Attached files
| file | filename |
|---|---|
| EX-21 - EX-21 - ODYSSEY HEALTHCARE INC | d71444exv21.htm |
| EX-32 - EX-32 - ODYSSEY HEALTHCARE INC | d71444exv32.htm |
| EX-31.1 - EX-31.1 - ODYSSEY HEALTHCARE INC | d71444exv31w1.htm |
| EX-31.2 - EX-31.2 - ODYSSEY HEALTHCARE INC | d71444exv31w2.htm |
| EX-23.1 - EX-23.1 - ODYSSEY HEALTHCARE INC | d71444exv23w1.htm |
| EX-10.10.4 - EX-10.10.4 - ODYSSEY HEALTHCARE INC | d71444exv10w10w4.htm |
| EX-10.11.2 - EX-10.11.2 - ODYSSEY HEALTHCARE INC | d71444exv10w11w2.htm |
Table of Contents
UNITED STATES SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form 10-K
(Mark One)
| þ | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2009
or
| o | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to
Commission file number 000-33267
Odyssey HealthCare, Inc.
(Exact name of registrant as specified in its charter)
| Delaware (State or other jurisdiction of incorporation or organization) |
43-1723043 (IRS Employer Identification Number) |
|
| 717 N. Harwood, Suite 1500 Dallas, Texas (Address of principal executive offices) |
75201 (Zip Code) |
Registrant’s telephone number, including area code:
(214) 922-9711
(214) 922-9711
Securities registered pursuant to Section 12(b) of the Act:
| Title of Each Class | Name of Each Exchange on Which Registered | |
| Common Stock, par value $0.001 per share | The NASDAQ Stock Market LLC |
Securities registered pursuant to Section 12(g) of the Act:
None
None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in
Rule 405 of the Securities Act. Yes o No þ
Indicate by check mark if the registrant is not required to file reports pursuant to Section
13 or Section 15(d) of the Act. Yes o No þ
Indicate by check mark whether the registrant (1) has filed all reports required to be filed
by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or
for such shorter period that the registrant was required to file such reports), and (2) has been
subject to such filing requirements for the past 90 days. Yes þ No o
Indicate by check mark whether the registrant has submitted electronically and posted on its
corporate Web site, if any, every Interactive Data File required to be submitted and posted
pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period
that the registrant was required to submit and post such files). Yes o No o
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation
S-K is not contained herein, and will not be contained, to the best of registrant’s knowledge, in
definitive proxy or information statements incorporated by reference in Part III of this Form 10-K
or any amendments to this Form 10-K. þ
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated
filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large
accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the
Exchange Act. (Check one):
| Large accelerated filer o | Accelerated filer þ | Non-accelerated filer o (Do not check if a smaller reporting company) |
Smaller reporting company o |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of
the Exchange Act). Yes o No þ
At June 30, 2009, there were 32,919,497 shares of the registrant’s Common Stock outstanding.
As of the same date, 31,515,199 shares of the registrant’s Common Stock were held by non-affiliates
of the registrant, having an aggregate market value of $324.0 million based on the last sale price
of a share of Common Stock on June 30, 2009 ($10.28), as reported on The NASDAQ Stock Market LLC
(formerly known as the Nasdaq National Market).
At
March 3, 2010, there were 33,411,290 shares of the registrant’s Common Stock outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the registrant’s Proxy Statement to be furnished to stockholders in connection
with the registrant’s 2010 Annual Meeting of Stockholders are incorporated by reference in Part III
of this Form 10-K.
FORM 10-K
ODYSSEY HEALTHCARE, INC.
For the Year Ended December 31, 2009
For the Year Ended December 31, 2009
TABLE OF CONTENTS
2
Table of Contents
FORWARD-LOOKING STATEMENTS
This Annual Report on Form 10-K includes forward-looking statements within the meaning of
Section 27A of the Securities Act of 1933 (as amended, the “Securities Act”) and Section 21E of the
Securities Exchange Act of 1934 (as amended, the “Exchange Act”). All statements other than
statements of historical facts contained in this report, including statements regarding our future
financial position and results of operations, business strategy and plans and objectives of
management for future operations and statements containing the words “believe,” “may,” “will,”
“estimate,” “continue,” “anticipate,” “intend,” “expect” and similar expressions, as they relate to
us, are forward-looking statements within the meaning of the federal securities laws. These
forward-looking statements are subject to known and unknown risks, uncertainties and assumptions,
which may cause our actual results, performance or achievements to differ materially from those
anticipated or implied by the forward-looking statements. Such risks, uncertainties and assumptions
include, but are not limited to the following:
| • | general market conditions; | ||
| • | adverse changes in reimbursement levels under Medicare and Medicaid programs; | ||
| • | government and private party legal proceedings and investigations; | ||
| • | adverse changes in the Medicare payment cap limits and increases in our estimated Medicare cap contractual adjustments; | ||
| • | decline in patient census growth; | ||
| • | increases in inflation including inflationary increases in patient care costs; | ||
| • | our ability to effectively implement our 2010 operations and development strategies; | ||
| • | our dependence on patient referral sources and potential adverse changes in patient referral practices of those referral sources; | ||
| • | our ability to successfully integrate and operate acquired hospice programs; | ||
| • | our ability to attract and retain healthcare professionals; | ||
| • | increases in our bad debt expense due to various factors, including an increase in the volume of pre-payment reviews by Medicare fiscal intermediaries; | ||
| • | adverse changes in the state and federal licensure and certification laws and regulations; | ||
| • | adverse results of regulatory surveys; | ||
| • | delays in licensure and/or certification of hospice programs and inpatient units; | ||
| • | cost of complying with the terms and conditions of our corporate integrity agreement; | ||
| • | adverse changes in the competitive environment in which we operate; | ||
| • | changes in state or federal income, franchise or similar tax laws and regulations; | ||
| • | adverse impact of natural disasters; and | ||
| • | changes in our estimate of additional share-based compensation expense. |
In light of these risks, uncertainties and assumptions, the forward-looking events and
circumstances discussed in this Annual Report on Form 10-K may not occur and actual results could
differ materially from those anticipated or
3
Table of Contents
implied in the forward-looking statements. Many of these factors are beyond our ability to
control or predict. Given these uncertainties, readers are cautioned not to place undue reliance on
such forward-looking statements, which reflect management’s views only as of the date hereof. We
undertake no obligation to revise or update any of the forward-looking statements or publicly
announce any updates or revisions to any of the forward-looking statements contained herein to
reflect any change in our expectations with regard thereto or any change in events, conditions,
circumstances or assumptions underlying such statements.
PART I
Item 1. Business
Overview and Business Strategy
Overview
We are one of the largest providers of hospice care in the United States in terms of both
average daily patient census and number of Medicare-certified hospice programs. We started in 1996
with a single hospice program; at year-end 2009 we provided care from 90 Medicare-certified hospice
programs in 29 states. On March 6, 2008, we completed our acquisition of VistaCare, Inc.
(“VistaCare”), which had a patient census of approximately 4,500 at the time of the acquisition.
Our average daily patient census for the year ended December 2009 was 12,380 compared to an average
daily patient census of 11,510 for the year ended December 2008, which was an increase of 7.6%.
Hospice services are designed to provide a wide range of care and services to terminally ill
patients and their families. The first hospice in the United States opened in 1974. In 1982,
Congress enacted legislation to create the Medicare hospice benefit, and hospice care became a
covered Medicare benefit in 1983. We are highly dependent on the Medicare program. Services
provided under the Medicare program represented approximately 93.1%, 92.5% and 92.4% of our net
patient service revenue for 2009, 2008 and 2007, respectively.
Under the Medicare hospice benefit, a patient is appropriate for hospice care if two
physicians determine that in their clinical judgment the patient’s life expectancy is six months or
less if the terminal illness runs its normal course and the patient agrees to forego curative
treatment for the patient’s terminal diagnosis. Medicare’s hospice benefit covers a broad range of
palliative (or comfort) services, including counseling and psychosocial services for terminally ill
patients and their families. Medicare beneficiaries who are hospice appropriate and elect to
receive hospice care have virtually all caregiving, medical equipment, supplies and drugs related
to the terminal illness covered by Medicare.
A central concept of hospice care involves the creation of an interdisciplinary group that
provides comprehensive management of the healthcare services and products needed by hospice
patients and their families. An interdisciplinary group is typically comprised of:
| • | a physician; | ||
| • | a patient care manager; | ||
| • | one or more registered nurses; | ||
| • | one or more certified home health aides; | ||
| • | a medical social worker; | ||
| • | a chaplain; | ||
| • | a homemaker; and | ||
| • | one or more specially trained volunteers. |
4
Table of Contents
We assign each of our hospice patients to an interdisciplinary group, which assesses the
clinical, psychosocial and spiritual needs of the patient and his or her family, develops a plan of
care and delivers, monitors and coordinates that plan of care with the goal of providing
appropriate care for the patient and his or her family. This interdisciplinary group approach
offers significant benefits to hospice patients, their families and payors, including:
| • | the provision of coordinated care and treatment; | ||
| • | clear accountability for clinical outcomes and cost of services; and | ||
| • | the potential reduction of stress and dysfunction on patients and their families. |
In contrast, the treatment of terminally ill patients outside the hospice setting often
results in the patient receiving medical services from physicians, hospitals, home health agencies,
skilled nursing facilities, and/or home infusion therapy companies, with little or no effective
coordination among the providers. This lack of coordination often results in a lack of clear
accountability for clinical outcomes and an increase in the cost of services provided. These
patients and their families also generally do not receive the psychosocial and bereavement
counseling services provided as part of the Medicare hospice benefit. For a complete description of
our hospice services, see “- Our Hospice Services and Centralized Support Center.”
Business Strategy
Our mission is “To Serve All People During the End of Life’s Journey.” For us, that means
providing quality, responsive care to all patients in our service areas who are appropriate for
hospice, regardless of diagnosis. It also means continuing to increase the number of patients and
families we serve in our existing service areas and expanding into other geographical areas. The
key components of our strategy for 2010 include:
Improve our organic growth: One of our areas of focus for 2010 is to further improve our
same store growth. Key elements of our strategy to improve same store growth are to continue to
improve our management tools used to monitor the productivity of our community education
representatives (“CERs”), and our training and development programs aimed at improving their
effectiveness. We will also continue the roll-out of our existing CareBeyond clinical programs,
which are disease specific clinical programs, as well as the development of additional
CareBeyond programs.
| Number of | ||||||||
| Medicare-Certified | ||||||||
| Hospice Programs | ||||||||
| for the Quarter | ||||||||
| Ended December 31, | ||||||||
| Average Daily Patient Census | 2009 | 2008 | ||||||
0-50 |
11 | 7 | ||||||
51-100 |
23 | 28 | ||||||
101-200 |
39 | 41 | ||||||
200+ |
17 | 18 | ||||||
Growth through selectively acquiring other hospices and other development activities: Our
development team identifies, evaluates and acquires hospices that complement our existing
geographic footprint. In 2008, there were approximately 3,389 Medicare-certified hospice
programs in the United States according to the Medicare Payment Advisory Commission’s (“MedPAC”)
publication “Report to Congress: Medicare Payment Policy — March 2010” (“2010 MedPAC Report”).
Approximately 35% of these programs were operated by non-profit organizations and approximately
52% of these programs were operated by for profit organizations, with the remainder being
government owned entities. We believe there are a significant number of potential acquisition
opportunities, although we intend to remain disciplined in our approach to evaluating those
opportunities. On December 31, 2009, we acquired a hospice operating in Westchester, Illinois
with an average daily patient census of approximately 50 patients. The acquisition of the
program in Westchester complements our existing program in Chicago, Illinois. In January 2010
we acquired a small hospice program in Overland
5
Table of Contents
Park, Kansas that will allow us to expand our service area in the Kansas City market. We
will continue to identify and evaluate strategic hospice acquisition opportunities in 2010.
Increase our efficiencies: We reduced our operating expenses per patient day during 2009.
Our operating expense per patient day for the year ending December 31, 2009 was $135.90, a
decrease of 1.2% from operating expense per patient day of $137.56 for same period in 2008. Our
operating expense per patient day for the fourth quarter of 2009 was $133.79, a decrease of 2.8%
from operating expense per patient day of $137.63 for the fourth quarter of 2008. We will
continue to look at ways to become more efficient in our provision of hospice services by
utilizing our scale to achieve savings in our cost of providing other services and supplies. We
also believe that we can become more efficient in providing support services to our hospice
programs, particularly in the areas of billing, collections as well as payroll processing.
Revenues and Industry Segments
The information required by Regulation S-K Items 101(b) and 101(d) related to financial
information about segments and financial information about net patient service revenue is contained
in note “19. Segment Information” of our consolidated financial statements, which are included
elsewhere in this Annual Report on Form 10-K.
Principal Office and State of Incorporation
Our corporate offices are located at 717 N. Harwood, Suite 1500, Dallas, Texas 75201. Our
telephone number is (214) 922-9711, and our website is www.odsyhealth.com. We were incorporated in
Delaware in August 1995 and began operations in January 1996.
Hospice Services and Payment
The Medicare hospice benefit covers the following services for palliative care, and we provide
each of these services directly or by contracted arrangement:
| • | Nursing care | ||
| • | Medical social services | ||
| • | Physician services | ||
| • | Patient counseling (dietary, spiritual and other) | ||
| • | General inpatient care | ||
| • | Medical supplies and equipment | ||
| • | Drugs for pain control and symptom management | ||
| • | Home health aide services | ||
| • | Homemaker services | ||
| • | Therapy (physical, occupational and speech) | ||
| • | Respite inpatient care | ||
| • | Family bereavement counseling |
Medicare is our largest payor for hospice services. For patients not eligible for Medicare,
many private insurance companies and most states with a Medicaid hospice benefit offer
substantially similar services for patients and families and substantially similar payment
schedules to hospice providers.
6
Table of Contents
The Medicare hospice benefit has always covered prescription drugs for palliative purposes.
Even though legislation added coverage for prescription drugs to Medicare, hospices are still
required to cover drugs for palliative care. Thus, beneficiaries in hospice care will continue to
be covered for symptom management of their terminal illness through the hospice benefit. Drugs for
conditions unrelated to the terminal illness may be covered through the optional Medicare drug
benefit.
While the Medicare hospice benefit is designed for patients with six months or less to live, a
patient’s hospice services can continue for more than six months as long as the patient remains
eligible. Initially, both the hospice medical director and the patient’s attending physician must
certify that in their clinical judgment the patient’s life expectancy is six months or less if the
terminal illness runs its normal course. The initial certification period is for 90 days. This
initial period is followed by an additional 90 day period and an unlimited number of 60 day periods
thereafter. At each recertification period, a physician, either our medical director or the
patient’s attending physician, must re-certify that the patient’s life expectancy is six months or
less on a forward looking basis, that is, not counting the days that have elapsed since the initial
certification or most recent recertification.
Medicare primarily makes per diem payments to hospices for each day a beneficiary is enrolled
for hospice care. The per diem payment structure is based on four levels of care (see below); the
majority of care provided by us is routine home care. Medicare per diem payments for each level of
care are subject to a wage index which varies based on the geographic location where the services
are provided.
| Reimbursement Range as of | ||||||||
| December 31, 2009 | ||||||||
| Level of Care | Description of Care | (Inclusive of Wage Index) | ||||||
| Routine Home Care | Hospice services provided in the
patient’s home or other residence.
Accounted for 97.4% and 97.5% of our
total days of care in 2009 and 2008,
respectively. |
$120.74-$215.00 | ||||||
| Continuous Home Care | Continuous care provided in the
patient’s home or other residence
during a period of crisis to manage
acute pain or other medical symptoms
for a minimum of eight hours per day,
with nursing care accounting for at
least half of the care provided. Paid
on an hourly basis. Accounted for 0.6%
of our total days of care in both 2009 and 2008. |
$704.68-$1,254.88 (per diem equivalent) |
||||||
| General Inpatient Care | Care provided in a hospital or other
inpatient facility to manage acute
pain and other medical symptoms that
cannot be managed effectively in a
home setting. Accounted for 1.8% and
1.7% of our total days of care in 2009
and 2008, respectively. |
$542.95-$934.52 | ||||||
| Respite Inpatient Care | Care provided for up to five days in a
hospital or other inpatient facility
to relieve the patient’s family or
other caregivers. Accounted for 0.2%
of our total days of care in both 2009
and 2008. |
$129.12-$206.58 | ||||||
Medicare base payment rates for hospice care are updated annually based on the hospital market
basket index computed by the Centers for Medicare and Medicaid Services (“CMS”), and are further
adjusted by a wage index to reflect healthcare labor costs across the country. The table below
lists Medicare hospice base payment rate increases for the past five years. These rate increases do
not include the effect of wage indexing.
| Percentage | ||||
| Effective Date of Rate Increase | Increase | |||
October 1, 2005 |
3.7 | % | ||
October 1, 2006 |
3.4 | % | ||
October 1, 2007 |
3.3 | % | ||
October 1, 2008 |
3.6 | % | ||
October 1, 2009 |
2.1 | % | ||
7
Table of Contents
Hospice Utilization and Market Opportunity
We believe that the following trends in hospice utilization and the aging population are
positive indicators for the hospice industry:
Increase in Hospice Use: The number of Medicare beneficiaries electing hospice care has
increased from approximately 513,000 in 2000 to approximately 1,055,000 in 2008, a 106%
increase, according to the 2010 MedPAC Report. According to the 2010 MedPAC Report, Medicare
spending for hospice care has grown from approximately $2.9 billion in 2000 to approximately
$11.2 billion in 2008. Although hospice use continues to rise, the annual growth rate in
Medicare spending has declined to 8.7% for the period 2007 to 2008 from the average annual
growth rate of 19.8% for the period 2000 to 2007 according to the 2010 MedPAC Report. Hospice
use has also increased considerably among Medicare patients with non-cancer diagnoses. According
to the 2010 MedPAC Report, patients with non-cancer diagnoses accounted for 69% of all hospice
patients in 2008, up from 47% in 1998. Approximately 70% and 69% of our 2009 and 2008
admissions, respectively, were patients with a non-cancer primary diagnosis.
Length of Stay: After several consecutive years of increase in average length of stay, the
average length of stay for hospice providers appears to have leveled off. According to the 2010
MedPAC Report, the average length of stay for Medicare hospice beneficiaries was 83 days in
2008, a slight increase of 3 days from 2007. The average length of stay for 2008 and 2007
represents, however, a significant increase over the average length of stay for 2000 of 54 days.
According to the 2010 MedPAC Report, even though the average length of stay for hospice
providers has increased significantly since 2000, the median length of stay has remained
relatively short at 17 days. Our average length of stay was 82 days for the year ended December
31, 2009 and 85 days for the years ended December 31, 2008 and 2007, respectively.
Aging Population in the United States: According to the 2000 census conducted by the United
States Census Bureau, an estimated 35.0 million persons, or approximately 12.4% of the total
United States population, were age 65 or over. The United States Census Bureau currently
projects that the population of persons age 65 and over will rise to an estimated 54.8 million,
or approximately 16.1% of the total United States population, by the year 2020.
Our Hospice Services and Centralized Support Center
Our Medicare-certified hospice programs are comprised of teams of caregivers, clinicians
responsible for assuring Medicare compliance, admissions coordinators, CERs and a small
administrative staff. Administrative functions such as human resources, payroll, employee benefits,
training, reimbursement, finance, accounting, legal and information systems are handled for all our
hospice programs at our centralized Support Center.
Caregivers: We provide a full range of hospice services (see “— Hospice Services and Payment”
for list of services and levels of care). At the time of admission to our hospice program, each
patient is assigned to an interdisciplinary group of caregivers including a physician, nurse, home
health aide, social worker and chaplain. In addition, we have trained volunteers, managed by a
volunteer coordinator, who provide non-medical support services such as running errands or
providing companionship to the patient. Our care is designed to provide pain and symptom relief for
the patient, but it extends beyond the patient’s physical needs: nurses counsel families and loved
ones on caring for patients and expectations as the terminal illness progresses; social workers and
spiritual care coordinators assist the patient and the family as appropriate; therapists,
dieticians and other disciplines are assigned as needed and bereavement coordinators provide
various support services to families and loved ones for at least 13 months after the patient’s
death. Our medical directors are physicians who are under contract with us to provide certain
clinical and administrative services, including oversight of patient care and weekly participation
in interdisciplinary group meetings to review our patients.
At the time of a patient’s admission, the nurse responsible for the patient develops a plan of
care, which delineates the services, supplies and medications the patient will receive. The plan of
care varies by patient and family situation and changes as the patient’s condition and needs
evolve. However, a typical plan of care would include visits by a nurse, home health aide, social
worker, chaplain and volunteers. Our services are available 24 hours a day, seven days a week.
Community Education Representatives: Each of our hospice programs has a team of CERs who
educate the healthcare community about hospice in general and our company specifically. Our CERs
work primarily with our referral sources, which include physicians, hospital discharge planners,
nursing homes, assisted living facilities and
8
Table of Contents
managed care and insurance companies. Our CERs utilize educational materials, most of which
are available in several different languages, prepared by our centralized training and education
staff. As of December 31, 2009, we had approximately 295 CERs.
Increasing Our Patient Census: The average daily patient census, which is an important
indicator of our financial results, is a function of our admissions and changes in our patients’
average length of stay. These factors are not only influenced by the quality of care we provide and
the work of our CERs with referral sources, but also by the aging population in this country and
the increasing acceptance and understanding of hospice. In 2009, our average daily patient census
was 12,380, an increase of 7.6% over 2008; admissions in 2009 were 49,513 an increase of 5.9% over
2008; and our average length of stay in 2009 was 82 days, a decrease of 2.7% from 2008. The
increases in average daily patient census and admissions are due primarily to the VistaCare
acquisition.
Where We Provide Our Care: Our patients reside in their own homes and in nursing homes and
other long-term care facilities, including assisted living facilities, that Medicare considers the
patient’s residence. We have contractual arrangements with these long-term care facilities to
provide hospice care to our patients who reside in those facilities.
Each of our hospice programs also has contracts with inpatient facilities, including hospitals
or skilled nursing facilities, to provide general inpatient care and respite inpatient care. In
addition, we operate our own inpatient hospice facilities where we provide general inpatient care
and respite inpatient care. We will continue to evaluate opportunities to develop additional
inpatient hospice facilities in select markets in 2010.
Medicare-Covered Care: The Medicare hospice benefit, which is similar to the benefits provided
under Medicaid and most commercial insurance, is designed to provide palliative care, that is, pain
and symptom relief, rather than curative care. In addition to hospice services provided by our
caregivers, we provide medical supplies (such as bandages and catheters), durable medical equipment
(such as hospital beds and wheelchairs), and drugs for pain and symptom relief related to the
terminal diagnosis.
Diagnoses: The following table lists the terminal diagnosis by disease for our admissions
during 2009, 2008 and 2007:
| Percentage of | ||||||||||||
| Patients Admitted | ||||||||||||
| by Primary Diagnosis | ||||||||||||
| Primary Diagnosis | 2009 | 2008 | 2007 | |||||||||
Cancer |
30 | % | 31 | % | 31 | % | ||||||
End-stage heart disease |
18 | 17 | 18 | |||||||||
Dementia |
13 | 14 | 17 | |||||||||
Debility |
9 | 12 | 9 | |||||||||
Lung disease |
9 | 9 | 8 | |||||||||
End-stage kidney disease |
3 | 3 | 3 | |||||||||
End-stage liver disease |
3 | 2 | 2 | |||||||||
Other |
15 | 12 | 12 | |||||||||
Totals |
100 | % | 100 | % | 100 | % | ||||||
9
Table of Contents
Hospice Programs, Inpatient Facilities and Support Center
Hospice Programs and Inpatient Facilities: Below is a listing of our 90 hospice programs that
were Medicare-certified as of December 31, 2009.
Alabama
|
Indiana | Rhode Island | ||
Birmingham
|
Evansville | Providence (Warwick) | ||
Mobile
|
Indianapolis | South Carolina | ||
Phenix City
|
New Albany | Charleston (North Charleston) | ||
Arizona
|
Terre Haute | Greenville | ||
Phoenix (two inpatient facilities)(1)
|
Louisiana | Tennessee | ||
Tucson (one inpatient facility)(1)
|
Lake Charles | Memphis | ||
Arkansas
|
New Orleans (Metairie) | Nashville | ||
Little Rock
|
Shreveport | Texas | ||
California
|
Massachusetts | Amarillo (one inpatient facility)(1) | ||
Bakersfield
|
Boston | Austin(2) | ||
Los Angeles (West Covina)
|
Michigan | Baytown | ||
Orange County (Garden Grove)
|
Detroit (Southfield) (one inpatient | Beaumont | ||
Palm Springs (Rancho Mirage) (one
|
facility)(1) | Brownsville | ||
inpatient facility)(1)
|
Flint (one inpatient facility)(1) | Conroe (one inpatient facility)(1) | ||
San Bernardino
|
Mississippi | Corpus Christi (one inpatient | ||
San Diego
|
Gulf Coast (Gulfport) | facility)(1)(2) | ||
San Jose (Campbell)
|
Jackson | Dallas(2) | ||
Colorado
|
Missouri | East Texas (Tyler) | ||
Colorado Springs
|
Kansas City | El Paso | ||
Denver
|
St. Louis | Fort Worth (one inpatient facility)(1) | ||
Delaware
|
Nebraska | Greenville | ||
Wilmington
|
Omaha | Houston (one inpatient facility)(1) | ||
Florida
|
Nevada | Houston North (one inpatient | ||
Miami
|
Las Vegas (one inpatient facility)(1) | facility)(1) | ||
Georgia
|
Reno (Sparks) | Lubbock (one inpatient facility)(1)(2) | ||
Athens (2)
|
New Jersey | San Angelo | ||
Atlanta (two inpatient facilities)(1)(2)
|
New Jersey (Piscataway) | San Antonio (one inpatient | ||
Augusta
|
New Mexico | facility)(1)(2) | ||
Columbus (one inpatient facility)(1)
|
Albuquerque (one inpatient | Temple | ||
Macon
|
facility)(1)(2) | Waxahachie | ||
Savannah
|
Hobbs | Utah | ||
Illinois
|
Ohio | Ogden | ||
Chicago — South (Chicago)
|
Cleveland (Mayfield Heights) | Salt Lake City | ||
Westchester
|
Columbus (one inpatient facility)(1) | Virginia | ||
| Toledo (Maumee) | Arlington (Vienna) | |||
| Oregon | Norfolk | |||
| Portland (Beaverton) | Richmond | |||
| Pennsylvania | Wisconsin | |||
| Harrisburg (Camp Hill) | Milwaukee (West Allis) | |||
| Philadelphia | ||||
| Pittsburgh |
| (1) | We had a total of 20 inpatient facilities as of December 31, 2009 with a total of 283 beds. | |
| (2) | Both Odyssey and VistaCare have a hospice program that is Medicare certified in the respective location. |
Support Center: Our corporate office in Dallas, Texas, which we call the Support Center,
provides centralized services and resources for each of our hospice programs, including financial
accounting systems such as billing, accounts payable and payroll; information and
telecommunications systems; clinical support services; human resources; regulatory compliance and
quality assurance; training; and legal support. We completed the process of transferring the
corporate functions of VistaCare to our Support Center during the fourth quarter of 2008.
We utilize a variety of software programs to manage our operations. Various electronic
management reports assist in labor utilization and productivity and show operating trends of our
various hospice programs. We utilize our intranet system to assist in standardizing our operational
procedures and for certain web-based training. We utilize a tracking system to manage contact and
relationship data associated with our CER’s and their referral networks. We regularly evaluate
relevant technology that could enhance our business processes and efficiency.
10
Table of Contents
Government Regulation and Payment Structure
The healthcare industry and our hospice programs are subject to extensive federal and state
regulation. Our hospice programs are licensed as required under the laws of the states where we
provide service as either hospices or home health agencies, or both. In addition, our hospice
programs must meet the Medicare conditions of participation to be eligible to receive payments
under the Medicare and Medicaid programs. Government regulation affects our business by controlling
growth, requiring licensing and certification of programs and facilities, regulating how facilities
are used and controlling payment for services provided. Further, the regulatory environment in
which we operate may change significantly in the future. While we believe we have structured our
agreements and operations in material compliance with applicable law, there can be no assurance
that we will be able to successfully address changes in the regulatory environment.
In addition to extensive existing government healthcare regulation, there have been numerous
initiatives on the federal and state levels for comprehensive reforms affecting the payment for and
availability of healthcare services. We believe that these healthcare reform initiatives will
continue during the foreseeable future. If adopted, some aspects of the proposed reforms, such as
further reductions in Medicare or Medicaid payments, could adversely affect us.
We believe that our business operations materially comply with applicable law. However, we
have not received a legal opinion from counsel or from any federal or state judicial or regulatory
authority to this effect, and many aspects of our business operations have not been the subject of
state or federal regulatory scrutiny or interpretation. Some of the laws applicable to us are
subject to limited or evolving interpretations; therefore, a review of our operations by a court or
law enforcement or regulatory authority might result in a determination that could have a material
adverse effect on us. Furthermore, the laws applicable to us may be amended or interpreted in a
manner that could have a material adverse effect on us. Our ability to conduct our business and to
operate profitably will depend in part upon obtaining and maintaining all necessary licenses,
Medicare and Medicaid certifications, certificates of need and other approvals, and complying with
applicable healthcare laws and regulations.
What are Medicare and Medicaid? Medicare is a federally funded and administered health
insurance program, primarily for individuals entitled to Social Security benefits who are 65 years
of age or older or who are disabled. Medicaid is a health insurance program jointly funded by state
and federal governments to provide medical assistance to qualifying low-income persons. All of the
29 states in which we currently operate offer Medicaid hospice services. Because of recent budget
pressures, several states have considered or are considering reducing or eliminating Medicaid
coverage for hospice services. We cannot assure you that the states that provide a Medicaid hospice
benefit will not change or eliminate their Medicaid hospice benefits nor can we assure you that
Congress will not change the Medicare hospice benefit.
Medicare Conditions of Participation. The Medicare program requires each of our hospice
programs to satisfy prescribed conditions of participation to be eligible to receive payments from
Medicare. These conditions of participation describe requirements associated with the management
and operations of our hospice programs. Compliance with the conditions of participation is
monitored by state survey agencies designated by the Medicare program. In some cases, failure to
comply with the conditions may result in payment denials, the imposition of fines or penalties or
the implementation of a corrective action plan. In extreme cases or cases in which there is a
history of repeat violations, a state survey agency may recommend a suspension of new admissions to
the hospice program or termination of the hospice program in its entirety. We believe that we are
in material compliance with the conditions of participation; however, we cannot predict how
surveyors will interpret all aspects of the Medicare conditions of participation.
The Medicare conditions of participation for hospice programs include the following:
| • | Governing Body. Each hospice must have a governing body that assumes full responsibility for the overall management and operations of a hospice and for ensuring that all services are provided in a manner consistent with accepted standards of practice. The governing body must designate one individual who is responsible for the day-to-day administrative operations of the hospice. The designated individual must be a hospice employee and must possess the education and experience required by the governing body. |
11
Table of Contents
| • | Direct Provision of Core Services. Medicare limits those services for which a hospice may use individual independent contractors or contract agencies to provide care to patients. Specifically, substantially all nursing, social work and counseling services must be provided directly by hospice employees meeting specific educational and professional standards. During periods of peak patient loads or under extraordinary circumstances, the hospice may be permitted to use contract workers, but the hospice must agree in writing to maintain professional, financial and administrative responsibility for the services provided by those individuals or entities. | ||
| • | Medical Director. Each hospice must have a medical director who is a physician and who assumes responsibility for overseeing the medical component of the hospice’s patient care program. When the medical director is not available, a physician designated by the hospice assumes the same responsibilities and obligations as the medical director. The medical director may be employed by or under contract with the hospice. | ||
| • | Professional Management of Non-Core Services. A hospice may arrange to have non-core services such as therapy services, home health aide services, medical supplies or drugs provided by a non-employee or outside entity. If the hospice elects to use an independent contractor to provide non-core services, then the hospice must do so through a written agreement, and must retain administrative and financial management and supervision over staff and services to ensure the provision of quality hospice care. Written agreements for arranged services must require that all services be authorized by hospice, furnished in a safe and effective manner by qualified personnel, and delivered in accordance with the patient’s plan of care. | ||
| • | Plan of Care. The hospice’s interdisciplinary group must establish an individualized written plan of care for each hospice patient in collaboration with the patient’s attending physician, the medical director or designated hospice physician, the patient or representative and the primary caregiver. The plan of care must be established prior to providing care to any hospice patient. The plan must assess the patient’s needs and specify the hospice care and services to be provided to meet those needs and also must be reviewed and updated at specified intervals. | ||
| • | Continuation of Care. A hospice may not discontinue or reduce care provided to a Medicare or Medicaid beneficiary if the individual becomes unable to pay for that care. | ||
| • | Admission to Hospice Care. A hospice shall admit a patient only on the recommendation of the medical director in consultation with, or with input from, the patient’s attending physician (if any). | ||
| • | Election of Hospice Benefit. An individual who meets the eligibility requirements for hospice care must file an election statement with the particular hospice that will provide care to the individual. If the individual is physically or mentally incapacitated, his or her representative must file the election statement. An individual or the individual’s representative may revoke the individual’s election of hospice care at any time. | ||
| • | Training. A hospice must provide orientation and ongoing training for its employees and contracted staff who have patient and family contact. A hospice must assess the skills and competency of all individuals furnishing care, including volunteers furnishing services, and provide in-service training and education programs where required. The hospice must have written policies and procedures describing its methods of assessment of competency and maintain a written description of the in-service training provided during the previous 12 months. | ||
| • | Quality Assessment and Performance Improvement. A hospice must develop, implement and maintain a hospice-wide quality assessment and performance improvement program involving all hospice services, including services provided under arrangement or contract. | ||
| • | Interdisciplinary Group. A hospice must designate an interdisciplinary group to provide or supervise hospice care services. The interdisciplinary group develops and updates plans of care, and establishes policies governing the day-to-day provision of hospice services. The interdisciplinary group must include at least a physician, registered nurse, social worker and spiritual or other counselor. A registered nurse must be designated to coordinate the plan of care and to ensure continuous assessment of each hospice patient’s and family’s needs. |
12
Table of Contents
| • | Volunteers. Hospice programs are required to recruit and train volunteers to provide patient care services or administrative services. Volunteer services must comprise at least five percent of the total patient care hours provided by all paid hospice employees and contract staff. | ||
| • | Licensure. Each hospice and all hospice personnel must be licensed, certified or registered in accordance with applicable federal, state and local laws and regulations. | ||
| • | Central Clinical Records. Hospice programs must maintain clinical records for each hospice patient that are organized in such a way that they may be easily retrieved. The clinical records must be complete and accurate and protected against loss, destruction and unauthorized use. The clinical records must be retained for 6 years after the death or discharge of the patient, unless state law stipulates a longer period of time. | ||
| • | Criminal Background Checks. Hospice programs must obtain a criminal background check on all hospice employees who have direct patient contact or access to patient records. Hospice contracts must require that all contracted entities obtain criminal background checks on contracted employees who have direct patient contact or access to patient records. |
In addition to the conditions of participation governing hospice services generally, Medicare
regulations also establish conditions of participation related to the provision of various services
and supplies that many hospice patients receive from us. These services include therapy services
(such as physical therapy, occupational therapy and speech-language pathology), home health aide
and homemaker services, pharmaceuticals, medical supplies, short-term general inpatient care and
respite inpatient care, among other services.
Surveys. Like many healthcare organizations, our hospice programs undergo surveys by federal
and state governmental authorities to assure compliance with both state licensing laws and
regulations and the Medicare conditions of participation. As is common in the healthcare community,
from time to time, we receive survey reports containing statements of deficiencies for alleged
failure to comply with the various regulatory requirements. We review these reports, prepare
responses and take appropriate corrective action, if required. The reviewing agency is generally
authorized to take various adverse actions against a hospice program found to be in non-compliance,
including the imposition of fines or suspension or revocation of a hospice program’s license. If
this adverse action were taken against any of our hospice programs, this action could materially
adversely affect that hospice program’s ability to continue to operate and to participate in the
Medicare and Medicaid programs. This could materially adversely affect our net patient service
revenue and profitability. None of our hospice programs has been suspended at any time from
participation in the Medicare or Medicaid programs or had its state licensure suspended or revoked.
One of the hospice programs that we acquired from VistaCare had previously been suspended from the
Medicare program. Prior to our acquisition of VistaCare, the suspended hospice program’s
participation in the Medicare program was restored.
Certificate of Need Laws and Other Restrictions. Some states have certificate of need (“CON”)
laws that require state approval prior to opening new healthcare facilities or expanding services
at existing healthcare facilities. Approval under CON laws is generally conditioned on the showing
of a demonstrable need for services in the community, and approximately 15 states have CON laws
that apply to hospice services. However, some states with CON requirements permit the transfer of a
CON from an existing provider to a new provider. We entered Nashville, Tennessee, in 1998, Little
Rock, Arkansas, in 2001 and Memphis, Tennessee, in 2003, by acquiring existing hospices that had
met the CON requirement in those states. In addition, we applied for and were awarded CONs in
Daytona, Miami and Ocala (Marion County), Florida and are currently operating hospice programs in
these cities. We have also received a CON to develop and operate a hospice program in Seattle,
Washington, which is currently the subject of a court challenge filed by several existing hospice
providers in the market. The development of our hospice program in Seattle, Washington is currently
stayed until the court challenge is resolved. The State of Alabama recently enacted a CON law that
applies to hospice home care programs. We currently operate three programs in Alabama. One of our
programs has received approval to operate under the non-substantive review process provided for
existing hospice providers in Alabama. Approval of our two remaining programs under this review
process is currently pending and we do not anticipate any issues with the approval of these two
programs. In the future, we may seek to develop or acquire hospice programs in states that have
CON laws. While several states have abolished CON laws and other states do not apply them to
hospice services, these laws could adversely affect
13
Table of Contents
our ability to expand services at our existing hospice programs or to make acquisitions or
develop hospices in new or existing geographic markets.
New York has additional laws that restrict the development and expansion of hospice programs.
Under New York law, a hospice cannot be owned by a corporation that has another corporation as a
stockholder. These laws may prevent us from being able to provide hospice services to residents of
New York.
Limits on the Acquisition or Conversion of Non-Profit HealthCare Organizations. An increasing
number of states require government review, public hearings and/or government approval of
transactions in which a for-profit entity proposes to purchase or otherwise assume the operations
of a non-profit healthcare facility. Heightened scrutiny of these transactions may significantly
increase the costs associated with future acquisitions of non-profit hospice programs in some
states and otherwise increase the difficulty in completing those acquisitions or prevent them
entirely. We cannot assure you that we will not encounter regulatory or governmental obstacles in
connection with our acquisition of non-profit hospice programs in the future.
State Licensure of Hospice. Most of our hospice programs must be licensed in the state in
which they operate. A few states do not have a hospice license requirement. State license rules
and regulations require our hospice programs to maintain certain standards and meet certain
requirements, which vary from state to state. We believe that our hospice programs are in material
compliance with applicable state licensure requirements. If one of our programs were found to be
out of compliance and actions were taken against our program, it could adversely affect our
program’s ability to continue to operate and to participate in the Medicare and Medicaid programs,
which could materially adversely affect us.
Overview of Government Payments
Substantially all of our net patient service revenue is attributable to payments received from
the Medicare and Medicaid programs. 97.0%, 96.6% and 97.0% of our net patient service revenue for
the years ended December 31, 2009, 2008 and 2007, respectively, were attributable to Medicare and
Medicaid payments.
As with most government programs, Medicare and Medicaid are subject to statutory and
regulatory changes, possible retroactive and prospective rate adjustments, administrative rulings,
and freezes and funding reductions, all of which may adversely affect payments to us. Base payment
rates for hospice services under the Medicare and Medicaid programs are indexed for inflation
annually; however, these increases have historically been less than actual inflation. These rates
are further adjusted geographically by the hospice wage index. On July 31, 2008, CMS published the
final rule that modified the hospice wage index by phasing out over a three year period the budget
neutrality adjustment factor. According to the final rule, the phase-out would occur over a three
year period beginning on October 1, 2008, with 25% of the phase-out becoming effective on October
1, 2008, 50% becoming effective on October 1, 2009 and the balance on October 1, 2010. As part of
the American Recovery and Reinvestment Act of 2009, the implementation of the phase-out of the
budget neutrality adjustment factor was delayed until October 1, 2009. CMS began paying providers
the estimated 1.1% increase in hospice rates from October 1, 2008 in the middle of 2009. On July
30, 2009, CMS issued a final rule to update the Medicare hospice wage index. The final rule also
re-implemented the phase-out of the budget neutrality adjustment factor beginning on October 1,
2009. The phase-out of the budget neutrality adjustment factor will now occur over a seven year
period, 10% in the first year and an additional 15% in each of the following six years. On October
1, 2009, payments to Medicare participating hospices increased by approximately 1.4%. This
increase includes the effect of the first year phase-out of the budget neutrality adjustment factor
used in computing the hospice wage index.
As part of its review of the Medicare hospice benefit, MedPAC recommended to Congress in its
Medicare publication “Report to Congress: Medicare Payment Policy — March 2009” (“2009 MedPAC
Report”) that Congress direct the Secretary of Health and Human Services to change the Medicare
payment system for hospices to:
| • | have relatively higher payments per day at the beginning of a patient’s hospice care and relatively lower payments per day as the duration of the hospice patient’s stay increases, | ||
| • | include relatively higher payments for the costs associated with patient death at the end of the hospice patient’s stay, and | ||
| • | implement the payment system changes in 2013, with a brief transitional period. |
14
Table of Contents
In its 2009 MedPAC Report, MedPAC estimated that these changes would result in a
reduction in aggregate payments to for-profit hospices of between 3.2% and 5.0%. Reductions or
changes in Medicare or Medicaid funding could significantly reduce our net patient service revenue
and our profitability.
On January 14, 2010, MedPAC voted to recommend that the Congress should reduce the annual
market basket update for hospice providers on October 1, 2010 by MedPAC’s adjustment for
productivity growth, which is estimated to be 1.3%. In addition, both the Senate and House of
Representatives have passed separate health care reform bills. Each of these bills include several
provisions that would adversely impact hospice providers, including a provision to reduce the
annual market basket update for hospice providers by a productivity adjustment. We cannot predict
at this time whether the recommendations included in the 2009 MedPAC Report, the recommendation
approved by MedPAC on January 14, 2010 or the provisions included in the various health care reform
proposals will be enacted or whether any additional healthcare reform initiatives will be
implemented or whether other changes in the administration of governmental healthcare programs or
interpretations of governmental policies or other changes affecting the healthcare system will
occur.
Laws and regulations governing the Medicare and Medicaid programs are complex and subject to
interpretation. We believe that we are in material compliance with all applicable laws and
regulations. Compliance with laws and regulations can be subject to future government review and
interpretation as well as significant regulatory action, including fines, penalties and exclusion
from the Medicare and Medicaid programs.
Medicare. Medicare pays us based on a prospective payment system under which we receive one of
four predetermined daily or hourly rates based on the level of care (See “- Hospice Services and
Payment”). The four levels of care are routine home care, continuous home care, general inpatient
care and respite inpatient care. As discussed above, these rates are currently subject to annual
adjustments for inflation and are also adjusted annually based on geographic location.
