Attached files
| file | filename |
|---|---|
| 10-K - 10-K - UNITED THERAPEUTICS Corp | a2196607z10-k.htm |
| EX-21 - EX-21 - UNITED THERAPEUTICS Corp | a2196607zex-21.htm |
| EX-23.1 - EX-23.1 - UNITED THERAPEUTICS Corp | a2196607zex-23_1.htm |
| EX-31.1 - EX-31.1 - UNITED THERAPEUTICS Corp | a2196607zex-31_1.htm |
| EX-32.2 - EX-32.2 - UNITED THERAPEUTICS Corp | a2196607zex-32_2.htm |
| EX-31.2 - EX-31.2 - UNITED THERAPEUTICS Corp | a2196607zex-31_2.htm |
| EX-32.1 - EX-32.1 - UNITED THERAPEUTICS Corp | a2196607zex-32_1.htm |
| EX-12.1 - EX-12.1 - UNITED THERAPEUTICS Corp | a2196607zex-12_1.htm |
| EX-10.46 - EX-10.46 - UNITED THERAPEUTICS Corp | a2196607zex-10_46.htm |
| EX-10.48 - EX-10.48 - UNITED THERAPEUTICS Corp | a2196607zex-10_48.htm |
Exhibit 10.47
DISTRIBUTION AGREEMENT
THIS DISTRIBUTION AGREEMENT (“Agreement”) is made as of August 17, 2009 (the “Effective Date”), by and between United Therapeutics Corporation (“UT”), a Delaware corporation, with offices at 1110 Spring Street, Silver Spring, Maryland and Accredo Health Group, Inc (“DISTRIBUTOR”), a Delaware corporation, with offices at 1640 Century Center Parkway, Memphis, Tennessee 38134.
Recitals
A. WHEREAS, DISTRIBUTOR has represented that it possesses the necessary expertise, financial resources and marketing organization to dispense and distribute UT Product (as hereinafter defined) and desires to acquire from UT the right to sell, market, distribute and maintain UT Product in the Territory (as hereinafter defined);
B. WHEREAS, UT is willing to appoint DISTRIBUTOR and DISTRIBUTOR is willing to accept appointment, as a distributor of UT Product in the Territory on the terms and conditions contained herein; and
C. WHEREAS, the Parties hereto believe that the business relationship regarding UT Product and related support will be mutually advantageous.
NOW, THEREFORE, in consideration of the mutual promises and covenants hereinafter set forth, the Parties agree as follows:
ARTICLE 1: INTRODUCTORY PROVISIONS
1.1 Defined Terms. The following terms, when used in capitalized form in this Agreement, shall have the meanings set forth below:
(a) “Agreement” shall mean this Distribution Agreement entered into by and between UT and DISTRIBUTOR as of the Effective Date.
(b) “Adverse Event” shall mean any “Adverse Drug Experience” as defined in 21 CFR 310.305, 21 CFR 314.80 and/or 21 CFR 600.80 (as applicable) or any replacements thereto.
(c) “Affiliate” when used with reference to either Party shall mean any corporation controlling, controlled by or under common control with the said Party and any officer, director or employee of such corporation, as the case may be. For purposes hereof, “control” shall mean ownership, directly or indirectly, of more than fifty percent (50%) of the securities having the right to vote for the election of directors, in the case of a corporation, and more than fifty percent (50%) of the beneficial interest in the capital, in the case of a business entity other than a corporation.
(d) “Applicable Laws” shall mean all laws, statutes, ordinances, codes, rules, and regulations that have been enacted by a government authority and which are in force as of the Effective Date or come into force during the term of this Agreement, in each case to the extent that the same are applicable to the performance by the Parties of their respective obligations under this Agreement, including, with respect to the United States, the Prescription Drug Marketing Act, the Federal Food, Drug and Cosmetics Act of 1938, as amended, the Health
Insurance Portability and Accountability Act, the Federal Anti-Kickback Statute, and any applicable FDA regulations.
(e) “Clean Prescription” shall mean a referral for which benefits have been verified and that includes a valid prescription that does not: (i) require physician, patient, or any third party intervention or information; (ii) involve backorder, short supply, allocation, or recall; or (iii) involve a referral that is subsequently canceled or requested to be held for future processing.
(f) “Commercially Reasonable Efforts” shall mean with respect to each Party, commercially reasonable efforts in accordance with the business, legal, medical and scientific judgment of a similarly situated company, and in accordance with the efforts and resources a similarly situated company would use taking into account reasonable commercial judgment and other relevant factors.
(g) “Confidential Information” shall mean all information disclosed by one Party (“Disclosing Party”) to the other Party (“Receiving Party”), regardless of the form in which it is disclosed, including information relating to the Disclosing Party’s markets, product specific payer policies, databases, customers, products, patents, inventions, procedures, methods, designs, strategies, plans, assets, liabilities, prices, costs, revenues, profits, organization, employees, agents, resellers or business in general, and with respect to UT as Disclosing Party, information embodied in UT Product. The following shall not be considered Confidential Information:
(i.) Information which is or becomes in the public domain through no fault or act of the Receiving Party;
(ii.) Information which was independently developed by the Receiving Party without the use of or reliance on Confidential Information;
(iii.) Information which was provided to the Receiving Party by a third party under no duty of confidentiality to the Disclosing Party; or
(iv.) Information that is required to be disclosed by Applicable Laws, provided, however, prompt prior notice thereof shall be given to the Disclosing Party.
(h) “Customer” shall mean any hospital, physician, health care company, Included Patient, distributor or other person or entity that is legally entitled to purchase the UT Product for use in the Territory.
(i) “Designated Shipment Location” shall mean the Designated Storage Location(s) to which UT has agreed to ship Units of UT Product as set forth in Attachment E attached hereto.
(j) “Designated Storage Location” shall mean the locations of DISTRIBUTOR’s facilities or pharmacies owned by DISTRIBUTOR or its Affiliate(s) for the storage of the Units of UT Product shipped to DISTRIBUTOR’s Designated Storage Locations as set forth in Attachment E attached hereto.
(k) “DISTRIBUTOR” shall mean Accredo Health Group, Inc. and its wholly owned subsidiaries.
(l) “Effective Date” shall mean the date first above written.
(m) “Force Majeure” shall mean any event, not existing as of the Effective Date and not reasonably within the control of the Parties as of such date, which, in whole or in material part, prevents or makes commercially unreasonable one Party’s performance of its obligations under this Agreement. Force Majeure shall include, without limitation: fire, storm, earthquake, flood, acts of state, war or civil unrest, labor dispute, inability to obtain labor or materials, and prolonged shortage of energy or any other supplies.
(n) “Good Distribution Practice” shall mean that practice of purchasing, storing and shipping a regulated pharmaceutical product and billing to and collecting from customers for a regulated pharmaceutical product in accordance with legal requirements and the standards and customary industry commercial practices.
(o) “Included Patient” shall mean an individual diagnosed with pulmonary arterial hypertension (“PAH”) who is prescribed UT Product.
(p) “Level 1 Appeal” shall mean an appeal of a reimbursement claim denial by a Third Party Payer due to an incomplete or improperly submitted reimbursement claim or other similar administrative oversight.
(q) “Level 2 Appeal” shall mean an appeal of a reimbursement claim denial by a Third Party Payer, whether such denial is first asserted upon verification of reimbursement or following submission of a reimbursement claim, because: (i) the applicable policy covering the Included Patient does not include UT Product as a covered benefit, or (ii) the Included Patient falls within a class of persons who are all denied coverage for UT Product as the result of the application of a general policy.
(r) “PAP Patient” shall mean any Included Patient who is enrolled in the Patient Assistance Program as established by UT from time to time and operated in accordance with Attachment C hereto. UT shall provide DISTRIBUTOR with the eligibility criteria for this program.
(s) “Price” shall mean the Wholesale Acquisition Cost for UT Product as set forth on Attachment A hereto.
(t) “UT Trademarks” shall mean any of the UT trademarks, logotypes and trade names listed on Attachment B hereto, as such attachment may be modified from time to time by UT during the term of this Agreement.
(u) “Parties” shall mean UT and DISTRIBUTOR collectively.
(v) “Party” shall mean either UT or DISTRIBUTOR.
(w) “Territory” shall mean the United States, including its territories and possessions, the fifty states and the District of Columbia only, unless otherwise expressly agreed in writing by the Parties.
