Attached files
| file | filename |
|---|---|
| EX-31.2 - China Yongxin Pharmaceuticals Inc. | v177274_ex31-2.htm |
| EX-31.1 - China Yongxin Pharmaceuticals Inc. | v177274_ex31-1.htm |
| EX-21.1 - China Yongxin Pharmaceuticals Inc. | v177274_ex21-1.htm |
| EX-32.1 - China Yongxin Pharmaceuticals Inc. | v177274_ex32-1.htm |
| EX-10.17 - China Yongxin Pharmaceuticals Inc. | v177274_ex10-17.htm |
| EX-10.14 - China Yongxin Pharmaceuticals Inc. | v177274_ex10-14.htm |
| EX-10.13 - China Yongxin Pharmaceuticals Inc. | v177274_ex10-13.htm |
| EX-10.15 - China Yongxin Pharmaceuticals Inc. | v177274_ex10-15.htm |
| EX-10.16 - China Yongxin Pharmaceuticals Inc. | v177274_ex10-16.htm |
UNITED
STATES
SECURITIES
AND EXCHANGE COMMISSION
WASHINGTON,
D.C. 20549
FORM
10-K
x
ANNUAL REPORT PURSUANT
TO SECTION 13 OR 15(D) OF
THE
SECURITIES EXCHANGE ACT OF 1934
FOR
THE FISCAL YEAR ENDED DECEMBER 31, 2009
OR
¨ TRANSITION REPORT PURSUANT TO SECTION
13 OR 15(D) OF
THE
SECURITIES EXCHANGE ACT OF 1934
FOR
THE TRANSITION PERIOD FROM _______ TO ___________
COMMISSION
FILE NO. 000-26293
CHINA
YONGXIN PHARMACEUTICALS INC.
(EXACT
NAME OF REGISTRANT AS SPECIFIED IN ITS CHARTER)
|
Delaware
|
20-1661391
|
|
|
(State
or other jurisdiction of incorporation or
organization)
|
(I.R.S.
Employer Identification No.)
|
|
|
927
Canada Court
City
of Industry, California
|
91748
|
|
|
(Address
of principal executive offices)
|
(Zip
Code)
|
REGISTRANT'S
TELEPHONE NUMBER, INCLUDING AREA CODE: (626) 581-9098
SECURITIES
REGISTERED PURSUANT TO SECTION 12(b) OF THE ACT:
None.
SECURITIES
REGISTERED PURSUANT TO SECTION 12(g) OF THE ACT:
Common
Stock, $.001 par value
Indicate by check mark if the
registrant is a well-known seasoned issuer, as defined in Rule 405 of the
Securities Act. Yes
¨ No x
Indicate
by check mark if the registrant is not required to file reports pursuant to
Section 13 or Section 15(d) of the Act. ¨
Indicate
by check mark whether the registrant (1) has filed all reports required to be
filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the
preceding 12 months (or for such shorter period that the registrant was required
to file such reports), and (2) has been subject to such filing requirements for
the past 90 days.
Yes x No ¨
Indicate
by check mark if disclosure of delinquent filers pursuant to Item 405 of
Regulation S-K (ss. 229.405 of this chapter) is not contained herein, and will
not be contained, to the best of registrant's knowledge, in definitive proxy or
information statements incorporated by reference in Part III of this Form 10-K
or any amendment to this Form 10-K. ¨
Indicate
by check mark whether the registrant is a large accelerated filer, an
accelerated filer, a non-accelerated filer or a smaller reporting company. See
the definitions of "large accelerated filer," "accelerated filer" and "smaller
reporting company" in Rule 12b-2 of the Exchange Act:
Large
accelerated filer ¨
Accelerated filer ¨
Non-accelerated filer ¨ Smaller reporting
company x
Indicate
by check mark whether the registrant is a shell company (as defined in Rule
12b-2 of the Act).
Yes ¨ No x
Indicate
by check mark whether the registrant has submitted electronically and posted on
its corporate Web site, if any, every Interactive Data File required to be
submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this
chapter) during the preceding 12 months (or for such shorter period that the
registrant was required to submit and post such files). Yes o No o
The
aggregate market value of the voting and non-voting common equity held by
non-affiliates computed by reference to the price at which the common equity was
sold, or the average bid and asked prices of such common equity, as of the last
business day of the registrant's most recently completed second fiscal quarter:
$1.7 million as of June 30, 2009.
There
were 57,348,923 shares outstanding of registrant's common stock, par value
$0.001 per share, as of March 10, 2010. The shares of the registrant's common
stock are currently quoted on the Over-the-Counter Bulletin Board, or
OTCBB.
Documents
Incorporated by Reference: None.
TABLE
OF CONTENTS
TO
ANNUAL REPORT ON FORM 10-K
FOR
THE FISCAL YEAR ENDED DECEMBER 31, 2009
|
Page
|
||
|
PART
I
|
||
|
Item
1.
|
Business.
|
4
|
|
Item
1A.
|
Risk
Factors.
|
13
|
|
Item
1B.
|
Unresolved
Staff Comments.
|
28
|
|
Item
2.
|
Properties.
|
28 |
|
Item
3.
|
Legal
Proceedings.
|
29 |
|
PART
II
|
||
|
Item
5.
|
Market
For Common Stock and Related Stockholder Matters
|
29 |
|
Item
6.
|
Selected
Financial Data
|
31 |
|
Item
7.
|
Management’s
Discussion and Analysis of Financial Condition and Results of
Operations
|
31 |
|
Item
7A.
|
Quantitative
and Qualitative Disclosures about Market Risk.
|
40 |
|
Item
8.
|
Financial
Statements and Supplementary Data.
|
40 |
|
Item
9.
|
Changes
in and Disagreements With Accountants on Accounting and
Financial Disclosure
|
40 |
|
Item
9A.
|
Controls
and Procedures
|
40 |
|
Item
9B.
|
Other
Information
|
41 |
|
PART
III
|
||
|
Item
10.
|
Directors,
Executive Officers and Corporate Governance
|
42 |
|
Item
11.
|
Executive
Compensation
|
45 |
|
Item
12.
|
Security
Ownership of Certain Beneficial Owners and
Management
|
47 |
|
Item
13.
|
Certain
Relationships and Related Transactions, and Director
Independence
|
49 |
|
Item
14
|
Principal
Accountant Fees and Services
|
49 |
|
PART
IV
|
||
|
Item
15.
|
Exhibits,
Financial Statement Schedules.
|
50 |
|
Signatures
|
53 |
2
CAUTIONARY
STATEMENT REGARDING FORWARD-LOOKING STATEMENTS
The
information contained in this annual report on Form 10-K (“Form 10-K”), includes
some statements that are not purely historical and that are "forward-looking
statements." Such forward-looking statements include, but are not limited to,
statements regarding our company's and our management's expectations, hopes,
beliefs, intentions or strategies regarding the future, including our financial
condition, results of operations, and the expected impact of the share exchange.
In addition, any statements that refer to projections, forecasts or other
characterizations of future events or circumstances, including any underlying
assumptions, are forward-looking statements. The words "anticipates,"
"believes," "continue," "could," "estimates," "expects," "intends," "may,"
"might," "plans," "possible," "potential," "predicts," "projects," "seeks,"
"should," "will," "would" and similar expressions, or the negatives of such
terms, may identify forward-looking statements, but the absence of these words
does not mean that a statement is not forward-looking.
The forward-looking statements contained in this Form 10-K are based on current
expectations and beliefs concerning future developments and the potential
effects on the parties and the transaction. There can be no assurance that
future developments actually affecting us will be those anticipated. These
forward-looking statements involve a number of risks, uncertainties (some of
which are beyond the parties' control) or other assumptions that may cause
actual results or performance to be materially different from those expressed or
implied by these forward-looking statements, including the
following:
|
Ÿ
|
The
market acceptance of the products we
sell;
|
|
|
Ÿ
|
Problems
that we may face in marketing and distributing the products we
sell;
|
|
|
Ÿ
|
Errors
in business planning attributable to insufficient market size or
segmentation data;
|
|
|
Ÿ
|
Exposure
to product liability and defect
claims;
|
|
|
Ÿ
|
Changes
in the laws of the People's Republic of China that affect our
operations;
|
|
|
Ÿ
|
Any
recurrence of health epidemics and other
outbreaks;
|
|
|
Ÿ
|
Our
ability to obtain and maintain all necessary government certifications
and/or licenses to conduct our
business;
|
|
|
Ÿ
|
Development
of a public trading market for our
securities;
|
|
|
Ÿ
|
Our
inability to raise additional capital when
needed;
|
|
|
Ÿ
|
Problems
with important suppliers and strategic business
partners;
|
|
|
Ÿ
|
The
cost of complying with current and future governmental regulations and the
impact of any changes in the regulations on our operations;
and
|
|
|
Ÿ
|
The
other factors referenced in this Prospectus, including, without
limitation, under the sections entitled "Risk Factors," "Financial
Information," "Management's Discussion and Analysis of Financial Condition
and Results of Operations," and
"Business."
|
These
risks and uncertainties, along with others, are also described above under the
heading "Risk Factors." Should one or more of these risks or uncertainties
materialize, or should any of the parties' assumptions prove incorrect, actual
results may vary in material respects from those projected in these
forward-looking statements. Moreover, we operate in a very competitive and
rapidly changing environment. New risk factors emerge from time to time and we
cannot predict all such risk factors, nor can we assess the impact of all such
risk factors on our business or the extent to which any factor, or combination
of factors, may cause actual results to differ materially from those contained
in any forward looking statements. We undertake no obligation to update or
revise any forward-looking statements, whether as a result of new information,
future events or otherwise, except as may be required under applicable
securities laws.
3
PART
I
Item
1. BUSINESS
With
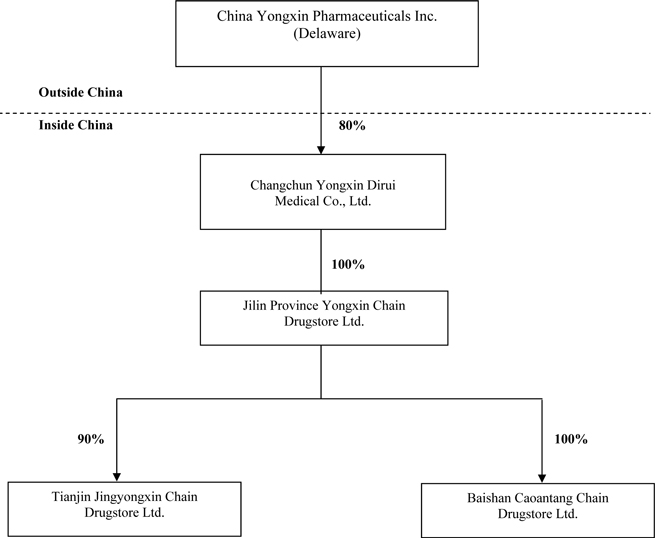
respect to this discussion, the terms, "we," "us," "our," and "Company" refer to
China Yongxin Pharmaceuticals Inc. (formerly Digital Learning Management
Corporation) (the “Company"), and its 80%-owned subsidiary, Changchun Yongxin
Dirui Medical Co., Ltd., a company organized under the laws of the PRC
("Yongxin"), and (i) Yongxin's wholly-owned subsidiary Jilin Province Yongxin
Chain Drugstore Ltd., a company organized under the laws of the PRC (“Yongxin
Drugstore”); (ii) Yongxin Drugstore's 90%-owned subsidiary, Tianjin Jingyongxin
Chain Drugstore Ltd., a company organized under the laws of the PRC
(“Jingyongxin Drugstore”); and (iii) Yongxin Drugstore's wholly-owned
subsidiary, Baishan Caoantang Chain Drugstore Ltd., a company organized under
the laws of the PRC (“Caoantang Drugstore”).
Through our Chinese subsidiaries, we are engaged in the wholesale
distribution of pharmaceuticals and medical-related products, and the sale and
distribution of pharmaceuticals, health and beauty products, ginseng and herbal
supplements, and other healthcare products through retail operations in the PRC.
Our corporate headquarters are located in City of Industry, California, but the
Company’s distribution operations are based in Changchun City, Jilin Province,
PRC. Substantially all of our employees are located in
China. At December 31, 2009, we had approximately 673 full time
employees, with 90 employees holding pharmaceutical licenses, and 87 of such
licensed pharmacists working at our retail drugstores. Our business
mainly operates in two segments: (i) the wholesale of pharmaceuticals and other
medical-related products and (ii) the operation of retail
drugstores.
HISTORY
AND CORPORATE STRUCTURE
The Company was originally incorporated
in Delaware on February 18, 1999 under the name of FreePCSQuote. On
December 21, 2006, Changchun Yongxin Dirui Medical Co., Ltd, a Chinese
corporation ("Yongxin") and all of the stockholders of Yongxin entered into a
share exchange transaction with the Company. On April 12, 2008, we
entered into a second amended Share Exchange Agreement with Yongxin, effective
November 16, 2007, in which the Company acquired from the original Yongxin
stockholders, and Yongxin stockholders transferred to the Company, 80% of the
equity interest of Yongxin in exchange for the issuance by the Company of an
aggregate of 21,000,000 shares of newly issued common stock and 5,000,000 shares
of Series A Convertible Preferred Stock to the original Yongxin stockholders
and/or their designees (the “Share Exchange Transaction”). The Series
A Convertible Preferred Stock is convertible over a 3 year period, into up to 30
million shares of common stock. For accounting purposes, this Share
Exchange Transaction was accounted for as a reverse merger, since the original
stockholders of Yongxin became the owners of a majority of the issued and
outstanding shares of common stock of the Company, and the directors and
executive officers of Yongxin became the directors and executive officers of the
Company. In connection with the Share Exchange Transaction, we
changed our name to “Nutradyne Group Inc.”
Yongxin was originally established in
1993. Yongxin’s business operations consist of wholesale and retail
sales of pharmaceuticals, medical equipment and other medical-related
products. Yongxin’s operations are based in Changchun City, Jilin
Province, China. In 2004,
Yongxin established Jilin Province Yongxin Chain Drugstore Ltd. (“Yongxin
Drugstore”) with an investment of RMB 2,500,000 (equivalent to $303,000) to
develop a customer-terminal network market. In July 2005, the Company
obtained the franchise rights in Jilin Province from American Medicine Shoppe
(Meixin International Medical Chains) and by then had developed 4 chains of
“Meixin Yongxin.” As of March 3, 2010, Yongxin Drugstore has
developed 21 retail chains drug stores in the name of Yongxin Drugstore which
collectively cover 3,373 M2 of retail space throughout Changchun
city in China. These drugstores sell over-the-counter western and
traditional Chinese medicines and other medical-related
products.
On March 16, 2007, Yongxin Drugstore
entered into various agreements with retail drug stores in Tianjin and
established Tianjin Jingyongxin Chain Drugstore Ltd. (“Jinyongxin Drugstore”)
with an investment of $116,868, in which the Company has 90% ownership of
Jinyongxin Drugstore. Jinyongxin Drugstore is located in Tianjin
City, China. As of March 3, 2010, Jinyongxin Drugstore has developed 26
retail chain drug stores with total retail space of 3,657 M2
throughout Tianjin City in China.
4
On May
15, 2007, Yongxin established Jilin Dingjian Natural Health Products Co., Ltd.
(“Dingjian”) with an investment of $116,868 whereby Yongxin acquired a 90%
ownership interest in Dingjian. The other 10% of Dingjian was held by
an individual named Jianwei Chen. Dingjian was formed under laws of
the People’s Republic of China and is located in Changchun City, Jilin Province.
Dingjian’s products include ginseng products, flower-flavored tea, rare raw
medicine materials and local specialty products. On November 21, 2009, Yongxin
disposed of its entire ownership interest in Dingjian pursuant to an Equity
Transfer Agreement (the “Agreement”) with Sun Shi Wei, an
individual. Pursuant to the Agreement, Yongxin transferred its 90%
ownership interest in Dingjian to Sun Shi Wei. No other consideration
was exchanged. As of the date of this Form 10-K, Yongxin holds no
ownership interest in Dingjian, and is not subject to any of its
liabilities.
On June 15, 2007, Yongxin Drugstore
established Baishan Caoantang Chain Drugstore Ltd. (“Caoantang Drugstore”) with
a total investment of $408,430. Caoantang Drugstore is a wholly-owned
subsidiary of Yongxin Drugstore. As of March 3, 2010, Caoantang
Drugstore operates a chain of 32 retail drugstores that collectively cover 2,804
M2of
retail space and sells over-the-counter western and traditional Chinese
medicines and other medical-related products.
On May 5, 2008, the Company changed its
name from Nutradyne Group, Inc to China Yongxin Pharmaceuticals
Inc.
On March 9, 2009, the Company formally
launched its Electronic Diagnosis System (the “System”), which enables its
customers to remotely receive a medical diagnosis and conveniently purchase
prescription drugs at the store. To date, the Company has installed
20 Systems in its Yongxin chain drugstores, all located in Changchun, Jilin
Province, China.
Since the beginning of 2009, the
Company has also signed 12 exclusive distribution agreements within the Jilin
province with several well known pharmaceutical manufacturers including Tianjin
Smith Kline & French Laboratones Ltd. As of March 3, 2010,
Yongxin has exclusive distribution rights for approximately 96 drugs in Jilin
province. This portfolio is a key component of its long term growth
strategy to leverage the large distribution center and channels established to
drive incremental future revenue growth. These agreements are typically
one year in duration and renewable.
On March 1, 2010, the Company sold its
digital e-learning business including its wholly-owned subsidiary, Digital
Learning Institute Inc., a Delaware corporation (“Digital Learning”); and (i)
Digital Learning’s wholly-owned subsidiary Software Education of America, Inc.,
a California corporation; (ii) Digital Learning’s wholly-owned subsidiary
McKinley Educational Services, Inc., a California corporation; (iii) Digital
Learning’s wholly-owned subsidiary Digital Knowledge Works, Inc., a Delaware
corporation; and (iv) Digital Learning’s wholly-owned subsidiary Coursemate,
Inc., a California corporation (referred to collectively herein as the "Digital
E-learning Business"). The Digital E-learning Business constituted a minimal
portion of our overall business and it has been our intention to divest
ourselves of the Digital E-learning Business to focus on the pharmaceutical
segment of our business.
The
following diagram illustrates our corporate structure as of the date of this
Form 10-K:
5
IMPORTANT
DISCLOSURES REGARDING OUR CORPORATE STRUCTURE
The
Company owns not all, but eighty percent (80%) of the outstanding equity
interest of Yongxin, a corporate entity organized and existing under the laws of
the PRC, which is a holding company for all other subsidiaries of the
Company. Yongxin Liu and Yongkui Liu individually own 11% and 9% of
Yongxin, respectively. On May 13, 2007, Yongxin Liu, Yongkui Liu and
the Company entered into an Equity Cooperative Joint Venture Contract dated May
13, 2007, pursuant to which Yongxin became an 80% subsidiary and a “joint
venture” under PRC law. At the time of formation, the parties sought
to form a joint venture as opposed to a wholly foreign owned entity (or WFOE),
due to certain PRC laws which made it advantageous for the Company, a
pharmaceutical business, to be constituted and classified as a joint
venture. Yongxin was granted a business license by the Administration
for Industry and Commerce of Changchun City on September 14, 2007, which
recognized and deemed it to be an equity joint venture, with 80% foreign
ownership and 20% domestic (PRC) ownership. Yongxin also
received recognition from, and was registered as an equity joint venture, by the
Jilin Branch of the State Administration of Foreign Exchange (SAFE) on September
20, 2007. Yongxin received MOFCOM certification pursuant to the
PRC’s Regulation for Mergers
with and Acquisitions of Domestic Enterprises by Foreign Investors and
other relevant laws on September 8, 2006.
As a
result of the corporate structure described above, we note several important
implications:
|
|
·
|
The
Company may not be able to exercise absolute control over Yongxin, because
it is not a 100% equityholder of Yongxin. The minority
shareholders, Yongxin Liu and Yonkui Liu, may hold certain minority rights
under PRC corporate law and pursuant to the equity joint venture
agreement, with respect to the control and governance of
Yongxin. Specifically,
Yongxin Liu and Yongkui Liu have the right to jointly appoint a director
and the Company has the right to appoint two
directors. Also,
under the equity joint venture agreement, Mr. Yongxin Liu has a
contractual right to appoint the General Manager of
Yongxin.
|
|
|
·
|
Since
Yongxin Liu is also the Chief Executive Officer and Chairman of the
Company and Yongkui Liu is also the Vice President and former Chief
Financial Officer and director of the Company, this may give rise to a
conflict of interest with the Company and the Company’s
shareholders. While the Company is not aware of any present
situation which involves a conflict of interest, in the future, a conflict
of interest may arise from the fact that Yongxin Liu and Yongkui Liu are
executive officers of the Company, and are also minority equityholders of
Yongxin. If there should ever be a divergence of interest of
the minority equityholders on the one hand, and the Company on the other
hand, Mr. Yongxin Liu and Yongkui Liu would have incentives to act to
protect their minority interests in Yongxin vis-à-vis the majority
controlling interest of the
Company.
|
|
|
·
|
Although
the Company consolidates the financial results of Yongxin and its
subsidiaries for financial reporting purposes, the Company will not
receive 100% of the economic benefit of the income and assets of Yongxin,
and in most cases the Company will receive only 80% of such economic
benefit. For example, if Yongxin were to distribute accumulated
earnings or assets, 20% of such distributed assets would be paid to the
minority equity holders, and 80% would be paid to the
Company.
|
|
|
·
|
For
financial reporting purposes, the Company also accounts for the 20%
interest as a non-controlling interest, which has the effect of lowering
reported earnings per share (as compared to a scenario in which the
Company owns 100% of Yongxin).
|
Please
also review our risk factors for a discussion of these and other factors for
your consideration.
INDUSTRY
China
represents one of the world's largest pharmaceutical markets. With its
population of over one billion people and a rapidly growing economy, China
presents significant potential for the pharmaceutical and retail drugstore
industry. The rise in disposable income of many Chinese residents has resulted
in greater demand and affordability of prescription and over-the-counter
medicines and other personal care products. The increasing population of people
over 65 in China has resulted in a stronger demand for medicines and other
healthcare-related products, because this demographic spends more money on these
products than younger people, on average. As living standards across China
improve and the Chinese population continues to age, we expect the demand for
healthcare-related products to continue to rise. The PRC government
has also recently attempted to regulate the pharmaceutical industry by enacting
a series of regulatory measures that are expected to favor non-hospital
drugstores more than hospital pharmacies.
In China,
consumers can purchase pharmaceutical and other related products at either
hospital pharmacies or non-hospital drugstores, including independent drugstores
and drugstore chains. Because hospital patients usually purchase
prescription medicines at hospital pharmacies, sales by hospital pharmacies have
traditionally accounted for a larger percentage of retail sales of prescription
medicines than non-hospital drugstores. However, most Chinese people
choose to purchase over-the-counter ("OTC"), non-prescription medicines from
non-hospital, retail pharmacies. Retail pharmacies in China include pharmacy
chains, individual stores, retail stores with OTC counters and other retailers
and supermarkets with OTC counters.
6
The
Chinese government owns and operates most of the hospitals throughout
China. The hospitals purchase their supplies of healthcare-related
products from wholesalers and distributors. A hospital's decision to purchase
individual products is typically based on a number of factors, and is sometimes
affected in part by corrupt practices, such as illegal
kickbacks. Recently, the PRC government has stepped-up its efforts to
combat corrupt practices, such as amending its criminal code in 2006 to increase
the penalties for corrupt business practices. We expect the increased
enforcement of such anti-corruption practices will create growth opportunities
for the Company as we can compete for business from hospitals on fair and equal
terms with other suppliers. The PRC Ministry of Health proposed regulations in
March 2007 to require hospitals to permit prescriptions to be filled at
non-hospital pharmacies, which we also expect will increase sales of
prescription medications at our retail drugstores.
Additionally,
reimbursement is available for sales of certain medicines by authorized
pharmacies to participants in the PRC national medical insurance
program. The provincial and municipal authorities responsible for the
administration of the social medical insurance funds to cover such
reimbursements have gradually increased funding in recent years. We
expect the funding to increase significantly in the future, which should help
boost our sales of products eligible for such reimbursements.
RECENT
DEVELOPMENTS
The
current economic conditions may affect our operations since many of our products
are discretionary and we depend to a significant extent upon a number of factors
relating to discretionary consumer spending in China. During economic downturns,
consumers tend to spend less on many of our products, including cosmetics,
organic products and health and nutritional
supplements.
Previously,
management believed that the government would pass certain favorable medical
policies (“National Medical Policy”) in the second half of 2009 to extend
medical insurance coverage to people who live in the rural areas or countryside
of China, which covers approximately 40% of the Chinese population. Management
believes the passage of the National Medical Policy would be highly beneficial
to our sales and operations. However, the National Medical Policy has
not been passed as of the date of the filing of this Form 10-K, and it is
unclear whether this policy will be passed in the near future, if at
all. Due to the uncertainty of the direction of the National Medical
Policy, the Company decided to make certain changes to the operation of its
business in the second half of 2009, including shifting its focus from the
wholesale sector to the retail sector of its business. The
Company will readjust its operations and sales strategy accordingly once the
status of the National Medical Policy becomes clear.
Since
last year, the Company has added products with higher profit margins to our
operations to increase our gross profit, including cosmetics and certain health
and nutritional products such as vitamins and
supplements. Management believes that the addition of such products
has increased our overall gross profit in 2009 and will increase our gross
profit margin for the next few years.
MARKET
FOCUS
Our business operates in two
segments: the wholesale distribution of pharmaceuticals and other
pharmacy-related products and the operation of retail drugstores. Prior to
November 2009, we operated another business known as Dingjian which cultivated
and processed ginseng. However, we transferred our ownership with all of its
assets and liabilities to an individual pursuant to an Equity Transfer Agreement
dated November 21, 2009. The following table reflects the revenue
contribution percentage from each of our business operations for the years ended
December 31, 2009, 2008 and 2007, respectively:
|
For the Years Ended
|
||||||||||||
|
December 31, 2009
|
December 31, 2008
|
December 31, 2007
|
||||||||||
|
Wholesale
Operations
|
70.08
|
%
|
81.6
|
%
|
84
|
%
|
||||||
|
Retail
Drugstore Operations
|
29.2
|
%
|
18.4
|
%
|
16
|
%
|
||||||
|
Ginseng
Processing and Manufacturing Operations
|
N/A
|
*
|
*
|
|||||||||
|
Total
Revenues
|
100
|
%
|
100
|
%
|
100
|
%
|
||||||
* less
than one percent.
Prior
to November 2009, we operated and owned 90% of another business known as
Dingjian which cultivated and processed ginseng. On November 21, 2009, Yongxin
transferred its 90% ownership interest in Dingjian to an individual pursuant to
an Equity Transfer Agreement. As of the date of this Form 10-K, we
hold no ownership interest in Dingjian.
7
WHOLESALE
OPERATIONS
Through
Yongxin, we engage in the wholesale distribution of pharmaceutical products,
medical products and equipment, herbal and nutritional supplements and cosmetics
to over 3,500 customers, which include hospitals, large clinics and retail
pharmacies. Yongxin has received the State Food and Drug
Administration approval and Good Supply Practices, or GSP,
certification.
We
opened our Logistics Center in January 2005 in Jilin Province which contains a
storage area of over 43,000 square meters, where we have the ability to store
our pharmaceutical inventory at various temperatures. The Logistics
Center is able to process over 30,000 orders per day from our customers and our
retail outlets.
Our
wholesale business has relationships with over 760 pharmaceutical
and pharmacy product manufacturers throughout
China. Typically, we enter into master agreements with our suppliers
at the beginning of each year, which provide the general terms, prices and
conditions for transactions for the supplier's products over the
year. We then enter into separate purchase agreements each time we
actually purchase products from a supplier. When we purchase the products from
our suppliers, we take title to the items and book them as
inventory. We then distribute the products to our wholesale
customers.
RETAIL
OPERATIONS
We
provide our retail drugstore customers with convenient and professional pharmacy
services. Each of our stores is staffed with a licensed pharmacist
and a staff trained to provide pharmacy services to our customers. Additionally,
we sell an assortment of other merchandise in our stores, including traditional
Chinese medicines, health and natural products, skin care products and
cosmetics. Our stores typically carry over 8,000 different types of
products. We frequently review and update the selection of products available in
our stores in response to changing consumer preferences.
In July
2005, Yongxin signed an agreement with American Medicine Shoppe International
("AMS") to become AMS' exclusive agent for AMS in the Jilin Province and to
develop a drug store franchise system under license from AMS within the Jilin
Province. We operate 4 drugstores pursuant to our relationship with
AMS under the "Meixin Yongxin" name.
Sales
made to retail customers are made by cash or debit or credit cards, or by
medical insurance cards under the PRC national medical insurance
program. We obtain reimbursement from the relevant government social
security bureaus, for sales made to eligible participants in the PRC national
medical insurance program on a monthly basis. As of March 1, 2010, 29
of our drugstores were designated stores under the PRC national medical
insurance program.
We
currently procure the merchandise for our stores from over 1,500 suppliers,
including both manufacturers and wholesalers. For the years ended
December 31, 2009, 2008 and 2007, our largest five suppliers accounted for
6.91%, 8.5% and 8.2% of our total purchases, respectively. We believe
that the products we carry in our stores are readily available from multiple
sources and do not anticipate any difficulties in continuing to procure such
products.
MARKETING
AND SALES
We have a
staff of approximately 150 employees dedicated to marketing and sales who design
our advertising campaigns and regional promotional activities. We
generate business by marketing directly to hospitals, retail drugstores and
medical clinics in China. Additionally, we advertise our business and
products to consumers through marketing activities and print advertisements in
newspapers to promote our brand and the other products available for sale in our
stores. In 2009 and 2008, we spent approximately $132,264 and
$26,124, respectively, on advertising.
8
RESEARCH
AND DEVELOPMENT
The
only significant research and development we conducted was in the cultivation
and processing of ginseng under Dingjian. The Company has not
conducted any significant research and development since we disposed of our
ownership interest in Dingjian in November 2009.
QUALITY
CONTROL
We
have stringent quality control systems that are implemented by more than 195
company-trained staff members, which represents approximately a third of our
total employees, to ensure quality control over our products, services and
production processes. The quality of the products we offer is of utmost
importance to our Company. Each of
our suppliers also has its own quality control program pursuant to the rules and
regulations under the Good Manufacture Practice (“G.M.P”) mandated by the World
Health Organization.
We
conduct random quality control testing of the products procured from our
suppliers in our wholesale and retail operations. We replace suppliers that do
not pass our quality inspections. Additionally, we monitor the
services provided in our drugstores by sending inspectors to observe the quality
of services provided by our drugstore pharmacists and staff.
Our
quality control procedures at our facilities are designed to maintain quality
standards and execute the following functions at our facilities:
|
|
·
|
setting
internal controls and regulations for
products;
|
|
|
|
|
|
·
|
implementing
sampling systems and sample files;
|
|
|
|
|
|
·
|
maintaining
quality of equipment and
instruments;
|
|
|
|
|
|
·
|
auditing
production records to ensure delivery of quality
products;
|
|
|
|
|
|
·
|
evaluating
the quality of products; and
|
|
|
|
|
|
·
|
articulating
the responsibilities of the quality control
staff.
|
COMPETITION
The
pharmaceutical distribution and retail drugstore industries in China are
fragmented and intensely competitive. While our primary competition
currently comes from other retail drugstore chains and drugstores, we face
increasing competition from discount and convenience stores and supermarkets for
our non-pharmaceutical products and services. We compete for
customers based on store location, selection of products and our brand
name.
Many of
our competitors are more established than we are, and have significantly greater
financial, technical, marketing and other resources than we
do. Additionally, many of them have greater name recognition and
larger customer bases than we do. These competitors may be able to
respond more quickly to changing consumer preferences and new business
opportunities than we do. Moreover, competition is expected to
increase due to the expected consolidation of the drugstore industry and new
store openings by our competitors. Our major competitors include:
China Nepstar Chain Drugstore Ltd., Shenzhen Accord Pharmacy Co., Ltd., Shenzhen
Associate Pharmacy Co., Ltd., Guangzhou Pharmaceutical Company, Jianmin Chain
Drugstore, Guangzhou Caizhilin Chain Drugstore, Liaoning Chengda Co., Ltd.,
Hangzhou Wulin Drugstore Co., Ltd. and Ningbo Siming Dayaofang Co.,
Ltd.
