Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Diadexus, Inc. | c01918e8vk.htm |
Exhibit 99.1

| Corporate Overview May 2010 Filed by VaxGen, Inc. Pursuant to Rule 425 Under the Securities Act of 1933 Subject Company: VaxGen, Inc. Commission File No. 0-26483 |

| Important Additional Information May Be Filed with the SEC In connection with the proposed merger transaction between VaxGen and diaDexus, VaxGen may file with the SEC a registration statement on Form S-4, which will include a prospectus of VaxGen and other relevant materials in connection with the proposed transactions, and may file with the SEC other documents regarding the proposed transaction. The final prospectus would be mailed to the stockholders of diaDexus. Investors and security holders of diaDexus are urged to read the prospectus (including any amendments or supplements thereto) and the other relevant material carefully in their entirety IF AND when they become available because they will contain important information about diaDexus, VaxGen and the proposed transaction. This communication shall not constitute an offer to sell or the solicitation of an offer to sell or the solicitation of an offer to buy any securities, nor shall there be any sale of securities in any jurisdiction in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such jurisdiction. No offering of securities shall be made except by means of a prospectus meeting the requirements of Section 10 of the Securities Act of 1933, as amended or an exemption therefrom. Upon filing, any prospectus and other relevant materials (when and if they become available), and any and all documents filed with the SEC, may be obtained free of charge at the SEC's web site at www.sec.gov. In addition, investors and security holders may obtain free copies of the documents filed with the SEC by VaxGen by directing a written request VaxGen, Inc., 379 Oyster Point Boulevard, Suite 10, South San Francisco, CA 94080, Attention: Investor Relations. |

| Forward Looking Statements This presentation contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 about diaDexus and VaxGen. Such statements include, but are not limited to, statements about the proposed transaction between VaxGen and diaDexus and its potential benefits to the diaDexus and VaxGen stockholders, the expected timing of the completion of the transaction, the combined company's plans, objectives, expectations and intentions with respect to future operations and products and other statements that are not historical in nature, particularly those that utilize terminology such as "will," "potential", "could," "can," "believe," "intends," "continue," "plans," "expects," "estimates" or comparable terminology. Forward-looking statements are based on current expectations and assumptions, and entail various known and unknown risks and uncertainties that could cause actual results to differ materially from those expressed in such forward-looking statements. Important factors known to diaDexus and VaxGen that could cause actual results to differ materially from those expressed in such forward-looking statements include the risk of general business and economic conditions; the failure of the diaDexus stockholders to approve the transaction; the failure of either party to meet any of the other conditions to the closing of the transaction; the failure to realize the anticipated benefits from the transaction or delay in realization thereof; diaDexus' need for and ability to obtain additional financing; the sufficiency of available capital to allow diaDexus to grow revenue or achieve profitability; the risk that diaDexus is unable to obtain required regulatory approvals and to commercially reintroduce its PLAC TIA test in a timely manner, or at all, or that diaDexus revenues are materially adversely affected by the issuance of a "do not use" letter to diaDexus customers with respect to the PLAC TIA test, the technical and commercial merits and potential of diaDexus' diagnostic products; and the difficulty of developing pharmaceutical and diagnostic products, obtaining regulatory and other approvals and achieving market acceptance. Additional factors that could cause VaxGen's results to differ materially from those described in the forward-looking statements can be found in VaxGen's most recent annual reports on Form 10-K and subsequent quarterly reports on Form 10-Q and other filings with the Securities and Exchange Commission, which are filed with the SEC and available at the SEC's web site at www.sec.gov and which discussions also are incorporated herein by reference. The information set forth herein speaks only as of the date hereof, and diaDexus and VaxGen disclaim any intention and do not assume any obligation to update or revise any forward looking statement, whether as a result of new information, future events or otherwise. |

| Presentation Outline Company Overview Product Overview Reimbursement Business Strategy Summary |

