Attached files
| file | filename |
|---|---|
| EXCEL - IDEA: XBRL DOCUMENT - JOHNSON & JOHNSON | Financial_Report.xls |
| EX-12 - EX-12 - JOHNSON & JOHNSON | y80744exv12.htm |
| EX-99 - EX-99 - JOHNSON & JOHNSON | y80744exv99.htm |
| EX-21 - EX-21 - JOHNSON & JOHNSON | y80744exv21.htm |
| EX-31.A - EX-31.A - JOHNSON & JOHNSON | y80744exv31wa.htm |
| EX-32.A - EX-32.A - JOHNSON & JOHNSON | y80744exv32wa.htm |
| EX-32.B - EX-32.B - JOHNSON & JOHNSON | y80744exv32wb.htm |
| EX-10.W - EX-10.W - JOHNSON & JOHNSON | y80744exv10ww.htm |
| EX-31.B - EX-31.B - JOHNSON & JOHNSON | y80744exv31wb.htm |
| 10-K - FORM 10-K - JOHNSON & JOHNSON | y80744e10vk.htm |
| EX-23 - EX-23 - JOHNSON & JOHNSON | y80744exv23.htm |
Table of Contents
| MANAGEMENT’S DISCUSSION AND ANALYSIS | ||
| 26 | ||
| 26 | ||
| 27 | ||
| 29 | ||
| 32 | ||
| 33 | ||
| 35 | ||
| AUDITED CONSOLIDATED FINANCIAL STATEMENTS | ||
| 36 | ||
| 37 | ||
| 38 | ||
| 39 | ||
| 40 | ||
| 64 | ||
| 65 | ||
| SUPPORTING SCHEDULES | ||
| 66 | ||
| 67 | ||
JOHNSON & JOHNSON 2009 ANNUAL REPORT
25
Table of Contents
Management’s Discussion and Analysis of Results of Operations and Financial Condition
Organization and Business Segments
DESCRIPTION OF THE COMPANY AND BUSINESS SEGMENTS
Johnson & Johnson and its subsidiaries (the “Company”) have approximately 115,500 employees
worldwide engaged in the research and development, manufacture and sale of a broad range of
products in the health care field. The Company conducts business in virtually all countries of the
world with the primary focus on products related to human health and well-being.
The Company is organized into three business segments: Consumer, Pharmaceutical and Medical
Devices and Diagnostics. The Consumer segment includes a broad range of products used in the baby
care, skin care, oral care, wound care and women’s health care fields, as well as nutritional and
over-the-counter pharmaceutical products. These products are marketed to the general public and
sold both to retail outlets and distributors throughout the world. The Pharmaceutical segment
includes products in the following therapeutic areas: anti-infective, antipsychotic,
cardiovascular, contraceptive, dermatology, gastrointestinal, hematology, immunology, neurology,
oncology, pain management, urology and virology. These products are distributed directly to
retailers, wholesalers and health care professionals for prescription use. The Medical Devices and
Diagnostics segment includes a broad range of products used principally in the professional fields
by physicians, nurses, therapists, hospitals, diagnostic laboratories and clinics. These products
include Cordis’ circulatory disease management products; DePuy’s orthopaedic joint reconstruction,
spinal care and sports medicine products; Ethicon’s surgical care, aesthetics and women’s health
products; Ethicon Endo-Surgery’s minimally invasive surgical products; LifeScan’s blood glucose
monitoring and insulin delivery products; Ortho-Clinical Diagnostics’ professional diagnostic
products and Vistakon’s disposable contact lenses.
The Company’s structure is based upon the principle of decentralized management. The Executive
Committee of Johnson & Johnson is the principal management group responsible for the operations and
allocation of the resources of the Company. This Committee oversees and coordinates the activities
of the Consumer, Pharmaceutical and Medical Devices and Diagnostics business segments.
In all of its product lines, the Company competes with companies both local and global,
located throughout the world. Competition exists in all product lines without regard to the number
and size of the competing companies involved. Competition in research, involving the development
and the improvement of new and existing products and processes, is particularly significant. The
development of new and innovative products is important to the Company’s success in all areas of
its business. This also includes protecting the Company’s portfolio of intellectual property. The
competitive environment requires substantial investments in continuing research and in maintaining
sales forces. In addition, the development and maintenance of customer demand for the Company’s
consumer products involves significant expenditures for advertising and promotion.
MANAGEMENT’S OBJECTIVES
A primary objective of the Company is to achieve superior levels of capital efficient profitable
growth. To accomplish this, the Company’s management operates the business consistent with certain
strategic principles that have proven successful over time. To this end, the Company participates
in growth areas in human health care and is committed to attaining leadership positions in these
growth areas through the development of innovative products and services. New products introduced
within the past five years accounted for approximately 25% of 2009 sales. In 2009, $7.0 billion, or
11.3% of sales, was invested in research and development. This investment reflects management’s
commitment to the importance of ongoing development of new and differentiated products and services
to sustain long-term growth.
With more than 250 operating companies located in 60 countries, the Company views its
principle of decentralized management as an asset and fundamental to the success of a broadly based
business. It also fosters an entrepreneurial spirit, combining the extensive resources of a large
organization with the ability to react quickly to local market changes and challenges.
The Company is committed to developing global business leaders who can drive growth
objectives. Businesses are managed for the long-term in order to sustain leadership positions and
achieve growth that provides an enduring source of value to our shareholders.
Unifying the management team and the Company’s dedicated employees in achieving these
objectives is Our Credo. Our Credo provides a common set of values and serves as a constant
reminder of the Company’s responsibilities to its customers, employees, communities and
shareholders. The Company believes that these basic principles, along with its overall mission of
improving the quality of life for people everywhere, will enable Johnson & Johnson to continue to
be among the leaders in the health care industry.
Results of Operations
ANALYSIS OF CONSOLIDATED SALES
In 2009, worldwide sales decreased 2.9% to $61.9 billion, compared to increases of 4.3% in 2008 and
14.6% in 2007. These sales changes consisted of the following:
| Sales (decrease)/increase due to: | 2009 | 2008 | 2007 | |||||||||
Volume |
(0.2 | )% | 1.1 | 10.1 | ||||||||
Price |
(0.1 | ) | 0.8 | 1.4 | ||||||||
Currency |
(2.6 | ) | 2.4 | 3.1 | ||||||||
Total |
(2.9 | )% | 4.3 | 14.6 | ||||||||
Sales by U.S. companies were $30.9 billion in 2009, $32.3 billion in 2008 and $32.4 billion in
2007. This represents a decrease of 4.4% in 2009, a decrease of 0.4% in 2008 and an increase of
9.0% in 2007. Sales by international companies were $31.0 billion in 2009, $31.4 billion in 2008
and $28.7 billion in 2007. This represents a decrease of 1.4% in 2009 and increases of 9.7% and
21.7% in 2008 and 2007, respectively.
JOHNSON & JOHNSON 2009 ANNUAL REPORT
26
Table of Contents
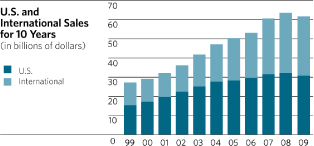
The five-year compound annual growth rates for worldwide, U.S. and international sales were
5.5%, 2.2% and 9.6%, respectively. The ten-year compound annual growth rates for worldwide, U.S.
and international sales were 8.5%, 7.1% and 10.1%, respectively.
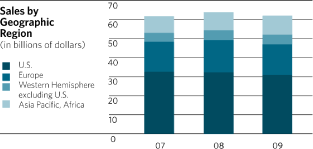
Sales in Europe experienced a decline of 5.1% including operational growth of 2.1% and a
negative impact from currency of 7.2%. Sales in the Western Hemisphere (excluding the U.S.)
experienced a decline of 0.3% including operational growth of 8.8% and a negative impact from
currency of 9.1%. Sales in the Asia-Pacific, Africa region achieved growth of 4.6%, including
operational growth of 4.4% and an increase of 0.2% related to the positive impact of currency.
In 2009, 2008 and 2007, the Company did not have a customer that represented 10% or more of
total consolidated revenues.
2009 results benefited from the inclusion of a 53rd week. (See Note 1 to the Consolidated
Financial Statements for Annual Closing Date details). The Company estimated that the fiscal year
2009 growth rate was enhanced by approximately 0.5%. While the additional week added a few days to
sales, it also added a full week’s worth of operating costs; therefore, the net earnings impact was
negligible.
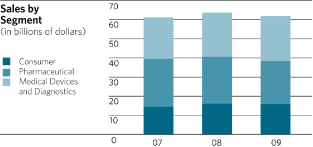
Analysis of Sales by Business Segments
CONSUMER SEGMENT
Consumer segment sales in 2009 were $15.8 billion, a decrease of 1.6% from 2008 with 2.0% of this
change due to operational growth and negative currency impact of 3.6%. U.S. Consumer segment sales
were $6.8 billion, a decrease of 1.4%. International sales were $9.0 billion, a decrease of 1.7%,
with growth of 4.7% achieved by operations and a decrease of 6.4% resulting from the negative
impact of currency fluctuations.
The Over-the-Counter (OTC) Pharmaceuticals and Nutritionals franchise sales were $5.6 billion,
a decrease of 4.5% from 2008. This was primarily due to the negative impact of currency and lower
sales of the over-the-counter ZYRTEC® allergy product line related to the
initial build of inventory by the trade during the 2008 launch year. This was partially offset by
sales growth in the SPLENDA® sweetener product line. The U.S. Food and Drug
Administration (FDA) is currently considering certain recommendations made by its advisory
committee for reducing the potential for overdose with acetaminophen, the active ingredient in
TYLENOL® brand products. The Company has provided the FDA with its own
recommendations and will continue to be actively engaged with the FDA on this topic. In December
2009, the Company announced a voluntary recall of all lots of TYLENOL®
Arthritis Pain 100 count with EZ-OPEN CAP following reports of an uncharacteristic smell; however,
there was an insignificant impact on sales. In January 2010, the Company has undertaken a broader
voluntary recall of TYLENOL® and certain OTC products as a precautionary
action.
The Skin Care franchise sales grew by 2.5% to $3.5 billion in 2009. The sales growth was
primarily due to the AVEENO®, NEUTROGENA®, and DABAO™
skin care lines. The Baby Care franchise sales were $2.1 billion, a decrease of 4.5% primarily due
to the negative impact of currency and lower sales for Babycenter.com as a result of exiting the
online retail business, partially offset by growth in the haircare product line. The Women’s Health
franchise sales were $1.9 billion, a decrease of 0.8% primarily due to the negative impact of
currency partially offset by increased sales associated with the acquisition of a joint venture
partner in France in the fiscal
Major Consumer Franchise Sales:
| % Change | ||||||||||||||||||||
| (Dollars in Millions) | 2009 | 2008 | 2007 | ’09 vs. ’08 | ’08 vs. ’07 | |||||||||||||||
OTC Pharmaceuticals & Nutritionals |
$ | 5,630 | 5,894 | 5,142 | (4.5) | % | 14.6 | |||||||||||||
Skin Care |
3,467 | 3,381 | 3,051 | 2.5 | 10.8 | |||||||||||||||
Baby Care |
2,115 | 2,214 | 1,982 | (4.5 | ) | 11.7 | ||||||||||||||
Women’s Health |
1,895 | 1,911 | 1,806 | (0.8 | ) | 5.8 | ||||||||||||||
Oral Care |
1,569 | 1,624 | 1,488 | (3.4 | ) | 9.1 | ||||||||||||||
Wound Care/Other |
1,127 | 1,030 | 1,024 | 9.4 | 0.6 | |||||||||||||||
Total |
$ | 15,803 | 16,054 | 14,493 | (1.6) | % | 10.8 | |||||||||||||
MANAGEMENT’S DISCUSSION AND ANALYSIS OF RESULTS OF OPERATIONS AND FINANCIAL CONDITION
27
Table of Contents
first quarter of 2009. Prior to the acquisition of the joint venture partner, sales by the joint
venture were not recorded as part of the Company’s sales to customers. The Oral Care franchise
sales were $1.6 billion, a decrease of 3.4% due to softness in the category in the U.S., partially
offset by growth of LISTERINE® mouthwash outside the U.S. The Wound Care/Other
franchise sales grew by 9.4% to $1.1 billion primarily due to the recent acquisitions in the
Wellness and Prevention platform and strong sales of PURELL® hand sanitizer.
Consumer segment sales in 2008 were $16.0 billion, an increase of 10.8% over 2007 with 8.3% of
this change due to operational growth and the remaining 2.5% due to positive currency fluctuations.
U.S. Consumer segment sales were $6.9 billion, an increase of 8.3%. International sales were $9.1
billion, an increase of 12.8%, with 8.3% as a result of operations and 4.5% due to currency
fluctuations over 2007.
PHARMACEUTICAL SEGMENT
Pharmaceutical segment sales in 2009 were $22.5 billion, a decrease of 8.3% from 2008, with an
operational decline of 6.1% and the remaining 2.2% due to the negative impact of currency
fluctuations. U.S. sales were $13.0 billion, a decrease of 12.1%. International sales were $9.5
billion, a decrease of 2.6%, which included 3.0% operational growth and a decrease of 5.6%
resulting from the negative impact of currency fluctuations.
REMICADE® (infliximab), a biologic approved for the treatment of a number
of immune mediated inflammatory diseases, achieved sales of $4.3 billion in 2009, with growth of
14.8% over the prior year primarily attributable to strong overall market growth.
REMICADE® is competing in a market which is experiencing increased competition
due to new entrants and the expansion of indications for existing competitors.
PROCRIT® (Epoetin alfa) and EPREX® (Epoetin alfa)
had combined sales of $2.2 billion in 2009, a decline of 8.7% compared to the prior year. Lower
sales of PROCRIT® and EPREX® were due to the declining
markets for Erythropoiesis Stimulating Agents (ESAs).
LEVAQUIN® (levofloxacin)/FLOXIN® (ofloxacin) sales
were $1.6 billion, a decline of 2.6% versus the prior year, due to competition in the category. The
patent for LEVAQUIN® (levofloxacin) in the U.S. will expire in December 2010.
A pediatric extension was granted by the FDA, which extends market exclusivity in the U.S. through
June 2011. The expiration of the product patent or loss of market exclusivity is likely to result
in a significant reduction in sales.
RISPERDAL® CONSTA® (risperidone), a long-acting
injectable for the treatment of schizophrenia, achieved sales of $1.4 billion in 2009, representing
an increase of 8.9% as compared to the prior year. The growth was due to a positive shift from
daily therapies to longer-acting RISPERDAL® CONSTA® and
the launch of RISPERDAL® CONSTA® in Japan earlier in the
year.
CONCERTA® (methylphenidate HCl), a product for the treatment of attention
deficit hyperactivity disorder (ADHD), achieved sales of $1.3 billion in 2009, representing an
increase of 6.3% over 2008. Sales results in 2008 were favorably impacted by approximately $115
million related to a change in the estimate of accrued sales reserves related to sales outside the
U.S. Although the original CONCERTA® patent expired in 2004, the FDA has not
approved any generic version that is substitutable for CONCERTA®. Parties have
filed Abbreviated New Drug Applications (ANDAs) for generic versions of
CONCERTA®, which are pending and may be approved at any time. An approval
would lead to a loss of exclusivity and is likely to result in a significant reduction in sales.
TOPAMAX® (topiramate), RISPERDAL® (risperidone), and
DURAGESIC®/Fentanyl Transdermal (fentanyl transdermal system) experienced
sales declines in 2009 of 57.9%, 57.7% and 14.3%, respectively, versus the prior year due to
generic competition. Market exclusivity in the U.S. expired for TOPAMAX®
(topiramate) in March 2009, RISPERDAL® oral in June 2008 and
DURAGESIC® in January 2005.
ACIPHEX®/PARIET® (rabeprazole sodium) experienced a
sales decline of 5.4% due to competition in the category.
In 2009, Other Pharmaceutical sales were $7.6 billion, representing a growth of 6.6% over the
prior year. Contributors to the increase were sales of VELCADE® (bortezomib),
a product for the treatment of multiple myeloma; PREZISTA® (darunavir), for
the treatment of HIV/AIDS patients; INTELENCE™ (etravirine), for HIV combination therapy and
INVEGA® (paliperidone), a once-daily atypical antipsychotic. The growth was
partially offset by the impact of a generic version of ORTHO TRI-CYCLEN® LO
shipped by a competitor. Subsequently, the generic manufacturer recognized the validity of the
patent, paid damages for its infringing sales and ceased further shipments of the product.
During 2009, the Company received regulatory approval for several new molecular entities
(NMEs), including STELARA™ (ustekinumab) in the U.S. and European Union (EU) for the treatment of
moderate-to-severe plaque psoriasis; INVEGA® SUSTENNA™ (paliperidone
palmitate) extended-release injectable suspension in the U.S. for the acute and maintenance
treatment of schizophrenia; SIMPONI™ (golimumab) in the U.S. and EU for the treatment of
moderate-to-severe, active rheumatoid arthritis (RA), active and progressive psoriatic arthritis
(PsA) and severe, active ankylosing spondylitis (AS); and PRILIGY™ (dapoxetine) in several
countries for the on-demand treatment of premature ejaculation. NUCYNTA™ (tapentadol) Immediate
Release Tablets, for relief
Major Pharmaceutical Product Revenues:
| % Change | ||||||||||||||||||||
| (Dollars in Millions) | 2009 | 2008 | 2007 | ’09 vs. ’08 | ’08 vs. ’07 | |||||||||||||||
REMICADE® (infliximab) |
$ | 4,304 | 3,748 | 3,327 | 14.8 | % | 12.7 | |||||||||||||
PROCRIT®/EPREX® (Epoetin alfa) |
2,245 | 2,460 | 2,885 | (8.7 | ) | (14.7 | ) | |||||||||||||
LEVAQUIN®/FLOXIN® (levofloxacin/ofloxacin) |
1,550 | 1,591 | 1,646 | (2.6 | ) | (3.3 | ) | |||||||||||||
RISPERDAL® CONSTA® (risperidone) |
1,425 | 1,309 | 1,128 | 8.9 | 16.0 | |||||||||||||||
CONCERTA® (methylphenidate HCl) |
1,326 | 1,247 | 1,028 | 6.3 | 21.3 | |||||||||||||||
TOPAMAX® (topiramate) |
1,151 | 2,731 | 2,453 | (57.9 | ) | 11.3 | ||||||||||||||
ACIPHEX®/PARIET® (rabeprazole sodium) |
1,096 | 1,158 | 1,357 | (5.4 | ) | (14.7 | ) | |||||||||||||
RISPERDAL® (risperidone) |
899 | 2,126 | 3,420 | (57.7 | ) | (37.8 | ) | |||||||||||||
DURAGESIC®/Fentanyl Transdermal (fentanyl transdermal system) |
888 | 1,036 | 1,164 | (14.3 | ) | (11.0 | ) | |||||||||||||
Other Pharmaceuticals |
7,636 | 7,161 | 6,458 | 6.6 | 10.9 | |||||||||||||||
Total |
$ | 22,520 | 24,567 | 24,866 | (8.3) | % | (1.2 | ) | ||||||||||||
JOHNSON & JOHNSON 2009 ANNUAL REPORT
28
Table of Contents
of moderate to severe acute pain, was also launched in the U.S. in 2009.
The Company also received approvals expanding the indications for several key products,
including INVEGA® (paliperidone) extended-release tablets in the U.S. for the
acute treatment of schizoaffective disorder; RISPERDAL®
CONSTA® (risperidone) Long-Acting Treatment in the U.S. as both monotherapy
and adjunctive therapy to lithium or valproate in the maintenance treatment of Bipolar I Disorder,
as well as for the treatment of schizophrenia in Japan; PREZISTA® (darunavir)
in the EU with low-dose ritonavir as part of combination therapy in treatment-naïve adults, as well
as for treatment-experienced pediatric patients with HIV.
The Company submitted a New Drug Application (NDA) to the FDA for tapentadol extended release
(ER) tablets, an investigational oral analgesic for the management of moderate to severe chronic
pain in patients 18 years of age or older. In addition, the Company also invested in a number of
new platforms for growth in Oncology, Alzheimer’s disease and vaccines for the treatment and
prevention of influenza and other infectious and non-infectious diseases.
Pharmaceutical segment sales in 2008 were $24.6 billion, a decrease of 1.2% from 2007, with an
operational decline of 3.1% and 1.9% increase due to the positive impact of currency fluctuations.
U.S. Pharmaceutical segment sales were $14.9 billion, a decrease of 4.9%. International
Pharmaceutical segment sales were $9.7 billion, an increase of 5.1%, which included 0.1% of
operational growth and 5.0% related to the positive impact of currency fluctuations.
MEDICAL DEVICES AND DIAGNOSTICS SEGMENT
The Medical Devices and Diagnostics segment achieved sales of $23.6 billion in 2009, representing
an increase of 1.9% over the prior year, with operational growth of 4.2% and a negative currency
impact of 2.3%. U.S. sales were $11.0 billion, an increase of 4.5% over the prior year.
International sales were $12.6 billion, a decrease of 0.2%, with growth of 4.0% from operations and
a decrease of 4.2% resulting from the negative impact of currency fluctuations.
The DePuy franchise achieved sales of $5.4 billion in 2009, a 4.6% increase over the prior
year. This was primarily due to growth in the spine, hip and knee product lines. Additionally, new
product launches in the Mitek sports medicine product line contributed to the growth.
The Ethicon Endo-Surgery franchise achieved sales of $4.5 billion in 2009, a 4.8% increase
over the prior year. This was attributable to growth in the endoscopy,
HARMONIC®, ENSEAL® and Advanced Sterilization product
lines.
The Ethicon franchise achieved sales of $4.1 billion in 2009, a 7.3% increase over the prior
year. This was attributable to growth in the sutures, biosurgical and mesh product lines in
addition to sales of newly acquired products from the acquisitions of Omrix Biopharmaceuticals,
Inc. and Mentor Corporation. The growth was partially offset by the divestiture of the Professional
Wound Care business of Ethicon, Inc. in the fiscal fourth quarter of 2008.
Sales in the Cordis franchise were $2.7 billion, a decline of 10.3% versus the prior year. The
decline reflects lower sales of the CYPHER® Sirolimus-eluting Coronary Stent
due to increased global competition. The decline was partially offset by growth of the Biosense
Webster business.
The Vision Care franchise achieved sales of $2.5 billion in 2009, a 0.2% increase over prior
year primarily related to growth in the Astigmatic contact lens product line offset by the negative
impact of currency.
Sales in the Diabetes Care franchise were $2.4 billion in 2009, a decline of 3.7% versus the
prior year. Declines in the LifeScan product line were partially offset by growth of the Animas
insulin delivery business resulting from new product launches and continued development in
international markets.
The Ortho-Clinical Diagnostics franchise achieved sales of $2.0 billion in 2009, a 6.6%
increase over the prior year primarily attributable to the recent launch of the
VITROS® 3600 and 5600 analyzers.
The Medical Devices and Diagnostics segment achieved sales of $23.1 billion in 2008,
representing an increase of 6.4% over the prior year, with operational growth of 3.5% and 2.9% due
to a positive impact from currency fluctuations. U.S. sales were $10.5 billion, an increase of
1.0%. International sales were $12.6 billion, an increase of 11.3%, with 5.8% from operations and a
positive currency impact of 5.5%.
Analysis of Consolidated Earnings Before Provision for Taxes on Income
Consolidated earnings before provision for taxes on income decreased by $1.1 billion to $15.8
billion in 2009 as compared to the $16.9 billion earned in 2008, a decrease of 6.9%. The decrease
was primarily related to lower sales, the negative impact of product mix, lower interest income due
to lower rates of interest earned and restructuring charges of $1.2 billion. This was partially
offset by lower selling, marketing and administrative expenses due to cost containment efforts
across all the businesses. 2008 included purchased in-process research and development (IPR&D)
charges of $0.2 billion and increased investment spending in selling, marketing and administrative
expenses utilized from the proceeds associated with the divestiture of the Professional Wound Care
business of Ethicon, Inc. The increase in 2008 of 27.4% over the $13.3 billion in 2007 was
primarily due to lower IPR&D charges of $0.6 billion, gains from divestitures of $0.5 billion and
higher litigation gains of $0.5 billion versus restructuring charges of $0.7 billion and the
write-down of the NATRECOR® intangible asset of $0.7 billion recorded in 2007.
As a percent to sales, consolidated
Major Medical Devices and Diagnostics Franchise Sales*:
| % Change | ||||||||||||||||||||
| (Dollars in Millions) | 2009 | 2008 | 2007 | ’09 vs. ’08 | ’08 vs. ’07 | |||||||||||||||
DEPUY® |
$ | 5,372 | 5,136 | 4,698 | 4.6 | % | 9.3 | |||||||||||||
ETHICON ENDO-SURGERY® |
4,492 | 4,286 | 3,834 | 4.8 | 11.8 | |||||||||||||||
ETHICON® |
4,122 | 3,840 | 3,603 | 7.3 | 6.6 | |||||||||||||||
CORDIS® |
2,679 | 2,988 | 3,314 | (10.3 | ) | (9.8 | ) | |||||||||||||
Vision Care |
2,506 | 2,500 | 2,209 | 0.2 | 13.2 | |||||||||||||||
Diabetes Care |
2,440 | 2,535 | 2,373 | (3.7 | ) | 6.8 | ||||||||||||||
ORTHO-CLINICAL DIAGNOSTICS® |
1,963 | 1,841 | 1,705 | 6.6 | 8.0 | |||||||||||||||
Total |
$ | 23,574 | 23,126 | 21,736 | 1.9 | % | 6.4 | |||||||||||||
| * | Prior year amounts have been reclassified to conform to current presentation. |
MANAGEMENT’S DISCUSSION AND ANALYSIS OF RESULTS OF OPERATIONS AND FINANCIAL CONDITION
29
Table of Contents
earnings before provision for taxes on income in 2009 was 25.4% versus 26.5% in 2008.
The sections that follow highlight the significant components of the changes in consolidated
earnings before provision for taxes on income.
Cost of Products Sold and Selling, Marketing and Administrative Expenses: Cost of products sold and selling, marketing and administrative expenses
as a percent to sales were as follows:
| % of Sales | 2009 | 2008 | 2007 | |||||||||
Cost of products sold |
29.8 | % | 29.1 | 29.1 | ||||||||
Percent point increase over the prior year |
0.7 | — | 0.9 | |||||||||
Selling, marketing and administrative expenses |
32.0 | 33.7 | 33.5 | |||||||||
Percent point (decrease)/increase over the
prior year |
(1.7 | ) | 0.2 | 0.8 | ||||||||
In 2009, cost of products sold as a percent to sales increased primarily due to the continued
negative impact of product mix and inventory write-offs associated with the restructuring activity.
Additionally, 2008 included some non-recurring positive items. There was a decrease in the percent
to sales of selling, marketing and administrative expenses in 2009 primarily due to cost
containment efforts across all the businesses and the annualized savings recognized from the 2007
restructuring program. Additionally, 2008 utilized the proceeds associated with the divestiture of
the Professional Wound Care business of Ethicon, Inc. to fund increased investment spending.
In 2008, cost of products sold as a percent to sales remained flat to the prior year. The
change in the mix of businesses, with higher sales growth in the Consumer business and a slight
sales decline in the Pharmaceutical business, had a negative impact on the cost of products sold as
a percent to sales. In 2008, this was offset by manufacturing efficiencies and non-recurring
positive items in 2008 and negative items in 2007. There was an increase in the percent to sales of
selling, marketing and administrative expenses in 2008 primarily due to the change in the mix of
businesses, whereby a greater proportion of sales were attributable to the Consumer segment, which
has higher selling, marketing and administrative spending. Additionally, in 2008 the Company
utilized the gain associated with the divestiture of the Professional Wound Care business of
Ethicon, Inc. to fund increased investment spending. This was partially offset by ongoing cost
containment efforts.
In 2007, there was an increase in the percent to sales of cost of products sold primarily due
to the impact of newly acquired consumer brands. There was an increase in the percent to sales of
selling, marketing and administrative expenses in 2007 primarily due to the impact of newly
acquired consumer brands partially offset by cost containment efforts.
Research and Development expense (excluding purchased in-process research and development
charges) by segment of business was as follows:
| 2009 | 2008 | 2007 | ||||||||||||||||||||||
| (Dollars in Millions) | Amount | % of Sales* | Amount | % of Sales* | Amount | % of Sales* | ||||||||||||||||||
Consumer |
$ | 632 | 4.0 | % | 624 | 3.9 | 564 | 3.9 | ||||||||||||||||
Pharmaceutical |
4,591 | 20.4 | 5,095 | 20.7 | 5,265 | 21.2 | ||||||||||||||||||
Medical Devices and Diagnostics |
1,763 | 7.5 | 1,858 | 8.0 | 1,851 | 8.5 | ||||||||||||||||||
Total research and development expense |
$ | 6,986 | 11.3 | % | 7,577 | 11.9 | 7,680 | 12.6 | ||||||||||||||||
Percent (decrease)/increase over the prior year |
(7.8 | )% | (1.3 | ) | 7.8 | |||||||||||||||||||
| * | As a percent to segment sales |
Research and Development Expense: Research and development activities represent a significant
part of the Company’s business. These expenditures relate to the development of new products,
improvement of existing products, technical support of products and compliance with governmental
regulations for the protection of consumers and patients.
In 2009 and 2008, the reduction in the Pharmaceutical research and development spending was
primarily due to increased efficiencies in Pharmaceutical research and development activities.
