Attached files
| file | filename |
|---|---|
| EX-24 - POWER OF ATTORNEY - Nano Magic Holdings Inc. | appliednano_ex24.htm |
| EX-11 - COMPUTATION OF (LOSS) PER COMMON SHARE - Nano Magic Holdings Inc. | appliednano_ex11.htm |
| EX-21 - SUBSIDIARIES - Nano Magic Holdings Inc. | appliednano_ex21.htm |
| EX-32.1 - CERTIFICATION - Nano Magic Holdings Inc. | appliednano_ex3201.htm |
| EX-31.1 - CERTIFICATION - Nano Magic Holdings Inc. | appliednano_ex3101.htm |
| EX-4.4 - FORM OF CONVERTIBLE NOTE PAYABLE - Nano Magic Holdings Inc. | appliednano_ex0404.htm |
UNITED
STATES
SECURITIES
AND EXCHANGE COMMISSION
Washington,
D.C. 20549
FORM
10-K
|
x
|
Annual
report under Section 13 or 15(d) of the Securities Exchange Act of 1934
for the fiscal year ended December 31, 2009;
or
|
|
o
|
Transition
report under Section 13 or 15(d) of the Securities Exchange Act of
1934
|
COMMISSION
FILE NO. 1-11602
APPLIED
NANOTECH HOLDINGS, INC.
(Exact
name of registrant as specified in its charter)
|
TEXAS
|
76-0273345
|
|
(State
of Incorporation)
|
(IRS
Employer Identification Number)
|
3006
Longhorn Boulevard, Suite 107, Austin, Texas 78758
(Address
of principal executive office, including Zip Code)
Registrant’s
telephone number, including area code: (512) 339-5020
Securities
registered pursuant to Section 12(b) of the Exchange Act:
|
Title
of each class
|
Name
of Each Exchange on Which Registered
|
|
Common
Stock, $0.001 par value
|
OTC
Bulletin Board
|
Securities
registered pursuant to Section 12(g) of the Exchange Act: None
Indicate
by check mark if the registrant is a well-known seasoned issuer as defined in
Rule 405 of the Securities Act. Yes o
No þ
Indicate
by check mark if the registrant is not required to file reports pursuant to
Section 13 or Section 15(d) of the Act. Yes o No
þ
Indicate
by check mark whether the registrant: (1) has filed all reports required to be
filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the
past 12 months (or for such shorter period that the registrant was required to
file such reports), and (2) has been subject to such filing requirements for the
past 90 days. Yes þ
No o
Indicate
by check mark if disclosure of delinquent filers in response to Item 405 of
Regulation S-K is not contained in this form and will not be contained, to the
best of registrant’s knowledge, in definitive proxy or information statements
incorporated by reference in Part III of this Form 10-K or any amendment to this
Form 10-K. o
Indicate
by check mark whether the registrant has submitted electronically and
posted on its corporate website, if any, every Interactive Data File required to
be submitted and posted pursuant to Rule 405 of Regulation S-T (232.405 of this
chapter) during the preceding 12 months (or for such shorter period that the
registrant was required to submit and post such files.
Yes o
No o
Indicate
by check mark whether the registrant is a large accelerated filer, an
accelerated filer, a non-accelerated filer, or a smaller reporting
company.
Large
Accelerated Filer o
Accelerated Filer o
Non-accelerated
Filer þ Smaller
Reporting Company o
Indicate
by check mark whether the registrant is a shell company (as defined in Rule
12b-2 of the Exchange Act).
Yes o
No þ
The
aggregate market value of the Common Stock held by non-affiliates of the
Registrant, based upon the average of the closing bid and asked price of the
Common Stock on the OTC Bulletin Board system on June 30, 2009 of $0.28, was
approximately $30 million. As of February 26. 2010, the registrant had
107,573,133 shares of Common Stock issued and outstanding.
Documents
Incorporated by Reference
No
documents are incorporated by reference into this annual report on Form
10-K
TABLE
OF CONTENTS
|
Page
|
|||
|
Part
I.
|
|||
|
Item
1.
|
Business
|
1
|
|
|
Item
1A.
|
Risk
Factors
|
11
|
|
|
Item
1B.
|
Unresolved
Staff Comments
|
16
|
|
|
Item
2.
|
Properties
|
17
|
|
|
Item
3.
|
Legal
Proceedings
|
17
|
|
|
PART
II
|
|||
|
Item
4.
|
Market
for Registrant’s Common Equity, Related Stockholder Matters and Issuer
Purchases of Equity Securities
|
18
|
|
|
Item
5.
|
Selected
Financial Data
|
20
|
|
|
Item
6.
|
Management’s
Discussion and Analysis of Financial Condition and Results of
Operations
|
21
|
|
|
Item
6A.
|
Quantitative
and Qualitative Disclosures About Market Risk
|
26
|
|
|
Item
7.
|
Financial
Statements and Supplementary Data
|
27
|
|
|
Item
8.
|
Changes
in and Disagreements with Accountants on Accounting and Financial
Disclosure
|
48
|
|
|
Item
8A(T).
|
Controls
and Procedures
|
48
|
|
|
Item
8B.
|
Other
Information
|
50
|
|
|
PART
III
|
|||
|
Item
9.
|
Directors,
Executive Officers and Corporate Governance
|
51
|
|
|
Item
10.
|
Executive
Compensation
|
53
|
|
|
Item
11.
|
Security
Ownership of Certain Beneficial Owners and Management and Related
Stockholder Matters
|
62
|
|
|
Item
12.
|
Certain
Relationships and Related Transactions, and Director
Independence
|
64
|
|
|
Item
13.
|
Principal
Accountant Fees and Services
|
65
|
|
|
PART
IV
|
|||
|
Item
14.
|
Exhibits
and Financial Statement Schedules
|
66
|
Important
Information Concerning Forward-Looking Statements
Our
disclosure and analysis in this report contains some forward-looking statements.
Forward-looking statements give our current expectations or forecasts of future
events. They use words such as “anticipate”, “believe”, “expect”, “estimate”,
“project”, “intend”, “plan”, and other words and terms of similar meaning in
connection with any discussion of future operating or financial performance. In
particular, these include statements relating to future actions, prospective
products or product approvals, future performance or results of current and
anticipated products, sales efforts, expenses, the outcome of contingencies such
as legal proceedings, and financial results. From time to time, we also may
provide oral or written forward-looking statements in other materials we release
to the public.
Any or
all of our forward-looking statements in this report and in any other public
statements we make may turn out to be wrong. They can be affected by inaccurate
assumptions we might make, or by known or unknown risks or uncertainties. Many
factors mentioned in the risk factors are important in determining future
results. Consequently, no forward-looking statement can be guaranteed. Actual
future results may vary materially.
We
undertake no obligation to publicly update any forward-looking statements,
whether as the result of new information, future events, or otherwise. You are
advised, however, to consult any further disclosures we make on related subjects
in our 10-Q, 8-K, and 10-K reports to the SEC. Also note that we include a
cautionary discussion of risks, uncertainties, and possibly inaccurate
assumptions relevant to our business. These are factors that we think could
cause our actual results to differ materially from expected and historical
results. Other factors besides those listed here could also adversely affect
us.
PART
I.
When used
in this document, the words “anticipate”, “believe”, “expect”, “estimate”,
“project”, “intend”, “plan”, and similar expressions are intended to identify
forward-looking statements. Such statements are subject to certain risks,
uncertainties, and assumptions. Should one or more of these risks or
uncertainties materialize, or should underlying assumptions prove incorrect,
actual results may vary materially from those anticipated, believed, expected,
estimated, projected, intended, or planned. For additional discussion of such
risks, uncertainties, and assumptions, see “Important Information Concerning
Forward-Looking Statements” included at the beginning of this report and “Risk
Factors” beginning on page 11 of this report.
Item
1. Business.
DESCRIPTION
OF BUSINESS
General
We are a
nanotechnology company engaged in research and development. Our research is
based on intellectual property and expertise that we have developed over the
past 20 years. Our research is aimed at solving problems at the molecular level
- in the area between the properties of matter that exist from the time several
molecules are bound together until the first aggregate of the same molecules is
created exhibiting the same chemical, physical and biological properties of the
bulk material. The challenge of nanotechnology is how to controllably assemble
fundamental nanoparticle building blocks in order to achieve new materials for
new applications. As part of our research we perform services for others and
develop products and materials based on our unique abilities in this area.
During this process we generate intellectual property, and our ultimate goal is
to generate sustainable recurring revenues by licensing this technology to
others. Our work is currently focused in the areas of nanomaterials,
nanoelectronics, sensors, nanoecology, and electron emission
activities.
We were
incorporated in Texas in 1987 and completed our initial public offering in 1993.
Our initial focus was on a next generation display technology called field
emission display (“FED”). Until late 2005, the majority of our research was
related to FED technology and other display technologies and activities. During
that time period we were at the forefront of the evolution of FED technology,
and we accumulated significant intellectual property along the way. However, the
display industry is dominated by a small number of large multinational
corporations, and we were entirely dependent on one of those companies
introducing a product using our technology. In late 2005, we
determined that we should diversify our activities to become less dependent on
those companies.
From 2006
to the present, we have expanded our research to many other areas to capitalize
on the knowledge that we gained during the first 18 years of our existence. Much
of this research was an outgrowth of our work in the FED area. This research was
either related to other display technologies, used processes learned while we
were working with FED technology, used raw materials used in our FED research,
or capitalized on other unique capabilities within our organization. As a result
we have developed significant intellectual property in other areas beyond that
solely related to the FED technology. At present, we have over 270 total U.S.
and foreign patents, including 90 issued, and over 180 pending.
Business
Model
At the
present time, we are first and foremost a research and development company. We
have an extensive portfolio of intellectual property that we have developed
throughout the last 20 years, and we intend to develop a portfolio of recurring
revenue streams by licensing our intellectual property to others. In addition,
for certain high potential products and technologies, we are exploring the
possibility of forming business units around some of these technologies to allow
us to participate in the commercialization process beyond simply licensing our
technology to others to manufacture products. We believe that, in some
instances, these business units will allow us to create greater value through
broader participation in the revenue stream and potentially decrease the time
required to bring products to market. In addition, having an ownership interest
in an entity that may be created related to the technology allows us to
participate in the value created as the technology gains market acceptance and
the entity grows. We have organized internal teams to focus on the development
of these high potential technologies and entities based on
them.
Page
1
Much of
our intellectual property and research relates to next-generation technologies
that are not in wide current use and as such, additional development work is
required before products can be manufactured using these technologies. Our
research and development efforts occur across a continuum moving from concept to
commercialization as follows:
Concept →
Laboratory → Development → Pilot /Introduction → Commercialization
To aid in
the process of moving our technologies from concept to commercialization, we
frequently perform funded research for both government entities and large
corporations. This enables us to focus our resources in areas that have the
highest level of interest to others, and thus the highest probability for
commercialization.
Research
and Development
As a
result of our focus on developing and protecting our intellectual property, we
spend significant amounts on research and development. We spent $3,662,323,
$4,614,644, and $4,526,166 on research and development in the years ended
December 31, 2009, 2008, and 2007, respectively. This represents approximately
59%, 54%, and 54% of our total operating costs and expenses in each of those
years. We expect to continue to invest heavily in research and development, and
we expect our research and development costs for 2010 to be in excess of 60% of
our operating costs. We seek to obtain funding for as much of our research as
possible.
Business
Segments
Our
operations currently consist of three reportable business segments.
Applied Nanotech,
Inc. ANI is the
entire focus of our current efforts. It was incorporated in January 1997 and is
developing our proprietary carbon nanotube and related technologies.
Accordingly, our research is focused in the broad area of carbon nanotube
technology and its application to the materials, electronics, sensor, medical,
display, and other industries. Our development plans for our technologies are
discussed later in this report.
Electronic
Billboard Technology, Inc. EBT was incorporated in
January 1997 and initially focused on developing sun-readable display products
for outdoor use. EBT expanded into other display areas, but in 2002, we
restructured EBT and limited ourselves to licensing EBT’s existing intellectual
property. In 2006, we sold EBT’s intellectual property in two simultaneous
transactions as discussed below. To the extent that EBT is basically inactive,
information relative to this segment may not be that meaningful.
Other. We also incur general
operating overhead that is not associated with any specific subsidiary or other
segment. This overhead is the approximate cost of being a public company, which
is the amount in excess of that which might be incurred by a private company
performing these same activities.
Following
is a summary of revenues, net loss from continuing operations, and total assets
for each segment for each of the last three years.
|
2009
|
2008
|
2007
|
||||||||||
|
Revenues
|
||||||||||||
|
ANI
|
$ | 4,052,746 | $ | 3,957,832 | $ | 3,989,803 | ||||||
|
EBT
|
$ | — | $ | — | $ | — | ||||||
|
Other
|
$ | — | $ | — | $ | — | ||||||
|
Total
Revenues
|
$ | 4,052,746 | $ | 3,957,832 | $ | 3,989,803 | ||||||
|
Net
income (loss) from continuing operations
|
||||||||||||
|
ANI
|
$ | (1,411,121 | ) | $ | (1,520,726 | ) | $ | (3,212,051 | ) | |||
|
EBT
|
$ | 5,459 | $ | (1,296 | ) | $ | (1,298 | ) | ||||
|
Other
|
$ | (746,943 | ) | $ | (1,163,845 | ) | $ | (1,043,542 | ) | |||
|
Total
|
$ | (2,152,605 | ) | $ | (2,685,867 | ) | $ | (4,256,891 | ) | |||
|
Assets
|
||||||||||||
|
ANI
|
$ | 780,848 | $ | 1,105,627 | $ | 778,863 | ||||||
|
EBT
|
$ | — | $ | — | $ | — | ||||||
|
Other
|
$ | 112,519 | $ | 686,862 | $ | 2,965,995 | ||||||
|
Total
|
$ | 893,367 | $ | 1,792,489 | $ | 3,744,858 | ||||||
Page
2
Applied
Nanotech, Inc.
Overall
We are a
nanotechnology company focusing our efforts on research, development of proof of
concepts for proposed products, and licensing our technology to others. We are
developing world-class technologies that generally fall under one of five
technology platforms. These platforms are:
|
|
·
|
Functional
nanomaterials, including composites and thermal
management;
|
|
|
·
|
Nanoelectronic
applications;
|
|
|
·
|
Sensor
technology;
|
|
|
·
|
Nanoecology;
and
|
|
|
·
|
Electron
emission activities, primarily in the display
area.
|
We intend
to license our technology to others to allow them to manufacture products using
our technology. We have no plans to establish any manufacturing facilities in
the foreseeable future, and manufacturing is not a part of our core
strategy. To the extent that manufacturing capabilities are needed for products
using our technology, we intend to use manufacturing partnerships, joint
ventures, or arrange to have products manufactured through contract
manufacturers. For certain high potential products and technologies, we are
exploring establishing business units that would enable us to participate in,
and profit from, the commercialization process beyond just
licensing.
Functional
Nanomaterials
Nanocomposites
We are in
the advanced stages of research into nanomaterials using carbon nanotube (“CNT”)
and other composites. We believe that some of the first widespread use of
nanotechnology by established companies will be in this area as they work to
improve existing products, materials, and processes. A significant opportunity
exists in this area for us to develop and license our technology. We are well
versed in harnessing the unique properties of CNTs for
a variety of applications. Our work with CNT composites has been in three main
areas – epoxies, nylons, and glass fibers.
Epoxies –
Epoxies are used in industries with huge worldwide markets, with applications
including adhesives, paints, coatings, and composites. The objective of our CNT
epoxy program has been to create composite materials to be used as an
alternative to traditional materials. Our goal is to reinforce epoxy with CNTs
to take advantage of CNTs mechanical properties while reducing the weight of
materials needed for specific applications. We have developed patented processes
and know-how which has enabled us to uniformly disperse CNTs throughout the
epoxy to create significant improvements in strength, toughness, durability,
vibration dampening, and other mechanical properties.
In
September 2005 we signed a development contract with a large sporting goods
manufacturer to develop nanocomposites to be used in sporting equipment. This
agreement culminated in a license agreement in October 2008 with this partner to
use our technology in its tennis and badminton racquets. We expect this
manufacturer to have products on the market in 2010 that will generate royalties
for us. This development agreement served as the foundation of our work in the
nanomaterials area.
Nylons –
The addition of CNTs to nylons can enhance certain mechanical properties and the
electrical conductivity of normally insulating material. Of all the types of
nylons available, Nylon 6 is the most common, with an estimated 60% of the world
volume and with uses in a variety of industries including textiles, carpeting,
safety belts, fishing lines, and a variety of other applications. We have
developed a patented process for coating nylon pellets with CNTs to improve
electrical conductivity. Nylon 6 with improved electrical conductivity can be
used for its anti-static qualities, electrostatic discharge, and
electromagnetic/RF shielding.
Page
3
Glass
Fibers – We have applied our knowledge in CNT enhanced composites to develop a
strengthened fiberglass that can be used for applications with long lifetime
requirements, such as wind turbine blades. Current wind blade research is
focused on lowering the weight of materials to reduce overall cost and increase
efficiency. Our early research has shown dramatic results in lowering the weight
of materials using CNT strengthened materials, and we are continuing to apply
resources with our partners, to further develop this technology.
Competition.
There is a wide range of companies working with composite materials,
including some working with CNT enhanced composites. Since each project is
unique, our competition would come from different companies for different
applications. Since we rely on patented technology, any competition would come
from different composites, or different formulations of CNT composites. Some of
the companies known to be working in the CNT composite area are Zyvex
Performance Materials in Columbus Ohio, GSI Creos in Tokyo, Japan, and Amroy
Europe, Ltd. in Finland.
Thermal
Management
With a
partner in Japan, we are marketing a unique patented thermal management material
called CarbAl™. In 2009, R&D magazine recognized Carbal™ as one of the 100
most significant product innovations of 2009 with its R&D 100 Award. The
inability to effectively control temperature is the number one cause of failure
for electronics, resulting in more failures than dust, humidity, vibration, and
other harmful conditions. CarbAl™ provides a passive thermal management solution
for temperature control issues that plague electronics manufacturers and has the
ability to reduce costs for, and extend the lives of many electronics
applications.
Carbal™
is a carbon based metal nanocomposite comprised of 80% carbonaceous matrix and a
dispersed metal component of 20% aluminum. Carbal™ has a unique combination of
low density, high thermal diffusivity, high thermal conductivity, and a low
coefficient of thermal expansion that results in a material that far exceeds the
capabilities of conventional passive thermal management materials. There is an
extensive worldwide market for thermal management materials and Carbal™ is ideal
for applications in the electronics industry, in LED displays, and for
concentrated photovoltaic solar cell systems.
Competition.
Because Carbal™ is a new patented material, its competition comes from
already existing active and passive thermal management systems. For example,
computer equipment includes fans, while LED Billboards include air conditioning
equipment. CPV solar cells are a developing product and thermal management
remains a significant problem.
Nanoelectronics
Applications
Conductive
Inks
As a
result of the move towards flexible electronics, soldering processes are slowly
disappearing, and new processes that are digital in nature are needed to allow a
move directly from design to the production line. One of the areas that we have
chosen to focus on is conductive inks. Nanotechnology will play an important
role in this process because only nanoparticles are capable of producing inks
that are compatible with the nozzles used in digital printing.
To
facilitate our development of conductive inks, we entered into a research and
development agreement with a leading industrial chemical products company
headquartered in Japan to develop conductive inks that can be deposited using an
additive process such as ink-jet printing or screen printing. The target market
for these technical inks includes printed circuit boards, flexible electronics
and displays, communications instrumentation, and Radio Frequency Identification
tags. Our work with this partner started with a feasibility study in early 2006
and culminated with a license agreement for copper inks and pastes in July 2009.
As a result of this partnership, we have received over $2.5 million in research
funding and $500,000 of a $1.5 million up-front license payment, with the
balance of $1.0 million due in June 2010.
We chose
to start this project by focusing on nanocopper inks using copper nanoparticles
because of copper’s superior electrical conductivity properties, low cost, and
because it is the material of choice in the printed circuit board industry. We
have been able to achieve electrical conductivity properties similar to that of
bulk copper with these inks. We have since expanded our work with conductive
inks to other inks including nickel, silver, and others, as well as to various
conductive pastes.
Page
4
Technical
Inks Printing Solution (TIPS)
Conductive Inks have the power to
revolutionize many types of electronics manufacturing. Our strategy is to
provide a comprehensive solution for end users by not only developing inks, but
assisting in the process from start to finish. As part of our concept, we have
done extensive research on raw materials (nanoparticles). We have developed
relationships with nanoparticle suppliers and are able to supply a variety of
nanoparticles as a result of these relationships. In addition, we have done
applications R&D on a variety of potential end user products to help develop
specifications for the inks. We have also formed relationships with hardware
manufacturers with the goal of providing seamless integration into high volume
manufacturing for companies wishing to use conductive inks in their
manufacturing processes.
We intend
to develop a revenue stream that includes revenue from the sale of
nano-particles, research revenue from applications development, commissions on
the sale of hardware, and royalties from the sale of inks.
Competition.
Numerous other companies are working with other technologies with the
goal of achieving results similar to the goals of our technology. The ultimate
success of products using our technology depends on the results of our research
compared with results achieved by others. There are silver inks on the market
today, but because of the high cost of silver relative to copper, a copper ink
is likely to open up additional markets.
Sensors
Overview.
We have greatly expanded our work and intellectual property in the area
of sensor technology. This is becoming an increasingly important part of our
business, and we expect it to become even more important in the future. Our
approach to sensor technology offers the unique advantage of manipulating
materials at the molecular level at which sensing events occur. We are pursuing
a multiplatform approach to address specific market needs. Some of the potential
applications are as follows:
Ion Mobility
Sensors. We are currently developing sensors based on Ion Mobility Sensor
technology and Differential Mobility Spectroscopy. These sensors are ideal for
use when both high sensitivity and high selectivity (low false positives) are
required. We are also improving on existing IMS and DMS technology by developing
nonradioactive gas ionization sources to replace the radioactive isotopes that
are currently used in these tools. We are currently involved in a project to
develop a highly sensitive Mercaptan sensor for use in the natural gas
industry.
We have
also submitted a significant proposal to the U.S. government for funding to
develop a breath analysis sensor for health monitoring using this technology.
Breath analysis has the potential to significantly lower health care costs by
providing early indications of potential changes in people’s health and
providing that information digitally to health professionals.
Hydrogen
sensors. These sensors are initially targeted for use in fuel cells for
automobiles and for remote monitoring of large power transformers. We developed
a hydrogen sensor for use in the measurement of hydrogen in power transformer
products. We are currently working with a large manufacturer of instruments,
controls, and monitoring systems used in the power transmission industry, and we
have an all encompassing agreement covering development, technology transfer,
and licensing with this partner. We expect this partner will introduce a product
using our sensor in 2010 and that it will begin generating royalties for
us.
Carbon Monoxide
Sensors. We have developed a low-power carbon monoxide sensor that can
last for 10,000 hours on a single battery. The sensor will be specific to carbon
monoxide with no cross sensitivity to other gases and elements and is also
easily portable and highly sensitive.
Biosensors.
Our carbon nanotube technology is ideally suited for use in biosensors. Sensors
based on carbon nanotubes or other nanomaterials can be used to detect chemical,
organic, or biological warfare agents, as well as explosives, hydrogen, ammonia
and numerous other chemicals. We have developed several proof of concepts
demonstrating the viability of our sensor technology, and are currently seeking
development partners to license the technology and integrate it into specific
products.
Other sensors.
We have demonstrated that carbon nanotubes can be used to develop sensors
for chemical, organic, and biological warfare agents. We have also demonstrated
that carbon nanotubes and other nanodetectors can be used for the remote
detection of explosives, sensors used in environmental monitoring, health care,
the food industry, biotech-biopharma applications, genetic biosensors, and
immunosensors. We are currently seeking funding to take our research in this
area to the next level of development, which would include proofs of concept,
and product development. Ideally, we would do this with a development partner
that would fund the development and license the technology for manufacturing
upon completion, or in conjunction with a development partner under a government
funding program. We most likely would have different development partners for
different sensors that may be used in different industries.
Page
5
Competition.
Our competition in the sensor area will come from a variety of
technologies and companies depending on the purpose and use of the sensor. There
are other technologies used in sensors; however, we believe carbon nanotube
based sensors and other nanodetectors are more versatile, can sense a broader
range of materials, and are more selective (sensitive) in their sensor results.
We believe that selecting the right strategic partners for development of proof
of concepts for our sensor technology is an important step in the market
acceptance of sensors using our technology.
Nano-ecology
Photoscrub®
Technology. We developed a concept called Photoscrub® which is based on
an air purification technology originally developed by one of our strategic
partners, Andes Electric Co., Ltd. The Photoscrub® is a thin film coating on a
flexible fiberglass cloth that decomposes pollutants at the molecular level in
liquids and gases. We began a one year project in the amount of roughly $950,000
to further develop this technology for the U.S. Army in November 2006. Based on
the results of this first phase, we were awarded a contract in the amount of
$1.45 million in January 2009 and are currently performing research under this
contract.
Electron Emission
Activities
Until
recent years, our main focus for virtually the entire life of Applied Nanotech
Holdings has been on electron emission activities. We have performed extensive
research and accumulated significant intellectual property in this
area. The bulk of that activity has centered on Field Emission
Display (“FED”) technology. FED is a next generation display technology that is
ideally suited for use in large flat screen televisions, with “large” being
defined as 50-inch diagonal or greater. We believe our intellectual property in
the electron emission area is mature and well developed. We are now dependent on
a manufacturer to introduce a product using our technology. As such, we are no
longer devoting any resources to further develop this technology, unless funded
for a specific application. We are, however, closely monitoring developments in
the industry to determine if a license is needed by a manufacturer for our
technology.