| Company Overview diaDexus is a privately held diagnostics company, located in South San Francisco, CA Focused on the development and commercialization of patent-protected in vitro diagnostic products addressing unmet needs in cardiovascular disease Formed in 1997 as a joint venture between SmithKline Beecham (now GSK) and Incyte GSK granted an exclusive license to certain GSK diagnostic IP, including Lp-PLA2 The PLAC(r) Test for Lp-PLA2 is the Company's first commercialized product The only blood test cleared by the FDA to assess risk for coronary heart disease and stroke Strong Lp-PLA2 IP portfolio; 22 issued and 11 pending patents Lp-PLA2 is an inflammatory enzyme implicated in the formation of rupture-prone plaque Over 65 published papers support Lp-PLA2 as a novel cardiovascular risk marker Test utilization limited by lack of universal reimbursement GSK has developed an Lp-PLA2 inhibitor, darapladib Phase III studies in progress 2009 revenues (unaudited) = $11.9 million 2009 net loss (unaudited) = $5.3 million |
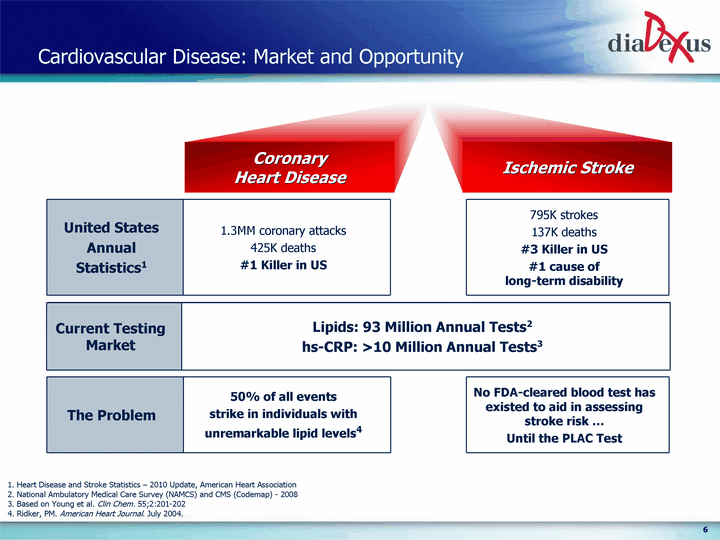
| Cardiovascular Disease: Market and Opportunity United States Annual Statistics1 1.3MM coronary attacks 425K deaths #1 Killer in US 795K strokes 137K deaths #3 Killer in US #1 cause of long-term disability Ischemic Stroke Coronary Heart Disease 1. Heart Disease and Stroke Statistics - 2010 Update, American Heart Association 2. National Ambulatory Medical Care Survey (NAMCS) and CMS (Codemap) - 2008 3. Based on Young et al. Clin Chem. 55;2:201-202 4. Ridker, PM. American Heart Journal. July 2004. The Problem 50% of all events strike in individuals with unremarkable lipid levels4 No FDA-cleared blood test has existed to aid in assessing stroke risk ... Until the PLAC Test Current Testing Market Lipids: 93 Million Annual Tests2 hs-CRP: >10 Million Annual Tests3 |

| Lp-PLA2 and The PLAC Test |

| Lp-PLA2 is an Inflammatory Enzyme Implicated in the Formation of Rupture-Prone Plaque Foam Cell Plaque Formation Macrophage SMC's Mildly Oxidized LDL Extensively Oxidized LDL Lumen Intima Media Adhesion Molecules Monocyte LDL Lyso- PtdCho Lp-PLA2 OxFA + Several lines of evidence suggest that oxidation of LDL plays a critical step in the development and progression of atherosclerosis Lp-PLA2 participates in the oxidative modification of LDL by hydrolyzing oxidized LDL generating lysophosphatidylcholine and oxidized free fatty acids, both of which are potent proinflammatory products that contribute to the formation of atherosclerotic plaques |
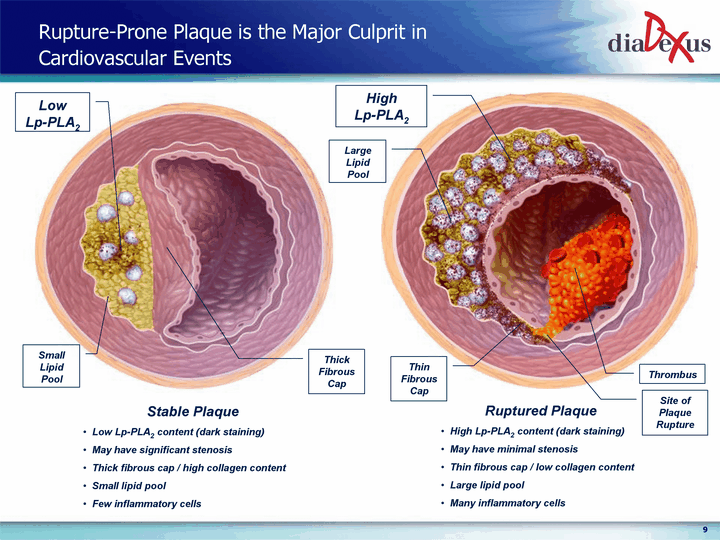
| Rupture-Prone Plaque is the Major Culprit in Cardiovascular Events Stable Plaque Low Lp-PLA2 content (dark staining) May have significant stenosis Thick fibrous cap / high collagen content Small lipid pool Few inflammatory cells Ruptured Plaque High Lp-PLA2 content (dark staining) May have minimal stenosis Thin fibrous cap / low collagen content Large lipid pool Many inflammatory cells |

