Attached files
| file | filename |
|---|---|
| EX-32.1 - Kun Run Biotechnology, Inc. | v178955_ex32-1.htm |
| EX-31.2 - Kun Run Biotechnology, Inc. | v178955_ex31-2.htm |
| EX-31.1 - Kun Run Biotechnology, Inc. | v178955_ex31-1.htm |
UNITED
STATES
SECURITIES
AND EXCHANGE COMMISSION
WASHINGTON,
D.C. 20549
Form
10-K
(Mark
One)
|
x
|
ANNUAL REPORT UNDER SECTION 13 OR
15(d) OF THE SECURITIES EXCHANGE ACT OF
1934
|
For the
fiscal year ended December 31, 2009.
|
o
|
TRANSITION REPORT UNDER SECTION
13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF
1934
|
For the
transition period from _______________ to ______________
Kun
Run Biotechnology, Inc.
(Exact name of registrant as specified in Charter)
|
Nevada
|
333-141384
|
98-0517550
|
||
|
(State
or other jurisdiction of
incorporation
or organization)
|
(Commission
File No.)
|
(IRS
Employee Identification
No.)
|
Free
Trade Zone
168
Nanhai Avenue, Haikou City
Hainan
Province, China 570216
(Address
of Principal Executive Offices)
86-898-6680-2207
(Issuer
Telephone number)
Securities
registered under Section 12(b) of the Exchange Act: None.
Securities
registered under Section 12(g) of the Exchange
Act:
|
Title of Each Class:
|
Name of Each Exchange on Which Registered
|
|
|
Common Stock, par value $0.001
|
None
|
Indicate
by check mark if the registrant is a well-known seasoned issuer, as defined in
Rule 405 of the Securities Act.
Yes o No þ
Indicate
by check mark if the registrant is not required to file reports pursuant to
Section 13 or Section 15(d) of the Act. Yes o No þ
Indicate
by check mark whether the registrant (1) has filed all reports required to
be filed by Sections 13 or 15(d) of the Securities Exchange Act of 1934
during the preceding 12 months (or for such shorter period that the
registrant was required to file such reports), and (2) has been subject to
such filing requirements for the past 90 days. Yes þ No o
Indicate by check mark whether the
registrant has submitted electronically and posted on its corporate Website, if
any, every Interactive Data File required to be submitted and posted pursuant to
Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12
months (or for such shorter period that the registrant was required to submit
and post such files). Yes o No o
Indicate
by check mark if disclosure of delinquent filers pursuant to Item 405 of
Regulation S-K is not contained herein, and will not be contained, to the
best of registrant’s knowledge, in definitive proxy or information statements
incorporated by reference in Part III of this Form 10-K or any
amendment to this Form 10-K.
o
Indicate
by check mark whether the registrant is a large accelerated filer, an
accelerated filer, a non-accelerated filer, or a smaller reporting company. See
the definitions of “large accelerated filer,” “accelerated filer” and “smaller
reporting company” in Rule 12b-2 of the Exchange Act.
|
Large accelerated filer o
|
|
Accelerated filer o
|
|
Non-accelerated filer o
(Do not check if a smaller
reporting company)
|
|
Smaller reporting
company þ
|
Indicate
by check mark whether the registrant is a shell company (as defined in Rule
12b-2 of the Act):
Yes o No þ
The aggregate market value of the
2,477,500 shares of voting and non-voting common equity stock held by
non-affiliates of the registrant was $ 2,973,000 as of June 30, 2009, the last
business day of the registrant’s most recently completed second fiscal quarter,
based on the last sale price of the registrant’s common stock on such date of
$1.2 per share, as reported by The Over-The-Counter Bulletin
Board.
As
of March 30, 2010, there were 25,000,000 shares of common stock of Kun Run
Biotechnology, Inc. outstanding.
TABLE
OF CONTENTS
|
Page
|
||
|
PART
I
|
||
|
Item
1
|
Business
|
2
|
|
Item
1A
|
Risk
Factors
|
17
|
|
Item
1B
|
Unresolved
Staff Comments
|
41
|
|
Item
2
|
Properties
|
41
|
|
Item
3
|
Legal
Proceedings
|
42
|
|
Item
4
|
Submission
of Matters to a Vote of Security Holders
|
42
|
|
PART
II
|
||
|
Item
5
|
Market
for Registrant’s Common Equity, Related Stockholder Matters and Issuer
Purchases of Equity Securities
|
42
|
|
Item
6
|
Selected
Financial Data
|
43
|
|
Item
7
|
Management’s
Discussion and Analysis of Financial Condition and Results of
Operation
|
45
|
|
Item
7A
|
Quantitative
and Qualitative Disclosures About Market Risk
|
61
|
|
Item
8
|
Financial
Statements and Supplementary Data
|
61
|
|
Item
9
|
Changes
in and Disagreements With Accountants on Accounting and Financial
Disclosure
|
61
|
|
Item
9A
|
Controls
and Procedures
|
61
|
|
Item
9B
|
Other
Information
|
63
|
|
PART
III
|
||
|
Item
10
|
Directors,
Executive Officers and Corporate Governance
|
63
|
|
Item
11
|
Executive
Compensation
|
65
|
|
Item
12
|
Security
Ownership of Certain Beneficial Owners and Management and Related
Stockholder Matters
|
67
|
|
Item
13
|
Certain
Relationships and Related Transactions, and Director
Independence
|
67
|
|
Item
14
|
Principal
Accounting Fees and Services
|
68
|
|
PART
IV
|
||
|
Item
15
|
Exhibits
and Financial Statement Schedules
|
68
|
1
Except as
otherwise indicated by the context, references in this document to “Company,”
“we,” “us,” or “our” are references to the combined business of Kun Run
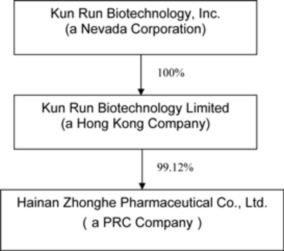
Biotechnology, Inc. (formerly, Aspen Racing Stables, Inc.) and its wholly-owned
subsidiaries, including Kun Run Biotechnology Ltd., a Hong Kong corporation and
Hainan Zhonghe Pharmaceutical Co., Ltd., a corporation organized under the laws
of the People’s Republic of China. References to “China” and “PRC” are
references to “People’s Republic of China.” References to "RMB" are to Renminbi,
the legal currency of China, and all references to “$” are to the legal currency
of the United States.
PART
I
Item
1. Business
We are
engaged, through Hainan Zhonghe Pharmaceutical Co., Ltd. (“Zhonghe”), our China
based indirect subsidiary, in the development, manufacture, marketing and sale
of prescription polypeptide drugs. Our principal products are polypeptide
derivatives as well as chemical products. Our products are sold primarily in
China and through Chinese domestic pharmaceutical distributors licensed by the
Chinese government. Our manufacturing and sales facilities are located in the
City of Haikou, Hainan Province.
Corporate
History
Kun Run
Biotechnology, Inc. (the “Company”) formerly known as Aspen Racing Stables, Inc.
(“Aspen”) was incorporated in the State of Nevada on March 10, 2006. The
Company’s shares are quoted for trading on the Over-The-Counter Bulletin Board
in the United States of America.
2
Kun Run
Biotechnology, Ltd., our non-operating Hong Kong holding subsidiary (“Kun Run”),
was incorporated on May 6, 2006 under the name Max Talent Industrial Ltd, which
changed to its present name on February 25, 2008. On March 24, 2008, Kun Run
completed its acquisition of 60.12% equity interest of Zhonghe, a company
organized under the laws of the People’s Republic of China (“PRC”) on April 17,
1995 and has since been engaged in the manufacture and sale of polypeptide
drugs. On May 27, 2008, Kun Run acquired an additional 39% equity interest of
Zhonghe, resulting in a 99.12% ownership of Zhonghe.
Thereafter, on August 21, 2008, Kun Run
entered into a Stock Purchase Agreement (the “Exchange Agreement”) with the
shareholders of the Company. The terms of the Exchange Agreement were
consummated and the acquisition was completed on September 16, 2008. As a result
of the transaction, the Company issued a total of 24,250,000 shares of its
common voting stock to Xueyun Cui (“Mr. Cui”) and Liqiong Yang, the shareholders
of Kun Run and their designees, in exchange for 100% of the capital stock of Kun
Run resulting in Kun Run becoming our wholly-owned subsidiary, and the
shareholders of Kun Run and their designees owning approximately 97% of the
issued and outstanding shares of the common stock of Aspen. In addition, Trixy
Asyniux-Walt, the original shareholder of Aspen, returned 1,000,000 of her
shares to the Company for cancellation and as of the closing owns 750,000 shares
of the Company’s common stock which constitutes approximately 3% of the
issued and outstanding shares of the Company’s common stock.
Organizational
Chart
Our
Industry
So far,
three types of molecules have been developed for the treatment of human
diseases: i) small molecules, ii) antibodies, and iii) peptides. Until recently,
the majority of the therapeutic molecules developed and marketed are small
molecules. However, with the recent development of several technologies in the
areas of peptide synthesis, screening, stabilization and modifications, peptides
are now recognized as leading molecules for therapeutics. Peptides play an
increasing role in the development of new treatments for cancer, diabetes,
auto-immune diseases, and more effective diagnostics due to its function as
chemical messengers and neurotransmitters. In the development of anti-cancer
drugs, peptides present the least side-effects. Unlike the present cocktail of
drugs used in chemotherapy, we believe that peptides provide the best prospects
because they can be target-specific and have the least lethal index (side
effects) and maximum therapeutic index (effectiveness of the
drug).
3
Peptides
are short strings of amino acids. Bioactive peptides, also called small
molecular weight active polypeptides, are peptides that are more active, and
bind more quickly, than regular peptides. Bioactive peptides have better
absorption and binding mechanisms than free amino acids.
A growing
area of biotechnology research is the discovery and development of new bioactive
peptides that can be used in medicine, health supplements, food and cosmetics.
Bioactive peptides can be divided into two groups: (i) therapeutic peptide
products, of which there are over 100 on the global market, and (ii)
peptides enhanced through the addition of bioactive peptides such as health
supplements and peptide-rich non-prescription medicines.
Their
role as mediators of key biological functions and their unique intrinsic
properties make them particularly attractive therapeutic agents. Peptides
show high biological activity associated with low toxicity and high specificity.
The benefits conferred by these characteristics include little unspecific
binding to molecular structures other than the desired target, minimization of
drug-drug interactions and less accumulation in tissues thus reducing risks of
complications due to intermediate metabolites. Additionally, compared to small
molecules, peptides offer valuable chemical and biological diversity on which
intellectual property is still widely available. As a result, even large
pharmaceutical companies, which traditionally focused on small molecules, are
increasingly including peptides in their pipelines. For example, Pfizer, GSK and
Eli Lilly have recently acquired peptide-based products.
The
therapeutic peptides market emerged in the 1970s, when Novartis launched
Lypressin, a vasopressin analogue. Since then, based on a report by Bionest
Partner in 2005 entitled “Therapeutic Peptides under the Spotlight,”
approximately 30 peptides have reached the market, representing a $5.3 billion
opportunity in 2003 (over 1.5 percent of the $325 billion global
pharmaceutical market). Among the different classes of peptides, GNRH/LHRH
agonists (leuprorelin, goserelin) account for almost 50 percent of the
market. Other key commercialized peptides include sandostatin
(somatostatin analogue, Novartis), glatiramer (immunomodulator peptide, Teva),
salmon calcitonin (Miacalcin, Novartis) and desmopressin (DDAVP,
Ferring).
The
worldwide market for therapeutic peptides was estimated to be $5.3 billion in
2003, and is expected to grow at a Compound Annual Growth Rate of 8.1% to $11.5
billion in 2013.
China’s
Peptide Market
China
possesses one fourth of the world’s population and has a rapidly growing
pharmaceuticals industry. In 2006, based on “China is Expected to Become the
Fifth Largest Drug Market in the World by 2010,” a report by Research and
Markets (http://www.researchandmarkets.com/reports), dated March 8, 2007, sales
in the Chinese pharmaceuticals industry reached $12 billion, an increase of 3.8
times the sales in 1998. The Chinese pharmaceuticals market is expected to grow
at a rate of 20 to 25 percent per year for the next five years. China is
forecasted to become the fifth largest drug market in the world by
2010.
4
A number
of factors contribute to an increasing demand for pharmaceuticals in
China:
|
|
·
|
Overall economic growth has led
to increased household income. Based on a report by Nankai University
dated April 3, 2007 entitled “Welfare effects of public health insurance
reform,” health awareness is increasing in a rapidly growing urban
population.
|
|
|
·
|
China’s current population is
aging and people who are 60 years of age or older will reach 9.0% of the
country’s population by 2010, up from 7.0% in 2003. Based on a report by
the China Social Protection Budget Committee entitled “China: Social
Pension System in China is Facing Harsh Challenges” dated April 21, 2005,
the Social Insurance Fund of China predicts that the aging population
(people older than 60) will reach 24.5% of China’s population in
2030.
|
|
|
·
|
Provincial and national health
insurance program expansion and reform are making healthcare services
available to more people, based on the Nankai University
report.
|
The
government has undertaken initiatives to regulate the domestic pharmaceuticals
industry to assure product quality and protect intellectual property rights.
These factors have led to increased direct foreign investment and rapid growth
in the industry.
The
Chinese therapeutic peptide market is growing rapidly. Research is focused on
developing new, more effective drugs that utilize bioactive peptides as active
pharmaceutical ingredients (APIs). Peptide medicines are used to cure diseases
and to strengthen the human immune system. The commercialization of research
findings is leading to rapid growth in this sector of the pharmaceutical
industry.
There are
only 26 therapeutic polypeptide medicines sold in China. The therapeutic
peptides industry in China is highly fragmented and dominated mainly by domestic
players. Only 11 foreign peptide products have obtained approvals from the
Chinese government to be sold across China, which only accounts for a small part
of the total market due to these foreign products’ weak market penetration and
high prices.
Competitive
Advantages
We
believe that we have the following competitive advantages:
1.
Focus on peptide products. Unlike other peptide manufacturers in
China, we focus on peptide drugs and seldom manufacture any other types of
drugs. Although we plan to manufacture certain generic drugs in the
future, the scope will be limited and used only as a temporary measure to
utilize our current surplus capacity.
5
2.
First Mover. We started to manufacture peptide drugs in 1997,
earlier than other peptide manufacturers in China, and we believe that we have
built more experience in manufacturing and marketing peptide drugs than any
other peptide manufacturer in China. In addition, we are the largest
manufacturer of TP-5 in China in terms of both volume and revenue. Based on a
survey conducted by Haihong Pharmaceutical Information Ltd. on 1410 hospitals
throughout China, we currently have a 65.5% market share of TP-5 in Beijing,
95.6% in Shanghai and 43.6% in Guangzhou.
3.
Broader Line of Products. Compared to most peptide manufacturers in China,
we offer more peptide products. Currently, we manufacture and market four
peptide drugs while most others only manufacture and market one or two peptide
products.
4.
More Products in the Pipeline. We currently have seven potential
peptide drugs at various stages of development and expect to launch several of
them in the next two to three years.
5. Better
Product Quality. Our peptide products have at least 99% purity compared to
95% purity of similar products of most other peptide manufacturers in China.
Higher purity leads to higher potency and lower side effects, the clinical value
sought by patients. Therefore, our products have a better name recognition
and can command higher prices.
6.
State-of-the-art Production Facility. Our production facility possesses a large
and efficient designed capacity to achieve economies of scale. It is one of the
largest bulk synthesized peptide manufacturing facilities in Asia and the
largest in China.
Our
Strategies
We plan
to adopt the following strategies:
1.
Strengthen Research and Development. We plan to strengthen and
expand our R&D capabilities to maintain our competitive advantages. In
order to achieve this objective, we plan to acquire the R&D facilities of
our affiliates if we are able to successfully raise sufficient funds in our
future financings. In addition, we will continue to strengthen our relationships
with universities and medical institutions in order to acquire new products, new
applications and new delivery mechanisms for peptides.
2.
Increase Revenue by Broadening Our Line of Products. Currently, we
manufacture four peptide products. To increase our revenue, we plan to add one
or two new peptide products per year for the next two to three years. We
plan to manufacture generic drugs or OEM manufacture for other pharmaceutical
companies at least for the short term due to extra capacity we have at our new
manufacturing facility: our phase II pharmaceutical plant, Injection Preparation
Plant obtained GMP approval on September 29, 2008 and went into production in
October of the same year. The Solid Oral Preparation Plant has been installed
and debugged, expecting to get GMP approval in May of 2010.
6
3.
Further Penetration of Market. Currently, we have distribution
agents in every province and municipality directly under the jurisdiction of
China’s State Council. In order to increase revenue, we plan to further
penetrate the market by expanding our distribution networks into China’s
secondary cities. In addition, we are testing a pilot program which awards
a distributor’s nationwide distributorship for certain products rather than
awards for distributorship within the geographic area of a province only.
We believe distributors may have more incentive to increase the sales of certain
peptide products through this new system.
4.
Expand into Overseas Market. In order to increase our revenue
growth, we plan to enter into markets in Southeast Asia, Africa and South
America. In addition, we have obtained a permit in Uzbekistan and are in
the process of applying for one in Korea. Our international department has
begun to receive orders from South Korea and is negotiating with distributors in
Uzbekistan and Indonesia.
5.
Maintain High Quality. Our products have better name recognition and
command higher prices because of their high quality. Our peptide products
have at least 99% purity compared to 95% purity of the similar products of most
other manufacturers in China. We plan to continue to maintain the high
quality of our products through utilizing advanced technology and equipment, and
quality control in our manufacturing process.
Principal
Products and Markets
Our
principal products are prescription polypeptide drugs used to treat immune
system malfunction and hyper function. Using various formulas, we produce a
number of peptide products with several forms of delivery including injections,
capsules and pills. We intend to concentrate our efforts for the next
several years on the development, production and sales of polypeptide
products.
Our
principal operations are in China, where we have manufacturing facilities and
sales distribution covering every province of China. We are engaging
mainly in manufacturing prescription polypeptide drugs. Currently, we
manufacture and market four polypeptide products across China: Thymopentin (
“TP-5”), Somatostatin (“SS”),Thymosin Alpha 1 (“Alpha 1”)and Desmopressin
Acetate ( “DDAVP”).
Thymopentin
(“TP-5”)
TP-5
is a two-phase immunostimulant and is used for treating tumor and hepatitis.
TP-5 has an extensive range of applications. Studies have indicated that TP-5
can significantly increase and promote the natural immune system’s ability to
defend against malignant diseases. In addition, it has been shown that TP-5 has
no toxicity and side effects, nor does it inter with other
drugs.
7
We
currently produce TP-5 in two forms: TP-5 freeze-dry powder and TP-5 pre-filled
injection.
Somatostatin
(“SS”)
Naturally
occurring somatostatin is mainly secreted in the inferior part of the
hypothalamus and the gastrointestinal tract. Somatostatin for injection is a
synthetic cyclic 14-amino acid peptide. It is entirely identical in structure
and activity to naturally occurring somatostatin. It is believed to be an
effective treatment for acute severe pancreatitis, upper gastrointestinal
hemorrhages, pancreatic fistulas, intestinal fistulas and biliary fistulas. It
inhibits endocrine and exocrine secretions of gastric somatostatin. Compared
with Octretide, Somatostatin binds perfectly with five subtypes of somatostatin
receptors, having 2 to 1,000 times more binding affinity than
Octreotide.
We
currently produce Somatostatin in two specifications: 0.25mg and 3mg.
Thymosin
Alpha 1 (“Alpha 1”)
Thymosin
Alpha 1 provides a safe and effective treatment for chronic hepatitis B when
used alone or in combination with interferon. Primary research indicates that
Thymosin Alpha 1 is also useful in treating a number of other diseases as well,
including hepatitis C, malignant tumors, melanoma and HIV/AIDS.
We
currently produce Thymosin Alpha 1 in a 1.6mg specification.
Desmopressin
Acetate Injection (“DDAVP”)
DDAVP is
a synthetic analogue of the natural pituitary hormone 8-arginine vasopressin
(ADH), an antidiuretic hormone affecting renal water conservation. It has been
shown to improve the level of platelet aggregation and is widely used before
surgeries to prevent bleeding. It has also been shown to be a treatment for
nocturnal enuresis, central diabetes insipidus, polyuria, polydipsia mild and
moderate forms of hemophilia A. Our DDAVP product is the only DDAVP product in
China that is without chlorobutanol, a type of preservative that widely used in
DDAVP products. It has been shown that the use of chlorobutanol may cause side
effect such as cardiovascular toxicity and disorders to nervous system. We
currently produce DDAVP in two specifications: 4ug and 15ug.
8
The
following tables exhibit the breakdown of our revenues for the two fiscal years
ended December 31, 2009 and 2008:


New
Products in the Pipeline
Through
our research and development, co-development and purchase from institutions, we
have secured a broad product pipeline with approximately 11 new products in
different stages from per-clinical research to SFDA application. We believe that
these new medicines will address some of the most widespread diseases including
diabetes, coagulant disease, hydrocephalus, tumor and other diseases as set
forth below:
9
|
No.
|
|
Indication
|
|
Category
|
|
Per-
clinical
|
|
Filed and
wait for
permits
|
|
soft
production
|
|
Expected
Launch
Day
|
|
1
|
Children
dwarfishness
|
New
peptide medicine
|
×
|
2011
|
||||||||
|
2
|
Diabetes
Type I
|
New
peptide medicine
|
×
|
2012
|
||||||||
|
3
|
Anticoagulant
|
New
peptide medicine
|
×
|
2011
|
||||||||
|
4
|
Antiplatelet
|
New
peptide medicine
|
×
|
2012
|
||||||||
|
5
|
Surgical
hemostasis, diabetes insipidus
|
Mimicry
peptide medicine
|
×
|
2010
|
||||||||
|
6
|
Gastrointestinal
hemorrhage
|
Mimicry
peptide medicine
|
×
|
2010
|
||||||||
|
7
|
Anti-premature
delivery
|
Mimicry
peptide medicine
|
×
|
2010
|
||||||||
|
8
|
Hyperlipidemia
|
Generic
medicine
|
×
|
2011
|
||||||||
|
9
|
Gastric
ulcer
|
Generic
medicine
|
×
|
2011
|
||||||||
|
10
|
Anti-Hepatitis
B Virus
|
Generic
medicine
|
×
|
2010
|
||||||||
|
11
|
Hypertension
|
Generic
medicine
|
×
|
2011
|
Among the new peptide products which
are launching in 2010, Entecavir (Item 10 in the above table)is a new oral
guanine nucleoside analog used in the treatment of anti-hepatitis B virus,
efficacious in inhibiting reverse transcription, DNA replication and
transcription in the viral replication process, suitable for antivirus therapy
of development trend of hepatitis B during different periods. Based on “The Dual
Protection of Strong Effectiveness and Low Drug Resistance to Insure the
Inhibition of Hepatitis B Virus Reinfection”, a report by China Medical Tribune
dated October 30th, 2008, Entecavir is the strongest antiviral nucleoside
analogue in latter days. Entecavir was researched and developed by a subsidiary
of Hainan Zhonghe Group, Co., Ltd. (Hainan Zhonghe Group), Hainan Zhonghe
Peptide Drugs Research & Development Co., Ltd. (Zhonghe Peptide). Entecavir
Dispersible Tablets have completed clinical trials and are expected to obtain
production approval in the second half of 2010.
We expect
the launch of these products will augment our product line and diversify our
product mix.
Research
and Development
We
currently conduct all of our research and development (“R&D”) activities
through our affiliates and through collaborative arrangements with universities
and research institutions in the PRC. Two of our affiliates have their own
research, development and laboratory facilities located near our headquarters in
the city of Haikou, Hainan Province. Additionally, we have established
several long-term partnerships with well-known universities and research
institutions in the PRC.
Sales
and Marketing
Currently,
we have 25 sales and marketing staff all based in our headquarters Haikou,
Hainan Province, PRC. They are responsible for managing our relationship
with all distributors and coordinating marketing activities. Our marketing
activities include advertising in medical magazines, conducting product seminars
at hospitals and medical institutions and sponsoring academic conferences.
We have also set up funds for research awards in the peptide medical
research areas.
10
Safety
and Quality Assurance
In
accordance with Good Manufacturing Practice (“GMP”) requirements, the Company
has written and implemented a quality assurance validation plan, procedures, and
a complete documentation system. The Company’s existing manufacturing facilities
has received the Certificate of Good Manufacturing Practices for Pharmaceutical
Products issued by Chinese State Food and Drug Administration (“SFDA”) in 2003
and renewed in early 2008. Our phase II pharmaceutical plant, Injection
Preparation Plant has obtained GMP approval on September 29, 2008 and went into
production in October of that year. The Solid Oral Preparation Plant has been
installed and debugged completely; expecting to get GMP approval in May of 2010
and begin production thereafter.
A strict
quality control system ensures that all products are produced in a
pollution-free, contamination-free and efficient production environment
following strict quality-oriented procedures. The warehouse for finished
products is adjacent to the production line, and is managed under the same
stringent hygienic requirements.
The
Company has a professional quality control team responsible for the supervision,
management and quality assurance of the whole production process and allocated a
general manager of the department to directly accountable for the quality of all
products.
Materials
and Suppliers
Raw
materials are sourced principally in the PRC and 90% of our raw materials
consist of amino acids and are generally available from a variety of suppliers.
We seek to mitigate the risk of a shortage of raw materials through
identification of alternative suppliers for the same or similar raw materials,
where available. We have purchasing staff with extensive knowledge of our
products who work with marketing, product research and development and
quality control personnel to source raw materials for products and other items.
Although one supplier accounts for more than 50% of our total raw material
purchases in 2009, we have identified various other suppliers if a change in
supplier is required as there are many manufacturers of amino acids in the
PRC.
|
Supplier accounting for more than 5% aggregate purchase amount in 2009
|
|||||||||
|
Name of Entity
|
Purchase Amount
in Current Year
(RMB)
|
Purchase Amount
in Current Year
(USD)
|
% to total amount
of purchase
amount
|
||||||
|
Sinopep Pharmaceutical Inc.,
|
14,778,100.00
|
2,166,469.46
|
53.6%
|
||||||
|
Zhejiang
Xinkang Pharmaceutical Glass Co., Ltd.
|
2,552,514.30
|
374,198.60
|
9.3%
|
||||||
|
Hubei
Huaqiang High-tech Co., Ltd.
|
1,941,265.00
|
284,589.45
|
7.0%
|
||||||
|
Shandong
Weigao Group Medical Polymer Company Limited
|
1,470,000.00
|
215,502.00
|
5.3%
|
||||||
|
Total
|
20,741,879.30
|
3,040,759.51
|
75.2%
|
||||||
11
Customers
and Distribution
Currently,
our products are sold primarily in the PRC through pharmaceutical distribution
companies that are licensed by the PRC government which in turn sell our
products to hospitals and medical institutions. We enter into distribution
arrangements with these distributors and provide them exclusivity for a period
of time within a particular geographical area.
Currently,
we utilize approximately 227 distributors throughout China with one major
distributor for each province, municipality directly under the jurisdiction of
the central PRC government and major city under the jurisdiction of provincial
governments.
The map
below exhibits the locations of our distributors as of December 31,
2009.