Our
electron emission activities can be divided into display activities and
non-display activities.
Display
Applications
The
display industry is, in general, dominated by large multinational corporations
primarily based in Asia and participation in manufacturing products for the
display market involves significant capital investments. While display
applications represent potentially huge markets for the use of our technology,
realization of this potential will require one of these companies to introduce a
product based on our technology. Our intellectual property in this area is well
developed, and we believe that any manufacturer that develops a product based on
electron emission activities involving carbon will require a license to our
technology; however, any products using this technology are still at present,
next generation products. We believe that the successful introduction of any
display products using this technology will require the participation of
existing display manufacturers or component suppliers. These manufacturers and
suppliers have a variety of interests and the introduction of new display
products may be affected by general economic conditions as well as the impact of
these new products on other areas of their business. Potential applications in
the display area include: large area CNT flat screen color field emission
displays, large area surface conduction color field emission displays such as
the one developed by Canon and Toshiba, backlights for displays, and picture
element tubes for medium resolution large area electronic
billboards.
Non-Display
Applications
There is
also a wide array of non-display related electron emission applications. These
potential applications are spread among a much larger group of potential
licensees, and the markets generally are not as large as the display
markets, although the markets are still very significant and would generate
substantial royalties if products are introduced. We have performed research
related to lighting applications, ion sources, traveling wave tubes, and are
exploring other electron emission applications. We believe these applications
may have the capability of generating license revenues sooner than display
applications, may be easier to integrate into existing products, and may be in
wide use sooner than display applications. While the breadth (number of patents)
of our display related emission activity patents is currently greater than for
non-display patents, our non-display Intellectual Property is growing and our
basic Raman patent is expected to cover all of our electron emission
activities.
Page
6
Key
Intellectual Property
Our most
significant patent in the electron emission area is a basic patent that we refer
to as the Raman Spectrum Patent (U.S. Patent 5869922) covering emissions from a
wide range of carbon forms. This patent covers field emission devices using
various forms of carbon; including carbon nanotubes (CNT), carbon films, and
other forms of carbon that fall within a particular range on the Untraviolet
Raman band. This basic patent is fundamental and has broad applicability and
wide geographic coverage, having been issued in the U.S., China, South Korea,
and has been validated in several European countries. The patent application is
also pending in Japan. In addition to this basic patent, we have a wide variety
of other patents that compliment this basic patent in specific situations. We
believe that any company developing a product that involves the emission of
electrons from carbon is likely to require a license to our
technology.
Competition.
Because of the strength of our intellectual property in the electron
emission area, our competition comes from other technologies, rather than from
other companies. For displays, this completion comes from LCD displays, Plasma
displays, LED displays, and color picture tubes.
EBT
Electronic
Display Products. EBT was formed to develop sun-readable display products
for outdoor use. We quickly expanded our focus to large area displays for indoor
use that would compete with Plasma and could be used as part of an overall point
of purchase advertising program and developed a patented product called the
E-Window™. We restructured EBT, stopped selling products directly, and instead
limited ourselves to licensing EBT’s intellectual property. As discussed below,
we sold EBT’s intellectual property in 2006.
Communication
patents. We
applied for patents covering a system of selling advertising for electronic
displays over the internet and other digital networks. The first of the patents,
which was filed in April 2000 with a priority date of April 1999, was issued in
2006. The allowed claims on this patent relate to methods, systems and computer
programs that facilitate displaying advertising information on multiple indoor
or outdoor electronic displays. There are also similar patents that we applied
for in Europe, Canada, Korea, and Japan. As discussed below, we sold this group
of patents in 2006.
Sale of
Intellectual Property. In 2006 we sold EBTs intellectual property in two
simultaneous transactions to Novus Communications Technologies, Inc. In the
first transaction, we sold our communications patents to Novus Partners, LLC, a
majority owned subsidiary of Novus Communications. We received an upfront
payment of $1,000,000 and the right to future royalties based on the revenue
received by Novus Partners. The agreement also contains certain provisions
related to future minimum royalty payments, which if not met, could allow us to
request a return of the patents. We received a total of $6,000 in payments in
2009 from Novus Partners.
In the
second transaction, we sold EBT’s remaining intellectual property and other
assets, which consisted solely of items related to the intellectual property,
such as drawings, etc. to Novus Displays, LLC, a newly formed organization owned
by Novus Communications. In exchange for the remaining intellectual property, we
received $500,000 and a 25% ownership interest in Novus Displays.
Future
Activities. EBT’s future activities
are limited to participation in the digital signage industry through Novus
Communications. Our communication patents are part of a larger package of
related patents held by Novus Partners and any future income that we may receive
is dependent on the ability of Novus Partners to license that patent package. We
have received a total of $6,000 in payments from Novus Partners since the time
of the initial payment in 2006, and we will not receive any additional royalties
from Novus Partners until such time as they have revenue producing agreements or
make minimum payments. While we anticipate receiving royalties in the future, we
have no control over, or input into, the licensing activities of Novus
Partners.
Page
7
Our
ownership interest in Novus Displays is a passive ownership interest, and we
have no active role in the day to day management. Our sale of our remaining
intellectual property to Novus Displays was done to facilitate the sale of our
communications patents to Novus Partners. We had no interest in devoting further
resources to develop that technology. The sale to Novus Displays provided us the
opportunity to receive additional future value from that technology, if it is
successfully deployed by Novus Displays. We will receive no income from Novus
Displays and only profit from an eventual sale of Novus Displays, if it occurs.
To the best of our knowledge, Novus Displays has not yet been capitalized beyond
its initial minimal capitalization, has not yet actively developed any of the
technology acquired from us in 2006, and is not carrying on any significant
business activities at the present time.
Technology
Agreements
Till Keesmann.
In May 2000, we licensed the rights to 6 carbon nanotube patents from
Till Keesmann in exchange for a payment of $250,000 payable in shares of our
common stock. Under the terms of the agreement, we are obligated to pay license
fees equal to 50% of any royalties received by us specifically related to these
patents. We are allowed to offset certain expenses, up to a maximum of $50,000
per year, against payments due under this agreement. The agreement also
contained provisions related to minimum license fee payments. These minimum
payments, totaling $1,000,000, have been made, and no further minimum payments
are due. We are allowed to offset these minimum payments against future royalty
payments; however, once these minimum payments and the expenses have been
offset, we may be liable for additional royalty payments.
The
Keesmann agreement was amended in 2008. As part of that amendment, the amount of
future offsets available for expenses incurred prior to June 2008 was fixed at
$300,000. In addition the amendment established licensing parameters under which
we can sublicense the patents without any additional approval by Mr. Keesmann.
The amendment also gave Mr. Keesmann the option of electing to receive an
additional advance of $100,000 against future royalties. In September 2008, Mr.
Keesmann elected to receive that advance, which is now also available for us to
offset against future royalties. Finally, the amendment contains provision which
would allow Mr. Keesmann to license the patents directly and remit 50% of the
royalties to us beginning in June 2010, if certain minimum royalties are not
achieved by that date. At this point, we have not made any additional payments
and it is unlikely that the minimum royalty payment will be achieved by May
2010.
Intellectual
Property Rights
An
important part of our product development strategy is to seek, when appropriate,
protection for our products and proprietary technology through the use of
various United States and foreign patents. Our patent portfolio consists of over
270 patents, including 90 issued patents and over 180 patent applications
pending before foreign and United States Patent and Trademark Offices. We also
have several unsubmitted patent applications in process. The patents, allowances
and applications relate to carbon nanotube field emission technology and other
technologies. In addition, there are foreign counterparts to certain United
States patents and applications. We consider our patent portfolio to be our most
valuable asset.
The
patenting of technology-related products and processes involves uncertain and
complex legal and factual questions. To date, no consistent policy has emerged
regarding the breadth of claims of such technology patents. Therefore, there is
no assurance that our pending United States and foreign applications will issue,
or what scope of protection any issued patents will provide, or whether any such
patents ultimately will be upheld as valid by a court of competent jurisdiction
in the event of a legal challenge. Interference proceedings, to determine
priority of invention, also could arise in any of our pending patent
applications. The costs of such proceedings would be significant and an
unfavorable outcome could result in the loss of rights to the invention at issue
in the proceedings. If we fail to obtain patents for our technology, and are
required to rely on unpatented proprietary technology, there is no assurance
that we can protect our rights in such unpatented proprietary technology, or
that others will not independently develop substantially equivalent proprietary
products and techniques, or otherwise gain access to our proprietary
technology.
Competitors
have filed applications for, or have been issued patents, and may obtain
additional patents and proprietary rights relating to products or processes used
in, necessary to, competitive with, or otherwise related to, our patents. The
scope and validity of these patents, the extent to which we may be required to
obtain licenses under these patents, or under other proprietary rights and the
cost and availability of licenses are unknown. This may limit our ability to
license our technology. Litigation concerning these or other patents could be
protracted and expensive. If suit were brought against us for patent
infringement, a challenge in the suit by us as to the validity of the other
patent would have to overcome a legal presumption of validity. There can be no
assurance that the validity of the patent would not be upheld by the court or
that, in such event, a license of the patent to us would be available. Moreover,
even if a license were available, the payments that would be required are
unknown and could materially reduce the value of our interest in the
affected products. We do, however, consider our patents to be very strong and
defendable in any action that may be brought against us. A major law firm has
reviewed our patent portfolio and agreed to handle litigation related to certain
of our patents on a contingency basis.
Page
8
We also
rely upon unpatented trade secrets. No assurances can be given that others will
not independently develop substantially equivalent proprietary information and
techniques or otherwise gain access to our trade secrets or disclose such
technology or that we can meaningfully protect our rights to our unpatented
trade secrets.
We
require our employees, directors, consultants, outside scientific collaborators,
sponsored researchers, and other advisors to execute confidentiality agreements
upon the commencement of employment or consulting relationships with us. These
agreements provide that all confidential information developed or made known to
the individual during the course of the relationship is to be kept confidential
and not disclosed to third parties except in specific circumstances. In the case
of employees, the agreements provide that all inventions conceived by the
individual shall be our exclusive property. There is no assurance, however, that
these agreements will provide meaningful protection for our trade secrets in the
event of unauthorized use or disclosure of such information.
Government
Regulation
Products
using our technology will be subject to extensive government regulation in the
United States and in other countries. In order to produce and market existing
and proposed products using our technology, our licensees must satisfy mandatory
safety standards established by the U.S. Occupational Safety and Health
Administration (“OSHA”), pollution control standards established by the U.S.
Environmental Protection Agency (“EPA”) and comparable state and foreign
regulatory agencies. We may also be subject to regulation under the Radiation
Control for Health and Safety Act administered by the Center for Devices and
Radiological Health (“CDRH”) of the U.S. Food and Drug Administration. We do not
believe that carbon nanotube field emission products will present any
significant occupational risks to the operators of such equipment. In addition,
the carbon nanotube field emission products are not expected to produce
significant hazardous or toxic waste that would require extraordinary disposal
procedures. Nevertheless, OSHA, the EPA, the CDRH and other
governmental agencies, both in the United States and in foreign countries, may
adopt additional rules and regulations that may affect us and products using our
technology. Additionally, our arrangements with our licensees and their
affiliates may subject products using our technology to export and import
control regulations of the U.S. and other countries. The cost of compliance with
these regulations has not been significant in the past and is not expected to be
material in the future.
A portion
of our revenue has consisted of reimbursement of expenditures under U.S.
government contracts. We recognized $1,694,082 of revenue in 2009, $2,295,887 in
2008, and $2,328,010 in 2007, related to government contracts. These
reimbursements represent all or a portion of the costs associated with such
contracts. As of December 31, 2009, we have several government contracts in
process that have approximately $1.8 million of revenue yet to be recognized.
Government contracts are subject to delays and risk of cancellation. Also,
government contractors generally are subject to various kinds of audits and
investigations by government agencies. These audits and investigations involve
review of a contractor’s performance on its contracts, as well as its pricing
practices, the costs it incurs and its compliance with all applicable laws,
regulations and standards. We are, and in the future expect to be, audited by
the government.
Employees
As of
February 26. 2010 we had 27 full-time employees, including 3 executive officers,
and 3 part time employees. Within the next twelve months, based on new
government contracts that we have received and expect to receive, we likely will
hire two to four additional employees to support our plans for increasing
research levels. We are not subject to any collective bargaining agreements, and
we consider our relations with our employees to be good.
Page
9
Available
Information
Our
website is http://www.appliednanotech.net.
Our periodic reports and all amendments to those reports required to be filed or
furnished pursuant to Section 13(a) or Section 15(d) of the Securities Exchange
Act of 1934 are available free of charge through its website. During the period
covered by this report, the Company made its periodic reports on Form 10-K, and
Form 10-Q and its current reports on Form 8-K and amendments to those documents
available on its website as soon as reasonably practicable after those reports
were filed with or furnished electronically to the Securities and Exchange
Commission. The Company will continue to make such reports and amendments to
those reports available on its website as soon as reasonably practicable after
those reports are filed with or furnished to the Securities and Exchange
Commission. Material contained on our website is not incorporated by reference
in this Annual Report on Form 10-K.
Page
10
|
Item
1A.
|
Risk
Factors
|
Our
success is dependent on our principal technologies
Our
technology platforms, which include nano-materials, nano-electronics, sensors,
electron emission activities, and nano-ecology, are emerging technologies. Our
financial condition and prospects are dependent upon licensing our intellectual
property to others and introduction of the technology into the marketplace.
Additional R&D needs to be conducted on some of our technologies before
others can produce products using this technology. Market acceptance of products
using our technology will be dependent upon acceptance within the industries of
those products of the quality, reliability, performance, efficiency, and breadth
of application and cost-effectiveness of the products. There can be no
assurances that these products will be able to gain commercial market
acceptance.
Products
using our technology may not be accepted by the market
Since our
inception, we have focused our product development and R&D efforts on
technologies that we believe will be a significant advancement over currently
available technologies. With any new technology, there is a risk that the market
may not appreciate the benefits or recognize the potential applications of the
technology. Market acceptance of products using our technology will depend, in
part, on the ability of our licensees to convince potential customers of the
advantages of such products as compared to competitive products. It will also
depend upon our ability to train manufacturers and others to use our
products.
Our
technology development is in its early stages and the outcome is
uncertain
Some of
our applications of nanotechnologies, and certain products that use these
technologies, will require significant additional development, engineering,
testing and investment prior to commercialization. We are exploring the use of
our technology in several different types of products. We have developed proof
of concepts of potential products based on our technologies. In some cases, we
are developing products jointly with others based on our technology. Upon
successful completion of the development process, our development partners will
likely be required to license our technology to produce and sell the products.
Our development partners retain all rights to any intellectual property that
they develop in the process.
If any of
the potential products that are being developed using our technologies are
successfully developed, it may not be possible for potential licensees to
produce these products in significant quantities at a price that is competitive
with other similar products. At the present time, the only significant revenue
that we receive related to our technology is related to reimbursed research
expenditures and development fees. These revenues are identified in our
quarterly filings on Form 10-Q and our annual filings on Form 10-K as revenues
of our Applied Nanotech, Inc. subsidiary in the related “Management’s Discussion
and Analysis of Financial Condition and Results of Operations” sections. We
anticipate receiving up-front license fees and royalties in 2010.
Our
development partners have certain rights to jointly developed property and to
license our technology
We have
committed to license our technology to our development partners upon completion
of certain development projects that are in process. The terms of all such
licenses have not yet been finalized. Our development partners usually also have
rights to any jointly developed property; however, any such jointly developed
property would likely be based, at least in part, on our underlying technology
which would require our partners to enter into a license agreement with us. See
also “Our technology development is in its early stages and the outcome is
uncertain” above for further discussion.
We
have limited resources and our focus on particular products may result in our
failure to capitalize on other opportunities
We have
limited resources available to successfully develop and commercialize our
technology. As of February 26. 2010, we had 30 full-time and part-time
employees. There is a wide array of potential applications for our technology
and our limited resources require us to focus on specific product areas, while
ignoring others. We focus our efforts on those projects for which we can obtain
external funding since the availability of funding provides an external
verification of the probability of commercial success of resulting
products.
Page
11
We
may not be able to provide system integration
In order
to prove that our technologies work and will produce a complete product, we may
be required to integrate a number of highly technical and complicated subsystems
into a fully integrated prototype. There is no assurance that we will be able to
successfully complete the development work on some of our proposed products or
that there will ultimately be any market for those products.
Many
products that may be developed using our technology will need to be integrated
into end-user products by manufacturers of those products. Although we intend to
develop products to be integrated into existing manufacturing capabilities,
manufacturers may be required to make modifications to, or expand their
manufacturing capabilities. Manufacturers may elect not to integrate products
using our technology into their end-user products, or they may not devote
adequate resources to modifying their manufacturing capabilities so that our
technologies can be successfully incorporated into their end-user products. The
complexity of integration may delay the introduction of products using our
technology.
Rapid
technological changes could render our technology obsolete; and we may not
remain competitive
The
industries in which we compete are highly competitive and are characterized by
rapid technological change. Our existing and proposed products will compete with
other existing products and may compete against other developing technologies.
Development by others of new or improved products, processes or technologies may
reduce the size of potential markets for our products. There is no assurance
that other products, processes or technologies will not render our proposed
products obsolete or less competitive. Many of our competitors have greater
financial, managerial, distribution, and technical resources than we do. We will
be required to devote substantial financial resources and effort to further
R&D. There is no assurance that we will successfully differentiate our
technology from our competitors’ technology, or that we will adapt to evolving
markets and technologies, develop new technologies, or achieve and maintain
technological advantages.
We
have limited manufacturing capacity and experience
We have
no established commercial manufacturing facilities; and we have no intention of
establishing a manufacturing facility on our own. We are focusing our efforts on
licensing our intellectual property to others for use in their manufacturing
processes. To the extent that any of the products that we develop require
manufacturing facilities, we intend to either contract with a qualified
manufacturer, or enter into a joint venture or other similar
arrangement.
The
health effects of nanotechnology are unknown
There is
no scientific agreement on the health effects of nanomaterials, but some
scientists believe that in some cases, nanomaterials may be hazardous to an
individual’s health or the environment. The science of nanotechnology is based
on arranging atoms in such a way as to modify or build materials not made in
nature; therefore, the effects are unknown. The Company takes appropriate
precautions for its employees working with carbon nanotubes and believes that
any health risks related to carbon nanotubes used in potential products can be
minimized. Future research into the effects of nanomaterials in general, and
carbon nanotubes in particular, on health and environmental issues may have an
adverse effect on products using our technology.
Public
perception(s) of ethical and social issues may discourage the use of
nanotechnology
Nanotechnology
has received both positive and negative publicity and is increasingly the
subject of public discussion and debate. Governments may, for social or health
purposes, prohibit or regulate the use of nanotechnology. This may restrict our
ability to license our technology, or the ability of our licensees to sell their
products.
The
loss of key personnel could adversely affect our business
Our
future success will depend on our ability to continue to attract and retain
highly qualified scientific, technical and managerial personnel. Competition for
such personnel may be intense. We may not be able to attract and retain all
personnel necessary for the development of our business. In addition, some of
the know-how and processes developed by us reside in our key scientific and
technical personnel. The loss of the services of key scientific, technical and
managerial personnel could have a material adverse effect on us until we are
able to replace those personnel.
Page
12
We
have a history of net losses
We have a
history of net losses, although these losses have been decreasing. From our
inception through December 31, 2009, we incurred net losses of approximately
$111 million. We have incurred net income and losses for the last ten years as
shown below:
|
Year
Ended December 31
|
Net
Income
(Loss)
|
|
|
2000
|
($9,471,279)
|
|
|
2001
|
($6,047,698)
|
|
|
2002
|
($5,452,890)
|
|
|
2003
|
($4,017,374)
|
|
|
2004
|
($7,139,109)
|
|
|
2005
|
($5,818,816)
|
|
|
2006
|
($6,593,892)
|
|
|
2007
|
($4,256,891)
|
|
|
2008
|
($2,685,867)
|
|
|
2009
|
($2,152,605)
|
Although
we expect to be profitable in the future, we may not be. Our profitability in
2010 is dependent upon a combination of signing of additional license agreements
and obtaining additional research funding. We may, however, continue to incur
additional operating losses for an extended period of time as we continue to
develop our technologies. We do, however, expect the magnitude of those losses,
if they continue, to decrease. We are primarily a contract research and
development organization and are dependent on license agreements and research
funding to achieve profitability. In order to continue development of our
technology, we anticipate that substantial research and development expenditures
will continue to be incurred. We have funded our operations to date primarily
through the proceeds from the sale of our equity securities and debt offerings.
Our auditors have included a going concern paragraph in their opinion on our
financial statements, which may impact our ability to obtain financing, if
additional financing is needed in 2010 beyond that which we have already
arranged as of the date of this filing.
We
have no current royalty agreements producing significant revenue
Our
strategy is dependent on licensing our technology to other companies and
obtaining royalties based on products that these licensees develop and sell. We
have no plans to manufacture and sell any products ourselves, and as such, we
have no product revenues. We may enter into joint ventures or other business
arrangements where we collaborate with others to sell or manufacture products.
While we do have existing licenses, none of the licensees are producing products
at the present time, and therefore none of the licenses are producing current
revenue. Successful implementation of our strategy requires royalty bearing
license agreements. We expect at least three of our licensees to introduce
products into the marketplace in 2010; however, there is no guarantee that
products will be introduced.
We expect
to license our technology to be used in many applications. See additional
discussion in the risk factor above entitled “Our technology
development is in its early stages and the outcome is uncertain”. It is our
intention that all future license agreements will include a provision that
requires the payment of ongoing royalties, although there is no assurance that
will occur.
We
are dependent on the availability of materials and suppliers
The
materials used in producing current and future products using our technology are
purchased from other vendors. We anticipate that the majority of raw materials
used in products to be developed by us will be readily available to
manufacturers. However, there is no assurance that the current availability of
these materials will continue in the future, or if available, will be procurable
at favorable prices.
Page
13
Our
revenues have been dependent on government contracts in the past
In many
years, a significant portion of our revenues were derived from contracts with
agencies of the United States government. Following is a summary of those
revenues for the past ten years:
|
Year
Ended December 31
|
Revenues
from
Government
Contracts
|
Percentage
of
Total
Revenue
|
||
|
2000
|
$352,341
|
13%
|
||
|
2001
|
$466,680
|
15%
|
||
|
2002
|
$254,152
|
18%
|
||
|
2003
|
$339,790
|
44%
|
||
|
2004
|
$305,721
|
80%
|
||
|
2005
|
$208,211
|
37%
|
||
|
2006
|
$583,236
|
52%
|
||
|
2007
|
$2,328,010
|
58%
|
||
|
2008
|
$2,295,887
|
58%
|
||
|
2009
|
$1,694,082
|
42%
|
We
currently have commitments for future government funding of approximately $1.8
million. We do not intend to seek any government funding unless it directly
relates to achievement of our strategic objectives.
Contracts
involving the United States government are, or may be, subject to various risks
including, but not limited to, the following:
|
|
·
|
Unilateral
termination for the convenience of the
government
|
|
|
·
|
Reduction
or modification in the event of changes in the government’s requirements
or budgetary constraints
|
|
|
·
|
Increased
or unexpected costs causing losses or reduced profits under fixed-price
contracts or unallowable costs under cost reimbursement
contracts
|
|
|
·
|
Potential
disclosure of our confidential information to third
parties
|
|
|
·
|
The
failure or inability of the prime contractor to perform its prime contract
in circumstances where we are a
subcontractor
|
|
|
·
|
The
failure of the government to exercise options provided for in the
contract.
|
|
|
·
|
The
right of the government to obtain a non-exclusive, royalty free,
irrevocable world-wide license to technology developed under contracts
funded by the government if we fail to continue to develop the
technology
|
We
may be exposed to litigation liability
We have
had lawsuits that arise in the normal course of business. We have been subject
to litigation in the past and have settled litigation in the past that has in
rare instances resulted in material payments. We expect all current lawsuits to
be resolved with no material negative impact on our financial statements, and we
are unaware of any other potential significant litigation. If we were to become
subject to a judgment that exceeds our ability to pay, that judgment would have
a material impact on our financial condition and could affect our ability to
continue in existence.
We
may have future capital needs and the source of that funding is
uncertain
We expect
to continue to incur substantial expenses for R&D, product testing, and
administrative overhead. The majority of R&D expenditures are for the
development of our technologies. Some of the proposed products using our
technology may not be available for commercial sale or routine use in the
immediate future. Commercialization of existing and proposed products that would
use our technology may require additional capital in excess of that currently
available to us. A shortage of capital could prevent us from achieving
profitability for an extended period of time. Because the timing and receipt of
revenues from the sale of products using our technology will be tied to the
achievement of certain product development, testing, manufacturing and marketing
objectives, which cannot be predicted with certainty, there may be substantial
fluctuations in our results of operations. If revenues do not increase as
rapidly as anticipated, or if product development and testing requires more
funding than anticipated, we may be required to curtail our activities and/or
seek additional financing from other sources. We may seek additional financing
through the offer of debt, equity, or any combination of the two at any
time.
Page
14
We have
developed a plan to allow us to maintain operations until we are able to sustain
ourselves. We have the existing resources to continue operations for a period
through at least the end of 2010 and well into 2011. Expected revenues,
excluding any new license agreements, will extend that period. Our plan is
primarily dependent on raising funds through the licensing of our technology and
revenue generated from performing contract research services. We expect to sign
significant license and development contracts within the next year, although
there is no assurance that this will occur.
