Attached files
| file | filename |
|---|---|
| EX-10 - HEALTHTRONICS, INC. | ex1032.htm |
| EX-10 - HEALTHTRONICS, INC. | exh1029.htm |
| EX-10 - HEALTHTRONICS, INC. | exh1030.htm |
| EX-10 - HEALTHTRONICS, INC. | exh1031.htm |
| EX-32 - HEALTHTRONICS, INC. | exh321.htm |
| EX-31 - HEALTHTRONICS, INC. | ex312.htm |
| EX-21 - HEALTHTRONICS, INC. | ex211.htm |
| EX-31 - HEALTHTRONICS, INC. | ex311.htm |
| EX-23 - HEALTHTRONICS, INC. | exh231.htm |
| EX-32 - HEALTHTRONICS, INC. | exh322.htm |
| EX-10 - HEALTHTRONICS, INC. | ex1034.htm |
| EX-10 - HEALTHTRONICS, INC. | exh1033.htm |
_______________________________________________________
UNITED STATES
SECURITIES AND EXCHANGE
COMMISSION
WASHINGTON, D.C. 20549
_____________________________
FORM 10-K
[X] Annual Report Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
For the fiscal year ended December 31, 2009
OR
[ ] Transition Report Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
For the transition period from ________ to ________
Commission File Number: 000-30406
_____________________________
HEALTHTRONICS, INC.
(Exact name of registrant as specified in its charter)
| GEORGIA
|
58-2210668
| ||||
|
(State or other jurisdiction of incorporation or organization) |
(I.R.S. Employer Identification No.) |
| 9825 Spectrum Drive, Building 3, Austin, Texas (Address of principal executive office) |
78717 (Zip Code) |
(512) 328-2892 (Registrant’s telephone number, including area code) |
Securities registered pursuant to Section 12(b) of the Act:
None
Securities registered pursuant to Section 12(g) of the Act:
Common Stock
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. YES __
NO X |
| Large accelerated filer __ | Accelerated filer X | Non-accelerated filer __ (do not check if a smaller reporting company) |
Smaller reporting company __ |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). YES __ NO X Aggregate Market Value at June 30, 2009: $ 61,728,000 |
| Indicate the number of shares outstanding of each of the registrant’s classes of common stock, as of the latest practicable date. |
|
Title of Each Class Common Stock, no par value |
Number of Shares Outstanding at
February 28, 2010 45,587,235 |
DOCUMENTS INCORPORATED BY REFERENCE
| Selected portions of the Registrant’s definitive proxy material for the 2010 annual meeting of stockholders are incorporated by reference into Part III of the Form 10-K. |
|
HEALTHTRONICS, INC. ANNUAL REPORT ON FORM 10-K FOR THE FISCAL YEAR ENDED DECEMBER 31, 2009 PART I |
|---|
ITEM 1. BUSINESS
|
General |
| 2 |
|
Radiation therapy services. We provide image guided radiation therapy (“IGRT”) technical services for
cancer treatment centers. Our IGRT technical services may relate to providing the technical (non-physician)
personnel to operate a physician practice group’s IGRT equipment, leasing IGRT equipment to a physician
practice group, providing services related to helping a physician practice group establish an IGRT treatment
center, or managing an IGRT treatment center. |
| • | Fees for urology treatments. A substantial majority of our revenue is derived from fees related to lithotripsy treatments performed using our lithotripters. For lithotripsy and prostate treatment services, we, through our partnerships and other entities, facilitate the use of our equipment and provide other support services in connection with these treatments at hospitals and other health care facilities. The professional fee payable to the physician performing the procedure is generally billed and collected by the physician. We recognize revenue for these services when the services are provided. IGRT technical services are billed monthly and the related revenues are recognized as the related services are provided. | |
| • | Fees for managing the operation of our lithotripters and prostate treatment devices. Through our partnerships and otherwise directly by us, we provide services related to operating our lithotripters and prostate treatment equipment and receive a management fee for performing these services. We recognize revenue for these services as the services are provided. |
| 3 |
|
• | Fees for maintenance services. We provide equipment maintenance services to our partnerships as well as outside parties. These services are billed either on a time and material basis or at a fixed contractual rate, payable monthly, quarterly, or annually. Revenues from these services are recorded when the related maintenance services are performed. |
| • | Fees for equipment sales, consumable sales and licensing applications. We manufacture and sell medical devices focused on minimally invasive technologies for tissue and tumor ablation through cryosurgery, and their related consumables. We also sell and maintain lithotripters and manufacture and sell consumables related to the lithotripters. We distribute the Revolix laser and consumables related to the laser. With respect to some lithotripter sales, in addition to the original sales price, we receive a licensing fee from the buyer of the lithotripter for each patient treated with such lithotripter. In exchange for this licensing fee, we provide the buyer of the lithotripter with certain consumables. All the sales for equipment and consumables are recognized when the related items are delivered. Revenues from licensing fees are recorded when the patient is treated. In some cases, we lease certain equipment to our partnerships as well as third parties. Revenues from these leases are recognized on a monthly basis or as procedures are performed. | |
| • | Fees for anatomical pathology services. We provide anatomical pathology services primarily to the urology community. Revenues from these services are recorded when the related laboratory procedures are performed. |
|
Revenues and Industry Segments |
| 4 |
|
Our policy is to sell our products and services under trademarks and to secure trademark protection in the United
States and elsewhere where we deem such protection important. |
| 5 |
|
We may also face product liability claims as a result of our medical device manufacturing. |
| • | the federal Medicare and Medicaid anti-kickback law, and state anti-kickback prohibitions; | |
| • | the federal physician self-referral prohibition commonly known as the Stark Law and the state law equivalents of the Stark Law; | |
| • | the federal False Claims Act; | |
| • | federal and state billing and claims submission laws and regulations; | |
| • | the federal Health Insurance Portability and Accountability Act of 1996 and state laws relating to patient privacy; | |
| • | state laws that prohibit the practice of medicine by non-physicians, and prohibit fee-splitting arrangements involving physicians; | |
| • | state and federal laws affecting the ownership and operation of the equipment we use and the manner in which we provide services; and | |
| • | federal and state laws governing the equipment we use in our business concerning patient safety and equipment operating specifications. | |
|
The federal anti-kickback law prohibits the knowing and willful offer, payment, solicitation or receipt of any form of remuneration in return for, or in order to induce, (i) the referral of a person for services, (ii) the furnishing or arranging for the furnishing of items or services, or (iii) the purchase, lease or order or arranging or recommending purchasing, leasing or ordering any item or service, in each case, reimbursable under any federal health care program. The Stark Law prohibits a physician from referring a Medicare patient for “designated health services,” or DHS, to an entity with which the physician has a direct or indirect financial relationship, whether in the nature of an ownership interest or a compensation arrangement, subject only to limited exceptions. The Stark Law also prohibits the recipient of a prohibited referral from billing for the DHS provided pursuant thereto. We contract with physicians under a variety of financial arrangements, and physicians have ownership interests in some entities in which we also have an interest. If our operations are found to be in violation of any of the laws and regulations to which we or our Customers are subject, we may be subject to the applicable penalty associated with the violation, including civil and criminal penalties, damages, fines, exclusion from the Medicare, Medicaid, and other governmental healthcare programs, loss of licenses, and the curtailment of our operations. While we believe that we are in compliance with all applicable laws, we cannot assure that our activities will be found to be in compliance with these laws if scrutinized by regulatory authorities. Any penalties, damages, fines or curtailment of our operations, individually or in the aggregate, could adversely affect our ability to operate our business and our financial results. The risks of us being found in violation of these laws and regulations is increased by the fact that many of the laws and regulations have not been fully interpreted by the regulatory authorities or in the courts, and their provisions are open to a variety of interpretations. Any action brought against us for violation of these laws or regulations, even if we were to successfully defend against it, could cause us to incur significant legal expenses and divert our management’s attention from the operation of our business. |
| 6 |
|
The Centers for Medicare & Medicaid Services (“CMS”) recently issued a rule that amended regulations
that implement the Stark Law. Under the rule, which went into effect October 1, 2009, certain physician-owned
ventures (e.g., laser, cryotherapy, and TUMT partnerships) are not able to contract with hospitals for the lease
of space or equipment under a per-procedure or per-click payment arrangement. Also under the rule, CMS
acknowledged that lithotripsy services performed under arrangements at hospitals is not a DHS under the Stark
Law. However, to the extent a physician with an ownership or compensation interest in one of our lithotripsy
partnerships otherwise refers patients to the hospital for DHS services, the Stark Law still applies and an
exception must be met. CMS has also issued an answer in the form of a “Frequently Asked Question” on
its website, where it indicates that the provision of lithotripsy services may be considered a service contract
and not a lease of space or equipment. Thus, according to the FAQ advice from CMS, our lithotripsy partnerships
may continue to contract with hospitals on a per-procedure payment basis so long as the contract is a service
arrangement rather than a leasing arrangement and the physician partners in the partnership do not otherwise
refer “designated health services” to the contracting hospitals (unless under an applicable
exception). If the partnership provides a technician and related support when providing lithotripsy services, we
believe such arrangement is a service arrangement. If a lithotripsy arrangement is considered a leasing
arrangement or if any of the physician partners in the lithotripsy arrangement refer “designated health
services” to a contracting hospital, then the fee arrangements between the partnership and the hospitals are
required to be in compliance with the new rule effective as of October 1, 2009. In addition, our prostate
treatment partnerships were required to be in compliance with the new rule effective as of October 1, 2009. We
restructured our prostate partnerships and/or their hospital contracts to comply with the amended regulations as
of October 1, 2009. Although we believe the prostate partnerships as restructured are in compliance with the
amended regulations, no assurance can be made that the prostate partnerships as restructured will be found to be
in compliance with the amended Stark law regulations if reviewed by regulatory authorities. If regulatory
authorities were to conclude that our prostate partnerships were not in compliance, any resulting penalties,
damages, fines or curtailment of our operations, individually or in the aggregate, could adversely effect our
ability to operate our business and affect our financial results. |
| 7 |
|
Generally, physician ownership and contracts with entities that provide ancillary medical services are a subject
of intensive legislative and regulatory attention. It is likely that additional legislation may be passed and
additional regulations will be promulgated that will further affect our business and our relationships with
physicians. The recently-passed American Recovery and Reinvestment Act of 2009 (commonly known as the Stimulus
Bill) contains a number of provisions affecting healthcare entities like us, including new requirements relating
to information technology, privacy, and data security. The U.S. President has stated that his administration
will make health care reform a major initiative, which may impact our provision of services and our
reimbursement. Potential changes that have been considered include controls on health care spending and price
controls. Any health care reform could have a material adverse effect on reimbursement for our services and
products and could negatively affect the commercial acceptance of our services and products, which could have a
material adverse effect on our operations financial position and results of operations. We are unable to state
with certainty the effect of any such pending legislation or regulation on our business. |
| • | quality system regulation, which requires manufacturers to follow design, testing, control, documentation and other quality assurance procedures during the manufacturing process; | |
| • | labeling regulations, which require specific information on product labels and in labeling, prohibit certain information, prohibit the promotion of products for unapproved or “off-label” uses and impose other restrictions on labeling; and | |
| • | medical device reporting regulations, which require that manufacturers report to the FDA if their device may have caused or contributed to a death or serious injury or malfunctioned in a way that would likely cause or contribute to a death or serious injury if it were to recur. | |
|
Failure to comply with applicable regulatory requirements can result in enforcement action by the FDA, which may include, but is not limited to, any of the following sanctions: |
| • | fines, injunctions, and civil penalties; | |
| • | recall or seizure of our products; | |
| • | operating restrictions, partial suspension or total shutdown of production; | |
| • | refusing our request for 510(k) clearance or premarket approval of new products; | |
| • | withdrawing 510(k) clearance or premarket approvals that are already granted; and | |
| • | criminal prosecution. | |
| 8 |
|
Equipment |
| 9 |
|
New and proposed federal and state laws and regulatory initiatives relating to various initiatives in health care
reform (such as improving privacy and the security of patient information and combating health care fraud) could
require us to expend substantial sums to appropriately respond to and comply with this broad variety of
legislation (such as acquiring and implementing new information systems for privacy and security protection),