Each of
the distributors is evaluated on an annual basis and a specific performance
target is set for each distributor that varies with the location and the size of
the market. Beijing took the place of Chongqing as the largest market for our
products in 2009. The following table sets sixth distributors that generated
more than 5% of our total sales in 2009:
12
Distributor
generating with more than 5% of our aggregate sales in 2009
|
Name of Distributor
|
Amount(RMB)
|
Amount(USD)
|
% to Total Sales
Revenue
|
||||||
|
Beijing
Yabaofangda Pharmaceutical Limited
|
6,859,487.22
|
1,005,600.83
|
7.6%
|
||||||
|
Chongqing
Dinghai Pharmaceutical Co., Ltd.
|
5,187,350.42
|
760,465.57
|
5.7%
|
||||||
|
Beijing
Xingshengyuan Pharmaceutical Ltd.
|
5,175,683.76
|
758,755.24
|
5.7%
|
||||||
|
Jiangxi
Jinsheng Pharmaceutical Ltd.
|
5,027,350.42
|
737,009.57
|
5.6%
|
||||||
|
Jinan
Shiqiang Pharmaceutical Limited
|
4,965,811.97
|
727,988.03
|
5.5%
|
||||||
|
Chengdu
Huashida Pharmaceutical Co., Ltd.
|
4,666,666.68
|
684,133.34
|
5.2%
|
||||||
|
Total
|
31,882,350.47
|
4,673,952.58
|
35.3%
|
||||||
Over the
past several years, we have continuously expanded our distribution channels for
our products at the province and major city levels. In the near future, we plan
to penetrate China’s secondary cities. We are also seeking to expand into
international markets and have been granted a permit in Uzbekistan and are in
the process of securing a permit in Korea.
Competition
Competition
in the polypeptide drug industry is intense in China and throughout the world.
We compete with various firms, many of which produce and market products
similar to our products, and many of which, especially international
competitors, have greater resources than us in terms of manufacturing and
marketing capabilities, management expertise and breadth, and financial
wherewithal. Some of these competitors are far larger, have more resources
than us and have stronger sales and distribution networks.
Our
direct competitors are domestic firms engaged in developing, manufacturing and
marketing prescription polypeptide products. There are numerous such companies
in the PRC. We believe most Chinese synthetic peptide manufacturers are
very small-scale operations that use dated biotechnologies and have high
operating costs. Furthermore, due to technological limitations, their peptide
drugs have a relatively lower efficacy rate and lower purity (compared to
Western counterparts), as well as a higher incidence of potential side effects.
For instance, cheaper peptide drugs has relatively low purity of around
95%, which is the required purity by SFDS of the PRC and means the active
component accounts for 95% with 5 % of impurity and water. In contrast, our
peptide products have at least 99% purity and we believe we deliver top
quality peptide drugs in PRC.
13
Compared
to our Chinese competitors, we have a wider range of peptide drugs to offer than
any other domestic competitors as shown below:
|
Manufacturer/Brand
|
TP-5 power
for Injection
|
TP-5 Per-filled
injection
|
Thymosin
alpha 1 for
Injection
|
Somatostatin for
Injection
|
Desmopressin
Acetate Injection
(DDAVP)
|
|||||
|
Zhonghe
|
Hexin
|
Hexin
|
Heri
|
Hening
|
Heyi
|
|||||
|
Shenzhen
Hanyu
|
Hanqiang
|
Hankang
|
Hangu
|
|||||||
|
Beijing
SL
|
O’ning
|
Shanting
|
||||||||
|
Wuhan
Hualong
|
Wutai
|
|||||||||
|
Hayao
Group
|
Taipuding
|
|||||||||
|
Beijing
Shiqiao
|
Tongda
|
|||||||||
|
Hainan
Shuangcheng
|
Jitai
|
|||||||||
|
Chengdu
Di’ao
|
Maipuxin
|
|||||||||
|
Nanjing
Changao
|
Lizhixue
|
We are
also facing competition from multinational drug manufacturers that are doing
business in China. These well-established biopharmaceutical giants have
better resources and a proven track record for successful product development
and commercialization. These competitors may be able to develop more proficient
and more affordable peptide products. However, we believe we have price
advantages compared with multinational manufacturers. Our peptide products are
usually only 60% of that of similar international brand products. The
following table shows the products that certain international competitors are
marketing in China:
|
Manufacturer/Brand
|
TP-5 power
for Injection
|
TP-5 Per-filled
injection
|
Thymosin a1
for Injection
|
Somatostatin for
Injection
|
Desmopressin
Acetate Injection
(DDAVP)
|
|||||
|
Zhonghe
|
Hexin
|
Hexin
|
Heri
|
Hening
|
Heyi
|
|||||
|
Ferring
|
Minirin
|
|||||||||
|
Siclone
|
Zadaxin
|
|||||||||
|
Swiss
Serono
|
Stilanmin
|
14
Although
we have enjoyed advantages in the polypeptide market in China, we expect that
the competition for peptide products in the PRC will become more intense over
the next few years both from existing competitors and new market entrants.
We will also face more competition from foreign companies who may have
established products, a strong proprietary pipeline and strong financial
resources. Our management believes that we have certain competitive
advantages in introducing new products to market due to key focus areas for
development, our existing distribution channels, research and development
capabilities and our relationship with certain universities and other research
institutions.
Employees
As of
December 31, 2009, we have approximately 149 employees with 62 in manufacturing,
12 in quality assurance and controls, 26 in research and development, 25 in
sales and marketing, and 24 in management. 84 of the employees have a
bachelor or higher degree.
As
required by applicable Chinese law, we have entered into employment
contracts with all the employees. Key employees in the Company are also required
to sign a confidentiality and non-compete agreement prohibiting them from
disclosing our trade secrets or using them for purposes other than benefiting
the Company.
Our
employees in China participate in a state pension program organized by
Chinese municipal and provincial governments. We are required to
contribute to the program at the rate of 20% of the average monthly
salary. In addition, we are required by Chinese law to cover employees in
China with other types of social insurance. Our total contribution may amount to
as much as 30% or more of the average employee’s monthly salary. We have
purchased social insurance for all of our employees. Social insurance expenses
were approximately $84,329 and $82,372 for fiscal year 2008 and 2009,
respectively. We believe we have paid for all social insurance due in
accordance with Chinese law.
Government
Regulation
Regulatory
Environment
Our
principal market is in the PRC. We are subject to the Pharmaceutical
Administrative Law of the PRC, which governs the licensing, manufacturing,
marketing and distribution of pharmaceutical products in the PRC, and sets
penalties for violations. Our business is subject to various regulations
and permit systems of the State Food and Drug Administration of China (“SFDA”).
Additionally, we are subject to government licensing rights and regulations
relating to our peptide drug permits which are granted on a non-exclusive basis
and limited for four to five years.
15
SFDA
Licenses
The SFDA
issues licenses and petitions for permission to manufacture and market
pharmaceutical products in the PRC. Our licenses relate primarily to
medical manufacturing licenses. Peptide drug products also require a
permit for sales, which permits are generally granted on a non-exclusive basis
for five years.
Foreign-owned
Enterprise Law
Because
our subsidiary in the PRC is majority foreign-owned enterprise, we are subject
to the law of foreign investment enterprises in the PRC, and the foreign company
provisions of the Company Law of China, which governs the conduct of our
wholly-owned subsidiaries and their officers and directors, and also limits our
ability to pay dividends.
Compliance
with Environmental Law
We must
comply with the Environmental Protection Law of the PRC, as well as applicable
local regulations. In addition to compliance with the PRC law and local
regulations, we consistently undertake active efforts to ensure the
environmental sustainability of our operations. Because the manufacturing
of herb and plant-based products does not generally cause significant damage or
pollution to the environment, the cost of complying with applicable
environmental laws is not material. In the event we fail to comply with
applicable laws, we may be subject to penalties.
Intellectual
Property
We regard
our service marks, trademarks, trade secrets, patents and similar intellectual
property (“IP”) as critical to our business. We have relied, and will
continue to rely, on patent, trademark and trade secret law, as well as
confidentiality and license agreements with certain of our employees,
consultants, customers and others, to protect our proprietary
rights
Under the
PRC law, medical products which have received approval from the SFDA, have
automatic protected IP rights for a five-year period from the date of grant of
such approval. The sixteen drugs we are currently manufacturing have been
granted such licenses. We have applied for extensions for four of the
licenses, as their original five-year period expired in 2009.
Trademarks
We have
numerous registered trademarks for our polypeptide products.
16
Item
1A. Risk Factors
An
investment in our securities is speculative and involves a high degree of risk.
You should carefully consider the risks described below and the other
information in this current report before purchasing any securities. The risks
and uncertainties described below are not the only ones facing us. Additional
risks and uncertainties may also adversely impair our business operations. If
any of the events described in the risk factors below actually occur, our
business, financial condition or results of operations could suffer
significantly. In such case, the value of your investment could decline and you
may lose all or part of the money you paid to buy the securities.
Risks
Related To The Company
Our product line is limited
to only four products, Thymopentin (TP-5), Desmopressin Acetate (DDAVP),
Somatostatin (SS), and Thymosin Alpha 1. Any adverse effects upon the
manufacturing, sales, or distribution channels of any one of these four products
could adversely affect our financial condition and results of
operations.
Our four
products, TP-5, DDAVP, SS and Thymosin Alpha 1, are our major sources of
revenues, approximately 99.05% in 2008 and 98.39% in 2009. In the near future,
these four products will continue to be the major sources of our revenues.
Because of our reliance upon these products, any adverse effects upon the
manufacturing, sales, or distribution channels will affect our ability to
deliver our products to our customers, or increase our costs, which in turn will
adversely affect the orders for our products. This could result in adverse
effects upon our financial results and conditions.
A
significant portion of our revenue is concentrated on a few large customers. If
we lose one or more of them, our results of operations may be adversely
impacted.
In 2009,
7.6% of our total sales was from one single customer. Because we changed our
sales distribution process and require up-front payments for sales of our drugs,
we do not have large amounts of collectibles from our customers. However, we
cannot assure you that we will not go back to the old way of sales on credit. As
a consequence, we may have large amounts of collectibles from these large
customers. If we lose one or more large customers like these, our
financial condition and results of operations may be adversely
affected.
17
The long-term effectiveness
of peptide drugs has not been proven.
Our
business focuses almost exclusively on the manufacture and marketing of peptide
drugs. Our peptide drugs, including, TP-5, DDAVP, SS and Thymosin Alpha 1,
are relatively new types therapy drugs. We sold our first drug in 1997. Although
current studies on the use of peptide drug for treatment have shown a reduction
in short-term side effects compared to other drugs and suggest an improvement in
long-term results, there are presently very few long-term studies of over 15
years on the effectiveness of using peptide technology for treatment. We plan to
continue our participation in future long-term studies of the effectiveness of
our products. These long-term studies include a large scale clinical study in
collaboration with the Ministry of Health of the People’s Republic of China, or
MOH, based on the conditional approval received for our Investigational Device
Exemption (IDE) application. If any of these studies fail to confirm the
effectiveness of our peptide drug or other peptide medical products, our sales
could decline. Moreover, there may be other clinical studies published on our
drug products of which we are not aware and which contain different conclusions
with respect to the safety, effectiveness or other aspects of our technologies.
Our customers and users of our products may conclude that our products are not
an acceptable treatment regimen, that the technologies underlying our products
are ineffective or unsafe, or that our products are less effective or safe than
other drugs. This could result in a decrease in our sales, which would have a
material adverse effect on our business, results of operations and financial
condition.
Our focus on acquisitions of
new products or technologies may result in integration costs, failures and
dilution to existing stockholders.
We have
been relying on our affiliates and academic institutions to develop new drugs.
We continue to seek attractive opportunities to acquire new products or
technologies, particularly those that could assist us in advancing our current
market penetration, or in expanding our product offerings. If we decide to
acquire another company or its assets in order to obtain its products or
technologies, we would face a number of risks including consummating the
acquisition on unfavorable terms and not obtaining adequate financing, which may
adversely affect our ability to develop new products and services and to compete
in our rapidly changing marketplace. These acquisitions could also require that
our management develop expertise in new areas, manage new business relationships
and trade models, and attract new customers. Successful management and
integration of acquisitions are subject to a number of risks, including
difficulties in assimilating acquired operations and managing remote operations,
potential loss of key employees, diversion of management’s attention from
existing business operations, assumption of contingent liabilities and
incurrence of potentially significant write-offs, which may adversely affect our
business or results of operations. In addition, if we consummate such an
acquisition through an exchange of our securities, our existing stockholders
could suffer dilution.
If we fail to effectively
manage our distribution network, our business, prospects and brand may be
materially affected by actions taken by our distributors.
We have a
limited ability to manage the activities of our distributors, who are
independent from us since we rely exclusively on these independent distributors.
Our distributors could take one or more of the following actions, any of which
could have a material adverse effect on our business, prospects and
brand:
|
|
·
|
sell products that compete with
our products in breach of their non-competition agreements with
us;
|
|
|
·
|
fail to adequately promote our
products;
|
|
|
·
|
fail to provide proper service to
our end-users; or
|
18
|
|
·
|
violate the anti-corruption laws
of China.
|
Failure
to adequately manage our distribution network or the non-compliance of our
distributors with their obligations under distribution agreements with us could
harm our corporate image among end users of our products and disrupt our sales,
resulting in a failure to meet our goals for sales. The PRC government has
increased its anti-bribery efforts in the healthcare sector to reduce improper
payments received by hospital administrators and doctors in connection with the
purchase of pharmaceutical products. We can not guarantee that our distributors
will not violate these laws or otherwise engage in illegal practices with
respect to their sales or marketing of our products.
Our business may suffer if
we are unable to collect payments from customers of our products on a timely
basis.
Before
2006, we permitted our distributors and other customers to distribute our
products on credit. Our distributors and other customers must make a significant
commitment of capital to purchase our products. Any downturn in the businesses
of our distributors and other customers of our products could reduce their
willingness or ability to pay us. Therefore, historically we have not been able
to collect all of our accounts receivable from our distributors and other
customers. After 2006, we required our distributors and other customers to make
payments before we deliver our products to them. Consequently, we have been able
to collect all of our receivable since 2006. However, we are still not able to
collect all our receivable before 2006. The failure of any of our distributors
and other customers of our products to make timely payments could require us to
recognize an allowance for doubtful accounts, which could have a material
adverse effect on our results of operations and financial
conditions.
We are subject to product
liability exposure and have limited insurance coverage.
Our
products are for the treatment of patients and we are exposed to potential
product liability claims in the event that the use of our products cause or are
alleged to have caused personal injuries or other adverse effects. A
successful product liability claim against us could require us to pay
substantial damages. Product liability claims against us, whether or not
successful, are costly and time-consuming to defend. Also, in the event that our
products proven to be defective, we may be required to recall or redesign such
products. We do not have any product liability insurance policy to cover
potential product liability arising from the use of our products. To date, we
have not been subject to any product liability claim yet, but we cannot assure
you that such claim will not be brought against us in the future. A product
liability claim, with or without merit, could result in significant adverse
publicity against us, and could have a material adverse effect on the
marketability of our products and our reputation, which in turn, could have a
material adverse effect on our business, financial condition and results of
operations.
19
Our limited operating
history makes evaluating our business and prospects
difficult.
We
commenced operations in 1995, and started to market and sell our products in
1997. Our limited operating history may not provide a meaningful basis for you
to evaluate our business, financial performance and prospects. We may not have
sufficient experience to address the risks frequently encountered by early-stage
companies, and as a result we may not be able to:
|
|
·
|
maintain
profitability;
|
|
|
·
|
preserve our leading position in
the market of peptide drug;
|
|
|
·
|
acquire and retain
customers;
|
|
|
·
|
attract, train, motivate and
retain qualified personnel;
|
|
|
·
|
keep up with evolving industry
standards and market
developments;
|
|
|
·
|
increase the market awareness of
our products;
|
|
|
·
|
respond to competitive market
conditions;
|
|
|
·
|
maintain adequate control of our
expenses;
|
|
|
·
|
manage our relationships with our
suppliers and distributors;
or
|
|
|
·
|
protect our proprietary
technologies.
|
If we are
unsuccessful in addressing any of these risks, our business may be materially
and adversely affected.
A significant interruption
in supply could prevent or limit our ability to accept and fulfill orders for
our products.
We
purchase all our materials from third-party suppliers. Currently, we do not have
any material long-term supply contracts with our suppliers. Our purchases
are made on a purchase order basis. We have one major supplier (>50%), thus
there is the significant risk that the supply of certain materials will be
interrupted. In that case, our manufacturing process would be delayed. We may be
unable to secure alternative sources of supply in a timely and cost-effective
manner, which could impair our ability to manufacture our products or decrease
our costs, harm our reputation and cause us to lose sales and orders for our
products. Any of these occurrences could have a material adverse impact on our
business, financial condition and results of operations.
20
We
generate a substantial portion of our revenues from sales of our Thymopentin
drug and a reduction in revenues of our Thymopentin drug would
cause our revenues to decline and could materially harm our
business.
We derive
a substantial percentage of our revenues from sales of the Thymopentin, or TP-5
drug. Our TP-5 drug accounted for 53% and 43% of our total revenues for the
fiscal years ended December 31, 2008 and December 31, 2009, respectively.
The segment income derived from the sale of TP-5 products was $6.17 million and
$5.75 million for the fiscal years ended December 31, 2008 and
December 31, 2009, respectively. Going forward, continued market
acceptance of our TP-5 drug will remain important to our success, and a
reduction in revenues from sales of our TP-5 drug will have a direct negative
impact on our business, financial condition and results of
operations.
Rapid growth and a rapidly
changing operating environment may strain our limited
resources.
Our
growth strategy includes our efforts to build our brand, develop new products,
and accelerate market acceptance of our products. This growth strategy requires
significant capital resources, and we may not generate an adequate return on our
investment. Our growth may involve the acquisition of new technologies,
businesses, products or services, the creation of strategic alliances in areas
in which we do not currently operate or the expansion of our distributor network
and direct sales force. This could require our management to develop expertise
in new areas, manage new business relationships and attract new types of
customers. We may also experience difficulties integrating these acquired
businesses, products or services into our existing business and operations. The
success of our growth strategy also depends in part on our ability to utilize
our financial, operational and management resources and to attract, train,
motivate and manage an increasing number of employees.
Our
drug-development program depends upon third-party research scientists who are
out of our control.
We depend
upon independent investigators and collaborators, such as universities and
medical institutions, to conduct our pre-clinical and clinical trials under
agreements with us. These collaborators are not our employees and we cannot
control the amount or timing of resources that they devote to our programs.
These investigators may not assign as great a priority to our programs or pursue
them as diligently as we would if we were undertaking such programs ourselves.
If outside collaborators fail to devote sufficient time and resources to our
drug-development programs, or if their performance is substandard, the approval
of our applications, if any, and our introduction of new drugs, if any, will be
delayed. These collaborators may also have relationships with other commercial
entities, some of whom may compete with us. If our collaborators assist our
competitors at our expense, our competitive position would be
harmed.
21
If we fail to increase
awareness and acceptance of our drugs in the medical community and among
patients, we will not be able to grow or even sustain the market for our peptide
drugs.
Our
peptide drugs, including, TP-5, DDAVP, SS, and Thymosin Alpha 1, use a
relatively new solid phase peptide synthesis technology and purification
technology. To achieve greater penetration of the potential market in China, we
must increase market awareness and use of our peptide drugs, which depend on,
among other things, the following:
|
|
·
|
the general levels of awareness
and acceptance in the medical community and among patients of peptide
drugs;
|
|
|
·
|
the amount of resources we have
available to increase product awareness and to educate potential
purchasers and users of our peptide
drugs;
|
|
|
·
|
our ability to provide good
technical support and customer service;
and
|
|
|
·
|
our ability to keep up with
technological changes and remain
competitive.
|
We may
not have the financial and operational resources required to promote awareness
and acceptance of our peptide drugs as widely or rapidly as is necessary to grow
or sustain the market for our peptide drugs. If we fail to increase awareness
and acceptance of our peptide drugs in the medical community and among patients,
we will not be able to grow, or even sustain, the market for our peptide drugs
as planned and our financial condition and results of operations will be harmed.
The amount of resources we have available to marketing our products is
approximately US $1 million.
We face fierce price
competitions after the exclusive licensing rights from Chinese SFDA of our drugs
expire and our financial conditions will be adversely
impacted.
Our drugs
face fierce price competitions from generic drugs manufactured by small to
mid-sized drug manufacturers. Even though the quality of these generic drugs is
inferior to our drugs, the price of these generic drugs is very
attractive. Once the exclusive licensing rights from Chinese SFDA for our
drugs expire, our financial results will be adversely affected because of
competitions from these generic drugs.
We may face significant
challenges in the progress toward our strategic objectives which may adversely
affect our financial results and conditions.
We face
significant obstacles in our quest for new markets, such as costs for
penetrating new markets, the hire and retention of sufficient qualified sales
and distribution staff members, implementation of overseas expansion efforts,
and establishment and maintenance of a model system. We cannot guarantee the
success of these strategies or objectives. We make our business plans and
strategies based on today’s situation and certain assumptions. There are
inherent risks and uncertainties within each stages of the development.
These significant obstacles could adversely affect our results of
operations and financial conditions.
22
If we fail to protect our
intellectual property rights, our competitors may take advantage of our
proprietary technology and know-how and compete directly against
us.
We have
not obtained any patent rights for our products in China yet. We applied for
patent rights in China for the following inventions: Acetic acid to
ammonia pressure injection, Thymopentin nasal spray’s preparation and
application and Thymopentin injection prescription. We have obtained the
approval of the Food and Drug Administration Bureau of South Korea for our TP-5
products in 2009. We are in the process of applying to Indonesia Food and Drug
Administration for certification.
Implementation
of PRC intellectual property-related laws has historically been lacking,
primarily because of ambiguities in the PRC laws and difficulties in
enforcement. Accordingly, intellectual property rights and confidentiality
protections in the PRC may not be as effective as in the United States
or other developed countries. Policing unauthorized use of proprietary
technology is difficult and expensive, and we might need to resort to litigation
to enforce our rights or defend us, or to determine the enforceability, scope
and validity of our proprietary rights or those of others. Such litigation may
require significant expenditure of cash and management efforts and could harm
our business, financial condition and results of operations. An adverse
determination in any such litigation will impair our intellectual property
rights and may harm our business, competitive position, and business
prospects.
We may be exposed to
intellectual property infringement and other claims by third parties, which, if
successful, could cause us to pay significant damage awards and incur other
costs.
While we
believe that the technology we use is not protected by any patent or
intellectual property rights, we face the risk of being the subject of
intellectual property infringement claims. The defense and prosecution of
intellectual property suits, patent opposition proceedings and related legal and
administrative proceedings can be both costly and time consuming and may
significantly divert the efforts and resources of our technical and management
personnel. An adverse determination in any such litigation or proceedings to
which we may become a party could subject us to significant liability, including
damage awards to third parties, require us to seek licenses from third parties,
to pay ongoing royalties, or to redesign our products or subject us to
injunctions preventing the manufacture and sale of our products. Protracted
litigation could also result in our customers or potential customers deferring
or limiting their purchase or use of our products until resolution of such
litigation.
23
Our operations might be
interrupted by the occurrence of a natural disaster or other catastrophic
events.
Almost
all of our manufacturing and research and development facilities are located in
a single location in Hainan Province, China. Typhoon happens frequently during
the summer in Hainan Province, which will possibly ruin our manufacturing
facilities. In addition, other natural disasters or catastrophic events,
including power interruptions, water shortages, storms, fires, earthquakes,
terrorist attacks and wars could disrupt our operations. Although we have some
spare facilities, we do not maintain all back-up facilities for the continued
operation of our business in the above circumstances. We might suffer losses as
a result of business interruptions and our operations and financial results
might be materially and adversely affected should these catastrophic events
occur. Moreover, any such event could delay our research and development
programs which will adversely impact our business operations and financial
results.
If we are unable to
successfully operate and manage our manufacturing operations, we may experience
a decrease in revenues.
As we
ramp up our manufacturing operations to accommodate our planned growth, we may
encounter difficulties associated with increasing production scale, including
shortages of qualified personnel to operate our equipment or manage
manufacturing operations, as well as shortages of key raw materials for our
products. In addition, we may also experience difficulties in producing
sufficient quantities of products or in achieving desired product quality. If we
are unable to successfully operate and manage our manufacturing operations to
meet our needs, we may not be able to provide our customers with the quantity or
quality of products they require in a timely manner. This could cause us to lose
customers and result in reduced revenues.
Our future capital needs are
uncertain and we may need to raise additional funds in the future.
We may
require additional cash resources in the future due to changed business
conditions or other future developments. We cannot assure you that our revenues
will be sufficient to meet our operational needs and capital requirements in the
future. In the past, we have not encountered difficulties in obtaining
financing. However, we cannot assure you that financing will be available in
amounts or on terms acceptable to us. Our future capital needs and other
business reasons could require us to sell additional equity or debt securities
or obtain a credit facility. The sale of additional equity or equity-linked
securities could result in additional dilution to our shareholders. The
incurrence of indebtedness would result in increased debt service obligations
and could result in operating and financing covenants that would restrict our
operations or our ability to pay dividends to our shareholders.
Risks
Related To Our Management and Internal Control
We may not be able to
achieve and maintain an effective system of internal
control over financial reporting, a failure of which may prevent us from
accurately reporting our financial results or detecting and preventing
fraud.
We are
constantly striving to establish and improve our business management and
internal control over financial reporting to forecast, budget and allocate our
funds. However, as a Chinese company that has recently become a US public
company, we face difficulties in hiring and retaining a sufficient
number of qualified employees to achieve and maintain an effective system of
internal control over financial reporting in a short period of time. As a
result, we may experience difficulty in collecting financial data and preparing
financial statements, books of account and corporate records, and instituting
business practices that meet international standards in a short
period.
24
We depend on key personnel
for our business operations, whose discontinuance could incur high replacement
costs.
Our
future success depends substantially on the continued services of our executive
officers, especially Mr. Xueyun Cui, our chairman, Mr. Xiaoqun Ye, our chief
executive officer, and chief quality control officer, Zhenhong Ling. Although we
have long-term employment contracts with management personnel, if one or more of
our key executive officers are unable or unwilling to continue in their present
positions, we may not be able to replace them readily, if at all. Therefore, our
business may be severely disrupted, and we may incur additional expenses
and require additional time to recruit and retain new
officers.
Risks
Related To The Industry
Disruptions in the capital
and credit markets related to the current national and worldwide financial
crisis, which may continue indefinitely or intensify, could adversely affect our results
of operations, cash flows and financial condition, or those of our customers and
suppliers.
The
current disruptions in the capital and credit markets may continue indefinitely
or intensify, and adversely impact our results of operations, cash flows and
financial condition, or those of our customers and suppliers. Disruptions in the
capital and credit markets as a result of uncertainty, changing or increased
regulation, reduced alternatives or failures of significant financial
institutions could adversely affect our access to liquidity needed to conduct or
expand our businesses or conduct acquisitions or make other discretionary
investments, as well as our ability to effectively hedge our currency or
interest rate. Such disruptions may also adversely impact the capital needs of
our customers and suppliers, which, in turn, could adversely affect our results
of operations, cash flows and financial condition.
In order to manufacture and
market our products, we are required to obtain various authorizations from
governmental regulatory authorities in China and other countries. If we fail to
obtain clearance or approvals in a timely fashion, our business may be
significantly affected.
The sale
and marketing of our products are subject to regulation in China. We are
required to obtain registrations with the State Food and Drug Administration (
the “SFDA”) and the regulatory authorities in charge of the approval in
countries where we plan to export. The process for obtaining regulatory
clearances or approvals can be lengthy and expensive, and the results are
unpredictable. In addition, the relevant regulatory authorities may introduce
additional requirements or procedures that have the effect of delaying or
prolonging the regulatory clearance or approval for our existing or new
products. If we are unable to obtain clearances or approvals needed to market
existing or new products, or obtain such clearances or approvals in a timely
fashion, our business could be significantly disrupted, and sales and
profitability could be materially and adversely affected.
25
We are
required to obtain registration certificates from the SFDA in order to sell our
drugs. We will need to renew the registration certificates once they expire. We
are also required to obtain production permits from the provincial level food
and drug administration before commencing the manufacture of our products. Once
our production permits for the manufacture of our products expire, we will need
to renew such production permits. We do not foresee any significant
difficulties in obtaining such renewal. But if we fail to obtain such renewal in
a timely fashion, our business may be adversely affected.
