Attached files
| file | filename |
|---|---|
| 10-K/A - LIGHTLAKE THERAPEUTICS INC. 10-K - OPIANT PHARMACEUTICALS, INC. | a6213093.htm |
| EX-3.I - EXHIBIT 3(I) - OPIANT PHARMACEUTICALS, INC. | a6213093ex3i.htm |
| EX-32 - EXHIBIT 32 - OPIANT PHARMACEUTICALS, INC. | a6213093ex32.htm |
| EX-10.5 - EXHIBIT 10.5 - OPIANT PHARMACEUTICALS, INC. | a6213093ex10_5.htm |
| EX-10.4 - EXHIBIT 10.4 - OPIANT PHARMACEUTICALS, INC. | a6213093ex10_4.htm |
| EX-10.6 - EXHIBIT 10.6 - OPIANT PHARMACEUTICALS, INC. | a6213093ex10_6.htm |
| EX-31.2 - EXHIBIT 31.2 - OPIANT PHARMACEUTICALS, INC. | a6213093ex31_2.htm |
| EX-31.1 - EXHIBIT 31.1 - OPIANT PHARMACEUTICALS, INC. | a6213093ex31_1.htm |
Exhibit
10.7
| (19) | [EUROPEAN PATENT OFFICE LOGO] |
[BAR
CODE]
(11) EP
1 681 057 B1
|
(12) EUROPEAN PATENT
SPECIFICATION
|
(45)
Date of publication and mention
|
(51)
Int Cl.:
|
|
|
of
the grant of the patent:
|
A61K 31/485 (2006.01)
|
A61P 25/30 (2006.01)
|
|
13.08.2008
Bulletin 2008/33
|
A61P 3/04 (2006.01)
|
(21) Application
number: 06396001.7
(22) Date of
filing: 10.01.2006
|
(54)
Use of naloxone for
treating eating disorders
|
Verwendung
von Naloxon zur Behandlung von Essstörungen
Utilisation
de Naloxone pour traiter les troubles alimentaires
(84)
Designated Contracting States:
AT
BE BG CH CY CZ DE DK EE ES FI FR GB GR HU IE IS IT LI LT LU LV MC NL PL PT RO SE
SI SK TR
(30)
Priority: 10.01.2005 US
31534
(43) Date
of publication of application: 19.07.2006 Bulletin
2006/29
(73) Proprietor:
Sinclair, John D. 02550
Evitskog (FI)
(72)
Inventor: Sinclair, John D.
02550 Evitskog (FI)
(74) Representative:
Hovi, Simo Pekka Tapani et al
Seppo Laine Oy,
Itämerenkatu
3 B
00180
Helsinki (FI)
(56)
References cited:
EP-A-
0 790
058 US-B1-
6 569 449
· FICHTER
M M: "DIE MEDIKAMENTOESE BEHANDLUNG VON ANOREXIA UND BULIMIA
NERVOSAEINEUEBERSICHTMEDICATIONFOR ANOREXIAAND BULIMIANERVOSA:A REVIEW"
NERVENARZT, SPRINGER VERLAG, BERLIN, DE, vol.64, no.1, 1993, pages21-35,
XP008016116 ISSN: 0028-2804
· DREWNOWSKI
A ET AL: "Naloxone, an opiate blocker, reduces the consumption of sweet high-fat
foods in obese and lean female binge eaters" AMERICAN JOURNAL OF CLINICAL
NUTRITION 1995 UNITED STATES, vol. 61, no. 6, 1995, pages 1206-1212, XP002376723
ISSN: 0002-9165
· "Drug
treatment for binge-eating disorders" JOURNAL OF THE AMERICAN DIETETIC
ASSOCIATION 1995 UNITED STATES, vol. 95, no. 11, 1995, page 1329, XP002376724
ISSN: 0002-8223
Note:
Within nine months of the publication of the mention of the grant of the
European patent in the European Patent Bulletin, any person may give notice to
the European Patent Office of opposition to that patent, in accordance with the
Implementing Regulations. Notice of opposition shall not be deemed to have been
filed until the opposition fee has been paid. (Art. 99(1) European Patent
Convention).
2
EP
1 681 057 B1
Description
Background of the
Invention
Field of the
Invention
[0001] The present invention
relates to the treatment of eating disorders. In particular, the invention
relates to the use of naloxone in methods of eating disorder
therapy.
Description of Related
Art
[0002] Various eating
disorders, including binge eating, bulimia, and stimulus-induced
over-eating, develop because the behaviors are reinforced by the opioidergic
system so often and so well that the person no longer can control the behavior.
Thus eating disorders resemble opiate addiction and alcoholism. Eating disorders
can-not, however, be treated effectively by continual daily ad-ministration of
opiate antagonists because normal healthy eating behavior is also reinforced by
the opioidergic system. Instead, a selective extinction method is needed
that weakens the eating disorder while strengthening healthy eating.
Extinction sessions in which the eating disorder responses are emitted while an
opiate antagonist blocks reinforcement must be interspersed with learning
sessions in which healthy eating responses are made while free of antagonist. In
between extinction and learning sessions there must be a wash-out period in
which the antagonist is allowed to be eliminated from the body, and during which
neither problem eating nor healthy eating should occur. Consequently,
preparations with long-lasting antagonists such as naltrexone and nalmefene with
wash-out periods of a day or more are not suitable, but naloxone with a half
life of only about an hour is excellent. Naloxone cannot be taken orally.
In-stead it is administered transdermally or by nasal inhalation. This
provides additional advantages with eating disorders: the purging with bulimia
does not affect the dosage; the gastrointestinal tract is not further disturbed
by the antagonist administration; and altering eating responses does not
affect taking the medication.
[0003] Opioid antagonists have
been patented for inducing anorexia (Smith, US Patent 4,217,353, 1980; US
Patent 4,477,457, 1984), and they also have been patented for treating
anorexia (Huebner, US Patent 4,546,103, 1985). Both results are valid. The
antagonists can also reduce binge eating and also the purging associated
with bulimia, but normal eating, too. Narrowly limited experiments have
found evidence for each of these effects. When put into long term practice,
however, the different effects counteract each other and cause
complications. For example, as Smith pointed out, the only clinical trial
using naloxone for anorexia was inconclusive because they coupled the treatment
with giving a hyper-caloric diet (Moore et al., 1981).
[0004] Unfortunately, the
methods used and previously
proposed for the treatment of eating disorders are unable to separate these
various actions. Consequently, the antagonists have produced mixed clinical
results, have not received FDA or equivalent European approval
|
|
5 for
use with eating disorders, and currently are not being used clinically for
such purposes.
|
[0005] In contrast, in the
field of alcoholism and drug addiction treatment, I proposed a method in which
the antagonists specifically remove the addictive behavior
|
|
10
(Sinclair, US Patent 4,882,335, Nov. 21, 1989; US Patent 5,587,381,
Dec. 24, 1996; EP Patent 0 346 830 B1, May 11, 1995). Our double-blind
placebo-controlled clinical trial has shown naltrexone is effective when
used in ac-cord with this method but not when used
otherwise
|
|
|
15
(Heinälä et al., 2001). Similar results have been obtained in
nearly all trials (Sinclair, 2001). Naltrexone has been approved by the
FDA for use in alcoholism treatment in 1995 and in Finland in 1996. Going
one step further, I improved the method into a procedure of "selective
ex-
|
|
|
20
tinction" that not only removes alcoholism and drug ad-diction but
also enhances other competing behaviors (Sinclair, US Patent 5,587,381,
1996; Sinclair et al., 1994; Sinclair, 2001). Especially in Finland where
naltrexone is widely used in this selective manner, it
has
|
|
|
25
become a major factor in the treatment of alcoholism. [0006] Extinguished
responses can be relearned; in-deed they are relearned more readily than
they were learned the first time. Subjects can be advised after a given
period of treatment to refrain henceforth from
mak-
|
|
|
30 ing
the extinguished response ever again in order to avoid relearning, but
they cannot avoid all responses reinforced through the opioidergic system.
One solution, used in alcoholism treatment, is to continue taking
antagonist in-definitely whenever there is a risk of drinking, or in
this
|
|
|
35 case
of making the eating disorder response again. Alternatively,
selective extinction can be used to "trim" of-fending responses that are
beginning to arise again be-fore they become harmfully strong. Like
finger-nails, the growth of responses is a useful natural process but
can
|
|
|
40
become harmful when left uncontrolled. Thus individuals with a
predilection for developing overly-strong responses might
periodically review their current activities and then trim those responses
that were beginning to get too strong -- as casually and almost as easily
as we trim our nails.
|
|
|
45
[0007] Perhaps the
greatest technological quest in this field since the discovery of the
opioid antagonists has been for preparations that would cause the
antagonists to remain in the body for longer periods of time.
Naltrex-
|
|
|
50 one
and nalmefene have been preferred over naloxone not only because they can
be taken orally but also be-cause of their much longer half-lives. Various
slow-release methods for naltrexone and nalmefene have been developed
over the passed two decades to
provide
|
|
|
55
weeks or months of constant blockade. Alkermes Inc. has recently
received FDA approval for Vivitrol, a long-acting, injectable formulation
of naltrexone.
|
3
EP 1 681 057 B1
Summary of the
Invention
[0008] The present invention
relates to a new and alternative way of treating eating disorders based on
the use of naloxone.
[0009] The above explained
quest, utilizing antagonists having a prolonged action and activity in the
body, is consistent with the previously proposed methods for treating bulimia
and binge eating with opioid antagonists. Their imagined mechanisms of action
would work best if the antagonists were always present, thus eliminating
supposed problems of patient compliance.
[0010] The present invention,
however, contemplates alternating periods when an opioid antagonist blocks the
opioid system (during which the eating disorder behaviors are emitted) with
periods when the patient’s body is free of antagonist (during which normal
healthy eating behaviors are made).