Restructuring: In 2009, the Company announced global restructuring initiatives that are expected to
generate pre-tax, annual cost savings of $1.4 – $1.7 billion when fully implemented in 2011, with
$0.8 – $0.9 billion expected to be achieved in 2010. The associated savings will provide additional
resources to invest in new growth platforms; ensure the successful launch of the Company’s many new
products and continued growth of the core businesses; and provide flexibility to adjust to the
changed and evolving global environment. In the fiscal fourth quarter of 2009 the Company recorded
a pre-tax charge of $1.2 billion, of which $113 million is included in cost of products sold.
The restructuring program announced in 2007 has been completed. See Note 22 to the
Consolidated Financial Statements for additional details related to the restructuring.
Purchased In-Process Research and Development: In 2009, in accordance with U.S. GAAP for business
combinations, purchased in-process research and development (IPR&D) is no longer expensed but
capitalized and tested for impairment. The Company capitalized $1.7 billion of IPR&D in 2009,
primarily associated with the acquisitions of Cougar Biotechnology, Inc. and substantially all of
the assets and rights of Elan’s Immunotherapy program.
In 2008, the Company recorded a charge for IPR&D of $181 million before and after tax related
to the acquisitions of Amic AB, SurgRx, Inc., HealthMedia, Inc. and Omrix Biopharmaceuticals, Inc.
HealthMedia, Inc, a privately held company that creates web-based behavior change interventions,
accounted for $7 million before tax of the IPR&D charges and was included in the operating profit
of the Consumer segment. The IPR&D charges for all of the following acquisitions were included in
the operating profit of the Medical Devices and Diagnostics segment. Amic AB, a Swedish developer
of in vitro diagnostic technologies for use in point-of-care and near-patient settings (outside the
physical facilities of the clinical laboratory), accounted for $40 million before tax of the IPR&D
charges. SurgRx, Inc., a privately held developer of the advanced bipolar tissue sealing system
used in the ENSEAL® family of devices, accounted for $7 million before tax of
the IPR&D charges. Omrix
Biopharmaceuticals, Inc., a fully integrated biopharmaceutical company that develops and markets
biosurgical and immunotherapy products, accounted for $127 million before tax of the IPR&D charges.
In 2007, the Company recorded a charge for IPR&D of $807 million before and after tax related
to the acquisition of Conor Medsystems, Inc. The IPR&D charge was included in the operating profit
of the Medical Devices and Diagnostics segment.
JOHNSON & JOHNSON 2009 ANNUAL REPORT
30
Table of Contents
Other (Income) Expense, Net: Other (income) expense, net includes gains and losses related to the
sale and write-down of certain investments in equity securities held by Johnson & Johnson
Development Corporation, gains and losses on the disposal of property, plant and equipment,
currency gains and losses, non-controlling interests, litigation settlements and liabilities and
royalty income. The unfavorable change of $0.5 billion in other (income) expense, net from 2009 to
2008 was primarily due to a gain of $0.5 billion from the divestiture of the Professional Wound
Care business of Ethicon, Inc. in 2008.
In 2008, other (income) expense, net included income from net litigation settlements and
awards of $0.5 billion and a gain of $0.5 billion from the divestiture of the Professional Wound
Care business of Ethicon, Inc. In 2007, other (income) expense, net included a charge of $0.7
billion before tax related to the NATRECOR® intangible asset write-down.
OPERATING PROFIT BY SEGMENT
Operating profits by segment of business were as follows:
| Percent of | ||||||||||||||||
| Segment Sales | ||||||||||||||||
| (Dollars in Millions) | 2009 | 2008 | 2009 | 2008 | ||||||||||||
Consumer |
$ | 2,475 | 2,674 | 15.7 | % | 16.7 | ||||||||||
Pharmaceutical |
6,413 | 7,605 | 28.5 | 31.0 | ||||||||||||
Med Devices and Diagnostics |
7,694 | 7,223 | 32.6 | 31.2 | ||||||||||||
Total (1) |
16,582 | 17,502 | 26.8 | 27.4 | ||||||||||||
Less: Expenses not allocated to segments (2) |
827 | 573 | ||||||||||||||
Earnings before provision for taxes on income |
$ | 15,755 | 16,929 | 25.4 | % | 26.5 | ||||||||||
| (1) | See Note 18 to the Consolidated Financial Statements for more details. | |
| (2) | Amounts not allocated to segments include interest (income) expense, non-controlling interests, and general corporate (income) expense. |
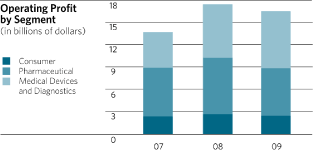
Consumer Segment: In 2009, Consumer segment operating profit decreased 7.4% from 2008. The
primary reasons for the decrease in operating profit was $369 million of restructuring charges,
partially offset by cost containment initiatives in 2009. In 2008, Consumer segment operating
profit increased 17.4% from 2007. Cost synergies, lower integration costs in 2008 related to the
acquisition of the Consumer Healthcare business of Pfizer Inc., and other cost containment
initiatives contributed to the increased operating profit in 2008.
Pharmaceutical Segment: In 2009, Pharmaceutical segment operating profit decreased 15.7% from 2008.
The primary reasons for the decrease in operating profit were $496 million of restructuring
charges, $92 million of litigation expense and negative product mix due to the loss of market
exclusivity for TOPAMAX® and RISPERDAL® oral. In 2008,
Pharmaceutical segment operating profit increased 16.3% from 2007. The primary driver of the
improved operating profit in 2008 was due to the restructuring charges of $429 million and $678
million for the NATRECOR® intangible asset write-down recorded in 2007.
Medical Devices and Diagnostics Segment: In 2009, the operating profit in the Medical Devices and
Diagnostics segment increased 6.5% from 2008. The improved operating profit was due to $478 million
gain from net litigation settlements, favorable product mix, manufacturing efficiencies and cost
containment initiatives related to selling, marketing and administrative expenses. This was
partially offset by $321 million in restructuring charges. In 2008, the operating profit in the
Medical Devices and Diagnostics segment increased 49.1% from 2007. The improved operating profit
was the result of the $429 million gain from net litigation settlements, favorable product mix,
manufacturing efficiencies and lower IPR&D charges of $174 million in 2008 versus $807 million in
2007. Additionally, $301 million of restructuring charges were recorded in 2007.
Interest (Income) Expense: Interest income in 2009 decreased by $271 million due to lower rates of
interest earned despite higher average cash balances. The cash balance, including marketable
securities, was $19.4 billion at the end of 2009, and averaged $15.6 billion as compared to the
$12.2 billion average cash balance in 2008. The increase in the average cash balance was primarily
due to cash generated from operating activities.
Interest expense in 2009 increased by $16 million due to a higher debt balance. The net debt
balance at the end of 2009 was $14.5 billion as compared to $11.9 billion at the end of 2008. The
higher average debt balance of $13.5 billion in 2009 versus $12.9 billion in 2008 was primarily
related to funding acquisitions and investments and the purchase of the Company’s Common Stock
under the ongoing Common Stock repurchase program announced on July 9, 2007.
Interest income in 2008 decreased by $91 million due to lower rates of interest earned despite
higher average cash balances. The cash balance, including marketable securities, was $12.8 billion
at the end of 2008, and averaged $12.2 billion as compared to the $6.6 billion average cash balance
in 2007. The increase in the average cash balance was primarily due to cash generated from
operating activities.
Interest expense in 2008 increased by $139 million due to a higher debt balance. In the second
half of 2007 the Company converted some of its short-term debt to fixed long-term debt at higher
interest rates. The net debt balance at the end of 2008 was $11.9 billion as compared to $9.5
billion at the end of 2007. The higher debt balance in 2008 was primarily due to the purchase of
the Company’s Common Stock under the ongoing Common Stock repurchase program announced on July 9,
2007 and to fund acquisitions.
Interest income in 2007 decreased by $377 million due to lower average cash balances. The
decline in the average cash balance was primarily due to the acquisition of the Consumer Healthcare
business of Pfizer Inc. on December 20, 2006.
Interest expense in 2007 increased by $233 million as compared to prior year due to a higher
average debt balance. The net debt balance at the end of 2007 was $9.5 billion as compared to $6.6
billion at the end of 2006. The higher debt balance in 2007 was due to the debt associated with the
acquisition of the Consumer Healthcare business of Pfizer Inc. and the Common Stock repurchase
program announced in 2007.
Provision for Taxes on Income: The worldwide effective income tax rate was 22.1% in 2009, 23.5% in
2008 and 20.4% in 2007. The 2009 tax rate decreased as compared to 2008 due to increases in taxable
income in lower tax jurisdictions relative to taxable income in higher tax jurisdictions. The 2008
tax rate increased as compared to 2007 due to increases in taxable income in higher tax
jurisdictions relative to taxable income in lower jurisdictions. In addition, the
MANAGEMENT’S DISCUSSION AND ANALYSIS OF RESULTS OF OPERATIONS AND FINANCIAL CONDITION
31
Table of Contents
2007 tax rate benefited from a one-time gain of $267 million related to a business restructuring of
certain international subsidiaries.
Liquidity and Capital Resources
LIQUIDITY & CASH FLOWS
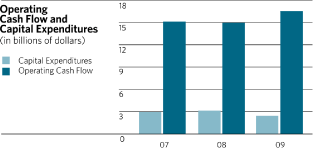
Cash and cash equivalents were $15.8 billion at the end of 2009 as compared with $10.8 billion at
the end of 2008. The primary sources of cash that contributed to the $5.0 billion increase versus
prior year were $16.6 billion of cash generated from operating activities and $2.5 billion net
proceeds from long and short-term debt. The major uses of cash were capital spending of $2.4
billion, acquisitions of $2.5 billion, net investment purchases of $2.8 billion, dividends to
shareholders of $5.3 billion and the repurchase of common stock, net of proceeds from the exercise
of options, of $1.2 billion.
Cash Flows from operations were $16.6 billion in 2009. The major sources of cash flow were net
income of $12.3 billion, adjusted for non-cash charges for depreciation, amortization and stock
based compensation of $3.4 billion, restructuring reserves of $1.1 billion and accounts receivable
and inventories of $0.5 billion. The remaining changes to operating cash flow were a use of funds
of $0.7 billion related to pension plan contributions and decreases in accounts payable partially
offset by decreases in other receivables, prepaid expenses and deferred taxes.
In 2009, the Company continued to have access to liquidity through the commercial paper
market. For additional details on borrowings, see Note 7 to the Consolidated Financial Statements.
The Company anticipates that operating cash flows, existing credit facilities and access to
the commercial paper markets will provide sufficient resources to fund operating needs in 2010.
FINANCING AND MARKET RISK
The Company uses financial instruments to manage the impact of foreign exchange rate changes on
cash flows. Accordingly, the Company enters into forward foreign exchange contracts to protect the
value of certain foreign currency assets and liabilities and to hedge future foreign currency
transactions primarily related to product costs. Gains or losses on these contracts are offset by
the gains or losses on the underlying transactions. A 10% appreciation of the U.S. Dollar from the
January 3, 2010 market rates would increase the unrealized value of the Company’s forward contracts
by $296 million. Conversely, a 10% depreciation of the U.S. Dollar from the January 3, 2010 market
rates would decrease the unrealized value of the Company’s forward contracts by $361 million. In
either scenario, the gain or loss on the forward contract would be offset by the gain or loss on
the underlying transaction and, therefore, would have no impact on future anticipated earnings and
cash flows.
The Company hedges the exposure to fluctuations in currency exchange rates, and the effect on
certain assets and liabilities in foreign currency, by entering into currency swap contracts. A 1%
change in the spread between U.S. and foreign interest rates on the Company’s interest rate
sensitive financial instruments would either increase or decrease the unrealized value of the
Company’s swap contracts by approximately $185 million. In either scenario, at maturity, the gain
or loss on the swap contract would be offset by the gain or loss on the underlying transaction and
therefore would have no impact on future anticipated cash flows.
The Company does not enter into financial instruments for trading or speculative purposes.
Further, the Company has a policy of only entering into contracts with parties that have at least
an “A” (or equivalent) credit rating. The counterparties to these contracts are major financial
institutions and there is no significant concentration of exposure with any one counterparty.
Management believes the risk of loss is remote.
The Company has access to substantial sources of funds at numerous banks worldwide. In
September 2009, the Company secured a new 364-day Credit Facility. Total credit available to the
Company approximates $10 billion, which expires September 23, 2010. Interest charged on borrowings
under the credit line agreements is based on either bids provided by banks, the prime rate or
London Interbank Offered Rates (LIBOR), plus applicable margins. Commitment fees under the
agreement are not material.
Total borrowings at the end of 2009 and 2008 were $14.5 billion and $11.9 billion,
respectively. The increase in borrowings between 2009 and 2008 was a result of financing general
corporate purposes and the continuation of the Common Stock repurchase program announced in 2007.
In 2009, net cash (cash and current marketable securities, net of debt) was $4.9 billion compared
to net cash of $1.0 billion in 2008. Total debt represented 22.3% of total capital (shareholders’
equity and total debt) in 2009 and 21.8% of total capital in 2008. Shareholders’ equity per share
at the end of 2009 was $18.37 compared with $15.35 at year-end 2008, an increase of 19.7%.
Johnson & Johnson continues to be one of a few industrial companies with a Triple A credit
rating. A summary of borrowings can be found in Note 7 to the Consolidated Financial Statements.
CONTRACTUAL OBLIGATIONS AND COMMITMENTS
The Company has contractual obligations, primarily lease, debt and unfunded retirement plans, with
no other significant obligations. To satisfy these obligations, the Company will use cash from
operations. The following table summarizes the Company’s contractual obligations and their
aggregate maturities as of January 3, 2010 (see Notes 7, 10 and 16 to the Consolidated Financial
Statements for further details):
| Long-term | Interest on | Unfunded | ||||||||||||||||||
| Debt | Debt | Retirement | Operating | |||||||||||||||||
| (Dollars in Millions) | Obligations | Obligations | Plans | Leases | Total | |||||||||||||||
2010 |
$ | 34 | 469 | 66 | 178 | 747 | ||||||||||||||
2011 |
35 | 465 | 65 | 150 | 715 | |||||||||||||||
2012 |
615 | 442 | 69 | 128 | 1,254 | |||||||||||||||
2013 |
507 | 410 | 73 | 103 | 1,093 | |||||||||||||||
2014 |
9 | 402 | 76 | 87 | 574 | |||||||||||||||
After 2014 |
7,057 | 4,525 | 474 | 94 | 12,150 | |||||||||||||||
Total |
$ | 8,257 | 6,713 | 823 | 740 | 16,533 | ||||||||||||||
For tax matters, see Note 8 to the Consolidated Financial Statements.
SHARE REPURCHASE AND DIVIDENDS
On July 9, 2007, the Company announced that its Board of Directors approved a stock repurchase
program, authorizing the Company to buy back up to $10.0 billion of the Company’s Common Stock. The
repurchase program has no time limit and may be suspended for periods or discontinued at any time.
Any shares acquired will be available for general corporate purposes. The Company funds the share
repurchase program through a combination of available cash and debt. As of January 3, 2010, the
Company repurchased an
JOHNSON & JOHNSON 2009 ANNUAL REPORT
32
Table of Contents
aggregate of 140.4 million shares of Johnson & Johnson Common Stock under the current repurchase
program at a cost of $8.9 billion. In addition, the Company has an annual program to repurchase
shares for use in employee stock and incentive plans.
The Company increased its dividend in 2009 for the 47th consecutive year. Cash dividends paid
were $1.930 per share in 2009, compared with dividends of $1.795 per share in 2008 and $1.620 per
share in 2007. The dividends were distributed as follows:
| 2009 | 2008 | 2007 | ||||||||||
First quarter |
$ | 0.460 | 0.415 | 0.375 | ||||||||
Second quarter |
0.490 | 0.460 | 0.415 | |||||||||
Third quarter |
0.490 | 0.460 | 0.415 | |||||||||
Fourth quarter |
0.490 | 0.460 | 0.415 | |||||||||
Total |
$ | 1.930 | 1.795 | 1.620 | ||||||||
On January 4, 2010, the Board of Directors declared a regular quarterly cash dividend of $0.490
per share, payable on March 9, 2010, to shareholders of record as of February 23, 2010. The Company
expects to continue the practice of paying regular cash dividends.
Other Information
CRITICAL ACCOUNTING POLICIES AND ESTIMATES
Management’s discussion and analysis of results of operations and financial condition are based on
the Company’s consolidated financial statements that have been prepared in accordance with
accounting principles generally accepted in the U.S. (GAAP). The preparation of these financial
statements requires that management make estimates and assumptions that affect the amounts reported
for revenues, expenses, assets, liabilities and other related disclosures. Actual results may or
may not differ from these estimates. The Company believes that the understanding of certain key
accounting policies and estimates are essential in achieving more insight into the Company’s
operating results and financial condition. These key accounting policies include revenue
recognition, income taxes, legal and self-insurance contingencies, valuation of long-lived assets,
assumptions used to determine the amounts recorded for pensions and other employee benefit plans
and accounting for stock options.
Revenue Recognition: The Company recognizes revenue from product sales when goods are shipped or
delivered, and title and risk of loss pass to the customer. Provisions for certain rebates, sales
incentives, trade promotions, coupons, product returns and discounts to customers are accounted for
as reductions in sales in the same period the related sales are recorded.
Product discounts granted are based on the terms of arrangements with direct, indirect and
other market participants, as well as market conditions, including prices charged by competitors.
Rebates, the largest being the Medicaid rebate provision, are estimated based on contractual terms,
historical experience, trend analysis and projected market conditions in the various markets
served. The Company evaluates market conditions for products or groups of products primarily
through the analysis of wholesaler and other third-party sell-through and market research data, as
well as internally generated information.
Sales returns are generally estimated and recorded based on historical sales and returns
information. Products that exhibit unusual sales or return patterns due to dating, competition or
other marketing matters are specifically investigated and analyzed as part of the accounting for
sales return accruals.
Sales returns allowances represent a reserve for products that may be returned due to
expiration, destruction in the field, or in specific areas, product recall. The returns reserve is
based on historical return trends by product and by market as a percent to gross sales.
The Company’s sales return reserves are accounted for in accordance with the U.S. GAAP guidance for
revenue recognition when right of return exists. Sales return reserves are recorded at full sales
value. Sales returns in the Consumer and Pharmaceutical segments are almost exclusively not
resalable. Sales returns for certain franchises in the Medical Devices and Diagnostics segment are
typically resalable but are not material. The Company rarely exchanges products from inventory for
returned products. The sales returns reserve for the total Company has ranged between 1.1% and 1.2%
of annual net trade sales during the prior three fiscal reporting years 2007-2009.
Promotional programs, such as product listing allowances and cooperative advertising
arrangements, are recorded in the year incurred. Continuing promotional programs include coupons
and volume-based sales incentive programs. The redemption cost of consumer coupons is based on
historical redemption experience by product and value. Volume-based incentive programs are based on
estimated sales volumes for the incentive period and are recorded as products are sold. The Company
also earns service revenue for co-promotion of certain products. For all years presented, service
revenues were less than 2% of total revenues and are included in sales to customers. Additionally,
these arrangements are evaluated to determine the appropriate amounts to be deferred.
In addition, the Company enters into collaboration arrangements, which contain multiple
revenue generating activities. The revenue for these arrangements is recognized as each activity is
performed or delivered, based on the relative fair value. Upfront fees received as part of these
arrangements are deferred and recognized as revenue earned over the obligation period.
See Note 1 to the Consolidated Financial Statements for additional disclosures on collaborations.
Reasonably likely changes to assumptions used to calculate the accruals for rebates, returns
and promotions are not anticipated to have a material effect on the financial statements. The
Company currently discloses the impact of changes to assumptions in the quarterly or annual filing
in which there is a material financial statement impact.
Below are tables which show the progression of accrued rebates, returns, promotions, reserve
for doubtful accounts and reserve for cash discounts by segment of business for the fiscal years
ended January 3, 2010 and December 28, 2008.
CONSUMER SEGMENT
| Balance at | Balance at | |||||||||||||||
| Beginning | Payments/ | End | ||||||||||||||
| (Dollars in Millions) | of Period | Accruals | Other | of Period | ||||||||||||
2009 |
||||||||||||||||
Accrued rebates(1) |
$ | 131 | 380 | (390 | ) | 121 | ||||||||||
Accrued returns |
115 | 134 | (122 | ) | 127 | |||||||||||
Accrued promotions |
202 | 1,996 | (1,926 | ) | 272 | |||||||||||
Subtotal |
$ | 448 | 2,510 | (2,438 | ) | 520 | ||||||||||
Reserve for doubtful accounts |
110 | 23 | (26 | ) | 107 | |||||||||||
Reserve for cash discounts |
22 | 285 | (286 | ) | 21 | |||||||||||
Total |
$ | 580 | 2,818 | (2,750 | ) | 648 | ||||||||||
2008 |
||||||||||||||||
Accrued rebates(1) |
$ | 217 | 300 | (386 | ) | 131 | ||||||||||
Accrued returns |
113 | 135 | (133 | ) | 115 | |||||||||||
Accrued promotions |
297 | 2,369 | (2,464 | ) | 202 | |||||||||||
Subtotal |
$ | 627 | 2,804 | (2,983 | ) | 448 | ||||||||||
Reserve for doubtful accounts |
71 | 41 | (2 | ) | 110 | |||||||||||
Reserve for cash discounts |
23 | 272 | (273 | ) | 22 | |||||||||||
Total |
$ | 721 | 3,117 | (3,258 | ) | 580 | ||||||||||
| (1) | Includes reserve for customer rebates of $46 million at January 3, 2010 and $73 million at December 28, 2008, recorded as a contra asset. |
MANAGEMENT’S DISCUSSION AND ANALYSIS OF RESULTS OF OPERATIONS AND FINANCIAL CONDITION
33
Table of Contents
PHARMACEUTICAL SEGMENT
| Balance at | Balance at | |||||||||||||||
| Beginning | Payments/ | End | ||||||||||||||
| (Dollars in Millions) | of Period | Accruals | Other | of Period | ||||||||||||
2009 |
||||||||||||||||
Accrued rebates(1) |
$ | 1,261 | 3,975 | (4,172 | ) | 1,064 | ||||||||||
Accrued returns |
490 | 147 | (295 | ) | 342 | |||||||||||
Accrued promotions |
107 | 330 | (353 | ) | 84 | |||||||||||
Subtotal |
$ | 1,858 | 4,452 | (4,820 | ) | 1,490 | ||||||||||
Reserve for doubtful accounts |
48 | 37 | (2 | ) | 83 | |||||||||||
Reserve for cash discounts |
23 | 462 | (437 | ) | 48 | |||||||||||
Total |
$ | 1,929 | 4,951 | (5,259 | ) | 1,621 | ||||||||||
2008 |
||||||||||||||||
Accrued rebates(1) |
$ | 1,249 | 3,331 | (3,319 | ) | 1,261 | ||||||||||
Accrued returns |
345 | 168 | (23 | ) | 490 | |||||||||||
Accrued promotions |
263 | 414 | (570 | ) | 107 | |||||||||||
Subtotal |
$ | 1,857 | 3,913 | (3,912 | ) | 1,858 | ||||||||||
Reserve for doubtful accounts |
26 | 24 | (2 | ) | 48 | |||||||||||
Reserve for cash discounts |
24 | 376 | (377 | ) | 23 | |||||||||||
Total |
$ | 1,907 | 4,313 | (2) | (4,291 | ) | 1,929 | |||||||||
| (1) | Includes reserve for customer rebates of $372 million at January 3, 2010 and $344 million at December 28, 2008, recorded as a contra asset. | |
| (2) | Includes $115 million adjustment related to previously estimated accrued sales reserves. |
MEDICAL DEVICES AND DIAGNOSTICS SEGMENT
| Balance at | Balance at | |||||||||||||||
| Beginning | Payments/ | End | ||||||||||||||
| (Dollars in Millions) | of Period | Accruals | Other | of Period | ||||||||||||
2009 |
||||||||||||||||
Accrued rebates(1) |
$ | 416 | 2,229 | (2,191 | ) | 454 | ||||||||||
Accrued returns |
189 | 74 | (43 | ) | 220 | |||||||||||
Accrued promotions |
47 | 120 | (94 | ) | 73 | |||||||||||
Subtotal |
$ | 652 | 2,423 | (2,328 | ) | 747 | ||||||||||
Reserve for doubtful accounts |
109 | 50 | (16 | ) | 143 | |||||||||||
Reserve for cash discounts |
34 | 416 | (418 | ) | 32 | |||||||||||
Total |
$ | 795 | 2,889 | (2,762 | ) | 922 | ||||||||||
2008 |
||||||||||||||||
Accrued rebates(1) |
$ | 336 | 1,947 | (1,867 | ) | 416 | ||||||||||
Accrued returns |
190 | 99 | (100 | ) | 189 | |||||||||||
Accrued promotions |
18 | 208 | (179 | ) | 47 | |||||||||||
Subtotal |
$ | 544 | 2,254 | (2,146 | ) | 652 | ||||||||||
Reserve for doubtful accounts |
96 | 36 | (23 | ) | 109 | |||||||||||
Reserve for cash discounts |
24 | 257 | (247 | ) | 34 | |||||||||||
Total |
$ | 664 | 2,547 | (2) | (2,416 | ) | 795 | |||||||||
| (1) | Includes reserve for customer rebates of $311 million at January 3, 2010 and $304 million at December 28, 2008, recorded as a contra asset. | |
| (2) | Includes $56 million adjustment related to previously estimated sales rebate reserve. |
Income Taxes: Income taxes are recorded based on amounts refundable or payable for the current
year and include the results of any difference between GAAP accounting and tax reporting, recorded
as deferred tax assets or liabilities. The Company estimates deferred tax assets and liabilities
based on current tax regulations and rates. Changes in tax laws and rates may affect recorded
deferred tax assets and liabilities in the future. Management believes that changes in these
estimates would not have a material effect on the Company’s results of operations, cash flows or
financial position.
In 2007, in accordance with U.S. GAAP the Company adopted the standard related to accounting
for uncertainty in income taxes. The Codification prescribes a recognition threshold and
measurement attribute for the financial statement recognition and measurement of a tax position
taken or expected to be taken in a tax return. The Codification also provides guidance on
derecognition, classification and other matters. See Note 8 to the Consolidated Financial
Statements for further information regarding income taxes.
At January 3, 2010 and December 28, 2008, the cumulative amounts of undistributed
international earnings were approximately $32.2 billion and $27.7 billion, respectively. The
Company intends to continue to reinvest its undistributed international earnings to expand its
international operations; therefore, no U.S. tax expense has been recorded with respect to the
undistributed portion not intended for repatriation.
Legal and Self Insurance Contingencies: The Company records accruals for various contingencies
including legal proceedings and product liability cases as these arise in the normal course of
business. The accruals are based on management’s judgment as to the probability of losses and,
where applicable, actuarially determined estimates. Additionally, the Company records insurance
receivable amounts from third-party insurers when recovery is probable. As appropriate, reserves
against these receivables are recorded for estimated amounts that may not be collected from
third-party insurers.
Long-Lived and Intangible Assets: The Company assesses changes in economic conditions and makes
assumptions regarding estimated future cash flows in evaluating the value of the Company’s
property, plant and equipment, goodwill and intangible assets. As these assumptions and estimates
may change over time, it may or may not be necessary for the Company to record impairment charges.
Employee Benefit Plans: The Company sponsors various retirement and pension plans, including
defined benefit, defined contribution and termination indemnity plans, which cover most employees
worldwide. These plans are based on assumptions for the discount rate, expected return on plan
assets, expected salary increases and health care cost trend rates. See Note 10 to the Consolidated
Financial Statements for further details on these rates and the effect a rate change would have on
the Company’s results of operations.
Stock Based Compensation: The Company recognizes compensation expense associated with the issuance
of equity instruments to employees for their services. The fair value of each award is estimated on
the date of grant using the Black-Scholes option valuation model and is expensed in the financial
statements over the vesting period. The input assumptions used in determining fair value are the
expected life, expected volatility, risk-free rate and the dividend yield. See Note 17 to the
Consolidated Financial Statements for additional information.
NEW ACCOUNTING PRONOUNCEMENTS
Refer to Note 1 to the Consolidated Financial Statements for recently adopted accounting
pronouncements and recently issued accounting pronouncements not yet adopted as of January 3, 2010.
ECONOMIC AND MARKET FACTORS
The Company is aware that its products are used in an environment where, for more than a decade,
policymakers, consumers and businesses have expressed concerns about the rising cost of health
care. In response to these concerns, the Company has a long-standing policy of pricing products
responsibly. For the period 1999-2009, in the United States, the weighted average compound annual
growth rate of the Company’s net price increases for health care products
JOHNSON & JOHNSON 2009 ANNUAL REPORT
34
Table of Contents
(prescription and over-the-counter drugs, hospital and professional products) was below the U.S.
Consumer Price Index (CPI).
Inflation rates continue to have an effect on worldwide economies and, consequently, on the
way companies operate. The Company will account for operations in Venezuela as highly inflationary
in 2010, as the prior three-year cumulative inflation rate has surpassed 100%. In the face of
increasing costs, the Company strives to maintain its profit margins through cost reduction
programs, productivity improvements and periodic price increases.