Direct patient care physician services delivered by physicians contracted with us are billed
separately by us to the Medicare fiscal intermediary and paid at the lesser of the actual charge or
100% of the Medicare allowable charge for these services. This payment is in addition to the daily
rates we receive for hospice care. We generally pay our contracted physicians 80% to 95% of the
Medicare allowable charge for these physician services. Payments for a patient’s attending
physician’s professional services, other than services furnished by physicians contracted with us,
are not paid to us, but rather are billed by and paid directly to the attending physician by the
Medicare carrier based on the Medicare physician fee schedule. Physician services represented 0.8%
and 0.7% of our gross patient service revenue for 2009 and 2008, respectively.
The Medicare Cap. Various provisions were included in the legislation creating the Medicare
hospice benefit to manage the cost to the Medicare program for hospice, including the patient’s
waiver of curative care requirement, the six-month terminal prognosis requirement and the Medicare
payment caps. The Medicare hospice benefit includes two fixed annual caps on payment, both of which
are assessed on a program-by-program basis. One cap is an absolute dollar amount limit, and the
other cap limits the number of days of inpatient care. None of our hospice programs exceeded the
payment limits on general inpatient care services for the years ended December 31, 2009, 2008 and
2007. The caps are calculated from November 1 through October 31 of each year.
Dollar Amount Cap. The Medicare revenue paid to a hospice program from November 1 to October
31 of the following year may not exceed the annual cap amount which is calculated by using the
following formula: the product of the number of admissions to the program of patients who are
electing to receive their Medicare hospice benefit for the first time, multiplied by the Medicare
cap amount, which for the November 1, 2008 through October 31, 2009 Medicare fiscal year was
$23,014. The Medicare cap amount is reduced proportionately for patients who transferred in or out
of our hospice services. The Medicare cap amount is annually adjusted for inflation, but is not
adjusted for geographic differences in wage levels, although hospice per diem payment rates are
wage indexed. The Medicare cap amount for the November 1, 2009 through October 31, 2010 cap year
has not yet been announced by the Medicare program. We currently estimate the Medicare cap amount
to be approximately $23,600 for the Medicare cap year ending October 31, 2010.
15
Table of Contents
The following table shows the Medicare cap amount for the past three years and the estimated
amount for the current year:
| Medicare Cap Year Ending October 31, | Medicare Cap Amount | |||
2007 |
$ | 21,410 | ||
2008 |
$ | 22,386 | ||
2009 |
$ | 23,014 | ||
2010 (estimated) |
$ | 23,600 | ||
The following table shows the amounts accrued and paid for the Medicare cap contractual
adjustments for the years ended December 31, 2007, 2008 and 2009, respectively:
| Accrued Medicare Cap Contractual Adjustments | ||||||||||||
| Year Ending December 31, | ||||||||||||
| 2007 | 2008 | 2009 | ||||||||||
| (in thousands) | ||||||||||||
Beginning balance — accrued Medicare
cap contractual adjustments |
$ | 26,679 | $ | 21,682 | $ | 23,719 | ||||||
Medicare cap contractual adjustments |
5,039 | (1) | 6,852 | (2) | 4,565 | (3) | ||||||
Medicare cap contractual adjustments —
discontinued operations |
2,651 | (4) | (27 | )(4) | (79 | )(4) | ||||||
Payments to Medicare fiscal intermediaries |
(12,687 | ) | (12,996 | ) | (9,407 | ) | ||||||
Balances acquired from VistaCare |
— | 8,208 | — | |||||||||
Ending balance — accrued Medicare cap
contractual adjustments |
$ | 21,682 | $ | 23,719 | $ | 18,798 | ||||||
| (1) | Includes additional accrual of $0.9 million related to the 2006 Medicare cap year. | |
| (2) | Includes additional accrual of $1.5 million related to the 2006 Medicare cap year. | |
| (3) | Includes an accrual reversal of $1.1 million related to the 2007 Medicare cap year. | |
| (4) | Medicare cap contractual adjustments reclassified to discontinued operations are related to all programs that were discontinued and sold during 2007, 2008 and 2009. |
The accuracy of our estimates of the Medicare cap contractual adjustment is affected by many
factors, including:
| • | the actual number of Medicare beneficiary patient admissions and discharges and the dates of occurrence of each; | ||
| • | changes in the average length of stay at our hospice programs; | ||
| • | fluctuations in admissions and discharges at our hospice programs; | ||
| • | possible enrollment of beneficiaries in our hospice programs who may have previously elected Medicare hospice coverage through another hospice program and whose Medicare cap amount is prorated for the days of service for the previous hospice admission; | ||
| • | possible enrollment of beneficiaries with another hospice program who had been on previous hospice service with one of our own hospice programs and discharged from our hospice program and whose Medicare cap amount is prorated between the programs for the days of service for the subsequent hospice admission; | ||
| • | fiscal intermediary disallowances of certain beneficiaries and changes in calculation methodology; | ||
| • | uncertainty surrounding length of patient stay in various patient groups, particularly with respect to non-cancer patients; and |
16
Table of Contents
| • | the fact that we are not advised of the Medicare cap amount that will be used by Medicare to calculate our Medicare cap contractual adjustment until the latter part of the Medicare cap year, requiring us to use an estimate of that amount throughout the year. |
Between 2003 and 2009, several of our hospice programs exceeded the Medicare cap amount. As a
result, we were required to repay a portion of payments previously received from Medicare. We
actively monitor the Medicare cap amount at each of our programs and seek to implement corrective
measures as necessary. We maintain what we believe are adequate allowances in the event that we
exceed the Medicare cap in any given fiscal year; however, because of the many variables involved
in estimating the Medicare cap contractual that are beyond our control, we cannot assure you that
we will not increase or decrease our estimated contractual allowance in the future. We cannot
assure you that one or more of our hospice programs will not exceed the Medicare cap amount in the
future.
Inpatient Care Cap. A hospice program’s inpatient care days, either general inpatient or
respite inpatient care and regardless of setting, may not exceed 20% of the program’s total patient
care days in the Medicare cap year. None of our hospice programs exceeded the payment limits on
general inpatient care services for the years ended December 31, 2009, 2008 and 2007. We cannot
assure you that one or more of our hospice programs will not exceed the Medicare inpatient care cap
in the future.
Fiscal Intermediary Reviews and other Billing Audits. Medicare contracts with fiscal
intermediaries to process hospice claims and periodically conduct targeted medical reviews and
other audits on hospice claims. During a typical review of one of our hospice programs, the fiscal
intermediary will request a small number of patient charts to review for hospice appropriateness
(that is, clinical documentation that supports the patient’s terminal prognosis) and various
required documents such as physician signatures and certifications. We routinely challenge claim
denials which we believe are unjustified. While we believe that our review results to date are
satisfactory, routine reviews and targeted medical reviews of our hospice programs could result in
material recoupments or denials of claims.
In addition to the denial of claims, reviews by fiscal intermediaries can impact our cash flow
and days outstanding in accounts receivable in two ways. First, in some cases we delay the bill
processing of claims undergoing a review by the fiscal intermediary. Second, Medicare has a claims
processing procedure known as sequential billing which prevents hospice programs from billing for a
period of service for a patient before the prior billed period has been reimbursed. These delays
can reduce our cash flow and increase our days outstanding in accounts receivable.
Medicare also contracts with other third parties, such as recovery audit contractors and
program integrity contractors, to perform post-payment reviews and audits. These contractors
typically request copies of patient charts and review the appropriateness of patients for hospice
services and compliance with the technical aspects of hospice billing. We believe our hospice
programs comply with all payor requirements at the time of billing. However, we cannot predict
whether future billing reviews or similar audits by these contractors will result in material
recoupments.
Medicare Six-Month Eligibility Rule and Waiver of Other Medicare Benefits. In order for a
Medicare beneficiary to qualify for the Medicare hospice benefit, two physicians must certify that
in their clinical judgment the beneficiary has less than six months to live, assuming the disease
runs its normal course. Beginning October 1, 2009, the hospice physician is required to include a
brief narrative explanation of the clinical findings that supports the determination that the
beneficiary has less than six months to live. In addition, the Medicare beneficiary must
affirmatively elect hospice care and waive any rights to other Medicare benefits related to the
terminal diagnosis. Medicare and other payor sources recognize that terminal illnesses are not
entirely predictable, and patients may continue to receive hospice service if the hospice medical
director or the patient’s attending physician recertify at time intervals prescribed by law that
the patient’s life expectancy, on a look-forward basis, continues to be less than six months. The
recertifications are required 90 and 180 days after admission and every 60 days thereafter. No
limits exist on the number of periods that a Medicare beneficiary may be recertified. A Medicare
beneficiary may revoke his or her election to receive hospice services at any time and resume
receiving regular Medicare benefits. The Medicare beneficiary may elect the hospice benefit again
at a later date provided that the beneficiary satisfies the six-month eligibility rule.
17
Table of Contents
In addition to the traditional Medicare fee-for-service program, the Medicare program also
offers a managed care benefit to electing Medicare beneficiaries. These managed care programs are
often referred to as Medicare Advantage programs. Our payments for services provided to Medicare
beneficiaries enrolled in Medicare Advantage programs are currently processed in the same way and
at the same rates as those of traditional Medicare fee-for-service beneficiaries. We cannot assure
you that hospice services will continue to be paid entirely under the Medicare fee-for-service
program.
Medicaid. Medicaid is a state-administered program financed by state funds and matching
federal funds to provide medical assistance to the indigent and certain other eligible persons. In
1986, hospice services became an optional state Medicaid benefit. For those states that elect to
provide a hospice benefit, Medicaid is required to pay us rates that are at least equal to the
hospice rates paid by Medicare. Approximately 45 states and the District of Columbia provide
hospice coverage to their Medicaid beneficiaries. Most of the states providing a Medicaid hospice
benefit pay us at rates equal to or greater than the rates provided under Medicare and those rates
are calculated using the same methodology as Medicare. States maintain flexibility to establish
their own hospice election procedures and to limit the number and duration of benefit periods for
which they will pay for hospice services. Several states, including states that we operate in, have
considered or are considering reducing or eliminating the Medicaid hospice benefit due to budgetary
issues. We cannot assure you that states, including states that we operate in, will not in the
future reduce or eliminate the Medicaid hospice benefit.
Long-Term Care Facility Residents. For our patients who receive nursing home care under state
Medicaid programs in states other than Arizona, Oregon and Pennsylvania, the applicable Medicaid
program pays us an amount equal to no more than 95% of the Medicaid per diem nursing home rate for
“room and board” services furnished to the patient by the nursing home. This room and board payment
is in addition to the applicable Medicare or Medicaid hospice per diem payment that we receive.
Pursuant to our standard agreements with nursing homes, we pay the nursing home for these “room and
board” services at a rate equal to 100% of the Medicaid per diem nursing home rate. See “Item 7.
Management’s Discussion and Analysis of Financial Condition and Results of Operations — Expenses.”
Other Healthcare Regulations
Fraud and Abuse Laws and Anti-Kickback Statute. Provisions of the Social Security Act,
commonly referred to as the fraud and abuse provisions, prohibit the filing of false or fraudulent
claims with Medicare or Medicaid and the payment or receipt of any form of remuneration in return
for the referral of Medicare or Medicaid patients or arranging for the referral of patients, or in
return for the recommendation, arrangement, purchase, lease or order of items or services that are
covered by the Medicare or Medicaid programs. Violation of these provisions could constitute a
felony criminal offense and applicable sanctions, including imprisonment of up to five years,
criminal fines of up to $25,000, civil monetary penalties of up to $50,000 per act plus three times
the amount claimed or three times the remuneration offered and exclusion from the Medicare and
Medicaid programs. Many states have adopted similar prohibitions against payments that are intended
to induce referrals of Medicaid and other third-party payor patients.
The Office of Inspector General, Department of Health and Human Services (“OIG”), has
published numerous “safe harbors” that exempt some practices from enforcement action under the
federal fraud and abuse laws. These safe harbors exempt specified activities, including bona fide
employment relationships, some contracts for the rental of space or equipment, some personal
service arrangements and management contracts, and certain joint ventures. While the failure to
satisfy all of the requirements of a particular safe harbor does not necessarily mean that the
arrangement is unlawful, arrangements that do not satisfy a particular safe harbor may be subject
to scrutiny by the OIG.
We are required under the Medicare conditions of participation and some state licensing laws
to contract with numerous healthcare providers and practitioners, including physicians, hospitals
and nursing homes, and to arrange for these individuals or entities to provide services to our
patients. In addition, we have contracts with other suppliers, including pharmacies, ambulance
services and medical equipment companies. Some of these individuals or entities may refer, or be in
a position to refer, patients to us, and we may refer, or be in a position to refer, patients to
these individuals or entities. These arrangements may not qualify for a safe harbor. We believe
that our contracts and arrangements with providers, practitioners and suppliers are not in
violation of applicable fraud and abuse laws.
18
Table of Contents
Pursuant to the Anti-Kickback Statute, and in an effort to reduce potential fraud and abuse
relating to federal healthcare programs, the federal government has announced a policy of increased
scrutiny of joint ventures and other transactions among healthcare providers. The OIG closely
scrutinizes healthcare joint ventures involving physicians and other referral sources. The OIG
published a fraud alert that outlined questionable features of “suspect” joint ventures in 1989 and
a Special Advisory Bulletin related to contractual joint ventures in 2003, and the OIG has
continued to rely on fraud alerts in later pronouncements. We currently operate four joint venture
hospice programs. Because one of our subsidiaries is an investor in each of our joint ventures, and
since one of our other subsidiaries provides management and other services to the joint venture
hospice program, our joint venture arrangements do not fit within the specific terms of the small
investment interest safe harbor or any other safe harbor. We believe, however, that our joint
venture arrangements do not fall within the activities prohibited by the Anti-Kickback Statute.
From time to time, various federal and state agencies, such as the OIG, issue a variety of
pronouncements, including fraud alerts, the OIG’s Annual Work Plan and other reports, identifying
practices that may be subject to heightened scrutiny. For example, in March 1998, the OIG issued a
special fraud alert titled “Fraud and Abuse in Nursing Home Arrangements with Hospices.” This
special fraud alert focused on payments received by nursing homes from hospices. The OIG also
issued a voluntary Compliance Program Guidance for Hospices in September 1999. We believe that we
are in material compliance with all applicable federal and state fraud and abuse laws. However, we
cannot assure you that these laws will not be interpreted in the future in such a way as to cause
us to be in violation of these laws.
HIPAA Fraud and Abuse Provisions. Portions of the Health Insurance Portability and
Accountability Act of 1996 (“HIPAA”) impose civil monetary penalties in cases involving the fraud
and abuse laws or contracting with excluded providers. In addition, HIPAA created new statutes
making it a felony to engage in fraud, theft, embezzlement, or the making of false statements with
respect to healthcare benefit programs, including private and government programs. In addition,
federal enforcement agencies can exclude from the Medicare and Medicaid programs any investors,
officers and managing employees associated with business entities that have committed healthcare
fraud, even if the individual had no first-hand knowledge of the fraud.
Civil Monetary Penalties Statute. The federal civil monetary penalties statute prohibits any
person or entity from knowingly submitting false or fraudulent claims, offering to or making
payments to a beneficiary to induce the beneficiary to use a particular provider or supplier, or
arranging or contracting with an individual or entity that the person or entity knows or should
know is excluded from the Medicare or Medicaid programs for the provision of items or services that
may be reimbursed, in whole or in part, by the Medicare or Medicaid programs. Violations can result
in civil monetary penalties ranging from $10,000 to $50,000 per claim or act, plus damages of not
more than three times the amount claimed for each such item or service.
False Claims Act. In addition to federal fraud and abuse laws, under separate statutes, the
submission of claims for items and services that are “not provided as claimed” may lead to civil
monetary penalties, criminal fines and imprisonment, and/or exclusion from participation in
federally funded healthcare programs, including the Medicare and Medicaid programs. These false
claims statutes include the Federal False Claims Act. Under the Federal False Claims Act, in
addition to actions being initiated by the federal government, a private party may bring an action
on behalf of the federal government. These private parties, are often referred to as qui tam
relators, and are entitled to share in any amounts recovered by the government. Both direct
enforcement activity by the government and qui tam actions have increased significantly in recent
years and have increased the risk that a healthcare company, like us, will have to defend a false
claims action, pay fines or be excluded from the Medicare and/or Medicaid programs as a result of
an investigation arising out of this type of an action. Many states have enacted similar laws
providing for the imposition of civil and criminal penalties for the filing of fraudulent claims.
We are currently defending an action filed by a qui tam relator under the Federal False Claims Act
and various state false claims acts. (See “Item 3. Legal Proceedings.”) Because of the complexity
of the government regulations applicable to our industry, we cannot assure that we will not be the
subject of additional actions under the False Claims Act or similar state law.
State False Claims Laws. The Deficit Reduction Act of 2005, or “DRA”, which was signed into
law on February 8, 2006, includes a provision encouraging states to adopt their own false claims
act provisions by increasing the states’ share of any recoveries related to Medicaid funds. The
majority of the states where we currently do business, have already adopted state false claims laws
that mirror to some degree the federal false claims laws. While these statutes vary in scope and
effect, the penalties for violating these false claims laws include administrative, civil
19
Table of Contents
and/or criminal fines and penalties, imprisonment and the imposition of multiple damages.
There has been an increase in enforcement activity by the states due in part to the implementation
of the DRA.
The Stark Law and State Physician Self-Referral Laws. Section 1877 of the Social Security Act,
commonly known as the “Stark Law,” prohibits physicians, subject to the exceptions described below,
from referring Medicare or Medicaid patients to any entity providing “designated health services”
in which the physician has an ownership or investment interest or with which the physician has
entered into a compensation arrangement. Persons who violate the Stark Law are subject to civil
monetary penalties and exclusion from the Medicare and Medicaid programs.
Hospice care is not specifically enumerated as a health service subject to this prohibition;
however, some of the ten designated health services under the Stark Law, including physical
therapy, pharmacy services and certain infusion therapies, are among the specific services
furnished by our hospice programs. Regulations interpreting the Stark Law currently provide that
compensation arrangements between referring physicians and a hospice will not violate the Stark
Law. We cannot assure you, however, that future regulatory changes will not result in us becoming
subject to the Stark Law’s prohibition in the future.
Many states have also enacted physician self-referral laws, which generally prohibit financial
relationships with referral sources that are not limited to services for which Medicare or Medicaid
payments may be made. Similar penalties, including loss of license or eligibility to participate in
government programs and civil and criminal fines, apply to violations of these state self-referral
laws. These laws vary from state to state and have seldom been interpreted by the courts or
regulatory agencies. We believe that our relationships with physicians do not violate these state
self-referral laws. However, we cannot assure you that these laws will not be interpreted in the
future in such a way as to call into question our relationships with physicians, including our
physician joint ventures.
Prohibition on Employing or Contracting with Excluded Providers. The Social Security Act and
federal regulations state that individuals or entities that have been convicted of a criminal
offense related to the delivery of an item or service under Medicare or Medicaid programs or that
have been convicted, under state and federal law, of a criminal offense relating to neglect or
abuse of residents in connection with the delivery of a healthcare item or service cannot
participate in any federal health care programs, including Medicare and Medicaid. Additionally,
individuals and entities convicted of fraud, that have had their licenses revoked or suspended, or
that have failed to provide services of adequate quality, also may be excluded from the Medicare
and Medicaid programs. Federal regulations prohibit Medicare providers, including hospice programs,
from submitting claims for items or services or their related costs if an excluded provider
furnished those items or services. The OIG maintains a list of excluded persons and entities.
Nonetheless, it is possible that we might unknowingly bill for services provided by an excluded
person or entity with whom it contracts. The penalty for contracting with an excluded provider may
range from civil monetary penalties of $50,000 and damages of up to three times the amount of
payment that was inappropriately received.
Corporate Practice of Medicine and Fee-Splitting. Most states have laws that restrict or
prohibit unlicensed persons or business entities, including corporations, from employing physicians
and/or prohibit direct or indirect payments or fee-splitting arrangements between physicians and
unlicensed persons or business entities. Possible sanctions for violations of these restrictions
include loss of a physician’s license, civil and criminal penalties and rescission of business
arrangements. These laws vary from state to state, are often vague and have seldom been interpreted
by the courts or regulatory agencies.
We employ or contract with physicians to provide medical direction and patient care services.
A state with these prohibitions could determine that the provision of patient care services by our
contracted physicians violates the corporate practice of medicine and/or fee-splitting
prohibitions. We structure our arrangements with healthcare providers to comply with the relevant
state law. However, we cannot assure you that government officials charged with the responsibility
for enforcing these laws will not assert that we, or transactions in which we are involved, are in
violation of these laws. These laws may also be interpreted by the courts in a manner inconsistent
with our interpretations. The determinations or interpretations by a state may require us to
restructure our arrangements with physicians in the applicable state.
20
Table of Contents
Regulation Governing the Privacy and Transmission of Healthcare Information
In addition to its antifraud provisions, HIPAA also requires improved efficiency in healthcare
delivery by standardizing electronic data interchange and by protecting the confidentiality and
security of individual health data. More specifically, HIPAA calls for:
| • | standardization of certain electronic patient health, administrative and financial data; | ||
| • | privacy standards protecting the privacy of individually identifiable health information; and | ||
| • | security standards protecting the confidentiality and integrity of electronically held individually identifiable health information. |
In August 2000, final regulations establishing standards for electronic data transactions and
code sets, as required under HIPAA, were released. These standards are designed to allow entities
within the healthcare industry to exchange medical, billing and other information and to process
transactions in a more timely and cost effective manner. Modifications to the electronic data
transactions and code sets standards were issued on February 20, 2003, and further modifications
were issued on March 10, 2003.
The HIPAA privacy standards are designed to protect the privacy of certain individually
identifiable health information. The privacy standards have required us to make certain updates to
our policies and procedures and conduct training for our employees surrounding these standards.
Sanctions for failing to comply with the HIPAA privacy rules could include civil monetary penalties
of $100 per incident, up to a maximum of $50,000 per person, per year, per standard, with maximum
penalties for additional violations in any one year ranging from $25,000 to $1.5 million. The final
rule also provides for criminal penalties of up to $50,000 and one year in prison for knowingly and
improperly obtaining or disclosing protected health information, up to $100,000 and five years in
prison for obtaining protected health information under false pretenses, and up to $250,000 and ten
years in prison for obtaining or disclosing protected health information with the intent to sell,
transfer or use such information for commercial advantage, personal gain or malicious harm.
The American Recovery and Reinvestment Act of 2009 made several significant changes to the
HIPAA privacy and security requirements. These changes include mandatory notification of breaches
of privacy and security involving protected health information to the affected individuals, the
Department of Health and Human Services and, in certain circumstances, the media. In addition,
several changes were made to increase enforcement of the HIPAA privacy and security requirements,
including giving state attorneys general new civil enforcement authority related to violations of
HIPAA’s privacy and security provisions and requiring the Department of Health and Human Services
to conduct periodic audits of covered entities. Because of the recent enactment of these changes
and the lack of regulatory guidance we cannot assure you that these changes will not have a
material adverse affect on us once they are fully implemented.
Additional Federal and State Healthcare Laws. The federal government and all states also
regulate other aspects of the hospice industry. In particular, our operations are subject to
federal and state laws covering professional services, the dispensing of drugs and other types of
hospice activities. Some of our employees are subject to state laws and regulations governing the
ethics and practice of medicine, respiratory therapy, pharmacy and nursing.
Surveys and Certification. Our operations are subject to periodic survey by government
entities to assure compliance with applicable state licensing and Medicare and Medicaid
certification. From time to time in the ordinary course of business, we, like other healthcare
companies, receive survey reports containing deficiencies for alleged failure to comply with
applicable requirements. We review these reports and take appropriate corrective action if
necessary. The failure to take corrective action or to obtain, renew or maintain any of the
required regulatory approvals, certifications or licenses could materially adversely affect our
business and could prevent our hospice programs involved therein from offering services to patients
or billing for those services. In addition, laws and regulations often are adopted to regulate new
products, services and industries. We cannot assure you that either the states or the federal
government will not impose additional regulations upon our activities that might adversely affect
us.
21
Table of Contents
Employment Laws and Regulations. As a large employer, we are subject to various federal and
state laws regulating employment practices. We are specifically subject to audits by various
federal and state agencies regarding our compliance with these laws. In addition, current and
former employees can initiate litigation alleging violations of federal and/or state wage and hour
laws and federal and state anti-discrimination and harassment laws. We believe that our employment
practices are in material compliance with applicable federal and state laws. However, we cannot
assure you that government officials charged with the responsibility of enforcing these laws or
current or former employees will not assert that we are in violation of these laws, or that these
laws will be interpreted by the courts in a manner consistent with our interpretations.
Compliance with Health Regulatory Laws. We maintain an internal corporate compliance program
and from time to time retain regulatory counsel for guidance on applicable laws and regulations.
However, we cannot assure you that our practices, if reviewed, would be found to be in compliance
with applicable federal and state laws, as the laws ultimately may be interpreted.
Compliance and Quality Assessment and Performance Improvement Programs
We have a comprehensive company-wide compliance program. Our compliance program provides for:
| • | a compliance officer and committee; | ||
| • | a corporate code of business conduct and ethics and standards of conduct; | ||
| • | employee education and training; | ||
| • | an internal system for reporting concerns on a confidential, anonymous basis; | ||
| • | ongoing internal auditing and monitoring programs; and | ||
| • | a means for enforcing the compliance program policies. |
As part of our ongoing internal auditing and monitoring programs, we conduct periodic
compliance reviews and internal regulatory audits and mock surveys at each of our
Medicare-certified hospice programs. If a program does not achieve a satisfactory rating, we
require it to prepare and implement a plan of correction. In certain situations we will perform a
follow-up audit and survey to verify that all deficiencies identified in the initial audit and
survey have been corrected.
On July 6, 2006, we entered into a five-year Corporate Integrity Agreement (“CIA”) with the
OIG. The CIA is structured to assure the federal government of our federal health care program
compliance and specifically covers clinical appropriateness of our hospice patients. The CIA
imposes certain auditing, self-reporting and training requirements that we must comply with. Under
the CIA, we have an affirmative obligation to report to the government probable violations of
applicable federal health care laws and regulations. This obligation could result in greater
scrutiny by regulatory authorities. Breach of the CIA could subject us to substantial monetary
penalties or affect our participation in the Medicare and Medicaid programs, or both. We have
agreed, during the five-year term of the CIA, to operate our compliance program in a manner that
meets the requirements of the CIA.
We have a quality assessment and performance improvement program in place. Our quality
assessment and performance improvement program involves:
| • | on-going education of staff and quarterly quality assessment and performance improvement meetings at each of our hospice programs and at our Support Center; | ||
| • | quarterly comprehensive audits of patient charts and site operations performed by each of our hospice programs; and | ||
| • | at least once a year, a comprehensive audit of patient charts and site operations performed on each of our hospice programs by our clinical compliance staff. |
22
Table of Contents
If a hospice program fails to achieve a satisfactory rating on a patient chart audit, we
require the program to prepare and implement a plan of correction. We then conduct a follow-up
patient chart audit to verify that appropriate action has been taken to prevent future
deficiencies.
We continually expand and refine our compliance and quality assessment and performance
improvement programs. Specific written policies, procedures, training and educational materials and
programs, as well as auditing and monitoring activities, have been prepared and implemented to
address the functional and operational aspects of our business. Our programs also address specific
problem areas identified through regulatory interpretation and enforcement activities. Our
policies, training, standardized documentation requirements, reviews and audits also specifically
address our financial arrangements with our referral sources, including fraud and abuse laws and
physician self-referral laws.
Competition
Hospice care in the United States is competitive. Because payments for hospice services are
generally paid on a per diem basis, we compete primarily on our ability to deliver quality,
responsive services. The hospice care market is highly fragmented, and we compete with a large
number of organizations, some of which have or may obtain significantly greater financial and
marketing resources than us. According to MedPAC, in 2008 there were 3,389 Medicare-certified
hospice programs, an increase of 4.0% over 2007. According to MedPAC, approximately 35% of existing
hospice programs are not-for-profit programs. Many hospice programs are small- and medium-sized
programs.
We also compete with a number of national and regional hospice providers, including Vitas
Healthcare which is a subsidiary of Chemed Corporation, hospitals, long-term care facilities, home
health agencies and other healthcare providers, including those with which we presently maintain
contractual relationships, that offer hospice and/or palliative care services such as Golden Living
(formerly Beverly Enterprises, Inc.) and Manor Care, Inc. Many of them offer home care to patients
who are terminally ill, and some actively market palliative care and “hospice-like” programs.
Relatively few barriers to entry exist, so other companies not currently providing hospice care may
enter the hospice markets that we serve and expand the variety of services they offer.
Insurance
We maintain primary general (occurrence basis) and professional (claims made basis) liability
coverage on a company-wide basis with limits of $1.0 million per occurrence and $3.0 million in the
aggregate, both with a deductible of $75,000 per occurrence or claim. We also maintain workers’
compensation coverage, except in Texas, at the statutory limits and an employer’s liability policy
with a $1.0 million limit per accident/employee, with a deductible of $500,000 per occurrence. In
Texas we do not subscribe to the state workers’ compensation program; instead, we maintain a
separate employer’s excess indemnity coverage in the amount of $5.0 million per accident/employee
and voluntary indemnity coverage in the amount of $5.0 million per accident/employee, with a $5.0
million aggregate limit. We also maintain a policy insuring hired and non-owned automobiles on a
company-wide basis with a $1.0 million limit of liability and a $250,000 deductible per occurrence.
In addition, we maintain umbrella coverage with a limit of $20.0 million excess over the general,
professional, hired and non-owned automobile and employer’s liability policies.
Employees
As of December 31, 2009, we had 5,891 full-time employees and 203 part-time employees.
Approximately 25% of our full-time employees and 20% of our part-time employees are registered
nurses. None of our employees are currently covered by collective bargaining agreements.
Available Information
We file reports with the Securities and Exchange Commission (“SEC”). We are a reporting
company and file an Annual Report on Form 10-K, Quarterly Reports on Form 10-Q and Current Reports
on Form 8-K when necessary. The public may read and copy any materials that we file with the SEC at
the SEC’s Public Reference Room at 100 F. Street, NE, Washington, D.C. 20549. The public may obtain
information on the operation of the Public Reference
23
Table of Contents
Room by calling the SEC at 1-800-SEC-0330. The SEC maintains an Internet site that contains
reports, proxy and information statements, and other information regarding issuers that file
electronically with the SEC. That website address is http://www.sec.gov.
We maintain a website with the address http://www.odsyhealth.com. We are not including the
information contained on our website as a part of, or incorporating it by reference into, this
Annual Report on Form 10-K. We make available free of charge through our website our Annual Reports
on Form 10-K, Quarterly Reports on Form 10-Q and Current Reports on Form 8-K, and amendments to
these reports, as soon as reasonably practicable after we electronically file such material with,
or furnish such material to, the SEC. These Annual Reports, Quarterly Reports and Current Reports
may be found on our website under the “Investor Relations” tab by clicking on the link titled “SEC
Filings.” Information relating to our corporate governance policies, including our Corporate Code
of Business Conduct and Ethics and Healthcare Compliance Program Standards of Conduct for our
directors, officers and employees and information concerning our Board committees, including
committee charters, is also available on our website at http://www.odsyhealth.com under the
“Investor Relations” tab by clicking on the link titled “Corporate Governance.” We will provide any
of the foregoing information free of charge upon written request to Investor Relations, Odyssey
HealthCare, Inc., 717 N. Harwood, Suite 1500, Dallas, Texas 75201. Reports of our executive
officers, directors and any other persons required to file securities ownership reports under
Section 16(a) of the Securities Exchange Act of 1934 are also available through our website under
the “Investor Relations” tab by clicking on the link titled “SEC Filings” and then clicking on the
link “View Section 16 Filings (3,4,5).”
Item 1A. Risk Factors
An investment in our common stock is subject to significant risks inherent in our business. As
such, you should consider carefully the risks and uncertainties described below and the other
information included in this Annual Report on Form 10-K. The occurrence of any of the events
described below could have a material adverse effect on our business. Additional risks and
uncertainties that we do not presently know or that we currently consider immaterial may also
impair our business operations. If any of the following risks occur, it could cause the trading
price of our common stock to decline, perhaps significantly.
If we fail to comply with the terms of our Corporate Integrity Agreement, we could be subject to
substantial monetary penalties or suspension or termination from participation in the Medicare and
Medicaid programs.
On July 6, 2006, we entered into a five-year Corporate Integrity Agreement (“CIA”) with the
Office of Inspector General of Health and Human Services. The CIA imposes certain auditing,
self-reporting and training requirements that we must comply with. If we fail to comply with the
terms of our CIA, we could be subject to substantial monetary penalties and/or suspension or
termination from participation in the Medicare and Medicaid programs. The imposition of monetary
penalties would adversely affect our profitability. A suspension or termination of our
participation in the Medicare and Medicaid programs would have a material adverse affect on our
profitability and financial condition as substantially all of our net patient service revenue is
attributable to payments received from the Medicare and Medicaid programs. 97.0% and 96.6% of our
net patient service revenue for the years ended December 31, 2009 and 2008, respectively, were
attributed to Medicare and Medicaid payments.
We are highly dependent on payments from Medicare and Medicaid. If there are changes in the rates
or methods governing these payments for our services, our net patient service revenue and profits
could materially decline.
We are highly dependent on payments from Medicare and Medicaid. Approximately 97.0%, 96.6% and
97.0% of our net patient service revenue for 2009, 2008 and 2007, respectively, consisted of
payments, paid primarily on a per diem basis, from the Medicare and Medicaid programs. Because we
generally receive fixed payments for our hospice care services based on the level of care provided
to our hospice patients, we are at risk for the cost of services provided to our hospice patients.
On July 31, 2008, CMS published the final rule that modified the hospice wage index by phasing out
over a three year period the budget neutrality adjustment factor. According to the final rule, the
phase-out would occur over a three year period beginning on October 1, 2008, with 25% of the
phase-out becoming effective on October 1, 2008, 50% becoming effective on October 1, 2009 and the
balance on October 1, 2010. As part of the American Recovery and Reinvestment Act of 2009, the
implementation of the phase-out of the budget neutrality adjustment factor was delayed until
October 1, 2009. CMS began paying providers the estimated
24
Table of Contents
1.1% increase in hospice rates from October 1, 2008 in the middle of 2009. On July 30, 2009,
CMS issued a final rule to update the Medicare hospice wage index. The final rule also
re-implemented the phase-out of the budget neutrality adjustment factor beginning on October 1,
2009. The phase-out of the budget neutrality adjustment factor will now occur over a seven year
period, 10% in the first year and an additional 15% in each of the following six years. On October
1, 2009, payments to Medicare participating hospices increased by approximately 1.4%. This
increase includes the effect of the first year phase-out of the budget neutrality adjustment factor
used in computing the hospice wage index.
As part of its review of the Medicare hospice benefit, MedPAC recommended to Congress in its
2009 MedPAC Report that Congress direct the Secretary of Health and Human Services to change the
Medicare payment system for hospice to:
| • | have relatively higher payments per day at the beginning of a patient’s hospice care and relatively lower payments per day as the length of the duration of the hospice patient’s stay increases, | ||
| • | include relatively higher payments for the costs associated with patient death at the end of the hospice patient’s stay, and | ||
| • | implement the payment system changes in 2013, with a brief transitional period. |
In its 2009 MedPAC Report, MedPAC estimated that these changes would result in a reduction in
aggregate payments to for-profit hospices of between 3.2% and 5.0%.
On January 14, 2010 MedPAC voted to recommend that the Congress should reduce the annual
market basket update for hospice providers on October 1, 2010 by MedPAC’s adjustment for
productivity growth, which is estimated to be 1.3%. In addition, both the Senate and House of
Representatives have passed separate health care reform bills. Each of these bills include several
provisions that would adversely impact hospice providers, including a provision to reduce the
annual market basket update for hospice providers by a productivity adjustment. We cannot predict
at this time whether the recommendations included in the 2009 MedPAC Report, the recommendation
approved by MedPAC on January 14, 2010 or the provisions included in the various health care reform
proposals will be enacted or whether any additional healthcare reform initiatives will be
implemented or whether other changes in the administration of governmental healthcare programs or
interpretations of governmental policies or other changes affecting the healthcare system will
occur.
Due to budgetary concerns, several states have considered or are considering reducing or
eliminating the Medicaid hospice benefit.
Reductions or changes in Medicare or Medicaid funding could significantly reduce our net
patient service revenue and our profitability. We cannot predict at this time whether the
recommendations included in the 2009 MedPAC Report or MedPAC’s January 14, 2010 recommendation will
be enacted or whether any additional healthcare reform initiatives will be implemented or whether
other changes in the administration of governmental healthcare programs, including the elimination
of Medicaid hospice benefits, or interpretations of governmental policies or other changes
affecting the healthcare system will occur. Reductions in amounts paid by government programs for
our services or changes in methods or regulations governing payments could cause our net patient
service revenue and profits to materially decline.
We are subject to a Medicare cap amount which is calculated by Medicare. Our net patient service
revenue and profitability could be adversely affected by limitations on Medicare payments.
Overall payments made by Medicare to us are subject to a cap amount calculated by the Medicare
fiscal intermediary at the end of the hospice cap period. The hospice cap period runs from November
1st of each year through October 31st of the following year. Total Medicare payments received by
each of our Medicare-certified programs during this period are compared to the cap amount for this
period. Payments in excess of the cap amount must be returned by us to Medicare. The cap amount is
calculated by multiplying the number of beneficiaries electing hospice care during the period by a
statutory Medicare cap amount that is indexed for inflation. The Medicare cap amount is reduced
proportionately for Medicare patients who transferred into or out of our hospice
25
Table of Contents
programs and either received or will receive hospice services from another hospice provider.
The Medicare cap amount for the twelve month period ending October 31, 2010 has not been
established by Medicare. Once published, the new Medicare cap amount will become effective
retroactively for all services performed since November 1, 2009. The hospice cap amount is computed
on a program-by-program basis. Our net patient service revenue for 2009 was reduced by
approximately $4.5 million as a result of our hospice programs exceeding the Medicare cap. Our
ability to comply with this limitation depends on a number of factors relating to a given hospice
program, including number of admissions, average length of stay, mix in level of care and Medicare
patients that transfer into and out of our hospice programs. Our revenue and profitability may be
materially reduced if we are unable to comply with this and other Medicare payment limitations. We
cannot assure you that additional hospice programs will not exceed the cap amount in the future or
that our estimate of the Medicare cap contractual adjustment will not materially differ from the
actual Medicare cap amount.
We operate in an industry that is subject to extensive federal, state and local regulation, and
changes in law and regulatory interpretations could reduce our net patient service revenue and
profitability.
The healthcare industry is subject to extensive federal, state and local laws, rules and
regulations relating to, among others:
| • | payment for services; | ||
| • | conduct of operations, including fraud and abuse, anti-kickback prohibitions, physician self-referral prohibitions and false claims; | ||
| • | privacy and security of medical records; | ||
| • | employment practices; and | ||
| • | facility and professional licensure, including certificates of need, surveys, certification and recertification requirements, and corporate practice of medicine prohibitions. |
In recent years, Congress and some state legislatures have introduced an increasing number of
proposals to make significant changes in the healthcare system. Changes in law and regulatory
interpretations could increase costs, reduce our net patient service revenue and profitability.
Recently, there have been heightened coordinated civil and criminal enforcement efforts by
both federal and state government agencies relating to the healthcare industry. There has also been
an increase in the filing of actions by private individuals on behalf of the federal government
against healthcare companies alleging the filing of false or fraudulent Medicare or Medicaid
claims. This heightened enforcement activity increases our potential exposure to damaging lawsuits,
investigations and other enforcement actions. Any such action could distract our management and
adversely affect our business reputation and profitability.
We were the subject of a civil investigation by the Civil Division of the United States
Department of Justice (“DOJ”). On July 6, 2006, we entered into a settlement agreement with the DOJ
to permanently settle the investigation. As part of the settlement of the investigation we entered
into a corporate integrity agreement on July 6, 2006 with the U.S. Department of Health and Human
Services, Office of Inspector General.
On February 14, 2008, we received a letter from the Medicaid Fraud Control Unit of the Texas
Attorney General’s office notifying us that it is conducting an investigation concerning Medicaid
hospice services provided by us, including our practices with respect to patient admission and
retention, and requesting medical records of approximately 50 patients served by our programs in
the State of Texas. Based on the preliminary stage of this investigation and the limited
information that we have at this time, we cannot predict the outcome of this investigation, the
Texas Attorney General’s views of the issues being investigated, any actions that the Texas
Attorney General may take or the impact, if any, that the investigation may have on our business,
results of operations, liquidity or capital resources. We believe that we are in material
compliance with the rules and regulations applicable to the Texas Medicaid hospice program. See
“Item 3. Legal Proceedings” and note “18. Commitments and Contingencies” to our consolidated
financial statements.
26
Table of Contents
On May 5, 2008, we received a letter from the United States Department of Justice (“DOJ”)
notifying us that it is conducting an investigation of VistaCare, Inc. and requesting that we
provide certain information and documents related to its investigation of claims submitted by
VistaCare to Medicare, Medicaid and TRICARE from January 1, 2003 through March 6, 2008, the date we
completed the acquisition of VistaCare. We have been informed by the DOJ and the Medicaid Fraud
Control Unit of the Texas Attorney General’s Office that they are reviewing allegations that
VistaCare may have billed the federal Medicare, Medicaid and TRICARE programs for hospice services
that were not reasonably or medically necessary or performed as claimed. The basis of the
investigation is a qui tam lawsuit filed in the United States District Court for the Northern
District of Texas by a former employee of VistaCare. The lawsuit was unsealed on October 5, 2009
and served on us on January 28, 2010. In connection with the unsealing of the complaint, the DOJ
filed a notice with the court declining to intervene in the qui tam action at this time. The Texas
Attorney General also filed a notice of non-intervention with the court. While these actions
should not be viewed as a final assessment by the DOJ or the Texas Attorney General of the merits
of this qui tam action, we consider them to be positive developments. We continue to cooperate
with the DOJ and the Texas Attorney General in their investigation. Based on the limited
information that we have at this time we cannot predict the outcome of the investigation, the DOJ’s
or Texas Attorney General’s views of the issues being investigated, other than the DOJ’s and Texas
Attorney General’s notice declining to intervene in the qui tam action at this time, any actions
that the DOJ or Texas Attorney General may take or the impact, if any, that the investigation may
have on our business, results of operations, liquidity or capital resources.
On January 5, 2009, we received a letter from the Georgia State Health Care Fraud Control Unit
notifying us that it is conducting an investigation concerning Medicaid hospice services provided
by VistaCare from 2003 through 2007 and requesting certain documents. We are cooperating with the
Georgia State Health Care Fraud Control Unit and have complied with the document request. Based on
the preliminary stage of this investigation and the limited information that we have at this time
we cannot predict the outcome of the investigation, the Georgia State Health Care Fraud Control
Unit’s views of the issues being investigated, any actions that the Georgia State Health Care Fraud
Control Unit may take or the impact, if any, that the investigation may have on our business,
results of operations, liquidity or capital resources.