[0011] The present invention
is, therefore based on the use of naloxone for the preparation of pharmaceutical
compositions for methods of treating eating disorders in mammals, including
human beings.
[0012] The method of treatment
preferred comprises "selective extinction". We have used a similar "selective
extinction" procedure extensively in treating alcoholism (Sinclair, 2001).
(Incidentally, there has been little problem here with compliance.
Alcoholics have difficulty complying if you tell them to refrain from
drinking. They do not have a problem, however, with obeying the instruction to
take a pill always before drinking.)
[0013] With alcoholics, we
include a wash-out period of about 48 hours for removal of the naltrexone.
During this time the patients should not drink alcohol and they also should not
engage in the alternative opioidergicallyreinforced behaviors that we wish
to strengthen. This is not a problem with alcoholism or drug
addiction.
[0014] In the case of eating
disorders, such long wash-out periods are not possible. For example, when
treating bulimia, the behavior we wish to extinguish is eating foods that
trigger bulimia. The alternative behavior we wish to strengthen is eating foods
that do not trigger bulimia. Obviously patients cannot be expected to avoid
both activities, that is, to refrain from all eating, for a 48 hour
wash-out period. Nalmefene is removed even more slowly.
[0015] Naloxone, however, has
a half-life of only 30 to 80 minutes in humans. A patient given naloxone on one
day would be free of it the following day.
[0016] The present invention
contemplates the use of the opiate antagonist naloxone for the preparation of a
pharmaceutical composition for the treatment of eating disorders. In particular
the present invention provides for the use of naloxone (or a similar opiate
antagonist having a half-life of less than about 2 hours, preferably less than
90 minutes).
[0017] In particular, the
present invention contemplates the use of naloxone in the formulation of a
pharmaceutical composition used in a method based on selective
extinction.
[0018] Particularly preferred
compositions are those which are suitable for transdermal or nasal
administration appropriate in a therapeutic method, utilizing the ability of
opiate antagonist to block positive reinforcement from
|
|
5
stimuli produced by highly-palatable foods, from purging, and from
anorexic behavior in order to extinguish bulimia and other eating
disorders while simultaneously strengthening normal healthy eating
behaviors and the consumption of foods conducive to
health.
|
|
|
10
[0019] The
subject suffering from one of these overly-strong eating disorder
responses makes the response repeatedly, in the presence of stimuli
similar to those to which the response had been learned, while active
quantities of naloxone are in his or her brain, thus
eventually
|
|
|
15
extinguishing the response and removing the desire to make the
response. These extinction sessions are separated by "learning
periods" when the subject is free of antagonist and can make other
responses but not the problem response, in order to restore the strength
of com-
|
|
|
20
peting responses. Thus the problem response is selectively
extinguished.
|
[0020] Considerable advantages
are obtained with the present invention.
[0021] The lifetime prevalence
of bulimia is 2.8 % for
|
|
25
women, and 5.7 % of women will show bulimia-like syndromes
(Kendler et al., 1992). The disorder was strongly influence by genetics,
with a heritability coefficient of 55 %. Comorbidity was reported between
bulimia and anorexia nervosa, alcoholism, panic disorder, generalized
anxiety disorder, phobia, and major
depression.
|
30
[0022] In most cases the
subject will suffer from several related problem responses: e.g.,
overly-strong eating responses for a dozen specific highly palatable food
items. Each will be extinguished separately. Further-
|
|
35
more, prior to extinguishing a particular response, the subject
will not be allowed to make that response for at least a week. The
resulting increased motivation to make the response after being deprived
of the opportunity ("deprivation effect") will assure that the subject
makes
|
|
|
40 that
response at the beginning of extinction and will in-crease the
effectiveness of extinction.
|
[0023] Depending upon the
severity and nature of the problem responses, provisions are made for using the
method within a treatment center, as an out-patient treatment, and as a
combination of the two.
45
Brief Description of the
Drawings
[0024]
50
Figure 1a
shows selective extinction (interspersing periods when alcohol was drunk daily
following naloxone injection with periods when saccharin was drunk with no
injection) strongly reduced alcohol
55 drinking,
while
Figure 1b
depicts increasing saccharin drinking in the same animals relative to intakes by
control animals injected with saline. Each data point is the
mean
4
5 EP 1 681 057
B1 6
of 1 to 4
days. The extremely low doses used from week 4 on have not previously been found
to be effective. * p<0.05 relative to saline controls.
Description of Preferred
Embodiments
[0025] The present invention
involves taking the selective extinction method for separating the actions
of opioid antagonists on different behaviors and contemplates applying it
to the treatment of eating disorders. Because the opioid antagonists
conventionally used in treating alcoholism are not suitable for treating eating
disorders, the present invention employs naloxone (or salts thereof) for use in
preparations that can be taken either transdermally or by nasal inhalation in a
manner suitable for selectively extinguishing eating disorders while reinforcing
healthy eating behaviors. In addition, several innovations are proposed to
optimize the method to the eating disorder field and which then differentiate
the method from all previously proposed treatments.
[0026] The key for how to
separate the actions of the antagonists comes from an understanding of how the
antagonists act in the nervous system to produce benefits.
[0027] There are two basic
processes through which long-term change is made in the organization of the
nervous system as a result of experience: one causes learning by
strengthening synapses; the other causes habituation and extinction by
weakening synapses (see Sinclair, 1981). Experimental results also show
that the two occur under different circumstances and follow different rules.
Thus, extinction is not simply learning to do some-thing else but rather a
separate phenomenon. It also is distinct from forgetting; it is an active
process for removing unsuccessful responses and requires the emission of
the response in the absence reinforcement.
[0028] Our preclinical
experimental results had shown that alcohol drinking is a learned behavior
(Sinclair, 1974), and that opioid antagonists suppress alcohol drinking by
mechanism of extinction (Sinclair, U.S. Patent 4,882,335, Nov. 21, 1989;
Sinclair, 1990). Extinction weakens only those responses that are made while
reinforcement is blocked. There the method I proposed for treating
alcoholism had the antagonist being administered just before the alcoholic
drank alcohol.
[0029] Others in the field,
however, believed that opioid antagonists block the craving for alcohol caused
by an imbalance, either a deficiency in opioid receptor activity (Tractenberg
and Blum, 1987; Volpicelli et al., 1990) or having too much opioid receptor
activity (Reid and Hub-bell, 1922). According to these theories, the antagonists
would be effective if given during abstinence; they would block craving and the
onset of drinking.
[0030] Our preclinical
experiments had shown that giving opioid antagonists during abstinence not
only failed to reduce subsequent drinking, but actually tended to in-crease
subsequent drinking above control levels (Sinclair et al., 2003). The same
result was found in our dual clinical trial
(Heinola et al., 2001). Naltrexone was effective when paired with alcohol
drinking, but naltrexone tended to be worse than placebo when given during
abstinence. Similar results can be seen in the other clinical trials
(Sin-
|
|
5
clair, 2001). The latest published count had 41 clinical trials
that obtained significant results from using opioid antagonists in a
manner allowing extinction; 37 trials using the antagonists in ways
precluding extinction, how-ever, got negative results; only 4 trials had
results con-
|
|
|
10
trary to this conclusion (Fantozzi and Sinclair, 2004). [0031] The mechanism
causing the increase in alcohol drinking when antagonists are administered
only during abstinence can be used to improve the efficacy of
treatment. It can increase the strength of behaviors other
than
|
|
|
15
alcohol drinking, of behaviors that can compete with drinking and
help fill the vacuum as drinking is extinguished. At the same time
other behaviors that are rein-forced by endorphins are protected from
extinction. One problem noted in some of the clinical alcohol trials is
a
|
|
|
20
reduction in the patients’ interest in sweets or
carbohydrates, or in sex (Bohn et al. 1994; Balldin et al., 1997).
This is probably caused by these behaviors being made while on naltrexone
and thus, along with alcohol drinking, being partially extinguished.
Naltrexone given to humans
|
|
|
25
reduces their preference for saccharin (Arbisi et al., 1999) [0032] The first step in
our clinical use of selective extinction in alcoholism treatment is
to have patients make a list of behaviors they find pleasurable. The
clinician identifies the behaviors on the list that are probably
re-
|
|
|
30
inforced by the opioidergic system and advises the patient to
avoid engaging in these activities on the days when taking naltrexone and
drinking. In the beginning of treatment, this is essentially every
day.
|
[0033] After the treatment has
reduced craving for al-
|
|
35
cohol, usually during the first month, the patient is advised to
have a weekend, starting with Friday evening, with no naltrexone and
drinking. Friday night and Saturday constitute a wash-out period for
naltrexone to be removed from the body. On Sunday afternoon, the patient
chooses
|
|
|
40 some
of the opioidergically-reinforced behaviors: eating a highly palatable
meal, jogging, having sex, cuddling, cards, etc. As expected, patients
usually report that the activities at this time are unusually
enjoyable.
|
[0034] The patients can return
to naltrexone and drink-
|
|
45 ing
on Monday. They are advised, however, to try the next week to have a
longer period without naltrexone and drinking but with the alternative
behaviors. A three-year follow-up showed that complying patients reported
a maximum of 1.5 ± 0.4 (SEM)
days per week (Sinclair et
|
50 al.,
2000).
[0035] The example included
here is a prior preclinical experiment
in which the alternative opioidergically-reinforced
behavior was saccharin drinking. Alcohol experienced rats
had continual access to food and water. Alcohol
solution was available for only an hour a day for 2 to 4 days.
On the next day or two, saccharin solution instead
was available. Naloxone (or saline for the control group) was
injected prior to the alcohol session. During
5
7 EP 1 681 057
B1 8
the first
three weeks when the naloxone doses were in the range previously found to be
effective, the alcohol drinking was practically abolished. Saccharin drinking in
the same animals was significantly increased.
