Attached files
| file | filename |
|---|---|
| EX-31.2 - EXHIBIT 31.2 - AXON ENTERPRISE, INC. | c97679exv31w2.htm |
| EX-32 - EXHIBIT 32 - AXON ENTERPRISE, INC. | c97679exv32.htm |
| EX-31.1 - EXHIBIT 31.1 - AXON ENTERPRISE, INC. | c97679exv31w1.htm |
| EX-23.1 - EXHIBIT 23.1 - AXON ENTERPRISE, INC. | c97679exv23w1.htm |
| EX-21.1 - EXHIBIT 21.1 - AXON ENTERPRISE, INC. | c97679exv21w1.htm |
| EX-10.19 - EXHIBIT 10.19 - AXON ENTERPRISE, INC. | c97679exv10w19.htm |
Table of Contents
UNITED STATES SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form 10-K
(Mark One)
| þ | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2009
or
| o | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to
Commission File Number: 001-16391
TASER International, Inc.
(Exact name of registrant as specified in its charter)
| Delaware (State or other jurisdiction of incorporation or organization) |
86-0741227 (I.R.S. Employer Identification Number) |
|
| 17800 N. 85th St. Scottsdale, AZ (Address of principal executive offices) |
85255 (Zip Code) |
(480) 991-0797
(Registrant’s telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act:
(Registrant’s telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class | Name of each exchange on which registered | |
| Common Stock, $0.00001 | The Nasdaq Global Select Market | |
| par value per share |
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of
the Securities Act. Yes o No þ
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or
Section 15(d) of the Act. Yes o No þ
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by
Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for
such shorter period that the registrant was required to file such reports), and (2) has been
subject to such filing requirements for the past 90 days.
Yes þ No o
Indicate by check mark whether the registrant has submitted electronically and posted on its
corporate Web site, if any, every Interactive Data File required to be submitted and posted
pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period
that the registrant was required to submit and post such files).
Yes o No o
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is
not contained herein, and will not be contained, to the best of registrant’s knowledge, in
definitive proxy or information statements incorporated by reference in Part III of this Form 10-K
or any amendment to this Form 10-K. þ
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a
non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated
filer” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act.
(Check one):
| Large accelerated filer o | Accelerated filer þ | Non-accelerated filer o | Smaller Reporting Company o | |||
| (Do not check if a smaller reporting company) |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of
the Act). Yes o No þ
The aggregate market value of the Common Stock held by non-affiliates of the issuer, based on the
last sales price of the issuer’s common stock on June 30, 2009, which was the last business day of
the registrant’s most recently completed second fiscal quarter, as reported by NASDAQ, was
$268,141,201.
The number of shares of the registrant’s common stock
outstanding as of March 10, 2010, was 62,455,900.
DOCUMENTS INCORPORATED BY REFERENCE
Parts of registrant’s definitive proxy statement to be prepared and filed with the Securities and
Exchange Commission not later than 120 days after December 31, 2009 are incorporated by reference
into Part III of this Form 10-K.
TASER INTERNATIONAL, INC.
ANNUAL REPORT ON FORM 10-K
Year Ended December 31, 2009
TABLE OF CONTENTS
ANNUAL REPORT ON FORM 10-K
Year Ended December 31, 2009
TABLE OF CONTENTS
| Page | ||||||||
| 3 | ||||||||
| 13 | ||||||||
| 21 | ||||||||
| 21 | ||||||||
| 21 | ||||||||
| 21 | ||||||||
| 21 | ||||||||
| 23 | ||||||||
| 24 | ||||||||
| 35 | ||||||||
| 36 | ||||||||
| 63 | ||||||||
| 63 | ||||||||
| 65 | ||||||||
| 66 | ||||||||
| 66 | ||||||||
| 66 | ||||||||
| 66 | ||||||||
| 66 | ||||||||
| 66 | ||||||||
| 68 | ||||||||
| Exhibit 10.19 | ||||||||
| Exhibit 21.1 | ||||||||
| Exhibit 23.1 | ||||||||
| Exhibit 31.1 | ||||||||
| Exhibit 31.2 | ||||||||
| Exhibit 32 | ||||||||
Table of Contents
PART I
The statements contained in this report that are not historical are “forward-looking
statements” within the meaning of Section 27A of the Securities Act of 1933, as amended (the
“Securities Act”), and Section 21E of the Securities Exchange Act of 1934, as amended (the
“Exchange Act”), including statements regarding our expectations, beliefs, intentions or strategies
regarding the future. We intend that such forward-looking statements be subject to the safe-harbor
provided by the Private Securities Litigation Reform Act of 1995. Such forward-looking statements
relate to, among other things:
| 1. | expected revenue and earnings growth; |
| 2. | estimates regarding the size of our target markets; |
| 3. | our ability to continue to successfully penetrate the law enforcement market; |
| 4. | growth expectations for existing accounts; |
| 5. | our ability and strategies to expand product sales to the international, Federal, military, corrections, private security and private citizen self-defense markets; |
| 6. | fluctuations in gross margins; |
| 7. | expansion of product capability and the sufficiency of our manufacturing capacity; |
| 8. | timing and expectations relating to new product and service introductions; |
| 9. | product safety; |
| 10. | our business model and strategy; |
| 11. | the automation of our production process; |
| 12. | plans to develop redundant tooling and capacity; |
| 13. | our insulation from competition and our competitive advantage; |
| 14. | the benefits and competitive advantages of our products and services; |
| 15. | our litigation strategy and the importance of favorable verdicts; |
| 16. | the outcome of legal proceedings we are involved in; |
| 17. | our intention to continue to participate in law enforcement trade shows; |
| 18. | our strategy to grow our international presence; |
| 19. | that we have readily available alternative materials and components suppliers; |
| 20. | the sufficiency and availability of our liquid assets and capital resources; |
| 21. | our plans to invest in data centers and upgrades to our technology and network infrastructure to support our EVIDENCE.COM service; |
| 22. | our intentions about future development efforts and activities, including that we anticipate a reduced level of investment in 2010 for our ECD hardware development, and increased level for our AXON and EVIDENCE.COM services; |
| 23. | trends and expectations relating to certain balance sheet accounts and working capital items; |
| 24. | our expectations, that we will renew our line of credit; |
| 25. | plans relating to our training programs; |
| 26. | the timing and resolution of, and trends relating to, unrecognized tax benefits and liabilities; and |
| 27. | anticipated capital expenditures. |
2
Table of Contents
These statements are qualified by important factors that could cause our actual results to
differ materially from those reflected by the forward-looking statements. Such factors include but
are not limited to those factors detailed in ITEM 1A of this annual report entitled “Risk Factors.”
The risks included in the foregoing list are not exhaustive. Other sections of this report may
include additional factors that could adversely affect our business and financial performance. New
risk factors emerge from time to time, and it is not possible for management to predict all such
factors, nor can it assess the impact of all such risk factors or the extent to which any factor,
or combination of factors, may cause actual results to differ materially from those contained in
any forward-looking statements. We undertake no obligation to update or revise any forward looking
statements to reflect changed assumptions, the occurrence of unanticipated events or changes to
expectations over time.
We own the following trademarks: ADVANCED TASER ®, CHECKLOK®, TASER®, XREP®, and the bolt on
West Hemisphere logo, all registered in the US. All other trademarks and service marks including
M18, M26, X26, X26C, C2, AXON, Shockwave, the bolt within circle logo, and designs belong to TASER
International, Inc., except as expressly indicated as belonging to another.
| Item 1. | Business |
Overview
TASER International, Inc.’s (the Company or TASER or we or our) core mission is to protect
life by providing less dangerous, more effective force options and technologies. We are a market
leader in the development, manufacture and sale of advanced Electronic Control Devices (ECDs)
designed for use in the law enforcement, military, corrections, private security and personal
defense markets. Since our inception in 1993, we have remained committed to providing solutions to
violent confrontation by developing devices with proprietary technology to incapacitate dangerous,
combative, or high-risk subjects who pose a risk to law enforcement officers, innocent citizens, or
themselves in a manner that is generally recognized as a safer alternative to other uses of force.
Our mission to protect life has also been extended to protect truth. We have learned that
bringing a subject into custody is not the end of the challenge for law enforcement. In fact, it
is typically just the beginning since a significant number of incidents that start as a physical
conflict, transition into a legal conflict. Whether it’s prosecuting and convicting the individual
arrested, or responding to excessive use of force allegations, the post-incident legal process is a
considerable part of the challenge law enforcement faces on a continual basis and can often take
years and millions of litigation dollars to resolve in the courtroom. To help law enforcement
address this challenge, we are developing a fully integrated hardware and software solution that
will provide our law enforcement customers the capabilities to capture, store, manage and analyze
video and other digital evidence.
Central to our strategy, we conduct research and develop advanced technologies for both the
creation of new, and the enhancement of existing, hardware and software products and services. We
believe that delivering breakthrough innovation and high-value solutions through our various
product platforms is the key to delivering compelling value propositions to meet our customers’
needs, and to our future growth. We place the highest level of importance on the safety and
appropriate use of our products and have established industry leading training services to provide
our users a comprehensive overview of the legal and policy issues, medical information and risk mitigation
relating to our ECDs and the use of force. Our products are sold through a network of
distribution channels developed for selling and marketing our products and services to law
enforcement agencies, primarily in North America with continuing focus and effort placed on
expanding these programs in international, military and other markets.
Our operations are comprised of one segment — the sale of advanced ECDs and accessories.
Information about sales by geographic region is included in footnote 1(p) of the consolidated
financial statements in Part II, Item 8 of this Annual Report on Form 10-K.
3
Table of Contents
Products
Electronic Control Devices (ECDs)
Our Technology
We make ECDs for two main types of market segments: (a) the law enforcement, military,
corrections and professional security markets, and (b) the consumer market. Our products use a
replaceable cartridge containing compressed nitrogen to deploy and propel two small probes that are
attached to the ECD by insulated conductive wires with lengths ranging from 15 to 35 feet. Our ECDs
transmit electrical pulses along the wires and into the body affecting the sensory and motor
functions of the peripheral nervous system. The energy can penetrate up to two cumulative inches of
clothing, or one inch per probe. The initial effect lasts five seconds for our law enforcement,
military and corrections products and up to thirty seconds for our consumer market models. This
effect can be extended, if necessary, by the operator.
Law Enforcement, Military, Corrections and Professional Security Products
For the law enforcement, military, corrections and professional security markets, we
manufacture three hand-held ECD product lines and have also incorporated our technology into
several other product line extensions.
Our most popular product is the TASER X26 with Shaped Pulse Technology, which we introduced in
2003. Shaped Pulse Technology is a refined energy pulse that concentrates a small portion of energy
to first penetrate any barriers, while the majority of the energy flows into the target freely
after the barrier has been penetrated. The TASER X26 product line consists of the TASER X26,
various cartridges (described below), a digital power magazine (DPM), download software and
equipment, extended warranties, and a number of holstering options and accessories. The TASER X26
product line (excluding sales of the consumer X26C product and individual cartridge sales)
accounted for approximately $53.4 million, or 51% of our net sales, for the year ended December 31,
2009 and for approximately $51.2 million, or 55%, of our net sales, for the year ended December 31,
2008.
In the third quarter of 2009, we introduced the TASER X3, which we believe represents a
significant advancement in capabilities and features over our existing devices. The X3 is a
revolutionary new multi-shot ECD that can engage multiple targets, display Warning Arcs while
loaded, and deliver a calibrated Neuro Muscular Incapacitation (NMI) pulse that results in improved
safety characteristics. While the X3 offers enhanced firepower over existing ECDs, it also
represents a significant leap in sensor and computation power — making it the most intelligent
hand-held force option ever developed.
Our third law enforcement product line is the ADVANCED TASER M26 which we originally launched
in November 1999. The ADVANCED TASER M26 product line consists of the ADVANCED TASER M26, various
cartridges (described below), rechargeable batteries, a battery charging system, data download
software and equipment, extended warranties, and a number of holstering options and accessories.
The ADVANCED TASER M26 product line (excluding individual cartridge sales) accounted for
approximately $3.3 million, or approximately 3%, of our net sales, for the year ended December 31,
2009 and for approximately $2.5 million, or 3%, of our net sales, for the year ended December 31,
2008.
ECD Product Line Extensions
Over the past several years, we have been developing more innovative ways to deploy our
proprietary NMI technology, increasing the capabilities of our systems and extending the range at
which they can be deployed. This resulted in two new products which were introduced to the market
in 2009.
The TASER XREP is a self-contained, wireless ECD that deploys from a 12-gauge pump-action
shotgun. It delivers a similar NMI bio-effect as our X26 handheld ECD, but can be delivered to a
maximum effective range of 100 feet (30.48 meters). The battery supply is fully integrated into the
chassis and provides the power to drive the XREP projectile engine. While the XREP can be fired
from a regular shotgun, we also partnered with Mossberg to develop the TASER X12 Less Lethal
Shotgun (LLS), which is a fully integrated less-lethal platform, utilizing a re-engineered Mossberg
500 pump-action shotgun that is optimized for the XREP. The TASER X12 includes Radial Ammunition
Key technology to prevent the system from deploying lethal 12-gauge rounds in order to remove the
possibility of end users accidentally firing a lethal round in a less-lethal system during high
stress situations.
4
Table of Contents
The TASER Shockwave system is the first generation of TASER Remote Area Denial (TRAD)
technology allowing for both increased safety and stand-off capability during hostile situations
through the use of our NMI technology. The Shockwave is designed as a fully modular system,
allowing the end user complete flexibility to deploy as needed to achieve the desired objective.
Multiple TASER Shockwave units can be stacked together either horizontally in order to extend area
coverage, or vertically to allow multiple salvo engagements; or daisy chained together to maximize
either area coverage or cartridge pattern density. These features provide the capability to project
Area Denial from a secure location. The system minimizes risk as the system can be activated with
the push of a button on the Control Box at a safe stand-off distance of up to 100 meters. The TASER
Shockwave unit deploys its cartridges up to 25-feet, to instantaneously incapacitate multiple
personnel within the field of deployment coverage.
Consumer Products
For the personal defense market our primary product is the TASER C2 consumer product, which we
introduced in 2007. This device is a compact system that provides the same proven NMI effectiveness
as our market leading TASER X26 but in a less intimidating, more compact form factor and at a price
point more attractive to private citizens. Our sale and marketing of the TASER C2 promotes
responsible ownership and aims to prevent misuse by keeping the device inactive until the owner has
successfully completed a background check either online or via a toll-free telephone number.
We also manufacture the TASER X26C, ADVANCED TASER M18 and ADVANCED TASER M18L devices for use
by consumers. The X26C was developed in conjunction with the law enforcement TASER X26 version;
however, its effect lasts longer allowing the owner more time to escape danger. The ADVANCED TASER
M18 and ADVANCED TASER M18L are designed after the law enforcement ADVANCED TASER M26 version;
however, the electrical pulse rate is lower. The ADVANCED TASER M18 and ADVANCED TASER M18L are
identical except that the ADVANCED TASER M18L has an integrated laser-aiming device. These three
product lines consist of the units themselves, air cartridges, batteries and digital power
magazines, and a number of holstering options and accessories.
Our total consumer products accounted for approximately $5.9 million, or 6% of our net sales,
for the year ended December 31, 2009 and for approximately $7.6 million, or 8% of our net sales for
the year ended December 31, 2008.
Cartridges and Other Accessories
We manufacture multiple cartridge types: a 15’ cartridge, a 21’ cartridge, a 25’ XP cartridge,
a 35’ cartridge, a 21’ training cartridge, a 15’ cartridge for the C2 and in 2009 we introduced the
new range of Smart Cartridges for use with the X3. The 15’ cartridge is capable of firing a
distance of 15’ and is sold primarily to the law enforcement market for training and the consumer
market for use in the ADVANCED TASER M18, ADVANCED TASER M18L, and TASER X26C devices. The C2 15’
cartridge is designed specifically for use in the TASER C2. The 21’, 25’ XP, 35’, and 21’ training
cartridge are sold only to the law enforcement, military, and corrections markets. The 25’ XP
cartridge is different from the 21’ cartridge in that it has a longer range and its probes are
longer and heavier, which allows it to penetrate a thicker clothing barrier. The training cartridge
contains non-conductive wiring, which allows law enforcement, military, and corrections trainers to
use the cartridge during training role-playing scenarios. The Smart Cartridges designed for the X3
come in a more compact form factor to accommodate the 3-in-1 multishot capability of the X3, with
ranges of 15’, 25’ and 35’. The Smart Cartridge communicates with the Fire Control System within the
X3, indicating the type of cartridge loaded in each bay and its deployment status. The new static
resistant propulsion system allows the X3 to display NMI arcs without firing the cartridge — which
also reduces the risk of accidental static discharge misfires.
All of our cartridges, with the exception of the training cartridge, contain numerous colored,
confetti-like tags bearing the cartridge’s serial number. These tags, referred to as Anti-Felon
Identification tags, or AFIDs, are scattered when one of our cartridges is fired. We require
sellers of our products to participate in the AFID program by registering buyers of our cartridges.
In many cases, we can use AFIDs to identify the registered owner of cartridges fired.
Individual cartridge sales accounted for approximately $27.9 million, or approximately 27% of
our net sales, for the year ended December 31, 2009 and for approximately $20.5 million, or
approximately 22% of our net sales, for the year ended December 31, 2008.
In 2009, we introduced The TASER Controlled Digital Power Magazine (CDPM), a new accessory for
the TASER X26 device. The CDPM has the same functionality as a regular DPM; however, the CDPM
features a disabling safety key and wrist strap designed to secure the device to the officer. If a
prisoner or suspect attempts to take the TASER X26 device away and breaks the safety key
connection, the system is designed to instantly deactivate the TASER X26.
5
Table of Contents
In 2006, we launched an accessory to the X26 called the TASER Cam. The TASER Cam is a video
recording device that captures both video and audio of potential and actual TASER use incidents.
The device captures video and audio before, during and after a TASER deployment, which provides law
enforcement with a greater level of accountability to support their use of TASER devices against a
resistant subject. The TASER Cam is capable of recording in zero light conditions through the use
of an infrared illuminator. A non audio version of the device is also available for agencies
operating in states where legislation prohibits the use of audio recordings.
In 2004, we introduced an accessory to the X26 that allows the X26 ECD to be attached to
military and law enforcement rifles via a Picatinny rail giving the user lethal and non-lethal
options on the same weapon.
AXON and EVIDENCE.COM
In 2009 we devoted significant resources to the design and development of our new end-to-end
digital evidence collection and management solution — AXON and EVIDENCE.COM.
The AXON is a tactical networkable computer combining advanced audio-video record/capture
capabilities worn by first responders. An audio-video earpiece, imager, speaker and microphone
integrates into the communications loop between existing radios and the communications headset,
recording video of critical incidents from the visual perspective of the officer. AXON
significantly changes officer efficiency by reducing report documentation workload while increasing
accuracy and accountability. EVIDENCE.COM is a virtual evidence warehouse, offering digital storage
in a highly secure, easily accessible environment. From EVIDENCE.COM, both agencies and legal
professionals may quickly access key evidence data without the difficult and sometimes-impossible
inventory searches common to existing storage methods.
We launched initial field trials of the AXON and EVIDENCE.COM in the fourth quarter of 2009
and we anticipate an increasing volume of similar trial programs in 2010. We believe these trial
programs are the best way for our customers to see the powerful capabilities and benefits of this
technology for themselves, and will help drive revenue in 2010.
Product Warranties
We offer a one year limited warranty on all of the TASER X3, TASER X26 and ADVANCED TASER
devices. After the warranty expires, if the device fails to operate properly for any reason, we
will replace the TASER X3 and X26 at a discounted price depending on when the product was placed in
service and replace the ADVANCED TASER device for a fee of $75. These fees are intended to cover
the handling and repair costs and include a profit. We believe this policy is attractive to our law
enforcement, military, and corrections agency customers. In particular, it avoids disputes
regarding the source or cause of any defect. Extended warranties which provide additional coverage
beyond the limited warranty, ranging from one to four years, are also offered for specified fees.
We offer a 90 day limited warranty on the TASER C2 and the X26C devices. Our TASER C2 and the
X26C are designed to disable an attacker for up to 30 seconds. We encourage private citizens to
leave the units and flee after firing them. As a result, we also provide free replacement units to
private citizens who follow this suggested procedure. To qualify for the replacement unit, users
must file a police report that describes the incident and confirms the use of the TASER C2 or the
X26C.
Markets
Law Enforcement and Corrections
Federal, state and local law enforcement agencies in the United States and throughout the
world currently represent the primary target market for our TASER X3, TASER X26 and ADVANCED TASER
device products. In the law enforcement market, more than 15,000 law enforcement agencies in over
50 countries have made initial purchases of our TASER brand devices for testing or deployment. In
addition, approximately 5,000 police departments have purchased or are in the process of purchasing
TASER devices to issue to all of their on duty patrol officers. In 2009, additional federal
agencies began or increased deployment of TASER devices. The U.S. Marshal Agency approved the TASER
X26 for use and decentralized procurement, which made it significantly easier for the regional U.S.
Marshal Offices to make their own purchases. The National Park Service made it mandatory to have
TASER devices in 2007 and 145 Park Service locations carry TASER X26 devices.
6
Table of Contents
We continue to deploy resources for educating correctional facility personnel as well as
parole and probation field officers in the benefits of using TASER brand products. We have
developed training programs and command staff demonstrations specific to the Corrections market and
we attended several Corrections tradeshows and conferences to expand our reach into the market. Our
TASER devices are deployed in county correctional facilities such as those operated by the Los
Angeles Custody Division and Maricopa County Sheriff (AZ). State correctional agencies deploying
TASER devices include Arizona, Arkansas, Colorado, Kentucky, Louisiana, Montana, Nevada, North
Dakota, Oregon, Tennessee, Utah, Washington and Wisconsin.
Military Forces, both United States and Foreign Allies
TASER devices continue to be deployed in support of key strategic military operations in
locations around the world. We continued our focus initiative on supporting our military customers.
We expanded our sales efforts by hiring the former head of the Military Joint Non Lethal Weapons
Directorate as our Vice President of Government and Military Programs in 2007. Additionally, during
2008, we met quarterly with our Senior Executive Advisory Group (SEAG) comprised of a team of
professionals with extensive military, homeland defense and law enforcement experience with the
purpose of advising on business models in support of military users. The business group (Federal
Programs) has concentrated on supporting military and other federal use of our existing products as
well as developing new technology through contracted support. In 2008, we entered into a science
and technology contract with the Joint Non-Lethal Weapons Directorate (JNLWD) of the U.S Department
of Defense to develop a 40mm projectile, compatible with already fielded weapons, which allows for
extension and improvement of the our existing eXtended Range Electronic Projectile (XREP)
technology. The development contract comprises three phases. Phase one commenced in 2008 and was
completed in early 2009. The second phase, contingent upon successful completion of the first, was
started in 2009 and was substantially completed by December 31, 2009. The third phase is optional
and contingent upon successful completion of phase two. In 2007, we received our first long term
Indefinite Quantity Indefinite Delivery (IDIQ) Military contract to provide up to $22.8 million of
product over a five year period through our GSA Distributor.
Private Security
We still continue to pursue opportunities for sales of TASER devices in private security
markets: however, we have made limited sales to date. Private security officers represent a broad
range of individuals, including contract security patrol, healthcare, gaming, retail security
employees and many others. Similar to our other emerging markets, we have developed training
programs and demonstrations specific to the industry by meeting with several large corporate and
private patrol security companies to discover their unique needs. We also attended several private
security tradeshows, conferences and industry association meetings to generate a presence in this
market space.
Private Citizen / Personal Protection
In July 2007, we introduced the TASER C2 personal protector, specifically designed for the
private citizen market. This consumer product contributed approximately 5%, 7% and 4% of our total
net sales in 2009, 2008 and 2007, respectively. While it has been a challenge trying to generate
product traction in a difficult economic climate for consumers, we believe private citizen sales
will continue to be a steady contributor to our business in 2010 as a result of various
distribution relationships and marketing strategies we have put in place to continue to promote
awareness of the TASER C2 in the consumer market.
Sales and Marketing
Law enforcement, federal / military, corrections and security agencies represent our primary
target markets. In each of these markets, the decision to purchase TASER devices is normally made
by a group of people, including the agency head, the agency’s training staff, and weapons experts.
Depending on the size and cost of the device deployment and local procurement rules and customs,
the decision may involve political decision-makers such as city council members or the federal
government. The decision-making process can take as little as a few weeks or as long as several
years. Although we have focused on three primary markets, we have been able to expand our customer
base to thousands of end users within these markets. We currently sell our products to more than
15,000 law enforcement agencies.
7
Table of Contents
Since the introduction of the ADVANCED TASER device in 1999, we have used multiple types of
media to communicate the benefits of acquiring and deploying our products. These campaigns have
included the development of personalized CD/DVD packages geared toward law enforcement leaders in
the community, advertisements in law enforcement publications, and the use of more than 2,400
training classes conducted around the world, and more recently in the case of the TASER X3 an
integrated online media launch including a dedicated website. We also target key regional and
national law enforcement trade shows where we can demonstrate the TASER devices to leading
departments. In 2009, we attended and exhibited at 75 regional, national and international law
enforcement trade shows. We also held our annual U.S. Tactical Conference for the trained master
instructors, and law enforcement training officers, the continued focus of which was to train the
officers in the use of all of the latest ECD’s and other new products.
We plan to continue investment in the area of law enforcement trade shows and conferences in
2010, as it provides us the ability to market our products to our target audience. We believe these
types of activities accelerate penetration of our TASER product lines in each market, which should
lead to increased visibility in both the private security and private citizen markets and reinforce
the value of non-lethal devices for self-defense.
United States Distribution
With the exception of several accounts to which we sell directly, the vast majority of our law
enforcement agency sales in the United States are made through our network of law enforcement
distributors. In addition, we have one military and federal government contracting distributor.
These distributors were selected based upon their reputation within their respective industries,
their contacts, and their distribution network. Our regional managers work closely with the
distributors in their territory to inform and educate the law enforcement communities. We continue
to monitor our law enforcement distributors closely to help ensure that our service standards are
achieved. We also reserve the right to take any large agency order directly to secure the agency’s
account balance with us.
Sales in the private citizen market are primarily made through our commercial distributors and
our web site. We have also established relationships selling to retail chains. We have implemented
a variety of marketing initiatives to support sales of the TASER C2 personal protector. We produced
an infomercial which aired in multiple markets during 2008, hired a professional advertising and
public relations company to assist us in media and press events, and editorial placements and
attended numerous tradeshows specifically to target the consumer market. We continue to sell all
other TASER citizen devices and products through web sales and our established commercial
distributors.
International Distribution
We market and distribute our products to foreign markets through a network of distributors.
For geographical and cultural reasons, our distributors usually have a territory defined by their
country’s borders. These distributors market both our law enforcement, military, and corrections
products, and our consumer products where allowed by law.
Our distributors work with local police, military, and corrections agencies in the same manner
as our domestic market distributors. For example, they perform demonstrations, attend industry
tradeshows, maintain country specific web sites, engage in print advertising, and arrange training
classes.
In 2009, we concentrated our international marketing on the countries that were furthest along
in the testing and purchasing process. These countries included the United Kingdom, Australia, New
Zealand and Brazil. We also plan to continue growing our international presence by expanding our
marketing efforts to a larger number of countries.
We shipped products to approximately 50 countries during fiscal 2009. As a percentage of total
sales, sales outside the U.S. increased to approximately 22% in 2009 from 18% in 2008 and 15% in
2007. Reference is made to Note 1(p) in the accompanying consolidated financial statements in Part
II, Item 8 of this Form 10-K for further information concerning our sales by geographic region.
Training Programs
Most law enforcement, military, security and corrections agencies will not purchase new
weapons until a training program is in place to instruct and certify personnel in their proper use.
We offer a 20 hour class that certifies law enforcement, military, corrections and security agency
trainers as instructors in the use of TASER ECDs. In 2009, we partnered with the Northeast
Wisconsin Technical College (NWTC) to provide an online learning opportunity for new and
re-certifying TASER instructors. As of December 31, 2009, approximately 48,000 law enforcement
officers around the world have been trained and certified as instructors in the proper use of TASER
brand devices. This includes approximately 43,500 officers in the United States and 4,500 in other
countries.
8
Table of Contents
Currently, 1,362 of our certified instructors have undergone further training and became
certified as master instructors. We authorize these individuals to train and certify other law
enforcement, military, corrections and professional security agency trainers as TASER instructors,
not just end-users within their own organization. The master instructors are independent
professional trainers, serve as local area TASER experts, and assist in conducting TASER
demonstrations at other police departments within their regions. In addition, 121 of our certified
instructors have completed the same training and were certified as advanced instructors. Advanced
instructors are authorized to certify others within their own agency as TASER instructors. Since
2001, TASER has held one annual Master Instructor School per year. Due to increased demand, we
conducted a second Master Instructor School in 2009, and are planning to continue hosting two
Master Instructor Schools each year with events on both the East and West coast to accommodate
customer requests. The fee for attending the Master Instructor school
is $500. Military personnel
are trained by our Chief Instructor. Approximately 170 of our master instructors have agreed to
conduct TASER device training classes on a regular basis. We provide logistical support for the
training classes. We charge a fee of $395 for each training attendee who is being certified for the
first time and $195 for recertification. We pay master instructors a per-session training fee for
each session they conduct. We conducted 362 training courses in 2009 and as of December 31, 2009,
we have conducted a cumulative 2,840 training courses during which we have trained more than 48,000
individuals as instructors for TASER ECD’s.