Our plan
is based on current development plans, current operating plans, the current
regulatory environment, historical experience in the development of electronic
products and general economic conditions. Changes could occur which would cause
certain assumptions on which this plan is based to be no longer valid. Our plan
is primarily dependent on increasing revenues, licensing our technology, and
raising additional funds through additional debt and equity offerings, only if
necessary. If adequate funds were not available from operations or additional
sources of financing, we may have to eliminate, or reduce substantially,
expenditures for research and development, and testing of our products. We may
have to obtain funds through arrangements with other entities that may require
us to relinquish rights to certain of our technologies or products. These
actions could materially and adversely affect us.
We
may be unable to enforce or defend our ownership and use of proprietary
technology
Our
ability to compete effectively with other companies will depend on our ability
to maintain the proprietary nature of our technology. Although we have been
awarded patents, have filed applications for patents, or have licensed
technology under patents that we do not own, the degree of protection offered by
these patents or the likelihood that pending patents will be issued is
uncertain. Competitors in both the United States and foreign countries, many of
which have substantially greater resources and have made substantial investment
in competing technologies, may already have, or may apply for and obtain patents
that will prevent, limit or interfere with our licensee’s ability to make and
sell our products using our technology. Competitors or potential licensees may
also intentionally infringe on our patents. The defense and prosecution of
patent suits is both costly and time-consuming, even if the outcome is favorable
to us.
In
foreign countries, the expenses associated with such proceedings can be
prohibitive. In addition, there is an inherent unpredictability in obtaining and
enforcing patents in foreign countries. An adverse outcome in the defense of a
patent suit could subject us to significant liabilities to third parties.
Although third parties have not asserted infringement claims against us, there
is no assurance that third parties will not assert such claims in the future. A
major law firm has reviewed our patent portfolio and agreed to handle litigation
related to certain of our patents on a contingency basis.
Changes
in patent laws could have a negative impact on us
New
legislation, regulations, or rules related to obtaining or enforcing patents
could significantly increase our operating costs and make it more difficult to
enforce or license our patents. If new legislation, regulations, or rules are
implemented by either Congress, the United States Patent and Trademark Office,
or the courts that impact the patent application process, the patent enforcement
process, or the rights of patent holders, these changes could negatively affect
our expenses and potential revenue.
We
rely on unpatented proprietary technology
We also
rely on unpatented proprietary technology, and there is no assurance that others
will not independently develop the same or similar technology, or otherwise
obtain access to our proprietary technology. To protect our rights in these
areas, we require employees, consultants, advisors and collaborators to enter
into confidentiality agreements. These agreements may not provide meaningful
protection for our trade secrets, know-how, or other proprietary information in
the event of any unauthorized use, misappropriation or disclosure of such trade
secrets, know-how, or other proprietary information. While we have attempted to
protect proprietary technology that we develop or acquire and will continue to
attempt to protect future proprietary technology through patents, copyrights and
trade secrets, we believe that our success will depend upon further innovation
and technological expertise.
Page
15
We
have technologies subject to licenses
In May
2000, we licensed the rights to 6 carbon nanotube patents from Till Keesmann in
exchange for a payment of $250,000 payable in shares of our common stock. Under
the terms of the agreement, we are obligated to pay license fees equal to 50% of
any royalties received by us specifically related to these patents. We are
allowed to offset certain expenses, up to a maximum of $50,000 per year, against
payments due under this agreement. The agreement also contained provisions
related to minimum license fee payments. These minimum payments, totaling
$1,000,000, have been made and no further minimum payments are due. We are
allowed to offset these minimum payments against future royalty payments;
however, once these minimum payments and the expenses have been offset, we may
be liable for additional royalty payments.
The
Keesmann agreement was amended in 2008. As part of that amendment, the amount of
future offsets available for expenses incurred prior to June 2008 was fixed at
$300,000. In addition the amendment established licensing parameters under which
we can sublicense the patents without any additional approval by Mr. Keesmann.
The amendment also gave Mr. Keesmann the option of electing to receive an
additional advance of $100,000 against future royalties. In September 2008, Mr.
Keesmann elected to receive that advance, which is now available for us to
offset against future royalties. Finally, the amendment contains provision which
would allow Mr. Keesmann to license the patents directly and remit 50% of the
royalties to us beginning in June 2010, if certain minimum royalties are not
achieved by that date. No additional royalties have been paid since then and it
is unlikely that the minimum royalties will be achieved by May
2010.
Our
business is subject to changing regulation of corporate governance and public
disclosure
Because
our common stock is publicly traded, we are subject to certain rules and
regulations of federal and state entities charged with the protection of
investors and the oversight of companies whose securities are publicly traded.
These entities have continued to develop additional regulations and requirements
in response to laws enacted by Congress, most notably the Sarbanes-Oxley Act of
2002. Complying with these new regulations has resulted in, and is likely to
continue to result in, increased general & administrative costs and a
diversion of management time and attention from revenue generating and other
business activities to compliance activities.
Our
business is subject to economic uncertainties
A portion
of our research revenues come from private sources, primarily large
multinational corporations. In addition, our strategy is dependent upon the
receipt of royalties related to the introduction of new products by these and
other companies. During times of extreme economic uncertainty, some companies
may cut back on spending on research projects, or delay the introduction of new
products.
|
Item
1B.
|
Unresolved Staff
Comments
|
None.
Page
16
|
Item
2.
|
Properties
|
We lease
a facility in Austin that is used for our corporate headquarters and research
and development activities under a lease expiring in February 2014. The lease
calls for payments of $12,505 through February 2010, $13,432 from March 2010
through February 2012, and $14,461 for the period from March 2012 through
February 2014.
We
believe that these facilities are suitable for our current needs and will be
adequate for our anticipated research, development, and administrative
activities, at least through the end of the lease period. We continuously
monitor our facility needs and may will move to a different facility at the end
of the lease period. We would expect a new facility to be similar in size to our
existing facility. If we embark on significant new research that requires
significant additional employees, we may have to establish additional
facilities.
We do not
currently invest in real estate or interests in real estate, real estate
mortgages, or securities of or interests in persons primarily engaged in real
estate activities. However, the Company has no policy, either for or against,
making such investments.
|
Item
3.
|
Legal
Proceedings
|
From time
to time the Company and its subsidiaries are also defendants in various lawsuits
that may arise related to minor matters. It is expected that any such lawsuits
would be settled for an amount no greater than the liability recorded in the
financial statements for such matters. If resolution of any of these suits
results in a liability greater than that recorded, it could have a material
impact on us.
Page
17
PART
II
|
Item
4.
|
Market for Registrant’s Common
Equity, Related Stockholder Matters and Issuer Purchases of Equity
Securities
|
Our
common stock, $0.001 par value, trades on the OTC Bulletin Board system under
the symbol “APNT”. The following table sets forth, on a per share basis for the
periods indicated, the high and low sale prices for the common stock as reported
by the OTC Bulletin Board system. These quotations reflect inter-dealer prices,
without retail mark-up, mark-down or commission and may not represent actual
transactions.
|
High
|
Low
|
||||||
|
2008
|
First
Quarter
|
$1.30
|
$0.86
|
||||
|
Second
Quarter
|
$1.30
|
$0.96
|
|||||
|
Third
Quarter
|
$1.15
|
$0.40
|
|||||
|
Fourth
Quarter
|
$0.55
|
$0.18
|
|||||
|
2009
|
First
Quarter
|
$0.37
|
$0.19
|
||||
|
Second
Quarter
|
$0.36
|
$0.22
|
|||||
|
Third
Quarter
|
$0.35
|
$0.19
|
|||||
|
Fourth
Quarter
|
$0.33
|
$0.19
|
|||||
|
2010
|
First
Quarter (through February 26. 2010)
|
$0.37
|
$0.20
|
As of
February 26. 2010, the closing sale price for our common stock as reported on
the OTC Bulletin Board system was $0.32. As of February 26. 2010, there were
approximately 350 shareholders of record for our common stock. This does not
include shareholders holding stock in street name in brokerage accounts. As of
our last record of total shareholders, including those holding stock in street
name, there were approximately 7,500 shareholders.
Cash
Dividends
We have
never paid cash dividends on our common stock, and it is unlikely that we will
pay any dividends in the foreseeable future. We currently intend to invest
future earnings, if any, to finance expansion of our business. Any payment of
cash dividends in the future will be dependent upon our earnings, financial
condition, capital requirements, and other factors deemed relevant by our board
of directors.
Page
18
Performance
Graph
The
following graph shows a comparison, since December 31, 2004, of cumulative total
return for Applied Nanotech Holdings, the NASDAQ Market Index, and an index for
Commercial Physical Research (SIC code 8731).
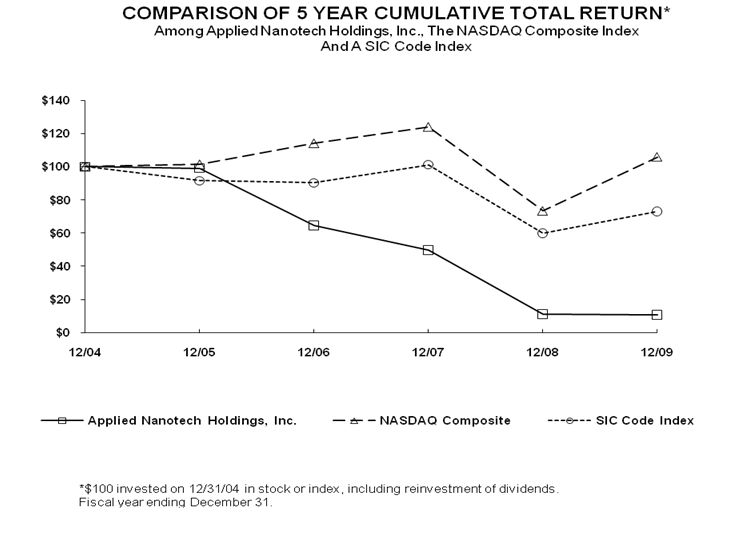
|
12/31/04
|
12/31/05
|
12/31/06
|
12/31/07
|
12/31/08
|
12/31/09
|
||
| Applied Nanotech Holdings, Inc |
100.00
|
99.08
|
64.52
|
49.77
|
11.06
|
10.60
|
|
|
NASDAQ
Market Index
|
100.00
|
101.41
|
114.05
|
123.93
|
73.43
|
105.89
|
|
|
Commercial
Physical Research (SIC Code 8731)
|
100.00
|
91.55
|
90.32
|
101.22
|
59.90
|
72.98
|
|
The
performance graph assumes $100 invested on December 31, 2004 in our common
stock, the NASDAQ Market Index, and an index based on a basket of stocks
composing SIC Code 8731. The total return assumes reinvestment of dividends for
the NASDAQ Market Index and the SIC Code Index. The total return is based on
historical results and is not intended to indicate future performance. These
dates represent arbitrary points in time and if different dates were presented,
different results would be obtained.
Recent
Sales of Unregistered Securities
We issued
no unregistered shares of our securities in the fiscal quarter ended December
31, 2009.
Page
19
Item
5. Selected Financial
Data
|
2009
|
2008
|
2007
|
2006
|
2005
|
||||||||||||||||
|
Revenues
|
$ | 4,052,746 | $ | 3,957,832 | $ | 3,989,803 | $ | 1,116,670 | $ | 565,660 | ||||||||||
|
Net
income (loss)
|
$ | (2,152,605 | ) | $ | (2,685,867 | ) | $ | (4,256,891 | ) | $ | (6,593,892 | ) | $ | (5,818,816 | ) | |||||
|
Income
(loss) per share
|
$ | (0.02 | ) | $ | (0.03 | ) | $ | (0.04 | ) | $ | (0.07 | ) | $ | (0.06 | ) | |||||
|
Total
assets
|
$ | 893,367 | $ | 1,792,489 | $ | 3,744,858 | $ | 2,693,442 | $ | 1,187,981 | ||||||||||
|
Long-term
obligations
|
$ | 196,958 | $ | 27,909 | $ | 30,004 | $ | — | $ | — | ||||||||||
|
Net
shareholders’ equity (deficit)
|
$ | (1,070,838 | ) | $ | 858,286 | $ | 2,828,902 | $ | 642,262 | $ | 859,339 | |||||||||
|
Cash
dividends per common share
|
$ | — | $ | — | $ | — | $ | — | $ | — | ||||||||||
Page
20
Item
6. Management’s Discussion and
Analysis of Financial Condition and Results of
Operations
The
following discussion should assist in understanding our financial condition,
liquidity, and capital resources as of December 31, 2009, and the results of
operations for the years ended December 31, 2009, 2008 and 2007. The Notes to
our Consolidated Financial Statements included later in this report contain
detailed information that should also be read in conjunction with this
discussion.
OVERVIEW
We are
engaged in research and development related to nanotechnology products.
Our main areas of focus in our research are on nanomaterials,
nanoelectronics, sensors, nanoecology, and electron emission activities. Our
technology, as well as many of its potential applications, is still new and in
the early stages of development. Our revenue has primarily consisted of
development projects involving either the U.S. government or large multinational
corporations. We have also received upfront payments related to royalty
agreements, but have not yet received royalties related to the sale of products
produced using our technology.
OUTLOOK
We expect
our total cash needs for 2010 to be approximately $6.2 million. We intend to
fund those needs through a combination of our cash on hand, sales,
reimbursements for research, and license agreements. We anticipate receiving
significantly more revenue in 2010 than was received in 2009. We have developed
a plan to enable us to achieve positive cash flow from operations with
approximately $6.5 million of revenue. As of February 26. 2010, we have
approximately $1.7 million in cash to cover potential revenue shortfalls that
may occur for the year and we believe this cash is adequate for our needs in
2010. We have also raised cash through a convertible debt offering as described
in the footnotes to the financial statements, and may raise additional cash
through this offering.
We have a
history of net operating losses. Our plan is to generate sufficient revenues in
2010 to achieve breakeven or better; however, losses may continue as we continue
to develop our technologies. There can be no assurances that we will be
profitable in 2010 or the future. Full commercial development of our technology
may require additional funds in the future. We expect to continue our
concentrated research and development of our technology in 2010.
We have
developed a plan to allow ourselves to maintain operations until we are able to
sustain ourselves on our own revenue. Our plan is primarily dependent on raising
funds through the licensing of our technology and through reimbursed research
arrangements. Our current cash balances, when combined with expected revenues
sources, are expected to be adequate to insure that we can maintain our
operations at the present level. We believe that we have the ability to continue
to secure short term funding, if needed, to enable us to continue operations
until our plan can be completed if this plan takes longer than anticipated. Our
auditors have included a going concern paragraph in their opinion on our
financial statements, which may impact our ability to obtain financing, if
financing is needed in 2010 beyond that which we have already arranged as of the
date of this filing.
This plan
is based on current development plans, current operating plans, the current
regulatory environment, historical experience in the development of electronic
products, and general economic conditions. Changes could occur which would cause
certain assumptions on which this plan is based to be no longer valid. Our plan
is primarily dependent on increasing revenues. Although we do not expect funding
our operations to be a problem, if adequate funds are not available from
operations, or additional sources of financing, we may have to eliminate, or
reduce substantially, expenditures for research and development, testing of our
products, or obtain funds through arrangements with other entities that may
require us to relinquish rights to certain technologies or products. Such
results could materially and adversely affect us.
Critical
Accounting Policies
Our
significant accounting policies are summarized in the footnotes to our financial
statements. Some of the most critical policies are also discussed
below.
Page
21
Stock based
compensation - We routinely grant stock
options to employees and others. We have granted options in the past and each
year all employees have the opportunity to earn additional performance-based
option awards. We account for these options using the fair value method of
accounting.
Other
- As a matter of policy,
we review our major assets for impairment. Our major operating assets are
accounts receivable, and property and equipment. We depreciate our property and
equipment over their estimated useful lives. We did not identify any impaired
items in 2007, 2008 or 2009. We have not experienced significant bad debts
expense, and we do not believe that we need an allowance for doubtful accounts
for any exposure to loss in our December 31, 2009 accounts
receivable.
LIQUIDITY
AND CAPITAL RESOURCES
Our cash
balance of $286,971 at December 31, 2009 was significantly lower than our cash
balance of $710,111 at December 31, 2008, as a result of our decision to not
raise additional equity in 2009. We had a working capital deficit of
approximately $1.2 million at December 31, 2009, compared with working capital
of roughly $0.5 million at December 31, 2008. Our current assets decreased by
approximately $900,000 as a result of our decrease in cash and a reduction in
accounts receivable. The December 31, 2008 accounts receivable balance included
a license payment receivable from our sporting goods partner that was received
in 2009. No similar receivable exists at December 31, 2009. Our current
liabilities increased by approximately $900,000 as a result of increases in
accounts payable and accrued expenses. The increase in accrued expenses was
primarily the result of deferral of officer salaries and board of director’s
compensation to conserve cash. The accounts payable increase was primarily the
result of working capital management.
The
principal source of our liquidity has been funds received from exempt offerings
of our common stock; however, in 2009 or 2008, we did not raise any equity from
private placements of our common stock. The last private placement of our common
stock was in April 2007 when we raised $6.0 million. We did receive proceeds of
approximately $60,000, and $300,000 from the exercise of stock options by
current and former employees in 2008, and 2007, respectively. No options were
exercised in 2009. We did receive $200,000 related to convertible notes in 2009
and an additional $1,550,000 subsequent to December 31, 2009.
As of
February 26. 2010, our cash balance is approximately $1.7 million. We believe
that our current cash level, when combined with known revenue is enough for us
to operate at least through the end of 2010 and well into 2011. We expect to
obtain additional revenue, beyond the known sources, and we expect to be able
raise additional, if needed. If neither of those two things occur, we intend to
cut expenses to a level to enable us to continue operations.
In the
event that we need additional funds in 2010 beyond the amount that we have
planned as of the date of this filing and those that we expect to receive as
revenues or from other sources, we may seek to sell additional debt or equity
securities. While we expect to be able to obtain any funds needed for
operations, there is no assurance that any financing alternatives can be
arranged on commercially acceptable terms. We believe that our success in
reaching profitability will be dependent upon the viability of products using
our technology and their acceptance in the marketplace.
Net cash
used in operating activities has decreased each of the last two years, to
approximately $561,000 in 2009, as compared with $2.3 million in 2008, and $5.3
million in 2007. The cash used in operations was primarily the result of the net
losses incurred in each year. Factors affecting this are discussed in the
Results of Operations section below. In 2009, a substantial portion of the net
loss was offset by management of working capital items, including reduction of
accounts receivable, increase in accounts payable, and deferral of officer and
board compensation resulting in an increase in accrued expenses. Payment of
legal fees related to the Keesmann litigation also significantly impacted the
cash used in operations in 2007. Approximately $1.1 million of legal fees
incurred in 2006 were paid in 2007, which had the overall effect of increasing
cash used in operations in 2007 by that amount. We have a plan to generate
positive cash flow from operations in 2010; however, since that will require
significantly increased revenues, there is no assurance that will be
achieved.
Cash used
in investing activities was the result of the purchase of capital assets in all
years. We would expect minimal cash to be used in investing activities in 2010.
No material commitments exist as of December 31, 2009, for future purchases of
capital assets.
Page
22
Our
contractual obligations as of December 31, 2009 consist of notes payable
(including interest), operating leases, and capital leases. The notes payable
are convertible into common stock at a rate of $0.20 per share and may be
converted before their due date in 2012. A summary of our obligations at
December 31, 2009 is as follows:
|
Total
|
2010
|
2011
|
2012
|
2013
|
2014
|
|||||||||||||||||||
|
Capital
leases
|
$ | 63,417 | $ | 27,691 | $ | 20,230 | $ | 9,787 | $ | 5,709 | — | |||||||||||||
|
Operating
leases
|
$ | 791,570 | $ | 187,179 | $ | 186,071 | $ | 193,589 | $ | 195,781 | $ | 28,950 | ||||||||||||
|
Convertible
notes Payable
|
$ | 220,000 | — | — | $ | 220,000 | — | — | ||||||||||||||||
We
believe that we will have the cash available to meet our cash requirements for
fiscal 2010.
RESULTS
OF OPERATIONS
Business Segments. We
have three reportable business segments, our Applied Nanotech, Inc. subsidiary,
our Electronic Billboard Technology, Inc. subsidiary, and our remaining
activities, primarily corporate overhead. Since EBT has only minimal activity,
our management discussion and analysis related to results of operations is much
more meaningful if done on a consolidated basis. To the extent that EBT activity
had a significant impact on consolidated activity, it will be discussed in the
context of the consolidated results.
Revenues. Following
is a summary of key revenue categories for the three years covered by this
report.
|
2009
|
2008
|
2007
|
||||||||||
|
Government
Revenues
|
$ | 1,694,082 | $ | 2,295,887 | $ | 2,328,010 | ||||||
|
Other
Contract Research
|
$ | 1,767,144 | $ | 824,358 | $ | 990,598 | ||||||
|
License
Fees and Royalties
|
$ | 500,000 | $ | 577,000 | $ | — | ||||||
|
Total
Revenues
|
$ | 4,052,746 | $ | 3,957,832 | $ | 3,989,803 | ||||||
|
Revenue
backlog at December 31
|
$ | 2,938,000 | $ | 3,272,000 | $ | 3,891,000 | ||||||
All of
the revenues during the 3-year period came from ANI. Our revenues were roughly
similar in all years and the majority of the revenues came from research
contracts. Our license revenue in 2008 came from the license
agreement with our sporting goods partner and in 2009 from our inks
partner.
The
revenue backlog as of December 31, 2009 results from several government programs
and private contracts and is similar to prior years. The majority of these
programs are currently in progress and generating revenue, with the remainder
related to contracts awarded, but not yet started. The overwhelming majority of
this backlog will be converted into revenue in 2010. We also have some highly
likely contracts that have not yet been finalized, and therefore, are not
included in the backlog numbers as of December 31, 2009. In addition, we expect
our inks partner to make a payment of $1.0 million in June 2010 to complete its
exclusive license. This expected payment is not included in our research
backlog.
We expect
revenue to be significantly higher in 2010 than in 2009, and we expect to
generate minimum revenues in 2010 of $6.0 million at ANI. Of that, $2.3 million
is already committed and in process, and the majority of the remaining $3.7
million has been specifically identified. There is no guarantee that these
revenues will be obtained; however, we think it is equally as likely that
revenues will exceed these numbers, as it is that they fall short of them.
Revenues could increase above these levels as a result of royalty agreements or
additional research revenues, but there is no assurance that this will occur, or
that even the projected minimum of $6.0 million in revenue will be achieved. We
are currently pursuing opportunities in several areas that could result in
additional revenue in 2010.
We expect
our revenues in all categories – government contracts, private research, and
license agreements, to increase in 2010 over 2009 levels.
Page
23
Cost of sales.
Because we do not ship products or provide homogenous services, we do not incur
costs of sales in the traditional sense. We do keep track of our costs on
individual projects, but because there is a wide variation in cost percentages,
presenting cost of sales information is not meaningful. Government sponsored
research has nominal or no gross margins and is primarily just a reimbursement
of costs. In some cases we charge nominal amounts for projects that have much
higher costs because we are proving a concept that will be helpful to us in
other areas, or are seeking a significantly larger follow up contract with the
customer. In other instances we may perform research contracts that have
significant positive margins because we are able to capitalize on work that we
have done and knowledge that we have gained in the past. At the present stage of
our development, it is more meaningful to look at total research and development
costs in conjunction with revenues than to look at solely internally funded
research projects and the cost of research associated with revenue producing
contracts.
Research and
development. Following is a summary of research and development
expenditures for the past three years.
|
2009
|
2008
|
2007
|
||||||||||
|
Total
Research and development
|
$ | 3,662,323 | $ | 4,614,644 | $ | 4,526,166 | ||||||
All
research and development expenses were incurred by Applied Nanotech, Inc. Our
level of research activity was roughly similar in 2008 and 2007, but down in
2009. In 2009, we continued to concentrate our efforts on funded projects and
reduce unfunded research and development. This resulted in a reduction of
research and development cost, even though revenues increased. In addition, our
mix of research revenue in 2009 was less heavily weighted toward government
contracts which frequently have lower margins.
We expect
to continue to incur substantial expenses in support of additional research and
development activities related to the commercial development of our
technologies. We expect to incur at least similar amounts of research related
expenditures in 2010 to those incurred in 2009. We may, however, incur more
research and development expense in 2010 than presently expected. We are
pursuing numerous opportunities for research contracts and depending upon the
nature of the contracts signed, we may require more research materials than
expected, or we may require additional personnel.
Selling, general, and
administrative. Following are some key components of selling, general,
and administrative expense:
|
2009
|
2008
|
2007
|
||||||||||
|
Stock
based compensation
|
$ | 131,962 | $ | 418,252 | $ | 22,705 | ||||||
|
Patent
Expense
|
$ | 731,422 | $ | 789,006 | $ | 545,643 | ||||||
|
Litigation
related expenses
|
$ | — | $ | 166,278 | $ | 1,110,946 | ||||||
|
Other
S, G, & A
|
$ | 1,677,432 | $ | 2,524,403 | $ | 2,179,426 | ||||||
|
Total
selling, general, and administrative
|
$ | 2,540,816 | $ | 3,897,939 | $ | 3,858,720 | ||||||
Total
selling, general and administrative expense was similar in 2007 and 2008, but
decreased substantially in 2009 as a result of the factors discussed
below.
One
significant component of selling, general, and administrative expense is stock
based compensation. Stock based compensation is significantly impacted by two
factors. First, the number of options vested, or expected to vest, each year.
Second, the fair value of the options vested. One of the factors impacting the
fair value is the underlying price of our stock. As a general rule, options
granted when the stock price is lower will have a lower fair value than options
granted when the stock price is higher. Stock based compensation expense was low
in 2007 as a result of a change in the estimate of the number of options related
to a multiyear goal program that were expected to vest, resulting in a reversal
of expenses booked in previous years. Stock based compensation was lower in 2009
than 2008, both because fewer options were granted and because our stock price
was lower. We will incur stock based compensation expense in 2010, but it is
difficult to predict the amount. The majority of options that we grant are
performance based options that vest upon the achievement of specified goals. The
amount of expense that we incur in 2010 will depend on the goals
achieved.