which could negatively impact our financial results. |
| 10 |
|
Regulatory approvals of medical products, if granted, may include significant limitations on the indicated uses
for which the medical products we manufacture may be marketed. In addition, to obtain such regulatory approvals,
the FDA and foreign regulatory authorities may impose numerous other requirements on us. FDA enforcement policy
prohibits the marketing of approved medical devices for unapproved uses. In addition, regulatory approvals can be
withdrawn for failure to comply with regulatory standards or as a result of unforeseen problems following initial
marketing. We may not be able to obtain or maintain regulatory approvals for our products on a timely basis, or
at all, and delays in receipt of or failure to receive such regulatory approvals, the loss of previously obtained
regulatory approvals or failure to comply with existing or future regulatory requirements could have a material
adverse effect on our business, financial condition, results of operations and cash flows. |
| 11 |
|
We may be subject to costly and time-consuming product liability actions that would materially harm our business. |
| 12 |
|
We may be required to modify our agreements, operations, marketing and expansion strategies in response to
changes in the statutory and regulatory environment. |
| • | future announcements concerning us, our competition or the health care services market generally; | |
| • | developments relating to our relationships with hospitals, other health care facilities, or physicians; | |
| • | developments relating to our sources of supply; | |
| • | claims made or litigation filed against us; | |
| • | changes in, or new interpretations of, government regulations; | |
| • | changes in operating results from quarter to quarter; | |
| • | sales of stock by insiders; | |
| • | news reports relating to trends in our markets; | |
| • | general economic conditions; | |
| • | economic conditions within our industry; |
| 13 |
| • | acquisitions and financings in our industry; and | |
| • | overall volatility of the stock market. |
|
Furthermore, stock prices for many companies fluctuate widely for reasons that may be unrelated to their
operating results. These fluctuations, coupled with changes in our results of operations and general economic,
political and market conditions, may adversely affect the market price of our common stock. |
| • | diversion of management’s attention; | |
| • | the need to integrate acquired operations; | |
| • | the risk that the expected cost savings and other synergies from the acquisition may not be fully realized, realized at all or take longer to realize than anticipated; | |
| • | potential loss of key employees of the acquired companies; and | |
| • | an increase in our expenses and working capital requirements. |
|
Any of these factors could adversely affect our ability to achieve anticipated levels of cash flows from our
acquired businesses or realize other anticipated benefits from those acquisitions. |
| 14 |
|
Our operations are partially dependent upon third-party suppliers, making us vulnerable to a supply shortage. |
| • | the need to comply with applicable federal Food and Drug Administration and foreign regulations relating to good manufacturing practices and medical device approval requirements, and with state licensing requirements; | |
| • | the need for special non-governmental certifications and registrations regarding product safety, product quality and manufacturing procedures in order to market products in the European Union; | |
| • | potential product liability claims for any defective goods that are distributed; and | |
| • | the need for research and development expenditures to develop or enhance products and compete in the equipment markets. | |
|
Our indebtedness may limit our financial and operating flexibility. |
| 15 |
| • | incur additional debt or liens; | |
| • | make payments in respect of or redeem or acquire any debt or equity issued by us; | |
| • | sell assets; | |
| • | make loans or investments; | |
| • | acquire or be acquired by other companies; and | |
| • | amend some of our contracts. | |
|
We currently have $44 million drawn on our $60 million revolving line of credit, which could have important consequences to you. For example, it could: |
| • | increase our vulnerability to general adverse economic and industry conditions; | |
| • | limit our ability to fund future working capital and capital expenditures, to engage in future acquisitions, or to otherwise realize the value of our assets and opportunities fully because of the need to dedicate a portion of our cash flow from operations to payments on our debt or to comply with any restrictive terms of our debt; | |
| • | limit our flexibility in planning for, or reacting to, changes in the industry in which we operate; and | |
| • | place us at a competitive disadvantage as compared to our competitors that have less debt. | |
|
In addition, if we fail to comply with the terms of any of our debt, our lenders will have the right to
accelerate the maturity of that debt and foreclose upon the collateral, if any, securing that debt. Realization
of any of these factors could adversely affect our business, financial condition and results of operations. |
| 16 |
|
We have in the past identified material weaknesses in our internal control over financial reporting, and the
identification of any significant deficiencies or material weaknesses in the future could affect our ability to
ensure timely and reliable financial reports. |
|
Executive Officers |
| Name | Age | Position | |
|---|---|---|---|
| James S.B. Whittenburg | 38 | Chief Executive Officer and President | |
| Richard A. Rusk | 48 | Chief Financial Officer and Treasurer | |
| Clint B. Davis | 37 | Senior Vice President, General Counsel and Secretary | |
| Clayton H. Duncan | 37 | Vice President-Radiation Therapy | |
| Scott A. Herz | 33 | Vice President-Corporate Development | |
| Laura A. Miller | 40 | President of Pathology Services | |
|
The foregoing does not include positions held in our subsidiaries. Our officers are elected for annual periods.
There are no family relationships between any of our executive officers and/or directors. |
| 17 |
|
Mr. Rusk was appointed Chief Financial Officer in November 2009. Mr. Rusk joined us in August 2000 as our
Corporate Controller and was named Vice President in June 2002. In June 2006, Mr. Rusk was named our Treasurer
and in September 2006, Mr. Rusk was named our Secretary. In September of 2008, Mr. Rusk was named Interim Chief
Financial Officer. Before joining us, Mr. Rusk, a CPA, was with KPMG LLP for approximately seventeen years, the
last ten years as a senior audit manager. |
| 18 |
|
We also make available free of charge on or through our website (http://www.healthtronics.com) our Annual Report
on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K, and, if applicable, amendments to
those reports filed or furnished pursuant to Section 13(a) of the Exchange Act as soon as reasonably practicable
after we electronically file such material with, or furnish it to, the SEC.
|
| 19 |
| PART II |
|---|
ITEM 5.
MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER
MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
|
The following table sets forth the high and low closing prices per share for our common stock on the Nasdaq Global Select Market for the years ended December 31, 2009 and 2008 (NASDAQ Symbol “HTRN”). |
| 2009 |
2008 |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| High |
Low |
High |
Low | ||||||||||||
| First Quarter | $ | 2 | .75 | $ | 1 | .28 | $ | 4 | .47 | $ | 3 | .12 | |||
| Second Quarter | $ | 2 | .01 | $ | 1 | .29 | $ | 4 | .30 | $ | 2 | .68 | |||
| Third Quarter | $ | 2 | .76 | $ | 1 | .77 | $ | 4 | .50 | $ | 2 | .83 | |||
| Fourth Quarter | $ | 2 | .64 | $ | 2 | .16 | $ | 3 | .04 | $ | 1 | .03 | |||
|
On February 20, 2010, we had 669 holders of record of our common stock. |
| Period |
Total Number of Shares Purchased |
Average Price Paid Per Share |
Total Number of Shares Purchased as Part of Publicly Announced Plans or Programs |
Maximum Number (or Approximate Dollar Value) of Shares That May Yet to be Purchased Under the Plans or Programs | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Month 1 (10/1/2009 – 10/31/2009) | 11,292 | (b) | $ | 2 | .23 | -- | ||||||||
| Month 2 (11/1/2009 – 11/30/2009) | 31,581 | (b) | $ | 2 | .44 | -- | ||||||||
| Month 3 (12/1/2009 – 12/31/2009) | -- | -- | -- | |||||||||||
| Total | 42,873 | $ | 2 | .38 | -- | $ 6,260,000 | ||||||||
|
_____________________ |
| (a) | On October 6, 2008, our Board of Directors authorized the repurchase of up to $10 million of our common stock. We anticipate that the stock will be repurchased through privately-negotiated transactions or on the open market. We intend to comply with the SEC’s Rule 10b-18, and the repurchases will be subject to market conditions, applicable legal requirements, and other factors. We are not obligated to repurchase shares under the program, and our Board of Directors may suspend or terminate the program at any time. The repurchase program has no expiration date. We have no repurchase plans or programs that expired during the period covered by the above table, and we have no repurchase plans or programs that we intend to terminate prior to expiration or under which we no longer intend to make further purchases. | |
| (b) | Represents shares used by 5 employees to pay their federal tax withholding obligation related to the vesting of certain restricted stock awards in October and November 2009. |
| 20 |
|
Equity Compensation Plan Information |
| (a) |
(b) |
(c) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
Plan Category |
Number of shares of our common stock to be issued upon exercise of outstanding options |
Weighted-average exercise price of outstanding options |
Number of shares of our common stock remaining available for future issuance under equity compensation plans (exceeding securities reflected in column (a)) | |||||||||
| Prime 1993 stock option plan | 65,666 | $ | 7 | .79 | -- | |||||||
| Prime 2003 stock option plan | 69,000 | $ | 5 | .61 | -- | |||||||
| HSS equity incentive plan and stock | ||||||||||||
| option plans | 2,653,283 | $ | 5 | .93 | 1,776,237 | |||||||
| Other equity compensation plans | ||||||||||||
| approved by our security holders | N/A | N/A | N/A | |||||||||
|
In 2004, in connection with the merger of HSS and Prime, we assumed in the merger stock options to acquire approximately 2,154,000 shares of common stock. |
| 21 |
|
Performance Graph |
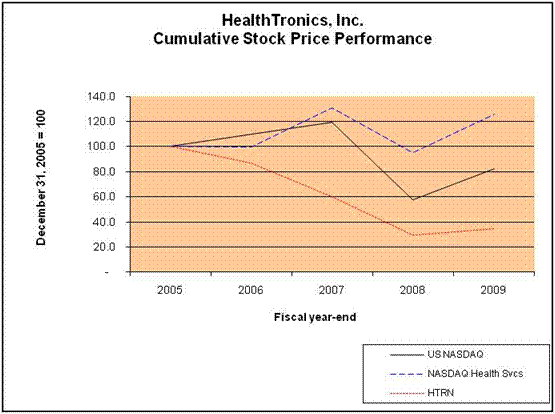
|
ITEM 6. SELECTED FINANCIAL DATA
|
The following tables set forth our summary consolidated historical financial information that has been derived from (a) our audited consolidated statements of income and cash flows for each of the years ended December 31, 2009, 2008, 2007, 2006 and 2005, (b) our audited consolidated balance sheets as of December 31, 2009, 2008, 2007, 2006, and 2005, and (c) our unaudited consolidated statements of income and cash flows for each of the three months ended December 31, September 30, June 30, and March 31, for 2009 and 2008. You should read this financial information in conjunction with “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our historical financial statements and notes thereto included elsewhere in this Annual Report on Form 10-K. The historical results are not necessarily indicative of results to be expected in any future period. |
| 22 |
| (In thousands, except per share data) | Years Ended December 31, |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2009 |
2008 |
2007 |
2006 |
2005 | |||||||||||||
| Revenues from continuing operations | $ | 185,330 | $ | 165,942 | $ | 140,418 | $ | 142,891 | $ | 152,267 | |||||||
| Income (loss) attributable to HealthTronics, Inc. from: | |||||||||||||||||
| Continuing Operations | $ | (3,916 | ) | $ | (128,693 | ) | $ | (14,485 | ) | $ | (16,446 | ) | $ | 10,933 | |||
| Discontinued Operations | -- | -- | (147 | ) | 25,129 | (1,745 | ) | ||||||||||
| Net income (loss) | $ | (3,916 | ) (1) | $ | (128,693 | )(2) | $ | (14,632 | )(3) | $ | 8,683 | (4) | $ | 9,188 | |||
| Diluted earnings (loss) per share | |||||||||||||||||
| attributable to HealthTronics, Inc.: | |||||||||||||||||
| Continuing Operations | $ | (0.10 | ) | $ | (3.53 | ) | $ | (0.41 | ) | $ | (0.47 | ) | $ | 0.31 | |||
| Discontinued Operations | -- | -- | -- | 0.72 | (0.05 | ) | |||||||||||
| Total | $ | (0.10 | ) | $ | (3.53 | ) | $ | (0.41 | ) | $ | 0.25 | $ | 0.26 | ||||
| Dividends per share | None | None | None | None | None | ||||||||||||
| Total assets | $ | 249,119 | $ | 234,386 | $ | 336,056 | $ | 346,733 | $ | 483,037 | |||||||
| Long-term obligations (a) | $ | 49,336 | $ | 45,662 | $ | 4,269 | $ | 6,063 | $ | 129,980 | |||||||
|
_____________________ |
| (a) | Includes long term debt, other long term obligations and deferred compensation liability. |
| Quarterly Data |
Quarter Ended |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (in thousands, except per share data) |
March 31 |
June 30 |
Sept. 30 |
Dec. 31 | ||||||||||
| 2009 |
(unaudited) |
|||||||||||||
| Revenues | $ | 43,612 | $ | 44,156 | $ | 47,283 | $ | 50,279 | ||||||
| Net income (loss) attibutable to HealthTronics, Inc. | $ | 390 | $ | 329 | $ | (2,302 | )(1) | $ | (2,333 | )(1) | ||||
| Per share amounts (basic): | ||||||||||||||
| Net income (loss) | $ | 0.01 | $ | 0.01 | $ | (0.06 | ) | $ | (0.05 | ) | ||||
| Weighted average shares outstanding | 35,892 | 36,006 | 41,043 | 43,491 | ||||||||||
| Per share amounts (diluted): | ||||||||||||||
| Net income (loss) | $ | 0.01 | $ | 0.01 | $ | (0.06 | ) | $ | (0.05 | ) | ||||
| Weighted average shares outstanding | 35,966 | 36,161 | 41,043 | 43,491 | ||||||||||
| 23 |
| Quarterly Data |
Quarter Ended |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (in thousands, except per share data) |
March 31 |
June 30 |
Sept. 30 |
Dec. 31 | ||||||||||
| 2008 |
(unaudited) |
|||||||||||||
| Revenues | $ | 33,954 | $ | 42,580 | $ | 44,771 | $ | 44,637 | ||||||
| Net income (loss) attributable to HealthTronics, Inc. | $ | 452 | $ | 710 | $ | 1,327 | $ | (131,182 | )(2) | |||||
| Per share amounts (basic): | ||||||||||||||
| Net income (loss) | $ | 0.01 | $ | 0.02 | $ | 0.04 | ($ 3.64 | ) | ||||||
| Weighted average shares outstanding | 35,425 | 37,059 | 37,503 | 36,004 | ||||||||||
| Per share amounts (diluted): | ||||||||||||||
| Net income (loss) | $ | 0.01 | $ | 0.02 | $ | 0.04 | ($ 3.64 | ) | ||||||
| Weighted average shares outstanding | 35,425 | 37,165 | 37,604 | 36,004 | ||||||||||
|
_____________________ |
|
(1) In the third quarter of 2009, in connection with our acquisition of Endocare, we booked approximately $3.9
million in acquisition related costs which are required to be expensed when incurred beginning in 2009. In the
fourth quarter of 2009, we booked an additional $4.8 million in costs related to the Endocare acquisition and the
restructuring of our partnerships as noted in the discussion of government regulation and supervision in Item 1. |
ITEM 7.