In 2005, we started a TASER Technician course to train agencies on proper care and
preventative maintenance of TASER devices. We charge a fee of $275 to each attendee. In 2009, we
hosted 28 Technician courses, including 18 in the United States and 10 internationally; 234
students attended the Technician course in the United States and 179 attended in other countries.
As of December 31, 2009, approximately 1,762 people have been trained and certified as TASER
Technicians.
In 2008, we started offering a TASER Evidence Collection and Analysis (“ECA”) course to teach
investigators how to collect and analyze TASER ECD related evidence at a crime scene. In 2009 we
conducted seven such ECA courses in the United States and trained 54 people. As of December 31,
2009, 143 have completed the ECA course. The fee for the ECA course is $150 per student. We have
also designed a training course for private citizen customers. Customers who purchase an X26C
device receive a certificate good for a one hour, one-on-one training session with an X26C
certified instructor. We have 843 instructors certified to give the X26C training. In the first
quarter of 2010 we plan to launch a new online training course for consumer instructors. This course
focuses on non-law enforcement private self-defense training schools that have expressed a desire
to include TAASER consumer products in their courses. The course fee will be $129.
In order to coordinate the growing demands of our training programs, we created a Training
Advisory Board. This board annually reviews the qualifications of the master instructors, and
provides retraining or certification as required. In addition, the Training Advisory Board oversees
the trainers and curriculum to ensure that new information is properly communicated and
implemented. The Training Advisory Board also gives input into new product development. We also
created the position of Senior Master Instructor. Twenty four experienced Master Instructors have
been promoted to this position based on their exemplary performance as Master Instructors. Their
primary duties are to perform quality control checks on Master Instructors during an instructor
course and to help instruct at the Master Instructor School. Additionally, we employee 3 staff
Instructors who are full time employees responsible for coordinating course delivery and
development. We also employ a Training Manager who oversees the law enforcement instructor course
and supervises the clerical staff.
Manufacturing
We perform light manufacturing and final assembly operations at our headquarters in
Scottsdale, Arizona and own substantially all of the equipment required to develop, prototype,
manufacture and assemble our finished products. This includes critical injection molds, schematics,
test equipment and prototypes utilized by our supply chain for the production of required raw
materials and sub-assemblies. We have implemented lean/six sigma methodologies to optimize all
direct and indirect resources within the organization which has helped to boost capacity for
existing products, as well as accommodate production of the new TASER products that were introduced
in 2009 and others scheduled for 2010 market release. We are currently operating a single
production shift; however, other capacity options, including the use of a second shift, will be
considered should we experience higher demand resulting from large orders of legacy or new product
releases. We continue to maintain our ISO 9001 certification.
Our XREP product is considered a firearm due to the propellant used to launch it from a
firearm. We have a Class 7 Federal Firearms license to manufacture, store and sell XREP and related
products. We lease facilities from a local third party who specializes in defense products and
provide facilities, ensuring compliance with required firearm and dangerous good standards.
9
Table of Contents
We continuously seek opportunities to invest in automated equipment for the continuous
improvement of product quality, and reduction of manufacturing costs. As a result, we have
implemented a number of equipment initiatives including the purchase and integration of robotic
equipment, computerized laboratory and medical testing equipment, machining and tooling equipment,
as well as sophisticated modeling equipment for our Research and Development Department. In the
fourth quarter of 2009, we completed an ambitious undertaking with the final installation of our
highly automated cartridge assembly line which we believe will significantly improve both our
production capacity and yields, while significantly improving efficiency over what was previously a
very labor intensive manufacturing process.
Our supplier base has and will continue to be a focus for us. We presently purchase finished
circuit boards and components primarily from suppliers located in the United States, along with
strategic relationships internationally. Although we currently obtain plastic components from an
outside supplier base, we own all the designs and tooling, with plans to develop redundant tooling
and capacity in other facilities. We believe there are readily available alternative suppliers in
most cases who can consistently meet our needs for these components. We continue to develop and
implement supply chain strategies to insure that both short and long term objectives are achieved,
while maintaining efficiencies at all levels within the organization.
Competition
Law Enforcement, Corrections and Private Security Markets
The primary competitive factors in the law enforcement and corrections market include a
weapon’s accuracy, effectiveness, safety, cost and ease of use. Stinger Systems, introduced an
electronic device in 2007 to compete with the TASER X26; however, to date, they have had limited
success with less than 1% market share. We believe that our strong relationship with customers, our
large installed base of products, and the significant amount of medical and safety testing already
performed on our products will provide us with a competitive advantage over our competition.
We also believe the ADVANCED TASER, TASER X26 and TASERX3 devices compete indirectly with a
variety of non-lethal alternatives. These alternatives include, but are not limited to, pepper
spray and impact weapons sold by companies such as Armor Holdings, Inc., and Pepperball
Technologies, Inc. We believe our TASER brand device’s advanced technology, versatility,
effectiveness, and low injury rate enable it to compete effectively against these non-lethal
alternatives.
Military Market
In the military markets, both in the United States and abroad, a wide variety of weapon
systems are utilized to accomplish the mission at hand. Conducted energy devices have gained
increased acceptance as a result of the policing role of military personnel in the conflicts in
both Iraq and Afghanistan. There has also been an increased awareness of the use of non-lethal
weapons to preserve human intelligence. TASER devices give our armed forces one means to capture or
immobilize targets without using lethal force. We are the only supplier providing ECDs to these
military agencies. There is indirect competition from pepper spray and impact weapons sold by
companies such as Armor Holdings, Inc. and Pepperball Technologies, Inc.
Private Citizen Market
Electronic control devices have gained limited acceptance in the private citizen market for
non-lethal weapons. These weapons compete with other non-lethal weapons such as batons, clubs, and
chemical sprays. The primary competitive factors in the private citizen market include a weapon’s
cost, effectiveness, safety and ease of use. We believe the widespread adoption of our TASER
devices by prominent law enforcement agencies will help us to further penetrate the private citizen
market.
Video Evidence Market
As we move into the video evidence capture and storage market segment, we are directly
competing in a highly fragmented and competitive market against companies with an established
presence such as the in-car video market. We believe our AXON product, which places the camera
directly on-officer, overcomes some of the inherent limitations that an in-car system brings. When
combined with our EVIDENCE.COM service to store, manage and analyze video events, we believe our
end-to-end solution is a compelling value proposition for law enforcement agencies to evaluate.
10
Table of Contents
Regulation
United States Regulation
The TASER X26, TASER X3, ADVANCED TASER, TASER C2, SHOCKWAVE and AIR TASER devices, as well as
the cartridges used by these devices, are subject to regulations; however, none are considered to
be a “firearm” by the U.S. Bureau of Alcohol, Tobacco, Firearms and Explosives. The TASER XREP,
however, does use a propellant system which falls under the
definition of a “firearm” and is,
therefore, subject to Federal firearms-related regulations specifically applying to the sale and
distribution of these devices within the United States. In the 1980s many states adopted
regulations restricting the sale and use of stun guns, inexpensive hand-held shock devices and
electronic weapons. We believe existing stun gun laws and regulations also apply to our devices.
In 2009, New Jersey’s Attorney General approved a supplemental use of force policy which
allows law enforcement officers in New Jersey to use electronic stun devices in limited
circumstances involving emotionally disturbed individuals. This policy also limits the number of
patrol officer per agency who carry electronic control devices. Until now, New Jersey was the only
state to prohibit the use of ECD’s and stun devices by law enforcement. In 2002 through 2004, we
worked with several law enforcement agencies, government agencies and distributors to overturn
prior legislation preventing the sale of TASER devices to law enforcement agencies in certain
regions of the U.S. These combined efforts were successful in changing the legislation in the
states of Hawaii, Massachusetts and Michigan.
In many cases, the law enforcement and corrections market is subject to different regulations
than the private citizen market. Where different regulations exist, we assume the regulations
affecting the private citizen market also apply to the private security markets except as the
applicable regulations otherwise specifically provide.
As of December 31, 2009, state and local codes prohibit the possession of stun guns, including
TASER ECDs by the general public in Hawaii, Wisconsin, Michigan, Massachusetts, Rhode Island, New
York, New Jersey and the District of Columbia as well as a number of counties, cities and towns.
We are also subject to environmental laws and regulations, including restrictions on the
presence of certain substances in electronic products. Reference is made to Section 1A, Risk
Factors under the heading “Environmental laws and regulations subject us to a number of risks and
could result in significant liabilities and costs”.
United States Export Regulation
Our ECDs are considered a crime control product by the U.S. Government. Accordingly, the
export of our devices is regulated under export administration regulations. As a result, we must
obtain export licenses from the Department of Commerce for all shipments to foreign countries other
than Canada. Most of our requests for export licenses have been granted, and the need to obtain
these licenses has not caused a material delay in our shipments. The need to obtain licenses,
however, has limited or impeded our ability to ship to certain foreign markets. Export regulations
also prohibit the further shipment of our products from foreign markets in which we hold a valid
export license to foreign markets in which we do not hold an export license for our products.
In addition, in 2000, the Department of Commerce adopted regulations restricting the export of
technology used in our devices. These regulations apply to both the technology incorporated in our
device systems and in the processes used to produce them. The technology export regulations do not
apply to production that takes place within the United States, but is applicable to all
sub-assemblies and controlled items manufactured outside the United States.
Foreign Regulation
Foreign regulations, which may affect our devices, are numerous and often unclear. We prefer
to work with a distributor who is familiar with the applicable import regulations in each of our
foreign markets. Experience with foreign distributors in the past indicates that restrictions may
prohibit certain sales of our products in a number of countries. The vast majority of countries
permit TASER devices to be sold and used by Law Enforcement. We rely on our distributors to inform
us of those countries where the TASER device is prohibited or restricted.
Previously, the United Kingdom was among the countries where TASER technologies were
prohibited. However, in January 2003, the British Police announced that the national government
would be backing a TASER pilot program for five police forces within the UK. After a prolonged
period of testing and review for operational effectiveness and medical safety, the British Home
Office announced its intention to authorize the deployment of electronic control devices by all
British police, including non-firearms officers, and in 2008 announced plans to fund 10,000 TASER
ECD’s for police officers in England and Wales, followed by significant purchases of ECDs and
cartridges in 2008 and 2009.
11
Table of Contents
Intellectual Property
We protect our intellectual property with U.S. and foreign patents and trademarks. Our patents
and pending patent applications relate to technology used by us in connection with our products. We
also rely on international treaties, organizations and foreign laws to protect our intellectual
property. As of December 31, 2009, we hold 39 United States patents and 51 foreign patents and also
have numerous patents and trademarks pending. Our patents expire at varying dates ranging between
2010 and 2026. Our earliest expiring United States patent generally covers projectile propellant
devices having a container of compressed gas in place of gunpowder as a propellant. We use this
technology in our cartridges. This patent expires in 2010. We continuously assess whether and where
to seek formal protection for particular innovations and technologies based on such factors as the
commercial significance of our operations and our competitors’ operations in particular countries
and regions; our strategic technology or product directions in different countries; and the degree
to which intellectual property laws exist and are meaningfully enforced in different jurisdictions.
Confidentiality agreements are used with employees, consultants and key suppliers to help
ensure the confidentiality of our trade secrets.
TASER has the exclusive rights to many internet domain names primarily including ‘taser.com’
and ‘evidence.com’.
Research and Development
Our research and development initiatives are conducted in two separate categories. The first
is internally funded research and development, and the second is research externally funded by
customers having requirements for specific capabilities. Both categories focus on next generation
technology, yet are differentiated by the anticipated breadth of the market opportunity, the time
to project completion and accounting treatment. Internally funded research has been primarily
focused on improvements to existing TASER products, or the development of new applications for
TASER technology that we believe generally will have broad market appeal. Externally funded work
focuses on specific packaging or delivery requirements of existing TASER technology that is of high
value to particular customers but may not be viable product solutions to other customers. These
projects generally represent product developments which are long-term in nature and require
external resources or expert consulting.
Research and development initiatives include bio-medical research and electrical, mechanical
and software engineering. We expect that future development projects will focus on extending the
range, improving the functionality and developing new delivery options for our ECD products. In
addition, during 2009 we devoted significant resources to the development of AXON and EVIDENCE.COM
and have established a dedicated software development team in Carpenteria, CA to plan, develop,
test and support the operation of our Software-as-a-Service (SaaS) product.
Our investment in internally funded research and development totaled approximately $20.0
million, $12.9 million and $4.4 million in 2009, 2008 and 2007, respectively. This funding allowed
our R&D department to expand to 62 engineers, technicians and specialists at the end of 2009, 26 of
whom form our software development team at TASER Virtual Systems. Our investment in research and
development staff and equipment continues to represent a significant increase from previous years
and reflects our commitment to maintaining and extending our current technology. Our return on that
investment is intended to be realized over the long term, although new systems and technologies
often have a more immediate impact on our business.
Employees
As of December 31, 2009, we had 358 full-time employees and 102 temporary employees. The
breakdown of our full time employees by department is as follows: 146 direct manufacturing
employees and 212 administrative and manufacturing support employees. Of the 212 administrative and
manufacturing support employees; 56 were involved in sales, marketing, communications and training;
72 were employed in research, development, TASER Virtual Systems and
engineering; 32 were employed
in administrative functions inclusive of executive management, legal, finance and accounting; 11
were employed in information systems technologies; 10 were employed
in quality control and 31 were
employed in manufacturing support functions. Our employees are not covered by any collective
bargaining agreement, and we have never experienced a work stoppage. We believe that our relations
with our employees are good.
Available Information
We were incorporated in Arizona in September 1993 as an ICER Corporation. We changed our name
to AIR TASER, Inc. in December 1993 and to TASER International, Incorporated in April 1998. In
January 2001, we reincorporated in Delaware as TASER International, Inc. Our website is located at
www.TASER.com. Our annual report on Form 10-K, quarterly reports on Form 10-Q, current reports on
Form 8-K and amendments to those reports filed or furnished pursuant
to Section 13(a) or 15(d) of
the Securities Exchange Act of 1934 are available on our website as soon as reasonably practicable
after we electronically file such material with, or furnish such material to, the SEC. Other
information that is not part of this Annual Report on Form 10-K can be accessed through our website
at www.TASER.com.
12
Table of Contents
| Item 1A. | Risk Factors |
Because of the following factors, as well as other variables affecting our operating results,
our past financial performance may not be a reliable indicator of our future performance and
historical trends should not be used to anticipate our results or trends in future periods.
We are materially dependent on acceptance of our products by the law enforcement, both domestic and
international, and federal markets. If law enforcement agencies do not continue to purchase our
products, our revenues will be adversely affected.
A substantial number of law enforcement and corrections agencies may not purchase our
electronic control devices. Law enforcement and corrections agencies may be influenced by claims or
perceptions that conducted energy weapons such as our products are unsafe or may be used in an
abusive manner. In addition, earlier generation conducted energy devices may have been perceived as
ineffective. Sales of our products to these agencies may also be delayed or limited by these claims
or perceptions.
Most of our end-user customers are subject to budgetary and political constraints that may delay or
prevent sales.
Most of our end-user customers are government agencies. These agencies often do not set their
own budgets and therefore have limited control over the amount of money they can spend. In
addition, these agencies experience political pressure that may dictate the manner in which they
spend money. As a result, even if an agency wants to acquire our products, it may be unable to
purchase them due to budgetary or political constraints. Currently, many governmental agencies are
experiencing severe budgetary stresses as a result of the ongoing worldwide recession. There can be
no assurance that the economic and budgeting issues will not worsen and adversely impact sales of
our products. Some government agency orders may also be canceled or substantially delayed due to
budgetary, political or other scheduling delays which frequently occur in connection with the
acquisition of products by such agencies and such cancellations may accelerate or be more severe
than we have experienced historically as a result of the current economic environment.
We may face personal injury, wrongful death and other liability claims that harm our reputation and
adversely affect our sales and financial condition.
Our products are often used in aggressive confrontations that may result in serious, permanent
bodily injury or death to those involved. Our products may be associated with these injuries. A
person injured in a confrontation or otherwise in connection with the use of our products may bring
legal action against us to recover damages on the basis of theories including personal injury,
wrongful death, negligent design, defective product or inadequate warning. We are currently subject
to a number of such lawsuits. We may also be subject to lawsuits involving allegations of misuse of
our products. If successful, personal injury, misuse and other claims could have a material adverse
effect on our operating results and financial condition and could result in negative publicity
about our products. Although we carry product liability insurance, we do incur significant legal
expenses within our self-insured retention in defending these lawsuits and significant litigation
could also result in a diversion of management’s attention and resources, negative publicity and a
potential award of monetary damages in excess of our insurance coverage. The outcome of any
litigation is inherently uncertain and there can be no assurance that our existing or any future
litigation will not have a material adverse effect on our revenues, our financial condition or
financial results.
We substantially depend on sales of our TASER X26 products, and if these products are not widely
accepted, our growth prospects will be diminished.
In the years ended December 31, 2009, 2008 and 2007, we derived our revenues predominantly
from sales of the TASER X26 brand devices and related cartridges, and expect to depend on sales of
these products for the foreseeable future. A decrease in the prices of or demand for these
products, or their failure to achieve broad market acceptance, would significantly harm our growth
prospects, operating results and financial condition.
13
Table of Contents
If we are unable to manage our growth, our prospects may be limited and our future profitability
may be
adversely affected.
We intend to expand our product and service lines and our manufacturing capacity as needed to
meet future demand. Any significant expansion may strain our managerial, financial and other
resources. If we are unable to manage our growth, our business, operating results and financial
condition could be adversely affected. We will need to continually improve our operations,
financial and other internal systems to manage our growth effectively, and any failure to do so may
lead to inefficiencies and redundancies, and result in reduced growth prospects and profitability.
To the extent demand for our products increases, our future success will be dependent upon our
ability to ramp manufacturing production capacity which will be accomplished by the implementation
of customized manufacturing automation equipment.
To the extent demand for our products increases significantly in future periods, one of our
key challenges will be to ramp our production capacity to meet sales demand, while maintaining
product quality. Our primary strategies to accomplish this include increasing the physical size of
our assembly facilities, the hiring of additional production staff, and the implementation of
customized automation equipment. The investments we made in this equipment may not yield the
anticipated labor and material efficiencies. Our inability to meet any future increase in sales
demand or effectively manage our expansion could have a material adverse affect on our revenues,
financial results and financial condition.
Pending litigation may subject us to significant litigation costs, judgments, fines and penalties
in excess of insurance coverage, and divert management attention from our business.
We are involved in numerous litigation matters relating to our products or the use of such
products, litigation against persons who we believe have defamed our products, litigation against
medical examiners who made errors in their autopsy reports, litigation against a competitor and
litigation against former employees. Such matters have resulted and are expected to continue to
result in substantial costs to us and some diversion of our management’s attention, which could
adversely affect our business, financial condition or operating results.
Our future success is dependent on our ability to expand sales through distributors and our
inability to recruit new distributors would negatively affect our sales.
Our distribution strategy is to pursue sales through multiple channels with an emphasis on
independent distributors. Our inability to establish relationships with and retain police equipment
distributors who can successfully sell our products would adversely affect our sales. In addition,
our arrangements with our distributors are generally short-term. If we do not competitively price
our products, meet the requirements of our distributors or end-users, provide adequate marketing
support, or comply with the terms of our distribution arrangements, our distributors may fail to
aggressively market our products or may terminate their relationships with us. These developments
would likely have a material adverse effect on our sales. Our reliance on the sales of our products
by others also makes it more difficult to predict our revenues, cash flow and operating results.
14
Table of Contents
If we are unable to design, introduce and sell new products or new product features successfully,
our business and financial results could be adversely affected.
Our future success will depend on our ability to develop new products or new product features
that achieve market acceptance in a timely and cost-effective manner. The development of new
products and new product features is complex, time consuming and expensive, and we may experience
delays in completing the development and introduction of new products. We cannot provide any
assurance that products that we may develop in the future will achieve market acceptance. If we
fail to develop new products or new product features on a timely basis that achieve market
acceptance, our business, financial results and competitive position could be adversely affected.
Delays in product development schedules may adversely affect our revenues.
The development of software products such as EVIDENCE.COM is a complex and time-consuming
process. New products and enhancements to existing products can require long development and
testing periods. Our increasing focus on our software-as-a-service platform also presents new and
complex development issues. Significant delays in new product or service releases or significant
problems in creating new products or services could adversely affect our revenue.
Acquisitions and joint ventures may have an adverse effect on our business.
We expect to make acquisitions or enter into joint ventures as part of our long-term business
strategy. These transactions involve significant challenges and risks including that the
transaction does not advance our business strategy, that we don’t realize a satisfactory return on
our investment, or that we experience difficulty in the integration of new employees, business
systems, and technology, or diversion of management’s attention from our other businesses. These
events could harm our operating results or financial condition.
We expend significant resources in anticipation of a sale due to our lengthy sales cycle and may
receive no revenue in return.
Generally, law enforcement and corrections agencies consider a wide range of issues before
committing to purchase our products, including product benefits, training costs, the cost to use
our products in addition to or in place of other non-lethal products, budget constraints and
product reliability, safety and efficacy. The length of our sales cycle may range from a few weeks
to as long as several years. Adverse publicity surrounding our products or the safety of such
products has in the past and could in the future lengthen our sales cycle with customers. In the
past, we believe we have experienced revenue decreases in part as the result of adverse effects on
our customers and potential customers of negative publicity surrounding our products or use of our
products. We may incur substantial selling costs and expend significant effort in connection with
the evaluation of our products by potential customers before they place an order. If these
potential customers do not purchase our products, we will have expended significant resources and
received no revenue in return.
Government regulation of our products may adversely affect sales.
Federal regulation of sales in the United States: Most of our devices are not firearms
regulated by the U.S. Bureau of Alcohol, Tobacco, Firearms and Explosives, but are consumer
products regulated by the U.S. Consumer Product Safety Commission. Although there are currently no
federal laws restricting sales of our devices in the United States, future federal regulation could
adversely affect sales of our products.
Federal regulation of international sales: Our devices are controlled as a “crime control”
product by the U.S. Department of Commerce, or DOC, for export directly from the United States.
Consequently, we must obtain an export license from the DOC for the export of our devices from the
United States other than to Canada. Our inability to obtain DOC export licenses on a timely basis
for sales of our devices to our international customers could significantly and adversely affect
our international sales.
15
Table of Contents
State and local regulation: Our devices are controlled, restricted or their use prohibited by
a number of state and local governments. Our devices are banned from private citizen sale or use in
seven states: New York, New Jersey, Rhode Island, Michigan, Wisconsin, Massachusetts and Hawaii.
Law enforcement use of our products is also prohibited in New Jersey. Some municipalities,
including Omaha, Nebraska and Washington, D.C., also prohibit private citizen use of our
products. Other jurisdictions may ban or restrict the sale of our products and our product sales
may be significantly affected by additional state, county and city governmental regulation.
Foreign regulation: Certain foreign jurisdictions prohibit the sale of conducted energy
devices such as our products, limiting our international sales opportunities.
Environmental laws and regulations subject us to a number of risks and could result in significant
liabilities and costs.
We may be subject to various state, federal and international laws and regulations governing
the environment, including restricting the presence of certain substances in electronic products
and making producers of those products financially responsible for the collection, treatment,
recycling and disposal of those products. Environmental legislation within the European Union (EU)
may increase our cost of doing business internationally and impact our revenues from EU countries
as we comply with and implement these requirements.
The EU has published Directives on the restriction of certain hazardous substances in
electronic and electrical equipment (the RoHS Directive) which became effective in July 2006, and
on electronic and electrical waste management (the WEEE Directive). The RoHS Directive restricts
the use of a number of substances, including lead. The WEEE Directive directs members of the
European Union to enact laws, regulations, and administrative provisions to ensure that producers
of electric and electronic equipment are financially responsible for the collection, recycling,
treatment and environmentally responsible disposal of certain products sold into the market after
August 15, 2005 and from products in use prior to that date that are being replaced. In addition,
similar environmental legislation has been or may be enacted in other jurisdictions, including the
U.S. (under federal and state laws) and other countries, the cumulative impact of which could be
significant.
We continue to monitor the impact of specific registration and compliance activities required
by the RoHS and WEEE Directives. We endeavor to comply with applicable environmental laws, yet
compliance with such laws could increase our operations and product costs; increase the
complexities of product design, procurement, and manufacturing; limit our ability to manage excess
and obsolete non-compliant inventory; limit our sales activities; and impact our future financial
results. Any violation of these laws can subject us to significant liability, including fines,
penalties, and prohibiting sales of our products into one or more states or countries, and result
in a material adverse effect on our financial condition.
If we are unable to protect our intellectual property, we may lose a competitive advantage or incur
substantial litigation costs to protect our rights.
Our future success depends upon our proprietary technology. Our protective measures, including
patents, trademarks and trade secret protection, may prove inadequate to protect our proprietary
rights. The right to stop others from misusing our trademarks and service marks in commerce depends
to some extent on our ability to show evidence of enforcement of our rights against such misuse in
commerce. Our efforts to stop improper use, if insufficient, may lead to loss of trademark and
service mark rights, brand loyalty and notoriety among our customers and prospective customers. Our
earliest expiring United States patent generally covers projectile propellant devices having a
container of compressed gas in place of gunpowder as a propellant. We use this technology in our
cartridges. This patent expires in 2010. The scope of any patent to which we have or may obtain
rights may not prevent others from developing and selling competing products. The validity and
breadth of claims covered in technology patents involve complex legal and factual questions, and
the resolution of such claims may be highly uncertain, lengthy and expensive. In addition, our
patents may be held invalid upon challenge, or others may claim rights in or ownership of our
patents.
We have a pending lawsuit in Federal District Court against Stinger Systems that alleges
infringement of three of our U.S. patents: 6,999,295; 7,102,870; and 7,234,262. In an infringement
case, the judge in a Markman hearing before trial begins can resolve disagreements on the meaning
of some of the terminology of the patent claims. In the pending case, the holding from the Markman
hearing largely adopted the meanings proposed by TASER. Nevertheless, Stinger Systems is expected
to challenge the validity of the patents at trial. If at trial the patents are upheld, the extent
of relief to TASER, including whether Stinger is enjoined and/or forced to pay damages, cannot be
predicted.
16
Table of Contents
We may be subject to intellectual property infringement claims, which could cause us to incur
litigation costs and divert management attention from our business.
Any intellectual property infringement claims against us, with or without merit, could be
costly and time-consuming to defend and divert our management’s attention from our business. If our
products were found to infringe a third party’s
proprietary rights, we could be forced to enter into costly royalty or licensing agreements in
order to be able to sell our products or discontinue use of the protected technology. Such royalty
and licensing agreements may not be available on terms acceptable to us or at all.
If we face competition in foreign countries, we can enforce patent rights only in the jurisdictions
in which our patent applications have been granted.
Our U.S. patents only protect us from imported infringing products coming into the U.S. from
abroad. Applications for patents in a few foreign countries have been made; however, these may be
inadequate to protect markets for our products in other foreign countries. Each foreign patent is
examined and granted according to the law of the country where it was filed independent of whether
a U.S. patent on similar technology was granted.
Our efforts to avoid the patent, trademark, and copyright rights of others may not provide notice
to us of potential infringements in time to avoid investing in product development and promotion
that must later be abandoned if suitable license terms cannot be reached.
There is no guarantee that our use of conventional technology searching and brand clearance
searching will identify all potential rights holders. Rights holders may demand payment for past
infringements and/or force us to accept costly license terms or discontinue use of protected
technology and/or works of authorship that may include for example photos, videos, and software.
Our current research and development focus on developing software-based products increases this
risk.
Government regulations applied to our products may affect our markets for these products.
We rely on the opinions of The Bureau of Alcohol Tobacco and Firearms, including the
determination that a device that has projectiles propelled by the release of compressed gas, in
place of the expanding gases from ignited gunpowder, are not classified as firearms. Changes in
statutes, regulations, and interpretation outside of our control may result in our products being
classified or reclassified as firearms. Our private citizen market could be substantially reduced
if consumers are required to obtain registration to own a firearm prior to purchasing our products.
Defects in our products could reduce demand for our products and result in a loss of sales, delay
in market acceptance and injury to our reputation.
Complex components and assemblies used in our products may contain undetected defects that are
subsequently discovered at any point in the life of the product. Defects in our products may result
in a loss of sales, delay in market acceptance and injury to our reputation and increased warranty
costs.
We
face risks associated with medical safety concerns and media
publicity concerning allegations of deaths and injuries occurring
after use of the TASER ECD and the negative effect this publicity
could have on our sales.
Law
enforcement personnel are frequently called upon to deal with
individuals in crisis who are psychologically compromised and are at
a heightened risk of serious injury or death, regardless of actions
taken by law enforcement. While the TASER ECD has been shown to be a
safer than traditional uses of force, it is not risk free and medical
concerns have been raised concerning its use. These concerns and the
associated negative publicity could have a negative impact on our
sales and customer relations.
