UNITED STATES SECURITIES AND
EXCHANGE COMMISSION
Washington, D.C.
20549
Form 10-K
| |
|
|
|
þ
|
|
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE
SECURITIES EXCHANGE ACT OF 1934
|
|
|
|
For the Fiscal Year Ended
December 31, 2009
|
|
or
|
|
o
|
|
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE
SECURITIES EXCHANGE ACT OF 1934
|
Commission File Number
000-23019
KENDLE INTERNATIONAL
INC.
| |
|
|
|
Ohio
|
|
31-1274091
|
(State or other jurisdiction
of incorporation or organization)
|
|
IRS Employer
ID No.
|
441 Vine Street, 500 Carew Tower
Cincinnati, Ohio 45202
513-381-5550
Securities Registered Pursuant to Section 12(b) of the
Act:
| |
|
|
|
Title of Each Class
|
|
Name of Each Exchange on Which Registered
|
|
|
|
Common Stock, no par value
|
|
The NASDAQ Stock Market LLC
(NASDAQ Global Select Market)
|
Securities Registered Pursuant to Section 12(g) of the
Act:
None
Indicate by check mark if the registrant is a well-known
seasoned issuer, as defined in Rule 405 of the Securities
Act. Yes o No þ
Indicate by check mark if the registrant is not required to file
reports pursuant to Section 13 or Section 15(d) of the
Act. Yes o No þ
Indicate by check mark whether the registrant (1) has filed
all reports required to be filed by Section 13 or 15(d) of
the Securities Exchange Act of 1934 during the preceding
12 months (or for such shorter period that the registrant
was required to file such reports), and (2) has been
subject to such filing requirements for the past
90 days. Yes þ No o
Indicate by check mark whether the registrant has submitted
electronically and posted on its corporate Web site, if any,
every Interactive Data File required to be submitted and posted
pursuant to Rule 405 of
Regulation S-T
(§ 232.405 of this chapter) during the preceding
12 months (or such shorter period that the registrant was
required to submit and post such
files.) Yes o No o
Indicate by check mark if disclosure of delinquent filers
pursuant to Item 405 of
Regulation S-K
(§ 229.405 of this chapter) is not contained herein,
and will not be contained, to the best of registrant’s
knowledge, in definitive proxy or information statements
incorporated by reference in Part III of this
Form 10-K
or any amendment to this
Form 10-K. o
Indicate by check mark whether the registrant is a large
accelerated filer, an accelerated filer, a non-accelerated
filer, or a smaller reporting company. See the definitions of
“large accelerated filer,” “accelerated
filer” and “smaller reporting company” in Rule
12b-2 of the
Exchange Act. (Check one):
|
|
|
|
| Large
accelerated
filer o
|
Accelerated
filer þ
|
Non-accelerated
filer o
|
Smaller
reporting
company o
|
(Do not check if a smaller reporting company)
Indicate by check mark whether the registrant is a shell company
(as defined in
Rule 12b-2
of the Exchange
Act). Yes o No þ
The aggregate market value of the Registrant’s Common Stock
at June 30, 2009, held by non-affiliates was $172,891,665
(based on the $12.24 closing price of the Company’s Common
Stock on The NASDAQ Global Select Market LLC on June 30,
2009).
As of March 3, 2010, 14,916,715 shares of no par value
Common Stock were issued and 14,893,663 shares of no par
value Common Stock were outstanding.
Documents
Incorporated by Reference
Portions of the Registrant’s Proxy Statement to be filed
with the Commission for its 2010 Annual Meeting of Shareholders
to be held May 20, 2010, are incorporated by reference into
Part III.
See Exhibit Index on page 46.
Kendle
International Inc.
Form 10-K
Annual Report
For the Fiscal Year Ended December 31, 2009
Table of
Contents
2
This Annual Report on
Form 10-K
contains certain statements that are “forward-looking
statements” within the meaning of the Private Securities
Litigation Reform Act of 1995. These statements are subject to a
number of assumptions, risks and uncertainties, many of which
are beyond the control of Kendle International Inc. (the Company
or Kendle). See “Item 1A — Risk
Factors” for further information.
The forward-looking statements speak as of the date made and are
not guarantees of future performance. Actual results or
developments may differ materially from the expectations
expressed or implied in the forward-looking statements, and the
Company undertakes no obligation to update any such statements.
PART I
General
Kendle International Inc., an Ohio corporation established in
1989, is a global clinical research organization (CRO) that
provides a broad range of Phase I-IV global clinical development
services to the biopharmaceutical industry. The Company augments
the research and development activities of biopharmaceutical
companies by offering high-quality, value-added clinical
research services and proprietary information technology
designed to reduce drug development time and expense. The
Company is managed in two reportable segments, Early Stage and
Late Stage. The Early Stage business currently focuses on the
Company’s Phase I operations, while Late Stage is comprised
of clinical development services related to Phases II
through IV.
Early
Stage
The Early Stage reportable segment is focused on the high-end
scientific exploratory medicine area, from
First-in-Human
studies through
proof-of-concept
stages, in the Company’s Toronto, Canada and Utrecht, The
Netherlands locations. The Company also provides full support
for Phase I studies in established compounds, including
bioequivalence and pharmacokinetics studies, at its Morgantown,
West Virginia location.
Late
Stage
The Late Stage reportable segment is organized slightly
differently than it was in 2008 but still includes four
reporting units: Clinical and Data Monitoring, Project
Management & Late Phase, Regulatory, Site and Medical
Affairs, and Biostatistics. This segment has a global reach that
enables the Company to meet its customers’ needs by
accessing specific patient populations in large urban areas as
well as emerging markets/regions all over the world.
The Clinical and Data Monitoring unit (C&DM) includes the
former Global Clinical Development (GCD) unit and conducts
Phase II through IV clinical trials worldwide.
C&DM provides a wide range of services including clinical
monitoring, investigator recruitment, patient recruitment, data
management and study reports to assist customers with their drug
development efforts.
The Project Management & Late Phase (PM &
LP) unit oversees all steps of a study, from award through close
out of a study. This unit also designs and conducts Phase IIIB
and IV studies worldwide. In addition, the Late Phase unit
also focuses on health economics and outcomes research,
observational studies, scientific events and medical education
services. Also included in PM & LP is the recently
formed group to provide services to the federal government and
health care foundation oriented organizations.
The Regulatory, Site and Medical Affairs unit provides
regulatory expertise and consulting services at every stage of
drug and device development, from early development to market
and post market. This unit designs, among other things, clinical
programs and clinical trial protocols, reviews programs and
provides gap analysis to assist sponsors in achieving their
clinical development strategies. The unit also provides
consulting services for nonclinical development for small
molecules, biologicals, vaccines and devices. This unit has
Chemistry, Manufacturing and Controls experts that assist with
the U.S. Food and Drug Administration application process.
Additionally, this unit has safety experts that assist customers
with the collection, analysis and reporting of accurate drug
safety data.
3
The Biostatistics and Statistical Programming unit offers full
service statistical support for Phase I to IV clinical
trials and submissions. The unit has biostatisticians that can
supply comprehensive statistical analysis plans on a turnkey
basis or on a consultant basis.
The Company believes that the outsourcing of drug development
activities by biopharmaceutical companies will increase as these
companies strive to grow revenues through an accelerated drug
development cycle while responding to cost containment
pressures. The CRO industry, by specializing in clinical trial
management, often performs the needed services with a higher
level of expertise or specialization at a faster pace and at a
lower cost than a biopharmaceutical company could perform such
services internally.
Acquisition
Activity
In June 2008, the Company completed its acquisition of
DecisionLine Clinical Research Corporation (DecisionLine) and
its related company. DecisionLine is a clinical research
organization located in Toronto, Ontario specializing in the
conduct of early phase studies. The acquisition supports the
overall goal of strategic business expansion, and, in
particular, expansion of Phase I studies. Please see
Note 13 to the Consolidated Financial Statements for
further detail regarding this acquisition.
Business
Strategy
The Company’s strategy is to continue to enhance its
reputation as a high-quality, global provider of a full range of
CRO services. This year has been a challenging one for the CRO
industry, for the Company and for its customers. The Company has
been affected by the repercussions of merger and acquisition
activity in the global biopharmaceutical industry and the
resultant reprioritization of their pipelines, search for cost
efficiencies and right sizing of their workforces resulting in
lower new business authorizations and higher than historical
cancellations. In the near term, the Company’s strategy is
to adapt to the current state of flux in the industry and bring
stability back to the Company’s operations. The
Company’s longer term strategy remains steadfast and
consists of the following strategic initiatives:
|
|
|
| |
•
|
Driving growth in the Company’s core competency in Phases I
through IV clinical trials.
|
| |
| |
•
|
Meeting the specific development pipeline needs for the
Company’s targeted customers.
|
| |
| |
•
|
Gaining share in the CRO market through further penetration in
the large (greater than $10 million in contract value)
trial sector as well as continued expansion in mid-sized trials.
|
| |
| |
•
|
Expanding the Company’s geographic footprint with a focus
on high-growth regions such as Latin America, Africa and
Asia/Pacific.
|
| |
| |
•
|
Setting the stage for business mix expansion, such as expansion
in support of Early Stage.
|
| |
| |
•
|
Building infrastructure (i.e. Enterprise Resource Planning) to
support the continued expansion and growth of the Company.
|
Customers
and Marketing
Net service revenues from the top five customers accounted for
approximately 27% of the Company’s total net service
revenues for both years ended December 31, 2009 and 2008.
No customer accounted for more than 10% of the Company’s
net service revenues for 2009 or 2008.
Segment and geographic information for the Company is contained
in Note 15 to the Consolidated Financial Statements.
Backlog
Backlog consists of anticipated net service revenue from
contracts, letters of intent and other forms of commitments
(collectively defined as backlog) that either have not started
but are anticipated to begin in the near future, or are in
process and have not been completed. Amounts included in backlog
represent anticipated future net service revenue and exclude net
service revenue that has been recognized previously in the
Consolidated Statements
4
of Operations. Once contracted work begins, net service revenue
is recognized in the Consolidated Statements of Operations.
Backlog at December 31, 2009, was approximately
$830 million compared to approximately $1.02 billion
at December 31, 2008. The majority of the Company’s
backlog is from large established biopharmaceutical firms with
annual revenues greater than $1 billion. Backlog arising
from contracts with small biotech firms with no revenues or
large biopharmaceutical partner are not material to the Company,
generally comprising 10% or less. In 2009, the Company and the
industry experienced higher than historical cancellations and
longer delays between proposals and awards due to merger and
acquisition activity in the industry, budget reductions and
pipeline reprioritizations at large pharmaceutical companies,
drug failures due to efficacy and safety, as well as,
contraction in the availability of credit to smaller lesser
capitalized companies, among other things. The average duration
of the contracts in backlog fluctuates from quarter to quarter
based on the contracts constituting backlog at any given time.
The Company generally experiences a longer period of time
between contract award and revenue recognition with respect to
large contracts covering global services. As the Company
competes for and enters into large contracts that are global in
nature, the Company expects the average duration of the
contracts in backlog to increase and may be affected by changes
in foreign exchange rates. Fluctuations in foreign exchange
rates caused backlog to increase by $8.0 million as of
December 31, 2009. Based on its experience in 2009, the
Company estimates that approximately 45% of its backlog at
December 31, 2009 will be recognized as net service revenue
in fiscal year 2010.
No assurance can be given that the Company will be able to
realize the net service revenues that are included in the
backlog. Backlog is not necessarily a meaningful indicator of
future results for a variety of reasons, including, but not
limited to, the following: (i) contracts vary in size and
duration, with revenue from some studies realized over a number
of years; (ii) the scope of contracts may change, either
increasing or decreasing the value of the contract; and
(iii) studies may be terminated or delayed at any time by
the study’s sponsor or by regulatory authorities due to
efficacy, safety or funding concerns, among other reasons.
Competition
The Company competes primarily against in-house research and
development departments of biopharmaceutical companies,
universities, teaching hospitals and other full-service CROs,
some of which possess substantially greater capital, technical
expertise and other resources than the Company. CROs generally
compete on the basis of past performance for a customer, medical
and scientific expertise in specific therapeutic areas, the
quality of services provided, the ability to manage large-scale
trials on a global basis, medical database management
capabilities, the ability to provide statistical and regulatory
services, the ability to recruit investigators, the ability to
recruit patients into studies, the ability to integrate
information technology with systems to improve the efficiency of
clinical research, an international presence with strategically
located facilities and financial viability and price.
The CRO industry is highly fragmented with hundreds of CROs
ranging from small, limited-service providers to full-service,
global drug development corporations. Some of the large
multi-national full-service CROs competing with the Company
include Covance, Inc., PAREXEL International Corporation,
Pharmaceutical Product Development, Inc., ICON plc, PRA
International, PharmaNet Development Group, Inc. and Quintiles
Transnational Corporation.
Government
Regulation
The Company’s clinical services are subject to industry
standards for the conduct of clinical research and development
studies that are contained in regulations for Good Clinical
Practice (GCP). The Food and Drug Administration (FDA) in the
United States and the European Agency for the Evaluation of
Medicinal Products in Europe (EMEA), along with other regulatory
bodies, require that clinical trial test results submitted to
the regulatory bodies be based on studies conducted in
accordance with GCP.
In addition, the International Conference on
Harmonization — Good Clinical Practice Guidelines
provides guidance on GCP. The Company implements and revises its
standard operating procedures to facilitate GCP compliance.
5
Employees
As of December 31, 2009, the Company employed approximately
3,640 associates, about 61% of whom were located outside the
United States. None of the Company’s employees are covered
by a collective bargaining agreement and the Company believes
its overall relations with its associates are good. Employees in
certain of the Company’s
non-U.S. locations
are represented by workers’ councils as required by local
laws.
Available
Information
The Company maintains a web site at the address
www.kendle.com. The Company is not including the
information contained on its web site as a part of, or
incorporating it by reference into, this Annual Report on
Form 10-K.
The Company makes available free of charge through its web site
its Annual Reports on
Form 10-K,
Quarterly Reports on
Form 10-Q
and Current Reports on
Form 8-K,
and amendments to these reports, as soon as reasonably
practicable after it electronically files such material with, or
furnishes such material to, the Securities and Exchange
Commission (SEC).
Required filings by the Company’s officers and directors
with respect to the Company furnished in electronic form are
also made available on its web site as is the Company’s
Proxy Statement for its annual meeting of shareholders. These
filings also may be read or copied at the SEC’s Public
Reference Room located in Washington, D.C. The SEC also
maintains an Internet site
(http://www.SEC.gov)
that contains reports, proxy statements and other information
regarding issuers that file electronically with the SEC.
Certain statements contained in this Annual Report on
Form 10-K
that are not historical facts constitute forward-looking
statements, within the meaning of the Private Securities
Litigation Reform Act of 1995, and are intended to be covered by
the safe harbors created by that Act. Reliance should not be
placed on forward-looking statements because they involve known
and unknown risks, uncertainties and other factors that may
cause actual results, performance or achievements to differ
materially from those expressed or implied. Forward-looking
statements generally contain the words “believe”,
“expect,” “may,” “anticipate,”
“intend,” “estimate,” “project,”
“plan,” “assume,” “seek to” or
other similar expressions, although not all forward-looking
statements contain these identifying words. Any forward-looking
statement speaks only as of the date made. The Company
undertakes no obligation to update any forward-looking
statements to reflect events or circumstances arising after the
date on which they are made.
Statements concerning, without limitation, expected financial
performance and results, plans and objectives, prospects,
on-going business strategies and possible future action which
the Company intends to pursue to achieve strategic objectives
constitute forward-looking information. Implementation of these
strategies and the achievement of such financial performance are
each subject to numerous conditions, uncertainties and risk
factors. The risk factors listed below are those deemed to be
material by the Company. Additional risks and uncertainties not
currently known may materially adversely affect the
Company’s business, financial condition
and/or
results of operations.
Risks
Related to Our Industry
The
Company depends on the biopharmaceutical industry for most of
its revenue.
The Company’s revenues depend on the outsourcing trends,
size of the drug-development pipeline and research and
development expenditures of the biopharmaceutical industry.
Economic factors and industry trends that affect companies in
the industry affect its business. A slowdown in research and
development spending or a reprioritization of the drug
development pipelines or limited access to capital to fund
projects in the biopharmaceutical industry could negatively
affect its net service revenues and results of operations.
Mergers and acquisitions in the biopharmaceutical industry and
the related rationalization of the drug-development pipelines
could result in delay or cancellation of certain existing
projects.
6
The
CRO industry is highly competitive.
The CRO industry is comprised of a wide range of competitors as
was previously mentioned, including small, niche providers as
well as large multi-national full-service global clinical
research organizations. These companies compete based on a
variety of factors, including reputation for quality,
performance, price, scope of service offerings and geographic
presence. Some of the Company’s competitors have greater
financial resources and a wider range of service offerings over
a greater geographic area. Additionally, the Company’s
customers have in-house capabilities to perform services that
are provided by CROs. These factors potentially could have a
negative impact on the Company’s ability to win business
awards.
Revenue
and earnings growth rates in the future may not be as robust as
in the past.
Current economic conditions including large pharmaceutical
company mergers, drug development pipeline reprioritization,
contraction of credit availability and cost containment efforts
by customers including reduction of research and development
spending, among other things, have had an impact on the CRO
industry and, in particular, the Company’s sales and
revenue growth rates. There can be no assurance that growth
rates will recover to the level experienced in the past.
Change
in government regulation or healthcare reform could adversely
affect the Company.
Government agencies regulate the drug development process
utilized by the Company in its work with biopharmaceutical
companies. Changes in regulations that simplify the drug
approval process or increases in regulatory requirements that
lessen the research and development efforts of the
Company’s customers could negatively affect the Company. In
addition, any failure on the Company’s part to comply with
existing regulations or in the adoption of new regulations could
impair the value of its services and result in the termination
of or additional costs under its contracts with customers.
Comprehensive healthcare reform could reduce the demand for
services which could reduce revenues. Legislation creating
downward pressure on the prices for drugs that pharmaceutical
and biotechnology companies can charge or the removal of these
drugs from reimbursement formularies could reduce the amount of
revenue the Company could earn from projects outsourced to it.
Healthcare reform outside the U.S. could also adversely
impact the Company’s revenues and profitability. Recent
activity contemplated by the U.S. federal government
related to health care reform and the funding for it raises
significant uncertainties for all businesses and could have a
material effect on the Company and its customers.
Changes
in tax legislation could adversely affect the
Company.
The current administration announced several proposals to reform
U.S. tax laws, including a proposal to limit foreign tax
credits and a proposal to defer tax deductions allocable to
non-U.S. earnings
until earnings are repatriated. It is unclear whether these
proposed tax reforms will be enacted or, if enacted, what the
scope of the reforms will be. Depending on the final content,
such reforms, if enacted, could have a material adverse effect
on our financial results.
Risks
Related to Our Business
The
current economic climate has caused the Company to rapidly
adjust from a robust growth strategy to a cost containment
strategy in a short period of time, which has placed, and is
expected to continue to place, significant demands on
it.
The Company has had to adapt to a rapidly changing economic and
industry environment. Historically, the Company had grown
rapidly, both organically and through acquisitions. In 2009, the
Company experienced lower new business authorizations than in
its recent past, combined with higher than historical levels of
cancellations. This placed significant stress upon the
organization to adjust from building toward expected future
growth to taking rapid actions to preserve profitability. As a
result, the Company embarked on reduction in force and cost
containment initiatives which could have an unfavorable impact
on the future operations should the environment quickly recover.
Additionally, certain geographic regions could grow at faster
rates than other geographic regions, resulting in the
7
Company hiring associates in those regions (i.e. Latin America
and Asia/Pacific) at a more accelerated pace than in other
regions.
The
Company’s contracts may be delayed, terminated or reduced
in scope with little or no notice.
Many of the Company’s contracts provide for services on a
fixed-price basis and may be terminated or reduced in scope with
little or no notice. Cancellations may occur for a variety of
reasons, including the failure of the product to satisfy safety
requirements, the customer’s inability to manufacture
sufficient quantities of the drug, unexpected results of the
product or the customer’s decision to terminate the
development of a product.
The loss, reduction in scope or delay of a large contract or the
delay of multiple contracts could have a material adverse effect
on the Company’s results of operations; although its
contracts entitle it to receive payments for work performed in
the event of a cancellation. Cancellation or delay of a large
contract or multiple contracts could leave the Company with
under-utilized resources and thereby negatively affect its net
service revenues and results of operations. The Company believes
its aggregate backlog is not necessarily a meaningful indicator
of future net service revenues and financial results.
The
fixed price nature of many of the Company’s contracts could
result in financial losses.
Because many of the Company’s contracts are structured as
fixed price, it is at financial risk if it initially underbids
the contract or overruns the initial cost estimates. Such
under-bidding or significant cost overruns could have a material
adverse effect on the Company’s business, results of
operations, financial condition and cash flows.
The
Company’s backlog may not be indicative of future
results.
The Company includes in backlog anticipated revenues from
projects currently in process plus written awards, signed
contracts, letters of intent and other awards believed to be
firm commitments. These contracts vary in size and duration and
may be subject to changes in scope, delays or cancellations. In
addition, the portion of backlog that may be performed in
non-U.S. subsidiaries
is exposed to fluctuations in the applicable foreign currencies
which could affect the amount of revenue ultimately recognized.
Fluctuations in foreign exchange rates caused backlog to
increase by approximately $8 million at December 31,
2009.
If the
Company is unable to attract suitable investigators and
volunteers for clinical trials, the Company’s business may
suffer.
The clinical research studies the Company operates rely upon the
accessibility and willing participation of physician
investigators and volunteer subjects. Investigators supervise
the administration of study drugs to patients during the course
of a clinical trial. Volunteer subjects generally include people
from the communities in which the studies are conducted. The
Company’s clinical research business could be adversely
affected if we are unable to attract suitable investigators or
clinical study volunteers on a consistent basis.
If the
Company is required to write off goodwill or other intangible
assets acquired in its business combinations, its financial
position and results of operations would be adversely
affected.
The Company had goodwill and other acquisition-related
intangible assets of approximately $258.8 million and
$255.4 million as of December 31, 2009 and
December 31, 2008, respectively, which constituted
approximately 48% and 46%, respectively, of its total assets at
these periods. The Company periodically (at least annually
unless triggering events occur that cause an interim
evaluation), evaluates goodwill and other acquired intangible
assets for impairment. Any future determination requiring the
write off of a portion of the Company’s goodwill or other
acquired intangible assets could adversely affect its results of
operations and financial condition. Should current economic
conditions continue, the annual or an interim evaluation (if
required as indicated above) could result in an impairment of
the Company’s goodwill, particularly Early Stage segment
goodwill. See Note 6 to the Consolidated Financial
Statements for further detail on goodwill or other intangible
assets.
8
The
Company’s indebtedness could adversely affect its business
and financial condition.
As of December 31, 2009, the Company had
$154.5 million (in par value) in convertible debt
outstanding and an additional $53.5 million of borrowing
capacity under a revolving line of credit as well as
approximately $86,000 of obligations outstanding under capital
leases. The Company also maintains a $5.0 million
multicurrency facility that is renewable annually and used in
connection with its European operations. In March 2010, the
Company terminated this line of credit and replaced it with a
revolving line of credit which provides approximately
$35 million in borrowing capacity. For a description of the
Company’s indebtedness and that of its subsidiaries, see
Liquidity and Capital Resources section of Management’s
Discussion and Analysis of Financial Condition and Results of
Operations.
The Company’s level of indebtedness will have several
important effects on its future operations. For example, the
Company will be required to use a portion of its cash flow from
operations for the payment of principal and interest due on its
outstanding indebtedness. In addition, the Company’s
outstanding indebtedness and leverage could increase the impact
of negative changes in general economic and industry conditions,
as well as competitive pressures. Finally, the level of the
Company’s outstanding indebtedness may affect its ability
to obtain additional financing for working capital, capital
expenditures or general corporate purposes.
General economic conditions as well as conditions affecting the
Company’s operations specifically, including, but not
limited to, financial and business conditions, many of which are
beyond its control, may affect its future performance. As a
result, these and other factors may affect the Company’s
ability to make principal and interest payments on its
indebtedness. The Company’s business might not continue to
generate cash flow at or above current levels, including levels
required to service its indebtedness. Moreover, if the Company
is required to repatriate foreign earnings to fund its debt
service, it may not be able to accomplish this in a “tax
efficient” manner and may, therefore, incur additional
income taxes. If the Company cannot generate sufficient cash
flow from operations in the future to service its indebtedness,
it may, among other things:
|
|
|
| |
•
|
Seek additional financing in the debt or equity markets;
|
| |
| |
•
|
Seek to refinance or restructure all or a portion of its
indebtedness;
|
| |
| |
•
|
Sell selected assets;
|
| |
| |
•
|
Reduce or delay planned capital expenditures
|
These measures might not be sufficient to enable the Company to
service its indebtedness. In addition, any financing,
refinancing or sale of assets might not be available on
economically favorable terms, if at all.
Furthermore, the Company’s credit facility contains certain
restrictive covenants which may affect, and in many respects
significantly limit, management’s choices in responding to
business, economic, regulatory and other competitive conditions.
The
Company’s access to funds under the Facility or its money
market fund holdings is dependent on the solvency and liquidity
of the participating lenders or fund sponsors.
The Company draws on its money market fund holdings or uses its
revolving credit facility to provide liquidity to fund its
operating needs. The balances fluctuate depending on the
Company’s needs and cash flows. If a party to the Facility
or the sponsors of the money market funds becomes insolvent or
unable to honor its commitments, the Company could have
insufficient cash to meet its obligations and may be required to
seek alternative forms of capital at rates and with terms and
conditions not as favorable as those under its current Facility.
The
Company may be exposed to risk from its various
counterparties.
The current global economy has shown signs of weakening and
continues to show signs of fragility; its impact may be far
reaching. As a result, the Company may be exposed to risks
related to defaults from its suppliers and customers, as well
as, from the counterparties to its purchased call options and
sold warrants that are beyond the Company’s control. Key
suppliers could fail to deliver agreed upon goods or services.
Customers may not be able to obtain financing for their clinical
trials with the Company, which may result in the delay or
cancellation of these trials. Additionally, customers may not be
able to pay or may pay receivables more slowly than in the past
resulting in bad debt expenses or poor cash
9
flows. The purchasers of the call options and sold warrants
could become insolvent resulting in an inability to honor their
commitments or in unanticipated dilution should the target
conversion price terms be met.
The
Company’s international operations are subject to numerous
risks.
The Company has international operations in many foreign
countries, including, but not limited to, South Africa,
India and countries in Eastern Europe, the Asia Pacific region
and Latin America. These operations are subject to risks and
uncertainties inherent in operating in these countries,
including government regulations, potential highly inflationary
economies, currency restrictions and other restraints,
burdensome taxes, government takeovers of assets and political
instability. These risks and uncertainties could negatively
impact the Company’s ability to, among other things,
perform large, global projects for its customers or repatriate
cash. Furthermore, the Company’s ability to deal with these
issues could be affected by applicable U.S. laws and the
need to protect its assets in those locations.
The
Company’s financial results are exposed to exchange rate
fluctuations.
The Company’s Consolidated Financial Statements are
denominated in U.S. dollars. For the year ended
December 31, 2009, approximately 57% of the Company’s
net service revenues were derived from operations outside the
United States compared to 55% in the same period of 2008. The
Company strives for contractual protection to limit the foreign
currency fluctuation risk exposure in contracts with its
customers where possible, but that protection is not always
achievable or adequate to insulate the Company from significant
fluctuations. Additionally, due to the uncertainties regarding
the timing of and currencies involved, it is impracticable to
implement hedging instruments to match the Company’s
foreign currency inflows and outflows. As a result, changes in
foreign currency exchange rates could significantly affect the
Company’s results of operations, financial position and
cash flows as well as its ability to finance large acquisitions
outside the United States.
The
Company’s quarterly operating results may
vary.
The Company’s operating results may vary significantly from
quarter to quarter and are influenced by a variety of factors,
such as:
|
|
|
| |
•
|
Exchange rate fluctuations;
|
| |
| |
•
|
Timing of contract amendments for changes in scope that could
affect the value of a contract and potentially impact the amount
of net service revenues from quarter to quarter;
|
| |
| |
•
|
Commencement, completion, execution or cancellation of large
contracts;
|
| |
| |
•
|
Collections of accounts receivable;
|
| |
| |
•
|
Progress of ongoing contracts and retention of customers;
|
| |
| |
•
|
Timing of and charges associated with completed acquisitions or
other events; and
|
| |
| |
•
|
Changes in the mix, both in terms of geography and type of
services.
|
The Company believes that operating results for any particular
quarter are not necessarily a meaningful indication of future
results. Although fluctuations in quarterly operating results
could negatively or positively affect the market price of the
Company’s common stock. These fluctuations may not be
indicative of future overall operating performance.
The
Company’s business depends on the continued effectiveness
and availability of its information technology infrastructure,
and failures of this infrastructure could limit its
operations.
To remain competitive in the Company’s industry, it must
employ information technologies that capture, manage, and
analyze the large streams of data generated during the clinical
trials we manage in compliance with applicable regulatory
requirements. In addition, because the Company provides services
on a global basis, it relies extensively on its technology to
allow the concurrent conduct of studies and work sharing around
the world. As with all information technology, the
Company’s systems could become vulnerable to potential
damage or interruptions from fires, blackouts,
telecommunications failures and other unexpected events, as well
as to break-ins, sabotage or intentional acts of
10
vandalism. Given the extensive reliance of the Company’s
business on this technology and the substantial investment in
new technology infrastructure, any substantial disruption or
resulting loss of data that is not avoided or corrected by its
backup measures could harm its business and operations. Although
the Company carries property and business interruption
insurance, the coverage may not be adequate to compensate for
all losses that may occur.
The
Company’s business depends on the successful implementation
and deployment of its enterprise wide reporting solution and
failure could harm the Company’s ability to manage its
business and obtain accurate financial reporting.
The Company has made a significant investment in an enterprise
resource planning solution which is scheduled to begin
deployment in 2010. This major initiative will integrate
pricing, sales, project accounting, time reporting, trial
management, human resources, billing and general ledger programs
to provide management and financial reporting. The delay in
implementation or failure of this project could result in, among
other things, an inability to manage our business, bill and
collect accounts receivable or accurately report our financial
results. This new technology will also rely on third parties for
processing and storing of data, which exposes the Company to
additional risks.
The
Company’s business could expose it to potential liability
for personal injury claims that could affect its financial
condition.
The Company’s business involves clinical trial management
which includes the testing of new drugs on human volunteers.
This business exposes the Company to the risk of liability for
personal injury or death to patients resulting from, among other
things, possible unforeseen adverse side effects or improper
administration of a drug or device. Many of these volunteers and
patients are already seriously ill and are at risk of further
illness or death. Any claim or liability could have a material
adverse effect on the Company’s financial position and its
reputation if, as a result, it were required to pay damages or
incur defense costs in connection with a claim and if:
(i) such claim is outside the scope of indemnification
agreements the Company has with customers and collaborative
partners, (ii) an indemnification agreement is not
performed in accordance with its terms or (iii) its
liability exceeds the amount of any applicable indemnification
limits or available insurance coverage. The Company might also
not be able to purchase adequate insurance for these risks at
reasonable rates in the future.
The
nature of the Company’s business exposes it to litigation
and regulatory risk.
The nature of the Company’s business exposes it to
litigation risk, and it is a party to lawsuits in the ordinary
course of its business. While the Company does not believe that
the resolution of any currently pending lawsuits against it
will, individually or in the aggregate, have a material adverse
effect on its business, financial condition or results of
operations, it is possible that one or more lawsuits to which it
is currently a party to or to which it subsequently becomes a
party to, could adversely affect it in the future. In addition,
failure to comply with applicable regulatory requirements can
result in actions that could adversely affect the Company’s
business and financial performance.
If the
Company fails to hire, retain and integrate qualified personnel,
it will be difficult for it to achieve its financial and
operational goals.
The Company’s success depends to a significant extent upon
the skills, experience and efforts of its senior management team
and its ability to hire qualified personnel in the geographic
regions and therapeutic areas in which it operates. The loss of
any of the Company’s executive officers or other key
employees, without a properly executed transition plan, could
have an adverse effect on it. In addition, there has been
substantial competition among both CROs and biopharmaceutical
companies for qualified personnel. Difficulty recruiting or
retaining qualified personnel
and/or
unexpected recruiting costs could affect the Company’s
ability to meet financial and operational goals.
11
New
standards or changes in existing accounting standards issued by
the Financial Accounting Standards Board (FASB), SEC or other
standard setting bodies may adversely affect the Company’s
financial statements and could entail significant expenditures.
The application of these standards often requires the use of
estimates and assumptions that may materially differ from actual
results.
The Company’s consolidated financial statements are
currently subject to the application of U.S. Generally
Accepted Accounting Principles (GAAP), which is periodically
revised
and/or
expanded. Accordingly, the Company is required to adopt new or
revised accounting standards issued by recognized authoritative
bodies, including the FASB. It is possible that future changes
in standards may change the current accounting treatment and
that such changes could have a material adverse effect on the
Company’s operating results and financial condition. (See
Note 1, Critical Accounting Policies, New Accounting
Pronouncements for known changes in standards.)
The preparation of the Company’s consolidated financial
statements in compliance with GAAP often requires management to
make estimates and assumptions based on available information at
that time. These estimates and assumptions may ultimately differ
from actual results and the impact could have a material adverse
effect on the Company’s financial position and results of
operations.
Risks
related to our Common Stock
Market
conditions have caused significant volatility in the
Company’s stock price.
The market price of the Company’s common stock has
historically experienced and is expected to continue to
experience some volatility because:
|
|
|
| |
•
|
The Company’s number of shares outstanding is significantly
less than that of its peers, causing relatively small
adjustments to have a substantial impact on earnings per share
and relatively small market activity to cause substantial
volatility.
|
| |
| |
•
|
General conditions in the economy and financial markets and
other developments affecting the Company or its competitors have
caused the market value of the Company’s common stock to
decline.
|
This volatility and valuation decline has affected securities
issued by many companies in many industries, in addition to the
Company’s common stock, often for reasons unrelated to
their operating performance. If the current valuation
declination continues, the Company’s total market
capitalization may be at a level which could result in an
impairment of the Company’s goodwill, particularly the
goodwill assigned to the Early Stage.
The
Company’s convertible note hedge and warrant transactions
may affect the trading price of its common stock.
In connection with the issuance of the Company’s
Convertible Notes (see Note 8, Debt), the Company entered
into convertible note hedge transactions with the participating
Underwriter and JP Morgan Chase (collectively, the
counterparties). The convertible note hedge transactions are
comprised of purchased call options and sold warrants. The
purchased call options are expected to reduce exposure to
potential dilution upon the conversion of the Convertible Notes.
The Company also entered into warrant transactions with such
counterparties. The sold warrants have an exercise price that is
approximately 70% higher than the closing price of the
Company’s common stock on the date the Convertible Notes
were priced. The warrants are expected to provide the Company
with some protection against increases in our stock price over
the conversion price per share. In connection with these
transactions, the counterparties, or their affiliates:
|
|
|
| |
•
|
May enter into various
over-the-counter
derivative transactions or purchase or sell the Company’s
common stock in secondary market transactions; and
|
| |
| |
•
|
May enter into, or may unwind, various
over-the-counter
derivatives or purchase or sell the Company’s common stock
in secondary market transactions, including during any
conversion reference period with respect to a conversion of the
Convertible Notes.
|
These activities may have the effect of increasing, or
preventing a decline in, the market price of the Company’s
common stock. In addition, any hedging transactions by the
counterparties, or their affiliates, including during any
conversion reference period, may have an adverse impact on the
trading price of the Company’s common
12
stock. The counterparties, or their affiliates, are likely to
modify their hedge positions from time to time prior to
conversion or maturity of the Convertible Notes by purchasing
and selling shares of the Company’s common stock or other
instruments, including
over-the-counter
derivative instruments, that they may wish to use in connection
with such hedging. In addition, the Company intends to exercise
its purchased call options whenever the Convertible Notes are
converted, although not required to do so. In order to unwind
any hedge positions with respect to the potential exercise of
the purchased call options, the counterparties or their
affiliates would expect to sell shares of common stock in
secondary market transactions or unwind various
over-the-counter
derivative transactions with respect to the Company’s
common stock during the conversion reference period for any
Convertible Notes that may be converted.