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION
AND RESULTS OF OPERATIONS
|
Forward-Looking Statements |
| 24 |
|
Statements that are predictive in nature, that depend upon or refer to future events or conditions, or that include words such as “will”, “would”, “should”, “plans”, “likely”, “expects”, “anticipates”, “intends”, “believes”, “estimates”, “thinks”, “may”, and similar expressions, are forward-looking statements. The following important factors, in addition to those discussed under “Risk Factors” under Part I, Item 1, could affect the future results of the health care industry in general, and us in particular, and could cause those results to differ materially from those expressed in such forward-looking statements. |
| • | uncertainties in our establishing or maintaining relationships with physicians and hospitals; | |
| • | the impact of current and future laws and governmental regulations; | |
| • | uncertainties inherent in third party payors’ attempts to limit health care coverages and levels of reimbursement; | |
| • | the effects of competition and technological changes; | |
| • | the availability (or lack thereof) of acquisition or combination opportunities; | |
| • | the integration of acquired business; and | |
| • | general economic, market or business conditions. |
|
General |
| 25 |
|
As the general partner of limited partnerships or the manager of other types of entities, we also provide
services relating to operating our lithotripters, including scheduling, staffing, training, quality assurance,
regulatory compliance, and contracting with payors, hospitals, and surgery centers. |
| 26 |
|
Revenue Recognition |
| • | Fees for urology treatments . A substantial majority of our revenue is derived from fees related to lithotripsy treatments performed using our lithotripters. For lithotripsy and prostate treatment services, we, through our partnerships and other entities, facilitate the use of our equipment and provide other support services in connection with these treatments at hospitals and other health care facilities. The professional fee payable to the physician performing the procedure is generally billed and collected by the physician. We recognize revenue for these services when the services are provided. IGRT technical services are billed monthly and the related revenues are recognized as the related services are provided. | |
| • | Fees for managing the operation of our lithotripters and prostate treatment devices. Through our partnerships and otherwise directly by us, we provide services related to operating our lithotripters and prostate treatment equipment and receive a management fee for performing these services. We recognize revenue for these services as the services are provided. | |
| • | Fees for maintenance services. We provide equipment maintenance services to our partnerships as well as outside parties. These services are billed either on a time and material basis or at a fixed contractual rate, payable monthly, quarterly, or annually. Revenues from these services are recorded when the related maintenance services are performed. | |
| • | Fees for equipment sales, consumable sales and licensing applications. We manufacture and sell medical devices focused on minimally invasive technologies for tissue and tumor ablation through cryosurgery, and their related consumables. We also sell and maintain lithotripters and manufacture and sell consumables related to the lithotripters. We distribute the Revolix laser and consumables related to the laser. With respect to some lithotripter sales, in addition to the original sales price, we receive a licensing fee from the buyer of the lithotripter for each patient treated with such lithotripter. In exchange for this licensing fee, we provide the buyer of the lithotripter with certain consumables. All the sales for equipment and consumables are recognized when the related items are delivered. Revenues from licensing fees are recorded when the patient is treated. In some cases, we lease certain equipment to our partnerships as well as third parties. Revenues from these leases are recognized on a monthly basis or as procedures are performed. | |
| • | Fees for anatomical pathology services. We provide anatomical pathology services primarily to the urology community. Revenues from these services are recorded when the related laboratory procedures are performed. | |
|
Recent Developments |
| 27 |
|
Critical Accounting Policies and Estimates |
| 28 |
|
Year ended December 31, 2009 compared to the year ended December 31, 2008 |
| 29 |
|
Year ended December 31, 2008 compared to the year ended December 31, 2007 |
| 30 |
|
Depreciation and amortization expense increased $1,256,000 in 2008 compared to 2007, primarily due to the
addition of our new AMPI entities and the amortization on our Ocean services agreement. |
| 31 |
|
Accounts receivable as of December 31, 2009 has increased $4,893,000 from December 31, 2008. This increase
relates primarily to our Endocare acquisition, which increased accounts receivable by approximately $3.4 million,
and significant growth across our Uropath and Claripath lab operations, as well as increases related primarily to
higher revenues and the timing of collections. |
| 32 |
|
General |
| Payments due by period |
||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Contractual Obligations |
Total |
Less than 1 year |
1-3 years |
3-5 years |
More than 5 years | |||||||||||||||
| Long Term Debt (1) | $ | 48,519 | $ | 2,556 | $ | 45,442 | $ | 136 | $ | 385 | ||||||||||
| Operating Leases (2) | 11,445 | 3,011 | 4,999 | 2,681 | 754 | |||||||||||||||
| Other contracts (3) | 274 | 148 | 126 | -- | -- | |||||||||||||||
| Total | $ | 60,238 | $ | 5,715 | $ | 50,567 | $ | 2,817 | $ | 1,139 | ||||||||||
|
_____________________ |
| (1) | Represents long term debt as discussed above. |
| (2) | Represents operating leases in the ordinary course of our business. |
| (3) | Represents non-compete obligations of $274, as described above. |
|
In addition, the scheduled principal repayments for all long term debt as of December 31, 2009 are payable as follows: |
| ($ in thousands) |
||||||
|---|---|---|---|---|---|---|
| 2010 | $ | 2,556 | ||||
| 2011 | 1,106 | |||||
| 2012 | 44,336 | |||||
| 2013 | 81 | |||||
| 2014 | 55 | |||||
| Thereafter | 385 | |||||
| Total | $ | 48,519 | ||||
|
Our primary sources of cash are cash flows from operations and borrowings under our senior credit facility. Our
cash flows from operations and therefore our ability to make scheduled payments of principal, or to pay the
interest on, or to refinance, our indebtedness, or to fund planned capital expenditures, will depend on our
future performance, which is subject to general economic, financial competitive, legislative, regulatory and
other factors discussed under “Risk Factors” under Part I. Likewise, our ability to borrow under our
senior credit facility will depend on these factors, which will affect our ability to comply with the covenants
in our facility and our ability to obtain waivers for, or otherwise address, any noncompliance with the terms of
our facility with our lenders. |
| 33 |
|
Inflation |
| 34 |
|
In April 2009, the FASB issued ASC 825, Financial Instruments (“ASC 825”), (formerly FASB Staff
Position 107-1, Interim Disclosures about Fair Value of Financial Instruments). ASC 825 requires disclosures
about fair value of financial instruments for interim reporting periods of publicly traded companies as well as
in annual financial statements. This standard also requires those disclosures in summarized financial information
at interim reporting periods beginning after March 15, 2009. The adoption of this ASC did not affect our
consolidated financial position, results of operations, or cash flows. |
| 35 |
|
In December 2007, the FASB issued accounting guidance contained within ASC 810, Consolidation (“ASC 810-10-65”), (formerly SFAS No. 160, Noncontrolling Interests in Consolidated Financial Statements) regarding noncontrolling interests. ASC 810-10-65 establishes accounting and reporting standards for the noncontrolling interest in a subsidiary and for the deconsolidation of a subsidiary. It clarifies that a noncontrolling interest in a subsidiary is an ownership interest in the consolidated entity that should be reported as equity in the consolidated financial statements. The adoption of ASC 810-10-65 revised our presentation of consolidated financial statements and further impact will continue to be dependent on our future changes in ownership in subsidiaries after the effective date. |
| 36 |
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
|
Interest Rate Risk |
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
|
The information required by this item is contained in Appendix A attached hereto. |
ITEM 9.
CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON
ACCOUNTING AND FINANCIAL DISCLOSURE
|
None. |
ITEM 9A. CONTROLS AND PROCEDURES
|
(a) Disclosure Controls and Procedures |
| 37 |
|
Management’s assessment of and conclusion on the effectiveness of internal control over financial reporting
did not include the internal controls of Endocare, Inc., which is included in the 2009 consolidated financial
statements of HealthTronics, Inc. and constituted 8% of total assets as of December 31, 2009 and 4% of revenues
for the year then ended. |
| 38 |
|
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM |
|
The Board of Directors and Shareholders |
/s/ Ernst & Young LLP |
|
Austin, Texas |
| 39 |
| PART III |
|---|
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
|
The information required by this item will be contained in our definitive proxy statement to be filed in connection with our 2010 annual meeting of stockholders, except for the information regarding our executive officers, which is presented in Part I of this Form 10-K. The information required by this item contained in our definitive proxy statement is incorporated herein by reference. |
ITEM 11. EXECUTIVE COMPENSATION
|
The information required by this item will be contained in our definitive proxy statement to be filed in connection with our 2010 annual meeting of stockholders and is incorporated herein by reference. |
ITEM 12.
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND
MANAGEMENT AND RELATED STOCKHOLDER MATTERS
|
The information required by this item will be contained in our definitive proxy statement to be filed in connection with our 2010 annual meeting of stockholders and is incorporated herein by reference. |
ITEM 13.