17
Table of Contents
Our dependence on third party suppliers for key components of our devices could delay shipment of
our products and reduce our sales.
We depend on certain domestic and foreign suppliers for the delivery of components used in the
assembly of our products. Our reliance on third-party suppliers creates risks related to our
potential inability to obtain an adequate supply of components or sub-assemblies and reduced
control over pricing and timing of delivery of components and sub-assemblies. Specifically, we
depend on suppliers of sub-assemblies, machined parts, injection molded plastic parts,
printed circuit boards, custom wire fabrications and other miscellaneous customer parts for our
products. We do not have long-term agreements with any of our suppliers and there is no guarantee
that supply will not be interrupted. Any interruption of supply for any material components of our
products could significantly delay the shipment of our products and have a material adverse effect
on our revenues, profitability and financial condition.
Component shortages could result in our inability to produce volume to adequately meet customer
demand, which could result in a loss of sales, delay in deliveries and injury to our reputation.
Single source components used in the manufacture of our products may become unavailable or
discontinued. Delays caused by industry allocations, or obsolescence may take weeks or months to
resolve. In some cases, parts obsolescence may require a product re-design to ensure quality
replacement components. These delays could cause significant delays in manufacturing and loss of
sales, leading to adverse effects significantly impacting our financial condition or results of
operations.
Our dependence on foreign suppliers for key components of our products could delay shipment of our
finished products and reduce our sales.
We depend on foreign suppliers for the delivery of certain components used in the assembly of
our products. Due to changes imposed for imports of foreign products into the United States, as
well as potential port closures and delays created by terrorist threats, public health issues or
national disasters, we are exposed to risk of delays caused by freight carriers or customs
clearance issues for our imported parts. Delays caused by our inability to obtain components for
assembly could have a material adverse effect on our revenues, profitability and financial
condition.
We may experience a decline in gross margins due to rising raw material and transportation costs
associated with a future increase in petroleum prices.
A significant number of our raw materials are comprised of petroleum based products, or incur
some form of landed cost associated with transporting the raw materials or components to our
facility. A significant rise in oil prices could adversely impact our ability to sustain current
gross margins, by increasing component pricing.
Catastrophic events may disrupt our business.
A disruption or failure of our systems or operations in the event of a major earthquake,
weather event, cyber-attack, terrorist attack, or other catastrophic event could cause delays in
completing sales, providing services, or performing other mission-critical functions. Our software
related research and development and primary EVIDENCE.COM data center are located in Southern
California, located near major earthquake faults. A catastrophic event that results in the
destruction or disruption of any of our critical business or information technology systems could
harm our ability to conduct normal business operations and our operating results.
We may experience outages and disruptions of our EVIDENCE.COM service if we fail to maintain an
adequate operations infrastructure.
We anticipate increasing user traffic related to the introduction of EVIDENCE.COM. The
complexity of this Software-as-a-Service (SaaS) product will demand significant computing power. We
have spent and expect to continue to spend substantial amounts to purchase or lease data centers
and equipment and to upgrade our technology and network infrastructure to handle an anticipated
increase in traffic. This expansion is expensive, complex, and could result in inefficiencies or
operational failures, which could diminish the quality of our products, services, and user
experience, resulting in damage to our reputation and loss of current and potential users,
subscribers, and advertisers, harming our operating results and financial condition.
18
Table of Contents
Our revenues and operating results may fluctuate unexpectedly from quarter to quarter, which may
cause our stock price to decline.
Our revenues and operating results have varied significantly in the past and may vary
significantly in the future due to various factors, including, but not limited to:
| • | budgetary cycles of municipal, state and federal law enforcement and corrections agencies | ||
| • | market acceptance of our products and services | ||
| • | the timing of large orders | ||
| • | the outcome of any existing or future litigation | ||
| • | adverse publicity surrounding our products, the safety of our products, or the use of our products | ||
| • | changes in our sales mix | ||
| • | new product introduction costs | ||
| • | increased raw material expenses | ||
| • | changes in our operating expenses | ||
| • | regulatory changes that may affect the marketability of our products |
As a result of these and other factors, we believe that period-to-period comparisons of our
operating results may not be meaningful in the short term, and our performance in a particular
period may not be indicative of our performance in any future period.
We may experience difficulties in the future in complying with Sarbanes-Oxley Section 404.
We are required to evaluate our internal controls under Section 404 of the Sarbanes-Oxley Act
of 2002. Beginning with our annual report on Form 10-K for the fiscal year ending December 31,
2005, we have been required to furnish a report by our management on our internal control over
financial reporting. Such report contains among other matters, an assessment of the effectiveness
of our internal control over financial reporting as of the end of our fiscal year, including a
statement as to whether or not our internal control over financial reporting is effective. Such
report also contains a statement that our independent registered public accounting firm has issued
an attestation report on management’s assessment of such internal controls. If we fail to maintain
proper and effective internal controls in future periods, it could adversely affect our operating
results, financial condition and our ability to run our business effectively and could cause
investors to lose confidence in our financial reporting. We expect to continue to incur expense and
to devote management resources to Section 404 compliance. In the event that our chief executive
officer, chief financial officer or our independent registered public accounting firm determine
that our internal control over financial reporting is not effective as defined under Section 404,
investor confidence in us may be adversely affected and could cause a decline in the market price
of our stock.
Foreign currency fluctuations may affect our competitiveness and sales in foreign markets.
The relative change in currency values creates fluctuations in our product pricing for
potential international customers. These changes in foreign end-user costs may result in lost
orders and reduce the competitiveness of our products in certain foreign markets. These changes may
also negatively affect the financial condition of some existing or potential foreign customers and
reduce or eliminate their future orders of our products.
19
Table of Contents
We maintain all of our cash, cash equivalent and short-term investment balances, some of which are
not insured, at one depository institution.
We maintain the majority of our cash and cash equivalent accounts at one depository
institution. As of December 31, 2009, our aggregate balances in such accounts were $45.2 million.
Of such amount, $250,000 was covered by Federal Deposit Insurance Corporation (FDIC) insurance,
while the remaining amounts were not insured as of the end of fiscal 2009.
Although we believe that the risk of loss associated with our uninsured deposit and investment
accounts is low given
the financial strength and reputation of our depository institution, we could suffer losses with
respect to the uninsured balances if the depositary institution failed and the institution’s assets
were insufficient to cover its deposits and/or the Federal government did not take actions to
support deposits in excess of existing FDIC insurance limits. Any such losses could have a material
adverse effect on our liquidity, financial condition and results of operations.
We face risks associated with rapid technological change and new competing products.
The technology associated with non-lethal devices is receiving significant attention and is
rapidly evolving. While we have patent protection in key areas of electro-muscular disruption
technology, it is possible that new non-lethal technology may result in competing products that
operate outside our patents and could present significant competition for our products.
We depend on our ability to attract and retain our key management and technical personnel.
Our success depends upon the continued service of our key management personnel. Our success
also depends on our ability to continue to attract, retain and motivate qualified technical
personnel. Although we have employment agreements with certain of our officers, the employment of
such persons is “at-will” and either we or the employee can terminate the employment relationship
at any time, subject to the applicable terms of the employment agreements. The competition for our
key employees is intense. The loss of the service of one or more of our key personnel could harm
our business.
20
Table of Contents
| Item 1B. | Unresolved Staff Comments |
None.
| Item 2. | Properties |
Our corporate headquarters and manufacturing facilities are based in a 100,000 square foot
facility in Scottsdale, Arizona, which we own. We also lease premises in Carpenteria, California
and Washington D.C. We believe our existing facilities are well maintained and in good operating
condition. We also believe we have adequate manufacturing capacity for our existing product lines
for the foreseeable future. To the extent that we introduce new products in the future, we will
likely need to acquire additional facilities to locate the associated production lines. However, we
believe we can acquire or lease such facilities on reasonable terms. The Company continues to make
investments in capital equipment as needed to meet anticipated demand for its products.
| Item 3. | Legal Proceedings |
See discussion of litigation in Note 7(c) to the consolidated financial statements included in Part
II, Item 8 of this annual report.
| Item 4. | Reserved |
PART II
| Item 5. | Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities |
Market Information
Our common stock is quoted under the symbol “TASR” on The NASDAQ Global Select Market. The
closing price of our common stock on March 10, 2010 was $7.07 per share.
The following table sets forth the high and low sales prices per share for our common stock as
reported by NASDAQ for each quarter of the last two fiscal years.
Common Stock “TASR”
| Fiscal Quarters Ended | High | Low | ||||||
March 31, 2008 |
$ | 14.06 | $ | 8.90 | ||||
June 30, 2008 |
$ | 10.27 | $ | 4.99 | ||||
September 30, 2008 |
$ | 7.48 | $ | 5.01 | ||||
December 31, 2008 |
$ | 6.94 | $ | 2.68 | ||||
March 31, 2009 |
$ | 5.74 | $ | 3.12 | ||||
June 30, 2009 |
$ | 5.40 | $ | 4.05 | ||||
September 30, 2009 |
$ | 5.58 | $ | 4.21 | ||||
December 31, 2009 |
$ | 4.67 | $ | 3.97 | ||||
Holders
As
of March 10, 2010, there were approximately 360 holders of record of our common stock.
Dividends
To date, we have not declared or paid cash dividends on our common stock. We do not intend to
pay cash dividends in
the foreseeable future and our revolving line of credit prohibits the payment of cash dividends.
21
Table of Contents
Issuer Purchases of Equity Securities
We did not repurchase any shares of our common stock in 2009.
Recent Sales of Unregistered Securities
No unregistered securities were sold by us in 2009. For more information about our stock
repurchase program, refer to Note 10(b) in Part II, Item 8 of this Annual Report.
Stock Performance Graph
The following stock performance graph compares the performance of our common stock to the
NASDAQ Stock Market (U.S.) and the Russell 3000 Index. The graph covers the period from December
31, 2004 to December 31, 2009. The graph assumes that the value of the investment in our stock and
in each index was $100 at December 31, 2004 and that all dividends were reinvested. We do not pay
dividends on our common stock.
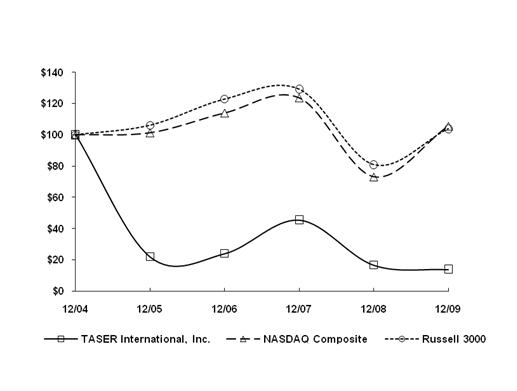
* $100 invested on 12/31/04 in stock & index-including reinvestment of dividends.
Fiscal year ending December 31.
| 12/04 | 12/05 | 12/06 | 12/07 | 12/08 | 12/09 | |||||||||||||||||||
TASER International, Inc. |
100.00 | 21.99 | 24.04 | 45.47 | 16.68 | 13.84 | ||||||||||||||||||
NASDAQ Composite |
100.00 | 101.33 | 114.01 | 123.71 | 73.11 | 105.61 | ||||||||||||||||||
Russell 3000 |
100.00 | 106.12 | 122.80 | 129.11 | 80.94 | 103.88 | ||||||||||||||||||
22
Table of Contents
| Item 6. | Selected Financial Data |
The following selected financial data should be read in conjunction with our consolidated
financial statements and the notes thereto, and with Item 7, “Management’s Discussion and Analysis
of Financial Condition and Results of Operations.” The statement of operations data for the years
ended December 31, 2009, 2008 and 2007 and the balance sheet data as of December 31, 2009 and 2008
have been derived from and should be read in conjunction with our audited consolidated financial
statements and the notes thereto included herein. The statement of operations data for the years
ended December 31, 2006 and 2005 and the balance sheet data as of December 31, 2007, 2006 and 2005
is derived from audited consolidated financial statements and the notes thereto which are not
included in this Annual Report on Form 10-K.
| For the Year Ended December 31, | ||||||||||||||||||||
| 2009 | 2008 | 2007 | 2006 | 2005 | ||||||||||||||||
Statement of Operations Data |
||||||||||||||||||||
Net sales |
$ | 104,251,560 | $ | 92,845,490 | $ | 100,727,191 | $ | 67,717,851 | $ | 47,694,181 | ||||||||||
Gross margin |
63,402,409 | 57,004,227 | 57,559,819 | 43,179,061 | 29,597,895 | |||||||||||||||
Sales, general and administrative expenses |
43,479,232 | 38,860,729 | 32,814,170 | 29,680,764 | 26,483,485 | |||||||||||||||
Research and development expenses |
20,002,351 | 12,918,161 | 4,421,596 | 2,704,521 | 1,574,048 | |||||||||||||||
Shareholder litigation settlement expense (a) |
— | — | — | 17,650,000 | — | |||||||||||||||
Income (loss) from operations |
(79,174 | ) | 5,225,337 | 20,324,053 | (6,856,224 | ) | 1,540,362 | |||||||||||||
Net income (loss) |
(1,106 | ) | 3,637,041 | 15,026,476 | (4,087,679 | ) | 1,056,516 | |||||||||||||
Income (loss) per common and common equivalent shares |
||||||||||||||||||||
Basic |
$ | (0.00 | ) | $ | 0.06 | $ | 0.24 | $ | (0.07 | ) | $ | 0.02 | ||||||||
Diluted |
$ | (0.00 | ) | $ | 0.06 | $ | 0.23 | $ | (0.07 | ) | $ | 0.02 | ||||||||
Weighted average number of common and common equivalent shares outstanding |
||||||||||||||||||||
Basic |
61,920,094 | 62,371,004 | 62,621,174 | 61,984,240 | 61,303,939 | |||||||||||||||
Diluted |
61,920,094 | 64,070,869 | 65,685,667 | 61,984,240 | 63,556,246 | |||||||||||||||
| As of December 31, | ||||||||||||||||||||
| 2009 | 2008 | 2007 | 2006 | 2005 | ||||||||||||||||
Balance Sheet Data |
||||||||||||||||||||
Working capital |
$ | 72,100,393 | $ | 80,642,516 | $ | 83,953,166 | $ | 37,813,576 | $ | 34,663,101 | ||||||||||
Total assets |
138,425,917 | 130,015,506 | 137,763,401 | 119,837,689 | 112,241,247 | |||||||||||||||
Total current liabilities |
13,784,853 | 10,956,199 | 12,473,616 | 18,302,688 | 7,586,701 | |||||||||||||||
Total long term obligations |
— | — | 11,695 | 230,973 | 76,188 | |||||||||||||||
Total stockholders’ equity |
$ | 117,701,196 | $ | 112,526,262 | $ | 120,636,750 | $ | 99,328,539 | $ | 103,738,375 | ||||||||||
| a) | In 2006 we reached an agreement to settle our securities class action and shareholder derivative lawsuits. |
23
Table of Contents
| Item 7. | Management’s Discussion and Analysis of Financial Condition and Results of Operations |
Management’s Discussion and Analysis of Financial Condition and Results of Operations (MD&A) is
designed to provide a reader of our consolidated financial statements with a narrative from the
perspective of our management on our financial condition, results of operations, liquidity and
certain other factors that may affect our future results. Our MD&A is presented in seven sections:
| • | Executive Overview and Key Strategic Initiatives |
| • | 2009 Overview |
| • | Results of Operations |
| • | Liquidity and Capital Resources |
| • | Contractual Obligations |
| • | Off Balance Sheet Arrangements |
| • | Critical Accounting Estimates |
Our MD&A should be read in conjunction with the other sections of this annual report on Form
10-K, including Part I, “Item 1A: Risk Factors”; Part II, “Item 6: Selected Financial Data”; and
Part II, “Item 8: Financial Statements and Supplementary Data.” The various sections of this MD&A
contain a number of forward-looking statements, all of which are based on our current expectations
and could be affected by the uncertainties and risk factors described throughout this filing.
Executive Overview and Key Strategic Initiatives
Our mission is to protect life by providing less dangerous, more effective use of force
options and technologies. We are a market leader in the development and manufacture of advanced
electronic control devices (ECDs) designed for use in the law enforcement, military, corrections,
private security and personal defense markets.
Our mission to protect life has also been extended to protect truth. We have learned that
bringing a subject into custody is not the end of the challenge for law enforcement. In fact, it
is typically just the beginning since a significant number of incidents that start as a physical
conflict, transition into a legal conflict. Whether it’s prosecuting and convicting the individual
arrested, or responding to excessive use of force allegations, the post incident legal process is a
considerable part of the challenge law enforcement faces on a continual basis and can often take
years and millions of litigation dollars to resolve in the courtroom. To help law enforcement
address these challenges, in AXON and EVIDENCE.Com, we have developed a fully integrated hardware
and software solution that will provide our law enforcement customers the capabilities to capture,
store, manage and analyze video and other digital evidence. This provides the Company entry into a
potentially significant new market space and the opportunity to diversify its sources of revenue.
Technological innovation is the foundation for our long term growth and we intend to maintain
our commitment to the research and development of our technology for both new and existing products
that further our mission. At the same time we have established industry leading training services
to provide our users a comprehensive overview of legal and policy issues, medical information and
risk mitigation relating to our ECDs and the use of force. We have built a network of distribution
channels for selling and marketing our products and services to law enforcement agencies, primarily
in North America, with ongoing focus and effort placed on expanding these programs in
international, military and other markets. Over 15,000 law enforcement agencies in over 50
countries have made initial purchases of our TASER brand devices for testing or deployment. To
date, we do not know of any significant sales of any competing ECD products.
24
Table of Contents
Our key strategies include:
| • | Increase market penetration in both the United States and international law enforcement markets. We believe that a large portion of these markets that do not currently use our products continue to present an opportunity for future growth, particularly with respect to international law enforcement agencies where there remains a significant opportunity for more widespread adoption. In recent years we have seen the international business become increasingly significant and we seek to maintain that trend as we can demonstrate the benefits of large scale adoptions of our ECDs, using countries such as the U.K and Australia as benchmarks of successful large scale programs. |
| • | Further develop our presence in federal government and military markets. We intend to continue to place a strong emphasis on supporting our military customers through our Government and Military Programs business group and our Senior Executive Advisory Group (SEAG) comprised of a team of professionals with extensive military, homeland defense and law enforcement experience with the purpose of advising on business development in support of military users. The primary focus of these groups is placed on supporting military use for our existing hardware as well as increasing technology development through contracted support. |
| • | Continued investment in development of innovative new products, which both complement and add to our existing platforms. These development efforts yielded multiple products such as X3, XREP and SHOCKWAVE in 2009. While we anticipate the level of new ECD hardware development to be at a reduced level in 2010, we are continuing to devote significant resources to bring AXON and EVIDENCE.COM to market. We launched initial field trials of the AXON and EVIDENCE.COM in the fourth quarter of 2009 and we anticipate an increasing volume of similar trial programs in 2010. We believe these trial programs are the best way for our customers to see the powerful capabilities and benefits of this technology for themselves, and will help drive revenue in 2010. |
| • | Operational excellence — leverage existing cost structure and human capital to move the Company towards the next phase of revenue growth and enhanced profitability. |
| • | Continued application for patents and intellectual property rights, both in the U.S and internationally, to protect key technology in our products and further attempt to protect our competitive position. |
| • | Continued aggressive litigation defense to protect our brand equity. We have assembled a team of world class medical experts and hired additional internal legal resources to provide an efficient means of defending the Company against product liability claims. Through March 12 2010, we have had a total of 106 cases dismissed or defense judgments in our favor. We view a continued record of successful litigation defense as a key factor for our long term growth and success. |
2009 Overview
Management believes that its ability to achieve a balance between growing our core business
and building the foundations for future growth is the key to increasing long-term shareholder
value. Our 2009 performance and the initiatives we have put in place reflect our continuing
commitment to achieving this balance. While domestic municipal spending was still negatively
impacted during 2009 as a result of the economic downturn, we were encouraged by the increasing
momentum in international adoption of our products and we experienced a breakthrough year in terms
of sales to our federal and military customers. In 2009, we remained focused on sustaining an
efficient operating environment to sustain gross margin levels and made significant investments in
our future by expanding our research and development programs for both hardware and software.
25
Table of Contents
Some 2009 highlights include the following:
| • | We achieved a record $104.3 million in sales, an increase of $11.4 million or 12.3% over 2008, driven primarily by growth in international and federal law enforcement and military markets. |
| • | We continued to focus our efforts on markets outside the U.S. and during 2009, our international sales continued to grow in significance, accounting for approximately 22% of our total sales compared to 18% in 2008 and 15% in 2007. In particular, 2009 international sales included significant follow-on orders from customers in the United Kingdom, Australia, New Zealand and Brazil. |
| • | Our strategy of developing a presence in federal law enforcement and military markets paid dividends with some significant orders including initial shipments under IDIQ contracts from the United States Army Garrison Rock Island Arsenal and U.S. Customs and Border Protection as well as other orders from the United States Marshals Service. Our federal and military business represented approximately 13% of net sales in 2009 compared to 5% in 2008. |
| • | We invested $20.0 million in research and development programs during 2009 with a concerted effort made to bring multiple new ECD products including the next generation X3, XREP and Shockwave products to market in 2009. This also included significant investment in the development of both hardware and software for our end-to-end digital evidence collection and management solution — AXON and EVIDENCE.COM, which commenced field trials in the fourth quarter of 2009. |
| • | Our strategy of vigorously defending against product liability lawsuits continues to be successful. Courts dismissed 23 lawsuits against us in 2009. We believe these dismissals serve to highlight the extensive medical and scientific evidence confirming the general safety of TASER technology. |
| • | We generated $10.1 million in cash from operations in 2009 and ended the year with $45.5 million in cash and cash equivalents with zero debt. In times of economic uncertainty, we feel our strong balance sheet allows us the flexibility to invest in our key strategic and growth initiatives, evidenced by the fact that our significant capital investments in 2009 were almost fully self funded from working capital resources. |
26
Table of Contents
Results of Operations
The following table presents data from our statements of operations as well as the percentage
relationship to total net revenues of items included in our statements of operations (dollars in
thousands):
| Year ended December 31, | ||||||||||||||||||||||||
| 2009 | 2008 | 2007 | ||||||||||||||||||||||
Net sales |
$ | 104,252 | 100 | % | $ | 92,845 | 100 | % | $ | 100,727 | 100 | % | ||||||||||||
Cost of products sold |
40,850 | 39 | % | 35,841 | 39 | % | 43,167 | 43 | % | |||||||||||||||
Gross margin |
63,402 | 61 | % | 57,004 | 61 | % | 57,560 | 57 | % | |||||||||||||||
Sales, general and administrative expenses |
43,479 | 42 | % | 38,861 | 42 | % | 32,814 | 33 | % | |||||||||||||||
Research and development expenses |
20,002 | 19 | % | 12,918 | 14 | % | 4,422 | 4 | % | |||||||||||||||
Income (loss) from operations |
(79 | ) | * | 5,225 | 6 | % | 20,324 | 20 | % | |||||||||||||||
Interest and other income, net |
170 | * | 1,718 | 2 | % | 2,202 | 2 | % | ||||||||||||||||
Income before income taxes |
91 | * | 6,943 | 7 | % | 22,526 | 22 | % | ||||||||||||||||
Provision (benefit) for income taxes |
92 | * | 3,306 | 4 | % | 7,500 | 7 | % | ||||||||||||||||
Net income |
$ | (1 | ) | * | $ | 3,637 | 4 | % | $ | 15,026 | 15 | % | ||||||||||||
| * | less than 1% |
Net Sales
For the years ended December 31, 2009, 2008 and 2007, sales by product line and by geography
were as follows (dollars in thousands):
| 2009 | 2008 | 2007 | ||||||||||||||||||||||
Sales by Product Line |
||||||||||||||||||||||||
TASER X26 |
$ | 53,996 | 52 | % | $ | 51,733 | 56 | % | $ | 61,638 | 61 | % | ||||||||||||
Single Cartridges |
27,908 | 27 | % | 20,526 | 22 | % | 25,250 | 25 | % | |||||||||||||||
TASER C2 |
4,929 | 5 | % | 6,127 | 7 | % | 3,983 | 4 | % | |||||||||||||||
TASER Cam |
3,087 | 3 | % | 3,304 | 4 | % | 4,012 | 4 | % | |||||||||||||||
ADVANCED TASER M26 & M18 |
3,693 | 4 | % | 3,422 | 4 | % | 2,412 | 2 | % | |||||||||||||||
TASER X3 |
746 | * | — | — | — | — | ||||||||||||||||||
TASER XREP |
549 | * | — | — | — | — | ||||||||||||||||||
Shockwave |
51 | * | — | — | — | — | ||||||||||||||||||
Other |
9,293 | 9 | % | 7,733 | 8 | % | 3,432 | 3 | % | |||||||||||||||
Total |
$ | 104,252 | 100 | % | $ | 92,845 | 100 | % | $ | 100,727 | 100 | % | ||||||||||||
| * | less than 1% (percentages may not add to 100% due to rounding) |
| 2009 | 2008 | 2007 | ||||||||||
Sales by Geographic Area |
||||||||||||
United States |
78 | % | 82 | % | 85 | % | ||||||
Other Countries |
22 | % | 18 | % | 15 | % | ||||||
Total |
100 | % | 100 | % | 100 | % | ||||||
Net sales increased $11.4 million, or 12%, to $104.3 million in 2009 compared to
$92.8 million in 2008. The growth in 2009 was primarily driven by significant international
shipments during the year and sales to federal law enforcement / military customers, including a
contract from the U.S. Customs and Border Patrol, the U.S Army and The United States Marshals
Service. The growth in international and Federal business offset a decline in domestic sales, which
we believe reflects lower municipal spending in the U.S. as agencies reassigned budget dollars due
to ongoing economic constraints. This increase in Federal and
international business resulted in higher sales of the TASER X26 product line which
increased $2.3 million, or 4%, to $54.0 million in 2009 compared to $51.7 million in 2008, and a
$7.4 million, or 36%, increase in single cartridge sales which grew to $27.9 million in 2009
compared to $20.5 million in 2008. Sales of the ADVANCED TASER increased by $0.3 million, while the
introduction of three new product lines, TASER X3, TASER XREP and Shockwave contributed $1.3
million in 2009. Offsetting these increases, sales of the TASER C2 consumer product declined $1.2
million, attributable to the adverse impact of the economic downturn on consumer spending. The
increase in other sales is primarily driven by growth in extended warranty revenues, out of
warranty repairs and the elimination of distributor discounts during 2008. Other sales also include
government grants, training and shipping revenues.
27
Table of Contents
Net sales for the year ended December 31, 2008 were $92.8 million, a decrease of $7.9 million,
or 8%, compared to $100.7 million in 2007. The decrease in 2008 was primarily driven by a decline
in sales of our core X26 product line and single cartridges which we believe reflected lower
municipal spending in the U.S. as agencies experienced constrained budgets due to prevailing
adverse economic conditions in 2008. This resulted in reduced sales of the TASER X26 product line
which decreased by $9.9 million, or 16%, to $51.7 million in 2008 compared to $61.6 million in
2007. Single cartridge sales also decreased by $4.7 million, or 19% to $20.5 million in 2008
compared to $25.2 million in 2007. Partially offsetting these decreases was a full year’s sales in
2008 of the TASER C2 product which began shipping in July 2007. Sales of the TASER C2 were $6.1
million in 2008, an increase of $2.1 million over the same period in 2007. Additionally, sales of
the ADVANCED TASER increased by $1.0 million mainly due to a large purchase made by an
international customer in the first quarter of 2008 and other sales grew $4.3 million due to a $2.0
million increase in out of warranty replacement and extended warranty sales as well as $2.1 million
reduction of cash and distributor discounts in 2008.
International sales for 2009 and 2008 represented approximately $22.7 million, or 22% of total
net sales, and $17.0 million or 18% of total net sales, respectively. International sales
represented approximately $15.1 million or 15% of total net sales in 2007. The growth in
international sales in both 2009 and 2008 reflects our continued commitment to marketing efforts in
countries outside the United States. In particular, 2009 international sales included significant
orders from the United Kingdom, Australia, New Zealand and Brazil.
Cost of Products Sold
Cost of products sold increased by $5.0 million, or 14%, to $40.8 million in 2009 compared to
$35.8 million in 2008. As a percentage of net sales, cost of products sold remained flat at 39% in
both 2009 and 2008. Direct manufacturing costs decreased slightly as a percentage of sales
primarily driven by negotiated supplier price reductions in certain raw material components being
partially offset by a less favorable sales mix weighted towards lower margin products. Direct labor
also decreased as a percentage of net sales due to the initial impact of automated cartridge
production in the fourth quarter of 2009. Offsetting the decrease in direct manufacturing costs,
indirect manufacturing expenses increased attributable to a lower absorption of indirect
manufacturing costs to inventory resulting from a decline in the overall number of production hours
in 2009 compared to 2008. Additionally, depreciation expense related to new automation equipment,
and freight costs increased. These increases in indirect manufacturing costs were offset by a
reduction in scrap, count discrepancies and engineering supplies.
