Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Vicor Technologies, Inc. | y03325e8vk.htm |
Exhibit 99.1
| Vicor Technologies, Inc. A Medical Diagnostic Company Dedicated to the Commercialization Of Breakthrough Risk Stratification Technology |

| Safe Harbor Statement This presentation contains forward-looking statements. Forward- looking statements are often signaled by forms of words such as should, could, will, might, plan, projection, forecast, expect, guidance, potential and developing. Actual results could vary materially from those expected due to a variety of risk factors, including whether the Company will continue as a going concern and successfully raise proceeds from financing activities sufficient to fund operations and clinical trials, the uncertainty of successful completion of any such clinical trial, the fact that the Company has not succeeded in commercializing any products and other factors identified in our Annual Report on Form 10-K for the fiscal year ended December 31, 2009 and subsequent filings with the Securities and Exchange Commission. The Company undertakes no obligation to update the results of these forward- looking statements to reflect events or circumstances after today or to reflect the occurrence of unanticipated events. |
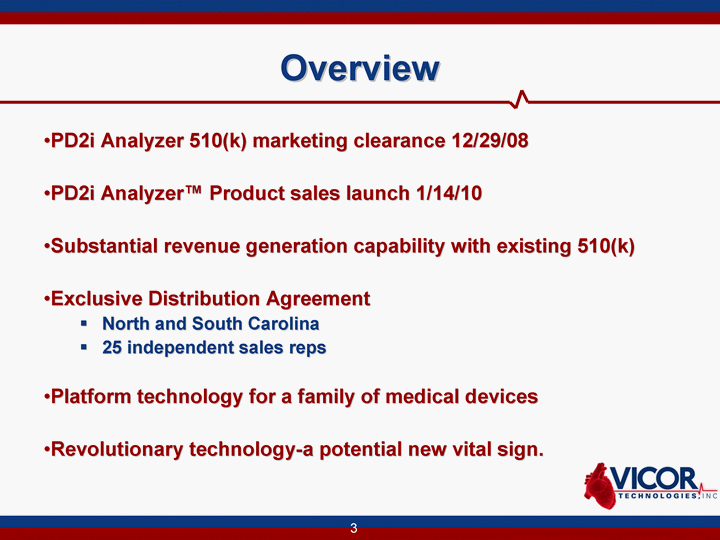
| Overview PD2i Analyzer 510(k) marketing clearance 12/29/08 PD2i Analyzer(tm) Product sales launch 1/14/10 Substantial revenue generation capability with existing 510(k) Exclusive Distribution Agreement North and South Carolina 25 independent sales reps Platform technology for a family of medical devices Revolutionary technology-a potential new vital sign. |
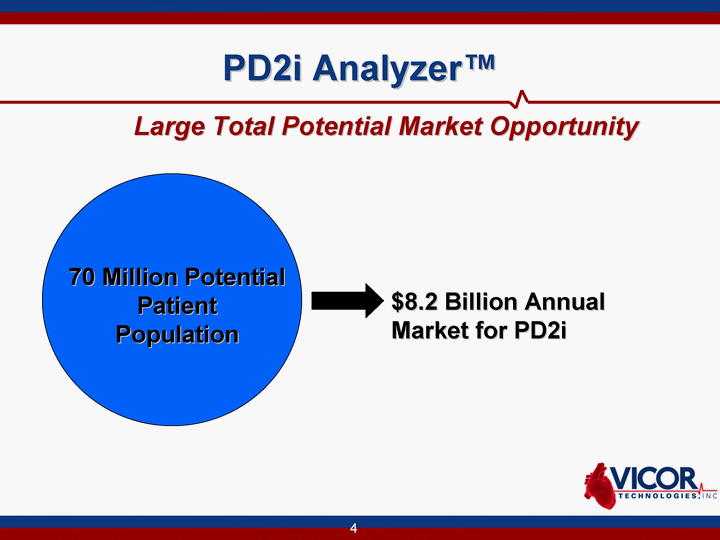
| PD2i Analyzer(tm) Large Total Potential Market Opportunity 70 Million Potential Patient Population $8.2 Billion Annual Market for PD2i |

| Overview PD2i Analyzer(tm) The Vicor PD2i Analyzer(tm) is intended to display and analyze electrocardiographic information and to measure heart rate variability (HRV) in patients at rest and in response to controlled exercise and paced respiration. These and other measurements are not intended for any specific clinical diagnosis. The clinical significance of HRV and other parameters must be determined by the physician. |