[0036] The opioidergic system
reinforces responses, not only when activated by an opiate or alcohol, but also
when certain types of stimuli are experienced. The stimuli cause a release of
opioids in the brain, reinforcing the responses that produced these stimuli.
Consequently, opioid antagonists have been shown in clinical trials to be
effective in treating compulsive gambling (Kim, US Patent 5,780,479, 1998; Kim
et al., 2001).
[0037] Opioidergic
reinforcement is well documented for food-related stimuli. On the basis of a
large body of data, Cooper and Kirkham (1990, p. 91) concluded that "ingested
items provide stimuli which lead to the release of endogenous opioidergic
peptides in the central nervous system". The system does not appear to be
involved in the reinforcement from eventually obtaining calories, but rather
with that from the pleasant stimulation. For example, opiate antagonists reduce
sham feeding of sucrose, and they suppress the eating of chocolate-coated
cookies by rats, but not the intake of normal rat chow. Similarly in humans, the
antagonist nalmefene suppress-es intake of highly palatable foods but not that
of less pleasant tasting ones. Another general finding is that antagonists
suppress food consumption (and alcohol drinking) only in the later parts of
the first session or later parts of the first eating binge but not at the
beginning.
[0038] Other workers in the
field interpret these results differently than I do. They suggest that
"endogenous opioids play a central role in the modulation of appetite"
(Jonas, 1990). The opioids released by food-related stimuli block satiety
effects and make food stimuli continue to be pleasant even after caloric needs
have been satisfied; thus the opioid release "contributes to the
maintenance of ingestional behavior" (Cooper and Kirkham, 1990) and is
"involved with processes associated with continuance of eating rather than
starting to eat" (Wild and Reid, 1990). In some people the opioid release is too
large or too long, and thus they do not stop eating (or alcohol drinking)
normally but rather have "out of control" binges. An opiate antagonist blocks
this opioid action; therefore, so long as the antagonist is present the
duration of a binge is shortened. Similarly with alcohol drinking,
"antagonists at opioceptors [sic] would reduce the propensity to continue to
drink once drinking has begun" (Hubbell and Reid, 1990). Another interpretation
was made by Huebner (US Patent 4,546,103, 1985). He saw endorphins providing
satisfaction and pleasure from purging for bulimic patients and from anorexic
behavior. Blocking the opioid system with endorphins would re-move the reason
for patients making the behaviors, and thus help them to stop.
[0039] Both of these
interpretations are best served by continual opioid blockade. If endorphins
cause normal eating to expand to a binge, then continual blockade would
continually prevent binges. Or if endorphins provide the
pleasure from purging, continual naltrexone would suppress purging at all times.
Therefore others have not proposed using only short periods of blockade
interspersed with periods when the opioid system was
5 functional,
as is done with the present invention.
[0040] I see the results not
as immediate effects of the opioids and the antagonists on appetite or satiety,
but rather as aftereffects produced by learning and extinction. When a
highly palatable food is consumed, opioids
|
|
10 are
released and as a result, after consolidation, the response is
stronger. In some people the responses are reinforced so often and so well
that they become extremely strong and cannot be controlled properly.
When the response is emitted while an opiate antagonist
blocks
|
|
|
15 the
reinforcement, the response is weakened. The effect can be seen even
during the first session, not at the very beginning but reducing intake in
the latter portions and thus terminating a binge earlier. The antagonists
can re-duce purging if the behavior is emitted while
reinforce-
|
20 ment is
blocked because of extinction.
[0041] Opiate antagonists have
been tested for eating disorders but the methods used were ones that would be
appropriate if the antagonists worked by directly increasing satiety or
reducing appetite. In particular, the subjects
|
|
25 were
kept continually on the antagonists in order to pre-vent all eating from
getting out of control and turning into a binge. For example, Alger et al.
(1990) gave patients suffering from binge eating initially 50 mg of the
longer lasting antagonist, naltrexone, once daily, then twice
dai-
|
|
|
30 ly,
and if that did not work, 3 times daily, apparently for the purpose of
making sure the patient was never free of naltrexone. Although some
patients seemed to benefit, over all the naltrexone treatment was not
significantly better than placebo. Similarly, although some
uncon-
|
|
|
35
trolled studies found benefits from naltrexone in the
treatment of bulimia, the one placebo-controlled study did not
(Jonas, 1990). A recent review of pharmacological treatments for
binge eating does not include opioid antagonists among the medicines
for which there is clinical sup-
|
40 port
(Carter et al., 2003).
[0042] According to the
extinction hypothesis, keeping a person continually on the antagonist is not
optimal for treating eating disorders. In the case of binge eating, it weakens
not only the binge-eating response but also all
|
|
45
other emitted responses reinforced through the opioidergic
system. This makes the procedure less effective be-cause the probability
of binge-eating is determined not by its absolute strength but rather by
its strength relative to all competing responses. Of particular
importance, eat-
|
50 ing in a
healthy manner is also extinguished.
[0043] As discussed above, the
present invention instead
employs the "selective extinction" procedure (Sinclair, US
Patent 5,587,381, 1996) which has the person take an
antagonist only before making the problem re
55 sponse but
free of the antagonist at times when the problem
response is not made. Thus extinction sessions, when
mainly the problem response is weakened, are interspersed
with "learning periods" when other competing
6
9 EP 1 681 057
B1 10
response
including healthy eating responses can regain their strength. In the treatment
of bulimia, only binge-eating of specific highly palatable food is weakened, but
other competing responses are not.
[0044] Experimental support
comes from my studies with alcohol drinking: keeping rats continually on an
antagonist (large doses of naltrexone or nalmefene in the food)
significantly lowered alcohol drinking but did not reduce it as completely as
selective extinction produced by 1 hour sessions daily when alcohol and the
short-acting antagonist, naloxone, were present, as shown in the example
here.
[0045] Support may also be
seen in the fact that the only blind, placebo-controlled experiment with humans
to obtain significant results involving binge eating and opioid antagonists was
an acute study in which naloxone significantly reduced the size of an eating
binge (Atkin-son, 1982).
[0046] There are two other
advantages of selective extinction. First, the continual presence of an
antagonist produces up-regulation of opioid receptors (Unterwald and Zukin,
1990; Parkes and Sinclair, 2000). Consequently, a problem response would
produce more reinforcement after the end of antagonist treatment, than it
did before. Up-regulation should be attenuated with the selective extinction
procedure because the antagonist is present only for relatively short sessions
interspersed with antagonist-free periods.
[0047] Second, although opiate
antagonists are considered safe, there are side-effects, such as liver
toxicity with naltrexone, elevated cortisol levels, and possible
immunosuppressive effects (Morgan and Kosten, 1990). These side-effects
should be greatly reduced or eliminated with only periodic administration
of the antagonist. The dysphoria sometimes reported with continual
administration might also be caused by the general blocking of pleasure
from a wide range of activities, and should be less of a problem with selective
extinction where the per-son is free to enjoy opioidergic reinforcement from
other responses during the learning periods.
[0048] Selective extinction
can be used for treating a variety of eating disorders. In addition to bulimia
and binge-eating, it could be used as a dieting aid. A contributing factor
to obesity for many people is overly-strong eating responses and cravings for a
few highly palatable and high-energy foods: chocolate, cookies, peanut but-ter,
etc. Losing weight and then maintaining a normal weight would be possible after
these specific responses were removed by selective extinction. Similarly,
selective extinction could be used by people who are not necessarily
overweight but have to restrict their intake of a particular substance
(e.g., sugar or sodium chloride) that can be identified with a specific stimulus
that activates the opioidergic system. (There is evidence that both sweet and
salty tastes are reinforcing through this system (Levine et al.,
1982).)
[0049] The present invention
takes advantage of a relationship between opiate antagonists and a
phenomenon R. J.
Senter and I discovered called the "alcohol-deprivation effects" (Sinclair and
Senter, 1967). Taking alcohol away after prolonged prior experience gradually
over several days increases the desire for it. When it is
|
|
5 first
returned, intense drinking begins immediately, probably accompanied
by intensified pleasure and reinforcement. Deprivation effects also
develop for saccharin and specific highly-palatable foods, as well as for
many habitual behaviors. Opiate antagonists have been found
to
|
|
|
10 be
more effective in suppressing alcohol drinking after deprivation (Kornet
et al., 1990). The probable reason is that extinction (unlike learning) is
most effective with "massed trials", i.e., when the response is made over
and over again, vigorously, without pausing (see
Sinclair,
|
|
|
15
1981). Therefore, the extinction of specific eating responses
will generally be done after several days of deprivation of the
specific food item. For example, if chocolate ice cream is listed by
patients as a triggering food for bulimia, these patients will be told to
abstain from
|
|
|
20
eating chocolate ice cream, plus ice cream in general and chocolate
in general, for a week before taking an opioid antagonists and getting
unlimited chocolate ice cream to eat and
purge.
|
[0050] There is evidence
linking anorexia nervosa to
|
|
25 the
opioidergic system. First, it may develop from bulimia (Kassett and
Gwirtsman, 1988). Second, there is some preliminary evidence from a small
study showing for improvement of anorexia nervosa from treatment with
an opiate antagonist (Luby et al., 1987). Marrazzi and
Luby
|
|
|
30
(1986) suggested that starvation causes the release of endorphins;
anorexic patients starve themselves supposedly to get elation from
their own opioids. I suspect the situation is somewhat more complicated.
