Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - OMNI BIO PHARMACEUTICAL, INC. | f8k_061110i701901.htm |
Exhibit 99.1

1
Jefferies
Global Life Sciences Conference
New York, NY
June 11, 2010
Omni Bio Pharmaceutical, Inc
(OTCBB:OMBP)

Forward-Looking Statements Except for the historical information contained herein, the matters discussed in this presentation are forward-looking statements. The forward-looking statements in this presentation are based on information available to us as of the date any such statements are made and we assume no obligation to update these forward-looking statements. These statements are subject to risks and uncertainties that could cause actual results to differ materially from those described in the statements. These risks and uncertainties include the risks described from time to time in our reports filed with the Securities and Exchange Commission, including our most recent annual report on Form 10-K and subsequent quarterly reports on Form 10-Q all of which are available at www.sec.gov. We refer you to the “Forward-Looking Statements” and “Risk Factors” sections of these filings.

3
Omni Bio Pharmaceutical, Inc.
Applying approved and novel products for the
treatment of health conditions such as Type 1 and
Type 2 diabetes, acute or chronic transplant
rejection and bacterial and viral infection
treatment of health conditions such as Type 1 and
Type 2 diabetes, acute or chronic transplant
rejection and bacterial and viral infection
3

4
Management
Acting Chief Executive Officer: Charles A. Dinarello, MD
Member of the National Academy of Sciences -Listed by Institute for
Scientific Information as the world’s fourth most cited scientist for 20 years
(1982-2003). Numerous international awards including 2009 Crafoord Prize
(Royal Swedish Academy), 2009 Albany Medical School Prize (largest prize
in medicine in USA), 2010 the Paul Ehrlich Prize (Germany’s most
prestigious prize in medicine and Novartis Prize in Immunology (2010).
MD, Yale University.
Scientific Information as the world’s fourth most cited scientist for 20 years
(1982-2003). Numerous international awards including 2009 Crafoord Prize
(Royal Swedish Academy), 2009 Albany Medical School Prize (largest prize
in medicine in USA), 2010 the Paul Ehrlich Prize (Germany’s most
prestigious prize in medicine and Novartis Prize in Immunology (2010).
MD, Yale University.
Chief Operating Officer-Edward C. Larkin
30 years experience in securities, corporate finance and regulatory matters
including contractual and negotiating experience. Past experience as a
director of a number of NASDAQ listed companies. MBA, University of
Denver. BS Finance, University of Colorado.
including contractual and negotiating experience. Past experience as a
director of a number of NASDAQ listed companies. MBA, University of
Denver. BS Finance, University of Colorado.
Chief Financial Officer-Robert C. Ogden, CPA:
20 years senior financial management experience, past experience as CPA
with major public accounting firm, and prior experience as CFO of microcap
companies. BS Commerce, University of Virginia.
with major public accounting firm, and prior experience as CFO of microcap
companies. BS Commerce, University of Virginia.
4

5
Objectives and Approach
Ø To develop, patent, and commercialize treatment of novel
indications with FDA approved drugs with large market
opportunities.
indications with FDA approved drugs with large market
opportunities.
Ø FDA approval for an existing drug means significantly
reduced time to market for new indications with limited
safety issues to overcome.
reduced time to market for new indications with limited
safety issues to overcome.
Ø FDA approval will likely focus on efficacy, and 505 (B)(2)
allowing such compounds to have a reduced regulatory
process time frame compared to a new drug with no safety
record.
allowing such compounds to have a reduced regulatory
process time frame compared to a new drug with no safety
record.
5

6
Type 1 Diabetes-15,000-18,000 Type 1 juvenile diabetics are diagnosed in the USA per
year (40 per day) No alternative to insulin therapy exists. Total Type 1 population
of 2 million patients including adults.
year (40 per day) No alternative to insulin therapy exists. Total Type 1 population
of 2 million patients including adults.
Type 2 Diabetes -(Total population currently estimated at 24 million in the USA)
Treatment of patients failing current therapy and/or high risk of cardiovascular
complications (2% or 480,000).
complications (2% or 480,000).
Influenza -Large perennial problem with unmet need for patients failing current
therapy who are at a high risk for serious complications (pulmonary distress
syndrome) and hospitalization.
therapy who are at a high risk for serious complications (pulmonary distress
syndrome) and hospitalization.
Transplant Rejection -Unmet medical need to reduce complications of immuno-
suppressive drugs and chronic rejection.
suppressive drugs and chronic rejection.
Biohazards -$5 billion in US government funding provided by Project Bioshield
(2004) for countermeasures. Major European Market Opportunities exist.
(2004) for countermeasures. Major European Market Opportunities exist.
6
Omni Target Market Populations

