Attached files
| file | filename |
|---|---|
| EX-23.1 - EX23-1 AUDITORS CONSENT - MICRON SOLUTIONS INC /DE/ | exhibit23-1.htm |
| EX-32.2 - EX32-2 CERTIFICATION OF CFO SEC906 SOX 2002 - MICRON SOLUTIONS INC /DE/ | exhibit32-2.htm |
| EX-32.1 - EX32-1 CERTIFICATION OF CEO SEC906 SOX 2002 - MICRON SOLUTIONS INC /DE/ | exhibit32-1.htm |
| EX-31.1 - EX31-1 CERTIFICATION OF CEO - MICRON SOLUTIONS INC /DE/ | exhibit31-1.htm |
| EX-31.2 - EX31-1 CERTIFICATION OF CFO - MICRON SOLUTIONS INC /DE/ | exhibit31-2.htm |
SECURITIES AND EXCHANGE COMMISSION
Washington,
D. C. 20549
FORM 10-K
[x] Annual report
pursuant to Section 13 or 15(d) of the Securities Exchange Act of
1934
For the fiscal year ended December 31,
2009
[ ] Transition report pursuant to Section
13 or 15(d) of the Securities Exchange Act of 1934
001-9731
(Commission
file number)
ARRHYTHMIA
RESEARCH TECHNOLOGY, INC.
(Name
of registrant as specified in its charter)
|
Delaware
(State
or other jurisdiction of incorporation of organization)
|
72-0925679
(IRS
Employer Identification Number)
|
|
25
Sawyer Passway, Fitchburg, MA
(Address
of principal executive offices)
|
01420
(Zip
Code)
|
(978)
345-5000
(Registrant's telephone
number)
Securities
Registered pursuant to Section 12 (b) of the Act:
|
Common
Stock, $.01 par value
(Title
of Each Class)
|
NYSE
AMEX
(Name
of each exchange on which
registered)
|
Securities
Registered Pursuant to Section 12 (g) of the Act:
None
Indicate by check mark if the
registrant is a well-known seasoned issuer, as defined in Rule 405 if the
Securities Act. Yes No X
Indicate by check mark if the
registrant is not required to file reports pursuant to Section 13 or 15(d) of
the Exchange Act. Yes No X
Indicate by check mark whether the
registrant (1) filed all reports required to be filed by Section 13 or 15(d) of
the Securities Exchange Act of 1934 during the preceding 12 months (or for such
shorter period that the registrant was required to file such reports), and (2)
has been subject to such filing requirements for the past 90
days. Yes X No X
Indicate by check mark if disclosure of
delinquent filers in response to Item 405 of Regulation S-K is not contained
herein, and will not be contained, to the best of registrant's knowledge, in
definitive proxy or information statements incorporated by reference in Part III
of this Form 10-K or any amendment to this Form 10-K. [ ]
Indicate by check mark whether the
registrant has submitted electronically and posted on its corporate Web site if
any, every interactive data file required to be submitted and posted pursuant to
Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter
period that the registrant was required to submit and post such
files). Yes _ No
_
Indicate by check mark whether the
registrant is a large accelerated filer, an accelerated filer, a non-accelerated
filer, or a smaller reporting company. See the definitions of “large accelerated
filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the
Exchange Act. (Check one):
Large
Accelerated filer [ ] Accelerated filer
[ ] Non-Accelerated filer
[ ] Smaller reporting company [ X
]
Indicate by check mark whether the
registrant is a shell company (as defined in Rule 12b-2 of the Exchange
Act). Yes __ No X
State the aggregate market value of the
voting and non-voting common equity held by non-affiliates computed by reference
to the price at which the common equity was last sold, or the average bid and
asked price of such common equity, as of the last business day of the
registrant’s most recently completed second fiscal quarter.
$7,409,313.
On March 3, 2010 there were 2,675,481
shares of the issuer's common stock, par value $.01, outstanding, which is the
only class of common or voting stock of the issuer.
DOCUMENTS
INCORPORATED BY REFERENCE
The registrant intends to file a
definitive proxy statement pursuant to Regulation 14A within 120 days of the end
of the fiscal year ended December 31, 2009. Portions of such proxy
statement are incorporated by reference into Part III of this Form
10-K.
|
Arrhythmia
Research Technology, Inc.
|
|||
|
TABLE
OF CONTENTS
|
|||
|
1
|
|||
|
|
7
|
||
|
10
|
|||
|
|
10
|
||
|
11
|
|||
|
|
11
|
||
|
12
|
|||
|
12
|
|||
|
18
|
|||
|
18
|
|||
|
18
|
|||
|
18
|
|||
|
19
|
|||
|
20
|
|||
|
20
|
|||
|
20
|
|||
|
20
|
|||
|
20
|
|||
|
20
|
|||
|
21
|
|||
OVERVIEW
Arrhythmia
Research Technology, Inc., a Delaware corporation ("ART"), is engaged in the
development of medical software, which analyzes electrical impulses of the heart
to detect and aid in the treatment of potentially lethal
arrhythmias. ART’s patented product consists of signal-averaging
electrocardiographic (SAECG) software named the PREDICTOR™
series.
Our SAECG
product is currently used in a National Institutes for Health (“NIH”) funded
investigation into “Risk Stratification in MADIT II Type
Patients”. At the completion of this study and assuming favorable
study results, ART expects to establish additional licensing contracts with
original equipment manufacturers for this product.
Sudden
cardiac death afflicts over 300,000 individuals in the United States each
year. Most sudden cardiac deaths are due to sustained ventricular
tachycardia (abnormally rapid heartbeat) or ventricular fibrillation (very fast,
completely irregular heartbeat). Ventricular late potentials may
indicate a risk of life-threatening ventricular arrhythmias. The
SAECG process enables late potentials to be amplified and enhanced, while
eliminating undesired electrical noise, allowing for clinical interpretation of
that risk. Rather than having a direct sales force, our efforts are
focused on marketing ART’s product through licensing to original equipment
manufacturers. Although, there were no sales or licensing of the
software in 2009 or 2008, ART licensed the PREDICTOR software in early
2010.
ART’s
wholly owned subsidiary, Micron Products, Inc., a Massachusetts corporation
(“Micron”), is a manufacturer and distributor of silver plated and non-silver
plated conductive resin sensors ("sensors") used in the manufacture of
disposable integrated electrodes constituting a part of electrocardiographic
diagnostic and monitoring instruments. Micron also acts as a
distributor of metal snap fasteners ("snaps"), another component used in the
manufacture of disposable electrodes. The sensors are a critical
component of the signal pathway in many different types of disposable
electrodes. For example, the disposable electrodes used to capture
the electric impulses of the heart and enable the analysis of late potentials
require sensors which provide for an accurate, low noise signal to be
transmitted to the monitoring device. Micron also manufactures and
sells or leases electrode assembly machines to its sensor and snap
customers.
| ART’s
wholly owned subsidiary, Micron Products, Inc., a Massachusetts
corporation (“Micron”), is a manufacturer and distributor of silver plated
and non-silver plated conductive resin sensors ("sensors") used in the
manufacture of disposable integrated electrodes constituting a part of
electrocardiographic diagnostic and monitoring instruments. Micron also
acts as a distributor of metal snap fasteners ("snaps"), another component
used in the manufacture of disposable electrodes. The sensors are a
critical component of the signal pathway in many different types of
disposable electrodes. For example, the disposable electrodes used to
capture the electric impulses of the heart and enable the analysis of late
potentials require sensors which provide for an accurate, low noise signal
to be transmitted to the monitoring device. Micron also manufactures and
sells or leases electrode assembly machines to its sensor and snap
customers.
Micron is one
of a few companies providing silver / silver-chloride sensors to the
medical device industry. Micron’s customers manufacture
monitoring and transmitting electrodes which are utilized in a variety of
bio-feedback and bio-stimulation applications including, among many
others, electrocardiograms (ECG’s), electroencephalograms (EEG’s),
electro-muscular stimulation (EMS), and thermo-electrical neural
stimulation (TENS). Micron also produces high volume precision
plastic products. These high volume products leverage the
production skills for the resin sensors while providing a diversification
from the dependence on a single product line. |
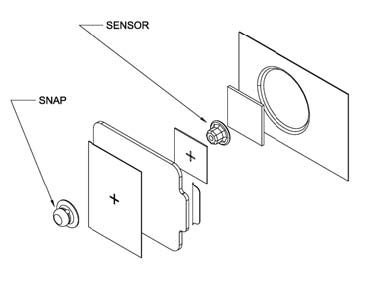 |
| Figure 1: Schematic of Integrated ECG Electrode |
Micron
Integrated Technologies (“MIT”), a division of Micron formed in January 2006,
specializes in the production of metal and plastic components and assemblies for
the medical and defense industries. In 2009, in order to better
leverage the high quality manufacturing of its New England Molders ("NEM")
division’s plastic production capacity and its Leominster Tool Division’s
(“LTD”) metal machining capabilities, Micron began marketing these divisions as
a complete source of custom manufacturing. The custom manufacturing
arm of Micron, MIT provides its customers with a comprehensive portfolio of
value-added manufacturing, design and engineering services, and complete product
life cycle management: from concept to product development, prototyping, and
volume production.
1
PRODUCTS
The
following table sets forth for the periods specified, the revenue derived from
the products of ART and its subsidiary Micron (collectively the
"Company"):
|
Year
Ended December 31,
|
||||||||||||||||
|
2009
|
%
|
2008
|
%
|
|||||||||||||
|
Sensors
|
$ | 8,837,180 | 42 | $ | 9,398,287 | 42 | ||||||||||
|
Subassembly
and metal component manufacturing
|
7,252,081 | 34 | 7,384,790 | 33 | ||||||||||||
|
Custom
injection molding
|
1,795,490 | 8 | 2,067,213 | 9 | ||||||||||||
|
Custom
manufactured metal medical devices
|
1,568,808 | 7 | 1,186,435 | 5 | ||||||||||||
|
Injection
molding tooling
|
629,595 | 3 | 1,478,970 | 7 | ||||||||||||
|
High
volume precision molded products
|
353,326 | 2 | 507,088 | 2 | ||||||||||||
|
Snaps
and snap machines
|
305,930 | 2 | 203,562 | 1 | ||||||||||||
|
Other
products
|
397,364 | 2 | 255,874 | 1 | ||||||||||||
|
Total
|
$ | 21,139,774 | 100 | $ | 22,482,219 | 100 | ||||||||||
|
|
Sensors
|
Micron is
a manufacturer and distributor of silver-plated and non-silver plated conductive
resin sensors for use in the manufacture of disposable electrodes for ECG
diagnostic, monitoring and related instrumentation. The type of
sensor manufactured by Micron consists of a molded plastic substrate plated with
a silver / silver chloride surface, which is a highly sensitive conductor of
electrical signals. Silver / silver chloride-plated disposable
electrodes are utilized in coronary care units, telemetry units, and for other
monitoring purposes. In addition to the traditional ECG tests,
disposable electrodes incorporating Micron’s sensors are used in connection with
stress tests, holter monitoring, and event recorders.
Micron
also manufactures sensors and conductive plastic studs used in the manufacture
of radio translucent electrodes. The radio translucent conductive
plastic studs are manufactured with uniquely engineered resin to enable
electrical conductivity between the sensor and the recording instrument without
the use of a metal snap. The radio translucent electrodes are
virtually invisible to X-rays and are preferred in some medical environments
such as nuclear medicine, cardiac catheterization laboratories, and certain
stress procedures. Micron also manufactures the mating conductive
resin snaps, which replace traditional metal snap fasteners in the radio
translucent applications.
Other
custom designed sensors are manufactured for specific unique applications in the
EEG, EMG or TENS markets. Recent growth in the volume of highly
engineered EEG sensors reflects the increasing demand for non-invasive measuring
of neurological impulses. Micron’s strength in design and low cost
manufacturing enables customers to grow into unique niche medical applications
and electrophysiological monitoring with custom designed sensors.
|
|
High
Volume Precision Molded Products
|
Micron
also sells high volume precision custom molded component parts. Sales
in these high volume molded products diversify the Company’s existing product
lines while utilizing previously unused manufacturing capacity. To
defray the customer’s upfront tooling costs and remain competitive with global
competition, some high volume customers require the financing of a customer
specific tool over several years. The cost of the tool is guaranteed
by the customer and repaid over time as the molded product is
shipped.
|
|
Snaps
and Snap Machines
|
Metal
Snap Fasteners
Metal
snap fasteners are used as an attachment and conductive connection between the
disposable electrode and the lead wires of an ECG machine. Micron
purchases the metal snap fasteners for resale from multiple suppliers and
performs additional quality assurance tests, repackages and stocks these snap
fasteners for its customers who purchase the snaps in addition to Micron’s
sensors.
2
High
Speed Electrode Assembly Machine
Certain
manufacturers of disposable medical electrodes use the Company’s attaching
machines in the assembly of sensors and snaps into disposable
electrodes. Manufacturing, leasing, selling, and providing
replacement parts to medical sensor and snap application machines provides
Micron with a complementary product to sell to existing sensor and snap
customers. As a value added service, a technician can be dispatched
to troubleshoot and improve the performance of the customers’ fully automated
electrode assembly production lines.
|
|
Other
Products and Services
|
Custom
Injection Molding
The
diversification of custom molding has increased production flexibility, and
dramatically expanded the capability to produce an increased size and complexity
of products. From consumable medical products to medical equipment
components, the MIT division has decreased Micron’s dependence on sensor
production for manufacturing growth. In order to leverage the
division’s thermoplastic injection molding capabilities, the division has
expanded into other value added services including packaging, assembly with
outsourced and internally produced metal components, clean room manufacturing,
and specialty coatings.
Defense
industry subassembly and metal component manufacturing
The MIT
division’s product life cycle management program is focused on the integration
of plastic and metal components into subassemblies. The value added
service of in house production capabilities combined with a network of
subcontracted specialty coatings, metallurgical treatments, and unique
production capabilities has diversified this product line to include defense
industry consumables and equipment subassemblies.
Injection
Molding Tooling
The
design, manufacture, and rehabilitation of injection molding tools for the
customer is part of the service package provided by the MIT
division. The division also provides cost savings to Micron by
vertically integrating mold making and repair into the structure of Micron’s
sensor and custom injection molding businesses. The Company’s
engineers and mold designers work with customers’ product development engineers
to design and produce unique tooling for their products. MIT’s
expertise in cost effective manufacturing creates a sustainable partnership with
the customers as prototyped parts move to full scale production. The
design and manufacture of tooling is a leading indicator of future product
revenue. The division continues to generate revenues from other
customers for similar industrial applications such as metal die casting molds,
investment casting wax molds, and thermoplastic injection/extrusion blow
molds.
Custom
Manufactured Metal Medical Devices
A climate
controlled medical machining cell was built for the custom computer aided design
and computer controlled metal machining of patient specific orthopedic medical
device components. The manufacturing space includes a machine
programming office with the latest technology in computer programming for 5-axis
machining with Computer Numerical Controlled (CNC) vertical milling machines and
a state of the art 5-axis machining center. These products involve
complex machining of wrought and cast cobalt-chromium-molybdenum alloy as well
as high molecular weight polymers into unique customized products. No
two components are identical and require precision manufacturing verified by
complex computer controlled automated coordinate measuring equipment that
measure up to 25 points per square inch. Additional capabilities
added to the cell include laser marking, passivation, automated polishing, and
ultra-sonic cleaning. The space can accommodate a 50% increase in
manufacturing capacity before reaching any physical constraints.