The effect, if any, of any of these transactions and activities
in connection with the Convertible Notes on the market price of
the Company’s common stock will depend in part on market
conditions and cannot be ascertained at this time, but any of
these activities could adversely affect the trading price of the
Company’s common stock and, as a result, the number of
shares and value of the common stock received upon conversion of
the Convertible Notes.
Anti-takeover
provisions in the Company’s charter documents and under
Ohio law may make an acquisition of the Company, which may be
beneficial to its stockholders, more difficult, which could
depress its stock price.
|
|
|
| |
•
|
Certain provisions of the Company’s Articles of
Incorporation and Code of Regulations and of Ohio law make it
difficult for a third party to acquire control of it without the
consent of its Board of Directors (Board). These anti-takeover
defenses may discourage, delay or prevent a transaction
involving a change in control of the Company, and, accordingly,
could limit the price that investors may be willing to pay for
its common stock, including transactions in which holders of
common stock might receive a premium for their shares over the
market price. In cases where Board approval is not obtained,
these provisions could also discourage proxy contests and make
it more difficult for existing shareholders to elect directors
of their choosing and cause the Company to take other corporate
actions they desire. These provisions include: The authorization
of undesignated preferred stock, the terms, rights, privileges
and restrictions of which may be established and shares of which
may be issued without shareholder approval;
|
| |
| |
•
|
Limitations on persons authorized to call a special meeting of
shareholders; and
|
| |
| |
•
|
Advance notice procedures required for shareholders to nominate
candidates for election as directors or to bring matters before
an annual meeting of shareholders.
|
In addition, the Company has adopted a shareholder rights plan
that may have anti-takeover effects, which will make its
acquisition by another company more difficult. The
Company’s shareholder rights plan provides that, in the
event any person or entity acquires 15% or more of its
outstanding common stock, its shareholders will be entitled to
purchase shares of common stock, or in certain instances, shares
of the acquirer, at a discounted price. The rights are intended
to discourage a significant share acquisition, merger or tender
offer involving the Company’s common stock by increasing
the cost of affecting any such transaction and, accordingly,
could have an adverse impact on a takeover attempt that a
shareholder might consider to be in the Company’s best
interests.
|
|
|
ITEM 1B.
|
UNRESOLVED
STAFF COMMENTS
|
None.
The Company leases all of its facilities with the exception of
the Company-owned facility in Ely, United Kingdom. The
Company’s principal executive offices are located in
Cincinnati, Ohio. Early in 2008, the Company entered into a
lease extension for these offices which extended the term of the
lease from 2009 to 2019 and increased the amount of space leased
from approximately 122,000 square feet to approximately
143,000 square feet. The lease extension also provides the
Company with an opportunity to lease additional space in the
future.
In addition, the Company leases substantial facilities in
Durham, North Carolina; Toronto, Canada; Munich, Germany;
Camberley, United Kingdom; Edinburgh, United Kingdom; Utrecht,
The Netherlands; and Mexico City,
13
Mexico. The Company’s Early Stage operations are located in
Morgantown, West Virginia, Toronto, Canada and Utrecht, The
Netherlands. The Company also maintains offices in various other
North American, European and Asia-Pacific, including Australian
and Indian locations, as well as in Latin America and South
Africa.
Management believes that such offices are sufficient to meet its
current needs and does not anticipate any difficulty in securing
additional space, as needed, on terms acceptable to the Company.
|
|
|
ITEM 3.
|
LEGAL
PROCEEDINGS
|
The Company is party to lawsuits and administrative proceedings
incidental to the normal course of business. The Company
currently is not a party to any pending material litigation,
nor, to the Company’s knowledge, is any material litigation
currently threatened against the Company.
|
|
|
ITEM 4.
|
REMOVED
AND RESERVED
|
PART II
|
|
|
ITEM 5.
|
MARKET
FOR REGISTRANT’S COMMON EQUITY, RELATED SHAREHOLDER MATTERS
AND ISSUER PURCHASES OF EQUITY SECURITIES
|
Shares of the Company’s Common Stock are listed on The
NASDAQ Global Select Market
LLC®
and are traded under the symbol “KNDL.” The following
table sets forth the high and low prices for shares of the
Company’s Common Stock for the periods indicated.
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2009 Quarterly
|
|
First
|
|
|
Second
|
|
|
Third
|
|
|
Fourth
|
|
|
|
|
Ranges of stock price
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
High
|
|
$
|
25.66
|
|
|
$
|
23.13
|
|
|
$
|
17.33
|
|
|
$
|
18.88
|
|
|
Low
|
|
|
15.05
|
|
|
|
8.28
|
|
|
|
9.57
|
|
|
|
14.71
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2008 Quarterly
|
|
First
|
|
|
Second
|
|
|
Third
|
|
|
Fourth
|
|
|
|
|
Ranges of stock price
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
High
|
|
$
|
51.60
|
|
|
$
|
46.71
|
|
|
$
|
52.00
|
|
|
$
|
45.90
|
|
|
Low
|
|
|
39.07
|
|
|
|
35.35
|
|
|
|
35.42
|
|
|
|
15.86
|
|
The number of holders of record of Kendle International Inc.
common stock was 145 as of March 3, 2010. This total
excludes shares held under beneficial ownership in nominee name
or within clearinghouse positions of brokerage firms or banks.
The Company has not paid dividends on its Common Stock since its
initial public offering in August 1997. The Company does not
currently intend to pay dividends in the foreseeable future and,
in any event, is restricted from paying dividends under the
terms and conditions of its credit facility.
14
Performance
Graph
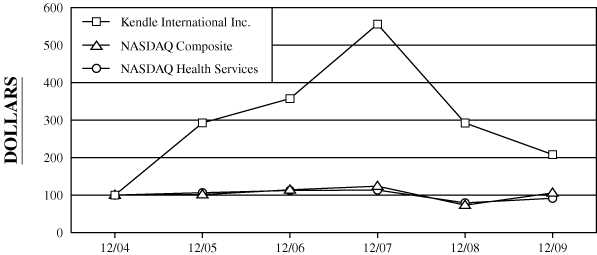
The following graph compares the five-year cumulative total
shareholder returns of the Company’s Common Stock with the
NASDAQ Composite Index and the NASDAQ Health Services Index.
COMPARISON
OF 5 YEAR CUMULATIVE TOTAL RETURN*
Among
Kendle International Inc., The NASDAQ Composite Index
And The NASDAQ Health Services Index
|
|
|
|
* |
|
$100 invested on
12/31/04 in
stock or index, including reinvestment of dividends. |
Fiscal year ending December 31.
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Dollar Value of $100 Investment at December 31,
|
|
|
|
2004
|
|
2005
|
|
2006
|
|
2007
|
|
2008
|
|
2009
|
|
Kendle International Inc.
|
|
|
100.00
|
|
|
|
292.50
|
|
|
|
357.39
|
|
|
|
555.91
|
|
|
|
292.27
|
|
|
|
208.07
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
NASDAQ Composite
|
|
|
100.00
|
|
|
|
101.33
|
|
|
|
114.01
|
|
|
|
123.71
|
|
|
|
73.11
|
|
|
|
105.61
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
NASDAQ Health Services
|
|
|
100.00
|
|
|
|
106.30
|
|
|
|
112.25
|
|
|
|
113.33
|
|
|
|
79.24
|
|
|
|
91.44
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Securities
Authorized Under Equity Compensation Plans:
The information required for Securities Authorized Under Equity
Compensation Plans can be found in Part III, Item 12,
Security Ownership of Certain Beneficial Owners and Management
of this Annual Report on
Form 10-K.
15
|
|
|
ITEM 6.
|
SELECTED
FINANCIAL DATA
|
CONSOLIDATED
STATEMENTS OF OPERATIONS
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
For the Years Ended December 31,
|
|
|
|
|
2009
|
|
|
2008(3)
|
|
|
2007(1)(3)
|
|
|
2006(2)
|
|
|
2005
|
|
|
|
|
(In thousands except per share data)
|
|
|
|
|
Net service revenues
|
|
$
|
416,688
|
|
|
$
|
475,092
|
|
|
$
|
397,584
|
|
|
$
|
283,471
|
|
|
$
|
202,032
|
|
|
Reimbursable
out-of-pocket
revenues
|
|
|
135,224
|
|
|
|
203,489
|
|
|
|
171,234
|
|
|
|
90,465
|
|
|
|
48,607
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total revenues
|
|
|
551,912
|
|
|
|
678,581
|
|
|
|
568,818
|
|
|
|
373,936
|
|
|
|
250,639
|
|
|
Cost and expenses:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Direct costs
|
|
|
210,586
|
|
|
|
247,436
|
|
|
|
204,161
|
|
|
|
152,826
|
|
|
|
108,582
|
|
|
Reimbursable
out-of-pocket
costs
|
|
|
135,224
|
|
|
|
203,489
|
|
|
|
171,234
|
|
|
|
90,465
|
|
|
|
48,607
|
|
|
Selling, general and administrative
|
|
|
144,659
|
|
|
|
155,577
|
|
|
|
125,744
|
|
|
|
91,796
|
|
|
|
68,216
|
|
|
Depreciation and amortization
|
|
|
15,712
|
|
|
|
15,253
|
|
|
|
14,865
|
|
|
|
10,403
|
|
|
|
7,991
|
|
|
Restructuring expenses
|
|
|
10,157
|
|
|
|
—
|
|
|
|
—
|
|
|
|
236
|
|
|
|
—
|
|
|
Intangible impairment charge
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
8,200
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total costs and expenses
|
|
|
516,338
|
|
|
|
621,755
|
|
|
|
516,004
|
|
|
|
353,926
|
|
|
|
233,396
|
|
|
Income from operations
|
|
|
35,574
|
|
|
|
56,826
|
|
|
|
52,814
|
|
|
|
20,010
|
|
|
|
17,243
|
|
|
Other income (expense):
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Interest income
|
|
|
579
|
|
|
|
760
|
|
|
|
1,466
|
|
|
|
1,939
|
|
|
|
1,019
|
|
|
Interest expense
|
|
|
(14,403
|
)
|
|
|
(15,891
|
)
|
|
|
(17,547
|
)
|
|
|
(6,781
|
)
|
|
|
(460
|
)
|
|
Write-off of deferred financing costs
|
|
|
—
|
|
|
|
—
|
|
|
|
(4,152
|
)
|
|
|
—
|
|
|
|
—
|
|
|
Other
|
|
|
4,034
|
|
|
|
(2,043
|
)
|
|
|
(4,816
|
)
|
|
|
(1,795
|
)
|
|
|
(287
|
)
|
|
Gain on debt extinguishment
|
|
|
2,887
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
300
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total other income (expenses)
|
|
|
(6,903
|
)
|
|
|
(17,174
|
)
|
|
|
(25,049
|
)
|
|
|
(6,637
|
)
|
|
|
572
|
|
|
Income before income taxes
|
|
|
28,671
|
|
|
|
39,652
|
|
|
|
27,765
|
|
|
|
13,373
|
|
|
|
17,815
|
|
|
Income taxes
|
|
|
13,434
|
|
|
|
16,509
|
|
|
|
11,755
|
|
|
|
4,843
|
|
|
|
7,141
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income
|
|
$
|
15,237
|
|
|
$
|
23,143
|
|
|
$
|
16,010
|
|
|
$
|
8,530
|
|
|
$
|
10,674
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
INCOME PER SHARE DATA
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income per share
|
|
$
|
1.03
|
|
|
$
|
1.57
|
|
|
$
|
1.10
|
|
|
$
|
0.60
|
|
|
$
|
0.79
|
|
|
Weighted average shares
|
|
|
14,862
|
|
|
|
14,751
|
|
|
|
14,520
|
|
|
|
14,323
|
|
|
|
13,572
|
|
|
Diluted:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income per share
|
|
$
|
1.02
|
|
|
$
|
1.54
|
|
|
$
|
1.08
|
|
|
$
|
0.58
|
|
|
$
|
0.76
|
|
|
Weighted average shares
|
|
|
14,992
|
|
|
|
14,993
|
|
|
|
14,889
|
|
|
|
14,762
|
|
|
|
14,120
|
|
CONSOLIDATED
BALANCE SHEET DATA(4)
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
As of December 31,
|
|
|
|
|
2009
|
|
|
2008(3)
|
|
|
2007(3)
|
|
|
2006
|
|
|
2005
|
|
|
|
|
Working capital
|
|
$
|
56,476
|
|
|
$
|
68,595
|
|
|
$
|
60,084
|
|
|
$
|
56,404
|
|
|
$
|
63,992
|
|
|
Total assets
|
|
|
539,723
|
|
|
|
554,888
|
|
|
|
498,675
|
|
|
|
455,072
|
|
|
|
184,759
|
|
|
Total short and long-term debt, including capital leases
|
|
|
138,394
|
|
|
|
172,159
|
|
|
|
165,673
|
|
|
|
200,099
|
|
|
|
4,572
|
|
|
Total shareholders’ equity
|
|
|
236,047
|
|
|
|
212,624
|
|
|
|
175,257
|
|
|
|
140,112
|
|
|
|
122,504
|
|
|
|
|
|
(1) |
|
Includes the effects of the January 1, 2007 adoption of
guidance for accounting for uncertainty in income taxes. |
| |
|
(2) |
|
Includes the effects of the January 1, 2006 adoption of
accounting guidance related to share-based payments. |
| |
|
(3) |
|
As adjusted due to the implementation of accounting guidance
related to convertible debt. See Note 2: Accounting Changes |
| |
|
(4) |
|
From 2005 to 2008, the Company made three acquisitions. See the
Acquisitions section of Managements’ Discussion and
Analysis of Financial Condition and Results of Operations. |
16
|
|
|
ITEM 7.
|
MANAGEMENT’S
DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF
OPERATIONS
|
Refer
to Risk Factors previously discussed in Part 1 Item 1A
and the Cautionary Statement for Forward-Looking Information
later in this
section.
Management’s
Discussion and Analysis of Financial Condition and Results of
Operations
The information set forth and discussed below in
Management’s Discussion and Analysis of Financial Condition
and Results of Operations (MD&A) is derived from the
Company’s Consolidated Financial Statements and the related
notes thereto, which are included herein, and should be read in
conjunction therewith.
Company
Overview
Kendle International Inc. (the Company or Kendle) is a global
clinical research organization (CRO) that delivers integrated
clinical development services, including clinical trial
management, clinical data management, statistical analysis,
medical writing, regulatory consulting and organizational
meeting management and publications services, among other
things, on a contract basis to the biopharmaceutical industry.
The Company operates in North America, Europe, Asia/Pacific,
Latin America and Africa. The Company operates its business in
two reportable operating segments, Early Stage and Late Stage.
The Early Stage business currently focuses on the Company’s
Phase I operations while Late Stage is comprised of clinical
development services related to Phase II through III
clinical trials conducted worldwide, late phase clinical
development services related to Phase IIIB and IV clinical
trials conducted worldwide, regulatory affairs and biometrics
offerings. The Company aggregates its clinical and data
monitoring reporting unit, project management and late phase
reporting unit, regulatory, site and medical affairs reporting
unit, and biostatistics and statistical programming reporting
unit into the Late Stage segment under the aggregation criteria
in accounting guidance related to segments. The aggregation
criteria met includes a similar nature of services provided, a
similar type of customer, similar methods used to distribute
services, similar economic characteristics and a similar
regulatory environment. In addition, the Company reports support
functions primarily composed of Human Resources, Information
Technology, Sales and Marketing and Finance under the Support
and Other category for purposes of segment reporting. A portion
of the costs incurred from the support units are allocated to
the Early and Late Stage reportable operating segments.
The Company’s revenue recognition process is described
later in this MD&A under “Critical Accounting Policies
and Estimates.”
Late
Stage Segment Contracts
The Company provides services to its customers primarily under
“full-service” contracts that include a broad range of
services in support of all aspects of a customer’s clinical
trial. These services typically include biostatistics, clinical
development services for Phase II through IV clinical
trials and regulatory affairs. The Company from time to time
provides a select number of these services under more narrow
contracts focusing on one or more specialty areas, referred to
as Functional Service Awards. The Company usually competes for
business awards in a competitive bidding process. In the bidding
process, the Company submits a bid that includes a price based
upon hourly billing rates for billable employees multiplied by
task hours the Company estimates will be necessary to achieve
the service assumptions. Upon receiving a business award, the
Company and its customer negotiate a contract to memorialize
these assumptions and the related price.
Service contracts usually are long-term arrangements that
require Company performance over several years. A contract
usually requires a portion of the contract fee to be paid at the
time of contract execution, and the balance is received in
specified installments or milestones over the contract’s
duration. Other methods for receiving payment include units
achieved and time and materials. During performance of the
services, any of the following events may occur and impact the
contract price:
|
|
|
| |
•
|
The customer may request a change in the assumptions;
|
| |
| |
•
|
The customer may increase or decrease the scope of services,
which requires a change to the service assumptions; and
|
17
|
|
|
| |
•
|
The Company may discover that, for a particular contract, the
assumptions are incorrect or insufficient to permit completion
of the contract.
|
In each of the foregoing situations, the Company enters into
negotiations for a contract amendment to reflect the change in
scope or assumptions and the related price. Depending on the
complexity of the amendment, the amendment process can take from
a few weeks for a simple adjustment, such as a timeline
extension, to several months for a complex amendment, such as a
change in patient enrollment strategy. Under the Company’s
policy, project teams are not authorized to engage in tasks
outside the scope of the contract without prior management
approval. In limited situations, management may authorize the
project team to commence work on activities outside the contract
scope while the Company and its customer negotiate and finalize
the contract amendment.
Contract amendments are commonplace within the industry and
occur on the majority of the Company’s contracts. At any
point in time, the Company will be in the process of discussing
numerous proposed amendments, the scope and value of which can
change significantly between time of proposal and final
agreement. The total value of these amendments primarily
represents future work and revenues.
In addition to full-service and functional service arrangements
described above, the Company provides consulting services to its
customers under contracts that generally are shorter-term in
nature than full-service contracts. Net service revenues from
these contracts represent less than 5% of the Company’s
consolidated net service revenues.
In connection with providing services, the Company incurs
pass-through costs, which include travel-related expenses for
Company employees performing services and fees payable to
third-party investigators or labs participating in, or
supporting, the customer’s clinical trial. The customer
agrees to reimburse the Company on a
dollar-for-dollar
basis for the costs incurred by the Company in accordance with
contractually specified parameters. The revenues and costs from
these pass-through and third-party costs are reflected in the
Company’s Consolidated Statements of Operations under the
line items titled “Reimbursable
out-of-pocket
revenues” and “Reimbursable
out-of-pocket
costs”, respectively.
The customer may terminate the contract at any time with little
or no advance notice to the Company. Customers, in particular,
may terminate a contract immediately for concerns related to the
efficacy or safety of a particular drug. Upon termination, the
customer is required to pay the Company for the value of work
completed up to termination as well as reimburse the Company for
its
out-of-pocket
costs incurred in accordance with the contract.
Although the majority of the Company’s contracts are
fixed-price and net service revenues are calculated on a
proportional performance or percentage of completion
methodology, the Company has seen increasing demand from its
customers to move toward a units-based contract methodology in
new contracts. It is the Company’s intent to structure more
of its contracts under a units-based methodology for calculating
net service revenues so the Company expects the percentage of
contracts under which net service revenues are recognized using
units-based methodology to increase in future periods. Under a
units-based contract methodology, amounts recognized as net
service revenues are calculated based on units completed in the
period multiplied by a unit value or selling price that is
outlined in the contract.
A contract amendment, which results in revisions to net service
revenues and cost estimates, is recognized in revenue
calculations beginning in the period in which the parties reach
written agreement to the amendment.
Early
Stage Segment Contracts
Early Stage segment business awards are also subject to a
competitive bidding process and, upon award, are memorialized in
a contract that includes terms and conditions that are
substantially similar to the Company’s contracts with its
Late Stage segment customers. Most of the revenue for the Early
Stage segment is recognized under units-based contracts by
multiplying units completed by the applicable contract
per-unit
price. In general, the Early Stage segment contract duration is
substantially less than that of the Late Stage segment. Because
the Early Stage business is also subject to the same
cancellation risk as the Late Stage contracts, the Company
attempts to require the customer to pay a cancellation fee if
the customer cancels a project award. Net service revenues from
these contracts generally represent less than 10% of the
Company’s consolidated net service revenues.
18
Acquisitions
In June 2008, the Company completed its acquisition of
DecisionLine Clinical Research Corporation (DecisionLine) and
its related company. DecisionLine (now Kendle Toronto) is a
clinical research organization located in Toronto, Ontario
specializing in the conduct of early phase studies. The
acquisition supports the overall goal of strategic business
expansion, and, in particular, expansion of early stage
capabilities. Please see Note 13 to the Consolidated
Financial Statements for further detail regarding this
acquisition.
The results of operations for acquisitions are included in the
Company’s Consolidated Statements of Operations from the
dates of acquisition.
Recent
Developments and CRO Marketplace
The CRO industry in general continues to be dependent on the
research and development efforts of the principal pharmaceutical
and biotechnology companies as major customers, and the Company
believes this dependence will continue. The loss of business
from any of its major customers could have a material adverse
effect on the Company. The current economic conditions have
created a very challenging climate causing the Company to
rapidly adjust its strategies and align the workforce to match
the demand for its services. The business climate has caused
customers to re-evaluate priorities resulting in increases in
contracts for the more promising projects, scaling back
and/or
canceling other projects and delaying decisions on others. The
biopharmaceutical industry is reducing costs and, often, their
workforce. The Company may benefit from increased outsourcing on
the part of its customers or it may be harmed by a reduction in
spending. The Company views the current conditions as an
opportunity to attract well qualified candidates to strengthen
and improve its operations and gain penetration and increase
market share as less research and development is done in-house
by our customers. Another industry trend is the decline in
prescription drug sales caused by cost conscious patients opting
for less expensive generic drugs or none at all. This is both an
opportunity and a challenge for the Company, as its customers
will need to find less costly, more efficient research options
often through the establishment of strategic alliances or
partnerships. The Company believes it is well positioned for
this development. The current economic conditions have also
impacted the credit environment, making the obtaining of
financing difficult for some customers. The Company is
proactively monitoring outstanding accounts receivable and
strives to remain in a cash positive position on its riskier
customers.
In addition, the volatility of currency exchange rate
fluctuations has a significant impact on the Company as more of
its business is earned outside the United States. Fluctuations
in exchange rates are not predictable with any degree of
accuracy or foreseeable.
Results
of Operations
Year
Ended December 31, 2009 (2009) Compared with Year
Ended December 31, 2008 (2008)
Net
Service Revenues
Information to be discussed regarding segment net service
revenues is outlined in the following table:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended December 31,
|
|
|
|
|
|
|
|
|
|
|
$ Increase
|
|
|
% Increase
|
|
|
|
|
2009
|
|
|
2008
|
|
|
(Decrease)
|
|
|
(Decrease)
|
|
|
|
|
Net service revenues
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Late stage
|
|
$
|
370,178
|
|
|
$
|
430,317
|
|
|
$
|
(60,139
|
)
|
|
|
(14.0
|
)%
|
|
Early stage(a)
|
|
|
37,473
|
|
|
|
35,199
|
|
|
|
2,274
|
|
|
|
6.5
|
%
|
|
Support & other(b)
|
|
|
9,037
|
|
|
|
9,576
|
|
|
|
(539
|
)
|
|
|
(5.6
|
)%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total net service revenues
|
|
$
|
416,688
|
|
|
$
|
475,092
|
|
|
$
|
(58,404
|
)
|
|
|
(12.3
|
)%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(a) |
|
The Early Stage segment results for the twelve months ended
December 31, 2008 include the June (acquisition date)
through December operating results of DecisionLine. |
| |
|
(b) |
|
Support and Other consists of revenues performed for
administrative services. |
19
Net service revenues decreased 12% to $416.7 million for
2009 from $475.1 million in 2008. The changes in currency
rates reduced net service revenues by approximately 6% for the
year 2009.
Net service revenues in the Late Stage segment decreased
approximately 14% to $370.2 million in 2009 compared to
$430.3 million in 2008. The declines were driven primarily
by changes in foreign currency exchange rates, reductions in the
scope of existing projects, cancellations of existing projects
and continued delays in the selling cycle, more specifically,
advancing contracts from the awarded status to the signed
contract status, which prevented the Company from commencing
work and revenue generating activities. As mentioned, the
Company also experienced a significantly higher than normal
cancellation rate on previously awarded studies. The Company
believes this situation is the result of biopharmaceutical
industry merger and acquisition activity, weakness in the
current global economy and reduced access to capital, among
other things. Additionally, recent pharmaceutical company
mergers as well as reduced prescription drug sales and
uncertainty in the global economy delayed customer decisions on
previously awarded contracts and slowed the contract signature
process as pharmaceutical companies re-evaluated their pipelines
and, in the case of newly merged customers, focused on
integration efforts rather than future development of products.
Net service revenues in the Early Stage segment increased
approximately 6.5%, or $2.3 million to approximately
$37.5 million in 2009 compared to $35.2 million in
2008. The majority of this increase is attributable to the
Kendle Toronto acquisition as 2009 results contain
12 months of net service revenues from Kendle Toronto
compared to 7 months of net service revenues in 2008. This
increase was partially offset by the impact of currency rate
fluctuations and decreased net service revenues at the Phase I
unit in the Netherlands as a result of the previously mentioned
project delays and cancellations.
A summary of net service revenues by geographic region for 2009
and 2008 is presented below:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
For the Year Ended December 31
|
|
|
|
|
|
|
|
|
|
|
$ Increase
|
|
|
% Increase
|
|
|
|
|
2009
|
|
|
2008
|
|
|
(Decrease)
|
|
|
(Decrease)
|
|
|
|
|
North America
|
|
$
|
200,866
|
|
|
$
|
229,346
|
|
|
$
|
(28,480
|
)
|
|
|
(12.4
|
)%
|
|
Europe
|
|
|
157,628
|
|
|
|
189,528
|
|
|
|
(31,900
|
)
|
|
|
(16.8
|
)%
|
|
Latin America
|
|
|
40,830
|
|
|
|
38,996
|
|
|
|
1,834
|
|
|
|
4.7
|
%
|
|
Asia-Pacific
|
|
|
17,364
|
|
|
|
17,222
|
|
|
|
142
|
|
|
|
0.8
|
%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total net service revenues
|
|
$
|
416,688
|
|
|
$
|
475,092
|
|
|
$
|
(58,404
|
)
|
|
|
(12.3
|
)%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
In 2009, the Company experienced a decline in both demand and
volume in North America and Europe as the Company’s
customers continue to look toward accessing the patient
populations in Latin America, Asia-Pacific and other lower cost
emerging regions to conduct clinical trials.
Net service revenues from the Company’s top five customers
accounted for approximately 27% of net service revenues in both
2009 and 2008. No customer accounted for more than 10% of total
net service revenues for 2009 or 2008.
Reimbursable
Out-of-Pocket
Revenues/Expenses
Reimbursable
out-of-pocket
revenues and expenses fluctuate from period to period due
primarily to the level of investigator activity in a particular
period. Reimbursable
out-of-pocket
revenues and expenses decreased 33% to $135.2 million in
2009 from $203.5 million in 2008. The decrease is due
primarily to a decrease in the number of studies in which the
Company is procuring investigator services.
20
Operating
Expenses
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
For the Year Ended December 31,
|
|
|
|
|
|
|
|
|
|
|
$ Increase
|
|
|
% Increase
|
|
|
|
|
2009
|
|
|
2008
|
|
|
(Decrease)
|
|
|
(Decrease)
|
|
|
|
|
Direct costs
|
|
$
|
210,586
|
|
|
$
|
247,436
|
|
|
$
|
(36,850
|
)
|
|
|
(14.9
|
)%
|
|
Reimbursable
out-of-pocket
costs
|
|
|
135,224
|
|
|
|
203,489
|
|
|
|
(68,265
|
)
|
|
|
(33.5
|
)%
|
|
SG&A expenses
|
|
|
144,659
|
|
|
|
155,577
|
|
|
|
(10,918
|
)
|
|
|
(7.0
|
)%
|
|
Restructuring expenses
|
|
|
10,157
|
|
|
|
—
|
|
|
|
10,157
|
|
|
|
|
|
|
Depreciation and amortization
|
|
|
15,712
|
|
|
|
15,253
|
|
|
|
459
|
|
|
|
3.0
|
%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total operating expenses
|
|
$
|
516,338
|
|
|
$
|
621,755
|
|
|
$
|
(105,417
|
)
|
|
|
(17.0
|
)%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Direct costs decreased in 2009 from 2008 by approximately
$36.9 million, or 15%. The decrease in direct costs in 2009
is attributable to the decline in net service revenues and
headcount reductions and other cost savings initiatives
initiated in the second quarter of 2009 as discussed in more
detail below.
Additionally, in the fourth quarter of 2008, the Company
identified a programming issue unique to one study and one
customer that required the Company to rework a large portion of
the project and additionally, to bear costs that would, under
normal circumstances, be absorbed by the customer. The Company
accrued $4.9 million related to these costs in the fourth
quarter of 2008. In 2009, as a result of ongoing discussions
with the customer and the insurance provider, the Company
increased the accrual for direct costs by $2.2 million to a
total of $7.1 million and received the insurance claim
recovery of $5.0 million. The net reduction in direct costs
in 2009 related to this programming issue and the insurance
claim recovery was approximately $2.8 million.
Direct costs expressed as a percentage of net service revenues
were 50.5% in 2009 compared to 52.1% in 2008. The decrease in
direct costs as a percentage of net service revenues in 2009 is
due in large part to the fluctuations in the accrual for rework
discussed in the preceding paragraph.
Selling, general and administrative (SG&A) expenses
decreased in 2009 from 2008 by approximately $10.9 million,
or 7%. The primary reason for the decrease in SG&A costs
relates to the cost savings initiatives undertaken in 2009, as
discussed in more detail below. Selling, general and
administrative expenses expressed as a percentage of net service
revenues were 34.7% in 2009 compared to 32.7% in 2008.
In the second quarter of 2009, the Company commenced several
initiatives to optimize its workforce and capacity and to reduce
operating expenses. These activities included a reduction of
discretionary spending, limiting previously planned headcount
additions, delay or elimination of salary merit increases,
reduction or elimination of certain other benefits, workforce
reductions or furloughs, and other cost savings in an attempt to
reduce expenses. The Company recorded a charge in the second
quarter of 2009 for severance-related and other expenses
(primarily related to facility closures), of approximately
$6.0 million. In the third quarter of 2009, the Company
revised its estimate of severance costs and expensed an
additional $380,000. As a result of these initiatives, the
Company realized savings of approximately $20 million in 2009.
In the fourth quarter of 2009, due to continued weakness in new
business awards and future net service revenue projections and
higher than historical cancellations, the Company committed to
additional headcount reductions expected to be initiated during
the first quarter of 2010 and recorded a charge in the fourth
quarter of 2009 for severance-related expenses of approximately
$3.8 million. Because the bulk of the Company’s
expenses are typically incurred in the Late Stage and Support
and Other reportable segments, the majority of the costs removed
from the business also affect those two segments. Headcount was
reduced from approximately 4,275 associates at the end of 2008
to 3,640 associates at the end of 2009. This reduction was
primarily in the U.S. and Western Europe and is expected to
result in savings of approximately $16 to $18 million in 2010.
When customer demand increases, the Company will be required to
increase staffing to deliver services and all of the estimated
savings may not materialize.
Depreciation and amortization expense increased by
$0.4 million, from $15.3 million in 2008 to
$15.7 million in 2009. The increase is primarily due to a
full year of amortization expense in 2009 on a customer
relationship asset from the Kendle Toronto acquisition versus
seven months in 2008.
21
Information to be discussed regarding segment operating income
is outlined in the below table:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended December 31,
|
|
|
|
|
|
|
|
|
|
|
Increase
|
|
|
% Increase
|
|
|
|
|
2009
|
|
|
2008
|
|
|
(Decrease)
|
|
|
(Decrease)
|
|
|
|
|
Operating Income/(Loss):
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Late stage
|
|
$
|
81,109
|
|
|
$
|
105,140
|
|
|
$
|
(24,031
|
)
|
|
|
(22.9
|
)%
|
|
Early stage(a)
|
|
|
4,187
|
|
|
|
6,177
|
|
|
|
(1,990
|
)
|
|
|
(32.2
|
)%
|
|
Support & other(b)
|
|
|
(49,722
|
)
|
|
|
(54,491
|
)
|
|
|
4,769
|
|
|
|
8.8
|
%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total operating income
|
|
$
|
35,574
|
|
|
$
|
56,826
|
|
|
$
|
(21,252
|
)
|
|
|
(37.4
|
)%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(a) |
|
The Early Stage segment results for the twelve months ended
December 31, 2008 include the June (acquisition date)
through December operating results of DecisionLine. |
| |
|
(b) |
|
Support and Other consists of unallocated corporate expenses,
primarily information technology, marketing and communications,
human resources, finance and legal, net of revenues earned. |
Income from operations in 2009 declined to $35.6 million,
or 8.5% of net service revenues, compared to $56.8 million,
or 12.0% of net service revenues in 2008. The overall decline in
operating income and operating income as a percentage of net
service revenues was primarily due to items discussed in more
detail in the preceding paragraphs, including the reduction in
net service revenues and the restructuring expense. Please see
section titled Non-GAAP Net Service Revenue, Operating
Income and EPS for further discussion.
Income from operations from Kendle’s Late Stage segment in
2009 was $81.1 million or 21.9% of net service revenues
compared to Late Stage income from operations of
$105.1 million, or 24.4% of net service revenues in 2008.
The decline in the Late Stage operating margin was due primarily
to a decline in net service revenues, primarily in the North
American and European regions, driven by the decline in new
business awards and new contracts, increased cancellations and
delays in existing studies leading to decreased utilization of
billable associates and excess capacity. Additionally, in 2009
the decline in Late Stage operating margin was due to the
accrual of costs related to the workforce capacity optimization
as discussed above.
Income from operations from Kendle’s Early Stage segment in
2009 was $4.2 million, or 11.2% of net service revenues
compared to Early Stage income from operations of
$6.2 million, or 17.5% of net service revenues in 2008. The
primary reason for the decline in operating income as a
percentage of net service revenues was due to low demand, for
reasons previously cited, at the Company’s Phase I unit in
the Netherlands.
Other
Income/(Expense)
Total other income (expense) was expense of $6.9 million in
2009 compared to expense of $17.2 million in 2008.
The components of Other Income/Expense were as follows for the
periods presented:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
For the Year Ended December 31,
|
|
|
|
|
2009
|
|
|
2008
|
|
|
Change
|
|
|
|
|
Other income (expense)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Interest income
|
|
$
|
579
|
|
|
$
|
760
|
|
|
$
|
(181
|
)
|
|
Interest expense
|
|
|
(14,403
|
)
|
|
|
(15,891
|
)
|
|
|
1,488
|
|
|
Gain on extinguishment of debt
|
|
|
2,887
|
|
|
|
—
|
|
|
|
2,887
|
|
|
Foreign currency gains/(losses)
|
|
|
5,350
|
|
|
|
(1,006
|
)
|
|
|
6,356
|
|
|
Other expenses
|
|
|
(1,316
|
)
|
|
|
(1,037
|
)
|
|
|
(279
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total other income (expense)
|
|
$
|
(6,903
|
)
|
|
$
|
(17,174
|
)
|
|
$
|
10,271
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
22
Interest
Income
Interest income decreased by approximately $181,000 in 2009 due
to lower returns on the invested cash.
Interest
Expense
The primary component of interest expense is related to the
Company’s 3.375% Convertible Notes issued in June
2007. The Company adopted new accounting guidance related to
convertible debt effective January 1, 2009 as it relates to
this issuance and as required by the new guidance has
retrospectively adjusted the prior periods for the effects of
the new guidance.