Page
24
The
second major component of selling, general, and administrative expense during
the periods presented is litigation related expense. We incurred significant
amounts of litigation related expense in 2007 as a result of our litigation
against both Canon, Inc. and Till Keesmann. The amount was significantly lower
in 2008 because both of these cases were completed. The Canon case went to trial
in 2007 and only minimal activity related to the appeal took place in 2008. The
Keesmann litigation was settled in 2008. As a result, our litigation expense
decreased significantly. We had no litigation in expense in 2009 and we
currently have no significant litigation in process and expect litigation
expense to be insignificant in 2010.
We also
spend a significant amount on patents. Our patent expense increased from
approximately $546,000 in 2007 to approximately $789,000 in 2008. This was the
result of an increase in the number of patents and a substantial rate increase
at our primary patent law firm. Our patent expense decreased to approximately
$731,000 in 2009 as a result of a switch in the law firm handling our patents in
the second half of the year. We expect patent expense to decrease further in
2010 as a result of this change. In addition, we are reviewing our patent
portfolio and eliminating or selling any patents we no longer consider useful to
us.
Other
selling, general, and administrative expense increased from 2007 to 2008. The
increase related to primarily to professional fees and royalty payments. Our
other professional fees increased by approximately $250,000 also as a result of
fees associated with the sale of portions of our intellectual property and
marketing efforts. Finally, we made an advance royalty payment of $100,000 to
Till Keesmann in connection with the amendment of our existing license agreement
with Mr. Keesmann.
Our other
selling, general, and administrative expenses decreased substantially from 2008
to 2009 as a result of significant cuts that we made. These were wide ranging
reductions covering most areas. We combined the CEO and CFO positions when our
previous CEO resigned, saving in excess of $200,000. We also reduced our
professional fees. We did not incur the previously mentioned fees associated
with the sale of intellectual property in 2009, or the royalties, that were
incurred in 2008.
We expect
selling, general and administrative expense in 2010 to be approximately $2.4
million, a slight reduction from 2009 levels as a result of the full year impact
of our cost reduction efforts in 2009. If revenues increase above
anticipated levels, or other unanticipated transactions occur, our selling,
general and administrative expenses could increase above expected
levels.
Gain on sale of intellectual
property and other assets. In 2008, we had total gains of $1,329,571
related to the sale of intellectual property and other assets. The majority of
that, $1,225,999, came from a sale of a portion of our future interest in
royalties from sublicensing the Keesmann patents. We also had a gain of $100,000
from the sale of certain patents which we were no longer using and which did not
relate to any of our current active technology platforms. The balance of the
gain in 2008 came from the sale of miscellaneous equipment.
In 2009,
the gain resulted from a payment received related to intellectual property sold
by our EBT subsidiary in 2006.
Other income.
Following is a summary of other income for the last three fiscal
years.
|
2009
|
2008
|
2007
|
||||||||||
|
Interest
expense
|
$ | (10,089 | ) | $ | (7,180 | ) | $ | (926 | ) | |||
|
Interest
Income
|
$ | 1,877 | $ | 46,493 | $ | 139,118 | ||||||
|
Litigation
settlement
|
$ | — | $ | 500,000 | $ | — | ||||||
Our
interest expense in all years was primarily associated with capital leases. We
expect our interest expense will increase in 2010 because of the convertible
notes payable that we issued in December 2009 and during 2010. Our interest
income is earned as a result of the investment of excess cash balances. Our
interest income was higher in 2007 as a result of the private placement of our
common stock that was completed in April 2007 and resulted in higher average
levels of invested funds throughout the remainder of the year. Our interest
income decreased in 2008 and 2009 as cash balances decreased and interest rates
decreased. We expect our interest income in 2010 to be negligible, given the
current interest rate environment. The litigation settlement in 2008 was income
from our settlement with the defendants in the Keesmann
litigation.
Page
25
Overview
The
largest single component of cost that we incur is payroll related expense.
Excluding the cost related to stock based compensation, we incurred payroll
related expense of approximately, $3.3 million in 2007, $3.5 million in 2008,
and $3.1 million in 2009. The reduction from the 2008 level results from a
decrease in the number of employees, as well as other cost reduction
initiatives. We expect payroll related expense in 2010 to remain at
approximately $3.1 million. We expect our burn rate for 2010, excluding any
revenue, to average slightly less than $500,000 per month. Based on this, we
believe we can reach breakeven at a revenue level of $6.0 million, but there is
no assurance that this will occur, or that we can achieve that level of revenue.
Our burn rate, excluding any revenue, as of the date of this filing is closer to
$425,000 per month. We expect expenditures to increase as additional revenue
producing projects are obtained. This expenditure level is based on anticipated
revenue levels. If these revenue levels are not attained, we will not incur many
of these expenses, and our expense level will also be lower than
anticipated.
SEASONALITY
AND INFLATION
Applied
Nanotech Holdings’ business is not seasonal in nature. Management believes that
Applied Nanotech Holdings’ operations have not been affected by
inflation.
ACCOUNTING
PRONOUNCEMENTS
In
June 2009, the Financial Accounting Standards Board (“FASB”) issued ASC 105
Accounting Standards Codification TM and Hierarchy of Generally Accepted
Accounting Principles. The FASB Accounting Standards Codification TM (the
“Codification”) has become the source of authoritative accounting principles
recognized by the FASB to be applied by nongovernmental entities in the
preparation of financial statements in accordance with Generally Accepted
Accounting Principles (“GAAP”). All existing accounting standard documents are
superseded by the Codification and any accounting literature not included in the
Codification will not be authoritative. Rules and interpretive releases of the
SEC issued under the authority of federal securities laws, however, will
continue to be the source of authoritative generally accepted accounting
principles for SEC registrants. Effective September 30, 2009, all references to
GAAP in our consolidated financial statements will include references to the new
Codification. The Codification does not change or alter existing GAAP and,
therefore, will not have an impact on our financial position, results of
operations, or cash flows.
In May
2009, the FASB issued ASC 855, Subsequent Events, which provides guidance on
events that occur after the balance sheet date but prior to the issuance of the
financial statements. ASC 855 distinguishes events requiring recognition in the
financial statements and those that may require disclosure in the financial
statements. Furthermore, ASC 855 requires disclosure of the date through which
subsequent events were evaluated. These requirements are effective for interim
and annual periods after June 15, 2009. We have adopted these requirements and
have evaluated subsequent events through February 26. 2010.
Item
6A. Quantitative and
Qualitative Disclosures About Market Risk
We do not
use any derivative financial instruments for hedging, speculative, or trading
purposes. Our exposure to market risk is currently immaterial.
Page
26
|
Item
7.
|
Financial Statements and
Supplementary Data
|
INDEX
TO CONSOLIDATED FINANCIAL STATEMENTS
OF
APPLIED NANOTECH HOLDINGS, INC.
CONSOLIDATED
FINANCIAL STATEMENTS
|
Independent
Auditor’s Report
|
28
|
|
Consolidated
Balance Sheets - December 31, 2009 and 2008
|
29
|
|
Consolidated
Statement of Operations - Years Ended December 31, 2009, 2008, and
2007
|
30
|
|
Consolidated
Statements of Shareholders’ Equity - Years Ended December 31, 2009, 2008,
and 2007
|
31
|
|
Consolidated
Statements of Cash Flows - Years Ended December 31, 2009, 2008, and
2007
|
32
|
|
Notes
to Consolidated Financial Statements
|
33
|
Page
27
REPORT
OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the
Board of Directors and Shareholders
Applied
Nanotech Holdings, Inc.
Austin,
Texas
We have
audited the accompanying consolidated balance sheets of Applied Nanotech
Holdings, Inc. and Subsidiaries (collectively, the “Company”) as of December 31,
2009 and 2008 and the related consolidated statements of operations,
shareholders’ equity and cash flows for each of the years in the three year
period ended December 31, 2009. These consolidated financial statements are the
responsibility of the Company’s management. Our responsibility is to express an
opinion on these consolidated financial statements based on our
audits.
We
conducted our audits in accordance with the standards of the Public Company
Accounting Oversight Board (United States). Those standards require that we plan
and perform the audit to obtain reasonable assurance about whether the financial
statements are free of material misstatement. An audit includes examining, on a
test basis, evidence supporting the amounts and disclosures in the financial
statements. An audit also includes assessing the accounting principles used and
significant estimates made by management, as well as evaluating the overall
financial statement presentation. We believe that our audits provide a
reasonable basis for our opinion.
In our
opinion, the consolidated financial statements referred to above present fairly,
in all material respects, the financial position of Applied Nanotech Holdings,
Inc. and Subsidiaries as of December 31, 2009 and 2008, and the results of their
operations and their cash flows for each of the years in the three year period
ended December 31, 2009 in conformity with accounting principles generally
accepted in the United States of America.
We also
have audited, in accordance with the standards of the Public Company Accounting
Oversight Board (United States), the Company’s internal control over financial
reporting as of December 31, 2009, based on criteria established in Internal
Control-Integrated Framework issued by the Committee of Sponsoring Organizations
of the Treadway Commission (COSO), and our report dated February 26. 2010
expressed an unqualified opinion on the effectiveness of internal control over
financial reporting.
The
accompanying consolidated financial statements have been prepared assuming that
the Company will continue as a going concern. As discussed in Note 1 to the
consolidated financial statements, the Company has experienced recurring losses
from operations and negative cash flow from operations. This raises substantial
doubt about the Company’s ability to continue as a going concern. Management’s
plan in regard to these matters is also described in Note 1 to the consolidated
financial statements. The consolidated financial statements do not include any
adjustments that might result from the outcome of this uncertainty.
/s/
Padgett, Stratemann & Co, L.L.P.
Padgett,
Stratemann & Co, L.L.P.
San
Antonio, Texas
February
26. 2010
Page
28
APPLIED
NANOTECH HOLDINGS, INC. AND SUBSIDIARIES
CONSOLIDATED
BALANCE SHEETS
|
ASSETS
|
||||||||
|
December
31,
|
||||||||
|
2009
|
2008
|
|||||||
|
Current
assets:
|
||||||||
|
Cash
and cash equivalents
|
$ | 286,971 | $ | 710,111 | ||||
|
Accounts
receivable, trade – net of allowance for doubtful accounts
|
249,164 | 661,704 | ||||||
|
Prepaid
expenses and other current assets
|
67,340 | 121,920 | ||||||
|
Total
current assets
|
603,475 | 1,493,735 | ||||||
|
Property
and equipment, net
|
267,008 | 278,853 | ||||||
|
Other
assets
|
22,884 | 19,901 | ||||||
|
Total
assets
|
$ | 893,367 | $ | 1,792,489 | ||||
|
LIABILITIES
AND SHAREHOLDERS’ EQUITY (DEFICIT)
|
||||||||
|
Current
liabilities:
|
||||||||
|
Accounts
payable
|
$ | 952,679 | $ | 438,878 | ||||
|
Obligations
under capital lease
|
21,939 | 35,012 | ||||||
|
Accrued
liabilities
|
482,629 | 207,809 | ||||||
|
Deferred
revenue
|
310,000 | 224,595 | ||||||
|
Total
current liabilities
|
1,767,247 | 906,294 | ||||||
|
Obligations
under capital lease, long-term
|
31,124 | 27,909 | ||||||
|
Convertible
Notes Payable
|
165,834 | — | ||||||
|
Total
liabilities
|
1,964,205 | 934,203 | ||||||
|
Commitments
and contingencies
|
— | — | ||||||
|
Shareholders’
equity (deficit) :
|
||||||||
|
Preferred
stock, $1.00 par value, 2,000,000 shares authorized; no
shares issued and outstanding
|
— | — | ||||||
|
Common
stock, 120,000,000 shares authorized, $.001 par value, 107,473,133
and
107,395,216 shares issued and outstanding, respectively
|
107,473 | 107,395 | ||||||
|
Additional
paid-in capital
|
109,518,998 | 109,295,595 | ||||||
|
Accumulated
deficit
|
(110,697,309 | ) | (108,544,704 | ) | ||||
|
Total
shareholders’ equity(deficit)
|
(1,070,838 | ) | 858,286 | |||||
|
Total
liabilities and shareholders’ equity (deficit)
|
$ | 893,367 | $ | 1,792,489 | ||||
See notes
to consolidated financial statements.
Page
29
APPLIED
NANOTECH HOLDINGS, INC. AND SUBSIDIARIES
CONSOLIDATED
STATEMENTS OF OPERATIONS
|
Year
ended December 31,
|
||||||||||||
|
|
2009
|
2008
|
2007
|
|||||||||
|
|
||||||||||||
|
Revenues
|
|
|
|
|||||||||
|
Contract
research
|
$ | 1,767,144 | $ | 824,358 | $ | 990,598 | ||||||
|
Government
contracts
|
1,694,082 | 2,295,887 | 2,328,010 | |||||||||
|
License
fees and royalties
|
500,000 | 577,000 | — | |||||||||
|
Other
|
91,250 | 260,587 | 671,195 | |||||||||
|
|
||||||||||||
|
Total
revenues
|
4,052,746 | 3,957,832 | 3,989,803 | |||||||||
|
Operating
costs
|
||||||||||||
|
Research
and development
|
3,662,323 | 4,614,644 | 4,526,166 | |||||||||
|
Selling,
general and administrative expenses
|
2,540,816 | 3,897,939 | 3,858,720 | |||||||||
|
Total
operating costs
|
6,203,139 | 8,512,583 | 8,384,886 | |||||||||
|
Gain
on sale of assets and other intellectual property
|
6,000 | 1,329,571 | — | |||||||||
|
|
||||||||||||
|
Loss
from operations
|
(2,144,393 | ) | (3,225,180 | ) | (4,395,083 | ) | ||||||
|
|
||||||||||||
|
Other
income (expense):
|
||||||||||||
|
Interest
income
|
1,877 | 46,493 | 139,118 | |||||||||
|
Interest
expense
|
(10,089 | ) | (7,180 | ) | (926 | ) | ||||||
|
Litigation
settlement
|
— | 500,000 | — | |||||||||
|
|
||||||||||||
|
Total
other income (expense)
|
(8,212 | ) | 539,313 | 138,192 | ||||||||
|
Loss
before taxes
|
(2,152,605 | ) | (2,685,867 | ) | (4,256,891 | ) | ||||||
|
|
||||||||||||
|
Provision
for taxes
|
— | — | — | |||||||||
|
|
||||||||||||
|
Net
loss applicable to common shareholders
|
$ | (2,152,605 | ) | $ | (2,685,867 | ) | $ | (4,256,891 | ) | |||
|
Earnings
(loss) per share
|
||||||||||||
|
Basic
and diluted
|
$ | (0.02 | ) | $ | (0.03 | ) | $ | (0.04 | ) | |||
|
Weighted
average common shares outstanding
|
||||||||||||
|
Basic
and diluted
|
107,427,877 | 107,292,686 | 106,321,400 | |||||||||
See notes
to consolidated financial statements.
Page
30
APPLIED
NANOTECH HOLDINGS, INC. AND SUBSIDIARIES
CONSOLIDATED
STATEMENTS OF SHAREHOLDERS’ EQUITY (DEFICIT)
|
Preferred
|
Common
|
Additional
|
Accumulated
|
|||||||||||||||||||||||||
|
Shares
|
Amount
|
Shares
|
Amount
|
Paid-In
Capital
|
Deficit
|
Total
|
||||||||||||||||||||||
|
Balance
December 31, 2006
|
- | $ | - | 104,257,607 | $ | 104,258 | $ | 102,139,950 | $ | (101,601,946 | ) | $ | 642,262 | |||||||||||||||
|
Issuance
of common stock options
as compensation
|
- | - | - | - | 128,957 | - | 128,957 | |||||||||||||||||||||
|
Issuance
of common stock as
a result of the exercise of employee
stock options
|
- | - | 307,244 | 307 | 304,116 | - | 304,423 | |||||||||||||||||||||
|
Issuance
of common stock for cash
|
- | - | 2,608,698 | 2,609 | 5,997,391 | - | 6,000,000 | |||||||||||||||||||||
|
Issuance
of restricted common stock
as compensation
|
- | - | - | - | 10,151 | - | 10,151 | |||||||||||||||||||||
|
Net
(loss)
|
- | - | - | - | - | (4,256,891 | ) | (4,256,891 | ) | |||||||||||||||||||
|
Balance
December 31, 2007
|
- | $ | - | 107,173,549 | $ | 107,174 | $ | 108,580,565 | $ | (105,858,837 | ) | $ | 2,828,902 | |||||||||||||||
|
Issuance
of common stock as
a result of the exercise of employee
stock options
|
- | - | 170,000 | 170 | 61,080 | - | 61,250 | |||||||||||||||||||||
|
Issuance
of common stock options
as compensation
|
- | - | - | - | 605,393 | - | 605,393 | |||||||||||||||||||||
|
Issuance
of restricted common stock
as compensation
|
- | - | 51,667 | 51 | 48,557 | - | 48,608 | |||||||||||||||||||||
|
Net
(loss)
|
- | - | - | - | - | (2,685,867 | ) | (2,685,867 | ) | |||||||||||||||||||
|
Balance
December 31, 2008
|
- | $ | - | 107,395,216 | $ | 107,395 | $ | 109,295,595 | $ | (108,544,704 | ) | $ | 858,286 | |||||||||||||||
|
Conversion
rights associated with issuance
of convertible notes
|
- | - | - | - | 35,000 | - | 35,000 | |||||||||||||||||||||
|
Issuance
of common stock options
as compensation
|
- | - | - | - | 160,505 | - | 160,505 | |||||||||||||||||||||
|
Issuance
of restricted common stock
as compensation
|
- | - | 77,917 | 78 | 27,898 | - | 27,976 | |||||||||||||||||||||
|
Net
(loss)
|
- | - | - | - | - | (2,152,605 | ) | (2,152,605 | ) | |||||||||||||||||||
|
Balance
December 31, 2009
|
- | $ | - | 107,473,133 | $ | 107,473 | $ | 109,518,998 | $ | (110,697,309 | ) | $ | (1,070,838 | ) | ||||||||||||||
See notes
to consolidated financial statements.
Page
31
APPLIED
NANOTECH HOLDINGS, INC. AND SUBSIDIARIES
CONSOLIDATED
STATEMENTS OF CASH FLOWS
|
Year
Ended
December
31,
|
||||||||||||
|
2009
|
2008
|
2007
|
||||||||||
|
Cash
flows from operating activities:
|
||||||||||||
|
Net
loss
|
$ | (2,152,605 | ) | $ | (2,685,867 | ) | $ | (4,256,891 | ) | |||
|
Adjustments
to reconcile net loss to net cash
used in operating activities:
|
||||||||||||
|
Depreciation
and amortization expense
|
64,353 | 70,536 | 50,498 | |||||||||
|
Stock
and options issued for services
|
188,481 | 654,001 | 139,108 | |||||||||
|
Amortization
of discount on debt
|
834 | — | — | |||||||||
|
Changes
in assets and liabilities:
|
||||||||||||
|
Accounts
receivable, trade
|
412,540 | (407,741 | ) | 110,755 | ||||||||
|
Prepaid
expenses and other assets
|
51,597 | 50,522 | (103,502 | ) | ||||||||
|
Accounts
payable
|
513,801 | (8,228 | ) | (1,115,382 | ) | |||||||
|
Accrued
expenses
|
274,820 | 104,806 | 15,766 | |||||||||
|
Deferred
Revenue and other current liabilities
|
85,405 | (81,832 | ) | (95,028 | ) | |||||||
|
Total
adjustments
|
1,591,831 | 382,064 | (997,785 | ) | ||||||||
|
Net
cash used in operating activities
|
(560,774 | ) | (2,303,803 | ) | (5,254,676 | ) | ||||||
|
Cash
flows from investing activities:
|
||||||||||||
|
Capital
expenditures
|
(20,901 | ) | (35,943 | ) | (112,682 | ) | ||||||
|
Net
cash used in investing activities
|
(20,901 | ) | (35,943 | ) | (112,682 | ) | ||||||
|
Cash
flows from financing activities:
|
||||||||||||
|
Proceeds
from issuance of common stock
|
— | 61,250 | 6,304,423 | |||||||||
|
Proceeds
of long term debt
|
200,000 | — | — | |||||||||
|
Repayment
of capitallease obligations
|
(41,465 | ) | (31,489 | ) | (2,307 | ) | ||||||
|
Net
cash provided by financing activities
|
158,535 | 29,761 | 6,302,116 | |||||||||
|
Net
increase (decrease) in cash and cash equivalents
|
(423,140 | ) | (2,309,985 | ) | 934,758 | |||||||
|
Cash
and cash equivalents, beginning of year
|
710,111 | 3,020,096 | 2,085,338 | |||||||||
|
Cash
and cash equivalents, end of year
|
$ | 286,971 | $ | 710,111 | $ | 3,020,096 | ||||||
See notes
to consolidated financial statements.
Page
32
APPLIED
NANOTECH HOLDINGS, INC. AND SUBSIDIARIES
NOTES
TO CONSOLIDATED FINANCIAL STATEMENTS
|
1.
|
Organization,
Operations, and Liquidity:
|
Applied
Nanotech Holdings, Inc. and its subsidiaries (“the Company”) are engaged in the
development of products for applications using nanomaterials, sensors,
nanoelectronics, and proprietary field emission technology as well as the
performance of significant research in those areas. We intend to obtain
development revenues for applying our technology to specific applications for
our development partners and to obtain royalty revenues from licensing this
technology to those partners and others. We have also developed patented
electronic sign technology and sold products using that technology, but have now
sold that technology. We may receive additional income from the sale of that
technology based on license revenues received by the purchaser of the
technology.
Until we
are able to operate profitably as a result of revenues from either reimbursed
research or license agreements, we may be required to seek additional funds
through the equity markets, or raise funds through debt instruments to allow us
to maintain operations. There is no assurance that license agreements will be
signed, that commercialization of our technology and products will result in
income from operations, or that funds will be available in the equity or debt
markets. Management believes it will continue to be able to secure additional
short term funding, if necessary, to allow the Company to continue operations
until we achieve profitability.
The
principal source of our liquidity since the time of our initial public offering
in 1993 has been from the funds received from exempt offerings of common stock,
preferred stock, and convertible debt securities, as well as license and
development revenues. We may receive additional funds from the exercise of
employee stock options. We may also seek to increase our liquidity through bank
borrowings or other financings, although this is not likely. There can be no
assurance that any of these financing alternatives can be arranged on
commercially acceptable terms. We believe that our success in reaching
profitability will depend on the viability of our technology and products using
that technology, their acceptance in the marketplace, and our ability to obtain
additional debt or equity financings in the future.
A portion
of our research and development has been funded by others. To the extent that
other funding is not available, research and development may be internally
funded by us; however, our primary objective is to focus our resources on
projects for which we receive funding.
The
Company has a history of net losses and negative cash flow from operations,
although these net losses and negative cash flows have been decreasing. These
factors may raise doubt with some about our ability to continue as a going
concern. The accompanying financial statements have been prepared in conformity
with generally accepted accounting principles, which contemplate continuation of
the Company as a going concern, and do not include any adjustments that may be
required if it were unable to continue as a going concern. Management believes
that actions currently being taken will allow it to achieve profitability and
allow the Company to continue as a going concern.
|
2.
|
Summary of Significant
Accounting Policies:
|
Principles
of consolidation
The
accompanying consolidated financial statements include the accounts of the
Company and its subsidiaries, Applied Nanotech, Inc. (“ANI”), and Electronic
Billboard Technology, Inc. (“EBT”), after the elimination of all significant
intercompany accounts and transactions. ANI is primarily involved in developing
products for applications using the Company’s proprietary field emission
technology, sensors, nanoelectronics, and nanomaterials which include
composites. EBT was primarily involved in the commercialization of electronic
digitized sign technology, but has now sold its technology.
Management’s
estimates
The
preparation of financial statements in conformity with generally accepted
accounting principles requires management to make estimates and assumptions that
affect the reported amounts of assets, liabilities, revenues, and expenses, as
well as the disclosure of contingent assets and liabilities. Actual results
could differ from those estimates. Significant estimates include NOL reserves,
bad debt reserves, assumptions used in calculating share based compensation,
depreciation, and litigation reserves.
Page
33
APPLIED
NANOTECH HOLDINGS, INC. AND SUBSIDIARIES
NOTES
TO CONSOLIDATED FINANCIAL STATEMENTS
2. Summary of Significant
Accounting Policies (continued):
Revenue recognition
Our
revenues include reimbursements under agreements to perform research and
development for government agencies and others. We do not perform research
contracts that are contingent upon successful results. Larger projects are
sometimes broken down in phases to allow the customer to determine at the end of
each phase if they wish to move to the next phase. The agreements with federal
government agencies generally provide that, upon completion of a technology
development program, the funding agency is granted a royalty-free license to use
any technology developed during the course of the program for its own purposes,
but not any preexisting technology that we use in connection with the program.
We retain all other rights to use, develop, and commercialize the technology and
recognize revenue when it is earned pursuant to the terms of the contract.
Agreements with nongovernmental entities generally allow the entity the first
opportunity to license the technology from us upon completion of the
project.
The
Company’s revenues also include royalties from licensing its technology, revenue
from the sale of products, and other miscellaneous revenues. Many of the
company’s projects may involve a combination of these types of revenues.