In April
2007, the SFDA announced a new regulation that was implemented on
October 1, 2007. Reagents used for IVD testing are divided into six
different categories, Classes I through VI, depending on the degree of risk
associated with each reagent, with the lower number of class representing longer
period of exclusive licensing right. We list our products below to
classify them into different categories.
|
Product Name
|
Category
|
Registration certificate expire date
|
||
|
Thymopentin
for Injection (TP-5)
|
Class
5
|
Dec
2010
|
||
|
Desmopressin
Acetate Injection (DDAVP)
|
Class
6
|
May
2011
|
||
|
Somatostatin
for Injection (SS)
|
Class
6
|
Nov
2014
|
||
|
Thymosin
a1 for Injection (Alpha 1)
|
Class
4
|
Sep.
2010
|
We are
required to obtain a registration certificate for each reagent prior to selling
that reagent for clinical use. However, a reagent that is used for research
purpose only is exempt from registration and/or approval. A reagent kit intended
for research use only must comply with the labeling requirements that present
the statement: “For research use only. Not for use in diagnostic procedures” on
the package. Before receiving the necessary registration certificates,
these products can be sold for research only. Thus, this may delay the surge of
sales in these products.
Outside
the PRC, our ability to market any of our potential products is contingent upon
receiving marketing authorizations from the appropriate foreign regulatory
authorities. These foreign regulatory approval processes include all of the
risks associated with the SFDA approval process described above and may include
additional risks.
26
We may not be able to comply
with applicable good manufacturing practice requirements and other regulatory
requirements, which could have a material adverse effect on our business,
financial condition and results of operations.
We are
required to comply with applicable good manufacturing practice regulations,
which include requirements relating to quality control and quality assurance as
well as corresponding maintenance, record-keeping and documentation standards.
Manufacturing facilities must be approved by governmental authorities
before we can use them to commercially manufacture our products and are subject
to inspection by regulatory agencies.
If we
fail to comply with applicable regulatory requirements at any stage during the
regulatory process, including following any product approval, we may be subject
to sanctions, including:
|
|
·
|
fines;
|
|
|
·
|
product recalls or
seizure;
|
|
|
·
|
injunctions;
|
|
|
·
|
refusal of regulatory agencies to
review pending market approval applications or supplements to approval
applications;
|
|
|
·
|
total or partial suspension of
production;
|
|
|
·
|
civil penalties;
and
|
|
|
·
|
withdrawals of previously
approved marketing
applications.
|
Unpredictable local policy
changes where our factories are located may adversely affect our
business.
Our
business activities are regulated by Chinese laws and local regulations
regarding food, nutritional products and medicine. Some of these local
regulations are related to administrative permits and approvals. The
expiration or restrictions on these permits or approvals for our drugs may
adversely affect our sales. Recent policy changes on drug sales
dramatically affect our drug sales price and sales entrance to various
hospitals. As a result, these changes may adversely affect our results of
operations and financial conditions.
New product development in
the medicine
and supply industry is both costly and labor-intensive and has a very low rate
of successful commercialization.
Our
success will depend in part on our ability to enhance our existing
products/drugs and to develop and acquire new drugs. The development process for
medical products is complex and uncertain, as well as time-consuming and costly.
Product development requires the accurate assessment of technological and market
trends as well as precise technological execution. We cannot assure you
that:
27
|
|
·
|
our product/drug development will
be successfully completed;
|
|
|
·
|
necessary regulatory clearances
or approvals will be granted by SFDA, or other regulatory bodies as
required on a timely basis, or at all;
or
|
|
|
·
|
any product/drug we develop can
be commercialized or will achieve market
acceptance.
|
Also, we
may be unable to locate suitable products/drugs to acquire or acquire such
products/drugs on commercially reasonable terms. Failure to develop or acquire,
obtain necessary regulatory clearances or approvals for, or successfully
commercialize or market potential new products/drugs could have a material
adverse effect on our financial condition and results of
operations.
Clinical trials are very
expensive, time-consuming and difficult to design and
implement.
Human
clinical trials are very expensive and difficult to design and implement, in
part because they are subject to rigorous regulatory requirements. The clinical
trial process is also time consuming. We estimate that clinical trials of our
product candidate will take at least several years to complete. Furthermore,
failure can occur at any stage of the trials, and we could encounter problems
that cause us to abandon or repeat clinical trials. The commencement and
completion of clinical trials may be delayed by several factors, including
with no limitation:
|
|
·
|
unforeseen safety
issues;
|
|
|
·
|
determination of dosing
issues;
|
|
|
·
|
lack of effectiveness during
clinical trials;
|
|
|
·
|
slower than expected rates of
patient recruitment;
|
|
|
·
|
inability to monitor patients
adequately during or after treatment;
and
|
|
|
·
|
inability or unwillingness of
medical investigators to follow our clinical
protocols.
|
In
addition, the SFDA, any SFDA-equivalent in foreign jurisdictions, may suspend
our clinical trials at any time if it appears that we are exposing participants
to unacceptable health risks or if the regulatory bodies find deficiencies in
our Investigational New Drug, or IND, submissions or the conduct of these
trials. Therefore, we cannot predict with any certainty the schedule for future
clinical trials.
28
The results of our clinical
trials may not support our product candidate claims.
Even if
our clinical trials are completed as planned, we cannot be certain that their
results will support our product candidate claims. Success in pre-clinical
testing and early clinical trials does not ensure that later clinical trials
will be successful, and we cannot be sure that the results of later clinical
trials will replicate the results of prior clinical trials and pre-clinical
testing. The clinical trial process may fail to demonstrate that our product
candidates are safe for humans and effective for indicated uses. This failure
would cause us to abandon a product candidate and may delay development of
other product candidates.
Physicians, patients and
other end consumers may abandon existing or chose not to accept and use our new
drugs.
Physicians
and patients may not accept and use our products. Acceptance and use of our
product will depend upon a number of factors including:
|
|
·
|
perceptions by members of the
health care community, including physicians, about the safety and
effectiveness of our
products;
|
|
|
·
|
post-effectiveness of our product
relative to competing products;
and
|
|
|
·
|
effectiveness of marketing and
distribution efforts by us and our licensees and distributors, if
any.
|
Because
we expect sales of our current and future products to generate substantially all
of our product revenues for the foreseeable future, the failure to find market
acceptance would harm our business and could require us to seek additional
financing.
Competition in the markets
in which we operate is expected to increase in the future.
Certain
of our existing and potential competitors have significantly greater financial,
research and development, sales and marketing, personnel resources and other
resources than we do. Competition will intensify as other companies enter our
markets. Competing companies may succeed in developing products that are more
effective or less costly than those that we may offer, and these companies may
also be more successful in marketing their products. Competing companies may
also introduce competitive pricing measures that adversely affect our sales
levels and margins. If we do not adequately address our competitive challenges,
we could lose sales and market share and fail to grow our business as planned,
which would have a material adverse effect on our financial condition, results
of operations and future growth.
In
addition, we believe that corrupt practices in the healthcare industry in China
still occur. In order to increase sales, certain manufacturers or distributors
of medical devices may pay kickbacks to hospital personnel who make procurement
decisions. We prohibit our employees from engaging in such practices and, to our
knowledge, none of our distributors engages in such practices. However, as
competition intensifies in the medical device and supplies industry in China, we
may lose sales, customers or contracts to competitors to the extent we or our
distributors refuse to engage in such practices.
29
We face competition from
multi-national corporations with more resources where we are at a
disadvantage.
In the
poly peptide drug industry, there are competitions from multi-national
conglomerates. With the fast-changing nature of the drug industry, we face
competitions from these multi-national corporations which have better resources.
These corporations have more diversified products, longer manufacturing and
sales history and better commercialization of their products. Competitions
from these international players may have adverse impacts on our business
conditions.
We face intense competition
that may prevent us from maintaining or increasing market share for our existing
products and gaining market acceptance of our future
products. Our competitors may develop or commercialize products before or more
successfully than us.
The
pharmaceutical market in China is intensely competitive, rapidly evolving and
highly fragmented. Our competitors may develop products that are superior to or
more affordable than ours or they may more effectively market products that
compete with ours. We face direct competition from manufacturers of other
medicines that are similar to our products. We also face competition from
western manufacturers of medicines, including multinational companies, that
manufacture medicines with similar curative effects and that can be used as
substitutes for our products. Many of our existing and potential competitors
have substantially greater financial, technical, manufacturing and other
resources than we do. Our competitors’ greater size in some cases provides them
with a competitive advantage with respect to manufacturing costs because of
their economies of scale and their ability to purchase raw materials at lower
prices. Many of our competitors also have better brand name recognition, more
established distribution networks and larger customer bases. In addition, many
of our competitors have extensive knowledge of our target markets. As a result,
they may be able to devote greater resources to the research, development,
promotion and sale of their products or respond more quickly to evolving
industry standards and changes in market conditions than we can. Our failure to
adapt to changing market conditions and to compete successfully with existing or
new competitors may materially and adversely affect our financial condition and
results of operations.
The production of our
products/drugs depends on the supply of quality medicinal raw
materials.
The
production of our products/drugs depends on the supply of raw materials of
suitable quality. The supply and market prices of these raw materials may be
adversely affected by various factors such as weather conditions and the
occurrence of natural disasters or sudden increases in demand that would impact
our costs of production. There is no assurance that we would be able to pass on
any resulting increase in costs to our customers and therefore any substantial
fluctuation in supply or the market prices of raw materials may adversely affect
our results of operations and profitability.
30
If we do not keep pace with
rapid technological change, we will be unable to capture and sustain a
meaningful market position.
The
pharmaceutical industry in China is characterized by rapid changes in
technology, constant enhancement of industrial know-how and the frequent
emergence of new products. Future technological improvements and continued
product developments in the pharmaceutical market may render our existing
products obsolete or affect their viability and competitiveness. Therefore, our
future success will largely depend on our ability to improve our existing
products, diversify our product range and develop new and competitively priced
products that can meet the requirements of the changing market. Should we fail
to respond to these frequent technological advances by improving our existing
products or developing new products in a timely manner, or should these products
do not achieve a desirable level of market acceptance, this may adversely affect
our business and profitability.
The ongoing anti-corruption
campaign initiated by the Chinese government targeting state-owned hospitals
could adversely affect our sales designated for hospitals.
The
Chinese government has recently launched a nationwide campaign against corrupt
practices that have been frequently engaged by state-owned hospitals in China,
including their acceptance of kickbacks or other illegal gains and benefits in
connection with their providing medical services and purchasing medical
equipment and medicines. In mid-2006, the PRC Ministry of Health ordered all
state-owned hospitals to review, among other things, their procurement policies
and procedures and self-correct problems and deficiencies, if any, by the end of
2006. As a result of this campaign, many state-owned hospitals have since
diverted a significant portion of their attention and resources to their
self-inspection and self-correction activities and are reviewing their
procurement policies. If the anti-corruption campaign becomes more
intensified, causing a significant change to the hospitals’ procurement policies
and procedures or otherwise resulting in a further delay for state-owned
hospitals to resume their normal procurement of our products, our sales
designated for hospitals, which account for a very substantial portion of our
total sales, could be adversely affected.
Risks
Related To Doing Business In China
We face the risk that
changes in the policies of the PRC government could have a significant
impact upon the business we may be able to conduct in the PRC and the
profitability of such business.
All of
our business operations are conducted in the PRC. Accordingly, our business,
financial condition, results of operations and prospects are affected
significantly by economic, political and legal developments in the PRC. The
Chinese economy differs from the economies of most developed countries in many
respects, including level of government involvement in economic activities,
stage of national development, and control of foreign exchange.
31
While the
Chinese economy has grown significantly in the past twenty years, the
growth has been uneven, both geographically and among various sectors of the
economy. Policies of the PRC government can have significant effects on the
economic conditions of the PRC. The PRC government has confirmed that economic
development will follow the model of a market economy. Under this direction, we
believe that the PRC will continue to strengthen its economic and trading
relationships with foreign countries and business development in the PRC will
follow market forces. While we believe that this trend will continue, we cannot
assure you that this will be the case. A change in policies by the PRC
government could adversely affect our interests by, among other factors: changes
in laws, regulations or the interpretation of laws and regulations, confiscatory
taxation, restrictions on currency conversion, imports or sources of supplies,
or the expropriation or nationalization of private enterprises. Although the PRC
government has been pursuing economic reform policies for more than two decades,
we cannot assure you that the government will continue to pursue such policies
or that such policies may not be significantly altered, especially in the
event of a change in leadership, social or political disruption, or other
circumstances affecting the PRC’s political, economic and social
life.
Recent PRC regulations
relating to the establishment of offshore companies by PRC residents may
subject our PRC resident shareholders to personal liability and limit our
ability to inject capital into our PRC subsidiaries, limit our
subsidiaries’ ability to distribute
profits to us or otherwise adversely affect us.
China
State Administration of Foreign Exchange, or the SAFE, issued a public circular
on October 21, 2005 concerning the acquisition by an offshore company
controlled by PRC residents of onshore assets in China. This circular requires
that (1) a PRC resident shall register with a local branch of the SAFE
before he or she establishes or controls an overseas special purpose vehicle, or
SPV, for the purpose of overseas equity financing (including convertible debt
financing); (2) when a PRC resident contributes the assets of or his
or her equity interests in a domestic enterprise to an SPV, or engages in
overseas financing after contributing assets or equity interests to an SPV, such
PRC resident must register his or her interest in the SPV and any changes in
such interest with a local branch of the SAFE; and (3) when the SPV
undergoes a material change outside of China, such as a change in share capital
or merger or acquisition, the PRC resident shall, within 30 days from the
occurrence of the event that triggers the change, register such change with a
local branch of the SAFE. Furthermore, PRC residents who are shareholders of
SPVs established before November 1, 2005 are required to register with a
local branch of the SAFE before March 31, 2006.
The
beneficial owners of our company who are PRC residents are required to update
their respective registrations with the local branch of the SAFE. However, we
cannot assure you that these beneficial owners will update their registrations
with the local branch of the SAFE in full compliance with the October 2005 SAFE
circular. The failure or inability of beneficial owners of our company who are
resident in the PRC to comply with the registration procedures set forth in the
October 2005 SAFE circular may subject these beneficial owners to fines and
legal sanctions and may also limit our ability to contribute additional capital
into our PRC subsidiaries, limit our PRC subsidiaries’ ability to distribute
dividends to us or otherwise adversely affect our business.
32
The approval of the China Securities
Regulatory Commission, or the CSRC, may be required in connection with this
transaction under a recently adopted PRC regulation.
On
August 8, 2006, six PRC regulatory agencies, the Ministry of Commerce, the
State-owned Assets Supervision and Administration Commission, or SASAC, the
State Administration for Taxation, the State Administration for Industry and
Commerce, the CSRC, and the SAFE, jointly adopted the Regulations on Mergers and
Acquisitions of Domestic Enterprises by Foreign Investors, or the New M&A
Rule, which became effective on September 8, 2006. This New M&A Rule
requires offshore SPVs that are controlled by PRC individuals and that have been
formed through acquisitions of PRC domestic companies for the purpose of seeking
a public listing on a stock exchange outside China to obtain CSRC approval prior
to publicly listing their securities on a stock exchange outside China.
Previously the CSRC approval was not needed for this type of listing on a stock
exchange outside China. On September 21, 2006, the CSRC published a notice
on its website specifying the documents and materials that SPVs are required to
submit when seeking CSRC approval for their listings outside of China. The
interpretation and application of the New M&A Rule remain unclear, and
we cannot assure you that this transaction does not require approval from the
CSRC. These uncertainties could inhibit or delay the completion of this
transaction. On the other hand, if CSRC approval is required for this
transaction, our failure to obtain or delay in obtaining the CSRC approval for
this transaction would subject us to sanctions imposed by the CSRC and other PRC
regulatory agencies. These sanctions could include fines and penalties on our
operations in China, restriction or limitation on our ability to pay dividend
outside of China, and other forms of sanctions that may cause a material and
adverse effect on our business, results of operations and financial
conditions.
The New
M&A Rule also established additional procedures and requirements that are
expected to make merger and acquisition activities by foreign investors more
time-consuming and complex, including requirements in some instances that the
Ministry of Commerce be notified in advance of any change-of-control transaction
in which a foreign investor takes control of a PRC domestic enterprise that owns
well-known trademarks or China’s traditional brands. We may grow our business in
part by acquiring other traditional Chinese medicine businesses. Complying
with the requirements of the New M&A Rule in completing this type of
transactions could be time-consuming, and any required approval processes,
including Ministry of Commerce approval, may delay or inhibit our ability to
complete such transactions, which could affect our ability to expand our
business or maintain our market share.
Your legal protection could
be limited. Our business is largely subject to the uncertain legal environment
in China.
The
Chinese legal system is a civil law system based on written statutes. Unlike
common law systems, it is a system in which precedents set in earlier legal
cases are not generally used. The overall effect of legislation enacted over the
past twenty years has been to enhance the protections afforded to foreign
invested enterprises in China. However, these laws, regulations and legal
requirements are relatively recent and are evolving rapidly, and their
interpretation and enforcement involves uncertainties. These uncertainties could
limit the legal protections available to foreign investors, such as the right of
foreign invested enterprises to hold licenses and permits such as requisite
business licenses. Laws, regulations or enforcement policies in China, including
those regulating medical devices and supplies, are evolving and subject to
future change.
33
You may experience
difficulties in effecting service of legal process and enforcing judgments
against us and our management in China.
Substantially
all of our assets and our subsidiaries are located in China. In addition, all of
our directors and officers reside within China, including our certified public
accountant (“CPA”). As a result, it may not be possible to affect service of
process within the United States or elsewhere outside of China upon most of our
directors and officers and our CPA, including with respect to matters arising
under the U.S. federal securities laws or applicable state securities laws.
Moreover, China is not a party to any treaties providing for reciprocal
enforcement of judgments of courts with the United States or most other western
jurisdictions. As a result, recognition and enforcement in China of judgments of
a court in the United States or any other jurisdictions mentioned above in
relation to any matter may be difficult or impossible. In addition, you
will have difficulties in bring an original action in a Chinese court to enforce
liabilities against our directors, officers and our CPA based upon the U.S.
federal securities laws.
The
Chinese government exerts substantial influence over the manner
in which we must conduct our business activities.
China
only recently has permitted provincial and local economic autonomy and private
economic activities. Chinese government has exercised and continues to exercise
substantial control over virtually every sector of the Chinese economy through
regulation and state ownership. Our ability to operate in China may be harmed by
changes in its laws and regulations, including those relating to taxation,
import and export tariffs, environmental regulations, land use rights, property
and other matters. We believe that our operations in China are in material
compliance with all applicable legal and regulatory requirements. However, the
central or local governments of these jurisdictions may impose new, stricter
regulations or interpretations of existing regulations that would require
additional expenditures and efforts on our part to ensure our compliance with
such regulations or interpretations. Accordingly, government actions in the
future, including any decision not to continue to support recent economic
reforms and to return to a more centrally planned economy, or regional or local
variations in the implementation of economic policies, could have a significant
effect on economic conditions in China or particular regions thereof, and could
require us to divest ourselves of any interest we hold in Chinese
properties.
Inflation in China may
inhibit our activity to conduct business in China.
In recent
years, the Chinese economy has experienced periods of rapid expansion and high
rates of inflation. During the past ten years, the rate of inflation in China
has been as high as 20.7% and as low as 2.2%. These factors have led to the
adoption by Chinese government, from time to time, of various corrective
measures designed to restrict the availability of credit or regulate growth and
contain inflation. While inflation has been more moderate since 1995, high
inflation may in the future cause Chinese government to impose controls on
credit and/or prices, or to take other action, which could inhibit economic
activity in China, and thereby harm the market for our
products.
34
Restrictions
on currency exchange may limit our ability to receive and use our revenues
effectively .
The
majority of our revenues will be settled in RMB or Chinese Yuan and U.S.
Dollars, and any future restrictions on currency exchanges may limit our ability
to use revenue generated in RMB to fund any future business activities outside
China or to make dividend or other payments in U.S. dollars. Although the
Chinese government introduced regulations in 1996 to allow greater
convertibility of the RMB for current account transactions, significant
restrictions still remain, including primarily the restriction that
foreign-invested enterprises may only buy, sell or remit foreign currencies
after providing valid commercial documents at those banks in China authorized to
conduct foreign exchange business. In addition, conversion of RMB for capital
account items, including direct investment and loans, is subject to governmental
approval in China, and companies are required to open and maintain separate
foreign exchange accounts for capital account items. We cannot be certain that
the Chinese regulatory authorities will not impose more stringent restrictions
on the convertibility of the RMB.
The fluctuation of the RMB
may materially and adversely affect your investment.
The value
of the RMB against the U.S. dollar and other currencies may fluctuate and is
affected by, among other things, changes in China’s political and economic
conditions. The conversion of RMB into foreign currencies, including U.S.
dollars, has historically been set by the People’s Bank of China. On
July 21, 2005, the PRC government changed its policy of pegging the value
of the RMB to the U.S. dollar. Under the new policy, the RMB is permitted to
fluctuate within a band against a basket of certain foreign currencies,
determined by the Bank of China, against which it can rise or fall by as much as
0.3% each day. This change in policy resulted in an approximately 17.5%
appreciation in the value of the RMB against the U.S. dollar between
July 21, 2005 andDecember 31, 2009. Since the adoption of this new
policy, the value of RMB against the U.S. dollar has fluctuated on a daily basis
within narrow ranges, but overall has further strengthened against the U.S.
dollar. There remains significant international pressure on the PRC government
to further liberalize its currency policy, which could result in a further and
more significant appreciation in the value of the RMB against the U.S. dollar.
As we import certain materials and supplies for reagent kits from the United
States, Finland and Sweden, fluctuations in the value of the RMB against the
currencies of those countries may increase the cost of our reagent kits.
For example, to the extent that we need to convert U.S. dollars we receive from
our initial public offering into RMB for our operations, appreciation of the RMB
against the U.S. dollar would have an adverse effect on the RMB amount we
receive from the conversion. Conversely, if we decide to convert our RMB into
U.S. dollars for the purpose of making payments for dividends on our ordinary
shares or for other business purposes, appreciation of the U.S. dollar against
the RMB would have a negative effect on the U.S. dollar amount available to us.
In addition, appreciation or depreciation in the value of the RMB relative to
the U.S. dollar would affect our financial results reported in U.S. dollar terms
without giving effect to any underlying change in our business or results
of operations.
35
We may
not be able to distribute our assets upon liquidation and dividend payment will
be subject to restrictions under Chinese foreign exchange rule
Our
assets are predominately located inside China. Under the laws governing foreign
investment enterprises in China, dividend distribution and liquidation are
allowed but subject to special procedures under the relevant laws and rules. Any
dividend payment will be subject to the decision of the board of directors
and subject to foreign exchange rules governing such repatriation. Any
liquidation is subject to both the relevant government agency’s approval and
supervision as well as the foreign exchange control. This may generate
additional risk for our investors in case of liquidation. See “Statutory
Reserves”.
A newly enacted PRC tax law
could increase the enterprise income tax rate applicable to our principal
subsidiaries in China, which could have a material adverse effect on our results
of operations.
The newly
enacted PRC Enterprise Income Tax Law, or the EIT Law, and the implementation
regulations for the EIT Law issued by the PRC State Council, became effective as
of January 1, 2008. The EIT Law adopts a uniform income tax rate of 25% for
most domestic enterprises and foreign investment enterprises. It provides a
five-year transition period from its effective date for enterprises established
before the promulgation date of the EIT Law which were entitled to a
preferential lower tax rate under the then effective tax laws or regulations.
The State Council issued the Notice on Implementation of the Transition Period
for Preferential Enterprise Income Tax, or the Transition Implementation Notice,
on December 26, 2007, which provides detailed rules on how preferential tax
rate under previous income tax laws or regulations would transition to the
uniform 25% EIT rate. Furthermore, under the EIT Law, entities that qualify as
“high and new technology enterprises” will enjoy a preferential EIT rate of 15%.
The Ministry of Science and Technology, the Ministry of Finance and the State
Administration of Taxation issued the Measures on Qualification of High and New
Technology Enterprises, or Circular 172, on April 14, 2008, which provides
detailed standards for “high and new technology enterprises”. In addition,
according to the Notice on Prepayment of Enterprise Income Tax issued by the
State Administration of Taxation, enterprises that have previously been
certified as a “high and new technology enterprise” shall pre-pay its EIT in the
rate of 25% temporarily until it is re-certified as a “high and new technology
enterprise” under Circular 172.
We have
applied for and have been recognized as Hainan “high and new technology
enterprises” under Circular 172 in December 2008 and enjoyed preferential rate
of 15% in 2008. The period of validity of the qualification is 3 years, counted
from the date of approval of qualification, therefore we will enjoy a further
two years preferential tax rate at 15% for 2009 and 2010. Enterprises may apply
for re-recognition according to the EIT Law 3 months in advance of expiration of
qualification, if the applicant fail to file an application in limited time or
meet the requirement of re-recognition, the hi-tech qualification will cease to
be effective automatically.
36
While we
may apply for re-recognition of our subsidiaries in China as hi-tech enterprises
to reduce our income tax expense, we cannot guarantee that our application will
be successful. In addition, if there are substantial changes taking place during
our operation or technology production (such as enterprise merging,
reorganization or business switch), we will probably fail to meet requirement
for hi-tech enterprise and our qualification will be terminated
accordingly.
In that
case, we expect our income tax expense to increase significantly in the coming
years.
If we receive dividends from
our operating subsidiaries located in the PRC, such dividends may be subject to
PRC withholding tax.
The
newly enacted EIT Law and the implementation regulations for the EIT Law issued
by the PRC State Council, became effective as of January 1, 2008. The EIT
Law provides that a maximum income tax rate of 20% may be applicable to
dividends payable to non-PRC investors that are “non-resident enterprises,”
to the extent such dividends are derived from sources within the PRC, the State
Council has reduced such rate to 10% through the implementation regulations. We
are a Nevada holding company and may receive dividends from our operating
subsidiaries located in the PRC. Thus, dividends paid to us by our subsidiaries
in China may be subject to the 10% income tax if we are considered as a
“non-resident enterprise” under the EIT Law. If we are required under the EIT
Law to pay income tax for any dividends we receive from our subsidiaries, our
income tax expenses will be increased and the amount of dividends, if any, we
may pay to our shareholders may be materially and adversely
affected.
We may be deemed a PRC
resident enterprise under the EIT Law and be
subject to the PRC taxation on our worldwide income.
The EIT
Law also provides that enterprises established outside of China whose “de facto
management bodies” are located in China are considered “resident enterprises”
and are generally subject to the uniform 25% enterprise income tax rate as to
their worldwide income. Under the implementation regulations for the EIT Law
issued by the PRC State Council, “de facto management body” is defined as a body
that has material and overall management and control over the manufacturing and
business operations, personnel and human resources, finances and treasury, and
acquisition and disposition of properties and other assets of an enterprise.
Although substantially all of our operational management is currently based in
the PRC, it is unclear whether PRC tax authorities would require (or permit) us
to be treated as a PRC resident enterprise. If we are treated as a resident
enterprise for PRC tax purposes, we will be subject to PRC tax on our worldwide
income at the 25% uniform tax rate, which could have an impact on our effective
tax rate and an adverse effect on our net income and results of operations,
although dividends distributed from our PRC subsidiaries to us could be exempt
from Chinese dividend withholding tax, since such income is exempted under
the new EIT Law to a PRC resident recipient.
37
We have
limited business insurance coverage in China, which could harm our
business.
We are
exposed to many risks, including equipment failures, natural disasters,
industrial accidents, power outages, and other business interruptions. We do not
carry business interruption insurance and as a result, we may be required to pay
for financial and other losses, damages and liabilities, including
those caused by natural disasters and other events beyond our control, out
of our own funds, which could have a material adverse effect on our business,
financial condition and results of operations.