MAJOR
CUSTOMERS
Our major
customers include government owned and operated hospitals, large clinics and
other retailers. No single customer accounted for 10% or more of the Company’s
net sales during the years ended December 31, 2009, 2008 and
2007. Our top five customers accounted for approximately 14.7%, 15%
and 13.9% of our net sales generated for the years ended December 31, 2009, 2008
and 2007, respectively. None of our directors, their associates or
any significant stockholder of the Company has any interest in any of our five
largest customers.
9
INTELLECTUAL
PROPERTY
Prior
to November 2009, we had four registered trademarks in China, which included
Longlife, Zinuo, Yongxintang, Gaoliyuan , under our subsidiary
Dingjian. However, since the discontinuation of Dingjian business
operation in November 2009, we no longer own any intellectual
property.
GOVERNMENT
REGULATIONS
As
a business operating in the PRC, we are subject to various regulations and
permit systems promulgated by the PRC government. These regulations
cover many of our products, including herbal products, over-the-counter
medicines and prescription medications.
Pharmaceutical
Product Distribution
We
sell and distribute pharmaceuticals, health and beauty products, dietary and
herbal supplements, and other healthcare products. Pharmaceuticals,
which have certain identified medical functions and are designed to treat a
specific illnesses or symptoms, can be available by prescription only or
over-the-counter and require the approval of China's State Food and Drug
Administration ("SFDA") before they can be sold. The herbal products
we sell, also known as dietary supplements or nutritional supplements, are
basically prophylactic or preventive in nature and are available
over-the-counter, and, in China, only require approval of the local government
for their production.
We
are subject to the Drug Administration Law of China, which governs the
licensing, manufacturing, marketing and distribution of pharmaceutical products
in China and sets penalties for violations of the law. Distributors of
pharmaceutical products are required to obtain permits from the appropriate
provincial or county level SFDA where the pharmaceutical distribution enterprise
is located. The granting of such permits is subject to an inspection
of a distributor's facilities, warehouses, hygienic environment, quality control
systems, personnel and equipment. Such permits have five year terms
and distributors must apply for renewal no later than six months prior to the
expiration date of the permit. We have a wholesale pharmaceutical
distribution permit which expires in March 2015. Each of our retail
locations also has a pharmaceutical distribution permit, which expire on various
dates. We do not have a permit to manufacture pharmaceutical
products.
Additionally, under the
Supervision and Administration Rules on Pharmaceutical Product Distribution
disseminated by the SFDA on January 31, 2007, and effective May 1, 2007, a
pharmaceutical product distributor is accountable for its procurement and sales
activities and is liable for the actions of its employees or agents in
connection with their conduct of distribution on behalf of the
distributor. Retail distributors may not sell prescription
pharmaceutical products, or Tier A over-the-counter pharmaceutical products,
listed in the national or provincial medical insurance catalogues without a
certified in-store pharmacist being present.
Foreign
Ownership of Wholesale or Retail Pharmaceutical Businesses in China
Under
current PRC law on foreign investment, foreign companies are allowed to
establish or invest in wholly-owned foreign enterprises or joint ventures that
engage in wholesale or retail sales of pharmaceuticals in
China. These regulations limit the number and size of retail pharmacy
outlets that a foreign investor may establish. If a foreign investor owns more
than 30 outlets that sell a variety of branded pharmaceutical products sourced
from different suppliers, the foreign investor's ownership interests in the
outlets are limited to 49.0%.
Nutritional
Supplements and Other Food Products
Distributors
of nutritional supplements and other food products must obtain a food hygiene
certificate from the appropriate provincial or local health regulatory
authorities pursuant to the PRC Food Hygiene Law and Rules on Food Hygiene
Certification. In order to obtain a certificate, a distributor's
facilities, warehouses, hygienic environment, quality control systems, personnel
and equipment are subject to inspection. Food hygiene certificates
are valid for four years, and must be renewed within six months prior to their
expiration.
10
The
Chinese Food Sanitation Law promulgates food sanitation standards. In
the PRC, only products manufactured at Government Good Manufacturing Practice
("GMP") certified facilities are available for sale in China. The
China Food and Drug Administration conducts the GMP inspections.
Good
Supply Practice Standards
We
are required to operate in accordance with Good Supply Practice (the "GSP")
standards that regulate wholesale and retail pharmaceutical product
distributors. The GSP standards ensure the quality of distribution of
pharmaceutical products in China. Pursuant to applicable GSP
standards, we must implement strict controls on the distribution of our
pharmaceutical products, including those concerning staff qualifications,
distribution premises, warehouses, inspection equipment and facilities,
management and quality control. Additionally, we are subject to
inspections organized by the local drug regulatory department of the people's
government of the province, autonomous region or municipality directly under the
PRC central government. We received a GSP Certificate from the Jilin
Province State Food and Drugs Administration Bureau with a term of five years,
which expired at the end of 2008. Such GSP Certificate was renewed at
the end of 2008 for another five year term, which shall expire at the end of
2013.
Insurance
Catalogue
Pursuant to the Decision of the
State Council on the Establishment of the State Basic Medical Insurance System
for Urban Employees and the Implementation Measures for the Administration of
the Scope of Medical Insurance Coverage for Pharmaceuticals for Urban Employees,
the Ministry of Labor and Social Security in China established the Insurance
Catalogue, in which the retail prices of certain pharmaceutical products are
listed and subject to price controls in the form of fixed prices or price
ceilings by the Chinese government. Manufacturers and distributors
are not permitted to set or change the retail price for any price-controlled
product above the applicable price ceiling or deviate from the applicable fixed
price imposed by the PRC government. The prices of other medicines
that are not subject to price controls are determined by the pharmaceutical
manufacturers, subject, in certain cases, to providing notice to the provincial
pricing authorities.
The Price Control Office of the NDRC, as well as provincial and
regional price control authorities, set the retail prices of products that are
subject to price controls. The wholesale price of the pharmaceutical
products subject to the price controls are generally determined by the set
retail price. The maximum prices of such medicine products are published by the
state and provincial administration authorities from time to time. Only the
pharmaceutical product manufacturer can apply for an increase in the retail
price of the product. While all of our pharmaceutical products are
subject to price controls, because our products are priced below the price
control level, price controls currently do not affect our sales of these
products.
The
medicines included in the Insurance Catalogue are selected by the Chinese
government authorities based on various factors including treatment
requirements, frequency of use, effectiveness and price. Medicines
included in the Insurance Catalogue are subject to price controls by the Chinese
government. The Insurance Catalogue is revised every two years. In connection
with each revision, the relevant provincial drug authority collects proposals
from relevant enterprises before organizing a comprehensive
appraisal. The SFDA then makes the final decision on any revisions
based on the preliminary opinion suggested by the provincial drug
administration.
The Insurance Catalogue is
divided into Parts A and B. The medicines included in Part A are
designated by the Chinese governmental authorities for general
application. Local governmental authorities may not adjust the
content of medicines in Part A. Although the medicines included in Part B are
designated by Chinese governmental authorities in the first instance, provincial
level authorities may make limited changes to the medicines included in Part B,
resulting in some regional variations in the medicines included in Part B from
region to region.
Patients
purchasing medicines included in Part A are entitled to reimbursement of the
costs of such medicines from the social medical fund in accordance with relevant
regulations in China. Patients purchasing medicines included in Part
B are required to pay a predetermined proportion of the costs of such
medicines.
For
fiscal years 2008 and 2007, approximately 24% of all the medicines distributed
by Yongxin were covered and reimbursable under the Insurance Catalogue issued by
the Chinese governmental authorities. However, the Insurance
Catalogue was revised in 2009 to include more categories and types of
medicines. As a result of these revisions, approximately 95.8% of all
the medicines distributed by Yongxin became listed in the Insurance Catalogue
during the fiscal year ended December 31, 2009, and management attributes part
of the Company’s increased revenues to these catalogue revisions. The
revenues attributable to sales of products covered under the Insurance Catalogue
during the fiscal years ended December 31, 2009, 2008 and 2007, represented
61.7%, 35% and 35%, respectively, of our total revenues for those
periods.
11
PRC
National Medical Insurance Program
Eligible
participants in the PRC national medical insurance program, mainly consisting of
urban residents, can purchase medicines in an authorized pharmacy by presenting
their medical insurance cards if the medicines purchased are included in the
national or provincial medical insurance catalogues. Authorized pharmacies can
generally either sell medicine on credit and obtain reimbursement from relevant
government social security bureaus on a monthly basis, or accept payments from
the participants at the of the purchase, and the participants in turn obtain
reimbursement from relevant government social security bureaus.
Purchases
of Tier A pharmaceutical products are generally fully reimbursable, except for
certain Tier A pharmaceutical products that are only reimbursable to the extent
the medicine is used the purposes stated in the Insurance Catalogue. Only a
portion of purchases of Tier-B pharmaceutical are reimbursable; participants
purchasing Tier B pharmaceutical products must make a co-payment which is not
reimbursable. Participants have varying amounts in their individual
accounts, which vary based on the contributions made by the participants and his
or her employer. Different regions in China have different requirements
regarding the caps of reimbursements in excess of the amounts in the individual
accounts.
Pharmaceutical
Product Advertisement
The
Standards for Examination and Publication of Advertisements of Pharmaceutical
Products and Rules for Examination of Advertisement of Pharmaceutical Products,
promulgated by the PRC State Administration of Industry and Commerce and the
SFDA, prevents the deceptive and misleading advertising of pharmaceutical
products. These regulations prohibit the advertisement of certain pharmaceutical
products and mandate that prescription pharmaceuticals only be advertised in
certain authorized medical magazines upon obtaining proper approval from the
local provincial level food and drug administration.
Environmental
Regulations
The
major environmental regulations applicable to us include the PRC Environmental
Protection Law, the PRC Law on the Prevention and Control of Water Pollution and
its Implementation Rules, the PRC Law on the Prevention and Control of Air
Pollution and its Implementation Rules, the PRC Law on the Prevention and
Control of Solid Waste Pollution, and the PRC Law on the Prevention and Control
of Noise Pollution.
We
have not been named as a defendant in any legal proceedings alleging violation
of environmental laws and have no reasonable basis to believe that there is any
threatened claim, action or legal proceedings against us that would have a
material adverse effect on our business, financial condition or results of
operations due to any non-compliance with environmental laws. To the
best knowledge of our management, we have been in full compliance with
environmental protection regulations during at least the past three
years.
Tax
Pursuant
to the Provisional Regulation of China on Value Added Tax ("VAT") and their
implementing rules, all entities and individuals that are engaged in the sale of
goods, the provision of repairs and replacement services and the importation of
goods in China are generally required to pay VAT at a rate of 17.0% of the gross
sales proceeds received, less any deductible VAT already paid or borne by the
taxpayer. Further, when exporting goods, the exporter is entitled to
a portion of or all the refund of VAT that it has already paid or
borne
12
Foreign
Currency Exchange
Under
the PRC foreign currency exchange regulations applicable to us, the Renminbi is
convertible for current account items, including the distribution of dividends,
interest payments, trade and service-related foreign exchange
transactions. Conversion of Renminbi for capital account items, such
as direct investment, loan, security investment and repatriation of investment,
however, is still subject to the approval of the PRC State Administration of
Foreign Exchange (“SAFE”). Foreign-invested enterprises may only buy,
sell and/or remit foreign currencies at those banks authorized to conduct
foreign exchange business after providing valid commercial documents and, in the
case of capital account item transactions, obtaining approval from SAFE. Capital
investments by foreign-invested enterprises outside of China are also subject to
limitations, which include approvals by the Ministry of Commerce, SAFE and the
State Reform and Development Commission.
Dividend
Distributions
Under
applicable PRC regulations, foreign-invested enterprises in China may pay
dividends only out of their accumulated profits, if any, determined in
accordance with PRC accounting standards and regulations. In addition,
foreign-invested enterprises in China are required to set aside at least 10.0%
of their after-tax profit based on PRC accounting standards each year to its
general reserves until the accumulative amount of such reserves reach 50.0% of
its registered capital. These reserves are not distributable as cash
dividends. The board of directors of a foreign-invested enterprise
has the discretion to allocate a portion of its after-tax profits to staff
welfare and bonus funds, which may not be distributed to equity owners except in
the event of liquidation. To date, we have not paid any dividends and we have no
plans to pay any dividends in the foreseeable future.
EMPLOYEES
Substantially
all of our employees are located in China. At March 1, 2009, we had
approximately 673 full time employees, with 90 employees holding pharmaceutical
licenses, and 87 of such licensed pharmacists working at our retail
drugstores. There are no collective bargaining contracts covering any
of our employees. We believe our relationship with our employees is
good.
Yongxin is required to contribute a
portion of its employees' total salaries to the Chinese government's social
insurance funds, including medical insurance, unemployment insurance and job
injuries insurance, and a housing assistance fund, in accordance with relevant
regulations. In the last three years, Yongxin contributed
approximately $174,006, $174,567 and $61,918 for the years ended December 31,
2009, 2008 and 2007, respectively. We expect the amount of Yongxin's
contribution to the government's social insurance funds to increase in the
future as Yongxin expands its workforce and
operations.
ITEM
1A. RISK FACTORS
Any
investment in our securities involves a high degree of risk. Potential investors
should carefully consider the material risks described below and all of the
information contained in this Form 10-K before deciding whether to purchase any
of our securities. Our business, financial condition or results of operations
could be materially adversely affected by these risks if any of them actually
occur. The shares of our common stock are currently quoted on the
Over-the-Counter Bulletin Board, or OTCBB under the symbol "CYXN." If and when
our securities are traded, the trading price could decline due to any of these
risks, and an investor may lose all or part of his or her investment. Some
of these factors have affected our financial condition and operating results in
the past or are currently affecting us. This report also contains
forward-looking statements that involve risks and uncertainties. Our actual
results could differ materially from those anticipated in these forward-looking
statements as a result of certain factors, including the risks faced described
below and elsewhere in this Form 10-K.
RISKS
RELATED TO OUR BUSINESS
THE
PURCHASE OF MANY OF OUR PRODUCTS IS DISCRETIONARY, AND MAY BE PARTICULARLY
AFFECTED BY ADVERSE TRENDS IN THE GENERAL ECONOMY; THEREFORE CHALLENGING
ECONOMIC CONDITIONS WILL MAKE IT MORE DIFFICULT FOR US TO GENERATE
REVENUE.
The current general economic recession
and crisis and any continuing unfavorable economic conditions may affect the
success of our operations since many of our products are discretionary and we
depend to a significant extent upon a number of factors relating to
discretionary consumer spending in China. These factors include
economic conditions and perceptions of such conditions by consumers, employment
rates, the level of consumers' disposable income, business conditions, interest
rates, consumer debt levels, availability of credit and levels of taxation in
regional and local markets in China where we sell our products. There
can be no assurance that consumer spending on many of our products, including
cosmetics, organic products and health and nutritional supplements, will not be
adversely affected by changes in general economic conditions in China and
globally.
13
THE
SUCCESS OF OUR BUSINESS DEPENDS ON OUR ABILITY TO MARKET AND ADVERTISE THE
PRODUCTS WE SELL EFFECTIVELY.
Our ability to establish effective
marketing and advertising campaigns is key to our success. Our
advertisements promote our corporate image, our merchandise and the pricing of
such products. If we are unable to increase awareness of our company
and our products, we may not be able to attract new customers. Our
marketing activities may not be successful in promoting our products or pricing
strategies or in retaining and increasing our customer base. We
cannot assure you that our marketing programs will be adequate to support our
future growth, which may result in a material adverse effect on our results of
operations.
WE
MAY BE UNABLE TO IDENTIFY AND RESPOND EFFECTIVELY TO SHIFTING CUSTOMER
PREFERENCES, AND WE MAY FAIL TO OPTIMIZE OUR PRODUCT OFFERING AND INVENTORY
POSITION.
Consumer preferences in the drugstore
industry change rapidly and are difficult to predict. The success of
our business depends on our ability to predict accurately and respond to future
changes in consumer preferences, carry the inventory demanded by customers,
deliver the appropriate quality of products, price products correctly and
implement effective purchasing procedures. We must optimize our
product selection and inventory positions based on consumer preferences and
sales trends. If we fail to anticipate, identify or react
appropriately to changes in consumer preferences and adapt our product selection
to these changing preferences, we could experience excess inventories, higher
than normal markdowns or an inability to sell our products, which, in turn,
could significantly reduce our revenue and have a material adverse effect on our
business, financial condition and results of operations.
IF
WE FAIL TO MAINTAIN OPTIMAL INVENTORY LEVELS, OUR INVENTORY HOLDING COSTS COULD
INCREASE OR CAUSE US TO LOSE SALES, EITHER OF WHICH COULD HAVE A MATERIAL
ADVERSE EFFECT ON OUR BUSINESS, FINANCIAL CONDITION AND RESULTS OF
OPERATIONS.
While we must maintain sufficient
inventory levels to operate our business successfully and meet our customers'
demands, we must be careful to avoid amassing excess
inventory. Changing consumer demands, manufacturer backorders and
uncertainty surrounding new product launches expose us to increased inventory
risks. Demand for products can change rapidly and unexpectedly,
including the time between when the product is ordered from the supplier to the
time it is offered for sale. We carry a wide variety of products and
must maintain sufficient inventory levels of our products. We may be
unable to sell certain products in the event that consumer demand
changes. Our inventory holding costs will increase if we carry excess
inventory. However, if we do not have a sufficient inventory of a
product to fulfill customer orders, we may lose orders or customers, which may
adversely affect our business, financial condition and results of
operations. We cannot assure you that we can accurately predict
consumer demand and events and avoid over-stocking or under-stocking
products.
IF WE ARE UNABLE TO MANAGE THE
DISTRIBUTION OF OUR PRODUCTS AT OUR LOGISTICS CENTER, WE MAY BE UNABLE TO MEET
CUSTOMER DEMAND.
Substantially all of our products are
distributed to our stores and our wholesale customers through our "Logistics
Center" located in our "Logistics Plaza" in Changchun, PRC. The
efficient operation and management of this facility is essential in order for us
to meet customer demands. Our business would suffer if we do not
successfully operate this distribution facility or if the operation of this
facility was disrupted for any reason, including disruptions caused by natural
disasters. Our failure to manage this facility properly could result
in higher distribution costs, excess or insufficient inventory, or an inability
to fulfill customer orders, each of which could result in a material adverse
effect on our results of operations.
14
DUE
TO THE GEOGRAPHIC CONCENTRATION OF OUR SALES IN THE NORTHEAST REGION OF CHINA,
OUR RESULTS OF OPERATIONS AND FINANCIAL CONDITION ARE SUBJECT TO FLUCTUATIONS IN
REGIONAL ECONOMIC CONDITIONS.
A significant percentage of our total
sales are made in the northeast region of China, particularly in Jilin
province. For the years ended December 31, 2009, 2008 and 2007,
approximately 86%, 85% and 80% of revenues, respectively, were generated from
this area. Our concentration of sales in this area heightens our
exposure to adverse developments related to competition, as well as economic and
demographic changes in this region. Our geographic concentration
might result in a material adverse effect on our business, financial condition
or results of operations in the future.
CERTAIN
DISRUPTIONS IN SUPPLY OF AND CHANGES IN THE COMPETITIVE ENVIRONMENT FOR OUR
PRODUCTS MAY ADVERSELY AFFECT OUR PROFITABILITY.
We carry a broad range of merchandise
in our stores, including pharmaceuticals, traditional Chinese medicines, herbal
and nutritional supplements and cosmetics. A significant disruption
in the supply of these products could decrease inventory levels and sales, and
materially adversely affect our business and financial
results. Shortages of products or interruptions in transportation
systems, labor strikes, work stoppages, war, acts of terrorism or other
interruptions or difficulties in the employment of labor or transportation in
the markets in which we purchase products may adversely affect our ability to
maintain sufficient inventories of our products to meet consumer
demand. If we were to experience a significant or prolonged shortage
of products from any of our suppliers and could not procure the products from
other sources, we would be unable to meet customer demand, which, in turn, would
adversely affect our sales, margins and customer relations.
OUR
OPERATIONS WOULD BE MATERIALLY ADVERSELY AFFECTED IF THIRD-PARTY CARRIERS WERE
UNABLE TO TRANSPORT OUR PRODUCTS ON A TIMELY BASIS.
All of our products are shipped through
third party carriers. If a strike or other event prevented or
disrupted these carriers from transporting our products, other carriers may be
unavailable or may not have the capacity to deliver our products to our
customers and to our retail stores. If adequate third party sources
to ship our products were unavailable at any time, our business would be
materially adversely affected.
THE
MARKET FOR OUR PRODUCTS AND SERVICES IS VERY COMPETITIVE AND, IF WE CANNOT
EFFECTIVELY COMPETE, OUR BUSINESS WILL BE HARMED.
The industries in which we operate are
highly fragmented and very competitive. We compete with local
drugstores and with large foreign multinational companies that offer products
that are similar to ours. Some of these competitors have larger local
or regional customer bases, more locations, more brand equity, and substantially
greater financial, marketing and other resources than we have. As a result, our
competitors may be in a stronger position to respond quickly to potential
acquisitions and other market opportunities, new or emerging technologies and
changes in customer tastes. We cannot assure you that we will be able
to maintain or increase our market share against the emergence of these or other
sources of competition. Failure to maintain and enhance our competitive position
could materially adversely affect our business and prospects.
WE
MAY NOT BE SUCCESSFUL IN COMPETING WITH OTHER WHOLESALERS AND DISTRIBUTORS OF
PHARMACEUTICAL PRODUCTS IN THE TENDER PROCESSES FOR THE PURCHASE OF MEDICINES BY
STATE-OWNED AND STATE-CONTROLLED HOSPITALS.
Our
wholesale business sells various pharmaceutical products to hospitals owned and
controlled by government authorities in the PRC. Government owned
hospitals purchase pharmaceutical products by using collective tender
processes. During a collective tender process, a hospital establishes
a committee of recognized pharmaceutical experts, which assesses bids submitted
by pharmaceutical manufacturers. The hospitals may only purchase
pharmaceuticals that win in collective tender processes. The
collective tender process for pharmaceuticals with the same chemical composition
must be conducted at least annually, and pharmaceuticals that have won in the
collective tender processes previously must participate and win in the
collective tender processes in the following period before hospitals may make
new purchases. If we are unable to win purchase contracts through the
collective tender processes in which we decide to participate, we will lose
market share to our competitors, and our sales and profitability will be
adversely affected.
15
COUNTERFEIT
PRODUCTS SOLD IN CHINA COULD NEGATIVELY IMPACT OUR REVENUES, BRAND REPUTATION,
BUSINESS AND RESULTS OF OPERATIONS.
Our products are also subject to
competition from counterfeit pharmaceuticals, which are pharmaceuticals
manufactured without proper licenses or approvals and are fraudulently
mislabeled with respect to their content and/or
manufacturer. Counterfeit pharmaceuticals are generally sold at lower
prices than authentic products due to their low production costs, and in some
cases are very similar in appearance to authentic
products. Counterfeit pharmaceuticals may or may not have the same
chemical content as their authentic counterparts. Although the PRC
government has recently been increasingly active in policing counterfeit
pharmaceuticals, there is a lack of effective counterfeit pharmaceutical
regulation control and enforcement systems in China. The
proliferation of counterfeit pharmaceuticals has grown in recent years and may
continue to grow in the future. Despite our implementation of quality
controls, we cannot assure you that we would not be distributing or selling
counterfeit products inadvertently. Any accidental sale or
distribution of counterfeit products can subject our company to fines,
administrative penalties, litigation and negative publicity, which could
negatively impact our revenues, brand reputation, business and results of
operations.
THE
RETAIL PRICES OF SOME OF OUR PRODUCTS ARE SUBJECT TO PRICE CONTROLS BY THE PRC
GOVERNMENT, WHICH MAY AFFECT BOTH OUR REVENUES AND NET INCOME.
The laws of the PRC permit the PRC
government to fix and adjust prices of certain pharmaceutical products,
including many of those listed in the Insurance Catalogue. Through
these price controls, the government can fix retail prices and set retail price
ceiling for certain of the pharmaceutical products we
sell. Additionally, the PRC government may periodically adjust the
retail prices of these products downward in order to make pharmaceuticals more
affordable to the general Chinese population. While our sales of
pharmaceutical products are not affected by the price controls because we
currently sell such products at prices below the price control level, we cannot
guarantee that our sales of these products will not be affected in the future,
as price controls may be increased or may affect additional
products. To the extent that we are subject to price controls, our
revenue, gross profit, gross margin and net income will be affected because the
revenue we derive from our sales will be limited and we may have limited ability
to control our costs. Further, if price controls affect both our
revenue and costs, our ability to be profitable and the extent of our
profitability will be effectively subject to determination by the applicable
regulatory authorities in the PRC. Any future price controls or price
reductions may reduce our revenue and profitability and have a material adverse
effect on our financial condition and results of operations.
IF
WE DO NOT COMPLY WITH THE APPLICABLE PRC LAWS AND REGULATIONS CONTROLLING THE
SALE OF MEDICINES UNDER THE PRC NATIONAL MEDICAL INSURANCE PROGRAM, WE MAY BE
SUBJECT TO FINES AND OTHER PENALTIES.
Persons eligible to participate in the
PRC National Medical Insurance Program can buy medicines that have been included
in the Insurance Catalogue using a medical insurance card in an authorized
pharmacy. The applicable PRC government social security bureau then
reimburses the pharmacy. PRC law also forbids pharmacies from selling
goods other than pre-approved medicines when purchases are made with medical
insurance cards. While we have established procedures to prevent our
drugstores from selling unauthorized goods to customers who make purchases with
medical insurance cards, we cannot assure you that these procedures will be
properly followed at all times in all of our stores. Violations of
this prohibition by any of our drugstores may result in the revocation of such
drugstore’s status as an authorized pharmacy. Additionally, we could
be subject to other fines or other penalties, and to negative publicity, which
could damage our company's reputation and have a material adverse effect on our
results of operations.
THE
REQUIRED CERTIFICATES, PERMITS, AND LICENSES RELATED TO OUR OPERATIONS ARE
SUBJECT TO GOVERNMENTAL CONTROL AND RENEWAL AND FAILURE TO OBTAIN RENEWAL WILL
CAUSE ALL OR PART OF OUR OPERATIONS TO BE TERMINATED.
We are subject to various PRC laws and
regulations pertaining to our wholesale and retail operations. We
have attained certificates, permits, and licenses required for the operation of
a pharmaceutical distributor and retailer. We cannot assure you that
we will have all necessary permits, certificates and authorizations for the
operation of our business at all times. Additionally, our
certifications, permits and authorizations are subject to periodic renewal by
the relevant government authorities. We intend to apply for renewal
of these certificates, permits and authorizations prior to their
expiration. During the renewal process, we will be re-evaluated by
the appropriate governmental authorities and must comply with the then
prevailing standards and regulations which may change from time to
time. In the event that we are not able to renew the certificates,
permits and licenses, all or part of our operations may be
terminated. Furthermore, if escalating compliance costs associated
with governmental standards and regulations restrict or prohibit any part of our
operations, it may adversely affect our operations and
profitability.
16
WE
MAY SUFFER AS A RESULT OF PRODUCT LIABILITY CLAIMS, PERSONAL INJURY CLAIMS OR
DEFECTIVE PRODUCTS.
Our pharmacies are exposed to risks
inherent in the packaging and distribution of pharmaceutical and other
healthcare products, such as with respect to improper filling of prescriptions,
labeling of prescriptions, adequacy of warnings, and the unintentional
distribution of counterfeit drugs. Furthermore, the applicable laws, rules and
regulations require our in-store pharmacists to offer counseling, without
additional charge, to our customers about medication, dosage, delivery systems,
common side effects and other information the in-store pharmacists deem
significant. Our in-store pharmacists may also have a duty to warn customers
regarding any potential negative effects of a prescription drug if the warning
could reduce or negate these effects and we may be liable for claims arising
from advice given by our in-store pharmacists. Further, we may sell products
which inadvertently have an adverse effect on the health of
individuals. Product liability claims may be asserted against us with
respect to any of the products we sell and as a retailer, we are required to pay
for damages for any successful product liability claim against us, although we
may have the right under applicable PRC laws, rules and regulations to recover
from the relevant manufacturer for compensation we paid to our customers in
connection with a product liability claim. Any product liability claim, product
recall, adverse side effects caused by improper use of the products we sell or
manufacturing defects may result in adverse publicity regarding us and the
products we sell, which would harm our reputation. If we are found
liable for product liability claims, we could be required to pay substantial
monetary damages. Furthermore, even if we successfully defend
ourselves against this type of claim, we could be required to spend significant
management, financial and other resources, which could disrupt our business, and
our reputation as well as our brand name may also suffer. We, like many other
similar companies in China, do not carry product liability insurance. As a
result, any imposition of product liability could materially harm our business,
financial condition and results of operations. In addition, we do not have any
business interruption insurance due to the limited coverage of any business
interruption insurance in China, and as a result, any business disruption or
natural disaster could severely disrupt our business and operations and
significantly decrease our revenue and profitability.
IF
OUR PRODUCTS ARE ALLEGED OR FOUND TO BE IN CONFLICT WITH PATENTS THAT HAVE BEEN
OR MAY BE GRANTED TO COMPETITORS OR OTHERS, OUR REPUTATION AND BUSINESS MAY BE
ADVERSELY AFFECTED.
While we currently do not own any
patents or license patents from third parties, other parties could bring legal
actions against us claiming damages and seeking to enjoin the marketing of our
products for allegedly conflicting with patents held by them. Any such
litigation could result in substantial cost to us and diversion of effort by our
management and technical personnel. If any such actions are
successful, in addition to any potential liability for damages, we could be
required to obtain a license in order to continue to market the affected
products. There can be no assurance that we would prevail in any such
action or that any license required under any such patent would be made
available on acceptable terms, if at all. Failure to obtain needed
patents, licenses or proprietary information held by others may have a material
adverse effect on our business. In addition, if we were to become
involved in such litigation, it could consume a substantial portion of our time
and resources. Also, with respect to licensed technology, there can
be no assurance that the licensor of the technology will have the resources,
financial or otherwise, or desire to defend against any challenges to the rights
of such licensor to its patents.
17
WE
RELY ON TRADE SECRET PROTECTIONS THROUGH CONFIDENTIALITY AGREEMENTS WITH OUR
EMPLOYEES, CUSTOMERS AND OTHER PARTIES; THE BREACH OF SUCH AGREEMENTS COULD
ADVERSELY AFFECT OUR BUSINESS ANDS RESULTS OF OPERATIONS.
We also rely on trade secrets, which we
seek to protect, in part, through confidentiality and non-disclosure agreements
with our employees, customers and other parties. There can be no
assurance that these agreements will not be breached, that we would have
adequate remedies for any such breach or that our trade secrets will not
otherwise become known to or independently developed by competitors. To the
extent that consultants, key employees or other third parties apply
technological information independently developed by them or by others to our
proposed projects, disputes may arise as to the proprietary rights to such
information that may not be resolved in our favor. We may be involved
from time to time in litigation to determine the enforceability, scope and
validity of our proprietary rights. Any such litigation could result in
substantial cost and diversion of effort by our management and technical
personnel.
THE
FAILURE TO MANAGE GROWTH EFFECTIVELY COULD HAVE AN ADVERSE EFFECT ON OUR
EMPLOYEE EFFICIENCY, PRODUCT QUALITY, WORKING CAPITAL LEVELS, AND RESULTS OF
OPERATIONS.
Any significant growth in the market
for our products or our entry into new markets may require an expansion of our
employee base for managerial, operational, financial, and other
purposes. As of March 3, 2010, we had approximately 673 full time
employees. During any growth, we may face problems related to our
operational and financial systems and controls, including quality control and
delivery and service capacities. We would also need to continue to
expand, train and manage our employee base. Continued future growth
will impose significant added responsibilities upon the members of management to
identify, recruit, maintain, integrate, and motivate new employees.
Aside
from increased difficulties in the management of human resources, we may also
encounter working capital issues, as we will need increased liquidity to finance
the purchase of raw materials and supplies, development of new products, and the
hiring of additional employees. For effective growth management, we
will be required to continue improving our operations, management, and financial
systems and controls. Our failure to manage growth effectively may
lead to operational and financial inefficiencies that will have a negative
effect on our profitability. We cannot assure investors that we will
be able to timely and effectively meet that demand and maintain the quality
standards required by our existing and potential customers.