Revenues are recognized as follows:
Government
Contracts - Revenue from government contracts is recognized when it is earned
pursuant to the terms of the contract. Long-term projects, such as SBIR Phase II
grants that usually range from $500,000 to $1,000,000 in total and usually
extend for a period of approximately two years, are generally based on
reimbursement of costs. These projects are usually billed monthly based on
costs, hours, or some other measure of activity during the month. As a general
rule, we recognize revenue on these contracts based on the activity level of the
contract during the period as compared with total estimated activity. This
generally would be a measure of proportional performance on the contract, such
as cost incurred compared with total expected cost. The recognition of revenue
may not correspond with the billings allowable under the contract. To the extent
that billings exceed revenue earned, a portion of the revenue is deferred until
such time as it is earned. Short-term projects, such as SBIR Phase I grants that
usually are less than $100,000 and usually extend for a period of approximately
6 months, are billed at periodic intervals as specified in the
contract.
Other
Research Contracts - Revenue from nongovernmental contracts is recognized when
it is earned pursuant to the terms of the contract. Each contract is unique and
tailored to the needs of the customer and goals of the project. Some contracts
may call for a monthly payment for a fixed period of time. Other contracts may
be for a fixed dollar amount with an unspecified time period, although there is
frequently a targeted completion date. These contracts generally involve some
sort of up front payment. Some contracts may call for the delivery of samples,
or may call for the transfer of equipment or other items developed during the
project to the customer. As a general rule, we recognize revenue on long term
contracts based on the activity level of the contract during the period as
compared with total estimated activity. This generally would be a measure of
proportional performance on the contract, such as cost incurred compared with
total expected cost. However, to the extent there are other significant contract
provisions such as the delivery of more than a nominal amount of samples or
delivery of equipment, we would modify this as appropriate. For other short term
contracts, generally less than $50,000, we recognize revenue when it is billed
under the terms of the contract.
Royalty
Revenue - The Company recognizes royalty revenues based on the shipment of
products by a licensee at the time the underlying product upon which the royalty
is based is shipped by the entity paying the royalty. For minimum royalty
payments paid by a licensee that are required for the licensee to maintain
exclusivity, royalty revenue is recognized at the time the minimum royalty
payment, is due, which normally corresponds somewhat with the time that the
payment is received. The Company recognizes license fees due at the time of the
signing of a royalty agreement when the licensee has an enforceable commitment
to pay, unless the terms of the agreement make it clear that the license fee is
a prepayment of future royalties. This normally corresponds with, or is
reasonably close to, the time of receipt of the payment.
Product
Sales - Revenue from product sales is recognized at the time the product
shipped. The Company’s primary business is research and development and the
licensing of its technology, not the sale of products. Product sales are
generally insignificant in number, and are usually limited to the sale of
samples, proofs of concepts, prototypes, or other items resulting from its
research.
Page
34
APPLIED
NANOTECH HOLDINGS, INC. AND SUBSIDIARIES
NOTES
TO CONSOLIDATED FINANCIAL STATEMENTS
|
2.
|
Summary of Significant
Accounting Policies
(continued):
|
Other
Revenue - Other miscellaneous revenue is recognized as deemed appropriate given
the facts of the situation and is generally not material.
Cash and
cash equivalents
The
Company considers all highly liquid investments purchased with an original
maturity of three months or less to be cash equivalents.
Accounts
receivable
The
Company occasionally sells products to others on credit; however most sales are
to large financially stable companies, or the Federal government. It is the
Company’s policy to record reserves for potential credit losses. Since
inception, the Company has experienced minimal credit losses. The Company
considered no reserves to be necessary for any of the years
presented.
Property
and equipment
Property
and equipment are recorded at cost, net of accumulated depreciation and
amortization. Depreciation is provided on the straight-line method over the
estimated useful lives of the assets, which range from three to seven years, or
the lease term for leasehold improvements, if less. Expenses for major renewals
and betterments that extend the original estimated economic useful lives of the
applicable assets are capitalized. Expenses for normal repairs and maintenance
are charged to operations as incurred. The cost and related accumulated
depreciation of assets sold or otherwise disposed of are removed from the
accounts, and any gain or loss is included in income.
Impairment
At each
balance sheet date, the Company evaluates the carrying amount and the
amortization period for its long-lived assets. If an indicator of impairment
exists, it is recorded at that time. There were no impairment charges recorded
in any of the years presented in these financial statements.
Income
taxes
The
Company accounts for income taxes using the liability method. Under this method,
deferred income taxes are recorded to reflect the tax consequences on future
years of temporary differences between the tax basis of the assets and
liabilities and their financial amounts at year-end. The Company provides a
valuation allowance to reduce deferred tax assets to their net realizable value.
The Company has determined that no reserve for uncertain tax positions is
required; however tax years 2006 through 2009 remain open for examination by the
U.S. Internal Revenue Service.
Research
and development expenses
Costs of
research and development for Company-sponsored projects are expensed as
incurred.
Disclosures
about fair value of financial instruments
The
following methods and assumptions were used to estimate the fair value of each
class of certain financial instruments for which it is practicable to estimate
that fair value. For cash equivalents and accounts receivable, the
carrying amount approximates fair value because of the short-term nature of
these instruments. The fair value of the Company’s capital lease obligations and
notes payable is estimated based on the quoted market prices for the same, or
similar issues, or on the current rates offered to the Company for obligations
of the same remaining maturities with similar collateral requirements. For all
years presented, the fair value of the Company’s capital lease obligations and
notes payable approximate their carrying values.
Page
35
APPLIED
NANOTECH HOLDINGS, INC. AND SUBSIDIARIES
NOTES
TO CONSOLIDATED FINANCIAL STATEMENTS
|
2.
|
Summary of Significant
Accounting Policies
(continued):
|
Income
(loss) per common share
Basic per
share amounts are computed, generally, by dividing net income or loss by the
weighted average number of common shares outstanding. Diluted per-share amounts
assume the conversion, exercise, or issuance of all potential common stock
instruments unless the effect is anti-dilutive, thereby reducing the loss or
increasing the income per common share. As described in Notes 8, 9 and 10, the
Company had options and warrants outstanding as indicated in the table below. In
addition, the Company has convertible notes payable, that if converted at
December 31, 2009, would have resulted in an additional 1,004,495 shares
outstanding. However, because the Company incurred losses in all years
presented, the inclusion of those potential common shares in the calculation of
diluted loss per-share would have an anti-dilutive effect. Therefore, basic and
diluted per-share amounts are the same in all years presented.
|
2009
|
2008
|
2007
|
||||
|
Options
|
4,430,392
|
7,889,897
|
6,897,180
|
|||
|
Warrants
|
1,304,353
|
1,304,353
|
1,304,353
|
|||
|
Weighted
average exercise price
|
$1.60
|
$1.52
|
$1.70
|
Share-based
payments
The
Company has three stock based compensation plans described in greater detail in
Note 9 to these financial statements. The Company uses the fair value method to
account for stock-based compensation. The fair value of each award is estimated
on the date of each grant. For restricted stock the fair market value is based
on the market value of the stock granted on the date of the grant. For options,
it is estimated using the Black Scholes option pricing model that uses the
assumptions noted in the following table. Estimated volatilities are based on
the historical volatility of the Company’s stock over the same period as the
expected term of the options. The expected term of options granted represents
the period of time that options granted are expected to be outstanding. The
Company uses historical data to estimate option exercise behavior and to
determine this term. The risk free rate used is based on the U.S. Treasury yield
curve in effect at the time of the grant using a time period equal to the
expected option term. The Company has never paid dividends and does not expect
to pay any dividends in the future.
|
2009
|
2008
|
2007
|
||||
|
Expected
dividend yield
|
0%
|
0%
|
0%
|
|||
|
Risk
Free Interest Rate
|
.37%-1.76%
|
1.1%-
3.3%
|
3.3%-
4.8%
|
|||
|
Expected
option term (in years)
|
2.0
- 3.5
|
0.75
- 3.5
|
3.5
- 5.0
|
|||
|
Turnover/Forfeiture
Rate
|
0%
|
0%
|
0%
|
|||
|
Expected
volatility
|
95%
- 100%
|
75%
- 89%
|
75%
- 82%
|
|||
|
Weighted-average
volatility
|
98%
|
82%
|
79%
|
The
Black-Scholes option valuation model and other existing models were developed
for use in estimating the fair value of traded options that have no vesting
restrictions and are fully transferable. These option valuation models require
the input of, and are highly sensitive to, subjective assumptions including the
expected stock price volatility. Applied Nanotech Holdings’ stock options have
characteristics significantly different from those of traded options, and
changes in the subjective input assumptions could materially affect the fair
value estimate.
Page
36
APPLIED
NANOTECH HOLDINGS, INC. AND SUBSIDIARIES
NOTES
TO CONSOLIDATED FINANCIAL STATEMENTS
|
2.
|
Summary of Significant
Accounting Policies
(continued):
|
Recently
issued accounting pronouncements
In June 2009, the Financial Accounting
Standards Board (“FASB”) issued ASC 105 Accounting Standards Codification TM and
Hierarchy of Generally Accepted Accounting Principles. The FASB Accounting
Standards Codification TM (the “Codification”) has become the source of
authoritative accounting principles recognized by the FASB to be applied by
nongovernmental entities in the preparation of financial statements in according
with Generally Accepted Accounting Principles (“GAAP”). All existing accounting
standard documents are superseded by the Codification and any accounting
literature not included in the Codification will not be authoritative. Rules and
interpretive releases of the SEC issued under the authority of federal
securities laws, however, will continue to be the source of authoritative
generally accepted accounting principles for SEC registrants. Effective
September 30, 2009, all references to GAAP in our consolidated financial
statements will include references to the new Codification. The Codification
does not change or alter existing GAAP and, therefore, will not have an impact
on our financial position, results of operations, or cash flows.
In May 2009, the FASB issued ASC 855,
Subsequent Events, which provides guidance on events that occur after the
balance sheet date but prior to the issuance of the financial statements. ASC
855 distinguishes events requiring recognition in the financial statements and
those that may require disclosure in the financial statements. Furthermore, ASC
855 requires disclosure of the date through which subsequent events were
evaluated. These requirements are effective for interim and annual periods after
June 15, 2009. We have adopted these requirements and have evaluated subsequent
events through February 26. 2010.
|
3.
|
Operating Lease
Obligations:
|
The
Company leases various facilities and equipment under operating lease agreements
having terms expiring at various dates through 2014. Rental expense was
$194,649, $221,787, and $179,594 for the years ended December 31,
2009, 2008, and 2007, respectively.
Future
minimum lease payments under operating leases that have initial or remaining
noncancelable lease terms in excess of one year at December 31, 2009, were as
follows:
|
2010
|
$
|
187,179
|
|
2011
|
186,071
|
|
|
2012
|
193,589
|
|
|
2013
|
195,781
|
|
|
2014
|
28,950
|
|
|
Total
future minimum lease payments
|
$
|
791,570
|
|
4.
|
Convertible Notes
Payable:
|
|
|
Notes
payable at December 31, 2009 consisted of notes payable to shareholders.
The notes are 30 month unsecured notes due in a lump sum in June 2012 that
bear interest at a rate of 8%. These notes, including any accrued
interest, are convertible into shares of common stock at the option of the
note holder at a rate of $0.20 per share. The face amount of the notes is
$200,000, however the conversion rights were valued at $35,000
and recorded as a discount to the note at the time of issuance. $834 of
that discount was amortized to interest expense as of December 31, 2009.
There were no notes payable outstanding as of December 31,
2008.
|
Page
37
APPLIED
NANOTECH HOLDINGS, INC. AND SUBSIDIARIES
NOTES
TO CONSOLIDATED FINANCIAL STATEMENTS
|
5.
|
Capital Lease
Obligations:
|
Capital
leases payable at December 31, 2009 and 2008 consisted of the
following:
|
|
2009
|
2008
|
|||||||
|
Capital
lease equipment due in monthly installments of $816 through
July 2013. The equipment value and lease obligation was
determined using a discount rate of 14.08%. The equipment is
included in plant and equipment at December 31, 2009 at a cost
of $42,040 and with accumulated amortization of $8,408.
|
$ | 35,072 | $ | 44,859 | |||||
|
Capital
lease equipment due in monthly installments of $2,883
through November 2009. The equipment value and lease obligation
was determined using a discount rate of 11.20%.
|
— | 31,709 | |||||||
|
Capital
lease equipment due in monthly installments of $1,492
through July 2011. The equipment value and lease obligation
was determined using a discount rate of 12.27%. The equipment
is included in office equipment at December 31, 2009 at
a cost of $47,120, with accumulated amortization of
$2,819.
|
28,345 | — | |||||||
|
Total
capital leases
|
63,417 | 76,568 | |||||||
|
Less
interest
|
10,354 | 13,647 | |||||||
|
Less
current portion
|
21,939 | 35,012 | |||||||
|
Capital
lease obligations, long-term
|
$ | 31,124 | $ | 27,909 | |||||
These
leases result in minimum payments of $27,691, $20,230, $9,787, and $5,709 from
2010 to 2013.
|
6.
|
Details of Certain
Balance Sheet Accounts:
|
Additional
information regarding certain balance sheet accounts at December 31, 2009 and
2008 is as follows:
|
December
31,
|
||||||||
|
2009
|
2008
|
|||||||
|
Property
and equipment:
|
||||||||
|
Plant
and equipment
|
$ | 1,088,216 | $ | 1,038,861 | ||||
|
Furniture
and office equipment
|
144,460 | 141,307 | ||||||
|
Leasehold
Improvements
|
19,019 | 19,019 | ||||||
|
Total
carrying cost
|
1,251,695 | 1,199,187 | ||||||
|
Less
accumulated depreciation
|
(984,687 | ) | (920,334 | ) | ||||
| $ | 267,008 | $ | 278,853 | |||||
|
Accrued
liabilities:
|
||||||||
|
Payroll
and related accruals
|
$ | 458,629 | $ | 183,809 | ||||
|
Other
|
24,000 | 24,000 | ||||||
|
Total
|
$ | 482,629 | $ | 207,809 | ||||
Depreciation
and amortization for the years ended December 31, 2009, 2008, and 2007 was
$64,353, $70,536, and $50,498, respectively. Equipment held under capital leases
and accumulated amortization on that equipment is included in these
totals.
Page
38
APPLIED
NANOTECH HOLDINGS, INC. AND SUBSIDIARIES
NOTES
TO CONSOLIDATED FINANCIAL STATEMENTS
|
7.
|
Income
Taxes:
|
The
components of deferred tax assets (liabilities) at December 31, 2009 and 2008,
were as follows:
|
December
31,
|
||||||||
|
2009
|
2008
|
|||||||
|
Deferred
tax assets:
|
||||||||
|
Net
operating loss carry forwards
|
$ | 30,435,000 | $ | 32,309,000 | ||||
|
Stock
based compensation
|
1,939,000 | 1,882,000 | ||||||
|
Research
and experimentation credits
|
125,000 | 270,000 | ||||||
|
Partnership
asset
|
39,000 | 48,000 | ||||||
|
Capitalized
intangible assets
|
37,000 | 74,000 | ||||||
|
Depreciation
assets
|
1,000 | 4,000 | ||||||
|
Accrued
expenses not deductible until paid
|
155,000 | 58,000 | ||||||
|
Total
deferred tax assets
|
32,731,000 | 34,645,000 | ||||||
|
Deferred
tax liabilities:
|
— | — | ||||||
|
Net
deferred tax assets before valuation allowance
|
32,731,000 | 34,645,000 | ||||||
|
Valuation
allowance
|
(32,731,000 | ) | (34,645,000 | ) | ||||
|
Net
deferred tax asset
|
||||||||
| $ | — | $ | — | |||||
The
following is a reconciliation of the amount of the income tax expense (benefit)
that would result from applying the statutory federal income tax rates to pretax
income (loss) and the reported amount of income tax expense (benefit) for the
periods ended December 31, 2009, 2008, and 2007.
|
|
December
31,
|
|||||||||||
|
2009
|
2008
|
2007
|
||||||||||
|
Expected
income tax expense (benefit)
|
$ | (732,000 | ) | $ | (914,000 | ) | $ | (1,447,000 | ) | |||
|
Non-deductible
expenses
|
11,000 | 3,000 | 10,000 | |||||||||
|
Expiration
of Tax Credit Carryforwards
|
145,000 | 190,000 | 8,000 | |||||||||
|
Expiration
of NOL Carryforwards
|
2,490,000 | 2,373,000 | 486,000 | |||||||||
|
Other
|
— | — | — | |||||||||
|
Increase
(Decrease) in Valuation Allowance
|
(1,914,000 | ) | (1,652,000 | ) | 943,000 | |||||||
|
Total
Tax
|
$ | — | $ | — | $ | — | ||||||
As of
December 31, 2009, the Company had net operating loss carry forwards of
approximately $90 million that expire from 2010 through 2029, that are available
to offset future taxable income. The majority of these carry forwards expire
after 2010. Additionally, the Company has tax credit carry forwards related to
research and development expenditures of approximately $125,000 that expire
through 2011.
The
Company’s IPO, completed in 1993, effected an ownership change under Internal
Revenue Code Section 382 which limited the Company’s ability to utilize carry
forwards from prior to the IPO. All net operating losses from 1993 and prior
have now been utilized or have expired. Any additional ownership
change resulting from stock issuances subsequent to the IPO will likely limit
the Company’s ability to utilize any net operating loss carry forwards or
credits generated before this change in ownership. These limitations tend to
relate to the timing of usage, rather than the loss of the ability to use these
net operating losses.
Page
39
APPLIED
NANOTECH HOLDINGS, INC. AND SUBSIDIARIES
NOTES
TO CONSOLIDATED FINANCIAL STATEMENTS
|
8.
|
Capital
Stock:
|
Preferred
stock
The
Company has authorization for the issuance of 2,000,000 shares of $1.00 par
value preferred stock. There were no shares of preferred stock outstanding for
any of the years presented.
Common
stock
No shares
were issued in private placements in 2009 or 2008; however, during 2007, the
Company issued shares of its common stock in a private placement in an exempt
offering under Regulation D of the Securities Act of 1933. These shares were
issued at prices that represented a slight discount to the market price of the
stock at the time of the offerings. All of these shares were registered to
enable the shareholder to be able to sell the shares, with the latest
registration statement declared effective June 19, 2007. A total of 2,608,698
were issued and proceeds of $6,000,000 were received.
At
December 31, 2009, common stock was reserved for the following
reasons:
|
Exercise
of stock warrants
|
1,304,353 | |||
|
Exercise
and future grants of stock options
|
9,513,633 | |||
|
Total
shares reserved
|
10,817,986 |
|
9.
|
Stock
Options:
|
The
Company sponsors three stock-based incentive compensation plans (the “Plans”),
which are described below. The compensation cost that has been charged against
income for these plans for the years ended December 31, 2009, 2008, and 2007 was
$188,481, $654,001, and $139,108, respectively. No income tax benefit was
recognized in the income statement and no compensation was capitalized in any of
the years presented.
The plans
allow the Company to grant incentive stock options, non-qualified stock options,
or restricted stock. The incentive stock options are exercisable for up to ten
years, at an option price per share not less than the fair market value on the
date the option is granted. The incentive stock options are limited to persons
who have been regular full-time employees of the Company or its present and
future subsidiaries for more than one (1) year and at the date of the grant of
any option are in the employ of the Company or its present and future
subsidiaries. Historically, the Company has not granted incentive stock options.
Non-qualified options may be granted to any person, including, but not limited
to, employees, independent agents, consultants and attorneys, who the Company’s
Compensation Committee believes have contributed, or will contribute, to the
success of the Company. Non-qualified options may be issued at option prices of
less than fair market value on the date of grant and are exercisable for up to
ten years from date of grant. The option vesting schedule for options granted is
determined by the Compensation Committee of the Board of Directors at the time
of the grant. The plans provide for accelerated vesting of unvested options if
there is a change in control, as defined in the plan.
In 1992,
the Company adopted the 1992 Employees Stock Option Plan (the “Employees Plan”)
and the 1992 Outside Directors’ Stock Option Plan (the “Directors
Plan”), for purposes of granting incentive or non-qualified stock
options. These plans were amended several times by the Company’s Board of
Directors to ultimately increase the number of shares authorized under the plans
to 6,500,000 for the Employees plan and 1,000,000 for the Directors Plan. Both
of these plans expired in 2002; however, options granted under these plans prior
to expiration remain outstanding until they are exercised, forfeited, or the
exercise period expires. At December 31, 2010, no shares remained available for
grant under either of these plans.
Page
40
APPLIED
NANOTECH HOLDINGS, INC. AND SUBSIDIARIES
NOTES
TO CONSOLIDATED FINANCIAL STATEMENTS
|
9.
|
Stock Options
(continued):
|
In
September 2002, the Board of Directors of the Company established the 2002
Equity Compensation Plan to replace the Employees Plan and the
Directors Plan, both of which expired in 2002, and reserved a total of 5,000,000
shares for issuance under the Plan. The plan was amended effective December 31,
2004 to increase the authorized shares to 8,000,000, and again effective
December 12, 2007 to increase the authorized shares to 10,000,000. A total of
5,083,241 shares remain available for grant under this at December 31,
2009.
The
company issues new shares for all options exercised. It does not expect to
repurchase any shares to facilitate future option exercises. The following table
summarizes information about stock options outstanding, some of which are not
expected to ultimately vest, and options currently exercisable under all three
stock option plans at December 31, 2009:
|
Options
Outstanding
|
Options
Exercisable
|
||||||
|
Range
of
Exercise
Prices
|
Number
Outstanding
at
12/31/09
|
Wgtd. Avg.
Remaining
Contractual
Life
|
Wgtd.
Avg.
Exercise
Price
|
Number
Exercisable
at
12/31/09
|
Wgtd. Avg.
Remaining
Contractual
Life
|
Wgtd.
Avg.
Exercise
Price
|
|
|
|
|||||||
|
$0.00
- $0.50
|
1,194,609
|
7.9
Years
|
$0.27
|
1,182,109
|
7.9
Years
|
$0.27
|
|
|
$0.51
- $1.00
|
688,917
|
2.2
Years
|
$0.86
|
688,917
|
2.2
Years
|
$0.86
|
|
|
$1.01
- $2.00
|
1,351,825
|
5.2
Years
|
$1.36
|
1,126,825
|
5.2
Years
|
$1.39
|
|
|
$2.01
- $3.00
|
1,195,041
|
4.7
Years
|
$2.42
|
1,195,041
|
4.7
Years
|
$2.42
|
|
|
|
|
||||||
|
Total
|
4,430,392
|
5.4
Years
|
$1.27
|
4,192,892
|
5.4
Years
|
$1.28
|
|
|
Aggregate
intrinsic value
|
$0
|
$0
|
|||||
Page
41
APPLIED
NANOTECH HOLDINGS, INC. AND SUBSIDIARIES
NOTES
TO CONSOLIDATED FINANCIAL STATEMENTS
|
9.
|
Stock Options
(continued):
|
The
following is a summary of stock option activity under all of the
plans:
|
Number
of
Shares
|
Wgtd.
Ave.
Exercise
Price
|
|||||||
|
Options
outstanding at December 31, 2006
|
7,713,912 | $1.58 | ||||||
|
Granted
|
1,831,196 | $1.20 | ||||||
|
Exercised
|
(307,244 | ) | $0.99 | |||||
|
Cancelled
|
(2,340,684 | ) | $1.52 | |||||
|
Options
outstanding at December 31, 2007
|
6,897,180 | $1.52 | ||||||
|
Granted
|
1,623,129 | $0.57 | ||||||
|
Exercised
|
(170,000 | ) | $0.36 | |||||
|
Cancelled
|
(460,412 | ) | $1.25 | |||||
|
Options
outstanding at December 31, 2008
|
7,889,897 | $1.36 | ||||||
|
Granted
|
658,065 | $0.45 | ||||||
|
Exercised
|
— | — | ||||||
|
Cancelled
|
(4,117,570 | ) | $1.31 | |||||
|
Options
outstanding at December 31, 2009
|
4,430,392 | $1.28 | ||||||
The
weighted-average grant-date fair value of options granted during the years ended
December 31, 2009, 2008, and 2007 was $0.13, $0.28, and $0.63, respectively. The
total intrinsic value of options exercised during the years ended December 31,
2008, and 2007 was $131,245, and $387,905 respectively. No options were
exercised in the year ended December 31, 2009. As of December 31, 2009, there
was a total of $157,408 of unrecognized compensation cost related to 237,500
non-vested options granted under the plan. Of these unvested options, 12,500
vest based on the passage of time and the remaining 225,000 options
vest upon the attainment of goals. The cost related to the time based options is
$2,090 and will all be recognized in 2010. It is unlikely that the goal based
options will vest, but if they do the entire expense will be recognized in 2010.
The fair value of shares vested during the years ended December 31, 2009, 2008,
and 2007 was $160,505, $605,393, and $299,519, respectively.
The 2002
Equity Compensation Plan also allows the issuance of restricted shares of common
stock. We issue shares of restricted stock in connection with our compensation
of outside Directors. We granted 59,167 shares, 45,000 shares, and 31,667
shares of restricted stock in 2009, 2008, and 2007, respectively. The
shares issued in 2007 and 2008 vested quarterly over a one year time period
starting with the date of the grant. A total of 70,417 of these shares have
vested and are issued and outstanding as of December 31, 2009. The remaining
6,250 shares did not vest and were forfeited. The shares granted in 2009 were
fully vested at the date of the grant are issued and outstanding at December 31,
2009. The shares granted in 2009 had a fair value of $15,383 and were granted at
a price of $0.26. The shares granted in 2008 had a fair value of $45,000 and
were granted at prices ranging from $0.91 to $1.15. The shares granted in 2007
had a fair value of $32,683 and were granted at prices ranging from $0.94 to
$1.19. We recognized expense in the financial statements of $27,976, $48,609,
and $10,150 in the years ended December 31, 2009, 2008, and 2007, respectively.