7
IP Portfolio
Omni is the licensee of 3 patent portfolios from
the University of Colorado comprising 1 issued
and 16 filed patents
the University of Colorado comprising 1 issued
and 16 filed patents
1. An issued patent, a notice of allowance and additional
patent applications for the treatment of viral diseases
including Influenza and HIV-1.
patent applications for the treatment of viral diseases
including Influenza and HIV-1.
2. Patent applications for the treatment of Cellular
Transplant Rejection, Graft vs. Host Disease, Transplant
Rejection and Diabetes.
Transplant Rejection, Graft vs. Host Disease, Transplant
Rejection and Diabetes.
3. Patent applications and a notice of allowance for the
treatment of bacterial disorders, including tuberculosis
and anthrax (biohazard).
treatment of bacterial disorders, including tuberculosis
and anthrax (biohazard).
7

8
Patent Portfolio and Business Approach
— Methods of use for alpha 1 antitrypsin (AAT)-based and
AAT Derivative based treatment of diabetes (Type 1 and
Type2).
AAT Derivative based treatment of diabetes (Type 1 and
Type2).
— Composition of matter and methods of use for AAT-based
treatment of viral and bacterial disorders.
treatment of viral and bacterial disorders.
— Methods of use for AAT-based and AAT derivative based
treatment of graft versus host disease, cellular transplant
and organ transplantation conditions.
treatment of graft versus host disease, cellular transplant
and organ transplantation conditions.
— Our objective is to sub-license the treatment of various
indications of the IP portfolio to one or more producers of
AAT, many of whom are major pharmaceutical companies.
indications of the IP portfolio to one or more producers of
AAT, many of whom are major pharmaceutical companies.
8


10
Alpha 1 Antitrypsin (AAT) and Omni
— AAT is a naturally occurring human serum protein. FDA approved and
currently is prescribed for emphysema and chronic obstructive pulmonary
disorder (COPD) in AAT deficient patients.
currently is prescribed for emphysema and chronic obstructive pulmonary
disorder (COPD) in AAT deficient patients.
— As a current regimen for the treatment of emphysema and COPD, AAT is
administered once a week by infusion. AAT has one of best safety records of
any biological.
administered once a week by infusion. AAT has one of best safety records of
any biological.
— Market for AAT replacement therapy of individuals in North America is
approximately $150 million.
approximately $150 million.
— The new indications that Omni is in the process of testing are based in part on
the method of use for markets that dwarf AAT's existing applications and
markets.
the method of use for markets that dwarf AAT's existing applications and
markets.
— An IND to conduct a clinical trial in patients with Type 1 diabetes has
received FDA clearance and we are currently enrolling patients and anticipate
infusing patients during the third calendar quarter of 2010.
received FDA clearance and we are currently enrolling patients and anticipate
infusing patients during the third calendar quarter of 2010.
10

11
AAT - A naturally occurring protein without the
side effects of other currently marketed agents
side effects of other currently marketed agents
AAT:
Inhibits destructive enzymes such as proteinase-3
Anti-migratory
Anti-Complement
Prevents maturation of antigen presenting cells
Induces T-regulatory cells
Inhibits responses to inflammatory cytokines
Increases production of anti-inflammatory molecules
11

