Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Ardea Biosciences, Inc./DE | a55405e8vk.htm |
| EX-99.1 - EX-99.1 - Ardea Biosciences, Inc./DE | a55405exv99w1.htm |
Exhibit 99.2

| Company Update March 8, 2010 |

| Safe Harbor Statement Statements contained in this presentation regarding matters that are not historical facts are "forward- looking statements" within the meaning of the Private Securities Litigation Reform Act of 1995. Because such statements are subject to risks and uncertainties, actual results may differ materially from those expressed or implied by such forward-looking statements. Such statements include, but are not limited to, statements regarding: Ardea's goals, its preclinical and clinical trial plans, timelines and milestones, its expectations about the size of its markets and commercial potential of its compounds, expected results of future clinical trials, expected properties of compounds under development, financial position, cash usage, licensing and partnering opportunities, liquidity and anticipated milestones. Risks that contribute to the uncertain nature of the forward-looking statements include: risks related to the outcomes of preclinical and clinical trials, risks related to regulatory approvals, delays in commencement of preclinical and clinical tests, costs associated with internal development, and the outcome of our business development activities, including collaboration or licensing agreements. These and other risks and uncertainties are described more fully in Ardea's most recently filed SEC documents, including its Annual Report on Form 10-K and Quarterly Reports on Form 10-Q, under the headings "Risk Factors." All forward-looking statements contained in this presentation speak only as of the date of this presentation, and Ardea undertakes no obligation to update such statements to reflect events that occur or circumstances that exist after the date hereof or otherwise. 2 |
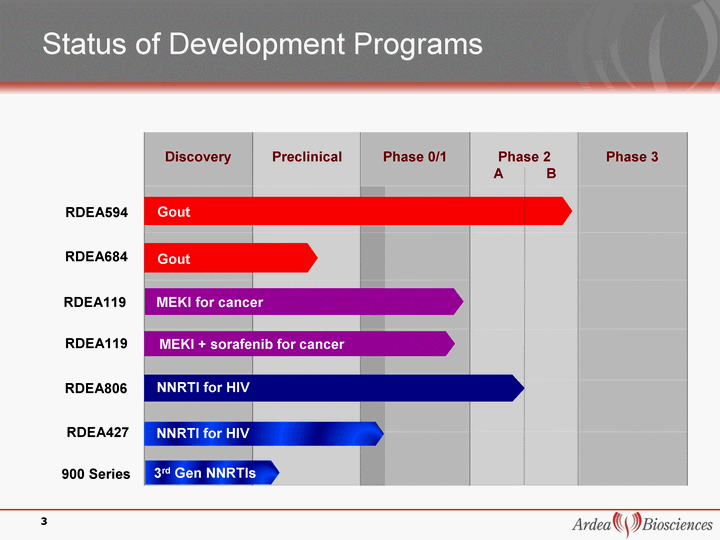
| Status of Development Programs RDEA427 RDEA806 900 Series Discovery Preclinical Phase 0/1 Phase 2 A B Phase 3 NNRTI for HIV NNRTI for HIV MEKI for cancer 3 Gout RDEA594 RDEA684 MEKI + sorafenib for cancer RDEA119 Gout RDEA119 3rd Gen NNRTIs |
