UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
(Mark One)
| |
|
|
| þ |
|
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2009
OR
| |
|
|
| o |
|
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to
Commission file number: 333-114396
General Nutrition Centers, Inc.
(Exact name of registrant as specified in its charter)
| |
|
|
DELAWARE
(state or other jurisdiction of
Incorporation or organization)
|
|
72-1575168
(I.R.S. Employer
Identification No.) |
| |
|
|
300 Sixth Avenue
Pittsburgh, Pennsylvania
(Address of principal executive offices)
|
|
15222
(Zip Code) |
Registrant’s telephone number, including area code: (412) 288-4600
Securities registered pursuant to section 12(b) of the Act: None
Securities registered pursuant to section 12(g) of the Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule
405 of the Securities Act. o Yes þ No
Indicate by check mark if the registrant is not required to file reports pursuant to Section
13 or 15(d) of the Act. þ Yes o No
Indicate by check mark whether the registrant (1) has filed all reports required to be filed
by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or
for such shorter period that the registrant was required to file such reports), and (2) has been
subject to such filing requirements for the past 90 days. o Yes þ No
Indicate by check mark whether the registrant has submitted electronically and posted on its
corporate Web site, if any, every Interactive Data File required to be submitted and posted
pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months
(or for such shorter period that the registrant was required to submit and post such files). o Yes o No
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation
S-K (229.405 of this chapter) is not contained herein, and will not be contained, to the best of
registrant’s knowledge, in definitive proxy or information statements incorporated by reference in
Part III of this Form 10-K or any amendment to this Form 10-K. þ
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated
filer, or a non-accelerated filer. See definition of “accelerated filer and large accelerated
filer” in Rule 12b-2 of the Exchange Act. (Check one):
| Large accelerated filer o | Accelerated filer o | Non-accelerated filer þ
(Do not check if a smaller reporting company) | Smaller reporting company o |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of
the Act). o Yes þ No
As of March 1, 2010, all of the registrant’s common equity was privately held, and there was
no public market for the registrant’s common equity nor any publicly available quotations for the
registrant’s common equity. As a result, the registrant is unable to calculate the aggregate
market value of the registrant’s common stock held by non-affiliates as of March 1, 2010. As of
March 1, 2010, 100 shares of the registrant’s common stock, par value $0.01 per share (the “Common
Stock”) were outstanding. All shares of our Common Stock are held by GNC Corporation.
FORWARD LOOKING STATEMENTS
This Form 10-K Report contains forward-looking statements within the meaning of the Private
Securities Litigation Reform Act of 1995 with respect to our financial condition, results of
operations and business. Forward-looking statements may relate to our plans, objectives, goals,
strategies, future events, future revenues or performance, capital expenditures, financing needs,
and other information that is not historical information. Discussions containing such
forward-looking statements may be found in Items 1, 2, 3, 7 and 7A hereof, as well as within this
report generally. In addition, when used in this report, the words “subject to,” “believe,”
“anticipate,” “plan,” “expect,” “intend,” “estimate,” “project,” “may,” “will,” “should,” “can,” or
the negative thereof, or variations thereon, or similar expressions, or discussions of strategy,
are intended to identify forward-looking statements, which are inherently uncertain.
All forward-looking statements, including, without limitation, our examination of historical
operating trends, are based upon our current expectations and various assumptions. We believe there
is a reasonable basis for our expectations and beliefs, but we may not realize our expectations and
our beliefs may not prove correct. Actual results could differ materially from those described or
implied by such forward-looking statements. Factors that may materially affect such forward-looking
statements include, among others:
| |
• |
|
uncertainty of the weakened economy; |
| |
| |
• |
|
significant competition in our industry; |
| |
| |
• |
|
unfavorable publicity or consumer perception of our products; |
| |
| |
• |
|
the incurrence of material product liability and product recall costs; |
| |
| |
• |
|
costs of compliance and our failure to comply with new and existing
governmental regulations; |
| |
| |
• |
|
the failure of our franchisees to conduct their operations profitably and
limitations on our ability to terminate or replace under-performing franchisees; |
| |
| |
• |
|
economic, political and other risks associated with our international
operations; |
| |
| |
• |
|
our failure to keep pace with the demands of our customers for new products and
services; |
| |
| |
• |
|
disruptions in our manufacturing system or losses of manufacturing
certifications; |
| |
| |
• |
|
the lack of long-term experience with human consumption of ingredients in some
of our products; |
| |
| |
• |
|
increases in the frequency and severity of insurance claims, particularly
claims for which we are self-insured; |
| |
| |
• |
|
loss or retirement of key members of management; |
| |
| |
• |
|
increases in the cost of borrowings and limitations on availability of
additional debt or equity capital; |
| |
| |
• |
|
the impact of our substantial debt on our operating income and our ability to
grow; |
| |
| |
• |
|
the failure to adequately protect or enforce our intellectual property rights
against competitors; |
| |
| |
• |
|
changes in applicable laws relating to our franchise operations; and |
| |
| |
• |
|
our inability to expand our franchise operations or attract new franchisees. |
Consequently, such forward-looking statements should be regarded solely as our current plans,
estimates and beliefs. You should not place undue reliance on forward-looking statements. We cannot
guarantee future results, events, levels of activity, performance or achievements. We do not
undertake and specifically decline any obligation to update, republish or revise forward-looking
statements to reflect future events or circumstances or to reflect the occurrences of unanticipated
events.
Industry data used throughout this report was obtained from industry publications and our
internal estimates. While we believe such information to be reliable, its accuracy has not been
independently verified and cannot be guaranteed.
ii
PART I
GNC
With our worldwide network of over 6,900 locations and our www.gnc.com website, we are the
leading global specialty retailer of health and wellness products, including vitamins, minerals and
herbal supplements (“VMHS”) products, sports nutrition products, and diet products. We believe that
the strength of our GNC® brand, which is distinctively associated with health and wellness,
combined with our stores and website, give us broad access to consumers and uniquely position us to
benefit from the favorable trends driving growth in the nutritional supplements industry and the
broader health and wellness sector. We derive our revenues principally from product sales through
our company-owned stores, franchise activities, and sales of products manufactured in our
facilities to third parties. Our broad and deep product mix, which is focused on high-margin,
value-added nutritional products, is sold under our GNC proprietary brands, including Mega Men®,
Ultra Mega®, WELLbeING®, Pro Performance®, Pro Performance® AMP and Preventive Nutrition®, and
under nationally recognized third-party brands.
Our domestic retail network, which is approximately nine times larger than the next largest
U.S. specialty retailer of nutritional supplements, provides a leading platform for our vendors to
distribute their products to their target consumer. This gives us leverage with our vendor partners
and has enabled us to negotiate product exclusives and first-to-market opportunities. In addition,
our in-house product development capabilities enable us to offer our customers proprietary
merchandise that can only be purchased through our stores or our website. As the nutritional
supplement consumer often requires knowledgeable customer service, we also differentiate ourselves
from mass and drug retailers with our well-trained sales associates. We believe that our expansive
retail network, our differentiated merchandise offering, and our quality customer service result in
a unique shopping experience.
Our principal executive offices are located at 300 Sixth Avenue, Pittsburgh, Pennsylvania
15222, and our telephone number is (412) 288-4600. We also maintain a website at www.gnc.com. The
contents of our website are not incorporated by reference in this report and shall not be deemed
“filed” under the Exchange Act.
In this report, unless the context requires otherwise, references to “we,” “us,” “our,”
“Company” or “GNC” refer to General Nutrition Centers, Inc. and its subsidiaries.
Corporate History
We are a holding company and all of our operations are conducted through our operating
subsidiaries.
On February 8, 2007, GNC Parent Corporation, our ultimate parent company at that time, entered
into an Agreement and Plan of Merger (the “Merger Agreement”) with GNC Acquisition Inc. and its
parent company, GNC Acquisition Holdings Inc. (“Holdings” or our “Parent”). On March 16, 2007, the
merger (the “Merger”) was consummated. Pursuant to the Merger Agreement, as amended, GNC
Acquisition Inc. was merged with and into GNC Parent Corporation, with GNC Parent Corporation as
the surviving corporation. Subsequently on March 16, 2007, GNC Parent Corporation was converted
into a Delaware limited liability company and renamed GNC Parent LLC.
1
As a result of the Merger, GNC Acquisition Holdings Inc. became the sole equity holder of GNC
Parent LLC and the ultimate parent company of both GNC Corporation, our direct parent company, and
us. The outstanding capital stock of GNC Acquisition Holdings Inc. is beneficially owned by Ares
Corporate Opportunities Fund II L.P. (“ACOF”), an affiliate of Ares Management LLC (“Ares”) Ontario
Teachers’ Pension Plan Board (“OTPP”), certain institutional investors, certain of our directors,
and certain former stockholders of GNC Parent Corporation, including members of our management.
Refer to Note 1, “Nature of Business,” and Item 12, “Security Ownership of Certain Beneficial
Owners and Management and Related Stockholders Matters,” to our consolidated financial statements
included in this report for additional information.
Holdings and each of its stockholders is party to a stockholders agreement. Among other
things, the stockholders agreement contains certain restrictions on transfer of shares of Holdings’
capital stock, and gives each of ACOF and OTPP the right to designate four members of Holdings’
board of directors (or, at the option of each, five members of the board of directors, one of which
shall be independent) for so long as they or their respective affiliates each own at least 10% of
the outstanding common stock of Holdings. For additional information, see Item 13, “Certain
Relationships and Related Transactions and Director Independence- Stockholders’ Agreement.”
GNC Parent Corporation was formed as a Delaware corporation in November 2006 to acquire all
the outstanding common stock of GNC Corporation.
General Nutrition Centers, Inc. was formed in October 2003 and GNC Corporation was formed as a
Delaware corporation in November 2003 by Apollo Management V, L.P. (“Apollo”), an affiliate of
Apollo Management V, L.P. and members of our management to acquire General Nutrition Companies,
Inc. from Numico USA, Inc., a wholly owned subsidiary of Koninklijke (Royal) Numico N.V.
(collectively, “Numico”). In December 2003, we purchased all of the outstanding equity interests
of General Nutrition Companies, Inc.
General Nutrition Companies, Inc. was founded in 1935 by David Shakarian who opened its first
health food store in Pittsburgh, Pennsylvania. Since that time, the number of stores has continued
to grow, and General Nutrition Companies, Inc. began producing its own vitamin and mineral
supplements as well as foods, beverages, and cosmetics. General Nutrition Companies, Inc. was
acquired in August 1999 by Numico Investment Corp. and, prior to its acquisition, was a publicly
traded company listed on the Nasdaq National Market.
Industry Overview
We operate within the large and growing U.S. nutritional supplements retail industry.
According to Nutrition Business Journal’s Supplement Business Report 2009, our industry
generated an estimated $25.2 billion in sales in 2008 and an estimated $26.6 billion in 2009,
and is projected to grow at an average annual rate of approximately 4% per year through 2017.
Our industry is also highly fragmented, and we believe this fragmentation provides large
operators, like us, the ability to compete more effectively due to scale advantages.
We expect several key demographic, healthcare, and lifestyle trends to drive the continued
growth of our industry. These trends include:
| • |
|
Increased Focus on Healthy Living. Consumers are leading more active lifestyles and
becoming increasingly focused on healthy living, nutrition, and supplementation. According
to the Nutrition Business Journal’s Supplement Business Report 2009, 15% of the U.S. adult
population were regular or heavy users of vitamins in 2008, up from 13% in 2007. We
believe that growth in the nutritional supplements industry will continue to be driven by
consumers who increasingly embrace health and wellness as a critical part of their
lifestyles. |
2
| • |
|
Aging Population. The average age of the U.S. population is increasing. U.S. Census
Bureau data indicates that the number of Americans age 65 or older is expected to increase
by approximately 52% from 2000 to 2015. We believe that these consumers are significantly
more likely to use nutritional supplements, particularly VMHS products, than younger
persons, and have higher levels of disposable income to pursue healthier lifestyles. |
| • |
|
Rising Healthcare Costs and Use of Preventive Measures. We believe that current
economic conditions are affecting health related choices being made by consumers. A report
released by the Kaiser Family Foundation and Health Research & Education Trust in 2009
found that, since 1999, the employee contribution for a family health insurance policy had
risen 128%. Additionally, according to the Nutrition Business Journal’s Supplement
Business Report 2009, a report by Information Resources Inc. reports that 25% of consumers
indicate that they are cutting back on doctor visits. We believe that as more people lost
their jobs and ability to pay for healthcare, many turned to supplements to remain healthy
and ward off expensive doctor visits and pharmaceutical drugs. We believe that these
measures, along with the rising cost of health care, will motivate consumers to purchase
more supplements. |
Participants in our industry include specialty retailers, supermarkets, drugstores, mass
merchants, multi-level marketing organizations, mail-order companies, and a variety of other
smaller participants. The nutritional supplements sold through these channels are divided into
four major product categories: VMHS; sports nutrition
products; diet products; and other wellness products. Most supermarkets, drugstores, and
mass merchants have narrow nutritional supplement product offerings limited primarily to simple
vitamins and herbs, with less knowledgeable sales associates than specialty retailers. We
believe that the market share of supermarkets, drugstores, and mass merchants over the last five
years has remained relatively constant.
3
Business Overview
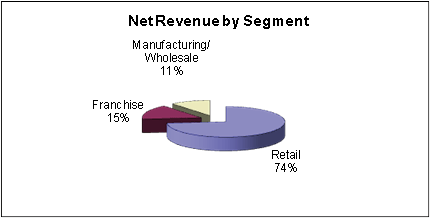
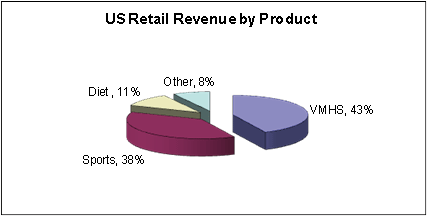
The following charts illustrate the percentage of our net revenue generated by our three
business segments and the percentage of our net U.S. retail supplement revenue generated by our
product categories:
Throughout 2009, we did not have a material concentration of sales from any single product or
product line.
Retail Locations
Our retail network represents the leading specialty retail store network in the nutritional
supplements industry according to Nutrition Business Journal’s Supplement Business Report 2009. As
of December 31, 2009, there were 6,917 GNC store locations globally, including:
| |
• |
|
2,665 company-owned stores in the United States (all 50 states, the District of
Columbia, and Puerto Rico); |
| |
| |
• |
|
167 company-owned stores in Canada; |
| |
| |
• |
|
909 domestic franchised stores; |
| |
| |
• |
|
1,307 international franchised stores in 47 countries; and |
| |
| |
• |
|
1,869 GNC franchised “store-within-a-store” locations under our strategic
alliance with Rite Aid Corporation (“Rite Aid”). |
4
Most of our company-owned and franchised U.S. stores are between 1,000 and 2,000 square feet
and are primarily located in shopping malls and strip shopping centers. We have approximately nine
times the domestic store base of our nearest U.S. specialty retail competitor.
Website. In December 2005 we started selling products through our website, www.gnc.com. This
additional sales channel has enabled us to market and sell our products in regions where we have
limited or no retail operations. Some of the products offered on our website may not be available
at our retail locations, enabling us to broaden the assortment of products available to our
customers. The ability to purchase our products through the internet also offers a convenient
method for repeat customers to evaluate and purchase new and existing products. To date, we believe
that most of the sales generated by our website are incremental to the revenues from our retail
locations.
Franchise Activities
We generate income from franchise activities primarily through product sales to franchisees,
royalties on franchise retail sales, and franchise fees. To assist our franchisees in the
successful operation of their stores and to protect our brand image, we offer a number of services
to franchisees including training, site selection, construction assistance, and accounting
services. We believe that our franchise program enhances our brand awareness and market presence
and will enable us to continue to expand our store base internationally with limited capital
expenditures. Over the last several years, we realigned our domestic franchise system with our
corporate strategies and re-acquired or closed unprofitable or non-compliant franchised stores in
order to improve the financial performance of the franchise system.
Franchised Store-Within-a-Store Locations
To increase brand awareness and promote access to customers who may not frequent specialty
nutrition stores, we entered into a strategic alliance in December 1998 with Rite Aid to open our
GNC franchised store-within-a-store locations. Through this strategic alliance, we generate
revenues from fees paid by Rite Aid for new store-within-a-store openings, sales to Rite Aid of our
products at wholesale prices, the manufacture of Rite Aid private label products, and retail sales
of certain consigned inventory. In 2007, we extended our alliance with Rite Aid through 2014 with a
five year option. At December 31, 2009, Rite Aid had opened 698 of an additional 1,125 stores that
Rite Aid committed to open by December 31, 2014.
Marketing
We market our proprietary brands of nutritional products through an integrated marketing
program that includes television, print, and radio media, storefront graphics, direct mailings to
members of our Gold Card loyalty program, and point of purchase promotional materials.
Manufacturing and Distribution
With our technologically sophisticated manufacturing and distribution facilities supporting
our retail stores, we are a vertically integrated producer and supplier of high-quality nutritional
supplements. By controlling the production and distribution of our proprietary products, we can
protect product quality, monitor delivery times, and maintain appropriate inventory levels.
5
Products
We offer a wide range of high-quality nutritional supplements sold under our GNC proprietary
brand names, including Mega Men, Ultra Mega, WELLbeING, Pro Performance, Pro Performance AMP and
Preventive Nutrition, and under nationally recognized third-party brand names. We report our sales
in four major nutritional supplement categories: VMHS; sports nutrition; diet; and other wellness.
We offer an extensive mix of brands and products, including over 1,800 active SKUs across multiple
categories. This variety is designed to provide our customers with a vast selection of products to
fit their specific needs. Sales of our proprietary brands at our company-owned stores represented
approximately 56% of our net retail product revenues for 2009, 51% for 2008, and 48% for 2007.
Consumers may purchase a GNC Gold Card in any U.S. GNC store or at www.gnc.com for $15.00. A
Gold Card allows a consumer to save 20% on all store and on-line purchases on the day the card is
purchased and during the first seven days of every month for a year. Gold Card members also receive
personalized mailings and e-mails with product news, nutritional information, and exclusive offers.
Products are delivered to our retail stores through our distribution centers located in
Leetsdale, Pennsylvania; Anderson, South Carolina; and Phoenix, Arizona. Our distribution centers
support our company-owned stores as well as franchised stores and Rite Aid locations. Our
distribution fleet delivers our finished goods and third-party products through our distribution
centers to our company-owned and domestic franchised stores on a weekly or biweekly basis depending
on the sales volume of the store. Each of our distribution centers has a quality control department
that monitors products received from our vendors to ensure they meet our quality standards.
Based on data collected from our point-of-sale systems, excluding certain required accounting
adjustments of $5.7 million for 2009, $4.7 million for 2008, $5.0 million for the period from March
16 to December 31, 2007, and $(0.6) million for the period from January 1 to March 15, 2007, below
is a comparison of our company-owned domestic store retail product sales by major product category
and the percentages of our company-owned domestic store retail product sales for the periods shown:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Successor |
|
|
Combined |
|
| |
|
Year ended December 31, |
|
|
|
|
|
|
|
|
|
| U.S Retail Product Categories: |
|
2009 |
|
|
2008 |
|
|
2007 |
|
| |
|
|
|
|
|
|
|
|
|
(dollars in millions) |
|
|
|
|
|
|
|
|
|
VMHS Products |
|
$ |
496.4 |
|
|
|
42.7 |
% |
|
$ |
465.2 |
|
|
|
41.3 |
% |
|
$ |
441.2 |
|
|
|
40.7 |
% |
Sports Nutrition Products |
|
|
443.4 |
|
|
|
38.2 |
% |
|
|
410.1 |
|
|
|
36.4 |
% |
|
|
387.0 |
|
|
|
35.7 |
% |
Diet Products |
|
|
128.0 |
|
|
|
11.0 |
% |
|
|
148.2 |
|
|
|
13.2 |
% |
|
|
156.7 |
|
|
|
14.6 |
% |
Other Wellness Products |
|
|
94.3 |
|
|
|
8.1 |
% |
|
|
102.0 |
|
|
|
9.1 |
% |
|
|
98.0 |
|
|
|
9.0 |
% |
| |
|
|
|
|
|
|
Total U.S. Retail revenues |
|
$ |
1,162.1 |
|
|
|
100.0 |
% |
|
$ |
1,125.5 |
|
|
|
100.0 |
% |
|
$ |
1,082.9 |
|
|
|
100.0 |
% |
| |
|
|
|
|
|
|
The data above represents the majority of the revenue reported for the domestic portion
of our retail segment. In addition to these sales, additional revenue and revenue adjustments are
recorded to ensure conformity with GAAP. This includes wholesale sales revenue (to our military
commissary locations), deferral of our Gold Card revenue to match the twelve month discount period
of the card, and a reserve for customer returns. These items are recurring in nature, and we
expect to record similar adjustments in the future.
VMHS
We sell vitamins and minerals in single vitamin and multi-vitamin form and in different
potency levels. Our vitamin and mineral products are available in liquid, tablets, soft gelatin,
and hard-shell capsules and powder forms, and are available in traditional bottle packaging form or
in customized daily packet form (“Vitapak”). Many of our special vitamin and mineral formulations,
such as Mega Men, Ultra Mega and WELLbeING are available only at GNC locations and on www.gnc.com.
In addition to our selection of VMHS products with unique formulations, we also offer the full
range of standard “alphabet”
6
vitamins. We sell herbal supplements in various solid dosage and soft
gelatin capsules, tea, and liquid
forms. We have consolidated our traditional herbal offerings under a single umbrella brand,
Herbal Plus®. In addition to the Herbal Plus line, we offer a full line of whole food-based
supplements and top selling herb and natural remedy products.
We also offer a variety of specialty products in our GNC and Preventive Nutrition product
lines. These products emphasize third-party research and literature regarding the positive benefits
from certain ingredients. These offerings include products designed to provide nutritional support
to specific areas of the body, such as joints, the heart and blood vessels, and the digestive
system.
Sports Nutrition Products
Sports nutrition products are designed to be taken in conjunction with an exercise and fitness
regimen. We typically offer a broad selection of sports nutrition products, such as protein and
weight gain powders, sports drinks, sports bars, and high potency vitamin formulations, including
GNC brands such as Pro Performance, Pro Performance AMP and popular third-party products.
Diet Products
Diet products consist of various formulas designed to supplement the diet and exercise plans
of weight conscious consumers. We typically offer a variety of diet products, including pills,
meal replacements, shakes, diet bars, and teas. Our retail stores offer our proprietary and
third-party products suitable for different diet and weight management approaches, including
low-carbohydrate diets, and products designed to increase thermogenesis (a change in the body’s
metabolic rate measured in terms of calories) and metabolism.
Other Wellness Products
Other wellness products is a comprehensive category that consists of sales of our Gold Card
preferred membership and sales of other nonsupplement products, including cosmetics, food items,
health management products, books, and DVD’s.
Product Development
We strongly believe that introduction of innovative, high quality, clinically proven, superior
performing products is a key driver to the business. Customers widely credit us as being leaders
in health products and rate the availability of a wide variety of products as one of our biggest
strengths. We identify shifting consumer trends through market research and through interactions
with our customers and leading industry vendors to assist in the development, manufacturing and
marketing of our new products. Our dedicated Innovation team independently drives the development
of proprietary products by collaborating with vendors to provide raw materials and clinical and
product development support for proprietary GNC branded products. We also work with our vendors to
ensure a steady flow of preferred products that are made available exclusively to our channel for a
period of time. During 2009, we targeted our product development efforts on specialty vitamins,
women’s nutrition, sports nutrition and condition specific products, resulting in the introduction
of the WELLbeING and AMP lines.
Research and Development
We have an internal research and development group that performs scientific research on
potential new products and enhancements to existing products, in part to assist our product
development team in creating new products, and in part to support claims that may be made as to the
purpose and function of the product. See Note 2, “Basis of Presentation and Summary of Significant
Accounting Policies,” to our consolidated financial statements included in this report.
7
Business Segments
We generate revenues from our three business segments, Retail, Franchise, and
Manufacturing/Wholesale. The following chart outlines our business segments and the historical
contribution to our consolidated revenues by those segments, after intercompany eliminations. For a
description of operating income (loss) by business segment, our total assets by business segment,
total revenues by geographic area, and total assets by geographic area, see Note 19, “Segments,” to
our consolidated financial statements included in this report.
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Successor |
|
|
Combined |
|
| |
|
|
|
|
|
Year ended December 31, |
|
|
|
|
|
| |
|
2009 |
|
|
2008 |
|
|
2007 |
|
| |
|
|
|
|
|
|
|
|
|
(dollars in millions) |
|
|
|
|
|
|
|
|
|
Retail |
|
$ |
1,256.3 |
|
|
|
73.6 |
% |
|
$ |
1,219.3 |
|
|
|
73.6 |
% |
|
$ |
1,168.6 |
|
|
|
75.3 |
% |
Franchise |
|
|
264.2 |
|
|
|
15.5 |
% |
|
|
258.0 |
|
|
|
15.6 |
% |
|
|
241.1 |
|
|
|
15.5 |
% |
Manufacturing/Wholesale
(Third Party) |
|
|
186.5 |
|
|
|
10.9 |
% |
|
|
179.4 |
|
|
|
10.8 |
% |
|
|
143.1 |
|
|
|
9.2 |
% |
| |
|
|
|
|
|
|
Total |
|
$ |
1,707.0 |
|
|
|
100.0 |
% |
|
$ |
1,656.7 |
|
|
|
100.0 |
% |
|
$ |
1,552.8 |
|
|
|
100.0 |
% |
| |
|
|
|
|
|
|
Retail
Our Retail segment generates revenues primarily from sales of products to customers at our
company-owned stores in the United States and Canada, and in the United States through our website,
www.gnc.com.
Locations
As of December 31, 2009, we operated 2,832 company-owned stores across all 50 states and in
Canada, Puerto Rico, and Washington, D.C. Most of our U.S. company-owned stores are between 1,000
and 2,000 square feet and are located primarily in shopping malls and strip shopping centers.
Traditional shopping mall and strip shopping center locations typically generate a large percentage
of our total retail sales. With the exception of our downtown stores, virtually all of our
company-owned stores follow one of two consistent formats, one for mall locations and one for strip
shopping center locations. Our store graphics are periodically redesigned to better identify with
our GNC customers and provide product information to allow the consumer to make educated decisions
regarding product purchases and usage. Our product labeling is consistent within our product lines
and the stores are designed to present a unified approach to packaging with emphasis on added
information for the consumer. As an ongoing practice, we continue to reset and upgrade all of our
company-owned stores to maintain a more modern and customer-friendly layout, while promoting our
GNC Live Well theme.
Franchise
Our Franchise segment is comprised of our domestic and international franchise operations. Our
Franchise segment generates revenues from franchise activities primarily through product sales to
franchisees, royalties on franchise retail sales, and franchise fees.
As a means of enhancing our operating performance and building our store base, we began
opening franchised locations in 1988. As of December 31, 2009, there were 2,216 franchised stores
operating, including 909 stores in the United States and 1,307 international franchised stores
operating in 47 countries. Approximately 89% of our franchised stores in the United States are in
strip shopping centers and are typically between 1,000 and 2,000 square feet. The international
franchised stores are typically smaller and, depending upon the country and cultural preferences,
are located in mall, strip center, street, or store-within-a-store locations. In addition, some
international franchisees sell on the internet in their respective countries. Typically, our
international stores have a store format and signage similar to our U.S. franchised stores.
To assist our franchisees in the successful operation of their stores and to protect our brand image,
we offer site selection, construction assistance,
8
accounting services, and
a three-part training program, which consists of classroom instruction and training in a
company-owned location, both of which occur prior to the franchised store opening, and actual
on-site training during the first week of operations of the franchised store. We believe we have
good relationships with our franchisees, as evidenced by our franchisee renewal rate of 93% between
2004 and 2009. We do not rely heavily on any single franchise operator in the United States, since
the largest franchisee owns and/or operates 13 store locations.
All of our franchised stores in the United States offer both our proprietary products and
third-party products, with a product selection similar to that of our company-owned stores. Our
international franchised stores are offered a more limited product selection than our franchised
stores in the United States with the product selection heavily weighted toward proprietary
products. Products are distributed to our franchised stores in the United States through our
distribution centers and transportation fleet in the same manner as our company-owned stores.
Products distributed to our international franchised stores are delivered to the franchisee’s
freight forwarder at the U.S. port of deportation, at which point our responsibility for the
delivery of the products ends.
Franchises in the United States
Revenues from our franchisees in the United States accounted for approximately 68% of our
total franchise revenues for the year ended December 31, 2009. In 2009, new franchisees in the
United States were required to pay an initial fee of $40,000 for a franchise license. Existing GNC
franchise operators may purchase an additional franchise license for a $30,000 fee. We typically
offer limited financing to qualified franchisees in the United States for terms up to five years.
Once a store begins operations, franchisees are required to pay us a continuing royalty of 6% of
sales and contribute 3% of sales to a national advertising fund. Our standard franchise agreements
for the United States are effective for a ten-year period with two five-year renewal options. At
the end of the initial term and each of the renewal periods, the renewal fee is generally 33% of
the franchisee fee that is then in effect. The franchisee renewal option is at our election for all
franchise agreements executed after December 1995. Franchisees must meet certain conditions in
order to exercise the franchisee renewal option. Our franchisees in the United States receive
limited geographical exclusivity and are required to follow the GNC store format.
Franchisees must meet certain minimum standards and duties prescribed by our franchise
operations manual and we conduct periodic field visit reports to ensure our minimum standards are
maintained. Generally, we enter into a five-year lease with one five-year renewal option with
landlords for our franchised locations in the United States. This allows us to secure space at
cost-effective rates, which we sublease to our franchisees at cost. By subleasing to our
franchisees, we have greater control over the location and have greater bargaining power for lease
negotiations than an individual franchisee typically would have. In addition, we can elect not to
renew subleases for underperforming locations. If a franchisee does not meet specified performance
and appearance criteria, the franchise agreement outlines the procedures under which we are
permitted to terminate the franchise agreement. In these situations, we may take possession of the
location, inventory, and equipment, and operate the store as a company-owned store or re-franchise
the location. The offering and sale of our franchises in the United States are regulated by the FTC
and various state authorities. See Item 1, “—Government Regulation—Franchise Regulation.”
International Franchises
Revenues from our international franchisees accounted for approximately 32% of our total
franchise revenues for the year ended December 31, 2009. In 2009, new international franchisees
were required to pay an initial fee of approximately $25,000 for a franchise license for each full
size store and average continuing royalty fees of approximately 5% of retail sales, with fees and
royalties varying depending on the country and the store type. Our franchise program has enabled
us to expand into international markets with limited capital expenditures. We expanded our
international presence from 746 international franchised locations at the end of 2004 to 1,307
international locations as of December 31, 2009 without incurring any capital expenditures related
to
9
this expansion. We typically generate less
revenue from franchises outside the United States due to lower international royalty rates and
the franchisees purchasing a smaller percentage of products from us compared to our domestic
franchisees.
Franchisees in international locations enter into development agreements with us for either
full-size stores, a store-within-a-store at a host location, wholesale distribution center
operations or internet distribution rights. The development agreement grants the franchisee the
right to develop a specific number of stores in a territory, often the entire country. The
international franchisee then enters into a franchise agreement for each location. The full-size
store franchise agreement has an initial ten-year term with two five-year renewal options. At the
end of the initial term and renewal periods, the international franchisee has the option to renew
the agreement at 33% of the franchise fee that is then in effect. Franchise agreements for
international store-within-a-store locations have an initial term of five years, with two five-year
renewal options. At the end of the initial term and each of the renewal periods, the international
franchisee of a store-within-a-store location has the option to renew the agreement for up to a
maximum of 50% of the franchise fee that is then in effect. Our international franchisees often
receive exclusive franchising rights to the entire country franchised, excluding U.S. military
bases. Our international franchisees must meet minimum standards and duties similar to our U.S.
franchisees. Our international franchise agreements and international operations may be regulated
by various country, local and international laws. See Item 1, “—Government Regulation—Franchise
Regulation.”
Manufacturing/Wholesale
Our Manufacturing/Wholesale segment is comprised of our manufacturing operations in South
Carolina and our wholesale sales business. This segment supplies our Retail and Franchise segments
as well as various third parties with finished products. Our Manufacturing/Wholesale segment
generates revenues through sales of manufactured products to third parties, and the sale of our
proprietary and third-party brand products to Rite Aid and www.drugstore.com. Our wholesale
operations, including our Rite Aid and www.drugstore.com wholesale operations, are supported
primarily by our Anderson, South Carolina distribution center.
Manufacturing
Our technologically sophisticated manufacturing and warehousing facilities support our Retail
and Franchise segments and enable us to control the production and distribution of our proprietary
products, better control costs, protect product quality, monitor delivery times, and maintain
appropriate inventory levels. We operate two manufacturing facilities, one in Greenville, South
Carolina and one in Anderson, South Carolina. We utilize our plants primarily for the production of
proprietary products. Our manufacturing operations are designed to allow low-cost production of a
variety of products of different quantities, sizes, and packaging configurations while maintaining
strict levels of quality control. Our manufacturing procedures are designed to promote consistency
and quality in our finished goods. We conduct sample testing on raw materials and finished
products, including weight, purity, and micro-bacterial testing. Our manufacturing facilities also
service our wholesale operations, including the manufacture and supply of Rite Aid private label
products for distribution to Rite Aid locations. We use our available capacity at these facilities
to produce products for sale to third-party customers.
The principal raw materials used in the manufacturing process are natural and synthetic
vitamins, herbs, minerals, and gelatin. We maintain multiple sources for the majority of our raw
materials, with the remaining being single-sourced due to the uniqueness of the material. During
2009, no one vendor supplied more than 10% of our raw materials. Our distribution fleet delivers
raw materials and components to our manufacturing facilities and also delivers our finished goods
and third-party products to our distribution centers.
10
Wholesale
Franchised Store-Within-a-Store Locations
To increase brand awareness and promote access to customers who may not frequent specialty
nutrition stores, we entered into a strategic alliance with Rite Aid to open GNC franchised
store-within-a-store locations. As of December 31, 2009, we had 1,869 store-within-a-store
locations. Through this strategic alliance, we generate revenues from sales to Rite Aid of our
products at wholesale prices, the manufacture of Rite Aid private label products, retail sales of
certain consigned inventory and license fees. We are Rite Aid’s sole supplier for the PharmAssure
vitamin brand and a number of Rite Aid private label supplements. In 2007, we extended our alliance
with Rite Aid through 2014 with a five year option. At December 31, 2009, Rite Aid had opened 698
of an additional 1,125 stores that Rite Aid has committed to open by December 31, 2014.
Distribution Agreement with drugstore.com
We are in the process of negotiating a three-year extension to our current internet
distribution agreement with drugstore.com, inc. which was renewed through June 2010. Through this
strategic alliance, www.drugstore.com was the exclusive internet retailer of our proprietary
products, the PharmAssure vitamin brand, and certain other nutritional supplements until June 2005,
when this exclusive relationship terminated. This continued alliance allows us to access a larger
base of customers, who may not otherwise live close to, or have the time to visit, a GNC store and
provides an internet distribution channel in addition to www.gnc.com. We generate revenues from the
distribution agreement with drugstore.com, inc. through sales of third-party products on a
wholesale basis and through retail sales of our proprietary products on a consignment basis.
Employees
As of December 31, 2009, we had a total of 5,271 full-time and 7,522 part-time employees, of
whom approximately 10,166 were employed in the domestic portion of our Retail segment; 40 were
employed in our Franchise segment; 1,371 were employed in our Manufacturing/Wholesale segment; 507
were employed in corporate support functions; and 709 were employed in Canada. None of our
employees belongs to a union or is a party to any collective bargaining or similar agreement. We
consider our relationships with our employees to be good.
Competition
The U.S. nutritional supplements retail industry is a large, highly fragmented, and growing
industry, with no single industry participant accounting for a majority of total industry retail
sales. Competition is based primarily on price, quality, and assortment of products, customer
service, marketing support, and availability of new products. In addition, the market is highly
sensitive to the introduction of new products.
We compete with publicly owned and privately owned companies, which are highly fragmented in
terms of geographical market coverage and product categories. We compete with other specialty
retailers, supermarkets, drugstores, mass merchants, multi-level marketing organizations,
mail-order companies, other internet sites, and a variety of other smaller participants. We believe
that the market is highly sensitive to the introduction of new products, including various
prescription drugs, which may rapidly capture a significant share of the market. In the United
States, many of our competitors have heavily advertised national brands manufactured by large
pharmaceutical and food companies and other retailers. Most supermarkets, drugstores, and mass
merchants have narrow product offerings limited primarily to simple vitamins and herbs and popular
third-party diet products. Our international competitors also include large international pharmacy
chains and major international supermarket chains as well as other large U.S.-based companies with
international operations. Our wholesale and manufacturing operations also compete with other
wholesalers and manufacturers of third-party nutritional supplements.
11
Trademarks and Other Intellectual Property
We believe trademark protection is particularly important to the maintenance of the recognized
brand names under which we market our products. We own or have rights to material trademarks or
trade names that we use in conjunction with the sale of our products, including the GNC brand name.
We also rely upon trade secrets, know-how, continuing technological innovations, and licensing
opportunities to develop and maintain our competitive position. We protect our intellectual
property rights through a variety of methods, including trademark, patent, and trade secret laws,
as well as confidentiality agreements and proprietary information agreements with vendors,
employees, consultants, and others who have access to our proprietary information. Protection of
our intellectual property often affords us the opportunity to enhance our position in the
marketplace by precluding our competitors from using or otherwise exploiting our technology and
brands. We are also a party to several intellectual property license agreements relating to certain
of our products. For example, we are a party to license agreements entered into in connection with
the Numico acquisition pursuant to which we license certain patent rights to Numico and Numico
licenses to us specific patent rights and proprietary information. These license agreements
generally continue until the expiration of the licensed patent, if applicable, or we elect to
terminate the agreement, or upon the mutual consent of the parties. The patents we own generally
have a term of 20 years from their filing date, although none of our owned or licensed patents are
currently associated with a material portion of our business. The duration of our trademark
registrations is generally 10, 15, or 20 years, depending on the country in which the marks are
registered, and the registrations can be renewed by us. The scope and duration of our intellectual
property protection varies throughout the world by jurisdiction and by individual product.
Insurance and Risk Management
We purchase insurance to cover standard risks in the nutritional supplements industry,
including policies to cover general products liability, workers’ compensation, auto liability, and
other casualty and property risks. Our insurance rates are dependent upon our safety record as well
as trends in the insurance industry. We also maintain workers’ compensation insurance and auto
insurance policies that are retrospective in that the cost per year will vary depending on the
frequency and severity of claims in the policy year. We currently maintain product liability
insurance and general liability insurance.
We face an inherent risk of exposure to product liability claims in the event that, among
other things, the use of products sold by GNC results in injury. With respect to product liability
coverage, we carry insurance coverage typical of our industry and product lines. Our coverage
involves self-insured retentions with primary and excess liability coverage above the retention
amount. We have the ability to refer claims to most of our vendors and their insurers to pay the
costs associated with any claims arising from such vendors’ products. In many cases, our insurance
covers such claims that are not adequately covered by a vendor’s insurance and provides for excess
secondary coverage above the limits provided by our product vendors.
We self-insure certain property and casualty risks based on our analysis of the risk, the
frequency and severity of a loss, and the cost of insurance for the risk. We believe that the
amount of self-insurance is not significant and will not have an adverse impact on our performance.
In addition, we may from time to time self-insure liability with respect to specific ingredients in
products that we may sell.
12
Government Regulation
Product Regulation
Domestic
The processing, formulation, manufacturing, packaging, labeling, advertising, and distribution
of our products are subject to regulation by one or more federal agencies, including the Food and
Drug Administration (the “FDA”), the Federal Trade Commission (the “FTC”), the Consumer Product
Safety Commission, the United States Department of Agriculture, and the Environmental Protection
Agency. These activities are also regulated by various agencies of the states and localities in
which our products are sold. Pursuant to the Federal Food, Drug, and Cosmetic Act (“FDCA”), the FDA
regulates the formulation, safety, manufacture, packaging, labeling, and distribution of dietary
supplements (including vitamins, minerals, and herbs), and over-the-counter drugs. The FTC has
jurisdiction to regulate the advertising of these products.
The FDCA has been amended several times with respect to dietary supplements. The Dietary
Supplement Health and Education Act of 1994 (“DSHEA”) established a new framework governing the
composition, safety, labeling and marketing of dietary supplements. “Dietary supplements” are
defined as vitamins, minerals, herbs, other botanicals, amino acids, and other dietary substances
for human use to supplement the diet, as well as concentrates, metabolites, constituents, extracts,
or combinations of such dietary ingredients. Generally, under DSHEA, dietary ingredients that were
marketed in the United States prior to October 15, 1994 may be used in dietary supplements without
notifying the FDA. “New” dietary ingredients (i.e., dietary ingredients that were “not marketed in
the United States before October 15, 1994”) must be the subject of a new dietary ingredient
notification submitted to the FDA unless the ingredient has been “present in the food supply as an
article used for food” without being “chemically altered.” A new dietary ingredient notification
must provide the FDA evidence of a “history of use or other evidence of safety” establishing that
use of the dietary ingredient “will reasonably be expected to be safe.” A new dietary ingredient
notification must be submitted to the FDA at least 75 days before the initial marketing of the new
dietary ingredient. The FDA may determine that a new dietary ingredient notification does not
provide an adequate basis to conclude that a dietary ingredient is reasonably expected to be safe.
Such a determination could prevent the marketing of such dietary ingredient. The FDA has announced
that it plans to issue a guidance governing notification of new dietary ingredients. While it is
not mandatory to comply with FDA guidance, it is a strong indication of the FDA’s current views on
the topic of the guidance, including its position on enforcement. Depending upon the
recommendations made in the guidance, particularly those relating to animal or human testing, such
guidance could make it more difficult for us to successfully provide notification of new dietary
ingredients.
The FDA issued a consumer warning in 1996, followed by proposed regulations in 1997, covering
dietary supplements that contain ephedrine alkaloids, which are obtained from the botanical species
ephedra and are commonly referred to as ephedra. In February 2003, the Department of Health and
Human Services announced a series of actions that the Department of Health and Human Services and
the FDA planned to execute with respect to products containing ephedra, including the solicitation
of evidence regarding the significant or unreasonable risk of illness or injury from dietary
supplements containing ephedra and the immediate execution of a series of actions against ephedra
products making unsubstantiated claims about sports performance enhancement. In addition, many
states proposed regulations and three states enacted laws restricting the promotion and
distribution of ephedra-containing dietary supplements. The botanical ingredient ephedra was
formerly used in several third-party and private label dietary supplement products. In January
2003, we began focusing our diet category on products that would replace ephedra products. In early
2003, we instructed all of our locations to stop selling products containing ephedra that were
manufactured by GNC or one of our affiliates. Subsequently, we instructed all of our locations to
stop selling any products containing ephedra by June 30, 2003. Sales of products containing ephedra
amounted to approximately $35.2 million or 3.3% of our retail sales in 2003 and $182.9 million, or
17.1% of our retail sales in 2002. In February 2004, the FDA issued a final regulation declaring
dietary supplements containing ephedra illegal under the FDCA because they present an unreasonable
risk of illness or injury under the conditions of use recommended
13
or suggested in labeling, or if no conditions of use are suggested or recommended in labeling,
under ordinary conditions of use. The rule took effect April 12, 2004 and banned the sale of
dietary supplement products containing ephedra. Similarly, the FDA issued a consumer advisory in
2002 with respect to dietary supplements that contain the ingredient Kava Kava, and the FDA is
currently investigating adverse effects associated with ingestion of this ingredient. One of our
then existing subsidiaries, Nutra Manufacturing, Inc., manufactured products containing Kava Kava
from December 1995 until August 2002. All stores were instructed to stop selling products
containing Kava Kava in December 2002. The FDA could take similar actions against other products or
product ingredients that in its determination present an unreasonable health risk to consumers.
DSHEA permits “statements of nutritional support” to be included in labeling for dietary
supplements without FDA pre-market approval. Such statements must be submitted to the FDA within 30
days of marketing, and dietary supplements bearing such claims must include the following label
disclosure: “This statement has not been evaluated by the Food and Drug Administration. This
product is not intended to diagnose, treat, cure, or prevent any disease.” Such statements may
describe how a particular dietary ingredient affects the structure, function, or general well-being
of the body, or the mechanism of action by which a dietary ingredient may affect body structure,
function, or well-being, but may not expressly or implicitly represent that a dietary supplement
will diagnose, cure, mitigate, treat, or prevent a disease. A company that uses a statement of
nutritional support in labeling must possess scientific evidence substantiating that the statement
is truthful and not misleading. If the FDA determines that a particular statement of nutritional
support is an unacceptable drug claim or an unauthorized version of a “health claim,” or, if the
FDA determines that a particular claim is not adequately supported by existing scientific data or
is false or misleading, we would be prevented from using the claim.
In addition, DSHEA provides that so-called “third-party literature,” e.g., a reprint of a
peer-reviewed scientific publication linking a particular dietary ingredient with health benefits,
may be used “in connection with the sale of a dietary supplement to consumers” without the
literature being subject to regulation as labeling. The literature: (1) must not be false or
misleading; (2) may not “promote” a particular manufacturer or brand of dietary supplement; (3)
must present a balanced view of the available scientific information on the subject matter; (4) if
displayed in an establishment, must be physically separate from the dietary supplements; and (5)
should not have appended to it any information by sticker or any other method. If the literature
fails to satisfy each of these requirements, we may be prevented from disseminating such literature
with our products, and any dissemination could subject our product to regulatory action as an
illegal drug.
On June 22, 2007, the FDA issued a final rule establishing regulations to require good
manufacturing practices (“GMPs”) for dietary supplements. The regulations establish the GMPs to
ensure quality throughout the manufacturing, packaging, labeling, and storing of dietary
supplements. The final rule includes requirements for establishing quality control procedures,
designing and constructing manufacturing plants, testing ingredients and the finished product,
recordkeeping, and handling consumer product complaints. As a companion document, the FDA also
issued an interim final rule that outlines a petition process for manufacturers to request an
exemption to the GMPs requirements for 100 percent identity testing of specific dietary ingredients
used in the processing of dietary supplements. Under the interim final rule, the manufacturer may
be exempted from the dietary ingredient identity testing requirement if it can provide sufficient
documentation that the reduced frequency of testing requested would still ensure the identity of
the dietary ingredient. Companies with more than 500 employees had until June 2008 to comply with
the new regulations, companies with less than 500 employees had until June 2009 to comply, and
companies with fewer than 20 employees have until June 2010 to comply. Our third-party suppliers
or vendors who are not already compliant may not be able to comply with the new rules without
incurring substantial additional expenses. In addition, if our third-party suppliers or vendors are
not able to timely comply with the new rules, we may experience increased costs or delays in
obtaining certain raw materials and third-party products.
14
In December 2006, Congress passed the Dietary Supplement and Nonprescription Consumer
Protection Act (S3546) (the “Act”). The Act, which became effective in December 2007, mandates
reporting of “serious adverse events” associated with dietary supplements and over-the-counter
drugs to FDA by a manufacturer, packer, or distributor whose name appears on the label of the
product. Records must be maintained of all adverse events for six years after receipt. The Act also
makes it illegal to submit a false report to the FDA.
The FDA has broad authority to enforce the provisions of the FDCA applicable to dietary
supplements, including powers to issue a public warning or notice of violation letter to a company,
publicize information about illegal products, detain products intended for import, request a recall
of illegal products from the market, and request the Department of Justice to initiate a seizure
action, an injunction action, or a criminal prosecution in the U.S. courts. The regulation of
dietary supplements may increase or become more restrictive in the future.
Legislation may be introduced which, if passed, would impose substantial new regulatory
requirements on dietary supplements. Although not yet reintroduced in this session of Congress,
bills have been repeatedly proposed in past sessions of Congress which would subject the dietary
ingredient dehydroepiandrosterone (“DHEA”) to the requirements of the Controlled Substances Act,
which would prevent our ability to sell products containing DHEA. In October 2004, legislation was
passed subjecting specified substances formerly used in some dietary supplements, such as
androstenedione or “andro,” to the requirements of the Controlled Substances Act. Under the 2004
law, these substances can no longer be sold as dietary supplements. Also, in March 2009, the
General Accounting Office (the “GAO”) issued a report that made four recommendations to enhance the
FDA’s oversight of dietary supplements. The GAO recommended that the Secretary of the Department
of Health and Human Services direct the Commissioner of the FDA to: (1) request authority to
require dietary supplement companies to identify themselves as a dietary supplement company as part
of the existing registration requirements and update this information annually, provide a list of
all dietary supplement products they sell and a copy of the labels and update this information
annually, and report all adverse events related to dietary supplements; (2) issue guidance to
clarify when an ingredient is considered a new dietary ingredient, the evidence needed to document
the safety of new dietary ingredients, and appropriate methods for establishing ingredient
identity; (3) provide guidance to industry to clarify when products should be marketed as
either dietary supplements or conventional foods formulated with added dietary ingredients; and
(4) coordinate with stakeholder groups involved in consumer outreach to identify
additional mechanisms for educating consumers about the safety, efficacy, and labeling of dietary
supplements, implement these mechanisms, and assess their effectiveness. These recommendations
could lead to increased regulation by the FDA or future legislation concerning dietary supplements.
The Dietary Supplement Safety Act (S 3002) was introduced in February 2010. The bill would
repeal a provision of DSHEA that permits the sale of all dietary ingredients sold in dietary
supplements marketed in the United States prior to October 15, 1994, and instead permit the sale of
only those dietary ingredients included on a list of Accepted Dietary Ingredients to be issued and
maintained by the FDA. The bill also would allow the FDA to: impose a fine of twice the
gross profits earned by a distributor on sales of any dietary supplement found to violate the law;
require a distributor to submit a yearly report on all non-serious Adverse Event Reports (“AERs”)
received during the year to the FDA; and allow the FDA to recall any dietary supplement it
determines with “a reasonable probability” would cause serious adverse health consequences or is
adulterated or misbranded. The bill also would require any dietary supplement distributor to
register with the FDA and submit a list of the ingredients in and copies of the labels of its
dietary supplements to the FDA and thereafter update such disclosures yearly and submit any new
dietary supplement product labels to the FDA before marketing any dietary supplement product. If
this bill becomes law, it could severely restrict the number of dietary supplements available for
sale and increase our costs and potential penalties associated with selling dietary supplements.
The FTC exercises jurisdiction over the advertising of dietary supplements and
over-the-counter drugs. In recent years, the FTC has instituted numerous enforcement actions
against dietary supplement companies for failure to have adequate substantiation for claims made in
advertising or for the use of false or misleading advertising claims. We continue to be subject to
three consent orders issued by the FTC. In 1984, the FTC instituted an investigation of General Nutrition, Incorporated,
15
one of
our then existing subsidiaries, alleging deceptive acts and practices in connection with the
advertising and marketing of certain of its products. General Nutrition, Incorporated accepted a
proposed consent order which was finalized in 1989, under which it agreed to refrain from, among
other things, making certain claims with respect to certain of its products unless the claims are
based on and substantiated by reliable and competent scientific evidence, and paid an aggregate of
$0.6 million to the American Diabetes Association, Inc., the American Cancer Society, Inc., and the
American Heart Association for the support of research in the fields of nutrition, obesity, or
physical fitness. We also entered into a consent order in 1970 with the FTC, which generally
addressed “iron deficiency anemia” type products. As a result of routine monitoring by the FTC,
disputes arose concerning its compliance with these orders and with regard to advertising for
certain hair care products. While we believe that General Nutrition, Incorporated, at all times,
operated in material compliance with the orders, it entered into a settlement in 1994 with the FTC
to avoid protracted litigation. As a part of this settlement, General Nutrition, Incorporated
entered into a consent decree and paid, without admitting liability, a civil penalty in the amount
of $2.4 million and agreed to adhere to the terms of the 1970 and 1989 consent orders and to abide
by the provisions of the settlement document concerning hair care products. We do not believe that
future compliance with the outstanding consent decrees will materially affect our business
operations. In 2000, the FTC amended the 1970 order to clarify language in it that was believed to
be ambiguous and outmoded.
The FTC continues to monitor our advertising and, from time to time, requests substantiation
with respect to such advertising to assess compliance with the various outstanding consent decrees
and with the Federal Trade Commission Act. Our policy is to use advertising that complies with the
consent decrees and applicable regulations. We review all products brought into our distribution
centers to assure that such products and their labels comply with the consent decrees. We also
review the use of third-party point of purchase materials such as store signs and promotional
brochures. Nevertheless, there can be no assurance that inadvertent failures to comply with the
consent decrees and applicable regulations will not occur. Some of the products sold by franchised
stores are purchased by franchisees directly from other vendors and these products do not flow
through our distribution centers. Although franchise contracts contain strict requirements for
store operations, including compliance with federal, state, and local laws and regulations, we
cannot exercise the same degree of control over franchisees as we do over our company-owned stores.
As a result of our efforts to comply with applicable statutes and regulations, we have from time to
time reformulated, eliminated, or relabeled certain of our products and revised certain provisions
of our sales and marketing program. We believe we are in material compliance
with the various consent decrees and with applicable federal, state, and local rules and
regulations concerning our products and marketing program. Compliance with the provisions of
national, state, and local environmental laws and regulations has not had a material effect upon
our capital expenditures, earnings, financial position, liquidity, or competitive position.
Foreign
Our products sold in foreign countries are also subject to regulation under various national,
local, and international laws that include provisions governing, among other things, the
formulation, manufacturing, packaging, labeling, advertising, and distribution of dietary
supplements and over-the-counter drugs. Government regulations in foreign countries may prevent or
delay the introduction, or require the reformulation, of certain of our products.
New Legislation or Regulation
We cannot determine what effect additional domestic or international governmental legislation,
regulations, or administrative orders, when and if promulgated, would have on our business in the
future. New legislation or regulations may require the reformulation of certain products to meet
new standards, require the recall or discontinuance of certain products not capable of
reformulation, impose additional record keeping, or require expanded documentation of the
properties of certain products, expanded or different labeling, or scientific substantiation.
16
Franchise Regulation
We must comply with regulations adopted by the FTC and with several state laws that regulate
the offer and sale of franchises. The FTC’s Trade Regulation Rule on Franchising and certain state
laws require that we furnish prospective franchisees with a franchise offering circular containing
information prescribed by the Trade Regulation Rule on Franchising and applicable state laws and
regulations.
We also must comply with a number of state laws that regulate some substantive aspects of the
franchisor-franchisee relationship. These laws may limit a franchisor’s business practices in a
number of ways, including limiting the ability to:
| |
• |
|
terminate or not renew a franchise without good cause; |
| |
| |
• |
|
interfere with the right of free association among franchisees; |
| |
| |
• |
|
disapprove the transfer of a franchise; |
| |
| |
• |
|
discriminate among franchisees with regard to franchise terms and charges, royalties,
and other fees; and |
| |
| |
• |
|
place new stores near existing franchises. |
To date, these laws have not precluded us from seeking franchisees in any given area and have
not had a material adverse effect on our operations. Bills intended to regulate certain aspects of
franchise relationships have been introduced into Congress on several occasions during the last
decade, but none have been enacted. Revisions to the FTC rule have also been proposed by the FTC
and currently are in the comment stage of the rulemaking process.
Our international franchise agreements and franchise operations are regulated by various
foreign laws, rules, and regulations. To date, these laws have not precluded us from seeking
franchisees in any given area and have not had a material adverse effect on our operations.
Environmental Compliance
In March 2008, the South Carolina Department of Health and Environmental Control (“DHEC”)
requested that we investigate our South Carolina facility for a possible source or sources of
contamination detected on an adjoining property. We have commenced the investigation at the
facility as requested by DHEC. After several phases of the investigation the possible source or
sources of contamination have not been sufficiently identified. We are continuing such
investigation. The proceedings in this matter have not yet progressed to a stage where it is
possible to estimate the timing and extent of any remedial action that may be required, the
ultimate cost of remediation, or the amount of our potential liability.
17
In addition to the foregoing, we are subject to numerous federal, state, local, and foreign
environmental and health and safety laws and regulations governing our operations, including the
handling, transportation, and disposal of our non-hazardous and hazardous substances and wastes, as
well as emissions and discharges from our operations into the environment, including discharges to
air, surface water, and groundwater. Failure to comply with such laws and regulations could result
in costs for remedial actions, penalties, or the imposition of other liabilities. New laws,
changes in existing laws or the interpretation thereof, or the development of new facts or changes
in our processes could also cause us to incur additional capital and operation expenditures to
maintain compliance with environmental laws and regulations and environmental permits. We also are
subject to laws and regulations that impose liability and cleanup responsibility for releases of
hazardous substances into the environment without regard to fault or knowledge about the condition
or action causing the liability. Under certain of these laws and regulations, such liabilities can
be imposed for cleanup of previously owned or operated properties, or for properties to which
substances or wastes that were sent in connection with current or former operations at our
facilities. The presence of contamination from such substances or wastes could also adversely
affect our ability to sell or lease our properties, or to use them as collateral for
financing. From time to time, we have incurred costs and obligations for correcting environmental
and health and safety noncompliance matters and for remediation at or relating to certain of our
properties or properties at which our waste has been disposed. We believe we have complied with,
and are currently complying with, our environmental obligations pursuant to environmental and
health and safety laws and regulations and that any liabilities for noncompliance will not have a
material adverse effect on our business or financial performance. However, it is difficult to
predict future liabilities and obligations, which could be material.
18
ITEM 1A. RISK FACTORS.
The following risk factors, among others, could cause our financial performance to differ
significantly from the goals, plans, objectives, intentions and expectations expressed in this
report. If any of the following risks and uncertainties or other risks and uncertainties not
currently known to us or not currently considered to be material actually occur, our business,
financial condition, or operating results could be harmed substantially.
Risks Relating to Our Business and Industry
General economic conditions, including a prolonged weakness in the economy, may affect consumer
purchases, which could adversely affect our sales and the sales of our business partners.
Our results, and those of our business partners to whom we sell, are dependent on a number of
factors impacting consumer spending, including general economic and business conditions; consumer
confidence; wages and employment levels; the housing market; consumer debt levels; availability of
consumer credit; credit and interest rates; fuel and energy costs; energy shortages; taxes; general
political conditions, both domestic and abroad; and the level of customer traffic within department
stores, malls and other shopping and selling environments. Consumer product purchases, including
purchases of our products, may decline during recessionary periods. A prolonged downturn or an
uncertain outlook in the economy may materially adversely affect our business and our revenues and
profits.
We operate in a highly competitive industry. Our failure to compete effectively could adversely
affect our market share, revenues, and growth prospects.
The U.S. nutritional supplements retail industry is large and highly fragmented. Participants
include specialty retailers, supermarkets, drugstores, mass merchants, multi-level marketing
organizations, on-line merchants, mail-order companies and a variety of other smaller participants.
We believe that the market is also highly sensitive to the introduction of new products, including
various prescription drugs, which may rapidly capture a significant share of the market. In the
United States, we also compete for sales with heavily advertised national brands manufactured by
large pharmaceutical and food companies, as well as other retailers. In addition, as some products
become more mainstream, we experience increased price competition for those products as more
participants enter the market. For example, when the trend in favor of low-carbohydrate products
developed, we experienced increased competition for our diet products from supermarkets, drug
stores, mass merchants and other food companies, which adversely affected sales of our diet
products. Our international competitors include large international pharmacy chains, major
international supermarket chains, and other large U.S.-based companies with international
operations. Our wholesale and manufacturing operations compete with other wholesalers and
manufacturers of third-party nutritional supplements. We may not be able to compete effectively and
our attempt to do so may require us to reduce our prices, which may result in lower margins.
Failure to effectively compete could adversely affect our market share, revenues, and growth
prospects.
Unfavorable publicity or consumer perception of our products and any similar products distributed
by other companies could cause fluctuations in our operating results and could have a material
adverse effect on our reputation, the demand for our products, and our ability to generate
revenues.
We are highly dependent upon consumer perception of the safety and quality of our products, as
well as similar products distributed by other companies. Consumer perception of products can be
significantly influenced by scientific research or findings, national media attention, and other
publicity about product use. A product may be received favorably, resulting in high sales
associated with that product that may not be sustainable as consumer preferences change. Future
scientific research or publicity could be unfavorable to our industry or any of our particular
products and may not be consistent with earlier favorable research or publicity. A future research
report or publicity that is perceived by our consumers as less favorable or that questions earlier
19
research or publicity could have a
material adverse effect on our ability to generate revenues. For example, sales of some of our VMHS
products, such as St. John’s Wort, Sam-e, and Melatonin, and more recently sales of Vitamin E, were
initially strong, but we believe decreased substantially as a result of negative publicity. As a
result of the above factors, our operations may fluctuate significantly from quarter to quarter.
Period-to-period comparisons of our results should not be relied upon as a measure of our future
performance. Adverse publicity in the form of published scientific research or otherwise, whether
or not accurate, that associates consumption of our products or any other similar products with
illness or other adverse effects, that questions the benefits of our or similar products, or that
claims that such products are ineffective could have a material adverse effect on our reputation,
the demand for our products, and our ability to generate revenues.
Our failure to appropriately respond to changing consumer preferences and demand for new products
could significantly harm our customer relationships and product sales.
Our business is particularly subject to changing consumer trends and preferences, especially
with respect to our diet products. For example, the recent trend in favor of low-carbohydrate diets
was not as dependent on diet products as many other dietary programs, which caused and may continue
to cause a significant reduction in sales in our diet category. Our continued success depends in
part on our ability to anticipate and respond to these changes, and we may not be able to respond
in a timely or commercially appropriate manner to these changes. If we are unable to do so, our
customer relationships and product sales could be harmed significantly.
Furthermore, the nutritional supplement industry is characterized by rapid and frequent
changes in demand for products and new product introductions. Our failure to accurately predict
these trends could negatively impact consumer opinion of our stores as a source for the latest
products. This could harm our customer relationships and cause losses to our market share. The
success of our new product offerings depends upon a number of factors, including our ability to:
| |
• |
|
accurately anticipate customer needs; |
| |
| |
• |
|
innovate and develop new products; |
| |
| |
• |
|
successfully commercialize new products in a timely manner; |
| |
| |
• |
|
price our products competitively; |
| |
| |
• |
|
manufacture and deliver our products in sufficient volumes and in a timely manner; and |
| |
| |
• |
|
differentiate our product offerings from those of our competitors. |
If we do not introduce new products or make enhancements to meet the changing needs of our
customers in a timely manner, some of our products could become obsolete, which could have a
material adverse effect on our revenues and operating results.
We depend on the services of key executives and changes in our management team could affect our
business strategy and adversely impact our performance and results of operations.
Some of our senior executives are important to our success because they have been instrumental
in setting our strategic direction, operating our business, identifying, recruiting and training
key personnel, identifying opportunities and arranging necessary financing. Losing the services of
any of these individuals could adversely affect our business until a suitable replacement could be
found. We believe that they could not quickly be replaced with executives of equal experience and
capabilities. We do not maintain key person life insurance policies on any of our executives.
20
Compliance with new and existing governmental regulations could increase our costs significantly
and adversely affect our results of operations.
The processing, formulation, manufacturing, packaging, labeling, advertising, and distribution
of our products are subject to federal laws and regulation by one or more federal agencies,
including the FDA, the FTC, the Consumer Product Safety Commission, the United States Department of
Agriculture, and the Environmental Protection Agency. These activities are also regulated by
various state, local, and international laws and agencies of the states and localities in which our
products are sold. Government regulations may prevent or delay the introduction, or require the
reformulation, of our products, which could result in lost revenues and increased costs to us. For
instance, the FDA regulates, among other things, the composition, safety, labeling, and marketing
of dietary supplements (including vitamins, minerals, herbs, and other dietary ingredients for
human use). The FDA may not accept the evidence of safety for any new dietary ingredient that we
may wish to market, may determine that a particular dietary supplement or ingredient presents an
unacceptable health risk, and may determine that a particular claim or statement of nutritional
value that we use to support the marketing of a dietary supplement is an impermissible drug claim,
is not substantiated, or is an unauthorized version of a “health claim.” See Item 1, “Business —
Government Regulation — Product Regulation” for additional information. Any of these actions
could prevent us from marketing particular dietary supplement products or making certain claims or
statements of nutritional support for them. The FDA could also require us to remove a particular
product from the market. Any future recall or removal would result in additional costs to us,
including lost revenues from any additional products that we are required to remove from the
market, any of which could be material. Any product recalls or removals could also lead to
liability, substantial costs, and reduced growth prospects.
Additional or more stringent regulations of dietary supplements and other products have been
considered from time to time. These developments could require reformulation of some products to
meet new standards, recalls or discontinuance of some products not able to be reformulated,
additional record-keeping requirements, increased documentation of the properties of some products,
additional or different labeling, additional scientific substantiation, adverse event reporting, or
other new requirements. Any of these developments could increase our costs significantly. For
example, the Dietary Supplement and Nonprescription Drug Consumer Protection Act (S3546) which was
passed by Congress in December 2006, imposes significant new regulatory requirements on dietary
supplements including reporting of “serious adverse events” to FDA and recordkeeping requirements.
This legislation could raise our costs and negatively impact our business. In June 2007, the FDA
adopted final regulations on GMPs in manufacturing, packaging, or holding dietary ingredients and
dietary supplements, which apply to the products we manufacture. These regulations require dietary
supplements to be prepared, packaged, and held in compliance with certain rules. These new
regulations could raise our costs and negatively impact our business. Additionally, our
third-party suppliers or vendors may not be able to comply with the new rules without incurring
substantial expenses. If our third-party suppliers or vendors are not able to timely comply with
the new rules, we may experience increased cost or delays in obtaining certain raw materials and
third-party products. Also, the FDA has announced that it plans to publish a guidance governing
the notification of new dietary ingredients. Although FDA guidance is not mandatory, it is a strong
indication of the FDA’s current views on the topic discussed in the guidance, including its
position on enforcement. Depending on its recommendations, particularly those relating to animal or
human testing, such guidance could also raise our costs and negatively impact our business. We may
not be able to comply with the new rules without incurring additional expenses, which could be
significant. For example, the Dietary Supplement Safety Act (S3002) was introduced in February
2010 and contains many restrictive provisions on the sale of dietary supplements, including, but
not limited to, provisions that limit the dietary ingredients acceptable for use in dietary
supplements, increased fines for violations of DSHEA, and increased registration and reporting
requirements with the FDA. If enacted, this bill could severely restrict the number of dietary
supplements available for sale and increase our costs and potential penalties associated with
selling dietary supplements.
21
Our failure to comply with FTC regulations and existing consent decrees imposed on us by the FTC
could result in substantial monetary penalties and could adversely affect our operating results.
The FTC exercises jurisdiction over the advertising of dietary supplements and has instituted
numerous enforcement actions against dietary supplement companies, including us, for failure to
have adequate substantiation for claims made in advertising or for the use of false or misleading
advertising claims. As a result of these enforcement actions, we are currently subject to three
consent decrees that limit our ability to make certain claims with respect to our products and
required us in the past to pay civil penalties and other amounts in the aggregate amount of $3.0
million. See Item 1, “Business — Government Regulation — Product Regulation” for more
information. Failure by us or our franchisees to comply with the consent decrees and applicable
regulations could occur from time to time. Violations of these orders could result in substantial
monetary penalties, which could have a material adverse effect on our financial condition or
results of operations.
We may incur material product liability claims, which could increase our costs and adversely affect
our reputation, revenues, and operating income.
As a retailer, distributor, and manufacturer of products designed for human consumption, we
are subject to product liability claims if the use of our products is alleged to have resulted in
injury. Our products consist of vitamins, minerals, herbs and other ingredients that are classified
as foods or dietary supplements and are not subject to pre-market regulatory approval in the United
States. Our products could contain contaminated substances, and some of our products contain
ingredients that do not have long histories of human consumption. Previously unknown adverse
reactions resulting from human consumption of these ingredients could occur. In addition,
third-party manufacturers produce many of the products we sell. As a distributor of products
manufactured by third parties, we may also be liable for various product liability claims for
products we do not manufacture. Although our purchase agreements with our third party vendors
typically require the vendor to indemnify us to the extent of any such claims, any such
indemnification is limited by its terms. Moreover, as a practical matter, any such indemnification
is dependent on the creditworthiness of the indemnifying party and its insurer, and the absence of
significant defenses by the insurers. To the extent we are unable to obtain full recovery from the
insurer or any third party in respect of any claims against us in connection with products
manufactured by such party, we could seek recovery directly from such party.
We have been and may be subject to various product liability claims, including, among others,
that our products include inadequate instructions for use or inadequate warnings concerning
possible side effects and interactions with other substances. For example, as of January 25, 2010,
we have been named as a defendant in twenty pending cases involving the sale of Hydroxycut diet
products, including six putative class action cases and eight personal injury cases. See Item 3,
“Legal Proceedings.” Any product liability claim against us could result in increased costs and
could adversely affect our reputation with our customers, which in turn could adversely affect our
revenues and operating income.
Compliance with the Iovate Hydroxycut recall has reduced our sales and margin and may adversely
affect our results of operations.
In May 2009, the FDA warned consumers to stop using Hydroxycut diet products, which are
produced by Iovate Health Sciences, Inc. (“Iovate”), which were sold in our stores. Iovate issued
a voluntary recall, with which we fully complied. Sales of the recalled Hydroxycut products
amounted to approximately $57.8 million, or 4.7% of our retail sales in 2008, and $18.8 million, or
4.2% of our retail sales in the first four months of 2009. We provided refunds or gift cards to
consumers who returned these products to our stores. In the second quarter we experienced a
reduction in sales and margin due to this recall as a result of accepting returns of products from
customers and a loss of sales as a replacement product was not available. Through December 31,
2009, we had refunded approximately $3.5 million to our retail customers and approximately $1.6
million to our wholesale customers for Hydroxycut product returns. A significant majority of the
retail refunds occurred in our second quarter; the
wholesale refunds were recognized in the early part of the third quarter. At the end of June,
Iovate launched new reformulated
22
Hydroxycut products that we began to sell in our stores. Although
sales in the second half of 2009 of the new reformulated Hydroxycut trailed pre-recall levels,
strong sales in our core sports, vitamins and herbs, along with other new third party diet
products, helped to mitigate the Hydroxycut sales decline.
Our operations are subject to environmental and health and safety laws and regulations that may
increase our cost of operations or expose us to environmental liabilities.
Our operations are subject to environmental and health and safety laws and regulations, and
some of our operations require environmental permits and controls to prevent and limit pollution of
the environment. We could incur significant costs as a result of violations of, or liabilities
under, environmental laws and regulations, or to maintain compliance with such environmental laws,
regulations, or permit requirements.
Because we rely on our manufacturing operations to produce nearly all of the proprietary products
we sell, disruptions in our manufacturing system or losses of manufacturing certifications could
adversely affect our sales and customer relationships.