12
Animal Studies - Diabetes
— At clinically relevant doses, AAT protects the insulin-producing beta cells against
toxic agents (Proceedings of the National Academy of Sciences , 2005 and 2008).
toxic agents (Proceedings of the National Academy of Sciences , 2005 and 2008).
— AAT protects HUMAN islet cells against the toxic effects of cytokines. Cytokines
are produced in Type 1 and Type 2 diabetes and are a major contributor to cell
death for the insulin-producing beta cells.
are produced in Type 1 and Type 2 diabetes and are a major contributor to cell
death for the insulin-producing beta cells.
— AAT prevents diabetes progression in an acceptable animal model, spontaneously
halting its development.
halting its development.
— Prolongs islet cell survival by protecting islet cells from destruction that is
common during transplantation or shortly thereafter.
common during transplantation or shortly thereafter.
12
13
13
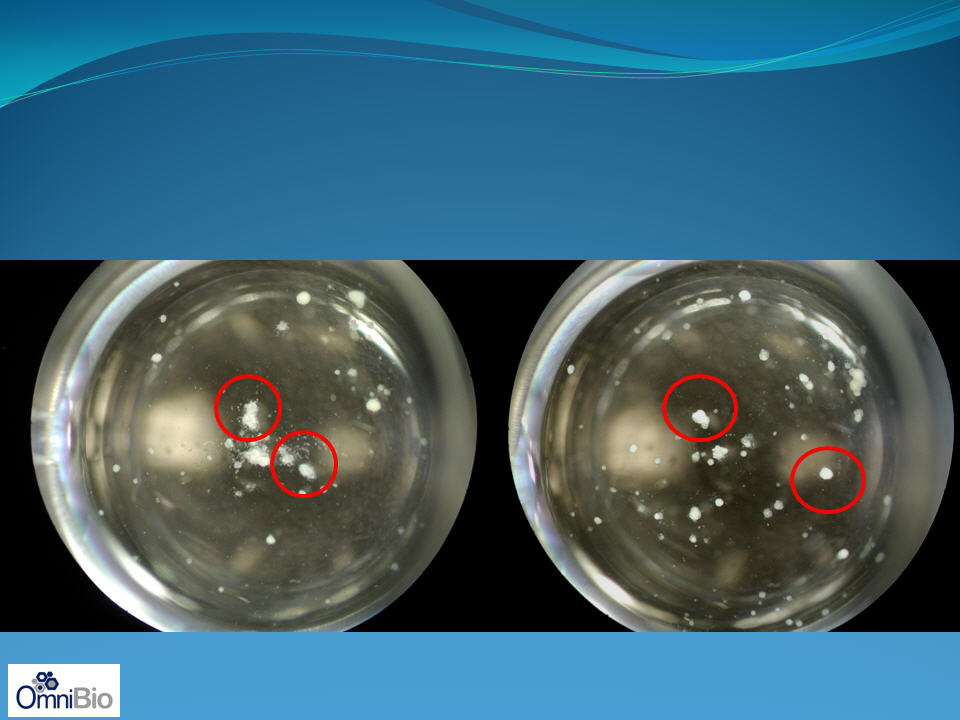
Human Islets: Effect of cytokine induced toxicity and
protection by AAT
protection by AAT
disintegrating, dying islets, as
takes place in vivo by immune attack
intact, living islets protected by AAT
during an immune attack
Presence of IL-1b/IFNg + Vehicle
Presence of IL-1b/IFNg + AAT