| Gout/Hyperuricemia 4 |
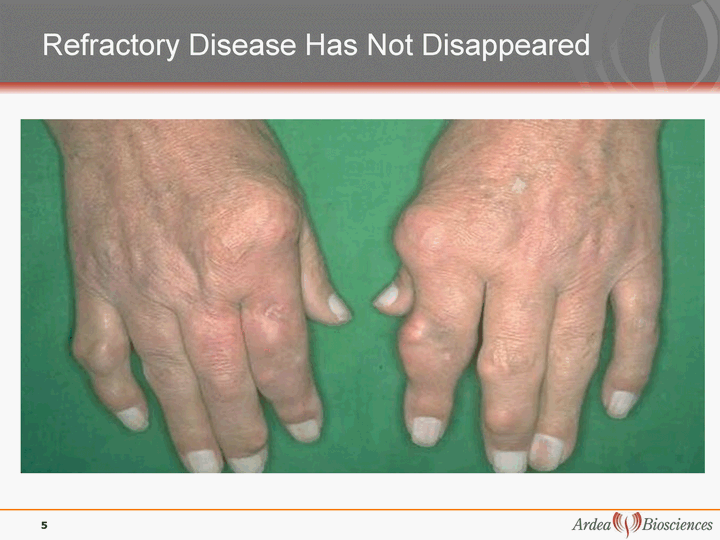
| Refractory Disease Has Not Disappeared 5 |
| Hyperuricemia/Gout - Unmet Medical Need Gout is caused by abnormally elevated levels of uric acid (>6.8 mg/dL) Painful and debilitating disease with attacks of severe pain/inflammation and disfiguring nodules (tophi) Increasing incidence and severity in U.S. (> 5,000,000 potential patients) with $11.2 billion hospitalization cost in 20051 Only one new drug available for hyperuricemia in last 40 years Hyperuricemia linked to elevated C-Reactive Protein, hypertension, increased mortality in Chronic Kidney Disease2 and possibly other cardiovascular risk factors3 with potentially more data coming soon: NHLBI sponsored study: Uric Acid and Hypertension in African Americans ~90% of patients are "under-excretors" of uric acid Defect in urate transporter recently found to be genetically linked to gout4 1Ann Rheum Dis 2008;67(Suppl II):96; 2Am J Kidney Dis 2009;53:796-803; 3 JAMA. 2008;300(8):924-932; 4Nature Genetics 40, 437 - 442 (2008) 6 |
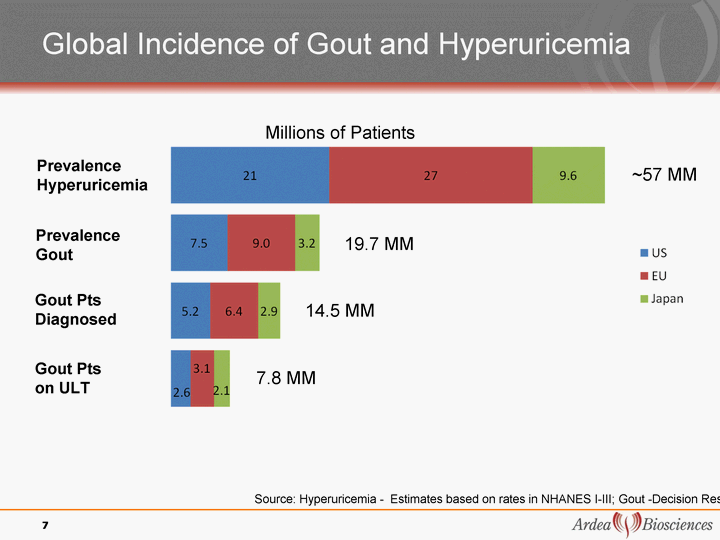
| Global Incidence of Gout and Hyperuricemia 7 Prevalence Hyperuricemia Prevalence Gout Gout Pts Diagnosed Gout Pts on ULT Source: Hyperuricemia - Estimates based on rates in NHANES I-III; Gout -Decision Resources ~57 MM 19.7 MM 14.5 MM 7.8 MM Millions of Patients |
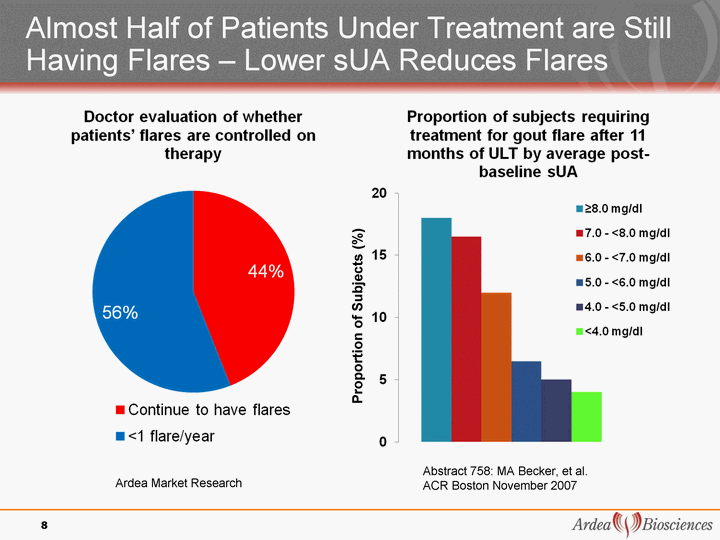
| Almost Half of Patients Under Treatment are Still Having Flares - Lower sUA Reduces Flares 8 8 Proportion of Subjects (%) Abstract 758: MA Becker, et al. ACR Boston November 2007 Ardea Market Research |
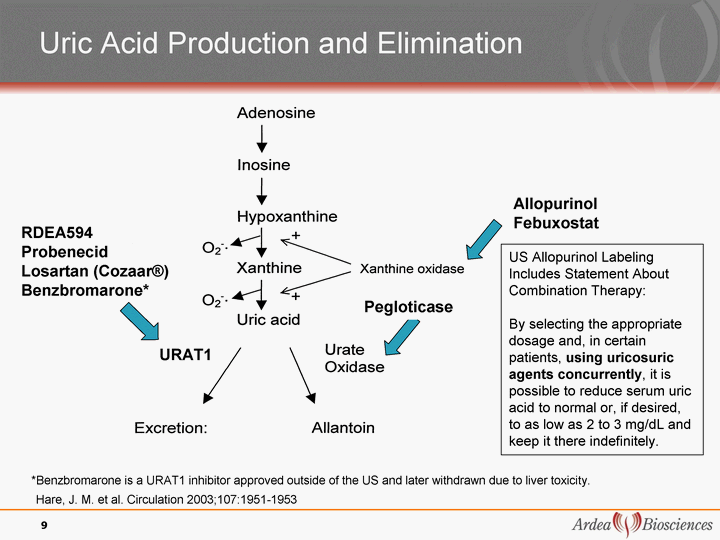
| Hare, J. M. et al. Circulation 2003;107:1951-1953 Uric Acid Production and Elimination Allopurinol Febuxostat RDEA594 Probenecid Losartan (Cozaar(r)) Benzbromarone* 9 *Benzbromarone is a URAT1 inhibitor approved outside of the US and later withdrawn due to liver toxicity. Pegloticase URAT1 US Allopurinol Labeling Includes Statement About Combination Therapy: By selecting the appropriate dosage and, in certain patients, using uricosuric agents concurrently, it is possible to reduce serum uric acid to normal or, if desired, to as low as 2 to 3 mg/dL and keep it there indefinitely. |