| INTERNAL MEDICINE 278.01 Morbid Obesity 279.3 Unspecified Immunity Deficiency 296.80 Bipolar Disease 311 Depression 300.00 Anxiety 307.4** Sleep Disorders 530.11 GERD 536.3 Gastroparesis 564.1 Irritable Bowel Syndrome 729.1 Fibromyalgia 309.81 Post-Traumatic Stress Syndrome 782.3 Edema ICD 9 CODING CARDIOLOGY 401.0 - 405.99 Hypertension 412 Post - MI 413.9 Angina 440.9 Atherosclerosis 424.0 Mitral Valve Prolapse Syndrome 425.4 Cardiomyopathy 427.9 Cardiac Dysrhythmias 428.0 Congestive Heart Failure ENDOCRINOLOGY 244.8 Acquired Hypothyroidism 246.9 Thyroid Disorders 256.31 Premature Menopausal Symptoms 627.2 Menopausal Syndromes NEPHROLOGY 402.90 Hypertensive Renal Disease 586 Renal Failure DIABETES 250 - 250.8** NEUROLOGY 307.01 Tension Headache 314.01 ADD/ADHD 332.0 Parkinsonism 333.0 Shy-Drager Syndrom (Orthostatic Hypotension with Multisystem Degeneration) 337.00 Idiopathic Peripheral Autonomic Neuropathy 358.1 Myasthenic Syndrome (Eaton-Lambert) 337.2 Chronic Regional Pain Syndrome or RSD 458.0 Orthostatic Hypotension 340 Multiple Sclerosis 458.1 Chronic Hypotension 346.0 - 346.9** Migraine 742.8 Other Specified Congenital Anomalies of the Nervous System 356.4 Idiopathic Progressive Polyneuropathy 780.2 Syncope and Collapse 356.8 Other Specified Idiopathic Peripheral Neuropathy 780.4 Dizziness, Light-headedness and Vertigo 356.9 Unspecified Peripheral Idiopathic Neuropathy 780.71 Chronic Fatigue Syndrome 357.2 Polyneuropathy in Diiabetes 780.0 Headache 357.4 Polyneuropathy in Other Disease Classified Elsewhere 785.0 Tachycardia (Postural) PULMONOLOGY 780.5 - 780.59 Sleep Disturbances 493.9 - 493.92** Asthma 493.2** COPD (Asthma with Chronic Obstructive Pulmonary Disease) This example was put together to help aid you on proper reimbursement procedures. If you have any questions regarding reimbursement, please feel free to contact David Fater toll-free at 800-998-9964 |