(x) “Third-Party Payers” shall mean managed care providers, health maintenance organizations, insurance companies, self-insurance programs of employers, third-
party administrators, the United States Medicare and Medicaid programs, and other similar entities.
(y) “Tyvaso Inhalation System” shall mean the Optineb® portable nebulizer system and all related supplies and accessories.
(z) “Unit of UT Product” shall mean the combination of UT Product, package insert and other items as may be determined and supplied by UT to DISTRIBUTOR, in each instance contained within a standard outer package supplied by UT and labeled in accordance with applicable legal requirements.
(aa) “UT Product” or “Product” shall mean treprostinil sodium for inhalation only, a pharmaceutical product administered by the Tyvaso Inhalation System for the treatment of PAH to be marketed in the Territory under the brand name Tyvaso®.
(bb) “WAC” shall mean the then-current Wholesale Acquisition Cost of UT Product as determined by UT.
1.2 Other Rules of Interpretation. Unless the context otherwise requires, (i) words of any gender include each other gender; (ii) words using the singular or plural number also include the plural or singular number, respectively; (iii) the terms “hereof,” “herein,” “hereby” and derivative or similar words refer to this entire Agreement; (iv) the terms “Article” or “Section” refer to the specified Article or Section of this Agreement; (v) the word “including” shall mean “including, without limitation;” (vi) the word “consent” shall mean “consent, not to be unreasonably withheld or delayed”; (vii) the word “or” shall be disjunctive but not exclusive; (viii) the words “made available” shall mean that the information referred to has been made available if requested by the party to whom such information is to be made available; and (ix) all references herein to “days” shall mean calendar days.
ARTICLE 2: MUTUAL REPRESENTATIONS AND WARRANTIES
2.1 Authority. Each Party represents and warrants that it possesses all corporate power and authority necessary to enter into this Agreement and to perform its obligations under this Agreement. All corporate acts and other proceedings required to be taken by or on the part of each Party to authorize it to perform its obligations under this Agreement have been duly and properly taken. This Agreement has been duly executed and delivered by each Party and constitutes legal, valid and binding obligations of each Party enforceable in accordance with its terms, subject to the application of general principles of equity.
2.2 No Conflicts. Each Party represents and warrants that the execution and performance of this Agreement will not conflict with or violate any other agreement or obligation binding on it.
2.3 Approvals. Except as expressly provided herein, each Party represents and warrants that no approval, authorization, consent or other order or action of or filing with any court, administrative agency or other governmental authority is required for the execution and delivery by such Party of this Agreement or its consummation of the transactions contemplated by this Agreement.
2.4 Debarment and Exclusion Certification Requirements. Each Party certifies that it has not been debarred under the provisions of the Generic Drug Enforcement Act of 1992, 21 U.S.C. § 335(a) and (b), and does not appear on the “list of excluded
individuals/entities” (“LEIE”) maintained by the Office of the Inspector General of the U.S. Department of Health & Human Services, available at http://oig.hhs.gov/fraud/exclusions/listofexcluded.html. In the event that, during the term of this Agreement, either Party (i) becomes debarred, (ii) is placed on the LEIE, or (iii) receives notice of an action or threat of an action with respect to its debarment or placement on the LEIE, such Party shall notify the other Party immediately. Each Party hereby certifies that it has not and will not use in any capacity the services of any individual, corporation, partnership or association that has been debarred under 21 U.S.C. § 335(a) and (b) or that appears on the LEIE. In the event that either Party becomes aware of the debarment, threatened debarment, appearance or threatened placement on the LEIE of any individual, corporation, partnership or association providing services to the other Party that directly or indirectly relate to activities under this Agreement, the other Party shall be immediately notified. In the event of an actual debarment or exclusion of DISTRIBUTOR or its owners during the term of this Agreement, this Agreement shall, as of, or prior to, the effective date of such debarment or exclusion, automatically terminate. In the event of an actual debarment or exclusion of any DISTRIBUTOR employee, agent or contractor during the term of this Agreement, such employee, agent or contractor must immediately cease providing any services to UT under this Agreement, and UT shall have the option of immediately terminating this Agreement.
ARTICLE 3: APPOINTMENT
3.1 Scope; Non-exclusive. UT hereby appoints DISTRIBUTOR, and DISTRIBUTOR hereby accepts such appointment, as a distributor of UT Product during the term of this Agreement, subject to the terms and conditions of this Agreement. This appointment is non-exclusive, and UT reserves the right to appoint additional distributors in the Territory and to distribute UT Product in the Territory on its own behalf.
3.2 Sub-distributors. DISTRIBUTOR shall not, without the prior written approval of UT, appoint any distributors or agents to act on behalf of DISTRIBUTOR (collectively, “Sub-distributors”) to distribute UT Product within the Territory, other than any of its Affiliates. DISTRIBUTOR shall at all times remain fully liable for the performance of any approved sub-distributors and DISTRIBUTOR shall provide UT with a written acknowledgement executed by each Sub-distributor that it has read this Agreement and agrees to be bound by its terms and conditions, including those contained in the attachments hereto.
3.3 Sales Outside the Territory. DISTRIBUTOR shall not distribute, sell or otherwise provide UT Product outside of the Territory and shall not advertise, promote or solicit customers for UT Product outside the Territory.
ARTICLE 4: OBLIGATIONS OF DISTRIBUTOR
4.1 Marketing. DISTRIBUTOR shall use Commercially Reasonable Efforts to fund and support ongoing marketing of its distribution of UT Product, consistent with DISTRIBUTOR’s normal funding and support for its overall distribution activities. In addition, DISTRIBUTOR shall use its Commercially Reasonable Efforts to fund and support ongoing sale of UT Product. Such Commercially Reasonable Efforts shall include, but not be limited to:
(a) Maintaining throughout the Territory adequate marketing, sales, and order-fulfillment staff who are adequately trained on PAH and UT Product. The
Parties acknowledge that this obligation requires DISTRIBUTOR to have the capability to provide the foregoing services throughout the Territory, but does not require DISTRIBUTOR to have a physical office within each jurisdiction within the Territory;
(b) Promptly responding to all inquiries from Customers, including responding to complaints, processing all orders and effecting all shipments of UT Product for Included Patients in accordance with the timelines and other terms and conditions contained within this Agreement;
(c) Providing UT Product to Included Patients pursuant to physician orders;
(i.) Exhibiting at conventions, trade shows, product fairs or other comparable events involving patients, Included Patients and/or physician specialists who have a high propensity to diagnose and treat patients suffering from PAH for the purpose of providing information on UT Product and/or educating attendees.
(d) Diligently investigating and pursuing all leads and inquiries of potential Customers referred to DISTRIBUTOR by UT and reporting within 7 days on the status of all such leads and inquiries. Notwithstanding the foregoing, nothing in this Agreement shall be construed as requiring DISTRIBUTOR to admit to its service, or provide UT Product to, any particular individual(s) or types of individual(s). Adding Included Patients to its service is in DISTRIBUTOR’s sole discretion.
4.2 Policies and Procedures. DISTRIBUTOR shall use Commercially Reasonable Efforts to comply with UT’s Policies and Procedures as provided and updated by UT from time to time and as accepted by DISTRIBUTOR. If any such Policies and Procedures contradict this Agreement, the terms of this Agreement shall control.
4.3 Written Assurance. DISTRIBUTOR hereby assures UT that DISTRIBUTOR shall not export UT Product from the Territory to any destination to which re-export requires a license under the United States Export Administration Regulations.
4.4 Product Specifications. DISTRIBUTOR shall store UT Product in accordance with all directions accompanying UT Product in order to maintain UT Product in accordance with UT- and FDA-approved specifications. DISTRIBUTOR shall dispense UT Product as prescribed, in accordance with all applicable pharmacy requirements. The Parties acknowledge that UT shall not have any rights, obligations, responsibilities, oversight or role of any kind or nature concerning DISTRIBUTOR’s practice of pharmacy in compliance with all applicable state pharmacy regulations and consistent with DISTRIBUTOR’s then current practices.
4.5 Pharmacy and Home Health Care Services. DISTRIBUTOR may create its own educational materials concerning UT Product or PAH (“Educational Materials”) for distribution by DISTRIBUTOR in accordance with this Agreement and DISTRIBUTOR’s obligations as a health care provider and pharmacy; provided, however, that all such Educational Materials shall: (i) be consistent with the contents of UT Product package insert approved by the FDA; (ii) comply with the conditions and requirements of all applicable state pharmacy regulations mandating the provision of patient educational materials on prescription drugs and their administration, and (iii) not be used by DISTRIBUTOR to promote, market or sell UT Product. Further, to the extent
that any UT Trademarks are included in such Educational Materials, then DISTRIBUTOR shall notify UT in writing prior to use of such materials.
