Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - AUXILIUM PHARMACEUTICALS INC | d8k.htm |
 1
June 2010
(NASDAQ: AUXL)
Exhibit 99.1 |
 2
Safe Harbor Statement
We will make various remarks during this presentation that constitute “forward-looking
statements” for purposes of the safe harbor provisions under The Private Securities Litigation
Reform Act of 1995, including statements regarding the opportunities to build shareholder value; the
potential for XIAFLEX to be a blockbuster opportunity; the amount of revenues that XIAFLEX may
generate; the potential for XIAFLEX to be used in multiple indications; the generation of cash through licensing of XIAFLEX in other territories and for new
indications; the Company’s ability to achieve its objectives for the U.S. launch of XIAFLEX for
Dupuytren’s in 2010; the size of the U.S. market for Dupuytren’s; the interpretation of
market research data; interpretation of clinical results, including the efficacy and tolerability of
the Company’s products and product candidates, and recurrence rates; the ability to obtain
reimbursement in the U.S. for XIAFLEX for the treatment of Dupuytren’s and the timing thereof; the timing of new reimbursement codes for XIAFLEX; the Company’s
intention to and the timing of announcing any XIAFLEX U.S. revenues; the Company’s intention to
provide XIAFLEX revenue guidance for the third quarter 2010 when it releases earnings for the
second quarter 2010; the timing of the end-of-phase II meeting and of the initiation of phase III for XIAFLEX for the treatment of Peyronie’s disease; the approval of
the Marketing Authorization Application for XIAFLEX for the treatment of Dupuytren’s contracture
in the European Union; and the Company’s development and operational goals and strategic
priorities for fiscal 2010. All remarks other than statements of historical facts made during this presentation, including but not limited to, statements regarding future
expectations, plans and prospects for the Company, statements regarding forward-looking financial
information and other statements containing the words “believe,” “may,” “could,”
“will,” “estimate,” “continue,” “anticipate,”
“intend,” “should,” “plan,” “expect,” and similar expressions, as they relate to the Company, constitute forward-looking statements. Actual
results may differ materially from those reflected in these forward-looking statements due to
various factors, including general financial, economic, regulatory and political condition
affecting the biotechnology and pharmaceutical industries and those discussed in Auxilium's Annual
Report on Form 10-K for the year ended December 31, 2009 and Auxilium’s Quarterly
Report on Form 10-Q for the period ended March 31, 2010 under the heading "Risk Factors“, which are on file with the Securities and Exchange Commission (the “SEC”)
and may be accessed electronically by means of the SEC’s home page on the Internet at
http://www.sec.gov or by means of the Company’s home page on the Internet at
http://www.auxilium.com under the heading “Investor Relations - SEC Filings.” There may
be additional risks that the Company does not presently know or that the Company currently
believes are immaterial which could also cause actual results to differ from those contained in the forward-looking statements. Given these risks and uncertainties, any or
all of these forward-looking statements may prove to be incorrect. Therefore, you should not rely
on any such factors or forward-looking statements. In addition, forward-looking statements provide the Company’s expectations, plans or
forecasts of future events and views as of the date of this presentation. The Company
anticipates that subsequent events and developments will cause the Company’s assessments to
change. However, while the Company may elect to update these forward-looking statements at
some point in the future, the Company specifically disclaims any obligation to do so. These forward-looking statements should not be relied upon as representing the
Company’s assessments as of any date subsequent to the date of this presentation.
|
 3
Opportunities to Build Shareholder Value
Note: Seeking partners for Transmucosal film product candidates
® |
 4 |
 5
XIAFLEX –
Unique, Late-Stage, Blockbuster
Opportunity
•
Potential to be the only effective non-surgical treatment for two high unmet
needs:
Approved on February 2, 2010 for Dupuytren’s Contracture in U.S.