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR
INDEPENDENCE
|
The information required by this item will be contained in our definitive proxy statement to be filed in connection with our 2010 annual meeting of stockholders and is incorporated herein by reference. |
ITEM 14. PRINCIPAL ACCOUNTANT FEES AND SERVICES
|
The information required by this item will be contained in our definitive proxy statement to be filed in connection with our 2010 annual meeting of stockholders and is incorporated herein by reference. |
| 40 |
| PART IV |
|---|
ITEM 15. EXHIBITS AND FINANCIAL STATEMENT SCHEDULES
|
(a) 1. Financial Statements. The information required by this item is contained in Appendix A attached hereto. |
(b) Exhibits. (1)
| 3.1 3.2 3.3 4.1 4.2 4.3 10.1 10.2 10.3* 10.4* 10.5* 10.6* 10.7* 10.8* 10.9* 10.10* 10.11* |
Amended and Restated Articles of Incorporation of the Company (incorporated by reference to Annex D to the Rule 424(b)(3) joint proxy statement/prospectus, dated October 6, 2004, filed by HealthTronics with the SEC on October 7, 2004). Amended and Restated Bylaws of the Company (incorporated by reference to Annex E to the Rule 424(b)(3) joint proxy statement/prospectus, dated October 6, 2004, filed by HealthTronics with the SEC on October 7, 2004). First Amendment to Bylaws of HealthTronics, Inc. (incorporated by reference to Exhibit 3.1 of HealthTronics’ Current Report Form 8-K filed on December 17, 2007). Specimen of Common Stock Certificate (filed as an Exhibit to the HSS Registration Statement on Form S-4 (Registration No. 33-56900)). Form of Series A Warrant (incorporated by reference to Exhibit 4.1 to Endocare, Inc.’s Current Report on Form 8-K filed on March 16, 2005). Form of Series B Warrant (incorporated by reference to Exhibit 4.2 to Endocare, Inc.’s Current Report on Form 8-K filed on March 16, 2005). Form of Indemnification Agreement dated October 11, 1993 between the Company and certain of its officers and directors (filed as an Exhibit to the Current Report on Form 8-K of Prime dated October 18, 1993). Release and Severance Agreement dated December 30, 2001 by and between Prime Medical Services Inc. and Kenneth S. Shifrin (filed as an Exhibit to the Annual Report on Form 10-K of Prime for the year ended December 31, 2001). Amended and Restated 1993 Stock Option Plan, as amended June 18, 2002 (filed as an Exhibit to the Annual Report on Form 10-K of Prime for the year ended December 31, 2002). Prime Medical Services, Inc., 2003 Stock Option Plan (incorporated by reference to Annex D of the Rule 424(b)(3) joint proxy statement/prospectus, dated January 7, 2004, filed by Prime with the SEC on January 8, 2004). HealthTronics 2004 Equity Incentive Plan (incorporated by reference to Exhibit 10.2 to HealthTronics’ Current Report on Form 8-K filed on January 25, 2005). Form of Board Service and Release Agreement by and between HealthTronics and Argil J. Wheelock, M.D. (filed as Exhibit 10.22 to the HSS Registration Statement on Form S-4 (Registration No. 333-117102)). Board Service, Amendment and Release Agreement by and between HealthTronics and Kenneth S. Shifrin (incorporated by reference to the HSS Registration Statement on Form S-4 (Registration No. 333-117102)). HSS Stock Option Plan - 2002 (incorporated by reference to Exhibit 10.1 of HealthTronics’ Current Report on Form 8-K filed on January 25, 2005). HSS Stock Option Plan - 2001 (incorporated by reference to Appendix A to HSS’ proxy statement filed with the SEC on April 18, 2001). HSS Stock Option Plan - 2000 (incorporated by reference to Appendix A to HSS’ proxy statement filed with the SEC on April 25, 2000). First Amendment to the HealthTronics, Inc. 2004 Equity Incentive Plan (incorporated by reference to Exhibit 10.1 to HealthTronics’ Quarterly Report on Form 10-Q for the quarter ended June 30, 2005, filed on August 5, 2005). |
| 41 |
|
10.12 10.13* 10.14* 10.15 10.16 10.17* 10.18 10.19 10.20 10.21* 10.22 10.23* 10.24* |
Form of Indemnification Agreement for directors and certain officers of HealthTronics (incorporated by reference to Exhibit 99.1 to HealthTronics’ Current Report on Form 8-K filed on June 1, 2005). First Amendment to Board Service and Release Agreement, dated as of March 2, 2006, by and between HealthTronics and Argil J. Wheelock, M.D. (incorporated by reference to Exhibit 10.1 of HealthTronics’ Current Report on Form 8-K filed on March 8, 2006). Second Amendment to the Company’s 2004 Equity Incentive Plan (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed with the Securities and Exchange Commission on June 14, 2006). Amended and Restated Distribution Agreement, dated as of February 28, 2007, by and among HealthTronics, Inc., Lisa Laser USA, Inc., and LISA laser products OHG (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed with the Securities and Exchange Commission on March 6, 2007). Stock Purchase Agreement, dated as of March 18, 2008, by and among HealthTronics, Inc., Litho Management, Inc., Advanced Medical Partners, Inc. and the stockholders of Advanced Medical Partners, Inc. (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed with the Securities and Exchange Commission on March 20, 2008). Third Amendment to the 2004 Equity Incentive Plan (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed with the Securities and Exchange Commission on May 14, 2008). Earnest Money Contract—Commercial Improved Property (Office Condominiums) dated as of April 1, 2008 (as amended on each of April 15, 2008 and May 12, 2008) by and between HealthTronics, Inc. and HPI Acquisition Company, LLC. (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed with the Securities and Exchange Commission on June 20, 2008). Lease Agreement dated May 19, 2008, by and between HealthTronics, Inc. and HEP- Davis Spring, L.P. (incorporated by reference to Exhibit 10.2 to the Company’s Current Report on Form 8-K filed with the Securities and Exchange Commission on June 20, 2008). Stock Purchase Agreement, dated as of October 10, 2008, by and between HealthTronics, Inc. and Atlantic Urological Associates, P.A (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed with the Securities and Exchange Commission on October 15, 2008). Executive Employment Agreement, effective August 15, 2007, by and between HealthTronics, Inc. and Clayton H. Duncan (incorporated by reference to Exhibit 10.35 of the Company’s 10-K filed with the Securities and Exchange Commission on March 10, 2009). Agreement and Plan of Merger, dated as of June 7, 2009, by and among Endocare, Inc., HT Acquisition, Inc., and HealthTronics, Inc. (incorporated by reference to Exhibit 2.1 to the Company’s Current Report on Form 8-K filed with the Securities and Exchange Commission on June 8, 2009). Termination and Consulting Agreement, dated June 16, 2009, by and between HealthTronics, Inc. and Robert A. Yonke (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed with the Securities and Exchange Commission on June 17, 2009). Executive Employment Agreement, effective July 30, 2009, by and between HealthTronics, Inc. and Clint B. Davis (incorporated by reference to Exhibit 10.1 of the Company’s 10-Q filed with the Securities and Exchange Commission on November 6, 2009). |
| 42 |
|
10.25 10.26* 10.27* 10.28 10.29* 10.30* 10.31* 10.32* 10.33* 10.34* 21.1 23.1 31.1 31.2 32.1 32.2 |
Second Amendment to Lease Agreement, dated as of August 20, 2009, between HEP-Davis Spring, L.P. as landlord and HealthTronics, Inc. as tenant (incorporated by reference to Exhibit 10.2 of the Company’s 10-Q filed with the Securities and Exchange Commission on November 6, 2009). Amended and Restated Executive Employment Agreement, dated as of November 5, 2009, by and between HealthTronics, Inc. and James Whittenburg (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed with the Securities and Exchange Commission on November 5, 2009). Executive Employment Agreement, dated as of November 30, 2009, by and between HealthTronics, Inc. and Richard A. Rusk (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed with the Securities and Exchange Commission on November 30, 2009). Credit Agreement, dated as of December 29, 2009, among HealthTronics, Inc., the lenders party thereto, JPMorgan Chase Bank, National Association, as Administrative Agent, J.P. Morgan Securities, Inc., as Arranger, and Bank of America, N.A., as Syndication Agent (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed with the Securities and Exchange Commission on December 29, 2009). Executive Employment Agreement, effective November 23, 2009, by and between HealthTronics, Inc. and Scott Herz (filed herewith). Executive Employment Agreement, effective December 21, 2009, by and between HealthTronics, Inc. and Laura Miller (filed herewith). Form of Incentive Stock Option Agreement (filed herewith). Form of Nonstatutory Stock Option Agreement (filed herewith). Form of Restricted Stock Award Agreement (filed herewith). Form of Director Restricted Stock Award Agreement (filed herewith). List of subsidiaries of the Company (filed herewith). Consent of Independent Registered Public Accounting Firm (filed herewith). Certification of Chief Executive Officer (filed herewith). Certification of Chief Financial Officer (filed herewith). Certification of Chief Executive Officer (filed herewith). Certification of Chief Financial Officer (filed herewith). |
|
_____________________ |
| (1) | The exhibits listed above will be furnished to any security holder upon written request for such exhibit to Richard A. Rusk, HealthTronics, Inc., 9825 Spectrum Drive, Austin, Texas 78717. The Securities and Exchange Commission (the “SEC”) maintains a website that contains reports, proxy and information statements and other information regarding registrants that file electronically with the SEC at “http://www.sec.gov”. |
| 43 |
|
SIGNATURES |
|
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized. |
| |
HEALTHTRONICS, INC. By: /s/ James S. B. Whittenburg James S. B. Whittenburg, Chief Executive Officer and President (Principal Executive Officer) Date: March 8, 2010 |
|
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated. |
|
By: Date: By: Date: By: Date: By: Date: By: Date: By: Date: |
/s/
James S. B. Whittenburg
James S. B. Whittenburg, Chief Executive Officer and President (Principal Executive Officer) and Director March 8, 2010 /s/ Richard A. Rusk Richard A. Rusk, Chief Financial Officer (Principal Financial and Accounting Officer) March 8, 2010 /s/ R. Steven Hicks R. Steven Hicks, Non-executive Chairman of the Board March 8, 2010 /s/ Donny R. Jackson Donny R. Jackson, Director March 8, 2010 /s/ Timothy J. Lindgren Timothy J. Lindgren, Director March 8, 2010 /s/ Ken S. Shifrin Ken S. Shifrin, Director March 8, 2010 |
| 44 |
|
By: Date: |
/s/
Argil J. Wheelock, M.D.