Cost of products sold decreased by $7.3 million, or 17%, to $35.8 million in 2008 compared to
$43.1 million in 2007. As a percentage of net sales, cost of products sold decreased to 39% in 2008
compared to 43% in 2007. The improvement in 2008 compared to 2007 was the result of a combination
of factors. The $2.1 million reduction in our cash and distributor sales discounts and the $2.0
million growth in extended warranty and out of warranty replacement revenue contributed to the
reduction in cost of products sold as a percentage of net sales. Total direct manufacturing costs
in 2008 decreased primarily as the result of a $2.5 million reduction in temporary labor and
overtime costs while product materials costs decreased due to improved supplier pricing negotiated
on various raw material components. Indirect manufacturing costs declined as a percentage of net
sales resulting from lower variable manufacturing costs including scrap expense, freight and
engineering supplies, a function of improved product quality and operating efficiencies as well as
reduced levels of production. In addition, our allocation of manufacturing overhead to inventory
increased due to a reduction in direct production hours during 2008 combined with an increase in
labor hours in finished goods inventory at December 31, 2008 compared to December 31, 2007.
Gross Margin
Gross margin increased $6.4 million, or 11%, to $63.4 million in 2009 compared to $57.0
million in 2008. As a percentage of net sales, gross margin remained flat at 61% for both 2009 and
2008.
Gross margin decreased $0.6 million to $57.0 million in 2008 compared to $57.6 million in
2007. As a percentage of net sales, gross margins increased to 61% in 2008 compared to 57% in 2007.
The improvement in gross margin in 2008 was attributable to the decrease in direct and indirect
manufacturing costs as a percentage of net sales for the reasons noted above under the discussion
of Cost of Products Sold.
28
Table of Contents
Sales, General and Administrative Expenses
For the years ended December 31, 2009, 2008 and 2007, sales, general and administrative
expenses were comprised as follows (dollars in thousands):
| Year Ended December 31, | Year Ended December 31, | |||||||||||||||||||||||||||||||
| $ | % | $ | % | |||||||||||||||||||||||||||||
| 2009 | 2008 | Change | Change | 2008 | 2007 | Change | Change | |||||||||||||||||||||||||
Salaries, benefits, and bonus |
$ | 11,335 | $ | 9,349 | $ | 1,986 | 21 | % | $ | 9,349 | $ | 8,148 | $ | 1,201 | 15 | % | ||||||||||||||||
Stock based compensation |
3,219 | 1,552 | 1,667 | 107 | % | 1,552 | 987 | 565 | 57 | % | ||||||||||||||||||||||
Legal, professional and accounting |
6,081 | 5,899 | 182 | 3 | % | 5,899 | 5,813 | 86 | 1 | % | ||||||||||||||||||||||
Sales and Marketing |
5,356 | 5,071 | 285 | 6 | % | 5,071 | 3,214 | 1,857 | 58 | % | ||||||||||||||||||||||
Consulting and lobbying services |
3,863 | 3,478 | 385 | 11 | % | 3,478 | 2,455 | 1,023 | 42 | % | ||||||||||||||||||||||
Travel and meals |
3,308 | 3,739 | (431 | ) | -12 | % | 3,739 | 3,762 | (23 | ) | -1 | % | ||||||||||||||||||||
Depreciation and amortization |
1,896 | 1,635 | 261 | 16 | % | 1,635 | 1,557 | 78 | 5 | % | ||||||||||||||||||||||
D&O and liability insurance |
1,812 | 2,191 | (379 | ) | -17 | % | 2,191 | 2,027 | 164 | 8 | % | |||||||||||||||||||||
Other |
6,609 | 5,947 | 662 | 11 | % | 5,947 | 4,851 | 1,096 | 23 | % | ||||||||||||||||||||||
Total |
$ | 43,479 | $ | 38,861 | $ | 4,618 | 12 | % | $ | 38,861 | $ | 32,814 | $ | 6,047 | 18 | % | ||||||||||||||||
Sales, general and administrative as percentage of net sales |
42 | % | 42 | % | 42 | % | 33 | % | ||||||||||||||||||||||||
Sales, general and administrative expenses were $43.5 million and $38.9 million in 2009 and 2008,
respectively, an increase of $4.6 million, or 12%. As a percentage of total net sales, sales,
general and administrative expenses remained flat at 42% for both 2009 and 2008. The dollar
increase during 2009 over the same period in 2008 is attributable to a $2.0 million growth in
salaries, benefits and bonus; related to an increase in personnel to support the expansion of our
business infrastructure as we introduce new products and enter new markets. Stock
based-compensation expense also increased $1.7 million related to a full year’s expense for some
large stock option grants during the third and fourth quarters of 2008 as well as new employee
stock option grants in 2009. Consulting and lobbying services increased $0.4 million primarily
related to strategic selling and marketing, advertising and IT process improvement related efforts
while
legal, professional and accounting fees remained flat as lower legal fees driven by the timing of
outstanding ligation in progress were offset by higher accounting fees. Sales and marketing
expenses also increased by a net amount of $0.3 million as increases in our tradeshow costs and our
selling costs in support of new product introductions were partially offset by a $1.3 million
decrease in general media advertising spend primarily due to $0.6 million of infomercial production
costs expensed in the first quarter of 2008 as well as reduced emphasis placed on consumer
marketing programs. The $0.7 million increase in other costs was primarily driven by a $0.4 million
settlement expense paid to a Board member in 2009 (see “Settlement agreement” in Note 11) as well
as increased computer licensing and maintenance costs.
Sales, general and administrative expenses were $38.9 million and $32.8 million in 2008 and
2007, respectively, an increase of $6.0 million, or 18%. As a percentage of total net sales, sales,
general and administrative expenses increased to 42% during 2008 compared to 33% in 2007. The
dollar increase during 2008 over 2007 is attributable to a combination of factors. Specifically,
salaries, benefits and bonus grew $1.2 million related to the addition of personnel to support the
expansion of our business infrastructure combined with an annual salary increase effective January
1, 2008 as well as higher cost of benefits. These increases were partially offset by a $0.8 million
decrease in bonuses due to the lower pre-tax income in 2008 as well as a program which allowed
employees to opt out of the cash based bonus program for two years in exchange for additional stock
options. Sales and marketing expense increased $1.9 million primarily due to expensing of $0.6
million in production costs of the TASER C2 infomercial as well as ongoing promotion and
infomercial airing costs and a $0.4 million increase in trade show expenses. Consulting and
lobbying services increased $1.0 million primarily attributable to strategic selling and marketing,
advertising and process improvement related efforts. In addition, stock based compensation
increased $0.6 million related to stock options granted in 2008 and D&O and liability insurance
costs were up $0.2 million from increased annual premiums. The
$1.1 million increase in other
expense is primarily attributable to a $0.5 million increase in recruiting and relocation expenses
driven by hiring of new vice presidents of sales, marketing, IT and HR and a $0.3 million increase
in computer licensing and maintenance fees.
29
Table of Contents
Research and Development Expenses
Research and development expenses increased $7.1 million, or 55%, to $20.0 million in 2009
compared to $12.9 million in 2008. The increase is driven by a $3.6 million increase in salary and
benefits as we have expanded our research and development headcount to support new product
development, including a dedicated SaaS development team. Stock-based compensation expenses
increased $0.8 million for stock options granted in the second half of 2008 and in 2009. Indirect
supplies and tooling costs increased $2.9 million primarily associated with the development of the
TASER X3, AXON and EVIDENCE.com. In particular, during the third quarter of 2009, we expedited the
build of AXON and X3 prototype display units for the TASER Tactical conference and the
International Association of Chiefs of Police. These increases are offset by the capitalization of
$2.3 million of internal salary and external consulting costs specifically related to the
development of EVIDENCE.com in 2009. We anticipate the level of new
ECD hardware development costs will be at a reduced level in 2010, however,
we will continue to devote the necessary resources to bring AXON and
EVIDENCE.com to market.
Research and development expenses increased $8.5 million, or 192%, to $12.9 million in 2008
compared to $4.4 million in 2007. The increase is driven by a $4.2 million increase in third party
consulting costs primarily associated with the development of AXON. In addition, there was $1.4
million growth in salary and benefit costs attributable to increased headcount combined with an
annual salary increase effective January 1, 2008, and a $1.3 million increase in indirect supplies
to support our continuing efforts to develop new products including AXON, XREP and Shockwave.
Interest and Other Income, Net
Interest and other income decreased by $1.5 million, or 90%, to $0.2 million in 2009 compared
to $1.7 million in 2008. The decrease is attributable to a significantly lower average yield on our
cash and investments as well as a lower average cash and investment balance in 2009. Our cash and
investment accounts earned interest at an approximate rate of 0.30% during 2009, down from 2.5% in
2008. Additionally, other income in 2008 included $0.4 million related to unused deferred insurance
settlement proceeds recognized upon the dismissal of all final appeals in a personal injury case.
Interest and other income decreased by $0.5 million, or 22%, to $1.7 million in 2008 compared
to $2.2 million in 2007. This was attributable to a $0.7 million decrease in interest income due to
lower average yields on our investments, partially offset by an increase in total average funds
invested during 2008 compared to 2007. Our cash and investment accounts earned interest at an
average rate of approximately 2.5% during 2008 compared to 4.2% in 2007. The decrease in interest
income was partially offset by other income of $0.4 million related to the unused deferred
insurance settlement proceeds recognized in the second quarter of 2008 upon the dismissal of all
final appeals in a personal injury case.
Provision for Income Taxes
The
provision for income taxes decreased by $3.2 million to $0.1 million in 2009 compared to
$3.3 million in 2008. The effective income tax rate for 2009 was
101% compared to 48% for 2008. The
2009 effective tax rate is driven by the impact of non-deductible
expenses for items such as incentive
stock option expense, meals and entertainment and lobbying expenses, making the income for tax
purposes higher than book pre-tax income which was close to break even for 2009.
The provision for income taxes decreased by $4.2 million to $3.3 million in 2008 compared to
$7.5 million in 2007. The effective income tax rate for 2008 was
48% compared to 33% for 2007.
Contributing to the increase in effective tax rate is the higher impact of certain non-deductible
items such as lobbying expenses against a lower taxable income for the year ended December 31,
2008. In addition, the 2008 effective tax rate is reduced by a reduced amount of research and
development tax credits ($608,000 in 2008 vs. $2.0 million in 2007), which was partially offset by
an increase in the liability for unrecognized tax benefits.
Net Income (Loss)
Net
income decreased by $3.6 million to break even (net loss of
$0.001 million) in 2009 compared to
net income of $3.6 million in 2008. Loss per basic and diluted share rounds to $0.00 for 2009
compared to income per basic and diluted share of $0.06 in 2008.
Net income decreased by $11.4 million to $3.6 million in 2008 compared to $15.0 million in
2007. Income per basic and diluted share was $0.06 for 2008. This compares to income per basic and
diluted share of $0.24 and $0.23, respectively, in 2007.
30
Table of Contents
Liquidity and Capital Resources
Summary
As of December 31, 2009, we had $45.5 million in cash and cash equivalents, a decrease of $1.4
million from the end of 2008 which is a function of cash generated from operations, offset by
investments in property and equipment. Specifically, we made some significant capital expenditures
for the purchase of our automated cartridge production equipment as well as server and networking
equipment to establish a data center for EVIDENCE.COM hosting, storage and network monitoring
operations.
Cash Flows
The following table summarizes our cash flows from operating, investing and financing activities
for each of the past three years ($ in thousands):
| Year ended December 31, | ||||||||||||
| 2009 | 2008 | 2007 | ||||||||||
Total cash provided by (used in): |
||||||||||||
Operating activities |
$ | 10,117 | $ | 8,118 | $ | 13,923 | ||||||
Investing activities |
(11,679 | ) | 8,117 | 7,006 | ||||||||
Financing activities |
187 | (12,156 | ) | 3,099 | ||||||||
(Decrease) increase in cash and cash equivalents |
$ | (1,375 | ) | $ | 4,079 | $ | 24,028 | |||||
Operating activities
Net cash provided by operating activities was $10.1 million in 2009, compared with $8.1
million in 2008 and $13.9 million in 2007.
Net cash provided by operating activities in 2009 of $10.1 million was driven by the net loss
for the period adjusted for the add back of non-cash expenses including stock-based compensation
expense of $5.0 million; depreciation and amortization expense of $3.6 million partially offset by
a $0.6 million net increase in deferred tax assets and provision for unrecognized tax benefits.
Changes in working capital include a $1.3 million decrease in accounts receivable, a function of
the timing of billing and collections; a $1.0 million decrease in prepaid and other assets due to a
difference in the timing of funding of our annual liability insurance premium; a $1.0 million
increase in accounts payable and accrued liabilities due to timing of year end check runs and a
$2.4 million increase in inventory, primarily driven by raw materials acquired for production of
new products.
Net cash provided by operating activities during 2008 reflects non-cash changes to net income
including, depreciation and amortization expense of $2.6 million, stock-based compensation expense
of $2.4 million, provision for warranty expense and excess and obsolete inventory of $1.0 million
and the $2.1 million utilization of deferred tax assets. In addition, prepaid and other assets
decreased $1.9 million due to i) the net receipt of insurance reimbursements of legal fees incurred
in excess of policy retention limits; ii) a decrease in prepaid advertising due to the expensing of
TASER C2 infomercial production costs and iii) amortization of prepaid liability and D&O insurance
premiums. Deferred revenue also increased $2.1 million driven by extended warranty sales in 2008.
Offsetting these items was a $5.2 million increase in accounts receivable due to timing of billing
and collections as well as a decrease in accounts payable and accrued liabilities of $2.3 million,
which reflects timing differences combined with a reduced rate of material purchasing as well as
the payment in January 2008 of $1.2 million for the second installment for automation equipment
which was accrued at December 31, 2007.
Investing activities
We used $11.7 million for investing activities in 2009 comprised of $13.7 million in
acquisitions of property and equipment related to new automated cartridge production equipment,
production equipment for new product lines, computer equipment to establish the first EVIDENCE.COM
data center and capitalized software development costs. In addition, we invested $0.5 million in
intangible assets, primarily consisting of patent applications. These net uses were partially
offset by $2.5 million in net proceeds from short term investments.
31
Table of Contents
Net cash provided by investing activities was $8.1 million during 2008 which was comprised of
$15.0 million in net proceeds from maturing investments over investment purchases partially offset
by the use of $6.1 million to purchase property and equipment mainly related to new automation
equipment and computer storage solutions. In addition, we invested $0.7 million in intangible
assets, primarily consisting of patent application costs.
Financing activities
During 2009, net cash provided by financing activities was $0.2 million, attributable to
proceeds from stock options exercised during the period.
During 2008, we utilized $12.2 million in financing activities, a function of the $12.5
million to repurchase 1.8 million shares of our common stock partially offset by $0.3 million of
proceeds attributable to stock options exercised in the year.
Liquidity
Our most significant sources of liquidity continue to be funds generated by operating
activities and available cash and cash equivalents. We believe funds generated from our expected
results of operation as well as available cash and cash equivalents will be sufficient to finance
our operations and strategic initiatives for 2010. We expect our investment in capital expenditures
in 2010 will be at a reduced level compared to 2009. In addition, our revolving credit facility is
available for additional working capital needs or investment opportunities. There can be no
assurance, however, that we will continue to generate cash flows at or above current levels or that
we will be able to maintain our ability to borrow under our revolving credit facility.
Capital Resources
We have a revolving line of credit with a domestic bank with a total availability of $10.0
million. The line is secured by substantially all of our assets, other than intellectual property,
and bears interest at varying rates, ranging from LIBOR plus 1.5% to prime. The line of credit
matures on June 30, 2010, and requires monthly payments of interest only. We expect to renew the
line of credit on similar terms upon its maturity. At December 31, 2009, there were no borrowings under the line and the
entire $10.0 million line was available based on the defined borrowing base, which is calculated
based on our eligible accounts receivable and inventory. Our agreement with the bank requires us to
comply with certain financial and other covenants including maintenance of minimum tangible net
worth and fixed charge coverage ratios. The ratio of total liabilities to tangible net worth can be
no greater than 1:1, and the fixed coverage charge ratio can be no less than 1.25:1, based upon a
trailing twelve month period. At December 31, 2009, the Company’s tangible net worth ratio was
18:1 and its fixed charge coverage ratio was 3.96:1. At December 31, 2009, we were in compliance
with those covenants.
Based on our strong balance sheet and the fact that we had no outstanding debt at December 31,
2009, we believe financing will be available, both through our existing credit line and possible
additional financing. However, there is no assurance that such funding will be available on terms
acceptable to us, or at all. Capital markets in the United States and throughout the world remain
disrupted and under stress. This disruption and stress is evidenced by a lack of liquidity in the
debt capital markets, the re-pricing of credit risk in the syndicated credit market, and the
failure of certain major financial institutions. This stress is compounded by the ongoing worldwide
recession. Reflecting this situation, many lenders and capital providers have reduced, and in some
cases ceased to provide, debt funding to borrowers. The resulting lack of available credit, lack of
confidence in the financial sector, increased volatility in the financial markets and reduced
business activity could materially and adversely affect our ability to obtain additional or
alternative financing should the need arise for us to access the debt markets.
32
Table of Contents
Contractual Obligations
The following table outlines our future contractual financial obligations by period in which
payment is expected, in thousands, as of December 31, 2009 (in thousands):
| Less than | More than | |||||||||||||||||||
| Total | 1 year | 1-3 years | 4-5 years | 5 years | ||||||||||||||||
Non-cancelable operating leases |
$ | 4,076 | $ | 1,237 | $ | 2,001 | $ | 838 | $ | — | ||||||||||
Joint
venture development funding a) |
$ | 1,425 | $ | 1,425 | $ | — | $ | — | $ | — | ||||||||||
Total contractual cash obligations |
$ | 5,501 | $ | 2,662 | $ | 2,001 | $ | 838 | $ | — | ||||||||||
| a) | On January 13, 2010 we entered into a Joint Venture agreement with RouteCloud LLC to establish TASER Protector Group. Under the agreement we will provide development funding of $1.425 million in 2010. Refer to note 13 of Part II, Item 8 of this annual report for more information. |
We are subject to U.S. federal income tax as well as income tax of multiple-state
jurisdictions. As of December 31, 2009, we had $2.3 million of gross unrecognized tax benefits
related to uncertain tax positions. The settlement period for our long-term income tax liabilities
cannot be determined: however, the liabilities are not expected to become due within the next
twelve months.
Off Balance Sheet Arrangements
We had no off balance sheet arrangements as of December 31, 2009.
Critical Accounting Estimates
We have identified the following accounting estimates as critical to our business operations
and the understanding of our results of operations. The preparation of this annual report on Form
10-K requires us to make estimates and assumptions that affect the reported amount of assets and
liabilities, disclosure of contingent assets and liabilities at the date of our consolidated
financial statements, and the reported amounts of revenue and expenses during the reporting period.
There can be no assurance that our actual results will not differ from those estimates. The effect
of these policies on our business operations is discussed below.
Standard Product Warranty Reserves
We warrant our law enforcement ECDs from manufacturing defects on a limited basis for a period
of one year after purchase, and thereafter will replace any defective TASER unit for a fee. We
warrant our TASER C2 product for 90 days. We track historical data related to returns and warranty
costs on a quarterly basis, and estimate future warranty claims based upon our historical
experience. We have also historically increased our reserve amount if we become aware of a
component failure that could result in larger than anticipated returns from our customers. As of
December 31, 2009, our reserve for warranty returns was $369,000 compared to a $615,000 reserve at
December 31, 2008. Our reserve for warranty returns has decreased, as the result of an improved
product returns experience, particularly in our X26 product line which we believe is a function of
continuing improvements made in the manufacturing and quality processes. In the event that product
returns under warranty differ from our estimates, changes to warranty reserves might become
necessary.
Inventory
Inventories are stated at the lower of cost or market, with cost determined using the weighted
average cost of raw materials, which approximates the first-in, first-out (FIFO) method, and an
allocation of manufacturing labor and overhead costs. The allocation of manufacturing labor and
overhead costs includes management judgments of what constitutes normal capacity of our production
facilities, and a determination of what costs are considered to be abnormal fixed production costs
which are expensed as current period charges. Provisions are made to reduce potentially excess,
obsolete or slow-moving inventories to their net realizable value. These provisions are based on
our best estimates after considering historical demand, projected future demand, inventory purchase
commitments, industry and market trends and conditions and other factors. Our reserve for excess
and obsolete inventory increased to $474,000 at December 31, 2009, compared to $130,000 at December
31, 2008. The increase was driven by some outdated C2 boards and excess parts resulting from the
introduction of the cartridge automation equipment. In the event that actual excess, obsolete or
slow-moving inventories differ from these estimates, changes to inventory reserves might become
necessary.
33
Table of Contents
Accounts Receivable
Sales are typically made on credit and we generally do not require collateral. We perform
ongoing credit evaluations of our customers’ financial condition and maintain an allowance for
estimated potential losses. Uncollectible accounts are written off when deemed uncollectible, and
accounts receivable are presented net of an allowance for doubtful accounts. This allowance
represents our best estimate and is based on our judgment after considering a number of factors
including third-party credit reports, actual payment history, customer-specific financial
information and broader market and economic trends and conditions. Our allowance for doubtful
accounts was $200,000 at December 31, 2009 and December 31, 2008. In the event that actual
uncollectible amounts differ from these estimates, changes in the allowance for doubtful accounts
might become necessary.
Valuation of Long-lived Assets
We review long-lived assets, such as property and equipment and intangible assets subject to
amortization, whenever events or changes in circumstances indicate that the carrying amount of such
assets may not be recoverable. We utilize a two-step approach to testing long-lived assets for
impairment. The first step tests for possible impairment indicators. If an impairment indicator is
present, the second step measures whether the asset is recoverable based on a comparison of the
carrying amount of the asset to the estimated undiscounted future cash flows expected to be
generated by the asset. If the carrying amount of an asset exceeds its estimated future cash flows,
an impairment charge is recognized by the amount by which the carrying amount of the asset exceeds
the fair value of the asset. Our review requires the use of judgment and estimates. Future events
or circumstances may result in a charge to earnings if we determine that the carrying value of a
long-lived asset is not recoverable.
Income Taxes
We recognize federal, state and foreign current tax liabilities or assets based on our
estimate of taxes payable or refundable in the current fiscal year by tax jurisdiction. We also
recognize federal, state and foreign deferred tax assets or liabilities, as appropriate, for our
estimate of future tax effects attributable to temporary differences and carryforwards.
We recognize the tax benefit from an uncertain tax position only if it is more likely than not
that the tax position will be sustained on examination by the taxing authorities, based on the
technical merits of the position. The tax benefits recognized in the consolidated financial
statements from such a position are measured based on the largest benefit that has a greater than
fifty percent likelihood of being realized upon ultimate resolution. Management must also assess
whether uncertain tax positions as filed could result in the recognition of a liability for
possible interest and penalties if any. We have completed research and development tax credit
studies which identified approximately $5.9 million in tax credits for Federal and Arizona income
tax purposes related to the 2003 through 2009 tax years, net of the federal benefit on the Arizona
research and development tax credits. Management made the determination that it was more likely
than not that the full benefit of the research and development tax credit would not be sustained on
examination and recorded a liability for unrecognized tax benefits of
$2.2 million as of December 31, 2009. Also included as part of the $2.3 million liability for unrecognized tax
benefits is a management estimate of $106,000 related to uncertain tax positions for certain state
income tax liabilities. As of December 31, 2009, management does not expect the amount of the unrecognized tax
benefit liability to increase or decrease significantly within the next 12 months. Should the
unrecognized tax benefit of $2.3 million be recognized, the Company’s effective tax rate would be
favorably impacted. Our estimates are based on the information available to us at the time we
prepare the income tax provisions. Our income tax returns are subject to audit by federal, state,
and local governments, generally years after the returns are filed. These returns could be subject
to material adjustments or differing interpretations of the tax laws.
Our calculation of current and deferred tax assets and liabilities is based on certain
estimates and judgments and involves dealing with uncertainties in the application of complex tax
laws. Our estimates of current and deferred tax assets and liabilities may change based, in part,
on added certainty or finality to an anticipated outcome, changes in accounting or tax laws in the
United States, or changes in other facts or circumstances. In addition, we recognize liabilities
for potential United States tax contingencies based on our estimate of whether, and the extent to
which, additional taxes may be due. If we determine that payment of these amounts is unnecessary or
if the recorded tax liability is less than our current assessment, we may be required to recognize
an income tax benefit or additional income tax expense in our consolidated financial statements.
34
Table of Contents
In preparing the Company’s consolidated financial statements, management assesses the
likelihood that its deferred tax assets will be realized from future taxable income. In evaluating
the Company’s ability to recover its deferred income tax assets, management considers all available
positive and negative evidence, including its operating results, ongoing tax planning and forecasts
of future taxable income on a jurisdiction by jurisdiction basis. A valuation allowance is
established if it is determined that it is more likely than not that some portion or all of the net
deferred tax assets will not be realized. Management exercises significant judgment in determining
its provisions for income taxes, its deferred tax assets and liabilities and its future taxable
income for purposes of assessing its ability to utilize any future tax benefit from its deferred
tax assets.
Although management believes that its tax estimates are reasonable, the ultimate tax
determination involves significant judgment that could become subject to audit by tax authorities
in the ordinary course of business, as well as the generation of sufficient future taxable income.
During 2009, management determined that it was more likely than not that its net operating loss
carryforwards for the state of Arizona, which would have expired in 2009, would be fully realized.
Accordingly, the valuation allowance of $200,000 the Company carried against its deferred tax
assets as of December 31, 2008, was reversed with the benefit recognized during 2009 as a reduction
of the current-year effective tax rate. Management believes that, as of December 31, 2009, based on
an evaluation and projections of future sales and profitability, no other valuation allowance was
deemed necessary. However, such deferred tax assets could be reduced in the future if projections
of future taxable income during the carryforward period are reduced.
Stock Based Compensation
We estimate the fair value of our stock based compensation by using the Black-Scholes-Merton
option pricing model which requires the input of highly subjective assumptions. These assumptions
include estimating the length of time employees will retain their stock options before exercising
them (expected term), the estimated volatility of our common stock price over the expected term and
the number of options that will ultimately not vest (forfeitures). We granted 825,800
performance-based stock options in 2008 and the first quarter of
2009, the vesting of which is
contingent upon the achievement of certain performance criteria including the successful
development and market acceptance of future product introductions as well as our future operating
performance. These options are vested and compensation expense is recognized based on management’s
best estimate of the probability of the performance criteria being satisfied using the most
currently available projections of future product adoption and operating performance, adjusted at
each balance sheet date. During 2009, 106,700 of these options were forfeited. Of the remaining
719,100 outstanding options, 286,708 are exercisable, and 432,392 are unvested. Changes in the
subjective and probability based assumptions can materially affect the estimate of fair value of
stock-based compensation and consequently, the related amount recognized on our statements of
operations. Refer to Note 10 to our consolidated financial statements for further discussion of how
we determined our valuation assumptions.
Contingencies
We are subject to the possibility of various loss contingencies including product related
litigation, arising in the ordinary course of business. We consider the likelihood of loss or
impairment of an asset or the incurrence of a liability, as well as our ability to reasonably
estimate the amount of loss in determining loss contingencies. An estimated loss contingency is
accrued when it is probable that an asset has been impaired or a liability has been incurred and
the amount of loss can be reasonably estimated. We regularly evaluate current information available
to us to determine whether such accruals should be adjusted and
whether new accruals are required. As of December 31, 2009 we have
not recorded any accruals for outstanding litigation.
| Item 7A. | Quantitative and Qualitative Disclosures About Market Risk |
Interest Rate Risk
We invest in
a limited number of financial instruments, which at December 31, 2009 consisted
entirely of investments in money market accounts, denominated in United States dollars.
At December 31, 2009, we did not hold any investments in fixed rate interest earning investments
which would be subject to market value adjustments resulting from fluctuations in interest rates.
Additionally, we have access to a line of credit borrowing facility which bears interest at
varying rates, ranging from LIBOR plus 1.5% to prime. At December 31, 2009, there was no amount
outstanding under the line of credit and the available borrowing under the line of credit was $10.0
million. We have not borrowed any funds under the line of credit since its inception, however,
should we need to do so in the future, such borrowings could be subject to adverse or favorable
changes in the underlying interest rate.
Exchange Rate Risk
We consider our direct exposure to foreign exchange rate fluctuations to be minimal.
Currently, sales to customers provide for pricing and payment in United States dollars, and
therefore, are not subject to exchange rate fluctuations. To date, we have not engaged in any
currency hedging activities, although we may do so in the future. Fluctuations in currency exchange
rates could harm our business in the future.
35
Table of Contents
| Item 8. | Financial Statements and Supplementary Data |
TASER INTERNATIONAL, INC.