Promising phase IIb results in Peyronie’s disease
•
Well-characterized mode of action
•
Worldwide rights support growth
Build company in North America
Partnered with Pfizer in EU for Dupuytren’s and Peyronie’s
Opportunity to add additional indications
Rights for other territories or indications could generate additional cash
•
We believe worldwide peak revenues for XIAFLEX could be in excess
of $1 Billion annually |
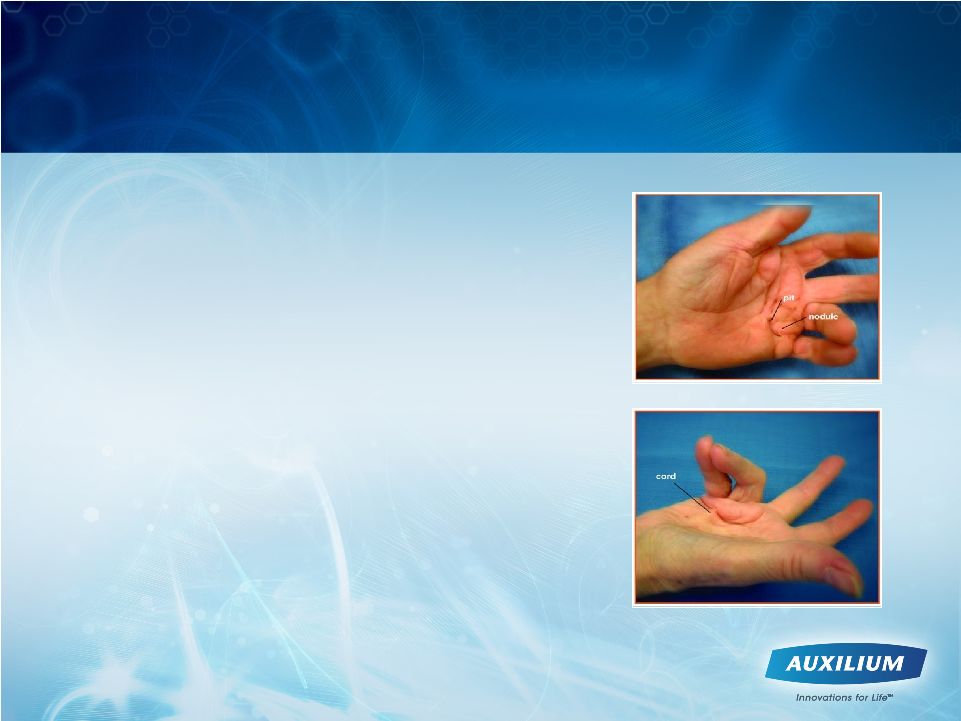 6
•
Excessive collagen deposition in
fascia of hand
•
Nodules represent early, active form
•
Cords develop over time, are palpable,
and result in contractures
•
Quality of life and daily activities can
be significantly affected
•
Surgery has been the current standard of
care and may be reserved for advanced
disease due to unpredictable results,
complications, long recovery and
recurrence/additional surgeries
Dupuytren’s Contracture is Debilitating for Patients
|
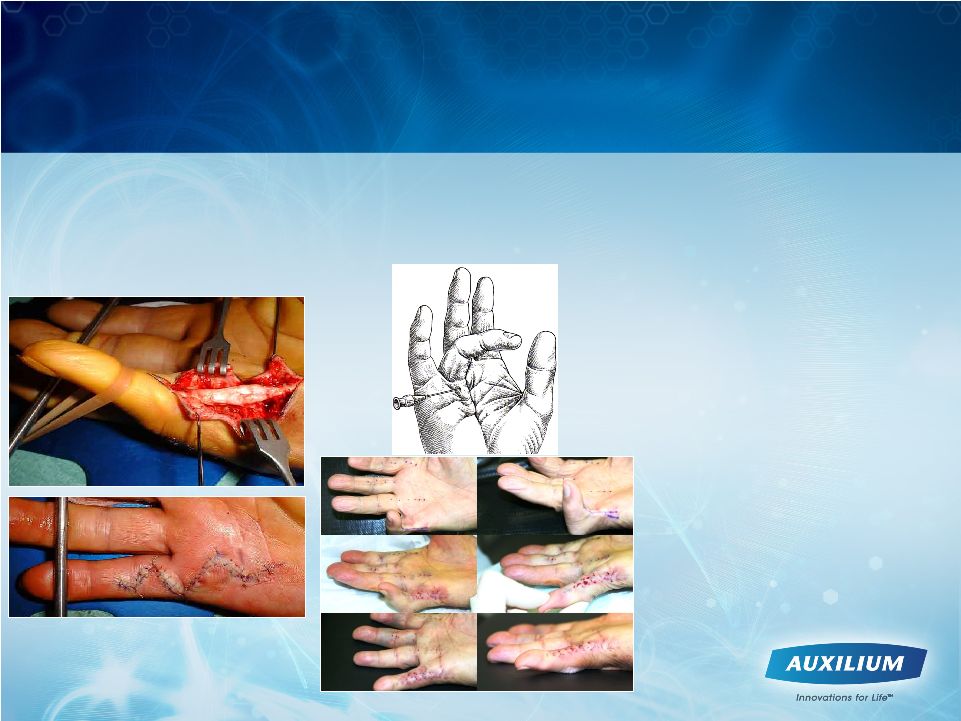 7
•
Surgery
•
Needle fasciotomy/
aponeurotomy
•
Amputation
Treatment Options Historically Limited to Invasive