On February 2, 2009, we received a subpoena from the OIG requesting certain documents related
to our provision of continuous care services from January 1, 2004 through February 2, 2009. On
September 9, 2009 we received a second subpoena from the OIG requesting medical records for certain
patients who had been provided continuous care services by us during the same time period. We are
cooperating with the OIG and are in the process of complying with the subpoena request. Based on
the preliminary stage of this investigation and the limited information that we have at this time
we cannot predict the outcome of the investigation, the OIG’s views of the issues being
investigated, any actions that the OIG may take or the impact, if any, that the investigation may
have on our business, results of operations, liquidity or capital resources.
On February 23, 2010, we received a subpoena from the OIG requesting various documents and
certain patient records of one our hospice programs relating to services performed from January 1,
2006 through December 31, 2009. We are cooperating with the OIG and are in the process of
complying with the subpoena request. Because of the preliminary stage of this investigation and
the limited information that we have at this time we cannot predict the outcome of the
investigation, the OIG’s views of the issues being investigated, any actions that the OIG may take
or the impact, if any, that the investigation may have on our business, results of operations,
liquidity or capital resources.
In the future, different interpretations or enforcement of laws, rules and regulations
governing the healthcare industry could subject our current business practices to allegations of
impropriety or illegality or could require us to make changes in our facilities, equipment,
personnel, services and capital expenditure programs, increase our operating expenses and distract
our management. If we fail to comply with these extensive laws and government regulations, we could
become ineligible to receive government program payments, suffer civil and criminal penalties or be
required to make significant changes to our operations. In addition, we could be forced to expend
considerable resources responding to an investigation or other enforcement action under these laws
or regulations. For a more detailed discussion of the regulatory environment in which we operate,
see “Item 1. Business — Government Regulation and Payment Structure.”
27
Table of Contents
Approximately 35% of our hospice patients reside in nursing homes. Changes in the laws and
regulations regarding payments for hospice services and “room and board” provided to our hospice
patients residing in nursing homes could reduce our net patient service revenue and profitability.
For our hospice patients receiving nursing home care under certain state Medicaid programs who
elect hospice care under Medicare or Medicaid, the state must pay us, in addition to the applicable
Medicare or Medicaid hospice per diem rate, an amount equal to at least 95% of the Medicaid per
diem nursing home rate for “room and board” furnished to the patient by the nursing home. We
contract with various nursing homes for the nursing homes’ provision of certain “room and board”
services that the nursing homes would otherwise provide Medicaid nursing home patients. We bill and
collect from the applicable state Medicaid program an amount equal to at least 95% of the amount
that would otherwise have been paid directly to the nursing home under the state’s Medicaid plan.
Under our standard nursing home contracts, we pay the nursing home for these “room and board”
services at 100% of the Medicaid per diem nursing home rate.
Government studies conducted in the last several years have suggested that the reimbursement
levels for hospice patients living in nursing homes may be excessive. In particular, the federal
government has expressed concern that hospice programs may provide fewer services to patients
residing in nursing homes than to patients living in other settings due to the presence of the
nursing home’s own staff to address problems that might otherwise be handled by hospice personnel.
Because hospice programs are paid a fixed per diem amount, regardless of the volume or duration of
services provided, the government is concerned that hospice programs may be increasing their
profitability by shifting the cost of certain patient care services to the nursing home.
The reduction or elimination of Medicare payments for hospice patients residing in nursing
homes would significantly reduce our net patient service revenue and profitability. In addition,
changes in the way nursing homes are reimbursed for “room and board” services provided to hospice
patients residing in nursing homes could affect our ability to obtain referrals from nursing homes.
A reduction in referrals from nursing homes would adversely affect our net patient service revenue
and profitability.
If we are unable to maintain relationships with existing patient referral sources or to establish
new referral sources, our growth and profitability could be adversely affected.
Our success is heavily dependent on referrals from physicians, nursing homes, assisted living
facilities, adult care centers, hospitals, managed care companies, insurance companies and other
patient referral sources in the communities that our hospice locations serve, as well as on our
ability to maintain good relations with these referral sources. Our referral sources are not
contractually obligated to refer hospice patients to us and may refer their patients to other
hospice care providers, or not at all. Our growth and profitability depend significantly on our
ability to provide good patient and family care, to establish and maintain close working
relationships with these patient referral sources and to increase awareness and acceptance of
hospice care by our referral sources and their patients. We cannot assure you that we will be able
to maintain our existing referral source relationships or that we will be able to develop and
maintain new relationships in existing or new markets. Our loss of existing relationships or our
failure to develop new relationships could adversely affect our ability to expand our operations
and operate profitably. Moreover, we cannot assure you that awareness or acceptance of hospice care
will increase.
Our growth strategy to develop new hospice programs in new and existing markets may not be
successful, which could adversely impact our growth and profitability.
An element of our growth strategy is expansion of our business by developing new hospice
programs in new markets and growth in our existing markets. This aspect of our growth strategy may
not be successful, which could adversely impact our growth and profitability. We cannot assure you
that we will be able to:
| • | identify markets that meet our selection criteria for new hospice programs; | ||
| • | hire and retain a qualified management team to operate each of our new hospice programs; | ||
| • | manage a large and geographically diverse group of hospice programs; | ||
| • | become Medicare and Medicaid certified in new markets; | ||
| • | generate sufficient hospice admissions in new markets to operate profitably in these new markets; or | ||
| • | compete effectively with existing programs in new markets. |
28
Table of Contents
Our growth strategy to acquire other hospices may not be successful and the integration of future
acquisitions may be difficult and disruptive to our ongoing business.
In addition to growing existing programs and developing new hospice programs, an element of
our growth strategy is expansion through the acquisition of other hospice programs. We cannot
assure you that our acquisition strategy will be successful. The success of our acquisition
strategy is dependent upon a number of factors, including:
| • | our ability to identify suitable acquisition candidates; | ||
| • | our ability to negotiate favorable acquisition terms, including purchase price, which may be adversely affected due to increased competition with other buyers; | ||
| • | the availability of financing on terms favorable to us, or at all; | ||
| • | our ability to integrate effectively the systems and operations of acquired hospices; | ||
| • | our ability to retain key personnel of acquired hospices; and | ||
| • | our ability to obtain required regulatory approvals. |
Acquisitions involve a number of other risks, including diversion of management’s attention
from other business concerns and the assumption of known or unknown liabilities of acquired
hospices, including liabilities for failure to comply with healthcare laws and regulations. The
integration of acquired hospices may place significant strains on our current operating and
financial systems and controls. We may not successfully overcome these risks or any other problems
encountered in connection with our acquisition strategy.
According to MedPAC, an estimated 35% of hospice programs in the United States are
not-for-profit programs. Accordingly, it is likely that a substantial number of acquisition
opportunities may involve hospices operated by not-for-profit entities. In recent years, several
states have increased review and oversight of transactions involving the sale of healthcare
facilities by not-for-profit entities. Although the level of review varies from state to state, the
current trend is to provide for increased governmental review, and in some cases approval, of
transactions in which a not-for-profit entity sells a healthcare facility or business. This
increased scrutiny may increase the difficulty in completing, or prevent the completion of,
acquisitions in some states in the future.
Our loss of key senior management personnel or our inability to hire and retain skilled employees
at a reasonable cost could adversely affect our business and our ability to increase patient
referrals.
Our future success depends, in significant part, upon the continued service of our key senior
management personnel. The loss of services of one or more of our key senior management personnel or
our inability to hire and retain new skilled employees could adversely affect our future operating
results. In addition, the loss of key CERs could negatively impact our ability to maintain or
increase patient referrals, a key aspect of our growth strategy.
Competition for skilled employees is intense, and the process of locating and recruiting
skilled employees with the combination of qualifications and attributes required to care
effectively for terminally ill patients and their families can be difficult and lengthy. We cannot
assure you that we will be successful in attracting, retaining or training highly skilled nursing,
management, CERs, administrative, admissions and other personnel. Our business could be disrupted
and our growth and profitability negatively impacted if we are unable to attract and retain skilled
employees.
29
Table of Contents
A nationwide shortage of qualified nurses could adversely affect our profitability and our ability
to grow and continue to provide quality, responsive hospice services to our patients as nursing
wages and benefits increase.
We currently employ approximately 1,800 full-time nurses and 50 part-time nurses. We depend on
qualified nurses to provide quality, responsive hospice services to our patients. There is
currently a nationwide shortage of qualified nurses that is being felt in some of the markets in
which we provide hospice services. In response to the shortage of qualified nurses in these
markets, we have increased and are likely to continue to increase our wages and benefits to recruit
and retain nurses or to engage contract nurses until we hire permanent staff nurses. Our inability
to attract and retain qualified nurses could adversely affect our ability to provide quality,
responsive hospice services to our patients and our ability to increase patient census in those
markets. In addition, because we operate in a fixed reimbursement environment, increases in the
wages and benefits that we must provide to attract and retain qualified nurses or an increase in
our reliance on contract nurses will negatively impact our profitability.
Medical reviews and audits by governmental and private payors could result in material payment
recoupments and payment denials, which could negatively impact our business.
Medicare fiscal intermediaries, other payors and government contractors periodically conduct
pre-payment and post-payment medical reviews and other audits of our reimbursement claims. In order
to conduct these reviews, the payor or contractor requests documentation from us and then reviews
that documentation to determine compliance with applicable rules and regulations, including the
eligibility of patients to receive hospice benefits, the appropriateness of the care provided to
those patients, and the documentation of that care. As a result of such reviews, we could be
required to return any amounts found to be overpaid, or amounts found to be overpaid could be
recouped through reductions in future payments. There is increasing pressure from state and
federal governments and other payors to scrutinize health care claims to determine their validity
and appropriateness. During the past several years our claims have been subject to reviews and
audits which have resulted in recoupments and offsets. We cannot predict whether medical reviews or
similar audits by federal or state agencies, commercial payors, or government contractors of our
hospice programs’ reimbursement claims will result in material recoupments or denials, which could
have a material adverse effect on our financial condition, cash flows and results of operations.
CMS has contracted with four Recovery Audit Contractors (“RACs”) to perform post-payment
reviews of health care providers. In January 2010, CMS announced that it has approved two issues
for the RACs to begin reviewing with respect to hospice providers. These initial hospice reviews
will focus on durable medical equipment services and other Medicare Part A and B services provided
to hospice patients that are related to a patient's terminal
prognosis and the financial obligation of the hospice provider to
determine whether the hospice provider arranged for and paid for the
services as required. We expect in the future that CMS will likely expand the scope of the reviews
conducted by the RACs. We cannot predict whether reviews by RACs of our hospice programs’
reimbursement claims will result in material recoupments, which could have a material adverse
affect on our financial condition and results of operations.
If any of our hospice programs fails to comply with the Medicare conditions of participation, that
program could be terminated from the Medicare program, thereby adversely affecting our net patient
service revenue and profitability.
Each of our hospice programs must comply with the extensive conditions of participation of the
Medicare hospice benefit. If any of our hospice programs fails to meet any of the Medicare
conditions of participation, that program may receive a notice of deficiency from the applicable
state surveyor. If that hospice program then fails to institute a plan of correction and correct
the deficiency within the correction period provided by the state surveyor, that program could be
terminated from receiving Medicare payments. For example, under the Medicare hospice program, each
of our hospice programs must demonstrate that volunteers provide administrative and direct patient
care services in an amount equal to at least five percent of the total patient care hours provided
by our employees and contract staff at the hospice program. If we are unable to attract a
sufficient number of volunteers at one of our hospice programs to meet this requirement, that
program could be terminated from the Medicare benefit if the program fails to address the
deficiency within the applicable correction period. Any termination of one or more of our hospice
programs from the Medicare program for failure to satisfy the volunteer or other conditions of
participation could adversely affect our net patient service revenue and profitability and
financial condition. We believe that we are in compliance with the conditions of participation;
however, we cannot predict how surveyors will interpret all aspects of the Medicare conditions of
participation.
30
Table of Contents
Many states have certificate of need laws or other regulatory provisions that may adversely impact
our ability to expand into new markets and thereby limit our ability to grow and to increase our
net patient service revenue.
Many states have enacted certificate of need laws that require prior state approval to open
new healthcare facilities or expand services at existing facilities. Those laws require some form
of state agency review or approval before a hospice may add new services or undertake significant
capital expenditures. New York has additional barriers to entry. New York places restrictions on
the corporate ownership of hospices. Accordingly, our ability to operate in New York is restricted.
These laws could adversely affect our ability to expand into new markets and to expand our services
and facilities in existing markets.
We may not be able to compete successfully against other hospice providers, and competitive
pressures may limit our ability to maintain or increase our market position and adversely affect
our profitability.
Hospice care in the United States is competitive. In many areas in which our hospice programs
are located, we compete with a large number of organizations, including:
| • | community-based hospice providers; | ||
| • | national and regional companies; | ||
| • | hospital-based hospice and palliative care programs; | ||
| • | nursing homes; and | ||
| • | home health agencies. |
Some of our current and potential competitors have or may obtain significantly greater
financial and marketing resources than us. Various healthcare companies have diversified into the
hospice market. For example, a few large healthcare providers, including Golden Living (formerly
Beverly Enterprises, Inc.) and Manor Care, Inc., have entered the hospice business directly or
through affiliates. Relatively few barriers to entry exist in our local markets. Accordingly, other
companies, including hospitals and other healthcare organizations that are not currently providing
hospice care, may expand their services to include hospice care or similar services. We may
encounter increased competition in the future that could negatively impact patient referrals to us,
limit our ability to maintain or increase our market position and adversely affect our
profitability.
If our costs were to increase more rapidly than the fixed payment adjustments we receive for our
hospice services from Medicare and Medicaid, our profitability could be negatively impacted.
We generally receive fixed payments for our hospice services based on the level of care we
provide to patients and their families. Accordingly, our profitability is largely dependent on our
ability to manage costs of providing hospice services. Medicare and Medicaid currently provide for
an annual adjustment of the various hospice payment rates based on the increase or decrease of the
medical care expenditure category of the Consumer Price Index; however, the increases have usually
been less than actual inflation. If this adjustment were eliminated or reduced, or if our costs of
providing hospice services, over one-half of which consist of labor costs, which have been rising,
increased more than the annual adjustment, our profitability could be negatively impacted. In
addition, cost pressures resulting from shorter patient lengths of stay and the use of more
expensive forms of palliative care, including drugs and drug delivery systems, could negatively
impact our profitability.
On July 31, 2008, CMS published the final rule that modified the hospice wage index by phasing
out over a three year period the budget neutrality adjustment factor. According to the final rule,
the phase-out would occur over a three year period beginning on October 1, 2008, with 25% of the
phase-out becoming effective on October 1, 2008, 50% becoming effective on October 1, 2009 and the
balance on October 1, 2010. As part of the American Recovery and Reinvestment Act of 2009, the
implementation of the phase-out of the budget neutrality adjustment factor was delayed until
October 1, 2009. CMS began paying providers the estimated 1.1% increase in hospice rates from
October 1, 2008 in the middle of 2009. On July 30, 2009, CMS issued a final rule to update the
Medicare hospice wage index. The final rule also re-implemented the phase-out of the budget
neutrality adjustment factor beginning
31
Table of Contents
on October 1, 2009. The phase-out of the budget neutrality adjustment factor will now occur
over a seven year period, 10% in the first year and an additional 15% in each of the following six
years. On October 1, 2009, payments to Medicare participating hospices increased by approximately
1.4%. This increase includes the effect of the first year phase-out of the budget neutrality
adjustment factor used in computing the hospice wage index.
On January 14, 2010 MedPAC voted to recommend that the Congress should reduce the annual
market basket update for hospice providers on October 1, 2010 by MedPAC’s adjustment for
productivity growth, which is estimated to be 1.3%. In addition, both the Senate and House of
Representatives have passed separate health care reform bills. Each of these bills include several
provisions that would adversely impact hospice providers, including a provision to reduce the
annual market basket update for hospice providers by a productivity adjustment.
Reductions or changes in Medicare or Medicaid funding could significantly reduce our net
patient service revenue and our profitability. We cannot predict at this time whether additional
payment changes or reductions in our payments will be enacted or whether any additional healthcare
reform initiatives will be implemented or whether other changes in the administration of
governmental healthcare programs or interpretations of governmental policies or other changes
affecting the healthcare system will occur.
Federal and state legislative and regulatory initiatives relating to patient privacy could require
us to expend substantial sums on acquiring and implementing new information systems.
There are currently numerous legislative and regulatory initiatives at both the state and
federal levels that address patient privacy concerns. In particular, HIPAA contains provisions that
have required us to implement new systems and business procedures designed to protect the privacy
and security of each of our patient’s individual health information. The Department of Health and
Human Services published final regulations addressing patient privacy on December 28, 2000,
transaction and code set final regulations on September 23, 2003, and final regulations addressing
the security of such health information on February 20, 2003. We believe we are in compliance with
the requirements of the privacy regulations, transaction and code set regulations, and security
regulations. We continue to evaluate and update our processes and procedures to meet the
requirements of the standards; however, we cannot assure you that all of the parties with whom we
do business will be in compliance with HIPAA. Additional legislative and regulatory initiatives and
changes in the interpretation of existing legislative and regulatory initiatives regarding patient
privacy could result in additional operating costs, which could materially adversely affect our
profitability.
The American Recovery and Reinvestment Act of 2009 made several significant changes to the
HIPAA privacy and security requirements. These changes include mandatory notification of breaches
of privacy and security involving protected health information to the affected individuals, the
Department of Health and Human Services and, in certain circumstances, the media. In addition,
several changes were made to increase enforcement of the HIPAA privacy and security requirements,
including giving state attorneys general new civil enforcement authority related to violations of
HIPAA’s privacy and security provisions and requiring the Department of Health and Human Services
to conduct periodic audits of covered entities. Because of the recent enactment of these changes
and the lack of regulatory guidance we cannot assure you that these changes will not have a
material adverse affect on us once they are fully implemented.
Our net patient service revenue and profitability may be constrained by cost containment
initiatives undertaken by insurers and managed care companies.
Initiatives undertaken by insurers and managed care companies to contain healthcare costs
affect the profitability of our hospice programs. We have a number of contractual arrangements with
insurers and managed care companies for providing hospice care for a fixed fee. These payors
attempt to control healthcare costs by contracting with hospices and other healthcare providers to
obtain services on a discounted basis. We believe that this trend may continue and may limit
payments for healthcare services, including hospice services. In addition, future changes in
Medicare related to Medicare Advantage programs could result in managed care companies becoming
financially responsible for providing hospice care. If such changes were to occur, managed care
companies could be responsible for payments to us out of their Medicare payments, and a greater
percentage of our net patient service revenue could come from managed care companies. As managed
care companies attempt to control hospice-related costs, they could reduce payments to us for
hospice services. These developments could negatively impact our net patient service revenue and
profitability.
32
Table of Contents
A significant reduction in the carrying value of our goodwill could have a material adverse effect
on our profitability.
A significant portion of our total assets consists of intangible assets, primarily goodwill.
Goodwill accounted for approximately 38.1% and 41.1% of our total assets as of December 31, 2009
and 2008, respectively. We perform our annual impairment testing as of November 30th
each year. In determining the fair value of our reporting units, we use multiples of earnings
before interest, taxes, depreciation and amortization (“EBITDA”). We believe using multiples of
EBITDA in determining the fair value of our reporting units is appropriate because it correlates
with what a market participant would be wiling to pay for that unit in today’s market. As of
November 30, 2009,, none of our reporting units failed step one and no reporting units were at risk
of failing step one. Furthermore, the fair values of each of our reporting units represented no
less than 185% of their carrying values. Prior to filing the Form 10-K, we evaluated whether any
events had occurred or any circumstances had changed since November 30, 2009 that would indicate
goodwill may have become impaired since the annual impairment testing. In this evaluation, we
considered both qualitative and quantitative factors such as any adverse change in the business
climate, current estimates of future profitability of reporting units, our current stock price and
our market capitalization compared to our book value. Based on this evaluation, we determined that
no indications of impairment had arisen since our annual goodwill impairment test. No impairment
charges have been recorded as of December 31, 2009, 2008 and 2007. Any future event that results
in a significant impairment of our goodwill, such as closure of a hospice program, changes in our
operating segments, sustained operating losses, or a significant decrease in our market
capitalization, could have a material adverse effect on our profitability.
Professional and general liability claims and hired and non-owned auto liability claims may have
an adverse effect on us either because our insurance coverage may be inadequate to cover the
losses or because claims against us, regardless of merit or eventual outcome, may adversely affect
our reputation, our ability to obtain patient referrals or our ability to expand our business.
In recent years, participants in the healthcare industry have become subject to an increasing
number of lawsuits, including allegations of medical malpractice. Many of these lawsuits involve
large claims and substantial defense costs. From time to time, we are subject to these types of
lawsuits. While we maintain professional and general liability insurance, some risks and
liabilities, including claims for punitive damages, are not covered by insurance. In addition, we
cannot assure you that our coverage will be adequate to cover potential losses. While we have been
able to obtain liability insurance in the past, insurance can be expensive and may not be available
in the future on terms acceptable to us, or at all. Claims, regardless of their merit or eventual
outcome, may also adversely affect our reputation, our ability to obtain patient referrals or our
ability to expand our business, as well as divert management resources from the operation of our
business.
We have a $250,000 deductible per occurrence under our hired and non-owned auto insurance
coverage. One or more severe auto accidents involving our employees could result in a significant
liability expense and corresponding reduction in profitability. We continue to evaluate our
insurance program for cost effective alternative insurance coverage. We cannot assure you that we
will be able to obtain cost effective insurance to adequately cover this risk.
An adverse ruling against us in certain litigation could have an adverse effect on our financial
condition and results of operations.
We are involved in litigation incidental to the conduct of our business currently and from
time to time. The damages claimed against us in some of these cases are substantial. See the “Item
3. Legal Proceedings” for a discussion of these litigation matters.
We cannot assure you that we will prevail in the pending cases. In addition to the possibility
of an adverse outcome, such litigation is costly to manage, investigate and defend, and the related
defense costs, diversion of management’s time and related publicity may adversely affect the
conduct of our business and the results of our operations.
33
Table of Contents
Because of conditions in the credit markets we may not be able to access our funds that are
currently invested in auction rate securities without incurring a substantial loss on the
disposition of such securities.
At December 31, 2009, we had $12.4 million in tax exempt auction rate securities (“ARS”) which
are carried at fair value and classified as long-term investments. The principal balance associated
with the ARS is $13.0 million. The ARS held by us are private placement securities for which the
interest rates are reset every 35 days. The reset dates have historically provided a liquid market
for these securities as investors historically could readily sell their investments. These types of
securities generally have not experienced payment defaults and are backed by student loans, which
carry guarantees as provided for under the Federal Family Education Loan Program of the U.S.
Department of Education and all were AAA/Aaa rated at December 31, 2009. To date we have collected
all interest payments on all of our ARS when due and expect to continue to do so in the future. We
intended to liquidate all of our ARS prior to the end of 2009. However, due to the problems
experienced in global credit and capital markets generally and the ARS market in particular, our
ability to liquidate our ARS this year has been impaired. We successfully liquidated $4.1 million
of ARS in October 2009, $8.0 million in July 2008, $8.0 million in June 2008, and $8.4 million in
January 2008, all at par. The remaining principal balance of $13.0 million associated with ARS will
not be accessible until successful ARS auctions occur, a buyer is found outside of the auction
process, the issuers establish a different form of financing to replace these securities, issuers
repay principal over time from cash flows prior to maturity, or final payments come due according
to contractual maturities ranging from 25 to 28 years.
If the uncertainties in the credit and capital markets continue or these markets deteriorate
further, these securities may not provide liquidity to us when needed or maintain the fair values
estimated by us. If we had to liquidate any ARS at this time, we could incur significant losses. We
currently believe that we have sufficient liquidity for our current needs without selling any ARS
and do not currently intend to attempt to liquidate these securities until market conditions
improve and it is not more likely than not that we will be required to liquidate any ARS before
recovery of our entire cost basis. If our currently available resources are not sufficient for our
needs and we are not able to liquidate any ARS on acceptable terms on a timely basis, it could have
a significant impact on our cash flows, financial condition and results of operations.
We may need additional capital to fund our operations and finance our growth, and we may not be
able to obtain it on terms acceptable to us, or at all.
In connection with our acquisition of VistaCare, we entered into a Second Amended and Restated
Credit Agreement (the “Credit Agreement”) on February 28, 2008 with General Electric Capital
Corporation and certain other lenders that provides us with a $130 million term loan (the “Term
Loan”) and a $30 million revolving line of credit. The Term Loan was used to pay a portion of the
purchase price and costs incurred with respect to the acquisition of VistaCare. We expect that our
existing funds, cash flows from operations and borrowings under the Credit Agreement will be
sufficient to fund our working capital needs, anticipated hospice development and acquisition
plans, debt service requirements, and other anticipated capital requirements for at least 12 months
following the date of this Annual Report on Form 10-K. Continued expansion of our business through
the development of new hospice programs, inpatient business development and acquisitions may
require additional capital, in particular if we were to accelerate our hospice program development
and acquisition plans. In the past, we have relied on funds raised through our initial public
offering and private issuances of debt and equity and also through bank financing and cash flows
from operations to support our growth. In the future, required financing may not be available or
may be available only on terms that are not favorable to us. If we are unable to raise additional
funds, we may have to delay or abandon some or all of our growth strategies. Further, if additional
funds are raised through the issuance of additional equity securities, the percentage ownership of
our stockholders would be diluted. Any new equity securities may have rights, preferences or
privileges senior to those of our common stock.
The Credit Agreement contains, and future debt agreements may contain, various covenants that
limit our discretion in the operation of our business.
The Credit Agreement and related documents contain, and the agreements and instruments
governing future credit facilities may contain, various restrictive covenants that, among other
things, require us to comply with or maintain certain financial tests and ratios, may require us to
make mandatory prepayments of principal and may restrict our ability to:
| • | incur more debt; |
34
Table of Contents
| • | redeem or repurchase stock, pay dividends or make other distributions; | ||
| • | make certain investments; | ||
| • | create liens; | ||
| • | enter into transactions with affiliates; | ||
| • | make certain acquisitions; | ||
| • | merge or consolidate; and | ||
| • | transfer or sell assets. |
In addition, events beyond our control could affect our ability to comply with and maintain
these financial tests and ratios. Any failure by us to comply with or maintain all applicable
financial tests and ratios and to comply with all applicable covenants could result in an event of
default under the Credit Agreement or any other future debt agreements. This could lead to the
acceleration of the maturity of any outstanding loans, the termination of the commitments to make
further extensions of credit and the enforcement of other rights and remedies. Even if we are able
to comply with all applicable covenants, the restrictions on our ability to operate our business at
our discretion could harm our business by, among other things, limiting our ability to take
advantage of financing, mergers, acquisitions and other corporate opportunities.
We are dependent on the proper functioning of our information systems to efficiently manage our
business.
Our information systems are essential for providing billing and accounts receivable functions.
Our systems are vulnerable to various disasters, including fire, storms, loss of power, physical or
software break-ins and other such events. If our systems fail or are unavailable for any reasons,
our ability to maintain billing records or to pay our staff in a timely manner could be
jeopardized.
Our inability to effectively integrate, manage and keep secure our information systems could
disrupt our operations.
Our business depends on effective and secure information systems that assist us in, among
other things, processing claims, reporting financial results, managing regulatory compliance
controls and maintaining operational efficiencies. These systems include software developed
in-house and systems provided by external contractors and other service providers. To the extent
that these external contractors or other service providers become insolvent or fail to support the
software or systems, our operations could be negatively affected. Our hospice programs also depend
upon our information systems for accounting, billing, collections, payroll and other information.
If we experience a reduction in the performance, reliability, or availability of our information
systems, our operations and ability to produce timely and accurate reports could be adversely
affected.
Our information systems and applications require continual maintenance, upgrading and
enhancement to meet our operational needs. Our acquisition activity requires transitions and
integration of various information systems. We regularly upgrade and expand our information
systems’ capabilities. If we experience difficulties with the transition and integration of
information systems or are unable to implement, maintain, or expand our systems properly, we could
suffer from, among other things, operational disruptions, regulatory problems and increases in
administrative expenses.
We may be required to expend significant capital and other resources to protect against the
threat of security breaches or to alleviate problems caused by breaches, including unauthorized
access to patient data stored in our information systems, and the introduction of computer viruses
to our systems. Our security measures may be inadequate to prevent security breaches and our
business operations would be negatively impacted by cancellation of contracts and loss of patients
if security breaches are not prevented.
35
Table of Contents
Provisions in our charter documents, under Delaware law, and in our stockholder rights plan could
discourage a takeover that stockholders may consider favorable.
Our certificate of incorporation and bylaws may discourage, delay or prevent a merger or
acquisition that a stockholder may consider favorable because they:
| • | authorize the issuance by the board of directors of preferred stock without the requirement of stockholder approval, which could make it more difficult for a third party to acquire a majority of our outstanding voting stock; | ||
| • | provide for a classified board of directors with staggered, three-year terms; | ||
| • | prohibit cumulative voting in the election of directors; | ||
| • | prohibit our stockholders from acting by written consent; | ||
| • | limit the persons who may call special meetings of stockholders; | ||
| • | prohibit our stockholders from amending our bylaws unless the amendment is approved by the holders of at least 80% of our shares of common stock; and | ||
| • | establish advance notice requirements for nominations for election to the board of directors or for proposing matters to be approved by stockholders at stockholder meetings. |
In addition, our certificate of incorporation prohibits the amendment by our stockholders of
many provisions of our certificate of incorporation unless the amendment is approved by the holders
of at least 80% of our shares of common stock.
Delaware law may also discourage, delay or prevent someone from acquiring or merging with us.
Under Delaware law, a corporation may not engage in a business combination with any holder of 15%
or more of its capital stock until the holder has held the stock for three years unless, among
other possibilities, the board of directors approves the transaction. Our board of directors could
use this provision to prevent or delay takeovers.
In addition, purchase rights distributed under our stockholder rights plan will cause
substantial dilution to any person or group that attempts to acquire us without conditioning the
offer on our redemption of the rights.
These provisions could discourage potential acquisition proposals and could delay or prevent a
change of control transaction. As a result, they may limit the price investors may be willing to
pay for our stock in the future.
Item 1B. Unresolved Staff Comments
None.
Item 2. Properties
Our executive offices and Support Center are located at 717 N. Harwood, Suite 1500, Dallas,
Texas 75201, where we currently lease approximately 70,000 square feet of space. We believe that
these facilities are adequate for our current uses and that additional space is available to
accommodate our anticipated growth. Our Medicare-certified hospice programs and alternative
delivery sites, including our inpatient units, and our three hospice programs under development are
in leased and owned facilities in 29 states with lease terms expiring at various times through
February 2017. We own the land and building for two of our 20 inpatient units. We believe these
facilities are in good operating condition and suitable for their intended purposes. Refer to “Item
1. Business — Hospice Programs, Inpatient Facilities and Support Center” for a complete listing of
the locations of our Medicare-certified hospice programs and inpatient facilities.
36
Table of Contents
Item 3. Legal Proceedings
On February 14, 2008, we received a letter from the Medicaid Fraud Control Unit of the Texas
Attorney General’s office notifying us that it is conducting an investigation concerning Medicaid
hospice services provided by us, including our practices with respect to patient admission and
retention, and requesting medical records of approximately 50 patients served by our programs in
the State of Texas. Based on the preliminary stage of this investigation and the limited
information that we have at this time, we cannot predict the outcome of this investigation, the
Texas Attorney General’s views of the issues being investigated, any actions that the Texas
Attorney General may take or the impact, if any, that the investigation may have on our business,
results of operations, liquidity or capital resources. We believe that we are in material
compliance with the rules and regulations applicable to the Texas Medicaid hospice program.
On May 5, 2008, we received a letter from the United States Department of Justice (“DOJ”)
notifying us that it is conducting an investigation of VistaCare, Inc. and requesting that we
provide certain information and documents related to its investigation of claims submitted by
VistaCare to Medicare, Medicaid and TRICARE from January 1, 2003 through March 6, 2008, the date we
completed the acquisition of VistaCare. We were informed that the DOJ and the Medicaid Fraud
Control Unit of the Texas Attorney General’s Office are reviewing allegations that VistaCare may
have billed the federal Medicare, Medicaid and TRICARE programs for hospice services that were not
reasonably or medically necessary or performed as claimed. The basis of the investigation is a qui
tam lawsuit filed in the United States District Court for the Northern District of Texas by a
former employee of VistaCare. The lawsuit was unsealed on October 5, 2009 and served to us on
January 28, 2010. In connection with the unsealing of the complaint, the DOJ filed a notice with
the court declining to intervene in the qui tam action at this time. The Texas Attorney General
also filed a notice of non-intervention with the court. While these actions should not be viewed
as a final assessment by the DOJ or the Texas Attorney General of the merits of this qui tam
action, we consider them to be positive developments. We continue to cooperate with the DOJ and the
Texas Attorney General in their investigation of us. Based on the limited information that we have
at this time, we cannot predict the outcome of the qui tam lawsuit or the related investigation,
the DOJ’s or Texas Attorney General’s views of the issues being investigated other than the DOJ’s
and Texas Attorney General’s notice declining to intervene in the qui tam action at this time, any
actions that the DOJ or the Texas Attorney General may take or the impact, if any, that the
investigation may have on our business, results of operations, liquidity or capital resources.
We have been named in a class action lawsuit filed on November 6, 2008, in Superior Court of
California, Los Angeles County by Charlia Cornish (“Cornish”) alleging class-wide wage and hour
issues at its California hospice programs. The suit alleges failure to provide overtime
compensation, meal and break periods, accurate itemized wage statements, and timely payment of
wages earned upon leaving employment. The purported class includes all persons employed by us in
California as an admission nurse, a case manager registered nurse, a licensed vocational nurse, a
registered nurse, a home health aide, a medical social worker, a triage coordinator, an office
manager, a patient care secretary or a spiritual counselor at anytime on or after November 6, 2004.
The lawsuit seeks payment of unpaid wages, damages, interest, penalties and reasonable attorneys’
fees and costs. In January 2009 we successfully moved the lawsuit to Federal District Court in the
Central District of California. On September 21, 2009, the court ruled in our favor denying
plaintiff’s request to amend and granting us motion for summary judgment dismissing the lawsuit in
its entirety. The plaintiff has elected not to appeal the decision dismissing the plaintiff’s
lawsuit.
On January 5, 2009, we received a letter from the Georgia State Health Care Fraud Control Unit
notifying us that it is conducting an investigation concerning Medicaid hospice services provided
by VistaCare from 2003 through 2007 and requesting certain documents. We are cooperating with the
Georgia State Health Care Fraud Control Unit and have complied with the document request. Based on
the preliminary stage of this investigation and the limited information that we have at this time,
we cannot predict the outcome of the investigation, the Georgia State Health Care Fraud Control
Unit’s views of the issues being investigated, any actions that the Georgia State Health Care Fraud
Control Unit may take or the impact, if any, that the investigation may have on our business,
results of operations, liquidity or capital resources.
On February 2, 2009, we received a subpoena from the United States Office of Inspector General
(“OIG”) requesting certain documents related to our provision of continuous care services from
January 1, 2004 through February 2, 2009. On September 9, 2009, we received a second subpoena from
the OIG requesting medical records
37
Table of Contents
for certain patients who had been provided continuous care services by us during the same time
period. We are cooperating with the OIG and are in the process of complying with the subpoena
requests. Based on the preliminary stage of this investigation and the limited information that we
have at this time we cannot predict the outcome of the investigation, the OIG’s views of the issues
being investigated, any actions that the OIG may take or the impact, if any, that the investigation
may have on our business, results of operations, liquidity or capital resources.
On March 5, 2009, we received a notice submitted on behalf of Ronaldo Ramos to the California
Labor & Workforce Development Agency regarding his intent to file a claim for penalties pursuant to
the California Private Attorney General Act for alleged violations of the California Labor Code.
Ramos is a former employee of ours and alleges that he and others similarly situated were
improperly paid for on-call hours. His notice indicates that he intends to seek to recover unpaid
wages, overtime, penalties, punitive damages, interest, and attorney’s fees. We are not aware of
him filing a lawsuit. We believe that we have complied with all regulations at issue, and intend to
vigorously defend against the claims asserted. Because the matter is in its early stage, we cannot
at this time estimate an amount or range of potential loss in the event of an unfavorable outcome.
We have been named in a class action lawsuit filed on January 25, 2010, in the United States
District Court Southern District of Texas Houston Division by Bobby Blevins, a former employee,
alleging failure to pay overtime to a purported class of similarly situated hourly-paid current and
former nurse employees. The plaintiff seeks to recover unpaid overtime compensation, damages and
attorney fees. We believe that we have complied with all regulations at issue, and intend to
vigorously defend against the claims asserted. Because of the early stage of this suit, we cannot
at this time estimate an amount or range of potential loss in the event of an unfavorable outcome.
On February 23, 2010, we received a subpoena from the OIG requesting various documents and
certain patient records of one our hospice programs relating to services performed from January 1,
2006 through December 31, 2009. We are cooperating with the OIG and are in the process of
complying with the subpoena request. Because of the preliminary stage of this investigation and
the limited information that we have at this time we cannot predict the outcome of the
investigation, the OIG’s views of the issues being investigated, any actions that the OIG may take
or the impact, if any, that the investigation may have on our business, results of operations,
liquidity or capital resources.
From time to time, we may be involved in other litigation matters relating to claims that
arise in the ordinary course of its business. Although the ultimate liability for these matters
cannot be determined, based on the information currently available to us, we do not believe that
the resolution of these other litigation matters to which we are currently a party will have a
material adverse effect on our business, results of operations or liquidity. Accrued legal fees and
other reserves at December 31, 2009 and 2008, were $2.8 million and $2.3 million, respectively,
which primarily related to these other litigation matters.
Item 4. (Removed and Reserved)
38
Table of Contents
PART II
Item 5. Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of
Equity Securities
Market for Common Stock. Our common stock has been quoted on The NASDAQ Stock Market LLC
(formerly known as the Nasdaq National Market) (the “NASDAQ”) under the symbol “ODSY” since October
31, 2001. Prior to that time there was no public market for our
common stock. As of March 3, 2010,
there were 17 record holders of our common stock. The following table sets forth the high and low
sales price per share of our common stock for the period indicated on the NASDAQ:
| High | Low | |||||||
2008 |
||||||||
First Quarter |
$ | 11.15 | $ | 8.27 | ||||
Second Quarter |
$ | 11.28 | $ | 8.57 | ||||
Third Quarter |
$ | 10.99 | $ | 8.38 | ||||
Fourth Quarter |
$ | 10.47 | $ | 6.76 | ||||
2009 |
||||||||
First Quarter |
$ | 12.27 | $ | 8.49 | ||||
Second Quarter |
$ | 11.50 | $ | 8.11 | ||||
Third Quarter |
$ | 13.52 | $ | 9.58 | ||||
Fourth Quarter |
$ | 15.98 | $ | 11.69 | ||||
Dividends. We have never declared or paid any cash dividends on our common stock and do not
anticipate paying cash dividends in the foreseeable future. We currently intend to retain future
earnings, if any, to fund our development and acquisition initiatives and working capital needs.
The payment of any future dividends will be at the discretion of our board of directors and
will depend on:
| • | any applicable contractual restrictions limiting our ability to pay dividends; | ||
| • | our earnings; | ||
| • | our financial condition; | ||
| • | our ability to fund capital requirements; and | ||
| • | other factors our board of directors deems relevant. |
Recent Sales of Unregistered Securities. We did not sell any of our equity securities in the
three year period ended December 31, 2009 that were not registered under the Securities Act of
1933.
Repurchases of Common Stock. On November 21, 2006 we announced the adoption of a stock
repurchase program to repurchase up to $10.0 million of our common stock over a twelve month
period. The timing and the amount of the repurchase of shares during the twelve-month period was
determined by management based on its evaluation of market conditions and other factors. We
completed this stock repurchase program in May 2007 and repurchased an aggregate of 801,683 shares
of our common stock at a total cost of $10.0 million (average cost of $12.47 per share). Of this
amount, 59,477 shares for approximately $0.8 million was repurchased during the second quarter of
2007. The stock repurchases were funded out of our working capital.
39
Table of Contents
On May 4, 2007, we announced the adoption of a stock repurchase program to repurchase up to
$50.0 million of our common stock over the twelve month period beginning on May 4, 2007 either in
the open market or through privately negotiated transactions, subject to market conditions and
other factors. The repurchased shares were added to our treasury shares and may be used for
employee stock plans and for other corporate purposes. The stock repurchases were funded out of
working capital. The stock repurchase program expired on May 4, 2008. We repurchased 1,056,623
shares of common stock for approximately $13.1 million (average cost of $12.42 per share) during
this repurchase program.
No shares were repurchased during 2008 or 2009. The terms of our credit agreement may restrict
our ability to repurchase additional stock in the future.
Common Stock Performance Graph. The following information in this Item 5 of this Annual Report
on Form 10-K is not deemed to be “soliciting material” or to be “filed” with the Securities
Exchange Commission or subject to Regulation 14A or 14C under the Securities Exchange Act of 1934
or to the liabilities of Section 18 of the Securities Exchange Act of 1934, and will not be deemed
to be incorporated by reference into any filing under the Securities Act of 1933 or the Securities
Exchange Act of 1934, except to the extent we specifically incorporate it by reference into such a
filing.
The graph and table below compare the cumulative total shareholder return on our common stock
from December 31, 2004 through December 31, 2009, against the cumulative total share holder return
of the NASDAQ Composite Index and the stocks making up an industry peer group.
COMPARISON OF 5 YEAR CUMULATIVE TOTAL RETURN*
Among Odyssey HealthCare, Inc., The NASDAQ Composite Index And A Peer Group
Among Odyssey HealthCare, Inc., The NASDAQ Composite Index And A Peer Group
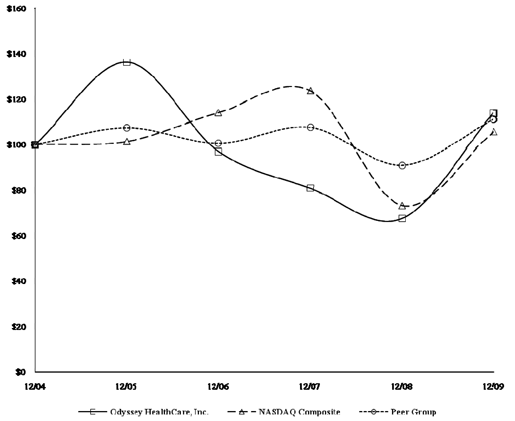
| * | $100 invested on 12/31/04 in stock or index, including reinvestment of dividends. Fiscal year ending December 31. |
40
Table of Contents
| Odyssey | Peer Group | NASDAQ Composite | ||||||||||
| HealthCare, Inc. | Index | Index | ||||||||||
12/31/05 |
$ | 136.26 | $ | 107.28 | $ | 101.33 | ||||||
12/31/06 |
96.93 | 100.54 | 114.01 | |||||||||
12/31/07 |
80.85 | 107.51 | 123.71 | |||||||||
12/31/08 |
67.62 | 90.82 | 73.11 | |||||||||
12/31/09 |
113.96 | 111.08 | 105.61 | |||||||||
The companies that comprise our Peer Group for purposes of stockholder return comparison are
as follows: Lincare Holdings, Inc., Amedisys, Inc., Getiva Health Services, Inc., and Chemed
Corporation. We include Chemed Corporation in our Peer Group, because Chemed Corporation’s
wholly-owned subsidiary, VITAS Healthcare Corporation, is one of the largest hospice providers in
the United States and is generally considered a peer by the investment community. Amedisys, Inc.
and Gentiva Health Services, Inc, are included in our Peer Group, because they provide hospice
services in addition to their core home health business, which is a non-facility based healthcare
service like hospice. Lincare Holdings, Inc. is included in our Peer Group, because it also
provides non-facility based healthcare services. We believe that our Peer Group is comparable to
us because they consist of primarily non-facility based healthcare services providers that are
generally characterized by relatively low levels of leverage, solid cash flow and multiple sources
of growth, including same store growth, de novo development and modest acquisition programs.
