notes payable required us to reserve $746,703 of our cash balance.
Our cash flow for the year ended March 31, 2010 does not reflect the financing that the company completed on April 28, 2010.
As of June 25, 2010, we had cash of $15,488,076. Our total current assets as of March 31, 2010 were $16,143,321 and our total current liabilities were 7,433,588 which resulted in a net working capital of $8,709,733 as of March 31, 2010.
Capital Resources
In April 28, 2010, we completed a public offering of 3.5 million shares of our common stock at a price of $5.00 per share resulting in net proceeds of $17.5 million. However, if we are to acquire another business or further expand our operations, we may need additional capital.
50
Contractual Obligations and Off-Balance Sheet Arrangements
Contractual Obligations
When we open store locations, we typically enter into lease agreements that are generally between four to five years. Our commitments for minimum rental payments under our leases for the next five years and thereafter are as follows:
|
Years ending March 31,
|
||||
|
2011
|
$ | 1,503,279 | ||
|
2012
|
1,239,488 | |||
|
2013
|
953,824 | |||
|
2014
|
690,934 | |||
|
2015
|
340,703 | |||
|
Thereafter
|
333,032 | |||
Logistics Services Commitments
We use a third party service provider, Zhejiang Yingte Logistics Co., Ltd., (“Yingte”) to accept goods from our suppliers and to deliver the goods to our store locations. On January 1, 2010 we entered into a one year agreement with Yingte and are obligated to pay 1% of the purchase price of the goods received from our suppliers by Yingte during the term of the agreement, from January 1, 2010 to December 31, 2010, with a contractual minimum of 2,900,000 RMB.
Off-balance Sheet Arrangements
We do not have any outstanding financial guarantees or commitments to guarantee the payment obligations of any third parties. We have not entered into any derivative contracts that are indexed to our shares and classified as stockholder’s equity or that are not reflected in our consolidated financial statements. Furthermore, we do not have any retained or contingent interest in assets transferred to an unconsolidated entity that serves as credit, liquidity or market risk support to such entity. We do not have any variable interest in any unconsolidated entity that provides financing, liquidity, market risk or credit support to us or engages in leasing, hedging or research and development services with us.
Exchange Rates
HJ Group maintains its books and records in Renminbi (“RMB”), the lawful currency of the PRC. In general, for consolidation purposes, the Company translates HJ Group’s assets and liabilities into U.S. Dollars using the applicable exchange rates prevailing at the balance sheet date, and the statement of income is translated at average exchange rates during the reporting period. Adjustments resulting from the translation of HJ Group’s financial statements are recorded as accumulated other comprehensive income.
Until July 21, 2005, RMB had been pegged to USD at the rate of RMB8.30: USD$1.00. On July 21, 2005, the PRC government reformed the exchange rate system into a managed floating exchange rate system based on market supply and demand with reference to a basket of currencies. In addition, the exchange rate of RMB to USD was adjusted to RMB8.11: USD$1.00 as of July 21, 2005. The People’s Bank of China announces the closing price of a foreign currency such as USD$ traded against RMB in the inter-bank foreign exchange market after the closing of the market on each working day, which will become the unified exchange rate for the trading against RMB on the following working day. The daily trading price of USD against RMB in the inter-bank foreign exchange market is allowed to float within a band of ±0.3% around the unified exchange rate published by the People’s Bank of China. This quotation of exchange rates does not imply free convertibility of RMB to other foreign currencies. All foreign exchange transactions continue to take place either through the Bank of China or other banks authorized to buy and sell foreign currencies at the exchange rates quoted by the People’s Bank of China. Approval of foreign currency payments by the Bank of China or other institutions required submitting a payment application form together with invoices, shipping documents and signed contracts.
51
The exchange rates used to translate amounts in RMB into US Dollars for the purposes of preparing the consolidated financial statements or otherwise stated in this report were as follows:
|
March 31,
2010
|
March 31,
2009
|
||||
|
Balance sheet items, except for the registered and paid-up capital, as of end of period/year
|
USD1:RMB
0.1467
|
USD1:RMB
0.1465
|
|||
|
Amounts included in the statement of operations, statement of changes in stockholders' equity and statement of cash flows for the period/ year ended
|
USD1:RMB
0.14664
|
USD1:RMB
0.14582
|
No representation is made that RMB amounts have been, or would be, converted into US$ at the above rates.
Inflation
We believe that inflation has not had a material effect on our operations to date.
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
Not applicable.
ITEM 8. FINANCIAL STATEMENTS
The consolidated financial statements and financial statement schedule are included in Part III, Item 15 (a) (1) and (2) of this Annual report on Form 10-K.
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURES
As reported in a Form 8-K Current Report filed with the SEC on December 2, 2009, we changed our independent accountants from Madsen & Associates CPA’s, Inc. to Moore Stephens Wurth Frazer and Torbet, LLP (“MSWFT”) effective November 11, 2009.
We were notified that, effective January 1, 2010, certain partners of MSWFT and Frost, PLLC (“Frost”) formed Frazer Frost, LLP (“Frazer Frost”), a new partnership. Pursuant to the terms of a combination agreement by and among MSWFT, Frazer Frost and Frost (the “Combination Agreement”), each of MSWFT and Frost contributed substantially all of their assets and certain of their liabilities to Frazer Frost, resulting in Frazer Frost assuming MSWFT’s engagement letter with us and becoming our new independent accounting firm. Frazer Frost is registered with the Public Company Accounting and Oversight Board (PCAOB).
ITEM 9A. CONTROLS AND PROCEDURES
Disclosure Controls and Procedures
We maintain disclosure controls and procedures that are designed to ensure that information required to be disclosed in our Exchange Act reports is recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms, and that such information is accumulated and communicated to our management, including its Chief Executive Officer and Chief Financial Officer, as appropriate, to allow timely decisions regarding required disclosure. In designing and evaluating the disclosure controls and procedures, management recognizes that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving the desired control objectives, and management necessarily is required to apply its judgment in evaluating the cost-benefit relationship of possible controls and procedures.
52
As of March 31, 2010, the end of the fiscal year covered by this report, our management, under the supervision and with the participation of our Chief Executive Officer and Chief Financial Officer, has performed an evaluation of the effectiveness of our disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) under the Securities Exchange Act of 1934).
Based on the evaluation, our Chief Executive Officer and Chief Financial Officer concluded that, as of the end of the period covered by this report, our disclosure controls and procedures were ineffective at the reasonable assurance level.
Management’s Report on Internal Control over Financial Reporting
We are responsible for establishing and maintaining adequate internal control over financial reporting (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)). Under the supervision and with the participation of our management, including our principal executive officer and principal financial officer, we conducted an evaluation of the effectiveness of our internal control over financial reporting based on the framework in Internal Control - Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). Based on our evaluation, management concluded that our internal control over financial reporting was not effective as of March 31, 2010 due to the following material weaknesses:
|
|
1.
|
Accounting and Finance Personnel Weaknesses - The current accounting staff is relatively inexperienced, and requires substantial training so as to meet with the higher demands necessary to fulfill the requirements of U.S. GAAP-based reporting and SEC rules and regulations.
|
Management’s assessment of the control deficiency over accounting and finance personnel as of March 31, 2010 considered the following factors including:
|
|
a.
|
the number of adjustments proposed by our independent auditors during our quarterly review and annual audit processes;
|
|
|
b.
|
the significance of the audit adjustments impact on the overall financial statements;
|
|
|
c.
|
how appropriately we complied with U.S. GAAP on transactions; and
|
|
|
d.
|
how accurately we prepared supporting information to provide to our independent auditors on a quarterly and annual basis.
|
Based on the above factors, management concluded that the control deficiency over accounting and finance personnel should be a material weakness as of March 31, 2010 and 2009, respectively, because the situation regarding the insufficient number of qualified resources in our U.S. reporting team remained the same in these two years.
|
|
2.
|
Lack of effective Internal Audit Function - We did not maintain effective controls over internal audit function due to the lack of qualified internal auditors who are familiar with internal audit and U.S. GAAP, and we did not implement adequate and proper supervisory review to ensure that significant internal control deficiencies can be detected or prevented.
|
The Company’s management has identified the steps it believes are necessary to address the weaknesses described above, and expect that we will satisfactorily address the control deficiencies and weaknesses relating to these matters by the end of our fiscal year ending March 31, 2011, although there can be no assurance that compliance will be achieved in this time frame.
This annual report does not include an attestation report of the Company’s independent registered accounting firm regarding internal control over financial reporting. The management’s report was not subject to attestation by the Company’s registered public accounting firm pursuant to temporary rules of the SEC that permit the Company to provide only management’s report in this annual report.
53
Changes in Internal Control over Financial Reporting
There have been no changes in our internal controls over financial reporting identified in connection with the evaluation required by paragraph (d) of Exchange Act Rule 13a-15 or Rule 15d-15 that occurred during the fourth quarter ended March 31, 2010 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
Limitations on Controls
Management does not expect that the Registrant’s disclosure controls and procedures or internal control over financial reporting will prevent or detect all error and fraud. Any control system, no matter how well designed and operated, is based upon certain assumptions and can provide only reasonable, not absolute, assurance that its objectives will be met. Further, no evaluation of controls can provide absolute assurance that misstatements due to error or fraud will not occur or that all control issues and instances of fraud, if any, within the Registrant have been detected.
ITEM 9B. OTHER INFORMATION
None.
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
The following table identifies our current executive officers and directors as of the date of this Form 10-K, their respective offices and positions, and their respective dates of election or appointment:
|
Name
|
Age
|
Position
|
Date of Appointment
|
|||
|
Lei Liu
|
45
|
Chief Executive Officer and Chairman of the Board of Directors
|
September 17, 2009
|
|||
|
Bennet P. Tchaikovsky
|
41
|
Chief Financial Officer
|
September 17, 2009
|
|||
|
Li Qi
|
37
|
Secretary and Director
|
October 23, 2009
|
|||
|
Chong’an Jin
|
46
|
Director
|
October 23, 2009
|
|||
|
Shike Zhu
|
47
|
Director
|
October 23, 2009
|
|||
|
Marc Thomas Serrio
|
51
|
Director
|
March 15, 2010
|
|||
|
Bowen Zhao
|
74
|
Director
|
March 15, 2010
|
|||
|
Yuehai Ke
|
38
|
Director
|
March 15, 2010
|
|||
|
Shuizhen Wu
|
60
|
Director
|
March 15, 2010
|
|||
|
Xiaomeng Yu
|
31
|
Director
|
March 15, 2010
|
Biographies
Lei Liu, Chief Executive Officer and Chairman of the Board of Directors
Mr. Liu is one of the three founders of HJ Group, and has been the executive director of Jiuzhou Pharmacy since September 2003 and the supervising director of Jiuzhou Service since November 2005. From December 1997 to August 2003, Mr. Liu worked in Tai He Drugstore as a general manager. From September 1992 to November 1997, Mr. Liu was an administration offical of Hangzhou Medical Junior College, his alma mater, where he was also a researcher and an anatomy instructor from September 1983 to July 1992. Mr. Liu has been a licensed researcher in the PRC since September 1988.
Bennet P. Tchaikovsky, Chief Financial Officer
Mr. Tchaikovsky is presently the chief financial officer of VLOV, Inc. which he performs on a part-time basis. From May 2008 through April 2010, Mr. Tchaikovsky served as the Chief Financial Officer of Skystar Bio-Pharmaceutical Company which he served on a part time basis. From March 2008 through November 2009, Mr. Tchaikovsky served as a director of Ever-Glory International Group. From December 2008 through November 2009, Mr. Tchaikovsky served as a director of Sino Clean Energy, Inc. From July 2004 through October 2007, Mr. Tchaikovsky served as the chief financial officer of Innovative Card Technologies, Inc. Mr. Tchaikovsky acted as a consultant to Innovative Card Technologies from November 2007 until July 2008. Mr. Tchaikovsky is a licensed Certified Public Accountant and an inactive member of the California State Bar. He received a B.A. in Business Economics from the University of California at Santa Barbara, and a J.D. from Southwestern University School of Law.
54
Li Qi, Secretary and Director
Ms. Qi is one of the three founders of HJ Group and is currently the general manager of both Jiuzhou Pharmacy and Jiuzhou Service. From January 2000 to June 2003, Ms. Qi worked in Zhejiang Yikang Drugstore as a general manager. From October 1991 to January 2000, Ms. Qi worked in the Branch Hospital of Hangzhou No. 1 People’s Hospital as a nurse. Ms. Qi is a licensed TCM pharmacist in the PRC and is a 1991 graduate of Hangzhou Nurse School.
Chong’an Jin, Director
Mr. Jin is one of the three founders of HJ Group and is presently the executive director of Jiuzhou Service and the managing general partner of Jiuzhou Clinic. From June 1996 to September 2003, Mr.Jin worked for Hangzhou Qiantang Medical Outpatient Clinic as a general manager. From December 1991 to October 1994, he worked in Hangzhou Hospital of Traditional Chinese Medicine as a physician of western medicine. From September 1988 to December 1991, Mr. Jin worked in Zhejiang Tumor Hospital as a physician of western medicine. In July 1988, Mr. Jin received a B.S. in Medicine from Sun Yat-sen Medical University, and is a licensed pharmacist in the PRC.
Shike Zhu, Director
Mr. Zhu is the chairman of Huai Nan Tian Rui Goods & Materials Co., Ltd., a post he has held since 2003. He is also the director of Tianri Rubber Products Co., Ltd. since 1994, where he was also the deputy general manager from 1994 to 1998. Since May 2008, Mr. Zhu has served as advisor to the chairman of China Wind Systems, Inc. From October 1988 to May 1994, Mr. Zhu was an official of Tiantai municipal government in Zhejiang Province, service as vice director of the Overseas Chinese Affairs Office and vice director of the Overseas Chinese Federation. Mr. Zhu is a graduate of Zhejiang TV University Engineering Management College.
Marc Thomas Serrio, Director
Mr. Serrio is founder and president of MTS Strategies, a position he has held since March 2008 which specializes in providing interim and part time financial executive services to early stage and middle market companies. Mr. Serrio has also been an independent executive consultant since January 1996, providing business and financial planning and analysis services to companies in various industries. Mr. Serrio was interim chief financial officer and interim chief operating officer of Kate Somerville LLC from July 2008 to March 2009, chief financial officer of Detection Logic, Inc. from November 2005 to March 2008, and chief financial officer of TriTech Software Systems from March 1999 to November 2005. Mr. Serrio is a graduate of the Marshall School of Business at the University of Southern California, with a B.S. in business administration in 1981 and a M.B.A. with emphasis on investment finance and business economics in 1985.