Signal-Averaging
Electrocardiographic (SAECG) Products - PREDICTOR™
In early
2010, the Company successfully converted its proprietary signal-averaged
electrocardiography (SAECG) software, PREDICTOR, that operates on a single
hardware based electrocardiogram acquisition platform, ART 1200-EPX, to a
customizable modular software product that is compatible with a variety of
hardware platforms. The conversion allows PREDICTOR to be used with
customer-specific electrocardiogram acquisition equipment to generate the
signal-averaged ECG analysis. The software can be customized to
interface with a variety of Original Equipment Manufacturer (“OEM”)
hardware. OEM customers can license PREDICTOR and bundle it with
other cardiac diagnostic software packages incorporated in their acquisition
equipment.
3
PREDICTOR
utilizes the unique, patented and proprietary algorithms which have been defined
as the “Standard” by the joint AHA/ACC/ESC task force on Signal-Averaging
Electrocardiography1. PREDICTOR is also capable of
incorporating additional signal processing capabilities included in the
Company’s software library for clinical research. This library
includes IntraSpect, a module that permits detection of ventricular late
potentials in patients with Bundle Branch Block, P-wave signal averaging which
helps predict patients at risk for atrial fibrillation and flutter and a Heart
Rate Variability module.
PREDICTOR
is currently being used in a NIH funded investigation into “Risk Stratification
in MADIT II Type Patients”. The primary objectives of this study
are: 1. To evaluate the predictive value of a multivariate model
consisting of pre-specified clinical and ECG parameters for predicting
arrhythmic events in Multicenter Automatic Defibrillator Implantation Trial II
(“MADIT II”) type post-infarction patients; 2. To develop
a multivariate risk-stratification model, based on a broader spectrum of
pre-specified clinical covariates and ECG parameters, and from it a risk-scoring
algorithm identifying high-risk and low-risk patient groups; this algorithm will
be validated by a cross-validation study. Such an algorithm will
enable an ordering of patients who may benefit most, and benefit least, from
implantable cardiac defibrillator (“ICD”) therapy. Results from this
investigation are expected in late 2011.
GENERAL
|
|
Customers
and Sales
|
During
the year ended December 31, 2009, there were three major customers, each of
which accounted for over 10% of the Company’s sales and a loss of this base may
have a material adverse effect on results. The three largest
customers accounted for 23%, 16%, and 12% of sales in 2009 as compared to 27%,
17%, and 12% of sales for the year ended December 31, 2008.
Micron
manufactures its sensors against purchase orders from electrode
manufacturers. The Company is aware of approximately 20 significant
manufacturers of disposable snap type, radio translucent and pre-wired
electrodes worldwide. Micron sells its sensors to most of these
manufacturers. Sales backlog is not material to Micron’s sensor
business due to the method of ordering employed by its customer base in this
competitive industry. Customers generally purchase on a single
purchase order basis without long-term commitments.
The
majority of the MIT divisions’ customers for injection molded thermoplastic
products are from the medical equipment, medical device and defense
industries. From single use medical or defense consumable products to
equipment components, the engineered production services provide quality design
and production capabilities which exceed the customers’ manufacturing
requirements. Certain customers require that an inventory of their
products be maintained at all times to enable just in time delivery
schedules. A commitment from customers is required by MIT to maintain
the higher level of finished goods inventory and raw material required for their
products. These agreements allow for a more flexible manufacturing
schedule with longer more cost effective production cycles. MIT’s
primary target customer is a medical product or device, defense related
contractor, manufacturer, or development company with a need for complete
product life cycle management from design to full production preferably
combining multiple manufacturing technologies such as plastic injection molding,
metalworking, assembly, and packaging.
The
following table sets forth, for the periods indicated, the approximate
consolidated revenues and percentages of revenues derived from the sales of all
of the Company's products in its geographic markets:
|
Revenues
for the Years Ended December 31,
|
||||||||||||||||
|
2009
|
%
|
2008
|
%
|
|||||||||||||
|
United
States
|
$ | 12,937,615 | 61 | $ | 13,290,098 | 59 | ||||||||||
|
Canada
|
3,684,087 | 17 | 5,118,913 | 23 | ||||||||||||
|
Europe
|
2,644,727 | 13 | 3,091,326 | 14 | ||||||||||||
|
Pacific
Rim
|
818,866 | 4 | 426,764 | 2 | ||||||||||||
|
Other
|
1,054,479 | 5 | 555,118 | 2 | ||||||||||||
|
Total
|
$ | 21,139,774 | 100 | $ | 22,482,219 | 100 | ||||||||||
While
some risks exist in foreign markets, the vast majority of the Company’s
customers are based in historically stable markets. To reduce the
risks associated with foreign shipment and currency exchange fluctuations, the
title to most of the products are transferred to the customers when shipped, and
payment is required in U.S. Dollars.
(1)
AHA/ACC/ESC Policy Statement: "Standards for the Analysis of Ventricular Late
Potentials Using High Resolution or Signal-Averaged Electrocardiography: A
Statement by a Task Force Committee of the European Society of Cardiology, the
American Heart Association and the American College of Cardiology. JACC Vol. 17,
No. 5, April 1991:999-1006
4
To help
offset the risk from fluctuations in the market price of silver, sensor
customers have generally been subject to a silver surcharge or discount based on
the market price of silver at the time of shipment. The Company is
sensitive to the impact of recent increases in silver cost, and continues to
explore options with the sensor customers to help mitigate the resulting
increases in surcharges.
|
|
Marketing
and Competition
|
Micron
sells its sensors to large, sophisticated OEM manufacturers of disposable snap
type and radio translucent ECG electrodes who compete internationally in the
electrode market against other OEM manufacturers as well as manufacturers of
tab-type electrodes. The Company has one major domestic competitor in
the sensor market along with an increasing number of minor competitors
worldwide. The sensor and snap market is extremely price sensitive
and barriers to entry are relatively low. The Company competes with
respect to its sensor products on the basis of pricing, technical capabilities,
quality of service and ability to meet customer requirements. With no
import restrictions, the Company’s foreign competitors with excess capacity can
be expected to expand sales in the U.S. In addition, many of the
major OEM customers, although not currently manufacturing silver-silver chloride
sensors, have the ability to do so with modest investment.
The
Company markets Micron and its MIT division as a highly specialized custom
injection thermoplastic molder to new and existing customers. The
Company believes it competes effectively based on its expertise in low cost
manufacturing of high volume precision products. The complex medical
products produced by the MIT division have expanded the existing customer base
and extensively diversified the product mix. It is the Company’s
intention to continue these efforts to market to the expanded customer base and
further diversify the product offerings. Global competition creates a
highly competitive environment. To meet this challenge, the MIT
division focuses its product development efforts on complex close tolerance
products not readily outsourced to offshore manufacturing. The
Company’s recent ISO 13485:2003 registration, the international quality standard
for medical devices, qualifies the Company to further expand into medical
products. The Company expects to become competitive in more markets
after completion of its registration as a U.S. Food and Drug Administration
(FDA) manufacturing facility in 2010. The Company’s International
Traffic in Arms Regulation (ITAR) registration with the US State Department
allows the Company to compete in defense applications restricted by export
controls and the Department of Defense.
After
success in early 2010, management is currently pursuing licensing arrangements
of its proprietary signal-averaged electrocardiography (SAECG) software,
PREDICTOR, to other Original Equipment Manufacturers for integration into
existing cardio diagnostic equipment. As previously stated, the SAECG
product is currently used in a NIH funded investigation into “Risk
Stratification in MADIT II Type Patients”.
|
|
Product
Suppliers and Manufacturing
|
Micron
manufactures its sensors at its Fitchburg, Massachusetts facility employing a
proprietary non-patented multi-step process. All employees sign
confidentiality agreements to protect this proprietary process. The
raw materials used by Micron are plastic resins used to mold the substrates and
silver-silver chloride chemical solutions for plating the molded plastic
substrates. Both the resins and the chemicals involved in the
silver-silver chloride process are available in adequate supply from multiple
commodity sources. As insulation against unanticipated price
increases, some resins and chemicals used in the production of sensors are
purchased in large quantities to lower or stabilize prices.
Resins
used by the MIT division are purchased for an individual customer order, with
most increases in resin costs passed on to the customer as orders are
acknowledged. Because the customer order determines the quantity of
material required, customers may, and have, guaranteed the purchase of specific
large quantities of product which allows the division to purchase raw material
at a more favorable cost thereby lowering the final cost to the
customer. The metal alloys are subject to the same customer order
limitations, and prices are fixed as the customer guarantees an
order.
Micron
distributes medical grade nickel-plated brass and stainless steel snap fasteners
purchased from multiple domestic and international sources. Micron
buys these snaps in bulk, performs additional quality assurance tests, and
stocks inventory to facilitate just-in-time shipments to its
customers. This business segment has decreased significantly in
revenue as price pressure has forced metal snap customers to buy direct from the
manufacturer to remain competitive.
The
Company’s 116,000 square foot manufacturing facilities are ITAR, ISO 9001:2001
and 13485:2003 registered. Micron’s injection molding machines
capacity ranges from 15 to 300 tons and includes a class 10,000 clean room used
for processes sensitive to environmental particulates. In addition,
this facility includes a climate-controlled space for the manufacture of metal
medical devices utilizing the latest in 5-axis CNC technology.
5
|
|
Inventory
Requirements
|
Larger
customers benefit from the Company’s ability to maintain an inventory of
standard sensors and snaps. This inventory policy allows for
predictable and planned production resulting in cost efficiencies that help to
offset price erosion in the marketplace.
Custom
manufactured product is completed on an order by order
basis. Finished goods inventory is product made in advance of an
acknowledged sales order, part of an annual blanket order quantity, or for a
specific safety stock requested by the customer.
|
|
Research
and Development
|
ART's
research and development efforts focus primarily on maintaining the software
library in the SAECG product lines in a compatible platform. The
Company continues to provide technical support to the NIH’s research project
utilizing ART’s software. Included in this expense is development
work to verify the integrity of the analytical algorithms, and improve the
stability and ease of customization of the software to be compatible with
various hardware and software platforms. For the fiscal years ended
December 31, 2009 and 2008, ART had research and development expenses
of approximately $21,527 and $69,779, respectively.
In 2009
and 2008, Micron’s research and development efforts resulted in $219,967 and
$250,261 of expense. These efforts include the development of a
unique process to eliminate certain hazardous materials from the manufacturing
processes. The 2008 expense included $52,000 for equipment tested in
a process improvement project for the sensor product line as well as the
impairment of equipment used for final product
testing. .
|
|
Patents
and Proprietary Technology
|
ART
acquired three patents related to time and frequency domain analysis of
electrocardiogram signals including U.S. Patent No. 5,117,833 entitled “Bi-Spectral Filtering of
Electrocardiogram Signals to Determine Selected QRS Potentials,” (the
“Bi-Spec Patent”) in 1993. These technologies are utilized in the
current version of PREDICTOR. In March 1997, the U.S. Patent Office
granted United States Patent No. 5,609,158 entitled “Apparatus and Method for Predicting
Cardiac Arrhythmia, by Detection of Micropotentials and Analysis of all ECG
Segments and Intervals” which covers a frequency domain analysis
technique for SAECG data.
The
Company believes that ART's products do not and will not infringe on patents or
violate proprietary rights of others. In the event that ART's
products infringe patents or proprietary rights of others, ART may be required
to modify the design of its products or obtain a license. There can
be no assurance that ART will be able to do so in a timely manner upon
acceptable terms and conditions. In addition, there can be no
assurance that ART will have the financial or other resources necessary to
enforce or defend a patent infringement or proprietary rights violation
action. Moreover, if ART's products infringe patents or proprietary
rights of others, ART could, under certain circumstances, become liable for
damages, which could have a material adverse effect on earnings.
Micron
employs a highly complex, proprietary non-patented multi-step manufacturing
process for its silver / silver chloride-plated sensors. To
maintain trade secrets associated with the manufacture of disposable electrode
sensors, all employees are required to sign non-disclosure and/or
non-competition agreements. Micron uses a patented material in the
production of some sensors. Micron paid $2,966 in 2009, and $4,288 in
2008 in royalties associated with this patent.
|
|
Government
Regulation
|
ART’s
software products are subject to, and ART believes currently comply with,
material clearance and distribution requirements from governmental regulatory
authorities, principally the FDA and the European Union (EU) equivalent
agency. These agencies promulgate quality system requirements under
which a medical device is to be developed, validated and
manufactured. The development of the product line will be managed in
accordance with applicable regulatory requirements.
Micron’s
sensor elements are components used in medical devices designed and manufactured
by original equipment manufacturers. As such, these elements are not
required to be listed with regulatory agencies and do not require regulatory
clearance for distribution. However, because Micron primarily
distributes sensors to manufacturers for use in finished medical devices, Micron
exercises as stringent controls over its manufacturing processes and finished
products as would be required if the sensors were considered medical
devices.
6
The MIT
division manufactures parts for invasive medical devices, components for medical
equipment, patented disposable medical laboratory products, and patented
military applications. Customers own the product designs and are,
therefore, subject to FDA, Department of Defense and EU
regulations. While such products are a part of a medical device or
other regulated equipment, customers are the regulated entity for the clearance
of those products. MIT exercises stringent controls over all their
manufacturing operations, and complies with any special controls required by
their customers.
|
|
Environmental
Regulation
|
Micron’s
operations involve use of hazardous and toxic materials, and generate hazardous,
toxic and other wastes. Its operations are subject to federal, state
and local laws and regulations governing the use, storage, handling and disposal
of such materials and certain waste products. Although management believes that
the safety procedures for using, handling, storing and disposing of such
materials comply with these standards required by state and federal laws and
regulations, the Company cannot completely eliminate the risk of accidental
contamination or injury from these materials.
Since its
inception, Micron has expended significant funds to train its personnel, install
waste treatment and recovery equipment and to retain an independent
environmental consulting firm to regularly review, monitor and upgrade its air
and waste water treatment activities. Management continues to
evaluate and test many possible technological advances that reduce or eliminate
the need for and use of hazardous materials in the manufacturing
processes. The acquisition of equipment to eliminate a hazardous
chemical from the process further emphasizes the commitment to the reduction and
elimination of certain hazardous processes. Costs of compliance are
not currently material to the Company’s operation. Micron believes
that the operation of its manufacturing facility is in compliance with currently
applicable safety, health and environmental laws and regulations.
|
|
Employees
|
As of
December 31, 2009, the Company had 89 full-time and 4 part-time
employees. The employees of the Company are not represented by a
union, and the Company believes its relationship with the employees is
satisfactory.
|
|
Periodic
Reporting and Financial Information
|
The
Company registered its common stock under the Securities Exchange Act of 1934,
as amended, or the Exchange Act, and have reporting obligations, including the
requirement that we file annual and quarterly reports with the
SEC. The public may read and copy materials the Company files with
the SEC at the SEC’s Public Reference Room at 100 F Street, NE, Washington, DC
20549, on official business days during the hours of 10:00 am to 3:00
pm. You may obtain information about the operation of the Public
Reference Room by calling the SEC at 1-800-SEC-0330. The SEC
maintains an Internet site that contains reports, proxy and information
statements, and other information regarding issuers that file electronically
with the SEC at http://www.sec.gov.
