Attached files
| file | filename |
|---|---|
| 10-K - VIRIDAX CORP | v188695_10k.htm |
| EX-31.1 - VIRIDAX CORP | v188695_ex31-1.htm |
| EX-32.2 - VIRIDAX CORP | v188695_ex32-2.htm |
| EX-32.1 - VIRIDAX CORP | v188695_ex32-1.htm |
| EX-31.2 - VIRIDAX CORP | v188695_ex31-2.htm |

E-15
ARTICLES
Article 1.
Definitions
|
l.l
|
ARS
means the United States Department of Agriculture, Agricultural
Research Service.
|
|
1.2
|
COOPERATOR
means Viridax Corporation (“Viridax"), 270 NW 3rd
Court, Boca Raton, FL 33432-3720.
|
|
1.3
|
Agreement
means this Cooperative Research and Development
Agreement.
|
|
1.4.
|
Confidential
Information means trade secrets or commercial or financial information
that is privileged or confidential under the meaning of 5 USC
552(b)(4).
|
|
1.5
|
Subject
Invention means any invention or other intellectual property conceived or
first reduced to practice under this Agreement which is patentable or
otherwise protectable under Title 35 of the United States Code, under 7
USC 2321, et seq., or under the patent laws of a foreign country. Specifically not included in
the definition of Subject Inventions are inventions made outside the Scope
of Agreement or prior to the execution of this
Agreement.
|
|
1.6
|
Scope
of Agreement means those activities set forth in Schedule 2, entitled
"Statement of Work."
|
|
1.7
|
Period
of Agreement means that period set forth under the Period of Agreement on
the ARS Office of Technology Transfer cover form for the
Agreement.
|
Article 2.
Publications
|
2.1
|
Subject
to the requirements of confidentiality and preservation of rights in
SubjectInventions,
either party may publish the results of this Agreement,
PROVIDED:
|
|
|
a.
|
The
other party is allowed to review the manuscript at least sixty (60) days
prior to submission for publication by submission to the Authorized
Agent.
|
|
|
b.
|
The
publication shall acknowledge this Agreement and the contributions of each
party’s personnel.
|
|
|
c.
|
The
final decision as to the publication content rests with the party that
writes the publication.
|
|
2.2
|
Publication
and/or other disclosure of the results of this Agreement shall be delayed
as necessary to preserve both United States of America and foreign patent
rights in a Subject Invention.
|
E-16
|
a.
|
Such
a delay will only be granted if requested in writing;
and
|
|
|
b.
|
The
requesting party demonstrates promptness and diligence in seeking patent
protection on the Subject
Invention.
|
Article 3.
Confidentiality
|
3.1
|
Confidential
Information, which is owned by one party to this Agreement and disclosed
to the other, shall be labeled "CONFIDENTIAL” by the submitter and shall
not be disclosed by the recipient without permission of the owner, EXCEPT
in accordance with Article 2.
|
|
3.2
|
To
the extent either party orally submits its Confidential information to the
other party, the submitting party will prepare a document marked
"CONFIDENTIAL" embodying or identifying in reasonable detail such orally
submitted Confidential Information and provide the document to the other
party within thirty (30) days of
disclosure.
|
|
3.3
|
Neither
party shall be bound by confidentiality if the Confidential Information
received from the other party:
|
a. Already
is available to the public or known to the recipient;
b. Becomes
available to the public through no fault of the recipient; or
|
|
c.
|
Is
non-confidentially received from another party legally entitled to
it.
|
Article
4. Meetings, Reports and Records
|
4.1
|
Frequent
and effective communication is essential to the successful accomplish-ment
of the objectives of this Agreement. To this end, the scientific
representatives of ARS and COOPERATOR shall meet (meetings need not be in
person if agreed upon) at least once every six (6) months to exchange
results, perform critiques, and make plans and recommendations. Written
progress reports shall be supplied by each party to the other at least
fifteen (I5) calendar days prior to each semi-annual
meeting.