Surgery with Significant Recuperation or Are
Unapproved and Ineffective
•
Non-surgical options
>
Splinting
>
Physical therapy
>
Corticosteroids |
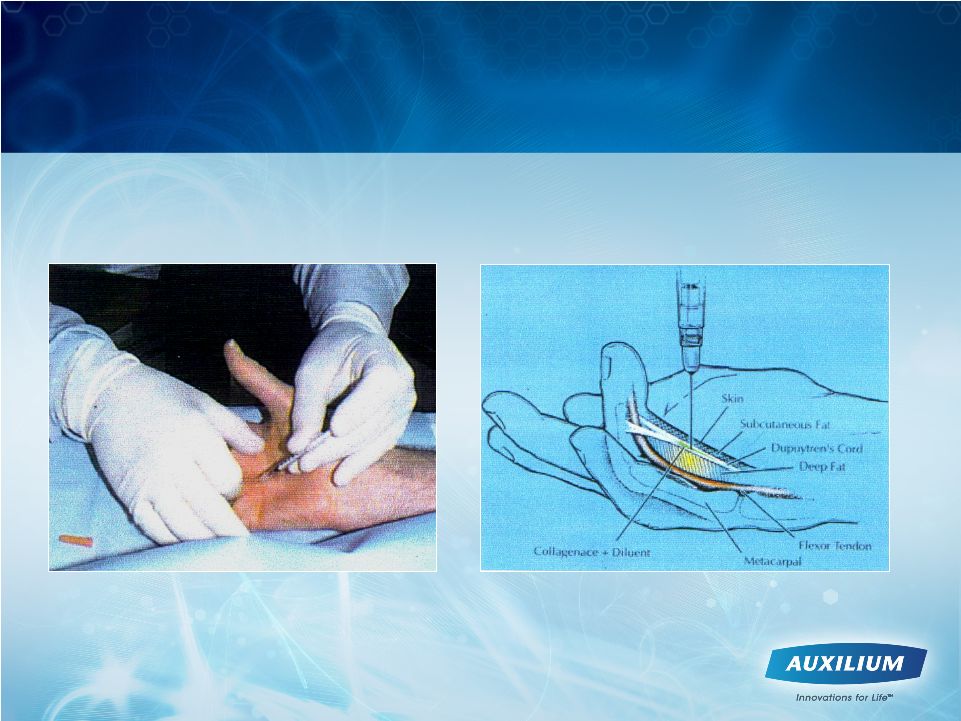 8
XIAFLEX Offers the First Nonsurgical Treatment
for Dupuytren’s Contracture |
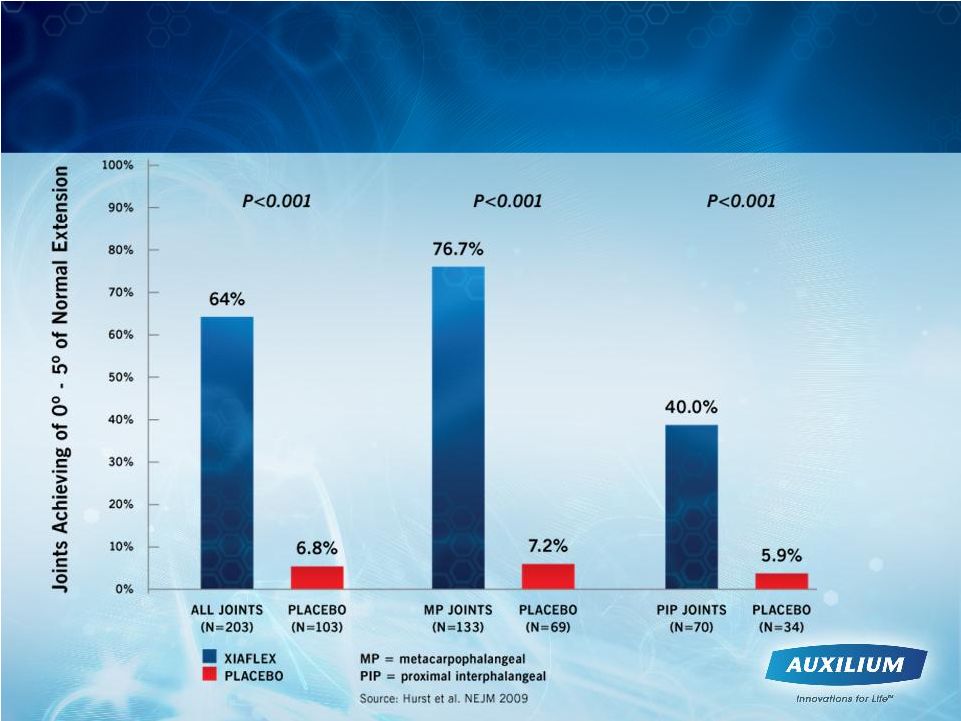 A
Significant Number of Dupuytren’s Contracture
Patients Benefited from Treatment with XIAFLEX
9 |
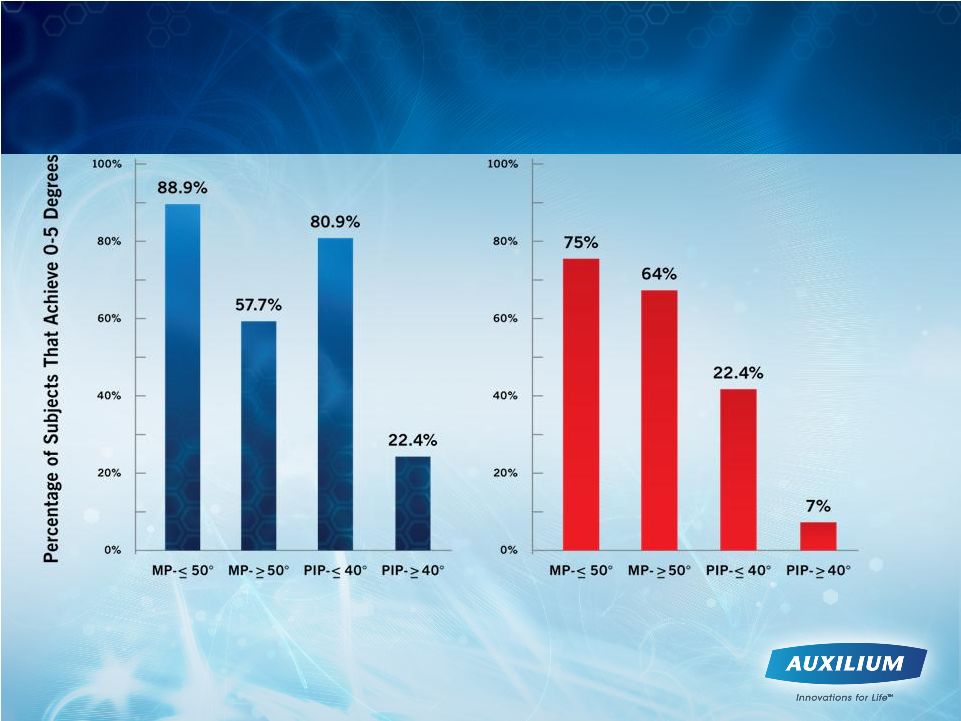 10
Early Intervention with XIAFLEX May Be the
Most Effective Treatment Approach
Hurst et al. NEJM 2009
N=248
N=125
Keith Denkler, M.D. Plast. Reconstr. Surg., 115802, 2005
Dupuytren’s
Fasciectomies
in 60 Consecutive Digits using lidocaine
with
epinepherine
and no tournequet |
 11
•
Most treatment related adverse events were mild or moderate in
intensity and resolved without intervention within a median of 10
days across studies
•
Serious Adverse Events possibly related to treatment were limited in
number (10 in total; 0.39% of >2600 injections)
–
4 SAEs
were tendon and ligament damage
•
No deaths, clinically meaningful changes in grip strength, arterial