WE
ARE DEPENDENT ON CERTAIN KEY PERSONNEL AND LOSS OF THESE KEY PERSONNEL COULD
HAVE A MATERIAL ADVERSE EFFECT ON OUR BUSINESS, FINANCIAL CONDITION AND RESULTS
OF OPERATIONS.
Our success is, to a certain extent,
attributable to the management, sales and marketing, and operational and
technical expertise of certain key personnel. Each of the named
executive officers, including Mr. Yongxin Liu, our Chief Executive Officer and
Chairman and Mr. Ning Liu, our President and Chief Operating Officer, perform
key functions in the operation of our business. The loss of a
significant number of these employees could have a material adverse effect upon
our business, financial condition, and results of operations. We do
not maintain key-man insurance for members of our management team because it is
not a customary practice in China. If we lose the services of any
senior management, we may not be able to locate suitable or qualified
replacements, and may incur additional expenses to recruit and train new
personnel, which could severely disrupt our business and prospects.
WE
ARE DEPENDENT ON A TRAINED WORKFORCE AND AN INABILITY TO RETAIN OR EFFECTIVELY
RECRUIT SUCH EMPLOYEES, INCLUDING IN-STORE PHARMACISTS FOR OUR STORES, COULD
HAVE A MATERIAL ADVERSE EFFECT ON OUR BUSINESS, FINANCIAL CONDITION AND RESULTS
OF OPERATIONS.
We must attract, recruit and retain a
sizeable workforce of qualified and trained staff, including in-store
pharmacists, in order to operate our retail drugstores. Applicable
PRC regulations require at least one qualified pharmacist to be stationed in
each drugstore to instruct or advise customers on prescription
medications. A shortage of pharmacists in the past few years has
occurred in the past few years due to increasing demand within the drugstore
industry as well as demand from other businesses in the healthcare industry. We
face competition for personnel from other drugstore chains, supermarkets, retail
chains, and pharmaceutical companies. We cannot assure you that we
will be able to attract, hire and retain sufficient numbers of in-store
pharmacists and other skilled employees that are necessary to continue to
develop and grow our business.
18
Our ability to implement effectively
our business strategy and expand our operations will depend upon, among other
factors, the successful recruitment and retention of additional highly skilled
and experienced pharmacists, managers and other technical and marketing
personnel. There is significant competition for technologically
qualified personnel in our business and we may not be successful in recruiting
or retaining sufficient qualified personnel consistent with our current and
future operational needs.
OUR
QUARTERLY RESULTS MAY FLUCTUATE BECAUSE OF MANY FACTORS AND, AS A RESULT,
INVESTORS SHOULD NOT RELY ON OUR OPERATING RESULTS AS INDICATIVE OF FUTURE
RESULTS.
Fluctuations in operating results or
the failure of operating results to meet the expectations of public market
analysts and investors may negatively impact the value of our
securities. Quarterly operating results may fluctuate in the future
due to a variety of factors that could affect revenues or expenses in any
particular quarter. Fluctuations in quarterly operating results could
cause the value of our securities to decline. Investors should not
rely on quarter-to-quarter comparisons of results of operations as an indication
of future performance. As a result of the factors listed below, it is
possible that in future periods results of operations may be below the
expectations of public market analysts and investors. This could
cause the market price of our securities to decline. Factors that may affect our
quarterly results include:
|
·
|
vulnerability
of our business to a general economic downturn in
China;
|
|
·
|
fluctuation
and unpredictability of the prices of the products we
sell;
|
|
·
|
seasonality
of our business;
|
|
·
|
changes
in the laws of the PRC that affect our
operations;
|
|
·
|
competition
from our competitors; and
|
|
·
|
our
ability to obtain necessary government certifications and/or licenses to
conduct our business.
|
OUR
STRATEGY TO ACQUIRE COMPANIES MAY RESULT IN UNSUITABLE ACQUISITIONS OR FAILURE
TO SUCCESSFULLY INTEGRATE ACQUIRED COMPANIES, WHICH COULD LEAD TO REDUCED
PROFITABILITY.
We may embark on a growth strategy
through acquisitions of companies or operations that complement existing product
lines, customers or other capabilities. We may be unsuccessful in
identifying suitable acquisition candidates, or may be unable to consummate a
desired acquisition. To the extent any future acquisitions are
completed, we may be unsuccessful in integrating acquired companies or their
operations, or if integration is more difficult than anticipated, we may
experience disruptions that could have a material adverse impact on future
profitability. Some of the risks that may affect our ability to
integrate, or realize any anticipated benefits from, acquisitions
include:
|
·
|
unexpected
losses of key employees or customer of the acquired
company;
|
|
·
|
difficulties
integrating the acquired company's standards, processes, procedures and
controls;
|
|
·
|
difficulties
coordinating new product and process
development;
|
|
·
|
difficulties
hiring additional management and other critical
personnel;
|
|
·
|
difficulties
increasing the scope, geographic diversity and complexity of our
operations;
|
|
·
|
difficulties
consolidating facilities, transferring processes and
know-how;
|
|
·
|
difficulties
reducing costs of the acquired company's
business;
|
19
|
·
|
diversion
of management's attention from our management;
and
|
|
·
|
adverse
impacts on retaining existing business relationships with
customers.
|
RISKS
RELATED TO US DOING BUSINESS IN CHINA
SUBSTANTIALLY
ALL OF OUR ASSETS ARE LOCATED IN THE PRC AND SUBSTANTIALLY ALL OF OUR REVENUES
ARE DERIVED FROM OUR OPERATIONS IN CHINA, AND CHANGES IN THE POLITICAL AND
ECONOMIC POLICIES OF THE PRC GOVERNMENT COULD HAVE A SIGNIFICANT IMPACT UPON THE
BUSINESS WE MAY BE ABLE TO CONDUCT IN THE PRC AND ACCORDINGLY ON THE RESULTS OF
OUR OPERATIONS AND FINANCIAL CONDITION.
Our business operations may be
adversely affected by the current and future political environment in the
PRC. The Chinese government exerts substantial influence and control
over the manner in which we must conduct our business activities. Our
ability to operate in China may be adversely affected by changes in Chinese laws
and regulations, including those relating to taxation, import and export
tariffs, raw materials, environmental regulations, land use rights, property and
other matters. Under the current government leadership, the
government of the PRC has been pursuing economic reform policies that encourage
private economic activity and greater economic
decentralization. There is no assurance, however, that the government
of the PRC will continue to pursue these policies, or that it will not
significantly alter these policies from time to time without
notice.
OUR
OPERATIONS ARE SUBJECT TO PRC LAWS AND REGULATIONS THAT ARE SOMETIMES VAGUE AND
UNCERTAIN. ANY CHANGES IN SUCH PRC LAWS AND REGULATIONS, OR THE
INTERPRETATIONS THEREOF, MAY HAVE A MATERIAL AND ADVERSE EFFECT ON OUR
BUSINESS.
The PRC's legal system is a civil law
system based on written statutes. Unlike the common law system prevalent in the
United States, decided legal cases have little value as precedent in China.
There are substantial uncertainties regarding the interpretation and application
of PRC laws and regulations, including but not limited to, the laws and
regulations governing our business, or the enforcement and performance of our
arrangements with customers in the event of the imposition of statutory liens,
death, bankruptcy or criminal proceedings. The Chinese government has been
developing a comprehensive system of commercial laws, and considerable progress
has been made in introducing laws and regulations dealing with economic matters
such as foreign investment, corporate organization and governance, commerce,
taxation and trade. However, because these laws and regulations are
relatively new, and because of the limited volume of published cases and
judicial interpretation and their lack of force as precedents, interpretation
and enforcement of these laws and regulations involve significant uncertainties.
New laws and regulations that affect existing and proposed future businesses may
also be applied retroactively.
Our principal operating subsidiary,
Yongxin, is considered a foreign invested enterprise under PRC laws, and as a
result is required to comply with PRC laws and regulations, including laws and
regulations specifically governing the activities and conduct of foreign
invested enterprises. We cannot predict what effect the
interpretation of existing or new PRC laws or regulations may have on our
businesses. If the relevant authorities find us in violation of PRC
laws or regulations, they would have broad discretion in dealing with such a
violation, including, without limitation:
|
·
|
levying
fines;
|
|
·
|
revoking
our business license, other licenses or
authorities;
|
|
·
|
requiring
that we restructure our ownership or operations;
and
|
|
·
|
requiring
that we discontinue any portion or all of our
business.
|
20
NEW
LABOR LAWS IN THE PRC MAY ADVERSLY AFFECT OUR RESULTS OF
OPERATIONS.
On
January 1, 2008, the PRC government promulgated the Labor Contract Law of
the PRC, or the New Labor Contract Law. The New Labor Contract Law
imposes greater liabilities on employers and significantly impacts the cost of
an employer’s decision to reduce its workforce. Further, it requires
certain terminations to be based upon seniority and not merit. In the
event we decide to significantly change or decrease our workforce, the New Labor
Contract Law could adversely affect our ability to enact such changes in a
manner that is most advantageous to our business or in a timely and cost
effective manner, thus materially and adversely affecting our financial
condition and results of operations.
THE
SCOPE OF OUR BUSINESS LICENSE IN CHINA IS LIMITED, AND WE MAY NOT EXPAND OR
CONTINUE OUR BUSINESS WITHOUT GOVERNMENT APPROVAL AND RENEWAL,
RESPECTIVELY.
Our principal operating subsidiary,
Yongxin, is a PRC foreign invested enterprise (“FIE”). An FIE can
only conduct business within its approved business scope, which ultimately
appears on its business license. Our license permits us to design,
manufacture, sell and market pharmaceutical products throughout the
PRC. Any amendment to the scope of our business requires further
application and government approval. In order for us to expand our
business beyond the scope of our license, it will be required to enter into
negotiations with the government authorities to obtain the approval that would
be required to expand the scope of our business. We cannot assure
investors that Yongxin will be able to obtain the necessary government approval
for any change or expansion of its business.
OUR
BUSINESS IS SUBJECT TO A VARIETY OF ENVIRONMENTAL LAWS AND REGULATIONS. OUR
FAILURE TO COMPLY WITH ENVIRONMENTAL LAWS AND REGULATIONS MAY HAVE A MATERIAL
ADVERSE EFFECT ON OUR BUSINESS AND RESULTS OF OPERATIONS.
We are subject to various environmental
laws and regulations that require us to obtain environmental permits and are
subject to registration and inspection by the SFDA. We have a
provincial license issued by the Business Administration Bureau, Jilin
Province. Although we are currently compliant with all provisions of
our registrations and licenses, we cannot assure you that at all times we will
be in compliance with environmental laws and regulations or our environmental
permits or that we will not be required to expend significant funds to comply
with, or discharge liabilities arising under, environmental laws, regulations
and permits. Additionally, we cannot guarantee you that our licenses and
registrations will be renewed. Any non-renewal of any of our required
permits and licenses could result in the termination of our business
operations.
PRC
REGULATIONS RELATING TO ACQUISITIONS OF PRC COMPANIES BY FOREIGN ENTITIES MAY
CREATE REGULATORY UNCERTAINTIES THAT COULD RESTRICT OR LIMIT OUR ABILITY TO
OPERATE, INCLUDING OUR ABILITY TO PAY DIVIDENDS.
The PRC State Administration of Foreign
Exchange (“SAFE”), issued a public notice in January 2005 concerning foreign
exchange regulations on mergers and acquisitions in China. The public
notice states that if an offshore company controlled by PRC residents intends to
acquire a PRC company, such acquisition will be subject to strict examination by
the relevant foreign exchange authorities. The public notice also
states that the approval of the relevant foreign exchange authorities is
required for any sale or transfer by the PRC residents of a PRC company's assets
or equity interests to foreign entities for equity interests or assets of the
foreign entities.
In April 2005, SAFE issued another
public notice further explaining the January notice. In accordance
with the April notice, if an acquisition of a PRC company by an offshore company
controlled by PRC residents has been confirmed by a Foreign Investment
Enterprise Certificate prior to the promulgation of the January notice, the PRC
residents must each submit a registration form to the local SAFE branch with
respect to their respective ownership interests in the offshore company, and
must also file an amendment to such registration if the offshore company
experiences material events, such as changes in the share capital, share
transfer, mergers and acquisitions, spin-off transaction or use of assets in
China to guarantee offshore obligations. The April notice also provides that
failure to comply with the registration procedures set forth therein may result
in restrictions on our PRC resident stockholders and subsidiaries. Pending the
promulgation of detailed implementation rules, the relevant government
authorities are reluctant to commence processing any registration or application
for approval required under the SAFE notices. On August 8, 2006, the
PRC Ministry of Commerce ("MOFCOM"), joined by the State-owned Assets
Supervision and Administration Commission of the State Council, the State
Administration of Taxation, the State Administration for Industry and Commerce,
the China Securities Regulatory Commission and SAFE, released a substantially
amended version of the Provisions for Foreign Investors to Merge with or Acquire
Domestic Enterprises (the "Revised M&A Regulations"), which took effect
September 8, 2006. These new rules significantly revised China's
regulatory framework governing onshore-to-offshore restructurings and foreign
acquisitions of domestic enterprises. These new rules signify greater
PRC government attention to cross-border merger, acquisition and other
investment activities, by confirming MOFCOM as a key regulator for issues
related to mergers and acquisitions in China and requiring MOFCOM approval of a
broad range of merger, acquisition and investment
transactions. Further, the new rules establish reporting requirements
for acquisition of control by foreigners of companies in key industries, and
reinforce the ability of the Chinese government to monitor and prohibit foreign
control transactions in key industries.
21
These
rules may significantly affect the means by which offshore-onshore
restructurings are undertaken in China in connection with offshore private
equity and venture capital financings, mergers and acquisitions. It
is expected that such transactional activity in China in the near future will
require significant case-by-case guidance from MOFCOM and other government
authorities as appropriate. It is anticipated that application of the
new rules will be subject to significant administrative interpretation, and we
will need to closely monitor how MOFCOM and other ministries apply the rules to
ensure its domestic and offshore activities continue to comply with PRC law.
Given the uncertainties regarding interpretation and application of the new
rules, we may need to expend significant time and resources to maintain
compliance. It is uncertain how our business operations or future
strategy will be affected by the interpretations and implementation of the SAFE
notices and new rules. Our business operations or future strategy could be
adversely affected by the SAFE notices and the new rules. For
example, we may be subject to more stringent review and approval processes with
respect to our foreign exchange activities.
THE
FOREIGN CURRENCY EXCHANGE RATE BETWEEN U.S. DOLLARS AND RENMINBI COULD ADVERSELY
AFFECT OUR FINANCIAL CONDITION.
To the extent that we need to convert
U.S. Dollars into Renminbi for our operational needs, our financial position and
the price of our common stock may be adversely affected should the Renminbi
appreciate against the U.S. Dollar at that time. Conversely, if we
decide to convert our Renminbi into U.S. Dollars for the operational needs or
paying dividends on our common stock, the dollar equivalent of our earnings from
our subsidiaries in China would be reduced should the U.S. Dollar appreciate
against the Renminbi.
Until 1994, the Renminbi experienced a
gradual but significant devaluation against most major currencies, including
dollars, and there was a significant devaluation of the Renminbi on January 1,
1994 in connection with the replacement of the dual exchange rate system with a
unified managed floating rate foreign exchange system. Since 1994,
the value of the Renminbi relative to the U.S. Dollar has remained stable and
has appreciated slightly against the U.S. Dollar. Countries, including the
United States, have argued that the Renminbi is artificially undervalued due to
China's current monetary policies and have pressured China to allow the Renminbi
to float freely in world markets. In July 2005, the PRC government
changed its policy of pegging the value of the Renminbi to the U.S.
Dollar. Under the new policy, the Renminbi is permitted to fluctuate
within a narrow and managed band against a basket of designated foreign
currencies. While the international reaction to the Renminbi
revaluation has generally been positive, there remains significant international
pressure on the PRC government to adopt an even more flexible currency policy,
which could result in further and more significant appreciation of the Renminbi
against the dollar.
FAILURE
TO COMPLY WITH THE UNITED STATES FOREIGN CORRUPT PRACTICES ACT COULD SUBJECT US
TO PENALTIES AND OTHER ADVERSE CONSEQUENCES.
As our ultimate holding company is a
Delaware corporation, we are subject to the United States Foreign Corrupt
Practices Act, which generally prohibits U.S. companies from engaging in bribery
or other prohibited payments to foreign officials for the purpose of obtaining
or retaining business. Foreign companies, including some that may
compete with us, are not subject to these prohibitions. Corruption,
extortion, bribery, pay-offs, theft and other fraudulent practices may occur
from time-to-time in the PRC. Although we specifically forbid our
employees from engaging in such corrupt practices, we can make no assurance that
our employees or other agents will not engage in such conduct for which we might
be held responsible. If our employees or other agents are found to
have engaged in such practices, we could suffer severe penalties and other
consequences that may have a material adverse effect on our business, financial
condition and results of operations.
22
IF
WE MAKE EQUITY COMPENSATION GRANTS TO PERSONS WHO ARE PRC CITIZENS, THEY MAY BE
REQUIRED TO REGISTER WITH THE STATE ADMINISTRATION OF FOREIGN EXCHANGE OF THE
PRC (“SAFE”). WE MAY ALSO FACE REGULATORY UNCERTAINTIES THAT COULD
RESTRICT OUR ABILITY TO ADOPT AN EQUITY COMPENSATION PLAN FOR OUR DIRECTORS AND
EMPLOYEES AND OTHER PARTIES UNDER PRC LAW.
On April 6, 2007, SAFE issued the
"Operating Procedures for Administration of Domestic Individuals Participating
in the Employee Stock Ownership Plan or Stock Option Plan of An Overseas Listed
Company, also know as "Circular 78." It is not clear whether Circular 78 covers
all forms of equity compensation plans or only those which provide for the
granting of stock options. For any plans which are so covered and are
adopted by a non-PRC listed company after April 6, 2007, Circular 78 requires
all participants who are PRC citizens to register with and obtain approvals from
SAFE prior to their participation in the plan. In addition, Circular
78 also requires PRC citizens to register with SAFE and make the necessary
applications and filings if they participated in an overseas listed company's
covered equity compensation plan prior to April 6, 2007. We intend to
adopt an equity compensation plan in the future and make option grants to our
officers and directors, most of who are PRC citizens. Circular 78 may
require our officers and directors who receive option grants and are PRC
citizens to register with SAFE. We believe that the registration and approval
requirements contemplated in Circular 78 will be burdensome and time
consuming. If it is determined that any of our equity compensation
plans are subject to Circular 78, failure to comply with such provisions may
subject us and participants of our equity incentive plan who are PRC citizens to
fines and legal sanctions and prevent us from being able to grant equity
compensation to our PRC employees. In that case, our ability to
compensate our employees and directors through equity compensation would be
hindered and our business operations may be adversely affected.
ANY
RECURRENCE OF SEVERE ACUTE RESPIRATORY SYNDROME (SARS), AVIAN FLU, OR ANOTHER
WIDESPREAD PUBLIC HEALTH PROBLEM, IN THE PRC COULD ADVERSELY AFFECT OUR
OPERATIONS.
A renewed outbreak of SARS, Avian Flu
or another widespread public health problem in China, where substantially all of
our businesses are located and where the substantial all of our sales occur,
could have a negative effect on our operations. Our businesses are
dependent upon our ability to continue to efficiently distribute and sell our
products. Such an outbreak could have an impact on our operations as a result
of:
|
·
|
quarantines
or closures of some of our drugstores or the closure of our Logistics
Center, which would severely disrupt our
operations,
|
|
·
|
the
sickness or death of our key officers and employees,
and
|
|
·
|
a
general slowdown in the Chinese
economy.
|
Any of
the foregoing events or other unforeseen consequences of public health problems
could adversely affect our operations.
A
DOWNTURN IN THE ECONOMY OF THE PRC MAY SLOW OUR GROWTH AND
PROFITABILITY.
The growth of the Chinese economy has
been uneven across geographic regions and economic sectors. There can
be no assurance that growth of the Chinese economy will be steady or that any
further downturn will not have a negative effect on our business, especially if
it results in either a decreased use of our products or in pressure on us to
lower our prices.
23
BECAUSE
OUR BUSINESS IS LOCATED IN THE PRC, WE MAY HAVE DIFFICULTY ESTABLISHING ADEQUATE
MANAGEMENT, LEGAL AND FINANCIAL CONTROLS, WHICH ARE REQUIRED IN ORDER TO COMPLY
WITH U.S. SECURITIES LAWS.
PRC companies have historically not
adopted a Western style of management and financial reporting concepts and
practices, which includes strong corporate governance, internal controls and,
computer, financial and other control systems. Most of our middle and
top management staff are not educated and trained in the Western system, and we
may have difficulty hiring new employees in the PRC with such
training. In addition, we may have difficulty in hiring and retaining
a sufficient number of qualified employees to work in the PRC. As a
result of these factors, we may experience difficulty in establishing
management, legal and financial controls, collecting financial data and
preparing financial statements, books of account and corporate records and
instituting business practices that meet Western
standards. Therefore, we may, in turn, experience difficulties in
implementing and maintaining adequate internal controls as required under
Section 404 of the Sarbanes-Oxley Act of 2002. This may result in
significant deficiencies or material weaknesses in our internal controls which
could impact the reliability of its financial statements and prevent us from
complying with SEC rules and regulations and the requirements of the
Sarbanes-Oxley Act of 2002. Any such deficiencies, weaknesses or lack
of compliance could have a materially adverse effect on our
business.
INVESTORS
MAY EXPERIENCE DIFFICULTIES IN EFFECTING SERVICE OF LEGAL PROCESS, ENFORCING
FOREIGN JUDGMENTS OR BRINGING ORIGINAL ACTIONS IN CHINA BASED UPON U.S. LAWS,
INCLUDING THE FEDERAL SECURITIES LAWS OR OTHER FOREIGN LAWS AGAINST US OR OUR
MANAGEMENT.
Most of our current business operations
are conducted in China. Moreover, most of our directors and officers
are nationals and residents of China or Hong Kong. All or
substantially all of the assets of these persons are located outside the United
States and in the PRC. As a result, it may not be possible to effect
service of process within the United States or elsewhere outside China upon
these persons. In addition, uncertainty exists as to whether the PRC
courts would recognize or enforce judgments of U.S. courts obtained against us
or such officers and/or directors predicated upon the civil liability provisions
of the securities laws of the United States or any state thereof, or be
competent to hear original actions brought in China against us or such persons
predicated upon the securities laws of the United States or any state
thereof.
IF
WE ARE FOUND TO BE IN VIOLATION OF CURRENT OR FUTURE PRC LAWS, RULES OR
REGULATIONS REGARDING THE LEGALITY OF FOREIGN INVESTMENT IN THE PRC WITH RESPECT
TO OUR OWNERSHIP STRUCTURE, WE COULD BE SUBJECT TO SEVERE
PENALTIES.
We currently conduct business
operations solely in the PRC through our wholly owned subsidiary,
Yongxin. We are a Delaware corporation and most of our direct and
indirect subsidiaries are companies organized under the laws of the
PRC. We are considered a foreign person or foreign invested
enterprise under PRC law. As a result, we are subject to PRC law
limitations on foreign ownership of Chinese companies. There are
substantial uncertainties regarding the interpretation and application of PRC
laws and regulations, including, but not limited to, the laws and regulations
governing our pharmaceutical distribution and retail drugstore
businesses.
Accordingly, it is possible that the
relevant PRC authorities could, at any time, assert that any portion of our
existing or future ownership structure and businesses violate existing or future
PRC laws, regulations or policies. It is also possible that the new
laws or regulations governing our business operations in the PRC that have been
adopted or may be adopted in the future will prohibit or restrict foreign
investment in, or other aspects of, any of our PRC subsidiaries' and our current
or proposed businesses and operations. The effectiveness of newly
enacted laws, regulations or amendments may be delayed, resulting in detrimental
reliance by foreign investors. New laws and regulations that affect
existing and proposed future businesses may also be applied
retroactively.
The PRC government has broad discretion
in dealing with violations of laws and regulations, including:
|
·
|
levying
fines;
|
|
·
|
confiscating
our income;
|
|
·
|
revoking
business and other licenses;
|
|
·
|
requiring
us to discontinue any portion or all of our
business;
|
|
·
|
requiring
us to restructure our ownership structure or operations;
and
|
24
|
·
|
requiring
actions necessary for compliance.
|
In particular, licenses and permits
issued or granted to us by relevant governmental bodies may be revoked at a
later time by higher regulatory bodies. We cannot predict the effect
of the interpretation of existing or new PRC laws or regulations on our
businesses. We cannot assure you that our current ownership and
operating structure would not be found in violation of any current or future PRC
laws or regulations. As a result, we may be subject to sanctions,
including fines, and could be required to restructure our operations or cease to
provide certain services. Any of these or similar actions could
significantly disrupt our business operations or restrict us from conducting a
substantial portion of our business operations, which, in turn, could materially
and adversely affect our business, financial condition and results of
operations.
WE
MAY BE ADVERSELY AFFECTED BY COMPLEXITY, UNCERTAINTIES AND CHANGES IN PRC
REGULATION OF PHARMACEUTICAL BUSINESSES AND DRUGSTORE COMPANIES, INCLUDING
LIMITATIONS ON OUR ABILITY TO OWN KEY ASSETS.
The PRC government regulates the
pharmaceutical and drugstore industries including foreign ownership of, and the
licensing and permit requirements pertaining to, companies operating in these
industries. These laws and regulations are relatively new and
evolving, and their interpretation and enforcement involve significant
uncertainty. As a result, in certain circumstances it may be
difficult to determine what actions or omissions may be deemed to be a violation
of applicable laws and regulations. Issues, risks and uncertainties
relating to PRC government regulation of the pharmaceutical industry include
those relating evolving licensing practices. Permits, licenses or operations at
our company may be subject to challenge, which may disrupt our business, or
subject us to sanctions, requirements to increase capital or other conditions or
enforcement, or compromise enforceability of related contractual arrangements,
or have other harmful effects on us. Although we believe we comply
with current PRC regulations, we cannot assure you that our ownership and
operating structure comply with PRC licensing, registration or other regulatory
requirements, with existing policies or with requirements or policies that may
be adopted in the future. If the PRC government determines that we do
not comply with applicable law, it could take other regulatory or enforcement
actions against us that could be harmful to our business.
RISKS
RELATED TO OUR CAPITAL STRUCTURE
IF THE PRC REGULATORY AUTHORITIES DETERMINE THAT OUR CURRENT CORPORATE STRUCTURE FOR OWNERSHIP AND OPERATION
OF OUR RETAIL DRUGSTORE
BUSINESS IN CHINA DO NOT COMPLY WITH PRC REGULATORY RESTRICTIONS ON FOREIGN
INVESTMENT IN THE DRUGSTORE INDUSTRY, WE COULD BE SUBJECT TO SEVERE
PENALTIES, OR MAY BE
REQUIRED TO UNDERTAKE A COSTLY AND/OR TIME-CONSUMING CORPORATE
RESTRUCTURING.
Under
current PRC law on foreign investment, foreign companies are allowed to
establish or invest in wholly-owned foreign enterprises or joint ventures that
engage in wholesale or retail sales of pharmaceuticals in
China. These regulations limit the number and size of retail pharmacy
outlets that a foreign investor may establish. If a foreign investor
owns more than 30 outlets that sell a variety of branded pharmaceutical products
sourced from different suppliers, the foreign investor's ownership interests in
the outlets may be limited to 49.0%. We currently control more than
30 retail pharmacy outlets through: (1) our 80% equity ownership interest in
Yongxin, which directly owns 21 outlets; (2) Yongxin’s 100% equity ownership in
Caoantang Drugstore, which, in turn, owns 32 outlets; and (3) Yongxin’s 90%
equity ownership interest in Jinyongxin Drugstore, which, in turn, owns 26
outlets. At the time of the establishment of Yongxin, our PRC equity
joint venture entity, in May 2007, Yongxin already controlled in excess of 30
retail pharmacy outlets and with this structure in place we obtained all
required approvals from the relevant governmental agencies in Jilin Province for
the joint venture. We have also been advised by our PRC counsel,
Jilin Zhong Yan Law Firm, that based on their understanding of the current PRC
laws, rules and regulations and the Company’s receipt of all relevant government
approvals for the equity joint venture, the structure under which we operate and
hold equity ownership in our retail pharmacy businesses (including our corporate
structure, our direct equity ownership interest in Yongxin, our indirect equity
ownership in Yongxin’s subsidiaries, Jinyongxin Drugstore and Caoantang
Drugstore, and the 79 retail pharmacy outlets that we control through this
structure) complies with all applicable PRC laws, rules and
regulations. However, there are uncertainties regarding the
interpretation and application of PRC laws, rules and regulations by PRC
government authorities, and we cannot assure you that such authorities will not
later issue a differing interpretation of the law and determine that our
corporate and/or ownership structure does not comply with PRC laws, rules and
regulations. In addition, new PRC laws, rules and regulations may be
introduced from time to time to impose additional requirements that may be
applicable to our corporate structure or our business operations. If
we and/or our PRC subsidiaries are determined to be in violation of any existing
or future PRC laws, rules or regulations, including laws applicable to foreign
investment in retail pharmacy outlets, or fail to obtain or maintain any of the
required governmental permits or approvals, the relevant PRC regulatory
authorities would have broad discretion in dealing with such violations,
including:
|
|
•
|
revoking
the business and operating licenses of our PRC consolidated
entities;
|
|
|
•
|
discontinuing
or restricting the operations of our PRC consolidated
entities;
|
|
|
•
|
imposing
conditions or requirements with which we or our PRC consolidated entities
may not be able to comply;
|
|
|
•
|
requiring
us or our PRC consolidated entities to restructure the relevant ownership
structure or operations;
|
|
|
•
|
restricting
or prohibiting our use of the proceeds from our financings to fund our
business and operations in
China; or
|
|
|
•
|
imposing
fines.
|
The
imposition of any of the above-described penalties and/or a restructuring of our
holding structure in order to comply with relevant PRC regulations could
severely disrupt our ability to conduct business and could have a material
adverse effect on our financial condition, results of operations and
prospects.
THERE
IS CURRENTLY A LIMITED TRADING MARKET FOR OUR COMMON STOCK, AND THERE IS NO
ASSURANCE OF A MORE ESTABLISHED PUBLIC TRADING MARKET, WHICH WOULD ADVERSELY
AFFECT THE ABILITY OF OUR INVESTORS TO SELL THEIR SECURITIES IN THE PUBLIC
MARKET.
While our common stock is currently
listed on the Over-the-Counter Bulletin Board ("OTCBB"), there is currently a
limited trading market for our common stock. The Financial Industry
Regulatory Authority has enacted changes that limit quotations on the OTCBB to
securities of issuers that are current in their reports filed with the
Securities and Exchange Commission. The effect on the OTCBB of these
rule changes and other proposed changes cannot be determined at this time. The
OTCBB is an inter-dealer, over-the-counter market that provides significantly
less liquidity than the NASDAQ Global Market (the "NASDAQ Global Market").
Quotes for stocks included on the OTCBB are not listed in the financial sections
of newspapers as are those for the NASDAQ Global Market. Therefore,
prices for securities traded solely on the OTCBB may be difficult to obtain and
holders of common stock may be unable to resell their securities at or near
their original offering price or at any price.
The market price for our common stock
is influenced by a number of factors, including:
|
|
·
|
our
ability to obtain additional financing and, if available, the terms and
conditions of the financing;
|
|
|
·
|
our
financial position and results of
operations;
|
|
|
·
|
concern
as to, or other evidence of, the reliability and safety of our products
and services or our competitors' products and
services;
|
|
|
·
|
announcements
of innovations or new products or services by us or our
competitors;
|
25
|
|
·
|
U.S.
federal and state governmental regulatory actions and the impact of such
requirements on our business;
|
|
|
·
|
Chinese
governmental regulatory actions and the impact of such requirements on our
business;
|
|
|
·
|
the
development of litigation against
us;
|
|
|
·
|
period-to-period
fluctuations in our operating
results;
|
|
|
·
|
changes
in estimates of our performance by any securities
analysts;
|
|
|
·
|
the
issuance of new equity securities pursuant to a future offering or
acquisition;
|
|
|
·
|
changes
in interest rates and/or foreign currency exchange
rates;
|
|
|
·
|
competitive
developments, including announcements by competitors of new products or
services or significant contracts, acquisitions, strategic partnerships,
joint ventures or capital
commitments;
|
|
|
·
|
investor
perceptions of us; and
|
|
|
·
|
general
economic and other national and international
conditions.
|
SHARES
ELIGIBLE FOR FUTURE SALE MAY ADVERSELY AFFECT THE MARKET PRICE OF OUR COMMON
STOCK, AS THE FUTURE SALE OF A SUBSTANTIAL AMOUNT OF OUTSTANDING STOCK IN THE
PUBLIC MARKETPLACE COULD REDUCE THE PRICE OF OUR COMMON STOCK.