|
|
4.2
|
Any
such plan or recommendation that is outside the Scope of Agreement shall
bereduced
to writing and referred to the Authorized Agent of each party for
appropriate action. Any such plan or recommendation so referred shall not
be binding upon either party unless incorporated into this Agreement by
written amendment.
|
|
4.3
|
Each
party shall keep complete records relating to this research. All such
records shall be available for inspection by either party at reasonable
times. The records, or true copies of them, shall be delivered to either
party upon request.
|
E-17
|
4.4
|
The
results of this Agreement and research data that are collected, compiled,
and evaluated under this Agreement shall be shared and mutually
interchanged by COOPERATOR and ARS.
|
|
4.5
|
A
final report summarizing all data shall be submitted by each party,
separately or jointly, to both party’s Authorized Agents within sixty (60)
days of the completion of this
Agreement.
|
Article
5. Research Exclusion
|
5.1
|
The
results of this Agreement owned or co-owned by the U.S. Government may be
made available to others by ARS for bona fide noncommercial research
purposes if:
|
a. Confidentiality
is not breached; or
b. Patent
or Plant Variety Protection Certificate rights are not compromised.
|
5.2
|
Plants
and animals, their genetic materials or information relating thereto, or
partsthereof,
covered by Plant Variety Protection Certificates, Plant Patents, or
Utility Patents, owned or co-owned by ARS, may be made available by ARS to
third parties for bona fide research purposes including the development of
new animals or plants.
|
Article
6. Ownership of Inventions
|
6.1
|
All
rights, title, and interest in any Subject Invention made solely by
employee(s) of ARS shall be owned by
ARS.
|
|
6.2
|
All
rights, title, and interest in any Subject Invention made jointly by at
least one (1) employee of ARS and at least one (1) employee of COOPERATOR
shall be jointly owned by ARS and
COOPERATOR.
|
|
6.3
|
All
rights, title, and interest in any Subject Invention made solely by
employees ofCOOPERATOR
shall be owned by COOPERATOR.
|
Article 7. Subject Invention
Licenses
|
7.1
|
COOPERATOR
is granted an option to negotiate an exclusive license in each Subject
Invention owned or co-owned by ARS for one or more field(s) of` use
encompassed by the Scope of Agreement. ARS does not conduct research on
human diagnostic and/or therapeutic technologies. In instances where a
Subject Invention has human diagnostic and/or therapeutic application,
COOPERATOR is granted an option to negotiate an exclusive license for such
uses. This license shall be consistent with the requirements of 35 USC
209(a), 209(b) (manufactured substantially in the U.S.), and 209(f) and
other such terms and conditions as may be reasonable under the
circumstances, as agreed upon through good faith negotiations between
COOPERATOR and ARS.
|
E-18
|
7.2
|
This
option shall terminate whenever COOPERATOR fails
to:
|
|
|
a.
|
Submit
a complete application for an exclusive license within one-hundred twenty
(120) days of being notified by ARS of an Invention’s availability for
licensing; or
|
|
|
b.
|
Submit
a good faith written response to a written proposal of licensing terms
within sixty (60) days of such
proposal.
|
|
7.3
|
COOPERATOR
grants ARS, on behalf of the U.S. Government, a royalty free,
nonexclusive, worldwide, irrevocable, nontransferable license for any
COOPERATOR solely owned Subject Invention. The purpose of this license
shall be to practice the Subject Invention or have it practiced, by or on
behalf of the U.S. Government, for I research or other U.S. Government
purposes. 15 USC 3710a(b)(2).
|
Article
8. Subject Invention Information
|
8.1
|
The
Authorized Agents or designees of each party shall promptly make written
disclosure to each other of each Subject
Invention.
|
|
8.2
|
This
information shall be treated in confidence by the receiving party, EXCEPT:
it may be shared with those having a need to
know.