Bowen Zhao, Director
Mr. Zhao is a senior economist who has dedicated the past 54 years toward the development of the pharmaceutical industry in Zhejiang Province. Mr. Zhao is currently the deputy president of Zhejiang Province Industry and Economic Association, Zhejiang Province Entrepreneur Association and China Commercial Pharmacy Association, positions he has held since December 1994. In September 1996, Mr. Zhao was instrumental in organizing Zhejiang Province Commercial Pharmacy Association (which became Zhejiang Province Pharmaceutical Industry Association in September 2002), and has served as its president since its founding. Mr. Zhao has been with Zhejiang Pharmaceutical Co., Ltd. since September 1983 and is currently its deputy manager, and has been with Zhejiang Province Pharmaceutical Administration since May 1995, and is currently its deputy director.
Yuehai Ke, Director
Dr. Ke is a professor of molecular genetics and cell signal transduction at the Department of Basic Medicine at Zhejiang University’s School of Medicine since September 2007, where he also advices doctorate candidates. Dr. Ke graduated from Zhejiang University in 1995, where he majored in biochemistry. After graduation, Dr. Ke joined the Chinese Center for Disease Control and Prevention from September 1995 to July 1998. Dr. Ke obtained his master degree in medicine in 1998 from Fudan University, where he studied genetic disease of human multiple genes, and his doctorate degree in 2001 also from Fudan University. In 2000, Dr. Ke was an exchange student at the School of Public Health at the University of Texas in Houston. From February 2002 to September 2007, Dr. Ke studied cell signal transduction at the Cancer and Stem Cell Research Center of the Burnham Medical Research Institute in California. From September 2005 to September 2007, Dr. Ke was an associate professor at the Chinese Academy of Medical Sciences & Peking Union Medical College, focusing his research and studies on the application development of cell kinetics models and genetic analysis.
55
Shuizhen Wu, Director
Dr. Wu has been with Zhejiang No. 1 Hospital, which is affiliated with Zhejiang University’s School of Medicine, since July 2005, where she is currently a researcher and senior management personnel. From July 1978 to May 1994, Dr. Wu served as deputy director of the medical faculty at Zhejiang Medical University. Dr. Wu is a 1978 graduate of Zhejiang Medical University.
Xiaomeng Yu, Director
Mr. Yu is president of China Mingsheng Bank’s Xiasha branch in Hangzhou, where he was previously its senior client manager from October 2005 to July 2008. From August, 2003 to September 2005, Mr. Yu was translator and site manager for Hangzhou Road Engineering Equipment Co., Ltd. Mr. Wu graduated from Japan’s Daito Bunka University in September 2003 with a degree in business management.
Family Relationships
There are no family relationships between or among any of the current directors, executive officers or persons nominated or charged to become directors or executive officers. There are no family relationships among our officers and directors and those of our subsidiaries and affiliated companies.
Involvement in Certain Legal Proceedings
There are no orders, judgments, or decrees of any governmental agency or administrator, or of any court of competent jurisdiction, revoking or suspending for cause any license, permit or other authority to engage in the securities business or in the sale of a particular security or temporarily or permanently restraining any of our officers or directors from engaging in or continuing any conduct, practice or employment in connection with the purchase or sale of securities, or convicting such person of any felony or misdemeanor involving a security, or any aspect of the securities business or of theft or of any felony. Nor are any of the officers or directors of any corporation or entity affiliated with us so enjoined.
Compliance with Section 16(a) of the Exchange Act
Based solely on review of the copies of such forms furnished to the Company, or written representations that no reports were required, the Company believes that for the fiscal year ended March 31, 2010, our directors, executive officers and holders of 10% or more of our common stock complied with Section 16(a) filing requirements applicable to them.
The Board of Directors and Committees
Our board of directors formally established separate audit, nominating and compensation committees comprising of independent directors on March 15, 2010.
Audit Committee
Our audit committee is made up of three independent directors: Marc Thomas Serrio, Yuehai Ke and Shuizhen Wu, who were appointed to the committee on March 15, 2010. Our board of directors has determined, based on information furnished by Mr. Serrio and other available information, that Mr. Serrio meets the requirements of an “audit committee financial expert” as such term is defined in the rules promulgated under the Securities Act and the Exchange Act, and has accordingly designated him as such as well as chairman of the committee.
56
The responsibilities of our audit committee include:
|
●
|
meeting with our management periodically to consider the adequacy of our internal control over financial reporting and the objectivity of our financial reporting;
|
|
●
|
appointing the independent registered public accounting firm, determining the compensation of the independent registered public accounting firm and pre-approving the engagement of the independent registered public accounting firm for audit and non-audit services;
|
|
●
|
overseeing the independent registered public accounting firm, including reviewing independence and quality control procedures and experience and qualifications of audit personnel that are providing us audit services;
|
|
●
|
meeting with the independent registered public accounting firm and reviewing the scope and significant findings of the audits performed by them, and meeting with management and internal financial personnel regarding these matters; and
|
|
●
|
reviewing our financing plans, the adequacy and sufficiency of our financial and accounting controls, practices and procedures, the activities and recommendations of the auditors and our reporting policies and practices, and reporting recommendations to our full board of directors for approval.
|
Compensation Committee
Our compensation committee is made up of three independent directors: Yuehai Ke, Bowen Zhao and Xiaomeng Yu, who were appointed to the committee on March 15, 2010. Dr. Ke is chairman of the committee. Our compensation committee will oversee and, as appropriate, making recommendations to the board of directors regarding the annual salaries and other compensation of our executive officers and our employees, and other policies, and provide assistance and recommendations with respect to our compensation policies and practices.
Nominating Committee
Our nominating committee is made up of three independent directors: Shuizhen Wu, Bowen Zhao and Xiaomeng Yu, who were appointed to the committee on March 15, 2010. Dr. Wu is chairwoman of the committee. Our compensation committee will assist in the selection of director nominees, approve director nominations to be presented for stockholder approval at our annual general meeting and fill any vacancies on our board of directors, consider any nominations of director candidates validly made by stockholders, and review and consider developments in corporate governance practices.
Director Independence
Based on the information submitted by Marc Thomas Serrio, Bowen Zhao, Yuehai Ke, Shuizhen Wu and Xiaomeng Yu, our board of directors has determined that each of them is independent under Rule 5605(a)(2) of The NASDAQ Listing Rules.
Code of Ethics
We have adopted a code of ethics that applies to our officers, directors and employees, including our chief executive officer, senior executive officers, principal accounting officer, and other senior financial officers. A copy of our code of ethics will also be provided to any person without charge, upon written request sent to us at our offices located at Room 507-513, 5th Floor, A Building, Meidu Plaza, Gongshu District, Hangzhou, Zhejiang Province, China.
ITEM 11. EXECUTIVE COMPENSATION
Summary of Compensation
The following summary compensation table indicates the cash and non-cash compensation earned during the fiscal years ended March 31, 2010 and 2009 by (i) our CEO (principal executive officer), (ii) our CFO (principal financial officer), (iii) the three most highly compensated executive officers other than our CEO and CFO who were serving as executive officers at the end of our last completed fiscal year, whose total compensation exceeded $100,000 during such fiscal year ends, and (iv) up to two additional individuals for whom disclosure would have been provided but for the fact that the individual was not serving as an executive officer at the end of our last completed fiscal year, whose total compensation exceeded $100,000 during such fiscal year ends.
57
|
SUMMARY COMPENSATION TABLE
|
||||||||||||||||||||||||
|
Name and Principal Position
|
Fiscal Year ended
March 31,
|
Salary
($)
|
Bonus
($)
|
Stock
Awards
( $)
|
Option
Awards
($)
|
Non-Equity
Incentive Plan
Compensation
($)
|
Nonqualified
Deferred
Compensation Earnings
($)
|
All Other
Compensation
( $)
|
Total
($)
|
|||||||||||||||
|
Lei Liu,
|
2010
|
21,942 |
-0-
|
-0-
|
-0-
|
-0-
|
-0-
|
-0-
|
21,942 | |||||||||||||||
|
current CEO (1)
|
2009
|
10,000
|
-0-
|
-0-
|
-0-
|
-0-
|
-0-
|
-0-
|
10,000
|
|||||||||||||||
|
Bennet P. Tchaikovsky
|
2010
|
-0-
|
-0-
|
-0-
|
-
|
-0-
|
-0-
|
-0-
|
-0-
|
-0-
|
||||||||||||||
|
Current CFO (2)
|
2009
|
-0-
|
-0-
|
-0-
|
-0-
|
-0-
|
-0-
|
-0-
|
-0-
|
|||||||||||||||
|
Li Qi, Current
|
2010
|
19,894 |
-0-
|
-0-
|
-0-
|
-0-
|
-0-
|
-0-
|
19,894 | |||||||||||||||
|
Secretary (3)
|
2009
|
10,000
|
-0-
|
-0-
|
-0-
|
-0-
|
-0-
|
-0-
|
10,000
|
|||||||||||||||
______________________
|
(1)
|
Mr. Liu was appointed as our chief executive officer on September 17, 2009, in connection with the Share Exchange. Accordingly, Mr. Liu’s compensation for the periods reported was paid to him by HJ Group. Mr. Liu’s compensation as reported is based on interbank exchange rate of RMB 6.83610 to $1.00 on March 31, 2010.
|
|
(2)
|
Mr. Tchaikovsky was appointed as our chief financial officer on September 17, 2009, in connection with the Share Exchange.
|
|
(3)
|
Ms. Qi was appointed as our secretary on September 17, 2009, in connection with the Share Exchange. Accordingly, Ms. Qi’s compensation for the periods reported was paid to her by HJ Group. Ms. Qi’s compensation as reported is based on interbank exchange rate of RMB 6.83610 to $1.00 on March 31, 2010.
|
Grants of Plan-Based Awards in Fiscal Year Ended March 31, 2010
None
Outstanding Equity Awards at Fiscal Year Ended March 31, 2010
None
Option Exercises and Stock Vested in Fiscal Year Ended March 31, 2010
None
Pension Benefits
None
Nonqualified Defined Contribution and Other Nonqualified Deferred Compensation Plans
None
Employment Agreements, Termination of Employment and Change-in-Control Arrangements
Except as described below, we currently have no employment agreements with any of our executive officers, nor any compensatory plans or arrangements resulting from the resignation, retirement or any other termination of any of our executive officers, from a change-in-control, or from a change in any executive officer’s responsibilities following a change-in-control.
58
Agreements for the Services of Bennet P. Tchaikovsky
On July 30, 2009, Jiuzhou Pharmacy entered into a CFO Services Agreement (the “CFO Agreement”) with Worldwide Officers, Inc., a California corporation (“WOI”), to retain the services of Bennet P. Tchaikovsky as its chief financial officer until the completion of a financing. Under the terms of the Loanout Agreement, Mr. Tchaikovsky performs his duties from the United States for compensation of $30,000 through the close of a financing. The CFO Agreement terminates at the closing of a financing, provided that Jiuzhou Pharmacy shall enter into a new agreement at that time with WOI to continue Mr. Tchaikovsky’s engagement as chief financial officer. Jiuzhou Pharmacy may also terminate the CFO Agreement upon a 30-day written notice to Mr. Tchaikovsky. On the other hand, Mr. Tchaikovsky may terminate the CFO Agreement upon a 90-day written notice to Jiuzhou Pharmacy.
On May 14, 2010, we entered into a Loanout Agreement with Worldwide Officers, Inc. (“WOI”) pursuant to which we engaged the services of Mr. Tchaikovsky as our Chief Financial Officer for a period of one year beginning May 14, 2010. Mr. Tchaikovsky has been serving as our Chief Financial Officer since September 2009 without a written agreement with us.
Under the Loanout Agreement, we will pay cash compensation of $100,000 to WOI in 4 installments of $25,000 upon the signing of the Loanout Agreement and on July 28, 2010, October 28, 2010 and January 28, 2011. We will also issue 10,000 restricted shares of our common stock to WOI as further compensation for Mr. Tchaikovsky’s services which will be held in escrow and vest in five installments and distributed to WOI or its designee at the end of the one year term. In addition, we will issue to WOI 4,000 shares of our common stock as bonus. The shares issuable to WOI are to be issued under a stock plan to be adopted by us, which we agree to use best efforts to adopt by August 31, 2010. In addition to the foregoing, WOI is also entitled to reimbursement of reasonable expenses incurred in connection with Mr. Tchaikovsky’s services, and Mr. Tchaikovsky is entitled to be included as an insured under a directors and officers’ insurance policy to be obtained by us.
Concurrently with the entry of the Loanout Agreement, we entered into an Indemnification Agreement with Mr. Tchaikovsky, pursuant to which we agree to hold Mr. Tchaikovsky harmless and indemnify him for and against any expense, liability or loss paid or incurred in connection with any action, suit or proceeding arising from or related to the fact that he is or was an officer of the Company, or serving in other capacities at the request of the Company, or anything done by him in such capacity.