We may file a registration statement to
register the shares issued to the Yongxin stockholders pursuant to the Share
Exchange Transaction. All of the shares included in an effective
registration statement as described above may be freely sold and transferred
except if subject to a lock up agreement.
In addition, the stockholders who
received shares of our common stock in the Share Exchange Transaction and/or
their designees may be eligible to sell all or some of our shares of common
stock by means of ordinary brokerage transactions in the open market pursuant to
Rule 144, promulgated under the Securities Act ("Rule 144"), subject to certain
limitations. In general, pursuant to Rule 144, a non-affiliate
stockholder (or stockholders whose shares are aggregated) who has satisfied a
six-month holding period, and provided that there is current public information
available, may sell all of its securities. Rule 144 also permits the
sale of securities, without any limitations, by a non-affiliate that has
satisfied a one-year holding period. Any substantial sale of common stock
pursuant to any resale prospectus or Rule 144 may have an adverse effect on the
market price of our common stock by creating an excessive supply.
THE
COMPANY MAY NOT BE ABLE TO EXERCISE ABSOLUTE CONTROL OVER
YONGXIN.
The
Company may not be able to exercise absolute control over Yongxin, because it is
not a 100% equityholder of Yongxin. The minority shareholders,
Yongxin Liu and Yonkui Liu, may hold certain minority rights under PRC corporate
law and pursuant to the equity joint venture agreement, with respect to the
control and governance of Yongxin. Specifically,
Yongxin Liu and Yongkui Liu have the right to jointly appoint a director and the
Company has the right to appoint two directors. Also,
under the equity joint venture agreement, Mr. Yongxin Liu has a contractual
right to appoint the General Manager of Yongxin.
OUR
CHIEF EXECUTIVE OFFICER AND CERTAIN RELATED COMPANY OFFICERS AND STOCKHOLDERS
HAVE SIGNIFICANT INFLUENCE OVER OUR COMPANYAND OUR CORPORATE
ACTIONS.
Our
current CEO and Chairman of the Board, Mr. Yongxin Liu and Company’s Vice
President Mr. Yongkui Liu (our CEO’s brother) and Ms. Yongmei Wang (spouse of
Yongkui Liu) collectively own and control a majority of our outstanding voting
capital shares as of December 31, 2009. If these officers and/or stockholders
were to act as a group, they would have a controlling influence in determining
the outcome of any corporate transaction or other matters submitted to our
stockholders for approval, including mergers, consolidations and the sale of all
or substantially all of our assets, election of directors, and other significant
corporate actions. Such officers and/or stockholders may also have the power to
prevent or cause a change in control. In addition, without the consent of these
stockholders, we could be prevented from entering into transactions that could
be beneficial to us. The interests of these stockholders may give rise to a
conflict of interest with the Company and the Company’s
shareholders. While the Company is not aware of any present situation
which involves a conflict of interest, in the future, a conflict of interest may
arise from the fact that Yongxin Liu and Yongkui Liu are executive officers of
the Company, and are also minority equityholders of Yongxin. If there
should ever be a divergence of interest of the minority equityholders on the one
hand, and the Company on the other hand, Mr. Yongxin Liu and Yongkui Liu would
have incentives to act to protect their minority interests in Yongxin vis-à-vis
the majority controlling interest of the Company.
THE
COMPANY WILL NOT RECEIVE 100% OF THE ECONOMIC BENEFIT OF THE INCOME AND ASSETS
OF YONGXIN.
Although
the Company consolidates the financial results of Yongxin and its subsidiaries
for financial reporting purposes, the Company will not receive 100% of the
economic benefit of the income and assets of Yongxin, and in most cases the
Company will receive only 80% of such economic benefit. For example,
if Yongxin were to distribute accumulated earnings or assets, 20% of such
distributed assets would be paid to the minority equity holders, and 80% would
be paid to the Company. For financial reporting purposes, the Company
also accounts for the 20% interest as a non-controlling interest, which has the
effect of lowering reported earnings per share (as compared to a scenario in
which the Company owns 100% of Yongxin).
26
IF
WE FAIL TO MAINTAIN EFFECTIVE INTERNAL CONTROLS OVER FINANCIAL REPORTING, THE
PRICE OF OUR COMMON STOCK MAY BE ADVERSELY AFFECTED.
We are required to establish and
maintain appropriate internal controls over financial
reporting. Failure to establish those controls, or any failure of
those controls once established, could adversely impact our public disclosures
regarding our business, financial condition or results of
operations. Any failure of these controls could also prevent us from
maintaining accurate accounting records and discovering accounting errors and
financial frauds. Rules adopted by the SEC pursuant to Section 404 of
the Sarbanes-Oxley Act of 2002 require annual assessment of our internal control
over financial reporting, and attestation of this assessment by our independent
registered public accountants. The SEC extended the compliance dates for
non-accelerated filers, as defined by the SEC. Accordingly, we
believe that the annual assessment of our internal controls requirement has
first applied to our annual report for the 2007 fiscal year and the attestation
requirement of management's assessment by our independent registered public
accountants will be first applied to our annual report for the 2010 fiscal
year. The standards that must be met for management to assess the
internal control over financial reporting as effective are new and complex, and
require significant documentation, testing and possible remediation to meet the
detailed standards. We may encounter problems or delays in completing
activities necessary to make an assessment of our internal control over
financial reporting. In addition, the attestation process by our
independent registered public accountants is new and we may encounter problems
or delays in completing the implementation of any requested improvements and
receiving an attestation of our assessment by our independent registered public
accountants. If we cannot assess our internal control over financial reporting
as effective, or our independent registered public accountants are unable to
provide an unqualified attestation report on such assessment, investor
confidence and share value may be negatively impacted.
In addition, management's assessment of
internal controls over financial reporting may identify weaknesses and
conditions that need to be addressed in our internal controls over financial
reporting or other matters that may raise concerns for investors. Any
actual or perceived weaknesses and conditions that need to be addressed in our
internal control over financial reporting, disclosure of management's assessment
of our internal controls over financial reporting, or disclosure of our public
accounting firm's attestation to or report on management's assessment of our
internal controls over financial reporting may have an adverse impact on the
price of our common stock.
THE
FOREIGN CURRENCY EXCHANGE RATE BETWEEN U.S. DOLLARS AND RENMINBI COULD ADVERSELY
AFFECT OUR FINANCIAL CONDITION.
To the extent that we need to convert
U.S. Dollars into Renminbi for our operational needs, our financial position and
the price of our common stock may be adversely affected should the Renminbi
appreciate against the U.S. Dollar at that time. Conversely, if we
decide to convert our Renminbi into U.S. Dollars for our operational needs or
paying dividends on our common stock, the U.S. Dollar equivalent of our earnings
from our subsidiaries in China would be reduced should the U.S. Dollar
appreciate against the Renminbi.
Until 1994, the Renminbi experienced a
gradual but significant devaluation against most major currencies, including
dollars, and there was a significant devaluation of the Renminbi on January 1,
1994 in connection with the replacement of the dual exchange rate system with a
unified managed floating rate foreign exchange system. Since 1994,
the value of the Renminbi relative to the U.S. Dollar has remained stable and
has appreciated slightly against the U.S. Dollar. Countries, including the
United States, have argued that the Renminbi is artificially undervalued due to
China's current monetary policies and have pressured China to allow the Renminbi
to float freely in world markets. In July 2005, the PRC government
changed its policy of pegging the value of the Renminbi to the
dollar. Under the new policy the Renminbi is permitted to fluctuate
within a narrow and managed band against a basket of designated foreign
currencies. While the international reaction to the Renminbi revaluation has
generally been positive, there remains significant international pressure on the
PRC government to adopt an even more flexible currency policy, which could
result in further and more significant appreciation of the Renminbi against the
dollar.
COMPLIANCE
WITH CHANGING REGULATION OF CORPORATE GOVERNANCE AND PUBLIC DISCLOSURE WILL
RESULT IN ADDITIONAL EXPENSES.
Changing laws, regulations and
standards relating to corporate governance and public disclosure, including the
Sarbanes-Oxley Act of 2002 and related SEC regulations, have created uncertainty
for public companies and significantly increased the costs and risks associated
with accessing the public markets and public reporting. Our
management team will need to invest significant management time and financial
resources to comply with both existing and evolving standards for public
companies, which will lead to increased general and administrative expenses and
a diversion of management time and attention from revenue generating activities
to compliance activities.
27
OUR
COMMON STOCK IS CONSIDERED A "PENNY STOCK," AND THEREBY IS SUBJECT TO ADDITIONAL
SALE AND TRADING REGULATIONS THAT MAY MAKE IT MORE DIFFICULT TO
SELL.
Our common stock is currently
considered to be a "penny stock" because it does not qualify for one of the
exemptions from the definition of "penny stock" under Section 3a51-1 of the
Securities Exchange Act for 1934, as amended (the "Exchange Act"). Our common
stock is considered a "penny stock" because it meets one or more of the
following conditions (i) the stock trades at a price less than $5.00 per share;
(ii) it is not traded on a "recognized" national exchange; (iii) it
is not quoted on the NASDAQ Capital Market, or even if so, has a price less
than $5.00 per share; or (iv) is issued by a company that has been in business
less than three years with net tangible assets less than $5
million.
The principal result or effect of being
designated a "penny stock" is that securities broker-dealers participating in
sales of our common stock will be subject to the "penny stock" regulations set
forth in Rules 15-2 through 15g-9 promulgated under the Exchange
Act. For example, Rule 15g-2 requires broker-dealers dealing in penny
stocks to provide potential investors with a document disclosing the risks of
penny stocks and to obtain a manually signed and dated written receipt of the
document at least two business days before effecting any transaction in a penny
stock for the investor's account. Moreover, Rule 15g-9 requires
broker-dealers in penny stocks to approve the account of any investor for
transactions in such stocks before selling any penny stock to that investor.
This procedure requires the broker-dealer to (i) obtain from the investor
information concerning his or her financial situation, investment experience and
investment objectives; (ii) reasonably determine, based on that information,
that transactions in penny stocks are suitable for the investor and that the
investor has sufficient knowledge and experience as to be reasonably capable of
evaluating the risks of penny stock transactions; (iii) provide the investor
with a written statement setting forth the basis on which the broker-dealer made
the determination in (ii) above; and (iv) receive a signed and dated copy of
such statement from the investor, confirming that it accurately reflects the
investor's financial situation, investment experience and investment
objectives. Compliance with these requirements may make it more
difficult and time consuming for holders of our common stock to resell their
shares to third parties or to otherwise dispose of them in the market or
otherwise.
WE
DO NOT FORESEE PAYING CASH DIVIDENDS IN THE FORESEEABLE FUTURE AND, AS A RESULT,
OUR INVESTORS' SOLE SOURCE OF GAIN, IF ANY, WILL DEPEND ON CAPITAL APPRECIATION,
IF ANY.
We do not plan to declare or pay any
cash dividends on our shares of common stock in the foreseeable future and
currently intend to retain any future earnings for funding growth. As
a result, investors should not rely on an investment in our securities if they
require the investment to produce dividend income. Capital
appreciation, if any, of our shares may be investors' sole source of gain for
the foreseeable future. Moreover, investors may not be able to resell
their shares of our common stock at or above the price they paid for
them.
ITEM
1B. UNRESOLVED STAFF COMMENTS
None.
ITEM
2. PROPERTIES
Our corporate headquarters are
located in the City of Industry, California at 927 Canada Court, City of
Industry, California 91748. We also lease office space in the in the Jilin
Province under a lease that expires on June 30, 2020.
We
currently operate one national distribution center, the Logistics Center, which
is located in Changchun that provides us with 43,000 square meters of storage
space. We lease the Logistics Center under a long-term lease
agreement that expires in 2020 and do not anticipate any material difficulties
in renewing our lease upon its expiration.
28
Currently,
all of our retail drugstores are located in the PRC. We lease substantially all
of our store locations from various third-parties under a total of 79 leases
with terms ranging from month-to-month to 5 years, which are renewed upon
expiration. The total combined space for our retail stores consisted of 9,834
square meters. We must negotiate with the landlords to extend our
leases or enter into new leases upon their expiration, at which time the
landlords may request a rent increase. Under applicable PRC law, we have
priority over other potential lessees with respect to the leased store space on
the same terms. Our retail stores are relatively small in size and are generally
easily movable to new locations. We do not expect our drugstore operations to be
adversely affected by any failure to renew or enter into new
leases.
ITEM
3. LEGAL PROCEEDINGS
On
or about October 17, 2008, in the legal proceeding titled Craig
Nagasugi v. Digital Learning Management Corporation, et al., a former officer initiated
an action in Los Angeles Superior Court, Central District, against the Company
alleging claims for damages related to an alleged employment
agreement. On December 29, 2008, the Company filed an Answer to the
Complaint. The Company strongly disputed the claims and diligently
defended against them. The matter went to trial on or about November 2, 2009 and
concluded as of November 9, 2009. The case resulted in a judgment
against the Company for $641,018. The
Company anticipates that the court will enter a revised judgment in the amount
of $746,000 against the Company in the second quarter of
2010. So far,
the judgment has not been paid.
Under
Allaudin
Jinnah v. China Yongxin Pharmaceuticals, Inc., filed in Los Angeles
Superior Court, Central District, on or about June 27, 2008, the Company
defended itself against claims for open account and intentional
misrepresentation. The Plaintiff sought past due attorneys’ fees for
services rendered in the amount of $193,100. The Plaintiff also
sought 67,000 shares of the Company’s common stock. The Plaintiff filed a motion
to enforce the Company’s settlement to receive up to $50,000 judgment and
200,000 to 400,000 shares of the Company’s common stock. At the hearing to
enforce the settlement, the court entered judgment against the Company for
$50,000 plus 200,000 shares of the Company’s common stock. The court
ordered the Company to issue an additional 200,000 shares of the Company’s
common stock as collateral for the $50,000. The said judgment was
satisfied in full by the Company in February 2010.
The
Company was also involved in a legal proceeding called Wells
Fargo Bank. N.A.. v. Software Education for America Inc. filed in Orange
County Superior Court on or about November 9, 2004. In this action,
the Cross-Complainant, Terry Koosed, sought to amend a $219,000 judgment he
obtained to include a subsidiary of the predecessor-in-interest of the Company,
which was not named or a participant in such lawsuit. On May 8, 2009,
the Orange County Superior Court rendered a decision to enter a judgment of
$219,000 against the Company. This judgment was satisfied in full by the
Company in February 2010.
Under
Adnan
Mann v. China Yongxin Pharmaceuticals, Inc., on or about March 10,
2009, a former employee filed claims for unpaid wages and penalties under
the California Labor Code and applicable Industrial Wage Orders. On July
10, 2009, the Company filed an Answer to the Complaint denying liability.
This matter is presently in the discovery stage and trial has been set for May
10, 2010.
PART
II
ITEM
5. MARKET FOR REGISTRANT’S COMMON STOCK, RELATED STOCKHOLDER MATTERS
AND ISSUER PURCHASES OF EQUITY SECURITIES
Our
common stock is currently quoted on the OTC Bulletin Board under the symbol
“CYXN.” The following table sets forth the high and low bid
information for the common stock for each quarter within the last two fiscal
years, as reported by the OTC Bulletin Board. The bid prices reflect
inter-dealer quotations, do not include retail markups, markdowns, or
commissions, and do not necessarily reflect actual
transactions.
29
|
Low
|
High
|
|||||||
|
2009
|
||||||||
|
Quarter
ended December 31, 2009
|
$
|
0.29
|
$
|
0.80
|
||||
|
Quarter
ended September 30, 2009
|
0.14
|
0.79
|
||||||
|
Quarter
ended June 30, 2009
|
0.11
|
0.32
|
||||||
|
Quarter
ended March 31, 2009
|
0.06
|
0.38
|
||||||
|
2008
|
||||||||
|
Quarter
ended December 31, 2008
|
$
|
0.05
|
$
|
0.59
|
||||
|
Quarter
ended September 30, 2008
|
0.41
|
1.40
|
||||||
|
Quarter
ended June 30, 2008
|
0.40
|
1.53
|
||||||
|
Quarter
ended March 31, 2008
|
0.93
|
1.72
|
||||||
The last
reported closing sales price for shares of our common stock was $0.61 per share
on the OTCBB on March 10, 2010. As of March 10, 2010, our stock was
held by approximately 211 stockholders. The price of our common stock
will likely fluctuate in the future. The stock market in general has experienced
extreme stock price fluctuations in the past few years. In some cases, these
fluctuations have been unrelated to the operating performance of the affected
companies. Many companies have experienced dramatic volatility in the market
prices of their common stock. We believe that a number of factors, both within
and outside our control, could cause the price of our common stock to fluctuate,
perhaps substantially. Factors such as the following could have a
significant adverse impact on the market price of our common stock:
|
|
·
|
our ability to obtain additional
financing and, if available, the terms and conditions of the
financing;
|
|
|
·
|
our financial position and
results of operations;
|
|
|
·
|
concern as to, or other evidence
of, the reliability and safety of our products and services or our
competitors’ products and
services;
|
|
|
·
|
announcements of innovations or
new products or services by us or our
competitors;
|
|
|
·
|
U.S. federal and state
governmental regulatory actions and the impact of such requirements on our
business;
|
|
|
·
|
Chinese governmental regulatory
actions and the impact of such requirements on our
business;
|
|
|
·
|
the outcome of legal claims
against us;
|
|
|
·
|
period-to-period fluctuations in
our operating results;
|
|
|
·
|
changes in estimates of our
performance by any securities
analysts;
|
|
|
·
|
the issuance of new equity
securities pursuant to a future offering or
acquisition;
|
|
|
·
|
changes in applicable interest
rates;
|
|
|
·
|
competitive developments,
including announcements by competitors of new products or services or
significant contracts, acquisitions, strategic partnerships, joint
ventures or capital
commitments;
|
|
|
·
|
investor perceptions of us;
and
|
|
|
·
|
general economic and other
national conditions.
|
DIVIDEND
POLICY
We do not
expect to declare or pay any cash dividends on our common stock in the
foreseeable future, and we currently intend to retain future earnings, if any,
to finance the expansion of our business. The decision whether to pay cash
dividends on our common stock will be made by our board of directors, in its
discretion, and will depend on our financial condition, operating results,
capital requirements and other factors that the board of directors considers
significant. We did not pay any cash dividends during the years ended
December 31, 2009 or 2008.
Under
applicable PRC regulations, foreign-invested enterprises in China may pay
dividends only out of their accumulated profits, if any, determined in
accordance with PRC accounting standards and regulations. In
addition, a foreign-invested enterprise in China are required to set aside at
least 10.0% of their after-tax profit based on PRC accounting standards each
year to its general reserves until the accumulative amount of such reserves
reach 50.0% of its registered capital. These reserves are not
distributable as cash dividends. The board of directors of a
foreign-invested enterprise has the discretion to allocate a portion of its
after-tax profits to staff welfare and bonus funds, which may not be distributed
to equity owners except in the event of liquidation.
30
RECENT
SALES OF UNREGISTERED SECURITIES
The
following is a summary of our transactions during the last three years involving
sales of our securities that were not registered under the Securities Act of
1933, as amended (the “Securities Act”) that have not been included in our
quarterly reports on Form 10-Q or in our current reports on Form
8-K:
On March
4, 2010, the Company consummated a private placement of its equity securities
with certain accredited investors pursuant to a Subscription Agreement for total
consideration of $125,000. The Company issued to the investors
secured convertible notes with a two year term, bearing 10% interest per annum,
convertible into common stock of the Company at a conversion price of $0.20 per
share, which is subject to adjustment for stock splits, recapitalizations and
other similar events, and will also be adjusted on a full-ratchet basis to equal
the price per share of any subsequent financing. The notes are
secured by a first priority interest in all current and future assets of the
Company, which will be cancelled upon repayment of the notes or upon conversion
of at least 50% of the principal amount of the notes into shares of Company
common stock. The notes may be redeemed by the Company at any time
for 110% of outstanding principal and interest. The note investors
also received, as a part of the financing, warrants for the purchase of up to
625,000 shares of our common stock with an exercise price $0.50 per share
(subject to adjustment for stock splits, recapitalizations and other similar
events) exercisable for a period of three years. The issuance of
these securities was exempt from registration pursuant to Rule 506 of Regulation
D promulgated under the Securities Act of 1933.
ITEM
6. SELECTED CONSOLIDATED FINANCIAL DATA
Not
applicable.
ITEM
7. MANAGEMENT'S DISCUSSION AND ANALYSIS AND RESULTS OF OPERATIONS
The
following discussion should be read in conjunction with our consolidated
financial statements and related notes included elsewhere in this
report. This report contains forward-looking
statements. The words “anticipated,” “believe,” “expect,” “plan,”
“intend,” “seek,” “estimate,” “project,” “could,” “may,” and similar expressions
are intended to identify forward-looking statements. These statements
include, among others, information regarding future operations, future capital
expenditures, and future net cash flow. Such statements reflect our
management’s current views with respect to future events and financial
performance and involve risks and uncertainties, including, without limitation,
general economic and business conditions, changes in foreign, political, social,
and economic conditions, regulatory initiatives and compliance with government
regulations, the ability to achieve further market penetration and additional
customer, and various other matters, any of which are beyond our
control. Should one or more of these risks and uncertainties occur,
or should underlying assumptions prove to be incorrect, actual results may vary
materially and adversely from those anticipated, believed, estimated or
otherwise indicated. Consequently, all of the forward-looking
statements made in this report are qualified by these cautionary statements and
there can be no assurance of the actual results or developments.
GENERAL
OVERVIEW
Our Company is engaged in the wholesale
distribution of pharmaceuticals and medical-related products, and the sale and
distribution of pharmaceuticals, beauty products, herbal and nutritional
supplements, and other health and medical-related products through retail
operations in the People’s Republic of China (“PRC” or “China”). The
products we sell include Western and Chinese traditional medicines,
pharmaceutical preparations, natural health products, health foods, cosmetics
and medical equipment. Our PRC operations began retail operations in
2004, and in 2005, we gained franchise rights from one of the world's largest
drug chains for China's Jilin Province. By the end of 2007, the
Company had become one of the fastest growing pharmaceutical companies in China
through our retail chain of drugstores as well as our wholesale distribution
operations in Northeastern China. Our corporate headquarters are
located in City of Industry, California, but the Company’s distribution
operations are based in Changchun City, Jilin Province, China, and our retail
drugstores are located throughout Jilin Province in Northeastern
China.
31
CRITICAL
ACCOUNTING POLICIES AND ESTIMATES
The SEC
defines critical accounting policies as those that are, in management's view,
most important to the portrayal of our financial condition and results of
operations and those that require significant judgments and
estimates. The preparation of these consolidated financial statements
requires our management to make estimates and assumptions that affect the
reported amounts of assets, liabilities, revenues and expenses, as well as the
disclosure of contingent assets and liabilities at the date of our financial
statements. We base our estimates on historical experience, actuarial
valuations and various other factors that we believe to be reasonable under the
circumstances, the results of which form the basis for making judgments about
the carrying value of assets and liabilities that are not readily apparent from
other sources. Some of those judgments can be subjective and complex
and, consequently, actual results may differ from these estimates under
different assumptions or conditions. While for any given estimate or assumption
made by our management there may be other estimates or assumptions that are
reasonable, we believe that, given the current facts and circumstances, it is
unlikely that applying any such other reasonable estimate or assumption would
materially impact the financial statements. The accounting principles
we utilized in preparing our consolidated financial statements conform in all
material respects to generally accepted accounting principles in the United
States of America. While our significant accounting policies are more
fully described in Note 2 to our consolidated financial statements, we
believe that the following accounting policies are the most critical to aid the
reader in fully understanding and evaluating this discussion and
analysis:
BASIS
OF PRESENTATION
The
accompanying financial statements have been prepared in conformity with
accounting principles generally accepted in the United States of America.
The functional currency of our operating subsidiary, Changchun Yongxin Dirui
Medical Co., Ltd is Chinese Renminbi (CNY); however the accompanying financial
statements have been translated and presented in United States Dollars
(USD).
TRANSLATION
ADJUSTMENT
As of
December 31, 2009 and 2008, the accounts of Yongxin were maintained, and its
financial statements were expressed, in CNY. Such financial statements were
translated into USD in accordance with Statement of Financial Accounts Standards
(“SFAS”) No. 52, “Foreign Currency Translation,” with the CNY as the functional
currency. According to the Statement, all assets and liabilities were translated
at the current exchange rate, stockholders’ equity are translated at the
historical rates and income statement items are translated at the average
exchange rate for the period. The resulting translation adjustments are reported
under other comprehensive income in accordance with SFAS No. 130 (ASC 220),
“Reporting Comprehensive Income” as a component of stockholders’
equity.
NON-CONTROLLING
INTEREST
The
accompanying consolidated financial statements have been prepared by the
Company, pursuant to the rules and regulations of the Securities and Exchange
Commission (the “SEC”) and in accordance with GAAP. The Company acquired 80% of
Yongxin, and Yongxin Liu and Yongkui Liu own 11% and 9% of Yongxin,
respectively. The 20% equity interest held by Yongxin Liu and Yongkui
Liu represents non-controlling interest amounting to $5,687,633 as at December
31, 2009 compared to $4,078,654 as at December 31, 2008.
The
Company owns a 90% ownership interest in Jinyongxin Drugstore. The remaining 10%
interest in Jinyongxin Drugstore is owned by third
parties.
ACCOUNTS
RECEIVABLE
The
Company maintains reserves for potential credit losses on accounts receivable.
Management reviews the composition of accounts receivable and analyzes
historical bad debts, customer concentrations, customer credit worthiness,
current economic trends and changes in customer payment patterns to evaluate the
adequacy of these reserves. Reserves are recorded primarily on a specific
identification basis. As of December 31, 2009, we made no allowance for doubtful
accounts. As of December 31, 2008, we made allowance for doubtful debts of
$112,452.
32
INVENTORIES
Inventories
are valued on a lower of weighted average cost or market basis.
Inventory includes product cost, inbound freight, warehousing costs and vendor
allowances not included as a reduction of advertising expense. The management
compares the cost of inventories with the market value and allowance is made for
writing down their inventories to market value, if lower. Work in process
inventories include the cost of raw materials and outsource processing
fees.
IMPAIRMENT
OF LONG-LIVED ASSETS
Effective
January 1, 2002, the Company adopted Statement of Financial Accounting Standards
No. 144, “Accounting for the Impairment or Disposal of Long-Lived Assets” (“SFAS
144”) (ASC 360), which addresses financial accounting and reporting for the
impairment or disposal of long-lived assets and supersedes SFAS No. 121,
“Accounting for the Impairment of Long-Lived Assets and for Long-Lived Assets to
be Disposed Of,” and the accounting and reporting provisions of APB Opinion No.
30, “Reporting the Results of Operations for a Disposal of a Segment of a
Business.” The Company periodically evaluates the carrying value of long-lived
assets to be held and used in accordance with SFAS 144 (ASC 360). SFAS 144 (ASC
360) requires impairment losses to be recorded on long-lived assets used in
operations when indicators of impairment are present and the undiscounted cash
flows estimated to be generated by those assets are less than the assets’
carrying amounts. In that event, a loss is recognized based on the amount by
which the carrying amount exceeds the fair market value of the long-lived
assets. Loss on long-lived assets to be disposed of is determined in a similar
manner, except that fair market values are reduced for the cost of
disposal.
REVENUE
RECOGNITION
The
Company’s revenue recognition policies are in compliance with Staff Accounting
Bulletin (“SAB”) 104 (ASC 605). Sales revenue is recognized at the date of
shipment to customers when a formal arrangement exists, the price is fixed or
determinable, the delivery is completed, no other significant obligations of the
Company exist and collectability is reasonably assured. The Company recognizes
revenue net of an allowance for estimated returns, at the time the merchandise
is sold or services performed. The allowance for sales returns is estimated
based on the Company’s historical experience. Sales taxes are presented on a net
basis (excluded from revenues and costs). Payments received before all of the
relevant criteria for revenue recognition are satisfied are recorded as unearned
revenue.
INCOME
TAXES
The
Company utilizes SFAS No. 109 (ASC 740), "Accounting for Income Taxes," which
requires the recognition of deferred tax assets and liabilities for the expected
future tax consequences of events that have been included in the financial
statements or tax returns. Under this method, deferred income taxes are
recognized for the tax consequences in future years of differences between the
tax basis of assets and liabilities and their financial reporting amounts at
each period end based on enacted tax laws and statutory tax rates applicable to
the periods in which the differences are expected to affect taxable income.
Valuation allowances are established, when necessary, to reduce deferred tax
assets to the amount expected to be realized.
The
Company accounts for income taxes using an asset and liability approach which
allows for the recognition and measurement of deferred tax assets based upon the
likelihood of realization of tax benefits in future years. Under the asset and
liability approach, deferred taxes are provided for the net tax effects of
temporary differences between the carrying amounts of assets and liabilities for
financial reporting purposes and the amounts used for income tax purposes. A
valuation allowance is provided for deferred tax assets if it is more likely
than not these items will either expire before the Company is able to realize
their benefits, or that future deductibility is uncertain.
The
Company records a valuation allowance for deferred tax assets, if any, based on
its estimates of its future taxable income as well as its tax planning
strategies when it is more likely than not that a portion or all of its deferred
tax assets will not be realized. If the Company is able to utilize more of its
deferred tax assets than the net amount previously recorded when unanticipated
events occur, an adjustment to deferred tax assets would increase the Company
net income when those events occur. The Company does not have any significant
deferred tax asset or liabilities in the PRC tax jurisdiction.
33
STOCK
BASED COMPENSATION
Effective
January 1, 2006, the Company adopted Statement No. 123R (ASC 718), Share-Based
Payment (SFAS 123R), which requires companies to measure and recognize
compensation expense for all stock-based payments at fair value. SFAS 123R is
being applied on the modified prospective basis. Prior to the adoption of SFAS
123R, the Company accounted for its stock-based compensation plans under the
recognition and measurement principles of Accounting Principles Board (APB)
Opinion No. 25, Accounting for Stock Issued to Employees, and related
interpretations, and accordingly, recognized no compensation expense related to
the stock-based plans. Under the modified prospective approach, SFAS 123R
applies to new awards and to awards that were outstanding on January 1, 2006
that are subsequently modified, repurchased or cancelled.
STATEMENT
OF CASH FLOWS
In
accordance with Statement of Financial Accounting Standards No. 95 (ASC 230),
"Statement of Cash Flows," cash flows from the Company's operations is
calculated based upon the local currencies. As a result, amounts related to
assets and liabilities reported on the statement of cash flows will not
necessarily agree with changes in the corresponding balances on the balance
sheet.
SEGMENT
REPORTING
Statement
of Financial Accounting Standards No. 131 ("SFAS 131") (ASC 250), "Disclosure
about Segments of an Enterprise and Related Information" requires use of the
"management approach" model for segment reporting. The management approach
model is based on the way a company's management organizes segments within the
company for making operating decisions and assessing performance. Reportable
segments are based on products and services, geography, legal structure,
management structure, or any other manner in which management disaggregates a
company. The Company allocates its resources and assesses the performance of its
sales activities based upon its products and services (see Note
20).
CONTINGENCIES
Certain
conditions may exist as of the date the financial statements are issued, which
may result in a loss to the Company but which will only be resolved when one or
more future events occur or fail to occur. The Company’s management and
legal counsel assess such contingent liabilities, and such assessment inherently
involves an exercise of judgment. In assessing loss contingencies related
to legal proceedings that are pending against the Company or unasserted claims
that may result in such proceedings, the Company’s legal counsel evaluates the
perceived merits of any legal proceedings or unasserted claims as well as the
perceived merits of the amount of relief sought or expected to be
sought.
If the
assessment of a contingency indicates that it is probable that a material loss
has been incurred and the amount of the liability can be estimated, then the
estimated liability would be accrued in the Company’s financial
statements. If the assessment indicates that a potential material loss
contingency is not probable but is reasonably possible, or is probable but
cannot be estimated, then the nature of the contingent liability, together with
an estimate of the range of possible loss if determinable and material would be
disclosed. Loss contingencies considered to be remote by management are
generally not disclosed unless they involve guarantees, in which case the
guarantee would be disclosed.