In
addition to the other information in this Form 10-K, the following factors
should be considered in evaluating the Company and its business. The
risks and uncertainties described below are not the only ones facing the
Company. Additional risks and uncertainties that the Company does not
presently know or currently deems immaterial may also impair the Company’s
business, results of operations and financial condition.
The
Company’s operating results may fluctuate significantly as a result of a variety
of factors.
The
Company’s operating results may fluctuate significantly in the future as a
result of a variety of factors, many of which are outside of our
control. These factors include:
|
·
|
the
ability to maintain the current pricing model and/or decrease the cost of
sales;
|
|
·
|
the
ability to increase sales of higher margin
products;
|
|
·
|
variations
in the mix of products sold;
|
|
·
|
the
level of demand for our products and services and those that the Comapny
may develop or acquire;
|
|
·
|
volatility
in commodity and energy prices and the ability to offset higher costs with
price increases;
|
|
·
|
variability
of customer delivery requirements;
|
|
·
|
continued
availability of supplies or materials used in manufacturing at competitive
prices;
|
|
·
|
the
amount and timing of investments in capital equipment, sales and
marketing, engineering and information technology resources;
and
|
|
·
|
general
economic conditions.
|
7
As a
strategic response to changes in the competitive environment, the Company may
from time to time make certain pricing, service, technology or marketing
decisions or business or technology acquisitions that could have a material
adverse effect on the quarterly and annual results. Due to all of
these factors, the operating results may fall below the expectations of
securities analysts, stockholders and investors in any future
period.
Large
OEM customers can change their demand on short notice, further adding to the
unpredictability of the quarterly sales and earnings.
The
quarterly results have in the past and may in the future vary due to the lack of
dependable long-term demand forecasts from the larger OEM
customers. In addition to this risk, many of the Company’s OEM
customers have the right to change their demand schedule, either up or down,
within a relatively short time horizon. These changes may result in
the Company incurring additional working capital costs and causing increased
manufacturing expenses due to these short-term fluctuations. In
particular, the quarterly operating results have in the past fluctuated as a
result of some of the larger OEM customers changing their orders within a fiscal
quarter. The expense levels and inventory, to a large extent, are
based on shipment expectations in the quarter. If sales levels fall
below these expectations, through a delay in orders or otherwise, operating
results are likely to be adversely affected. Although the Company
continues to attempt to lessen the dependency on a few large customers, it can
provide no assurance that it will be able to materially alter this dependency in
the immediate future, if at all.
A
significant portion of our revenues are derived from the sale of a single
product line.
In fiscal
years 2009 and 2008, the Company derived 42% of its revenues from medical
electrode sensors for use in disposable electrodes. While the
technology in electrode sensors has been used for many years, there is no
assurance that a new patented or unpatented technology might not replace the
existing disposable electrode sensors. Any substantial technological
advance that eliminates the Company’s products will have a material adverse
effect on the operating results.
The
Company is dependent on a limited number of customers.
In the
fiscal years 2009 and 2008, 51% and 56%, respectively, of the Company’s revenues
were derived from individual customers with 10% or more of the total
sales. The loss of any one or more of these customers might have an
immediate significant adverse effect on our financial results. In an
effort to maintain this customer base, more favorable terms than might otherwise
be agreed to could be granted. Currently, the Company generally does
not receive purchase volume commitments extending beyond several months. Large
corporations can shift focus away from a need for the Company’s products with
little or no warning.
Failure
to comply with Quality System Regulations or industry standards could result in
a material adverse effect on the business and results of
operations.
The
Company’s Quality Management System complies with the requirements of ISO
9001:2000 and ISO 13485:2005. If the Company were not able to comply
with the Quality Management System or industry-defined standards, the Company
may not be able to fill customer orders to the satisfaction of the
customers. Failure to produce products compliant with these standards
could lead to a loss of customers which would have an adverse impact on the
business and results of operations.
If
trade secrets are not kept confidential, the secrets may be used by others to
compete against the Company.
Micron
relies on unpatented trade secrets to protect its proprietary processes and
there are no assurances that others will not independently develop or acquire
substantially equivalent technologies or otherwise gain access to the
proprietary process. Ultimately the meaningful protection of such
unpatented proprietary technology cannot be guaranteed. The Company
relies on confidentiality agreements with its employees. Remedies for
any breach by a party of these confidentiality agreements may not be adequate to
prevent such actions. Failure to maintain trade secret protection,
for any reason, could have a material adverse effect on the
Company.
The
initiatives that the Company is implementing in an effort to improve our
manufacturing productivity could be unsuccessful, which could harm its business
and results of operations.
In an
effort to improve manufacturing productivity, the Company has implemented
several strategic initiatives focusing on improving the manufacturing processes
and procedures. Management believes these initiatives should improve
customer satisfaction as well as revenue and income. However, in the
event these initiatives are not successful, due to systemic failure to fully
embrace the concepts and maximize the benefits of the investments of equipment
and technology, the results of operations will not improve as
expected.
8
If
the Company is unable to keep up with rapid technological changes, the
processes, products or services may become obsolete and
unmarketable.
The
medical device and medical software industries are characterized by
technological change over time. Although the Company attempts to
expand technological capabilities in order to remain competitive, discoveries by
others may make the Company’s processes or products obsolete. If the
Company cannot compete effectively in the marketplace, the potential for
profitability and financial position will suffer.
General
economic conditions, largely out of the Company’s control, may adversely affect
the Company’s financial condition and results of operations.
The
Company’s business may be affected by changes in general economic conditions,
both nationally and internationally. Recessionary economic cycles,
higher interest rates, higher fuel and other energy costs, inflation, higher
levels of unemployment, changes in the laws or industry regulations or other
economic factors may adversely affect the demand for the Company’s
products. Additionally, these economic factors, as well as higher tax
rates, increased costs of labor, insurance and healthcare, and changes in other
laws and regulations may increase the Company’s cost of sales and operating
expenses, which may adversely affect the Company’s financial condition and
results of operations.
The
Company is subject to stringent environmental regulations.
The
Company is subject to a variety of federal, state and local requirements
governing the protection of the environment. These environmental
regulations include those related to the use, storage, handling, discharge and
disposal of toxic or otherwise hazardous materials used in or resulting from the
Company’s manufacturing processes. Failure to comply with
environmental law could subject the Company to substantial liability or force us
to significantly change our manufacturing operations. In addition,
under some of these laws and regulations, the Company could be held financially
responsible for remedial measures if its properties are contaminated, even if it
did not cause the contamination.
A
product liability suit could adversely affect our operating
results.
The
testing, manufacture, marketing and sale of medical devices of the customers
entail the inherent risk of liability claims or product recalls. If the
customers are involved in a lawsuit, it is foreseeable that the Company would
also be named. Although the Company maintains product liability
insurance, coverage may not be adequate. Product liability insurance is
expensive, and in the future may not be available on acceptable terms, if at
all. A successful product liability claim or product recall could have a
material adverse effect on the business, financial condition, and ability to
market product in the future.
The
Company could become involved in litigation over intellectual property
rights.
The
medical device industry has been characterized by extensive litigation regarding
patents and other intellectual property rights. Litigation, which would likely
result in substantial cost to us, may be necessary to enforce any patents issued
or licensed to us and/or to determine the scope and validity of others'
proprietary rights. In particular, competitors and other third
parties hold issued patents, which may result in claims of infringement against
the Comapny or other patent litigation. The Company also may have to
participate in interference proceedings declared by the United States Patent and
Trademark Office, which could result in substantial cost, to determine the
priority of inventions.
The
Company may make acquisitions of companies, products or technologies that may
disrupt the business and divert management’s attention, adversely impacting our
results of operations and financial condition.
The
Company may make acquisitions of complementary companies, products or
technologies from time to time. Any acquisitions will require the
assimilation of the operations, products and personnel of the acquired
businesses and the training and motivation of these
individuals. Management may be unable to maintain and improve upon
the uniform standards, controls, procedures and policies if the Company fails in
this integration. Acquisitions may cause disruptions in operations
and divert management’s attention from day-to-day operations, which could impair
our relationships with current employees, customers and strategic
partners. The Company also may have to, or choose to, incur debt or
issue equity securities to pay for any future acquisitions. The
issuance of equity securities for an acquisition could be substantially dilutive
to our stockholders’ holdings. In addition, profitability may suffer
because of such acquisition-related costs or amortization costs for other
intangible assets. If management is unable to fully integrate
acquired businesses, products, technologies or personnel with existing
operations, the Company may not receive the intended benefits of such
acquisitions. The Company is not currently party to any agreements,
written or oral, for the acquisition of any company, product or
technology.
9
Healthcare
policy changes, including pending proposals to reform the U.S. healthcare
system, may have a material adverse effect on the results.
Healthcare
costs have risen significantly over the past decade. There have been
and continue to be proposals by legislators, regulators and third-party payers
to keep these costs down. Certain proposals, if passed, would impose
limitations on the prices we will be able to charge for our products, or the
amounts of reimbursement available for our products from governmental agencies
or third-party payers. These limitations could have a material
adverse effect on the Company’s financial position and results of
operations.
Changes
in the health care industry in the U.S. and elsewhere could adversely affect the
demand for the products as well as the way in which the Company conducts
business. Significantly, the new administration and Congressional and
state leaders have expressed a strong desire to reform the U.S. healthcare
system. Recently, President Obama and members of Congress have
proposed significant reforms. On November 7, 2009, the House of
Representatives passed and, on December 24, 2009, the Senate passed health
reform legislation which if enacted would require most individuals to have
health insurance, establish new regulations on health plans, create insurance
pooling mechanisms and a government health insurance option to compete with
private plans, and other expanded public health care measures. This
legislation also would reduce Medicare spending on services provided by
hospitals and other providers and the House bill proposes a 2.5 percent tax on
the first taxable sale of any medical device. The Senate bill
included a $2 billion annual fee or excise tax on the medical device
manufacturing sector. If the excise taxes are enacted into law, the
Company’s results of operations may be materially and adversely
affected.
The
Company may be exposed to potential risks relating to internal control over
financial reporting and the ability to have those controls attested to by the
independent registered public accounting firm.
As
directed by Section 404 of the Sarbanes-Oxley Act of 2002 (“SOX 404”), the
Securities and Exchange Commission adopted rules requiring public companies to
include a report of management on the Company’s internal control over financial
reporting in their annual reports, including Form 10-K. In addition,
the independent registered public accounting firm auditing a company’s financial
statements must also attest to and report on the Company’s assessment of the
effectiveness of the company’s internal control over financial reporting as well
as the operating effectiveness of the company’s internal
controls. The Company was subject to the management evaluation and
review portion of these requirements for the fiscal year ended December 31,
2009. Management is evaluating the Company’s internal control systems
in order to allow the independent auditors attest to, management’s internal
controls, as a required part of the Annual Report on Form 10-K beginning with
the report for the fiscal year ended December 31, 2010.
In the
event the Company no longer qualifies as a smaller reporting company at the end
of 2010, it may be subject to more stringent requirements under SOX
404. Accordingly, there can be no assurance that the Company will
receive any required attestation from the independent registered public
accounting firm. In the event the independent register public
accounting firm identifies significant deficiencies or material weaknesses in
the Company’s internal controls that management cannot remediate in a timely
manner or is unable to receive an attestation from the independent registered
public accounting firm with respect to the Company’s internal controls,
investors and others may lose confidence in the reliability of the financial
statements and the Company’s ability to obtain equity or debt financing could
suffer.
None
The
manufacturing facility and offices of the Company are located in two buildings
in an industrial area in Fitchburg, Massachusetts. The first
building, which was purchased in April 1994, consists of a 22,000 square foot,
six story building. The second building, which was purchased in
September 1996, is over 94,000 square feet, including an antique brick three
story mill building. Commencing in 2003, the 40,000 square foot
“Mill” building portion of the second building underwent major renovations to
preserve and create functional space from a previously unusable section of the
facility. The renovations created space currently occupied by the MIT
division. From October 2006 to February 2007, a third building of
approximately 40,000 square feet, a fourth building of 12,000 square feet and
vacant parcel between the buildings that abut the complex were acquired without
any specific requirement for space. The Company believes the
acquisition of the adjacent property positions the Company for continued growth
in its current location. The Company believes its current facilities
are sufficient to meet current and future production needs through fiscal year
ending December 31, 2010.
10
The
Company is from time to time subject to legal proceedings, threats of legal
action and claims which arise in the ordinary course of our
business. Management believes the resolution of these matters will
not have a material adverse effect on the results of operations or financial
condition.
|
|
ART's
Common Stock has been listed on the NYSE AMEX, formerly the American Stock
Exchange, since March 3, 1992 and trades under the ticker symbol
HRT.
The
following table sets forth, for the period indicated, the high and low sale
prices per share for ART's Common Stock as quoted by the NYSE AMEX.
|
High
|
Low
|
|||||||
|
Year
Ended December 31, 2009
|
||||||||
|
1st
Quarter
|
$ | 2.65 | $ | 1.38 | ||||
|
2nd
Quarter
|
3.46 | 2.10 | ||||||
|
3rd
Quarter
|
4.60 | 2.61 | ||||||
|
4th
Quarter
|
4.84 | 3.26 | ||||||
|
Year
Ended December 31, 2008
|
||||||||
|
1st
Quarter
|
$ | 9.30 | $ | 5.00 | ||||
|
2nd
Quarter
|
7.00 | 5.40 | ||||||
|
3rd
Quarter
|
6.03 | 3.25 | ||||||
|
4th
Quarter
|
3.98 | 1.70 | ||||||
As of
March 1, 2010 the number of record holders of ART's common stock is estimated to
be 300 not including beneficial holders of our common stock held in street
name.
|
|
Dividend
Policy
|
No
dividends were declared in 2009 or 2008.
On
January 15, 2010, the Board of Directors declared a cash dividend of $0.06 per
share. The dividend was paid March 1, 2010.
Future
determination as to the payment of cash dividends, if any, will be at the
discretion of the Board of Directors and will be dependent upon the Company’s
financial condition, results of operations, capital requirements, potential
acquisitions, and other such factors as the Board of Directors may deem
relevant, including any restrictions under any credit facilities in place now or
in the future. The Company's demand line of credit agreement contains
conditions including prior notification of the payment of
dividends.