14
Type 1 Diabetes Trial - Preventing the destruction
of insulin-producing islet cells
of insulin-producing islet cells
— Barbara Davis Center for Childhood Diabetes at the University of Colorado
Hospital-Denver, Anschutz Medical Campus
Hospital-Denver, Anschutz Medical Campus
— Recently diagnosed Type 1 diabetics
— 8 weekly infusions of AAT at the commencement of the trial, then the
patients symptoms will be monitored for two years
patients symptoms will be monitored for two years
— Recognized endpoints (same as for immunosuppressive depleting antibodies)
— 2 Year trial period (from enrollment) with data reviewed at least quarterly
14
15
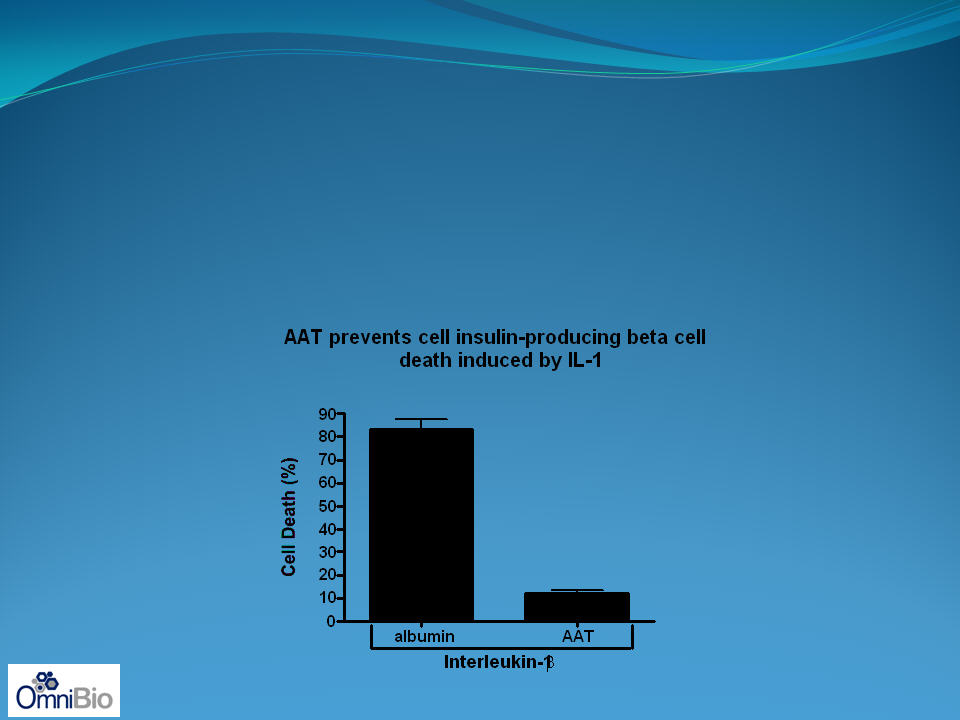
AAT in salvaging the loss of the insulin-producing
beta-cell in Type 2 diabetes
In Type 2 diabetes, progressive loss of functional insulin-producing beta-cells
takes place due to IL-1b produced in the fat that enters the islet
Blocking IL-1b restores function and lowers hyperglycemia
AAT blocks IL-1b induced cell death of the beta cell

16
Leland Shapiro, MD , FACP
— Associate Professor of Medicine University of Colorado
— Certified by American Board of Internal Medicine
— University of Massachusetts School of Medicine
— Broadly published researcher
16
Principal Investigator at the University of
Colorado, Anschutz Medical Campus
Colorado, Anschutz Medical Campus