The Company is exposed to fluctuations in currency exchange rates. A 1% change in the value of
the U.S. dollar as compared to all foreign currencies in which the Company had sales, income or
expense in 2009 would have increased or decreased the translation of foreign sales by $300 million
and income by $50 million.
The Company faces various worldwide health care changes that may continue to result in pricing
pressures that include health care cost containment and government legislation relating to sales,
promotions and reimbursement.
Changes in the behavior and spending patterns of purchasers of health care products and
services, including delaying medical procedures, rationing prescription medications, reducing the
frequency of physician visits and foregoing health care insurance coverage, as a result of the
current global economic downturn may continue to impact the Company’s businesses.
The Company also operates in an environment which has become increasingly hostile to
intellectual property rights. Generic drug firms have filed Abbreviated New Drug Applications
(ANDAs) seeking to market generic forms of most of the Company’s key pharmaceutical products, prior
to expiration of the applicable patents covering those products. In the event the Company is not
successful in defending the patent claims challenged in ANDA filings, the generic firms will then
introduce generic versions of the product at issue, resulting in the potential for substantial
market share and revenue losses for that product. For further information see the discussion on
“Litigation Against Filers of Abbreviated New Drug Applications” in Note 21 to the Consolidated
Financial Statements.
LEGAL PROCEEDINGS
The Company is involved in numerous product liability cases in the United States, many of which
concern alleged adverse reactions to drugs and medical devices. The damages claimed are
substantial, and while the Company is confident of the adequacy of the warnings and instructions
for use which accompany such products, it is not feasible to predict the ultimate outcome of
litigation. However, the Company believes that if any liability results from such cases, it will be
substantially covered by existing amounts accrued in the Company’s balance sheet under its
self-insurance program and by third-party product liability insurance.
The Company is also involved in a number of patent, trademark and other lawsuits, as well as
investigations, incidental to its business. The ultimate legal and financial liability of the
Company in respect to all claims, lawsuits and proceedings referred to above cannot be estimated
with any certainty. However, in the Company’s opinion, based on its examination of these matters,
its experience to date and discussions with counsel, the ultimate outcome of legal proceedings, net
of liabilities already accrued in the Company’s balance sheet, is not expected to have a material
adverse effect on the Company’s financial condition, although the resolution in any reporting
period of one or more of these matters could have a significant impact on the Company’s results of
operations and cash flows for that period.
See Note 21 to the Consolidated Financial Statements for further information regarding legal
proceedings.
COMMON STOCK MARKET PRICES
The Company’s common stock is listed on the New York Stock Exchange under the symbol JNJ. The
composite market price ranges for Johnson & Johnson common stock during 2009 and 2008 were:
| 2009 | 2008 | |||||||||||||||
| High | Low | High | Low | |||||||||||||
First quarter |
$ | 61.00 | 46.25 | 68.85 | 61.17 | |||||||||||
Second quarter |
56.65 | 50.12 | 68.32 | 63.40 | ||||||||||||
Third quarter |
62.47 | 55.71 | 72.76 | 63.10 | ||||||||||||
Fourth quarter |
65.41 | 58.78 | 69.86 | 52.06 | ||||||||||||
| Year-end close | $64.41 |
58.56 |
||||||||||||||
Cautionary Factors That May Affect Future Results
This Annual Report contains forward-looking statements. Forward-looking statements do not
relate strictly to historical or current facts and anticipate results based on management’s plans
that are subject to uncertainty. Forward-looking statements may be identified by the use of words
such as “plans,” “expects,” “will,” “anticipates,” “estimates” and other words of similar meaning
in conjunction with, among other things, discussions of future operations, financial performance,
the Company’s strategy for growth, product development, regulatory approval, market position and
expenditures.
Forward-looking statements are based on current expectations of future events. The Company
cannot guarantee that any forward-looking statement will be accurate, although the Company believes
that it has been reasonable in its expectations and assumptions. Investors should realize that if
underlying assumptions prove inaccurate or that unknown risks or uncertainties materialize, actual
results could vary materially from the Company’s expectations and projections. Investors are
therefore cautioned not to place undue reliance on any forward-looking statements. The Company does
not undertake to update any forward-looking statements as a result of new information or future
events or developments.
Risks and uncertainties include general industry conditions and competition; economic
conditions, such as interest rate and currency exchange rate fluctuations; technological advances,
new products and patents attained by competitors; challenges inherent in new product development,
including obtaining regulatory approvals; challenges to patents; U.S. and foreign health care
reforms and governmental laws and regulations; trends toward health care cost containment;
increased scrutiny of the health care industry by government agencies; product efficacy or safety
concerns resulting in product recalls or regulatory action.
The Company’s report on Form 10-K for the year ended January 3, 2010 includes, in Exhibit 99,
a discussion of additional factors that could cause actual results to differ from expectations. The
Company notes these factors as permitted by the Private Securities Litigation Reform Act of 1995.
MANAGEMENT’S DISCUSSION AND ANALYSIS OF RESULTS OF OPERATIONS AND FINANCIAL CONDITION
35
Table of Contents
Johnson & Johnson and Subsidiaries
Consolidated Balance Sheets
| At January 3, 2010 and December 28, 2008 (Dollars in Millions Except Share and Per Share Data) (Note 1) | 2009 | 2008 | ||||||
Assets |
||||||||
Current assets |
||||||||
Cash and cash equivalents (Notes 1 and 2) |
$ | 15,810 | 10,768 | |||||
Marketable securities (Notes 1 and 2) |
3,615 | 2,041 | ||||||
Accounts receivable trade, less allowances for doubtful accounts $333 (2008, $268) |
9,646 | 9,719 | ||||||
Inventories (Notes 1 and 3) |
5,180 | 5,052 | ||||||
Deferred taxes on income (Note 8) |
2,793 | 3,430 | ||||||
Prepaid expenses and other receivables |
2,497 | 3,367 | ||||||
Total current assets |
39,541 | 34,377 | ||||||
Property, plant and equipment, net (Notes 1 and 4) |
14,759 | 14,365 | ||||||
Intangible assets, net (Notes 1 and 5) |
16,323 | 13,976 | ||||||
Goodwill (Notes 1 and 5) |
14,862 | 13,719 | ||||||
Deferred taxes on income (Note 8) |
5,507 | 5,841 | ||||||
Other assets |
3,690 | 2,634 | ||||||
Total assets |
$ | 94,682 | 84,912 | |||||
Liabilities and Shareholders’ Equity |
||||||||
Current liabilities |
||||||||
Loans and notes payable (Note 7) |
$ | 6,318 | 3,732 | |||||
Accounts payable |
5,541 | 7,503 | ||||||
Accrued liabilities |
5,796 | 5,531 | ||||||
Accrued rebates, returns and promotions |
2,028 | 2,237 | ||||||
Accrued salaries, wages and commissions |
1,606 | 1,432 | ||||||
Accrued taxes on income |
442 | 417 | ||||||
Total current liabilities |
21,731 | 20,852 | ||||||
Long-term debt (Note 7) |
8,223 | 8,120 | ||||||
Deferred taxes on income (Note 8) |
1,424 | 1,432 | ||||||
Employee related obligations (Notes 9 and 10) |
6,769 | 7,791 | ||||||
Other liabilities |
5,947 | 4,206 | ||||||
Total liabilities |
44,094 | 42,401 | ||||||
Shareholders’ equity |
||||||||
Preferred stock — without par value (authorized and unissued 2,000,000 shares) |
— | — | ||||||
Common stock — par value $1.00 per share (Note 12) (authorized 4,320,000,000 shares; issued 3,119,843,000 shares) |
3,120 | 3,120 | ||||||
Accumulated other comprehensive income (Note 13) |
(3,058 | ) | (4,955 | ) | ||||
Retained earnings |
70,306 | 63,379 | ||||||
| 70,368 | 61,544 | |||||||
Less: common stock held in treasury, at cost (Note 12) (365,522,000 shares and 350,665,000 shares) |
19,780 | 19,033 | ||||||
Total shareholders’ equity |
50,588 | 42,511 | ||||||
Total liabilities and shareholders’ equity |
$ | 94,682 | 84,912 | |||||
See Notes to Consolidated Financial Statements
JOHNSON & JOHNSON 2009 ANNUAL REPORT
36
Table of Contents
Johnson & Johnson and Subsidiaries
Consolidated Statements of Earnings
| (Dollars in Millions Except Per Share Figures) (Note 1) | 2009 | 2008 | 2007 | |||||||||
Sales to customers |
$ | 61,897 | 63,747 | 61,095 | ||||||||
Cost of products sold |
18,447 | 18,511 | 17,751 | |||||||||
Gross profit |
43,450 | 45,236 | 43,344 | |||||||||
Selling, marketing and administrative expenses |
19,801 | 21,490 | 20,451 | |||||||||
Research expense |
6,986 | 7,577 | 7,680 | |||||||||
Purchased in-process research and development (Note 20) |
— | 181 | 807 | |||||||||
Interest income |
(90 | ) | (361 | ) | (452 | ) | ||||||
Interest expense, net of portion capitalized (Note 4) |
451 | 435 | 296 | |||||||||
Other (income) expense, net |
(526 | ) | (1,015 | ) | 534 | |||||||
Restructuring (Note 22) |
1,073 | — | 745 | |||||||||
Earnings before provision for taxes on income |
15,755 | 16,929 | 13,283 | |||||||||
Provision for taxes on income (Note 8) |
3,489 | 3,980 | 2,707 | |||||||||
Net earnings |
$ | 12,266 | 12,949 | 10,576 | ||||||||
Basic net earnings per share (Notes 1 and 15) |
$ | 4.45 | 4.62 | 3.67 | ||||||||
Diluted net earnings per share (Notes 1 and 15) |
$ | 4.40 | 4.57 | 3.63 | ||||||||
Cash dividends per share |
$ | 1.930 | 1.795 | 1.620 | ||||||||
Basic average shares outstanding (Notes 1 and 15) |
2,759.5 | 2,802.5 | 2,882.9 | |||||||||
Diluted average shares outstanding (Notes 1 and 15) |
2,789.1 | 2,835.6 | 2,910.7 | |||||||||
See Notes to Consolidated Financial Statements
CONSOLIDATED FINANCIAL STATEMENTS
37
Table of Contents
Johnson & Johnson and Subsidiaries
Consolidated Statements of Equity
| Accumulated | ||||||||||||||||||||||||
| Other | Treasury | |||||||||||||||||||||||
| Comprehensive | Retained | Comprehensive | Common Stock | Stock | ||||||||||||||||||||
| (Dollars in Millions) (Note 1) | Total | Income | Earnings | Income | Issued Amount | Amount | ||||||||||||||||||
Balance, December 31, 2006 |
$ | 39,318 | 49,290 | (2,118 | ) | 3,120 | (10,974 | ) | ||||||||||||||||
Net earnings |
10,576 | 10,576 | 10,576 | |||||||||||||||||||||
Cash dividends paid |
(4,670 | ) | (4,670 | ) | ||||||||||||||||||||
Employee compensation and stock option plans |
2,311 | 131 | 2,180 | |||||||||||||||||||||
Conversion of subordinated debentures |
9 | (4 | ) | 13 | ||||||||||||||||||||
Repurchase of common stock |
(5,607 | ) | (5,607 | ) | ||||||||||||||||||||
Adoption of FIN 48 |
(19 | ) | (19 | ) | ||||||||||||||||||||
Other |
(24 | ) | (24 | ) | ||||||||||||||||||||
Other comprehensive income, net of tax: |
||||||||||||||||||||||||
Currency translation adjustment |
786 | 786 | 786 | |||||||||||||||||||||
Unrealized gains on securities |
23 | 23 | 23 | |||||||||||||||||||||
Employee benefit plans |
670 | 670 | 670 | |||||||||||||||||||||
Losses on derivatives & hedges |
(54 | ) | (54 | ) | (54 | ) | ||||||||||||||||||
Reclassification adjustment |
(5 | ) | ||||||||||||||||||||||
Total comprehensive income |
11,996 | |||||||||||||||||||||||
Balance, December 30, 2007 |
$ | 43,319 | 55,280 | (693 | ) | 3,120 | (14,388 | ) | ||||||||||||||||
Net earnings |
12,949 | 12,949 | 12,949 | |||||||||||||||||||||
Cash dividends paid |
(5,024 | ) | (5,024 | ) | ||||||||||||||||||||
Employee compensation
and stock option plans |
2,180 | 175 | 2,005 | |||||||||||||||||||||
Conversion of subordinated debentures |
— | (1 | ) | 1 | ||||||||||||||||||||
Repurchase of common stock |
(6,651 | ) | (6,651 | ) | ||||||||||||||||||||
Other comprehensive income, net of tax: |
||||||||||||||||||||||||
Currency translation adjustment |
(2,499 | ) | (2,499 | ) | (2,499 | ) | ||||||||||||||||||
Unrealized losses on securities |
(59 | ) | (59 | ) | (59 | ) | ||||||||||||||||||
Employee benefit plans |
(1,870 | ) | (1,870 | ) | (1,870 | ) | ||||||||||||||||||
Gains on derivatives & hedges |
166 | 166 | 166 | |||||||||||||||||||||
Reclassification adjustment |
(27 | ) | ||||||||||||||||||||||
Total comprehensive income |
8,660 | |||||||||||||||||||||||
Balance, December 28, 2008 |
$ | 42,511 | 63,379 | (4,955 | ) | 3,120 | (19,033 | ) | ||||||||||||||||
Net earnings |
12,266 | 12,266 | 12,266 | |||||||||||||||||||||
Cash dividends paid |
(5,327 | ) | (5,327 | ) | ||||||||||||||||||||
Employee compensation and stock option plans |
1,402 | 25 | 1,377 | |||||||||||||||||||||
Conversion of subordinated debentures |
2 | (4 | ) | 6 | ||||||||||||||||||||
Repurchase of common stock |
(2,130 | ) | (2,130 | ) | ||||||||||||||||||||
Other |
(33 | ) | (33 | ) | ||||||||||||||||||||
Other comprehensive income, net of tax: |
||||||||||||||||||||||||
Currency translation adjustment |
1,363 | 1,363 | 1,363 | |||||||||||||||||||||
Unrealized losses on securities |
(55 | ) | (55 | ) | (55 | ) | ||||||||||||||||||
Employee benefit plans |
565 | 565 | 565 | |||||||||||||||||||||
Gains on derivatives & hedges |
24 | 24 | 24 | |||||||||||||||||||||
Total comprehensive income |
14,163 | |||||||||||||||||||||||
Balance, January 3, 2010 |
$ | 50,588 | 70,306 | (3,058 | ) | 3,120 | (19,780 | ) | ||||||||||||||||
See Notes to Consolidated Financial Statements
JOHNSON & JOHNSON 2009 ANNUAL REPORT
38
Table of Contents
Johnson & Johnson and Subsidiaries
Consolidated Statements of Cash Flows
| (Dollars in Millions) (Note 1) | 2009 | 2008 | 2007 | |||||||||
Cash flows from operating activities |
||||||||||||
Net earnings |
$ | 12,266 | 12,949 | 10,576 | ||||||||
Adjustments to reconcile net earnings to cash flows from
operating activities: |
||||||||||||
Depreciation and amortization of property and intangibles |
2,774 | 2,832 | 2,777 | |||||||||
Stock based compensation |
628 | 627 | 698 | |||||||||
Purchased in-process research and development |
— | 181 | 807 | |||||||||
Intangible asset write-down (NATRECOR®) |
— | — | 678 | |||||||||
Deferred tax provision |
(436 | ) | 22 | (1,762 | ) | |||||||
Accounts receivable allowances |
58 | 86 | 22 | |||||||||
Changes in assets and liabilities, net of effects from acquisitions: |
||||||||||||
Decrease/(increase) in accounts receivable |
453 | (736 | ) | (416 | ) | |||||||
Decrease/(increase) in inventories |
95 | (101 | ) | 14 | ||||||||
(Decrease)/increase in accounts payable and accrued liabilities |
(507 | ) | (272 | ) | 2,642 | |||||||
Decrease/(increase) in other current and non-current assets |
1,209 | (1,600 | ) | (1,578 | ) | |||||||
Increase in other current and non-current liabilities |
31 | 984 | 564 | |||||||||
Net cash flows from operating activities |
16,571 | 14,972 | 15,022 | |||||||||
Cash flows from investing activities |
||||||||||||
Additions to property, plant and equipment |
(2,365 | ) | (3,066 | ) | (2,942 | ) | ||||||
Proceeds from the disposal of assets |
154 | 785 | 457 | |||||||||
Acquisitions, net of cash acquired (Note 20) |
(2,470 | ) | (1,214 | ) | (1,388 | ) | ||||||
Purchases of investments |
(10,040 | ) | (3,668 | ) | (9,659 | ) | ||||||
Sales of investments |
7,232 | 3,059 | 7,988 | |||||||||
Other (primarily intangibles) |
(109 | ) | (83 | ) | (368 | ) | ||||||
Net cash used by investing activities |
(7,598 | ) | (4,187 | ) | (5,912 | ) | ||||||
Cash flows from financing activities |
||||||||||||
Dividends to shareholders |
(5,327 | ) | (5,024 | ) | (4,670 | ) | ||||||
Repurchase of common stock |
(2,130 | ) | (6,651 | ) | (5,607 | ) | ||||||
Proceeds from short-term debt |
9,484 | 8,430 | 19,626 | |||||||||
Retirement of short-term debt |
(6,791 | ) | (7,319 | ) | (21,691 | ) | ||||||
Proceeds from long-term debt |
9 | 1,638 | 5,100 | |||||||||
Retirement of long-term debt |
(219 | ) | (24 | ) | (18 | ) | ||||||
Proceeds from the exercise of stock options/excess tax benefits |
882 | 1,486 | 1,562 | |||||||||
Net cash used by financing activities |
(4,092 | ) | (7,464 | ) | (5,698 | ) | ||||||
Effect of exchange rate changes on cash and cash equivalents |
161 | (323 | ) | 275 | ||||||||
Increase in cash and cash equivalents |
5,042 | 2,998 | 3,687 | |||||||||
Cash and cash equivalents, beginning of year (Note 1) |
10,768 | 7,770 | 4,083 | |||||||||
Cash and cash equivalents, end of year (Note 1) |
$ | 15,810 | 10,768 | 7,770 | ||||||||
Supplemental cash flow data |
||||||||||||
Cash paid during the year for: |
||||||||||||
Interest |
$ | 533 | 525 | 314 | ||||||||
Income taxes |
2,363 | 4,068 | 4,099 | |||||||||
Supplemental schedule of noncash investing and financing activities |
||||||||||||
Treasury stock issued for employee compensation and stock
option plans, net of cash proceeds |
$ | 541 | 593 | 738 | ||||||||
Conversion of debt |
2 | — | 9 | |||||||||
Acquisitions |
||||||||||||
Fair value of assets acquired |
$ | 3,345 | 1,328 | 1,620 | ||||||||
Fair value of liabilities assumed and non-controlling interests |
(875 | ) | (114 | ) | (232 | ) | ||||||
Net cash paid for acquisitions |
$ | 2,470 | 1,214 | 1,388 | ||||||||
See Notes to Consolidated Financial Statements
CONSOLIDATED FINANCIAL STATEMENTS
39
Table of Contents
JOHNSON & JOHNSON
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
1. Summary of Significant Accounting Policies
PRINCIPLES OF CONSOLIDATION
The consolidated financial statements include the accounts of Johnson & Johnson and
subsidiaries (the “Company”). Inter-company accounts and transactions are eliminated.
DESCRIPTION OF THE COMPANY AND BUSINESS SEGMENTS
The Company has approximately 115,500 employees worldwide engaged in the research and
development, manufacture and sale of a broad range of products in the health care field. The
Company conducts business in virtually all countries of the world and its primary focus is on
products related to human health and well-being.
The Company is organized into three business segments: Consumer, Pharmaceutical and Medical
Devices and Diagnostics. The Consumer segment manufactures and markets a broad range of products
used in the baby care, skin care, oral care, wound care and women’s health care fields, as well as
nutritional and over-the-counter pharmaceutical products. These products are marketed to the
general public and sold both to retail outlets and distributors throughout the world. The
Pharmaceutical segment includes products in the following therapeutic areas: anti-infective,
antipsychotic, cardiovascular, contraceptive, dermatology, gastrointestinal, hematology,
immunology, neurology, oncology, pain management, urology and virology. These products are
distributed directly to retailers, wholesalers and health care professionals for prescription use.
The Medical Devices and Diagnostics segment includes a broad range of products used principally in
the professional fields by physicians, nurses, therapists, hospitals, diagnostic laboratories and
clinics. These products include Cordis’ circulatory disease management products; DePuy’s
orthopaedic joint reconstruction, spinal care and sports medicine products; Ethicon’s surgical
care, aesthetics and women’s health products; Ethicon Endo-Surgery’s minimally invasive surgical
products; LifeScan’s blood glucose monitoring and insulin delivery products; Ortho-Clinical
Diagnostics’ professional diagnostic products and Vistakon’s disposable contact lenses.
NEW ACCOUNTING PRONOUNCEMENTS
RECENTLY ADOPTED ACCOUNTING PRONOUNCEMENTS
RECENTLY ADOPTED ACCOUNTING PRONOUNCEMENTS
During the fiscal fourth quarter of 2009, in accordance with U.S. GAAP, the Company adopted the
authoritative guidance for employers’ disclosures about postretirement benefit plan assets to
enhance the disclosure regarding the types of assets and associated risks in an employer’s defined
benefit pension or other postretirement plan, as well as, events in the economy and markets that
could have a significant effect on the value of the plan assets. The adoption of this standard did
not have a material impact on the Company’s results of operations, cash flows or financial
position. See Note 10 for enhanced disclosures.
During the fiscal third quarter of 2009, the Company adopted The FASB Accounting Standards CodificationTM (ASC or Codification) and the
Hierarchy of Generally Accepted Accounting Principles (GAAP) which establishes the Codification as
the sole source for authoritative U.S. GAAP and will supersede all accounting standards in U.S.
GAAP, aside from those issued by the SEC. The adoption of the Codification did not have an impact
on the Company’s results of operations, cash flows or financial position. Since the adoption of the
Accounting Standards Codification (ASC) the Company’s notes to the consolidated financial
statements will no longer make reference to Statement of Financial Accounting Standards (SFAS) or
other U.S. GAAP pronouncements.
During the fiscal second quarter of 2009, in accordance with U.S. GAAP, the Company adopted
the standards on subsequent events. This pronouncement establishes standards of accounting for and
disclosure of events that occur after the balance sheet date but before financial statements are
issued. See Note 23 for related disclosure.
During the fiscal first quarter of 2009, in accordance with U.S. GAAP, the Company adopted the
standards on business combinations and non-controlling interests in Consolidated Financial
Statements. These standards aim to improve, simplify, and converge internationally, the accounting
for business combinations and the reporting of non-controlling interests in consolidated financial
statements. These standards have an impact on the manner in which the Company accounts for
acquisitions beginning in the fiscal year 2009. Significant changes include the capitalization of
purchased in-process research and development (IPR&D), expensing of acquisition related
restructuring actions and transaction related costs and the recognition of contingent purchase
price consideration at fair value at the acquisition date. In addition, changes in accounting for
deferred tax asset valuation allowances and acquired income tax uncertainties after the measurement
period will be recognized in earnings rather than as an adjustment to the cost of acquisition. This
accounting treatment for taxes is applicable to acquisitions that occurred both prior and
subsequent to the adoption of the standard. Operating profit attributable to non-controlling
interests is reported in Other (Income) Expense, net and the related tax impact to the Provision
for Taxes. Additionally, equity attributable to non-controlling interests is recorded in Other
Non-Current liabilities. Non-controlling interests as related to the Company’s financial statements
are immaterial and therefore, not separately disclosed.
During the fiscal first quarter of 2009, in accordance with U.S. GAAP, the Company adopted the
standard related to disclosures about derivative instruments and hedging activities, which enhanced
the disclosure regarding the Company’s derivative and hedging activities. The adoption of this
standard did not have a material impact on the Company’s results of operations, cash flows or
financial position. See Note 6 for enhanced disclosures.
During the fiscal first quarter of 2009, in accordance with U.S. GAAP, the Company adopted the
standard on collaborative arrangements related to the development and commercialization of
intellectual property. This standard addresses the income statement classification of payments made
between parties in a collaborative arrangement. The impact of the adoption of this standard related
to all collaboration agreements that existed as of January 3, 2010 and December 28, 2008 was
immaterial to the Company’s results of operations, cash flows or financial position.
During the fiscal first quarter of 2009, in accordance with U.S. GAAP, the Company adopted the
standard related to defensive intangible assets. This standard applies to acquired intangible
assets in situations in which an entity does not intend to actively use the asset but intends to
hold the asset to prevent others from obtaining access to the asset, except for intangible assets
that are used in research and development activities. The adoption of this standard did not have a
material impact on the Company’s results of operations, cash flows or financial position.
RECENTLY ISSUED ACCOUNTING STANDARDS, NOT ADOPTED AS OF JANUARY 3, 2010
The FASB issued guidance and amendments to the criteria for separating consideration in
multiple-deliverable revenue arrangements.
40
Table of Contents
JOHNSON & JOHNSON
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
The guidance and amendments are expected to: (a) provide principles and application guidance on
whether multiple deliverables exist, how the arrangement should be separated, and the consideration
allocated; (b) require an entity to allocate revenue in an arrangement using estimated selling
prices of deliverables if a vendor does not have vendor-specific objective evidence or third-party
evidence of selling price; and (c) eliminate the use of the residual method and require an entity
to allocate the revenue using the relative selling price method. The guidance significantly expands
the disclosure requirements for multiple-deliverable revenue arrangements. This guidance is
effective prospectively for revenue arrangements entered into or materially modified in fiscal
years beginning on or after June 15, 2010. Early adoption is permitted. The Company adopted this
guidance in the first fiscal quarter of 2010. The adoption will not have a material impact on the
Company’s results of operations, cash flows or financial position; however, it will expand the
disclosures for such arrangements.
The FASB issued a standard to improve financial reporting by enterprises involved with
variable interest entities. This statement is effective for the Company beginning with the fiscal
year 2010. Earlier application is prohibited. The adoption of this standard will not have a
material impact on the Company’s results of operations, cash flows or financial position.
CASH EQUIVALENTS
The Company considers securities with maturities of three months or less, when purchased, to be
cash equivalents.
INVESTMENTS
Short-term marketable securities are carried at cost, which approximates fair value.
Investments classified as available-for-sale are carried at estimated fair value with unrealized
gains and losses recorded as a component of accumulated other comprehensive income. Long-term debt
securities that the Company has the ability and intent to hold until maturity are carried at
amortized cost. Management determines the appropriate classification of its investment in debt and
equity securities at the time of purchase and re-evaluates such determination at each balance sheet
date. The Company periodically reviews its investments in equity securities for impairment and
adjusts these investments to their fair value when a decline in market value is deemed to be other
than temporary. If losses on these securities are considered to be other than temporary, the loss
is recognized in earnings.
PROPERTY, PLANT AND EQUIPMENT AND DEPRECIATION
Property, plant and equipment are stated at cost. The Company utilizes the straight-line method
of depreciation over the estimated useful lives of the assets:
Building and building equipment |
20-40 years | |||
Land and leasehold improvements |
10-20 years | |||
Machinery and equipment |
2-13 years | |||
The Company capitalizes certain computer software and development costs, included in machinery and
equipment, when incurred in connection with developing or obtaining computer software for internal
use. Capitalized software costs are amortized over the estimated useful lives of the software,
which generally range from 3 to 8 years.
The Company reviews long-lived assets to assess recoverability using undiscounted cash flows.
When certain events or changes in operating or economic conditions occur, an impairment assessment
may be performed on the recoverability of the carrying value of these assets. If the asset is
determined to be impaired, the loss is measured based on the difference between the asset’s fair
value and its carrying value. If quoted market prices are not available, the Company will estimate
fair value using a discounted value of estimated future cash flows.
REVENUE RECOGNITION
The Company recognizes revenue from product sales when the goods are shipped or delivered and
title and risk of loss pass to the customer. Provisions for certain rebates, sales incentives,
trade promotions, coupons, product returns and discounts to customers are accounted for as
reductions in sales in the same period the related sales are recorded.
Product discounts granted are based on the terms of arrangements with direct, indirect and
other market participants, as well as market conditions, including prices charged by competitors.
Rebates, the largest being the Medicaid rebate provision, are estimated based on contractual terms,
historical experience, trend analysis and projected market conditions in the various markets
served. The Company evaluates market conditions for products or groups of products primarily
through the analysis of wholesaler and other third-party sell-through and market research data, as
well as internally generated information.
Sales returns are generally estimated and recorded based on historical sales and returns
information. Products that exhibit unusual sales or return patterns due to dating, competition or
other marketing matters are specifically investigated and analyzed as part of the accounting for
sales return accruals. Sales returns allowances represent a reserve for products that may be
returned due to expiration, destruction in the field, or in specific areas, product recall. The
returns reserve is based on historical return trends by product and by market as a percent to gross
sales. In accordance with the Company’s accounting policies, the Company generally issues credit to
customers for returned goods. The Company’s sales return reserves are accounted for in accordance
with U.S. GAAP guidance regarding revenue recognition when right of return exists. Sales return
reserves are recorded at full sales value. Sales returns in the Consumer and Pharmaceutical
segments are almost exclusively not resalable. Sales returns for certain franchises in the Medical
Devices and Diagnostics segment are typically resalable but are not material. The Company rarely
exchanges products from inventory for returned products. The sales returns reserve for the total
Company has ranged between 1.1% and 1.2% of annual net trade sales during the prior three fiscal
reporting years 2007-2009.