4.6 Complaints., DISTRIBUTOR shall process any and all complaints received from Customers in the Territory regarding the UT Product in accordance with Section 9.3 of this Agreement.
4.7 Inventory. DISTRIBUTOR shall maintain at all times adequate inventory of Units of UT Product (the “Inventory”) as are mutually considered by UT and DISTRIBUTOR to be sufficient to meet Customers’ anticipated demands for UT Product. Such requirements may be adjusted by UT and DISTRIBUTOR from time to time. Notwithstanding the foregoing, DISTRIBUTOR shall maintain a minimum Inventory level at all times between the following minimum and maximum:
(a) At a minimum: no less than thirty (30) days’ inventory on hand at any time based on current demand and usage of UT Product by DISTRIBUTOR’s customers; and
(b) At a maximum no greater than seventy-five (75) days’ inventory on hand based on current demand and usage of UT Product by DISTRIBUTOR’s customers; and
(c) calculations of inventory levels shall be based on the current monthly average usage of UT Product by Included Patients (“Usage”). Usage shall be equal to the rolling average number of Units of UT Product distributed by DISTRIBUTOR each month for the previous three (3) months.
DISTRIBUTOR shall ensure that it purchases enough Inventory each month to meet Usage demand for UT Product in addition to the thirty (30) day minimum Inventory level requirement. From time to time, UT and DISTRIBUTOR may mutually agree to reasonably change the above-listed minimum and maximum requirements and DISTRIBUTOR shall adjust its Inventory accordingly.
4.8 Storage of UT Product. DISTRIBUTOR shall store and maintain UT Product solely at the Designated Storage Locations described in Attachment E hereto. DISTRIBUTOR shall store, maintain and handle the Product in accordance with Good Distribution Practice, Applicable Laws, the UT Product package insert and UT’s written instructions, including any requirements with respect to racking, temperature, light, darkness, vibration and rotation. The UT Product must be stored at the temperature range specified by UT to ensure safety and reliability, and rotated so that the oldest unexpired Units of UT Product are shipped before newer unexpired Units of UT Product, unless UT specifies otherwise. DISTRIBUTOR shall promptly notify UT of any material or significant change in its storage conditions or shipping procedures for UT Product. DISTRIBUTOR shall maintain complete and accurate records for inspection by UT or its representatives, upon ten (10) business days’ prior notice during regular business hours, of all movements and transactions involving UT Product. Such records shall reflect unit, lot number and Customer information, including defective or returned Units of UT Product, such that the Units of UT Product may be traced for purposes of stock reconciliation, recall and general marketing and shipping review. UT shall also have the right to inspect DISTRIBUTOR’s storage conditions and shipping procedures for UT Product upon ten (10) business days’ prior notice, during regular business hours. DISTRIBUTOR shall not manufacture, mix, process, combine or incorporate UT Product alone or into any other substance.
4.9 Distributor Expenses. DISTRIBUTOR shall bear all of its own costs and expenses incurred in carrying out its obligations under this Agreement, including, but not limited to, all rents, salaries, commissions, demonstration, travel and accommodation.
4.10 Distributor Reporting. DISTRIBUTOR shall complete a series of regular reports as described in Attachment F hereto. The reports are due no later than the 10th of each month following the end of the respective reporting periods and constitute Confidential Information of DISTRIBUTOR. The Parties acknowledge that Applicable Laws or existing contractual relationships with Third-Party Payers may restrict DISTRIBUTOR’s ability to collect, use, include and/or disclose as Data certain patient- and physician-specific data. DISTRIBUTOR warrants that it will not provide patient- and physician-specific data and information where so limited by such existing contractual relationships or Applicable Laws. New contracts with Third Party Payers and the enactment of new Applicable Laws which further limit the disclosure of patient- and physician-specific data and information shall not be deemed to be a change in a law for purposes of this Agreement. Neither Party may resell data to IMS, Wolters Kluwer, or any other data aggregation service without the express written consent of the other Party.
4.11 Distributor Representations.
(a) DISTRIBUTOR acknowledges that UT Product constitutes a sensitive therapeutic drug, and that distribution and handling of the UT Product requires specialized training and dedication to Customer needs. DISTRIBUTOR represents and warrants that it will train and deploy its agents and employees in the manner necessary to meet these special requirements.
(b) DISTRIBUTOR represents and warrants that it and its officers, directors, agents and/or employees as applicable are qualified to perform the services and activities described in this Agreement and that all licenses and/or approvals necessary to conduct such services and activities have been obtained and shall be maintained throughout the term of this Agreement.
4.12 DISTRIBUTOR provides appropriate pharmacy services as required by Applicable Laws. DISTRIBUTOR shall also perform the following activities in support of the distribution of the UT Product:
(a) Inhalation Device: DISTRIBUTOR shall ensure that its personnel are trained on the use of the Tyvaso Inhalation System and capable of providing such training to Customers. Training and education for use must comply with the technical and administrative requirements specified in the package insert for Tyvaso and the instructions for use manual for the Tyvaso Inhalation System.
(b) Included Patient Benefit Verification: DISTRIBUTOR shall handle Included Patient enrollment, initial processing, insurance eligibility and benefits verification. If DISTRIBUTOR is unable to service a patient, then DISTRIBUTOR shall immediately, i.e., no more than five (5) business days from the initial receipt of the referral, re-direct the referral to an appropriate specialty pharmacy participating in the Tyvaso distribution network.
(i.) Upon receipt of a prescription for UT Product, DISTRIBUTOR shall immediately fax the prescribing physician to confirm receipt of the prescription. No more than one (1) business day from receipt of the prescription, DISTRIBUTOR shall perform verification of insurance
coverage for UT Product. If the prescription is received after 2 p.m. Eastern time, DISTRIBUTOR may have until the end of the next business day to perform verification of insurance coverage for UT Product.
(ii.) DISTRIBUTOR shall take all necessary actions to verify, or assist Customers in verifying, insurance coverage for UT Product including, without limitation, researching and attempting to determine: (1) all Customer information and coverage parameters, including all relevant clinical documentation; (2) if UT Product is covered, under what type of plan (e.g., a “medical plan” or a “pharmacy plan”), the Included Patient cost share amount, if any, and the rate of reimbursement, if available; (3) whether prior authorization is required for reimbursement; (4) if prior authorization is required, what information the Customer must submit in order to receive such authorization; and (5) whether any other activities, submissions or approvals are required to obtain reimbursement promptly and to the fullest extent permitted by the Third-Party Payer. During the process of benefit verification, DISTRIBUTOR shall communicate with the referral source and provide information to the prescribing physician in a time and manner sufficient for the circumstances.
(iii.) DISTRIBUTOR shall record the results of its research on the foregoing and shall report such information to the Customer within one (1) business day from receipt. [Rachel & Mike are OK with this]
(iv.) If the Third-Party Payer requires prior authorization, then DISTRIBUTOR shall, within one (1) business day, notify and assist the Customer with questions relating to the requirements for prior authorization.
(v.) If, prior to the submission of a claim for reimbursement, a Third-Party Payer informs DISTRIBUTOR that the Customer or UT Product is not eligible for coverage, then, within one (1) business day, DISTRIBUTOR shall make such inquiries of the Third-Party Payer as shall be necessary to determine the requirements for submission of an appeal of the denial of coverage. DISTRIBUTOR shall promptly record the results of this inquiry and report such information to the Customer and to the UT managed markets designee.
(vi.) If a Customer notifies DISTRIBUTOR of a denial of coverage and DISTRIBUTOR determines that an appeal of the denial of coverage would require a Level 1 Appeal, then DISTRIBUTOR shall immediately notify the Customer. The Customer, at its option, may elect to pursue the Level 1 Appeal directly or request DISTRIBUTOR’s assistance. If the Customer elects to have DISTRIBUTOR assist with the Level 1 Appeal, DISTRIBUTOR, at its cost, shall use reasonable efforts to assist Customer, and if an Included Patient is pursuing the Level 1 Appeal on his/her own behalf, DISTRIBUTOR, at its cost, shall promptly initiate (at the latest within one (1) business day) and pursue such Level 1 Appeal in accordance with the Third-Party Payer’s processes. Upon request, UT shall provide reasonable assistance to DISTRIBUTOR, including assistance with preparing applications and participation in telephone conferences and meetings with representatives of the Third-
Party Payer. DISTRIBUTOR shall notify the Customer immediately following any interim and final determinations by the Third Party Payer in response to any Level 1 Appeal. All documents prepared as part of a Level 1 Appeal, and any information obtained in connection therewith, shall be promptly recorded.
