Attached files
| file | filename |
|---|---|
| 8-K - 8-K - AIkido Pharma Inc. | a10-8768_18k.htm |
Exhibit 99.1
|
|
Spherix Incorporated Overview Presentation April 27, 2010 Robert Lodder, Ph.D., President Robert Clayton, Chief Financial Officer Leisa Dennehy, MBA, Corporate and Commercial Development Advisor |
|
|
Forward-Looking Statement This presentation contains forward-looking statements that involve risks and uncertainties, including those described in the Company’s Annual Report for the year ended December 31, 2009 filed on Form 10-K. |
|
|
Today’s Objectives Provide background on Spherix and tagatose development Explore the Company's short-term and long-term options for funding sources and partnering strategies Learn more about your Company’s experience and service capabilities |
|
|
Agenda Spherix Company Overview Diabetes Market and Unmet Needs Tagatose Rationale for Use in Diabetes Tagatose Clinical Development Plan Status of “Market Readiness” Activities Company Financials |
|
|
Spherix Highlights Spherix is a public company (NASDAQ: SPEX; since 1967) Core expertise began as chiral chemistry of carbohydrates - applied to NASA missions, food uses and pharmaceuticals Company has two subsidiaries: Biospherics: Pharmaceutical development division Spherix Consulting: Scientific consulting on food and drug approvals for US and overseas clients Biospherics is focused on development of tagatose and related pipeline agents for the treatment of diabetes and metabolic disorders |
|
|
Spherix Pharmaceutical Development Lead product, D-tagatose, currently in global phase 3 clinical trial as monotherapy treatment of “mild” Type 2 diabetes (HbA1c between 6.6 -9.0%) D-tagatose patented as a pharmaceutical agent for glycemic control in diabetes Designation of GRAS (Generally Recognized as Safe) by US FDA; evaluated for safety by WHO/JECFA (ADI not specified) originally being developed as a sweetening agent for foods and beverages when medical benefits were observed Spherix primarily self-funded development of D-tagatose Corporate objective to sell or license commercial rights to tagatose as an Rx therapy in US and Europe |
|
|