Equity
Compensation Plan Information
The
following table provides information, as of December 31, 2009, with respect to
our equity compensation plans:
|
Plan
Category
|
Number
of securities to be issued upon exercise of outstanding options, warrants
and rights
|
Weighted-average
exercise price of outstanding options, warrants and rights
|
Number
of securities remaining available for future issuance under equity
compensation plans (excluding securities reflected in column
(a))
|
|
(a)
|
(b)
|
(c)
|
|
|
Equity
compensation plans approved by security holders
|
179,000
|
$ 9.29
|
268,000
(1)
|
|
Equity
compensation plans not approved by security holders
|
-
|
-
|
-
|
|
Total
|
179,000
|
$ 9.29
|
268,000
(1)
|
|
(1)
|
Includes
168,000 shares available under the 2001 Stock Option Plan and 100,000
shares available under the 2005 Stock Award
Plan.
|
11
Recent
Sales of Unregistered Securities
None
|
|
Purchases
of Equity Securities
|
On
October 3, 2008, our Board of Directors authorized the repurchase in the open
market from time to time of up to $650,000 of our common
stock. Repurchases totaling 12,810 and 23,389 shares were made in
2009 and 2008, respectively, for a total cost of $33,188 and
$53,975. No repurchases were made during the fourth quarter of
2009.
The Company’s purchases are subject to
trading restrictions including average volume of shares traded over the previous
four weeks, which greatly reduced our ability to repurchase shares.
Not Applicable
The
following discussions of the Company’s results of operations and financial
condition should be read in conjunction with the consolidated financial
statements and notes pertaining to them that appear elsewhere in this Form
10-K.
Any
forward-looking statements made herein are based on current expectations of the
Company that involve a number of risks and uncertainties and should not be
considered as guarantees of future performance. These statements are
made under the Safe Harbor Provisions of the Private Securities Litigation
Reform Act of 1995. Forward looking statements may be identified by
the use of words such as “expect,” “anticipate,” “believe,” “intend,” “plans,”
“predict,” or “will”.
Although
the Company believes that expectations are based on reasonable assumptions,
management can give no assurance that the expectations will
materialize. Many factors could cause actual results to differ
materially from our forward looking statements. Several of these
factors include, in addition to those contained in “Factors that may affect
future operating results,” without limitation:
|
·
|
the
ability to maintain our current pricing model and/or decrease the cost of
sales;
|
|
·
|
a
stable interest rate market and/or a stable currency rate environment in
the world, and specifically the countries where the Company is doing
business in or plans to do business
in;
|
|
·
|
continued
availability of supplies or materials used in manufacturing at competitive
prices;
|
|
·
|
volatility
in commodity and energy prices and the Company’s ability to offset higher
costs with price increases;
|
|
·
|
adverse
regulatory developments in the United States or any other country the
Company plans to do business in;
|
|
·
|
entrance
of competitive products in the Company’s
markets;
|
|
·
|
the
ability of management to execute plans and motivate personnel in the
execution of those plans;
|
|
·
|
no
adverse publicity related to the Company and or its
products;
|
|
·
|
no
adverse claims relating to the Company’s intellectual
property;
|
|
·
|
the
adoption of new, or changes in, accounting
principles;
|
|
·
|
the
passage of new, or changes in, regulations; legal
proceedings;
|
|
·
|
the
ability to maintain compliance with the NYSE AMEX requirements for
continued listing of the common
stock;
|
|
·
|
the
costs inherent with complying with statutes and regulations applicable to
public reporting companies, such as the Sarbanes-Oxley Act
of 2002;
|
|
·
|
the
ability to efficiently integrate future acquisitions and other new lines
of business that the Company may enter in the future, if any;
and
|
|
·
|
other
risks referenced from time to time elsewhere in this report and in the
Company’s filings with the SEC.
|
The
Company is under no obligation and does not intend to update, revise or
otherwise publicly release any revisions to these forward-looking statements to
reflect events or circumstances after the date hereof or to reflect the
occurrence of any unanticipated events.
12
Results
of Operations
The
Company’s primary source of revenue relates to the manufacturing of components,
devices and equipment primarily for the medical and defense
industries. The single largest category of revenue relates to
Micron’s production and sale of silver/silver chloride coated and conductive
resin sensors used as component parts in the manufacture of integrated
disposable electrophysiological sensors. These disposable medical
devices are used worldwide in the monitoring of electrical signals in various
medical applications. In an effort to leverage current skills, the
Company has expanded into custom thermoplastic injection molded products and
product life cycle management. This sector includes revenues from
both high volume precision injection molding and custom injection
molding. With the addition of a medical machining cell, the Company
began production of patient specific metal medical
devices. Management continues to identify complementary and/or
synergistic products, technologies and lines of business in an effort to broaden
the Company’s offerings.
The
following table sets forth for the periods indicated, the percentages of the net
sales represented by certain items reflected in the Company's statements of
operations.
|
Years
ended December 31,
|
||||||||
|
2009
|
2008
|
|||||||
|
Net
sales
|
100.0 % | 100.0 % | ||||||
|
Cost of sales
|
83.1 | 81.0 | ||||||
|
Gross
profit
|
16.9 | 19.0 | ||||||
|
Selling and marketing
|
3.2 | 3.5 | ||||||
|
General and
administrative
|
10.0 | 11.3 | ||||||
|
Research and development
|
1.2 | 1.4 | ||||||
|
Income
before income tax provision
|
2.5 | 2.8 | ||||||
|
Income tax provision
|
0.7 | 1.1 | ||||||
|
Net
income
|
1.8 % | 1.7 % | ||||||
Net
Sales
Net sales
for 2009 were $21,139,774, a decrease of $1,342,445 or 6%, when compared to the
total net sales of $22,482,219 in 2008. The disposable electrode sensor business
continues to experience extreme price pressure in an increasingly competitive
market. The revenue associated with the sensor business, including silver
surcharge, decreased by $561,107 as a result of price erosion in Canada and
Europe. The complementary metal snap business increased by
$102,368. The MIT division’s products experienced a net decrease in
revenue of $871,434, this includes the loss of $3,718,000 of sales
related to imported forgings that were phased out in the first three months of
2009. Due to a change in customer demands, the precision molded
products from Micron decreased approximately $153,762 when compared to
2008. The remaining increase of $141,490 was related to the snap
assembly machine business and other miscellaneous revenues, including $54,484 in
non-recurring silver reclaim. There were no sales of SAECG software
in either 2009 or 2008.
Cost
of Sales
Cost of
sales was $17,558,140 (83.1% of net sales) in 2009 compared to $18,204,526 (81%
of net sales) in 2008 a decrease of $646,386 or 3.6%. A significant
portion of the increase in the percentage of cost of sales in relation to net
sales can be attributed to material costs. Gross margin is negatively
affected by the increase in material costs as not all increases can be passed
along to the customer in the form of price increases or
surcharges. Silver pricing has generally been passed on to our
customers in the form of a surcharge, but this does not preclude the Company
from being pressured to reduce its margins as the price continues to
climb. Silver surcharge collected from our customers is approximately
13% of total net sales for years ended December 31, 2009 and 2008,
respectively. The stabilization of manufacturing costs continues to
be a major focus of management efforts. All current products,
services and programs, including those in development, continue to be evaluated
for contribution and value to our overall business strategy and
results. Products, services and programs that are underperforming
from an overall contribution standpoint and not expected to improve will be
phased out or discontinued so that the Company’s resources can be put to use in
developing those of more strategic value.
13
Selling
and Marketing
Selling
and marketing expenses decreased to $682,568 (3.2% of net sales) in 2009 from
$781,456 (3.5% of net sales) in 2008, a decrease of $98,888, or
12.7%. This decrease in selling and marketing expense is mainly
attributable to the reduction in sales and business development personnel being
offset by increased travel and trade show costs incurred. Management
believes this decrease to be nominal and expects the expense as a percentage of
sales to remain stable in the foreseeable future.
General
and Administrative Expenses
General
and administrative expenses were $2,102,461 (10.0% of net sales) in 2009 as
compared to $2,536,648 (11.3% of net sales) in 2008, a decrease of $434,187 or
17.1%. Included in the expense for the year ended December 31, 2008
is a onetime charge of $250,000 for costs associated with a terminated
acquisition following due diligence. Although the delay by the SEC
for outside auditor attestation requirements of SOX Section 404 limited a
previously expected increase in audit fees for 2009, the costs associated with
the related internal control documentation with outside consultants was $46,646
and $88,258 for the twelve months in 2009 and 2008, respectively.
Research
and Development
Research
and development costs decreased to $241,494 (1.2% of net sales) in 2009 from
$320,040 (1.4% of net sales) in 2008, a decrease of $78,546, or
25%. For the fiscal years ended December 31, 2009, and
2008, ART had research and development expenses of approximately $21,527 and
$69,779, respectively. Expenses include the technical support of a
NIH research project utilizing ART’s proprietary Signal Averaged ECG
product. In 2009 and 2008, Micron’s research and development efforts
resulted in $219,967 and $250,261 of expense. The expense is for
process improvements on the Micron sensor and snap product lines and new
processes and capabilities within MIT. The twelve months ended
December 31, 2008 included $52,000 of expense for equipment tested in a process
improvement project with the sensor product line as well as the impairment of
equipment used for final product testing.
Interest
Expense
Interest
expense was $31,699 in 2009 compared to $46,230 in 2008, a decrease of $14,531,
or 31%. This expense is a result of the acquisition note and an
equipment loan, which were paid in full in 2008 and 2009,
respectively. The Company does not incur an unused borrowing base fee
under our unsecured credit facility.
Other
Income
Other
income was $2,757 in 2009 compared to $29,464 in 2008, a decrease of $26,707, or
91%. The majority of other income was bank interest of $12,082 and $29,861, in
2009 and 2008, respectively. Lower average balances and a decrease in
the rate of return account for the decrease in interest income. The
remainder of other income was from miscellaneous expense items including a loss
in the disposal of assets, and currency losses relating to a foreign
government’s import taxes and the timing of the reimbursement.
Income
Taxes
The
Company’s combined federal and state effective income tax rate was 29% and 42%
in 2009 and 2008, respectively. The effective rates in 2009 were
lower than the statutory rates primarily due to the reductions in tax from state
and federal research and development and investment tax credits.
Goodwill
As of
December 31, 2009, the Company’s goodwill of $1,564,966 is related to three
reporting units, $1,244,000 associated with the acquisition of Micron Products,
Inc. in 1992, $235,727 associated with the acquisition of Shrewsbury Molders,
Inc. in 2004, and $85,239 associated with the acquisition of Leominster Tool Co.
Inc. in December 2006. There was no impairment to the goodwill
associated with or expected in any acquisition based on the first quarter annual
impairment test in 2009.
Earnings
Per Share
The basic
earnings per share is $0.14 in 2009 as compared to $0.13 in 2008, an increase of
$0.01, or 8%. The earnings per share for 2008 included non-recurring
charges totaling $302,000, related to an acquisition and research and
development activities. This charge, net of tax, decreased basic
earnings per share for 2008 by $0.07.
14
Off-Balance
Sheet Arrangements
The
Company entered in to a sale lease-back transaction for certain equipment
purchased during 2009 totaling $677,810. A five year operating lease
obligation for the equipment began December 31, 2009 with the first payment due
February 1, 2010. The transaction includes an additional $322,190 of
lease line capacity. The operating lease requires payments totaling
$146,867 in 2010, and $139,690 for each year following until 2014.
Liquidity
and Capital Resources
Working
capital was $8,922,328 as of December 31, 2009 as compared to
$7,440,721 as the same date in 2008. Operating results produced
positive cash flows of $2,386,186 of which $361,195 was spent on capital asset
investment. Cash and cash equivalents were $3,674,179 and $2,320,467
at December 31, 2009, and 2008, respectively. Substantially all of
these funds are invested in fixed rate bank deposit accounts.
Inventories
decreased to $2,956,682 at the end of 2009, a decrease of $770,810 from the end
of 2008. The decreased inventory was primarily the result of the lean
manufacturing programs. These efforts focused production on reducing
inventory in production. The increased unit cost of silver offset the
decreases in volume of sensors in inventory.
Net
capital equipment expenditures were $361,195 in 2009 as compared to $1,015,702
in 2008. In 2009, the majority of the expenditures were for
production equipment. Not included in the net capital expenditures
was production automation equipment for the sensor line costing
$677,810. This equipment was put into service under an operating
lease. In 2008, the majority of the expenditures were for the
acquisition of additional production machinery and equipment, including upgrades
in and replacement of existing machinery and tooling. A climate
controlled mold manufacturing space built for LTD and the addition of an
ultrasonic cleaning production line cost $306,000 and $55,000,
respectively. The majority of remaining capital expenditures related
to manufacturing equipment replacements and additions including computer
controlled inspection equipment.
An
unsecured $1,000,000 credit facility was available in 2009 and
2008. The agreement provides for borrowings up to 80% of eligible
accounts receivable plus 50% of raw material and finished goods
inventories. This facility has no borrowing base
charge. There were no outstanding borrowings on our line of credit as
of December 31, 2009 and 2008. The agreement contains covenants that
apply upon drawing on the line. The covenants relate to various matters
including notice prior to executing further borrowings and security interests,
merger or consolidation, acquisitions, guarantees, sales of assets other than in
the normal course of business, leasing, changes in ownership and payment of
dividends. As of January 15, 2010, the credit facility was increased
to $2,000,000.
The
Company had a one year term note secured by equipment with a limit of
$813,000. The loan was drawn down by $383,000 for equipment delivered
and installed in October 2007. A second payment of $383,000 was made
in January of 2008 for this equipment. In the third quarter of 2008
the equipment note was extended for one year with a decrease in the fixed rate
from 6.75% to 6.5% per annum. The equipment note was paid in full on
September 15, 2009.
On
December 31, 2009, the Company received a reimbursement of $677,810 for a sale
lease-back transaction related to new production equipment installed during the
second half of 2009. This arrangement included a lease line with a
credit limit of $1,000,000, and the Company expects to use the remaining
$322,190 for the purchase of certain production equipment in the beginning of
2010.
Funding
for future research and development is expected to be provided by ongoing
operations, and at this time there are no plans for projects that would require
outside funding.
In
October 2008, the Company’s Board of Directors authorized the repurchase in the
open market from time to time of up to $650,000 of the Company’s outstanding
stock. An aggregate of 23,389 shares were purchased in the fourth
quarter of 2008 under the program for an aggregate of $53,975. In
2009, the Company repurchased 12,810 shares for an aggregate of
$33,188.
Inflation
The
Company believes that inflation in the United States or international markets
has not had a significant effect on its results of operations except for the
impact of the increase in volatility of materials and energy prices particularly
the cost of silver.
15
Recent
Accounting Pronouncements
In June
2009, the FASB issued ASC 105-10 (formerly SFAS No. 168), “Accounting Standards
Codification™ and the Hierarchy of Generally Accepted Accounting Principles
(GAAP).” The Financial Accounting Standards Board (FASB) Accounting Standards
Codification (“Codification”) has become the source of authoritative accounting
principles recognized by the FASB to be applied by nongovernmental entities in
the preparation of financial statements in accordance with GAAP. All existing
accounting standard documents are superseded by the Codification and any
accounting literature not included in the Codification will not be
authoritative. However, rules and interpretive releases of the SEC
issued under the authority of federal securities laws will continue to be the
source of authoritative generally accepted accounting principles for SEC
registrants. Effective September 30, 2009, all references made to
GAAP in our consolidated financial statements will include the new Codification
numbering system along with original references. The Codification
does not change or alter existing GAAP and, therefore, will not have an impact
on our financial position, results of operations or cash flows.