(vii.) If DISTRIBUTOR determines that an appeal of the denial of coverage would require a Level 2 Appeal, DISTRIBUTOR shall notify the Customer, the Included Patient, and UT (if DISTRIBUTOR deems necessary and if the Included Patient consents) immediately of such determination. The Included Patient, at his or her option, may elect to pursue the Level 2 Appeal directly or to request that Customer assist with pursuit of the Level 2 Appeal. If Customer assists with the pursuit of a Level 2 Appeal, DISTRIBUTOR shall provide reasonable assistance to Customer, including assistance with preparing applications and participation in telephone conferences and meetings with representatives of the Third-Party Payer.
(c) Dispensing Activities:
(i.) Upon completion of benefits investigation and, if necessary, after prior authorization, DISTRIBUTOR shall process Customer’s order for UT Product if Customer chooses to place an order. If Customer elects not to place an order at the time that Included Patient benefits are reported, DISTRIBUTOR shall attempt to determine the reason for Customer’s choice (e.g., “Included Patient to receive UT Product at an alternate facility”, “physician elected not to order UT Product”, or “Included Patient elected not to receive UT Product”). DISTRIBUTOR shall immediately record this information.
(ii.) When the prescriber is the Customer, DISTRIBUTOR shall attempt to contact the Included Patient on the same day that the benefit verification has been completed for the Included Patient in order to inform the Included Patient of his or her cost share amount, if any, and to make arrangements with the Included Patient for collection such cost share amount, if any, and to introduce the Included Patient to the DISTRIBUTOR’s services. DISTRIBUTOR may delay shipment of UT Product until the Included Patient’s cost share amount is satisfied in full. DISTRIBUTOR shall be solely responsible for submitting claims for reimbursement directly to the Third-Party Payer for the applicable reimbursable amount (deducting any Included Patient cost share amount).
(iii.) DISTRIBUTOR shall dispense the Unit(s) of UT Product (along with a current package insert) to Included Patients pursuant to a valid prescription and in accordance with Applicable Laws. Upon receipt of a Clean Prescription, DISTRIBUTOR shall ensure that the Included Patient receives UT Product within one (1) business day from receipt of such prescription, or at such other time as the Included Patient may request.
(d) Follow up Activity Generally: Unless DISTRIBUTOR is otherwise required to contact Customer sooner or more often, DISTRIBUTOR shall contact Customer
two (2) business days after receipt of a prescription/referral and every two (2) business days thereafter to update Customer on the status of a benefits investigation/prior authorization/appeal or other related matter. When required to obtain additional information to complete a valid prescription/coverage determination/prior authorization/appeal or related matter, DISTRIBUTOR shall communicate all required information to the appropriate party and continue to contact such party every business day until the needed information is received or the matter is otherwise closed.
(a) Social Services: DISTRIBUTOR shall engage in patient advocacy and upon receipt of inquiries from or Customers, provide notice to such Customers of alternate funding sources, certain hardship reimbursement support, and certain indigent and patient assistance programs, including UT’s PAP as described in Attachment C hereto. DISTRIBUTOR shall send an application to all eligible Included Patients who request to participate in the PAP within one (1) business day from the date of such request, with notice to the referral source (via fax, email or mail) as well.
(b) Product & Ancillary Supply Distribution: DISTRIBUTOR shall make available and/or dispense with UT Product, as necessary and appropriate for the applicable site of service (e.g., health care provider/physician office, clinic, hospital outpatient setting, pharmacy-owned facility, home), the contents of the UT Product package and supplies necessary for UT Product administration.
(c) Education: DISTRIBUTOR shall provide its standard educational support (including the provision of any UT Materials or Educational Materials) regarding UT Product administration and safety to Customers and caregivers involved in treating Included Patients. DISTRIBUTOR shall promptly respond to questions from managed care organizations and other Third-Party Payers about UT Product. Notwithstanding the foregoing, the provision of such educational services shall be performed in accordance with the obligations contained in this Agreement including those with respect to training.
(e) Nursing Services:
(i.) DISTRIBUTOR shall make available on an as-needed basis its standard telephonic nursing services in accordance with its standard policies and procedures. If DISTRIBUTOR receives requests for administration for UT Product, it shall facilitate such requests in accordance with its standard business practices. DISTRIBUTOR’s standard telephonic nursing services shall be rendered by nurses who have the requisite and necessary training, experience, licenses and permits in accordance with Applicable Laws. DISTRIBUTOR may not seek reimbursement for its standard telephonic nursing services directly from UT or from the Included Patient.
(i.) the Parties shall work together in good faith to develop an integrated nursing program to adequately support UT Product, Included Patients and Customers with the following elements:
(a) All nurses shall be trained by DISTRIBUTOR with respect to UT Product and PAH prior to any interaction with an Included Patient or Customer. All nurses (including per diem nurses) shall
pass competency testing on the following topics (at a minimum): PAH and PAH drug classes; UT Product; Patient needs whether naïve or experienced; Administration of UT Product; Training patients on administration of UT Product; Relevant nursing standards of care for administration of UT Product; Any and all devices/pumps that are to be used with UT Product; An “ideal patient encounter”; and HIPAA, patient privacy and any other applicable legal requirements;
(b) DISTRIBUTOR shall provide updated training as necessary for nurses to excel in the foregoing competency areas;
(c) DISTRIBUTOR shall update and refresh training and require regularly updated certification testing when new information becomes available or when a nurse has not provided services for an extended period of time;
(d) DISTRIBUTOR shall make available to UT upon request, for UT’s review and comment, training materials related to UT Product and the administration and support of UT Product;
(e) DISTRIBUTOR shall make available to UT records of completion of related training upon UT’s request;
(f) DISTRIBUTOR shall manage nonperformance of nurses (including per diem nurses) through appropriate measures, including re-training, discipline or removal; and
(g) DISTRIBUTOR shall reasonably provide nurses who are able to speak the same language as the Included Patient or a translation service.
(f) Additional Performance Requirements: As part of the overall activities performed in support of the distribution of UT Product, DISTRIBUTOR agrees to keep careful records of the following data points and maintain the requisite levels of competency for each data point and shall provide such data in reports to UT as UT reasonably requests, but no less than quarterly:
(i.) ASA: meaning the average speed DISTRIBUTOR takes to answer a call measured over a calendar month. DISTRIBUTOR shall use its Best Efforts to ensure that the ASA does not exceed 15 seconds, and in any event, at least 95% of all calls to DISTRIBUTOR shall be answered by a live person within fifteen (15) seconds;
(ii.) Calls Dropped: meaning the percentage of calls that are dropped before being answered over the course of a calendar month. DISTRIBUTOR shall use its Best Efforts to ensure that the Calls Dropped does not exceed 2%; and
(iii.) AHT: meaning the average hold time experienced by a caller as measured over the course of a calendar month. DISTRIBUTOR shall use its Best Efforts to ensure that the AHT does not exceed 45 seconds,
and in any event, at least 95% of calls placed on hold will be on hold for less than forty-five (45) seconds.
4.13 Tyvaso Continuing Patient Compliance, Support and Education Program. As set forth in Attachment G hereto and in accordance with this Section 4.13, DISTRIBUTOR agrees to perform all required services under the Continuing Patient Compliance, Support and Education Program for Tyvaso (“CPCSEP Services”) and UT agrees to pay DISTRIBUTOR a fee for such CPCSEP Services (“Service Fee”).
(a) DISTRIBUTOR warrants that it will perform the CPCSEP Services in a professional manner, in compliance with industry standards, and in accordance with the descriptions and representations set forth on Attachment G hereto or as otherwise mutually agreed by the Parties from time to time.
(b) DISTRIBUTOR shall submit detailed monthly invoices to UT setting forth a description of the CPCSEP Services actually performed and the corresponding Service Fee due. The foregoing invoices are due to UT within ten (10) days of the end of each calendar month. UT’s shall pay DISTRIBUTOR within sixty (60) days from receipt of each invoice.