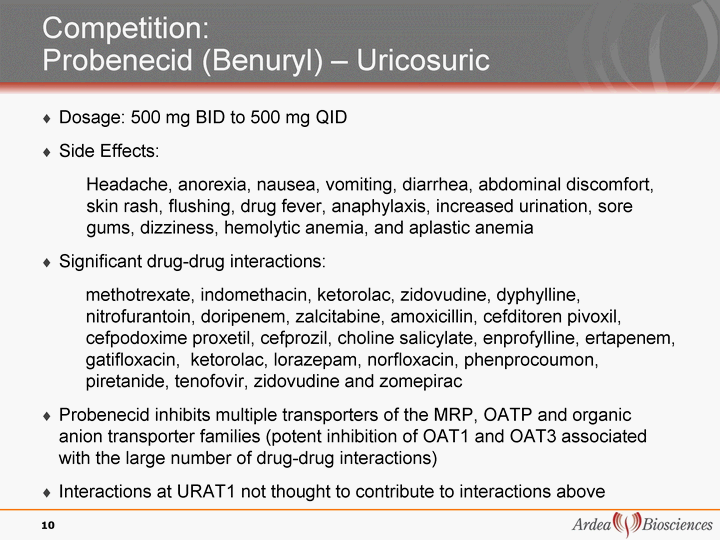
| Competition: Probenecid (Benuryl) - Uricosuric Dosage: 500 mg BID to 500 mg QID Side Effects: Headache, anorexia, nausea, vomiting, diarrhea, abdominal discomfort, skin rash, flushing, drug fever, anaphylaxis, increased urination, sore gums, dizziness, hemolytic anemia, and aplastic anemia Significant drug-drug interactions: methotrexate, indomethacin, ketorolac, zidovudine, dyphylline, nitrofurantoin, doripenem, zalcitabine, amoxicillin, cefditoren pivoxil, cefpodoxime proxetil, cefprozil, choline salicylate, enprofylline, ertapenem, gatifloxacin, ketorolac, lorazepam, norfloxacin, phenprocoumon, piretanide, tenofovir, zidovudine and zomepirac Probenecid inhibits multiple transporters of the MRP, OATP and organic anion transporter families (potent inhibition of OAT1 and OAT3 associated with the large number of drug-drug interactions) Interactions at URAT1 not thought to contribute to interactions above 10 |
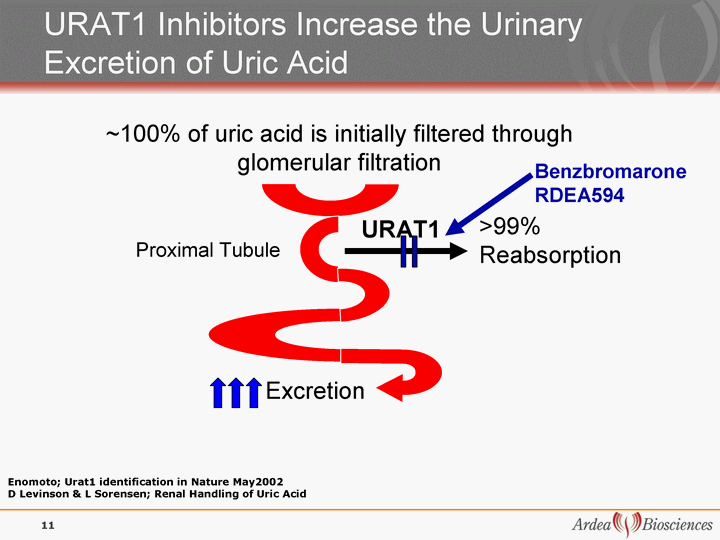
| >99% Reabsorption Excretion ~100% of uric acid is initially filtered through glomerular filtration URAT1 Inhibitors Increase the Urinary Excretion of Uric Acid URAT1 Enomoto; Urat1 identification in Nature May2002 D Levinson & L Sorensen; Renal Handling of Uric Acid Proximal Tubule Benzbromarone RDEA594 11 |
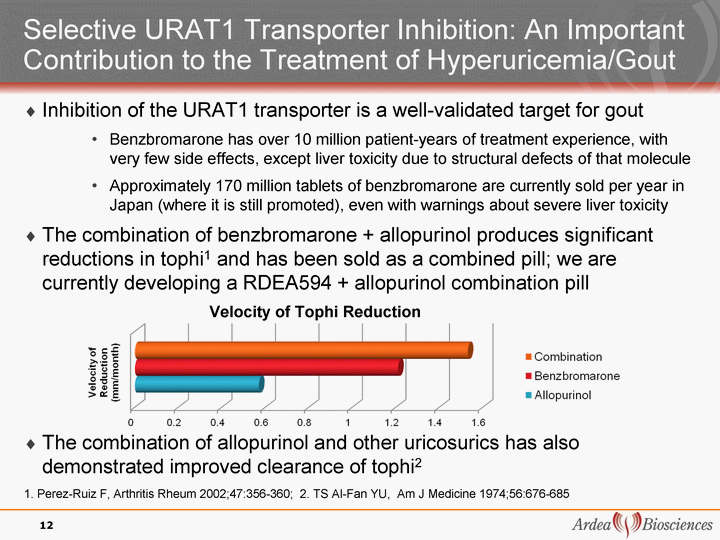
| 12 Selective URAT1 Transporter Inhibition: An Important Contribution to the Treatment of Hyperuricemia/Gout Inhibition of the URAT1 transporter is a well-validated target for gout Benzbromarone has over 10 million patient-years of treatment experience, with very few side effects, except liver toxicity due to structural defects of that molecule Approximately 170 million tablets of benzbromarone are currently sold per year in Japan (where it is still promoted), even with warnings about severe liver toxicity The combination of benzbromarone + allopurinol produces significant reductions in tophi1 and has been sold as a combined pill; we are currently developing a RDEA594 + allopurinol combination pill The combination of allopurinol and other uricosurics has also demonstrated improved clearance of tophi2 1. Perez-Ruiz F, Arthritis Rheum 2002;47:356-360; 2. TS Al-Fan YU, Am J Medicine 1974;56:676-685 Velocity of Tophi Reduction |