injuries or nerve injuries related to XIAFLEX reported
•
No clinically meaningful changes in laboratory values
•
No clinically meaningful systemic allergic reactions
XIAFLEX Was Well-Tolerated in Phase III Studies |
 12
Contracture Recurrence Affects Patients
•
The earliest reports of recurrence for surgery were seen in 22% of
female
and
19%
of
male
Dupuytren’s
contracture
patients
at
a
mean
of
12
months
following
fasciectomy
1
.
Additionally,
a
rate
of
up
to
34%
has
been
reported
within
the
first
2
years
following
surgery
2
.
•
Abstract
for
a
prospective
trial
comparing
needle
fasciotomy
to
open
fasciectomy
reported
a
recurrence
rate
for
needle
fasciotomy
of
85%
at
a mean
of
2.3
years
3
.
•
XIAFLEX Long term extension study of phase III trials will follow
recurrence rates up to 5 years. 2 year data now available.
(1) Anwar et al., The Journal of Hand Surgery, Vol. 32A No. 9 November 2007 ;
1423-1428 (2) Leclercq
C. Epidemiology. In: Tubiana
R, Leclercq
C, Hurst LC, Badalamente
MA, Mackin
EJ, eds, Dupuytren’s
disease.
Martin Dunitz
Ltd, London, 2000;239-249.