(c) The Parties agree and acknowledge that the Service Fee paid hereunder has been determined through good faith and arms-length negotiation to be the fair market value of the CPCSEP Services to be rendered. No amount paid or reimbursed hereunder is intended to be, nor shall it be construed as, an offer or payment made, whether directly or indirectly, to induce the referral of patients, the purchase, lease or order of any item or service, or the recommending of the purchase, lease or order of any item or service.
(d) By accepting payment of the Service Fee from UT, DISTRIBUTOR represents and warrants to UT that DISTRIBUTOR has actually performed the CPCSEP Services as invoiced and was obligated to perform the CPCSEP Services (or any substantially similar activity) solely pursuant to the obligations in this Section 4.13 and Attachment G hereto. The Parties each agree that the Service Fee is not a discount but instead represents a fair amount in consideration for the CPCSEP Services described in this Section 4.13 and Attachment G hereto. Further, in recognition of the foregoing, DISTRIBUTOR warrants that it will retain the Service Fee for its own account.
(e) The CPCSEP Services and corresponding Service Fees will be evaluated by UT on an annual basis and UT may terminate all or a portion of the CPCSEP Services at any time upon thirty (30) days prior written notice to DISTRIBUTOR without any additional fee or expense.
4.14 DISTRIBUTOR agrees to make available all personnel responsible for overseeing/managing the activities related to the distribution of UT Product for quarterly meetings with UT personnel at reasonably agreed upon times and places in order to review and assess DISTRIBUTOR performance relative to the various obligations described in this Article 4 and elsewhere in this Agreement.
ARTICLE 5: OBLIGATIONS OF UT
5.1 Training. UT may in its discretion provide training to DISTRIBUTOR for UT Product at a time and in a manner as determined by DISTRIBUTOR.
5.2 UT Materials. UT shall provide DISTRIBUTOR, upon DISTRIBUTOR’s request, with reasonable quantities of sales and marketing materials for UT Product as they are developed by UT, including but not limited to reprints, brochures, package inserts, peer reviewed articles and other scientific and medical information regarding UT Product, informational material and other marketing literature (“UT Materials”), for use and distribution by DISTRIBUTOR in accordance with this Agreement. DISTRIBUTOR shall use the UT Materials in accordance with UT’s written directions, including providing the package insert to Customers until such time as the package insert is included with UT Product. DISTRIBUTOR shall not revise, alter, change, supplement or reproduce in any manner the UT Materials and their content as provided by UT without UT’s advance written permission. Nothing in this provision requires UT to create any specific materials.
ARTICLE 6: ORDERS FOR PRODUCTS
6.1 Purchase Orders. DISTRIBUTOR shall submit written purchase orders to UT by electronic mail or in accordance with written instructions provided by UT. Purchase Orders shall be submitted once per month by the 10th day of the month. Each such order shall set forth: (a) the package reference for the UT Product ordered (i.e. “Starter Kit”, “Re-Supply Kit”, or “Supplemental Refill”), including item numbers; (b) quantities in multiples of ten (10) per package reference; (c) requested delivery dates; (d) specific shipping instructions; and (e) if applicable, any relevant export control information or documentation to enable UT to comply with Applicable Laws. Except as otherwise agreed by UT, DISTRIBUTOR shall submit such purchase orders at least five (5) business days prior to the requested delivery dates. DISTRIBUTOR is responsible for good Inventory management processes and subsequent purchases should not deviate negatively by more than 15% from the previous PO unless unexpected events occur and are communicated to UT in advance in writing. DISTRIBUTOR may only purchase UT Product from UT or through the acquisition of all or part of a Pharmacy authorized to dispense Product. DISTRIBUTOR may only sell UT Product for use by an Included Patient and may not sell, transfer or distribute UT Product to any entity that DISTRIBUTOR knows is likely to resell the UT Product.
6.2 Acceptance of Orders. Each purchase order shall be governed by the terms and conditions set forth in this Agreement with respect to such order to the exclusion of any additional or contrary terms set forth in the DISTRIBUTOR purchase order. Any terms or conditions of such purchase order that conflict with the terms and conditions of this Agreement shall be null and void. Notwithstanding the foregoing, in the event of exigent circumstances, UT shall use its Best Efforts to accept an emergency purchase order from DISTRIBUTOR two (2) business days prior to the requested delivery date
6.3 Delivery Terms. Units of UT Product ordered by DISTRIBUTOR and accepted by UT shall be packed for shipment and storage in accordance with UT’s standard commercial shipping practices. UT shall use its best efforts to deliver Units of UT Product into the possession of a common carrier for delivery within a reasonable period of time after acceptance of a purchase order by UT. Unless mutually agreed upon by DISTRIBUTOR and UT, no UT Product shall be shipped on a Friday, Saturday or Sunday. Each order may only be shipped, and shall be addressed for shipment, to the Designated Shipment
Location specified in Attachment E. Unless UT and DISTRIBUTOR otherwise agree in writing, all deliveries of UT Product shall be F.O.B. DISTRIBUTOR’s Designated Shipment Location. UT shall insure each shipment of UT Product with a reputable insurer for the full invoice price of such shipment. Risk of loss and title to UT Product shall pass to DISTRIBUTOR upon delivery at its Designated Shipment Location. UT shall have no liability for any loss, theft, destruction or damage to the Units of UT Product once they have been delivered to a Designated Shipment Location and the exterior has been inspected by DISTRIBUTOR for visible damagewithout necessity of opening . Each individual package of UT Product. shall be inspected within five (5) business days of delivery to DISTRIBUTOR’s Designated Shipment Locations. DISTRIBUTOR shall, at its sole cost and expense, insure the Products from the time of delivery at DISTRIBUTOR’s Designated Shipment Location until delivery of the Units of UT Product by DISTRIBUTOR to Customer has been completed. In each case such insurance or self-insurance shall be for the UT Product’s full replacement value (i.e., market value) against fire, theft, loss or destruction, and such other risks as are customarily insured against by prudent persons in a similar line of business. At UT’s request, DISTRIBUTOR shall furnish to UT certificates of insurance evidencing the types and amounts of coverage.
6.4 Modification of Orders. No accepted purchase order shall be modified or canceled except upon the written agreement of both Parties.
6.5 Change Order Charges. If DISTRIBUTOR requests modifications to an accepted order prior to the scheduled delivery date provided in such order, then, in consideration for accepting such change order, UT may extend the scheduled delivery date and/or require DISTRIBUTOR to pay a change order charge equal to the sum of the actual documented non-recoverable costs incurred by UT by reason of such change order.
6.6 Product Changes. Subject to applicable regulatory approval, UT reserves the right, in its sole discretion and without incurring any liability to DISTRIBUTOR except as otherwise provided in this Agreement, to: (a) alter UT Product; (b) discontinue the manufacture of UT Product; or (c) commence the manufacture and sale of new products having features which make UT Product obsolete. UT also reserves the right, in its sole discretion and without incurring any liability to DISTRIBUTOR except as otherwise provided in this Agreement, immediately to alter the specifications or the manufacturing process for UT Product for reasons of health or safety. UT shall fill all accepted purchase orders from DISTRIBUTOR for altered or discontinued UT Product for which manufacturing and commercial deliveries have commenced prior to the effective date of such a change but otherwise shall have no obligation to do so unless the delivery date requested in the relevant purchase order is prior to the effective date of such a change. UT shall notify DISTRIBUTOR in writing when such modifications or changes occur.
6.7 Rolling Forecasts. DISTRIBUTOR shall provide UT with an annual, non-binding twelve (12) month forecast projecting DISTRIBUTOR’s intended purchases of UT Product for the coming twelve (12) months, as well as such other mutually agreeable information. UT shall receive this annual forecast no later than January 10th of each calendar year. DISTRIBUTOR shall also update UT on a rolling basis each calendar quarter, and each updated forecast shall be received by UT no later than the 10th day of the month following the end of each calendar quarter.