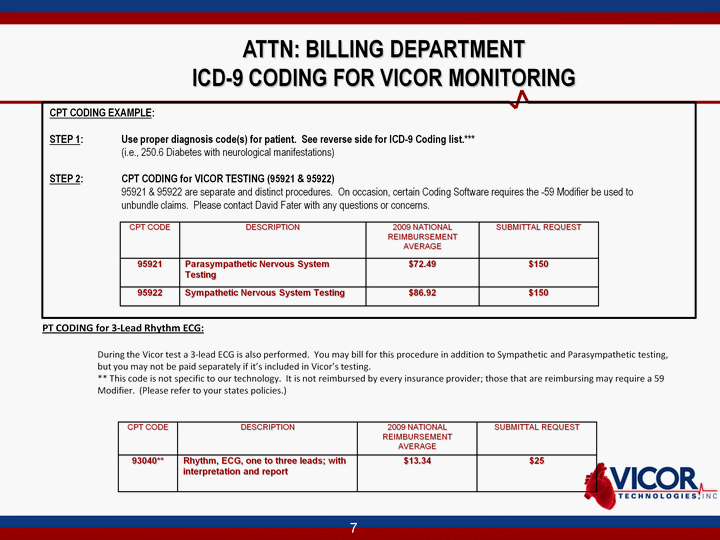
| ATTN: BILLING DEPARTMENT ICD-9 CODING FOR VICOR MONITORING CPT CODING EXAMPLE: STEP 1: Use proper diagnosis code(s) for patient. See reverse side for ICD-9 Coding list.*** (i.e., 250.6 Diabetes with neurological manifestations) STEP 2: CPT CODING for VICOR TESTING (95921 & 95922) 95921 & 95922 are separate and distinct procedures. On occasion, certain Coding Software requires the -59 Modifier be used to unbundle claims. Please contact David Fater with any questions or concerns. CPT CODE DESCRIPTION 2009 NATIONAL REIMBURSEMENT AVERAGE SUBMITTAL REQUEST 95921 Parasympathetic Nervous System Testing $72.49 $150 95922 Sympathetic Nervous System Testing $86.92 $150 PT CODING for 3-Lead Rhythm ECG: During the Vicor test a 3-lead ECG is also performed. You may bill for this procedure in addition to Sympathetic and Parasympathetic testing, but you may not be paid separately if it's included in Vicor's testing. ** This code is not specific to our technology. It is not reimbursed by every insurance provider; those that are reimbursing may require a 59 Modifier. (Please refer to your states policies.) CPT CODE DESCRIPTION 2009 NATIONAL REIMBURSEMENT AVERAGE SUBMITTAL REQUEST 93040** Rhythm, ECG, one to three leads; with interpretation and report $13.34 $25 |
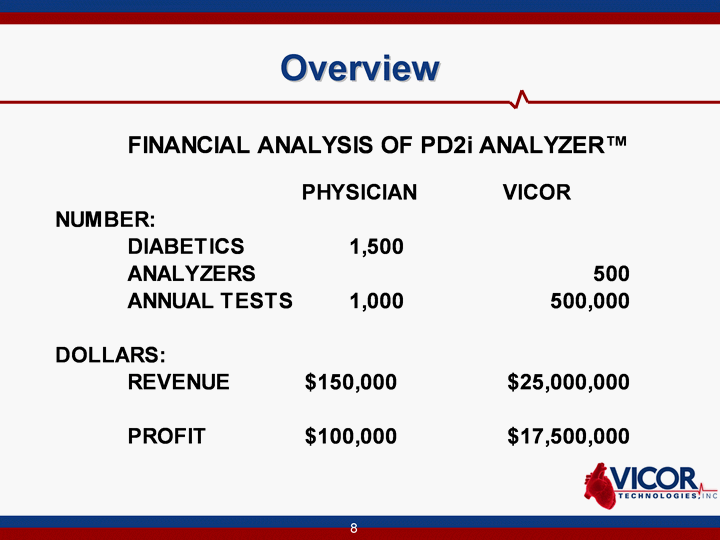
| Overview |
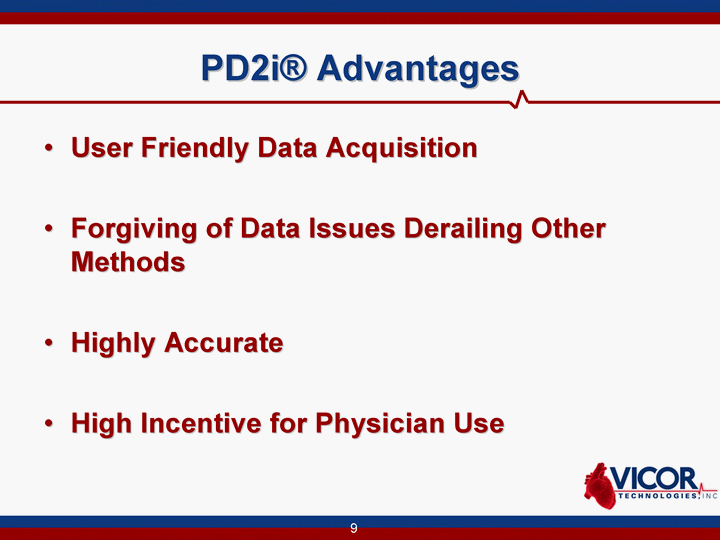
| PD2i(r) Advantages User Friendly Data Acquisition Forgiving of Data Issues Derailing Other Methods Highly Accurate High Incentive for Physician Use |
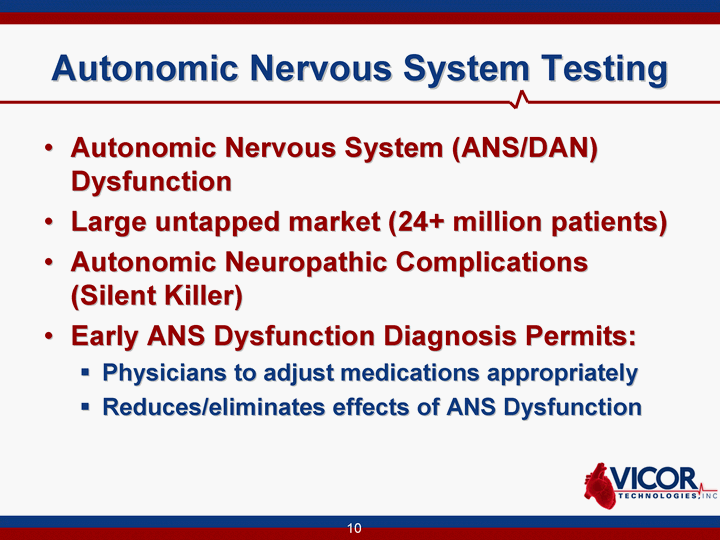
| Autonomic Nervous System Testing Autonomic Nervous System (ANS/DAN) Dysfunction Large untapped market (24+ million patients) Autonomic Neuropathic Complications (Silent Killer) Early ANS Dysfunction Diagnosis Permits: Physicians to adjust medications appropriately Reduces/eliminates effects of ANS Dysfunction |