The specific anorexic behaviors may be reinforced by the opioid
sys-
|
|
|
35 tem,
but a major factor contributing to the condition is the extinction of
normal eating response. During the developmental phase, the patients
make all of the normal eating responses: going to the table, taking
the food, pushing it around with a fork, but then willfully withholding
the
|
|
|
40
responses of tasting and swallowing the food. Thus the preliminary
responses are made but do not get reinforcement from taste or from
removal of hunger, and as a result are extinguished. In any case, it is
clear that the solution is a strengthening of normal eating
behaviors,
|
|
|
45 and
extinction of the responses maintaining anorexia. This should be
accomplished by administering opioid antagonists while the patient is
not eating, interspersed with antagonist-free periods when the patient
does in fact eat a small amount of highly palatable
food
|
|
|
50
[0051] The
selective extinction method here for treating eating disorders
comprises selectively extinguishing the behaviors causing the disorder
while strengthening normal health eating behaviors. It preferably
comprises the steps of:
|
55
|
|
- repeatedly
administering naloxone in a dosage sufficient to block the effects of
opiate agonists to a subject suffering from an eating disorder caused
by one
|
7
11 EP 1 681 057
B1 12
or more
related problem responses;
|
|
-
while the amount of naloxone in the subject’s body is sufficient to block
opiate effects, having the subject make one of the problem responses from
which the subject suffers in the presence of stimuli similar to those to
which it had been learned,
|
|
|
-
after the amount of naloxone is no longer sufficient to block opiate
effects, having the subject make healthy eating responses to food items
that do not trigger the problem responses;
and
|
|
|
-
continuing the steps of administration of naloxone and having one after
another of the problem responses made, followed by having a
naloxone-free period in which healthy eating occurs, until the problem
responses are extinguished.
|
[0052] Based on the above, the
invention comprises, i.a., the following preferred embodiments:
In the
first, naloxone is used for the preparation of a pharmaceutical composition to
be Administered simultaneously with the patient making eating disorder
responses but in a manner so that effective levels of naloxone are not present
in the body when the patient makes healthy eating responses, and
continuing the alternating between extinction of eating disorder
responses with naloxone and reinforcement of healthy eating behaviors until the
eating disorder responses are weak enough to be controlled. Typically, in
the method, the patient makes said "healthy eating responses" a few hours,
preferably about 0.2 to 12 hours, in particular about 0.5 to 6 hours, typically
about 0.8 to 4 hours, after the first eating responses.
In a
second embodiment, naloxone is used transdermally and in a dose per day
amounting to about 0.001 mg to 50 mg.
[0053] The present invention
provides a means, or de-vice, suitable for the rapid, easy and foolproof
transdermal delivery of the opiate antagonist. The device is a package
containing a fixed dose of antagonist, a vehicle and a permeation enhancer to
assure rapid systemic de-livery of the antagonist. The package contemplated is a
container, such as a capsule, sachet, or squeeze tube, holding a fixed volume of
an ointment containing the antagonist, vehicle and enhancer. An important
consideration for the formulation that has not been mentioned previously is
the ease and rapidity of removing the transdermal composition and thus
terminating further naloxone administration.
[0054] For the transdermal
administration, naloxone can be in the form of the acid, the base, or the salts
there-of. The concentrations of naloxone in the ointment can range from 1 mg/ml
up to or in excess of the solubility limit of the vehicle. Possible vehicles
include propylene glycol, isopropanol, ethanol, oleic acid,
N-methylpyrrolidone, sesame oil, olive oil, wood alcohol ointments,
vaseline,
a triglyceride gel sold under the trade name Softisan 378, and the
like.
[0055] Possible permeation
enhancers include saturated and unsaturated fatty acids and their esters,
alco-
|
|
5 hols,
acetates, monoglycerides, diethanolamides and N, N-dimethylamides, such as
linolenic acid, linolenyl alcohol, oleic acid, oleyl alcohol, stearic
acid, stearyl alcohol, palmitic acid, palmityl alcohol, myristic acid,
myristyl alcohol, 1-dodecanol, 2-dodecanol, lauric acid,
decanol,
|
|
|
10
capric acid, octanol, caprylic acid,
1-dodecylazacycloheptan-2-one sold under the trade name Azone by
Nelson Research and Development, ethyl caprylate, isopropyl
myristate, hexamethylene lauramide, hexamethylene palmitate, capryl
alcohol, decyl methyl sulfoxide,
|
|
|
15
dimethyl sulfoxide, salicylic acid and derivatives,
N,N-diethyl-m-toluamide, crotamiton, 1-substituted
azacycloalkan-2-ones, polyethylene glycol manolaurate, and other
compounds compatible with the package and the antagonist, and having
transdermal permeation activity. In
|
|
|
20
accord with patent EPA-0282156, corticosteroid or other agents to
lessen skin irritation could also be included. A preferred vehicle is
propylene glycol and a preferred enhancer is linolenic acid (10
%).
|
[0056] Further details on
transdermal compositions 25 will
appear from US Patent No. 5,096,715.
[0057] In a third embodiment,
naloxone is given by intranasal inhalation and the dose per day is 0.001 mg
to 50 mg.
[0058] The intranasal
formulations can be formulated
|
|
30 with
naloxone in the form of the acid, the base, or the salts thereof together
with a stabilizer and a surfactant. Among pharmaceutically acceptable
surfactants the following can be mentioned: Polyoxyethylene castor
oil derivatives; mono-fatty acid esters of polyoxyethylene
(20)
|
|
|
35
sorbitan and sorbitan esters (TWEEN 20 and TWEEN 80),
polyoxyethylene monostearate (TWEEN 60), polyoxyethylene (20)
sorbitan monopalmitate (TWEEN 40), and polyoxyethylene 20 sorbitan
monolaurate (TWEEN 20); polyglyceryl esters, and polyoxyethylated kernel
oil.
|
|
|
40
Preferably, the surfactant will be between about 0.01 % and 10% by
weight of the pharmaceutical composition. [0059] The
pharmaceutically useful stabilizers include antioxidants, such as sodium
sulfite and metabisulfite, sodium thiosulfate and formaldehyde,
sulfoxylate, sulfur
|
|
|
45
dioxide, ascorbic acid, isoascorbic acid, thioglycerol,
thioglycolic acid, cysteine hydrochloride, acetyl cysteine,
hydroquinone, propyl gallate, nordihydroguaiaretic acid, butylated
hydroxytoluene, butylated hydroxyanisole, alpha-tocopherol and
lecithin. The stabilizer will
preferably
|
|
|
50 be
present in a concentration of about 0.01% and 5% by weight of the
intranasal composition.
|
[0060] Naturally, the
compositions may contain other components, as well (e.g. chelating agents and
fluidizing agents).
|
|
55
[0061] Each
of the above embodiments can be carried out by a method in which the dose
of naloxone is started at a high level of 5 to 50 mg and then is
progressively reduced over the days of
treatment.
|
8
13 EP 1 681 057
B1 14
[0062] By the above
embodiments, various eating disorders, typically selected from the group
comprising, binge eating, bulimia, bulimia-like syndrome, anorexia nervosa, and
habitual over-eating stimulated by specific stimuli including certain foods,
situations, or moods, can be successfully treated.
[0063] The method can also be
used in situations wherein the patient must lower intake of a particular class
of dietary substances including sodium chloride, sugars, cholesterols or
low-density cholesterols, and the responses to be selectively extinguished
are the eating of particular foods with high amounts of these substances. [0064] It should be noted that
the method can be used with subjects diagnosed as suffering from maladaptive
overly-strong responses reinforced by stimulation-induced release of
opioids and resulting in eating disorders. It cannot be used for patients
for whom the opiate antagonist is contraindicated. In particularly, patients who
are physiologically dependent upon opiates must be excluded.
[0065] Specific details for
the use of selective extinction with each of the different varieties of
problem responses are presented below. The initial steps, however, in each
case are similar. First, detailed information is obtained about the
patient’s responses: the particular responses that cause the patient
problems, the situations in which they have typically been emitted, and
particularly the foods that trigger the behavior. Second, the patient is checked
for alcoholism, drug addiction, or other contraindications. Third, if there
is any possibility of an active opiate addiction despite denials by the patient,
a small dose of opiate antagonist is administered under close medical
supervision.
[0066] Naloxone is metabolized
so rapidly in the liver that all of it is removed during the first pass after
oral administration. Consequently, it usually is injected, as was the case in a
previous test for treating anorexia (Huebner, US Patent 4,546,103,
1985).
[0067] Transdermal
administration of naloxone, how-ever, is much better suited for repeated
self-administration. I previously proposed a transdermal devise for
ad-ministering a fixed dose of an opioid antagonist, including naloxone, for use
in alcoholism treatment (Sinclair, US Patent 5,096,715, 1992). Recent
experiments (Panchagnula et al., 2001) have shown this devise with 33 %
propylene glycol as the vehicle and ethanol as the permeation enhancer
is even more effective for transdermal de-livery of naloxone than I had
anticipated: "theoretically blood levels well above the therapeutic
concentration of naloxone can be achieved" with a transdermal patch of a
convenient size. An intranasal spray has also been shown suitable for rapid
administration of naloxone for the majority of subjects and could also be used,
probably in combination with transdermal administration (Loimer et al.,
1992).
[0068] Avoiding the oral route
also has distinct advantages for selective extinction of eating disorders.
First, there is the problem that some of an orally administered medication
would be lost by purging, or not taken by anorexic patients. Second,
troubles with the gastrointestinal tract are common complications with eating
disorders. Oral administration itself irritates the throat and it
directs
|
|
5 the
highest concentration of the medication to intestines where it interacts
with opioidergically controlled motility. Third, the response of taking an
oral medication is similar to the eating responses we are trying to alter
with the treatment, thus adding a possible complication to the
pro-
|
10
cedure.