RECENT
ACCOUNTING PRONOUNCEMENTS
In September 2009, the Financial
Accounting Standards Board (“FASB”) issued guidance related to revenue
recognition for multiple element deliverables which eliminates the requirement
that all undelivered elements must have objective and reliable evidence of fair
value before a company can recognize the portion of the consideration that is
attributable to items that already have been delivered. Under the new guidance,
the relative selling price method is required to be used in allocating
consideration between deliverables and the residual value method will no longer
be permitted. This guidance is effective prospectively for revenue arrangements
entered into or materially modified in 2011 although early adoption is
permitted. A company may elect, but will not be required, to adopt the
amendments retrospectively for all prior periods. The Company is currently
evaluating this guidance and has not yet determined the impact, if any, that it
will have on the consolidated financial statements.
34
In June 2009, the FASB issued ASC 105
(previously SFAS No. 168, “The FASB Accounting Standards Codification and the
Hierarchy of Generally Accepted Accounting Principles ("GAAP")” - a replacement
of FASB Statement No. 162), which will become the source of authoritative
accounting principles generally accepted in the United States recognized by the
FASB to be applied to nongovernmental entities.
In June 2009, the FASB issued ASC 855
(previously SFAS No. 165, “Subsequent Events”), which establishes general
standards of accounting for and disclosures of events that occur after the
balance sheet date but before the financial statements are issued or available
to be issued. It is effective for interim and annual periods ending after June
15, 2009. There was no material impact upon the adoption of this standard on the
Company’s consolidated financial statements.
In June 2009, the FASB issued ASC
860 (previously SFAS No. 166, “Accounting for Transfers of Financial
Assets”), which requires additional information regarding transfers of financial
assets, including securitization transactions, and where companies have
continuing exposure to the risks related to transferred financial assets. SFAS
166 eliminates the concept of a “qualifying special-purpose entity,” changes the
requirements for derecognizing financial assets, and requires additional
disclosures. SFAS 166 is effective for fiscal years beginning after
November 15, 2009. The Company does not believe this pronouncement will
impact its financial statements.
In
June 2009, the FASB issued ASC 810 (previously SFAS No. 167) for
determining whether to consolidate a variable interest entity. These amended
standards eliminate a mandatory quantitative approach to determine whether a
variable interest gives the entity a controlling financial interest in a
variable interest entity in favor of a qualitatively focused analysis, and
require an ongoing reassessment of whether an entity is the primary beneficiary.
These amended standards are effective for us beginning in the first quarter of
fiscal year 2010 and we are currently evaluating the impact that adoption will
have on our consolidated financial statements.
In August 2009, the FASB issued
Accounting Standards Update (“ASU”) 2009-05, which amends ASC Topic 820,
Measuring Liabilities at Fair Value, which provides additional guidance on the
measurement of liabilities at fair value. These amended standards clarify that
in circumstances in which a quoted price in an active market for the identical
liability is not available, we are required to use the quoted price of the
identical liability when traded as an asset, quoted prices for similar
liabilities, or quoted prices for similar liabilities when traded as assets. If
these quoted prices are not available, we are required to use another valuation
technique, such as an income approach or a market approach. These amended
standards are effective for us beginning in the fourth quarter of fiscal year
2009 and are not expected to have a significant impact on our consolidated
financial statements.
RESULTS
OF OPERATIONS
Comparison of Years Ended December
31, 2009 and December 31, 2008.
The
following table sets forth the results of our operations for the periods
indicated:
|
|
For
the Years ended
|
|
||||||
|
|
December 31,
|
|
|
December 31,
|
|
|||
|
|
2009
|
|
|
2008
|
|
|||
|
Net
Revenue
|
$
|
47,589,280
|
|
|
$
|
59,116,534
|
|
|
|
Cost
of Revenue
|
31,271,463
|
47,226,275
|
||||||
|
Gross
Profit
|
16,317,817
|
11,890,259
|
||||||
|
Selling
Expenses
|
3,543,383
|
3,521,147
|
||||||
|
General
& Administrative Expenses
|
3,575,059
|
2,500,366
|
||||||
|
Total
Operating Expenses
|
7,118,442
|
6,021,513
|
||||||
|
Income
from Operations
|
9,199,376
|
5,868,745
|
||||||
|
Other
Income
|
278,846
|
690,516
|
||||||
|
Operating
Income Before Tax and Non-Controlling Interest
|
9,349,545
|
6,400,113
|
||||||
|
Provision
for Income Tax
|
(2,594,483
|
) |
(1,009,643
|
) | ||||
|
Net
Income Before Non-Controlling Interest
|
$
|
6,724,111
|
$
|
5,305,619
|
||||
|
Non-Controlling
Interest
|
(1,599,122
|
) |
(1,239,480
|
) | ||||
|
Net
Income
|
5,124,989
|
4,066,139
|
||||||
|
Basic
Earnings Per Share
|
0.15
|
0.13
|
||||||
|
Diluted
Earnings Per Share
|
0.15
|
0.13
|
||||||
|
Basic
Weighted Average Shares Outstanding
|
33,240,797
|
31,150,819
|
||||||
|
Diluted
Weighted Average Shares Outstanding
|
35,070,051
|
31,150,819
|
||||||
35
NET
REVENUES. For the year ended December 31, 2009, our net revenues decreased
approximately 19.5% from $59,116,534 in 2008 to $47,589,280 in
2009. The decrease was attributable to a decrease of sales from the
wholesale sector of our business, which included sales to hospitals, medical
facilities and other retailers which represented approximately 70% of our total
net revenues. Sales volume from the wholesale sector of our business
decreased due to the uncertainty of the direction of the National Medical
Policy, as described in the recent development section of this Form
10-K. And as a result, we have reduced our focus on the wholesale
sector of our business and we are shifting our focus to increase the retail
sector of our business, which currently represents approximately 30% of our
total net revenues. The overall policy for our drug retail business
has not changed. The Company will readjust its operations and sales
strategy accordingly once the direction of the National Medical Policy becomes
clear. The revenues for our retail operations, increased from $10,865,100 in
2008 to 13,898,110 in 2009, or approximately 27.9%. The increase in
our retail segment is attributable to the addition of seven new retail
drugstores in 2009. These new retail stores are located in prime
locations in the center of the city and each generates a high volume of
sales. As of November 2009, we discontinued our ginseng and health
products sector because we want to focus on the retail segment of our
business.
COST
OF SALES. Cost of sales to net sales percentage decreased from $47,226,275, or
approximately 79.9% of net revenues for the year ended December 31, 2008, to
$31,271,463, or approximately 65.7% of net sales for the year ended December 31,
2009. The cost of sales decreased by $15,954,812 or approximately
33.8%, and such decrease corresponded with the decrease in sales
volume. However, we were able to reduce the cost of sales to net
sales percentage from 79.9% in 2008 to 65.7% in 2009 due to a restructure of our
product mix. We increased the proportion of products, brands and
types of medicines which had lower actual costs and higher gross profit margins
which resulted in a lower cost of sales for the Company. Our other
operating costs, such as utilities, labor and transportation remained stable and
only decreased in proportion to the decrease of our sales.
GROSS
PROFIT. Gross profit increased approximately 37.2% from $11,890,259 for the year
ended December 31, 2008 to $16,317,817 for the year ended December 31,
2009. This increase in gross profit was primarily due to the change
of our product mix in which the proportion of products with higher profit
margins increased, such as cosmetics and certain health and nutritional
products. Management believes that the addition of such products will
increase our overall gross profit margin for the next few
years.
SELLING
EXPENSES. Selling expenses, which consist of advertising and promotion expenses,
freight charges and salaries, stayed approximately the same from $3,521,147 for
the year ended December 31, 2008 to $3,543,383 for the same period in
2009. Even though we opened seven new stores in 2009, our selling
expenses remained approximately unchanged because we controlled our selling
expenses through certain cost-cutting efforts such as the reduction of utilities
usage, cutback of office supplies and changes in the packaging of our
supplies.
GENERAL
AND ADMINISTRATIVE EXPENSES. General and administrative expenses were $3,575,059
for the year ended December 31, 2009, as compared to $2,500,366 for the year
ended December 31, 2008, an increase of 43.0%. This increase was largely
due to an increase in bad debt expense and increases in accrued litigation
fees.
OTHER INCOME. Other income
decreased 59.6% from $690,516 in 2008 to $278,846 in
2009. The Company
received approximately $180,000 in government subsidies in 2008, which were not
received in 2009. The decrease was also attributable to
the change
of our accounting method at the end of fiscal 2008. With the
new
accounting method in 2009, Yongxin Drugstore calculated its cost by the actual
cost instead of the sales price so the cost was recorded at a lower price under
other income.
36
NET
INCOME. Net income increased approximately 26.0% from a net income of $4,066,139
for the year ended December 31, 2008 to a net income of $5,124,989 for the year
ended December 31, 2009. The increase was largely due to the increase
in our gross profit due to the change of our product mix, in which the
proportion of products with higher profit margins increased, which resulted in
higher gross profit margins for the company.
37
LIQUIDITY
Cash
Flows
Net cash
flow provided by operating activities was $4.3 million for the year ended
December 31, 2009 and $5.9 million in operating activities for the year ended
December 31, 2008. For the year ended December 31, 2009, the increase
in cash flows provided by operating activities was primarily attributable to net
income and increase in accrued expenses offset by increases in accounts
receivable and other receivables. For the year ended December 31,
2008, the net cash flows provided by operating activities was attributable to a
decrease in accounts receivable of $954,908, an increase in advances to
suppliers of $53,084, an increase in notes receivable by $1.3 million, an
increase in inventory by $1.1 million, a decrease in accounts payable by $2.2
million, an increase in advance from customers by $1.8 million, and an increase
in taxes by $0.9 million.
The
Company incurred cash outflows of $3.0 million from investing activities during
the year ended December 31, 2009, as compared to cash outflows of $6.7 million
for the same period in 2008. The significant decrease in cash
outflows was mainly attributable to the purchase of fixed assets and increase in
the amounts due from related parties. The cash outflows of $6.7
million in investing activities during the year ended December 31, 2008 was
mainly attributable to remodeling and construction expenses. In
conjunction with the 2008 Olympics, certain governmental policies were enacted
in an effort to make the commercial areas of Beijing more environmentally
friendly and improve the appearance and function of retail stores. We
renovated our offices and retail drugstores to comply with these governmental
policies in 2008. We also remodeled some of our stores to sublet to
other tenants to bring in extra income. We also invested in the
development of ERP software to link sales to our accounting and finance
department in 2008.
Net cash
flows provided by financing activities was $201,153 for the year ended December
31, 2008 compared to net cash flows used in financing activities of $367,325 for
the year ended December 31, 2009. The decrease was mainly
attributable to a decrease in financing activities in 2009 as compared to
2008.
38
CAPITAL
RESOURCES
The
Company believes that the existing cash and cash equivalents, and cash generated
from operating activities will be sufficient to meet the needs of its current
operations, including anticipated capital expenditures and scheduled debt
repayments, for the next twelve months. The Company may also seek
additional financing to meet the needs of its long-term strategic
plan.
We had
certain material commitments for capital expenditures due to the remodeling and
construction of our offices and retail drugstores and the development of our ERP
software in 2009. The total capital expenditure budget for 2009 was
$6,477,630, and the full expenditure has been paid as of December 31,
2009. Other than working capital and loans, we presently have no
other alternative source of working capital. We may need to raise additional
working capital to complete the projects. We may seek to raise
additional capital through the sale of equity securities. No
assurances can be given that we will be successful in obtaining additional
capital, or that such capital will be available in terms acceptable to our
company. At this time, we have no commitments or plans to obtain additional
capital.
Notwithstanding
the above, we may seek to raise additional cash to fund future investments or
acquisitions we may decide to pursue. To the extent it becomes
necessary to raise additional cash in the future, we may seek to raise it
through the sale of debt or equity securities, funding from joint-venture or
strategic partners, debt financing or loans, issuance of common stock, or a
combination of the foregoing. We currently do not have any binding
commitments for, or readily available sources of, additional
financing. We cannot provide any assurances that we will be able to
secure the additional cash or working capital we may require to continue our
operations.
CONTRACTUAL
OBLIGATIONS
This
table summarizes our known contractual obligations and commercial commitments at
December 31, 2009.
|
|
Payments Due by Period
|
|||||||||||||||||||
|
|
Total
|
Less than 1
year
|
1-3 Years
|
3-5 Years
|
5 Years +
|
|||||||||||||||
|
Contractual Obligations :
|
|
|
|
|
|
|||||||||||||||
|
Bank
Indebtedness
|
$
|
2,421,184
|
$
|
1,100,884
|
$
|
1,320,300
|
$
|
-
|
$
|
-
|
||||||||||
|
Other
Indebtedness
|
$
|
13,992,257
|
$
|
13,992,257
|
$
|
-
|
$
|
-
|
$
|
-
|
||||||||||
|
Capital
Lease Obligations
|
$
|
105,559
|
$
|
105,559
|
$
|
-
|
$
|
-
|
$
|
-
|
||||||||||
|
Operating
Leases
|
$
|
2,401,718
|
$
|
1,058,624
|
$
|
1,247,543
|
$
|
95,551
|
$
|
-
|
||||||||||
|
Purchase
Obligations
|
$
|
4,151,219
|
$
|
4,151,219
|
$
|
-
|
$
|
-
|
$
|
-
|
||||||||||
|
Total
Contractual Obligations:
|
$
|
23,021,937
|
$
|
20,408,543
|
$
|
2,567,843
|
$
|
95,551
|
$
|
-
|
||||||||||
Off-Balance
Sheet Arrangements
We have
not entered into any other financial guarantees or other commitments to
guarantee the payment obligations of any third parties. We have not
entered into any derivative contracts that are indexed to our shares and
classified as stockholder's equity or that are not reflected in our consolidated
financial statements. Furthermore, we do not have any retained or
contingent interest in assets transferred to an unconsolidated entity that
serves as credit, liquidity or market risk support to such entity. We do not
have any variable interest in any unconsolidated entity that provides financing,
liquidity, market risk or credit support to us or engages in leasing or hedging
services with us.
39
ITEM
7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
We do not
use derivative financial instruments in our investment portfolio and have no
foreign exchange contracts. Our financial instruments consist of cash and cash
equivalents, trade accounts receivable, accounts payable and long-term
obligations. We consider investments in highly-liquid instruments purchased with
a remaining maturity of 90 days or less at the date of purchase to be cash
equivalents.
Interest Rates. Our exposure
to market risk for changes in interest rates relates primarily to our short-term
obligations; thus, fluctuations in interest rates would not have a material
impact on the fair value of these securities. At December 31, 2009, we had
approximately $1,805,271 in cash and cash equivalents. A hypothetical 10%
increase or decrease in interest rates would not have a material impact on our
earnings or loss, or the fair market value or cash flows of these
instruments.
Foreign Exchange Rate. We use
the United States Dollar (“U.S. Dollars”) for financial reporting purposes but
all of our sales and inputs are transacted in Renminbi (“RMB”). As a result,
changes in the relative values of U.S. Dollars and RMB affect our reported
levels of revenues and profitability as the results are translated into U.S.
Dollars for reporting purposes. However, since we conduct our sales and purchase
inputs in RMB, fluctuations in exchange rates are not expected to significantly
affect our financial stability, or gross and net profit margins. We do not
currently expect to incur significant foreign exchange gains or losses, or gains
or losses associated with any foreign operations. During the years
ended December 31, 2009 and 2008, we recorded net foreign currency gains of
$123,209 and $824,961, respectively.
ITEM
8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
The
information required by this Item 8 is incorporated by reference to information
begins on Page F-1 of this Form 10-K.
ITEM
9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL
DISCLOSURE
None.
ITEM
9A. CONTROLS AND PROCEDURES
Evaluation
of Disclosure Controls and Procedures
Disclosure
controls and procedures are controls and other procedures that are designed to
ensure that information required to be disclosed by us in the reports that we
file or submit under the Exchange Act is recorded, processed, summarized and
reported, within the time periods specified in the SEC's rules and forms.
Disclosure controls and procedures include, without limitation, controls and
procedures designed to ensure that information required to be disclosed by the
Company in the reports that we file or submit under the Exchange Act is
recorded, processed, summarized and reported within the time periods specified
in the SEC's rules and forms and that information required to be disclosed by us
in the reports that we file or submit under the Exchange Act is accumulated and
communicated to our management, including our principal executive and financial
officers, as appropriate to allow timely decisions regarding required
disclosure.
As of the
end of the period covered by this Form 10-K, we conducted an evaluation, under
the supervision and with the participation of our Chief Executive Officer and
Chief Financial Officer, of our disclosure controls and procedures (as defined
in Rule 13a-15(e) and Rule 15d-15(e) of the Exchange Act). Based upon
this evaluation, our Chief Executive Officer and Chief Financial Officer
concluded that the Company's disclosure controls and procedures are
effective.
40
Management’s
Annual Report on Internal Control over Financial Reporting
Our
management is responsible for establishing and maintaining adequate internal
control over financial reporting. Internal control over financial
reporting, as defined in Exchange Act Rule 13a-15(f), is a process designed by,
or under the supervision of, our principal executive and principal financial
officers and effected by our board of directors, management and other personnel,
to provide reasonable assurance regarding the reliability of financial reporting
and the preparation of financial statements for external purposes in accordance
with generally accepted accounting principles and includes those policies and
procedures that:
|
|
·
|
Pertain
to the maintenance of records that in reasonable detail accurately and
fairly reflect the transactions and dispositions of our
assets;
|
|
|
·
|
Provide
reasonable assurance that transactions are recorded as necessary to permit
preparation of financial statements in accordance with generally accepted
accounting principles, and that our receipts and expenditures are being
made only in accordance with authorizations of our management and
directors; and
|
|
|
·
|
Provide
reasonable assurance regarding prevention or timely detection of
unauthorized acquisition, use of disposition of our assets that could have
a material effect on the financial
statements.
|
Because
of its inherent limitations, internal control over financial reporting may not
prevent or detect misstatements. Projections of any evaluation of effectiveness
to future periods are subject to the risk that controls may become inadequate
because of changes in conditions, or that the degree of compliance with the
policies or procedures may deteriorate. All internal control systems, no matter
how well designed, have inherent limitations. Therefore, even those systems
determined to be effective can provide only reasonable assurance with respect to
financial statement preparation and presentation.
Our
management assessed the effectiveness of our internal control over financial
reporting as of December 31, 2009. In making this assessment, our
management used the criteria set forth by the Committee of Sponsoring
Organizations of the Treadway Commission (COSO) in Internal Control-Integrated
Framework. Based on this assessment, management believes that as of December 31,
2009, our internal control over financial reporting is effective based on those
criteria.
This Form
10-K does not include an attestation report of the Company's registered public
accounting firm regarding internal control over financial reporting.
Management's report was not subject to attestation by the Company's registered
public accounting firm pursuant to temporary rules of the SEC that permit the
Company to provide only management's report in this Form 10-K.
Changes
in Internal Control
There
were no changes in our internal control over financial reporting (as defined in
Rules 13a-15(f) and 15d-15(f) under the Securities Exchange Act of 1934, as
amended) during the quarter ended December 31, 2009 that have materially
affected, or are reasonably likely to materially affect, our international
control over financial reporting.
ITEM
9B. OTHER INFORMATION
None.
41
PART
III
ITEM
10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE
GOVERNANCE
Departure
and Appointment of Officers and Directors
Departure
of Officers
Effective December 4, 2009, Yongkui Liu
voluntarily resigned as the Chief Financial Officer of the
Company. The decision by Mr. Liu to resign from his position was not
the result of any material disagreement with the Company on any matter relating
to the Company’s operations, policies or practices.
Appointment
of Officers
Effective December 4, 2009, Harry Zhang
was appointed as the Chief Financial Officer of the Company.
Departure
of Directors
Effective
November 8, 2009, Umesh Patel voluntarily resigned as a director on the board of
directors of the Company. Effective March 3, 2010, Mr. Yongkui Liu
and Ms. Yongmei Wang each voluntarily resigned as directors on the board of
directors of the Company. The decision by each of these directors to
resign from their positions was not the result of any material disagreement with
the Company on any matter relating to the Company’s operations, policies or
practices. Mr. Yongkui Liu is presently the Vice President of the
Company, and will continue to serve in this position. Ms. Yongmei
Wang is presently the Vice President and Treasurer of the Company,
and continues to serve in this position.
Appointment
of Directors
Effective
November 8, 2009, the board of directors of the Company appointed Mr. Harry
Zhang to fill the vacancy created by the resignation of Mr. Umesh
Patel. Effective March 3, 2010, the board of directors of the Company
appointed Mr. Hal Lieberman, Dr. Laura Philips, Mr. Jingang Wang and Mr. Bing Li
to fill the vacancies created by the resignations of Mr. Yongkui Liu and Ms.
Yongmei Wang. Based upon information submitted to the board by Mr. Hal
Lieberman, Dr. Laura Philips, Mr. Jingang Wang and Mr. Bing Li, the board of
Directors has determined that they are each “independent” under the listing
standards of both the NYSE Amex Exchange and the NASDAQ Stock
Market. None of the four appointees has participated in the
preparation of the Company’s financial statements or any current subsidiary at
any time during the past three years and each of them are able to read and
understand fundamental financial statements.
Current Management
The
following table identifies our current executive officers and directors, their
respective offices and positions, and their respective dates of election or
appointment:
|
Name
|
Age
|
Position
|
Effective Date of
Appointment
|
|||
|
Yongxin
Liu
|
41
|
Chairman
of the Board and Chief Executive Officer
|
November
16, 2007
|
|||
|
Harry
Zhang
|
46
|
Chief
Financial Officer and Director
|
December
4, 2009 (CFO)
November
8, 2009 (Director)
|
|||
|
Ning
Liu
|
47
|
President,
Chief Operating Officer and Director
|
November
16, 2007
|
|||
|
Hal
Lieberman
|
60
|
Director
|
March
3, 2010
|
|||
|
Laura
Philips
|
52
|
Director
|
March
3, 2010
|
|||
|
Jingang
Wang
|
41
|
Director
|
March
3, 2010
|
|||
|
Bing
Li
|
43
|
Director
|
March
3, 2010
|
Each
director will hold office until the next annual meeting of stockholders and
until his or her successor has been elected and qualified.
Yongxin
Liu has been the Chairman of Changchun Yongxin Dirui Medical Co., Ltd.
(“Yongxin”) since 2003 and Jilin Province Yongxin Chain Drugstore Co., Ltd.
(“Yongxin Drugstore”) since 2001. From August 1998 to 2003, Mr. Liu
served as the General Manager of Yongxin Drugstore. From 1984 to June
2006, Mr. Liu was employed by Changchun Medical Materials Marketing Co., Ltd.
(“Changchun Medical”), serving as the Assistant Manager of Business and
Vice-manager. From July 1991 to July 1994, Mr. Liu studied at
Northeast Normal University, majoring in Management. In August 2004,
he received an MBA from Beijing University.
42
Harry
Zhang also serves as the chief Financial officer and vice president of
Surplus Elegant Investment Ltd. in addition to being our Chief Financial Officer
and member of our board of directors. From March 2004 to August 2006, Mr.
Zhang served as the Chief Financial Officer of AXM Pharma, Inc. Mr. Zhang
worked as an auditor at Deloitte Touche Tohmatsu from May 1995 to March
2004. Mr. Zhang worked at the Beijing Municipal Audit Bureau from 1988 to
1995. Mr. Zhang earned his Bachelor’s degree from Nanjing Agricultural
University in 1988 and received his Master of Business Administration degree
from University of Northern Virginia in June 2007. Mr. Zhang is also a
certified public accountant in China.
Ning Liu
has been the president of Succeed Group Inc., a media company, since
2003. Prior to his service at Succeed Group, Inc., Mr. Liu was the
president of Super Nu-Life Products Inc., a nutraceuticals manufacturer from
1994 to 2003. From 1992 to 1994, Mr. Liu was the president of
Goldenrise Development Inc. Additionally, from 1986 TO 2002, Mr. Liu
served as president of Accords System Inc. Mr. Liu is active in
founding, organizing and managing a number of foreign investment projects
in China, and he counsels Chinese companies in doing business in the United
States, and in mergers with public companies in the United
States. Mr. Liu graduated from Beijing University with a Masters of
Arts degree in 1985.
Hal
Lieberman also serves as the president and chief executive officer of
HemoTherapeutics, Inc., and has held such position from 2006 to
the present. From 1998 to 2006, Mr. Lieberman acted as a consultant,
provided interim management and served on the boards of a variety of health care
and medical device companies. In 1988, Mr. Lieberman served as the
president and chief executive officer of HemaCare Corporation. Mr.
Lieberman acted as the vice president for MEDIQ Imaging Services from 1981 to
1988. Mr. Lieberman earned his Master’s degree in Health Care
Administration from George Washington University. Mr. Lieberman is
also a member of American College of Health Care Administrators, The
Entrepreneurship Institute, Southern California Biomedical Council and served as
an advisor to U.S. Defense Advanced Research Projects Agency
(DARPA).
Laura
Philips also serves on the Boards of Directors of Delcath Systems, Inc,
Wellgen Inc., and the Boyce Thompson Institute. From 2003 to 2006, she was Chief
Operating Officer and Acting Chief Financial Officer of NexGenix
Pharmaceuticals. Prior to that, she was Vice President, Program Management for
the AMDeC Foundation. Dr. Philips worked at Corning Incorporated from 1997 to
2002, where she held several positions including Program Director of the Fuel
Cells Division. From 1994 to 1996 Dr. Philips held various government positions
in Washington, D.C., most recently in a Presidential appointment as Senior
Policy Advisor to Secretary of Commerce Ronald Brown. Dr. Philips was on the
faculty of Cornell University in the Department of Chemistry from 1987 to 1994
and was an NIH Post-Doctoral Fellow at the University of Chicago. She received a
B.A. in Chemistry from Williams College, a Ph.D. in Physical Chemistry from the
University of California Berkeley and an MBA with Distinction from Cornell
University’s Johnson School of Management.
Jingang
Wang also serves as the executive director for CoSci Med-Tech Co., Ltd.,
and has held such position since May 2003 to the present. From June
2002 to May 2003, Mr. Wang worked as the executive director in Beijing
Lingtainbicheng Medicine Technology Co., Ltd. Mr. Wang served as the
deputy general manager and assistant general manager of Mudanjiang Lingtai
Pharmaceuticals Co., Ltd from October 1998 to June 2002. Mr. Wang
acted as the deputy chief in Mudanjiang’s Food and Drugs Administration from
August 1990 to October 1998. Mr. Wang was an assistant engineer in
Mudanjiang No. 3 Pharmaceutical Factory from September 1998 to August
1990. Mr. Wang received his Bachelor’s degree in Medical Research and
Development from Heilongjian Chinese Medical University in 1998.
Bing Li
also serves as the general manager of Jilin Province Fire Fighting Engineering
Co., Ltd., the general manager of Jilin Province Yinjian Small & Medium
Enterprises Credit Guaranty Co., Ltd., the vice-chairman of the board of
directors of Changchun JLU Technology Park Construction and Development Co.,
Ltd, the deputy managing director of Jilin Province Intelligentization
Committee, and the general manager of Beijing Kunshanjinhai Film & TV
Cultural Investment Co., Ltd, and has held such positions from 1999 to the
present. From March 1990 to June 1990, Mr. Li served as the manager
of Export Department of Jilin Provincial Forestry Import & Export
Company. Prior to March 1990, Mr. Li worked on various foreign
affairs for Jilin Provincial Forestry Department. Mr. Li received his
Bachelor’s degree in English from Changchun University in 1988.
43
Family
Relationships
Mr. Yongxin Liu and Mr. Yongkui Liu are
brothers. Additionally, Mr. Yongkui Liu, our Vice President and
former Chief Financial Officer and director, is the spouse of Ms. Yongmei Wang,
our Treasurer and Vice President and former director.
Involvement
in Certain Legal Proceedings
None.
Board
Committees
Effective
March 3, 2010, the board established an audit committee, a nominating committee
and a compensation committee and has adopted charters for these committees.
The board has determined that in its judgment, Mr. Hal Lieberman, Ms.
Laura Philips, Mr. Jingang Wang and Mr. Bing Li are each “independent” under the
listing standards of both the NYSE Amex Exchange and the NASDAQ
Stock Market .
Compliance with Section 16(a) of the
Securities Exchange Act of 1934
Section
16(a) of the Securities Exchange Act of 1934 requires the Company's directors
and executive officers, and persons who own more than 10% of the Company's
Common Stock, to file with the SEC initial reports of beneficial ownership and
reports of changes in beneficial ownership of Common Stock of the Company.
Officers, directors and greater than 10% stockholders are required by the SEC to
furnish the Company with copies of all section 16(a) reports they
file. Based solely on our review of the Section 16 filings furnished
to us, Yongkui Liu and Yongxin Liu filed delinquent Form 4 reports on January 5,
2010 for transactions that occurred on December 16, 2009, Yongxin Liu, Yongkui
Liu and Yongmei Wang filed delinquent Form 3 reports on March 12, 2010 for
transactions that occurred on November 16, 2007, and Ning Liu filed a delinquent
Form 3 report on March 11, 2010 for a transaction that occurred on November 16,
2007.
Code
of Conduct
We
adopted a Code of Ethics that applies to our principal executive officer,
principal financial officer, principal accounting officer or controller or
persons performing similar functions. Anyone who would like a copy of
our Code of Conduct may do so by writing to the Company at its principal place
of business at 927 Canada Court, City of Industry, California
91748.
Audit
Committee
The board
of directors adopted and approved a charter for the Audit Committee on March 3,
2010. Currently, three directors comprise the Audit Committee: Hal
Lieberman, Jingang Wang and Bing Li. Mr. Lieberman serves as Chairman
of the Audit Committee. The members of the Audit Committee are currently
“independent directors” as that term is defined under the listing standards of
both the NYSE Amex Exchange and the NASDAQ Stock
Market . Mr. Lieberman also qualifies as an “audit committee
financial expert” as defined by the rules of the SEC. Mr. Hal
Lieberman has the requisite attributes of an “audit committee financial expert”
as defined by regulations promulgated by the SEC and that such attributes were
acquired through relevant education and experience.
Our Audit
Committee is responsible, in accordance with the Audit Committee charter,
recommending our independent auditors, and overseeing our audit activities and
certain financial matters to protect against improper and unsound practices and
to furnish adequate protection to all assets and records.
Our Audit
Committee pre-approves all audit and non-audit services provided by our
independent auditors. These services may include audit services, audit-related
services, tax services and other services. Pre-approval is generally provided
for up to one year and any pre-approval is detailed as to particular service or
category of services and is generally subject to a specific budget. The Audit
Committee has delegated pre-approval authority to its Chairman when expedition
of services is necessary. The independent auditors and management are required
to periodically report to the full Audit Committee regarding the extent of
services provided by the independent auditor in accordance with this
pre-approval, and the fees for the services performed to date.
44
Compensation
Committee
The board
of directors adopted and approved a charter for the Compensation Committee on
March 3, 2010. The Compensation Committee currently consists of
Jingang Wang, Bing Li and Laura Philips. Mr. Wang serves as Chairman of the
Compensation Committee. The members of the Compensation Committee are currently
“independent directors” under the listing standards of both the NYSE
Amex Exchange and the NASDAQ Stock Market .
In
accordance with the Compensation Committee’s charter, the Compensation Committee
is responsible for overseeing and, and as appropriate, making recommendations to
the board regarding the annual salaries and other compensation of the Company’s
executive officers and general employees and other polices, providing assistance
and recommendations with respect to the compensation policies and practices of
the Company.
Nominating
Committee
The board
of directors adopted and approved a charter for the Nominating Committee on
March 3, 2010. The Nominating Committee currently consists of Bing
Li, Jingang Wang and Hal Lieberman. Mr. Li serves as the Chairman of
the Nominating Committee. The members of the Nominating Committee are
currently “independent directors” under the listing standards of both
the NYSE Amex Exchange and the NASDAQ Stock
Market .
In
accordance with the Nominating Committee’s charter, the Nominating Committee is
responsible for proposing to the board a slate of nominees for election by the
stockholders at the Annual Meeting of Stockholders, to periodically review and
develop criteria for selection of new directors and nominees for vacancies on
the board, to review the desired experience and qualities to assure appropriate
board composition, and to recommend to the board qualified candidates for the
board.