(3) A.L. Van Rijssen, 2010 International Symposium on Dupuytren’s
Disease |
 13
Two Year Recurrence Rate of 19.3% for
XIAFLEX
®
in Dupuytren’s
Contracture
All Joints
MP Joints
PIP Joints
Patients from All Phase III Studies (n=950)
1,568
920
648
Patients Enrolled in Extension Study (n=634)
1,065
641
424
Patients Successfully Treated and Enrolled in Extension
Study (n=474)
619*
449*
170
Joints with Recurrence (n/%)
(119/618)
19.3%
(61/448)
13.6%
(58/170)
34.1%
Note: No patients at Year 2 had been retreated with commercial XIAFLEX
* One patient had unrelated hand surgery and post-operative bandaging prevented an
accurate assessment of recurrence at 2 years |
 14
Launch Update Through
May 2010 |
 15
•
Position XIAFLEX as a paradigm changing non-surgical
treatment alternative for physicians, payers and patients
•
Achieve high awareness among targeted physicians and
diagnosed patients
•
Get
targeted
physicians
through
the
sales
cycle
–
from
interest
to
injection to reimbursement
•
Educate and assist physician offices with unfamiliar
reimbursement processes at each step of the way
Key Objectives for U.S. XIAFLEX Launch in 2010 |
 16
First Commercial Patient Injected
Patient with 50 degree
MP cord pre injection
Post manipulation –
Able to lay hand flat
on table
Post injection and
pre manipulation
Individual results may vary.
See Hurst et al. NEJM 2009 |
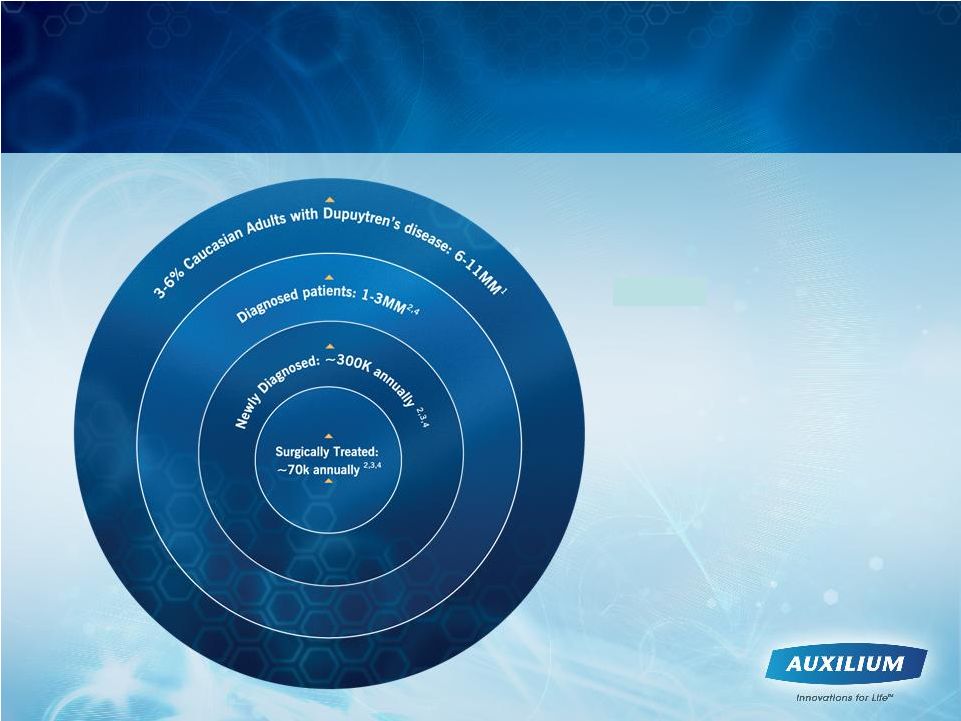 1.
Dupuytren’s
Disease
–
Tubiana,
LeClerq,
Hurst, Badalamente, Mackin
2.
SDI Claims Data Based Projections
3.
Medicare Data Based Projections (BESS
database used, Medicare 5% database also
used to validate numbers)
4.
Auxilium
Research
(Patient Segmentation,
Forecast Research, WK/AMA Databases)
Sources:
We Believe that Targeting the Eligible Patient
Population Should Drive Development of the U.S.