Binge-eating and
bulimia
[0069] Severe cases should be
handled initially in a
|
|
15
treatment center to assure compliance, to increase motivation,
to monitor health, and to provide counseling and training concerning
correct eating habits. The information obtained includes a list of the
patient’s "trigger foods", i.e., those highly palatable foods that
precipitate binges,
|
|
|
20 are
frequently included in binges, are greatly craved, or give the patient
intense pleasure when the first bite is eaten. A list is also prepared for
the patient of "healthy foods", i.e., nutritious foods that do meet any of
the above characteristics for trigger
foods.
|
|
|
25
[0070] The
patient is kept initially on diet specifically excluding a particular
trigger food and foods with similar characteristics for a week prior to
treatment. (In the afore-mentioned example, if the trigger is chocolate
ice cream, the patient avoids not only chocolate ice cream but
all
|
|
|
30 ice
cream and all chocolate.) Naloxone is then administered, perhaps
first by nasal spray and then transdermally, and then while active
quantities are present in the system, the patient is presented with the
trigger food and encouraged to have an eating binge of it. If possible,
the
|
|
|
35
situation in which the food is eaten should be similar to that in
which the patient usually has had eating binges. The response set should
also be similar; e.g., if the patient typically has purged after an
eating binge previously, purging should occur also in the extinction
session. No
|
40 healthy
foods should be available.
The
duration of an extinction session should match the patient’s previous binging
behavior. If binging normally continued for several days, the same should occur
in treatment, with additional transdermal administrations of
45 naloxone
being given as needed.
[0071] At the end of the
extinction session, the transdermal administration is stopped and the skin area
involved is washed thoroughly.
[0072] The extinction session
is followed the next day 50 by a
"learning period" of one day or more when no antagonist
is given and only healthy foods are available. Not only
are trigger foods not available, but also all stimuli related to
them; the patient should not see them or smell them, nor
should they be discussed in counseling. The
55 safe foods
can be restricted to meal times, but the patient can eat as
much as desired then: no attempt at dieting should
occur during the learning periods. Learning of alternative
behaviors can be encouraged, but care should
9
15 EP 1 681 057
B1 16
be used
with regard to responses reinforced through the opioid system. For example,
greater than normal intake of alcohol should not be allowed.
[0073] In subsequent
extinction sessions, the patient binges on other trigger foods that have not
been included in the previous sessions. In severe case being handled at a
clinical center, treatment continues until binge eating with most of the
patient’s trigger foods has been extinguished and the person has gained
greater control over his or her eating habits. Thereafter, an out-patient mode
of selective extinction treatment can be used. The subject is given take-home
doses of the opiate antagonist and told to take one whenever there is a high
probability that unsafe foods will be eaten in the next few hours. The
instructions state that the patients should go ahead and have an eating binge if
they feel like it, but only after taking naloxone. Under no circumstance should
they binge without taking the antagonist. The antagonist should not be taken
otherwise, i.e., when the patient thinks there is little chance of eating
trigger foods.
Dietary aid for
stimulus-bound overeating
[0074] An out-patient mode of
selective extinction is used for patients with less severe eating problems and
high motivation and ability for compliance. It can be used with subjects who are
obese or only moderately over-weight whose weight problem is not due to
glandular anomalies but rather is caused by eating more than more than caloric
needs in response to specific stimuli. The stimuli can be specific
highly-palatable ("trigger") food items, situations, or moods. Examples of
trigger food items would be chocolate, mayonnaise, peanut butter, potato chips,
cream, butter, and cheese. Examples of trigger situations are watching
television, fast food and other restaurants, parties, holidays, and "midnight
snack" excursions. Examples of moods are premenstrual syndrome (PMS),
post-traumatic stress (PTS), anxiety in anticipation of a stress situation,
and celebration euphoria. [0075] The procedure is the
same as that with binge-eating and bulimia except the subject is not kept in a
treatment center but rather conducts his or her own extinction sessions in
the outside world. The subject is given clear, precise instructions (similar to
those specified above for binge eating and bulimia) on how to extinguish the
problem eating responses (e.g., 1. create a list of trigger stimuli, 2. choose
one, 3. refrain from it for one week, 4. arrange for the trigger stimulus to be
present, 5. self-administer naloxone, 6. what to do during intervening
"learning periods" when the antagonist is not taken and the trigger stimuli
are avoided as much as possible.
Dietary aid for limiting
intake of specific substances (sugar, salt,
etc.)
[0076] Selective extinction
can be used for people who are not necessarily overweight but must reduce their
intake of a
particular substance that is closely associated with a distinct stimulus that
causes opioid release.
[0077] One example is with
people who need to reduce their intake of sodium chloride. Sodium chloride is
closely
|
|
5
associated with salty tastes, and there is evidence showing
that a salty taste produces reinforcement through the opioidergic system
(Levine et al., 1982). The person is given a series of extinction sessions
on naloxone and learning periods off of the antagonist. During the
extinc-
|
|
|
10 tion
sessions a variety of salty-tasting foods are eaten. If necessary, the
salty taste could be produced by a salt substitute, but sodium chloride
should be used if there is no medical danger from short-term intake of the
sub-stance. During the learning sessions, salty-tasting
foods
|
|
|
15 are
omitted from the diet completely. The responses of eating salty foods are
thus selectively extinguished, while the responses of eating non-salty
foods are not weakened and may be enhanced. This will reduce the
desire for salty foods and make it easier for the person to
stay
|
20 on a
low salt diet.
[0078] A similar procedure
could be used with people who need to restrict their intake of sugars. Sweet
foods are eaten during the extinction sessions and non-sweet ones during the
learning periods. The sweet taste could
|
|
25 be
produced with artificial sweeteners, but sugar should be used if there is
no medical danger from such limited
intake.
|
[0079] The method also can be
used with people who need to restrict their intake of cholesterols or
specifically
|
|
30
low-density cholesterols. Although there probably is no specific
taste stimuli associated with cholesterols, they tend to be present in
highest amounts in particular highly-palatable foods. Consequently, during
the extinction sessions the person eats these particular foods and
during
|
|
|
35 the
learning session the person eats foods with low amounts of cholesterol or
low-density cholesterols. [0080] This procedure
could be used either in a treatment center or on an out-patient basis
depending upon the person’s ability to comply and the severity of the
ail-
|
40 ment
requiring the dietary limitations.
Anorexia
nervosa
[0081] The patient is kept
continually on a transdermal
|
|
45 opiate
antagonist for a period (probably 2 days or more) while intravenous
nutrients are supplied. Naltrexone or nalmefene could be used initially
but naloxone should be used in the last
day.
|
[0082] Antagonist
administration is then abruptly ter-
|
|
50
minated. During the next day (a learning period when the system is
free of active levels of antagonist), the patient is given small portions
of a variety of highly-palatable foods and strongly encouraged to eat at
least a small amount. The rebound supersensitivity of the opioid
sys-
|
|
|
55 tem
should help to reinforce the eating responses that are
made.
|
[0083] The next day the
patient is placed again on the antagonists and fed intravenously. The pattern of
extinc-
10
17 EP 1 681 057
B1 18
tion
sessions and learning periods continues. New highly-palatable foods are
introduced on each antagonist-free day, with at least a week between duplication
of the same food item in order to allow deprivation effects to increase the
reinforcement. After the first sessions, in-creasing attention is paid to
providing a well-rounded, nutritious variety of highly-palatable foods.
Pharmacological potentiation of the opioidergic response, e.g., with
moderate amounts of alcohol, can be employed.
[0084] During extinction
session days on the antagonist, the patient is encouraged to make the most
common responses from his or her own list of previously-learned competing
anorexic responses (e.g., vigorous exercise) that are probably reinforced
through the opioid system. In most cases, the aim should be weakening these
responses only to a normal level.
EXAMPLE
[0085] Selective extinction:
weakening of one behavior and at the same time strengthening another. Selective
extinction is produced by pairing the response we want to decrease (alcohol
drinking by rats in this example) with an opioid antagonist and pairing the
response we want to become more powerful (saccharin drinking here) with times
when the antagonist is not in the body.
[0086] The example
demonstrates that selective extinction can be produced with naloxone (i.e.,
the antagonist best suited for treating eating disorders.) It also shows
that selective extinction works with eating behaviors like saccharin
drinking that are normally reinforced by the flavor causing endorphins to be
released.
Methods
[0087] Male Wistar rats (n=26)
were individually housed with daily access to 10 % ethanol, with food and water
always present. After 2 months prior experience, the rats were switched to
having 2-4 alcohol-access days interspersed with 1 or 2 days when saccharin
solution (1 g/l) was available for 1 hr. The rats were then divided into 2
matched groups, one always receiving a subcutaneous dose of naloxone prior to
alcohol access and a control group receiving a similar injection of saline prior
to alcohol access. No injections were made prior to saccharin access. In
addition, the naloxone dose was progressively reduced from 10.000 to 0.005
mg/kg.
Results
[0088] The naloxone injections
significantly reduced alcohol drinking in comparison with both the alcohol
in-take by the controls and in comparison with their own prior levels (see Fig.
la). The alcohol drinking continued to be significantly reduced for 8 weeks;
many of these weeks involved doses far lower than previously found to be
effective. Alcohol drinking was reduced to nearly zero for most rats for 6
weeks. The suppression of drinking of alcohol
drinking appears greater than in previous experiments in which both alcohol
drinking and antagonist ad-ministration occurred every day and specifically
greater than in studies aimed at maintaining a continual presence
|
|
5 of
the antagonist by using longer lasting naltrexone or nalmefene and mixing
them with the food.
|
[0089] In contrast to the
sharp reduction in alcohol drinking, saccharin drinking was consistently higher
in the naloxone treated rats than in the controls and signif-
|
|
10
icantly so during the first three weeks when doses of naloxone
previously shown to be effective were used (see Fig.
1b).
|
References
15
[0090]
"Naloxone
in the Treatment of Anorexia Nervosa: Effect on Weight Gain and Lipolysis" R.