|
|
8.3
|
Each
party shall provide, when requested by the other, all information in its
possession, or true copies thereof, pertaining to a Subject Invention
which may be necessary or useful in the preparation, filing, and
prosecution of patent or Plant Variety Protection Certificate applications
covering the Subject Invention.
|
Article
9. Intellectual Property Protection
Applications
|
9.1
|
ARS
and COOPERATOR agree to cooperate with the other in the preparation,
filing, and prosecution of Patent or Plant Variety Protection Certificate
applications on Subject Inventions in the United States of America and any
other country.
|
|
9.2
|
ARS
shall provide COOPERATOR’S Authorized Agent or their designee with a copy
of any such application on a Subject Invention within fourteen (14)
calendar days of filing.
|
|
9.3
|
ARS
shall have the first option to prepare and prosecute patent or Plant
Variety Protection Certificate applications on Subject Inventions that are
owned or co-owned by the U.S. Government, which option may be waived in
whole or in part.
|
Article
10. Use of Name or Endorsements
COOPERATOR
shall not in any way state or imply that this Agreement or the results of this
Agreement are an endorsement of its organizational units, employees, products,
or services except to the extent permission is specifically granted by
ARS.
E-19
Article
11. Regulatory Compliance with Government Rules &
Regulations
|
11.1
|
COOPERATOR
is responsible for obtaining appropriate opinions, permits, or licenses
from Federal or State agencies, which regulate research materials, or
commercial products that may arise from the research work performed within
the Scope of agreement.
|
|
11.2
|
In
carrying out its responsibilities under this Article, COOPERATOR
shall:
|
|
a.
|
Consult
and coordinate regulatory approval actions with ARS;
and
|
|
b.
|
Give
ARS’ Authorized Agent or designee a copy of any applications
andopinions,
permits, or licenses issued.
|
|
11.3
|
Both
parties acknowledge and agree to comply with all applicable laws and
regulations of the Animal Plant Health and Inspection Service, the Center
for Disease Control, and or Export Control Administration pertaining to
possession or transference of technical information, biological materials,
pathogens, toxins, genetic elements, genetically engineered
microorganisms, vaccines, and the
like.
|
Article
12. Liability
It is
understood and agreed that neither party to this Agreement shall be responsible
for any damages or injuries arising out of the conduct of activities governed by
this Agreement, except to the extent that such damages and/or injuries were
caused by the negligent or wrongful acts or omissions of its employees, agents
or officers. ARS’ liability shall be limited by the Federal Tort Claims Act, 28
USC 2671, et seq.
Article 13.
Termination
|
13.1
|
Either
party may unilaterally terminate this entire Agreement at any time by
giving the other party written notice not less then sixty (60) calendar
days prior to the desired termination date. In the event that ARS
unilaterally terminates the Agreement not-for-cause and does not continue
the research project, and assuming that there is not yet technology
available for licensing, then, if COOPERATOR so requests, ARS will provide
non-exclusive access to transfer the research materials and associated
know how for such materials to COOPERATOR to finish the research
project.
|
|
13.2
|
Article
2. “Publications", 3. "Confidentiality", 6. "Ownership", 7. "Subject
Invention Licenses”, 10. "Use of Name or Endorsements", and 12.
"Liability" shall survive the expiration or termination of this
Agreement.
|
|
13.3
|
If
either party unilaterally terminates this Agreement pursuant to Article
13.1, each party shall return to the other or destroy, as shall be then
agreed, any and all data and materials originated or provided by one party
to the other that is still in the receiving party’s possession within 30
days of termination.
|
E-20
Article
14. Availability of Appropriations
The
continuance of this Agreement is subject to the passage by the Congress of the
United States of an appropriation of funds from which expenditures may legally
be made
to cover
ARS’ contributions.