Market to Its Full Potential
17 |
 18
XIAFLEX Launch Strategy is Tailored to
Customer Needs
•
~ 7,000 target physicians
–
Hand surgeons, plastic surgeons, orthopedic surgeons, general
surgeons, rheumatologists
•
~ 100 field based personnel
–
Sales reps, sales managers, and reimbursement specialists
•
Medical science liaisons
–
Key opinion leader and regional opinion leader support
|
 19
Access to Physicians
•
Since launch in late March, majority of physicians called
upon receptive to meeting with our sales reps
•
Significant time being spent with office staff –
Calls can
typically run from 30 minutes to 3 hours
•
Changing a treatment paradigm takes time |
 Unique Sales Cycle for Targeted Physicians
Physician Interest,
Training & Enrollment
Successful XIAFLEX
XPERIENCE
20 |
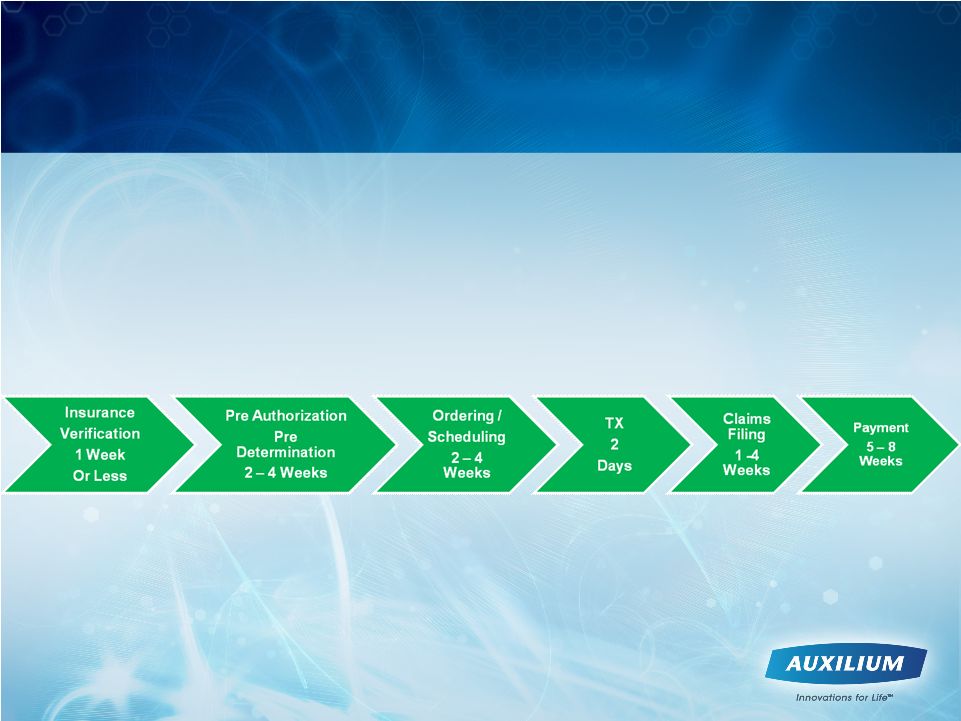 21
Changing Treatment Paradigm and Reimbursement
Cycle May Drive Physician Adoption Rate
Prior to treatment decision Physician must go through:
•Awareness
•Interest
•Training / Enrollment into XIAFLEX XPERIENCE program
•Reimbursement education / comfort
•Patient Identification / counseling
11 –
21 weeks from treatment decision through receipt of payment
possible |
 22
•
Accounts need customized support every step of the way
•
Miscellaneous J and CPT codes being used; component