Our
property and equipment insurance does not cover the full value of our property
and equipment, which leaves us with exposure in the event of loss or damage to
our properties or claims filed against us. We currently do not carry any
product liability or other similar insurance. We cannot assure you that we
would not face liability in the event of the failure of any of our
products. This is particularly true given our plan to significantly expand our
sales into international markets, like the United States, where product
liability claims are more prevalent.
Except
for property and automobile insurance, we do not have other insurance such as
business liability or disruption insurance coverage for our operations in the
PRC. We do not maintain a reserve fund for warranty or defective products
claims. Our costs could substantially increase if we experience a
significant number of warranty claims. We have not established any reserve funds
for potential warranty claims since historically we have experienced few
warranty claims for our products so that the costs associated with our warranty
claims have been low. If we experience an increase in warranty claims
significantly, it would have a material adverse effect on our financial
condition and results of operations.
Any future outbreak of
severe acute respiratory syndrome or avian influenza in
China, or similar adverse public health developments, may severely disrupt our
business and operations.
A renewed
outbreak of severe acute respiratory syndrome, the Avian Flu or another
widespread public health problem in China, where all of our manufacturing
facilities are located and where all of our revenues are derived from, could
have a negative effect on our operations. In addition, there have been confirmed
human cases of avian influenza in PRC, Vietnam, Iraq, Thailand, Indonesia,
Turkey, Cambodia and other countries which have proven fatal in some instances.
If such an outbreak or any other similar epidemic were to spread in China, where
our operations are located, it may adversely affect our business and operating
results. Such an outbreak could have an impact on our operations as a result of
quarantines or closures of our manufacturing facilities or the retail outlets,
which would severely disrupt our operations, the sickness or death of our key
officers and employees, and a general slowdown in the Chinese
economy.
38
Risks
Related To The Market For Our Stock
Our Common Stocks subject to
price volatility and may result in losses for investors.
The stock
market has experienced significant price and volume fluctuations that have
particularly affected the trading prices of equity securities of many companies
that have business operations exclusively in China. These fluctuations have
often been unrelated or disproportionate to the operating performance of many of
these companies. Any negative change in the public perception of these companies
could decrease our stock price regardless of our operating results. We expect
our stock price to be subject to fluctuations as a result of a variety of
factors, including factors beyond our control.
These
factors include without limitation actual or anticipated variations in our
quarterly operating results, announcements of technological innovations or new
products or services by us or our competitors, announcements relating to
strategic relationships or acquisitions, additions or terminations of coverage
of our common stock by securities analysts, statements by securities analysts
regarding us or our industry, conditions or trends in the our industry, and
changes in the economic performance and/or market valuations of other medical
product companies.
The
prices at which our common stock trades will affect our ability to raise
capital, which may have an adverse affect on our ability to fund our
operations.
Our common stock may be
considered to
be a “penny
stock” and, as
such, the market for our common stock may be further limited by certain SEC
rules applicable to penny stocks.
To the
extent the price of our common stock remains below $5.00 per share or we have
net tangible assets of $2,000,000 or less, our common shares will be subject to
certain “penny stock” rules promulgated by the SEC. Those rules impose certain
sales practice requirements on brokers who sell penny stock to persons other
than established customers and accredited investors (generally institutions with
assets in excess of $5,000,000 or individuals with net worth in excess of
$1,000,000). For transactions covered by the penny stock rules, the broker must
make a special suitability determination for the purchaser and receive the
purchaser’s written consent to the transaction prior to the sale. Furthermore,
the penny stock rules generally require, among other things, that brokers
engaged in secondary trading of penny stocks provide customers with written
disclosure documents, monthly statements of the market value of penny stocks,
disclosure of the bid and asked prices, disclosure of the compensation to the
brokerage firm, and disclosure of the sales person working for the brokerage
firm. These rules and regulations adversely affect the ability of brokers to
sell our common shares and limit the liquidity of our securities.
We do not intend to pay cash
dividends in the foreseeable future.
We do not
anticipate paying any cash dividends in the foreseeable future. We currently
intend to retain all available funds and any future earnings for use in the
operation and expansion of our business. In addition, the terms of any future
debt or credit facility may preclude us from paying any dividends. As a result,
capital appreciation, if any, of our common stock will be your sole source of
potential gain in your investment for the foreseeable future.
39
Our chief executive officer
could exert significant influence over our significant corporate decisions and
may act in a manner that advances his best
interests and not necessarily those of other stockholders.
Our
Chairman, Xueyun Cui, beneficially own approximately 90.09% of our common stock.
As a result, Mr. Cui may be able to influence significantly the outcome of
all matters requiring stockholder approval, including the election and removal
of directors and any merger, consolidation, or sale of all or substantially all
of our assets and he may act in a manner that advances his best interests and
not necessarily those of other stockholders, including investors in this
offering, by, among other things: delaying, deferring or preventing a change in
control of us; entrenching our management and/or our board of directors;
impeding a merger, consolidation, takeover or other business combination
involving us; discouraging a potential acquirer from making a tender offer or
otherwise attempting to obtain control of us; or causing us to enter into
transactions or agreements that are not in the best interests of all
stockholders.
There is currently a very
limited trading market for our common stock
Our
common stock is quoted on the OTCBB. However, our bid and asked quotations have
not regularly appeared on the OTCBB for any consistent period of time. There is
no established trading market for our common stock and our common stock may
never be included for trading on any stock exchange or through any other
quotation system (including, without limitation, the NASDAQ Stock Market). You
may not be able to sell your shares due to the absence of a trading
market.
We will incur increased
costs as a result of changes in laws and regulations relating to corporate
governance matters.
As a
public reporting company, we will need to comply with the Sarbanes-Oxley Act of
2002 and the related rules and regulations adopted by the SEC, including
expanded disclosures, accelerated reporting requirements and more complex
accounting rules. Compliance with Section 404 of the Sarbanes-Oxley Act of 2002
and other requirements will increase our costs and require additional management
resources. Additionally, these laws and regulations could make it more difficult
or more costly for us to obtain certain types of insurance, including director
and officer liability insurance, and we may be forced to accept reduced policy
limits and coverage or incur substantially higher costs to obtain the same or
similar coverage. The impact of these events could also make it more difficult
for us to attract and retain qualified persons to serve on our board of
directors, our board committees or as executive officers. We are presently
evaluating and monitoring developments with respect to these laws and
regulations and cannot predict or estimate the amount or timing of additional
costs we may incur to respond to their requirements.
40
We may require additional
capital, which may not be available on commercially reasonable terms, or at
all.
Capital
raise through the sale of equity securities may result in dilution to our
shareholders. The incurrence of indebtedness would result in increased debt
service obligations and could result in operating and financing covenants that
would restrict our operations. Financing may be unavailable in amounts or on
terms acceptable to us, or at all. Failure to obtain such additional capital
could have an adverse impact on our business strategies and growth
prospects.
Our future capital raising
and the conversion of our outstanding shares of preferred stock and warrants may
dilute our shareholders’
equities.
If we
need to obtain external financing, our capital raising could require us to sell
additional equity or debt securities or obtain credit facilities. The sale of
additional equity or equity-linked securities could result in additional
dilution to our then existing shareholders.
Item 1B Unresolved Staff
Comments
None.
Item
2. Properties
All our
properties are located in Haikou City, Hainan Province, PRC. Under Chinese
law, the government owns all of the land in the PRC and companies and
individuals are authorized to use the land only through land use rights granted
by the PRC government. We have land use rights for two pieces of
land with an area approximately 39,300 square meters and 28,477 square meters,
respectively and with expiration dates in 2064 and 2063, respectively. The
structure on the land covers an area of approximately 18,324,square meters
including two manufacturing facilities, an office building, and a warehouse. One
of the pieces of land has been under the title of Zhonghe and the other piece of
land was acquired from Hainan Zhonghe Group, which was pledged to the bank for
the loans granted to Zhonghe. We expect to transfer the legal title from Hainan
Zhonghe Group by June 30, 2010 after the loans are fully settled in early
2010.
One
facility covers an area of 7,787 square meters and utilizes world advanced solid
phase peptide synthesis (SPPS) and purifying technology with an annual capacity
to synthesize 10 kg TP-5, 1 kg DDAVP, 1.5 kg Somatostatin for injection and 4kg
TA for injection in bulk and manufacture 10 million bottles of freeze dry power
of TP-5, DDAVP, SS and TA. We believe it is the largest synthesis plant in Asia.
The other facility was only completed in July 2008 with an area of 7762 square
meters. Its designed annual capacity is 150 million tablets, 120 million
capsules, 30 million granules bags, 20 million freeze-dried powder bottles, 30
million bottles small volume injection (resistant bottles), and 10 million
small-capacity injection (pre-filling syringes). Our phase II pharmaceutical
plant, Injection Preparation Plant has obtained GMP approval on September 29,
2008 and went into production in October of that year. The Solid Oral
Preparation Plant has been installed and debugged completely; expecting to get
GMP approval in May of 2010 and begin production thereafter.
41
Our main
manufacturing equipment was imported from abroad and our auxiliary equipment was
made in China. They include pumps, dryers, compressors, air conditioning units,
synthesizers, automatic liquid injection assembly, and testing instruments such
spectrometers. We believe that all our properties and equipment have been
adequately maintained, are generally in good condition, and are suitable and
adequate for our business.
Item
3. Legal Proceedings
We are
not a party to any material pending legal proceedings, and to the best of our
knowledge, no such proceedings by or against the Company have been
threatened.
Item
4. Submission of Matters to a Vote of Security Holders
None.
Part
II
Item
5. Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer
Purchases of Equity Securities Market Price of and Dividends on the Registrants
Common Equity and Related Stockholder Matters
Market
Information
Our
common stock is traded on the NASD's Over-the-Counter Bulletin Board under the
symbol "KURU.OB" since November 6, 2008.
The
following table sets forth, for the quarters indicated, the range of closing
high and low bid prices of our common stock as reported by the NASD
Over-the-Counter Bulletin Board, as adjusted for all previously effected stock
splits.
The
quotations reflect inter-dealer prices without retail mark-up, mark-down or
commission, and may not represent actual transactions.
42
|
Quarter Ended:
|
High
|
Low
|
||||
|
October
31, 2007
|
1.01
|
0.65
|
||||
|
January
31, 2008
|
1.35
|
1.01
|
||||
|
April
30, 2008
|
1.01
|
0.8
|
||||
|
July
31, 2008
|
1.01
|
0.8
|
||||
|
October
31, 2008
|
3.9
|
3.9
|
||||
|
December
31, 2008
|
1.3
|
1.3
|
||||
|
March
31, 2009
|
1.3
|
1.3
|
||||
|
June
30, 2009
|
1.2
|
1.2
|
||||
|
September
30, 2009
|
3.0
|
3.0
|
||||
|
December
31, 2009
|
2.2
|
2.2
|
||||
|
Until
March 22, 2010
|
2.0
|
2.0
|
||||
*The
reverse merger occurred on September 16, 2008 and there was no or minimal
trading of our common stock prior to the reverse merger.
Holders
As of
March 30, 2010, we had 13 stockholders of record holding an aggregate of
25,000,000 issued and outstanding shares of our common stock.
Dividends
During
fiscal year ended December 31, 2009, the Company did not distribute any stock or
cash dividends and the Company does not anticipate to distribute any stock or
cash dividends on our common stock in the foreseeable
future.
Item
6. Selected Financial Data
You
should read the following selected consolidated financial data in conjunction
with “Management's Discussion and Analysis of Financial Condition and Results of
Operations,” the financial statements and related notes, and the other financial
information included in this annual report on Form 10-K.
43
|
The year ended
Dec. 31, 2009
|
The year ended
Dec. 31, 2008
|
The year ended
Dec. 31, 2007
|
||||||||||
|
Consolidated Statement of
Operations
|
($)
|
($)
|
($)
|
|||||||||
|
(audited)
|
(audited)
|
(audited)
|
||||||||||
|
Sales
(net of discounts, returns and allowances)
|
13,235,756
|
11,622,503
|
7,471,326
|
|||||||||
|
Other
sales
|
||||||||||||
|
Cost
of sales
|
(3,980,328)
|
(3,410,891
|
)
|
(3,134,608
|
)
|
|||||||
|
Gross
profit
|
9,255,428
|
8,211,612
|
4,336,718
|
|||||||||
|
Selling
expenses
|
(387,536)
|
(687,949
|
)
|
(1,416,469
|
)
|
|||||||
|
General
and administrative expenses
|
(907,804)
|
(1,184,864
|
)
|
(967,121
|
)
|
|||||||
|
Research
and development expenses
|
(336,730)
|
(271,476)
|
(276,138)
|
|||||||||
|
Unusual
charge-Make good provision
|
-
|
(1,300,000)
|
-
|
|||||||||
|
Income
from operations
|
7,623,358
|
4,767,323
|
1,676,990
|
|||||||||
|
Interest
expense, net
|
(423,966)
|
(495,557
|
)
|
(543,400
|
)
|
|||||||
|
Other
income
|
482,885
|
2,670,040
|
70,077
|
|||||||||
|
Income
before taxation
|
7,682,277
|
6,941,806
|
1,203,667
|
|||||||||
|
Income
tax
|
(1,130,854)
|
(907,439)
|
56,796
|
|||||||||
|
Minority
interest
|
(57,664)
|
(64,658
|
)
|
(11,095
|
)
|
|||||||
|
Net
income
|
6,493,759
|
5,969,709
|
1,249,368
|
|||||||||
|
Basic
earnings per share
|
0.26
|
0.24
|
0.05
|
|||||||||
|
Diluted
earnings per share
|
0.26
|
0.24
|
0.05
|
|||||||||
|
|
As of
|
As of
|
As of
|
|||||||||
|
|
Dec. 31, 2009
|
Dec. 31, 2008
|
Dec. 31, 2007
|
|||||||||
|
Consolidated Balance Sheets
|
($)
|
($)
|
($)
|
|||||||||
|
(audited)
|
(audited)
|
(audited)
|
||||||||||
|
Current
Assets
|
14,699,876
|
14,624,269
|
6,369,578
|
|||||||||
|
Total
Assets
|
36,930,010
|
28,641,878
|
19,762,486
|
|||||||||
|
Current
Liabilities
|
11,287,283
|
3,302,601
|
3,490,290
|
|||||||||
|
Total
Liabilities
|
11,634,573
|
9,839,006
|
9,282,765
|
|||||||||
|
Minority
Interest
|
226,846
|
169,145
|
103,365
|
|||||||||
|
Total
Stockholders’ Equity
|
25,068,591
|
18,633,727
|
10,376,356
|
|||||||||
44
Item
7 Management’s Discussion and Analysis of Financial Conditions and Results of
Operations
Introduction
The
following discussion of the results of our operations and financial condition
should be read in conjunction with consolidated financial statements of the
Company and the related notes thereto appearing elsewhere herein. The following
discussion includes forward-looking statements regarding business prospects,
financial trends and accounting policies that may affect our future operating
results, financial position and cash flows. We use words such as “will,’’
“anticipate,’’ “estimate,’’ “expect,’’ “project,’’ “intend,’’ “plan,’’
“believe,’’ “forecast’’ and other words and terms of similar meaning in
connection with any discussion of future operating or financial performance.
Actual results may differ materially from results anticipated in these
forward-looking statements. We base the forward-looking statements on
information currently available to us, and we assume no obligation to update
them.
MD&A
is organized as follows:
Overview This
section provides a general description of our corporate structure and the scope
of our operation.
Critical Accounting Policies and
Estimates This section identifies those accounting policies
and accounting estimates that are considered important to the Company’s results
of operations and financial condition, require significant judgment and require
estimates on the part of management in application.
Results of
Operations This section provides an analysis of Kun Run’s
results of operations for the two years ending December 31, 2009 and
2008.
Liquidity and Capital
Resources This section provides an analysis of Kun Run’s cash
flows for the two years ended December 31, 2009 and 2008, as well as a
discussion of the Company’s outstanding debt and commitments that existed as of
December 31, 2009.
Off-Balance Sheet
Arrangements This section discloses any off-balance sheet
arrangements that have or are reasonably likely to have a current or future
effect on Camelot’s financial condition.
Recently issued accounting
pronouncements This section provides a quick look at the new
guidance affected the accounting issued by the Financial Accounting Standard
Board (“FASB”).
Overview
We are
engaged, through Hainan Zhonghe Pharmaceutical Co., Ltd., our China based
subsidiary (99.12% ownership), in the development, manufacture, marketing and
sale of prescription polypeptide drugs. Our principal products are polypeptide
derivatives. Our products are sold primarily in China through Chinese domestic
pharmaceutical distributors licensed by the Chinese government. Our
manufacturing and sales facilities are located in the City of Haikou, Hainan
Province.
45
Critical Accounting Policies
and Estimates
Use of
estimates
In
preparing financial statements in conformity with accounting principles
generally accepted in the United States of America, management makes estimates
and assumptions that affect the reported amounts of assets and liabilities and
disclosures of contingent assets and liabilities at the dates of the financial
statements, as well as the reported amounts of revenues and expenses during the
reporting periods. These accounts and estimates include, but are not limited to,
the valuation of accounts receivable, inventories, deferred income taxes and the
estimation on useful lives and residual values of property, plant and equipment
and pharmaceutical licenses and patents. Actual results could differ from those
estimates.
Allowance for doubtful
debts
The
Company establishes an allowance for doubtful debts based on management’s
assessment of the ability to collect trade receivables. A considerable amount of
judgment is required in assessing the amount of the allowance. The Company
considers the historical level of credit losses and applies percentages to aged
receivable categories. The Company makes judgments about the creditworthiness of
each customer based on ongoing credit evaluations, and monitors current economic
trends that might impact the level of credit losses in the future. If the
financial condition of the customers were to deteriorate, resulting in their
inability to make payments, a larger allowance may be required.
Based on
the above assessment, during the reporting periods, the management establishes
the following rates of general provision provided on gross amount of trade
receivables:
|
|
Rate
|
|||
|
Aged
within 1 year
|
10 | % | ||
|
Aged
over 1 year but within 2 years
|
40 | % | ||
|
Aged
over 2 years but within 3 years
|
80 | % | ||
|
More
than 3 years
|
100 | % | ||
Additional
specific provision is made against trade receivables whenever they are
considered to be doubtful.
Bad debts
are fully written off when identified or the trade receivable has aged more than
3 years. The Company extends unsecured credit to customers ranging from three to
six months in the normal course of business. The Company does not accrue
interest on trade accounts receivable.
46
(Recovery
of)/provision for doubtful debts of $(142,673) and $281,698 are included in
other income and selling expenses for two years ended December 31, 2009 and 2008
respectively.
Property, plant and
equipment
Property,
plant and equipment are stated at cost less accumulated depreciation. Cost
represents the purchase price of the asset and other costs incurred to bring the
asset into its existing use.
Depreciation
is provided on straight-line basis over their estimated useful lives. The
principal depreciation rates are as follows:
|
|
Annual rate
|
Residual value
|
||||||
|
Buildings
|
2.5 - 5 | % | 5 | % | ||||
|
Plant
and machinery
|
10 - 20 | % | 3 | % | ||||
|
Motor
vehicles
|
14 - 20 | % | 5 | % | ||||
|
Furniture,
fixtures and equipment
|
20 | % | 3 | % | ||||
|
Leasehold
improvements
|
20 | % |
Nil
|
|||||
Construction
in progress mainly represents expenditures in respect of Zhonghe’s new offices
and factories under construction. All direct costs relating to the acquisition
or construction of Zhonghe’s new office and factories are capitalized as
construction in progress. No depreciation is provided in respect of construction
in progress.
Maintenance
or repairs are charged to expense as incurred. Upon sale or disposition, the
applicable amounts of asset cost and accumulated depreciation are removed from
the accounts and the net amount less proceeds from disposal is charged or
credited to income.
Impairment of long-lived
assets
Long-lived
assets are tested for impairment in accordance with ASC 360-10-45 “Impairment or
Disposal of Long-Lived Assets” (previously Statement of Financial Accounting
Standards (“SFAS”) No. 144), Accounting for the impairment or disposal of
long-lived assets, respectively. The Company periodically evaluates
potential impairment whenever events or changes in circumstances indicate that
the carrying amount of the assets may not be recoverable. The Company
recognizes impairment of long-lived assets and investment in an affiliate in the
event that the net book values of such assets exceed the future undiscounted
cashflows attributable to such assets. During the reporting periods,
the Company has not identified any indicators that would require testing for
impairment.
47
Revenue
recognition
Revenue
from sales of the Company’s products is recognized when the significant risks
and rewards of ownership have been transferred to the buyer at the time of
delivery, the sales price is fixed or determinable and collection is reasonably
assured.
Stock-based
compensation
The
Company adopted the provisions of ASC 718 (previously SFAS 123(R)), which
requires the use of the fair value method of accounting for share-based
compensation. Under the fair value based method, compensation cost related to
employee stock options or similar equity instruments which are equity-classified
awards, is measured at the grant date based on the value of the award and is
recognized over the requisite service period, which is usually the vesting
period. ASC 718 also requires measurement of cost of a liability-classified
award based on its current fair value.
Income
taxes
The
Company uses the asset and liability method of accounting for income taxes
pursuant to ASC 740 “Income Taxes” (previously SFAS No. 109). Under
the asset and liability method of ASC 740, deferred tax assets and liabilities
are recognized for the future tax consequences attributable to temporary
differences between the financial statements carrying amounts of existing assets
and liabilities and loss carryforwards and their respective tax
bases. Deferred tax assets and liabilities are measured using enacted
tax rates expected to apply to taxable income in the years in which those
temporary differences are expected to be recovered or settled.
Dividends
Dividends
are recorded in the Company’s financial statements in the period in which they
are declared.
Off-balance sheet
arrangements
The
Company does not have any off-balance sheet arrangements.
Fair value of financial
instruments
The
Company adopted ASC 820 (previously SFAS No. 157) on January 1, 2008. The
adoption of ASC 820 did not materially impact the Company’s financial position,
results of operations or cash flows.
ASC 820
requires the disclosure of the estimated fair value of financial instruments
including those financial instruments for which fair value option was not
elected. Except for secured borrowings disclosed as below, the carrying amounts
of the financial assets and liabilities approximate to their fair values due to
short maturities or the applicable interest rates approximate the current market
rates :
48
|
As of December 31, 2009
|
As of December 31, 2008
|
|||||||||||||||
|
Carrying
|
Carrying
|
|||||||||||||||
|
amount
|
Fair value
|
amount
|
Fair value
|
|||||||||||||
|
Secured
borrowings
|
$ | 6,381,450 | $ | 6,362,757 | $ | 6,528,150 | $ | 6,407,755 | ||||||||
The fair
values of secured borrowings are estimated using discounted cash flow analysis,
based on the Company’s current incremental borrowing rates for similar types of
borrowing arrangements.
Results
of Operations
Fiscal
Year 2009 Compared to Fiscal Year 2008
|
Year ended December 31,
|
||||||||||||||||||||
|
2009
|
% as of total
sales
|
2008
|
% as of total
sales
|
Variance
%
|
||||||||||||||||
|
REVENUES
|
$ | 13,235,756 | 100 | % | $ | 11,622,503 | 100 | % | 14 | % | ||||||||||
|
LESS:
COST OF SALES
|
(3,980,328 | ) | 30 | % | (3,410,891 | ) | 29 | % | 17 | % | ||||||||||
|
GROSS
PROFIT
|
9,255,428 | 70 | % | 8,211,612 | 71 | % | 13 | % | ||||||||||||
|
GROSS
PROFIT RATIO
|
70 | % | 71 | % | ||||||||||||||||
|
OPERATING
EXPENSES
|
||||||||||||||||||||
|
SELLING
EXPENSES
|
(387,536 | ) | 3 | % | (687,949 | ) | 6 | % | -44 | % | ||||||||||
|
ADMINISTRATIVE
EXPENSES
|
(907,804 | ) | 7 | % | (1,184,864 | ) | 10 | % | -23 | % | ||||||||||
|
RESEARCH
AND DEVELOPMENT COSTS
|
(336,730 | ) | 3 | % | (271,476 | ) | 2 | % | 24 | % | ||||||||||
|
UNUSUAL
CHARGE-MAKE GOOD PROVISION
|
- | - | (1,300,000 | ) | 11 | % | -100 | % | ||||||||||||
|
INCOME
FROM OPERATIONS
|
7,623,358 | 4,767,323 | 60 | % | ||||||||||||||||
|
SUBSIDY
INCOME
|
129,217 | 1 | % | 31,003 | 0 | % | 317 | % | ||||||||||||
|
INTEREST
INCOME
|
178,830 | 1 | % | 50,438 | 0 | % | 255 | % | ||||||||||||
|
(LOSS)/GAIN
ON DISPOSAL OF PROPERTY, PLANT AND EQUIPMENT
|
(183 | ) | - | 2,416,110 | 21 | % | -100 | % | ||||||||||||
|
OTHER
INCOME
|
353,851 | 3 | % | 222,927 | 2 | % | 59 | % | ||||||||||||
|
FINANCE
COSTS
|
(602,796 | ) | 5 | % | (545,995 | ) | 5 | % | 10 | % | ||||||||||
|
PROFITS
BEFORE TAXATION
|
7,682,277 | 58 | % | 6,941,806 | 60 | % | 11 | % | ||||||||||||
|
INCOME
TAX
|
(1,130,854 | ) | 9 | % | (907,439 | ) | 8 | % | 25 | % | ||||||||||
|
MINORITY
INTEREST SHARE OF PROFITS
|
(57,664 | ) | 0 | % | (64,658 | ) | 1 | % | -11 | % | ||||||||||
|
NET
INCOME
|
6,493,759 | 49 | % | 5,969,709 | 51 | % | 9 | % | ||||||||||||
|
FOREIGN
CURRENCY TRANSACTION ADJUSTMENTS
|
6,173 | 0 | % | 858,953 | 7 | % | ||||||||||||||
|
TOTAL
COMPREHENSIVE INCOME
|
$ | 6,499,932 | 49 | % | $ | 6,828,662 | 59 | % | ||||||||||||
|
EARNINGS
PER SHARE: BASIC AND DILUTED
|
$ | 0.26 | $ | 0.24 | 8 | % | ||||||||||||||
|
WEIGHTED
AVERAGE NUMBER OF SHARES OUTSTANDING: BASIC AND DILUTED
|
25,000,000 | 24,467,808 | ||||||||||||||||||
49
Revenues
|
Year ended December 31,
|
||||||||||||||||||||
|
2009
|
As of total
sales %
|
2008
|
As of total
sales %
|
Variance
%
|
||||||||||||||||
|
REVENUES
|
13,235,756 | 100 | % | 11,622,503 | 100 | % | 14 | % | ||||||||||||
|
COST
OF SALES
|
(3,980,328 | ) | -30 | % | (3,410,891 | ) | -29 | % | 17 | % | ||||||||||
|
GROSS
PROFIT
|
9,255,428 | 70 | % | 8,211,612 | 71 | % | 13 | % | ||||||||||||
|
GROSS
PROFIT RATIO
|
70 | % | 71 | % | ||||||||||||||||
Revenues
for the year ended December 31, 2009 were $13.2 million, an increase of $1.6
million, or 14% over revenues for the year ended December 31, 2008. Revenues by
product categories were as follows:
|
Year ended December 31,
2009
|
Year ended December 31,
2008
|
|
||||||||||||||||||
|
Amount
|
%
|
Amount
|
%
|
|
||||||||||||||||
|
Product
|
(USD)
|
as of total
sales
|
(USD)
|
as of total
sales
|
Variance
%
|
|||||||||||||||
|
TP-5
Products
|
||||||||||||||||||||
|
TP-5
powder for injection (1mg)
|
1,660,720 | 13 | % | 1,940,855 | 17 | % | -14 | % | ||||||||||||
|
TP-5
powder for injection (10mg)
|
877,190 | 6 | % | 681,321 | 6 | % | 29 | % | ||||||||||||
|
TP-5
pre-filled injection (1ml:1mg)
|
889,644 | 7 | % | 1,185,292 | 10 | % | -25 | % | ||||||||||||
|
TP-5
pre-filled injection (1ml:10mg)
|
2,318,748 | 17 | % | 2,364,188 | 20 | % | -2 | % | ||||||||||||
|
Sub-total
( TP-5 products)
|
5,746,302 | 43 | % | 6,171,656 | 53 | % | -7 | % | ||||||||||||
|
Other
products
|
||||||||||||||||||||
|
SS
for injection 3mg
|
838,860 | 6 | % | 961,266 | 8 | % | -13 | % | ||||||||||||
|
Thymosin
á1 for injection 1.6mg
|
4,790,671 | 36 | % | 3,247,576 | 28 | % | 48 | % | ||||||||||||
|
DDAVP
Injection 1ml:4ug
|
1,254,974 | 10 | % | 800,680 | 7 | % | 57 | % | ||||||||||||
|
DDAVP
Injection 1ml:15ug
|
391,194 | 3 | % | 330,934 | 3 | % | 18 | % | ||||||||||||
|
Granisetron
Hydrochloride Injection 3ml:3mg
|
130,109 | 1 | % | 82,147 | 1 | % | 58 | % | ||||||||||||
|
Ozagrel
Sodium for Injection 80mg/40mg
|
52,540 | 1 | % | 23,807 | 0 | % | 121 | % | ||||||||||||
|
Others
|
31,106 | 0 | % | 4,437 | 0 | % | 601 | % | ||||||||||||
|
In
Total
|
13,235,756 | 100 | % | 11,622,503 | 100% | % | 14 | % | ||||||||||||
50
TP-5, our
major product, accounted for 43% of total revenues and contributed $5.7 million
of sales for the year ended December 31, 2009, a decrease of $425,354, or 7%, as
compared to 2008. This decrease was due to increased market competition which
resulted in lower total TP-5 sales volumes and average selling
price.