Moore, I.H
|
20
|
Mills,
A. Forster, Journal of the Royal Society of Medicine 1981, 74,
129-31.
|
"Targeted
Use of Naltrexone Without Prior Detoxification in the Treatment of Alcohol
Dependence: A Factorial Double-Blind Placebo-Controlled Trial." P
|
|
25
Heinälä, H. Alho, K. Kiianmaa, J. Lönnqvist, K. Kuoppasalmi,
and J.D. Sinclair, Journal of Clinical Psychopharmacology, 2001. 21,
287-292.
|
"Evidence
about the Use of Naltrexone and for Different Ways of Using It in the
Treatment of Alcohol-
|
30
|
ism"
J. D. Sinclair, Alcohol and Alcoholism 2001,36,
2-10.
|
"The Rest
Principle: A Neurophysiological Theory of Behavior" J. D. Sinclair, Lawrence
Erlbaum Associates, Hillsdale, NJ, 1981.
|
35
|
"Rats
Learning to Work for Alcohol" J. D. Sinclair, Nature 1974, 249,
590-592.
|
"Drugs to
Decrease Alcohol Drinking", J.D.Sinclair, Annals of Medicine, 1990, 22,
357-362.
"Alcohol
and Opioid Peptides: Neuropharmacologi-
|
|
40 cal
Rational for Physical Craving of Alcohol" M.C. Tractenberg, and K. Blum.
American Journal of Drug and Alcohol Abuse 1987,13,
365-372.
|
"Naltrexone
and the Treatment of Alcohol Dependence" J.R. Volpicelli, C.P.O’Brien,
A.I.Alterman, and
|
|
45 M.
Hayashida, In Opioids, Bulimia, and Alcohol Abuse & Alcoholism,
L.D.Reid, ed. Springer-Verlag, New York, 1990, pp
195-214.
|
"Opioids
Modulate Rats’ Propensities to Take Alcoholic Beverages" L. D. Reid, and C.
L. Hubbell, in
|
|
50 Novel
Pharmacological Interventions for Alcoholism. C.A. Naranjo and E.M.
Sellers (eds) New York: Springer-Verlag,
pp.121-134,1992
|
"Uso
Efficace del Naltrexone: Ciò Che Non è Stato Detto a Medici e Pazienti"
(Effective use of naltrex-
|
|
55 one:
What doctors and patients have not been told) D. Sinclair, F. Fantozzi,
and J. Yanai, The Italian Journal of the Addictions, 2003,
41,15-21.
|
"La
Ricaduta Nell’Alcol: un Concetto Vincente, Ma
11
19 EP 1 681 057
B1 20
in Via di
Estinzione?" F. Fantozzi, and D. Sinclair, Personalit Dipendenze 2004,10,
219-243. "Naltrexone and Brief Counselling to Reduce Heavy Drinking" M. J. Bohn,
H.R. Kranzler, D. Beazoglou, and B.A. Staehler, The American Journal on
Addictions 1994, 3, 91-99.
"A
Randomized 6 Month Double-Blind Placebo-Controlled Study of Naltrexone and
Coping Skills Education Programme" J. Balldin, M. Berglund, S. Borg, M.
Månsson, P. Berndtsen, J. Franck, L. Gustafsson, J. Halldin, C. Hollstedt, L-H.
Nilsson, and G. Stolt, Alcohol and Alcoholism 1997, 32, 325.
"The
Effect of Naltrexone on Taste Detection and Recognition Threshold" P.A. Arbisi,
C.J. Billington, A.S. Levine, Appetite 1999, 32, 241-249. "Long-Term Follow Up
of Continued Naltrexone Treatment" J. D. Sinclair, K. Sinclair, and H. Alho,
Alcoholism: Clinical and Experimental Research 2000, 24, suppl.
182A.
"Double-Blind
Naltrexone and Placebo Comparison Study in The Treatment of Pathological
Gambling" S. W. Kim, J. E. Grant, D. E. Adson, and Y. C. Shin, Biological
Psychiatry 2001, 49:914-921.
"Basic
Mechanisms of Opioids’ Effects on Eating and Drinking", S.J. Cooper and T.C.
Kirkham, in Opioids, Bulimia, and Alcohol Abuse & Alcoholism, L.D. Reid, ed.
Springer-Verlag, New York, 1990, 91-110.
"Naltrexone
and Bulimia: Initial Observations", J.M. Jonas, in Opioids, Bulimia, and Alcohol
Abuse & Alcoholism, L.D. Reid, ed., Springer-Verlag, New York, 1990,
123-130.
"Obesity,
Anorexia Nervosa, and Bulimia: A General Overview", K.D. Wild and L.D. Reid,in
Opioids, Bulimia, and Alcohol Abuse & Alcoholism, L.D. Reid, ed.,
Springer-Verlag, New York, 1990, 3-21.
"Opioids
Modulate Rats’ Intake of Alcoholic Bever-ages", C.L. Hubbell and L.D. Reid, in
Opioids, Bulimia, and Alcohol Abuse & Alcoholism, L.D. Reid, ed.,
Springer-Verlag, New York, 1990, 145-174.
"Using
Drugs to Manage Binge-Eating among Obese and Normal Weight Patients", S.A.Alger,
M.J.Schwalberg, J.M. Bigaoutte, L.J. Howard, and L.D. Reid, in: Opioids,
Bulimia, and Alcohol Abuse & Alcoholism, L.D.Reid, ed., Springer-Verlag, New
York, 1990, 131-142.
"Pharmacologic
Treatment Of Binge Eating Disorder" W.P. Carter, J.I. Hudson, J.K. Lalonde,
L. Pindyck, S.L. McElroy, and H.G. Pope Jr., International Journal of
Eating Disorders 2003, 34, Suppl:S74-88. "Naloxone Decreases Food Intake in
Obese Hu-mans", R. L. Atkinson, Journal of Clinical Endocrinology and
Metabolism, 1982, 55, 196-198.
"The
Endogenous Opioidergic Systems" E.M.Unterwald and R.S.Zukin, in, Opioids,
Bulimia, and Alcohol Abuse & Alcoholism, L.D. Reid, ed.,
Springer-Verlag, New York, 1990, 49-72.
"Reduction
of Alcohol Drinking and Upregulation of Opioid Receptors by Oral Naltrexone in
AA Rats" J.H. Parkes
and J.D.Sinclair. Alcohol, 2000, 21, 215-221.
"Potential
Toxicities of High Doses of Naltrexone in Patients with Appetitive Disorders",
C.J. Morgan and
|
|
5 T.R.
Kosten, in Opioids, Bulimia, and Alcohol Abuse & Alcoholism, L.D.
Reid, ed. Springer-Verlag, New York, 1990,
261-273.
|
"Flavor
Enhances the Antidipsogenic Effect of Naloxone", A. S. Levine, S. S. Murray, J.
Kneip, M.
|
10
|
Grace
and J. E. Morley, Physiology and Behavior, 1982, 28,
23-25.
|
"Increased
preference for ethanol in rats following alcohol deprivation", J.D. Sinclair,
and R.J.Senter, Psychonomic Science 1967, 8, 11-12.
|
|
15 "The
Effect of Naltrexone on Alcohol Consumption after Alcohol Deprivation in
Rhesus Monkeys", M. Kornet, C. Goosen, and J.M. Van Ree, Abstracts of the
XXth Nordic Meeting on Biological Alcohol Re-search, Espoo, Finland, May
13-15, 1990, abstract
|
20 20
"Pattern
of Onset of Bulimic Symptoms in Anorexia Nervosa", J.A. Kassett, H.E. Gwirtsman,
W.H. Kaye, H.A. Brandt, and D.C. Jimerson, American Journal of Psychiatry, 1988,
145, 1287-1288.
|
|
25
"Case Reports: Treatment of Chronic Anorexia Nervosa with
Opiate Blockade", E.D. Luby, M.A. Marrazzi, and J. Kinzie, Journal of
Clinical Psychopharmacolgy, 1987, 7,
52-53.
|
"An
Auto-Addiction Opioid Model of Chronic Anorex-
|
|
30 ia
Nervosa", M.A. Marrazzi and E.D. Luby, International Journal of
Eating Disorders, 1986, 5, 191-208. "Transdermal Delivery of Naloxone:
Effect of Water, Propylene Glycol, Ethanol and Their Binary
Combinations on Permeation Through Rat Skin" R.
Pan-
|
|
|
35
chagnula, P.S. Salve, N.S. Thomas, A.K. Jain, and P. Ramarao,
International Journal of Pharmacology 2001, 219,
95-105.
|
"Nasal
Administration of Naloxone is as Effective as the Intravenous Route in Opiate
Addicts" N. Loimer,
|
40
|
P.
Hofmann, and H.R. Chaudhry, International Journal of Addictions,
1994, 29, 819-827.
|
"The
Genetic Epidemiology of Bulimia Nervosa" K.S. Kendler, C. MacLean, M. Neale, R.
Kessler, A. Heath, and L. Eaves, American Journal of Psychia-
45 try,
1991,148, 1627-1637.
"Selective
Extinction of Alcohol Drinking in Rats with Decreasing Doses of Opioid
Antagonists" J.D. Sinclair, L. Vilamo, and B. Jakobson. Alcoholism:
Clinical and Experimental Research 1994, 18, 489.
50
Claims
1. Use of naloxone for the
preparation of a pharmaceu-
|
|
55
tical composition for treating eating disorders by a method based
on selective extinction comprising the
steps:
|
12
21 EP 1 681 057
B1 22
- identification of the
specific responses in the patient’s eating behavior that are unhealthy or
otherwise inappropriate,
-
administering the pharmaceutical composition containing naloxone just before the
patient 5
makes these unhealthy responses,
- having
the patient make healthy eating responses only when the effective levels of
naloxone are no longer present in the body, and
- having
the patient to alternate between making 10 unhealthy
eating responses when effective levels of naloxone are present and making
healthy eating responses when effective levels of naloxone are not present, so
long as the patient
still
wants to make the unhealthy eating respons 15
es.
|
2. Use
according to claim 1, wherein the eating disorder is selected from the
group comprising binge eating, bulimia, bulimia-like syndrome, anorexia
nervosa, and habitual over-eating stimulated by specific stimuli
including certain foods, situations, or moods.