Item 6. Selected Financial Data
The selected consolidated statement of operations data set forth below for the years ended
December 31, 2009, 2008 and 2007 and the consolidated balance sheet data as of December 31, 2009
and 2008 are derived from our consolidated financial statements that have been audited by Ernst &
Young LLP, and that are included elsewhere in this Annual Report on Form 10-K, and are qualified by
reference to those consolidated financial statements. The selected consolidated statement of
operations data set forth below for the years ended December 31, 2006 and 2005
and the consolidated balance sheet data as of December 31, 2007, 2006 and 2005 are derived
from our consolidated financial statements that have been audited by Ernst & Young LLP, but are not
included in this Annual Report on Form 10-K.
The historical results presented below are not necessarily indicative of the results to be
expected for any future period. Prior periods are not comparable to the current period due to the
acquisition of VistaCare on March 6, 2008. Prior periods have been reclassified for discontinued
operations. You should read the selected financial information set forth below in conjunction with
“Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operation” and
our consolidated financial statements and the notes thereto appearing elsewhere in this Annual
Report on Form 10-K.
41
Table of Contents
| Year Ended December 31, | ||||||||||||||||||||
| 2009 | 2008 | 2007 | 2006 | 2005 | ||||||||||||||||
| (In thousands, except per share amounts) | ||||||||||||||||||||
Statements of Operations Data: |
||||||||||||||||||||
Net patient service revenue |
$ | 686,438 | $ | 616,050 | $ | 398,232 | $ | 379,218 | $ | 348,592 | ||||||||||
Operating expenses: |
||||||||||||||||||||
Direct hospice care |
396,774 | 361,445 | 233,664 | 222,496 | 193,823 | |||||||||||||||
General and administrative(1) |
199,069 | 199,292 | 131,788 | 115,771 | 102,980 | |||||||||||||||
Government settlement |
— | — | — | — | 13,000 | |||||||||||||||
Provision for uncollectible accounts |
11,490 | 10,907 | 5,344 | 4,007 | 4,023 | |||||||||||||||
Depreciation and amortization |
6,725 | 7,868 | 5,723 | 5,080 | 4,002 | |||||||||||||||
Total operating expenses |
614,058 | 579,512 | 376,519 | 347,354 | 317,828 | |||||||||||||||
Income from continuing operations before other
income (expense) |
72,380 | 36,538 | 21,713 | 31,864 | 30,764 | |||||||||||||||
Other income (expense): |
||||||||||||||||||||
Interest income |
479 | 1,968 | 2,509 | 2,576 | 1,341 | |||||||||||||||
Interest expense |
(6,574 | ) | (7,430 | ) | (208 | ) | (188 | ) | (198 | ) | ||||||||||
| (6,095 | ) | (5,462 | ) | 2,301 | 2,388 | 1,143 | ||||||||||||||
Income from continuing operations before
provision for income taxes |
66,285 | 31,076 | 24,014 | 34,252 | 31,907 | |||||||||||||||
Provision for income taxes |
24,583 | 11,141 | 8,001 | 12,124 | 13,263 | |||||||||||||||
Income from continuing operations |
41,702 | 19,935 | 16,013 | 22,128 | 18,644 | |||||||||||||||
Loss from discontinued operations, net of
income taxes(2) |
(498 | ) | (5,252 | ) | (3,888 | ) | (2,399 | ) | (88 | ) | ||||||||||
Net income |
41,204 | 14,683 | 12,125 | 19,729 | 18,556 | |||||||||||||||
Less: Net income attributable to
noncontrolling interests |
613 | 257 | 14 | — | — | |||||||||||||||
Net income attributable to Odyssey stockholders |
$ | 40,591 | $ | 14,426 | $ | 12,111 | $ | 19,729 | $ | 18,556 | ||||||||||
Income (loss) per common share: |
||||||||||||||||||||
Basic: |
||||||||||||||||||||
Continuing operations attributable to
Odyssey stockholders |
$ | 1.25 | $ | 0.60 | $ | 0.48 | $ | 0.65 | $ | 0.54 | ||||||||||
Discontinued operations attributable to
Odyssey stockholders |
$ | (0.02 | ) | $ | (0.16 | ) | $ | (0.12 | ) | $ | (0.07 | ) | $ | 0.00 | ||||||
Net income attributable to Odyssey
stocholders |
$ | 1.23 | $ | 0.44 | $ | 0.36 | $ | 0.58 | $ | 0.54 | ||||||||||
Diluted: |
||||||||||||||||||||
Continuing operations attributable to
Odyssey stockholders |
$ | 1.24 | $ | 0.59 | $ | 0.48 | $ | 0.64 | $ | 0.53 | ||||||||||
Discontinued operations attributable to
Odyssey stockholders |
$ | (0.02 | ) | $ | (0.16 | ) | $ | (0.12 | ) | $ | (0.07 | ) | $ | 0.00 | ||||||
Net income attributable to Odyssey
stocholders |
$ | 1.22 | $ | 0.43 | $ | 0.36 | $ | 0.57 | $ | 0.53 | ||||||||||
Weighted average shares outstanding: |
||||||||||||||||||||
Basic |
32,935 | 32,674 | 33,029 | 34,145 | 34,384 | |||||||||||||||
Diluted |
33,225 | 33,188 | 33,188 | 34,529 | 34,935 | |||||||||||||||
42
Table of Contents
| Year Ended December 31, | ||||||||||||||||||||
| 2009 | 2008 | 2007 | 2006 | 2005 | ||||||||||||||||
| (Unaudited) | ||||||||||||||||||||
| (Dollars in thousands) | ||||||||||||||||||||
Operating Data: |
||||||||||||||||||||
Number of
Medicare-certified
hospice programs(3) |
90 | 94 | 67 | 66 | 63 | |||||||||||||||
Admissions(4) |
49,513 | 46,772 | 32,246 | 32,001 | 30,972 | |||||||||||||||
Days of care(5) |
4,518,617 | 4,212,771 | 2,791,780 | 2,747,888 | 2,607,854 | |||||||||||||||
Average daily census(6) |
12,380 | 11,510 | 7,649 | 7,528 | 7,145 | |||||||||||||||
Cash flows provided by
operating activities |
$ | 81,650 | $ | 21,049 | $ | 12,814 | $ | 32,623 | $ | 58,171 | ||||||||||
Cash flows (used in)
provided by investing
activities |
$ | (4,083 | ) | $ | (97,187 | ) | $ | 4,391 | $ | (27,183 | ) | $ | (52,845 | ) | ||||||
Cash flows (used in)
provided by financing
activities |
$ | (4,978 | ) | $ | 119,795 | $ | (12,391 | ) | $ | (13,051 | ) | $ | (14,994 | ) | ||||||
| As of December 31, | ||||||||||||||||||||
| 2009 | 2008 | 2007 | 2006 | 2005 | ||||||||||||||||
| (Dollars in thousands) | ||||||||||||||||||||
Balance Sheet Data: |
||||||||||||||||||||
Working capital |
$ | 100,280 | $ | 82,429 | $ | 75,275 | $ | 70,555 | $ | 62,639 | ||||||||||
Total assets |
$ | 503,004 | $ | 460,951 | $ | 275,209 | $ | 269,986 | $ | 244,967 | ||||||||||
Total long-term debt, including current portion |
$ | 115,202 | $ | 123,075 | $ | 1 | $ | 3 | $ | 9 | ||||||||||
Total Odyssey stockholders’ equity |
$ | 248,751 | $ | 200,071 | $ | 182,837 | $ | 179,596 | $ | 167,298 | ||||||||||
| (1) | Includes share-based compensation of $5.1 million, $4.3 million, $3.8 million, $5.6 million and $0.7 million for the years ended December 31, 2009, 2008, 2007, 2006 and 2005, respectively. Also, general and administrative expenses include expenses for hospice care and support center. | |
| (2) | See the notes to our consolidated financial statements included elsewhere in this Annual Report on Form 10-K for a discussion of loss from discontinued operations, net of income taxes. | |
| (3) | Number of Medicare-certified hospice programs at end of each respective year. | |
| (4) | Represents the total number of patients admitted into our hospice programs during the period. | |
| (5) | Represents the total days of care provided to our patients during the period. | |
| (6) | Represents the average number of patients for whom we provided hospice care each day during the period and is computed by dividing days of care by the number of days during the period. |
43
Table of Contents
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations
The following discussion of our financial condition and results of operations should be read
in conjunction with our selected consolidated financial and operating data and the consolidated
financial statements and related notes included elsewhere in this Annual Report on Form 10-K.
Overview
We are one of the largest providers of hospice care in the United States in terms of both
average daily patient census and number of Medicare-certified hospice programs. As of December 31,
2009, we operated 90 Medicare-certified hospice programs, serving patients and their families in 29
states. We operate all of our hospice programs through our operating subsidiaries. Our net patient
service revenue of $686.4 million in 2009 represents an increase of 11.4% over net patient service
revenue of $616.0 million in 2008, and an increase of 72.4% over net patient service revenue of
$398.2 million in 2007. In 2009, 2008 and 2007, we reported net income attributable to Odyssey
stockholders of $40.6 million, $14.4 million and $12.1 million, respectively.
On March 6, 2008, we completed our acquisition of VistaCare. The transaction substantially
extended our industry leadership and geographic reach. We believe the transaction created
additional visibility that adds value to our marketing, recruiting and development activities.
Following the completion of this transaction, we had approximately 100 Medicare-certified hospice
programs in 30 states and an average daily census of more than 12,000 patients. During 2008 and
2009, we consolidated some markets in which both Odyssey and VistaCare had programs in the same
location. The operations of VistaCare were included in our results of operations beginning
February 29, 2008.
On November 21, 2006, we announced the adoption of a new stock repurchase program to
repurchase up to $10.0 million of our common stock over a twelve-month period. The timing and the
amount of any repurchase of shares during the twelve-month period was determined by management
based on its evaluation of market conditions and other factors. We completed this stock repurchase
program in May 2007 and repurchased an aggregate of 801,683 shares of common stock at a total cost
of $10.0 million (average cost of $12.47 per share). Of this amount, 59,477 shares for
approximately $0.8 million was repurchased in 2007. The stock repurchases were funded out of
working capital.
On May 4, 2007, we announced the adoption of a stock repurchase program to repurchase up to
$50.0 million of our common stock over the twelve month period beginning on May 4, 2007 either in
the open market or through privately negotiated transactions, subject to market conditions and
other factors. The repurchased shares were added to our treasury shares and may be used for
employee stock plans and for other corporate purposes. The stock repurchases were funded out of
working capital. The stock repurchase program expired on May 4, 2008. We repurchased 1,056,623
shares of common stock for approximately $13.1 million (average cost of $12.42 per share) during
this program.
No shares were repurchased during 2008 or 2009. The terms of our credit agreement may
restrict our ability to repurchase additional stock in the future.
Developed Hospices
We have developed the following hospice programs since January 1, 2007:
During 2007, we received Medicare certification for our Boston, Massachusetts; Ventura County,
California; and Fort Wayne, Indiana hospice programs. We continued the development of hospice
programs in Dayton, Ohio; Augusta, Georgia; and Alameda, California.
During 2008, we received Medicare certification for our Augusta, Georgia and Dayton, Ohio
programs. During the third quarter of 2008, we converted our Dayton, Ohio program to an alternate
delivery site of our Columbus, Ohio program. We continued the development of hospice programs in
Alameda, California and Salem, Oregon.
During 2009, we were still awaiting Medicare certification for our hospice programs in
Alameda, California and Salem, Oregon.
44
Table of Contents
Once a hospice becomes Medicare certified, the process is started to obtain Medicaid
certification. This process takes approximately six months and varies from state to state.
Acquisitions
During 2008, as discussed above, we completed the acquisition of VistaCare on March 6, 2008
for approximately $149.5 million which includes $2.4 million in transaction costs. We financed the
VistaCare acquisition primarily with a $130 million term loan from General Electric Capital
Corporation. See note “12. Borrowings” to our consolidated financial statements included elsewhere
in this Annual Report on Form 10-K.
On December 31, 2008, we acquired a hospice program in Flint, Michigan for approximately $0.5
million.
On December 31, 2009, we acquired a hospice program in Westchester, Illinois for approximately
$3.2 million.
As part of our ongoing acquisition strategy, we are continually evaluating other potential
acquisition opportunities.
Discontinued Operations
We conduct ongoing strategic reviews of our hospice programs and evaluate whether to sell or
close certain hospice programs based on these reviews. Our results of operations and statistics for
prior periods have been restated to reflect the reclassification of these programs to discontinued
operations. No programs were held for sale as of December 31, 2009. The Oklahoma City program and
the Oklahoma City inpatient unit were held for sale as of December 31, 2008.
During the first quarter of 2007, we announced that we would exit the Tulsa, Oklahoma hospice
market which was located in our Central region and in February 2007, we sold our Tulsa hospice
program. As part of the sale, the purchaser assumed the office lease and purchased certain assets
such as furniture/fixtures, equipment, deposits and licenses. We recognized an immaterial pretax
loss during the first quarter of 2007 related to the sale of the program.
During the second quarter of 2007, we decided to sell our Valdosta, Georgia; Columbia, South
Carolina; St. George, Utah; Rockford, Illinois; and Allentown, Pennsylvania hospice programs and
the Huntsville, Alabama alternate delivery site (“ADS”). We completed the sale of our Valdosta and
Columbia programs which were located in our Southeast region in June 2007 and recognized an
immaterial pretax loss in the second quarter on the sale of the programs. We completed the sale of
our Huntsville ADS and our St. George and Allentown programs which were located in our Southeast,
Mountain and Midwest regions, respectively, during the third quarter of 2007 and recognized an
immaterial pretax loss in the third quarter for the disposition of the programs. We completed the
sale of the Rockford program which was located in our Midwest region during the fourth quarter of
2007 and recognized an immaterial pretax gain in the fourth quarter on the sale of the Rockford
program.
During the fourth quarter of 2007, we decided to sell our Odessa, Texas; Big Spring, Texas;
Cincinnati, Ohio; and Wichita, Kansas hospice programs. We completed the sale of the Odessa and Big
Spring programs which were located in our Mountain region on January 1, 2008 and recognized an
immaterial pretax loss during the fourth quarter of 2007 related to these programs. We completed
the sale of the Cincinnati and Wichita programs, which were located in our Midwest and South
Central regions, respectively, during the first quarter of 2008 and no material amounts were
recorded as a result.
During the first quarter of 2008, we decided to sell our Baton Rouge, Louisiana; Ventura,
California; Fort Wayne, Indiana; and Oklahoma City, Oklahoma hospice programs, which were located
in our Southeast, West, Midwest and South Central regions, respectively. We also decided to close
the Bryan/College Station, Texas hospice program and the Dallas, Texas inpatient unit. The closures
of the Bryan/College Station program and Dallas inpatient unit, which were located in our Texas and
South Central regions, respectively, resulted in a pretax loss of $1.5 million during the first
quarter of 2008, which included an accrual of $1.2 million for future lease costs related to the
closed programs.
During the second quarter of 2008, we decided to close the Colorado Springs, Colorado
inpatient unit and the Tucson, Arizona VistaCare hospice program. The closures, which were located
in our Mountain and VistaCare West
45
Table of Contents
regions, respectively, resulted in a pretax loss of $2.3 million during the second quarter of
2008, which included an accrual of $2.1 million for future lease costs related to the closed
programs.
During the third quarter of 2008, we completed the sale of the Baton Rouge hospice program,
which was located in our Southeast region, and no material amounts were recorded as a result of the
sale.
During the fourth quarter of 2008, we completed the sale of the Ventura and Fort Wayne hospice
programs which were located in the West and Midwest regions, respectively, and recognized an
immaterial pretax gain for each of these programs.
During the second quarter of 2009, we recorded a pretax loss of approximately $0.6 million,
which was a result of the writedown of assets from $2.1 million to $1.5 million for the Oklahoma
City program, including the related inpatient unit. We completed the sale of the Oklahoma City
program, including the related inpatient unit, on July 13, 2009. The Oklahoma City program and
inpatient unit were located in our South Central region. Net proceeds from the sale were
approximately $1.5 million. The $1.5 million received in net proceeds was paid to our lenders as a
mandatory prepayment of principal.
Net Patient Service Revenue
Net patient service revenue is the estimated net realizable revenue (exclusive of our
provision for uncollectible accounts) from Medicare, Medicaid, commercial insurance, managed care
payors, patients and others for services rendered to our patients. To determine net patient service
revenue, we adjust gross patient service revenue for estimated contractual adjustments based on
historical experience and estimated Medicare cap contractual adjustments. Net patient service
revenue also does not include charity care or the Medicaid room and board payments. (See “Item 1.
Business — Government Regulation and Payment Structure- Overview of Government Payments”). We
recognize net patient service revenue in the month in which our services are delivered. Services
provided under the Medicare program represented approximately 93.1%, 92.5% and 92.4% of our net
patient service revenue for the years ended December 31, 2009, 2008 and 2007, respectively.
Services provided under Medicaid programs represented approximately 3.9%, 4.1% and 4.6% of our net
patient service revenue for the years ended December 31, 2009, 2008 and 2007, respectively. The
payments we receive from Medicare and Medicaid are calculated using daily or hourly rates for each
of the four levels of care we deliver and are adjusted based on geographic location.
The four main levels of care we provide are routine home care, general inpatient care,
continuous home care and inpatient respite care. We also receive reimbursement for physician
services, self-pay and non-governmental room and board. Routine home care is the largest component
of our gross patient service revenue, representing 89.2%, 89.7% and 88.5% of gross patient service
revenue for the years ended December 31, 2009, 2008 and 2007, respectively. General inpatient care
represented 7.6%, 7.2% and 7.4% of gross patient service revenue for the years ended December 31,
2009, 2008 and 2007, respectively. Continuous home care represented 2.1%, 2.1% and 3.2% of gross
patient service revenue for the years ended December 31, 2009, 2008 and 2007, respectively.
Inpatient respite care and reimbursement for physician services, self pay and non-governmental room
and board represents the remaining 1.1%, 1.0% and 0.9% of gross patient service revenue for these
periods, respectively.
The principal factors that impact net patient service revenue are our average daily census,
levels of care, annual changes in Medicare and Medicaid payment rates due to adjustments for
inflation and estimated Medicare cap contractual adjustments. Average daily census is affected by
the number of patients referred and admitted into our hospice programs and average length of stay
of those patients once admitted. Average length of stay is impacted by patients’ decisions of when
to enroll in hospice care after diagnoses of terminal illnesses and, once enrolled, the length of
the terminal illnesses. Our average length of stay was 82 days for the year ended December 31, 2009
and 85 days for the years ended December 31, 2008 and 2007, respectively.
Base payment rates for hospice services under the Medicare and Medicaid programs are indexed
for inflation annually; however, these increases have historically been less than actual inflation.
These rates are further adjusted geographically by the hospice wage index. On October 1, 2009 and
2008, the base Medicare payment rates for hospice care increased by approximately 2.1% and 3.6%,
respectively, over the base rates previously in effect. On July 31, 2008, CMS published the final
rule that modified the hospice wage index by phasing out over a three year period the budget
neutrality adjustment factor. According to the final rule the phase-out would occur over a three
46
Table of Contents
year period beginning on October 1, 2008, with 25% of the phase-out becoming effective on
October 1, 2008, 50% becoming effective on October 1, 2009 and the balance on October 1, 2010. As
part of the American Recovery and Reinvestment Act of 2009, the implementation of the phase-out of
the budget neutrality adjustment factor was delayed until October 1, 2009. CMS began paying
providers the estimated 1.1% increase in hospice rates from October 1, 2008 in the middle of 2009.
This increase resulted in additional revenues for 2009 from Medicare and Medicaid of $1.4 million
related to services performed from October 1, 2008 through December 31, 2008. On July 30, 2009,
CMS issued a final rule to update the Medicare hospice wage index. The final rule also
re-implemented the phase-out of the budget neutrality adjustment factor beginning on October 1,
2009. The phase-out of the budget neutrality adjustment factor will occur over a seven year
period, 10% in the first year and an additional 15% in each of the following six years. On October
1, 2009, payments to Medicare participating hospices increased by approximately 1.4%. This
increase includes the effect of the first year phase-out of the budget neutrality adjustment factor
used in computing the hospice wage index.
Expenses
Because payments for hospice services are primarily paid on a per diem basis, our
profitability is largely dependent on our ability to manage the expenses of providing hospice
services. We recognize expenses as incurred and classify expenses as either direct hospice care
expenses or general and administrative expenses. Direct hospice care expenses primarily include
direct patient care salaries, payroll taxes, employee benefits, pharmaceuticals, medical equipment
and supplies, inpatient costs and reimbursement of mileage for our patient caregivers. Length of
stay impacts our direct hospice care expenses as a percentage of net patient service revenue
because, if lengths of stay decline, direct hospice care expenses, which are often highest during
the earliest and latter days of care for a patient, are spread against fewer days of care. Expenses
are generally higher during the earliest days because of increased labor expense to evaluate the
patient and determine the medical and social services needs of the family. Expenses are also
normally higher during the last days of care because patients generally require greater hospice
services including drugs, medical equipment and nursing care at that time due to their
deteriorating medical condition. In addition, cost pressures resulting from the use of more
expensive forms of palliative care, including drugs and drug delivery systems, and increasing
direct patient care salaries and employee benefit costs will negatively impact our profitability.
For our patients receiving nursing home care under a state Medicaid program who elect hospice
care under Medicare or Medicaid, we contract with nursing homes for room and board services. The
state must pay us, in addition to the applicable Medicare or Medicaid hospice daily or hourly rate,
an amount equal to at least 95% of the Medicaid daily nursing home rate for room and board
furnished to the patient by the nursing home. Under our standard nursing home contracts, we pay the
nursing home for these room and board services at 100% of the Medicaid daily nursing home rate. We
refer to these costs, net of Medicaid payments, as “nursing home costs, net.”
General and administrative expenses for hospice care primarily include non-patient care
salaries (including salaries for our executive directors, directors of patient services, patient
care managers, community education representatives and other non-patient care staff), payroll
taxes, employee benefits for our employees at our hospice programs, office leases and other
operating costs.
General and administrative expenses for our support center primarily include salaries, payroll
taxes and employee benefits for employees located at our support center. These expenses also
include our share-based compensation, office lease, professional fees and other operating costs.
47
Table of Contents
The following table sets forth the percentage of net patient service revenue represented by
the items included in direct hospice care expenses and general and administrative expenses for the
periods indicated:
| Year Ended December 31, | ||||||||||||
| 2009 | 2008 | 2007 | ||||||||||
Direct hospice care expenses: |
||||||||||||
Salaries, benefits and payroll taxes |
38.0 | % | 38.3 | % | 39.1 | % | ||||||
Pharmaceuticals |
4.8 | 4.8 | 5.2 | |||||||||
Medical equipment and supplies |
5.4 | 5.8 | 5.3 | |||||||||
Inpatient costs |
1.9 | 2.2 | 2.2 | |||||||||
Other (including medical director fees, contracted patient care
services, nursing home costs and mileage) |
7.7 | 7.6 | 6.9 | |||||||||
Total |
57.8 | % | 58.7 | % | 58.7 | % | ||||||
General and administrative expenses — hospice care: |
||||||||||||
Salaries, benefits and payroll taxes |
13.1 | % | 14.0 | % | 14.5 | % | ||||||
Leases |
2.7 | 2.8 | 2.9 | |||||||||
Other (including insurance, recruiting, travel, telephone and printing) |
3.8 | 4.1 | 4.0 | |||||||||
Total |
19.6 | % | 20.9 | % | 21.4 | % | ||||||
General and administrative expenses — support center: |
||||||||||||
Salaries, benefits and payroll taxes |
4.8 | % | 5.9 | % | 4.6 | % | ||||||
Share-based compensation |
0.7 | 0.7 | 1.0 | |||||||||
Leases |
0.3 | 0.4 | 0.4 | |||||||||
Legal and accounting fees |
1.1 | 1.1 | 1.9 | |||||||||
Other (including insurance, recruiting, travel, telephone and printing) |
2.5 | 3.4 | 3.8 | |||||||||
Total |
9.4 | % | 11.5 | % | 11.7 | % | ||||||
The following table sets forth the cost per day of care represented by the items included in direct
hospice care expenses and general and administrative expenses for hospice care for the years ended
December 31, 2009, 2008 and 2007, respectively:
| Year Ended December 31, | ||||||||||||
| 2009 | 2008 | 2007 | ||||||||||
Direct hospice care expenses: |
||||||||||||
Salaries, benefits and payroll taxes |
$ | 57.68 | $ | 56.06 | $ | 55.76 | ||||||
Pharmaceuticals |
7.27 | 7.05 | 7.43 | |||||||||
Medical equipment and supplies |
8.23 | 8.44 | 7.53 | |||||||||
Inpatient costs |
2.95 | 3.18 | 3.09 | |||||||||
Other (including medical director fees, contracted patient care
services, nursing home costs and mileage) |
11.68 | 11.07 | 9.89 | |||||||||
Total |
$ | 87.81 | $ | 85.80 | $ | 83.70 | ||||||
General and administrative expenses — hospice care: |
||||||||||||
Salaries, benefits and payroll taxes |
$ | 19.92 | $ | 20.52 | $ | 20.69 | ||||||
Leases |
4.10 | 4.04 | 4.14 | |||||||||
Other (including insurance, recruiting, travel, telephone and printing) |
5.71 | 5.99 | 5.73 | |||||||||
Total |
$ | 29.73 | $ | 30.55 | $ | 30.56 | ||||||
48
Table of Contents
Share-based Compensation Charges
We account for share-based compensation in accordance with Financial Accounting Standards
(“FASB”) Accounting Standards Codification (“ASC”) Topic 718 “Compensation — Stock Compensation.”
Under FASB ASC Topic 718, we recognize share-based compensation ratably using the straight-line
attribution method over the requisite service period. Share-based compensation is measured based
on the grant date fair value of the respective award. The fair value of option awards is estimated
at the date of grant using the Black-Scholes valuation model. We estimate forfeitures based on
historical experience and future expectations. Share-based compensation expense is included within
the “general and administrative — support center” line item in our consolidated statements of
income. We recorded $5.1 million, $4.3 million and $3.8 million in share-based compensation
expense for the years ended December 31, 2009, 2008 and 2007, respectively.
In February 2008, the Compensation Committee of the Board of Directors (the “Committee”)
approved, for certain executive officers, the exchange of selected “underwater” stock options for
restricted stock. The Committee was concerned that the underwater stock options provided little or
no financial or retention incentives to the executive officers. The Committee believes that the
exchange of the underwater stock options for the restricted stock adequately addressed those
concerns. Stock option awards of 685,000 shares, with a weighted average exercise price of $17.35,
were exchanged for 126,146 shares of restricted stock. Of the stock option awards exchanged,
287,500 shares were unvested. The shares of restricted stock had a fair value of $8.72 per share
and vest ratably over a three year period beginning February 12, 2009. There was not a material
change to our share-based compensation expense from the exchange.
Provision for Income Taxes
Our provision for income taxes consists of current and deferred federal and state income tax
expenses. We estimate that our effective tax rate will be approximately 37.5% during 2010.
Critical Accounting Policies
Our significant accounting policies are more fully described in note “2. Summary of
Significant Accounting Policies” to our consolidated financial statements included elsewhere in
this Annual Report on Form 10-K. Certain of our accounting policies are particularly important to
the portrayal of our financial position and results of operations included elsewhere in this Annual
Report on Form 10-K and require the application of significant judgment by us; as a result, they
are subject to an inherent degree of uncertainty. In applying these policies, we use our judgment
to determine the appropriate assumptions to be used in the determination of certain estimates.
These estimates are based on our historical payment experience, our observance of trends in the
industry and information available from other outside sources, as appropriate.
Net Patient Service Revenue and Allowance for Uncollectible Accounts
We report net patient service revenue at the estimated net realizable amounts (exclusive of
our provision for uncollectible accounts) from Medicare, Medicaid, commercial insurance, managed
care payors, patients and others for services rendered to our patients. Regarding commercial,
managed care and other payors, payments are subject to usual and customary rates. To determine net
patient service revenue, we adjust gross patient service revenue for estimated contractual
adjustments based on historical experience and estimated Medicare cap contractual adjustments. Net
patient service revenue also does not include charity care or the Medicaid room and board payments.
We recognize net patient service revenue in the month in which our services are delivered. Due to
the complexity of the laws and regulations affecting Medicare and Medicaid, a reasonable
possibility exists that recorded estimates could change by a material amount in the future.
We maintain a policy for reserving for uncollectible accounts. We calculate the allowance for
uncollectible accounts based on a formula tied to the aging of accounts receivable by payor class.
We also reserve for specific accounts that are determined to be uncollectible. Accounts are written
off when all collection efforts are exhausted.
49
Table of Contents
Medicare Regulation and Contractual Adjustments
The Medicare Cap. Various provisions were included in the legislation creating the Medicare
hospice benefit to manage the cost to the Medicare program for hospice, including the patient’s
waiver of curative care requirement, the six-month terminal prognosis requirement and the Medicare
payment caps. The Medicare hospice benefit includes two fixed annual caps on payment, both of which
are assessed on a program-by-program basis. One cap is an absolute dollar amount; the other limits
the number of days of inpatient care. The caps are calculated from November 1 through October 31 of
each year.
Dollar Amount Cap. The Medicare revenue paid to a hospice program from November 1 to October
31 of the following year may not exceed the annual cap amount which is calculated by using the
following formula: the product of the number of admissions to the program by patients who are
electing to receive their Medicare hospice benefit for the first time, multiplied by the Medicare
cap amount, which for the November 1, 2008 through October 31, 2009 Medicare fiscal year was
$23,014. The Medicare cap amount is reduced proportionately for patients who transferred in or out
of our hospice services. The Medicare cap amount is annually adjusted for inflation, but is not
adjusted for geographic differences in wage levels, although hospice per diem payment rates are
wage indexed. The Medicare cap amount for the November 1, 2009 through October 31, 2010 cap year
has not yet been announced by the Medicare program. We currently estimate the Medicare cap amount
to be approximately $23,600 for the Medicare cap year ending October 31, 2010.
Inpatient Care Cap. A hospice program’s inpatient care days, either general inpatient or
respite inpatient care and regardless of setting, may not exceed 20% of the program’s total patient
care days in the Medicare cap year. None of our hospice programs exceeded the payment limits on
general inpatient care services for the years ended December 31, 2009, 2008 and 2007.
The following table shows the amount accrued and paid for the Medicare cap contractual
adjustments for the years ended December 31, 2007, 2008 and 2009, respectively:
| Accrued Medicare Cap Contractual Adjustments | ||||||||||||
| Year Ending December 31, | ||||||||||||
| 2007 | 2008 | 2009 | ||||||||||
| (in thousands) | ||||||||||||
Beginning balance — accrued Medicare
cap contractual adjustments |
$ | 26,679 | $ | 21,682 | $ | 23,719 | ||||||
Medicare cap contractual adjustments |
5,039 | (1) | 6,852 | (2) | 4,565 | (3) | ||||||
Medicare cap contractual adjustments —
discontinued operations |
2,651 | (4) | (27 | )(4) | (79 | )(4) | ||||||
Payments to Medicare fiscal intermediaries |
(12,687 | ) | (12,996 | ) | (9,407 | ) | ||||||
Balances acquired from VistaCare |
— | 8,208 | — | |||||||||
Ending balance — accrued Medicare cap
contractual adjustments |
$ | 21,682 | $ | 23,719 | $ | 18,798 | ||||||
| (1) | Includes additional accrual of $0.9 million related to the 2006 Medicare cap year. | |
| (2) | Includes additional accrual of $1.5 million related to the 2006 Medicare cap year. | |
| (3) | Includes an accrual reversal of $1.1 million related to the 2007 Medicare cap year. | |
| (4) | Medicare cap contractual adjustments reclassified to discontinued operations are related to all programs that were discontinued and sold during 2007, 2008 and 2009. |
Laws and regulations governing the Medicare and Medicaid programs are complex and subject to
interpretation. We believe that we are in compliance with all applicable laws and regulations.
Compliance with laws and regulations can be subject to future government review and interpretation
as well as significant regulatory action including fines, penalties and exclusion from the Medicare
and Medicaid programs.
50
Table of Contents
Insurance Risks
General and professional liability costs for the healthcare industry have increased and become
more difficult to estimate. In addition, insurance coverage for patient care liabilities and other
risks has become more difficult to obtain. Insurance carriers often require companies to increase
their liability retention levels and pay higher policy premiums for reduced coverage. Hired and
non-owned auto liability costs are a significant risk area for us, because almost all of our
services are provided where our patients reside rather than in facilities that we operate. We
require our employees to maintain the state required minimum liability coverage on their vehicles.
Our current hired and non-owned auto liability coverage has a deductible of $250,000 per claim. We
continue to evaluate options to address this insurance risk area; however, we cannot assure you
that we will be able to find cost effective insurance coverage to address this insurance risk area.
In our consolidated financial statements, we reserve for potential contingencies associated with
the uninsured portion of our general and professional liability risks and hired and non-owned auto
liability risks, based on our experience, consultation with our attorneys and insurers and our
existing insurance coverage.
Goodwill and Indefinite-lived Intangible Assets
Goodwill is the excess of the purchase price over the fair value of identifiable assets
acquired. Indefinite-lived intangible assets are comprised of license agreements and trademarks.
Under FASB ASC Topic 350, “Intangibles — Goodwill and Other,” (formerly Statement of Financial
Accounting Standards (“SFAS”) No. 142 “Goodwill and Other Intangible Assets”), goodwill and
intangible assets with indefinite lives are not amortized, but tested for impairment annually or
more frequently if certain indications of impairment arise. Goodwill is reviewed at the reporting
unit level, which is defined as an operating segment or one level below an operating segment. We
have defined our reporting units at the operating segment level.
Goodwill impairment is determined using a two-step process. The first step is to identify if a
potential impairment exists by comparing the fair value of a reporting unit with its carrying
amount, including goodwill. If the fair value of a reporting unit exceeds its carrying amount,
goodwill of the reporting unit is not considered to have a potential impairment and the second step
of the impairment test is not necessary. However, if the carrying amount of a reporting unit
exceeds its fair value, the second step is performed to determine if goodwill is impaired and to
measure the amount of impairment loss to recognize, if any. The second step compares the implied
fair value of goodwill with the carrying amount of goodwill. If the implied fair value of goodwill
exceeds the carrying amount, then goodwill is not considered impaired. However, if the carrying
amount of goodwill exceeds the implied fair value, an impairment loss is recognized in an amount
equal to that excess. The implied fair value of goodwill is determined in the same manner as the
amount of goodwill recognized in a business combination (i.e., the fair value of the reporting unit
is allocated to all the assets and liabilities, including any unrecognized intangible assets, as if
the reporting unit had been acquired in a business combination and the fair value of the reporting
unit was the purchase price paid to acquire the reporting unit). The amount of the impairment would
be the difference between the carrying amount of the goodwill and the implied fair value of the
goodwill.
Our 2009 annual goodwill impairment testing was performed as of November 30, 2009. In
determining the fair value of our reporting units, we used multiples of earnings before interest,
taxes, depreciation and amortization (“EBITDA”). We believe using multiples of EBITDA in
determining the fair value of reporting units is appropriate because it correlates with what a
market participant would be willing to pay for that reporting unit in today’s market. As of the
date of our annual impairment testing, none of the reporting units failed step one and no reporting
units were at risk of failing step one. Furthermore, the fair values of each of our reporting
units represented no less than 185% of their carrying values. We evaluated whether any events had
occurred or any circumstances had changed since November 30, 2009 that would indicate goodwill may
have become impaired since the annual impairment testing. In this evaluation, we considered both
qualitative and quantitative factors such as any adverse change in the business climate, current
estimates of future profitability of reporting units, our current stock price and our market
capitalization compared to our book value. Based on this evaluation, we determined that no
indications of impairment had arisen since our annual goodwill impairment test. No impairment
charges have been recorded as of December 31, 2009, 2008 and 2007. We cannot predict whether we
will incur impairment charges in the future or whether any impairment charges recorded will
negatively impact our results of operations or financial position in the future.
51
Table of Contents
Income Taxes
In preparing financial statements, we exercise significant judgment in determining our
provision for income taxes, deferred tax assets and liabilities, and the valuation allowance. We
account for income taxes using the liability method as required by ASC Topic 740, “Income Taxes”
(formerly SFAS No. 109 — “Accounting for Income Taxes”). Under the liability method, deferred taxes
are determined based on differences between financial reporting and tax basis of assets and
liabilities and are measured using the enacted tax laws that will be in effect when the differences
are expected to reverse. We provide a valuation allowance for any net deferred tax assets when it
is more likely than not that a portion of such net deferred tax assets will not be recovered.
On January 1, 2007, we adopted a new accounting pronouncement issued by the FASB located under
ASC Topic 740, “Income Taxes” (formerly FASB FIN No. 48 — “Accounting for Uncertainty in Income
Taxes”), which clarifies the accounting for uncertainty in income taxes. This pronouncement
prescribes a recognition threshold and measurement attribute for the financial statement
recognition and measurement of a tax position taken or expected to be taken in a tax return and
also provides guidance on various related matters such as derecognition, classification, interest
and penalties, accounting in interim periods, disclosures and transition.
Results of Operations
The following table sets forth selected consolidated financial information as a percentage of
net patient service revenue for the periods indicated:
| Year Ended December 31, | ||||||||||||
| 2009 | 2008 | 2007 | ||||||||||
Net patient service revenue |
100 | % | 100 | % | 100 | % | ||||||
Operating expenses: |
||||||||||||
Direct hospice care |
57.8 | 58.7 | 58.7 | |||||||||
General and administrative — hospice care |
19.6 | 20.9 | 21.4 | |||||||||
General and administrative — support center |
9.4 | 11.5 | 11.7 | |||||||||
Provision for uncollectible accounts |
1.7 | 1.8 | 1.3 | |||||||||
Depreciation and amortization |
1.0 | 1.2 | 1.4 | |||||||||
| 89.5 | 94.1 | 94.5 | ||||||||||
Income from continuing operations before other income (expense) |
10.5 | 5.9 | 5.5 | |||||||||
Other income (expense), net |
(0.8 | ) | (0.9 | ) | 0.5 | |||||||
Income from continuing operations before provision for income taxes |
9.7 | 5.0 | 6.0 | |||||||||
Provision for income taxes |
(3.6 | ) | (1.8 | ) | (2.0 | ) | ||||||
Income from continuing operations |
6.1 | 3.2 | 4.0 | |||||||||
Loss from discontinued operations, net of income taxes |
(0.1 | ) | (0.8 | ) | (1.0 | ) | ||||||
Net income |
6.0 | 2.4 | 3.0 | |||||||||
Less: Net income attributable to noncontrolling interests |
(0.1 | ) | (0.1 | ) | 0.0 | |||||||
Net income attributable to Odyssey stockholders |
5.9 | % | 2.3 | % | 3.0 | % | ||||||
52
Table of Contents
Year Ended December 31, 2009 Compared to Year Ended December 31, 2008
The following table summarizes and compares our results of operations for the years ended
December 31, 2009 and 2008 respectively:
| Year Ended December 31, | ||||||||||||||||
| 2009 | 2008 | $ Change | % Change | |||||||||||||
| (In thousands, except % change) | ||||||||||||||||
Net patient service revenue |
$ | 686,438 | $ | 616,050 | $ | 70,388 | 11.4 | % | ||||||||
Operating expenses: |
||||||||||||||||
Direct hospice care |
396,774 | 361,445 | 35,329 | 9.8 | ||||||||||||
General and administrative — hospice care |
134,335 | 128,718 | 5,617 | 4.4 | ||||||||||||
General and administrative — support center |
64,734 | 70,574 | (5,840 | ) | (8.3 | ) | ||||||||||
Provision for uncollectible accounts |
11,490 | 10,907 | 583 | 5.3 | ||||||||||||
Depreciation and amortization |
6,725 | 7,868 | (1,143 | ) | (14.5 | ) | ||||||||||
| 614,058 | 579,512 | 34,546 | 6.0 | |||||||||||||
Income from continuing operations before other income
(expense) |
72,380 | 36,538 | 35,842 | 98.1 | ||||||||||||
Other expense |
(6,095 | ) | (5,462 | ) | (633 | ) | (11.6 | ) | ||||||||
Income from continuing operations before provision for
income taxes |
66,285 | 31,076 | 35,209 | 113.3 | ||||||||||||
Provision for income taxes |
24,583 | 11,141 | 13,442 | 120.7 | ||||||||||||
Income from continuing operations |
41,702 | 19,935 | 21,767 | 109.2 | ||||||||||||
Loss from discontinued operations, net of income taxes |
(498 | ) | (5,252 | ) | 4,754 | 90.5 | ||||||||||
Net income |
41,204 | 14,683 | 26,521 | 180.6 | ||||||||||||
Less: Net income attributable to noncontrolling interests |
613 | 257 | 356 | 138.5 | ||||||||||||
Net income attributable to Odyssey stockholders |
$ | 40,591 | $ | 14,426 | $ | 26,165 | 181.4 | % | ||||||||
Net Patient Service Revenue
Net patient service revenue increased $70.4 million, or 11.4%, to $686.4 million for the year
ended December 31, 2009 from $616.0 million for the year ended December 31, 2008, due primarily to
the incremental net patient service revenue generated from VistaCare operations of approximately
$50.3 million. Net patient service revenue for the year ended December 31, 2009, includes a full
twelve months of VistaCare operations as compared to the year ended December 31, 2008, which
includes ten full months of VistaCare operations. In addition, the year ended December 31, 2009
includes $5.8 million in net service patient revenue related to a new hospice program that was
acquired on December 31, 2008. There was also an additional $1.4 million of net patient service
revenue recorded during the three months ended March 31, 2009 related to the fourth quarter of
2008. This additional $1.4 million in net patient service revenue was due to the delay in
implementing the phase-out of the budget neutrality adjustment factor, as previously discussed,
which was delayed until October 1, 2009. Net patient service revenue per day of care was $151.91
and $146.23 for the years ended December 31, 2009 and 2008, respectively. This increase was
primarily due to overall increases in Medicare payment rates for our hospice services of
approximately 2.1% and 3.6% on October 1, 2009 and 2008, respectively. In addition, Medicare cap
expense decreased $2.3 million to $4.6 million for the year ended December 31, 2009 from $6.9
million for the year ended December 31, 2008. Medicare revenues represented 93.1% and 92.5% of our
net patient service revenue for the years ended December 31, 2009 and 2008, respectively. Medicaid
revenues represented 3.9% and 4.1% of our net patient service revenue for the years ended December
31, 2009 and 2008, respectively.