For 2009, the amount of interest expense recognized for the
contractual interest rate was $6.1 million and the amount
recognized for discount amortization was $6.5 million for a
total of $12.6 million. Additionally, $1.3 million of
interest expense was recognized for amortization of deferred
issuance costs related to the debt offering and the facility it
maintained. For 2008, the amount of interest expense recognized
for the contractual interest rate was $6.8 million and the
amount recognized for discount amortization was
$6.6 million for a total of $13.4 million.
Additionally, $1.2 million of interest expense was
recognized for amortization of deferred issuance costs related
to the debt offering and the facility it maintained. The decline
in interest expense in 2009 is due to the open market
repurchases during the year of a portion of the convertible
notes outstanding.
Gain
on Extinguishment of Debt
During 2009, the Company repurchased in several transactions on
the open market $45.5 million in par value of Convertible
Notes for cash in the amount of $36.5 million. The carrying
value of these Convertible Notes at the time of repurchase was
approximately $40.0 million and a pretax gain on
extinguishment of debt of approximately $2.9 million was
recorded. As part of the repurchase transactions, the
proportionate share of debt issuance costs in the amount of
$840,000 were included in the determination of the gain amount
and were written off.
See also the Liquidity and Capital Resources section.
Foreign
Currency
In the first quarter of 2007, the Company entered into foreign
currency hedge arrangements to hedge foreign currency exposure
related to intercompany notes outstanding. The hedging
transactions were designed to mitigate the Company’s
exposure related to two intercompany notes between the
Company’s U.S. subsidiary, as lender, and the
Company’s subsidiary in each of the United Kingdom and
Germany. The derivative arrangements were not designated for
hedge accounting treatment and mark to market adjustments on
these arrangements were recorded in the Company’s
Consolidated Statements of Operations.
In the first quarter of 2009, the Company eliminated a
substantial portion of the note payable between the
U.S. subsidiary and the U.K. subsidiary, referenced above,
and the entire amount of the note payable between the
U.S. subsidiary and German subsidiary. In connection with
these transactions, the Company also terminated its foreign
currency hedge arrangements referenced in the preceding
paragraph.
In 2009, losses related to the exchange rate fluctuations on the
intercompany notes and related derivative instruments
(pre-settlement) were approximately $1.0 million compared
to gains of approximately $2.4 million in 2008 on the
intercompany notes and derivatives instruments.
In addition to the gains on the intercompany notes and foreign
currency hedge arrangements discussed above, the Company
recorded foreign exchange rate gains of approximately
$6.3 million in 2009 compared to losses of approximately
$3.4 million in 2008. The foreign exchange gain in 2009
mostly occurred in the first six months of 2009 and was
primarily due to the strengthening of the British pound against
the Euro and the strengthening of the U.S. dollar against
both the British pound and the Euro during that time period. As
mentioned below, the Company has implemented procedures intended
to mitigate the impact of foreign currency exchange rate
fluctuations and those procedures have helped to reduce the
impact in the second half of 2009. The foreign exchange losses
in 2008 were due primarily to the weakening of the British pound
against the Euro as the Company had a large amount of euro
denominated payables in countries that have a functional
currency of the British pound.
23
The exchange rate transaction gains and losses typically occur
when the Company holds assets
and/or
liabilities in a currency other than the functional currency of
the reporting location. Due to uncertainties regarding the
timing of and currencies involved in the majority of the
Company’s foreign exchange rate transactions, it is
impracticable to implement hedging instruments to match the
Company’s foreign currency inflows and outflows. In 2009,
the Company implemented procedures intended to mitigate the
impact of foreign currency exchange rate fluctuations including
an intercompany procedure to allow for regular settlement of
intercompany balances. The Company will continue to evaluate
ways to mitigate the risk of this impact in the future.
Income
Taxes
The Company recorded tax expense at an effective rate of
approximately 46.8% in 2009 compared to approximately 41.6% in
2008. The tax expense of $13.4 million for 2009 includes
$4.4 million in tax expense for the settlement of foreign
currency hedges and related intercompany notes and a
$1.0 million item for the tax related to the taxable gain
from the extinguishment of debt in connection with the open
market repurchases. These items are partially offset by a tax
benefit recorded in the third quarter of 2009 related to
revisions in estimates for certain items related to the
Company’s 2008 U.S. federal tax return and an
additional tax benefit of approximately $1.0 million
recorded during 2009 related to the usage of certain deferred
tax assets for which a valuation allowance had been previously
recorded. In 2008, the Company recorded an additional tax
benefit of approximately $3.3 million related to the
recognition of tax benefits that were previously unrecognized in
accordance with accounting guidance related to accounting for
uncertainty in income taxes.
Net
Income
The net income for 2009 was approximately $15.2 million, or
$1.02 per diluted share and $1.03 per basic share.
The net income for 2008 was approximately $23.1 million, or
$1.54 per diluted share and $1.57 per basic share.
Non-GAAP Net
Service Revenue, Operating Income and EPS
Non-GAAP net service revenues, non-GAAP operating income and
non-GAAP EPS are alternative views of the Company’s
performance used by management that the Company is providing
because management believes this information enhances
investors’ understanding of the Company’s results.
Non-GAAP measures exclude certain items because of the nature of
these items and the impact that they have on the analysis of
underlying business performance and trends. The excluded items
are constant currency adjustments related to the impact of
exchange rate changes on net service revenues and income from
operations, restructuring charges, a gain on extinguishment of
debt, additional interest expense related to the adoption of ASC
470, a write-off of deferred financing costs and a discrete tax
item. The excluded items are significant components in
understanding and assessing financial performance. Therefore,
the information on non-GAAP net service revenue, operating
income and EPS should be considered in addition to, but not in
lieu of, net service revenue, operating income and earnings per
share prepared in accordance with generally accepted accounting
principles in the United States (“GAAP”).
Additionally, since non-GAAP net service revenue, operating
income and EPS are not measures determined in accordance with
GAAP, they have no standardized meaning prescribed by GAAP and,
therefore, may not be comparable to the calculation of similar
measures of other companies.
24
A reconciliation between GAAP financial measures and non-GAAP
financial measures is as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
For the Years Ended December 31,
|
|
|
|
|
2009
|
|
|
2008
|
|
|
2007
|
|
|
|
|
(In thousands except per share data)
|
|
|
|
|
Reconciliation of non-GAAP net service revenues:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net service revenues, as reported
|
|
$
|
416,688
|
|
|
$
|
475,092
|
|
|
|
397,584
|
|
|
Constant currency adjustment
|
|
|
27,617
|
|
|
|
(910
|
)
|
|
|
(19,051
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Non-GAAP net service revenues
|
|
$
|
444,305
|
|
|
$
|
474,182
|
|
|
$
|
378,533
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Reconciliation of non-GAAP income from operations:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Income from operations, as reported
|
|
$
|
35,574
|
|
|
$
|
56,826
|
|
|
$
|
52,814
|
|
|
Restructuring charge, as reported
|
|
|
10,157
|
|
|
|
—
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Subtotal
|
|
|
45,731
|
|
|
|
56,826
|
|
|
|
52,814
|
|
|
Constant currency adjustment
|
|
|
7,086
|
|
|
|
(2,693
|
)
|
|
|
(2,228
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Non-GAAP income from operations
|
|
$
|
52,817
|
|
|
$
|
54,133
|
|
|
$
|
50,586
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Reconciliation of non-GAAP net income:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income, as reported
|
|
$
|
15,237
|
|
|
$
|
23,143
|
|
|
$
|
16,010
|
|
|
Additional interest expense related to convertible debt
|
|
|
6,456
|
|
|
|
6,630
|
|
|
|
2,873
|
|
|
Restructuring expense, net of tax
|
|
|
6,799
|
|
|
|
—
|
|
|
|
—
|
|
|
Gain on extinguishment of debt, net of tax
|
|
|
(1,887
|
)
|
|
|
—
|
|
|
|
—
|
|
|
Write-off of deferred financing costs, net of tax
|
|
|
—
|
|
|
|
—
|
|
|
|
2,636
|
|
|
Discrete tax item related to foreign currency hedge
|
|
|
4,441
|
|
|
|
—
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Non-GAAP net income
|
|
$
|
31,046
|
|
|
$
|
29,773
|
|
|
$
|
21,519
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income per share, as reported:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic
|
|
$
|
1.03
|
|
|
$
|
1.57
|
|
|
$
|
1.10
|
|
|
Diluted
|
|
$
|
1.02
|
|
|
$
|
1.54
|
|
|
$
|
1.08
|
|
|
Weighted Average Shares Outstanding:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic
|
|
|
14,862
|
|
|
|
14,751
|
|
|
|
14,520
|
|
|
Diluted
|
|
|
14,992
|
|
|
|
14,993
|
|
|
|
14,889
|
|
|
Non-GAAP net income per share:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic
|
|
$
|
2.09
|
|
|
$
|
2.02
|
|
|
$
|
1.48
|
|
|
Diluted
|
|
$
|
2.07
|
|
|
$
|
1.99
|
|
|
$
|
1.45
|
|
Constant
Currency Adjustments
Non-GAAP net service revenue and non-GAAP operating income
present net service revenue and operating income by translating
current year results from local currency to U.S. dollars
using the exchange rates in effect during the previous year.
Restructuring
Costs
Non-GAAP operating income and non-GAAP net income and EPS
exclude restructuring costs, primarily costs related to facility
closures and employee severance costs. Restructuring costs have
occurred in past periods and may occur in future periods, and
therefore should not be considered to be non-recurring. However,
management excludes these amounts from non-GAAP operating income
and non-GAAP net income and EPS because it believes it is
helpful for understanding the Company’s performance.
25
Additional
Interest Expense Related to Convertible Debt
Non-GAAP net income and EPS exclude additional interest expense
related to the retrospective adoption of FASB ASC 470.
Gain
on Extinguishment of Debt
Non-GAAP net income and EPS exclude gains recorded on the
partial repurchases of the Company’s convertible notes that
occurred during 2009.
Write-off
of deferred financing costs
Non-GAAP net income and EPS exclude a write-off of deferred
financing costs in 2007 that related to deferred financing costs
incurred in relation to a term note that was repaid in 2007 with
proceeds from the issuance of Convertible Notes.
Discrete
tax item related to foreign currency hedge
Non-GAAP net income and EPS exclude a discrete tax item related
to the unwinding of a foreign currency hedge instrument.
Year
Ended December 31, 2008 (2008) Compared with Year
Ended December 31, 2007 (2007)
Net
Service Revenues
Information to be discussed regarding segment net service
revenues is outlined in the below table:
Revenues
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended December 31,
|
|
|
|
|
|
|
|
|
|
|
Increase
|
|
|
% Increase
|
|
|
|
|
2008
|
|
|
2007
|
|
|
(Decrease)
|
|
|
(Decrease)
|
|
|
|
|
Net service revenues
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Late stage
|
|
$
|
430,317
|
|
|
$
|
366,379
|
|
|
$
|
63,938
|
|
|
|
17.5
|
%
|
|
Early stage(a)
|
|
|
35,199
|
|
|
|
21,373
|
|
|
|
13,826
|
|
|
|
64.7
|
%
|
|
Support & other(b)
|
|
|
9,576
|
|
|
|
9,832
|
|
|
|
(256
|
)
|
|
|
(2.6
|
)%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total net service revenues
|
|
$
|
475,092
|
|
|
$
|
397,584
|
|
|
|
77,508
|
|
|
|
19.5
|
%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(a) |
|
The Early Stage segment results for the twelve months ended
December 31, 2008 include the June (acquisition date)
through December operating results of DecisionLine. |
| |
|
(b) |
|
Support and Other consists of primarily of revenues for
administrative services. |
Net service revenues increased 19% to $475.1 million for
2008 from $397.6 million in 2007. Exchange rate
fluctuations had a minimal impact on net service revenues for
the full year.
Net service revenues in the Late Stage segment increased
approximately 17% to $430.3 million in 2008 compared to
$366.4 million in 2007.
Net service revenues in the Early Stage segment increased
approximately $13.8 million to approximately
$35.2 million in 2008 compared to $21.4 million in
2007. The majority of this increase is attributable to the
DecisionLine acquisition as net service revenues from
DecisionLine were approximately $12.8 million for the
period from June 2008, date of acquisition, through the end of
the year. Net service revenues at the Company’s Phase I
unit in Morgantown, West Virginia decreased by approximately
$0.1 million in 2008 compared to 2007 while net service
revenues at the Phase I unit in the Netherlands increased by
approximately $1.1 million in 2008 compared to 2007. The
decline in net service revenue at the Company’s Early Stage
unit in Morgantown was due partially to a third quarter of 2008
contract delay of two studies. The majority of the work on these
contracts was completed in the first quarter of 2009.
26
A summary of net service revenues by geographic region for 2008
and 2007 is presented below:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
For the Year Ended December 31,
|
|
|
|
|
|
|
|
|
|
|
$ Increase
|
|
|
% Increase
|
|
|
|
|
2008
|
|
|
2007
|
|
|
(Decrease)
|
|
|
(Decrease)
|
|
|
|
|
North America
|
|
$
|
229,346
|
|
|
$
|
198,242
|
|
|
$
|
31,104
|
|
|
|
15.7%
|
|
|
Europe
|
|
|
189,528
|
|
|
|
165,195
|
|
|
|
24,333
|
|
|
|
14.7%
|
|
|
Latin America
|
|
|
38,996
|
|
|
|
20,784
|
|
|
|
18,212
|
|
|
|
87.6%
|
|
|
Asia-Pacific
|
|
|
17,222
|
|
|
|
13,363
|
|
|
|
3,859
|
|
|
|
28.9%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total net service revenues
|
|
$
|
475,092
|
|
|
$
|
397,584
|
|
|
$
|
77,508
|
|
|
|
19.5%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net service revenues in North America and Europe increased by
approximately 16% and 15%, respectively, in 2008 compared to
2007 primarily due to larger projects awarded to the Company.
Net service revenues in Latin America increased 88% to
$39.0 million as the Company’s customers continue to
look toward Latin America, Asia-Pacific and other lower cost
emerging regions to conduct clinical trials. Net service
revenues in Asia-Pacific increased by approximately 29% in 2008
compared to 2007. Although the Company expects strong demand for
clinical trial services in Latin America and Asia-Pacific to
continue in the future, the Company does not expect the rate of
net service revenue increase to continue at the levels
experienced in 2008.
Net service revenues from the Company’s top five customers
accounted for approximately 27% and 25% of net service revenues
in 2008 and 2007, respectively. No customer accounted for more
than 10% of total net service revenues for 2008 or 2007.
Reimbursable
Out-of-Pocket
Revenues/Expenses
Reimbursable
out-of-pocket
revenues and expenses fluctuate from period to period due
primarily to the level of investigator activity in a particular
period. Reimbursable
out-of-pocket
revenues and expenses increased 19% to $203.5 million in
2008 from $171.2 million in 2007. The increase was due
primarily to an increase in the number of studies in which the
Company procured investigator services as well as to an increase
in the size of those studies.
Operating
Expenses
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
For the Year Ended December 31,
|
|
|
|
|
|
|
|
|
|
|
Increase
|
|
|
% Increase
|
|
|
|
|
2008
|
|
|
2007
|
|
|
(Decrease)
|
|
|
(Decrease)
|
|
|
|
|
Direct costs
|
|
$
|
247,436
|
|
|
$
|
204,161
|
|
|
$
|
43,275
|
|
|
|
21.2%
|
|
|
Reimbursable
out-of-pocket
costs
|
|
|
203,489
|
|
|
|
171,234
|
|
|
|
32,255
|
|
|
|
18.8%
|
|
|
SG&A expenses
|
|
|
155,577
|
|
|
|
125,744
|
|
|
|
29,833
|
|
|
|
23.7%
|
|
|
Depreciation and amortization
|
|
|
15,253
|
|
|
|
14,865
|
|
|
|
388
|
|
|
|
2.6%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total operating expenses
|
|
$
|
621,755
|
|
|
$
|
516,004
|
|
|
$
|
105,751
|
|
|
|
20.5%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Direct costs increased by 21% from $204.2 million in 2007
to $247.4 million in 2008. The increase in direct costs was
attributable to the increased hiring of billable employees to
support the growth in the overall business. Direct costs
expressed as a percentage of net service revenues were 52.1% in
2008 compared to 51.4% in 2007. The slight increase in direct
costs as a percentage of net service revenues in 2008 was due in
part to additional direct costs of approximately
$4.9 million recorded in the fourth quarter of 2008 due to
a programming issue unique to one study and one customer. As a
result of the programming issue, the Company needed to rework
the project, resulting in additional direct costs accrued at
December 31, 2008. At December 31, 2008, the Company
was in the process of working with its insurance provider to
recover direct cost amounts that might be covered under the
terms of the Company’s insurance coverage. Accordingly, no
receivable had been recorded at December 31, 2008 related
to potential insurance recovery. Any insurance proceeds received
would serve to reduce direct costs in future periods.
Selling, general and administrative expenses increased
$29.9 million, or 24%, from $125.7 million in 2007 to
$155.6 million in 2008. The increase was primarily due to
increases in employee-related costs from the Company’s
27
increase in headcount. Average headcount, which includes both
billable and nonbillable associates, increased by 20% in 2008
when compared with 2007. The headcount increase is in line with
the Company’s efforts to build infrastructure to support
the Company’s growth. The increase in employee-related
costs was comprised of general salary increases and
corresponding payroll tax and benefit increases including
increased health care costs. Selling, general and administrative
expenses expressed as a percentage of net service revenues were
32.7% in 2008 compared to 31.6% in 2007.
Depreciation and amortization expense increased by
$0.4 million, from $14.9 million in 2007 to
$15.3 million in 2008. The increase was primarily due to
increased amortization expense of approximately $952,000 related
to amortization of a customer relationship asset acquired in the
June 2008 acquisition of DecisionLine offset partially by a
decline in amortization expense on certain finite-lived
intangible assets acquired in the 2006 acquisition of the Phase
II-IV Clinical Services business of Charles River Laboratories
International, Inc. Finite-lived intangibles are amortized in a
manner consistent with the underlying expected future cash flows
from the customers, resulting in higher amortization expense in
the initial year of acquisition. In addition, depreciation
expense increased as a result of new asset purchases in 2008 and
the related depreciation thereon.
Information to be discussed regarding segment operating income
is outlined in the below table:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended December 31,
|
|
|
|
|
|
|
|
|
|
|
Increase
|
|
|
% Increase
|
|
|
|
|
2008
|
|
|
2007
|
|
|
(Decrease)
|
|
|
(Decrease)
|
|
|
|
|
Operating Income/(Loss):
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Late stage
|
|
$
|
105,140
|
|
|
$
|
85,971
|
|
|
$
|
19,169
|
|
|
|
22.3
|
%
|
|
Early stage(a)
|
|
|
6,177
|
|
|
|
2,941
|
|
|
|
3,236
|
|
|
|
110.0
|
%
|
|
Support & other(b)
|
|
|
(54,491
|
)
|
|
|
(36,098
|
)
|
|
|
(18,393
|
)
|
|
|
(51.0
|
)%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total operating income
|
|
$
|
56,826
|
|
|
$
|
52,814
|
|
|
|
4,012
|
|
|
|
7.6
|
%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(a) |
|
The Early Stage segment results for the twelve months ended
December 31, 2008 include the June (acquisition date)
through December operating results of DecisionLine. |
| |
|
(b) |
|
Support and Other consists of unallocated corporate expenses,
primarily information technology, marketing and communications,
human resources, finance and legal, net of revenues. |
Income from operations in 2008 increased to $56.8 million,
or 12.0% of net service revenues, compared to
$52.8 million, or 13.3% of net service revenues in 2007.
The overall decline in operating income as a percentage of net
service revenues was due in part to a revenue reduction of
$2.3 million and additional direct costs of
$4.9 million due to the programming issue on one project
and the related rework discussed above as well as an overall
decline in the utilization of billable associates in 2008
compared to 2007. The revenue reduction and additional direct
costs due to the programming issue is included in the
Company’s Support and Other category for purposes of
segment reporting.
Income from operations from Kendle’s Late Stage segment in
2008 was $105.1 million or 24.4% of net service revenues
compared to Late Stage income from operations of
$86.0 million, or 23.5% of net service revenues in 2007.
The increase in income from operations as a percentage of net
services revenues for the Late Stage segment for the year ended
December 31, 2008 compared to the same period in 2007 is
primarily due to the growth in the Company’s Late Stage
operations in lower-cost, emerging markets in Latin America and
Asia-Pacific.
Income from operations from Kendle’s Early Stage segment in
2008 was $6.2 million, or 17.5% of net service revenues
compared to Early Stage income from operations of
$2.9 million, or 13.8% of net service revenues in 2007. The
primary reason for the increase in operating margin in Early
Stage in 2008 was due to operating income from Kendle Toronto of
approximately $2.2 million and an increase in operating
income at the Company’s Phase I unit in the Netherlands.
28
Other
Income/(Expense)
Total other income (expense) was expense of $17.2 million
in 2008 compared to expense of $25.0 million in 2007.
The components of Other Income/Expense were as follows for the
periods presented:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
For the Year Ended December 31,
|
|
|
|
|
2008
|
|
|
2007
|
|
|
Change
|
|
|
|
|
Other income (expense)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Interest income
|
|
$
|
760
|
|
|
$
|
1,466
|
|
|
$
|
(706
|
)
|
|
Interest expense
|
|
|
(15,891
|
)
|
|
|
(17,547
|
)
|
|
|
1,656
|
|
|
Write-off of deferred financing costs
|
|
|
—
|
|
|
|
(4,152
|
)
|
|
|
4,152
|
|
|
Foreign currency (losses)
|
|
|
(1,006
|
)
|
|
|
(4,513
|
)
|
|
|
3,507
|
|
|
Other expenses
|
|
|
(1,037
|
)
|
|
|
(303
|
)
|
|
|
(734
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total other income (expense)
|
|
$
|
(17,174
|
)
|
|
$
|
(25,049
|
)
|
|
$
|
7,875
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Interest
Income
Interest income decreased by approximately $706,000 in 2008 due
to lower cash and investment balances, as well as a decline in
interest rates, in 2008 compared to 2007. In addition, the
Company used available cash to finance its June 2008 acquisition
of DecisionLine.
Interest
Expense
The primary component of interest expense is related to the
Company’s 3.375% Convertible Notes issued in June
2007. The Company adopted new accounting guidance related to
convertible debt effective January 1, 2009 as it relates to
this issuance and as required by the new guidance has
retrospectively adjusted the prior periods for the effects of
the new guidance.
For 2008, the total amount of interest expense was
$15.9 million including $6.8 million of interest
expense recognized at the contractual rate and discount
amortization of $6.6 million. A total of $1.2 million
of interest expense was recognized for amortization of deferred
issuance costs related to the debt offering and the facility it
maintained. In 2007, the Company incurred interest expense of
approximately $17.5 million, including $3.1 million of
interest expense at the contractual rate and $2.9 million
of interest expense recognized for discount amortization on the
convertible notes. The remainder of the interest expense related
to the term notes prior to their retirement.
In the first quarter of 2007, the Company entered into interest
rate swap and collar arrangements to fix the rate on a portion
of its then outstanding term debt. The derivative arrangements
were not designed for hedge accounting treatment and mark to
market adjustments on these arrangements are recorded in the
Company’s Consolidated Statements of Operations. In the
fourth quarter of 2007, the Company terminated the interest rate
swap and paid $881,000 to reflect the amounts due upon
termination of the swap. Total 2007 losses related to the
interest rate swap/collar arrangements, including the $881,000
paid upon termination of the swap arrangement were approximately
$1.3 million. Losses related to the interest rate
swap/collar arrangements are reflected in interest expense in
the Company’s Consolidated Statements of Operations.
Write-off
of deferred financing costs
In the third quarter of 2007, the Company issued
$200.0 million in 3.375% Convertible Notes. In
conjunction with issuance and sale of the Convertible Notes, the
Company made a mandatory prepayment on its term debt and
subsequently paid off the balance of the term note in the third
quarter of 2007. Consequently, in the third quarter of 2007, the
Company wrote-off approximately $4.2 million in deferred
financing costs related to the term note.
29
Foreign
Currency
In the first quarter of 2007, the Company entered into foreign
currency hedge arrangements to hedge foreign currency exposure
related to intercompany notes outstanding. The derivative
arrangements were not designated for hedge accounting treatment
and mark to market adjustments on these arrangements are
recorded in the Company’s Consolidated Statements of
Operations. In 2008, the Company recorded gains of approximately
$2.4 million related to exchange rate fluctuations on these
intercompany notes and the related derivative instruments
compared to gains of approximately $1.4 million related to
the intercompany notes and derivative instruments in 2007.
In addition to the gains on the intercompany notes and foreign
currency hedge arrangements discussed above, the Company
recorded foreign exchange rate losses of approximately
$3.4 million in 2008 compared to losses of approximately
$5.9 million in 2007. The foreign exchange losses in 2008
were due primarily to the weakening of the British pound against
the Euro as the Company has a large amount of euro denominated
payables in countries that have a functional currency of the
British pound. The foreign exchange loss in 2007 was due to the
weakening of the British pound against the Euro and the
weakening of the U.S. dollar against both the British pound
and the Euro. As the Company increases its global contracts, it
is increasingly exposed to fluctuations in exchange rates.
The exchange rate transaction gains and losses typically occur
when the Company holds assets
and/or
liabilities in a currency other than the functional currency of
the reporting location. With the exception of the hedge
arrangements on intercompany notes referred to above, the
Company does not currently have hedges in place to mitigate
exposure due to foreign exchange rate fluctuations. Due to
uncertainties regarding the timing of and currencies involved in
the majority of the Company’s foreign exchange rate
transactions, it is impracticable to implement hedging
instruments to match the Company’s foreign currency inflows
and outflows.
Income
Taxes
The Company recorded tax expense at an effective rate of
approximately 41.6% in 2008 compared to approximately 42.3% in
2007. The decrease in the effective income tax rate in 2008 is
primarily due to the distribution of income among the
Company’s
non-U.S. subsidiaries.
In addition, in 2008 the Company recorded an additional tax
benefit of approximately $3.3 million related to the
recognition of tax benefits that were previously unrecognized in
accordance with accounting guidance related to accounting for
uncertainty in income taxes. In 2007, the Company had recorded
additional tax expense of approximately $416,000 related to that
same pronouncement.
Net
Income
The net income for 2008 was approximately $23.1 million or
$1.54 per diluted share and $1.57 per basic share.
The net income for 2007 was approximately $16.0 million, or
$1.08 per diluted share and $1.10 per basic share.
Liquidity
and Capital Resources
The Company had cash and cash equivalents of approximately
$52.1 million at December 31, 2009 compared to
approximately $35.2 million at December 31, 2008. In
2009, cash and cash equivalents increased by $16.9 million
as a result of cash provided by operating activities of
$54.3 million offset by cash used in investing and
financing activities of $4.5 million and
$36.9 million, respectively. Foreign exchange rates
positively impacted cash and cash equivalents by approximately
$4.0 million. In addition, the Company has restricted cash
of approximately $1.1 million at December 31, 2009
compared with $884,000 at December 31, 2008, which
represents cash received from customers that is segregated in a
separate Company bank account and available for use only for
specific project-related expenses, primarily investigator fees,
upon authorization from the customer. Because this restricted
cash is directly related to operation of the Company’s
business, the activity is included in operating cash flows as
opposed to financing cash flows.
Net cash provided by operating activities for the year consisted
primarily of net income adjusted for noncash expenses combined
with a reduction in net accounts receivable. Total noncash
depreciation, amortization, and debt issuance cost amortization,
net of the noncash gain on debt extinguishment totaled
$20.6 million for the year ended December 31, 2009.
Additionally, in June 2009, the Company received cash payments
totaling $4.5 million for
30
tenant improvement allowances as per the terms of the lease for
the Company’s headquarters. In 2009, changes in operating
assets and liabilities provided net cash in the amount of
$10.5 million, driven by collections of accounts
receivable. In 2008, the opposite was the case as the change in
net operating assets used $6.0 million in cash primarily
driven by an increase in accounts receivable partially offset by
an increase in advanced billings. Fluctuations in accounts
receivable and advance billings occur on a regular basis as
services are performed, milestones or other billing criteria are
achieved, invoices are sent to customers and payments for
outstanding accounts receivable are collected from customers.
Accounts receivable, net of advance billings, decreased from
$63.4 million at December 31, 2008, to
$49.1 million at December 31, 2009. The Company has
been vigilant in monitoring and collecting its accounts
receivable. The decrease in accounts receivable in 2009 was
primarily due to increased collections particularly in the
U.S. and the European subsidiaries, as well as, the decline
in revenues.
Cash flows used for investing activities for the year ended
December 31, 2009 included the use of $19.0 million
for property and equipment additions and $2.9 million for
acquisition of businesses (primarily partial payment of accrued
contingent consideration) combined with the receipt of
$17.3 million from the termination of the existing hedge
agreements. Cash flows used for investing activities for the
year ended December 31, 2008 consisted primarily of capital
expenditures of $27.1 million, the acquisition of
DecisionLine for $18.1 million and a $1.1 million
payment to terminate the interest rate collar.
Cash flows used in financing activities for the year ended
December 31, 2009 were $36.9 million, of which
$36.5 million was used to repurchase $45.5 million in
par value of the Company’s Convertible Notes on the open
market. Cash flows provided by financing activities for the year
ended December 31, 2008 excluding the offsetting effects of
drawdowns and repayments of the credit facility it maintained
consisted primarily of $2.2 million in proceeds from stock
option activity.
Cash used for capital expenditures was $19.0 million,
$27.1 million and $15.3 million in 2009, 2008 and
2007, respectively, and were primarily related to information
technology initiatives.
In March 2010, the Company terminated its existing credit
agreement (including all amendments and related agreements, the
“Old Facility”) and entered into a new credit
agreement (the “Facility”). The Facility is comprised
of a $35 million revolving loan commitment, with up to
$10 million of such commitment available for issuance of
letters of credit and up to $5 million of such commitment
available for same-day swing line loans. At the Company’s
request and with the consent of the current lender or additional
lenders, the total commitment may be increased by, or
incremental term loans may be obtained for, up to an additional
$15 million. At the Company’s election, loans under
the Facility are available either at (i) an adjusted base
rate plus an applicable margin or (ii) an adjusted LIBO
rate plus an applicable margin. The applicable margin for each
interest rate is calculated in accordance with the terms of the
Facility.
The Facility matures in March 2015, which maturity will
accelerate to January 15, 2012 in the event that five
percent (5%) or more of the currently outstanding principal
amount of the Convertible Notes discussed below have not been
redeemed or repaid in full on or prior to January 15, 2012.
Borrowings under the Facility are collateralized by
substantially all of the Company’s assets pursuant to the
terms of a Pledge and Security Agreement and obligations
incurred under the Facility are secured by a Guaranty in favor
of various secured parties. The Facility contains various
affirmative and negative covenants including those regarding
limitations on the Company’s ability to incur certain
indebtedness, limitations on certain investments, limitations on
capital expenditures in any fiscal year and limitations on
certain acquisitions and asset sales outside the ordinary course
of business as well as financial covenants regarding limitations
on the Company’s total leverage ratio, senior secured
leverage ratio and interest coverage ratio. The Company is in
compliance with the covenants as of the date it entered into the
Facility.
In connection with the termination of the Old Facility, the
Company intends to write off approximately $665,000 in
unamortized fees during the first quarter of 2010.
The Company also maintains an existing $5.0 million
Multicurrency Facility that is renewable annually and is used in
connection with the Company’s European operations.
In July, 2007, the Company issued $200 million of
5-year
Convertible Notes with a maturity date of July 15, 2012.
The Convertible Notes bear interest at an annual rate of 3.375%,
payable semi-annually in arrears on January 15 and July 15 of
each year. The Convertible Notes are convertible at the option
of the holder into cash and, if
31
applicable, shares of the Company’s common stock at an
initial conversion price of $47.71 per share (approximating
20.9585 shares per $1,000 principal amount of the
Convertible Notes), upon the occurrence of certain events. In
addition, upon events defined as a “fundamental
change” under the Convertible Note Indenture, holders of
the Convertible Notes may require the Company to repurchase the
Convertible Notes. If upon the occurrence of such events in
which the holders of the Convertible Notes exercise the
conversion provisions of the Convertible Notes, the Company will
need to remit the principal balance of the Convertible Notes to
the holders in cash. As such, the Company would be required to
classify the entire amount outstanding of the Convertible Notes
as a current liability in the following quarter. The evaluation
of the classification of amounts outstanding associated with the
Convertible Notes occurs every quarter. As of December 31,
2009, the Convertible Notes are classified as long-term in the
accompanying Consolidated Balance Sheets.
Concurrent with the sale of the Convertible Notes, the Company
purchased convertible bond hedges from two counterparties which
are designed to mitigate potential dilution from the conversion
of the Convertible Notes in the event that the market value per
share of the Company’s common stock at the time of exercise
is greater than approximately $47.71. In addition, the Company
issued warrants to the counterparties that could require the
Company to issue shares of the Company’s common stock at a
strike price of $61.22 per share. The Convertible Note hedge and
warrant transactions generally have the effect of increasing the
conversion price of the Convertible Notes to approximately
$61.22 per share of Kendle common stock, representing
approximately a 70% premium based on the closing sale price as
reported on The NASDAQ Global Market on July 10, 2007, of
$36.01 per share.
In 2009, the Company repurchased $45.5 million in par value
of its Convertible Notes on the open market for cash of
$36.5 million. The amortized carrying value of these
repurchased notes was $40.0 million and a non cash net gain
of $2.9 million was recorded. As part of the repurchase
transaction, the proportionate share of debt issuance costs in
the amount of $840,000 was written off. This transaction is
expected to result in reduced cash interest expense and noncash
discount amortization of $10.9 million over the remaining
term of the Convertible Notes. Of the consideration paid to
repurchase the Convertible Notes, $204,000 was allocated to the
equity component. This is considered to be a reacquisition of a
portion of the equity component of the Convertible Notes and as
such is recorded as a reduction of additional paid in capital.
Additionally, the related bond hedge and warrant transactions
were proportionately unwound at a cost of $97,000.
As of December 31, 2009, $154.5 million in par value
was outstanding under the Convertible Notes, no amounts were
outstanding under the revolving line of credit the Company
maintained and no amounts were outstanding under the
Multicurrency Facility.
The Company’s primary cash needs on both a short-term and
long-term basis are for the payment of salaries and fringe
benefits, hiring and recruiting expenses, business development
costs, capital expenditures, acquisitions and facility-related
expenses. The Company believes that its existing capital
resources, together with cash flows from operations and
borrowing capacity under the Facility and the Multicurrency
Facility, will be sufficient to meet its foreseeable cash needs
for both the short and long term.
The Company has not historically experienced regular liquidity
or collections issues with the large majority of its customers.
However, the Company does have contracts with biotechnology and
small pharmaceutical companies, some of which are dependent upon
external financing to fund their contractual commitments. The
Company is continuing to monitor the financial status of its
customers.
As more of the Company’s revenues are generated in
locations other than the U.S., repatriation of those cash flows
in a tax efficient manner is increasingly challenging.
In the future, the Company will continue to consider the
acquisition of businesses to enhance its service offerings,
therapeutic base and global presence. Any such acquisitions may
require additional external financings and the Company may from
time to time seek to obtain funds from public or private
issuances of equity or debt securities. There can be no
assurance that such financings will be available on terms
acceptable to the Company.