Article
15. Disputes
|
15.1
|
Any
dispute arising under this Agreement, which cannot be readily resolved,
shall be submitted jointly to the Authorized Agents, identified in Article
16.
|
|
15.2
|
Each
party agrees to seek in good faith to resolve the issue through
negotiation or other forms of nonbinding dispute resolution processes
mutually acceptable to the parties.
|
|
15.3
|
Pending
the resolution of any dispute or claim pursuant to Artic1e 15, the parties
agree that performance of all obligations shall be pursued
diligently.
|
Article
16. Notices and Authorized Agents
Notices
between the parties and copies of correspondence among the scientific and/or
technical
representatives of each party that interpret or may have a bearing on the legal
effect of
this Agreement’s terms and conditions shall be sent to the Authorized Agents.
Referencing
Agreement Number 58-3K95-8-1246 thereon, send copies to:
|
ARS’
Authorized Agent
|
Cooperator’s
Authorized Agent
|
|
Martha
B. Steinbock
|
Ledyard
H. DeWees, JD
|
|
USDA-ARS-OTT
|
Corporate
Secretary & General Counsel
|
|
5601
Sunnyside Ave.
|
270
NW 3rd Court
|
|
Beltsville,
Maryland 20705-5131
|
Boca
Raton, Fl 33432-3720
|
|
Tel.:
301-504-6905
|
561-368-1427
|
|
Fax:
301-504-5060
|
Fax:
561-395-8312
|
|
E-mail:
crada.ott@nps.ars.usda.gov
|
E-mail:
idewees@gmail.com
|
Article
17. Limitation on ARS’ Scientific Representative’s
Authority
ARS’
Scientific Representative, also known as the Authorized Departmental Officer’s
Designated Representative (“‘ADODR"), is authorized to perform the research and
development falling within the Scope of Agreement. This individual is not
authorized to change or interpret with authority the terms and conditions of
this Agreement.
E-21
Article
18. Assignments
|
18.1
|
Neither
this Agreement nor any rights or obligations of the parties hereto shall
beassigned
or otherwise transferred by either party without the prior written consent
of the other party, which consent shall not be unreasonably
withheld.
|
|
18.2
|
In
no case shall COOPERATOR assign or transfer this Agreement to a party not
a citizen or legal resident of the United
States.
|
|
18.3
|
ARS
is an agency of the U.S. Government and any rights or obligations created
under this Agreement are freely transferable within the U.S. Government
and shall not be deemed an "assignment” as contemplated by this Article
18.
|
Article
19. Relationship of Parties
|
19.1
|
ARS
and COOPERATOR act in their independent capacities in the performance of
their respective functions under this Agreement and neither party is to be
considered the officer, agent, or employee of the
other.
|
|
19.2
|
Each
party shall allow, consistent with policies and procedures of ARS and
theCOOPERATOR,
access to their facilities, as
needed.
|
|
19.3
|
Each
party shall separately assign personnel, equipment, supplies,
transportation, and facilities, as needed and available to meet respective
responsibilities hereunder, such resources to remain the property of the
assignor.
|
Article
20. Force Majeure
|
20.1
|
Neither
party shall be liable for any unforeseeable event beyond its reasonable
control not caused by the fault or negligence of such
party:
|
|
a.
|
Which
causes the party to be unable to perform its obligations under
thisAgreement;
and
|
|
b.
|
Which
it has been unable to overcome by the exercise of due
diligence.
|
|
c.
|
This
includes, but is not limited to, flood, drought, earthquake, storm,
fire,pestilence,
lightning and other natural catastrophes, epidemic, war, riot, civil
disturbance or disobedience, strikes, labor dispute, failure, or sabotage
of either party’s facilities or any order or injunction made by a court or
public agency.
|
|
20.2
|
In
the event of the occurrence of such force majeure event, the party unable
to perform shall promptly notify the other party. It shall
also:
|
|
|
a.
|
Use
its best efforts to resume performance as quickly as
possible;
|
E-22
|
|
b.
|
Suspend
performance only for such period of time as is necessary as a result of
the force majeure event.
|
Article
21. Amendment
|
21.1
|
If
either party desires a modification in this Agreement, the parties shall
confer in good faith to determine the desirability of such
modification.
|
|
21.2
|
Such
modification shall not be effective until a written amendment is signed by
the
Authorized Agents of both
parties.
|
Article
22. Severability
The
illegality or invalidity of any provision of this Agreement shall not impair,
affect, or invalidate
the other provisions of this Agreement.