Director Compensation
The following table provides compensation information for our directors during the fiscal year ended March 31, 2010:
|
Name
|
Fiscal
Year ended March 31, |
Fees
Earned
or
Paid in
Cash
($)
|
Stock
Awards
($)(1)
|
Option
Awards
($)(1)
|
Non-Equity
Incentive Plan
Compensation
($)
|
Nonqualified
Deferred
Compensation
Earnings ($)
|
All Other
Compensation
($)
|
Total
($)
|
||||||||||||
|
Lei Liu (2)
|
2010
|
-0-
|
-0-
|
-0-
|
-0-
|
-0-
|
-0-
|
-0-
|
||||||||||||
|
Li Qi (2)
|
2010
|
-0-
|
-0-
|
-0-
|
-0-
|
-0-
|
-0-
|
-0-
|
||||||||||||
|
Chong’an Jin
|
2010
|
-0-
|
-0-
|
-0-
|
-0-
|
-0-
|
-0-
|
-0-
|
||||||||||||
|
Shike Zhu
|
2010
|
-0-
|
-0-
|
-0-
|
-0-
|
-0-
|
-0-
|
-0-
|
||||||||||||
|
Marc Thomas Serrio
|
2010
|
-0-
|
1,862
|
-0-
|
-0-
|
-0-
|
-0-
|
1,862
|
||||||||||||
|
Bowen Zhao
|
2010
|
-0-
|
-0-
|
-0-
|
-0-
|
-0-
|
-0-
|
-0-
|
||||||||||||
|
Yuehai Ke
|
2010
|
-0-
|
-0-
|
-0-
|
-0-
|
-0-
|
-0-
|
-0-
|
||||||||||||
|
Shuizhen Wu
|
2010
|
-0-
|
-0-
|
-0-
|
-0-
|
-0-
|
-0-
|
-0-
|
||||||||||||
|
Xiaomeng Yu
|
2010
|
-0-
|
-0-
|
-0-
|
-0-
|
-0-
|
-0-
|
-0-
|
||||||||||||
|
(1)
|
Reflects dollar amount expensed by the Company during the applicable fiscal year for financial statement reporting purposes.
|
|
(2)
|
This individual's compensation is reflected in the Summary Compensation Table on page 58 above.
|
We do not currently have an established policy to provide compensation to members of our board of directors for their services in that capacity, although we have entered into certain agreements with a director as described below. We intend to develop such a policy in the near future.
Agreements with Directors
We entered into an agreement with Mr. Serrio in the form of a director offer letter, pursuant to which we have agreed to compensate him $40,000 annually for his services as a director and audit committee financial expert and chairman. Mr. Serrio’s compensation will be in the form of 6,897 restricted shares of our common stock, payable in four quarterly installments beginning with the quarter ending March 31, 2010. Additionally, we are obligated to obtain and maintain a directors and officers’ insurance policy, and to include Mr. Serrio as an insured under such policy.
59
Concurrently with the director offer letter, we also entered into an indemnification agreement with Mr. Serrio, pursuant to which we agree to hold Mr. Serrio harmless and indemnify him from and against any expense, liability or loss paid or incurred in connection with any action, suit or proceeding arising from or related to the fact that Mr. Serrio is or was a director of the Company or anything done by him in such capacity.
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
Equity Compensation Plan Information
None
Security Ownership of Certain Beneficial Owners and Management
The following table sets forth certain information regarding our common stock beneficially owned on June 25, 2010 for (i) each stockholder known to be the beneficial owner of 5% or more of our outstanding common stock, (ii) each executive officer and director, and (iii) all executive officers and directors as a group. To the best of our knowledge, subject to community and marital property laws, all persons named have sole voting and investment power with respect to such shares, except as otherwise noted.
|
Common Stock Beneficially Owned
|
||||||||
|
Executive officers and directors: (1)
|
|
Number of
Shares
beneficially
owned (2)
|
|
|
Percentage of
class beneficially
owned (3)
|
|||
|
Lei Liu, chief executive officer and chairman of the board of directors (4)
|
6,030,000
|
44.7
|
%
|
|||||
|
Bennet P. Tchaikovsky, chief financial officer (5)
|
106,000
|
0.8
|
%
|
|||||
|
Li Qi, Secretary and Director (4)
|
6,030,000
|
44.7
|
%
|
|||||
|
Chong’an Jin, Director (4)
|
6,030,000
|
44.7
|
%
|
|||||
|
Shike Zhu, Director (6)
|
250,000
|
1.9
|
%
|
|||||
|
Marc Thomas Serrio (7)
|
3,450
|
*
|
||||||
|
Bowen Zhao (8)
|
0
|
0
|
%
|
|||||
|
Yuehai Ke (9)
|
0
|
0
|
%
|
|||||
|
Shuizhen Wu (10)
|
0
|
0
|
%
|
|||||
|
Xiaomeng Yu (11)
|
0
|
0
|
%
|
|||||
|
All directors and executive officers as a group (5 persons)
|
6,389,450
|
47.3
|
%
|
|||||
|
5% Shareholders: (1)
|
||||||||
|
Super Marvel Limited (4)
|
6,030,000
|
44.7
|
%
|
|||||
|
*
|
Less than 1%.
|
|
|
(1)
|
Unless otherwise noted, the address for each of the named beneficial owners is: Room 507-513, 5th Floor, A Building, Meidu Plaza, Gongshu District, Hangzhou, Zhejiang Province, China.
|
|
(2)
|
Under Rule 13d-3, a beneficial owner of a security includes any person who, directly or indirectly, through any contract, arrangement, understanding, relationship, or otherwise has or shares: (i) voting power, which includes the power to vote, or to direct the voting of shares; and (ii) investment power, which includes the power to dispose or direct the disposition of shares. Certain shares may be deemed to be beneficially owned by more than one person (if, for example, persons share the power to vote or the power to dispose of the shares). In addition, shares are deemed to be beneficially owned by a person if the person has the right to acquire the shares (for example, upon exercise of an option) within 60 days of the date as of which the information is provided. In computing the percentage ownership of any person, the amount of shares outstanding is deemed to include the amount of shares beneficially owned by such person (and only such person) by reason of these acquisition rights. As a result, the percentage of outstanding shares of any person as shown in this table does not necessarily reflect the person's actual ownership or voting power with respect to the number of shares of common stock actually outstanding.
|
60
|
(3)
|
Unless otherwise noted, the number and percentage of outstanding shares of common stock is based upon 13,500,002 shares outstanding as of June 25, 2010.
|
|
(4)
|
The address of Super Marvel Limited (“Super Marvel”) address is P.O. Box 957, Offshore Incorporations Centre, Road Town, Tortola, British Virgin Islands. The owners of Super Marvel are Lei Liu (39%), who is also its executive director, and Li Qi (30%) and Chong’an Jin (31%), who are also its directors. As such, they are deemed to have or share investment control over Super Marvel’s portfolio. The numbers of shares of common stock reported herein as beneficially owned by Mr. Liu, Ms. Qi and Mr. Jin are held by Super Marvel, which they in turn own indirectly through their respective ownership of Super Marvel.
|
|
|
(5)
|
Bennet P. Tchaikovsky’s address is: 6571 Morningside Drive, Huntington Beach, CA 92648. Includes 6,000 shares that Mr. Tchaikovsky has the right to acquire beneficial ownership of within 60 days of June 25, 2010.
|
|
(6)
|
Shike Zhu’s address is: Citigroup Tower, 24/F, 33 Hua Yuan Shi Qiao Road, Pudong New Area, Shanghai, China 200120.
|
|
|
(7)
|
Marc Thomas Serrio’s address is: P.O. Box 91836, Pasadena, California 91109. Includes 3,450 shares to which Mr. Serrio has the right to acquire within 60 days of June 25, 2010.
|
|
(8)
|
Bowen Zhao’s address is: Room 1315, Hualong Business Building, No. 110 N. Ganshan Road, Hangzhou, China 310000.
|
|
|
(9)
|
Yuehai Ke’s address is: 388 Yuhangtang Road, Hangzhou, China 310058.
|
|
(10)
|
Shuizhen Wu’s address is: Room 2302, #20 Building, Hanlinguan Daxue Road, Hangzhou, China 310000.
|
|
|
(11)
|
Xiaomeng Yu’s address is: Wen Hui Guan Quen Fang 7-2, No. 3 Street, Baiyang Street, Economic Commercial and Technological Development Area, Hangzhou, China 310018.
|
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS
Share Exchange Agreement
On September 17, 2009, we executed the Exchange Agreement with Renovation and the Renovation Stockholders.
On the Closing Date of the Exchange Agreement, we issued 7,900,000 shares of our common stock to the Renovation Stockholders in exchange for 100% of the issued and outstanding common stock of Renovation. Immediately upon the closing of the Share Exchange, we had a total of 10,000,000 shares of common stock outstanding , with the Renovation Stockholders collectively owning approximately 79% of our total issued and outstanding common shares.
As a result of the Share Exchange, the Renovation Stockholders became our controlling shareholders and Renovation became our wholly owned subsidiary.
Other Related Party Transactions
Set forth below are the related party transactions us and our officers and/or directors as of the dates set forth on the table:
|
|
March 31,
2010
|
|
March 31,
2009
|
|||||
|
Amounts due from directors (1):
|
$
|
-
|
$
|
2,432
|
||||
|
Amount due to director (2):
|
$
|
935,000
|
$
|
326,715
|
||||
|
Advances to supplier (3):
|
$
|
-
|
$
|
1,797,104
|
||||
|
(1)
|
Represents interest free loans to two directors of the Company, Li Qi and Chong’an Jin. The loans are due upon demand. There is no written documentation for these loans, which represent cash advances for out-of-pocket expenses for Ms. Qi and Mr. Jin, who are responsible for submitting receipts for these amounts or refunding the balance.
|
61
|
(2)
|
As of March 31, 2010, the amount due to directors represents contribution from directors, Li Qi, Chong 'an Jin, and Lei Liu to Jiuxin Management to enable Jiuxin Management to meet its approved PRC registered capital requirements. Such contributions are to be returned to the directors upon demand. At March 31, 2009, the amounts due to directors represent leasehold improvement expenses paid by a director of the Company, Lei Liu, on behalf of the Company and are due upon demand.
|
|
(3)
|
Represents prepayment for inventory purchase made to a supplier, which was formerly owned by some of the Company’s directors. The Company will collect inventory from the supplier. The Company’s purchases from the related party supplier amounted to $3,785,728 and $1,970,444 for the years ended March 31, 2010 and 2009, respectively.
|
The Company also leases a retail space and the corporate office space from Mr. Liu under long-term operating lease agreements beginning August 2008 to August 2010 and from January 2008 to March 2012, respectively. The rent for the retail and the corporate office space are $175,968 and $170,123 for years ended March 31, 2010 and 2009, respectively. For the year ended March 31, 2010, $175,968 was paid to Mr. Liu for these leases. For the year ended March 31, 2009, $171,360 was paid to Mr. Liu.
ITEM 14. PRINCIPAL ACCOUNTING FEES AND SERVICES
Audit Fees
The aggregate fees billed by our independent auditors, Frazer Frost LLP (successor entity of Moore Stephens Wurth Frazer and Torbet, LLP), for the fiscal years ended March 31, 2010 and 2009, for professional services rendered for the audit of the Company’s annual financial statements and review of the Company’s quarterly financial statements were $235,000 and $155,000, respectively.
Audited-Related Fees
For the fiscal years ended March 31, 2010 and 2009, there were no fees billed by our independent auditors for services reasonably related to the performance of the audit or review of the financial statements outside of those fees disclosed above under “Audit Fees,”
Tax Fees
The aggregate fees billed by Frazer Frost for the fiscal years ended March 31, 2010 and 2009 for services rendered for tax compliance, tax advice and tax planning work to the Company were $0 and $0, respectively.
All Other Fees
For the fiscal years ended March 31, 2010 and 2009, there were no other fees billed by our independent auditors for products and services outside of those fees disclosed above under “Audit Fees”, “Audit-Related Fees” and “Tax Fees”.
ITEM 15. EXHIBITS AND FINANCIAL STATEMENT SCHEDULES
(1) Financial Statements
The following consolidated financial statements of China Jo-Jo are included in Part II, Item 8 of this Report:
Report of Independent Registered Public Accounting Firm
Consolidated Balance Sheets at March 31, 2010 and 2009
Consolidated Statements of Operations and Comprehensive Income for the Years Ended March 31, 2010 and 2009
Consolidated Statements of Shareholders’ Equity for the Years Ended March 31, 2010 and 2009
62
Consolidated Statements of Cash Flows for the Years Ended March 31, 2010 and 2009
Notes to Consolidated Financial Statements
(2) Financial Statement Schedules
Schedules are omitted because the required information is not present or is not present in amounts sufficient to require submission of the schedule or because the information required is given in the consolidated financial statements or the notes thereto.