Argil J. Wheelock, M.D., Director March 8, 2010 |
| 45 |
APPENDIX A |
|
INDEX |
| Page | |
|---|---|
|
Report of Independent Registered Public Accounting Firm Consolidated Financial Statements: Consolidated Statements of Income for the years ended December 31, 2009, 2008 and 2007. Consolidated Balance Sheets at December 31, 2009 and 2008. Consolidated Statements of Stockholders’ Equity for the years ended December 31, 2009, 2008 and 2007. Consolidated Statements of Cash Flows for the years ended December 31, 2009, 2008 and 2007. Notes to Consolidated Financial Statements. | A-2 A-3 A-4 A-6 A-7 A-10 |
| A-1 |
|
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM |
|
The Board of Directors and Stockholders /s/: Ernst & Young LLP
Austin, Texas |
| A-2 |
|
HEALTHTRONICS, INC. AND SUBSIDIARIES CONSOLIDATED STATEMENTS OF INCOME |
|---|
| ($ in thousands, except per share data) |
Years Ended December 31, |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2009 |
2008 |
2007 | |||||||||
| Revenues | $ | 185,330 | $ | 165,942 | $ | 140,418 | |||||
| Cost of revenues (exclusive of depreciation and | |||||||||||
| amortization shown separately below) | 90,660 | 75,679 | 64,715 | ||||||||
| Gross profit | 94,670 | 90,263 | 75,703 | ||||||||
| Operating expenses: | |||||||||||
| Selling, general and administrative | 24,046 | 20,006 | 15,884 | ||||||||
| Impairment charges | -- | 144,000 | 20,800 | ||||||||
| Depreciation and amortization | 14,544 | 12,363 | 11,107 | ||||||||
| Total operating expenses | 38,590 | 176,369 | 47,791 | ||||||||
| Operating income (loss) | 56,080 | (86,106 | ) | 27,912 | |||||||
| Other income (expenses): | |||||||||||
| Interest and dividends | 101 | 1,233 | 1,146 | ||||||||
| Interest expense | (1,576 | ) | (1,077 | ) | (829 | ) | |||||
| (1,475 | ) | 156 | 317 | ||||||||
| Income (loss) from continuing operations before provision | |||||||||||
| for income taxes | 54,605 | (85,950 | ) | 28,229 | |||||||
| Provision for income taxes | 2,837 | (11,516 | ) | (2,854 | ) | ||||||
| Consolidated net income (loss) from continuing operations | 51,768 | (74,434 | ) | 31,083 | |||||||
| Loss from discontinued operations, net of tax | -- | -- | (147 | ) | |||||||
| Consolidated net income (loss) | 51,768 | (74,434 | ) | 30,936 | |||||||
| Less: Net income attributable to noncontrolling interest | (55,684 | ) | (54,259 | ) | (45,568 | ) | |||||
| Net loss attributable to HealthTronics, Inc. | $ | (3,916 | ) | $ | (128,693 | ) | $ | (14,632 | ) | ||
| Basic earnings per share attributable to HealthTronics, Inc.: | |||||||||||
| Loss from continuing operations | $ | (0.10 | ) | $ | (3.53 | ) | $ | (0.41 | ) | ||
| Loss from discontinued operations | -- | -- | -- | ||||||||
| Net loss attributable to HealthTronics, Inc. | $ | (0.10 | ) | $ | (3.53 | ) | $ | (0.41 | ) | ||
| Weighted average shares outstanding | 39,135 | 36,499 | 35,421 | ||||||||
| Diluted earnings per share attributable to HealthTronics, Inc.: | |||||||||||
| Loss from continuing operations | $ | (0.10 | ) | $ | (3.53 | ) | $ | (0.41 | ) | ||
| Loss from discontinued operations | -- | -- | -- | ||||||||
| Net loss attributable to HealthTronics, Inc. | $ | (0.10 | ) | $ | (3.53 | ) | $ | (0.41 | ) | ||
| Weighted average shares outstanding | 39,135 | 36,499 | 35,421 | ||||||||
|
See accompanying notes to consolidated financial statements. |
|
A-3 |
|
HEALTHTRONICS, INC. AND SUBSIDIARIES
CONSOLIDATED BALANCE SHEETS |
| December 31, |
||||||||
|---|---|---|---|---|---|---|---|---|
| ($ in thousands) |
2009 |
2008 | ||||||
| ASSETS | ||||||||
| Current assets: | ||||||||
| Cash and cash equivalents | $ | 8,412 | $ | 22,854 | ||||
| Accounts receivable, less allowance for doubtful | ||||||||
| accounts of $2,722 in 2009 and $2,485 in 2008 | 32,580 | 27,687 | ||||||
| Other receivables | 1,207 | 1,410 | ||||||
| Prepaid expenses and other current assets | 3,306 | 2,895 | ||||||
| Inventory | 12,498 | 8,843 | ||||||
| Total current assets | 58,003 | 63,689 | ||||||
| Property and equipment: | ||||||||
| Equipment, furniture and fixtures | 60,696 | 55,050 | ||||||
| Building and leasehold improvements | 9,139 | 8,254 | ||||||
| 69,835 | 63,304 | |||||||
| Less accumulated depreciation and | ||||||||
| amortization | (36,954 | ) | (30,535 | ) | ||||
| Property and equipment, net | 32,881 | 32,769 | ||||||
| Other investments | 1,850 | 1,819 | ||||||
| Goodwill | 103,282 | 93,620 | ||||||
| Intangible assets, net | 48,993 | 40,278 | ||||||
| Other noncurrent assets | 4,110 | 2,211 | ||||||
| $ | 249,119 | $ | 234,386 | |||||
|
See accompanying notes to consolidated financial statements. |
|
A-4 |
|
HEALTHTRONICS, INC. AND SUBSIDIARIES
CONSOLIDATED BALANCE SHEETS (continued) |
| ($ in thousands, except share data) |
December 31, |
|||||||
|---|---|---|---|---|---|---|---|---|
| 2009 |
2008 | |||||||
| LIABILITIES | ||||||||
| Current liabilities: | ||||||||
| Current portion of long-term debt | $ | 2,556 | $ | 2,490 | ||||
| Accounts payable | 4,679 | 6,468 | ||||||
| Accrued expenses | 13,130 | 9,316 | ||||||
| Total current liabilities | 20,365 | 18,274 | ||||||
| Long-term debt, net of current portion | 45,963 | 43,897 | ||||||
| Other long term obligations | 3,373 | 1,765 | ||||||
| Deferred income taxes | 6,002 | 3,355 | ||||||
| Total liabilities | 75,703 | 67,291 | ||||||
| STOCKHOLDERS' EQUITY | ||||||||
| Preferred stock, $.01 par value, 30,000,000 shares authorized: none outstanding | ||||||||
| Common stock, no par value, 70,000,000 shares authorized: 47,556,430 shares | ||||||||
| issued and 45,584,108 shares outstanding in 2009 and 39,494,314 shares | ||||||||
| issued and 37,618,206 shares outstanding in 2008 | 226,722 | 211,667 | ||||||
| Accumulated deficit | (91,768 | ) | (87,852 | ) | ||||
| Treasury stock, at cost, 1,972,322 shares in 2009 and 1,876,108 shares in 2008 | (4,564 | ) | (4,443 | ) | ||||
| Total HealthTronics, Inc. shareholders' equity | 130,390 | 119,372 | ||||||
| Noncontrolling interest | 43,026 | 47,723 | ||||||
| Total Equity | 173,416 | 167,095 | ||||||
| $ | 249,119 | $ | 234,386 | |||||
|
See accompanying notes to consolidated financial statements. |
|
A-5 |
|
HEALTHTRONICS, INC. AND SUBSIDIARIES CONSOLIDATED STATEMENTS OF STOCKHOLDERS' EQUITY For the years ended December 31, 2009, 2008 and 2007 |
|---|
| ($ in thousands, except share data) | Issued Common Stock |
Treasury Stock |
|||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Shares |
Amount |
Accumulated Earnings (Deficit) |
Shares |
Amount |
Non- Controlling Interest |
Total | |||||||||||||||||
| Balance, December 31, 2006 | 35,475,236 | $ | 200,941 | $ | 55,473 | (95,405 | ) | $ | (897 | ) | $ | 30,104 | $ | 285,621 | |||||||||
| Net Income (loss) | -- | -- | (14,632 | ) | -- | -- | 45,568 | 30,936 | |||||||||||||||
| Distributions paid to noncontrolling interest | -- | -- | -- | -- | -- | (35,210 | ) | (35,210 | ) | ||||||||||||||
| Sale of subsidiary interest to | |||||||||||||||||||||||
| noncontrolling interest | -- | -- | -- | -- | -- | 3,253 | 3,253 | ||||||||||||||||
| Purchase of subsidiary interest from | |||||||||||||||||||||||
| noncontrolling interest | -- | -- | -- | -- | -- | (2,062 | ) | (2,062 | ) | ||||||||||||||
| Contribution of treasury stock | -- | -- | -- | 45,266 | 425 | -- | 425 | ||||||||||||||||
| Share-based compensation | 135,000 | 1,108 | -- | -- | -- | -- | 1,108 | ||||||||||||||||
| Balance, December 31, 2007 | 35,610,236 | 202,049 | 40,841 | (50,139 | ) | (472 | ) | 41,653 | 284,071 | ||||||||||||||
| Net Income (loss) | -- | -- | (128,693 | ) | -- | -- | 54,259 | (74,434 | ) | ||||||||||||||
| Distributions paid to noncontrolling interest | -- | -- | -- | -- | -- | (53,626 | ) | (53,626 | ) | ||||||||||||||
| Sale of subsidiary interest to | |||||||||||||||||||||||
| noncontrolling interest | -- | -- | -- | -- | -- | 6,725 | 6,725 | ||||||||||||||||
| Purchase of subsidiary interest from | |||||||||||||||||||||||
| noncontrolling interest | -- | -- | -- | -- | -- | (1,288 | ) | (1,288 | ) | ||||||||||||||
| Issuance of stock for acquisitions | 1,987,486 | 6,737 | -- | -- | -- | -- | 6,737 | ||||||||||||||||
| Purchase of treasury stock | -- | -- | -- | (1,763,580 | ) | (3,971 | ) | -- | (3,971 | ) | |||||||||||||
| Share-based compensation | 1,896,592 | 2,881 | -- | (62,389 | ) | -- | -- | 2,881 | |||||||||||||||
| Balance, December 31, 2008 | 39,494,314 | 211,667 | (87,852 | ) | (1,876,108 | ) | (4,443 | ) | 47,723 | 167,095 | |||||||||||||
| Net Income (loss) | -- | -- | (3,916 | ) | -- | -- | 55,684 | 51,768 | |||||||||||||||
| Distributions paid to noncontrolling interest | -- | -- | -- | -- | -- | (62,388 | ) | (62,388 | ) | ||||||||||||||
| Sale of subsidiary interest to | |||||||||||||||||||||||
| noncontrolling interest | -- | -- | -- | -- | -- | 5,324 | 5,324 | ||||||||||||||||
| Purchase of subsidiary interest from | |||||||||||||||||||||||
| noncontrolling interest | -- | (1,162 | ) | -- | -- | -- | (3,317 | ) | (4,479 | ) | |||||||||||||
| Issuance of stock for acquisitions | 7,398,396 | 13,217 | -- | -- | -- | -- | 13,217 | ||||||||||||||||
| Purchase of treasury stock | -- | -- | -- | (51,714 | ) | (121 | ) | -- | (121 | ) | |||||||||||||
| Share-based compensation | 663,720 | 3,000 | -- | (44,500 | ) | -- | -- | 3,000 | |||||||||||||||
| Balance, December 31, 2009 | 47,556,430 | $ | 226,722 | $ | (91,768 | ) | (1,972,322 | ) | $ | (4,564 | ) | $ | 43,026 | $ | 173,416 | ||||||||
|
See accompanying notes to consolidated financial statements. |
|
A-6 |
|
HEALTHTRONICS, INC. AND SUBSIDIARIES CONSOLIDATED STATEMENTS OF CASH FLOWS |
|---|
| Years Ended December 31, |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ($ in thousands) |
2009 |
2008 |
2007 | ||||||||
| CASH FLOWS FROM OPERATING ACTIVITIES: | |||||||||||
| Fee and other revenue collected | $ | 187,828 | $ | 167,865 | $ | 144,821 | |||||
| Cash paid to employees, suppliers of goods and others | (112,618 | ) | (95,939 | ) | (82,361 | ) | |||||
| Cash paid for acquisition related costs | (10,458 | ) | -- | -- | |||||||
| Interest received | 101 | 1,233 | 1,146 | ||||||||
| Interest paid | (1,517 | ) | (990 | ) | (835 | ) | |||||
| Taxes paid | (1,396 | ) | (1,324 | ) | (538 | ) | |||||
| Discontinued operations | -- | -- | (356 | ) | |||||||
| Net cash provided by operating activities | 61,940 | 70,845 | 61,877 | ||||||||
| CASH FLOWS FROM INVESTING ACTIVITIES: | |||||||||||
| Purchase of entities, net of cash acquired | (3,528 | ) | (49,487 | ) | (11,829 | ) | |||||
| Purchases of equipment and leasehold improvements | (11,702 | ) | (11,779 | ) | (9,469 | ) | |||||
| Proceeds from sales of assets | 1,125 | 9,165 | 1,224 | ||||||||
| Other | (31 | ) | (210 | ) | (18 | ) | |||||
| Discontinued operations | -- | -- | 1,335 | ||||||||