Article
23. Ambiguities
ARS and
COOPERATOR agree that each party has reviewed this Agreement and that any rule
of construction to the effect that ambiguities are to be resolved against the
drafting party shall not apply to the interpretation of this
Agreement.
Article
24. Officials Not To Benefit
|
24.1
|
No
Delegate to or Member of the Congress of the United States of America
shall have a part of or benefit from this
Agreement.
|
|
24.2
|
This
requirement does not include corporations if this Agreement is entered
into for the corporation’s general
benefit.
|
Article
25. Subcontracting Approval
|
25.1
|
A
party hereto desiring to obtain and use the services of a third party via
contract or otherwise shall give prior notice to the other party,
including details of the contract or other
arrangement.
|
|
25.2
|
This
requirement is to assure that confidentiality is not breached and rights
in Subject. Inventions are not
compromised.
|
Article
26. Governing Law
The
construction, validity, performance, and effect of this entire Agreement shall
be governed by the laws applicable to the Government of the United States of
America as practiced in the Federal Courts located in the District of
Columbia.
E-23
Article
27. Entire Agreement
|
27.1
|
This
Agreement constitutes the entire agreement between COOPERATOR and ARS and
supersedes all prior agreements and understandings between them with
respect to its subject matter.
|
|
27.2
|
Any
representations, promise, or condition in connection with such subject
matter, which is not incorporated in this Agreement, shall not be binding
upon either party.
|
|
27.3
|
No
modification, renewal, extension, waiver, or termination of this Agreement
or any of its provisions shall be binding upon the party against whom
enforcement of such modification, renewal, extension, waiver, or
termination is sought, unless made in writing and signed on behalf of such
party by that party’s Authorized
Agent.
|
|
27.4
|
As
used herein, the word "termination" includes any and all means of bringing
to an end prior to its expiration by its own terms of this Agreement, or
any provision thereof, whether by release, discharge, abandonment, or
otherwise.
|
E-24
SCHEDULE
1
CERTIFICATIONS
COOPERATOR
certifies that it:
1. x is, o is not, a small
business.
2. o is, x is not, a minority
business.
3.
Operates as:
|
o
|
an
individual
|
|
o
|
a
partnership
|
|
x
|
a
corporation
|
|
o
|
limited
liability corporation
|
|
o
|
public
institution
|
|
o
|
private
institution
|
|
o
|
educational
institution;
|
|
4.
|
Has
not paid or agreed to pay any company or person (other than a bona fide
employee working solely for COOPERATOR) any fee, commission, percentage,
or brokerage fee, contingent upon the award of this Agreement, and if so,
agrees to furnish information relating thereto, as requested, by the
Authorized Departmental Officer.
|
|
5.
|
Has
not employed or retained any company or person (other than a full-time
bona fide employee working solely for COOPERATOR) to solicit or secure
this Agreement.
|
|
6.
|
Its
Principal Officers are not listed on the U.S. Governments list of debarred
and suspended organizations and individuals; shall notify the Authorized
Departmental Officer if so listed; and shall not subcontract or otherwise
award to any organization or individual so
listed.
|
|
7.
|
Agrees
to comply with the provisions of the Civil Rights Act of 1964, as amended,
and Executive Order 11246, addressing equal opportunity and affirmative
action.
|
|
8.
|
Agrees
to comply with the provisions of Title IX of the Education Amendment of
1972, 20 USC 1681, et seq.; Section 504 of the Rehabilitation Act of 1973,
as amended, 29 USC 794; Age Discrimination Act of 1975, 42 USC 6101-6107;
Clean Air Act, 42 USC 7401, et seq.; and Drug-Free Workplace Act of 1988,
41 USC 701, et seq.
|
|
9.