| PD2i CA(tm) SCD not a Heart Attack Heart Attack-"Internal Plumbing Problem" SCD - breakdown of the heart-brain axis SCD - swift and unexpected 95% of SCD victims die pre-hospital SCD often asymptomatic Sudden Cardiac Death |
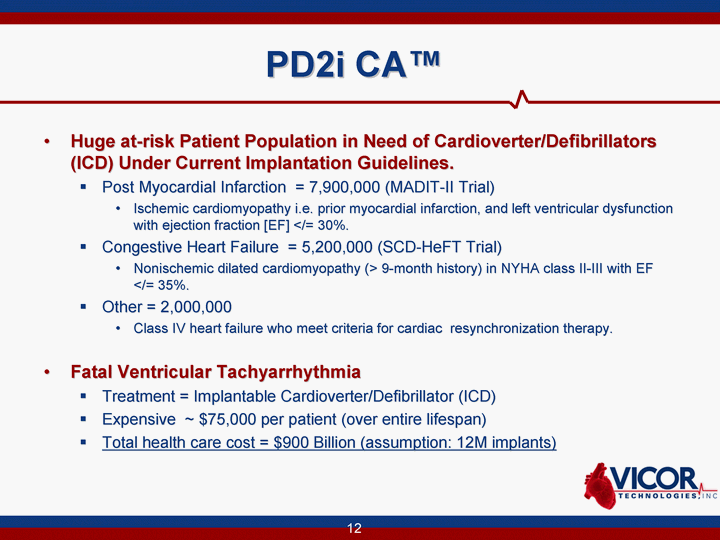
| PD2i CA(tm) Huge at-risk Patient Population in Need of Cardioverter/Defibrillators (ICD) Under Current Implantation Guidelines. Post Myocardial Infarction = 7,900,000 (MADIT-II Trial) Ischemic cardiomyopathy i.e. prior myocardial infarction, and left ventricular dysfunction with ejection fraction [EF] </= 30%. Congestive Heart Failure = 5,200,000 (SCD-HeFT Trial) Nonischemic dilated cardiomyopathy (> 9-month history) in NYHA class II-III with EF </= 35%. Other = 2,000,000 Class IV heart failure who meet criteria for cardiac resynchronization therapy. Fatal Ventricular Tachyarrhythmia Treatment = Implantable Cardioverter/Defibrillator (ICD) Expensive ~ $75,000 per patient (over entire lifespan) Total health care cost = $900 Billion (assumption: 12M implants) |
| PD2i CA(tm) Over-Implantation of ICDs 76% of ICDs Implanted Never Fired Appropriately (SCD-HeFT)1,2 80% of SCD victims DO NOT meet current criteria for ICD Currently 20 ICD's Used to Save One Life 1. Moss AJ, Zareba W, Hall WJ, et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med 2002;346:877-83. 2. Bardy GH, Lee KL, Mark DB, et al. Amiodarone or a Implantable Cardioverter-Defibrillator for Congestive Heart Failure. N Engl J Med 2005;352:225-37. |
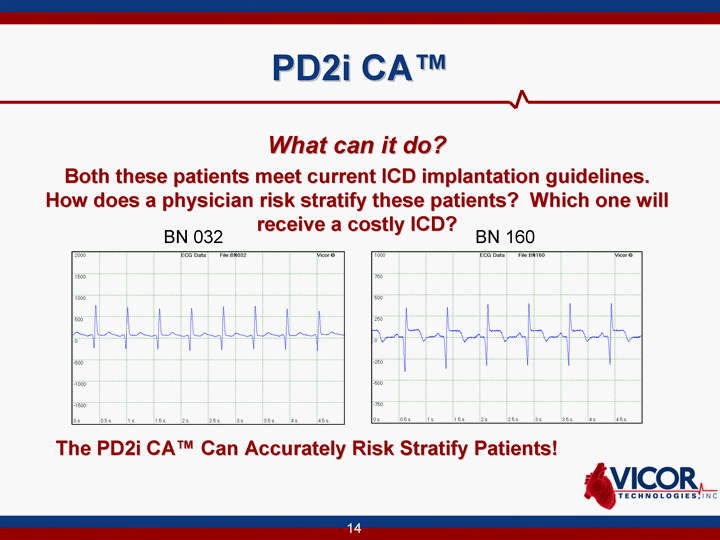
| PD2i CA(tm) What can it do? Both these patients meet current ICD implantation guidelines. How does a physician risk stratify these patients? Which one will receive a costly ICD? BN 032 BN 160 The PD2i CA(tm) Can Accurately Risk Stratify Patients! |
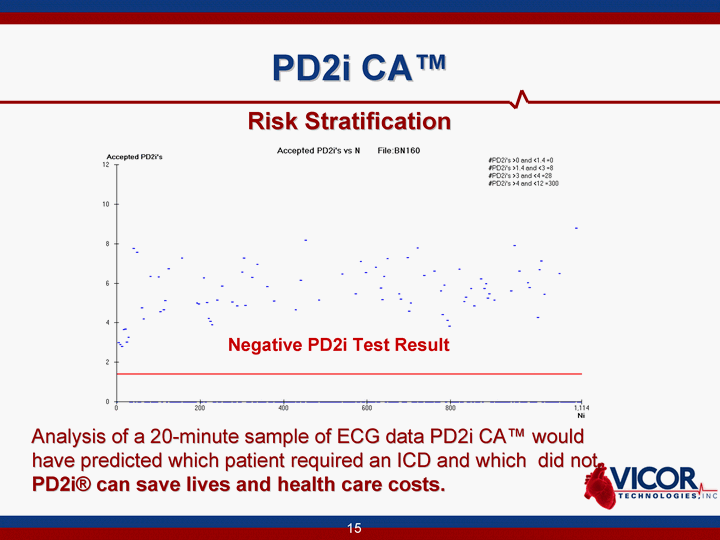
| PD2i CA(tm) Analysis of a 20-minute sample of ECG data PD2i CA(tm) would have predicted which patient required an ICD and which did not. PD2i(r) can save lives and health care costs. Negative PD2i Test Result Risk Stratification |
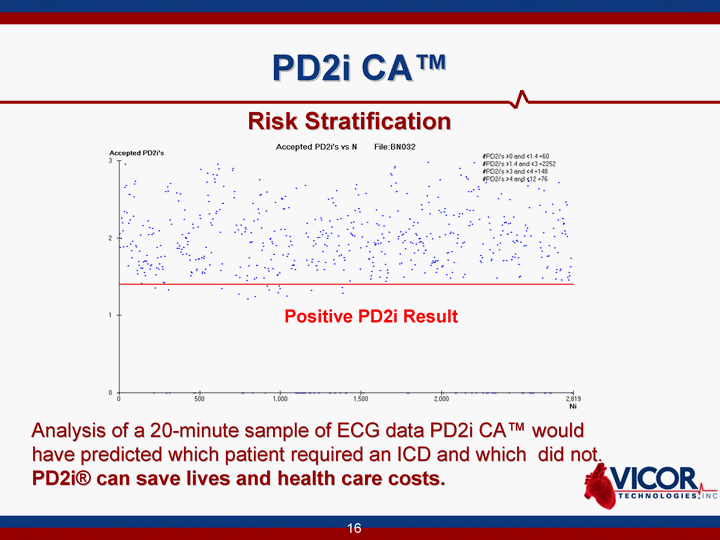
| PD2i CA(tm) Analysis of a 20-minute sample of ECG data PD2i CA(tm) would have predicted which patient required an ICD and which did not. PD2i(r) can save lives and health care costs. Positive PD2i Result Risk Stratification |