and bundled approaches are being accepted
•
C-code for institutional use will be effective July 1, 2010
•
CMS has recommended a J-Code for XIAFLEX which will
be effective January 1, 2011
•
CPT code specific to XIAFLEX may come in 2012
Reimbursement Processes Are New to Target
Physicians |
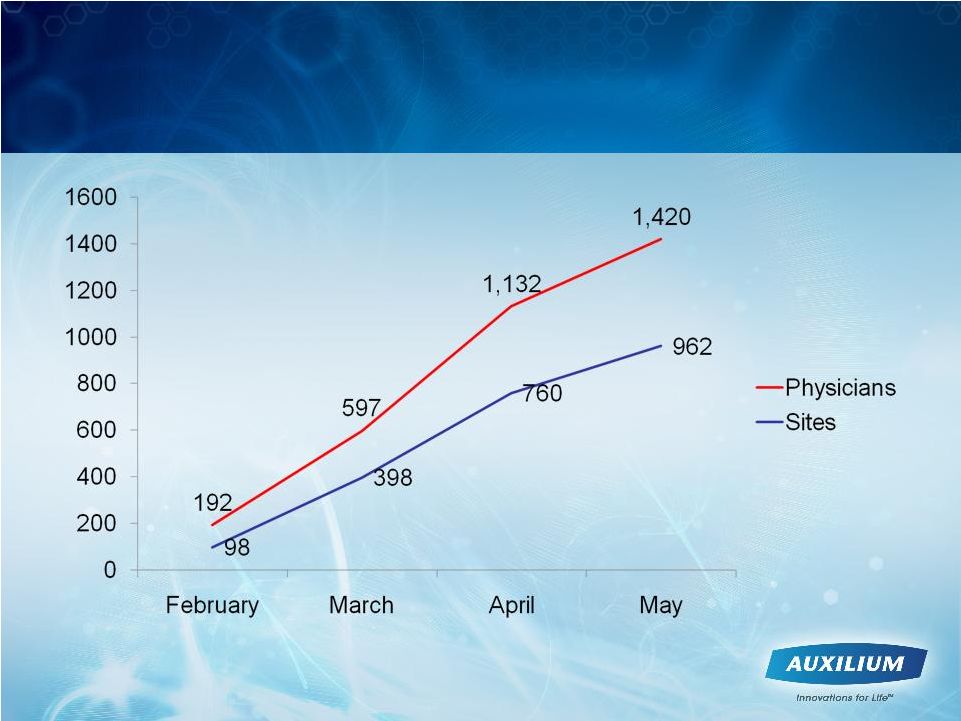 23
Leading Indicators Trending Positively
-
Over 1,400 physicians and 900 sites have enrolled in XIAFLEX
XPERIENCE program |
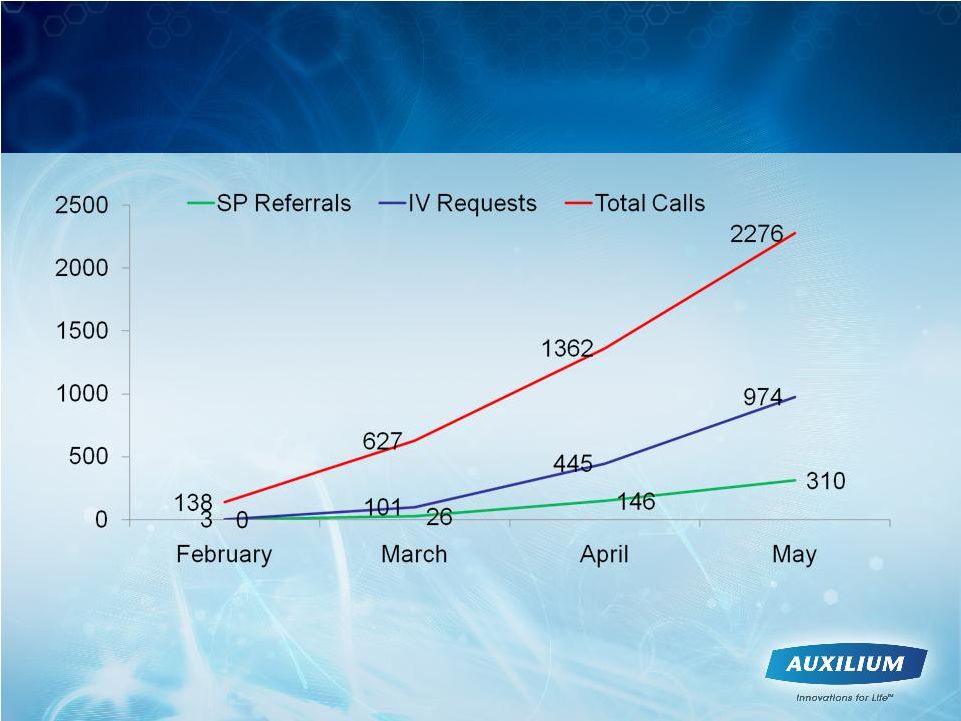 24
Leading Indicators Trending Positively
-
Cumulative Call Center Volume
Note: SP = Specialty Pharmacy; IV = Insurance Verification
|
 25
Leading Indicators Trending Positively
-
Sites that have ordered XIAFLEX |
 26
Approximately 87% of insured lives have access
to XIAFLEX |
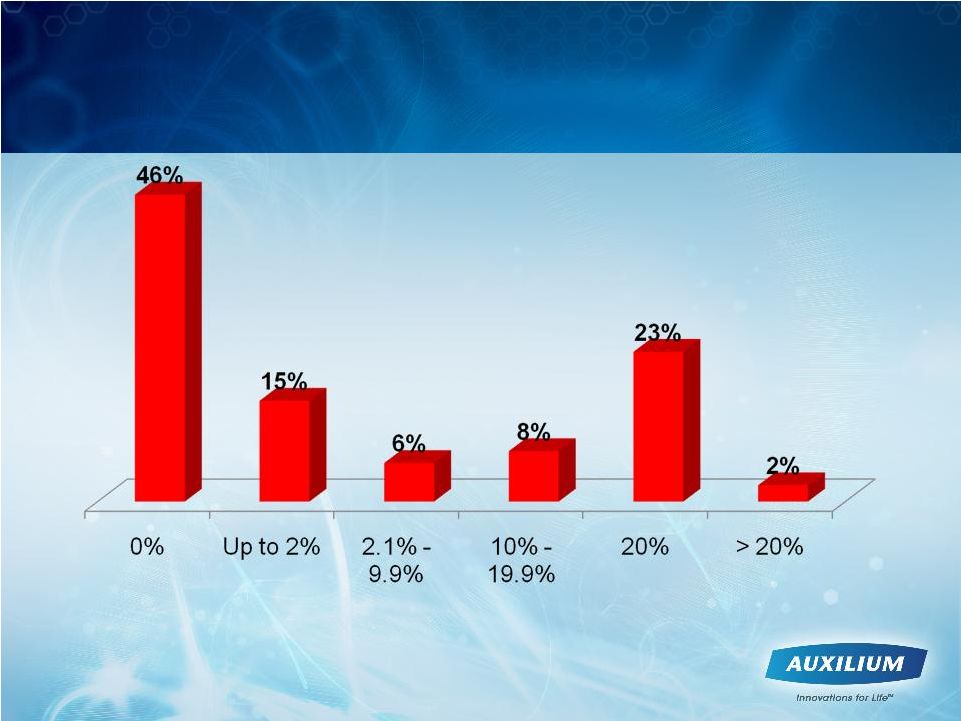 27
Out of Pocket Costs In Line With Our
Expectations
Source: Data processed by AUXL call center through May 31, 2010
A significant portion of patients paying
20% and above have a secondary
insurance, which may cover a portion of
this cost
% Of Treatment Cost Paid by Patient |
 28
Distribution Network is Operating Smoothly |
 29
We Believe Launch Strategy Is Sound:
•
Product efficacy and safety are consistent with clinical data
•
Access to targeted physicians is good.