Our manufacturing operations produced approximately 35% and 34% of the products we sold for
years ended December 31, 2009 and 2008, respectively. Other than powders and liquids, nearly all of
our proprietary products are produced in our manufacturing facility located in Greenville, South
Carolina. During 2009, no one vendor supplied more than 10% of our raw materials. In the event any
of our third-party suppliers or vendors becomes unable or unwilling to continue to provide raw
materials in the required volumes and quality levels or in a timely manner, we would be required to
identify and obtain acceptable replacement supply sources. If we are unable to obtain alternative
supply sources, our business could be adversely affected. Any significant disruption in our
operations at our Greenville, South Carolina facility for any reason, including regulatory
requirements and loss of certifications, power interruptions, fires, hurricanes, war or other force
of nature, could disrupt our supply of products, adversely affecting our sales and customer
relationships.
If we fail to protect our brand name, competitors may adopt trade names that dilute the value of
our brand name.
We have invested significant resources to promote our GNC brand name in order to obtain the
public recognition that we have today. However, we may be unable or unwilling to strictly enforce
our trademark in each jurisdiction in which we do business. In addition, because of the differences
in foreign trademark laws concerning proprietary rights, our trademark may not receive the same
degree of protection in foreign countries as it does in the United States. Also, we may not always
be able to successfully enforce our trademark against competitors or against challenges by others.
For example, a third party is currently challenging our right to register in the United States
certain marks that incorporate our “GNC Live Well” trademark. This third party initiated
proceedings in the United States Patent and Trademark Office to cancel four registrations for our
“GNC Live Well” mark. Subsequently, we permitted three of these registrations to lapse. Other third
parties are also challenging our “GNC Live Well” trademark in foreign jurisdictions. Our failure to
successfully protect our trademark could diminish the value and effectiveness of our past and
future marketing efforts and could cause customer confusion. This could in turn adversely affect
our revenues and profitability.
Intellectual property litigation and infringement claims against us could cause us to incur
significant expenses or prevent us from manufacturing, selling, or using some aspect of our
products, which could adversely affect our revenues and market share.
We are currently and may in the future be subject to intellectual property litigation and
infringement claims, which could cause us to incur significant expenses or prevent us from
manufacturing, selling or using some aspect of our products. Claims of intellectual property
infringement also may require us to enter into costly royalty or license agreements. However, we
may be unable to
obtain royalty or license agreements on terms acceptable to us or at all. Claims that our
technology or
23
products infringe on intellectual property rights could be costly and would divert
the attention of management and key personnel, which in turn could adversely affect our revenues
and profitability.
A substantial amount of our revenues are generated from our franchisees, and our revenues could
decrease significantly if our franchisees do not conduct their operations profitably or if we fail
to attract new franchisees.
As of both December 31, 2009 and December 31, 2008, approximately 32% of our retail locations
were operated by franchisees. Our franchise operations generated approximately 15.5% and 15.6% of
our revenues for the years ended December 31, 2009 and 2008, respectively. Our revenues from
franchised stores depend on the franchisees’ ability to operate their stores profitably and adhere
to our franchise standards. In the twelve months ended December 31, 2009, a net 45 domestic
franchise stores were closed. The closing of franchised stores or the failure of franchisees to
comply with our policies could adversely affect our reputation and could reduce the amount of our
franchise revenues. These factors could have a material adverse effect on our revenues and
operating income.
If we are unable to attract new franchisees or to convince existing franchisees to open
additional stores, any growth in royalties from franchised stores will depend solely upon increases
in revenues at existing franchised stores, which could be minimal. In addition, our ability to open
additional franchised locations is limited by the territorial restrictions in our existing
franchise agreements as well as our ability to identify additional markets in the United States and
other countries that are not currently saturated with the products we offer. If we are unable to
open additional franchised locations, we will have to sustain additional growth internally by
attracting new and repeat customers to our existing locations.
Our operating results and financial condition could be adversely affected by the financial and
operational performance of Rite Aid.
As of December 31, 2009, Rite Aid operated 1,869 GNC franchised “store-within-a-store”
locations and has committed to open additional franchised “store-within-a-store” locations.
Revenue from sales to Rite Aid (including license fee revenue for new store openings) represented
approximately 3.3% of total revenue for the year ended December 31, 2009. Any liquidity and
operational issues that Rite Aid may experience could impair its ability to fulfill its obligations
and commitments to us, which would adversely affect our operating results and financial condition.
Economic, political, and other risks associated with our international operations could adversely
affect our revenues and international growth prospects.
As of December 31, 2009, we had 167 company-owned Canadian stores and 1,307 international
franchised stores in 47 countries. We derived 10.2% of our revenues for the year ended December 31,
2009 and 10.1% of our revenues for 2008 from our international operations. As part of our business
strategy, we intend to expand our international franchise presence. Our international operations
are subject to a number of risks inherent to operating in foreign countries, and any expansion of
our international operations will increase the effects of these risks. These risks include, among
others:
| |
• |
|
political and economic instability of foreign markets; |
| |
| |
• |
|
foreign governments’ restrictive trade policies; |
| |
| |
• |
|
inconsistent product regulation or sudden policy changes by foreign agencies or governments; |
| |
| |
• |
|
the imposition of, or increase in, duties, taxes, government royalties, or non-tariff trade
barriers; |
| |
| |
• |
|
difficulty in collecting international accounts receivable and potentially longer payment
cycles; |
| |
| |
• |
|
increased costs in maintaining international franchise and marketing efforts; |
24
| |
• |
|
difficulty in operating our manufacturing facility abroad and procuring supplies from
overseas suppliers; |
| |
| |
• |
|
exchange controls; |
| |
| |
• |
|
problems entering international markets with different cultural bases and consumer
preferences; and |
| |
| |
• |
|
fluctuations in foreign currency exchange rates. |
Any of these risks could have a material adverse effect on our international operations and
our growth strategy.
Franchise regulations could limit our ability to terminate or replace under-performing franchises,
which could adversely impact franchise revenues.
Our franchise activities are subject to federal, state, and international laws regulating the
offer and sale of franchises and the governance of our franchise relationships. These laws impose
registration, extensive disclosure requirements, and bonding requirements on the offer and sale of
franchises. In some jurisdictions, the laws relating to the governance of our franchise
relationship impose fair dealing standards during the term of the franchise relationship and
limitations on our ability to terminate or refuse to renew a franchise. We may, therefore, be
required to retain an under-performing franchise and may be unable to replace the franchisee, which
could adversely impact franchise revenues. In addition, we cannot predict the nature and effect of
any future legislation or regulation on our franchise operations.
We are not insured for a significant portion of our claims exposure, which could materially and
adversely affect our operating income and profitability.
We have procured insurance independently for the following areas: (1) general liability; (2)
product liability; (3) directors and officers liability; (4) property insurance; (5) workers’
compensation insurance; and (6) various other areas. We are self-insured for other areas,
including: (1) medical benefits; (2) workers’ compensation coverage in New York, with a stop loss
of $250,000; (3) physical damage to our tractors, trailers, and fleet vehicles for field personnel
use; and (4) physical damages that may occur at company-owned stores. We are not insured for some
property and casualty risks due to the
frequency and severity of a loss, the cost of insurance, and the overall risk analysis. In
addition, we carry product liability insurance coverage that requires us to pay
deductibles/retentions with primary and excess liability coverage above the deductible/retention
amount. Because of our deductibles and self-insured retention amounts, we have significant exposure
to fluctuations in the number and severity of claims. We currently maintain product liability
insurance with a retention of $3.0 million per claim with an aggregate cap on retained loss of
$10.0 million. As a result, our insurance and claims expense could increase in the future.
Alternatively, we could raise our deductibles/retentions, which would increase our already
significant exposure to expense from claims. If any claim exceeds our coverage, we would bear the
excess expense, in addition to our other self-insured amounts. If the frequency or severity of
claims or our expenses increase, our operating income and profitability could be materially
adversely affected. See Item 3, “Legal Proceedings.”
25
The controlling stockholders of our Parent may take actions that conflict with the interests of
other stockholders and investors. This control may have the effect of delaying or preventing
changes of control or changes in management.
An affiliate of Ares and OTPP, and certain of our directors and members of our management
indirectly beneficially own substantially all of the outstanding equity of our Parent and, as a
result, have the indirect power to elect our directors, to appoint members of management, and to
approve all actions requiring the approval of the holders of our common stock, including adopting
amendments to our certificate of incorporation and approving mergers, acquisitions, or sales of all
or substantially all of our assets. The interests of our ultimate controlling stockholders might
conflict with the interests of other stockholders or the holders of our debt. Our ultimate
controlling stockholders also may have an interest in pursuing acquisitions, divestitures,
financings or other transactions that, in their judgment, could enhance their equity investment,
even though such transactions might involve risks to the holders of our debt.
Risks Related to Our Substantial Indebtedness
Our substantial debt could adversely affect our results of operations and financial condition and
otherwise adversely impact our operating income and growth prospects.
As of December 31, 2009, our total consolidated long-term debt (including current portion) was
approximately $1,059.8 million, and we had an additional $52.1 million available for borrowing on a
collateralized basis under our $60.0 million senior revolving credit facility after giving effect
to the use of $7.9 million of the Revolving Credit Facility to secure letters of credit. In
September 2008, Lehman Brothers Holdings Inc. (“Lehman”), whose subsidiaries have a $6.3 million
credit commitment under our Revolving Credit Facility, filed for bankruptcy. We do not expect that
Lehman will fund its pro rata share of the borrowing as required under the facility. If other
financial institutions that have extended credit commitments to us are adversely affected by the
condition of the U.S. and international capital markets, they may become unable to fund borrowings
under the Revolving Credit Facility, which could have a material and adverse impact on our
financial condition and our ability to borrow additional funds, if needed, for working capital,
capital expenditures, acquisitions, and other corporate purposes. The borrowing proceeds were paid
back in May 2009.
If other financial institutions that have extended credit commitments to us are adversely
affected by the conditions of the U.S. and international capital markets, they may become unable to
fund borrowings under the Revolving Credit Facility, which could have a material and adverse impact
on our financial condition and our ability to borrow additional funds, if needed, for working
capital, capital expenditures, acquisitions, and other corporate purposes.
All of the debt under our senior credit facility bears interest at variable rates. Our
unhedged debt is subject to additional interest expense if these rates increase significantly,
which could also reduce our ability to borrow additional funds.
Our substantial debt could have material consequences on our financial condition. For
example, it could:
| |
• |
|
make it more difficult for us to satisfy our obligations with respect to the
Senior Toggle Notes and the 10.75% Senior Subordinated Notes; |
| |
| |
• |
|
increase our vulnerability to general adverse economic and industry conditions; |
| |
| |
• |
|
require us to use all or a large portion of our cash flow from operations to pay
principal and interest on our debt, thereby reducing the availability of our cash flow
to fund working capital, research and development efforts, capital expenditures, and
other business activities; |
| |
| |
• |
|
increase our vulnerability to general adverse economic and industry conditions; |
26
| |
• |
|
limit our flexibility in planning for, or reacting to, changes in our business
and the industry in which we operate; |
| |
| |
• |
|
restrict us from making strategic acquisitions or exploiting business
opportunities; |
| |
| |
• |
|
place us at a competitive disadvantage compared to our competitors that have less
debt; and |
| |
| |
• |
|
limit our ability to borrow additional funds, dispose of assets, or pay cash
dividends. |
For additional information regarding the interest rates and maturity dates of our existing
debt, see Item 7, “Management Discussion and Analysis of Financial Condition and Results of
Operations—Liquidity and Capital Resources.”
Despite our current significant level of debt, we may still be able to incur additional debt, which
could increase the risks described above, adversely affect our financial health, or prevent us from
fulfilling our obligations under the Senior Toggle Notes and the 10.75% Senior Subordinated Notes.
We and our subsidiaries may be able to incur additional debt in the future, including
collateralized debt. Although the 2007 Senior Credit Facility and the indentures governing the
Senior Toggle Notes and the 10.75% Senior Subordinated Notes contain restrictions on the incurrence
of additional debt, these restrictions are subject to a number of qualifications and exceptions. If
additional debt is added to our current level of debt, the risks described above would increase.
We require a significant amount of cash to service our debt. Our ability to generate cash depends
on many factors beyond our control and, as a result, we may not be able to make payments on our
debt obligations.
We may be unable to generate sufficient cash flow from operations, to realize anticipated cost
savings and operating improvements on schedule or at all, or to obtain future borrowings under our
credit facilities or otherwise in an amount sufficient to enable us to pay our debt or to fund our
other liquidity needs. In addition, because we conduct our operations through our operating
subsidiaries, we depend on those entities for dividends and other payments to generate the funds
necessary to meet our financial obligations, including payments on our debt. Under certain
circumstances, legal and contractual restrictions, as well as the financial condition and operating
requirements of our subsidiaries, may limit our ability to obtain cash from our subsidiaries. If we
do not have sufficient liquidity, we may need to refinance or restructure all or a portion of our
debt on or before maturity, sell assets, or borrow more money. We may not be able to do so on terms
satisfactory to us or at all.
If we are unable to meet our obligations with respect to our debt, we could be forced to
restructure or refinance our debt, seek equity financing, or sell assets. If we are unable to
restructure, refinance, or sell assets in a timely manner or on terms satisfactory to us, the
trading price of the Senior Toggle Notes and the 10.75% Senior Subordinated Notes could decline and
we may default under our obligations. As of December 31, 2009 substantially all of our debt was
subject to acceleration clauses. A default on any of our debt obligations could trigger these
acceleration clauses and cause those and our other obligations to become immediately due and
payable. Upon an acceleration of any of our debt, we may not be able to make payments under our
debt.
We may not have the ability to raise the funds necessary to finance the change of control offer
required by the indentures, which could cause us to default on our debt obligations, including the
Senior Notes and the Senior Subordinated Notes.
Upon certain “change of control” events, as that term is defined in the indentures governing
the Senior Toggle Notes and the 10.75% Senior Subordinated Notes, we will be required to make an
offer to repurchase all or any part of each holder’s notes at a price equal to 101% of the
principal thereof, plus accrued interest to the date of repurchase. Because we do not have
27
access to the cash flow of
our subsidiaries, we will likely not have sufficient funds available at the time of any change of
control event to repurchase all tendered notes pursuant to this requirement. Our failure to offer
to repurchase notes or to repurchase notes tendered following a change of control would result in a
default under the indentures. Accordingly, prior to repurchasing the notes upon a change of control
event, we must refinance all of our outstanding indebtedness. We may be unable to refinance all of
our outstanding indebtedness on terms acceptable to us or at all. If we were unable to refinance
all such indebtedness, we would remain effectively prohibited from offering to repurchase the
notes.
Restrictions in the agreements governing our existing indebtedness may prevent us from taking
actions that we believe would be in the best interest of our business.
The agreements governing our existing indebtedness contain customary restrictions on us or our
subsidiaries, including covenants that restrict us or our subsidiaries, as the case may be, from:
| |
• |
|
incurring additional indebtedness and issuing preferred stock; |
| |
| |
• |
|
granting liens on our assets; |
| |
| |
• |
|
making investments; |
| |
| |
• |
|
consolidating or merging with, or acquiring, another business; |
| |
| |
• |
|
selling or otherwise disposing our assets; |
| |
| |
• |
|
paying dividends and making other distributions to our parent entities; |
| |
| |
• |
|
entering into transactions with our affiliates; and |
| |
| |
• |
|
incurring capital expenditures in excess of limitations set within the agreement. |
Our ability to comply with these covenants and other provisions of the 2007 Senior Credit
Facility and the indentures governing the Senior Toggle Notes and the 10.75% Senior Subordinated
Notes may be affected by changes in our operating and financial performance, changes in general
business and economic conditions, adverse regulatory developments, or other events beyond our
control. The breach of any of these covenants could result in a default under our debt, which could
cause those and other obligations to become immediately due and payable. If any of our debt is
accelerated, we may not be able to repay it.
The senior credit facility also requires that we meet specified financial ratios, including,
but not limited to, maximum total leverage ratios. These restrictions may prevent us from taking
actions that we believe would be in the best interest of our business and may make it difficult for
us to successfully execute our business strategy or effectively compete with companies that are not
similarly restricted.
ITEM 1B. UNRESOLVED STAFF COMMENTS.
None.
28
ITEM 2. PROPERTIES.
As of December 31, 2009, there were 6,917 GNC store locations globally. In our Retail segment,
all but one of our company-owned stores are located on leased premises that typically range in size
from 1,000 to 2,000 square feet. In our Franchise segment, primarily all of our franchised stores
in the United States and Canada are located on premises we lease and then sublease to our
respective franchisees. All of our franchised stores in the remaining international markets are
owned or leased directly by our franchisees. No single store is material to our operations.
As of December 31, 2009, our company-owned and franchised stores in the United States and Canada
(excluding store-within-a-store locations) and our other international franchised stores consisted
of:
29
| |
|
|
|
|
|
|
|
|
| |
|
Company- |
|
|
| United States and Canada |
|
Owned Retail |
|
Franchise |
Alabama |
|
|
34 |
|
|
|
10 |
|
Alaska |
|
|
7 |
|
|
|
5 |
|
Arizona |
|
|
50 |
|
|
|
8 |
|
Arkansas |
|
|
20 |
|
|
|
6 |
|
California |
|
|
229 |
|
|
|
124 |
|
Colorado |
|
|
63 |
|
|
|
10 |
|
Connecticut |
|
|
40 |
|
|
|
4 |
|
Delaware |
|
|
14 |
|
|
|
4 |
|
District of Columbia |
|
|
6 |
|
|
|
1 |
|
Florida |
|
|
219 |
|
|
|
96 |
|
Georgia |
|
|
91 |
|
|
|
47 |
|
Hawaii |
|
|
21 |
|
|
|
0 |
|
Idaho |
|
|
7 |
|
|
|
5 |
|
Illinois |
|
|
97 |
|
|
|
48 |
|
Indiana |
|
|
55 |
|
|
|
20 |
|
Iowa |
|
|
27 |
|
|
|
4 |
|
Kansas |
|
|
24 |
|
|
|
6 |
|
Kentucky |
|
|
39 |
|
|
|
9 |
|
Louisiana |
|
|
39 |
|
|
|
10 |
|
Maine |
|
|
8 |
|
|
|
0 |
|
Maryland |
|
|
52 |
|
|
|
21 |
|
Massachusetts |
|
|
59 |
|
|
|
6 |
|
Michigan |
|
|
77 |
|
|
|
37 |
|
Minnesota |
|
|
60 |
|
|
|
10 |
|
Mississippi |
|
|
21 |
|
|
|
9 |
|
Missouri |
|
|
43 |
|
|
|
20 |
|
Montana |
|
|
4 |
|
|
|
3 |
|
Nebraska |
|
|
11 |
|
|
|
11 |
|
Nevada |
|
|
19 |
|
|
|
11 |
|
New Hampshire |
|
|
15 |
|
|
|
5 |
|
New Jersey |
|
|
79 |
|
|
|
34 |
|
New Mexico |
|
|
19 |
|
|
|
2 |
|
New York |
|
|
160 |
|
|
|
37 |
|
North Carolina |
|
|
97 |
|
|
|
24 |
|
North Dakota |
|
|
6 |
|
|
|
0 |
|
Ohio |
|
|
108 |
|
|
|
40 |
|
Oklahoma |
|
|
29 |
|
|
|
11 |
|
Oregon |
|
|
23 |
|
|
|
5 |
|
Pennsylvania |
|
|
142 |
|
|
|
33 |
|
Puerto Rico |
|
|
23 |
|
|
|
0 |
|
Rhode Island |
|
|
13 |
|
|
|
1 |
|
South Carolina |
|
|
29 |
|
|
|
22 |
|
South Dakota |
|
|
5 |
|
|
|
0 |
|
Tennessee |
|
|
42 |
|
|
|
26 |
|
Texas |
|
|
198 |
|
|
|
80 |
|
Utah |
|
|
24 |
|
|
|
5 |
|
Vermont |
|
|
4 |
|
|
|
0 |
|
Virginia |
|
|
84 |
|
|
|
21 |
|
Washington |
|
|
48 |
|
|
|
13 |
|
West Virginia |
|
|
20 |
|
|
|
2 |
|
Wisconsin |
|
|
56 |
|
|
|
3 |
|
Wyoming |
|
|
5 |
|
|
|
0 |
|
Canada |
|
|
167 |
|
|
|
2 |
|
|
|
|
|
|
|
|
|
|
Total |
|
|
2,832 |
|
|
|
911 |
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
| International |
|
Franchise |
Aruba |
|
|
2 |
|
Australia |
|
|
40 |
|
Azerbaijan |
|
|
1 |
|
Bahamas |
|
|
3 |
|
Bahrain |
|
|
3 |
|
Bolivia |
|
|
3 |
|
Brunei |
|
|
2 |
|
Bulgaria |
|
|
4 |
|
Cayman Islands |
|
|
2 |
|
Chile |
|
|
135 |
|
Costa Rica |
|
|
14 |
|
Cyprus |
|
|
3 |
|
Dominican Republic |
|
|
18 |
|
Ecuador |
|
|
14 |
|
El Salvador |
|
|
9 |
|
Ghana |
|
|
1 |
|
Guam |
|
|
1 |
|
Guatemala |
|
|
32 |
|
Honduras |
|
|
4 |
|
Hong Kong |
|
|
52 |
|
India |
|
|
36 |
|
Indonesia |
|
|
35 |
|
Israel |
|
|
2 |
|
Kuwait |
|
|
5 |
|
Lebanon |
|
|
6 |
|
Malaysia |
|
|
49 |
|
Mexico |
|
|
322 |
|
Mongolia |
|
|
3 |
|
Nigeria |
|
|
2 |
|
Oman |
|
|
2 |
|
Pakistan |
|
|
6 |
|
Panama |
|
|
5 |
|
Peru |
|
|
44 |
|
Philippines |
|
|
35 |
|
Qatar |
|
|
4 |
|
Saudi Arabia |
|
|
49 |
|
Singapore |
|
|
58 |
|
South Korea |
|
|
136 |
|
Spain |
|
|
2 |
|
Taiwan |
|
|
29 |
|
Thailand |
|
|
30 |
|
Trinidad |
|
|
1 |
|
Turkey |
|
|
57 |
|
UAE |
|
|
6 |
|
Ukraine |
|
|
1 |
|
Venezuela |
|
|
37 |
|
|
|
|
|
|
Total |
|
|
1,305 |
|
|
|
|
|
|
30
In our Manufacturing/Wholesale segment, we lease facilities for manufacturing, packaging,
warehousing, and distribution operations. We manufacture a majority of our proprietary products at
an approximately 300,000 square-foot facility in Greenville, South Carolina. We also lease an
approximately 630,000 square-foot complex located in Anderson, South Carolina, for packaging,
materials receipt, lab testing, warehousing, and distribution. Both the Greenville and Anderson
facilities are leased on a long-term basis pursuant to “fee-in-lieu-of-taxes” arrangements with the
counties in which the facilities are located, but we retain the right to purchase each of the
facilities at any time during the lease for $1.00, subject to a loss of tax benefits. We lease a
210,000 square-foot distribution center in Leetsdale, Pennsylvania and a 112,000 square-foot
distribution center in Phoenix, Arizona. We also lease space at a distribution center in Canada.
We lease three small regional sales offices in Fort Lauderdale, Florida; Tustin, California;
and Mississauga, Ontario. None of the regional sales offices is larger than 6,500 square feet. Our
253,000 square-foot corporate headquarters in Pittsburgh, Pennsylvania, is owned by Gustine Sixth
Avenue Associates, Ltd., a Pennsylvania limited partnership, of which we are a limited partner
entitled to share in 75% of the partnership’s profits or losses. The partnership’s ownership of the
land and buildings, and the partnership’s interest in the ground lease to us, are all encumbered by
a mortgage in the original principal amount of $17.9 million, with an outstanding balance of $7.2
million as of December 31, 2009. This partnership is included in our consolidated financial
statements.
|
|
|
| ITEM 3. |
|
LEGAL PROCEEDINGS. |
We are engaged in various legal actions, claims and proceedings arising in the normal course
of business, including claims related to breach of contracts, products liabilities, intellectual
property matters and employment-related matters resulting from our business activities. As with
most actions such as these, an estimation of any possible and/or ultimate liability cannot always
be determined. We continue to assess the requirement to account for additional contingencies in
accordance with the standard on contingencies. If we are required to make a payment in connection
with an adverse outcome in these matters, it could have a material impact on our financial
condition and operating results.
As a manufacturer and retailer of nutritional supplements and other consumer products that are
ingested by consumers or applied to their bodies, we have been and are currently subjected to
various product liability claims. Although the effects of these claims to date have not been
material to the Company, it is possible that current and future product liability claims could have
a material adverse impact on its business or financial condition. We currently maintain product
liability insurance with a deductible/retention of $3.0 million per claim with an aggregate cap on
retained loss of $10.0 million. We typically seek and have obtained contractual indemnification
from most parties that supply raw materials for our products or that manufacture or market products
we sell. We also typically seek to be added, and have been added, as an additional insured under
most of such parties’ insurance policies. We are also entitled to indemnification by Numico for
certain losses arising from claims related to products containing ephedra or Kava Kava sold prior
to December 5, 2003. However, any such indemnification or insurance is limited by its terms and any
such indemnification, as a practical matter, is limited to the creditworthiness of the indemnifying
party and its insurer, and the absence of significant defenses by the insurers. We may incur
material products liability claims, which could increase its costs and adversely affect our
reputation, revenues and operating income.
Pro-Hormone/Androstenedione Cases. We are currently defending six lawsuits (the “Andro
Actions”) relating to the sale by GNC of certain nutritional products alleged to contain the
ingredients commonly known as Androstenedione, Androstenediol, Norandrostenedione, and
Norandrostenediol (collectively, “Andro Products”). Five of these lawsuits were filed in
California, New York, New Jersey, Pennsylvania, and Florida. The most recent case was filed in
Illinois (see Stephens and Pio matter discussed below).
In each of the six cases, plaintiffs sought, or are seeking, to certify a class and obtain
damages on behalf of the class representatives and all those similarly-situated who purchased from
us certain nutritional supplements alleged to contain one or more Andro Products.
31
In April 2006, we filed pleadings seeking to remove the then-pending Andro Actions to the
respective federal district courts for the districts in which the respective Andro Actions were
pending. At the same time, we filed motions seeking to transfer the then-pending Andro Actions to
the U.S. District Court, Southern District of New York based on “related to” bankruptcy
jurisdiction, as one of the manufacturers supplying it with Andro Products, and from whom it sought
indemnity, MuscleTech Research and Development, Inc. (“MuscleTech”), had filed for bankruptcy. We
were successful in removing the New Jersey, New York, Pennsylvania, and Florida Andro Actions to
federal court and transferring these actions to the U.S. District Court, Southern District of New
York based on bankruptcy jurisdiction. The California case, Guzman v. General Nutrition Companies,
Inc., was not removed and remains pending in the Superior Court of the State of California for the
County of Los Angeles.
Following the conclusion of the MuscleTech bankruptcy case, in September 2007, plaintiffs
filed a stipulation dismissing all claims related to the sale of MuscleTech products in the four
cases then-pending in the U.S. District Court, Southern District of New York (New Jersey, New York,
Pennsylvania, and Florida). Additionally, plaintiffs filed motions with the Court to remand those
actions to their respective state courts, asserting that the federal court had been divested of
jurisdiction because the MuscleTech bankruptcy action was no longer pending. That motion was never
ruled upon and has been rendered moot by the disposition of the case, discussed below.
On June 4, 2008, the U.S. District Court, Southern District of New York (on its own motion)
set a hearing for the purpose of hearing argument as to why the New Jersey, New York, Pennsylvania,
and Florida cases should not be dismissed for failure to prosecute in conformity to the Court’s
Case Management Order. Following the hearing, the Court advised that all four cases would be
dismissed with prejudice and issued an order to that effect. In August 2008, plaintiffs appealed
the dismissal of the four cases to U.S. Court of Appeals for the Second Circuit, and oral argument
was heard on October 14, 2009. The Second Circuit reversed the dismissal and remanded the case to
the U.S. District Court, Southern District of New York.
In the Guzman case in California, plaintiffs’ Motion for Class Certification was denied on
September 8, 2008. Plaintiffs appealed on October 31, 2008. Oral arguments took place on January
15, 2010 and the court reversed the order denying class certification.
On October 3, 2008, the plaintiffs in the five other Andro Actions filed another suit related
to the sale of Andro Products in state court in Illinois. Stephens and Pio v. General Nutrition
Companies, Inc. (Case No. 08 CH 37097, Circuit Court of Cook County, Illinois, County Department,
Chancery Division). The allegations are substantially similar to all of the other Andro Actions.
As any liabilities that may arise from these cases are not probable or reasonably estimable at
this time, no liability has been accrued in the accompanying financial statements.
California Wage and Break Claim. On November 4, 2008, ninety-eight plaintiffs filed
individual claims against the Company in the Superior Court of the State of California for the
County of Orange, which was removed to the U.S. District Court, Central District of California on
February 17, 2009. Each of the plaintiffs had previously been a member of a purported class in a
lawsuit filed against the Company in 2007 and resolved in September 2009. The plaintiffs allege
that they were not provided all of the rest and meal periods to which they were entitled under
California law, and further allege that we failed to pay them split shift and overtime compensation
to which they were entitled under California law. Discovery in this case is ongoing and we are
vigorously defending these matters. The court has developed a mediation procedure for handling the
pending claims and has ordered the parties to mediate with small groups of plaintiffs and stayed
the case as to the plaintiffs not participating in the mediations. The first of the mediation
sessions occurred February 10, 2010 and March 4, 2010 and did not result in any settlements. Any
liabilities that may arise from these matters are not probable or reasonably estimable at this
time.
32
Franchise Class Action. On November 7, 2006, Abdul Ahussain, on behalf of himself and all
others similarly situated, sued GNC Franchising, LLC and General Nutrition Corporation in the U.S.
District Court, Central District of California, Western Division. Plaintiff alleged that GNC
engages in unfair business practices designed to earn a profit at its franchisees’ expense, among
other things, in violation of California Business & Professions Code, §§ 17200 et seq. (the
“CBPC”). These alleged practices include: (1) requiring its franchises to carry slow moving
products, which cannot be returned to GNC after expiration, with the franchisee bearing the loss;
(2) requiring franchised stores to purchase new or experimental products, effectively forcing the
franchisees to provide free market research; (3) using our Gold Card program to collect information
on franchised store customers and then soliciting business from such customers; (4) underselling
its franchised stores by selling products through the GNC website at prices below or close to the
wholesale price, thereby forcing franchises to sell the same products at a loss; and (5)
manipulating prices at which franchised stores can purchase products from third-party suppliers, so
as to maintain GNC’s favored position as a product wholesaler. Plaintiffs are seeking damages in
an unspecified amount and equitable and injunctive relief. On March 19, 2008, the court certified
a class as to only plaintiffs’ claim under the CBPC. The class consists of all persons or entities
who are or were GNC franchisees in the State of California from November 13, 2002 to the date of
adjudication. Plaintiff’s individual claims were settled and dismissed. On March 18, 2009, our
motion for summary judgment was granted as to the CBPC class claim. In April 2009, GNC filed a
motion for court costs and attorneys’ fees and the court ordered the plaintiffs to pay
approximately $0.4 million to GNC for its fees and costs. Plaintiff’s judgment was satisfied in
full by October 6, 2009, and a Satisfaction of Judgment was filed with the court on that date.
Jackson Claim. On November 10, 2008, Grady Jackson, on behalf of himself and all others
similarly situated, filed a complaint against General Nutrition Corporation and us in the Superior
Court of the State of California for the County of Alameda. On December 15, 2008, the matter was
removed to the U.S. District Court, Northern District of California. This consumer class and
representative action brought under California Unfair Competition and False Advertising Law
asserts, among other things, that the non-GNC product “Nikki Haskell’s Star Caps,” contained a
prescription diuretic ingredient that was not disclosed on the label. On March 31, 2009, GNC filed
a motion to dismiss. By order dated June 10, 2009, the court dismissed three of the seven counts
asserted by plaintiffs. In September 2009, a settlement was reached, contemplating payment of an
immaterial amount of attorneys’ fees to putative class counsel by GNC, and distribution of GNC
discount coupons to certain putative class members. The court signed the stipulation of dismissal
on October 27, 2009.
DiMauro Claim. On December 18, 2008, plaintiffs Laura and Charles DiMauro filed a personal
injury complaint against General Nutrition Corporation in Circuit Court for Miami-Dade County
Florida. Plaintiffs allege that Laura DiMauro’s use and consumption of a non-GNC product called
“Up Your Gas” resulted in liver failure that required a liver transplant in August 2007.
Plaintiffs assert, among other things, claims for strict liability, negligence, and fraud and seek
unspecified monetary damages. GNC has moved to dismiss this case on the grounds of forum non
conveniens. In December 2009, a settlement was reached, contemplating payment of an immaterial
amount by GNC.
Romero Claim. On April 27, 2009, plaintiff J.C. Romero, a professional baseball player, filed
a complaint against, among others, General Nutrition Centers, Inc. in Superior Court of New Jersey
(Law Division/Camden County). Plaintiff alleges that he purchased from a GNC store and consumed
6-OXO Extreme, which is manufactured by a third party, and in August 2008, was alleged to have
tested positive for a banned substance. Plaintiff served a 50 game suspension imposed by Major
League Baseball. The seven count complaint asserts, among other things, claims for negligence,
strict liability, misrepresentation, breach implied warranty and violations of the New Jersey
Consumer Fraud Act, and seeks unspecified monetary damages. GNC tendered the claim to the
insurance company of the franchisee whose GNC store sold and allegedly misrepresented the product.
On or about October 9, 2009, GNC answered plaintiff’s first amended complaint and cross-claimed
against co-defendants Proviant Technologies and Ergopharm. Discovery in this case is ongoing and we
are vigorously defending the matter. Any liabilities that may arise from this matter are not
probable or reasonably estimable at this time.
33
Ciavarra Claim. On November 19, 2008, Ryan Ciavarra filed a personal injury lawsuit against,
among others, General Nutrition Corporation, in the District Court of Harris County, Texas.
Plaintiff alleges that his use and consumption of the diet product Hydroxycut, which is
manufactured by a third party and was, until recently, sold in our stores, caused severe liver
damage, jaundice and elevated liver enzymes. Plaintiff asserts claims for strict liability,
negligence and breach of warranty and seeks unspecified monetary damages. Any liabilities that may
arise from this matter are not probable or reasonably estimable at this time.
Hydroxycut Claims. On May 1, 2009, the FDA issued a warning on several Hydroxycut-branded
products manufactured by Iovate Health Sciences U.S.A., Inc. (“Iovate”). The FDA warning was based
on 23 reports of liver injuries from consumers who claimed to have used the products between 2002
and 2009. As a result, Iovate voluntarily recalled fourteen Hydroxycut-branded products. Following
the recall, GNC was named, among other defendants, in 22 lawsuits in several states (note that
prior to May 1, 2009, GNC was a co-defendant in one Hydroxycut case, Ciavarra (see
“Ciavarra Claim” entry above)). Iovate previously accepted GNC’s tender request for defense and
indemnification under its purchasing agreement with GNC and, as such, Iovate has accepted GNC’s
request for defense and indemnification in the new Hydroxycut matters. GNC’s ability to obtain
full recovery in respect of any claims against GNC in connection with products manufactured by
Iovate under the indemnity is dependent on Iovate’s insurance coverage and the creditworthiness of
its insurer, and the absence of significant defenses by the insurer. To the extent GNC was not
fully compensated by Iovate’s insurer, it could seek recovery directly from Iovate. GNC’s ability
to fully recover such amounts would be limited by the creditworthiness of Iovate.
GNC has been named in 23 lawsuits related to Hydroxycut: 17 individual, largely personal
injury claims (including Ciavarra discussed above) and 6 putative class actions, generally
inclusive of claims of consumer fraud, misrepresentation, strict liability, and breach of warranty.
The following 16 personal injury matters were filed by individuals claiming injuries from use
and consumption of Hydroxycut-branded products:
| |
• |
|
Michael Owens and Donna Owens v. Iovate Health Sciences USA, Inc., et al., Superior
Court of the State of California, County of Los Angeles, BC413006 (filed May 1, 2009); |
| |
| |
• |
|
Eva M. Stasiak v. Iovate Health Sciences USA, Inc., et al., Superior Court of the
State of California, County of Los Angeles, BC413201 (filed May 11, 2009); |
| |
| |
• |
|
Jaime Ruben Perez v. Gerald Brandt, individually and d/b/a/ Breakthru Products, et
al., 229th Judicial District, Duval County, Texas (filed May 29, 2009); |
| |
| |
• |
|
Juan A. Noyola, II v. Iovate Health Sciences USA, Inc., et al., U.S. District Court,
Southern District of New York, 09CV6740 (filed July 29, 2009); |
| |
| |
• |
|
Christopher and Dana Hamilton v. Iovate Health Sciences USA, Inc., et al., U.S.
District Court, Northern District of Ohio, 09CV1944 (filed August 18, 2009); |
| |
| |
• |
|
Hector Manuel Abarca and Diana Curiel v. Iovate Health Sciences USA, Inc., et al.,
U.S. District Court, Northern District of California, 09CV3861 (filed August 21, 2009); |
| |
| |
• |
|
Jessica Rogoff v. General Nutrition Centers, Inc., et al., Superior Court of the
State of California, County of Los Angeles, BC422842 (filed September 29, 2009); |
| |
| |
• |
|
Pattie Greenwood, et al. v. Iovate Health Sciences, USA, Inc., et al., State Court
of Oklahoma County, CJ-2009-10759 (filed October 30, 2009); |
| |
| |
• |
|
Lucretia Ballou v. Muscletech Research and Development, Inc., et al., U.S. District
Court, Western District of Louisiana, 09CV1996 (filed December 3, 2009); |
| |
| |
• |
|
Clinton Davis v. GNC Corporation, et al., U.S. District Court, Eastern District of
Pennsylvania, 09CV5055 (filed November 11, 2009); |
| |
| |
• |
|
Michael Fyalka v. Iovate Health Sciences, et al., U.S. District Court, Southern
District of Illinois, 09CV944 (filed November 10, 2009); |
| |
| |
• |
|
Monica Fay Stepter v. Iovate Health Sciences, USA, Inc., et al., 17th
Judicial District Court, Parish of LaFourche, Louisiana (filed November 25, 2009); |
34
| |
• |
|
Andrew Nolley v. Muscletech Research and Development, et al., U.S. District Court,
Northern District of Mississippi, 09CV140 (filed December 18, 2009); |
| |
| |
• |
|
Kerry Donald v. Iovate Health Sciences Group, et al., Supreme Court of the State of
New York, Kings County (filed January 22, 2010); |
| |
| |
• |
|
Casey Slyter v. GNC Corporation, et al., U.S. District Court, District of Kansas,
10CV2065 (filed January 29, 2010); and |
| |
| |
• |
|
Debra Rutherford, et al. v. Muscletech Research and Development, Inc., U.S. District
Court, Northern District of Alabama, 10CV370 (filed February 19, 2010). |
Plaintiffs in the Owens and Stasiak cases dismissed those cases without prejudice in June 2009.
Plaintiff Perez filed a Notice of Non-suit without prejudice which was granted by court order on
November 18, 2009. Plaintiff Noyola amended the original complaint in December 2009 and did not
identify GNC as a defendant.
The following six putative class actions generally include claims of consumer fraud,
misrepresentation, strict liability, and breach of warranty:
| |
• |
|
Andrew Dremak, et al. v. Iovate Health Sciences Group, Inc., et al., U.S. District
Court, Southern District of California, 09CV1088 (filed May 19, 2009); |
| |
| |
• |
|
Enjoli Pennier, et al. v. Iovate Health Sciences, et al., U.S. District Court,
Eastern District of Louisiana, 09CV3533 (filed May 13, 2009); |
| |
| |
• |
|
Alejandro M. Jimenez, et al. v. Iovate Health Sciences, Inc., et al., U.S. District
Court, Eastern District of California, 09CV1473 (filed May 28, 2009); |
| |
| |
• |
|
Amy Baker, et al. v. Iovate Health Sciences USA, Inc., et al., U.S. District Court,
Northern District of Alabama, 09CV872 (filed May 4, 2009); |
| |
| |
• |
|
Kyle Davis and Sara Carreon, et al. v. Iovate Health Sciences USA, Inc., et al.,
U.S. District Court, Northern District of Alabama, 09CV896 (filed May 7, 2009); and |
| |
| |
• |
|
Lenny Charles Gunn, Tonya Rhoden, and Nicholas Atelevich, et al., v. Iovate Health
Sciences Group, Inc., et al., U.S. District Court, Southern District of California,
09CV2337 (filed October 24, 2009). |
By court order dated October 6, 2009, the United States Judicial Panel on Multidistrict Litigation
consolidated pretrial proceedings of many of the pending actions (including the above-listed GNC
class actions) in the Southern District of California (In re: Hydroxycut Marketing and Sales
Practices Litigation, MDL No. 2087). Any liabilities that may arise from these matters are not
probable or reasonably estimable at this time.
Bedell Claim. On May 1, 2009, plaintiff Eugene Bedell, Jr. filed a personal injury complaint
against, among others, General Nutrition Centers, Inc. and GNC Corporation, in Circuit Court of
Loudoun County, Virginia. Plaintiff alleges that his use and consumption of a non-GNC product
called “Nitro T3” caused him to have a stroke. Plaintiff makes certain allegations regarding the
risks of using and consuming Nitro T3 and GNC’s alleged failure to warn consumers of those risks.
Plaintiff seeks unspecified monetary damages. Under its purchasing agreement with the vendor,
WellNx, GNC tendered the matter to WellNx for defense and indemnification. WellNx has accepted
GNC’s request for defense and indemnification. Any liabilities that may arise from this matter are
not probable or reasonably estimable at this time.
Chen Claim. On April 30, 2009, plaintiff Yuging “Phillis” Chen filed a Third Amended Complaint
against, among others, Nutra Manufacturing, Inc., in the Superior Court of California for the
County of Los Angeles. Plaintiff alleges that her use and consumption of various products,
including Mega Garlic Plus and Herbalifeline, manufactured by Nutra Manufacturing, caused personal
injuries. Plaintiff asserts, among other things, claims for strict liability, negligence, and
fraud, and seeks unspecified monetary damages. Any liabilities that may arise from this matter are
not probable or reasonably estimable at this time.
35
California False Labeling and Consumer Fraud Claims. Beginning in May 2009, a series of false
labeling and consumer fraud cases (listed below) were filed in California in connection with label
claims of non-GNC products sold in the Company’s stores.
| |
• |
|
Michael Gonzales and Zia Nawabi, et al. v. Maximum Human Performance, Inc., et al., U.S.
District Court, Central District of California, 09CV532 (filed May 5, 2009) (“Musclemeds”); |
| |
| |
• |
|
Michael Campos and Michael Gonzales, et al. v. LG Sciences, LLC, et al., Superior Court
of California, for the County of Orange, removed to U.S. District Court, Central District
of California, 09CV663 (filed May 20, 2009) (“LG Sciences”); |
| |
| |
• |
|
Nicole Forlenza and Shaiden Monroe, et al. v. Dynakor Pharmacal, LLC, et al., U.S.
District Court, Central District of California, 09CV3730 (filed May 26, 2009) (“Basic
Research — Akävar”); |
| |
| |
• |
|
Vance Monroe and Mac Gonzales, et al. v. Biotab Nutraceuticals, Inc., et al., U.S.
District Court, Southern District of California, 09CV1207 (filed June 3, 2009); |
| |
| |
• |
|
Richard Johnson, et al. v. Vianda, LLC, et al., Superior Court of California, for the
County of Los Angeles, BC 423825 (filed October 20, 2009); and |
| |
| |
• |
|
Michael Flores, et al. v. EP2. Inc., et al., U.S. District Court, Central District of
California, 09CV7872 (filed October 28, 2009). |
The LG Sciences matter was settled and dismissed in August 2009. A settlement was reached and
approved by the Court in December 2009 in the Musclemeds case. Plaintiffs in the Basic Research -
Akävar case dismissed GNC with prejudice in January 2010, after which a similar action, Shalena
Dysthe, et al., v. Basic Research, et al., U.S. District Court, Central District of California,
09CV8013 (filed November 2, 2009) was filed by a different law firm.
While only seven lawsuits of this type have been filed to date, GNC expects the number of these
types of cases to grow, as we have received four additional demand notices of similar claims. In
all instances, the GNC vendors of the products at issue have agreed to defend and indemnify GNC.
Any liabilities that may arise from these matters are not probable or reasonably estimable at this
time.
|
|
|
| ITEM 4. |
|
SUBMISSION OF MATTERS TO A VOTE OF SECURITY HOLDERS. |
None.
36
PART II
|
|
|
| ITEM 5. |
|
MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF
SECURITIES. |
There is no established public trading market for our common stock. As of March 1, 2010, 100
shares of our Common Stock were outstanding, all of which are held by our Parent. As of December
31, 2009, there was one holder of our common stock and there were 39 holders of our ultimate
Parent’s common stock. See Item 12, “Security Ownership of Certain Beneficial Owners and
Management and Related Stockholder Matters” included in this report.
Dividends
In July 2009, our board of directors declared a $13.6 million dividend to our direct parent
company, GNC Corporation, with a payment date of August 30, 2009. GNC Acquisition Holdings, Inc.,
our ultimate parent company, was the final recipient of this dividend. The dividend was paid with
cash generated from operations.
Any dividends that we may pay in the future will be subject to the determination and approval
of our board of directors. We are subject to certain restrictions on our ability to pay dividends
under the terms of the Senior Credit Facility, Senior Notes, and Senior Subordinated Notes.
Securities Authorized for Issuance under Equity Compensation Plans
Upon completion of the Merger, our Parent adopted the GNC Acquisition Holdings Inc. 2007 Stock
Incentive Plan (the “2007 Plan”). The purpose of the 2007 Plan is to enable us to attract, retain,
and reward highly qualified personnel who will contribute to our success. The 2007 Plan provides
for the granting of stock options, restricted stock, and certain other stock-based awards. Awards
under the 2007 Plan may be granted to certain eligible employees, directors, consultants, or
advisors as determined by the administering committee of our ultimate Parent’s board of directors.
As of December 31, 2009 the total number of shares of our ultimate Parent’s Class A common stock
reserved and available under the 2007 Plan is 10,419,178 shares. Stock options granted under the
2007 Plan are granted at not less than fair market value (or, in the case of persons holding more
than 10% of the voting power of us, our ultimate Parent, or our subsidiaries, less than 110% of
fair market value), generally vest over a five-year vesting schedule, and expire after ten years
from date of grant. If any award granted under the 2007 Plan expires, terminates, is canceled, or
is forfeited for any reason, the number of shares underlying such award will become available for
future awards under the 2007 Plan. No awards other than stock options have been granted under the
2007 Plan.
| |
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
Number of |
| |
|
|
|
|
|
|
|
|
|
securities remaining |
| |
|
|
|
|
|
|
|
|
|
available for future |
| |
|
|
|
|
|
|
|
|
|
issuance under |
| |
|
|
|
|
|
|
|
|
|
equity |
| |
|
Number of securities |
|
Weighted-average |
|
compensation plans |
| |
|
to be issued upon |
|
exercise price of |
|
(excluding |
| |
|
exercise of |
|
outstanding |
|
securities |
| |
|
outstanding options, |
|
options, warrants |
|
reflected in first |
| Plan category |
|
warrants and rights |
|
and rights |
|
column) |
Equity compensation
plans approved by
security holders |
|
|
9,263,640 |
1 |
|
$ |
7.27 |
|
|
|
1,155,538 |
|
Equity compensation
plans not approved
by security holders |
|
|
12,750 |
|
|
$ |
5.00 |
2 |
|
|
— |
|
Total |
|
|
9,276,390 |
|
|
$ |
7.27 |
|
|
|
1,155,538 |
|
|
|
|
| (1) |
|
Consists of options to purchase shares of our Parent’s Class A common stock granted
pursuant to the GNC Acquisition Holdings Inc. 2007 Stock Incentive Plan. |
| |
| (2) |
|
Plus accrued and unpaid dividends through the date of exercise. |
37
On September 1, 2009, David Berg joined the Company as its Executive Vice President of
Global Business Development and Chief Operating Officer, International. In connection with his
employment, our Parent’s Compensation Committee granted Mr. Berg certain options (“Preferred Stock
Options”) to purchase 12,750 shares of our ultimate Parent’s Series A Cumulative Preferred Stock,
par value $0.001 per share (“Preferred Stock”), pursuant to the terms of a Preferred Stock Option
Agreement. The Preferred Stock Options are not governed by the 2007 Plan, and the grant was not
approved by our or our Parent’s security holders. The Preferred Stock Options have an exercise
price equal to $5.00 per share (plus accrued and unpaid dividends through the date of exercise). A
portion of the Preferred Stock Options vest on the first anniversary of employment; the balance
vest on the second anniversary; provided, that Mr. Berg is permitted to exercise his vested
Preferred Stock Options only within the seven day period following each vesting date. If Mr. Berg
does not exercise his vested Preferred Stock Options within such exercise period, then such vested
Preferred Stock Options terminate and expire. See Item 11, “Executive Compensation — Employment
and Separation Agreements.”
38
ITEM 6. SELECTED FINANCIAL DATA.
The selected consolidated financial data presented below as of and for the years ended
December 31, 2009 and 2008 and as of December 31, 2007 (the Successor Periods), for the period
March 16 to December 31, 2007, and for the period January 1, 2007 to March 15, 2007 (the
Predecessor Period) are derived from our audited consolidated financial statements and their notes
included in this report. The selected consolidated financial data presented below as of and for
the years ended December 31, 2006 and 2005, are derived from our audited consolidated financial
statements and their notes, which are not included in this report. The selected consolidated
financial data as of March 15, 2007, December 31, 2006, and 2005 and for the period from January 1,
2007 to March 15, 2007 and for the years ended December 31, 2006 and 2005 represent the period
during which General Nutrition Centers, Inc. was owned by Apollo.
On February 8, 2007, our parent corporation entered into an Agreement and Plan of Merger with
GNC Acquisition Inc. and its parent company, GNC Acquisition Holdings, Inc., pursuant to which GNC
Acquisition Inc. agreed to merge with and into GNC Parent Corporation, and as a result GNC Parent
Corporation would continue as the surviving corporation and a wholly owned subsidiary of GNC
Acquisition Holdings Inc. This Merger was accounted for under the purchase method of accounting.
As a result, the financial data presented as of December 31, 2007, and for the period from March
16, 2007 to December 31, 2007 represents the period of operations after the Merger.
As a result of the Merger, the consolidated statement of operations for the successor period
includes the following: interest and amortization expense resulting from the issuance of the Senior
Floating Rate Toggle Notes and the 10.75% Senior Subordinated Notes; and amortization of intangible
assets related to the Merger. Further, as a result of purchase accounting, the fair values of our
assets on the date of the Merger became their new cost basis.
You should read the following financial information together with the information under Item
7, “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our
consolidated financial statements and their related notes.
39
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Successor |
|
|
|
Predecessor |
|
| |
|
Year Ended |
|
|
Year Ended |
|
|
March 16- |
|
|
|
January 1- |
|
|
Year Ended |
|
|
Year Ended |
|
| |
|
December 31, |
|
|
December 31, |
|
|
December 31, |
|
|
|
March 15, |
|
|
December 31, |
|
|
December 31, |
|
| (dollars in millions) |
|
2009 |
|
|
2008 |
|
|
2007 |
|
|
|
2007 |
|
|
2006 |
|
|
2005 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Statement of Operations Data: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Revenue: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Retail |
|
$ |
1,256.3 |
|
|
$ |
1,219.3 |
|
|
$ |
909.3 |
|
|
|
$ |
259.3 |
|
|
$ |
1,122.7 |
|
|
$ |
989.4 |
|
Franchising |
|
|
264.2 |
|
|
|
258.0 |
|
|
|
193.9 |
|
|
|
|
47.2 |
|
|
|
232.3 |
|
|
|
212.8 |
|
Manufacturing/Wholesale |
|
|
186.5 |
|
|
|
179.4 |
|
|
|
119.8 |
|
|
|
|
23.3 |
|
|
|
132.1 |
|
|
|
115.5 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total revenue |
|
|
1,707.0 |
|
|
|
1,656.7 |
|
|
|
1,223.0 |
|
|
|
|
329.8 |
|
|
|
1,487.1 |
|
|
|
1,317.7 |
|
Cost of sales, including costs of warehousing,
distribution and occupancy |
|
|
1,116.4 |
|
|
|
1,082.6 |
|
|
|
814.2 |
|
|
|
|
212.2 |
|
|
|
983.5 |
|
|
|
898.7 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Gross profit |
|
|
590.6 |
|
|
|
574.1 |
|
|
|
408.8 |
|
|
|
|
117.6 |
|
|
|
503.6 |
|
|
|
419.0 |
|
Compensation and related benefits |
|
|
263.0 |
|
|
|
249.8 |
|
|
|
195.8 |
|
|
|
|
64.3 |
|
|
|
260.8 |
|
|
|
228.6 |
|
Advertising and promotion |
|
|
50.0 |
|
|
|
55.1 |
|
|
|
35.0 |
|
|
|
|
20.5 |
|
|
|
50.7 |
|
|
|
44.7 |
|
Other selling, general and administrative |
|
|
96.5 |
|
|
|
98.7 |
|
|
|
71.3 |
|
|
|
|
17.3 |
|
|
|
92.4 |
|
|
|
76.2 |
|
Other expense(income)(1) |
|
|
(0.1 |
) |
|
|
0.7 |
|
|
|
(0.4 |
) |
|
|
|
(0.1 |
) |
|
|
0.5 |
|
|
|
(3.1 |
) |
Merger related costs |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
34.6 |
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating income (loss) |
|
|
181.2 |
|
|
|
169.8 |
|
|
|
107.1 |
|
|
|
|
(19.0 |
) |
|
|
99.2 |
|
|
|
72.6 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Interest expense, net |
|
|
70.0 |
|
|
|
83.0 |
|
|
|
75.5 |
|
|
|
|
43.0 |
|
|
|
39.6 |
|
|
|
43.1 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Income (loss) before income taxes |
|
|
111.2 |
|
|
|
86.8 |
|
|
|
31.6 |
|
|
|
|
(62.0 |
) |
|
|
59.6 |
|
|
|
29.5 |
|
Income tax expense (benefit) |
|
|
41.6 |
|
|
|
32.0 |
|
|
|
12.6 |
|
|
|
|
(10.7 |
) |
|
|
22.2 |
|
|
|
10.9 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income (loss) |
|
$ |
69.6 |
|
|
$ |
54.8 |
|
|
$ |
19.0 |
|
|
|
$ |
(51.3 |
) |
|
$ |
37.4 |
|
|
$ |
18.6 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| (1) |
|
Other expense (income) includes foreign currency (gain) loss for all of the periods
presented. Other expense (income) for the year ended December 31, 2006 included $1.2 million
loss on the sale of our Australian manufacturing facility. Other expense (income) for the
year ended December 31, 2005 included $2.5 million transaction fee income related to the
transfer of our GNC Australian franchise rights to an existing franchisee. |
40
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Successor |
|
|
|
Predecessor |
|
| |
|
Year Ended |
|
|
Year Ended |
|
|
March 16- |
|
|
|
January 1- |
|
|
Year Ended |
|
|
Year Ended |
|
| |
|
December 31, |
|
|
December 31, |
|
|
December 31, |
|
|
|
March 15, |
|
|
December 31, |
|
|
December 31, |
|
| (dollars in millions) |
|
2009 |
|
|
2008 |
|
|
2007 |
|
|
|
2007 |
|
|
2006 |
|
|
2005 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance Sheet Data: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents |
|
$ |
75.1 |
|
|
$ |
42.3 |
|
|
$ |
28.9 |
|
|
|
$ |
9.5 |
|
|
$ |
24.1 |
|
|
$ |
86.0 |
|
Working capital (2) |
|
|
382.6 |
|
|
|
305.1 |
|
|
|
258.1 |
|
|
|
|
233.6 |
|
|
|
249.5 |
|
|
|
298.7 |
|
Total assets |
|
|
2,303.6 |
|
|
|
2,292.0 |
|
|
|
2,239.6 |
|
|
|
|
974.1 |
|
|
|
968.8 |
|
|
|
1,025.6 |
|
Total current and non-current long-term debt |
|
|
1,059.8 |
|
|
|
1,084.7 |
|
|
|
1,087.0 |
|
|
|
|
10.7 |
|
|
|
431.4 |
|
|
|
473.4 |
|
Stockholder’s equity |
|
|
717.5 |
|
|
|
652.1 |
|
|
|
608.7 |
|
|
|
|
680.8 |
|
|
|
312.3 |
|
|
|
340.9 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Other Data: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net cash provided by (used in) operating activities |
|
|
114.0 |
|
|
|
77.4 |
|
|
|
92.0 |
|
|
|
|
(50.9 |
) |
|
|
73.6 |
|
|
|
65.1 |
|
Net cash used in investing activities |
|
|
(42.2 |
) |
|
|
(60.4 |
) |
|
|
(1,671.4 |
) |
|
|
|
(6.2 |
) |
|
|
(23.4 |
) |
|
|
(21.5 |
) |
Net cash (used in) provided by financing
activities |
|
|
(39.3 |
) |
|
|
(3.4 |
) |
|
|
1,598.7 |
|
|
|
|
42.8 |
|
|
|
(112.2 |
) |
|
|
(42.6 |
) |
Capital expenditures |
|
$ |
28.7 |
|
|
$ |
48.7 |
|
|
$ |
28.9 |
|
|
|
$ |
5.7 |
|
|
$ |
23.8 |
|
|
$ |
20.8 |
|
Number of stores (at end of period): |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Company-owned stores (3) |
|
|
2,832 |
|
|
|
2,774 |
|
|
|
2,745 |
|
|
|
|
2,699 |
|
|
|
2,688 |
|
|
|
2,650 |
|
Franchised stores (3) |
|
|
2,216 |
|
|
|
2,144 |
|
|
|
2,056 |
|
|
|
|
2,018 |
|
|
|
2,007 |
|
|
|
2,014 |
|
Franchised store-within-a-store locations (3) |
|
|
1,869 |
|
|
|
1,712 |
|
|
|
1,358 |
|
|
|
|
1,266 |
|
|
|
1,227 |
|
|
|
1,149 |
|
|
|
|
| (2) |
|
Working capital represents current assets less current liabilities. |
41
|
|
|
| (3) |
|
The following table summarizes our stores for the periods indicated: |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Successor |
|
|
|
Predecessor |
| |
|
Year Ended |
|
Year Ended |
|
March 16- |
|
|
January 1- |
|
Year Ended |
|
Year Ended |
| |
|
December 31, |
|
December 31, |
|
December 31, |
|
|
March 15, |
|
December 31, |
|
December 31, |
| |
|
2009 |
|
2008 |
|
2007 |
|
|
2007 |
|
2006 |
|
2005 |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Company-owned stores |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Beginning of period balance |
|
|
2,774 |
|
|
|
2,745 |
|
|
|
2,699 |
|
|
|
|
2,688 |
|
|
|
2,650 |
|
|
|
2,642 |
|
New store openings |
|
|
45 |
|
|
|
71 |
|
|
|
64 |
|
|
|
|
18 |
|
|
|
54 |
|
|
|
35 |
|
Franchise conversions (a)(b) |
|
|
53 |
|
|
|
33 |
|
|
|
44 |
|
|
|
|
17 |
|
|
|
80 |
|
|
|
102 |
|
Store closings(b) |
|
|
(40 |
) |
|
|
(75 |
) |
|
|
(62 |
) |
|
|
|
(24 |
) |
|
|
(96 |
) |
|
|
(129 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
End of period balance |
|
|
2,832 |
|
|
|
2,774 |
|
|
|
2,745 |
|
|
|
|
2,699 |
|
|
|
2,688 |
|
|
|
2,650 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Franchised stores |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Domestic |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Beginning of period balance |
|
|
954 |
|
|
|
978 |
|
|
|
1,022 |
|
|
|
|
1,046 |
|
|
|
1,156 |
|
|
|
1,290 |
|
Store openings (b) |
|
|
31 |
|
|
|
41 |
|
|
|
16 |
|
|
|
|
4 |
|
|
|
5 |
|
|
|
17 |
|
Store closings (c) |
|
|
(76 |
) |
|
|
(65 |
) |
|
|
(60 |
) |
|
|
|
(28 |
) |
|
|
(115 |
) |
|
|
(151 |
) |
End of period balance |
|
|
909 |
|
|
|
954 |
|
|
|
978 |
|
|
|
|
1,022 |
|
|
|
1,046 |
|
|
|
1,156 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
International |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Beginning of period balance |
|
|
1,190 |
|
|
|
1,078 |
|
|
|
996 |
|
|
|
|
961 |
|
|
|
858 |
|
|
|
746 |
|
Store openings |
|
|
187 |
|
|
|
198 |
|
|
|
115 |
|
|
|
|
44 |
|
|
|
169 |
|
|
|
132 |
|
Store closings |
|
|
(70 |
) |
|
|
(86 |
) |
|
|
(33 |
) |
|
|
|
(9 |
) |
|
|
(66 |
) |
|
|
(20 |
) |
End of period balance |
|
|
1,307 |
|
|
|
1,190 |
|
|
|
1,078 |
|
|
|
|
996 |
|
|
|
961 |
|
|
|
858 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Store-within-a-store (Rite Aid) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Beginning of period balance |
|
|
1,712 |
|
|
|
1,358 |
|
|
|
1,266 |
|
|
|
|
1,227 |
|
|
|
1,149 |
|
|
|
1,027 |
|
Store openings |
|
|
177 |
|
|
|
401 |
|
|
|
101 |
|
|
|
|
39 |
|
|
|
80 |
|
|
|
130 |
|
Store closings |
|
|
(20 |
) |
|
|
(47 |
) |
|
|
(9 |
) |
|
|
|
— |
|
|
|
(2 |
) |
|
|
(8 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
End of period balance |
|
|
1,869 |
|
|
|
1,712 |
|
|
|
1,358 |
|
|
|
|
1,266 |
|
|
|
1,227 |
|
|
|
1,149 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total Stores |
|
|
6,917 |
|
|
|
6,630 |
|
|
|
6,159 |
|
|
|
|
5,983 |
|
|
|
5,922 |
|
|
|
5,813 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| (a) |
|
Stores that were acquired from franchisees and subsequently converted into
company-owned stores. |
| |
| (b) |
|
Includes corporate store locations acquired by franchisees. |
| |
| (c) |
|
Includes franchised stores closed and acquired by us. |
42
|
|
|
| ITEM 7. |
|
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATION. |
You should read the following discussion in conjunction with Item 6, “Selected Financial Data”
and our consolidated financial statements and accompanying notes included in this report. The
discussion in this section contains forward-looking statements that involve risks and
uncertainties. See Item 1A, “Risk Factors” in this report for a discussion of important factors
that could cause actual results to differ materially from those described or implied by the
forward-looking statements contained herein. Please refer to “Forward Looking Statements” included
elsewhere in this report.
Business Overview
We are a leading global specialty retailer of nutritional supplements, which include VMHS,
sports nutrition products, diet products, and other wellness products. We derive our revenues
principally from product sales through our company-owned stores and online through www.gnc.com,
franchise activities, and sales of products manufactured in our facilities to third parties. We
sell products through a worldwide network of more than 6,900 locations operating under the GNC
brand name.
Revenues and Operating Performance from our Business Segments
We measure our operating performance primarily through revenues and operating income from our
three business segments, Retail, Franchise, and Manufacturing/Wholesale, and through the management
of unallocated costs from our warehousing, distribution and corporate segments, as follows:
| |
• |
|
Retail revenues are generated by sales to consumers at our company-owned stores
and online through www.gnc.com. Although we believe that our retail and franchise
businesses are not seasonal in nature, historically we have experienced, and expect to
continue to experience, a variation in our net sales and operating results from quarter
to quarter, with the first half of the year being stronger than the second half of the
year. As a leader in our industry, we expect our retail revenue growth to be consistent
with projected industry growth as a result of our disproportionate market share, scale
economies in purchasing and advertising, strong brand awareness, and vertical
integration. |
| |
| |
• |
|
Franchise revenues are generated primarily from: |
| |
(1) |
|
product sales to our franchisees; |
| |
| |
(2) |
|
royalties on franchise retail sales; and |
| |
| |
(3) |
|
franchise fees, which are charged for initial franchise awards, renewals,
and transfers of franchises. |
Since we do not anticipate the number of our domestic franchised stores to grow substantially,
we expect our domestic franchise revenue growth will be generated by royalties on increased
franchise retail sales and product sales to our existing franchisees. We expect that an increase in
the number of our international franchised stores over the next five years will result in increased
initial franchise fees associated with new store openings and increased revenues from product sales
to new franchisees. As international franchise trends continue to improve, we also anticipate that
franchise revenue from international operations will be driven by increased product sales to our
franchisees. Since our international franchisees pay royalties to us in U.S. dollars, any
strengthening of the U.S. dollar relative to our franchisees’ local currency may offset some of the
growth in royalty revenue.
| |
• |
|
Manufacturing/wholesale revenues are generated through sales of manufactured
products to third parties, generally for third-party private label brands, and the sale
of our proprietary and third-party products to and through Rite Aid and
www.drugstore.com. License fee revenue from the opening of GNC franchised
store-within-a-store locations within Rite Aid stores is also recorded in this
segment. Our revenues generated by our manufacturing and wholesale operations are subject
to our available manufacturing capacity. |
43
A significant portion of our business infrastructure is comprised of fixed operating costs.
Our vertically integrated distribution network and manufacturing capacity can support higher sales
volume without significant incremental costs. We therefore expect our operating expenses to grow at
a lesser rate than our revenues, resulting in significant operating leverage in our business.
The following trends and uncertainties in our industry could positively or negatively affect
our operating performance:
| |
• |
|
broader consumer awareness of health and wellness issues and rising healthcare
costs; |
| |
| |
• |
|
interest in, and demand for, condition-specific products based on scientific
research; |
| |
| |
• |
|
significant effects of favorable and unfavorable publicity on consumer demand; |
| |
| |
• |
|
lack of a single product or group of products dominating any one product
category; |
| |
| |
• |
|
lack of long-term experience with human consumption of ingredients of some of
our products; |
| |
| |
• |
|
volatility in the diet category; |
| |
| |
• |
|
rapidly evolving consumer preferences and demand for new products; |
| |
| |
• |
|
costs associated with complying with new and existing governmental regulation;
and |
| |
| |
• |
|
a change in disposable income available to consumers, as a result of current
economic conditions. |
Executive Overview
Our recent results of operations continue the favorable trend seen in recent years. In the
retail segment, our domestic retail comparable store sales, including e-commerce, increased 2.8%
for 2009 as compared to 2008. Included in this increase is a 29.9% increase in our www.gnc.com
business. We achieved these results in a difficult economic environment, when many other retailers
were posting negative comparable store sales, and despite a short-term reduction in revenue that we
experienced in 2009 due to the Hydroxycut recall. Our core product categories of VMHS and Sports
Nutrition have delivered strong consistent performance. Another reason for our strong results is
the emphasis we place on innovation and the introduction of new products. For example, clinical
testing and research provided the framework for the 2009 introduction of Pro Performance AMP, a
product line targeted at avid athletes. Additionally, the WELLbeING product line launched in
early 2009 provides formulations and vitamin products uniquely designed for women. These products
were introduced with the intention of attracting new customers to our stores.
Our www.gnc.com business continues to grow, with a 29.9% increase in revenue in 2009 compared
with 2008. We redesigned our website in the latter part of 2009, enhancing the site’s
functionality through improved search capabilities, navigation and expanded content. We expect
these enhancements to allow us to continue to grow our revenue through increased traffic and
conversion rates.
Our domestic franchise business is aligned with our corporate operating standards, and key
performance indicators to our domestic franchise stores are consistent with our company store
results. We continue to see growth in sales of our GNC brand products, particularly in the higher
margin Pro Performance brand and in sales of our proprietary Vitapaks, which has helped to
strengthen the franchise system. Without committing our own capital resources, our international
franchise system has continued
44
to grow and strengthen our presence globally, with the addition of 117 and 112 net new locations
in 2009 and 2008, respectively. For 2009, the international franchise business recognized a 9.8%
increase in revenue, compared with 2008, primarily on the strength of higher product sales.
Our manufacturing strategy is designed to provide our stores with proprietary products at the
lowest possible cost, and utilize additional capacity to promote production efficiencies and
enhance our position in the third party contract business. Under this strategy, our third party
manufacturing sales grew 38.7% and 3.8% in 2008 and 2009, respectively, over the prior year
periods. We expect continuing steady performance from our manufacturing plant utilizing this
strategy.
We believe that the strength of our brand and our leadership position in the health and
wellness sector provide significant future opportunities to capitalize on favorable demographics
and consumer trends. In our experience, our customers have continued to focus on their personal
health and well-being during economic downturns; nonetheless, a continued downturn or an uncertain
outlook in the economy may materially and adversely affect our business and financial results.
While our 2009 results do not reflect a negative impact due to the general downturn of the
economy, the downturn could affect our business and operating results in the future. Our results
depend on a number of factors impacting consumer spending, including, but not limited to, general
economic and business conditions, consumer confidence, consumer debt levels, availability of
consumer credit, and the level of customer traffic within malls and other shopping environments.
Consumer purchases of products, including ours and those of our partners, may decline during
recessionary periods.
If consumer purchases of products decline, we could be impacted in the following ways:
| |
• |
|
retail sales at our company stores and through our website could decline; |
| |
| |
• |
|
demand for our branded products produced at our manufacturing plant could
decline; |
| |
| |
• |
|
demand for products produced for distributors and other retailers / wholesalers
could diminish; |
| |
| |
• |
|
our domestic franchisees may choose not to renew their franchise licenses,
which in turn would lower our franchise product revenue; and |
| |
| |
• |
|
our international franchisees may experience decreased revenue resulting in
lower royalties and product revenue to us; additionally, a strengthening of the U.S.
dollar may impact us, as our international franchisees purchase inventory from and pay
royalties to us in U.S. dollars. |
In May 2009, the FDA warned consumers to stop using certain Hydroxycut products, produced by
Iovate Health Sciences, Inc., which were sold in our stores. Iovate issued a voluntary recall,
with which we immediately fully complied. Sales of the recalled Hydroxycut products amounted to
approximately $57.8 million, or 4.7% of our retail sales in 2008 and $18.8 million, or 4.2% of our
retail sales in the first four months of 2009. We provided refunds or gift cards to consumers who
returned these products to our stores. In the second quarter, we experienced a reduction in sales
and margin due to the recall as a result of accepting returns of products from customers and a loss
of sales as a Hydroxycut replacement product was not available. Through December 31, 2009, we had
refunded approximately $3.5 million to our retail customers and approximately $1.6 million to our
wholesale customers for Hydroxycut product returns. A significant majority of the retail refunds
occurred in our second quarter; the wholesale refunds were recognized in the early part of the
third quarter. All returns of product by our customers were recognized as a reduction in sales in
the period when the return occurred. At the end of June, Iovate launched new reformulated
Hydroxycut products that we began to sell in our stores. Although post-recall sales of the new
reformulated Hydroxycut trailed pre-recall levels, strong sales in our core sports, vitamins and
herbs products, along with other new third party diet products, helped to mitigate the decrease in
sales from the Hydroxycut product line.