Experienced Senior Management Team Claire L. Kruger, Ph.D., DABT, Chief Executive Officer Toxicologist with 20 years of consulting experience; primary area of expertise is in pharmaceuticals, consumer products and foods, where she provides scientific, regulatory, and strategic support to clients in both the US and international regulatory arenas Robert A. Lodder, Ph.D., President Founder of InfraReDx, Inc. and Prescient Medical, Inc., Professor of Pharmaceutical Sciences at the College of Pharmacy, University of Kentucky Medical Center Robert L. Clayton, CPA, Chief Financial Officer 16 years of experience in finance and accounting, including 5 years in public accounting; previously served as Director of Finance and Controller for Spherix Leisa Dennehy, M.B.A., Commercial and Corporate Development Advisor >20 years in pharma commercial roles; previously with PandG, Glaxo/GW, IntraBiotics, Peninsula, Protez, Talecris. Experience in new product market planning, product launches , asset valuation, and pharma partnering Randy Brown, B.S., Chief of Operations 20 years in the pharmaceutical industry; previously with Pfizer, Eli Lilly and Genentech; experience in managing global trials in roughly 30 different countries including new emerging areas such as India Ram Nimmagudda, Ph.D., Director of New Business Development 15 years of experience in business development, nutrition and nutraceutical industries, with particular expertise in novel food ingredients; previous positions of Director of New Business Development for South Asia at DSM Functional Foods, Director and Sr. Principal Technologist, External Technologies at Wm. Wrigley Jr. Co. and Vice President of Technology Growth at Char. Hansen, Inc. |
|
|
Spherix Company Overview Diabetes Market and Unmet Needs Tagatose Rationale for Use in Diabetes Tagatose Clinical Development Plan Status of “Market Readiness” Activities Company Financials Agenda |
|
|
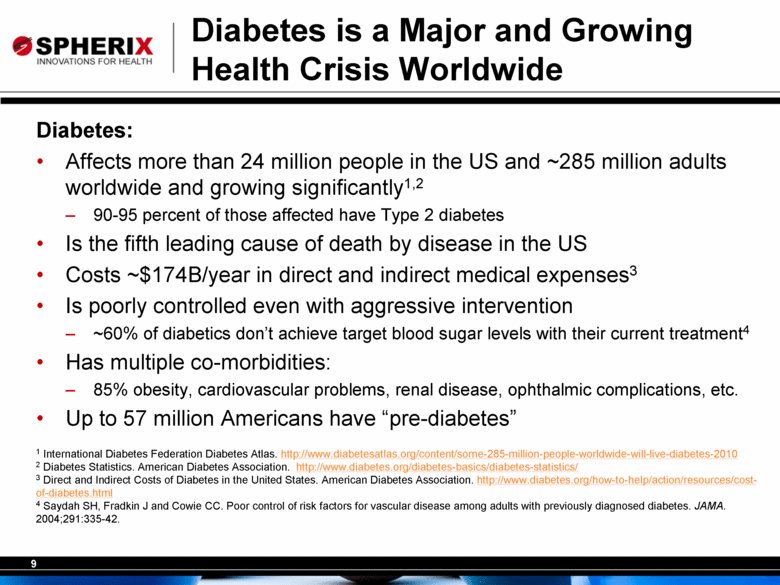