| Staining Intensity for Lp-PLA2 Correlates with Progression to Plaque Rupture Early plaque with lipid pool Thick cap with small necrotic lipid core "stable plaque" Thin cap "rupture-prone" plaque Ruptured plaque with thrombus in lumen Reddish-brown staining shows presence of Lp-PLA2 Kolodgie et al., Arterioscler Thromb Vasc Biol. 2006; 26:2523-2529. |

| 65+ Studies and Abstracts Over 65 studies and abstracts support Lp-PLA2 as a novel cardiovascular risk marker providing new information, over and above traditional risk factors The PLAC Test's Substantial Body of Evidence |
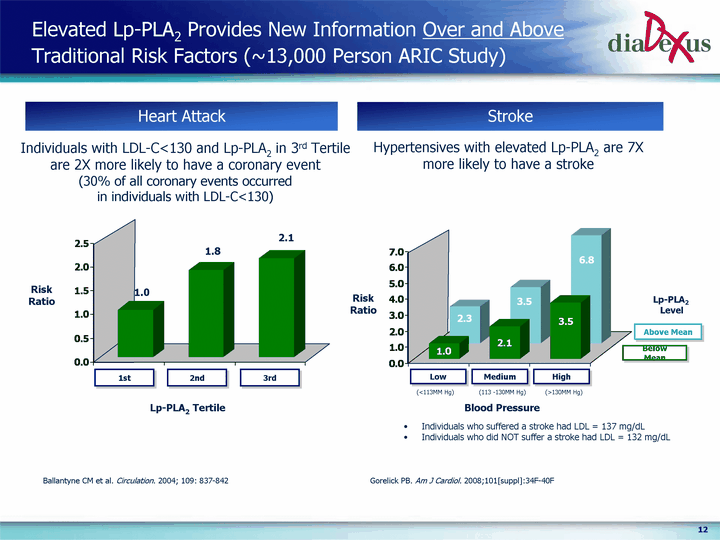
| Elevated Lp-PLA2 Provides New Information Over and Above Traditional Risk Factors (~13,000 Person ARIC Study) Gorelick PB. Am J Cardiol. 2008;101[suppl]:34F-40F 1st 2nd 3rd 1 1.83 2.08 Heart Attack Heart Attack Heart Attack Stroke Hypertensives with elevated Lp-PLA2 are 7X more likely to have a stroke Risk Ratio 1st 2nd 3rd 1.0 1.8 2.1 Lp-PLA2 Tertile Lp-PLA2 Level * < 113 mm Hg 113-130 mm Hg > 130 mm Hg Low (below median) 1 2.05 3.52 High (above median) 2.29 3.53 6.75 Low (<113MM Hg) Medium (113 -130MM Hg) High (>130MM Hg) Below Mean Above Mean Individuals with LDL-C<130 and Lp-PLA2 in 3rd Tertile are 2X more likely to have a coronary event (30% of all coronary events occurred in individuals with LDL-C<130) Blood Pressure Risk Ratio Individuals who suffered a stroke had LDL = 137 mg/dL Individuals who did NOT suffer a stroke had LDL = 132 mg/dL Ballantyne CM et al. Circulation. 2004; 109: 837-842 |