3. Use
of naloxone for the preparation of a pharmaceutical composition for
improving compliance among patients who must lower intake of a particular
class of restricted food including dietary substances such as sodium
chloride, sugars, cholesterols or low-density cholesterols by a
method based on selective extinction comprising the
steps:
|
20
25
30
-
administering the pharmaceutical composition containing naloxone just before the
patients eats the restricted food,
- having
the patient eat other non-restneted food 35 only when
the effective levels of naloxone are no longer present in the body,
- having
the patient alternate between occasion-ally eating the restricted food when the
effective levels of naloxone are present and eating non- 40 restricted
food when effective levels of naloxone are not present, so long as the patient
still wants to eat the restricted food.
-
Identifizieren der spezifischen Reaktionen im Essverhalten des Patienten, welche
ungesund oder anderweitig ungünstig sind,
|
Patentansprüche
1. Verwendung von
Naloxon zur Herstellung eines Arzneimittels zur Behandlung von
Essstörungen durch ein Verfahren beruhend auf selektiver Löschung,
umfassend die Schritte:
|
-
Verabreichen des Arzneimittels enthaltend Naloxon kurz bevor der Patient
diese ungesunden Reaktionen ausführt,
- den
Patienten dazu bringen, die gesunden Essreaktionen nur dann auszuführen, wenn
die wirksamen Spiegel von Naloxon nicht länger im Körper vorherrschen,
und
- den
Patienten dazu bringen, zwischen dem Ausführen von ungesunden Essreaktionen,
wenn wirksame Spiegel von Naloxon vorherrschen, und dem Ausführen von
gesunden Essreaktionen, wenn wirksame Spiegel von Naloxon nicht
vorherrschen, zu wechseln, so lange der Patient die ungesunden Essreaktionen
immer noch ausführen will.
|
4. The
use according to any of claims 1 to 3, wherein 45 naloxone
is given transdermally and the dose per day is 0.001 mg to 50
mg.
5. The
use according to any of claims 1 to 3, wherein naloxone is given by
intranasal inhalation and the 50 dose
per day is 0.001 mg to 50 mg.
6. The
use in accordance with any of the preceding claims, wherein the dose of
naloxone is started at a high level of 5 to 50 mg and then is
progressively 55
reduced over the days of treatment.
|
|
2. Verwendung
nach Anspruch 1, wobei die Essstörung ausgewählt ist aus Binge
Eating, Bulimie, bulimieähnlichem Syndrom, Anorexia nervosa und
gewohnheitsmäßigem Über-Essen stimuliert durch spezifische Stimuli
einschließlich bestimmter Nahrungsmittel, Situationen oder
Gemütszustände.
3. Verwendung
von Naloxon zur Herstellung eines Arzneimittels zur Verbesserung der
Therapietreue unter Patienten, welche die Aufnahme von einer
bestimmten Klasse an begrenzten Nahrungsmitteln herabsetzen
müssen, einschließlich Diätsubstanzen wie Natriumchlorid, Zucker,
Cholesterine oder LDLCholesterine, durch ein Verfahren beruhend auf
selektiver Löschung umfassend die
Schritte:
|
-
Verabreichen des Arzneimittels enthaltend Naloxon kurz bevor der Patient
das begrenzte Nahrungsmittel isst,
- den
Patienten dazu bringen, andere, nicht-begrenzte Nahrungsmittel nur dann zu
essen, wenn die wirksamen Spiegel von Naloxon nicht länger im Körper
vorherrschen, und
- den
Patienten dazu bringen, zwischen dem gelegentlichen Essen des begrenzten
Nahrungsmittels, wenn wirksame Spiegel von Naloxon vorherrschen, und dem
Essen von nicht-begrenzten Nahrungsmitteln, wenn wirksame Spiegel von
Naloxon nicht vorherrschen, zu wechseln, so lange der Patient das begrenzte
Nahrungsmittel immer noch essen will.
13
23 EP 1 681 057
B1 24
|
4.
|
Verwendung
gemäß einem der Ansprüche 1 bis 3, wobei Naloxon transdermal verabreicht
wird und die Tagesdosis 0,001 mg bis 50 mg
beträgt.
|
5
|
5.
|
Verwendung
gemäß einem der Ansprüche 1 bis 3, wobei Naloxon durch intranasale
Inhalation verabreicht wird und die Tagesdosis 0,001 mg bis 50 mg
beträgt.
|
|
6.
|
Verwendung
gemäß einem der vorangegangenen 10
Ansprüche, wobei die Dosis an Naloxon bei einem hohen Spiegel von 5
bis 50 mg gestartet und dann allmählich über die Tage der Behandlung
reduziert wird.
|
-
administrer la composition pharmaceutique contenant la naloxone juste avant que
le patient n’ait ces réponses morbides,
- faire
que le patient ait des réponses alimentaires saines seulement lorsque les
taux efficaces de naloxone ne sont plus présents dans le corps,
et
- faire
que le patient alterne entre manger occasionnellement des aliments
interdits lorsque les taux efficaces de naloxone sont présents et manger des
aliments non interdits lorsque les taux efficaces de naloxone ne sont plus
présents, tant que le patient veut encore manger les aliments
interdits.
15
Revendications
|
1.
|
Utilisation
de naloxone pour la préparation d’une composition pharmaceutique pour le
traitement de troubles alimentaires par un procédé basé sur une extinction
sélective comprenant les étapes de
:
|
-
identifier les réponses spécifiques dans le comportement alimentaire d’un
patient qui sont morbides, ou inappropriées,
-
administrer la composition pharmaceutique contenant la naloxone juste avant que
le patient n’ait ces réponses morbides,
- faire
que le patient ait des réponses alimentaires saines seulement lorsque les
taux efficaces de naloxone ne sont plus présents dans le corps,
et
- faire
que le patient alterne entre les réponses alimentaires morbides lorsque les taux
efficaces de naloxone sont présents et des réponses alimentaires saines
lorsque les taux efficaces de naloxone ne sont plus présents, tant que le
patient veut encore avoir des réponses alimentaires
morbides.
|
2.
|
Utilisation
selon la revendication 1, dans laquelle le trouble alimentaire est choisi
dans le groupe comprenant l’hyperphagie boulimique, la boulimie, un
syndrome similaire à la boulimie, l’anorexie mentale, et l’hyperphagie
ordinaire stimulée par des stimulus spécifiques incluant certain(e)s
aliments, situations, ou humeurs.
|
|
3.
|
Utilisation
de naloxone pour la préparation d’une composition pharmaceutique pour
améliorer l’observance des patients qui doivent réduire la
consommation d’une classe particulière d’aliments interdits incluant
des substances alimentaires telles que le chlorure de sodium, les sucres,
les cholestérols ou les cholestérols de basse densité par un procédé basé
sur l’extinction sélective comprenant les étapes de
:
|
|
4.
|
Utilisation
selon l’une quelconque des revendications 1 à 3, dans laquelle la
naloxone est administrée par voie transdermique et la dose quotidienne est
de 0,001 mg à 50 mg.
|
20
|
5.
|
Utilisation
selon l’une quelconque des revendications 1 à 3, dans laquelle la
naloxone est administrée par inhalation intranasale et la dose quotidienne
est de 0,001 mg à 50 mg.
|
25
|
6.
|
Utilisation
selon l’une quelconque des revendications précédentes, dans laquelle
la dose de naloxone est initiée à un taux élevé de 5 à 50 mg puis est
progressivement réduite pendant le
traitement.
|
30
35
40
45
50
55
14
EP 1 681 057
B1
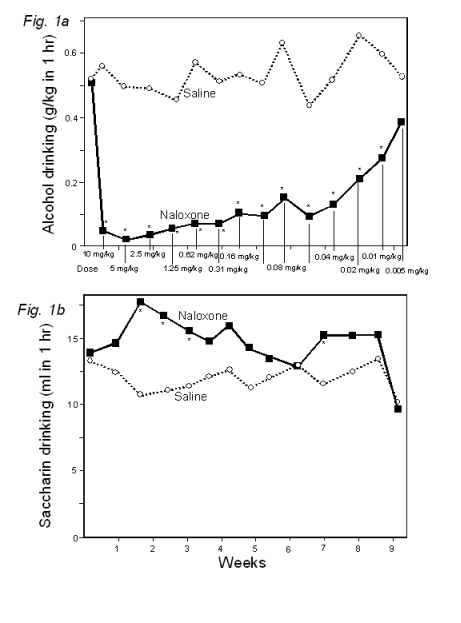
15
EP 1 681 057 B1
REFERENCES
CITED IN THE DESCRIPTION
This
list of references cited by the applicant is for the reader’s convenience only. It
does not form part of the
European patent document.
Even though great care has been taken in compiling the references, errors or
omissions cannot be excluded and the EPO disclaims all liability in this
regard.
Patent
documents cited in the description
|
· US
4217353 A [0003]
|
· • US 5587381 A [0005] [0005]
[0043]
|
|
· US
4477457 A [0003]
|
· • EP 0346830 B1 [0005]
|
|
· US
4546103 A [0003] [0038]
[0066]
|
· • US 5780479 A [0036]
|
|
· US
4882335 A [0005]
[0028]
|
· • US 5096715 A [0056]
[0067]
|
Non-patent
literature cited in the description
|
·
|
R. MOORE ; I.H MILLS ; A.
FORSTER. Naloxone in the Treatment of Anorexia Nervosa: Effect on
Weight Gain and Lipolysis. Journal of the Royal Society
of Medicine, 1981, vol. 74, 129-31 [0090]
|
|
·
|
P HEINÄLÄ ; H. ALHO ; K.