Diabetes is a Major and Growing Health Crisis Worldwide Diabetes: Affects more than 24 million people in the US and ~285 million adults worldwide and growing significantly1,2 90-95 percent of those affected have Type 2 diabetes Is the fifth leading cause of death by disease in the US Costs ~$174B/year in direct and indirect medical expenses3 Is poorly controlled even with aggressive intervention ~60% of diabetics don’t achieve target blood sugar levels with their current treatment4 Has multiple co-morbidities: 85% obesity, cardiovascular problems, renal disease, ophthalmic complications, etc. Up to 57 million Americans have “pre-diabetes” 1 International Diabetes Federation Diabetes Atlas. http://www.diabetesatlas.org/content/some-285-million-people-worldwide-will-live-diabetes-2010 2 Diabetes Statistics. American Diabetes Association. http://www.diabetes.org/diabetes-basics/diabetes-statistics/ 3 Direct and Indirect Costs of Diabetes in the United States. American Diabetes Association. http://www.diabetes.org/how-to-help/action/resources/cost-of-diabetes.html 4 Saydah SH, Fradkin J and Cowie CC. Poor control of risk factors for vascular disease among adults with previously diagnosed diabetes. JAMA. 2004;291:335-42. |
|
|
Complex and Aggressive Treatment Pathway Suggests High Medical Need AACE/ACE Consensus Panel for Type 2 Diabetes Endocrine Practice 2009;25:540-559 |
|
|
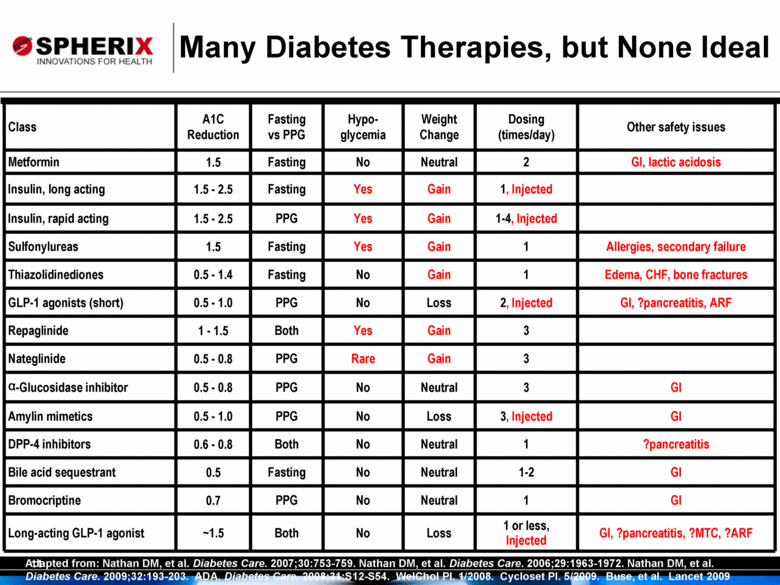
Class A1C Reduction Fasting vs PPG Hypo- glycemia Weight Change Dosing (times/day) Other safety issues Metformin 1.5 Fasting No Neutral 2 GI, lactic acidosis Insulin, long acting 1.5 - 2.5 Fasting Yes Gain 1, Injected Insulin, rapid acting 1.5 - 2.5 PPG Yes Gain 1-4, Injected Sulfonylureas 1.5 Fasting Yes Gain 1 Allergies, secondary failure Thiazolidinediones 0.5 - 1.4 Fasting No Gain 1 Edema, CHF, bone fractures GLP-1 agonists (short) 0.5 - 1.0 PPG No Loss 2, Injected GI, ?pancreatitis, ARF Repaglinide 1 - 1.5 Both Yes Gain 3 Nateglinide 0.5 - 0.8 PPG Rare Gain 3 a-Glucosidase inhibitor 0.5 - 0.8 PPG No Neutral 3 GI Amylin mimetics 0.5 - 1.0 PPG No Loss 3, Injected GI DPP-4 inhibitors 0.6 - 0.8 Both No Neutral 1 ?pancreatitis Bile acid sequestrant 0.5 Fasting No Neutral 1-2 GI Bromocriptine 0.7 PPG No Neutral 1 GI Long-acting GLP-1 agonist ~1.5 Both No Loss 1 or less, Injected GI, ?pancreatitis, ?MTC, ?ARF Adapted from: Nathan DM, et al. Diabetes Care. 2007;30:753-759. Nathan DM, et al. Diabetes Care. 2006;29:1963-1972. Nathan DM, et al. Diabetes Care. 2009;32:193-203. ADA. Diabetes Care. 2008;31:S12-S54. WelChol PI. 1/2008. Cycloset PI. 5/2009. Buse, et al. Lancet 2009 Many Diabetes Therapies, but None Ideal |