2009 saw
a large growth of revenue from the Company’s Other products. Other
products accounted for 57% of total revenues, contributing $7.5 million of
sales, representing an increase of 37% from 2008.
Sales of
Somatostatin for injection (3mg) accounted for 6% of total sales in 2009,
decreasing 13% to $838,860 in 2009 from $961,266 in 2008. The main
reason for this decrease is lower sales volumes and sales price as a result of
greater market competition.
Thymosin
Alpha 1 injection (1.6mg) revenues have grown steadily since it was introduced
to the market in 2005. The sales volume of Alpha 1 injection significantly
increased in 2009. The Company benefited from the fact that there were few
competitors in this drug category. Meanwhile, our Thymosin Alpha 1 for injection
(1.6mg) continued its popularity in the market. The strong market demand on
Thymosin Alpha 1 injection (1.6mg) made it the Company’s best selling product in
2009, which contributed $4.8 million in revenue (36% of the total sales) for the
year ended December 31, 2009, representing a 48% increase from
2008.
Desmopressin
acetate injection (DDAVP) also experienced significant sales growth to
approximately $1.6 million for 2009 from $1.1 million in 2008,
representing a 45% increase.
The TP-5
market is mature and is highly competitive while the markets for Somatostatin,
DDAVP and Thymosin á1 are still developing with greater market potential and
less pricing pressure. Thus, the Company’s product portfolio has broadened from
one single flagship product, TP-5, to four peptide products and will make
major contributions to revenue . The Company expects its current
product mix to be sustained in the foreseeable future as brand recognition and
product efficacy drive product acceptance in the market.
Cost of Goods Sold and Gross
Profit
Cost of
revenues for year ended December 31, 2009 was approximately $ 4.0 million,
compared with $3.4 million for the same period of 2008, an increase of 17%. The
gross margin for year ended December 31, 2009 was 70%, slightly decreased from
71% for the same period of 2008.
51
The
increase in cost of revenue was mainly due to the increase in revenue. As a
percentage of revenue, the cost of goods sold increased slightly to 30% for the
year ended December 31, 2009 compared to 29% for 2008.
Operating
Expenses
|
Year ended December 31,
|
||||||||||||||||||||
|
2009
|
%
|
2008
|
%
|
Variance
|
||||||||||||||||
|
as of total sales
|
As of total sales
|
%
|
||||||||||||||||||
|
OPERATING
EXPENSES
|
||||||||||||||||||||
|
SELLING
EXPENSES
|
387,536 | 3 | % | 687,949 | 6 | % | -44 | % | ||||||||||||
|
ADMINISTRATIVE
EXPENSES
|
907,804 | 7 | % | 1,184,864 | 10 | % | -23 | % | ||||||||||||
|
RESEARCH
AND DEVELOPMENT COSTS
|
336,730 | 3 | % | 271,476 | 2 | % | 24 | % | ||||||||||||
|
UNUSUAL
CHARGE-MAKE GOOD PROVISION
|
- | - | 1,300,000 | 11 | % | -100 | % | |||||||||||||
| 1,632,070 | 13 | % | 3,444,289 | 29 | % | -53 | % | |||||||||||||
Selling
Expenses
|
Year ended December 31,
|
Variance
|
|||||||||||||||
|
2009
|
2008
|
Amount
|
%
|
|||||||||||||
|
SELLING
EXPENSES
|
||||||||||||||||
|
Marketing
and Advertising
|
107,741 | 101,562 | 6,179 | 6 | % | |||||||||||
|
Traveling
and Transportation
|
140,546 | 79,550 | 60,996 | 77 | % | |||||||||||
|
Salaries
|
43,889 | 49,155 | (5,266 | ) | -11 | % | ||||||||||
|
Provision
for doubtful debts
|
- | 281,698 | (281,698 | ) | -100 | % | ||||||||||
|
Other
Selling Expenses
|
95,360 | 175,984 | (80,624 | ) | -46 | % | ||||||||||
| 387,536 | 687,949 | (300,413 | ) | -44 | % | |||||||||||
52
Selling
expenses stood at $387,536, or 3% of total sales, in the year ended December 31,
2009, decreased 44% from $687,949 for 2008. The major contributor to the
decrease in expenses, year over year, was Provision for doubtful
debts. Due to efficient management of the Accounts Receivable,
no additional provision was incurred in 2009.
Administrative
Expenses
Our
administrative expenses were approximately $907,804 (7% of total sales) and $1.2
million (10% of total sales) for the years ended December 31, 2009 and 2008,
respectively. The details of general and administrative expenses were as
follows:
|
Year ended December 31,
|
Variance
|
|||||||||||||||
|
2009
|
2008
|
Amount
|
%
|
|||||||||||||
|
ADMINISTRATIVE
EXPENSES
|
||||||||||||||||
|
Consultancy
fee
|
80,943 | 377,996 | (297,053 | ) | -79 | % | ||||||||||
|
Audit
fee
|
77,746 | 83,203 | (5,457 | ) | -7 | % | ||||||||||
|
Traveling
and Transportation
|
12,179 | 36,654 | (24,475 | ) | -67 | % | ||||||||||
|
Entertainment
|
11,007 | 12,311 | (1,304 | ) | -11 | % | ||||||||||
|
Office
supplies
|
4,817 | 15,210 | (10,393 | ) | -68 | % | ||||||||||
|
Depreciation
|
184,645 | 124,340 | 60,305 | 49 | % | |||||||||||
|
Salaries
|
136,902 | 205,437 | (68,535 | ) | -33 | % | ||||||||||
|
Others
|
399,565 | 329,713 | 69,852 | 21 | % | |||||||||||
| 907,804 | 1,184,864 | (277,060 | ) | -23 | % | |||||||||||
|
|
1)
|
The
main contributor to the decrease in Administrative Expenses in 2009
compared to 2008 was Consultancy fees. In 2008, the Company
conducted a reverse merger and incurred extraordinary Consultancy
fees. Consultancy fees paid under the Stock Purchase Agreement
(see COMPENSATION CHARGES of this section) were $377,996. In 2009, the Company
resumed normal business practice in regards to use of
Consultancy.
|
|
|
2)
|
Overall cost control of general
and administrative procedure also contributed to the decrease in
Administrative Expenses in
2009.
|
53
Research and Development
Costs
Research
and development expenses were $336,730 in 2009, representing an increase as
compared to $271,476 in 2008. In regards to percentage of Revenue, there was
only a slight increase in Research and development expenses from 2008 to
2009. 2009 expenses were 3% of revenue as compared to 2% in
2008. Research and development costs were mainly costs for clinical
analysis, registration and consumption of test specimens for our new
products.
Income from
Operations
Income
from operations increased 60% to $7.6 million in 2009 from $4.8 million in 2008.
The sharp increase in Income from operations was largely due to our strong
marketing and sales efforts and efficient cost controls resulting in increased
Sales and decreased expenses. Moreover, 2008 Income from operations included a
Make Good Provision expense of $1.3 million which was not incurred in
2009.
The Make
Good Provision is a result of a Make Good Escrow Agreement entered between Mr.
Xueyun Cui and the escrow agent on August 15, 2008. 1,000,000 of the shares
issued to Mr. Cui were put into an escrow. The Escrow Shares were
pledged to secure the Company’s commitment to achieve the 2008 Guaranteed
After-Tax Net Income.
Following
the achievement of the 2008 performance target, the Escrow Shares to be released
back to Mr. Cui was treated as an expense for the amount of the market value of
the shares (i.e. $1.30) as of the date of the performance goals are met, i.e.
December 31, 2008. The total expense recognized for the fiscal year 2008 was
$1,300,000 ($1.30×1,000,000).
Interest
Income
Interest
income for 2009 was $178,830, as compared to $50,438 for 2008. The increased
income was accrued from the principal amount of $4.2 million due from Hainan
Zhonghe Group at an interest rate of 5.31% per annum, unsecured and repayable on
demand.
The loan
was mainly used by a subsidiary of Hainan Zhonghe Group, Hainan Zhonghe Peptide
Drugs Research & Development Co., Ltd., to research and develop the new
product, Entecavir, which is expected to launch in 2010.
Other
Income
|
Year ended December 31,
|
||||||||||||||||||||
|
2009
|
As of total
sales %
|
2008
|
As of total
sales %
|
Variance
%
|
||||||||||||||||
|
OTHER
INCOME
|
||||||||||||||||||||
|
Recovery
of doubtful debts
|
$ | 142,673 | 1 | % | $ | - | - | 100 | % | |||||||||||
|
Rental
income
|
211,104 | 2 | % | 222,515 | 2 | % | -5 | % | ||||||||||||
|
Others
|
74 | 0 | % | 412 | 0 | % | -82 | % | ||||||||||||
| $ | 353,851 | 3 | % | $ | 222,927 | 2 | % | 59 | % | |||||||||||
54
Rental
Income
Rental
income was $211,104 in 2009, as compared to $222,515 in 2008. Our tenant,
Sinopep Pharmaceutical Inc., rented our plant to use as their
workshop.
Finance
Costs
|
Year ended December 31,
|
||||||||||||||||||||
|
2009
|
As of total
sales
|
2008
|
As of total
sales
|
Variance
%
|
||||||||||||||||
|
FINANCE
COSTS
|
%
|
%
|
||||||||||||||||||
|
Interest
Expenses
|
$ | 600,629 | 5 | % | $ | 495,832 | 4 | % | 21 | % | ||||||||||
|
Bills
Discounting Charges
|
8,116 | 0 | % | 47,177 | 0 | % | -83 | % | ||||||||||||
|
Bank
Charges and Net Exchange Loss
|
(5,949 | ) | 0 | % | 2,986 | 0 | % | -299 | % | |||||||||||
| $ | 602,796 | 5 | % | $ | 545,995 | 4 | % | 10 | % | |||||||||||
Interest
Expenses
Interest
expenses were accrued from long-term borrowings which balance remained unchanged
between 2008 and 2009.
Net Income
Net
income increased 9% to approximately $6.5 million for the year ended December
31, 2009. This was an improvement of approximately $0.5 million, from
approximately $6.0 million for the same period of 2008.
Excluding
the effects of two 2008 extraordinary items, the $1.3 million of Unusual
Charge-Make Good Provision and $2.4 million of Gain on Disposal of Property,
Plant and Equipment, the net income of 2009 increased 13% compared to
2008. 2008 net income excluding the extraordinary items was $5.8
million.
55
This
increase was in line with our revenue growth and margin
improvement.
Also,
among the Company’s phase II pharmaceutical plant, the Solid Oral Preparations
Plant has completed installation and debugging, passed on-site inspection and is
expecting to obtain GMP approval in May of 2010. We believe the increased
production capabilities will support our sales and marketing efforts and improve
our profitability in the future.
Earnings per
share
Earnings
per share for the year ended December 31, 2009 were $0.26 per share (both basic
and diluted), compared with $0.24 per share (both basic and diluted) for the
same period in 2008. The increase was contributed by our increased profitability
in 2009.
LIQUIDITY
AND CAPITAL RESOURCES
CASH
At
December 31, 2009, we had cash and cash equivalents totaling $810,809. Our
working capital was $3.4 million, representing a decrease of $7.9 million as
compared to working capital of $11.3 million at December 31, 2008. Net
cash provided by operating activities was approximately $7.0 million for the
year ended December 31, 2009.
CASH
FLOW
The
following table provides detailed information about our net cash flow for all
financial statement periods presented in this report.
(All
amounts in thousands of U.S. dollars)
|
Year Ended December 31,
|
||||||||
|
Cash Flow
|
2009
|
2008
|
||||||
|
Net
cash provided by operating activities
|
6,968
|
5,790
|
||||||
|
Net
cash used in investing activities
|
(6,466
|
)
|
(4,937
|
)
|
||||
|
Net
cash used in financing activities
|
(126
|
)
|
(1,129)
|
|||||
|
Net
cash flow
|
377
|
(237)
|
||||||
56
Operating
Activities
Net cash
provided by operating activities was approximately $7.0 million for the year
ended December 31, 2009, an increase of approximately $1.2 million from the net
cash used in operating activities of approximately $5.8 million for the same
period in 2008. The increased cash flow was due primarily to the increase of our
revenue by $1.6 million in 2009 compared with 2008.
We
currently generate positive cash flow through operations. We believe that our
cash flow generated from operations will be sufficient to sustain operations for
at least the next twelve months.
Investing
Activities
Net cash
used in investing activities in the year 2009 was $6.5 million,
representing an increase of $1.5 million from
net cash used in investing activities of $4.9 million in 2008. A portion of the
cash went to acquiring property, plant and equipment. We also lent $7.6 million
to related companies at a fixed interest rate; the interest earned thereby will
provide an inflow of cash during the next few years starting in
2010.
Financing
Activities
Net cash
used in financing activities for the year ended December 31, 2009 was $125,710,
compared with net cash provided from financing activities of $1.1 million during
the same period of 2008. During this period, we repaid $3.1 million in loans to
a bank.
Off-Balance
Sheet Arrangements
The
Company does not have any off-balance sheet arrangements that have or are
reasonably likely to have a current or future effect on our financial condition,
changes in financial condition, revenues or expenses, results or operations,
liquidity, capital expenditures or capital resources that is material to
investors.
Recently
issued accounting pronouncements
FASB
Accounting Standards Codification (Accounting Standards Update “ASU” 2009-1). In
June 2009, the Financial Accounting Standard Board (“FASB”) approved its
Accounting Standards Codification (“Codification”) as the single source of
authoritative United States accounting and reporting standards applicable for
all non-governmental entities, with the exception of the SEC and its staff. The
Codification is effective for interim or annual financial periods ending after
September 15, 2009 and impacts our financial statements as all future references
to authoritative accounting literature will be referenced in accordance with the
Codification. There have been no changes to the content of our financial
statements or disclosures as a result of implementing the
Codification.
57
As a
result of our implementation of the Codification during the quarter ended
September 30, 2009, previous references to new accounting standards and
literature are no longer applicable. In the current financial statements, we
will provide reference to both new and old guidance to assist in understanding
the impacts of recently adopted accounting literature, particularly for guidance
adopted since the beginning of the current fiscal year but prior to the
Codification.
Noncontrolling
Interests (Included in amended Topic ASC 810 “Consolidation”, previously SFAS
No. 160 “Noncontrolling Interests in Consolidated Financial Statements”, an
amendment of ARB No. 51). The amended topic establishes accounting and reporting
standards for the noncontrolling interest in a subsidiary and for the
deconsolidation of a subsidiary. It clarifies that a noncontrolling interest in
a subsidiary is an ownership interest in the consolidated entity that should be
reported as equity in the consolidated financial statements. We adopted the
amended topic on January 1, 2009. As a result, we have reclassified financial
statement line items within our Consolidated Balance Sheets and Statements of
Income and Comprehensive Income for the prior period to conform to this
amended topic.
Business
Combinations (Included in amended Topic ASC 805 “Business Combinations”,
previously SFAS No. 141(R)). This ASC guidance addresses the accounting and
disclosure for identifiable assets acquired, liabilities assumed, and
noncontrolling interests in a business combination. The adoption of this amended
topic has no material impact on the Company’s financial statements.
Intangibles-Goodwill
and Other (Included in amended Topic ASC 350”, previously FASB staff position
(“FSP”) FAS 142-3, Determination of the Useful Life of Intangible Assets). The
amended topic amends the factors an entity should consider in developing renewal
or extension assumptions used in determining the useful life of recognized
intangible assets under FASB Statement No. 142, “Goodwill and Other
Intangible Assets”. This new guidance applies prospectively to intangible assets
that are acquired individually or with a group of other assets in business
combinations and asset acquisitions. The amended topic is effective for
financial statements issued for fiscal years and interim periods beginning after
December 15, 2008. Early adoption is prohibited. The adoption of this
amended topic has no material effect on the Company's financial
statements.
Business
Combinations (Included in amended Topic ASC 805, previously FSP No. 141R-1
“Accounting for Assets Acquired and Liabilities Assumed in a Business
Combination That Arise from Contingencies”). Amended topic ASC 805 amends the
requirements for the provisions in FASB Statement 141R for the initial
recognition and measurement, subsequent measurement and accounting, and
disclosures for assets and liabilities arising from contingencies in business
combinations. The amended topic eliminates the distinction between contractual
and non-contractual contingencies, including the initial recognition and
measurement criteria and instead carries forward most of the provisions for
acquired contingencies. The amended topic is effective for contingent assets and
contingent liabilities acquired in evaluating the impact. The adoption of this
amended topic has no material impact on the Company’s financial
statements.
58
Fair
Value Measurements and Disclosures (Included in amended Topic ASC 820,
previously FSP No. 157-4, “Determining Whether a Market is Not Active and a
Transaction Is Not Distressed”.) The amended topic clarifies when markets are
illiquid or that market pricing may not actually reflect the “real” value of an
asset. If a market is determined to be inactive and market price is reflective
of a distressed price then an alternative method of pricing can be used, such as
a present value technique to estimate fair value. The amended topic identifies
factors to be considered when determining whether or not a market is inactive.
The amended topic would be effective for interim and annual periods ending after
June 15, 2009, with early adoption permitted for periods ending after March 15,
2009 and shall be applied prospectively. The adoption of this amended topic has
no material effect on the Company's financial statements.
Investments
- Debt and Equity Securities - Overall - Transition and Open Effective Date
Information (Included in amended Topic ASC 320, previously FASB Staff Position
No. 115-2 and Statement of Financial Accounting Standards No. 124-2,
“Recognition and Presentation of Other-Than-Temporary Impairments”). The amended
topic amends the other-than-temporary impairment guidance in U.S. GAAP for debt
securities through increased consistency in the timing of impairment recognition
and enhanced disclosures related to the credit and noncredit components of
impaired debt securities that are not expected to be sold. In addition,
increased disclosures are required for both debt and equity securities regarding
expected cash flows, credit losses, and securities with unrealized losses. The
adoption of this amended topic has no material impact on the Company’s financial
statements.
Interim
Disclosures about Fair Value of Financial Instruments (Included in amended Topic
ASC 825 “Financial Instruments”, previously FSP SFAS No. 107-1). This guidance
requires that the fair value disclosures required for all financial instruments
be included in interim financial statements. This guidance also requires
entities to disclose the method and significant assumptions used to estimate the
fair value of financial instruments on an interim and annual basis and to
highlight any changes from prior periods. The amended topic was effective for
interim periods ending after September 15, 2009. The adoption of this amended
topic has no material impact on the Company’s financial statements.
Subsequent
Events (Included in amended Topic ASC 855 “Subsequent Events”, previously SFAS
No. 165). The amended topic establishes accounting and disclosure requirements
for subsequent events. The amended topic details the period after the balance
sheet date during which we should evaluate events or transactions that occur for
potential recognition or disclosure in the financial statements, the
circumstances under which we should recognize events or transactions occurring
after the balance sheet date in its financial statements and the required
disclosures for such events. We adopted this amended topic effective June 1,
2009.
Accounting
for Transfers of Financial Assets (Included in amended Topic ASC 860 “Transfers
and Servicing”, previously SFAS No. 166, “Accounting for Transfers of Financial
Assets - an Amendment of FASB Statement No. 140.”). The amended topic addresses
information a reporting entity provides in its financial statements about the
transfer of financial assets; the effects of a transfer on its financial
position, financial performance, and cash flows; and a transferor’s continuing
involvement in transferred financial assets. Also, the amended topic removes the
concept of a qualifying special purpose entity, limits the circumstances in
which a transferor derecognizes a portion or component of a financial asset,
defines participating interest and enhances the information provided to
financial statement users to provide greater transparency. The amended
topic is effective for the first annual reporting period beginning after
November 15, 2009 and will be effective for us as of January 1, 2010. The
management is in the process of evaluating the impact of adopting this amended
topic on the Company’s financial statements.
59
Consolidation
of Variable Interest Entities – Amended (Included in amended Topic ASC 810
“Consolidation”, previously SFAS 167 “Amendments to FASB Interpretation No.
46(R)”). The amended topic requires an enterprise to perform an analysis to
determine the primary beneficiary of a variable interest entity; to require
ongoing reassessments of whether an enterprise is the primary beneficiary of a
variable interest entity and to eliminate the quantitative approach previously
required for determining the primary beneficiary of a variable interest entity.
The amended topic also requires enhanced disclosures that will provide users of
financial statements with more transparent information about an enterprise’s
involvement in a variable interest entity. The amended topic is effective for
the first annual reporting period beginning after November 15, 2009 and will be
effective for us as of January 1, 2010. The management is in the process of
evaluating the impact of adopting this amended topic on the Company’s financial
statements.
In August
2009, the FASB issued Accounting Standards Update (“ASU”) No. 2009-05 (“ASU
Update 2009-05”), an update to ASC 820, Fair Value Measurements and Disclosures.
This update provides amendments to reduce potential ambiguity in financial
reporting when measuring the fair value of liabilities. Among other provisions,
this update provides clarification that in circumstances in which a quoted price
in an active market for the identical liability is not available, a reporting
entity is required to measure fair value using one or more of the valuation
techniques described in ASU Update 2009-05. ASU Update 2009-05 became effective
for the Company’s annual financial statements for the year ended December 31,
2009. The adoption of this ASU update has no material impact on the Company’s
financial statements.
The FASB
issued ASU No. 2009-13, Revenue Recognition (Topic 605): Multiple Deliverable
Revenue Arrangements - A Consensus of the FASB Emerging Issues Task Force.” This
update provides application guidance on whether multiple deliverables exist, how
the deliverables should be separated and how the consideration should be
allocated to one or more units of accounting. This update establishes a selling
price hierarchy for determining the selling price of a deliverable. The
selling price used for each deliverable will be based on vendor-specific
objective evidence, if available, third-party evidence if vendor-specific
objective evidence is not available, or estimated selling price if neither
vendor-specific or third-party evidence is available. The Company will be
required to apply this guidance prospectively for revenue arrangements entered
into or materially modified after January 1, 2011; however, earlier application
is permitted. The management is in the process of evaluating the impact of
adopting this ASU update on the Company’s financial statements.
The FASB
issued ASU-2010-09 (Topic 855) to amend guidance on subsequent events to remove
the requirement for SEC filers (as defined in ASU 2010-09) to disclose the date
through which an entity has evaluated subsequent events. This change alleviates
potential conflicts with current SEC guidance. An SEC filer is still required to
evaluate subsequent events through the date financial statements are issued, but
disclosure of that date is no longer required. The amendments in ASU 2010-09
became effective upon issuance of the guidance.
60
Item
7A. Quantitative and Qualitative Disclosures about Market Risk.
Not
required for Smaller Reporting Company.
Item
8. Financial Statements and Supplementary Data.
Please
see Financial Statements to this Annual Report on Form 10-K.
Item
9. Changes in and Disagreements with Accountants on Accounting and Financial
Disclosure.
There are
no such reportable events as required by Item 304(b) of Regulation
S-K.
Item
9A. Controls and Procedures.
(a)
Management’s annual report on disclosure controls and procedures.
As
required by Exchange Act Rule 15d-15(b), our management has carried out an
evaluation, under the supervision of our Chief Executive Officer and Principal
Accounting Officer, of the effectiveness of the design and operation of our
disclosure controls and procedures as of December 31, 2009.
Disclosure
controls and procedures refer to controls and other procedures designed to
ensure that information required to be disclosed in the reports we file or
submit under the Securities Exchange Act is recorded, processed, summarized and
reported within the time periods specified in the rules and forms of the SEC and
that such information is accumulated and communicated to our management,
including our Chief Executive Officer and Principal Accounting Officer, as
appropriate, to allow timely decisions regarding required
disclosure.
Based on
that evaluation, the Chief Executive Officer and Principal Accounting Officer
have concluded that these disclosure controls and procedures are
effective.
(b)
Management’s annual report on internal control over financial
reporting.
As
required by Exchange Act Rule 15d-15(c), our management has carried out an
evaluation, under the supervision of our Chief Executive Officer and Principal
Accounting Officer, of the effectiveness of the design and operation of our
internal control over financial reporting as of December 31,
2009.
61
Management
is responsible for establishing and maintaining adequate internal control over
financial reporting. The Company's internal control over financial reporting is
a process that is designed to provide reasonable assurance regarding the
reliability of financial reporting and the preparation of financial
statements for external purposes in accordance with generally accepted
accounting principles, and includes those policies and procedures
that:
|
|
·
|
Pertain to the maintenance of
records that, in reasonable detail, accurately and fairly reflect the
transactions and dispositions of assets of the
Company,
|
|
|
·
|
Provide reasonable assurance that
transactions are recorded as necessary to permit preparation of financial
statements in accordance with generally accepted accounting principles,
and that receipts and expenditures are being made only in accordance with
authorizations of management and the board of directors of the Company,
and
|
|
|
·
|
Provide reasonable assurance
regarding prevention or timely detection of unauthorized acquisition, use,
or disposition of the Company's assets that could have a material effect
on the financial statements.
|
Because
of its
inherent limitations, internal control over financial reporting may not prevent
or detect misstatements. Also, projections of any evaluation of effectiveness to
future periods are subject to the risk that controls may become inadequate
because of changes in conditions, or that the degree of compliance with the
policies and procedures may deteriorate.
Management
performed an assessment of the effectiveness of the Company's internal control
over financial reporting as of December 31, 2009. In making this assessment, we
used the criteria set forth by the Committee of Sponsoring Organizations of the
Treadway Commission (COSO) in Internal Control - Integrated Framework. Based on
our assessment, we determined that, as of December 31, 2009, our internal
control over financial reporting was effective.
This
Annual Report does not include an attestation report of the Company’s registered
public accounting firm regarding internal control over financial
reporting. Management’s report was not subject to attestation by the
Company’s registered public accounting firm pursuant to temporary rules of the
Securities and Exchange Commission that permit the Company to provide only
management’s report in this Annual Report.
(c) Changes
in Internal Controls over Financial Reporting
During
the fourth quarter of the year ended
December 31, 2009, there was no change in our internal controls over
financial reporting that has materially affected, or that is reasonably
likely to materially affect, our internal control over financial
reporting.
62
Item
9B. Other Information.
None.