6.8 Chargeback Pricing. Subject to UT’s reimbursement of the “Chargebacks” (as described below) DISTRIBUTOR shall provide wholesale distribution to certain entities eligible for discounted government pricing (e.g., FSS, VA, PHS (340B)) (“Discounted
Entity”) as described herein. The discounted government pricing is less than the price at which DISTRIBUTOR purchases UT Product (i.e., less than the Price set forth in Attachment A). DISTRIBUTOR shall create an account for each Discounted Entity purchasing UT Product from DISTRIBUTOR. As part of this process, DISTRIBUTOR shall use commercially reasonable efforts to identify whether the proposed Discounted Entity is eligible for discounted government pricing through direct documentation from the proposed Discounted Entity or through review of data on the HRSA eligibility website or other database resource. As an order for Product is received from the Discounted Entity, DISTRIBUTOR shall provide Product to the Discounted Entity at the discounted government price. The difference between the discounted government price and the List Price for the Product is referred to as the “Chargeback.” The Chargeback shall be paid by UT to DISTRIBUTOR by check. When submitting a Chargeback request to UT, DISTRIBUTOR shall include the following information: (i) date of sale to Discounted Entity, (ii) the Discounted Entity’s name and address, (iii) product(s) purchased from DISTRIBUTOR (iv) UT’s price to DISTRIBUTOR for the Product, (v) DISTRIBUTOR’s price to the Discounted Entity for the Product, and (vi) the amount of Chargeback requested. Chargeback request(s) shall be submitted to UT by the 10th of each month for all activity in the previous calendar month. UT shall process Chargeback credits due DISTRIBUTOR within thirty (30) days of receipt of the Chargeback submission. DISTRIBUTOR shall not set off Chargebacks owed by UT against any amounts owed by DISTRIBUTOR to UT. Upon termination of this Agreement, if there are any unapplied credits for a Chargeback, UT shall issue a check in the amount thereof to DISTRIBUTOR. Chargebacks paid hereunder constitute reimbursement to DISTRIBUTOR for debits incurred in administering UT discounts to Discounted Entities, and are not, and should not be construed as, remuneration intended to induce DISTRIBUTOR to purchase, order, lease, or recommend any UT product.
ARTICLE 7: PRICES AND PAYMENTS
7.1 Prices. DISTRIBUTOR shall pay the Prices for UT Product purchased under this Agreement that are in effect at the time of submission of a relevant purchase order by DISTRIBUTOR, except as provided in Section 7.2 below.
7.2 Price Changes. At any time during the term of this Agreement, UT may increase or decrease its Prices for UT Product with notice to DISTRIBUTOR of the effective date of the price change. Any such price change shall not apply to purchase orders submitted prior to the effective date of the applicable price change.
7.3 Costs. All costs related to shipping, insuring, packing, handling and delivering UT Product to DISTRIBUTOR’s facility shall be at the sole expense of UT. All such costs incurred after the instant of delivery to the Designated Shipment Location shall be the responsibility of DISTRIBUTOR. Notwithstanding anything to the contrary in this Agreement, UT may, in its sole discretion, charge DISTRIBUTOR for any and all shipping, packing, handling or delivery charges associated with emergency purchase orders, or if DISTRIBUTOR places three or more orders in a one month period.
7.4 Payment Terms; Invoices. DISTRIBUTOR shall make payments for UT Product within sixty (60) days of its receipt of an applicable invoice from UT. DISTRIBUTOR shall be eligible for a two percent (2%) prompt pay discount if
payment is received by UT within thirty (30) days of the date of invoice. All payments shall be made in United States Dollars.
7.5 Provision of Invoices to Government Payers. Upon the request of any federal or state agency with jurisdiction over claims for reimbursement of UT Product, DISTRIBUTOR may provide such agency with invoices received from UT that accurately reflect the actual charge to DISTRIBUTOR for UT Product purchased pursuant to this Agreement with prompt written notice to UT of such request.
7.6 Overdue Payments. If and for so long as any payment from DISTRIBUTOR to UT or UT to DISTRIBUTOR under this Agreement shall be overdue:
(a) Interest shall be due and payable at the rate of twelve percent (12%) per annum, or such lower rate as may be the maximum legally permissible rate of interest, on all balances outstanding from the first date such payment is due until fully paid;
(b) Each Party shall have the right to recover its collection costs and expenses (including reasonable attorneys’ fees) for late payments. UT reserves the right to withhold or suspend shipment of UT Product if there is any unsettled or outstanding balance owed or caused by DISTRIBUTOR to UT and to revoke any credit terms it may offer DISTRIBUTOR; and
(c) Overdue payments automatically forfeit any prompt pay discounts referenced in Section 7.3.
7.7 Tax Payments. Each Party shall pay all taxes, duties, import deposits, assessments and other governmental charges, however designated, that are now or hereafter imposed upon such Party by any governmental authority or agency in connection with the performance of its obligations under this Agreement.
7.8 Resale Prices. The Parties acknowledge that DISTRIBUTOR may offer the UT Product in the Territory at such prices or discounts as DISTRIBUTOR, in its sole discretion, may determine.
7.9 Drug Formulary. The Parties acknowledge and agree that no payment made pursuant to this Agreement is intended in any way as a payment related to a drug formulary or drug formulary activities. The Parties acknowledge and agree that no drug formulary or drug formulary activities have been negotiated or discussed between the Parties in connection with this Agreement.
ARTICLE 8: ACCEPTANCE, WARRANTY AND PRODUCTS SUPPORT
8.1 Acceptance of UT Product. DISTRIBUTOR shall promptly inspect each shipment of UT Product. In the event of any shortage, damage, expiration or discrepancy in a shipment of UT Product on the exterior of the shipment of UT Product that is patently obvious, DISTRIBUTOR shall promptly report the same to UT and furnish such written evidence or other documentation as UT may reasonably request. DISTRIBUTOR shall be deemed to have accepted a shipment and UT shall not be liable for any such shortage, damage, expiration or discrepancy in such shipment unless DISTRIBUTOR provides UT with such notice and substantiating evidence within five (5) days of receipt of the UT Product at DISTRIBUTOR’s Designated Shipment Location. Upon receipt of reasonable substantiating evidence of such shortage, damage or discrepancy, UT shall promptly provide additional UT Product or substitute products to DISTRIBUTOR.
8.2 Product Warranty. UT hereby authorizes DISTRIBUTOR to pass on the UT standard warranty set forth in Attachment D to DISTRIBUTOR’s Customers in the Territory, which may be revised by UT upon written notice to DISTRIBUTOR.
8.3 Excluded Claims. UT shall not have any additional warranty obligations to DISTRIBUTOR or Customers under Section 8.2 above or otherwise to the extent that DISTRIBUTOR has made any warranties, oral or written, beyond those expressly set forth in the standard UT warranty, set forth in Attachment D hereto. DISTRIBUTOR shall not offer its customers any warranties different from or in addition to those given by UT hereunder.
8.4 Limited Warranty. THE WARRANTIES SET FORTH IN THE UT WARRANTY, ATTACHMENT D HERETO, AND THE OTHER TERMS AND CONDITIONS OF THIS AGREEMENT, ARE IN LIEU OF ALL OTHER WARRANTIES, EXPRESS OR IMPLIED, WHICH ARE HEREBY DISCLAIMED AND EXCLUDED BY UT, INCLUDING, WITHOUT LIMITATION, ANY WARRANTY OF MERCHANTABILITY OR FITNESS FOR A PARTICULAR PURPOSE OR USE. THE SOLE AND EXCLUSIVE REMEDIES FOR BREACH OF UT’S STANDARD WARRANTIES SHALL BE LIMITED TO THE REMEDIES PROVIDED IN UT’S STANDARD WARRANTIES SET FORTH ON ATTACHMENT D HERETO AND AS OTHERWISE PROVIDED IN THIS AGREEMENT.
8.5 Limited Remedy. UT SHALL NOT BE LIABLE TO DISTRIBUTOR OR ANY OF ITS CUSTOMERS FOR LOSS OR DAMAGE CAUSED BY DISTRIBUTOR’s DELAY IN FURNISHING THE PRODUCTS UNDER THIS AGREEMENT. UT SHALL NOT BE LIABLE TO DISTRIBUTOR OR ANY OF ITS AFFILIATES, EMPLOYEES, AGENTS OR CONTRACTORS FOR ANY SPECIAL, INDIRECT, INCIDENTAL OR CONSEQUENTIAL LOSSES OR DAMAGES, EVEN IF UT SHALL HAVE BEEN ADVISED OF THE POSSIBILITY OF SUCH POTENTIAL LOSS OR DAMAGE BY DISTRIBUTOR OR SUCH THIRD PARTY. NOTWITHSTANDING THE FOREGOING, IN CASE OF ANY CONFLICT BETWEEN THE PROVISIONS OF THIS SECTION AND SECTION 12.3, SECTION 12.3 AND SECTION 12.4 SHALL CONTROL.