| Net cash used in investing activities | (14,136 | ) | (52,311 | ) | (18,757 | ) | |||||
| CASH FLOWS FROM FINANCING ACTIVITIES: | |||||||||||
| Borrowings on notes payable | 12,997 | 51,747 | 2,546 | ||||||||
| Payments on notes payable, exclusive of interest | (10,986 | ) | (14,508 | ) | (5,760 | ) | |||||
| Distributions to noncontrolling interest | (62,020 | ) | (53,757 | ) | (42,736 | ) | |||||
| Contributions by noncontrolling interest, net of buyouts | (2,116 | ) | (389 | ) | 389 | ||||||
| Purchase of treasury stock | (121 | ) | (3,971 | ) | -- | ||||||
| Discontinued operations | -- | -- | (20 | ) | |||||||
| Net cash used in financing activities | (62,246 | ) | (20,878 | ) | (45,581 | ) | |||||
| NET DECREASE IN CASH AND CASH EQUIVALENTS | (14,442 | ) | (2,344 | ) | (2,461 | ) | |||||
| Cash and cash equivalents, beginning of period, includes cash | |||||||||||
| from discontinued operations of $(198) for 2007 | 22,854 | 25,198 | 27,659 | ||||||||
| Cash and cash equivalents, end of period | $ | 8,412 | $ | 22,854 | $ | 25,198 | |||||
|
See accompanying notes to consolidated financial statements. |
|
A-7 |
|
HEALTHTRONICS, INC. AND SUBSIDIARIES CONSOLIDATED STATEMENTS OF CASH FLOWS (continued) |
|---|
| ($ in thousands) |
Years Ended December 31, |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2009 |
2008 |
2007 | |||||||||
| Reconciliation of net income (loss) to net cash provided by operating activities: | |||||||||||
| Net income (loss) | $ | 51,768 | $ | (74,434 | ) | $ | 30,936 | ||||
| Adjustments to reconcile net income (loss) to cash provided | |||||||||||
| by operating activities: | |||||||||||
| Depreciation and amortization | 14,544 | 12,363 | 11,107 | ||||||||
| Provision for uncollectible accounts | 522 | 145 | (108 | ) | |||||||
| Provision for deferred income taxes | 2,647 | (12,180 | ) | (624 | ) | ||||||
| Non-cash share-based compensation | 3,000 | 2,881 | 1,534 | ||||||||
| Impairment charges | -- | 144,000 | 20,800 | ||||||||
| Other | 295 | (1,112 | ) | 70 | |||||||
| Discontinued Operations | -- | -- | (117 | ) | |||||||
| Changes in operating assets and liabilities, | |||||||||||
| net of effect of purchase transactions: | |||||||||||
| Accounts receivable | (526 | ) | (1,360 | ) | 1,008 | ||||||
| Other receivables | 250 | 1,399 | (1,124 | ) | |||||||
| Inventory | 3,344 | 242 | 1,282 | ||||||||
| Other assets | 608 | 900 | 744 | ||||||||
| Accounts payable | (5,665 | ) | (1,314 | ) | (579 | ) | |||||
| Accrued expenses | (8,847 | ) | (685 | ) | (3,052 | ) | |||||
| Total adjustments | 10,172 | 145,279 | 30,941 | ||||||||
| Net cash provided by operating activities | $ | 61,940 | $ | 70,845 | $ | 61,877 | |||||
|
See accompanying notes to consolidated financial statements. |
|
A-8 |
|
HEALTHTRONICS, INC. AND SUBSIDIARIES CONSOLIDATED STATEMENTS OF CASH FLOWS (continued) |
|---|
| ($ in thousands) |
Years Ended December 31, |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2009 |
2008 |
2007 | |||||||||
| SUPPLEMENTAL INFORMATION OF NON-CASH | |||||||||||
| INVESTING AND FINANCING ACTIVITIES: | |||||||||||
| At December 31, the Company had accrued distributions payable | |||||||||||
| to noncontrolling interests. The effect of this transaction was as follows: | |||||||||||
| Current liabilities increased by | $ | 40 | $ | 95 | $ | 226 | |||||
| Noncontrolling interest decreased by | 40 | 95 | 226 | ||||||||
| In 2009, the Company acquired three cryosurgical partnerships | |||||||||||
| and a company that manufactures devices, consumables and | |||||||||||
| provides related services for the cryosurgical industry. The net assets | |||||||||||
| and liabilities acquired were as follows: | |||||||||||
| Current assets increased by | 14,766 | -- | -- | ||||||||
| Noncurrent assets increased by | 13,160 | -- | -- | ||||||||
| Goodwill increased by | 9,662 | -- | -- | ||||||||
| Current liabilities increased by | 16,810 | -- | -- | ||||||||
| Other long-term obligations increased by | 1,443 | -- | -- | ||||||||
| In 2008, the Company acquired three lithotripsy partnerships, | |||||||||||
| a cryosurgical company, a pathology lab and an IGRT | |||||||||||
| services operation. The net assets and | |||||||||||
| liabilities acquired were as follows: | |||||||||||
| Current assets increased by | -- | 6,456 | -- | ||||||||
| Noncurrent assets increased by | -- | 3,812 | -- | ||||||||
| Goodwill increased by | -- | 26,995 | -- | ||||||||
| Current liabilities increased by | -- | 4,941 | -- | ||||||||
| Other long-term obligations increased by | -- | 906 | -- | ||||||||
| Noncontrolling interest increased by | -- | 5,825 | -- | ||||||||
| In 2007, the Company acquired two lithotripsy partnerships and sold | |||||||||||
| three lithotripsy partnerships. The net assets and | |||||||||||
| liabilities acquired/sold were as follows: | |||||||||||
| Current assets increased by | -- | -- | 580 | ||||||||
| Noncurrent assets increased by | -- | -- | 1,816 | ||||||||
| Goodwill increased by | -- | -- | 9,044 | ||||||||
| Current liabilities increased by | -- | -- | 380 | ||||||||
| Other long-term obligations decreased by | -- | -- | 354 | ||||||||
| Noncontrolling interest increased by | -- | -- | 802 | ||||||||
|
See accompanying notes to consolidated financial statements. |
|
A-9 |
|
HEALTHTRONICS, INC. AND SUBSIDIARIES NOTES TO CONSOLIDATED FINANCIAL STATEMENTS |
|---|
|
A.
ORGANIZATION AND OPERATION OF THE COMPANY |
|
A-10 |
|
HEALTHTRONICS, INC. AND SUBSIDIARIES NOTES TO CONSOLIDATED FINANCIAL STATEMENTS – (Continued) |
|---|
|
We are headquartered in Austin, Texas and provide urology services in approximately 46 states. |
|
A-11 |
|
HEALTHTRONICS, INC. AND SUBSIDIARIES NOTES TO CONSOLIDATED FINANCIAL STATEMENTS – (Continued) |
|---|
|
Impairment of long-lived assets |
|
A-12 |
|
HEALTHTRONICS, INC. AND SUBSIDIARIES NOTES TO CONSOLIDATED FINANCIAL STATEMENTS – (Continued) |
|---|
|
We provide anatomical pathology services primarily to the urology market place. Revenues from these services are
recorded when the related laboratory procedures are performed. IGRT technical services are billed monthly and
the related revenues are recognized as the related services are provided. |
|
A-13 |
|
HEALTHTRONICS, INC. AND SUBSIDIARIES NOTES TO CONSOLIDATED FINANCIAL STATEMENTS – (Continued) |
|---|
| ($ in thousands) |
Balance at Beginning of Year |
Costs and Expenses |
Deductions |
Other |
Balance at End of Year | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Allowance for Doubtful Accounts: |
||||||||||||||||||||
| 2009 | $ | 2,485 | $ | 771 | $ | (249 | ) | $ | (285 | ) | $ | 2,722 | ||||||||
| 2008 | $ | 2,368 | $ | 285 | $ | (140 | ) | $ | (28 | ) | $ | 2,485 | ||||||||
| 2007 | $ | 2,166 | $ | 329 | $ | (437 | ) | $ | 310 | $ | 2,368 | |||||||||
|
Inventory |
| ($ in thousands) |
December 31, 2009 |
December 31, 2008 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Raw Materials | $ | 7,381 | $ | 5,993 | |||||||
| Work in process | 414 | -- | |||||||||
| Finished Goods | 4,703 | 2,850 | |||||||||
| $ | 12,498 | $ | 8,843 | ||||||||
|
Stock-Based Compensation |
|
A-14 |
|
HEALTHTRONICS, INC. AND SUBSIDIARIES NOTES TO CONSOLIDATED FINANCIAL STATEMENTS – (Continued) |
|---|
|
Debt Issuance Costs |
| (In thousands, except per share data) |
Net Loss |
Wtd. Avg. No. of Shares |
Per Share Amounts | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| For the year ended December 31, 2009 | ||||||||||||||
| Basic | $ | (3,916 | ) | 39,135 | $ | (0.10 | ) | |||||||
| Effect of dilutive securities: | ||||||||||||||
| Options and non-vested shares | -- | -- | ||||||||||||
| Diluted | $ | (3,916 | ) | 39,135 | $ | (0.10 | ) | |||||||
| For the year ended December 31, 2008 | ||||||||||||||
| Basic | $ | (128,693 | ) | 36,499 | $ | (3.53 | ) | |||||||
| Effect of dilutive securities: | ||||||||||||||
| Options and non-vested shares | -- | -- | ||||||||||||
| Diluted | $ | (128,693 | ) | 36,499 | $ | (3.53 | ) | |||||||
| For the year ended December 31, 2007 | ||||||||||||||
| Basic | $ | (14,632 | ) | 35,421 | $ | (0.41 | ) | |||||||
| Effect of dilutive securities: | ||||||||||||||
| Options and non-vested shares | -- | -- | ||||||||||||
| Diluted | $ | (14,632 | ) | 35,421 | $ | (0.41 | ) | |||||||
|
Unexercised employee stock options and non-vested shares to purchase 3,699,000, 2,783,000 and 3,329,000 shares of our common stock as of December 31, 2009, 2008 and 2007, respectively, were not included in the computations of diluted EPS because the exercise prices were greater than the average market price of our common stock during the respective periods or we had a net loss in the respective period. |
|
A-15 |
|
HEALTHTRONICS, INC. AND SUBSIDIARIES NOTES TO CONSOLIDATED FINANCIAL STATEMENTS – (Continued) |
|---|
|
Recently Issued Pronouncements |
|
A-16 |
|
HEALTHTRONICS, INC. AND SUBSIDIARIES NOTES TO CONSOLIDATED FINANCIAL STATEMENTS – (Continued) |
|---|
|
In April 2009, the FASB issued ASC 825, Financial Instruments (“ASC 825”), (formerly FASB Staff
Position 107-1, Interim Disclosures about Fair Value of Financial Instruments). ASC 825 requires disclosures
about fair value of financial instruments for interim reporting periods of publicly traded companies as well as
in annual financial statements. This standard also requires those disclosures in summarized financial information
at interim reporting periods beginning after March 15, 2009. The adoption of this ASC did not affect our
consolidated financial position, results of operations, or cash flows. |
|
A-17 |
|
HEALTHTRONICS, INC. AND SUBSIDIARIES NOTES TO CONSOLIDATED FINANCIAL STATEMENTS – (Continued) |
|---|
|
C. GOODWILL AND OTHER INTANGIBLE ASSETS
|
| (in thousands) |
Total | |||||||
|---|---|---|---|---|---|---|---|---|
| Balance, December 31, 2007 | $ | 217,505 | ||||||
| Additions | 26,995 | |||||||
| Deletions | (6,880 | ) | ||||||
| Impairments | (144,000 | ) | ||||||
| Balance, December 31, 2008 | $ | 93,620 | ||||||
| Additions | 9,662 | |||||||
| Deletions | -- | |||||||
| Impairments | -- | |||||||
| Balance, December 31, 2009 | $ | 103,282 | ||||||
|
In the fourth quarter of 2008, in connection with our annual goodwill impairment test, we recorded a goodwill
impairment to our urology services reporting unit totaling $144 million. Although our core operations remain
stable and reflect growth over the prior year, we adjusted certain assumptions in our discounted cash flow model
to address the recent declines in our market capitalization, which had fallen significantly below our
consolidated net assets. In addition, the market comparables component of our impairment test was negatively
impacted by the current global economic crisis and global decline in the stock markets. |
|
A-18 |
|
HEALTHTRONICS, INC. AND SUBSIDIARIES NOTES TO CONSOLIDATED FINANCIAL STATEMENTS – (Continued) |
|---|
|