| hsCRP top hsCRP bottom hs-CRP bottom 1.2 1 hs-CRP top 4.2 1.35 hsCRP top hsCRP bottom Lp-PLA2 bottom 5.74 1 Lp-PLA2 top 11.4 5.37 Ballantyne et al, Circulation. 2004; 109: 837-842 and personal communication 4.2 1.2 1.4 1.0 Risk Ratio Heart Attack Stroke 11.4 5.8 5.5 1.0 Risk Ratio Ballantyne et al, Arch Intern Med. 2005; 165: 2479-2484 Lp-PLA2 top Lp-PLA2 bottom Lp-PLA2 top Lp-PLA2 bottom hs-CRP top hs-CRP bottom Lp-PLA2 When Used in Combination with hs-CRP Identifies Individuals at Even Higher Risk for Heart Attack and Stroke hs-CRP top hs-CRP bottom (n>12,000, 6-8 yr f/u, 194 ischemic strokes) (n>12,000, 6-8 yr f/u, 203 coronary events) Risk ratios compare top tertiles with bottom tertiles |

| Risk Assessment Drives LDL-C Goals per ATP III Guidelines1; Further Testing is Required to Find Hidden Cardiovascular Risk Low CV Risk 0-1 risk factors Moderate CV Risk 2+ risk factors and FRS ^ 20% Very High CV Risk2 LDL-C Goal < 160 mg/dL LDL-C Goal < 70 mg/dL LDL-C Goal < 130 mg/dL LDL-C Goal < 100 mg/dL Assess Treat Test Low Inflammatory Marker Testing High 1. National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143-3421. 2. Grundy SM, et al. Implications of recent clinical trails for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation. 2004;110(2):227-239. - Very High CV Risk defined as established CVD PLUS multiple risk factors, OR severe and poorly controlled risk factors, OR metabolic syndrome, OR ACS. ATP III guidelines proposed that emerging risk factors, including inflammatory markers, "can be taken into consideration according to clinical judgment as optional modifiers of therapy but they should be used only as an adjunct to adjust the estimate of absolute risk status obtained with the major risk factors" High CV Risk CHD, or CHD Risk Equivalent or FRS > 20% |
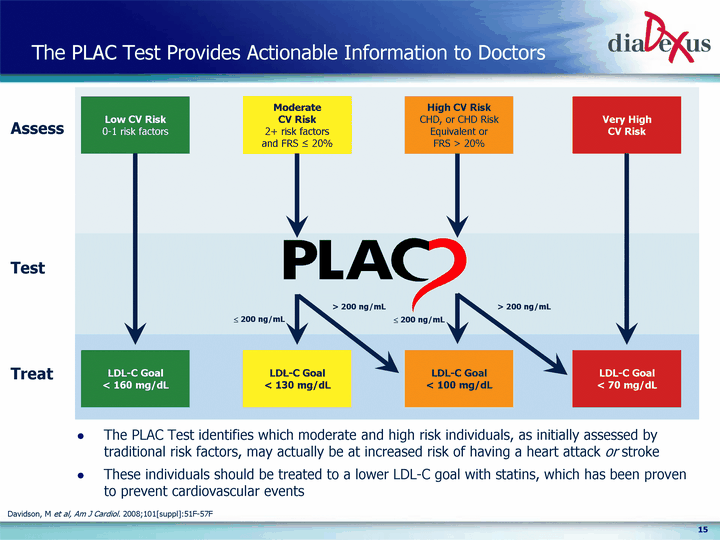
| The PLAC Test Provides Actionable Information to Doctors Low CV Risk 0-1 risk factors Moderate CV Risk 2+ risk factors and FRS ^ 20% High CV Risk CHD, or CHD Risk Equivalent or FRS > 20% Very High CV Risk LDL-C Goal < 160 mg/dL LDL-C Goal < 70 mg/dL LDL-C Goal < 130 mg/dL LDL-C Goal < 100 mg/dL ? 200 ng/mL ? 200 ng/mL > 200 ng/mL > 200 ng/mL The PLAC Test identifies which moderate and high risk individuals, as initially assessed by traditional risk factors, may actually be at increased risk of having a heart attack or stroke These individuals should be treated to a lower LDL-C goal with statins, which has been proven to prevent cardiovascular events Assess Treat Test Davidson, M et al, Am J Cardiol. 2008;101[suppl]:51F-57F |

| Consensus Recommendation for Lp-PLA2 Testing Published In American Journal of Cardiology Supplement (June 2008) Advances in the Detection of Rupture-Prone Plaque: The Role of Lp-PLA2 in Cardiovascular Risk Assessment Michael Davidson MD Clinical Prof., Head of Preventive Cardiology, Univ. of Chicago Mark Alberts MD Prof. of Neurology, Northwestern Univ. Jeff Anderson MD Prof. of Medicine (Cardiology), Univ. of Utah Marshall Corson MD Assoc. Prof. of Medicine, Univ. of Washington Philip Gorelick MD Chair Neurology, Univ. of Illinois Amir Lerman MD Prof. of Medicine (Cardiology), Mayo Clinic Peter Jones MD Prof. & Director Lipid Clinic, Baylor Univ. Joseph McConnell PhD Cardiovascular Lab Director, Mayo Howard Weintraub, MD Clinical Prof. Lipid, Treatment & Research Center, New York Univ. Publication of this supplement was supported by an unrestricted educational grant from diaDexus, Inc. |