In
December 2007, the FASB issued ASC 810 (formerly “SFAS No. 160”),
“Non-controlling Interests in Consolidated Financial Statements - an amendment
of ARB No. 51.” ASC 810 establishes accounting and reporting
standards for the noncontrolling interest in a subsidiary for the
deconsolidation of a subsidiary. ASC 810 is effective for financial
statements issued for fiscal years beginning after December 15, 2008, and
interim statements within those fiscal years. The Company does not
currently have any noncontrolling interests.
In
December 2007, the FASB issued ASC 805-10 (formerly “SFAS No. 141(R)”),
“Business Combinations”, which retains the purchase method of accounting for
acquisitions, but requires a number of changes, including changes in the way
assets and liabilities are recognized in the purchase method of accounting. It
changes the recognition of assets acquired and liabilities assumed arising from
contingencies, requires the capitalization of in-process research and
development at fair value, and requires the expensing of acquisition-related
costs as incurred. The provisions of this standard will apply prospectively to
business combinations occurring in our fiscal year beginning January 1, 2009 and
the adoption did not have an impact on our financial position or results of
operations; however, it could impact future transactions entered into by the
Company.
In March
2008, the FASB issued ASC 815 (formerly “SFAS No. 161”), “Disclosures about
Derivative Instruments and Hedging Activities—an amendment of FASB Statement No.
133.” ASC 815 amends and expands the disclosure requirements to
provide improved transparency into the uses and financial statement impact of
derivative instruments and hedging activities. The adoption of ASC
815 did not have a material impact on our Financial Statements.
In April
2008, the FASB issued ASC 350-30, (formerly “FSP FAS 142-3”), “General
Intangibles Other Than Goodwill,” which details the factors that should be
considered in developing the useful lives for intangible assets with renewal or
extension provisions. ASC 350-30 requires an entity to consider its
own historical experience in renewing or extending similar arrangements,
regardless of whether those arrangements have explicit renewal or extension
provisions, when determining the useful life of an intangible
asset. In the absence of such experience, an entity shall consider
the assumptions that market participants would use about renewal or extension,
adjusted for entity-specific factors. ASC 350-30 also requires an
entity to disclose information regarding the extent to which the expected future
cash flows associated with an intangible asset are affected by the entity’s
intent and/or ability to renew or extend the arrangement. ASC 350-30
will be effective for qualifying intangible assets acquired by the Company on or
after July 1, 2009. The application of ASC 350-30 did not have a
material impact on the Company’s results of operations, cash flows or financial
positions; however, it could impact future transactions entered into by the
Company.
In May
2009, the FASB issued ASC 855-10 (formerly “SFAS No. 165”), “Subsequent Events,”
which establishes general standards of accounting for and disclosure of events
that occur after the balance sheet date but before financial statements are
issued or are available to be issued. We adopted this standard upon
issuance with no impact on our financial position or results of
operations.
In
October 2009, the FASB issued Accounting Standards Update (“ASU”) No. 2009-13,
“Multiple-Deliverable Revenue Arrangements” (“ASU 2009-13”). ASU
2009-13 establishes the accounting and reporting guidance for arrangements
including multiple revenue-generating activities, and provides amendments to the
criteria for separating deliverables, measuring and allocating arrangement
consideration to one or more units of accounting. The amendments in
ASU 2009-13 also establish a selling price hierarchy for determining the selling
price of a deliverable. Significantly enhanced disclosures are also
required to provide information about a vendor’s multiple-deliverable revenue
arrangements, including information about the nature and terms, significant
deliverables, and its performance within arrangements. The amendments
also require providing information about the significant judgments made and
changes to those judgments and about how the application of the relative
selling-price method affects the timing or amount of revenue
recognition. The amendments in ASU 2009-13 are effective
prospectively for revenue arrangements entered into or materially modified in
the fiscal years beginning on or after June 15, 2010. Early
application is permitted. The Company is currently evaluating the
potential impact, if any, the adoption of ASU 2009-13 will have on its financial
position or results of operations.
16
Critical
Accounting Policies
The
preparation of financial statements and related disclosures in conformity with
generally accepted accounting principles requires management to make judgments,
assumptions and estimates that affect the amounts reported. Note 2 of
Notes to Consolidated Financial Statements describe the significant accounting
policies used in the preparation of the consolidated financial
statements. Some of these significant accounting policies are
considered to be critical accounting policies, as defined below.
A
critical accounting policy is defined as one that is both material to the
presentation of the Company’s financial statements and requires management to
make difficult, subjective or complex judgments that could have a material
effect on the Company’s financial condition and results of
operations. Specifically, critical accounting estimates have the
following attributes: 1) the Company is required to make assumptions about
matters that are highly uncertain at the time of the estimate; and 2) different
estimates the Company could reasonably have used, or changes in the estimate
that are reasonably likely to occur, would have a material effect on the
Company’s financial condition or results of operations.
Estimates
and assumptions about future events and their effects cannot be determined with
certainty. The Company bases its estimates on historical experience
and on various other assumptions believed to be applicable and reasonable under
the circumstances. These estimates may change as new events occur, as
additional information is obtained and as the Company’s operating environment
changes. These changes have historically been minor and have been
included in the consolidated financial statements as soon as they became
known. In addition, management is periodically faced with
uncertainties, the outcomes of which are not within its control and will not be
known for prolonged periods of time. These uncertainties are
discussed in the section above entitled “Factors that may affect future
operating results.” Based on a critical assessment of its
accounting policies and the underlying judgments and uncertainties affecting the
application of those policies, management believes that the Company‘s
consolidated financial statements are fairly stated in accordance with generally
accepted accounting principles, and present a meaningful presentation of the
Company’s financial condition and results of operations.
Management
believes that the following are critical accounting policies:
Revenue
Recognition and Accounts Receivable
The
Company recognizes revenue upon product shipment, provided that there exists
persuasive evidence of an arrangement, the fee is fixed or determinable, and
collectability of the related receivable is reasonably assured.
The
financing of customer purchased tooling utilizes the direct financing method of
revenue recognition. This requires the gain or loss on the sale of
the tooling to be recorded at the time the tool is put into service while the
customer’s stream of payments is reflected as a lease receivable.
Based on
management’s on-going analysis of accounts receivable balances, and after the
initial recognition of the revenue, as to any event that adversely affects the
ultimate ability to collect the related receivable, management will record an
allowance for bad debts. Bad debts have not had a significant impact
on the Company’s financial position, results of operations and cash
flows.
Inventory
and Inventory Reserves
The
Company values its inventory at the lower of average cost or
market. The Company reviews its inventory for quantities in excess of
production requirements, obsolescence and for compliance with internal quality
specifications. Any adjustments to inventory would be equal to the
difference between the cost of inventory and the estimated net market value
based upon assumptions about future demand, market conditions and expected cost
to distribute those products to market.
The
Company maintains some reserve for excess, slow moving, and obsolete
inventory. A review of inventory on hand is made at least annually
and some obsolete inventory is scrapped and/or recycled. The review
is based on several factors including a current assessment of future product
demand, historical experience, and product expiration.
17
Deferred
Tax Assets
The
Company assesses its deferred tax assets based upon a more likely than not to be
realized criteria. The Company considers future taxable income and
ongoing prudent and feasible tax planning strategies in assessing the need for
the valuation allowance. In accordance with ASC 740 we recognize the
benefits of a tax position if that position is more likely than not to be
sustained on audit, based on the technical merit of the position.
Asset
Impairment – Goodwill
The
Company reviews the valuation of goodwill and intangible assets to assess
potential impairments. Management reassesses the useful lives of
other intangible assets with identifiable useful lives in accordance with the
guidelines set forth in ASC 350, “Intangible
Assets”. The value assigned to intangible assets is determined
by a valuation based on estimates and judgment regarding expectations for the
success and life cycle of products previously acquired or others likely to be
acquired in the future. If the actual sale of product and market
acceptance differs significantly from the estimates, management may be required
to record an impairment charge to write down the asset to its realizable
value. To test for impairment, a present value of an estimate of
future cash flows related to goodwill or intangible assets with indefinite lives
are calculated and compared to the value of the intangible asset during the
first quarter annually. When impairment exists it could have a
material adverse effect on the Company’s business, financial condition and
results of operations.
Asset
Impairment – Long Lived Assets
The
Company assesses the impairment of long-lived assets and intangible assets with
finite lives whenever events or changes in circumstances indicate that the
carrying value may not be fully recoverable. When the Company’s
management determines that the carrying value of such assets may not be
recoverable, management generally measures any impairment on a projected
discounted cash flow method using a discount rate determined by management to be
commensurate with the risk inherent in its current business model.
Not Applicable
The
information required by this item may be found on pages F-1 through F-19 of this
Annual Report on Form 10-K.
Not Applicable
Evaluation
of Disclosure Controls and Procedures
As of the
end of the period covered by this annual report the Company’s management, with
the participation of the Company’s Chief Executive Officer and Chief Financial
Officer (“the Certifying Officer”), conducted evaluations of the Company’s
disclosure controls and procedures. As defined under Sections 13a –
15(e) and 15d – 15(e) of the Securities Exchange Act of 1934, as amended (the
“Exchange Act”), the term “disclosure controls and procedures” means controls
and other procedures of an issuer that are designed to ensure that information
required to be disclosed by the issuer in the reports that it files or submits
under the Exchange Act is recorded, processed, summarized and reported, within
the time periods specified in the Commission’s rules and
forms. Disclosure controls and procedures include without limitation,
controls and procedures designed to ensure that information required to be
disclosed by an issuer in the reports that it files or submits under the
Exchange Act is accumulated and communicated to the issuer’s management,
including the Certifying Officer, to allow timely decisions regarding required
disclosure. Based on this evaluation, the Certifying Officer has
concluded that the Company’s disclosure controls and procedures were effective
to ensure that material information is recorded, processed, summarized and
reported by management of the Company on a timely basis in order to comply with
the Company’s disclosure obligations under the Exchange Act and the rules and
regulations promulgated thereunder.
18
Changes
in Internal Control Over Financial Reporting
Further,
there were no changes in the Company’s internal control over financial reporting
during the Company’s last fiscal quarter that materially affected, or are
reasonably likely to materially affect, the Company’s internal control over
financial reporting.
Management’s
Report on Internal Control Over Financial Reporting
Our Chief
Executive Officer (“CEO”) and Chief Financial Officer (“CFO”) are responsible
for establishing and maintaining adequate internal control over financial
reporting, as such term is defined in Rule 13a-15(f) of the Securities Exchange
Act of 1934, as amended (the “Exchange Act”).
Internal
control over financial reporting is defined in Rule 13a-15(f) promulgated under
the Exchange Act as a process designed by, or under the supervision of, our CEO
and CFO and effected by our board of directors, management and other personnel,
to provide reasonable assurance regarding the reliability of financial reporting
and the preparation of financial statements for external purposes in accordance
with generally accepted accounting principles and includes those policies and
procedures that:
|
·
|
pertain
to the maintenance of records that in reasonable detail accurately and
fairly reflect the transactions and dispositions of our
assets;
|
|
·
|
provide
reasonable assurance that transactions are recorded as necessary to permit
preparation of financial statements in accordance with generally accepted
accounting principles, and that our receipts and expenditures are being
made only in accordance with authorizations of our management and
directors; and
|
|
·
|
provide
reasonable assurance regarding prevention or timely detection of
unauthorized acquisition or disposition of our assets that could have a
material effect on the financial
statements.
|
Readers
are cautioned that internal control over financial reporting, no matter how well
designed, has inherent limitations and may not prevent or detect misstatements.
Therefore, even effective internal control over financial reporting can only
provide reasonable assurance with respect to the financial statement preparation
and presentation.
Our
management, under the supervision and with the participation of our CEO and CFO,
has evaluated the effectiveness of our disclosure controls and procedures as
defined in Exchange Act Rules 13a-15(e) and 15d-15(e) as of the end of the
period covered by this Report based upon the framework in Internal Control
Integrated Framework issued by the Committee of Sponsoring Organizations of the
Treadway Commission (“COSO”). Based on such evaluation, our management has made
an assessment that our internal control over financial reporting is effective as
of December 31, 2009.
This
annual report does not include an attestation report of the Company’s
independent registered public accounting firm regarding internal control over
financial reporting. Management’s report was not subject to
attestation by the Company’s independent registered public accounting firm due
to a transition period established by the rules of the Securities and Exchange
Commission that permit the Company to provide only management’s report in this
annual report.
None.
19
The
information with respect to directors and executive officers required under this
item is incorporated by reference to the applicable information set forth in our
Proxy Statement for our 2010 Annual Meeting of Shareholders.
The
information required under this item is incorporated by reference to the
applicable information in our Proxy Statement for our 2010 Annual Meeting of
Shareholders.
The
information required under this item is incorporated by reference to the
applicable information in our Proxy Statement for our 2010 Annual Meeting of
Shareholders.
The
information required under this item is incorporated by reference to the
applicable information in our Proxy Statement for our 2010 Annual Meeting of
Shareholders.
The
information required under this item is incorporated by reference to the
applicable information in our Proxy Statement for our 2010 Annual Meeting of
Shareholders.
The
Company hereby furnishes the exhibits listed on the attached exhibit
index. Exhibits, which are incorporated herein by reference, may be
inspected and copied at the public reference facilities maintained by the SEC at
Room 1580, Washington, D.C. 20549. Copies of such material may be
obtained by mail from the Public Reference Section of the SEC at 100 F Street,
N.E., Washington, D.C. 20549, at prescribed rates. The SEC also
maintains a website that contains reports, proxy and information statements and
other information regarding registrants that file electronically with the SEC at
the address “http://www.sec.gov”. The Company maintains a web site
that contains reports, proxy and information statements and other information
electronically at the address “http://www.arthrt.com”. Information
on our website is not a part of this Annual Report on Form 10-K.
20
Pursuant
to the requirements of Section 13 or 15(d) of the Securities Exchange Act of
1934, the registrant caused this report to be signed on its behalf by the
undersigned, thereunto duly authorized.
ARRHYTHMIA
RESEARCH TECHNOLOGY, INC.
By: /s/
James E Rouse
James E.
Rouse,
President and Chief Executive
Officer
March 10, 2010
Pursuant
to the requirements of the Securities Exchange Act of 1934, this report has been
signed below by the following persons on behalf of the registrant and in the
capacities and on the dates indicated.
|
Signature
|
Capacity
|
Date
|
||
|
/s/
James E. Rouse
|
President,
Chief Executive Officer and
|
March
10, 2010
|
||
|
James
E. Rouse
/s/
David A. Garrison
|
Director
(Principal Executive Officer)
Executive
Vice President of Finance
and
Chief Financial Officer
|
March
10, 2010
|
||
|
David
A. Garrison
/s/
E. P. Marinos
|
(Principal
Financial and Accounting Officer)
Chairman
of the Board
|
March
10, 2010
|
||
|
E.
P. Marinos
/s/
Julius Tabin
|
Director
|
March
10, 2010
|
||
|
Julius
Tabin
/s/
Paul F. Walter
|
Director
|
March
10, 2010
|
||
|
Paul
F. Walter
/s/
Jason R. Chambers
|
Director
|
March
10, 2010
|
||
|
Jason
R. Chambers
|
||||
21
Arrhythmia
Research Technology, Inc.