CONSOLIDATED BALANCE SHEETS
December 31,
CONSOLIDATED BALANCE SHEETS
December 31,
| 2009 | 2008 | |||||||
Assets |
||||||||
Current assets: |
||||||||
Cash and cash equivalents |
$ | 45,505,049 | $ | 46,880,435 | ||||
Short-term investments |
— | 2,498,998 | ||||||
Accounts receivable, net of allowance of $200,000 in 2009 and 2008, respectively |
15,384,993 | 16,793,553 | ||||||
Inventory |
15,085,750 | 13,467,117 | ||||||
Prepaid expenses and other assets |
1,461,539 | 2,528,539 | ||||||
Deferred income tax assets, net |
8,447,915 | 9,430,073 | ||||||
Total current assets |
85,885,246 | 91,598,715 | ||||||
Property and equipment, net |
38,673,065 | 27,128,032 | ||||||
Deferred income tax assets, net |
10,997,093 | 8,826,778 | ||||||
Intangible assets |
2,765,701 | 2,447,011 | ||||||
Other long-term assets |
104,812 | 14,970 | ||||||
Total assets |
$ | 138,425,917 | $ | 130,015,506 | ||||
Liabilities and stockholders’ equity |
||||||||
Current liabilities: |
||||||||
Accounts payable |
$ | 6,357,195 | $ | 3,856,961 | ||||
Accrued liabilities |
4,252,577 | 4,275,907 | ||||||
Current portion of deferred revenue |
2,819,155 | 2,510,645 | ||||||
Customer deposits |
355,926 | 312,686 | ||||||
Total current liabilities |
13,784,853 | 10,956,199 | ||||||
Deferred revenue, net of current portion |
4,675,089 | 4,840,965 | ||||||
Liability for unrecognized tax benefits |
2,264,779 | 1,692,080 | ||||||
Total liabilities |
20,724,721 | 17,489,244 | ||||||
Commitments and contingencies |
||||||||
Stockholders’ equity |
||||||||
Preferred stock, $0.00001 par value per share; 25 million shares authorized; no shares issued and outstanding at December 31, 2009 and 2008 |
— | — | ||||||
Common stock, $0.00001 par value per share; 200 million shares authorized; 62,119,063 and 61,795,712 shares issued and outstanding at December 31, 2009 and 2008, respectively |
642 | 638 | ||||||
Additional paid-in capital |
92,839,165 | 87,663,129 | ||||||
Treasury stock, 2,091,600 shares at December 31, 2009 and 2008 |
(14,708,237 | ) | (14,708,237 | ) | ||||
Retained earnings |
39,569,626 | 39,570,732 | ||||||
Total stockholders’ equity |
117,701,196 | 112,526,262 | ||||||
Total liabilities and stockholders’ equity |
$ | 138,425,917 | $ | 130,015,506 | ||||
The accompanying notes are an integral part of these consolidated financial statements.
36
Table of Contents
TASER INTERNATIONAL, INC.
CONSOLIDATED STATEMENTS OF OPERATIONS
For The Years Ended December 31,
CONSOLIDATED STATEMENTS OF OPERATIONS
For The Years Ended December 31,
| 2009 | 2008 | 2007 | ||||||||||
Net sales |
$ | 104,251,560 | $ | 92,845,490 | $ | 100,727,191 | ||||||
Cost of products sold: |
||||||||||||
Direct manufacturing expense |
29,578,304 | 26,756,080 | 31,507,727 | |||||||||
Indirect manufacturing expense |
11,270,847 | 9,085,183 | 11,659,645 | |||||||||
Total cost of products sold |
40,849,151 | 35,841,263 | 43,167,372 | |||||||||
Gross margin |
63,402,409 | 57,004,227 | 57,559,819 | |||||||||
Sales, general and administrative expenses |
43,479,232 | 38,860,729 | 32,814,170 | |||||||||
Research and development expenses |
20,002,351 | 12,918,161 | 4,421,596 | |||||||||
Income (loss) from operations |
(79,174 | ) | 5,225,337 | 20,324,053 | ||||||||
Interest and other income, net |
170,547 | 1,717,967 | 2,202,187 | |||||||||
Income before provision for income taxes |
91,373 | 6,943,304 | 22,526,240 | |||||||||
Provision for income taxes |
92,479 | 3,306,263 | 7,499,764 | |||||||||
Net income (loss) |
$ | (1,106 | ) | $ | 3,637,041 | $ | 15,026,476 | |||||
Income (loss) per common and common equivalent shares |
||||||||||||
Basic |
$ | (0.00 | ) | $ | 0.06 | $ | 0.24 | |||||
Diluted |
$ | (0.00 | ) | $ | 0.06 | $ | 0.23 | |||||
Weighted average number of common and common equivalent shares outstanding |
||||||||||||
Basic |
61,920,094 | 62,371,004 | 62,621,174 | |||||||||
Diluted |
61,920,094 | 64,070,869 | 65,685,667 | |||||||||
The accompanying notes are an integral part of these consolidated financial statements.
37
Table of Contents
TASER INTERNATIONAL, INC.
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY
For the Years Ended December 31, 2009, 2008 and 2007
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY
For the Years Ended December 31, 2009, 2008 and 2007
| Additional | Total | |||||||||||||||||||||||||||
| Common Stock | Paid-in | Treasury Stock | Retained | Stockholders’ | ||||||||||||||||||||||||
| Shares | Amount | Capital | Shares | Amount | Earnings | Equity | ||||||||||||||||||||||
Balance, December 31, 2006 |
61,939,974 | $ | 622 | $ | 80,629,659 | 300,000 | $ | (2,208,957 | ) | $ | 20,907,215 | $ | 99,328,539 | |||||||||||||||
Exercise of stock options |
1,107,574 | 11 | 3,143,758 | — | — | — | 3,143,769 | |||||||||||||||||||||
Shareholder derivate settlement |
216,355 | 2 | 1,749,998 | — | — | — | 1,750,000 | |||||||||||||||||||||
Stock-based compensation expense |
— | — | 1,387,966 | — | — | — | 1,387,966 | |||||||||||||||||||||
Net income |
— | — | — | — | — | 15,026,476 | 15,026,476 | |||||||||||||||||||||
Balance, December 31, 2007 |
63,263,903 | 635 | 86,911,381 | 300,000 | (2,208,957 | ) | 35,933,691 | 120,636,750 | ||||||||||||||||||||
Exercise of stock options |
323,409 | 3 | 342,818 | — | — | — | 342,821 | |||||||||||||||||||||
Deferred tax asset correction (see footnote 8) |
— | — | (2,014,955 | ) | — | — | — | (2,014,955 | ) | |||||||||||||||||||
Stock-based compensation expense |
— | — | 2,423,885 | — | — | — | 2,423,885 | |||||||||||||||||||||
Purchase of treasury stock |
(1,791,600 | ) | — | — | 1,791,600 | (12,499,280 | ) | — | (12,499,280 | ) | ||||||||||||||||||
Net income |
— | — | — | — | — | 3,637,041 | 3,637,041 | |||||||||||||||||||||
Balance, December 31, 2008 |
61,795,712 | 638 | 87,663,129 | 2,091,600 | (14,708,237 | ) | 39,570,732 | 112,526,262 | ||||||||||||||||||||
Exercise of stock options |
323,351 | 4 | 155,540 | — | — | — | 155,544 | |||||||||||||||||||||
Stock-based compensation expense |
— | — | 4,988,837 | — | — | — | 4,988,837 | |||||||||||||||||||||
Tax benefit from stock options exercised |
— | — | 31,659 | — | — | — | 31,659 | |||||||||||||||||||||
Net loss |
— | — | — | — | — | (1,106 | ) | (1,106 | ) | |||||||||||||||||||
Balance, December 31, 2009 |
62,119,063 | $ | 642 | $ | 92,839,165 | 2,091,600 | $ | (14,708,237 | ) | $ | 39,569,626 | $ | 117,701,196 | |||||||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
38
Table of Contents
TASER INTERNATIONAL, INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS
For the Years Ended December 31,
CONSOLIDATED STATEMENTS OF CASH FLOWS
For the Years Ended December 31,
| 2009 | 2008 | 2007 | ||||||||||
Cash Flows from Operating Activities: |
||||||||||||
Net income (loss) |
$ | (1,106 | ) | $ | 3,637,041 | $ | 15,026,476 | |||||
Adjustments to reconcile net income (loss) to net cash provided by operating activities: |
||||||||||||
Depreciation and amortization |
3,634,412 | 2,637,773 | 2,521,237 | |||||||||
Loss on disposal of assets |
66,829 | 171,098 | 49,949 | |||||||||
Provision for doubtful accounts |
82,251 | 78,010 | 80,835 | |||||||||
Provision for excess and obsolete inventory |
821,983 | 640,655 | 206,335 | |||||||||
Provision for warranty |
92,278 | 368,521 | 1,030,291 | |||||||||
Stock-based compensation expense |
4,988,837 | 2,423,885 | 1,387,966 | |||||||||
Deferred insurance settlement proceeds |
— | (404,848 | ) | (104,219 | ) | |||||||
Deferred income taxes |
(1,188,157 | ) | 2,060,623 | 5,831,783 | ||||||||
Provision for unrecognized tax benefits |
572,699 | 592,007 | 1,100,073 | |||||||||
Tax benefit
from stock-based compensation, net |
(31,659 | ) | — | — | ||||||||
Change in assets and liabilities: |
||||||||||||
Accounts receivable |
1,326,309 | (5,180,010 | ) | (1,704,339 | ) | |||||||
Inventory |
(2,440,616 | ) | (600,968 | ) | (4,455,393 | ) | ||||||
Prepaids and other assets |
976,156 | 1,856,355 | (2,235,862 | ) | ||||||||
Accounts payable and accrued liabilities |
1,031,196 | (2,323,792 | ) | 1,069,483 | ||||||||
Deferred revenue |
142,634 | 2,115,699 | 2,222,981 | |||||||||
Accrued litigation settlement |
— | — | (8,000,000 | ) | ||||||||
Other liabilities |
— | — | (199,999 | ) | ||||||||
Customer deposits |
43,240 | 45,958 | 95,236 | |||||||||
Net cash provided by operating activities |
10,117,286 | 8,118,007 | 13,922,833 | |||||||||
Cash Flows from Investing Activities: |
||||||||||||
Purchases of investments |
— | (43,887,640 | ) | (138,203,034 | ) | |||||||
Proceeds from investments |
2,500,000 | 58,895,113 | 149,731,426 | |||||||||
Purchases of property and equipment |
(13,708,177 | ) | (6,144,425 | ) | (4,067,579 | ) | ||||||
Purchases of intangible assets |
(471,698 | ) | (745,622 | ) | (454,403 | ) | ||||||
Net cash (used in) provided by investing activities |
(11,679,875 | ) | 8,117,426 | 7,006,410 | ||||||||
Cash Flows from Financing Activities: |
||||||||||||
Proceeds from options exercised |
155,544 | 342,821 | 3,143,769 | |||||||||
Tax benefit
from stock-based compensation, net |
31,659 | — | — | |||||||||
Repurchase of common stock |
— | (12,499,280 | ) | — | ||||||||
Payments under capital leases |
— | — | (45,236 | ) | ||||||||
Net cash provided by (used in) financing activities |
187,203 | (12,156,459 | ) | 3,098,533 | ||||||||
Net (decrease) increase in cash and cash equivalents |
(1,375,386 | ) | 4,078,974 | 24,027,776 | ||||||||
Cash and cash equivalents, beginning of period |
46,880,435 | 42,801,461 | 18,773,685 | |||||||||
Cash and cash equivalents, end of period |
$ | 45,505,049 | $ | 46,880,435 | $ | 42,801,461 | ||||||
Supplemental Disclosure: |
||||||||||||
Cash paid for income taxes — net |
$ | 894,563 | $ | 523,950 | $ | 475,000 | ||||||
Cash paid for interest |
$ | — | $ | — | $ | 5,186 | ||||||
Non Cash Transactions- |
||||||||||||
Property and equipment purchases in accounts payable |
$ | 1,385,089 | $ | — | $ | 1,198,891 | ||||||
Deferred tax asset correction (see footnote 8) |
$ | — | $ | 2,014,955 | $ | — | ||||||
Common stock issued for shareholder derivative lawsuit settlement |
$ | — | $ | — | $ | 1,750,000 | ||||||
The accompanying notes are an integral part of these consolidated financial statements.
39
Table of Contents
TASER INTERNATIONAL, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
1. Organization and Summary of Significant Accounting Policies
TASER International, Inc. (“TASER” or the “Company”) is a developer and manufacturer of advanced
electronic control devices (“ECDs”) designed for use in law enforcement, military, corrections,
private security and personal defense. In addition, the Company is developing full technology
solutions for the capture, storage and management of video/audio evidence as well as other tactical
capabilities for use in law enforcement. The Company sells its products worldwide through its
direct sales force, distribution partners, online store and third party resellers. The Company was
incorporated in Arizona in September 1993 and reincorporated in Delaware in January 2001. The
Company’s corporate headquarters and manufacturing facilities are located in Scottsdale, Arizona.
The Company’s internet services and software development division facilities are located in
Carpenteria, California.
The accompanying consolidated financial statements include the accounts of the Company, and
its wholly owned subsidiary, TASER International Europe SE (“TASER Europe”). TASER Europe was
established in the third quarter of 2009 to facilitate sales and provide customer service to our
customers in the European region. All material intercompany accounts, transactions, and profits
have been eliminated.
a. Basis of Presentation and Use of Estimates
The accompanying consolidated financial statements have been prepared in conformity with
accounting principles generally accepted in the United States of America. The preparation of these
consolidated financial statements requires management to make estimates and assumptions that affect
the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities
at the date of the consolidated financial statements and the reported amounts of revenues and
expenses during the reporting period. Significant estimates in these consolidated financial
statements include allowances for doubtful accounts receivable, inventory valuation reserves,
product warranty reserves, valuation of long lived assets, deferred income taxes, stock-based
compensation and contingencies. Actual results could differ from those estimates.
b. Cash, Cash Equivalents and Investments
Cash and cash equivalents include cash and money market funds on hand and short-term
investments with original maturities of three months or less. Short-term investments include
securities having maturities of 90 days to one year. Long-term investments include securities
having original maturities of more than one year. At December 31, 2009, the entire $45.5 million of
the Company’s cash and cash equivalents was comprised of cash and money market funds. Previously,
the Company’s short and long-term investments were invested in government sponsored mortgage-backed
securities and were classified as held to maturity. In February 2009, the Company’s remaining
short-term investment in a government sponsored entity was called at par value by the issuing
agency.
The Company valued its cash equivalents in money market accounts using observable inputs that
reflect quoted prices for securities with identical characteristics, and accordingly, management
classified the valuation techniques that use these inputs as Level 1.
The Company’s
cash and investment accounts earned interest at an approximate rate of 0.30%
during 2009, down from 2.5% in 2008. Included in the Company’s cash balances are deposits,
including money market funds, with a single bank of $45.2 million, which is in excess of the FDIC
insurance coverage limit of $250,000.
c. Inventory
Inventories are stated at the lower of cost or market. Cost is determined using the weighted
average cost of raw materials which approximates the first-in, first-out (FIFO) method and includes
allocations of manufacturing labor and overhead. Provisions are made to reduce potentially excess,
obsolete or slow-moving inventories to their net realizable value. These provisions are based on
management’s best estimate after considering historical demand, projected future demand, inventory
purchase commitments, industry and market trends and conditions and other factors. Management
evaluates inventory costs for abnormal costs due to excess production capacity and treats such
costs as period costs.
d. Property and Equipment
Property and equipment are stated at cost net of accumulated depreciation. Additions and
improvements are capitalized while ordinary maintenance and repair expenditures are charged to
expense as incurred. Depreciation is calculated using the straight-line method over the estimated
useful lives of the assets.
40
Table of Contents
TASER INTERNATIONAL, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
e. Capitalized software development costs
For development costs related to EVIDENCE.COM, the Company’s Software as a Service (SaaS)
product, the Company capitalizes qualifying computer software costs that are incurred during the
application development stage. Costs related to preliminary project planning activities and
post-implementation activities are expensed as incurred. At December 31, 2009 and 2008, capitalized
software development costs were approximately $2.4 million and $0, respectively. The Company will
begin amortizing capitalized software development costs over an estimated useful life of 36 months
once all final product testing is substantially complete, which is expected to be during 2010.
f. Impairment of Long-Lived Assets
Management evaluates whether events and circumstances have occurred that indicate the
remaining estimated useful life of long-lived assets and identifiable intangible assets may warrant
revision or that the remaining balance of these assets may not be recoverable. In performing the
review for recoverability, management estimates the future undiscounted cash flows expected to
result from the use of the assets and their eventual disposition. The amount of the impairment
loss, if impairment exists, would be calculated based on the excess of the carrying amounts of the
assets over their estimated fair value computed using discounted cash flows. No impairment losses
were recorded in 2009, 2008 or 2007.
g. Customer Deposits
The Company requires certain deposits in advance of shipment for certain customer sales
orders. Customer deposits are recorded as a current liability on the accompanying consolidated
balance sheets.
h. Revenue Recognition and Accounts Receivable
The Company recognizes revenues when persuasive evidence of an arrangement exists, delivery
has occurred or services have been rendered, title has transferred, the price is fixed and
collectability is reasonably assured. In most instances, sales of the Company’s products are final
and its customers do not have a right to return the product. The Company’s consumer product, the
TASER C2, has a 30 day right of return for inactivated units purchased direct from the Company. The
historical product return rate is used to determine the return reserve.
During 2008, the Company began selling the TASER C2 product through certain retailers who do
not assume title, risk of loss to the inventory or credit risk. The Company therefore recognizes
revenue from such retailers on a sell-through method using information provided by the retailer.
The revenue and related costs are deferred until the product has been sold by the retailer.
The Company offers customers the right to purchase extended warranties that include additional
services and coverage beyond the limited warranty on the TASER X26 and ADVANCED TASER products.
Revenue for extended warranty purchases is deferred at the time of sale and recognized over the
warranty period commencing on the date of sale. The extended warranties range from one to four
years. At December 31, 2009 and 2008, $7.5 million and $7.4 million was deferred under this
program, respectively. The current portion of deferred revenue represents the extended warranty
revenue which will be recognized in 2010.
Sales are typically made on credit and the Company generally does not require collateral.
Management performs ongoing credit evaluations of its customers’ financial condition and maintains
an allowance for estimated potential losses. Uncollectible accounts are written off when deemed
uncollectible, and accounts receivable are presented net of an allowance for doubtful accounts.
This allowance represents our best estimate and is based on our judgment after considering a number
of factors, including third-party credit reports, actual payment history, cash discounts,
customer-specific financial information and broader market and economic trends and conditions.
41
Table of Contents
TASER INTERNATIONAL, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
Included as a component of revenue is development funding provided by the Joint Non-Lethal
Weapons Directorate (JNLWD) of the U.S Department of Defense under a cost-plus fixed fee contract.
Periodically, an invoice summarizing the reimbursable expenses is submitted to JNLWD for payment.
The payment request submitted by the Company to the JNLWD details the costs incurred in the period
plus a nominal contracted profit margin. The total amount of revenue recognized for this work in
the years ended December 31, 2009 and 2008 was $1.3 million and $0.9 million, respectively.
Certain of the Company’s customers are charged shipping fees, which are recorded as a
component of net sales. Sales tax collected on sales is netted against government remittances and
thus, recorded on a net basis. Training revenue is recorded as the service is provided.
i. Cost of Products Sold
Cost of products sold represents manufacturing costs, consisting of materials, labor and
overhead related to finished goods and components. Shipping costs incurred related to product
delivery are also included in cost of products sold.
j. Advertising Costs
The Company expenses advertising costs in the period in which they are incurred with the
exception of commercial advertising production costs which are expensed at the time the first
commercial is shown on television. The Company incurred advertising costs of $681,000, $2,085,000,
and $931,000 in 2009, 2008 and 2007, respectively. At December 31, 2009, the Company had $79,000 of
prepaid advertising costs. There were no prepaid advertising costs at December 31, 2008.
Advertising costs are included in sales, general and administrative expenses in the accompanying
statements of operations.
k. Warranty Costs
The Company warrants its X3 and X26 products from manufacturing defects on a limited basis for
a period of one year after purchase, and thereafter will replace any defective unit for a fee. The
C2 product is warranted for a period of 90 days after purchase. The Company also sells extended
warranties for periods of up to four years after the expiration of the limited one year warranty.
Management tracks historical data related to returns and warranty costs on a quarterly basis, and
estimates future warranty claims by applying the estimated weighted average return rate to the
product sales for the period. If management becomes aware of a component failure that could result
in larger than anticipated returns from its customers, the reserve would be increased. After the
one year warranty expires, if the device fails to operate properly for any reason, the Company will
replace the TASER X26 for a prorated discounted price depending on when the product was placed into
service and replace the ADVANCED TASER device for a fee of $75. These fees are intended to cover
the handling and repair costs and include a profit. The following table summarizes the changes in
the estimated product warranty liabilities for the years ended December 31, 2009, 2008 and 2007:
| 2009 | 2008 | 2007 | ||||||||||
Balance at Beginning of Period |
$ | 615,031 | $ | 919,254 | $ | 713,135 | ||||||
Utilization of Accrual |
(337,998 | ) | (672,744 | ) | (824,172 | ) | ||||||
Warranty Expense |
92,278 | 368,521 | 1,030,291 | |||||||||
Balance at End of the Period |
$ | 369,311 | $ | 615,031 | $ | 919,254 | ||||||
l. Research and Development Expenses
The Company expenses research and development costs as incurred. The Company incurred product
development expense of approximately $20.0 million, $12.9 million, and $4.4 million in 2009, 2008
and 2007, respectively.
42
Table of Contents
TASER INTERNATIONAL, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
m. Income Taxes
Income taxes are accounted for under the asset and liability method. Deferred tax assets and
liabilities are recognized for the future tax consequences attributable to differences between the
financial statement amounts of assets and liabilities and their respective tax bases and operating
loss and tax credit carry forwards. Deferred tax assets and liabilities are measured using enacted
tax rates expected to apply to taxable income in future years in which those temporary differences
are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a
change in tax rate is recognized in income in the period that includes the enactment date. Deferred
tax assets are reduced through the establishment of a valuation allowance at the time, based upon
available evidence, it becomes more likely than not that the deferred tax assets will not be
realized. Income tax-related interest and penalties are recorded as a component of the provision
for income taxes.
The Company recognizes the tax benefit from an uncertain tax position only if it is more
likely than not that the tax position will be sustained on examination by the taxing authorities,
based on the technical merits of the position. The tax benefits recognized in the consolidated
financial statements from such a position are measured based on the largest benefit that has a
greater than fifty percent likelihood of being realized upon ultimate resolution. Management also
assesses whether uncertain tax positions as filed could result in the recognition of a liability
for possible interest and penalties. The Company’s policy is to include interest and penalties
related to unrecognized tax benefits as a component of income tax expense. Refer to Note 8 for
additional information regarding the change in unrecognized tax benefits.
n. Concentration of Credit Risk and Major Customers / Suppliers
Financial instruments that potentially subject the Company to concentrations of credit risk
consist of accounts receivable. Sales are typically made on credit and the Company generally does
not require collateral. Management performs ongoing credit evaluations of its customers’ financial
condition and maintains an allowance for estimated losses. Uncollectible accounts are written off
when deemed uncollectible, and accounts receivable are presented net of an allowance for doubtful
accounts. The allowance for bad debts totaled $200,000 as of December 31, 2009 and 2008.
Historically, the Company has experienced a low level of write offs related to doubtful accounts.
The Company sells its products primarily through a network of unaffiliated distributors. The
Company also reserves the right to sell directly to the end user to secure the customer’s account.
In 2009, one distributor represented approximately 12% of total sales. No other customer exceeded
10% of total sales in 2009. In 2008, one distributor represented approximately 11% of total sales.
No other customer exceeded 10% of total sales in 2008. In 2007, one distributor represented
approximately 16% of total sales. No other customer exceeded 10% of total sales in 2007.
At December 31, 2009, the Company had accounts receivable from one customer comprising 12.5%
of the aggregate accounts receivable balance. At December 31, 2008, the Company had accounts
receivable from two customers comprising 30% and 12% of the aggregate accounts receivable balance.
The customer comprising the 30% balance is the result of a large individual sale made to the U.K.
Government in December 2008, which was paid in full in February 2009. These customers are
unaffiliated distributors of the Company’s products.
The Company currently purchases finished circuit boards and injection-molded plastic
components from suppliers located in the United States. Although the Company currently obtains many
of these components from single source suppliers, the Company owns the injection molded component
tooling used in their production. As a result, management believes it could obtain alternative
suppliers in most cases without incurring significant production delays. The Company also purchases
small, machined parts from a vendor in Taiwan, custom cartridge assemblies from a proprietary
vendor in the United States, and electronic components from a variety of foreign and domestic
distributors. Management believes that there are readily available alternative suppliers in most
cases who can consistently meet its needs for these components. The Company acquires most of its
components on a purchase order basis and does not have long-term contracts with suppliers.
43
Table of Contents
TASER INTERNATIONAL, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
o. Fair Value of Financial Instruments
The Company uses the fair value framework for measuring financial assets and liabilities
measured on a recurring basis. The Company does not have any
nonfinancial assets or nonfinancial liabilities that it recognizes or
discloses at fair value in its consolidated financial statements on a
recurring basis.
Fair value is defined as the price that would be received from the sale of an asset or paid to
transfer a liability (an exit price) on the measurement date in an orderly transaction between
market participants in the principal or most advantageous market for the asset or liability. The
fair value framework specifies a hierarchy of valuation techniques, which is based on whether the
inputs into the valuation technique are observable or unobservable. The hierarchy is as follows:
| • | Level 1 — Valuation techniques in which all significant inputs are unadjusted quoted prices from active markets for assets or liabilities that are identical to the assets or liabilities being measured. | ||
| • | Level 2 — Valuation techniques in which significant inputs include quoted prices from active markets for assets or liabilities that are similar to the assets or liabilities being measured and/or quoted prices for assets or liabilities that are identical or similar to the assets or liabilities being measured from markets that are not active. Also, model-derived valuations in which all significant inputs and significant value drivers are observable in active markets are Level 2 valuation techniques. | ||
| • | Level 3 — Valuation techniques in which one or more significant inputs or significant value drivers are unobservable. Unobservable inputs are valuation technique inputs that reflect our own assumptions about the assumptions that market participants would use in pricing an asset or liability. |
Following is a description of the valuation techniques that the Company uses to measure the
fair value of assets and liabilities that it measures and reports on its balance sheet at fair
value on a recurring basis:
| • | Cash Equivalents. As of December 31, 2009 and 2008, the Company’s cash equivalents consisted of money market mutual funds. The Company valued its cash equivalents using observable inputs that reflect quoted prices for securities with identical characteristics, and accordingly, the Company classifies the valuation techniques that use these inputs as Level 1. | ||
| • | Investments. As of December 31, 2008, the Company’s investments consisted of a federal government sponsored entity security. The Company’s investments are held to maturity and stated at amortized cost, which approximates fair value. Information about the fair value of the Company’s investments is included in Note 2. |
The Company’s financial instruments also include accounts receivable, accounts payable and
accrued liabilities. Due to the short-term nature of these instruments, their fair value
approximates their carrying value on the balance sheet as of December 31, 2009 and 2008.
p. Segment Information
Management has determined that its operations are comprised of one reportable segment — the
sale of advanced electronic control devices and accessories, and will evaluate reportable segments
related to new product offerings expected to be launched in 2010. For the years ended December 31,
2009, 2008 and 2007, sales by geographic area were as follows:
| 2009 | 2008 | 2007 | ||||||||||
Sales by Geographic Area |
||||||||||||
United States |
78 | % | 82 | % | 85 | % | ||||||
Other Countries |
22 | % | 18 | % | 15 | % | ||||||
Total |
100 | % | 100 | % | 100 | % | ||||||
44
Table of Contents
TASER INTERNATIONAL, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
Sales to customers outside of the United States are denominated in U.S. dollars and are attributed
to each country based on the billing address of the distributor or customer. To date, no individual
country outside the U.S. has represented a material amount of total net revenue. Substantially all
assets of the Company are located in the United States.
q. Stock-Based Compensation
The Company calculates the fair value of stock-based awards using the Black-Scholes-Merton option
valuation model, which incorporates various assumptions including volatility, expected life, and
interest rates. The assumptions used for the years ended December 31, 2009, 2008 and 2007 and the
resulting estimates of weighted-average fair value per share of options granted during those
periods are as follows:
| 2009 | 2008 | 2007 | ||||||||||
Weighted average / range of volatility |
70 | % | 70 | % | 60 | % | ||||||
Risk-free interest rate |
1.9 | % | 2.2 | % | 4.7 | % | ||||||
Dividend rate |
0.0 | % | 0.0 | % | 0.0 | % | ||||||
Expected life of options |
4.5 years | 4.0 years | 4.0 years | |||||||||
Weighted average fair value of options granted |
$ | 2.56 | $ | 2.89 | $ | 5.22 | ||||||
The expected life of the options represents the estimated period of time until
exercise and is based on historical experience of similar awards, giving consideration to the
contractual terms, vesting schedules and expectations of future employee behavior. Expected stock
price volatility is based on a combination of historical volatility of the Company’s stock and the
one-year implied volatility of its publicly traded options for the related vesting periods. The
risk-free interest rate is based on the implied yield available on U.S. Treasury zero-coupon issues
with an equivalent remaining term. The Company has not paid dividends in the past and does not plan
to pay any dividends in the near future. The estimated fair value of stock-based compensation
awards and other options is amortized to expense on a straight line basis over the relevant vesting
period. As share-based compensation expense recognized is based on awards ultimately expected to
vest, it is reduced for estimated forfeitures. Forfeitures are estimated at the time of grant and
revised, if necessary, in subsequent periods if actual forfeitures differ from those estimates. The
Company forfeiture rate was calculated based on its historical experience of awards which
ultimately vested. See Note 10 for further discussion of the Company’s stock-based compensation.
r. Income (Loss) Per Common Share
Basic income per share is computed by dividing net income (loss) by the weighted average
number of common shares outstanding during the periods presented. Diluted income per share reflects
the potential dilution that could occur if outstanding stock options were exercised utilizing the
treasury stock method. The calculation of the weighted average number of shares outstanding and
earnings per share are as follows:
| For the Year Ended December 31, | ||||||||||||
| 2009 | 2008 | 2007 | ||||||||||
Numerator for basic and diluted earnings per share |
||||||||||||
Net (loss) income |
$ | (1,106 | ) | $ | 3,637,041 | $ | 15,026,476 | |||||
Denominator for basic earnings per share — weighted average shares outstanding |
61,920,094 | 62,371,004 | 62,621,174 | |||||||||
Dilutive effect of shares issuable under stock options outstanding |
— | 1,699,865 | 3,064,493 | |||||||||
Denominator for diluted earnings per share — adjusted weighted average shares outstanding |
61,920,094 | 64,070,869 | 65,685,667 | |||||||||
Net (loss) income per common share |
||||||||||||
Basic |
$ | (0.00 | ) | $ | 0.06 | $ | 0.24 | |||||
Diluted |
$ | (0.00 | ) | $ | 0.06 | $ | 0.23 | |||||
45
Table of Contents
TASER INTERNATIONAL, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
Basic income (loss) per share is based upon the weighted average number of common shares
outstanding during the period. Diluted income per share includes the dilutive effect of potential
stock option exercises, calculated using the treasury stock method. As a result of the net loss for
the year ended December 31, 2009, 8,056,927 shares of potential dilutive securities were considered
anti-dilutive and excluded from the calculation as their effect would have been to reduce the net
loss per share. For the years ended December 31, 2008 and 2007, the effects of 2,205,861 and
315,764, respectively, were excluded from the calculation of diluted income per share as their
effect would have been anti-dilutive and increased the net income per share.
s. Recently adopted accounting guidance
On July 1, 2009, the Company adopted the authoritative guidance issued by the Financial
Accounting Standards Board (“FASB”) that the Accounting Standard Codification (“ASC”) be recognized
as the source of authoritative U.S. generally accepted accounting principles (“U.S. GAAP”). The
Codification did not change U.S. GAAP, but instead introduced a new structure that combines all
authoritative standards into a comprehensive, topically organized single source of authoritative
GAAP. Adoption of the new guidance did not have a material impact on the Company’s consolidated
financial statements.