Promotional programs, such as product listing allowances and cooperative advertising
arrangements, are recorded in the year incurred. Continuing promotional programs include coupons
and volume-based sales incentive programs. The redemption cost of consumer coupons is based on
historical redemption experience by product and value. Volume-based incentive programs are based on
the estimated sales volumes for the incentive period and are recorded as products are sold. The
Company also earns service revenue for co-promotion of certain products and includes it in sales to
customers. These arrangements are evaluated to determine the appropriate amounts to be deferred.
SHIPPING AND HANDLING
Shipping and handling costs incurred were $964 million, $1,017 million and $934 million in
2009, 2008 and 2007, respectively, and are included in selling, marketing and administrative
expense. The amount of revenue received for shipping and handling is less than 0.5% of sales to
customers for all periods presented.
INVENTORIES
Inventories are stated at the lower of cost or market determined by the first-in, first-out
method.
41
Table of Contents
JOHNSON & JOHNSON
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
INTANGIBLE ASSETS AND GOODWILL
The authoritative literature on U.S. GAAP requires that goodwill and intangible assets with
indefinite lives be assessed annually for impairment. The Company completed the annual impairment
test for 2009 in the fiscal fourth quarter and no impairment was determined. Future impairment
tests will be performed annually in the fiscal fourth quarter, or sooner if a triggering event
occurs.
Intangible assets that have finite useful lives continue to be amortized over their useful
lives, and are reviewed for impairment when warranted by economic conditions. See Note 5 for
further details on Intangible Assets and Goodwill.
FINANCIAL INSTRUMENTS
As required by U.S. GAAP all derivative instruments are recorded on the balance sheet at fair
value. Changes in the fair value of derivatives are recorded each period in current earnings or
other comprehensive income, depending on whether the derivative is designated as part of a hedge
transaction, and if so, the type of hedge transaction.
The Company documents all relationships between hedged items and derivatives. The overall risk
management strategy includes reasons for undertaking hedge transactions and entering into
derivatives. The objectives of this strategy are: (1) minimize foreign currency exposure’s impact
on the Company’s financial performance; (2) protect the Company’s cash flow from adverse movements
in foreign exchange rates; (3) ensure the appropriateness of financial instruments; and (4) manage
the enterprise risk associated with financial institutions. See Note 6 for additional information
on Financial Instruments.
PRODUCT LIABILITY
Accruals for product liability claims are recorded, on an undiscounted basis, when it is
probable that a liability has been incurred and the amount of the liability can be reasonably
estimated based on existing information. The accruals are adjusted periodically as additional
information becomes available. As a result of cost and availability factors, effective November 1,
2005, the Company ceased purchasing third-party product liability insurance. Based on the
availability of prior coverage, receivables for insurance recoveries related to product liability
claims are recorded on an undiscounted basis, when it is probable that a recovery will be realized.
RESEARCH AND DEVELOPMENT
Research and development expenses are expensed as incurred. Upfront and milestone payments made
to third-parties in connection with research and development collaborations are expensed as
incurred up to the point of regulatory approval. Payments made to third-parties subsequent to
regulatory approval are capitalized and amortized over the remaining useful life of the related
product. Amounts capitalized for such payments are included in other intangibles, net of
accumulated amortization.
The Company enters into collaborative arrangements, typically with other pharmaceutical or
biotechnology companies, to develop and commercialize drug candidates or intellectual property.
These arrangements typically involve two (or more) parties who are active participants in the
collaboration and are exposed to significant risks and rewards dependent on the commercial success
of the activities. These collaborations usually involve various activities by one or more parties,
including research and development, marketing and selling and distribution. Often, these
collaborations require upfront, milestone and royalty or profit share payments, contingent upon the
occurrence of certain future events linked to the success of the asset in development. Amounts due
from collaborative partners related to development activities are generally reflected as a
reduction of research and development expense because the performance of contract development
services is not central to the Company’s operations. In general, the income statement presentation
for these collaborations is as follows:
| Nature/Type of Collaboration | Statement of Earnings Presentation | |||
Third-party sale of product |
Sales to customers | |||
Royalties/milestones paid to collaborative partner (post-regulatory approval)* |
Cost of goods sold | |||
Royalties received from collaborative partner |
Other income (expense), net | |||
Upfront payments & milestones paid to collaborative partner (pre-regulatory approval) |
Research expense | |||
Research and development payments to collaborative partner |
Research expense | |||
Research and development payments received from collaborative partner |
Reduction of Research expense | |||
| * | Milestones are capitalized as intangible assets and amortized to cost of goods sold over the useful life. |
ADVERTISING
Costs associated with advertising are expensed in the year incurred and are included in the
selling, marketing and administrative expenses. Advertising expenses worldwide, which are comprised
of television, radio, print media and Internet advertising, were $2.4 billion in 2009, $2.9 billion
in 2008 and $2.7 billion in 2007.
INCOME TAXES
The Company intends to continue to reinvest its undistributed international earnings to expand
its international operations; therefore, no U.S. tax expense has been recorded with respect to the
undistributed portion not intended for repatriation. At January 3, 2010 and December 28, 2008, the
cumulative amount of undistributed international earnings were approximately $32.2 billion and
$27.7 billion, respectively.
Deferred income taxes are recognized for tax consequences of temporary differences by applying
enacted statutory tax rates, applicable to future years, to differences between the financial
reporting and the tax basis of existing assets and liabilities.
NET EARNINGS PER SHARE
Basic earnings per share is computed by dividing net earnings available to common shareholders
by the weighted average number of common shares outstanding for the period. Diluted earnings per
share reflects the potential dilution that could occur if securities were exercised or converted
into common stock using the treasury stock method.
USE OF ESTIMATES
The preparation of consolidated financial statements in conformity with accounting principles
generally accepted in the U.S. requires management to make estimates and assumptions that affect
the amounts reported. Estimates are used when accounting for sales discounts, rebates, allowances
and incentives, product liabilities, income taxes, depreciation, amortization, employee benefits,
contingencies and intangible asset and liability valuations. For instance, in determining annual
pension and post-employment benefit
costs, the Company estimates the rate of return on plan assets, and the cost of future health care
benefits. Actual results may or may not differ from those estimates.
42
Table of Contents
JOHNSON & JOHNSON
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
ANNUAL CLOSING DATE
The Company follows the concept of a fiscal year, which ends on the Sunday nearest to the end
of the month of December. Normally each fiscal year consists of 52 weeks, but every five or six
years the fiscal year consists of 53 weeks, as was the case in 2009 and will be the case again in
2014.
RECLASSIFICATION
Certain prior period amounts have been reclassified to conform to current year presentation.
2. Cash, Cash Equivalents and Current Marketable Securities
| January 3, 2010 | December 28, 2008 | |||||||||||||||||||||||
| Amortized | Unrealized | Estimated | Amortized | Unrealized | Estimated | |||||||||||||||||||
| (Dollars in Millions) | Cost | Gains/(Losses) | Fair Value | Cost | Gains/(Losses) | Fair Value | ||||||||||||||||||
Current Investments |
||||||||||||||||||||||||
Cash |
$ | 2,517 | — | 2,517 | 3,276 | — | 3,276 | |||||||||||||||||
Government securities and obligations |
13,370 | 1 | 13,371 | 7,486 | 4 | 7,490 | ||||||||||||||||||
Corporate debt securities |
426 | — | 426 | 627 | 1 | 628 | ||||||||||||||||||
Money market funds |
1,890 | — | 1,890 | 813 | — | 813 | ||||||||||||||||||
Time deposits |
1,222 | — | 1,222 | 607 | — | 607 | ||||||||||||||||||
Total cash, cash equivalents and
current marketable securities |
$ | 19,425 | 1 | 19,426 | 12,809 | 5 | 12,814 | |||||||||||||||||
As of January 3, 2010, current marketable securities consist of $3,434 million and $181 million
of government securities and obligations and corporate debt securities, respectively.
As of December 28, 2008, current marketable securities consist of $1,663 million, $342 million
and $36 million of government securities and obligations, corporate debt securities and time
deposits, respectively.
Fair value of government securities and obligations and corporate debt securities were
estimated using quoted broker prices in active markets.
The Company invests its excess cash in both deposits with major banks throughout the world and
other high-quality money market instruments. The Company has a policy of making investments only
with commercial institutions that have at least an A (or equivalent) credit rating.
3. Inventories
At the end of 2009 and 2008, inventories were comprised of:
| (Dollars in Millions) | 2009 | 2008 | ||||||
Raw materials and supplies |
$ | 1,144 | 839 | |||||
Goods in process |
1,395 | 1,372 | ||||||
Finished goods |
2,641 | 2,841 | ||||||
| $ | 5,180 | 5,052 | ||||||
4. Property, Plant and Equipment
At the end of 2009 and 2008, property, plant and equipment at cost and accumulated depreciation
were:
| (Dollars in Millions) | 2009 | 2008 | ||||||
Land and land improvements |
$ | 714 | 886 | |||||
Buildings and building equipment |
8,863 | 7,720 | ||||||
Machinery and equipment |
17,153 | 15,234 | ||||||
Construction in progress |
2,521 | 3,552 | ||||||
| 29,251 | 27,392 | |||||||
Less accumulated depreciation |
14,492 | 13,027 | ||||||
| $ | 14,759 | 14,365 | ||||||
The Company capitalizes interest expense as part of the cost of construction of facilities and
equipment. Interest expense capitalized in 2009, 2008 and 2007 was $101 million, $147 million and
$130 million, respectively.
Depreciation expense, including the amortization of capitalized interest in 2009, 2008 and
2007, was $2.1 billion, $2.0 billion and $1.9 billion, respectively.
Upon retirement or other disposal of property, plant and equipment, the costs and related
amounts of accumulated depreciation or amortization are eliminated from the asset and accumulated
depreciation accounts, respectively. The difference, if any, between the net asset value and the
proceeds are recorded in earnings.
5. Intangible Assets and Goodwill
At the end of 2009 and 2008, the gross and net amounts of intangible assets were:
| (Dollars in Millions) | 2009 | 2008 | ||||||
Intangible assets with definite lives: |
||||||||
Patents and trademarks — gross |
$ | 5,697 | 5,119 | |||||
Less accumulated amortization |
2,177 | 1,820 | ||||||
Patents and trademarks — net |
$ | 3,520 | 3,299 | |||||
Other intangibles — gross |
$ | 7,808 | 7,376 | |||||
Less accumulated amortization |
2,680 | 2,433 | ||||||
Other intangibles — net |
$ | 5,128 | 4,943 | |||||
Total intangible assets with definite lives — gross |
$ | 13,505 | 12,495 | |||||
Less accumulated amortization |
4,857 | 4,253 | ||||||
Total intangible assets with definite lives — net |
$ | 8,648 | 8,242 | |||||
Intangible assets with indefinite lives: |
||||||||
Trademarks |
$ | 5,938 | 5,734 | |||||
Purchased in-process research and development* |
1,737 | — | ||||||
Total intangible assets with indefinite lives |
$ | 7,675 | 5,734 | |||||
Total intangible assets — net |
$ | 16,323 | 13,976 | |||||
| * | Purchased in-process research and development will be accounted for as an indefinite-lived intangible asset until the underlying project is completed or abandoned. |
43
Table of Contents
JOHNSON & JOHNSON
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Goodwill as of January 3, 2010 and December 28, 2008, as allocated by segment of business is as
follows:
| Med Dev | ||||||||||||||||
| (Dollars in Millions) | Consumer | Pharm | and Diag | Total | ||||||||||||
Goodwill at December 30, 2007 |
$ | 8,125 | 964 | 5,034 | 14,123 | |||||||||||
Acquisitions |
191 | — | 286 | 477 | ||||||||||||
Currency translation/other |
(842 | ) | (1 | ) | (38 | ) | (881 | ) | ||||||||
Goodwill at December 28, 2008 |
$ | 7,474 | 963 | 5,282 | 13,719 | |||||||||||
Acquisitions |
— | 271 | 401 | 672 | ||||||||||||
Currency translation/other* |
600 | 10 | (139 | ) | 471 | |||||||||||
Goodwill at January 3, 2010 |
$ | 8,074 | 1,244 | 5,544 | 14,862 | |||||||||||
| * | Includes reclassification between segments. |
The weighted average amortization periods for patents and trademarks and other intangible
assets are 17 years and 28 years, respectively. The amortization expense of amortizable assets for
the fiscal years ended January 3, 2010, December 28, 2008 and December 30, 2007 was $675 million,
$788 million and $844 million before tax, respectively. Certain patents and intangible assets were
written down to fair value during fiscal years 2009, 2008 and 2007, with the resulting charge
included in amortization expense.
The estimated amortization expense for the five succeeding years approximates $700 million
before tax, per year. Substantially all of the amortization expense is included in cost of products
sold.
6. Fair Value Measurements
During the fiscal first quarter of 2009, in accordance with U.S. GAAP the Company adopted the
standard related to disclosures about derivative instruments and hedging activities. This standard
requires qualitative disclosures about objectives and strategies for using
derivatives, quantitative disclosures about fair value amounts of gain and losses on derivative
instruments, and disclosures about credit-risk-related contingent features in derivative
agreements.
The Company uses forward exchange contracts to manage its exposure to the variability of cash
flows, primarily related to the foreign exchange rate changes of future intercompany product and
third-party purchases of raw materials denominated in foreign currency. The Company also uses cross
currency interest rate swaps to manage currency risk primarily related to borrowings. Both types of
derivatives are designated as cash flow hedges. The Company also uses forward exchange contracts to
manage its exposure to the variability of cash flows for repatriation of foreign dividends. These
contracts are designated as net investment hedges. Additionally, the Company uses forward exchange
contracts to offset its exposure to certain foreign currency assets and liabilities. These forward
exchange contracts are not designated as hedges and therefore, changes in the fair values of these
derivatives are recognized in earnings, thereby offsetting the current earnings effect of the
related foreign currency assets and liabilities. The Company does not enter into derivative
financial instruments for trading or speculative purposes, or contain credit risk related
contingent features or requirements to post collateral. On an ongoing basis the Company monitors
counterparty credit ratings. The Company considers credit non-performance risk to be low, because
the Company enters into agreements with commercial institutions that have at least an A (or
equivalent) credit rating. As of January 3, 2010, the Company had notional amounts outstanding for
forward foreign exchange contracts and cross currency interest rate swaps of $21 billion and $4
billion, respectively.
As required by U.S. GAAP for derivative instruments and hedging activities, all derivative
instruments are to be recorded on the balance sheet at fair value. Changes in the fair value of
derivatives are recorded each period in current earnings or other comprehensive income, depending
on whether the derivative is designated as part of a hedge transaction, and if so, the type of
hedge transaction.
The designation as a cash flow hedge is made at the entrance date into the derivative
contract. At inception, all derivatives are expected to be highly effective. Changes in the fair
value of a derivative that is designated as a cash flow hedge and is highly effective are recorded
in accumulated other comprehensive income until the underlying transaction affects earnings, and
are then reclassified to earnings in the same account as the hedged transaction. Gains/losses on
net investment hedges are accounted for through the currency translation account and are
insignificant. On an ongoing basis, the Company assesses whether each derivative continues to be
highly effective in offsetting changes in the cash flows of hedged items. If and when a derivative
is no longer expected to be highly effective, hedge accounting is discontinued. Hedge
ineffectiveness, if any, is included in current period earnings in other (income) and expense, net,
and was insignificant for the fiscal year ended January 3, 2010 and December 28, 2008. Refer to
Note 13 for disclosures of movements in Accumulated Other Comprehensive Income.
As of January 3, 2010, the balance of deferred net gains on derivatives included in
accumulated other comprehensive income was $145 million after-tax. For additional information, see
Note 13. The Company expects that substantially all of the amount related to foreign exchange
contracts will be reclassified into earnings over the next 12 months as a result of transactions
that are expected to occur over that period. The maximum length of time over which the Company is
hedging transaction exposure is 18 months excluding interest rate swaps. The amount ultimately
realized in earnings will differ as foreign exchange rates change. Realized gains and losses are
ultimately determined by actual exchange rates at maturity of the derivative.
The following table is a summary of the activity for the fiscal year ended January 3, 2010
related to designated derivatives as defined in the Codification:
| Gain/(Loss) | Gain/(Loss) | |||||||||||
| Gain/(Loss) | reclassed from | recognized | ||||||||||
| recognized in | Accumulated | in Other | ||||||||||
| Cash Flow Hedges | Accumulated | OCI into | Income/ | |||||||||
| (Dollars in Millions) | OCI(1) | income(1) | Expense(2) | |||||||||
Foreign exchange contracts |
$ | (63 | ) | (47 | )(A) | 1 | ||||||
Foreign exchange contracts |
(173 | ) | 70 | (B) | (1 | ) | ||||||
Foreign exchange contracts |
5 | 13 | (C) | — | ||||||||
Cross currency interest rate swaps |
241 | (16 | )(D) | — | ||||||||
Foreign exchange contracts |
28 | (6 | )(E) | (12 | ) | |||||||
Total |
$ | 38 | 14 | (12 | ) | |||||||
| (1) | Effective portion | |
| (2) | Ineffective portion | |
| (A) | Included in Sales to customer | |
| (B) | Included in Cost of products sold | |
| (C) | Included in Research expense | |
| (D) | Included in Interest (Income)/Interest Expense, net | |
| (E) | Included in Other (Income)/Expense, net |
For the fiscal year ended January 3, 2010, a gain of $21 million was recognized in Other
(income)/expense, net, relating to foreign exchange contracts not designated as hedging instruments
under the Codification.
44
Table of Contents
JOHNSON & JOHNSON
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
During the fiscal first quarter of 2008, in accordance with U.S. GAAP, the Company
adopted the standard related to fair value measurements except for non-financial assets and
liabilities recognized or disclosed at fair value on a non-recurring basis, which became effective
during the first fiscal quarter of 2009. The effect of adoption on December 29, 2008 of this
standard for non-financial assets and liabilities recorded at fair value on a non-recurring basis
did not have a material impact on the Company’s financial position and results of operations. This
standard defines fair value, establishes a framework for measuring fair value and expands
disclosures about fair value measurements. During the fiscal first quarter of 2008, the Company
adopted the standard related to fair value option for financial assets and financial liabilities.
This standard permits the Company to measure certain financial assets and financial liabilities at
fair value. The Company assessed the fair value option made available upon adopting this standard,
and has elected not to apply the fair value option to any financial instruments that were not
already recognized at fair value.
U.S. GAAP defines fair value as the exit price that would be received to sell an asset or paid
to transfer a liability. Fair value is a market-based measurement that should be determined using
assumptions that market participants would use in pricing an asset or liability. The authoritative
literature establishes a three-level hierarchy to prioritize the inputs used in measuring fair
value. The levels within the hierarchy are described in the table below with level 1 having the
highest priority and level 3 having the lowest.
The fair value of a derivative financial instrument (i.e. forward exchange contract, currency
swap) is the aggregation by currency of all future cash flows discounted to its present value at
the prevailing market interest rates and subsequently converted to the U.S. dollar at the current
spot foreign exchange rate. The Company does not believe that fair values of these derivative
instruments materially differ from the amounts that could be realized upon settlement or maturity,
or that the changes in fair value will have a material effect on the Company’s results of
operations, cash flows or financial position.
The Company also holds equity investments which are classified as level 1 since they are
traded in an active exchange market.
During 2009, the Company acquired substantially all of the
assets and rights of Elan’s Alzheimer’s Immunotherapy Program through a newly formed company,
JANSSEN Alzheimer Immunotherapy (JAI), of which the Company owns 50.1% and Elan owns 49.9%. In
addition, the Company purchased approximately 107 million newly issued American Depositary Receipts
(ADRs) of Elan, representing 18.4% of Elan’s outstanding ordinary shares. As part of this
transaction, the Company paid $885 million to Elan and committed to fund up to $250 million of
Elan’s share of research and development spending by JAI. Of this total consideration of $1,135
million, $793 million represents the fair value of the 18.4% investment in Elan based on Elan’s
share price in an actively traded market as of the date of this transaction. The IPR&D related to
this transaction was $679 million and is associated with bapineuzumab, a potential first-in-class
treatment that is being evaluated for slowing the progression of Alzheimer’s Disease. The value of
the IPR&D was calculated using cash flow projections discounted for the risk inherent in such
projects. Probability of success factors ranging from 40-50% were used to reflect inherent clinical
and regulatory risk. The discount rate applied was 26%. The non-controlling interest related to
this transaction was $590 million, which the Company has recorded in other non-current liabilities.
During 2009, the Company entered into a strategic collaboration with Crucell N.V. which will
focus on the discovery, development and commercialization of monoclonal antibodies and vaccines for
the treatment and prevention of influenza and other infectious and non-infectious diseases. In
addition, the Company, through its affiliate, purchased approximately 18% of Crucell’s outstanding
ordinary shares for an aggregate purchase price of $448 million. Of the total consideration paid,
$329 million represents the fair value of the investment based on Crucell’s share price in an
actively traded market as of the date of the transaction with the excess recorded to research and
development expense in 2009.
The Company did not have any other significant financial assets or liabilities which would
require revised valuations under this standard that are recognized at fair value.
The Company’s significant financial assets and liabilities measured at fair value as of January 3,
2010 and December 28, 2008 were as follows:
| Significant | ||||||||||||||||||||
| Quoted prices in | other | Significant | ||||||||||||||||||
| active markets for | observable | unobservable | ||||||||||||||||||
| identical assets | inputs | inputs | 2009 | 2008 | ||||||||||||||||
| (Dollars in Millions) | Level 1 | Level 2 | Level 3 | Total | Total* | |||||||||||||||
Derivatives designated as hedging instruments: |
||||||||||||||||||||
Assets: |
||||||||||||||||||||
Foreign exchange contracts |
$ | — | 436 | — | 436 | 1,238 | ||||||||||||||
Cross currency interest rate swaps |
— | 126 | ** | — | 126 | 110 | ||||||||||||||
Total |
— | 562 | — | 562 | 1,348 | |||||||||||||||
Liabilities: |
||||||||||||||||||||
Foreign exchange contracts |
— | 608 | — | 608 | 1,298 | |||||||||||||||
Cross currency interest rate swaps |
— | 571 | *** | — | 571 | 1,033 | ||||||||||||||
Total |
— | 1,179 | — | 1,179 | 2,331 | |||||||||||||||
Derivatives not designated as hedging instruments: |
||||||||||||||||||||
Assets: |
||||||||||||||||||||
Foreign exchange contracts |
— | 33 | — | 33 | 84 | |||||||||||||||
Liabilities: |
||||||||||||||||||||
Foreign exchange contracts |
— | 40 | — | 40 | 47 | |||||||||||||||
Other investments |
$ | 1,134 | — | — | 1,134 | 41 | ||||||||||||||
| * | 2008 assets and liabilities are all classified as Level 2 with the exception of other investments of $41 million which are classified as Level 1. | |
| ** | Includes $119 million of non-current assets. | |
| *** | Includes $517 million of non-current liabilities. |
See Notes 2 and 7 for financial assets and liabilities held at
carrying amount on the Consolidated Balance Sheet.
45
Table of Contents
JOHNSON & JOHNSON
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
7. Borrowings
The components of long-term debt are as follows:
| Effective | Effective | |||||||||||||||
| (Dollars in Millions) | 2009 | Rate % | 2008 | Rate % | ||||||||||||
6.625% Notes due 2009 |
— | — | 199 | 6.80 | ||||||||||||
5.15% Debentures due 2012 |
$ | 599 | 5.18 | % | 599 | 5.18 | ||||||||||
3.80% Debentures due 2013 |
500 | 3.82 | 500 | 3.82 | ||||||||||||
5.55% Debentures due 2017 |
1,000 | 5.55 | 1,000 | 5.55 | ||||||||||||
5.15% Debentures due 2018 |
898 | 5.15 | 898 | 5.15 | ||||||||||||
4.75% Notes due 2019 (1B Euro 1.4382)(2)/(1B Euro 1.4000)(3) |
1,429 | (2) | 5.35 | 1,390 | (3) | 5.35 | ||||||||||
3% Zero Coupon Convertible Subordinated Debentures due 2020 |
188 | 3.00 | 183 | 3.00 | ||||||||||||
6.73% Debentures due 2023 |
250 | 6.73 | 250 | 6.73 | ||||||||||||
5.50% Notes due 2024 (500MM GBP1.6189)(2)/(500MM GBP1.4759)(3) |
803 | (2) | 5.71 | 731 | (3) | 5.71 | ||||||||||
6.95% Notes due 2029 |
294 | 7.14 | 294 | 7.14 | ||||||||||||
4.95% Debenture due 2033 |
500 | 4.95 | 500 | 4.95 | ||||||||||||
5.95% Notes due 2037 |
995 | 5.99 | 995 | 5.99 | ||||||||||||
5.86% Debentures due 2038 |
700 | 5.86 | 700 | 5.86 | ||||||||||||
Other (Includes Industrial Revenue Bonds) |
101 | 102 | ||||||||||||||
| 8,257 | (4) | 5.42 | (1) | 8,341 | (4) | 5.46 | (1) | |||||||||
Less current portion |
34 | 221 | ||||||||||||||
| $ | 8,223 | 8,120 | ||||||||||||||
| (1) | Weighted average effective rate. | |
| (2) | Translation rate at January 3, 2010. | |
| (3) | Translation rate at December 28, 2008. | |
| (4) | The excess of the fair value over the carrying value of debt was $0.8 billion in 2009 and $1.4 billion in 2008. |
Fair value of the non-current debt was estimated using market prices, which were corroborated
by quoted broker prices in active markets.
The Company has access to substantial sources of funds at numerous banks worldwide. In
September 2009, the Company secured a new 364-day Credit Facility. Total credit available to the
Company approximates $10 billion which expires September 23, 2010. Interest charged on borrowings
under the credit line agreements is based on either bids provided by banks, the prime rate or
London Interbank Offered Rates (LIBOR), plus applicable margins. Commitment fees under the
agreements are not material.
On July 28, 2000, ALZA Corporation, a subsidiary of the Company, completed a private offering
of the 3% Zero Coupon Convertible Subordinated Debentures, which were issued at a price of $551.26
per $1,000 principal amount at maturity. Under the terms of the 3% Debentures, holders are entitled
to convert their debentures into approximately 15.0 million shares of Johnson & Johnson stock at a
price of $40.102 per share. Approximately 11.4 million shares have been issued as of January 3,
2010, due to voluntary conversions by note holders. At the option of the holder, the 3% Debentures
may be repurchased by the Company on July 28, 2013, at a purchase price equal to the issue price
plus accreted original issue discount to such purchase date. The Company, at its option, may also
redeem any or all of the 3% Debentures after July 28, 2003 at the issue price plus accreted
original issue discount.
Throughout 2009 the Company continued to have access to liquidity through the commercial paper
market. Short-term borrowings and the current portion of long-term debt amounted to approximately
$6.3 billion at the end of 2009, of which $5.8 billion was borrowed under the Commercial Paper
Program. The remainder represents principally local borrowing by international subsidiaries.
The Company filed a shelf registration with the Securities and Exchange Commission that became
effective March 11, 2008 which enables the Company to issue an unlimited aggregate principal amount
in debt securities and warrants to purchase debt securities.
Aggregate maturities of long-term obligations commencing in 2009 are:
| (Dollars in Millions) | After | |||||||||
| 2010 | 2011 | 2012 | 2013 | 2014 | 2014 | |||||
$34 |
35 | 615 | 507 | 9 | 7,057 | |||||
8. Income Taxes
The provision for taxes on income consists of:
| (Dollars in Millions) | 2009 | 2008 | 2007 | |||||||||
Currently payable: |
||||||||||||
U.S. taxes |
$ | 2,410 | 2,334 | 2,990 | ||||||||
International taxes |
1,515 | 1,624 | 1,479 | |||||||||
| 3,925 | 3,958 | 4,469 | ||||||||||
Deferred: |
||||||||||||
U.S. taxes |
187 | 126 | (722 | ) | ||||||||
International taxes |
(623 | ) | (104 | ) | (1,040 | ) | ||||||
| (436 | ) | 22 | (1,762 | ) | ||||||||
| $ | 3,489 | 3,980 | 2,707 | |||||||||
A comparison of income tax expense at the U.S. statutory rate of 35% in 2009, 2008 and 2007, to
the Company’s effective tax rate is as follows:
| (Dollars in Millions) | 2009 | 2008 | 2007 | |||||||||
U.S. |
$ | 7,141 | 6,579 | 5,237 | ||||||||
International |
8,614 | 10,350 | 8,046 | |||||||||
Earnings before taxes on income: |
$ | 15,755 | 16,929 | 13,283 | ||||||||
Tax rates: |
||||||||||||
U.S. statutory rate |
35.0 | % | 35.0 | 35.0 | ||||||||
Ireland and Puerto Rico operations |
(5.1 | ) | (6.8 | ) | (8.8 | ) | ||||||
Research and orphan drug tax credits |
(0.6 | ) | (0.6 | ) | (0.8 | ) | ||||||
U.S. state and local |
1.8 | 1.6 | 2.1 | |||||||||
International subsidiaries excluding Ireland |
(6.7 | ) | (5.6 | ) | (7.3 | ) | ||||||
U.S. manufacturing deduction |
(0.4 | ) | (0.4 | ) | (0.3 | ) | ||||||
In-process research and development (IPR&D) |
0.0 | 0.4 | 2.1 | |||||||||
U.S. Tax international income |
(1.6 | ) | (0.5 | ) | (1.9 | ) | ||||||
All other |
(0.3 | ) | 0.4 | 0.3 | ||||||||
Effective tax rate |
22.1 | % | 23.5 | 20.4 | ||||||||
The Company has subsidiaries manufacturing in Ireland under an incentive tax rate. In addition,
the Company has subsidiaries operating in Puerto Rico under various tax incentive grants. The
decrease in the 2009 tax rate was primarily due to increases in taxable income in lower tax
jurisdictions relative to taxable income in higher tax jurisdictions. The increase in the 2008 tax
rate was mainly attributed to increases in taxable income in higher tax jurisdictions relative to
taxable income in lower jurisdictions, as well as a business restructuring of certain international
subsidiaries in 2007, resulting in a one-time benefit of $267 million, which reduced the 2007
effective tax rate by 2%.