|
|
Significant Unmet Market Need Demands New Therapeutic Options Managing disease progression from ‘pre-diabetes’ to diabetes Managing disease progression from Type 2 to Type 1 Beta-cell sparing approaches Greater clinical efficacy from pharmacotherapy Fewer than ½ of patients are well controlled (NHANES study) Better glycemic control, especially post-prandial hyper- and hypoglycemia Improved long-term safety and risk/benefit Increased patient compliance and adherence to therapy |
|
|
Spherix Company Overview Diabetes Market and Unmet Needs Tagatose Rationale for Use in Diabetes Tagatose Clinical Development Plan Status of “Market Readiness” Activities Company Financials Agenda |
|
|
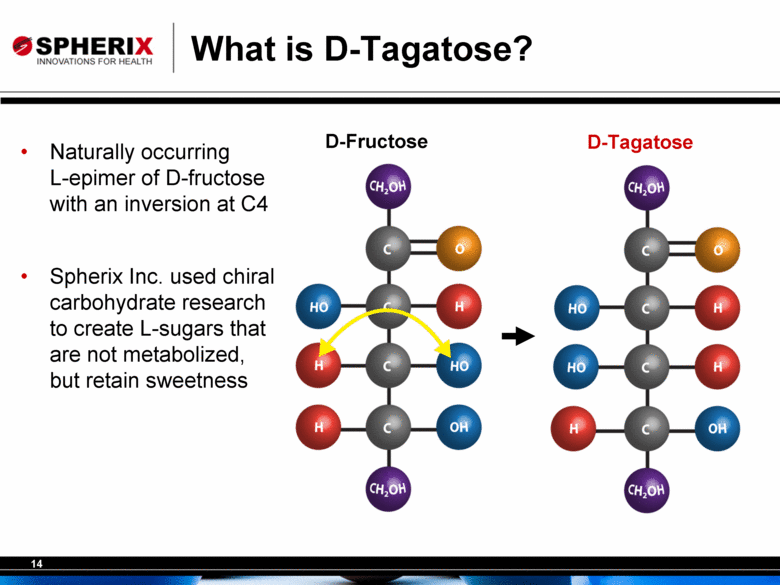
What is D-Tagatose? D-Tagatose D-Fructose Naturally occurring L-epimer of D-fructose with an inversion at C4 Spherix Inc. used chiral carbohydrate research to create L-sugars that are not metabolized, but retain sweetness |
|
|
Overview of Tagatose Originally developed as reduced calorie sugar substitute 92% as sweet as sucrose with only 38% of the calories Manufacturing economics not efficient for food industry D-tagatose was approved as safe for use in foods Approved for use as a low calorie sweetener in foods Approved in the US as an excipient in non-chronic OTC drugs (throat lozenges, cough syrup), toothpaste, mouthwash and cosmetics US FDA (GRAS) since 2001 WHO JECFA since June 2004 EU since 2006 Beneficial health effects discovered during pre-clinical studies Currently being evaluated in Phase 2 and Phase 3 clinical trials as a new diabetic treatment |
|
|
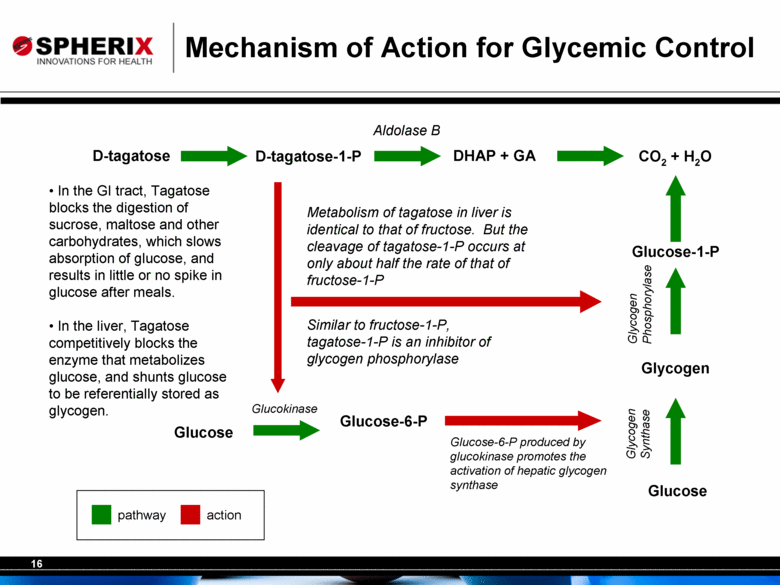
Mechanism of Action for Glycemic Control D-tagatose D-tagatose-1-P DHAP + GA CO2 + H2O Glucose Glucose-6-P Glucose Glycogen Aldolase B Glucokinase Metabolism of tagatose in liver is identical to that of fructose. But the cleavage of tagatose-1-P occurs at only about half the rate of that of fructose-1-P Glycogen Synthase Glucose-6-P produced by glucokinase