The weighted average fair value of shares granted and vested during 2009 was
$0.26. There were no shares unvested at December 31, 2009. The weighted average
fair value of shares granted during 2008, vested during 2008, and unvested at
December 31, 2008 was $1.00, $1.02, and $1.01, respectively. The weighted
average fair value of shares granted during 2007, vested during 2007, and
unvested at December 31, 2007 was $1.03, $0.94, and $1.05,
respectively.
Page
42
APPLIED
NANOTECH HOLDINGS, INC. AND SUBSIDIARIES
NOTES
TO CONSOLIDATED FINANCIAL STATEMENTS
|
9.
|
Stock Options
(continued):
|
In
November 2009, we offered a voluntary option exchange program to all non-officer
employees of the Company. Employees were able to exchange higher priced options
for options with an exercise price of $0.30 share. Employees received a fraction
of the number of shares exchanged in a ratio equal to $0.30 divided by the
original exercise price of the options. A total of 889,917 options were
exchanged for 222,768 shares with an exercise price of $0.30 per share. This
resulted in a reduction of option expense of $25,664.
During
2008, we repriced the exercise price of 200,000 options held by the Company’s
then CEO, from $1.19 per share to $0.75 per share in connection with a
simultaneous reduction in his compensation. The expense associated with this
repricing was $10,510 and was being recognized over the vesting term of the
options. A total of $2,366 was recognized in 2008. An additional $2,366 in
expense was recognized up until the time of his resignation in May 2009. The
balance was associated with options that never vested and therefore will not be
recognized.
The
Company also adjusted the price of certain non-officer options issued in 2008.
The Company has a performance based option program covering all non-officer
employees. The options are granted early in the year and vest as of December 31,
if certain goals are achieved. A total of 229,997 were granted under this
program in 2008. The original exercise price of these options was to be $1.19
per share, however the exercise price was adjusted to be equal to the market
price of the stock on the date of vesting, December 31, 2008. The effect of this
adjustment in exercise price, which effectively amounts to a repricing, was an
expense of $18,114, which was fully recorded in 2008.
During
the year ended December 31, 2007, we extended the contractual life of 20,000
options granted to a consultant by two years, and as a result, we recognized
additional compensation expense of $5,990. We account for options issued to
consultants using the same assumptions as for employees.
|
10.
|
Stock
Warrants:
|
Common
stock warrants
In 1997,
in connection with fundraising activities, we issued 75,000 ten
year warrants that enabled the holder to purchase shares
of the our common stock at a price of $1.00. A total of 15,000 of these warrants
were exercised in 1999. The remaining warrants expired unexercised.
In 2007,
we issued 1,304,353 warrants in connection with a private placement of the
Company’s stock. These warrants enable the holder to purchase shares of the
Company’s common stock at a price of $2.50 per share through the
earlier of April 2011, or the date that the shares acquired in the private
placement are sold by the shareholder.
The
following is a summary of outstanding warrants:
|
Number
of
Shares
|
Exercise
Price
|
|||||||
|
Warrants
outstanding at January 1, 2007
|
60,000 | $1.00-2.00 | ||||||
|
Issued
|
1,304,353 | $2.50 | ||||||
|
Expired
|
(60,000 | ) | $2.00 | |||||
|
|
||||||||
|
Warrants
outstanding at December 31, 2007
|
1,304,353 | $2.50 | ||||||
|
Issued
|
— | — | ||||||
|
Expired
|
— | — | ||||||
|
Warrants
outstanding at December 31, 2008
|
1,304,353 | $2.50 | ||||||
|
Expired
|
— | — | ||||||
|
|
||||||||
|
Warrants
outstanding at December 31, 2009
|
1,304,353 | $2.50 | ||||||
Page
43
APPLIED
NANOTECH HOLDINGS, INC. AND SUBSIDIARIES
NOTES
TO CONSOLIDATED FINANCIAL STATEMENTS
|
11.
|
Supplemental Cash Flow
Information:
|
Cash paid
for interest was $8,356, $7,180, and $926 for 2009, 2008, and 2007,
respectively. The following non-cash transactions have been excluded from the
accompanying consolidated statement of cash flows:
|
2009
|
2008
|
2007
|
||||||||||
|
Non-cash
financing activities:
|
||||||||||||
|
Capital
lease transaction
|
$ | 31,607 | $ | 34,990 | $ | 61,727 | ||||||
|
12.
|
Retirement
Plan:
|
The
Company sponsors a defined contribution 401(k) profit sharing plan. Company
contributions are discretionary and no company contributions were made in any of
the years presented.
|
13.
|
Commitments and
Contingencies:
|
Till
Keesmann Agreement
In May
2000, we licensed the rights to 6 carbon nanotube patents from Till Keesmann in
exchange for a payment of $250,000 payable in shares of our common stock. Under
the terms of the agreement, we are obligated to pay license fees equal to 50% of
any royalties received by us specifically related to these patents. We are
allowed to offset certain expenses, up to a maximum of $50,000 per year, against
payments due under this agreement. The agreement also contained provisions
related to minimum license fee payments. These minimum payments, totaling
$1,000,000, have been made and no further minimum payments are due. We are
allowed to offset these minimum payments against future royalty payments;
however, once these minimum payments and the expenses have been offset, we may
be liable for additional royalty payments.
The
Keesmann agreement was amended in 2008. As part of that amendment, the amount of
future offsets available for expenses incurred prior to June 2008 was fixed at
$300,000. In addition the amendment established licensing parameters under which
we can sublicense the patents without any additional approval by Mr. Keesmann.
The amendment also gave Mr. Keesmann the option of electing to receive an
additional advance of $100,000 against future royalties. In September 2008, Mr.
Keesmann elected to receive that advance, which is now available for us to
offset against future royalties. Finally, the amendment contains provision which
would allow Mr. Keesmann to license the patents directly and remit 50% of the
royalties to us beginning in June 2010, if certain minimum royalties are not
achieved by that date.
In 2008,
we also sold a portion of our potential future royalty stream related to the
Keesmann patents to IP Verwertungs GmbH (“IPV”) for $1.4 million. A total of
$1.226 million has been received and the remaining $174,000 will be offset
against future royalties due IPV. IPV will receive 25% of our portion of the
Keesmann royalties until we have received and retained $1.8 million. After that
level has been reached, IPV will receive 50% of our portion of the Keesmann
royalties, or approximately 25% of the total royalties on the Keesmann
patents.
Research
and development commitments
As of
December 31, 2009, the Company had several research contracts pending and in
process. The total amount of those contracts is $6,038,263. Of that total,
$3,100,466 has been recognized as revenue and $2,937,796 will be recognized in
the future. The revenue to be recognized from these research contracts in
2010 is expected to exceed the cost of this research.
Page
44
APPLIED
NANOTECH HOLDINGS, INC. AND SUBSIDIARIES
NOTES
TO CONSOLIDATED FINANCIAL STATEMENTS
|
13.
|
Commitments and
Contingencies (continued):
|
Government
contracts
Governmental
contractors are subject to many levels of audit and investigation. Among United
States agencies that oversee contract performance are: the Defense Contract
Audit Agency, the Inspector General, the Defense Criminal Investigative Service,
the General Accounting Office, the Department of Commerce, the Department of
Justice and Congressional Committees. The Company’s management believes that an
audit or investigation, if any, as a result of such oversight would not have any
material adverse effect upon the Company’s financial condition or results of
operations.
Legal
proceedings
On July
20, 1998, TFI Telemark, Inc. filed a complaint in the County Court at Law No. 2
of Travis County, Texas against the Company for debts of its now defunct
subsidiary, Plasmatron. The Company was served with notice of this suit on
August 5, 1998. The Company believes that no amounts are due to TFI; however,
all amounts claimed as owing by TFI are recorded as liabilities in the
consolidated financial statements of the Company. There has been no activity on
this case in the last year. The Company believes the ultimate resolution of this
matter will not have a material impact on the consolidated financial statements
of the Company.
From time
to time the Company and its subsidiaries are also defendants in various lawsuits
that may arise related to minor matters. It is expected that all such lawsuits
will be settled for an amount no greater than the liability recorded in the
financial statements for such matters. If resolution of any of these suits
results in a liability greater than that recorded, it could have a material
impact on us.
|
14.
|
Research and
Development Contracts:
|
The
Company makes significant expenditures for research and development. We seek
funding for our research and development costs to reduce the cost of such
expenditures to us. We only seek funding for projects that fit within our
strategic vision. A substantial portion of our funded research has been from
government contracts. Under government contracts, the government has the right
to utilize the results for its purposes and we have the right to utilize the
technology for commercial purposes. Generally, when we contract with other
entities, the entity is also conducting its own internal research related to
application of our technology to its products and such expenditures by the
entity frequently exceeds the amount of funding provided to the Company. Usually
the entity has the first opportunity to license the technology at the conclusion
of the project, if they desire. The costs of a particular research program may
exceed the funding received, however since the goal of the research is to
ultimately lead to a license, our willingness to share part of the development
cost is evaluated on a case by case basis.
The
following schedule summarizes certain information with respect to research and
development contracts:
|
2009
|
2008
|
2007
|
||||||||||
|
Contract
research revenues
|
$ | 3,461,226 | $ | 3,120,245 | $ | 3,318,608 | ||||||
|
Costs
incurred charged to operations included in research and
development
|
$ | 1,595,216 | $ | 2,200,603 | $ | 2,222,473 | ||||||
|
Amount
of additional funding commitments at December 31
|
$ | 2,937,796 | $ | 3,271,805 | $ | 3,890,842 | ||||||
Page
45
APPLIED
NANOTECH HOLDINGS, INC. AND SUBSIDIARIES
NOTES
TO CONSOLIDATED FINANCIAL STATEMENTS
|
15.
|
Quarterly Financial
Information (Unaudited):
|
|
First
|
Second
|
Third
|
Fourth
|
Total
|
||||||||||||||||
|
Quarter
|
Quarter
|
Quarter
|
Quarter
|
Year
|
||||||||||||||||
|
2009
|
||||||||||||||||||||
|
Revenues
|
$ | 824,525 | $ | 922,097 | $ | 1,506,673 | $ | 799,451 | $ | 4,052,746 | ||||||||||
|
Operating
income (loss)
|
(831,466 | ) | (652,735 | ) | (2,750 | ) | (657,442 | ) | (2,144,393 | ) | ||||||||||
|
Net
(loss)
|
(833,114 | ) | (654,027 | ) | (4,435 | ) | (661,029 | ) | (2,152,605 | ) | ||||||||||
|
Earnings
(loss) per share
|
||||||||||||||||||||
|
Basic
and Diluted
|
(0.01 | ) | (0.01 | ) | (0.00 | ) | (0.01 | ) | (0.02 | ) | ||||||||||
|
2008
|
||||||||||||||||||||
|
Revenues
|
$ | 870,014 | $ | 853,234 | $ | 891,853 | $ | 1,342,731 | $ | 3,957,832 | ||||||||||
|
Operating
income (loss)
|
(1,267,391 | ) | (1,357,688 | ) | (79,386 | ) | (520,715 | ) | (3,225,180 | ) | ||||||||||
|
Net
(loss)
|
(747,579 | ) | (1,345,041 | ) | (74,577 | ) | (518,670 | ) | (2,685,867 | ) | ||||||||||
|
Earnings
(loss) per share
|
||||||||||||||||||||
|
Basic
and Diluted
|
(0.01 | ) | (0.01 | ) | (0.00 | ) | (0.01 | ) | (0.03 | ) | ||||||||||
|
2007
|
||||||||||||||||||||
|
Revenues
|
$ | 956,867 | $ | 916,038 | $ | 1,049,749 | $ | 1,067,149 | $ | 3,989,803 | ||||||||||
|
Operating
income (loss)
|
(1,357,317 | ) | (1,281,562 | ) | (790,693 | ) | (965,511 | ) | (4,395,083 | ) | ||||||||||
|
Net
(loss)
|
(1,345,887 | ) | (1,229,787 | ) | (759,910 | ) | (921,307 | ) | (4,256,891 | ) | ||||||||||
|
Earnings
(loss) per share
|
||||||||||||||||||||
|
Basic
and Diluted
|
(0.01 | ) | (0.01 | ) | (0.01 | ) | (0.01 | ) | (0.04 | ) | ||||||||||
Annual
Earnings (loss) per share may not equal the sum of the four quarterly amounts
due to rounding.
|
16.
|
Concentrations of
Credit Risk:
|
The
Company’s financial instruments that are exposed to concentrations of credit
risk consist of cash and cash equivalents and receivables. The Company places
its cash and cash equivalents with high credit quality financial institutions;
however for periods of time during the year, bank balances on deposit were in
excess of the Federal Deposit Insurance Corporation insurance limit. No amounts
in excess of the FDIC limit were held in bank accounts in any year presented. At
December 31, 2007 and 2008, the Company held $2,414,958 and $103,603,
respectively, in excess of the Securities Investor Protection Corporation limits
in an account at Charles Schwab & Co. Inc.
The
Company’s receivables are uncollateralized and result primarily from its
research and development projects performed primarily for U.S. Federal
Government Agencies and services performed for large U.S. and multinational
corporations. The Company has not incurred any material losses on these
receivables.
|
17.
|
Significant
Customers:
|
Applied
Nanotech, Inc. received research and development revenues from the U.S.
Government in the three years as disclosed on the income statement. In addition
to the U.S. Government, the Company had three customers from which it has
received in excess of 10% of its consolidated revenues in one or more of the
past three years. We received revenues of $233,333, and $416,787 from Mitsui
& Co., Ltd. in the years ended December 31, 2008 and 2007,
respectively. We also had revenues from Ishihara Chemical Company, Ltd. of
$1,675,000, $633,250 and $685,963 in the years ended December 31, 2009, 2008,
and 2007, respectively. Finally, we recognized revenues of $180,000, $663,108
and $348,448 in the years ended December 31, 2009, 2008 and 2007 respectively
from Yonex Co. Ltd.
Page
46
APPLIED
NANOTECH HOLDINGS, INC. AND SUBSIDIARIES
NOTES
TO CONSOLIDATED FINANCIAL STATEMENTS
|
18.
|
Gain on Sale of
Intellectual Property and Other
Assets:
|
In 2008,
we had a gain of $100,000 from the sale of patents which did not relate to any
of our current active technology platforms. We had an additional gain of
$1,225,999 from the sale of a portion of our interest in any future royalties
received from sublicensing the Keesmann patents as described in more detail in
Note 13.
|
19.
|
Segment
Information:
|
The
Company’s operations are classified into three principal reportable
segments.
|
ANI
|
EBT
|
All
Other
|
Total
|
|||||||||||||
|
2009
|
||||||||||||||||
|
Revenue
|
$ | 4,052,746 | $ | — | $ | — | $ | 4,052,746 | ||||||||
|
Interest
Expense
|
8,356 | — | 1,733 | 10,089 | ||||||||||||
|
Depreciation
and Amortization
|
63,931 | — | 423 | 64,354 | ||||||||||||
|
Research
and Development
|
3,662,323 | — | — | 3,662,323 | ||||||||||||
|
Net
Loss
|
(1,411,121 | ) | 5,459 | (746,943 | ) | (2,152,605 | ) | |||||||||
|
Assets
|
780,848 | — | 112,519 | 893,367 | ||||||||||||
|
Capital
Expenditures
|
50,816 | — | 1,692 | 52,508 | ||||||||||||
|
2008
|
||||||||||||||||
|
Revenue
|
$ | 3,957,832 | $ | — | $ | — | $ | 3,957,832 | ||||||||
|
Interest
Expense
|
7,180 | — | — | 7,180 | ||||||||||||
|
Depreciation
and Amortization
|
70,141 | — | 395 | 70,536 | ||||||||||||
|
Research
and Development
|
4,614,644 | — | — | 4,614,644 | ||||||||||||
|
Net
Loss
|
(1,520,726 | ) | (1,296 | ) | (1,163,845 | ) | (2,685,867 | ) | ||||||||
|
Assets
|
1,105,627 | — | 686,862 | 1,792,489 | ||||||||||||
|
Capital
Expenditures
|
70,933 | — | — | 70,933 | ||||||||||||
|
2007
|
||||||||||||||||
|
Revenue
|
$ | 3,989,803 | $ | — | $ | — | $ | 3,989,803 | ||||||||
|
Interest
Expense
|
926 | — | — | 926 | ||||||||||||
|
Depreciation
and Amortization
|
49,575 | — | 923 | 50,498 | ||||||||||||
|
Research
and Development
|
4,526,166 | — | — | 4,526,166 | ||||||||||||
|
Net
Income (Loss)
|
(3,212,051 | ) | (1,298 | ) | (1,043,542 | ) | (4,256,891 | ) | ||||||||
|
Assets
|
778,863 | — | 2,965,995 | 3,744,858 | ||||||||||||
|
Capital
Expenditures
|
174,409 | — | — | 174,409 | ||||||||||||
Financial
information is furnished to the chief operating officer for review regarding
each subsidiary of the Company. The ANI segment consists of the activities of
ANI and includes license revenues and contract research revenues related to
ANI’s technology. The Company’s EBT subsidiary previously sold electronic
display products, but sold its technology in 2006. All other segments include
the Company’s general overhead. The accounting policies applied by each of the
segments are the same as those used by the Company.
|
20.
|
Subsequent
events:
|
From
January 1, 2010 through February 26. 2010, we received cash related to
the issuance of notes payable in the face amount of $1,550,000 with
the same terms as described in Note 4 to the financial statements. We also
issued 100,000 shares of common stock in payment of an accounts payable in the
amount of $30,000.
Page
47
Item
8. Changes in and Disagreements with
Accountants on Accounting and Financial Disclosure.
None
Item
8A(T). Controls and
Procedures.
Management
is responsible for establishing and maintaining effective disclosure controls
and procedures, as defined under Rule 13a-15 of the Securities Exchange Act
of 1934, that are designed to cause the material information required to be
disclosed by Applied Nanotech Holdings in the reports it files or submits under
the Securities Exchange Act of 1934 to be recorded, processed, summarized, and
reported to the extent applicable within the time periods required by the
Securities and Exchange Commission’s rules and forms. In designing and
evaluating the disclosure controls and procedures, management recognized that a
control system, no matter how well designed and operated, can provide only
reasonable, not absolute, assurance that the objectives of the control system
are met. Because of the inherent limitations in all control systems, no
evaluation of controls can provide absolute assurance that all control issues
and instances of fraud, if any, with a company have been detected.
As of the
end of the period covered by this report, Applied Nanotech Holdings performed an
evaluation under the supervision and with the participation of management,
including the Chief Executive Officer and Chief Financial Officer, of the
effectiveness of the design and operation of its disclosure controls and
procedures pursuant to Rule 13a-15 of the Securities Exchange Act of 1934.
Based upon that evaluation, the Chief Executive Officer and Chief Financial
Officer concluded that the disclosure controls and procedures were effective at
the reasonable assurance level.
Report
on Management’s Assessment of Internal Control over Financial
Reporting
The
management of Applied Nanotech Holdings, Inc. is responsible for establishing
and maintaining adequate internal control over financial reporting. Internal
control over financial reporting is defined under applicable Securities and
Exchange Commission rules as a process designed under the supervision of the
Company’s Chief Executive Officer and Chief Financial Officer and effected by
the Company’s Board of Directors, management and other personnel to provide
reasonable assurance regarding the reliability of financial reporting and the
preparation of the Company’s financial statements for external purposes in
accordance with U.S. generally accepted accounting principles. The Company’s
internal control over financial reporting includes those policies and procedures
that:
|
|
·
|
Pertain
to the maintenance of records that, in reasonable detail, accurately and
fairly reflect the transactions and dispositions of the assets of the
Company;
|
|
|
·
|
Provide
reasonable assurance that transactions are recorded as necessary to permit
preparation of financial statements in accordance with U.S. generally
accepted accounting principles, and that receipts and expenditures of the
Company are being made only in accordance with authorizations of
management and the directors of the Company;
and
|
|
|
·
|
Provide
reasonable assurance regarding prevention or timely detection of
unauthorized acquisition, use or disposition of the Company’s assets that
could have a material effect on the financial
statements.
|
Because
of its inherent limitations, internal control over financial reporting may not
prevent or detect misstatements. Also, projections of any evaluation of
effectiveness to future periods are subject to the risk that controls may become
inadequate because of changes in conditions, or that the degree of compliance
with the policies or procedures may deteriorate.
As of
December 31, 2009, management, with the participation of the Company’s
Chief Executive Officer and Chief Financial Officer, assessed the effectiveness
of the Company’s internal control over financial reporting based on the criteria
for effective internal control over financial reporting established in “Internal
Control — Integrated Framework,” issued by the Committee of Sponsoring
Organizations (COSO) of the Treadway Commission. Based on the assessment,
management determined that the Company’s internal control over financial
reporting was effective as of December 31, 2009. Our independent auditors,
Padgett Stratemann & Co., LLC have issued an attestation report, which is
included below, on our internal control.
Page
48
Changes
in Internal Control over Financial Reporting
No
changes were made to the Company’s internal control over financial reporting (as
defined in Rule 13a-15 under the Securities Exchange Act of 1934) during
the last fiscal quarter that materially affected, or are reasonably likely to
materially affect, the Company’s internal control over financial
reporting.
Attestation
Report of Independent Registered Public Accounting Firm
To the
Board of Directors and Stockholders of Applied Nanotech Holdings,
Inc.:
We have
audited the internal control over financial reporting of Applied Nanotech
Holdings, Inc. (the Company) as of December 31, 2009, based on criteria
established in Internal Control—Integrated Framework issued by the Committee of
Sponsoring Organizations of the Treadway Commission (COSO). The
Company’s management is responsible for maintaining effective internal control
over financial reporting and for its assessment of the effectiveness of internal
control over financial reporting included in the accompanying Report on
Management’s Assessment of Internal Control over Financial
Reporting. Our responsibility is to express an opinion on the
Company's internal control over financial reporting based on our
audit.
We
conducted our audit in accordance with the standards of the Public Company
Accounting Oversight Board (United States). Those standards require
that we plan and perform the audit to obtain reasonable assurance about whether
effective internal control over financial reporting was maintained in all
material respects. Our audit included obtaining an understanding of internal
control over financial reporting, assessing the risk that a material weakness
exists, and testing and evaluating the design and operating effectiveness of
internal control based on the assessed risk. Our audit also included
performing such other procedures as we considered necessary in the
circumstances. We believe that our audit provides a reasonable basis
for our opinion.
A
company's internal control over financial reporting is a process designed to
provide reasonable assurance regarding the reliability of financial reporting
and the preparation of financial statements for external purposes in accordance
with generally accepted accounting principles. A company's internal
control over financial reporting includes those policies and procedures that (1)
pertain to the maintenance of records that, in reasonable detail, accurately and
fairly reflect the transactions and dispositions of the assets of the company;
(2) provide reasonable assurance that transactions are recorded as necessary to
permit preparation of financial statements in accordance with generally accepted
accounting principles, and that receipts and expenditures of the company are
being made only in accordance with authorizations of management and directors of
the company; and (3) provide reasonable assurance regarding prevention or timely
detection of unauthorized acquisition, use, or disposition of the company's
assets that could have a material effect on the financial
statements.
Because
of its inherent limitations, internal control over financial reporting may not
prevent or detect misstatements. Also, projections of any evaluation
of effectiveness to future periods are subject to the risk that controls may
become inadequate because of changes in conditions, or that the degree of
compliance with the policies or procedures may deteriorate.
In our
opinion, Applied Nanotech Holdings, Inc. maintained, in all material respects,
effective internal control over financial reporting as of December 31, 2009,
based on criteria established in Internal Control—Integrated Framework issued by
the Committee of Sponsoring Organizations of the Treadway Commission
(COSO).
We have
also audited, in accordance with the standards of the Public Company Accounting
Oversight Board (United States), the consolidated balance sheets and the related
consolidated statements of operations, shareholders equity and cash flows of
Applied Nanotech Holdings, Inc and our report dated February 26. 2010 expressed
an unqualified opinion with an explanatory paragraph about the Company’s ability
to continue as a going concern.
Padgett,
Stratemann & Co., L.L.P.
San
Antonio, TX
February
26. 2010
Page
49
Item 8B. Other
Information.
None
Page
50
PART
III
Item
9. Directors, Executive
Officers and Corporate Governance
The
following sets forth the names, ages and certain information concerning the
Directors and Executive Officers of Applied Nanotech Holdings.
|
Name
|
Age
|
Position
|
Director/Officer Since
|
Term Expires
|
|
Dr. Zvi
Yaniv
|
63
|
Director,
President,
Chief
Operating Officer
|
July
1996
|
2010
|
|
Douglas
P. Baker
|
53
|
Director,
Chief Executive
Officer,
Chief Financial Officer
|
June
1996
|
2010
|
|
Ronald
J. Berman
|
53
|
Director
|
May
1996
|
2010
|
|
Dr.
Robert Ronstadt
|
68
|
Director,
Chairman
|
January
2003
|
2010
|
|
Howard
Westerman
|
57
|
Director
|
May
2007
|
2010
|
|
Tracy
K. Bramlett
|
54
|
Director
|
September
2007
|
2010
|
|
Paul
F. Rocheleau
|
56
|
Director,
Vice Chairman
|
May 2009
|
2010
|
|
Clinton
J. Everton
|
36
|
Director
|
October
2008
|
2010
|
|
Dr.
Richard Fink
|
50
|
Vice
President
|
January
2008
|
N/A
|
______________
Dr. Zvi
Yaniv has served as the Company’s President and Chief Operating Officer and a
Director since July 29, 1996. Dr. Yaniv has degrees in physics,
mathematics, and electro-optics as well as a Ph.D. in Physics. Prior to joining
the Company, in May 1996, Dr. Yaniv operated a consulting practice and
previously was President and CEO of Optical Imaging Systems Inc., a supplier of
flat panel color liquid crystal displays to the avionics and defense
industries.