On January 1, 2009, the Company adopted the authoritative guidance issued by the FASB on
business combinations. The guidance expands the definition of a business and a business
combination; requires the acquirer to recognize the assets acquired, liabilities assumed and
non-controlling interests (including goodwill), measured at fair value at the acquisition date;
requires acquisition-related expenses and restructuring costs to be recognized separately from the
business combination; requires assets acquired and liabilities assumed to be recognized at their
acquisition-date fair values with subsequent changes recognized in earnings; and requires
in-process research and development to be capitalized at fair value as an indefinite-lived
intangible asset. This guidance will impact new acquisitions closed after January 1, 2009.
On January 1, 2009, the Company adopted the authoritative guidance issued by the FASB that
changes the accounting and reporting for non-controlling interests. Non-controlling interests are
to be reported as a component of equity separate from the parent’s equity, and purchases or sales
of equity interests that do not result in a change in control are to be accounted for as equity
transactions. In addition, net income or loss attributable to a non-controlling interest is to be
included in net income and, upon a loss of control, the interest sold, as well as any interest
retained, is to be recorded at fair value with any gain or loss recognized in net income. Adoption
of the new guidance did not have a material impact on the Company’s consolidated financial
statements.
On January 1, 2009, the Company adopted the authoritative guidance on fair value measurement
for nonfinancial assets and liabilities, except for items that are recognized or disclosed at fair
value in the financial statements on a recurring basis (at least annually). Adoption of the new
guidance did not have a material impact on the Company’s consolidated financial statements, as
management has not adopted the fair value option for any non-financial assets of liabilities.
On January 1, 2009, the Company adopted the authoritative guidance on the determination of the
useful life of intangible assets which amended the factors that should be considered in developing
renewal or extension assumptions used to determine the useful life of a recognized intangible asset
under previously issued goodwill and intangible assets guidance. This change was intended to
improve the consistency between the useful life of a recognized intangible asset and the period of
expected cash flows used to measure the fair value of the asset under topics related to business
combinations and other U.S. GAAP. Adoption of the new guidance did not have a material impact on
the Company’s consolidated financial statements.
46
Table of Contents
TASER INTERNATIONAL, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
Recent accounting guidance not yet adopted
In October 2009, the FASB issued authoritative guidance on revenue recognition that will
become effective for the Company beginning January 1, 2011, with earlier adoption permitted. Under
the new guidance on arrangements that include software elements, tangible products that have
software components that are essential to the functionality of the tangible product will no longer
be within the scope of the software revenue recognition guidance, and software-enabled products
will now be subject to other relevant revenue recognition guidance. Additionally, the FASB issued
authoritative guidance on revenue arrangements with multiple deliverables that are outside the
scope of the software revenue recognition guidance. Under the new guidance, when vendor specific
objective evidence or third party evidence for deliverables in an arrangement cannot be determined,
a best estimate of the selling price is required to separate deliverables and allocate arrangement
consideration using the relative selling price method. The new guidance includes new disclosure
requirements on how the application of the relative selling price method affects the timing and
amount of revenue recognition. Management is evaluating the impact that adoption of this new
guidance will have on the Company’s consolidated financial statements.
In June 2009, the FASB issued authoritative guidance on the consolidation of variable interest
entities, which is effective for the Company beginning January 1, 2010. The new guidance requires
revised evaluations of whether entities represent variable interest entities, ongoing assessments
of control over such entities, and additional disclosures for variable interests. Management
believes adoption of this new guidance will not have a material impact on the Company’s
consolidated financial statements.
2. Cash, cash equivalents and investments
Cash and cash equivalents include funds on hand and short-term investments with maturities of
three months or less. Short-term investments include securities having maturities of 90 days to one
year. The following is a summary of cash, cash equivalents and held-to-maturity securities as
distributed by type at December 31:
| 2009 | 2008 | |||||||||||||||||||||||||||||||
| Gross | Gross | Gross | Gross | |||||||||||||||||||||||||||||
| Unrealized | Unrealized | Unrealized | Unrealized | |||||||||||||||||||||||||||||
| Cost | Gains | Losses | Fair Value | Cost | Gains | Losses | Fair Value | |||||||||||||||||||||||||
Cash and money market funds |
$ | 45,505,049 | $ | — | $ | — | $ | 45,505,049 | $ | 46,880,435 | $ | — | $ | — | $ | 46,880,435 | ||||||||||||||||
Government sponsored entity securities |
— | — | — | — | 2,498,998 | 12,876 | — | 2,511,874 | ||||||||||||||||||||||||
| $ | 45,505,049 | $ | — | $ | — | $ | 45,505,049 | $ | 49,379,433 | $ | 12,876 | $ | — | $ | 49,392,309 | |||||||||||||||||
In February 2009, the Company’s remaining investment in a government sponsored entity was
called at par value by the issuing agency.
3. Inventory
Inventories consisted of the following at December 31:
| December 31, 2009 | December 31, 2008 | |||||||
Raw materials and work-in-process |
$ | 10,387,229 | $ | 7,371,608 | ||||
Finished goods |
5,172,595 | 6,225,409 | ||||||
Reserve for excess and obsolete inventory |
(474,074 | ) | (129,900 | ) | ||||
Total Inventory |
$ | 15,085,750 | $ | 13,467,117 | ||||
47
Table of Contents
TASER INTERNATIONAL, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
4. Property and Equipment
Property and equipment consisted of the following at December 31:
| Estimated | ||||||||||||
| Useful Life | 2009 | 2008 | ||||||||||
Land |
$ | 2,899,962 | $ | 2,899,962 | ||||||||
Building |
39 Years | 14,115,446 | 13,764,555 | |||||||||
Production equipment |
3-7 Years | 18,353,467 | 3,957,227 | |||||||||
Telephone equipment |
5 Years | 9,283 | — | |||||||||
Computer equipment |
3-5 Years | 9,148,440 | 5,400,861 | |||||||||
Furniture and office equipment |
5-7 Years | 3,035,410 | 2,714,562 | |||||||||
Vehicles |
5 Years | 503,872 | 503,872 | |||||||||
Website development costs |
3 Years | 807,006 | 605,581 | |||||||||
Capitalized software development costs |
3 Years | 2,349,724 | — | |||||||||
Construction in process |
n/a | 40,358 | 6,324,612 | |||||||||
Total Cost |
51,262,968 | 36,171,232 | ||||||||||
Less: Accumulated depreciation |
(12,589,903 | ) | (9,043,200 | ) | ||||||||
Net Property and Equipment |
$ | 38,673,065 | $ | 27,128,032 | ||||||||
Construction in process includes new product production equipment which was not in
service at December 31, 2009. Depreciation and amortization expense for the years ended December
31, 2009, 2008 and 2007 was $3.5 million, $2.6 million, and $2.5 million, respectively.
5. Intangible Assets
Intangible assets consisted of the following at December 31:
| 2009 | 2008 | |||||||||||||||||||||||||||
| Gross | Gross | |||||||||||||||||||||||||||
| Carrying | Accumulated | Net Carrying | Carrying | Accumulated | Net Carrying | |||||||||||||||||||||||
| Useful Life | Amount | Amortization | Amount | Amount | Amortization | Amount | ||||||||||||||||||||||
Amortized intangible assets |
||||||||||||||||||||||||||||
Domain names |
5 Years | $ | 230,991 | $ | 60,000 | $ | 170,991 | $ | 117,756 | $ | 60,000 | $ | 57,756 | |||||||||||||||
Issued patents |
4 to 15 Years | 869,309 | 206,907 | 662,402 | 677,808 | 156,297 | 521,511 | |||||||||||||||||||||
Issued trademarks |
9 to 11 Years | 131,058 | 19,183 | 111,875 | 46,283 | 9,888 | 36,395 | |||||||||||||||||||||
Non compete agreement |
5 to 7 Years | 150,000 | 106,429 | 43,571 | 150,000 | 79,286 | 70,714 | |||||||||||||||||||||
| 1,381,358 | 392,519 | 988,839 | 991,847 | 305,471 | 686,376 | |||||||||||||||||||||||
Unamortized intangible assets |
||||||||||||||||||||||||||||
TASER Trademark |
900,000 | 900,000 | 900,000 | 900,000 | ||||||||||||||||||||||||
Patents and trademarks pending |
876,862 | 876,862 | 860,635 | 860,635 | ||||||||||||||||||||||||
| 1,776,862 | 1,776,862 | 1,760,635 | 1,760,635 | |||||||||||||||||||||||||
Total intangible assets |
$ | 3,158,220 | $ | 392,519 | $ | 2,765,701 | $ | 2,752,482 | $ | 305,471 | $ | 2,447,011 | ||||||||||||||||
Amortization expense for the years ended December 31, 2009, 2008 and 2007 was
$88,000, $80,000, and $62,000, respectively. Estimated amortization for intangible assets with
definite lives for the next five years ended December 31, and thereafter is as follows:
2010 |
$ | 90,919 | ||
2011 |
81,367 | |||
2012 |
61,367 | |||
2013 |
61,367 | |||
2014 |
62,137 | |||
Thereafter |
631,682 | |||
| $ | 988,839 | |||
48
Table of Contents
TASER INTERNATIONAL, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
6. Accrued Liabilities
Accrued liabilities were comprised as follows at December 31:
| 2009 | 2008 | |||||||
Accrued salaries and benefits |
$ | 1,691,303 | $ | 1,145,634 | ||||
Accrued expenses |
2,191,963 | 2,249,193 | ||||||
Accrued warranty expense |
369,311 | 615,031 | ||||||
Accrued income tax |
— | 266,049 | ||||||
Total |
$ | 4,252,577 | $ | 4,275,907 | ||||
7. Commitments and Contingencies
a. Lease Obligations
The Company has entered into operating leases for various office space, storage facilities and
equipment. Rent expense under all operating leases, including both cancelable and non-cancelable
leases, was $1,040,312, $223,000, and $136,000 for the years ended December 31, 2009, 2008, and
2007, respectively. Future minimum lease payments under non-cancelable operating leases having
remaining terms in excess of one year as of December 31, 2009, are as follows:
| Year ending December 31, | ||||
2010 |
$ | 1,237,420 | ||
2011 |
1,163,233 | |||
2012 |
837,418 | |||
2013 |
574,240 | |||
2014 |
263,190 | |||
Total |
$ | 4,075,501 | ||
b. Purchase Commitments
On July 2, 2007, the Company entered into a contract with Automation Tooling Systems Inc. for
the purchase of equipment at a total cost of approximately $8.4 million. The equipment was
delivered and installed at the Company’s facility during 2009. Installment payments of $2.7
million, $3.0 million, and $1.5 million were paid in 2009, 2008, and 2007, respectively. The
remaining balance of $1.2 million is due in 2010 and was recorded in accounts payable at December
31, 2009.
c. Litigation
Product Litigation
The Company is currently named as a defendant in 42 lawsuits in which the plaintiffs allege
either wrongful death or personal injury in situations in which the TASER device was used (or
present) by law enforcement officers or during training exercises. Companion cases arising from the
same incident have been combined into one for reporting purposes.
In
addition, 106 other lawsuits have been dismissed or judgment entered in favor of the
Company which are not included in this number. An appeal was filed by the plaintiff in the
Mann (GA) litigation, Thompson (MI) litigation, Neal-Lomax (NV) and Rosa (CA) cases where judgment
was entered in favor of the Company. These cases are not included in
this number.
49
Table of Contents
TASER INTERNATIONAL, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
Also not included in the number of pending lawsuits is the Heston lawsuit in which a jury
verdict was entered against the Company on June 6, 2008, and judgment was entered against the
Company on January 30, 2009 in the amount of $153,150 as compensatory damages, $1,423,127 as
attorney fees, and $182,000 as costs. These damages, fees and costs are covered by the Company’s
insurance policies. The jury found that Mr. Heston’s own actions were 85 percent responsible for
his death. The jury assigned 15 percent of the responsibility to TASER for a “negligent failure to
warn” that extended or multiple TASER ECD applications could cause muscle contractions that could
potentially contribute to acidosis to a degree that could cause cardiac arrest. The jury
inappropriately awarded $5,200,000 in punitive damages against TASER, which were subsequently
disallowed by the Court on October 24, 2008. The Court denied the balance of the Company’s motion
for judgment as a matter of law on all other grounds. The Company has filed a notice of appeal with
respect to the judgment and plaintiffs have filed a notice of cross appeal.
With respect to each of the pending 42 lawsuits, the following table lists the name of
plaintiff, the date the Company was served with process, the jurisdiction in which the case is
pending, the type of claim and the status of the matter. While the facts vary from case to case,
the product liability claims are typically based on an alleged product defect resulting in injury
or death, usually involving a failure to warn, and the plaintiffs are seeking monetary damages.
This table also lists those cases that were dismissed or judgment entered during the most recent
fiscal quarter. Cases that were dismissed or judgment entered in prior fiscal quarters are not
included in this table. In each of the pending lawsuits, the plaintiff is seeking monetary damages
from the Company. The claims and in some instances, the defense of each of these lawsuits has been
submitted to our insurance carriers that maintained insurance coverage during these applicable
periods and we continue to maintain product liability insurance coverage with varying limits and
deductibles. Our product liability insurance coverage during these periods ranged from $5,000,000
to $10,000,000 in coverage limits and from $10,000 to $1,000,000 in per incident deductibles. We
are defending each of these lawsuits vigorously and do not expect these to individually and in the
aggregate, materially affect our business, results of operations or financial condition.
50
Table of Contents
TASER INTERNATIONAL, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
| Month | ||||||||
| Plaintiff | Served | Jurisdiction | Claim Type | Status | ||||
Glowczenski
|
Oct-04 | US District Court, ED NY | Wrongful Death | Trial rescheduled, date to be determined | ||||
Washington
|
May-05 | US District Court, ED CA | Wrongful Death | Discovery Phase | ||||
Sanders
|
May-05 | US District Court, ED CA | Wrongful Death | Dismissed | ||||
Graff
|
Sep-05 | Maricopa Superior Court, AZ | Wrongful Death | Discovery Phase-trial scheduled June 2010 | ||||
Heston
|
Nov-05 | US District Court, ND CA | Wrongful Death | Plaintiff Jury Verdict, punitive damages thrown out, judgment entered against TASER for $153,150 compensatory damages, $1,423,127 attorney fees and $182,000 costs, appeal filed | ||||
Rosa
|
Nov-05 | US District Court, ND CA | Wrongful Death | Dismissed, Summary Judgment Granted, Appeal Pending | ||||
Yeagley
|
Nov-05 | Hillsborough County Circuit County, FL | Wrongful Death | Discovery Phase | ||||
Neal-Lomax
|
Dec-05 | US District Court, NV | Wrongful Death | Dismissed, Appeal Pending | ||||
Mann
|
Dec-05 | US District Court, ND GA, Rome Div | Wrongful Death | Dismissed, Appeal Pending | ||||
Bagnell
|
Jul-06 | Supreme Court for British Columbia, Canada | Wrongful Death | Discovery Phase | ||||
Hollman
|
Aug-06 | US District Court, ED NY | Wrongful Death | Discovery Phase | ||||
Oliver
|
Sep-06 | US District Court, MD FL, Orlando Division | Wrongful Death | Motion Phase | ||||
Teran/LiSaola
|
Oct-06 | US District Court, ND CA | Wrongful Death | Dismissed | ||||
Augustine
|
Jan-07 | 11th Judicial Circuit Court, Miami-Dade, FL | Wrongful Death | Discovery Phase | ||||
Wendy Wilson, Estate of Ryan Wilson
|
Aug-07 | District Court Boulder County, CO | Wrongful Death | Motion Phase | ||||
Jack Wilson, Estate of Ryan Wilson (Companion to Wendy Wilson)
|
Nov-07 | District Court Boulder County, CO | Wrongful Death | Motion Phase | ||||
Marquez
|
Jun-08 | US District Court, AZ | Wrongful Death | Motion Phase | ||||
Salinas
|
Aug-08 | US District Court, Northern District CA | Wrongful Death | Discovery Phase, Trial Scheduled February 2011 | ||||
Thomas (Pike)
|
Oct-08 | US District Court, WD Louisiana, Alexandria | Wrongful Death | Case Stayed | ||||
Dwyer
|
Nov-08 | US District Court, ED TX, Marshall Division | Wrongful Death | Discovery Phase | ||||
Carroll
|
Mar-09 | US District Court, Southern District TX | Wrongful Death | Discovery Phase | ||||
Shrum
|
May-09 | Allen County District Court, Iola, KS | Wrongful Death | Discovery Phase | ||||
Athetis
|
May-09 | Maricopa Superior Court, AZ | Wrongful Death | Discovery Phase | ||||
Hagans
|
May-09 | Common Pleas Court, Franklin County, OH | Wrongful Death | Discovery Phase-trial scheduled August 2011 | ||||
Martinez
|
May-09 | US District Court, SD CA | Wrongful Death | Dismissed | ||||
Bartley
|
Jun-09 | US District Court, ED LA | Wrongful Death | Dismissed | ||||
Sapinoso
|
Jul-09 | CA Superior Court, Los Angeles, S Central Dist. | Wrongful Death | Dismissed | ||||
Abrahams
|
Jul-09 | CA Superior Court, Yolo County | Wrongful Death | Discovery Phase-trial scheduled Oct — 2010 | ||||
Humphreys
|
Oct-09 | CA Superior Court, San Joaquin County | Wrongful Death | Pleading Phase | ||||
Forbes
|
Dec-09 | US District Court, MS | Wrongful Death | Discovery Phase-trial scheduled Sep-2010 | ||||
Terriquez
|
Feb-10 | Superior Court of CA, Orange County | Wrongful Death | Pleading Phase | ||||
Rich
|
Feb-10 | US District Court, Nevada | Wrongful Death | Pleading Phase | ||||
McKenzie
|
Feb-10 | US Disctrict Court, ED CA | Wrongful Death | Pleading Phase | ||||
Turner
|
Feb-10 | General Court of Justice, Superior Court Div, Mecklenburg County, NC | Wrongful Death | Pleading Phase | ||||
Dang
|
Mar-10 | CA Superior Court, Orange County | Wrongful Death | Pleading Phase | ||||
Stewart
|
Oct-05 | Circuit Court for Broward County, FL | Training Injury | Discovery Phase | ||||
Lewandowski
|
Jan-06 | US District Court, NV | Training Injury | Motion Phase | ||||
Peterson
|
Jan-06 | US District Court, NV | Training Injury | Dismissed | ||||
Husband
|
Mar-06 | British Columbia Supreme Court, Canada | Training Injury | Discovery Phase | ||||
Perry
|
Jul-08 | US District Court CO | Training Injury | Dismissed | ||||
Grable
|
Aug-08 | FL 6th Judicial Circuit Court, Pinellas County | Training Injury | Discovery Phase | ||||
Koon
|
Dec-08 | 17th Judicial Circuit Court, Broward County, FL | Training Injury | Discovery Phase | ||||
Bickle
|
Mar-09 | 18th Judicial District Court, Gallatin County, MT | Training Injury | Discovery Phase | ||||
Foley
|
Mar-09 | US District Court, MA | Training Injury | Discovery Phase | ||||
Peppler
|
Apr-09 | Circuit Court 5th Judicial Dist., Sumter City, FL | Training Injury | Discovery Phase | ||||
Kandt
|
Jun-09 | US District Court, ND NY | Training Injury | Discovery Phase | ||||
Wieffenbach
|
Jun-06 | Circuit Court of 12th Judicial District, Will County, II | Injury During Arrest | Dismissed | ||||
Payne
|
Oct-06 | Circuit Court of Cook County, IL | Injury During Arrest | Dismissed | ||||
Butler
|
Sep-08 | CA Superior Court, Santa Cruz County | Injury During Arrest | Motion Phase, trial scheduled Mar — 2010 | ||||
Reston
|
Apr-09 | Circuit Court 4th Judicial Dist., Duval Cty, FL | Injury During Arrest | Discovery Phase, trial scheduled Sep — 2010 | ||||
Lucas
|
Jun-09 | US District Court, ED CA | Injury During Arrest | Discovery Phase | ||||
Spence
|
Jul-09 | CA Superior Court, Marin County | Injury During Arrest | Pleading Phase | ||||
Wheat
|
Jul-09 | CA Superior Court, Los Angeles County | Injury During Arrest | Discovery Phase, trial scheduled Aug — 2010 | ||||
Fahy
|
Dec-09 | Circuit Court of City of St. Louis | Injury During Arrest | Discovery Phase | ||||
Tylecki
|
Jan-10 | US District Court, DE | Injury During Arrest | Pleading Phase | ||||
Derbyshire
|
Nov-09 | Ontario Superior Court of Justice | Officer Injury | Pleading Phase |
51
Table of Contents
TASER INTERNATIONAL, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
Other Litigation
In November 2006, we filed a lawsuit against the Chief Medical Examiner of Summit County, OH,
in the Court of Common Pleas of Summit County Ohio, to seek the correction of erroneous cause of
death determinations relating to autopsy reports prepared by medical examiner, Dr. Lisa Kohler,
which determined that the TASER device was a contributing factor in the deaths of Richard Holcomb,
Dennis Hyde and Mark McCullaugh. We asked the Court to order a hearing on the appropriate causes of
death of Messrs. Hyde, Holcomb and McCullaugh, and to order changes in the medical examiner’s cause
of death determinations for Messrs. Hyde, Holcomb and McCullaugh removing all references to any
TASER device causing or contributing to the causes of death for Messrs. Hyde, Holcomb and
McCullaugh. Defendant filed a motion to dismiss for lack of standing and that motion was denied by
the Court in January 2007. The City of Akron joined this lawsuit as a co-plaintiff. This case went
to trial in April 2008 and on May 2, 2008, the Court entered an order ruling in favor of TASER and
the City of Akron and ordered the medical examiner to remove any reference to the TASER device as a
cause of death and to change the manner of death for Holcomb and Hyde to accidental and for
McCullaugh to undetermined. Defendant filed an appeal and on March 30, 2009, the Ohio’s 9th
Judicial District Court of Appeals entered an order affirming the trial court’s order. On May 15,
2009, Defendant filed an appeal to the Ohio Supreme Court, and the Company and the City of Akron
have filed responsive briefs. On August 28, 2009 the Ohio Supreme Court declined to hear Dr.
Kohler’s appeal.
In January 2007, we filed a lawsuit in the U.S. District Court for Arizona against Stinger
Systems, Inc. alleging patent infringement, patent false marking, and false advertising. Defendant
filed an answer and counterclaim for false advertising and punitive damages. More specifically, the
counterclaim seeks judgment; invalidating U.S. Patent 7,145,762; holding patent 7,145,762 not
infringed; granting permanent injunction to prohibit false advertising and labeling; granting
unspecified punitive damages for false advertising and/or unfair competition and injuries
proximately caused; and to pay defendants reasonable attorneys’ fees. Discovery has begun and no
trial date has been set. On February 2, 2009, the court issued an order based on a Markman hearing
(patent claims construction hearing) held on May 7, 2008 in which the Court adopted TASER’s claim
construction on the disputed patent claim term in TASER’s U.S. patent number 7,102,870 and all of
TASER’s claim construction on all of the disputed patent claim terms in TASER’s U.S. patent number
7,234,262. In addition, the Court adopted TASER’s claim construction on one of the disputed patent
claim terms in TASER’s U.S. patent number 6,999,295 and rejected both parties’ claim construction
in the other disputed claim term in this patent. Discovery is ongoing and no trial date has been
sent. Both parties have filed motions for summary judgment.
In October 2007, we filed a lawsuit in Arizona Superior Court for Maricopa County against
Steve Ward and Mark Johnson, both former TASER employees and VIEVU LLC, et. al. for breach of duty
of loyalty, breach of contract, breach of fiduciary duty, and conversion. This lawsuit does not
involve our electronic control device business and we do not expect this litigation to have a
material impact on our financial results. Defendants Ward and VIEVU LLC filed an answer and
counterclaim for declaration of non-infringement, tortious interference with contractual relations,
tortious interference with business expectancy, and abuse of process. The lawsuit seeks
compensatory damages, constructive trust, exemplary damages, injunctive relief attorneys’ fees,
costs and disbursements. Discovery has begun and no trial date has been set. Cross motions for
summary judgment were filed and on March 4, 2009, the Court denied Defendants’ motion for summary
judgment on the trade secret claim and on April 9, 2009, the Court granted TASER’s motion for
summary judgment against Ward on the breach of fiduciary duty and the breach of duty of loyalty
claims. We filed a Motion to Extend Discovery Period by and to reconvene the Deposition of Steve
Ward, and Defendants have filed Defendant’s Response in Opposition to this motion. In addition,
Defendants Steve Ward and VIEVU LLC have filed a Motion for Reconsideration or in the alternative
to make the Court’s Ruling a Final Judgment and Stay Proceeding Pending Outcome of Appeal. The
Court denied the Motion for Reconsideration, but granted the motion to make the Court’s Ruling a
Final Judgment and Stayed the Proceeding Pending Outcome of Appeal. An appeal has been filed by
Defendants to the Arizona State Court of Appeals.
In June 2008, we filed an amended complaint in the State Court of Fulton County, Georgia
joining as a plaintiff in an existing lawsuit previously filed by certain current and former
stockholders of the Company against Morgan Stanley & Co., Inc., and ten other brokerage firms
alleging a conspiracy to unlawfully, deceptively, and fraudulently manipulate the price of the
Company’s common stock in the context of illegal naked shorting. Specifically, the amended
complaint alleges that the defendants committed conspiracy and endeavored to violate the Georgia
Racketeer Influenced and Corrupt Organization Act; Securities Fraud; Theft By Taking; Theft By
Deception; Violation of The Georgia Computer Systems Protection Act; Violation of the Georgia
Securities Act; Violation of the Georgia Computer Systems Protection Act; and Conversion. The
lawsuit seeks compensatory and punitive damages as well as expenses of litigation including
attorneys’ fees and costs. Defendants have filed motions to dismiss or alternatively a motion for a
more definite statement and, on July 29, 2009, the Court entered an order denying Defendants’
motion to dismiss and alternatively a motion for a more definite statement. Discovery has begun in
this litigation.