46
Table of Contents
JOHNSON & JOHNSON
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Temporary differences and carry forwards for 2009 and 2008 are as follows:
| 2009 | 2008 | |||||||||||||||
| Deferred Tax | Deferred Tax | |||||||||||||||
| (Dollars in Millions) | Asset | Liability | Asset | Liability | ||||||||||||
Employee related obligations |
$ | 2,153 | 2,615 | |||||||||||||
Stock based compensation |
1,291 | 1,296 | ||||||||||||||
Depreciation |
(661 | ) | (523 | ) | ||||||||||||
Non-deductible intangibles |
(2,377 | ) | (1,791 | ) | ||||||||||||
International R&D capitalized for tax |
1,989 | 1,914 | ||||||||||||||
Reserves & liabilities |
1,014 | 688 | ||||||||||||||
Income reported for tax purposes |
648 | 629 | ||||||||||||||
Net operating loss carryforward international |
615 | 393 | ||||||||||||||
Miscellaneous international |
1,474 | (110 | ) | 964 | (251 | ) | ||||||||||
Miscellaneous U.S. |
799 | 1,828 | ||||||||||||||
Total deferred income taxes |
$ | 9,983 | (3,148 | ) | 10,327 | (2,565 | ) | |||||||||
The difference between the net deferred tax on income per the balance sheet and the net
deferred tax above is included in taxes on income on the balance sheet. The 2009 and 2008 deferred
tax Miscellaneous U.S. includes current year tax receivables. The Company has a wholly-owned
international subsidiary which has cumulative net losses. The Company believes that it is more
likely than not that the subsidiary will realize future taxable income sufficient to utilize these
deferred tax assets.
The following table summarizes the activity related to unrecognized tax benefits:
| (Dollars in Millions) | 2009 | 2008 | 2007 | |||||||||
Beginning of year |
$ | 1,978 | 1,653 | 1,262 | ||||||||
Increases related to current year tax positions |
555 | 545 | 487 | |||||||||
Increases related to prior period tax positions |
203 | 87 | 77 | |||||||||
Decreases related to prior period tax positions |
(163 | ) | (142 | ) | (117 | ) | ||||||
Settlements |
(87 | ) | (137 | ) | (14 | ) | ||||||
Lapse of statute of limitations |
(83 | ) | (28 | ) | (42 | ) | ||||||
End of year |
$ | 2,403 | 1,978 | 1,653 | ||||||||
The Company had $2.4 billion and $2.0 billion of unrecognized tax benefits, as of January 3,
2010 and December 28, 2008, respectively. All of the unrecognized tax benefits of $2.4 billion at
January 3, 2010, if recognized, would affect the Company’s annual effective tax rate. The Company
conducts business and files tax returns in numerous countries and currently has tax audits in
progress with a number of tax authorities. The U.S. Internal Revenue Service (IRS) has completed
its audit for the tax years through 2002. In other major jurisdictions where the Company conducts
business, the years remain open generally back to the year 2002 with some jurisdictions remaining
open as far back as 1995. The Company does not expect that the total amount of unrecognized tax
benefits will significantly change over the next twelve months. The Company believes that it is
possible that within the next twelve months, the IRS may complete its audit of the tax years
2003-2005. The close of the audit may result in the reduction of unrecognized tax benefits. The
Company is not able to provide a reasonably reliable estimate of the timing of any other future tax
payments relating to uncertain tax positions.
The Company classifies liabilities for unrecognized tax benefits and related interest and
penalties as long-term liabilities. Interest expense and penalties related to unrecognized tax
benefits are classified as income tax expense. During the fiscal year ended January 3, 2010, the
Company recognized $85 million of interest expense and $30 million of interest income with an
after-tax impact of $36 million expense. For the fiscal year ended December 28, 2008, the Company
recognized $106 million of interest expense with an after-tax impact of $69 million. For the fiscal
year ended December 30, 2007, the Company recognized $58 million of interest expense and $42
million of interest income with an after-tax impact of $10 million expense. The total amount of
accrued interest was $309 million and $227 million in 2009 and 2008, respectively.
9. Employee Related Obligations
At the end of 2009 and 2008, employee related obligations recorded on the Consolidated Balance
Sheet were:
| (Dollars in Millions) | 2009 | 2008 | ||||||
Pension benefits |
$ | 2,792 | 4,382 | |||||
Postretirement benefits |
2,245 | 2,217 | ||||||
Postemployment benefits |
1,504 | 870 | ||||||
Deferred compensation |
790 | 772 | ||||||
Total employee obligations |
7,331 | 8,241 | ||||||
Less current benefits payable |
562 | 450 | ||||||
Employee related obligations — long-term |
$ | 6,769 | 7,791 | |||||
Prepaid employee related obligations of $266 million and $136 million for 2009 and 2008,
respectively, are included in other assets on the consolidated balance sheet.
10. Pensions and Other Benefit Plans
The Company sponsors various retirement and pension plans, including defined benefit, defined contribution and termination indemnity plans, which cover most employees worldwide. The Company also provides postretirement benefits, primarily health care, to all U.S. retired employees and their dependents.
The Company sponsors various retirement and pension plans, including defined benefit, defined contribution and termination indemnity plans, which cover most employees worldwide. The Company also provides postretirement benefits, primarily health care, to all U.S. retired employees and their dependents.
Many international employees are covered by government-sponsored programs and the cost to the
Company is not significant.
Retirement plan benefits are primarily based on the employee’s compensation during the last
three to five years before retirement and the number of years of service. International
subsidiaries have plans under which funds are deposited with trustees, annuities are purchased
under group contracts, or reserves are provided.
The Company does not fund retiree health care benefits in advance and has the right to modify
these plans in the future.
The Company uses the date of its consolidated financial statements (January 3, 2010 and
December 28, 2008, respectively) as the measurement date for all U.S. and international retirement
and other benefit plans.
In accordance with U.S. GAAP the Company has adopted the recent standards related to
employers’ accounting for defined benefit pension and other postretirement plans.
47
Table of Contents
JOHNSON & JOHNSON
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Net periodic benefit costs for the Company’s defined benefit retirement plans and
other benefit plans for 2009, 2008 and 2007 include the following components:
| Retirement Plans | Other Benefit Plans | |||||||||||||||||||||||
| (Dollars in Millions) | 2009 | 2008 | 2007 | 2009 | 2008 | 2007 | ||||||||||||||||||
Service cost |
$ | 511 | 545 | 597 | $ | 137 | 142 | 140 | ||||||||||||||||
Interest cost |
746 | 701 | 656 | 174 | 166 | 149 | ||||||||||||||||||
Expected return on plan assets |
(934 | ) | (876 | ) | (809 | ) | (1 | ) | (2 | ) | (2 | ) | ||||||||||||
Amortization of prior service cost |
13 | 10 | 10 | (5 | ) | (4 | ) | (7 | ) | |||||||||||||||
Amortization of net transition asset |
1 | 2 | 1 | — | — | — | ||||||||||||||||||
Recognized actuarial losses |
155 | 62 | 186 | 55 | 64 | 66 | ||||||||||||||||||
Curtailments and settlements |
(11 | ) | 7 | 5 | (1 | ) | — | — | ||||||||||||||||
Net periodic benefit cost |
$ | 481 | 451 | 646 | $ | 359 | 366 | 346 | ||||||||||||||||
The net periodic benefit cost attributable to U.S. retirement plans was $286 million, $220
million and $379 million in 2009, 2008 and 2007, respectively.
Amounts expected to be recognized in net periodic benefit cost in the coming year for the
Company’s defined benefit retirement plans and other postretirement plans:
| (Dollars in Millions) | ||||
Amortization of net transition obligation |
$ | 1 | ||
Amortization of net actuarial losses |
296 | |||
Amortization of prior service cost |
5 | |||
Unrecognized gains and losses for the U.S. pension plans are amortized over the average
remaining future service for each plan. For plans with no active employees, they are amortized over
the average life expectancy. The amortization of gains and losses for the other U.S. benefit plans
is determined by using a 10% corridor of the greater of the market value of assets or the projected
benefit obligation. Total unamortized gains and losses in excess of the corridor are amortized over
the average remaining future service.
Prior service costs/benefits for the U.S. pension plans are amortized over the remaining
future service of plan participants at the time of the plan amendment. Prior service cost/benefit
for the other U.S. benefit plans is amortized over the average remaining service to full
eligibility age of plan participants at the time of the plan amendment.
The weighted-average assumptions in the following table represent the rates used to develop
the actuarial present value of projected benefit obligation for the year listed and also the net
periodic benefit cost for the following year.
| Retirement Plans | Other Benefit Plans | |||||||||||||||||||||||
| (Dollars in Millions) | 2009 | 2008 | 2007 | 2009 | 2008 | 2007 | ||||||||||||||||||
U.S. Benefit Plans |
||||||||||||||||||||||||
Discount rate |
6.50 | % | 6.50 | 6.50 | 6.50 | % | 6.50 | 6.50 | ||||||||||||||||
Expected long-term rate of return on plan assets |
9.00 | 9.00 | 9.00 | 9.00 | 9.00 | 9.00 | ||||||||||||||||||
Rate of increase in compensation levels |
4.50 | 4.50 | 4.50 | 4.50 | 4.50 | 4.50 | ||||||||||||||||||
International Benefit Plans |
||||||||||||||||||||||||
Discount rate |
5.75 | % | 6.00 | 5.50 | 6.75 | % | 7.25 | 6.50 | ||||||||||||||||
Expected long-term rate of return on plan assets |
8.00 | 8.00 | 8.25 | — | — | — | ||||||||||||||||||
Rate of increase in compensation levels |
4.00 | 4.00 | 4.00 | 4.75 | 4.50 | 4.50 | ||||||||||||||||||
The Company’s discount rates are determined by considering current yield curves representing
high quality, long-term fixed income instruments. The resulting discount rates are consistent with
the duration of plan liabilities.
The expected long-term rate of return on plan assets assumption is determined using a building
block approach, considering historical averages and real returns of each asset class. In certain
countries, where historical returns are not meaningful, consideration is given to local market
expectations of long-term returns.
The following table displays the assumed health care cost trend rates, for all individuals:
| Health Care Plans | 2009 | 2008 | ||||||
Health care cost trend rate assumed for next year |
8.00 | % | 9.00 | |||||
Rate to which the cost trend rate is assumed to decline (ultimate trend) |
5.00 | % | 5.00 | |||||
Year the rate reaches the ultimate trend rate |
2017 | 2015 | ||||||
A one-percentage-point change in assumed health care cost trend rates would have the following
effect:
| One-Percentage- | One-Percentage- | |||||||
| (Dollars in Millions) | Point Increase | Point Decrease | ||||||
Health Care Plans |
||||||||
Total interest and service cost |
$ | 34 | $ | (28 | ) | |||
Postretirement benefit obligation |
315 | (254 | ) | |||||
48
Table of Contents
JOHNSON & JOHNSON
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
The following table sets forth information related to the benefit obligation and the fair value
of plan assets at year-end 2009 and 2008 for the Company’s defined benefit retirement plans and
other postretirement plans:
| Retirement Plans | Other Benefit Plans | |||||||||||||||
| (Dollars in Millions) | 2009 | 2008 | 2009 | 2008 | ||||||||||||
Change in Benefit Obligation |
||||||||||||||||
Projected benefit obligation — beginning of year |
$ | 11,923 | 12,002 | $ | 2,765 | 2,721 | ||||||||||
Service cost |
511 | 545 | 137 | 142 | ||||||||||||
Interest cost |
746 | 701 | 174 | 166 | ||||||||||||
Plan participant contributions |
50 | 60 | — | — | ||||||||||||
Amendments |
3 | 10 | — | 1 | ||||||||||||
Actuarial losses (gains) |
412 | (318 | ) | 51 | (124 | ) | ||||||||||
Divestitures & acquisitions |
15 | — | 13 | (2 | ) | |||||||||||
Curtailments & settlements & restructuring |
(3 | ) | (2 | ) | 748 | — | ||||||||||
Benefits paid from plan |
(570 | ) | (535 | ) | (313 | ) | (122 | ) | ||||||||
Effect of exchange rates |
362 | (540 | ) | 15 | (17 | ) | ||||||||||
Projected benefit obligation — end of year* |
$ | 13,449 | 11,923 | $ | 3,590 | 2,765 | ||||||||||
Change in Plan Assets |
||||||||||||||||
Plan assets at fair value — beginning of year |
$ | 7,677 | 10,469 | $ | 17 | 29 | ||||||||||
Actual return (loss) on plan assets |
2,048 | (2,787 | ) | 4 | (7 | ) | ||||||||||
Company contributions |
1,354 | 978 | 308 | 117 | ||||||||||||
Plan participant contributions |
50 | 60 | — | — | ||||||||||||
Settlements |
— | (1 | ) | — | — | |||||||||||
Benefits paid from plan assets |
(570 | ) | (535 | ) | (313 | ) | (122 | ) | ||||||||
Effect of exchange rates |
364 | (507 | ) | — | — | |||||||||||
Plan assets at fair value — end of year |
$ | 10,923 | 7,677 | $ | 16 | 17 | ||||||||||
Funded status at — end of year* |
$ | (2,526 | ) | (4,246 | ) | $ | (3,574 | ) | (2,748 | ) | ||||||
Amounts Recognized in the Company’s Balance Sheet consist of the following: |
||||||||||||||||
Non-current assets |
$ | 266 | 136 | $ | — | — | ||||||||||
Current liabilities |
(53 | ) | (45 | ) | (484 | ) | (212 | ) | ||||||||
Non-current liabilities |
(2,739 | ) | (4,337 | ) | (3,090 | ) | (2,536 | ) | ||||||||
Total recognized in the consolidated balance sheet — end of year |
$ | (2,526 | ) | (4,246 | ) | $ | (3,574 | ) | (2,748 | ) | ||||||
Amounts Recognized in Accumulated Other Comprehensive Income consist of the
following: |
||||||||||||||||
Net actuarial loss |
$ | 3,415 | 4,209 | $ | 924 | 1,006 | ||||||||||
Prior service cost (credit) |
47 | 43 | (23 | ) | (29 | ) | ||||||||||
Unrecognized net transition obligation |
5 | 6 | — | — | ||||||||||||
Total before tax effects |
$ | 3,467 | 4,258 | $ | 901 | 977 | ||||||||||
Accumulated Benefit Obligations — end of year* |
$ | 11,687 | 10,357 | |||||||||||||
Changes in Plan Assets and Benefit Obligations Recognized in Other Comprehensive
Income |
||||||||||||||||
Net periodic benefit cost |
$ | 481 | 451 | $ | 359 | 366 | ||||||||||
Net actuarial (gain) loss |
(704 | ) | 3,344 | 48 | 60 | |||||||||||
Amortization of net actuarial loss |
(134 | ) | (68 | ) | (131 | ) | (65 | ) | ||||||||
Prior service cost |
3 | 10 | — | 1 | ||||||||||||
Amortization of prior service cost |
(13 | ) | (11 | ) | 5 | 6 | ||||||||||
Effect of exchange rates |
57 | (102 | ) | 2 | (1 | ) | ||||||||||
Total recognized in other comprehensive income, before tax |
$ | (791 | ) | 3,173 | $ | (76 | ) | 1 | ||||||||
Total recognized in net periodic benefit cost and other comprehensive income |
$ | (310 | ) | 3,624 | $ | 283 | 367 | |||||||||
| * | The Company does not fund certain plans, as funding is not required. $1.2 billion of the projected benefit obligation and $1.2 billion of the underfunded status for each of the fiscal years 2009 and 2008 relates to the unfunded pension plans. $1.0 billion and $0.9 billion of the accumulated benefit obligation for the fiscal years 2009 and 2008, respectively, relate to these unfunded pension plans. |
Plans with accumulated benefit obligations in excess of plan assets consist of the following:
| Retirement Plans | ||||||||
| (Dollars in Millions) | 2009 | 2008 | ||||||
Accumulated benefit obligation |
$ | (4,065 | ) | (9,885 | ) | |||
Projected benefit obligation |
(4,663 | ) | (11,379 | ) | ||||
Plan assets at fair value |
2,564 | 7,021 | ||||||
49
Table of Contents
JOHNSON & JOHNSON
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
The following table displays the projected future benefit payments from the Company’s
retirement and other benefit plans:
| (Dollars in Millions) | 2010 | 2011 | 2012 | 2013 | 2014 | 2015-2019 | ||||||||||||||||||
Projected future benefit payments |
||||||||||||||||||||||||
Retirement plans |
$ | 558 | 553 | 582 | 604 | 636 | 3,925 | |||||||||||||||||
Other benefit plans — gross |
$ | 209 | 198 | 196 | 198 | 197 | 995 | |||||||||||||||||
Medicare rebates |
(9 | ) | — | — | — | — | — | |||||||||||||||||
Other benefit plans — net |
$ | 200 | 198 | 196 | 198 | 197 | 995 | |||||||||||||||||
In 2009, the Company contributed $839 million and $515 million to its U.S. and international
pension plans, respectively. In addition, the Company funded $500 million to its U.S. plans in the
first month of 2010.
In 2006, Congress passed the Pension Protection Act of 2006. The Act amended the Employee
Retirement Income Security Act (ERISA) for plan years beginning after 2007 and established new
minimum funding standards for U.S. employer defined benefit plans.
The Company plans to continue to fund its U.S. defined benefit plans to comply with the Act.
International plans are funded in accordance with local regulations. Additional discretionary
contributions are made when deemed appropriate to meet the long-term obligations of the plans. For
certain plans, funding is not a common practice, as funding provides no economic benefit.
Consequently the Company has several pension plans that are not funded.
The following table displays the projected future minimum contributions to the Company’s U.S.
and international unfunded retirement plans. These amounts do not include any discretionary
contributions that the Company may elect to make in the future.
| (Dollars in Millions) | 2010 | 2011 | 2012 | 2013 | 2014 | 2015-2019 | ||||||||||||||||||
Projected future contributions |
||||||||||||||||||||||||
Unfunded U.S. retirement plans |
$ | 34 | 36 | 38 | 40 | 44 | 288 | |||||||||||||||||
Unfunded International retirement plans |
$ | 32 | 29 | 31 | 33 | 32 | 186 | |||||||||||||||||
Each pension plan is overseen by a local committee or board that is responsible for the overall
administration and investment of the pension plans. In determining investment policies, strategies
and goals, each committee or board considers factors including local pension rules and regulations;
local tax regulations; availability of investment vehicles (separate accounts, commingled accounts,
insurance funds, etc.); funded status of the plans; ratio of actives to retirees; duration of
liabilities; and other relevant factors including diversification, liquidity of local markets and
liquidity of base currency. A majority of the Company’s pension funds are open to new entrants and
are expected to be on-going plans. Permitted investments are primarily liquid and/or listed, with
little reliance on illiquid and non-traditional investments such as hedge funds. An asset
allocation of 75% equities and 25% fixed income is generally pursued unless local regulations and
illiquidity require otherwise.
The Company’s retirement plan asset allocation at the end of 2009 and 2008 and target
allocations for 2010 are as follows:
| Percent of | Target | |||||||||||
| Plan Assets | Allocation | |||||||||||
| 2009 | 2008 | 2010 | ||||||||||
U.S. Retirement Plans |
||||||||||||
Equity securities |
76 | % | 70 | % | 75 | % | ||||||
Debt securities |
24 | 30 | 25 | |||||||||
Total plan assets |
100 | % | 100 | % | 100 | % | ||||||
International Retirement Plans |
||||||||||||
Equity securities |
65 | % | 61 | % | 65 | % | ||||||
Debt securities |
34 | 38 | 34 | |||||||||
Real estate and other |
1 | 1 | 1 | |||||||||
Total plan assets |
100 | % | 100 | % | 100 | % | ||||||
The Company’s other benefit plans are unfunded except for U.S. life insurance contract assets
of $16 million and $17 million at January 3, 2010 and December 28, 2008, respectively.
The fair value of Johnson & Johnson common stock directly held in plan assets was $469 million
(4.3% of total plan assets) at January 3, 2010 and $416 million (5.4% of total plan assets) at
December 28, 2008.
DETERMINATION OF FAIR VALUE
The Plan has an established and well-documented process for determining fair values. Fair value
is based upon quoted market prices, where available. If listed prices or quotes are not available,
fair value is based upon models that primarily use, as inputs, market-based or independently
sourced market parameters, including yield curves, interest rates, volatilities, equity or debt
prices, foreign exchange rates and credit curves.
While the Plan believes its valuation methods are appropriate and consistent with other market
participants, the use of different methodologies or assumptions to determine the fair value of
certain financial instruments could result in a different estimate of fair value at the reporting
date.
50
Table of Contents
JOHNSON & JOHNSON
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
VALUATION HIERARCHY
The authoritative literature establishes a three-level hierarchy to prioritize the inputs used
in measuring fair value. The levels within the hierarchy are described in the table below with
Level 1 having the highest priority and Level 3 having the lowest.
A financial instrument’s categorization within the valuation hierarchy is based upon the lowest
level of input that is significant to the fair value measurement.
Following is a description of the valuation methodologies used for the investments measured at
fair value.
• Short-term investments — Cash and quoted short-term instruments are valued at the closing
price or the amount held on deposit by the custodian bank. Other investments are through investment
vehicles valued using the Net Asset Value (NAV) provided by the administrator of the fund. The NAV
is based on the value of the underlying assets owned by the fund, minus its liabilities, and then
divided by the number of shares outstanding. The NAV is a quoted price in a market that is not
active and classified as Level 2.
• Government and agency securities — A limited number of these investments are valued at the
closing price reported on the major market on which the individual securities are traded. Where
quoted prices are available in an active market, the investments are classified within Level 1 of
the valuation hierarchy. If quoted market prices are not available for the specific security, then
fair values are
estimated by using pricing models, quoted prices of securities with similar characteristics or
discounted cash flows. When quoted market prices for a security are not available in an active
market, they are classified as Level 2.
• Debt instruments — A limited number of these investments are valued at the closing price
reported on the major market on which the individual securities are traded. Where quoted prices are
available in an active market, the investments are classified as Level 1.
If quoted market prices
are not available for the specific security, then fair values are estimated by using pricing
models, quoted prices of securities with similar characteristics or discounted cash flows and are
classified as Level 2. Level 3 debt instruments are priced based on unobservable inputs.
• Equity securities — Common stocks are valued at the closing price reported on the major
market on which the individual securities are traded. Substantially all common stock is classified
within Level 1 of the valuation hierarchy.
• Commingled funds — The investments are public investment vehicles valued using the NAV
provided by the fund administrator. The NAV is based on the value of the underlying assets owned by
the fund, minus its liabilities, and then divided by the number of shares outstanding. Assets in
the Level 2 category have a quoted market price in a market that is not active.
• Insurance contracts — The instruments are issued by insurance companies. The fair value is
based on negotiated value and the underlying investments held in separate account portfolios as
well as considering the credit worthiness of the issuer. The underlying investments are government,
asset-backed and fixed income securities. In general, insurance contracts are classified as Level 3
as there are no quoted prices nor other observable inputs for pricing.
• Other assets — Other assets are represented primarily by limited partnerships and real
estate investments, as well as commercial loans and commercial mortgages that are not classified as
corporate debt. Other assets that are exchange listed and actively traded are classified as Level 1
while inactively traded assets are classified as Level 2. Most limited partnerships represent
investments in private equity and similar funds that are valued by the general partners. These, as
well as any other assets valued using unobservable inputs, are classified as Level 3.
The following table sets forth the trust investments measured at fair value as of January 3, 2010:
| Quoted Prices | Significant | |||||||||||||||
| in Active | Other | Significant | ||||||||||||||
| Markets for | Observable | Unobservable | ||||||||||||||
| Identical Assets | Inputs | Inputs | ||||||||||||||
| (Dollars in Millions) | (Level 1) | (Level 2) | (Level 3) | Total Assets | ||||||||||||
Short-term investment funds |
$ | 91 | 358 | — | 449 | |||||||||||
Government and agency securities |
— | 1,165 | — | 1,165 | ||||||||||||
Debt instruments |
3 | 1,145 | 5 | 1,153 | ||||||||||||
Equity securities |
5,068 | 58 | 15 | 5,141 | ||||||||||||
Commingled funds |
— | 2,673 | 26 | 2,699 | ||||||||||||
Insurance contracts |
— | — | 32 | 32 | ||||||||||||
Other assets |
31 | 171 | 82 | 284 | ||||||||||||
Trust investments at fair value |
$ | 5,193 | 5,570 | 160 | 10,923 | |||||||||||
LEVEL 3 GAINS AND LOSSES
The table below sets forth a summary of changes in the fair value of the Plan’s Level 3 assets
for the year ended January 3, 2010:
| Debt | Equity | Commingled | Insurance | Other | Total | |||||||||||||||||||
| (Dollars in Millions) | Instruments | Securities | Funds | Contracts | Assets | Level 3 | ||||||||||||||||||
Balance December 28, 2008 |
$ | 7 | 15 | 15 | 29 | 85 | 151 | |||||||||||||||||
Realized gains (losses) |
— | — | — | 3 | — | 3 | ||||||||||||||||||
Unrealized gains (losses) |
2 | (2 | ) | (2 | ) | — | (3 | ) | (5 | ) | ||||||||||||||
Purchases, sales, issuances and settlements, net |
(4 | ) | 2 | 13 | — | — | 11 | |||||||||||||||||
Balance January 3, 2010 |
$ | 5 | 15 | 26 | 32 | 82 | 160 | |||||||||||||||||
51
Table of Contents
JOHNSON & JOHNSON
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
11. Savings Plan
The Company has voluntary 401 (k) savings plans designed to enhance the existing retirement
programs covering eligible employees. The Company matches a percentage of each employee’s
contributions consistent with the provisions of the plan for which he/she is eligible. Total
Company matching contributions to the plans were $163 million, $166 million and $169 million in
2009, 2008 and 2007, respectively.
12. Capital and Treasury Stock
Changes in treasury stock were:
| (Amounts in Millions Except Treasury Stock | Treasury Stock | |||||||
| Number of Shares in Thousands) | Shares | Amount | ||||||
Balance at December 31, 2006 |
226,612 | $ | 10,974 | |||||
Employee compensation and stock option plans |
(33,296 | ) | (2,180 | ) | ||||
Conversion of subordinated debentures |
(194 | ) | (13 | ) | ||||
Repurchase of common stock |
86,498 | 5,607 | ||||||
Balance at December 30, 2007 |
279,620 | 14,388 | ||||||
Employee compensation and stock option plans |
(29,906 | ) | (2,005 | ) | ||||
Conversion of subordinated debentures |
(19 | ) | (1 | ) | ||||
Repurchase of common stock |
100,970 | 6,651 | ||||||
Balance at December 28, 2008 |
350,665 | 19,033 | ||||||
Employee compensation and stock option plans |
(22,161 | ) | (1,377 | ) | ||||
Conversion of subordinated debentures |
(96 | ) | (6 | ) | ||||
Repurchase of common stock |
37,114 | 2,130 | ||||||
Balance at January 3, 2010 |
365,522 | $ | 19,780 | |||||
Aggregate shares of Common Stock issued were approximately 3,120 million shares at the end of
2009, 2008 and 2007.
Cash dividends paid were $1.930 per share in 2009, compared with dividends of $1.795 per share
in 2008 and $1.620 per share in 2007.