| PD2i CA(tm) Skinner, Pratt, Vybiral (1993) American Heart Journal, 125:731- 743. 37 Total Enrolled, 7 Rejections, 30 Completed Study 2. Prospective Human Clinical Study (Emergency Room, 5 Tertiary Care Hospitals, Philadelphia, Newark, Camden, Detroit, Allentown). 918 Total Enrolled, 173 Rejections, 745 Completed Study. 3. MIT-BI PhysioBank Study. 44 Total , 11 Rejections, 33 Analyzed. True Positive (7) False Positive (4) False Negative (0) True Negative (19) Sensitivity = 100% Specificity = 83% True Positive (23) False Positive (104) False Negative (1) True Negative (617) Sensitivity = 96% Specificity = 86% True Positive (17) False Positive (3) False Negative (0) True Negative (13) Sensitivity = 100% Specificity = 81% Confirmatory Clinical Studies Data |
| PD2i CA(tm) The "MUSIC" Data "Prognostic Significance of Point Correlation Dimension Algorithm (PD2i) in Chronic Heart Failure Patients" MERTE SUBITA EN INSUFFICIENCIA CARDIACA 651 Class II/III Congestive Heart Failure Patients 52 Verified Arrhythmic Deaths Patients Followed for a Mean of 44 Months Digital ECG and Holter Recordings made on enrollment The PD2i CA(tm) analyzed Initial ECG and Holter Recordings Completed analysis submitted to the University of Rochester Findings will be the basis for FDA 510(k) submission in Q2 2010 |
| PD2i CA(tm) Positive Regulatory/Reimbursement Climate CMS-CAG-00175R3 March 2006 Might be Mandated High Margin = High Profitability Software product Razor/Razorblade Business Model Development costs already incurred Not Driven Solely by Large Reimbursement Pricing Flexibility Strong Business Model |
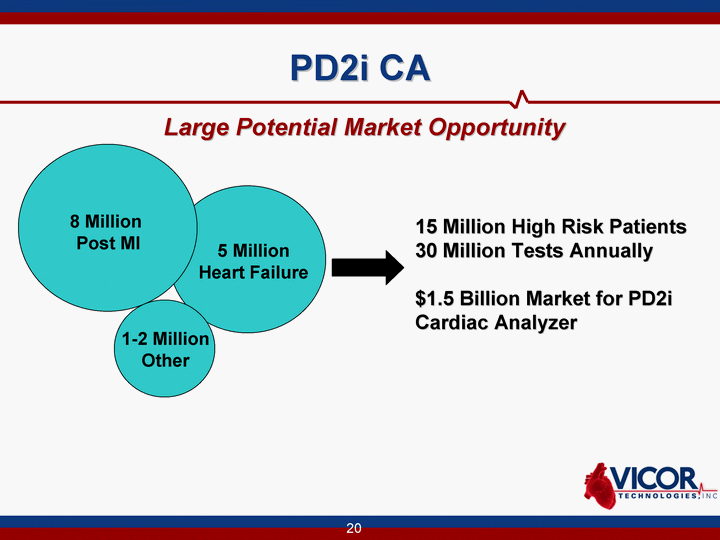
| PD2i CA Large Potential Market Opportunity 5 Million Heart Failure 1-2 Million Other 15 Million High Risk Patients 30 Million Tests Annually $1.5 Billion Market for PD2i Cardiac Analyzer 8 Million Post MI |