ITEM 11. EXECUTIVE
COMPENSATION
Summary
Compensation Tables
The
following table sets forth information concerning the compensation for the
fiscal years ended December 31, 2009 and 2008 of the principal executive
officer, principal financial officer, in addition to our three most highly
compensated officers whose annual compensation exceeded $100,000, and up to two
additional individuals for whom disclosure would have been required but for the
fact that the individual was not serving as an executive officer of the
registrant at the end of the last fiscal year.
|
Name and Position
|
Year
|
Salary
|
Bonus
|
Total
|
||||||||||
|
Yongxin
Liu,
|
2009
|
$
|
87,976
|
$
|
0
|
$
|
87,976
|
|||||||
|
Chief
Executive Officer and Chairman of the Board
|
2008
|
$
|
82,392
|
$
|
5,633
|
$
|
88,025
|
|||||||
|
Yongkui
Liu,
|
2009
|
$
|
70,381
|
$
|
0
|
$
|
70,381
|
|||||||
|
Former
Chief Financial Officer and Director
|
2008
|
$
|
64,787
|
$
|
5,633
|
$
|
70,420
|
|||||||
Grants
of Plan-Based Awards in 2009
There
were no option grants as of December 31, 2009.
Outstanding
Equity Awards at 2009 Fiscal Year End
There
were no outstanding equity awards as of December 31, 2009.
45
Option
Exercises and Stock Vested in Fiscal 2009
There
were no option exercises or stock vested as of December 31, 2009.
Pension
Benefits
There
were no pension benefit plans in effect as of December 31, 2009.
Nonqualified
defined contribution and other nonqualified deferred compensation
plans
There was
no nonqualified defined contribution or other nonqualified deferred compensation
plans in effect as of December 31, 2009.
Employment
Agreements
We have
no employment agreements with any of our executive officers as of December 31,
2009.
Director
Compensation
For the
year ended December 31, 2009, none of the members of our board of directors
received compensation for his or her service as a director. Effective
March 3, 2010, Hal Lieberman, Laura Philips, Jingang Wang and Bing Li have each
have executed and delivered a director offer letter with the Company. Under the
terms of the agreements, Ms. Philips, Mr. Wang and Mr. Li shall be entitled to
the annual compensation of $20,000 and eligible to receive either restricted
stock or a stock option award for the purchase of up to 200,000 shares (subject
to appropriate adjustment in the event of reverse stock splits or similar
events) of the Company’s common stock through an incentive stock option plan to
be adopted by the Company. Mr. Hal Lieberman, who will not only serve
as a director, but also as Audit Committee Chairman, shall be entitled to the
annual compensation of $30,000 and eligible to receive a stock option award for
the purchase of up to 200,000 shares of the Company’s common stock.
Outstanding
Equity Awards at Fiscal Year-End
None of
our executive officers were granted or otherwise received any option, stock or
equity incentive plan awards during 2009 and there were no outstanding
unexercised options previously awarded to our officers and directors, at the
fiscal year end, December 31, 2009.
Indemnifications
of Directors And Executive Officers And Limitations of Liability
Under
Section 145 of the General Corporation Law of the State of Delaware, we can
indemnify our directors and officers against liabilities they may incur in such
capacities, including liabilities under the Securities Act of 1933, as amended
(the “Securities Act”). Our certificate of incorporation provides that, pursuant
to Delaware law, our directors shall not be liable for monetary damages for
breach of the directors’ fiduciary duty of care to us and our stockholders. This
provision in the certificate of incorporation does not eliminate the duty of
care, and in appropriate circumstances equitable remedies such as injunctive or
other forms of nonmonetary relief will remain available under Delaware law. In
addition, each director will continue to be subject to liability for breach of
the director’s duty of loyalty to us or our stockholders, for acts or omissions
not in good faith or involving intentional misconduct or knowing violations of
the law, for actions leading to improper personal benefit to the director, and
for payment of dividends or approval of stock repurchases or redemptions that
are unlawful under Delaware law. The provision also does not affect a director’s
responsibilities under any other law, such as the federal securities laws or
state or federal environmental laws.
Our
bylaws provide for the indemnification of our directors to the fullest extent
permitted by the Delaware General Corporation Law. Our bylaws further provide
that our board of directors has discretion to indemnify our officers and other
employees. We are required to advance, prior to the final disposition of any
proceeding, promptly on request, all expenses incurred by any director or
executive officer in connection with that proceeding on receipt of an
undertaking by or on behalf of that director or executive officer to repay those
amounts if it should be determined ultimately that he or she is not entitled to
be indemnified under the bylaws or otherwise. We are not, however, required to
advance any expenses in connection with any proceeding if a determination is
reasonably and promptly made by our board of directors by a majority vote of a
quorum of disinterested board members that (i) the party seeking an advance
acted in bad faith or deliberately breached his or her duty to us or our
stockholders and (ii) as a result of such actions by the party seeking an
advance, it is more likely than not that it will ultimately be determined that
such party is not entitled to indemnification pursuant to the applicable
sections of our bylaws.
46
We have
been advised that in the opinion of the SEC, insofar as indemnification for
liabilities arising under the Securities Act may be permitted to our directors,
officers and controlling persons pursuant to the foregoing provisions, or
otherwise, such indemnification is against public policy as expressed in the
Securities Act and is therefore unenforceable. In the event a claim for
indemnification against such liabilities (other than our payment of expenses
incurred or paid by our director, officer or controlling person in the
successful defense of any action, suit or proceeding) is asserted by such
director, officer or controlling person in connection with the securities being
registered, we will, unless in the opinion of our counsel the matter has been
settled by controlling precedent, submit to a court of appropriate jurisdiction
the question of whether such indemnification by us is against public policy as
expressed in the Securities Act and will be governed by the final adjudication
of such issue.
We may
enter into indemnification agreements with each of our directors and officers
that are, in some cases, broader than the specific indemnification provisions
permitted by Delaware law, and that may provide additional procedural
protection. We have not entered into any indemnification agreements with our
directors or officers, but may choose to do so in the future. Such
indemnification agreements may require us, among other things, to:
|
|
·
|
indemnify
officers and directors against certain liabilities that may arise because
of their status as officers or
directors;
|
|
|
·
|
advance
expenses, as incurred, to officers and directors in connection with a
legal proceeding, subject to limited exceptions;
or
|
|
|
·
|
obtain
directors’ and officers’ insurance.
|
ITEM
12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT
AND RELATED STOCKHOLDER MATTERS
The
following table sets forth information regarding the beneficial ownership of our
common stock as of March 10, 2010 for each of our directors and officers; all
directors and officers as a group; and each person known by us to beneficially
own five percent or more of our common stock.
Beneficial ownership is determined
in accordance with SEC rules. Unless otherwise indicated in the
table, the persons and entities named in the table have sole voting and sole
investment power with respect to the shares set forth opposite the stockholder’s
name. Unless otherwise indicated, the address of each stockholder
listed in the table is c/o China Yongxin Pharmaceuticals Inc., 927 Canada Court,
City of Industry, California 91748.
47
|
Name and Address
of Beneficial Owner
|
Title
|
Beneficially
Owned
|
Percent of
Class
|
|||||||
|
Directors
and Executive Officers
|
||||||||||
|
Yongxin
Liu
|
Chief
Executive Officer and Chairman of the Board
|
12,000,000
|
(1)
|
20.9
|
%
|
|||||
|
Ning
Liu (2)
|
President, Chief
Operating Officer and Director
|
—
|
—
|
|||||||
|
Harry
Zhang
|
Chief
Financial Officer and Director
|
—
|
—
|
|||||||
|
Hal
Lieberman
|
Director
|
—
|
—
|
|||||||
|
Laura
Philips
|
Director
|
—
|
—
|
|||||||
|
Jingang
Wang
|
Director
|
—
|
—
|
|||||||
|
Harry
Zhang
|
Director
|
—
|
—
|
|||||||
|
Officers
and Directors as a Group (total of 7 persons)
|
12,000,000
|
20.9
|
%
|
|||||||
|
5%
Holders
|
||||||||||
|
Accord
Success Ltd., BVI
|
5,400,000
|
(3)
|
9.4
|
%
|
||||||
|
Boom
Day Investments, Ltd., BVI
|
13,399,998
|
(4)
|
23.4
|
%
|
||||||
|
Yongkui
Liu
|
14,599,998
|
(5)
|
25.5
|
%
|
||||||
|
Yongmei
Wang
|
14,599,998
|
(5)
|
25.5
|
%
|
||||||
|
Master
Power Holdings Coup Ltd., BVI
|
4,200,000
|
(6)
|
7.3
|
%
|
||||||
|
Misala
Holdings Inc., BVI
|
12,000,000
|
(1)
|
20.9
|
%
|
|
|
(1)
|
Represents
shares of common stock in our company held by Misala Holdings Inc., a
British Virgin Islands corporation (“Misala Holdings”), over which Mr.
Yongxin Liu may be deemed to have voting and investment control. The
12,000,000 shares of common stock were converted from 2,000,000 shares of
Series A Convertible Preferred Stock, which were each convertible into 6
shares of common stock, upon the Company meeting certain required net
income amounts for conversion for the fiscal years ending 2007 and 2008,
held by Misala Holdings Inc. Misala Holdings also holds an
additional 1,000,000 shares of Series A Preferred Stock, each share of
which is convertible into six shares of common stock upon certain
conditions being met pursuant to the Certificate of
Incorporation.
|
|
|
(2)
|
Mr.
Ning Liu’s address is 22128 Steeplechase Lane, Diamond Bar, CA
91765.
|
|
|
(3)
|
Mr.
Tao Wang has voting and investment control over the shares owned by this
entity.
|
|
|
(4)
|
Mr.
Yongkui Liu has voting and investment control over the shares owned by
this entity. Includes 5,400,000 shares of common stock held by Boom
Day Investments Ltd., British Virgin Islands corporation (“Boom Day
Investments”) over which Mr. Yongkui Liu may be deemed to have voting and
investment control, and 7,999,998 shares of common stock converted from
1,333,333 shares of Series A Convertible Preferred Stock, which were each
convertible into 6 shares of common stock, upon the Company meeting
certain required net income amounts for conversion for the fiscal years
ending 2007 and 2008, held by Boom Day Investments. Boom Day Investments
also holds an additional 666,667 shares of Series A Preferred Stock, each
share of which is convertible into six shares of common stock upon certain
conditions being met pursuant to the Certificate of
Incorporation.
|
48
|
|
(5)
|
Includes
13,399,998 shares held directly by Boom Day Investments, over which Mr.
Yongkui Liu, the sole director and sole shareholder of Boom Day
Investments, has voting and investment control, and over which Mrs.
Yongmei Wang, the spouse of Mr. Yongkui Liu, may be deemed to have shared
voting and investment control. Also includes 1,200,000 shares
of common stock held by Perfect Sum Investment Ltd., a British Virgin
Islands corporation (“Perfect Sum”), over which Mr. Yongkui Liu’s spouse,
Mrs. Yongmei Wang, may be deemed to have voting and investment control and
over which Mr. Yongkui Liu may be deemed to have shared voting and
dispositive control.
|
|
|
(6)
|
Mr.
Yong Liu has voting and investment control over the shares owned by this
entity.
|
Equity
Compensation Plan Information
We have
not adopted any equity compensation plan as of December 31,
2009.
ITEM
13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR
INDEPENDENCE
Transactions
with Related Persons
Changchun
Yongxin Dirui Medical Co., Ltd.
Changchun
Yongxin Dirui Medical Co., Ltd. (“Yongxin”) and all of the
shareholders of Yongxin entered into an amended share exchange agreement with
the Company on June 15, 2007. On November 16, 2007, Yongxin and the
Company closed on the share exchange under the Amended Exchange Agreement. In
accordance with the Amended Exchange Agreement, the Company issued 21,000,000
shares of newly issued common stock and 5,000,000 shares of Series A Preferred
Stock to the Yongxin shareholders or their designees, representing, immediately
following closing, 70% of the total issued and outstanding shares of common
stock of the Company in exchange for 80% shares of Yongxin. Yongxin is our
80%-owned subsidiary and has interlocking executive and director positions with
us. Mr. Yongxin Liu, the Chairman and Chief Executive Officer of the
Company, is also the President of Yongxin. Mr. Yongkui Liu, the Vice
President of the Company, is also the Vice President of Yongxin.
Loans
from Related Parties
On
December 1, 2005, Mr. Aurangzeb Bhatti, the former Chief Executive Officer of
our predecessor company, Digital Learning Management Corporation, made an
unsecured loan to the Company with a principal of US$184,662 interest free and
due on demand. As of the date of this Form 10-K, such loan is still
outstanding.
Director
Independence
See
Item 10, “Directors, Executive Officers and Corporation Governance” for a
discussion of board member independence.
ITEM 14. PRINCIPAL ACCOUNTING
FEES AND SERVICES
The
following table sets forth fees billed to us by our independent registered
accounting firm, Kabani & Company, Inc. during the fiscal years ended
December 31, 2009 and 2008 for: (i) services rendered for the audit of our
annual financial statements and the review of our quarterly financial
statements, (ii) services by our auditor that are reasonably related to the
performance of the audit or review of our financial statements and that are not
reported as Audit Fees, and (iii) services rendered in connection with tax
compliance, tax advice and tax planning. We did not engage our
auditors for any other services during 2009 and 2008.
49
|
December 31,
2009
|
December 31,
2008
|
|||||||
|
(i)
Audit Fees(1)
|
$
|
160,000
|
(2)
|
$
|
115,000
|
(2)
|
||
|
(ii)
Audit Related Fees
|
-
|
-
|
||||||
|
(iii)
Tax Fees
|
-
|
-
|
||||||
|
(iv)
All Other Fees
|
-
|
-
|
||||||
|
Total
fees
|
$
|
160,000
|
(2)
|
$
|
115,000
|
(2)
|
||
|
|
(1)
|
These
are fees for professional services performed by Kabani & Company, Inc.
for the audit of our annual financial statements and review of our
quarterly reports.
|
|
|
(2)
|
Includes
$40,000 in fees related to services provided to Digital Learning
Management Corporation and $75,000 in fees related to services provided to
Yongxin.
|
Pre-Approval
Policy
Effective
March 3, 2010, our Audit Committee pre-approves all audit and non-audit services
provided by our independent auditors. These services may include
audit services, audit-related services, tax services and other
services. Pre-approval is generally provided for up to one year and
any pre-approval is detailed as to particular service or category of services
and is generally subject to a specific budget. The Audit Committee
has delegated pre-approval authority to its Chairman when expedition of services
is necessary. The independent auditors and management are required to
periodically report to the full Audit Committee regarding the extent of services
provided by the independent auditor in accordance with this pre-approval, and
the fees for the services performed to date.
PART
IV
ITEM
15. EXHIBITS AND FINANCIAL STATEMENT SCHEDULES
Financial
Statements and Schedules
Our consolidated financial statements
for the fiscal years ending December 31, 2009 and 2008 begin on page F-1 of this
Form 10-K. We are not required to file any financial statement
schedules.
Exhibits
The Exhibit Index lists those documents
that we are required to file with this Form 10-K.
EXHIBIT
INDEX
|
Exhibit
|
||
|
Number
|
Description
|
|
|
2.1
|
Exchange Agreement by and between
Digital Learning Management Corporation and Changchun Yongxin Dirui
Medical Co., Ltd dated December 21, 2006 (incorporated by reference to
Exhibit 10.1 to the Current Report on Form 8-K filed with the SEC on
December 28, 2006).
|
|
|
2.2
|
First Amendment to Share Exchange
Agreement, dated as of June 15, 2007, by and among Digital Learning
Management Corporation, Chanchun Yongxin Dirui Medical Co., Ltd.
(“Yongxin”) and the stockholders of Yongxin (incorporated by reference to
Exhibit B to the Definitive Proxy Statement on Schedule 14A filed with the
SEC on September 14, 2007)
|
|
|
2.3
|
Second Amendment to the Share
Exchange Agreement, dated as of April 12, 2008,and effective as of
November 16, 2007, by and among Nutradyne Group, Inc., Chanchun Yongxin
Dirui Medical Co., Ltd. (“Yongxin”) and the stockholders of Yongxin
(incorporated by reference to Exhibit 10.1 to the Current Report on Form
8-K filed with the SEC on April 15,
2008)
|
50
|
3.1
|
Certificate of Incorporation
(incorporated by reference to Exhibit 3 to the Company’s General Form For
Registration of Securities of Small Business Issuers on Form 10-SB, filed
with the SEC on November 5, 1999).
|
|
|
3.2
|
Certificate of Amendment of
Articles of Incorporation of the Company (incorporated by reference to
Exhibit A of the Company’s definitive information statement on Schedule
14C filed with the SEC on February 25, 2004).
|
|
|
3.3
|
Bylaws of the Company
(incorporated by reference to Exhibit 3.2 to the Company’s Current Report
on Form 8-K filed with the SEC on September 27,
2004).
|
|
|
3.4
|
Certificate of Ownership and
Merger Merging China Yongxin Pharmaceuticals Inc. and Nutradyne Group,
Inc. (incorporated by reference to Exhibit 3.1 to the Current Report on
Form 8-K Filed with the SEC on May 9, 2008).
|
|
|
10.1
|
Summary
English Translation of the Company’s Form Lease Agreement for its
Retail Drugstores (incorporated by reference to Exhibit 10.1 to the Annual
Report on Form 10-K Filed with the SEC on April 15,
2009).
|
|
|
10.2
|
Stock
Purchase Warrant (incorporated by reference to Exhibit 10.1 to the Current
Report on Form 8-K Filed with the SEC on September 25,
2009).
|
|
|
10.3
|
Corporate
Communications Consulting Agreement (incorporated by reference to Exhibit
10.1 to the Current Report on Form 8-K Filed with the SEC on December 23,
2009).
|
|
|
10.4
|
Form
of Subscription Agreement (incorporated by reference to Exhibit 10.1 to
the Current Report on Form 8-K Filed with the SEC on January 26,
2010).
|
|
|
10.5
|
Form
of Note (incorporated by reference to Exhibit 10.1 to the Current Report
on Form 8-K Filed with the SEC on January 26, 2010).
|
|
|
10.6
|
Form
of Warrant (incorporated by reference to Exhibit 10.1 to the Current
Report on Form 8-K Filed with the SEC on January 26,
2010).
|
|
|
10.7
|
Form
of Stock Pledge Agreement (incorporated by reference to Exhibit 10.1 to
the Current Report on Form 8-K Filed with the SEC on January 26,
2010).
|
|
|
10.8
|
Form
of Subsidiary Guaranty Agreement (incorporated by reference to Exhibit
10.1 to the Current Report on Form 8-K Filed with the SEC on January 26,
2010).
|
|
|
10.9
|
Form
of Lock Up Agreement (incorporated by reference to Exhibit 10.1 to the
Current Report on Form 8-K Filed with the SEC on January 26,
2010).
|
|
|
10.10
|
Form
of Leakout Agreement (incorporated by reference to Exhibit 10.1 to the
Current Report on Form 8-K Filed with the SEC on January 26,
2010).
|
|
|
10.11
|
Form
of Collateral Agent Agreement (incorporated by reference to Exhibit 10.1
to the Current Report on Form 8-K Filed with the SEC on January 26,
2010).
|
|
|
10.12
|
Form
of Director Offer and Acceptance Letter (incorporated by reference to
Exhibit 10.1 to the Current Report on Form 8-K Filed with the SEC on March
4, 2010).
|
|
|
10.13
|
Equity
Transfer Agreement by and between Yongxin and Sun Shi Wei dated November
21, 2009.*
|
|
|
10.14
|
Stock
Purchase Agreement between the Company and PmMaster Beijing Software Co.,
Ltd. dated March 1,
2010.*
|
51
|
10.15
|
Amended
and Restated Director’s Offer and Acceptance Letter dated March 15,
2010.*
|
|
| 10.16 |
Share
Purchase Agreement by and among Digital Learning Management Corp., Yongxin
Liu and Yongkui Liu dated May 13,
2007.*
|
|
| 10.17 |
Sino-Foreign
Joint Venture Operation Agreement by and among Digital Learning Management
Corp., Yongxin Liu and Yongkui Liu dated May 13,
2007.*
|
|
|
21.1
|
List
of Subsidiaries.*
|
|
|
31.1
|
Certification
of Chief Executive Officer pursuant to Item 601(b)(31) of Regulation S-K,
as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of
2002.*
|
|
|
31.2
|
Certification
of Chief Financial Officer pursuant to Item 601(b)(31) of Regulation S-K,
as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of
2002.*
|
|
|
32.1
|
Certifications
of Chief Executive Officer and Chief Financial Officer pursuant to 18
U.S.C. Section 1350 as adopted pursuant to Section 906 of the
Sarbanes-Oxley Act of
2002.*
|
* Filed
herewith.
52
SIGNATURES
Pursuant
to the requirements of Section 13 or 15(d) of the Securities Exchange Act of
1934, the Registrant has duly caused this report to be signed on its behalf by
the undersigned, thereunto duly authorized, in the City of Changchun, People’s
Republic of China, on March 30, 2010.
|
CHINA
YONGXIN PHARMACEUTICALS INC.
|
|||
|
(Registrant)
|
|||
|
Dated:
March 30, 2010
|
By:
|
/s/ Yongxin Liu
|
|
|
By: Yongxin
Liu
|
|||
|
Chief
Executive Officer and
|
|||
|
Chairman
of the Board
|
|||
|
(Principal
Executive Officer)
|
|||
|
Dated:
March 30, 2010
|
By:
|
/s/ Harry
Zhang
|
|
|
By: Harry
Zhang
|
|||
|
Chief
Financial Officer
|
|||
|
(Principal
Financial and Accounting Officer)
|
|||
Pursuant
to the requirements of the Securities Exchange Act of 1934, this report has been
signed below by the following persons on behalf of the Company in the capacities
and on the dates indicated.
|
Signature
|
Capacity
|
Date
|
||
|
/s/ Yongxin
Liu
|
Chief
Executive Officer and
|
March
30, 2010
|
||
|
By: Yongxin
Liu
|
Chairman
of the Board
(Principal
Executive Officer)
|
|||
|
/s/ Harry
Zhang
|
Chief
Financial Officer and Director
|
March
30, 2010
|
||
|
By: Harry
Zhang
|
(Principal
Financial and Accounting Officer)
|
|||
|
/s/ Ning
Liu
|
President,
Chief Operating Officer and Director
|
March
30, 2010
|
||
|
By:
Ning Liu
|
||||
|
/s/ Hal
Lieberman
|
Director
|
March
30, 2010
|
||
|
By:
Hal Lieberman
|
||||
|
/s/ Laura
Philips
|
Director
|
March
30, 2010
|
||
|
By:
Laura Philips
|
|
/s/ Jingang
Wang
|
Director
|
March
30, 2010
|
||
|
By:
Jingang Wang
|
|
/s/ Bing
Li
|
Director
|
March
30, 2010
|
||
|
By:
Bing Li
|
53
CHINA
YONGXIN PHARMACEUTICALS INC.
CONSOLIDATED
FINANCIAL STATEMENTS
DECEMBER
31, 2009
TABLE
OF CONTENTS
|
F-2
|
||
|
Consolidated
Balance Sheets as of December 31, 2009 and December 31,
2008
|
F-3
|
|
|
Consolidated
Statements of Income for the years ended December 31, 2009 and
2008
|
F-4
|
|
|
Consolidated
Statements of Cash Flows for the years ended December 31, 2009 and
2008
|
F-5
|
|
|
F-6
|
||
|
Notes
to Consolidated Financial Statements
|
F-7 – F-24
|
F-1
REPORT OF INDEPENDENT REGISTERED
PUBLIC ACCOUNTING FIRM
To the
Board of Directors and Stockholders
China
Yongxin Pharmaceuticals Inc.
We have
audited the accompanying consolidated balance sheets of China Yongxin
Pharmaceuticals Inc. and its subsidiaries (the “Company”) as of December
31, 2009 and 2008, and the related consolidated statements of income,
stockholders' equity, and cash flows for the years then ended. These
consolidated financial statements are the responsibility of the Company's
management. Our responsibility is to express an opinion on these consolidated
financial statements based on our audits.
We
conducted our audits of these statements in accordance with the standards of the
Public Company Accounting Oversight Board (United States). Those standards
require that we plan and perform the audit to obtain reasonable assurance about
whether the financial statements are free of material
misstatement. An audit includes examining, on a test basis, evidence
supporting the amounts and disclosures in the financial statements. An audit
also includes assessing the accounting principles used and significant estimates
made by management, as well as evaluating the overall financial statement
presentation. We believe that our audits provide a reasonable basis for our
opinion.
In our
opinion, the consolidated financial statements referred to above present fairly,
in all material respects, the consolidated financial position of China Yongxin
Pharmaceuticals Inc. and its subsidiaries as of December 31, 2009 and 2008,
and the results of its consolidated statements of operations, stockholders'
equity, and its cash flows for the years then ended, in conformity with
accounting principles generally accepted in the United States of
America.
/s/
KABANI & COMPANY, INC.