ARTICLE 9: REGULATORY APPROVALS, COMPLIANCE AND AUDITS
9.1 Compliance with Applicable Laws. UT shall be solely responsible for, and comply with, Applicable Laws governing the regulation of the manufacture, importation, design, testing, inspection, labeling, sale, warning and instructions for use of UT Product in the Territory, or otherwise applicable to the performance of its obligations under this Agreement. DISTRIBUTOR shall comply with all Applicable Laws governing its distribution and sale of UT Product in the Territory, or otherwise applicable to the performance of its obligations hereunder. Each Party shall comply with Applicable Laws intended to prevent fraud, waste and abuse in federal health care programs, including but not limited to Medicare and Medicaid, and shall conduct its activities hereunder in an ethical and professional manner.
9.2 Government Inquiries. In the event that DISTRIBUTOR receives an inquiry, or similar notice from a government agency or entity for information or an inspection (a “Notice”) which relates to UT Product or this Agreement, DISTRIBUTOR shall: (a) notify and provide a copy to UT of such Notice promptly within three (3) business days of receipt of such Notice; (b) unless expressly prohibited by the Notice, consult with UT regarding its response to the Notice to determine, among other things, whether any of UT’s
Confidential Information shall be disclosed (which in all events shall be subject to DISTRIBUTOR’s obligations specified in Article 10 of this Agreement); (c) keep UT informed of the progress of any inspection and provide UT with prior notice of any documents related to UT Product or UT to be provided to such government entity; and (d) provide UT with a copy of any documents related to UT Product or UT ultimately produced pursuant to such Notice. Further, DISTRIBUTOR shall provide UT with a summary of the results of any inspection and such actions, if any, taken to remedy conditions cited in such inspections. DISTRIBUTOR further agrees to cooperate with any inspection of a shipment of UT Product by a governmental agency.
9.3 Adverse Event Reporting. DISTRIBUTOR shall not be responsible for FDA reporting of adverse events. DISTRIBUTOR shall attempt to warm transfer a caller with potential Adverse Event information to a phone number designated by UT. Otherwise, DISTRIBUTOR shall notify UT by fax to (919) 313-1297 or e-mail at drugsafety@unither.com immediately, or at the latest within three (3) business days, of any complaint of a potential Adverse Event from a third party being reported to DISTRIBUTOR. As directed by UT, such fax or e-mail report sent by DISTRIBUTOR shall include information as required by UT in order to adequately report such Adverse Event to FDA.
9.4 Withdrawal or Recall of Product. Any recalls of UT Product shall be conducted in compliance with FDA requirements and the UT standard operating procedure for recalls (“UT Recall SOP”) as provided to and accepted by DISTRIBUTOR. DISTRIBUTOR shall prepare and maintain a written standard operating procedure that provides processes for conducting recall-related activities for UT Product as directed by UT and in accordance with the UT Recall SOP. The decision to recall UT Product shall be made solely by UT, unless otherwise dictated by a governmental authority. UT shall be responsible for the expenses related to recall activities as described below, unless the recall results from a breach of any of DISTRIBUTOR’s representations and warranties under this Agreement or DISTRIBUTOR’s negligence or willful misconduct, in which event DISTRIBUTOR shall be responsible for all of recall-related expenses. For purposes of this Agreement, the expenses of the activities shall be: (i) the reasonable expenses of notification and return or destruction (if authorized by UT) of UT Product, (ii) the cost to replace UT Product, (iii) the costs directly associated with the distribution of replacement UT Product including pharmacist and dispensing labor, cold packs and labels; (iv.) reasonable communications to Included Patients such as patient letters, patient phone calls and follow-up customer service; (v.) labor associated with managing the recall process; (vi.) any expenses associated with dispensing activity missed by DISTRIBUTOR as a result of UT’s provision of product to Included Patients beyond that is necessary to replace recalled product; and (vii.) shipping and insurance costs associated with returning recalled UT Product. DISTRIBUTOR and UT shall cooperate fully with one another in conducting any activity contemplated by this Section 9.4. Destruction of recalled product shall be conducted in accordance with the recall plan, as approved by UT under the UT Recall SOP and by any applicable governmental authorities. If instructed by UT, DISTRIBUTOR may return recalled UT Product to UT at UT’s expense within thirty (30) days from completion of the recall and UT shall replace the UT Product recalled or refund the cost of such returned UT Product. Any UT Product returned to UT under this Section 9.4 shall be shipped by common carrier in a manner that preserves the integrity of the UT Product shipped, as instructed by UT. Title to the recalled UT Product and risk of loss, theft, destruction or damage to UT Product during shipment as described above shall pass from DISTRIBUTOR to UT upon delivery of recalled UT Product at UT’s facility. DISTRIBUTOR’s obligation to insure UT
Product shall continue with respect to recalled UT Product until UT’s receipt of such recalled UT Product.
9.5 Visits by Parties. DISTRIBUTOR shall permit UT to visit its place of business and inspect its records, inventories and other relevant materials and records relating solely to its performance of this Agreement, upon reasonable advance notice and during normal business hours.
9.6 No Returns. UT will not accept the return of any UT Product, unless agreed in writing by UT, except if returned pursuant to a recall under Section 9.4 above.
ARTICLE 10: PROPERTY OWNERSHIP; CONFIDENTIALITY
All Confidential Information and other proprietary materials, documents, information, databases, complete and incomplete case report forms and all data that one Party (“Disclosing Party”) supplies to the other Party (“Receiving Party”) shall be the sole and exclusive property of the Disclosing Party (“Disclosing Party Property”). All Confidential Information shall be deemed confidential and proprietary to the Disclosing Party. During the term of this Agreement and for a period of five (5) years following thereafter, the Receiving Party shall: (a) not disclose or provide any Confidential Information to any third party, and (b) take reasonable measures to prevent any unauthorized disclosure of Confidential Information by its employees, agents, contractors or consultants during the term hereof including advising such individuals of applicable confidentiality obligations. Upon termination of this Agreement, the Receiving Party shall return or destroy to the Disclosing Party, at the Disclosing Party’s request and expense, all unused Disclosing Party Property, except the Receiving Party may keep one (1) copy of such Disclosing Party Property for legal archival purposes.
ARTICLE 11: TRADEMARKS
11.1 Trademark License Grant. UT hereby grants to DISTRIBUTOR, and DISTRIBUTOR hereby accepts from UT, a nonexclusive, nontransferable, and royalty-free right and license, during the term of this Agreement, to reproduce and use the UT Trademarks in connection with the distribution, marketing and sale or other distribution of UT Product in the Territory and in accordance with UT’s standards and instructions and for no other purpose. DISTRIBUTOR shall not use any other marks or trade names in connection with the marketing and distribution of UT Product, except that DISTRIBUTOR may use its marks or trade names in a manner consistent with its normal course of business, such as adding a label on the packaging identifying DISTRIBUTOR as a distributor of UT Product, and such use shall not confer on UT any rights or license in DISTRIBUTOR’s marks or trade names. UT may inspect and monitor DISTRIBUTOR’s use of the UT Trademarks. DISTRIBUTOR shall not remove or alter any UT trade names, trademarks, copyright notices, serial numbers, labels, tags or other identifying marks, symbols or legends affixed to any UT Product, documentation or containers or packages.
11.2 Registration. In its sole discretion, UT may register the UT Trademarks in the Territory if UT determines that registration is necessary or useful to the successful distribution of UT Product. In addition, if UT believes that it is advisable to effect any filing or obtain any governmental approval or sanction for the use by DISTRIBUTOR of any of UT Trademarks pursuant to this Agreement, the Parties shall cooperate to do so. All expenses relating to the registration of the UT Trademarks in the Territory as well as the making of any filing or obtaining any governmental approvals for the use by DISTRIBUTOR of the Trademarks shall be borne by UT.
11.3 Termination of Use. Immediately upon termination of this Agreement, DISTRIBUTOR’s license and right granted in Section 11.1 shall be revoked and DISTRIBUTOR shall cease and desist from use of any UT Trademark in any manner, other than to liquidate its then-existing inventory of UT Product within six months of such termination. DISTRIBUTOR hereby grants to UT or its designee, in the event of such termination, full power of attorney, with the right of substitution, to cancel, revoke or withdraw any governmental registration or authorization permitting DISTRIBUTOR to use any UT Trademark in the Territory, and DISTRIBUTOR shall provide such further documentation and assistance as UT may reasonably request in connection therewith.