promotes the activation of hepatic glycogen synthase Glucose-1-P Glycogen Phosphorylase Similar to fructose-1-P, tagatose-1-P is an inhibitor of glycogen phosphorylase pathway action In the GI tract, Tagatose blocks the digestion of sucrose, maltose and other carbohydrates, which slows absorption of glucose, and results in little or no spike in glucose after meals. In the liver, Tagatose competitively blocks the enzyme that metabolizes glucose, and shunts glucose to be referentially stored as glycogen. |
|
|
In other words. D-Tagatose depresses elevations of blood sugar levels by increasing glycogen synthesis while decreasing glycogen utilization, resulting in effective control of blood sugar and modulation of HbA1c Blood sugar Glycogen synthesis Glycogen utilization = Controlled Blood Sugar and HbA1c Mechanism of Action for Glycemic Control |
|
|
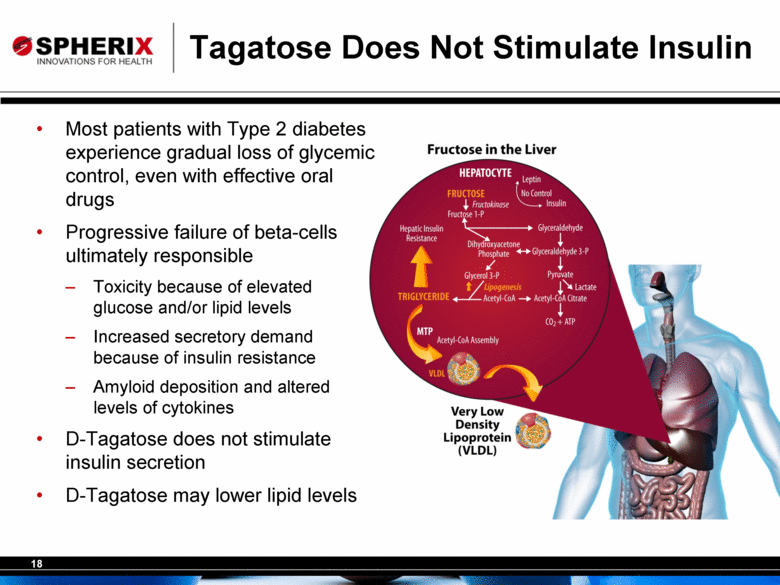
Tagatose Does Not Stimulate Insulin Most patients with Type 2 diabetes experience gradual loss of glycemic control, even with effective oral drugs Progressive failure of beta-cells ultimately responsible Toxicity because of elevated glucose and/or lipid levels Increased secretory demand because of insulin resistance Amyloid deposition and altered levels of cytokines D-Tagatose does not stimulate insulin secretion D-Tagatose may lower lipid levels |
|
|
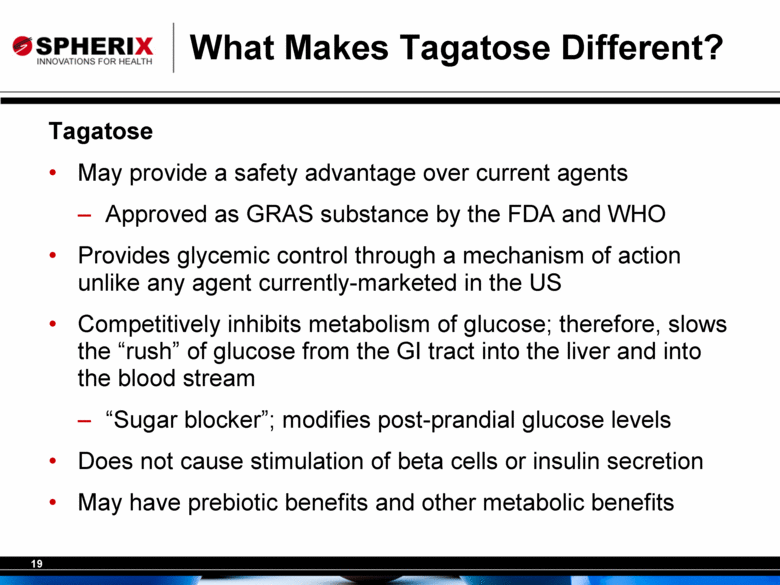
What Makes Tagatose Different? Tagatose May provide a safety advantage over current agents Approved as GRAS substance by the FDA and WHO Provides glycemic control through a mechanism of action unlike any agent currently-marketed in the US Competitively inhibits metabolism of glucose; therefore, slows the “rush” of glucose from the GI tract into the liver and into the blood stream “Sugar blocker”; modifies post-prandial glucose levels Does not cause stimulation of beta cells or insulin secretion May have prebiotic benefits and other metabolic benefits |
|
|
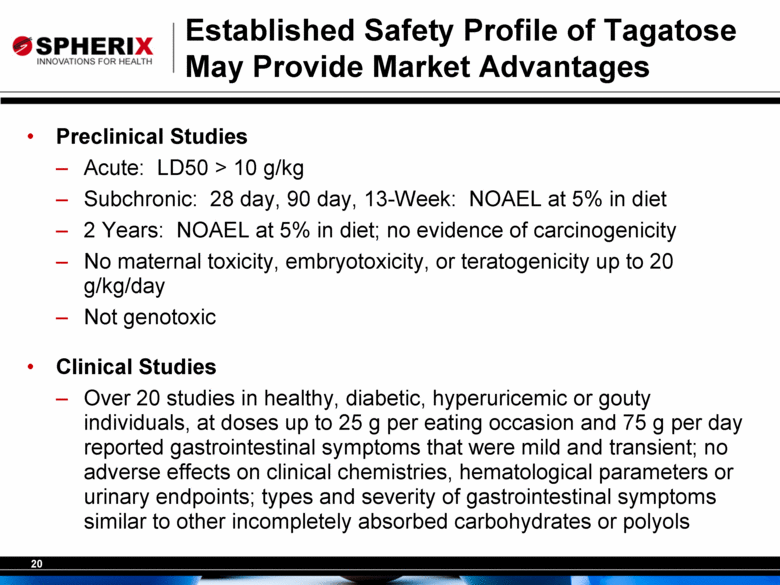