52
Table of Contents
TASER INTERNATIONAL, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
In July 2008, we were served with a summons and complaint in the lawsuit entitled Proformance
Vend USA vs. TASER International, Inc. which was filed in Arizona Superior Court for Maricopa
County alleging breach of contract of a vending machine contract and seeking money damages,
including tort damages, attorney’s fees and costs. We have filed an answer to this complaint.
Discovery has begun and no trial date has been set.
In February 2009, we filed a complaint in the United States District Court for the District of
Nevada against James F. McNulty, Jr., Robert Gruder, and Stinger Systems, Inc. alleging securities
fraud under 15 U.S.C. § 78j, trade libel, unfair competition under the Lanham Act, 15 U.S.C. §
1125, abuse of process, and deceptive trade practices. Our complaint seeks compensatory damages,
punitive damages, injunctive relief, attorneys’ fees and costs. Motions to dismiss are pending.
In
December 2009 we filed a complaint in Maricopa County Superior Court,
Arizona against Interwoven Inc. et. al. alleging breach of contract,
misrepresentation and fraud for failure to comply with a settlement
agreement regarding an e-discovery services dispute. Our complaint
seeks compensatory damages, attorneys’ fees and costs. Defendant
has filed a motion to compel arbitration which is pending.
In
February 2010 we were served with a summons and complaint in the
lawsuit entitled General Employment vs. TASER International, Inc.
which was filed in Arizona Superior Court for Maricopa County
alleging breach of contract of a recruiting contract and seeking
money damages, including attorney’s fees and costs. We have
filed an answer to this complaint. Discovery has begun and no trial
date has been set.
General
From time to time, the Company is notified that it may be a party to a lawsuit or that a claim
is being made against it. It is the Company’s policy to not disclose the specifics of any claim or
threatened lawsuit until the summons and complaint are actually served on the Company. After
carefully assessing the claim, and assuming we determine that we are not at fault, we vigorously
defend and pursue any lawsuit filed against or by the Company. Although we do not expect the
outcome in any pending individual case to be material, the outcome of any litigation is inherently
uncertain and there can be no assurance that any expense, liability or damages that may ultimately
result from the resolution of these matters will be covered by our insurance or will not be in
excess of amounts provided by insurance coverage and will not have a material adverse effect on our
business, operating results or financial condition. In addition, the Company has seven lawsuits
where the costs of legal defense incurred are in excess of its liability insurance deductibles. As
of December 31, 2009, the Company has been fully reimbursed by its insurance company for these
legal costs. The Company may settle a lawsuit in situations where a settlement can be obtained for
nuisance value and for an amount that is expected to be less than the cost of defending a lawsuit.
The number of product liability lawsuits dismissed includes a small number of police officer
training injury lawsuits that were settled by the Company and dismissed in cases where the
settlement economics to the Company were significantly less than the cost of litigation. Due to the
confidentiality of our litigation strategy and the confidentiality agreements that are executed in
the event of a settlement, the Company does not identify or comment on which specific lawsuits have
been settled or the amount of any settlement.
d. Employment Agreements
The Company has employment agreements with its Chairman, Chief Executive Officer, Chief
Strategy Officer, President-Hardware Group, Chief Financial Officer, Vice President of Research and
Development, Vice President Operations and Executive Vice President of Human Capital. The Company
may terminate the agreements with or without cause. Should the Company terminate the agreements
without cause, or upon a change of control of the Company or death of the employee, the employees
are entitled to additional compensation. Under these circumstances, these officers and employees
may receive the amounts remaining under their contracts upon termination, which would total $1.7
million in the aggregate at December 31, 2009.
53
Table of Contents
TASER INTERNATIONAL, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
8. Income Taxes
Significant components of the Company’s deferred income tax assets and liabilities are as
follows:
| December 31, | ||||||||
| 2009 | 2008 | |||||||
Deferred income tax assets |
||||||||
Net operating loss carryforward |
$ | 92,262 | $ | 4,114,090 | ||||
Deferred revenue |
1,898,388 | 1,379,071 | ||||||
Reserves and accruals |
1,326,190 | 1,132,988 | ||||||
Non-employee stock option expense |
313,631 | 312,218 | ||||||
Non-qualified stock option expense |
1,535,105 | 587,795 | ||||||
Capitalized R&D |
12,982,041 | 6,718,001 | ||||||
Minimum tax credit carryforward |
1,555,075 | 1,111,541 | ||||||
R&D tax credit |
3,787,498 | 4,361,353 | ||||||
Inventory
costs capitalized |
422,839 | — | ||||||
Deferred income tax assets |
23,913,029 | 19,717,057 | ||||||
Deferred income tax liabilities |
||||||||
Depreciation |
(4,134,443 | ) | (1,183,910 | ) | ||||
Amortization |
(110,799 | ) | (76,296 | ) | ||||
Change in
inventory accounting method |
(222,779 | ) | — | |||||
Deferred income tax liabilities |
(4,468,021 | ) | (1,260,206 | ) | ||||
Net deferred income tax assets before valuation allowance |
19,445,008 | 18,456,851 | ||||||
Less: Valuation allowance |
— | (200,000 | ) | |||||
Net deferred income tax assets |
$ | 19,445,008 | $ | 18,256,851 | ||||
Reported as: |
||||||||
Current deferred tax assets |
$ | 8,447,915 | $ | 9,430,073 | ||||
Long-term deferred tax assets |
10,997,093 | 8,826,778 | ||||||
| $ | 19,445,008 | $ | 18,256,851 | |||||
In July 2000, the Company granted 136,364 warrants to acquire Company stock at an
exercise price of $0.55 per share to a member of its Board of Directors as additional consideration
for a $1.5 million loan. In October 2004, the stock warrants were exercised with an intrinsic value
of $5,233,650. The Board member was incorrectly provided tax forms that indicated the award was
taxable income to him. The Company included the $5,233,650 as stock compensation expense in its
2004 tax return and, because the Company had a net operating loss carryforward, recorded a
$2,014,955 deferred tax asset on the balance sheet and a corresponding increase to additional paid
in capital. The Company’s 2004 tax return was audited by the IRS in 2007 with no adjustment made to
the stock compensation expense deduction recorded by the Company. During a tax examination of the
Board member’s tax return it was determined that the exercise of the warrant should not have
created taxable income and that the inclusion of the intrinsic value of the warrant in the
director’s Form 1099 was in fact, an error. Accordingly, in the second quarter of 2008, the Company
reduced its deferred tax asset balance and additional paid in capital by $2,014,955. The adjusting
entry was a balance sheet only adjustment and had no impact on retained earnings and was not
considered material to the associated account balances or the balance sheet as a whole.
The
Company fully utilized its remaining federal NOL for financial
reporting purposes of $10.0 million during 2009. The federal NOL
for tax purposes remaining at December 31, 2009 is
estimated to be $7.0 million after current year utilization and
differs from the NOL for financial reporting purposes as a result of
stock compensation deductions. The Company
recognizes the income tax benefits associated with certain stock compensation deductions only when
such deductions produce a reduction to the company’s actual tax liability. Accordingly, in 2009 the
Company recognized a cash tax benefit of $32,000 attributable to
stock compensation deductions, which
was recorded as a credit to additional paid in capital. The Company’s federal NOL carryforward expires in 2024. The
Company’s remaining deferred tax assets of $92,000 related to state NOL’s expire at various dates
beginning in 2014 through 2025. The Company has federal and Arizona state research and development credit carryovers of
$1.6 million and $2.2 million, respectively which expire at various dates beginning in 2023 for federal purposes and 2018 for state purposes.
The Company has a minimum tax credit carryover of $1.6 million which does not expire.
54
Table of Contents
TASER INTERNATIONAL, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
In preparing the Company’s consolidated financial statements, management has assessed the
likelihood that its deferred income tax assets will be realized from future taxable income. In
evaluating the ability to recover its deferred income tax assets, management considers all
available evidence, positive and negative; including the Company’s operating results, ongoing tax
planning and forecasts of future taxable income on a jurisdiction by jurisdiction basis. A
valuation allowance is established if it is determined that it is more likely than not that some
portion or all of the net deferred income tax assets will not be realized. Management exercises
significant judgment in determining the Company’s provisions for income taxes, its deferred income
tax assets and liabilities and its future taxable income for purposes of assessing its ability to
utilize any future tax benefit from its deferred income tax assets. Although management believes
that its tax estimates are reasonable, the ultimate tax determination involves significant
judgments that could become subject to audit by tax authorities in the ordinary course of business.
During 2009, management determined that it was more likely than not that its net operating loss
carryforwards for the state of Arizona, which would have expired in 2009, would be fully realized.
Accordingly, the valuation allowance of $200,000 the Company carried against its deferred tax
assets as of December 31, 2008, was reversed with the benefit recognized during 2009 as a reduction
of the current-year effective tax rate. Management believes that, other than as previously
described, as of December 31, 2009, based on an evaluation and projections of future sales and
profitability, no other valuation allowance was deemed necessary as management concluded that it is
more likely than not that the Company’s net deferred income tax assets will be realized. However,
the deferred tax asset could be reduced in the near-term if estimates of future taxable income
during the carryforward period are reduced.
Significant components of the provision (benefit) for income taxes are as follows:
| For the Year Ended December 31, | ||||||||||||
| 2009 | 2008 | 2007 | ||||||||||
Current |
||||||||||||
Federal |
$ | 417,722 | $ | 414,094 | $ | 518,682 | ||||||
State |
290,215 | 239,539 | 49,226 | |||||||||
Total Current |
707,937 | 653,633 | 567,908 | |||||||||
Deferred |
||||||||||||
Federal |
(427,332 | ) | 2,520,677 | 6,014,663 | ||||||||
State |
(760,825 | ) | (460,054 | ) | (182,880 | ) | ||||||
Total Deferred |
(1,188,157 | ) | 2,060,623 | 5,831,783 | ||||||||
Tax provision recorded as an increase in liability for unrecorded tax benefits |
572,699 | 592,007 | 1,100,073 | |||||||||
Provision for income taxes |
$ | 92,479 | $ | 3,306,263 | $ | 7,499,764 | ||||||
A reconciliation of the Company’s effective income tax rate to the federal statutory rate
follows:
| For the Year Ended December 31, | ||||||||||||
| 2009 | 2008 | 2007 | ||||||||||
Federal income tax at the statutory rate |
31,981 | 2,430,156 | 7,884,184 | |||||||||
State income taxes, net of federal benefit (a) |
(502,186 | ) | 333,279 | 810,945 | ||||||||
Permanent differences (b) |
1,097,908 | 853,450 | 813,184 | |||||||||
Research and development |
(990,867 | ) | (729,047 | ) | (3,108,621 | ) | ||||||
Change in liability for unrecognized tax benefits |
572,699 | 592,007 | 1,100,073 | |||||||||
Change in valuation allowance |
(200,000 | ) | (48,603 | ) | — | |||||||
Other |
82,944 | (124,979 | ) | — | ||||||||
Income tax expense |
92,479 | 3,306,263 | 7,499,764 | |||||||||
Effective tax rate |
101.2 | % | 47.6 | % | 33.3 | % | ||||||
| a) | The 2009 credit provision for the state income taxes is primarily driven by current year utilization of Arizona research and development tax credits. | |
| b) | Permanent differences include certain expenses which are not deductible for tax purposes including lobbying fees and stock-based compensation expense related to incentive stock options. |
55
Table of Contents
TASER INTERNATIONAL, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
The Company has
completed research and development tax credit studies which identified
approximately $5.9 million in tax credits for Federal and Arizona income tax purposes
related to the 2003 through 2009 tax years, net of the federal benefit on the Arizona research
and development tax credits. Management has made the determination that it is more likely than
not that the full benefit of the research and development tax credit will not be sustained on
examination and recorded a liability for unrecognized tax benefits of
$2.2 million as of December 31, 2009. Also included in the
liability for unrecongnized tax benefits, management accrued
approximately $106,000 for estimated uncertain tax positions related
to certain state income tax liabilities. As of December 31, 2009, management does not
expect the amount of the unrecognized tax benefit liability to increase or decrease significantly
within the next 12 months. Should the unrecognized tax benefit of $2.3 million be
recognized, the Company’s effective tax rate would be favorably impacted.
The
following presents a rollforward of our liability for unrecognized tax benefits as of
December 31:
| 2009 | 2008 | |||||||
Balance at January 1, |
$ | 1,692,080 | $ | 1,100,073 | ||||
Increase in prior year tax positions |
— | — | ||||||
Increase in current year tax positions |
477,717 | 640,850 | ||||||
Increase (decrease) related to adjustment of previous estimates of activity |
94,982 | (48,843 | ) | |||||
Decrease related to settlements with taxing authorities |
— | — | ||||||
Decrease related to lapse in statute of limitations |
— | — | ||||||
Balance at December 31, |
$ | 2,264,779 | $ | 1,692,080 | ||||
An examination by the United States Internal Revenue Service (the “IRS”) for 2006
was concluded in the third quarter of 2008 with no significant adjustment required by the IRS.
Federal income tax returns for 2006, 2007and 2008 remain open to examination by the IRS, while
state and local income tax returns for 2002 through 2008 also remain open to examination.
9. Line of Credit
The Company has a line of credit agreement with a total availability facility of $10 million.
The line is secured primarily by the Company’s accounts receivable and inventory and bears interest
at varying rates, ranging from LIBOR plus 1.5% to prime. The availability under this line is
computed on a monthly borrowing base, which is based on the Company’s eligible accounts receivable
and inventory. The line of credit matures on June 30, 2010 and requires monthly payments of
interest only. The Company expects to renew the line on similar terms upon its maturity. At
December 31, 2009, there was no amount outstanding under the line of credit and the available
borrowing under the existing line of credit was $10.0 million. There were no borrowings under the
line during the year ended December 31, 2009.
The Company’s agreement with the bank requires the Company to comply with certain financial
and other covenants including maintenance of minimum tangible net worth and fixed charge coverage.
The ratio of total liabilities to tangible net worth can be no greater than 1:1, and the fixed
coverage charge ratio can be no less than 1.25:1, based upon a trailing twelve month period. At
December 31, 2009, the Company’s tangible net worth ratio was .18:1 and its fixed charge coverage
ratio was 3.96:1. At December 31, 2009, the Company was in compliance with the covenants.
56
Table of Contents
TASER INTERNATIONAL, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
10. Stockholders’ Equity
a. Common Stock and Preferred Stock
The Company has authorized the issuance of two classes of stock designated as “common stock” and
“preferred stock,” each having a par value of $0.00001 per share. The Company is authorized` to
issue is 200 million shares of common stock and 25 million shares of preferred stock.
b. Stock Repurchase
In April 2008, TASER’s Board of Directors authorized a stock repurchase program to acquire up
to $12.5 million of the Company’s outstanding common stock subject to stock market conditions and
corporate considerations. During 2008, the Company repurchased 1.79 million shares at a weighted
average cost of $6.98 per share and a total cost of $12.5 million.
c. Stock Option Plans
The Company has historically issued stock options to various equity owners and key employees
as a means of attracting and retaining quality personnel. The option holders have the right to
purchase a stated number of shares at the market price on the grant date. The options issued under
the Company’s 1999 Stock Option Plan (the “1999 Plan”) generally vest over a three-year period and
have a contractual maturity of ten years. The options issued under the Company’s 2001 Stock Option
Plan (the “2001 Plan”) generally vest over a three-year period and have a contractual maturity of
ten years. The options issued under the Company’s 2004 Stock Option Plan (the “2004 Plan”)
generally vest over a three-year period and have a contractual maturity of ten years, however the
majority of options issued under this plan within fiscal 2005 had vesting terms of one year. The
shares issuable under each of the plans were registered on Form S-8 with the United States
Securities and Exchange Commission.
On March 31, 2009, the Company’s Board of Directors approved, subject to stockholder approval,
the 2009 Stock Incentive Plan (“the 2009 Plan”), under which the Company reserved 1,000,000 shares
of common stock available for future grants. The 2009 Stock Incentive Plan was approved at the
Annual Meeting of Stockholders on May 28, 2009. Options issued under the 2009 Plan generally vest
over a three-year period and have a contractual maturity of ten years.
The total number of shares registered under these plans was as follows: 9,952,500 under the
1999 Plan, 6,600,000 under the 2001 Plan, 6,800,000 under the 2004 Plan and 1,000,000 under the
2009 Plan. These plans provide for officers, key employees, directors and consultants to receive
nontransferable stock options to purchase an aggregate of 24,352,500 shares of the Company’s common
stock. As of December 31, 2009, 1,708,192 options remain available for future grants.
Stock Option Activity
A summary of the Company’s stock options at December 31, 2009, 2008 and 2007 and for the years
then ended is presented in the table below:
| 2009 | 2008 | 2007 | ||||||||||||||||||||||
| Weighted | Weighted | Weighted | ||||||||||||||||||||||
| Average | Average | Average | ||||||||||||||||||||||
| Options | Exercise Price | Options | Exercise Price | Options | Exercise Price | |||||||||||||||||||
Options outstanding, beginning of year |
9,108,930 | $ | 5.87 | 5,234,072 | $ | 6.06 | 5,902,182 | $ | 5.13 | |||||||||||||||
Granted |
551,270 | $ | 4.56 | 4,285,671 | $ | 5.38 | 533,404 | $ | 10.42 | |||||||||||||||
Exercised |
(323,351 | ) | $ | 0.48 | (323,409 | ) | $ | 1.06 | (1,107,574 | ) | $ | 2.84 | ||||||||||||
Expired/terminated |
(556,782 | ) | $ | 6.36 | (87,404 | ) | $ | 11.29 | (93,940 | ) | $ | 10.52 | ||||||||||||
Options outstanding, end of year |
8,780,067 | $ | 5.94 | 9,108,930 | $ | 5.87 | 5,234,072 | $ | 6.06 | |||||||||||||||
Exercisable at end of year |
5,988,159 | $ | 6.23 | 4,901,483 | $ | 6.02 | 4,683,066 | $ | 5.58 | |||||||||||||||
Options available for grant at end of year |
1,708,192 | 702,680 | 4,900,947 | |||||||||||||||||||||
Weighted average fair value of options granted during the year |
$ | 2.56 | $ | 2.89 | $ | 5.22 | ||||||||||||||||||
57
Table of Contents
TASER INTERNATIONAL, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
The following table summarizes information about stock options outstanding and exercisable as of
December 31, 2009:
| Options Outstanding | Options Exercisable | |||||||||||||||||||
| Weighted | ||||||||||||||||||||
| Weighted | Average | Weighted | ||||||||||||||||||
| Average | Remaining | Average | ||||||||||||||||||
| Number | Exercise | Contractual | Number | Exercise | ||||||||||||||||
| Range of Exercise Price | Outstanding | Price | Life Life | Exercisable | Price | |||||||||||||||
| $0.28 – $0.99 | 673,241 | $ | 0.36 | 3.1 | 673,241 | $ | 0.36 | |||||||||||||
| $1.03 – $2.41 | 817,578 | $ | 1.57 | 2.5 | 817,578 | $ | 1.57 | |||||||||||||
| $3.53 – $9.93 | 6,403,828 | $ | 6.12 | 7.4 | 3,698,919 | $ | 6.83 | |||||||||||||
| $10.07 – $19.76 | 831,020 | $ | 12.18 | 5.7 | 744,021 | $ | 12.31 | |||||||||||||
| $20.12 – $29.98 | 54,400 | $ | 24.32 | 4.4 | 54,400 | $ | 24.32 | |||||||||||||
| $0.28 – $29.98 | 8,780,067 | $ | 5.94 | 6.5 | 5,988,159 | $ | 6.23 | |||||||||||||
The total fair value of options exercisable was approximately $19.3 million, $15.4
million, and $13.6 million at December 31, 2009, 2008 and 2007, respectively. The aggregate
intrinsic value of options outstanding and options exercisable at December 31, 2009 was $5.3
million and $5.1 million, respectively. The aggregate intrinsic value of unvested options at
December 31, 2009 was approximately $203,000. Aggregate intrinsic value represents the difference
between the Company’s closing stock price on the last trading day of the fiscal period, which was
$4.38 as of December 31, 2009, and the exercise price of the option multiplied by the number of
options outstanding. Total intrinsic value of options exercised was $1.3 million, $2.4 million, and
$9.7 million for the years ended December 31, 2009, 2008 and 2007, respectively.
At December 31, 2009, the Company had 2,791,908 unvested options outstanding with a weighted
average exercise price of $5.34 per share, weighted average fair value of $2.84 per share and
weighted average remaining contractual life of 8.7 years. Of the unvested options outstanding at
December 31, 2009, the Company expects that 2,673,810 options will ultimately vest based on its
historical experience.
Stock-based Compensation Expense
The Company accounts for share-based compensation using the fair-value method. Reported
share-based compensation was classified as follows for the years ended December 31:
| 2009 | 2008 | 2007 | ||||||||||
Stock-based compensation was allocated as follows: |
||||||||||||
Indirect manufacturing expense |
$ | 349,243 | $ | 257,964 | $ | 187,585 | ||||||
Sales, general and administrative expenses |
3,218,735 | 1,552,411 | 986,616 | |||||||||
Research and development expenses |
1,420,859 | 613,510 | 213,765 | |||||||||
| $ | 4,988,837 | $ | 2,423,885 | $ | 1,387,966 | |||||||
As of December 31, 2009, total unrecognized stock-based compensation expense related
to non-vested stock options was approximately $8.0 million, which is expected to be recognized over
a total weighted average period of approximately 12 months. Approximately $4.7 million of this
expense is expected to be recognized in 2010.
Total share-based compensation expense recognized in the statement of operations for the years
ended December 31, 2009, 2008 and 2007 includes $2,550,000, $1,438,000 and $1,094,000,
respectively, related to Incentive Stock Options (“ISO“s) for which no tax benefit is recognized.
The total deferred tax benefits related to non-qualified stock options were approximately $1.5
million and $0.6 million for the years ended December 31, 2009 and 2008, respectively. In 2009 the
Company recorded a tax benefit of $32,000 to offset taxes payable related to the non-qualified
disposition of ISOs exercised and sold. The total unrecognized tax benefit related to the
non-qualified disposition of stock options in 2009, 2008 and 2007 was approximately $1.3 million,
$1.2 million and $3.0 million, respectively.
58
Table of Contents
TASER INTERNATIONAL, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
The Company granted 825,800 performance-based stock options in 2008 and the first half of
2009, the vesting of which is contingent upon the achievement of certain performance criteria
related to the successful and timely development and market acceptance of future product
introductions, as well as the future operating performance of the Company. Compensation expense is
recognized over the implicit service period (the date the performance condition is expected to be
achieved) based on management’s estimate of the probability of the performance criteria being
satisfied, adjusted at each balance sheet date. During 2009, 106,700 of
these options were forfeited. Of the remaining 719,100 outstanding options, 286,708 options are
exercisable and 432,392 are unvested. The fair value of the unvested performance-based
options outstanding and still expected to vest was estimated to be $1.1 million. The Company
recognized $0.8 million, and $0.1 million of related stock based compensation expense during 2009
and 2008, respectively.
11. Related Party Transactions
Aircraft charter
The Company reimburses Thomas P. Smith, Chairman of the Company’s Board of Directors, and
Patrick W. Smith, Chief Executive Officer, for business use of their personal aircraft. For the
years ended December 31, 2009, 2008 and 2007, the Company incurred expenses of approximately
$274,000, $197,000, and $394,000, respectively, to Thomas P. Smith. For the years ended December
31, 2009, 2008, and 2007, the Company incurred expenses of approximately $10,000, $107,000 and
$54,000, respectively, to Patrick W. Smith. At December 31, 2009 and 2008, the Company had
outstanding payables of approximately $15,000 and $0, respectively, to Thomas P. Smith. At December
31, 2009 and 2008, the Company had no outstanding payables due to Patrick W. Smith. Management
believes that the rates charged by Thomas P. Smith and Patrick W. Smith are equal to or below
commercial rates the Company would pay to charter similar aircraft from independent charter
companies.
TASER Foundation
In November 2004, the Company established the TASER Foundation. The TASER Foundation is an
Internal Revenue Code Section 501(c)(3) non-profit corporation and has been granted tax exempt
status by the IRS. The TASER Foundation’s mission is to honor the service and sacrifice of local
and federal law enforcement officers in the United States and Canada lost in the line of duty by
providing financial support to their families. Over half of the initial $1 million endowment was
contributed directly by the Company’s employees. The Company bears all administrative costs of the
TASER Foundation in order to ensure 100% of all donations are distributed to the families of fallen
officers. For the years ended December 31, 2009, 2008 and 2007, the Company incurred approximately
$265,000, $233,000, and $179,000, respectively, in such administrative costs. For the years ended
December 31, 2009, 2008 and 2007, the Company contributed $35,000, $25,000, and $300,000,
respectively, to the TASER Foundation. At December 31, 2009, the Company had approximately $1,200
in accrued salaries payable to the Foundation. At December 31, 2008, the Company had no outstanding
payable amounts to the Foundation.
Consulting services
The Company engages Mark Kroll, a member of the Board of Directors, to provide consulting
services. The expenses related to these services for the years ended December 31, 2009, 2008 and
2007 were approximately $251,000, $293,000, and $227,000, respectively. At December 31, 2009 and
2008, the Company had accrued liabilities of approximately $14,000 and $23,000, respectively,
related to these services.
59
Table of Contents
TASER INTERNATIONAL, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
Settlement agreement
On May 15, 2009, Bruce and Donna Culver, husband and wife (the “Culvers”), and the Company,
entered into a settlement and release agreement (the “Agreement”), the background and material
terms of which are described below. Mr. Culver has served as a director of the Company since
January 1994. In addition, he currently chairs the Nominating Committee of the Board.
In July 2000, the Culvers provided a loan to the Company in exchange for a promissory note and
warrants to purchase 136,364 shares of the Company’s common stock for $0.55 per share. In October
2004, the Culvers exercised the warrants, and the Company issued them a Form 1099, which included
the in-the-money value of the warrants as stock compensation based upon the advice of the Company’s
then-current outside tax advisors. In 2007, the Culvers informed the Company that their personal
tax advisors had determined that the 2004 Form 1099 was not the proper tax treatment for the
transaction, and that the value of the warrants should not have been included as compensation
because the warrants were issued in connection with the loan rather than services. The Company
responded by issuing an amended Form 1099 excluding the value of the warrants, and the Culvers
filed an amended 2004 federal tax return seeking a refund. The Culvers are also seeking a refund
with respect to their 2004 California tax return.
The parties entered into the Agreement to settle any disputes that the Culvers might have with
the Company in connection with the original Form 1099 that was issued in October 2004 and the
Culvers’ resulting tax liability. Pursuant to the Agreement, the Company agreed to pay the Culvers
$350,000 upon execution in exchange for a full release, which is recorded in sales, general and
administrative expense for the year ended December 31, 2009. The Agreement also contains a
claw-back provision, pursuant to which the Culvers agreed to pay to the Company the amount of any
refund they receive from the federal government and/or the State of California, up to the $350,000
amount of the settlement payment. The Culvers will be entitled to keep 100% of any refund(s) they
receive in excess of $350,000. As of December 31, 2009 the Culvers had not received any tax
refunds, however a refund was received in February 2010. The Company
will record the $350,000 due under the claw-back provision as income
once the cash has been received.
12. Employee Benefit Plan
The Company has a defined contribution profit sharing 401(k) plan (the “Plan”) for eligible
employees, which is qualified under Sections 401(a) and 401(k) of the Internal Revenue Code of
1986, as amended. Employees are entitled to make tax-deferred contributions of up to the maximum
allowed by law of their eligible compensation, but not exceeding $16,500 in 2009. The Company
matches 100% of the first 3% of eligible compensation contributed to the Plan by each participant
and 50% of the next 2% of eligible compensation contributed to the Plan by each participant.
Beginning January 1, 2008, the Company’s matching contributions are immediately vested. The
Company’s matching contributions to the Plan for the years ended December 31, 2009, 2008 and 2007
were approximately $475,000, $398,000, and $250,000, respectively. Future matching or profit
sharing contributions to the Plan are at the Company’s sole discretion.
13. Subsequent events
On January 13, 2010, the Company entered into a Joint Venture agreement with RouteCloud LLC to
establish TASER Protector Group to exclusively develop, market, sell and support a new suite of
products that give parents the ability to manage their children’s mobile phone usage and driving
behaviors though a simple to use interface on a mobile phone, computer or TV. Under the agreement
the Company will provide RouteCloud development funding up to
$1.725 million ($0.3 million of which was funded in the
fourth quarter of 2009 under a letter of understanding between the
parties) and will provide
various marketing and administrative support functions upon launch of the products. RouteCloud is
responsible for development of all products as well as various administrative, finance and billing
functions. The Company has also received warrants to purchase non-voting membership interests
constituting 20% (subject to anti-dilution provisions) of RouteCloud’s equity for an aggregate
exercise price of $1.0 million. If unexercised, the warrants terminate one year after the first
Protector Product launch. Should the Company exercise the warrants, the Company is entitled to one
board seat on RouteCloud’s Board of Directors.