And
Subsidiary
Contents
Report of Independent Registered
Public Accounting
Firm F-2
Consolidated
Financial Statements:
Balance
sheets F-3, F-4
Statements
of
income F-5
Statements
of changes in shareholders’
equity F-6
Statements
of cash
flows
F-7
Notes to
consolidated financial
statements
F-8 to F-19
F-1
Report of Independent
Registered Public Accounting Firm
To the
Board of Directors and the Shareholders of
Arrhythmia
Research Technology, Inc.
Fitchburg,
Massachusetts
We have
audited the accompanying consolidated balance sheets of Arrhythmia Research
Technology, Inc. and Subsidiary as of December 31, 2009 and 2008, and the
related consolidated statements of income, changes in shareholders’ equity and
cash flows for the years then ended. These financial statements are
the responsibility of the Company’s management. Our responsibility is to express
an opinion on these financial statements based on our audits.
We
conducted our audits in accordance with standards of the Public Company
Accounting Oversight Board (United States). Those standards require
that we plan and perform the audit to obtain reasonable assurance about whether
the financial statements are free of material misstatement. The
Company is not required to have, nor were we engaged to perform, an audit of its
internal control over financial reporting. Our audits included
consideration of internal control over financial reporting as a basis for
designing audit procedures that are appropriate in the circumstances, but not
for the purpose of expressing an opinion on the effectiveness of the Company's
internal control over financial reporting. Accordingly, we express no
such opinion. An audit also includes examining, on a test basis,
evidence supporting the amounts and disclosures in the financial statements,
assessing the accounting principles used and significant estimates made by
management, as well as evaluating the overall financial statement
presentation. We believe that our audits provide a reasonable basis
for our opinion.
In our
opinion, the consolidated financial statements referred to above present fairly,
in all material respects, the financial position of Arrhythmia Research
Technology, Inc. and Subsidiary as of December 31, 2009 and 2008, and the
results of their operations and their cash flows for the years then ended, in
conformity with accounting principles generally accepted in the United States of
America.
/s/ CCR
LLP
Westborough,
Massachusetts
March 10,
2010
F-2
Arrhythmia
Research Technology, Inc.
and
Subsidiary
Consolidated
Balance Sheets
|
December
31,
|
2009
|
2008
|
||||||
|
Assets
|
||||||||
|
Current
assets:
Cash and cash
equivalents
|
$ | 3,674,179 | $ | 2,320,467 | ||||
|
Trade accounts receivable, net of
allowance
for doubtful accounts
of $49,976 and $45,619
|
3,818,538 | 2,705,145 | ||||||
|
Inventories (Note
3)
|
2,956,682 | 3,727,492 | ||||||
|
Deferred income taxes (Note
6)
|
22,500 | 21,000 | ||||||
|
Prepaid tax
|
123,789 | 309,000 | ||||||
|
Deposits, prepaid expenses and
other current assets
|
147,243 | 392,209 | ||||||
|
Total current
assets
|
10,742,931 | 9,475,313 | ||||||
|
Property, plant and equipment,
net (Note 4)
|
6,343,575 | 7,305,278 | ||||||
|
Goodwill (Note
2)
|
1,564,966 | 1,564,966 | ||||||
|
Other
intangible assets, net
|
95,887 | 143,010 | ||||||
|
Total assets
|
$ | 18,747,359 | $ | 18,488,567 | ||||
See
accompanying notes to consolidated financial statements.
F-3
Arrhythmia
Research Technology, Inc.
and
Subsidiary
Consolidated
Balance Sheets
(Continued)
|
December
31,
|
2009
|
2008
|
||||||
|
Liabilities
and Shareholders’ Equity
|
||||||||
|
Current
liabilities:
Accounts payable
|
$ | 1,543,700 | $ | 1,106,974 | ||||
|
Accrued expenses
|
276,903 | 289,527 | ||||||
|
Current note payable (Note
5)
|
- | 638,091 | ||||||
|
Total current
liabilities
|
1,820,603 | 2,034,592 | ||||||
|
Long
term liabilities:
|
||||||||
|
Long term deferred tax liability
(Note 6)
|
350,000 | 315,500 | ||||||
|
Long term portion of deferred gain
on Lease (Note 8)
|
22,347 | - | ||||||
|
Total long term
liabilities
|
372,347 | 315,500 | ||||||
|
Total liabilities
|
2,192,950 | 2,350,092 | ||||||
|
Commitments and
contingencies (Note 8):
|
||||||||
|
Shareholders’ equity
(Notes 7 and 10):
|
||||||||
|
Common stock, $.01 par value;
10,000,000 shares authorized;
3,926,491 issued, 2,675,481 and
2,688,291 outstanding respectively
|
39,265 | 39,265 | ||||||
|
Additional
paid-in-capital
|
10,317,403 | 10,243,568 | ||||||
|
Treasury stock at cost, 1,251,010
and 1,238,200 shares respectively
|
(3,413,742 | ) | (3,380,554 | ) | ||||
|
Retained earnings
|
9,611,483 | 9,236,196 | ||||||
|
Total shareholders’
equity
|
16,554,409 | 16,138,475 | ||||||
|
Total liabilities and
shareholders’ equity
|
$ | 18,747,359 | $ | 18,488,567 | ||||
See
accompanying notes to consolidated financial statements.
F-4
Arrhythmia
Research Technology, Inc.
and
Subsidiary
Consolidated
Statements of Income
|
Years
ended December 31,
|
2009
|
2008
|
||||||
|
Net
sales
|
$ | 21,139,774 | $ | 22,482,219 | ||||
|
Cost
of sales
|
17,558,140 | 18,204,526 | ||||||
|
Gross profit
|
3,581,634 | 4,277,693 | ||||||
|
Selling
and marketing
|
682,568 | 781,456 | ||||||
|
General
and administrative
|
2,102,461 | 2,536,648 | ||||||
|
Research
and development
|
241,494 | 320,040 | ||||||
|
Income from
operations
|
555,111 | 639,549 | ||||||
|
Other
income (expense):
|
||||||||
|
Interest expense
|
(31,699 | ) | (46,230 | ) | ||||
|
Other income
|
2,757 | 29,464 | ||||||
|
Total other
expense
|
(28,942 | ) | (16,766 | ) | ||||
|
Income
before income taxes
|
526,169 | 622,783 | ||||||
|
Income tax provision
(Note 6)
|
152,000 | 261,400 | ||||||
|
Net
income
|
$ | 374,169 | $ | 361,383 | ||||
|
Earnings per share (Note
2):
|
||||||||
|
Basic
|
$ | 0.14 | $ | 0.13 | ||||
|
Diluted
|
$ | 0.14 | $ | 0.13 | ||||
See
accompanying notes to consolidated financial statements.
F-5
Arrhythmia
Research Technology, Inc.
and
Subsidiary
Consolidated
Statements of Changes in Shareholder’s Equity
(Notes 2,7
and 10)
|
Common
Stock
|
Additional
|
Treasury
|
Retained
|
||||||||
|
Shares
|
Amount
|
Paid-in
Capital
|
Stock
|
Earnings
|
Total
|
||||||
|
December
31, 2007
|
3,926,491
|
$
|
39,265
|
$
|
10,143,339
|
$
|
(3,326,579)
|
$
|
8,874,813
|
$
|
15,730,838
|
|
Share based
compensation
|
100,229
|
100,229
|
|||||||||
|
Treasury stock repurchased 23,389
shares
|
(53,975)
|
(53,975)
|
|||||||||
|
Net income
|
361,383
|
361,383
|
|||||||||
|
December
31, 2008
|
3,926,491
|
$
|
39,265
|
$
|
10,243,568
|
$
|
(3,380,554)
|
$
|
9,236,196
|
$
|
16,138,475
|
|
Share based
compensation
|
73,835
|
73,835
|
|||||||||
|
Treasury stock repurchased
12,810 shares
|
(33,188)
|
(33,188)
|
|||||||||
|
Cash dividends
(adjustment)
|
1,118
|
1,118
|
|||||||||
|
Net income
|
374,169
|
374,169
|
|||||||||
|
December
31, 2009
|
3,926,491
|
$
|
39,265
|
$
|
10,317,403
|
$
|
(3,413,742)
|
$
|
9,611,483
|
$
|
16,554,409
|
See
accompanying notes to consolidated financial statements.
F-6
Arrhythmia
Research Technology, Inc.
and
Subsidiary
Consolidated
Statements of Cash Flows
(Note
9)
|
Years
ended December 31,
|
2009
|
2008
|
|||
|
Cash
flows from operating activities:
|
|||||
|
Net income
|
$
|
374,169
|
$
|
361,383
|
|
|
Adjustments to reconcile net
income to net cash provided
by operating
activities:
|
|||||
|
Depreciation and
amortization
|
1,394,604
|
1,399,154
|
|||
|
Provision for doubtful
accounts
|
4,357
|
(4,211)
|
|||
|
Deferred income tax
provision
|
33,000
|
184,000
|
|||
|
Share based
compensation
|
73,835
|
100,229
|
|||
|
Changes in operating assets and
liabilities:
|
|||||
|
Trade accounts
receivable
|
(1,117,750)
|
58,557
|
|||
|
Inventories
|
770,810
|
(725,972)
|
|||
|
Deposits, prepaid expenses and
other assets
|
430,177
|
153,346
|
|||
|
Accounts payable and accrued
expenses
|
422,984
|
410,894
|
|||
|
Net cash provided by operating
activities
|
2,386,186
|
1,937,380
|
|||
|
Cash
flows from investing activities:
|
|||||
|
Capital expenditures, net of
disposals
|
(361,195)
|
(1,015,702)
|
|||
|
Net cash used in investing
activities
|
(361,195)
|
(1,015,702)
|
|||
|
Cash
flows from financing activities:
|
|||||
|
Payments to notes
payable
|
(638,091)
|
(231,647)
|
|||
|
Repurchase of
stock
|
(33,188)
|
(53,975)
|
|||
|
Net cash used in financing
activities
|
(671,279)
|
(285,622)
|
|||
|
Net
increase in cash and cash equivalents
|
1,353,712
|
636,056
|
|||
|
Cash and cash
equivalents, beginning of year
|
2,320,467
|
1,684,411
|
|||
|
Cash and cash
equivalents, end of year
|
$
|
3,674,179
|
$
|
2,320,467
|
|
See
accompanying notes to consolidated financial statements.
F-7
1. Description of
Business
Arrhythmia Research Technology, Inc. (“ART”) is engaged in
the licensing of medical software, which acquires data and analyzes electrical
impulses of the heart to detect and aid in the treatment of potentially lethal
arrhythmias. Micron Products, Inc. (“Micron”), a wholly owned
subsidiary, is the primary source of consolidated revenues. Micron
manufactures disposable electrode sensors used as a component part in the
manufacture of integrated disposable electro-physiological
sensors. These disposable medical devices are used worldwide in the
monitoring of electric signals in various medical
applications. Micron has expanded into custom plastic injection
molded products and product life cycle management. Revenues in this
sector are primarily custom injection molding, and end-to-end product life cycle
management through a comprehensive portfolio of value-added services such as
design, engineering, prototyping, manufacturing, machining, assembly and
packaging.
2. Accounting
Policies
Principles of Consolidation
The consolidated financial statements include the accounts
of ART and Micron (collectively the “Company”). All intercompany
balances and transactions have been eliminated in consolidation.
Revenue Recognition
The Company recognizes revenue upon product shipment,
provided that there exists persuasive evidence of an arrangement, the fee is
fixed or determinable, and collectability of the related receivable is
reasonably assured.
Financing Customer Purchased Tooling
In order to lessen the impact of the initial cost of a
custom mold, Micron provides a tooling financing package for select
customers. The cost of the tool is charged in conjunction with the
product shipments over the first 3 or 4 years of the agreed upon purchasing
program. The customer agrees to pay for the tool in full upon any
delay or termination in the program. The income is recognized
utilizing the direct financing method.
Cash and Cash Equivalents
Cash and cash equivalents consist of cash on hand and on
deposit in high quality financial institutions. The Company considers
all highly liquid debt instruments with original maturities of three months or
less to be cash equivalents.
Inventories
Inventories are stated at the lower of average cost or
market. Cost of inventories is determined by the first-in, first-out
method.
Concentration of Credit Risk
Financial instruments which potentially expose the Company
to concentrations of credit risk, as defined by Accounting Standards
Codification (“ASC”) 310 “Receivables”, (formerly Statement of Financial
Accounting Standard (“SFAS”) No. 105 “Disclosure of Information about
Financial Instruments with Off-Balance-Sheet Risk and Financial Instruments with
Concentrations of Credit Risk”), consist primarily of trade accounts receivable
and cash and cash equivalents.
Accounts receivable are customer obligations due under
normal trade terms. A large portion of Micron’s products are sold to
large diversified medical and defense product manufacturers. The
Company does not generally require collateral for its sales; however, the
Company believes that its terms of sale provide adequate protection against
significant credit risk.
Senior management regularly reviews accounts receivable to
determine if any receivables will potentially be uncollectible. The
Company includes any accounts receivable balances that are determined to be
uncollectible, along with a general reserve, in our overall allowance for
doubtful accounts. After all attempts to collect a receivable have
failed, the receivable is written off against the allowance. Based on
the information available to us, management believes the allowance for doubtful
accounts as of December 31, 2009 is adequate.
F-8
|
|
2.
|
Accounting
Policies (Continued)
|
Concentration of Credit
Risk (Continued)
It is the Company’s policy to place its cash and cash
equivalents in high quality financial institutions. The Company does
not believe significant credit risk exists above federally insured limits with
respect to these institutions.
Property, Plant and Equipment
Property, plant and equipment are recorded at cost and
include expenditures which substantially extend their useful
lives. Depreciation on property, plant and equipment is calculated
using the straight-line method over the estimated useful lives of the
assets. Expenditures for maintenance and repairs are charged to
earnings as incurred. When equipment is retired or sold, the
resulting gain or loss is reflected in earnings.
Goodwill
The Company accounts for goodwill and intangibles in
accordance with ASC 350 “Intangibles – Goodwill and other”, (formerly
SFAS No. 142 “Goodwill and Other Intangible Assets”). ASC
350 requires that companies test goodwill for impairment at least
annually. In addition, ASC 350 requires that the Company identify
reporting units for the purpose of assessing potential future impairments of
goodwill, reassess the useful lives of other existing recognized intangible
assets, and cease amortization of intangible assets with an indefinite useful
life. An intangible asset with an indefinite useful life should be
tested for impairment in accordance with the guidelines in ASC
350. ASC 350 is required to be applied to all goodwill and other
intangible assets regardless of when those assets were initially
recognized.
There was no impairment to goodwill as of first quarter of
2009 and no indicators have arisen to require the Company to review goodwill in
the interim period. The Company performs its annual impairment
testing for the goodwill valuation during the first quarter of the fiscal
year.
Long-Lived Assets
The Company accounts for long lived assets in accordance
with ASC 350 (formerly SFAS No. 144, “Accounting for the Impairment or
Disposal of Long-Lived Assets”). A long lived asset used in research
and development was impaired for $22,378 as of December 31, 2008.