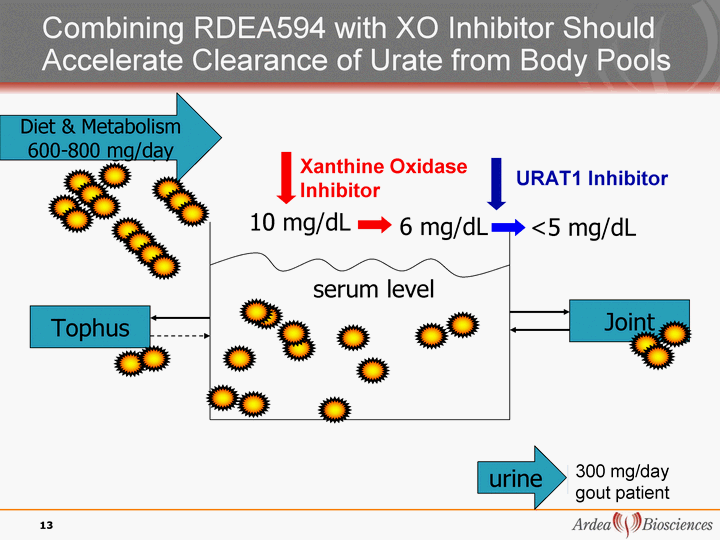
| Diet & Metabolism <400 mg/day Diet & Metabolism 600-800 mg/day Joint Tophus 10 mg/dL 6 mg/dL serum level Xanthine Oxidase Inhibitor URAT1 Inhibitor <5 mg/dL Combining RDEA594 with XO Inhibitor Should Accelerate Clearance of Urate from Body Pools urine urine 200 mg/day 13 600 mg/day 300 mg/day gout patient |
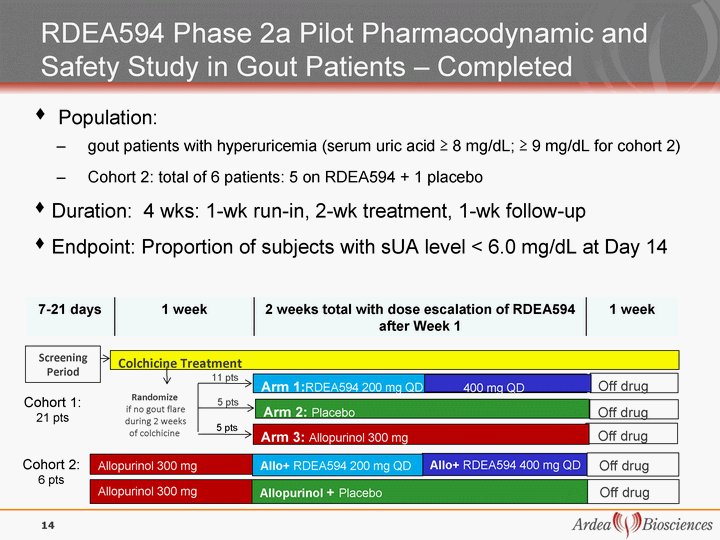
| Arm 1:RDEA594 200 mg QD Screenin g Period Colchicine Treatment Randomize if no gout flare during 2 weeks of colchicine Off drug Off drug Arm 2: Placebo 400 mg QD Off drug Arm 3: Allopurinol 300 mg 5 pts 5 pts 11 pts 7-21 days 1 week 2 weeks total with dose escalation of RDEA594 after Week 1 1 week Population: gout patients with hyperuricemia (serum uric acid ^ 8 mg/dL; ^ 9 mg/dL for cohort 2) Cohort 2: total of 6 patients: 5 on RDEA594 + 1 placebo Duration: 4 wks: 1-wk run-in, 2-wk treatment, 1-wk follow-up Endpoint: Proportion of subjects with sUA level < 6.0 mg/dL at Day 14 RDEA594 Phase 2a Pilot Pharmacodynamic and Safety Study in Gout Patients - Completed Allopurinol 300 mg Allo+ RDEA594 400 mg QD Allo+ RDEA594 200 mg QD Off drug Cohort 2: 6 pts 14 Allopurinol 300 mg Off drug Allopurinol + Placebo Cohort 1: 21 pts |
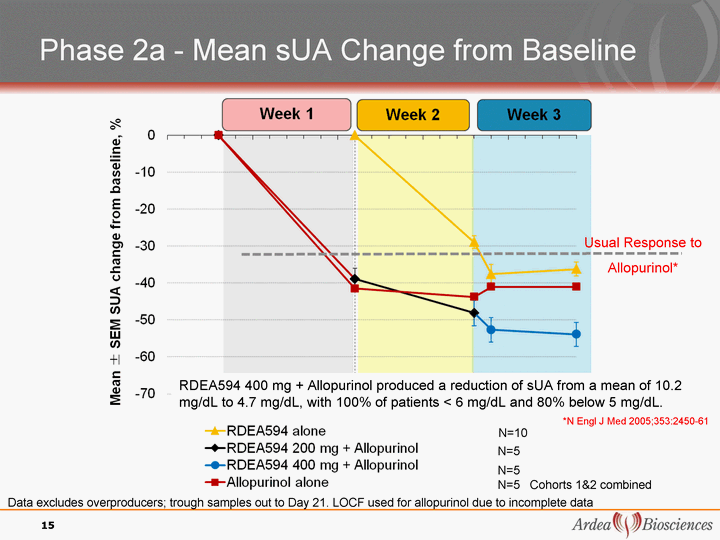
| Phase 2a - Mean sUA Change from Baseline N=5 N=5 N=5 Cohorts 1&2 combined Data excludes overproducers; trough samples out to Day 21. LOCF used for allopurinol due to incomplete data N=10 RDEA594 400 mg + Allopurinol produced a reduction of sUA from a mean of 10.2 mg/dL to 4.7 mg/dL, with 100% of patients < 6 mg/dL and 80% below 5 mg/dL. Usual Response to Allopurinol* 15 *N Engl J Med 2005;353:2450-61 |
| RDEA594 and Allopurinol Combination Product Under Development Allopurinol 300 mg (Zyloprim) RDEA594 400 mg RDEA594 400 mg and Allopurinol 300 mg Reference Product, Tylenol (r) GelCap 16 |
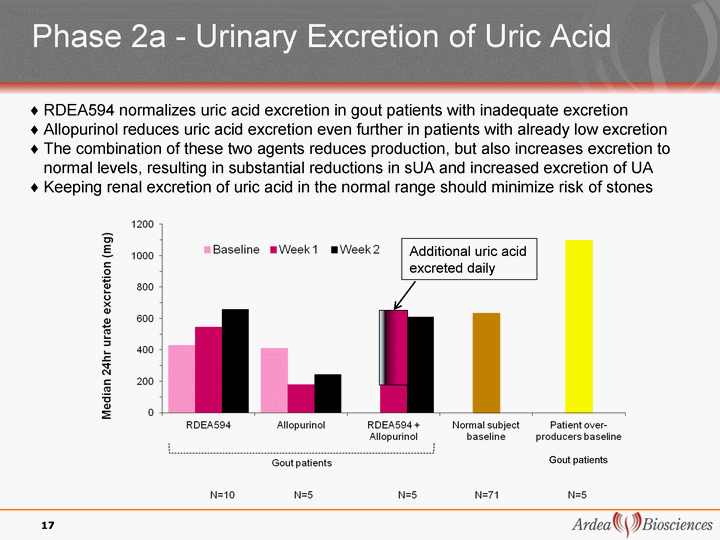
| Phase 2a - Urinary Excretion of Uric Acid Gout patients RDEA594 normalizes uric acid excretion in gout patients with inadequate excretion Allopurinol reduces uric acid excretion even further in patients with already low excretion The combination of these two agents reduces production, but also increases excretion to normal levels, resulting in substantial reductions in sUA and increased excretion of UA Keeping renal excretion of uric acid in the normal range should minimize risk of stones 17 Additional uric acid excreted daily |