| PD2i VS(tm) Collaboration with the U.S. Army (USAISR) Assess the severity of injury and imminent death Civilian sector opportunity: 38,000,000 annual events). Anticipated FDA 510(k) Marketing Clearance in 2010 |

| PD2i VS(tm) USAISR Study #1 USE OF HEART-RATE COMPLEXITY TO DETERMINE THE NEED FOR LIFE SAVING INTERVENTIONS IN COMBAT CASUALTIES Abnormal HRC values exhibited in all patients Those requiring LSI's had Min PD2i values < 1 Findings suggest utility of PD2i for diagnosis of need for LSI |
| PD2i VS(tm) USAISR Trial "Assessment of the PD2i(r) Score in Adult Severe Trauma" Study of 325 trauma patients 20 deaths All were identified by low PD2i(r) value 14 deaths all had Low PD2i(r) value and NO life saving intervention performed in field |
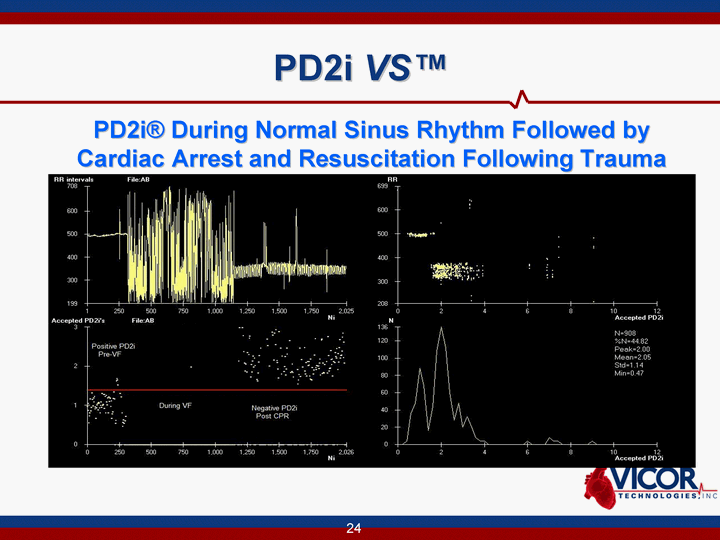
| PD2i VS(tm) PD2i VS(tm) PD2i(r) During Normal Sinus Rhythm Followed by Cardiac Arrest and Resuscitation Following Trauma |

| PD2i VS(tm) PD2i VS(tm) PD2i VS(tm) Monitor During Transport with Stable Patient With 3 Minute Initial Evaluation and 1 Minute Updates |

| PD2i(r) Marketing Plan PD2i Analyzer(tm) (ANS Testing) Product launch 1/14/10 Initial 25 Independent sales reps in NC and SC in January Targeted marketing of shareholder practices- Q2-2010 Roll out in Q2 and Q3 2010 in other states Sales personnel Other Distributors Sudden Cardiac Death Beta tests in several practices- Cedars Sinai Medical Group-Los Angeles Jackson Memorial Hospital North-Miami 510(k) submission Q2 2010 and 510(k) clearance thereafter Use of 25 sales reps in NC and SC initially Thought Leader seminars- Q3 2010 Roll out in Q3 2010 of other states |