Income Taxes
The Company accounts for income taxes in accordance with
ASC 740 “Income Taxes,” (formerly SFAS No. 109 “Accounting for Income
Taxes”), which requires recognition of deferred tax liabilities and assets for
the expected future tax consequences of events that have been included in the
financial statements or tax returns. Under this method, deferred tax
liabilities and assets are determined based on the difference between the
financial statement and tax bases of assets and liabilities using tax rates in
effect for the year in which the differences are expected to
reverse.
In accordance with ASC 740, (formerly FIN 48), the Company
recognizes the benefits of a tax position if that position is more likely than
not of being sustained on audit, based on the technical merit of the
position. Management believes it be more likely than not that the
Company can sustain management’s tax positions on audit.
Fair Value of Financial Instruments
The carrying amount reported in the balance sheets for cash
and cash equivalents, accounts receivable, accounts payable and accrued
liabilities approximate their fair value due to the immediate or short-term
maturity of such instruments.
Use of Estimates
The preparation of financial statements in conformity with
generally accepted accounting principles requires management to make estimates
and assumptions that affect the reported amounts of assets and liabilities and
disclosure of contingent assets and liabilities at the date of the financial
statements and the reported amounts of revenue and expenses during the reporting
periods. Actual results could differ from those
estimates.
F-9
|
|
2.
|
Accounting
Policies (Continued)
|
Earnings Per Share Data
The Company follows the provisions of ASC 260 “Earnings Per
Share,” (formerly SFAS No. 128 “Earnings Per Share”), which requires
the Company to present its basic earnings per share and diluted earnings per
share, and certain other earnings per share disclosures for each year
presented. Basic earnings per share is computed by dividing income
available to common shareholders by the weighted average number of common shares
outstanding. The computation of diluted earnings per share is similar
to the computation of basic earnings per share except that the denominator is
increased to include the average number of additional common shares that would
have been outstanding if the dilutive potential common shares had been
issued. In addition, the numerator is adjusted for any changes in
income that would result from the assumed conversions of those potential
shares.
Basic and diluted EPS computations are as
follows:
|
Years ended December 31,
|
2009
|
2008
|
||||||
|
Net income available to common
shareholders
|
$ | 374,169 | $ | 361,283 | ||||
|
Weighted average common shares
outstanding
|
2,680,394 | 2,709,382 | ||||||
|
Basic EPS
|
$ | 0.14 | $ | 0.13 | ||||
|
Diluted EPS:
|
||||||||
|
Net income available to common
shareholders
|
$ | 374,169 | $ | 361,283 | ||||
|
Weighted average common shares outstanding,
basic
|
2,680,394 | 2,709,382 | ||||||
|
Assumed conversion of net common shares issuable
under stock option plans
|
- | 1,638 | ||||||
|
Weighted average common and common equivalent
shares outstanding, diluted
|
2,680,394 | 2,711,020 | ||||||
|
Diluted EPS
|
$ | 0.14 | $ | 0.13 | ||||
Stock-Based Compensation
The Company accounts for share based compensation under the
provisions of ASC 718 “Stock Compensation,” (formerly
SFAS No. 123(R) “Share Based Payment”), which establishes accounting
for equity instruments exchanged for employee services. Under
ASC 718, share-based compensation cost is measured at the grant date, based
on the fair value of the award, and is recognized as an expense over the
employee’s requisite service period (generally the vesting period of the equity
grant).
Comprehensive Income
The Company follows the provisions of ASC 220
“Comprehensive Income,” (formerly SFAS No. 130 “Reporting
Comprehensive Income”), which establishes standards for reporting and display of
comprehensive income, its components, and accumulated
balances. Comprehensive income is defined to include all changes in
equity except those resulting from investments by owners and distributions to
owners. The Company did not have any components of comprehensive
income, exclusive of net income, for the years ended December 31, 2009
and 2008.
Preferred Stock
The Company has 2,000,000 shares of $1 par value preferred
stock authorized. No shares have been issued.
Shipping and Handling Costs
Shipping and handling costs are classified as a cost of
sales in the consolidated statements of income. The custom
manufacturing divisions as a normal course of business charge their customer
base for shipping and handling, and therefore classify the amounts billed as
revenue in the consolidated statements of income.
F-10
|
|
2.
|
Accounting
Policies (Continued)
|
Industry Segments
The Company follows the provisions of ASC 280 “Segment
Reporting,” (formerly SFAS No. 131 “Disclosure about Segments of an
Enterprise and Related Information”), which requires reporting of selected
information about operating segments in interim and annual financial statements
issued to the public. It also establishes standards for disclosures
regarding products and services, geographic areas, and major
customers. ASC 280 defines operating segments as components of
an enterprise about which separate financial information is available that is
evaluated regularly by the chief operating decision maker in deciding how to
allocate resources and in assessing performance.
Research and Development
Research
and development expenses include costs directly attributable to the conduct of
research and development programs primarily related to the development of our
software products and improving the efficiency and capabilities of our
manufacturing processes. Such costs include salaries, payroll taxes,
employee benefit costs, materials, supplies, depreciation on research equipment,
and services provided by outside contractors. All costs associated
with research and development programs are expensed as incurred.
Recent Accounting Pronouncements
In
December 2007, the Financial Accounting Standards Board (FASB) issued ASC 810
(formerly “SFAS No. 160”), “Non-controlling Interests in
Consolidated Financial Statements - an amendment of ARB No.
51.” ASC 810 establishes accounting and reporting standards
for the noncontrolling interest in a subsidiary for the deconsolidation of a
subsidiary. ASC 810 is effective for financial statements issued for
fiscal years beginning after December 15, 2008, and interim statements
within those fiscal years. The Company does not currently have any
noncontrolling interests.
In
December 2007, the FASB issued ASC 805-10 (formerly “SFAS No. 141(R)”),
“Business Combinations,” which retains the purchase method of accounting for
acquisitions, but requires a number of changes, including changes in the way
assets and liabilities are recognized in the purchase method of accounting. It
changes the recognition of assets acquired and liabilities assumed arising from
contingencies, requires the capitalization of in-process research and
development at fair value, and requires the expensing of acquisition-related
costs as incurred. The provisions of this standard will apply prospectively to
business combinations occurring in our fiscal year beginning January 1, 2009 and
the adoption did not have an impact on our financial position or results of
operations; however, it could it future transactions entered into by the
Company.
In March
2008, the FASB issued ASC 815 (formerly “SFAS No. 161”), “Disclosures about Derivative
Instruments and Hedging Activities—an amendment of FASB Statement
No.133.” ASC 815
amends and expands the disclosure requirements to provide improved transparency
into the uses and financial statement impact of derivative instruments and
hedging activities. The adoption of ASC 815 did not have a material
impact on our Financial Statements.
In April
2008, the FASB issued ASC 350-30, (formerly “FSP FAS 142-3”), “General
Intangibles Other Than Goodwill,” which details the factors that should be
considered in developing the useful lives for intangible assets with renewal or
extension provisions. ASC 350-30 requires an entity to consider its
own historical experience in renewing or extending similar arrangements,
regardless of whether those arrangements have explicit renewal or extension
provisions, when determining the useful life of an intangible
asset. In the absence of such experience, an entity shall consider
the assumptions that market participants would use about renewal or extension,
adjusted for entity-specific factors. ASC 350-30 also requires an
entity to disclose information regarding the extent to which the expected future
cash flows associated with an intangible asset are affected by the entity’s
intent and/or ability to renew or extend the arrangement. ASC 350-30
will be effective for qualifying intangible assets acquired by the Company on or
after July 1, 2009. The application of ASC 350-30 did not have a
material impact on the Company’s results of operations, cash flows or financial
positions; however, it could impact future transactions entered into by the
Company.
F-11
|
|
2.
|
Accounting
Policies (Continued)
|
Recent accounting
pronouncements (Continued)
In May
2009, the FASB issued ASC 855-10 (formerly SFAS No. 165), “Subsequent Events,”
which establishes general standards of accounting for and disclosure of events
that occur after the balance sheet date but before financial statements are
issued or are available to be issued. We adopted this standard upon
issuance with no impact on our financial position or results of
operations.
In June
2009, the FASB issued ASC 105-10 (formerly SFAS No. 168), “Accounting Standards
Codification™ and the Hierarchy of Generally Accepted Accounting Principles
(GAAP).” The FASB Accounting Standards Codification (“Codification”) has become
the source of authoritative accounting principles recognized by the FASB to be
applied by nongovernmental entities in the preparation of financial statements
in accordance with GAAP. All existing accounting standard documents are
superseded by the Codification and any accounting literature not included in the
Codification will not be authoritative. However, rules and
interpretive releases of the SEC
issued under the authority of federal securities laws will continue to be
the source of authoritative generally accepted accounting principles for SEC
registrants. Effective September 30, 2009, all references made to
GAAP in our consolidated financial statements will include the new Codification
numbering system along with original references. The Codification
does not change or alter existing GAAP and, therefore, will not have an impact
on our financial position, results of operations or cash flows.
In
October 2009, the FASB issued Accounting Standards Update (“ASU”) No. 2009-13,
“Multiple-Deliverable Revenue Arrangements” (ASU 2009-13). ASU
2009-13 establishes the accounting and reporting guidance for arrangements
including multiple revenue-generating activities, and provides amendments to the
criteria for separating deliverables, measuring and allocating arrangement
consideration to one or more units of accounting. The amendments in
ASU 2009-13 also establish a selling price hierarchy for determining the selling
price of a deliverable. Significantly enhanced disclosures are also
required to provide information about a vendor’s multiple-deliverable revenue
arrangements, including information about the nature and terms, significant
deliverables, and its performance within arrangements. The amendments
also require providing information about the significant judgments made and
changes to those judgments and about how the application of the relative
selling-price method affects the timing or amount of revenue
recognition. The amendments in ASU 2009-13 are effective
prospectively for revenue arrangements entered into or materially modified in
the fiscal years beginning on or after June 15, 2010. Early
application is permitted. The Company is currently evaluating the
potential impact, if any, the adoption of ASU 2009-13 will have on its financial
position or results of operations.
3. Inventories
Inventories consist of the following:
|
December
31,
|
2009
|
2008
|
||||||
|
Raw
materials
|
$ | 1,043,228 | $ | 1,099,876 | ||||
|
Work-in-process
|
234,360 | 773,245 | ||||||
|
Finished
goods
|
1,679,094 | 1,854,371 | ||||||
|
Total
|
$ | 2,956,682 | $ | 3,727,492 | ||||
F-12
|
|
4.
|
Property, Plant and Equipment,
Net
|
Property, plant and equipment consist of the
following:
|
December
31,
|
Asset
Lives
|
2009
|
2008
|
||||||
|
Machinery
and equipment
|
5
to 15 years
|
$ | 10,729,220 | $ | 10,532,555 | ||||
|
Equipment
held for lease
|
10
years
|
69,400 | 69,400 | ||||||
|
Building
and improvements
|
20
years
|
4,073,702 | 4,038,610 | ||||||
|
Vehicles
|
3
to 5 years
|
158,908 | 158,908 | ||||||
|
Furniture,
fixtures, computers and software
|
3
to 5 years
|
1,345,607 | 1,319,551 | ||||||
|
Land
|
202,492 | 202,492 | |||||||
|
Construction
in progress
|
126,174 | 94,829 | |||||||
|
Total
property, plant and equipment
|
16,705,503 | 16,416,345 | |||||||
|
Less:
accumulated depreciation
|
(10,361,928 | ) | (9,111,067 | ) | |||||
|
Property,
plant and equipment, net
|
$ | 6,343,575 | $ | 7,305,278 | |||||
The Company leases attaching machines to customers under
operating leases for periods of up to one year with renewable
terms. The cost of the leased equipment is depreciated on a
straight-line basis over ten years. Accumulated depreciation on
leased equipment was $142,950 at December 31, 2009 and 2008. The
Company sold two leased machines to its customers in 2008 and none in
2009.
|
|
5.
|
Debt
|
The Company had a note payable resulting from the
acquisition of Leominster Tool Co. Inc. of approximately $200,000 with a balance
of $0 at December 31, 2009 and 2008. This note was paid in full
during the first three months of 2008.
The Company had a one year term note secured by equipment
for a maximum of $813,000. In the third quarter of 2008, the
equipment note was extended for one year with a decrease in the fixed rate from
6.75% to 6.5% per annum. The equipment note was amortized over 6
years with a balloon payment for the remaining balance at September 15,
2009. This note was paid in full on September 15, 2009.
The Company has an unsecured $1,000,000 renewable credit
facility which provides for borrowings up to 80% of eligible accounts receivable
plus 50% of raw material and finished goods inventories up to a $300,000
maximum. This facility has no borrowing base charge. There
are no outstanding borrowings on the line of credit at December 31, 2009 and
2008. As of January 15, 2010, this line of credit was increased to a
limit of $2,000,000 at a rate of 2% over LIBOR.
The agreement contains covenants that apply upon drawing on
the line. The covenants relate to various matters including notice prior to
executing further borrowings and security interests, merger or consolidation,
acquisitions, guarantees, sales of assets other than in the normal course of
business, leasing, changes in ownership and payment of dividends.
|
|
6.
|
Income Taxes
|
The income tax provision consists of the
following:
|
Years
Ended December 31,
|
2009
|
2008
|
||||||
|
Current:
Federal
|
$ | 114,000 | $ | 73,400 | ||||
|
State
|
5,000 | 4,000 | ||||||
| 119,000 | 77,400 | |||||||
|
Deferred:
Federal
|
44,000 | 171,000 | ||||||
|
State
|
(9,000 | ) | 13,000 | |||||
| 33,000 | 184,000 | |||||||
|
Total
income tax provision
|
$ | 152,000 | $ | 261,400 | ||||
F-13
|
|
6.
|
Income
Taxes (Continued)
|
The components of deferred income taxes are as
follows:
|
Years
Ended December 31,
|
2009
|
2008
|
||||||
|
Deferred
income taxes:
Inventories
|
$ | 8,000 | $ | 7,000 | ||||
|
Other current
|
14,500 | 14,000 | ||||||
|
Total current deferred tax
assets
|
22,500 | 21,000 | ||||||
|
Property, plant and
equipment
|
(376,000 | ) | (345,500 | ) | ||||
|
Patents and
intangibles
|
26,000 | 30,000 | ||||||
|
Total long term deferred tax
liability
|
(350,000 | ) | (315,500 | ) | ||||
|
Deferred
income taxes, net
|
$ | (327,500 | ) | $ | (294,500 | ) | ||
The Company files a consolidated federal income tax
return. The actual income tax provision differs from the federal
statutory income tax rate (34%) as follows:
|
Years
Ended December 31,
|
2009
|
2008
|
||||||
|
Tax
provision computed at statutory rate
|
$ | 179,000 | $ | 212,000 | ||||
|
Increases
(reductions) due to:
State income taxes, net of
federal benefit
|
33,000 | 39,000 | ||||||
|
Permanent
differences
|
16,000 | 28,000 | ||||||
|
Tax credits (Federal &
State)
|
(72,000 | ) | (68,400 | ) | ||||
|
Other
|
(4,000 | ) | 50,800 | |||||
|
Income
tax expense
|
$ | 152,000 | $ | 261,400 | ||||
7. Employee
Benefit Plans
The Company sponsors an Employee Savings and Investment
Plan under Section 401(k) of the Internal Revenue Code covering all eligible
employees of the Company. Employees can contribute up to 90% of their
eligible compensation to the maximum allowable by the IRS. The
Company’s matching contributions are at the discretion of the
Company. The Company’s matching contributions in 2009 and 2008 were
$28,861 and $30,747, respectively.