60
Table of Contents
TASER INTERNATIONAL, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
14. Selected Quarterly Financial Data (unaudited)
Selected quarterly financial data for years ended December 31, 2009 and 2008 follows (in
thousands except for per share data):
| Quarter Ended | ||||||||||||||||
| Mar. 31, 2009 | Jun. 30, 2009 | Sep. 30, 2009 | Dec. 31, 2009 | |||||||||||||
| (a) | (a) | |||||||||||||||
Net sales |
$ | 24,604,780 | $ | 21,833,398 | $ | 23,310,457 | $ | 34,502,925 | ||||||||
Gross margin |
$ | 14,629,251 | $ | 13,738,728 | $ | 13,265,812 | $ | 21,768,618 | ||||||||
Net income (loss) |
$ | (467,759 | ) | $ | (723,404 | ) | $ | (3,176,016 | ) | $ | 4,366,073 | |||||
Basic net income (loss) per share |
$ | (0.01 | ) | $ | (0.01 | ) | $ | (0.05 | ) | $ | 0.07 | |||||
Diluted net income (loss) per share |
$ | (0.01 | ) | $ | (0.01 | ) | $ | (0.05 | ) | $ | 0.07 | |||||
| Quarter Ended | ||||||||||||||||
| Mar. 31, 2008 | Jun. 30, 2008 | Sep. 30, 2008 | Dec. 31, 2008 | |||||||||||||
Net sales |
$ | 22,486,504 | $ | 21,101,309 | $ | 22,859,459 | $ | 26,398,218 | ||||||||
Gross margin |
$ | 12,763,318 | $ | 13,605,023 | $ | 13,894,793 | $ | 16,741,093 | ||||||||
Net income — Note (b) |
$ | 1,216,587 | $ | (2,015,736 | ) | $ | 650,377 | $ | 3,785,813 | |||||||
Basic net income per share |
$ | 0.02 | $ | (0.03 | ) | $ | 0.01 | $ | 0.06 | |||||||
Diluted net income per share |
$ | 0.02 | $ | (0.03 | ) | $ | 0.01 | $ | 0.06 | |||||||
| a) | For the quarter ended September 30, 2009, the Company deferred $3.5 million of revenue from X26 sales related to a trade-in program allowing agencies to upgrade to the TASER X3. The deferred revenue of $3.5 million was subsequently recognized in the quarter ended December 31, 2009 either when the trade-in occurred or upon the expiration of the offer on December 31, 2009. | |
| b) | For the quarter ended June 30, 2008, the Company recorded a $5.2 million non-cash charge for punitive damages following an adverse jury verdict in a litigation trial. The $5.2 million was reversed in the fourth quarter of 2008 following a court order that all punitive damages be disregarded. Refer to Note 7c. |
61
Table of Contents
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
Board of Directors and Stockholders of
TASER International, Inc.
We have audited the accompanying consolidated balance sheets of TASER International, Inc. (a
Delaware corporation) and subsidiary as of December 31, 2009 and 2008, and the related consolidated
statements of operations, stockholders’ equity, and cash flows for each of the three years in the
period ended December 31, 2009. Our audits of the basic financial statements included the
supplementary financial statement schedule listed in the index appearing under Item 15(a)(2). These
financial statements and financial statement schedule are the responsibility of the Company’s
management. Our responsibility is to express an opinion on these financial statements and financial
statement schedule based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight
Board (United States). Those standards require that we plan and perform the audit to obtain
reasonable assurance about whether the financial statements are free of material misstatement. An
audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the
financial statements. An audit also includes assessing the accounting principles used and
significant estimates made by management, as well as evaluating the overall financial statement
presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all
material respects, the financial position of TASER International, Inc. and subsidiary as of
December 31, 2009 and 2008, and the results of their operations and their cash flows for each of
the three years in the period ended December 31, 2009, in conformity with accounting principles
generally accepted in the United States of America. Also in our opinion, the related financial
statement schedule, when considered in relation to the basic financial statements taken as a whole,
presents fairly, in all material respects, the information set forth therein.
We also audited, in accordance with the standards of the Public Company Accounting Oversight Board
(United States), TASER International, Inc.’s internal control over financial reporting as of
December 31, 2009, based on criteria established in Internal Control — Integrated Framework issued
by the Committee of Sponsoring Organizations of the Treadway Commission (COSO) and our report dated
March 15, 2010, expressed an unqualified opinion.
/s/ GRANT THORNTON LLP
Phoenix, Arizona
March 15, 2010
62
Table of Contents
| Item 9. | Changes in and Disagreements With Accountants on Accounting and Financial Disclosure |
None.
| Item 9A. | Controls and Procedures |
Attached as exhibits to this Form 10-K are certifications of the Company’s Chief Executive
Officer (“CEO”) and Chief Financial Officer (“CFO”), which are required in accordance with Rule
13a-14 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”). This “Controls and
Procedures” section includes information concerning the controls and controls evaluation referred
to in the certifications. This section should be read in conjunction with the certifications and
the Grant Thornton attestation report for a more complete understanding of the topics presented.
Evaluation of disclosure controls and procedures
As of the end of the period covered by this Annual Report on Form 10-K, we evaluated under the
supervision of our CEO and our CFO, the effectiveness of our disclosure controls and procedures (as
defined in Rules 13a-15(e) or 15d-15(e) of the Exchange Act). Based on this evaluation, our CEO and
our CFO have concluded that as of December 31, 2009 our disclosure controls and procedures are
effective to ensure that information we are required to disclose in reports that we file or submit
under the Exchange Act (i) is recorded, processed, summarized and reported within the time periods
specified in Securities and Exchange Commission rules and forms, and (ii) is accumulated and
communicated to our management, including our CEO and our CFO, as appropriate to allow timely
decisions regarding required disclosure.
Management report on internal control over financial reporting
Our management is responsible for establishing and maintaining adequate internal control over
financial reporting to provide reasonable assurance regarding the reliability of our financial
reporting and the preparation of consolidated financial statements for external purposes in
accordance with U.S. generally accepted accounting principles. Internal control over financial
reporting includes those policies and procedures that:
| (i) | Pertain to the maintenance of records that in reasonable detail accurately and fairly reflect the transactions and dispositions of the assets of the company; | ||
| (ii) | Provide reasonable assurance that transactions are recorded as necessary to permit preparation of consolidated financial statements in accordance with U.S. generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and | ||
| (iii) | Provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of the company’s assets that could have a material effect on the consolidated financial statements. |
Management assessed our internal control over financial reporting as of December 31, 2009, the
end of our fiscal year. Management based its assessment on criteria established in Internal
Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway
Commission. Management’s assessment included evaluation of such elements as the design and
operating effectiveness of key financial reporting controls, process documentation, accounting
policies and our overall control environment. This assessment is supported by testing and
monitoring performed by our Internal Audit organization.
Based on our assessment, management has concluded that our internal control over financial
reporting was effective as of the end of the fiscal year to provide reasonable assurance regarding
the reliability of financial reporting and the preparation of consolidated financial statements for
external reporting purposes in accordance with generally accepted accounting principles. We
reviewed the results of management’s assessment with the Audit Committee of our Board of Directors.
Our independent registered public accounting firm, Grant Thornton LLP, who also audited our
consolidated financial statements, assessed the effectiveness of our internal control over
financial reporting. Grant Thornton LLP has issued their attestation report, which is included
herein.
Changes in internal control over financial reporting
During the three months ended December 31, 2009, there was no change in our internal control
over financial reporting identified in connection with the evaluation required by paragraph (d) of
Rule 13a-15 or Rule 15d-15 that has materially affected, or is reasonably likely to materially
affect, our internal control over financial reporting.
63
Table of Contents
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
Board of Directors and Stockholders of
TASER International, Inc.
TASER International, Inc.
We have audited TASER International, Inc. (a Delaware Corporation) and subsidiary’s internal
control over financial reporting as of December 31, 2009, based on criteria established in Internal
Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway
Commission (COSO). TASER International Inc.’s management is responsible for maintaining effective
internal control over financial reporting and for its assessment of the effectiveness of internal
control over financial reporting, included in the accompanying “Management report on internal
control over financial reporting” in Item 9A, Controls and Procedures. Our responsibility is to
express an opinion on TASER International Inc.’s internal control over financial reporting based on
our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight
Board (United States). Those standards require that we plan and perform the audit to obtain
reasonable assurance about whether effective internal control over financial reporting was
maintained in all material respects. Our audit included obtaining an understanding of internal
control over financial reporting, assessing the risk that a material weakness exists, testing and
evaluating the design and operating effectiveness of internal control based on the assessed risk,
and performing such other procedures as we considered necessary in the circumstances. We believe
that our audit provides a reasonable basis for our opinion.
A company’s internal control over financial reporting is a process designed to provide reasonable
assurance regarding the reliability of financial reporting and the preparation of consolidated
financial statements for external purposes in accordance with generally accepted accounting
principles. A company’s internal control over financial reporting includes those policies and
procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately
and fairly reflect the transactions and dispositions of the assets of the company;
(2) provide reasonable assurance that transactions are recorded as necessary to permit preparation
of consolidated financial statements in accordance with generally accepted accounting principles,
and that receipts and expenditures of the company are being made only in accordance with
authorizations of management and directors of the company; and (3) provide reasonable assurance
regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the
company’s assets that could have a material effect on the consolidated financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or
detect misstatements. Also, projections of any evaluation of effectiveness to future periods are
subject to the risk that controls may become inadequate because of changes in conditions, or that
the degree of compliance with the policies or procedures may deteriorate.
In our opinion, TASER International, Inc. maintained, in all material respects, effective internal
control over financial reporting as of December 31, 2009, based on criteria established in Internal
Control—Integrated Framework issued by COSO.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight
Board (United States), the consolidated balance sheets of TASER International, Inc. as of December
31, 2009 and 2008, and the related consolidated statements of operations, stockholders’ equity, and
cash flows for each of the three years in the period ended
December 31, 2009, and our report dated March 15, 2010, expressed an unqualified opinion.
/s/ GRANT THORNTON LLP
Phoenix, Arizona
March 15, 2010
March 15, 2010
64
Table of Contents
| Item 9B. | Other Information |
Item 1.01 Entry into a Material Definitive Agreement
On January 13, 2010, the Company entered into a Joint Venture Agreement (the
“Protector Group Agreement”) with William D. Kennedy, WDK Enterprises, LLC, and RouteCloud, LLC
(“RouteCloud”) to establish TASER Protector Group to exclusively develop, market, sell and support
a new suite of products that give parents the ability to manage their children’s mobile phone usage
and driving behaviors though a simple to use interface on a mobile phone, computer or TV. Under
the Protector Group Agreement the Company will provide RouteCloud development funding up to $1.725
million and will provide various marketing and administrative support functions upon launch of the
products. RouteCloud is responsible for development of all products as well as various
administrative, finance and billing functions. Royalty revenue will be split between the Company
and RouteCloud. The Company has also received warrants to purchase non-voting membership interests
constituting 20% (subject to anti-dilution provisions) of RouteCloud’s equity for $1.0 million. If
unexercised, the warrants terminate one year after the first Protector Product Launch. Should the
Company exercise the warrants, the Company is entitled to one board seat on RouteCloud’s Board of
Directors.
A copy of the Protector Group Agreement is filed as Exhibit 10.19 to this Form 10-K.
65
Table of Contents
PART III
| Item 10. | Directors, Executive Officers and Corporate Governance |
The information required to be disclosed by this item is incorporated herein by reference to our
definitive proxy statement for the 2010 Annual Meeting of Stockholders (the 2010 Proxy Statement)
which proxy statement we expect to file with the Securities and Exchange Commission within 120 days
after the end of our fiscal year ended December 31, 2009.
| Item 11. | Executive Compensation |
The information required to be disclosed by this item is incorporated herein by reference to our
2010 Proxy Statement.
| Item 12. | Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters |
The information required to be disclosed by this item is incorporated herein by reference to our
2010 Proxy Statement.
Equity Compensation Plan Information
The following table provides details of our equity compensation plans at December 31, 2009:
| Number of | ||||||||||||||||
| Number of | Securities | |||||||||||||||
| Securities | to be Issued upon | Number of | ||||||||||||||
| Authorized for | Exercise of | Weighted Average | Securities | |||||||||||||
| Issuance | Outstanding | Exercise Price of | Remaining | |||||||||||||
| Under the | Options, | Outstanding | Available for | |||||||||||||
| Plan Category | Plan | Warrants or Rights | Options | Future Issuance | ||||||||||||
Equity compensation plans approved by security holders |
24,352,500 | 8,780,067 | $ | 5.94 | 1,708,192 | |||||||||||
Equity compensation plans not approved by security holders |
— | — | $ | — | — | |||||||||||
Total |
24,352,500 | 8,780,067 | $ | 5.94 | 1,708,192 | |||||||||||
Refer to note 10(c) to the consolidated financial statements in Part II, Item 8 of this annual
report for more information on the Company’s equity compensation plans.
| Item 13. | Certain Relationships and Related Transactions, and Director Independence |
The information required to be disclosed by this item is incorporated herein by reference to our
2010 Proxy Statement.
| Item 14. | Principal Accountant Fees and Services |
The information required to be disclosed by this item is incorporated by reference to our 2010
Proxy Statement.
PART IV
| Item 15. | Exhibits and Financial Statement Schedules |
| (a) | The following documents are filed as part of this report: | |
| 1. | Consolidated financial statements: | |
| All consolidated financial statements as set forth under Part II, Item 8 of this report. | ||
| 2. | Supplementary Financial Statement Schedules: | |
| Schedule II — Valuation and Qualifying Accounts |
Other schedules have not been included because they are not applicable or because the information
is included elsewhere in this report.
66
Table of Contents
3. Exhibits:
| Exhibit | ||||
| Number | Description | |||
| 3.1 | Certificate of Incorporation, as amended (incorporated by reference to Exhibit 3.1 to Registration Statement on Form SB-2, effective May 11, 2001 (Registration
No. 333-55658), as
amended) |
|||
| 3.2 | Bylaws, as amended (incorporated by reference to Exhibit 3.2 to Registration Statement on Form SB-2, effective May 11, 2001 (Registration No. 333-55658), as
amended) |
|||
| 3.3 | Certificate of Amendment to Certificate of Incorporation dated September 1, 2004 (incorporated by reference to Exhibit 3.3 to the Annual Report on Form 10-KSB, filed
March 31, 2005) |
|||
| 4.1 | Form of Common Stock Certificate (incorporated by reference to Exhibit 4.2 to Registration Statement on Form SB-2, effective May 11, 2001 (Registration
No. 333-55658), as amended) |
|||
| 10.1 | * | Executive Employment Agreement with Patrick W. Smith, dated July 1, 1998 (incorporated by reference to Exhibit 10.1 to Registration Statement on Form SB-2 effective
May 11, 2001
(Registration No. 333-55658), as amended) |
||
| 10.2 | * | Executive Employment Agreement with Thomas P. Smith, dated November 15, 2000 (incorporated by reference to Exhibit 10.2 to Registration Statement on Form SB-2
effective May 11,
2001 (Registration No. 333-55658), as amended) |
||
| 10.3 | * | Form of Indemnification Agreement between the Company and its directors (incorporated by reference to Exhibit 10.4 to Registration Statement on Form SB-2 effective
May 11, 2001
(Registration No. 333-55658), as amended) |
||
| 10.4 | * | Form of Indemnification Agreement between the Company and its officers (incorporated by reference to Exhibit 10.5 to Registration Statement on Form SB-2 effective
May 11, 2001
(Registration No. 333-55658), as amended) |
||
| 10.5 | * | 1999 Stock Option Plan (incorporated by reference to Exhibit 10.6 to Registration Statement on Form SB-2, effective May 11, 2001 (Registration No. 333-55658), as
amended) |
||
| 10.6 | * | 2001 Stock Option Plan (incorporated by reference to Exhibit 10.7 to Registration Statement on Form SB-2, effective May 11, 2001 (Registration No. 333-55658), as
amended) |
||
| 10.7 | Form of Sales Representative Agreement with respect to services by and between the Company and Sales Representatives (incorporated by reference to Exhibit 10.15 to the Annual
Report on Form 10-KSB, filed March 18, 2002) |
|||
| 10.8 | * | Executive Employment Agreement with Douglas E. Klint, dated December 15, 2002 (incorporated by referenced to Exhibit 10.17 to the Annual Report on Form 10-KSB, filed
March 14, 2003) |
||
| 10.9 | Credit Agreement dated June 22, 2004, between the Company and Bank One (incorporated by reference to Exhibit 10.13 to the Annual Report on Form 10-KSB, filed
March 31, 2005) |
|||
| 10.10 | Amendment to Credit Agreement dated as of October 31, 2006 between the Company and JP Morgan Chase Bank, N.A. (incorporated by reference to Exhibit 10.17 to Form 8-K,
filed
November 1, 2006) |
|||
| 10.11 | * | Executive Employment Agreement with Daniel Behrendt, dated April 28, 2004 (incorporated by reference to Exhibit 10.14 to the Annual Report on Form 10-KSB, filed
March 31, 2005) |
||
| 10.12 | * | 2004 Stock Option Plan (incorporated by reference to Exhibit 10.15 to the Annual Report on Form 10-KSB, filed March 31, 2005) |
||
| 10.13 | * | 2004 Outside Director Stock Option Plan, as amended. (incorporated by reference to exhibit 10.16 to the Annual Report on Form 10-KSB, filed March 31, 2005) |
||
| 10.14 | 2009 Stock Incentive Plan (incorporated by reference to Appendix A to the Company’s 2009 Proxy Statement, filed April 15, 2009) |
|||
| 10.15 | Agreement with Automation Tooling Systems Inc. for purchase of equipment (incorporated by reference to Exhibit 10.18 to the Quarterly Report on Form 10-Q, filed
August 9, 2007) |
|||
| 10.16 | * | Executive Employment Agreement with Steven Mercier, dated February 11, 2008 (incorporated by reference to Exhibit 10.16 to the Annual Report on Form 10-K, filed
February 29, 2008) |
||
| 10.17 | * | Executive Employment Agreement with Jas Dhillon, dated August 1, 2008 (incorporated by reference to Exhibit 10.17 to the Annual Report on Form 10-K filed on
March 16, 2009) |
||
| 10.18 | Settlement and Release Agreement with Bruce and Donna Culver, dated May 15, 2009 (incorporated by reference to Exhibit 10.1 to Quarterly report on Form 10-Q, filed
August 7, 2009) |
|||
| 10.19 | Joint Venture Agreement with RouteCloud LLC, William D. Kenedy and WDK Enterprises, LLC, dated January 13, 2010 |
|||
| 14.1 | Code of Business Conduct and Ethics, as adopted by the Company’s Board of Directors (incorporated by reference to Exhibit 14.1 to the Annual Report on Form 10-KSB,
filed March 31,
2005) |
|||
| 21.1 | List of Subsidiaries |
|||
| 23.1 | Consent of Grant Thornton, LLP, independent registered public accounting firm |
|||
| 31.1 | Principal Executive Officer Certification pursuant to Rule 13a-14(a) or Rule 15d-14(a) |
|||
| 31.2 | Principal Financial Officer Certification pursuant to Rule 13a-14(a) or Rule 15d-14(a) |
|||
| 32.0 | Principal Executive Officer and Principal Financial Officer Certification pursuant to 18 U.S.C. Section 1350 as adopted pursuant to Section 906 of the Sarbanes-Oxley Act
of 2002 |
|||
| * | Management contract or compensatory plan or arrangement |
67
Table of Contents
SIGNATURES
In accordance with the requirements of the Securities Exchange Act of 1934, the registrant has duly
caused this report on Form 10-K to be signed on its behalf by the undersigned, thereunto duly
authorized.
TASER INTERNATIONAL, INC.
Date:
March 15, 2010
| By: | /s/ PATRICK W. SMITH | |||
| Chief Executive Officer | ||||
Date:
March 15, 2010
| By: | /s/ DANIEL M. BEHRENDT | |||
| Chief Financial Officer | ||||
| (Principal Financial and Accounting Officer) | ||||
KNOW ALL PERSONS BY THESE PRESENTS, that each person whose signature appears below constitutes and
appoints Patrick W. Smith his or her true and lawful attorney-in-fact and agent, with full power of
substitution and resubstitution, for him or her and in his or her name, place and stead, in any and
all capacities, to sign any and all amendments to this Annual Report on Form 10-K, and to file the
same, with all exhibits thereto and other documents in connection therewith the Securities and
Exchange Commission, granting unto said attorney-in-fact and agent full power and authority to do
and perform each and every act and thing requisite and necessary to be done in and about the
premises, as fully and to all intents and purposes as he or she might or could do in person hereby
ratifying and confirming all that said attorney-in-fact and agent, or his substitute or
substitutes, may lawfully do or cause to be done by virtue hereof.
In accordance with the Exchange Act, this report has been signed by the following persons on behalf
of the registrant and in the capacities and on the dates indicated.
| Date | ||||
/s/ PATRICK W. SMITH
|
Director | March 15, 2010 | ||
/s/ THOMAS P. SMITH
|
Director | March 15, 2010 | ||
/s/ MATTHEW R. MCBRADY
|
Director | March 15, 2010 | ||
/s/ BRUCE R. CULVER
|
Director | March 15, 2010 | ||
/s/ JUDY MARTZ
|
Director | March 15, 2010 | ||
/s/ MARK W. KROLL
|
Director | March 15, 2010 | ||
/s/ MICHAEL GARNREITER
|
Director | March 15, 2010 | ||
/s/ JOHN S. CALDWELL
|
Director | March 15, 2010 | ||
/s/ RICHARD H. CARMONA
|
Director | March 15, 2010 |
68
Table of Contents
SCHEDULE II — VALUATION AND QUALIFYING ACCOUNTS
| Balance at | Charged | Charged to | Balance | |||||||||||||||||
| beginning | to costs | other | at end of | |||||||||||||||||
| Description | of period | and expenses | accounts | Deductions | period | |||||||||||||||
Allowance for doubtful accounts |
||||||||||||||||||||
Year ended December 31, 2009 |
$ | 200,000 | $ | 82,251 | $ | $ | (82,251 | ) | $ | 200,000 | ||||||||||
Year ended December 31, 2008 |
$ | 189,977 | $ | 78,010 | $ | $ | (67,987 | ) | $ | 200,000 | ||||||||||
Year ended December 31, 2007 |
$ | 110,052 | $ | 80,835 | $ | — | $ | (910 | ) | $ | 189,977 | |||||||||
Allowance for excess and obsolete inventory |
||||||||||||||||||||
Year ended December 31, 2009 |
$ | 129,900 | $ | 821,983 | $ | — | $ | (477,809 | ) | $ | 474,074 | |||||||||
Year ended December 31, 2008 |
$ | 320,555 | $ | 640,655 | $ | — | $ | (831,310 | ) | $ | 129,900 | |||||||||
Year ended December 31, 2007 |
$ | 223,201 | $ | 206,335 | $ | — | $ | (108,981 | ) | $ | 320,555 | |||||||||
Warranty Reserve |
||||||||||||||||||||
Year ended December 31, 2009 |
$ | 615,031 | $ | 92,278 | $ | — | $ | (337,998 | ) | $ | 369,311 | |||||||||
Year ended December 31, 2008 |
$ | 919,254 | $ | 368,521 | $ | — | $ | (672,744 | ) | $ | 615,031 | |||||||||
Year ended December 31, 2007 |
$ | 713,135 | $ | 1,030,291 | $ | — | $ | (824,172 | ) | $ | 919,254 | |||||||||
69
Table of Contents
| Exhibit | ||||
| Number | Description | |||
| 3.1 | Certificate of Incorporation, as amended (incorporated by reference to Exhibit 3.1 to Registration Statement on Form SB-2, effective May 11, 2001 (Registration
No. 333-55658), as
amended) |
|||
| 3.2 | Bylaws, as amended (incorporated by reference to Exhibit 3.2 to Registration Statement on Form SB-2, effective May 11, 2001 (Registration No. 333-55658), as
amended) |
|||
| 3.3 | Certificate of Amendment to Certificate of Incorporation dated September 1, 2004 (incorporated by reference to Exhibit 3.3 to the Annual Report on Form 10-KSB, filed
March 31, 2005) |
|||
| 4.1 | Form of Common Stock Certificate (incorporated by reference to Exhibit 4.2 to Registration Statement on Form SB-2, effective May 11, 2001 (Registration
No. 333-55658), as amended) |
|||
| 10.1 | * | Executive Employment Agreement with Patrick W. Smith, dated July 1, 1998 (incorporated by reference to Exhibit 10.1 to Registration Statement on Form SB-2 effective
May 11, 2001
(Registration No. 333-55658), as amended) |
||
| 10.2 | * | Executive Employment Agreement with Thomas P. Smith, dated November 15, 2000 (incorporated by reference to Exhibit 10.2 to Registration Statement on Form SB-2
effective May 11,
2001 (Registration No. 333-55658), as amended) |
||
| 10.3 | * | Form of Indemnification Agreement between the Company and its directors (incorporated by reference to Exhibit 10.4 to Registration Statement on Form SB-2 effective
May 11, 2001
(Registration No. 333-55658), as amended) |
||
| 10.4 | * | Form of Indemnification Agreement between the Company and its officers (incorporated by reference to Exhibit 10.5 to Registration Statement on Form SB-2 effective
May 11, 2001
(Registration No. 333-55658), as amended) |
||
| 10.5 | * | 1999 Stock Option Plan (incorporated by reference to Exhibit 10.6 to Registration Statement on Form SB-2, effective May 11, 2001 (Registration No. 333-55658), as
amended) |
||
| 10.6 | * | 2001 Stock Option Plan (incorporated by reference to Exhibit 10.7 to Registration Statement on Form SB-2, effective May 11, 2001 (Registration No. 333-55658), as
amended) |
||
| 10.7 | Form of Sales Representative Agreement with respect to services by and between the Company and Sales Representatives (incorporated by reference to Exhibit 10.15 to the Annual
Report on Form 10-KSB, filed March 18, 2002) |
|||
| 10.8 | * | Executive Employment Agreement with Douglas E. Klint, dated December 15, 2002 (incorporated by referenced to Exhibit 10.17 to the Annual Report on Form 10-KSB, filed
March 14, 2003) |
||
| 10.9 | Credit Agreement dated June 22, 2004, between the Company and Bank One (incorporated by reference to Exhibit 10.13 to the Annual Report on Form 10-KSB, filed
March 31, 2005) |
|||
| 10.10 | Amendment to Credit Agreement dated as of October 31, 2006 between the Company and JP Morgan Chase Bank, N.A. (incorporated by reference to Exhibit 10.17 to Form 8-K,
filed
November 1, 2006) |
|||
| 10.11 | * | Executive Employment Agreement with Daniel Behrendt, dated April 28, 2004 (incorporated by reference to Exhibit 10.14 to the Annual Report on Form 10-KSB, filed
March 31, 2005) |
||
| 10.12 | * | 2004 Stock Option Plan (incorporated by reference to Exhibit 10.15 to the Annual Report on Form 10-KSB, filed March 31, 2005) |
||
| 10.13 | * | 2004 Outside Director Stock Option Plan, as amended. (incorporated by reference to exhibit 10.16 to the Annual Report on Form 10-KSB, filed March 31, 2005) |
||
| 10.14 | 2009 Stock Incentive Plan (incorporated by reference to Appendix A to the Company’s 2009 Proxy Statement, filed April 15, 2009) |
|||
| 10.15 | Agreement with Automation Tooling Systems Inc. for purchase of equipment (incorporated by reference to Exhibit 10.18 to the Quarterly Report on Form 10-Q, filed
August 9, 2007) |
|||
| 10.16 | * | Executive Employment Agreement with Steven Mercier, dated February 11, 2008 (incorporated by reference to Exhibit 10.16 to the Annual Report on Form 10-K, filed
February 29, 2008) |
||
| 10.17 | * | Executive Employment Agreement with Jas Dhillon, dated August 1, 2008 (incorporated by reference to Exhibit 10.17 to the Annual Report on Form 10-K filed on
March 16, 2009) |
||
| 10.18 | Settlement and Release Agreement with Bruce and Donna Culver, dated May 15, 2009 (incorporated by reference to Exhibit 10.1 to Quarterly report on Form 10-Q, filed
August 7, 2009) |
|||
| 10.19 | Joint Venture Agreement with RouteCloud LLC, William D. Kenedy and WDK Enterprises, LLC, dated January 13, 2010 |
|||
| 14.1 | Code of Business Conduct and Ethics, as adopted by the Company’s Board of Directors (incorporated by reference to Exhibit 14.1 to the Annual Report on Form 10-KSB,
filed March 31,
2005) |
|||
| 21.1 | List of Subsidiaries |
|||
| 23.1 | Consent of Grant Thornton, LLP, independent registered public accounting firm |
|||
| 31.1 | Principal Executive Officer Certification pursuant to Rule 13a-14(a) or Rule 15d-14(a) |
|||
| 31.2 | Principal Financial Officer Certification pursuant to Rule 13a-14(a) or Rule 15d-14(a) |
|||
| 32.0 | Principal Executive Officer and Principal Financial Officer Certification pursuant to 18 U.S.C. Section 1350 as adopted pursuant to Section 906 of the Sarbanes-Oxley Act
of 2002 |
|||
| * | Management contract or compensatory plan or arrangement |
70