Our discounted cash flow projections for each reporting unit were based on five-year financial forecasts. The
five-year forecasts and all amounts and assumptions used in preparing the financial forecasts were based on
annual financial forecasts developed internally by management for use in managing our business. Our independent
valuation firm utilized this information to assist us in calculating the fair value of the company. For our
December 31, 2009 forecast, the significant assumptions of these five-year forecasts included annual revenue
growth rates ranging from 3.5% to 8.2% and from 5.0% to 116% for the urology services and anatomical pathology
reporting units, respectively. The future cash flows were discounted to present value using a mid-year
convention and a discount rate of 13% for the urology services and 15.0% for the anatomical pathology reporting
unit. Terminal values for the reporting units were calculated using a Gordon growth methodology with a long-term
growth rate of 3.0% for the urology services reporting unit and 4.0% for the anatomical pathology reporting
unit. The future terminal values of the urology services and anatomical pathology reporting units were $253
million and $39 million respectively at December 31, 2009. |
|
A-19 |
|
HEALTHTRONICS, INC. AND SUBSIDIARIES NOTES TO CONSOLIDATED FINANCIAL STATEMENTS – (Continued) |
|---|
| Weighted Average Life (years) |
Gross Carrying Amount |
Accumulated Amortization |
Net |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| December 31, 2009 | ||||||||||||||
| Finite life intangibles: | ||||||||||||||
| IGRT services contract | 20 | .0 | $ | 35,245 | $ | 2,195 | $ | 33,050 | ||||||
| Endocare brand name, | ||||||||||||||
| patents, and other | 12 | .8 | 10,845 | 391 | 10,454 | |||||||||
| Non-compete agreements, hospital | ||||||||||||||
| contracts, and patents | 2 | .1 | 3,914 | 2,425 | 1,489 | |||||||||
| Total | $ | 50,004 | $ | 5,011 | $ | 44,993 | ||||||||
| December 31, 2008 | ||||||||||||||
| Finite life intangibles: | ||||||||||||||
| IGRT services contract | 20 | .0 | $ | 35,245 | $ | 617 | $ | 34,628 | ||||||
| Non-compete agreements, hospital | ||||||||||||||
| contracts, and patents | 4 | .3 | 3,751 | 2,101 | 1,650 | |||||||||
| Total | $ | 38,996 | $ | 2,718 | $ | 36,278 | ||||||||
|
Amortization expense for other intangible assets with finite lives was $2,866,000, $1,286,000 and $1,028,000 for the years ended December 31, 2009, 2008 and 2007, respectively. We estimate annual amortization expense for each of the five succeeding fiscal years as follows (in thousands): |
| Year |
Amount | |||||||
|---|---|---|---|---|---|---|---|---|
| 2010 | $ | 3,410 | ||||||
| 2011 | 3,116 | |||||||
| 2012 | 2,913 | |||||||
| 2013 | 2,770 | |||||||
| 2014 | 2,742 | |||||||
| Thereafter | 30,042 | |||||||
|
D. ACQUISITIONS |
|
A-20 |
|
HEALTHTRONICS, INC. AND SUBSIDIARIES NOTES TO CONSOLIDATED FINANCIAL STATEMENTS – (Continued) |
|---|
| (in thousands) |
Endocare | |||||||
|---|---|---|---|---|---|---|---|---|
| Current assets | $ | 14,447 | ||||||
| Property and equipment | 1,506 | |||||||
| Intangible assets | 11,161 | |||||||
| Goodwill | 6,751 | |||||||
| Other noncurrent assets | 72 | |||||||
| Total assets acquired | 33,937 | |||||||
| Current liabilities | 16,630 | |||||||
| Long-term liabilities | 93 | |||||||
| Total liabilities assumed | 16,723 | |||||||
| Net assets acquired | $ | 17,214 | ||||||
|
The factors contributing to the recognition of goodwill are based upon several strategic and synergistic benefits
that are expected to be realized from the combination of HealthTronics and Endocare. These benefits include the
expectation that HealthTronics’ complementary products and technologies will broaden the urology platform
and increase prominence in the urology market and increase HealthTronics’ position as a leading provider of
urology services. HealthTronics also expects to realize substantial synergies and operating efficiencies. The
combination should enable the combined company to operate on a more cost efficient and effective basis with
anticipated cost reductions in administration, facilities, and services. The acquisition provides HealthTronics
broader coverage within the United States, as well as increased scale and scope for further expanding operations
through product development and complementary strategic transactions. |
|
A-21 |
|
HEALTHTRONICS, INC. AND SUBSIDIARIES NOTES TO CONSOLIDATED FINANCIAL STATEMENTS – (Continued) |
|---|
|
On July 15, 2008, we acquired UroPath, LLC (“UroPath”) for $7.5 million in cash. Founded in 2003,
UroPath is a leading provider of anatomical pathology laboratory services in the U.S. with locations in Florida,
Texas, and Pennsylvania. Based on our allocation of the purchase price, we recorded approximately $7.4 million
of goodwill related to this transaction, all of which is tax deductible. |
| ($ in thousands, except per share data) |
2009 |
2008 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total revenues | $ | 197,797 | $ | 207,735 | |||||||
| Total expenses | 207,853 | 340,086 | |||||||||
| Net loss attributable to HealthTronics, Inc. | $ | (10,056 | ) | $ | (132,351 | ) | |||||
| Diluted earnings per share | $ | (0.22 | ) | $ | (2.98 | ) | |||||
|
E. FAIR VALUE OF FINANCIAL INSTRUMENTS |
| 2009 |
2008 |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ( $ in thousands) |
Carrying Amount |
Fair Value |
Carrying Amount |
Fair Value | |||||||||||||
| Financial assets: | |||||||||||||||||
| Cash and cash equivalents | $ | 8,412 | $ | 8,412 | $ | 22,854 | $ | 22,854 | |||||||||
| Warrants/Common Stock | -- | -- | 290 | 290 | |||||||||||||
| Financial liabilities: | |||||||||||||||||
| Debt | $ | 48,519 | $ | 48,519 | $ | 46,387 | $ | 46,387 | |||||||||
| Other long-term obligations | 3,373 | 3,305 | 1,765 | 1,727 | |||||||||||||
|
The following methods and assumptions were used by us in estimating our fair value disclosures for financial
instruments. |
|
A-22 |
|
HEALTHTRONICS, INC. AND SUBSIDIARIES NOTES TO CONSOLIDATED FINANCIAL STATEMENTS – (Continued) |
|---|
|
Cash and Cash Equivalents
|
|
A-23 |
|
HEALTHTRONICS, INC. AND SUBSIDIARIES NOTES TO CONSOLIDATED FINANCIAL STATEMENTS – (Continued) |
|---|
|
F. ACCRUED EXPENSES |
| December 31, |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ($ in thousands) |
2009 |
2008 | |||||||||
| Accrued group insurance costs | $ | 421 | $ | 304 | |||||||
| Compensation and payroll related expense | 7,120 | 3,339 | |||||||||
| Accrued interest | 152 | 94 | |||||||||
| Accrued taxes | 1,024 | 613 | |||||||||
| Accrued professional fees | 246 | 317 | |||||||||
| Unearned revenues | 1,213 | 1,181 | |||||||||
| Deferred Rent | 226 | 396 | |||||||||
| Accrued distributions | 109 | 95 | |||||||||
| Other | 2,619 | 2,977 | |||||||||
| $ | 13,130 | $ | 9,316 | ||||||||
|
G. INDEBTEDNESS |
| ($ in thousands) | December 31, |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Interest Rates |
Maturities |
2009 |
2008 |
||||||||||||||
| Floating | 2009-2012 | $ | 44,591 | $ | 41,528 | ||||||||||||
| 4%-9% | 2009-2012 | 3,928 | 4,859 | ||||||||||||||
| $ | 48,519 | $ | 46,387 | ||||||||||||||
| Less current portion of long-term debt | 2,556 | 2,490 | |||||||||||||||
| $ | 45,963 | $ | 43,897 | ||||||||||||||
|
Senior Credit Facility |
|
A-24 |
|
HEALTHTRONICS, INC. AND SUBSIDIARIES NOTES TO CONSOLIDATED FINANCIAL STATEMENTS – (Continued) |
|---|
|
Other long term debt |
| Year |
Amount | |||||||
|---|---|---|---|---|---|---|---|---|
| 2010 | $ | 2,556 | ||||||
| 2011 | 1,106 | |||||||
| 2012 | 44,336 | |||||||
| 2013 | 81 | |||||||
| 2014 | 55 | |||||||
| Thereafter | 385 | |||||||
|
H. COMMITMENTS AND CONTINGENCIES |
| ($ in thousands) Year |
Amount | |||||||
|---|---|---|---|---|---|---|---|---|
| 2010 | $ | 2,785 | ||||||
| 2011 | 2,327 | |||||||
| 2012 | 2,080 | |||||||
| 2013 | 1,129 | |||||||
| 2014 | 849 | |||||||
| Thereafter | 480 | |||||||
|
I. STOCK BASED COMPENSATION |
|
A-25 |
|
HEALTHTRONICS, INC. AND SUBSIDIARIES NOTES TO CONSOLIDATED FINANCIAL STATEMENTS – (Continued) |
|---|
|
Under ASC 718, nonvested stock awards are awards that the employee has not yet earned the right to sell, and are
subject to forfeiture if the terms of service are not satisfied. These awards should be measured based on the
market prices of otherwise identical (i.e., identical except for the vesting condition) common stock at the grant
date. A nonvested equity share awarded to an employee shall be measured at its fair value as if it were vested
and issued on the grant date. The vesting restrictions are taken into account by recognizing compensation cost
only for awards for which the employee has rendered the requisite service (i.e., vested). |
|
A-26 |
|
HEALTHTRONICS, INC. AND SUBSIDIARIES NOTES TO CONSOLIDATED FINANCIAL STATEMENTS – (Continued) |
|---|
| (a) |
(b) |
(c) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
Plan Category |
Number of shares of our common stock to be issued upon exercise of outstanding options |
Weighted-average exercise price of outstanding options |
Number of shares of our common stock remaining available for future issuance under equity compensation plans (exceeding securities reflected in column (a)) | |||||||||
| Prime 1993 stock option plan | 65,666 | $ | 7 | .79 | -- | |||||||
| Prime 2003 stock option plan | 69,000 | $ | 5 | .61 | -- | |||||||
| HSS equity incentive plan and stock | ||||||||||||
| option plans | 2,653,283 | $ | 5 | .93 | 1,776,237 | |||||||
| Other equity compensation plans | ||||||||||||
| approved by our security holders | N/A | N/A | N/A | |||||||||
|
To calculate the compensation cost that was recognized under ASC 718 for the three years ended December 31, 2009,
2008, and 2007, we used the Black-Scholes option-pricing model with the following weighted-average assumptions
for equity awards granted. For December 31, 2009 and 2007, respectively: risk-free interest rates were 0.65% and
4.6%; dividend yields were 0%; volatility factors of the expected market price of our common stock were 65% and
47%; and a weighted-average expected life of the option of 6 years. There were no options granted in the year
ended December 31, 2008. |
|
A-27 |
|
HEALTHTRONICS, INC. AND SUBSIDIARIES NOTES TO CONSOLIDATED FINANCIAL STATEMENTS – (Continued) |
|---|
|
In 2008, we granted a total of 1,896,592 of non-vested shares under our 2004 Equity Incentive Plan. 170,449 shares vest 25% on each of the first four anniversaries of the grant date. 1,121,993 shares vest based on the achievement of the performance targets outlined below. 604,150 shares vest 25% on each of the first four anniversaries of the grant date; however, their vesting can be accelerated if the following performance targets are reached. 735,136 of the shares that vest per the performance targets below have a two year service requirement regardless of the performance targets being met. |