In light of these matters, we continue to focus on our core strategies. In the near term, we
will concentrate on our primary strategies, in particular:
45
| |
• |
|
Driving top-line performance in each of our business segments by attracting new
customers through product innovation and the introduction of new, scientifically
backed products,
improved product assortment, effective marketing campaigns designed to increase
traffic and awareness of the GNC brand, and price competitiveness; |
| |
| |
• |
|
Investing in key infrastructure areas for future growth, including e-commerce
and international development; and |
| |
| |
• |
|
Generating efficiencies and cost savings in the everyday operations of the
business that will allow us to leverage profit margins on modest revenue growth. |
We will continue to seek improvements in each of the business segments and position ourselves
for long term growth.
Related Parties
Management Services Agreement. Upon consummation of the Merger, the Company entered into a
management services agreement with our Parent. Under the agreement, our Parent provides the
Company and its subsidiaries with certain services in exchange for an annual fee of $1.5 million,
as well as customary fees for services rendered in connection with certain major financial
transactions, plus reimbursement of expenses and a tax gross-up relating to a non-tax deductible
portion of the fee. The Company provides customary indemnifications to our Parent and its
affiliates and those providing services on its behalf. In addition, upon consummation of the
Merger, the Company incurred an aggregate fee of $10.0 million, plus reimbursement of expenses,
payable to our Parent for services rendered in connection with the Merger. For each of the years
ended December 31, 2008 and 2009, $1.5 million was paid pursuant to this agreement.
Credit Facility. Upon consummation of the Merger, the Company entered into a $735.0 million
credit agreement, under which various fund portfolios related to one of our sponsors, Ares, are
lenders. As of December 31, 2009, certain affiliates of Ares held approximately $62.1 million of
term loans under the Company’s Senior Credit Facility.
Lease Agreements. General Nutrition Centres Company, a wholly owned subsidiary of the
Company, is party to 21 lease agreements, as lessee, with Cadillac Fairview Corporation, as lessor,
with respect to properties located in Canada (the “Lease Agreements”). Cadillac Fairview
Corporation is a direct, wholly owned subsidiary of OTPP, one of the principal stockholders of
Parent. For the years ended December 31, 2009 and 2008, the Company paid $2.4 million and $2.5
million, respectively, under the Lease Agreements. Each lease was negotiated in the ordinary
course of business on an arm’s length basis.
Product Purchases. During our 2009 fiscal year, we purchased certain fish oil and probiotics
products manufactured by Lifelong Nutrition, Inc. (“Lifelong”) for resale under our proprietary
brand name WELLbeING. Carmen Fortino, who serves as one of our directors, is the Managing
Director, a member of the Board of Directors and a stockholder of Lifelong. The aggregate value of
the products we purchased from Lifelong was $3.3 million for the 2009 fiscal year.
Results of Operations
The following information presented as of December 31, 2009, 2008, and 2007 and for the years
ended December 31, 2009 and 2008, for the period March 16, 2007 to December 31, 2007, and for the
period January 1 to March 15, 2007 was derived from our audited consolidated financial statements
and accompanying notes.
The following information may contain financial measures other than in accordance with
generally accepted accounting principles, and should not be considered in isolation from or as a
substitute for our historical consolidated financial statements. In addition, the adjusted
combined operating results may not reflect the actual results we would have achieved absent the
adjustments and may not be predictive of future results of operations. We present this information
because we use it to monitor and evaluate our ongoing operating results and trends, and believe it
provides investors with an understanding of our operating performance over comparative periods.
46
As discussed in the “Segment” note to our consolidated financial statements, we evaluate
segment operating results based on several indicators. The primary key performance indicators are
revenues and operating income or loss for each segment. Revenues and operating income or loss, as
evaluated by management, exclude certain items that are managed at the consolidated level, such as
warehousing and transportation costs, impairments, and other corporate costs. The following
discussion compares the revenues and the operating income or loss by segment, as well as those
items excluded from the segment totals.
Same store sales growth reflects the percentage change in same store sales in the period
presented compared to the prior year period. Same store sales are calculated on a daily basis for
each store and exclude the net sales of a store for any period if the store was not open during the
same period of the prior year. Beginning in the first quarter of 2006, we also included our
internet sales, as generated through www.gnc.com and www.drugstore.com, in our domestic
company-owned same store sales calculation. When a store’s square footage has been changed as a
result of reconfiguration or relocation in the same mall or shopping center, the store continues to
be treated as a same store. If, during the period presented, a store was closed, relocated to a
different mall or shopping center, or converted to a franchised store or a company-owned store,
sales from that store up to and including the closing day or the day immediately preceding the
relocation or conversion are included as same store sales as long as the store was open during the
same period of the prior year. We exclude from the calculation sales during the period presented
from the date of relocation to a different mall or shopping center and from the date of a
conversion. In the second quarter of 2006, we modified the calculation method for domestic
franchised same store sales consistent with this description, which has been the method
historically used for domestic company-owned same store sales.
Results of Operations
(Dollars in millions and percentages expressed as a percentage of total net revenues)
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Successor |
|
|
Predecessor |
|
| |
|
Year Ended December 31, |
|
|
Year Ended December 31, |
|
|
March 16 - December 31, |
|
|
January 1 - March 15, |
|
| |
|
2009 |
|
|
2008 |
|
|
2007 |
|
|
2007 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Revenues: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Retail |
|
$ |
1,256.3 |
|
|
|
73.6 |
% |
|
$ |
1,219.3 |
|
|
|
73.6 |
% |
|
$ |
909.3 |
|
|
|
74.3 |
% |
|
$ |
259.3 |
|
|
|
78.6 |
% |
Franchise |
|
|
264.2 |
|
|
|
15.5 |
% |
|
|
258.0 |
|
|
|
15.6 |
% |
|
|
193.9 |
|
|
|
15.9 |
% |
|
|
47.2 |
|
|
|
14.3 |
% |
Manufacturing / Wholesale |
|
|
186.5 |
|
|
|
10.9 |
% |
|
|
179.4 |
|
|
|
10.8 |
% |
|
|
119.8 |
|
|
|
9.8 |
% |
|
|
23.3 |
|
|
|
7.1 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total net revenues |
|
|
1,707.0 |
|
|
|
100.0 |
% |
|
|
1,656.7 |
|
|
|
100.0 |
% |
|
|
1,223.0 |
|
|
|
100.0 |
% |
|
|
329.8 |
|
|
|
100.0 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating expenses: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cost of sales, including warehousing,
distribution and occupancy costs |
|
|
1,116.4 |
|
|
|
65.4 |
% |
|
|
1,082.6 |
|
|
|
65.3 |
% |
|
|
814.2 |
|
|
|
66.5 |
% |
|
|
212.2 |
|
|
|
64.4 |
% |
Compensation and related benefits |
|
|
263.0 |
|
|
|
15.4 |
% |
|
|
249.8 |
|
|
|
15.1 |
% |
|
|
195.8 |
|
|
|
16.0 |
% |
|
|
64.3 |
|
|
|
19.5 |
% |
Advertising and promotion |
|
|
50.0 |
|
|
|
2.9 |
% |
|
|
55.1 |
|
|
|
3.3 |
% |
|
|
35.0 |
|
|
|
2.9 |
% |
|
|
20.5 |
|
|
|
6.2 |
% |
Other selling, general and administrative
expenses |
|
|
86.7 |
|
|
|
5.1 |
% |
|
|
87.8 |
|
|
|
5.3 |
% |
|
|
62.1 |
|
|
|
5.1 |
% |
|
|
16.5 |
|
|
|
5.0 |
% |
Amortization expense |
|
|
9.8 |
|
|
|
0.6 |
% |
|
|
10.9 |
|
|
|
0.7 |
% |
|
|
9.2 |
|
|
|
0.7 |
% |
|
|
0.8 |
|
|
|
0.2 |
% |
Foreign currency loss (gain) |
|
|
(0.1 |
) |
|
|
0.0 |
% |
|
|
0.7 |
|
|
|
0.0 |
% |
|
|
(0.4 |
) |
|
|
0.0 |
% |
|
|
(0.1 |
) |
|
|
0.0 |
% |
Transaction related costs |
|
|
— |
|
|
|
0.0 |
% |
|
|
— |
|
|
|
0.0 |
% |
|
|
— |
|
|
|
0.0 |
% |
|
|
34.6 |
|
|
|
10.5 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total operating expenses |
|
|
1,525.8 |
|
|
|
89.4 |
% |
|
|
1,486.9 |
|
|
|
89.7 |
% |
|
|
1,115.9 |
|
|
|
91.2 |
% |
|
|
348.8 |
|
|
|
105.8 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating income: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Retail |
|
|
153.1 |
|
|
|
9.0 |
% |
|
|
140.9 |
|
|
|
8.5 |
% |
|
|
106.5 |
|
|
|
8.8 |
% |
|
|
28.2 |
|
|
|
8.6 |
% |
Franchise |
|
|
80.8 |
|
|
|
4.7 |
% |
|
|
80.8 |
|
|
|
4.9 |
% |
|
|
55.0 |
|
|
|
4.5 |
% |
|
|
14.5 |
|
|
|
4.4 |
% |
Manufacturing / Wholesale |
|
|
73.5 |
|
|
|
4.3 |
% |
|
|
67.4 |
|
|
|
4.1 |
% |
|
|
38.9 |
|
|
|
3.2 |
% |
|
|
10.3 |
|
|
|
3.1 |
% |
Unallocated corporate and other
costs: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Warehousing and distribution costs |
|
|
(53.6 |
) |
|
|
-3.1 |
% |
|
|
(54.2 |
) |
|
|
-3.3 |
% |
|
|
(40.7 |
) |
|
|
-3.3 |
% |
|
|
(10.7 |
) |
|
|
-3.2 |
% |
Corporate costs |
|
|
(72.6 |
) |
|
|
-4.3 |
% |
|
|
(65.1 |
) |
|
|
-3.9 |
% |
|
|
(52.6 |
) |
|
|
-4.4 |
% |
|
|
(26.7 |
) |
|
|
-8.2 |
% |
Merger related costs |
|
|
— |
|
|
|
0.0 |
% |
|
|
— |
|
|
|
0.0 |
% |
|
|
— |
|
|
|
0.0 |
% |
|
|
(34.6 |
) |
|
|
-10.5 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Subtotal unallocated corporate and
other costs, net |
|
|
(126.2 |
) |
|
|
-7.4 |
% |
|
|
(119.3 |
) |
|
|
-7.2 |
% |
|
|
(93.3 |
) |
|
|
-7.7 |
% |
|
|
(72.0 |
) |
|
|
-21.9 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total operating income (loss) |
|
|
181.2 |
|
|
|
10.6 |
% |
|
|
169.8 |
|
|
|
10.3 |
% |
|
|
107.1 |
|
|
|
8.8 |
% |
|
|
(19.0 |
) |
|
|
-5.8 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Interest expense, net |
|
|
70.0 |
|
|
|
|
|
|
|
83.0 |
|
|
|
|
|
|
|
75.5 |
|
|
|
|
|
|
|
43.0 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Income (loss) before income taxes |
|
|
111.2 |
|
|
|
|
|
|
|
86.8 |
|
|
|
|
|
|
|
31.6 |
|
|
|
|
|
|
|
(62.0 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Income tax expense (benefit) |
|
|
41.6 |
|
|
|
|
|
|
|
32.0 |
|
|
|
|
|
|
|
12.6 |
|
|
|
|
|
|
|
(10.7 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income (loss) |
|
$ |
69.6 |
|
|
|
|
|
|
$ |
54.8 |
|
|
|
|
|
|
$ |
19.0 |
|
|
|
|
|
|
$ |
(51.3 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Note: The numbers in the above table have been rounded to millions. All calculations
related to the Results of Operations for the year-over-year comparisons below were derived from
unrounded data and could occasionally differ immaterially if you were to use the table above for
these calculations.
47
Comparison of the Years Ended December 31, 2009 and 2008
Revenues
Our consolidated net revenues increased $50.3 million, or 3.0%, to $1,707.0 million for the
year ended December 31, 2009 compared to $1,656.7 million for the same period in 2008. The
increase was the result of increased sales in all of our segments.
Retail. Revenues in our Retail segment increased $37.0 million, or 3.0%, to $1,256.3 million
for the year ended December 31, 2009 compared to $1,219.3 million for the same period in 2008. The
increase from 2008 to 2009 included an increase of $10.8 million for sales through www.gnc.com.
Sales increases occurred in the major product categories of VMHS and sports nutrition. Sales in
the diet category were negatively impacted by the Hydroxycut recall that occurred in May 2009. Our
domestic company-owned same store sales, including our internet sales, improved by 2.8% for the
year ended December 31, 2009. Our Canadian company-owned stores had improved same store sales of
0.8%, in local currency, for the year ended December 31, 2009 but declined when measured in U.S.
dollars due to the volatility of the U.S. dollar. Our company-owned store base increased by 51
domestic stores to 2,665 compared to 2,614 at December 31, 2008, primarily due to new store
openings and franchise store acquisitions, and by 7 Canadian stores to 167 at December 31, 2009
compared to 160 at December 31, 2008.
Franchise. Revenues in our Franchise segment increased $6.2 million, or 2.4%, to $264.2
million for the year ended December 31, 2009 compared to $258.0 million for the same period in
2008. Domestic franchise revenue decreased by $1.4 million for the year ended December 31, 2009 as
increased product sales were more than offset by lower franchise fee revenue. Domestic royalty
income was flat despite operating 45 fewer stores during 2009 as compared to 2008. There were 909
stores at December 31, 2009 compared to 954 stores at December 31, 2008. International franchise
revenue increased by $7.6 million
for the year ended December 31, 2009 as a result of increases in product sales, partially
offset by lower franchise fee revenue. International royalty income increased $0.5 million for the
2009 period compared to the 2008 period as sales increases in our franchisees’ respective local
currencies were impacted by the strengthening of the U.S. dollar from 2008 to 2009. Our
international franchise store base increased by 117 stores to 1,307 at December 31, 2009 compared
to 1,190 at December 31, 2008.
Manufacturing/Wholesale. Revenues in our Manufacturing/Wholesale segment, which includes
third-party sales from our manufacturing facility in South Carolina, as well as wholesale sales to
Rite Aid and www.drugstore.com, increased $7.1 million, or 4.0%, to $186.5 million for the year
ended December 31, 2009 compared to $179.4 million for 2008. Sales increased in the South Carolina
plant by $4.4 million, and revenues associated with Rite Aid increased by $1.4 million. This
increase was due to increases in wholesale and consignment sales to Rite Aid of $4.6 million,
partially offset by lower initial and renewal license fee revenue of $3.2 million as a result of
Rite Aid opening 197 fewer franchise store-within-a-stores in 2009 as compared to 2008. In
addition, sales to www.drugstore.com increased by $1.3 million in 2009 as compared to 2008.
Cost of Sales
Consolidated cost of sales, which includes product costs, costs of warehousing and
distribution and occupancy costs, increased $33.8 million, or 3.1%, to $1,116.4 million for the
year ended December 31, 2009 compared to $1,082.6 million for the same period in 2008.
Consolidated cost of sales, as a percentage of net revenue, was 65.4% for the year ended December
31, 2009 as compared to 65.3% for the year ended December 31, 2008.
48
Product costs. Product costs increased $29.5 million, or 3.6%, to $840.0 million for the
year ended December 31, 2009 compared to $810.5 million for the same period in 2008 as a result of
increased sales volumes and raw materials costs. Consolidated product costs, as a percentage of
net revenue, were 49.2% for the year ended December 31, 2009 as compared to 48.9% for the year
ended December 31, 2008.
Warehousing and distribution costs. Warehousing and distribution costs increased $0.1 million,
or 0.2%, to $57.6 million for the year ended December 31, 2009 compared to $57.5 million for the
same period in 2008. The increase was primarily due to increased internet fulfillment wages offset
by decreases in fuel costs. Consolidated warehousing and distribution costs, as a percentage of
net revenue, were 3.4% and 3.5% for the year ended December 31, 2009 and 2008, respectively.
Occupancy costs. Occupancy costs increased $4.2 million, or 2.0%, to $218.8 million for the
year ended December 31, 2009 compared to $214.6 million for the same period in 2008. This increase
was the result of increases in depreciation expense of $2.9 million and lease related costs of $1.4
million, offset by $0.1 million decrease in other costs. Consolidated occupancy costs, as a
percentage of net revenue, were 12.8% and 13.0% for the year ended December 31, 2009 and 2008,
respectively.
Selling, General and Administrative (“SG&A”) Expenses
Our consolidated SG&A expenses, including compensation and related benefits, advertising and
promotion expense, other selling, general and administrative expenses, and amortization expense,
increased $5.9 million, or 1.5%, to $409.5 million, for the year ended December 31, 2009 compared
to $403.6 million for the same period in 2008. These expenses, as a percentage of net revenue,
were 24.0% for the year ended December 31, 2009 compared to 24.4% for the year ended December 31,
2008.
Compensation and related benefits. Compensation and related benefits increased $13.2 million,
or 5.3%, to $263.0 million for the year ended December 31, 2009 compared to $249.8 million for the
same period in 2008. The increase was due to: (1) $8.5 million in base wages to support our
increased store base and sales volume and to comply with the increases in minimum wage rates; (2)
$1.4 million in health insurance; (3) $1.2 million in commissions and incentive expense; and (4)
other wage related expenditures of $1.0 million. In addition, 2008 expenses included a $1.1
million reduction in base wages due to a change in our vacation policy effective March 31, 2008.
Advertising and promotion. Advertising and promotion expenses decreased $5.1 million, or
9.1%, to $50.0 million for the year ended December 31, 2009 compared to $55.1 million during the
same period in 2008. Advertising expense decreased primarily as a result of decreases in media
costs of $2.3 million and print advertising costs of $3.4 million, partially offset by increases in
other advertising costs of $0.6 million.
Other SG&A. Other SG&A expenses, including amortization expense, decreased $2.2 million or
2.3%, to $96.5 million for the year ended December 31, 2009 compared to $98.7 million for the year
ended December 31, 2008. Decreases in bad debt expense of $2.3 million, amortization expense of
$1.2 million and other selling expenses of $0.3 million were partially offset by increases in
telecommunications expenses of $1.9 million due to the installation of a new point of sale (“POS”)
register system in 2008 and professional fees of $0.8 million. In addition, 2009 other SG&A
includes a $1.1 million gain from proceeds received from the Visa/Mastercard antitrust litigation
settlement.
Foreign Currency (Loss) Gain
Foreign currency loss/gain for the year ended December 31, 2009 and 2008, resulted primarily
from accounts payable activity with our Canadian subsidiary. We recognized income of $0.1 million
for the year ended December 31, 2009 and a loss of $0.7 million for the year ended December 31,
2008.
49
Operating Income
As a result of the foregoing, consolidated operating income increased $11.4 million or 6.7% to
$181.2 million for the year ended December 31, 2009 compared to $169.8 million for the same period
in 2008. Operating income, as a percentage of net revenue, was 10.6% for the year ended December
31, 2009 and 10.3% for the year ended December 31, 2008.
Retail. Operating income increased $12.2 million, or 8.7%, to $153.1 million for the year
ended December 31, 2009 compared to $140.9 million for the same period in 2008. The increase was
primarily the result of higher dollar margins on increased sales volumes and reduced advertising
spending, partially offset by increases in occupancy costs, compensation costs and other SG&A
expenses.
Franchise. Operating income is unchanged at $80.8 million for each of the years ended December
31, 2009 and 2008.
Manufacturing/Wholesale. Operating income increased $6.1 million, or 9.0%, to $73.5 million
for the year ended December 31, 2009 compared to $67.4 million for the same period in 2008. This
increase was primarily the result of increased margins from our South Carolina manufacturing
facility, partially offset by decreases in Rite Aid license fee revenue.
Warehousing and Distribution Costs. Unallocated warehousing and distribution costs decreased
$0.6 million, or 1.3%, to $53.6 million for the year ended December 31, 2009 compared to $54.2
million for the same period in 2008. The decrease was primarily due to decreases in fuel costs.
Corporate Costs. Corporate overhead costs increased $7.5 million, or 11.7%, to $72.6 million
for the year ended December 31, 2009 compared to $65.1 million for the same period in 2008. The
increase was primarily due to an increase in compensation expense and professional fees in 2009.
In addition, 2008 compensation expense includes a $1.1 million reduction due to a change in our
vacation policy effective March 31, 2008.
Interest Expense
Interest expense decreased $13.0 million, or 15.7%, to $70.0 million for the year ended
December 31, 2009 compared to $83.0 million for the same period in 2008. This decrease was
primarily attributable to decreases in interest rates on our variable rate debt in 2009 as compared
to 2008 and $25.3 million in principal payments during 2009.
Income Tax Expense
We recognized $41.6 million of income tax expense (or 37.4% of pre-tax income) during the year
ended December 31, 2009 compared to $32.0 million (or 36.9% of pre-tax income) for the same period
of 2008. For the year ended December 31, 2009, a $0.5 million discrete tax benefit was recorded
while a $2.0 million discrete tax benefit was recorded for the year ended December 31, 2008.
Net Income
As a result of the foregoing, consolidated net income increased $14.8 million to $69.6 million
for the year ended December 31, 2009 compared to $54.8 million for the same period in 2008.
50
Comparison of the Year Ended December 31, 2008 and the Successor Period 2007 — March 16, 2007 to
December 31, 2007
The following discussion compares the results of operations of the year ended December 31, 2008 to
the Successor Period March 16, 2007 to December 31, 2007 (“2007 Successor Period”). Our results of
operations from period to period may not be comparable because the 2007 Sucessor Period was only
291 days.
Revenues
Our consolidated net revenues increased $433.7 million, or 35.5%, to $1,656.7 million for the
year ended December 31, 2008 compared to $1,223.0 million for the 2007 Successor Period, due to
increased sales in each of our segments, and a result of comparing a 366 day period to a 291 day
period.
Retail. Revenues in our Retail segment increased $310.0 million, or 34.1%, to $1,219.3
million for the year ended December 31, 2008 compared to $909.3 million for the 2007 Successor
Period. The increase from the 2007 Successor Period to the year ended December 31, 2008 included
an increase of $14.4 million of sales through www.gnc.com. Sales increases occurred in each of the
major product categories of VMHS and sports nutrition. Our company-owned store base increased by
16 domestic stores to 2,614 compared to 2,598 at December 31, 2007, primarily due to new store
openings and franchise store acquisitions, and by 13 Canadian stores to 160 at December 31, 2008
compared to 147 at December 31, 2007.
Franchise. Revenues in our Franchise segment increased $64.1 million, or 33.1%, to $258.0
million for the year ended December 31, 2008 compared to $193.9 million for the 2007 Successor
Period. The increase is primarily due to comparing a 366 day period to a 291 day period. Domestic
franchise revenue increased $42.1 million as a result of increased product sales despite operating
24 fewer stores during 2008 compared to 2007. There were 954 stores at December 31, 2008 compared
to 978 stores at December 31, 2007. International franchise revenue increased $22.0 million as a
result of increased product sales and royalties. Our international franchise store base increased
by 112 stores to 1,190 at December 31, 2008 compared to 1,078 at December 31, 2007.
Manufacturing/Wholesale. Revenues in our Manufacturing/Wholesale segment, which includes
third-party sales from our manufacturing facilities in South Carolina, as well as wholesale sales
to Rite Aid and www.drugstore.com, increased $59.6 million, or 49.7%, to $179.4 million for the
year ended December 31, 2008 compared to $119.8 million for the 2007 Successor Period. Sales
increased in the South Carolina plant by $46.6 million, and revenues associated with Rite Aid
increased by $11.4 million due primarily due to increased license fees as a result of Rite Aid
opening 401 stores for the year ended December 31, 2008 as opposed to 101 stores for the 2007
Successor Period.
Cost of Sales
Consolidated cost of sales, which includes product costs, costs of warehousing and
distribution and occupancy costs, increased $268.4 million, or 33.0%, to $1,082.6 million for the
year ended December 31, 2008 compared to $814.2 million for the 2007 Successor Period. The
increase is primarily due to comparing a 366 day period to a 291 day period. Consolidated cost of
sales, as a percent of net revenue, decreased to 65.3% in the year ended December 31, 2008 compared
to 66.5% for the 2007 Successor Period.
Product costs. Product costs increased $201.4 million, or 33.1%, to $810.5 million for the
year ended December 31, 2008 compared to $609.1 million for the 2007 Successor Period. The
increase is primarily due to comparing a 366 day period to a 291 day period. To a lesser extent,
the increased product costs were the result of increased sales volumes and rising raw material
prices. Product costs for the year ended December 31, 2007 include $15.5 million of non-cash
expense from amortization of inventory step up to fair value due to the Merger. Consolidated
product costs, as a percentage of net revenue, declined to 48.9% for the year ended December 31,
2008 compared to 49.8% for the 2007 Successor Period.
51
Warehousing and distribution costs. Warehousing and distribution costs increased $14.6
million, or 34.1%, to $57.5 million for the year ended December 31, 2008 compared to $42.9 million
for the 2007 Successor Period. The increase is primarily due to comparing a 366 day period to a
291 day period. To a lesser extent, the increase was due to increases in shipping and fuel costs,
and an increase in wages to support internet fulfillment. Consolidated warehousing and
distribution costs, as a percentage of net revenue, were 3.5% for both the year ended December 31,
2008 and the 2007 Successor Period.
Occupancy costs. Occupancy costs increased $52.4 million, or 32.3%, to $214.6 million for the
year ended December 31, 2008 compared to $162.2 million for the 2007 Successor Period. The
increase is primarily due to comparing a 366 day period to a 291 day period. Additionally, we
recognized higher lease related costs, primarily as a result of scheduled increases in our store
leases, and the addition of 29 corporate stores since December 31, 2007, and an increase in
depreciation expense related to these same additional stores. Consolidated occupancy costs, as a
percentage of net revenue, were 13.0% for the year ended December 31, 2008 compared to 13.3% for
the 2007 Successor Period.
Selling, General and Administrative (“SG&A”) Expenses
Our consolidated SG&A expenses, including compensation and related benefits, advertising and
promotion expense, other selling, general and administrative expenses, and amortization expense
increased $101.5 million, or 33.6%, to $403.6 million for the year ended December 31, 2008 compared
to $302.1 million for the 2007 Successor Period. The increase is primarily due to comparing a 366
day period to a 291 day period. These expenses, as a percentage of net revenues, were 24.4% for
the year ended December 31, 2008 compared to 24.7% for the 2007 Successor Period.
Compensation and related benefits. Compensation and related benefits increased $54.0 million,
or 27.6%, to $249.8 million for the year ended December 31, 2008 compared to $195.8 million for the
2007 Successor Period. The increase is primarily due to comparing a 366 day period to a 291 day
period. Secondarily, full-time and part-time wages increased to support an increased sales volume
and store base. Compensation and related benefits, as a percentage of net revenues, was 15.1% for
the year ended December 31, 2008 compared to 16.0% for the 2007 Successor Period.
Advertising and promotion. Advertising and promotion expenses increased $20.1 million, or
57.3%, to $55.1 million for the year ended December 31, 2008 compared to $35.0 million for the 2007
Successor Period. The increase is primarily due to comparing a 366 day period to a 291 day period.
Advertising and promotion costs, as a percentage of net revenues, were 3.3% for the year ended
December 31, 2008 compared to 2.9% for the 2007 Successor Period. This increase is primarily
attributable to an increase in agency fees and media advertising.
Other SG&A. Other SG&A expenses, including amortization expense, increased $27.4 million, or
38.6%, to $98.7 million for the year ended December 31, 2008 compared to $71.3 million for the 2007
Successor Period. Other SG&A expenses, as a percentage of net revenues were 6.0% for the year
ended December 31, 2008 compared to 5.8% for the 2007 Successor Period. The increase is due to
comparing a 366 day period to a 291 day period. Additionally, the increase is attributable to
additional third party commission expense on higher commission sales, higher telecommunications
expense due to the installation of a new POS register system, and higher bad debt expense. Other
SG&A expenses, as a percentage of net revenues were 6.0% for the year ended December 31, 2008
compared to 5.8% for the 2007 Successor Period.
Foreign Currency (Loss) Gain
Foreign currency loss/gain for the year ended December 31, 2008 and the 2007 Successor Period
resulted primarily from accounts payable activity with our Canadian subsidiary. We incurred a loss
of $0.7 million for the year ended December 31, 2008 compared to a gain of $0.4 million for the
2007 Successor Period.
52
Operating Income
As a result of the foregoing, consolidated operating income increased $62.7 million, or 58.5%,
to $169.8 million for the year ended December 31, 2008 compared to $107.1 million for the 2007
Successor Period. Operating income, as a percentage of net revenue, was 10.3% for the year ended
December 31, 2008 compared to 8.8% for the 2007 Successor Period.
Retail. Operating income increased $34.4 million, or 32.4%, to $140.9 million for the year
ended December 31, 2008 compared to $106.5 million for the 2007 Successor Period. Included in the
2007 Successor Period was $9.6 million of amortization of inventory and lease step up adjustments
as a result of the Merger. The increase in operating income was primarily the result of comparing
a 366 day period to a 291 day period. Additionally, higher dollar margins on increased sales
volumes and lower operating expenses, as a percentage of revenue, contributed to the increase in
operating income.
Franchise. Operating income increased $25.8 million, or 46.9%, to $80.8 million for the year
ended December 31, 2008 compared to $55.0 million for the 2007 Successor Period. Included in the
2007 Successor Period was $0.1 million of amortization of inventory step up adjustments as a result
of the Merger. The increase in operating income was primarily the result of comparing a 366 day
period to a 291 day period. Additionally, the increase was due to an increase in margins related
to higher wholesale sales to our international and domestic franchisees, offset by increases in
intangible amortization as a result of the Merger, and higher bad debt expense.
Manufacturing/Wholesale. Operating income increased $28.5 million, or 73.1%, to $67.4 million
for the year ended December 31, 2008 compared to $38.9 million for the 2007 Successor Period.
Included in the 2007 Successor Period was $5.7 million of amortization of inventory step up
adjustments as a result of the Merger. The increase in operating income was primarily the result
of comparing a 366 day period to a 291 day period. Additionally, the increase in operating income
was the result of improved margins on our third-party contract sales and increases in Rite Aid
license fee revenue.
Warehousing and distribution costs. Unallocated warehousing and distribution costs increased
$13.5 million, or 33.3%, to $54.2 million for the year ended December 31, 2008 compared to $40.7
million for the 2007 Successor Period. The increase in costs was primarily the result of
comparing a 366 day period to a 291 day period. Additionally, the increase in cost was due to
increases in fuel and shipping costs, and additional wages to support our internet fulfillment
business.
Corporate costs. Corporate costs increased $12.5 million, or 23.8%, to $65.1 million for the
year ended December 31, 2008 compared to $52.6 million for the 2007 Successor Period. The
increase in costs was primarily the result of comparing a 366 day period to a 291 day period.
Interest Expense
Interest expense increased $7.5 million, or 9.9%, to $83.0 million for the year ended December
31, 2008 compared to $75.5 million for the 2007 Successor Period. This increase is primarily
the result of comparing a 366 day period to a 291 day period.
Income Tax Expense
We recognized $32.0 million of consolidated income tax expense (or 36.9% of pre-tax income)
during the year ended December 31, 2008 compared to $12.6 million (or 39.9% of pre-tax income) for
the 2007 Successor Period. The effective tax rate for the year ended December 31, 2008 was
approximately 36.9%, which includes discrete tax benefits of $2.0 million. The effective tax rate
for the 2007 Successor Period was approximately 39.9%.
53
Net Income
As a result of the foregoing, consolidated net income increased $35.8 million, or 188.5%, to
$54.8 million for the year ended December 31, 2008 compared to $19.0 million for the 2007 Successor
Period.
Predecessor Period 2007 — January 1, 2007 to March 15, 2007
Because the basic operations of the Company did not change as a result of the Merger, there were no
significant differences in the Company’s financial results of operations and operational trends for
the predecessor period of January 1, 2007 to March 15, 2007, except for the following:
| |
• |
|
Compensation and related benefits included $3.8 million of non-cash stock based
compensation generated as a result of the cancellation of all stock options at the merger
date; $9.6 million in accelerated discretionary payments made to vested option holders
(including the associated payroll taxes), and $1.9 million in incentives related to the
Merger. |
| |
| |
• |
|
Merger related costs included costs incurred by our parent, and recognized by us, in
relation to the Merger, of $34.6 million. These costs were comprised of selling-related
expenses of $26.4 million, a contract termination fee paid to our previous owner of $7.5
million, and other costs of $0.7 million. |
| |
| |
• |
|
Interest expense included the write-off of $34.8 million of call premiums and deferred
fee write-offs as a result of the Merger. |
As a result of the additional Merger related expenses described above, we recognized a $10.7
million tax benefit for the period from January 1, 2007 to March 15, 2007.
Liquidity and Capital Resources
At
December 31, 2009, we had $75.1 million in cash and cash equivalents and $382.6 million in
working capital compared with $42.3 million in cash and cash equivalents and $305.1 million in
working capital at December 31, 2008. The $77.5 million increase in our working capital was driven
by increases in our inventory and cash and a decrease in our accrued interest, accounts payable and
current portion of long-term debt. This was offset by a decrease in our deferred taxes.
We expect to fund our operations through internally generated cash and, if necessary, from
borrowings under our $60.0 million revolving credit facility (the “Revolving Credit Facility”). At
December 31, 2009, we had $52.1 million available under the Revolving Credit Facility, after giving
effect to $7.9 million utilized to secure letters of credit. In September 2008, Lehman Brothers
Holdings Inc. (“Lehman”), whose subsidiaries have a $6.3 million credit commitment under our
Revolving Credit Facility, filed for bankruptcy. We do not expect that Lehman will fund its pro
rata share of the borrowing as required under the facility. If other financial institutions that
have extended credit commitments to us are adversely affected by the condition of the U.S. and
international capital markets, they may become unable to fund borrowings under the Revolving Credit
Facility, which could have a material and adverse impact on our financial condition and our ability
to borrow additional funds, if needed, for working capital, capital expenditures, acquisitions, and
other corporate purposes.
We expect our primary uses of cash in the near future will be debt service requirements,
capital expenditures and working capital requirements. In July 2009, our board of directors
declared a $13.6 million dividend to our direct parent company, GNC Corporation, with a payment
date of August 30, 2009. GNC Acquisition Holdings, Inc., our ultimate parent company, was the
final recipient of this dividend. The dividend was paid with cash generated from operations.
We currently anticipate that cash generated from operations, together with amounts available
under the Revolving Credit Facility (excluding Lehman’s commitment), will be sufficient for the
term of the facility, which matures on March 15, 2012, to meet our operating expenses, capital
expenditures and debt service
obligations as they become due. However, our ability to make scheduled payments of principal
on, to pay interest on, or to refinance our debt and to satisfy our other debt obligations
54
will
depend on our future operating performance, which will be affected by general economic, financial
and other factors beyond our control. We are currently in compliance with our debt covenant
reporting and compliance obligations under the Revolving Credit Facility.
Cash Provided by Operating Activities
Cash provided by operating activities was $114.0 million, $77.4 million, $92.0 million and
($50.9) million during the years ended December 31, 2009 and 2008, the 2007 Successor Period, and
the period from January 1, 2007 to March 15, 2007, respectively. The primary reason for the changes
in each year was the change in net income between each of the periods and changes in working
capital accounts. Net income increased $14.8 million for the year ended December 31, 2009 compared
with the same period in 2008. Net income increased $35.8 million for the year ended December 31,
2008 compared with the 2007 Successor Period; however, inventory increases during 2008 offset the
increase in net income.
For the year ended December 31, 2009, inventory increased $15.7 million, as a result of
increases in our finished goods and a decrease in our reserves. Accounts payable decreased $28.1
million, primarily due to the timing of disbursements. Accrued liabilities increased by $0.8
million, primarily the result of increased deferred revenue.
For the year ended December 31, 2008, inventory increased $48.2 million, as a result of
increases in our finished goods and work in process inventories. Accounts payable increased $22.0
million, primarily the result of increases in inventory. Accrued liabilities decreased by $16.1
million, primarily the result of decreases in accrued payroll related to the timing of the pay with
year end.
For the 2007 Successor Period, inventory decreased $4.0 million, as a result of increases in
our finished goods and a decrease in our reserves. Franchise notes receivable decreased $2.6
million for the 2007 Successor Period, as a result of payments on existing notes, a reduction in
our receivable portfolio, fewer company-financed franchise store openings and more store closings.
Accrued liabilities increased by $29.0 million, primarily the result of increases in accrued
interest on debt and increases in accrued payroll.
Cash Used in Investing Activities
We used cash from investing activities of $42.2 million, $60.4 million, $1,671.3 million, and
$6.2 million for the years ended December 31, 2009 and 2008, the 2007 Successor Period, and the
period from January 1, 2007 to March 15, 2007, respectively. We used cash of $11.3 million, $10.8
million and $1,642.1 million during the years ended December 31, 2009 and 2008, and the 2007
Successor Period, respectively, related to the Merger. Capital expenditures, which were primarily
for improvements to our retail stores and our South Carolina manufacturing facility and which
represent the majority of our remaining cash used in investing activities, were $28.7 million,
$48.7 million, $28.8 million and $5.7 million, during the years ended December 31, 2009 and 2008,
the 2007 Successor Period, and the period from January 1, 2007 to March 15, 2007, respectively. In
2008, we invested $1.0 million in the purchase of certain intangible assets from a third party.
Cash Used in Financing Activities
We used cash of $39.3 million in 2009 for payments on long-term debt, including $3.8 million
for an excess cash payment in March 2009 under the requirements of the 2007 Senior Credit Facility.
This payment was calculated based on 2008 financial results as defined by the 2007 Senior Credit
Facility. In addition, we repaid the outstanding $5.4 million balance on the Revolving Credit
Facility in May 2009 and made $14.0 million in optional repayments on the 2007 Senior Credit
facility ($9.0 million in June 2009 and $5.0 million in December 2009). A $13.6 million dividend
declared in July 2009 was paid in August 2009 to GNC Corporation, our direct parent.
We used cash from financing activities of $8.0 million in 2008 for required payments on long
term
debt and received $5.4 million from borrowings on the Revolving Credit Facility.
55
We received cash from financing activities of $1,598.7 million in the 2007 Successor Period.
The primary uses of this cash were: (1) proceeds from the issuance of the Senior Notes and Senior
Subordinated Notes, (2) borrowings under the Senior Credit Facility, and (3) issuance of new
equity.
$735.0 Million Senior Credit Facility. The Senior Credit Facility consists of a $675.0
million term loan facility and a $60.0 million Revolving Credit Facility. As of December 31, 2009
and 2008, $7.9 million and $6.2 million was pledged to secure letters of credit, respectively. The
term loan facility will mature in September 2013. The Revolving Credit Facility will mature in
March 2012. The Senior Credit Facility permits us to prepay a portion or all of the outstanding
balance without incurring penalties (except LIBOR breakage costs). Subject to certain exceptions,
commencing in fiscal 2008, the Credit Agreement requires that 100% of the net cash proceeds from
certain asset sales, casualty insurance, condemnations and debt issuances, and a specified
percentage (ranging from 50% to 0% based on a defined leverage ratio) of excess cash flow (as
defined in the agreement) for each fiscal year must be used to pay down outstanding borrowings.
GNC Corporation, our direct parent company, and our existing and future direct and indirect
domestic subsidiaries have guaranteed our obligations under the Senior Credit Facility. In
addition, the Senior Credit Facility is collateralized by first priority pledges (subject to
permitted liens) of our equity interests and the equity interests of our domestic subsidiaries.
All borrowings under the Senior Credit Facility bear interest, at our option, at a rate per
annum equal to (i) the higher of (x) the prime rate (as publicly announced by JPMorgan Chase Bank,
N.A. as its prime rate in effect) and (y) the federal funds effective rate, plus 0.50% per annum
plus, at December 31, 2008, in each case, applicable margins of 1.25% per annum for the term loan
facility and 1.0% per annum for the revolving credit facility or (ii) adjusted LIBOR plus 2.25% per
annum for the term loan facility and 2.0% per annum for the revolving credit facility. In addition
to paying interest on outstanding principal under the Senior Credit Facility, we are required to
pay a commitment fee to the lenders under the Revolving Credit Facility in respect of unutilized
revolving loan commitments at a rate of 0.50% per annum.
The Senior Credit Facility contains customary covenants, including incurrence covenants and
certain other limitations on the ability of GNC Corporation, us, and our subsidiaries to incur
additional debt, guarantee other obligations, grant liens on assets, make investments or
acquisitions, dispose of assets, make optional payments or modifications of other debt instruments,
pay dividends or other payments on capital stock, engage in mergers or consolidations, enter into
sale and leaseback
transactions, enter into arrangements that restrict our and our subsidiaries’ ability to pay
dividends or grant liens, engage in transactions with affiliates, and change the passive holding
company status of GNC Corporation.
The Senior Credit Facility contains events of default, including (subject to customary cure
periods and materiality thresholds) defaults based on (1) the failure to make payments under the
senior credit facility when due, (2) breaches of covenants, (3) inaccuracies of representations and
warranties, (4) cross-defaults to other material indebtedness, (5) bankruptcy events, (6) material
judgments, (7) certain matters arising under the Employee Retirement Income Security Act of 1974,
as amended, (8) the actual or asserted invalidity of documents relating to any guarantee or
security document, (9) the actual or asserted invalidity of any subordination terms supporting the
Senior Credit Facility, and (10) the occurrence of a change in control. If any such event of
default occurs, the lenders would be entitled to accelerate the facilities and take various other
actions, including all actions permitted to be taken by a collateralized creditor. If certain
bankruptcy events occur, the facilities will automatically accelerate.
Senior Toggle Notes. In connection with the Merger, we completed a private offering of $300.0
million of our Senior Floating Rate Toggle Notes due 2014 (the “Senior Notes”). The Senior Notes
are our senior non collateralized obligations and are effectively subordinated to all of our
existing and future collateralized debt, including the Senior Credit Facility, to the extent of the
assets securing such debt, rank equally with all our existing and future non collateralized senior
debt and rank senior to all our existing and future senior subordinated debt, including the Senior
Subordinated Notes (as defined below). The Senior Notes are guaranteed on a senior non
collateralized basis by each of our existing and future domestic subsidiaries (as defined in the
Senior Notes indenture). If we fail to make payments on the Senior Notes, the
notes guarantors must make them instead.
56
We may elect in our sole discretion to pay interest on the Senior Notes in cash, entirely by
increasing the principal amount of the Senior Notes or issuing Senior Notes (“PIK interest”), or on
50% of the outstanding principal amount of the Senior Notes in cash and on 50% of the outstanding
principal amount of the Senior Notes by increasing the principal amount of the Senior Notes or by
issuing Senior Notes (“partial PIK interest”). Cash interest on the Senior Notes accrues at
six-month LIBOR plus 4.5% per annum, and PIK interest, if any, accrues at six-month LIBOR plus
5.25% per annum. If we elect to pay PIK interest or partial PIK interest, it will increase the
principal amount of the Senior Notes or issue Senior Notes in an aggregate principal amount equal
to the amount of PIK interest for the applicable interest payment period (rounded up to the nearest
$1,000) to holders of the Senior Notes on the relevant record date. To date, we have elected to
pay cash interest. The Senior Notes are treated as having been issued with original issue discount
for U.S. federal income tax purposes.
We may redeem some or all of the Senior Notes at any time, at specified redemption prices. If
we experience certain kinds of changes in control, we must offer to purchase the Senior Notes at
101% of par plus accrued interest to the purchase date.
The Senior Notes indenture contains certain limitations and restrictions on our and our
restricted subsidiaries’ ability to incur additional debt beyond certain levels, pay dividends,
redeem or repurchase our stock or subordinated indebtedness or make other distributions, dispose of
assets, grant liens on assets, make investments or acquisitions, engage in mergers or
consolidations, enter into arrangements that restrict our ability to pay dividends or grant liens,
and engage in transactions with affiliates. In addition, the Senior Notes indenture restricts our
and certain of our subsidiaries’ ability to declare or pay dividends to our or their stockholders.
10.75% Senior Subordinated Notes. In connection with the Merger, we completed a private
offering of $110.0 million of our 10.75% Senior Subordinated Notes due 2015 (the “Senior
Subordinated Notes”). The Senior Subordinated Notes are our senior subordinated non collateralized
obligations and are subordinated to all our existing and future senior debt, including our Senior
Credit Facility and the Senior Notes and rank equally with all of our existing and future senior
subordinated debt and rank senior to all our existing and future subordinated debt. The Senior
Subordinated Notes are guaranteed on a senior subordinated non collateralized basis by each of our
existing and future domestic subsidiaries (as defined in the Senior Subordinated Notes indenture).
If we fail to make payments on the Senior Subordinated Notes, the notes guarantors must make them
instead. Interest on the Senior Subordinated Notes accrues at the rate of 10.75% per year from
March 16, 2007 and is payable semi-annually in arrears on March 15 and September 15 of each year,
beginning on September 15, 2007.
We may redeem some or all of the Senior Subordinated Notes at any time, at specified
redemption prices. If we experience certain kinds of changes in control, we must offer to purchase
the Senior Subordinated Notes at 101% of par plus accrued interest to the purchase date.
The Senior Subordinated Notes indenture contains certain limitations and restrictions on our
and our restricted subsidiaries’ ability to incur additional debt beyond certain levels, pay
dividends, redeem or repurchase our stock or subordinated indebtedness or make other distributions,
dispose of assets, grant liens on assets, make investments or acquisitions, engage in mergers or
consolidations, enter into arrangements that restrict our ability to pay dividends or grant liens,
and engage in transactions with affiliates. In addition, the Senior Subordinated Notes indenture
restricts our and certain of our subsidiaries’ ability to declare or pay dividends to our
stockholders.
57
Contractual Obligations
The following table summarizes our future minimum non-cancelable contractual obligations at
December 31, 2009:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Payments due by period |
| |
|
|
|
|
|
Less than |
|
|
|
|
|
|
| (in millions) |
|
Total |
|
1 year |
|
1-3 years |
|
4-5 years |
|
After 5 years |
| |
Long-term debt obligations (1) |
|
$ |
1,061.8 |
|
|
$ |
1.5 |
|
|
$ |
11.7 |
|
|
$ |
938.6 |
|
|
$ |
110.0 |
|
Scheduled interest payments (2) |
|
|
221.4 |
|
|
|
61.3 |
|
|
|
101.4 |
|
|
|
56.3 |
|
|
|
2.4 |
|
Operating lease obligations (3) |
|
|
426.7 |
|
|
|
106.5 |
|
|
|
154.6 |
|
|
|
85.0 |
|
|
|
80.6 |
|
Purchase commitments (4)(5) |
|
|
20.6 |
|
|
|
9.0 |
|
|
|
5.1 |
|
|
|
3.1 |
|
|
|
3.4 |
|
| |
|
|
|
|
$ |
1,730.5 |
|
|
$ |
178.3 |
|
|
$ |
272.8 |
|
|
$ |
1,083.0 |
|
|
$ |
196.4 |
|
| |
|
|
|
|
|
| (1) |
|
These balances consist of the following debt obligations: (a) $644.6
million for the Senior Credit Facility based on a variable interest
rate; (b) $300.0 million for our Senior Notes based on a variable
interest rate; (c) $110.0 million for our Senior Subordinated Notes
with a fixed interest rate; and (d) $7.2 million for our mortgage with
a fixed interest rate. Repayment of the Senior Credit Facility is
based on the current amortization schedule and does not take into
account any unscheduled payments that may occur due to our future cash
positions. |
| |
| (2) |
|
The interest that will accrue on the long-term obligations includes
variable rate payments, which are estimated using the associated LIBOR
index as of December 31, 2009. The Senior Credit Facility uses the
three month LIBOR index while the Senior Notes uses the six month
LIBOR index. Also included in the scheduled interest payments is the
activity associated with our interest rate swap agreements which also
use the three month LIBOR index and the six month LIBOR index. |
| |
| (3) |
|
These balances consist of the following operating leases: (a) $407.3
million for company-owned retail stores; (b) $62.4 million for
franchise retail stores, which is offset by $62.4 million of sublease
income from franchisees; and (c) $19.4 million relating to various
leases for tractors/trailers, warehouses, automobiles, and various
equipment at our facilities. |
| |
| (4) |
|
These balances consist of $6.8 million of advertising, $0.5 million in
inventory commitments, $2.5 million of required spending for website
redesign and $10.8 million related to a management services agreement.
In connection with the Merger, we entered into a management services
agreement with our parent, GNC Acquisition Holdings Inc., pursuant to
which we agreed to pay an annual fee of $1.5 million in consideration
for certain management and advisory services. See Item 13, “Certain
Relationships and Related Transactions — Management Services
Agreement.” |
| |
| (5) |
|
We are unable to make a reasonably reliable estimate as to when cash
settlement with taxing authorities may occur for our unrecognized tax
benefits. Also, certain other long term liabilities, included in our
consolidated balance sheet relate principally to the fair value of our
interest rate swap agreement, and rent escalation liabilities, and we
are unable to estimate the timing of these payments. Therefore, these
long term liabilities are not included in the table above. See Note
5, “Income Taxes,” and Note 14, “Other Long Term Liabilities,” to the
Consolidated Financial Statements for additional information. |
In addition to the obligations scheduled above, we have entered into employment agreements
with
certain executives that provide for compensation and certain other benefits. Under certain
circumstances, including a change of control, some of these agreements provide for severance or
other payments, if those circumstances would ever occur during the term of the employment
agreement.
58
Off Balance Sheet Arrangements
As of December 31, 2009 and 2008, we had no relationships with unconsolidated entities or
financial partnerships, such as entities often referred to as structured finance or special purpose
entities, which would have been established for the purpose of facilitating off balance sheet
arrangements, or other contractually narrow or limited purposes. We are, therefore, not materially
exposed to any financing, liquidity, market, or credit risk that could arise if we had engaged in
such relationships.
We had a balance of unused barter credits on account with a third-party barter agency, which
were generated by exchanging inventory with a third-party barter vendor. In exchange, the barter
vendor supplied us with barter credits. We did not record a sale on the transaction as the
inventory sold was for expiring products that were previously fully reserved for on our balance
sheet. In accordance with the standard on nonmonetary transactions, a sale is recorded based on
either the value given up or the value received, whichever is more easily determinable. The value
of the inventory was determined to be zero, as the inventory was fully reserved. Therefore, these
credits were not recognized on the balance sheet and would only have been realized if we purchased
services or products through the bartering company. The barter credits expired as of March 31,
2009.
Effect of Inflation
Inflation generally affects us by increasing costs of raw materials, labor, and equipment. We
do not believe that inflation had any material effect on our results of operations in the periods
presented in our consolidated financial statements.
Critical Accounting Estimates
You should review the significant accounting policies described in the notes to our
consolidated financial statements under the heading ‘Basis of Presentation and Summary of
Significant Accounting Policies’’ included elsewhere in this report.
Use of Estimates
Certain amounts in our financial statements require management to use estimates, judgments,
and assumptions that affect the reported amounts of assets and liabilities at the date of the
financial statements and the reported amounts of revenues and expenses during the periods
presented. Our accounting policies are described in the notes to our consolidated financial
statements under the heading ‘Basis of Presentation and Summary of Significant Accounting
Policies’’ included elsewhere in this report. Our critical accounting policies and estimates are
described in this section. An accounting estimate is considered critical if:
| |
• |
|
the estimate requires management to make assumptions about matters that were
uncertain at the time the estimate was made; |
| |
| |
• |
|
different estimates reasonably could have been used; or |
| |
| |
• |
|
changes in the estimate that would have a material impact on our financial condition
or our results of operations are likely to occur from period to period. |
Management believes that the accounting estimates used are appropriate and the resulting
balances are reasonable. However, actual results could differ from the original estimates,
requiring adjustments to these balances in future periods.
59
Revenue Recognition
We operate primarily as a retailer, through company-owned stores, franchised stores, and to a
lesser extent, as a wholesaler. On December 28, 2005, we started recognizing revenue through
product sales on our website, www.gnc.com. We apply the provisions of the standard on revenue
recognition, which requires the following:
| |
• |
|
Persuasive evidence of an arrangement exists. |
| |
| |
• |
|
Delivery has occurred or services have been rendered. |
| |
| |
• |
|
The price is fixed or determinable. |
| |
| |
• |
|
Collectability is reasonably assured. |
We recognize revenues in our Retail segment at the moment a sale to a customer is recorded.
Gross revenues are reduced by actual customer returns and a provision for estimated future customer
returns, which is based on management’s estimates after a review of historical customer returns. We
recognize revenues on product sales to franchisees and other third parties when the risk of loss,
title and insurable risks have transferred to the franchisee or third-party. We recognize revenues
from franchise fees at the time a franchised store opens or at the time of franchise renewal or
transfer, as applicable.
Inventories
Where necessary, we provide estimated allowances to adjust the carrying value of our inventory
to the lower of cost or net realizable value. These estimates require us to make approximations
about the future demand for our products in order to categorize the status of such inventory items
as slow moving, obsolete, or in excess of need. These future estimates are subject to the ongoing
accuracy of management’s forecasts of market conditions, industry trends, and competition. We are
also subject to volatile changes in specific product demand as a result of unfavorable publicity,
government regulation, and rapid changes in demand for new and improved products or services.
Accounts Receivable and Allowance for Doubtful Accounts
The majority of our retail revenues are received as cash or cash equivalents. The majority of
our franchise revenues are billed to the franchisees with varying terms for payment. We offer
financing to qualified domestic franchisees with the initial purchase of a franchise location. The
notes are demand notes, payable monthly over periods of five to seven years. We generate a
significant portion of our revenue from ongoing product sales to franchisees and third-party
customers. An allowance for doubtful accounts is established based on regular evaluations of our
franchisees’ and third-party customers’ financial health, the current status of trade receivables,
and any historical write-off experience. We maintain both specific and general reserves for
doubtful accounts. General reserves are based upon our historical bad debt experience, overall
review of our aging of accounts receivable balances, general economic conditions of our industry,
or the geographical regions and regulatory environments of our third-party customers and
franchisees.
Impairment of Long-Lived Assets
Long-lived assets, including fixed assets and intangible assets with finite useful lives, are
evaluated periodically by us for impairment whenever events or changes in circumstances indicate
that the carrying amount of any such asset may not be recoverable. If the sum of the undiscounted
future cash flows is less than the carrying value, we recognize an impairment loss, measured as the
amount by which the carrying value exceeds the fair value of the asset. These estimates of cash
flow require significant management judgment and certain assumptions about future volume, revenue
and expense growth rates, foreign exchange rates, devaluation and inflation. As such, this estimate
may differ from actual cash flows.
60
Self-Insurance
We have procured insurance for such areas as: (1) general liability; (2) product liability;
(3) directors and officers liability; (4) property insurance; and (5) ocean marine insurance. We
are self-insured for such areas as: (1) medical benefits; (2) workers’ compensation coverage in the
State of New York with a stop loss of $250,000; (3) physical damage to our tractors, trailers and
fleet vehicles for field personnel use; and (4) physical damages that may occur at the corporate
store locations. We are not insured for certain property and casualty risks due to the frequency
and severity of a loss, the cost of insurance and the overall risk analysis. Our associated
liability for this self-insurance was not significant as of December 31, 2009 and 2008. Prior to
the Numico acquisition, General Nutrition Companies, Inc. was included as an insured under several
of Numico’s global insurance policies.
We carry product liability insurance with a retention of $3.0 million per claim with an
aggregate cap on retained losses of $10.0 million. We carry general liability insurance with
retention of $110,000 per claim with an aggregate cap on retained losses of $600,000. The majority
of the Company’s workers’ compensation and auto insurance are in a deductible/retrospective plan.
We reimburse the insurance company for the workers compensation and auto liability claims, subject
to a $250,000 and $100,000 loss limit per claim, respectively.
As part of the medical benefits program, we contract with national service providers to
provide benefits to our employees for all medical, dental, vision and prescription drug services.
We then reimburse these service providers as claims are processed from our employees. We maintain a
specific stop loss provision of $250,000 per incident with a maximum limit up to $2.0 million per
participant, per benefit year, respectively. We have no additional liability once a participant
exceeds the $2.0 million ceiling. Our liability for medical claims is included as a component of
accrued benefits as described in Note 10, “Accrued Payroll and Related Liabilities,” to our
consolidated financial statements included in this report, and was $2.0 million and $2.2 million as
of December 31, 2009 and 2008, respectively.
Goodwill and Indefinite-Lived Intangible Assets
On an annual basis, we perform a valuation of the goodwill and indefinite lived intangible
assets associated with our operating segments. To the extent that the fair value associated with
the goodwill and indefinite-lived intangible assets is less than the recorded value, we write down
the value of the asset. The valuation of the goodwill and indefinite-lived intangible assets is
affected by, among other things, our business plan for the future, and estimated results of future
operations. Changes in the business plan or operating results that are different than the estimates
used to develop the valuation of the assets may result in an impact on their valuation.
Historically, we have recognized impairments to our goodwill and intangible assets based on
declining financial results and market conditions. The most recent valuation was performed at
October 1, 2009, and no impairment was found. There was also no impairment found during 2009 or
2008. See the “Goodwill and Intangible Assets” note to our consolidated financial statements
included elsewhere in this report. Based upon our improved capitalization of our financial
statements subsequent to the Numico acquisition, the stabilization of our financial condition, our
anticipated future results based on current estimates and current market conditions, we do not
currently expect to incur additional impairment charges in the near future, however, recent global
events could have a negative effect on our business and operating results which could affect the
valuation of our intangibles.
Leases
We have various operating leases for company-owned and franchised store locations and
equipment. Store leases generally include amounts relating to base rental, percent rent and other
charges such as common area maintenance fees and real estate taxes. Periodically, we receive
varying amounts of reimbursements from landlords to compensate us for costs incurred in the
construction of stores. We amortize these reimbursements as an offset to rent expense over the
life of the related lease. We determine the period used for the straight-line rent expense for
leases with option periods and conform it to the term used for amortizing improvements.
61
Income Taxes
We are required to estimate income taxes in each of the jurisdictions in which we operate.
This process involves estimating the actual current tax liability together with assessing temporary
differences in recognition of income (loss) for tax and accounting purposes. These differences
result in deferred tax assets and liabilities, which are included in our consolidated balance
sheet. We then assess the likelihood that the deferred tax assets will be recovered from future
taxable income and, to the extent we believe that recovery is not likely, we establish a valuation
allowance against the deferred tax asset. Further, we operate within multiple taxing jurisdictions
and are subject to audit in these jurisdictions. These audits can involve complex issues which may
require an extended period of time to resolve and could result in additional assessments of income
tax. We believe adequate provisions for income taxes have been made for all periods.
We adopted the update to the standard on income taxes at the beginning of fiscal 2007. As a
result of the adoption of this standard, we recognize liabilities for uncertain tax positions based
on the two-step process prescribed by the interpretation. The first step requires us to determine
if the weight of available evidence indicates that the tax position has met the threshold for
recognition; therefore, we must evaluate whether it is more likely than not that the position will
be sustained on audit, including resolution of any related appeals or litigation processes. The
second step requires us to measure the tax benefit of the tax position taken, or expected to be
taken, in an income tax return as the largest amount that is more than 50% likely of being realized
upon ultimate settlement. This measurement step is inherently difficult and requires subjective
estimations of such amounts to determine the probability of various possible outcomes. We
reevaluate the uncertain tax positions each quarter based on factors including, but not limited to,
changes in facts or circumstances, changes in tax law, effectively settled issues under audit, and
new audit activity. Such a change in recognition or measurement would result in the recognition of
a tax benefit or an additional charge to the tax provision in the period.
Although we believe the measurement of our liabilities for uncertain tax positions is
reasonable, no assurance can be given that the final outcome of these matters will not be different
than what is reflected in the historical income tax provisions and accruals. If additional taxes
are assessed as a result of an audit or litigation, it could have a material effect on our income
tax provision and net income in the period or periods for which that determination is made.
Recently Issued Accounting Pronouncements
In June 2009, the Financial Accounting Standards Board (the “FASB”) issued a standard on
Generally Accepted Accounting Principles. This standard establishes the FASB Accounting Standards
Codification (the “Codification”) as the single source of authoritative nongovernmental accounting
principles generally accepted in the United States of America (“U.S. GAAP”). The Codification is
effective for interim and annual periods ending after September 15, 2009. The adoption of this
standard did not have any impact on our financial statements.
In June 2009, the SEC issued a staff accounting bulletin (“SAB”) that revises or rescinds
portions of the interpretive guidance included in the codification of SABs in order to make the
interpretive guidance consistent with U.S. GAAP. The principal revisions include deletion of
material no longer necessary or that has been superseded because of the issuance of new standards.
We adopted this SAB during the second quarter of 2009; the adoption did not have any impact on our
consolidated financial statements.
Fair Value
In September 2006, the FASB issued new standards on fair value measurements and disclosures.
These new standards define fair value, establish a framework for measuring fair value in accordance
with U.S. GAAP, and expand disclosures about fair value measurements. The new standard applies
under other accounting pronouncements that require or permit fair value measurements and,
accordingly, does not require any new fair value measurements. The original standard was initially
62
effective as of
January 1, 2008, but in February 2008 the FASB delayed the effectiveness date for applying this
standard to nonfinancial assets and nonfinancial liabilities that are not currently recognized or
disclosed at fair value in the financial statements. The standard was effective for fiscal years
beginning after November 15, 2007, except for nonfinancial assets and liabilities that are
recognized or disclosed at fair value in the financial statements on a nonrecurring basis, for
which application has been deferred for one year. We adopted this new standard in the first
quarter of fiscal 2008 for financial assets and liabilities. See Note 23, “Fair Value
Measurements,” to our consolidated financial statements included in this report. We evaluated the
impact of the standard on the valuation of all nonfinancial assets and liabilities, including those
measured at fair value in goodwill, brands, and indefinite lived intangible asset impairment
testing; the adoption had no impact on our consolidated financial statements for the year ended
December 31, 2009.
In February 2007, the FASB issued a new standard on financial instruments that amends a
previously issued standard. This standard expands the use of fair value accounting but does not
affect existing standards which require assets or liabilities to be carried at fair value. The
objective of the standard is to improve financial reporting by providing companies with the
opportunity to mitigate volatility in reported earnings caused by measuring related assets and
liabilities differently without having to apply complex hedge accounting provisions. Under the
standard, a company may elect to use fair value to measure eligible items at specified election
dates and report unrealized gains and losses on items for which the fair value option has been
elected in earnings at each subsequent reporting date. Eligible items include, but are not limited
to, accounts and loans receivable, available-for-sale and held-to-maturity securities, equity
method investments, accounts payable, guarantees, issued debt and firm commitments. The new
standard was effective for fiscal years beginning after November 15, 2007. The adoption of this
standard did not affect the financial statements as we did not elect to use the fair value option.
In October 2008, the FASB issued a new standard on determining the fair value of a financial
asset. This standard clarifies the application of accounting standards in a market that is not
active and provides an example to illustrate key considerations in determining the fair value of a
financial asset when the market for that financial asset is not active. The new standard was
effective upon issuance for financial statements that have not been issued. The adoption of this
new standard did not have any impact on our financial assets and liabilities.
In August 2009, the FASB issued an update to the standard on fair value measurements and
disclosures. This update provides guidance on the manner in which the fair value of liabilities
should be determined. This update is effective for annual periods ending after September 15, 2009.
The adoption of this standard did not have any impact on our financial statements.
Business Combinations and Consolidations
In December 2007, the FASB issued a new standard on business combinations. This new standard
establishes principles and requirements for how an acquirer in a business combination recognizes
and measures in its financial statements the identifiable assets acquired, the liabilities assumed
and any noncontrolling interest in the acquiree at the acquisition date fair value. The new
standard significantly changes the accounting for business combinations in a number of areas,
including the treatment of contingent consideration, preacquisition contingencies, transaction
costs, in-process research and development and restructuring costs. In addition, under this
standard, changes in an acquired entity’s deferred tax assets and uncertain tax positions after the
measurement period will impact the acquirer’s income tax expense. The standard provides guidance
regarding what information to disclose to enable users of the financial statements to evaluate the
nature and financial effects of the business combination. The original standard became effective
for fiscal years beginning after December 15, 2008 with early application prohibited and amends the
standard on income taxes such that adjustments made to deferred taxes and acquired tax
contingencies after January 1, 2009, even for business combinations completed before this date,
will impact net income. We adopted this new standard during the first quarter of fiscal 2009; the
adoption did not have any impact on our consolidated financial statements.
63
In December 2007, the FASB issued a new standard on consolidation. The issuance of this
new standard changes the accounting and reporting for minority interests, which have been
recharacterized as noncontrolling interests and classified as a component of equity. This new
consolidation method significantly changes the accounting for transactions with minority interest
holders. The standard was effective for fiscal years beginning after December 15, 2008 with early
application prohibited. We adopted this new standard during the first quarter of fiscal 2009; the
adoption did not have any impact on our consolidated financial statements.
In June 2009, the FASB issued an update to the standard on consolidations. The standard is
intended to improve financial reporting by providing additional guidance to companies involved with
variable interest entities and by requiring additional disclosures about a company’s involvement in
variable interest entities. This standard is effective for interim and annual periods ending after
January 1, 2010. The adoption of this standard will not have any impact on our financial
statements.
Other
In March 2008, the FASB issued a new standard on derivatives and hedging. The new standard
requires companies with derivative instruments to disclose information that should enable financial
statement users to understand how and why a company uses derivative instruments, how derivative
instruments and related hedged items are accounted for and how derivative instruments and related
hedged items affect a company’s financial position, financial performance, and cash flows. The new
standard was effective for interim and annual periods beginning on or after November 15, 2008. We
adopted this new standard during the first quarter of 2009; the adoption had no impact on our
consolidated financial statements.
In April 2008, the FASB issued a new standard on goodwill and intangibles which amends the
factors that should be considered in developing renewal or extension assumptions used to determine
the useful life of a recognized intangible. The intent of this new standard is to improve the
consistency between the useful life of a recognized intangible asset and the period of expected
cash flows used to measure the fair value of the asset. We adopted this new standard during the
first quarter of fiscal 2009; the adoption did not have a any impact on our consolidated financial
statements.
In April 2009, the FASB issued a new standard on the disclosure of financial instruments.
This standard brings the interpretive guidance into alignment with the changes in U.S. GAAP. We
adopted this standard during the second quarter of fiscal 2009; the adoption did not have any
impact on our consolidated financial statements.
In May 2009, the FASB issued a standard on subsequent events which establishes general
standards of accounting for and disclosing of events that occur after the balance sheet date but
before financial statements are issued or are available to be issued. The intent of this standard
is to incorporate accounting guidance that originated as auditing standards into the body of
authoritative literature issued by the FASB which is consistent with the FASB’s objective to codify
all authoritative U.S. accounting guidance related to a particular topic in one place. We adopted
this standard during the second quarter of 2009; the adoption did not have any impact on our
consolidated financial statements.In September 2009, the FASB issued an update to the standard on
income taxes. This update adds to the definition of a tax position of an entity’s status,
including its status as a pass-through entity, eliminates certain disclosure requirements for
non-public entities, and provides application for pass-through entities. The adoption of this
standard did not have any impact on our financial statements.
64
|
|
|
| ITEM 7A. |
|
QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK. |
Market risk represents the risk of changes in the value of market risk sensitive instruments
caused by fluctuations in interest rates, foreign exchange rates and commodity prices. Changes in
these factors could cause fluctuations in the results of our operations and cash flows. In the
ordinary course of business, we are primarily exposed to foreign currency and interest rate risks.
We do not use derivative financial instruments in connection with these commodity market risks.
We are exposed to market risks from interest rate changes on our variable rate debt. Although
changes in interest rates do not impact our operating income, the changes could affect the fair
value of our interest rate swaps and interest payments. As of December 31, 2009, we had fixed rate
debt of $117.2 million and variable rate debt of $942.6 million. In conjunction with the Merger,
we entered into interest rate swaps, effective April 2, 2007, which effectively convert a portion
of the variable LIBOR component of the effective interest rate on two $150.0 million notional
portions of our debt under our $675.0 million Senior Credit Facility to a fixed rate over a
specified term. Each of these $150.0 million notional amounts has a three month LIBOR tranche
conforming to the interest payment dates on the term loan. During September 2008, we entered into
two new forward agreements with start dates of the expiration dates of the pre-existing interest
rate swap agreements (April 2009 and April 2010). In September 2008, we also entered into a new
interest rate swap agreement with an effective date of September 30, 2008 that effectively
converted an additional notional amount of $100.0 million of debt from a floating to a fixed
interest rate. The $100.0 million notional amount has a three month LIBOR tranche conforming to the
interest payment dates on the term loan. In June 2009 we entered into a new swap agreement with an
effective date of September 15, 2009. The $150.0 million notional amount has a six month LIBOR
tranche conforming to the interest payment dates on the Senior Notes.
These agreements are summarized in the following table:
| |
|
|
|
|
|
|
|
|
| |
|
Total Notional |
|
|
|
|
|
|
| Derivative |
|
Amount |
|
Term |
|
Counterparty Pays |
|
Company Pays |
Interest Rate Swap |
|
$150.0 million |
|
April 2007-April 2010 |
|
3 month LIBOR |
|
4.90% |
Interest Rate Swap |
|
$150.0 million |
|
April 2009-April 2011 |
|
3 month LIBOR |
|
3.07% |
Forward Interest Rate Swap |
|
$150.0 million |
|
April 2010-April 2011 |
|
3 month LIBOR |
|
3.41% |
Interest Rate Swap |
|
$100.0 million |
|
September 2008-September 2011 |
|
3 month LIBOR |
|
3.31% |
Interest Rate Swap |
|
$150.0 million |
|
September 2009-September 2012 |
|
6 month LIBOR |
|
2.68% |
Based on our variable rate debt balance as of December 31, 2009, a 1% change in interest rates
would increase or decrease our annual interest cost by $3.9 million.
Foreign Exchange Rate Risk
We are subject to the risk of foreign currency exchange rate changes in the conversion from
local currencies to the U.S. dollar of the reported financial position and operating results of our
non-U.S. based subsidiaries. We are also subject to foreign currency exchange rate changes for
purchases of goods and services that are denominated in currencies other than the U.S. dollar. The
primary currency to which we are exposed to fluctuations is the Canadian Dollar. The fair value of
our net foreign investments and our foreign denominated payables would not be materially affected
by a 10% adverse change in foreign currency exchange rates for the periods presented.