KIIANMAA ; J. LÖNNQVIST ; K. KUOPPASALMI ; J.D. SINCLAIR.
Targeted Use of Naltrexone Without Prior Detoxification in the
Treatment of Alcohol Dependence: A Factorial Double-Blind
Placebo-Controlled Trial. Journal of Clinical
Psychopharmacology,
2001, vol. 21, 287-292 [0090]
|
|
·
|
J. D. SINCLAIR. Evidence
about the Use of Naltrexone and for Different Ways of Using It in the
Treatment of Alcoholism. Alcohol and Alcoholism,
2001, vol. 36, 2-10 [0090]
|
|
·
|
J. D. SINCLAIR. The Rest
Principle: A Neurophysiological Theory of Behavior. Lawrence Erlbaum
Associates, 1981 [0090]
|
|
·
|
J. D. SINCLAIR. Rats
Learning to Work for Alcohol. Nature, 1974, vol. 249,
590-592 [0090]
|
|
·
|
J.D.SINCLAIR. Drugs to
Decrease Alcohol Drinking. Annals of Medicine,
1990, vol. 22, 357-362 [0090]
|
|
·
|
M.C. TRACTENBERG ; K. BLUM.
Alcohol and Opioid Peptides: Neuropharmacological Rational for
Physical Craving of Alcohol. American Journal
of Drug and
Alcohol Abuse, 1987, vol. 13, 365-372 [0090]
|
|
·
|
Naltrexone
and the Treatment of Alcohol Dependence. J.R. VOLPICELLI ; C.P.O’BRIEN ;
A.I.ALTERMAN ; M. HAYASHIDA. Opioids, Bulimia, and Alcohol
Abuse & Alcoholism. Springer-Verlag, 1990, 195-214 [0090]
|
|
·
|
Opioids
Modulate Rats’ Propensities to Take Alcoholic Beverages. Novel
Pharmacological Interventions for Alcoholism. Springer-Verlag, 1992,
121-134 [0090]
|
|
·
|
Uso
Efficace del Naltrexone: Ciò Che Non è Stato Detto a Medici e Pazienti.
D. SINCLAIR ; F. FANTOZZI
; J. YANAI. The Italian Journal of the Addictions. 2003, vol.
41, 15-21 [0090]
|
|
·
|
F. FANTOZZI ; D. SINCLAIR.
La Ricaduta Nell’Alcol: un Concetto Vincente, Ma in Via di
Estinzione. Personalit Dipendenze,
2004, vol. 10, 219-243 [0090]
|
|
·
|
M. J. BOHN ; H.R. KRANZLER ; D.
BEAZOGLOU ; B.A.
STAEHLER. Naltrexone and Brief Counselling to Reduce Heavy
Drinking. The American
Journal on
Addictions,
1994, vol. 3, 91-99 [0090]
|
|
·
|
J. BALLDIN ; M. BERGLUND ; S.
BORG ; M. MÅNSSON ; P. BERNDTSEN ; J. FRANCK ; L. GUSTAFSSON ; J. HALLDIN
; C. HOLLSTEDT ; L-H. NILSSON. A Randomized 6 Month
Double-Blind Placebo-Controlled Study of Naltrexone and Coping Skills
Education Programme. Alcohol and Alcoholism,
1997, vol. 32, 325 [0090]
|
|
·
|
P.A. ARBISI ; C.J. BILLINGTON ;
A.S. LEVINE. The Effect of Naltrexone on Taste Detection and
Recognition Threshold. Appetite, 1999, vol.
32, 241-249 [0090]
|
|
·
|
J. D. SINCLAIR ; K. SINCLAIR ;
H. ALHO. Long-Term Follow Up of Continued Naltrexone
Treatment. Alcoholism: Clinical and
Experimental Re-search, 2000, vol. 24 (182A [0090]
|
|
·
|
S. W. KIM ; J. E. GRANT ; D. E.
ADSON ; Y. C. SHIN. Double-Blind Naltrexone and Placebo
Comparison Study in The Treatment of Pathological Gambling.
Biological Psychiatry,
2001, vol. 49, 914-921 [0090]
|
|
·
|
S.J. COOPER ; T.C. KIRKHAM.
Basic Mechanisms of Opioids’ Effects on Eating and Drinking. Opioids, Bulimia, and Alcohol Abuse
& Alcoholism, 1990, 91-110 [0090]
|
|
·
|
Naltrexone
and Bulimia: Initial Observations. J.M. JONAS. Opioids,
Bulimia, and Alcohol Abuse & Alcoholism. Springer-Verlag, 1990,
123-130 [0090]
|
|
·
|
Obesity,
Anorexia Nervosa, and Bulimia: A General Overview. K.D. WILD ; L.D. REID.
Opioids, Bulimia, and Alcohol Abuse & Alcoholism.
Springer-Verlag, 1990, 3-21 [0090]
|
16
EP 1 681 057 B1
|
·
|
Opioids
Modulate Rats’ Intake of Alcoholic Beverages. C.L. HUBBELL ; L.D. REID.
Opioids, Bulimia, and Alcohol Abuse & Alcoholism.
Springer-Verlag, 1990, 145-174 [0090]
|
|
·
|
Using
Drugs to Manage Binge-Eating among Obese and Normal Weight Patients. S.A.ALGER ; M.J.SCHWALBERG ;
J.M. BIGAOUTTE ; L.J. HOWARD ; L.D. REID.
Opioids, Bulimia, and Alcohol Abuse & Alcoholism.
Springer-Verlag, 1990, 131-142 [0090]
|
|
·
|
Pharmacologic
Treatment Of Binge Eating Disorder. W.P. CARTER ; J.I. HUDSON ;
J.K. LALONDE ; L. PINDYCK ; S.L. MCELROY ; H.G.
POPE JR. Inter-national Journal of Eating Disorders. 2003, vol. 34,
S74-88 [0090]
|
|
·
|
R. L. ATKINSON. Naloxone
Decreases Food Intake in Obese Humans. Journal of Clinical
Endocrinology and
Metabolism, 1982, vol. 55, 196-198 [0090]
|
|
·
|
The
Endogenous Opioidergic Systems. E.M.UNTERWALD ; R.S.ZUKIN.
Opioids, Bulimia, and Alcohol Abuse & Alcoholism.
Springer-Verlag, 1990, 49-72 [0090]
|
|
·
|
J. H. PARKES ; J.D.SINCLAIR.
Reduction of Alcohol Drinking and Upregulation of Opioid
Receptors by Oral Naltrexone in AA Rats. Alcohol, 2000, vol. 21,
215-221 [0090]
|
|
·
|
Potential
Toxicities of High Doses of Naltrexone in Patients with Appetitive
Disorders. C.J. MORGAN ;
T.R. KOSTEN. Opioids, Bulimia, and Alcohol Abuse & Alcoholism.
Springer-Verlag, 1990, 261-273 [0090]
|
|
·
|
A. S. LEVINE ; S. S. MURRAY ;
J. KNEIP ; M. GRACE ; J. E. MORLEY. Flavor Enhances the
Antidipsogenic Effect of Naloxone. Physiology and Behavior,
1982, vol. 28, 23-25 [0090]
|
|
·
|
J.D. SINCLAIR ; R.J.SENTER.
Increased preference for ethanol in rats following alcohol
deprivation. Psychonomic
Science, 1967, vol. 8, 11-12 [0090]
|
|
·
|
M. KORNET ; C. GOOSEN ; J.M.
VAN REE. The Effect of Naltrexone on Alcohol Consumption after
Alcohol Deprivation in Rhesus Monkeys. Abstracts of the XXth Nordic
Meeting on Biological Alcohol Re-search, 13 May 1990 [0090]
|
|
·
|
J.A. KASSETT ; H.E. GWIRTSMAN ;
W.H. KAYE ; H.A. BRANDT ; D.C. JIMERSON. Pattern of Onset of
Bulimic Symptoms in Anorexia Nervosa. American Journal of Psychiatry,
1988, vol. 145, 1287-1288 [0090]
|
|
·
|
E.D. LUBY ; M.A. MARRAZZI ; J.
KINZIE. Case Re-ports: Treatment of Chronic Anorexia Nervosa with
Opiate Blockade. Journal
of Clinical Psychopharmacolgy, 1987, vol. 7, 52-53 [0090]
|
|
·
|
M.A. MARRAZZI ; E.D. LUBY.
An Auto-Addiction Opioid Model of Chronic Anorexia Nervosa. International
Journal
of Eating
Disorders, 1986, vol. 5, 191-208 [0090]
|
|
·
|
R. PANCHAGNULA ; P.S. SALVE ;
N.S. THOMAS ; A.K.
JAIN ; P. RAMARAO. Transdermal Delivery of Naloxone: Effect of
Water, Propylene Glycol, Ethanol and Their Binary Combinations on
Permeation Through Rat Skin. International Journal of
Pharmacology,
2001, vol. 219, 95-105 [0090]
|
|
·
|
N. LOIMER ; P. HOFMANN ; H.R.
CHAUDHRY. Nasal Administration of Naloxone is as Effective as
the Intravenous Route in Opiate Addicts. International Journal of
Addictions, 1994, vol. 29, 819-827 [0090]
|
|
·
|
K.S. KENDLER ; C. MACLEAN ; M.
NEALE ; R. KESSLER
; A. HEATH ; L. EAVES. The Genetic Epidemiology of Bulimia
Nervosa. American
Journal
of Psychiatry,
1991, vol. 148, 1627-1637 [0090]
|
|
·
|
J.D. SINCLAIR ; L. VILAMO ; B.
JAKOBSON. Selective Extinction of Alcohol Drinking in Rats
with De-creasing Doses of Opioid Antagonists. Alcoholism: Clinical and
Experimental Research, 1994, vol. 18, 489 [0090]
|
17