Direct Hospice Care Expenses
Direct hospice care expenses increased $35.4 million, or 9.8%, to $396.8 million for the year
ended December 31, 2009 from $361.4 million for the year ended December 31, 2008, due primarily to
the incremental direct hospice care expenses incurred from the VistaCare operations of
approximately $28.1 million for the year ended December 31, 2009 compared to the year ended
December 31, 2008. The direct hospice care expenses for the year ended December 31, 2009 includes
a full twelve months of VistaCare operations as compared to the year ended December
53
Table of Contents
31, 2008, which includes ten full months of VistaCare operations. In addition, we incurred
approximately $3.9 million in direct hospice care expenses related to a new hospice program that
was acquired on December 31, 2008. Salaries, benefits and payroll tax expense for Odyssey programs
increased $3.3 million, or 2.0%, to $168.6 million for the year ended December 31, 2009 from $165.3
million for the year ended December 31, 2008. As a percentage of net patient service revenue, our
direct hospice care expenses were 57.8% and 58.7% for the years ended December 31, 2009 and 2008,
respectively.
General and Administrative Expenses — Hospice Care
General and administrative expenses — hospice care increased $5.6 million, or 4.4%, to $134.3
million for the year ended December 31, 2009 from $128.7 million for the year ended December 31,
2008, due primarily to the incremental general and administrative expenses incurred from the
VistaCare operations. The general and administrative expenses — hospice care for the year ended
December 31, 2009 includes a full twelve months of VistaCare operations as compared to the year
ended December 31, 2008, which includes ten full months of VistaCare operations. In addition, we
incurred approximately $1.8 million in general and administrative expense related to a new hospice
program that was acquired on December 31, 2008. As a percentage of net patient service revenue,
our general administrative expenses — hospice care were 19.6% and 20.9% for the years ended
December 31, 2009 and 2008, respectively.
General and Administrative Expenses — Support Center
General and administrative expenses — support center decreased $5.9 million, or 8.3%, to $64.7
million for the year ended December 31, 2009 from $70.6 million for the year ended December 31,
2008. For the year ended December 31, 2008, we incurred $7.9 million in ramp down and integration
expenses related to the VistaCare acquisition compared to no ramp down and integration expenses for
the year ended December 31, 2009. Excluding the ramp down and integration expenses, our general
and administrative expenses increased $2.0 million of which our salaries, benefits, and payroll tax
expenses increased approximately $1.9 million to $38.0 million for the year ended December 31, 2009
from $36.1 million for the year ended December 31, 2008. As a percentage of net patient service
revenue, our general and administrative expenses — support center were 9.4% and 11.5% for the
years ended December 31, 2009 and 2008, respectively.
Provision for Uncollectible Accounts
Our provision for uncollectible accounts increased $0.6 million, or 5.3%, to $11.5 million for
the year ended December 31, 2009 from $10.9 million for the year ended December 31, 2008. As a
percentage of net patient service revenue, our provision for uncollectible accounts was 1.7% and
1.8% for the years ended December 31, 2009 and 2008, respectively.
Depreciation and Amortization Expense
Depreciation and amortization expense decreased $1.2 million, or 14.5%, to $6.7 million for
the year ended December 31, 2009 from $7.9 million for the year ended December 31, 2008. This
decrease was primarily due to an adjustment of depreciation expense of $0.6 million related to
capitalized computer software costs and certain assets becoming fully depreciated. As a percentage
of net patient service revenue, depreciation and amortization expense was 1.0% and 1.3% for the
years ended December 31, 2009 and 2008, respectively.
Other Expense
Other expense increased $0.6 million to $6.1 million for the year ended December 31, 2009 from
$5.5 million for the year ended December 31, 2008. Interest expense decreased $0.8 million to $6.6
million for the year ended December 31, 2009 from $7.4 million for the year ended December 31, 2008
primarily due to lower interest rates on our term loan and lower borrowings outstanding during
2009. Borrowings outstanding at December 31, 2009 and 2008 were $115.2 million and $123.1 million,
respectively. In addition, interest income decreased $1.5 million to $0.5 million for the year
ended December 31, 2009 from $2.0 million for the year ended December 31, 2008, which was primarily
due to a decrease in interest earned on our money market and ARS accounts.
54
Table of Contents
Provision for Income Taxes
Our provision for income taxes increased $13.5 million, or 120.7%, to $24.6 million for the
year ended December 31, 2009 from $11.1 million for the year ended December 31, 2008. We had an
effective income tax rate of approximately 37.4% and 36.1% for the years ended December 31, 2009
and 2008, respectively. The increase in the effective tax rate for 2009 is related to a higher
percentage of taxable earnings due to our increasing income before taxes and our lower tax-exempt
interest income earned, which is due to lower interest rates.
Year Ended December 31, 2008 Compared to Year Ended December 31, 2007
The following table summarizes and compares our results of operations for the years ended
December 31, 2008 and 2007, respectively:
| Year Ended December 31, | ||||||||||||||||
| 2008 | 2007 | $ Change | % Change | |||||||||||||
| (In thousands, except % change) | ||||||||||||||||
Net patient service revenue |
$ | 616,050 | $ | 398,232 | $ | 217,818 | 54.7 | % | ||||||||
Operating expenses: |
||||||||||||||||
Direct hospice care |
361,445 | 233,664 | 127,781 | 54.7 | ||||||||||||
General and administrative — hospice care |
128,718 | 85,304 | 43,414 | 50.9 | ||||||||||||
General and administrative — support center |
70,574 | 46,484 | 24,090 | 51.8 | ||||||||||||
Provision for uncollectible accounts |
10,907 | 5,344 | 5,563 | 104.1 | ||||||||||||
Depreciation and amortization |
7,868 | 5,723 | 2,145 | 37.5 | ||||||||||||
| 579,512 | 376,519 | 202,993 | 53.9 | |||||||||||||
Income from continuing operations before other income
(expense) |
36,538 | 21,713 | 14,825 | 68.3 | ||||||||||||
Other income (expense) |
(5,462 | ) | 2,301 | (7,763 | ) | (337.4 | ) | |||||||||
Income from continuing operations before provision for
income taxes |
31,076 | 24,014 | 7,062 | 29.4 | ||||||||||||
Provision for income taxes |
11,141 | 8,001 | 3,140 | 39.2 | ||||||||||||
Income from continuing operations |
19,935 | 16,013 | 3,922 | 24.5 | ||||||||||||
Loss from discontinued operations, net of income taxes |
(5,252 | ) | (3,888 | ) | 1,364 | 35.1 | ||||||||||
Net income |
14,683 | 12,125 | 2,558 | 21.1 | % | |||||||||||
Less: Net income attributable to noncontrolling interests |
257 | 14 | 243 | 1,735.7 | ||||||||||||
Net income attributable to Odyssey stockholders |
$ | 14,426 | $ | 12,111 | $ | 2,315 | 19.1 | % | ||||||||
Net Patient Service Revenue
Net patient service revenue increased $217.8 million, or 54.7%, to $616.0 million from $398.2
million for the years ended December 31, 2008 and 2007, respectively, primarily due to the net
patient service revenue of approximately $185.8 million generated from VistaCare operations from
the date we acquired control of VistaCare which was February 28, 2008 through December 2008. Net
patient service revenue per day of care was $146.23 and $142.64 for the years ended December 31,
2008 and 2007, respectively. This increase was primarily due to overall increases in Medicare
payment rates for our hospice services of approximately 2.5% and 3.3% on October 1, 2008 and 2007,
respectively. In addition, our same store census increased by approximately 247 patients, or 3.8%,
to 6,736 patients for the year ended December 31, 2008 from 6,489 patients for the year ended
December 31, 2007, which resulted in increased billable days of approximately 96,891. The increase
in net patient service revenue was offset by the Medicare cap contractual adjustment of $6.9
million and $5.0 million for the years ended December 31, 2008 and 2007, respectively. Medicare
revenues represented 92.5% and 92.4% of our net patient service revenue for the years ended
December 31, 2008 and 2007, respectively. Medicaid revenues represented 4.1% and 4.6% of our net
patient service revenue for the year ended December 31, 2008 and 2007, respectively. Our net
patient service revenue for 2008 does not include any benefit from the delay in the phase out of
the budget neutrality adjustment factor from October 1, 2008 to October 1, 2009 as a result of the
enactment of the American Recovery and Reinvestment Act of 2009.
55
Table of Contents
Direct Hospice Care Expenses
Direct hospice care expenses increased $127.8 million, or 54.7%, to $361.4 million for the
year ended December 31, 2008 from $233.7 million for the year ended December 31, 2007, primarily
due to our VistaCare operations’ direct hospice care expenses of approximately $110.6 million from
the date of acquisition through December 2008. Salaries, benefits and payroll tax expense for
legacy Odyssey operations increased approximately $9.6 million, or 6.2%, to $165.3 million for the
year ended December 31, 2008 from $155.7 million for the year ended December 31, 2007. This
increase is primarily due to annual salary increases and an increase related to a change in the
estimate of our workers’ compensation accrual based on an updated analysis of our workers’
compensation claims history and due to our increased headcount. As a percentage of net patient
service revenue, our direct hospice care expenses were 58.7% for both of the years ended December
31, 2008 and 2007.
General and Administrative Expenses — Hospice Care
General and administrative expenses — hospice care increased $43.4 million, or 50.9%, to
$128.7 million for the year ended December 31, 2008, from $85.3 million for the year ended December
31, 2007, primarily due to VistaCare’s operations’ general and administrative expenses for hospice
care of approximately $33.6 million from the date of acquisition through December 2008. Salaries,
benefits and payroll tax expense for legacy Odyssey operations increased approximately $8.2
million, or 14.2%, to $66.0 million for the year ended December 31, 2008, from $57.8 million for
the year ended December 31, 2007. This increase is primarily due to annual salary increases and an
increase related to a change in the estimate of our workers’ compensation accrual based on an
updated analysis of our workers’ compensation claims history and due to our increased headcount. As
a percentage of net patient service revenue, our general administrative expenses — hospice care
were 20.9% and 21.4% for the years ended December 31, 2008 and 2007, respectively.
General and Administrative Expenses — Support Center
General and administrative — support center expenses increased $24.1 million, or 51.8%, to
$70.6 million for the year ended December 31, 2008, from $46.5 million for the year ended December
31, 2007. During the year ended December 31, 2008, we incurred approximately $7.9 million in
expenses related to the ramp down of VistaCare’s corporate office and integration of VistaCare’s
operations. We also incurred approximately $5.1 million in expenses related to ramping up the
Dallas Support Center to accommodate the acquisition. Salaries, benefits and payroll tax expense
related to Odyssey’s Support Center increased $10.8 million, or 59%, to $29.1 million for the year
ended December 31, 2008 from $18.3 million for the year ended December 31, 2007. As a percentage of
net patient service revenue, our general and administrative expenses for support center were 11.5%
and 11.7% for the years ended December 31, 2008 and 2007, respectively.
Provision for Uncollectible Accounts
Our provision for uncollectible accounts increased $5.6 million, or 104.1%, to $10.9 million
for the year ended December 31, 2008 from $5.3 million for the year ended December 31, 2007, due to
an increase in the number of additional document requests (“ADRs”) from our Medicare fiscal
intermediaries, which resulted in an increase in our aging, denials and additional write-offs of
patient accounts and our acquisition of VistaCare, which increased our provision for uncollectible
accounts in accordance with our bad debt reserve policy. As a percentage of net patient service
revenue, our provision for uncollectible accounts was 1.8% and 1.3% for the years ended December
31, 2008 and 2007, respectively.
Depreciation and Amortization Expense
Depreciation and amortization expense increased $2.2 million, or 37.5%, to $7.9 million for
the year ended December 31, 2008 from $5.7 million for the year ended December 31, 2007. This
increase was primarily due to depreciation expense of approximately $1.3 million related to assets
acquired in our acquisition of VistaCare. As a percentage of net patient service revenue,
depreciation and amortization expense was 1.2% and 1.4% for the years ended December 31, 2008 and
2007, respectively.
56
Table of Contents
Other Income (Expense)
Other expense increased $7.8 million to $5.5 million for the year ended December 31, 2008 from
$2.3 million in other income for the year ended December 31, 2007. Interest expense increased $7.2
million for the year ended December 31, 2008 as a result of borrowings related to our acquisition
of VistaCare.
Provision for Income Taxes
Our provision for income taxes increased $3.1 million, or 39.2%, to $11.1 million for the year
ended December 31, 2008 from $8.0 million for the year ended December 31, 2007. We had an effective
income tax rate of approximately 36.1% and 33.3% for the years ended December 31, 2008 and 2007,
respectively. The 2007 effective income tax rate is lower primarily due to the 2007 federal tax
credit related to Hurricane Katrina and higher tax exempt interest income for 2007.
Liquidity and Capital Resources
As of December 31, 2009, we had cash and cash equivalents of $128.6 million and working
capital of $100.2 million. At such date, we also had $12.4 million in long term investments or ARS
which we plan to liquidate in an orderly manner. Our principal liquidity requirements are for debt
service, Medicare cap contractual adjustments, working capital, new hospice program and inpatient
development, hospice acquisitions, and other capital expenditures. We finance these requirements
primarily with existing funds, cash flows from operating activities, borrowings under our revolving
line of credit, operating leases, and normal trade credit terms.
Cash provided by operating activities and discontinued operations was $81.7 million, $21.0
million and $12.8 million for the years ended December 31, 2009, 2008 and 2007, respectively, and
represented net income generated, non-cash charges related to depreciation, amortization,
share-based compensation and taxes, and increases and decreases in working capital. We paid $9.4
million, $13.0 million and $15.4 million for the years ended December 31, 2009, 2008 and 2007,
respectively, for Medicare cap contractual adjustments. Our days outstanding in accounts receivable
was 50 days, 60 days and 55 days as of December 31, 2009, 2008 and 2007, respectively. The increase
in days outstanding from 2007 to 2008 is due primarily to an increase in accounts receivable at the
VistaCare sites that were converted to our billing system and an increase in accounts receivable as
a result of delay in collections due to additional document requests received from our Medicare
fiscal intermediaries. The decrease in days outstanding from 2008 to 2009 is primarily due to the
Company’s strong cash collections on VistaCare accounts receivable balances during the year.
Investing activities, consisting primarily of cash paid for acquisitions, purchases of
property and equipment and to purchase or sell investments, used cash of $4.1 million and $97.2
million for the years ended December 31, 2009 and 2008, respectively. During the year ended
December 31, 2009, the use of cash was due primarily to our purchases of hospice programs in Flint,
Michigan and Westchester, Illinois and purchases of property and equipment offset by sales of
long-term investments. During the year ended December 31, 2008, the use of cash was due primarily
to our purchase of VistaCare offset by the sales of long-term investments. See note “3.
Acquisitions” to our consolidated financial statements included elsewhere in this annual report on
Form 10-K. During the year ended December 31, 2007, we generated cash of $4.4 million which was
primarily due to the sale of short-term investments offset by purchases of property and equipment.
Net cash used in financing activities was $5.0 million for the year ended December 31, 2009
compared to net cash provided by financing activities of $119.8 million for the same period in
2008. During the year ended December 31, 2009, the use of cash was primarily due to payments on
our credit facility. The cash generated from financing activities for the year ended December 31,
2008 represented proceeds of $130 million from borrowings under our credit facility partially
offset by payments on our credit facility of $6.9 million and payments of debt issue costs of $4.4
million in connection with the acquisition of VistaCare. Net cash used in financing activities was
$12.4 million during the year ended December 31, 2007, primarily due to the purchase of treasury
stock.
In connection with our acquisition of VistaCare, we entered into a Second Amended and Restated
Credit Agreement (the “Credit Agreement”) on February 28, 2008 with General Electric Capital
Corporation and certain other lenders that provides us with a $130.0 million term loan (the “Term
Loan”) and a $30.0 million revolving line
57
Table of Contents
of credit. The Term Loan was used to pay a portion of the purchase price and costs incurred
with respect to the acquisition of VistaCare and to pay certain fees and expenses incurred in
connection with the Credit Agreement. The revolving line of credit may be used to fund future
acquisitions, working capital, capital expenditures and for general corporate purposes. The
borrowing capacity under the credit agreement is reduced by any outstanding letters of credit and
payments under the term loan. At December 31, 2009, outstanding letters of credit totaled $8.8
million and are used as collateral for our insurance policies. As of December 31, 2009, the
borrowing capacity under the credit agreement was $21.2 million, of which no amounts were drawn.
Borrowings under the Term Loan and revolving line of credit bear interest at an applicable
margin above an Index Rate (based on the higher of the prime rate or 50 basis points over the
federal funds rate) or above LIBOR. At December 31, 2009, both the applicable term loan margin and
the applicable revolver margin for LIBOR loans were 2.50% and for Index Rate loans were 1.50%. At
December 31, 2008, both the applicable term loan margin and the applicable revolver margin for
LIBOR loans were 3.0% and for Index Rate loans were 2.0%. These margins are based on our leverage
ratio and can vary from 2.50% to 3.25% for LIBOR loans and 1.50% to 2.25% for Index Rate loans.
In April 2008, we entered into two interest rate swap agreements described below that
effectively convert a notional amount of $60.0 million of floating rate borrowings to fixed rate
borrowings.
Borrowings outstanding at December 31, 2009 were $115.2 million and carried a weighted-average
interest rate of 4.3%, including the effect of the interest rate swaps. At December 31, 2009,
$53.6 million of the Term Loan carried interest at LIBOR plus 2.50% (2.73%) while $40.0 million of
the Term Loan carried interest at a fixed rate of 5.45% and $20.0 million of the Term Loan carried
interest at a fixed rate of 5.92% as a result of interest rate swap agreements. The remaining $1.6
million of the Term Loan carried interest at the Index Rate plus 1.50% (4.75%).
Borrowings outstanding at December 31, 2008 were $123.1 million and carried a weighted-average
interest rate of 5.7%, including the effect of the interest rate swaps. At December 31, 2008,
$61.7 million of the Term Loan carried interest at LIBOR plus 3.00% (ranging from 5.15% to 5.34%)
while $40.0 million of the Term Loan carried interest at a fixed rate of 5.95% and $20.0 million of
the Term Loan carried interest at a fixed rate of 6.42% as a result of interest rate swap
agreements. The remaining $1.4 million of the Term Loan carried interest at the Index Rate plus
2.00% (5.25%).
The final installment of the Term Loan will be due on February 28, 2014 and the revolving line
of credit will expire on February 28, 2013. The revolving line of credit has an unused facility fee
of 0.25% per annum. In connection with the acquisition of VistaCare, all of the subsidiaries of
VistaCare (together with us, and certain of our subsidiaries, including VistaCare, the “Odyssey
Obligors”) have become guarantors of the obligations under the Credit Agreement and have granted
security interests in substantially all of their existing and after-acquired personal property. The
Term Loan and the revolving line of credit are secured by substantially all of the Odyssey
Obligors’ existing and after-acquired personal property, including the stock of certain
subsidiaries owned by the Odyssey Obligors but not party to the Credit Agreement. The Odyssey
Obligors are subject to affirmative and negative covenants under the Credit Agreement, including
financial covenants consisting of a maximum leverage ratio and a minimum fixed charge coverage
ratio. At both December 31, 2009 and 2008, we were in compliance with our financial covenants. We
are subject to mandatory prepayments based on cash proceeds received from the sale of partnership
interests and property. During the third quarter of 2009, we paid $1.5 million related to
mandatory prepayments of principal, which were based on cash proceeds received from the sale of the
Oklahoma City program. We paid approximately $2.1 million related to mandatory prepayments of
principal during the year ended December 31, 2008. In addition, we are subject to an annual excess
cash flow requirement, which may result in us having to make additional principal payments on its
Term Loan. For the year ended December 31, 2009, we were obligated to make an excess cash flow
payment of $29.3 million, which will be paid during the second quarter of 2010. No such payment
was required to be made for the year ended December 31, 2008. In the future, we may be required to
make additional principal payments related to the excess cash flow requirement.
In connection with the execution of the Second Amended and Restated Credit Agreement, we
incurred approximately $4.4 million of loan costs during 2008 which are being amortized using the
effective interest method over the life of the Credit Agreement. Deferred loan costs totaled $3.0
million and $3.8 million (net of accumulated amortization) at December 31, 2009 and 2008,
respectively.
58
Table of Contents
On November 7, 2008, our subsidiaries Odyssey HealthCare Operating A, LP, a Delaware limited
partnership, Odyssey HealthCare Operating B, LP, a Delaware limited partnership, Hospice of the
Palm Coast, Inc., a Florida not for profit corporation, and VistaCare, Inc., a Delaware
corporation, entered into an Amendment No. 1 to Second Amended and Restated Credit Agreement with
General Electric Capital Corporation and the other lenders signatory thereto. This amendment
permits our existing investments in ARS, which otherwise would have been required to be liquidated
on or prior to November 24, 2008, to be retained indefinitely.
In April 2008, we entered into an interest rate swap agreement, which effectively converts a
notional amount of $40.0 million of floating rate borrowings to fixed rate borrowings. The term of
the interest rate swap expires in April 2011. Under the terms of the interest rate swap agreement,
we receive from the counterparty interest on the $40.0 million notional amount based on three-month
LIBOR and pay to the counterparty a fixed rate of 2.95%. We entered into a second interest rate
swap agreement in April 2008, which effectively converts a notional amount of $20.0 million of
floating rate borrowings to fixed rate borrowings. The term of the second interest rate swap also
expires in April 2011. Under the terms of this second interest rate swap agreement, we receive from
the counterparty interest on the $20.0 million notional amount based on three-month LIBOR and pay
to the counterparty a fixed rate of 3.42%. We account for the interest rate swaps as a cash flow
hedge. These swaps effectively converted $60.0 million of our variable-rate borrowings to
fixed-rate borrowings beginning in April 2008 and through April 2011. We believe the interest rate
swaps will be highly effective in achieving our goal of minimizing the volatility of cash flows
associated with changes in interest rates on its variable debt.
FASB ASC Topic 815, “Derivatives and Hedging” (formerly SFAS No. 133 — “Accounting for
Derivative Instruments and Hedging Activities”), requires companies to recognize all derivative
instruments as either assets or liabilities at fair value on the balance sheet. In accordance with
ASC Topic 815, we have designated this derivative instrument as a cash flow hedge. As such, changes
in the fair value of the derivative instrument are recorded as a component of other comprehensive
income or loss (“OCI”) to the extent of effectiveness. The ineffective portion of the change in
fair value of the derivative instrument is recognized in interest expense.
We are exposed to credit losses in the event of nonperformance by the counterparties to the
two interest rate swap agreements. Management believes that the counterparties are creditworthy and
anticipates that the counterparties and us will satisfy all obligations under the contracts. Hedge
effectiveness testing for the year ended December 31, 2009 indicates that the swaps are highly
effective hedges and as such, there is no amount related to hedging ineffectiveness to expense. As
of December 31, 2009, we do not expect any amounts to be reclassified within the next twelve months
to earnings from accumulated other comprehensive loss related to these cash flow hedges.
As of December 31, 2009 and 2008, we had long-term investments totaling $12.4 million and
$16.7 million, respectively, consisting of tax exempt auction rate securities (“ARS”). The ARS
held by us are private placement securities for which the interest rates are reset every 35 days.
The reset dates have historically provided a liquid market for these securities as investors
historically could readily sell their investments. These types of securities generally have not
experienced payment defaults and are backed by student loans, which carry guarantees as provided
for under the Federal Family Education Loan Program of the U.S. Department of Education. All of the
securities were AAA/Aaa rated at December 31, 2009. To date we have collected all interest payments
on all of our ARS when due and expect to continue to do so in the future. We intended to liquidate
all of our ARS prior to the end of 2009. However, due to the problems experienced in global credit
and capital markets generally and the ARS market in particular, our ability to liquidate our ARS
this year has been impaired. We successfully liquidated $4.1 million of ARS in October 2009, $8.0
million in July 2008, $8.0 million of ARS in June 2008, and $8.4 million of ARS in January 2008,
all at par. The remaining principal of $13.0 million associated with ARS will not be accessible
until successful ARS auctions occur, a buyer is found outside of the auction process, the issuers
establish a different form of financing to replace these securities, issuers repay principal over
time from cash flows prior to maturity, or final payments come due according to contractual
maturities from 25 to 28 years. We expect that we will receive the principal associated with these
ARS through one of these means. We have classified these ARS as long-term investments.
We prepared a discounted cash flow analysis for our ARS using an estimated maturity of one
year, which is when we estimate we will be able to liquidate these securities at par. We used a
discount rate to reflect the current
59
Table of Contents
reduced liquidity of these securities. The discount rate was calculated by taking the existing
interest rate being earned on the ARS as of December 31, 2009 and including a liquidity risk
premium rate, which was calculated based on treasury yields applicable to the ARS maturity dates as
of December 31, 2009. During the years ended December 31, 2009 and 2008, we reduced the fair value
of the ARS by $0.1 million and $0.4 million (before taxes) based on this analysis. Changes in the
fair value of the ARS are recognized, net of tax in accumulated other comprehensive income (loss).
If the uncertainties in the credit and capital markets continue or these markets deteriorate
further, these securities may not provide liquidity to us when needed or maintain the fair values
estimated by us. If we had to liquidate any ARS at this time, we could incur significant losses. We
currently believe that we have sufficient liquidity for our current needs without selling any ARS
and do not currently intend to attempt to liquidate these securities until market conditions
improve and it is not more likely than not that we will be required to liquidate any ARS before
recovery of our entire cost basis. If our currently available resources are not sufficient for our
needs and we are not able to liquidate any ARS on acceptable terms on a timely basis, it could have
a significant adverse impact on the our cash flows, financial condition and results of operations.
We expect that our principal liquidity requirements will be for debt service, Medicare cap
contractual adjustments, working capital, new hospice program development, hospice acquisitions,
and other capital expenditures. We expect that our existing funds, cash flows from operating
activities, operating leases, normal trade credit terms and our existing revolving line of credit
under the Credit Agreement will be sufficient to fund our principal liquidity requirements for at
least 12 months following the date of this Annual Report on Form 10-K. Our future liquidity
requirements and the adequacy of our available funds will depend on many factors, including receipt
of payments for our services, changes in the Medicare per beneficiary cap amount, changes in
Medicare payment rates, regulatory changes and compliance with new regulations, expense levels,
capital expenditures, development of new hospices and acquisitions, government and private party
legal proceedings and investigations and our ability to enter into a new credit agreement on terms
satisfactory to us. We do not depend on cash flows from discontinued operations to provide for
future liquidity.
Contractual Obligations
We have various contractual obligations as of December 31, 2009 that could impact our
liquidity as summarized below:
| Payments Due by Period | ||||||||||||||||||||
| Less Than | More than | |||||||||||||||||||
| Total | 1 Year | 1-3 Years | 3-5 Years | 5 Years | ||||||||||||||||
| (In thousands) | ||||||||||||||||||||
Long-Term Debt |
$ | 115,202 | $ | 38,675 | $ | 20,291 | $ | 56,236 | $ | — | ||||||||||
Operating Leases |
64,420 | 17,931 | 26,114 | 15,865 | 4,510 | |||||||||||||||
Total Contractual Obligations |
$ | 179,622 | $ | 56,606 | $ | 46,405 | $ | 72,101 | $ | 4,510 | ||||||||||
Off-Balance Sheet Arrangements
As of December 31, 2009, we do not have any off-balance sheet arrangements.
Interest Rate and Foreign Exchange Risk
Interest Rate Risk. Changes in interest rates would affect the fair value of our fixed rate
debt instruments, but would not have an impact on our earnings or cash flow. At December 31, 2009,
we had $115.2 million of debt instruments of which $60.0 million were fixed rate debt instruments.
A fluctuation of 100 basis points in interest rates on our variable rate debt instruments, which
are tied to the LIBOR, would affect our earnings and cash flows by $0.6 million (pre-tax) per year,
but would not affect the fair value of the variable rate debt.
Foreign Exchange. We operate our business within the United States and execute all
transactions in U.S. dollars.
60
Table of Contents
Recent Accounting Pronouncements
In September 2006, the FASB issued a new accounting pronouncement regarding fair value
(formerly SFAS No. 157 — “Fair Value Measurements”). This pronouncement, located under FASB ASC
Topic 820, “Fair Value Measurements and Disclosures,” defines fair value, establishes a framework
for measuring fair value under GAAP, and expands disclosures about fair value measurements. This
pronouncement does not require any new fair value measurements in financial statements, but
standardizes its definition and guidance in GAAP. We adopted this pronouncement effective beginning
on January 1, 2008 for financial assets and financial liabilities which did not have a material
impact on our financial statements. In February 2008, the FASB delayed by one year the effective
date of this pronouncement for all nonfinancial assets and nonfinancial liabilities, except those
that are recognized or disclosed at fair value in the financial statements on a recurring basis (at
least annually). We adopted this pronouncement effective beginning on January 1, 2009 for
nonfinancial assets and nonfinancial liabilities, which did not have any impact on our financial
statements.
In December 2007, the FASB issued a new accounting pronouncement regarding business
combinations (formerly SFAS No. 141 (revised 2007) — “Business Combinations”). This pronouncement,
located under FASB ASC Topic 805, “Business Combinations,” was issued to improve the relevance,
representational faithfulness, and comparability of information in financial statements about a
business combination and its effects. This pronouncement retains the purchase method of accounting
for business combinations, but requires a number of changes including contingent consideration,
such as earn-outs, will be recognized at its fair value on the acquisition date and, for certain
arrangements, changes in fair value will be recognized in earnings until settled;
acquisition-related transaction and restructuring costs will be expensed as incurred;
previously-issued financial information will be revised for subsequent adjustments made to finalize
the purchase price accounting; reversals of valuation allowances related to acquired deferred tax
assets and changes to acquired income tax uncertainties will be recognized in earnings, except in
certain situations. ASC Topic 805 also requires an acquirer to recognize at fair value, an asset
acquired or a liability assumed in a business combination that arises from a contingency provided
the asset or liability’s fair value can be determined on the date of acquisition. We adopted this
pronouncement on a prospective basis effective beginning on January 1, 2009. For business
combinations completed on or subsequent to the adoption date, the application of this pronouncement
may have a significant impact on our financial statements, the magnitude of which will depend on
the specific terms and conditions of the transactions. We did not have any material business
combinations during 2009.
In December 2007, the FASB issued a new accounting pronouncement regarding noncontrolling
interests and the deconsolidation of a subsidiary (formerly SFAS No. 160 — “Noncontrolling
Interests in Consolidated Financial Statements — an amendment of ARB No. 51”). This pronouncement,
located under FASB ASC Topic 810, “Consolidation,” was issued to improve the relevance,
comparability, and transparency of financial information provided in financial statements by
establishing accounting and reporting standards for the noncontrolling interest in a subsidiary and
for the deconsolidation of a subsidiary. We adopted this pronouncement effective beginning on
January 1, 2009. As a result of this adoption, we present noncontrolling interests (previously
shown as minority interests in consolidated subsidiaries) as a component of equity on the
consolidated balance sheets. Minority interest expense is no longer separately reported as a
reduction in net income on the consolidated statements of income, but is instead shown below net
income under the heading “net income attributable to non controlling interests.” Total provision
for income taxes remains unchanged; however, our effective tax rate as calculated from the balances
shown on the consolidated statements of income have changed as net income attributable to
noncontrolling interests is no longer included as a deduction in the determination of income from
continuing operations. The adoption of this pronouncement did not have a material impact on our
financial statements other than the presentation requirements previously mentioned.
In March 2008, the FASB issued a new accounting pronouncement regarding derivative and hedging
activities (formerly SFAS No. 161 — “Disclosures about Derivative Instruments and Hedging
Activities — an amendment of FASB Statement No. 133”). This pronouncement, located under FASB ASC
Topic 815, “Derivatives and Hedging,” was issued to improve transparency of financial information
provided in financial statements by requiring expanded disclosures about an entity’s derivative and
hedging activities. This pronouncement requires entities to provide expanded disclosures about: how
and why an entity uses derivative instruments; how derivative instruments and related hedged items
are accounted for; and how derivative instruments and related hedged items affect an entity’s
61
Table of Contents
financial position, financial performance, and cash flows. We adopted this pronouncement
effective beginning on January 1, 2009. The adoption of this pronouncement did not have any impact
on our financial statements as it contains only disclosure requirements.
In April 2009, the FASB issued a new accounting pronouncement regarding interim disclosures
about fair value of financial instruments (formerly FSP FAS 107-1 and Accounting Principles Board
(“APB”) Opinion No. 28-1 — “Interim Disclosures about Fair Value of Financial Instruments”). This
pronouncement, located under FASB ASC Topic 825, “Financial Instruments,” increases the frequency
of fair value disclosures by requiring both interim and annual disclosures. We adopted this
pronouncement on a prospective basis effective beginning on April 1, 2009. The adoption of this
pronouncement did not have any impact on our financial statements as it contains only disclosure
requirements.
In April 2009, the FASB issued a new accounting pronouncement regarding fair value (formerly
Staff Position No. 157-4, “Determining Fair Value When the Volume and Level of Activity for the
Asset or Liability Have Significantly Decreased and Identifying Transactions That Are Not
Orderly”). This pronouncement, located under FASB ASC Topic 820, “Fair Value Measurements and
Disclosures,” provides additional guidance for estimating fair value when the volume and level of
activity for the asset or liability have significantly decreased. This pronouncement also includes
guidance on identifying circumstances that indicate a transaction is not orderly. We adopted this
pronouncement on a prospective basis effective beginning on April 1, 2009. The adoption of this
pronouncement did not have any impact on our financial statements.
In April 2009, the FASB issued a new accounting pronouncement regarding other-than-temporary
impairments (formerly FASB Staff Position No. FAS 115-2 and 124-2, “Recognition and Presentation of
Other-Than-Temporary Impairments”). This pronouncement, located under FASB ASC Topic 320,
“Investments — Debt and Equity Securities,” amends the other-than-temporary impairment guidance
for debt securities to make the guidance more operational and to improve the presentation and
disclosure of other-than-temporary impairments on debt and equity securities in the financial
statements. This pronouncement does not amend existing recognition and measurement guidance related
to other-than-temporary impairments of equity securities and does not require disclosures for
earlier periods presented for comparative purposes at initial adoption. In periods after initial
adoption, this pronouncement requires comparative disclosures only for periods ending after initial
adoption. We adopted this pronouncement during the second quarter of 2009 and it did not have any
impact on our financial statements.
In May 2009, the FASB issued a new accounting pronouncement regarding subsequent events
(formerly SFAS No. 165 — “Subsequent Events”). This pronouncement, located under FASB ASC Topic
855, “Subsequent Events,” was issued to establish general standards of accounting for and
disclosure of events that occur after the balance sheet date but before financial statements are
issued or are available to be issued. This pronouncement requires entities to disclose the date
through which subsequent events have been evaluated, as well as whether that date is the date the
financial statements were issued or the date the financial statements were available to be issued.
We adopted this pronouncement on a prospective basis effective beginning on April 1, 2009. The
adoption of this pronouncement did not have any impact on our financial statements. In February
2010, the FASB amended its guidance on subsequent events to remove the requirement for public
companies to disclose the date through which an entity has evaluated subsequent events.
In June 2009, the FASB issued a new accounting pronouncement regarding authoritative GAAP
(formerly SFAS No. 168 — “The FASB Accounting Standards Codification and the Hierarchy of
Generally Accepted Accounting Principles”). This pronouncement, located under FASB ASC Topic 105,
“Generally Accepted Accounting Principles,” establishes the FASB Accounting Standards Codification
(“Codification”) as the source of authoritative GAAP recognized by the FASB for nongovernmental
entities. Rules and interpretive releases of the SEC under federal securities laws are also sources
of authoritative GAAP for SEC registrants. All guidance contained in the Codification carries an
equal level of authority. All other nongrandfathered non-SEC accounting literature not included in
the Codification is nonauthoritative. We adopted this pronouncement effective beginning on July 1,
2009. The adoption of this pronouncement did not have any impact on our financial statements other
than the manner in which new accounting guidance is referenced.
In June 2009, the FASB issued a new accounting pronouncement regarding variable interest
entities (formerly SFAS No. 167, “Amendments to FASB Interpretation No. 46(R)”). This
pronouncement, located under FASB ASC
62
Table of Contents
Topic 810, “Consolidation,” was issued to improve financial reporting by enterprises involved
with variable interest entities and to provide more relevant and reliable information to users of
financial statements. ASC Topic 810 requires an enterprise to perform an ongoing analysis to
determine whether the enterprise has a controlling financial interest in a variable interest
entity. This pronouncement will be effective for us beginning on January 1, 2010. We do not expect
the adoption of this pronouncement to have a significant impact on our future financial position,
results of operations, earnings per share, and cash flows.
Payment, Legislative and Regulatory Changes
We are highly dependent on payments from the Medicare and Medicaid programs. These programs
are subject to statutory and regulatory changes, possible retroactive and prospective rate
adjustments, administrative rulings, rate freezes and funding reductions. Reductions in amounts
paid by these programs for our services or changes in methods or regulations governing payments for
our services could materially adversely affect our net patient service revenue and profitability.
For the year ended December 31, 2009, Medicare and Medicaid services constituted 93.1% and 3.9% of
our net patient service revenue, respectively.
Inflation
The healthcare industry is labor intensive. Wages and other expenses increase during periods
of inflation and when labor shortages occur in the marketplace. In addition, suppliers pass along
rising costs to us in the form of higher prices. We have implemented cost control measures designed
to curb increases in operating expenses. However, our operating expenses are increasing more
rapidly due to expected inflationary pressures than our rate increases and growth in patient
census. This dynamic is putting increasing pressure on our operating margins. We cannot predict our
ability to cover or offset future cost increases.
Item 7A. Quantitative and Qualitative Disclosures About Market Risk
Changes in interest rates would affect the fair value of our fixed rate debt instruments, but
would not have an impact on our earnings or cash flow. We currently have $115.2 million of debt
instruments of which $60.0 million are fixed rate debt instruments. A fluctuation of 100 basis
points in interest rates on our variable rate debt instruments, which are tied to the LIBOR, would
affect our earnings and cash flows by $0.6 million (pre-tax) per year, but would not affect the
fair value of the variable rate debt.
Item 8. Financial Statements and Supplementary Data
Reference is made to the Index to Consolidated Financial Statements on page F-1 of this Annual
Report on Form 10-K for a listing of our consolidated financial statements and related notes
thereto. All financial statement schedules are omitted because the required information is not
present, not present in material amounts or is presented within the consolidated financial
statements.
Item 9. Changes in and Disagreements With Accountants on Accounting and Financial Disclosure
None.
63
Table of Contents
| Item 9A. | Controls and Procedures |
As required by Rule 13a-15(b) of the Securities Exchange Act of 1934 (the “Exchange Act”), we
have evaluated, under the supervision and with the participation of our management, including our
principal executive officer and principal financial officer, the effectiveness of the design and
operation of our disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e)
under the Exchange Act) as of December 31, 2009. Our disclosure controls and procedures are
designed to provide reasonable assurance that the information required to be disclosed by us in
reports that we file under the Exchange Act is accumulated and communicated to our management,
including our principal executive officer and principal financial officer, as appropriate, to allow
timely decisions regarding required disclosure and is recorded, processed, summarized and reported
within the time periods specified in the rules and forms of the Securities Exchange Commission.
Based upon the evaluation, our principal executive officer and principal financial officer have
concluded that our disclosure controls and procedures were effective as of December 31, 2009 at the
reasonable assurance level.
There have been no changes in our internal control over financial reporting (as defined in
Rules 13a-15(f) and 15a-15(f) under the Securities Exchange Act of 1934) that occurred during the
quarter ended December 31, 2009, that have materially affected, or are reasonably likely to
materially affect, our internal control over financial reporting.
Management’s Report on Internal Control over Financial Reporting.
Management of the Company is responsible for establishing and maintaining effective internal
control over financial reporting as defined in Rule 13a-15(f) under the Securities Exchange Act of
1934. The Company’s internal control over financial reporting is designed to provide reasonable
assurance to the Company’s management and board of directors regarding the preparation and fair
presentation of published financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent
or detect misstatements. Therefore, even those systems determined to be effective can provide only
reasonable assurance with respect to financial statement preparation and presentation.
Management assessed the effectiveness of the Company’s internal control over financial
reporting as of December 31, 2009. In making this assessment, management used the criteria set
forth by the Committee of Sponsoring Organizations of the Treadway Commission in Internal Control —
Integrated Framework. Based on our assessment, we believe that, as of December 31, 2009, the
Company’s internal control over financial reporting is effective based on those criteria.
The effectiveness of internal control over financial reporting as of December 31, 2009, has
been audited by Ernst & Young LLP, the independent registered public accounting firm who has
audited the Company’s consolidated financial statements. Ernst & Young’s attestation report on the
effectiveness of the Company’s internal control over financial reporting appears on page 65 hereof.
64
Table of Contents
Report of Ernst & Young LLP, Independent Registered Public Accounting Firm
The Board of Directors and Shareholders of Odyssey HealthCare, Inc.
We have audited Odyssey HealthCare, Inc.
and subsidiaries’ internal control over financial reporting
as of December 31, 2009, based on criteria established in Internal Control-Integrated Framework
issued by the Committee of Sponsoring Organizations of the Treadway Commission (the COSO criteria).
Odyssey HealthCare, Inc. and subsidiaries’ management is responsible for maintaining effective
internal control over financial reporting, and for its assessment of the effectiveness of internal
control over financial reporting included in the accompanying, “Management’s Report on Internal
Control Over Financial Reporting.” Our responsibility is to express an opinion on the Company’s
internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight
Board (United States). Those standards require that we plan and perform the audit to obtain
reasonable assurance about whether effective internal control over financial reporting was
maintained in all material respects. Our audit included obtaining an understanding of internal
control over financial reporting, assessing the risk that a material weakness exists, testing and
evaluating the design and operating effectiveness of internal control based on the assessed risk,
and performing such other procedures as we considered necessary in the circumstances. We believe
that our audit provides a reasonable basis for our opinion.
A company’s internal control over financial reporting is a process designed to provide reasonable
assurance regarding the reliability of financial reporting and the preparation of financial
statements for external purposes in accordance with generally accepted accounting principles. A
company’s internal control over financial reporting includes those policies and procedures that (1)
pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the
transactions and dispositions of the assets of the company; (2) provide reasonable assurance that
transactions are recorded as necessary to permit preparation of financial statements in accordance
with generally accepted accounting principles, and that receipts and expenditures of the company
are being made only in accordance with authorizations of management and directors of the company;
and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized
acquisition, use, or disposition of the company’s assets that could have a material effect on the
financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or
detect misstatements. Also, projections of any evaluation of effectiveness to future periods are
subject to the risk that controls may become inadequate because of changes in conditions, or that
the degree of compliance with the policies or procedures may deteriorate.