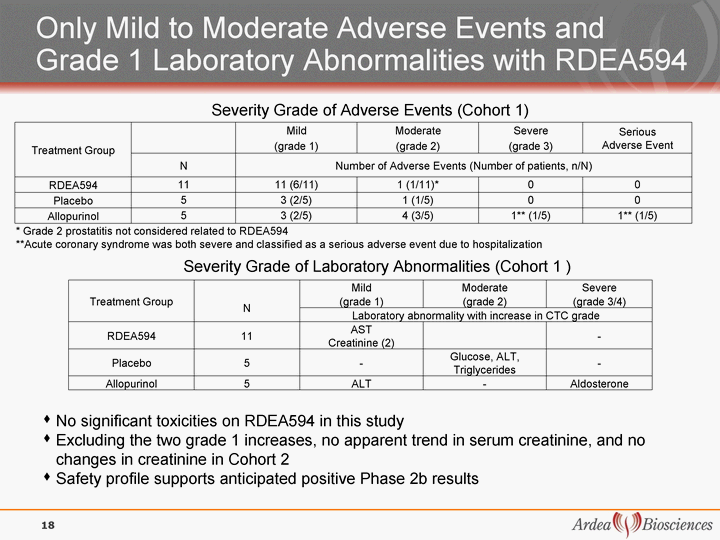
| Treatment Group Mild Moderate Severe Serious Adverse Event Treatment Group (grade 1) (grade 2) (grade 3) Serious Adverse Event Treatment Group N Number of Adverse Events (Number of patients, n/N) Number of Adverse Events (Number of patients, n/N) Number of Adverse Events (Number of patients, n/N) Number of Adverse Events (Number of patients, n/N) RDEA594 11 11 (6/11) 1 (1/11)* 0 0 Placebo 5 3 (2/5) 1 (1/5) 0 0 Allopurinol 5 3 (2/5) 4 (3/5) 1** (1/5) 1** (1/5) * Grade 2 prostatitis not considered related to RDEA594 **Acute coronary syndrome was both severe and classified as a serious adverse event due to hospitalization * Grade 2 prostatitis not considered related to RDEA594 **Acute coronary syndrome was both severe and classified as a serious adverse event due to hospitalization * Grade 2 prostatitis not considered related to RDEA594 **Acute coronary syndrome was both severe and classified as a serious adverse event due to hospitalization * Grade 2 prostatitis not considered related to RDEA594 **Acute coronary syndrome was both severe and classified as a serious adverse event due to hospitalization * Grade 2 prostatitis not considered related to RDEA594 **Acute coronary syndrome was both severe and classified as a serious adverse event due to hospitalization * Grade 2 prostatitis not considered related to RDEA594 **Acute coronary syndrome was both severe and classified as a serious adverse event due to hospitalization Treatment Group N Mild Moderate Severe Treatment Group N (grade 1) (grade 2) (grade 3/4) Treatment Group N Laboratory abnormality with increase in CTC grade Laboratory abnormality with increase in CTC grade Laboratory abnormality with increase in CTC grade RDEA594 11 AST Creatinine (2) - Placebo 5 - Glucose, ALT, Triglycerides - Allopurinol 5 ALT - Aldosterone Only Mild to Moderate Adverse Events and Grade 1 Laboratory Abnormalities with RDEA594 Severity Grade of Adverse Events (Cohort 1) Severity Grade of Laboratory Abnormalities (Cohort 1 ) No significant toxicities on RDEA594 in this study Excluding the two grade 1 increases, no apparent trend in serum creatinine, and no changes in creatinine in Cohort 2 Safety profile supports anticipated positive Phase 2b results 18 |