|
Is
in a position to undertake, perform, and complete this Agreement and will
diligently perform work in accordance with its
provisions.
|
E-25
SCHEDULE
2
STATEMENT
OF WORK
A. Introduction/Background
In this
age of ever-increasing antimicrobial resistance development among the most
severe pathogens in modern health care, food-safety and veterinary health, it
has become essential to develop agents to kill or prevent bacterial growth. Most
importantly, these agents should be refractory to resistance development in the
target pathogen. It is also important to avoid the use of broad range
antimicrobials in order to have a minimal impact on the commensal bacterial
community and thus avoid the acquisition by commensal bacteria of DNA elements
harboring resistance genes.
The
Agricultural Research Service’s Animal Biosciences and Biotechnology Laboratory
(ABBL), Beltsville, Maryland has previously developed and protected novel
technology to create triple acting peptidoglycan hydrolase fusion antimicrobials
against the single genus Staphylococcus that are predicted to be refractory to
resistance development. ARS will partner with Viridax Corporation
("Cooperator”), to evaluate the ARS technology and the Cooperator will
commercially develop the technology.
It is
understood that ABBL is also working on research projects that relate to but are
not subject to the terms and conditions of this agreement and that ABBL will
continue to verify the level of lytic activity in previously reported proteins
(for example, but not limited to: Ply700, B30 endolysin, LambdaSa2 endolysin,
LysK, Lysostaphin, and phil l endolysin) that can contain either two or just one
active lytic domain (turbidity assay, MIC assay) to determine the relative
efficacy of each.
B. Objective
The
overall objectives are to join the resources and expertise of ARS
and Cooperator to evaluate (ARS), and commercialize (Cooperator)
previously ARS-developed novel, multi-domain, antimicrobials that will target
Staphylococcus aureus.
The objective is to target S.
aureus for treating bovine mastitis and (human respiratory infections)
(Cooperator).
C. Approach
|
|
a.
|
Fusion
antimicrobials will be evaluated for their effect on pathogenic Staphylococcus aureus.
The fusion antimicrobials were created by ARS by fusing three
peptidoglycan hydrolase lytic domains that each can individually target a
unique staphylococcal cell wall peptidoglycan bond and cause cell lysis
(hereinafter referred to as "Background Invention” and described in Fig.
l). The Background Invention harbors three cell lytic activities that each
target a different bond of the peptidoglycan. The three existing fusion
proteins (LysK-lyso, 390LysK-lyso, & Lyso-390LysK) are described in
Fig. l.
|
E-26
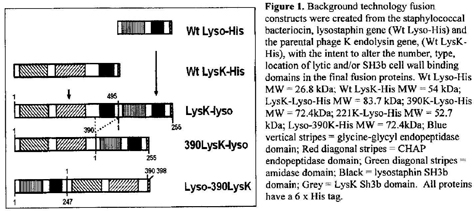
|
|
b.
|
Verification
that all three lytic domains are functional in each triple
fusion construct is currently being conducted under an ARS
project plan objective.
|
|
|
c.
|
This
CRADA may be amended for up to four years to include additional
research.
|
D. ARS
Responsibilities
|
|
l.
|
Conduct
these portions of the research project or perform the following
tasks:
|
|
|
a.
|
Evaluate
the level of antimicrobial activity of the fusion antimicrobials in a
variety of in vitro assays (turbidity reduction assays, MIC assays, plate
lysis assays) against Ventilator Associated Pneumonia (VAP) S. aureus clinical
isolates (broth-grown cultures).
|
|
|
b.
|
Cooperate
with Cooperator employee(s) in the testing of the Background Invention for
purity, stability and other parameters deemed necessary for the use of
these proteins in VAP animal
models.
|
|
|
c.