Douglas
P. Baker has been with the Company since June 17, 1996, a Director since May
2006, and CEO since May 2009. Mr. Baker is a Certified Public Accountant
and has both a Bachelors in Business Administration and a Masters in Business
Administration. Immediately prior to joining Applied Nanotech Holdings, Inc.,
Mr. Baker was a divisional controller for MascoTech, Inc. from 1991 to
1996. Mr. Baker also has prior experience in public accounting and as CFO
of a privately held company. Mr. Baker is also Chairman of the Board of
Directors of Total Health Care, Inc., a non-profit Health Maintenance
Organization and has been a member of the Board of Directors of that
organization since 1987.
Ronald J.
Berman has been a Director since May 1996. Mr. Berman co-founded BEG
Enterprises, Inc. and was its President from 1989 until 1998. Mr. Berman
currently is President of R.J. Berman Enterprises, Ltd., a real estate
development company, Inergi Fitness, and Walkers Warehouse. Mr. Berman
earned a Juris Doctor degree in 1980 from the University of
Detroit.
Dr. Robert
Ronstadt has been a Director since January 2003 and Chairman of the Board of
Directors since May 2009. Dr. Ronstadt was Vice President of
Technology Commercialization for Boston University from June 2003 through 2005.
At the same time, he became the Director of Boston University’s Technology
Commercialization Institute. He was special advisor to the Chancellor of Boston
University from January to May 2003. Prior to that, from 1998 to 2002, he
was Director of the IC2 Institute at the University of Texas in Austin and
the J. Marion West Chair of Constructive Capitalism. Dr. Ronstadt was a
professor of entrepreneurship at the Pepperdine University School of Business
Management from 1992 to 1998 and Babson College in Wellesley Massachusetts from
1975 to 1985. From 1986 to 1992, he was the CEO of a software
enterprise.
Howard
Westerman is the Chief Executive Officer of JW Operating Company, a privately
held energy development and energy services company headquartered in Dallas,
Texas. Mr. Westerman joined JW Operating Company in 1978 and became CEO in 1999.
Under his leadership as CEO, the Company’s revenues increased from approximately
$70 million to $1 Billion. Mr. Westerman is also a member of the Board of
Directors of Peerless Manufacturing Company, a global provider of environmental
and separation filtration products, listed on the NASDAQ Global Market Exchange.
Mr. Westerman also serves on numerous charitable and community
boards.
Page
51
Tracy K.
Bramlett is President of Industrial Hygiene and Safety Technology, Inc. (IHST),
a full service industrial hygiene consulting company that he formed in 1987.
IHST specializes in Indoor Environmental Quality issues. Prior to forming IHST,
Mr. Bramlett was a corporate industrial hygienist for Burlington Northern
Railroad.
Paul F.
Rocheleau has been a Director of the Company since May 2009, and Vice Chairman
of the Board since October 2009. He is Chairman of the Board and Chief
Investment Officer of Virginia Biosciences Commercialization Center. Prior to
that he was a managing director at Cary Street Partners, a regional investment
banking firm based in Richmond, Virginia. Mr. Rocheleau also serves on the Board
of Directors of Nanochemonics, a specialty nanomaterials company and the
advisory Board of Apex systems, an IT staffing company.
Clinton
J. Everton has been a Director of the Company since October 2008, and is
currently President of the Margrave Group, a company that he founded in 2008.
Prior to that, from 2001 to 2007, he held a variety of roles at Thompson NETg,
including Vice President, Chief Technology Officer, and SVP Product operations
before becoming President in 2005. Prior to Thompson NETg, Mr. Everton was
President of Knowledge Communication, a pioneer in the e-learning
field.
Dr.
Richard Fink is Vice President of Engineering for Applied Nanotech, Inc.; a
subsidiary of Applied Nanotech Holdings, Inc. Dr. Fink has a Bachelor in Science
degree from South Dakota State University and a Masters in Science and Ph.D. in
Physics from the University of Illinois and has worked for the Company since
1995. Dr. Fink is also the co-founder of the Nanomaterials Applications Center
at Texas State University – San Marcos, and has been chairman of the Texas
Chapter of the Society for Information Display since 2004.
All
members of the Board of Directors attended greater than 75% of the meetings of
the board of directors and meetings of the committees of the board on which he
served with the exception of Mr. Rocheleau, who attended less than 75% of such
meetings.
Shareholder
Director Nominating Procedures
The
Company has a procedure in place for holders of the Company’s common stock to
recommend nominees to the Company’s Board of Directors. These procedures are set
forth in Article 9(b) of the Company’s Restated Articles of Incorporation (the
“Restated Articles”), a copy of which is filed as Exhibit 3(I) to this Annual
Report on Form 10-K. Nominations of persons for election to the Board of
Directors of the Company may be made at a meeting of shareholders by or at the
direction of the Board of Directors or by any shareholder of the Company
entitled to vote for the election of Directors at the meeting who complies with
the notice procedures set forth in Article 9(b). Such nominations, other than
those made by or at the direction of the Board of Directors, shall be made
pursuant to timely notice in writing to the Secretary of the Corporation. To be
timely, a shareholder’s notice shall be delivered to or mailed and received at
the principal executive offices of the Company not less than 60 days nor more
than 90 days prior to the meeting; provided, however, that in the event that
less than 70 days’ notice or prior public disclosure of the date of the meeting
is given or made to shareholders, notice by the shareholder to be timely must be
so received not later than the close of business on the 10th day following the
date on which such notice of the date of the meeting was mailed or such public
disclosure was made. Such shareholder’s notice shall set forth (i) as to each
person whom the shareholder proposes to nominate for election or re-election as
a Director, (A) the name, age, business address and residence address of such
person, (B) the principal occupation or employment of such person, (C) the class
and number of shares of the Company which are beneficially owned by such person,
and (D) any other information relating to such person that is required to be
disclosed in solicitations of proxies for election of Directors, or is otherwise
required, in each case pursuant to Regulation 14A under the Securities Exchange
Act of 1934, as amended (including without limitation such person’s written
consent to being named in the proxy statement as a nominee and to serve as a
Director if elected); and (ii) as to the shareholder giving the notice, (1) the
name and address, as they appear on the Company’s books, of such shareholder and
(2) the class and number of shares of the Company which are beneficially owned
by such shareholder. No person shall be eligible for election as a Director of
the Company unless nominated in accordance with the procedures set forth in
Article 9(b) of the Restated Articles. The Chairman of the meeting shall, if the
facts warrant, determine and declare to the meeting that a nomination was not
made in accordance with the procedures prescribed herein, and if he should so
determine, he shall so declare to the meeting and the defective nomination shall
be disregarded.
Page
52
Committees
The Board
of Directors has three formal committees. The audit committee consists of
Mr. Everton, Mr. Westerman and Mr. Rocheleau. The audit committee operates
without a chairman and met twice in 2009. The compensation committee consists of
Mr. Berman, Mr. Bramlett, and Mr. Rocheleau. Mr. Berman is chairman of the
compensation committee, which met four times in 2009. The nominating committee
consists of Mr. Rocheleau, Mr. Westerman, and Mr. Bramlett. The nominating
committee operates without a chairman and met once during 2009. The Board also
established an advisory committee in 2009 which is not a formal committee of the
Board. This advisory committee is the business development advisory committee.
The committee is chaired by Mr. Rocheleau and includes Mr. Everton, Mr. Baker,
and Dr. Yaniv.
Audit
Committee Financial Expert
The Board
of Directors has determined that both Mr. Westerman and Mr. Rocheleau
qualify as an “audit committee financial expert” under applicable SEC rules and
that all members of our audit committee qualify as “independent” as defined
under applicable SEC rules.
Code
of Ethics
We have
adopted a Code of Ethics within the meaning of Item 406(b) of Regulation S-K of
the Securities Exchange Act of 1934. This Code of Ethics applies to all
directors, officers, and employees of the Company. A copy of this Code of Ethics
is publicly available on our website at www.appliednanotech.net.
Section
16(a) Beneficial Ownership Reporting Compliance
Section
16(a) of the Securities of Exchange Act of 1934 requires Applied Nanotech
Holdings’ officers, Directors, and persons who beneficially own more than 10 %
of a registered class of Applied Nanotech Holdings’ common stock, to file
reports of ownership and changes in ownership with the Securities and Exchange
Commission. Officers, Directors, and beneficial owners of more than 10% of
Applied Nanotech Holdings’ common stock are required by the Securities and
Exchange Commission regulations to furnish Applied Nanotech Holdings with copies
of all Section 16(a) forms that they file.
Based
solely on review of the copies of such reports furnished to us, or written
representations that no reports were required, we believe that for the period
from January 1, 2009 through December 31, 2009, all Officers, Directors, and
greater than 10% beneficial owners complied with all Section 16(a) filing
requirements applicable to them except that Director Rocheleau filed his initial
Form 3 after the due date. The form was filed on June 9, 2009, but due May 15,
2009.
Item
10. Executive Compensation
Compensation
Discussion and Analysis
Objectives
of Compensation Program
The
primary objective of our compensation program for employees, including our
compensation program for executive officers, is to attract, retain, and motivate
qualified individuals and reward them in a manner that is fair to all
stakeholders. We strive to provide incentives for every employee that rewards
them for their contribution to the Company, while at the same time promoting an
ownership mentality.
Elements
of Compensation
There are
three main components to our compensation package - base salaries, bonuses, and
stock based compensation. A fourth, less significant component is other benefits
and perquisites. Our compensation program is designed to be competitive with
other employment opportunities and to align the interests of all employees,
including executive officers, with the long-term interests of our shareholders.
For our executive officers, we link a much higher percentage of total
compensation to incentive compensation such as bonus and stock based
compensation than we do for other employees.
Page
53
Base
Salaries
We
provide our executive officers with a level of cash compensation that is
designed to facilitate an appropriate lifestyle and provide a reasonable minimum
compensation. We make this determination based on a variety of factors including
professional accomplishments, level of education, past experience and scope of
responsibilities. The actual amount of base salary earned by each named
executive officer is set forth in the Summary Compensation Table included later
in this section.
As
discussed in more detail below, the following base salary amounts for these
executive officers are currently in effect: Dr. Yaniv - $275,000, Mr.
Baker - $275,000, and Dr. Fink - $125,000. However; the executive
officers are voluntarily deferring a substantial portion of their salaries to
preserve cash for the Company. Mr. Baker is deferring 35% of his salary and Drs.
Yaniv and Fink are each deferring 15% of their salaries.
The
salary level for our executive officers was basically frozen from 2005 to 2007.
During that time period, the CEO and COO, each had a salary of approximately
$250,000 per year and the CFO had a salary of $180,000 per year. As the result
of a study discussed below, the following base salary amounts became effective
January 1, 2008: CEO, Mr. Bijou - $300,000; COO, Dr. Yaniv - $275,000: and CFO,
Mr. Baker $225,000. In addition, effective December 31, 2007, Dr. Richard Fink,
Vice President became a named executive officer. Dr. Fink’s salary became
$125,000 effective January 1, 2008. Effective August 1, 2008, executive officers
began voluntarily deferring a portion of their salaries. Mr. Baker’s salary was
adjusted to $275,000 on May 11, 2009 as a result of his appointment to CEO.
However; Mr. Baker deferred the entire amount of the salary increase to assist
the company with managing its cash flow.
Bonuses
We have a
formula bonus plan covering all employees, including executive officers. This
plan was originally established in 2004 and is based solely on the profitability
of the company. This plan is designed to reward all employees when we are
successful in reaching profitability. No bonuses have ever been paid under this
plan, since we have incurred losses in each of the years since adoption of the
plan. The maximum bonuses payable under this plan are $250,000 for the chief
executive officer, $200,000 for the chief operating officer, and $150,000 for
the chief financial officer. The maximum amounts would be payable if our net
income is equal to, or exceeds $10 million. For purposes of this plan, net
income is calculated using the accounting principles in effect at the time the
plan was adopted, meaning stock based compensation using fair value is excluded
from the calculation. There are no minimum amounts payable under the plan and
the target amounts are equal to the maximum amounts payable. The compensation
committee of the board of the directors also has the power to award
discretionary bonuses; however, no such bonuses have been granted since 2002 in
the case of the CEO and the CFO. The COO received a discretionary bonus of
$115,000 in 2007.
Stock
Based Compensation
All of
our employees participate in our stock based compensation plans and receive
awards of non-qualified stock options annually. We use non-qualified options
because of the favorable tax treatment to us and the near universal expectation
by employees in our industry that they will receive stock options. The
overwhelming majority of these awards are performance based awards that only
vest upon achievement of specific goals. For non-executive officer employees,
these goals tend to be operational oriented goals relating to specific projects
or potential projects. For executive officers, these goals are broad in nature
and involve more substantial accomplishments. Following is a discussion of the
option grants to executive officers.
In
2007, the compensation committee adopted a multiyear option program for
executive officers covering the years 2008 to 2010. This program included a
mixture of time based option and performance based options. The majority of
these grants were performance based options with goals related to modified
cashflow from operations and various earnings per share targets. Dr. Fink
received options as part of this program; however, as previously indicated, Dr.
Fink became an executive officer effective December 31, 2007 and was not an
executive officer at the time of the grant.
A portion
of the goals related to options granted in 2007 were tied to cash flow goals for
2008. These options expired unvested as of December 31, 2008. The executive
officers were granted new options on December 31, 2008 to replace the expiring
options. These new options vest based on cash flow goals for 2009 and were
priced 15% above the market price of the common stock on the date of grant. The
options granted in 2008 did not vest and have since expired.
Page
54
For
purposes of this discussion of performance based option goals, we consider the
goals related to earnings per share targets to be part of our confidential
strategic plans, and as such we do not disclose the specifics of the goals at
this time. Attainment of these goals will require the company to achieve
financial results never before achieved in the history of the Company. We
consider these goals achievable, but they represent a substantial stretch and
are considered essential to proving our business model. They align the interests
of the executive officers with those of the shareholders.
At the
present time, we have no formal policy related to stock ownership for executive
officers, other than for those officers that are also members of the Board of
Directors and are covered under the Board policy, which is described later in
this section. In establishing grant levels, we do not consider the equity
ownership levels of the executive officer. In general, we do not consider the
existence of fully vested prior awards when establishing new grants. However,
with newer executive officers, we may consider the lack of prior awards in
establishing a higher level of new grants.
Timing of
Option Grants
We do not
have a formal written policy related to the timing of option grants; however we
do have certain time periods when options are normally granted. At the present
time, we do not have any analysts that follow our stock and the release of our
quarterly financial reports normally has no noticeable impact on the price of
our stock. As such, we do not have trading windows, nor do we limit option
grants to any sort of windows. There are two normal situations where options are
granted. The first would be at the time a new employee, including executive
officers, is hired. If a new employee receives options as part of starting
employment, those options are granted either at, or shortly after, the
employment start date.
The
majority of options are performance based awards granted on an annual basis as
part of a budgeting/goal setting process. For executive officers, the
compensation committee meets annually to establish compensation levels,
including salary, bonus, and options, for the year. This meeting normally occurs
in late November or early December prior to the start of the new year - for
example in December 2008 for 2009 compensation. It could, however, occur as
early as November or as late as January. For all other employees, the goal
setting process starts in December, but since it involves many more distinct
goals and many more individuals; it is a longer process and as a result usually
is not ready for submission to the Compensation Committee until January or
later. All performance based awards for employees other than executive officers
are annual awards that must either vest by the end of the calendar year, or they
will expire unvested. At the time of the proposed award, we consider whether
there are any known upcoming significant events, and have in the past delayed
awards as a result of expected positive events.
All
option grants for employees are approved by the compensation committee of the
Board of Directors. The compensation committee has authorized the executive
officers to grant limited amounts of options to new hires without seeking
additional compensation committee approval. The compensation committee does not
delegate any of its powers for granting options to others, except that it has
granted the CEO the power to grant up to 20,000 options to new hires without the
specific approval of the compensation committee.
Other
Benefits and Perquisites
Since we
have not yet reached profitability on a consistent basis, we take a relatively
bare-bones approach to benefits for all employees, including executive officers.
There are no benefit plans available to executive officers that are not
available to all employees. Executive officers participate in the same benefit
plans covering other employees. These benefits include limited health and dental
insurance, and group term life insurance. The only retirement plan that we
maintain is a 401K plan funded entirely by employee elective deferrals. We have
no company funded retirement plans or deferred compensation plans. We also do
not provide any of the perquisites common at larger companies. The only perq
that we provide is an auto allowance. Our CEO and COO receive an auto allowance
of $1,000 per month and our CFO received an auto allowance of $500 per month up
until the time that he became CEO. The amounts paid as auto allowances are
considered in setting the overall level of compensation for the executive
officer.
Page
55
Compensation
Approval Process
The
Compensation Committee of the Board of Directors approves all compensation and
awards to executive officers. The CEO provides recommendations to the
compensation committee for the other executive officers, all of which directly
or indirectly report to him, and regarding most compensation matters, including
executive compensation, the CFO in consultation with the CEO, provides
information to the Compensation Committee. However, the Compensation Committee
does not delegate any of its functions to others in setting compensation. We did
not make formal use of any compensation consultants in determining executive
compensation levels for any of the executive officers.
In 2006
we used an executive search firm in connection with our search for a new CEO.
That firm, Christian & Timbers, indicated that at the time our CEO salary
was at the low end of the acceptable range for similar companies, although when
considering an extensive option package, total compensation fell within the
normal range of CEO compensation. In November 2007, we performed a compensation
analysis to benchmark our compensation package against other similar companies.
We did not use an outside compensation consultant for this study, but rather
performed the analysis internally. We selected the following companies: Nanogen
(NGEN), Nanosphere (NSPH), Harris & Harris (TINY), Nanosys (NNSY), Acacia
Research/Acacia Technologies (ACTG), Arrowhead Research (ARWR), and Symyx
Technologies (SMMX). In selecting these companies, we considered such factors as
nanotechnology involvement, market capitalization, revenue levels,
profitability, and line of business. While no particular company is a perfect
match, we believe that overall this is a representative mix of companies to use
as a comparison. We gathered data on these companies from publicly available
data, including SEC filings. In general, there was a lag related to these
filings, so the most recent data available for these companies was from 2006 or
prior.
The
results of our benchmarking study showed that the compensation paid to executive
officers at Applied Nanotech Holdings, Inc. was well below average for the peer
group selected. Salaries were at or near the bottom of the range. In
the case of the CEO and COO, all but one of the comparison companies paid higher
salaries and in the case of the CFO, all of the comparison companies paid higher
salaries. The majority of the comparison companies paid bonuses despite the
existence of net operating losses. While the Applied Nanotech Holdings officers
had the potential for larger option grants, based on options actually vested, on
average the comparison companies also had higher levels of options
granted.
When
setting compensation levels for 2008 and future years, we considered the result
of this study. Salaries were set at the previously disclosed levels based on
this study and in consideration of the fact that salary levels had been
unchanged for three years. These new salary levels are, in general, still below
average for the comparison companies. Until such time as the company attains
profitability, we believe it reasonable for salaries to continue to be below
average. When the company reaches profitability, it is anticipated that salaries
will be adjusted to market levels. Our bonus plan based on profitability remains
in place and it is anticipated that future bonuses will not be paid until such
time as we have reached profitability. Finally, as previously described, we
granted options to the executive officers in 2007, and 2008. These options
included both time based and performance based options, although the majority of
the options were performance based. This split was determined, in part based on
the option vesting history over the past several years. No options were granted
to executive officers in 2009, but we anticipate grants in 2010.
We
updated the previously mentioned compensation study at the end of 2008 and found
that in general, compensation levels had increased at the comparison companies.
We, however, left compensation unchanged for our executive officers for 2009.
Since we had no intention of increasing salaries in 2010, we did not update our
compensation study. Salaries for 2010 remain at 2009 levels.
We
believe our total compensation package mitigates unreasonable risk-taking by our
senior executives. In this regard, we strike a balance between short-term and
long-term cash and equity awards. A significant portion of our executives’ pay
is linked to the achievement of financial goals directly aligned to stockholder
interests. Our long-term equity awards incent executives to take
a long term view of the company and to assume reasonable risks to develop new
products, explore new markets and expand existing business.
Compensation
Committee Report
We have
reviewed and discussed with management certain Compensation Discussion and
Analysis provisions to be included in the Company’s 2009 Annual Report on Form
10-K for the fiscal year ended December 31, 2009, filed pursuant to Section 13
or 15(d) of the Securities Exchange Act of 1934 (“Form 10-K”). Based on the
reviews and discussions referred to above, we recommend to the Board of
Directors that the Compensation Discussion and Analysis referred to above be
included in the Company’s Form 10-K.
Compensation
Committee
Ronald
J. Berman, Chairman; Paul F. Rocheleau; Tracy Bramlett
Page
56
The
following table sets forth the total cash compensation paid or to be paid, as
well as certain other compensation paid or accrued, for services rendered during
the fiscal years ended December 31, 2009, 2008 and 2007 by all individuals that
served as Chief Executive Officer during 2009, the Chief Financial Officer, all
individuals that were Named Executive Officers as of the end of the previous
year, and all executive officers whose total annual salary and bonus exceeded
$100,000 for the fiscal year ended December 31, 2009 (the “Named Executive
Officers”):
SUMMARY
COMPENSATION TABLE
|
Name &
Principal Position
|
Year
|
Salary (1)
|
Bonus
|
Option Awards (2)
|
All
Other Compensation
(5)
|
Total
|
|||||||||||||||
|
Thomas
F. Bijou (3)
|
2009
|
$ | 73,775 | $ | 0 | $ | 0 | $ | 5,000 | $ | 78,775 | ||||||||||
|
Chief
Executive Officer
|
2008
|
$ | 284,167 | $ | 0 | $ | 37,876 | $ | 12,000 | $ | 334,043 | ||||||||||
|
2007
|
$ | 288,000 | $ | 0 | $ | 211,824 | $ | 0 | $ | 499,824 | |||||||||||
|
Dr.
Zvi Yaniv
|
2009
|
$ | 275,000 | $ | 0 | $ | 0 | $ | 12,000 | $ | 287,000 | ||||||||||
|
Chief
Operating Officer
|
2008
|
$ | 275,000 | $ | 0 | $ | 13,680 | $ | 12,000 | $ | 300,680 | ||||||||||
|
2007
|
$ | 250,000 | $ | 115,000 | $ | 105,912 | $ | 12,000 | $ | 470,912 | |||||||||||
|
Douglas
P. Baker (4)
|
2009
|
$ | 251,956 | $ | 0 | $ | 0 | $ | 9,833 | $ | 261,789 | ||||||||||
|
Chief
Executive Officer
|
2008
|
$ | 225,000 | $ | 0 | $ | 8,550 | $ | 6,000 | $ | 239,550 | ||||||||||
|
Chief
Financial Officer
|
2007
|
$ | 180,000 | $ | 0 | $ | 66,195 | $ | 6,000 | $ | 246,195 | ||||||||||
|
Dr.
Richard Fink
|
2009
|
$ | 125,000 | $ | 0 | $ | 0 | $ | 0 | $ | 125,000 | ||||||||||
|
2008
|
$ | 125,000 | $ | 0 | $ | 3,420 | $ | 0 | $ | 128,420 | |||||||||||
|
2007
|
$ | 100,000 | $ | 0 | $ | 38,423 | $ | 0 | $ | 138,423 | |||||||||||
(1)
Salary amounts for 2008 and 2009 for all officers reflect amounts earned during
the year. Effective August 1, 2008, the officers began deferring a portion of
their salaries as part of the Company’s cost reduction/cash conservation
initiatives. The amount of 2008 and 2009 salaries still payable as of December
31, 2009 are $85,641 for Mr. Baker, $58,443 for Dr. Yaniv, and $26,568 for Dr.
Fink. Mr. Baker’s salary was increased to $275,000 on an annual basis effective
with his appointment to CEO on May 11, 2009, however the entire amount of the
increase was deferred and is still payable at December 31, 2009
(2)
Amounts included in the option awards column are calculated using fair value
accounting. See Note 9 of the consolidated financial statements included in this
annual report for the assumptions underlying valuation of equity awards. The
amounts are calculated based on options granted during the year that are
expected to vest. As discussed in the Compensation Discussion and Analysis, the
majority of options granted are performance based options associated with
specific goals. To the extent that the goals are not achieved, the options do
not vest. All options granted to Mr. Bijou in all years expired unexercised as a
result of his resignation in 2009. In 2007 each of the officers were granted
performance based options for which the likely of vesting was remote and the
options were not expected to vest at the time of the grant. The number of
options and grant date fair value of these performance based options not
included in the table for each officer in 2007 was as follows: Mr. Bijou –
440,000 options with a fair value of $293,533; Mr. Yaniv – 220,000 options with
a fair value of $141,676; Mr. Baker – 137,500 options with a fair value of
$88,548; and Dr. Fink – 55,000 options with a fair value of $35,419. The options
issued in 2008 to all officers were performance based options that were expected
to vest at the time of grant; however, all options granted to officers in 2008
expired unvested in December 2009.
Page
57
(3)
Mr. Bijou terminated employment as CEO on May 7, 2009. His compensation was
a combination of salary and management fee. As part of the management fee, he
was responsible for certain expenses such as employee benefits and secretarial
services. Both the management fee and salary are included under the heading of
salary in this table.
(4) Mr.
Baker became CEO, effective May 11, 2009 upon the resignation of Mr.
Bijou.
(5) The
amounts included in the “All Other Compensation” column represent automobile
allowances in all years.