On December 16, 2009, the Board of Directors, after a
recommendation from management and approval by the Compensation Committee,
granted 75,500 incentive stock options and non-qualified stock options to vest
over five years with an effective grant date of January 4, 2010 priced at the
average closing price for the prior ten trading days. Forty percent
of the options were granted to non-executive management. These
options were granted from the shareholder approved 2001 stock option plan
described in Note 10.
On April 28, 2005, the Company’s Board of Directors adopted
the 2005 Stock Award Plan. The Board’s objective in adopting the
Plan, based on the recommendation of management and approved by the Compensation
Committee, was to assist the Company in attracting and retaining the services of
certain employees, directors, and consultants deemed to be key and to secure the
benefits of the incentive inherent in ownership of the Company’s
securities. An aggregate of 100,000 shares were available for
issuance to employees, directors, and consultants. No awards have
been granted under the Stock Award Plan.
8. Commitments and
Contingencies
Legal Matters
The Company is from time to time subject to legal
proceedings, threats of legal action and claims which arise in the ordinary
course of our business. Management believes the resolution of these
matters will not have a material adverse effect on our results of operations or
financial condition.
F-14
8. Commitments
and Contingencies (Continued)
Environmental Groundwater
Like many industrial processes, the Micron manufacturing
process utilizes hazardous and non-hazardous chemicals, the treatment and
disposal of which are subject to federal and state regulation. Since
its inception, Micron has expended significant funds to train its personnel,
install waste treatment and recovery equipment and retain an independent
environmental consulting firm to constantly review, monitor and upgrade its air
and waste water treatment activities. As a result, Micron believes
that the operations of its manufacturing facility are in compliance with
currently applicable safety, health and environmental laws and
regulations.
Based on the Company’s analyses and subject to the
difficulty in estimating these future costs, the Company does not expect future
costs in connection with environmental matters to have a material adverse effect
on its financial condition, result of operations or liquidity.
Employment Agreements
The Company has employment agreements with two executives
extending through October 5, 2011 and
January 1, 2012. The agreements provide for a base
compensation and certain other benefits. The agreements also contain
other terms and conditions of employment, including termination payments under
certain circumstances.
Operating Leases
The Company leases vehicles and equipment under
non-cancelable lease arrangements. Lease expense under all operating
leases was approximately $8,100 and $10,700 in 2009 and 2008,
respectively.
On December 31, 2009, the company received a payment of
$677,810 for a sale lease-back transaction related to new production equipment
installed during the second half of 2009. This arrangement included a
lease line with a credit limit of $1,000,000, and the Company expects to use the
remaining $322,190 for the purchase of certain production equipment in the
beginning of 2010. This transaction created a long term deferred gain
on the sale of assets of $22,347, which will be amortized over the life of the
lease.
Future minimum operating lease payments as of December 31,
2009 are approximately as follows:
|
Year
|
Amount
|
|||
|
2010
|
$ | 146,867 | ||
|
2011
|
139,690 | |||
|
2012
|
139,690 | |||
|
2013
|
139,690 | |||
|
2014
|
139,690 | |||
|
Total
|
$ | 705,627 | ||
9. Supplemental Cash
Flow Information
Cash paid for income taxes and interest for the years ended
December 31 are as follows:
|
2009
|
2008
|
|||||||
|
Income taxes
|
$ | - | $ | 109,000 | ||||
|
Interest
|
31,699 | 47,826 | ||||||
In 2009, installation of production equipment costing
$677,810 financed with an operating lease did not require a cash outlay from the
Company.
F-15
10. Stock
Options
The Company accounts for non-cash share based compensation
under ASC 718 “Stock Compensation,” which establishes accounting for equity
instruments exchanged for employee services. Under ASC 718,
share-based compensation cost is measured at the grant date, based on the fair
value of the award, and is recognized as an expense over the employee’s
requisite service period (generally the vesting period of the equity
grant).
For the year ended December 31, 2009 and 2008, share-based
compensation included in general and administrative expenses amounted to $73,835
and $100,229, respectively.
The fair value of each stock option granted is estimated on
the date of grant using the Black-Scholes option-pricing model. Key
assumptions used to estimate the fair value of the stock options include the
exercise price of the award, the expected option term, and the expected
volatility of the Company’s stock over the option’s expected term, the risk free
interest rate over the option’s expected term, and the Company’s expected annual
dividend yield. The Company believes that the valuation technique and
the approach utilized to develop the underlying assumptions are appropriate in
calculating the fair values of the Company’s stock options for the year ended
December 31, 2009 and 2008. Estimates of fair values are not intended
to predict actual future events or the value ultimately realized by persons who
receive equity awards.
The fair value of the option grant in 2008 was estimated on
the grant date using the Black-Scholes option pricing model with the following
assumptions:
|
2008
|
|
|
Expected option term (1)
|
4.5 years
|
|
Expected volatility factor (2)
|
41%
|
|
Risk-free interest rate (3)
|
3.3%
|
|
Expected annual dividend yield
|
0.0%
|
|
(1)
|
The option life was determined using the simplified
method for estimated expected option life, which qualifies as
“plain-vanilla” options.
|
|
(2)
|
The stock volatility for each grant is determined
based on the review of the experience of the weighted average of
historical daily price changes of the Company’s common stock over the most
recent year.
|
|
(3)
|
The risk-free interest rate for periods equal to the
expected term of the share option is based on the U.S. Treasury yield
curve in effect at the time of
grant.
|
Share-Based Incentive Plan
At December 31, 2009, the Company had one stock option plan
that includes both incentive and non-qualified stock options to be granted to
certain eligible employees, non-employee directors, or
consultants. The maximum number of shares reserved for issuance is
400,000 shares. The options granted have six-year contractual terms
and either vest immediately or vest annually over a five-year term.
At December 31, 2009, there were 168,000 shares available
for future grants under the above stock option plan.
F-16
10. Stock
Options (Continued)
The following table sets forth the stock option
transactions for the year ended December 31, 2009:
|
Number of shares
|
Weighted average Exercise Price
|
Weighted average remaining contractual
term
|
Aggregate Intrinsic Value
|
||||||||||
|
Outstanding at December 31, 2007
|
127,000 | $ | 10.45 |
3.8 years
|
|||||||||
|
Granted
|
107,500 | 7.15 | |||||||||||
|
Exercised
|
- | - | |||||||||||
|
Cancelled/expired
|
(26,500) | 10.39 | |||||||||||
|
Outstanding at December 31, 2008
|
208,000 | $ | 10.45 |
3.7 years
|
|||||||||
|
Granted
|
- | - | |||||||||||
|
Exercised
|
- | - | |||||||||||
|
Cancelled/expired
|
(29,000) | $ | 5.45 | ||||||||||
|
Outstanding at December 31, 2009
|
179,000 | $ | 9.29 |
3.1 years
|
$ | - | |||||||
|
Exercisable at end of year
|
92,200 | $ | 10.04 |
2.5 years
|
$ | - | |||||||
The weighted average fair value of stock options granted
during 2008 was $2.73.
During the year ended December 31, 2009 and 2008,
no options were exercised. At December 31, 2009 and 2008,
the intrinsic value of the exercisable options is $0.
The following table sets forth the status of the Company’s
non-vested options for the year ended December 31, 2009:
|
Number of shares
|
Weighted average Fair Value
|
|||||||
|
Non-vested at December 31, 2008
|
111,000 | $ | 3.46 | |||||
|
Granted
|
- | - | ||||||
|
Vested (with an intrinsic value of
$0)
|
(23,200) | 3.66 | ||||||
|
Cancelled/expired
|
(1,000) | 2.74 | ||||||
|
Non-vested at December 31, 2009
|
86,800 | $ | 3.42 | |||||
The following table presents the average price and
contractual life information about options outstanding and exercisable at
December 31, 2009:
|
Exercise Price
|
Number of Outstanding Shares
|
Weighted Average Remaining Contractual Life
(years)
|
Options Currently Exercisable
|
|||||||||||
| 7.15 | 96,000 | 4.01 | 19,200 | |||||||||||
| 9.86 | 63,000 | 1.97 | 63,000 | |||||||||||
| 12.42 | 10,000 | 2.59 | 6,000 | |||||||||||
| 23.10 | 10,000 | 3.18 | 4,000 | |||||||||||
As of December 31, 2009, there was $221,301 of unrecognized
compensation cost related to non-vested share based compensation arrangements
granted under the stock option plan. This cost is expected to be
recognized over a weighted average period of 3.99 years.
As of December 31, 2008, there was $297,169 of unrecognized
compensation cost related to non-vested share based compensation arrangements
granted under the stock option plan. This cost is expected to be
recognized over a weighted average period of 5.13 years.
F-17
11. Industry and
Geographic Segments
The Company’s operations are classified into two business
segments: medical electrode components and plastic molding, and computerized
medical instruments.
The following table shows sales, operating income (loss)
and other financial information by industry segment as of and for the years
ended December 31, 2009 and 2008:
|
Year ended December 31, 2009
|
Medical Electrode Components and Plastic
Molding
|
Computerized Medical Instruments
|
Corporate
|
Consolidated
|
||||||||||||
|
Sales
|
$ | 21,139,774 | $ | - | $ | - | $ | 21,139,774 | ||||||||
|
Operating income (loss)
|
$ | 1,714,964 | $ | (35,721 | ) | $ | (1,124,132 | ) | $ | 555,111 | ||||||
|
Capital Expenditures
|
$ | 361,195 | $ | - | $ | - | $ | 361,195 | ||||||||
|
Depreciation and Amortization
|
$ | 1,310,772 | $ | - | $ | 83,832 | $ | 1,394,604 | ||||||||
|
Total Assets at December 31, 2009
|
$ | 14,372,218 | $ | 50,927 | $ | 4,324,214 | $ | 18,747,359 | ||||||||
|
Year ended December 31, 2008
|
Medical Electrode Components and Plastic
Molding
|
Computerized Medical Instruments
|
Corporate
|
Consolidated
|
||||||||||||
|
Sales
|
$ | 22,482,219 | $ | - | $ | - | $ | 22,482,219 | ||||||||
|
Operating income (loss)
|
$ | 2,185,345 | $ | (375,832 | ) | $ | (1,169,964 | ) | $ | 639,549 | ||||||
|
Capital Expenditures
|
$ | 991,646 | $ | - | $ | 24,056 | $ | 1,015,702 | ||||||||
|
Depreciation and Amortization
|
$ | 1,316,097 | $ | - | $ | 83,057 | $ | 1,399,154 | ||||||||
|
Total Assets at December 31,
2008
|
$ | 15,590,769 | $ | 72,217 | $ | 2,825,581 | $ | 18,488,567 | ||||||||
The following table sets forth the geographic distribution
of the Company’s net sales:
|
2009
|
2008
|
|||||||
|
United States
|
$ | 12,937,615 | $ | 13,290,098 | ||||
|
Canada
|
3,684,087 | 5,118,913 | ||||||
|
Europe
|
2,644,727 | 3,091,326 | ||||||
|
Pacific Rim
|
818,866 | 426,764 | ||||||
|
Other
|
1,054,479 | 555,118 | ||||||
|
Net Sales
|
$ | 21,139,774 | $ | 22,482,219 | ||||
The following table sets forth the percentage of net sales
to significant customers of the medical electrode and injection molded component
segment in relation to total segment sales:
|
Customers
|
2009
|
2008
|
||||||||
| A | 23% | 27% | ||||||||
| B | 16% | 12% | ||||||||
| C | - | 17% | ||||||||
| D | 12% | - | ||||||||
F-18
12. Quarterly
Financial Data
|
(unaudited)
|
First
Quarter
|
Second
Quarter
|
Third
Quarter
|
Fourth
Quarter
|
||||||||||||
|
2009
Net
Sales
|
$ | 4,683,454 | $ | 5,371,439 | $ | 5,457,377 | $ | 5,627,504 | ||||||||
|
Gross
Profit
|
944,322 | 875,133 | 881,309 | 880,870 | ||||||||||||
|
Net
Income
|
81,774 | 76,920 | 108,214 | 107,261 | ||||||||||||
|
Basic
Earnings per share
|
0.03 | 0.03 | 0.04 | 0.04 | ||||||||||||
|
Diluted
Earnings per share
|
0.03 | 0.03 | 0.04 | 0.04 | ||||||||||||
|
2008
Net
Sales
|
$ | 5,459,742 | $ | 6,426,120 | $ | 5,838,390 | $ | 4,757,967 | ||||||||
|
Gross
Profit
|
1,111,438 | 1,346,471 | 841,560 | 978,224 | ||||||||||||
|
Net
Income
|
149,372 | 134,119 | 68,139 | 9,753 | ||||||||||||
|
Basic
Earnings per share
|
0.06 | 0.05 | 0.03 | 0.00 | ||||||||||||
|
Diluted
Earnings per share
|
0.05 | 0.05 | 0.03 | 0.00 | ||||||||||||
F-19
EXHIBIT
INDEX
|
Exhibit
Number
|
Description
of Exhibit
|
Page
|
||
|
3.0
|
Articles
of Incorporation
|
(a)
|
||
|
3.1
|
Amended
and Restated By-laws
|
(c)
|
||
|
4.0
|
Form
of Certificate evidencing shares of the Company's Common Stock.
|
(a)
|
||
|
4.6*
|
2001
Stock Option Plan
|
(b)
|
||
|
4.8*
|
2005
Stock Award Plan
|
(d)
|
||
|
10.43*
|
Employment
agreement between James E. Rouse and the Company dated
December 26th, 2006.
|
(e)
|
||
|
10.44*
|
Employment
agreement between David A. Garrison and the Company dated
January 1, 2007.
|
(e)
|
||
|
21.0
|
Subsidiaries
|
(f)
|
||
|
X-1
|
||||
|
X-2
|
||||
|
X-3
|
||||
|
X-4
|
||||
|
X-5
|
||||
*
Indicates a management contract or compensatory plan required to be filed as an
exhibit.
|
(a)
|
Incorporated
by reference to the Company’s Registration Statement on Form S-18 as filed
with the Commission in April 1988, Registration Statement No.
33-20945-FW.
|
|
(b)
|
Incorporated
by reference to the Company’s Form 10-K for fiscal year ended December 31,
2001 as filed with the Commission in March 2002.
|
|
(c)
|
Incorporated
by reference to the Company’s Current Report on Form 8-K as filed with the
Commission in December 2007.
|
|
(d)
|
Incorporated
by reference to the Company’s Registration Statement on Form S-8 as filed
with the Commission in December 2005, Registration Statement No.
333-130678.
|
|
(e)
|
Incorporated
by reference to the Company’s Form 10-KSB for fiscal year ended December
31, 2006, as filed with the Commission in March 2007.
|
|
(f)
|
Incorporated
by reference to the Company’s Form 10-K for the fiscal year ended December
31, 2007 as filed with the Commission in March
2008.
|