(3) Exhibits
EXHIBIT INDEX
|
Exhibit
Number
|
Description
|
|
|
2
|
Share Exchange Agreement among Kerrisdale Mining Corporation (“Kerrisdale”), certain of its stockholders, Renovation Investment (Hong Kong) Co., Ltd. (“Renovation”) and its shareholders dated September 17, 2009 (1)
|
|
|
3.1
|
Articles of Incorporation of Kerrisdale (2)
|
|
|
3.2
|
Certificate of Amendment to Articles of Incorporation of Kerrisdale filed with the Nevada Secretary of State on July 14, 2008 (3)
|
|
|
3.3
|
Articles of Merger between Kerrisdale Mining and China Jo-Jo Drugstores, Inc. filed with the Nevada Secretary of State on September 22, 2009 (1)
|
|
|
3.4
|
Bylaws (2)
|
|
|
3.5
|
Text of Amendments to the Bylaws (3)
|
|
|
3.6
|
Certificate of Change Pursuant to NRS 78.209 with an effective date of April 9, 2010 (9)
|
|
|
4
|
Specimen of Common Stock Certificate (2)
|
|
|
10.1
|
Consulting Services Agreement between Zhejiang Jiuxin Investment Management Co., Ltd. (“Jiuxin Management”) and Hangzhou Jiuzhou Grand Pharmacy Chain Co., Ltd. (“Jiuzhou Pharmacy”) dated August 1, 2009 (1)
|
|
|
10.2
|
Operating Agreement among Jiuxin Management, Jiuzhou Pharmacy and its owners dated August 1, 2009 (1)
|
|
|
10.3
|
Equity Pledge Agreement among Jiuxin Management, Jiuzhou Pharmacy and its owners dated August 1, 2009 (1)
|
|
|
10.4
|
Option Agreement among Jiuxin Management, Jiuzhou Pharmacy and its owners dated August 1, 2009 (1)
|
|
|
10.5
|
Voting Rights Proxy Agreement among Jiuxin Management, Jiuzhou Pharmacy and its owners dated August 1, 2009 (1)
|
|
|
10.6
|
Consulting Services Agreement between Jiuxin Management and Hangzhou Jiuzhou Clinic of Integrated Traditional and Western Medicine (General Partnership) (“Jiuzhou Clinic”) dated August 1, 2009 (1)
|
|
|
10.7
|
Operating Agreement among Jiuxin Management, Jiuzhou Clinic and its owners dated August 1, 2009 (1)
|
|
|
10.8
|
Equity Pledge Agreement among Jiuxin Management, Jiuzhou Clinic and its owners dated August 1, 2009 (1)
|
|
|
10.9
|
Option Agreement among Jiuxin Management, Jiuzhou Clinic and its owners dated August 1, 2009 (1)
|
|
|
10.10
|
Voting Rights Proxy Agreement among Jiuxin Management, Jiuzhou Clinic and its owners dated August 1, 2009 (1)
|
|
|
10.11
|
Consulting Services Agreement between Jiuxin Management and Hangzhou Jiuzhou Medical & Public Health Service Co., Ltd. (“Jiuzhou Service”) dated August 1, 2009 (1)
|
|
|
10.12
|
Operating Agreement among Jiuxin Management, Jiuzhou Service and its owners dated August 1, 2009 (1)
|
|
|
10.13
|
Equity Pledge Agreement among Jiuxin Management, Jiuzhou Service and its owners dated August 1, 2009 (1)
|
|
|
10.14
|
Option Agreement among Jiuxin Management, Jiuzhou Service and its owners dated August 1, 2009 (1)
|
|
|
10.15
|
Voting Rights Proxy Agreement among Jiuxin Management, Jiuzhou Service and its owners dated August 1, 2009 (1)
|
63
|
10.16
|
Amendment to Consulting Services Agreement between Jiuxin Management and Jiuzhou Pharmacy dated October 27, 2009 (4)
|
|
|
10.17
|
Amendment to Operating Agreement between Jiuxin Management and Jiuzhou Pharmacy dated October 27, 2009 (4)
|
|
|
10.18
|
Amendment to Option Agreement between Jiuxin Management and Jiuzhou Pharmacy dated October 27, 2009 (4)
|
|
|
10.19
|
Amendment to Voting Rights Proxy Agreement between Jiuxin Management and Jiuzhou Pharmacy dated October 27, 2009 (4)
|
|
|
10.20
|
Amendment to Consulting Services Agreement between Jiuxin Management and Jiuzhou Clinic dated October 27, 2009 (4)
|
|
|
10.21
|
Amendment to Operating Agreement between Jiuxin Management and Jiuzhou Clinic dated October 27, 2009 (4)
|
|
|
10.22
|
Amendment to Option Agreement between Jiuxin Management and Jiuzhou Clinic dated October 27, 2009 (4)
|
|
|
10.23
|
Amendment to Voting Rights Proxy Agreement between Jiuxin Management and Jiuzhou Clinic dated October 27, 2009 (4)
|
|
|
10.24
|
Amendment to Consulting Services Agreement between Jiuxin Management and Jiuzhou Service dated October 27, 2009 (4)
|
|
|
10.25
|
Amendment to Operating Agreement between Jiuxin Management and Jiuzhou Service dated October 27, 2009 (4)
|
|
|
10.26
|
Amendment to Option Agreement between Jiuxin Management and Jiuzhou Service dated October 27, 2009 (4)
|
|
|
10.27
|
Amendment to Voting Rights Proxy Agreement between Jiuxin Management and Jiuzhou Service dated October 27, 2009 (4)
|
|
|
10.28
|
Director Offer Letter with Marc Thomas Serrio dated March 15, 2010 (7)
|
|
|
10.29
|
Indemnification Agreement with Marc Thomas Serrio dated March 15, 2010 (7)
|
|
|
10.30
|
Loanout Agreement with Worldwide Officers, Inc. dated May 14, 2010 (11)
|
|
|
10.31
|
Indemnification Agreement with Mr. Bennet Tchaikovsky dated May 14, 2010 (11)
|
|
|
14
|
Code of Ethics (7)
|
|
|
16
|
Letter from Madsen & Associates CPA’s, Inc. dated April 15, 2010. (10)
|
|
|
21
|
List of subsidiaries (5)
|
|
|
23
|
Consent of Frazer Frost, LLP (successor entity of Moore Stephens Wurth Frazer and Torbet, LLP) *
|
|
|
31.1
|
Section 302 Certification by the Corporation’s Chief Executive Officer *
|
|
|
31.2
|
Section 302 Certification by the Corporation’s Chief Financial Officer *
|
|
|
32.1
|
Section 906 Certification by the Corporation’s Chief Executive Officer *
|
|
|
32.2
|
Section 906 Certification by the Corporation’s Chief Financial Officer *
|
|
|
99.1
|
Agreement between Jiuzhou Pharmacy and Yingte Logistics Co., Ltd. (“Yingte Logistics”) dated January 1, 2009 (1)
|
|
|
99.2
|
Form of CFO Services Agreement entered into between Jiuzhou Pharmacy and Worldwide Officers, Inc. on July 30, 2009 (6)
|
|
|
99.3
|
Agreement between Jiuzhou Pharmacy and Yingte Logistics dated January 1, 2010 (8)
|
|
| 99.4 |
Project Agreement between The People's Government of Qianhong Village, Lin'an, Zhejiang Province (the "Qianhong Local Government") and Jiuzhou Pharmacy dated February 27, 2010 *
|
|
| 99.5 |
Security Deposit Agreement between the Qianhong Local Government and Jiuzhou Pharmacy dated February 27, 2010 *
|
|
*
|
Filed herewith
|
|
(1)
|
Incorporated by reference from the Registrant’s Registration Statement on Form SB-2 filed on November 28, 2007.
|
|
(2)
|
Incorporated by reference from the Registrant’s Current Report on Form 8-K filed on July 15, 2008.
|
|
(3)
|
Incorporated by reference from the Registrant’s Current Report on Form 8-K filed on September 24, 2009.
|
|
(4)
|
Incorporated by reference from the Registrant’s Current Report on Form 8-K filed on October 30, 2009.
|
|
(5)
|
Incorporated by reference from the Registrant’s Registration Statement on Form S-1 filed on December 21, 2009.
|
|
(6)
|
Incorporated by reference from the Registrant’s Registration Statement on Form S-1/A filed on January 27, 2010.
|
|
(7)
|
Incorporated by reference from the Registrant’s Current Report on Form 8-K filed on March 16, 2010.
|
|
(8)
|
Incorporated by reference from the Registrant’s Registration Statement on Form S-1/A filed on March 23, 2010
|
|
(9)
|
Incorporated by reference from the Registrant’s Current Report on Form 8-K filed on April 14, 2010.
|
|
(10)
|
Incorporated by reference from the Registrant’s Current Report on Form 8-K filed on April 15, 2010.
|
|
(11)
|
Incorporated by reference from the Registrant’s Current Report filed on May 17, 2010.
|
64
Pursuant to the requirements of section 13 or 15 (d) of the Securities Exchange Act of 1934, the Registrant has duly caused this Report to be signed on its behalf by the undersigned, thereunto duly authorized.
|
CHINA JO-JO DRUGSTORES, INC.
(Registrant)
|
||||
|
Date: June 29, 2010
|
By:
|
/s/ Lei Liu
|
||
|
Lei Liu
Chief Executive Officer
|
||||
|
Date: June 29, 2010
|
By:
|
/s/ Bennet P. Tchaikovsky
|
||
|
Bennet P. Tchaikovsky
Chief Financial Officer
|
In accordance with the Exchange Act, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
|
Name
|
Title
|
Date
|
||
|
/s/ Lei Liu
|
Chief Executive Officer / Director
|
June 29, 2010
|
||
|
Lei Liu
|
||||
|
/s/ Bennet P. Tchaikovsky
|
Chief Financial Officer
|
June 29, 2010
|
||
|
Bennet P. Tchaikovsky
|
||||
|
/s/ Li Qi
|
Secretary / Director
|
June 29, 2010
|
||
|
Li Qi
|
||||
|
/s/ Chong’an Jin
|
Director
|
June 29, 2010
|
||
|
Chong’an Jin
|
||||
|
/s/ Shike Zhu
|
Director
|
June 29, 2010
|
||
|
Shike Zhu
|
||||
|
/s/ Yuehai Ke
|
Director
|
June 29, 2010
|
||
|
Yuehai Ke
|
||||
|
/s/ Marc Thomas Serrio
|
Director
|
June 29, 2010
|
||
|
Marc Thomas Serrio
|
||||
|
/s/ Shuizhen Wu
|
Director
|
June 29, 2010
|
||
|
Shuizhen Wu
|
|
/s/ Xiaomeng Yu
|
Director
|
June 29, 2010
|
||
|
Xiaomeng Yu
|
||||
|
/s/ Bowen Zhao
|
Director
|
June 29, 2010
|
||
|
Bowen Zhao
|
65
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders of
China Jo-Jo Drugstores, Inc and Subsidiaries
We have audited the accompanying consolidated balance sheets of China Jo-Jo Drugstores, Inc. and subsidiaries as of March 31, 2010 and 2009, and the related consolidated statements of income and other comprehensive income, shareholders’ equity and cash flows for each of the years in the two-year period ended March 31, 2010. China Jo-Jo Drugstores, Inc. and subsidiaries’ management is responsible for these financial statements. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. The company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audit included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of China Jo-Jo Drugstores, Inc. and subsidiaries as of March 31, 2010 and 2009, and the results of its operations and its cash flows for each of the years in the two-year period ended March 31, 2010 in conformity with accounting principles generally accepted in the United States of America.
/s/ Frazer Frost, LLP (Successor Entity of Moore Stephens Wurth Frazer and Torbet, LLP, see Form 8-K filed on January 7, 2010)
Brea, California
June 29, 2010
|
CHINA JO-JO DRUGSTORES, INC AND SUBSIDIARIES
|
||||||||
|
(FORMERLY KNOWN AS KERRISDALE MINING CORPORATION)
|
||||||||
|
CONSOLIDATED BALANCE SHEETS
|
||||||||
|
AS OF MARCH 31, 2010 AND MARCH 31, 2009
|
||||||||
|
ASSETS
|
||||||||
|
2010
|
2009
|
|||||||
|
CURRENT ASSETS
|
||||||||
|
Cash
|
$ | 801,593 | $ | 996,302 | ||||
|
Restricted cash
|
746,703 | - | ||||||
|
Accounts receivable, trade
|
1,228,294 | 1,265,110 | ||||||
|
Inventories
|
3,770,411 | 2,793,101 | ||||||
|
Other receivables
|
282,073 | 67,079 | ||||||
|
Other receivables - related parties
|
- | 2,432 | ||||||
|
Advances to suppliers
|
6,850,240 | 5,485,113 | ||||||
|
Advances to supplier - related party
|
- | 1,797,104 | ||||||
|
Other current assets
|
2,464,007 | 564,379 | ||||||
|
Total current assets
|
16,143,321 | 12,970,620 | ||||||
|
PROPERTY AND EQUIPMENT, net
|
1,186,292 | 979,432 | ||||||
|
OTHER ASSETS:
|
||||||||
|
Long term deposit
|
2,539,424 | 2,015,149 | ||||||
|
Prepaid rent - noncurrent
|
4,254,300 | - | ||||||
|
Total other assets
|
6,793,724 | 2,015,149 | ||||||
|
Total assets
|
$ | 24,123,337 | $ | 15,965,201 | ||||
|
LIABILITIES AND SHAREHOLDERS' EQUITY
|
||||||||
|
CURRENT LIABILITIES
|
||||||||
|
Short term loans
|
$ | 880,200 | $ | 1,465,000 | ||||
|
Notes payable
|
1,464,241 | - | ||||||
|
Accounts payable, trade
|
2,330,317 | 5,939,237 | ||||||
|
Other payables
|
331,656 | 404,731 | ||||||
|
Other payables - related parties
|
935,000 | 326,715 | ||||||
|
Taxes payable
|
1,235,083 | 811,316 | ||||||
|
Accrued liabilities
|
257,091 | 360,655 | ||||||
|
Total liabilities
|
7,433,588 | 9,307,654 | ||||||
|
COMMITMENTS AND CONTINGENCIES
|
||||||||
|
SHAREHOLDERS' EQUITY
|
||||||||
|
Common stock; $0.001 par value; 250,000,000 shares authorized;
|
||||||||
|
10,000,000 and 7,900,000 shares issued and outstanding
|
||||||||
|
as of March 31, 2010 and March 31, 2009, respectively
|
10,000 | 7,900 | ||||||
|
Paid-in capital
|
877,884 | 669,700 | ||||||
|
Statutory reserves
|
1,309,109 | 1,309,109 | ||||||
|
Retained earnings
|
14,855,016 | 5,033,275 | ||||||
|
Accumulated other comprehensive loss
|
(362,260 | ) | (362,437 | ) | ||||
|
Total shareholders' equity
|
16,689,749 | 6,657,547 | ||||||
|
Total liabilities and shareholders' equity
|
$ | 24,123,337 | $ | 15,965,201 | ||||
See report of independent registered public accounting firm. The accompanying notes are an integral part of these consolidated financial statements.
F-1
|
CHINA JO-JO DRUGSTORES, INC AND SUBSIDIARIES
|
||||||||
|
(FORMERLY KNOWN AS KERRISDALE MINING CORPORATION)
|
||||||||
|
CONSOLIDATED STATEMENTS OF INCOME AND OTHER COMPREHENSIVE INCOME
|
||||||||
|
FOR YEARS ENDED MARCH 31, 2010 AND 2009
|
||||||||
|
2010
|
2009
|
|||||||
|
REVENUES
|
$ | 55,174,929 | $ | 44,776,652 | ||||
|
COST OF GOODS SOLD
|
38,276,889 | 32,607,741 | ||||||
|
GROSS PROFIT
|
16,898,040 | 12,168,911 | ||||||
|
SELLING EXPENSES
|
2,630,332 | 1,712,474 | ||||||
|
GENERAL & ADMINISTRATIVE EXPENSES
|
1,510,154 | 1,399,305 | ||||||
|
OPERATING EXPENSES
|
4,140,486 | 3,111,779 | ||||||
|
INCOME FROM OPERATIONS
|
12,757,554 | 9,057,132 | ||||||
|
OTHER INCOME (EXPENSE), NET
|
(55,520 | ) | 17,369 | |||||
|
INCOME BEFORE INCOME TAXES
|
12,702,034 | 9,074,501 | ||||||
|
PROVISION FOR INCOME TAXES
|
2,880,293 | 2,260,985 | ||||||
|
NET INCOME
|
9,821,741 | 6,813,516 | ||||||
|
OTHER COMPREHENSIVE INCOME
|
||||||||
|
Foreign currency translation adjustments
|
177 | 27,688 | ||||||
|
COMPREHENSIVE INCOME
|
$ | 9,821,918 | $ | 6,841,204 | ||||
|
BASIC AND DILUTED WEIGHTED AVERAGE NUMBER OF SHARES
|
9,025,000 | 7,900,000 | ||||||
|
BASIC AND DILUTED EARNING PER SHARE
|
$ | 1.09 | $ | 0.86 | ||||
See report of independent registered public accounting firm. The accompanying notes are an integral part of these consolidated financial statements.