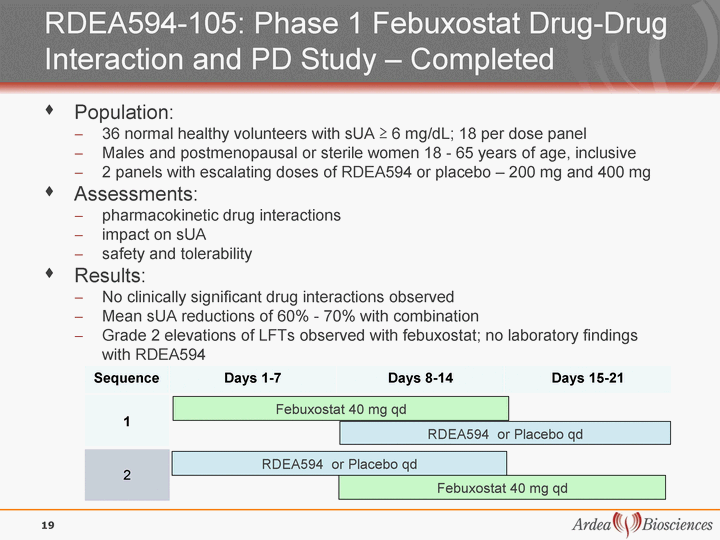
| RDEA594-105: Phase 1 Febuxostat Drug-Drug Interaction and PD Study - Completed Population: 36 normal healthy volunteers with sUA ^ 6 mg/dL; 18 per dose panel Males and postmenopausal or sterile women 18 - 65 years of age, inclusive 2 panels with escalating doses of RDEA594 or placebo - 200 mg and 400 mg Assessments: pharmacokinetic drug interactions impact on sUA safety and tolerability Results: No clinically significant drug interactions observed Mean sUA reductions of 60% - 70% with combination Grade 2 elevations of LFTs observed with febuxostat; no laboratory findings with RDEA594 Febuxostat 40 mg qd RDEA594 or Placebo qd Sequence Days 1-7 Days 8-14 Days 15-21 RDEA594 or Placebo qd Febuxostat 40 mg qd 1 2 19 |
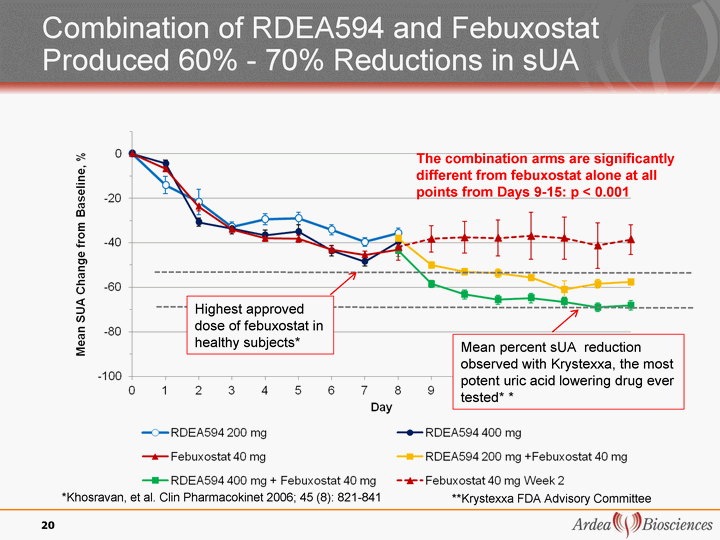
| Combination of RDEA594 and Febuxostat Produced 60% - 70% Reductions in sUA *Khosravan, et al. Clin Pharmacokinet 2006; 45 (8): 821-841 20 The combination arms are significantly different from febuxostat alone at all points from Days 9-15: p < 0.001 Mean percent sUA reduction observed with Krystexxa, the most potent uric acid lowering drug ever tested* * **Krystexxa FDA Advisory Committee Highest approved dose of febuxostat in healthy subjects* |
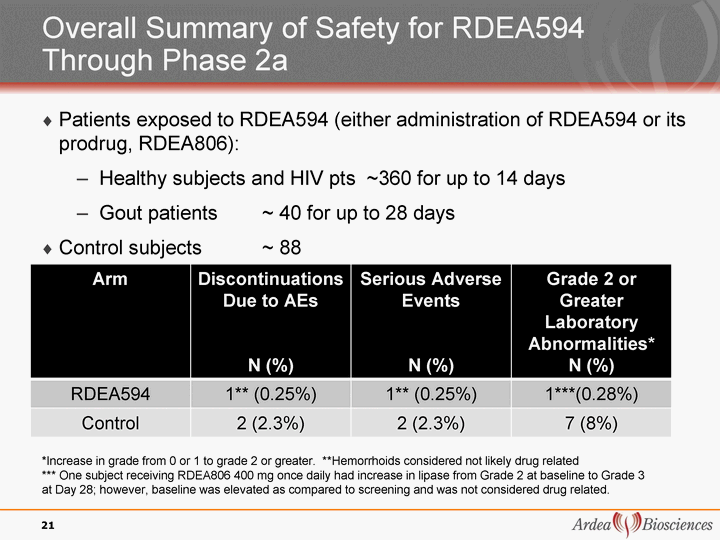
| Overall Summary of Safety for RDEA594 Through Phase 2a Patients exposed to RDEA594 (either administration of RDEA594 or its prodrug, RDEA806): Healthy subjects and HIV pts ~360 for up to 14 days Gout patients ~ 40 for up to 28 days Control subjects ~ 88 21 Arm Discontinuations Due to AEs N (%) Serious Adverse Events N (%) Grade 2 or Greater Laboratory Abnormalities* N (%) RDEA594 1** (0.25%) 1** (0.25%) 1***(0.28%) Control 2 (2.3%) 2 (2.3%) 7 (8%) *Increase in grade from 0 or 1 to grade 2 or greater. **Hemorrhoids considered not likely drug related *** One subject receiving RDEA806 400 mg once daily had increase in lipase from Grade 2 at baseline to Grade 3 at Day 28; however, baseline was elevated as compared to screening and was not considered drug related. |