65
|
|
|
| ITEM 8. |
|
FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA. |
TABLE OF CONTENTS
| |
|
|
|
|
| |
|
Page |
|
|
|
|
|
|
|
|
|
67 |
|
|
|
|
|
|
|
|
|
|
|
As of December 31, 2009 and December 31, 2008 |
|
|
70 |
|
|
|
|
|
|
|
|
|
|
|
For the years ended December 31, 2009 and 2008, for the period from
March 16 to December 31, 2007, and for the period from January 1 to
March 15, 2007 |
|
|
71 |
|
|
|
|
|
|
|
|
|
|
|
For the years ended December 31, 2009 and 2008, for the period from
March 16 to December 31, 2007, and for the period from January 1 to
March 15, 2007 |
|
|
72 |
|
|
|
|
|
|
|
|
|
|
|
For the years ended December 31, 2009 and 2008, for the period from
March 16 to December 31, 2007, and for the period from January 1
to March 15, 2007 |
|
|
73 |
|
|
|
|
|
|
|
|
|
74 |
|
66
Report of Independent Registered Public Accounting Firm
To the Shareholder and Board of Directors of General Nutrition Centers, Inc.:
In our opinion, the accompanying consolidated balance
sheets and the related consolidated
statements of operations, of stockholder’s equity and comprehensive income (loss) and of cash flows
present fairly, in all material respects, the financial position of General Nutrition Centers, Inc.
and its subsidiaries at December 31, 2009 and 2008, and the results of its operations and its cash
flows for each of the two years in the period ended December 31, 2009 and the period from March 16,
2007 through December 31, 2007 in conformity with accounting principles generally accepted in the
United States of America. In addition, in our opinion, the financial statement schedule listed in
the index appearing under item 15(a)(2), present fairly, in all material respects, the information
set forth therein when read in conjunction with the related consolidated financial statements.
Also in our opinion, the Company maintained, in all material respects, effective internal control
over financial reporting as of December 31, 2009, based on criteria established in Internal Control
— Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway
Commission (COSO). The Company’s management is responsible for these financial statements and
financial statement schedule, for maintaining effective internal control over financial reporting
and for its assessment of the effectiveness of internal control over financial reporting, included
in Management’s Report on Internal Control over Financial Reporting appearing under Item 9A. Our
responsibility is to express opinions on these financial statements, on the financial statement
schedule, and on the Company’s internal control over financial reporting based on our audits (which
was an integrated audit in 2009). We conducted our audits in accordance with the standards of the
Public Company Accounting Oversight Board (United States). Those standards require that we plan
and perform the audits to obtain reasonable assurance about whether the financial statements are
free of material misstatement and whether effective internal control over financial reporting was
maintained in all material respects. Our audits of the financial statements included examining, on
a test basis, evidence supporting the amounts and disclosures in the financial statements,
assessing the accounting principles used and significant estimates made by management, and
evaluating the overall financial statement presentation. Our audit of internal control over
financial reporting included obtaining an understanding of internal control over financial
reporting, assessing the risk that a material weakness exists, and testing and evaluating the
design and operating effectiveness of internal control based on the assessed risk. Our audits also
included performing such other procedures as we considered necessary in the circumstances. We
believe that our audits provide a reasonable basis for our opinions.
As discussed in Note 2 to the consolidated financial statements, the Company changed the manner in
which it accounts for uncertain tax positions in 2007.
A company’s internal control over financial reporting is a process designed to provide reasonable
assurance regarding the reliability of financial reporting and the preparation of financial
statements for external purposes in accordance with generally accepted accounting principles. A
company’s internal control over financial reporting includes those policies and procedures that (i)
pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the
transactions and dispositions of the assets of the company; (ii) provide reasonable assurance that
transactions are recorded as necessary to permit preparation of financial statements in accordance
with generally accepted accounting principles, and that receipts and expenditures of the company
are being made only in accordance with authorizations of management and directors of the company;
and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized
acquisition, use, or disposition of the company’s assets that could have a material effect on the
financial statements.
67
Because of its inherent limitations, internal control over financial reporting may not prevent or
detect misstatements. Also, projections of any evaluation of effectiveness to future periods are
subject to the risk that controls may become inadequate because of changes in conditions, or that
the degree of compliance with the policies or procedures may deteriorate.
/s/ PricewaterhouseCoopers LLP
Pittsburgh, Pennsylvania
March 11, 2010
68
Report of Independent Registered Public Accounting Firm
To the Shareholder and Board of Directors of General Nutrition Centers, Inc.:
In our opinion, the accompanying consolidated
statements of operations, of stockholder’s equity and comprehensive income (loss) and of cash flows present fairly, in all material respects, the
financial position of General Nutrition Centers, Inc. and its subsidiaries (the “Company”) for the
period from January 1, 2007 to March 15, 2007 in conformity with accounting principles generally
accepted in the United States of America. In addition, in our opinion, the financial statement
schedule listed in the index appearing under item 15(a)(2), presents fairly, in all material
respects, the information set forth therein when read in conjunction with the related consolidated
financial statements. These financial statements and the financial statement schedule are the
responsibility of the Company’s management. Our responsibility is to express an opinion on these
financial statements and the financial statement schedule based on our audits. We conducted our
audits of these statements in accordance with the standards of the Public Company Accounting
Oversight Board (United States). Those standards require that we plan and perform the audit to
obtain reasonable assurance about whether the financial statements are free of material
misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and
disclosures in the financial statements, assessing the accounting principles used and significant
estimates made by management, and evaluating the overall financial statement presentation. We
believe that our audits provide a reasonable basis for our opinion.
As discussed in Note 2 to the consolidated financial statements, the Company changed the manner in
which it accounts for uncertain tax positions in 2007.
/s/ PricewaterhouseCoopers LLP
Pittsburgh, Pennsylvania
July 30, 2007
69
GENERAL NUTRITION CENTERS, INC. AND SUBSIDIARIES
Consolidated Balance Sheets
(in thousands, except share data)
| |
|
|
|
|
|
|
|
|
| |
|
December 31, |
|
|
December 31, |
|
| |
|
2009 |
|
|
2008 |
|
Current assets: |
|
|
|
|
|
|
|
|
Cash and cash equivalents |
|
$ |
75,089 |
|
|
$ |
42,307 |
|
Receivables, net (Note 3) |
|
|
94,355 |
|
|
|
89,413 |
|
Inventories, net (Note 4) |
|
|
370,492 |
|
|
|
363,654 |
|
Deferred tax assets, net (Note 5) |
|
|
— |
|
|
|
11,455 |
|
Prepaids and other current assets (Note 6) |
|
|
42,219 |
|
|
|
47,952 |
|
|
|
|
|
|
|
|
Total current assets |
|
|
582,155 |
|
|
|
554,781 |
|
|
|
|
|
|
|
|
|
|
Long-term assets: |
|
|
|
|
|
|
|
|
Goodwill (Note 7) |
|
|
624,753 |
|
|
|
622,909 |
|
Brands (Note 7) |
|
|
720,000 |
|
|
|
720,000 |
|
Other intangible assets, net (Note 7) |
|
|
154,370 |
|
|
|
163,176 |
|
Property, plant and equipment, net (Note 8) |
|
|
199,581 |
|
|
|
206,154 |
|
Deferred financing fees, net (Note 2) |
|
|
18,411 |
|
|
|
22,470 |
|
Other long-term assets (Note 9) |
|
|
4,332 |
|
|
|
2,518 |
|
|
|
|
|
|
|
|
Total long-term assets |
|
|
1,721,447 |
|
|
|
1,737,227 |
|
|
|
|
|
|
|
|
Total assets |
|
$ |
2,303,602 |
|
|
$ |
2,292,008 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Current liabilities: |
|
|
|
|
|
|
|
|
Accounts payable |
|
$ |
95,904 |
|
|
$ |
123,577 |
|
Accrued payroll and related liabilities (Note 10) |
|
|
22,277 |
|
|
|
22,582 |
|
Accrued interest (Note 12) |
|
|
14,552 |
|
|
|
15,745 |
|
Current portion, long-term debt (Note 12) |
|
|
1,724 |
|
|
|
13,509 |
|
Deferred revenue and other current liabilities (Note 11) |
|
|
65,130 |
|
|
|
74,309 |
|
|
|
|
|
|
|
|
Total current liabilities |
|
|
199,587 |
|
|
|
249,722 |
|
|
|
|
|
|
|
|
|
|
Long-term liabilities: |
|
|
|
|
|
|
|
|
Long-term debt (Note 12) |
|
|
1,058,085 |
|
|
|
1,071,237 |
|
Deferred tax liabilities, net (Note 5) |
|
|
288,894 |
|
|
|
278,003 |
|
Other long-term liabilities (Note 13) |
|
|
39,520 |
|
|
|
40,984 |
|
|
|
|
|
|
|
|
Total long-term liabilities |
|
|
1,386,499 |
|
|
|
1,390,224 |
|
|
|
|
|
|
|
|
Total liabilities |
|
|
1,586,086 |
|
|
|
1,639,946 |
|
|
|
|
|
|
|
|
|
|
Stockholder’s equity: |
|
|
|
|
|
|
|
|
Common stock, $0.01 par value,
1,000 shares authorized, 100 shares issued and outstanding (Note 17) |
|
|
— |
|
|
|
— |
|
Paid-in-capital |
|
|
594,932 |
|
|
|
592,355 |
|
Retained earnings |
|
|
129,783 |
|
|
|
73,764 |
|
Accumulated other comprehensive loss (Note 17) |
|
|
(7,199 |
) |
|
|
(14,057 |
) |
|
|
|
|
|
|
|
Total stockholder’s equity |
|
|
717,516 |
|
|
|
652,062 |
|
|
|
|
|
|
|
|
Total liabilities and stockholder’s equity |
|
$ |
2,303,602 |
|
|
$ |
2,292,008 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The accompanying notes are an integral part of the consolidated financial statements.
70
GENERAL NUTRITION CENTERS, INC. AND SUBSIDIARIES
Consolidated Statements of Operations
(in thousands)
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Successor |
|
|
|
Predecessor |
|
| |
|
Year ended |
|
|
Year ended |
|
|
March 16- |
|
|
|
January 1- |
|
| |
|
December 31, |
|
|
December 31, |
|
|
December 31, |
|
|
|
March 15, |
|
| |
|
2009 |
|
|
2008 |
|
|
2007 |
|
|
|
2007 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Revenue |
|
$ |
1,707,007 |
|
|
$ |
1,656,729 |
|
|
$ |
1,222,987 |
|
|
|
$ |
329,829 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cost of sales, including costs of warehousing,
distribution and occupancy |
|
|
1,116,437 |
|
|
|
1,082,630 |
|
|
|
814,238 |
|
|
|
|
212,175 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Gross profit |
|
|
590,570 |
|
|
|
574,099 |
|
|
|
408,749 |
|
|
|
|
117,654 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Compensation and related benefits |
|
|
263,046 |
|
|
|
249,793 |
|
|
|
195,792 |
|
|
|
|
64,311 |
|
Advertising and promotion |
|
|
50,034 |
|
|
|
55,060 |
|
|
|
35,062 |
|
|
|
|
20,473 |
|
Other selling, general and administrative |
|
|
96,454 |
|
|
|
98,732 |
|
|
|
71,213 |
|
|
|
|
17,396 |
|
Foreign currency loss (gain) |
|
|
(155 |
) |
|
|
733 |
|
|
|
(424 |
) |
|
|
|
(154 |
) |
Merger-related costs (Note 1) |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
34,603 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating income (loss) |
|
|
181,191 |
|
|
|
169,781 |
|
|
|
107,106 |
|
|
|
|
(18,975 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Interest expense, net (Note 12) |
|
|
69,953 |
|
|
|
83,000 |
|
|
|
75,522 |
|
|
|
|
43,036 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Income (loss) before income taxes |
|
|
111,238 |
|
|
|
86,781 |
|
|
|
31,584 |
|
|
|
|
(62,011 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Income tax expense (benefit) (Note 5) |
|
|
41,619 |
|
|
|
32,001 |
|
|
|
12,600 |
|
|
|
|
(10,697 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income (loss) |
|
$ |
69,619 |
|
|
$ |
54,780 |
|
|
$ |
18,984 |
|
|
|
$ |
(51,314 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The accompanying notes are an integral part of the consolidated financial statements.
71
GENERAL NUTRITION CENTERS, INC. AND SUBSIDIARIES
Consolidated Statement of Stockholder’s Equity and Comprehensive Income (Loss)
(in thousands, except share data)
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accumulated |
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Other |
|
|
Total |
|
| |
|
Common Stock |
|
|
|
|
|
|
Retained |
|
|
Comprehensive |
|
|
Stockholder’s |
|
| Predecessor |
|
Shares |
|
|
Dollars |
|
|
Paid-in-Capital |
|
|
Earnings |
|
|
Income/(Loss) |
|
|
Equity |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance at December 31, 2006 |
|
|
100 |
|
|
$ |
— |
|
|
$ |
261,899 |
|
|
$ |
49,108 |
|
|
$ |
1,324 |
|
|
$ |
312,331 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Comprehensive income (loss): |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(51,314 |
) |
|
|
— |
|
|
|
(51,314 |
) |
Foreign
currency translation adjustments |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(283 |
) |
|
|
(283 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Comprehensive loss |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(51,597 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Adoption of FIN 48 |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(418 |
) |
|
|
— |
|
|
|
(418 |
) |
Cancellation of stock options |
|
|
— |
|
|
|
— |
|
|
|
(47,018 |
) |
|
|
— |
|
|
|
— |
|
|
|
(47,018 |
) |
Non-cash stock-based compensation |
|
|
— |
|
|
|
— |
|
|
|
4,124 |
|
|
|
— |
|
|
|
— |
|
|
|
4,124 |
|
Capital contribution from selling shareholder |
|
|
— |
|
|
|
— |
|
|
|
463,393 |
|
|
|
— |
|
|
|
— |
|
|
|
463,393 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance at March 15, 2007 |
|
|
100 |
|
|
$ |
— |
|
|
$ |
682,398 |
|
|
$ |
(2,624 |
) |
|
$ |
1,041 |
|
|
$ |
680,815 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Successor |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Comprehensive income (loss): |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
18,984 |
|
|
|
— |
|
|
|
18,984 |
|
Unrealized loss on derivatives designated and qualified
as cash flow hedges, net of tax of $2,051 |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(3,584 |
) |
|
|
(3,584 |
) |
Foreign
currency translation adjustments |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
2,732 |
|
|
|
2,732 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Comprehensive income |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
18,132 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
GNC Corporation investment in
General Nutrition Centers, Inc. |
|
|
100 |
|
|
|
— |
|
|
|
589,000 |
|
|
|
— |
|
|
|
— |
|
|
|
589,000 |
|
Return of capital to GNC Corporation |
|
|
— |
|
|
|
— |
|
|
|
(314 |
) |
|
|
— |
|
|
|
— |
|
|
|
(314 |
) |
Non-cash stock-based compensation |
|
|
— |
|
|
|
— |
|
|
|
1,907 |
|
|
|
— |
|
|
|
— |
|
|
|
1,907 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance at December 31, 2007 |
|
|
100 |
|
|
$ |
— |
|
|
$ |
590,593 |
|
|
$ |
18,984 |
|
|
$ |
(852 |
) |
|
$ |
608,725 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Comprehensive income (loss): |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
54,780 |
|
|
|
— |
|
|
|
54,780 |
|
Unrealized loss on derivatives designated and qualified
as cash flow hedges, net of tax of $4,829 |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(8,438 |
) |
|
|
(8,438 |
) |
Foreign currency translation adjustments |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(4,767 |
) |
|
|
(4,767 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Comprehensive income |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
41,575 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Return of capital to GNC Corporation |
|
|
— |
|
|
|
— |
|
|
|
(832 |
) |
|
|
— |
|
|
|
— |
|
|
|
(832 |
) |
Non-cash stock-based compensation |
|
|
— |
|
|
|
— |
|
|
|
2,594 |
|
|
|
— |
|
|
|
— |
|
|
|
2,594 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance at December 31, 2008 |
|
|
100 |
|
|
$ |
— |
|
|
$ |
592,355 |
|
|
$ |
73,764 |
|
|
$ |
(14,057 |
) |
|
$ |
652,062 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Comprehensive income (loss): |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
69,619 |
|
|
|
— |
|
|
|
69,619 |
|
Unrealized gain on derivatives designated and qualified
as cash flow hedges, net of tax of $1,537 |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
2,686 |
|
|
|
2,686 |
|
Foreign currency translation adjustments |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
4,172 |
|
|
|
4,172 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Comprehensive income |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
76,477 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Return of capital to GNC Corporation |
|
|
— |
|
|
|
— |
|
|
|
(278 |
) |
|
|
— |
|
|
|
— |
|
|
|
(278 |
) |
Non-cash stock-based compensation |
|
|
— |
|
|
|
— |
|
|
|
2,855 |
|
|
|
— |
|
|
|
— |
|
|
|
2,855 |
|
Dividend payment |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(13,600 |
) |
|
|
— |
|
|
|
(13,600 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance at December 31, 2009 |
|
|
100 |
|
|
$ |
— |
|
|
$ |
594,932 |
|
|
$ |
129,783 |
|
|
$ |
(7,199 |
) |
|
$ |
717,516 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The accompanying notes are an integral part of the consolidated financial statements.
72
GENERAL NUTRITION CENTERS, INC. AND SUBSIDIARIES
Consolidated Statements of Cash Flows
(in thousands)
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Successor |
|
|
|
Predecessor |
|
| |
|
Year ended |
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
March 16- |
|
|
|
January 1- |
|
| |
|
December 31, |
|
|
December 31, |
|
|
December 31, |
|
|
|
March 15, |
|
| |
|
2009 |
|
|
2008 |
|
|
2007 |
|
|
|
2007 |
|
CASH FLOWS FROM OPERATING ACTIVITIES: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income |
|
$ |
69,619 |
|
|
$ |
54,780 |
|
|
$ |
18,984 |
|
|
|
$ |
(51,314 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Adjustments to reconcile net income (loss) to net cash provided
by (used in) operating activities: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Depreciation expense |
|
|
36,906 |
|
|
|
31,562 |
|
|
|
20,810 |
|
|
|
|
6,510 |
|
Deferred fee writedown — early debt extinguishment |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
11,680 |
|
Amortization of intangible assets |
|
|
9,759 |
|
|
|
10,891 |
|
|
|
9,191 |
|
|
|
|
866 |
|
Amortization of deferred financing fees |
|
|
4,104 |
|
|
|
3,907 |
|
|
|
2,921 |
|
|
|
|
589 |
|
Amortization of original issue discount |
|
|
374 |
|
|
|
339 |
|
|
|
247 |
|
|
|
|
— |
|
Increase in provision for inventory losses |
|
|
11,151 |
|
|
|
14,406 |
|
|
|
10,400 |
|
|
|
|
2,247 |
|
Non-cash stock-based compensation |
|
|
2,855 |
|
|
|
2,594 |
|
|
|
1,907 |
|
|
|
|
4,124 |
|
(Decrease) increase in provision for losses on accounts receivable |
|
|
(2,540 |
) |
|
|
253 |
|
|
|
(335 |
) |
|
|
|
(39 |
) |
Decrease (increase) in net deferred taxes |
|
|
21,431 |
|
|
|
24,371 |
|
|
|
9,303 |
|
|
|
|
(3,874 |
) |
Changes in assets and liabilities: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(Increase) decrease in receivables |
|
|
(3,488 |
) |
|
|
(5,371 |
) |
|
|
(10,752 |
) |
|
|
|
1,676 |
|
Increase in inventory, net |
|
|
(15,661 |
) |
|
|
(48,248 |
) |
|
|
(6,377 |
) |
|
|
|
(2,128 |
) |
(Increase) decrease in franchise note receivables, net |
|
|
(314 |
) |
|
|
1,008 |
|
|
|
2,587 |
|
|
|
|
912 |
|
Increase (decrease) in accrued income taxes |
|
|
2,777 |
|
|
|
(10,495 |
) |
|
|
12,619 |
|
|
|
|
(4,967 |
) |
Decrease (increase) in other assets |
|
|
4,187 |
|
|
|
(6,259 |
) |
|
|
(7,474 |
) |
|
|
|
3,394 |
|
(Decrease) increase in accounts payable |
|
|
(28,119 |
) |
|
|
22,075 |
|
|
|
(986 |
) |
|
|
|
(387 |
) |
(Decrease) increase in interest payable |
|
|
(1,193 |
) |
|
|
(2,365 |
) |
|
|
18,110 |
|
|
|
|
(7,531 |
) |
Increase (decrease) in accrued liabilities |
|
|
2,109 |
|
|
|
(16,083 |
) |
|
|
10,882 |
|
|
|
|
(12,682 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net cash provided by (used in) operating activities |
|
|
113,957 |
|
|
|
77,365 |
|
|
|
92,037 |
|
|
|
|
(50,924 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CASH FLOWS FROM INVESTING ACTIVITIES: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Capital expenditures |
|
|
(28,682 |
) |
|
|
(48,666 |
) |
|
|
(28,851 |
) |
|
|
|
(5,693 |
) |
Acquisition of the Company |
|
|
(11,268 |
) |
|
|
(10,842 |
) |
|
|
(1,642,061 |
) |
|
|
|
— |
|
Franchise store conversions |
|
|
239 |
|
|
|
404 |
|
|
|
77 |
|
|
|
|
— |
|
Acquisition of intangibles |
|
|
— |
|
|
|
(1,000 |
) |
|
|
— |
|
|
|
|
— |
|
Store acquisition costs |
|
|
(2,463 |
) |
|
|
(321 |
) |
|
|
(489 |
) |
|
|
|
(555 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net cash used in investing activities |
|
|
(42,174 |
) |
|
|
(60,425 |
) |
|
|
(1,671,324 |
) |
|
|
|
(6,248 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CASH FLOWS FROM FINANCING ACTIVITIES: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Issuance of new equity |
|
|
— |
|
|
|
— |
|
|
|
552,291 |
|
|
|
|
— |
|
Return of capital to Parent company |
|
|
(278 |
) |
|
|
(832 |
) |
|
|
(314 |
) |
|
|
|
— |
|
Contribution from selling shareholders |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
463,393 |
|
Dividend payment |
|
|
(13,600 |
) |
|
|
— |
|
|
|
— |
|
|
|
|
— |
|
Borrowings from new revolving credit facility |
|
|
— |
|
|
|
5,375 |
|
|
|
10,500 |
|
|
|
|
— |
|
Payments on new revolving credit facility |
|
|
(5,375 |
) |
|
|
— |
|
|
|
(10,500 |
) |
|
|
|
— |
|
Borrowings from new senior credit facility |
|
|
— |
|
|
|
— |
|
|
|
675,000 |
|
|
|
|
— |
|
Proceeds from issuance of new senior sub notes |
|
|
— |
|
|
|
— |
|
|
|
110,000 |
|
|
|
|
— |
|
Proceeds from issuance of new senior notes |
|
|
— |
|
|
|
— |
|
|
|
297,000 |
|
|
|
|
— |
|
Redemption of 8 5/8% senior notes |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
(150,000 |
) |
Redemption of 8 1/2% senior notes |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
(215,000 |
) |
Payment of 2003 senior credit facility |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
(55,290 |
) |
Payments on long-term debt |
|
|
(19,952 |
) |
|
|
(7,974 |
) |
|
|
(6,021 |
) |
|
|
|
(334 |
) |
Financing fees |
|
|
(45 |
) |
|
|
— |
|
|
|
(29,298 |
) |
|
|
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net cash (used in) provided by financing activities |
|
|
(39,250 |
) |
|
|
(3,431 |
) |
|
|
1,598,658 |
|
|
|
|
42,769 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Effect of exchange rate on cash |
|
|
249 |
|
|
|
(56 |
) |
|
|
(29 |
) |
|
|
|
(165 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net increase (decrease) in cash |
|
|
32,782 |
|
|
|
13,453 |
|
|
|
19,342 |
|
|
|
|
(14,568 |
) |
Beginning balance, cash |
|
|
42,307 |
|
|
|
28,854 |
|
|
|
9,512 |
|
|
|
|
24,080 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Ending balance, cash |
|
$ |
75,089 |
|
|
$ |
42,307 |
|
|
$ |
28,854 |
|
|
|
$ |
9,512 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The accompanying notes are an integral part of the consolidated financial statements.
73
GENERAL NUTRITION CENTERS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
NOTE 1. NATURE OF BUSINESS
General Nature of Business. General Nutrition Centers, Inc. (“GNC” or the “Company”), a
Delaware corporation, is a leading specialty retailer of nutritional supplements, which include:
vitamins, minerals and herbal supplements (“VMHS”), sports nutrition products, diet products and
other wellness products.
The Company’s organizational structure is vertically integrated as the operations consist of
purchasing raw materials, formulating and manufacturing products and selling the finished products
through its retail, franchising and manufacturing/wholesale segments. The Company operates
primarily in three business segments: Retail; Franchising; and Manufacturing/Wholesale. Corporate
retail store operations are located in North America and Puerto Rico and in addition the Company
offers products domestically through www.gnc.com and www.drugstore.com. Franchise stores are
located in the United States and 47 international countries. The Company operates its primary
manufacturing facilities in South Carolina and distribution centers in Arizona, Pennsylvania and
South Carolina. The Company manufactures the majority of its branded products, but also
merchandises various third-party products. Additionally, the Company licenses the use of its
trademarks and trade names.
The processing, formulation, packaging, labeling and advertising of the Company’s products are
subject to regulation by one or more federal agencies, including the Food and Drug Administration
(“FDA”), Federal Trade Commission (“FTC”), Consumer Product Safety Commission, United States
Department of Agriculture and the Environmental Protection Agency. These activities are also
regulated by various agencies of the states and localities in which the Company’s products are
sold.
Merger of the Company. On February 8, 2007, GNC Parent Corporation, our ultimate parent
company at the time, entered into an Agreement and Plan of Merger with GNC Acquisition Inc. and its
parent company, GNC Acquisition Holdings Inc. (“Parent”), pursuant to which GNC Acquisition Inc.
agreed to merge with and into GNC Parent Corporation, and as a result GNC Parent Corporation would
continue as the surviving corporation and a wholly owned subsidiary of GNC Acquisition Holdings
Inc. (the “Merger”). The purchase equity contribution was made by Ares Corporate Opportunities
Fund II, L.P. (“ACOF”) and Ontario Teachers’ Pension Plan Board (“OTPP”), together with additional
institutional investors and certain management of the Company. The transaction closed on March 16,
2007 and was accounted for under the purchase method of accounting. The transaction occurred
between unrelated parties and no common control existed. The merger consideration (excluding
acquisition costs of $13.7 million) totaled $1.65 billion, including the repayment of existing debt
and other liabilities, and was funded with a combination of equity contributions and the issuance
of new debt. The following reconciles the total merger consideration to the cash purchase price:
| |
|
|
|
|
| |
|
March 16, 2007 |
|
| |
|
(in thousands) |
|
Merger consideration |
|
$ |
1,650,000 |
|
Acquisition costs |
|
|
13,732 |
|
Debt assumed by buyer |
|
|
(10,773 |
) |
|
|
|
|
Fair value of net assets acquired |
|
|
1,652,959 |
|
Non-cash rollover of shares |
|
|
(36,709 |
) |
|
|
|
|
Cash paid at acquisition |
|
$ |
1,616,250 |
|
|
|
|
|
The Company was subject to certain tax adjustments that were settled upon filing of the
predecessor’s final tax return, and receipt of the tax refund associated with that return. Also,
pursuant to the Merger agreement, the Company agreed to pay additional consideration for amounts
refunded from tax returns. During the period from March 16 to December 31, 2007, the Company paid
$25.9 million for total cash paid for the Merger of $1,642.1 million. In September 2008, pursuant
to the Merger agreement, $10.8 million of additional consideration was paid as a result of the
Company filing its March 16, 2007 to December 31, 2007 consolidated federal tax return. In the
fourth quarter of 2009, pursuant to the Merger
74
GENERAL NUTRITION CENTERS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
agreement, $11.3 million of additional consideration
was paid as a result of the Company filing its 2008 consolidated federal tax return. The Merger agreement requires payments to former
shareholders and optionholders in lieu of income tax payments made for utilizing net operating
losses (“NOL’s”) created as a result of the Merger.
In connection with the Merger on March 16, 2007, the Company issued $300.0 million aggregate
principal amount of Senior Floating Rate Toggle Notes due 2014 and $110.0 million aggregate
principal amount of 10.75% Senior Subordinated Notes due 2015. In addition, the Company obtained a
senior credit facility comprised of a $675.0 million term loan facility and a $60.0 million
revolving credit facility. The Company borrowed the entire $675.0 million under the term loan
facility and $10.5 million under the revolving credit facility to fund a portion of the acquisition
price. The Company utilized proceeds from the new debt to repay its December 2003 senior credit
facility, its 8 5/8% senior notes issued in January 2005, and its 8 1/2% senior subordinated notes
issued in December 2003. The Company contributed the remainder of the debt proceeds, after payment
of fees and expenses, to a newly formed, wholly owned subsidiary, which then loaned such net
proceeds to GNC Parent Corporation. GNC Parent Corporation used those proceeds, together with the
equity contributions, to repay GNC Parent Corporation’s outstanding floating rate senior PIK notes
issued in November 2006, pay the merger consideration, and pay fees and expenses related to the
Merger transactions.
In connection with the Merger, the Company recognized charges of $34.6 million in the period
ending March 15, 2007. In addition, the Company recognized compensation charges associated with
the Merger of $15.3 million in the period ending March 15, 2007.
Pursuant to the Merger agreement, as amended, GNC Acquisition Inc. was merged with and into
GNC Parent Corporation with GNC Parent Corporation surviving the Merger. Subsequently on March 16,
2007, GNC Parent was converted into a Delaware limited liability company and renamed GNC Parent
LLC.
In conjunction with the Merger, final fair value adjustments were made to the Company’s
financial statements as of March 16, 2007. As a result of the Merger and the final fair values
assigned, the financial statements as of December 31, 2007 reflected these adjustments made in
accordance with the standard on business combinations. The following table summarizes the fair
values assigned to the Company’s assets and liabilities in connection with the Merger.
| |
|
|
|
|
| |
|
March 16, 2007 |
|
| |
|
(in thousands) |
|
|
|
|
|
|
Assets: |
|
|
|
|
Current assets |
|
$ |
480,230 |
|
Goodwill |
|
|
626,259 |
|
Other intangible assets |
|
|
901,661 |
|
Property, plant and equipment |
|
|
181,765 |
|
Other assets |
|
|
16,813 |
|
|
|
|
|
Total assets |
|
$ |
2,206,728 |
|
|
|
|
|
|
|
|
|
|
Liabilities: |
|
|
|
|
Current liabilities |
|
|
232,943 |
|
Long-term debt |
|
|
10,773 |
|
Deferred tax liability |
|
|
257,732 |
|
Other liabilities |
|
|
52,321 |
|
|
|
|
|
Total liabilities |
|
$ |
553,769 |
|
|
|
|
|
|
|
|
|
|
Fair value of net assets acquired |
|
$ |
1,652,959 |
|
|
|
|
|
The above table does not include Merger consideration related to payments based on utilization
of net operating losses for 2008 and 2009.
75
GENERAL NUTRITION CENTERS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
NOTE 2. BASIS OF PRESENTATION AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Basis of Presentation
The accompanying consolidated financial statements and footnotes have been prepared by the
Company in accordance with accounting principles generally accepted in the United States of America
(“U.S. GAAP”) and with the instructions to Form 10-K and Regulation S-X. The Company’s normal
reporting period is based on a calendar year.
The financial statements as of December 31, 2009, 2008 and 2007 reflect periods subsequent to
the Merger and include the accounts of the Company and its wholly owned subsidiaries. Included for
the year ended 2009 and 2008 and for the period from March 16, 2007 through December 31, 2007 are
fair value adjustments to assets and liabilities, including inventory, goodwill, other intangible
assets and property, plant and equipment. Accordingly, the accompanying financial statements for
the periods prior to the Merger are labeled as “Predecessor” and the periods subsequent to the
Merger are labeled as “Successor”.
Summary of Significant Accounting Policies
Principles of Consolidation. The consolidated financial statements include the accounts of
the Company and all of its subsidiaries and a variable interest entity. All material intercompany
transactions have been eliminated in consolidation.
The Company has no relationships with unconsolidated entities or financial partnerships, such
as entities often referred to as structured finance or special purpose entities, which would have
been established for the purpose of facilitating off balance sheet arrangements, or other
contractually narrow or limited purposes.
Use of Estimates. The preparation of financial statements in conformity with U.S. GAAP
principles requires management to make estimates and assumptions. Accordingly, these estimates and
assumptions affect the reported amounts of assets and liabilities and disclosure of contingent
assets and liabilities at the date of the financial statements, and the reported amounts of
revenues and expenses during the reporting period. Some of the most significant estimates
pertaining to the Company include the valuation of inventories, the allowance for doubtful
accounts, income tax valuation allowances and the recoverability of long-lived assets. On a
regular basis, management reviews its estimates utilizing currently available information, changes
in facts and circumstances, historical experience and reasonable assumptions. After such reviews
and if deemed appropriate, those estimates are adjusted accordingly. Actual results could differ
from those estimates.
Cash and Cash Equivalents. The Company considers cash and cash equivalents to include all cash
and liquid deposits and investments with a maturity of three months or less. The majority of
payments due from banks for third-party credit cards process within 24-48 hours, except for
transactions occurring on a Friday, which are generally processed the following Monday. All credit
card transactions are classified as cash and the amounts due from these transactions totaled $2.1
million at December 31, 2009 and $2.2 million at December 31, 2008.
Book overdrafts of $0.7 million and $4.2 million as of December 31, 2009 and 2008,
respectively, represent checks issued that had not been presented for payment to the banks and are
classified as accounts payable in the Company’s consolidated balance sheet. The Company typically
funds these overdrafts through normal collections of funds or transfers from bank balances at other
financial institutions. Under the terms of the Company’s facilities with its banks, the respective
financial institutions are not legally obligated to honor the book overdraft balances as of
December 31, 2009 and 2008, or any balance on any given date.
76
GENERAL NUTRITION CENTERS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
Inventories. Inventory components consist of raw materials, finished product and packaging
supplies. Inventories are stated at the lower of cost or market on a first in/first out basis.
Cost is determined using a standard costing system which approximates actual costs. The Company
regularly reviews its inventory levels in order to identify slow moving and short dated products,
expected length of time for product sell through and future expiring product. Upon analysis, the
Company has established certain valuation allowances to reserve for such inventory. When
allowances are considered necessary, after such reviews, the inventory balances are adjusted and
reflected net in the accompanying financial statements.
Accounts Receivable and Allowance for Doubtful Accounts. The Company sells product to its
franchisees and, to a lesser extent, various third parties. See Note 3, “Receivables”, for the
components of accounts receivable. To determine the allowance for doubtful accounts in accordance
with the standard on impairment of receivables, factors that affect collectability from the
Company’s franchisees or third-party customers include their financial strength, payment history,
reported sales and the overall retail economy. The Company establishes an allowance for doubtful
accounts for franchisees based on an assessment of the franchisees’ operations which includes
analysis of their operating cash flows, sales levels, and status of amounts due to the Company,
such as rent, interest and advertising. In addition, the Company considers the franchisees’
inventory and fixed assets, which the Company can use as collateral in the event of a default by
the franchisee. An allowance for international franchisees is calculated based on unpaid, non
collateralized amounts associated with their receivable balance. An allowance for receivable
balances due from third parties is recognized, if considered necessary, based on facts and
circumstances. These allowances are deducted from the related receivables and reflected net in the
accompanying financial statements.
Notes Receivable. The Company offers financing to qualified franchisees in connection with the
initial purchase of a franchise store. The notes offered by the Company to its franchisees are
demand notes, payable monthly over a period ranging from five to seven years. Interest accrues
principally at an annual rate that ranges from 8.0% to 13.75%, based on the amount of initial
deposit, and is payable monthly. Allowances for these receivables are recognized in accordance
with the Company’s policy described in the Accounts Receivable and Allowance for Doubtful Accounts
above.
Property, Plant and Equipment. Property, plant and equipment expenditures are recorded at
cost. As a result of the Merger, the remaining estimated useful lives of already-existing property
and equipment were reevaluated on a prospective basis using the fair values determined at the date
of the Merger. These remaining useful lives ranged from one year to sixteen years across all asset
classes with the exception of buildings, whose useful lives ranged from fifteen to thirty seven
years. Depreciation and amortization are recognized using the straight-line method over the
estimated useful life of the property. Fixtures are depreciated over three to fifteen years, and
equipment is generally depreciated over ten years. Computer equipment and software costs are
generally depreciated over three to five years. Amortization of improvements to retail leased
premises is recognized using the straight-line method over the estimated useful life of the
improvements, or over the life of the related leases including renewals that are reasonably
assured, whichever period is shorter. Buildings are depreciated over forty years and building
improvements are depreciated over the remaining useful life of the building. The Company records
tax depreciation in conformity with the provisions of applicable tax law.
Expenditures that materially increase the value or clearly extend the useful life of property,
plant and equipment are capitalized in accordance with the policies outlined above. Repair and
maintenance costs incurred in the normal operations of business are expensed as incurred. Gains
from the sale of property, plant and equipment are recognized in current operations.
The Company recognized depreciation expense of property, plant and equipment of $36.9 million
for the year ended December 31, 2009, $31.6 million for the year ended December 31, 2008, $20.8
million for the period March 16 to December 31, 2007, and $6.5 million for the period January 1 to
March 15, 2007.
Goodwill and Intangible Assets. Goodwill represents the excess of purchase price over the
fair value of identifiable net assets of acquired entities. Goodwill and intangible assets with
indefinite useful lives are not amortized, but instead are tested for impairment at least annually.
The Company completes its annual impairment test in the fourth quarter. The Company records
goodwill and franchise rights upon the acquisition of franchisee stores when the consideration
given to the franchisee
77
GENERAL NUTRITION CENTERS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
exceeds the fair value of the identifiable assets acquired and liabilities assumed of the store. This goodwill is accounted
for in accordance with the above policy. See Note 7, “Goodwill, Brands, and Other Intangible
Assets, Net.”
Long-lived Assets. The Company periodically performs reviews of underperforming businesses
and other long-lived assets, including amortizable intangible assets, for impairment pursuant to
the provisions of the standard related to the accounting for impairment on disposal of long lived
assets. Factors the Company considers important that may trigger an impairment review include:
significant changes in the manner of its use of assets of the strategy for its overall business;
significant negative industry or economic trends; store closings; or under-performing business
trends. These reviews may include an analysis of the current operations and capacity utilization,
in conjunction with an analysis of the markets in which the businesses are operating. A comparison
is performed of the undiscounted projected cash flows of the current operating forecasts to the net
book value of the related assets. If it is determined that the full value of the assets may not be
recoverable, an appropriate charge to adjust the carrying value of the long-lived assets to fair
value may be required.
Revenue Recognition. The Company operates predominately as a retailer, through Company-owned
stores, franchised stores and sales through its website, www.gnc.com and to a lesser extent through
wholesale operations. For all years and periods presented herein, the Company has complied with and
adopted the standard on revenue recognition.
The Retail segment recognizes revenue at the moment a sale to a customer is recorded. These
revenues are recorded via the Company’s point of sale system. Gross revenues are netted against
actual customer returns and an allowance for expected customer returns. The Company records a
reserve for expected customer returns based on management’s estimate, which is derived from
historical return data. Revenue is deferred on sales of the Company’s Gold Cards and subsequently
amortized over 12 months. The length of the amortization period is determined based on matching
the discounts associated with the Gold Card program to the revenue deferral during the twelve month
membership period. For an annual fee, the card provides customers with a 20% discount on all
products purchased, both on the date the card is purchased and certain specified days of every
month.
The Company also sells gift cards to its customers. Revenue from gift cards is recognized
when the gift card is redeemed. These gift cards do not have expiration dates. Based upon
historical redemption rates, a small percentage of gift cards will never be redeemed, referred to
as “breakage.” The Company first sold gift cards in late 2001 and the Company began to recognize
gift card breakage revenue in 2008, when the likelihood of redemption became remote and amounts
were not escheatable. Total revenue for 2009 and 2008 includes $0.3 million and $0.6 million,
respectively, related to recognition of gift card breakage revenue.
The Franchise segment generates revenues through product sales to franchisees, royalties,
franchise fees and interest income on the financing of the franchise locations. See Note 20,
“Franchise Revenue.” These revenues are netted by actual franchisee returns and an allowance for
projected returns. The franchisees purchase a majority of the products they sell from the Company
at wholesale prices. Revenue on product sales to franchisees is recognized when risk of loss, title
and insurable risks have transferred to the franchisee. Franchise fees are recognized by the
Company at the time of a franchise store opening. Interest on the financing of franchisee notes
receivable is recognized as it becomes due and payable. Gains from the sale of company-owned
stores to franchisees are recognized in accordance with the standard on accounting for sales of
real estate. This standard requires gains on sales of corporate stores to franchisees to be
deferred until certain criteria are satisfied regarding the collectability of the related
receivable and the seller’s remaining obligations. Remaining sources of franchise income,
including royalties, are recognized as earned.
The Manufacturing/Wholesale segment sells product primarily to the other Company segments and
third-party customers. Revenue is recognized when risk of loss, title and insurable risks have
transferred to the customer, net of estimated returns and allowances. The Company also has a
consignment arrangement with certain customers and revenue is recognized when products are sold to
the ultimate customer.
78
GENERAL NUTRITION CENTERS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
Cost of Sales. The Company purchases products directly from third party manufacturers as well
as manufactures its own products. The Company’s cost of sales includes product costs, costs of
warehousing and distribution and occupancy costs. The cost of manufactured products includes
depreciation expense related to the manufacturing facility and related equipment.
Vendor Allowances. The Company enters into two main types of arrangements with certain
vendors, the most significant of which results in the Company receiving credits as sales rebates
based on arrangements with such vendors. The Company also enters into arrangements with certain
vendors through which the Company receives rebates for purchases during the year typically based on
volume discounts. As the right of offset exists under these arrangements, rebates received under
both arrangements are recorded as a reduction in the vendors’ accounts payable balances on the
balance sheet and represent the estimated amounts due to GNC under the rebate provisions of such
contracts. Rebates are presented as a reduction in accounts payable. The corresponding rebate
income is recorded as a reduction of cost of goods sold based on inventory turnover, in accordance
with the provisions of the standard on accounting by a reseller for cash consideration received
from a vendor. For volume rebates, the appropriate level of such income is derived from the level
of actual purchases made by GNC from suppliers. The amount recorded as a reduction to cost of goods
sold was $34.1 million for the year ended December 31, 2009, $29.3 million for the year ended
December 31, 2008, $20.9 million for the period from March 16 to December 31, 2007 and $6.6 million
for the period from January 1 to March 15, 2007.
Distribution and Shipping Costs. The Company bills franchisees and third-party customers
shipping and transportation costs and reflects these charges in revenue. The unreimbursed costs
that are associated with these costs are included in cost of sales.
Research and Development. Research and development costs arising from internally generated
projects are expensed by the Company as incurred. The Company recognized $0.4 million for the year
ended December 31, 2009, $0.9 million for the year ended December 31, 2008, $0.5 million for the
period from March 16 to December 31, 2007, and $0.1 million in research and development costs for
the period January 1 to March 15, 2007. These costs are included in Other SG&A costs in the
accompanying financial statements.
Advertising Expenditures. The Company recognizes advertising, promotion and marketing program
costs the first time the advertising takes place with exception to the costs of producing
advertising, which are expensed as incurred during production. The Company administers national
advertising funds on behalf of its franchisees. In accordance with the franchisee contracts, the
Company collects advertising fees from the franchisees and utilizes the proceeds to coordinate
various advertising and marketing campaigns. The Company recognized $50.0 million for the year
ended December 31, 2009, $55.1 million for the year ended December 31, 2008, $35.0 million for the
period March 16 to December 31, 2007, and $20.5 million for the period January 1 to March 15, 2007,
net of approximately $11.0 million annually from the national advertising fund.
Merger Related Costs. For the period January 1 to March 15, 2007, Merger related costs of
$34.6 million includes costs incurred by GNC Parent LLC, and recognized by us, in relation to the
Merger. These costs were comprised of selling-related expenses of $26.4 million, a contract
termination fee paid to our previous owner of $7.5 million and other costs of $0.7 million.
Leases. The Company has various operating leases for company-owned and franchised store
locations and equipment. Store leases generally include amounts relating to base rental, percent
rent and other charges such as common area maintenance fees and real estate taxes. Periodically,
the Company receives varying amounts of reimbursements from landlords to compensate the Company for
costs incurred in the construction of stores. These reimbursements are amortized by the Company as
an offset to rent expense over the life of the related lease. The Company determines the period
used for the straight-line rent expense for leases with option periods and conforms it to the term
used for amortizing improvements.
79
GENERAL NUTRITION CENTERS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
The Company leases an approximately 300,000 square-foot-facility in Greenville, South Carolina
where the majority of its proprietary products are manufactured. The Company also leases a 630,000
square foot complex located in Anderson, South Carolina, for packaging, materials receipt, lab
testing, warehousing, and distribution. Both the Greenville and Anderson facilities are leased on
a long-term basis pursuant to “fee-in-lieu-of-taxes” arrangements with the counties in which the
facilities are located, but the Company retains the right to purchase each of the facilities at any
time during the lease for $1.00, subject to a loss of tax benefits. As part of a tax incentive
arrangement, the Company assigned the facilities to the counties and leases them back under
operating leases. The Company leases the facilities from the counties where located, in lieu of
paying local property taxes. Upon exercising its right to purchase the facilities back from the
counties, the Company will be subject to the applicable taxes levied by the counties. In
accordance with the standards on the accounting for leases, the purchase option in the lease
agreements prevent sale-leaseback accounting treatment. As a result, the original cost basis of
the facilities remains on the balance sheet and continues to be depreciated.
The Company leases a 210,000 square foot distribution center in Leetsdale, Pennsylvania and a
112,000 square foot distribution center in Phoenix, Arizona. The Company also has operating leases
for its fleet of distribution tractors and trailers and fleet of field management vehicles. In
addition, the Company also has a minimal amount of leased office space in California, Florida, and
Canada. The expense associated with leases that have escalating payment terms is recognized on a
straight-line basis over the life of the lease. See Note 15, “Long-Term Lease Obligations.”
Contingencies. In accordance with the standards on contingencies the Company accrues a loss
contingency if it is probable and can be reasonably estimated or a liability had been incurred at
the date of the financial statements if those financial statements have not been issued. If both of
the conditions above are not met, or if an exposure to loss exists in excess of the amount accrued,
disclosure of the contingency shall be made when there is at least a reasonable possibility that a
loss or an additional loss may have been incurred. The Company accrues costs that are part of
legal settlements when the settlement is probable.
Pre-Opening Expenditures. The Company recognizes the cost associated with the opening of new
stores as incurred. These costs are charged to expense and are not material for the periods
presented. Franchise store pre-opening costs are incurred by the franchisees.
Deferred Financing Fees. Costs related to the financing of the Senior Subordinated Notes
issued in December 2003, 8 5/8% Senior Notes and the December 2003 senior credit facility were
capitalized and were being amortized over the term of the respective debt. As of March 15, 2007,
the remaining deferred financing fees were written off as the debt was extinguished as a part of
the Merger. In conjunction with the Merger, $29.3 million in costs related to the financing of new
debt were capitalized and are being amortized over the life of the new debt. Accumulated
amortization as of December 31, 2009 and 2008 were $10.9 million and $6.8 million, respectively.
Income Taxes. The Company accounts for income taxes in accordance with the standards on
income taxes. As prescribed by these standards, the Company utilizes the asset and liability
method of accounting for income taxes. Under the asset and liability method, deferred tax assets
and liabilities are recognized for the estimated future tax consequences attributable to
differences between the financial statements carrying amounts of existing assets and liabilities
and their respective tax bases. Deferred tax assets and liabilities are measured using enacted tax
rates in effect for the year in which those temporary differences are expected to be recovered or
settled. See Note 5, “Income Taxes.”
For the year ended December 31, 2009 the Company will file a consolidated federal income tax
return. For state income tax purposes, the Company will file on both a consolidated and separate
return basis in the states in which it conducts business. The Company filed in a consistent manner
in 2008 and 2007.
The Company adopted the updates on the provisions of the standards on income taxes on January
1, 2007 related to the accounting for uncertainty in income taxes. As a result of the
implementation, the Company recognized an adjustment of $0.4 million to retained earnings for the liability for
unrecognized income tax benefits, net of the deferred tax effect. It is the
80
GENERAL NUTRITION CENTERS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
Company’s policy to
recognize interest and penalties related to uncertain tax positions as a component of income tax
expense. See Note 5, “Income Taxes,” for additional information regarding the change in
unrecognized tax benefits.
Self-Insurance. The Company has procured insurance for such areas as: (1) general liability;
(2) product liability; (3) directors and officers liability; (4) property insurance; and (5) ocean
marine insurance. The Company is self-insured for such areas as: (1) medical benefits; (2)
worker’s compensation coverage in the State of New York with a stop loss of $250,000; (3) physical
damage to the Company’s tractors, trailers and fleet vehicles for field personnel use; and (4) physical damages that may occur
at the corporate store locations. The Company is not insured for certain property and casualty
risks due to the frequency and severity of a loss, the cost of insurance and the overall risk
analysis.
The Company carries product liability insurance with a retention of $3.0 million per claim
with an aggregate cap on retained losses of $10.0 million. The Company carries general liability
insurance with retention of $110,000 per claim with an aggregate cap on retained losses of
$600,000. The majority of the Company’s workers’ compensation and auto insurance are in a
deductible/retrospective plan. The Company reimburses the insurance company for the workers
compensation and auto liability claims, subject to a $250,000 and $100,000 loss limit per claim,
respectively.
As part of the medical benefits program, the Company contracts with national service providers
to provide benefits to its employees for all medical, dental, vision and prescription drug
services. The Company then reimburses these service providers as claims are processed from Company
employees. The Company maintains a specific stop loss provision of $250,000 per individual per plan
year with a maximum lifetime benefit limit of $2.0 million per individual. The Company has no
additional liability once a participant exceeds the $2.0 million ceiling. The Company’s liability
for medical claims is included as a component of accrued benefits in Note 10, “Accrued Payroll and
Related Liabilities,” and was $2.0 million and $2.2 million as of December 31, 2009 and 2008,
respectively.
Stock Compensation. The Company utilizes the Black-Scholes model to calculate the fair value
of options under this standard, which is consistent with disclosures previously included in prior
year financial statements under the original stock compensation standard. The resulting
compensation cost is recognized in the Company’s financial statements over the option vesting
period.
Foreign Currency. For all foreign operations, the functional currency is the local currency.
In accordance with the standard on foreign currency matters, assets and liabilities of those
operations, denominated in foreign currencies, are translated into U.S. dollars using period-end
exchange rates, and income and expenses are translated using the average exchange rates for the
reporting period. Gains or losses resulting from foreign currency transactions are included in
results of operations.
Financial Instruments and Derivatives. On January 1, 2009, the Company adopted the revised
accounting standards on disclosure of derivative instruments and hedging activities. This new
standard expands the current disclosure requirements. This new standard provides for an enhanced
understanding of (1) how and why an entity uses derivative instruments, (2) how derivative
instruments and related hedged items are accounted for under previous standards and their related
interpretations, and (3) how derivative instruments affect an entity’s financial position,
financial performance, and cash flows.
As part of the Company’s financial risk management program, it uses certain derivative
financial instruments. The Company does not enter into derivative transactions for speculative
purposes and holds no derivative instruments for trading purposes. The Company uses derivative
financial instruments to reduce its exposure to market risk for changes in interest rates primarily
in respect of its long term debt obligations. The Company tries to manage its interest rate risk
in order to balance its exposure to both fixed and floating rates while minimizing its borrowing
costs. Floating-to-fixed interest rate swap agreements, designated as cash flow hedges of interest
rate risk, are entered into from time to time to hedge our exposure to interest rate changes on a
portion of the Company’s floating rate debt. These interest rate swap agreements convert a portion
of the Company’s floating rate debt to fixed rate debt. Interest rate floors designated as cash
flow hedges involve the receipt of variable-rate amounts from a counterparty if interest rates fall below the strike rate on the contract in exchange for an upfront premium. The
Company records
81
GENERAL NUTRITION CENTERS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
the fair value of these contracts as an asset or a liability, as applicable, in the
balance sheet, with the offset to accumulated other comprehensive income (loss), net of tax. The
Company measures hedge effectiveness by assessing the changes in the fair value or expected future
cash flows of the hedged item. The ineffective portions, if any, are recorded in interest expense
in the current period.
Derivatives designated as hedging instruments have been recorded in the consolidated balance
sheet at fair value as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
Fair Value |
|
| |
|
Balance Sheet Location |
|
|
December 31, 2009 |
|
|
December 31, 2008 |
|
| |
|
|
|
|
|
(in thousands) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Interest Rate
Products |
|
Other long-term liabilities |
|
$ |
(14,679 |
) |
|
$ |
(18,902 |
) |
|
|
|
|
|
|
|
|
|
|
|
The Company has interest rate swap agreements outstanding that effectively converted notional
amounts of an aggregate $550.0 million of debt from floating to fixed interest rates. The five
outstanding agreements mature between April 2010 and September 2012. During the second quarter of
2009, the Company entered into a derivative contract that consisted of an interest rate swap with a
bought floor that effectively converted a notional amount of $150.0 million of the senior toggle
notes from a floating to a fixed rate, effective September 2009. The floor is intended to replicate
the optionality present in the original debt agreement, providing an equivalent offset in the
interest payments.
Amounts reported in accumulated other comprehensive income related to derivatives will be
reclassified to interest expense as interest payments are made on the Company’s variable-rate debt.
During the twelve months ending December 31, 2010, the Company estimates that an additional $12.0
million will be reclassified as an increase to interest expense.
Components of gains and losses recorded in the consolidated balance sheet and consolidated
income statements for the year ended December 31, 2009, are as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
Location of Gain or |
|
|
|
|
| |
|
Amount of Gain or |
|
|
(Loss) Reclassified from |
|
|
Amount of Gain or (Loss) |
|
| Derivatives in Cash |
|
(Loss) Recognized in |
|
|
Accumulated OCI into |
|
|
Reclassified from |
|
| Flow Hedging |
|
OCI on Derivative |
|
|
Income (Effective |
|
|
Accumulated OCI into |
|
| Relationships |
|
(Effective Portion) |
|
|
Portion) |
|
|
Income (Effective Portion) |
|
| (in thousands) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Interest Rate Products |
|
$ |
(9,024 |
) |
|
Interest income/ (expense) |
|
$ |
(13,247 |
) |
|
|
|
|
|
|
|
|
|
|
|
During the year ended December 31, 2009, there was no amount recorded as ineffective from
accumulated other comprehensive income.
Under the Company’s agreements with its derivative counterparty, if the Company defaults on
any of its indebtedness, including default where repayment of the indebtedness has not been
accelerated by the lender, then the Company could also be declared in default on its derivative
obligations.
As of December 31, 2009, the fair value of derivatives in a net liability position related to
these agreements was $18.1 million, including accrued interest of $3.4 million but excluding
adjustments for nonperformance risk. If the Company had breached any of these provisions at
December 31, 2009, it could have been required to settle its obligations under the agreements at
their full termination value, which approximates the fair value of derivatives including accrued
interest.
82
GENERAL NUTRITION CENTERS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
Recently Issued Accounting Pronouncements
In June 2009, the Financial Accounting Standards Board (“FASB”) issued a standard on Generally
Accepted Accounting Principles. This standard establishes the FASB Accounting Standards
Codification (the “Codification”) as the single source of authoritative nongovernmental accounting
principles generally accepted in the United States of America (“U.S. GAAP”). The Codification is
effective for interim and annual periods ending after September 15, 2009. The adoption of this
standard did not have any impact on the Company’s financial statements.
In June 2009, the SEC issued a staff accounting bulletin (“SAB”) that revises or rescinds
portions of the interpretive guidance included in the codification of SABs in order to make the
interpretive guidance consistent with U.S. GAAP. The principal revisions include deletion of
material no longer necessary or that has been superseded because of the issuance of new standards.
The Company adopted this SAB during the second quarter of 2009; the adoption did not have any
impact on its consolidated financial statements.
Fair Value
In September 2006, the FASB issued new standards on fair value measurements and disclosures.
These new standards define fair value, establish a framework for measuring fair value in U.S. GAAP,
and expand disclosures about fair value measurements. The new standard applies under other
accounting pronouncements that require or permit fair value measurements and, accordingly, does not
require any new fair value measurements. The original standard was initially effective as of
January 1, 2008, but in February 2008 the FASB delayed the effectiveness date for applying this
standard to nonfinancial assets and nonfinancial liabilities that are not at fair value in the
financial statements. The standard was effective for fiscal years beginning after November 15,
2007, except for nonfinancial assets and liabilities that are recognized or disclosed at fair value
in the financial statements on a nonrecurring basis, for which application has been deferred for
one year. The Company adopted this new standard in the first quarter of fiscal 2008 for financial
assets and liabilities. See Note 23, “Fair Value Measurements,” to our consolidated financial
statements included in this report.
In February 2007, the FASB issued a new standard on financial instruments that amends a
previously issued standard. This standard expands the use of fair value accounting but does not
affect existing standards which require assets or liabilities to be carried at fair value. The
objective of the standard is to improve financial reporting by providing companies with the
opportunity to mitigate volatility in reported earnings caused by measuring related assets and
liabilities differently without having to apply complex hedge accounting provisions. Under the
standard, a company may elect to use fair value to measure eligible items at specified election
dates and report unrealized gains and losses on items for which the fair value option has been
elected in earnings at each subsequent reporting date. Eligible items include, but are not limited
to, accounts and loans receivable, available-for-sale and held-to-maturity securities, equity
method investments, accounts payable, guarantees, issued debt and firm commitments. The new
standard was effective for fiscal years beginning after November 15, 2007. The adoption of this
standard did not affect the financial statements as the Company did not elect to use the fair value
option.
In October 2008, the FASB issued a new standard on determining the fair value of a financial
asset. This standard clarifies the application of accounting standards in a market that is not
active and provides an example to illustrate key considerations in determining the fair value of a
financial asset when the market for that financial asset is not active. The new standard was
effective upon issuance for financial statements that have not been issued. The adoption of this
new standard did not have any impact on the Company’s financial assets and liabilities.
In August 2009, the FASB issued an update to the standard on fair value measurements and
disclosures. This update provides guidance on the manner in which the fair value of liabilities
should be determined. This update is effective for annual periods ending after September 15, 2009.
The adoption of this standard did not have any impact on the Company’s financial statements.
83
GENERAL NUTRITION CENTERS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
Business Combinations and Consolidation
In December 2007, the FASB issued a new standard on business combinations. This new standard
establishes principles and requirements for how an acquirer in a business combination recognizes
and measures in its financial statements the identifiable assets acquired, the liabilities assumed
and any noncontrolling interest in the acquiree at the acquisition date fair value. The new
standard significantly changes the accounting for business combinations in a number of areas,
including the treatment of contingent consideration, preacquisition contingencies, transaction
costs, in-process research and development and restructuring costs. In addition, under this
standard, changes in an acquired entity’s deferred tax assets and uncertain tax positions after the
measurement period will impact the acquirer’s income tax expense. The standard provides guidance
regarding what information to disclose to enable users of the financial statements to evaluate the
nature and financial effects of the business combination. The original standard became effective
for fiscal years beginning after December 15, 2008 with early application prohibited and amends the
standard on income taxes such that adjustments made to deferred taxes and acquired tax
contingencies after January 1, 2009, even for business combinations completed before this date,
will impact net income. The Company adopted this new standard during the first quarter of fiscal
2009; the adoption did not have any impact on its consolidated financial statements.
In December 2007, the FASB issued a new standard on consolidation. The issuance of this
new standard changes the accounting and reporting for minority interests, which have been
recharacterized as noncontrolling interests and classified as a component of equity. This new
consolidation method significantly changes the accounting for transactions with minority interest
holders. The standard was effective for fiscal years beginning after December 15, 2008 with early
application prohibited. The Company adopted this new standard during the first quarter of fiscal
2009; the adoption did not have any impact on its consolidated financial statements.
In June 2009, the FASB issued an update to the standard on consolidations. The standard is
intended to improve financial reporting by providing additional guidance to companies involved with
variable interest entities and by requiring additional disclosures about a company’s involvement in
variable interest entities. This standard is effective for interim and annual periods ending after
January 1, 2010. The adoption of this standard will not have any impact on the Company’s financial
statements.
Other
In March 2008, the FASB issued a new standard on derivatives and hedging. The new standard
requires companies with derivative instruments to disclose information that should enable financial
statement users to understand how and why a company uses derivative instruments, how derivative
instruments and related hedged items are accounted for and how derivative instruments and related
hedged items affect a company’s financial position, financial performance, and cash flows. The new
standard was effective for interim and annual periods beginning on or after November 15, 2008. The
Company adopted this standard during the first quarter of 2009; the adoption had no impact on its
consolidated financial statements.
In April 2008, the FASB issued a new standard on goodwill and intangibles which amends the
factors that should be considered in developing renewal or extension assumptions used to determine
the useful life of a recognized intangible. The intent of this new standard is to improve the
consistency between the useful life of a recognized intangible asset and the period of expected
cash flows used to measure the fair value of the asset. The Company adopted this new standard
during the first quarter of fiscal 2009; the adoption did not have any impact on its consolidated
financial statements.
In April 2009, the FASB issued a new standard on the disclosure of financial instruments.
This new standard brings the interpretive guidance into alignment with the changes in U.S. GAAP.
The Company adopted this new standard during the second quarter of fiscal 2009; the adoption did
not have any impact on its consolidated financial statements.
84
GENERAL NUTRITION CENTERS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
In May 2009, the FASB issued a standard on subsequent events which establishes general
standards of accounting for and disclosing of events that occur after the balance sheet date but
before financial statements are issued or are available to be issued. The intent of this standard
is to incorporate accounting guidance that originated as auditing standards into the body of
authoritative literature issued by the FASB which is consistent with the FASB’s objective to codify
all authoritative U.S. accounting guidance related to a particular topic in one place. The Company
adopted this standard during the second quarter of 2009; the adoption did not have any impact on
its consolidated financial statements. In September 2009, the FASB issued an update to the
standard on income taxes. This update adds to the definition of a tax position of an entity’s
status, including its status as a pass-through entity, eliminates certain disclosure requirements
for non-public entities, and provides application for pass-through entities. The adoption of this
standard did not have any impact on the Company’s financial statements.
85
GENERAL NUTRITION CENTERS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
NOTE 3. RECEIVABLES
Receivables at each respective period consisted of the following:
| |
|
|
|
|
|
|
|
|
| |
|
December 31, |
|
|
December 31, |
|
| |
|
2009 |
|
|
2008 |
|
| |
|
(in thousands) |
|
Trade receivables |
|
$ |
90,832 |
|
|
$ |
88,913 |
|
Other |
|
|
4,889 |
|
|
|
4,278 |
|
Allowance for doubtful accounts |
|
|
(1,789 |
) |
|
|
(4,147 |
) |
Related party |
|
|
423 |
|
|
|
369 |
|
|
|
|
|
|
|
|
|
|
$ |
94,355 |
|
|
$ |
89,413 |
|
|
|
|
|
|
|
|
NOTE 4. INVENTORIES, NET
Inventories at each respective period consisted of the following:
| |
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
December 31, 2009 |
|
| |
|
|
|
|
|
|
|
|
|
Net Carrying |
|
| |
|
Gross cost |
|
|
Reserves (a) |
|
|
Value |
|
| |
|
(in thousands) |
|
Finished product ready for sale |
|
$ |
319,688 |
|
|
$ |
(8,266 |
) |
|
$ |
311,422 |
|
Work-in-process, bulk product and raw materials |
|
|
54,803 |
|
|
|
(1,288 |
) |
|
|
53,515 |
|
Packaging supplies |
|
|
5,555 |
|
|
|
— |
|
|
|
5,555 |
|
|
|
|
|
|
|
|
|
|
|
|
|
$ |
380,046 |
|
|
$ |
(9,554 |
) |
|
$ |
370,492 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
December 31, 2008 |
|
| |
|
|
|
|
|
|
|
|
|
Net Carrying |
|
| |
|
Gross cost |
|
|
Reserves (a) |
|
|
Value |
|
| |
|
(in thousands) |
|
Finished product ready for sale |
|
$ |
311,218 |
|
|
$ |
(9,275 |
) |
|
$ |
301,943 |
|
Work-in-process, bulk product and raw materials |
|
|
57,995 |
|
|
|
(1,111 |
) |
|
|
56,884 |
|
Packaging supplies |
|
|
4,827 |
|
|
|
— |
|
|
|
4,827 |
|
|
|
|
|
|
|
|
|
|
|
|
|
$ |
374,040 |
|
|
$ |
(10,386 |
) |
|
$ |
363,654 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| (a) |
|
Reserves primarily consist of amounts recorded for valuation and obsolescence. |
86
GENERAL NUTRITION CENTERS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
NOTE 5. INCOME TAXES
Deferred income taxes reflect the net tax effects of temporary differences between the
carrying amount of assets and liabilities for financial reporting purposes and the amounts used for
income tax purposes.
Significant components of the Company’s deferred tax assets and liabilities at each respective
period consisted of the following:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
December 31, 2009 |
|
|
December 31, 2008 |
|
| |
|
(in thousands) |
|
| |
|
Assets |
|
|
Liabilities |
|
|
Net |
|
|
Assets |
|
|
Liabilities |
|
|
Net |
|
Deferred tax: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Current assets (liabilities): |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating reserves |
|
$ |
2,906 |
|
|
$ |
— |
|
|
$ |
2,906 |
|
|
$ |
4,510 |
|
|
$ |
— |
|
|
$ |
4,510 |
|
Deferred revenue |
|
|
1,727 |
|
|
|
— |
|
|
|
1,727 |
|
|
|
12,002 |
|
|
|
— |
|
|
|
12,002 |
|
Prepaid expenses |
|
|
— |
|
|
|
(10,170 |
) |
|
|
(10,170 |
) |
|
|
— |
|
|
|
(10,292 |
) |
|
|
(10,292 |
) |
Accrued worker compensation |
|
|
2,167 |
|
|
|
— |
|
|
|
2,167 |
|
|
|
1,845 |
|
|
|
— |
|
|
|
1,845 |
|
Foreign tax credits |
|
|
1,035 |
|
|
|
— |
|
|
|
1,035 |
|
|
|
2,858 |
|
|
|
— |
|
|
|
2,858 |
|
Other |
|
|
3,401 |
|
|
|
(1,688 |
) |
|
|
1,713 |
|
|
|
562 |
|
|
|
(30 |
) |
|
|
532 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total current |
|
$ |
11,236 |
|
|
$ |
(11,858 |
) |
|
$ |
(622 |
) |
|
$ |
21,777 |
|
|
$ |
(10,322 |
) |
|
$ |
11,455 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Non-current assets (liabilities): |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Intangibles |
|
$ |
— |
|
|
$ |
(308,724 |
) |
|
$ |
(308,724 |
) |
|
$ |
— |
|
|
$ |
(305,644 |
) |
|
$ |
(305,644 |
) |
Fixed assets |
|
|
5,255 |
|
|
|
— |
|
|
|
5,255 |
|
|
|
9,945 |
|
|
|
— |
|
|
|
9,945 |
|
Stock compensation |
|
|
2,705 |
|
|
|
— |
|
|
|
2,705 |
|
|
|
1,638 |
|
|
|
— |
|
|
|
1,638 |
|
Net operating loss carryforwards |
|
|
8,100 |
|
|
|
— |
|
|
|
8,100 |
|
|
|
12,743 |
|
|
|
— |
|
|
|
12,743 |
|
Interest rate swap |
|
|
5,343 |
|
|
|
— |
|
|
|
5,343 |
|
|
|
6,880 |
|
|
|
— |
|
|
|
6,880 |
|
Foreign tax credits. |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
3,950 |
|
|
|
— |
|
|
|
3,950 |
|
Other |
|
|
5,957 |
|
|
|
— |
|
|
|
5,957 |
|
|
|
4,475 |
|
|
|
— |
|
|
|
4,475 |
|
Valuation allowance |
|
|
(7,530 |
) |
|
|
— |
|
|
|
(7,530 |
) |
|
|
(11,990 |
) |
|
|
— |
|
|
|
(11,990 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total non-current |
|
$ |
19,830 |
|
|
$ |
(308,724 |
) |
|
$ |
(288,894 |
) |
|
$ |
27,641 |
|
|
$ |
(305,644 |
) |
|
$ |
(278,003 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total net deferred taxes |
|
$ |
31,066 |
|
|
$ |
(320,582 |
) |
|
$ |
(289,516 |
) |
|
$ |
49,418 |
|
|
$ |
(315,966 |
) |
|
$ |
(266,548 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
As of December 31, 2009 and 2008, the Company had deferred tax assets relating to state
NOLs in the amount of $8.1 million and $12.7 million, respectively. With the exception of $0.6
million and $0.8 million of deferred tax assets as of December 31, 2009 and 2008, respectively, a
valuation allowance was provided for all the state NOLs as the Company currently believes that
these NOLs, with lives ranging from five to twenty years, may not be realizable prior to their
expiration.
Deferred income taxes were not provided on cumulative undistributed earnings of international
subsidiaries, at December 31, 2009 and 2008, as unremitted earnings of the Company’s non-U.S.
subsidiaries were determined to be permanently reinvested.