F-2
|
CHINA JO-JO DRUGSTORES, INC AND SUBSIDIARIES
|
||||||||||||||||||||||||||||
|
(FORMERLY KNOWN AS KERRISDALE MINING CORPORATION)
|
||||||||||||||||||||||||||||
|
CONSOLIDATED STATEMENTS OF SHAREHOLDERS' EQUITY
|
||||||||||||||||||||||||||||
|
FOR YEARS ENDED MARCH 31, 2010 AND 2009
|
||||||||||||||||||||||||||||
|
Accumulated
|
||||||||||||||||||||||||||||
|
Common Stock
|
Retained Earnings
|
other
|
||||||||||||||||||||||||||
|
Number of
|
Paid-in
|
Statutory
|
comprehensive
|
|||||||||||||||||||||||||
|
shares
|
Amount
|
capital
|
reserves
|
Unrestricted
|
income/(loss)
|
Totals
|
||||||||||||||||||||||
|
BALANCE, March 31, 2008
|
7,900,000 | $ | 7,900 | $ | 669,700 | $ | 606,665 | $ | (1,077,797 | ) | $ | (390,125 | ) | $ | (183,657 | ) | ||||||||||||
|
Net income
|
6,813,516 | 6,813,516 | ||||||||||||||||||||||||||
|
Adjustment of statutory reserves
|
702,444 | (702,444 | ) | - | ||||||||||||||||||||||||
|
Foreign currency translation adjustments
|
27,688 | 27,688 | ||||||||||||||||||||||||||
|
BALANCE, March 31, 2009
|
7,900,000 | $ | 7,900 | $ | 669,700 | $ | 1,309,109 | $ | 5,033,275 | $ | (362,437 | ) | $ | 6,657,547 | ||||||||||||||
|
Shares issued for reorganization on
|
||||||||||||||||||||||||||||
|
September 17, 2009
|
2,100,000 | 2,100 | (2,100 | ) | - | |||||||||||||||||||||||
|
Stock-based compensation
|
202,120 | 202,120 | ||||||||||||||||||||||||||
|
Net income
|
9,821,741 | 9,821,741 | ||||||||||||||||||||||||||
|
Shareholder contribution
|
8,164 | 8,164 | ||||||||||||||||||||||||||
|
Foreign currency translation adjustments
|
177 | 177 | ||||||||||||||||||||||||||
|
BALANCE, March 31, 2010
|
10,000,000 | $ | 10,000 | $ | 877,884 | $ | 1,309,109 | $ | 14,855,016 | $ | (362,260 | ) | $ | 16,689,749 | ||||||||||||||
See report of independent registered public accounting firm. The accompanying notes are an integral part of these consolidated financial statements.
F-3
|
CHINA JO-JO DRUGSTORES, INC AND SUBSIDIARIES
|
||||||||
|
(FORMERLY KNOWN AS KERRISDALE MINING CORPORATION)
|
||||||||
|
CONSOLIDATED STATEMENTS OF CASH FLOWS
|
||||||||
|
FOR THE YEARS ENDED MARCH 31, 2010 AND 2009
|
||||||||
|
2010
|
2009
|
|||||||
|
CASH FLOWS FROM OPERATING ACTIVITIES:
|
||||||||
|
Net income
|
$ | 9,821,741 | $ | 6,813,516 | ||||
|
Adjustments to reconcile net income to net cash
|
||||||||
|
provided by (used in) operating activities:
|
||||||||
|
Depreciation and amortization
|
396,574 | 439,464 | ||||||
|
Stock compensation
|
126,325 | - | ||||||
|
Change in operating assets
|
||||||||
|
Accounts receivable, trade
|
38,527 | (407,133 | ) | |||||
|
Inventories
|
(973,099 | ) | 127,466 | |||||
|
Other receivables
|
(214,815 | ) | 367,791 | |||||
|
Other receivables - related parties
|
2,435 | 167,234 | ||||||
|
Advances to suppliers
|
(1,357,084 | ) | (4,793,517 | ) | ||||
|
Advances to suppliers - related parties
|
1,798,822 | (751,251 | ) | |||||
|
Other current assets
|
(1,898,290 | ) | (36,279 | ) | ||||
|
Long term deposit
|
(521,310 | ) | (2,005,795 | ) | ||||
| Prepaid rent - noncurrent | (4,252,560 | ) | - | |||||
|
Change in operating liabilities
|
||||||||
|
Accounts payable, trade
|
(2,151,905 | ) | (227,834 | ) | ||||
|
Other payables and accrued liabilities
|
(103,512 | ) | (95,177 | ) | ||||
|
Other payables-related parties
|
(327,027 | ) | (21,952 | ) | ||||
|
Customer deposits
|
- | (87,492 | ) | |||||
|
Taxes payable
|
422,487 | 279,969 | ||||||
|
Net cash provided by (used in) operating activities
|
807,309 | (230,990 | ) | |||||
|
CASH FLOWS FROM INVESTING ACTIVITIES:
|
||||||||
|
Purchase of equipment
|
(74,516 | ) | (35,906 | ) | ||||
|
Additions to leasehold improvements
|
(527,709 | ) | (438,166 | ) | ||||
|
Net cash used in investing activities
|
(602,225 | ) | (474,072 | ) | ||||
|
CASH FLOWS FROM FINANCING ACTIVITIES:
|
||||||||
|
Restricted cash
|
(746,703 | ) | - | |||||
|
Other payables-related parties
|
935,000 | - | ||||||
|
Payments on short-term loans
|
(2,932,400 | ) | (512,400 | ) | ||||
|
Proceeds from short-term loans
|
2,343,600 | 1,465,600 | ||||||
|
Distribution to shareholders
|
- | (148,007 | ) | |||||
|
Net cash (used in) provided by financing activities
|
(400,503 | ) | 805,193 | |||||
|
EFFECT OF EXCHANGE RATE ON CASH
|
710 | 17,223 | ||||||
|
(DECREASE) INCREASE IN CASH
|
(194,709 | ) | 117,354 | |||||
|
CASH, beginning of year
|
996,302 | 878,948 | ||||||
|
CASH, end of year
|
$ | 801,593 | $ | 996,302 | ||||
See report of independent registered public accounting firm. The accompanying notes are an integral part of these consolidated financial statements.
F-4
CHINA JO-JO DRUGSTORES, INC. AND SUBSIDIARIES
(FORMERLY KNOWN AS KERRISDALE MINING CORPORATION)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
MARCH 31, 2010
Note 1 – DESCRIPTION OF BUSINESS AND ORGANIZATION
China Jo-Jo Drugstores, Inc. (“Jo-Jo Drugstores” or the “Company”), was incorporated in Nevada on December 19, 2006, originally under the name “Kerrisdale Mining Corporation.” On September 24, 2009, the Company changed its name to “China Jo-Jo Drugstores, Inc.” in connection with a share exchange transaction as described below.
On September 17, 2009, the Company completed a share exchange transaction with Renovation Investment (Hong Kong) Co., Ltd. (“Renovation HK”), and Renovation HK became a wholly-owned subsidiary of the Company. On the closing date, the Company issued 7,900,000 of its common stock (taking into account the 1-for-2 reverse stock split effected on April 9, 2010) to Renovation HK’s stockholders in exchange for 100% of the capital stock of Renovation HK. Prior to the share exchange transaction, the Company had 2,100,000 shares of common stock issued and outstanding (taking into account the 1-for-2 reverse stock split effected on April 9, 2010). After the share exchange transaction, the Company had 10,000,000 shares of common stock outstanding (taking into account the 1-for-2 reverse stock split effected on April 9, 2010) and Renovation HK’s stockholders owned 79% of the issued and outstanding shares. The management members of Renovation HK became the directors and officers of the Company. The share exchange transaction was accounted for as a reverse acquisition and recapitalization whereby Renovation HK is deemed to be the accounting acquirer (legal acquiree) and the Company the accounting acquiree (legal acquirer) and, as a result, the consolidated financial statements of the Company (the legal acquirer) is, in substance, those of Renovation HK (the accounting acquirer), with the assets and liabilities, and revenues and expenses of the Company being included effective from the date of the share exchange transaction. As the share exchange transaction was accounted for as a reverse acquisition and recapitalization, there was no gain or loss recognized on the transaction. The historical financial statements for periods prior to September 17, 2009 are those of Renovation HK except that the equity section and earnings per share have been retroactively restated to reflect the reverse acquisition.
Renovation HK was incorporated on September 2, 2008, under the laws of Hong Kong Special Administrative Region (“Hong Kong”). Renovation HK has no substantive operations of its own except for its holding of Zhejiang Jiuxin Investment Management Co., Ltd. (“Jiuxin Management”), its wholly owned subsidiary. Through Jiuxin Management, the Company controls three companies through certain exclusive agreements discussed below, that operate a chain of pharmacies in the People’s Republic of China (“PRC” or “China”), namely, Hangzhou Jiuzhou Grand Pharmacy Chain Co., Ltd. (“Jiuzhou Pharmacy”), Hangzhou Jiuzhou Clinic of Integrated Traditional and Western Medicine (“Jiuzhou Clinic”) and Hangzhou Jiuzhou Medical and Public Health Service Co., Ltd. (“Jiuzhou Service”, and collectively with Jiuzhou Pharmacy and Jiuzhou Clinic as “HJ Group”).
Jiuxin Management was established in the People’s Republic of China (“PRC” or “China”) by Renovation HK on October 14, 2008, as a wholly foreign owned enterprise (“WFOE”), with registered capital of $4,500,000. Renovation HK is required by the PRC company law to pay 15% of the registered capital by December 31, 2009, and the balance within two years. Jiuxin Management has no substantive operations of its own except for entering into certain exclusive agreements with HJ Group and performing its obligations thereunder.
Jiuzhou Pharmacy is a PRC limited liability company established in Zhejiang Province on September 9, 2003 with registered capital of $605,000 (RMB 5,000,000), engages in the retail sales of prescription and non prescription drugs, traditional Chinese medicine and general merchandise in the PRC.
Jiuzhou Clinic is a PRC general partnership established in Zhejiang Province on October 10, 2003. It is engaged in providing both traditional and western medical services in the PRC.
See report of independent registered public accounting firm.
F-5
Jiuzhou Service is a PRC limited liability company established in Zhejiang Province on November 2, 2005 with registered capital of $60,500 (RMB 500,000). It is engaged in providing medical-related management consulting services in the PRC.
All three HJ Group companies have been under the common control of the same three owners (the “Owners”) since their respective establishment dates, pursuant to agreements amongst the Owners to vote their interests in concert as memorialized in a voting agreement. Based on such voting agreement, the Company has determined that common control exists among the three HJ Group companies in accordance with the guidance of definition of common control accounting standard. Operationally, the Owners have operated the three HJ Group companies in conjunction with one another since each company’s respective establishment date. Each HJ Group company holds the licenses and approvals necessary to operate its business in China.
The paid-in capital of all three HJ Group companies was funded by the majority shareholders of Renovation HK. PRC law currently has limits on foreign ownership of companies in certain industries, including those of HJ Group. To comply with these foreign ownership restrictions, on August 1, 2009, Jiuxin Management entered into following exclusive agreements with HJ Group and the Owners (collectively the “Contractual Arrangements”).
The Company has concluded that Jiuzhou Pharmacy, Jiuzhou Clinic and Jiuzhou Service are each a Variable Interest Entity (VIE) and that the Company’s wholly owned subsidiary, Jiuxin Management, absorb a majority of the risk of loss from the activities of these three companies, and enables the Company, through Jiuxin Management, to receive a majority of their respective expected residual returns. Such conclusion is based on the terms of the Contractual Arrangements as follows:
|
(1)
|
Under the Consulting Services Agreement, Jiuxin Management will provide exclusive consulting and services to HJ Group for a quarterly fee in the amount of each HJ Group company’s quarterly net income after tax. The Company has the right to receive the expected residual gains of each HJ Group company, and there is no cap on such expected residual gains. The Company is also obligated to absorb the risk of loss from the activities of each HJ Group company. The Company is not protected from such risk of loss and is not guaranteed a return by HJ Group or by other parties involved with HJ Group. The consulting services agreement shall remain in effect for the maximum period of time permitted by law, unless sooner terminated by Jiuxin Management or if either Jiuxin Management or an HJ Group company becomes bankrupt or insolvent, or otherwise dissolves or ceases business operations.
|
|
(2)
|
Under the Equity Pledge Agreement, the Owners have pledged their rights, title and equity interest in HJ Group as security for Jiuxin Management to collect its fees from each HJ Group company under the Consulting Services Agreement. On May 18, 2010, all the owners effectively completed the registration of their equity pledge to Zheijiang Management Co., Ltd.
|
|
(3)
|
Under the Operating Agreement, Jiuxin Management has exclusive authority of all decision-making relating to HJ Group’s ongoing major operations, including establishing compensation levels and hiring and firing key personnel. In order to ensure HJ Group’s normal operation, Jiuxin Management agrees to act as the guarantor for HJ Group in any contract, agreement or transaction with third parties relating to HJ Group’s operations, and to guarantee HJ Group’s performance of such contract, agreement or transaction. As a counter guarantee, each HJ Group company agrees to pledge all of its assets including receivables to Jiuxin Management which have not been pledged to any third parties at the execution date of this agreement. The operating agreement shall remain in effect unless terminated by Jiuxin Management.
|
|
(4)
|
Under the Proxy Agreement, the Owners have authorized any designee of Jiuxin Management to exercise all of their respective voting rights as owners of HJ Group. The voting rights proxy agreement shall remain in effect for the maximum period of time permitted by law.
|
|
(5)
|
Under the Option Agreement, the Owners have granted Jiuxin Management the exclusive right and option to acquire all of their equity interests in HJ Group. The option agreement shall remain in effect for the maximum period time permitted by law.
|
See report of independent registered public accounting firm.