13. Accumulated Other Comprehensive Income
Components of other comprehensive income/(loss) consist of the following:
| Total | ||||||||||||||||||||
| Gains/ | Accumulated | |||||||||||||||||||
| Foreign | Gains/ | (Losses) on | Other | |||||||||||||||||
| Currency | (Losses) on | Employee | Derivatives | Comprehensive | ||||||||||||||||
| (Dollars in Millions) | Translation | Securities | Benefit Plans | & Hedges | Income/(Loss) | |||||||||||||||
December 31, 2006 |
$ | (158 | ) | 61 | (2,030 | ) | 9 | (2,118 | ) | |||||||||||
2007 changes |
||||||||||||||||||||
Unrealized gain (loss) |
— | 28 | — | (78 | ) | |||||||||||||||
Net amount reclassed to net earnings |
— | (5 | ) | — | 24 | |||||||||||||||
Net 2007 changes |
786 | 23 | 670 | (54 | ) | 1,425 | ||||||||||||||
December 30, 2007 |
$ | 628 | 84 | (1,360 | ) | (45 | ) | (693 | ) | |||||||||||
2008 changes |
||||||||||||||||||||
Unrealized gain (loss) |
— | (32 | ) | — | 94 | |||||||||||||||
Net amount reclassed to net earnings |
— | (27 | ) | — | 72 | |||||||||||||||
Net 2008 changes |
(2,499 | ) | (59 | ) | (1,870 | ) | 166 | (4,262 | ) | |||||||||||
December 28, 2008 |
$ | (1,871 | ) | 25 | (3,230 | ) | 121 | (4,955 | ) | |||||||||||
2009 changes |
||||||||||||||||||||
Unrealized gain (loss) |
— | (52 | ) | — | 38 | |||||||||||||||
Net amount reclassed to net earnings |
— | (3 | ) | — | (14 | ) | ||||||||||||||
Net 2009 changes |
1,363 | (55 | ) | 565 | 24 | 1,897 | ||||||||||||||
January 3, 2010 |
$ | (508 | ) | (30 | ) | (2,665 | ) | 145 | (3,058 | ) | ||||||||||
The tax effect on the unrealized gains/(losses) on the equity securities was income of $14
million in 2009 and expense of $14 million and $46 million in 2008 and 2007, respectively. The tax
effect related to employee benefit plans was $302 million, $1,090 million and $349 million in 2009,
2008 and 2007, respectively. The tax effect on the gains/(losses) on derivatives and hedges was
expense of $78 million and $70 million in 2009 and 2008, respectively, and income of $24 million in
2007. See Note 6 for additional information relating to derivatives and hedging.
The currency translation adjustments are not adjusted for income taxes as they relate to
permanent investments in international subsidiaries.
52
Table of Contents
JOHNSON & JOHNSON
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
14. International Currency Translation
For translation of its subsidiaries operating in non-U.S. Dollar currencies, the Company has
determined that the local currencies of its international subsidiaries are the functional
currencies except those in highly inflationary economies, which are defined as those which have had
compound cumulative rates of inflation of 100% or more during the past three years, or where a
substantial portion of its cash flows are not in the local currency.
In consolidating international subsidiaries, balance sheet currency effects are recorded as a
component of accumulated other comprehensive income. This equity account includes the results of
translating all balance sheet assets and liabilities at current exchange rates, except for those
located in highly inflationary economies. The translation of balance sheet accounts for highly
inflationary economies are reflected in the operating results.
An analysis of the changes during 2009, 2008 and 2007 for foreign currency translation
adjustments is included in Note 13.
Net currency transaction and translation gains and losses included in other (income) expense
were losses of $210 million, $31 million and $23 million in 2009, 2008 and 2007, respectively.
15. Earnings Per Share
The following is a reconciliation of basic net earnings per share to diluted net earnings per
share for the fiscal years ended January 3, 2010, December 28, 2008 and December 30, 2007:
| (Shares in Millions Except Per Share Data) | 2009 | 2008 | 2007 | |||||||||
Basic net earnings per share |
$ | 4.45 | 4.62 | 3.67 | ||||||||
Average shares outstanding — basic |
2,759.5 | 2,802.5 | 2,882.9 | |||||||||
Potential shares exercisable under stock option plans |
118.0 | 179.0 | 178.6 | |||||||||
Less: shares repurchased under treasury stock method |
(92.0 | ) | (149.6 | ) | (154.5 | ) | ||||||
Convertible debt shares |
3.6 | 3.7 | 3.7 | |||||||||
Adjusted average shares outstanding — diluted |
2,789.1 | 2,835.6 | 2,910.7 | |||||||||
Diluted net earnings per share |
$ | 4.40 | 4.57 | 3.63 | ||||||||
The diluted net earnings per share calculation includes the dilutive effect of convertible debt
that is offset by the related reduction in interest expense of $4 million after-tax for years 2009,
2008 and 2007.
Diluted net earnings per share excludes 121 million, 59 million and 64 million shares
underlying stock options for 2009, 2008 and 2007, respectively, as the exercise price of these
options was greater than their average market value, which would result in an anti-dilutive effect
on diluted earnings per share.
16. Rental Expense and Lease Commitments
Rentals of space, vehicles, manufacturing equipment and office and data processing equipment
under operating leases were approximately $322 million in 2009, $309 million in 2008 and $302
million in 2007.
The approximate minimum rental payments required under operating leases that have initial or
remaining non-cancelable lease terms in excess of one year at January 3, 2010 are:
| (Dollars in Millions) | After | ||||||||||||||||||||||
| 2010 | 2011 | 2012 | 2013 | 2014 | 2014 | Total | |||||||||||||||||
| $178 | 150 | 128 | 103 | 87 | 94 | 740 | |||||||||||||||||
Commitments under capital leases are not significant.
17. Common Stock, Stock Option Plans and Stock Compensation Agreements
STOCK OPTIONS
At January 3, 2010, the Company had 11 stock-based compensation plans. The shares outstanding
are for contracts under the Company’s 1995 and 2000 Stock Option Plans, the 2005 Long-Term
Incentive Plan, the 1997 Non-Employee Director’s Plan and the ALZA, Inverness, and Scios Stock
Option Plans. During 2009, no options or restricted shares were granted under any of these plans
except under the 2005 Long-Term Incentive Plan.
The compensation cost that has been charged against income for these plans was $628 million,
$627 million and $698 million for 2009, 2008 and 2007, respectively. The total income tax benefit
recognized in the income statement for share-based compensation costs was $210 million, $210
million and $238 million for 2009, 2008 and 2007, respectively. Share-based compensation costs
capitalized as part of inventory were insignificant in all periods.
Stock options expire 10 years from the date of grant and vest over service periods that range
from six months to five years. All options are granted at the average of the high and low prices of
the Company’s common stock on the New York Stock Exchange on the date of grant. Under the 2005
Long-Term Incentive Plan, the Company may issue up to 260 million shares of common stock. Shares
available for future grants under the 2005 Long-Term Incentive Plan were 139.7 million at the end
of 2009.
The Company settles employee stock option exercises with treasury shares. Treasury shares are
replenished throughout the year for the number of shares used to settle employee stock option
exercises.
The fair value of each option award was estimated on the date of grant using the Black-Scholes
option valuation model that uses the assumptions noted in the following table. Expected volatility
represents a blended rate of 4-year daily historical average volatility rate, and a 5-week average
implied volatility rate based on at-the-money traded Johnson & Johnson options with a life of 2
years. Historical data is used to determine the expected life of the option. The risk-free rate was
based on the U.S. Treasury yield curve in effect at the time of grant.
53
Table of Contents
JOHNSON & JOHNSON
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
The average fair value of options granted was $8.35, $7.66, and $11.67 in 2009, 2008,
and 2007, respectively. The fair value was estimated based on the weighted average assumptions of:
| 2009 | 2008 | 2007 | |||||||||||
Risk-free rate |
2.71 | % | 2.97 | % | 4.78 | % | |||||||
Expected volatility |
19.5 | % | 15.0 | % | 14.7 | % | |||||||
Expected life |
6.0 | yrs | 6.0 | yrs | 6.0 | yrs | |||||||
Dividend yield |
3.30 | % | 2.90 | % | 2.50 | % | |||||||
A summary of option activity under the Plan as of January 3, 2010, December 28, 2008, and December
30, 2007 and changes during the years ending on those dates is presented below:
| Aggregate | ||||||||||||
| Weighted | Intrinsic | |||||||||||
| Outstanding | Average | Value | ||||||||||
| (Shares in Thousands) | Shares | Exercise Price | (Dollars in Millions) | |||||||||
Shares at December 31, 2006 |
242,927 | $ | 54.57 | $ | 2,788 | |||||||
Options granted |
26,789 | 65.61 | ||||||||||
Options exercised |
(33,224 | ) | 45.92 | |||||||||
Options canceled/forfeited |
(7,863 | ) | 63.00 | |||||||||
Shares at December 30, 2007 |
228,629 | 56.83 | $ | 2,411 | ||||||||
Options granted |
22,428 | 61.80 | ||||||||||
Options exercised |
(30,033 | ) | 50.27 | |||||||||
Options canceled/forfeited |
(5,525 | ) | 61.90 | |||||||||
Shares at December 28, 2008 |
215,499 | 58.14 | $ | 597 | ||||||||
Options granted |
21,576 | 58.32 | ||||||||||
Options exercised |
(18,225 | ) | 50.97 | |||||||||
Options canceled/forfeited |
(6,131 | ) | 61.85 | |||||||||
Shares at January 3, 2010 |
212,719 | $ | 58.66 | $ | 1,310 | |||||||
The total intrinsic value of options exercised was $184 million, $506 million, and $625 million in
2009, 2008 and 2007, respectively. The total unrecognized compensation cost was $612 million as of
January 3, 2010, $632 million as of December 28, 2008 and $652 million as of December 30, 2007. The
weighted average period for this cost to be recognized was 1.16 years, 1.06 years and 1.01 years
for 2009, 2008, and 2007, respectively.
The following table summarizes stock options outstanding and exercisable at January 3, 2010:
| Outstanding | Exercisable | |||||||||||||||||||
| (Shares in Thousands) | Average | Average | ||||||||||||||||||
| Exercise | Average | Exercise | Exercise | |||||||||||||||||
| Price Range | Options | Life | (1) | Price | Options | Price | ||||||||||||||
$ 7.33 - $28.09 |
104 | 1.5 | $ | 22.89 | 104 | $ | 22.89 | |||||||||||||
$31.27- $40.08 |
131 | 0.3 | 35.83 | 131 | 35.83 | |||||||||||||||
$41.26- $49.86 |
1,024 | 1.2 | 47.09 | 1,024 | 47.09 | |||||||||||||||
$50.52- $52.11 |
17,328 | 0.8 | 50.70 | 17,328 | 50.70 | |||||||||||||||
$52.13- $53.77 |
22,193 | 3.1 | 52.22 | 22,152 | 52.22 | |||||||||||||||
$53.93- $54.89 |
26,155 | 4.0 | 53.93 | 26,156 | 53.93 | |||||||||||||||
$55.01- $58.25 |
26,332 | 2.1 | 57.30 | 26,328 | 57.30 | |||||||||||||||
$58.33- $65.10 |
63,805 | 7.7 | 59.48 | 21,367 | 58.48 | |||||||||||||||
$65.62- $68.37 |
55,647 | 5.8 | 65.97 | 33,759 | 66.19 | |||||||||||||||
| 212,719 | 5.0 | $ | 58.66 | 148,349 | $ | 57.26 | ||||||||||||||
| (1) | Average contractual life remaining in years. |
Stock options exercisable at December 28, 2008 and December 30, 2007 were 144,962 at an average
price of $56.25 and an average life of 5.3 years and 137,310 at an average price of $52.33 and an
average life of 5.6 years, respectively.
RESTRICTED SHARE UNITS
The Company grants restricted share units with a vesting period of three years. The Company
settles employee stock issuance with treasury shares. Treasury shares are replenished throughout
the year for the number of shares used for employee stock issuances.
A summary of share activity under the Plan as of January 3, 2010:
| Outstanding | ||||
| (Shares in Thousands) | Shares | |||
Shares at December 31, 2006 |
6,885 | |||
Shares granted |
8,029 | |||
Shares issued |
(33 | ) | ||
Shares canceled/forfeited |
(1,220 | ) | ||
Shares at December 30, 2007 |
13,661 | |||
Shares granted |
10,105 | |||
Shares issued |
(40 | ) | ||
Shares canceled/forfeited |
(1,468 | ) | ||
Shares at December 28, 2008 |
22,258 | |||
Shares granted |
11,172 | |||
Shares issued |
(5,714 | ) | ||
Shares canceled/forfeited |
(1,392 | ) | ||
Shares at January 3, 2010 |
26,324 | |||
The average fair value of the restricted share units granted was $52.79, $56.70 and $60.86 in 2009,
2008 and 2007, respectively, using the fair market value at the date of grant. The fair value of
restricted share units was discounted for dividends, which are not paid on the restricted share
units during the vesting period. The fair value of restricted share units settled was $308.4
million, $2.5 million and $1.8 million in 2009, 2008 and 2007, respectively.
54
Table of Contents
JOHNSON & JOHNSON
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
18. Segments of Business(1) and Geographic Areas
| Sales to Customers(2) | ||||||||||||
| (Dollars in Millions) | 2009 | 2008 | 2007 | |||||||||
Consumer — |
||||||||||||
United States |
$ | 6,837 | 6,937 | 6,408 | ||||||||
International |
8,966 | 9,117 | 8,085 | |||||||||
Total |
15,803 | 16,054 | 14,493 | |||||||||
Pharmaceutical — |
||||||||||||
United States |
13,041 | 14,831 | 15,603 | |||||||||
International |
9,479 | 9,736 | 9,263 | |||||||||
Total |
22,520 | 24,567 | 24,866 | |||||||||
Medical Devices and Diagnostics — |
||||||||||||
United States |
11,011 | 10,541 | 10,433 | |||||||||
International |
12,563 | 12,585 | 11,303 | |||||||||
Total |
23,574 | 23,126 | 21,736 | |||||||||
Worldwide total |
$ | 61,897 | 63,747 | 61,095 | ||||||||
| Operating Profit | Identifiable Assets | |||||||||||||||||||||||
| (Dollars in Millions) | 2009(5) | 2008(6) | 2007(7) | 2009 | 2008 | 2007 | ||||||||||||||||||
Consumer |
$ | 2,475 | 2,674 | 2,277 | $ | 24,671 | 23,765 | 26,550 | ||||||||||||||||
Pharmaceutical |
6,413 | 7,605 | 6,540 | 21,460 | 19,544 | 19,780 | ||||||||||||||||||
Medical Devices and Diagnostics |
7,694 | 7,223 | 4,846 | 22,853 | 20,779 | 19,978 | ||||||||||||||||||
Total |
16,582 | 17,502 | 13,663 | 68,984 | 64,088 | 66,308 | ||||||||||||||||||
Less: Expense not allocated to segments(3) |
827 | 573 | 380 | |||||||||||||||||||||
General corporate(4) |
25,698 | 20,824 | 14,646 | |||||||||||||||||||||
Worldwide total |
$ | 15,755 | 16,929 | 13,283 | $ | 94,682 | 84,912 | 80,954 | ||||||||||||||||
| Additions to Property, | Depreciation and | |||||||||||||||||||||||
| Plant & Equipment | Amortization | |||||||||||||||||||||||
| (Dollars in Millions) | 2009 | 2008 | 2007 | 2009 | 2008 | 2007 | ||||||||||||||||||
Consumer |
$ | 439 | 499 | 504 | $ | 513 | 489 | 472 | ||||||||||||||||
Pharmaceutical |
535 | 920 | 1,137 | 922 | 986 | 1,033 | ||||||||||||||||||
Medical Devices and Diagnostics |
1,114 | 1,251 | 919 | 1,124 | 1,146 | 1,080 | ||||||||||||||||||
Segments total |
2,088 | 2,670 | 2,560 | 2,559 | 2,621 | 2,585 | ||||||||||||||||||
General corporate |
277 | 396 | 382 | 215 | 211 | 192 | ||||||||||||||||||
Worldwide total |
$ | 2,365 | 3,066 | 2,942 | $ | 2,774 | 2,832 | 2,777 | ||||||||||||||||
| Sales to Customers(2) | Long-Lived Assets(8) | |||||||||||||||||||||||
| (Dollars in Millions) | 2009 | 2008 | 2007 | 2009 | 2008 | 2007 | ||||||||||||||||||
United States |
$ | 30,889 | 32,309 | 32,444 | $ | 22,399 | 21,674 | 21,685 | ||||||||||||||||
Europe |
15,934 | 16,782 | 15,644 | 17,347 | 14,375 | 15,578 | ||||||||||||||||||
Western Hemisphere excluding U.S. |
5,156 | 5,173 | 4,681 | 3,540 | 3,328 | 3,722 | ||||||||||||||||||
Asia-Pacific, Africa |
9,918 | 9,483 | 8,326 | 1,868 | 1,898 | 1,261 | ||||||||||||||||||
Segments total |
61,897 | 63,747 | 61,095 | 45,154 | 41,275 | 42,246 | ||||||||||||||||||
General corporate |
790 | 785 | 702 | |||||||||||||||||||||
Other non long-lived assets |
48,738 | 42,852 | 38,006 | |||||||||||||||||||||
Worldwide total |
$ | 61,897 | 63,747 | 61,095 | $ | 94,682 | 84,912 | 80,954 | ||||||||||||||||
| (1) | See Note 1 for a description of the segments in which the Company operates. | |
| (2) | Export sales are not significant. In 2009, 2008 and 2007, the Company did not have a customer that represented 10% of total revenues. | |
| (3) | Amounts not allocated to segments include interest (income) expense, non-controlling interests and general corporate (income) expense. | |
| (4) | General corporate includes cash and marketable securities. | |
| (5) | Includes $1,186 million of restructuring expense, comprised of $369 million, $496 million, and $321 million for the Consumer, Pharmaceutical, and Medical Devices and Diagnostics segments, respectively. Includes $386 million of fourth quarter net litigation gain, comprised of a $92 million expense in the Pharmaceutical segment and a gain of $478 million in the Medical Devices and Diagnostics segment. | |
| (6) | Includes $7 million and $174 million of IPR&D for the Consumer and Medical Devices and Diagnostics segments, respectively. Includes $379 million of fourth quarter net litigation gain, comprised of a $50 million expense in the Consumer segment and a gain of $429 million in the Medical Devices and Diagnostics segment. The Medical Devices and Diagnostics segment also includes $536 million gain on the divestiture of the Professional Wound Care business of Ethicon, Inc. | |
| (7) | Includes $745 million of restructuring expense, comprised of $15 million, $429 million, and $301 million for the Consumer, Pharmaceutical, and Medical Devices and Diagnostics segments, respectively. The Medical Devices and Diagnostics segment includes $807 million of IPR&D. The Pharmaceutical segment also includes $678 million for the write-down of the NATRECOR ® intangible asset. | |
| (8) | Long-lived assets include property, plant and equipment, net for 2009, 2008 and 2007 of $14,759, $14,365 and $14,185, respectively, and intangible assets and goodwill, net for 2009, 2008 and 2007 of $31,185, $27,695 and $28,763, respectively. |
55
Table of Contents
JOHNSON & JOHNSON
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
19. Selected Quarterly Financial Data (unaudited)
Selected unaudited quarterly financial data for the years 2009 and 2008 are summarized below:
| 2009 | 2008 | |||||||||||||||||||||||||||||||
| First | Second | Third | Fourth | First | Second | Third | Fourth | |||||||||||||||||||||||||
| (Dollars in Millions Except Per Share Data) | Quarter | Quarter | Quarter | Quarter | (1) | Quarter | Quarter | (2) | Quarter | Quarter | (3) | |||||||||||||||||||||
Segment sales to customers |
||||||||||||||||||||||||||||||||
Consumer |
$ | 3,711 | 3,854 | 3,989 | 4,249 | 4,064 | 4,036 | 4,099 | 3,855 | |||||||||||||||||||||||
Pharmaceutical |
5,780 | 5,498 | 5,249 | 5,993 | 6,429 | 6,340 | 6,113 | 5,685 | ||||||||||||||||||||||||
Med Devices & Diagnostics |
5,535 | 5,887 | 5,843 | 6,309 | 5,701 | 6,074 | 5,709 | 5,642 | ||||||||||||||||||||||||
Total sales |
$ | 15,026 | 15,239 | 15,081 | 16,551 | 16,194 | 16,450 | 15,921 | 15,182 | |||||||||||||||||||||||
Gross profit |
10,775 | 10,789 | 10,647 | 11,239 | 11,580 | 11,699 | 11,147 | 10,810 | ||||||||||||||||||||||||
Earnings before provision for taxes on income |
4,643 | 4,263 | 4,245 | 2,604 | 4,747 | 4,375 | 4,290 | 3,517 | ||||||||||||||||||||||||
Net earnings |
3,507 | 3,208 | 3,345 | 2,206 | 3,598 | 3,327 | 3,310 | 2,714 | ||||||||||||||||||||||||
Basic net earnings per share |
$ | 1.27 | 1.16 | 1.21 | 0.80 | 1.27 | 1.18 | 1.19 | 0.98 | |||||||||||||||||||||||
Diluted net earnings per share |
$ | 1.26 | 1.15 | 1.20 | 0.79 | 1.26 | 1.17 | 1.17 | 0.97 | |||||||||||||||||||||||
| (1) | The fourth quarter of 2009 includes an after-tax charge of $852 million for restructuring and $212 million after-tax of income from net litigation. | |
| (2) | The second quarter of 2008 includes an after-tax charge of $40 million for IPR&D. | |
| (3) | The fourth quarter of 2008 includes an after-tax charge of $141 million for IPR&D, $229 million after-tax of income from net litigation and $331 million after-tax gain on the divestiture of the Professional Wound Care business of Ethicon, Inc. The gain from the divestiture of the Professional Wound Care business of Ethicon, Inc. was reinvested in the business. |
20. Business Combinations and Divestitures
Certain businesses were acquired for $2,470 million in cash and $875 million of liabilities
assumed and non-controlling interests during 2009. These acquisitions were accounted for by the
purchase method and, accordingly, results of operations have been included in the financial
statements from their respective dates of acquisition.
The 2009 acquisitions included: Mentor Corporation, a leading supplier of medical products for
the global aesthetics market; Cougar Biotechnology, Inc., a development stage biopharmaceutical
company with a specific focus on oncology; Finsbury Orthopaedics Limited, a privately held UK-based
manufacturer and global distributor of orthopaedic implants; Gloster Europe, a privately held
developer of innovative disinfection processes and technologies to prevent healthcare-acquired
infections and substantially all of the assets and rights of Elan’s Alzheimer’s Immunotherapy
Program through a newly formed company, of which the Company owns 50.1% and Elan owns 49.9%.
The excess of purchase price over the estimated fair value of tangible assets acquired
amounted to $2,940 million and has been assigned to identifiable intangible assets, with any
residual recorded to goodwill. Of this amount, approximately $1,737 million has been identified as
the value of IPR&D primarily associated with the acquisitions of Cougar Biotechnology, Inc. and
substantially all of the assets and rights of Elan’s Alzheimer’s Immunotherapy Program.
Additionally, approximately $1,107 million has been identified as the value of other intangible
assets, including patents & technology and customer relationships primarily associated with the
acquisition of Mentor Corporation.
The IPR&D related to the acquisition of Cougar Biotechnology, Inc. was $971 million and is
associated with abiraterone acetate, a late stage, first-in-class compound for the treatment of
prostate cancer. The value of the IPR&D was calculated using cash flow projections discounted for
the risk inherent in such projects. Probability of success factors ranging from 60-85% were used to
reflect inherent clinical and regulatory risk. The discount rate applied was 23.5%.
Refer to Note 6 for information related to the Elan transaction.
Certain businesses were acquired for $1,214 million in cash and $114 million of liabilities
assumed during 2008. These acquisitions were accounted for by the purchase method and, accordingly,
results of operations have been included in the financial statements from their respective dates of
acquisition.
The 2008 acquisitions included: Amic AB, a privately held Swedish developer of in vitro
diagnostic technologies for use in point-of-care and near-patient settings; Beijing Dabao Cosmetics
Co., Ltd., a company that sells personal care brands in China; SurgRx, Inc., a privately held
developer of the advanced bipolar tissue sealing system used in the ENSEAL®
family of devices; HealthMedia, Inc., a privately held company that creates web-based behavior
change interventions; LGE Performance Systems, Inc., a privately held company known as Human
Performance Institute™, which develops science-based training programs to improve employee
engagement and productivity and Omrix Biopharmaceuticals, Inc., a fully integrated
biopharmaceutical company that develops and markets biosurgical and immunotherapy products.
The excess of purchase price over the estimated fair value of tangible assets acquired
amounted to $891 million and has been assigned to identifiable intangible assets, with any residual
recorded to goodwill. Approximately $181 million has been identified as the value of IPR&D
associated with the acquisitions of Omrix Biopharmaceuticals, Inc., Amic AB, SurgRx, Inc. and
HealthMedia, Inc.
The IPR&D charge related to the acquisition of Omrix Biopharmaceuticals, Inc. was $127 million
and is associated with stand-alone and combination biosurgical technologies used to achieve
hemostasis. The value of the IPR&D was calculated using cash flow projections discounted for the
risk inherent in such projects. Probability of success factors ranging from 60-90% were used to
reflect inherent clinical and regulatory risk. The discount rate applied was 14%. As of the end of
the 2008 fiscal year, 97.8% of the outstanding shares of Common Stock of Omrix Biopharmaceuticals,
56
Table of Contents
JOHNSON & JOHNSON
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Inc. had been tendered by stockholders. Excluding shares that were tendered subject to
guaranteed delivery procedures, 90.2% of the outstanding shares of Common Stock had been tendered.
On December 30, 2008 the Company completed the acquisition of Omrix Biopharmaceuticals, Inc.
The IPR&D charge related to the acquisition of Amic AB was $40 million and is associated with
point-of-care device and 4CAST Chip technologies. The value of the IPR&D was calculated using cash
flow projections discounted for the risk inherent in such projects. The discount rate applied was
20%.
The IPR&D charge related to the acquisition of SurgRx, Inc. was $7 million and is associated
with vessel cutting and sealing surgical devices. The value of the IPR&D was calculated using cash
flow projections discounted for the risk inherent in such projects. Probability of success factors
ranging from 90-95% were used to reflect inherent clinical and regulatory risk. The discount rate
applied was 18%.
The IPR&D charge related to the acquisition of HealthMedia, Inc. was $7 million and is
associated primarily with process enhancements to software technology. The value of the IPR&D was
calculated using cash flow projections discounted for the risk inherent in such projects. A
probability of success factor of 90% was used to reflect inherent risk. The discount rate applied
was 14%.
Certain businesses were acquired for $1,388 million in cash and $232 million of liabilities
assumed during 2007. These acquisitions were accounted for by the purchase method and, accordingly,
results of operations have been included in the financial statements from their respective dates of
acquisition.
The 2007 acquisitions included: Conor Medsystems, Inc., a cardiovascular device company, with
new drug delivery technology; Robert Reid, Inc., a Japanese orthopedic product distributor; and
Maya’s Mom, Inc., a social media company.
The excess of purchase price over the estimated fair value of tangible assets acquired
amounted to $636 million and has been assigned to identifiable intangible assets, with any residual
recorded to goodwill. Approximately $807 million has been identified as the value of IPR&D
associated with the acquisition of Conor Medsystems, Inc.
The IPR&D charge related to the acquisition of Conor
Medsystems, Inc. was $807 million and is associated with research related to the discovery and
application of the stent technology. The value of the IPR&D was calculated using cash flow
projections discounted for the risk inherent in such projects. The discount rate applied was 19%.
Supplemental pro forma information for 2009, 2008 and 2007 in accordance with U.S. GAAP
standards related to business combinations, and goodwill and other intangible assets, is not
provided, as the impact of the aforementioned acquisitions did not have a material effect on the
Company’s results of operations, cash flows or financial position.
With the exception of the divestiture of the Professional Wound Care business of Ethicon,
Inc., which resulted in a gain of $536 million before tax, and is recorded in other (income)
expense, net, in 2008, divestitures in 2009, 2008 and 2007 did not have a material effect on the
Company’s results of operations, cash flows or financial position.
Note 21 — Legal Proceedings
PRODUCT LIABILITY
The Company’s subsidiaries are involved in numerous product liability cases in the United States,
many of which concern alleged adverse reactions to drugs and medical devices. The damages claimed
are substantial, and while the Company is confident of the adequacy of the warnings and
instructions for use that accompany such products, it is not feasible to predict the ultimate
outcome of litigation. However, the Company believes that if any product liability results from
such cases, it will be substantially covered by existing amounts accrued in the Company’s balance
sheet and, where available, by third-party product liability insurance.
Multiple products of Johnson & Johnson subsidiaries are subject to numerous product liability
claims and lawsuits. There are a significant number of claimants who have pending lawsuits or
claims regarding injuries allegedly due to ORTHO EVRA®,
RISPERDAL®, LEVAQUIN®, DURAGESIC®,
the CHARITÉ™ Artificial Disc and CYPHER® Stent. These claimants seek
substantial compensatory and, where available, punitive damages.
With respect to RISPERDAL ®, the Attorneys General of eight states and
the Office of General Counsel of the Commonwealth of Pennsylvania have filed actions seeking
reimbursement of Medicaid or other public funds for RISPERDAL® prescriptions
written for off-label use, compensation for treating their citizens for alleged adverse reactions
to RISPERDAL®, civil fines or penalties, punitive damages, or other relief.
The Attorney General of Texas has joined a qui tam action in that state seeking similar relief.
Certain of these actions also seek injunctive relief relating to the promotion of
RISPERDAL®. The Attorneys General of more than 40 other states have indicated
a potential interest in pursuing similar litigation against the Company’s subsidiary, Janssen
Pharmaceutica Inc. (Janssen) (now Ortho-McNeil-Janssen Pharmaceuticals Inc. (OMJPI)), and have
obtained a tolling agreement staying the running of the statute of limitations while they inquire
into the issues. In addition, there are six cases filed by union health plans seeking damages for
alleged overpayments for RISPERDAL®, several of which seek certification as
class actions. In the case brought by the Attorney General of West Virginia, based on claims for
alleged consumer fraud as to DURAGESIC® as well as
RISPERDAL®, Janssen (now OMJPI) was found liable and damages were assessed at
$4.5 million. OMJPI has filed an appeal.