CERTIFIED
PUBLIC ACCOUNTANTS
Los
Angeles, California
March
30, 2010
F-2
CHINA
YONGXIN PHARMACEUTICALS INC. AND SUBSIDIARIES
CONSOLIDATED
BALANCE SHEETS
AS
OF DECEMBER 31, 2009 AND 2008
|
2009
|
2008
|
|||||||
|
ASSETS
|
||||||||
|
Current
Assets:
|
||||||||
|
Cash
and cash equivalents
|
$ | 1,805,271 | $ | 600,432 | ||||
|
Restricted
cash
|
467,369 | - | ||||||
|
Accounts
receivable, net
|
12,305,103 | 6,027,340 | ||||||
|
Notes
receivable
|
903,867 | 1,334,078 | ||||||
|
Other
receivable, net
|
1,931,084 | 351,488 | ||||||
|
Advances
to suppliers
|
5,056,246 | 6,185,388 | ||||||
|
Prepaid
expenses
|
534,769 | 342,441 | ||||||
|
Inventory,
net
|
7,811,628 | 7,713,209 | ||||||
|
Due
from related party
|
1,199,628 | - | ||||||
|
Current
assets of discontinued operations
|
- | 173,201 | ||||||
|
Total
Current Assets
|
32,014,966 | 22,727,579 | ||||||
|
Property
and Equipment, net
|
8,751,813 | 2,673,909 | ||||||
|
Construction
In Progress
|
1,551 | 6,066,249 | ||||||
|
Intangible
Assets, net
|
987,332 | 72,680 | ||||||
|
Non-current
assets of Discontinued Operations
|
- | 7,305 | ||||||
|
Total
Assets
|
$ | 41,755,662 | $ | 31,547,722 | ||||
|
LIABILITIES AND STOCKHOLDERS'
EQUITY
|
||||||||
|
Current
Liabilities:
|
||||||||
|
Accounts
payable
|
$ | 4,151,219 | $ | 3,171,826 | ||||
|
Accrued
expenses & other payable
|
5,170,786 | 2,406,602 | ||||||
|
Advances
from customers
|
2,055,602 | 2,579,997 | ||||||
|
Taxes
payable
|
1,421,434 | 1,245,649 | ||||||
|
Loans
from related parties
|
184,662 | 184,662 | ||||||
|
Short-term
loan payable
|
1,100,884 | 1,945,179 | ||||||
|
Deferred
income
|
419,277 | 273,753 | ||||||
|
Shares
to be issued
|
65,000 | 35,000 | ||||||
|
Liabilities
of discontinued operations
|
628,837 | 735,289 | ||||||
|
Total
Current Liabilities
|
15,197,700 | 12,577,957 | ||||||
|
Long
term loan
|
1,320,300 | 1,320,390 | ||||||
|
Commitments
and Contingencies
|
- | - | ||||||
|
Stockholders'
Equity:
|
||||||||
|
Preferred
stock, $0.001 par value; 5,000,000 shares authorized; 1,666,667 shares
issued and outstanding as of December 31, 2009 and 5,000,000 shares issued
and outstanding as of December 31, 2008
|
1,667 | 5,000 | ||||||
|
Common
stock; $0.001 par value; 75,000,000 shares authorized; 56,448,923 shares
issued and outstanding as of December 31, 2009 and 31,400,540 shares
issued and outstanding as of December 31, 2008
|
56,449 | 31,401 | ||||||
|
Additional
paid in capital
|
1,165,899 | 615,906 | ||||||
|
Deferred
consulting expense - issuance of warrants
|
(4,740 | ) | (72,815 | ) | ||||
|
Prepaid
consulting - issuance of shares
|
(5,000 | ) | (68,750 | ) | ||||
|
Receivable
from a related party
|
(50,000 | ) | (50,000 | ) | ||||
|
Statutory
reserve
|
2,630,329 | 1,841,241 | ||||||
|
Other
comprehensive income
|
1,807,859 | 1,684,649 | ||||||
|
Retained
earnings
|
13,920,649 | 9,563,803 | ||||||
|
Non-controlling
interest
|
5,714,550 | 4,098,940 | ||||||
|
Total
Stockholders' Equity
|
25,237,662 | 17,649,375 | ||||||
|
Total
Liabilities and Stockholders' Equity
|
$ | 41,755,662 | $ | 31,547,722 | ||||
The
accompanying notes are an integral part of these consolidated financial
statements
F-3
CHINA
YONGXIN PHARMACEUTICALS INC. AND SUBSIDIARIES
CONSOLIDATED
STATEMENTS OF INCOME
FOR
THE YEARS ENDED DECEMBER 31, 2009 AND 2008
|
2009
|
2008
|
|||||||
|
Net
Revenues
|
$ | 47,589,280 | $ | 59,116,534 | ||||
|
Cost
of Goods Sold
|
(31,271,463 | ) | (47,226,275 | ) | ||||
|
Gross
profit
|
16,317,817 | 11,890,259 | ||||||
|
Operating
Expenses:
|
||||||||
|
Selling
expenses
|
3,543,383 | 3,521,147 | ||||||
|
General
and administrative expenses
|
3,575,059 | 2,500,366 | ||||||
|
Total
operating expenses
|
7,118,442 | 6,021,513 | ||||||
|
Income
From Operations
|
9,199,376 | 5,868,745 | ||||||
|
Other
Income (Expense):
|
||||||||
|
Other
income
|
278,846 | 690,516 | ||||||
|
Other
expense
|
(137,849 | ) | (152,469 | ) | ||||
|
Interest
income (expense)
|
9,173 | (6,679 | ) | |||||
|
Total
other income
|
150,170 | 531,368 | ||||||
|
Operating
Income Before Income Tax and Non controlling Interest
|
9,349,545 | 6,400,113 | ||||||
|
Provision
for income tax
|
(2,594,483 | ) | (1,009,643 | ) | ||||
|
Net
Income Before Non controlling Interest and Discontinued
operations
|
6,755,062 | 5,390,470 | ||||||
|
Loss
from discontinued operations
|
(30,951 | ) | (84,850 | ) | ||||
|
Net
Income Before Non controlling Interest
|
6,724,111 | 5,305,619 | ||||||
|
Non
controlling interest
|
(1,599,122 | ) | (1,239,480 | ) | ||||
|
Net
Income
|
5,124,989 | 4,066,139 | ||||||
|
Other
Comprehensive Item:
|
||||||||
|
Foreign
exchange translation gain
|
123,209 | 824,961 | ||||||
|
Net
Comprehensive Income
|
$ | 5,248,198 | $ | 4,891,100 | ||||
|
Earning
per share
|
||||||||
|
Basic
|
$ | 0.15 | $ | 0.13 | ||||
|
Diluted
|
$ | 0.15 | $ | 0.13 | ||||
|
Weighted
average number of shares outstanding
|
||||||||
|
Basic
|
33,240,797 | 31,150,819 | ||||||
|
Diluted
|
35,070,051 | 31,150,819 | ||||||
The
accompanying notes are an integral part of these consolidated financial
statements
F-4
CHINA
YONGXIN PHARMACEUTICALS INC. AND SUBSIDIARIES
CONSOLIDATED
STATEMENTS OF CASH FLOWS
FOR
THE YEARS ENDED DECEMBER 31, 2009 AND 2008
|
2009
|
2008
|
|||||||
|
CASH
FLOWS FROM OPERATING ACTIVITIES
|
||||||||
|
Net
Income
|
5,124,989 | 4,066,139 | ||||||
|
Adjustments
to reconcile net income to net cash provided by operating
activities:
|
||||||||
|
Provision
for bad debts
|
(138,997 | ) | - | |||||
|
Stocks
and warrants issued for services
|
94,599 | 424,700 | ||||||
|
Depreciation
and amortization
|
447,689 | 310,443 | ||||||
|
Amortization
of prepaid & deferred consulting cost
|
141,565 | - | ||||||
|
Non-controlling
interest
|
1,599,122 | 1,239,480 | ||||||
|
(Increase)
/ decrease in current assets:
|
||||||||
|
Accounts
receivable
|
(6,135,319 | ) | 954,908 | |||||
|
Notes
receivable
|
429,856 | (1,310,799 | ) | |||||
|
Other
receivable
|
(1,535,598 | ) | (169,668 | ) | ||||
|
Advances
to suppliers
|
1,128,028 | (53,084 | ) | |||||
|
Prepaid
expenses
|
(192,233 | ) | (5,074 | ) | ||||
|
Inventory
|
(98,884 | ) | (1,083,197 | ) | ||||
|
Increase
/ (decrease) in current liabilities:
|
||||||||
|
Accounts
payable
|
895,698 | (2,164,344 | ) | |||||
|
Accrued
expenses and other payable
|
2,763,133 | 910,098 | ||||||
|
Tax
payable
|
175,762 | 924,337 | ||||||
|
Shares
to be issued
|
30,000 | - | ||||||
|
Advances
from customers
|
(523,898 | ) | 1,821,020 | |||||
|
Deferred
income
|
- | 16,679 | ||||||
|
Total
Adjustments
|
(919,476 | ) | 1,815,498 | |||||
|
Net
cash provided by operating activities from continuing
operations
|
4,205,513 | 5,881,637 | ||||||
|
Net
cash provided by/ (used in) operating activities of discontinued
operations
|
88,974 | 13,323 | ||||||
|
Net
cash provided by operating activities
|
4,294,486 | 5,894,960 | ||||||
|
CASH
FLOWS FROM INVESTING ACTIVITIES
|
||||||||
|
Restricted
cash
|
(467,369 | ) | - | |||||
|
Investment
|
- | (115,309 | ) | |||||
|
Acquistion
of property & equipment, net
|
(1,375,577 | ) | (786,486 | ) | ||||
|
Due
from related party
|
(1,198,892 | ) | - | |||||
|
Additions
to construction in progress
|
- | (5,960,396 | ) | |||||
|
Contribution
from Non controlling Interest
|
- | 11,532 | ||||||
|
Net
cash used in investing activities from continuing
operations
|
(3,041,838 | ) | (6,850,659 | ) | ||||
|
Net
cash provided by investing activities of discontinued
operations
|
16,284 | 139,039 | ||||||
|
Net
cash used in investing activities
|
(3,025,555 | ) | (6,711,620 | ) | ||||
|
CASH
FLOWS FROM FINANCING ACTIVITIES
|
||||||||
|
Receipt
of loans/ (payment of loans) from non-related parties
|
(729,437 | ) | 203,797 | |||||
|
Stock
issued for cash
|
467,369 | - | ||||||
|
Receipts
of loan from related parties, net
|
- | 64,868 | ||||||
|
Net
cash provided by/ (used in) financing activities from continuing
operations
|
(262,068 | ) | 268,664 | |||||
|
Net
cash used in financing activities of discontinued
operations
|
(105,257 | ) | (67,511 | ) | ||||
|
Net
cash provided by/ (used in) financing activities
|
(367,325 | ) | 201,153 | |||||
|
NET
INCREASE/ (DECREASE) IN CASH AND CASH EQUIVALENTS
|
901,606 | (615,507 | ) | |||||
|
EFFECT
OF EXCHANGE RATE CHANGES ON CASH AND CASH EQUIVALENTS
|
303,232 | 69,292 | ||||||
|
CASH
AND CASH EQUIVALENTS, BEGINNING BALANCE
|
600,432 | 1,146,648 | ||||||
|
CASH
AND CASH EQUIVALENTS, ENDING BALANCE
|
$ | 1,805,271 | $ | 600,432 | ||||
|
SUPPLEMENTAL
DISCLOSURES:
|
||||||||
|
Cash
paid during the year for:
|
||||||||
|
Interest
|
$ | 170,726 | $ | 167,156 | ||||
|
Income
tax
|
$ | 2,497,591 | $ | 15,927 | ||||
The
accompanying notes are an integral part of these consolidated financial
statements
F-5
CHINA
YONGXIN PHARMACEUTICALS INC. AND SUBSIDIARIES
CONSOLIDATED
STATEMENT OF STOCKHOLDERS' EQUITY
FOR
THE YEARS ENDED DECEMBER 31, 2009 AND 2008
|
Additional
|
Other
|
Deferred
consulting
|
Prepaid
consulting
|
Receivable
from
|
Non
|
Total
|
||||||||||||||||||||||||||||||||||||||||||||||
|
Common
Stock
|
Preferred
Stock
|
Paid-in
|
Comprehensive
|
Statutory
|
expense-
|
issuance
|
related
|
controlling
|
Retained
|
Stockholders’
|
||||||||||||||||||||||||||||||||||||||||||
|
Shares
|
Amount
|
Shares
|
Amount
|
Capital
|
Income
|
Reserve
|
warrants
|
of shares
|
party
|
Interest
|
Earnings
|
Equity
|
||||||||||||||||||||||||||||||||||||||||
|
Balance
as of December 31, 2007
|
31,041,845 | $ | 31,042 | 5,000,000 | $ | 5,000 | $ | - | $ | 859,688 | $ | 1,341,600 | $ | - | $ | - | $ | - | $ | 2,623,303 | $ | 5,997,306 | $ | 10,857,937 | ||||||||||||||||||||||||||||
|
Issuance
of shares
|
108,695 | 109 | - | - | 49,891 | - | - | - | - | (50,000 | ) | - | - | |||||||||||||||||||||||||||||||||||||||
|
Stock
and warrants issued for consulting
|
250,000 | 250 | - | - | 566,015 | - | - | (291,265 | ) | (275,000 | ) | - | - | - | ||||||||||||||||||||||||||||||||||||||
|
Amortization
of prepaid consulting
|
- | - | - | - | - | - | - | 218,450 | 206,250 | - | - | 424,700 | ||||||||||||||||||||||||||||||||||||||||
| - | ||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Foreign
exchange translation gain
|
- | - | - | - | - | 1,031,202 | - | - | - | - | - | 1,031,202 | ||||||||||||||||||||||||||||||||||||||||
| - | ||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Transfer
to statutory reserve
|
- | - | - | - | - | - | 499,641 | - | - | - | (499,641 | ) | - | |||||||||||||||||||||||||||||||||||||||
| - | ||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Net
income for the year
|
- | - | - | - | - | - | - | - | - | - | 5,305,619 | 5,305,619 | ||||||||||||||||||||||||||||||||||||||||
|
Transfer
to Non-controlling interest
|
29,916 | 29,916 | ||||||||||||||||||||||||||||||||||||||||||||||||||
|
Transfer
to non- controlling interest
|
- | - | - | - | - | (206,241 | ) | - | - | - | - | 1,445,721 | (1,239,480 | ) | - | |||||||||||||||||||||||||||||||||||||
|
Balance
as of December 31, 2008
|
31,400,540 | 31,401 | 5,000,000 | 5,000 | 615,906 | 1,684,649 | 1,841,241 | (72,815 | ) | (68,750 | ) | (50,000 | ) | 4,098,940 | 9,563,804 | 17,649,375 | ||||||||||||||||||||||||||||||||||||
|
Contribution
by Non-controlling interest
|
- | - | - | - | - | - | - | - | - | - | 6,631 | - | 6,631 | |||||||||||||||||||||||||||||||||||||||
|
Warrants
issued for consulting
|
- | - | - | - | 28,439 | - | - | (4,740 | ) | - | - | - | - | 23,699 | ||||||||||||||||||||||||||||||||||||||
|
Amortization
of prepaid consulting
|
- | - | - | - | - | - | - | 72,815 | 68,750 | - | - | - | 141,565 | |||||||||||||||||||||||||||||||||||||||
|
Shares
issued for consulting
|
710,000 | 710 | - | - | 75,190 | - | - | - | (5,000 | ) | - | - | - | 70,900 | ||||||||||||||||||||||||||||||||||||||
|
Shares
issued under private placement
|
4,338,383 | 4,338 | - | - | 463,031 | - | - | - | - | - | - | - | 467,369 | |||||||||||||||||||||||||||||||||||||||
|
Conversion
of preferred stock to common stock
|
20,000,000 | 20,000 | (3,333,333 | ) | (3,333 | ) | (16,667 | ) | - | - | - | - | - | - | - | - | ||||||||||||||||||||||||||||||||||||
|
Foreign
exchange translation gain
|
- | - | - | - | - | 154,011 | - | - | - | - | - | - | 154,011 | |||||||||||||||||||||||||||||||||||||||
|
Transfer
to statutory reserve
|
- | - | - | - | - | - | 789,088 | - | - | - | - | (789,088 | ) | - | ||||||||||||||||||||||||||||||||||||||
|
Net
income for the year
|
- | - | - | - | - | - | - | - | - | - | - | 6,724,111 | 6,724,111 | |||||||||||||||||||||||||||||||||||||||
|
Transfer
to non controlling interest
|
- | - | - | - | - | (30,802 | ) | - | - | - | - | 1,608,979 | (1,578,177 | ) | - | |||||||||||||||||||||||||||||||||||||
|
Balance
as of December 31, 2009
|
56,448,923 | $ | 56,449 | 1,666,667 | $ | 1,667 | $ | 1,165,899 | $ | 1,807,859 | $ | 2,630,329 | $ | (4,740 | ) | $ | (5,000 | ) | $ | (50,000 | ) | $ | 5,714,550 | $ | 13,920,650 | $ | 25,237,661 | |||||||||||||||||||||||||
The
accompanying notes are an integral part of these consolidated financial
statements
F-6
CHINA
YONGXIN PHARMACEUTICALS INC.
NOTES TO
CONSOLIDATED FINANCIAL STATEMENTS
NOTE 1 -
ORGANIZATION
China
Yongxin Pharmaceuticals Inc. (formerly Digital Learning Management Corporation
and Nutradyne Group, Inc.) (the “Company") was incorporated in Delaware on
February 18, 1999. The Company through its Chinese subsidiaries is engaged in
the pharmaceutical medicines and appliances wholesale distribution, pharmacy
retail drug stores and ginseng product sales.
On
December 21, 2006, Changchun Yongxin Dirui Medical Co., Ltd, a Chinese
corporation ("Yongxin") and all of the stockholders of Yongxin entered into a
share exchange agreement (“Share Exchange Agreement”) with the Company. The
Share Exchange Agreement was amended on June 15, 2007 (the “Amended Exchange
Agreement”). On November 16, 2007, Yongxin and the Company closed on the share
exchange under the Amended Exchange Agreement. On April 12, 2008, we
entered into a second amended Share Exchange Agreement with Yongxin, effective
November 16, 2007, in which the Company acquired from the original Yongxin
stockholders, and Yongxin stockholders transferred to the Company, 80% of the
equity interest of Yongxin in exchange for the issuance by the Company of an
aggregate of 21,000,000 shares of newly issued common stock and 5 million shares
of Series A Preferred Stock to the Yongxin stockholders and/or their designees,
representing, immediately following closing, 70% of the total issued and
outstanding shares of common stock of the Company in exchange for 80% shares of
Yongxin (“Share Exchange Transaction”). The Series A Convertible Preferred Stock
is convertible over a 3 year period, into up to 30 million shares of common
stock.
For
accounting purposes, this transaction was accounted for as a reverse merger,
since the stockholders of Yongxin own a majority of the issued and outstanding
shares of common stock of the Company, and the directors and executive officers
of Yongxin became the directors and executive officers of the
Company. This acquisition was accounted for at historical cost in a
manner similar to that in the pooling of interests method since after the
acquisition, the former stockholders of Yongxin acquired a majority of the
outstanding shares of the Company.
Yongxin
was originally established in 1993. The Company is engaged in wholesale and
retail of medicines. The Company’s operations are based in Changchun City, Jilin
Province, China.
In 2004,
Yongxin established Jilin Province Yongxin Chain Drugstore Ltd. (“Yongxin
Drugstore”) with an investment of RMB 2,500,000 (equivalent to $303,000) to
develop a customer-terminal network market. In July 2005, the Company obtained
the franchise rights in Jilin Province from American Medicine Shoppe (Meixin
International Medical Chains) and sells over-the-counter western and traditional
Chinese medicines and other medical-related products.
On March
16, 2007, Jilin Province Yongxin Chain Drugstore Ltd. entered into various
agreements with retail drug stores in Tianjin, and established Tianjin
Jingyongxin Chain Drugstore Ltd. (“Jinyongxin Drugstore”) with an investment of
$116,868, in which the Company has the 90% ownership of the Jinyongxin
Drugstore. The Company is located in Tianjin City,
China.
On May
15, 2007, Yongxin established Jilin Dingjian Natural Health Products Co., Ltd.
(“Dingjian”) with an investment of $116,868 whereby the stockholders of the
company have 90% ownership of Dingjian. Dingjian was formed under laws of the
People's Republic of China and is located in Changchun City, Jilin
Province.
On June
15 2007, Yongxin Drugstore established Baishan Caoantang Chain Drugstore Ltd. (
“Caoantang Drugstore”) with an investment of $328,430, including $144,509 in
cash and $183,921 to purchase the property and equipment and Yongxin agreed to
pay $80,076 evenly over the next 32 months for this investment. Caoantang
Drugstore is a 100% owned subsidiary of Yongxin Drugstore. Caoantang
Drugstore operates a chain of 32 chain retail drugstores and covers a business
area of 2,804 M2, which
sell over-the-counter western and traditional Chinese medicines and other
medical-related products.
F-7
On May 5,
2008 the Company changed its name from Nutradyne Group, Inc to China Yongxin
Pharmaceuticals Inc.
In
November 2009, Dingjian entered into an Equity Transfer Agreement (the
“Agreement”) with Sun Shi Wei, an individual, to transfer 90% of ownership with
all its assets and liabilities to Sun Shi Wei. The 10%
non-controlling interest remained unchanged. Both parties agreed that
Sun Shi Wei assumed the net liabilities. No other consideration was exchanged.
Dingjian would be liable for any undiscovered liability other than the liability
assumed by Sun Shi Wei, pursuant to the Agreement.
NOTE
2 - SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
BASIS OF
PRESENTATION
The
accompanying financial statements have been prepared in conformity with
accounting principles generally accepted in the United States of America. The
functional currency of our operating subsidiary, Changchun Yongxin Dirui Medical
Co., Ltd is Chinese Renminbi (“CNY”); however the accompanying financial
statements have been translated and presented in United States Dollars
(“USD”).
TRANSLATION
ADJUSTMENT
As of
December 31, 2009 and 2008, the accounts of Yongxin were maintained, and its
financial statements were expressed, in Chinese Yuan Renminbi. Such financial
statements were translated into USD in accordance with Statement of Financial
Accounts Standards (“SFAS”) No. 52, “Foreign Currency Translation,” with the CNY
as the functional currency. According to the Statement, all assets and
liabilities were translated at the current exchange rate, stockholders’ equity
are translated at the historical rates and income statement items are translated
at the average exchange rate for the period. The resulting translation
adjustments are reported under other comprehensive income in accordance with
SFAS No. 130 (ASC 220), “Reporting Comprehensive Income” as a component of
stockholders’ equity.
USE OF
ESTIMATES
The
preparation of financial statements in conformity with generally accepted
accounting principles in the United States (“GAAP”) requires management to make
certain estimates and assumptions that affect the reported amounts of assets and
liabilities and disclosure of contingent assets and liabilities at the date of
the financial statements and the reported amounts of revenues and expenses
during the reporting period. Actual results could differ from those
estimates. Significant estimates include collectability of accounts
receivable, accounts payable, sales returns and recoverability of long-term
assets.
PRINCIPLES OF
CONSOLIDATION
The
consolidated financial statements include the accounts of China Yongxin
Pharmaceuticals Inc. and its subsidiaries collectively referred to within as the
“Company”. All material inter-company accounts, transactions and
profits have been eliminated in consolidation.
NON-CONTROLLING
INTEREST
The
accompanying consolidated financial statements have been prepared by the
Company, pursuant to the rules and regulations of the Securities and Exchange
Commission (the “SEC”) and in accordance with GAAP. The Company acquired 80% of
Yongxin, and Yongxin Liu and Yongkui Liu own 11% and 9% of Yongxin,
respectively. The 20% equity interest held by Yongxin Liu and Yongkui
Liu represents non-controlling interest amounting to $5,687,633 as at December
31, 2009 compared to $4,078,654 as at December 31, 2008.
The
Company owns a 90% ownership interest in Jinyongxin Drugstore. The remaining 10%
interest in Jinyongxin Drugstore is owned by third
parties.
CASH AND CASH
EQUIVALENTS
Cash and
cash equivalents consist primarily of cash in banks and highly liquid
investments with original maturities of 90 days or less.
F-8
ACCOUNTS
RECEIVABLE
The
Company maintains reserves for potential credit losses on accounts receivable.
Management reviews the composition of accounts receivable and analyzes
historical bad debts, customer concentrations, customer credit worthiness,
current economic trends and changes in customer payment patterns to evaluate the
adequacy of these reserves. Reserves are recorded primarily on a specific
identification basis. As of December 31, 2009, we made no allowance
for doubtful debts. As of December 31, 2008, we made allowance of doubtful debts
of $112,452.
ADVANCES TO
SUPPLIERS
The
Company advances to certain vendors for purchase of its material. The
advances to suppliers are interest free and unsecured. As of December 31, 2009
and December 31, 2008, advance to suppliers amounted to $5,056,246 and
$6,186,269, respectively.
INVENTORIES
Inventories
are valued on a lower of weighted average cost or market
basis. Inventory includes product cost, inbound freight,
warehousing costs and vendor allowances not included as a reduction of
advertising expense. The management compares the cost of inventories with the
market value and allowance is made for writing down their inventories to market
value, if lower. Work in process inventories include the cost of raw materials
and outsource processing fees.
PROPERTY AND
EQUIPMENT
Property
and equipment are recorded at cost. Depreciation is computed using the
straight-line method over useful lives ranging from 5 to 10 years. Expenditures
for maintenance and repairs are charged to earnings as incurred; additions,
renewals and betterments are capitalized. When property and equipment are
retired or otherwise disposed of, the related cost and accumulated depreciation
are removed from the respective accounts, and any gain or loss is included in
operations. Assets held under capital leases are recorded at the lesser of the
present value of the future minimum lease payments or the fair value of the
leased property. Expenditures for maintenance and repairs are charged to
operations as incurred. Depreciation of property and equipment is provided using
the straight-line method for substantially all assets with estimated lives
of:
|
Buildings
|
20
years
|
|
|
Infrastructures
and leasehold improvements
|
10
years
|
|
|
Equipment
(including electronic facilities, sports, education and recreation
facilities)
|
10
years
|
|
|
Automobiles
|
10
years
|
|
|
Furniture
and fixtures
|
5
years
|
|
|
Computer
hardware and software
|
|
5
years
|
IMPAIRMENT OF LONG-LIVED
ASSETS
Effective
January 1, 2002, the Company adopted Statement of Financial Accounting Standards
No. 144, “Accounting for the Impairment or Disposal of Long-Lived Assets” (“SFAS
144”) (ASC 360), which addresses
financial accounting and reporting for the impairment or disposal of long-lived
assets and supersedes SFAS No. 121, “Accounting for the Impairment of Long-Lived
Assets and for Long-Lived Assets to be Disposed Of,” and the accounting and
reporting provisions of APB Opinion No. 30, “Reporting the Results of Operations
for a Disposal of a Segment of a Business.” The Company periodically evaluates
the carrying value of long-lived assets to be held and used in accordance with
SFAS 144 (ASC 360). SFAS 144 (ASC 360) requires impairment losses to be recorded
on long-lived assets used in operations when indicators of impairment are
present and the undiscounted cash flows estimated to be generated by those
assets are less than the assets’ carrying amounts. In that event, a loss is
recognized based on the amount by which the carrying amount exceeds the fair
market value of the long-lived assets. Loss on long-lived assets to be disposed
of is determined in a similar manner, except that fair market values are reduced
for the cost of disposal.
REVENUE
RECOGNITION
The
Company’s revenue recognition policies are in compliance with Staff Accounting
Bulletin (“SAB”) 104 (ASC 605). Sales revenue is recognized at the date of
shipment to customers when a formal arrangement exists, the price is fixed or
determinable, the delivery is completed, no other significant obligations of the
Company exist and collectability is reasonably assured. The Company recognizes
revenue net of an allowance for estimated returns, at the time the merchandise
is sold or services performed. The allowance for sales returns is estimated
based on the Company’s historical experience. Sales taxes are presented on a net
basis (excluded from revenues and costs). Payments received before all of the
relevant criteria for revenue recognition are satisfied are recorded as unearned
revenue.
F-9
ADVERTISING
Advertising
expenses consist primarily of costs of promotion for corporate image and product
marketing and costs of direct advertising. The Company expenses all
advertising costs as incurred. The advertising expense for the years
ended December 31, 2009 and 2008 was $132,264 and $26,124,
respectively.
VENDOR
ALLOWANCES
Vendor
allowances are principally received as a result of purchase levels, sales or
promotion of vendors' products. Allowances are generally recorded as
a reduction of inventory and are recognized as a reduction of cost of sales when
the related merchandise is sold. Those allowances received for
promoting vendors' products are offset against advertising expense and result in
a reduction of selling, occupancy and administration expenses to the extent of
advertising costs incurred, with the excess treated as a reduction of inventory
costs.
INCOME
TAXES
The
Company utilizes SFAS No. 109 (ASC 740), "Accounting for Income Taxes," which
requires the recognition of deferred tax assets and liabilities for the expected
future tax consequences of events that have been included in the financial
statements or tax returns. Under this method, deferred income taxes are
recognized for the tax consequences in future years of differences between the
tax basis of assets and liabilities and their financial reporting amounts at
each period end based on enacted tax laws and statutory tax rates applicable to
the periods in which the differences are expected to affect taxable income.
Valuation allowances are established, when necessary, to reduce deferred tax
assets to the amount expected to be realized.
The
Company accounts for income taxes using an asset and liability approach which
allows for the recognition and measurement of deferred tax assets based upon the
likelihood of realization of tax benefits in future years. Under the asset and
liability approach, deferred taxes are provided for the net tax effects of
temporary differences between the carrying amounts of assets and liabilities for
financial reporting purposes and the amounts used for income tax purposes. A
valuation allowance is provided for deferred tax assets if it is more likely
than not these items will either expire before the Company is able to realize
their benefits, or that future deductibility is uncertain.
The
Company records a valuation allowance for deferred tax assets, if any, based on
its estimates of its future taxable income as well as its tax planning
strategies when it is more likely than not that a portion or all of its deferred
tax assets will not be realized. If the Company is able to utilize more of its
deferred tax assets than the net amount previously recorded when unanticipated
events occur, an adjustment to deferred tax assets would increase the Company
net income when those events occur. The Company does not have any significant
deferred tax asset or liabilities in the PRC tax jurisdiction.
FAIR VALUE OF FINANCIAL
INSTRUMENTS
Statement
of Financial Accounting Standard No. 107, Disclosures about Fair Value of
Financial Instruments, requires that the Company disclose estimated fair values
of financial instruments. The carrying amounts reported in the statements of
financial position for current assets and current liabilities qualifying as
financial instruments are a reasonable estimate of fair value.
STOCK BASED
COMPENSATION
Effective
January 1, 2006, the Company adopted Statement No. 123R (ASC 718),
Share-Based Payment (SFAS 123R), which requires companies to measure and
recognize compensation expense for all stock-based payments at fair value. SFAS
123R is being applied on the modified prospective basis. Prior to the adoption
of SFAS 123R, the Company accounted for its stock-based compensation plans under
the recognition and measurement principles of Accounting Principles Board
(APB) Opinion No. 25, Accounting for Stock Issued to Employees, and
related interpretations, and accordingly, recognized no compensation expense
related to the stock-based plans. Under the modified prospective approach, SFAS
123R applies to new awards and to awards that were outstanding on
January 1, 2006 that are subsequently modified, repurchased or
cancelled.
F-10
BASIC AND DILUTED EARNINGS
PER SHARE
Earnings
per share are calculated in accordance with the Statement of Financial
Accounting Standards No. 128 (SFAS No. 128) (ASC 260), “Earnings per share”.
SFAS No. 128 (ASC 260) superseded Accounting Principles Board Opinion No.15 (APB
15). Net income (loss) per share for all periods presented has been restated to
reflect the adoption of SFAS No. 128. Basic earnings per share are computed by
dividing net income by the weighted average number of common shares outstanding
during the period. Diluted earnings per share are computed by dividing net
income by the weighted average number of common shares and dilutive common
equivalent shares (restricted stock awards and stock options) outstanding during
the period. Weighted average number of common shares was calculated in
accordance with the Statement of Financial Accounting Standards No. 141R (SFAS
No. 141R) (ASC 805), “Business Combinations”. Basic and diluted
earnings per share were $0.15 and $0.13 for the years ended December 31, 2009
and 2008, respectively.
STATEMENT OF CASH
FLOWS
In
accordance with Statement of Financial Accounting Standards No. 95 (ASC 230),
"Statement of Cash Flows," cash flows from the Company's operations is
calculated based upon the local currencies. As a result, amounts related to
assets and liabilities reported on the statement of cash flows will not
necessarily agree with changes in the corresponding balances on the balance
sheet.
SEGMENT
REPORTING
Statement
of Financial Accounting Standards No. 131 ("SFAS 131") (ASC 250), "Disclosure
about Segments of an Enterprise and Related Information" requires use of the
"management approach" model for segment reporting. The management
approach model is based on the way a company's management organizes segments
within the company for making operating decisions and assessing performance.
Reportable segments are based on products and services, geography, legal
structure, management structure, or any other manner in which management
disaggregates a company. The Company allocates its resources and assesses the
performance of its sales activities based upon its products and services (see
Note 20).
RISKS AND
UNCERTAINTIES
The
Company is subject to substantial risks from, among other things, intense
competition associated with the industry in general, other risks associated with
financing, liquidity requirements, rapidly changing customer requirements,
limited operating history, foreign currency exchange rates and the volatility of
public markets.
The
Company’s operations are carried out in the PRC. Accordingly, the Company’s
business, financial condition and results of operations may be influenced by the
political, economic and legal environments in the PRC, by the general state of
PRC’s economy. The Company’s business may be influenced by changes in
governmental policies with respect to laws and regulations, anti-inflationary
measures, currency conversion and remittance abroad, and rates and methods of
taxation, among other things.
Under
current PRC law on foreign investment, foreign companies are allowed to
establish or invest in wholly-owned foreign enterprises or joint ventures that
engage in wholesale or retail sales of pharmaceuticals in China. These
regulations limit the number and size of retail pharmacy outlets that a foreign
investor may establish. If a foreign investor owns more than 30 outlets that
sell a variety of branded pharmaceutical products sourced from different
suppliers, the foreign investor's ownership interests in the outlets may be
limited to 49.0%. The Company currently controls more than 30 retail pharmacy
outlets through its 80% equity ownership interest in Yongxin, 100% equity
ownership in Caoantang Drugstore and 90% equity ownership interest in Jinyongxin
Drugstore. At the time of the establishment of Yongxin, Yongxin already
controlled in excess of 30 retail pharmacy outlets and with this structure in
place it obtained all required approvals from the relevant governmental agencies
in Jilin Province for the joint venture. The Company has been advised by its PRC
counsel, that based on their understanding of the current PRC laws, rules and
regulations and the Company’s receipt of all relevant government approvals for
the equity joint venture, the structure under which it operates and holds equity
ownership in its retail pharmacy businesses complies with all applicable PRC
laws, rules and regulations. However, there are uncertainties regarding the
interpretation and application of PRC laws, rules and regulations by PRC
government authorities, and there is a risk that such authorities may later
issue a differing interpretation of the law and determine that the Company’s
corporate and/or ownership structure does not comply with PRC laws, rules and
regulations. In addition, new PRC laws, rules and regulations may be introduced
from time to time to impose additional requirements that may be applicable to
the Company’s corporate structure or its business operations. If the Company
and/or its PRC subsidiaries are determined to be in violation of any existing or
future PRC laws, rules or regulations, including laws applicable to foreign
investment in retail pharmacy outlets, or fail to obtain or maintain any of the
required governmental permits or approvals, the relevant PRC regulatory
authorities would have broad discretion in dealing with such violations,
including: The imposition of penalties and/or a restructuring of the Company’s
holding structure in order to comply with relevant PRC regulations could
severely disrupt the Company’s ability to conduct business and could have a
material adverse effect on the Company’s financial condition, results of
operations and prospects and also could result in:
|
•
|
revoking
the business and operating licenses of the Company’s PRC consolidated
entities;
|
|
•
|
discontinuing
or restricting the operations of the Company’s PRC consolidated
entities;
|
|
•
|
imposing
conditions or requirements with which the Company or its PRC consolidated
entities may not be able to
comply;
|
|
•
|
requiring
the Company or its PRC consolidated entities to restructure the relevant
ownership structure or
operations;
|
|
•
|
restricting
or prohibiting the Company’s use of the proceeds from its financings to
fund its business and operations in China;
or
|
|
•
|
imposing
fines.
|
CONCENTRATIONS OF CREDIT
RISK
Financial
instruments that potentially subject the Company to concentrations of credit
risk are cash, accounts receivable and other receivables arising from its normal
business activities. The Company places its cash in what it believes
to be credit-worthy financial institutions. The Company has a
diversified customer base, most of which are in China. The Company
controls credit risk related to accounts receivable through credit approvals,
credit limits and monitoring procedures. The Company routinely
assesses the financial strength of its customers and, based upon factors
surrounding the credit risk, establishes an allowance, if required, for
uncollectible accounts and, as a consequence, believes that its accounts
receivable credit risk exposure beyond such allowance is limited.
F-11
CONTINGENCIES
Certain
conditions may exist as of the date the financial statements are issued, which
may result in a loss to the Company but which will only be resolved when one or
more future events occur or fail to occur. The Company’s management
and legal counsel assess such contingent liabilities, and such assessment
inherently involves an exercise of judgment. In assessing loss
contingencies related to legal proceedings that are pending against the Company
or unasserted claims that may result in such proceedings, the Company’s legal
counsel evaluates the perceived merits of any legal proceedings or unasserted
claims as well as the perceived merits of the amount of relief sought or
expected to be sought.
If the
assessment of a contingency indicates that it is probable that a material loss
has been incurred and the amount of the liability can be estimated, then the
estimated liability would be accrued in the Company’s financial
statements. If the assessment indicates that a potential material
loss contingency is not probable but is reasonably possible, or is probable but
cannot be estimated, then the nature of the contingent liability, together with
an estimate of the range of possible loss if determinable and material would be
disclosed. Loss contingencies considered to be remote by management are
generally not disclosed unless they involve guarantees, in which case the
guarantee would be disclosed.
RECENT ACCOUNTING
PRONOUNCEMENTS
In
September 2009, the Financial Accounting Standards Board (“FASB”) issued
guidance related to revenue recognition for multiple element deliverables which
eliminates the requirement that all undelivered elements must have objective and
reliable evidence of fair value before a company can recognize the portion of
the consideration that is attributable to items that already have been
delivered. Under the new guidance, the relative selling price method is required
to be used in allocating consideration between deliverables and the residual
value method will no longer be permitted. This guidance is effective
prospectively for revenue arrangements entered into or materially modified in
2011 although early adoption is permitted. A company may elect, but will not be
required, to adopt the amendments retrospectively for all prior periods. The
Company is currently evaluating this guidance and has not yet determined the
impact, if any, that it will have on the consolidated financial
statements.
In June
2009, the FASB issued ASC 105 (previously SFAS No. 168, “The FASB Accounting
Standards Codification and the Hierarchy of Generally Accepted Accounting
Principles ("GAAP")” - a replacement of FASB Statement No. 162), which will
become the source of authoritative accounting principles generally accepted in
the United States recognized by the FASB to be applied to nongovernmental
entities.
In June
2009, the FASB issued ASC 855 (previously SFAS No. 165, “Subsequent Events”),
which establishes general standards of accounting for and disclosures of events
that occur after the balance sheet date but before the financial statements are
issued or available to be issued. It is effective for interim and annual periods
ending after June 15, 2009. There was no material impact upon the adoption of
this standard on the Company’s consolidated financial statements.