F-6
Accordingly, the Company accounts for each of these three companies as a VIE.
As a result of the Contractual Arrangements, which obligate the Company to absorb all of the risk of loss from HJ Group’s activities and enable the Company to receive all of HJ Group’s expected residual returns, the Company accounts for each HJ Group company as a variable interest entity (“VIE”) under Financial Accounting Standards Board (FASB)’s accounting standard. Accordingly, the financial statements of HJ Group are consolidated into the financial statements of the Company.
Note 2 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
The reporting entities
The Company’s consolidated financial statements of reflect the activities of the Company and the following subsidiary and VIEs:
|
Subsidiaries
|
Incorporated in
|
Percentage of
Ownership
|
||
|
Renovation HK
|
Hong Kong
|
100%
|
||
|
Jiuxin Management
|
PRC
|
100%
|
||
|
Jiuzhou Pharmacy
|
PRC
|
VIE by Contractual Arrangements
|
||
|
Jiuzhou Clinic
|
PRC
|
VIE by Contractual Arrangements
|
||
|
Jiuzhou Service
|
PRC
|
VIE by Contractual Arrangements
|
Basis of presentation and consolidation
The accompanying consolidated financial statements are prepared in accordance with accounting principles generally accepted in the United States of America. The consolidated financial statements include the financial statements of the Company, its wholly-owned subsidiary and its VIEs. All significant inter-company transactions and balances between the Company, its subsidiary and VIEs are eliminated upon consolidation.
Consolidation of variable interest entities
In accordance with the consolidation of Variable Interest Entities ("FIN 46R") standards, variable interest entities (VIEs) are generally entities that lack sufficient equity to finance their activities without additional financial support from other parties or whose equity holders lack adequate decision making ability. All VIEs with which the Company is involved must be evaluated to determine the primary beneficiary of the risks and rewards of the VIE. The primary beneficiary is required to consolidate the VIE for financial reporting purposes.
The Company has concluded that Jiuzhou Pharmacy, Jiuzhou Clinic and Jiuzhou Service are each a VIE and that the Company’s wholly owned subsidiary, Jiuxin Management, absorb a majority of the risk of loss from the activities of these three companies, and enable the Company, through Jiuxin Management, to receive a majority of their respective expected residual returns. Such conclusion is based on the terms of the Contractual Arrangements as they are stating in the Note 1.
Accordingly, the Company accounts for each of these three companies as a VIE.
Additionally, as the three HJ Group companies are under common control, the consolidated financial statements have been prepared as if the transactions had occurred retroactively as to the beginning of the reporting period of these consolidated financial statements.
Control is defined under the accounting standard defining common control as “an individual, enterprise, or immediate family members who hold more than 50 percent of the voting ownership interest of each entity.” Because Lei Liu, Li Qi, and Chong’an Jin collectively own 100% of HJ Group and have agreed to vote their interests in concert since the establishment of each HJ Group company as memorialized in a voting agreement, the Company believes that these three individuals collectively have control of HJ Group in accordance with EITF02-05. Accordingly, the Company believes that Jiuzhou Pharmacy, Jiuzhou Clinic and Jiuzhou Service were constructively held under common control by Jiuxin Management as of the time the Contractual Agreements were entered into, establishing Jiuxin Management as their primary beneficiary. Jiuxin Management, in turn, is owned by Renovation HK.
See report of independent registered public accounting firm.
F-7
Although the Company recognizes the consolidation of VIEs standard but does not provide for retroactive accounting treatment, HJ Group in substance were controlled by the same three individuals on September 9, 2003, October 10, 2003 and November 2, 2005, the establishment dates of Jiuzhou Pharmacy, Jiuzhou Clinic and Jiuzhou Service, respectively. Such common control condition resulted in the share exchange transaction to be a capital transaction in substance, reflected as a recapitalization, and the Company has accordingly recorded the consolidation of Renovation HK at its historical cost.
Use of estimates
The preparation of consolidated financial statements in conformity with generally accepted accounting principles requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, disclosure of contingent assets and liabilities at the date of the financial statements, and the reported amounts of revenues and expenses during the reporting period. For example, the Company estimates its allowance for doubtful accounts and useful lives of plant and equipment. Because of the use of estimates inherent in the financial reporting process, actual results could differ from those estimates.
Fair values of financial instruments
The accounting standards regarding fair value of financial instruments and related fair value measurements defines fair value, establishes a three-level valuation hierarchy for disclosures of fair value measurement and enhances disclosures requirements for fair value measures. The carrying amounts reported in the balance sheets for current receivables, payables, notes payables and short term loans qualify as financial instruments and are a reasonable estimate of fair value because of the short period of time between the origination of such instruments and their expected realization and their current market rate of interest. The three levels are defined as follow:
|
●
|
Level 1 inputs to the valuation methodology are quoted prices (unadjusted) for identical assets or liabilities in active markets.
|
|
●
|
Level 2 inputs to the valuation methodology include quoted prices for similar assets and liabilities in active markets, and inputs that are observable for the assets or liability, either directly or indirectly, for substantially the full term of the financial instruments.
|
|
●
|
Level 3 inputs to the valuation methodology are unobservable and significant to the fair value.
|
The Company did not identify any assets and liabilities that are required to be presented on the balance sheet at fair value.
Revenue recognition
Revenue from sales of prescription medicine at the drugstores is recognized when the prescription is filled and the customer picks up and pays for the prescription.
Revenue from sales of other merchandise at the drugstores is recognized at the point of sale, which is when the customer pays for and receives the merchandise.
Revenue from medical services is recognized after the service has been rendered to the customer.
Revenue from sales of merchandise to non-retail customers is recognized when the following conditions are met: 1) persuasive evidence of an arrangement exists (sales agreements and customer purchase orders are used to determine the existence of an arrangement); 2) delivery of goods has occurred and risks and benefits of ownership have been transferred, which is when the goods are received by the customer at its designated location in accordance with the sales terms; 3) the sales price is fixed or determinable; and 4) collectability is probable. Historically, sales returns have been immaterial.
See report of independent registered public accounting firm.
F-8
The Company’s revenue is net of value added tax (“VAT”) collected on behalf of tax authorities in respect of the sale of merchandise. VAT collected from customers, net of VAT paid for purchases, is recorded as a liability in the balance sheet until it is paid to the tax authorities.
Cash
Cash consists of cash on hand, cash in the drugstores and cash at banks.
Restricted cash
The Company’s restricted cash consists of cash in a bank as security for its notes payable. The Company has notes payable outstanding with the bank and is required to keep certain amounts on deposit that are subject to withdrawal restrictions. The notes payable are generally short term in nature due to their short maturity period of six to nine months; thus restricted cash is classified as a current asset. As of March 31, 2010, restricted cash and notes payable amounted to $746,703 and $1,464,241, respectively. There were no notes payable and restricted cash as of March 31, 2009.
Accounts receivable
Accounts receivable represent amounts due from banks relating to retail sales that are paid or settled by the customers’ debit or credit cards, amounts due from government social security bureaus relating to retail sales of drugs, prescription medicine, and medical services that are paid or settled by the customers’ medical insurance cards, and amounts due from non-retail customers for sales of merchandise.
Management regularly reviews aging of receivables and changes in payment trends by its customers, and records a reserve when they believe collection of amounts due are at risk. Accounts considered uncollectible are written off. Historically, the amount of bad debt write-off has been immaterial and the Company has been able to collect substantially all amounts due from these parties. At March 31, 2010 and 2009, management concluded all amounts are collectible and allowance for doubtful accounts deemed not necessary.
Inventories
Inventories are stated at the lower of cost or market. Cost is determined using the weighted average cost method. Market is the lower of replacement cost or net realizable value. The Company carries out physical inventory counts on a monthly basis at each store and warehouse location to ensure that the amounts reflected in the consolidated financial statements at each reporting period are properly stated and valued. The Company records write-downs to inventories for shrinkage losses and damaged merchandise that are identified during the inventory counts.
Property and equipment
Property and equipment are stated at cost, net of accumulated depreciation or amortization. Depreciation is calculated on the straight-line method over the following estimated useful lives of the assets, taking into consideration the assets’ estimated residual value. Leasehold improvements are amortized over the shorter of 5 years or the lease term of the underlying assets. Following are the estimated useful lives of the Company’s other property and equipment:
|
Estimated Useful Life
|
||
|
Leasehold improvements
|
5 years
|
|
|
Motor vehicles
|
5 years
|
|
|
Office equipment & furniture
|
3-5 years
|
Maintenance, repairs and minor renewals are charged to expense as incurred. Major additions and betterment to property and equipment are capitalized.
See report of independent registered public accounting firm.
F-9
Impairment of long lived assets
The Company evaluates long lived tangible and intangible assets for impairment, at least annually, but more often whenever events or changes in circumstances indicate that the carrying value may not be recoverable from its estimated future cash flows. Recoverability is measured by comparing the asset’s net book value to the related projected undiscounted cash flows from these assets, considering a number of factors including past operating results, budgets, economic projections, market trends and product development cycles. If the net book value of the asset exceeds the related undiscounted cash flows, the asset is considered impaired, and a second test is performed to measure the amount of impairment loss. Based on its review, the Company believes that, as of March 31, 2010 and 2009, there was no impairment.
Income taxes
The Company records income taxes pursuant to the accounting standards for income taxes. The accounting standards for income taxes require the recognition of deferred income tax liabilities and assets for the expected future tax consequences of temporary differences between income tax basis and financial reporting basis of assets and liabilities. Provision for income taxes consists of taxes currently due plus deferred taxes.
A tax position is recognized as a benefit only if it is “more likely than not” that the tax position would be sustained in a tax examination, with a tax examination being presumed to occur. The amount recognized is the largest amount of tax benefit that is greater than 50% likely of being realized on examination. For tax positions not meeting the “more likely than not” test, no tax benefit is recorded. The accounting standard also provides guidance on de-recognition, classification, interest and penalties, accounting in interim periods, disclosures, and transition. The adoption had no affect on the Company’s financial statements. There were no deferred tax amounts at March 31, 2010 and 2009.
The charge for taxation is based on the results for the year as adjusted for items, which are non-assessable or disallowed. It is calculated using tax rates that have been enacted or substantively enacted by the balance sheet date.
Deferred tax is accounted for using the balance sheet liability method in respect of temporary differences arising from differences between the carrying amount of assets and liabilities in the financial statements and the corresponding tax basis used in the computation of assessable tax profit. In principle, deferred tax liabilities are recognized for all taxable temporary differences, and deferred tax assets are recognized to the extent that it is probable that taxable profit will be available against which deductible temporary differences can be utilized. Deferred tax is calculated at the tax rates that are expected to apply to the period when the asset is realized or the liability is settled. Deferred tax is charged or credited in the income statement, except when related items are credited or charged directly to equity, in which case the deferred tax is also dealt with in equity.
Value Added Tax (VAT)
Sales revenue represents the invoiced value of goods, net of a value-added tax (VAT). All of the Company’s products are sold in the PRC are subject to a Chinese value-added tax on the gross sales price. The value-added tax rate vary from 0% to 17%, depending on the type of products sold. This VAT may be offset by VAT paid by the Company on raw materials and other materials included in the cost of producing or acquiring its finished products. The Company recorded VAT payable and VAT receivable net of payments in the accompanying financial statements. The VAT tax return is filed offsetting the payables against the receivables.
Stock Based Compensation
The Company accounts for equity instruments issued in exchange for the receipt of goods or services from other than employees in accordance with FASB’s accounting standards regarding accounting for stock-based compensation and accounting for equity instruments that are issued to other than employees for acquiring or in conjunction with selling goods or services. Costs are measured at the estimated fair market value of the consideration received or the estimated fair value of the equity instruments issued, whichever is more reliably determinable. The value of equity instruments issued for consideration other than employee services is determined on the earlier of a performance commitment or completion of performance by the provider of goods or services as defined by these accounting standards. In the case of equity instruments issued to consultants, the fair value of the equity instrument is recognized over the term of the consulting agreement.
See report of independent registered public accounting firm.
F-10
Advertising and promotion costs
Advertising and promotion costs are expensed as incurred. Advertising and promotion costs amounted to $455,543 and $232,902 for the years ended March 31, 2010 and 2009, respectively. Advertising costs consist primarily of print and television advertisements.
Pre-opening costs
Expenditures related to the opening of new drugstores, other than expenditures for property and equipment, are expensed as incurred.
Vendor allowances
The Company accounts for vendor allowances by reducing the carrying value of inventories and are subsequently transferring the amounts to cost of goods sold when the inventories are sold, unless those allowances are specifically identified as reimbursements for advertising, promotion and other services, in which case they are recognized as a reduction of the related advertising and promotion or other service costs.
For the years ended March 31, 2010 and 2009, the Company recognized vendor allowances of $236,084 and $264,845 in cost of goods sold, respectively.
Distribution costs
Distribution costs represent the costs of transporting merchandise from warehouse to stores. These costs are expensed as incurred and are included in sales, marketing and other operating expenses.
Operating leases
The Company leases premises for retail drugstores, warehouse, offices, and land under non-cancelable operating leases. Operating lease payments are expensed on a straight-line basis over the term of lease. A majority of the Company’s retail drugstore leases have a 3 to 5-year term with a renewal option upon the expiration of the lease. The Company has historically been able to renew a majority of its drugstores leases. Under the terms of the lease agreements, the Company has no legal or contractual asset retirement obligations at the end of the lease. Land leased from the local government is amortized on a straight-line basis over a 30-year term.
Commitments and contingencies
Liabilities for loss contingencies arising from claims, assessments, litigation, fines and other sources are recorded when it is probable that a liability has been incurred and the amount of the assessment can be reasonably estimated. Historically, the Company has experienced no product liability or malpractice claims.
Foreign currency translation
The Company uses the United States dollar (“U.S. dollars” or “USD”) for financial reporting purposes. The Company’s subsidiaries and VIEs maintain their books and records in their functional currency, being the primary currency of the economic environment in which their operations are conducted.
In general, for consolidation purposes, the Company translates the subsidiaries' and VIEs’ assets and liabilities into U.S. dollars using the applicable exchange rates prevailing at the balance sheet date, and the statement of income and cash flows are translated at average exchange rates during the reporting period. As a result, amounts related to assets and liabilities reported on the statement of cash flows will not necessarily agree with changes in the corresponding balances on the balance sheet. Equity accounts are translated at historical rates. Adjustments resulting from the translation of the subsidiaries’ and VIEs’ financial statements are recorded as accumulated other comprehensive income.