PART
III
Item
10. Directors, Executive Officers and Corporate Governance.
The
following sets forth the name and position of each of our current executive
officers and directors.
|
Name
|
Age
|
Position Held
|
||
|
Xueyun
Cui
|
49
|
Chairman
|
||
|
Xiaoqun
Ye
|
43
|
Chief
Executive Officer
|
||
|
Yan
Lin
|
38
|
Chief
Accounting Officer
|
The
following is a summary of the biographical information of our
directors and officers:
Xueyun Cui Mr. Cui, was
elected the sole director of Kun Run Biotechnology, Inc. on September 16, 2008.
As the Chairman of the Company, he joined the Company in April 1995
and serves as Chairman from April 1995 to present. Before joining
the Company, Mr. Cui had been General Manager of Hainan Business Chemical Group
for 5 years.
Mr. Cui
Xueyun has more than 10 years of operational management experience in the
pharmaceutical industry to qualify as a director. As Chairman of
Hainan Zhonghe, our subsidiary, Mr. Cui led the team and produced Thymopentin,
the first peptide pharmaceutical drug in China. Mr. Cui has also served as Vice
President of the pharmaceutical industry association in Hainan Province since
2004.
Xiaoqun Ye Mr. Ye was
elected the Chief Executive Officer of Kun Run Biotechnology, Inc. on September
16, 2008. He joined the Company in April 2002 as General Manager. He obtained
his bachelor degree in Electronics from Shaanxi faculty of
engineering.
Since entering the
pharmaceutical industry in 1997, Mr. Ye Xiaoqun has gained extensive experience
serving in engineering, plant management and general management. In 2005, he was
named one of the “top ten pharmaceutical industry talents” of Hainan Province.
Mr. Ye’s outstanding pharmaceutical management and market development
experience, along with his 8-year tenure at Hainan Zhonghe made him the best
choice for CEO of Kun Run Bio-Technology Co., Ltd.
Yan Lin, Ms. Lin was
elected Chief Accounting Officer of Kun Run Biotechnology, Inc. on September 16,
2008. She joined the Company in October 2001 as Financial Manager. Ms. Lin
graduated from Central Finance University with a bachelor degree in
accounting.
63
There are
no agreements or understandings for any of our executive officers or directors
to resign at the request of another person and no officer or director is acting
on behalf of, nor will any of them act at the direction of any other
person.
Directors
are elected until their successors are duly elected and qualified.
Our
directors and executive officers have not, during the past ten
years:
|
|
·
|
had any bankruptcy petition filed
by or against any business of which was a general partner or executive
officer, either at the time of the bankruptcy or within two years prior to
that time,
|
|
|
·
|
been convicted in a criminal
proceeding and is not subject to a pending criminal
proceeding,
|
|
|
·
|
been subject to any order,
judgment or decree, not subsequently reversed, suspended or vacated, of
any court of competent jurisdiction, permanently or temporarily enjoining,
barring, suspending or otherwise limiting his involvement in any type of
business, securities, futures, commodities or banking activities;
or
|
|
|
·
|
been found by a court of
competent jurisdiction (in a civil action), the Securities Exchange
Commission or the Commodity Futures Trading Commission to have violated a
federal or state securities or commodities law, and the judgment has not
been reversed, suspended or
vacated.
|
Board
Composition and Committees
Prior to
closing of the reverse merger, the board of directors was composed of only one
member, Trixy Sasyniuk-Walt. Ms. Sasyniuk-Walt has submitted her resignation as
our director on September 16, 2008. Mr. Xueyun Cui was appointed as our sole
director upon the resignation of Ms. Sasyniuk-Walt.
We
currently do not have standing audit, nominating or compensation committees.
Currently, our entire board of directors is responsible for the functions that
would otherwise be handled by these committees. We intend, however, to establish
an audit committee and a compensation committee of our board of directors as
soon as practicable. We envision that the audit committee will be primarily
responsible for reviewing the services performed by our independent
auditors, evaluating our accounting policies and our system of internal
controls. The compensation committee will be primarily responsible for reviewing
and approving our salary and benefits policies (including stock options) and
other compensation of our executive officers.
Our board
of directors has not made a determination as to whether any member of our board
is an audit committee financial expert. Upon the establishment of an audit
committee, the board will determine whether any of the directors qualify as an
audit committee financial expert.
64
Compensation
of Directors.
We have
no formal or informal arrangements or agreements to compensate our directors for
services they provide as directors. We plan to implement a compensation program
for our independent directors, as and when they are appointed, which we
anticipate will include such elements as an annual retainer, meeting attendance
fees and stock options. The details of such compensation program will be
negotiated with each such director .
Family
Relationships
There are
no family relationships among our directors or officers.
Section 16(a) Beneficial Ownership
Reporting Compliance
Section
16(a) of the Exchange Act requires our executive officers, directors and
beneficial owners of more than ten percent (10%) to report their beneficial
ownership of equity interests in the company to the SEC. Their initial reports
are required to be filed using the SEC's Form 3, and they are required to report
subsequent purchases, sales, and other changes using the SEC's Form 4, which
must be filed within two business days of most transactions. Officers,
directors, and persons owning more than 10% of our capital shares are required
by SEC regulations to furnish us with copies of all of reports they file
pursuant to Section 16(a).
According
to our records, all Section 16(a) forms were filed in a timely
manner.
Code
of Ethics
We have
not adopted the Code of Ethics yet. We are in the process of finalize such Code
of Ethics.
Item
11. Executive Compensation.
The
following is a summary of the compensation we paid to our now Chief Executive
Officer, Chief Financial Officer and Mr. Xueyun Cui, for the two years ended
December 31, 2009, and 2008, respectively. No executive officer received
compensation in excess of $100,000 for any of these two years.
65
|
Name and
Principal Position
|
Fiscal
Year
|
Salary
($)
|
Bonus
($)
|
Stock
Awards
($)
|
Option
Awards
($)
|
Non-equity
Incentive Plan
Compensation
($)
|
Change in Pension
Value and Nonqualified
Deferred Compensation
Earnings
($)
|
All Other
Compensation
($)
|
Total
($)
|
||||||||||||||||||||||||||
|
Xueyun
Cui
|
2009
|
15,844 | 0 | 0 | 0 | 0 | 0 | 0 | 15,844 | ||||||||||||||||||||||||||
|
(Chairman)(1)
|
2008
|
15,574 | 15,574 | ||||||||||||||||||||||||||||||||
|
Xiaoqun
Ye (CEO) (2)
|
2009
2008
|
11,443 11,248 | 0 | 0 | 0 | 0 | 0 | 0 |
11,443
11,248
|
||||||||||||||||||||||||||
|
Yan
Lin (CAO)(3)
|
2009
|
7,130
|
0 | 0 | 0 | 0 | 0 | 0 |
7,130
|
||||||||||||||||||||||||||
|
2008
|
6,534 |
6,534
|
|||||||||||||||||||||||||||||||||
|
|
(1)
|
Mr. Xueyun Cui was appointed the
sole director on September 16,
2008.
|
|
|
(2)
|
Mr. Xiaoqun Ye was appointed
Chief Executive Officer on September 16,
2008.
|
|
|
(3)
|
Ms. Yan Lin was appointed Chief
Accounting Officer on September 16,
2008.
|
Bonuses
and Deferred Compensation
We do not
have any bonus, deferred compensation or retirement plan. We do not have a
compensation committee; all decisions regarding compensation are determined by
our entire board of directors.
Stock
Option and Stock Appreciation Rights
We do not
currently have a Stock Option Plan or Stock Appreciation Rights Plan. No stock
options or stock appreciation rights were awarded during the fiscal year ended
December 31, 2009.
Employment
Agreements
We
currently have long-term employment agreements with our executive
officers.
The
material terms are as follows:
We have
signed a 5-year employment agreement with Xiaoqun Ye, our CEO. The agreement is
from January 1, 2007 to December 31, 2011. Salary is based on performance
subject to our productions and operations. Hours are 8 hours per day, 40 hours
per week. We may arrange Mr. Ye to work overtime (no more than 3 hours a day, no
more than 36 hours overtime a month) provided that Mr. Ye is paid overtime
salary in accordance with the Chinese laws and regulations. Mr. Ye is eligible
for standard employee benefits under Chinese laws and regulations.
We have
signed a 5-year employment agreement with Yan Lin, our Chief Accounting Officer.
The agreement is from January 1, 2009 to December 31, 2013. Salary is
$3,519 per year with bonuses based on performance subject to our productions and
operations. Ms. Lin is eligible for standard employee benefits under Chinese
laws and regulations.
We do not
have any employment agreement with Mr. Xueyun Cui, our sole
director.
66
Item
12. Security Ownership of Certain Beneficial Owners and Management and Related
Stockholder Matters
The
following table sets forth certain information with respect to the beneficial
ownership of our voting securities on March 27, 2010 by (i) each person who owns
beneficially more than 5% of the outstanding shares of our common stock, (ii)
each of our directors, (iii) our chief executive officer, and (iv) all of our
executive officers and directors as a group.
|
Name and Address of Beneficial Owner *
|
No. of Shares
|
Percentage of
Shares
Outstanding
|
||||
|
Xueyun
Cui
|
22,522,500
|
90.09%
|
||||
|
Xiaoqun
Ye
|
0
|
0%
|
||||
|
Yan
Lin
|
0
|
0%
|
||||
|
Directors
and officers as a group (3 persons):
|
22,522,500
|
90.09%
|
||||
The
address for officers and directors is Hainan Zhonghe Pharmaceutical Co., Ltd.,
Free Trade Zone, 168 Nanhai Avenue, Haikou, Hainan Province, P. R.
China.
Item
13. Certain Relationships and Related Transactions, and Director
Independence
The
Company has entered into the following transactions with related
parties:
|
Related party relationship
|
Type of transaction
|
Year ended December 31,
|
||||||||
|
|
|
2009
|
2008
|
|||||||
|
Related
company with the same management
personnel
|
Sales/
Hainan Heyi Pharmaceutical Ltd
|
$
|
310,191
|
$
|
725,856
|
|||||
|
Related
company with the same management personnel
|
Interest
expenses under agreement
|
$
|
177,527
|
$
|
48,604
|
|||||
In
addition, as of December 31, 2009, the aggregate amount due from Hainan Zhonghe
Group is $4,239,232. The aggregate amount due from Hainan Heyi
Pharmaceutical Co., Ltd. is $417,569. These amounts are for purchase of new
drugs and R & D. Mr. Xueyun Cui, our sole director, is the controlling
shareholder of Hainan Zhonghe Group. Hainan Peptide and Hainan
Heyi.
67
On
March 23, 2009, Zhonghe entered into an agreement with Zhonghe Peptide to
acquire a technology know-how in relation to the production of a new drug at a
total consideration of RMB60 million. As of September 30, 2009, RMB54 million
(equivalent to $7.92 million) was paid to Zhonghe Peptide as a deposit, which
was settled by offsetting the amount due from related companies of $7.59 million
and by cash of $0.33 million. The transaction is expected to be completed in of
2010.
Item
14. Principal Accounting Fees And Services
PKF has
audited our financial statements annually for the 2009 and 2008 fiscal
year. All of the services described below were approved by our board and audit
committee prior to performance. The board has determined that the payments made
to its independent accountant for these services are compatible with maintaining
such auditor's independence.
Audit
Fees
The
aggregate fees for professional services rendered by PKF in connection with its
audit of our annual consolidated financial statements in our Form 10-K for the
fiscal year ended December 31, 2009 and December 31, 2008 totaled
approximately $77,698 and $184,576, respectively.
Audit-Related
Fees
No fees
were paid or accrued by us for assurance and related services rendered by PKF in
connection with their audit and review of our financial statements for the
fiscal years ended December 31, 2009 and December 31, 2008.
Tax
Fees
No fees
were paid or accrued by us for professional services rendered by PKF
tax advice and tax planning for the fiscal years December 31, 2009 and December
31, 2008, respectively.
All
Other Fees
No fees
were paid or accrued by us for other services rendered by PKF for the
fiscal years ended December 31, 2009 and December 31, 2008.
PART
IV
Item
15. Exhibits and Financial Statement Schedules
68
Kun
Run Biotechnology, Inc.
Consolidated
Financial Statements
For the
year ended December 31, 2009
(Stated
in US dollars)
69
Kun Run
Biotechnology, Inc.
Consolidated
Financial Statements
Index to
Consolidated Financial Statements
|
Pages
|
||
|
Report
of Independent Registered Public Accounting Firm
|
1
|
|
|
Consolidated
Statements of Income and Comprehensive Income
|
2
|
|
|
Consolidated
Balance Sheets
|
3 -
4
|
|
|
Consolidated
Statements of Cash Flows
|
5 -
6
|
|
|
Consolidated
Statements of Equity
|
7
|
|
|
Notes
to Consolidated Financial Statements
|
8 -
28
|

Report of Independent Registered Public Accounting Firm
To the
Board of Directors and Stockholders of
Kun Run
Biotechnology, Inc.
We have
audited the accompanying consolidated balance sheets of Kun Run Biotechnology,
Inc. (the “Company”) and its subsidiaries as of December 31, 2009 and 2008, and
the related consolidated statements of income and comprehensive income, equity
and cash flows for each of the two years in the period ended December 31,
2009. These financial statements are the responsibility of the
Company’s management. Our responsibility is to express an opinion on these
financial statements based on our audits.
We
conducted our audits in accordance with the standards of the Public Company
Accounting Oversight Board (United States). Those standards require
that we plan and perform the audit to obtain reasonable assurance about whether
the financial statements are free of material misstatement. An audit
includes examining, on a test basis, evidence supporting the amounts and
disclosures in the financial statements. An audit also includes
assessing the accounting principles used and significant estimates made by
management, as well as evaluating the overall financial statement
presentation. We believe that our audits provide a reasonable basis
for our opinion.
In our
opinion, the financial statements referred to above present fairly, in all
material respects, the consolidated financial position of the Company and its
subsidiaries as of December 31, 2009 and 2008, and the consolidated results of
their operations and their cash flows for the each of the two years in the
period ended December 31, 2009 in conformity with accounting principles
generally accepted in the United States of America.

PKF
Certified
Public Accountants
Hong
Kong, China
March 30,
2010
|
Tel
852 2806 3822 | Fax 852 2806 3712
E-mail
info@pkf-hk.com | www.pkf-hk.com
PKF
| 26/F, Citicorp Centre | 18 Whitfield Road
| Causeway Bay | Hong Kong
PKF
Hong Kong is a member firm of the PKF International Limited network of
legally independent firms and doe not accept any responsibility or
liability for the actions or inactions on the part of any other individual
member firm or firms
|
 |
- 1
-
Kun
Run Biotechnology, Inc.
Consolidated
Statements of Income and Comprehensive Income
(Stated
in US Dollars)
|
Year ended December 31,
|
||||||||
|
2009
|
2008
|
|||||||
|
Sales
revenue
|
$ | 13,235,756 | $ | 11,622,503 | ||||
|
Cost
of sales
|
3,980,328 | 3,410,891 | ||||||
|
Gross
profit
|
9,255,428 | 8,211,612 | ||||||
|
Operating
expenses
|
||||||||
|
Administrative
expenses
|
907,804 | 1,184,864 | ||||||
|
Research
and developments expenses
|
336,730 | 271,476 | ||||||
|
Selling
expenses
|
387,536 | 687,949 | ||||||
|
Unusual
charge - make good provision
|
- | 1,300,000 | ||||||
| 1,632,070 | 3,444,289 | |||||||
|
Income
from operations
|
7,623,358 | 4,767,323 | ||||||
|
Interest
income
|
178,830 | 50,438 | ||||||
|
Other
income - Note 4
|
353,851 | 222,927 | ||||||
|
Government
subsidy income
|
129,217 | 31,003 | ||||||
|
(Loss)/gain
on disposal of property, plant and equipment
|
(183 | ) | 2,416,110 | |||||
|
Net
finance costs - Note 5
|
(602,796 | ) | (545,995 | ) | ||||
|
Income
before income taxes and noncontrolling interest
|
7,682,277 | 6,941,806 | ||||||
|
Income
taxes - Note 6
|
(1,130,854 | ) | (907,439 | ) | ||||
|
Net
income before noncontrolling interest
|
6,551,423 | 6,034,367 | ||||||
|
Net
income attributable to noncontrolling interest - Note 7
|
(57,664 | ) | (64,658 | ) | ||||
|
Net
income attributable to Kun Run Biotechnology, Inc. common
stockholders
|
$ | 6,493,759 | $ | 5,969,709 | ||||
|
Net
income before noncontrolling interests
|
$ | 6,551,423 | $ | 6,034,367 | ||||
|
Other
comprehensive income Foreign currency translation
adjustments
|
6,210 | 860,075 | ||||||
|
Comprehensive
income
|
6,557,633 | 6,894,442 | ||||||
|
Comprehensive
income attributable to noncontrolling interest
|
(57,701 | ) | (65,780 | ) | ||||
|
Comprehensive
income attributable to Kun Run Biotechnology, Inc. common
stockholders
|
$ | 6,499,932 | $ | 6,828,662 | ||||
|
Earnings
per share attributable to Kun Run Biotechnology, Inc. common
stockholders: basic and diluted - Note 8
|
$ | 0.26 | $ | 0.24 | ||||
|
Weighted
average number of shares Outstanding: basic and diluted
|
25,000,000 | 24,467,808 | ||||||
See the
accompanying notes to consolidated financial statements.
- 2
-
Kun
Run Biotechnology, Inc.
Consolidated
Balance Sheets
(Stated
in US Dollars)
|
As of December 31,
|
||||||||
|
2009
|
2008
|
|||||||
|
ASSETS
|
||||||||
|
Current
assets
|
||||||||
|
Cash
and cash equivalents
|
$ | 810,809 | $ | 433,599 | ||||
|
Trade
receivables, net - Note 9
|
4,284,515 | 4,732,750 | ||||||
|
Bills
receivable
|
360,360 | 117,360 | ||||||
|
Other
receivables, prepayments and deposits - Note 10
|
2,338,971 | 985,683 | ||||||
|
Receivables
from disposal of properties - Note 11
|
- | 2,061,793 | ||||||
|
Inventories
- Note 12
|
2,248,420 | 689,415 | ||||||
|
Amounts
due from related companies - Note 13
|
4,656,801 | 5,595,307 | ||||||
|
Deferred
taxes - Note 6
|
- | 8,362 | ||||||
|
Total
current assets
|
14,699,876 | 14,624,269 | ||||||
|
Intangible
assets - Note 14
|
86,551 | 111,004 | ||||||
|
Property,
plant and equipment, net - Note 15
|
10,098,529 | 9,685,374 | ||||||
|
Land
use rights - Note 16
|
3,704,660 | 3,775,540 | ||||||
|
Deposit
for acquisition of property, plant and equipment
|
418,594 | 445,691 | ||||||
|
Deposit
paid to a related company for acquisition of an intangible asset - Note
17
|
7,921,800 | - | ||||||
|
TOTAL
ASSETS
|
$ | 36,930,010 | $ | 28,641,878 | ||||
See the
accompanying notes to consolidated financial statements.
- 3
-
Kun
Run Biotechnology, Inc.
Consolidated
Balance Sheets (Cont’d)
(Stated
in US Dollars)
|
As
of December 31,
|
||||||||
|
2009
|
2008
|
|||||||
|
LIABILITIES
AND EQUITY
|
||||||||
|
LIABILITIES
|
||||||||
|
Current
liabilities
|
||||||||
|
Trade
payables
|
$ | 452,139 | $ | 966,937 | ||||
|
Other
payables and accrued expenses - Note 18
|
3,961,125 | 1,672,500 | ||||||
|
Dividend
payable to Zhonghe’s former/existing noncontrolling
stockholders
|
7,209 | 7,209 | ||||||
|
Income
tax payable
|
815,435 | 655,019 | ||||||
|
Amount
due to a related company - Note 13
|
- | 936 | ||||||
|
Secured
borrowings - Note 19
|
6,051,375 | - | ||||||
|
Total
current liabilities
|
11,287,283 | 3,302,601 | ||||||
|
Deferred
taxes - Note 6
|
17,215 | 8,255 | ||||||
|
Secured
long-term borrowings - Note 19
|
330,075 | 6,528,150 | ||||||
|
TOTAL
LIABILITIES
|
11,634,573 | 9,839,006 | ||||||
|
COMMITMENTS AND CONTINGENCIES
- Note 20
|
||||||||
|
STOCKHOLDERS’
EQUITY
|
||||||||
|
Preferred
stock : par value of $0.001 per share, authorized 10,000,000 shares
in 2009 and 2008; none issued and outstanding
|
- | - | ||||||
|
Common
stock : par value $0.001 per share Authorized 100,000,000 shares in 2009
and 2008; issued and outstanding 25,000,000 shares in 2009 and
2008
|
25,000 | 25,000 | ||||||
|
Additional
paid-in capital
|
8,903,965 | 8,969,033 | ||||||
|
Statutory
and other reserves - Note 21
|
3,743,028 | 2,820,850 | ||||||
|
Accumulated
other comprehensive income
|
1,607,518 | 1,601,345 | ||||||
|
Retained
earnings
|
10,789,080 | 5,217,499 | ||||||
|
TOTAL
KUN RUN BIOTECHNOLOGY, INC. STOCKHOLDERS’ EQUITY
|
25,068,591 | 18,633,727 | ||||||
|
NONCONTROLLING
INTEREST
|
226,846 | 169,145 | ||||||
|
TOTAL
EQUITY
|
25,295,437 | 18,802,872 | ||||||
|
TOTAL
LIABILITIES AND EQUITY
|
$ | 36,930,010 | $ | 28,641,878 | ||||
See the
accompanying notes to consolidated financial statements.
- 4
-
Kun
Run Biotechnology, Inc.
Consolidated
Statements of Cash Flows
(Stated
in US Dollars)
|
Year ended December 31,
|
||||||||
|
2009
|
2008
|
|||||||
|
Cash
flows from operating activities
|
||||||||
|
Net income before
noncontrolling interest
|
$ | 6,551,423 | $ | 6,034,367 | ||||
|
Adjustments
to reconcile net income before noncontrolling interests to net cash
provided by operating activities :-
|
||||||||
|
Depreciation
|
796,045 | 661,747 | ||||||
|
Amortization
of land use rights and intangible assets
|
95,268 | 72,845 | ||||||
|
Deferred
taxes
|
17,305 | 28,524 | ||||||
|
Loss
(gain) on disposal of property, plant and equipment
|
183 | (2,416,110 | ) | |||||
|
(Recovery
of) provision for doubtful debts
|
(142,673 | ) | 281,698 | |||||
|
Unusual
charge - make good provision
|
- | 1,300,000 | ||||||
|
Changes
in operating assets and liabilities :-
|
||||||||
|
Trade
receivables
|
591,044 | (1,294,173 | ) | |||||
|
Bills
receivable
|
(242,742 | ) | (115,360 | ) | ||||
|
Other
receivables, prepayments and deposits
|
(1,370,853 | ) | 523,286 | |||||
|
Inventories
|
(1,558,272 | ) | (123,050 | ) | ||||
|
Amounts
due from related companies
|
298,565 | (629,852 | ) | |||||
|
Trade
payables
|
(514,508 | ) | 347,897 | |||||
|
Other
payables and accrued expenses
|
2,287,387 | 268,500 | ||||||
|
Income
tax recoverable
|
- | 206,218 | ||||||
|
Income
tax payable
|
160,287 | 643,856 | ||||||
|
Net
cash flows provided by operating activities
|
6,968,459 | 5,790,393 | ||||||
|
Cash
flows from investing activities
|
||||||||
|
Payments
to acquire and for deposit for acquisition of property, plant and
equipment
|
(601,176 | ) | (1,140,333 | ) | ||||
|
Proceeds
from sales of property, plant and equipment
|
2,059,306 | 1,211,104 | ||||||
|
Deposit
for acquisition of intangible assets
|
(322,520 | ) | - | |||||
|
Payment
to acquire patents
|
- | (77,147 | ) | |||||
|
Cash
acquired from RTO
|
- | 149,665 | ||||||
|
Amounts
due from related parties
|
(7,601,210 | ) | (5,079,840 | ) | ||||
|
Net
cash flows used in investing activities
|
$ | (6,465,600 | ) | $ | (4,936,551 | ) | ||
See the
accompanying notes to consolidated financial statements.
- 5
-
Kun
Run Biotechnology, Inc.
Consolidated
Statements of Cash Flows (Cont’d)
(Stated
in US Dollars)
|
Year ended December 31,
|
||||||||
|
2009
|
2008
|
|||||||
|
Cash
flows from financing activities
|
||||||||
|
Proceeds
from secured borrowings
|
$ | 2,932,000 | $ | 324,450 | ||||
|
Repayment
of secured borrowings
|
(3,080,500 | ) | (1,442,000 | ) | ||||
|
Dividend
paid to Zhonghe’s former stockholder - Hainan Hekun Bronze Art Co., Ltd.
(“Hainan Hekun”)
|
(935 | ) | - | |||||
|
Dividend
paid to existing noncontrolling stockholders
|
- | (11,885 | ) | |||||
|
Amount
due to a related party
|
23,725 | - | ||||||
|
Net
cash flows used in financing activities
|
(125,710 | ) | (1,129,435 | ) | ||||
|
Effect
of foreign currency translation on cash and cash
equivalents
|
61 | 38,273 | ||||||
|
Net
increase (decrease) in cash and cash equivalents
|
377,210 | (237,320 | ) | |||||
|
Cash
and cash equivalents - beginning of year
|
433,599 | 670,919 | ||||||
|
Cash
and cash equivalents - end of year
|
$ | 810,809 | $ | 433,599 | ||||
|
Supplemental
disclosures for cash flow information :-
|
||||||||
|
Cash
paid for :-
|
||||||||
|
Interest
and bill discounting charges
|
$ | 608,745 | $ | 543,009 | ||||
|
Income
taxes
|
$ | 953,215 | $ | 28,840 | ||||
|
Non-cash
investing and financing activities:
|
||||||||
|
Dividend
payable to Zhonghe’s former stockholders settled by offsetting amounts due
from related companies and other payables
|
$ | - | $ | 125,948 | ||||
|
Deposit
for acquisition of an intangible asset by offsetting amounts due from
related companies - Note 13(b) and 17
|
7,589,000 | - | ||||||
|
Acquisition
of a building by offsetting the amount due from Hainan Zhonghe Group -
Note 13(b)
|
$ | 651,254 | $ | - | ||||
See the
accompanying notes to consolidated financial statements.
- 6
-
Kun
Run Biotechnology, Inc.
Consolidated
Statements of Equity
(Stated
in US Dollars)
|
Kun Run Biotechnology, Inc. stockholders
|
||||||||||||||||||||||||||||||||
|
Statutory
|
Accumulated
|
|||||||||||||||||||||||||||||||
|
Additional
|
and other
|
other
|
||||||||||||||||||||||||||||||
|
Common stock
|
paid-in
|
reserves
|
comprehensive
|
Retained
|
Noncontrolling
|
|||||||||||||||||||||||||||
|
No. of shares
|
Amount
|
capital
|
(Note
21 )
|
income
|
earnings
|
interest
|
Total
|
|||||||||||||||||||||||||
|
Balance,
January 1, 2008
|
24,250,000 | $ | 24,250 | $ | 7,541,074 | $ | 1,725,313 | $ | 742,392 | $ | 343,327 | $ | 103,365 | $ | 10,479,721 | |||||||||||||||||
|
Recapitalisation
|
750,000 | 750 | 127,959 | - | - | - | - | 128,709 | ||||||||||||||||||||||||
|
Net
income
|
- | - | - | - | - | 5,969,709 | 64,658 | 6,034,367 | ||||||||||||||||||||||||
|
Foreign
currency translation adjustments
|
- | - | - | - | 858,953 | - | 1,122 | 860,075 | ||||||||||||||||||||||||
|
Appropriation
to reserves
|
- | - | - | 1,095,537 | - | (1,095,537 | ) | - | - | |||||||||||||||||||||||
|
Unusual
charge - make good provision
|
- | - | 1,300,000 | - | - | - | - | 1,300,000 | ||||||||||||||||||||||||
|
Balance,
December 31, 2008
|
25,000,000 | 25,000 | 8,969,033 | 2,820,850 | 1,601,345 | 5,217,499 | 169,145 | 18,802,872 | ||||||||||||||||||||||||
|
Net
income
|
- | - | - | - | - | 6,493,759 | 57,664 | 6,551,423 | ||||||||||||||||||||||||
|
Foreign
currency translation adjustments
|
- | - | - | - | 6,173 | - | 37 | 6,210 | ||||||||||||||||||||||||
|
Appropriation
to reserves
|
- | - | - | 922,178 | - | (922,178 | ) | - | - | |||||||||||||||||||||||
|
Capital
contribution
|
- | - | 23,725 | - | - | - | - | 23,725 | ||||||||||||||||||||||||
|
Distribution
of earning and special profits - Note 22
|
- | - | (88,793 | ) | - | - | - | - | (88,793 | ) | ||||||||||||||||||||||
|
Balance,
December 31, 2009
|
25,000,000 | $ | 25,000 | $ | 8,903,965 | $ | 3,743,028 | $ | 1,607,518 | $ | 10,789,080 | $ | 226,846 | $ | 25,295,437 | |||||||||||||||||
See the
accompanying notes to consolidated financial statements.