Income before income taxes consisted of the following components:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Successor |
|
|
|
Predecessor |
|
| |
|
Year ended |
|
|
Year ended |
|
|
March 16- |
|
|
|
January 1- |
|
| |
|
December 31, |
|
|
December 31, |
|
|
December 31, |
|
|
|
March 15, |
|
| |
|
2009 |
|
|
2008 |
|
|
2007 |
|
|
|
2007 |
|
| |
|
(in thousands) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Domestic |
|
$ |
104,155 |
|
|
$ |
79,840 |
|
|
$ |
25,607 |
|
|
|
$ |
(63,672 |
) |
Foreign |
|
|
7,083 |
|
|
|
6,941 |
|
|
|
5,977 |
|
|
|
|
1,661 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total income (loss) before income taxes |
|
$ |
111,238 |
|
|
$ |
86,781 |
|
|
$ |
31,584 |
|
|
|
$ |
(62,011 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
87
GENERAL NUTRITION CENTERS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
| |
|
Income tax expense/(benefit) for all periods consisted of the following components: |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Successor |
|
|
|
Predecessor |
|
| |
|
Year ended |
|
|
Year ended |
|
|
March 16 - |
|
|
|
January 1 |
|
| |
|
December 31, |
|
|
December 31, |
|
|
December 31, |
|
|
|
- March 15, |
|
| |
|
2009 |
|
|
2008 |
|
|
2007 |
|
|
|
2007 |
|
| |
|
(in thousands) |
|
Current: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Federal |
|
$ |
10,373 |
|
|
$ |
3,082 |
|
|
$ |
— |
|
|
|
$ |
(21,547 |
) |
State |
|
|
6,704 |
|
|
|
3,391 |
|
|
|
462 |
|
|
|
|
(279 |
) |
Foreign |
|
|
3,111 |
|
|
|
1,157 |
|
|
|
2,835 |
|
|
|
|
444 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
20,188 |
|
|
|
7,630 |
|
|
|
3,297 |
|
|
|
|
(21,382 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Deferred: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Federal |
|
|
20,548 |
|
|
|
22,753 |
|
|
|
8,266 |
|
|
|
|
9,984 |
|
State |
|
|
883 |
|
|
|
1,618 |
|
|
|
1,037 |
|
|
|
|
701 |
|
Foreign |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
21,431 |
|
|
|
24,371 |
|
|
|
9,303 |
|
|
|
|
10,685 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Income tax expense (benefit) |
|
$ |
41,619 |
|
|
$ |
32,001 |
|
|
$ |
12,600 |
|
|
|
$ |
(10,697 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
For the year ended December 31, 2009, a net $0.5 million discrete tax benefit was
recorded while a $2.0 million discrete tax benefit was recorded for the year ended December 31,
2008.
The following table summarizes the differences between the Company’s effective tax rate for
financial reporting purposes and the federal statutory tax rate.
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Successor |
|
|
|
Predecessor |
|
| |
|
Year ended |
|
|
Year ended |
|
|
March 16 |
|
|
|
January 1- |
|
| |
|
December 31, |
|
|
December 31, |
|
|
December 31, |
|
|
|
March 15, |
|
| |
|
2009 |
|
|
2008 |
|
|
2007 |
|
|
|
2007 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Percent of pretax earnings: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Statutory federal tax rate |
|
|
35.0 |
% |
|
|
35.0 |
% |
|
|
35.0 |
% |
|
|
|
35.0 |
% |
Increase (reduction) resulting from: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
State income tax, net of federal tax benefit |
|
|
2.6 |
% |
|
|
2.6 |
% |
|
|
2.3 |
% |
|
|
|
0.6 |
% |
Other permanent differences |
|
|
0.9 |
% |
|
|
1.2 |
% |
|
|
2.0 |
% |
|
|
|
-17.4 |
% |
International operations, net of foreign tax credits |
|
|
(0.6 |
%) |
|
|
0.0 |
% |
|
|
0.0 |
% |
|
|
|
0.0 |
% |
Federal tax credits and income deductions |
|
|
(1.4 |
%) |
|
|
(2.5 |
%) |
|
|
(0.2 |
%) |
|
|
|
0.1 |
% |
Tax impact of uncertain tax positions and other |
|
|
0.9 |
% |
|
|
0.6 |
% |
|
|
0.8 |
% |
|
|
|
-1.0 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Effective income tax rate |
|
|
37.4 |
% |
|
|
36.9 |
% |
|
|
39.9 |
% |
|
|
|
17.3 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
In June 2006, the FASB issued a new standard on income taxes which applies to all open
tax positions accounted for in accordance with previously issued standards on income taxes. This
interpretation is intended to result in increased relevance and comparability in financial
reporting of income taxes and to provide more information about the uncertainty in income tax
assets and liabilities.
The Company adopted the provisions of this standard on January 1, 2007. As a result of the
implementation of the standard, the Company recognized a $0.4 million increase in the liability for
unrecognized tax benefits which was accounted for as a reduction to the January 1, 2007 balance of
retained earnings. Additionally, as a result of the implementation of the standard, the Company
recorded
88
GENERAL NUTRITION CENTERS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
$15.0 million of unrecognized tax benefits related to a balance sheet reclassification
that did not impact retained earnings. A total of $11.3 million of this reclassification relates
to the gross presentation of certain tax positions related to periods that are subject to the
indemnification provisions of the purchase agreement between the Company and Numico. Under these
provisions Numico is responsible for the satisfaction of these claims, and, as such the Company
recorded a corresponding receivable of $11.3 million. The remaining $3.7 million related to tax
positions previously categorized as current liabilities.
After the recognition of these items in connection with the implementation of the standard on
income taxes, the total liability for unrecognized tax benefits at January 1, 2007 was $14.2
million. At December 31, 2009 and 2008, the Company had a liability of $6.8 million and $5.5
million, respectively, for unrecognized tax benefits. The Company recognizes interest and penalties
accrued related to unrecognized tax benefits in income tax expense. Accrued interest and penalties
were $2.2 million and $1.2 million as of December 31, 2009 and 2008, respectively.
As of December 31, 2009, the Company is not aware of any positions for which it is reasonably
possible that the total amounts of unrecognized tax benefits will significantly increase or
decrease within the next 12 months.
The Company files a consolidated federal tax return and various consolidated and separate tax
returns as prescribed by the tax laws of the state and local jurisdictions in which it and its
subsidiaries operate. The Company has been audited by the Internal Revenue Service, (“IRS”),
through its March 15, 2007 tax year. The IRS commenced an examination of the Company’s 2005, 2006
and short period 2007 federal income tax returns in February 2008. The IRS issued an examination
report in the second quarter of 2009, the Company received notification from the IRS that the Joint
Committee of Taxation had completed its review and had taken no exceptions to the conclusions
reached by the IRS. As such the Company recorded a discrete tax benefit of $0.9 million for the
reduction of its liability of unrecognized tax benefits. The Company has various state and local
jurisdiction tax years open to examination (earliest open period 2004), and the Company also has
certain state and local jurisdictions currently under audit. As of December 31, 2009, the Company
believes that it is appropriately reserved for any potential federal and state income tax
exposures.
A reconciliation of the beginning and ending amount of unrecognized tax benefits is as
follows:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Successor |
|
|
|
Predecessor |
|
| |
|
Year Ended |
|
|
Year Ended |
|
|
|
|
|
|
|
|
| |
|
December 31, |
|
|
December 31, |
|
|
March 16 - |
|
|
|
January 1- |
|
| |
|
2009 |
|
|
2008 |
|
|
December 31, 2007 |
|
|
|
March 15, 2007 |
|
| |
|
(in thousands) |
|
|
|
|
|
|
Balance of unrecognized tax benefits at beginning of period |
|
$ |
5,542 |
|
|
$ |
6,871 |
|
|
$ |
15,771 |
|
|
|
$ |
14,190 |
|
Additions for tax positions taken during current period |
|
|
1,881 |
|
|
|
1,620 |
|
|
|
617 |
|
|
|
|
1,581 |
|
Additions for tax positions taken during prior periods |
|
|
2,108 |
|
|
|
— |
|
|
|
— |
|
|
|
|
— |
|
Reductions for tax positions taken during prior periods |
|
|
(2,264 |
) |
|
|
(2,059 |
) |
|
|
(235 |
) |
|
|
|
— |
|
Settlements |
|
|
(491 |
) |
|
|
(890 |
) |
|
|
(9,282 |
) |
|
|
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance of unrecognized tax benefits at end of period |
|
$ |
6,776 |
|
|
$ |
5,542 |
|
|
$ |
6,871 |
|
|
|
$ |
15,771 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
At December 31, 2009, the amount of unrecognized tax benefits that, if recognized, would
affect the effective tax rate is $5.9 million. While it is often difficult to predict the final
outcome or the timing of resolution of any particular uncertain tax position, we believe that
our unrecognized tax benefits reflect the most likely outcome. We adjust these unrecognized tax
benefits, as well as the related interest, in light of changing facts and circumstances. Settlement
of any particular position could require the use of cash. Favorable resolution would be recognized
as a reduction to our effective income tax rate in the period of resolution.
89
GENERAL NUTRITION CENTERS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
NOTE 6. PREPAIDS AND OTHER CURRENT ASSETS
Other current assets at each respective period consisted of the following:
| |
|
|
|
|
|
|
|
|
| |
|
December 31, |
|
|
December 31, |
|
| |
|
2009 |
|
|
2008 |
|
| |
|
(in thousands) |
|
Current portion of franchise note receivables |
|
$ |
718 |
|
|
$ |
871 |
|
Less: allowance for doubtful accounts |
|
|
— |
|
|
|
(240 |
) |
Prepaid rent |
|
|
13,397 |
|
|
|
12,992 |
|
Prepaid insurance |
|
|
4,452 |
|
|
|
5,590 |
|
Prepaid income tax |
|
|
9,737 |
|
|
|
11,138 |
|
Prepaid payroll tax |
|
|
923 |
|
|
|
3,684 |
|
Other current assets |
|
|
12,992 |
|
|
|
13,917 |
|
|
|
|
|
|
|
|
|
|
$ |
42,219 |
|
|
$ |
47,952 |
|
|
|
|
|
|
|
|
NOTE 7. GOODWILL, BRANDS, AND OTHER INTANGIBLE ASSETS, NET
Management utilized various resources in arriving at its final fair value adjustments that
were made to the Company’s financial statements as of March 16, 2007. In connection with the
Merger, final fair values were assigned to various other intangible assets. The Company’s brands
were assigned a final fair value representing the longevity of the Company name and general
recognition of the product lines. The Gold Card program was assigned a final fair value
representing the underlying customer listing, for both the Retail and Franchise segments. The
retail agreements were assigned a final fair value reflecting the opportunity to expand the Company
stores within a major drug store chain and on military facilities. A final fair value was assigned
to the agreements with the Company’s franchisees, both domestic and international, to operate
stores for a contractual period. Final fair values were assigned to the Company’s manufacturing
and wholesale segments for production and continued sales to certain customers.
For the year ended December 31, 2009 and 2008, the Company acquired 53 and 33 franchise
stores, respectively. These acquisitions are accounted for utilizing the purchase method of
accounting and the Company records the acquired inventory, fixed assets, franchise rights and
goodwill, with an applicable reduction to receivables and cash. For the year ended December 31,
2009, the total purchase prices associated with these acquisitions was $9.3 million, of which $2.5
million was paid in cash. For the year ended December 31, 2008, the total purchase price
associated with these acquisitions was $1.7 million, of which $0.3 million was paid in cash.
90
GENERAL NUTRITION CENTERS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
The following table summarizes the Company’s goodwill activity:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
Manufacturing/ |
|
|
|
|
| |
|
Retail |
|
|
Franchising |
|
|
Wholesale |
|
|
Total |
|
| |
|
(in thousands) |
|
Balance at December 31, 2007 |
|
$ |
306,126 |
|
|
$ |
117,303 |
|
|
$ |
202,841 |
|
|
$ |
626,270 |
|
Acquired franchise stores |
|
|
351 |
|
|
|
— |
|
|
|
— |
|
|
|
351 |
|
Other adjustments |
|
|
(3,712 |
) |
|
|
— |
|
|
|
— |
|
|
|
(3,712 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance at December 31, 2008 |
|
$ |
302,765 |
|
|
$ |
117,303 |
|
|
$ |
202,841 |
|
|
$ |
622,909 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Acquired franchise stores |
|
|
1,844 |
|
|
|
— |
|
|
|
— |
|
|
|
1,844 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance at December 31, 2009 |
|
$ |
304,609 |
|
|
$ |
117,303 |
|
|
$ |
202,841 |
|
|
$ |
624,753 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
During the year ended December 31, 2008, the Company recorded adjustments of $(3.7)
million related to income taxes. The 2008 adjustment relates principally to foreign tax credits
from amended federal returns.
Intangible assets other than goodwill consisted of the following at each respective period:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
Retail |
|
|
Franchise |
|
|
Operating |
|
|
Franchise |
|
|
|
|
| |
|
Gold Card |
|
|
Brand |
|
|
Brand |
|
|
Agreements |
|
|
Rights |
|
|
Total |
|
| |
|
(in thousands) |
Balance at December 31, 2007 |
|
$ |
6,041 |
|
|
$ |
500,000 |
|
|
$ |
220,000 |
|
|
$ |
165,702 |
|
|
$ |
1,129 |
|
|
$ |
892,872 |
|
Acquired franchise stores |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
195 |
|
|
|
195 |
|
Other additions |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
1,000 |
|
|
|
— |
|
|
|
1,000 |
|
Amortization expense |
|
|
(3,585 |
) |
|
|
— |
|
|
|
— |
|
|
|
(6,683 |
) |
|
|
(623 |
) |
|
|
(10,891 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance at December 31, 2008 |
|
$ |
2,456 |
|
|
$ |
500,000 |
|
|
$ |
220,000 |
|
|
$ |
160,019 |
|
|
$ |
701 |
|
|
$ |
883,176 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
Acquired franchise stores |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
953 |
|
|
|
953 |
|
Other additions |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
Amortization expense |
|
|
(2,081 |
) |
|
|
— |
|
|
|
— |
|
|
|
(6,943 |
) |
|
|
(735 |
) |
|
|
(9,759 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance at December 31, 2009 |
|
$ |
375 |
|
|
$ |
500,000 |
|
|
$ |
220,000 |
|
|
$ |
153,076 |
|
|
$ |
919 |
|
|
$ |
874,370 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The following table represents the gross carrying amount and accumulated amortization for
each major intangible asset:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Estimated |
|
|
December 31, 2009 |
|
December 31, 2008 |
| |
|
Life |
|
|
|
|
|
|
Accumulated |
|
|
Carrying |
|
|
|
|
|
|
Accumulated |
|
|
Carrying |
|
| |
|
in years |
|
|
Cost |
|
|
Amortization |
|
|
Amount |
|
|
Cost |
|
|
Amortization |
|
|
Amount |
|
| |
|
(in thousands) |
|
Brands — retail |
|
|
— |
|
|
$ |
500,000 |
|
|
$ |
— |
|
|
$ |
500,000 |
|
|
$ |
500,000 |
|
|
$ |
— |
|
|
$ |
500,000 |
|
Brands — franchise |
|
|
— |
|
|
|
220,000 |
|
|
|
— |
|
|
|
220,000 |
|
|
|
220,000 |
|
|
|
— |
|
|
|
220,000 |
|
Gold card — retail |
|
|
3 |
|
|
|
3,500 |
|
|
|
(3,354 |
) |
|
|
146 |
|
|
|
3,500 |
|
|
|
(2,545 |
) |
|
|
955 |
|
Gold card — franchise |
|
|
3 |
|
|
|
5,500 |
|
|
|
(5,271 |
) |
|
|
229 |
|
|
|
5,500 |
|
|
|
(3,999 |
) |
|
|
1,501 |
|
Retail agreements |
|
|
25-35 |
|
|
|
31,000 |
|
|
|
(3,090 |
) |
|
|
27,910 |
|
|
|
31,000 |
|
|
|
(2,038 |
) |
|
|
28,962 |
|
Franchise agreements |
|
|
25 |
|
|
|
70,000 |
|
|
|
(7,817 |
) |
|
|
62,183 |
|
|
|
70,000 |
|
|
|
(5,016 |
) |
|
|
64,984 |
|
Manufacturing agreements |
|
|
25 |
|
|
|
70,000 |
|
|
|
(7,817 |
) |
|
|
62,183 |
|
|
|
70,000 |
|
|
|
(5,017 |
) |
|
|
64,983 |
|
Other intangibles |
|
|
5 |
|
|
|
1,150 |
|
|
|
(350 |
) |
|
|
800 |
|
|
|
1,150 |
|
|
|
(60 |
) |
|
|
1,090 |
|
Franchise rights |
|
|
1-5 |
|
|
|
3,061 |
|
|
|
(2,142 |
) |
|
|
919 |
|
|
|
2,108 |
|
|
|
(1,407 |
) |
|
|
701 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ |
904,211 |
|
|
$ |
(29,841 |
) |
|
$ |
874,370 |
|
|
$ |
903,258 |
|
|
$ |
(20,082 |
) |
|
$ |
883,176 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
91
GENERAL NUTRITION CENTERS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
The following table represents future estimated amortization expense of intangible assets
with finite lives:
| |
|
|
|
|
| |
|
Estimated |
|
| |
|
amortization |
|
| Years ending December 31, |
|
expense |
|
| |
|
(in thousands) |
|
2010 |
|
|
7,710 |
|
2011 |
|
|
7,071 |
|
2012 |
|
|
6,976 |
|
2013 |
|
|
6,920 |
|
2014 |
|
|
6,679 |
|
Thereafter |
|
|
119,014 |
|
|
|
|
|
Total |
|
$ |
154,370 |
|
|
|
|
|
NOTE 8. PROPERTY, PLANT AND EQUIPMENT
Property, plant and equipment at each respective period consisted of the following:
| |
|
|
|
|
|
|
|
|
| |
|
December 31, |
|
|
December 31, |
|
| |
|
2009 |
|
|
2008 |
|
| |
|
(in thousands) |
|
Land, buildings and improvements |
|
$ |
61,572 |
|
|
$ |
59,718 |
|
Machinery and equipment |
|
|
82,273 |
|
|
|
71,230 |
|
Leasehold improvements |
|
|
72,284 |
|
|
|
63,701 |
|
Furniture and fixtures |
|
|
44,963 |
|
|
|
38,682 |
|
Software |
|
|
18,035 |
|
|
|
12,537 |
|
Construction in progress |
|
|
4,974 |
|
|
|
9,301 |
|
|
|
|
|
|
|
|
Total property, plant and equipment |
|
$ |
284,101 |
|
|
$ |
255,169 |
|
Less: accumulated depreciation |
|
|
(84,520 |
) |
|
|
(49,015 |
) |
|
|
|
|
|
|
|
Net property, plant and equipment |
|
$ |
199,581 |
|
|
$ |
206,154 |
|
|
|
|
|
|
|
|
The Company is a 50% limited partner in a partnership that owns and manages the building that
houses the Company’s corporate headquarters. The Company occupies the majority of the available
lease space of the building. The general partner is responsible for the operation and management
of the property and reports the results of the partnership to the Company. The Company has
consolidated the limited partnership, net of elimination adjustments, in the accompanying financial
statements.
92
GENERAL NUTRITION CENTERS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
NOTE 9. OTHER LONG-TERM ASSETS
Other assets at each respective period consisted of the following:
| |
|
|
|
|
|
|
|
|
| |
|
December 31, |
|
|
December 31, |
|
| |
|
2009 |
|
|
2008 |
|
| |
|
(in thousands) |
|
Long-term franchise notes receivables |
|
$ |
2,646 |
|
|
$ |
1,197 |
|
Long-term deposit |
|
|
517 |
|
|
|
511 |
|
Other |
|
|
1,169 |
|
|
|
810 |
|
|
|
|
|
|
|
|
|
|
$ |
4,332 |
|
|
$ |
2,518 |
|
|
|
|
|
|
|
|
Annual maturities of the Company’s long term and current (see current portion in Note 6,
“Prepaids and Other Current Assets”) franchise notes receivable at December 31, 2009 are as
follows:
| |
|
|
|
|
| Years ending December 31, |
|
Receivables |
|
| |
| |
|
(in thousands) |
|
2010 |
|
|
718 |
|
2011 |
|
|
692 |
|
2012 |
|
|
721 |
|
2013 |
|
|
666 |
|
2014 |
|
|
567 |
|
|
|
|
|
Total |
|
$ |
3,364 |
|
|
|
|
|
NOTE 10. ACCRUED PAYROLL AND RELATED LIABILITIES
Accrued payroll and related liabilities at each respective period consisted of the following:
| |
|
|
|
|
|
|
|
|
| |
|
December 31, |
|
|
December 31, |
|
| |
|
2009 |
|
|
2008 |
|
| |
|
(in thousands) |
|
Accrued payroll |
|
$ |
17,255 |
|
|
$ |
14,592 |
|
Accrued taxes and benefits |
|
|
5,022 |
|
|
|
7,990 |
|
|
|
|
|
|
|
|
Total |
|
$ |
22,277 |
|
|
$ |
22,582 |
|
|
|
|
|
|
|
|
93
GENERAL NUTRITION CENTERS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
NOTE 11. DEFERRED REVENUE AND OTHER CURRENT LIABILITIES
Other current liabilities at each respective period consisted of the following:
| |
|
|
|
|
|
|
|
|
| |
|
December 31, |
|
|
December 31, |
|
| |
|
2009 |
|
|
2008 |
|
| |
|
(in thousands) |
|
Deferred revenue |
|
$ |
33,837 |
|
|
$ |
32,465 |
|
Payable to former shareholders |
|
|
2,625 |
|
|
|
12,954 |
|
Accrued occupancy |
|
|
4,882 |
|
|
|
4,219 |
|
Accrued worker compensation |
|
|
5,892 |
|
|
|
5,069 |
|
Accrued taxes |
|
|
5,683 |
|
|
|
5,111 |
|
Deferred tax liability (see Note 5) |
|
|
622 |
|
|
|
— |
|
Accrued income tax |
|
|
404 |
|
|
|
— |
|
Other current liabilities |
|
|
11,185 |
|
|
|
14,491 |
|
|
|
|
|
|
|
|
Total |
|
$ |
65,130 |
|
|
$ |
74,309 |
|
|
|
|
|
|
|
|
Deferred revenue consists primarily of Gold Card and gift card deferrals.
94
GENERAL NUTRITION CENTERS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
NOTE 12. LONG-TERM DEBT / INTEREST
In conjunction with the Merger, the Company repaid certain of its existing debt and issued new
debt. The new debt, which was entered into or issued on the closing, consisted of a senior credit
facility comprised of a $675.0 million term loan facility and a $60.0 million revolving credit
facility (the “2007 Senior Credit Facility”), $300.0 million aggregate principal amount of Senior
Floating Rate Toggle Notes due 2014 (the “Senior Toggle Notes”), and $110.0 million aggregate
principal amount of 10.75% Senior Subordinated Notes due 2015 (the “10.75% Senior Subordinated
Notes”). The Company utilized proceeds from the new debt to repay its December 2003 senior credit
facility, its 8 5/8% Senior Notes issued in January 2005, and its 8 1/2% Senior
Subordinated Notes issued in December 2003.
Long-term debt at each respective period consisted of the following:
| |
|
|
|
|
|
|
|
|
| |
|
December 31, |
|
|
December 31, |
|
| |
|
2009 |
|
|
2008 |
|
| |
|
(in thousands) |
|
|
|
|
|
|
|
|
|
|
2007 Senior credit facility |
|
$ |
644,619 |
|
|
$ |
668,563 |
|
Senior Toggle Notes |
|
|
297,959 |
|
|
|
297,585 |
|
10.75% Senior Subordinated Notes |
|
|
110,000 |
|
|
|
110,000 |
|
Mortgage |
|
|
7,184 |
|
|
|
8,557 |
|
Capital leases |
|
|
47 |
|
|
|
41 |
|
Less: current maturities |
|
|
(1,724 |
) |
|
|
(13,509 |
) |
|
|
|
|
|
|
|
Total |
|
$ |
1,058,085 |
|
|
$ |
1,071,237 |
|
|
|
|
|
|
|
|
At December 31, 2009, the Company’s total debt principal maturities are as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
Senior |
|
|
10.75% Senior |
|
|
|
|
|
|
|
| Years Ending |
|
2007 Senior |
|
|
Toggle |
|
|
Subordinated |
|
|
Mortgage Loan/ |
|
|
|
|
| December 31, |
|
Credit Facility |
|
|
Notes (a) |
|
|
Notes |
|
|
Capital Leases |
|
|
Total |
|
| |
|
(in thousands) |
|
2010 |
|
$ |
237 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
1,487 |
|
|
$ |
1,724 |
|
2011 |
|
|
1,681 |
|
|
|
— |
|
|
|
— |
|
|
|
1,582 |
|
|
|
3,263 |
|
2012 |
|
|
6,750 |
|
|
|
— |
|
|
|
— |
|
|
|
1,695 |
|
|
|
8,445 |
|
2013 |
|
|
635,951 |
|
|
|
— |
|
|
|
— |
|
|
|
1,812 |
|
|
|
637,763 |
|
2014 |
|
|
— |
|
|
|
300,000 |
|
|
|
— |
|
|
|
655 |
|
|
|
300,655 |
|
Thereafter |
|
|
— |
|
|
|
— |
|
|
|
110,000 |
|
|
|
— |
|
|
|
110,000 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ |
644,619 |
|
|
$ |
300,000 |
|
|
$ |
110,000 |
|
|
$ |
7,231 |
|
|
$ |
1,061,850 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| (a) |
|
The Senior Toggle Notes include the balance of the initial original issue discount
of $3.0 million. |
95
GENERAL NUTRITION CENTERS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
The Company’s net interest expense for each respective period is as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Successor |
|
|
|
Predecessor |
|
| |
|
Year ended |
|
|
March 16- |
|
|
|
January 1- |
|
| |
|
December 31, |
|
|
December 31, |
|
|
December 31, |
|
|
|
March 15, |
|
| |
|
2009 |
|
|
2008 |
|
|
2007 |
|
|
|
2007 |
|
| |
|
(in thousands) |
|
|
|
|
|
|
|
|
2007 Senior credit facility |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Term Loan |
|
$ |
32,775 |
|
|
$ |
43,302 |
|
|
$ |
41,165 |
|
|
|
$ |
— |
|
Revolver |
|
|
489 |
|
|
|
482 |
|
|
|
374 |
|
|
|
|
— |
|
Senior Toggle Notes |
|
|
20,003 |
|
|
|
23,671 |
|
|
|
23,455 |
|
|
|
|
— |
|
10.75% Senior Subordinated Notes |
|
|
11,825 |
|
|
|
11,825 |
|
|
|
9,361 |
|
|
|
|
— |
|
Deferred financing fees |
|
|
4,104 |
|
|
|
3,907 |
|
|
|
2,922 |
|
|
|
|
589 |
|
Mortgage |
|
|
544 |
|
|
|
643 |
|
|
|
680 |
|
|
|
|
392 |
|
OID amortization |
|
|
374 |
|
|
|
339 |
|
|
|
247 |
|
|
|
|
— |
|
Interest income |
|
|
(161 |
) |
|
|
(1,169 |
) |
|
|
(2,682 |
) |
|
|
|
(336 |
) |
2003 Senior credit facility |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Term Loan |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
918 |
|
Revolver |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
132 |
|
8 1/2% Senior Subordinated Notes |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
2,695 |
|
8 5/8% Senior Notes |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
3,807 |
|
Deferred fee writedown-early extinguishment |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
11,680 |
|
Call premiums |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
23,159 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
Interest expense, net |
|
$ |
69,953 |
|
|
$ |
83,000 |
|
|
$ |
75,522 |
|
|
|
$ |
43,036 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
Accrued interest at each respective period consisted of the following:
| |
|
|
|
|
|
|
|
|
| |
|
December 31, |
|
|
December 31, |
|
| |
|
2009 |
|
|
2008 |
|
| |
|
(in thousands) |
|
|
|
|
|
|
|
|
|
|
2007 Senior credit facility |
|
$ |
5,350 |
|
|
$ |
5,564 |
|
Senior Toggle Notes |
|
|
5,720 |
|
|
|
6,700 |
|
10.75% Senior Subordinated Notes |
|
|
3,482 |
|
|
|
3,481 |
|
|
|
|
|
|
|
|
Total |
|
$ |
14,552 |
|
|
$ |
15,745 |
|
|
|
|
|
|
|
|
96
GENERAL NUTRITION CENTERS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
Description of Debt:
2007 Senior Credit Facility. The 2007 Senior Credit Facility consists of a $675.0 million
term loan facility and a $60.0 million revolving credit facility. The term loan facility will
mature in September 2013. The revolving credit facility will mature in March 2012. The 2007 Senior
Credit Facility permits the Company to prepay a portion or all of the outstanding balance without
incurring penalties (except LIBOR breakage costs). Subject to certain exceptions, the credit
agreement requires that 100% of the net cash proceeds from certain asset sales, casualty insurance,
condemnations and debt issuances, and a specified percentage (ranging from 50% to 0% based on a
defined leverage ratio) of excess cash flow (as defined in the agreement) for each fiscal year must
be used to pay down outstanding borrowings. GNC Corporation, the Company’s direct parent company,
and the Company’s existing and future direct and indirect domestic subsidiaries have guaranteed the
Company’s obligations under the 2007 Senior Credit Facility. In addition, the 2007 Senior Credit
Facility is collateralized by first priority pledges (subject to permitted liens) of the Company’s
equity interests and the equity interests of the Company’s domestic subsidiaries.
All borrowings under the 2007 Senior Credit Facility bear interest, at the Company’s option,
at a rate per annum equal to (i) the higher of (x) the prime rate (as publicly announced by JP
Morgan Chase Bank, N.A. as its prime rate in effect) and (y) the federal funds effective rate, plus
0.50% per annum plus, at December 31, 2009, applicable margins of 1.25% per annum for the term loan
facility and 1.0% per annum for the revolving credit facility or (ii) adjusted LIBOR plus 2.25% per
annum for the term loan facility and 2.0% per annum for the revolving credit facility. In addition
to paying interest on outstanding principal under the 2007 Senior Credit Facility, the Company is
required to pay a commitment fee to the lenders under the revolving credit facility in respect of
unutilized revolving loan commitments at a rate of 0.50% per annum.
The 2007 Senior Credit Facility contains customary covenants, including incurrence covenants
and certain other limitations on the ability of GNC Corporation, the Company, and its subsidiaries
to incur additional debt, guarantee other obligations, grant liens on assets, make investments or
acquisitions, dispose of assets, make optional payments or modifications of other debt instruments,
pay dividends or other payments on capital stock, engage in mergers or consolidations, enter into
sale and leaseback transactions, enter into arrangements that restrict the Company’s and its
subsidiaries’ ability to pay dividends or grant liens, engage in transactions with affiliates, and
change the passive holding company status of GNC Corporation.
The 2007 Senior Credit Facility contains events of default, including (subject to customary
cure periods and materiality thresholds) defaults based on (1) the failure to make payments under
the 2007 Senior Credit Facility when due, (2) breach of covenants, (3) inaccuracies of
representations and warranties, (4) cross-defaults to other material indebtedness, (5) bankruptcy
events, (6) material judgments, (7) certain matters arising under the Employee Retirement Income
Security Act of 1974, as amended, (8) the actual or asserted invalidity of documents relating to
any guarantee or security document, (9) the actual or asserted invalidity of any subordination
terms supporting the 2007 Senior Credit Facility, and (10) the occurrence of a change in control.
If any such event of default occurs, the lenders would be entitled to accelerate the facilities and
take various other actions, including all actions permitted to be taken by a collateralized
creditor. If certain bankruptcy events occur, the facilities will automatically accelerate.
The Company issues letters of credit as a guarantee of payment to third-payment vendors in
accordance with specified terms and conditions. It also issues letters of credit for various
insurance contracts. The revolving credit facility allows for $25.0 million of the $60.0 million
revolving credit facility to be used as collateral for outstanding letters of credit.
97
GENERAL NUTRITION CENTERS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
The Company pays interest based on the aggregate available amount of the revolving credit
facility at a per annum rate equal to 0.5%. The Company pays interest on outstanding borrowings on
the revolving credit facility at a Eurodollar rate or Adjusted Base Rate (“ABR”) plus the
applicable margin in effect. As of both December 31, 2009 and 2008, the ABR was 4.25%. The
Company also pays an additional interest rate of 2.25% per annum on all outstanding letters of
credit issued. As of December 31, 2009 and 2008, $7.9 million and $6.2 million, respectively, of
the revolving credit facility was utilized to secure letters of credit. The Company had
outstanding ABR borrowings under the revolving credit facility of $5.4 million at December 31,
2008. No amounts were outstanding at December 31, 2009.
Senior Toggle Notes. In connection with the Merger, the Company completed a private offering
of $300.0 million of Senior Floating Rate Toggle Notes due 2014 at 99% of par value. The Senior
Toggle Notes are the Company’s senior non collateralized obligations and are effectively
subordinated to all of the Company’s existing and future collateralized debt, including the 2007
Senior Credit Facility, to the extent of the assets securing such debt, rank equally with all the
Company’s existing and future non collateralized senior debt and rank senior to all the Company’s
existing and future senior subordinated debt, including the 10.75% Senior Subordinated Notes. The
Senior Toggle Notes are guaranteed on a senior non collateralized basis by each of the Company’s
existing and future domestic subsidiaries (as defined in the Senior Toggle Notes indenture). If the
Company fails to make payments on the Senior Toggle Notes, the notes guarantors must make them
instead.
The Company may elect in its sole discretion to pay interest on the Senior Toggle Notes in
cash, entirely by increasing the principal amount of the Senior Toggle Notes or issuing new Senior
Toggle Notes (“PIK interest”), or on 50% of the outstanding principal amount of the Senior Toggle
Notes in cash and on 50% of the outstanding principal amount of the Senior Toggle Notes by
increasing the principal amount of the Senior Toggle Notes or by issuing new Senior Toggle Notes
(“partial PIK interest”). Cash interest on the Senior Toggle Notes accrues at six-month LIBOR plus
4.5% per annum, and PIK interest, if any, accrues at six-month LIBOR plus 5.25% per annum. If the
Company elects to pay PIK interest or partial PIK interest, it will increase the principal amount
of the Senior Toggle Notes or issue new Senior Toggle Notes in an aggregate principal amount equal
to the amount of PIK interest for the applicable interest payment period (rounded up to the nearest
$1,000) to holders of the Senior Toggle Notes on the relevant record date. The Senior Toggle Notes
are treated as having been issued with original issue discount for U.S. federal income tax
purposes. Interest on the Senior Toggle Notes is payable semi-annually in arrears on March 15 and
September 15 of each year.
The Company may redeem some or all of the Senior Toggle Notes at any time at specified
redemption prices. If the Company experiences certain kinds of changes in control, it must offer
to purchase the notes at 101% of par plus accrued interest to the purchase date.
The Senior Toggle Notes indenture contains certain limitations and restrictions on the
Company’s and the Company’s restricted subsidiaries’ ability to incur additional debt beyond
certain levels, pay dividends, redeem or repurchase the Company’s stock or subordinated
indebtedness or make other distributions, dispose of assets, grant liens on assets, make
investments or acquisitions, engage in mergers or consolidations, enter into arrangements that
restrict the Company’s ability to pay dividends or grant liens, and engage in transactions with
affiliates. In addition, the Senior Toggle Notes indenture restricts the Company’s and certain of
the Company’s subsidiaries’ ability to declare or pay dividends to its stockholders.
In accordance with the terms of the Senior Toggle Notes purchase agreement and the offering
memorandum, these notes were required to be exchanged for publicly registered exchange notes within
210 days after the sale of these notes. As required, these notes were registered and the exchange
offer was completed on September 28, 2007.
10.75% Senior Subordinated Notes. In connection with the Merger, the Company completed a
private offering of $110.0 million of its 10.75% Senior Subordinated Notes due 2015. The
10.75% Senior Subordinated Notes are the Company’s senior subordinated non collateralized
obligations and are subordinated to all the Company’s existing and future senior debt, including
the Company’s 2007 Senior Credit Facility and the Senior Toggle Notes, rank equally with all of the
98
GENERAL NUTRITION CENTERS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
Company’s
existing and future senior subordinated debt, and rank senior to all the Company’s existing and
future subordinated debt. The 10.75% Senior Subordinated Notes are guaranteed on a senior
subordinated non collateralized basis by each of the Company’s existing and future domestic
subsidiaries (as defined in the 10.75% Senior Subordinated Notes indenture). If the Company fails
to make payments on the 10.75% Senior Subordinated Notes, the notes guarantors must make them
instead. Interest on the 10.75% Senior Subordinated Notes accrues at the rate of 10.75% per year
from March 16, 2007 and is payable semi-annually in arrears on March 15 and September 15 of each
year.
The Company may redeem some or all of the 10.75% Senior Subordinated Notes at any time at
specified redemption prices. If the Company experiences certain kinds of changes in control, it
must offer to purchase the 10.75% Senior Subordinated Notes at 101% of par plus accrued interest to
the purchase date.
The 10.75% Senior Subordinated Notes indenture contains certain limitations and restrictions
on the Company’s and its restricted subsidiaries’ ability to incur additional debt beyond certain
levels, pay dividends, redeem or repurchase the Company’s stock or subordinated indebtedness or
make other distributions, dispose of assets, grant liens on assets, make investments or
acquisitions, engage in mergers or consolidations, enter into arrangements that restrict the
Company’s ability to pay dividends or grant liens, and engage in transactions with affiliates. In
addition, the 10.75% Senior Subordinated Notes indenture restricts the Company’s and certain of the
Company’s subsidiaries’ ability to declare or pay dividends to the Company’s stockholders.
In accordance with the terms of the 10.75% Senior Subordinate Notes purchase agreement and the
offering memorandum, these notes were required to be exchanged for publicly registered exchange
notes within 210 days after the sale of these notes. As required, these notes were registered and
the exchange offer was completed on September 28, 2007.
The Company expects to fund its operations through internally generated cash and, if
necessary, from borrowings under the amount remaining available under the Company’s $60.0 million
revolving credit facility. The Company expects its primary uses of cash in the near future will be
debt service requirements, capital expenditures and working capital requirements. The Company
anticipates that cash generated from operations, together with amounts available under the
Company’s revolving credit facility, will be sufficient to meet its future operating expenses,
capital expenditures and debt service obligations as they become due. However, the Company’s
ability to make scheduled payments of principal on, to pay interest on, or to refinance the
Company’s indebtedness and to satisfy the Company’s other debt obligations will depend on the
Company’s future operating performance, which will be affected by general economic, financial and
other factors beyond the Company’s control. The Company believes that it has complied with the
Company’s covenant reporting and compliance in all material respects for the year ended December
31, 2009.
Predecessor Debt:
Senior Credit Facility. In 2003, the Company entered into a senior credit facility with a
syndicate of lenders. Our then-parent and our domestic subsidiaries guaranteed our obligations
under the senior credit facility. The senior credit facility at December 31, 2004 consisted of a
$285.0 million term loan facility and a $75.0 million revolving credit facility. This facility was
subsequently amended in December 2004. In
January 2005, as a stipulation of the December 2004 amendment, the Company used the net
proceeds of its Senior Notes (as described below) offering of $145.6 million, together with $39.4
million of cash on hand, to repay a portion of the indebtedness under the prior $285.0 million term
loan facility. In conjunction with the Merger, the Company repaid certain of its existing debt, and
issued new debt. The Company utilized proceeds from the new debt to repay its December 2003 senior
credit facility.
The Company issues letters of credit as a guarantee of payment to third-party vendors in
accordance with specified terms and conditions. It also issues letters of credit for various
insurance contracts. The revolving credit facility allows for $50.0 million of the $75 million
revolving credit facility to be used as collateral for outstanding letters of credit. As of
December 31, 2006, $9.3 million of the revolving credit facility was utilized to secure letters of
credit.
99
GENERAL NUTRITION CENTERS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
Senior Notes. In January 2005, the Company issued $150.0 million of its Senior Notes.
The Senior Notes were scheduled to mature on January 15, 2011, and bore interest at the rate of 8
5/8% per annum, which was payable semi-annually in arrears on January 15 and July 15 of each year.
In conjunction with the Merger, the Company repaid certain of its existing debt, and issued new
debt. The Company utilized proceeds from the new debt to redeem in full its 8 5/8% Senior Notes
issued in January 2005.
Senior Subordinated Notes. In 2003, the Company issued $215.0 million of its Senior
Subordinated Notes. The Senior Subordinated Notes were mature on December 1, 2010, and bore
interest at the rate of 8 1/2% per annum, which was payable semi-annually in arrears on June 1 and
December 1 of each year. In conjunction with the Merger, the Company repaid certain of its
existing debt, and issued new debt. The Company utilized proceeds from the new debt to redeem in
full its 8 1/2% Senior Subordinated Notes issued in December 2003.
NOTE 13. OTHER LONG TERM LIABILITIES
Other long term liabilities at each respective period consisted of the following:
| |
|
|
|
|
|
|
|
|
| |
|
December 31, |
|
|
December 31, |
|
| |
|
2009 |
|
|
2008 |
|
| |
|
(in thousands) |
|
Fair value of interest rate swap agreements |
|
$ |
14,679 |
|
|
$ |
18,902 |
|
Payable to former shareholders |
|
|
— |
|
|
|
767 |
|
Liability for unrecognized tax benefits |
|
|
6,776 |
|
|
|
5,542 |
|
Rent escalations |
|
|
10,569 |
|
|
|
10,089 |
|
Other |
|
|
7,496 |
|
|
|
5,684 |
|
|
|
|
|
|
|
|
Total |
|
$ |
39,520 |
|
|
$ |
40,984 |
|
|
|
|
|
|
|
|
100
GENERAL NUTRITION CENTERS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
NOTE 14. FINANCIAL INSTRUMENTS
At December 31, 2009 and 2008, the Company’s financial instruments consisted of cash and cash
equivalents, receivables, franchise notes receivable, accounts payable, certain accrued liabilities
and long-term debt. The carrying amount of cash and cash equivalents, receivables, accounts payable
and accrued liabilities approximates their fair value because of the short maturity of these
instruments. Based on the interest rates currently available and their underlying risk, the
carrying value of the franchise notes receivable approximates their fair value. These fair values
are reflected net of reserves, which are recognized according to Company policy. The Company
determined the estimated fair values of its debt by using currently available market information
and estimates and assumptions where appropriate. Accordingly, as considerable judgment is required
to determine these estimates, changes in the assumptions or methodologies may have an effect on
these estimates. The actual and estimated fair values of the Company’s financial instruments are
as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
December 31, 2009 |
|
December 31, 2008 |
| |
|
Carrying |
|
Fair |
|
Carrying |
|
Fair |
| |
|
Amount |
|
Value |
|
Amount |
|
Value |
| |
|
(in thousands) |
Cash and cash equivalents |
|
$ |
75,089 |
|
|
$ |
75,089 |
|
|
$ |
42,307 |
|
|
$ |
42,307 |
|
Receivables |
|
|
94,355 |
|
|
|
94,355 |
|
|
|
89,413 |
|
|
|
89,413 |
|
Franchise notes receivable |
|
|
3,364 |
|
|
|
3,364 |
|
|
|
1,828 |
|
|
|
1,828 |
|
Accounts payable |
|
|
95,904 |
|
|
|
95,904 |
|
|
|
123,577 |
|
|
|
123,577 |
|
Long term debt |
|
|
1,059,809 |
|
|
|
977,718 |
|
|
|
1,084,746 |
|
|
|
702,392 |
|
NOTE 15. LONG-TERM LEASE OBLIGATIONS
The Company enters into operating leases covering its retail store locations. The Company is
the primary lessor of the majority of all leased retail store locations and sublets the locations
to individual franchisees. The leases generally provide for an initial term of between five and
ten years, and may include renewal options for varying terms thereafter. The leases require minimum
monthly rental payments and a pro rata share of landlord allocated common operating expenses. Most
retail leases also require additional rentals based on a percentage of sales in excess of specified
levels (“Percent Rent”). According to the individual lease specifications, real estate taxes,
insurance and other related costs may be included in the rental payment or charged in addition to
rent. Other lease expenses relate to and include distribution facilities, transportation
equipment, data processing equipment and automobiles.
As the Company is the primary lessee for the majority of the franchise store locations, it is
ultimately liable for the lease payments to the landlord. The Company makes the payments to the
landlord directly, and then bills the franchisee for reimbursement of this cost. If a franchisee
defaults on its sub-lease and its sub-lease is terminated, the Company has in the past converted,
and expects in the future to, convert any such franchise store into a corporate store and fulfill
the remaining lease obligation.
101
GENERAL NUTRITION CENTERS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
The composition of the Company’s rental expense for all periods presented included the
following components:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Successor |
|
|
|
Predecessor |
|
| |
|
Year Ended |
|
|
Year Ended |
|
|
March 16- |
|
|
|
January 1- |
|
| |
|
December 31, |
|
|
December 31, |
|
|
December 31, |
|
|
|
March 15, |
|
| |
|
2009 |
|
|
2008 |
|
|
2007 |
|
|
|
2007 |
|
| |
|
|
|
(in thousands) |
|
|
|
Retail stores: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Rent on long-term operating leases,
net of sublease income |
|
$ |
110,365 |
|
|
$ |
109,199 |
|
|
$ |
83,867 |
|
|
|
$ |
20,887 |
|
Landlord related taxes |
|
|
16,498 |
|
|
|
15,987 |
|
|
|
12,138 |
|
|
|
|
2,987 |
|
Common operating expenses |
|
|
29,398 |
|
|
|
31,435 |
|
|
|
24,659 |
|
|
|
|
6,364 |
|
Percent rent |
|
|
15,899 |
|
|
|
14,159 |
|
|
|
9,880 |
|
|
|
|
2,863 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
172,160 |
|
|
|
170,780 |
|
|
|
130,544 |
|
|
|
|
33,101 |
|
Truck fleet |
|
|
4,740 |
|
|
|
4,363 |
|
|
|
3,441 |
|
|
|
|
904 |
|
Other |
|
|
11,189 |
|
|
|
11,331 |
|
|
|
6,847 |
|
|
|
|
4,031 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ |
188,089 |
|
|
$ |
186,474 |
|
|
$ |
140,832 |
|
|
|
$ |
38,036 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Minimum future obligations for non-cancelable operating leases with initial or remaining
terms of at least one year in effect at December 31, 2009 are as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Company |
|
|
Franchise |
|
|
|
|
|
|
|
|
|
|
|
| |
|
Retail |
|
|
Retail |
|
|
|
|
|
|
Sublease |
|
|
|
|
| |
|
Stores |
|
|
Stores |
|
|
Other |
|
|
Income |
|
|
Total |
|
| |
|
(in thousands) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2010 |
|
$ |
100,660 |
|
|
$ |
21,421 |
|
|
$ |
5,812 |
|
|
$ |
(21,421 |
) |
|
$ |
106,472 |
|
2011 |
|
|
83,393 |
|
|
|
17,312 |
|
|
|
5,025 |
|
|
|
(17,312 |
) |
|
|
88,418 |
|
2012 |
|
|
63,061 |
|
|
|
11,983 |
|
|
|
3,091 |
|
|
|
(11,983 |
) |
|
|
66,152 |
|
2013 |
|
|
46,572 |
|
|
|
6,702 |
|
|
|
2,318 |
|
|
|
(6,702 |
) |
|
|
48,890 |
|
2014 |
|
|
33,914 |
|
|
|
2,274 |
|
|
|
2,180 |
|
|
|
(2,274 |
) |
|
|
36,094 |
|
Thereafter |
|
|
79,652 |
|
|
|
2,718 |
|
|
|
964 |
|
|
|
(2,718 |
) |
|
|
80,616 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ |
407,252 |
|
|
$ |
62,410 |
|
|
$ |
19,390 |
|
|
$ |
(62,410 |
) |
|
$ |
426,642 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
NOTE 16. COMMITMENTS AND CONTINGENCIES
Litigation
The Company is engaged in various legal actions, claims and proceedings arising in the normal
course of business, including claims related to breach of contracts, products liabilities,
intellectual property matters and employment-related matters resulting from the Company’s business
activities. As with most actions such as these, an estimation of any possible and/or ultimate
liability cannot always be determined. The Company continues to assess the requirement to account
for additional contingencies in accordance with the standard on contingencies. If the Company is
required to make a payment in connection with an adverse outcome in these matters, it could have a
material impact on its financial condition and operating results.
As a manufacturer and retailer of nutritional supplements and other consumer products that are
ingested by consumers or applied to their bodies, the Company has been and is currently subjected
to various product liability claims. Although the effects of these claims to date have not been
material to the
Company, it is possible that current and future product liability claims could have a material
adverse impact on its business or financial condition. The Company currently maintains product
liability insurance with a deductible/retention of $3.0 million per claim with an aggregate cap on
retained loss of $10.0 million. The Company typically seeks and has obtained contractual
indemnification from most parties that supply raw materials for its products or that manufacture or
market products it sells. The Company also typically seeks to be added, and has been added, as an additional
102
GENERAL NUTRITION CENTERS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
insured under most of such parties’
insurance policies. The Company is also entitled to indemnification by Numico for certain losses
arising from claims related to products containing ephedra or Kava Kava sold prior to December 5,
2003. However, any such indemnification or insurance is limited by its terms and any such
indemnification, as a practical matter, is limited to the creditworthiness of the indemnifying
party and its insurer, and the absence of significant defenses by the insurers. The Company may
incur material products liability claims, which could increase its costs and adversely affect its
reputation, revenues and operating income.
Pro-Hormone/Androstenedione Cases. The Company is currently defending six lawsuits (the “Andro
Actions”) relating to the sale by GNC of certain nutritional products alleged to contain the
ingredients commonly known as Androstenedione, Androstenediol, Norandrostenedione, and
Norandrostenediol (collectively, “Andro Products”). Five of these lawsuits were filed in
California, New York, New Jersey, Pennsylvania, and Florida. The most recent case was filed in
Illinois (see Stephens and Pio matter discussed below).
In each of the six cases, plaintiffs sought, or are seeking, to certify a class and obtain
damages on behalf of the class representatives and all those similarly-situated who purchased from
the Company certain nutritional supplements alleged to contain one or more Andro Products.
In April 2006, the Company filed pleadings seeking to remove the then-pending Andro Actions to
the respective federal district courts for the districts in which the respective Andro Actions were
pending. At the same time, the Company filed motions seeking to transfer the then-pending Andro
Actions to the U.S. District Court, Southern District of New York based on “related to” bankruptcy
jurisdiction, as one of the manufacturers supplying it with Andro Products, and from whom it sought
indemnity, MuscleTech Research and Development, Inc. (“MuscleTech”), had filed for bankruptcy. The
Company was successful in removing the New Jersey, New York, Pennsylvania, and Florida Andro
Actions to federal court and transferring these actions to the U.S. District Court, Southern
District of New York based on bankruptcy jurisdiction. The California case, Guzman v. General
Nutrition Companies, Inc., was not removed and remains pending in the Superior Court of the State
of California for the County of Los Angeles.
Following the conclusion of the MuscleTech bankruptcy case, in September 2007, plaintiffs
filed a stipulation dismissing all claims related to the sale of MuscleTech products in the four
cases then-pending in the U.S. District Court, Southern District of New York (New Jersey, New York,
Pennsylvania, and Florida). Additionally, plaintiffs filed motions with the Court to remand those
actions to their respective state courts, asserting that the federal court had been divested of
jurisdiction because the MuscleTech bankruptcy action was no longer pending. That motion was never
ruled upon and has been rendered moot by the disposition of the case, discussed below.
On June 4, 2008, the U.S. District Court, Southern District of New York (on its own motion)
set a hearing for the purpose of hearing argument as to why the New Jersey, New York, Pennsylvania,
and Florida cases should not be dismissed for failure to prosecute in conformity to the Court’s
Case Management Order. Following the hearing, the Court advised that all four cases would be
dismissed with prejudice and issued an order to that effect. In August 2008, plaintiffs appealed
the dismissal of the four cases to U.S. Court of Appeals for the Second Circuit, and oral argument
was heard on October 14, 2009.
The Second Circuit reversed the dismissal and remanded the case to the U.S. District Court,
Southern District of New York.
In the Guzman case in California, plaintiffs’ Motion for Class Certification was denied on
September 8, 2008. Plaintiffs appealed on October 31, 2008. Oral arguments took place on January
15, 2010 and the court reversed the order denying class certification.
On October 3, 2008, the plaintiffs in the five other Andro Actions filed another suit related
to the sale of Andro Products in state court in Illinois. Stephens and Pio v. General Nutrition
Companies, Inc. (Case No. 08 CH 37097, Circuit Court of Cook County, Illinois, County Department,
Chancery Division). The allegations are substantially similar to all of the other Andro Actions.
103
GENERAL NUTRITION CENTERS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As any liabilities that may arise from these cases are not probable or reasonably
estimable at this time, no liability has been accrued in the accompanying financial statements.
California Wage and Break Claim. On November 4, 2008, ninety-eight plaintiffs filed
individual claims against the Company in the Superior Court of the State of California for the
County of Orange, which was removed to the U.S. District Court, Central District of California on
February 17, 2009. Each of the plaintiffs had previously been a member of a purported class in a
lawsuit filed against the Company in 2007 and resolved in September 2009. The plaintiffs allege
that they were not provided all of the rest and meal periods to which they were entitled under
California law, and further allege that we failed to pay them split shift and overtime compensation
to which they were entitled under California law. Discovery in this case is ongoing and we are
vigorously defending these matters. The court has developed a mediation procedure for handling the
pending claims and has ordered the parties to mediate with small groups of plaintiffs and stayed
the case as to the plaintiffs not participating in the mediations. The first of the mediation
sessions occurred February 10, 2010 and March 4, 2010 and did not result in any settlements. Any
liabilities that may arise from these matters are not probable or reasonably estimable at this
time.
Franchise Class Action. On November 7, 2006, Abdul Ahussain, on behalf of himself and all
others similarly situated, sued GNC Franchising, LLC and General Nutrition Corporation in the U.S.
District Court, Central District of California, Western Division. Plaintiff alleged that GNC
engages in unfair business practices designed to earn a profit at its franchisees’ expense, among
other things, in violation of California Business & Professions Code, §§ 17200 et seq. (the
“CBPC”). These alleged practices include: (1) requiring its franchises to carry slow moving
products, which cannot be returned to GNC after expiration, with the franchisee bearing the loss;
(2) requiring franchised stores to purchase new or experimental products, effectively forcing the
franchisees to provide free market research; (3) using our Gold Card program to collect information
on franchised store customers and then soliciting business from such customers; (4) underselling
its franchised stores by selling products through the GNC website at prices below or close to the
wholesale price, thereby forcing franchises to sell the same products at a loss; and (5)
manipulating prices at which franchised stores can purchase products from third-party suppliers, so
as to maintain GNC’s favored position as a product wholesaler. Plaintiffs are seeking damages in
an unspecified amount and equitable and injunctive relief. On March 19, 2008, the court certified
a class as to only plaintiffs’ claim under the CBPC. The class consists of all persons or entities
who are or were GNC franchisees in the State of California from November 13, 2002 to the date of
adjudication. Plaintiff’s individual claims were settled and dismissed. On March 18, 2009, the
Company’s motion for summary judgment was granted as to the CBPC class claim. In April 2009, GNC
filed a motion for court costs and attorneys’ fees and the court ordered the plaintiffs to pay
approximately $0.4 million to GNC for its fees and costs. Plaintiff’s judgment was satisfied in
full by October 6, 2009, and a Satisfaction of Judgment was filed with the court on that date.
Jackson Claim. On November 10, 2008, Grady Jackson, on behalf of himself and all others
similarly
situated, filed a complaint against General Nutrition Corporation and the Company in the
Superior Court of the State of California for the County of Alameda. On December 15, 2008, the
matter was removed to the U.S. District Court, Northern District of California. This consumer
class and representative action brought under California Unfair Competition and False Advertising
Law asserts, among other things, that the non-GNC product “Nikki Haskell’s Star Caps” contained a
prescription diuretic ingredient that was not disclosed on the label. On March 31, 2009, GNC filed
a motion to dismiss. By order dated June 10, 2009, the court dismissed three of the seven counts
asserted by plaintiffs. In September 2009, a settlement was reached, contemplating payment of an
immaterial amount of attorneys’ fees to putative class counsel by GNC, and distribution of GNC
discount coupons to certain putative class members. The court signed the stipulation of dismissal
on October 27, 2009.
DiMauro Claim. On December 18, 2008, plaintiffs Laura and Charles DiMauro filed a personal
injury complaint against General Nutrition Corporation in Circuit Court for Miami-Dade County
Florida. Plaintiffs allege that Laura DiMauro’s use and consumption of a non-GNC product called
“Up Your Gas” resulted in liver failure that required a liver transplant in August 2007.
Plaintiffs assert, among other things, claims for strict liability, negligence, and fraud and seek
unspecified monetary damages. GNC has moved to dismiss this case on the grounds of forum non
conveniens. In December 2009, a settlement was reached, contemplating payment of an immaterial
amount by GNC.
104
GENERAL NUTRITION CENTERS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
Romero Claim. On April 27, 2009, plaintiff J.C. Romero, a professional baseball player,
filed a complaint against, among others, General Nutrition Centers, Inc. in Superior Court of New
Jersey (Law Division/ Camden County). Plaintiff alleges that he purchased from a GNC store and
consumed 6-OXO Extreme, which is manufactured by a third party, and in August 2008, was alleged to
have tested positive for a banned substance. Plaintiff served a 50 game suspension imposed by
Major League Baseball. The seven count complaint asserts, among other things, claims for
negligence, strict liability, misrepresentation, breach implied warranty and violations of the New
Jersey Consumer Fraud Act, and seeks unspecified monetary damages. GNC tendered the claim to the
insurance company of the franchisee whose GNC store sold and allegedly misrepresented the product.
On or about October 9, 2009, GNC answered plaintiff’s first amended complaint and cross-claimed
against co-defendants Proviant Technologies and Ergopharm. Discovery in this case is ongoing and
the Company is vigorously defending the matter. Any liabilities that may arise from this matter are
not probable or reasonably estimable at this time.
Ciavarra Claim. On November 19, 2008, Ryan Ciavarra filed a personal injury lawsuit against,
among others, General Nutrition Corporation, in the District Court of Harris County, Texas.
Plaintiff alleges that his use and consumption of the diet product Hydroxycut, which is
manufactured by a third party and was, until recently, sold in the Company’s stores, caused severe
liver damage, jaundice and elevated liver enzymes. Plaintiff asserts claims for strict liability,
negligence and breach of warranty and seeks unspecified monetary damages. Any liabilities that may
arise from this matter are not probable or reasonably estimable at this time.
Hydroxycut Claims. On May 1, 2009, the FDA issued a warning on several Hydroxycut-branded
products manufactured by Iovate Health Sciences U.S.A., Inc. (“Iovate”). The FDA warning was based
on 23 reports of liver injuries from consumers who claimed to have used the products between 2002
and 2009. As a result, Iovate voluntarily recalled fourteen Hydroxycut-branded products. Following
the recall, GNC was named, among other defendants, in 22 lawsuits in several states (note that
prior to May 1, 2009, GNC was a co-defendant in one Hydroxycut case, Ciavarra (see
“Ciavarra Claim” entry above)). Iovate previously accepted GNC’s tender request for defense and
indemnification under its purchasing agreement with GNC and, as such, Iovate has accepted GNC’s
request for defense and indemnification in the new Hydroxycut matters. GNC’s ability to obtain
full recovery in respect of any claims against GNC in connection with products manufactured by
Iovate under the indemnity is dependent on Iovate’s insurance coverage and the
creditworthiness of its insurer, and the absence of significant defenses by the insurer. To the
extent GNC was not fully compensated by Iovate’s insurer, it could seek recovery directly from
Iovate. GNC’s ability to fully recover such amounts would be limited by the creditworthiness of
Iovate.
GNC has been named in 23 lawsuits related to Hydroxycut: 17 individual, largely personal
injury claims (including Ciavarra discussed above) and 6 putative class actions, generally
inclusive of claims of consumer fraud, misrepresentation, strict liability, and breach of warranty.
The following 16 personal injury matters were filed by individuals claiming injuries from use
and consumption of Hydroxycut-branded products:
| |
• |
|
Michael Owens and Donna Owens v. Iovate Health Sciences USA, Inc., et al., Superior
Court of the State of California, County of Los Angeles, BC413006 (filed May 1, 2009); |
| |
| |
• |
|
Eva M. Stasiak v. Iovate Health Sciences USA, Inc., et al., Superior Court of the
State of California, County of Los Angeles, BC413201 (filed May 11, 2009); |
| |
| |
• |
|
Jaime Ruben Perez v. Gerald Brandt, individually and d/b/a/ Breakthru Products, et
al., 229th Judicial District, Duval County, Texas (filed May 29, 2009); |
| |
| |
• |
|
Juan A. Noyola, II v. Iovate Health Sciences USA, Inc., et al., U.S. District Court,
Southern District of New York, 09CV6740 (filed July 29, 2009); |
| |
| |
• |
|
Christopher and Dana Hamilton v. Iovate Health Sciences USA, Inc., et al., U.S.
District Court, Northern District of Ohio, 09CV1944 (filed August 18, 2009); |
105
GENERAL NUTRITION CENTERS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
| |
• |
|
Hector Manuel Abarca and Diana Curiel v. Iovate Health Sciences USA, Inc., et
al., U.S. District Court, Northern District of California, 09CV3861 (filed August 21,
2009); |
| |
| |
• |
|
Jessica Rogoff v. General Nutrition Centers, Inc., et al., Superior Court of the
State of California, County of Los Angeles, BC422842 (filed September 29, 2009); |
| |
| |
• |
|
Pattie Greenwood, et al. v. Iovate Health Sciences, USA, Inc., et al., State Court
of Oklahoma County, CJ-2009-10759 (filed October 30, 2009); |
| |
| |
• |
|
Lucretia Ballou v. Muscletech Research and Development, Inc., et al., U.S. District
Court, Western District of Louisiana, 09CV1996 (filed December 3, 2009); |
| |
| |
• |
|
Clinton Davis v. GNC Corporation, et al., U.S. District Court, Eastern District of
Pennsylvania, 09CV5055 (filed November 11, 2009); |
| |
| |
• |
|
Michael Fyalka v. Iovate Health Sciences, et al., U.S. District Court, Southern
District of Illinois, 09CV944 (filed November 10, 2009); |
| |
| |
• |
|
Monica Fay Stepter v. Iovate Health Sciences, USA, Inc., et al., 17th
Judicial District Court, Parish of LaFourche, Louisiana (filed November 25, 2009); |
| |
| |
• |
|
Andrew Nolley v. Muscletech Research and Development, et al., U.S. District Court,
Northern District of Mississippi, 09CV140 (filed December 18, 2009); |
| |
| |
• |
|
Kerry Donald v. Iovate Health Sciences Group, et al., Supreme Court of the State of
New York, Kings County (filed January 22, 2010); |
| |
| |
• |
|
Casey Slyter v. GNC Corporation, et al., U.S. District Court, District of Kansas,
10CV2065 (filed January 29, 2010); and |
| |
| |
• |
|
Debra Rutherford, et al. v. Muscletech Research and Development, Inc., U.S. District
Court, Northern District of Alabama, 10CV370 (filed February 19, 2010). |
Plaintiffs in the Owens and Stasiak cases dismissed those cases without prejudice in June 2009.
Plaintiff Perez filed a Notice of Non-suit without prejudice which was granted by court order on
November 18, 2009. Plaintiff Noyola amended the original complaint in December 2009 and did not
identify GNC as a defendant.
The following six putative class actions generally include claims of consumer fraud,
misrepresentation, strict liability, and breach of warranty:
| |
• |
|
Andrew Dremak, et al. v. Iovate Health Sciences Group, Inc., et al., U.S. District
Court, Southern District of California, 09CV1088 (filed May 19, 2009); |
| |
| |
• |
|
Enjoli Pennier, et al. v. Iovate Health Sciences, et al., U.S. District Court,
Eastern District of Louisiana, 09CV3533 (filed May 13, 2009); |
| |
| |
• |
|
Alejandro M. Jimenez, et al. v. Iovate Health Sciences, Inc., et al., U.S. District
Court, Eastern District of California, 09CV1473 (filed May 28, 2009); |
| |
| |
• |
|
Amy Baker, et al. v. Iovate Health Sciences USA, Inc., et al., U.S. District Court,
Northern District of Alabama, 09CV872 (filed May 4, 2009); |
| |
| |
• |
|
Kyle Davis and Sara Carreon, et al. v. Iovate Health Sciences USA, Inc., et al.,
U.S. District Court, Northern District of Alabama, 09CV896 (filed May 7, 2009); and |
| |
| |
• |
|
Lenny Charles Gunn, Tonya Rhoden, and Nicholas Atelevich, et al., v. Iovate Health
Sciences Group, Inc., et al., U.S. District Court, Southern District of California,
09CV2337 (filed October 24, 2009). |
By court order dated October 6, 2009, the United States Judicial Panel on Multidistrict Litigation
consolidated pretrial proceedings of many of the pending actions (including the above-listed GNC
class actions) in the Southern District of California (In re: Hydroxycut Marketing and Sales
Practices Litigation, MDL No. 2087). Any liabilities that may arise from these matters are not
probable or reasonably estimable at this time.
Bedell Claim. On May 1, 2009, plaintiff Eugene Bedell, Jr. filed a personal injury complaint
against, among others, General Nutrition Centers, Inc. and GNC Corporation, in Circuit Court of
Loudoun County, Virginia. Plaintiff alleges that his use and consumption of a non-GNC product
called “Nitro T3” caused him to have a stroke. Plaintiff makes certain allegations regarding the
risks of using and consuming Nitro T3 and GNC’s alleged failure to warn consumers of those risks.
Plaintiff seeks unspecified monetary
106
GENERAL NUTRITION CENTERS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
damages. Under its purchasing agreement with the vendor, WellNx, GNC tendered the matter
to WellNx for defense and indemnification. WellNx has accepted GNC’s request for defense and
indemnification. Any liabilities that may arise from this matter are not probable or reasonably
estimable at this time.
Chen Claim. On April 30, 2009, plaintiff Yuging “Phillis” Chen filed a Third Amended Complaint
against, among others, Nutra Manufacturing, Inc., in the Superior Court of California for the
County of Los Angeles. Plaintiff alleges that her use and consumption of various products,
including Mega Garlic Plus and Herbalifeline, manufactured by Nutra Manufacturing, caused personal
injuries. Plaintiff asserts, among other things, claims for strict liability, negligence, and
fraud, and seeks unspecified monetary damages. Any liabilities that may arise from this matter are
not probable or reasonably estimable at this time.
California False Labeling and Consumer Fraud Claims. Beginning in May 2009, a series of false
labeling and consumer fraud cases (listed below) were filed in California in connection with label
claims of non-GNC products sold in the Company’s stores.
| |
• |
|
Michael Gonzales and Zia Nawabi, et al. v. Maximum Human Performance, Inc., et al., U.S.
District Court, Central District of California, 09CV532 (filed May 5, 2009) (“Musclemeds”); |
| |
| |
• |
|
Michael Campos and Michael Gonzales, et al. v. LG Sciences, LLC, et al., Superior Court
of California, for the County of Orange, removed to U.S. District Court, Central District
of California, 09CV663 (filed May 20, 2009) (“LG Sciences”); |
| |
| |
• |
|
Nicole Forlenza and Shaiden Monroe, et al. v. Dynakor Pharmacal, LLC, et al., U.S.
District Court, Central District of California, 09CV3730 (filed May 26, 2009) (“Basic
Research — Akävar”); |
| |
| |
• |
|
Vance Monroe and Mac Gonzales, et al. v. Biotab Nutraceuticals, Inc., et al., U.S.
District Court, Southern District of California, 09CV1207 (filed June 3, 2009); |
| |
| |
• |
|
Richard Johnson, et al. v. Vianda, LLC, et al., Superior Court of California, for the
County of Los Angeles, BC 423825 (filed October 20, 2009); and |
| |
| |
• |
|
Michael Flores, et al. v. EP2. Inc., et al., U.S. District Court, Central District of
California, 09CV7872 (filed October 28, 2009). |
The LG Sciences matter was settled and dismissed in August 2009. A settlement was reached and
approved by the Court in December 2009 in the Musclemeds case. Plaintiffs in the Basic Research -
Akävar case dismissed GNC with prejudice in January 2010, after which a similar action, Shalena
Dysthe, et al., v. Basic Research, et al., U.S. District Court, Central District of California,
09CV8013 (filed November 2, 2009) was filed by a different law firm.
Commitments
The Company maintains certain purchase commitments with various vendors to ensure its
operational needs are fulfilled of approximately $20.5 million. The future purchase commitments
consisted of $9.7 million of advertising, inventory commitments and spending for website redesign,
$10.8 million management services agreement and bank fees. Other commitments related to the
Company’s business operations cover varying periods of time and are not significant. All of these
commitments are expected to be fulfilled with no adverse consequences to the Company’s operations
of financial condition.
Environmental Compliance
In March 2008, the South Carolina Department of Health and Environmental Control (“DHEC”)
requested that the Company investigate its South Carolina facility for a possible source or sources
of contamination detected on an adjoining property. The Company has commenced the investigation at
the facility as requested by DHEC. After several phases of the investigation the possible source
or sources of contamination have not been sufficiently identified. The Company is continuing such
investigation. The proceedings in this matter have not yet progressed to a stage where it is
possible to estimate the timing and extent of any remedial action that may be required, the
ultimate cost of remediation, or the amount of the Company’s potential liability.
107
GENERAL NUTRITION CENTERS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
In addition to the foregoing, the Company is subject to numerous federal, state, local, and
foreign environmental and health and safety laws and regulations governing its operations,
including the handling, transportation, and disposal of the Company’s non-hazardous and hazardous
substances and wastes, as well as emissions and discharges from its operations into the
environment, including discharges to air, surface water, and groundwater. Failure to comply with
such laws and regulations could result in costs for remedial actions, penalties, or the imposition
of other liabilities. New laws, changes in existing laws or the interpretation thereof, or the
development of new facts or changes in their processes could also cause the Company to incur
additional capital and operation expenditures to maintain compliance with environmental laws and
regulations and environmental permits. The Company also is subject to laws and regulations that
impose liability and cleanup responsibility for releases of hazardous substances into the
environment without regard to fault or knowledge about the condition or action causing the
liability. Under certain of these laws and regulations, such liabilities can be imposed for cleanup
of previously owned or operated properties, or for properties to which substances or wastes that
were sent in connection with current or former operations at its facilities. The presence of
contamination from such substances or wastes could also adversely affect the Company’s ability to
sell or lease its properties, or to use them as collateral for financing. From time to time, the
Company has incurred costs and obligations for correcting environmental and health and safety
noncompliance matters and for remediation at or relating to certain of its properties or properties
at which its waste has been disposed. The Company believes it has complied with, and is currently
complying with, its environmental obligations pursuant to environmental and health and safety laws
and regulations and that any liabilities for noncompliance will not have a material adverse effect
on its business or financial performance. However, it is difficult to predict future liabilities
and obligations, which could be material.
NOTE 17. STOCKHOLDER’S EQUITY
At December 31, 2009 there were 100 shares of Common Stock, par value $.01 per share,
outstanding. All of our outstanding stock was owned by GNC Corporation, our direct parent, at
December 31, 2009.