17
— In the U.S. each year:
— 10-20% of population infected (30-60 million people)
— Children and the Elderly are at risk groups
— 114,000 hospitalizations
— 36,000 deaths
— Annual Economic Burden of approximately $3-15 billion.
17
Influenza
18
18
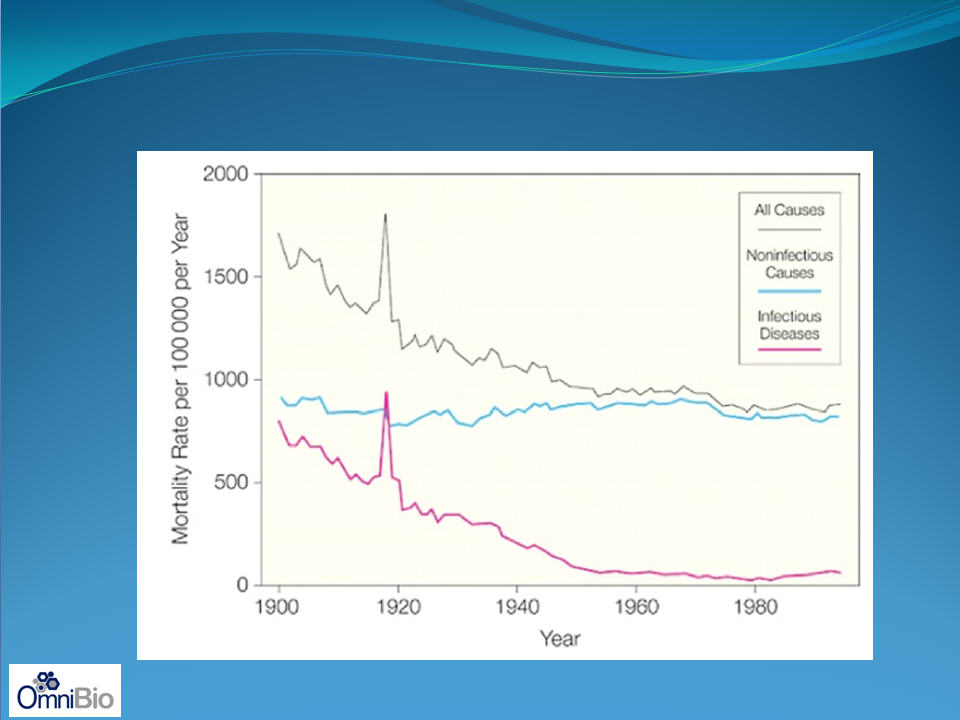
Effect of 1918 Pandemic Flu on Population
19
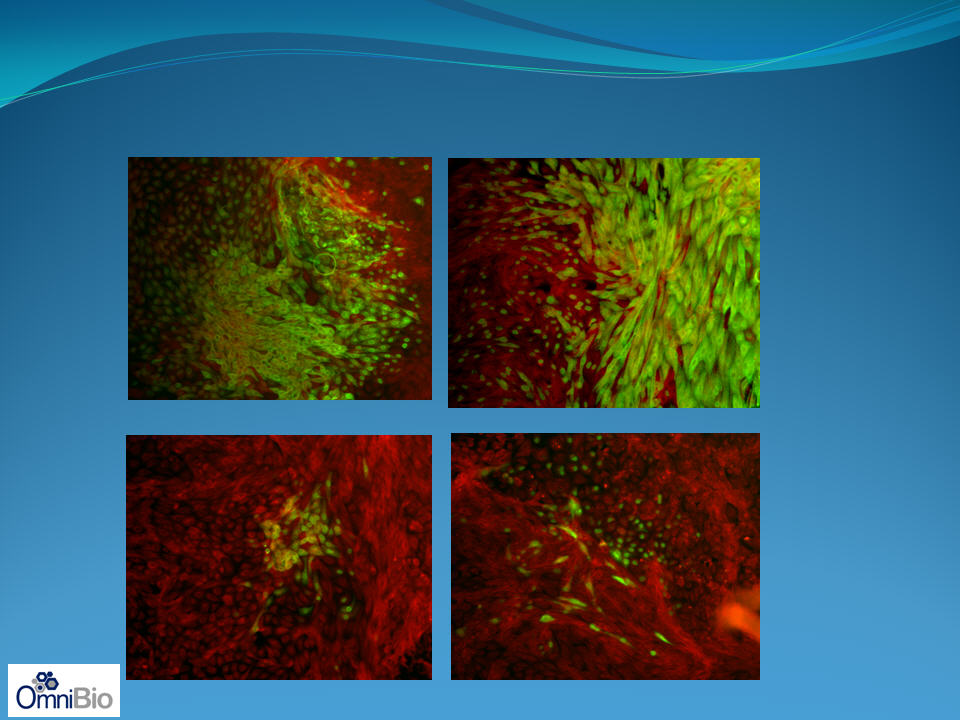
Influenza
alone
Influenza
+ AAT
19
AAT protects cells from influenza infection