Established Safety Profile of Tagatose May Provide Market Advantages Preclinical Studies Acute: LD50 > 10 g/kg Subchronic: 28 day, 90 day, 13-Week: NOAEL at 5% in diet 2 Years: NOAEL at 5% in diet; no evidence of carcinogenicity No maternal toxicity, embryotoxicity, or teratogenicity up to 20 g/kg/day Not genotoxic Clinical Studies Over 20 studies in healthy, diabetic, hyperuricemic or gouty individuals, at doses up to 25 g per eating occasion and 75 g per day reported gastrointestinal symptoms that were mild and transient; no adverse effects on clinical chemistries, hematological parameters or urinary endpoints; types and severity of gastrointestinal symptoms similar to other incompletely absorbed carbohydrates or polyols |
|
|
Spherix Company Overview Diabetes Market and Unmet Needs Tagatose Rationale for Use in Diabetes Tagatose Clinical Development Plan Status of “Market Readiness” Activities Company Financials Agenda |
|
|
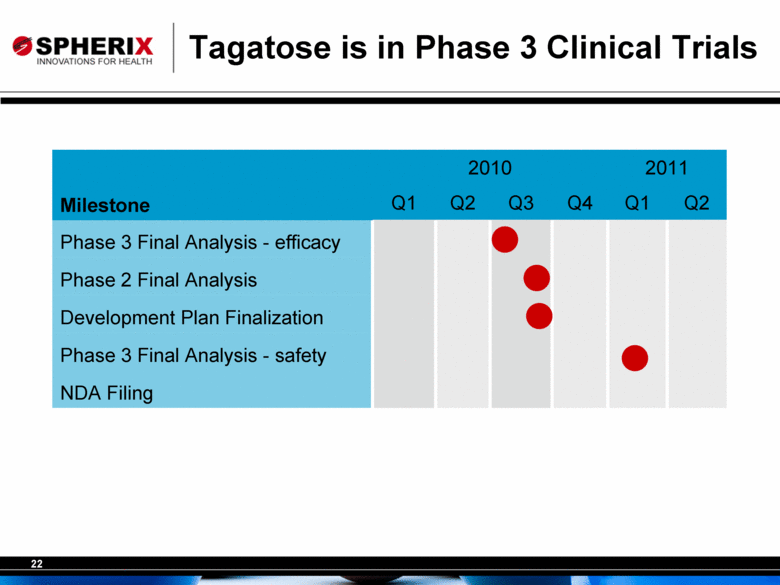
Tagatose is in Phase 3 Clinical Trials 2010 2011 Milestone Q1 Q2 Q3 Q4 Q1 Q2 Phase 3 Final Analysis - efficacy Phase 2 Final Analysis Development Plan Finalization Phase 3 Final Analysis - safety NDA Filing |
|
|
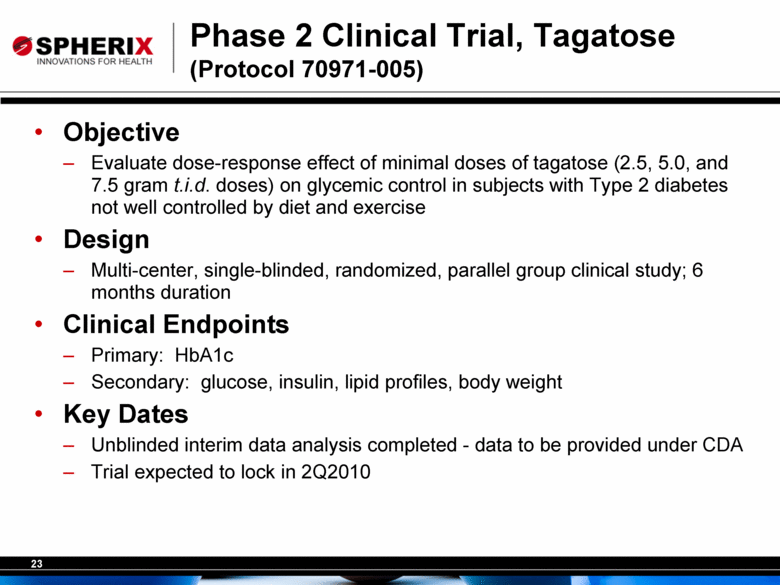
Phase 2 Clinical Trial, Tagatose (Protocol 70971-005) Objective Evaluate dose-response effect of minimal doses of tagatose (2.5, 5.0, and 7.5 gram t.i.d. doses) on glycemic control in subjects with Type 2 diabetes not well controlled by diet and exercise Design Multi-center, single-blinded, randomized, parallel group clinical study; 6 months duration Clinical Endpoints Primary: HbA1c Secondary: glucose, insulin, lipid profiles, body weight Key Dates Unblinded interim data analysis completed - data to be provided under CDA Trial expected to lock in 2Q2010 |
|
|
Phase 3 Clinical Trial, Tagatose NEET Study (Protocol 70971-004) Objective Evaluate effect of 15-gram, t.i.d dose of D-tagatose on glycemic control in subjects with Type 2 diabetes not well controlled by diet and exercise Design Multi-center, double blind, randomized, parallel group study 443 patients randomized 30 sites in US; 32 sites in India Powered to detect 0.5 percentage point change in HbA1c Clinical Endpoints Primary: HbA1c Secondary: glucose, insulin, lipid profiles, body weight Key Dates Blinded, interim data analysis done 4Q09; no statistical penalty Efficacy portion of trial expected to close by 2Q2010 NDA could be filed as early as mid-2011 |