The accumulated balances of other comprehensive income and their related tax effects included
as part of the consolidated financial statements are as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
Tax Benefit |
|
|
|
|
| |
|
Before tax amount |
|
|
(Expense) |
|
|
Net Other Comprehensive Income (Loss) |
|
| |
|
|
|
|
|
Unrealized |
|
|
|
|
|
|
|
|
|
|
Unrealized |
|
|
|
|
| |
|
Foreign |
|
|
Gain/(Loss) |
|
|
Unrealized |
|
|
Foreign |
|
|
Gain/(Loss) |
|
|
|
|
| |
|
currency |
|
|
on |
|
|
Gain/(Loss) on |
|
|
currency |
|
|
on |
|
|
|
|
| $ in thousands |
|
translation |
|
|
Derivatives |
|
|
Derivatives |
|
|
translation |
|
|
Derivatives |
|
|
Total |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance at December 31, 2007 |
|
$ |
(852 |
) |
|
$ |
— |
|
|
$ |
— |
|
|
$ |
(852 |
) |
|
$ |
— |
|
|
$ |
(852 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Foreign currency translation adjustment |
|
|
(4,767 |
) |
|
|
— |
|
|
|
— |
|
|
|
(4,767 |
) |
|
|
— |
|
|
|
(4,767 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Unrealized loss on derivatives designated as
cash flow hedge, net of tax |
|
|
— |
|
|
|
(13,267 |
) |
|
|
4,829 |
|
|
|
— |
|
|
|
(8,438 |
) |
|
|
(8,438 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance at December 31, 2008 |
|
|
(5,619 |
) |
|
|
(13,267 |
) |
|
|
4,829 |
|
|
|
(5,619 |
) |
|
|
(8,438 |
) |
|
|
(14,057 |
) |
Foreign currency translation adjustment |
|
|
4,172 |
|
|
|
— |
|
|
|
— |
|
|
|
4,172 |
|
|
|
— |
|
|
|
4,172 |
|
Unrealized gain on derivatives designated as
cash flow hedge, net of tax |
|
|
— |
|
|
|
4,223 |
|
|
|
(1,537 |
) |
|
|
— |
|
|
|
2,686 |
|
|
|
2,686 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance at December 31, 2009 |
|
$ |
(1,447 |
) |
|
$ |
(9,044 |
) |
|
$ |
3,292 |
|
|
$ |
(1,447 |
) |
|
$ |
(5,752 |
) |
|
$ |
(7,199 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
108
GENERAL NUTRITION CENTERS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
NOTE 18. STOCK-BASED COMPENSATION PLANS
Stock Options
The Company utilizes the Black-Scholes model to calculate the fair value of options under the
standard, which is consistent with disclosures previously included in prior year financial
statements under the previous standard for stock compensation. The resulting compensation cost is
recognized in the Company’s financial statements over the option vesting period. At December 31,
2009, the net unrecognized compensation cost was $8.8 million and is expected to be recognized over
a weighted average period of approximately 2.4 years.
In 2007, the Board of Directors of the Parent (the “Board”) and Parent’s stockholders approved
and adopted the GNC Acquisition Holdings Inc. 2007 Stock Incentive Plan (the “2007 Plan”). The
purpose of the Plan is to enable the Parent to attract and retain highly qualified personnel who
will contribute to the success of the Company. The Plan provides for the granting of stock
options, restricted stock, and other stock-based awards. The Plan is available to certain eligible
employees, directors, consultants or advisors as determined by the administering committee of the
Board. The total number of shares of our Parent’s Class A common stock reserved and available for
the 2007 Plan is 10.4 million shares. Stock options under the Plan generally are granted with
exercise prices at or above fair market value, typically vest over a four or five-year period and
expire ten years from date of grant. No stock appreciation rights, restricted stock, deferred
stock or performance shares have been granted under the Plan.
The following table outlines our Parent’s total stock options activity:
| |
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
Weighted |
| |
|
|
|
|
|
Average |
| |
|
Total Options |
|
Exercise Price |
|
|
|
|
|
|
|
|
|
Outstanding at December 31, 2008 |
|
|
8,883,692 |
|
|
$ |
7.10 |
|
Granted |
|
|
806,850 |
|
|
|
10.16 |
|
Exercised |
|
|
— |
|
|
|
— |
|
Forfeited |
|
|
(338,715 |
) |
|
|
6.25 |
|
Expired |
|
|
(88,187 |
) |
|
|
6.25 |
|
|
|
|
|
|
|
|
|
|
Outstanding at December 31, 2009 |
|
|
9,263,640 |
|
|
$ |
7.27 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Exercisable at December 31, 2009 |
|
|
3,173,710 |
|
|
$ |
6.65 |
|
|
|
|
|
|
|
|
|
|
The standard on stock compensation requires that the cost resulting from all share-based
payment transactions be recognized in the financial statements. Stock-based compensation expense
for the years ended December 31, 2009 and 2008 and for the period from March 16, 2007 to December
31, 2007 was $2.9 million, $2.6 million and $1.9 million, respectively.
As of December 31, 2009, the weighted average remaining contractual life of outstanding
options was 7.7 years. At December 31, 2009, the weighted average remaining contractual life of
exercisable options was 7.9 years. The weighted average fair value of options granted during 2009,
2008, and 2007, was $3.19, $1.17, and $1.61, respectively.
The Black-Scholes model utilizes the following assumptions in determining a fair value: price
of underlying stock, option exercise price, expected option term, risk-free interest rate, expected
dividend yield, and expected stock price volatility over the option’s expected term. As the Company
has had minimal exercises of stock options through December 31, 2009, 2008 and 2007 option term has
been estimated by considering both the vesting period, which is typically for the successor and
predecessor plans, five and four years, respectively, and the contractual term of ten and seven
years, respectively. As the Company’s underlying stock is not publicly traded on an open market,
the Company utilized its current peer group
109
GENERAL NUTRITION CENTERS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
average to estimate the expected volatility. The
assumptions used in the Company’s Black-Scholes valuation related to stock option grants made
during the year ended December 31, 2009, 2008 and 2007 were as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
2009 |
|
2008 |
|
2007 |
| |
Dividend yield |
|
|
0.00 |
% |
|
|
0.00 |
% |
|
|
0.00 |
% |
Expected option life |
|
7.5 years |
|
7.5 years |
|
7.5 years |
Volatility factor percentage of market price |
|
|
34.20%-44.60 |
% |
|
|
26.00%-28.40 |
% |
|
|
23.00%-25.00 |
% |
Discount rate |
|
|
0.43%-3.28 |
% |
|
|
3.08%-3.64 |
% |
|
|
4.16%-4.96 |
% |
As the Black-Scholes option valuation model utilizes certain estimates and assumptions, the
existing models do not necessarily represent the definitive fair value of options for future
periods.
Predecessor
In 2006, the Board of Directors of the Company and GNC Corporation approved and adopted the
GNC Corporation 2006 Omnibus Stock Incentive Plan (the “2006 Plan”). In 2003 the boards approved
and adopted the GNC Corporation (f/k/a General Nutrition Centers Holding Company) 2003 Omnibus
Stock Incentive Plan (the “2003 Plan” and, together with the 2006 Plan, the “Plans”). The purpose
of the Plans was to enable the Company to attract and retain highly qualified personnel who would
contribute to the success of the Company. The Plans provided for the granting of stock options,
stock appreciation rights, restricted stock, deferred stock and performance shares. The Plans were
available to certain eligible employees, directors, consultants or advisors as determined by the
administering committee of the boards. The total number of shares of GNC Corporation’s common
stock reserved and available for the 2006 Plan was 3.8 million shares and under the 2003 Plan was
4.0 million shares. Stock options under the Plans generally were granted at fair market value,
vested over a four-year vesting schedule and expired after seven years from date of grant. If
stock options were granted at an exercise price that was less than fair market value at the date of
grant, compensation expense was recognized immediately for the intrinsic value. No stock
appreciation rights, restricted stock, deferred stock or performance shares were granted under the
Plans as of December 31, 2006.
The standard on stock compensation requires that the cost resulting from all share-based
payment transactions be recognized in the financial statements. For the period from January 1, 2007
to March 15, 2007, the Company recognized total compensation expense of $4.1 million, of which $3.8
million related to the acceleration of the vesting of these options. The Company recorded $47.0
million as a reduction in equity on March 15, 2007 related to the cancellation of these options.
110
GENERAL NUTRITION CENTERS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
NOTE 19. SEGMENTS
The Company has three operating segments, each of which is a reportable segment. The
operating segments represent identifiable components of the Company for which separate financial
information is available. This information is utilized by management to assess performance and
allocate assets accordingly. The Company’s management evaluates segment operating results based on
several indicators. The primary key performance indicators are sales and operating income or loss
for each segment. Operating income or loss, as evaluated by management, excludes certain items
that are managed at the consolidated level, such as distribution and warehousing, impairments and
other corporate costs. The following table represents key financial information for each of the
Company’s business segments, identifiable by the distinct operations and management of each:
Retail, Franchising, and Manufacturing/Wholesale. The Retail segment includes the Company’s
corporate store operations in the United States, Canada and its www.gnc.com business. The
Franchise segment represents the Company’s franchise operations, both domestically and
internationally. The Manufacturing/Wholesale segment represents the Company’s manufacturing
operations in South Carolina and the Wholesale sales business. This segment supplies the Retail
and Franchise segments, along with various third parties, with finished products for sale. The
Warehousing and Distribution, Corporate Costs, and Other Unallocated Costs represent the Company’s
administrative expenses. The accounting policies of the segments are the same as those described in
the “Basis of Presentation and Summary of Significant Accounting Policies”.
111
GENERAL NUTRITION CENTERS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
The following table represents key financial information of the Company’s business segments:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Successor |
|
|
|
Predecessor |
|
| |
|
Year Ended |
|
|
Year Ended |
|
|
March 16 - |
|
|
|
January 1 - |
|
| |
|
December 31, |
|
|
December 31, |
|
|
December 31, |
|
|
|
March 15, |
|
| |
|
2009 |
|
|
2008 |
|
|
2007 |
|
|
|
2007 |
|
| |
|
(in thousands) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Revenue: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Retail |
|
$ |
1,256,314 |
|
|
$ |
1,219,305 |
|
|
$ |
909,264 |
|
|
|
$ |
259,313 |
|
Franchise |
|
|
264,168 |
|
|
|
258,020 |
|
|
|
193,896 |
|
|
|
|
47,237 |
|
Manufacturing/Wholesale: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Intersegment (1) |
|
|
201,306 |
|
|
|
180,070 |
|
|
|
133,051 |
|
|
|
|
35,477 |
|
Third Party |
|
|
186,525 |
|
|
|
179,404 |
|
|
|
119,827 |
|
|
|
|
23,279 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Sub total Manufacturing/Wholesale |
|
|
387,831 |
|
|
|
359,474 |
|
|
|
252,878 |
|
|
|
|
58,756 |
|
Sub total segment revenues |
|
|
1,908,313 |
|
|
|
1,836,799 |
|
|
|
1,356,038 |
|
|
|
|
365,306 |
|
Intersegment elimination (1) |
|
|
(201,306 |
) |
|
|
(180,070 |
) |
|
|
(133,051 |
) |
|
|
|
(35,477 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total revenue |
|
$ |
1,707,007 |
|
|
$ |
1,656,729 |
|
|
$ |
1,222,987 |
|
|
|
$ |
329,829 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(1) Intersegment revenues are eliminated from consolidated revenue. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating income (loss): |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Retail |
|
$ |
153,142 |
|
|
$ |
140,916 |
|
|
$ |
106,448 |
|
|
|
$ |
28,249 |
|
Franchise |
|
|
80,800 |
|
|
|
80,816 |
|
|
|
55,000 |
|
|
|
|
14,518 |
|
Manufacturing/Wholesale |
|
|
73,450 |
|
|
|
67,378 |
|
|
|
38,915 |
|
|
|
|
10,267 |
|
Unallocated corporate and other costs: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Warehousing and distribution costs |
|
|
(53,557 |
) |
|
|
(54,266 |
) |
|
|
(40,697 |
) |
|
|
|
(10,667 |
) |
Corporate costs |
|
|
(72,644 |
) |
|
|
(65,063 |
) |
|
|
(52,560 |
) |
|
|
|
(26,739 |
) |
Merger-related costs |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
(34,603 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Sub total unallocated corporate and
other costs |
|
|
(126,201 |
) |
|
|
(119,329 |
) |
|
|
(93,257 |
) |
|
|
|
(72,009 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total operating income (loss) |
|
|
181,191 |
|
|
|
169,781 |
|
|
|
107,106 |
|
|
|
|
(18,975 |
) |
Interest expense, net |
|
|
69,953 |
|
|
|
83,000 |
|
|
|
75,522 |
|
|
|
|
43,036 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Income before income taxes |
|
|
111,238 |
|
|
|
86,781 |
|
|
|
31,584 |
|
|
|
|
(62,011 |
) |
Income tax expense (benefit) |
|
|
41,619 |
|
|
|
32,001 |
|
|
|
12,600 |
|
|
|
|
(10,697 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income (loss) |
|
$ |
69,619 |
|
|
$ |
54,780 |
|
|
$ |
18,984 |
|
|
|
$ |
(51,314 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
112
GENERAL NUTRITION CENTERS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Successor |
|
|
|
Predecessor |
|
| |
|
Year Ended |
|
|
Year Ended |
|
|
March 16 - |
|
|
|
January 1 - |
|
| |
|
December 31, |
|
|
December 31, |
|
|
December 31, |
|
|
|
March 15, |
|
| |
|
2009 |
|
|
2008 |
|
|
2007 |
|
|
|
2007 |
|
| |
|
(in thousands) |
|
Depreciation and amortization: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Retail |
|
$ |
24,164 |
|
|
$ |
21,449 |
|
|
$ |
14,806 |
|
|
|
$ |
4,114 |
|
Franchise |
|
|
4,081 |
|
|
|
5,001 |
|
|
|
4,025 |
|
|
|
|
365 |
|
Manufacturing / Wholesale |
|
|
10,926 |
|
|
|
9,783 |
|
|
|
7,014 |
|
|
|
|
1,714 |
|
Corporate / Other |
|
|
7,494 |
|
|
|
6,220 |
|
|
|
4,156 |
|
|
|
|
1,183 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total depreciation and amortization |
|
$ |
46,665 |
|
|
$ |
42,453 |
|
|
$ |
30,001 |
|
|
|
$ |
7,376 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Capital expenditures: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Retail |
|
$ |
20,640 |
|
|
$ |
33,074 |
|
|
$ |
18,347 |
|
|
|
$ |
4,778 |
|
Franchise |
|
|
2 |
|
|
|
7 |
|
|
|
4 |
|
|
|
|
— |
|
Manufacturing / Wholesale |
|
|
4,527 |
|
|
|
11,108 |
|
|
|
6,694 |
|
|
|
|
285 |
|
Corporate / Other |
|
|
3,513 |
|
|
|
4,477 |
|
|
|
3,806 |
|
|
|
|
630 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total capital expenditures |
|
$ |
28,682 |
|
|
$ |
48,666 |
|
|
$ |
28,851 |
|
|
|
$ |
5,693 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total assets |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Retail |
|
$ |
1,262,755 |
|
|
$ |
1,263,229 |
|
|
$ |
1,242,999 |
|
|
|
$ |
472,131 |
|
Franchise |
|
|
468,949 |
|
|
|
471,247 |
|
|
|
476,685 |
|
|
|
|
273,348 |
|
Manufacturing / Wholesale |
|
|
423,884 |
|
|
|
436,018 |
|
|
|
426,250 |
|
|
|
|
129,438 |
|
Corporate / Other |
|
|
149,240 |
|
|
|
121,514 |
|
|
|
93,698 |
|
|
|
|
106,348 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total assets |
|
$ |
2,304,828 |
|
|
$ |
2,292,008 |
|
|
$ |
2,239,632 |
|
|
|
$ |
981,265 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Geographic areas |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total revenues: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
United States |
|
$ |
1,618,452 |
|
|
$ |
1,567,641 |
|
|
$ |
1,156,806 |
|
|
|
$ |
314,804 |
|
Foreign |
|
|
88,555 |
|
|
|
89,088 |
|
|
|
66,181 |
|
|
|
|
15,025 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total revenues |
|
$ |
1,707,007 |
|
|
$ |
1,656,729 |
|
|
$ |
1,222,987 |
|
|
|
$ |
329,829 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Long-lived assets: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
United States |
|
$ |
193,762 |
|
|
$ |
201,787 |
|
|
$ |
189,416 |
|
|
|
$ |
181,617 |
|
Foreign |
|
|
10,151 |
|
|
|
6,885 |
|
|
|
6,526 |
|
|
|
|
3,323 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total long-lived assets |
|
$ |
203,913 |
|
|
$ |
208,672 |
|
|
$ |
195,942 |
|
|
|
$ |
184,940 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The following table represents sales by general product category. The category “Other”
includes other wellness products sales from the Company’s point of sales system and certain
required accounting adjustments of $5.7 million for 2009, $4.7 million for 2008, $5.0 million for
the period from March 16 to December 31, 2007, and ($0.6) million for the period from January 1 to
March 15, 2007.
113
GENERAL NUTRITION CENTERS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Successor |
|
|
|
Predecessor |
|
| |
|
Year ended |
|
|
Year ended |
|
|
March 16- |
|
|
|
January 1- |
|
| |
|
December 31, |
|
|
December 31, |
|
|
December 31 |
|
|
|
March 15, |
|
| U.S Retail Product Categories: |
|
2009 |
|
|
2008 |
|
|
2007 |
|
|
|
2007 |
|
| |
|
(in thousands) |
|
VMHS |
|
$ |
496,427 |
|
|
$ |
465,245 |
|
|
$ |
342,731 |
|
|
|
$ |
98,447 |
|
Sports Nutrition Products |
|
|
443,408 |
|
|
|
410,133 |
|
|
|
299,035 |
|
|
|
|
87,983 |
|
Diet and Weight Management Products |
|
|
128,039 |
|
|
|
148,158 |
|
|
|
120,099 |
|
|
|
|
36,647 |
|
Other Wellness Products |
|
|
99,886 |
|
|
|
106,681 |
|
|
|
81,218 |
|
|
|
|
21,211 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total U.S. Retail revenues |
|
|
1,167,759 |
|
|
|
1,130,217 |
|
|
|
843,083 |
|
|
|
|
244,288 |
|
Canada retail revenues (1) |
|
|
88,555 |
|
|
|
89,088 |
|
|
|
66,181 |
|
|
|
|
15,025 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total Retail Revenue |
|
$ |
1,256,314 |
|
|
$ |
1,219,305 |
|
|
$ |
909,264 |
|
|
|
$ |
259,313 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| (1) |
|
Canada sales are presented in total not by category as product sales for Canada are managed in local currency. |
The data above represents the majority of the revenue reported for the domestic portion of
the Company’s retail segment. In addition to these sales, additional revenue and revenue
adjustments are recorded to ensure conformity with GAAP. This includes wholesale revenue (to the
Company’s military commissary locations), deferral of our Gold Card revenue to match the twelve
month discount period of the card, and a reserve for customer returns. These items are recurring
in nature, and the Company expects to record similar adjustments in the future.
In addition to the Retail product categories discussed above, Franchise revenues are primarily
generated from (1) product sales to franchisees, (2) royalties from franchise retail sales and (3)
franchise fees, and Manufacturing/ Wholesale sales are generated from sales of manufactured
products to third parties, primarily in the VMHS product category.
NOTE 20. FRANCHISE REVENUE
The Company’s Franchise segment generates revenues through product sales to franchisees,
royalties, franchise fees and interest income on the financing of the franchise locations. The
Company enters into franchise agreements with initial terms of ten years. The Company charges
franchisees three types of flat franchise fees associated with stores: initial, transfer and
renewal. The initial franchise fee is payable prior to the franchise store opening as
consideration for the initial franchise rights and services performed by the Company. Transfer fees
are paid as consideration for the same rights and services as the initial fee and occur when a
former franchisee transfers ownership of the franchise location to a new franchisee. This is
typically a reduced fee compared to the initial franchise fee. The renewal franchise fee is
charged to existing franchisees upon renewal of the franchise contract. This fee is similar to,
but typically less than the initial fee.
Once the franchised store is opened, transferred or renewed, the Company has no further
obligations under these fees to the franchisee. Therefore, all initial, transfer and renewal
franchise fee revenue is recognized in the period in which a franchise store is opened, transferred
or date the contract period is renewed. The Company recognized initial franchise fees of $2.4
million for the year ended December 31, 2009, $3.3 million for the year ended December 31,
2008, $1.4 million for the period March 16 to December 31, 2007, and $0.3 million for the
period from January 1 to March 15, 2007.
114
GENERAL NUTRITION CENTERS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
The following is a summary of our franchise revenue by type:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Successor |
|
|
|
Predecessor |
|
| |
|
Year Ended |
|
|
Year Ended |
|
|
March 16 - |
|
|
|
January 1 - |
|
| |
|
December 31, |
|
|
December 31, |
|
|
December 31, |
|
|
|
March 15, |
|
| |
|
2009 |
|
|
2008 |
|
|
2007 |
|
|
|
2007 |
|
| |
|
(in thousands) |
|
Product sales |
|
$ |
217,920 |
|
|
$ |
209,662 |
|
|
$ |
160,665 |
|
|
|
$ |
38,409 |
|
Royalties |
|
|
35,561 |
|
|
|
35,147 |
|
|
|
25,990 |
|
|
|
|
7,102 |
|
Franchise fees |
|
|
4,570 |
|
|
|
5,676 |
|
|
|
3,013 |
|
|
|
|
810 |
|
Other |
|
|
6,117 |
|
|
|
7,535 |
|
|
|
4,228 |
|
|
|
|
916 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total franchise revenue |
|
$ |
264,168 |
|
|
$ |
258,020 |
|
|
$ |
193,896 |
|
|
|
$ |
47,237 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
NOTE 21. SUPPLEMENTAL CASH FLOW INFORMATION
The Company remitted cash payments for federal and state income taxes of $16.0 million, $18.1
million, and $1.2 million for the years ended December 31, 2009 and 2008, and for the period
January 1 to March 15, 2007, respectively. The Company received cash refunds of $19.7 million, net
of tax payments for the period from March 16 to December 31, 2007.
The Company remitted cash payments for interest expense related to outstanding debt of $66.7
million, $80.1 million, $56.8 million, and $38.7 million, for the years ended December 31, 2009 and
2008, the period from March 16 to December 31, 2007, and the period from January 1 to March 15,
2007.
In September 2009, the Company converted a short term receivable of $1.2 million from a
franchisee into a long term note receivable.
115
GENERAL NUTRITION CENTERS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
NOTE 22. RETIREMENT PLANS
The Company sponsors a 401(k) defined contribution savings plan covering substantially all
employees. Full time employees who have completed 30 days of service and part time employees who
have completed 1,000 hours of service are eligible to participate in the plan. The plan provides
for employee contributions of 1% to 80% of individual compensation into deferred savings, subject
to IRS limitations. The plan provides for Company contributions upon the employee meeting the
eligibility requirements. The Company match consists of both a fixed and a discretionary match
which is based on a specified financial target for all participants in the plan. The fixed match
is 50% on the first 3% of the salary that an employee defers and the discretionary match could be
up to an additional 100% match on the 3% deferral. A discretionary match can be approved at any
time by the Company.
An employee becomes vested in the Company match portion as follows:
| |
|
|
|
|
| |
|
Percent |
| Years of Service |
|
Vested |
0-1 |
|
|
0 |
% |
1-2 |
|
|
33 |
% |
2-3 |
|
|
66 |
% |
3+ |
|
|
100 |
% |
The Company made cash contributions of $1.2 million for the years ended December 31, 2009
and 2008, and $0.9 million for the period March 16 to December 31, 2007, and $0.3 million for the
period January 1 to March 15, 2007. In addition, the Company made a discretionary match for the
2007 plan year of $0.6 million in April 2008, for the 2008 plan year of $0.6 million in March 2009
and for the 2009 plan year of $0.6 million in February 2010.
The Company has a Non-qualified Executive Retirement Arrangement Plan that covers key
employees. Under the provisions of this plan, certain eligible key employees are granted cash
compensation, which in the aggregate was not significant for any year presented.
The Company has a Non-qualified Deferred Compensation Plan that provides benefits payable to
certain qualified key employees upon their retirement or their designated beneficiaries upon death.
This plan allows participants the opportunity to defer pretax amounts ranging from 2% to 100% of
their base compensation plus bonuses. The plan is funded entirely by elective contributions made by
the participants. The Company has elected to finance any potential plan benefit obligations using
corporate owned life insurance policies.
NOTE 23. FAIR VALUE MEASUREMENTS
As described in Note 2, the Company adopted the provisions of the new standard on fair
value measurements and disclosures as of January 1, 2008. This standard defines fair value,
establishes a consistent framework for measuring fair value, and expands disclosures for each major
asset and liability category measured at fair value on either a recurring or nonrecurring basis.
The standard clarifies that fair value is an exit price, representing the amount that would be
received to sell an asset or paid to transfer a liability in an orderly transaction between market
participants. As such, fair value is a market-based measurement that should be determined based on
assumptions that market participants would use in pricing an asset or liability. As a basis for
considering such assumptions, the standard establishes a three-tier fair value hierarchy which
prioritizes the inputs used in measuring fair value as follows:
116
GENERAL NUTRITION CENTERS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
| |
Level 1 — |
|
observable inputs such as quoted prices in active markets for identical
assets and liabilities; |
| |
| |
Level 2 — |
|
observable inputs such as quoted prices for similar assets or
liabilities in active markets, quoted prices for identical or similar assets or
liabilities in markets that are not active, other inputs that are observable, or can
be corroborated by observable market data; and |
| |
| |
Level 3 — |
|
unobservable inputs for which there are little or no market data, which
require the reporting entity to develop its own assumptions. |
The following table presents our financial assets and liabilities that were accounted for at
fair value on a recurring basis as of December 31, 2009 by level within the fair value hierarchy:
| |
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Fair Value Measurements Using |
| |
|
Level 1 |
|
Level 2 |
|
Level 3 |
| |
|
(in thousands) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Other long-term liabilities |
|
$ |
2,337 |
|
|
$ |
14,679 |
|
|
$ |
— |
|
The following is a description of the valuation methodologies used for these items,
as well as the general classification of such items pursuant to the fair value hierarchy of the
standard on Fair Value Measurements and Disclosures:
Other long-term liabilities — Other long-term liabilities classified as Level 1 consist of
liabilities related to the Company’s non-qualified deferred compensation plan. The liabilities
related to these plans are adjusted based on changes in the fair value of the underlying
employee-directed investment choices. Since the employee-directed investment choices are exchange
traded equity indexes with quoted prices in active markets, the liabilities are classified as
within Level 1 on the fair value hierarchy. Other long-term liabilities classified as Level 2
consist of the Company’s interest rate swaps. The derivatives are a pay-variable, receive-fixed
interest rate swap based on a LIBOR rate. Fair value is based on a model-derived valuation using
the LIBOR rate, which is an observable input in an active market. Therefore, the Company’s
derivative is classified as Level 2 on the fair value hierarchy.
In addition to the above table, the Company’s financial instruments also consist of cash and
cash equivalents, accounts receivable, accounts payable and long-term debt. The Company did not
elect to value its long-term debt with the fair value option in accordance with the standard on
Financial Instruments. The Company believes that the recorded values of all of its other financial
instruments approximate their fair values because of their nature and respective durations.
NOTE 24. RELATED PARTY TRANSACTIONS
Successor:
Management Services Agreement. Upon consummation of the Merger, the Company entered into a
services agreement with its ultimate Parent, GNC Acquisition Holdings Inc (“Holdings”). Under the
agreement, Holdings agreed to provide the Company and its subsidiaries with certain services in
exchange for an annual fee of $1.5 million, as well as customary fees for services rendered in
connection with certain major financial transactions, plus reimbursement of expenses and a tax
gross-up relating to a non-tax deductible portion of the fee. The company agreed to provide
customary indemnifications to Holdings and its affiliates and those providing services on its
behalf. In addition, upon consummation of the Merger, the Company incurred an aggregate fee of
$10.0 million, plus reimbursement of expenses, payable to Holdings for services rendered in
connection with the Merger. As of December 31, 2009, $4.2 million had been paid pursuant to this
agreement.
117
GENERAL NUTRITION CENTERS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
Credit Facility. Upon consummation of the Merger, the Company entered into a $735.0
million credit agreement, of which various Ares fund portfolios, which are related to one of our
sponsors, are investors. As of December 31, 2009 and 2008, certain affiliates of Ares Management
LLC held approximately $62.1 million and $63.9 million, respectively of term loans under the
Company’s 2007 Senior Credit Facility.
Stock Purchase. During the third and fourth quarters of 2008, Axcel Partners III, LLC, of
which an officer and director of the Company is a member, purchased 273,215 shares of Common Stock
of Holdings at a price of $6.82 per share, for an aggregate purchase price of $1.9 million and
45,478 shares of Common Stock of Holdings at a price of $7.08 per share, for an aggregate purchase
price of $0.3 million, respectively and 110,151 and 18,710 shares of Preferred Stock of Holdings at
a price of $5.00 per share plus accrued and unpaid dividends through the dates of purchase, for an
aggregate purchase price of $0.6 million and $0.1 million, respectively.
Lease Agreements. General Nutrition Centres Company, a wholly owned subsidiary of the
Company, is party to 21 lease agreements, as lessee, with Cadillac Fairview Corporation, as lessor,
with respect to properties located in Canada. Cadillac Fairview Corporation is a direct, wholly
owned subsidiary of OTPP, one of the principal stockholders of Holdings. The aggregate value of
the leases is approximately $12.4 million, together with certain future landlord related costs, of
which $2.4 million was paid for the year ended December 31, 2009, $2.5 million for the year ended
December 31, 2008 and $2.0 million for the period March 16 to December 31, 2007. Each lease was
negotiated in the ordinary course of business on an arm’s length basis.
Product Purchases. During the Company’s 2009 fiscal year, it purchased certain fish oil and
probiotics products manufactured by Lifelong Nutrition, Inc. (“Lifelong”) for resale under the
Company’s proprietary brand name WELLbeING. Carmen Fortino, who serves as one of the directors of
the Company and its Parent, is the Managing Director, a member of the Board of Directors and a
stockholder of Lifelong. The aggregate value of the products the Company purchased from Lifelong
was $3.3 million for the 2009 fiscal year.
Predecessor:
Management Service Fees. As of December 5, 2003, the Company and Parent entered into a
management services agreement with Apollo Management V. The agreement provides that Apollo
Management V furnish certain investment banking, management, consulting, financial planning, and
financial advisory services on an ongoing basis and for any significant financial transactions that
may have been undertaken. The length of the agreement was ten years. There was an annual general
services fee of $1.5 million, which was payable in monthly installments. There were also major
transaction services fees for services that Apollo Management V provided based on normal and
customary fees of like kind. In addition, the Company reimbursed expenses that were incurred and
paid by Apollo Management V on behalf of the Company. For the period from January 1, 2007 to March
15, 2007, $0.4 million was paid to Apollo Management V under the terms of this agreement. In
addition, as a result of the Merger, for the period from January 1, 2007 to March 15, 2007, $7.5
million was paid to Apollo Management V as a one-time payment for the termination of the management
services agreement.
118
GENERAL NUTRITION CENTERS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
NOTE 25. SUPPLEMENTAL GUARANTOR INFORMATION
As of December 31, 2009 the Company’s debt included its 2007 Senior Credit Facility, Senior
Toggle Notes and 10.75% Senior Subordinated Notes. The 2007 Senior Credit Facility has been
guaranteed by GNC Corporation and the Company’s existing and future direct and indirect material
domestic subsidiaries. The Senior Toggle Notes are general non collateralized obligations of the
Company, are effectively subordinated to the Company’s 2007 Senior Credit Facility to the extent of
the value of the collateral securing the 2007 Senior Credit Facility and are senior in right of
payment to all existing and future subordinated obligations of the Company, including its 10.75%
Senior Subordinated Notes. The Senior Toggle Notes are unconditionally guaranteed on a non
collateralized basis by all of the Company’s existing and future direct and indirect material
domestic subsidiaries. The 10.75% Senior Subordinated Notes are general non collateralized
obligations and are guaranteed on a senior subordinated basis by the Company’s existing and future
direct and indirect material domestic subsidiaries and rank junior in right of payment to the
Company’s 2007 Senior Credit Facility and Senior Toggle Notes. The guarantors are the same for the
2007 Senior Credit Facility, Senior Toggle Notes and 10.75% Senior Subordinated Notes.
Non-guarantor subsidiaries include the remaining direct and indirect foreign subsidiaries. The
subsidiary guarantors are 100% owned, directly or indirectly, by the Company. The guarantees are
full and unconditional and joint and several. Investments in subsidiaries are accounted for under
the equity method of accounting.
Presented below are condensed consolidated financial statements of the Company as the
parent/issuer, and the combined guarantor and non-guarantor subsidiaries as of December 31, 2009
and 2008, and for the year ended December 31, 2009 and 2008, the period from March 16, 2007 to
December 31, 2007, and the period ended March 15, 2007. Intercompany balances and transactions
have been eliminated.
The Company reorganized its corporate structure effective January 1, 2009. Certain guarantor
subsidiaries were merged into General Nutrition Centers, Inc. (the “Parent/Issuer”), which remained
the Parent/Issuer after the reorganization; certain other guarantor subsidiaries were merged into
each other. Supplemental guarantor information for periods prior to January 1, 2009 reflect the
corporate structure that existed prior to the reorganization.
119
GENERAL NUTRITION CENTERS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
Supplemental Condensed Consolidating Balance Sheets
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
Combined |
|
|
Combined |
|
|
|
|
|
|
|
| |
|
Parent/ |
|
|
Guarantor |
|
|
Non-Guarantor |
|
|
|
|
|
|
|
| December 31, 2009 |
|
Issuer |
|
|
Subsidiaries |
|
|
Subsidiaries |
|
|
Eliminations |
|
|
Consolidated |
|
| |
|
|
|
|
|
|
|
|
|
(in thousands) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Current assets |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents |
|
$ |
77,797 |
|
|
$ |
(4,801 |
) |
|
$ |
2,093 |
|
|
$ |
— |
|
|
$ |
75,089 |
|
Receivables, net |
|
|
895 |
|
|
|
92,273 |
|
|
|
1,187 |
|
|
|
— |
|
|
|
94,355 |
|
Intercompany receivables |
|
|
139,168 |
|
|
|
— |
|
|
|
— |
|
|
|
(139,168 |
) |
|
|
— |
|
Inventories, net |
|
|
— |
|
|
|
339,975 |
|
|
|
30,517 |
|
|
|
— |
|
|
|
370,492 |
|
Prepaids and other current assets |
|
|
19,308 |
|
|
|
14,409 |
|
|
|
8,502 |
|
|
|
— |
|
|
|
42,219 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total current assets |
|
|
237,168 |
|
|
|
441,856 |
|
|
|
42,299 |
|
|
|
(139,168 |
) |
|
|
582,155 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Goodwill |
|
|
— |
|
|
|
624,285 |
|
|
|
468 |
|
|
|
— |
|
|
|
624,753 |
|
Brands |
|
|
— |
|
|
|
720,000 |
|
|
|
— |
|
|
|
— |
|
|
|
720,000 |
|
Property, plant and equipment, net |
|
|
7,409 |
|
|
|
163,882 |
|
|
|
28,290 |
|
|
|
— |
|
|
|
199,581 |
|
Investment in subsidiaries |
|
|
1,550,708 |
|
|
|
(7,687 |
) |
|
|
— |
|
|
|
(1,543,021 |
) |
|
|
— |
|
Other assets |
|
|
28,876 |
|
|
|
157,018 |
|
|
|
— |
|
|
|
(8,781 |
) |
|
|
177,113 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total assets |
|
$ |
1,824,161 |
|
|
$ |
2,099,354 |
|
|
$ |
71,057 |
|
|
$ |
(1,690,970 |
) |
|
$ |
2,303,602 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Current liabilities |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Current liabilities |
|
$ |
34,129 |
|
|
$ |
154,435 |
|
|
$ |
11,023 |
|
|
$ |
— |
|
|
$ |
199,587 |
|
Intercompany payables |
|
|
— |
|
|
|
113,359 |
|
|
|
25,809 |
|
|
|
(139,168 |
) |
|
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total current liabilities |
|
|
34,129 |
|
|
|
267,794 |
|
|
|
36,832 |
|
|
|
(139,168 |
) |
|
|
199,587 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Long-term debt |
|
|
1,052,341 |
|
|
|
32 |
|
|
|
14,493 |
|
|
|
(8,781 |
) |
|
|
1,058,085 |
|
Deferred tax liabilities |
|
|
(4,754 |
) |
|
|
294,087 |
|
|
|
(439 |
) |
|
|
— |
|
|
|
288,894 |
|
Other long-term liabilities |
|
|
24,929 |
|
|
|
14,129 |
|
|
|
462 |
|
|
|
— |
|
|
|
39,520 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total liabilities |
|
|
1,106,645 |
|
|
|
576,042 |
|
|
|
51,348 |
|
|
|
(147,949 |
) |
|
|
1,586,086 |
|
Total stockholder’s equity (deficit) |
|
|
717,516 |
|
|
|
1,523,312 |
|
|
|
19,709 |
|
|
|
(1,543,021 |
) |
|
|
717,516 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total liabilities and stockholder’s
equity (deficit) |
|
$ |
1,824,161 |
|
|
$ |
2,099,354 |
|
|
$ |
71,057 |
|
|
$ |
(1,690,970 |
) |
|
$ |
2,303,602 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
120
GENERAL NUTRITION CENTERS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
Supplemental Condensed Consolidating Balance Sheets
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
Combined |
|
|
Combined |
|
|
|
|
|
|
|
| |
|
Parent/ |
|
|
Guarantor |
|
|
Non-Guarantor |
|
|
|
|
|
|
|
| December 31, 2008 |
|
Issuer |
|
|
Subsidiaries |
|
|
Subsidiaries |
|
|
Eliminations |
|
|
Consolidated |
|
| |
|
|
|
|
|
|
|
|
|
(in thousands) |
|
|
|
|
|
|
|
|
|
Current assets |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents |
|
$ |
— |
|
|
$ |
40,077 |
|
|
$ |
2,230 |
|
|
$ |
— |
|
|
$ |
42,307 |
|
Receivables, net |
|
|
369 |
|
|
|
88,972 |
|
|
|
72 |
|
|
|
— |
|
|
|
89,413 |
|
Intercompany receivables |
|
|
— |
|
|
|
87,554 |
|
|
|
— |
|
|
|
(87,554 |
) |
|
|
— |
|
Inventories, net |
|
|
— |
|
|
|
342,085 |
|
|
|
21,569 |
|
|
|
— |
|
|
|
363,654 |
|
Prepaids and other current assets |
|
|
(82 |
) |
|
|
55,520 |
|
|
|
3,969 |
|
|
|
— |
|
|
|
59,407 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total current assets |
|
|
287 |
|
|
|
614,208 |
|
|
|
27,840 |
|
|
|
(87,554 |
) |
|
|
554,781 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Goodwill |
|
|
— |
|
|
|
622,441 |
|
|
|
468 |
|
|
|
— |
|
|
|
622,909 |
|
Brands |
|
|
— |
|
|
|
720,000 |
|
|
|
— |
|
|
|
— |
|
|
|
720,000 |
|
Property, plant and equipment, net |
|
|
— |
|
|
|
180,494 |
|
|
|
25,660 |
|
|
|
— |
|
|
|
206,154 |
|
Investment in subsidiaries |
|
|
1,797,306 |
|
|
|
10,482 |
|
|
|
— |
|
|
|
(1,807,788 |
) |
|
|
— |
|
Other assets |
|
|
22,470 |
|
|
|
174,475 |
|
|
|
— |
|
|
|
(8,781 |
) |
|
|
188,164 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total assets |
|
$ |
1,820,063 |
|
|
$ |
2,322,100 |
|
|
$ |
53,968 |
|
|
$ |
(1,904,123 |
) |
|
$ |
2,292,008 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Current liabilities |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Current liabilities |
|
$ |
20,644 |
|
|
$ |
220,120 |
|
|
$ |
8,958 |
|
|
$ |
— |
|
|
$ |
249,722 |
|
Intercompany payables |
|
|
69,244 |
|
|
|
— |
|
|
|
18,310 |
|
|
|
(87,554 |
) |
|
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total current liabilities |
|
|
89,888 |
|
|
|
220,120 |
|
|
|
27,268 |
|
|
|
(87,554 |
) |
|
|
249,722 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Long-term debt |
|
|
1,064,024 |
|
|
|
30 |
|
|
|
15,964 |
|
|
|
(8,781 |
) |
|
|
1,071,237 |
|
Deferred tax liabilities |
|
|
(4,813 |
) |
|
|
282,816 |
|
|
|
— |
|
|
|
— |
|
|
|
278,003 |
|
Other long-term liabilities |
|
|
18,902 |
|
|
|
21,828 |
|
|
|
254 |
|
|
|
— |
|
|
|
40,984 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total liabilities |
|
|
1,168,001 |
|
|
|
524,794 |
|
|
|
43,486 |
|
|
|
(96,335 |
) |
|
|
1,639,946 |
|
Total stockholder’s equity (deficit) |
|
|
652,062 |
|
|
|
1,797,306 |
|
|
|
10,482 |
|
|
|
(1,807,788 |
) |
|
|
652,062 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total liabilities and stockholder’s
equity (deficit) |
|
$ |
1,820,063 |
|
|
$ |
2,322,100 |
|
|
$ |
53,968 |
|
|
$ |
(1,904,123 |
) |
|
$ |
2,292,008 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
121
GENERAL NUTRITION CENTERS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
Supplemental Condensed Consolidating Statements of Operations
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
Combined |
|
|
Combined |
|
|
|
|
|
|
|
| |
|
Parent/ |
|
|
Guarantor |
|
|
Non-Guarantor |
|
|
|
|
|
|
|
| Year ended December 31, 2009 |
|
Issuer |
|
|
Subsidiaries |
|
|
Subsidiaries |
|
|
Eliminations |
|
|
Consolidated |
|
| |
|
|
|
|
|
|
|
|
|
(in thousands) |
|
|
|
|
|
|
|
|
|
Revenue |
|
$ |
— |
|
|
$ |
1,622,085 |
|
|
$ |
102,092 |
|
|
$ |
(17,170 |
) |
|
$ |
1,707,007 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cost of sales, including costs of warehousing,
distribution and occupancy |
|
|
— |
|
|
|
1,060,619 |
|
|
|
72,988 |
|
|
|
(17,170 |
) |
|
|
1,116,437 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Gross profit |
|
|
— |
|
|
|
561,466 |
|
|
|
29,104 |
|
|
|
— |
|
|
|
590,570 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Compensation and related benefits |
|
|
41,713 |
|
|
|
205,190 |
|
|
|
16,143 |
|
|
|
— |
|
|
|
263,046 |
|
Advertising and promotion |
|
|
— |
|
|
|
49,280 |
|
|
|
754 |
|
|
|
— |
|
|
|
50,034 |
|
Other selling, general and administrative |
|
|
33,111 |
|
|
|
63,431 |
|
|
|
(88 |
) |
|
|
— |
|
|
|
96,454 |
|
Subsidiary (income) expense |
|
|
(75,141 |
) |
|
|
997 |
|
|
|
— |
|
|
|
74,144 |
|
|
|
— |
|
Other (income) expense |
|
|
(71,075 |
) |
|
|
66,915 |
|
|
|
4,005 |
|
|
|
— |
|
|
|
(155 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating income (loss) |
|
|
71,392 |
|
|
|
175,653 |
|
|
|
8,290 |
|
|
|
(74,144 |
) |
|
|
181,191 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Interest expense, net |
|
|
4,204 |
|
|
|
64,569 |
|
|
|
1,180 |
|
|
|
— |
|
|
|
69,953 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Income (loss) before income taxes |
|
|
67,188 |
|
|
|
111,084 |
|
|
|
7,110 |
|
|
|
(74,144 |
) |
|
|
111,238 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Income tax (benefit) expense |
|
|
(2,431 |
) |
|
|
41,973 |
|
|
|
2,077 |
|
|
|
— |
|
|
|
41,619 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income (loss) |
|
$ |
69,619 |
|
|
$ |
69,111 |
|
|
$ |
5,033 |
|
|
$ |
(74,144 |
) |
|
$ |
69,619 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Supplemental Condensed Consolidating Statements of Operations
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
Combined |
|
|
Combined |
|
|
|
|
|
|
|
| Successor |
|
Parent/ |
|
|
Guarantor |
|
|
Non-Guarantor |
|
|
|
|
|
|
|
| Year ended December 31, 2008 |
|
Issuer |
|
|
Subsidiaries |
|
|
Subsidiaries |
|
|
Eliminations |
|
|
Consolidated |
|
| |
|
|
|
|
|
|
|
|
|
(in thousands) |
|
|
|
|
|
|
|
|
|
Revenue |
|
$ |
— |
|
|
$ |
1,566,054 |
|
|
$ |
102,018 |
|
|
$ |
(11,343 |
) |
|
$ |
1,656,729 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cost of sales, including costs of warehousing,
distribution and occupancy |
|
|
— |
|
|
|
1,020,402 |
|
|
|
73,571 |
|
|
|
(11,343 |
) |
|
|
1,082,630 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Gross profit |
|
|
— |
|
|
|
545,652 |
|
|
|
28,447 |
|
|
|
— |
|
|
|
574,099 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Compensation and related benefits |
|
|
— |
|
|
|
234,188 |
|
|
|
15,605 |
|
|
|
— |
|
|
|
249,793 |
|
Advertising and promotion |
|
|
— |
|
|
|
54,351 |
|
|
|
709 |
|
|
|
— |
|
|
|
55,060 |
|
Other selling, general and administrative |
|
|
2,215 |
|
|
|
92,893 |
|
|
|
3,624 |
|
|
|
— |
|
|
|
98,732 |
|
Subsidiary (income) expense |
|
|
(58,977 |
) |
|
|
(5,565 |
) |
|
|
— |
|
|
|
64,542 |
|
|
|
— |
|
Other (income) expense |
|
|
— |
|
|
|
126 |
|
|
|
607 |
|
|
|
— |
|
|
|
733 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating income (loss) |
|
|
56,762 |
|
|
|
169,659 |
|
|
|
7,902 |
|
|
|
(64,542 |
) |
|
|
169,781 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Interest expense, net |
|
|
4,242 |
|
|
|
77,579 |
|
|
|
1,179 |
|
|
|
— |
|
|
|
83,000 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Income (loss) before income taxes |
|
|
52,520 |
|
|
|
92,080 |
|
|
|
6,723 |
|
|
|
(64,542 |
) |
|
|
86,781 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Income tax (benefit) expense |
|
|
(2,260 |
) |
|
|
33,103 |
|
|
|
1,158 |
|
|
|
— |
|
|
|
32,001 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income (loss) |
|
$ |
54,780 |
|
|
$ |
58,977 |
|
|
$ |
5,565 |
|
|
$ |
(64,542 |
) |
|
$ |
54,780 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
122
GENERAL NUTRITION CENTERS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
Supplemental Condensed Consolidating Statements of Operations
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
Combined |
|
|
Combined |
|
|
|
|
|
|
|
| Successor |
|
Parent/ |
|
|
Guarantor |
|
|
Non-Guarantor |
|
|
|
|
|
|
|
| Period from March 16, 2007 to December 31, 2007 |
|
Issuer |
|
|
Subsidiaries |
|
|
Subsidiaries |
|
|
Eliminations |
|
|
Consolidated |
|
| |
|
|
|
|
|
|
|
|
|
(in thousands) |
|
|
|
|
|
|
|
|
|
Revenue |
|
$ |
— |
|
|
$ |
1,158,143 |
|
|
$ |
75,180 |
|
|
$ |
(10,336 |
) |
|
$ |
1,222,987 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cost of sales, including costs of warehousing,
distribution and occupancy |
|
|
— |
|
|
|
770,261 |
|
|
|
54,313 |
|
|
|
(10,336 |
) |
|
|
814,238 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Gross profit |
|
|
— |
|
|
|
387,882 |
|
|
|
20,867 |
|
|
|
— |
|
|
|
408,749 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Compensation and related benefits |
|
|
— |
|
|
|
183,901 |
|
|
|
11,891 |
|
|
|
— |
|
|
|
195,792 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Advertising and promotion |
|
|
— |
|
|
|
34,560 |
|
|
|
502 |
|
|
|
— |
|
|
|
35,062 |
|
Other selling, general and administrative |
|
|
1,356 |
|
|
|
67,315 |
|
|
|
2,542 |
|
|
|
— |
|
|
|
71,213 |
|
Subsidiary (income) expense |
|
|
(24,467 |
) |
|
|
(2,612 |
) |
|
|
— |
|
|
|
27,079 |
|
|
|
— |
|
Other (income) expense |
|
|
— |
|
|
|
(77 |
) |
|
|
(347 |
) |
|
|
— |
|
|
|
(424 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating income (loss) |
|
|
23,111 |
|
|
|
104,795 |
|
|
|
6,279 |
|
|
|
(27,079 |
) |
|
|
107,106 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Interest expense, net |
|
|
7,080 |
|
|
|
67,611 |
|
|
|
831 |
|
|
|
— |
|
|
|
75,522 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Income (loss) before income taxes |
|
|
16,031 |
|
|
|
37,184 |
|
|
|
5,448 |
|
|
|
(27,079 |
) |
|
|
31,584 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Income tax (benefit) expense |
|
|
(2,953 |
) |
|
|
12,717 |
|
|
|
2,836 |
|
|
|
— |
|
|
|
12,600 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income (loss) |
|
$ |
18,984 |
|
|
$ |
24,467 |
|
|
$ |
2,612 |
|
|
$ |
(27,079 |
) |
|
$ |
18,984 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Supplemental Condensed Consolidating Statements of Operations
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
Combined |
|
|
Combined |
|
|
|
|
|
|
|
| Predecessor |
|
|
|
|
|
Guarantor |
|
|
Non-Guarantor |
|
|
|
|
|
|
|
| Period ended March 15, 2007 |
|
Issuer |
|
|
Subsidiaries |
|
|
Subsidiaries |
|
|
Eliminations |
|
|
Consolidated |
|
| |
|
|
|
|
|
|
|
|
|
(in thousands) |
|
|
|
|
|
|
|
|
|
Revenue |
|
$ |
— |
|
|
$ |
314,632 |
|
|
$ |
17,489 |
|
|
$ |
(2,292 |
) |
|
$ |
329,829 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cost of sales, including costs of warehousing,
distribution and occupancy |
|
|
— |
|
|
|
201,973 |
|
|
|
12,494 |
|
|
|
(2,292 |
) |
|
|
212,175 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Gross profit |
|
|
— |
|
|
|
112,659 |
|
|
|
4,995 |
|
|
|
— |
|
|
|
117,654 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Compensation and related benefits |
|
|
— |
|
|
|
61,615 |
|
|
|
2,696 |
|
|
|
— |
|
|
|
64,311 |
|
Advertising and promotion |
|
|
— |
|
|
|
20,435 |
|
|
|
38 |
|
|
|
— |
|
|
|
20,473 |
|
Other selling, general and administrative |
|
|
86 |
|
|
|
17,514 |
|
|
|
(204 |
) |
|
|
— |
|
|
|
17,396 |
|
Subsidiary (income) expense |
|
|
(12,958 |
) |
|
|
(1,581 |
) |
|
|
— |
|
|
|
14,539 |
|
|
|
— |
|
Other (income) expense |
|
|
34,603 |
|
|
|
— |
|
|
|
(154 |
) |
|
|
— |
|
|
|
34,449 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating income (loss) |
|
|
(21,731 |
) |
|
|
14,676 |
|
|
|
2,619 |
|
|
|
(14,539 |
) |
|
|
(18,975 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Interest expense, net |
|
|
42,981 |
|
|
|
(539 |
) |
|
|
594 |
|
|
|
— |
|
|
|
43,036 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Income (loss) before income taxes |
|
|
(64,712 |
) |
|
|
15,215 |
|
|
|
2,025 |
|
|
|
(14,539 |
) |
|
|
(62,011 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Income tax (benefit) expense |
|
|
(13,398 |
) |
|
|
2,257 |
|
|
|
444 |
|
|
|
— |
|
|
|
(10,697 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income (loss) |
|
$ |
(51,314 |
) |
|
$ |
12,958 |
|
|
$ |
1,581 |
|
|
$ |
(14,539 |
) |
|
$ |
(51,314 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
123
GENERAL NUTRITION CENTERS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
Supplemental Condensed Consolidating Statements of Cash Flows
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
Combined |
|
|
Combined |
|
|
|
|
| Successor |
|
Parent/ |
|
|
Guarantor |
|
|
Non-Guarantor |
|
|
|
|
| Year ended December 31, 2009 |
|
Issuer |
|
|
Subsidiaries |
|
|
Subsidiaries |
|
|
Consolidated |
|
| |
|
|
|
|
|
(in thousands) |
|
|
|
|
|
NET CASH PROVIDED BY OPERATING ACTIVITIES: |
|
$ |
— |
|
|
$ |
109,200 |
|
|
$ |
4,757 |
|
|
$ |
113,957 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CASH FLOWS FROM INVESTING ACTIVITIES: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Capital expenditures |
|
|
(2,446 |
) |
|
|
(22,470 |
) |
|
|
(3,766 |
) |
|
|
(28,682 |
) |
Investment/distribution |
|
|
129,379 |
|
|
|
(129,379 |
) |
|
|
— |
|
|
|
— |
|
Acquisition of the Company |
|
|
(11,268 |
) |
|
|
— |
|
|
|
— |
|
|
|
(11,268 |
) |
Other investing |
|
|
— |
|
|
|
(2,224 |
) |
|
|
— |
|
|
|
(2,224 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Net cash provided by (used in) investing activities |
|
|
115,665 |
|
|
|
(154,073 |
) |
|
|
(3,766 |
) |
|
|
(42,174 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CASH FLOWS FROM FINANCING ACTIVITIES: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
GNC Corporation investment in
General Nutrition Centers, Inc |
|
|
(278 |
) |
|
|
— |
|
|
|
— |
|
|
|
(278 |
) |
Dividend payment |
|
|
(13,600 |
) |
|
|
— |
|
|
|
— |
|
|
|
(13,600 |
) |
Financing fees |
|
|
(45 |
) |
|
|
— |
|
|
|
— |
|
|
|
(45 |
) |
Other financing |
|
|
(23,945 |
) |
|
|
(5 |
) |
|
|
(1,377 |
) |
|
|
(25,327 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Net cash used in financing activities |
|
|
(37,868 |
) |
|
|
(5 |
) |
|
|
(1,377 |
) |
|
|
(39,250 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Effect of exchange rate on cash |
|
|
— |
|
|
|
— |
|
|
|
249 |
|
|
|
249 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net increase (decrease) in cash |
|
|
77,797 |
|
|
|
(44,878 |
) |
|
|
(137 |
) |
|
|
32,782 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Beginning balance, cash |
|
|
— |
|
|
|
40,077 |
|
|
|
2,230 |
|
|
|
42,307 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Ending balance, cash |
|
$ |
77,797 |
|
|
$ |
(4,801 |
) |
|
$ |
2,093 |
|
|
$ |
75,089 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
124
GENERAL NUTRITION CENTERS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
Supplemental Condensed Consolidating Statements of Cash Flows
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
Combined |
|
|
Combined |
|
|
|
|
| Successor |
|
Parent/ |
|
|
Guarantor |
|
|
Non-Guarantor |
|
|
|
|
| Year ended December 31, 2008 |
|
Issuer |
|
|
Subsidiaries |
|
|
Subsidiaries |
|
|
Consolidated |
|
| |
|
|
|
|
|
(in thousands) |
|
|
|
|
|
NET CASH PROVIDED BY OPERATING ACTIVITIES: |
|
$ |
— |
|
|
$ |
71,618 |
|
|
$ |
5,638 |
|
|
$ |
77,256 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CASH FLOWS FROM INVESTING ACTIVITIES: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Capital expenditures |
|
|
— |
|
|
|
(43,767 |
) |
|
|
(4,899 |
) |
|
|
(48,666 |
) |
Investment/distribution |
|
|
13,056 |
|
|
|
(13,056 |
) |
|
|
— |
|
|
|
— |
|
Acquisition of intangible |
|
|
— |
|
|
|
(1,000 |
) |
|
|
— |
|
|
|
(1,000 |
) |
Acquisition of the Company |
|
|
(10,842 |
) |
|
|
— |
|
|
|
— |
|
|
|
(10,842 |
) |
Other investing |
|
|
— |
|
|
|
83 |
|
|
|
— |
|
|
|
83 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net cash provided by (used in) investing activities |
|
|
2,214 |
|
|
|
(57,740 |
) |
|
|
(4,899 |
) |
|
|
(60,425 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CASH FLOWS FROM FINANCING ACTIVITIES: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
GNC Corporation investment in
General Nutrition Centers, Inc |
|
|
(832 |
) |
|
|
— |
|
|
|
— |
|
|
|
(832 |
) |
Other financing |
|
|
(1,382 |
) |
|
|
109 |
|
|
|
(1,217 |
) |
|
|
(2,490 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Net cash provided by (used in) financing activities |
|
|
(2,214 |
) |
|
|
109 |
|
|
|
(1,217 |
) |
|
|
(3,322 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Effect of exchange rate on cash |
|
|
— |
|
|
|
— |
|
|
|
(56 |
) |
|
|
(56 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net increase (decrease) in cash |
|
|
— |
|
|
|
13,987 |
|
|
|
(534 |
) |
|
|
13,453 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Beginning balance, cash |
|
|
— |
|
|
|
26,090 |
|
|
|
2,764 |
|
|
|
28,854 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Ending balance, cash |
|
$ |
— |
|
|
$ |
40,077 |
|
|
$ |
2,230 |
|
|
$ |
42,307 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
125
GENERAL NUTRITION CENTERS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
Supplemental Condensed Consolidating Statements of Cash Flows
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
Combined |
|
|
Combined |
|
|
|
|
| Successor |
|
Parent/ |
|
|
Guarantor |
|
|
Non-Guarantor |
|
|
|
|
| Period from March 16, 2007 to December 31, 2007 |
|
Issuer |
|
|
Subsidiaries |
|
|
Subsidiaries |
|
|
Consolidated |
|
| |
|
|
|
|
|
(in thousands) |
|
|
|
|
|
NET CASH PROVIDED BY OPERATING ACTIVITIES: |
|
$ |
1,567 |
|
|
$ |
80,795 |
|
|
$ |
5,551 |
|
|
$ |
87,913 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CASH FLOWS FROM INVESTING ACTIVITIES: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Capital expenditures |
|
|
— |
|
|
|
(25,098 |
) |
|
|
(3,753 |
) |
|
|
(28,851 |
) |
Investment/distribution |
|
|
40,878 |
|
|
|
(40,878 |
) |
|
|
— |
|
|
|
— |
|
Acquisition of the Company |
|
|
(1,642,061 |
) |
|
|
— |
|
|
|
— |
|
|
|
(1,642,061 |
) |
Other investing |
|
|
— |
|
|
|
(412 |
) |
|
|
— |
|
|
|
(412 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Net cash used in investing activities |
|
|
(1,601,183 |
) |
|
|
(66,388 |
) |
|
|
(3,753 |
) |
|
|
(1,671,324 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CASH FLOWS FROM FINANCING ACTIVITIES: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
GNC Corporation investment in
General Nutrition Centers, Inc |
|
|
(314 |
) |
|
|
— |
|
|
|
— |
|
|
|
(314 |
) |
Issuance of new equity |
|
|
552,291 |
|
|
|
— |
|
|
|
— |
|
|
|
552,291 |
|
Borrowings from new senior credit facility |
|
|
675,000 |
|
|
|
— |
|
|
|
— |
|
|
|
675,000 |
|
Proceeds from issuance of new senior sub notes |
|
|
110,000 |
|
|
|
— |
|
|
|
— |
|
|
|
110,000 |
|
Proceeds from issuance of new senior notes |
|
|
297,000 |
|
|
|
— |
|
|
|
— |
|
|
|
297,000 |
|
Financing fees |
|
|
(29,298 |
) |
|
|
— |
|
|
|
— |
|
|
|
(29,298 |
) |
Other financing |
|
|
(5,063 |
) |
|
|
4,124 |
|
|
|
(958 |
) |
|
|
(1,897 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Net cash provided by (used in) financing activities |
|
|
1,599,616 |
|
|
|
4,124 |
|
|
|
(958 |
) |
|
|
1,602,782 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Effect of exchange rate on cash |
|
|
— |
|
|
|
— |
|
|
|
(29 |
) |
|
|
(29 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net increase in cash |
|
|
— |
|
|
|
18,531 |
|
|
|
811 |
|
|
|
19,342 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Beginning balance, cash |
|
|
— |
|
|
|
7,559 |
|
|
|
1,953 |
|
|
|
9,512 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Ending balance, cash |
|
$ |
— |
|
|
$ |
26,090 |
|
|
$ |
2,764 |
|
|
$ |
28,854 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
126
GENERAL NUTRITION CENTERS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
Supplemental Condensed Consolidating Statements of Cash Flows
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
Combined |
|
|
Combined |
|
|
|
|
| Predecessor |
|
Parent/ |
|
|
Guarantor |
|
|
Non-Guarantor |
|
|
|
|
| Period ended March 15, 2007 |
|
Issuer |
|
|
Subsidiaries |
|
|
Subsidiaries |
|
|
Consolidated |
|
| |
|
|
|
|
|
(in thousands) |
|
|
|
|
|
NET CASH USED IN OPERATING ACTIVITIES: |
|
$ |
(43,103 |
) |
|
$ |
(3,102 |
) |
|
$ |
(583 |
) |
|
$ |
(46,788 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CASH FLOWS FROM INVESTING ACTIVITIES: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Capital expenditures |
|
|
— |
|
|
|
(5,117 |
) |
|
|
(576 |
) |
|
|
(5,693 |
) |
Investment/distribution |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
Other investing |
|
|
— |
|
|
|
(555 |
) |
|
|
— |
|
|
|
(555 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Net cash used in investing activities |
|
|
— |
|
|
|
(5,672 |
) |
|
|
(576 |
) |
|
|
(6,248 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CASH FLOWS FROM FINANCING ACTIVITIES: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Contribution from selling shareholders |
|
|
463,393 |
|
|
|
— |
|
|
|
— |
|
|
|
463,393 |
|
Redemption of 8 5/8% senior notes |
|
|
(150,000 |
) |
|
|
— |
|
|
|
— |
|
|
|
(150,000 |
) |
Redemption of 8 1/2% senior notes |
|
|
(215,000 |
) |
|
|
— |
|
|
|
— |
|
|
|
(215,000 |
) |
Payment of 2003 senior credit facility |
|
|
(55,290 |
) |
|
|
— |
|
|
|
— |
|
|
|
(55,290 |
) |
Other financing |
|
|
— |
|
|
|
(4,136 |
) |
|
|
(334 |
) |
|
|
(4,470 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Net cash provided by (used in) financing activities |
|
|
43,103 |
|
|
|
(4,136 |
) |
|
|
(334 |
) |
|
|
38,633 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Effect of exchange rate on cash |
|
|
— |
|
|
|
— |
|
|
|
(165 |
) |
|
|
(165 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net decrease in cash |
|
|
— |
|
|
|
(12,910 |
) |
|
|
(1,658 |
) |
|
|
(14,568 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Beginning balance, cash |
|
|
— |
|
|
|
20,469 |
|
|
|
3,611 |
|
|
|
24,080 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Ending balance, cash |
|
$ |
— |
|
|
$ |
7,559 |
|
|
$ |
1,953 |
|
|
$ |
9,512 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
NOTE 26. SUBSEQUENT EVENTS
Management has considered all other subsequent events.
127
|
|
|
| ITEM 9. |
|
CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE. |
None.
|
|
|
| ITEM 9A. |
|
CONTROLS AND PROCEDURES. |
Evaluation of Disclosure Controls and Procedures
Our management, with the participation of our Chief Executive Officer (“CEO”) and Chief
Financial Officer (“CFO”), has evaluated the effectiveness of our disclosure controls and
procedures (as such term is defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act) as of
the end of the period covered by this report. Disclosure controls and procedures are designed to
provide reasonable assurance that the information required to be disclosed in the reports that we
file or submit under the Exchange Act has been appropriately recorded, processed, summarized and
reported on a timely basis and are effective in ensuring that such information is accumulated and
communicated to the Company’s management, including our CEO and CFO, as appropriate to allow timely
decisions regarding required disclosure. Based on such evaluation, our CEO and CFO have concluded
that, as of December 31, 2009, our disclosure controls and procedures are effective at the
reasonable assurance level.
Management’s Report on Internal Control Over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over
financial reporting as defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act. Our
internal control over financial reporting is designed to provide reasonable assurance regarding the
reliability of financial reporting and the preparation of financial statements for external
purposes in accordance with generally accepted accounting principles. Because of its inherent
limitations, internal control over financial reporting may not prevent or detect misstatements.
Our management, with the participation of our Chief Executive Officer and Chief Financial
Officer, has assessed the effectiveness of our internal control over financial reporting based on
the framework and criteria established in Internal Control-Integrated Framework issued by the
Committee of Sponsoring Organizations of the Treadway Commission. Based on this assessment, our
management has concluded that, as of December 31, 2009, our internal control over financial
reporting was effective based on that framework.
Our independent registered public accounting firm, PricewaterhouseCoopers LLP, has audited the
effectiveness of our internal control over financial reporting as of December 31, 2009, as stated
in their report, which is included in Item 8, “Financial Statements and Supplementary Data” of this
Annual Report on Form 10-K.
Changes in Internal Control Over Financial Reporting
There were no changes in our internal control over financial reporting during the quarter
ended December 21, 2009 that have materially affected, or are reasonably likely to materially
affect, our internal control over financial reporting.
|
|
|
| ITEM 9B. |
|
OTHER INFORMATION. |
None.
128
PART III
|
|
|
| ITEM 10. |
|
DIRECTORS AND EXECUTIVE OFFICERS OF THE REGISTRANT AND CORPORATE GOVERNANCE |
The following table sets forth certain information regarding our directors and executive officers
as of February 2, 2010.
| |
|
|
|
|
|
|
| Name |
|
Age |
|
Position |
Joseph Fortunato
|
|
|
56 |
|
|
Director and Chief Executive Officer |
Beth J. Kaplan
|
|
|
51 |
|
|
Director, President and Chief Merchandising and Marketing Officer |
Michael M. Nuzzo
|
|
|
39 |
|
|
Executive Vice President, Chief Financial Officer |
David Berg
|
|
|
48 |
|
|
Executive Vice President of Global Business Development, Chief
Operating Officer, International |
Tom Dowd
|
|
|
46 |
|
|
Executive Vice President of Store Operations and Development |
J. Kenneth Fox
|
|
|
59 |
|
|
Senior Vice President and Treasurer |
Gerald J. Stubenhofer, Jr.
|
|
|
41 |
|
|
Senior Vice President, Chief Legal Officer and Secretary |
Lee Karayusuf
|
|
|
59 |
|
|
Senior Vice President of Distribution and Transportation |
Michael Locke
|
|
|
64 |
|
|
Senior Vice President of Manufacturing |
Robert Kral
|
|
|
56 |
|
|
Senior Vice President of Merchandising |
Robert Chessen
|
|
|
59 |
|
|
Senior Vice President of Human Resources |
Christine Clark
|
|
|
50 |
|
|
Senior Vice President of eCommerce |
Victoria Binau
|
|
|
53 |
|
|
Senior Vice President of Marketing |
Darryl Green
|
|
|
49 |
|
|
Senior Vice President of Merchandising |
Guru Ramanathan
|
|
|
46 |
|
|
Senior Vice President, Chief Innovation Officer |
Anthony Phillips
|
|
|
42 |
|
|
Senior Vice President, Business Analytics and Inventory
Management |
Norman Axelrod
|
|
|
57 |
|
|
Chairman of the Board of Directors |
Andrew Claerhout
|
|
|
38 |
|
|
Director |
Carmen Fortino
|
|
|
51 |
|
|
Director |
Michael Hines
|
|
|
53 |
|
|
Director |
David B. Kaplan
|
|
|
42 |
|
|
Director |
Romeo Leemrijse
|
|
|
39 |
|
|
Director |
Jeffrey B. Schwartz
|
|
|
35 |
|
|
Director |
Joseph Fortunato became one of our directors in March 2007 upon consummation of the Merger.
Additionally, Mr. Fortunato has served as our Chief Executive Officer since January 2008. In
November 2005, Mr. Fortunato became President and Chief Executive Officer of General Nutrition
Companies, Inc. Mr. Fortunato served as Senior Executive Vice President and Chief Operating
Officer from June 2005 until November 2005. Beginning in November 2001 until June 2005, Mr.
Fortunato served as Executive Vice President and Chief Operating Officer of General Nutrition
Companies, Inc. From October 2000 until November 2001, he served as its Executive Vice President
of Retail Operations and Store Development. Mr. Fortunato began his employment with General
Nutrition Companies, Inc. in October 1990 and has held various positions, including Senior Vice
President of Financial Operations from 1997 to 1998, and Director of Financial Operations from 1990
to 1997. From 1984 to 1988, Mr. Fortunato was President of Fortunato & Associates Financial
Consulting Group. From 1975 to 1984, Mr. Fortunato was the Controller of Motor Coils Manufacturing
Company, a manufacturer of traction motors for locomotives and oil drilling rigs.
129
Beth J. Kaplan became one of our directors in February 2008. Additionally, Ms. Kaplan has
served as our President and Chief Marketing and Merchandising Officer since January 2008. From
March 2005 to December 2007, Ms. Kaplan served as Managing Member for Axcel Partners, LLC, a
venture capital
firm. From June 2002 to March 2005, Ms. Kaplan was Executive Vice President of Bath & Body
Works. Previous to this, Ms. Kaplan worked at Rite Aid Corporation as Senior Executive Vice
President, and at Procter & Gamble Co. Ms. Kaplan also serves on the Board of Directors of
Blackboard Inc.
Michael M. Nuzzo became our Executive Vice President and Chief Financial Officer in September
2008. Prior to joining GNC, Mr. Nuzzo was Senior Vice President — Finance at Abercrombie & Fitch.
From 1999 to 2008, Mr. Nuzzo served in various senior level finance and retail operations and
strategic planning roles with Abercrombie & Fitch. Prior to his work in the retail sector, Mr.
Nuzzo was a senior consultant with William M. Mercer and Medimetrix Group. Mr. Nuzzo earned his
undergraduate degree in Economics at Kenyon College in 1992 and also received his MBA in Finance
and Accounting from the University of Chicago in 1998.
David Berg became our Executive Vice President of Global Business Development and Chief
Operating Officer International in August 2009. Prior to joining GNC, Mr. Berg was the Executive
Vice President and Chief Operating Officer for Best Buy international from 2002 to 2009. From 2001
to 2002, he was the President and Chief Operating Officer, International Division for the United
Kingdom based office equipment and solutions company Danka Business Systems. In the later part of
the 90’s Mr. Berg had served as Danka’s Executive Vice President overseeing worldwide legal,
corporate development, human resources and real estate function for the US operation. In the early
part of this decade, Mr. Berg served as Senior Vice President and board member for Comdial
Corporation a telecommunications manufacturer, franchisor and distributor; he also served as
President and Chief Operating Officer for iPool.com. From 1994 to 1997, Mr. Berg was Senior Vice
President for Nordic Track, Inc. Mr. Berg started his career with Bell South Corporation where for
eight years he was their Corporate Attorney.