See report of independent registered public accounting firm.
F-11
The accounting standard, “Reporting Comprehensive Income”, requires disclosure of all components of comprehensive income and loss on an annual and interim basis. Comprehensive income and loss is defined as the change in equity of a business enterprise during a period from transactions and other events and circumstances from non-owner sources. The Company’s accumulated other comprehensive income and loss only consist of changes in foreign currency exchange rates. Accumulated other comprehensive loss in the statement of shareholders’ equity amounted to $362,263 and $362,437 as of March 31, 2010 and 2009, respectively. The balance sheet amounts with the exception of equity at March 31, 2010 and 2009 were translated at 1 RMB to $0.14670 USD and at 1 RMB to $0.1465 USD, respectively. The average translation rates applied to income and cash flow statement amounts for the years ended March 31, 2010 and 2009 were at 1 RMB to $0.14664 USD and at 1 RMB to $0.14582 USD, respectively.
Concentrations and credit risk
The Company’s operations are all carried out in the PRC. Accordingly, the Company’s business, financial condition and results of operations may be influenced by the political, economic and legal environments in the PRC, and by the general state of the PRC’s economy. The Company’s operations in the PRC are subject to specific considerations and significant risks not typically associated with companies in the North America and Western Europe. These include risks associated with, among others, the political, economic and legal environments and foreign currency exchange. The Company’s results may be adversely affected by changes in governmental policies with respect to laws and regulations, anti-inflationary measures, currency conversion and remittance abroad, and rates and methods of taxation, among other things.
Certain financial instruments, which subject the Company to concentration of credit risk, consist of cash and restricted cash. Balances at financial institutions or state-owned banks within the PRC are not covered by insurance. As of March 31, 2010 and 2009, the Company had deposits totaling $1,533,175 and $995,448 that are not covered by insurance, respectively. The Company has not experienced any losses in such accounts and believes it is not exposed to any significant risks on its cash in bank accounts.
For the fiscal years ended March 31, 2010 and 2009, all of the Company’s sales and purchases arose in the PRC. No major customers accounted for more than 10% of the Company’s total revenues for the years ended March 31, 2010 and 2009, respectively.
For the fiscal year ended March 31, 2010, two vendors collectively accounted for 23% of the Company’s total purchases and 10% of total inventory prepayment. For the fiscal year ended March 31, 2009, two vendors collectively accounted for 32% of the Company’s total purchases and 1% of total accounts payable.
Recently issued accounting pronouncements
In December 2009, FASB issued ASU No. 2009-16, Accounting for Transfers of Financial Assets. This Accounting Standards Update amends the FASB Accounting Standards Codification for the issuance of FASB Statement No. 166, Accounting for Transfers of Financial Assets—an amendment of FASB Statement No. 140.The amendments in this Accounting Standards Update improve financial reporting by eliminating the exceptions for qualifying special-purpose entities from the consolidation guidance and the exception that permitted sale accounting for certain mortgage securitizations when a transferor has not surrendered control over the transferred financial assets. In addition, the amendments require enhanced disclosures about the risks that a transferor continues to be exposed to because of its continuing involvement in transferred financial assets. Comparability and consistency in accounting for transferred financial assets will also be improved through clarifications of the requirements for isolation and limitations on portions of financial assets that are eligible for sale accounting. The Company does not expect the adoption of this ASU to have a material impact on its consolidated financial statements.
In December, 2009, FASB issued ASU No. 2009-17, Improvements to Financial Reporting by Enterprises Involved with Variable Interest Entities. This Accounting Standards Update amends the FASB Accounting Standards Codification for the issuance of FASB Statement No. 167, Amendments to FASB Interpretation No. 46(R). The amendments in this Accounting Standards Update replace the quantitative-based risks and rewards calculation for determining which reporting entity, if any, has a controlling financial interest in a variable interest entity with an approach focused on identifying which reporting entity has the power to direct the activities of a variable interest entity that most significantly impact the entity’s economic performance and (1) the obligation to absorb losses of the entity or (2) the right to receive benefits from the entity. An approach that is expected to be primarily qualitative will be more effective for identifying which reporting entity has a controlling financial interest in a variable interest entity. The amendments in this Update also require additional disclosures about a reporting entity’s involvement in variable interest entities, which will enhance the information provided to users of financial statements. The adoption of this ASU did not have a material impact on its consolidated financial statements.
See report of independent registered public accounting firm.
F-12
In January 2010, FASB issued ASU No. 2010-01- Accounting for Distributions to Shareholders with Components of Stock and Cash. The amendments in this Update clarify that the stock portion of a distribution to shareholders that allows them to elect to receive cash or stock with a potential limitation on the total amount of cash that all shareholders can elect to receive in the aggregate is considered a share issuance that is reflected in EPS prospectively and is not a stock dividend for purposes of applying Topics 505 and 260 (Equity and Earnings Per Share). The amendments in this update are effective for interim and annual periods ending on or after December 15, 2009, and should be applied on a retrospective basis. The Company adopted this standard and has determined the standard did not have material effect on the Company’s consolidated financial statements.
In January 2010, FASB issued ASU No. 2010-02 – Accounting and Reporting for Decreases in Ownership of a Subsidiary – a Scope Clarification. The amendments in this Update affect accounting and reporting by an entity that experiences a decrease in ownership in a subsidiary that is a business or nonprofit activity. The amendments also affect accounting and reporting by an entity that exchanges a group of assets that constitutes a business or nonprofit activity for an equity interest in another entity. The amendments in this update are effective beginning in the period that an entity adopts SFAS No. 160, “Non-controlling Interests in Consolidated Financial Statements – An Amendment of ARB No. 51.” If an entity has previously adopted SFAS No. 160 as of the date the amendments in this update are included in the Accounting Standards Codification, the amendments in this update are effective beginning in the first interim or annual reporting period ending on or after December 15, 2009. The amendments in this update should be applied retrospectively to the first period that an entity adopted SFAS No. 160. The Company adopted this standard and has determined the standard did not have material effect on the Company’s consolidated financial statements.
In January 2010, FASB issued ASU No. 2010-06 – Improving Disclosures about Fair Value Measurements. This update provides amendments to Subtopic 820-10 that requires new disclosure as follows: 1) Transfers in and out of Levels 1 and 2. A reporting entity should disclose separately the amounts of significant transfers in and out of Level 1 and Level 2 fair value measurements and describe the reasons for the transfers. 2) Activity in Level 3 fair value measurements. In the reconciliation for fair value measurements using significant unobservable inputs (Level 3), a reporting entity should present separately information about purchases, sales, issuances, and settlements (that is, on a gross basis rather than as one net number).This update provides amendments to Subtopic 820-10 that clarify existing disclosures as follows: 1) Level of disaggregation. A reporting entity should provide fair value measurement disclosures for each class of assets and liabilities. A class is often a subset of assets or liabilities within a line item in the statement of financial position. A reporting entity needs to use judgment in determining the appropriate classes of assets and liabilities. 2) Disclosures about inputs and valuation techniques. A reporting entity should provide disclosures about the valuation techniques and inputs used to measure fair value for both recurring and nonrecurring fair value measurements. Those disclosures are required for fair value measurements that fall in either Level 2 or Level 3.The new disclosures and clarifications of existing disclosures are effective for interim and annual reporting periods beginning after December 15, 2009, except for the disclosures about purchases, sales, issuances, and settlements in the roll forward of activity in Level 3 fair value measurements. Those disclosures are effective for fiscal years beginning after December 15, 2010, and for interim periods within those fiscal years. The Company adopted the disclosures standards, except for the disclosures to be adopted for fiscal year beginning after December 15, 2010, and has determined the standard did not have material effect on the Company’s consolidated financial statements.
See report of independent registered public accounting firm.
F-13
In February 2010, the FASB issued ASU 2010-09, “Subsequent Events (Topic 855): Amendments to Certain Recognition and Disclosure Requirements,” or ASU 2010-09. ASU 2010-09 primarily rescinds the requirement that, for listed companies, financial statements clearly disclose the date through which subsequent events have been evaluated. Subsequent events must still be evaluated through the date of financial statement issuance; however, the disclosure requirement has been removed to avoid conflicts with other SEC guidelines. ASU 2010-09 was effective immediately upon issuance and was adopted in February 2010.
In March 2010, FASB issued ASU No. 2010-10 –Amendments for Certain Investment Funds. This update defers the effective date of the amendments to the consolidation requirements made by FASB Statement 167 to a reporting entity’s interest in certain types of entities. The deferral will mainly impact the evaluation of reporting enterprises’ interests in mutual funds, private equity funds, hedge funds, real estate investment entities that measure their investment at fair value, real estate investment trusts, and venture capital funds. The ASU also clarifies guidance in Statement 167 that addresses whether fee arrangements represent a variable interest for all service providers and decision makers. The ASU is effective for interim and annual reporting periods in fiscal year beginning after November 15, 2009. The Company is currently evaluating the impact of this ASU, however, the Company does not expect the adoption of this ASU to have a material impact on its consolidated financial statements
Note 3 – SUPPLEMENTAL DISCLOSURE OF CASH FLOW INFORMATION
Interest paid for the years ended March 31, 2010 and 2009, amounted to $65,011 and $32,035, respectively.
Income taxes paid for the years ended March 31, 2010 and 2009 amounted to $2,606,347 and $2,148,692, respectively.
Note 4 – OTHER CURRENT ASSETS
Other current assets consist of the following:
|
March 31,
2010
|
March 31,
2009
|
|||||||
|
Prepaid rental expense
|
$ | 1,205,881 | $ | 475,864 | ||||
| Refundable deposit (1) | 1,026,900 | - | ||||||
|
Lease rights transfer fees (2)
|
92,021 | - | ||||||
|
Prepaid advertisement expenses
|
55,051 | 14,721 | ||||||
|
Prepaids and other assets
|
84,154 | 73,794 | ||||||
|
Total
|
$ | 2,464,007 | $ | 564,379 | ||||
| (1) | Refundable deposit is a payment made to a local government in connection with entering into a 30-year operating land lease agreement (see Note 8) and is refundable. As of March 31, 2010 and 2009, the refundable deposit amounted to $1,026,900 and $0, respectively. |
|
(2)
|
Lease rights transfer fees are money paid by the Company to secure store rentals in coveted areas. These additional costs of acquiring the right to lease the new stores are capitalized and amortized over the period of the initial lease term.
|
Note 5 – PROPERTY AND EQUIPMENT
Property and equipment for years ended March 31, 2010 and 2009 consists of the following:
| March 31, | ||||||||
|
2010
|
2009
|
|||||||
|
Leasehold improvements
|
$
|
2,588,627
|
$
|
2,057,892
|
||||
|
Office equipment and furniture
|
379,668
|
304,709
|
||||||
|
Motor vehicles
|
162,665
|
162,443
|
||||||
|
Total
|
3,130,960
|
2,525,044
|
||||||
|
Less: Accumulated depreciation and amortization
|
1,944,668
|
1,545,612
|
||||||
|
Property and equipment, net
|
$
|
1,186,292
|
$
|
979,432
|
||||
See report of independent registered public accounting firm.
F-14
Total depreciation expense for property and equipment for the years ended March 31, 2010 and 2009 was $396,574 and $439,464, respectively. No depreciation expense was included in cost of goods sold for the years presented because the Company’s business does not involve manufacturing of merchandise and the amount of depreciation of property and equipment utilized in acquiring, warehousing and transporting the merchandise to locations ready for sale is not material.
Note 6 – ADVANCES TO SUPPLIERS
Advances to suppliers and advances to suppliers-related parties are monies deposited or advanced to outside vendors for future inventory purchases. Most of the Company’s vendors require a certain amount of money to be deposited with them as a guarantee that the Company will receive their purchase on a timely basis. This amount is refundable and bears no interest. As of March 31, 2010 and 2009, the advances to suppliers amounted to $6,850,240 and 7,282,217, respectively.
Note 7 – LONG TERM DEPOSITS
Long term deposits includes monies deposited or advanced to landlords for securing retail store leases for which the Company does not anticipate applying or being returned within the next twelve months. Most of the Company’s landlords require a minimum of six months’ rent being paid up front plus additional deposits. As of March 31, 2010 and 2009, the long term deposit for retail leases amounted to $2,539,424 and $2,015,149, respectively.
Note 8 – PREPAID RENT - NONCURRENT
Prepaid rent - noncurrent is a prepayment to a local government in connection with entering into a 30-year operating land lease agreement. As of March 31, 2010 and 2009, the prepayment for the land lease amounted to $4,254,300 and $0, respectively.
Note 9 – STATUORY RESERVE
Statutory reserves represent restricted retained earnings. Based on their legal formation, all PRC entities are required to set aside 10% of their net incomes as reported in their statutory accounts on an annual basis to the Statutory Surplus Reserve Fund (the “Reserve Fund”). Once the total set-aside in the Reserve Fund reaches 50% of an entity’s registered capital, further appropriations are discretionary. The Reserve Fund can be used to increase the entity’s registered capital upon approval by relevant government authorities and eliminate its future losses under PRC GAAP upon a resolution by its board of directors. The Reserve Fund is not distributable to shareholders except in the event of liquidation.
Appropriations to the above statutory reserves are accounted for as a transfer from unrestricted earnings to statutory reserves. During the years ended March 31, 2010 and 2009, the Company made total appropriations to these statutory reserves of $0 and $702,444, respectively. The Company fulfilled the 50% registered capital requirement as of March 31, 2009. Therefore, during the year ended March 31, 2010, no additional appropriations to the statutory reserves were made from unrestricted earnings.
There are no legal requirements in the PRC to fund these statutory reserves by transfer of cash to any restricted accounts, and the Company does not do so. These statutory reserves are not distributable as cash dividends.
Note 10 – TAXES
Income Tax
The Company is registered in Nevada whereas its subsidiary, Renovation HK, is registered in Hong Kong, and conducts all of its business through Renovation HK’s subsidiary, Jiuxin Management, and the three PRC VIEs, Jiuzhou Pharmacy, Jiuzhou Clinic and Jiuzhou Service. Jiuxin Management and the VIEs are subject to PRC tax laws.