Numerous claims and lawsuits in the United States relating to the drug
PROPULSID®, withdrawn from general sale by the Company’s Janssen (now OMJPI)
subsidiary in 2000, have been resolved or are currently enrolled in settlement programs with an
aggregate cap below $100 million. Similar litigation concerning PROPULSID® is
pending in Canada, where a national class action of persons alleging adverse reactions to the drug
has been certified and a settlement program instituted with an aggregate cap below $10 million.
AFFIRMATIVE STENT PATENT LITIGATION
In patent infringement actions tried in Delaware Federal District Court in late 2000, Cordis
Corporation (Cordis), a subsidiary of Johnson & Johnson, obtained verdicts of infringement and
patent validity, and damage awards against Boston Scientific Corporation (Boston Scientific) and
Medtronic AVE, Inc. (Medtronic) based on a number of Cordis vascular stent patents. In December
2000, the jury in the damage action against Boston Scientific returned a verdict of $324 million
and the jury in the Medtronic action returned a verdict of $271 million. The Court of Appeals for
the Federal Circuit has upheld liability in these cases, and on September 30, 2008, the district
court entered judgments, including interest, in the amounts of $702 million and $521 million
against Boston Scientific and
57
Table of Contents
JOHNSON & JOHNSON
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Medtronic, respectively. Medtronic paid $472 million in October 2008, representing the
judgment, net of amounts exchanged in settlement of a number of other litigations between the
companies. The net settlement of $472 million was recorded as a credit to other (income) expense,
net in the 2008 consolidated statement of earnings. In September 2009, Cordis settled this case
with Boston Scientific together with the Kastenhofer/Fontirroche and Ding cases described below,
for a net payment of $716 million. As part of that settlement Boston Scientific received a paid up
license to the Fontirroche family of patents worldwide and Cordis received a paid license to the
Kastenhofer and Ding families of patents worldwide and the parties settled all pending lawsuits
worldwide relating to these patents. The receipt of $716 million, less the impact of other
litigation matters, resulted in a credit to other (income) expense, net of $386 million in the
fiscal fourth quarter of 2009. In addition, in May 2009, Medtronic paid $270 million to settle
additional patent infringement claims asserted by Cordis based on its vascular stent patents, which
was recorded as a credit to other (income) expense, net in the fiscal second quarter of 2009.
In January 2003, Cordis filed a patent infringement action against Boston Scientific in
Delaware Federal District Court accusing its Express2™, Taxus® and
Liberte® stents of infringing the Palmaz patent that expired in November 2005.
The Liberte® stent was also accused of infringing Cordis’ Gray patent that
expires in 2016. In June 2005, a jury found that the Express2™, Taxus® and
Liberte® stents infringed the Palmaz patent and that the
Liberte® stent also infringed the Gray patent. On March 31, 2009, the U.S.
Court of Appeals for the Federal Circuit affirmed this judgment. The case was remanded to the
district court for a trial on damages and willfulness. Cordis also filed a lawsuit in Delaware
Federal District Court in October of 2008 alleging that Boston Scientific’s sales of
Taxus® and Liberte® after June of 2005 infringes Cordis’
Gray patent. On January 29, 2010, these cases together with the Jang case referred to in the
paragraph below, were settled. Under the terms of the settlement, Boston Scientific paid Cordis
$1.0 billion on February 1, 2010, and will pay Cordis an additional $725 million plus interest on
January 3, 2011. Cordis granted Boston Scientific a paid up worldwide license under the Palmaz and
Gray patents and Boston Scientific granted Cordis a paid up worldwide license under the Jang
patents for all stents sold by Cordis except the 2.25mm size Cypher.
Cordis has several pending lawsuits in New Jersey and Delaware Federal District Court against
Guidant Corporation (Guidant), Abbott Laboratories, Inc. (Abbott), Boston Scientific and Medtronic
alleging that the Xience V™ (Abbott), Promus™ (Boston Scientific) and
Endeavor® (Medtronic) drug eluting stents infringe several patents owned by or
licensed to Cordis. In one of the cases against
Boston Scientific, alleging that sales of their Promus™ stent infringed Wright and Falotico
patents, on January 20, 2010 the District Court in Delaware found the Wright/Falotico patent
invalid for lack of written description and/or lack of enablement. Cordis intends to appeal this
ruling.
PATENT LITIGATION AGAINST VARIOUS JOHNSON & JOHNSON SUBSIDIARIES
The products of various Johnson & Johnson subsidiaries are the subject of various patent
lawsuits, the outcomes of which could potentially adversely affect the ability of those
subsidiaries to sell those products, or require the payment of past damages and future royalties.
In July 2005, a jury in Federal District Court in Delaware found that the Cordis
CYPHER® Stent infringed Boston Scientific’s Ding ‘536 patent and that the
Cordis CYPHER® and BX VELOCITY® Stents also infringed
Boston Scientific’s Jang ‘021 patent. The jury also found both of those patents valid. In January
2009, the Court of
Appeals for the Federal Circuit held the Ding patent invalid and a judgment in favor of Cordis in
that case has been entered. In March 2009, the Court of Appeals for the Federal Circuit upheld the
judgment that Cordis’ CYPHER® Stent infringed Boston Scientific’s Jang patent.
The case has been remanded for a trial on the issues of damages and willfulness. The Jang case has
been dismissed as part of the January 2010 settlement described in the paragraph above relating to
the Express2™, Taxus® and Liberte® stents.
In Germany, Boston Scientific had several actions based on its Ding patents pending against
the Cordis CYPHER® Stent. Boston Scientific also had brought actions in
Belgium, the Netherlands, Germany, France and Italy under its Kastenhofer patent, which purports to
cover two-layer catheters such as those used to deliver the CYPHER® Stent.
These cases have been settled as part of the September 2009 settlement described above.
Trial in Boston Scientific’s U.S. case based on the Kastenhofer patent in Federal District
Court in California concluded in October 2007 with a jury finding that the patent was invalid. The
jury also found for Cordis on its counterclaim that sale by Boston Scientific of its balloon
catheters and stent delivery systems infringe Cordis’ Fontirroche patent. The Court has denied
Boston Scientific’s post trial motions. This case was settled as part of the September 2009
settlement described above.
In May 2008, Centocor, Inc. (Centocor) (now Centocor Ortho Biotech Inc. (COBI)) filed a
lawsuit against Genentech, Inc. (Genentech) in U.S. District Court for the Central District of
California seeking to invalidate the Cabilly II patent. Prior to filing suit, Centocor had a
sublicense under this patent from Celltech (who was licensed by Genentech) for
REMICADE® and had been paying royalties to Celltech. Centocor has terminated
that sublicense and stopped paying royalties. Genentech has filed a counterclaim alleging that
REMICADE® infringes its Cabilly II patents and that the manufacture of
REMICADE®, STELARA™, SIMPONI™ and ReoPro® also infringes
one of its other patents relating to the purification of antibodies made through recombinant DNA
techniques. The court has scheduled a hearing for Summary Judgment Motions in August 2010.
In April 2009, a bench trial was held before the Federal District Court for the Middle
District of Florida on the liability phase of Ciba’s patent infringement lawsuit alleging that
Johnson & Johnson Vision Care, Inc.’s (JJVC) ACUVUE® OASYS™ lenses infringe
three of their Nicholson patents. In August 2009, the District Court found two of these patents
valid and infringed and entered judgment against JJVC. JJVC has appealed that judgment to the Court
of Appeals for the Federal Circuit. On March 22, 2010, the District Court will hold a hearing on
Ciba’s motion for a permanent injunction. If the judgment is upheld on appeal the Court will
schedule another trial to determine damages and willfulness.
In May 2009, Abbott Biotechnology Ltd. filed a patent infringement lawsuit against Centocor
(now COBI) in the United States District Court for the District of Massachusetts. The suit alleges
that Centocor’s SIMPONI™ product, a human anti-TNF alpha antibody, infringes Abbott’s ‘394 patent
(the Salfeld patent). The case has been stayed pending the resolution of an arbitration filed by
Centocor directed to its claim that it is licensed under the ‘394 patent. The arbitration is
scheduled for March 2010.
In August 2009, Abbott GmbH & Co. (Abbott GmbH) and Abbott Bioresearch Center filed a patent
infringement lawsuit against COBI in the United States District Court for the District of
Massachusetts. The suit alleges that COBI’s STELARA™ product infringes two U.S. patents assigned to
Abbott GmbH. In August 2009, COBI filed a complaint for a declaratory judgment of non-infringement
and invalidity of the Abbott GmbH patents in the United States District Court for the District of
Columbia. On the same date, also in the United States District Court for the District of
58
Table of Contents
JOHNSON & JOHNSON
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Columbia, COBI filed a Complaint for Review of a Patent Interference Decision granting priority
of invention on one of the two asserted patents to Abbott GmbH. In August 2009, Abbott GmbH and
Abbott Laboratories Limited brought a patent infringement suit in Canada alleging that STELARA™
infringes Abbott GmbH’s Canadian patent. The cases filed by COBI in the District of Columbia have
been transferred to the District of Massachusetts.
In August 2009, Bayer Healthcare LLC filed suit against COBI in Massachusetts District Court
alleging infringement by COBI’s SIMPONI™ product of its patent relating to human anti-TNF
antibodies. Bayer has also filed suit under its European counterpart to these patents in Germany
and the Netherlands.
In June 2009, Centocor’s (now COBI) lawsuit alleging that Abbott’s HUMIRA anti-TNF alpha
product infringes Centocor’s ‘775 patent went to trial in Federal District Court in the Eastern
District of Texas. On June 28, 2009 a jury returned a verdict finding the patent valid and
willfully infringed, and awarded Centocor damages of approximately $1.7 billion. A bench trial on
Abbott’s defenses, of inequitable conduct and prosecution laches, was held in August 2009, and the District Court decided these
issues in favor of Centocor. All of Abbott’s post trial motions have been denied except that the
District Court granted Abbott’s motion to overturn the jury finding of willfulness. Judgment in the
amount of $1.9 billion was entered in favor of Centocor in December 2009 and Abbott has filed an
appeal to the Court of Appeals for the Federal Circuit. The Company has not reflected any of the
$1.9 billion in its consolidated financial statements. Centocor has also filed a new lawsuit in the
Eastern District of Texas seeking damages for infringement of the ‘775 patent attributable to sales
of HUMIRA subsequent to the jury verdict in June 2009.
The following chart summarizes various patent lawsuits concerning products of the Company’s
subsidiaries that have yet to proceed to trial:
| J&J | Plaintiff/ | |||||||||||
| Product | Company | Patents | Patent Holder | Court | Trial Date** | Date Filed | ||||||
CYPHER® Stent
|
Cordis | Wall | Wall | E.D. TX | Q2/11 | 11/07 | ||||||
CYPHER® Stent
|
Cordis | Saffran | Saffran | E.D. TX | Q2/11 | 10/07 | ||||||
Blood Glucose Meters and Strips
|
LifeScan | Wilsey | Roche Diagnostics | D. DE | * | 11/07 | ||||||
REMICADE®, ustekinumab,
|
Centocor/COBI | Cabilly II | Genentech | C.D. CA | * | 05/08 | ||||||
golimumab, ReoPro® |
||||||||||||
SIMPONI™
|
Centocor/COBI | Salfeld | Abbott Laboratories | MA | * | 05/09 | ||||||
SIMPONI™
|
Centocor/COBI | Boyle | Bayer Healthcare | MA | * | 08/09 | ||||||
STELARA™
|
Centocor/COBI | Salfeld | Abbott GmbH | MA/DC | * | 08/09 | ||||||
| * | Trial date to be scheduled. | |
| ** | Q reflects the Company’s fiscal quarter. |
LITIGATION AGAINST FILERS OF ABBREVIATED NEW DRUG APPLICATIONS (ANDAs)
The following chart indicates lawsuits pending against generic firms that filed Abbreviated New
Drug Applications (ANDAs) seeking to market generic forms of products sold by various subsidiaries
of the Company prior to expiration of the applicable patents covering those products. These ANDAs
typically include allegations of non-infringement, invalidity and unenforceability of these
patents. In the event the subsidiary of the Company involved is not successful in these actions, or
the statutory 30-month stay expires before a ruling from the district court is obtained, the firms
involved will have the ability, upon FDA approval, to introduce generic versions of the product at
issue resulting in very substantial market share and revenue losses for the product of the
Company’s subsidiary.
As noted in the following chart, 30-month stays expired during 2009, and will expire in 2010,
2011 and 2012 with respect to ANDA challenges regarding various products:
| Brand Name | Patent/NDA | Generic | Trial | Date | 30-Month | |||||||
| Product | Holder | Challenger | Court | Date** | Filed | Stay Expiration | ||||||
CONCERTA®
|
McNeil-PPC | Andrx | D. DE | Q4/07 | 09/05 | None | ||||||
18, 27, 36 and 54 mg controlled
release tablet
|
ALZA | KUDCO | D. DE | * | 01/10 | 05/12 | ||||||
LEVAQUIN® 250, 500, 750 mg tablet
|
Ortho-McNeil | Lupin | D. NJ | * | 10/06 | 03/09 | ||||||
ORTHO TRI-CYCLEN® LO
|
Ortho-McNeil | Watson | D. NJ | * | 10/08 | 03/11 | ||||||
0.18 mg/0.025 mg, 0.215 mg/0.025 mg and 0.25 mg/0.025 mg |
Sandoz | D. NJ D. NJ |
* * |
06/09 | 10/11 06/12 |
|||||||
ULTRAM ER® 100, 200, 300 mg tablet
|
Ortho-McNeil/Biovail | Par | D. DE | Q2/09 | 05/07 | 09/09 | ||||||
| 06/07 | 11/09 | |||||||||||
| 10/07 | 03/10 | |||||||||||
ULTRAM ER® 100, 200, 300 mg tablet
|
Ortho-McNeil/Biovail | Impax | D. DE | Q2/10 | 08/08 | 01/11 | ||||||
| 11/08 | 03/11 | |||||||||||
ULTRAM ER® 100, 200, 300 mg tablet
|
Ortho-McNeil/Biovail | Paddock | D.DRD. Minn. | * | 09/09 | 01/12 | ||||||
ULTRAM ER® 100, 200, 300 mg tablet
|
Ortho-McNeil/Biovail | Cipher | D. DE | * | 10/09 | 03/12 | ||||||
ULTRAM ER® 100, 200, 300 mg tablet
|
Ortho-McNeil/Biovail | Lupin | D. DE | * | 01/10 | 06/12 | ||||||
| * | Trial date to be scheduled. | |
| ** | Q reflects the Company’s fiscal quarter. |
59
Table of Contents
JOHNSON & JOHNSON
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
In the action against Barr Pharmaceuticals, Inc. (Barr) (now a wholly-owned
subsidiary of Teva Pharmaceutical Industries LTD.) regarding ORTHO TRI-CYCLEN®
LO, in January 2008, the Company’s subsidiary Ortho Women’s Health & Urology, a Division of
Ortho-McNeil-Janssen Pharmaceuticals, Inc. (OMJPI), and Barr agreed to a non-binding term sheet to
settle the litigation, which settlement discussions are still underway. The trial court postponed
the January 2008 trial without setting a new trial date. In June 2009, Barr launched its generic
product “at risk” before trial. OMJPI sought a preliminary injunction and recall of Barr product
which the Court
granted in July 2009. In July 2009, the parties entered into a definitive agreement to settle the
lawsuit. Under the terms of the settlement, Barr obtained a release for its sales of its generic
product in exchange for an undisclosed royalty payment. Barr also obtained a non-exclusive,
royalty-bearing license to re-enter the market on December 31, 2015, or earlier in certain limited
circumstances.
In October 2008, the Company’s subsidiary OMJPI filed suit in Federal District Court in New
Jersey against Watson Laboratories, Inc. (Watson) in response to Watson’s ANDA regarding ORTHO
TRI-CYCLEN® LO. In June 2009, the Company’s subsidiary OMJPI filed suit in
Federal District Court in New Jersey against Sandoz Laboratories, Inc. (Sandoz) in response to
Sandoz’s ANDA regarding ORTHO TRI-CYCLEN® LO. The Sandoz and Watson cases have
been consolidated.
In January 2010, the Company’s subsidiary OMJPI filed suit in Federal District Court in New
Jersey against Lupin Ltd. and Lupin Pharmaceuticals, Inc. (collectively “Lupin”) in response to
Lupin’s ANDA regarding ORTHO TRI-CYCLEN® LO.
In the action against Barr and AlphaPharm with respect to their ANDA challenges to the
RAZADYNE® patent that Janssen (now OMJPI) licenses from Synaptech, Inc.
(Synaptech), a four-day non-jury trial was held in the Federal District Court in Delaware in May
2007. In August 2008, the court held that the patent was invalid because it was not enabled.
Janssen (OMJPI) and Synaptech have appealed the decision. Since the court’s decision, multiple
generic companies have received final approvals for their products and have launched “at risk”
pending appeal. Additional generic approvals and launches could occur at any time. In September
2009, the Court of Appeals affirmed the judgment that the patent is invalid.
In the action by McNEIL-PPC, Inc. (McNeil-PPC) and ALZA Corporation (ALZA) against Andrx
Corporation (Andrx) with respect to its ANDA challenge to the CONCERTA®
patents, a five-day non-jury trial was held in the Federal District Court in Delaware in December
2007. In March 2009, the court ruled that one CONCERTA® patent would not be
infringed by Andrx’s proposed generic product and that the patent was invalid because it was not
enabled. The court dismissed without prejudice Andrx’s declaratory judgment suit on a second patent
for lack of jurisdiction. McNeil-PPC and ALZA filed an appeal in May 2009. The appeals court heard
argument on February 3, 2010. A decision is pending.
ALZA and OMJPI filed a second suit in Federal District Court in Delaware against
Kremers-Urban, LLC and KUDCO Ireland, Ltd. (KUDCO) in January 2010 in response to KUDCO’s ANDA
challenge regarding CONCERTA® tablets. In its notice letter, KUDCO contends
that two ALZA patents for CONCERTA® are invalid and not infringed by a KUDCO
generic.
In the RAZADYNE® ER cases, a lawsuit was filed against Barr on the
RAZADYNE® use patent that Janssen (now OMJPI) licenses from Synaptech in June
2006. In September 2008, the above-discussed Delaware decision invalidating the
RAZADYNE® use patent resulted in entry of judgment for Barr on that patent,
but the case will be reopened if Janssen (now OMJPI) and Synaptech win on appeal. Barr has received
FDA approval of its product and has launched “at risk.” In September 2009, the Federal Circuit
affirmed
the Delaware decision invalidating the RAZADYNE® use patent. As a result, this
case will not be reopened.
In the action against Lupin Pharmaceuticals, Inc. (Lupin) regarding its ANDA concerning
LEVAQUIN®, Lupin contends that the U.S. Patent and Trademark Office improperly
granted a patent term extension to the patent that Ortho-McNeil (now Ortho-McNeil-Janssen
Pharmaceuticals, Inc. (OMJPI)) licenses from Daiichi Pharmaceuticals, Inc. (Daiichi). Lupin alleges
that the active ingredient in LEVAQUIN® was the subject of prior marketing,
and therefore was not eligible for the patent term extension. Lupin concedes validity and that its
product would violate the patent if marketed prior to the expiration of the original patent term.
Summary judgment against Lupin was granted in May 2009 and Lupin appealed. Oral argument was held
in September 2009. A decision is pending.
In the ULTRAM® ER actions, Ortho-McNeil Pharmaceutical, Inc.
(Ortho-McNeil) (now OMJPI), filed lawsuits (each for different dosages) against Par
Pharmaceuticals, Inc. and Par Pharmaceuticals Companies, Inc. (Par) in May, June and October 2007
on two Tramadol ER formulation patents owned by Purdue Pharma Products L.P. (Purdue) and Napp
Pharmaceutical Group Ltd. (Napp). OMJPI also filed lawsuits (each for different dosages) against
Impax Laboratories, Inc. (Impax) on a Tramadol ER formulation patent owned by Purdue and Napp in
August and November 2008. Purdue, Napp and Biovail Laboratories International SRL (Biovail) (the
NDA holder) joined as co-plaintiffs in the lawsuits against Par and Impax, but Biovail and OMJPI
were subsequently dismissed for lack of standing. The trial against Par took place in April 2009.
In August 2009, the Court issued a decision finding the patents-in-suit invalid. Purdue has
appealed that decision. The trial against Impax is scheduled for June 2010. In November 2009, the
case against Impax was stayed with the consent of all parties. In September and October 2009,
respectively, Purdue filed suits against Paddock Laboratories, Inc. (Paddock) and Cipher
Pharmaceuticals Inc. (Cipher) on its Tramadol ER formulation patents.
In January 2010, Purdue filed a suit against Lupin Ltd. (Lupin) on its Tramadol ER formulation
patents.
In September 2009, Centocor Ortho Biotech Products, L.P. (COBI, LP) intervened in an
inventorship dispute between Kansas University Center for Research (KUCR) involving certain U.S.
government-owned VELCADE® formulation patents. KUCR brought this action
against the U.S. government in the District of Kansas seeking to add two Kansas University
scientists to the patents. The U.S. government licensed the patents (and their foreign
counterparts) to Millennium Pharmaceuticals, Inc., who in turn sublicensed the patents (and their
foreign counterparts) to COBI,LP for commercial marketing outside the U.S. If KUCR succeeds in its
co-inventorship claim and establishes co-ownership in the U.S. VELCADE®
formulation patents, we anticipate that KUCR will initiate actions to establish co-inventorship and
co-ownership with respect to the foreign counterpart patents in the countries where COBI, LP has
commercial marketing rights. If KUCR in Kansas is successful, this may adversely affect COBI, LP’s
license rights in those countries.
AVERAGE WHOLESALE PRICE (AWP) LITIGATION
Johnson & Johnson and several of its pharmaceutical subsidiaries, along with numerous other
pharmaceutical companies, are defendants in a series of lawsuits in state and federal courts
involving allegations that the pricing and marketing of certain pharmaceutical products amounted to
fraudulent and otherwise actionable conduct because, among other things, the companies allegedly
reported an inflated Average Wholesale Price (AWP) for the drugs at issue. Many of these cases,
both federal actions and state actions
60
Table of Contents
JOHNSON & JOHNSON
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
removed to federal court, have been consolidated for pre-trial purposes in a Multi-District
Litigation (MDL) in Federal District Court in Boston, Massachusetts. The plaintiffs in these cases
include classes of private persons or entities that paid for any portion of the purchase of the
drugs at issue based on AWP, and state government entities that made Medicaid payments for the
drugs at issue based on AWP.
The MDL Court identified classes of Massachusetts-only private insurers providing “Medi-gap”
insurance coverage and private payers for physician-administered drugs where payments were based on
AWP (“Class 2” and “Class 3”), and a national class of individuals who made co-payments for
physician-administered drugs covered by Medicare (“Class 1”). A trial of the two Massachusetts-only
class actions concluded before the MDL Court in December 2006. In June 2007, the MDL Court issued
post-trial rulings, dismissing the Johnson & Johnson defendants from the case regarding all claims
of Classes 2 and 3, and subsequently of Class 1 as well. Plaintiffs appealed the Class 1 judgment
and, in September 2009, the Court of Appeals vacated the judgment and remanded for further
proceedings in the District Court. AWP cases brought by various Attorneys General have proceeded to
trial against other manufacturers. One state case against certain of the Company’s subsidiaries has
been set for trial in late 2010, and other state cases are likely to be set for trial thereafter.
OTHER
In July 2003, Centocor (now COBI), a Johnson & Johnson subsidiary, received a request that it
voluntarily provide documents and information to the criminal division of the U.S. Attorney’s
Office, District of New Jersey, in connection with its investigation into various Centocor
marketing practices. Subsequent requests for documents have been received from the U.S. Attorney’s
Office. Both the Company and Centocor have responded to these requests for documents and
information.
In December 2003, Ortho-McNeil (now OMJPI) received a subpoena from the U.S. Attorney’s Office
in Boston, Massachusetts seeking documents relating to the marketing, including alleged off-label
marketing, of the drug TOPAMAX® (topiramate). Additional subpoenas for
documents have been received, and current and former employees have testified before a grand jury.
Discussions are underway in an effort to resolve this matter, but whether agreement can be reached
and on what terms is uncertain.
In January 2004, Janssen (now OMJPI) received a subpoena from the Office of the Inspector
General of the U.S. Office of Personnel Management seeking documents concerning sales and marketing
of, any and all payments to physicians in connection with sales and marketing of, and clinical
trials for, RISPERDAL® (risperidone) from 1997 to 2002. Documents subsequent
to 2002 have also been requested. An additional subpoena seeking information about marketing of and
adverse reactions to RISPERDAL® was received from the U.S. Attorney’s Office
for the Eastern District of Pennsylvania in November 2005. Subpoenas seeking testimony from various
witnesses before a grand jury have also been received. Janssen is cooperating in responding to
ongoing requests for documents and witnesses. The government is continuing to actively investigate
this matter. In February 2010, the government served Civil Investigative Demands seeking additional
information relating to sales and marketing of RISPERDAL® and sales and
marketing of INVEGA®.
In September 2004, Ortho Biotech Inc. (Ortho Biotech) (now COBI), received a subpoena from the
U.S. Office of Inspector General’s Denver, Colorado field office seeking documents directed to the
sales and marketing of PROCRIT® (Epoetin alfa) from 1997 to the present, as
well as to dealings with U.S. Oncology Inc., a healthcare services network for oncologists. Ortho
Biotech (now COBI) has responded to the subpoena.
In September 2004, plaintiffs in an employment discrimination litigation initiated against the
Company in 2001 in Federal District Court in New Jersey moved to certify a class of all African
American and Hispanic salaried employees of the Company and its affiliates in the U.S., who were
employed at any time from November 1997 to the present. Plaintiffs seek monetary damages for the
period 1997 through the present (including punitive damages) and equitable relief. The Court denied
plaintiffs’ class certification motion in December 2006 and their motion for reconsideration in
April 2007. Plaintiffs sought to appeal these decisions and, in April 2008, the Court of Appeals
ruled that plaintiffs’ appeal of the denial of class certification was untimely. In July 2009,
plaintiffs filed a motion for certification of a modified class, which the Company is opposing.
Plaintiffs are engaged in further discovery of individual plaintiffs’ claims. The hearing on
plaintiffs’ motion for class certification is scheduled for July 2010.
In March 2005, DePuy Orthopaedics, Inc. (DePuy), a Johnson & Johnson subsidiary, received a
subpoena from the U.S. Attorney’s Office, District of New Jersey, seeking records concerning
contractual relationships between DePuy and surgeons or surgeons-in-training involved in hip and
knee replacement and reconstructive surgery. This investigation was resolved by DePuy and the four
other leading suppliers of hip and knee implants in late September 2007 by agreements with the U.S.
Attorney’s Office for the District of New Jersey. The settlements included an 18-month Deferred
Prosecution Agreement (DPA), acceptance by each company of a monitor to assure compliance with the
DPA and, with respect to four of the five companies, payment of settlement monies and entry into
five year Corporate Integrity Agreements. DePuy paid $85 million as its settlement. The term of the
Monitor-ship under the Deferred Prosecution Agreement concluded on March 27, 2009, and an order
dismissing all charges was entered on March 30, 2009.
In November 2007, the Attorney General of the Commonwealth of Massachusetts issued a Civil
Investigative Demand to DePuy seeking information regarding financial relationships between a
number of Massachusetts-based orthopedic surgeons and providers and DePuy. DePuy is responding to
Massachusetts’ additional requests.
In July 2005, Scios Inc. (Scios), a Johnson & Johnson subsidiary, received a subpoena from the
U.S. Attorney’s Office, District of Massachusetts, seeking documents related to the sales and
marketing of NATRECOR®. Scios responded to the subpoena. In early August 2005,
Scios was advised that the investigation would be handled by the U.S. Attorney’s Office for the
Northern District of California in San Francisco. Additional requests for documents have been
received and responded to and former Scios employees have testified before a grand jury in San
Francisco. The qui tam complaints were unsealed on February 19, 2009. The U.S. government
has intervened in one of the qui tam actions, and filed a complaint against Scios and the Company
in June 2009. Scios and Johnson & Johnson have filed a motion to dismiss the qui tam complaint
filed by the government, and that motion was denied. The criminal investigation is continuing and
discussions are underway in an effort to settle this matter. Whether a settlement can be reached
and on what terms is uncertain.
In September 2005, the Company received a subpoena from the U.S. Attorney’s Office, District
of Massachusetts, seeking documents related to sales and marketing of eight drugs to Omnicare,
Inc., a manager of pharmaceutical benefits for long-term care facilities. The Johnson & Johnson
subsidiaries involved responded to the subpoena. Several employees of the Company’s pharmaceutical
subsidiaries have been subpoenaed to testify before a grand jury in connection with this
investigation. In April 2009, the Company was served with the complaints in two civil qui tam cases
related to
61
Table of Contents
JOHNSON & JOHNSON
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
marketing of prescription drugs to Omnicare, Inc. On January 15, 2010, the government filed a
complaint intervening in the cases. The complaint asserts claims under the federal False Claims Act
and a related state law claim in connection with the marketing of several drugs to Omnicare.
In November 2005, Amgen Inc. (Amgen) filed suit against Hoffmann-LaRoche, Inc. (Roche) in the
U.S. District Court for the District of Massachusetts seeking a declaration that the Roche product
CERA, which Roche has indicated it would seek to introduce into the United States, infringes a
number of Amgen patents concerning EPO. Amgen licenses EPO for sale in the United States to Ortho
Biotech (now COBI) for non-dialysis indications. Trial in this action concluded in October 2007
with a verdict in Amgen’s favor, finding the patents valid and infringed. The judge issued a
preliminary injunction blocking the CERA launch, and subsequently made the injunction permanent.