J. Kenneth Fox became our Senior Vice President and Treasurer in December 2006, and served as
our Interim Chief Financial Officer from March 2008 to September 2008. Previously, he served as our
Vice President and Treasurer from June 1997 December 2006. Mr. Fox began his employment with GNC
as Manager of Corporate Accounting in July 1985 and has served in various Accounting and Finance
positions, including Manager Accounting/Budgets, Assistant Corporate Controller and Assistant
Treasurer.
Gerald J. Stubenhofer, Jr. became our Senior Vice President, Chief Legal Officer and Secretary
in September 2007. From January 2005 to September 2007, Mr. Stubenhofer worked at the law firm of
McGuireWoods, LLP as a Partner and member of the Complex Commercial Litigation Department. From
April 2002 to January 2005, Mr. Stubenhofer worked at McGuireWoods, LLP as an Associate. From June
1997 to November 1999, Mr. Stubenhofer served as our Assistant General Counsel.
Tom Dowd became Executive Vice President of Store Operations and Development in May 2007
(retroactive to April 2007), having served as Senior Vice President and General Manager of Retail
Operations of General Nutrition Corporation since December 2005 and as Senior Vice President of
Stores since March 2003. From March 2001 until March 2003, Mr. Dowd was President of Healthlabs,
LLC, an unaffiliated contract supplement manufacturing and product consulting company. Mr. Dowd
was Senior Vice President of Retail Sales from May 2000 until March 2001, and Division Three Vice
President of General Nutrition Corporation from December 1998 to May 2000.
Lee Karayusuf became Senior Vice President of Distribution and Transportation of General
Nutrition Companies, Inc. in December 2000 with additional responsibility for its then affiliates,
Rexall Sundown and Unicity. Mr. Karayusuf served as Manager of Transportation of General Nutrition
Companies, Inc. from December 1991 until March 1994 and Vice President of Transportation and
Distribution from 1994 until December 2000.
Michael Locke became Senior Vice President of Manufacturing of Nutra Manufacturing, Inc. in
June 2003. From January 2000 until June 2003, Mr. Locke served as the head of North American
Manufacturing Operations for Numico, the former parent company of General Nutrition Companies, Inc.
From 1994 until 1999, he served as Senior Vice President of Manufacturing of Nutra Manufacturing,
Inc. (f/k/a General Nutrition Products, Inc.), and from 1991 until 1993, he served as Vice
President of Distribution. From 1986 until 1991, Mr. Locke served as Director of Distribution of General
Nutrition Distribution Company, our indirect subsidiary.
130
Robert Kral became our Senior Vice President of Merchandising in July 2009. Prior to joining
GNC, Mr. Kral operated a consulting business from 2007 to 2009, and created and worked on several
entrepreneurial opportunities, including a pay as you go broadband wireless business. Prior to
2007, Mr. Kral held various positions at Walgreen’s; he served as Senior Vice President of
Merchandising from 2006 to 2007; Vice President of Merchandising Strategies from 2002 to 2006;
Operations Vice President from 2000 to 2002; District Manager from 1994 to 1998; and store manager
from 1982 to 1998. Mr. Kral started his career as a Pharmacist at Walgreen’s in 1977.
Robert Chessen became Senior Vice President of Human Resources in August 2008. Prior to
joining GNC, Mr. Chessen served as Vice President of Human Resources for Charming Shoppes, Inc.
from 2002 to July 2008. Mr. Chessen held senior HR positions both in Charming’s corporate offices
and its Crosstown Traders division. From 2000 to 2002, Mr. Chessen was Vice President of Human
Resources for Weis Markets. From 1993 to 2000, Mr. Chessen served in various positions with Gart
Sports, including Senior Vice President of Human Resources. Mr. Chessen began his career with May
Department Stores and held various positions in management, merchandising and human resources
positions from 1973 to 1993. He is a graduate of Northwestern University.
Christine Clark became Senior Vice President of eCommerce in August 2008. From 2003 to 2008,
Ms. Clark was President/Founder of MCR Strategies, a multi-faceted consulting company focusing on
electronic retailing opportunities. From 2000 to 2003, Ms. Clark was Senior Vice President and
General Manager of HSN.com, a division of the Home Shopping Network. Prior to 2000, Ms. Clark held
various Senior Merchandising/Management positions at Fogdog.com, Vitalcast.com, Coast to Coast
Apparel Services, Macy’s West and Bloomingdales NY.
Victoria Binau became our Senior Vice President of Marketing in February 2009. Prior to
joining GNC, Ms. Binau was the Senior Vice President, Marketing for the Babies ‘R’ Us division of
Toys ‘R’ Us Inc. from 2007 to 2009. In 2006, she led the Marketing Research and Visual
Merchandising functions at RadioShack Corp. A member of the U.S. Marketing Leadership Team, Ms.
Binau held a variety of key Marketing positions for the McDonald’s Corp. in both national and
global marketing concepts. From 1996 to 1998, Ms. Binau was the Vice President of Account Services
for Falgren Advertising in Columbus, OH. From 1986 to 1989, Ms. Binau worked for the McDonald’s
Corp in various Regional Field positions. From 1979 to 1986, she worked for Nationwise Autoparts,
WCOL/WXGT and Lazarus in advertising, media, communications and broadcast roles. Ms. Binau is a
graduate of Ohio State University.
Darryl Green became our Senior Vice President of Domestic Franchising in August 2005, having
served as Vice President of Retail Operations for the Southeast United States since November 2003.
Mr. Green began his employment with GNC in 1983 and has served in various retail, marketing and
franchising positions with the Company, including Division Merchandise Manager and Vice President
of Retail Sales.
Guru Ramanathan Ph.D., became our Senior Vice President of Product and Package Innovation in
February 2008, having previously served as Senior Vice President of Scientific Affairs since April
2007 and Vice President of Scientific Affairs since December 2003. Dr. Ramanathan began his
employment as Medical Director of General Nutrition Corporation in April 1998. Between August 2000
and December 2003, he also provided scientific and clinical trials oversight for the North American
subsidiaries of Royal Numico, the former parent company of General Nutrition Corporation. Prior to
joining General Nutrition Corporation, Dr. Ramanathan worked as Medical Director and Secretary for
the Efamol subsidiary of Scotia Pharmaceuticals in Boston. Between 1984 and 1998, in his capacity
as a pediatric dentist and dental surgeon, Dr. Ramanathan held various industry consulting and
management roles, as well as clinical, research and teaching appointments in Madras, India, and
Tufts University and New England Medical Center in Boston, Massachusetts.
131
Anthony T. Phillips became our Senior Vice President of Business Analysis and Strategic
Project Management in October 2008, having previously served as Senior Vice President, Business
Analysis and Inventory Management from December 2006 to September 2008. Beginning December 2005
until November 2006, Mr. Phillips served as Vice President, Business Analysis. Mr. Phillips served
as Senior Director Merchandise Planning and Analysis from September 2003 to November 2005, and from
October 2000 to August 2003, he served as Senior Director of Retail Analysis. Mr. Phillips first
joined GNC in March 1993, as an analyst in our merchandising and sales department.
Norman Axelrod became Chairman of our Board of Directors in March 2007 upon consummation of
the Merger. Mr. Axelrod was Chief Executive Officer and Chairman of the Board of Directors of
Linens ‘n Things, Inc. until its acquisition in February 2006. Mr. Axelrod joined Linens ‘n Things
as Chief Executive Officer in 1988 and was elected to the additional position of Chairman of the
Board in 1997. From 1976 to 1988, Mr. Axelrod held various management positions at Bloomingdale’s,
ending with Senior Vice President, General Merchandise Manager. Mr. Axelrod also serves on the
Boards of Directors of Maidenform Brands, Inc. and Jaclyn, Inc. Mr. Axelrod has worked with Ares
Management LLC as an operating partner since 2007.
Andrew Claerhout became one of our directors in July 2009. In April 2005, Mr. Claerhout joined
Teachers’ Private Capital from EdgeStone Capital Partners. Previously, Mr. Claerhout worked at
Pacific Equity Partners in Australia and Bain & Company in Canada and in Hong Kong. Mr. Claerhout
has been involved in a number of transactions while at Teachers’, including the take-private of
Alexander Forbes Limited, the creation of Actera Partners and several minority co-investments,
including Kabel Deutschland, Grupo Corporativo Ono and Valentino Fashion Group. Mr. Claerhout
currently sits on the board of AOT Bedding (Serta), Easton-Bell Sports and the advisory boards of
multiple private equity funds. Mr. Claerhout received an HBA from the Richard Ivey School of
Business at the University of Western Ontario and has completed the Stanford Executive Program at
the Graduate School of Business, Stanford University.
Carmen Fortino became one of our directors in July 2007. Mr. Fortino has been Chief Executive
Officer of Seroyal International Inc., a natural pharmaceutical company based in Richmond Hill,
Ontario, since May 2007 and also serves on its board of directors. He also serves as Managing
Director and a member of the board of directors of Lifelong Nutrition, Inc. From 2003 to January
2007, Mr. Fortino held the positions of Executive Vice President-Ontario Region, and Officer for
Loblaw Companies Ltd. From 2000 to 2003, Mr. Fortino was Executive Vice President of Zehrmart
Limited in Cambridge, Ontario. Prior to 2000, Mr. Fortino held several management positions for
Loblaw Companies Ltd., including Senior Vice President — Supply Chain & Logistics.
Michael F. Hines became one of our directors in November 2009. Mr. Hines was Executive Vice
President and Chief Financial Officer of Dick’s Sporting Goods, Inc. from 1995 to March 2007. From
1990 to 1995, he held management positions with Staples, Inc., most recently as Vice President,
Finance. Earlier, he spent 12 years in public accounting, the last eight years with the accounting
firm Deloitte & Touche, LLP in Boston. Mr. Hines also serves on the Board of TJX Companies, which
he joined in 2007. Previously he served on the Board of Yankee Candle, Inc. from 2003 to 2007,
when the company went private.
David B. Kaplan became one of our directors in March 2007 upon consummation of the Merger. Mr.
Kaplan has been a Senior Partner in the Private Equity Group of Ares Management LLC, an alternative
asset investment management firm, since April 2003 and serves on the Executive Committee of Ares
Management. Mr. Kaplan joined Ares Management from Shelter Capital Partners, LLC, where he was a
Senior Principal from 2000 to 2003. From 1991 through 2000, Mr. Kaplan was affiliated with, and a
Senior Partner of, Apollo Management, L.P. and its affiliates, during which time he completed
multiple private equity investments from origination through exit. Prior to Apollo, Mr. Kaplan was
a member of the Investment Banking Department at Donaldson, Lufkin & Jenrette Securities Corp. Mr.
Kaplan has over 20 years of experience managing investments in, and serving on the boards of
directors of, companies operating in various industries, including in the retail and consumer
products industries. Mr. Kaplan
currently serves on the boards of directors of Maidenform Brands, Inc., Stream Global
Services, Inc., and
132
Orchard Supply Hardware Corporation. Mr. Kaplan also serves on the Board of
Governors of Cedars-Sinai Medical Center and is a Trustee, Treasurer and Chairman of the Investment
Committee of the Center for Early Education. Mr. Kaplan graduated with High Distinction, Beta Gamma
Sigma, from the University of Michigan, School of Business Administration with a B.B.A.
concentrating in Finance.
Romeo Leemrijse became one of our directors in May 2009. Mr. Leemrijse joined Teachers’ in
2006 having previously worked at EdgeStone Capital Partners and CIBC World Markets. Mr. Leemrijse
has participated in a number of transactions while at Teachers’, including the acquisition of New
Zealand Yellow Pages Group, the publisher of the print and online directories in New Zealand, and
several minority co-investments including Valentino Fashion Group, Intelsat and Select Service
Partners. Mr. Leemrijse currently sits on the board of directors of AOT Bedding (Serta) and
previously sat on the advisory boards of a number of leading international private equity funds.
Mr. Leemrijse received a Bachelor of Commerce from the University of Calgary and is a CFA
charterholder.
Jeffrey B. Schwartz became one of our directors in March 2007 upon consummation of the Merger.
Mr. Schwartz is a Principal in the Ares Private Equity Group. Mr. Schwartz joined Ares in 2004 from
Lehman Brothers Inc. where he served as a Vice President in the Financial Sponsors Group and
specialized in providing acquisition advice to financial sponsors on potential leveraged buyouts.
Prior to Lehman Brothers Inc., Mr. Schwartz was with the Wasserstein Perella Group where he
specialized in mergers and acquisitions and leveraged finance. Mr. Schwartz also currently serves
on the Boards of Directors of Stream Global Services, Inc. and WCA Waste Corporation and served as
a director of Samsonite Corporation from September 2005 until May 2007. Mr. Schwartz graduated from
University of Pennsylvania’s Wharton School of Business with a BS in Economics.
Board Composition and Terms
As of February 15, 2009, our board of directors was composed of ten directors. Each director
serves for annual terms or until his or her successor is elected and qualified. Pursuant to a
stockholders agreement, as amended and restated on February 12, 2008, two of our Parent’s principal
stockholders each have the right to designate four members of our Parent’s board of directors (or,
at the sole option of each, five members of the board of directors, one of which shall be
independent) for so long as they or their respective affiliates each own at least 10% of the
outstanding common stock of our Parent. The stockholders agreement also provides for election of
our Parent’s then-current chief executive officer to our Parent’s board of directors. Our Parent’s
board of directors intends for our board of directors and the board of directors of GNC Corporation
to have the same composition. Effective February 12, 2008, our Parent’s board of directors
approved an amendment to our Parent’s by-laws that expanded the maximum size of its board of
directors from nine to eleven members, the exact number of which will be set from time to time by
our Parent’s board of directors.
Board Committees
The board of directors has the authority to appoint committees to perform certain management
and administration functions. Our board of directors historically had an audit committee and a
compensation committee, which had the same members as the audit committee and compensation
committee of our direct and ultimate parent companies. In connection with the Merger, our board
of directors formed and appointed members to the audit committee and the compensation committee.
Audit Committee
The audit committee selects on behalf of our board of directors an independent public
accounting firm to be engaged to audit our financial statements, discusses with the independent
auditors their independence, approves the compensation of the independent public accounting firm,
reviews and discusses the audited financial statements with the independent auditors and management
and will recommend to our board of directors whether the audited financials should be included in
our Annual Reports on Form 10-K to be filed with the SEC. The audit committee also oversees the
Company’s internal audit function. The audit committee members are Jeffrey Schwartz, Romeo Leemrijse and
Michael Hines; there is one vacant position on the audit
133
committee. The board of directors has
determined that Mr. Schwartz is an “audit committee financial expert” as defined by Item 405(d)(ii)
of Regulation S-K.
Compensation Committee
The compensation committee reviews and either approves, on behalf of our board and our
Parent’s board of directors, or recommends to the board of directors for approval the annual
salaries and other compensation of our executive officers and individual stock and stock option
grants. The compensation committee also provides assistance and recommendations with respect to our
compensation policies and practices and assists with the administration of our compensation plans.
The compensation committee members are Andrew Claerhout, Norman Axelrod, David Kaplan and Romeo
Leemrijse.
|
|
|
| ITEM 11. |
|
EXECUTIVE COMPENSATION |
Compensation Discussion and Analysis
Overview
Our compensation structure and policies for our executive officers are subject to review and
approval by the compensation committee of the board of directors of our Parent (the “Compensation
Committee”). This Compensation Discussion and Analysis reflects our compensation structure and
policies currently in effect.
Generally, the Compensation Committee is empowered to review and approve on an annual basis:
| |
• |
|
the corporate goals and objectives with respect to compensation for our Chief
Executive Officer; |
| |
| |
• |
|
the evaluation process and compensation structure for our other executive
officers; and |
| |
| |
• |
|
the compensation structure and annual compensation for the directors on our
board of directors and the board of directors of our Parent (together, the “Company
Board”) and committee service by non-employee directors. |
In addition, the Compensation Committee has the authority to review our incentive compensation
plans, to recommend changes to such plans to the Company Board as needed and to exercise all the
authority of the Company Board with respect to the administration of such plans.
The primary objective of our compensation program is to attract and retain qualified employees
who are enthusiastic about our mission and culture. A further objective of our compensation program
is to provide incentives and to reward each employee for his or her contribution to us. In
addition, we strive to promote an ownership mentality among our key leaders and directors.
Finally, we intend for our compensation structure to be perceived as fair to our employees,
stockholders and noteholders. The foregoing objectives are applicable to the compensation of our
principal executive officer, principal financial officer and three other most highly compensated
executive officers (collectively, “Named Executive Officers”).
Our compensation program is designed to reward the Named Executive Officers for their
individual contributions, incentivize them for future performance and recognize our positive growth
and financial performance. The Compensation Committee considers numerous factors, including the
Named Executive Officers’ experience in conjunction with the level and complexity of the position
in setting executive compensation. Regarding the compensation program and structure generally and all
aspects
134
of executive compensation, our management, principally our Chief Executive Officer,
provides recommendations to the Compensation Committee; however, the Compensation Committee does
not delegate any of its functions to others in setting compensation. Our Chief Executive Officer
does not provide recommendations with respect to his own compensation. We do not generally engage
any consultants related to executive or director compensation matters; however, in December 2008
our Compensation Committee reviewed a comparative analysis of our top nine executives’ total
compensation packages prepared by the Hay Consulting Group to determine whether the compensation
packages of our top nine executives are at market levels. Although our Compensation Committee
reviewed this report, which generally indicated that our top nine executives receive market
compensation, the Compensation Committee did not rely on this report or use it for benchmarking
purposes in determining the current or future compensation of our Named Executive Officers. Our
Compensation Committee does, however, regularly refer to survey and other compensation data, as
described more fully below.
Elements of the Company’s Executive Compensation
Annual compensation for our Named Executive Officers is provided under employment agreements.
We have employment agreements with all of our Named Executive Officers.
Generally, annual compensation for our Named Executive Officers consists of the following
components:
| |
1. |
|
Base salary. The Compensation Committee uses base salary to attract and retain
a strong motivated leadership team at levels that are commensurate with other specialty
retailers of comparable size to us. |
| |
| |
2. |
|
Annual incentive compensation. Annual incentive compensation is used to reward
our Named Executive Officers for our growth and financial performance based on
achievement of criteria approved by the Compensation Committee. Our Compensation
Committee receives input from our Human Resources Department and Chief Executive
Officer about our Named Executive Officers’ performance and business goals and
objectives, and considers prevailing market practices based on, among other things,
survey comparisons from Mercer Human Resource Consulting LLC, Western Management Group,
and Watson Wyatt Worldwide to determine the compensation and criteria for particular
positions and seniority levels. However, these surveys are not used to benchmark
compensation. As additional cash compensation that is contingent on our annual
financial performance, annual incentive compensation augments the base salary component
while being tied directly to financial performance. Annual incentive compensation is
documented in an annual plan, which is adopted by the Compensation Committee prior to
or during the beginning of the applicable year. |
| |
| |
3. |
|
Stock options. Stock options, which are discussed in more detail under
“—Stock Awards,” are granted to recognize and incentivize performance. Stock options
provide a non-cash compensation component to drive performance, but with a long-term
horizon, since value to the Named Executive Officer is dependent on continued
employment and appreciation in our overall value. |
| |
| |
4. |
|
Benefits and perquisites. Our Named Executive Officers participate in employee
benefits generally available to all employees, as well as any benefits generally made
available to our executive officers. In addition, the Named Executive Officers receive
certain perquisites, which are primarily based on level of position. Such perquisites
may include insurance and parking, or additional cash compensation to meet specific
goals, such as car allowance and professional assistance. We believe such perquisites
are a necessary component for a competitive compensation package. In addition, we
maintain a non-qualified deferred compensation plan in which certain of our Named
Executive Officers are eligible to
participate. |
135
| |
5. |
|
Severance compensation. Our Chief Executive Officer and our other Named
Executive Officers with employment agreements are entitled to severance compensation,
including: |
| |
• |
|
a payment based on the Named Executive Officer’s base salary upon termination
because of death or disability, termination by us without cause, or termination by
the Named Executive Officer for good reason; |
| |
| |
• |
|
a prorated payment of annual incentive compensation for the year in which
employment is terminated if a bonus would have been payable had the Named Executive
Officer been employed at the end of the year; and |
| |
| |
• |
|
reimbursement of the cost of continuation coverage under COBRA to the extent it
exceeds the amount the Named Executive Officer was paying for health insurance
premiums while employed for a period following the termination of the Named
Executive Officer’s employment. |
See “— Employment Agreements with our 2009 Named Executive Officers” and “—Potential
Termination or Change-in-Control Payments” for a discussion of the severance payments and benefits
our Chief Executive Officer and the other Named Executive Officers may be entitled to receive upon
a termination of employment.
We believe that a competitive executive compensation program is needed in order both to
attract and retain qualified Named Executive Officers.
Stock Awards
All of our employees, and the employees of direct and indirect subsidiaries and other
affiliates, including our Named Executive Officers, are eligible for awards of stock options,
restricted stock, and/or other stock-based awards under the GNC Acquisition Holdings Inc. 2007
Stock Incentive Plan (the “2007 Stock Plan”), which are intended to recognize and incentivize
performance. We believe that through a broad-based plan the economic interests of our employees,
including our Named Executive Officers, are more closely aligned to ownership interests.
Under the terms of the 2007 Stock Plan, the Compensation Committee of our Parent (the “Parent
Compensation Committee”) is responsible for administering the 2007 Stock Plan and making any award
determinations. The Parent Compensation Committee does not delegate any function of the stock
option grants. The Compensation Committee intends for stock option grants generally to be
considered on an annual basis, except for new hires, promotions, and special performance
recognition. Awards are generally granted only after the release of material information, such as
quarterly or annual earnings, or at other times if the circumstances of the grant are evidenced and
no action is taken with respect to the date of the grant that would constitute, or create the
appearance of, a manipulation of the award exercise price.
The Parent Compensation Committee sets the exercise price per share for stock option grants at
an amount greater than or equal to the fair market value per share of our common stock. However,
our ultimate parent company’s common stock has not been and is not publicly traded. The Parent
Compensation Committee has used a valuation methodology in which the fair market value of the
common stock is based on our business enterprise value and, in situations deemed appropriate by the
Parent Compensation Committee, may be discounted to reflect the lack of marketability associated
with the common stock.
136
How We Chose Amounts and/or Formulas for Each Element
Base Salary. The Compensation Committee intends to set the base salary for our Named
Executive Officers at a level to attract and retain a strong motivated leadership team, but not so
high that it creates a negative perception with our employees generally, noteholders or
stockholders. Each Named Executive Officer’s current and prior compensation is considered in
setting future compensation. In addition, we review the compensation practices of other companies.
Base salary amounts are determined by complexity and level of position as well as market
comparisons.
Each year, we perform a market analysis with respect to the compensation of all of our Named
Executive Officers. Although we do not use compensation consultants, we participate in various
surveys and use the survey data for market comparisons. Currently, we use surveys with both base
salary and other short-term compensation data, including incentive compensation and fringe
benefits, that are available from Mercer Human Resource Consulting LLC, Western Management Group,
and Watson Wyatt Worldwide in the specialty retail and non-durable manufacturing categories. In
addition to focusing our analysis on the specific executive positions, we break down the survey
information based on corporate and/or average store revenue and geographic location of comparable
companies to ensure that we are using valid comparisons. We also use internal value comparisons;
however, we do not have any specific point system or rating structure for internal values.
We have not historically used, and do not intend to use, the information in the surveys for
benchmarking purposes or in our process for setting compensation. Rather, the Compensation
Committee sets compensation levels and then uses the information in the surveys to confirm and
demonstrate to management that the compensation being paid by us is consistent with market levels.
Effective January 1, 2010, the Compensation Committee granted merit-based increases to the
annual base salaries of Mr. Fortunato and Ms. Kaplan, and effective December 6, 2009, the
Compensation Committee granted merit-based increases to the annual base salaries of Messrs. Nuzzo
and Dowd. The annual base salaries of Mr. Fortunato, Ms. Kaplan and Messrs. Nuzzo and Dowd were
increased to $886,000, $716,000, $409,400 and $350,000, respectively. The increases ranged from 2% to
6.2% as a percentage of previous salary levels and were based upon the Named Executive Officers’
performance for the year ended December 31, 2009. In addition, effective October 29, 2009, the
Compensation Committee granted Mr. Stubenhofer a merit-based increase in his annual base salary to
$320,000.
Annual Incentive Compensation. Our Named Executive Officers are entitled to annual
performance bonuses pursuant to the terms of their employment agreements. The annual performance
bonus for each Named Executive Officer has target and maximum bonus amounts expressed as a
percentage of his or her annual base salary. The respective percentages are determined by position
and level of responsibility and are stated in the annual incentive plan adopted by the Compensation
Committee. The employment agreements of our Chief Executive Officer and President provide that
their targets will not be less than 75% of their respective base salaries with a maximum of 125% of
their respective base salaries. The target and/or maximum amounts may be increased for any Named
Executive Officer by the terms of an employment agreement entered into after the adoption of an
annual incentive plan.
The following table sets forth the target and maximum bonus amounts for each level of
executive officer with respect to the 2009 incentive plan adopted in February 2009 (the “2009
Incentive Plan”), the 2008 incentive plan adopted in February 2008 (the “2008 Incentive Plan”) and
the 2007 incentive plan adopted in June 2007 (which replaced and superseded the 2007 incentive plan
adopted in December 2006) (the “2007 Incentive Plan”):
137
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
2009 Incentive Plan |
|
2008 Incentive Plan |
|
2007 Incentive Plan |
| |
|
Target |
|
Maximum |
|
Target |
|
Maximum |
|
Target |
|
Maximum |
| Level |
|
Amount |
|
Amount |
|
Amount |
|
Amount |
|
Amount |
|
Amount |
CEO |
|
|
75 |
% |
|
|
125 |
% |
|
|
75 |
% |
|
|
125 |
% |
|
|
75 |
% |
|
|
125 |
% |
President |
|
|
75 |
% |
|
|
125 |
% |
|
|
75 |
% |
|
|
125 |
% |
|
|
— |
|
|
|
— |
|
Executive Vice President |
|
|
45 |
% |
|
|
100 |
% |
|
|
45 |
% |
|
|
100 |
% |
|
|
45 |
% |
|
|
100 |
% |
Senior Vice President |
|
|
40 |
% |
|
|
75 |
% |
|
|
40 |
% |
|
|
75 |
% |
|
|
40 |
% |
|
|
75 |
% |
Each annual incentive plan establishes thresholds, expressed as a percentage of the target
amount or the maximum amount, based on the achievement of certain financial performance goals. The
target bonus is designed to provide Named Executive Officers with a normal target bonus if we
perform to expectation. The threshold bonus is designed to provide Named Executive Officers with
some bonus opportunity, but less than the target opportunity if we do not achieve our expected
budgeted performance. If we exceed our budgeted performance, Named Executive Officers will be paid
a maximum bonus in excess of the target in order to reward them for our outstanding performance.
For 2007, 2008 and 2009, the goal is based on budgeted EBITDA subject to certain adjustments for
non-recurring items as determined by the Company Board. The following table sets forth the
thresholds and related goals with respect to both the 2007 Incentive Plan, the 2008 Incentive Plan
and the 2009 Incentive Plan:
| |
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
2009 Incentive Plan |
|
2008 Incentive Plan |
|
2007 Incentive Plan |
| Thresholds |
|
Budgeted EBITDA |
|
Budgeted EBITDA |
|
Budgeted EBITDA |
First threshold—33% of
target |
|
|
95 |
% |
|
|
95 |
% |
|
|
95 |
% |
Second
threshold—66% of
target |
|
|
— |
|
|
|
97 |
% |
|
|
97 |
% |
Target |
|
|
100 |
% |
|
|
100 |
% |
|
|
100 |
% |
Maximum |
|
|
106.5 |
% |
|
|
108 |
% |
|
|
108 |
% |
As in 2008, for the 2009 Incentive Plan, the payment amount will be pro rated for budgeted
EBITDA achieved between the Target and Maximum levels.
The annual incentive plan for 2010 performance (the “2010 Incentive Plan”) was adopted by the
Compensation Committee on February 4, 2010. It provides for the same target and maximum bonus
amounts for the CEO, President, Executive Vice President and Senior Vice Presidents as the 2009
Incentive Plan. In addition, the 2010 Incentive Plan’s goal is based on budgeted EBITDA subject to
certain adjustments for non-recurring items as determined by the Company Board. The thresholds and
related goals with respect to the 2010 Incentive Plan are as follows:
| |
|
|
|
|
| |
|
2010 Incentive Plan |
| Thresholds |
|
Budgeted EBITDA |
First threshold—33% of target |
|
|
91.5 |
% |
Second threshold—66% of target |
|
|
— |
|
Target |
|
|
100 |
% |
Maximum |
|
|
103.9 |
% |
We do not disclose our internal budget for results of operations, including budgeted EBITDA
(as determined by the Company Board). This amount constitutes confidential financial information,
and we believe that disclosure of this amount, whether with respect to historical periods or future
periods, would cause us competitive harm by disclosing to competitors a key element of our internal
projections.
138
The Compensation Committee sets the EBITDA target at a level it believes is both challenging
and achievable. By establishing a target that is challenging, the Compensation Committee believes
that performance of its employees, and therefore the Company, is maximized. By setting a target
that is also achievable, the Compensation Committee believes that employees remain motivated to
perform at the high level required to achieve the target. While the Company has experienced
success in meeting the established EBIDTA targets, the Compensation Committee may determine in a
particular year that based upon other factors than financial performance the awarding of full or
partial bonuses is appropriate. In 2007, 2008, and 2009 the Company successfully met established
EBITDA targets and bonus payments were made.
The Compensation Committee may, in its discretion, amend the foregoing levels on an individual
basis if it determines that competitive considerations and/or circumstances require the Company to
make exceptions to the foregoing levels to retain qualified executives.
Based on our financial performance in 2009, we achieved a goal that exceeded target, but was
less than the maximum threshold as described in the table above. As a result, in February 2010 each
of our Named Executive Officers was paid an amount above the target, but less than the maximum
possible annual incentive compensation under the 2009 Incentive Plan. Management believes that
achieving 100%, or more, of the goal of meeting or exceeding 100% of budgeted EBITDA set in the
2010 Incentive Plan, while possible to achieve for our Named Executive Officers, will present a
significant challenge.
Generally, an annual performance bonus is payable only if the Named Executive Officer is
employed by us on the date payment is made.
Stock Options. We believe that equity-based awards are an important factor in aligning the
long-term financial interest of our Named Executive Officers and stockholders. The Parent
Compensation Committee continually evaluates the use of equity-based awards and intends to continue
to use such awards in the future as part of designing and administering the Company’s compensation
program. See “— Stock Awards” above for more information regarding our stock option grants.
We follow a practice of granting equity incentives in the form of stock options in order to
grant awards that contain both substantial incentive and retention characteristics. These awards
are designed to provide emphasis on providing significant incentives for continuing growth in
stockholder value. Stock options are generally granted on an annual basis, except for new
employees on the commencement of their employment and to existing employees following a significant
change in job responsibilities or to recognize special performance. Stock options generally are
subject to vesting in annual installments on the first five anniversaries of the date of grant and
have a term of ten years. However, stock options granted to our Chief Executive Officer and
President are subject to vesting in annual installments on the first four anniversaries of the date
of grant and have a term of ten years.
139
The Parent Compensation Committee determines stock option grant awards in accordance with
the Named Executive Officer’s performance and level of position. The executive officer level
positions within the company hierarchy are as follows: Chief Executive Officer, President,
Executive Vice President, Senior Vice President, and Vice President. All stock option grants to
executive officers are determined by the Parent Compensation Committee. Since January 2008, we
have consistently applied the following ranges of stock option grants for individuals at the
executive officer level:
Chief Executive Officer: 1,000,000 shares (minimum level)
President: 750,000 shares (minimum level)
Executive Vice President: 300,000 to 350,000 shares
Senior Vice President: 70,000 to 135,000 shares
Vice President: 20,000 to 30,000 shares
Within a given range, the size of the stock option award is determined based on the executive
officer’s duties and the Company’s interest in attracting, retaining and providing significant
incentives for the executive officer.
We seek to provide employees, including all executive officers, with overall compensation and
incentive packages that are commensurate with their respective functions and levels of seniority,
and that are competitive within the retail industry. The Compensation Committee has determined
that the foregoing grant levels are appropriate within the overall compensation and incentive
package applicable to the various executive officer positions.
As the Chief Executive Officer and President are unique offices, each filled by a single
individual, the Compensation Committee has established minimum stock grant levels only. This
enables the Compensation Committee to craft a total compensation package necessary to recruit and
retain top talent to attract and retain individuals at these levels. All other officer level
positions have multiple individuals who share the same title and level within the Company.
Stock option grant awards made at the time of the Merger were based, in part, on the length of
service and performance of the Named Executive Officer through the date of the Merger. Following
the Merger, stock option grant awards have been made at or about the time that a Named Executive
Officer begins service with GNC. Since a Named Executive Officer generally has little or no record
of service prior to receiving stock option grant awards, elements of individual performance are not
taken into account when making such stock option grant awards. To the extent that the Compensation
Committee or our Company Board determines, at a future date, that it is appropriate to grant stock
option awards to executive officers based on performance, the Compensation Committee or Company
Board, as applicable, will establish standards for making such awards at that time.
On May 14, 2009, the Compensation Committee approved a change to the exercise prices of
non-qualified stock options to purchase shares of Class A common stock of the Company, par value
$0.001, that were previously granted to Mr. Nuzzo and certain other executives. The Compensation
Committee repriced the exercise prices in order to preserve the incentive intended to be afforded
by the grant of stock options, and such repricings were approved by stockholders. For more
information, see “Summary Compensation Table — Option Repricing.”
Benefits and Perquisites. We provide a fringe benefit package for our Named Executive
Officers. Generally, our Named Executive Officers are entitled to participate in, and to receive
benefits under, any benefit plans, arrangements, or policies available to employees generally or to
our executive officers generally. The fringe benefits for our Chief Executive Officer and
President were negotiated in connection with their employment agreements and in some respects were
set at a higher level as a matter of policy based on the position. The basic fringe benefits
package for our Named Executive
140
Officers who are senior vice presidents generally consists of the following items:
| |
• |
|
health insurance in accordance with our health insurance plan or program in
effect from time to time; |
| |
| |
• |
|
prescription drug coverage in accordance with our health insurance plan or
program, or separate prescription drug coverage plan or program, in effect from
time to time; |
| |
| |
• |
|
dental insurance in accordance with our dental insurance plan or program in
effect from time to time; |
| |
| |
• |
|
long-term disability insurance in accordance with our long-term disability
insurance plan or program in effect from time to time; |
| |
| |
• |
|
short-term disability insurance in accordance with our short-term disability
insurance plan or program in effect from time to time; |
| |
| |
• |
|
life insurance coverage in accordance with our life insurance program in effect
from time to time, which for our Chief Executive Officer will be an amount equal to
2 times his base salary, not to exceed the maximum coverage limit provided from
time to time in accordance with our employee benefits plan; |
| |
| |
• |
|
an automobile allowance in an annual amount equal to $5,000; |
| |
| |
• |
|
an allowance for professional assistance in an annual amount equal to $3,500; |
| |
| |
• |
|
a supplemental retirement allowance in an annual amount equal to $10,000
($25,000 for our Chief Executive Officer); |
| |
| |
• |
|
a financial planning and tax preparation allowance in an annual amount equal to
$3,000 ($8,000 for our Chief Executive Officer); and |
| |
| |
• |
|
for senior vice presidents located at our headquarters in Pittsburgh,
Pennsylvania, a downtown Pittsburgh parking lease with an annual value in an amount
equal to $2,640. |
Named Executive Officers at the executive vice president level receive additional fringe
benefits, which generally consist of some of the allowances listed, but at higher amounts (car
allowance of $11,500; professional assistance allowance of $7,500; and, if applicable, a Pittsburgh
parking lease with a $3,300 value). In addition to the basic package, we have Named Executive
Officers who have historically received some of these allowances in greater amounts and have been
grandfathered at those levels even though the current basic package is set at lower amounts.
Messrs. Fortunato and Dowd receive an additional benefit on a grandfathered basis as follows: a
supplemental medical allowance of $6,000 per year. In addition, Mr. Dowd received a car allowance
in a greater amount than other executive officers of the same level of position on a grandfathered
basis. Although Mr. Dowd and Mr. Nuzzo are Executive Vice Presidents, Mr. Dowd received a car
allowance of $11,500, whereas Mr. Nuzzo received a car allowance of only $6,500.
In addition to the fringe benefits set forth above, the fringe benefits package for our Chief
Executive Officer also consists of an allowance for country club dues and expenses incurred for
business reasons in an annual amount equal to $15,000, plus a one-time membership fee of $10,000
for a business club; and first class air travel for all business trips. In lieu of the individual
allowances set forth above for our Named Executive Officers, our President receives $50,000 of
additional fringe benefits to cover professional assistance, supplemental retirement, financial
planning and automotive expenses.
141
Under certain circumstances, management may recommend and the Compensation Committee may
approve more limited benefits or additional benefits, such as relocation expenses for new
executives. Benefits and perquisites may be limited or expanded based on the needs of an executive
officer or the circumstances of such executive officer’s employment. For example, parking
allowances are provided only to those executive officers whose places of employment require parking
licenses, and housing allowances are provided to our most senior executives only where management
and the compensation committee determine that such benefits are necessary to attract, retain or
enhance the performance of the executives.
While the Compensation Committee in its discretion may revise, amend or add to Named Executive
Officers’ benefits if it deems it advisable, we have no current plans to change the levels of
benefits currently provided to our Named Executive Officers. We annually review these fringe
benefits and make adjustments as warranted based on competitive practices, our performance and the
individual’s responsibilities and performance. The Compensation Committee has approved these other
benefits as a reasonable component of our executive compensation program. Please see the “All
Other Compensation” column in the Summary Compensation Table for further information regarding
these fringe benefits.
We also maintain a 401(k) plan for eligible employees that permits each participant to make
voluntary pre-tax contributions and provides that the Company may make matching contributions;
however, none of our current Named Executive Officers are currently eligible to participate in the
401(k) plan.
The Company maintains the GNC Live Well Later Non-qualified Deferred Compensation Plan for the
benefit of a select group of management or highly compensated employees. Under the deferred
compensation plan, an eligible employee of such subsidiary or a participating affiliate may elect
to defer a portion of his or her future compensation under the plan by electing such deferral prior
to the beginning of the calendar year during which the deferral amount would be earned. Mr. Dowd
is the only Named Executive Officer who made contributions to the plan in 2009. Please see the
Non-qualified Deferred Compensation Table for more information regarding the deferred compensation
plan.
Employment Agreements and Severance Compensation. We have employment agreements with all of
our Named Executive Officers. Please see “— Employment Agreements with our 2009 Named Executive
Officers” for more information regarding the employment agreements with our Named Executive
Officers as in effect in 2009, and “—Potential Termination or Change-in-Control Payments” for more
information regarding termination and payments made in connection with a change in control. We
will continue to determine appropriate employment agreement and severance packages for our Named
Executive Officers in a manner that we believe will attract and retain qualified executive
officers.
Chief Executive Officer Compensation
Mr. Fortunato’s annual compensation is weighted towards variable, performance-based
compensation, with the Company’s financial performance as the primary determinant of value. For
2009, Mr. Fortunato’s compensation consisted of:
| |
• |
|
$860,000 base salary, |
| |
| |
• |
|
no stock option awards, |
| |
| |
• |
|
annual performance compensation under the 2009 Incentive Plan of $948,580, |
| |
| |
• |
|
a discretionary bonus of $100,000 for meeting additional performance targets,
including personnel initiatives (as described below), and |
| |
| |
• |
|
other compensation, including fringe benefits, equal to $72,576. |
142
At the beginning of 2008, the Compensation Committee established a development plan to be
implemented by Mr. Fortunato. Mr. Fortunato’s objectives under the plan were in addition to his
ongoing responsibilities as Chief Executive Officer. Among Mr. Fortunato’s primary objectives were
the completion of the restructuring and development of the Company’s senior management team through
the attraction, hiring and retention of qualified personnel and development of leadership
capabilities. At the conclusion of 2008, the Compensation Committee determined that Mr.
Fortunato’s performance met a significant number of the pre-determined objectives and awarded Mr.
Fortunato a bonus of $90,000.
During the first quarter of 2009, the Compensation Committee determined to implement a similar
incentive program for Mr. Fortunato for fiscal year 2009. Under this program, Mr. Fortunato was
eligible to receive an additional discretionary bonus of up to $200,000 based on the achievement of
certain non-financial objectives. Among the primary objectives was ongoing executive leadership
development, including through enhanced time management strategies and the implementation of
collaborative approaches, and achievement of the Company’s strategic initiatives. Throughout the
year, Mr. Fortunato provided reports to, and received input and feedback from, the Compensation
Committee regarding his efforts to achieve the established objectives and complete the required
actions. At the conclusion of 2009, the Compensation Committee determined that Mr. Fortunato’s
performance met a significant number of the pre-determined objectives and awarded Mr. Fortunato a
bonus of $100,000.
During the first quarter of 2010, the Compensation Committee determined that, following the
conclusion of fiscal year 2010, it will evaluate Mr. Fortunato’s performance for fiscal year 2010
and determine whether any discretionary bonus is warranted. In addition, effective January 1, 2010,
the Compensation Committee granted Mr. Fortunato a merit-based increase in his annual base salary
to $886,000.
See the Summary Compensation Table for more information regarding Mr. Fortunato’s
compensation.
Accounting and Tax Considerations
As a general matter, the Compensation Committee reviews and considers the various tax and
accounting implications of compensation vehicles utilized by the Company.
Our Parent Company’s stock option grant policies have been impacted by the implementation of
Financial Accounting Standards Board Accounting Standards Codification Topic 718 (“FASB ASC 718”)
(formerly known as FAS 123R), which it adopted in the first quarter of fiscal year 2006. Under this
accounting pronouncement, we are required to value unvested stock options granted prior to our
adoption of FASB ASC 718 under the fair value method and expense those amounts in our income
statement over the stock option’s remaining vesting period.
Since neither our equity securities nor the equity securities of our direct or indirect parent
companies are publicly traded, we are not currently subject to any limitations under Internal
Revenue Code Section 162(m). While we are not required to do so, we have structured our
compensation programs in a manner to generally comply with Internal Revenue Code Section 162(m).
Under Section 162(m) of the Internal Revenue Code, a limitation was placed on tax deductions of any
publicly traded corporation for individual compensation to certain executives of such corporation
exceeding $1,000,000 in any taxable year, unless the compensation is performance-based. Had we
been subject to Section 162(m) in 2007, we might have been subject to deduction limitations with
respect to some of our Named Executive Officers because of discretionary bonus payments paid in
March 2007 to all optionholders whose options vested in 2007 entitling them to receive payment
pursuant to the terms of a November 2006 dividend and bonuses paid in March 2007 in connection with
the completion of the Merger. These bonus payments were not performance-based.
143
Compensation Committee Interlocks and Insider Participation
In the year ended December 31, 2009, none of our Named Executive Officers served as a director
or member of the compensation committee of another entity whose executive officers served on our
Company Board or Compensation Committee.
Compensation Committee Report
The members of the Compensation Committee have reviewed and discussed with management the
Compensation Discussion and Analysis. Based on their review and the discussions between members of
the Compensation Committee with members of management, the members of the Compensation Committee
recommended to our board of directors that the Compensation Discussion and Analysis be included in
this annual report on Form 10-K.
| |
|
|
|
|
| |
Compensation Committee of the Board of Directors:
|
|
| |
|
|
| |
Andrew Claerhout (Chair) |
|
| |
Norman Axelrod
David B. Kaplan
Romeo Leemrijse |
|
| |
Notwithstanding any SEC filing by the Company that includes or incorporates by reference other SEC
filings in their entirety, this Compensation Committee Report shall not be deemed to be “filed”
with the SEC except as specifically provided otherwise therein.
144
Summary Compensation Table
The following table sets forth information concerning compensation we paid to our principal
executive officer, principal financial officer and three other most highly compensated executive
officers who were serving as executive officers as of December 31, 2009 (collectively, the “2009
Named Executive Officers”), for services rendered in all capacities to us during fiscal year 2009.
In accordance with SEC rules, the compensation described in this table does not include medical or
group life insurance received by the 2009 Named Executive Officers that are available generally to
all salaried employees of the Company.
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Change in |
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Pension |
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Value and |
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Non-Equity |
|
Non-qualified |
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Stock |
|
Option |
|
Incentive Plan |
|
Deferred |
|
All Other |
|
|
| Name and |
|
|
|
|
|
Salary |
|
Bonus |
|
Awards |
|
Awards |
|
Compensation |
|
Compensation |
|
Compensation |
|
Total |
| Principal Position |
|
Year |
|
($) |
|
($)1 |
|
($) |
|
($)2 |
|
($)3 |
|
Earnings ($)4 |
|
($)5,6 |
|
($) |
Joseph Fortunato |
|
|
2009 |
|
|
|
860,000 |
|
|
|
100,000 |
|
|
|
— |
|
|
|
— |
|
|
|
948,580 |
|
|
|
— |
|
|
|
72,576 |
|
|
|
1,981,156 |
|
Chief Executive Officer |
|
|
2008 |
|
|
|
855,769 |
|
|
|
216,573 |
|
|
|
— |
|
|
|
— |
|
|
|
928,509 |
|
|
|
— |
|
|
|
70,753 |
|
|
|
2,071,604 |
|
|
|
|
2007 |
|
|
|
787,500 |
|
|
|
1,909,384 |
|
|
|
— |
|
|
|
4,083,556 |
|
|
|
700,875 |
|
|
|
— |
|
|
|
7,260,110 |
|
|
|
14,741,425 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Beth J. Kaplan |
|
|
2009 |
|
|
|
696,154 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
767,857 |
|
|
|
— |
|
|
|
138,755 |
|
|
|
1,602,766 |
|
President and Chief Merchandising and Marketing Officer |
|
|
2008 |
|
|
|
675,000 |
|
|
|
250,000 |
|
|
|
— |
|
|
|
1,822,120 |
|
|
|
732,375 |
|
|
|
— |
|
|
|
119,770 |
|
|
|
3,599,265 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Michael M. Nuzzo |
|
|
2009 |
|
|
|
400,000 |
|
|
|
— |
|
|
|
— |
|
|
|
553,442 |
|
|
|
335,200 |
|
|
|
— |
|
|
|
125,321 |
|
|
|
1,413,963 |
|
Executive Vice President and Chief Financial Officer |
|
|
2008 |
|
|
|
98,462 |
|
|
|
— |
|
|
|
— |
|
|
|
462,250 |
|
|
|
80,542 |
|
|
|
— |
|
|
|
14,992 |
|
|
|
656,246 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Thomas Dowd |
|
|
2009 |
|
|
|
330,154 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
276,669 |
|
|
|
— |
|
|
|
44,015 |
|
|
|
650,838 |
|
Executive Vice |
|
|
2008 |
|
|
|
332,500 |
|
|
|
17,404 |
|
|
|
— |
|
|
|
— |
|
|
|
271,985 |
|
|
|
— |
|
|
|
44,015 |
|
|
|
665,904 |
|
President of Store Operations and Development |
|
|
2007 |
|
|
|
293,077 |
|
|
|
226,708 |
|
|
|
— |
|
|
|
727,139 |
|
|
|
168,702 |
|
|
|
— |
|
|
|
1,028,078 |
|
|
|
2,443,704 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Gerald Stubenhofer7 |
|
|
2009 |
|
|
|
294,310 |
|
|
|
15,000 |
|
|
|
— |
|
|
|
— |
|
|
|
190,418 |
|
|
|
— |
|
|
|
22,280 |
|
|
|
522,008 |
|
Senior Vice President and Chief Legal Officer |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| (1) |
|
Reflects the entire amount set forth under “Bonus” for the 2009 Named Executive Officers: |
| |
(a) |
|
For 2007: (i) discretionary payments we made in March 2007 to Messrs. Fortunato and
Dowd whose options vested in 2007 entitling them to receive payment pursuant to the terms
of a November 2006 dividend to the indirect parent company’s common stockholders in the
amount of $5.42 per share, and which were determined based on the per share amount of the
dividend and the number of outstanding vested option shares held by each optionholder as of
December 15, 2006, and (ii) one time cash success bonuses upon the completion of the Merger
paid on March 16, 2007 in the following amounts: Mr. Fortunato — $500,000 and Mr. Dowd —
$50,000. |
145
| |
(b) |
|
For 2008: (i) payments we made in September 2008 pursuant to the Merger agreement of
additional consideration in lieu of income tax payments in respect of net operating losses
created as a result of the Merger to each of Messrs. Fortunato and Dowd, based on the number
of outstanding vested option shares held by Messrs. Fortunato and Dowd as of the Merger;
(ii) a one-time discretionary bonus in respect of performance in 2008 in the following
amounts: Mr. Fortunato — $90,000; and (iii) a one-time signing bonus to Ms. Kaplan of
$250,000. |
| |
(c) |
|
For 2009: represents (i) a discretionary bonus paid to Mr. Fortunato for meeting
additional performance targets, including personnel initiatives as described in
“Compensation Discussion and Analysis — Chief Executive Officer Compensation” and (ii) a
one-time discretionary bonus paid to Mr. Stubenhofer in October 2009. |
|
|
|
| (2) |
|
Reflects the aggregate grant date fair value of option awards granted during the fiscal years
ended December 31, 2008 and December 31, 2007 which have been computed in accordance with FASB
ASC Topic 718. No stock options were granted during the fiscal year ended December 31, 2009. |
| |
| |
|
On May 14, 2009, the Compensation Committee repriced the exercise prices of 150,000 of Mr.
Nuzzo’s stock options from $9.57 to $7.70 per share and 150,000 of his stock options from $14.35
to $11.55 per share. The incremental fair value of such stock options is reported in this
column in accordance with FASB ASC Topic 718. For more information, please see “Option
Repricing” below. |
| |
| |
|
For additional information, see Note 18 under the heading “Stock-Based Compensation Plans” of
the Notes to Consolidated Financial Statements included in the Company’s Annual Report on Form
10-K for the fiscal year ended December 31, 2009. The amounts reflect the accounting expense
for these awards and do not correspond to the actual value that may be recognized by such
persons with respect to these awards. |
| |
| (3) |
|
Reflects, as applicable, annual incentive compensation paid in March 2008 with respect to
performance in 2007 pursuant to the 2007 Incentive Plan, annual incentive compensation paid in
February 2009 with respect to performance in 2008 pursuant to our 2008 Incentive Plan and
annual incentive compensation paid in February 2010 with respect to performance in 2009
pursuant to our 2009 Incentive Plan. Our results of operations for 2007, 2008 and 2009
exceeded the target goals for the target bonus payable for each applicable year, but were less
than the maximum goal thresholds for the maximum bonus payable, to each 2007 Named Executive
Officer under the 2007 Incentive Plan, each 2008 Named Executive Officer under the 2008
Incentive Plan and each 2009 Named Executive Officer under the 2009 Incentive Plan,
respectively. See “Compensation Discussion and Analysis—How We Chose Amounts and/or Formulas
for Each Element” for information about the Incentive Plans. |
| |
| (4) |
|
Represents the above-market or preferential portion of the change in value of the executive
officer’s account under our GNC Live Well Later Non-qualified Deferred Compensation Plan. See
“Non-qualified Deferred Compensation” under the Non-qualified Deferred Compensation Table for
a description of our deferred compensation plan. |
| |
| (5) |
|
The components of all other compensation for the 2009 Named Executive Officers are set forth
in the following table: |
146
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
Imputed Value for |
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
Life Insurance |
|
Payment for |
|
|
| Named Executive |
|
|
|
|
|
Perquisites |
|
Premiums |
|
Cancelled Optionsa |
|
Total |
| Officer |
|
Year |
|
($) |
|
($) |
|
($) |
|
($) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Joseph Fortunato |
|
|
2009 |
|
|
|
71,562 |
|
|
|
1,014 |
|
|
|
— |
|
|
|
72,576 |
|
|
|
|
2008 |
|
|
|
69,739 |
|
|
|
1,014 |
|
|
|
— |
|
|
|
70,753 |
|
|
|
|
2007 |
|
|
|
94,437 |
|
|
|
552 |
|
|
|
7,165,121 |
|
|
|
7,260,110 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Beth J. Kaplan |
|
|
2009 |
|
|
|
138,203 |
|
|
|
552 |
|
|
|
— |
|
|
|
138,755 |
|
|
|
|
2008 |
|
|
|
119,374 |
|
|
|
396 |
|
|
|
— |
|
|
|
119,770 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Michael M. Nuzzo |
|
|
2009 |
|
|
|
125,108 |
|
|
|
213 |
|
|
|
— |
|
|
|
125,321 |
|
|
|
|
2008 |
|
|
|
14,992 |
|
|
|
— |
|
|
|
— |
|
|
|
14,992 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Thomas Dowd |
|
|
2009 |
|
|
|
43,660 |
|
|
|
355 |
|
|
|
— |
|
|
|
44,015 |
|
|
|
|
2008 |
|
|
|
43,660 |
|
|
|
355 |
|
|
|
— |
|
|
|
44,015 |
|
|
|
|
2007 |
|
|
|
42,643 |
|
|
|
240 |
|
|
|
985,195 |
|
|
|
1,028,078 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Gerald Stubenhofer |
|
|
2009 |
|
|
|
22,040 |
|
|
|
240 |
|
|
|
— |
|
|
|
22,280 |
|
| |
(a) |
|
Reflects payments made to certain 2009 Named Executive Officers pursuant to the terms of
the Merger on March 16, 2007, for outstanding options canceled in connection with the Merger
in an amount equal to the excess, if any, of the per share merger consideration paid in the
Merger over the exercise price per share of the option, multiplied by the number of shares of
GNC Parent Corporation common stock subject to the option and subject to reduction for
required withholding tax. |
|
|
|
| (6) |
|
Perquisites include cash amounts received by certain 2009 Named Executive Officers for, or in
reimbursement of, supplemental medical, supplemental retirement, parking, professional
assistance, car allowance, financial services assistance and the imputed value of life
insurance premiums. With respect to our Chief Executive Officer, perquisites also include
reimbursement of country club dues and expenses. With respect to our President, perquisites
also include reimbursement of housing, commuting expenses and director parking. With respect
to our Chief Financial Officer, perquisites also include reimbursement of certain state taxes. |
| |
| |
|
For 2008, the following perquisites exceeded the greater of $25,000 or 10% of each 2009 Named
Executive Officer’s total perquisites: |
| |
• |
|
Mr. Fortunato: supplemental retirement — $25,000, professional assistance -
$10,500, car allowance — $11,500, club dues — $8,079 and financial services — $8,000; |
| |
| |
• |
|
Ms. Kaplan: supplemental retirement — $20,000, housing allowance — $17,857 and
commuting expenses — $51,517; |
| |
| |
• |
|
Mr. Nuzzo: supplemental retirement — $2,222, professional assistance — $1,670 and
reimbursement of certain state taxes — $8,545; and |
| |
| |
• |
|
Mr. Dowd: supplemental medical — $6,000, supplemental retirement — $10,000,
professional assistance — $7,500, car allowance — $11,500 and financial services -
$8,000. |
For 2009, the following perquisites exceeded the greater of $25,000 or 10% of each 2009 Named
Executive Officer’s total perquisites:
| |
• |
|
Ms. Kaplan: housing allowance — $44,845 and commuting expenses — $43,166; and |
| |
| |
• |
|
Mr. Nuzzo: reimbursement of certain state taxes — $95,568. |
|
|
|
| (7) |
|
Mr. Stubenhofer was not a named executive officer for the fiscal years ended December
31, 2007 and December 31, 2008 based on the level of his total compensation in such years.
Effective October 29, 2009, Mr. Stubenhofer’s base salary was increased from $288,000 to $320,000
per year. |
147
Option Repricing
On May 14, 2009, the Compensation Committee approved a change to the exercise prices of
non-qualified stock options to purchase shares of Class A common stock of the Company, par value
$0.001, that were previously granted to Mr. Nuzzo on October 21, 2008. The Compensation Committee
determined that,
as of May 14, 2009, the exercise prices of the options were substantially greater than the
fair market value of the common stock. In order to preserve the incentive intended to be afforded
by the grant of stock options, the Compensation Committee repriced the exercise prices of 150,000
of Mr. Nuzzo’s stock options from $9.57 to $7.70 per share and an additional 150,000 of Mr. Nuzzo’s
stock options from $14.35 to $11.55 per share. Such repricings were approved by our stockholders
and were structured in a way intended to comply with Section 409A of the Internal Revenue Code.
All other terms of the stock options remain unchanged.
Grants of Plan-Based Awards
The following table sets forth information concerning awards under the Company’s non-equity
incentive plans granted to each of the 2009 Named Executive Officers during the fiscal year ended
December 31, 2009. Assumptions used in the calculation of certain dollar amounts are included in
Note 18 under the heading “Stock-Based Compensation Plans” of the Notes to the Company’s
Consolidated Financial Statements included in the Company’s Annual Report on Form 10-K for the
fiscal year ended December 31, 2009.
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
All Other |
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Option |
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Awards: |
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Number |
|
|
|
|
|
Grant Date |
| |
|
|
|
|
|
Estimated Possible Payouts under Non-equity |
|
of |
|
Exercise or |
|
Fair Value of Stock |
| |
|
|
|
|
|
Incentive Plan Awards (1) |
|
Securities |
|
Base Price |
|
and |
| |
|
|
|
|
|
Threshold |
|
Threshold |
|
|
|
|
|
Underlying |
|
of Option |
|
Option |
| |
|
Grant |
|
#1 |
|
#2 |
|
Target |
|
Maximum |
|
Options |
|
Awards |
|
Awards |
| Name |
|
Date |
|
($) |
|
($) |
|
($) |
|
($) |
|
(#) |
|
($/Sh) |
|
($) |
| |
Joseph Fortunato |
|
|
— |
|
|
|
212,850 |
|
|
|
425,700 |
|
|
|
645,000 |
|
|
|
1,075,000 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Beth J. Kaplan |
|
|
— |
|
|
|
172,298 |
|
|
|
344,597 |
|
|
|
522,116 |
|
|
|
870,193 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Michael M. Nuzzo |
|
|
— |
|
|
|
59,400 |
|
|
|
118,800 |
|
|
|
180,000 |
|
|
|
400,000 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
May 14, 2009 |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
150,000 |
|
|
|
7.70 |
|
|
|
277,306 |
(2) |
|
|
May 14, 2009 |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
150,000 |
|
|
|
11.55 |
|
|
|
276,136 |
(3) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Thomas Dowd |
|
|
— |
|
|
|
49,028 |
|
|
|
98,056 |
|
|
|
148,569 |
|
|
|
330,154 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Gerald Stubenhofer |
|
|
— |
|
|
|
38,849 |
|
|
|
77,698 |
|
|
|
117,724 |
|
|
|
220,733 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
| (1) |
|
The amounts represent the threshold, target and maximum potential amounts that might have
been payable based on the targets approved for the 2009 Named Executive Officers under the
2009 Incentive Plan. See “Compensation Discussion and Analysis—How We Chose Amounts and/or
Formulas for Each Element” for more information regarding the thresholds under the 2009
Incentive Plan. See “Summary Compensation Table—Non-Equity Incentive Plan Compensation” and
footnote 3 to the Summary Compensation Table for information regarding the actual amounts paid
in February 2010 to the 2009 Named Executive Officers under the 2009 Incentive Plan. |
148
|
|
|
| (2) |
|
Represents the incremental fair value of Mr. Nuzzo’s stock options computed in accordance
with FASB ASC Topic 718 with respect to which the exercise prices were repriced. On May 14,
2009, the Compensation Committee repriced the exercise prices of 150,000 of Mr. Nuzzo’s
options that were granted on October 21, 2008 from $9.57 to $7.70 per share. For more
information, please see “Option Repricing” above. For additional information, see Note 18
under the heading “Stock-Based Compensation Plans” of the Notes to Consolidated Financial
Statements included in the Company’s Annual Report on Form 10-K for the fiscal year ended
December 31, 2009. |
| |
| (3) |
|
Represents the incremental fair value of Mr. Nuzzo’s stock options computed in accordance
with FASB ASC Topic 718 with respect to which the exercise prices were repriced. On May 14,
2009, the
Compensation Committee repriced the exercise prices of 150,000 of Mr. Nuzzo’s options that were
granted on October 21, 2008 from $14.35 to $11.55 per share. For more information, please see
“Option Repricing” above. For additional information, see Note 18 under the heading
“Stock-Based Compensation Plans” of the Notes to the Consolidated Financial Statements included
in the Company’s Annual Report on Form 10-K for the fiscal year ended December 31, 2009. |
Outstanding Equity Awards at Fiscal Year-End
The table below sets forth information regarding exercisable and unexercisable option awards
granted to the 2009 Named Executive Officers under our 2007 Stock Plan and held as of December 31,
2009.
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Option Awards |
| |
|
Number of Securities Underlying Unexercised |
|
Option |
|
|
| |
|
Options (#)(1) |
|
Exercise Price |
|
|
| Name |
|
Exercisable |
|
Unexercisable |
|
($) |
|
Option Expiration Date |
| |
Joseph Fortunato |
|
|
40,000 |
|
|
|
40,000 |
|
|
|
5.00 |
|
|
|
3/16/2017 |
|
|
|
|
591,438 |
|
|
|
591,439 |
|
|
|
5.00 |
|
|
|
3/16/2017 |
|
|
|
|
631,438 |
|
|
|
631,439 |
|
|
|
7.50 |
|
|
|
3/16/2017 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Beth J. Kaplan |
|
|
175,000 |
|
|
|
700,000 |
|
|
|
6.93 |
|
|
|
1/2/2018 |
|
|
|
|
175,000 |
|
|
|
700,000 |
|
|
|
10.39 |
|
|
|
1/2/2018 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Michael M. Nuzzo |
|
|
30,000 |
|
|
|
120,000 |
|
|
|
7.70 |
|
|
|
10/21/2018 |
|
|
|
|
30,000 |
|
|
|
120,000 |
|
|
|
11.55 |
|
|
|
10/21/2018 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Thomas Dowd |
|
|
70,817 |
|
|
|
106,277 |
|
|
|
5.00 |
|
|
|
3/16/2017 |
|
|
|
|
70,817 |
|
|
|
106,277 |
|
|
|
7.50 |
|
|
|
3/16/2017 |
|
|
|
|
19,182 |
|
|
|
28,774 |
|
|
|
5.00 |
|
|
|
5/4/2017 |
|
|
|
|
19,182 |
|
|
|
28,774 |
|
|
|
7.50 |
|
|
|
5/4/2017 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Gerald Stubenhofer |
|
|
25,000 |
|
|
|
37,500 |
|
|
|
5.00 |
|
|
|
11/1/2017 |
|
|
|
|
25,000 |
|
|
|
37,500 |
|
|
|
7.50 |
|
|
|
11/1/2017 |
|
|
|
|
| (1) |
|
Time-based stock option awards made under the 2007 Stock Plan, which awards vest subject
to continuing employment, other than the stock options granted to Mr. Fortunato and Ms.
Kaplan, in five equal annual installments commencing on the first anniversary of the date of
grant. For the stock options granted to Mr. Fortunato and Ms. Kaplan, such stock options vest
in four equal annual installments commencing on the first anniversary of the date of grant. |
Option Exercises and Stock Vested
No stock options were exercised in 2009. We have not issued, nor are there any outstanding,
shares of restricted stock.
149
Pension Benefits
The Company did not have a pension plan in effect for the benefit of its 2009 Named Executive
Officers for the fiscal year ending December 31, 2009.
Non-qualified Deferred Compensation
The Company maintains the GNC Live Well Later Non-qualified Deferred Compensation Plan for the
benefit of a select group of management or highly compensated employees. Under the deferred
compensation plan, an eligible employee of such subsidiary or a participating affiliate may elect
to defer a portion of his or her future compensation under the plan by electing such deferral prior
to the beginning of the calendar year during which the deferral amount would be earned (or, if
applicable, within 30 days of the date on which the employee first becomes eligible to participate
in the plan). The minimum amount of salary that may be deferred by an eligible employee for a
calendar year is $200, subject to a maximum of 25% of the employee’s salary otherwise payable for
the year and the minimum amount of bonus that may be deferred by an eligible employee for a
calendar year is $2,000, subject to a maximum of 25% of the employee’s bonus otherwise payable for
the year. The employers participating in the plan may in their discretion elect to make a matching
contribution to the plan for a calendar year, based on amounts deferred by eligible employees for
that year. An eligible employee may elect at the time amounts are deferred under the plan to have
such amounts credited to an in-service account, which is payable (subject to certain special
elections for 2006 and 2007 pursuant to Section 409A of the Internal Revenue Code of 1986, as
amended (the “Code”) on a future date selected by the employee at the time the employee first
elects to defer compensation under the plan, or to a retirement account, which is payable (subject
to the special elections described above) upon the employee’s retirement (as defined in the plan).
Payments will be made earlier than the dates described above as a result of the death or
disability of an employee participating in the plan. If a participating employee dies before
retirement, a death benefit will be paid to the employee’s beneficiaries in certain cases. For
purposes of applying the provisions of the Code and the Employee Retirement Income Security Act
(ERISA) to the plan, the plan is intended to be an unfunded arrangement.
The following table identifies the 2009 Named Executive Officers who participate in the plan,
their contributions, our contributions and the earnings in 2009, and their aggregate balance at the
end of 2009.
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Executive |
|
Registrant |
|
Aggregate |
|
Aggregate |
|
Aggregate |
| |
|
Contributions in |
|
Contributions in |
|
Earnings in Last |
|
Withdrawals/ |
|
Balance at Last |
| |
|
Last Fiscal Year |
|
Last Fiscal Year |
|
Fiscal Year |
|
Distributions |
|
Fiscal Year-End |
| Name |
|
($)(1) |
|
($) |
|
($) |
|
($) |
|
($)(2) |
Joseph Fortunato |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
Beth J. Kaplan |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
Michael M. Nuzzo |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
Thomas Dowd |
|
|
23,504 |
|
|
|
— |
|
|
|
19,919 |
|
|
|
— |
|
|
|
89,246 |
|
Gerald Stubenhofer |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
| (1) |
|
The amounts reported in this column reflect deferrals under the GNC Live Well Later
Non-qualified Deferred Compensation Plan of base salary and bonus earned by and paid to Mr. Dowd in
the fiscal year ended December 31, 2009. A portion of the amount reported as salary and/or bonus
in the Summary Compensation Table, column (c) and (g), respectively were deferred by Mr. Dowd in
the fiscal year ended December 31, 2009 as follows: $9,905 of salary and $13,599 of bonus. |
| |
| (2) |
|
The amounts reported in this column include previously earned, but deferred, salary and bonus
that were reported in our Summary Compensation Table in previous years as follows: (i) $10,372 in
2008 and (ii) $32,864 in 2007. |
150
Employment Agreements with our 2009 Named Executive Officers
Chief Executive Officer
On March 16, 2007, we entered into an employment agreement with Mr. Fortunato that provides
for a five-year term with automatic annual one-year renewals thereafter unless we or Mr. Fortunato
provide at least one-year advance notice of termination, and an annual base salary of not less than
$800,000, subject to certain upward adjustments. Effective January 1, 2009, Mr. Fortunato’s
employment agreement was amended to comply with Code Section 409A. Effective January 1, 2010, the
Compensation Committee granted Mr. Fortunato a merit-based
increase in his annual base salary to $886,000. The employment agreement provides for an annual performance bonus with a target bonus of 75%
and a maximum bonus of 125% of Mr. Fortunato’s annual base salary based upon our attainment of
annual goals established by the Company Board or the Compensation Committee. The employment
agreement also provides that Mr. Fortunato will receive certain fringe benefits and perquisites
similar to those provided to our other executive officers. The employment agreement provides that
upon a change in control all of Mr. Fortunato’s stock options will fully vest and become
immediately exercisable and all restrictions with respect to restricted stock, if any, granted to
Mr. Fortunato will lapse.
Upon Mr. Fortunato’s termination for death or total disability we will be required to pay to
him (or his guardian or personal representative):
| |
• |
|
a lump sum equal to one times his base salary plus the annualized value of his
perquisites; and |
| |
| |
• |
|
a prorated share of the annual bonus he would have received had he worked the
full year, provided bonus targets are met for such year. |
We will also pay the monthly cost of COBRA coverage for Mr. Fortunato to the same extent we paid
for such coverage prior to the termination date for the period permitted by COBRA or, in the case
of disability, until Mr. Fortunato obtains other employment offering substantially similar or
improved group health benefits. In addition, Mr. Fortunato’s outstanding stock options will vest
and restrictions on restricted stock awards will lapse as of the date of termination, in each case,
assuming he had continued employment during the calendar year in which termination occurs and for
the year following such termination.
If Mr. Fortunato’s employment is terminated without cause, he resigns for good reason or we
decline to renew the employment term for reasons other than those that would constitute cause after
the initial five-year employment term, then, subject to Mr. Fortunato’s execution of a release:
| |
• |
|
Mr. Fortunato will receive payment of a lump sum amount of two times his base
salary and the annualized value of his perquisites; |
| |
| |
• |
|
Mr. Fortunato will receive payment of a lump sum amount of two times his
average annual bonus paid or payable with respect to the most recent three fiscal
years; |
| |
| |
• |
|
we will pay the monthly cost of COBRA coverage for Mr. Fortunato to the same
extent we paid for such coverage prior to the termination date for the period
permitted by COBRA or until Mr. Fortunato obtains other employment offering
substantially similar or improved group health benefits; and |
| |
| |
• |
|
Mr. Fortunato’s outstanding stock options will vest and restrictions on
restricted stock awards will lapse if they would have otherwise done so in the 24
months following the termination had Mr. Fortunato continued to be employed (36
months if such termination
occurs in anticipation of a change in control, or within the six months prior to,
or at any time following, an initial public offering of our Parent’s common stock). |
151
If such termination occurs in anticipation of or during the two-year period following a change in
control, or within six months prior to or at any time following the completion of an initial public
offering of our Parent’s common stock, the multiple of base salary and annualized perquisites and
of average annual bonus will increase from two times to three times. A termination of Mr.
Fortunato’s employment will be deemed to have been in anticipation of a change in control if such
termination occurs at any time from and after the period beginning six months prior to a change in
control and such termination occurs (i) after we or our Parent enter into a definitive agreement
that provides for a change in control or (ii) at the request of an unrelated third party who has
taken steps reasonably calculated to effect a change in control.