32
Contractual
Obligations
Future minimum payments for all contractual obligations for
years subsequent to December 31, 2009 are as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year
|
|
|
Years
|
|
|
Years
|
|
|
Years
|
|
|
|
|
|
|
|
2010
|
|
|
2011-2012
|
|
|
2013-2014
|
|
|
After 2015
|
|
|
Total
|
|
|
|
|
(In thousands)
|
|
|
|
|
Capital lease obligations (including interest)
|
|
$
|
55
|
|
|
$
|
35
|
|
|
$
|
—
|
|
|
$
|
—
|
|
|
$
|
90
|
|
|
Operating Leases
|
|
|
18,476
|
|
|
|
28,736
|
|
|
|
16,695
|
|
|
|
25,091
|
|
|
|
88,998
|
|
|
Debt payments(1)
|
|
|
—
|
|
|
|
154,500
|
|
|
|
—
|
|
|
|
—
|
|
|
|
154,500
|
|
|
Interest on debt(2)
|
|
|
5,214
|
|
|
|
8,039
|
|
|
|
—
|
|
|
|
—
|
|
|
|
13,253
|
|
|
Reserve for uncertain tax positions, including interest and
penalties(3)
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total
|
|
$
|
23,745
|
|
|
$
|
191,310
|
|
|
$
|
16,695
|
|
|
$
|
25,091
|
|
|
$
|
256,841
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Short-term obligations arising in the ordinary course of
business are excluded from the above table.
|
|
|
|
(1) |
|
Under the terms of the Convertible Notes, the Convertible Notes
are convertible into shares of the Common Stock upon the
occurrence of various factors described in the Liquidity and
Capital Resources section of Management’s Discussion and
Analysis. The above table assumes no conversion with the
remaining principal amount of the Convertible Notes paid at the
maturity date of July 15, 2012. |
| |
|
(2) |
|
The interest rate used in the calculation of interest was 3.375%
which represents the cash coupon obligation. |
| |
|
(3) |
|
Reserves for uncertain tax positions of $1.8 million have
not been reflected in the above table due to the inherent
uncertainty as to the amount and timing of settlement, which is
contingent upon the occurrence of possible future events, such
as examinations and determinations by various tax authorities. |
Critical
Accounting Policies and Estimates
The preparation of financial statements in conformity with
accounting principles generally accepted in the United States of
America requires management to make significant estimates and
assumptions that affect the reported Consolidated Financial
Statements for a particular period. Actual results could differ
from those estimates.
Revenue
Recognition
The majority of the Company’s net service revenues are
based on fixed-price contracts calculated on a proportional
performance basis (also referred to herein as
“percentage-of-completion”)
based upon assumptions regarding the estimated total costs for
each contract. The Company also recognizes revenue under
units-based contracts by multiplying units completed by the
applicable contract
per-unit
price. Additionally, work is performed under
time-and-materials
contracts, recognizing revenue as hours are worked based on the
hourly billing rate for each contract.
Percentage-of-Completion
With respect to certain fixed price contracts, costs are
incurred for performance of each contract and compared to the
estimated budgeted costs for that contract to determine a
percentage of completion on the contract. The percentage of
completion is then multiplied by the contract value to determine
the amount of revenue recognized. The contract value equals the
value of the services to be performed under the contract as
determined by aggregating the labor hours estimated to be
incurred to perform the tasks in the contract at the agreed
rates. Contract value excludes the value of third-party and
other pass-through costs. As the work progresses, original
estimates might be changed as a result of management’s
regular contract review process.
Management regularly reviews the budget on each contract to
determine if the budgeted costs accurately reflect the costs
that the Company will incur for contract performance. The
Company reviews each contract’s
33
performance to date, current cost trends and circumstances
specific to each contract. The Company estimates its remaining
costs to complete the contract based on a variety of factors,
including:
|
|
|
| |
•
|
Actual costs incurred to date and the work completed as a result
of incurring the actual costs;
|
| |
| |
•
|
The remaining work to be completed based on the timeline of the
contract as well as the number of incomplete tasks in the
contract; and
|
| |
| |
•
|
Factors that could change the rate of progress of future
contract performance.
|
Examples of factors included in the review process that could
change the rate of progress of future contract performance are:
patient enrollment rate, changes in the composition of staff on
the project or other customer requirements, among other things.
Based on these contract reviews, the Company adjusts its cost
estimates. Adjustments to net service revenue resulting from
changes in cost estimates are recorded on a cumulative basis in
the period in which the revisions are made. When estimates
indicate a loss, such loss is provided in the current period in
its entirety. While the Company routinely adjusts cost estimates
on individual contracts, the Company’s estimates and
assumptions historically have been accurate in all material
respects in the aggregate. The Company expects the estimates and
assumptions to remain accurate in all material respects in the
aggregate in future periods.
A contract amendment, which results in revisions to net service
revenues and cost estimates, is recognized in the
percentage-of-completion
calculations beginning in the period in which the parties reach
written agreement to the amendment. (See also Company Overview
section of MD&A for a description of the contract amendment
process.) Historically, the aggregate value of contract
amendments signed in any year represents 15% to 20% of annual
sales. Although the majority of the Company’s contract
amendments relate to future services, the Company and its
customers may execute contract amendments for services that the
Company already has performed. In these circumstances, net
service revenue from past services performed is recognized in
the current period. Historically, the impact of such amendments
on results of operations has not been material.
Under the Company’s policy, project teams are not
authorized to engage in tasks outside the scope of the contract
without prior management approval. In limited situations,
management may authorize the project team to commence work on
activities outside the contract scope while the Company and its
customer negotiate and finalize the contract amendment. When
work progresses on unsigned, unprocessed contract amendments,
the Company reviews the direct costs incurred, and, where
material defers such costs on the balance sheet. In addition,
the impact of such costs on the estimates to complete is
considered and, where material, the estimates are adjusted.
Historically, neither the deferred costs nor the impact on
estimates have been material.
The Company believes that total costs constitute the most
appropriate indicator of the performance of fixed price
contracts because the costs relate primarily to the amount of
labor hours incurred to perform the contract. The customer
receives the benefit of the work performed throughout the
contract term and is obligated to pay for services once
performed. Accordingly, the Company believes that an input
measure of cost is a reasonable surrogate for an output measure
under the proportional performance model and is consistent with
the revenue recognition concepts issued by the SEC.
Units-Based
Although the majority of the Company’s contracts are
fixed-price and net service revenues are calculated on a
percentage of completion methodology as discussed above, the
Company has seen increasing demand from its customers to move to
a units-based contract methodology in new contracts. It is the
Company’s intent to structure more of its contracts under a
units-based methodology for calculating net service revenues so
the Company expects the percentage of contracts under which net
service revenues are recognized using units-based methodology to
increase in future periods. Under a units-based contract
methodology, amounts recognized as net service revenues are
calculated based on units completed in the period multiplied by
a unit value or selling price that is outlined in the contract.
For a units-based contract, a typical unit could include such
things as completion of a monitoring visit, monthly site
management units or case report form pages entered. The Company
tracks the units completed for each unit
34
category included in the contract. Net service revenue is
recognized monthly based on the units actually completed in the
period at the agreed upon unit value or selling price. Net
service revenue is recognized only up to the number of units
contained in each contract. If the Company completes or expects
to complete units over and above the number of units initially
estimated and contained in the contract, a contract amendment is
generated to reflect the additional units needed.
A contract amendment, which results in revisions to net service
revenues and expected units or unit values, is recognized in the
unit-based net service revenue calculations beginning in the
period in which the parties agree to the amendment. (See also
Company Overview section of MD&A for a description of the
contract amendment process.)
The Company believes that under certain types of contracts the
value of the work performed is best captured by calculating net
service revenues using the value of units completed. The Company
believes that units-based revenue recognition is consistent with
the revenue recognition concepts issued by the SEC.
As the Company provides services on projects, it also incurs
third-party and other pass-through costs, which are reimbursable
by its customers pursuant to the contract. The revenues and
costs from these third-party and other pass-through costs are
reflected in the Company’s Consolidated Statements of
Operations under the line items titled “Reimbursable
out-of-pocket
revenues” and “Reimbursable
out-of-pocket
costs”, respectively.
Direct
Costs
Direct costs consist of compensation and related fringe benefits
for project-related associates, unreimbursed project-related
costs and an allocated portion of indirect costs, which
primarily includes facilities-related costs and information
systems costs. Labor costs represent over 80% of total direct
costs with the allocated portion of indirect costs and other
expenses representing the remainder. To determine the allocated
portion of indirect costs, the Company calculates an allocation
percentage based on the relationship between billable associate
salaries and total salaries. The remaining indirect costs are
allocated to SG&A.
Because the Company’s business is labor intensive, direct
costs historically have increased with an increase in net
service revenues. The Company, however, has not experienced any
material variations in the relationship between direct costs and
net service revenues for the fiscal years ended 2009, 2008, and
2007. The following factors, among others, may cause direct
costs to decrease as a percentage of net service revenues:
|
|
|
| |
•
|
Higher utilization rates for billable employees; and
|
| |
| |
•
|
The ability to complete contracted work more efficiently than
estimated by the Company.
|
The following factors, among others, will cause direct costs to
increase as a percentage of net service revenues:
|
|
|
| |
•
|
The occurrence of cost overruns from increased time to complete
contract performance;
|
| |
| |
•
|
Lower utilization rates for billable employees;
|
| |
| |
•
|
Increased costs due to higher-paid employees or contractors
performing contract services; and
|
| |
| |
•
|
Pricing pressure from increased competition.
|
Other
Costs
Selling, general and administrative expenses consist of
compensation and related fringe benefits for sales and
administrative employees and professional services, as well as
unallocated costs related to facilities, information systems and
other costs.
Depreciation and amortization expenses consist of depreciation
and amortization costs recorded on a straight-line method over
the estimated useful life of the property or equipment and
internally developed software. Finite-lived intangible assets
are generally amortized on an accelerated basis based on the
discounted cash flow calculations used in the valuation of the
asset.
35
Accounts
Receivable/Allowance for Doubtful Accounts
Billed accounts receivable represent amounts for which invoices
have been issued to customers. Unbilled accounts receivable are
amounts recognized as revenue for which invoices have not yet
been issued to customers. Advance billings represent amounts
billed or payment received for which revenues have not yet been
earned. The Company maintains an allowance for doubtful accounts
receivable based on historical evidence of accounts receivable
collections and specific identification of accounts receivable
that might pose collection problems. The bad debt reserve is
monitored on a regular basis and adjusted as circumstances
warrant. The Company’s allowance for doubtful accounts has
been sufficient to cover any bad debt write-offs. The Company
will continue to monitor its bad debt exposure and adjust bad
debt reserves as necessary.
If the Company is unable to collect all or part of its
outstanding receivables, there could be a material impact to the
Company’s Consolidated Statements of Operations or
financial position.
Goodwill
and Intangible Assets
The Company analyzes goodwill and other finite-lived intangible
assets to determine any potential impairment loss annually in
the fourth quarter, unless conditions exist that require an
updated analysis on an interim basis. During 2009, management
concluded that there was no evidence of events triggering an
interim evaluation.
A fair value approach is used to test goodwill for impairment.
The fair value approach compares estimates related to the fair
value of the reporting unit with the unit’s carrying
amount, including goodwill. The fair value is determined using
both the market approach and the income approach using projected
discounted cash flows. If the carrying amount of the reporting
unit exceeds the fair value, the amount of the impairment loss
must be measured. The Company has five reporting units that were
tested for impairment: Early Stage, and the Late Stage reporting
units consisting of: Clinical and Data Monitoring, Project
Management and Late Phase Services, Regulatory, Site and Medical
Affairs and Biostatistics and Statistical Programming. The
Company made some changes to its four Late Stage segment
reporting units in 2009 in conjunction with its internal
reorganization. The Company’s Late Stage reporting units
have historically had, individually and in the aggregate, a
significant amount of fair value in excess of carrying value.
The Company evaluated the goodwill for the Late Stage reporting
units under the previous reporting unit structure and determined
that there was no indication of impairment at the time the
reporting units were reorganized. The Company determined its
reporting units using the guidance related to accounting for
goodwill. This guidance defines a reporting unit as the
components of operating segments for which discrete financial
information is available and regularly reviewed by management.
At December 31, 2009 and 2008, the fair value of all
reporting units exceeded the carrying value, resulting in no
goodwill impairment charge. As of December 31, 2009, the
goodwill assigned to each unit as well as the percentage by
which their fair values exceeded their carrying values are as
follows:
| |
|
|
|
|
|
|
|
|
|
|
|
Goodwill
|
|
|
Fair Value in Excess of
|
|
|
|
|
Allocated
|
|
|
Carrying Value
|
|
|
|
|
(In thousands)
|
|
|
|
|
Clinical and Data Monitoring
|
|
$
|
160,229
|
|
|
|
80
|
%
|
|
Project Management & Late Phase
|
|
|
24,124
|
|
|
|
65
|
%
|
|
Regulatory, Site and Medical Affairs
|
|
|
27,984
|
|
|
|
50
|
%
|
|
Biostatistics and Statistical Programming
|
|
|
12,939
|
|
|
|
80
|
%
|
|
Early Stage
|
|
|
18,313
|
|
|
|
40
|
%
|
|
|
|
|
|
|
|
|
|
|
|
Total
|
|
$
|
243,589
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The estimate of fair value is inherently subjective and requires
the Company to make a number of assumptions and projections.
These assumptions and projections relate to revenue growth
rates, profit margin percentages, discount rates, perpetuity
growth rates, future capital expenditures and future market
conditions, among other things. The most significant assumptions
used in the 2009 goodwill impairment testing were revenue growth
rates and the weighted average cost of capital (WACC). The
revenue growth rates are forward looking and by their nature are
uncertain. The Company may or may not be able to achieve the
estimated growth rates and/or margin rates. The rates used were,
in general, approximately the same for the Early Stage reporting
unit and more conservative than
36
have been used in the past for the Late Stage reporting units
because of the current economy and reduced long term outlook for
the pharmaceutical and CRO industries. The WACC rate for the
Early Stage reporting unit is higher than the rate used in 2008.
The WACC rates used for the Late Stage units are not materially
different with those used in prior year evaluations. If factors
change and the Company employs different assumptions in
estimating fair value of its long-lived assets, the estimated
fair value of these assets could change and result in impairment
charges. See Item 1A, Risk Factors for discussion of risks
and potential risks that may impact future net service revenues,
among other things, of the Company.
Internally
Developed Software
Pursuant to the accounting guidance related to the
capitalization of software developed or obtained for internal
use, the Company capitalizes costs incurred to internally
develop software used primarily in the Company’s
proprietary clinical trial and data management systems, and
amortizes these costs over the useful life of the product, not
to exceed five years. Internally developed software represents
software in the application development stage, and there is no
assurance that the software development process will produce a
final product for which the fair value exceeds its carrying
value. Internally developed software is an intangible asset
subject to impairment write-downs whenever events indicate that
the carrying value of the software may not be recoverable. As
with other long-lived assets, this asset is reviewed at least
annually to determine the appropriateness of the carrying value
of the asset and the estimated useful lives. Assessing the fair
value of the internally developed software requires estimates
and judgment on the part of management. As discussed in
Note 1 to the Consolidated Financial Statements, internally
developed software is amortized over its estimated useful life
of five years. The Company believes the useful life established
remains reasonable.
Tax
Valuation Allowance and Tax Liabilities
The Company follows the guidance related to accounting for
uncertainties in income taxes. This guidance requires
significant judgment in determining what constitutes an
individual tax position as well as assessing the outcome of each
tax position. Changes in judgments as to recognition or
measurement of tax positions can materially affect the estimate
of the effective tax rate, and, consequently, the Company’s
operating results. The Company considers many factors when
evaluating and estimating tax positions and tax benefits, which
may require periodic adjustments and which may not accurately
anticipate actual outcomes. In addition, the calculation of tax
liabilities involves dealing with uncertainties in the
application of complex tax regulations in a multitude of
jurisdictions. At December 31, 2009, the Company has
recorded a gross liability for uncertain income tax positions of
$1.8 million. If events occur and the payment of these
amounts ultimately proves to be unnecessary, the reversal of
liabilities would result in tax benefits being recognized in the
period when it is determined the liabilities are no longer
necessary. If the calculation of liability related to uncertain
tax positions proves to be more or less than the ultimate
assessment, a tax expense or benefit to expense, respectively,
would result.
The Company provides for income taxes on all transactions that
have been recognized in the Consolidated Financial Statements in
accordance with accounting guidance related to accounting for
income taxes. Specifically, the Company estimates its tax
liability based on current tax laws in the statutory
jurisdictions in which it operates. Because the Company conducts
business on a global basis, its effective tax rate has and will
continue to depend upon the geographic distribution of its
pre-tax earnings (losses) among jurisdictions with varying tax
rates. These estimates include judgments about deferred tax
assets and liabilities resulting from temporary differences
between assets and liabilities recognized for financial
reporting purposes and such amounts recognized for tax purposes.
The Company has assessed the realization of deferred tax assets
and a valuation allowance has been established based on an
assessment that it is more likely than not that realization
cannot be assured. The ultimate realization of this tax benefit
is dependent upon the generation of sufficient pretax income in
the respective tax jurisdictions. If estimates prove inaccurate
or if the tax laws change unfavorably, significant revisions in
the valuation allowance may be required in the future.
Stock-based
Compensation
The Company follows the guidance related to accounting for
share-based payments. The Company generally uses the
straight-line method of recording compensation expense relative
to share-based payment, unless awards
37
have graded vesting features and the expense recorded under the
straight-line method is not adequate. In those situations,
compensation cost is recognized separately over each tranche.
Stock-based compensation expense is recorded primarily in
general and administrative expenses in the Company’s
Consolidated Statements of Operations as the majority of the
stock option expense relates to options granted to executives.
If factors change and the Company employs different assumptions
in the calculation of stock-based compensation in future
periods, the compensation expense that the Company records may
differ significantly from the expense recorded in the current
period.
New
Accounting Pronouncements
For a discussion of New Accounting Pronouncements, see
Note 1 to the Company’s Consolidated Financial
Statements.
Cautionary
Statement for Forward-Looking Information
Certain statements contained in this Annual Report on
Form 10-K
that are not historical facts constitute forward-looking
statements within the meaning of the Private Securities
Litigation Reform Act of 1995 and are intended to be covered by
the safe harbors created by that Act. Reliance should not be
placed on forward-looking statements because they involve known
and unknown risks, uncertainties and other factors that may
cause actual results, performance or achievements to differ
materially from those expressed or implied. Any forward-looking
statement speaks only as of the date made. The Company
undertakes no obligation to update any forward-looking
statements to reflect events or circumstances arising after the
date on which they are made.
Statements concerning expected financial performance, on-going
business strategies and possible future action which the Company
intends to pursue to achieve strategic objectives constitute
forward-looking information. Implementation of these strategies
and the achievement of such financial performance are each
subject to numerous conditions, uncertainties and risk factors.
Factors that could cause actual performance to differ materially
from these forward-looking statements include those Risk Factors
set forth in Item 1A of this Annual Report on
Form 10-K.
|
|
|
ITEM 7A.
|
QUANTITATIVE
AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
|
Interest
Rates
The Company is exposed to changes in interest rates on its
amounts outstanding under the Facility and Multicurrency
Facility. At December 31, 2009, no amounts were outstanding
under either the Old Facility or the Multicurrency Facility and
as of the date of this
Form 10-K,
no amounts are outstanding under the Facility.
Foreign
Currency
The Company operates on a global basis and is therefore exposed
to various types of currency risks. Two specific transaction
risks arise from the nature of the contracts the Company
executes with its customers. From time to time contracts are
denominated in a currency different than the particular local
currency. This contract currency denomination issue is
applicable only to a portion of the contracts executed by the
Company. The first risk occurs as revenue recognized for
services rendered is denominated in a currency different from
the currency in which the subsidiary’s expenses are
incurred. As a result, the subsidiary’s net service
revenues and resultant net income or loss can be affected by
fluctuations in exchange rates.
The second risk results from the passage of time between the
invoicing of customers under these contracts and the ultimate
collection of customer payments against such invoices. Because
the contract is denominated in a currency other than the
subsidiary’s local currency, the Company recognizes a
receivable at the time of invoicing at the local currency
equivalent of the foreign currency invoice amount. Changes in
exchange rates from the time the invoice is prepared until the
payment from the customer is received will result in the Company
receiving either more or less in local currency than the local
currency equivalent of the invoice amount at the time the
invoice was prepared and the receivable established. This
difference is recognized by the Company as a foreign currency
38
transaction gain or loss, as applicable, and is reported in
Other Income (Expense) in the Consolidated Statements of
Operations.
A third type of transaction risk arises from transactions
denominated in multiple currencies between any two of the
Company’s various subsidiary locations. For each
subsidiary, the Company maintains an intercompany receivable and
payable, which is denominated in multiple currencies. Changes in
exchange rates from the time the intercompany receivable/payable
balance arises until the balance is settled or measured for
reporting purposes, results in exchange rate gains and losses.
This intercompany receivable/payable arises when work is
performed by a Kendle location in one country on behalf of a
Kendle location in a different country under contract with the
customer. Additionally, there are occasions when funds are
transferred between subsidiaries for working capital purposes.
Where possible, the Company settles these intercompany balances
frequently to reduce this exposure. The foreign currency
transaction gain or loss is reported in Other Income (Expense)
in the Consolidated Statements of Operations.
In 2009, the Company recorded total foreign exchange gains of
$6.3 million compared to losses of approximately
$3.4 million in 2008 related to the risks described above.
The Company’s Consolidated Financial Statements are
denominated in U.S. dollars. Accordingly, changes in
exchange rates between the applicable foreign currency and the
U.S. dollar will affect the translation of each foreign
subsidiary’s financial results into U.S. dollars for
purposes of reporting the Consolidated Financial Statements. The
Company’s foreign subsidiaries translate their financial
results from local currency into U.S. dollars as follows:
income statement accounts are translated at average exchange
rates for the period; balance sheet asset and liability accounts
are translated at end of period exchange rates; and equity
accounts are translated at historical exchange rates.
Translation of the balance sheet in this manner affects the
shareholders’ equity account referred to as the foreign
currency translation adjustment account. This account exists
only in the foreign subsidiaries’ U.S. dollar balance
sheet and is necessary to keep the foreign subsidiaries’
balance sheet stated in U.S. dollars in balance. Cumulative
foreign currency translation adjustments, which are reported as
a separate component of Shareholders’ Equity, were
approximately $22.2 million and $14.5 million at
December 31, 2009 and 2008, respectively.
Foreign
Currency Hedges
In 2007, the Company entered into foreign currency hedging
transactions to mitigate exposure in movements between the
U.S. dollar and British Pounds Sterling and
U.S. dollar and Euro. The hedging transactions were
designed to mitigate the Company’s exposure related to two
intercompany notes between the Company’s
U.S. subsidiary, as lender, and the Company’s
subsidiaries in each of the United Kingdom and Germany. The note
between the Company’s U.S. subsidiary and United
Kingdom subsidiary is denominated in Pounds Sterling and had an
outstanding principal amount of approximately $41.4 million
at December 31, 2008. In January 2009, the Company
eliminated a substantial portion of this note leaving a
remaining balance outstanding at December 31, 2009 of
$6.6 million. The hedge agreement related to this loan was
terminated in January resulting in $17.3 million of cash
proceeds. The Company recorded foreign exchange gains of
$997,000 related to the Pound Sterling loan in 2009. Prior to
the termination of the Pound Sterling hedge in January 2009, the
Company recorded foreign exchange losses of $1.3 million.
The note between the Company’s U.S. subsidiary and
German subsidiary was denominated in Euros and had an
outstanding principal amount of approximately $22.8 million
at December 31, 2008. This note was repaid in full in March
2009, and the related hedge agreement was terminated at the same
time. Foreign exchange losses of $1.5 million and gains of
$741,000 were recorded related to the Euro note and the Euro
hedge agreement, respectively, prior to their repayment and
termination in March 2009. The hedge agreements were not
designated for hedge accounting treatment under accounting
guidance related to derivatives and all changes in the fair
market value of the hedge were recorded in the Company’s
Consolidated Statements of Operations.
In 2008, the Company recorded gains of approximately
$1.4 million on the Euro hedge transactions and gains of
approximately $18.0 million on the Pound Sterling
transaction related to the changes in the fair market value of
the hedge. The gains on the fair market value of the hedge were
offset by foreign exchange losses of approximately
$1.3 million on the change in value of the Euro
intercompany note and $15.7 million on the change in value
of the Pound Sterling intercompany note.
39
Fair
Value of Derivative Transactions
The notional amount and fair value of the Company’s foreign
currency hedges at December 31, 2008 were $77,955 and
$17,853, respectively. As discussed above, the foreign currency
hedge agreements were terminated in 2009 and are no longer
outstanding.
|
|
|
ITEM 8.
|
FINANCIAL
STATEMENTS AND SUPPLEMENTARY DATA
|
The Financial Statements and Supplementary Data called for by
this Item are set forth in pages F-1 to F-36, which are
incorporated herein by reference.
|
|
|
ITEM 9.
|
CHANGES
IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND
FINANCIAL DISCLOSURE
|
None to report.
|
|
|
ITEM 9A.
|
CONTROLS
AND PROCEDURES
|
Management’s
Evaluation of Disclosure Controls and Procedures
Based on the Company’s most recent evaluation, which was
completed as of the end of the period covered by this Annual
Report on
Form 10-K,
our CEO/Chairman (principal executive officer) and Chief
Financial Officer (principal financial and accounting officer)
concluded that the Company’s disclosure controls and
procedures (as defined in
Rules 13a-15(e)
and
15d-15(e) of
the Securities Exchange Act of 1934, as amended) are effective.
(a) Management’s
Report on Internal Control over Financial
Reporting
The Company’s management is responsible for establishing
and maintaining adequate internal controls designed to provide
reasonable assurance regarding the reliability of financial
reporting and the preparation of financial statements for
external purposes in accordance with accounting principles
generally accepted in the United States of America
(“GAAP”). The Company’s internal control over
financial reporting includes those policies and procedures that:
1. pertain to the maintenance of records that, in
reasonable detail, accurately and fairly reflect the
transactions and dispositions of assets of the Company;
2. provide reasonable assurance that transactions are
recorded as necessary to permit preparation of consolidated
financial statements in accordance with GAAP and that receipts
and expenditures of the Company are being made only in
accordance with the authorizations of management and the
Directors of the Company; and
3. provide reasonable assurance regarding prevention or
timely detection of unauthorized acquisition, use or disposition
of the Company’s assets that could have a material effect
on the Consolidated Financial Statements.
All internal control systems, no matter how well designed, have
inherent limitations, including the possibility of human error,
collusion and the improper overriding of controls by management.
Accordingly, even those systems determined to be effective can
provide only reasonable, but not absolute assurance with respect
to financial statement preparation and presentation. Further,
because of changes in conditions, the effectiveness of internal
control may vary over time.
As required by Section 404 of the Sarbanes Oxley Act of
2002, management assessed the effectiveness of the
Company’s internal control over financial reporting as of
December 31, 2009. Management’s assessment is based on
the criteria established in the Internal Control —
Integrated Framework issued by the Committee of Sponsoring
Organizations of the Treadway Commission. Based upon our
assessment, management concludes that the Company maintained
effective internal control over financial reporting as of
December 31, 2009. Deloitte and Touche LLP, the
Company’s independent registered public accounting firm,
has issued an attestation report on the Company’s internal
control over financial reporting and as of December 31,
2009. This report appears below.
40
(b) Changes
in Internal Control Over Financial Reporting
There have been no changes in the Company’s internal
control over financial reporting during the most recently
completed fiscal quarter ended December 31, 2009 that have
materially affected, or are reasonably likely to materially
affect the Company’s internal control over financial
reporting.
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
/s/ Candace
Kendle
Candace
Kendle, PharmD
|
|
Chairman of the Board of Directors, Chief Executive Officer and
Principal Executive Officer
|
|
March 16, 2010
|
|
|
|
|
|
|
|
/s/ Keith
A. Cheesman
Keith
A. Cheesman
|
|
Senior Vice President, Chief Financial Officer and Principal
Financial Accounting Officer
|
|
March 16, 2010
|
41
REPORT
OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Shareholders of
Kendle International Inc.
Cincinnati, Ohio
We have audited the internal control over financial reporting of
Kendle International Inc. and subsidiaries (the
“Company”) as of December 31, 2009, based on
criteria established in Internal Control — Integrated
Framework issued by the Committee of Sponsoring Organizations of
the Treadway Commission. The Company’s management is
responsible for maintaining effective internal control over
financial reporting and for its assessment of the effectiveness
of internal control over financial reporting, included in the
accompanying Management’s Report on Internal Control over
Financial Reporting. Our responsibility is to express an opinion
on the Company’s internal control over financial reporting
based on our audit.
We conducted our audit in accordance with the standards of the
Public Company Accounting Oversight Board (United States). Those
standards require that we plan and perform the audit to obtain
reasonable assurance about whether effective internal control
over financial reporting was maintained in all material
respects. Our audit included obtaining an understanding of
internal control over financial reporting, assessing the risk
that a material weakness exists, testing and evaluating the
design and operating effectiveness of internal control based on
the assessed risk, and performing such other procedures as we
considered necessary in the circumstances. We believe that our
audit provides a reasonable basis for our opinion.
A company’s internal control over financial reporting is a
process designed by, or under the supervision of, the
company’s principal executive and principal financial
officers, or persons performing similar functions, and effected
by the company’s board of directors, management, and other
personnel to provide reasonable assurance regarding the
reliability of financial reporting and the preparation of
financial statements for external purposes in accordance with
generally accepted accounting principles. A company’s
internal control over financial reporting includes those
policies and procedures that (1) pertain to the maintenance
of records that, in reasonable detail, accurately and fairly
reflect the transactions and dispositions of the assets of the
company; (2) provide reasonable assurance that transactions
are recorded as necessary to permit preparation of financial
statements in accordance with generally accepted accounting
principles, and that receipts and expenditures of the company
are being made only in accordance with authorizations of
management and directors of the company; and (3) provide
reasonable assurance regarding prevention or timely detection of
unauthorized acquisition, use, or disposition of the
company’s assets that could have a material effect on the
financial statements.
Because of the inherent limitations of internal control over
financial reporting, including the possibility of collusion or
improper management override of controls, material misstatements
due to error or fraud may not be prevented or detected on a
timely basis. Also, projections of any evaluation of the
effectiveness of the internal control over financial reporting
to future periods are subject to the risk that the controls may
become inadequate because of changes in conditions, or that the
degree of compliance with the policies or procedures may
deteriorate.
In our opinion, the Company maintained, in all material
respects, effective internal control over financial reporting as
of December 31, 2009, based on the criteria established in
Internal Control — Integrated Framework issued by the
Committee of Sponsoring Organizations of the Treadway Commission.
We have also audited, in accordance with the standards of the
Public Company Accounting Oversight Board (United States), the
consolidated financial statements as of and for the year ended
December 31, 2009 of the Company and our report dated
March 16, 2010 expressed an unqualified opinion on those
financial statements and included an explanatory paragraph
relating to the retrospective application of the new accounting
guidance for convertible debt instruments.
/s/ DELOITTE &
TOUCHE LLP
Cincinnati, Ohio
March 16, 2010
42
|
|
|
ITEM 9B.
|
OTHER
INFORMATION
|
On March 15, 2010, the Company terminated its existing
credit agreement with the several lenders from time to time
party thereto and UBS Securities AG, Stamford Branch, as
Administrative Agent (including all amendments and related
agreements, the “Old Facility”) and entered into a
Credit Agreement (the “Credit Agreement”) with various
lenders and JPMorgan Chase Bank, N.A. (“JPMorgan”), a
Guaranty (the “Guaranty”) executed by various
subsidiaries of the Company in favor of JPMorgan and a Pledge
and Security Agreement (the “Security Agreement”) with
various subsidiaries of the Company and JPMorgan.
See Liquidity and Capital Resources section of Management’s
Discussion and Analysis of Financial Condition and Results of
Operations on page 31 under Item 7 of this
Form 10-K
for a discussion regarding the termination of the Old Facility
and a description of the Credit Agreement, Guaranty and Security
Agreement (collectively referred to throughout this
Form 10-K
as the “Facility”), which description is qualified in
its entirety by reference to the Credit Agreement, Guaranty and
Security Agreement, filed herewith as Exhibits 10.14, 10.15
and 10.16, respectively, and incorporated herein by reference.
PART III
|
|
|
ITEM 10.
|
DIRECTORS,
EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
|
DIRECTORS
OF THE COMPANY
The name, age and background information for each of the
Company’s Directors is set forth in the section titled
“Election of Directors” contained in the
Company’s definitive Proxy Statement to be filed with the
SEC and is incorporated herein by reference.
AUDIT
COMMITTEE
Information regarding the members of the audit committee and the
audit committee financial expert is set forth in the sections
titled “Board of Directors” and “Audit
Committee” under the heading “Governance of the
Company” in the Company’s definitive Proxy Statement
to be filed with the SEC. The information in these sections is
incorporated herein by reference.
EXECUTIVE
OFFICERS OF THE COMPANY
The Executive Officers of the Company at March 1, 2010,
were as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Executive
|
|
Name
|
|
Age
|
|
Position
|
|
Officer Since
|
|
|
|
Candace Kendle, PharmD
|
|
63
|
|
Chief Executive Officer and Chairman of the Board of Directors
|
|
1989
|
|
Christopher C. Bergen
|
|
59
|
|
Executive Vice President and Chief Administrative Officer
|
|
1989
|
|
Keith A. Cheesman
|
|
50
|
|
Senior Vice President and Chief Financial Officer
|
|
2009
|
|
Simon S. Higginbotham
|
|
49
|
|
Senior Vice President and Chief Marketing Officer
|
|
2004
|
|
Stephen A. Cutler, PhD
|
|
50
|
|
Senior Vice President and Chief Operating Officer
|
|
2009
|
Background information regarding Dr. Kendle and
Mr. Bergen is set forth in the sections titled
“Election of Directors” and “Securities Ownership
of Management” in the Company’s definitive Proxy
Statement to be filed with the SEC. Background information
regarding Mr. Cheesman, Mr. Higginbotham and
Dr. Cutler is set forth in the notes to the table within
the section titled “Securities Ownership of
Management” contained in the Company’s definitive
Proxy Statement to be filed with the SEC. This information is
incorporated herein by reference.
43
SECTION 16(A)
BENEFICIAL OWNERSHIP REPORTING COMPLIANCE
Information on compliance with Section 16(a) of the
Exchange Act is set forth in the section titled
“Section 16(a) Beneficial Ownership Reporting
Compliance” under the heading entitled “Securities
Ownership” in the Company’s definitive Proxy Statement
to be filed with the SEC and is incorporated herein by reference.
CODE OF
ETHICS
The Company has adopted a Code of Ethics and Conduct, which
applies to the Company’s employees, including its Chief
Executive Officer and its principal financial and accounting
officer. The Code of Ethics and Conduct is available on the
Company’s web site at www.kendle.com. Amendments to
the Code of Ethics and Conduct will be posted to the
Company’s web site. In addition, the Company will make
available, free of charge, to any person, a copy of its Code of
Ethics and Conduct upon written request submitted to the
Company. This written request should be addressed to the
Company’s Secretary at the Company’s principal
executive offices.
|
|
|
ITEM 11.
|
EXECUTIVE
COMPENSATION
|
Information required by this report is set forth in the
following sections under the heading titled “Governance of
the Company” in the Company’s definitive Proxy
Statement to be filed with the SEC, which information is
incorporated herein by reference:
|
|
|
| |
•
|
“Compensation Committee Interlocks and Insider
Participation”; and
|
| |
| |
•
|
“Compensation of Directors”.
|
Information required by this report is set forth in the
“Executive Compensation” section in the Company’s
definitive Proxy Statement to be filed with the SEC. The
“Executive Compensation” section includes information
under the following headings, which information is incorporated
herein by reference.
|
|
|
| |
•
|
“Compensation Discussion and Analysis:;
|
| |
| |
•
|
“Compensation Committee Report”;
|
| |
| |
•
|
“Summary Compensation Table”;
|
| |
| |
•
|
“Grants of Plan-Based Awards”;
|
| |
| |
•
|
“Outstanding Equity Awards at Fiscal Year End;
|
| |
| |
•
|
“Option Exercises and Stock Vested”;
|
| |
| |
•
|
“Nonqualified Deferred Compensation; and
|
| |
| |
•
|
“Potential Payments Upon Termination or
Change-in-Control”.
|
|
|
|
ITEM 12.
|
SECURITY
OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND
RELATED STOCKHOLDER MATTERS
|
Information on the number of shares beneficially owned by each
Director and by all Directors and Executive Officers as a group
is set forth in the section titled “Securities Ownership of
Management” under the heading titled “Securities
Ownership” in the Company’s definitive Proxy Statement
to be filed with the SEC. The information set forth in such
section is incorporated herein by reference.