The Federal Circuit upheld the entry of a permanent injunction. This matter has been settled
pursuant to an agreement between the parties.
In February 2006, the Company received a subpoena from the U.S. Securities & Exchange
Commission (SEC) requesting documents relating to the participation by several Johnson & Johnson
subsidiaries in the United Nations Iraq Oil for Food Program. The subsidiaries are cooperating with
the SEC and U.S. Department of Justice (DOJ) in producing responsive documents.
In February 2007, the Company voluntarily disclosed to the DOJ and the SEC that subsidiaries
outside the United States are believed to have made improper payments in connection with the sale
of medical devices in two small-market countries, which payments may fall within the jurisdiction
of the Foreign Corrupt Practices Act (FCPA). In the course of continuing dialogues with the
agencies, other issues potentially rising to the level of FCPA violations in additional markets
have been brought to the attention of the agencies by the Company. The Company has provided and
will continue to provide additional information to the DOJ and SEC, and will cooperate with the
agencies’ reviews of these matters. Law enforcement agencies of a number of other countries are
also pursuing investigations of matters voluntarily disclosed by the Company to the DOJ and SEC.
Discussions are underway in an effort to resolve these matters, and the Iraq Oil for Food matter
referenced above, but whether agreement can be reached and on what terms is uncertain.
In March 2007, the Company received separate subpoenas from the U.S. Attorney’s Office in
Philadelphia, the U.S. Attorney’s Office in Boston and the U.S. Attorney’s Office in San Francisco.
The subpoenas relate to investigations by these three offices referenced above concerning,
respectively, sales and marketing of RISPERDAL® by Janssen (now OMJPI),
TOPAMAX® by Ortho-McNeil (now OMJPI) and NATRECOR® by
Scios. The subpoenas request information regarding the Company’s corporate supervision and
oversight of these three subsidiaries, including their sales and marketing of these drugs. The
Company responded to these requests. In addition, the U.S. Attorney’s Office in Boston has issued
subpoenas for grand jury testimony to several employees of Johnson & Johnson.
In May 2007, the New York State Attorney General issued a subpoena seeking information
relating to the marketing and safety of PROCRIT®. The Company is responding to
these requests.
In April 2007, the Company received two subpoenas from the Office of the Attorney General of
the State of Delaware. The subpoenas seek documents and information relating to nominal pricing
agreements. For purposes of the subpoenas, nominal pricing agreements are defined as agreements
under which the Company agreed to provide a pharmaceutical product for less than ten percent of the
Average Manufacturer Price for the product. The Company responded to these requests.
In January 2008, the European Commission (“EC”) began an industry-wide antitrust inquiry
concerning competitive conditions within the pharmaceutical sector. Because this is a sector
inquiry, it is not based on any specific allegation that the Company has violated EC competition
law. The inquiry began with unannounced raids of a substantial number of pharmaceutical companies
throughout Europe, including Johnson & Johnson affiliates. In March 2008, the EC issued detailed
questionnaires to approximately 100 companies, including Johnson & Johnson affiliates. In November
2008, the EC issued a preliminary report summarizing its findings. The final report was issued on
July 8, 2009.
In March 2008, the Company received a letter request from the Attorney General of the State of
Michigan. The request seeks documents and information relating to nominal price transactions. The
Company responded to the request and will cooperate with the inquiry.
In June 2008, the Company received a subpoena from the United States Attorney’s Office for the
District of Massachusetts relating to the marketing of biliary stents by the Company’s Cordis
subsidiary. Cordis is cooperating in responding to the subpoena.
In September 2008, Multilan AG (Multilan), an indirect subsidiary of Schering-Plough
Corporation, commenced arbitration against Janssen Pharmaceutica NV for an alleged wrongful
termination of an agreement relating to payments in connection with termination of certain
marketing rights. Multilan seeks declaratory relief, specific performance and damages. This case
was recently settled and a charge was recorded to other income (expense), net, in the fiscal fourth
quarter of 2009.
In February 2009, Basilea Pharmaceutica AG (Basilea) brought an arbitration against the
Company and various affiliates alleging that the Company breached the 2005 License Agreement for
cefto-biprole by, among other things, failing to secure FDA approval of the cSSSI (skin) indication
and allegedly failing to properly develop the pneumonia indication. Basilea is seeking to recover
damages and a declaration that the Company materially breached the agreement. This matter has been
scheduled for an arbitration hearing commencing in June 2010 followed by post-trial submissions.
In April 2009, the Company received a HIPPA subpoena from the U.S. Attorney’s Office for the
District of Massachusetts (Boston) seeking information regarding the Company’s financial
relationship with several psychiatrists. The Company is responding to this request.
In April 2009, Ortho-Clinical Diagnostics, Inc. (OCD) received a grand jury subpoena from the
U.S. Department of Justice, Antitrust Division, requesting documents and information for the period
beginning September 1, 2000 through the present, pertaining to an investigation of alleged
violations of the antitrust laws in the blood reagents industry. The Company is in the process of
complying with the subpoena. In the weeks following the public announcement that OCD had received a
subpoena from the Antitrust Division, multiple class action complaints were filed. The various
cases were consolidated for pre-trial purposes in the Eastern District of Pennsylvania.
In May 2009, the New Jersey Attorney General issued a subpoena to DePuy Orthopaedics, Inc.,
seeking information regarding the financial interest of clinical investigators who performed
clinical studies for DePuy Orthopaedics, Inc. and DePuy Spine, Inc. The Company is responding to
these requests.
In May 2009, COBI commenced an arbitration proceeding before the American Arbitration
Association against Schering-Plough Corporation and its subsidiary Schering-Plough (Ireland)
Company (collectively, Schering-Plough). COBI and Schering-Plough are parties to a series of
agreements (the Distribution
62
Table of Contents
JOHNSON & JOHNSON
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Agreements) that grant Schering-Plough the exclusive right to distribute the drugs
REMICADE® and SIMPONI™ worldwide, except within the United States, Japan,
Taiwan, Indonesia, and the People’s Republic of China (including Hong Kong) (the “Territory”). COBI
distributes REMICADE® and SIMPONI™, the next generation treatment, within the
United States. In the arbitration, COBI seeks a declaration that the agreement and merger between
Merck & Co., Inc. (Merck) and Schering-Plough constitutes a change of control under the terms of
the Distribution Agreements that permits COBI to terminate the Agreements. The termination of the
Distribution Agreements would return to COBI the right to distribute REMICADE®
and SIMPONI™ within the Territory. Schering-Plough has filed a response to COBI’s arbitration
demand that denies that it has undergone a change of control. The arbitrators have been selected
and the matter will be proceeding to arbitration in late September 2010.
In December 2009, the State of Israel (Sheba Medical Center) filed a lawsuit against three
Omrix entities. In the lawsuit, the State claimed that an employee of a government-owned hospital
was the inventor on several patents related to fibrin glue technology, that he developed while he
was a government employee. The State claims that he had no right to transfer any intellectual
property to Omrix because it belongs to the State. The State is seeking damages plus royalty on
QUIXIL™ and EVICEL™ or, alternatively, transfer of the patents to the State.
In recent years the Company has received numerous requests from a variety of United States
Congressional Committees to produce information relevant to ongoing congressional inquiries. It is
the Company’s policy to cooperate with these inquiries by producing the requested information.
With respect to all the above matters, the Company and its subsidiaries are vigorously
contesting the allegations asserted against them and otherwise pursuing defenses to maximize the
prospect of success. The Company and its subsidiaries involved in these matters continually
evaluate their strategies in managing these matters and, where appropriate, pursue settlements and
other resolutions where those are in the best interest of the Company.
The Company is also involved in a number of other patent, trademark and other lawsuits
incidental to its business. The ultimate legal and financial liability of the Company in respect to
all claims, lawsuits and proceedings referred to above cannot be estimated with any certainty.
However, in the Company’s opinion, based on its examination of these matters, its experience to
date and discussions with counsel, the ultimate outcome of legal proceedings, net of liabilities
accrued in the Company’s balance sheet, is not expected to have a material adverse effect on the
Company’s financial condition, although the resolution in any reporting period of one or more of
these matters could have a significant impact on the Company’s results of operations and cash flows
for that period.
22. Restructuring
In the fourth quarter of 2009, the Company announced global restructuring initiatives designed
to strengthen the Company’s position as one of the world’s leading global health care companies.
This program will allow the Company to invest in new growth platforms; ensure the successful launch
of its many new products and continued growth of its core businesses; and provide flexibility to
adjust to the changed and evolving global environment.
During the fiscal fourth quarter of 2009, the Company recorded $1.2 billion in related pre-tax
charges of which, approximately $830 million of the pre-tax restructuring charges are expected to
require cash payments. The $1.2 billion of restructuring charges consists of
severance costs of $748 million, asset write-offs of $362 million and $76 million related to
leasehold and contract obligations. The $362 million of asset write-offs relate to inventory of
$113 million (recorded in cost of products sold), property, plant and equipment of $107 million,
intangible assets of $81 million and other assets of $61 million. Additionally, as part of this
program the Company plans to eliminate approximately 7,500 positions of which approximately 700
have been eliminated since the restructuring was announced.
The following table summarizes the severance charges and the associated spending for the
fiscal year ended 2009:
| Asset | ||||||||||||||||
| (Dollars in Millions) | Severance | Write-Offs | Other | Total | ||||||||||||
2009 restructuring charge |
$ | 748 | 362 | 76 | 1,186 | |||||||||||
Current year activity |
(62 | ) | (149 | ) | (28 | ) | (239 | ) | ||||||||
Reserve balance, January 3, 2010* |
$ | 686 | 213 | 48 | 947 | |||||||||||
| * | Cash outlays for severance are expected to be substantially paid out over the next 12 to 18 months in accordance with the Company’s plans and local laws. |
For additional information on the restructuring as it relates to the segments, see Note 18.
In the third quarter of 2007, the Company announced restructuring initiatives in an effort to
improve its overall cost structure. This action was taken to offset the anticipated negative
impacts associated with generic competition in the Pharmaceutical segment and challenges in the
drug-eluting stent market. The Company’s Pharmaceuticals segment has reduced its cost base by
consolidating certain operations, while continuing to invest in recently launched products and its
late-stage pipeline of new products. The Cordis franchise has moved to a more integrated business
model to address the market changes underway with drug-eluting stents and to better serve the broad
spectrum of its patients’ cardiovascular needs, while reducing its cost base. The Company
accelerated steps to standardize and streamline certain aspects of its enterprise-wide functions
such as human resources, finance and information technology to support growth across the business,
while also leveraging its scale more effectively in areas such as procurement to benefit its
operating companies. Additionally, as part of this program the Company eliminated approximately
4,600 positions.
The Company recorded $745 million in related pre-tax charges during the fiscal third quarter
of 2007, of which, approximately $500 million of the pre-tax restructuring charges required cash
payments. The $745 million of restructuring charges consists of severance costs of $450 million,
asset write-offs of $272 million and $23 million related to leasehold obligations. The $272 million
of asset write-offs relate to property, plant and equipment of $166 million, intangible assets of
$48 million and other assets of $58 million. The restructuring initiative announced in 2007 has
been completed.
23. Subsequent Events
On January 20, 2010, the Company completed the acquisition of Acclarent Inc. for a net purchase
price of approximately $785 million. Acclarent Inc. is a medical technology company dedicated to
designing, developing and commercializing devices that address conditions affecting the ear, nose
and throat.
price of approximately $785 million. Acclarent Inc. is a medical technology company dedicated to
designing, developing and commercializing devices that address conditions affecting the ear, nose
and throat.
The Company has performed an evaluation of subsequent events through March 1, 2010, the date
the Company issued these financial statements.
63
Table of Contents
Report of Independent Registered Public Accounting Firm
To the Shareholders and Board of Directors of Johnson & Johnson:
In our opinion, the accompanying consolidated balance sheets and the related consolidated
statements of earnings, statements of equity, and statements of cash flows present fairly, in all
material respects, the financial position of Johnson & Johnson and its subsidiaries (“the Company”)
at January 3, 2010 and December 28, 2008, and the results of their operations and their cash flows
for each of the three years in the period ended January 3, 2010 in conformity with accounting
principles generally accepted in the United States of America. Also in our opinion, the Company
maintained, in all material respects, effective internal control over financial reporting as of
January 3, 2010, based on criteria established in Internal Control — Integrated Framework issued by
the Committee of Sponsoring Organizations of the Treadway Commission (COSO). The Company’s
management is responsible for these financial statements, for maintaining effective internal
control over financial reporting and for its assessment of the effectiveness of internal control
over financial reporting, included in the accompanying, “Management’s Report on Internal Control
over Financial Reporting.” Our responsibility is to express opinions on these financial statements
and on the Company’s internal control over financial reporting based on our integrated audits. We
conducted our audits in accordance with the standards of the Public Company Accounting Oversight
Board (United States). Those standards require that we plan and perform the audits to obtain
reasonable assurance about whether the financial statements are free of material misstatement and
whether effective internal control over financial reporting was maintained in all material
respects. Our audits of the financial statements included examining, on a test basis, evidence
supporting the amounts and disclosures in the financial statements, assessing the accounting
principles used and significant estimates made by management, and evaluating the overall financial
statement presentation. Our audit of internal control over financial reporting included obtaining
an understanding of internal control over financial reporting, assessing the risk that a material
weakness exists, and testing and evaluating the design and operating effectiveness of internal
control based on the assessed risk. Our audits also included performing such other procedures as we
considered necessary in the circumstances. We believe that our audits provide a reasonable basis
for our opinions.
As discussed in Note 1 to the Consolidated Financial Statements, the Company changed the
manner in which it accounts for business combinations in 2009.
A company’s internal control over financial reporting is a process designed to provide
reasonable assurance regarding the reliability of financial reporting and the preparation of
financial statements for external purposes in accordance with generally accepted accounting
principles. A company’s internal control over financial reporting includes those policies and
procedures that (i) pertain to the maintenance of records that, in reasonable detail, accurately
and fairly reflect the transactions and dispositions of the assets of the company; (ii) provide
reasonable assurance that transactions are recorded as necessary to permit preparation of financial
statements in accordance with generally accepted accounting principles, and that receipts and
expenditures of the company are being made only in accordance with authorizations of management and
directors of the company; and (iii) provide reasonable assurance regarding prevention or timely
detection of unauthorized acquisition, use, or disposition of the company’s assets that could have
a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent
or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are
subject to the risk that controls may become inadequate because of changes in conditions, or that
the degree of compliance with the policies or procedures may deteriorate.
New York, New York
March 1, 2010
JOHNSON & JOHNSON 2009 ANNUAL REPORT
64
Table of Contents
Management’s Report on Internal Control Over Financial Reporting
Under Section 404 of the Sarbanes-Oxley Act of 2002, management is required to assess the
effectiveness of the Company’s internal control over financial reporting as of the end of each
fiscal year and report, based on that assessment, whether the Company’s internal control over
financial reporting is effective.
Management of the Company is responsible for establishing and maintaining adequate internal
control over financial reporting. The Company’s internal control over financial reporting is
designed to provide reasonable assurance as to the reliability of the Company’s financial reporting
and the preparation of external financial statements in accordance with generally accepted
accounting principles.
Internal controls over financial reporting, no matter how well designed, have inherent
limitations. Therefore, internal control over financial reporting determined to be effective can
provide only reasonable assurance with respect to financial statement preparation and may not
prevent or detect all misstatements. Moreover, projections of any evaluation of effectiveness to
future periods are subject to the risk that controls may become inadequate because of changes in
conditions, or that the degree of compliance with the policies or procedures may deteriorate.
The Company’s management has assessed the effectiveness of the Company’s internal control over
financial reporting as of January 3, 2010. In making this assessment, the Company used the criteria
established by the Committee of Sponsoring Organizations of the Treadway Commission (COSO) in
“Internal Control-Integrated Framework.” These criteria are in the areas of control environment,
risk assessment, control activities, information and communication, and monitoring. The Company’s
assessment included extensive documenting, evaluating and testing the design and operating
effectiveness of its internal controls over financial reporting.
Based on the Company’s processes and assessment, as described above, management has concluded
that, as of January 3, 2010, the Company’s internal control over financial reporting was effective.
The effectiveness of the Company’s internal control over financial reporting as of January 3,
2010 has been audited by PricewaterhouseCoopers LLP, an independent registered public accounting
firm, as stated in their report, which appears herein.
|
 |
|
William C. Weldon
|
Dominic J. Caruso | |
Chairman, Board of Directors, and
|
Vice President, Finance, and | |
Chief Executive Officer
|
Chief Financial Officer |
MANAGEMENT’S REPORT ON INTERNAL CONTROL OVER FINANCIAL REPORTING
65
Table of Contents
Summary of Operations and Statistical Data 1999-2009
| (Dollars in Millions Except Per Share Figures) | 2009 | 2008 | 2007 | 2006 | 2005 | 2004 | 2003 | 2002 | 2001 | 2000 | 1999 | |||||||||||||||||||||||||||||||||
Sales to customer — U.S. |
$ | 30,889 | 32,309 | 32,444 | 29,775 | 28,377 | 27,770 | 25,274 | 22,455 | 19,825 | 17,316 | 15,532 | ||||||||||||||||||||||||||||||||
Sales to customer— International |
31,008 | 31,438 | 28,651 | 23,549 | 22,137 | 19,578 | 16,588 | 13,843 | 12,492 | 11,856 | 11,825 | |||||||||||||||||||||||||||||||||
Total sales |
61,897 | 63,747 | 61,095 | 53,324 | 50,514 | 47,348 | 41,862 | 36,298 | 32,317 | 29,172 | 27,357 | |||||||||||||||||||||||||||||||||
Cost of products sold |
18,447 | 18,511 | 17,751 | 15,057 | 14,010 | 13,474 | 12,231 | 10,498 | 9,622 | 8,987 | 8,559 | |||||||||||||||||||||||||||||||||
Selling, marketing and administrative expenses |
19,801 | 21,490 | 20,451 | 17,433 | 17,211 | 16,174 | 14,463 | 12,520 | 11,510 | 10,675 | 10,182 | |||||||||||||||||||||||||||||||||
Research expense |
6,986 | 7,577 | 7,680 | 7,125 | 6,462 | 5,344 | 4,834 | 4,094 | 3,704 | 3,186 | 2,821 | |||||||||||||||||||||||||||||||||
Purchased in-process research and development |
— | 181 | 807 | 559 | 362 | 18 | 918 | 189 | 105 | 66 | — | |||||||||||||||||||||||||||||||||
Interest income |
(90 | ) | (361 | ) | (452 | ) | (829 | ) | (487 | ) | (195 | ) | (177 | ) | (256 | ) | (456 | ) | (429 | ) | (266 | ) | ||||||||||||||||||||||
Interest expense, net of portion capitalized |
451 | 435 | 296 | 63 | 54 | 187 | 207 | 160 | 153 | 204 | 255 | |||||||||||||||||||||||||||||||||
Other (income) expense, net |
(526 | ) | (1,015 | ) | 534 | (671 | ) | (214 | ) | 15 | (385 | ) | 294 | 185 | (94 | ) | 119 | |||||||||||||||||||||||||||
Restructuring |
1,073 | — | 745 | — | — | — | — | — | — | — | — | |||||||||||||||||||||||||||||||||
| 46,142 | 46,818 | 47,812 | 38,737 | 37,398 | 35,017 | 32,091 | 27,499 | 24,823 | 22,595 | 21,670 | ||||||||||||||||||||||||||||||||||
Earnings before provision for taxes on income |
15,755 | 16,929 | 13,283 | 14,587 | 13,116 | 12,331 | 9,771 | 8,799 | 7,494 | 6,577 | 5,687 | |||||||||||||||||||||||||||||||||
Provision for taxes on income |
3,489 | 3,980 | 2,707 | 3,534 | 3,056 | 4,151 | 2,923 | 2,522 | 2,089 | 1,813 | 1,554 | |||||||||||||||||||||||||||||||||
Net earnings |
12,266 | 12,949 | 10,576 | 11,053 | 10,060 | 8,180 | 6,848 | 6,277 | 5,405 | 4,764 | 4,133 | |||||||||||||||||||||||||||||||||
Percent of sales to customers |
19.8 | 20.3 | 17.3 | 20.7 | 19.9 | 17.3 | 16.4 | 17.3 | 16.7 | 16.3 | 15.1 | |||||||||||||||||||||||||||||||||
Diluted net earnings per share of common stock |
$ | 4.40 | 4.57 | 3.63 | 3.73 | 3.35 | 2.74 | 2.29 | 2.06 | 1.75 | 1.55 | 1.34 | ||||||||||||||||||||||||||||||||
Percent return on average shareholders’ equity |
26.4 | 30.2 | 25.6 | 28.3 | 28.2 | 27.3 | 27.1 | 26.4 | 24.0 | 25.3 | 26.0 | |||||||||||||||||||||||||||||||||
Percent increase (decrease) over previous year: |
||||||||||||||||||||||||||||||||||||||||||||
Sales to customers |
(2.9 | ) | 4.3 | 14.6 | 5.6 | 6.7 | 13.1 | 15.3 | 12.3 | 10.8 | 6.6 | 14.9 | ||||||||||||||||||||||||||||||||
Diluted net earnings per share |
(3.7 | ) | 25.9 | (2.7 | ) | 11.3 | 22.3 | 19.7 | 11.2 | 17.7 | 12.9 | 15.7 | 34.0 | |||||||||||||||||||||||||||||||
Supplementary expense data: |
||||||||||||||||||||||||||||||||||||||||||||
Cost of materials and services(1) |
$ | 27,651 | 29,346 | 27,967 | 22,912 | 22,328 | 21,053 | 18,568 | 16,540 | 15,333 | 14,113 | 13,922 | ||||||||||||||||||||||||||||||||
Total employment costs |
14,587 | 14,523 | 14,571 | 13,444 | 12,364 | 11,581 | 10,542 | 8,942 | 8,153 | 7,376 | 6,727 | |||||||||||||||||||||||||||||||||
Depreciation and amortization |
2,774 | 2,832 | 2,777 | 2,177 | 2,093 | 2,124 | 1,869 | 1,662 | 1,605 | 1,592 | 1,510 | |||||||||||||||||||||||||||||||||
Maintenance and repairs(2) |
567 | 583 | 483 | 506 | 510 | 462 | 395 | 360 | 372 | 327 | 322 | |||||||||||||||||||||||||||||||||
Total tax expense(3) |
5,052 | 5,558 | 4,177 | 4,857 | 4,285 | 5,215 | 3,890 | 3,325 | 2,854 | 2,517 | 2,221 | |||||||||||||||||||||||||||||||||
Supplementary balance sheet data: |
||||||||||||||||||||||||||||||||||||||||||||
Property, plant and equipment, net |
14,759 | 14,365 | 14,185 | 13,044 | 10,830 | 10,436 | 9,846 | 8,710 | 7,719 | 7,409 | 7,155 | |||||||||||||||||||||||||||||||||
Additions to property, plant and equipment |
2,365 | 3,066 | 2,942 | 2,666 | 2,632 | 2,175 | 2,262 | 2,099 | 1,731 | 1,689 | 1,822 | |||||||||||||||||||||||||||||||||
Total assets |
94,682 | 84,912 | 80,954 | 70,556 | 58,864 | 54,039 | 48,858 | 40,984 | 38,771 | 34,435 | 31,163 | |||||||||||||||||||||||||||||||||
Long-term debt |
8,223 | 8,120 | 7,074 | 2,014 | 2,017 | 2,565 | 2,955 | 2,022 | 2,217 | 3,163 | 3,429 | |||||||||||||||||||||||||||||||||
Operating cash flow |
16,571 | 14,972 | 15,022 | 14,248 | 11,799 | 11,089 | 10,571 | 8,135 | 8,781 | 6,889 | 5,913 | |||||||||||||||||||||||||||||||||
Common stock information |
||||||||||||||||||||||||||||||||||||||||||||
Dividends paid per share |
$ | 1.930 | 1.795 | 1.620 | 1.455 | 1.275 | 1.095 | 0.925 | 0.795 | 0.700 | 0.620 | 0.550 | ||||||||||||||||||||||||||||||||
Shareholders’ equity per share |
$ | 18.37 | 15.35 | 15.25 | 13.59 | 13.01 | 10.95 | 9.25 | 7.79 | 8.05 | 6.82 | 5.73 | ||||||||||||||||||||||||||||||||
Market price per share (year-end close) |
$ | 64.41 | 58.56 | 67.38 | 66.02 | 60.10 | 63.42 | 50.62 | 53.11 | 59.86 | 52.53 | 46.63 | ||||||||||||||||||||||||||||||||
Average shares outstanding (millions) — basic |
2,759.5 | 2,802.5 | 2,882.9 | 2,936.4 | 2,973.9 | 2,968.4 | 2,968.1 | 2,998.3 | 3,033.8 | 2,993.5 | 2,978.2 | |||||||||||||||||||||||||||||||||
— diluted |
2,789.1 | 2,835.6 | 2,910.7 | 2,961.0 | 3,002.8 | 2,992.7 | 2,995.1 | 3,049.1 | 3,089.3 | 3,075.2 | 3,090.4 | |||||||||||||||||||||||||||||||||
Employees (thousands) |
115.5 | 118.7 | 119.2 | 122.2 | 115.6 | 109.9 | 110.6 | 108.3 | 101.8 | 100.9 | 99.8 | |||||||||||||||||||||||||||||||||
| (1) | Net of interest and other income. | |
| (2) | Also included in cost of materials and services category. | |
| (3) | Includes taxes on income, payroll, property and other business taxes. |
SUMMARY OF OPERATIONS AND STATISTICAL DATA
66
Table of Contents
Shareholder Return Performance Graphs
Set forth below are line graphs comparing the cumulative total shareholder return on the
Company’s Common Stock for periods of five years and ten years ending December 31, 2009, against
the cumulative total return of the Standard & Poor’s 500 Stock Index, the Standard & Poor’s
Pharmaceutical Index and the Standard & Poor’s Health Care Equipment Index. The graphs and tables
assume that $100 was invested on December 31, 2004 and December 31,1999 in each of the Company’s
Common Stock, the Standard & Poor’s 500 Stock Index, the Standard & Poor’s Pharmaceutical Index and
the Standard & Poor’s Health Care Equipment Index and that all dividends were reinvested.
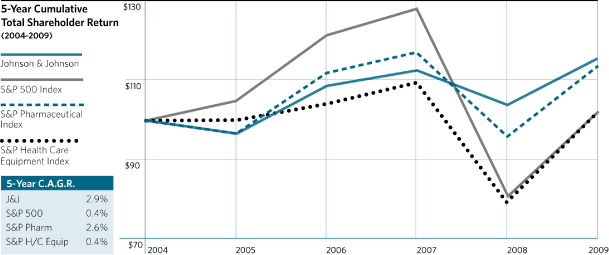
| 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | |||||||||||||||||||||||||||
Johnson & Johnson |
$ | 100.00 | 96.64 | 108.67 | 112.59 | 103.84 | 115.55 | |||||||||||||||||||||||||
S&P 500 Index |
$ | 100.00 | 104.91 | 121.48 | 128.15 | 80.74 | 102.11 | |||||||||||||||||||||||||
S&P Pharmaceutical Index |
$ | 100.00 | 96.64 | 111.96 | 117.17 | 95.85 | 113.68 | |||||||||||||||||||||||||
S&P Health Care Equipment Index |
$ | 100.00 | 100.05 | 104.18 | 109.52 | 79.25 | 102.06 | |||||||||||||||||||||||||
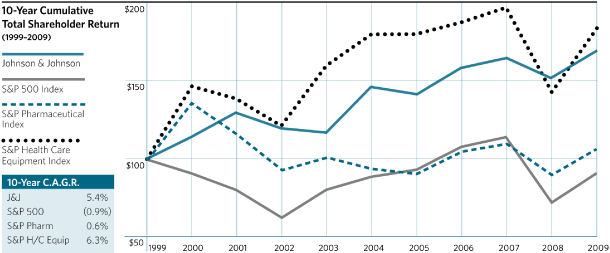
| 1999 | 2000 | 2001 | 2002 | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | |||||||||||||||||||||||||||||||||||||||||||||||
Johnson & Johnson |
$ | 100.00 | 114.20 | 130.20 | 119.94 | 117.41 | 146.95 | 142.02 | 159.69 | 165.46 | 152.60 | 169.81 | |||||||||||||||||||||||||||||||||||||||||||||
S&P 500 Index |
$ | 100.00 | 90.92 | 80.11 | 62.41 | 80.31 | 89.04 | 93.42 | 108.17 | 114.11 | 71.89 | 90.92 | |||||||||||||||||||||||||||||||||||||||||||||
S&P Pharmaceutical Index |
$ | 100.00 | 136.20 | 116.39 | 93.07 | 101.23 | 93.71 | 90.56 | 104.92 | 109.80 | 89.82 | 106.54 | |||||||||||||||||||||||||||||||||||||||||||||
S&P Health Care Equipment Index |
$ | 100.00 | 146.64 | 139.21 | 121.61 | 160.57 | 180.84 | 180.93 | 188.39 | 198.06 | 143.31 | 184.56 | |||||||||||||||||||||||||||||||||||||||||||||
SHAREHOLDER RETURN PERFORMANCE GRAPHS
67