•
Usage is characterized by “test driving”, which is typical for
paradigm changing treatment
•
Payers willing to provide access to drug and procedures
•
All distribution channels active and operating as expected
•
Leading indicators trending positively |
 30
Auxilium’s Plan to Provide Visibility on
XIAFLEX Launch
•
We will pre-release Q2 revenues in early July
•
On Q2 earnings call we will provide revenue
guidance for Q3
•
We will continue to provide quarterly revenue
guidance through 2010 |
 31
Strategic Corporate Priorities in 2010
•
Execute the launch in the U.S. for XIAFLEX in Dupuytren’s
contracture;
•
Support Pfizer in the ongoing regulatory review of the EMA
Dupuytren’s submission and prepare for their potential EU
approval;
•
Commence phase III for XIAFLEX in Peyronie’s disease,
assuming a successful End-of phase 2 meeting scheduled with
FDA in June;
•
Prioritize our development of additional pipeline indications for
XIAFLEX; and,
•
Continue to maximize Testim revenues while vigorously
defending our intellectual property. |
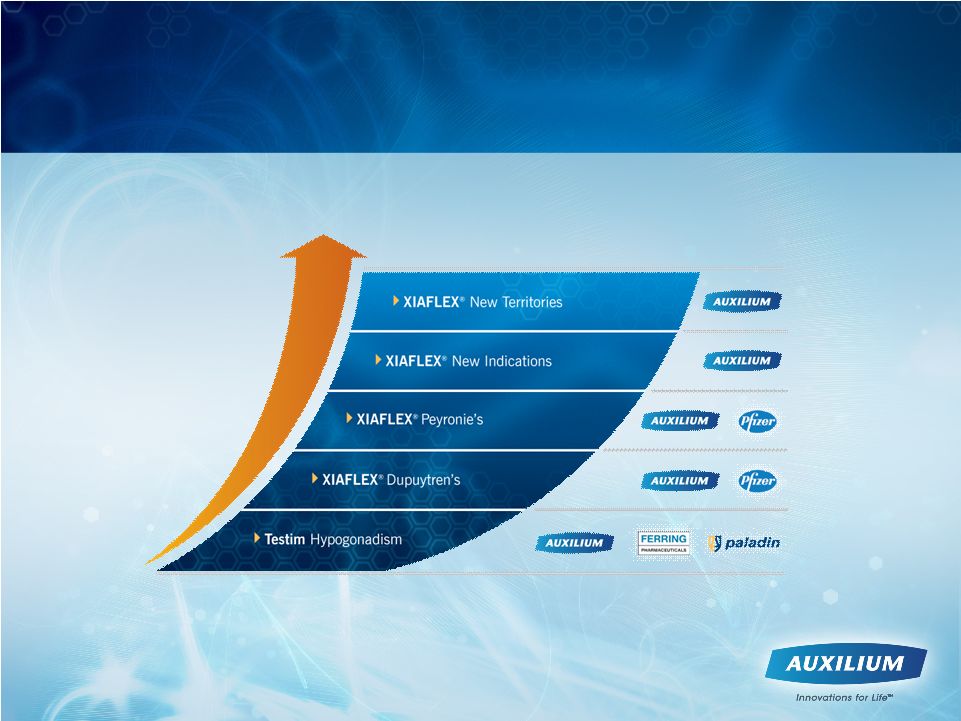 32
Opportunities to Build Shareholder Value
Note: Seeking partners for Transmucosal film product candidates
®
® |