Information on the number of shares beneficially owned by any
person who is known to the Company to be the beneficial owner of
more than five percent of the Company’s Common Stock is set
forth in the section titled “Principal Shareholders”
under the heading titled “Securities Ownership” in the
Company’s definitive Proxy Statement to be filed with the
SEC. The information set forth in such section is incorporated
herein by reference.
44
The following table presents summary information at
December 31, 2009, with respect to all of the
Company’s equity compensation plans.
Equity
Compensation Plan Information
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(c)
|
|
|
|
|
|
|
|
|
|
|
Number of Securities
|
|
|
|
|
(a)
|
|
|
|
|
|
Remaining Available
|
|
|
|
|
Number of
|
|
|
|
|
|
for Future Issuance
|
|
|
|
|
Securities to be
|
|
|
(b)
|
|
|
Under Equity
|
|
|
|
|
Issued upon
|
|
|
Weighted-Average
|
|
|
Compensation Plans
|
|
|
|
|
Exercise of
|
|
|
Exercise Price
|
|
|
(Excluding
|
|
|
|
|
Outstanding
|
|
|
of Outstanding
|
|
|
Securities
|
|
|
|
|
Options, Warrants
|
|
|
Options, Warrants
|
|
|
Reflected in Column
|
|
|
Plan Category
|
|
and Rights(1)
|
|
|
and Rights(1)
|
|
|
(a))(2)
|
|
|
|
|
Equity compensation plans approved by security holders
|
|
|
379,875
|
|
|
$
|
16.44
|
|
|
|
769,300
|
|
|
Equity compensation plans not approved by security holders
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
Total
|
|
|
379,875
|
|
|
$
|
16.44
|
|
|
|
769,300
|
|
|
|
|
|
(1) |
|
Excludes the 2003 Directors’ Compensation Plan under
which no options, warrants or rights are granted. This plan has
been approved by shareholders for up to 75,000 shares. |
| |
|
(2) |
|
Represents shares available for issuance under the 2007 Stock
Incentive Plan. |
|
|
|
ITEM 13.
|
CERTAIN
RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR
INDEPENDENCE
|
TRANSACTIONS
WITH RELATED PERSONS
Information with respect to transactions with related persons,
if any, is contained under the caption “Review and Approval
of Transactions with Related Persons” under the heading
titled “Governance of the Company” in the
Company’s definitive Proxy Statement to be filed with the
SEC. The information set forth in such section is incorporated
herein by reference.
REVIEW,
APPROVAL OR RATIFICATION OF TRANSACTIONS WITH RELATED
PERSONS
Information with respect to the review, approval or ratification
of transactions with related persons is contained under the
caption “Review and Approval of Transactions with Related
Persons” under the heading titled “Governance of the
Company” in the Company’s definitive Proxy Statement
to be filed with the SEC. The information set forth in such
section is incorporated herein by reference.
PROMOTERS
AND CERTAIN CONTROL PERSONS
Not applicable.
DIRECTOR
INDEPENDENCE
The name of each Director who is independent is contained in the
section titled “Director Independence” under the
heading titled “Governance of the Company” in the
Company’s definitive Proxy Statement to be filed with the
SEC. The information set forth in such section is incorporated
herein by reference.
|
|
|
ITEM 14.
|
PRINCIPAL
ACCOUNTANT FEES AND SERVICES
|
Information required by this Item is contained in the sections
titled “Audit Committee’s Pre-Approval Policies and
Procedures” and “Fees Paid to Registered Public
Accounting Firm” under the heading titled
“Ratification of Appointment of Registered Public
Accounting Firm” in the Company’s definitive Proxy
Statement to be filed with the SEC and is incorporated herein by
reference.
45
PART IV
|
|
|
ITEM 15.
|
EXHIBITS AND
FINANCIAL STATEMENT SCHEDULES
|
(a) (1) and (2) — All financial statements
and schedules required to be filed by Item 8 of this Annual
Report on
Form 10-K
and included in this report are listed beginning on
page F-1.
No additional financial statements or schedules are being filed
as the required information is not applicable or because the
information is required and is included in the respective
financial statements or notes thereto.
(3) Exhibits — Exhibits set forth below that are
on file with the SEC are incorporated by reference as exhibits
hereto.
| |
|
|
|
|
Exhibit
|
|
|
|
Number
|
|
Description of Exhibit
|
|
|
|
|
2
|
.1
|
|
Stock Purchase Agreement between the Company and Charles River
Laboratories International, Inc. dated as of May 9, 2006
(Incorporated by reference to the
Form 8-K
filed with the Commission on May 12, 2006)
|
|
|
2
|
.2
|
|
First Amendment to the Stock Purchase Agreement dated as of
August 16, 2006 between the Company and Charles River
Laboratories, Inc. (Incorporated by reference to the
Company’s Current Report on
Form 8-K
filed with the Commission on August 18, 2006)
|
|
|
3
|
.1
|
|
Restated and Amended Articles of Incorporation. Incorporated by
reference to the Company’s Registration Statement
No. 333-30581
filed under the Securities Act of 1933.
|
|
|
3
|
.2
|
|
Amended and Restated Code of Regulations (Incorporated by
reference to the Company’s Current Report on
Form 8-K
filed with the Commission on November 16, 2009)
|
|
|
3
|
.3
|
|
Amendment of the Restated and Amended Articles of Incorporation
to Increase the Authorized Shares (Incorporated by reference to
the Company’s definitive Proxy Statement on
Schedule 14A filed with the Commission on April 14,
1999)
|
|
|
4
|
|
|
Specimen Common Stock Certificate (Incorporated by reference to
the Company’s Registration Statement
No. 333-30581
filed under the Securities Act of 1933)
|
|
|
4
|
.1
|
|
Shareholder Rights Agreement dated August 14, 2009 between
the Company and American Stock Transfer &
Trust Company LLC, as Rights Agent (Incorporated by
reference to the Company’s filing on
Form 8-A
filed with the Commission on August 20, 2009)
|
|
|
4
|
.2
|
|
Indenture dated March 31, 2007 between the Company and
LaSalle Bank National Association (Incorporated by reference to
the Company’s
Form S-3
filed with the Commission on April 9, 2007)
|
|
|
4
|
.3
|
|
Supplemental Indenture No. 1 dated July 16, 2007
between the Company and LaSalle Bank National Association
(Incorporated by reference to the Company’s Current Report
on
Form 8-K
filed with the Commission on July 16, 2007)
|
|
|
10
|
.1
|
|
Indemnity Agreement dated June 21, 1996 by and between the
Company and Candace Kendle Bryan (Incorporated by reference to
the Company’s Registration Statement
No. 333-30581
filed under the Securities Act of 1933)
|
|
|
10
|
.2
|
|
Indemnity Agreement dated June 21, 1996 by and between the
Company and Christopher C. Bergen (Incorporated by reference to
the Company’s Registration Statement
No. 333-30581
filed under the Securities Act of 1933)
|
|
|
10
|
.3
|
|
Indemnity Agreement dated June 21, 1996 by and between the
Company and Timothy M. Mooney (Incorporated by reference to the
Company’s Registration Statement
No. 333-30581
filed under the Securities Act of 1933)
|
|
|
10
|
.4
|
|
Indemnity Agreement dated May 14, 1997 by and between the
Company and Charles A. Sanders (Incorporated by reference to the
Company’s Annual Report on
Form 10-K
for the year ended December 31, 1997)
|
|
|
10
|
.5
|
|
Indemnity Agreement dated May 14, 1997 by and between the
Company and Philip E. Beekman (Incorporated by reference to the
Company’s Annual Report on
Form 10-K
for the year ended December 31, 1997)
|
|
|
10
|
.6
|
|
Indemnity Agreement dated December 10, 1998 by and between
the Company and Robert Buck (Incorporated by reference to the
Company’s Annual Report on
Form 10-K
for the year ended December 31, 1998)
|
46
| |
|
|
|
|
Exhibit
|
|
|
|
Number
|
|
Description of Exhibit
|
|
|
|
|
10
|
.7
|
|
Indemnity Agreement dated December 10, 1998 by and between
the Company and Mary Beth Price (Incorporated by reference to
the Company’s Annual Report on
Form 10-K
for the year ended December 31, 1998)
|
|
|
10
|
.8
|
|
Form of Indemnity Agreement by and between the Company and each
member of the Company’s Board of Directors, except for
those Indemnity Agreements noted above (Incorporated by
reference to the Company’s Annual Report on
Form 10-K
for the year ended December 31, 2003)
|
|
|
10
|
.9
|
|
Confirmation of Convertible Bond Hedge Transaction, dated
July 10, 2007, by and between the Company and UBS AG,
London Branch (Incorporated by reference to the Company’s
Current Report on
Form 8-K
filed with the Commission on July 10, 2007)
|
|
|
10
|
.10
|
|
Confirmation of Convertible Bond Hedge Transaction, dated
July 10, 2007, by and between the Company and JPMorgan
Chase Bank, National Association, London Branch (Incorporated by
reference to the Company’s Current Report on
Form 8-k
filed with the Commission on July 10, 2007)
|
|
|
10
|
.11
|
|
Confirmation of Issuer Warrant Transaction, dated July 10,
2007, by and between the Company and UBS AG, London Branch
(Incorporated by reference to the Company’s Current Report
on
Form 8-k
filed with the Commission on July 10, 2007)
|
|
|
10
|
.12
|
|
Confirmation of Issuer Warrant Transaction, dated July 10,
2007, by and between the Company and JPMorgan Chase Bank,
National Association, London Branch (Incorporated by reference
to the Company’s Current Report on
Form 8-k
filed with the Commission on July 10, 2007)
|
|
|
10
|
.13
|
|
Lease Agreement dated February 27, 2008 by and between the
Company and Carew Realty, Inc. (Incorporated by reference to the
Company’s Quarterly Report on
Form 10-Q
for the quarterly period ended March 31, 2008)
|
|
|
10
|
.14
|
|
Credit Agreement dated as of March 15, 2010 by and among
the Company, various Lenders and JPMorgan Chase Bank, N.A., as
Administrative Agent (Filed herewith)
|
|
|
10
|
.15
|
|
Guaranty dated as of March 15, 2010 by and among various
Subsidiaries of the Company in favor of JPMorgan Chase Bank,
N.A., as Administrative Agent (Filed herewith)
|
|
|
10
|
.16
|
|
Pledge and Security Agreement dated as of March 15, 2010 by
and among the Company, various Subsidiaries of the Company and
JPMorgan Chase Bank. N.A., as Administrative Agent (Filed
herewith)
|
|
|
10
|
.17
|
|
MANAGEMENT CONTRACTS AND COMPENSATION PLANS
|
|
|
|
|
|
(a) 1995 Stock Option and Stock Incentive Plan
(Incorporated by reference to the Company’s Registration
Statement
No. 333-30581
filed under the Securities Act of 1933)
|
|
|
|
|
|
(b) 1995 Stock Option and Stock Incentive Plan —
Individual Stock Option Agreement for Incentive Stock Option
(contained in Exhibit 10.20(a)) (Incorporated by reference
to the Company’s Registration Statement
No. 333-30581
filed under the Securities Act of 1933)
|
|
|
|
|
|
(c) 1997 Stock Option and Stock Incentive Plan
(Incorporated by reference to the Company’s Registration
Statement
No. 333-30581
filed under the Securities Act of 1933)
|
|
|
|
|
|
(c)(1) Amendment No. 1 to 1997 Stock Option and Stock
Incentive Plan (Incorporated by reference to the Company’s
Annual Report on
Form 10-K
for the year ended December 31, 2002)
|
|
|
|
|
|
(c)(2) Amendment No. 2 to 1997 Stock Option and Stock
Incentive Plan (Incorporated by reference to the Company’s
definitive Proxy Statement on Schedule 14A filed with the
Commission on April 12, 2000)
|
|
|
|
|
|
(c)(3) Amendment No. 3 to 1997 Stock Option and Stock
Incentive Plan (Incorporated by reference to the Company’s
Annual Report on
Form 10-K
for the year ended December 31, 2002)
|
|
|
|
|
|
(c)(4) Form of Restricted Stock Award Agreement (Incorporated by
reference to the Company’s Annual Report on
Form 10-K
for the year ended December 31, 2004)
|
|
|
|
|
|
(d) Form of Protective Compensation and Benefit Agreement.
Incorporated by reference to the Company’s Registration
Statement
No. 333-30581
filed under the Securities Act of 1933)
|
|
|
|
|
|
(e) 2007 Stock Incentive Plan (Incorporated by reference to
the Company’s definitive Proxy Statement on
Schedule 14A filed with the Commission on April 9,
2007)
|
|
|
|
|
|
(e)(1) Form of Performance Based Stock Unit Agreement
(Incorporated by reference to the Company’s Quarterly
Report on
Form 10-Q
for the quarterly period ended March 31, 2008)
|
47
| |
|
|
|
|
Exhibit
|
|
|
|
Number
|
|
Description of Exhibit
|
|
|
|
|
|
|
|
(f) Annual Incentive Plan (Incorporated by reference to the
Company’s Current Report on
Form 8-K
filed with the Commission on May 20, 2008)
|
|
|
|
|
|
(g) Nonqualified Deferred Compensation Plan (Incorporated
by reference to the Company’s Current Report on
Form 8-K
filed with the Commission on May 20, 2008)
|
|
|
|
|
|
(h) 2003 Directors Compensation Plan (Incorporated by
reference to the Company’s Quarterly Report on
Form 10-Q
for the quarterly period ended June 30, 2003)
|
|
|
12
|
.1
|
|
Computation of Ratio of Earnings to Fixed Charges (Filed
herewith)
|
|
|
14
|
|
|
Code of Ethics (Available on the Company’s Web site at
www.kendle.com)
|
|
|
21
|
|
|
List of Subsidiaries (Filed herewith)
|
|
|
23
|
.1
|
|
Consent of Deloitte & Touche LLP (Filed herewith)
|
|
|
24
|
|
|
Powers of Attorney (Filed herewith)
|
|
|
31
|
.1
|
|
Certification of the Chief Executive Officer pursuant to
Rule 13a-14(a)
(Filed herewith)
|
|
|
31
|
.2
|
|
Certification of the Chief Financial Officer pursuant to
Rule 13a-14(a)
(Filed herewith)
|
|
|
32
|
.1
|
|
Certification Pursuant to 18 U.S.C Section 1350 as Adopted
Pursuant to Section 906 of the Sarbanes-Oxley Act of
2002 — Chief Executive Office (Filed herewith)
|
|
|
32
|
.2
|
|
Certification Pursuant to 18 U.S.C Section 1350 as Adopted
Pursuant to Section 906 of the Sarbanes-Oxley Act of
2002 — Chief Financial Officer (Filed herewith)
|
48
Kendle
International Inc.
Index to
Consolidated Financial Statements
| |
|
|
|
|
|
|
|
Page
|
|
|
|
|
|
|
F-2
|
|
|
|
|
|
F-3
|
|
|
|
|
|
F-4
|
|
|
|
|
|
F-5
|
|
|
|
|
|
F-6
|
|
|
|
|
|
F-7
|
|
F-1
REPORT
OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Shareholders of
Kendle International Inc.
Cincinnati, Ohio
We have audited the accompanying consolidated balance sheets of
Kendle International Inc. and subsidiaries (the
“Company”) as of December 31, 2009 and 2008, and
the related consolidated statements of operations,
shareholders’ equity, and cash flows for each of the three
years in the period ended December 31, 2009. These
financial statements are the responsibility of the
Company’s management. Our responsibility is to express an
opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the
Public Company Accounting Oversight Board (United States). Those
standards require that we plan and perform the audit to obtain
reasonable assurance about whether the financial statements are
free of material misstatement. An audit includes examining, on a
test basis, evidence supporting the amounts and disclosures in
the financial statements. An audit also includes assessing the
accounting principles used and significant estimates made by
management, as well as evaluating the overall financial
statement presentation. We believe that our audits provide a
reasonable basis for our opinion.
In our opinion, such consolidated financial statements present
fairly, in all material respects, the financial position of
Kendle International Inc. and subsidiaries as of
December 31, 2009 and 2008, and the results of their
operations and their cash flows for each of the three years in
the period ended December 31, 2009, in conformity with
accounting principles generally accepted in the United States of
America.
As discussed in Note 2, the consolidated financial
statements have been adjusted for the retrospective application
of the new accounting guidance for convertible debt instruments,
which became effective January 1, 2009.
We have also audited, in accordance with the standards of the
Public Company Accounting Oversight Board (United States), the
Company’s internal control over financial reporting as of
December 31, 2009, based on the criteria established in
Internal Control — Integrated Framework issued by the
Committee of Sponsoring Organizations of the Treadway Commission
and our report dated March 16, 2010 expressed an
unqualified opinion on the Company’s internal control over
financial reporting.
/s/ DELOITTE &
TOUCHE LLP
Cincinnati, Ohio
March 16, 2010
F-2
CONSOLIDATED
STATEMENTS OF OPERATIONS
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
For the Years Ended December 31,
|
|
|
|
|
2009
|
|
|
2008(1)
|
|
|
2007(1)
|
|
|
|
|
(In thousands except per share data)
|
|
|
|
|
Net service revenues
|
|
$
|
416,688
|
|
|
$
|
475,092
|
|
|
$
|
397,584
|
|
|
Reimbursable
out-of-pocket
revenues
|
|
|
135,224
|
|
|
|
203,489
|
|
|
|
171,234
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total revenues
|
|
|
551,912
|
|
|
|
678,581
|
|
|
|
568,818
|
|
|
Costs and expenses:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Direct costs
|
|
|
210,586
|
|
|
|
247,436
|
|
|
|
204,161
|
|
|
Reimbursable
out-of-pocket
costs
|
|
|
135,224
|
|
|
|
203,489
|
|
|
|
171,234
|
|
|
Selling, general and administrative
|
|
|
144,659
|
|
|
|
155,577
|
|
|
|
125,744
|
|
|
Depreciation and amortization
|
|
|
15,712
|
|
|
|
15,253
|
|
|
|
14,865
|
|
|
Restructuring expenses
|
|
|
10,157
|
|
|
|
—
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total costs and expenses
|
|
|
516,338
|
|
|
|
621,755
|
|
|
|
516,004
|
|
|
Income from operations
|
|
|
35,574
|
|
|
|
56,826
|
|
|
|
52,814
|
|
|
Other income (expense):
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Interest income
|
|
|
579
|
|
|
|
760
|
|
|
|
1,466
|
|
|
Interest expense
|
|
|
(14,403
|
)
|
|
|
(15,891
|
)
|
|
|
(17,547
|
)
|
|
Write-off of deferred financing costs
|
|
|
—
|
|
|
|
—
|
|
|
|
(4,152
|
)
|
|
Gain on extinguishment of debt
|
|
|
2,887
|
|
|
|
—
|
|
|
|
—
|
|
|
Other
|
|
|
4,034
|
|
|
|
(2,043
|
)
|
|
|
(4,816
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total other income (expenses)
|
|
|
(6,903
|
)
|
|
|
(17,174
|
)
|
|
|
(25,049
|
)
|
|
Income before income taxes
|
|
|
28,671
|
|
|
|
39,652
|
|
|
|
27,765
|
|
|
Income taxes
|
|
|
13,434
|
|
|
|
16,509
|
|
|
|
11,755
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income
|
|
$
|
15,237
|
|
|
$
|
23,143
|
|
|
$
|
16,010
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Income per share data:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income per share
|
|
$
|
1.03
|
|
|
$
|
1.57
|
|
|
$
|
1.10
|
|
|
Weighted average shares
|
|
|
14,862
|
|
|
|
14,751
|
|
|
|
14,520
|
|
|
Diluted:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income per share
|
|
$
|
1.02
|
|
|
$
|
1.54
|
|
|
$
|
1.08
|
|
|
Weighted average shares
|
|
|
14,992
|
|
|
|
14,993
|
|
|
|
14,889
|
|
|
|
|
|
(1) |
|
As adjusted due to the implementation of accounting guidance
related to convertible debt. See Note 2: Accounting Changes. |
The accompanying notes are an integral part of these
Consolidated Financial Statements.
F-3
CONSOLIDATED
BALANCE SHEETS
| |
|
|
|
|
|
|
|
|
|
As of December 31,
|
|
2009
|
|
|
2008(1)
|
|
|
|
|
(In thousands except share data)
|
|
|
|
|
ASSETS
|
|
Current assets:
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents
|
|
$
|
52,103
|
|
|
$
|
35,169
|
|
|
Restricted cash
|
|
|
1,112
|
|
|
|
884
|
|
|
Accounts receivable, net
|
|
|
125,287
|
|
|
|
157,971
|
|
|
Deferred tax asset — current
|
|
|
8,147
|
|
|
|
14,077
|
|
|
Other current assets
|
|
|
20,676
|
|
|
|
18,439
|
|
|
|
|
|
|
|
|
|
|
|
|
Total current assets
|
|
|
207,325
|
|
|
|
226,540
|
|
|
Property and equipment, net
|
|
|
53,539
|
|
|
|
44,578
|
|
|
Goodwill
|
|
|
243,589
|
|
|
|
236,329
|
|
|
Other finite-lived intangible assets, net
|
|
|
15,164
|
|
|
|
19,031
|
|
|
Long-term deferred tax asset
|
|
|
12,716
|
|
|
|
1,346
|
|
|
Other assets
|
|
|
7,390
|
|
|
|
27,064
|
|
|
|
|
|
|
|
|
|
|
|
|
Total assets
|
|
$
|
539,723
|
|
|
$
|
554,888
|
|
|
|
|
|
|
|
|
|
|
|
|
LIABILITIES AND SHAREHOLDERS’ EQUITY
|
|
Current liabilities:
|
|
|
|
|
|
|
|
|
|
Current portion of obligations under capital leases
|
|
$
|
52
|
|
|
$
|
188
|
|
|
Trade payables
|
|
|
14,928
|
|
|
|
19,015
|
|
|
Advance billings
|
|
|
76,202
|
|
|
|
94,561
|
|
|
Other accrued liabilities
|
|
|
59,667
|
|
|
|
44,181
|
|
|
|
|
|
|
|
|
|
|
|
|
Total current liabilities
|
|
|
150,849
|
|
|
|
157,945
|
|
|
Obligations under capital leases, less current portion
|
|
|
34
|
|
|
|
123
|
|
|
Convertible notes
|
|
|
138,308
|
|
|
|
171,848
|
|
|
Deferred income tax liabilities
|
|
|
7,508
|
|
|
|
4,424
|
|
|
Non-current income taxes payable
|
|
|
1,872
|
|
|
|
2,123
|
|
|
Other liabilities
|
|
|
5,105
|
|
|
|
5,801
|
|
|
|
|
|
|
|
|
|
|
|
|
Total liabilities
|
|
|
303,676
|
|
|
|
342,264
|
|
|
Commitments and contingencies
|
|
|
|
|
|
|
|
|
|
Shareholders’ equity:
|
|
|
|
|
|
|
|
|
|
Preferred stock — no par value; 100,000 shares
authorized; none issued and outstanding
|
|
|
|
|
|
|
|
|
|
Common stock — no par value; 45,000,000 shares
authorized; 14,912,327 and 14,861,518 shares issued and
14,889,275 and 14,838,466 outstanding at December 31, 2009
and 2008, respectively
|
|
|
75
|
|
|
|
75
|
|
|
Additional paid-in capital
|
|
|
180,534
|
|
|
|
180,020
|
|
|
Retained earnings
|
|
|
33,736
|
|
|
|
18,499
|
|
|
Accumulated other comprehensive income:
|
|
|
|
|
|
|
|
|
|
Foreign currency translation adjustment
|
|
|
22,194
|
|
|
|
14,522
|
|
|
Less: Cost of common stock held in treasury, 23,052 shares
at December 31, 2009 and 2008, respectively
|
|
|
(492
|
)
|
|
|
(492
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Total shareholders’ equity
|
|
|
236,047
|
|
|
|
212,624
|
|
|
|
|
|
|
|
|
|
|
|
|
Total liabilities and shareholders’ equity
|
|
$
|
539,723
|
|
|
$
|
554,888
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(1) |
|
As adjusted due to the implementation of accounting guidance
related to convertible debt. See Note 2: Accounting Changes. |
The accompanying notes are an integral part of these
Consolidated Financial Statements.
F-4
CONSOLIDATED
STATEMENTS OF SHAREHOLDERS’ EQUITY
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accumulated
|
|
|
|
|
|
|
|
|
|
|
Common Stock
|
|
|
Common
|
|
|
Additional
|
|
|
|
|
|
Retained
|
|
|
Other
|
|
|
Total
|
|
|
|
|
|
|
|
Number of
|
|
|
Stock
|
|
|
Paid-in
|
|
|
Treasury
|
|
|
Earnings
|
|
|
Comprehensive
|
|
|
Shareholders’
|
|
|
Comprehensive
|
|
|
|
|
Shares
|
|
|
Amount
|
|
|
Capital(1)
|
|
|
Stock
|
|
|
(Deficit)(1)
|
|
|
Income
|
|
|
Equity(1)
|
|
|
Income(1)
|
|
|
|
|
(In thousands except share data)
|
|
|
|
|
Balance at January 1, 2007
|
|
|
14,422,341
|
|
|
$
|
75
|
|
|
$
|
154,641
|
|
|
$
|
(492
|
)
|
|
$
|
(16,392
|
)
|
|
$
|
2,280
|
|
|
$
|
140,112
|
|
|
|
|
|
|
Net income
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
16,010
|
|
|
|
|
|
|
|
16,010
|
|
|
|
16,010
|
|
|
Other comprehensive income:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Foreign currency translation adjustment
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
1,518
|
|
|
|
1,518
|
|
|
|
1,518
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Comprehensive income
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
17,528
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Shares issued under stock plans
|
|
|
235,636
|
|
|
|
|
|
|
|
2,560
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2,560
|
|
|
|
|
|
|
Stock option expense
|
|
|
|
|
|
|
|
|
|
|
860
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
860
|
|
|
|
|
|
|
Income tax benefit from exercise of stock options
|
|
|
|
|
|
|
|
|
|
|
188
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
188
|
|
|
|
|
|
|
Note hedge transaction
|
|
|
|
|
|
|
|
|
|
|
(42,880
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(42,880
|
)
|
|
|
|
|
|
Issuance of warrants
|
|
|
|
|
|
|
|
|
|
|
24,740
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
24,740
|
|
|
|
|
|
|
Adoption of new guidance related to tax uncertainties
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(4,262
|
)
|
|
|
|
|
|
|
(4,262
|
)
|
|
|
|
|
|
Equity component of convertible notes(1)
|
|
|
|
|
|
|
|
|
|
|
36,412
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
36,412
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance at December 31, 2007
|
|
|
14,657,977
|
|
|
$
|
75
|
|
|
$
|
176,521
|
|
|
$
|
(492
|
)
|
|
$
|
(4,644
|
)
|
|
$
|
3,798
|
|
|
$
|
175,258
|
|
|
|
|
|
|
Net income
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
23,143
|
|
|
|
|
|
|
|
23,143
|
|
|
|
23,143
|
|
|
Other comprehensive income:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Foreign currency translation adjustment
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10,724
|
|
|
|
10,724
|
|
|
|
10,724
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Comprehensive income
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
33,867
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Shares issued under stock plans
|
|
|
180,489
|
|
|
|
|
|
|
|
2,294
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2,294
|
|
|
|
|
|
|
Stock compensation expense
|
|
|
|
|
|
|
|
|
|
|
1,030
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
1,030
|
|
|
|
|
|
|
Income tax benefit from exercise of stock options
|
|
|
|
|
|
|
|
|
|
|
175
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
175
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance at December 31, 2008
|
|
|
14,838,466
|
|
|
$
|
75
|
|
|
$
|
180,020
|
|
|
$
|
(492
|
)
|
|
$
|
18,499
|
|
|
$
|
14,522
|
|
|
$
|
212,624
|
|
|
|
|
|
|
Net income
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
15,237
|
|
|
|
|
|
|
|
15,237
|
|
|
|
15,237
|
|
|
Other comprehensive income:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Foreign currency translation adjustment
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
7,672
|
|
|
|
7,672
|
|
|
|
7,672
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Comprehensive income
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
22,909
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Share based compensation plans and other
|
|
|
50,809
|
|
|
|
|
|
|
|
815
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
815
|
|
|
|
|
|
|
Repurchase of equity component of convertible notes
|
|
|
|
|
|
|
|
|
|
|
(204
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(204
|
)
|
|
|
|
|
|
Partial retirement of note hedges and warrants
|
|
|
|
|
|
|
|
|
|
|
(97
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(97
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance at December 31, 2009
|
|
|
14,889,275
|
|
|
$
|
75
|
|
|
$
|
180,534
|
|
|
$
|
(492
|
)
|
|
$
|
33,736
|
|
|
$
|
22,194
|
|
|
$
|
236,047
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(1) |
|
As adjusted due to the implementation of accounting guidance
related to convertible debt. See Note 2: Accounting Changes. |
The accompanying notes are an integral part of these
Consolidated Financial Statements.
F-5
CONSOLIDATED
STATEMENTS OF CASH FLOWS
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
For the Years Ended December 31,
|
|
|
|
|
2009
|
|
|
2008(1)
|
|
|
2007(1)
|
|
|
|
|
(In thousands)
|
|
|
|
|
Cash flows from operating activities
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income
|
|
$
|
15,237
|
|
|
$
|
23,143
|
|
|
$
|
16,010
|
|
|
Adjustments to reconcile net income to cash provided by
operating activities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Depreciation and amortization
|
|
|
15,712
|
|
|
|
15,253
|
|
|
|
14,865
|
|
|
Deferred income taxes
|
|
|
4,138
|
|
|
|
(6,745
|
)
|
|
|
(5,234
|
)
|
|
Income tax benefit from stock option exercises
|
|
|
(2
|
)
|
|
|
(175
|
)
|
|
|
(188
|
)
|
|
Compensation expense on stock grants
|
|
|
679
|
|
|
|
1,030
|
|
|
|
879
|
|
|
Debt issue cost amortization
|
|
|
7,729
|
|
|
|
7,783
|
|
|
|
8,081
|
|
|
Foreign currency exchange (gain) loss
|
|
|
(2,691
|
)
|
|
|
4,321
|
|
|
|
3,770
|
|
|
Gain on extinguishment of debt
|
|
|
(2,887
|
)
|
|
|
—
|
|
|
|
—
|
|
|
Other
|
|
|
1,333
|
|
|
|
(1,564
|
)
|
|
|
172
|
|
|
Changes in operating assets and liabilities, net of effects from
acquisitions:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Restricted cash
|
|
|
(82
|
)
|
|
|
(85
|
)
|
|
|
1,587
|
|
|
Accounts receivable
|
|
|
37,786
|
|
|
|
(28,036
|
)
|
|
|
(16,138
|
)
|
|
Other current assets
|
|
|
(1,344
|
)
|
|
|
(3,493
|
)
|
|
|
1,507
|
|
|
Other assets
|
|
|
(31
|
)
|
|
|
(317
|
)
|
|
|
(486
|
)
|
|
Trade payables
|
|
|
(4,395
|
)
|
|
|
1,010
|
|
|
|
2,632
|
|
|
Advance billings
|
|
|
(22,401
|
)
|
|
|
19,613
|
|
|
|
18,814
|
|
|
Accrued liabilities and other
|
|
|
5,508
|
|
|
|
5,318
|
|
|
|
14,712
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net cash provided by operating activities
|
|
|
54,289
|
|
|
|
37,056
|
|
|
|
60,983
|
|
|
Cash flows from investing activities
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Proceeds from termination of foreign currency hedges
|
|
|
17,312
|
|
|
|
—
|
|
|
|
—
|
|
|
Acquisitions of property and equipment
|
|
|
(18,966
|
)
|
|
|
(27,097
|
)
|
|
|
(15,255
|
)
|
|
Acquisitions of businesses, less cash acquired
|
|
|
(2,875
|
)
|
|
|
(18,053
|
)
|
|
|
2,154
|
|
|
Other
|
|
|
32
|
|
|
|
(1,143
|
)
|
|
|
(722
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net cash used in investing activities
|
|
|
(4,497
|
)
|
|
|
(46,293
|
)
|
|
|
(13,823
|
)
|
|
Cash flows from financing activities
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Proceeds from issuance of long-term debt
|
|
|
—
|
|
|
|
37,500
|
|
|
|
200,000
|
|
|
Payments of long-term debt
|
|
|
—
|
|
|
|
(37,500
|
)
|
|
|
(199,500
|
)
|
|
Repurchase of convertible notes
|
|
|
(36,463
|
)
|
|
|
—
|
|
|
|
—
|
|
|
Purchase of note hedges
|
|
|
(97
|
)
|
|
|
—
|
|
|
|
(42,880
|
)
|
|
Proceeds from the issuance of warrants
|
|
|
—
|
|
|
|
—
|
|
|
|
24,740
|
|
|
Proceeds from issuance of common stock
|
|
|
247
|
|
|
|
2,241
|
|
|
|
2,623
|
|
|
Income tax benefit from stock option exercises
|
|
|
2
|
|
|
|
175
|
|
|
|
188
|
|
|
Accounts payable — book overdraft
|
|
|
133
|
|
|
|
465
|
|
|
|
(22
|
)
|
|
Payments on capital lease obligations
|
|
|
(228
|
)
|
|
|
(234
|
)
|
|
|
(196
|
)
|
|
Debt issue costs
|
|
|
(482
|
)
|
|
|
—
|
|
|
|
(6,946
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net cash provided by (used in) financing activities
|
|
|
(36,888
|
)
|
|
|
2,647
|
|
|
|
(21,993
|
)
|
|
Effects of exchange rates on cash and cash equivalents
|
|
|
4,030
|
|
|
|
(3,753
|
)
|
|
|
428
|
|
|
Net increase (decrease) in cash and cash equivalents
|
|
|
16,934
|
|
|
|
(10,343
|
)
|
|
|
25,595
|
|
|
Cash and cash equivalents
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Beginning of year
|
|
|
35,169
|
|
|
|
45,512
|
|
|
|
19,917
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
End of year
|
|
$
|
52,103
|
|
|
$
|
35,169
|
|
|
$
|
45,512
|
|
|
Supplemental disclosure of cash flow information
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash paid during the year for interest
|
|
$
|
7,312
|
|
|
$
|
8,087
|
|
|
$
|
10,107
|
|
|
Cash paid during the year for income taxes
|
|
$
|
14,382
|
|
|
$
|
25,931
|
|
|
$
|
4,870
|
|
|
Supplemental schedule of noncash investing and financing
activities
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Acquisition of equipment under capital leases
|
|
$
|
—
|
|
|
$
|
—
|
|
|
$
|
173
|
|
|
Accrued equipment purchases
|
|
$
|
(1,307
|
)
|
|
$
|
(1,090
|
)
|
|
$
|
(3,574
|
)
|
|
Acquisitions of businesses:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Fair value of assets acquired via cash
|
|
$
|
5,039
|
|
|
$
|
25,432
|
|
|
$
|
(2,154
|
)
|
|
Fair value of liabilities assumed or incurred
|
|
$
|
(2,164
|
)
|
|
$
|
(7,379
|
)
|
|
$
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net cash payments/(inflows)
|
|
$
|
2,875
|
|
|
$
|
18,053
|
|
|
$
|
(2,154
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(1) |
|
As adjusted due to the implementation of accounting guidance
related to convertible debt. See Note 2: Accounting Changes. |
The accompanying notes are an integral part of these
Consolidated Financial Statements.
F-6
NOTES TO
CONSOLIDATED FINANCIAL STATEMENTS
|
|
|
1.
|
NATURE OF
BUSINESS AND SIGNIFICANT ACCOUNTING POLICIES:
|
Nature
of Business
Kendle International Inc. (the Company or Kendle), an Ohio
corporation established in 1989, is a global clinical research
organization (CRO) that provides a broad range of Phase I-IV
global clinical development services to the biopharmaceutical
industry. The Company has operations in North America, Europe,
Asia/Pacific, Latin America and Africa.
Principles
of Consolidation and Organization
The Consolidated Financial Statements include the financial
information of Kendle International Inc. and its wholly-owned
subsidiaries. Investments in unconsolidated companies that are
at least 20% owned and in which the Company can exercise
significant influence, but not control, are carried at cost plus
equity in undistributed earnings since acquisition. Investments
in unconsolidated companies that are less than 20% owned and in
which the Company cannot exercise significant influence are
carried at cost. There are no significant amounts on the
Consolidated Balance Sheets related to investments in
unconsolidated companies.