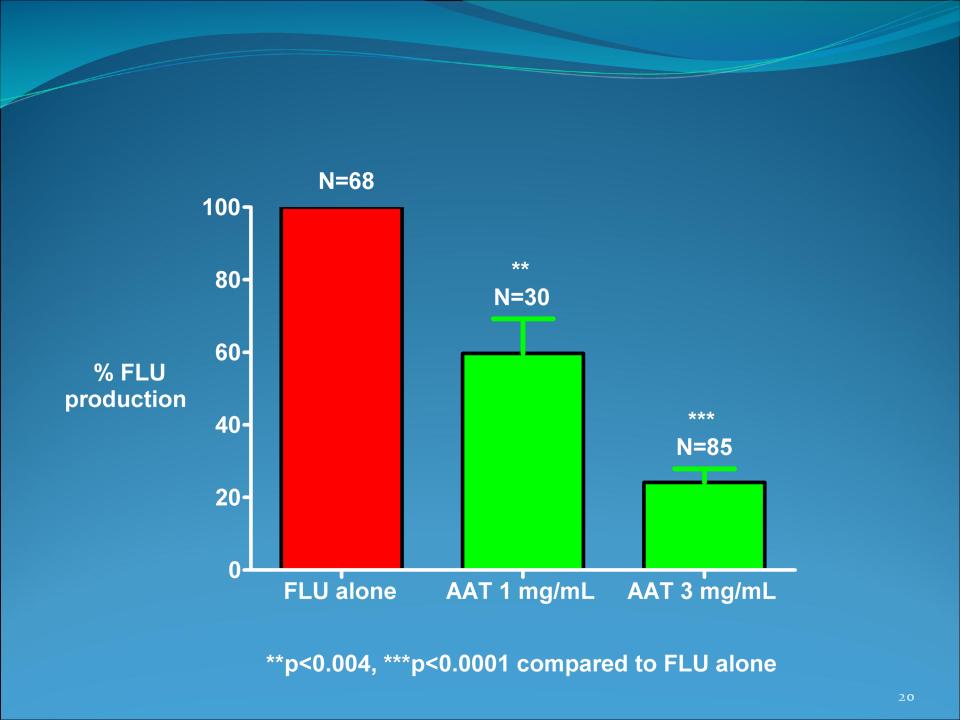
AAT inhibits influenza production in
infected cells (10 experiments + replicates)
infected cells (10 experiments + replicates)
21
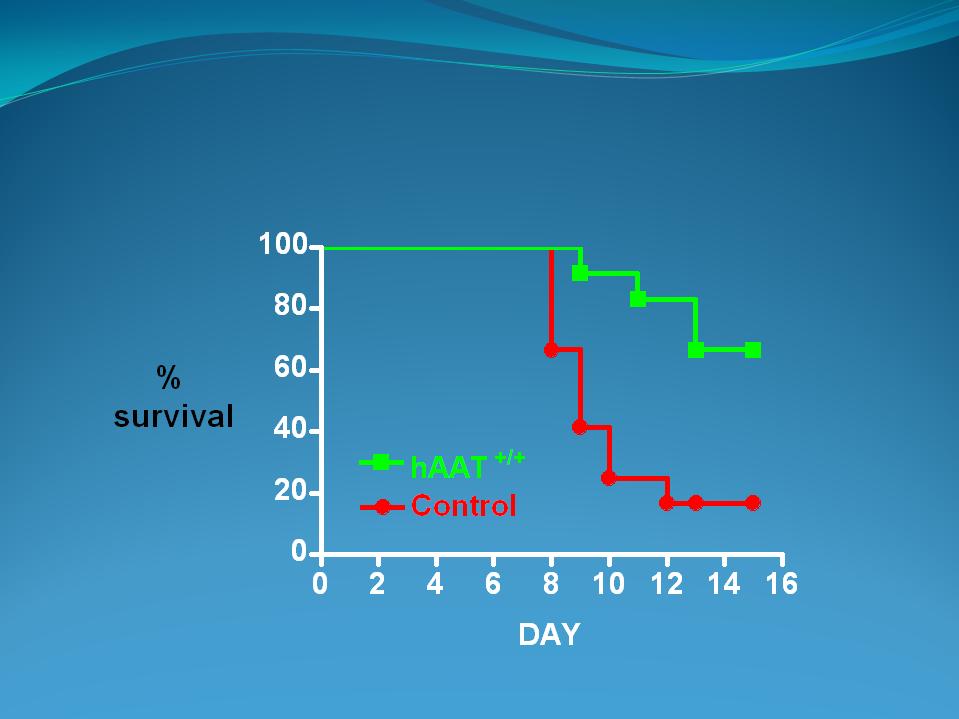
Survival in AAT transgenic mice
challenged with live influenza
(p=0.0007)
challenged with live influenza
(p=0.0007)
22
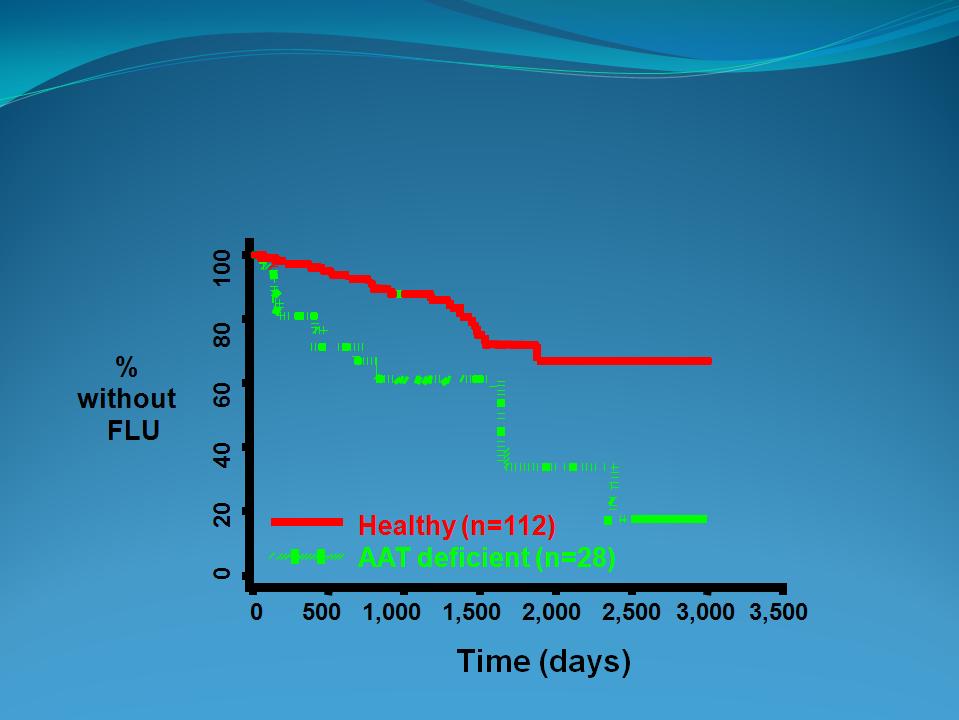
AAT deficiency is a risk factor for
FLU infection in humans
p=0.0012
FLU infection in humans
p=0.0012