In our opinion, Odyssey HealthCare, Inc. and subsidiaries maintained, in all material respects,
effective internal control over financial reporting as of December 31, 2009, based on the COSO
criteria.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight
Board (United States), the consolidated balance sheets of Odyssey
HealthCare, Inc. and subsidiaries as of December
31, 2009 and 2008, and the related consolidated statements of income, stockholders’ equity, and
cash flows for each of the three years in the period ended December 31, 2009 and our report dated
March 10, 2010, expressed an unqualified opinion thereon.
| /s/ Ernst & Young LLP | ||||
Dallas, Texas
March 10, 2010
65
Table of Contents
| Item 9A(T). Controls and Procedures | ||
Not applicable.
| Item 9B. Other Information | ||
None.
PART III
| Item 10. Directors, Executive Officers and Corporate Governance | ||
The information set forth under the headings “Proposal One — Election of Class III Directors,”
“Directors,” “Corporate Governance — Standing Committees of our Board,” “Corporate Governance —
Director Nomination Process,” “Corporate Governance — Code of Ethics,” “Corporate Governance — Our
Board,” “Executive Officers” and “Stock Ownership Matters — Section 16(a) Beneficial Ownership
Reporting Compliance” contained in our definitive Proxy Statement to be filed pursuant to
Regulation 14A of the Securities Exchange Act of 1934 (the “Exchange Act”) in connection with our
2010 Annual Meeting of Stockholders is incorporated herein by reference.
| Item 11. Executive Compensation | ||
The information set forth under the headings “Corporate Governance — Standing Committees of
our Board — Compensation Committee,” “Director Compensation,” “Compensation Committee Interlocks
and Insider Participation,” “Compensation Discussion and Analysis,” “Executive Compensation” and
“Compensation Committee Report” contained in our definitive Proxy Statement to be filed pursuant to
Regulation 14A of the Exchange Act in connection with our 2010 Annual Meeting of Stockholders is
incorporated herein by reference.
| Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters | ||
The information set forth under the heading “Stock Ownership Matters — Security Ownership of
Principal Stockholders and Management” contained in our definitive Proxy Statement to be filed
pursuant to Regulation 14A of the Exchange Act in connection with our 2010 Annual Meeting of
Stockholders is incorporated herein by reference.
Equity-Based Compensation Plans. The following table provides information, as of December 31,
2009, about our common stock that may be issued upon the exercise of options or vesting of
restricted stock awards under the Odyssey HealthCare, Inc. Stock Option Plan and the 2001
Equity-Based Compensation Plan:
EQUITY COMPENSATION PLAN INFORMATION
| (c) | ||||||||||||
| (a) | Number of Securities | |||||||||||
| Number of Securities | Remaining Available | |||||||||||
| to be Issued | (b) | for Future Issuance | ||||||||||
| Upon Exercise of | Weighted-Average | Under Equity Compensation | ||||||||||
| Outstanding Options, | Exercise Price of | Plans (Excluding | ||||||||||
| Warrants, Awards | Outstanding Options, | Securities | ||||||||||
| Plan Category | and Rights | Warrants, and Rights | Reflected in Column(a)) | |||||||||
| (In thousands, except weighted average exercise price) | ||||||||||||
Equity
Compensation Plans
Approved by
Stockholders |
2,826 | (1) | $ | 16.01 | 1,473 | |||||||
Equity Compensation
Plans Not Approved
by Stockholders |
— | — | — | |||||||||
Total |
2,826 | $ | 16.01 | 1,473 | ||||||||
66
Table of Contents
| (1) | Includes 1,433,726 options outstanding and 1,392,202 unvested restricted stock awards and units at December 31, 2009. Restricted stock awards and units are not included in the calculation of the weighted-average exercise price since there is no exercise price associated with the award. |
| Item 13. Certain Relationships and Related Transactions, and Director Independence | ||
The information set forth under the headings “Transactions With Related Persons” and
“Corporate Governance — Our Board — Board Size; Director Independence” contained in our definitive
Proxy Statement to be filed pursuant to Regulation 14A of the Exchange Act in connection with our
2010 Annual Meeting of Stockholders is incorporated herein by reference.
| Item 14. Principal Accountant Fees and Services | ||
The information set forth under the heading “Audit Committee Matters — Fees Paid to
Independent Auditors” contained in our definitive Proxy Statement to be filed pursuant to
Regulation 14A of the Exchange Act in connection with our 2010 Annual Meeting of Stockholders is
incorporated herein by reference.
PART IV
| Item 15. Exhibits and Financial Statement Schedules | ||
The following documents are filed as part of this Annual Report on Form 10-K:
(1) The financial statements filed as part of this Annual Report on Form 10-K are listed in
the Index to Consolidated Financial Statements on page F-1 of this Annual Report on Form 10-K.
(2) All financial statement schedules are omitted because the required information is not
present, not present in material amounts or is presented within the financial statements.
(3) The following documents are filed or incorporated by reference as exhibits to this
Annual Report on Form 10-K:
| Exhibit | ||
| Number | Description | |
2.1 —
|
Agreement and Plan of Merger, dated January 15, 2008, among Odyssey HealthCare Holding Company, OHC Investment, Inc. and VistaCare, Inc. (incorporated by reference to Exhibit 2.1 to Odyssey HealthCare, Inc’s (the “Company”) Current Report on Form 8-K as filed with the Securities and Exchange Commission (the “Commission”) on January 15, 2008)(1) | |
2.2 —
|
Form of Stockholder Agreement, dated January 15, 2008, among Odyssey HealthCare Holding Company, OHC Investment, Inc. and each of the following directors and executive officers of VistaCare, Inc.: Richard R. Slager, John Crisci, Stephen Lewis, Roseanne Berry, Henry Hirvela, James T. Robinson, James C. Crews, Jon M. Donnell, Jack A. Henry, Geneva B. Johnson, Pete A. Klisares and Brian S. Tyler (incorporated by reference to Exhibit 2.2 to the Company’s Current Report on Form 8-K as filed with the Commission on January 15, 2008) | |
3.1 —
|
Fifth Amended and Restated Certificate of Incorporation (incorporated by reference to Exhibit 3.1 to the Company’s Amendment No. 2 to Registration Statement on Form S-1 (Registration No. 333-51522) as filed with the Commission on September 13, 2001) | |
3.2 —
|
Second Amended and Restated Bylaws (incorporated by reference to Exhibit 3.2 to the Company’s Registration Statement on Form S-1 (Registration No. 333-51522) as filed with the Commission on December 8, 2000) | |
3.3 —
|
First Amendment to the Second Amended and Restated Bylaws of Odyssey HealthCare, Inc., effective as of December 20, 2007 (incorporated by reference to Exhibit 3.2 to the Company’s Current Report on Form 8-K as filed with the Commission on December 21, 2007) | |
3.4 —
|
Second Amendment to the Second Amended and Restated Bylaws of Odyssey HealthCare, Inc., effective as of May 20, 2008 (incorporated by reference to Exhibit 3.1 to the Company’s Current |
67
Table of Contents
| Exhibit | ||
| Number | Description | |
| Report on Form 8-K as filed with the Commission on May 20, 2008) | ||
4.1 —
|
Form of Common Stock Certificate (incorporated by reference to Exhibit 4.1 to the Company’s Amendment No. 1 to Registration Statement on Form S-1 (Registration No. 333-51522) as filed with the Commission on August 2, 2001) | |
4.2 —
|
Rights Agreement (the “Rights Agreement”) dated November 5, 2001, between Odyssey HealthCare, Inc. and Rights Agent (incorporated by reference to Exhibit 4.1 to the Company’s Registration Statement on Form 8-A as filed with the Commission on December 8, 2001) | |
4.3 —
|
Form of Certificate of Designation of Series A Junior Participating Preferred Stock (included as Exhibit A to the Rights Agreement (Exhibit 4.3 hereto)) | |
10.1.1 —
|
Second Amended and Restated Credit Agreement, dated February 28, 2008, by and among General Electric Capital Corporation, a Delaware corporation, individually as Lender and as Agent for the Lenders, the other Lenders signatory thereto, Odyssey HealthCare Operating A, LP, a Delaware limited partnership, Odyssey HealthCare Operating B, LP, a Delaware limited partnership, Hospice of the Palm Coast, Inc., a Florida not for profit corporation, OHC Investment Inc., a Delaware corporation, and the other Credit Parties signatory thereto (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K as filed with the Commission on March 4, 2008) | |
10.1.2 —
|
Amendment No. 1 to Second Amended and Restated Credit Agreement, dated November 7, 2008, by and among General Electric Capital Corporation, a Delaware corporation, individually as Lender and as Agent for the Lenders, the other Lenders signatory thereto, Odyssey HealthCare Operating A, LP, a Delaware limited partnership, Odyssey HealthCare Operating B, LP, a Delaware limited partnership, Hospice of the Palm Coast, Inc., a Florida not for profit corporation, and VistaCare, Inc., a Delaware corporation (incorporated by reference to Exhibit 10.1 to the Company’s Quarterly Report on Form 10-Q as filed with the Commission on November 10, 2008) | |
10.2† —
|
Amended and Restated Employment Agreement, by and between Odyssey HealthCare, Inc. and Robert A. Lefton, effective as of October 11, 2005 (unless otherwise specified therein) (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K as filed with the Commission on December 24, 2008) | |
10.3† —
|
Amended and Restated Employment Agreement, by and between Odyssey HealthCare, Inc. and Brenda A. Belger, effective as of August 1, 2005 (unless otherwise specified therein) (incorporated by reference to Exhibit 10.4 to the Company’s Current Report on Form 8-K as filed with the Commission on December 24, 2008) | |
10.4† —
|
Amended and Restated Employment Agreement, by and between Odyssey HealthCare, Inc. and W. Bradley Bickham, effective as of August 1, 2005 (unless otherwise specified therein) (incorporated by reference to Exhibit 10.3 to the Company’s Current Report on Form 8-K as filed with the Commission on December 24, 2008) |
68
Table of Contents
| Exhibit | ||
| Number | Description | |
10.5† —
|
Amended and Restated Employment Agreement, by and between Odyssey HealthCare, Inc. and R. Dirk Allison, effective as of October 30, 2006 (unless otherwise specified therein) (incorporated by reference to Exhibit 10.2 to the Company’s Current Report on Form 8-K as filed with the Commission on December 24, 2008) | |
10.6.1† —
|
Amended and Restated Employment Agreement, by and between Odyssey HealthCare, Inc. and Craig P. Goguen, effective as of August 20, 2007 (unless otherwise specified therein) (incorporated by reference to Exhibit 10.5 to the Company’s Current Report on Form 8-K as filed with the Commission on December 24, 2008) | |
10.7† —
|
Employment Agreement, by and between Odyssey HealthCare, Inc. and Frank Anastasio, dated March 17, 2008 (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K as filed with the Commission on March 21, 2008) | |
10.8† —
|
Agreement by and among Odyssey HealthCare, Inc. and Richard R. Burnham, effective as of January 1, 2007 (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K as filed with the Commission on January 5, 2007) | |
10.9.1† —
|
Odyssey HealthCare, Inc. Stock Option Plan (the “Stock Option Plan”) (incorporated by reference to Exhibit 10.5 to the Company’s Registration Statement on Form S-1 (Registration No. 333-51522) as filed with the Commission on December 8, 2000) | |
10.9.2† —
|
First Amendment to the Stock Option Plan, dated January 31, 2001 (incorporated by reference to Exhibit 10.5.2 to the Company’s Amendment No. 2 to Registration Statement on Form S-1 (Registration No. 333-51522) as filed with the Commission on September 13, 2001) | |
10.10.1† —
|
2001 Equity-Based Compensation Plan (incorporated by reference to Exhibit 10.6 to the Company’s Amendment No. 2 to Registration Statement on Form S-1 (Registration No. 333-51522) as filed with the Commission on September 13, 2001) | |
10.10.2† —
|
First Amendment to the 2001 Equity-Based Compensation Plan, dated May 5, 2005 (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K as filed with the Commission On May 5, 2005) | |
10.10.3† —
|
Second Amendment to the 2001 Equity-Based Compensation Plan, dated May 5, 2005 (incorporated by reference to Exhibit 10.2 to the Company’s Quarterly Report on Form 10-Q as filed with the Commission on August 8, 2005) | |
10.10.4† —
|
Form of Restricted Stock Award Agreement pursuant to the 2001 Equity — Based Compensation Plan Management Stock Option Agreement* | |
10.10.5† —
|
Odyssey HealthCare, Inc. Equity-Based Compensation Plan Management Stock Option Agreement, dated October 11, 2005, by and between Odyssey HealthCare, Inc. and Robert A. Lefton (incorporated by reference to Exhibit 10.2 to the Company’s Current Report on Form 8-K as filed with the Commission on October 12, 2005) | |
10.10.6† —
|
Form of Restricted Stock Unit Award Agreement under the Odyssey HealthCare Inc. 2001 Equity Based Compensation Plan — Time Based RSU Award (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K as filed with the Commission on February 26, 2007) | |
10.10.7† —
|
Form Restricted Stock Unit Award Agreement under the Odyssey HealthCare Inc. 2001 Equity Based Compensation Plan — Additional Incentive Based RSU Award (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K as filed with the Commission on February 26, 2007) | |
10.10.8 —
|
Form of Restricted Stock Award Agreement under the Odyssey HealthCare, Inc. 2001 Equity-Based Compensation Plan — Non-Employee Director Award (incorporated by reference to Exhibit 10.4 to the Company’s Quarterly Report on Form 10-Q as filed with the Commission on November 9, 2007) | |
10.11.1 —
|
Employee Stock Purchase Plan (incorporated by reference to Exhibit 10.7 to the Company’s Amendment No. 2 to Registration Statement on Form S-1 (Registration No. 333-51522) as filed with the Commission on September 13, 2001) | |
10.11.2 —
|
First Amendment to Employee Stock Purchase Plan, dated March 6, 2002* | |
10.12† —
|
Form of Indemnification Agreement between Odyssey HealthCare, Inc. and its directors and officers (incorporated by reference to Exhibit 10.8 to the Company’s Registration Statement on Form S-1 (Registration No. 333-51522) as filed with the Commission on December 8, 2000) |
69
Table of Contents
| Exhibit | ||
| Number | Description | |
10.13 —
|
Settlement Agreement, dated July 6, 2006, among the United States of America acting through the entities named therein, JoAnn Russell and Odyssey HealthCare, Inc. (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K as filed with the Commission on July 12, 2006) | |
10.14 —
|
Corporate Integrity Agreement, dated July 6, 2006, between the Office of Inspector General of the Department of Health and Human Services and Odyssey HealthCare, Inc. (incorporated by Reference to Exhibit 10.2 to the Company’s Current Report on Form 8-K as filed with the Commission on July 12, 2006) | |
21 —
|
Subsidiaries of Odyssey HealthCare, Inc.* | |
23.1 —
|
Consent of Ernst & Young LLP* | |
31.1 —
|
Certification required by Rule 13a-14(a), dated March 10, 2010, by Robert A. Lefton, Chief Executive Officer* | |
31.2 —
|
Certification required by Rule 13a-14(a), dated March 10, 2010, by R. Dirk Allison, Chief Financial Officer* | |
32 —
|
Certification required by Rule 13a-14(b), dated March 10, 2010, by Robert A. Lefton, Chief Executive Officer, and R. Dirk Allison, Chief Financial Officer** |
| † | Management contract or compensatory plan or arrangement. | |
| * | Filed herewith. | |
| ** | Furnished herewith. | |
| (1) | The schedules and exhibits to the Agreement and Plan of Merger have been omitted from this filing pursuant to Item 601(b)(2) of Regulation S-K. The Company will furnish copies of any such schedules and exhibits to the SEC upon request. |
70
Table of Contents
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934,
the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto
duly authorized.
| ODYSSEY HEALTHCARE, INC. |
||||
| By: | /s/ ROBERT A. LEFTON | |||
| Robert A. Lefton | ||||
| President and Chief Executive Officer | ||||
Date: March 10, 2010
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been
signed below by the following persons on behalf of registrant and in the capacities and on the
dates indicated:
| Signature | Title | Date | ||
/s/ ROBERT A. LEFTON
|
President, Chief Executive Officer, and Director (Principal Executive Officer) |
March 10, 2010 | ||
/s/ R. DIRK ALLISON
|
Senior Vice President, Chief Financial Officer, Assistant Secretary and Treasurer (Principal Financial and Accounting Officer) |
March 10, 2010 | ||
/s/ RICHARD R. BURNHAM
|
Chairman of the Board | March 10, 2010 | ||
/s/ JAMES E. BUNCHER
|
Director | March 10, 2010 | ||
/s/ JOHN K. CARLYLE
|
Director | March 10, 2010 | ||
/s/ DAVID W. CROSS
|
Director | March 10, 2010 | ||
/s/ PAUL J. FELDSTEIN
|
Director | March 10, 2010 | ||
/s/ ROBERT A. ORTENZIO
|
Director | March 10, 2010 | ||
/s/ SHAWN S. SCHABEL
|
Director | March 10, 2010 | ||
/s/ DAVID L. STEFFY
|
Director | March 10, 2010 |
71
Table of Contents
ODYSSEY HEALTHCARE, INC. AND SUBSIDIARIES
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
| Page | ||||
Odyssey HealthCare, Inc. |
||||
| F-2 | ||||
| F-3 | ||||
| F-4 | ||||
| F-5 | ||||
| F-6 | ||||
| F-7 | ||||
F-1
Table of Contents
Report of Ernst & Young LLP, Independent Registered Public Accounting Firm
The Board of Directors and Shareholders of Odyssey HealthCare, Inc.
We have audited the accompanying consolidated balance sheets of Odyssey HealthCare, Inc. and
subsidiaries as of December 31, 2009 and 2008, and the related consolidated statements of income,
stockholders’ equity, and cash flows for each of the three years in the period ended December 31,
2009. These financial statements are the responsibility of the Company’s management. Our
responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight
Board (United States). Those standards require that we plan and perform the audit to obtain
reasonable assurance about whether the financial statements are free of material misstatement. An
audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the
financial statements. An audit also includes assessing the accounting principles used and
significant estimates made by management, as well as evaluating the overall financial statement
presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material
respects, the consolidated financial position of Odyssey HealthCare, Inc. and subsidiaries at
December 31, 2009 and 2008, and the consolidated results of their operations and their cash flows
for each of the three years in the period ended December 31, 2009, in conformity with U.S.
generally accepted accounting principles.
As discussed in Note 2 to the consolidated financial statements, the Company adopted revisions to
U.S. generally accepted accounting principles and changed its method of accounting and financial
statement presentation of noncontrolling interests in equity of consolidated subsidiaries,
effective January 1, 2009.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight
Board (United States), Odyssey HealthCare, Inc. and subsidiaries’ internal control over financial reporting as of
December 31, 2009, based on criteria established in Internal Control-Integrated Framework issued by
the Committee of Sponsoring Organizations of the Treadway Commission and our report dated March 10,
2010 expressed an unqualified opinion thereon.
| /s/ Ernst & Young LLP | ||||
Dallas, Texas
March 10, 2010
F-2
Table of Contents
ODYSSEY HEALTHCARE, INC. AND SUBSIDIARIES
CONSOLIDATED BALANCE SHEETS
CONSOLIDATED BALANCE SHEETS
| December 31, | ||||||||
| 2009 | 2008 | |||||||
| (In thousands, except | ||||||||
| share and per share amounts) | ||||||||
ASSETS |
||||||||
Current assets: |
||||||||
Cash and cash equivalents |
$ | 128,632 | $ | 56,043 | ||||
Accounts receivable from patient services, net of
allowance for uncollectible accounts of $12,462 and
$9,789 at December 31, 2009 and 2008, respectively |
110,593 | 127,922 | ||||||
Income taxes receivable |
352 | 66 | ||||||
Deferred tax assets |
10,235 | 13,319 | ||||||
Prepaid expenses and other current assets |
6,017 | 7,906 | ||||||
Assets of discontinued operations |
— | 2,067 | ||||||
Total current assets |
255,829 | 207,323 | ||||||
Property and equipment, net of accumulated depreciation |
20,700 | 22,816 | ||||||
Deferred loan costs, net |
3,033 | 3,761 | ||||||
Long-term investments |
12,425 | 16,659 | ||||||
Intangibles, net of accumulated amortization |
19,251 | 19,644 | ||||||
Goodwill |
191,766 | 189,521 | ||||||
Other assets |
— | 1,227 | ||||||
Total assets |
$ | 503,004 | $ | 460,951 | ||||
LIABILITIES AND EQUITY |
||||||||
Current liabilities: |
||||||||
Accounts payable |
$ | 4,016 | $ | 4,906 | ||||
Accrued compensation |
31,729 | 27,493 | ||||||
Accrued nursing home costs |
18,144 | 16,478 | ||||||
Accrued Medicare cap contractual adjustments |
18,798 | 23,719 | ||||||
Other accrued expenses |
44,187 | 45,904 | ||||||
Current maturities of long-term debt |
38,675 | 6,394 | ||||||
Total current liabilities |
155,549 | 124,894 | ||||||
Long-term debt, less current maturities |
76,527 | 116,681 | ||||||
Deferred tax liabilities |
15,171 | 13,610 | ||||||
Other liabilities |
4,597 | 3,233 | ||||||
Commitments and contingencies |
— | — | ||||||
Equity: |
||||||||
Odyssey stockholders’ equity: |
||||||||
Common stock, $.001 par value: 75,000,000 shares authorized — 38,549,833 and 38,137,834 shares issued at December 31, 2009 and 2008, respectively |
39 | 38 | ||||||
Additional paid-in capital |
125,716 | 117,732 | ||||||
Retained earnings |
194,431 | 153,840 | ||||||
Accumulated other comprehensive loss, net of income taxes |
(1,481 | ) | (1,585 | ) | ||||
Treasury stock, at cost, 5,347,072 shares held at
December 31, 2009 and 2008, respectively |
(69,954 | ) | (69,954 | ) | ||||
Total Odyssey stockholders’ equity |
248,751 | 200,071 | ||||||
Noncontrolling interests |
2,409 | 2,462 | ||||||
Total equity |
251,160 | 202,533 | ||||||
Total liabilities and equity |
$ | 503,004 | $ | 460,951 | ||||
F-3
Table of Contents
ODYSSEY HEALTHCARE, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF INCOME
CONSOLIDATED STATEMENTS OF INCOME
| Year Ended December 31, | ||||||||||||
| 2009 | 2008 | 2007 | ||||||||||
| (In thousands, except per share amounts) | ||||||||||||
Net patient service revenue |
$ | 686,438 | $ | 616,050 | $ | 398,232 | ||||||
Operating expenses: |
||||||||||||
Direct hospice care |
396,774 | 361,445 | 233,664 | |||||||||
General and administrative — hospice care |
134,335 | 128,718 | 85,304 | |||||||||
General and administrative — support center |
64,734 | 70,574 | 46,484 | |||||||||
Provision for uncollectible accounts |
11,490 | 10,907 | 5,344 | |||||||||
Depreciation |
6,333 | 7,437 | 5,480 | |||||||||
Amortization |
392 | 431 | 243 | |||||||||
| 614,058 | 579,512 | 376,519 | ||||||||||
Income from continuing operations before other income (expense) |
72,380 | 36,538 | 21,713 | |||||||||
Other income (expense): |
||||||||||||
Interest income |
479 | 1,968 | 2,509 | |||||||||
Interest expense |
(6,574 | ) | (7,430 | ) | (208 | ) | ||||||
| (6,095 | ) | (5,462 | ) | 2,301 | ||||||||
Income from continuing operations before provision for income taxes |
66,285 | 31,076 | 24,014 | |||||||||
Provision for income taxes |
24,583 | 11,141 | 8,001 | |||||||||
Income from continuing operations |
41,702 | 19,935 | 16,013 | |||||||||
Loss from discontinued operations, net of income taxes |
(498 | ) | (5,252 | ) | (3,888 | ) | ||||||
Net income |
41,204 | 14,683 | 12,125 | |||||||||
Less: Net income attributable to noncontrolling interests |
613 | 257 | 14 | |||||||||
Net income attributable to Odyssey stockholders |
$ | 40,591 | $ | 14,426 | $ | 12,111 | ||||||
Income (loss) per common share: |
||||||||||||
Basic: |
||||||||||||
Continuing operations attributable to Odyssey stockholders |
$ | 1.25 | $ | 0.60 | $ | 0.48 | ||||||
Discontinued operations attributable to Odyssey stockholders |
(0.02 | ) | (0.16 | ) | (0.12 | ) | ||||||
Net income attributable to Odyssey stockholders |
$ | 1.23 | $ | 0.44 | $ | 0.36 | ||||||
Diluted: |
||||||||||||
Continuing operations attributable to Odyssey stockholders |
$ | 1.24 | $ | 0.59 | $ | 0.48 | ||||||
Discontinued operations attributable to Odyssey stockholders |
(0.02 | ) | (0.16 | ) | (0.12 | ) | ||||||
Net income attributable to Odyssey stockholders |
$ | 1.22 | $ | 0.43 | $ | 0.36 | ||||||
Weighted average shares outstanding: |
||||||||||||
Basic |
32,935 | 32,674 | 33,029 | |||||||||
Diluted |
33,225 | 33,188 | 33,188 | |||||||||
F-4
Table of Contents
ODYSSEY HEALTHCARE, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY
| Additional | Accumulated Other | Total | ||||||||||||||||||||||||||||||
| Common Stock | Paid-in | Retained | Comprehensive | Treasury | Noncontrolling | Stockholders’ | ||||||||||||||||||||||||||
| Shares | Amount | Capital | Earnings | Loss | Stock | Interests | Equity | |||||||||||||||||||||||||
| (Amounts in thousands) | ||||||||||||||||||||||||||||||||
Balance at January 1, 2007 |
37,870 | $ | 38 | $ | 108,682 | $ | 126,921 | $ | — | $ | (56,045 | ) | $ | — | $ | 179,596 | ||||||||||||||||
Share-based compensation |
9 | — | 3,829 | — | — | — | — | 3,829 | ||||||||||||||||||||||||
Income tax benefit on
share-based compensation |
— | — | 119 | — | — | — | — | 119 | ||||||||||||||||||||||||
Exercise of stock options |
161 | — | 472 | — | — | — | — | 472 | ||||||||||||||||||||||||
Issuance of shares under
Employee Stock Purchase Plan |
23 | — | 237 | — | — | — | — | 237 | ||||||||||||||||||||||||
Purchase of treasury stock,
at cost |
— | — | — | — | — | (13,909 | ) | — | (13,909 | ) | ||||||||||||||||||||||
Cumulative effect of change
in accounting for
uncertainties in income taxes |
— | — | — | 382 | — | — | — | 382 | ||||||||||||||||||||||||
Transactions between Odyssey
and noncontrolling interests |
— | — | — | — | — | — | 881 | 881 | ||||||||||||||||||||||||
Net income |
— | — | — | 12,111 | — | — | 14 | 12,125 | ||||||||||||||||||||||||
Balance at December 31, 2007 |
38,063 | 38 | 113,339 | 139,414 | — | (69,954 | ) | 895 | 183,732 | |||||||||||||||||||||||
Comprehensive income: |
||||||||||||||||||||||||||||||||
Net income |
— | — | — | 14,426 | — | — | 257 | 14,683 | ||||||||||||||||||||||||
Unrealized loss on interest
rate swaps, net of income
taxes |
— | — | — | — | (1,303 | ) | — | — | (1,303 | ) | ||||||||||||||||||||||
Unrealized loss on auction
rate securities, net of
income taxes |
— | — | — | — | (282 | ) | — | — | (282 | ) | ||||||||||||||||||||||
Total comprehensive income,
net of income taxes |
13,098 | |||||||||||||||||||||||||||||||
Transactions between Odyssey
and noncontrolling interests |
— | — | — | — | — | — | 1,310 | 1,310 | ||||||||||||||||||||||||
Share-based compensation |
80 | — | 4,347 | — | — | — | — | 4,347 | ||||||||||||||||||||||||
Income tax benefit on
share-based compensation |
— | — | 150 | — | — | — | — | 150 | ||||||||||||||||||||||||
Shares redeemed for employee
tax withholdings |
(35 | ) | — | (336 | ) | — | — | — | — | (336 | ) | |||||||||||||||||||||
Exercise of stock options |
11 | — | 50 | — | — | — | — | 50 | ||||||||||||||||||||||||
Issuance of shares under
Employee Stock Purchase Plan |
19 | — | 182 | — | — | — | — | 182 | ||||||||||||||||||||||||
Balance at December 31, 2008 |
38,138 | 38 | 117,732 | 153,840 | (1,585 | ) | (69,954 | ) | 2,462 | 202,533 | ||||||||||||||||||||||
Comprehensive income: |
||||||||||||||||||||||||||||||||
Net income |
— | — | — | 40,591 | — | — | 613 | 41,204 | ||||||||||||||||||||||||
Unrealized gain on interest
rate swaps, net of income
taxes |
— | — | — | — | 185 | — | — | 185 | ||||||||||||||||||||||||
Unrealized loss on auction
rate securities, net of
income taxes |
— | — | — | — | (81 | ) | — | — | (81 | ) | ||||||||||||||||||||||
Total comprehensive income,
net of income taxes |
41,308 | |||||||||||||||||||||||||||||||
Transactions between Odyssey
and noncontrolling interests |
— | — | — | — | — | — | (666 | ) | (666 | ) | ||||||||||||||||||||||
Share-based compensation |
138 | — | 5,083 | — | — | — | — | 5,083 | ||||||||||||||||||||||||
Income tax benefit on
share-based compensation |
— | — | 1,345 | — | — | — | — | 1,345 | ||||||||||||||||||||||||
Shares redeemed for employee
tax withholdings |
— | — | (658 | ) | — | — | — | — | (658 | ) | ||||||||||||||||||||||
Exercise of stock options |
274 | 1 | 2,214 | — | — | — | — | 2,215 | ||||||||||||||||||||||||
Balance at December 31, 2009 |
38,550 | $ | 39 | $ | 125,716 | $ | 194,431 | $ | (1,481 | ) | $ | (69,954 | ) | $ | 2,409 | $ | 251,160 | |||||||||||||||
F-5
Table of Contents
ODYSSEY HEALTHCARE, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CASH FLOWS
CONSOLIDATED STATEMENTS OF CASH FLOWS
| Year Ended December 31, | ||||||||||||
| 2009 | 2008 | 2007 | ||||||||||
| (In thousands) | ||||||||||||
Operating Activities: |
||||||||||||
Net income attributable to Odyssey stockholders |
$ | 40,591 | $ | 14,426 | $ | 12,111 | ||||||
Adjustments to reconcile net income to net cash provided by
operating activities and discontinued operations: |
||||||||||||
Loss from discontinued operations, net of taxes |
498 | 5,252 | 3,888 | |||||||||
Net income attributable to noncontrolling interests |
613 | 257 | 14 | |||||||||
Loss on disposal of property and equipment |
410 | 150 | 211 | |||||||||
Depreciation and amortization |
6,725 | 7,868 | 5,723 | |||||||||
Amortization of deferred loan costs |
728 | 892 | 113 | |||||||||
Share-based compensation expense |
5,083 | 4,347 | 3,829 | |||||||||
Deferred income taxes |
4,588 | (2,233 | ) | (1,408 | ) | |||||||
Provision for uncollectible accounts |
11,490 | 10,907 | 5,344 | |||||||||
Changes in operating assets and liabilities, net of acquisitions: |
||||||||||||
Accounts receivable from patient services |
7,240 | (21,980 | ) | (18,770 | ) | |||||||
Prepaid expenses and other current assets |
2,919 | 3,108 | 458 | |||||||||
Accounts payable, accrued nursing home costs, accrued
Medicare cap contractual adjustments and other accrued
expenses |
765 | (1,945 | ) | 1,301 | ||||||||
Net cash provided by operating activities |
81,650 | 21,049 | 12,814 | |||||||||
Investing Activities: |
||||||||||||
Cash paid for acquisitions, net of cash acquired |
(3,035 | ) | (126,796 | ) | 724 | |||||||
Cash received from the sale of hospice programs and property |
1,490 | 1,344 | 698 | |||||||||
Purchases of short-term and long-term investments |
— | (9,000 | ) | (49,053 | ) | |||||||
Sales of short-term and long-term investments |
4,100 | 41,693 | 61,650 | |||||||||
Purchases of property and equipment, net |
(6,638 | ) | (4,428 | ) | (9,628 | ) | ||||||
Net cash (used in) provided by investing activities |
(4,083 | ) | (97,187 | ) | 4,391 | |||||||
Financing Activities: |
||||||||||||
Proceeds from exercise of stock options |
2,215 | 46 | 877 | |||||||||
Cash (paid) received from sale of partnership interests and
partnership distributions |
(665 | ) | 893 | 881 | ||||||||
Tax benefit from share-based compensation |
1,345 | 150 | 119 | |||||||||
Purchase of treasury stock |
— | — | (13,909 | ) | ||||||||
Payments of debt issue costs |
— | (4,368 | ) | (357 | ) | |||||||
Proceeds from borrowings under credit facility |
— | 130,000 | — | |||||||||
Payments on credit facility |
(7,873 | ) | (6,926 | ) | (2 | ) | ||||||
Net cash (used in) provided by financing activities |
(4,978 | ) | 119,795 | (12,391 | ) | |||||||
Net increase in cash and cash equivalents |
72,589 | 43,657 | 4,814 | |||||||||
Cash and cash equivalents, beginning of year |
56,043 | 12,386 | 7,572 | |||||||||
Cash and cash equivalents, end of year |
$ | 128,632 | $ | 56,043 | $ | 12,386 | ||||||
Supplemental cash flow information: |
||||||||||||
Cash interest paid |
$ | 5,875 | $ | 5,529 | $ | 95 | ||||||
Income taxes paid |
$ | 20,363 | $ | 1,754 | $ | 5,389 | ||||||
F-6
Table of Contents
ODYSSEY HEALTHCARE, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Years Ended December 31, 2009, 2008 and 2007
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Years Ended December 31, 2009, 2008 and 2007
1. Description of Business
Odyssey HealthCare, Inc. and its subsidiaries (the “Company”) provide hospice care, with a
goal of improving the quality of life of terminally ill patients and their families. Hospice
services focus on palliative care for patients with life-limiting illnesses, which is care directed
at managing pain and other discomforting symptoms and addressing the psychosocial and spiritual
needs of patients and their families. The Company provides for all medical, psychosocial care and
certain other support services related to the patient’s terminal illness.
The Company was incorporated on August 29, 1995 in the state of Delaware and, as of December
31, 2009, had 90 Medicare-certified hospice providers serving patients and their families in 29
states, with significant operations in Texas, California and Arizona.
The Company completed its acquisition of VistaCare on March 6, 2008. The acquisition described
in note “3. Acquisitions” significantly affects the comparability of the financial information for
the years ended December 31, 2009, 2008 and 2007.
During 2008 and 2007, the Company offered equity interests in its Savannah, Georgia; Augusta,
Georgia; Kansas City, Missouri and Brownsville, Texas hospice programs to third party investors of
approximately 40%, 40%, 20% and 40%, respectively. The Company received $1.6 million and $0.9
million in proceeds during 2008 and 2007, respectively, from its investors in the offerings, which
is recorded in noncontrolling interests on the Company’s consolidated balance sheets.
2. Summary of Significant Accounting Policies
Basis of Presentation and Principles of Consolidation
The accompanying consolidated financial statements reflect the results of operations and cash
flows for the years ended December 31, 2009, 2008 and 2007. Certain amounts reported in previous
years have been reclassified to conform to the 2009 presentation. In the period that a component
of an entity has been disposed of or classified as held for sale, the results of operations for
current and prior periods are reclassified in a single caption titled discontinued operations. The
consolidated financial statements include the accounts of Odyssey HealthCare, Inc., its
wholly-owned subsidiaries, and all subsidiaries and entities controlled by the Company through its
direct ownership of a majority voting interest. All material intercompany accounts and transactions
have been eliminated in consolidation.
The Company’s consolidated financial statements include the accounts and operations of the
Company and its subsidiaries and noncontrolling interests in which it owns more than a 50 percent
interest. Noncontrolling interests, previously shown as minority interests, are reported below net
income under the heading “Net income attributable to noncontrolling interests” in the consolidated
statements of income and shown as a component of equity in the consolidated balance sheets.
Use of Estimates
The preparation of financial statements in conformity with U.S. generally accepted accounting
principles requires management to make estimates and assumptions that affect the amounts reported
in the consolidated financial statements and accompanying notes. Management estimates include an
allowance for uncollectible accounts, accrued compensation, accrued Medicare cap contractual
allowances, other contractual allowances, accrued nursing home costs, accrued workers’
compensation, accrued patient care costs, accrued income taxes, accrued professional fees, accrued
legal settlements, goodwill and intangible asset impairment and share-based compensation expense
related to performance based awards. Actual results could differ from those estimates and such
difference could be material.
F-7
Table of Contents
Cash and Cash Equivalents
Cash and cash equivalents include currency, checks on hand, money market funds and overnight
repurchase agreements of government securities.
Long-term Investments
As of December 31, 2009 and 2008, the Company had long-term investments totaling $12.4 million
and $16.7 million, respectively, consisting of tax exempt auction rate securities (“ARS”). The ARS
held by the Company are private placement securities for which the interest rates are reset every
35 days. The reset dates have historically provided a liquid market for these securities as
investors historically could readily sell their investments. These types of securities generally
have not experienced payment defaults and are backed by student loans, which carry guarantees as
provided for under the Federal Family Education Loan Program of the U.S. Department of Education.
All of the securities were AAA/Aaa rated at December 31, 2009. To date the Company has collected
all interest payments on all of its ARS when due and expects to continue to do so in the future.
The Company intended to liquidate all of its ARS prior to the end of 2009. However, due to the
problems experienced in global credit and capital markets generally and the ARS market in
particular, the Company’s ability to liquidate its ARS this year has been impaired. The Company
successfully liquidated $4.1 million of ARS in October 2009, $8.0 million in July 2008, $8.0
million of ARS in June 2008, and $8.4 million of ARS in January 2008, all at par. The remaining
principal of $13.0 million associated with ARS will not be accessible until successful ARS auctions
occur, a buyer is found outside of the auction process, the issuers establish a different form of
financing to replace these securities, issuers repay principal over time from cash flows prior to
maturity, or final payments come due according to contractual maturities from 25 to 28 years. The
Company expects that it will receive the principal associated with these ARS through one of these
means. The Company has classified these ARS as long-term investments.
The Company prepared a discounted cash flow analysis for its ARS using an estimated maturity
of one year, which is when the Company estimates it will be able to liquidate these securities at
par. The Company used a discount rate to reflect the current reduced liquidity of these securities.
The discount rate was calculated by taking the existing interest rate being earned on the ARS as of
December 31, 2009 and including a liquidity risk premium rate, which was calculated based on
treasury yields applicable to the ARS maturity dates as of December 31, 2009. During the years
ended December 31, 2009 and 2008, the Company reduced the fair value of the ARS by $0.1 million and
$0.4 million (before taxes) based on this analysis. Changes in the fair value of the ARS are
recognized, net of tax, in accumulated other comprehensive income (loss).
If the uncertainties in the credit and capital markets continue or these markets deteriorate
further, these securities may not provide liquidity to the Company when needed or maintain the fair
values estimated by the Company. If the Company had to liquidate any ARS at this time, it could
incur significant losses. The Company currently believes that it has sufficient liquidity for its
current needs without selling any ARS and does not currently intend to attempt to liquidate these
securities until market conditions improve and it is not more likely than not that the Company will
be required to liquidate any ARS before recovery of their entire cost basis. If the Company’s
currently available resources are not sufficient for its needs and it is not able to liquidate any
ARS on acceptable terms on a timely basis, it could have a significant adverse impact on the
Company’s cash flows, financial condition and results of operations.
Fair Value of Financial Instruments
The carrying amount of the Company’s financial instruments, including cash and cash
equivalents, accounts receivable, accounts payable, and accrued liabilities approximate fair value
because of their generally short maturities. The Company also carries its ARS at fair value. See
note, “14. Fair Value of Financial Instruments” for additional information.
F-8
Table of Contents
Accounts Receivable
Accounts receivable represents amounts due from patients, third-party payors (principally the
Medicare and Medicaid programs), and others for services rendered based on payment arrangements
specific to each payor. Approximately 89.2% and 90.9% of the gross accounts receivable as of
December 31, 2009 and 2008, respectively, represent amounts due from the Medicare and Medicaid
programs. The Company maintains a policy for reserving for uncollectible accounts. The Company
calculates the allowance for uncollectible accounts based on a formula tied to the aging of
accounts receivable by payor class. The Company may also reserve for specific accounts that are
determined to be uncollectible. Accounts are written off when all collection efforts are exhausted.
Medicare fiscal intermediaries and other payors periodically conduct pre-payment and
post-payment medical reviews and other audits of the Company’s reimbursement claims. In order to
conduct these reviews, the payor requests documentation in the form of additional document requests
from the Company and then reviews that documentation to determine compliance with applicable rules
and regulations, including the eligibility of patients to receive hospice benefits, the
appropriateness of the care provided to those patients, and the documentation of that care. The
Company cannot predict whether medical reviews or similar audits by federal or state agencies or
commercial payors of the Company’s hospice programs’ reimbursement claims will result in material
recoupments or denials, which could have a material adverse effect on the Company’s financial
condition, results of operations and cash flows.
Goodwill and Indefinite-lived Intangible Assets
Goodwill is the excess of the purchase price over the fair value of identifiable assets
acquired. Indefinite-lived intangible assets are comprised of license agreements and trademarks.
Under FASB ASC Topic 350, “Intangibles — Goodwill and Other,” (formerly Statement of Financial
Accounting Standards (“SFAS”) No. 142 “Goodwill and Other Intangible Assets”), goodwill and
intangible assets with indefinite lives are not amortized, but tested for impairment annually or
more frequently if certain indications of impairment arise. Goodwill is reviewed at the reporting
unit level, which is defined as an operating segment or one level below an operating segment. The
Company has defined their reporting units at the operating segment level.
Goodwill impairment is determined using a two-step process. The first step is to identify if a
potential impairment exists by comparing the fair value of a reporting unit with its carrying
amount, including goodwill. If the fair value of a reporting unit exceeds its carrying amount,
goodwill of the reporting unit is not considered to have a potential impairment and the second step
of the impairment test is not necessary. However, if the carrying amount of a reporting unit
exceeds its fair value, the second step is performed to determine if goodwill is impaired and to
measure the amount of impairment loss to recognize, if any. The second step compares the implied
fair value of goodwill with the carrying amount of goodwill. If the implied fair value of goodwill
exceeds the carrying amount, then goodwill is not considered impaired. However, if the carrying
amount of goodwill exceeds the implied fair value, an impairment loss is recognized in an amount
equal to that excess. The implied fair value of goodwill is determined in the same manner as the
amount of goodwill recognized in a business combination (i.e., the fair value of the reporting unit
is allocated to all the assets and liabilities, including any unrecognized intangible assets, as if
the reporting unit had been acquired in a business combination and the fair value of the reporting
unit was the purchase price paid to acquire the reporting unit). The amount of the impairment would
be the difference between the carrying amount of the goodwill and the implied fair value of the
goodwill.
The Company’s 2009 annual goodwill impairment testing was performed as of November 30, 2009.