|
|
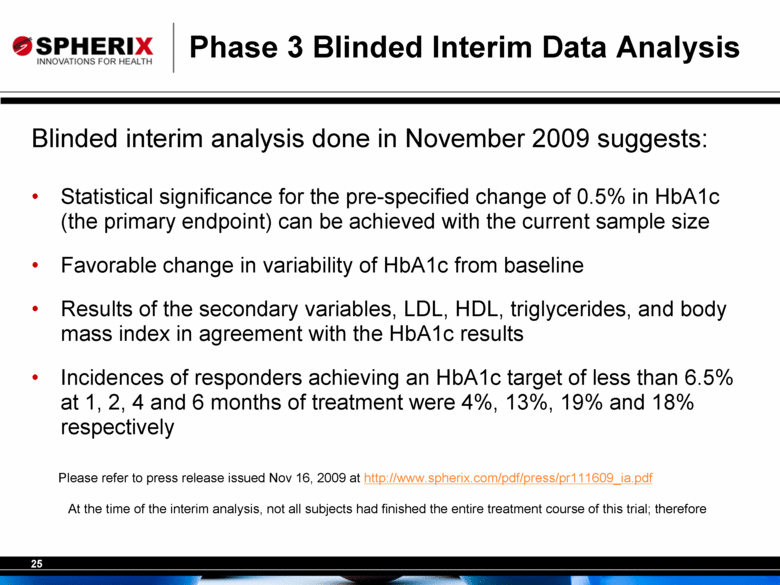
Phase 3 Blinded Interim Data Analysis Blinded interim analysis done in November 2009 suggests: Statistical significance for the pre-specified change of 0.5% in HbA1c (the primary endpoint) can be achieved with the current sample size Favorable change in variability of HbA1c from baseline Results of the secondary variables, LDL, HDL, triglycerides, and body mass index in agreement with the HbA1c results Incidences of responders achieving an HbA1c target of less than 6.5% at 1, 2, 4 and 6 months of treatment were 4%, 13%, 19% and 18% respectively Please refer to press release issued Nov 16, 2009 at http://www.spherix.com/pdf/press/pr111609_ia.pdf At the time of the interim analysis, not all subjects had finished the entire treatment course of this trial; therefore the number of responders was different for different months of therapy. |
|
|
Spherix Company Overview Diabetes Market and Unmet Needs Tagatose Rationale for Use in Diabetes Tagatose Clinical Development Plan Status of “Market Readiness” Activities Company Financials Agenda |
|
|
Manufacturing at Full Scale with Commercial Capacity Secured Inalco S.p.A. of Italy DMF 22715 and LoA One metric ton batches In use in phase 3 trial Supply agreement signed for full commercial scale quantities |
|
|
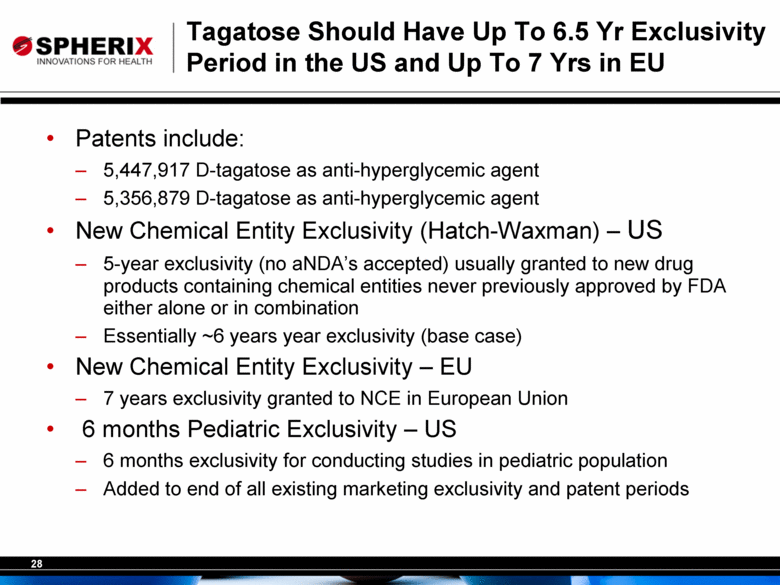
Tagatose Should Have Up To 6.5 Yr Exclusivity Period in the US and Up To 7 Yrs in EU Patents include: 5,447,917 D-tagatose as anti-hyperglycemic agent 5,356,879 D-tagatose as anti-hyperglycemic agent New Chemical Entity Exclusivity (Hatch-Waxman) – US 5-year exclusivity (no aNDA’s accepted) usually granted to new drug products containing chemical entities never previously approved by FDA either alone or in combination Essentially ~6 years year exclusivity (base case) New Chemical Entity Exclusivity – EU 7 years exclusivity granted to NCE in European Union 6 months Pediatric Exclusivity – US 6 months exclusivity for conducting studies in pediatric population Added to end of all existing marketing exclusivity and patent periods |
|
|