| RDEA594 Phase 2 Program Focuses on Important Commercial Opportunities Phase 2b monotherapy dose-response, safety and efficacy study (n~140) Supports use in patients who are intolerant to allopurinol, inadequate responders to allopurinol, and first-line treatment Phase 2b allopurinol add-on study in gout patients stable on allopurinol 300 mg QD with sUA > 6 mg/dL (n=72) Preliminary results from ongoing Phase 2a - Cohort 2 supports the dose selection in the Phase 2b allopurinol combination study Targets use in 60% of allopurinol patients that do not achieve adequate reduction in uric acid with standard dose, and provides data for fixed combination product Renal impairment and pharmacodynamic study (n=12) Safety, tolerability, pharmacokinetics and impact on sUA in subjects with moderate renal insufficiency with or without allopurinol Febuxostat drug-drug interaction and pharmacodynamic study (n=36) Results strongly support use of combination in patients with high baseline levels and tophaceous gout patients 22 |
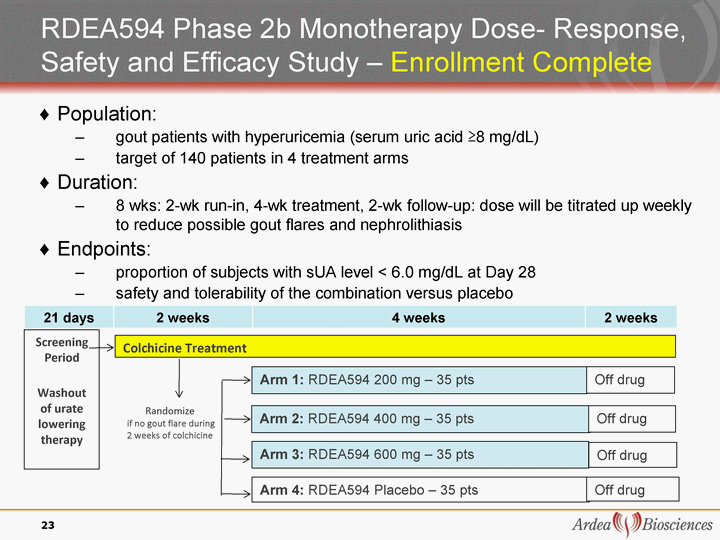
| RDEA594 Phase 2b Monotherapy Dose- Response, Safety and Efficacy Study - Enrollment Complete 21 days 2 weeks 4 weeks 2 weeks Population: gout patients with hyperuricemia (serum uric acid ^8 mg/dL) target of 140 patients in 4 treatment arms Duration: 8 wks: 2-wk run-in, 4-wk treatment, 2-wk follow-up: dose will be titrated up weekly to reduce possible gout flares and nephrolithiasis Endpoints: proportion of subjects with sUA level < 6.0 mg/dL at Day 28 safety and tolerability of the combination versus placebo Arm 1: RDEA594 200 mg - 35 pts Screenin g Period Washout of urate lowering therapy Colchicine Treatment Randomize if no gout flare during 2 weeks of colchicine Off drug Arm 2: RDEA594 400 mg - 35 pts Off drug Arm 4: RDEA594 Placebo - 35 pts Off drug Arm 3: RDEA594 600 mg - 35 pts Off drug 23 |
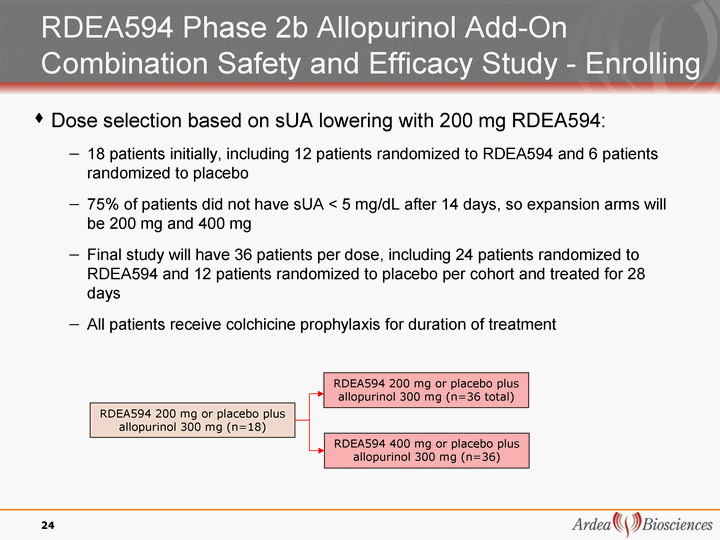