In determining the fair value of reporting units, the Company uses multiples of earnings before
interest, taxes, depreciation and amortization (“EBITDA”). The Company believes using multiples of
EBITDA in determining the fair value of reporting units is appropriate because it correlates with
what a market participant would be willing to pay for that reporting unit in today’s market. As of
the date of the Company’s annual impairment testing, none of the reporting units failed step one
and no reporting units were at risk of failing step one. Furthermore, the fair values of each of
the Company’s reporting units represented no less than 185% of their carrying values. The Company
evaluated whether any events had occurred or any circumstances had changed since November 30, 2009
that would indicate goodwill may have become impaired since the annual impairment testing. In this
evaluation, the Company considered both qualitative and quantitative factors such as any adverse
change in the business climate, current estimates of future profitability of reporting units, the
Company’s current stock price and its market capitalization compared to the Company’s book value.
Based on this evaluation, the Company determined that no indications of
F-9
Table of Contents
impairment have arisen since the annual goodwill impairment test. No impairment charges have
been recorded as of December 31, 2009, 2008 and 2007.
The Company’s total cumulative amortizable goodwill for tax purposes was $79.4 million and
$81.6 million as of December 31, 2009 and 2008, respectively. The goodwill and intangibles expected
to be deductible for tax purposes is $6.1 million and $5.3 million for the tax years ended December
31, 2009 and 2008, respectively.
Net Patient Service Revenue
Net patient service revenue is reported at the estimated net realizable amounts (exclusive of
the provision for uncollectible accounts) from Medicare, Medicaid, commercial insurance and managed
care payors, patients and others for services rendered to patients. To determine net patient
service revenue, management adjusts gross patient service revenue for estimated contractual
adjustments based on historical experience and estimated Medicare cap contractual adjustments. Net
patient service revenue does not include charity care or the Medicaid room and board payments. Net
patient service revenue is recognized in the month in which services are delivered. The percentage
of net patient service revenue derived under the Medicare and Medicaid programs was 97.0%, 96.6%
and 97.0% for the years ended December 31, 2009, 2008 and 2007, respectively.
The Company is subject to two limitations on Medicare payments for services. With one
limitation, if inpatient days of care provided to patients at a hospice exceeds 20% of the total
days of hospice care provided for an annual period beginning on November 1, then payment for days
in excess of this limit are paid for at the routine home care rate. None of the Company’s hospice
programs exceeded the payment limits on inpatient services for the years ended December 31, 2009,
2008 and 2007.
With the other limitation, overall payments made by Medicare to the Company on a per hospice
program basis are also subject to a cap amount calculated by the Medicare fiscal intermediary at
the end of the hospice cap period. The Medicare revenue paid to a hospice program from November 1
to October 31 of the following year may not exceed the annual cap amount which is calculated by
using the following formula: Number of admissions to the program by patients who are electing to
receive their Medicare hospice benefit for the first time multiplied by the Medicare cap amount,
which for the November 1, 2008 through October 31, 2009 Medicare cap year was $23,014. The Medicare
cap amount is reduced proportionately for patients who transferred in or out of our hospice
services. The Medicare cap amount is annually adjusted for inflation, but is not adjusted for
geographic differences in wage levels, although hospice per diem payment rates are wage indexed.
The Medicare cap amount for the November 1, 2009 through October 31, 2010 cap year has not yet been
announced by the Medicare program. The Company currently estimates the Medicare cap amount to be
approximately $23,600 for the Medicare cap year ending October 31, 2010.
F-10
Table of Contents
The following table shows the amounts accrued and paid for the Medicare cap contractual
adjustments for the years ended December 31, 2007, 2008 and 2009, respectively:
| Accrued Medicare Cap Contractual Adjustments | ||||||||||||
| Year Ending December 31, | ||||||||||||
| 2007 | 2008 | 2009 | ||||||||||
| (in thousands) | ||||||||||||
Beginning balance — accrued Medicare
cap contractual adjustments |
$ | 26,679 | $ | 21,682 | $ | 23,719 | ||||||
Medicare cap contractual adjustments |
5,039 | (1) | 6,852 | (2) | 4,565 | (3) | ||||||
Medicare cap contractual adjustments —
discontinued operations |
2,651 | (4) | (27 | )(4) | (79 | )(4) | ||||||
Payments to Medicare fiscal intermediaries |
(12,687 | ) | (12,996 | ) | (9,407 | ) | ||||||
Balances acquired from VistaCare |
— | 8,208 | — | |||||||||
Ending balance — accrued Medicare cap
contractual adjustments |
$ | 21,682 | $ | 23,719 | $ | 18,798 | ||||||
| (1) | Includes additional accrual of $0.9 million related to the 2006 Medicare cap year. | |
| (2) | Includes additional accrual of $1.5 million related to the 2006 Medicare cap year. | |
| (3) | Includes an accrual reversal of $1.1 million related to the 2007 Medicare cap year. | |
| (4) | Medicare cap contractual adjustments reclassified to discontinued operations are related to all programs that were discontinued and sold during 2007, 2008 and 2009. |
The Company reviews the adequacy of its accrued estimated Medicare cap contractual adjustments
on a quarterly basis. Because of the many variables involved in estimating the Medicare cap
contractual there is at least a reasonable possibility that recorded estimates will change by a
material amount in the near term.
Laws and regulations governing the Medicare and Medicaid programs are complex and subject to
interpretation. The Company believes that it is in material compliance with all applicable laws and
regulations. Compliance with laws and regulations can be subject to future government review and
interpretation as well as significant regulatory action including fines, penalties and exclusion
from the Medicare and Medicaid programs.
Charity Care
The Company provides charity care to patients without charge when management of the hospice
program determines that the patient does not have the financial capability to pay, which is
determined at or near the time of admission. Because the Company does not pursue collection of
amounts determined to qualify as charity care, they are not reported as revenue.
Charity care, based on established charges, amounted to $6.1 million, $5.9 million and $4.4
million for the years ended December 31, 2009, 2008 and 2007, respectively.
Direct Hospice Care Expenses
Direct hospice care expenses consist primarily of direct patient care salaries, employee
benefits, payroll taxes, and travel costs associated with hospice care providers. Direct hospice
care expenses also include the cost of pharmaceuticals, medical equipment and supplies, inpatient
arrangements, net nursing home costs, medical director fees, purchased services such as ambulance,
infusion and radiology and reimbursement for mileage for the Company’s patient caregivers.
F-11
Table of Contents
Property and Equipment and Other Intangible Assets
Property and equipment, including improvements to existing facilities, are recorded at cost.
Depreciation and amortization are calculated primarily using the straight-line method over the
estimated useful lives of the assets. Estimated useful lives for major asset categories are three
to five years for equipment and computer software, five years for office furniture and twenty years
for buildings. Leasehold improvements are amortized over the shorter of the lease term or the
asset’s useful life, generally three to five years. Routine repairs and maintenance are charged to
expense as incurred. Certain costs associated with developing computer software for internal use
are capitalized.
Identifiable intangible assets with finite lives are amortized over their estimated useful
lives. These intangible assets are comprised of non-compete agreements, favorable leases and
capitalized Certificate of Need (“CON”) costs. The non-compete agreements are being amortized based
on the terms of their respective agreements ranging from two to seven years. The CON costs are
related to CON’s obtained in Florida and are being amortized over 20 years. The favorable leases
are being amortized over their respective lease terms, ranging from two to six years.
When events, circumstances and operating results indicate that the carrying value of certain
property, equipment, and other intangible assets might be impaired, the Company prepares
projections of the undiscounted future cash flows expected to result from the use of the assets and
their eventual disposition. If the projections indicate that the recorded amounts are not expected
to be recoverable, such amounts are reduced to estimated fair value. Indicators of potential
impairment are typically beyond the control of management. If market conditions become less
favorable than those projected by management, impairments may be required.
Share-Based Compensation
The Company accounts for share-based compensation in accordance with FASB ASC Topic 718
“Compensation — Stock Compensation.” Under FASB ASC Topic 718, the Company recognizes share-based
compensation ratably using the straight-line attribution method over the requisite service period.
Share-based compensation is measured based on the grant date fair value of the respective awards.
The fair value of option awards is estimated at the date of grant using the Black-Scholes valuation
model. The Company estimates forfeitures based on historical experience and future expectations.
Share-based compensation expense is included within the “general and administrative — support
center” line item in the Company’s consolidated statements of income.
Net Income Per Common Share
Basic net income per common share is computed by dividing net income by the weighted average
number of common shares outstanding during the period. Diluted net income per common share is
computed by dividing the net income by the weighted average number of common shares outstanding
during the period plus the effect of dilutive securities, giving effect to the conversion of
employee stock options, restricted stock awards and outstanding warrants (using the treasury stock
method and considering the effect of unrecognized deferred compensation charges).
Discontinued Operations
Discontinued operations represent a component of an entity that has been disposed of or is
classified as held for sale and has operations and cash flows that can be clearly distinguished
from the rest of the entity. In the period that a component of an entity has been disposed of or
classified as held for sale, the results of operations for current and prior periods are
reclassified in a single caption titled discontinued operations.
Income Taxes
The Company accounts for income taxes using the liability method as required by ASC Topic 740,
“Income Taxes” (formerly SFAS No. 109 — “Accounting for Income Taxes”). Under the liability method,
deferred taxes are determined based on differences between financial reporting and tax basis of
assets and liabilities and are measured using the enacted tax laws that will be in effect when the
differences are expected to reverse. Management provides a valuation allowance for any net
deferred tax assets when it is more likely than not that a portion of such net deferred tax assets
will not be recovered.
F-12
Table of Contents
On January 1, 2007, the Company adopted a new accounting pronouncement issued by the FASB
located under ASC Topic 740, “Income Taxes” (formerly FASB FIN No. 48 — “Accounting for Uncertainty
in Income Taxes”), which clarifies the accounting for uncertainty in income taxes. The cumulative
effect of applying the provisions of this pronouncement is reported as an adjustment to the opening
balance of retained earnings. This pronouncement prescribes a recognition threshold and measurement
attribute for the financial statement recognition and measurement of a tax position taken or
expected to be taken in a tax return and also provides guidance on various related matters such as
derecognition, classification, interest and penalties, accounting in interim periods, disclosures
and transition.
Self-Insured Liability Insurance
The Company maintains general (occurrence basis) and professional (claims made basis)
liability insurance coverage on a company-wide basis with limits of liability of $1.0 million per
occurrence and $3.0 million in the aggregate, both with a deductible of $75,000 per occurrence or
claim. The Company also maintains workers’ compensation coverage, except in Texas, at the statutory
limits and an employer’s liability policy with a $1.0 million limit per accident/employee, with a
deductible of $500,000 per occurrence. In Texas the Company does not subscribe to the state
workers’ compensation program, instead the Company maintains a separate employer’s excess indemnity
coverage in the amount of $5.0 million per accident/employee and voluntary indemnity coverage in
the amount of $5.0 million per accident/employee, with a $5.0 million aggregate limit. The Company
also maintains a policy insuring hired and non-owned automobiles with a $1.0 million limit of
liability and a $250,000 deductible per occurrence. In addition, the Company maintains umbrella
coverage with a limit of $20.0 million excess over the general, professional, hired and non-owned
automobile and employer’s liability policies. The Company has accrued $10.2 million and $8.9
million for workers’ compensation claims as of December 31, 2009 and 2008, respectively.
Nursing Home Costs
For patients receiving nursing home care under a state Medicaid program who elect hospice care
under Medicare or Medicaid, the Company contracts with nursing homes for the nursing homes to
provide patients room and board services. The state must pay the Company, in addition to the
applicable Medicare or Medicaid hospice daily or hourly rate, an amount equal to at least 95% of
the Medicaid daily nursing home rate for room and board furnished to the patient by the nursing
home. Under the Company’s standard nursing home contracts, the Company pays the nursing home for
these room and board services at the Medicaid daily nursing home rate. Nursing home costs are
offset by nursing home net revenue, and the net amount is included in direct hospice care expenses.
Nursing home costs totaled $133.1 million, $119.2 million and $84.7 million for the years ended
December 31, 2009, 2008 and 2007, respectively. Nursing home net revenue totaled $125.3 million,
$112.0 million and $80.1 million for the years ended December 31, 2009, 2008 and 2007,
respectively. This resulted in net nursing home costs of $7.8 million, $7.2 million and $4.6
million for the years ended December 31, 2009, 2008 and 2007, respectively.
Advertising Costs
The Company expenses all advertising costs as incurred, which totaled $1.0 million, $1.3
million and $0.7 million for the years ended December 31, 2009, 2008 and 2007, respectively.
Deferred Rent Liability
Payments under operating leases are recognized as rent expense on a straight-line basis over
the term of the related lease. The difference between the rent expense recognized for financial
reporting purposes and the actual payments made in accordance with the lease agreements is
recognized as a deferred rent liability. Deferred rent at December 31, 2009 and 2008 was $5.8
million and $6.6 million, respectively.
Subsequent Events
The Company has evaluated subsequent events for recognition and disclosure in the financial
statements and has determined that no additional disclosures are necessary.
F-13
Table of Contents
Recent Accounting Pronouncements
In September 2006, the FASB issued a new accounting pronouncement regarding fair value
(formerly SFAS No. 157 — “Fair Value Measurements”). This pronouncement, located under FASB ASC
Topic 820, “Fair Value Measurements and Disclosures,” defines fair value, establishes a framework
for measuring fair value under GAAP, and expands disclosures about fair value measurements. This
pronouncement does not require any new fair value measurements in financial statements, but
standardizes its definition and guidance in GAAP. The Company adopted this pronouncement effective
beginning on January 1, 2008 for financial assets and financial liabilities, which did not have a
material impact on its financial statements. In February 2008, the FASB delayed by one year the
effective date of this pronouncement for all nonfinancial assets and nonfinancial liabilities,
except those that are recognized or disclosed at fair value in the financial statements on a
recurring basis (at least annually). The Company adopted this pronouncement effective beginning on
January 1, 2009 for nonfinancial assets and nonfinancial liabilities, which did not have any impact
on the Company’s financial statements.
In December 2007, the FASB issued a new accounting pronouncement regarding business
combinations (formerly SFAS No. 141 (revised 2007) — “Business Combinations”). This pronouncement,
located under FASB ASC Topic 805, “Business Combinations,” was issued to improve the relevance,
representational faithfulness, and comparability of information in financial statements about a
business combination and its effects. This pronouncement retains the purchase method of accounting
for business combinations, but requires a number of changes including contingent consideration,
such as earn-outs, will be recognized at its fair value on the acquisition date and, for certain
arrangements, changes in fair value will be recognized in earnings until settled;
acquisition-related transaction and restructuring costs will be expensed as incurred;
previously-issued financial information will be revised for subsequent adjustments made to finalize
the purchase price accounting; reversals of valuation allowances related to acquired deferred tax
assets and changes to acquired income tax uncertainties will be recognized in earnings, except in
certain situations. ASC Topic 805 also requires an acquirer to recognize at fair value, an asset
acquired or a liability assumed in a business combination that arises from a contingency provided
the asset or liability’s fair value can be determined on the date of acquisition. The Company
adopted this pronouncement on a prospective basis effective beginning on January 1, 2009. For
business combinations completed on or subsequent to the adoption date, the application of this
pronouncement may have a significant impact on the Company’s financial statements, the magnitude of
which will depend on the specific terms and conditions of the transactions. The Company did not
have any material business combinations during 2009.
In December 2007, the FASB issued a new accounting pronouncement regarding noncontrolling
interests and the deconsolidation of a subsidiary (formerly SFAS No. 160 — “Noncontrolling
Interests in Consolidated Financial Statements — an amendment of ARB No. 51”). This pronouncement,
located under FASB ASC Topic 810, “Consolidation,” was issued to improve the relevance,
comparability, and transparency of financial information provided in financial statements by
establishing accounting and reporting standards for the noncontrolling interest in a subsidiary and
for the deconsolidation of a subsidiary. The Company adopted this pronouncement effective beginning
on January 1, 2009. As a result of this adoption, the Company presents noncontrolling interests
(previously shown as minority interests in consolidated subsidiaries) as a component of equity on
the consolidated balance sheets. Minority interest expense is no longer separately reported as a
reduction in net income on the consolidated statements of income, but is instead shown below net
income under the heading “net income attributable to non controlling interests.” Total provision
for income taxes remains unchanged; however, the Company’s effective tax rate as calculated from
the balances shown on the consolidated statements of income have changed as net income attributable
to noncontrolling interests is no longer included as a deduction in the determination of income
from continuing operations. The adoption of this pronouncement did not have a material impact on
the Company’s financial statements other than the presentation requirements previously mentioned.
In March 2008, the FASB issued a new accounting pronouncement regarding derivative and hedging
activities (formerly SFAS No. 161 — “Disclosures about Derivative Instruments and Hedging
Activities — an amendment of FASB Statement No. 133”). This pronouncement, located under FASB ASC
Topic 815, “Derivatives and Hedging,” was issued to improve transparency of financial information
provided in financial statements by requiring expanded disclosures about an entity’s derivative and
hedging activities. This pronouncement requires entities to provide expanded disclosures about: how
and why an entity uses derivative instruments; how derivative instruments and related hedged items
are accounted for; and how derivative instruments and related hedged items affect an entity’s
financial position, financial performance, and cash flows. The Company adopted this pronouncement
effective
F-14
Table of Contents
beginning on January 1, 2009. The adoption of this pronouncement did not have any impact on
the Company’s financial statements as it contains only disclosure requirements.
In April 2009, the FASB issued a new accounting pronouncement regarding interim disclosures
about fair value of financial instruments (formerly FSP FAS 107-1 and Accounting Principles Board
(“APB”) Opinion No. 28-1 — “Interim Disclosures about Fair Value of Financial Instruments”). This
pronouncement, located under FASB ASC Topic 825, “Financial Instruments,” increases the frequency
of fair value disclosures by requiring both interim and annual disclosures. The Company adopted
this pronouncement on a prospective basis effective beginning on April 1, 2009. The adoption of
this pronouncement did not have any impact on the Company’s financial statements as it contains
only disclosure requirements.
In April 2009, the FASB issued a new accounting pronouncement regarding fair value (formerly
Staff Position No. 157-4, “Determining Fair Value When the Volume and Level of Activity for the
Asset or Liability Have Significantly Decreased and Identifying Transactions That Are Not
Orderly”). This pronouncement, located under FASB ASC Topic 820, “Fair Value Measurements and
Disclosures,” provides additional guidance for estimating fair value when the volume and level of
activity for the asset or liability have significantly decreased. This pronouncement also includes
guidance on identifying circumstances that indicate a transaction is not orderly. The Company
adopted this pronouncement on a prospective basis effective beginning on April 1, 2009. The
adoption of this pronouncement did not have any impact on the Company’s financial statements.
In April 2009, the FASB issued a new accounting pronouncement regarding other-than-temporary
impairments (formerly FASB Staff Position No. FAS 115-2 and 124-2, “Recognition and Presentation of
Other-Than-Temporary Impairments”). This pronouncement, located under FASB ASC Topic 320,
“Investments — Debt and Equity Securities,” amends the other-than-temporary impairment guidance
for debt securities to make the guidance more operational and to improve the presentation and
disclosure of other-than-temporary impairments on debt and equity securities in the financial
statements. This pronouncement does not amend existing recognition and measurement guidance related
to other-than-temporary impairments of equity securities and does not require disclosures for
earlier periods presented for comparative purposes at initial adoption. In periods after initial
adoption, this pronouncement requires comparative disclosures only for periods ending after initial
adoption. The Company adopted this pronouncement during the second quarter of 2009 and it did not
have any impact on the Company’s financial statements.
In May 2009, the FASB issued a new accounting pronouncement regarding subsequent events
(formerly SFAS No. 165 — “Subsequent Events”). This pronouncement, located under FASB ASC Topic
855, “Subsequent Events,” was issued to establish general standards of accounting for and
disclosure of events that occur after the balance sheet date but before financial statements are
issued or are available to be issued. This pronouncement requires entities to disclose the date
through which subsequent events have been evaluated, as well as whether that date is the date the
financial statements were issued or the date the financial statements were available to be issued.
The Company adopted this pronouncement on a prospective basis effective beginning on April 1, 2009.
The adoption of this pronouncement did not have any impact on the Company’s financial statements.
In February 2010, the FASB amended its guidance on subsequent events to remove the requirement for
public companies to disclose the date through which an entity has evaluated subsequent events.
In June 2009, the FASB issued a new accounting pronouncement regarding authoritative GAAP
(formerly SFAS No. 168 — “The FASB Accounting Standards Codification and the Hierarchy of
Generally Accepted Accounting Principles”). This pronouncement, located under FASB ASC Topic 105,
“Generally Accepted Accounting Principles,” establishes the FASB Accounting Standards Codification
(“Codification”) as the source of authoritative GAAP recognized by the FASB for nongovernmental
entities. Rules and interpretive releases of the SEC under federal securities laws are also sources
of authoritative GAAP for SEC registrants. All guidance contained in the Codification carries an
equal level of authority. All other nongrandfathered non-SEC accounting literature not included in
the Codification is nonauthoritative. The Company adopted this pronouncement effective beginning on
July 1, 2009. The adoption of this pronouncement did not have any impact on the Company’s financial
statements other than the manner in which new accounting guidance is referenced.
In June 2009, the FASB issued a new accounting pronouncement regarding variable interest
entities (formerly SFAS No. 167, “Amendments to FASB Interpretation No. 46(R)”). This
pronouncement, located under FASB ASC Topic 810, “Consolidation,” was issued to improve financial
reporting by enterprises involved with variable interest
F-15
Table of Contents
entities and to provide more relevant and reliable information to users of financial
statements. ASC Topic 810 requires an enterprise to perform an ongoing analysis to determine
whether the enterprise has a controlling financial interest in a variable interest entity. This
pronouncement will be effective for the Company beginning on January 1, 2010. The Company does not
expect the adoption of this pronouncement to have a significant impact on its future financial
position, results of operations, earnings per share, and cash flows.
3. Acquisitions
On March 6, 2008, the Company completed its acquisition of Scottsdale, Arizona-based
VistaCare, Inc. (“VistaCare”) for $8.60 per share, or approximately $147.1 million, plus $2.4
million in transaction costs. The transaction was structured as a two-step acquisition including a
cash tender offer for all outstanding shares of VistaCare common stock followed by a cash merger in
which the Company acquired all of the remaining outstanding shares of VistaCare common stock. The
transaction substantially extended the Company’s industry leadership and geographic reach. The
Company also believes that the transaction created additional visibility that adds value in its
marketing, recruiting and development activities. Following the completion of this transaction, the
Company had approximately 100 Medicare-certified hospice locations in 30 states and an average
daily census of more than 12,000 patients. During 2008 and 2009, the Company consolidated some
markets in which both Odyssey and VistaCare had programs in the same location. As of December 31,
2009, the Company had 90 Medicare-certified programs in 29 states. The operations of VistaCare were
included in the Company’s results of operations beginning February 29, 2008.
The purchase price was allocated to assets acquired and liabilities assumed based on estimated
fair values. The Company obtained independent appraisals of identifiable intangible assets and
their remaining useful lives. The Company also reviewed and determined the fair value of other
assets and liabilities assumed. The final estimated fair values of the assets acquired and
liabilities assumed relating to the VistaCare acquisition are summarized below (in thousands):
Cash |
$ | 22,617 | ||
Other current assets |
46,390 | |||
Property and equipment |
4,959 | |||
Other assets |
13,056 | |||
Licenses |
8,982 | |||
Trademarks |
7,235 | |||
Other intangible assets |
456 | |||
Goodwill |
90,980 | |||
Total assets acquired |
194,675 | |||
Current liabilities |
44,024 | |||
Other liabilities |
1,155 | |||
Net assets acquired |
$ | 149,496 | ||
The Company recorded $91.0 million of goodwill in connection with this acquisition. No amount
is expected to be deductible for tax purposes. Any future adjustments to the acquired assets and
liabilities will be recorded as a component of net income.
Prior to the acquisition, the Company determined that it would transition the VistaCare
corporate functions to the Company’s corporate office. During the third quarter of 2008, the
Company substantially completed the transition of the VistaCare corporate functions to its Dallas
Support Center and the transition of all the VistaCare program sites to its information systems.
During the fourth quarter of 2008, the Company completed the process of ramping up its Support
Center operations. Estimated liabilities of $6.1 million for severance costs, $1.9 million for
lease termination costs, $0.3 million related to a buyout of a non-compete agreement and $0.2
million for bonuses related to the transition were recorded as part of the purchase price
allocation. All estimated liabilities have been paid as of December 31, 2009 except for the lease
termination costs which has a remaining balance of $0.5 million.
On December 31, 2008, the Company acquired a hospice program in Flint, Michigan for
approximately $0.5 million.
On December 31, 2009, the Company acquired a hospice program in Westchester, Illinois for
approximately $3.2 million.
F-16
Table of Contents
The following unaudited pro forma data summarizes the results of operations for the
periods indicated as if the VistaCare and Flint, Michigan acquisitions noted above had been
completed as of the beginning of the periods presented. The 2009 acquisition was not included in
the pro forma results as it was not a material acquisition. The pro forma results of operations
gives effect to actual operating results prior to the acquisitions, adjusted to include the pro
forma effect of the acquisitions. The pro forma results do not purport to be indicative of the
results that would have actually been obtained if the acquisitions occurred as of the beginning of
the periods presented or that may be obtained in the future.
| For the years | ||||||||
| ended December 31, | ||||||||
| 2008 | 2007 | |||||||
| (in millions, except per share data) | ||||||||
Revenues |
$ | 660.4 | $ | 647.0 | ||||
Income from continuing operations |
14.3 | 15.7 | ||||||
Net income |
9.5 | 12.1 | ||||||
Income per common share: |
||||||||
Basic: |
||||||||
Continuing operations |
$ | 0.44 | $ | 0.48 | ||||
Net |
0.29 | 0.37 | ||||||
Diluted: |
||||||||
Continuing operations |
$ | 0.43 | $ | 0.47 | ||||
Net |
0.29 | 0.36 | ||||||
4. Goodwill and Intangible Assets
The table below sets forth the changes in the carrying amount of goodwill by segment for the
years ended December 31, 2009 and 2008:
| South | VistaCare | VistaCare | VistaCare | |||||||||||||||||||||||||||||||||||||||||||||||||
| Northeast | Southeast | Central | Midwest | Texas | Mountain | West | South | Southwest | South | Central | West/North | Total | ||||||||||||||||||||||||||||||||||||||||
January 1, 2008 |
$ | 3,397 | $ | 12,314 | $ | 17,346 | $ | 3,734 | $ | 21,775 | $ | 31,983 | $ | 7,630 | $ | — | $ | — | $ | — | $ | — | $ | — | $ | 98,179 | ||||||||||||||||||||||||||
Acquisition |
— | — | 50 | — | 312 | — | — | — | — | 12,988 | 19,432 | 58,560 | 91,342 | |||||||||||||||||||||||||||||||||||||||
December 31, 2008 |
3,397 | 12,314 | 17,396 | 3,734 | 22,087 | 31,983 | 7,630 | — | — | 12,988 | 19,432 | 58,560 | 189,521 | |||||||||||||||||||||||||||||||||||||||
Acquisition |
— | — | — | 2,245 | — | — | — | — | — | — | — | — | 2,245 | |||||||||||||||||||||||||||||||||||||||
Transfers |
5,973 | (3,404 | ) | 7,176 | 16,619 | 15,480 | 12,905 | — | 12,913 | 23,318 | (12,988 | ) | (19,432 | ) | (58,560 | ) | — | |||||||||||||||||||||||||||||||||||
December 31, 2009 |
$ | 9,370 | $ | 8,910 | $ | 24,572 | $ | 22,598 | $ | 37,567 | $ | 44,888 | $ | 7,630 | $ | 12,913 | $ | 23,318 | $ | — | $ | — | $ | — | $ | 191,766 | ||||||||||||||||||||||||||
During 2009, the Company reorganized its operating segments to better align its hospice
programs. In accordance with ASC Topic 350, the Company reallocated goodwill from the old segment
to the new segment based on the relative fair value of the hospice program transferred. In
determining the fair value of a hospice program, the Company used multiples of EBITDA, which the
Company believes correlates with what a market participant would be willing to pay for that program
in today’s market.
Intangible assets as of December 31, 2009 and 2008 consisted of the following:
| December 31, 2009 | December 31, 2008 | |||||||||||||||
| Gross | Gross | |||||||||||||||
| Carrying | Accumulated | Carrying | Accumulated | |||||||||||||
| Amount | Amortization | Amount | Amortization | |||||||||||||
Non-competition agreements |
$ | 2,305 | $ | 2,185 | $ | 2,305 | $ | 2,072 | ||||||||
Licenses |
11,195 | — | 11,295 | — | ||||||||||||
Trademarks |
7,235 | — | 7,235 | — | ||||||||||||
Other |
1,188 | 487 | 1,188 | 307 | ||||||||||||
Totals |
$ | 21,923 | $ | 2,672 | $ | 22,023 | $ | 2,379 | ||||||||
F-17
Table of Contents
Intangible assets amortization expense was $0.4 million, $0.4 million and $0.2 million for the
years ended December 31, 2009, 2008 and 2007, respectively. Estimated intangible assets
amortization expense is $0.1 million for 2010, 2011, 2012, 2013, and 2014. Actual future
amortization expense could differ from these estimated amounts as a result of future acquisitions
and other factors.
5. Repurchases of Common Stock
On November 21, 2006, the Company announced the adoption of an open market stock repurchase
program to repurchase up to $10.0 million of the Company’s common stock over a twelve-month period.
The timing and the amount of any repurchase of shares during the twelve-month period was determined
by management based on its evaluation of market conditions and other factors. The Company completed
this stock repurchase program in May 2007 and repurchased an aggregate of 801,683 shares of the
Company’s common stock at a total cost of $10.0 million (average cost of $12.47 per share). Of this
amount, 59,477 shares for approximately $0.8 million was repurchased in 2007. The stock repurchases
were funded out of working capital.
On May 4, 2007, the Company announced the adoption of a stock repurchase program to repurchase
up to $50.0 million of the Company’s common stock over the twelve month period beginning on May 4,
2007 either in the open market or through privately negotiated transactions, subject to market
conditions and other factors. The repurchased shares were added to the treasury shares of the
Company and may be used for employee stock plans and for other corporate purposes. The stock
repurchases were funded out of working capital. The stock repurchase program expired on May 4,
2008. The Company repurchased 1,056,623 shares of its common stock for approximately $13.1 million
(average cost of $12.42 per share) during this program.
No shares were repurchased during 2008 or 2009. The terms of the Company’s credit agreement
may restrict the Company’s ability to repurchase additional stock in the future.
6. Stock Options and Restricted Stock Awards
During 2001, the Company adopted the 2001 Equity-Based Compensation Plan (“Compensation
Plan”). Awards of stock options and restricted stock under the Compensation Plan shall not exceed
the lesser of 225,000,000 shares or 10% of the total number of shares of common stock then
outstanding, assuming the exercise of all outstanding options, warrants and the conversion or
exchange or exercise of all securities convertible into or exchangeable or exercisable for common
stock. In May 2005, shareholders of the Company approved an amendment to increase the number of
common shares reserved and available for issuance from inception of the Compensation Plan to a
total of 6,149,778 shares under the Compensation Plan. The Company no longer grants options under
the Odyssey HealthCare, Inc. Stock Option Plan (“Stock Option Plan”). There were 1,473,480,
1,811,516 and 1,466,883 shares available for issuance under the Compensation Plan at December 31,
2009, 2008 and 2007, respectively.
At December 31, 2009, there were 12,614 and 1,421,112 options outstanding under the Stock
Option Plan and the Compensation Plan, respectively, with exercise prices ranging from $1.38 to
$30.64 per share. Most options granted have five to ten year terms and vest ratably over a four or
five year term, with the exception of certain options for which the Company accelerated the
vesting.
F-18
Table of Contents
A summary of outstanding stock options under the Company’s stock compensation plans at
December 31, 2009 is presented below:
| Weighted- | ||||||||||||||||
| Average | ||||||||||||||||
| Remaining | ||||||||||||||||
| Weighted- | Contractual | Aggregate | ||||||||||||||
| Outstanding | Average | Term | Intrinsic | |||||||||||||
| Range of exercise prices | Options | Exercise Price | (in years) | Value | ||||||||||||
$1.38 to $9.93 |
242,614 | $ | 9.50 | 7.39 | 1,477,967 | |||||||||||
$9.94 to $15.53 |
612,724 | $ | 13.36 | 4.35 | 1,369,530 | |||||||||||
$15.54 to $19.72 |
313,204 | $ | 17.98 | 5.57 | — | |||||||||||
$19.73 to $22.33 |
139,936 | $ | 22.22 | 3.55 | — | |||||||||||
$22.34 to $30.64 |
125,248 | $ | 29.76 | 3.98 | — | |||||||||||
Totals |
1,433,726 | $ | 16.01 | 5.02 | $ | 2,847,497 | ||||||||||
At December 31, 2009, there were 1,392,202 restricted stock awards and restricted stock unit
(“RSU’s”) awards outstanding under the Compensation Plan that are described in more detail below.
In addition to time-based awards, the Company will grant incentive-based RSUs to certain
employees from time to time. The total number and vesting of the incentive-based RSUs that are
eligible for each award recipient is based upon the Company attaining certain specified earnings
per share (“EPS”) from continuing operations targets in the year granted. Provided the award
recipient remains an employee continuously from the date of grant through the applicable vesting
date, one-fourth of the incentive-based RSUs eligible for vesting for each award recipient, based
on the satisfaction of the applicable EPS target, will vest on the date the Compensation Committee
(“Committee”) certifies that the EPS target has been met. The remaining three-fourths of the
incentive-based RSUs eligible for vesting for each award recipient, based on the satisfaction of
the applicable EPS target, will vest in three equal, annual installments.
In February 2008, the Committee approved, for certain executive officers, the exchange of
selected “underwater” stock options for time-based RSUs. The Committee was concerned that the
underwater stock options provided little or no financial or retention incentives to the executive
officers. The Committee believes that the exchange of the underwater stock options for the
time-based RSUs adequately addressed those concerns. Stock option awards of 685,000 shares, with a
weighted average exercise price of $17.35, were exchanged for 126,146 shares of time-based RSUs. Of
the stock option awards exchanged, 287,500 shares were unvested. The shares of time based RSUs had
a fair value of $9.18 per share and will vest ratably over a three year period beginning February
12, 2009. There was no material charge to share-based compensation expense from the exchange.
The Company recorded $5.1 million, $4.3 million
and $3.8 million in share-based compensation
expense for the years ended December 31, 2009, 2008 and 2007, respectively, for awards under the
Compensation Plan. The tax benefit on share-based compensation expense was $1.3 million, $0.2
million and $0.1 million for the years ended December 31, 2009, 2008 and 2007, respectively.
The deemed fair value for options was estimated at the date of grant using the Black-Scholes
Model, which considers volatility. The following table illustrates the weighted average assumptions
for the years ended December 31:
| 2007 | 2008 | |||||||
Risk-free interest rate |
4.57 | % | 2.84 | % | ||||
Expected life |
5 years | 5 years | ||||||
Expected volatility |
0.496 | 0.495 | ||||||
Expected dividend yield |
— | — | ||||||
F-19
Table of Contents
A summary of stock option activity under the Company’s stock compensation plans at December
31, 2009 is presented below:
| Weighted- | ||||||||||||||||
| Average | ||||||||||||||||
| Remaining | ||||||||||||||||
| Weighted- | Contractual | Aggregate | ||||||||||||||
| Average | Term | Intrinsic | ||||||||||||||
| Options | Exercise Price | (in years) | Value | |||||||||||||
Outstanding at January 1, 2009 |
2,252,766 | $ | 16.12 | |||||||||||||
Granted |
— | $ | — | |||||||||||||
Exercised |
(274,346 | ) | $ | 8.07 | ||||||||||||
Cancelled |
(544,694 | ) | $ | 20.46 | ||||||||||||
Outstanding at December 31, 2009 |
1,433,726 | $ | 16.01 | 5.02 | $ | 2,847,497 | ||||||||||
Exercisable at December 31, 2009 |
1,252,676 | $ | 16.72 | 4.67 | $ | 1,996,408 | ||||||||||
The weighted average deemed fair value of the options granted was $4.64 and $5.14 for the
years ended December 31, 2008 and 2007, respectively. The total aggregate intrinsic value of
options exercised was $1.6 million, $48,000 and $1.7 million during the years ended December 31,
2009, 2008 and 2007, respectively. Cash received from option exercises under stock-based payment
arrangements during the year ended December 31, 2009 was $2.2 million.
A summary of the Company’s restricted stock activity for the year ended December 31, 2009 is
presented below:
| Compensation Plan | ||||||||
| Weighted- | ||||||||
| Average | ||||||||
| Grant-Date | ||||||||
| Shares | FairValue | |||||||
Non-vested at January 1, 2009 |
744,449 | $ | 9.42 | |||||
Granted |
925,368 | $ | 10.49 | |||||
Vested |
(234,415 | ) | $ | 9.51 | ||||
Cancelled |
(43,200 | ) | $ | 8.93 | ||||
Non-vested at December 31, 2009 |
1,392,202 | $ | 10.12 | |||||
The total fair value of restricted stock that vested during the year ended December 31, 2009
was $2.5 million.
As of
December 31, 2009, there was $11.5 million (pretax) of
total unrecognized share-based
compensation expense related to the Company’s non-vested share-based compensation plans, which is
expected to be recognized over a weighted-average period of 2.6 years.
F-20
Table of Contents
7. Earnings Per Share
The following table presents the calculation of basic and diluted net income attributable to
Odyssey stockholders per common share:
| Year Ended December 31, | ||||||||||||
| 2009 | 2008 | 2007 | ||||||||||
| (In thousands, except per share amounts) | ||||||||||||
Numerator: |
||||||||||||
Numerator for net income per share |
||||||||||||
Income from continuing operations |
$ | 41,702 | $ | 19,935 | $ | 16,013 | ||||||
Loss from discontinued operations, net of tax |
(498 | ) | (5,252 | ) | (3,888 | ) | ||||||
Less: net income (loss) attributable to noncontrolling interests |
613 | 257 | 14 | |||||||||
Net income attributable to Odyssey stockholders |
$ | 40,591 | $ | 14,426 | $ | 12,111 | ||||||
Denominator: |
||||||||||||
Denominator for basic net income per share — weighted average shares |
32,935 | 32,674 | 33,029 | |||||||||
Effect of dilutive securities: |
||||||||||||
Employee stock options and restricted stock awards |
290 | 512 | 157 | |||||||||
Series B Preferred Stock Warrants convertible to common stock |
— | 2 | 2 | |||||||||
Denominator for diluted net income per share — adjusted weighted
average shares and assumed or actual conversions |
33,225 | 33,188 | 33,188 | |||||||||
Income (loss) per common share: |
||||||||||||
Basic: |
||||||||||||
Continuing operations attributable to Odyssey stockholders |
$ | 1.25 | $ | 0.60 | $ | 0.48 | ||||||
Discontinued operations attributable to Odyssey stockholders |
(0.02 | ) | (0.16 | ) | (0.12 | ) | ||||||
Net income attributable to Odyssey stockholders |
$ | 1.23 | $ | 0.44 | $ | 0.36 | ||||||
Diluted: |
||||||||||||
Continuing operations attributable to Odyssey stockholders |
$ | 1.24 | $ | 0.59 | $ | 0.48 | ||||||
Discontinued operations attributable to Odyssey stockholders |
(0.02 | ) | (0.16 | ) | (0.12 | ) | ||||||
Net income attributable to Odyssey stockholders |
$ | 1.22 | $ | 0.43 | $ | 0.36 | ||||||
For the years ended December 31, 2009, 2008 and 2007, options outstanding of 1,119,707,
2,040,052 and 3,014,219, respectively, were not included in the computation of diluted earnings per
share because either the exercise prices of the options were greater than the average market price
of the common stock or the total assumed proceeds under the treasury stock method resulted in
negative incremental shares, and thus the inclusion would have been anti-dilutive. In addition,
there were 22,430, 44,860 and 112,788 anti-dilutive shares relating to restricted stock awards for
the years ended December 31, 2009, 2008 and 2007, respectively.
8. Discontinued Operations
The Company conducts an ongoing strategic review of its hospice programs and evaluates whether
to sell or close certain hospice programs based on this strategic review. No programs were held for
sale as of December 31, 2009. The Oklahoma City program and the Oklahoma City inpatient unit were
held for sale as of December 31, 2008.
During the first quarter of 2007, the Company announced that it would exit the Tulsa, Oklahoma
hospice market which was located in the Company’s Central region and in February 2007, the Company
sold the Tulsa hospice program. As part of the sale, the purchaser assumed the office lease and
purchased certain assets such as furniture/fixtures, equipment, deposits and licenses. The Company
recognized an immaterial pretax loss during the first quarter of 2007 related to the sale of the
program.
During the second quarter of 2007, the Company decided to sell its Valdosta, Georgia;
Columbia, South Carolina; St. George, Utah; Rockford, Illinois; and Allentown, Pennsylvania hospice
programs and the Huntsville,
F-21
Table of Contents
Alabama alternate delivery site (“ADS”). The Company completed the sale of its Valdosta and
Columbia programs which were located in the Company’s Southeast region in June 2007 and recognized
an immaterial pretax loss in the second quarter on the sale of the programs. The Company completed
the sale of its Huntsville ADS and its St. George and Allentown programs which were located in the
Company’s Southeast, Mountain and Midwest regions, respectively, during the third quarter of 2007
and recognized an immaterial pretax loss in the third quarter for the disposition of the programs.
The Company completed the sale of the Rockford program which was located in the Company’s Midwest
region during the fourth quarter of 2007 and recognized an immaterial pretax gain in the fourth
quarter on the sale of the Rockford program.
During the fourth quarter of 2007, the Company decided to sell its Odessa, Texas; Big Spring,
Texas; Cincinnati, Ohio; and Wichita, Kansas hospice programs. The Company completed the sale of
the Odessa and Big Spring programs which were located in the Company’s Mountain region on January
1, 2008 and recognized an immaterial pretax loss during the fourth quarter of 2007 related to these
programs. The Company completed the sale of the Cincinnati and Wichita programs, which were located
in the Company’s Midwest and South Central regions, respectively, during the first quarter of 2008
and no material amounts were recorded as a result.
During the first quarter of 2008, the Company decided to sell its Baton Rouge, Louisiana;
Ventura, California; Fort Wayne, Indiana; and Oklahoma City, Oklahoma hospice programs, which were
located in the Company’s Southeast, West, Midwest and South Central regions, respectively. The
Company also decided to close the Bryan/College Station, Texas hospice program and the Dallas,
Texas inpatient unit. The closures of the Bryan/College Station program and Dallas inpatient unit,
which were located in the Company’s Texas and South Central regions, respectively, resulted in a
pretax loss of $1.5 million during the first quarter of 2008, which included an accrual of $1.2
million for future lease costs related to the closed programs.
During the second quarter of 2008, the Company decided to close the Colorado Springs, Colorado
inpatient unit and the Tucson, Arizona VistaCare hospice program. The closures, which were located
in the Company’s Mountain and VistaCare West regions, respectively, resulted in a pretax loss of
$2.3 million during the second quarter of 2008, which included an accrual of $2.1 million for
future lease costs related to the closed programs.