All intercompany accounts and transactions have been eliminated.
The results of operations of the Company’s wholly-owned
subsidiaries have been included in the Consolidated Financial
Statements of the Company from the respective dates of
acquisition.
Business
Combinations
Accounting guidance related to business combinations requires
assets acquired and liabilities assumed in a business
combination to be recorded at fair value. Fair values are
generally determined by using comparisons to market value
transactions and present value techniques. The use of a
discounted cash flow technique requires significant judgments
with respect to expected cash flows to be derived from the
assets, the estimated period of time the assets will produce
those cash flows and the selection of an appropriate discount
rate. Changes in such estimates could change the amounts
allocated to individual identifiable assets, the lives over
which the assigned values are amortized and the amounts
allocated to goodwill. While the Company believes its
assumptions are reasonable, if different assumptions were made,
the purchase price allocation and the estimated useful lives of
amortizable assets could differ substantially from the reported
amounts.
Results of operations for acquired entities are included in the
Company’s results of operations from the date of
acquisition.
Foreign
Currency Translation
Assets and liabilities of the Company’s wholly-owned
subsidiaries are translated into U.S. dollars at year-end
exchange rates. Income statement accounts are translated at
average exchange rates for the period. These translation
adjustments are recorded as a separate component of
Shareholders’ Equity. Foreign currency transaction gains
and losses are included in the Consolidated Statements of
Operations in Other income (expense).
Cash
and Cash Equivalents, Including Restricted Cash
Cash and cash equivalents consist of demand deposits and money
market funds held with financial institutions, with an initial
maturity of three months or less.
The Company maintains its demand deposits with certain financial
institutions. The balance of one account from
time-to-time
exceeds the maximum U.S. federally insured amount.
Additionally, there is no state insurance coverage on bank
balances held in The Netherlands.
At December 31, 2009, the Company held cash of
approximately $1,112,000 that is restricted as to its use as
compared to approximately $884,000 at December 31, 2008.
The restricted cash represents cash received from
F-7
customers that is segregated in a separate Company bank account
and available for use only for specific
project-related
expenses, primarily investigator fees, upon authorization from
the customer.
Revenue
Recognition
The majority of the Company’s net service revenues are
based on fixed-price contracts calculated on a proportional
performance basis (also referred to herein as
“percentage-of-completion”)
based upon assumptions regarding the estimated total costs for
each contract. The Company also recognizes revenue under
units-based contracts by multiplying units completed by the
applicable contract
per-unit
price. Additionally, work is performed under
time-and-materials
contracts, recognizing revenue as hours are worked based on the
hourly billing rate for each contract.
Percentage-of-Completion
With respect to fixed price contracts, costs are incurred for
performance of each contract and compared to the estimated
budgeted costs for that contract to determine a percentage of
completion on the contract. The percentage of completion is then
multiplied by the contract value to determine the amount of
revenue recognized. The contract value equals the value of the
services to be performed under the contract as determined by
aggregating the labor hours estimated to be incurred to perform
the tasks in the contract at the agreed rates. Contract value
excludes the value of third-party and other pass-through costs.
As the work progresses, original estimates might be changed as a
result of management’s regular contract review process.
Management regularly reviews the budget on each contract to
determine if the budgeted costs accurately reflect the costs
that the Company will incur for contract performance. The
Company reviews each contract’s performance to date,
current cost trends and circumstances specific to each contract.
The Company estimates its remaining costs to complete the
contract based on a variety of factors, including:
|
|
|
| |
•
|
Actual costs incurred to date and the work completed as a result
of incurring the actual costs;
|
| |
| |
•
|
The remaining work to be completed based on the timeline of the
contract as well as the number of incomplete tasks in the
contract; and
|
| |
| |
•
|
Factors that could change the rate of progress of future
contract performance.
|
Examples of factors included in the review process that could
change the rate of progress of future contract performance are;
patient enrollment rate, changes in the composition of staff on
the project or other customer requirements, among other things.
Based on these contract reviews, the Company adjusts its cost
estimates. Adjustments to net service revenue resulting from
changes in cost estimates are recorded on a cumulative basis in
the period in which the revisions are made. When estimates
indicate a loss, such loss is provided in the current period in
its entirety. While the Company routinely adjusts cost estimates
on individual contracts, the Company’s estimates and
assumptions historically have been accurate in all material
respects in the aggregate. The Company expects the estimates and
assumptions to remain accurate in all material respects in the
aggregate in future periods.
A contract amendment, which results in revisions to net service
revenues and cost estimates, is recognized in the
percentage-of-completion
calculations beginning in the period in which the parties reach
written agreement to the amendment. Historically the aggregate
value of contract amendments signed in any year represents 15%
to 20% of annual sales, and, like sales, represents future net
service revenues. Although the majority of the Company’s
contract amendments relate to future services, the Company and
its customers may execute contract amendments for services that
the Company already has performed. In these circumstances, net
service revenue from these services is recognized in the current
period. Historically, the impact of such amendments on results
of operations has not been material.
Under the Company’s policy, project teams are not
authorized to engage in tasks outside the scope of the contract
without prior management approval. In limited situations,
management may authorize the project team to commence work on
activities outside the contract scope while the Company and its
customer negotiate and finalize the contract amendment. When
work progresses on unsigned, unprocessed contract amendments,
the Company
F-8
reviews the direct costs incurred, and, where material, defers
such costs on the balance sheet. In addition, the impact of such
costs on the estimates to complete is considered and, where
material, the estimates are adjusted. Historically, neither the
deferred costs nor the impact on estimates have been material.
The Company believes that total costs are an appropriate
indicator of the performance of fixed price contracts because
the costs relate primarily to the amount of labor hours incurred
to perform the contract. The customer receives the benefit of
the work performed throughout the contract term and is obligated
to pay for services once performed. Accordingly, the Company
believes that an input measure of cost is a reasonable surrogate
for an output measure under the proportional performance model
and is consistent with the revenue recognition concepts issued
by the Securities and Exchange Commission.
Units-Based
Although the majority of the Company’s contracts are
fixed-price and net service revenues are calculated on a
percentage of completion methodology as discussed above, the
Company has seen increasing demand from its customers to move
toward a units-based contract methodology in new contracts. It
is the Company’s intent to structure more of its contracts
under a units-based methodology for calculating net service
revenues so the Company expects the percentage of contracts
under which net service revenues are recognized using
units-based methodology to increase in future periods. Under a
units-based contract methodology, amounts recognized as net
service revenues are calculated based on units completed in the
period multiplied by a unit value or selling price that is
outlined in the contract.
For a units-based contract, a typical unit could include such
things as completion of a monitoring visit, monthly site
management units or case report form pages entered. The Company
tracks the units completed for each unit category included in
the contract. Net service revenue is recognized monthly based on
the units actually completed in the period at the agreed upon
unit value or selling price. Net service revenue is recognized
only up to the number of units contained in each contract. If
the Company completes or expects to complete units over and
above the number of units initially estimated and contained in
the contract, a contract amendment is generated to reflect the
additional units needed.
A contract amendment, which results in revisions to net service
revenues and cost estimates, is recognized in the unit-based net
service revenue calculations beginning in the period in which
the parties agree to the amendment.
The Company believes that under certain types of contracts the
value of the work performed is best captured by calculating net
service revenues using the value of units completed. The Company
believes that units-based revenue recognition is consistent with
the revenue recognition concepts issued by the Securities and
Exchange Commission.
As the Company provides services on projects, it also incurs
third-party and other pass-through costs, which are reimbursable
by its customers pursuant to the contract. The revenues and
costs from these third-party and other pass-through costs are
reflected in the Company’s Consolidated Statements of
Operations under the line items titled “Reimbursable
out-of-pocket
revenues” and “Reimbursable
out-of-pocket
costs”, respectively.
Concentration
of Credit Risk
Accounts receivable represent amounts due from customers that
are concentrated mainly in the biopharmaceutical industry. The
concentration of credit risk is subject to the financial and
industry conditions of the Company’s customers. The Company
does not require collateral or other securities to support
customer receivables. The Company monitors the creditworthiness
of its customers. Refer to Note 15, Segment Information for
additional information regarding revenue concentration.
Long-Lived
Assets
Property and equipment are stated at cost, net of accumulated
depreciation. Depreciation is computed over the estimated useful
lives, ranging from five to fifty years using the straight-line
method. Leasehold improvements are amortized over the lesser of
the estimated useful life of the improvement or the remaining
term of the underlying lease. Repairs and maintenance are
charged to expense as incurred. Upon disposition, the asset and
the related
F-9
accumulated depreciation are relieved and any gains or losses
are reflected in the Consolidated Statements of Operations.
Useful lives by asset category can vary based on the nature of
the asset purchased. The following represents the maximum
estimated useful lives for the below categories of assets:
| |
|
|
|
Furniture and Fixtures
|
|
10 years
|
|
Computer and Software
|
|
5 years
|
|
Leasehold Improvements
|
|
Lesser of estimated life of asset or lease term
|
|
Buildings
|
|
50 years
|
|
Capital Lease Assets
|
|
Lesser of estimated life of asset or lease term
|
Equipment under capital leases is recorded at the present value
of future minimum lease payments and is amortized over the
estimated useful lives of the assets, not to exceed the terms of
the related leases. Accumulated amortization on equipment under
capital leases was approximately $399,000 at December 31,
2009 compared to $1.4 million at December 31, 2008.
The Company capitalizes costs such as design, configuration
activities, coding and testing activities incurred internally to
develop software used primarily in the Company’s
proprietary clinical trial and data management systems, and
amortizes these costs on a straight-line basis over the
estimated useful life of the product, not to exceed five years.
The Company does not capitalize costs that are precluded from
capitalization in the authoritative guidance such as preliminary
project phase costs, planning, oversight, process re-engineering
costs, training costs, or data conversion costs. Internally
developed software costs included in the Consolidated Balance
Sheets at December 31, 2009, and 2008 were
$18.0 million and $17.4 million, respectively. The
related accumulated amortization at December 31, 2009, and
2008 was $16.2 million and $15.6 million, respectively.
In accordance with guidance related to accounting for impairment
or disposal of long-lived assets, long-lived assets such as
property, plant and equipment, and software are reviewed for
impairment whenever facts and circumstances indicate that the
carrying value may not be recoverable. When required, impairment
losses on assets to be held and used are recognized based on the
fair value of the asset. The fair value is determined based on
estimates of future cash flows, market value of similar assets,
if available, or independent appraisals, if required. If the
carrying amount of the long-lived asset is not recoverable from
its undiscounted cash flows, an impairment loss is recognized
for the difference between the carrying amount and fair value of
the asset.
Goodwill
In accordance with the provisions of guidance related to
accounting for goodwill and other intangible assets, the Company
discontinued the amortization of goodwill and other identifiable
intangible assets that have indefinite useful lives. Intangible
assets that have finite useful lives will continue to be
amortized over their estimated useful lives.
Goodwill is evaluated annually in the fourth quarter for
impairment at the reporting unit level unless conditions exist
that require an updated analysis on an interim basis. Such
evaluation is based on a two-step test starting with a
comparison of the carrying amount of the reporting unit to the
fair value of the reporting unit. If the carrying amount of the
reporting unit exceeds the fair value, the second phase of the
test measures the impairment. The Company has five reporting
units that are tested for impairment: Early Stage, and four
reporting units that are aggregated into the Late Stage segment
for segment reporting purposes. The four Late Stage reporting
units were slightly reorganized in 2009 and are Clinical and
Data Monitoring, Project Management and Late Phase Services,
Regulatory, Site and Medical Affairs and Biostatistics and
Statistical Programming.
In 2009 and 2008, the Company analyzed goodwill for impairment
by comparing the carrying amounts of the reporting units to the
fair values of the reporting units. The fair values of the
reporting units were calculated based on the income approach
which uses discounted cash flows as well as public information
regarding the market capitalization of the Company.
The Company completed the testing in the fourth quarter of 2009.
The fair value of the Early Stage unit and each of the four Late
Stage reporting units exceeded the carrying value, resulting in
no goodwill impairment for 2009. The analysis in the fourth
quarter of 2008 resulted in no goodwill impairment for 2008.
F-10
Derivatives
From time to time, the Company may use derivative instruments to
manage exposure to interest rates and foreign currency.
Derivatives meeting the hedge criteria established in guidance
related to accounting for derivative instruments and hedging
activities, are recorded in the Consolidated Balance Sheets at
fair value at each balance sheet date. When the derivative is
entered into, the Company designates whether or not the
derivative instrument is an effective hedge of an asset,
liability or firm commitment and classifies the hedge as a cash
flow hedge or a fair value hedge. If the hedge is determined to
be an effective cash flow hedge, changes in the fair value of
the derivative instrument are recorded as a component of other
comprehensive income (loss). Changes in the value of fair value
hedges are recorded in results of operations.
In 2007, the Company entered into foreign currency hedging
transactions to mitigate exposure in movements between the
U.S. dollar and British Pounds Sterling and
U.S. dollar and Euro. The hedging transactions were
designed to mitigate the Company’s exposure related to two
intercompany notes between the Company’s
U.S. subsidiary, as lender, and the Company’s
subsidiaries in each of the United Kingdom and Germany. The note
between the Company’s U.S. subsidiary and United
Kingdom subsidiary is denominated in Pounds Sterling and had an
outstanding principal amount of approximately $41.4 million
at December 31, 2008. In January 2009, the Company
eliminated a substantial portion of this note leaving a
remaining balance outstanding at December 31, 2009 of
$6.6 million. The hedge agreement related to this loan was
terminated in January resulting in $17.3 million of cash
proceeds. The Company recorded foreign exchange gains of
$997,000 related to the Pound Sterling loan in 2009. Prior to
the termination of the Pound Sterling hedge in January 2009, the
Company recorded foreign exchange losses of $1.3 million.
The note between the Company’s U.S. subsidiary and
German subsidiary was denominated in Euros and had an
outstanding principal amount of approximately $22.8 million
at December 31, 2008. This note was repaid in full in March
2009, and the related hedge agreement was terminated at the same
time. Foreign exchange losses of $1.5 million and gains of
$741,000 were recorded related to the Euro note and the Euro
hedge agreement, respectively, prior to their repayment and
termination in March 2009. The hedge agreements were not
designated for hedge accounting treatment under accounting
guidance related to derivatives and all changes in the fair
market value of the hedge were recorded in the Company’s
Consolidated Statements of Operations.
In 2008, the Company recorded gains of approximately
$1.4 million on the Euro hedge transactions and gains of
approximately $18.0 million on the Pound Sterling
transaction related to the changes in the fair market value of
the hedge. The gains on the fair market value of the hedge were
offset by foreign exchange losses of approximately
$1.3 million on the change in value of the Euro
intercompany note and $15.7 million on the change in value
of the Pound Sterling intercompany note.
Marketing
and Advertising Costs
Marketing and advertising costs include costs incurred to
promote the Company’s business. Marketing and advertising
costs are expensed as incurred. Advertising expense incurred by
the Company was $2.1 million for each of the years ended
December 31, 2009 and 2008 and was $2.4 million for
the year ended December 31, 2007.
Investigator
and Project Costs
In addition to various contract costs previously described, the
Company incurs costs, in excess of contract amounts, which are
reimbursable by its customers. Accounting guidance related to
income statement characterization of reimbursements received for
out-of-pocket
expenses incurred requires the Company to include amounts paid
to investigators and other
out-of-pocket
costs as reimbursable
out-of-pocket
revenues and reimbursable
out-of-pocket
expenses in the Consolidated Statements of Operations. In
certain contracts, these costs are fixed by the contract terms.
Accordingly, the Company recognizes these costs as part of net
service revenues and direct costs.
Net
Income Per Share Data
Net income per basic share is computed using the weighted
average common shares outstanding. Net income per diluted share
is computed using the weighted average common shares and
potential common shares outstanding.
F-11
The weighted average shares used in computing net income per
diluted share have been calculated as follows:
Net
Income Per Share Data
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2009
|
|
|
2008
|
|
|
2007
|
|
|
|
|
(In thousands)
|
|
|
|
|
Weighted average common shares outstanding
|
|
|
14,862
|
|
|
|
14,751
|
|
|
|
14,520
|
|
|
Stock options and non-vested restricted shares
|
|
|
130
|
|
|
|
242
|
|
|
|
369
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted average shares
|
|
|
14,992
|
|
|
|
14,993
|
|
|
|
14,889
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
In 2009, 149,000 of outstanding options were antidilutive. No
options were antidilutive in 2008 or 2007.
Under accounting guidance related to contingently convertible
instruments effect on diluted earnings per share because of the
Company’s obligation to settle the par value of its
Convertible Notes (defined in Note 8, Debt) in cash, the
Company is not required to include any shares underlying the
Convertible Notes in its weighted average shares outstanding
used in calculating diluted earnings per share until the average
stock price per share for the quarter exceeds the $47.71
conversion price and only to the extent of the additional shares
that the Company may be required to issue in the event that the
Company’s conversion obligation exceeds the principal
amount of the Convertible Notes converted. These conditions have
not been met as of December 31, 2009. At any such time in
the future that these conditions are met, only the number of
shares that would be issuable (under the “treasury”
method of accounting for the share dilution) will be included,
which is based upon the amount by which the average stock price
exceeds the conversion price. The following table provides
examples of how changes in the Company’s stock price will
require the inclusion of additional shares in the denominator of
the weighted average shares outstanding — assuming
dilution calculation. The table also reflects the impact on the
number of shares that the Company would expect to issue upon
concurrent settlement of the Convertible Notes and the bond
hedges and warrants mentioned below:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Incremental Shares
|
|
|
|
|
|
|
|
Total Treasury
|
|
Shares Due to the
|
|
Issued by the
|
|
|
|
Convertible Notes
|
|
|
|
Method Incremental
|
|
Company under Note
|
|
Company Upon
|
|
Share Price
|
|
Shares
|
|
Warrant Shares
|
|
Shares(1)
|
|
Hedges(2)
|
|
Conversion(2)
|
|
|
|
$40.00
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
$45.00
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
$50.00
|
|
|
148,094
|
|
|
|
—
|
|
|
|
148,094
|
|
|
|
(148,094
|
)
|
|
|
—
|
|
|
$55.00
|
|
|
429,003
|
|
|
|
—
|
|
|
|
429,003
|
|
|
|
(429,003
|
)
|
|
|
—
|
|
|
$60.00
|
|
|
663,094
|
|
|
|
—
|
|
|
|
663,094
|
|
|
|
(663,094
|
)
|
|
|
—
|
|
|
$65.00
|
|
|
861,171
|
|
|
|
188,457
|
|
|
|
1,049,628
|
|
|
|
(861,171
|
)
|
|
|
188,457
|
|
|
$70.00
|
|
|
1,030,951
|
|
|
|
406,288
|
|
|
|
1,437,240
|
|
|
|
(1,030,951
|
)
|
|
|
406,288
|
|
|
|
|
|
(1) |
|
Represents the number of incremental shares that must be
included in the calculation of fully diluted shares under U.S.
Generally Accepted Accounting Principles. |
| |
|
(2) |
|
Represents the number of incremental shares to be issued by the
Company upon conversion of the Convertible Notes, assuming
concurrent settlement of the bond hedges and warrants. |
Income
Taxes
The Company and its U.S. subsidiaries file a consolidated
U.S. federal income tax return. Other subsidiaries of the
Company file tax returns in their local jurisdictions.
The Company provides for income taxes on all transactions that
have been recognized in the Consolidated Financial Statements in
accordance with accounting guidance related to income taxes.
Accordingly, the impact of changes in income tax laws on
deferred tax assets and deferred tax liabilities are recognized
in net earnings in the period during which such changes are
enacted.
F-12
The Company records deferred tax assets and liabilities based on
temporary differences between the financial statement and tax
bases of assets and liabilities and for tax benefit
carryforwards using enacted tax rates in effect in the year in
which the differences are expected to reverse. Management
provides valuation allowances against deferred tax assets for
amounts that are not considered more likely than not to be
realized. The valuation of the deferred tax asset is dependent
on, among other things, the ability of the Company to generate a
sufficient level of future taxable income. In estimating future
taxable income, the Company has considered both positive and
negative evidence, such as historical and forecasted results of
operations, and has considered the implementation of prudent and
feasible tax planning strategies.
The Company follows accounting guidance related to accounting
for uncertainty in income taxes which requires significant
judgment in determining what constitutes an individual tax
position as well as assessing the outcome of each tax position.
Changes in judgments as to recognition or measurement of tax
positions can materially affect the estimate of the effective
tax rate, and, consequently, the Company’s operating
results. The Company considers many factors when evaluating and
estimating tax positions and tax benefits, which may require
periodic adjustments and which many not accurately anticipate
actual outcomes. In addition, the calculation of tax liabilities
involves dealing with uncertainties in the application of
complex tax regulations in a multitude of jurisdictions. The
Company determines its liability for uncertain tax positions
globally. At December 31, 2009, the Company has recorded a
gross liability for uncertain tax positions of
$1.8 million. If events occur and the payment of these
amounts ultimately proves to be unnecessary, the reversal of
liabilities would result in tax benefits being recognized in the
period when it is determined the liabilities are no longer
necessary. If the calculation of liability related to uncertain
tax positions proves to be more or less than the ultimate
assessment, a tax expense or benefit to expense, respectively,
would result.
In December 2007, the FASB issued accounting guidance related to
business combinations. The Company expects this guidance to have
an impact on its consolidated financial statements, but the
impact will depend on the nature, terms and size of the
acquisitions consummated. One of the changes in accounting for
business combinations is the treatment of previously unresolved
or settled tax related items. These items no longer adjust
goodwill, but are recorded in the current period tax expense. An
immaterial portion of the $1.8 million liability for
unrecognized tax benefits as of December 31, 2009 relates
to tax positions of acquired entities taken prior to their
acquisition by the Company. If such liabilities reverse, they
will affect the income tax provision in the period of reversal.
In 2009, the Company received revised information pertaining to
an acquisition made in 2006. As a result of this new
information, the Company determined that it is able to claim
additional net operating loss deductions on an amended 2006
federal income tax return. After claiming these additional net
operating loss deductions, the Company will then have available
for use approximately $6.9 million of foreign tax credits
to offset the Company’s future U.S. tax liabilities.
Accordingly, the Company has recorded a gross deferred tax asset
for these foreign tax credits. However, due to the uncertainty
associated with the ultimate realizability of those additional
deferred tax assets, the Company has determined that a valuation
allowance is required to be recorded against 100% of the
additional deferred tax assets, thus resulting in no change to
the Company’s tax expense for the period. A change in
judgment regarding the realizability of these additional
deferred tax assets in a subsequent period could have a material
effect on the Company’s tax expense in such future period.
Stock
Options
The Company follows the accounting guidance related to
accounting for share-based payments. The Company generally uses
the straight-line method of recording compensation expense
relative to share-based payment, unless awards have graded
vesting features and the expense recorded under the
straight-line method is not adequate. In those situations,
compensation cost is recognized separately over each tranche.
Stock-based compensation expense is recorded primarily in
general and administrative expenses in the Company’s
Consolidated Statements of Operations as the majority of the
stock option expense related to options granted to executives.
Restricted/Unrestricted
Stock
Non-vested stock (referred to as “restricted stock” in
the 1997 Plan) may also be granted pursuant to the 2007 Plan,
which replaced the 1997 Plan (defined in Note 9).
Non-vested shares typically vest ratably over a three-year
period, with shares restricted from transfer until vesting. In
2009 and 2008, the Company granted 86,250 and
F-13
24,200 shares of restricted stock, respectively. No such
shares were granted in 2007. If a participant ceases to be an
eligible employee prior to the lapsing of transfer restrictions,
such shares return to the Company without consideration.
Unrestricted stock also may be granted to key employees under
the 2007 Plan, which replaced the 1997 Plan. Unrestricted shares
vest immediately. The Company granted 3,000 shares of
unrestricted Common Stock in 2009. No shares of unrestricted
stock were granted in 2008 or 2007.
Use of
Estimates
The preparation of Consolidated Financial Statements in
conformity with accounting principles generally accepted in the
United States requires management to make estimates and
assumptions that affect the reported amounts of assets and
liabilities and disclosure of contingent assets and liabilities
at the date of the Consolidated Financial Statements and the
reported amounts of revenues and expenses during the reporting
period. Actual results could differ from those estimates.
Subsequent
Events
Effective June 30, 2009, the Company adopted new accounting
guidance related to subsequent events which requires entities to
evaluate events or transactions occurring after the balance
sheet date through the date of issuance of the condensed
consolidated financial statements to determine whether events
require recognition or nonrecognition and disclosure.
Recognition is required for the effects of all subsequent events
that provide additional evidence about conditions that existed
at the date of the balance sheet, including estimates inherent
in the process of preparing financial statements. Nonrecognized
subsequent events may require disclosure. The Company has
evaluated subsequent events through the issuance date. See
Note 8 for subsequent event related to the Company’s
revolving credit agreements.
New
Accounting Pronouncements
In October 2009, the Financial Accounting Standards Board (FASB)
issued FASB ASC
2009-13,
Revenue Recognition (Topic 605) related to revenue
recognition for multiple deliverable revenue arrangements. The
guidance must be adopted no later than the beginning of the
first fiscal year beginning on or after June 15, 2010
(January 1, 2011 for the Company). The Company is currently
evaluating the impact, if any, of this guidance on its
consolidated financial statements.
Effective January 1, 2009, the Company retrospectively
adopted new accounting guidance related to the accounting for
convertible debt instruments contained in FASB ASC 470. This
guidance requires the liability and equity components of
convertible debt instruments that may be settled in cash upon
conversion to be separately accounted for in a manner that
reflects the issuer’s nonconvertible debt borrowing rate.
The Company applied the guidance to its $200 million (par
value) 3.375% Convertible Notes issuance. The Company
calculated the initial fair value of the debt component of the
Convertible Notes as of the issuance date of July 16, 2007
to be $162.3 million and the resulting value of the
conversion option or equity component to be $37.7 million
based on an interest rate for comparable nonconvertible debt of
8.02%. Additionally, the initial debt issuance costs of
$6.6 million were allocated on a proportional basis
consistent with the debt instrument into debt issuance costs of
$5.4 million and
F-14
equity issuance costs of $1.2 million. The following
financial statement line items for the years ended
December 31, 2008 and 2007 were affected by this accounting
change (in thousands):
Balance
Sheet
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
As of December 31, 2008
|
|
|
|
As Reported
|
|
Adjustments
|
|
As Adjusted
|
|
|
|
Before
|
|
due to
|
|
After
|
|
|
|
ASC 470
|
|
ASC 470
|
|
ASC 470
|
|
|
|
Other assets
|
|
$
|
27,735
|
|
|
$
|
(671
|
)
|
|
$
|
27,064
|
|
|
Total assets
|
|
|
555,559
|
|
|
|
(671
|
)
|
|
|
554,888
|
|
|
Convertible notes
|
|
|
200,000
|
|
|
|
(28,152
|
)
|
|
|
171,848
|
|
|
Total liabilities
|
|
|
370,416
|
|
|
|
(28,152
|
)
|
|
|
342,264
|
|
|
Additional paid in capital
|
|
|
143,608
|
|
|
|
36,412
|
|
|
|
180,020
|
|
|
Retained earnings
|
|
|
27,430
|
|
|
|
(8,931
|
)
|
|
|
18,499
|
|
|
Total shareholders’ equity
|
|
|
185,143
|
|
|
|
27,481
|
|
|
|
212,624
|
|
|
Total liabilities and shareholders’ equity
|
|
|
555,559
|
|
|
|
(671
|
)
|
|
|
554,888
|
|
Statement
of Operations
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
For the Year Ended December 31, 2008
|
|
|
|
As Reported
|
|
Adjustments
|
|
As Adjusted
|
|
|
|
Before
|
|
due to
|
|
After
|
|
|
|
ASC 470
|
|
ASC 470
|
|
ASC 470
|
|
|
|
Interest expense
|
|
$
|
(9,637
|
)
|
|
$
|
(6,254
|
)
|
|
$
|
(15,891
|
)
|
|
Total other income (expense)
|
|
|
(10,920
|
)
|
|
|
(6,254
|
)
|
|
|
(17,174
|
)
|
|
Income before taxes
|
|
|
45,906
|
|
|
|
(6,254
|
)
|
|
|
39,652
|
|
|
Net income
|
|
|
29,397
|
|
|
|
(6,254
|
)
|
|
|
23,143
|
|
|
Earnings per share
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic
|
|
|
1.99
|
|
|
|
(0.42
|
)
|
|
|
1.57
|
|
|
Diluted
|
|
|
1.96
|
|
|
|
(0.42
|
)
|
|
|
1.54
|
|
|
Comprehensive income
|
|
|
40,121
|
|
|
|
(6,254
|
)
|
|
|
33,867
|
|
Statement
of Operations
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
For the Year Ended December 31, 2007
|
|
|
|
As Reported
|
|
Adjustments
|
|
As Adjusted
|
|
|
|
Before
|
|
due to
|
|
After
|
|
|
|
ASC 470
|
|
ASC 470
|
|
ASC 470
|
|
|
|
Interest expense
|
|
$
|
(14,870
|
)
|
|
$
|
(2,677
|
)
|
|
$
|
(17,547
|
)
|
|
Total other income/(expense)
|
|
|
(22,372
|
)
|
|
|
(2,677
|
)
|
|
|
(25,049
|
)
|
|
Income before taxes
|
|
|
30,442
|
|
|
|
(2,677
|
)
|
|
|
27,765
|
|
|
Net income
|
|
|
18,687
|
|
|
|
(2,677
|
)
|
|
|
16,010
|
|
|
Earnings per share
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic
|
|
|
1.29
|
|
|
|
(0.19
|
)
|
|
|
1.10
|
|
|
Diluted
|
|
|
1.26
|
|
|
|
(0.18
|
)
|
|
|
1.08
|
|
|
Comprehensive income
|
|
|
20,205
|
|
|
|
(2,677
|
)
|
|
|
17,528
|
|
F-15
|
|
|
3.
|
FAIR
VALUE OF FINANCIAL INSTRUMENTS:
|
The Company follows accounting guidance related to fair value
measurements which defines fair value and requires that assets
and liabilities carried at fair value be classified and
disclosed in one of the following three categories:
Level 1: Quoted market prices in active
markets for identical assets or liabilities.
Level 2: Observable market based inputs
or unobservable inputs that are corroborated by market data.
Level 3: Unobservable inputs that are not
corroborated by market data.
The Company generally applies fair value techniques on a
non-recurring basis associated with valuing potential impairment
loss related to goodwill, and valuing potential impairment loss
related to long-lived assets.
The following table summarizes the carrying amounts and fair
values of certain financial assets and liabilities at
December 31, 2009:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Quoted Prices in
|
|
|
|
|
|
|
|
|
|
Active Markets for
|
|
Significant Other
|
|
Significant
|
|
|
|
Carrying
|
|
Identical Assets
|
|
Observable Inputs
|
|
Unobservable Inputs
|
|
|
|
Amount
|
|
(Level 1)
|
|
(Level 2)
|
|
(Level 3)
|
|
|
|
(In thousands)
|
|
|
|
Money Market Accounts
|
|
$
|
16,627
|
|
|
$
|
16,627
|
|
|
$
|
—
|
|
|
$
|
—
|
|
The following table summarizes the carrying amounts and fair
values of certain financial assets and liabilities at
December 31, 2008:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Quoted Prices in
|
|
|
|
|
|
|
|
|
|
Active Markets for
|
|
Significant Other
|
|
Significant
|
|
|
|
Carrying
|
|
Identical Assets
|
|
Observable Inputs
|
|
Unobservable Inputs
|
|
|
|
Amount
|
|
(Level 1)
|
|
(Level 2)
|
|
(Level 3)
|
|
|
|
(In thousands)
|
|
|
|
Foreign Currency Hedges
|
|
$
|
17,853
|
|
|
$
|
—
|
|
|
$
|
17,853
|
|
|
$
|
—
|
|
|
Money Market Accounts
|
|
$
|
16,937
|
|
|
$
|
16,937
|
|
|
$
|
—
|
|
|
$
|
—
|
|
The fair values of derivative assets and liabilities traded in
the
over-the-counter
market are determined using quantitative models that require the
use of multiple inputs including interest rates, prices and
indices to generate pricing and volatility factors, which are
used to value the position. The predominant market inputs are
actively quoted and can be validated through external sources,
including brokers, market transactions and third-party pricing
services.
The Company adopted accounting guidance related to fair value
option for financial assets and liabilities and elected not to
apply the fair value option to any eligible financial
instruments. The carrying amounts of cash, accounts receivable,
accounts payable and accruals approximate fair value. The fair
value of the Company’s Convertible Notes was approximately
89% and 76% of the par value at December 31, 2009 and 2008,
respectively. The bond hedges and warrants associated with the
Convertible Notes currently have no value.
Accounts receivable are billed when certain milestones defined
in customer contracts are achieved. All unbilled accounts
receivable are expected to be collected within one year.
| |
|
|
|
|
|
|
|
|
|
|
|
December 31,
|
|
|
|
|
2009
|
|
|
2008
|
|
|
|
|
(In thousands)
|
|
|
|
|
Billed (net of allowance)
|
|
$
|
79,207
|
|
|
$
|
94,860
|
|
|
Unbilled
|
|
|
46,080
|
|
|
|
63,111
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$
|
125,287
|
|
|
$
|
157,971
|
|
|
|
|
|
|
|
|
|
|
|
F-16
The Company maintains an allowance for doubtful accounts
receivable based on historical evidence of accounts receivable
collections and specific identification of accounts receivable
that might cause collection problems. The balance in allowance
for doubtful accounts receivable was as follows:
| |
|
|
|
|
|
Balance at December 31, 2006
|
|
$
|
752
|
|
|
Invoice write-offs
|
|
|
(34
|
)
|
|
Additional expense
|
|
|
236
|
|
|
|
|
|
|
|
|
Balance at December 31, 2007
|
|
$
|
954
|
|
|
Invoice write-offs
|
|
|
(896
|
)
|
|
Additional expense
|
|
|
3,150
|
|
|
Foreign currency adjustments
|
|
|
(141
|
)
|
|
|
|
|
|
|
|
Balance at December 31, 2008
|
|
$
|
3,067
|
|
|
Invoice write-offs
|
|
|
(1,317
|
)
|
|
Additional expense
|
|
|
847
|
|
|
Foreign currency adjustments
|
|
|
86
|
|
|
|
|
|
|
|
|
Balance at December 31, 2009
|
|
$
|
2,683
|
|
|
|
|
|
|
|
Due to the economic climate in the second-half of 2008 and the
tightening of the credit markets, the Company increased its bad
debt reserve, primarily to cover exposure from a limited number
of customers that rely on outside sources to fund their
operations. The Company will continue to monitor its bad debt
exposure and adjust bad debt reserves as necessary.
|
|
|
5.
|
PROPERTY
AND EQUIPMENT:
|
Property and equipment is summarized as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
December 31,
|
|
|
|
|
2009
|
|
|
2008
|
|
|
|
|
(In thousands)
|
|
|
|
|
Furnishings, equipment and other
|
|
$
|
95,786
|
|
|
$
|
85,628
|
|
|
Construction in process
|
|
|
18,395
|
|
|
|
9,862
|
|
|
Equipment under capital leases
|
|
|
467
|
|
|
|
1,616
|
|
|
Less: accumulated depreciation and amortization
|
|
|
(61,109
|
)
|
|
|
(52,528
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Property and equipment, net
|
|
$
|
53,539
|
|
|
$
|
44,578
|
|
|
|
|
|
|
|
|
|
|
|
Depreciation expense for the years ended December 31, 2009,
2008 and 2007 was $10.5 million, $9.8 million and
$8.9 million, respectively.