|
Develop
purification, stabilization, and formulation protocols that are compatible
with testing in vitro (turbidity reduction assays, MIC assays, plate lysis
assays) and in vivo (VAP animal
models).
|
|
|
d.
|
Evaluate
and optimize storage and assay conditions (e. g. pH, salt, osmolytes,
heavy metals) for maintaining optimal activity of the fusion
constructs.
|
|
|
e.
|
Via
nickel-chromatography, produce sufficient quantities, of the His-tagged,
stabilized, purified, fusion antimicrobials for testing in the VAP animal
models. (Exact amounts will be determined by activity testing
described above but is not expected to exceed a cost of $6,000 in
production supplies.) These protein preparations will be tested via SDS
PAGE for purity determinations and other assays, mutually agreed upon by
Cooperator and ARS (e.g. endotoxin assay), as deemed necessary for
optimizing their use in animal
models.
|
E-27
|
|
f.
|
If
deemed necessary for optimization of the preparation, alternative
purification strategies and new constructs lacking His-tags will be
attempted for the purpose of removing the endotoxin and/or the
His-tag.
|
|
|
2.
|
Provide
laboratory space in Building 230, utilities, services, and general support
to COOPERATOR'S personnel, as needed and available at the ARS
location.
|
E. COOPERATOR'S
Responsibilities
|
1.
|
Perform
these portions of the research
effort:
|
|
|
a.
|
Provide
supplies, and facility costs, including but not limited to: use of
MALDI-TOF services at USDA, DNA sequencing services via outside
contractor, PCR primer synthesis via outside contractor, space charges,
computer purchase, and computer and internet access at
USDA.
|
|
|
b.
|
Identify
a collaborating laboratory with expertise in VAP animal model(s) and
oversee these collaborators in the testing of the ARS-produced
fusion
|
antimicrobials.
|
|
c.
|
Provide
VAP S. aureus
clinical isolates for testing in the ARS in vitro
assays.
|
|
2.
|
Pay
$
150,000 (first year) to
ARS.
|
|
|
a.
|
The
payment schedule is: Three payments of $50,000 each, one every 4 months
with the first payment due within 30 days of final signature on the
agreement.
|
|
|
b.
|
Make
checks or money orders out to the "Agricultural Research Service," cite
Agreement No. 58-3K95-9-1339 thereon, and send
to:
|
USDA,
ARS, BA, Budget and Fiscal Office
10300
Baltimore Ave., Bldg. 003, Room 301
Beltsville,
MD 20705
F.
ARS & COOPERATOR’S Joint or Mutual Responsibilities
|
l.
|
Perform
these portions of the effort
jointly:
|
|
|
a.
|
Jointly
identify a Postdoctoral Fellow to perform the work (USDA
employee).
|
|
|
b.
|
Communicate
goals and jointly decide on the strategy to identify the optimization
protocols for the models/assays with which they should be
tested.
|
E-28
SCHEDULE
3
ESTIMATED
BUDGET
TOTAL
YEARS (one)
|
ARS Receives
Funds for
|
ARS In-House
|
Cooperator
In-House
|
||||||||||
|
A. Salaries
and Wages
|
85,000.00 | 6,000.00 | 7,000.00 | |||||||||
|
B. Equipment
|
||||||||||||
|
C. Materials
and Supplies
|
15,000.00 | 5,000.00 | 4,000.00 | |||||||||
| D. Travel | ||||||||||||
|
1.
Domestic
|
||||||||||||
|
2.
Foreign
|
3,000.00 | |||||||||||
|
E. Facilities
|
10,000.00 | 8,000.00 | 7,000.00 | |||||||||
|
F. Other
Direct Costs
|
10,000.00 | |||||||||||
|
G. TOTAL
DIRECT
|
120,000.00 | 19,000.00 | ||||||||||
|
H. Indirect
Costs
|
30,000.00 | |||||||||||
|
I. TOTAL
COSTS
|
$ | 150,000.00 | 19,000.00 | 21,000.00 | ||||||||
E-29