| Percent of Grant Vesting |
Performance Target (% increase over grant price) | |||||||
|---|---|---|---|---|---|---|---|---|
| 25% | 15 | % | ||||||
| 25% | 30 | % | ||||||
| 25% | 45 | % | ||||||
| 25% | 60 | % | ||||||
|
On May 5, 2008, the first performance target related to one of the grants was met and as a result 92,862 of the
non-vested stock awards vested, which resulted in the recognition of approximately $307,000 in share based
compensation cost. On August 25, 2008, the second performance target was met resulting in the vesting of an
additional 92,862 shares and the recognition of approximately $118,000 in share based compensation cost. |
| 2009 |
2008 |
2007 |
||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Options (000) |
Weighted Average Exercise Price |
Options (000) |
Weighted Average Exercise Price |
Options (000) |
Weighted Average Exercise Price | |||||||||||||||
| Outstanding at beginning of year | 2,559 | $ | 7.03 | 3,194 | $ | 7.07 | 3,908 | $ | 7.32 | |||||||||||
| Granted | 650 | 2.44 | -- | -- | 245 | 5.82 | ||||||||||||||
| Exercised | -- | -- | -- | -- | -- | -- | ||||||||||||||
| Cancelled | (387 | ) | 7.26 | (399 | ) | 7.60 | (552 | ) | 8.07 | |||||||||||
| Forfeited | (34 | ) | 3.98 | (236 | ) | 6.61 | (407 | ) | 7.38 | |||||||||||
| Outstanding at end of year | 2,788 | $ | 5.97 | 2,559 | $ | 7.03 | 3,194 | $ | 7.07 | |||||||||||
| Exercisable at end of year | 2,029 | $ | 7.04 | 2,182 | $ | 7.13 | 2,049 | $ | 7.35 | |||||||||||
| Weighted-average fair value of | ||||||||||||||||||||
| options granted during the period | $1.45 | N/A | $ | 2.99 | ||||||||||||||||
|
During the year ended December 31, 2009, there were no exercises of options to purchase common stock and the
total fair value of shares vested during 2009 was $793,000. During the year ended December 31, 2008, there were
no exercises of options to purchase common stock and the total fair value of shares vested during 2008 was
$1,153,000. During the year ended December 31, 2007, there were no exercises of options to purchase common stock
and the total fair value of shares vested during 2007 was $966,000. |
|
A-28 |
|
HEALTHTRONICS, INC. AND SUBSIDIARIES NOTES TO CONSOLIDATED FINANCIAL STATEMENTS – (Continued) |
|---|
|
Additional information regarding options outstanding for all plans as of December 31, 2009, is as follows: |
| Outstanding Options |
Exercisable Options |
|||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Range of Exercise Prices |
Options (000) |
Weighted Average Remaining Contractual Life |
Weighted Average Exercise Price |
Options (000) |
Weighted Average Remaining Contractual Life |
Weighted Average Exercise Price | ||||||||||||||
| $0.00 - $4.24 | 633 | 9.9 years | $ | 2 | .44 | -- | N/A | $ | -- | |||||||||||
| $4.25 - $6.49 | 254 | 5.2 years | 5 | .87 | 209 | 4.8 years | 5 | .94 | ||||||||||||
| $6.50 - $6.99 | 1,242 | 4.7 years | 6 | .64 | 1,185 | 4.6 years | 6 | .64 | ||||||||||||
| $7.00 - $7.50 | 355 | 5.7 years | 7 | .38 | 331 | 5.7 years | 7 | .39 | ||||||||||||
| $7.51 - $9.50 | 140 | 4.1 years | 7 | .85 | 140 | 4.1 years | 7 | .85 | ||||||||||||
| $9.51 - $13.69 | 164 | 3.3 years | 9 | .93 | 164 | 3.3 years | 9 | .93 | ||||||||||||
| 2,788 | $ | 5 | .97 | 2,029 | $ | 7 | .04 | |||||||||||||
| Aggregate intrinsic value (in thousands) | $ | 127 | $ | -- | ||||||||||||||||
|
The aggregate intrinsic value in the table above is based on our closing stock price of $2.64 per share as of
December 31, 2009. |
| Nonvested Shares |
Shares (000) |
Weighted-Average Grant-Date Fair Value |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Nonvested at January 1, 2007 | -- | $ | -- | ||||||||
| Granted | 135 | 4.75 | |||||||||
| Vested | -- | -- | |||||||||
| Forfeited | -- | -- | |||||||||
| Nonvested at December 31, 2007 | 135 | $ | 4.75 | ||||||||
| Granted | 1,897 | 2.52 | |||||||||
| Vested | (220 | ) | 3.52 | ||||||||
| Forfeited | (62 | ) | 3.22 | ||||||||
| Nonvested at December 31, 2008 | 1,750 | $ | 2.55 | ||||||||
| Granted | 619 | 1.93 | |||||||||
| Vested | (278 | ) | 2.50 | ||||||||
| Forfeited | (50 | ) | 3.34 | ||||||||
| Nonvested at December 31, 2009 | 2,041 | $ | 2.34 | ||||||||
|
J. INCOME TAXES |
|
A-29 |
|
HEALTHTRONICS, INC. AND SUBSIDIARIES NOTES TO CONSOLIDATED FINANCIAL STATEMENTS – (Continued) |
|---|
|
Income tax expense (benefit) consists of the following: |
| Years Ended December 31, |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ($ in thousands) |
2009 |
2008 |
2007 | |||||||||||
| Federal: | ||||||||||||||
| Current | $ | -- | $ | -- | $ | -- | ||||||||
| Deferred | 2,803 | (8,147 | ) | (2,878 | ) | |||||||||
| State: | ||||||||||||||
| Current | 190 | 202 | 212 | |||||||||||
| Deferred | (193 | ) | (3,643 | ) | (401 | ) | ||||||||
| Foreign | ||||||||||||||
| Current | -- | -- | -- | |||||||||||
| Deferred | 37 | 72 | 213 | |||||||||||
| $ | 2,837 | $ | (11,516 | ) | $ | (2,854 | ) | |||||||
|
A reconciliation between the statutory rate and the effective tax rate on income from operations for the years ended December 31, 2009, 2008 and 2007 is as follows: |
| Years Ended December 31, |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2009 |
2008 |
2007 | ||||||||||||
| Stautory Rate | 35.0 | % | 35.0 | % | 35.0 | % | ||||||||
| Foreign jurisdiction statutory income tax rate | 0.1 | % | -- | (0.3 | )% | |||||||||
| State taxes, net of federal tax effect | -- | 2.6 | % | -- | ||||||||||
| Change in valuation allowance | 5.1 | % | (17.3 | )% | -- | |||||||||
| Goodwill impairment | -- | (28.7 | )% | 12.7 | % | |||||||||
| Other | 0.5 | % | 0.2 | % | (1.0 | )% | ||||||||
| Effective tax rate (excluding noncontrolling interests) | 40.7 | % | (8.2 | )% | 46.4 | % | ||||||||
| (Income) Loss attributable to noncontrolling interests | (35.5 | )% | 21.6 | % | (56.5 | )% | ||||||||
| 5.2 | % | 13.4 | % | (10.1 | )% | |||||||||
|
The tax effects of temporary differences that give rise to significant portions of the deferred tax assets and deferred tax liabilities are as follows: |
| December 31, |
||||||||
|---|---|---|---|---|---|---|---|---|
| ($ in thousands) |
2009 |
2008 | ||||||
| Deferred tax assets: | ||||||||
| Net operating loss carryforward | $ | 44,804 | $ | 34,395 | ||||
| Capital loss carryforward | 180 | -- | ||||||
| Allowance for bad debts | 21 | 32 | ||||||
| State deferred taxes | 3,826 | 3,792 | ||||||
| FAS 123(R) expense | 1,771 | 1,129 | ||||||
| AMT Credit | 789 | 789 | ||||||
| HTRN acquired built-in losses | 2,787 | 4,331 | ||||||
| Capitalized costs | 4,536 | 1,384 | ||||||
| Accrued expenses deductible for tax purposes when paid | 1,591 | 733 | ||||||
| Total gross deferred tax assets | 60,305 | 46,585 | ||||||
| Less valuation allowance | (54,275 | ) | (46,585 | ) | ||||
| Net deferred tax assets | 6,030 | -- | ||||||
| Deferred tax liabilities: | ||||||||
| Property and equipment, principally due to differences in depreciation | (2,982 | ) | (1,212 | ) | ||||
| Intangible assets, principally due to differences in amortization periods for tax purposes | (9,050 | ) | (2,143 | ) | ||||
| Total gross deferred tax liability | (12,032 | ) | (3,355 | ) | ||||
| Net deferred tax liability | $ | (6,002 | ) | $ | (3,355 | ) | ||
|
A-30 |
|
HEALTHTRONICS, INC. AND SUBSIDIARIES NOTES TO CONSOLIDATED FINANCIAL STATEMENTS – (Continued) |
|---|
|
In assessing the realizablity of deferred tax assets, management considers whether it is more likely than not
that some portion or all of the deferred tax assets will not be realized. The ultimate realization of deferred
tax assets is dependent upon the generation of future taxable income during the periods in which those temporary
differences become deductible. Management considers the scheduled reversals of deferred tax liabilities
(including the impact of available carry back and carry forward periods), projected future taxable income and tax
planning strategies in making this assessment. In the fourth quarter of 2008, primarily as a result of a
goodwill impairment charge, the Company incurred a cumulative book loss for the most recent three year period.
Management considers a cumulative loss for the most recent three year period to be a negative indicator as to the
realizability of deferred tax assets based upon future taxable income. In 2009, the Company continued to have a
cumulative book loss for the most recent three year period. Based on these criteria, management believes it will
not realize the benefits of these deductible differences; accordingly, we have recorded a full valuation
allowance at December 31, 2009 and 2008. |
|
A-31 |
|
HEALTHTRONICS, INC. AND SUBSIDIARIES NOTES TO CONSOLIDATED FINANCIAL STATEMENTS – (Continued) |
|---|
|
Effective January 1, 2007, we adopted ASC 740-10, Accounting for Uncertainty in Income Taxes (“ASC
740-10”), (formerly FIN No. 48 Accounting for Uncertainty in Income Taxes). ASC 740-10 specifies the way
public companies are to account for uncertainties in income tax reporting, and prescribes a methodology for
recognizing, reversing, and measuring the tax benefits of a tax position taken, or expected to be taken, in a tax
return. Adoption of ASC 740-10 on January 1, 2007 did not result in a cumulative effect adjustment to our
retained earnings. |
|
A-32 |
|
HEALTHTRONICS, INC. AND SUBSIDIARIES NOTES TO CONSOLIDATED FINANCIAL STATEMENTS – (Continued) |
|---|
|
The following table details selected financial information included in loss from discontinued operations in the consolidated statements of income for December 31, 2007. |
| Consolidated Statements of Income |
|
|||||||
|---|---|---|---|---|---|---|---|---|
| ($ in thousands) |
2007 |
|||||||
| For the Year Ended December 31 | ||||||||
| Revenue | ||||||||
| Rocky Mountain Prostate Thermotherapies | $ | 3,268 | ||||||
| HIFU | -- | |||||||
| Cost of services | ||||||||
| Rocky Mountain Prostate Thermotherapies | (3,739 | ) | ||||||
| HIFU | (216 | ) | ||||||
| Depreciation and amortization | ||||||||
| Rocky Mountain Prostate Thermotherapies | -- | |||||||
| HIFU | (3 | ) | ||||||
| Income (loss) from discontinued operations | $ | (690 | ) | |||||
| |
||||||||
|---|---|---|---|---|---|---|---|---|
| ($ in thousands) |
2007 |
|||||||
| Gain on Sale of Rocky Mountain Prostate Thermotherapy | $ | 451 | ||||||
| Loss from discontinued operations | (690 | ) | ||||||
| Income tax benefit on discontinued operations | 92 | |||||||
| Loss from discontinued operations, net of tax | $ | (147 | ) | |||||
|
The gain on sale of Rocky Mountain Prostate Thermotherapy, noted above, includes a charge to goodwill of $3.25
million. |
|
A-33 |
|
HEALTHTRONICS, INC. AND SUBSIDIARIES NOTES TO CONSOLIDATED FINANCIAL STATEMENTS – (Continued) |
|---|
|
N. RELATED PARTY TRANSACTIONS |
|
A-34 |