| PD2i(r) International Marketing Plan Active discussions with distribution partners in: Canada South America Israel Far East |
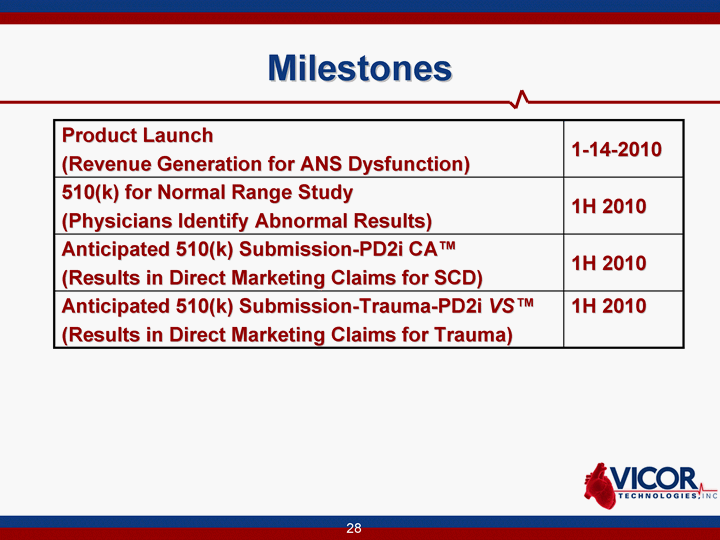
| Milestones Product Launch (Revenue Generation for ANS Dysfunction) 1-14-2010 510(k) for Normal Range Study (Physicians Identify Abnormal Results) 1H 2010 Anticipated 510(k) Submission-PD2i CA(tm) (Results in Direct Marketing Claims for SCD) 1H 2010 Anticipated 510(k) Submission-Trauma-PD2i VS(tm) (Results in Direct Marketing Claims for Trauma) 1H 2010 |
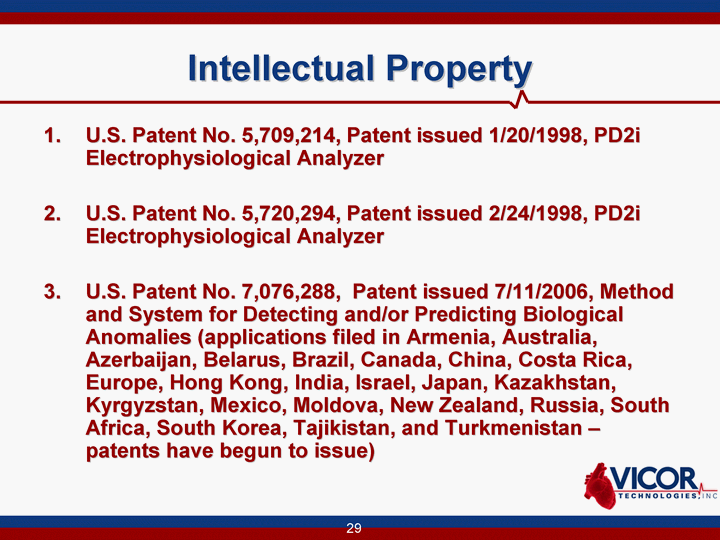
| Intellectual Property U.S. Patent No. 5,709,214, Patent issued 1/20/1998, PD2i Electrophysiological Analyzer U.S. Patent No. 5,720,294, Patent issued 2/24/1998, PD2i Electrophysiological Analyzer 3. U.S. Patent No. 7,076,288, Patent issued 7/11/2006, Method and System for Detecting and/or Predicting Biological Anomalies (applications filed in Armenia, Australia, Azerbaijan, Belarus, Brazil, Canada, China, Costa Rica, Europe, Hong Kong, India, Israel, Japan, Kazakhstan, Kyrgyzstan, Mexico, Moldova, New Zealand, Russia, South Africa, South Korea, Tajikistan, and Turkmenistan - patents have begun to issue) |
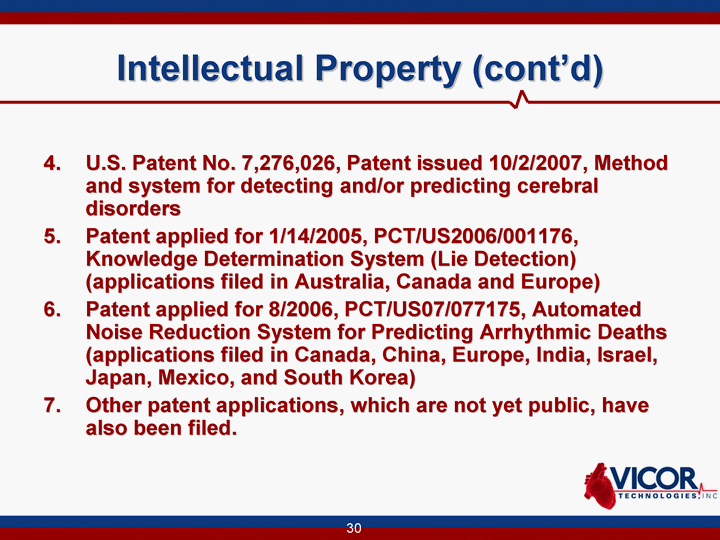
| Intellectual Property (cont'd) 4. U.S. Patent No. 7,276,026, Patent issued 10/2/2007, Method and system for detecting and/or predicting cerebral disorders 5. Patent applied for 1/14/2005, PCT/US2006/001176, Knowledge Determination System (Lie Detection) (applications filed in Australia, Canada and Europe) 6. Patent applied for 8/2006, PCT/US07/077175, Automated Noise Reduction System for Predicting Arrhythmic Deaths (applications filed in Canada, China, Europe, India, Israel, Japan, Mexico, and South Korea) 7. Other patent applications, which are not yet public, have also been filed. |