See report of independent registered public accounting firm.
F-15
Beginning January 2008, the Chinese Enterprise Income Tax (“EIT”) law replaced China’s former income tax laws. The standard EIT rate of 25% replaced the 33% rate previously applicable to enterprises. Therefore, starting from January 2008 Jiuzhou Pharmacy has been subject to an effective tax rate of 25%.
Jiuzhou Clinic and Jiuzhou Service are subject to the regular income tax rate of 25% in calendar year 2009 and 2010.
Penalties and interest incurred related to underpayment of income tax are classified as income tax expense in the year incurred. No significant penalties or interest relating to income taxes have been incurred during the three months ended March 31, 2010 and 2009. GAAP also provides guidance on de-recognition, classification, interest and penalties, accounting in interim periods, disclosures and transition.
The following table reconciles the US statutory rates to the Company's effective tax rate for the years ended March 31, 2010 and 2009:
|
2010
|
2009
|
|||||||
|
U.S. Statutory rates
|
34 | % | 34 | % | ||||
|
Foreign income not recognized in USA
|
(34 | ) | (34 | ) | ||||
|
China income taxes
|
25 | 25 | ||||||
|
Other (a)
|
(2 | ) | 0 | |||||
|
Effective tax rate
|
23 | % | 25 | % | ||||
|
(a)
|
The 2% represents the expenses (income) incurred by the Company that are not subjected to PRC income tax.
|
Value Added Tax
Sales of products are subject to VAT ranging from 0% to 17%. VAT on sales and on purchases amounted to $8,985,061 and $6,245,139 for the year ended March 31, 2010, respectively, and $7,300,265 and $5,172,307 for the year ended March 31, 2009, respectively.
Sales and purchases are recorded net of VAT collected and paid as the Company acts as an agent for the government. VAT taxes are not impacted by the income tax holiday.
Taxes payable at March 31, 2010 and 2009 consisted of the following:
|
2010
|
2009
|
|||||||
|
VAT
|
$
|
341,989
|
$
|
196,784
|
||||
|
Income tax
|
875,868
|
588,681
|
||||||
|
Others
|
17,226
|
25,851
|
||||||
|
Total taxes payable
|
$
|
1,235,083
|
$
|
811,316
|
||||
Note 11 – OTHER PAYABLES
Other payables at March 31, 2010 and 2009 consisted of the following:
|
2010
|
2009
|
|||||||
|
Cash advance from third party (a)
|
$
|
-
|
$
|
314,975
|
||||
|
Other
|
331,656
|
89,756
|
||||||
|
Total
|
$
|
331,656
|
$
|
404,731
|
||||
Notes:
|
(a)
|
Represents short term cash advance from a non-related third party, Hangzhou Today Real Estate. The advance is non-interest bearing and due upon request.
|
See report of independent registered public accounting firm.
F-16
Note 12 – SHORT TERM LOANS
Short term loans represent amounts due to various banks and are normally due on demand or within one year. These loans generally can be renewed with the banks. Short term bank loans at March 31, 2010 and 2009, consisted of the following:
|
2010
|
2009
|
|||||||
|
Hangzhou Bank, due August 2009 annual interest at 4.86%, secured by the personal properties of the Company’s shareholders
|
|
$
|
586,000
|
|||||
|
Hangzhou Bank, due September 2010 annual interest at 4.86%, secured by the personal properties of the Company’s shareholders
|
$
|
586,800
|
||||||
|
Hangzhou Bank, due September 2009 annual interest at 4.86%, secured by the personal properties of the Company’s shareholders
|
|
$
|
879,000
|
|||||
|
Hangzhou Bank, due July 2010 annual interest at 4.86%, secured by the personal properties of the Company’s shareholders
|
$
|
293,400
|
||||||
|
Total
|
$
|
880,200
|
$
|
1,465,000
|
||||
Interest expense for the years ended March 31, 2010 and 2009 amounted to $65,011 and $32,035, respectively.
Note 13 – POSTRETIREMENT BENEFITS
Regulations in the PRC require the Company to contribute to a defined contribution retirement plan for all permanent employees. The contribution is based on a percentage required by the local government and the employees' current compensation. The Company contributed $155,543 and $57,776 in employment benefits and pension in the years ended March 31, 2010 and 2009, respectively.
Note 14 – RELATED PARTY TRANSACTIONS AND ARRANGEMENTS
Amounts receivable from and payable to related parties are summarized as follows:
|
March 31,
2010
|
March 31,
2009
|
|||||||
|
Amounts due from directors (1):
|
$
|
-
|
$
|
2,432
|
||||
|
Amount due to directors (2):
|
$
|
935,000
|
$
|
326,715
|
||||
|
Advances to supplier (3):
|
$
|
-
|
$
|
1,797,104
|
||||
|
(1)
|
Represents interest free loans to two of the Company’s directors, Li Qi and Chong’an Jin. The loans are due upon demand.
|
|
(2)
|
As of March 31, 2010, the amount due to directors represents contribution from directors, Li Qi ,Chong’an Jin, and Lei Liu to Jiuxin Management to enable Jiuxin Management to meet its approved PRC registered capital requirements. Such contributions are to be returned to the directors upon demand. At March 31, 2009, the amounts due to directors represent leasehold improvement expenses paid by a director of the Company, Lei Liu, on behalf of the Company and are due upon demand.
|
|
(3)
|
Represents prepayment for inventory purchase made to a supplier, which was formerly owned by the Company’s directors.
|
The Company also leases its facilities from a director. See Note 16 below for details.
See report of independent registered public accounting firm.
F-17
Note 15 – EARNINGS PER SHARE and CAPITAL TRANSACTIONS
Stock-based compensation
On September 1, 2009, pursuant to agreements entered on July 30, 2009, shareholders of Renovation HK sold shares of Renovation HK to service providers including the Company’s chief financial officer and legal counsel which shares, after the share exchange transaction between the Company and Renovation HK on September 17, 2009, collectively represent 2.50% of the Company’s issued and outstanding common stock.
Using the fair value of services provided as of March 31, 2010, the Company estimated that the total stock compensation expense to be recognized from these transactions was $202,120. The Company recognized $126,325 as stock compensation expense for the year ended March 31, 2010. The remaining $75,795 was applied to accrued legal fees, which were expensed during the year ended March 31, 2009.
On March 15, 2010, the Company agreed to issue 6,897 shares (taking into account the 1-for-2 reverse stock split effected on April 9, 2010) of restricted common stock to a director. The shares are payable in quarterly installments beginning on March 31, 2010 and $1,862 was recorded as compensation expense of the director for the year ended March 31, 2010.
Shareholders Contribution
On June 8, 2009, the three Owners of Jiuzhou Pharmacy acquired 100% equity interests of Kuaileren Pharmacy Co., Ltd. (“Kuaileren”) from its owner for stock consideration. On August 21, 2009, the three Owners contributed their 100% equity interests of Kuaileren to Jiuzhou Pharmacy, and Kuaileren became a wholly-owned subsidiary of Jiuzhou Pharmacy. The registered capital of Kuaileren is $15,000 (RMB 100,000). The transfer of the equity interest has been treated as a contribution to owner’s equity and has been valued at $8,164, the estimated fair value of the equity interest transferred.
Earnings per share
The Company reports earnings per share in accordance with the provisions of FASB’s related accounting standard. This standard requires presentation of basic and diluted earnings per share in conjunction with the disclosure of the methodology used in computing such earnings per share. Basic earnings per share excludes dilution and is computed by dividing income available to common stockholders by the weighted average common shares outstanding during the period. Diluted earnings per share takes into account the potential dilution that could occur if securities or other contracts to issue common stock were exercised and converted into common stock.
The following is a reconciliation of the basic and diluted earnings per share computation:
|
Years Ended March 31,
|
||||||||
|
2010
|
2009
|
|||||||
|
Net income for earnings per share
|
$
|
9,821,741
|
$
|
6,813,516
|
||||
|
Weighted average shares used in basic computation
|
||||||||
|
Basic and diluted
|
9,025,000
|
7,900,000
|
||||||
|
Earnings per share
|
||||||||
|
Basic and diluted
|
$
|
1.09
|
$
|
0.86
|
||||
All share and per share amounts used in the Company’s consolidated financial statements and notes thereto have been retroactively restated to reflect the 1-for-2 reverse stock split effected on April 9, 2010.
Note 16 – SEGMENTS
The Company sells prescription and over the counter medicines, traditional Chinese medicines, which are medicines derived from Chinese herbs, Chinese herbs, dietary supplement, medical instruments,, and sundry items. The class of customer, selling practice and distribution process are the same for all products. The Company’s chief operating decision-makers (i.e. chief executive officer and his direct reports) review financial information presented on a consolidated basis, accompanied by disaggregated information about revenues by product lines for purposes of allocating resources and evaluating financial performance. There are no segment managers who are held accountable for operations, operating results and plans for levels or components below the consolidated unit level. Based on qualitative and quantitative criteria established by the accounting standard, “Disclosures about Segments of an Enterprise and Related Information”, the Company considers itself to be operating within one reportable segment.
See report of independent registered public accounting firm.
F-18
The Company does not have long-lived assets located in foreign countries. In accordance with the enterprise-wide disclosure requirements of FASB’s accounting standard, the Company's net revenue from external customers by main segment is as follows:
|
Years Ended March 31,
|
||||||||
|
2010
|
2009
|
|||||||
|
Prescription Drugs
|
$
|
21,210,967
|
$
|
16,316,977
|
||||
|
Over The Counter (OTC) Drugs
|
19,042,200
|
12,358,624
|
||||||
|
Nutritional Supplements
|
5,137,890
|
7,189,776
|
||||||
|
Traditional Chinese Medicine Products
|
6,407,200
|
5,449,671
|
||||||
|
Sundry Products
|
2,260,710
|
2,047,534
|
||||||
|
Medical Devices
|
1,115,962
|
1,414,070
|
||||||
|
Total
|
$
|
55,174,929
|
$
|
44,776,652
|
||||
Note 17 – COMMITMENTS AND CONTINGENCIES
Operating lease commitments
The Company recognizes lease expense on a straight line basis over the term of the lease in accordance to the accounting standard. The Company has entered into various tenancy agreements for the lease of store premises and land leased from a local government to be used potentially for the cultivation of Chinese medicinal herbs. The Company’s leases do not contain any escalating lease payments or contingent rental payments terms.
One of the Company's retail spaces and the corporate office space are leased from Lei Liu, a director of the Company under long-term operating lease agreements from August 2008 to August 2010 and from January 2008 to March 2012, respectively. The rent expense for the retail space and the corporate office are $175,968 and $170,123 for the years ended March 31, 2010 and 2009, respectively. For the years ended March 31, 2010 and 2009, rent paid to Mr. Liu amounted to $175,968 and $171,360, respectively.
The Company’s commitments for minimum rental payments under its leases for the next five years and thereafter are as follows:
|
Years ending March 31,
|
Amount
|
|||
|
2011
|
$
|
1,649,979
|
||
|
2012
|
1,386,188
|
|||
|
2013
|
1,100,524
|
|||
|
2014
|
837,634
|
|||
|
2015
|
487,403
|
|||
|
Thereafter
|
4,000,532
|
|||
Rental expense for the years ended March 31, 2010 and 2009 amounted to $1,301,497 and $963,879, respectively.
As of March 31, 2010 and 2009, prepayment on rental expense amounted to $5,460,181 and $475,864, respectively.
Logistics Services Commitments
The Company renewed its agreement for logistics services (“Logistics Agreement”) with Zhejiang Yingte Logistics Co., Ltd. (“Yingte”) for the period January 1, 2010 to December 31, 2010 to provide logistics and other related services. Pursuant to the Logistics Agreement, Yingte accepts goods from the Company’s suppliers, stores the goods and then delivers the goods to the Company’s store locations as directed by the Company and the Company is required to pay Yingte 1% of the purchase price of the delivered goods. The Company is obligated to pay a minimum of 2,900,000 RMB annually (1% of 290 million RMB: the total minimum amount of goods to be delivered under the Logistics Agreement).
See report of independent registered public accounting firm.
F-19
As of March 31, 2010 and 2009, the Company did not have any contingent liabilities.
Legal Proceedings
In December 2009, Jiuzhou Pharmacy filed suit against The Ventana Group, LLC and Michael Hom in the California Superior Court for the County of San Mateo, alleging breach of contract of an agreement entered into with the defendants in 2008 and seeking damages of $25,000. The suit was subsequently amended to remove Michael Hom as a defendant. In May 2010, Jiuzhou Pharmacy sought for default judgment against the defendants, which remains pending.
Note 18 – SUBSEQUENT EVENT
On April 9, 2010, the Company effected a 1-for-2 reverse split of its issued and outstanding common shares and a proportional reduction of its authorized common shares. Therefore, all share and per share amounts used in the Company’s consolidated financial statements and notes thereto have been retroactively restated to reflect the 1-for-2 reverse stock split.
On April 28, 2010, the Company closed a public offering of 3.5 million shares of common stock at $5.00 per share with gross proceeds, prior to deducting underwriting discounts, commissions and offering expenses, of approximately $17.5 million and net proceeds of approximately $15.2 million. In connection with the public offering, the Company also issued 525,000 overallotment shares at $5.00 per share.
On May 14, 2010, the Company entered into a Loan-out Agreement with Worldwide Officers, Inc. pursuant to which the Company formally engaged the services of the Company’s chief financial officer, Mr. Bennet P. Tchaikovsky. Under the Loan-out Agreement, the Company engages the services of Mr. Tchaikovsky as its chief financial officer for a term of one year beginning May 14, 2010 for a cash compensation of $100,000 payable to Worldwide Officers, Inc., in four installments. In addition, the Company agrees, as further compensation for Mr. Tchaikovsky’s services, to issue 10,000 restricted shares of the Company’s common stock to be held in escrow and vest in five installments. The Company also agrees to issue 4,000 shares of the Company’s common stock as bonus for his services.
On May 18, 2010, the three Owners of Jiuzhou Pharmacy completed the registration of their pledge obligations under the Equity Pledge Agreement with the local State Administration of Industry and Commerce.
The Company has performed an evaluation of subsequent events through the date these consolidated financial statements were issued.
See report of independent registered public accounting firm.
F-20