- 7
-
|
1.
|
Corporate
information
|
Kun Run
Biotechnology, Inc. (the “Company”) was incorporated in the State of Nevada on
March 10, 2006. The Company’s shares are quoted for trading on the
Over-The-Counter Bulletin Board in the United States of America.
On
September 8, 2008, the Company implemented a reverse split of which the issued
common shares of the Company decreased from 6,000,000 to 1,750,000 with par
value remaining unchanged at $0.001 each. On September 16, 2008,
1,000,000 common shares, which are held by the stockholders of the Company of
$0.001 each were cancelled.
On August
21, 2008, the Company entered into an agreement with the stockholders of Kun Run
Biotechnology Limited (“Kun Run”) to acquire their issued and outstanding common
stocks in Kun Run by issuing 24,250,000 common shares (representing the number
of shares after the reverse split) at par value of $0.001 each. The
acquisition, which was completed on September 16, 2008, constituted a reverse
takeover transaction (“RTO”) and thereafter Kun Run became a wholly owned
subsidiary of the Company.
Kun Run,
which changed its name from Max Talent Industrial Ltd. on February 25, 2008, was
incorporated in Hong Kong on May 6, 2006 as a limited liability company with
registered share capital of HK$10,000, divided into 10,000 common shares of HK$1
par value each. Kun Run was dormant since its incorporation until March 24,
2008, which is the date Kun Run entered into a share purchase agreement with
Hainan Zhonghe (Group) Co., Ltd. (“Hainan Zhonghe Group”), Hainan Zhonghe
Peptide Drugs Research & Development Co., Ltd. (“Zhonghe Peptide”) and
Hainan Hekun to acquire their respective 60%, 0.083% and 0.033% interests in
Hainan Zhonghe Pharmaceutical Co., Ltd. (“Zhonghe”) at a total cash
consideration of $5,205,906. On May 27, 2008, the Company entered into another
share purchase agreement with Hainan Zhonghe Group to acquire its 39% interests
in Zhonghe at a cash consideration of $3,416,563. The acquisitions (the
“Acquisition”) were completed on June 26, 2008. Upon the completion of the
Acquisition, Zhonghe became a 99.12% owned subsidiary of Kun Run. Zhonghe is a
high-technology domestic company established in Hainan Province, the PRC on
April 17, 1995 with registered capital of RMB11 million.
Mr.
Xueyun Cui (“Mr. Cui”) is the ultimate controlling party of Hainan Zhonghe
Group, Zhonghe Peptide, Hainan Hekun, Kun Run and the Company. It is therefore
defined that Hainan Zhonghe Group and Zhonghe Peptide were the related parties
for the Company due to under the common control of Mr. Cui.
Following
the Acquisition and RTO, the Company commenced to be engaged in manufacture,
marketing, sale and distribution of polypeptide drugs which could be used to
treat immunity dysfunction and hyperfunction, as well as some high quality
chemical drugs mainly in the People’s Republic of China (“PRC”).
|
2.
|
Basis
of presentation
|
The
Acquisition and RTO and have been accounted for as a recapitalization of the
Company whereby the historical financial statements and operations of Kun Run
and its subsidiary, Zhonghe, become the historical financial statements of the
Company, with no adjustment to the carrying value of the assets and
liabilities. The 750,000 common shares (representing the number of
shares after the reverse split) of the Company outstanding prior to the RTO are
accounted for at $128,709 of net book value at the time of the
RTO. The accompanying consolidated financial statements reflect
the recapitalization of the stockholders equity as if the transaction occurred
as of the beginning of the first period presented.
- 8
-
|
3.
|
Summary
of significant accounting policies
|
Principles of
consolidation
The
consolidated financial statements include the accounts of the Company and its
subsidiaries. All significant inter-company accounts and transactions
have been eliminated in consolidation.
Use of
estimates
In
preparing the consolidated financial statements in conformity with accounting
principles generally accepted in the United States of America, management makes
estimates and assumptions that affect the reported amounts of assets and
liabilities and disclosures of contingent assets and liabilities at the dates of
the consolidated financial statements, as well as the reported amounts of
revenues and expenses during the reporting periods. These accounts
and estimates include, but are not limited to, the valuation of accounts
receivable, inventories, deferred income taxes, estimation on useful lives and
residual values of property, plant and equipment and intangible
assets. Actual results could differ from those
estimates.
Concentrations of credit
risk
Financial
instruments that potentially subject the Company to significant concentrations
of credit risk consist principally of cash and cash equivalents, trade and bills
receivables and amount due from related companies. As of December 31,
2009 and 2008, substantially all of the Company’s cash and cash equivalents were
held by major financial institutions located in the PRC, which management
believes are of high credit quality. With respect to trade and bills
receivables, the Company extends credit based on an evaluation of the customer’s
financial condition. The Company generally does not require
collateral for trade receivables and maintains an allowance for doubtful
accounts of trade receivables.
During
the reporting periods, no customers representing 10% or more of the Company’s
consolidated sales.
Details
of customers for 10% or more of the Company's trade receivables are
:-
|
As of December 31,
|
||||||||
|
2009
|
2008
|
|||||||
|
Beijing
Xingshengyuan Pharmaceutical Ltd.
|
$ | 742,888 | $ | 1,240,606 | ||||
|
Changqin
Dinghai Pharmaceutical Ltd.
|
774,139 | 308,870 | ||||||
|
Hainan
Heyi Pharmaceutical Ltd.
|
359,493 | 714,548 | ||||||
| $ | 1,876,520 | $ | 2,264,024 | |||||
Cash and cash
equivalents
Cash and
cash equivalents include all cash, deposits in banks and other highly liquid
investments with initial maturities of three months or less. As of
December 31, 2009 and 2008, almost all the cash and cash equivalents were
denominated in Renminbi (“RMB”) and were placed with banks in the
PRC. They are not freely convertible into foreign currencies and the
remittance of these funds out of the PRC is subject to exchange control
restrictions imposed by the PRC government. The remaining
insignificant balance of cash and cash equivalents were denominated in Hong Kong
dollars and US dollars.
- 9
-
|
3.
|
Summary
of significant accounting policies
(Cont’d)
|
Allowance for doubtful
debts
The
Company establishes an allowance for doubtful debts based on management’s
assessment of the collectibility of trade receivables. As a
considerable amount of judgment is required in assessing the amount of the
allowance, the Company considers the historical level of credit losses and
applies percentages to aged receivable categories. The Company makes
judgments about the creditworthiness of each customer based on ongoing credit
evaluations, and monitors current economic trends that might impact the level of
credit losses in the future. If the financial condition of the
customers were to deteriorate, resulting in their inability to make payments, a
larger allowance may be required.
Based on
the above assessment, during the reporting years, the management establishes the
following rates of general provision provided on gross amount of trade
receivables :-
|
Rate
|
||||
|
Aged
within 1 year
|
10 | % | ||
|
Aged
over 1 year but within 2 years
|
40 | % | ||
|
Aged
over 2 years but within 3 years
|
80 | % | ||
|
More
than 3 years
|
100 | % | ||
Additional
specific provision is made against trade receivables whenever they are
considered to be doubtful.
Bad debts
are fully written off when identified or the trade receivable aged more than 3
years. The Company extends unsecured credit to customers ranging from
three to six months in the normal course of business. The Company
does not accrue interest on trade accounts receivable.
(Recovery
of)/provision for doubtful debts of $(142,673) and $281,698 are included in
other income and selling expenses for two years ended December 31, 2009 and 2008
respectively.
Inventories
Inventories
are stated at the lower of cost or market value. Cost is determined
on weighted average basis and includes all expenditures incurred in bringing the
goods to the point of sale and putting them in a saleable
condition. In assessing the ultimate realization of inventories, the
management makes judgments as to future demand requirements compared to current
or committed inventory levels. The Company’s reserve requirements
generally increase with its projected demand requirements decrease due to market
conditions, product life cycle changes. The Company estimates the demand
requirements based on market conditions, forecasts prepared by its customers,
sales contracts and orders in hand. Inventory that is obsolete or expired is
written down to its market value if lower than cost. Inventory quantities are
regularly reviewed and provisions for excess or obsolete inventory are recorded
primarily based on the Company’s forecast of future demand and market
conditions.
- 10
-
|
3.
|
Summary
of significant accounting policies
(Cont’d)
|
Property, plant and
equipment
Property,
plant and equipment are stated at cost less accumulated
depreciation. Cost represents the purchase price of the asset and
other costs incurred to bring the asset into its existing use.
Depreciation
is provided on straight-line basis over their estimated useful
lives. The principal depreciation rates are as follows
:-
|
Annual rate
|
Residual value
|
|||||||
|
Buildings
|
2.5 - 5 | % | 5 | % | ||||
|
Plant
and machinery
|
10 - 20 | % | 3 | % | ||||
|
Motor
vehicles
|
14 - 20 | % | 5 | % | ||||
|
Furniture,
fixtures and equipment
|
20 | % | 3 | % | ||||
|
Leasehold
improvements
|
20 | % |
Nil
|
|||||
Construction
in progress mainly represents expenditures in respect of new offices and
factories under construction of Zhonghe. All direct costs relating to
the acquisition or construction of Zhonghe’s new office and factories are
capitalized as construction in progress. No depreciation is provided in respect
of construction in progress.
Maintenance
or repairs are charged to expense as incurred. Upon sale or
disposition, the applicable amounts of asset cost and accumulated depreciation
are removed from the accounts and the net amount less proceeds from disposal is
charged or credited to income.
Intangible
assets
They
represent pharmaceutical licenses and patents and are stated at cost less
accumulated amortization. Amortization is provided on a straight-line basis over
their useful lives of 5 years.
Land use
rights
Land use
rights are stated at cost less accumulated amortization. Amortization
is provided using the straight-line method over the terms of the leases of 55
and 56 years obtained from the relevant PRC land authority.
Impairment of long-lived
assets
Long-lived
assets are tested for impairment in accordance with ASC 360-10-45 “Impairment or
Disposal of Long-Lived Assets” (previously Statement of Financial Accounting
Standards (“SFAS”) No. 144), Accounting for the impairment or disposal of
long-lived assets, respectively. The Company periodically evaluates
potential impairment whenever events or changes in circumstances indicate that
the carrying amount of the assets may not be recoverable. The Company
recognizes impairment of long-lived assets and investment in an affiliate in the
event that the net book values of such assets exceed the future undiscounted
cashflows attributable to such assets. During the reporting periods,
the Company has not identified any indicators that would require testing for
impairment.
Noncontrolling
interests
Noncontrolling
interests resulted from the consolidation of Zhonghe, a 99.12% owned
subsidiary.
- 11
-
|
3.
|
Summary
of significant accounting policies
(Cont’d)
|
Government subsidy
income
Government
subsidy income was $129,217 for the year ended December 31, 2009 (2008 :
$31,003). It mainly consisted of receipt of funds granted to subsidize the
research and development activities in relation to the Zhonghe’s qualification
of new and high technology. The amount is un-conditional,
non-refundable and without any restrictions on usage at the time of grant to and
receipt by the Company. Such grant is recognised as income at time of
receipt for the year.
Revenue
recognition
Revenue
from sales of the Company’s products is recognized when the significant risks
and rewards of ownership have been transferred to the buyer at the time of
delivery, the sales price is fixed or determinable and collection is reasonably
assured.
Advertising, transportation
and research and development expenses
Advertising,
transportation and research and development expenses are charged to expense as
incurred.
Advertising
expenses amounting to $58,105 and $69,591 for two years ended December 31, 2009
and 2008 respectively are included in selling expenses.
Transportation
expenses amounting to $57,801 and $50,623 for two years ended December 31, 2009
and 2008 respectively are included in selling expenses.
Research
and development expenses consist primarily of remuneration for research and
development staff and material costs for research and development.
Cost of
sales
Cost of
sales consists primarily of material costs, freight charges, purchasing and
receiving costs, inspection costs, wages, employee compensation, depreciation
and related costs, which are directly attributable to the production of
products. Write-down of inventory to lower of cost or market is also
recorded in cost of sales.
Selling
expenses
Selling
expenses mainly consists of advertising, commission, entertainment, salaries,
transportation cost and traveling expense which are incurred during the selling
activities.
General and administrative
expenses
General
and administrative expenses consist of rent paid, office expenses, staff
welfare, consumables, labor protection and salaries and wage which are incurred
at the administrative level and exchange difference.
- 12
-
|
3.
|
Summary
of significant accounting policies
(Cont’d)
|
Stock-based
compensation
The
Company adopted the provisions of ASC 718 (previously SFAS 123(R)), which
requires the use of the fair value method of accounting for share-based
compensation. Under the fair value based method, compensation cost related to
employee stock options or similar equity instruments which are equity-classified
awards, is measured at the grant date based on the value of the award and is
recognized over the requisite service period, which is usually the vesting
period. ASC 718 also requires measurement of cost of a liability-classified
award based on its current fair value.
Income
taxes
The
Company uses the asset and liability method of accounting for income taxes
pursuant to ASC 740 “Income Taxes” (previously SFAS No. 109). Under
the asset and liability method of ASC 740, deferred tax assets and liabilities
are recognized for the future tax consequences attributable to temporary
differences between the financial statements carrying amounts of existing assets
and liabilities and loss carryforwards and their respective tax
bases. Deferred tax assets and liabilities are measured using enacted
tax rates expected to apply to taxable income in the years in which those
temporary differences are expected to be recovered or settled.
Dividends
Dividends
are recorded in Company’s financial statements in the period in which they are
declared.
Off-balance sheet
arrangements
The
Company does not have any off-balance sheet arrangements.
Comprehensive
income
The
Company has adopted ASC 220, “Comprehensive Income”, which establishes standards
for reporting and display of comprehensive income, its components and
accumulated balances. Components of comprehensive income include net
income and foreign currency translation adjustments.
Foreign currency
translation
The
functional currency of the Company is RMB and RMB is not freely convertible into
foreign currencies. The Company maintains its financial statements in
the functional currency. Monetary assets and liabilities denominated
in currencies other than the functional currency are translated into the
functional currency at rates of exchange prevailing at the balance sheet
date. Transactions denominated in currencies other than the
functional currency are translated into the functional currency at the exchange
rates prevailing at the dates of the transaction. Exchange gains or
losses arising from foreign currency transactions are included in the
determination of net income for the respective periods.
- 13
-
|
3.
|
Summary
of significant accounting policies
(Cont’d)
|
Foreign currency translation
(Cont’d)
For
financial reporting purposes, the financial statements of the Company which are
prepared using the functional currency have been translated into United States
dollars. Assets and liabilities are translated at the exchange rates
at the balance sheet dates and revenue and expenses are translated at the
average exchange rates and stockholders’ equity is translated at historical
exchange rates. Any translation adjustments resulting are not included in
determining net income but are included in foreign exchange adjustment to other
comprehensive income, a component of stockholders’ equity. The
exchange rates in effect at December 31, 2009 and 2008 were both RMB1 $0.1467.
There is no significant fluctuation in exchange rate for the conversion of RMB
to US dollars after the balance sheet date.
Basic and diluted earnings
per share
The
Company reports basic earnings per share in accordance with ASC 260, “Earnings
Per Share” (previously SFAS No. 128). Basic earnings per share are
computed using the weighted average number of shares outstanding during the
periods presented. The weighted average number of shares of the
Company represents the common stock outstanding during the reporting
periods.
Fair value of financial
instruments
The
Company adopted ASC 820 (previously SFAS No. 157) on January 1, 2008. The
adoption of ASC 820 did not materially impact the Company’s financial position,
results of operations or cash flows.
ASC 820
requires the disclosure of the estimated fair value of financial instruments
including those financial instruments for which fair value option was not
elected. Except for secured borrowings disclosed as below, the carrying amounts
of the financial assets and liabilities approximate to their fair values due to
short maturities or the applicable interest rates approximate the current market
rates :-
|
As of December 31, 2009
|
As of December 31, 2008
|
|||||||||||||||
|
Carrying
|
Carrying
|
|||||||||||||||
|
amount
|
Fair
value
|
amount
|
Fair
value
|
|||||||||||||
|
Secured
borrowings
|
$ | 6,381,450 | $ | 6,362,757 | $ | 6,528,150 | $ | 6,407,755 | ||||||||
The fair
values of secured borrowings are estimated using discounted cash flow analysis,
based on the Company’s current incremental borrowing rates for similar types of
borrowing arrangements.
- 14
-
|
3.
|
Summary
of significant accounting policies
(Cont’d)
|
Recently issued accounting
pronouncements
FASB
Accounting Standards Codification (Accounting Standards Update “ASU” 2009-1). In
June 2009, the Financial Accounting Standard Board (“FASB”) approved its
Accounting Standards Codification (“Codification”) as the single source of
authoritative United States accounting and reporting standards applicable for
all non-governmental entities, with the exception of the SEC and its staff. The
Codification is effective for interim or annual financial periods ending after
September 15, 2009 and impacts our financial statements as all future references
to authoritative accounting literature will be referenced in accordance with the
Codification. There have been no changes to the content of our financial
statements or disclosures as a result of implementing the
Codification.
As a
result of our implementation of the Codification during the quarter ended
September 30, 2009, previous references to new accounting standards and
literature are no longer applicable. In the current financial statements, we
will provide reference to both new and old guidance to assist in understanding
the impacts of recently adopted accounting literature, particularly for guidance
adopted since the beginning of the current fiscal year but prior to the
Codification.
Noncontrolling Interests (Included
in amended Topic ASC 810 “Consolidation”, previously SFAS No. 160
“Noncontrolling Interests in Consolidated Financial Statements”, an amendment of
ARB No. 51). The amended topic establishes accounting and reporting
standards for the noncontrolling interest in a subsidiary and for the
deconsolidation of a subsidiary. It clarifies that a noncontrolling interest in
a subsidiary is an ownership interest in the consolidated entity that should be
reported as equity in the consolidated financial statements. We adopted the
amended topic on January 1, 2009. As a result, we have reclassified financial
statement line items within our Consolidated Balance Sheets and Statements of
Income and Comprehensive Income for the prior period to conform to this amended
topic.
Business Combinations (Included in
amended Topic ASC 805 “Business Combinations”, previously SFAS No. 141(R)).
This ASC guidance addresses the accounting and disclosure for
identifiable assets acquired, liabilities assumed, and noncontrolling interests
in a business combination. The adoption of this amended topic has no material
impact on the Company’s financial statements.
Intangibles-Goodwill and Other
(Included in amended Topic ASC 350, previously FASB staff position (“FSP”) FAS
142-3, Determination of the Useful Life of Intangible Assets). The
amended topic amends the factors an entity should consider in developing renewal
or extension assumptions used in determining the useful life of recognized
intangible assets under FASB Statement No. 142, “Goodwill and Other
Intangible Assets”. This new guidance applies prospectively to intangible assets
that are acquired individually or with a group of other assets in business
combinations and asset acquisitions. The amended topic is effective for
financial statements issued for fiscal years and interim periods beginning after
December 15, 2008. Early adoption is prohibited. The adoption of this
amended topic has no material effect on the Company's financial
statements.
- 15
-
|
3.
|
Summary
of significant accounting policies
(Cont’d)
|
Recently issued accounting
pronouncements (Cont’d)
Business Combinations (Included in
amended Topic ASC 805, previously FSP No. 141R-1 “Accounting for Assets Acquired
and Liabilities Assumed in a Business Combination That Arise from
Contingencies”). Amended topic ASC 805 amends the requirements for the
provisions in FASB Statement 141R for the initial recognition and measurement,
subsequent measurement and accounting, and disclosures for assets and
liabilities arising from contingencies in business combinations. The amended
topic eliminates the distinction between contractual and non-contractual
contingencies, including the initial recognition and measurement criteria and
instead carries forward most of the provisions for acquired contingencies. The
amended topic is effective for contingent assets and contingent liabilities
acquired in evaluating the impact. The adoption of this amended topic has no
material impact on the Company’s financial statements.
Fair Value Measurements and
Disclosures (Included in amended Topic ASC 820, previously FSP No. 157-4,
“Determining Whether a Market is Not Active and a Transaction Is Not
Distressed”.) The amended topic clarifies when markets are illiquid or
that market pricing may not actually reflect the “real” value of an asset. If a
market is determined to be inactive and market price is reflective of a
distressed price then an alternative method of pricing can be used, such as a
present value technique to estimate fair value. The amended topic identifies
factors to be considered when determining whether or not a market is inactive.
The amended topic would be effective for interim and annual periods ending after
June 15, 2009, with early adoption permitted for periods ending after March 15,
2009 and shall be applied prospectively. The adoption of this amended topic has
no material effect on the Company's financial statements.
Investments - Debt and Equity
Securities - Overall - Transition and Open Effective Date Information (Included
in amended Topic ASC 320, previously FASB Staff Position No. 115-2 and Statement
of Financial Accounting Standards No. 124-2, “Recognition and Presentation of
Other-Than-Temporary Impairments”). The amended topic amends the
other-than-temporary impairment guidance in U.S. GAAP for debt securities
through increased consistency in the timing of impairment recognition and
enhanced disclosures related to the credit and noncredit components of impaired
debt securities that are not expected to be sold. In addition, increased
disclosures are required for both debt and equity securities regarding expected
cash flows, credit losses, and securities with unrealized losses. The adoption
of this amended topic has no material impact on the Company’s financial
statements.
Interim Disclosures about Fair Value
of Financial Instruments (Included in amended Topic ASC 825 “Financial
Instruments”, previously FSP SFAS No. 107-1). This guidance requires that
the fair value disclosures required for all financial instruments be included in
interim financial statements. This guidance also requires entities to disclose
the method and significant assumptions used to estimate the fair value of
financial instruments on an interim and annual basis and to highlight any
changes from prior periods. The amended topic was effective for interim periods
ending after September 15, 2009. The adoption of this amended topic has no
material impact on the Company’s financial statements.
Subsequent Events (Included in
amended Topic ASC 855 “Subsequent Events”, previously SFAS No. 165). The
amended topic establishes accounting and disclosure requirements for subsequent
events. The amended topic details the period after the balance sheet date during
which we should evaluate events or transactions that occur for potential
recognition or disclosure in the financial statements, the circumstances under
which we should recognize events or transactions occurring after the balance
sheet date in its financial statements and the required disclosures for such
events. We adopted this amended topic effective June 1, 2009.
- 16
-
|
3.
|
Summary
of significant accounting policies
(Cont’d)
|
Recently issued accounting
pronouncements (Cont’d)
Accounting for Transfers of
Financial Assets (Included in amended Topic ASC 860 “Transfers and Servicing”,
previously SFAS No. 166, “Accounting for Transfers of Financial Assets - an
Amendment of FASB Statement No. 140.”). The amended topic addresses
information a reporting entity provides in its financial statements about the
transfer of financial assets; the effects of a transfer on its financial
position, financial performance, and cash flows; and a transferor’s continuing
involvement in transferred financial assets. Also, the amended topic removes the
concept of a qualifying special purpose entity, limits the circumstances in
which a transferor derecognizes a portion or component of a financial asset,
defines participating interest and enhances the information provided to
financial statement users to provide greater transparency. The amended topic is
effective for the first annual reporting period beginning after November 15,
2009 and will be effective for us as of January 1, 2010. The management is in
the process of evaluating the impact of adopting this amended topic on the
Company’s financial statements.
Consolidation of Variable Interest
Entities – Amended (Included in amended Topic ASC 810 “Consolidation”,
previously SFAS 167 “Amendments to FASB Interpretation No. 46(R)”). The
amended topic requires an enterprise to perform an analysis to determine the
primary beneficiary of a variable interest entity; to require ongoing
reassessments of whether an enterprise is the primary beneficiary of a variable
interest entity and to eliminate the quantitative approach previously required
for determining the primary beneficiary of a variable interest entity. The
amended topic also requires enhanced disclosures that will provide users of
financial statements with more transparent information about an enterprise’s
involvement in a variable interest entity. The amended topic is effective for
the first annual reporting period beginning after November 15, 2009 and will be
effective for us as of January 1, 2010. The management is in the process of
evaluating the impact of adopting this amended topic on the Company’s financial
statements
In August
2009, the FASB issued Accounting Standards Update (“ASU”) No. 2009-05 (“ASU
Update 2009-05”), an update to ASC 820, Fair Value Measurements and Disclosures.
This update provides amendments to reduce potential ambiguity in financial
reporting when measuring the fair value of liabilities. Among other provisions,
this update provides clarification that in circumstances in which a quoted price
in an active market for the identical liability is not available, a reporting
entity is required to measure fair value using one or more of the valuation
techniques described in ASU Update 2009-05. ASU Update 2009-05 became effective
for the Company’s annual financial statements for the year ended December 31,
2009. The adoption of this ASU update has no material impact on the Company’s
financial statements.
The FASB
issued ASU No. 2009-13, Revenue Recognition (Topic 605): Multiple Deliverable
Revenue Arrangements - A Consensus of the FASB Emerging Issues Task Force.” This
update provides application guidance on whether multiple deliverables exist, how
the deliverables should be separated and how the consideration should be
allocated to one or more units of accounting. This update establishes a selling
price hierarchy for determining the selling price of a deliverable. The selling
price used for each deliverable will be based on vendor-specific objective
evidence, if available, third-party evidence if vendor-specific objective
evidence is not available, or estimated selling price if neither vendor-specific
or third-party evidence is available. The Company will be required to apply this
guidance prospectively for revenue arrangements entered into or materially
modified after January 1, 2011; however, earlier application is permitted. The
management is in the process of evaluating the impact of adopting this ASU
update on the Company’s financial statements.
- 17
-
|
3.
|
Summary
of significant accounting policies
(Cont’d)
|
Recently issued accounting
pronouncements (Cont’d)
The FASB
issued ASU-2010-09 (Topic 855) to amend guidance on subsequent events to remove
the requirement for SEC filers (as defined in ASU 2010-09) to disclose the date
through which an entity has evaluated subsequent events. This change alleviates
potential conflicts with current SEC guidance. An SEC filer is still required to
evaluate subsequent events through the date financial statements are issued, but
disclosure of that date is no longer required. The amendments in ASU 2010-09
became effective upon issuance of the guidance.
|
4.
|
Other
income
|
|
Year ended December 31,
|
||||||||
|
2009
|
2008
|
|||||||
|
Recovery
of doubtful debts
|
$ | 142,673 | $ | - | ||||
|
Rental
income
|
211,104 | 222,515 | ||||||
|
Others
|
74 | 412 | ||||||
| $ | 353,851 | $ | 222,927 | |||||
|
5.
|
Net
finance costs
|
|
Year ended December 31,
|
||||||||
|
2009
|
2008
|
|||||||
|
Interest
expenses
|
$ | 600,629 | $ | 495,832 | ||||
|
Bills
discounting charges
|
8,116 | 47,177 | ||||||
|
Bank
charges
|
1,930 | 2,675 | ||||||
|
Net
exchange (gain)/loss
|
(7,879 | ) | 311 | |||||
| $ | 602,796 | $ | 545,995 | |||||
|
6.
|
Income
taxes
|
United
States
Kun Run
Biotechnology, Inc. is subject to the United States Federal and state income tax
at a statutory rate of 35%. No provision for the U.S. Federal income
taxes have been made as the Company had no taxable income in this jurisdiction
for the reporting periods.