| Vicor Management Employees and Consultants David Fater - President and CEO Over 13 years experience as a senior executive with 3 public healthcare companies. Spent 24 years as a senior international partner with Ernst & Young. Dr. James Skinner, Ph.D. - Director of Grant R&D Extensive experience as a scientist and manager of large research and development projects. Professor of Medicine at Baylor College of Medicine. Recipient of many research grants. Principal investigator of a program that operated 5 laboratories and 3 core facilities. Dr. Jerry Anchin, Ph.D. - Director of R&D 25 year's accumulated experience in the biotechnology diagnostic and medical device sector. Actively involved in immunology, molecular biology, and drug discovery. |

| Vicor Management Employees and Consultants Dr. Daniel N. Weiss, M.D.,F.A.C.C. - CMO Extensive experience as a practicing cardiologist and electrophysiologist. Partner of Florida Arrhythmia Consultants. Director of the Jim Moran Heart and Vascular Center since 1994. Consultant to Medtronics, St. Jude Medical, and Guidant. Dr. Richard M. Cohen, Ph.D. - Business Development 30 year's experience in worldwide operations and sourcing. COO of four health care companies, including two medical device companies. Thomas J. Bohannon-CPA-Chief Accounting Officer 40 year's experience in accounting and financial reporting. |
| Vicor Management Employees and Consultants Lloyd Chesney-Chief Technology Officer Michael Harwitz-Senior Systems Architect 25+ year's experience in developing web-based enterprise systems for health care Specialized Software and Programming: Southwest Research Institute Keith Bartels, Ph.D David Tong, Ph.D. Extensive experience with medical devices and biomedical engineering Jules Mitchel, Ph.D., MBA - Director of Regulatory Affairs President of Target Health, Inc. |
| Scientific Advisory Board Mark E. Josephson, M.D. Chief of Cardiology at Beth Israel Deaconness Medical Center. Author of "Clinical Cardiac Electrophysiology". Scientific Advisor to over 20 companies. Hein J. J. Wellens, M.D. Professor & Chairman of Department of Cardiology Academisch Ziekenhuis Maastricht. Director of Interuniversity Cardiology Institute of the Netherlands. Richard M. Luceri, M.D., F.A.C.C. Director Interventional Arrhythmia Center, Holy Cross Hospital. Clinical investigator in MADIT II Trial. Clinical investigator & Principal Author SCD-HeFT Trial. Jules Mitchel, Ph.D., MBA Founder and President/CEO Target Health Inc. NYC. |

| Scientific Advisory Board Robert G. Hauser, M.D., F.A.C.C., FHRS Senior Consulting Cardiologist - Minneapolis Heart Institute Chairman Cardiovascular Services Division at Abbott Northwestern Hospital Chief Executive Officer of Cardiac Pacemakers, Inc -acquired by Guidant Jonathan Kaplan, M.D., M.P.H. Medical Director of Fidelis Care in New York Former Medical Director for Excellus Blue Cross Blue Shield David Chazanovitz Former CEO of Cambridge Heart, Inc. Edward F. Lundy, M.D., Ph.D. Chief of Cardiothoracic Surgery at Good Samaritan Hospital Ph.D. in Physiology with focus on altered-state physiologies (hibernation) |

| Ongoing Clinical Trials & Market Potential Trial Name Market Indication Patient Market Size (Millions) MUSIC SCD 15 Normal Range Study Diabetic Neuropathy 18 BIRST Neuro-ICU 0.5 CASA SCD-Student Athletes US NCAA Population USAISR Trauma Armed Forces Population Kidney Failure SCD-Dialysis 2 CARE-e Cardiac Rehab 15 CARE-T Cardiac Evaluation 15 UMMC Trauma ICU/Mobile Triage 38 Mississippi Blood Services Internal Bleeding 38 MGH Trauma Trauma Triage 38 |
| PD2i Analyzer Large Total Potential Market Opportunity 70 Million Potential Patient Population $8.2 Billion Market for PD2i |

| Investment Highlights Life saving technology Addresses substantial medical markets, U.S. and International Substantial cost savings to public and private insurers High margin, high operating profit products Beginning revenue ramp in first quarter of 2010 Attractive business model Highly experienced management team; world-class Scientific Advisory Board |

| Thank You. Vicor Technologies, Inc. 2300 NW Corporate Blvd.,Suite 123 Boca Raton, FL 33431 Phone: (877) 528-PD2i (7324) Fax: (561) 995-2449 www.vicortech.com |