Market Planning for Tagatose Has Been Done with Pharma Partner in Mind Market Research Exploratory and product profile research: 2007/2008 Product profile and key message testing: 2010 Product Positioning Early (“mild”) diabetes represents large market segment Product Valuation Forecast to be developed from market input Medical Advisory Boards Two advisory board meetings held: October 2009 and April 2010 Trade name and other brand development activities Minimal activity done, assuming pharma partner prefers to define brand Not yet on critical path for launch Plans being developed to pursue pre-launch activities if decision is made to go without a partner |
|
|
Spherix Company Overview Diabetes Market and Unmet Needs Tagatose Rationale for Use in Diabetes Tagatose Clinical Development Plan Status of “Market Readiness” Activities Company Financials Agenda |
|
|
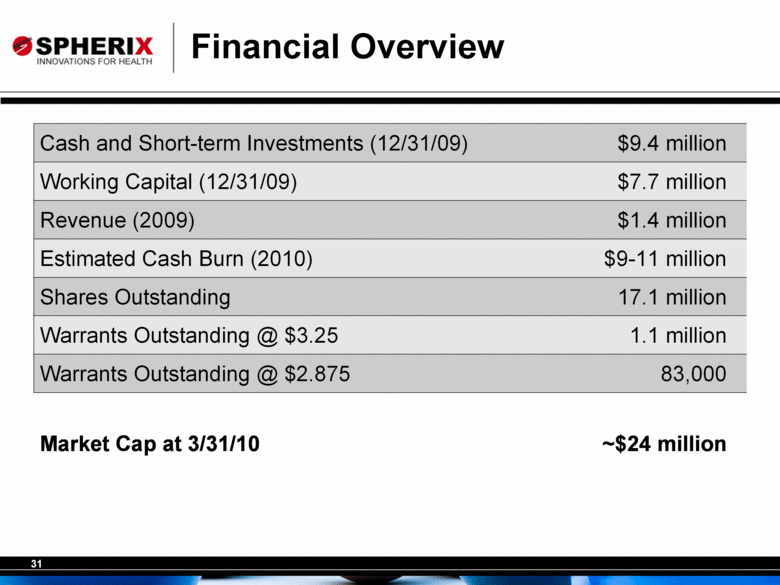
Financial Overview Cash and Short-term Investments (12/31/09) $9.4 million Working Capital (12/31/09) $7.7 million Revenue (2009) $1.4 million Estimated Cash Burn (2010) $9-11 million Shares Outstanding 17.1 million Warrants Outstanding @ $3.25 1.1 million Warrants Outstanding @ $2.875 83,000 Market Cap at 3/31/10 ~$24 million |
|
|
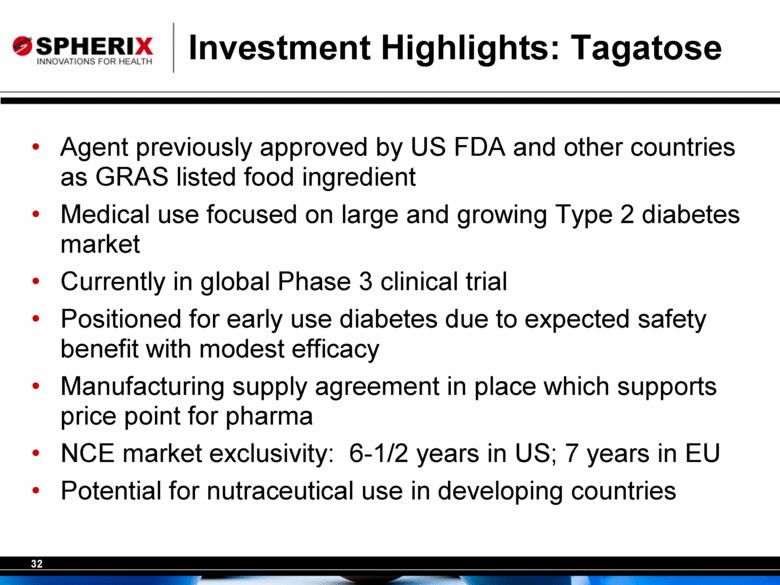
Investment Highlights: Tagatose Agent previously approved by US FDA and other countries as GRAS listed food ingredient Medical use focused on large and growing Type 2 diabetes market Currently in global Phase 3 clinical trial Positioned for early use diabetes due to expected safety benefit with modest efficacy Manufacturing supply agreement in place which supports price point for pharma NCE market exclusivity: 6-1/2 years in US; 7 years in EU Potential for nutraceutical use in developing countries |
|
|
Robert Lodder, Ph.D. Leisa Dennehy President Commercial and Corporate Development rlodder@spherix.com ldennehy@spherix.com Phone: 301-476-0705 Phone: 301-897-0617 Robert Clayton Spherix Incorporated Chief Financial Officer www.spherix.com rclayton@spherix.com NASDAQ: SPEX Phone: 301-897-0616 6430 Rockledge Dr Westmoreland Building #503 Bethesda, MA 20817 Phone: 301-897-2540 Toll-free: 1-866-SPHERIX For More Information, Please Contact: |