For purposes of Mr. Fortunato’s employment agreement, “cause” generally means any of the following
events as determined in good faith by a 2/3 vote of the Parent Board, Mr. Fortunato’s:
| |
• |
|
conviction of, or plea of nolo contendere to, a crime which constitutes a
felony; |
| |
| |
• |
|
willful disloyalty or deliberate dishonesty with respect to the Company or our
Parent that is injurious to our or our Parent’s financial condition, business or
reputation; |
| |
| |
• |
|
commission of an act of fraud or embezzlement against us or our Parent; |
| |
| |
• |
|
material breach of any provision of his employment agreement or any other
written contract or agreement with us or our Parent that is not cured; or |
| |
| |
• |
|
willful and continued failure to materially perform his duties or his continued
failure to substantially perform duties requested or prescribed by the Parent Board
or the Company Board which is not cured. |
For purposes of Mr. Fortunato’s employment agreement, “good reason” generally means, without Mr.
Fortunato’s consent:
| |
• |
|
our failure to comply with any material provision of his employment agreement
which is not cured; |
| |
| |
• |
|
a material adverse change in his responsibilities, duties or authority which,
in the aggregate, causes his positions to have less responsibility or authority; |
| |
| |
• |
|
removal from his current positions or failure to elect (or appoint) him to, or
removal of him from the Parent Board or the Company Board; |
| |
| |
• |
|
a material reduction in his base salary; or |
| |
| |
• |
|
a relocation of his principal place of business of more than 75 miles. |
For purposes of Mr. Fortunato’s employment agreement, “change in control” generally means:
| |
• |
|
an acquisition representing 50% or more of either our Parent’s common stock or
the combined voting power of the securities of our Parent entitled to vote
generally in the election of the Parent Board; |
| |
| |
• |
|
a change in 2/3 of the members of Parent Board from the members on the
effective date of his employment agreement, unless approved by (i) 2/3 of the
members of the Parent Board on the effective date of his employment agreement or
(ii) members nominated by such members; |
152
| |
• |
|
the approval by Parent stockholders of (i) a complete liquidation or
dissolution of our Parent or the Company or (ii) the sale or other disposition
(other than a merger or consolidation) of all or substantially all of the assets
of our Parent and its subsidiaries; or |
| |
| |
• |
|
we cease to be a direct or indirect wholly owned subsidiary of our Parent. |
President and Chief Merchandising and Marketing Officer
On December 19, 2007, we entered into an employment agreement with Ms. Kaplan in connection
with her appointment as President and Chief Merchandising and Marketing Officer. The employment
agreement was amended, effective January 1, 2009, to comply with Code Section 409A. The employment
agreement provides for an employment term through January 2, 2010, subject to automatic one-year
renewals unless we or Ms. Kaplan provide at least one year advance notice and an annual base salary
of not less than $675,000, subject to certain upward adjustments. Effective January 1, 2010, the
Compensation Committee granted Ms. Kaplan a merit-based increase in her annual base salary to
$716,000. Ms. Kaplan is also entitled to an annual performance bonus with a target bonus of 75%
and a maximum bonus of 125% of her annual base salary, based upon the attainment of certain goals
established jointly in good faith by the Chief Executive Officer and Ms. Kaplan. The employment
agreement also provides that Ms. Kaplan will receive certain fringe benefits and perquisites
similar to those provided to our other executive officers. Upon a change in control, all of Ms.
Kaplan’s stock options will fully vest and become immediately exercisable and all restrictions with
respect to restricted stock, if any, granted to Ms. Kaplan will lapse.
Upon Ms. Kaplan’s death or total disability, we will be required to pay her (or her guardian
or personal representative):
| |
• |
|
a lump sum equal to her base salary plus the annualized value of her
perquisites; and |
| |
| |
• |
|
a prorated share of the annual bonus she would have received had she worked the
full year, provided bonus targets are met for such year. |
We will also pay the monthly cost of COBRA coverage for Ms. Kaplan to the same extent we paid
for such coverage prior to the termination date for the period permitted by COBRA or, in the case
of disability, until Ms. Kaplan obtains other employment offering substantially similar or improved
group health benefits. In addition, Ms. Kaplan’s outstanding stock options will vest and
restrictions on restricted stock awards will lapse as of the date of termination, in each case,
assuming she had continued employment during the calendar year in which termination occurs and for
the year following such termination.
If Ms. Kaplan’s employment is terminated without cause, she resigns for good reason, or we
decline to renew the employment term for reasons other than those that would constitute cause after
the initial two-year employment term, then, subject to Ms. Kaplan’s execution of a release:
| |
• |
|
Ms. Kaplan will receive payment of a lump sum amount equal to 18 months of her base
salary; |
| |
| |
• |
|
Ms. Kaplan will receive payment of a lump sum amount equal to her average annual bonus
paid or payable with respect to the most recent three fiscal years; and |
| |
| |
• |
|
Ms. Kaplan will be responsible for payment of the monthly cost of COBRA coverage, but we
will reimburse Ms. Kaplan for any portion of the monthly cost of COBRA coverage that
exceeds the amount of monthly health insurance premium (with respect to Ms. Kaplan’s
coverage and any eligible dependent coverage) payable by Ms. Kaplan immediately prior to
such termination, such reimbursements to continue through the expiration of the agreement
term or the severance period. |
153
If such termination occurs in anticipation of or during the two-year period following a change in
control, or
within six months prior to or at any time following the completion of an initial public offering of
our Parent’s common stock, then Ms. Kaplan will receive payment of a lump sum amount equal to two
times her base salary and the annualized value of her perquisites and the average annual bonus will
increase to two times. A termination of Ms. Kaplan’s employment will be deemed to have been in
anticipation of a change in control if such termination occurs at any time from and after the
period beginning six months prior to a change in control and such termination occurs (i) after we
or our Parent enter into a definitive agreement that provides for a change in control or (ii) at
the request of an unrelated third party who has taken steps reasonably calculated to effect a
change in control.
For purposes of Ms. Kaplan’s employment agreement, “cause” generally means Ms. Kaplan’s:
| |
• |
|
conviction of, or plea of nolo contendere to, a crime which constitutes a felony; |
| |
| |
• |
|
willful disloyalty or deliberate dishonesty with respect to the Company or our Parent
that is injurious to our or our Parent’s financial condition, business or reputation; |
| |
| |
• |
|
commission of an act of fraud or embezzlement against us or our Parent; |
| |
| |
• |
|
material breach of any provision of her employment agreement or any other written
contract or agreement with us or our Parent that is not cured; or |
| |
| |
• |
|
willful and continued failure to materially perform her duties or her continued failure
to substantially perform duties requested or prescribed by the Parent Board or the Company
Board which is not cured. |
For purposes of Ms. Kaplan’s employment agreement, “good reason” generally means, without Ms.
Kaplan’s consent:
| |
• |
|
our failure to comply with any material provision of her employment agreement which is
not cured; |
| |
| |
• |
|
a material adverse change in her responsibilities, duties or authority which, in the
aggregate, causes her positions to have less responsibility or authority; |
| |
| |
• |
|
removal from her current positions or failure to elect (or appoint) her to, or removal
of her from, the Parent Board or the Company Board; |
| |
| |
• |
|
a material reduction in her base salary; |
| |
| |
• |
|
a relocation of her principal place of business of more than 100 miles; or |
| |
| |
• |
|
our failure to appoint her Chief Executive Officer in the event Mr. Fortunato ceases to
serve as Chief Executive Officer of us or our Parent. |
For purposes of Ms. Kaplan’s employment agreement, “change in control” generally means:
| |
• |
|
an acquisition representing 50% or more of either our Parent’s common stock or the
combined voting power of the securities of our Parent entitled to vote generally in the
election of the Parent Board; |
| |
| |
• |
|
a change in 2/3 of the members of Parent Board from the members on the effective date of
her employment agreement, unless approved by (i) 2/3 of the members of the Parent Board on
the effective date of her employment agreement or (ii) members nominated by such members; |
154
| |
• |
|
the approval by Parent stockholders of (i) a complete liquidation or dissolution of our
Parent or the Company or (ii) the sale or other disposition (other than a merger or
consolidation) of all or substantially all of the assets of our Parent and its
subsidiaries; or |
| |
| |
• |
|
we cease to be a direct or indirect wholly owned subsidiary of our Parent. |
Other 2009 Named Executive Officers
On October 31, 2008, we entered into an employment agreement with Mr. Nuzzo in connection with
his appointment as Executive Vice President and Chief Financial Officer. On April 21, 2008, we
entered into an employment agreement with Mr. Dowd, our Executive Vice President of Store
Operations and Development, and on October 1, 2007, we entered into an employment agreement with
Mr. Stubenhofer, our Senior Vice President Chief Legal Officer and Secretary. These employment
agreements were amended, effective January 1, 2009, to comply with Code Section 409A.
The employment agreements contain substantially the same terms. Each agreement provides for a
two-year term with automatic one-year renewals thereafter unless we or the executive provide at
least 30 days’ advance notice of termination. Pursuant to their employment agreements, Messrs.
Nuzzo, Dowd and Stubenhofer are entitled to a base salary in the amount equal to $400,000, $320,000
and $275,000, respectively, in each case subject to annual review by the Company Board or the
Compensation Committee. Effective December 6, 2009, the Compensation Committee granted Messrs.
Nuzzo and Dowd merit-based increases in their annual base salaries to $409,400 and $350,000,
respectively. Effective October 29, 2009, the Compensation Committee granted Mr. Stubenhofer a
merit-based increase in his annual base salary to $320,000. The employment agreements also entitle
the executives to annual performance bonuses payable if we exceed the annual goals determined by
the Company Board or the Compensation Committee, and to certain fringe benefits and perquisites
similar to those provided to our other executive officers.
The employment agreements also provide for certain benefits upon termination of employment.
Upon death or disability, the executives (or their estates) are entitled to their current base
salary for the remainder of the employment period, and, subject to the discretion of the Company
Board or the Compensation Committee, a pro rata share of the annual bonus based on actual
employment, provided bonus targets are met. Upon termination of employment by us without cause or
voluntarily by the executive for good reason, subject to the execution of a written release, the
executive is also entitled to:
| |
• |
|
salary continuation generally for the remainder of the agreement term (unless the
termination occurs during the initial term in which case, Mr. Nuzzo is entitled to salary
continuation for one year and Mr. Dowd is entitled to salary continuation for six months),
or two years if the termination occurs upon or within six months following a change in
control; |
| |
| |
• |
|
subject to the discretion of the Company Board or the Compensation Committee, a pro rata
share of the annual bonus based on actual employment; and |
| |
| |
• |
|
reimbursement for any portion of the monthly cost of COBRA coverage that exceeds the
amount of monthly health insurance premium (with respect to the executive’s coverage and
any eligible dependent coverage) payable by the executive immediately prior to such
termination, such reimbursements to continue through the expiration of the agreement term
or the severance period. |
For purposes of the employment agreements, “cause” generally means the executive’s:
| |
• |
|
failure to comply with any obligation imposed by his employment agreement; |
| |
| |
• |
|
being indicted for any felony or any misdemeanor that causes or is likely to cause harm
or embarrassment to the Company, in the reasonable judgment of the board;
|
155
| |
• |
|
theft, embezzlement or fraud in connection with the performance of duties; |
| |
| |
• |
|
engaging in any activity that gives rise to a material conflict of interest with the
Company; |
| |
| |
• |
|
misappropriation by the executive of any material business opportunity of the Company; |
| |
| |
• |
|
any failure to comply with, observe or carry out the Company’s or the Company Board’s
rules, regulations, policies or codes of ethics or conduct; |
| |
| |
• |
|
substance abuse or illegal use of drugs that, in the reasonable judgment of the Company
Board, impairs the executive’s performance or causes or is likely to cause harm or
embarrassment to the Company; or |
| |
| |
• |
|
engagement in conduct that the executive knows or should know is injurious to the
Company. |
For purposes of the employment agreements, “good reason” generally means, without the executive’s
prior written consent:
| |
• |
|
the Company’s failure to comply with material obligations under his employment
agreement; |
| |
| |
• |
|
a change of the executive’s position; or |
| |
| |
• |
|
a material reduction in the executive’s base salary. |
For purposes of the employment agreements, “change in control” generally means:
| |
• |
|
an acquisition representing 50% or more of either our Parent’s common stock or the
combined voting power of the securities of our Parent entitled to vote generally in the
election of the Parent Board; |
| |
| |
• |
|
a change in 2/3 of the members of Parent Board from the members on the effective date of
the executive’s employment agreement, unless approved by (i) 2/3 of the members of the
Parent Board on the effective date of the executive’s employment agreement or (ii) members
nominated by such members; |
| |
| |
• |
|
the approval by Parent stockholders of (i) a complete liquidation or dissolution of our
Parent or the Company or (ii) the sale or other disposition (other than a merger or
consolidation) of all or substantially all of the assets of our Parent and its
subsidiaries; or |
| |
| |
• |
|
we cease to be a direct or indirect wholly owned subsidiary of our Parent. |
Under all circumstances, all of Messrs. Nuzzo’s, Dowd’s and Stubenhofer’s unvested equity
awards will be forfeited as of the date of the executive’s termination.
156
General
The employment agreements for all of our 2009 Named Executive Officers contain:
| |
• |
|
terms of confidentiality concerning trade secrets and confidential or
proprietary information which may not be disclosed by the executive except as
required by court order or applicable law; and |
| |
| |
• |
|
certain non-competition and non-solicitation provisions which restrict the
executive and certain relatives from engaging in activities against our interests
or those of our parent companies during the term of employment and, in the case of
Mr. Fortunato and Ms. Kaplan, eighteen months following the termination of
employment, and in the case of the other 2009 Named Executive Officers, for the
longer of the first anniversary of the date of termination of employment or the
period during which the executive receives termination payments. |
157
Potential Termination or Change-in-Control Payments
The following tables summarize the value of the compensation that 2009 Named Executive
Officers would have received if they had terminated employment on December 31, 2009 under the
circumstances shown or if we had undergone a change in control on such date. The tables exclude
(1) compensation amounts accrued through December 31, 2009 that would be paid in the normal course
of continued employment, such as accrued but unpaid salary, and (2) vested account balances under
our 401(k) Plan that are generally available to all of our salaried employees. Where applicable,
the amounts reflected for the prorated annual incentive compensation in 2009 are the amounts that
were actually paid to the 2009 Named Executive Officers in February 2010 under the 2009 Incentive
Plan, since the hypothetical termination date is the last day of the fiscal year for which the
bonus is to be determined.
Where applicable, the information in the tables uses a fair market value per share of $6.50 as
of December 31, 2009 for GNC Parent Corporation’s common stock. Since the Merger, the Compensation
Committee has used a valuation methodology in which the fair market value of the common stock is
based on our business enterprise value and, in situations deemed appropriate by the Parent
Compensation Committee, may be discounted to reflect the lack of marketability associated with the
common stock.
The termination and change in control arrangements for our 2009 Named Executive Officers and
other senior employees are generally based on form employment agreements. As such, these
arrangements generally are uniform and not highly negotiated. The amounts payable in connection
with termination and change in control events are tied to our officers’ respective base salaries
and annual bonuses, and therefore are proportionately higher for the more senior and highly
compensated officers. Similarly, the termination and change in control arrangements for our Chief
Executive Officer and President generally provide for higher payments than those for other
officers. These provisions were negotiated with our most senior officers, and deemed appropriate
by the Compensation Committee, to both attract and retain the individuals and to ensure that their
long-term interests are aligned with those of the Company. Specifically, the change in control
provisions are designed to reflect the expectations of the Company Board with respect to the manner
in which the Company will be operated over the life of the employment agreements and to be
consistent with our peer companies. Similarly, the termination provisions, which provide for lump
sum payments of salary and bonus, and in some instances, acceleration of stock options, are
designed to preserve the value of the long-term compensation arrangements for Mr. Fortunato and Ms.
Kaplan to ensure the continued alignment of their interests with those of the Company.
Because the amounts payable in connection with termination and change in control events are
generally based on the formula set forth in the form employment agreements, the Compensation
Committee does not generally consider the amounts when establishing the compensation of its named
executive officers. The Compensation Committee, together with the Company, established the terms
of the foregoing arrangements to address and conform to our overall compensation objectives in
attracting and retaining the caliber of executives that are integral to our growth: market
competitiveness; maintaining management continuity, particularly through periods of uncertainty
related to change in control events; providing our key personnel with the assurance of fair and
equitable treatment following a change in management control and other events; and ensuring that
management is held to high standards of integrity and performance.
158
Chief Executive Officer
Joseph Fortunato
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Termination |
|
|
|
|
|
Termination |
|
|
|
|
|
|
|
|
| |
|
w/o |
|
Termination |
|
w/o |
|
|
|
|
|
|
|
|
| |
|
cause or |
|
w/o |
|
cause or |
|
Termination |
|
|
|
|
|
|
| |
|
for Good |
|
cause or |
|
for Good |
|
w/o |
|
|
|
|
|
|
| |
|
Reason or |
|
for Good |
|
Reason in |
|
Cause or |
|
|
|
|
|
|
| |
|
Non- |
|
Reason |
|
anticipation |
|
for Good |
|
|
|
|
|
|
| |
|
renewal of |
|
upon a |
|
of a |
|
Reason in |
|
|
|
|
|
|
| |
|
the |
|
Change in |
|
Change in |
|
Connection |
|
Voluntary |
|
Death or |
|
Change of |
| |
|
Agreement |
|
Control |
|
Control |
|
with an IPO |
|
Termination |
|
Disability |
|
Control |
| Benefit |
|
($) |
|
($) |
|
($) |
|
($) |
|
($) |
|
($) |
|
($) |
| |
Lump Sum Base Salary |
|
|
1,720,000 |
|
|
|
2,580,000 |
|
|
|
2,580,000 |
|
|
|
2,580,000 |
|
|
|
— |
|
|
|
860,000 |
|
|
|
— |
|
Lump Sum Annual Incentive Compensation |
|
|
1,718,643 |
|
|
|
2,577,964 |
|
|
|
2,577,964 |
|
|
|
2,577,964 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
Lump Sum Annualized Value or Perquisites |
|
|
143,124 |
|
|
|
214,686 |
|
|
|
214,686 |
|
|
|
214,686 |
|
|
|
— |
|
|
|
71,562 |
|
|
|
— |
|
Prorated Annualized Incentive Compensation |
|
|
948,580 |
|
|
|
948,580 |
|
|
|
948,580 |
|
|
|
948,580 |
|
|
|
— |
|
|
|
948,580 |
|
|
|
— |
|
Health & Welfare Benefits |
|
|
14,469 |
|
|
|
14,469 |
|
|
|
14,469 |
|
|
|
14,469 |
|
|
|
— |
|
|
|
14,469 |
|
|
|
— |
|
Accelerated Vesting of Stock Options |
|
|
947,158 |
|
|
|
947,158 |
|
|
|
947,158 |
|
|
|
947,158 |
|
|
|
— |
|
|
|
473,579 |
|
|
|
947,158 |
|
Payment Reduction |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
| |
Net Value |
|
|
5,491,973 |
|
|
|
7,282,856 |
|
|
|
7,282,856 |
|
|
|
7,282,856 |
|
|
|
— |
|
|
|
2,368,190 |
|
|
|
947,158 |
|
| |
159
Other 2009 Named Executive Officers
Beth J. Kaplan
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Termination |
|
|
|
|
|
Termination |
|
|
|
|
|
|
|
|
| |
|
w/o |
|
Termination |
|
w/o |
|
|
|
|
|
|
|
|
| |
|
cause or |
|
w/o |
|
cause or |
|
Termination |
|
|
|
|
|
|
| |
|
for Good |
|
cause or |
|
for Good |
|
w/o |
|
|
|
|
|
|
| |
|
Reason or |
|
for Good |
|
Reason in |
|
Cause or |
|
|
|
|
|
|
| |
|
Non- |
|
Reason |
|
Anticipation |
|
for Good |
|
|
|
|
|
|
| |
|
renewal of |
|
upon a |
|
of a |
|
Reason in |
|
|
|
|
|
|
| |
|
the |
|
Change in |
|
Change in |
|
Connection |
|
Voluntary |
|
Death or |
|
Change of |
| |
|
Agreement |
|
Control |
|
Control |
|
with an IPO |
|
Termination |
|
Disability |
|
Control |
| Benefit |
|
($) |
|
($) |
|
($) |
|
($) |
|
($) |
|
($) |
|
($) |
| |
Lump Sum Base Salary |
|
|
1,042,500 |
|
|
|
1,390,000 |
|
|
|
1,390,000 |
|
|
|
1,390,000 |
|
|
|
— |
|
|
|
695,000 |
|
|
|
— |
|
Lump Sum Annual Incentive Compensation |
|
|
750,116 |
|
|
|
1,500,232 |
|
|
|
1,500,232 |
|
|
|
1,500,232 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
Lump Sum Annualized Value or Perquisites |
|
|
— |
|
|
|
100,000 |
|
|
|
100,000 |
|
|
|
100,000 |
|
|
|
— |
|
|
|
50,000 |
|
|
|
— |
|
Prorated Annualized Incentive Compensation |
|
|
767,857 |
|
|
|
767,857 |
|
|
|
767,857 |
|
|
|
767,857 |
|
|
|
— |
|
|
|
767,857 |
|
|
|
— |
|
Health & Welfare Benefits |
|
|
13,312 |
|
|
|
13,312 |
|
|
|
13,312 |
|
|
|
13,312 |
|
|
|
— |
|
|
|
13,312 |
|
|
|
— |
|
Accelerated Vesting of Stock Options |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
Payment Reduction |
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
| |
Net Value |
|
|
2,573,785 |
|
|
|
3,771,401 |
|
|
|
3,771,401 |
|
|
|
3,771,401 |
|
|
|
— |
|
|
|
1,526,169 |
|
|
|
— |
|
| |
Michael M. Nuzzo
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
Termination w/o |
|
|
|
|
|
|
| |
|
|
|
|
|
cause or for |
|
|
|
|
|
|
| |
|
|
|
|
|
Good Reason |
|
|
|
|
|
|
| |
|
Termination w/o |
|
within 6 Months |
|
|
|
|
|
|
| |
|
cause or for |
|
after a Change in |
|
Voluntary |
|
Death or |
|
Change of |
| |
|
Good Reason |
|
Control |
|
Termination |
|
Disability |
|
Control |
| Benefit |
|
($) |
|
($) |
|
($) |
|
($) |
|
($) |
| |
Base Salary Continuation |
|
|
400,000 |
|
|
|
800,000 |
|
|
|
— |
|
|
|
400,000 |
|
|
|
— |
|
Pro Rata Bonus |
|
|
335,200 |
|
|
|
335,200 |
|
|
|
— |
|
|
|
335,200 |
|
|
|
— |
|
Health & Welfare Benefits |
|
|
7,396 |
|
|
|
17,749 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
Accelerated Vesting of Stock Options |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
Payment Reduction |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
| |
Net Value |
|
|
742,596 |
|
|
|
1,152,950 |
|
|
|
— |
|
|
|
735,200 |
|
|
|
— |
|
| |
160
Thomas Dowd
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
Termination w/o |
|
|
|
|
|
|
| |
|
|
|
|
|
cause or for |
|
|
|
|
|
|
| |
|
|
|
|
|
Good Reason |
|
|
|
|
|
|
| |
|
Termination w/o |
|
within 6 Months |
|
|
|
|
|
|
| |
|
cause or for |
|
after a Change in |
|
Voluntary |
|
Death or |
|
Change of |
| |
|
Good Reason |
|
Control |
|
Termination |
|
Disability |
|
Control |
| Benefit |
|
($) |
|
($) |
|
($) |
|
($) |
|
($) |
| |
Base Salary Continuation |
|
|
164,800 |
|
|
|
659,200 |
|
|
|
— |
|
|
|
100,235 |
|
|
|
— |
|
Pro Rata Bonus |
|
|
276,669 |
|
|
|
276,669 |
|
|
|
— |
|
|
|
276,669 |
|
|
|
— |
|
Health & Welfare Benefits |
|
|
2,930 |
|
|
|
17,578 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
Accelerated Vesting of Stock Options |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
Payment Reduction |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
| |
Net Value |
|
|
444,399 |
|
|
|
953,447 |
|
|
|
— |
|
|
|
376,904 |
|
|
|
— |
|
| |
Gerald Stubenhofer
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
Termination w/o |
|
|
|
|
|
|
| |
|
|
|
|
|
cause or for |
|
|
|
|
|
|
| |
|
|
|
|
|
Good Reason |
|
|
|
|
|
|
| |
|
Termination w/o |
|
within 6 Months |
|
|
|
|
|
|
| |
|
cause or for |
|
after a Change |
|
Voluntary |
|
Death or |
|
Change of |
| |
|
Good Reason |
|
in Control |
|
Termination |
|
Disability |
|
Control |
| Benefit |
|
($) |
|
($) |
|
($) |
|
($) |
|
($) |
| |
Base Salary Continuation |
|
|
240,000 |
|
|
|
640,000 |
|
|
|
— |
|
|
|
240,000 |
|
|
|
— |
|
Pro Rata Bonus |
|
|
190,418 |
|
|
|
190,418 |
|
|
|
— |
|
|
|
190,418 |
|
|
|
— |
|
Health & Welfare Benefits |
|
|
13,183 |
|
|
|
17,578 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
Accelerated Vesting of Stock Options |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
Payment Reduction |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
| |
Net Value |
|
|
443,601 |
|
|
|
847,996 |
|
|
|
— |
|
|
|
430,418 |
|
|
|
— |
|
| |
We have employment agreements with our 2009 Named Executive Officers. See “Employment
Agreements with Our 2009 Named Executive Officers” for a description of the severance and change in
control benefits provided under these employment agreement.
The employment agreements provide that if any payment would have been subject to or result in
the imposition of the excise tax imposed by Code Section 4999, then the amount of such payment or
payments would have been reduced to the highest amount that may be paid by us without subjecting
such payment to the excise tax. Mr. Fortunato’s and Ms. Kaplan’s employment agreements provide
that the reduction will not apply if he would, on a net after-tax basis, receive less compensation
than if the payment were not so reduced. Based on a hypothetical change in control on December 31,
2009, none
161
of the 2009 Named Executive Officers would have been subject to a reduction payment if
their employment had been terminated at the time of a December 31, 2009 change in control or on
December 31, 2009 in anticipation of a change in control or a change in control without an
employment termination. For purposes of calculating any hypothetical reduction payment as a result
of change in control payments, we have assumed that the change in control payments for any of the
2009 Named Executive Officers would have included the amount of 2009 annual incentive compensation,
and the value of any options granted in 2009. To the extent any of these amounts were paid prior
to December 31, 2009, they are not reflected in the tables above. The calculation of the payment
reduction amounts does not include a valuation of the non-competition covenant in the 2009 Named
Executive Officer’s employment agreements. A portion of the severance payments payable to the 2009
Named Executive Officers may be attributable to reasonable compensation for the non-competition
covenant and could eliminate or reduce the reduction amount.
The employment agreements for Mr. Fortunato and Ms. Kaplan provide for accelerated vesting of
stock options on a change in control. The 2007 Stock Plan provides that, in the event of a change
in control, unvested stock options generally may be fully vested, cancelled for fair value or
substituted for awards that substantially preserve the applicable terms of the stock options. We
have assumed for purposes of the table that upon a change in control of the Company, Messrs.
Nuzzo’s, Dowd’s and Stubenhofer’s unvested stock options would be substituted for awards that
substantially preserve the
applicable terms of the stock options. In the event that in the exercise of discretion by the
Compensation Committee, Messrs. Nuzzo’s, Dowd’s and Stubenhofer’s unvested stock options would have
become vested in connection with a change in control on December 31, 2009, the value of their
vested options as of such would have been: Mr. Nuzzo — $—; Mr. Dowd — $337,500; and Mr.
Stubenhofer — $93,750.
Finally, although there is no requirement to do so or guarantee that it would have been paid,
we have assumed that, in the exercise of discretion by the Compensation Committee, the 2009 Named
Executive Officers would have been paid their prorated annual incentive compensation for the year
in which their employment was terminated based on a hypothetical termination date of the end of
that year, other than in the case of voluntary termination without good reason or a termination by
the Company for cause.
Upon a termination of employment on December 31, 2009, the shares of our Parent’s common stock
owned by 2009 Named Executive Officers other than Mr. Fortunato and Ms. Kaplan would have been
subject to repurchase by us or our designee for a period of 180 days (270 days upon termination
because of death or disability) following the termination based on fair value as determined by the
Company Board.
Director Compensation
Pursuant to our director compensation policy, effective as of August 15, 2007, we compensate
our directors as follows: (i) our non-employee chairman receives an annual retainer of $200,000 and
(ii) our non-employee directors receive an annual retainer of $40,000. Directors are not entitled
to any additional cash compensation such as fees for attending meetings. However, each
non-employee director is entitled to receive a grant of non-qualified stock options to purchase a
minimum of 36,176 shares of Parent’s common stock. Any director or chairman who is employed by
ACOF, OTPP and other purchasers in connection with the Merger is not entitled to any retainers or
stock option grants.
The table below sets forth information with respect to compensation for our directors for
2009.
Norman Axelrod, David B. Kaplan, Jeffery B. Schwartz, Lee Sienna, Josef Prosperi and Michele
J. Buchignani were each appointed as members of the Company Board effective as of March 16, 2007.
As stated above, any employee employed by ACOF or OTPP is not entitled to any additional
compensation for serving as director. Accordingly, Messrs. Kaplan, Schwartz, Sienna and Prosperi
and Ms. Buchignani are not listed in the table below. On May 14, 2009, Andrew Claerhout and Romeo
Leemrijse were elected to fill vacancies created by the resignations of Mr. Sienna and Ms.
Buchignani. Mr. Prosperi also resigned effective July 29, 2009.
162
Richard D. Innes and Carmen Fortino were elected as directors to the Company Board
effective July 17, 2007. Effective October 21, 2009, Mr. Innes resigned as a director of the
Company Board.
On October 21, 2009, Michael Hines was elected a director to the Company Board effective
October 21, 2009. In connection with his election, the Compensation Committee granted Mr. Hines a
non-qualified stock option to purchase 29,800 shares of Class A common stock of the Company, par
value $0.001 at an exercise price of $8.42 per share, and 29,800 options to purchase shares of such
common stock at an exercise price of $12.63 per share. Each stock option (i) has a term of 10 years
from the election date and (ii) becomes vested and exercisable in five equal installments on each
anniversary of the election date, subject to Mr. Hines’ continued service as a director until the
applicable vesting date.
Mr. Fortunato and Ms. Kaplan serve as members of the Company Board, but neither receives any
compensation for serving as a director.
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Change in |
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Pension Value |
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
and Non- |
|
|
|
|
| |
|
Fees |
|
|
|
|
|
|
|
|
|
Non-Equity |
|
qualified |
|
|
|
|
| |
|
Earned or |
|
|
|
|
|
|
|
|
|
Incentive |
|
Deferred |
|
|
|
|
| |
|
Paid in |
|
Stock |
|
Option |
|
Plan |
|
Compensation |
|
All other |
|
|
| |
|
Cash |
|
Awards |
|
Awards |
|
Compensation |
|
Earnings |
|
Compensation |
|
Total |
| Name |
|
($) |
|
($) |
|
($)1,2 |
|
($) |
|
($) |
|
($) |
|
($) |
Norman Axelrod |
|
|
200,000 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
200,000 |
|
Richard D. Innes |
|
|
40,000 |
3 |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
40,000 |
|
Carmen Fortino |
|
|
40,000 |
3 |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
40,000 |
|
Michael Hines |
|
|
10,000 |
|
|
|
— |
|
|
|
185,356 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
195,356 |
|
|
|
|
| (1) |
|
Reflects the aggregate grant date fair value of option awards granted during the fiscal
year ended December 31, 2009 computed in accordance with FASB ASC Topic 718. For additional
information, see Note 18 under the heading “Stock-Based Compensation Plans” of the Notes to
Consolidated Financial Statements included in the Company’s Annual Report on Form 10-K for the
fiscal year ended December 31, 2009. The amounts reflect the accounting expense for these
awards and do not correspond to the actual value that may be recognized by such persons with
respect to these awards. The grant date fair value was $3.11 per share, calculated in
accordance with FASB ASC Topic 718. |
| |
| (2) |
|
The table below sets forth information regarding exercisable and unexercisable stock
options granted to the listed directors and held as of March 1, 2010. No other stock awards
were made to the directors, and no stock options were exercised by the directors in 2009. |
| |
|
|
|
|
|
|
|
|
| |
|
Option Awards Outstanding on March 1, 2010 |
| Name |
|
Exercisable |
|
Unexercisable |
Norman Axelrod |
|
|
146,156 |
|
|
|
219,236 |
|
Richard D. Innes |
|
|
— |
|
|
|
— |
|
Carmen Fortino |
|
|
14,470 |
|
|
|
21,706 |
|
Michael Hines |
|
|
— |
|
|
|
29,800 |
|
|
|
|
| (3) |
|
Messrs, Innes and Fortino each received payment in Canadian Dollars in the amount of CAN
$46,569. The amount set forth in the table above reflect such amounts reflected in U.S.
Dollars based on an average conversion rate of 1.164. |
163
|
|
|
| ITEM 12. |
|
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER
MATTERS |
The following table sets forth, as of March 1, 2010 (the “Ownership Date”), the number of
shares of our Parent’s common stock beneficially owned by (1) each person or group known by us to
own beneficially more than 5% of the outstanding shares of our Parent’s common stock, (2) each
director, (3) each named executive officer, and (4) all directors and executive officers as a
group.
Percentage ownership is based on a total of 86,742,639 shares of our Parent’s Class A common
stock and Class B common stock and 29,964,165 shares of our Parent’s Series A preferred stock
outstanding as of the Ownership Date, subject to the assumptions described below.
Unless otherwise indicated in the footnotes to the table, and subject to community property
laws where applicable, the following persons have sole voting and investment control with respect
to the shares beneficially owned by them. In accordance with SEC rules, if a person has a right to
acquire beneficial ownership of any shares of our Parent’s common stock, on or within 60 days of
the Ownership Date, upon exercise of outstanding options or otherwise, the shares are deemed
beneficially owned by that person and are deemed to be outstanding solely for the purpose of
determining the percentage of shares that person beneficially owns. These shares are not included
in the computations of percentage ownership for any other person.
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Beneficial Ownership |
| |
|
Class A |
|
Class B |
|
|
|
|
|
Series A |
|
|
| |
|
Common |
|
Common |
|
|
|
|
|
Preferred |
|
|
| Name of Beneficial Owner |
|
Shares |
|
Shares |
|
Percentage |
|
Shares |
|
Percentage |
| |
Directors and Named Executive
Officers1,2: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Norman Axelrod3 |
|
|
205,782 |
|
|
|
— |
|
|
|
* |
|
|
|
20,374 |
|
|
|
* |
|
Andrew Claerhout4,5 |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
Thomas Dowd |
|
|
302,542 |
|
|
|
— |
|
|
|
* |
|
|
|
17,674 |
|
|
|
* |
|
Carmen Fortino5 |
|
|
14,470 |
|
|
|
— |
|
|
|
* |
|
|
|
— |
|
|
|
* |
|
Joseph Fortunato |
|
|
2,142,809 |
|
|
|
— |
|
|
|
2.4 |
% |
|
|
84,907 |
|
|
|
* |
|
Michael Hines1 |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
Beth J. Kaplan6 |
|
|
700,000 |
|
|
|
— |
|
|
|
* |
|
|
|
— |
|
|
|
— |
|
David B. Kaplan3,7 |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
Romeo Leemrijse4,5 |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
Michael M. Nuzzo |
|
|
60,000 |
|
|
|
— |
|
|
|
* |
|
|
|
— |
|
|
|
— |
|
Jeffrey B. Schwartz3,8 |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
Gerald J. Stubenhofer |
|
|
50,000 |
|
|
|
— |
|
|
|
* |
|
|
|
— |
|
|
|
* |
|
All directors and executive
officers as a group
(23 persons) |
|
|
4,435,786 |
|
|
|
— |
|
|
|
4.9 |
% |
|
|
187,610 |
|
|
|
* |
|
164
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Beneficial Ownership |
| |
|
Class A |
|
Class B |
|
|
|
|
|
Series A |
|
|
| |
|
Common |
|
Common |
|
|
|
|
|
Preferred |
|
|
| Name of Beneficial Owner |
|
Shares |
|
Shares |
|
Percentage |
|
Shares |
|
Percentage |
| |
Beneficial Owners of 5% or More
of Outstanding Parent’s Common
Stock : |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Ares Corporate Opportunities
Fund II, L.P9 |
|
|
33,539,898 |
|
|
|
— |
|
|
|
38.7 |
% |
|
|
11,460,102 |
|
|
|
38.2 |
% |
KL Holdings LLC10 |
|
|
4,605,028 |
|
|
|
— |
|
|
|
5.3 |
% |
|
|
1,573,472 |
|
|
|
5.3 |
% |
Ontario Teachers’ Pension Plan
Board11 |
|
|
14,581,393 |
|
|
|
28,168,561 |
|
|
|
49.3 |
% |
|
|
14,607,046 |
|
|
|
48.7 |
% |
|
|
|
| * |
|
Less than 1% of the outstanding shares. |
| |
| (1) |
|
The address of Mr. Hines and each current executive officer is c/o General Nutrition
Centers, Inc., 300 Sixth Avenue, Pittsburgh, Pennsylvania 15222. |
| |
| (2) |
|
On March 16, 2007, in connection with the Merger, our Parent entered into a
stockholders agreement with each of our stockholders. Pursuant to the stockholders
agreement, as amended February 12, 2008, our Parent’s principal stockholders each have the
right to designate four members of our Parent’s board of directors (or, at the sole option
of each, five members of the board of directors, one of which shall be independent), for so
long as they or their respective affiliates each own at least 10% of the outstanding common
stock of our Parent. As a result, each of ACOF and OTPP may be deemed to be the beneficial
owner of the shares of our Parent’s common stock and preferred stock held by the other
parties to the stockholders’ agreement. ACOF and OTPP each expressly disclaims beneficial
ownership of such shares of our Parent’s common stock and preferred stock. |
| |
| (3) |
|
The address of each of Messrs. Axelrod, Kaplan and Schwartz is c/o Ares Corporate
Opportunities Fund II, L.P., 2000 Avenue of the Stars, 12th Floor, Los Angeles,
California 90067. |
| |
| (4) |
|
Mr. Claerhout is the Vice President and Mr. Leemrijse is a Director of the private
equity group of OTPP. Each of Messrs. Claerhout and Leemrijse disclaims beneficial
ownership of the shares of our Parent’s common stock and preferred stock owned by OTPP. |
| |
| (5) |
|
The address for each of Messrs. Claerhout, Fortino and Leemrijse is c/o Ontario
Teachers’ Pension Plan Board, 5650 Yonge Street, Toronto, Ontario M2M 4H5. |
| |
| (6) |
|
Ms. Kaplan is a member of Axcel Managers LLC, the managing member of Axcel Partners III
LLC, and of SK Limited Partnership, a member of Axcel Partners III LLC. Axcel Partners III
LLC holds 318,693 shares of our Class A common stock and 128,861 shares of our Series A
preferred stock. Ms. Kaplan disclaims beneficial ownership of the shares owned by Axcel
Partners III LLC, except to the extent of any pecuniary interest therein. The address of
each of Axcel Managers LLC, SK Limited Partnership and Axcel Partners III LLC is 10955
Nacirema Lane, Stevenson, MD 21153. |
165
|
|
|
| (7) |
|
Mr. Kaplan is a Senior Partner in the Private Equity Group of Ares and member of Ares
Partners Management Company LLC, both of which indirectly control ACOF. Mr. Kaplan disclaims beneficial ownership of the shares owned by ACOF, except to the extent of any
pecuniary interest therein. |
| |
| (8) |
|
Mr. Schwartz is a Principal in the Private Equity Group of Ares, which indirectly
controls ACOF. Mr. Schwartz disclaims beneficial ownership of the shares owned by ACOF,
except to the extent of any pecuniary interest therein. |
| |
| (9) |
|
Reflects shares owned by ACOF. The general partner of ACOF is ACOF Management II, L.P.
(“ACOF Management II”) and the general partner of ACOF Management II is ACOF Operating
Manager II, L.P. (“ACOF Operating Manager II”). ACOF Operating Manager II is indirectly
owned by Ares which, in turn, is indirectly controlled by Ares Partners Management Company,
LLC (“APMC” and, together with ACOF, ACOF Management II, ACOF Operating Manager II and Ares
the “Ares Entities”). Antony P. Ressler is the Manager of APMC. Each of Mr. Ressler, the
Ares Entities (other than ACOF, with respect to the shares owned by ACOF) and the partners,
members and managers thereof, disclaims beneficial ownership of these shares, except to the
extent of any pecuniary interest therein. The address of each Ares Entity is 2000 Avenue of
the Stars, 12th Floor, Los Angeles, CA 90067. |
| |
| (10) |
|
The address of KL Holdings LLC is 1250 Fourth Street, Santa Monica, California 90401. |
| |
| (11) |
|
The address of Ontario Teachers’ Pension Plan Board is 5650 Yonge Street, Toronto,
Ontario M2M 4H5. |
|
|
|
| ITEM 13. |
|
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS AND DIRECTOR INDEPENDENCE. |
Management Services Agreement
Upon completion of the Merger, we entered into a management services agreement with our
Parent. Under the agreement, our Parent agreed to provide us and our subsidiaries with certain
services in exchange for an annual fee of $1.5 million, as well as customary fees for services
rendered in connection with certain major financial transactions, plus reimbursement of expenses
and a tax gross-up relating to a non-tax deductible portion of the fee. Under the terms of the
management services agreement, we have agreed to provide customary indemnification to our Parent
and its affiliates and those providing services on its behalf. In addition, upon completion of the
Merger, we incurred an aggregate fee of $10.0 million, plus reimbursement of expenses, payable to
our Parent for services rendered in connection with the Merger. In 2009, we paid $1.5 million
under this agreement.
Stockholders’ Agreement
Upon completion of the Merger, our Parent entered into a stockholders agreement with each of
its stockholders, which includes certain of our directors, employees, and members of our management
and our principal stockholders. The stockholders agreement was amended and restated as of February
12, 2008. Through a voting agreement, the amended and restated stockholders agreement gives each
of ACOF and OTPP, our Parent’s principal stockholders, the right to designate four members of our
Parent’s board of directors (or, at the sole option of each, five members of the board of
directors, one of which shall be independent) for so long as they or their respective affiliates
each own at least 10% of the outstanding common stock of our Parent. The voting agreement also
provides for election of our Parent’s then-current chief executive officer to our Parent’s board of
directors. Under the terms of the amended and restated stockholders agreement, certain significant
corporate actions require the approval of a majority of directors on the board of directors,
including a majority of the directors designated by ACOF and a majority of the directors designated
by OTPP. The amended and restated stockholders agreement also contains significant transfer
restrictions and certain rights of first offer, tag-along, and drag-along rights. In addition, the
amended and restated stockholders agreement contains registration rights that require our Parent to
register common stock held by the stockholders who are parties to the stockholders agreement in the
event our Parent registers for sale, either for its own account or for the account of others,
shares of its common stock.
166
Lease Agreements
General Nutrition Centres Company, a wholly owned subsidiary of the Company, is party to 21
lease agreements, as lessee, with Cadillac Fairview Corporation, as lessor, with respect to
properties located in Canada. Cadillac Fairview Corporation is a direct, wholly owned subsidiary
of OTPP, one of the principal stockholders of our parent. See Item 12, “—Security Ownership of
Certain Beneficial Owners and Management and Related Stockholder Matters”. The aggregate value of
the leases is approximately $12.4 million, together with certain future landlord related costs, of
which $2.4 million was paid during the 2009 fiscal year. Each lease was negotiated in the ordinary
course of business on an arm’s length basis.
Product Purchases
During our 2009 fiscal year, we purchased certain fish oil and probiotics products
manufactured by Lifelong Nutrition, Inc. (“Lifelong”) for resale under our proprietary brand name
WELLbeING. Carmen Fortino, who serves as one of our directors, is the Managing Director, a member
of the Board of Directors and a stockholder of Lifelong. The aggregate value of the products we
purchased from Lifelong was $3.3 million for the 2009 fiscal year.
Stock Purchase
During the third and fourth quarters of 2008, Axcel Partners III, LLC purchased 273,215 shares
of Common Stock of our parent at a price of $6.82 per share, for an aggregate purchase price of
$1.9 million, and 45,478 shares of Common Stock of our parent at a price of $7.08 per share, for an
aggregate purchase price of $0.3 million, respectively, and 110,151 and 18,710 shares of Preferred
Stock of our parent at a price of $5.00 per share plus accrued and unpaid dividends through the
dates of purchase, for an aggregate purchase price of $0.6 million and $0.1 million, respectively.
Ms. Kaplan, who serves as a director and as our President and Chief Merchandising and Marketing
Officer, is a member of Axcel Managers LLC, the managing member of Axcel Partners III LLC, and of
SK Limited Partnership, a member of Axcel Partners III LLC.
Stock Purchase Agreement
In February 2010, Holdings, GNC and Guru Ramanathan, Senior Vice President, Chief Innovation
Officer of GNC, entered into a Stock Purchase Agreement in connection with Mr. Ramanathan’s
previous purchase, in July 2008, of 14,885 shares of Common Stock of Holdings at a price of $6.93
per share, for an aggregate purchase price of $103,153, and 4,961 shares of Preferred Stock of
Holdings at a price of $5.6637 per share, for an aggregate purchase price of $28,097.62.
Director Independence
Through a voting agreement, our Parent’s stockholders agreement gives each of our Parent’s
principal stockholders, the right to designate three members of our Parent’s board of directors
(or, at the sole option of each, four members of the board of directors, one of which shall be
independent) for so long as they or their respective affiliates each own at least 10% of the
outstanding common stock of our Parent. The voting agreement also provides for election of our
Parent’s then-current chief executive officer to our Parent’s board of directors. Our Parent’s
board of directors intends for our board of directors and the board of directors of GNC Corporation
to have the same composition, which was put into place effective March 16, 2007 following the
closing of the Merger. Each member of the current board of directors is either an affiliate of one
of our Parent’s principal stockholders or one of our executive officers.
Our board of directors has historically had an audit committee and a compensation committee,
which have had the same members as the audit committee and compensation committee of our direct and
ultimate parent companies. In connection with the Merger, our board of directors appointed members
to the audit committee and the compensation committee, which are the same members as the
audit committee and compensation committee of our direct and ultimate parent companies.
167
Code of Ethics
The Company has adopted a Code of Ethics applicable to the Company’s directors, executive
officers, including Chief Executive Officer, and senior financial officers. In addition, the
Company has adopted a Code of Ethical Business Conduct for all employees. Our Code of Ethics is
posted on our website at www.gnc.com on the Corporate Governance page of the Investor Relations
section of the website.
Although we have not adopted formal procedures for the review, approval or ratification of
transactions with related persons, our Board reviews potential transactions with those parties we
have identified as related parties prior to the consummation of the transaction, and we adhere to
the general policy that such transactions should only be entered into if they are approved by our
Board, in accordance with applicable law, and on terms that, on the whole, are no more or loss
favorable than those available from unaffiliated third parties.
168
|
|
|
| ITEM 14. |
|
PRINCIPAL ACCOUNTANT FEES AND SERVICES. |
Aggregate fees for professional services rendered for the Company by PricewaterhouseCoopers
LLP (“PricewaterhouseCoopers”) for the years ended December 31, 2009 and 2008, were:
| |
|
|
|
|
|
|
|
|
| Type of Fee |
|
2009 |
|
|
2008 |
|
| |
|
(in thousands) |
|
|
|
|
|
|
|
|
|
|
Audit Fees |
|
$ |
862 |
|
|
$ |
767 |
|
Audit-Related Fees |
|
|
15 |
|
|
|
254 |
|
Tax Fees |
|
|
207 |
|
|
|
49 |
|
Other Fees |
|
|
17 |
|
|
|
1 |
|
|
|
|
|
|
|
Total |
|
$ |
1,101 |
|
|
$ |
1,071 |
|
|
|
|
|
|
|
|
Audit Fees
The Audit Fees for the year ended December 31, 2009 relate to professional services rendered
by PricewaterhouseCoopers for the integrated audit of our consolidated annual financial statements
and our internal control over financial reporting.
The Audit Fees for the year ended December 31, 2008 relate to professional services rendered
for the audit of our consolidated financial statements.
The Audit Fees for the years ended December 31, 2009 and 2008, also include the review of the
quarterly consolidated financial statements, issuance of consents and statutory audits.
Audit-Related Fees
Audit-Related Fees for the year ended December 31, 2009 relate to professional services
rendered for accounting related matters by PricewaterhouseCoopers.
Audit-Related Fees for the year ended December 31, 2008 include the assessment of the
Company’s internal control over financial reporting by PricewaterhouseCoopers.
Tax Fees
The Tax Fees for the years ended December 31, 2009 and 2008 include services rendered by
PricewaterhouseCoopers relating to tax compliance.
The Tax Fees for the year ended December 31, 2009 also include other tax planning services.
Other Fees
The Other Fees for the year ended December 31, 2009 relate to professional services rendered
for payment security compliance by PricewaterhouseCoopers.
In accordance with policies adopted by our audit committee, all audit and non-audit related
services to be performed by our independent public accountants must be approved in advance by the
audit committee or by a designated member of the audit committee. All services rendered by
PricewaterhouseCoopers were approved by our audit committee.
169
PART IV
|
|
|
| ITEM 15. |
|
EXHIBITS, FINANCIAL STATEMENT SCHEDULES. |
(a) Documents filed as part of this report:
| |
(1) |
|
Financial statements filed in Part II, Item 8 of this report: |
| |
• |
|
Report of Independent Registered Public Accounting Firm. |
| |
| |
• |
|
Consolidated Balance Sheets
As of December 31, 2009 and December 31, 2008. |
| |
| |
• |
|
Consolidated Statements of Operations
For the year ended December 31, 2009 and 2008, for the period
from March 16 to
December 31, 2007, and for the period from January 1 to March 15, 2007. |
| |
| |
• |
|
Consolidated Statements of Stockholder’s (Deficit) Equity and Comprehensive Income (Loss)
For the year ended December 31,2009 and2008, for the period
from March 16 to
December 31, 2007, and for the period from January 1 to March 15, 2007. |
| |
| |
• |
|
Consolidated Statements of Cash Flows
For the year ended December 31,2009 and 2008, for the period
from March 16 to
December 31, 2007, and for the period from January 1 to March 15, 2007. |
| |
| |
• |
|
Notes to Consolidated Financial Statements |
| |
(2) |
|
Financial statement schedule: |
170
SCHEDULE II- VALUATION AND QUALIFYING ACCOUNTS
General Nutrition Centers, Inc. and Subsidiaries
Valuation and Qualifying Accounts
Allowance for Doubtful Accounts (1)
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Successor |
|
|
|
Predecessor |
|
| |
|
Year ended |
|
|
Year ended |
|
|
March 16- |
|
|
|
January 1- |
|
| |
|
December 31, |
|
|
December 31, |
|
|
December 31, |
|
|
|
March 15, |
|
| |
|
2009 |
|
|
2008 |
|
|
2007 |
|
|
|
2007 |
|
| |
|
|
|
|
|
(in thousands) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance at beginning of period |
|
$ |
4,388 |
|
|
$ |
3,875 |
|
|
$ |
4,266 |
|
|
|
$ |
4,382 |
|
Additions-charged to costs and expense |
|
|
3,442 |
|
|
|
4,025 |
|
|
|
1,973 |
|
|
|
|
929 |
|
Deductions |
|
|
(6,041 |
) |
|
|
(3,512 |
) |
|
|
(2,364 |
) |
|
|
|
(1,045 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance at end of period |
|
$ |
1,789 |
|
|
$ |
4,388 |
|
|
$ |
3,875 |
|
|
|
$ |
4,266 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Inventory Reserves
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Successor |
|
|
|
Predecessor |
|
| |
|
Year ended |
|
|
Year ended |
|
|
March 16- |
|
|
|
January 1- |
|
| |
|
December 31, |
|
|
December 31, |
|
|
December 31, |
|
|
|
March 15, |
|
| |
|
2009 |
|
|
2008 |
|
|
2007 |
|
|
|
2007 |
|
| |
|
|
|
|
|
(in thousands) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance at beginning of period |
|
$ |
10,386 |
|
|
$ |
9,859 |
|
|
$ |
10,487 |
|
|
|
$ |
10,796 |
|
Additions-charged to costs and expense |
|
|
2,917 |
|
|
|
3,318 |
|
|
|
2,541 |
|
|
|
|
747 |
|
Deductions |
|
|
(3,749 |
) |
|
|
(2,791 |
) |
|
|
(3,169 |
) |
|
|
|
(1,056 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance at end of period |
|
$ |
9,554 |
|
|
$ |
10,386 |
|
|
$ |
9,859 |
|
|
|
$ |
10,487 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Tax Valuation Allowances
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Successor |
|
|
|
Predecessor |
|
| |
|
Year ended |
|
|
Year ended |
|
|
March 16- |
|
|
|
January 1- |
|
| |
|
December 31, |
|
|
December 31, |
|
|
December 31, |
|
|
|
March 15, |
|
| |
|
2009 |
|
|
2008 |
|
|
2007 |
|
|
|
2007 |
|
| |
|
|
|
|
|
(in thousands) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance at beginning of period |
|
$ |
11,990 |
|
|
$ |
10,955 |
|
|
$ |
7,191 |
|
|
|
$ |
13,231 |
|
Additions-charged at cost and expense |
|
|
264 |
|
|
|
2,344 |
|
|
|
3,764 |
|
|
|
|
130 |
|
Deductions |
|
|
(4,724 |
) |
|
|
(1,309 |
) |
|
|
— |
|
|
|
|
(6,170 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance at end of period |
|
$ |
7,530 |
|
|
$ |
11,990 |
|
|
$ |
10,955 |
|
|
|
$ |
7,191 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| (1) |
|
These balances are the total allowance for doubtful accounts for trade accounts receivable and the current and long-term franchise note
receivable and also includes our returns for our wholesale customers. |
171
Listed below are all exhibits filed as part of this report. Certain exhibits are
incorporated by reference from statements and reports previously filed by the Company or our
Parent with the SEC pursuant to Rule 12b-32 under the Exchange Act:
3.1 Certificate of Incorporation of General Nutrition Centers, Inc. (f/k/a Apollo GNC Holding,
Inc.) (the “Company”). (Incorporated by reference to Exhibit 3.1 to the Company’s Registration
Statement on Form S-4 (File No. 333-114502), filed April 15, 2004.)
3.2 Certificate of Amendment to the Certificate of Incorporation of the Company. (Incorporated by
reference to Exhibit 3.2 to the Company’s Registration Statement on Form S-4 (File No. 333-114502),
filed April 15, 2004.)
3.3 By-Laws of the Company. (Incorporated by reference to Exhibit 3.3 to the Company’s Registration
Statement on Form S-4 (File No. 333-114502), filed April 15, 2004.)
3.4 Second Amended and Restated Bylaws of the Company. *
4.1 Amended and Restated Stockholders Agreement, dated February 12, 2008, by and among GNC
Acquisition Holdings Inc. (“Holdings”), Ares Corporate Opportunities Fund II, L.P., Ontario
Teachers’ Pension Plan Board and the other stockholders party thereto. (Incorporated by reference
to Exhibit 4.1 to the Company’s Annual Report on Form 10-K (File No. 333-144396), filed March 14,
2008.)
4.2 Indenture, dated as of March 16, 2007, among the Company, the Guarantors named therein and
LaSalle Bank National Association, as trustee, governing the Senior Floating Rate Toggle Notes due
2014. (Incorporated by reference to Exhibit 4.9 to the Company’s Registration Statement on Form S-4
(File No. 333-144396), filed July 6, 2007.)
4.3 Form of Senior Floating Rate Toggle Note due 2014. (Incorporated by reference to Exhibit 4.2
above.)
4.4 Indenture, dated as of March 16, 2007, among the Company, the Guarantors named therein and
LaSalle Bank National Association, as trustee, governing the 10.75% Senior Subordinated Notes due
2015. (Incorporated by reference to Exhibit 4.11 to the Company’s Registration Statement on Form
S-4 (File No. 333-144396), filed July 6, 2007.)
4.5 Form of 10.75% Senior Subordinated Note due 2015. (Incorporated by reference to Exhibit 4.4
above.)
4.6 Registration Rights Agreement, dated as of March 16, 2007, by and among the Company, the
Guarantors named therein and J.P. Morgan Securities Inc., Goldman, Sachs & Co. and Lehman Brothers
Inc. with respect to the Senior Floating Rate Toggle Notes due 2014. (Incorporated by reference to
Exhibit 4.13 to the Company’s Registration Statement on Form S-4 (File No. 333-144396), filed July
6, 2007.)
4.7 Registration Rights Agreement, dated as of March 16, 2007, by and among the Company, the
Guarantors named therein and J.P. Morgan Securities Inc., Goldman, Sachs & Co. and Lehman Brothers
Inc. with respect to the 10.75% Senior Subordinated Notes due 2015. (Incorporated by reference to
Exhibit 4.14 to the Company’s Registration Statement on Form S-4 (File No. 333-144396), filed July
6, 2007.)
10.1 Mortgage, Assignment of Leases, Rents and Contracts, Security Agreement and Fixture Filing,
dated March 23, 1999, from Gustine Sixth Avenue Associates, Ltd., as Mortgagor, to Allstate Life
Insurance Company, as Mortgagee. (Incorporated by reference to Exhibit 10.5 to the Company’s
Registration Statement on Form S-4 (File No. 333-114502), filed April 15, 2004.)
172
10.2 Patent License Agreement, dated December 5, 2003, by and between N.V. Nutricia and General
Nutrition Investment Company. (Incorporated by reference to Exhibit 10.6 to the Company’s
Registration Statement on Form S-4 (File No. 333-114502), filed April 15, 2004.)
10.3 Patent License Agreement, dated December 5, 2003, by and between N.V. Nutricia and General
Nutrition Investment Company. (Incorporated by reference to Exhibit 10.7 to the Company’s
Registration Statement on Form S-4 (File No. 333-114502), filed April 15, 2004.)
10.4 Patent License Agreement, dated December 5, 2003, by and between N.V. Nutricia and General
Nutrition Investment Company. (Incorporated by reference to Exhibit 10.8 to the Company’s
Registration Statement on Form S-4 (File No. 333-114502), filed April 15, 2004.)
10.5 Patent License Agreement, dated December 5, 2003, by and between General Nutrition Corporation
and N.V. Nutricia. (Incorporated by reference to Exhibit 10.9 to the Company’s Registration
Statement on Form S-4 (File No. 333-114502), filed April 15, 2004.)
10.6 Know-How License Agreement, dated December 5, 2003, by and between N.V. Nutricia and General
Nutrition Corporation. (Incorporated by reference to Exhibit 10.10 to the Company’s Registration
Statement on Form S-4 (File No. 333-114502), filed April 15, 2004.)
10.7 Know-How License Agreement, dated December 5, 2003, by and between Numico Research B.V. and
General Nutrition Investment Company. (Incorporated by reference to Exhibit 10.11 to the Company’s
Registration Statement on Form S-4 (File No. 333-114502), filed April 15, 2004.)
10.8 Know-How License Agreement, dated December 5, 2003, by and between N.V. Nutricia and General
Nutrition Corporation. (Incorporated by reference to Exhibit 10.12 to the Company’s Registration
Statement on Form S-4 (File No. 333-114502), filed April 15, 2004.)
10.9 Patent License Agreement, dated December 5, 2003, by and between General Nutrition Investment
Company and Numico Research B.V. (Incorporated by reference to Exhibit 10.13 to the Company’s
Registration Statement on Form S-4 (File No. 333-114502), filed April 15, 2004.)
10.10 GNC Live Well Later Non-Qualified Deferred Compensation Plan, effective February 1, 2002.
(Incorporated by reference to Exhibit 10.14 to the Company’s Registration Statement on Form S-4
(File No. 333-114502), filed April 15, 2004.)
10.11 GNC Acquisition Holdings Inc. 2007 Stock Incentive Plan, adopted March 16, 2007.
(Incorporated by reference to Exhibit 10.12 to the Company’s Pre-Effective Amendment No. 1 to its
Registration Statement on Form S-4 (File No. 333-114396), filed August 10, 2007.)
10.12 Amendment No. 1 to the GNC Acquisition Holdings Inc. 2007 Stock Incentive Plan, dated as of
February 12, 2008. (Incorporated by reference to Exhibit 10.11 to the Company’s Annual Report on
Form 10-K (File No. 333-144396), filed March 14, 2008.)
10.13 Form of Non-Qualified Stock Option Agreement Pursuant to the GNC Acquisition Holdings Inc.
2007 Stock Incentive Plan, dated as of March 16, 2007. (Incorporated by reference to Exhibit 10.13
to the Company’s Pre-Effective Amendment No. 1 to its Registration Statement on Form S-4 (File No.
333-144396), filed August 10, 2007.)
10.14 Form of Incentive Stock Option Agreement Pursuant to the GNC Acquisition Holdings Inc. 2007
Stock Incentive Plan. (Incorporated by reference to Exhibit 10.13 to the Company’s Annual Report on
Form 10-K (File No. 333-114502), filed March 14, 2008.)
10.15 Amended and Restated Employment Agreement, dated as of February 16, 2009, by and among the
Company, Holdings and Joseph M. Fortunato. (Incorporated by reference to Exhibit 10.15 to the
Company’s Annual Report on Form 10-K (File No. 333-114396), filed March 19, 2009.)
173
10.16.1 Employment Agreement, dated as of October 31, 2008, by and between the Company and Michael
M. Nuzzo. (Incorporated by reference to Exhibit 10.1 to the Company’s Form 8-K (File No.
333-144396), filed November 4, 2008.)
10.16.2 Amendment No.1 to Employment Agreement, dated as of March 3, 2009, by and between the
Company and Michael M. Nuzzo. (Incorporated by reference to Exhibit 10.16.2 to the Company’s Annual
Report on Form 10-K (File No. 333-114396), filed March 19, 2009.)
10.17.1 Employment Agreement, dated as of December 19, 2007, by and among the Company, Holdings and
Beth J. Kaplan. (Incorporated by reference to Exhibit 10.16 to the Company’s Annual Report on Form
10-K (File No. 333-144396), filed March 14, 2008.)
10.17.2 Amendment No.1 to Employment Agreement, dated as of March 3, 2009, by and among the
Company, Holdings and Beth J. Kaplan. (Incorporated by reference to Exhibit 10.17.2 to the
Company’s Annual Report on Form 10-K (File No. 333-114396), filed March 19, 2009.)
10.18.1 Employment Agreement, dated as of April 21, 2008, by and between the Company and Thomas
Dowd. (Incorporated by reference to Exhibit 10.1 to the Company’s Quarterly Report on Form 10-Q
(File No. 333-144396), filed May 9, 2008.)
10.18.2 Amendment No.1 to Employment Agreement, dated as of March 3, 2009, by and between the
Company and Thomas Dowd. (Incorporated by reference to Exhibit 10.18.2 to the Company’s Annual
Report on Form 10-K (File No. 333-114396), filed March 19, 2009.)
10.19.1 Employment Agreement, dated as of October 1, 2007, by and between the Company and Gerald J.
Stubenhofer. *
10.19.2 Amendment No.1 to Employment Agreement, dated as of March 3, 2009, by and between the
Company and Gerald J. Stubenhofer. *
10.20 GNC/Rite Aid Retail Agreement, dated as of December 8, 1998, by and between General Nutrition
Sales Corporation and Rite Aid Corporation. (Incorporated by reference to Exhibit 10.24 to the
Company’s Pre-Effective Amendment No. 1 to its Registration Statement on Form S-4 (File No.
333-114502), filed August 9, 2004.) †
10.21 Amendment to the GNC/Rite Aid Retail Agreement, dated as of December 8, 1998, by and between
General Nutrition Sales Corporation and Rite Aid Hdqtrs Corp. (Incorporated by reference to Exhibit
10.25 to the Company’s Pre-Effective Amendment No. 1 to its Registration Statement on Form S-4
(File No. 333-114502), filed August 9, 2004.) †
10.22 Amendment to the GNC/Rite Aid Retail Agreement, effective as of May 1, 2004, between General
Nutrition Sales Corporation and Rite Aid Hdqtrs Corp. (Incorporated by reference to Exhibit 10.26
to the Company’s Pre-Effective Amendment No. 1 to its Registration Statement on Form S-4 (File No.
333-114502), filed August 9, 2004.) †
10.23.1 Form of Indemnification Agreement for directors. (Incorporated by reference to Exhibit
10.22.1 to the Company’s Pre-Effective Amendment No. 1 to its Registration Statement on Form S-4
(File No. 333-144396), filed August 10, 2007.)
10.23.2 Form of Indemnification Agreement for executive officers. (Incorporated by reference to
Exhibit 10.22.2 to the Company’s Pre-Effective Amendment No. 1 to its Registration Statement on
Form S-4 (File No. 333-144396), filed August 10, 2007.)
10.24 Amended and Restated Stock Purchase Agreement, dated as of November 21, 2006, by and between
GNC Parent Corporation and GNC Corporation. (Incorporated by reference to Exhibit 10.1 to the
Company’s Form 8-K (File No. 333-114502), filed November 28, 2006.)
174
10.25 Management Services Agreement, dated as of March 16, 2007, by and between Holdings and the
Company. (Incorporated by reference to Exhibit 10.25 to the Company’s Pre-Effective Amendment No. 1
to its Registration Statement on Form S-4 (File No. 333-144396), filed August 10, 2007.)
10.26 Credit Agreement, dated as of March 16, 2007, among GNC Corporation, the Company, the lenders
party thereto, J.P. Morgan Securities Inc. and Goldman Sachs Credit Partners L.P., as joint lead
arrangers, Goldman Sachs Credit Partners L.P., as syndication agent, Merrill Lynch Capital
Corporation and Lehman Commercial Paper Inc., as documentation agents, and JPMorgan Chase Bank,
N.A., as administrative agent. (Incorporated by reference to Exhibit 10.31 to the Company’s
Pre-Effective Amendment No. 1 to its Registration Statement on Form S-4 (File No. 333-144396),
filed August 10, 2007.)
10.27 Guarantee and Collateral Agreement, dated as of March 16, 2007, by GNC Corporation, the
Company and the Guarantors party thereto in favor of JPMorgan Chase Bank, N.A., as administrative
agent. (Incorporated by reference to Exhibit 10.32 to the Company’s Pre-Effective Amendment No. 1
to its Registration Statement on Form S-4 (File No. 333-144396), filed August 10, 2007).
10.28 Form of Intellectual Property Security Agreement, dated as of March 16, 2007, by GNC
Corporation, the Company and the Guarantors party thereto in favor of JPMorgan Chase Bank, N.A., as
administrative agent. (Incorporated by reference to Exhibit 10.27 above.)
10.29 Amended and Restated GNC/Rite Aid Retail Agreement, dated as of July 31, 2007, by and between
Nutra Sales Corporation (f/k/a General Nutrition Sales Corporation) and Rite Aid Hdqtrs. Corp.
(Incorporated by reference to Exhibit 10.34 to the Company’s Pre-Effective Amendment No. 1 to its
Registration Statement on Form S-4 (File No. 333-144396), filed August 10, 2007). †
12.1 Statement re: Computation of Ratio of Earnings to Fixed Charges. *
21.1 Subsidiaries of the Company. (Incorporated by reference to Exhibit 21.1 to the Company’s
Annual Report on Form 10-K (File No. 333-114396), filed March 19, 2009.)
31.1 Certification of Chief Executive Officer pursuant to Exchange Act Rule 13a-14(a) and Rule
15d-14(a), as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. *
31.2 Certification of Chief Financial Officer pursuant to Exchange Act Rule 13a-14(a) and Rule
15d-14(a), as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. *
32.1 Certification of Chief Executive Officer and Chief Financial Officer pursuant to 18 U.S.C.
Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. *
|
|
|
| * |
|
Filed herewith |
| |
| † |
|
Portions of this exhibit have been omitted pursuant to a request for confidential treatment. The
omitted portions have been separately filed with the SEC. |
175
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934,
the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto
duly authorized.
| |
|
|
|
|
GENERAL NUTRITION CENTERS, INC.
|
|
|
| By: |
/s/ Joseph Fortunato
|
|
|
| |
Joseph Fortunato |
|
|
| |
Chief Executive Officer
Dated: March 11, 2010 |
|
|
| |
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been
signed below by the following persons on behalf of the registrant and in the capacities and on the
dates indicated.
| |
|
|
|
|
| |
|
|
| By: |
/s/ Joseph Fortunato
|
|
|
| |
Joseph Fortunato |
|
|
| |
Director and Chief Executive Officer (principal executive officer)
Dated: March 11, 2010 |
|
|
| |
| |
|
|
|
|
| |
|
|
| By: |
/s/ Michael M. Nuzzo
|
|
|
| |
Michael M. Nuzzo |
|
|
| |
Chief Financial Officer (principal financial and accounting officer)
Dated: March 11, 2010 |
|
|
| |
| |
|
|
|
|
| |
|
|
| By: |
/s/ Norman Axelrod
|
|
|
| |
Norman Axelrod |
|
|
| |
Chairman of the Board of Directors
Dated: March 7, 2010 |
|
|
| |
| |
|
|
|
|
| |
|
|
| By: |
/s/ Andrew Claerhout
|
|
|
| |
Andrew Claerhout |
|
|
| |
Director
Dated: March 8, 2010 |
|
|
| |
| |
|
|
|
|
| |
|
|
| By: |
/s/ Carmen Fortino
|
|
|
| |
Carmen Fortino |
|
|
| |
Director
Dated: March 9, 2010 |
|
|
| |
| |
|
|
|
|
| |
|
|
| By: |
/s/ Michael F. Hines
|
|
|
| |
Michael F. Hines |
|
|
| |
Director
Dated: March 9, 2010 |
|
|
| |
| |
|
|
|
|
| |
|
|
| By: |
/s/ Beth J. Kaplan
|
|
|
| |
Beth J. Kaplan |
|
|
| |
Director
Dated: March 9, 2010 |
|
|
176
| |
|
|
|
|
| |
|
|
| By: |
/s/ David B. Kaplan
|
|
|
| |
David B. Kaplan |
|
|
| |
Director
Dated: March 8, 2010 |
|
|
| |
| |
|
|
|
|
| |
|
|
| By: |
/s/ Romeo Leemrijse
|
|
|
| |
Romeo Leemrijse |
|
|
| |
Director
Dated: March 8, 2010 |
|
|
| |
| |
|
|
|
|
| |
|
|
| By: |
/s/ Jeffrey B. Schwartz
|
|
|
| |
Jeffrey B. Schwartz |
|
|
| |
Director
Dated: March 8, 2010 |
|
|
| |
177