| Reimbursement |

| Current Coverage Level Limits Adoption Early Test Utilization United CMS Cigna BCBS Aetna Physician Adopters Mid Late Coverage has to reach a "tipping-point" before test utilization shifts |

| diaDexus' Plan to Achieve Broader Reimbursement Coverage Clinical dossier summarizing science and clinical utility distributed to >100 private payers Request positive coverage policies through key opinion leaders Communicate the value of the test in face-to-face meetings with medical directors Drive test utilization to increase pressure on private payers Lobby for the inclusion of the PLAC Test into national guidelines While some payers have decided to cover the test based on the published epidemiological data, other payers require randomized clinical studies demonstrating that individuals with elevated Lp-PLA2 levels, when treated with statins (or other therapies) compared to placebo, have better clinical outcomes diaDexus is currently addressing this issue by testing banked samples from two prospective, statin clinical studies GSK is addressing the question of whether inhibiting Lp-PLA2 with darapladib improves cardiovascular outcomes in two large Phase III studies Broader Reimbursement Coverage May Drive Further Adoption of the PLAC Test |

| Coverage Progress for the PLAC Test Cumulative Covered Lives Published coverage policy in place, or coverage verified directly by diaDexus Covered Lives |

| Business Strategy |
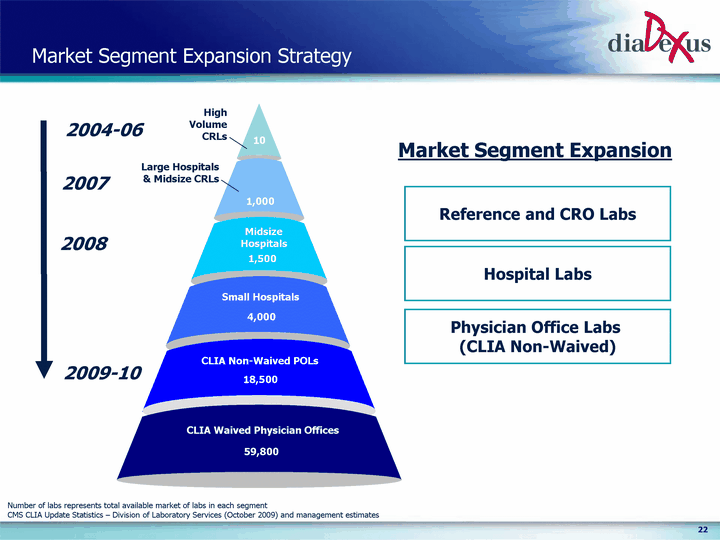
| Market Segment Expansion Strategy High Volume CRLs 10 1,000 Large Hospitals & Midsize CRLs 1,500 Midsize Hospitals 4,000 Small Hospitals 18,500 CLIA Non-Waived POLs 59,800 CLIA Waived Physician Offices Reference and CRO Labs Hospital Labs Physician Office Labs (CLIA Non-Waived) Market Segment Expansion 2004-06 2007 2008 2009-10 Number of labs represents total available market of labs in each segment CMS CLIA Update Statistics - Division of Laboratory Services (October 2009) and management estimates |
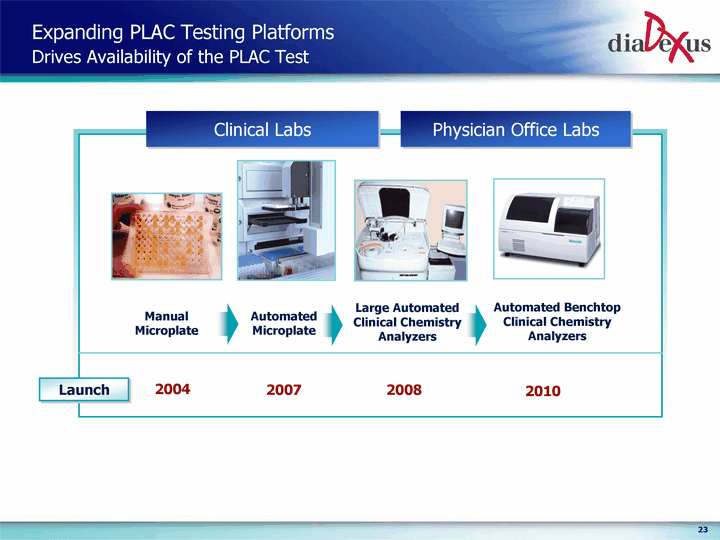
| Expanding PLAC Testing Platforms Drives Availability of the PLAC Test Physician Office Labs Clinical Labs Launch Manual Microplate 2004 Automated Microplate 2007 2008 2010 Large Automated Clinical Chemistry Analyzers Automated Benchtop Clinical Chemistry Analyzers |