F-17
|
|
|
6.
|
GOODWILL
AND OTHER INTANGIBLE ASSETS:
|
Non-amortizable
intangible assets at December 31, 2009, and
December 31, 2008, are comprised of:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Early Stage
|
|
|
Late Stage
|
|
|
Goodwill Total
|
|
|
|
|
(In thousands)
|
|
|
|
|
Balance at December 31, 2007
|
|
$
|
4,083
|
|
|
$
|
226,085
|
|
|
$
|
230,168
|
|
|
Additional amounts acquired
|
|
|
9,073
|
|
|
|
—
|
|
|
|
9,073
|
|
|
Additional adjustments
|
|
|
—
|
|
|
|
81
|
|
|
|
81
|
|
|
Tax benefit to reduce goodwill
|
|
|
—
|
|
|
|
(338
|
)
|
|
|
(338
|
)
|
|
Foreign currency fluctuations
|
|
|
(1,609
|
)
|
|
|
(1,046
|
)
|
|
|
(2,655
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance at December 31, 2008
|
|
$
|
11,547
|
|
|
$
|
224,782
|
|
|
$
|
236,329
|
|
|
Contingent consideration paid
|
|
|
5,039
|
|
|
|
—
|
|
|
|
5,039
|
|
|
Foreign currency fluctuations
|
|
|
1,727
|
|
|
|
494
|
|
|
|
2,221
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance at December 31, 2009
|
|
$
|
18,313
|
|
|
$
|
225,276
|
|
|
$
|
243,589
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The goodwill balances presented above are net of accumulated
amortization taken prior to the change in accounting guidance
related to goodwill and an impairment recorded in 2002 totaling
$78 million. The Company acquired approximately
$14.1 million of goodwill as a result of its acquisition of
DecisionLine, see Note 13, Acquisition. The goodwill and
the finite-lived intangible assets acquired in the acquisition
are not deductible for income tax purposes.
Goodwill and other intangible assets consisted of the following:
| |
|
|
|
|
|
|
|
|
|
|
|
As of December 31,
|
|
|
As of December 31,
|
|
|
|
|
2009
|
|
|
2008
|
|
|
|
|
(In thousands)
|
|
|
|
|
Goodwill
|
|
$
|
243,589
|
|
|
$
|
236,329
|
|
|
|
|
|
|
|
|
|
|
|
|
Amortizable intangible assets:
|
|
|
|
|
|
|
|
|
|
Carrying amount:
|
|
|
|
|
|
|
|
|
|
Customer relationships
|
|
$
|
22,169
|
|
|
$
|
22,007
|
|
|
Non-compete agreements
|
|
|
—
|
|
|
|
460
|
|
|
Completed technology
|
|
|
2,600
|
|
|
|
2,600
|
|
|
Backlog
|
|
|
6,200
|
|
|
|
6,200
|
|
|
Internally developed software(a)
|
|
|
17,963
|
|
|
|
17,427
|
|
|
|
|
|
|
|
|
|
|
|
|
Total carrying amount
|
|
$
|
48,932
|
|
|
$
|
48,694
|
|
|
Accumulated Amortization:
|
|
|
|
|
|
|
|
|
|
Customer relationships
|
|
$
|
(8,098
|
)
|
|
$
|
(5,003
|
)
|
|
Non-compete agreements
|
|
|
—
|
|
|
|
(460
|
)
|
|
Completed technology
|
|
|
(1,771
|
)
|
|
|
(1,252
|
)
|
|
Backlog
|
|
|
(5,936
|
)
|
|
|
(5,521
|
)
|
|
Internally developed software(a)
|
|
|
(16,187
|
)
|
|
|
(15,569
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Total accumulated amortization
|
|
$
|
(31,992
|
)
|
|
$
|
(27,805
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Net amortizable intangible assets
|
|
$
|
16,940
|
|
|
$
|
20,889
|
|
|
|
|
|
|
|
|
|
|
|
|
Total goodwill and intangible assets
|
|
$
|
260,529
|
|
|
$
|
257,218
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(a) |
|
Internally developed software is included in Other Assets in the
Company’s Consolidated Balance Sheets. |
F-18
Amortizable intangible assets at December 31, 2009, and
December 31, 2008, are composed of:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(a)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Internally
|
|
|
|
|
Customer
|
|
|
Completed
|
|
|
|
|
|
Developed
|
|
|
|
|
Relationships
|
|
|
Technology
|
|
|
Backlog
|
|
|
Software
|
|
|
|
|
(In thousands)
|
|
|
|
|
Balance at December 31, 2007
|
|
$
|
16,025
|
|
|
$
|
1,864
|
|
|
$
|
1,575
|
|
|
$
|
1,360
|
|
|
Additional amounts acquired
|
|
|
4,930
|
|
|
|
—
|
|
|
|
—
|
|
|
|
1,187
|
|
|
Foreign currency fluctuations
|
|
|
(923
|
)
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
2008 amortization
|
|
|
(3,028
|
)
|
|
|
(516
|
)
|
|
|
(896
|
)
|
|
|
(689
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance at December 31, 2008
|
|
$
|
17,004
|
|
|
$
|
1,348
|
|
|
$
|
679
|
|
|
$
|
1,858
|
|
|
Additional amounts acquired
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
536
|
|
|
Foreign currency fluctuations
|
|
|
662
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
2009 amortization
|
|
|
(3,595
|
)
|
|
|
(519
|
)
|
|
|
(415
|
)
|
|
|
(618
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance at December 31, 2009
|
|
$
|
14,071
|
|
|
$
|
829
|
|
|
$
|
264
|
|
|
$
|
1,776
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(a) |
|
Internally developed software is included in Other Assets in the
Company’s Consolidated Balance Sheets. |
The weighted-average useful life of the Company’s Customer
Relationship intangible assets is approximately 13 years.
Completed technology represents proprietary technology acquired
in the Company’s August 2006 acquisition of CRL Clinical
Services. Value was assigned to the completed technology based
on the technology directly related to revenue generation or
profit enhancement. The value was calculated using an income
approach, which assumes that the value of the technology is
equivalent to the present value of the future stream of economic
benefits that can be derived from its ownership. A useful life
of five years for the intangible asset was determined by
estimating the remaining useful life of the technology acquired.
Backlog represents backlog acquired in the Company’s August
2006 acquisition of CRL Clinical Services. Value was assigned to
backlog by evaluating the expected future economic operating
income generated by the backlog. The useful life of the backlog
of approximately seven years was determined by evaluating the
remaining life of the contracts that compose the backlog
acquired.
Internally-developed software is included in Other Assets within
the Consolidated Financial Statements. The Company typically
amortizes internally-developed software over a five year useful
life.
Amortization expense for the next five years relating to these
amortizable intangible assets is estimated to be as follows:
| |
|
|
|
|
|
|
|
(In thousands)
|
|
|
|
|
2010:
|
|
$
|
3,938
|
|
|
2011:
|
|
|
3,351
|
|
|
2012:
|
|
|
2,261
|
|
|
2013:
|
|
|
1,445
|
|
|
2014:
|
|
|
906
|
|
|
Thereafter:
|
|
|
5,039
|
|
|
|
|
|
|
|
|
Total
|
|
$
|
16,940
|
|
|
|
|
|
|
|
For further detail regarding the amortizable assets acquired in
2008, see Note 13, Acquisition.
F-19
|
|
|
7.
|
OTHER
ACCRUED LIABILITIES:
|
Other accrued liabilities at December 31, 2009 and 2008
consisted of the following:
| |
|
|
|
|
|
|
|
|
|
|
|
December 31,
|
|
|
|
|
2009
|
|
|
2008
|
|
|
|
|
(In thousands)
|
|
|
|
|
Accrued compensation and related payroll withholdings and taxes
|
|
$
|
22,428
|
|
|
$
|
16,640
|
|
|
Income tax payable
|
|
|
4,369
|
|
|
|
2,895
|
|
|
Interest payable
|
|
|
2,390
|
|
|
|
3,113
|
|
|
Other
|
|
|
30,480
|
|
|
|
21,533
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$
|
59,667
|
|
|
$
|
44,181
|
|
|
|
|
|
|
|
|
|
|
|
Through March 2010, the Company was party to a credit agreement
(including all amendments, the “Old Facility”). The
Old Facility was comprised of a revolving loan commitment with a
maximum borrowing capacity of $53.5 million that expires in
August 2011. The Facility contained various affirmative and
negative covenants including financial covenants regarding
maximum leverage ratio, minimum interest coverage ratio and
limitations on capital expenditures. In the first quarter of
2009, the Company sought and received an amendment to the Old
Facility to adjust certain covenants at a cost of $482,000.
These costs were deferred and are being amortized over the
remaining life of the Old Facility.
In March 2010, the Company terminated the Old Facility and
entered into a new credit agreement (the “Facility”).
The Facility is comprised of a $35 million revolving loan
commitment. At the Company’s request and with the consent
of the current lender or additional lenders, the total
commitment may be increased by, or incremental term loans may be
obtained for, up to an additional $15 million. At the
Company’s election, loans under the Facility are available
either at (i) an adjusted base rate plus an applicable
margin or (ii) an adjusted LIBO rate plus an applicable
margin. The applicable margin for each interest rate is
calculated in accordance with the terms of the Facility.
The Facility matures in March 2015, which maturity will
accelerate to January 15, 2012 in the event that five
percent (5%) or more of the currently outstanding principal
amount of the Convertible Notes discussed below have not been
redeemed or repaid in full on or prior to January 15, 2012.
The Facility contains various affirmative and negative covenants
including those regarding limitations on the Company’s
ability to incur certain indebtedness, limitations on certain
investments, limitations on capital expenditures in any fiscal
year and limitations on certain acquisitions and asset sales
outside the ordinary course of business as well as financial
covenants regarding limitations on the Company’s total
leverage ratio, senior secured leverage ratio and interest
coverage ratio. The Company is in compliance with the covenants
as of the date it entered into the Facility.
In connection with the termination of the Old Facility, the
Company intends to write off approximately $665,000 in
unamortized fees during the first quarter of 2010.
The Company also maintains an existing $5.0 million
Multicurrency Facility that is renewable annually and is used in
connection with the Company’s European operations.
No amounts were outstanding under the revolving credit loan
portion of the Old Facility or the Multicurrency Facility at
December 31, 2009 and 2008.
In July 2007, the Company entered into a Purchase Agreement with
UBS Securities LLC (the Underwriter) for the issuance and sale
by the Company of $200 million, including a
$25 million over-allotment of the Company’s
Convertible Notes (Convertible Notes). The Convertible Notes
have a maturity date of July 15, 2012 and were sold to the
Underwriters at a price of $1,000 per Convertible Note, less an
underwriting discount of 3% per Convertible Note. In connection
with this issuance, the Company entered into convertible note
hedge transactions with the participating Underwriter and JP
Morgan Chase (collectively, the counterparties). The convertible
note hedge transactions are comprised of purchased call options
and sold warrants. The purchased call options are expected to
F-20
reduce exposure to potential dilution upon the conversion of the
Convertible Notes. The Company also entered into warrant
transactions with such counterparties. The sold warrants have an
exercise price that is approximately 70% higher than the closing
price of the Company’s common stock on the date the
Convertible Notes were priced. The warrants are expected to
provide the Company with some protection against increases in
our stock price over the conversion price per share.
The discount on the Convertible Notes and the adjusted debt
issuance costs are being amortized into interest expense over
the term of the Convertible Notes using the effective interest
rate method. The adoption of new accounting guidance related to
accounting for convertible debt instruments (see
Note 2-Accounting
Changes) resulted in an adjusted liability amount of
$162.3 million, $165.2 million, and
$171.8 million as of July 16, 2007 (issuance date),
December 31, 2007, and December 31, 2008,
respectively. Additionally, the adoption of the new guidance
resulted in an increase to additional paid in capital for the
conversion option, net of equity issuance costs, of
$36.4 million as of the issuance date. Interest expense for
the years ended December 31, 2007 and December 31,
2008 increased by $2.7 million and $6.3 million,
respectively, over previously reported amounts.
In 2009, the Company repurchased on the open market a portion of
its outstanding Convertible Notes with a par value of
$45.5 million for cash in the amount of $36.5 million.
The amortized book value of the repurchased debt was
$40.0 million, resulting in a net gain of
$2.9 million. Debt issuance costs with a carrying value of
$840,000 were written off in conjunction with all of these
transactions. These transactions resulted in the allocation to
equity of $204,000 of the consideration given for the
repurchase, which was recorded as a reduction to additional paid
in capital. For the entire repurchase program, the related bond
hedges and warrant agreements were proportionately reduced at a
net cost of $97,000 and were also recorded as a reduction to
additional paid in capital.
The carrying amounts of the equity and debt components were as
follows:
| |
|
|
|
|
|
|
|
|
|
|
|
As of December 31,
|
|
|
As of December 31,
|
|
|
|
|
2009
|
|
|
2008
|
|
|
|
|
(In thousands)
|
|
|
|
|
Equity component, carrying amount
|
|
$
|
36,207
|
|
|
$
|
36,412
|
|
|
|
|
|
|
|
|
|
|
|
|
Principal component, at par
|
|
$
|
154,500
|
|
|
$
|
200,000
|
|
|
Unamortized discount
|
|
|
(16,192
|
)
|
|
|
(28,152
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Principal component, carrying amount
|
|
$
|
138,308
|
|
|
$
|
171,848
|
|
|
|
|
|
|
|
|
|
|
|
The net carrying amounts of the Convertible Notes are classified
as long-term in the accompanying Consolidated Balance Sheets.
The debt discount is being amortized, using the effective
interest rate method, over the term of the Convertible Notes
which mature on July 15, 2012. Interest expense on the
Convertible Notes has been recorded at the effective rate of
8.02%.
Interest expense recognized related to the Convertible Notes was
as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
For the Years Ended December 31,
|
|
|
|
|
2009
|
|
|
2008
|
|
|
2007
|
|
|
|
|
(In thousands)
|
|
|
|
|
Interest cost at coupon rate
|
|
$
|
6,096
|
|
|
$
|
6,750
|
|
|
$
|
3,094
|
|
|
Discount amortization
|
|
|
6,456
|
|
|
|
6,631
|
|
|
|
2,873
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total interest expense recognized
|
|
$
|
12,552
|
|
|
$
|
13,381
|
|
|
$
|
5,967
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The Company adopted, effective January 1, 2009, new
accounting guidance related to freestanding contracts that are
indexed to an entity’s own stock. No changes were required
to the Company’s consolidated financial statements as a
result of this pronouncement.
F-21
|
|
|
9.
|
EMPLOYEE
BENEFIT PLANS:
|
401(k)
Plan
The Company maintains a 401(k) retirement plan covering
substantially all U.S. associates that meet minimum age
requirements. The Company historically has made a matching
contribution of 50% of each participant’s contribution of
up to 6% of salary, however this match was temporarily suspended
beginning in July 2009. The Company’s matching
contributions to this plan totaled approximately $1,063,000,
$1,902,000 and $1,873,000 for the years ended December 31,
2009, 2008 and 2007, respectively.
Stock
Option and Stock Incentive Plan
In 1997, the Company established the 1997 Stock Option and Stock
Incentive Plan (including amendments, the “1997 Plan”)
that, as amended, provided for the issuance of up to
3,000,000 shares of the Company’s Common Stock,
including both incentive and non-qualified stock options,
restricted and unrestricted shares, and stock appreciation
rights. The 1997 Plan expired by its terms on August 14,
2007. See below for a discussion of the successor plan.
Participation in the 1997 Plan was at the discretion of the
Board of Directors’ Management Development and Compensation
Committee. Prior to August 2002, the 1997 Plan was administered
by the Board of Directors’ Compensation Subcommittee. The
exercise price of incentive stock options granted under the 1997
Plan must be no less than the fair market value of the Common
Stock, as determined under the 1997 Plan provisions, at the date
the option is granted (110% of fair market value for
shareholders owning more than 10% of the Company’s Common
Stock). The exercise price of non-qualified stock options must
be no less than 95% of the fair market value of the Common Stock
at the date the option is granted. The vesting provisions of the
options granted under the 1997 Plan are determined at the
discretion of the Management Development and Compensation
Committee. The options generally expire either 90 days
after termination of employment or, if earlier, ten years after
date of grant. No options under this 1997 plan could be granted
after its expiration date in August 2007.
Non-vested stock (referred to as “restricted stock” in
the 1997 Plan) may also be granted pursuant to the 1997 Plan.
Restricted shares typically vest ratably over a three year
period, with shares restricted from transfer until vesting. If a
participant ceases to be an eligible employee prior to the
lapsing of transfer restrictions, such shares return to the
Company without consideration. Unrestricted stock may also be
granted to key employees under the 1997 Plan. Unrestricted
shares vest immediately.
At the Annual Meeting of Shareholders on May 10, 2007,
shareholders of the Company approved the 2007 Stock Incentive
Plan (the “2007 Plan”). Under the 2007 Plan, all
employees of the Company and its subsidiaries will be eligible
to receive awards. The 2007 Plan is an “omnibus” stock
plan that provides a variety of equity award vehicles to
maintain flexibility. The 2007 Plan will permit the grant of
stock options, stock appreciation rights, restricted stock
awards, restricted stock units and stock awards. A maximum of
one million shares will be available for grants of all equity
awards under the 2007 Plan. No shares of Common Stock were
granted in 2007 under the 2007 Plan.
In the first quarter of 2008, the Company issued
1,850 shares of non-vested stock which vests over a
12-month
period. Under the terms of the 2007 Plan, the associates that
received the 1,850 shares are considered “retirement
eligible”, which is defined as at least 55 years of
age and 10 years of service with the Company or
65 years of age. Therefore, the entire expense for these
shares was recorded in the first quarter of 2008. The Company
did not issue any shares of non-vested stock in 2009.
Unrestricted stock may also be granted to key employees under
the 2007 Plan. Unrestricted shares vest immediately. In the
third quarter of 2009, the Company granted 3,000 shares of
Common Stock.
In 2008, the Company issued 7,100 shares of time-vested
restricted stock units (RSUs) under the 2007 Plan. The RSUs vest
over a variety of periods ranging from immediate to
36 months. Of the 7,100 shares awarded, 2,400 were
issued to retirement eligible associates and resulted in
immediate vesting and expense recognition. The expense
recognition for the remaining balance of RSUs issued will be
recorded on a straight line basis over the term of the specific
vesting period. In 2009, the Company issued 53,050 shares
of time-vested RSUs. Of this award, 49,050 shares vest in
their entirety after 18 months and the remaining
4,000 shares vest in their entirety after 60 months.
Of the 53,050 shares awarded, 5,000 RSUs were issued to
retirement eligible associates and resulted in
F-22
immediate vesting and expense recognition. The expense for the
remaining RSUs will be recorded on a straight-line basis over
the vesting period.
Also, in the first six months of 2008, the Company issued
15,250 shares of performance-based RSUs under the 2007
Plan. The vesting of these shares is dependent upon a
performance condition; the Company meeting a certain EPS target
for 2008, and a service condition; as 33% vest on
March 15th 2009, 33% vest on January 1, 2010, and
33% vest on January 1, 2011. If the performance condition
is not met at least at the 90% of target level, the award does
not vest. If the performance condition is met at greater than
90% of target level but below 100% of target level, the number
of shares is adjusted to 50% of the original award. Of the total
of 15,250 shares issued, 8,550 were issued in the first
quarter of 2008 to retirement eligible associates. Under the
terms of the award, regardless of whether or not the performance
condition is achieved, should an event similar to retirement
occur during the first half of 2008, one-half of the RSUs
granted would immediately vest. Similarly, should an event
similar to retirement occur during the second half of 2008, the
full amount of the RSUs granted would immediately vest. The
Company therefore recorded one-half of the expense for the
retirement eligible associates in the first quarter of 2008 and
recorded the second half of the expense in the third quarter of
2008. Expense for non retirement-eligible associates is being
recorded over the vesting period (33 months). In the fourth
quarter of 2008, the Company determined that the performance
conditions were not met and the retirement eligible associates
had not retired. Accordingly, none of the granted shares will
vest and therefore all previously recorded expense was reversed.
In 2009, the Company issued 33,200 shares of
performance-based RSUs. Of this award, the vesting of 27,200 of
these shares is dependent upon a performance condition, the
Company meeting a certain EPS target for 2009, and a service
condition, as 33% vest in March 2010, 33% vest in January 2011,
and 33% vest in January 2012. If the performance condition is
not met at least at the 90% of target level, the award does not
vest. If the performance condition is met at greater than 90% of
target level but below 100% of target level, the number of
shares is adjusted to 50% of the original award. Of the total of
33,200 shares issued, 18,000 were issued to retirement
eligible associates. Under the terms of the award, regardless of
whether or not the performance condition is achieved, should an
event similar to retirement occur during the first half of 2009,
one-half of the RSUs granted would immediately vest. Similarly,
should an event similar to retirement occur during the second
half of 2009, the full amount of the RSUs granted would
immediately vest. The Company therefore recorded one-half of the
expense for the retirement eligible associates in the first
quarter of 2009 and recorded the second half of the expense in
the third quarter of 2009. Expense for non retirement-eligible
associates is being recorded over the vesting period. In the
fourth quarter of 2009, the Company determined that the
performance conditions were not met and the retirement eligible
associates had not retired. Accordingly, none of the granted
shares will vest and therefore all previously recorded expense
was reversed.
Stock-based compensation expense related to stock options was
approximately $329,000, $786,000 and $860,000 in 2009, 2008 and
2007, respectively. In 2009, stock-based compensation expense
caused net income to decrease by approximately $256,000.
Stock-based compensation expense in 2008 and 2007 caused net
income to decrease by approximately $626,000 and $690,000,
respectively. Stock-based compensation expense is recorded
primarily in general and administrative expenses in the
Company’s Consolidated Statements of Operations as the
majority of the stock option expense relates to options granted
to executives.
The weighted average fair value of the options granted in 2009,
2008 and 2007 was estimated as $6.03, $18.65 and $12.79,
respectively, on the date of grant using the Black-Scholes
option-pricing model with the following assumptions:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2009
|
|
|
2008
|
|
|
2007
|
|
|
|
|
Expected dividend yield
|
|
|
0
|
%
|
|
|
0
|
%
|
|
|
0
|
%
|
|
Risk-free interest rate
|
|
|
2.2
|
%
|
|
|
3.6
|
%
|
|
|
4.7
|
%
|
|
Expected volatility
|
|
|
70.9
|
%
|
|
|
52.0
|
%
|
|
|
41.9
|
%
|
|
Expected holding period
|
|
|
3.2 years
|
|
|
|
5.0 years
|
|
|
|
4.5 years
|
|
The expected volatility is based on the Company’s stock
price over a historical period which approximates the expected
term of the option as well as a comparison to volatility for
other companies in the Company’s industry and expectations
of future volatility. The risk free interest rate is based on
the implied yield in U.S. Treasury issues with
F-23
a remaining term approximating the expected term of the option.
The expected option term is calculated as the historic weighted
average life of similar awards.
As of December 31, 2009, there was approximately $899,000
of total unrecognized compensation cost, $503,000 of which
relates to options and $396,000 of which relates to non-vested
stock. The cost is expected to be recognized over a weighted
average period of 2.2 years for options and 1.3 years
for non-vested stock.
In addition, stock compensation accounting guidance also
requires the benefits of tax deductions in excess of recognized
compensation expense to be reported as a financing cash flow,
rather than as an operating cash flow. This requirement reduced
net operating cash flows and increased net financing cash flows
by approximately $2,000, $175,000 and $188,000 in 2009, 2008 and
2007, respectively.
Aggregate stock option activity during 2009, 2008 and 2007 was
as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted
|
|
|
|
|
|
|
|
|
|
|
Weighted
|
|
|
Average
|
|
|
Aggregate
|
|
|
|
|
|
|
|
Average
|
|
|
Remaining
|
|
|
Intrinsic
|
|
|
|
|
Shares
|
|
|
Exercise Price
|
|
|
Contractual Life
|
|
|
Value
|
|
|
|
|
($ in thousands)
|
|
|
|
|
Options outstanding at Dec. 31, 2006
|
|
|
797,597
|
|
|
$
|
11.55
|
|
|
|
|
|
|
|
|
|
|
Granted
|
|
|
40,000
|
|
|
|
32.50
|
|
|
|
|
|
|
|
|
|
|
Canceled
|
|
|
(55,056
|
)
|
|
|
10.23
|
|
|
|
|
|
|
|
|
|
|
Exercised
|
|
|
(239,534
|
)
|
|
|
10.98
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Options outstanding at Dec. 31, 2007
|
|
|
543,007
|
|
|
|
13.33
|
|
|
|
|
|
|
|
|
|
|
Granted
|
|
|
68,000
|
|
|
|
38.32
|
|
|
|
|
|
|
|
|
|
|
Canceled
|
|
|
(17,290
|
)
|
|
|
24.94
|
|
|
|
|
|
|
|
|
|
|
Exercised
|
|
|
(179,040
|
)
|
|
|
12.52
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Options outstanding at Dec. 31, 2008
|
|
|
414,677
|
|
|
|
17.35
|
|
|
|
|
|
|
|
|
|
|
Granted
|
|
|
54,250
|
|
|
|
12.37
|
|
|
|
|
|
|
|
|
|
|
Canceled
|
|
|
(53,275
|
)
|
|
|
25.79
|
|
|
|
|
|
|
|
|
|
|
Exercised
|
|
|
(35,777
|
)
|
|
|
6.95
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Options outstanding at Dec. 31, 2009
|
|
|
379,875
|
|
|
|
16.44
|
|
|
|
5.01
|
|
|
$
|
2,328
|
|
|
Exercisable at Dec. 31, 2009
|
|
|
336,425
|
|
|
|
15.33
|
|
|
|
4.53
|
|
|
$
|
2,239
|
|
The intrinsic value of options exercised was approximately
$268,000 in 2009, $6.2 million in 2008 and
$6.7 million in 2007.
At December 31, 2008, the aggregate intrinsic value of
options outstanding was $4.4 million and the aggregate
intrinsic value of options exercisable was $3.8 million.
Intrinsic value for stock options is calculated based on the
difference between the exercise price of the underlying awards
and the quoted price of the Company’s Common Stock as of
the reporting date. Substantially all of the outstanding options
are expected to vest.
F-24
Options Outstanding
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted Average
|
|
|
Weighted
|
|
|
|
|
Outstanding at
|
|
|
Remaining
|
|
|
Average
|
|
|
Range of Exercise Price
|
|
December 31, 2009
|
|
|
Contractual Life
|
|
|
Exercise Price
|
|
|
|
|
$ 3.46 - $ 6.92
|
|
|
44,560
|
|
|
|
3.56
|
|
|
$
|
5.92
|
|
|
$ 6.93 - $10.38
|
|
|
146,290
|
|
|
|
4.49
|
|
|
|
8.40
|
|
|
$10.39 - $13.83
|
|
|
33,750
|
|
|
|
5.10
|
|
|
|
11.81
|
|
|
$13.84 - $17.29
|
|
|
15,500
|
|
|
|
7.18
|
|
|
|
16.21
|
|
|
$17.30 - $20.75
|
|
|
42,775
|
|
|
|
1.63
|
|
|
|
19.82
|
|
|
$20.76 - $24.21
|
|
|
8,000
|
|
|
|
9.11
|
|
|
|
22.28
|
|
|
$24.22 - $27.67
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
$27.68 - $31.13
|
|
|
12,000
|
|
|
|
6.98
|
|
|
|
30.06
|
|
|
$31.14 - $34.59
|
|
|
37,000
|
|
|
|
6.57
|
|
|
|
32.76
|
|
|
$34.60 - $60.00
|
|
|
40,000
|
|
|
|
8.37
|
|
|
|
37.60
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
379,875
|
|
|
|
5.01
|
|
|
|
16.44
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Options Exercisable
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted
|
|
|
|
|
|
|
|
Exercisable at
|
|
|
Average
|
|
|
|
|
|
Range of Exercise Price
|
|
December 31, 2009
|
|
|
Exercise Price
|
|
|
|
|
|
|
|
$ 3.46 - $ 6.92
|
|
|
44,560
|
|
|
$
|
5.92
|
|
|
|
|
|
|
$ 6.93 - $10.38
|
|
|
146,290
|
|
|
|
8.40
|
|
|
|
|
|
|
$10.39 - $13.83
|
|
|
24,000
|
|
|
|
11.56
|
|
|
|
|
|
|
$13.84 - $17.29
|
|
|
5,000
|
|
|
|
16.95
|
|
|
|
|
|
|
$17.30 - $20.75
|
|
|
42,775
|
|
|
|
19.82
|
|
|
|
|
|
|
$20.76 - $24.21
|
|
|
5,600
|
|
|
|
22.45
|
|
|
|
|
|
|
$24.22 - $27.67
|
|
|
—
|
|
|
|
—
|
|
|
|
|
|
|
$27.68 - $31.13
|
|
|
7,200
|
|
|
|
30.06
|
|
|
|
|
|
|
$31.14 - $34.59
|
|
|
37,000
|
|
|
|
32.76
|
|
|
|
|
|
|
$34.60 - $60.00
|
|
|
24,000
|
|
|
|
37.48
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
336,425
|
|
|
$
|
15.33
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
At December 31, 2008, 325,977 options were exercisable with
a weighted-average exercise price of $14.93. At
December 31, 2007, 421,687 options were exercisable with a
weighted-average exercise price of $13.62.
Restricted/Unrestricted
Stock
The Company has granted awards of restricted shares to certain
executives pursuant to the 1997 Plan. Such shares generally vest
ratably over a three-year period, with shares restricted from
transfer until vesting. If a participant ceases to be an
eligible employee prior to the lapsing of transfer restrictions,
such shares return to the Company without consideration.
F-25
Compensation expense related to restricted and unrestricted
stock awards was as follows:
Compensation Expense
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended December 31,
|
|
|
|
|
2009
|
|
|
2008
|
|
|
2007
|
|
|
|
|
(In thousands)
|
|
|
|
|
Restricted stock
|
|
$
|
315
|
|
|
$
|
244
|
|
|
$
|
19
|
|
|
Unrestricted stock
|
|
|
35
|
|
|
|
—
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total stock based compensation
|
|
$
|
350
|
|
|
$
|
244
|
|
|
$
|
19
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
A summary of restricted stock activity is as follows:
Restricted Stock
| |
|
|
|
|
|
|
|
Shares
|
|
|
|
Outstanding at December 31, 2006
|
|
|
6,250
|
|
|
Granted
|
|
|
—
|
|
|
Vested
|
|
|
(6,125
|
)
|
|
Canceled
|
|
|
—
|
|
|
|
|
|
|
|
|
Outstanding at December 31, 2007
|
|
|
125
|
|
|
Granted
|
|
|
24,200
|
|
|
Vested
|
|
|
(125
|
)
|
|
Canceled
|
|
|
(15,250
|
)
|
|
|
|
|
|
|
|
Outstanding at December 31, 2008
|
|
|
8,950
|
|
|
Granted
|
|
|
86,250
|
|
|
Vested
|
|
|
(6,084
|
)
|
|
Canceled
|
|
|
(36,800
|
)
|
|
|
|
|
|
|
|
Outstanding at December 31, 2009
|
|
|
52,316
|
|
|
|
|
|
|
|
The weighted-average per share fair value of restricted shares
vested was $43.32 in 2009, $24.47 in 2008 and $8.63 in 2007. The
weighted-average per share fair value of restricted shares
granted was $15.02 in 2009 and $42.49 in 2008.
In the second and third quarters of 2009, the Company initiated
a series of measures to achieve operating efficiencies and
reduce its cost structure. These measures include workforce
reductions, furloughs, reduction in work week, wage and hiring
freezes, elimination of a portion of employee benefits, strict
controls over discretionary spending and facilities closures,
among other items. The Company recorded $6.4 million of
expense for these restructuring costs. Of this amount,
approximately $1.5 million relates to closure or
consolidation of facilities, net of expected sublease income.
The accrual for the facilities related costs provides for
remaining lease and other contractual payments and will be paid
out over the remaining lease terms. Approximately $518,000 was
charged against the accrual for facilities related costs in
2009, resulting in a remaining accrual of $968,000 as of
December 31, 2009. The remaining $4.9 million in
restructuring costs related to severance, employee benefits and
outplacement expenses for approximately 9% of the Company’s
workforce as of the beginning of the second quarter, all of
which has been paid out as of December 31, 2009. The
employees affected were primarily located in the U.S. and
Western Europe.
Late in the fourth quarter, the Company determined more
reductions were needed, primarily in the U.S. and Western
Europe, to more appropriately match the workforce to the
anticipated demand and workload. As a result,
F-26
the Company committed to an additional workforce action
resulting in $3.8 million in additional severance costs
were accrued for the reduction of an additional 9% of the
workforce as of December 31, 2009.
Of the total restructuring program, almost 60% related to the
Late Stage segment and 40% related to the Support and Other
segment. An immaterial amount related to the Early Stage segment.
|
|
|
11.
|
COMMITMENTS
AND CONTINGENCIES:
|
Leases:
The Company leases facilities, office equipment and computers
under agreements that are classified as either capital or
operating leases. The leases have initial terms that range from
two to seven years, with eight facility leases that have
provisions to extend the leases for an additional three to five
years. Future minimum payments, by year and in the aggregate,
under non-cancelable capital and operating leases with initial
or remaining terms of one year or more, are as follows at
December 31, 2009:
| |
|
|
|
|
|
|
|
|
|
|
|
Capital
|
|
|
Operating
|
|
|
|
|
Leases
|
|
|
Leases
|
|
|
|
|
(In thousands)
|
|
|
|
|
2010
|
|
$
|
55
|
|
|
$
|
18,476
|
|
|
2011
|
|
|
35
|
|
|
|
15,698
|
|
|
2012
|
|
|
—
|
|
|
|
13,038
|
|
|
2013
|
|
|
—
|
|
|
|
9,295
|
|
|
2014
|
|
|
—
|
|
|
|
7,400
|
|
|
Thereafter
|
|
|
—
|
|
|
|
25,091
|
|
|
|
|
|
|
|
|
|
|
|
|
Total minimum lease payments
|
|
|
90
|
|
|
$
|
88,998
|
|
|
|
|
|
|
|
|
|
|
|
|
Amounts representing interest
|
|
|
(4
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Present value of net minimum lease payments
|
|
|
86
|
|
|
|
|
|
|
Current portion
|
|
|
52
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Obligations under capital leases, less current portion
|
|
$
|
34
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Rental expense under operating leases for 2009, 2008 and 2007
was $18.7 million, $16.9 million and
$14.7 million, respectively.
Early in 2008, the Company entered into a lease extension
related to office space at the Company’s principal
executive offices in Cincinnati, Ohio. The agreement extended
the term of the lease from 2009 to 2019 and increased the amount
of space leased from approximately 122,000 square feet to
approximately 143,000 square feet. The new lease extension
terms provide for scheduled rent increases based on the Consumer
Price Index. The Company is accounting for this rent expense
consistent with the accounting guidance related to contingent
rentals. The lease extension provides for up to
$5.2 million in tenant improvement allowances to be used in
connection with leasehold improvement construction, of which
$4.5 million was received in 2009. These allowances will be
amortized as a reduction of rent expense over the term of the
lease.
Protective
Compensation and Benefit Agreements:
The Company has entered into Protective Compensation and Benefit
Agreements with certain associates, including all Executive
Officers of the Company. These Agreements, subject to annual
review by the Company’s Board of Directors, expire on the
last day of the fiscal year, and are automatically extended in
one-year increments unless canceled by the Company. These
Agreements provide for specified benefits in the event of a
change in control, as defined in the Agreements. At
December 31, 2009, the maximum amount which could be
required to be paid under these Agreements, if such events
occur, is approximately $9.8 million.
F-27
Other:
In the fourth quarter of 2008, the Company identified a
programming issue unique to one study and one customer that
required the Company to rework a large portion of the project
and additionally, to bear costs that would, under normal
circumstances, be absorbed by the customer. As a result, in the
fourth quarter of 2008, the Company recorded an accrual for
estimated additional direct costs of approximately
$4.9 million and reduced net service revenues by
approximately $2.3 million. In 2009, based on information
provided by the customer, the Company increased the accrual for
estimated additional direct costs by $2.2 million to a
total of $7.1 million and received an insurance claim
recovery of $5.0 million.
Legal
Proceedings:
In the normal course of business, the Company is a party to
various claims and legal proceedings. The Company records a
reserve for these matters when an adverse outcome is probable
and the amount of the potential liability is reasonably
estimable. Although the ultimate outcome of these matters is
currently not determinable, management of the Company, after
consultation with legal counsel, does not believe that the
resolution of these matters will have a material effect upon the
Company’s financial condition, results of operations or
cash flows for an interim or annual period.