During the third quarter of 2008, the Company completed the sale of the Baton Rouge hospice
program, which was located in the Company’s Southeast region, and no material amounts were recorded
as a result of the sale.
During the fourth quarter of 2008, the Company completed the sale of the Ventura and Fort
Wayne hospice programs which were located in the West and Midwest regions, respectively, and
recognized an immaterial pretax gain for each of these programs.
During the second quarter of 2009, the Company recorded a pretax loss of approximately $0.6
million, which was a result of the writedown of assets from $2.1 million to $1.5 million for the
Oklahoma City program, including the related inpatient unit. The Company completed the sale of the
Oklahoma City program, including the related inpatient unit, on July 13, 2009. The Oklahoma City
program and inpatient unit were located in the Company’s South Central region. Net proceeds from
the sale were approximately $1.5 million. The $1.5 million received in net proceeds was paid to the
Company’s lenders as a mandatory prepayment of principal.
The assets of these entities included in discontinued operations are presented in the
consolidated balance sheets under the captions “Assets of discontinued operations.” The carrying
amounts of these assets were as follows:
| December 31, | December 31, | |||||||
| 2009 | 2008 | |||||||
| (In thousands) | (In thousands) | |||||||
Prepaid expenses and other current assets |
$ | — | $ | 15 | ||||
Property and equipment, net |
— | 2,052 | ||||||
Total assets of discontinued operations |
$ | — | $ | 2,067 | ||||
F-22
Table of Contents
Net revenue and losses for these entities and the write-down of assets sold were included in
the consolidated statement of operations as “Loss from discontinued operations, net of income
taxes,” for all periods presented. The amounts are as follows (in thousands):
| Year Ended December 31, | ||||||||||||
| 2009 | 2008 | 2007 | ||||||||||
Net patient service revenue |
$ | 2,230 | $ | 6,200 | $ | 22,766 | ||||||
Pre-tax loss from operations |
$ | (230 | ) | $ | (8,031 | ) | $ | (5,960 | ) | |||
Benefit for income taxes |
88 | 3,072 | 1,988 | |||||||||
Loss from discontinued operations |
$ | (142 | ) | $ | (4,959 | ) | $ | (3,972 | ) | |||
Gain (loss) on sale of assets, net of income taxes |
(356 | ) | (293 | ) | 84 | |||||||
Loss from discontinued operations, net of income taxes |
$ | (498 | ) | $ | (5,252 | ) | $ | (3,888 | ) | |||
Loss per diluted share |
$ | (0.02 | ) | $ | (0.16 | ) | $ | (0.12 | ) | |||
9. Allowance for Uncollectible Accounts
The allowance for uncollectible accounts for patient accounts receivable is as follows:
| Balance at | Provision for | Write-Offs, | Balance at | |||||||||||||
| Beginning of | Uncollectible | Net of | End of | |||||||||||||
| Year | Accounts | Recoveries | Year | |||||||||||||
| (In thousands) | ||||||||||||||||
Year ended December 31, 2007 |
$ | 2,501 | $ | 5,344 | $ | (3,482 | ) | $ | 4,363 | |||||||
Year ended December 31, 2008 |
$ | 4,363 | $ | 10,907 | $ | (5,481 | ) | $ | 9,789 | |||||||
Year ended December 31, 2009 |
$ | 9,789 | $ | 10,842 | $ | (8,169 | ) | $ | 12,462 | |||||||
10. Property and Equipment
Property and equipment at December 31, 2009 and 2008 are detailed below:
| December 31, | ||||||||
| 2009 | 2008 | |||||||
| (In thousands) | ||||||||
Office furniture |
$ | 10,015 | $ | 9,676 | ||||
Computer hardware |
6,604 | 6,870 | ||||||
Computer software |
11,467 | 12,676 | ||||||
Equipment |
2,998 | 3,640 | ||||||
Motor vehicles |
369 | 369 | ||||||
Land |
1,098 | 1,098 | ||||||
Buildings |
4,529 | 4,534 | ||||||
Leasehold improvements |
13,958 | 11,288 | ||||||
Construction in progress |
509 | 393 | ||||||
Property and equipment |
51,547 | 50,544 | ||||||
Less accumulated depreciation and amortization |
(30,847 | ) | (27,728 | ) | ||||
Property and equipment, net |
$ | 20,700 | $ | 22,816 | ||||
Depreciation expense for property and equipment was $6.3 million, $7.4 million and $5.5
million for the year ended December 31, 2009, 2008 and 2007, respectively. The Company had $2.7
million and $4.6 million in unamortized computer software costs as of December 31, 2009 and 2008,
respectively. The Company recorded depreciation expense related to the amortization of computer
software costs of $0.9 million, $1.8 million and $1.8 million for the years ended December 31,
2009, 2008 and 2007, respectively.
F-23
Table of Contents
11. Other Accrued Expenses
Other accrued expenses at December 31, 2009 and 2008 are as follows:
| December 31, | ||||||||
| 2009 | 2008 | |||||||
| (In thousands) | ||||||||
Workers’ compensation |
$ | 10,172 | $ | 8,895 | ||||
Inpatient |
6,705 | 7,432 | ||||||
Deferred rent |
5,821 | 6,581 | ||||||
Pharmacy |
851 | 728 | ||||||
Medical supplies and durable medical equipment |
3,691 | 3,507 | ||||||
Property taxes |
357 | 487 | ||||||
Medical director fees |
671 | 661 | ||||||
Professional fees |
3,721 | 3,144 | ||||||
New billing system |
— | 2,024 | ||||||
Interest |
1,123 | 1,195 | ||||||
Federal taxes payable |
1,504 | 3,285 | ||||||
Accounts receivable credit balances |
2,338 | 1,813 | ||||||
Other |
7,233 | 6,152 | ||||||
| $ | 44,187 | $ | 45,904 | |||||
12. Borrowings
Borrowings consisted of the following at December 31, 2009 and 2008:
| December 31, | ||||||||
| 2009 | 2008 | |||||||
| (In thousands) | ||||||||
Term loan due between 2008 and 2014 |
$ | 115,202 | $ | 123,075 | ||||
Less current maturities |
38,675 | 6,394 | ||||||
Long-term debt, less current maturities |
$ | 76,527 | $ | 116,681 | ||||
In connection with the Company’s acquisition of VistaCare, it entered into a Second Amended
and Restated Credit Agreement (the “Credit Agreement”) on February 28, 2008 with General Electric
Capital Corporation and certain other lenders that provides the Company with a $130.0 million term
loan (the “Term Loan”) and a $30.0 million revolving line of credit. The Term Loan was used to pay
a portion of the purchase price and costs incurred with respect to the acquisition of VistaCare and
to pay certain fees and expenses incurred in connection with the Credit Agreement. The revolving
line of credit may be used to fund future acquisitions, working capital, capital expenditures and
for general corporate purposes. The borrowing capacity under the credit agreement is reduced by any
outstanding letters of credit and payments under the term loan. At December 31, 2009, outstanding
letters of credit totaled $8.8 million and are used as collateral for insurance policies. As of
December 31, 2009, the borrowing capacity under the credit agreement was $21.2 million, of which no
amounts were drawn.
Borrowings under the Term Loan and revolving line of credit bear interest at an applicable
margin above an Index Rate (based on the higher of the prime rate or 50 basis points over the
federal funds rate) or above LIBOR. At December 31, 2009, both the applicable term loan margin and
the applicable revolver margin for LIBOR loans were 2.50% and for Index Rate loans were 1.50%. At
December 31, 2008, both the applicable term loan margin and the applicable revolver margin for
LIBOR loans were 3.0% and for Index Rate loans were 2.0%. These margins are based on the Company’s
leverage ratio and can vary from 2.50% to 3.25% for LIBOR loans and 1.50% to 2.25% for Index Rate
loans.
In April 2008, the Company entered into two interest rate swap agreements described in note
“13. Derivative Instrument and Hedging Activity” that effectively convert a notional amount of
$60.0 million of floating rate borrowings to fixed rate borrowings.
F-24
Table of Contents
Borrowings outstanding at December 31, 2009 were $115.2 million and carried a weighted-average
interest rate of 4.3%, including the effect of the interest rate swaps. At December 31, 2009,
$53.6 million of the Term Loan carried interest at LIBOR plus 2.50% (2.73%) while $40.0 million of
the Term Loan carried interest at a fixed rate of 5.45% and $20.0 million of the Term Loan carried
interest at a fixed rate of 5.92% as a result of interest rate swap agreements. The remaining $1.6
million of the Term Loan carried interest at the Index Rate plus 1.50% (4.75%).
Borrowings outstanding at December 31, 2008 were $123.1 million and carried a weighted-average
interest rate of 5.7%, including the effect of the interest rate swaps. At December 31, 2008,
$61.7 million of the Term Loan carried interest at LIBOR plus 3.00% (ranging from 5.15% to 5.34%)
while $40.0 million of the Term Loan carried interest at a fixed rate of 5.95% and $20.0 million of
the Term Loan carried interest at a fixed rate of 6.42% as a result of interest rate swap
agreements. The remaining $1.4 million of the Term Loan carried interest at the Index Rate plus
2.00% (5.25%).
The final installment of the Term Loan will be due on February 28, 2014 and the revolving line
of credit will expire on February 28, 2013. The revolving line of credit has an unused facility fee
of 0.25% per annum. In connection with the acquisition of VistaCare, all of the subsidiaries of
VistaCare (together with the Company, and certain of the Company’s subsidiaries, including
VistaCare, the “Odyssey Obligors”) have become guarantors of the obligations under the Credit
Agreement and have granted security interests in substantially all of their existing and
after-acquired personal property. The Term Loan and the revolving line of credit are secured by
substantially all of the Odyssey Obligors’ existing and after-acquired personal property, including
the stock of certain subsidiaries owned by the Odyssey Obligors but not party to the Credit
Agreement. The Odyssey Obligors are subject to affirmative and negative covenants under the Credit
Agreement, including financial covenants consisting of a maximum leverage ratio and a minimum fixed
charge coverage ratio. At both December 31, 2009 and 2008, the Company was in compliance with its
financial covenants. The Company is subject to mandatory prepayments based on cash proceeds
received from the sale of partnership interests and property. During the third quarter of 2009,
the Company paid $1.5 million related to mandatory prepayments of principal, which were based on
cash proceeds received from the sale of the Oklahoma City program. The Company paid approximately
$2.1 million related to mandatory prepayments of principal during the year ended December 31, 2008.
In addition, the Company is subject to an annual excess cash flow requirement, which may result in
the Company having to make additional principal payments on its Term Loan. For the year ended
December 31, 2009, the Company was obligated to make an excess cash flow payment of $29.3 million,
which will be paid during the second quarter of 2010. No such payment was required to be made for
the year ended December 31, 2008. In the future, the Company may be required to make additional
principal payments related to the excess cash flow requirement.
The debt maturity schedule of the Term Loan is as follows and reflects the $29.3 million
estimated cash flow payment that will be made during the second
quarter of 2010 (in thousands):
2010 |
$ | 38,675 | ||
2011 |
9,276 | |||
2012 |
11,015 | |||
2013 |
13,334 | |||
2014 |
42,902 | |||
Thereafter |
— | |||
Total |
$ | 115,202 | ||
In connection with the execution of the Second Amended and Restated Credit Agreement, the
Company incurred approximately $4.4 million of loan costs during 2008 which are being amortized
using the effective interest method over the life of the Credit Agreement. Deferred loan costs
totaled $3.0 million and $3.8 million (net of accumulated amortization) at December 31, 2009 and
2008, respectively.
On November 7, 2008, the Company’s subsidiaries Odyssey HealthCare Operating A, LP, a Delaware
limited partnership, Odyssey HealthCare Operating B, LP, a Delaware limited partnership, Hospice of
the Palm Coast, Inc., a Florida not for profit corporation, and VistaCare, Inc., a Delaware
corporation, entered into an Amendment No. 1
F-25
Table of Contents
to Second Amended and Restated Credit Agreement with General Electric Capital Corporation and
the other lenders signatory thereto. This amendment permits the Company’s existing investments in
ARS, which otherwise would have been required to be liquidated on or prior to November 24, 2008, to
be retained indefinitely.
13. Derivative Instrument and Hedging Activity
The Company entered into an interest rate swap agreement during April 2008, which effectively
converts a notional amount of $40.0 million of floating rate borrowings to fixed rate borrowings.
The term of the interest rate swap expires in April 2011. Under the terms of the interest rate swap
agreement, the Company receives from the counterparty interest on the $40.0 million notional amount
based on three-month LIBOR and pays to the counterparty a fixed rate of 2.95%. The Company entered
into a second interest rate swap agreement in April 2008, which effectively converts a notional
amount of $20.0 million of floating rate borrowings to fixed rate borrowings. The term of the
second interest rate swap also expires in April 2011. Under the terms of the interest rate swap
agreement, the Company receives from the counterparty interest on the $20.0 million notional amount
based on three-month LIBOR and pays to the counterparty a fixed rate of 3.42%. The Company accounts
for the interest rate swaps as a cash flow hedge. These swaps effectively converted $60.0 million
of the Company’s variable-rate borrowings to fixed-rate borrowings beginning in April 2008 and
through April 2011. The Company believes the interest rate swaps will be highly effective in
achieving the Company’s goal of minimizing the volatility of cash flows associated with changes in
interest rates on its variable debt.
FASB ASC Topic 815, “Derivatives and Hedging” (formerly SFAS No. 133 — “Accounting for
Derivative Instruments and Hedging Activities”), requires companies to recognize all derivative
instruments as either assets or liabilities at fair value on the balance sheet. In accordance with
ASC Topic 815, the Company has designated this derivative instrument as a cash flow hedge. As such,
changes in the fair value of the derivative instrument are recorded as a component of other
comprehensive income or loss (“OCI”) to the extent of effectiveness. The ineffective portion of the
change in fair value of the derivative instrument is recognized in interest expense.
The Company is exposed to credit losses in the event of nonperformance by the counterparties
to the two interest rate swap agreements. Management believes that the counterparties are
creditworthy and anticipates that the counterparties and the Company will satisfy all obligations
under the contracts. Hedge effectiveness testing for the year ended December 31, 2009 indicates
that the swaps are highly effective hedges and as such, there is no amount related to hedging
ineffectiveness to expense. As of December 31, 2009, the Company does not expect any amounts to be
reclassified within the next twelve months to earnings from accumulated other comprehensive loss
related to these cash flow hedges.
The fair values of the Company’s interest rate swap agreements as presented in the
consolidated balance sheets are as follows (in thousands):
| Liability Derivatives | ||||||||||||||||
| December 31, 2009 | December 31, 2008 | |||||||||||||||
| Balance Sheet | Balance Sheet | |||||||||||||||
| Location | Fair Value | Location | Fair Value | |||||||||||||
Derivatives designated as
hedging instruments: |
||||||||||||||||
| Other Long Term | Other Long Term | |||||||||||||||
Interest rate swap agreements |
Liabilities | $ | 1,747 | Liabilities | $ | 2,042 | ||||||||||
The effect of the interest rate swap agreements on the Company’s consolidated
comprehensive loss, net of related taxes, for the year ended December 31, is as follows (in
thousands):
| Amount of Income/(Loss) | ||||||||||||||||
| Recognized | Income/(Loss) Reclassified from | |||||||||||||||
| in Other Comprehensive | Accumulated Other Comprehensive | |||||||||||||||
| Income/(Loss) | Loss to Earnings (effective portion) | |||||||||||||||
| 2009 | 2008 | 2009 | 2008 | |||||||||||||
Derivatives designated as cash flow hedges: |
||||||||||||||||
Interest rate swap agreements |
$ | 185 | $ | (1,303 | ) | $ | — | $ | — | |||||||
F-26
Table of Contents
14. Fair Value of Financial Instruments
The fair value of financial instruments is the amount at which the instrument could be
exchanged in a current transaction between willing parties. The fair values of the long-term debt
are estimated using discounted cash flow analysis, based on the Company’s incremental borrowing
rates for similar types of borrowing arrangements. Management estimates that the carrying amounts
of cash and cash equivalents, accounts receivable, accounts payable, long-term debt and certain
other assets are not materially different from their fair values.
The Company categorizes its assets and liabilities recorded at fair value based upon the
following fair value hierarchy established by the FASB.
| • | Level 1 valuations use quoted prices in active markets for identical assets or liabilities that are accessible at the measurement date. An active market is a market in which transactions for the asset or liability occur with sufficient frequency and volume to provide pricing information on an ongoing basis. | ||
| • | Level 2 valuations use inputs other than actively quoted market prices included within Level 1 that are observable for the asset or liability, either directly or indirectly. Level 2 inputs include: (a) quoted prices for similar assets or liabilities in active markets, (b) quoted prices for identical or similar assets or liabilities in markets that are not active, (c) inputs other than quoted prices that are observable for the asset or liability such as interest rates and yield curves observable at commonly quoted intervals and (d) inputs that are derived principally from or corroborated by observable market data by correlation or other means. | ||
| • | Level 3 valuations use unobservable inputs for the asset or liability. Unobservable inputs are used to the extent observable inputs are not available, thereby allowing for situations in which there is little, if any, market activity for the asset or liability at the measurement date. |
The table below sets forth our fair value hierarchy for our ARS and interest rate swaps
measured at fair value as of December 31, 2009.
| Quoted Prices | ||||||||||||||||
| in Active | Significant | |||||||||||||||
| Markets for | Other | Significant | ||||||||||||||
| Identical | Observable | Unobservable | ||||||||||||||
| Assets | Inputs | Inputs | ||||||||||||||
| (Level 1) | (Level 2) | (Level 3) | Total | |||||||||||||
Asset: |
||||||||||||||||
Auction rate securities |
$ | — | $ | — | $ | 12,425 | $ | 12,425 | ||||||||
Liabilities: |
||||||||||||||||
Interest rate swaps |
$ | — | $ | — | $ | 1,747 | $ | 1,747 | ||||||||
The table below sets forth our fair value hierarchy for our ARS and interest rate swaps
measured at fair value as of December 31, 2008.
| Quoted Prices | ||||||||||||||||
| in Active | Significant | |||||||||||||||
| Markets for | Other | Significant | ||||||||||||||
| Identical | Observable | Unobservable | ||||||||||||||
| Assets | Inputs | Inputs | ||||||||||||||
| (Level 1) | (Level 2) | (Level 3) | Total | |||||||||||||
Asset: |
||||||||||||||||
Auction rate securities |
$ | — | $ | — | $ | 16,659 | $ | 16,659 | ||||||||
Liabilities: |
||||||||||||||||
Interest rate swaps |
$ | — | $ | — | $ | 2,042 | $ | 2,042 | ||||||||
F-27
Table of Contents
The Company prepares a discounted cash flow analysis for its ARS to estimate a fair value each
quarter. The assumptions used include an estimated maturity of one year, which is when the Company
estimates it will be able to liquidate these ARS at par and a discount rate to reflect the current
reduced liquidity of these ARS. The discount rate was calculated by taking the existing interest
rate being earned on the ARS as of December 31, 2009 and including a liquidity risk premium rate,
which was calculated based on the treasury yields applicable to the ARS maturity dates as of
December 31, 2009. During the years ended December 31, 2009 and 2008, the Company reduced the fair
value of the ARS by $0.1 million and $0.4 million (before taxes) based on this analysis. Changes in
the fair value of the ARS are recognized, net of tax in accumulated other comprehensive income
(loss). During October 2009, the Company liquidated one ARS at par for $4.1 million.
Also, at December 31, 2009, the Company had liabilities related to its interest rate swaps of
approximately $1.7 million, before income taxes, that were measured at fair value on a recurring
basis using the Level 3 valuation methodology. The fair value reflects the contractual terms of the
derivatives, including the period to maturity, and uses observable market-based inputs, including
interest rate curves and implied volatilities along with estimates of current credit spreads, to
evaluate the likelihood of default by the Company or its counterparties.
The Company’s cash and cash equivalents are measured at fair value using the Level 1 valuation
methodology.
The following table presents the changes in fair value of the Company’s Level 3 assets and
liabilities for the years ended December 31, 2009 and 2008 (in thousands):
| Significant Unobservable | ||||||||
| Inputs | ||||||||
| (Level 3) | ||||||||
| Interest | ||||||||
| ARS | Rate Swaps | |||||||
Balance at January 1, 2008 |
$ | — | $ | — | ||||
Transfers from Level 1 |
41,450 | — | ||||||
Transfers to Level 1 |
(24,350 | ) | ||||||
Unrealized loss included in other comprehensive loss (before tax) |
(441 | ) | (2,042 | ) | ||||
Balance at December 31, 2008 |
16,659 | (2,042 | ) | |||||
Transfers to Level 1 |
(4,100 | ) | — | |||||
Unrealized income (loss) included in other comprehensive loss (before tax) |
(134 | ) | 295 | |||||
Balance at December 31, 2009 |
$ | 12,425 | $ | (1,747 | ) | |||
15. Comprehensive Income
The FASB established guidelines for reporting changes in equity during a period from
transactions and other events and circumstances from non-owner sources. Comprehensive income
attributable to Odyssey stockholders includes the net change in the fair value of ARS and interest
rate swaps, net of income tax, and are included as a component of Odyssey stockholders’ equity.
The components of comprehensive income, net of income tax, are as follows (in thousands):
| For the years ended December 31, | ||||||||||||
| 2009 | 2008 | 2007 | ||||||||||
Net income |
$ | 41,204 | $ | 14,683 | $ | 12,125 | ||||||
Other comprehensive income (loss), net of tax: |
||||||||||||
Unrealized income (loss) on interest rate swaps(1) |
185 | (1,303 | ) | — | ||||||||
Unrealized loss on ARS(2) |
(81 | ) | (282 | ) | — | |||||||
Total other comprehensive income (loss), net of tax |
104 | (1,585 | ) | — | ||||||||
Comprehensive income |
41,308 | 13,098 | 12,125 | |||||||||
Less: comprehensive income attributable to noncontrolling interests |
613 | 257 | 14 | |||||||||
Comprehensive income attributable to Odyssey stockholders |
$ | 40,695 | $ | 12,841 | $ | 12,111 | ||||||
| (1) | Unrealized income (loss) on interest rate swaps is recorded net of tax expense (benefit) of $0.1 million and $(0.7) million for the years ended December 31, 2009 and 2008, respectively. | |
| (2) | Unrealized loss on ARS is recorded net of tax (benefit) of $(0.1) million and $(0.2) million for the years ended December 31, 2009 and 2008, respectively. |
F-28
Table of Contents
The components of accumulated other comprehensive loss are as follows:
| As of December 31, | ||||||||
| 2009 | 2008 | |||||||
Unrealized loss on interest rate swaps |
$ | (1,118 | ) | $ | (1,303 | ) | ||
Unrealized loss on ARS |
(363 | ) | (282 | ) | ||||
Accumulated other comprehensive |
$ | (1,481 | ) | $ | (1,585 | ) | ||
16. Income Taxes
Significant components of the Company’s deferred tax assets and liabilities are as follows:
| December 31, | ||||||||
| 2009 | 2008 | |||||||
| (In thousands) | ||||||||
Current deferred tax assets: |
||||||||
Accounts receivable |
$ | 2,954 | $ | 2,919 | ||||
Insurance |
441 | 255 | ||||||
Accrued compensation |
2,081 | 1,930 | ||||||
Workers’ compensation |
4,020 | 3,402 | ||||||
Accrued Medicare cap contractual adjustments |
452 | 4,491 | ||||||
Other |
287 | 322 | ||||||
| 10,235 | 13,319 | |||||||
Non-current deferred tax assets and liabilities: |
||||||||
Deferred compensation |
5,949 | 4,314 | ||||||
Federal net operating loss |
168 | 3,211 | ||||||
Accrued rent |
2,485 | 1,519 | ||||||
Amortizable and depreciable assets |
(25,335 | ) | (24,254 | ) | ||||
Interest rate swaps and ARS |
841 | 897 | ||||||
Other |
721 | 703 | ||||||
| (15,171 | ) | (13,610 | ) | |||||
Net deferred tax liabilities |
$ | (4,936 | ) | $ | (291 | ) | ||
The components of the Company’s income tax expense (benefit) are as follows:
| Year Ended December 31, | ||||||||||||
| 2009 | 2008 | 2007 | ||||||||||
| (In thousands) | ||||||||||||
Current: |
||||||||||||
Federal |
$ | 16,025 | $ | 12,268 | $ | 6,416 | ||||||
State |
3,219 | 1,683 | 1,067 | |||||||||
| 19,244 | 13,951 | 7,483 | ||||||||||
Deferred: |
||||||||||||
Federal |
5,192 | (2,011 | ) | 466 | ||||||||
State |
147 | (799 | ) | 52 | ||||||||
| 5,339 | (2,810 | ) | 518 | |||||||||
| $ | 24,583 | $ | 11,141 | $ | 8,001 | |||||||
F-29
Table of Contents
The reconciliation of income tax expense computed at the federal statutory tax rate to income
tax expense is as follows:
| Year Ended December 31, | ||||||||||||||||||||||||
| 2009 | 2008 | 2007 | ||||||||||||||||||||||
| Amount | Percent | Amount | Percent | Amount | Percent | |||||||||||||||||||
| (Dollars in thousands) | ||||||||||||||||||||||||
Tax at federal statutory rate |
$ | 22,985 | 35 | % | $ | 10,787 | 35 | % | $ | 8,342 | 35 | % | ||||||||||||
State income tax, net of federal benefit |
2,213 | 3 | 852 | 3 | 727 | 4 | ||||||||||||||||||
Municipal interest income not included
in taxable income |
(149 | ) | — | (567 | ) | (2 | ) | (739 | ) | (4 | ) | |||||||||||||
Income tax credits |
(307 | ) | (1 | ) | (279 | ) | (1 | ) | (313 | ) | (2 | ) | ||||||||||||
Other non-deductible expenses and other |
(159 | ) | — | 348 | 1 | (16 | ) | — | ||||||||||||||||
| $ | 24,583 | 37 | % | $ | 11,141 | 36 | % | $ | 8,001 | 33 | % | |||||||||||||
In July 2006, the FASB issued guidance regarding accounting for uncertainty in income taxes
(formerly FASB Interpretation No. 48, “Accounting for Uncertainty in Income Taxes”), which became
effective for the Company on January 1, 2007. As a result of the application of this guidance, the
Company recorded an adjustment of $0.4 million to its opening balance of retained earnings and
reclassified $1.3 million from deferred tax liabilities to other liabilities for uncertain tax
positions. If these liabilities are settled favorably, it would impact the Company’s effective tax
rate. The only periods still subject to audit for the Company’s federal tax return are the 2006
through 2009 tax years. The Company will classify interest and penalties in the provision for
income taxes. The Company has recorded an accrual of $46,000 and $0.1 million for interest in the
provision for income taxes during the years ended December 31, 2009 and 2008, respectively.
The activity of the liability for uncertain tax positions is as follows (in thousands):
Balance January 1, 2008 |
$ | 1,322 | ||
Accrual of interest |
81 | |||
Balance December 31, 2008 |
1,403 | |||
Accrual of interest |
46 | |||
Balance December 31, 2009 |
$ | 1,449 | ||
The Company does not expect a significant increase or decrease to the liability for uncertain
tax positions over the next twelve months.
The Company had federal and state net operating loss (“NOL”) carryforwards of $0.5 million and
$1.4 million, respectively, at December 31, 2009. However, due to the uncertainty in recognizing
the state NOLs, a full valuation allowance has been established for the state NOLs. All NOLs were
acquired from VistaCare. The Company expects to utilize the federal NOLs by 2011.
17. Retirement Plan
The Company sponsors a 401(k) plan, which is available to substantially all employees after
meeting certain eligibility requirements. After the acquisition of VistaCare and termination of the
VistaCare 401(k) plan, the employees of VistaCare were able to enroll in the Company’s 401(k) plan
subject to meeting certain eligibility requirements. The plan provides for contributions by the
employees based on a percentage of their income. The Company, at its discretion, may make
contributions. Matching contributions totaled $1.5 million, $1.2 million and $0.9 million for the
years ended December 31, 2009, 2008 and 2007, respectively.
F-30
Table of Contents
18. Commitments and Contingencies
Leases
The Company leases office space and equipment at its various locations. Most of the Company’s
lease terms have escalation clauses and renewal options, typically equal to the original lease
term. Total rental expense was $21.1 million, $20.1 million and $13.3 million for the years ended
December 31, 2009, 2008 and 2007, respectively.
Future minimum rental commitments under noncancelable operating leases for the years
subsequent to December 31, 2009, are as follows (in thousands):
2010 |
$ | 17,931 | ||
2011 |
14,396 | |||
2012 |
11,718 | |||
2013 |
9,409 | |||
2014 |
6,456 | |||
Thereafter |
4,510 | |||
| $ | 64,420 | |||
Contingencies
On February 14, 2008, the Company received a letter from the Medicaid Fraud Control Unit of
the Texas Attorney General’s office notifying the Company that it is conducting an investigation
concerning Medicaid hospice services provided by the Company, including the Company’s practices
with respect to patient admission and retention, and requesting medical records of approximately 50
patients served by the Company’s programs in the State of Texas. Based on the preliminary stage of
this investigation and the limited information that the Company has at this time, the Company
cannot predict the outcome of this investigation, the Texas Attorney General’s views of the issues
being investigated, any actions that the Texas Attorney General may take or the impact, if any,
that the investigation may have on the Company’s business, results of operations, liquidity or
capital resources. The Company believes that it is in material compliance with the rules and
regulations applicable to the Texas Medicaid hospice program.
On May 5, 2008, the Company received a letter from the United States Department of Justice
(“DOJ”) notifying the Company that it is conducting an investigation of VistaCare, Inc. and
requesting that the Company provide certain information and documents related to its investigation
of claims submitted by VistaCare to Medicare, Medicaid and TRICARE from January 1, 2003 through
March 6, 2008, the date the Company completed the acquisition of VistaCare. The Company was
informed that the DOJ and the Medicaid Fraud Control Unit of the Texas Attorney General’s Office
are reviewing allegations that VistaCare may have billed the federal Medicare, Medicaid and TRICARE
programs for hospice services that were not reasonably or medically necessary or performed as
claimed. The basis of the investigation is a qui tam lawsuit filed in the United States District
Court for the Northern District of Texas by a former employee of VistaCare. The lawsuit was
unsealed on October 5, 2009 and served on the Company on January 28, 2010. In connection with the
unsealing of the complaint, the DOJ filed a notice with the court declining to intervene in the qui
tam action at this time. The Texas Attorney General also filed a notice of non-intervention with
the court. While these actions should not be viewed as a final assessment by the DOJ or the Texas
Attorney General of the merits of this qui tam action, the Company considers them to be positive
developments. The Company continues to cooperate with the DOJ and the Texas Attorney General in
their investigation of the Company. Based on the limited information that the Company has at this
time, it cannot predict the outcome of the qui tam lawsuit or the related investigation, the DOJ’s
or Texas Attorney General’s views of the issues being investigated other than the DOJ’s and Texas
Attorney General’s notice declining to intervene in the qui tam action at this time, any actions
that the DOJ or the Texas Attorney General may take or the impact, if any, that the investigation
may have on the Company’s business, results of operations, liquidity or capital resources.
The Company has been named in a class action lawsuit filed on November 6, 2008 in Superior
Court of California, Los Angeles County by Charlia Cornish (“Cornish”) alleging class-wide wage and
hour issues at its
F-31
Table of Contents
California hospice programs. The suit alleges failure to provide overtime compensation, meal
and break periods, accurate itemized wage statements, and timely payment of wages earned upon
leaving employment. The purported class includes all persons employed by the Company in California
as an admission nurse, a case manager registered nurse, a licensed vocational nurse, a registered
nurse, a home health aide, a medical social worker, a triage coordinator, an office manager, a
patient care secretary or a spiritual counselor at anytime on or after November 6, 2004. The
lawsuit seeks payment of unpaid wages, damages, interest, penalties and reasonable attorneys’ fees
and costs. In January 2009 the Company successfully moved the lawsuit to Federal District Court in
the Central District of California. On September 21, 2009, the court ruled in the Company’s favor
denying plaintiff’s request to amend and granting the Company’s motion for summary judgment
dismissing the lawsuit in its entirety. The plaintiff has elected not to appeal the decision
dismissing the plaintiff’s lawsuit.
On January 5, 2009, the Company received a letter from the Georgia State Health Care Fraud
Control Unit notifying the Company that it is conducting an investigation concerning Medicaid
hospice services provided by VistaCare from 2003 through 2007 and requesting certain documents. The
Company is cooperating with the Georgia State Health Care Fraud Control Unit and has complied with
the document request. Based on the preliminary stage of this investigation and the limited
information that the Company has at this time, the Company cannot predict the outcome of the
investigation, the Georgia State Health Care Fraud Control Unit’s views of the issues being
investigated, any actions that the Georgia State Health Care Fraud Control Unit may take or the
impact, if any, that the investigation may have on the Company’s business, results of operations,
liquidity or capital resources.
On February 2, 2009, the Company received a subpoena from the United States Office of
Inspector General (“OIG”) requesting certain documents related to the Company’s provision of
continuous care services from January 1, 2004 through February 2, 2009. On September 9, 2009, the
Company received a second subpoena from the OIG requesting medical records for certain patients who
had been provided continuous care services by the Company during the same time period. The Company
is cooperating with the OIG and is in the process of complying with the subpoena requests. Based on
the preliminary stage of this investigation and the limited information that the Company has at
this time the Company cannot predict the outcome of the investigation, the OIG’s views of the
issues being investigated, any actions that the OIG may take or the impact, if any, that the
investigation may have on the Company’s business, results of operations, liquidity or capital
resources.
On March 5, 2009, the Company received a notice submitted on behalf of Ronaldo Ramos to the
California Labor & Workforce Development Agency regarding his intent to file a claim for penalties
pursuant to the California Private Attorney General Act for alleged violations of the California
Labor Code. Ramos is a former employee of the Company and alleges that he and others similarly
situated were improperly paid for on-call hours. His notice indicates that he intends to seek to
recover unpaid wages, overtime, penalties, punitive damages, interest, and attorney’s fees. The
Company is not aware of him filing a lawsuit. The Company believes that it has complied with all
regulations at issue, and it intends to vigorously defend against the claims asserted. Because the
matter is in its early stage, the Company cannot at this time estimate an amount or range of
potential loss in the event of an unfavorable outcome.
The Company has been named in a class action lawsuit filed on January 25, 2010, in the United
States District Court Southern District of Texas Houston Division by Bobby Blevins, a former
employee, alleging failure to pay overtime to a purported class of similarly situated hourly-paid
current and former nurse employees. The plaintiff seeks to recover unpaid overtime compensation,
damages and attorney fees. The Company believes that it has complied with all regulations at
issue, and intends to vigorously defend against the claims asserted. Because of the early stage of
this suit, the Company cannot at this time estimate an amount or range of potential loss in the
event of an unfavorable outcome.
On February 23, 2010, the Company received a subpoena from the OIG requesting various
documents and certain patient records of one its hospice programs relating to services performed
from January 1, 2006 through December 31, 2009. The Company is cooperating with the OIG and is in
the process of complying with the subpoena request. Because of the preliminary stage of this
investigation and the limited information that the Company has at this time the Company cannot
predict the outcome of the investigation, the OIG’s views of the issues being investigated, any
actions that the OIG may take or the impact, if any, that the investigation may have on the
Company’s business, results of operations, liquidity or capital resources.
F-32
Table of Contents
From time to time, the Company may be involved in other litigation matters relating to claims
that arise in the ordinary course of its business. Although the ultimate liability for these
matters cannot be determined, based on the information currently available to the Company, the
Company does not believe that the resolution of these other litigation matters to which the Company
is currently a party will have a material adverse effect on the Company’s business, results of
operations or liquidity. Accrued legal fees and other reserves at December 31, 2009 and 2008, were
$2.8 million and $2.3 million, respectively, which primarily related to these other litigation
matters.
F-33
Table of Contents
19. Segment Information
The Company currently evaluates performance and allocates resources primarily on the basis of
cost per day of care and income from continuing operations. During 2009, the Company reorganized
the regions and restated the financial information presented below for current and prior periods.
Prior periods have also been restated for the reclassification of discontinued programs to
discontinued operations. The distribution by regions of the Company’s net patient service revenue,
direct hospice care expenses, income (loss) from continuing operations before other income
(expense) (which is used by management for operating performance review), average daily census and
total assets are summarized in the following tables:
| Year Ended December 31, | ||||||||||||
| 2009 | 2008 | 2007 | ||||||||||
| (In thousands) | ||||||||||||
Net patient service revenue: |
||||||||||||
Northeast |
$ | 62,474 | $ | 56,987 | $ | 38,869 | ||||||
Southeast |
56,860 | 53,098 | 48,340 | |||||||||
South Central |
69,286 | 59,098 | 47,911 | |||||||||
Midwest |
105,766 | 87,263 | 56,302 | |||||||||
Texas |
127,855 | 114,278 | 62,716 | |||||||||
Mountain |
74,607 | 79,261 | 58,389 | |||||||||
West |
75,183 | 70,984 | 60,708 | |||||||||
South |
56,264 | 48,267 | 13,042 | |||||||||
Southwest |
57,495 | 47,591 | 12,816 | |||||||||
Odyssey Support Center |
648 | (777 | ) | (861 | ) | |||||||
| $ | 686,438 | $ | 616,050 | $ | 398,232 | |||||||
Direct hospice care expenses: |
||||||||||||
Northeast |
$ | 34,577 | $ | 32,687 | $ | 21,511 | ||||||
Southeast |
34,370 | 32,021 | 30,912 | |||||||||
South Central |
42,817 | 38,254 | 30,057 | |||||||||
Midwest |
60,585 | 51,395 | 32,551 | |||||||||
Texas |
77,914 | 71,400 | 39,677 | |||||||||
Mountain |
41,788 | 43,704 | 32,434 | |||||||||
West |
38,640 | 35,791 | 31,481 | |||||||||
South |
33,462 | 28,856 | 8,183 | |||||||||
Southwest |
33,494 | 28,261 | 6,986 | |||||||||
Odyssey Support Center |
(873 | ) | (924 | ) | (128 | ) | ||||||
| $ | 396,774 | $ | 361,445 | $ | 233,664 | |||||||
Income (loss) from continuing operations before other income (expense): |
||||||||||||
Northeast |
$ | 15,433 | $ | 11,617 | $ | 8,233 | ||||||
Southeast |
9,871 | 7,799 | 5,745 | |||||||||
South Central |
11,702 | 6,175 | 6,981 | |||||||||
Midwest |
23,798 | 16,277 | 10,837 | |||||||||
Texas |
22,100 | 17,397 | 7,400 | |||||||||
Mountain |
18,626 | 19,982 | 14,243 | |||||||||
West |
21,144 | 20,273 | 15,056 | |||||||||
South |
8,251 | 6,218 | 1,218 | |||||||||
Southwest |
12,292 | 9,273 | 2,231 | |||||||||
VistaCare Support Center |
752 | (12,522 | ) | — | ||||||||
Odyssey Support Center |
(71,589 | ) | (65,951 | ) | (50,231 | ) | ||||||
| $ | 72,380 | $ | 36,538 | $ | 21,713 | |||||||
F-34
Table of Contents
| Year Ended December 31, | ||||||||||||
| 2009 | 2008 | 2007 | ||||||||||
Average Daily Census (unaudited): |
||||||||||||
Northeast |
1,149 | 1,084 | 768 | |||||||||
Southeast |
1,117 | 1,064 | 1,067 | |||||||||
South Central |
1,228 | 1,125 | 949 | |||||||||
Midwest |
1,938 | 1,689 | 1,104 | |||||||||
Texas |
2,389 | 2,188 | 1,227 | |||||||||
Mountain |
1,257 | 1,366 | 1,031 | |||||||||
West |
1,166 | 1,124 | 991 | |||||||||
South |
1,039 | 926 | 236 | |||||||||
Southwest |
1,097 | 944 | 276 | |||||||||
| 12,380 | 11,510 | 7,649 | ||||||||||
| December 31, | ||||||||
| 2009 | 2008 | |||||||
| (In thousands) | ||||||||
Total Assets: |
||||||||
Northeast |
$ | 20,800 | $ | 20,954 | ||||
Southeast |
20,199 | 32,935 | ||||||
South Central |
40,484 | 32,369 | ||||||
Midwest |
54,033 | 46,016 | ||||||
Texas |
66,128 | 72,181 | ||||||
Mountain |
59,609 | 58,591 | ||||||
West |
19,196 | 21,305 | ||||||
South |
27,353 | 25,648 | ||||||
Southwest |
38,293 | 35,769 | ||||||
Odyssey Support Center |
156,909 | 115,183 | ||||||
| $ | 503,004 | $ | 460,951 | |||||
20. Unaudited Quarterly Financial Information
The quarterly interim financial information shown below has been prepared by the Company’s
management and is unaudited. It should be read in conjunction with the audited consolidated
financial statements appearing herein:
| 2009 Calendar Quarters | ||||||||||||||||
| First | Second | Third | Fourth | |||||||||||||
| (In thousands, except per share amounts) | ||||||||||||||||
Total net revenues |
$ | 167,532 | $ | 170,295 | $ | 175,234 | $ | 173,377 | ||||||||
Net income |
8,855 | 8,600 | 11,780 | 11,967 | ||||||||||||
Less: Net income attributable to noncontrolling interests |
136 | 81 | 199 | 196 | ||||||||||||
Net income attributable to Odyssey stockholders |
$ | 8,719 | $ | 8,519 | $ | 11,581 | $ | 11,771 | ||||||||
Net income attributable to Odyssey stockholders per share: |
||||||||||||||||
Basic |
$ | 0.27 | $ | 0.26 | $ | 0.35 | $ | 0.36 | ||||||||
Diluted |
$ | 0.26 | $ | 0.26 | $ | 0.35 | $ | 0.35 | ||||||||
Weighted average shares outstanding — Basic |
32,801 | 32,905 | 32,932 | 33,098 | ||||||||||||
Weighted average shares outstanding — Diluted |
32,950 | 33,059 | 33,332 | 33,647 | ||||||||||||
F-35
Table of Contents
| 2008 Calendar Quarters | ||||||||||||||||
| First | Second | Third | Fourth | |||||||||||||
| (In thousands, except per share amounts) | ||||||||||||||||
Total net revenues |
$ | 122,808 | $ | 160,716 | $ | 165,241 | $ | 167,284 | ||||||||
Net income |
1,499 | 1,666 | 6,022 | 5,496 | ||||||||||||
Less: Net (loss) income attributable to noncontrolling
interests |
(33 | ) | 13 | 135 | 142 | |||||||||||
Net income attributable to Odyssey stockholders |
$ | 1,532 | $ | 1,653 | $ | 5,887 | $ | 5,354 | ||||||||
Net income attributable to Odyssey stockholders per share: |
||||||||||||||||
Basic |
$ | 0.05 | $ | 0.05 | $ | 0.18 | $ | 0.16 | ||||||||
Diluted |
$ | 0.05 | $ | 0.05 | $ | 0.18 | $ | 0.16 | ||||||||
Weighted average shares outstanding — Basic |
32,639 | 32,660 | 32,670 | 32,724 | ||||||||||||
Weighted average shares outstanding — Diluted |
32,802 | 32,872 | 33,052 | 32,936 | ||||||||||||
F-36