| Two Test Formats: PLAC TIA & PLAC ELISA Two Test Formats: PLAC TIA & PLAC ELISA Two Test Formats: PLAC TIA & PLAC ELISA PLAC TIA Test for Automated Clinical Chemistry Analyzers PLAC ELISA Test The PLAC TIA Test format employs turbidimetric immunoassay (TIA) technology for the quantitative determination of Lp-PLA2 Used on automated clinical chemistry analyzers The PLAC ELISA Test format employs enzyme linked immunoassay (ELISA) technology for the quantitative determination of Lp-PLA2 Used on automated microplate systems |
| PLAC TIA Product Suspension and Status Product Suspension On May 10, 2010, diaDexus began notifying its customers that it had suspended commercialization of the PLAC TIA product due to heterophilic interference observed in a relatively small subset of samples tested The PLAC ELISA test format remains available for sale PLAC TIA customers can either switch to the PLAC ELISA format or send their Lp-PLA2 samples to labs performing the PLAC ELISA test In accordance with applicable regulatory requirements, diaDexus has informed FDA of its suspension of the PLAC TIA product and the company's associated instructions to customers Status diaDexus plans to submit a new 510(k) to FDA by the end of Q2 2010 seeking clearance of an enhanced PLAC TIA product that addresses the heterophilic interference observed in the current PLAC TIA product Year-to-date, PLAC TIA sales accounted for approximately two thirds of the company's product revenue, however the majority of high volume PLAC TIA testing labs have already converted to the PLAC ELISA format, enabling the company to retain ^75% of the PLAC TIA revenue Separately, a number of labs who cannot test by ELISA method have decided to send their Lp-PLA2 samples to labs performing PLAC ELISA testing |

| Expanding Reach through Sales Force Partnership 8 Physician Sales Force 2 Medical Science Liaisons Physicians Clinical/CRO Labs 4 Lab Service Reps Physician Office Labs (POL) U.S. Lab Distribution: Fisher HealthCare INOVA Diagnostics International Markets 1 International Director U.S. POL Distribution: PSS World Medical Int'l Distribution: 22 Distributors/30 Countries Partners Fisher HealthCare distributes the PLAC immunoturbidimetric product and INOVA distributes the PLAC ELISA product |

| GSK's Lp-PLA2 Drug Could Increase Lp-PLA2 Testing GSK has developed an Lp-PLA2 inhibitor, darapladib GSK commenced Phase III clinical development in Dec 2008 First Phase III study (STABILITY, n=15,500 patients) passed first interim safety check in Sep 2009 Second Phase III study (SOLID, n=11,500 patients) commenced in Dec 2009 Ongoing Phase III studies may drive awareness of Lp-PLA2 |

| Summary Cardiovascular disease is the leading cause of death in the U.S. Traditional risk factors do not adequately identify individuals at high risk The PLAC Test identifies individuals who may be at increased risk of having a heart attack or stroke, over and above traditional risk factors These individuals should be treated more aggressively, including the use of statins to lower LDL-C, which has been proven to prevent cardiovascular events The PLAC Test is the only FDA-cleared blood test for assessing risk for heart attack and ischemic stroke Backed by significant clinical validation, including >65 published papers PLAC ELISA test format currently available for sale New 510(k) for PLAC TIA test format planned for submission by end of Q2 2010 We believe adoption of the PLAC Test is currently constrained by lack of universal reimbursement coverage We expect growth will be driven by: Broadening reimbursement coverage Sales and marketing investment Product portfolio expansion Prospective launch of GSK's Lp-PLA2 inhibitor |