Anti-takeover
Provisions:
The Company has adopted a shareholder rights plan that may have
anti-takeover effects which will make an acquisition of the
Company by another company more difficult. The Company’s
shareholder rights plan provides that, in the event any person
or entity acquires 15% or more of the Company’s outstanding
Common Stock, shareholders of the Company will be entitled to
purchase shares of Common Stock, or in certain instances shares
of the acquirer, at a discounted price. The rights are intended
to discourage a significant share acquisition, merger or tender
offer involving the Company’s Common Stock by increasing
the cost of effecting any such transaction and, accordingly,
could have an adverse impact on a takeover attempt that a
shareholder might consider to be in its best interests.
The provision for income taxes for the years ended
December 31, 2009, 2008, and 2007, is as follows:
Expense/(Benefit)
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2009
|
|
|
2008
|
|
|
2007
|
|
|
|
|
(In thousands)
|
|
|
|
|
Current:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Federal
|
|
$
|
(4,519
|
)
|
|
$
|
2,908
|
|
|
$
|
1,946
|
|
|
State and local
|
|
|
(586
|
)
|
|
|
324
|
|
|
|
510
|
|
|
Foreign
|
|
|
14,401
|
|
|
|
19,702
|
|
|
|
14,195
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Subtotal
|
|
|
9,296
|
|
|
|
22,934
|
|
|
|
16,651
|
|
|
Deferred:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Federal
|
|
|
4,131
|
|
|
|
(3,394
|
)
|
|
|
(3,091
|
)
|
|
State and local
|
|
|
164
|
|
|
|
(828
|
)
|
|
|
(755
|
)
|
|
Foreign
|
|
|
(157
|
)
|
|
|
(2,523
|
)
|
|
|
(1,388
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Subtotal
|
|
|
4,138
|
|
|
|
(6,745
|
)
|
|
|
(5,234
|
)
|
|
Benefit applied to reduce goodwill
|
|
|
—
|
|
|
|
320
|
|
|
|
338
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total provision
|
|
$
|
13,434
|
|
|
$
|
16,509
|
|
|
$
|
11,755
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
F-28
The sources of income (loss) before income taxes are presented
as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2009
|
|
|
2008
|
|
|
2007
|
|
|
|
|
(In thousands)
|
|
|
|
|
United States
|
|
$
|
(19,124
|
)
|
|
$
|
4,406
|
|
|
$
|
(2,157
|
)
|
|
Foreign
|
|
|
47,795
|
|
|
|
35,246
|
|
|
|
29,922
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Income before income taxes
|
|
$
|
28,671
|
|
|
$
|
39,652
|
|
|
$
|
27,765
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The Company’s consolidated effective income tax rate
differed from the U.S. Federal statutory income tax rate of
34% in 2009, 2008 and 2007 as set forth below:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2009
|
|
|
2008
|
|
|
2007
|
|
|
|
|
Income tax expense at the U.S. Federal statutory rate
|
|
|
34.0
|
%
|
|
|
34.0
|
%
|
|
|
34.0
|
%
|
|
Effects of foreign taxes, net of foreign tax credits and
deductions
|
|
|
(6.5
|
)
|
|
|
2.0
|
|
|
|
3.3
|
|
|
State and local income taxes, net of Federal benefit
|
|
|
(1.4
|
)
|
|
|
(1.8
|
)
|
|
|
(0.7
|
)
|
|
Original issue discount-Convertible Notes
|
|
|
(0.9
|
)
|
|
|
0.1
|
|
|
|
0.1
|
|
|
Effects of cross currency hedge unwind
|
|
|
14.2
|
|
|
|
0.0
|
|
|
|
0.0
|
|
|
Effects of deemed foreign dividend
|
|
|
7.8
|
|
|
|
8.3
|
|
|
|
2.6
|
|
|
Other
|
|
|
(0.4
|
)
|
|
|
(1.0
|
)
|
|
|
3.0
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total
|
|
|
46.8
|
%
|
|
|
41.6
|
%
|
|
|
42.3
|
%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
A provision has not been made for U.S. or additional
foreign taxes on the undistributed portion of earnings of
foreign subsidiaries as those earnings have been permanently
reinvested. The undistributed earnings of foreign subsidiaries
approximately $84.7 million. It is not practicable to
determine the amount of the additional taxes that would result
if these earnings were repatriated.
F-29
Components of the Company’s net deferred tax asset and
liability included in the Consolidated Balance Sheets at
December 31, 2009, and 2008 are as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
2009
|
|
|
2008
|
|
|
|
|
(In thousands)
|
|
|
|
|
Deferred tax assets:
|
|
|
|
|
|
|
|
|
|
Compensation and employee benefits
|
|
$
|
1,346
|
|
|
$
|
3,221
|
|
|
Accrued expenses and other future deductible items
|
|
|
5,412
|
|
|
|
4,833
|
|
|
Foreign operating loss carryforward
|
|
|
17,965
|
|
|
|
17,256
|
|
|
State and local operating loss carryforward
|
|
|
2,317
|
|
|
|
1,616
|
|
|
Federal operating loss carryforward
|
|
|
2,873
|
|
|
|
737
|
|
|
Deferred state income taxes
|
|
|
182
|
|
|
|
279
|
|
|
Contributions carryforward
|
|
|
68
|
|
|
|
67
|
|
|
Capital loss carryforward
|
|
|
18
|
|
|
|
18
|
|
|
Foreign tax credit carryforward
|
|
|
10,552
|
|
|
|
3,678
|
|
|
Unrealized foreign exchange losses
|
|
|
3,377
|
|
|
|
533
|
|
|
Stock option expense
|
|
|
455
|
|
|
|
—
|
|
|
Other
|
|
|
3,141
|
|
|
|
2,956
|
|
|
|
|
|
|
|
|
|
|
|
|
Total deferred tax assets
|
|
|
47,706
|
|
|
|
35,194
|
|
|
Deferred tax liabilities:
|
|
|
|
|
|
|
|
|
|
Intangible assets
|
|
|
(1,673
|
)
|
|
|
(1,247
|
)
|
|
Depreciation and software costs
|
|
|
(3,930
|
)
|
|
|
(521
|
)
|
|
Open market repurchase
|
|
|
(995
|
)
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
Total deferred tax liability
|
|
|
(6,598
|
)
|
|
|
(1,768
|
)
|
|
Valuation allowance
|
|
|
(28,080
|
)
|
|
|
(22,368
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Total net deferred tax asset/(liability)
|
|
$
|
13,028
|
|
|
$
|
11,058
|
|
|
|
|
|
|
|
|
|
|
|
As a result of certain realization requirements, the table of
deferred tax assets and liabilities shown above does not include
certain deferred tax assets at December 31, 2009 and 2008
that arose directly from tax deductions related to equity
compensation in excess of compensation recognized for financial
reporting. Equity will be increased by approximately
$3.1 million if and when such deferred tax assets are
ultimately realized. The Company uses tax law ordering for
purposes of determining when excess tax benefits have been
realized.
The Company has a Federal operating loss carryforward of
approximately $8.4 million with a recognized tax benefit of
approximately $2.9 million that will expire in 2027.
The deferred tax asset for state and local operating loss
carryforward of approximately $2.3 million relates to
amounts that expire at various times from 2010 to 2029. The
amount that will expire in 2010 is approximately $24,000. A
valuation allowance has been established for approximately
$505,000 of this tax asset based upon an assessment that it is
more likely than not that realization cannot be assured in
certain tax jurisdictions.
The Company has foreign operating loss carryforwards of
approximately $5.9 million with a recognized tax benefit of
approximately $1.9 million that can be carried forward
indefinitely.
The Company has foreign operating loss carryforwards of
approximately $58.5 million with a tax benefit of
approximately $16.0 million for which a valuation allowance
has been established based upon an assessment that it is more
likely than not that realization cannot be assured. The ultimate
realization of this tax benefit is dependent upon the generation
of sufficient operating income in the respective tax
jurisdictions. Of this benefit, approximately $540,000 will
expire at various times from 2010 to 2020 and approximately
$15.5 million can be carried forward indefinitely.
F-30
The Company has a Federal foreign tax credit carryforward of
approximately $10.5 million that will expire in 2016. A
valuation allowance has been recorded against approximately
$8.5 million of this asset based upon the assessment that
it is more likely than not that realization will not be assured
before expiration.
A valuation allowance has been established for other deferred
tax assets of approximately $3.0 million related to
operations in foreign tax jurisdictions based upon an assessment
that it is more likely than not that realization cannot be
assured.
Yearly activity related to the Company’s valuation
allowance is as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2009
|
|
|
2008
|
|
|
2007
|
|
|
|
|
(In thousands)
|
|
|
|
|
Beginning balance
|
|
$
|
22,368
|
|
|
$
|
19,341
|
|
|
$
|
18,118
|
|
|
Additions charged to expense
|
|
|
1,025
|
|
|
|
9,080
|
|
|
|
3,050
|
|
|
Additions attributable to acquisitions
|
|
|
6,874
|
|
|
|
—
|
|
|
|
—
|
|
|
Reductions from utilization, reassessments and expirations
|
|
|
(2,187
|
)
|
|
|
(6,053
|
)
|
|
|
(1,827
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Ending balance
|
|
$
|
28,080
|
|
|
$
|
22,368
|
|
|
$
|
19,341
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Under current accounting requirements, at December 31,
2009, approximately $12.4 million of the valuation
allowance relates to prior year business combinations. If this
deferred tax asset is subsequently recognized, the Company will
be required to credit the current period’s tax provision
rather than goodwill.
There were no income tax costs (benefits) related to unrealized
gains and losses in Other Comprehensive Income components of
Shareholders’ Equity for any period presented. Where
appropriate, the components of Other Comprehensive Income are
presented net of an approximate 40% tax rate.
At both December 31, 2009 and 2008, the unrealized tax
benefit was approximately $1.8 million, gross of federal
tax benefit. A reconciliation of the beginning and ending amount
of unrecognized tax benefit is as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2009
|
|
|
2008
|
|
|
2007
|
|
|
|
|
(In thousands)
|
|
|
|
|
Beginning Balance
|
|
$
|
1,764
|
|
|
$
|
5,447
|
|
|
$
|
—
|
|
|
Unrecognized tax benefit — adoption
|
|
|
—
|
|
|
|
—
|
|
|
|
6,828
|
|
|
Gross increases — tax positions in prior period
|
|
|
—
|
|
|
|
230
|
|
|
|
167
|
|
|
Gross decreases — tax positions in prior period
|
|
|
—
|
|
|
|
(2,747
|
)
|
|
|
(352
|
)
|
|
Gross increases — tax positions in current period
|
|
|
451
|
|
|
|
384
|
|
|
|
1,663
|
|
|
Gross decreases — tax positions in current period
|
|
|
—
|
|
|
|
—
|
|
|
|
(71
|
)
|
|
Settlements
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
Lapse of statute of limitations
|
|
|
(447
|
)
|
|
|
(1,550
|
)
|
|
|
(2,788
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Unrecognized tax benefit balance at December 31
|
|
$
|
1,768
|
|
|
$
|
1,764
|
|
|
$
|
5,447
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Included in the balance at December 31, 2009, are
approximately $1.8 million of unrecognized tax benefits,
net of federal tax benefit that, if recognized, would affect the
effective tax rate.
The liabilities for unrecognized tax benefits are carried in
other non-current liabilities on the Consolidated Balance Sheets
because the payment of cash is not anticipated within one year
of the balance sheet date for any significant amounts.
Interest and penalties associated with uncertain tax positions
are recognized as components of income tax expense on the
Consolidated Statements of Operations. The Company reduced tax
related interest and penalties in the amount of approximately
$93,000 in its Consolidated Statements of Operations during 2009
and at December 31, 2009, has recorded a liability of
approximately $144,000 for interest and penalties.
The Company files income tax returns in the U.S. federal
jurisdiction, and various states and foreign jurisdictions. With
few exceptions, the Company is no longer subject to
U.S. federal examinations by tax
F-31
authorities for years before 2006 and state and local or foreign
income tax examinations by tax authorities for years before 2005.
The tax years that remain subject to examination for the
Company’s major tax jurisdictions are shown below:
| |
|
|
|
|
|
Jurisdiction
|
|
Open Years
|
|
|
|
United States
|
|
|
2006 - 2009
|
|
|
Germany
|
|
|
2007 - 2009
|
|
|
United Kingdom
|
|
|
2006 - 2009
|
|
|
Netherlands
|
|
|
2008 - 2009
|
|
The Company operates in various state and local jurisdictions.
Open tax years for state and local jurisdictions approximate the
open years reflected above for the United States.
Details pertaining to the Company’s 2008 acquisition are
listed below. The Company had no acquisitions in 2009 or 2007.
The acquisition occurring in 2008 has been accounted for using
the purchase method of accounting.
Acquisition
of DecisionLine Clinical Research Corporation and related
company:
In June 2008, the Company completed its acquisition of 100% of
the outstanding common stock of DecisionLine Clinical Research
Corporation (DecisionLine), an Ontario corporation, and its
related company. DecisionLine, previously privately owned, is a
clinical research organization (CRO) located in Toronto, Ontario
specializing in the conduct of early phase studies. The
acquisition supports the Company’s overall goal of
strategic business expansion and diversification into areas with
high growth opportunities such as Phase I studies. DecisionLine
was integrated into the Company as part of the Company’s
Early Stage segment.
The aggregate purchase price was approximately $18,355,000 in
cash, including acquisition costs, plus net adjustments for
working capital and other items in accordance with the terms and
conditions of the Share Purchase Agreement of $1,276,000. In
addition, there was an earnout provision, as well as an
additional contingent payment upon receipt of certain tax
credits arising from pre-acquisition operations. In March 2009,
the above mentioned payment of certain tax credits was received,
and accordingly recorded as additional goodwill in the amount of
approximately $720,000 (equivalent of $900,000 in Canadian
dollars at the then current exchange rates). Additionally, in
May 2009, an agreement was reached to settle the outstanding
earnout provision for $4.3 million (equivalent of
$5 million in Canadian dollars at the then current exchange
rates) and accelerate the payment date for a portion of the
amount. This additional goodwill was recorded in the second
quarter of 2009.
F-32
The following table summarizes the estimated fair values of the
assets acquired and liabilities assumed at the date of
acquisition.
| |
|
|
|
|
|
|
|
(In thousands at the
|
|
|
|
|
prevailing exchange rate
|
|
|
|
|
at June 2, 2008,
|
|
|
|
|
except contingent
|
|
|
|
|
consideration recorded at appropriate
|
|
|
Purchase Price Allocation
|
|
exchange rate)
|
|
|
|
|
Cash
|
|
$
|
1,562
|
|
|
Accounts receivable
|
|
|
5,922
|
|
|
Other current assets
|
|
|
1,398
|
|
|
Property, plant and equipment
|
|
|
2,913
|
|
|
Other long-term assets
|
|
|
1,196
|
|
|
Intangible assets
|
|
|
4,930
|
|
|
Goodwill
|
|
|
14,112
|
|
|
|
|
|
|
|
|
Total assets acquired
|
|
|
32,033
|
|
|
Advance billings
|
|
|
(3,503
|
)
|
|
Other current liabilities
|
|
|
(2,956
|
)
|
|
Other long-term liabilities
|
|
|
(898
|
)
|
|
|
|
|
|
|
|
Total liabilities assumed
|
|
|
(7,357
|
)
|
|
|
|
|
|
|
|
Net assets acquired
|
|
$
|
24,676
|
|
|
|
|
|
|
|
For the acquisition discussed above, results of operations are
included in the Company’s Consolidated Statements of
Operations from the date of acquisition.
The following unaudited pro forma results of operations assume
the acquisition of DecisionLine occurred at the beginning of
2008:
| |
|
|
|
|
|
|
|
Twelve Months Ended
|
|
|
|
December 31, 2008(1)
|
|
|
|
(In thousands, except per share data)
|
|
|
|
Net service revenues
|
|
$
|
485,778
|
|
|
Income before income taxes
|
|
$
|
41,866
|
|
|
Net income
|
|
$
|
24,302
|
|
|
Net income per diluted share
|
|
$
|
1.62
|
|
|
Weighted average shares
|
|
|
14,993
|
|
|
|
|
|
(1) |
|
As adjusted due to the implementation of accounting guidance
related to the convertible debt. See Note 2: Accounting
Changes. |
Pro forma results for the twelve months ended December 31,
2008 reflect historical restructuring costs of approximately of
$480,000 ($307,000 net of tax).
The pro forma financial information is not necessarily
indicative of the operating results that would have occurred had
the acquisition been consummated as of January 1, 2008, nor
is it necessarily indicative of future operating results.
The Company has a 50%-owned joint venture investment in Beijing
KendleWits Medical Consulting Co., Ltd. (KendleWits), a company
located in China. This investment is accounted for under the
equity method. To date, the Company has contributed
approximately $750,000 for the capitalization of KendleWits. In
2003, the Company determined that its investment in KendleWits
was permanently impaired and as a result recorded a non-cash
charge to reduce the carrying value of its investment to zero.
Future capital investment needs will be dependent upon the
on-going capitalization needs of KendleWits and the
Company’s willingness to provide additional capital. The
Company is not obligated to make any additional investment in
KendleWits and currently has no plans to do so.
F-33
The Company operates its business in two reportable segments,
Early Stage and Late Stage. The Early Stage business currently
focuses on the Company’s Phase I operations, while Late
Stage is comprised of contract services related to Phase II
through IV clinical trials, regulatory affairs and
biometrics offerings. The Late Stage segment changed its
reporting unit structure in 2009. The Late Stage reporting units
still meet the criteria for aggregation and there is no change
to segment reporting as a result of the reorganization. Support
and Other consists of unallocated corporate expenses, primarily
information technology, marketing and communications, human
resources, finance and legal, net of revenues earned for
billable administrative services performed.
Segment information for the years ended December 31, 2009,
2008 and 2007 is as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Early
|
|
|
Late
|
|
|
Support
|
|
|
|
|
|
|
|
Stage
|
|
|
Stage
|
|
|
& Other
|
|
|
Total
|
|
|
|
|
(In thousands)
|
|
|
|
|
Twelve months ended December 31, 2009
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net service revenues
|
|
$
|
37,473
|
|
|
$
|
370,178
|
|
|
$
|
9,037
|
|
|
$
|
416,688
|
|
|
Reimbursable
out-of-pocket
revenues
|
|
|
—
|
|
|
|
135,224
|
|
|
|
—
|
|
|
|
135,224
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total revenues
|
|
$
|
37,473
|
|
|
$
|
505,402
|
|
|
$
|
9,037
|
|
|
$
|
551,912
|
|
|
Operating income (loss)
|
|
$
|
4,187
|
|
|
$
|
81,109
|
|
|
$
|
(49,722
|
)
|
|
$
|
35,574
|
|
|
Total assets
|
|
$
|
48,070
|
|
|
$
|
406,832
|
|
|
$
|
84,821
|
(a)
|
|
$
|
539,723
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Early
|
|
|
Late
|
|
|
Support
|
|
|
|
|
|
|
|
Stage(b)
|
|
|
Stage
|
|
|
& Other
|
|
|
Total
|
|
|
|
|
(In thousands)
|
|
|
|
|
Twelve months ended December 31, 2008
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net service revenues
|
|
$
|
35,199
|
|
|
$
|
430,317
|
|
|
$
|
9,576
|
|
|
$
|
475,092
|
|
|
Reimbursable
out-of-pocket
revenues
|
|
|
—
|
|
|
|
203,489
|
|
|
|
—
|
|
|
|
203,489
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total revenues
|
|
$
|
35,199
|
|
|
$
|
633,806
|
|
|
$
|
9,576
|
|
|
$
|
678,581
|
|
|
Operating income (loss)
|
|
$
|
6,177
|
|
|
$
|
105,140
|
|
|
$
|
(54,491
|
)
|
|
$
|
56,826
|
|
|
Total assets
|
|
$
|
46,431
|
|
|
$
|
423,785
|
|
|
$
|
84,672
|
(a)
|
|
$
|
554,888
|
(c)
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Early
|
|
|
Late
|
|
|
Support
|
|
|
|
|
|
|
|
Stage
|
|
|
Stage
|
|
|
& Other
|
|
|
Total
|
|
|
|
|
(In thousands)
|
|
|
|
|
Twelve months ended December 31, 2007
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net service revenues
|
|
$
|
21,373
|
|
|
$
|
366,379
|
|
|
$
|
9,832
|
|
|
$
|
397,584
|
|
|
Reimbursable
out-of-pocket
revenues
|
|
|
—
|
|
|
|
171,234
|
|
|
|
—
|
|
|
|
171,234
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total revenues
|
|
$
|
21,373
|
|
|
$
|
537,613
|
|
|
$
|
9,832
|
|
|
$
|
568,818
|
|
|
Operating income (loss)
|
|
$
|
2,941
|
|
|
$
|
85,971
|
|
|
$
|
(36,098
|
)
|
|
$
|
52,814
|
|
|
|
|
|
(a) |
|
Primarily comprised of cash and tax-related assets. |
| |
|
(b) |
|
The Early Stage segment results for the twelve months ended
December 31, 2008 include the June (acquisition date)
through December operating results of DecisionLine. |
| |
|
(c) |
|
As adjusted due to the implementation of accounting guidance
related to the convertible debt. See Note 2: Accounting
Changes. |
F-34
Financial information by geographic area is as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
For the Years Ended December 31,
|
|
|
Net Service Revenues
|
|
2009
|
|
|
2008
|
|
|
2007
|
|
|
|
|
(In thousands)
|
|
|
|
|
North American Region:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
United States
|
|
$
|
179,700
|
|
|
$
|
214,935
|
|
|
$
|
196,588
|
|
|
Other
|
|
|
21,166
|
|
|
|
14,411
|
|
|
|
1,654
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$
|
200,866
|
|
|
$
|
229,346
|
|
|
$
|
198,242
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
European Region:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Germany
|
|
$
|
40,475
|
|
|
$
|
56,661
|
|
|
$
|
41,822
|
|
|
United Kingdom
|
|
|
37,792
|
|
|
|
42,303
|
|
|
|
44,145
|
|
|
Other
|
|
|
79,362
|
|
|
|
90,564
|
|
|
|
79,228
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$
|
157,629
|
|
|
$
|
189,528
|
|
|
$
|
165,195
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Latin American Region(1):
|
|
$
|
40,829
|
|
|
$
|
38,996
|
|
|
$
|
20,784
|
|
|
Asia/Pacific Region:
|
|
$
|
17,364
|
|
|
$
|
17,222
|
|
|
$
|
13,363
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total Net Service Revenues
|
|
$
|
416,688
|
|
|
$
|
475,092
|
|
|
$
|
397,584
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(1) |
|
Latin American region includes Mexico |
| |
|
|
|
|
|
|
|
|
|
|
|
For the Years Ended
|
|
|
|
|
December 31,
|
|
|
Identifiable Long-Lived Assets
|
|
2009
|
|
|
2008
|
|
|
|
|
(In thousands)
|
|
|
|
|
North American Region:
|
|
|
|
|
|
|
|
|
|
United States
|
|
$
|
41,711
|
|
|
$
|
33,119
|
|
|
Other
|
|
|
2,414
|
|
|
|
2,275
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$
|
44,125
|
|
|
$
|
35,394
|
|
|
|
|
|
|
|
|
|
|
|
|
European Region:
|
|
|
|
|
|
|
|
|
|
Germany
|
|
$
|
743
|
|
|
$
|
780
|
|
|
United Kingdom
|
|
|
3,606
|
|
|
|
3,344
|
|
|
Other
|
|
|
3,107
|
|
|
|
3,332
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$
|
7,456
|
|
|
$
|
7,456
|
|
|
|
|
|
|
|
|
|
|
|
|
Latin American Region:(1)
|
|
$
|
2,635
|
|
|
$
|
2,507
|
|
|
Asia/Pacific Region:
|
|
$
|
1,099
|
|
|
$
|
1,079
|
|
|
|
|
|
|
|
|
|
|
|
|
Total long-lived assets
|
|
$
|
55,315
|
|
|
$
|
46,436
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(1) |
|
Latin American region includes Mexico |
No other country is material for separate presentation for any
period presented.
In 2009 and 2008, there were no sponsors who individually
accounted for more than 10% of the Company’s consolidated
net service revenues.
F-35
|
|
|
16.
|
QUARTERLY
FINANCIAL DATA (unaudited):
|
Earnings per basic share as presented on the 2009 and 2008
income statements do not equal the sum of earnings per share for
each quarter presented below due to rounding differences in each
quarter.
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Quarter
|
|
First
|
|
|
Second
|
|
|
Third
|
|
|
Fourth
|
|
|
|
|
(In thousands, except per share data)
|
|
|
|
|
2009
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net service revenues
|
|
$
|
108,103
|
|
|
$
|
107,351
|
|
|
$
|
104,588
|
|
|
$
|
96,646
|
|
|
Gross profit
|
|
|
50,126
|
|
|
|
52,147
|
|
|
|
55,674
|
|
|
|
48,155
|
|
|
Income from operations
|
|
|
8,137
|
|
|
|
6,978
|
|
|
|
15,533
|
|
|
|
4,926
|
|
|
Net income
|
|
|
887
|
|
|
|
3,193
|
|
|
|
8,826
|
|
|
|
2,331
|
|
|
Net income per diluted share
|
|
|
0.06
|
|
|
|
0.21
|
|
|
|
0.59
|
|
|
|
0.16
|
|
|
Net income per basic share
|
|
|
0.06
|
|
|
|
0.22
|
|
|
|
0.59
|
|
|
|
0.16
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Quarter
|
|
First
|
|
|
Second
|
|
|
Third
|
|
|
Fourth
|
|
|
|
|
2008
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net service revenues
|
|
$
|
114,124
|
|
|
$
|
126,989
|
|
|
$
|
124,828
|
|
|
$
|
109,151
|
|
|
Gross profit
|
|
|
54,954
|
|
|
|
63,466
|
|
|
|
60,758
|
|
|
|
48,478
|
|
|
Income from operations
|
|
|
13,975
|
|
|
|
16,080
|
|
|
|
15,869
|
|
|
|
10,902
|
|
|
Net income(1)
|
|
|
4,104
|
|
|
|
6,303
|
|
|
|
9,381
|
|
|
|
3,355
|
|
|
Net income per diluted share(1)
|
|
|
0.27
|
|
|
|
0.42
|
|
|
|
0.62
|
|
|
|
0.22
|
|
|
Net income per basic share(1)
|
|
|
0.28
|
|
|
|
0.43
|
|
|
|
0.64
|
|
|
|
0.23
|
|
|
|
|
|
(1) |
|
As adjusted due to the implementation of accounting guidance
related to convertible debt. See Note 2: Accounting Changes. |
F-36
SIGNATURES
Pursuant to the requirements of Section 13 of the
Securities Exchange Act of 1934, the Registrant has duly caused
this report to be signed on its behalf by the undersigned,
thereunto duly authorized.
KENDLE INTERNATIONAL INC.
Candace Kendle, PharmD
Chairman, CEO and Principal Executive Officer
DATE SIGNED: March 16, 2010
Pursuant to the requirements of the Securities Exchange Act of
1934, this report has been signed by the following persons on
behalf of the Registrant and in the capacities and on the dates
indicated.
| |
|
|
|
|
|
|
|
Signature
|
|
Capacity
|
|
Date
|
|
|
|
|
|
|
|
|
|
/s/ Candace
Kendle
Candace
Kendle, PharmD
|
|
Chairman of the Board of Directors, Chief Executive Officer and
Principal Executive Officer
|
|
March 16, 2010
|
|
|
|
|
|
|
|
*
Christopher
C. Bergen
|
|
Executive Vice President and
Chief Administrative Officer
|
|
March 16, 2010
|
|
|
|
|
|
|
|
/s/ Keith
A. Cheesman
Keith
A. Cheesman
|
|
Senior Vice President, Chief Financial Officer and Principal
Financial Accounting Officer
|
|
March 16, 2010
|
|
|
|
|
|
|
|
*
G.
Steven Geis, Ph.D., M.D.
|
|
Director
|
|
March 16, 2010
|
|
|
|
|
|
|
|
*
Donald
C. Harrison, M.D.
|
|
Director
|
|
March 16, 2010
|
|
|
|
|
|
|
|
*
Timothy
E. Johnson, Ph.D.
|
|
Director
|
|
March 16, 2010
|
|
|
|
|
|
|
|
*
Frederick
A. Russ, Ph.D.
|
|
Director
|
|
March 16, 2010
|
|
|
|
|
|
|
|
*
Robert
R. Buck
|
|
Director
|
|
March 16, 2010
|
|
|
|
|
|
|
|
*
Timothy
M. Mooney
|
|
Director
|
|
March 16, 2010
|
|
|
|
|
|
|
|
* /s/ Jarrod
B. Pontius
Jarrod
B. Pontius
|
|
as Attorney In-Fact
|
|
March 16, 2010
|
EXHIBIT INDEX
| |
|
|
|
|
|
|
Exhibit
|
|
|
|
|
|
Number
|
|
Description of Exhibit
|
|
Filing Status
|
|
|
|
|
2
|
.1
|
|
Stock Purchase Agreement between the Company and Charles River
Laboratories International, Inc. dated as of May 9, 2006
|
|
*
|
|
|
2
|
.2
|
|
First Amendment to the Stock Purchase Agreement dated as of
August 16, 2006 between the Company and Charles River
Laboratories, Inc.
|
|
*
|
|
|
3
|
.1
|
|
Restated and Amended Articles of Incorporation
|
|
*
|
|
|
3
|
.2
|
|
Amended and Restated Code of Regulations
|
|
*
|
|
|
3
|
.3
|
|
Amendment of the Restated and Amended Articles of Incorporation
to Increase the Authorized Shares
|
|
*
|
|
|
4
|
|
|
Specimen Common Stock Certificate
|
|
*
|
|
|
4
|
.1
|
|
Shareholder Rights Agreement dated August 14, 2009 between the
Company and American Stock Transfer & Trust Company LLC,
as Rights Agent
|
|
*
|
|
|
4
|
.2
|
|
Indenture dated March 31, 2007 between the Company and LaSalle
Bank National Association
|
|
*
|
|
|
4
|
.3
|
|
Supplemental Indenture No. 1 dated July 16, 2007 between the
Company and LaSalle Bank National Association
|
|
*
|
|
|
10
|
.1
|
|
Indemnity Agreement dated June 21, 1996 by and between the
Company and Candace Kendle Bryan
|
|
*
|
|
|
10
|
.2
|
|
Indemnity Agreement dated June 21, 1996 by and between the
Company and Christopher C. Bergen
|
|
*
|
|
|
10
|
.3
|
|
Indemnity Agreement dated June 21, 1996 by and between the
Company and Timothy M. Mooney
|
|
*
|
|
|
10
|
.4
|
|
Indemnity Agreement dated May 14, 1997 by and between the
Company and Charles A. Sanders
|
|
*
|
|
|
10
|
.5
|
|
Indemnity Agreement dated May 14, 1997 by and between the
Company and Philip E. Beekman
|
|
*
|
|
|
10
|
.6
|
|
Indemnity Agreement dated December 10, 1998 by and between the
Company and Robert Buck
|
|
*
|
|
|
10
|
.7
|
|
Indemnity Agreement dated December 10, 1998 by and between the
Company and Mary Beth Price
|
|
*
|
|
|
10
|
.8
|
|
Form of Indemnity Agreement by and between the Company and each
member of the Company’s Board of Directors, except for
those Indemnity Agreements noted above and filed previously.
|
|
*
|
|
|
10
|
.9
|
|
Confirmation of Convertible Bond Hedge Transaction, dated July
10, 2007, by and between the Company and UBS AG, London Branch
|
|
*
|
|
|
10
|
.10
|
|
Confirmation of Convertible Bond Hedge Transaction, dated July
10, 2007, by and between the Company and JPMorgan Chase Bank,
National Association, London Branch
|
|
*
|
|
|
10
|
.11
|
|
Confirmation of Issuer Warrant Transaction, dated July 10, 2007,
by and between the Company and UBS AG, London Branch
|
|
*
|
|
|
10
|
.12
|
|
Confirmation of Issuer Warrant Transaction, dated July 10, 2007,
by and between the Company and JPMorgan Chase Bank, National
Association, London Branch
|
|
*
|
|
|
10
|
.13
|
|
Lease Agreement dated February 27, 2008 by and between the
Company and Carew Realty, Inc.
|
|
*
|
|
|
10
|
.14
|
|
Credit Agreement dated as of March 15, 2010 by and among
the Company, various Lenders and JPMorgan Chase Bank, N.A., as
Administrative Agent
|
|
Filed herewith
|
|
|
10
|
.15
|
|
Guaranty dated as of March 15, 2010 by and among various
Subsidiaries of the Company in favor of JPMorgan Chase Bank,
N.A., as Administrative Agent
|
|
Filed herewith
|
|
|
10
|
.16
|
|
Pledge and Security Agreement dated as of March 15, 2010 by
and among the Company, various Subsidiaries of the Company and
JPMorgan Chase Bank. N.A., as Administrative Agent
|
|
Filed herewith
|
| |
|
|
|
|
|
|
Exhibit
|
|
|
|
|
|
Number
|
|
Description of Exhibit
|
|
Filing Status
|
|
|
|
|
10
|
.17
|
|
MANAGEMENT CONTRACTS AND COMPENSATION PLANS
|
|
*
|
|
|
|
|
|
(a) 1995 Stock Option and Stock Incentive Plan
|
|
*
|
|
|
|
|
|
(b) 1995 Stock Option and Stock Incentive Plan —
Individual Stock Option Agreement for Incentive Stock Option
(contained in Exhibit 10.20(a))
|
|
*
|
|
|
|
|
|
(c) 1997 Stock Option and Stock Incentive Plan
|
|
*
|
|
|
|
|
|
(c)(1) Amendment No. 1 to 1997 Stock Option and Stock Incentive
Plan
|
|
*
|
|
|
|
|
|
(c)(2) Amendment No. 2 to 1997 Stock Option and Stock Incentive
Plan
|
|
*
|
|
|
|
|
|
(c)(3) Amendment No. 3 to 1997 Stock Option and Stock Incentive
Plan
|
|
*
|
|
|
|
|
|
(c)(4) Form of Restricted Stock Award Agreement
|
|
*
|
|
|
|
|
|
(d) Form of Protective Compensation and Benefit Agreement
|
|
*
|
|
|
|
|
|
(e) 2007 Stock Incentive Plan
|
|
*
|
|
|
|
|
|
(e)(1) Form of Performance-Based Stock Unit Agreement
|
|
*
|
|
|
|
|
|
(f) Annual Incentive Plan
|
|
*
|
|
|
|
|
|
(g) Nonqualified Deferred Compensation Plan
|
|
*
|
|
|
|
|
|
(h) 2003 Directors Compensation Plan
|
|
*
|
|
|
12
|
.1
|
|
Computation of Ratio of Earnings to Fixed Charges
|
|
Filed herewith
|
|
|
14
|
|
|
Code of Ethics
|
|
Available on
the Company’s
website
|
|
|
21
|
|
|
List of Subsidiaries
|
|
Filed herewith
|
|
|
23
|
.1
|
|
Consent of Deloitte & Touche LLP
|
|
Filed herewith
|
|
|
24
|
|
|
Powers of Attorney
|
|
Filed herewith
|
|
|
31
|
.1
|
|
Certification of the Chief Executive Officer pursuant to Rule
13a-14(a)
|
|
Filed herewith
|
|
|
31
|
.2
|
|
Certification of the Chief Financial Officer pursuant to Rule
13a-14(a)
|
|
Filed herewith
|
|
|
32
|
.1
|
|
Certification Pursuant to 18 U.S.C Section 1350 as Adopted
Pursuant to Section 906 of the Sarbanes-Oxley Act of
2002 — Chief Executive Officer
|
|
Filed herewith
|
|
|
32
|
.2
|
|
Certification Pursuant to 18 U.S.C Section 1350 as Adopted
Pursuant to Section 906 of the Sarbanes-Oxley Act of
2002 — Chief Financial Officer
|
|
Filed herewith
|