In
June 2009, the FASB issued ASC 860 (previously SFAS No. 166,
“Accounting for Transfers of Financial Assets”) , which requires additional
information regarding transfers of financial assets, including securitization
transactions, and where companies have continuing exposure to the risks related
to transferred financial assets. SFAS 166 eliminates the concept of a
“qualifying special-purpose entity,” changes the requirements for derecognizing
financial assets, and requires additional disclosures. SFAS 166 is effective for
fiscal years beginning after November 15, 2009. The Company does
not believe this pronouncement will impact its financial
statements.
In
June 2009, the FASB issued ASC 810 (previously SFAS No. 167) for
determining whether to consolidate a variable interest entity. These amended
standards eliminate a mandatory quantitative approach to determine whether a
variable interest gives the entity a controlling financial interest in a
variable interest entity in favor of a qualitatively focused analysis, and
require an ongoing reassessment of whether an entity is the primary
beneficiary. These amended standards are effective for us beginning
in the first quarter of fiscal year 2010 and we are currently evaluating the
impact that adoption will have on our consolidated financial
statements.
In August
2009, the FASB issued Accounting Standards Update (“ASU”) 2009-05, which amends
ASC Topic 820, Measuring Liabilities at Fair Value, which provides additional
guidance on the measurement of liabilities at fair value. These amended
standards clarify that in circumstances in which a quoted price in an active
market for the identical liability is not available, we are required to use the
quoted price of the identical liability when traded as an asset, quoted prices
for similar liabilities, or quoted prices for similar liabilities when traded as
assets. If these quoted prices are not available, we are required to use another
valuation technique, such as an income approach or a market
approach. These amended standards became effective for us beginning
in the fourth quarter of fiscal year 2009 and are not expected to have a
significant impact on our consolidated financial statements.
F-12
NOTE
3 –OTHER RECEIVABLE
Other
receivables as of December 31, 2009 and 2008 are summarized as follows. The
receivables are interest free, unsecured, and due on demand.
|
2009
|
2008
|
|||||||
|
Advance
to employees
|
$ | 26,493 | $ | 92,368 | ||||
|
Advances
to store employees
|
15,037 | 2,685 | ||||||
|
Advances
to third parties
|
- | 93,364 | ||||||
|
Rent
receivable
|
79,218 | 79,223 | ||||||
|
Deposits
|
765,925 | 7,619 | ||||||
|
Sponsorship
from customers
|
987,174 | - | ||||||
|
Others
|
57,237 | 76,229 | ||||||
|
Total
|
$ | 1,931,084 | $ | 351,488 | ||||
NOTE
4 – PREPAID EXPENSES
The
balance of Company prepaid expenses as of December 31, 2009 and 2008 comprised
of the following:
|
2009
|
2008
|
|||||||
|
Prepaid
rent
|
$ | 18,087 | $ | 273,484 | ||||
|
Rent
|
489,156 | - | ||||||
|
Other
prepaid expenses
|
27,525 | 68,957 | ||||||
|
Total
|
$ | 534,769 | $ | 342,441 | ||||
NOTE
5 - INVENTORIES
As of
December 31, 2009 and 2008, inventory consisted of the following:
|
2009
|
2008
|
|||||||
|
Packaging
Materials
|
$ | 200,007 | $ | 342,832 | ||||
|
Finished
Goods
|
7,611,621 | 7,370,377 | ||||||
|
Total
inventory
|
7,811,628 | 7,713,209 | ||||||
|
Net
inventory
|
$ | 7,811,628 | $ | 7,713,209 | ||||
F-13
NOTE
6 - PROPERTIES AND EQUIPMENT
As of
December 31, 2009 and 2008 the property and equipment of the Company consisted
of the following:
|
2009
|
2008
|
|||||||
|
Office
furniture and fixtures
|
$ | 930,962 | $ | 998,730 | ||||
|
Vehicles
|
392,557 | 441,921 | ||||||
|
Buildings
|
8,637,138 | 2,079,690 | ||||||
|
Total
property and equipment
|
9,960,657 | 3,520,341 | ||||||
|
Less:
Accumulated depreciation
|
(1,200,420 | ) | (846,432 | ) | ||||
|
Net
value of property and equipment
|
$ | 8,751,813 | $ | 2,673,909 | ||||
The
Company had depreciation expense of $409,245 and $293,632for as of December 31,
2009 and 2008, respectively.
NOTE
7 - CONSTRUCTION IN PROGRESS & SOFTWARE DEVELOPMENT
As of
December 31, 2009 and 2008, construction in progress, representing
infrastructures improvement and software development, amounted to $1,551 and
$6,066,249, respectively. The amount of capitalized interest included
in construction in progress as of December 31, 2009 and 2008 is $0 and $311,702,
respectively. The constructions were finished at the end of December
and were transferred to fixed assets.
As of
December 31, 2009 and 2008, the construction in progress of the Company
consisted of the following:
|
2009
|
2008
|
|||||||
|
Infrastructures
improvement
|
$ | - | $ | 4,841,430 | ||||
|
Capitalized
interest
|
- | 913,117 | ||||||
|
Total
infrastructures improvement
|
- | 5,754,547 | ||||||
|
Software
development
|
1,551 | 311,702 | ||||||
|
Total
construction in progress
|
$ | 1,551 | $ | 6,066,249 | ||||
NOTE
8- INTANGIBLE ASSETS
As of
December 31, 2009 and December 31, 2008, the intangible assets of the Company
consisted of the following:
|
2009
|
2008
|
|||||||
|
Trademark
|
$ | - | $ | 1,174 | ||||
|
Software
|
1,102,893 | 108,286 | ||||||
|
Total
intangible assets
|
1,102,893 | 109,460 | ||||||
|
Less:
Accumulated amortization
|
(115,561 | ) | (36,780 | ) | ||||
|
Net
value of intangible assets
|
$ | 987,332 | $ | 72,680 | ||||
F-14
The
amortization expense for as of December 31, 2009 and December 31, 2008 amounted
to $37,370 and $18,360, respectively.
The
amortization expenses for intangible assets for next five years after December
31, 2009 are as follows:
|
December
31, 2010
|
$ | 197,466 | ||
|
December
31, 2011
|
197,466 | |||
|
December
31, 2012
|
197,466 | |||
|
December
31, 2013
|
197,466 | |||
|
December
31, 2014
|
197,466 | |||
|
Total
|
$ | 987,432 |
NOTE
9 - ACCRUED EXPENSES AND OTHER PAYABLES
The other
payable represents the deposits made by the sales representatives and sales
distributors for the right to sell products for the Company. Other
payables and accrued expenses consist of the following as of December 31, 2009
and 2008:
|
2009
|
2008
|
|||||||
|
Accrued
compensation
|
$ | 1,091,299 | $ | 998,824 | ||||
|
Accrued
rent expense
|
124,874 | 247,573 | ||||||
|
Accrued
professional fees
|
86,026 | 60,806 | ||||||
|
Accrued
litigation
|
987,515 | 311,685 | ||||||
|
Accrued
interest
|
8,133 | 78,473 | ||||||
|
Accrued
payable
|
2,539,032 | 435,135 | ||||||
|
Accrued
education& employee funds
|
- | 29,088 | ||||||
|
Other
accrued expense
|
112,151 | 43,099 | ||||||
|
Sales
agent deposits
|
113,265 | 84,668 | ||||||
|
Other
payable
|
108,491 | 117,251 | ||||||
| $ | 5,170,786 | $ | 2,406,602 | |||||
NOTE
10 - ADVANCE FROM CUSTOMERS
The
advances from customers amounted to $2,055,602 and $2,579,997, respectively as
of December 31, 2009 and December 31, 2008, represent the deposits made by
customers to purchase inventory from the Company.
F-15
NOTE
11 - DEFERRED INCOME
A portion
of the Company’s net revenue is derived directly from government-sponsored
healthcare programs, and the Company is therefore subject to government
regulations on reimbursement on the sales made through the healthcare
programs. The Jilin Province Social Insurance Bureau and Changchun
City Insurance Bureau reimburse 90% of the sales that the Company’s pharmacy
retail stores made through the healthcare program networks in the following
month, and retain 10% of the sales until the following year. The
amount will be repaid proportionally based on the level of evaluation made by
the Insurance Bureaus in the following year. The Company classified
10% of the sales made through the healthcare program networks as deferred income
as the collectability of these sales is uncertain. As of December 31, 2009 and
December 31, 2008, the Company has deferred income of $420,277 and $273,753,
respectively.
NOTE
12 - SHARES TO BE ISSUED
The
Company classifies all amounts, against which shares have not been issued, as
shares to be issued. Once the Company issues shares, the amounts are
classified as Common stock. As of December 31, 2009, the Company has
total 500,000 shares to be issued with balance of $35,000 pursuant to an
agreement with a software consultant entered into by the Company in 2005, and
the amount is included in the accrued expenses.
During
the year ended December 31, 2009, the Company entered into an agreement with an
investor relations firm for services. The term of services is one
year and the Company is obligated to issue 600,000 shares to the investor
relations firms. As of December 31, 2009, only 300,000 shares were
issued to the investor relations firm and the balance is still to be
issued. The Company has recorded the fair market value of the 300,000
shares of $36,000 as shares to be issued. The unamortized portion of the fee of
$6,000 has been recorded as a contra amount and netted out.
NOTE
13 -TAXES PAYABLE
Tax
payable comprised of the following taxes as of December 31, 2009 and
2008:
|
2009
|
2008
|
|||||||
|
VAT
|
$ | 7,874 | $ | 14,247 | ||||
|
Business
Tax
|
94,785 | 166,817 | ||||||
|
City
Construction Tax
|
6,658 | 6,660 | ||||||
|
Education
Tax
|
5,356 | 5,357 | ||||||
|
Income
Tax
|
1,305,906 | 1,051,642 | ||||||
|
Others
|
855 | 1,326 | ||||||
|
Total
|
$ | 1,421,434 | $ | 1,245,649 | ||||
The
Company is registered in the State of Delaware and has operations in primarily
two tax jurisdictions; the PRC and the United States. For certain
operations in the U.S., the Company has incurred net accumulated operating
losses for income tax purposes. The Company believes that it is more
likely than not that these net accumulated operating losses will not be utilized
in the future. Therefore, the Company has provided full valuation
allowance for the deferred tax assets arising from the losses at these locations
as of December 31, 2009. Accordingly, the Company has no net deferred
tax assets.
The
provision for income taxes from continuing operations on income consists of the
following for as of December 31, 2009 and December 31, 2008:
|
2009
|
2008
|
|||||||
|
Current
income tax expense
|
||||||||
|
US
Federal
|
- | - | ||||||
|
US
State
|
- | - | ||||||
|
PRC
current income tax expense
|
$ | 2,594,483 | $ | 1,009,643 | ||||
|
Total
Provision for Income Tax
|
$ | 2,594,483 | $ | 1,009,643 | ||||
F-16
The
following is a reconciliation of the provision for income taxes at the U.S.
federal income tax rate to the income taxes reflected in the Statement of
Operations:
|
2009
|
2008
|
|||||||
|
Tax
expense (credit) at statutory rate - federal
|
34 | % | 34 | % | ||||
|
State
tax expense net of federal tax
|
6 | % | 6 | % | ||||
|
Changes
in valuation allowance
|
(40 | )% | (40 | )% | ||||
|
Foreign
income tax - PRC
|
25 | % | 25 | % | ||||
|
Exempt
from income tax
|
- | - | ||||||
|
Temporary
difference
|
0.24 | % | 2 | % | ||||
|
Tax
expense at actual rate
|
25.24 | % | 27 | % | ||||
United States of
America
The
Company has significant income tax net operating losses (“NOL”) carried forward
from prior years. Due to the change in ownership of more than fifty
percent, the amount of NOL which may be used in any one year will be subject to
a restriction under section 382 of the Internal Revenue Code. Due to
the uncertainty of the realizability of the related deferred tax assets of
$4,473,105, a reserve equal to the amount of deferred income taxes has been
established at December 31, 2009.
People’s Republic of China
(“PRC”)
Pursuant
to the PRC Income Tax Laws, the Company's subsidiary is generally subject to
Enterprise Income Taxes ("EIT") at a statutory rate of 25%.
The
following table sets forth the significant components of the provision for
income taxes for operation in PRC as of December 31, 2009 and 2008.
|
2009
|
2008
|
|||||||
|
Net
taxable income
|
$ | 10,475,363 | $ | 7,245,543 | ||||
|
Income
tax @ 25.24% and 27%
|
$ | 2,594,483 | $ | 1,009,643 | ||||
F-17
NOTE
14 - SHORT-TERM LOANS PAYABLE
The
Company had loans payable amounting to $1,100,884 as of December 31, 2009 and
$1,967,185 as of December 31, 2008. The loans are secured by personal
properties of a main stockholder of the Company. The loans payable at
December 31, 2009 comprised of the following:
|
2009
|
2008
|
|||||||
|
Loan
payable to a non-related party, interest free, due by December 31,
2009
|
249,406 | |||||||
|
Loan
payable to a non-related party, interest free, due by December 31,
2009
|
772,156 | |||||||
|
Loan
payable to a non-related party, interest free, due by December 31,
2009
|
558,642 | |||||||
|
Loan
payable to a non-related party, interest free, due by December 31,
2009
|
234,736 | |||||||
|
Loan
payable to a non-related party, interest at 1.5% annually, due by December
31, 2009
|
237,146 | - | ||||||
|
Loan
payable to Jilin Bank, interest at 6.9% annually, due by January 22,
2010
|
733,500 | |||||||
|
Various
loans, interest free, unsecured and due on demand
|
130,238 | 130,238 | ||||||
|
Total
|
$ | 1,100,884 | $ | 1,945,179 | ||||
NOTE
15 - LONG-TERM LOAN PAYABLE
The
Company had long term loans payable amounting to $1,320,300 as of December 31,
2009 and $1,320,390 as of December 31, 2008. The loans are secured by
personal properties of a significant stockholder of the Company. The
loans payable at December 31, 2009 comprised of the following:
The
following is the future payment schedule of the long term loan:
|
2009
|
2008
|
|||||||
|
Loan
Payable to Runfeng Agriculture Credit Union, annual interest at 8.748% and
11.02%, respectively, due by January 26, 2011
|
$ | 1,320,300 | $ | 1,320,390 | ||||
The
following is the future payment schedule of the long term loan:
|
Due
January 26, 2011
|
$ | 1,320,300 |
NOTE
16 - LOANS FROM RELATED
PARTIES
As of
December 31, 2009 and December 31, 2008, the loans from related parties were
comprised of the following:
|
2009
|
2008
|
|||||||
|
Loans
payable to officers, interest free, due on demand, and
unsecured
|
$ | 184,662 | $ | 184,662 | ||||
|
Total
|
$ | 184,662 | $ | 184,662 | ||||
F-18
NOTE
17 - STOCKHOLDERS' EQUITY
The
Series A Convertible Preferred Stock is convertible over a 3 year period, into
up to 30 million shares of common stock. In particular, the holder of any shares
of Series A Convertible Preferred Stock shall have the right, at its option, to
convert, any such shares of Series A Convertible Preferred Stock into such
number of fully paid and nonassessable shares of Common Stock on a six (6) for
one (1) basis. The conversion formula is conditioned on the Company earning no
less than $3 million of net income in for the fiscal year ending December 31,
2007; $4 million of net income in the fiscal year ending December 31, 2008 and
$5 million of net income in the fiscal year ending December 31, 2009. In the
event that in any of the three fiscal years, the Company earns less than
required net income amounts for conversion, then the conversion right shall be
proportionately reduced by the amount of the shortfall below the required net
income amount, with the "catch-up" right to convert additional shares to the
extent that the net income exceeds $3 million; $4 million and $5 million
respectively in each of the three consecutive years. In no event shall this
conversion right allow for the conversion of the Series A Preferred Stock into
more than 6 common shares for each share of Series A Preferred Stock over the
course of the aforementioned three calendar years. The net income requirements
shall be based upon an audit of the revenues for each fiscal year. All
conversions shall be made within 30 days of the completion of such
audit. During the year ended December 31, 2009, holders of 3,333,333
preferred shares opted to convert the preferred shares into 20,000,000 common
shares.
As of
December 31, 2009 and December 31, 2008, the Company had 56,448,923 and
31,400,540 shares of common stock issued and outstanding,
respectively.
During
the year ended December 31, 2009, the Company issued 10,000 shares for website
designing services and 100,000 shares for legal services. The shares were valued
at the fair market value of $9,900 and expensed during the year ended December
31, 2009 in the accompanying consolidated financial statements.
During
the year ended December 31, 2009, the Company entered into a consulting
agreement with an investor relations firm to provide investor relations and
public relations services. The agreement is for a period of 1 year and the
Company agreed to issue 300,000 shares of restricted common stock and 300,000
warrants at exercise prices ranging from $1 per share to $2 per share, to the
investor relations firm. The Company valued the shares at the fair market value
of $30,000 and expensed $23,700 during the year ended December 31, 2009 in the
consolidated financial statements.
During
the year ended December 31, 2009, the Company entered into a consulting
agreement with an investor relations firm to provide investor relations and
public relations services. The agreement is for a period of 1 year
and the Company agreed to issue 600,000 shares of restricted common stock to the
investor relation firm. The Company valued the shares at the fair
market value of $72,000 and expensed $66,000 during the year ended December 31,
2009 in the consolidated financial statements. As of December 31, 2009, 300,000
of such shares are still not issued and are included in the shares to be issued.
The unamortized part of the fee has been netted out of the amount of shares to
be issued.
On
September 25, 2009, the Company closed a private placement of its equity
securities. We issued a total of 3,338,383 shares of our common
stock, restricted in accordance with Rule 144, to 157 accredited investors, for
total consideration of $467,369. In addition, we issued to the
investors warrants to acquire another 3,338,385 shares of our common stock at
$0.35 per share, exercisable for a period of three years. In relation
to this private placement, the Company also issued 1,000,000 shares of common
stock and paid $35,000 cash. The warrants granted to the investors were valued
using the Black-Scholes option-pricing model.
During
the year ended December 31, 2009, holders of 3,333,333 preferred shares opted to
convert the preferred shares into 20,000,000 common shares.
On April
1, 2008, the Company issued to Investor Relations International (“IRI”) 250,000
restricted common stocks valued at $275,000, to render investor relations and
financial communication services. The Company amortized the prepaid
consulting over 1 year period based upon the terms of the
agreement.
As of
October 30, 2008, the Company sold 108,695 shares to an unrelated party for
$50,000. The amount was received directly by the related party, and
the Company shows a receivable from the related party for such
amount. The related party receivable is interest free, due on demand,
unsecured and has been reflected in the equity section in the accompanying
consolidated financial statements.
NOTE
18 – WARRANTS
Following
is a summary of the warrant activity for the period ended December 31,
2009:
|
Outstanding,
December 31, 2008
|
2,022,080 | |||
|
Granted
during the year
|
3,638,385 | |||
|
Expired
during the year
|
(472,080 | ) | ||
|
Exercised
during the year
|
- | |||
|
Outstanding,
December 31, 2009
|
5,188,385 |
F-19
Following
is a summary of the status of warrants outstanding at December 31,
2009:
|
Outstanding
Warrants
|
Exercisable
Warrants
|
|||||||||||||||||||
|
Exercise
Price
|
Number
of
Warrants
|
Average
Remaining
Contractual
Life
|
Average
Exercise
Price
|
Number
of
Warrants
|
Intrinsic
Value
|
|||||||||||||||
|
$0.5
- $4
|
5,188,385 | 2.87 | $ | 0.59 | 5,188,385 | 1,189,016 | ||||||||||||||
The
assumptions used in calculating the fair value of options granted using the
Black-Scholes option-pricing model are as follows:
The
3,638,385 warrants granted during the year ended December 31, 2009:
|
Risk-free
interest rate
|
2.63 | % | ||
|
Expected
life of the warrants
|
3-5
years
|
|||
|
Expected
volatility
|
215%-217 | % | ||
|
Expected
dividend yield
|
0 | |||
During
the year ended December 31, 2009 the Company granted 300,000 warrants at
exercise prices ranging from $1 per share to $2 per share, to an investor
relation firm. The Black-Scholes fair market value of the warrants was $28,439.
The Company recorded an expense of $30,300 during the year ended December 31,
2009 in the consolidated financial statements for the warrants.
On
September 25, 2009, the Company closed a private placement of our equity
securities. We issued a total of 3,338,385 shares of our common
stock, restricted in accordance with Rule 144, to 157 accredited investors, for
total consideration of $467,369. In addition, we issued to the
investors warrants to acquire another 3,338,385 shares of our common stock at
$0.35 per share, exercisable for a period of three years. The fair
market value of the warrants was calculated using the Black-Scholes option
pricing model and was netted against the net proceeds of the private
placement.
NOTE
19 – COMMITMENTS AND CONTINGENCIES
Consulting
agreements
On April
1, 2008, the Company signed a letter of engagement with Investor Relations
International (“IRI”). According to the terms of the agreement, IRI
agreed to perform investor relations and financial communication
services. The agreement was for a twelve-month period and the Company
agreed to pay $10,000 per month to IRI, to issue 250,000 shares of restricted
common stock, and to issue 300,000 warrants at an exercise price from $1.50 to
$4.00 per share. During the year ended December 31, 2009, the Company
expensed $141,566 in the consolidated financial statements.
As of
December 31, 2009, the Company entered into a consulting agreement with an
investor relations firm to provide investor relations and public relations
services. The agreement is for a period of 1 year and the Company
agreed to issue 600,000 shares of restricted common stock to the investor
relations firm. The Company recorded an expense of $66,000 during the year ended
December 31, 2009 in the consolidated financial statements. As of
December 31, 2009, 300,000 of such shares are still not issued and are included
in the shares to be issued. The unamortized part of the fee has been
netted out of the amount of shares to be issued.
Leases
The
Company leases its operating locations. Initial terms are typically 5
to 10 years, followed by additional terms containing priority of renewal options
at the maturity of the lease agreements, and may include rent escalation
clauses. The company recognizes rent expense on a straight-line basis
over the term of the lease.
F-20
Minimum
rental commitments at December 31, 2009, under all leases having an initial or
remaining non-cancelable term of more than one year are shown:
|
2010
|
1,058,624 | |||
|
2011
|
945,708 | |||
|
2012
|
301,835 | |||
|
2013
|
95,551 | |||
|
2014
|
- | |||
|
Total
minimum lease payments
|
2,401,718 |
The
company sub-leases its building to an unrelated company. The lease
term is one year. The Company recognizes rent income on a
straight-line basis over the term of the lease.
Legal
proceedings
On or
about October 17, 2008, a former officer initiated an action in the Superior
Court for the State of California, County of Los Angeles, Central District,
against the Company alleging claims for damages related to an alleged employment
agreement. On December 29, 2008, the Company filed an Answer to the
Complaint. The Company strongly disputed the claims and diligently
defended against them. The Company was defending itself against claims for open
account and intentional misrepresentation. The Plaintiff sought past
due attorneys’ fees for services rendered in the amount of
$193,100. The case was settled in October, 2009 for $50,000 cash and
400,000 shares of common stock. The court also ordered interest at
the rate of 10% on $50,000 from June 20, 2009 till the date the amount is paid
off. The Company has accrued an aggregate sum of $127,397 for the
cash to be paid and for the fair market value of the shares to be
issued.
The
Company was also involved in an ongoing legal proceeding filed in Orange County
Superior Court on or about November 9, 2004. In this action, the
Cross-Complainant, Terry Koosed, sought to amend a $219,000 judgment he obtained
to include a subsidiary of the predecessor-in-interest of the Company, which was
not named or a participant in such lawsuit. The Company strongly
disputed the lawsuit and aggressively defended such action. The Company has
accrued $219,000 in the accompanying financials statements.
A former
employee of the Company, the plaintiff, brought a lawsuit against the Company
seeking unpaid wages, bonuses, benefits, penalties and interest. The
case went into trial in November 2009 and the trial court thereafter issued a
judgment for plaintiff and against the Company in amount of
$641,016. As of December 31, 2009, the Company has not paid the
judgment amount and the same has been accrued in the accompanying financials as
accrued litigation.
A former
employee of the Company, the plaintiff, brought a lawsuit against the Company
seeking unpaid wages that accrued during his employment from 2005 to 2008 as
well as penalties, interest and attorney fees. The breakdown of
plaintiff’s damage claim is still unknown at this time. The parties are in
discovery and trial has been set for May 10, 2010.
NOTE
20 – SEGMENT INFORMATION
The
Company operates in two business segments: retail drug stores, pharmaceutical
medicine wholesales sales. These segments were identified based on their
separate and distinct products and services, technology, marketing strategies
and management reporting. Management evaluates the segments’ operating
performance separately and allocates resources based on their respective
financial condition, results of operations and cash flows. Inter-segment
transactions and balances are eliminated in consolidation.
The
retail drug store segment is complemented by such core front-end categories as
over-the-counter medications, health and beauty products, and other
items. As of December 31, 2009, the retail drug store segment
operated 79 retail stores with business area of 9,834 square meters in three
cities in China.
The
pharmaceutical medicine wholesales segment, operated through Yongxin Medical,
provides logistics wholesale distribution of over-the-counter and prescribed
medicines to hospitals, clinics, medical institutions and retail drug
stores.
F-21
The
following table summarizes significant financial information by
segment:
|
2009
|
2008
|
|||||||
|
Revenues
from unaffiliated customers:
|
||||||||
|
Retail
drug stores
|
$ | 13,898,119 | 10,865,100 | |||||
|
Pharmaceutical
medicine wholesales
|
38,832,521 | 53,117,095 | ||||||
|
Unallocated
|
1,000 | |||||||
|
Revenues
from inter-company sales
|
(5,141,360 | ) | (4,866,661 | ) | ||||
|
Consolidated
Totals
|
$ | 47,589,280 | 59,116,534 | |||||
|
Net
income:
|
||||||||
|
Retail
drug stores
|
$ | 1,084,679 | 599,589 | |||||
|
Pharmacy
wholesales
|
5,255,550 | 4,482,397 | ||||||
|
Unallocated
|
1,217,460 | (930,279 | ) | |||||
|
Net
income from inter-company
|
(19,823 | ) | (85,568 | ) | ||||
|
Consolidated
Totals
|
$ | 5,124,989 | 4,066,139 | |||||
|
Depreciation
and amortization:
|
||||||||
|
Retail
drug stores
|
60,869 | 167,680 | ||||||
|
Pharmacy
wholesales
|
316,390 | 142,182 | ||||||
|
Unallocated
|
70,430 | 581 | ||||||
|
Consolidated
Totals
|
447,689 | 310,443 | ||||||
|
Interest
income:
|
||||||||
|
Retail
drug stores
|
6,803 | 2,470 | ||||||
|
Pharmacy
wholesales
|
8,509 | - | ||||||
|
Unallocated
|
- | - | ||||||
|
Consolidated
Totals
|
15,312 | 2,470 | ||||||
|
Interest
expense:
|
||||||||
|
Retail
drug stores
|
- | - | ||||||
|
Pharmacy
wholesales
|
6,138 | 720 | ||||||
|
Unallocated
|
- | 8,428 | ||||||
|
Consolidated
Totals
|
6,138 | 9,148 | ||||||
|
Capital
expenditures:
|
||||||||
|
Retail
drug stores
|
1,123,242 | 1,350,129 | ||||||
|
Pharmacy
wholesales
|
245,034 | 5,399,303 | ||||||
|
Unallocated
|
7,300 | 112,759 | ||||||
|
Consolidated
Totals
|
1,375,577 | 6,862,191 | ||||||
|
Identifiable
assets:
|
||||||||
|
Retail
drug stores
|
29,782,442 | 8,333,213 | ||||||
|
Pharmacy
wholesales
|
11,505,850 | 23,206,845 | ||||||
|
Unallocated
|
467,370 | 7,664 | ||||||
|
Consolidated
Totals
|
$ | 41,755,662 | 31,547,722 | |||||
F-22
NOTE
21 – STATUTORY RESERVE
As
stipulated by the Company Law of the People’s Republic of China (PRC), net
income after taxation can only be distributed as dividends after appropriation
has been made for the following:
|
|
i.
|
Making
up cumulative prior years’ losses, if
any;
|
|
|
ii.
|
Allocations
to the “Statutory Surplus Reserve” of at least 10% of income after tax, as
determined under PRC accounting rules and regulations, until the fund
amounts to 50% of the Company’s registered
capital;
|
|
|
iii.
|
Allocations
of 5% to 10% of income after tax, as determined under PRC accounting rules
and regulations, to the Company’s “Statutory Common Welfare Fund”, which
is established for the purpose of providing employee facilities and other
collective benefits to the Company’s employees; and statutory common
welfare fund is no longer required per the new cooperation law executed in
2006.
|
|
|
iv.
|
Allocations
to the discretionary surplus reserve, if approved in the stockholders’
general meeting.
|
NOTE
22 - DISCONTINUED OPERATIONS
On
September 30, 2005, Software Education of America, Inc., subsidiary of
Nutradyne, filed a petition in bankruptcy under Chapter 7 of the U.S. Bankruptcy
Code. The petition was necessitated because SEA was unable to continue to meet
its financial obligations. SEA is presented in the accompanying financial
statements as discontinued operations.
In
November 2009, a subsidiary of the Company, Jilin Dingjian Natural
Health Products Co., Ltd, entered into an agreement (the “Agreement”)
with Sun Shi Wei, an individual, to transfer 90% of ownership with all its
assets and liabilities to Sun Shi Wei. The 10% minority interest
remained unchanged. Both parties agreed that Sun Shi Wei assumed the
net liability. No other money was exchanged. The Agreement also
indicated that the Company would be liable for any undiscovered
liability.
Because
the buyer assumed the net liability, the Company recorded a journal entry to
record a gain from disposal of assets and liabilities at November 30,
2009. Jilin Dingjian Natural Health Products Co., Ltd is presented
in the accompanying
financial statements as discontinued operations.
Balance
Sheet information for the discontinued subsidiaries as of December 31, 2009
and 2008 is as follows:
|
|
2009
|
2008
|
||||||
|
Assets:
|
||||||||
|
Cash
|
$ | - | $ | 8,989 | ||||
|
Accounts
receivables, net
|
3,534 | |||||||
|
Other
receivables
|
5,965 | |||||||
|
Prepaid
expenses
|
3,244 | |||||||
|
Inventory
|
- | 151,468 | ||||||
|
Total
current assets
|
- | 173,201 | ||||||
|
Property,
Plant & Equipment, net
|
- | 6,298 | ||||||
|
Intangible
Assets, net
|
- | 1,007 | ||||||
|
Total
assets
|
$ | - | $ | 180,507 | ||||
|
Liabilities:
|
||||||||
|
Accounts
payable
|
$ | 227,590 | $ | 227,590 | ||||
|
Accrued
expenses
|
238,581 | 239,704 | ||||||
|
Loans
payable
|
162,666 | 267,995 | ||||||
|
Total
liabilities
|
$ | 628,837 | $ | 735,289 | ||||
|
Net
liabilities of discontinued operations
|
$ | 628,837 | $ | 554,783 | ||||
F-23
NOTE
23 - SUBSEQUENT EVENT
On March
4, 2010, the Company consummated a private placement of its equity securities
with certain accredited investors pursuant to a Subscription Agreement for total
consideration of $125,000. The Company issued to the investors secured
convertible notes with a two year term, bearing 10% interest per annum,
convertible into common stock of the Company at a conversion price of $0.20 per
share, which is subject to adjustment for stock splits, recapitalizations and
other similar events, and will also be adjusted on a full-ratchet basis to equal
the price per share of any subsequent financing. The notes are secured by
pledged stock, and by a first priority interest in all current and future assets
of the Company, which will be cancelled upon repayment of the notes or upon
conversion of at least 50% of the principal amount of the notes into shares of
Company common stock. The notes may be redeemed by the Company at any time for
110% of outstanding principal and interest. The note investors also received, as
a part of the financing, warrants for the purchase of up to 625,000 shares of
our common stock with an exercise price $0.50 per share (subject to adjustment
for stock splits, recapitalizations and other similar events) exercisable for a
period of three years.
In
addition, subsequent to year end, the Company issued a total of 900,000 shares
of common stock for the settlement of liability that was accrued on the balance
sheet as of December 31, 2009.
F-24