GRANTS
OF PLAN-BASED AWARDS TABLE
No grants plan-based awards were
made to the named executive officers in 2009.
Page
58
OUTSTANDING
EQUITY AWARDS AT FISCAL YEAR-END TABLE
The
following table sets forth information concerning the outstanding equity awards
held by the Named Executive Officers at December 31, 2009.
|
Option
Awards
|
|||||||||||||
|
Name
|
Number
of Securities Underlying Unexercised Options - Number Exercisable
|
Equity
Incentive Plan Awards: Number of Securities Underlying Unexercised
Unearned Options
|
Option
Exercise Price
|
Option
Expiration
Date
|
|||||||||
|
Dr.
Zvi Yaniv
|
30,000 | - | $1.50 |
02/02/2010
|
|||||||||
| 200,000 | - | $1.50 |
06/27/2010
|
||||||||||
| 30,000 | - | $0.96 |
07/28/2013
|
||||||||||
| 250,000 | - | $2.73 |
12/31/2013
|
||||||||||
| 250,000 | - | $2.17 |
12/31/2014
|
||||||||||
| 180,000 | - | $1.19 |
12/03/2017
|
||||||||||
| - | 120,000 | $1.19 |
12/03/2017
|
||||||||||
|
Douglas
P. Baker (1)
|
30,000 | - | $1.50 |
02/02/2010
|
|||||||||
| 50,000 | - | $1.50 |
06/27/2010
|
||||||||||
| 60,000 | - | $0.63 |
03/02/2011
|
||||||||||
| 100,000 | - | $0.92 |
07/16/2011
|
||||||||||
| 150.000 | - | $0.73 |
12/05/2011
|
||||||||||
| 13,000 | - | $0.96 |
07/28/2013
|
||||||||||
| 200,000 | - | $2.73 |
12/31/2013
|
||||||||||
| 150,000 | - | $2.17 |
12/31/2014
|
||||||||||
| 112,500 | - | $1.19 |
12/03/2017
|
||||||||||
| - | 75,000 | $1.19 |
12/03/2017
|
||||||||||
|
Dr.
Richard Fink
|
2.500 | - | $1.50 |
01/03/2010
|
|||||||||
| 2,500 | - | $1.50 |
02/15/2010
|
||||||||||
| 2,000 | - | $0.58 |
02/16/2011
|
||||||||||
| 32,750 | - | $1.00 |
12/31/2012
|
||||||||||
| 6,875 | - | $0.50 |
03/20/2013
|
||||||||||
| 21,000 | - | $0.56 |
04/16/2013
|
||||||||||
| 19,881 | - | $2.50 |
03/10/2014
|
||||||||||
| 15,906 | - | $2.17 |
01/01/2015
|
||||||||||
| 3,487 | - | $2.17 |
02/14/2016
|
||||||||||
| 10,112 | - | $2.25 |
04/11/2016
|
||||||||||
| 16,713 | - | $1.28 |
01/29/2017
|
||||||||||
| 45,000 | - | $1.19 |
12/03/2017
|
||||||||||
| - | 30,000 | $1.19 |
12/03/2017
|
||||||||||
______________________
(1)
Includes options still outstanding that were previously transferred by gift and
reported on Form 4 by the Named Executive Officer. For Mr. Baker, these options
are the 60,000 options expiring 03/02/2011.
OPTION
EXERCISE AND STOCK VESTED TABLE
Named
executive officers exercised no options and received no vested stock awards in
2009.
Page
59
PENSION
BENEFITS TABLE
We
maintain no retirement plans covering Named Executive Officers or other
employees, except for a 401K plan funded solely by elective employee
contributions. As such no pension benefits table is included.
NON-QUALIFIED
DEFERRED COMPENSATION TABLE
We do not
maintain any non-qualified deferred compensation plans covering Named Executive
Officers or other employees. As such, no deferred compensation table is
included.
POTENTIAL
PAYMENTS UPON TERMINATION OR CHANGE IN CONTROL
None of
the named executive officers have formal written employment contracts, and
therefore, there are no formal payments due on a change in control or other
employment termination. Our 2002 Equity Compensation Plan, which includes all
employees, including executive officers, includes a provision which accelerates
the vesting of all unvested options upon certain change in control events.
Unvested options held by Named Executive Officers as of December 31, 2009 are
reflected in the Outstanding Equity Awards at Fiscal Year-End Table included in
this item.
It is our
policy to pay severance upon termination when termination is initiated by us and
is for other than cause. We have no formal guidelines, but rather each case is
handled on an individual basis. Factors considered include position, length of
service, reason for termination, possible future relationships, as well as other
potential factors. Payments may be made in a lump sum or in periodic
installments and are usually accompanied by a severance agreement that includes
a release, a non-disparagement clause, and possibly a non-compete agreement. In
the case of executive officers, the minimum severance due, unless alternative
amounts are negotiated, is one month of severance for each year or
partial year of service at the Company, with a minimum of six months. In the
case of the existing executive officers, that would equate to fourteen months
for Dr. Yaniv and Mr. Baker, and fifteen months for Dr. Fink. This could be
viewed as the minimum potential pay out upon termination; however, upon mutual
agreement of the parties, a lower amount could be negotiated.
DIRECTOR
COMPENSATION
|
Name
|
Fees
Earned or Paid in Cash (1)
|
Stock
Awards
(2)
|
Total
|
|||
|
Howard
Westerman
|
$ 12,000
|
$ 2,600
|
$
14,600
|
|||
|
Ronald
J. Berman
|
$ 18,000
|
$ 2,600
|
$
20,600
|
|||
|
Tracy
K. Bramlett
|
$ 12,000
|
$ 2,600
|
$
14,600
|
|||
|
Dr. Robert
Ronstadt
|
$ 31,817
|
$ 2,600
|
$
34,417
|
|||
|
Patrick
V. Stark (3)
|
-
|
$ 2,600
|
$ 2,600
|
|||
|
Clinton
J. Everton (4)
|
$ 12,000
|
$ 1,950
|
$
13,950
|
|||
|
Paul
F. Rocheleau(5)
|
$ 8,000
|
$ 433
|
$ 8,433
|
|
(1)
|
Amounts
included in this column represent fees earned. All fees were deferred by
Board Members and no fees were paid in
cash.
|
|
(2)
|
Amounts
included in the restricted stock awards column are calculated based on the
total fair market value of the shares granted on the date of the grant
based on the closing price of $0.26 per
share.
|
|
(3)
|
Director
Stark resigned in August
2009.
|
|
(4)
|
Director
Everton became a Director in October
2008.
|
|
(5)
|
Director
Rocheleau became a Director in May
2009.
|
Page
60
We paid
our outside Directors with a combination of cash and restricted stock from 2007
through 2009. We believe this mixture of cash and equity was appropriate at our
current level of development and provided the Directors with current
compensation for services rendered while still aligning the interests of the
Directors with the shareholders. Reasonable expenses incurred by each Director
in connection with his duties as a Director are also reimbursed by the
company.
The
Directors each received an annual retainer of $12,000, paid quarterly. Each
committee chairman received an additional annual retainer of $6,000, paid
quarterly, and the Board Chairman, if not an employee, received an additional
annual retainer of $8,000. As part of our cost reduction/cash conservation
initiative, all outside Directors deferred their cash compensation for the
3rd an
4th
quarters of 2008 and all of 2009. Until the resignation of Mr. Bijou in May
2009, the company had no independent Chairman. Dr. Ronstadt became chairman and
in addition to the regular fee of $8,000 per year, an additional fee of $1,583
per month related to the transition was approved for the time from when he
became Chairman in May through December 2009, bringing the independent chairman
fee to $2.250 per month. The fee reverts to the standard fee of $8,000 per year
in 2010. Dr. Ronstadt deferred the entire amount of the fee as part of the
deferral of all cash fees by Directors.
In
addition to the cash payments, each outside Director received restricted stock.
Up until 2008, a quarterly grant of 2,500 shares of restricted stock was granted
on the last day of January, April, July, and October. These grants were prorated
if a Director only serves a portion of the quarter and the grants vest quarterly
over a one year period starting on the date of the grant. This policy continued
until July 2008, at which time it was changed to a single annual grant of 10,000
fully vested shares on the last day of July each year. The first such grant was
in July 2009 and this annual grant was prorated for partial years of
service.
In
January 2010, the Board of Directors changed its compensation policy for Outside
Directors for 2010. Compensation will revert to an option based program to
replace the current mix of cash and restricted stock. Each Director will receive
an annual grant of 45,000 options in July. Committee chairman and the Vice
Chairman will receive an additional 5,000 options and the Chairman will receive
an additional 10,000 options. Meeting fees will be set at $100 per meeting per
Director and an Independent Chairman will receive an annual fee of $9,000. The
cash portion of all Director’s fees will continue to be deferred until the
Company believes it can pay both officers and Directors their full compensation.
The Board believes this option based compensation will be easier for the Company
from a cash standpoint and better compensate Directors for their
efforts.
All of
the Directors have retained the right to pursue additional business activities
that are not competitive with the business of Applied Nanotech Holdings, and do
not adversely affect their performance as Directors. If conflicts of interest
arise, the nature of the conflict must be fully disclosed to the Board of
Directors, and the person who is subject to the conflict must abstain from
participating in any decision that may impact on his conflict of interest.
Except for this disclosure and abstention policy, the Directors will not be in
breach of any fiduciary duties owed to Applied Nanotech Holdings or the
shareholders by virtue of their participation in such additional business
activities.
Page
61
Director
Ownership Requirements
We also
have a policy related to stock ownership requirements that covers all Directors
- both outside Directors and employee Directors. All Directors are required to
own a minimum of 20,000 shares of Applied Nanotech Holdings, Inc. common stock.
There is no time limit in which a new director must meet those requirements;
however, until a Director owns a minimum of 20,000 shares, the Director is not
allowed to sell any shares. Furthermore, if a Director owns in excess of 20,000
shares, that Director is not allowed to sell shares, whether owned or received
as a result of the exercise of options, if at the completion of the transaction,
it will result in an ownership position of less than 20,000 shares. All current
Directors currently meet this ownership requirement with the exception of
Director Rocheleau.
In
January 2010, the Board adopted a policy whereby Directors are prohibited from
selling shares of the Company’s stock, except during two window periods that
will occur each year in the last two full calendar weeks in March and the first
two calendar weeks in October, or pursuant to an adopted 10b-5(1)
plan.
Compensation
Committee Interlocks and Insider Participation
The
Compensation Committee currently consists of Mr. Berman, Mr. Bramlett, and
Mr. Rocheleau. Dr. Ronstadt was a member of the compensation committee until he
became Chairman of the Board in May 2009. None of them is or has been an officer
or employee of Applied Nanotech Holdings, Inc. nor do any of them have any
relationships requiring disclosure under Item 404 of Regulation S-K. No
interlocking relationship existed during the fiscal year ended December 31,
2009, between Applied Nanotech Holdings’ Board of Directors or Compensation
Committee and the board of directors or compensation committee of any other
company.
Item
11. Security Ownership of
Certain Beneficial Owners and Management and Related Stockholder
Matters.
CERTAIN
BENEFICIAL OWNERS
The only
persons or entities known to be the beneficial owner of 5% or more of the
outstanding voting stock of the common stock of Applied Nanotech Holdings, Inc.
stock as of February 26. 2010, are listed below. This information is based on
public filings as of December 31, 2009. For the purposes of this Annual Report
on Form 10-K, beneficial ownership of securities is defined in accordance with
the rules of the SEC to mean generally the power to vote or dispose of
securities, regardless of any economic interest therein.
|
Beneficial
Ownership
|
Percent
of Outstanding
Common Stock
|
|||
|
Pinnacle
Fund, L.P.
|
6,242,438
|
5.81%
|
||
|
Barry
Kitt, General Partner
4965
Preston Park Blvd., Suite 240
Plano,
TX 75093
|
Page
62
SECURITY
OWNERSHIP OF MANAGEMENT
Set forth
below is certain information with respect to beneficial ownership of Applied
Nanotech Holdings’ common stock as of February 26. 2010, by each Director, each
Named Executive Officer and by the directors and executive officers as a group.
Unless otherwise indicated, each person or member of the group listed has sole
voting and investment power with respect to the shares of common stock
listed.
|
Name
|
Options
Included
in
Beneficial
Ownership
(1)
|
Common
Shares
Owned
|
Common
Stock
Beneficial
Ownership
|
Percentage
of
Class
|
||||||||||||
|
Douglas
P. Baker
|
835,500 | 59,500 | 895,000 | * | ||||||||||||
|
Dr. Zvi
Yaniv
|
910,000 | 160,000 | 1,070,000 | * | ||||||||||||
|
Dr.
Richard Fink
|
173,724 | - | 173,724 | * | ||||||||||||
|
Ronald
J. Berman
|
395,000 | 569,092 | 964,092 | * | ||||||||||||
|
Howard
Westerman
|
- | 208,707 | 208,707 | * | ||||||||||||
|
Dr. Robert
Ronstadt
|
175,000 | 40,617 | 215,617 | * | ||||||||||||
|
Clinton
J. Everton
|
- | 37,500 | 37,500 | * | ||||||||||||
|
Tracy
Bramlett
|
- | 28,333 | 28,333 | * | ||||||||||||
|
Paul
F. Rocheleau
|
- | 1,667 | 1,667 | * | ||||||||||||
|
All
Executive Officers and
Directors
as a group (9 persons)
|
2,489,224 | 1,105,416 | 3,594,640 | 3.27 | % | |||||||||||
|
*
|
Less
than 1%
|
|
(1)
|
This
column lists shares that are subject to options exercisable within sixty
(60) days of February 26. 2010, and are included in common stock
beneficial ownership pursuant to Rule 13d-3(d)(1) of the Exchange
Act.
|
SECURITIES
AUTHORIZED FOR ISSUANCE UNDER EQUITY COMPENSATION PLANS
|
Equity
Compensation
Plans
Not Approved by
the
Shareholders of
Applied
Nanotech Holdings
|
Number
of Securities to
be
issued upon exercise
of
outstanding options
|
Weighted-average
exercise
price of
outstanding
options
|
Number
of Securities
remaining
available
for future
issuance under
equity
compensation
plans
(4)
|
|
(a)
|
(b)
|
I
|
|
|
1992
Employee Plan (1)
|
627,000
|
$1.14
|
-
|
|
1992
Outside Directors Plan
(2)
|
170,000
|
$1.17
|
-
|
|
2002
Equity
Compensation
Plan (3)
|
3,633,392
|
$1.30
|
5,083,241
|
|
Total
|
4,430,392
|
$1.28
|
5,083,241
|
|
(1)
|
The
1992 Employee Plan was originally approved by shareholders and authorized
3.0 million shares. The plan was subsequently amended twice by the Board
to increase the authorized number of shares and is therefore classified as
a plan not approved by our
shareholders.
|
|
(2)
|
The
1992 Outside Directors Plan was originally approved by shareholders and
authorized 500,000 shares. The plan was subsequently amended by the Board
to increase the authorized number of shares and is therefore classified as
a plan not approved by our
shareholders
|
|
(3)
|
The
2002 Equity Compensation Plan was overwhelmingly approved by a majority of
the shareholders actually casting votes at both the 2008 and 2007 annual
meeting of shareholders. However, since less than 50% of the shares
eligible to vote actually cast votes, the plan does not fall into the
category of plans approved by shareholders under SEC
rules.
|
Page
63
|
(4)
|
This
column excludes securities reflected in column
(a)
|
There are
no equity compensation plans approved by shareholders at the present
time.
The 1992
Employee Plan was created in 1992 for the purpose of granting incentive or
non-qualified stock options to employees of, or contractors for, the Company. A
total of 6.5 million shares were authorized under the plan. All options granted
under this plan were priced at the fair market value of our common stock on the
date of grant, or greater, and have a life of ten (10) years from their date of
grant, subject to earlier termination as set forth in such plan. The plan
expired in 2002; however, options granted prior to such plan’s expiration remain
exercisable, subject to the terms of the respective option grants.
The 1992
Outside Directors’ Plan was established in 1992 for the purpose of granting
non-qualified options to non-employee Directors of the Company. A total of 1.0
million options were authorized under the plan. All options granted under this
plan were priced at the fair market value of our common stock or greater on the
date of grant and have a life of ten (10) years from their date of grant,
subject to earlier termination as set forth in such plan. The plan expired in
2002; however, options granted prior to such plan’s expiration remain
exercisable, subject to the terms of the respective option grants.
In 2002,
the Company’s Board of Directors established the 2002 Equity Compensation Plan
for the purpose of granting incentive or non-qualified stock options to
employees or directors of the Company. All options granted under this plan were
priced at the fair market value of our common stock, or greater, on the date of
grant and have a life of up to ten (10) years from their date of grant, subject
to earlier termination as set forth in such plan. A total of 5,000,000 options
were initially authorized under this plan. This plan was amended December 31,
2004 to increase the authorized shares by 3,000,000 to a total of 8,000,000
shares and again on December 12, 2007 to increase the authorized shares by
another 2,000,000 to a total of 10,000,000 shares.
For a
further description of each of the stock option plans described above, please
see Note 9 to the Consolidated Financial Statements herein.
Item
12. Certain Relationships and
Related Transactions, and Director Independence
It is our written policy that all
material related party transactions be approved by the Board of Directors, with
any member of the Board affect by the related party transaction abstaining from
the vote.
Page
64
Item
13. Principal Accountant Fees
and Services
Audit
Fees
The
aggregate fees billed to the Company by Padgett, Stratemann & Co., L.L.P.
for the audit of Applied Nanotech Holdings’ annual financial statements and for
the review of the financial statements included in its quarterly reports on Form
10-Q for the Fiscal Years ended December 31, 2009 and 2008 were $60,500 and
$62,500. No fees were billed to the Company in 2007 by the Company’s current
auditor. Our prior auditor, Sprouse & Anderson, L.L.P. merged with Padgett,
Stratemann & Co., L.L.P. in 2007 and no longer exists. The aggregate fees
billed to the Company by Sprouse & Anderson, L.L.P. for the audit of Applied
Nanotech Holdings’ annual financial statements and for the review of the
financial statements included in its quarterly reports on Form 10-Q for the
Fiscal Years ended December 31, 2007 totaled $56,609.
Audit-Related
Fees
Applied
Nanotech Holdings did not incur or pay any fees to either Padgett, Stratemann
& Co. L.L.P. or Sprouse & Anderson, L.L.P., and neither Padgett,
Stratemann & Co. L.L.P. or Sprouse & Anderson; L.L.P. provided any
services related to audit-related fees in the last two fiscal
years.
Tax
Fees
There
were no fees billed to Applied Nanotech Holdings by either Padgett, Stratemann
& Co. L.L.P. or Sprouse & Anderson, L.L.P. for services rendered to
Applied Nanotech Holdings during the last two fiscal years for tax compliance,
tax advice, or tax planning.
All
Other Fees
There
were no fees billed to Applied Nanotech Holdings by either Padgett, Stratemann
& Co. L.L.P. or Sprouse & Anderson, L.L.P. for services rendered to
Applied Nanotech Holdings during the last two fiscal years, other than the
services described above under “Audit Fees.”
It is the
audit committee’s policy to pre-approve all services provided by the Company’s
auditors. All services provided by Padgett Stratemann & Co. L.L.P. in 2009
and 2008 and by Sprouse & Anderson, L.L.P. during the year ended December
31, 2007 were pre-approved by the audit committee.
As of the
date of this filing, Applied Nanotech Holdings current policy is to not engage
Padgett, Stratemann & Co., L.L.P. to provide, among other things,
bookkeeping services, appraisal or valuation services, or internal audit
services. The policy provides that Applied Nanotech Holdings engage Padgett,
Stratemann & Co., L.L.P. to provide audit, tax, and other assurance
services, such as review of SEC reports or filings.
The Audit
Committee considered and determined that the provision of the services other
than the services described under “Audit Fees” is compatible with maintaining
the independence of the independent auditors.
Page
65
PART
IV
Item
14. Exhibits and Financial
Statement Schedules
|
(a)
|
The
following documents are filed as part of this Annual Report on Form
10-K:
|
|
|
(1)
|
All Financial
Statements
|
The
response to this portion of Item 14 is set forth in Item 7 of Part II
hereof.
|
|
(2)
|
Financial Statement
Schedules
|
Schedules
for which provision is made in the applicable accounting regulations of the
Securities and Exchange Commission are not required under the related
instructions or are inapplicable, and therefore have been omitted.
|
|
(3)
|
Exhibits
|
See
accompanying Index to Exhibits on page 68 for a descriptive response to this
item. The Company will furnish to any shareholder, upon written request, any
exhibit listed in the accompanying Index to Exhibits upon payment by such
shareholder of the Company’s reasonable expenses in furnishing any such
exhibit.
|
(b)
|
Reference
is made to Item 14(a)(3)
above.
|
|
(c)
|
Reference
is made to Item 14(a)(2)
above.
|
Page
66
SIGNATURES
Pursuant
to the requirements of Section 13 or 15(d) of the Securities Exchange Act of
1934, the registrant has caused this report to be signed on its behalf by the
undersigned, thereunto duly authorized.
|
APPLIED
NANOTECH HOLDINGS, INC.
|
|||
|
By:
|
/s/
Douglas P. Baker
|
||
|
Douglas
P. Baker, Chief Executive Officer,
Chief
Financial Officer
March
4, 2010
|
|||
Pursuant
to the requirements of the Securities Exchange Act of 1934, this report has been
signed by the following persons on behalf of the registrant and in the
capacities and on the dates indicated.
|
Signature
|
Title
|
Date
|
|
/s/
Douglas P. Baker
Douglas
P. Baker
|
Chief
Executive Officer and Chief Financial Officer
(Principal
Executive Officer. Principal Financial Officer, Principal
Accounting Officer, and Director)
|
March
4, 2010
|
|
Dr. Robert
Ronstadt*
Clinton
J. Everton*
Ronald
J. Berman*
Dr. Zvi
Yaniv*
Howard
Westerman*
Tracy
Bramlett*
Paul
F. Rocheleau*
|
Directors
|
March
4, 2010
|
|
*By:
|
/s/
Douglas P. Baker
|
|||
|
(Douglas
P. Baker,
Attorney-in-Fact)
|
||||
Page
67
INDEX TO
EXHIBITS
The
exhibits indicated by an asterisk (*) have been previously filed with the
Securities
and
Exchange Commission and are incorporated herein by reference.
|
EXHIBIT
NUMBER
|
DESCRIPTION
OF EXHIBIT
|
|
3(i).1*
|
Restated
Articles of Incorporation of Company, as filed with the Secretary of State
for the State of Texas. (Exhibit 3(i).1 to the Company’s Current Report on
Form 8-K dated as of December 12, 2007).
|
|
3(ii).1*
|
Amended
and Restated Bylaws of the Company. (Exhibit 3(ii).1 to the Company’s
Current Report on Form 8-K dated as of December 12,
2007).
|
|
4.1
*
|
Form
of Certificate for shares of the Company’s common stock (Exhibit 4.1 to
the Company’s Registration Statement on Form SB-2[No.33-51446-FW] dated
January 7, 1993).
|
|
4.2*
|
Amended
and Restated Rights Agreement dated as of November 16, 2000, between the
Company and American Securities Transfer, Incorporated, as Rights Agent,
which includes as Exhibit A the form of Statement of Resolution
establishing and designating series of preferred stock as “Series H Junior
Participating Preferred Stock” and fixing and determining the relative
rights and preferences thereof, as Exhibit B the form of Rights
Certificate, and as Exhibit C the Summary of Rights to Purchase Preferred
Shares. (Exhibit 4.1 to the Company’s Current Report on Form 8-K dated as
of November 16, 2000).
|
|
4.3*
|
Form
of Term sheet for Convertible Notes Payable (Exhibit 99.1 to the Company’s
Current Report on Form 8-K dated as of February 19,
2010)
|
|
4.4
|
Form
of Convertible Note Payable
|
|
10.1*
|
Amended
and Restated 1992 Outside Directors’ Stock Option Plan (Exhibit 4.2 to the
Company’s Registration Statement on Form S-8 [No. 333-56547] dated June 9,
1998).
|
|
10.2*
|
Amended
and Restated 1992 Stock Option Plan (Exhibit 4.1 to the Company’s
Registration Statement on Form S-8 [No. 333-56457] dated June 9,
1998)
|
|
10.3*
|
Amended
and Restated 2002 Equity Compensation Plan. (Exhibit 99.1 to the Company’s
Current Report on Form 8-K dated as of December 12,
2007).
|
|
10.4*
|
Applied
Nanotech Holdings, Inc. Audit Committee Charter (Exhibit 10.23 to the
Company’s Annual Report on Form 10-KSB for the fiscal year ended December
31, 2002)
|
|
10.5*
|
Applied
Nanotech Holdings, Inc. Compensation Committee Charter (Exhibit 10.18 to
the Company’s Current Report on Form 10-K for the fiscal year ended
December 31, 2005).
|
|
10.6*
|
Applied
Nanotech Holdings, Inc. Nominating Committee Charter (Exhibit 10.19 to the
Company’s Current Report on Form 10-K for the fiscal year ended December
31, 2005).
|
|
11
|
Computation
of (Loss) per Common Share
|
|
14
*
|
Applied
Nanotech Holdings, Inc. Code of Ethics (Exhibit 14 to the Company’s Annual
Report on Form 10-KSB for the fiscal year ended December 31,
2004)
|
|
21
|
Subsidiaries
of the Company
|
|
24
|
Powers
of Attorney
|
|
31.1
|
Rule
13a-14(a)/15d-14(a) Certificate of Douglas P. Baker, Chief Executive
Officer and Chief Financial Officer
|
|
32.1
|
Section
1350 Certificate of Douglas P. Baker, Chief Executive Officer and Chief
Financial Officer
|
Page
68