| RDEA594 Phase 2b Allopurinol Add-On Combination Safety and Efficacy Study - Enrolling Dose selection based on sUA lowering with 200 mg RDEA594: 18 patients initially, including 12 patients randomized to RDEA594 and 6 patients randomized to placebo 75% of patients did not have sUA < 5 mg/dL after 14 days, so expansion arms will be 200 mg and 400 mg Final study will have 36 patients per dose, including 24 patients randomized to RDEA594 and 12 patients randomized to placebo per cohort and treated for 28 days All patients receive colchicine prophylaxis for duration of treatment 24 RDEA594 200 mg or placebo plus allopurinol 300 mg (n=18) RDEA594 200 mg or placebo plus allopurinol 300 mg (n=36 total) RDEA594 400 mg or placebo plus allopurinol 300 mg (n=36) |
| Cancer 25 MEK program licensed to Bayer HealthCare on April 28, 2009 $35 million upfront license fee Payments could total $407 million, excluding royalties Low double-digit royalties on worldwide sales Ardea responsible for completing two dose-finding studies (expected completion to MTD in 1H10) |
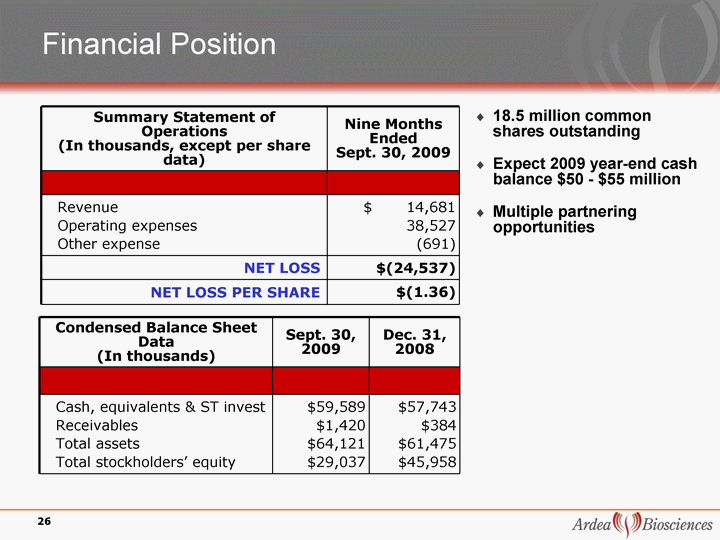
| Financial Position Summary Statement of Operations (In thousands, except per share data) Nine Months Ended Sept. 30, 2009 Revenue Operating expenses Other expense $ 14,681 38,527 (691) NET LOSS $(24,537) NET LOSS PER SHARE $(1.36) 18.5 million common shares outstanding Expect 2009 year-end cash balance $50 - $55 million Multiple partnering opportunities Condensed Balance Sheet Data (In thousands) Sept. 30, 2009 Dec. 31, 2008 Cash, equivalents & ST invest Receivables Total assets Total stockholders' equity $59,589 $1,420 $64,121 $29,037 $57,743 $384 $61,475 $45,958 26 |
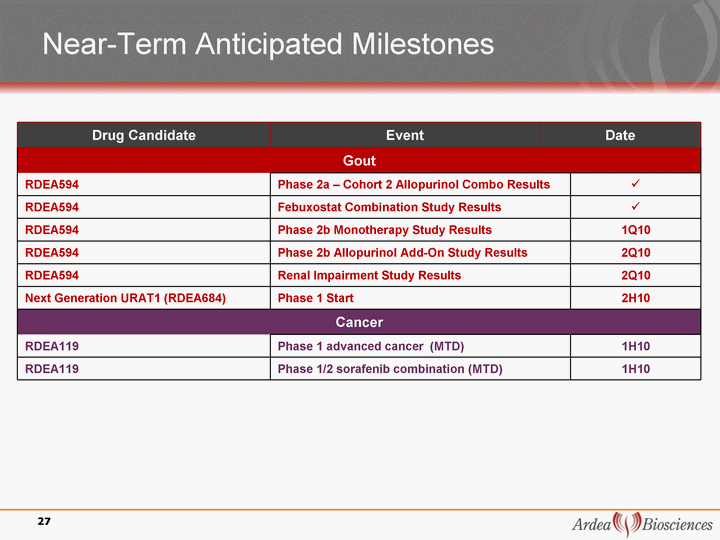
| Near-Term Anticipated Milestones Drug Candidate Event Date Date Gout Gout Gout Gout RDEA594 Phase 2a - Cohort 2 Allopurinol Combo Results Phase 2a - Cohort 2 Allopurinol Combo Results ? RDEA594 Febuxostat Combination Study Results Febuxostat Combination Study Results ? RDEA594 Phase 2b Monotherapy Study Results Phase 2b Monotherapy Study Results 1Q10 RDEA594 Phase 2b Allopurinol Add-On Study Results Phase 2b Allopurinol Add-On Study Results 2Q10 RDEA594 Renal Impairment Study Results Renal Impairment Study Results 2Q10 Next Generation URAT1 (RDEA684) Phase 1 Start Phase 1 Start 2H10 Cancer Cancer Cancer Cancer RDEA119 Phase 1 advanced cancer (MTD) Phase 1 advanced cancer (MTD) 1H10 RDEA119 Phase 1/2 sorafenib combination (MTD) Phase 1/2 sorafenib combination (MTD) 1H10 27 |