Hong
Kong
Kun Run
Biotechnology Ltd. was incorporated in Hong Kong and subject to Hong Kong
profits tax at a tax rate of 16.5%. No provision for Hong Kong profits tax has
been made as the company has no taxable income during the reporting
periods.
- 18
-
|
6.
|
Income
taxes (Con’t)
|
PRC
The
Company’s subsidiary, Zhonghe, is registered and operates in Hainan Province,
the PRC, and recognized as “Manufacturing Enterprise Located in Special Economic
Zone”. As a result, Zhonghe is entitled to a preferential corporate
income tax (“CIT”) rate of 15% up to December 31, 2007.
The PRC’s
legislative body, the National People’s Congress, adopted the unified CIT Law on
March 16, 2007. This new tax law replaces the existing separate
income tax laws for domestic enterprises and foreign-invested enterprises and
became effective on January 1, 2008. Under the new tax law, a
unified income tax rates is set at 25% for both domestic enterprises and
foreign-invested enterprises. However, there will be a transition
period for enterprises, whether foreign-invested or domestic, that are currently
receiving preferential tax treatments granted by relevant tax
authorities. Enterprises that are subject to an enterprise income tax
rate lower than 25% may continue to enjoy the lower rate and will transit into
the new tax rate over a five year period beginning on the effective date of the
CIT Law. In accordance with the guideline, enterprise is subject to the tax rate
of 18% for 2008, 20% for 2009, 22% for 2010, 24% for 2011 and 25% for 2012
respectively during the transition period. Enterprises that are currently
entitled to exemptions for a fixed term will continue to enjoy such treatment
until the exemption term expires.
As
approved by the relevant tax authority in the PRC, Zhonghe, being a Foreign
Investment Enterprise ("FIE"), engaged in an advanced technology industry, was
approved to enjoy a further three years' preferential tax rate at 15% for 2008,
2009 and 2010.
The
components of the provision for income taxes are :-
|
Year ended December 31,
|
||||||||
|
2009
|
2008
|
|||||||
|
Current
taxes
|
$ | 1,113,549 | $ | 878,915 | ||||
|
Deferred
taxes
|
17,305 | 28,524 | ||||||
| $ | 1,130,854 | $ | 907,439 | |||||
The
effective income tax expenses differ from the PRC statutory income tax rate of
25% (2008 : 18%) in the PRC as follows :-
|
Year ended December 31,
|
||||||||
|
2009
|
2008
|
|||||||
|
Provision
for income taxes at PRC statutory income tax rate of 25% (2008 :
18%)
|
$ | 1,920,569 | $ | 1,249,525 | ||||
|
Non-deductible
items for tax
|
42,705 | 253,477 | ||||||
|
Income
not subject to tax
|
(21,135 | ) | (24,433 | ) | ||||
|
Advanced
Technology Industry Tax Benefit
|
(781,940 | ) | (251,687 | ) | ||||
|
Underprovision
for prior year
|
(29,345 | ) | - | |||||
|
Tax
Concession
|
- | (319,443 | ) | |||||
| $ | 1,130,854 | $ | 907,439 | |||||
- 19
-
|
6.
|
Income
taxes (Con’t)
|
PRC
(Cont’d)
During
the two years ended December 31, 2009 and 2008, the aggregate amounts of
benefits from Advanced Technology Industry Tax Benefit and Tax Concession were
$781,940 and $571,130 and the respective effect on earnings per share were $0.03
and $0.02 respectively.
In July
2006, the FASB issued ASC 740-10-25 (previously Interpretation No. 48,
“Accounting for Uncertainty in Income Taxes”). This interpretation
requires recognition and measurement of uncertain income tax positions using a
“more-likely-than-not” approach. The Company adopted this ASC
740-10-25 on January 1, 2007. Under the new CIT Law which became
effective on January 1, 2008, the Company may be deemed to be a resident
enterprise by the PRC tax authorities. If the Company was deemed to
be resident enterprise, the Company may be subject to the CIT at 25% on the
worldwide taxable income and dividends paid from PRC subsidiaries to their
overseas holding companies may be exempted from 10% PRC withholding
tax. Except for certain immaterial interest income from a bank
deposit placed with a financial institution outside the PRC, all of the
Company’s income is generated from the PRC operation. Given the
immaterial amount of income generated from outside the PRC and the PRC’s
subsidiaries do not intend to pay dividends in the foreseeable future, the
management considers that the impact arising from resident enterprise on the
Company’s financial position is not significant. The management evaluated the
Company’s overall tax positions and considered that no additional provision for
uncertainty in income taxes is necessary as of December 31, 2009.
Deferred
tax assets (liabilities) as of December 31, 2009 and 2008 are composed of the
following :-
|
As of December 31,
|
||||||||
|
2009
|
2008
|
|||||||
|
PRC
|
||||||||
|
Current
deferred tax assts:-
|
||||||||
|
Accrued
liabilities
|
$ | - | $ | 8,362 | ||||
|
PRC
|
||||||||
|
Non
current deferred tax assts (liabilities):-
|
||||||||
|
Depreciation
of property, plant and equipment
|
$ | (13,305 | ) | $ | (5,959 | ) | ||
|
Amortisation
of land use right
|
(3,910 | ) | (2,379 | ) | ||||
|
Amortisation
of patents
|
- | 83 | ||||||
| $ | (17,215 | ) | $ | (8,255 | ) | |||
|
7.
|
Noncontrolling
interest
|
Noncontrolling
interest in the consolidated statements of income and comprehensive income of
$57,664 and $64,658 for the year ended December 31, 2009 and 2008 respectively
represents the noncontrolling stockholders’ proportionate share of the net
income of Zhonghe.
|
8.
|
Earnings
per share
|
During
the reporting periods, the Company had no dilutive
instruments. Accordingly, the basic and diluted earnings per share
are the same.
- 20
-
|
9.
|
Trade
receivables, net
|
|
As of December 31,
|
||||||||
|
2009
|
2008
|
|||||||
|
Trade
receivables
|
$ | 5,134,430 | $ | 5,769,370 | ||||
|
Less
: allowance for doubtful accounts
|
(849,915 | ) | (1,036,620 | ) | ||||
| $ | 4,284,515 | $ | 4,732,750 | |||||
An
analysis of the allowance for doubtful accounts for the years ended December 31,
2009 and 2008 is as follows :-
|
Year ended December 31,
|
||||||||
|
2009
|
2008
|
|||||||
|
Balance
at beginning of year
|
$ | 1,036,620 | $ | 741,880 | ||||
|
(Reversal)
addition of bad debt expense, net
|
(142,673 | ) | 281,698 | |||||
|
Bad
debts written off
|
(44,032 | ) | (43,043 | ) | ||||
|
Translation
adjustments
|
- | 56,085 | ||||||
|
Balance
at end of year
|
$ | 849,915 | $ | 1,036,620 | ||||
|
10.
|
Other
receivables, prepayments and
deposits
|
|
As of December 31,
|
||||||||
|
2009
|
2008
|
|||||||
|
Deposits
paid
|
$ | 5,486 | $ | 65,808 | ||||
|
Prepayments
|
596,198 | 688,746 | ||||||
|
Other
receivables
|
302,272 | 176,694 | ||||||
|
Paid
in advance to suppliers
|
1,435,015 | 54,435 | ||||||
| $ | 2,338,971 | $ | 985,683 | |||||
|
11.
|
Receivables
from disposal of properties
|
The
amounts represent receivables from disposal of properties at considerations of
totalling $3,237,761 of which $1,175,968 was received as of December 31, 2008.
The remaining balance of $2,061,793 was subsequently settled in April
2009.
|
12.
|
Inventories
|
|
As of December 31,
|
||||||||
|
2009
|
2008
|
|||||||
|
Raw
materials
|
$ | 471,577 | $ | 284,837 | ||||
|
Work-in-progress
|
1,302,181 | 260,504 | ||||||
|
Finished
goods
|
474,662 | 144,074 | ||||||
| $ | 2,248,420 | $ | 689,415 | |||||
- 21
-
|
13.
|
Amounts
due from / to related companies
|
|
As
of December 31,
|
||||||||
|
2009
|
2008
|
|||||||
|
Amounts
due from related parties:-
|
||||||||
|
Hainan
Zhonghe Group - Note 13(b)
|
$ | 4,239,232 | $ | 4,352,639 | ||||
|
Zhonghe
Peptide - Note 13(c)
|
- | 528,120 | ||||||
|
Hainan
Heyi Pharmaceutical Co., Ltd. (“Hainan Heyi”)
|
||||||||
|
-
Note 13(d)
|
417,569 | 714,548 | ||||||
| $ | 4,656,801 | $ | 5,595,307 | |||||
|
Amount
due to a related party:-
|
||||||||
|
Dividend
payable to Zhonghe’s former stockholder
|
||||||||
|
Hainan
Hekun - Note 13(d)
|
$ | - | $ | 936 | ||||
Notes
:-
|
|
(a)
|
Mr.
Cui, the Company’s Chairman, sole director and the beneficial owner of
approximately 90.09% of the Company’s outstanding common stock, is the
ultimate controlling party of Hainan Zhonghe Group, Zhonghe Peptide,
Hainan Heyi and Hainan Hekun.
|
|
|
(b)
|
The
amounts are interest bearing at 5.31% per annum, unsecured and repayable
on demand. During the year of 2009, an advance of $6,776,756 was made to
Hainan Zhonghe Group, $6,236,426 was transferred to Zhonghe Peptide in
accordance with an agreement for acquisition of an intangible asset from
Zhonghe Peptide (Note 17) and $651,254 was offset with the consideration
of acquisition of a building from Hainan Zhonghe
Group.
|
|
|
(c)
|
The
amount is interest bearing at a benchmark rate in the PRC per annum,
unsecured. During the year of 2009, an advance of $824,454 was made to
Zhonghe Peptide. Together with $6,236,426 transferred from Hainan Zhonghe
Group, the accumulated balance of $7,589,000 was offset with the deposit
payable to Zhonghe Peptide for acquisition of an intangible asset (Note
17).
|
|
(d)
|
The
amounts are interest free, unsecured and repayable on
demand.
|
|
14.
|
Intangible
assets
|
|
As of December 31,
|
||||||||
|
2009
|
2008
|
|||||||
|
Pharmaceutical
licenses and patents
|
||||||||
|
Costs
|
$ | 124,738 | $ | 124,738 | ||||
|
Accumulated
amortization
|
(38,187 | ) | (13,734 | ) | ||||
|
Net
|
$ | 86,551 | $ | 111,004 | ||||
During
the two years ended December 31, 2009 and 2008 amortization charge was $24,435
and $4,695 respectively.
- 22
-
|
14.
|
Intangible
assets (Cont’d)
|
The
estimated aggregate amortization expenses for patents for the five succeeding
years is as follows :-
|
Year
|
||||
|
2010
|
$ | 22,370 | ||
|
2011
|
22,005 | |||
|
2012
|
22,005 | |||
|
2013
|
20,171 | |||
|
2014
|
- | |||
| $ | 86,551 | |||
|
15.
|
Property,
plant and equipment, net
|
|
As of December 31,
|
||||||||
|
2009
|
2008
|
|||||||
|
Costs
:-
|
||||||||
|
Buildings
|
$ | 6,825,521 | $ | 5,892,393 | ||||
|
Plant
and machinery
|
5,439,929 | 4,233,317 | ||||||
|
Furniture,
fixtures and equipment
|
290,140 | 273,587 | ||||||
|
Leasehold
improvements
|
118,645 | 118,645 | ||||||
|
Motor
vehicles
|
714,281 | 714,281 | ||||||
| 13,388,516 | 11,232,223 | |||||||
|
Accumulated
depreciation
|
(3,675,288 | ) | (2,882,383 | ) | ||||
|
Construction
in progress - Note 15(c)
|
385,301 | 1,335,534 | ||||||
|
Net
|
$ | 10,098,529 | $ | 9,685,374 | ||||
|
|
(a)
|
An
analysis of buildings pledged to banks for bank and other loans (Note
19(b)(i)) is as follows :-
|
|
Year ended December 31,
|
||||||||
|
2009
|
2008
|
|||||||
|
Costs
|
$ | 3,224,955 | $ | 3,224,955 | ||||
|
Accumulated
depreciation
|
(296,654 | ) | (128,793 | ) | ||||
|
Net
|
$ | 2,928,301 | $ | 3,096,162 | ||||
As of
December 31, 2009, buildings with carrying value of $2,928,301 acquired from
Hainan Zhonghe Group on December 31, 2007 have been pledged for bank loan
granted to Zhonghe (Note 19b(i)). Accordingly the legal title of the
buildings cannot be transferred to Zhonghe until the bank loan is fully repaid
in January 2010. Pursuant to the separate trust agreement, both
parties agreed that Hainan Zhonghe Group will continue to hold the legal title
of the abovementioned pledged buildings for Zhonghe until the full settlement of
related bank loans has been made by Zhonghe.
- 23
-
|
15.
|
Property,
plant and equipment, net (Cont’d)
|
|
|
(b)
|
During
the reporting periods, depreciation is included in
:-
|
|
As of December 31,
|
||||||||
|
2009
|
2008
|
|||||||
|
Cost
of sales and overheads of inventories
|
$ | 546,187 | $ | 537,407 | ||||
|
Administrative
expenses
|
249,858 | 124,340 | ||||||
| $ | 796,045 | $ | 661,747 | |||||
During
the years ended December 31, 2009 and 2008, property, plant and equipment with
carrying amounts of $183 and $821,651 were disposed of at considerations of $Nil
and $3,237,761 resulting in loss/(gain) of $183 and $(2,416,110)
respectively.
|
|
(c)
|
Construction
in Progress
|
Construction
in progress mainly comprises capital expenditure for construction of the
Company’s factories.
|
16.
|
Land
use rights
|
|
As of December 31,
|
||||||||
|
2009
|
2008
|
|||||||
|
Land
use rights
|
$ | 3,847,536 | $ | 3,847,536 | ||||
|
Accumulated
amortization
|
(142,876 | ) | (71,996 | ) | ||||
| $ | 3,704,660 | $ | 3,775,540 | |||||
The
carrying amount of land use rights as of December 31, 2009 represents two
separate land use rights acquired from Hainan Zhonghe Group on September 29,
2007 and December 31, 2007 respectively. The land use right with
carrying value of $2,248,339 as of December 31, 2009 was pledged to the bank for
the loans granted to Zhonghe (Note 19b(ii)). The legal title of the
pledged land use right has not been transferred to Zhonghe after the acquisition
as such transfer can only be done after the related bank loans granted to
Zhonghe will be fully settled in 2010. Pursuant to a trust agreement,
both parties agreed that Hainan Zhonghe Group will continue to hold the legal
title of the pledged land for Zhonghe until the full settlement of related bank
loans has been made by Zhonghe.
During
the two years ended December 31, 2009 and 2008, amortization amounted to $70,833
and $68,150 respectively.
- 24
-
|
16.
|
Land
use rights (Cont’d)
|
The
estimated aggregate amortization expenses for land use rights for the five
succeeding years is as follows :-
|
Year
|
||||
|
2010
|
$ | 68,580 | ||
|
2011
|
68,580 | |||
|
2012
|
68,580 | |||
|
2013
|
68,580 | |||
|
2014
|
68,580 | |||
| $ | 342,900 | |||
|
17.
|
Deposit
paid to a related company for acquisition of an intangible
asset
|
On March
23, 2009, Zhonghe entered into an agreement with Zhonghe Peptide to acquire a
technology know-how in relation to the production of a new drug at a total
consideration of RMB60 million. As of September 30, 2009, RMB54 million
(equivalent to $7.92 million) was paid to Zhonghe Peptide as a deposit, which
was settled by offsetting the amount due from Zhonghe Peptide (Note13 (c)) of
$7.59 million and by cash of $0.33 million. The transaction is expected to be
completed in 2010.
|
18.
|
Other
payables and accrued expenses
|
|
As of December 31,
|
||||||||
|
2009
|
2008
|
|||||||
|
Accrued
audit fee
|
$ | 63,081 | $ | 55,746 | ||||
|
Deposits
from sales agents and other deposit
|
2,490,138 | 518,080 | ||||||
|
Other
accrued expenses
|
70,043 | 70,427 | ||||||
|
Other
tax payable
|
463,087 | 780,705 | ||||||
|
Payable
for acquisition of property, plant and equipment
|
412,003 | 128,091 | ||||||
|
Sales
receipt in advance from customers
|
411,396 | 47,955 | ||||||
|
Staff
welfare payable (Note)
|
- | 59,917 | ||||||
|
Salary
payable
|
51,377 | 11,579 | ||||||
| $ | 3,961,125 | $ | 1,672,500 | |||||
Note
:-
Staff
welfare payable represents accrued staff medical, industry injury claims, labor
and unemployment insurances. All of which are third parties insurance
and the insurance premiums are based on certain percentage of
salaries. The obligations of the Company are limited to those
premiums contributed by the Company.
- 25
-
|
19.
|
Secured
borrowings
|
|
As of December 31,
|
||||||||
|
2009
|
2008
|
|||||||
|
Short-term
loan
|
||||||||
|
Bank
loan and other loan
|
||||||||
|
Long-term
loans - current portion
|
$ | 6,051,375 | $ | - | ||||
| 6,051,375 | - | |||||||
|
Long-term
loans
|
||||||||
|
Bank
loan - (Note c)
|
||||||||
|
-
due 2010, interest bearing at 8.28% per annum
|
5,721,300 | 5,868,000 | ||||||
|
Other
loan (Note d)
|
||||||||
|
-
due 2010, interest - free
|
330,075 | 330,075 | ||||||
|
-
due 2011, interest - free
|
330,075 | 330,075 | ||||||
| 6,381,450 | 6,528,150 | |||||||
|
Less:
current maturities
|
(6,051,375 | ) | - | |||||
| 330,075 | 6,528,150 | |||||||
| $ | 6,381,450 | $ | 6,528,150 | |||||
|
|
(a)
|
The
weighted-average interest rate for short-term loans as of December 31,
2009 was 7.71% per annum.
|
|
|
(b)
|
As
of December 31, 2009, the above bank loans were secured by the following
:-
|
|
|
(i)
|
Buildings
with carrying value of $2,928,301 (Note 15(a));
and
|
|
|
(ii)
|
Land
use right with carrying value of $2,248,339 (Note
16).
|
|
|
(c)
|
The
long-term bank loans as of December 31, 2009 and 2008 were at fixed rate
of 8.28% per annum.
|
|
|
(d)
|
The
other loans, which were granted to Zhonghe by the PRC local government
authority, is interest-free and secured by the buildings disposed at
considerations of $442,233 in 2008 to a third party. The other
loans were not discounted to their present values as the effect of
discounting is immaterial. The legal title of the pledged
buildings has not been transferred to the third party as the related other
loans granted to Zhonghe has not been settled until
2011. Pursuant to a trust agreement, both parties agreed that
Zhonghe will continue to hold the legal title of the pledged buildings
until the full settlement of related other loans has been made by
Zhonghe.
|
During
the reporting periods, there was no covenant requirement under the facilities
granted to the Company.
|
20.
|
Commitments
and contingencies
|
|
|
(a)
|
Capital
commitment
|
As of
December 31, 2009, the Company had capital commitments amounting to $1,159,633
in respect of the acquisition of property, plant and equipment and an intangible
asset which were contracted for but not provided in the consolidated financial
statements.
- 26
-
|
20.
|
Commitments
and contingencies (Cont’d)
|
|
|
(b)
|
Operating lease
arrangement
|
As of
December 31, 2009, the Company had no non-cancelable operating
lease.
|
|
(c)
|
Contingencies
|
As of
December 31, 2009, the Company had no contingencies.
|
21.
|
Statutory
and other reserves
|
The
statutory and other reserves comprise of the following :-
|
As of December 31,
|
||||||||
|
2009
|
2008
|
|||||||
|
Statutory
surplus reserve
|
$ | 3,743,028 | $ | 2,820,850 | ||||
In
accordance with the relevant laws and regulations of the PRC, it is required
that not less than 10% of its net income (the percentage is upon approval from
the board of directors’ meeting), after offsetting any prior years’ losses, for
PRC tax reporting purpose to the statutory reserve.
When the
balance of such reserve reaches 50% of the registered capital, any further
appropriation is optional. Upon approval from the board of directors
of the Zhonghe, the statutory reserve can mainly be used to offset accumulated
losses.
|
22.
|
Special
distribution of earning and profits
|
On
December 8, 2009, Zhonghe entered into an agreement with Hainan Zhonghe Group to
acquire a building with carrying amount of $562,461 located in Special Economic
Zone, at a total consideration of $651,254. Special distribution of
earning and profits represents the excess consideration of $88,793 paid for the
acquired building by offsetting the amount due from the Hainan Zhonghe
Group.
|
23.
|
Defined
contribution plan
|
Pursuant
to the relevant PRC regulations, the Company is required to make contributions
at a rate of 29% of the average salaries for the latest fiscal year-end of
Hainan Province Haikou Shi to a defined contribution retirement scheme organized
by a state-sponsored social insurance plan in respect of the retirement benefits
for the Company's employees in the PRC. The only obligation of the
Company with respect to retirement scheme is to make the required contributions
under the plan. No forfeited contribution is available to reduce the
contribution payable in the future years. The defined contribution
plan contributions were charged to the consolidated statements of income and
other comprehensive income. The Company contributed $57,168 and
$55,241 for the year ended December 31, 2009 and 2008
respectively.
- 27
-
|
24.
|
Segment
information
|
The
Company is solely engaged in the manufacturing, marketing, sale and distribution
of drugs. Since the nature of the products, their production
processes, the type of their customers and their distribution methods are
substantially similar, they are considered as a single reportable segment under
ASC 280 (Previously SFAS 131), “Segments Reporting”.
All of
the Company’s long-lived assets and revenues classified based on the customers
are located in the PRC.
|
25.
|
Related
party transactions
|
Apart
from the transactions as disclosed in notes 13, 15, 16, 17 and 22 to the
financial statements, the Company has entered into the following transactions
with related parties:-
|
Year ended December 31,
|
||||||||||
|
Related parties
|
Type of transaction
|
2009
|
2008
|
|||||||
|
Hainan
Heyi
|
Sales
|
$ | 310,191 | $ | 725,856 | |||||
|
Hainan
Zhonghe Group
|
Interest
income
|
$ | 177,527 | $ | 48,604 | |||||
|
26.
|
Subsequent
events
|
The
Company evaluated all events or transactions that occurred after December 31,
2009 and has determined that there is no material recognizable nor subsequent
events or transactions which would require recognition or disclosure in the
financial statements, other than noted herein.
- 28
-
Index to
Exhibits
|
Number
|
Description of Exhibit
|
|
|
3.1
|
Amended
and Restated Articles of Incorporation of the Company incorporated by
reference to exhibit 1 of Form 8-A12G filed on April 13,
2009
|
|
|
3.2
|
Amended
and Restated Bylaws of the Company incorporated by reference to exhibit 2
of Form 8-A12G filed on April 13, 2009.
|
|
|
4.1
(1)
|
Stock
Purchase Agreement by and among Aspen Racing Stables, Inc., Trixy
Sasyniuk-Walt, Kun Run Biotechnology Ltd., Xueyun Cui and Liqiong Yang
dated August 21, 2008
|
|
|
4.2
(1)
|
Make
Good Escrow Agreement by and among Aspen Racing Stables, Inc., Xueyun Cui,
Trixy Sasyniuk-Walt, and Securities Transfer Corporation dated September
16, 2008
|
|
|
10.1
(1)
|
Consulting
Agreement by and between Halter Capital Corporation and Kun Run
Biotechnology Ltd. dated September 16, 2008
|
|
|
10.2
(2)
|
Real
Property Purchase and Sale Agreement between Hainan Zhonghe Pharmaceutical
Co., Ltd. and Hainan Twenty First Media Co., Ltd. dated October 9,
2008
|
|
|
10.3
(2)
|
Supplemental
Contract to exhibit 10.2 dated October 27, 2008
|
|
|
10.4
(2)
|
Real
Property Purchase and Sale Agreement between Hainan Zhonghe Pharmaceutical
Co., Ltd. and Hainan Xike Real Estates Co., Ltd. dated October 6,
2008
|
|
|
10.5
(2)
|
Power
Cable Purchase Agreement between Hainan Zhonghe Pharmaceutical Co. Ltd.
and Hainan Delixi Electric Power Facility Company dated March 27,
2008
|
|
|
10.6
(2)
|
Power
Cable Purchase Agreement between Hainan Delixi Electro-mechanical
Equipment Mfg.Co.Ltd and Hainan Zhonghe Pharmaceutical Co. Ltd. dated
March 27, 2008
|
|
|
10.7
|
General
Agent Agreement between Hainan Zhonghe Pharmaceutical Co. Ltd. and Beijing
Yabaofangda Pharmaceutical Limited dated November 1, 2008 incorporated by
reference to same exhibit number of our annual report on Form 10-K/A filed
on July 30, 2009.
|
|
|
10.8
(2)
|
Employment
Agreement between the Company and Yan Lin dated January 1,
2009
|
|
|
10.9
(2)
|
Employment
Agreement between the Company and Xiaoqun Ye dated January 1,
2007
|
|
|
10.10
|
Assignment
Contract by and between Hainan Zhonghe Peptide Drugs Research &
Development Co., Ltd. and Hainan Zhonghe Pharmaceutical Co., Ltd., dated
as of March 23, 2009 incorporated by reference to exhibit 99.1 of our
current report on Form 8-K/A filed on July 30, 2009.
|
|
|
21.1
|
Subsidiaries
of the Company (incorporated by reference to same exhibit number of our
annual report on Form 10-K/A filed on April 3, 2009)
|
|
|
31.1*
|
Certification
of Chief Executive Officer under Section 302 of the Sarbanes-Oxley Act of
2002
|
|
|
31.2*
|
Certification
of Principal Financial Officer under Section 302 of the Sarbanes-Oxley Act
of 2002
|
|
|
32.1*
|
|
Certifications
Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002, 18 U.S.C.
Section 1350
|
|
(1)
|
Incorporated
by reference to same exhibit number of our current report on Form 8-K
filed on September 22, 2008
|
|
(2)
|
Incorporated
by reference to same exhibit number of our annual report on Form 10-K
filed on March 31, 2009.
|
|
*
|
Filed
herewith.
|
- 29
-
SIGNATURE
Pursuant
to the requirements of the Securities Exchange Act of 1934, the Registrant has
duly caused this Annual Report on Form 10-K to be signed on its
behalf by the undersigned hereunto duly authorized.
|
Kun
Run Biotechnology, Inc.
|
||
|
(Registrant)
|
||
|
Dated:
March 30, 2010
|
||
|
By
/s/ Xiaoqun Ye
|
||
|
Name:
|
Xiaoqun
Ye
|
|
|
Title:
|
Chief
Executive
Officer
|
|
Pursuant
to the requirements of the Securities Exchange Act of 1934, this Annual Report
on Form 10-K has been signed by the following persons in the capacities
indicated as of March 30, 2010.
|
Signature
|
Title
|
|
|
/s/
Xueyun
Cui
|
Director
|
|
|
Xueyun
Cui
|
||
|
/s/Yan Lin
|
Chief
Accounting Officer
|
|
|
Yan
Lin
|
- 30
-