23
23
• Both a treatment and preventative product
• Potentially broad spectrum treatment for different types of influenza including
“bird FLU”, “swine Flu (H1N1)”, and “weaponized” FLU
“bird FLU”, “swine Flu (H1N1)”, and “weaponized” FLU
• Impervious to mutation-induced resistance: AAT is still effective even if the
virus changes or mutates
virus changes or mutates
• Compatible with existing influenza treatments and immunizations
• Inhaled delivery places AAT in the lungs where initial infection and damage
occurs
occurs
AAT Impact on Influenza

24
24
|
Indication
|
Laboratory
studies in cells |
Animal
studies |
Human
studies |
Clinical trial
|
|
Anthrax
|
+
|
+
|
=
|
NA
|
|
Influenza
|
+
|
+
|
+
|
=
|
|
Diabetes Type 1
|
+
|
+
|
+
|
Pending
|
|
Diabetes Type 2
|
+
|
+
|
=
|
=
|
|
Graft rejection
|
+
|
+
|
+
|
=
|
|
Tuberculosis
(TB) |
+
|
+
|
=
|
=
|
AAT-based studies: Immediate
Opportunities and Status
Opportunities and Status
+ = completed or in process; - = has not been completed

25
Business Summary
Ø Broad Intellectual Property Position In Major Markets
Diabetes
Influenza and Bacterial diseases
3 Licenses
16 Patents Pending, 1 US Patent Issued
Ø Upcoming AAT Clinical Trials in Diabetes
FDA approved pharmaceutical applied to new and large markets
Outsourced business model requiring low overhead, capital
requirements primarily focused on sponsored research and
protection of intellectual property
requirements primarily focused on sponsored research and
protection of intellectual property
25

26
Omni Bio Pharmaceutical, Inc
Corporate Information:
OTCBB Symbol: OMBP
Fiscal Year End: March 31
Outstanding Shares as of March 31, 2010: 28 Million
Fiscal Year End: March 31
Outstanding Shares as of March 31, 2010: 28 Million
Common Stock Equivalents (3/31/2010): 11.5 Million
Corporate and IP Counsel: Faegre & Benson
Corporate and IP Counsel: Faegre & Benson
Auditors: Hein and Associates

27
Thank you for your interest
27