11.4 Reservation of Rights.
(a) DISTRIBUTOR acknowledges UT’s proprietary rights in and to any UT Trademark, subject to the license and right granted in Section 11.1. DISTRIBUTOR shall not adopt, use or register any words, phrases or symbols that are identical to or confusingly similar to any UT Trademark and shall not use any UT Trademark as part of DISTRIBUTOR’s corporate or trade name or permit any third party to do so.
(b) UT acknowledges DISTRIBUTOR’s proprietary rights in and to any of DISTRIBUTOR’s trademarks. UT shall not adopt, use or register any words, phrases or symbols that are identical to or confusingly similar to any of DISTRIBUTOR’s trademarks and shall not use any such trademark as part of UT’s corporate or trade name or permit any third party to do so.
11.5 Infringements. Each Party shall promptly notify the other Party in writing if it becomes aware of any use in the Territory by any third party of trademark or of any similar mark, which may constitute an infringement of a UT Trademark or DISTRIBUTOR’s trademarks. Subject to the provisions of this Article 11, Each Party shall have the exclusive right, in its sole discretion, to institute proceedings against third-party infringers of its trademarks.
ARTICLE 12: INSURANCE AND INDEMNIFICATION
12.1 Insurance. DISTRIBUTOR shall maintain in effect during the term of this Agreement a comprehensive general liability policy (which may be in the form of primary or excess coverage) in an amount not less than Two Million Dollars ($2,000,000) per occurrence and Three Million Dollars ($3,000,000) in the aggregate. The deductible for such policy shall be no more than One Hundred Thousand Dollars ($100,000) DISTRIBUTOR agrees to provide UT with a certificate of insurance evidencing compliance with this section upon written request of UT.
12.2 Claims. For the purposes of this Article 12 a “Claim” shall mean any liabilities, damages, costs or expenses, including, without limitation, reasonable attorneys’ fees arising from any claim, lawsuit, demand or other action by a third party.
12.3 DISTRIBUTOR Indemnification of UT. Except as provided in Section 12.4, DISTRIBUTOR shall indemnify, defend and hold harmless UT, its Affiliates, and their respective officers, directors, employees, agents, successors and assigns from and against any Claim to the extent such Claim relates to or is based on: (a) property damage, personal injury or death resulting from DISTRIBUTOR’s negligent or reckless provision or maintenance of UT Product (except to the extent the same results from any wrongful
act or omission of UT); (b) DISTRIBUTOR’s violation of Applicable Laws; or (c) any breach by DISTRIBUTOR of any of its representations, warranties, covenants or agreements under this Agreement.
12.4 UT Indemnification of DISTRIBUTOR for UT Product. Except as provided in Section 12.3, UT shall indemnify, defend and hold harmless DISTRIBUTOR and its Affiliates, and their respective officers, directors, employees, agents and successors and assigns from and against any Claim to the extent such Claim relates to or is based on: (a) property damage, personal injury or death resulting from use of UT Product (except to the extent the same results from any wrongful action or omission of DISTRIBUTOR); (b) UT’s violation of Applicable Laws; or (c) any breach by UT of any of its representations, warranties, covenants or agreements under this Agreement.
12.5 Indemnification Procedure. A Party seeking indemnification under this Article 12 (“Indemnified Party”) shall give prompt written notice to the indemnifying Party (“Indemnifying Party”) of any Claim covered by the indemnification obligations hereunder; provided, however, that a delay in such notice shall not terminate the Indemnifying Party’s indemnification obligations hereunder, unless such delay shall have materially impaired the defense of such Claim. Such Indemnifying Party shall have sole and exclusive control of the defense of any such Claim, including the choice and direction of any legal counsel; provided, however, if Indemnifying Party’s choice of legal counsel would be subject to a material conflict of interest under the applicable rules of professional conduct governing such counsel, the Indemnified Party shall not be obligated to waive such conflict and may request separate legal counsel at the Indemnifying Party’s expense. The Indemnifying Party may not settle or compromise any such Claim without the written consent of the Indemnified Party, which consent shall not be unreasonably withheld.
12.6 Litigation Support. In the event and for so long as an Indemnifying Party actively is contesting or defending against any Claim under this Article 12, the Indemnified Party shall cooperate with the Indemnifying Party and its legal counsel in the contest or defense of such Claim, make available its personnel, and provide such testimony and access to its books and records as shall be reasonably necessary in connection with the contest or defense of such Claim, all at the sole cost and expense of the Indemnifying Party.
12.7 Subrogation. The Indemnifying Party shall be subrogated to the rights of the Indemnified Party against any third party bringing a Claim, and such Indemnified Party hereby assigns to the Indemnifying Party all claims, causes of action and other rights that the Indemnified Party may then have against such third party. Conversely, and without in any way limiting the obligation of either Party to indemnify the other Party as herein provided, to the extent that an Indemnifying Party fails to perform its indemnification obligations under Section 12.3 or Section 12.4 above, the Indemnifying Party hereby assigns to the Indemnified Party all claims, causes of action and other rights which the Indemnifying Party may then have against any third party with respect to any Claim for which indemnification is provided hereunder.
ARTICLE 13: ARTICLE 13 JOINT PUBLICITY
13.1 Public Disclosure. If either Party wishes to make a public disclosure concerning this Agreement or the relationship established hereunder and such disclosure mentions the other Party by name or description, such other Party shall be provided with an advance copy of the disclosure and shall have two (2) business days within which to approve or
disapprove such use or its name of description (including mention of the name of the Product); provided, however: (a) approval shall not be unreasonably withheld by either Party; (b) failure to respond within two (2) business days shall be deemed approval; and (c) if approval is denied, no disclosure shall use the name of or otherwise describe such Party except to the extent required by Applicable Laws, or the extent that the description of the other Party is limited to public information about the availability of UT Product.
13.2 Filings with Securities and Exchange Commission. Notwithstanding the foregoing, each Party acknowledges that both Parties are, or are affiliates of, a publicly traded company and each Party hereby consents to the disclosure of this Agreement and the relationship between the Parties in their respective filings with the Securities and Exchange Commission and disclosures to their stockholders; provided, however, that each Party shall use commercially reasonable efforts not to disclose the specific financial terms and conditions of this Agreement except when such disclosure is required by Applicable Laws or by this Agreement.
ARTICLE 14: FORCE MAJEURE
14.1 Notice. A Party affected by an event of Force Majeure shall promptly provide the other Party with written notice describing the event, its cause and foreseeable duration, and its possible consequences upon performance under this Agreement.
14.2 Suspension of Performance. After an affected Party has given notice under Section 15.1, that Party shall be relieved of any performance obligation under this Agreement for obligations which the Force Majeure event prevents, but only to the extent and only for so long as the Force Majeure prevents performance. The other Party may likewise suspend the performance of all or part of its obligations, except for the obligation to pay any amount due and owing and those obligations specified in Section 16.4(c) of this Agreement. Notwithstanding the foregoing, UT shall use Commercially Reasonable Efforts to allocate available UT Product to DISTRIBUTOR at least in proportion to DISTRIBUTOR’s historical purchases.
14.3 Substitute Performance. If DISTRIBUTOR is delayed by an event of Force Majeure, UT shall, at its sole option, allow a third party to cover the services related to the distribution of UT Product that DISTRIBUTOR was unable to complete due to its delay and such third party shall receive the fees DISTRIBUTOR would have received during its period of delay.
14.4 Termination. If the period of Force Majeure continues for more than sixty (60) days, either Party may terminate this Agreement upon giving notice to the other Party without incurring liability other than the obligation to make payments due up to and including such date of termination.
ARTICLE 15: TERM AND TERMINATION
15.1 Term. The initial term of this Agreement shall begin on the Effective Date and shall continue in force for one (1) year from the Effective Date. Thereafter, this Agreement shall automatically renew for additional periods of one (1) year each, unless either of the Parties shall have given the other Party written notice of its non-renewal of this Agreement no later than ninety (90) days prior to the end of the initial or any renewal term hereof.
15.2 Termination. This Agreement may be terminated prior to the expiration of the then current term as follows:
(a) Either Party may terminate this Agreement immediately upon written notice to the other Party if the other Party files a petition of any type as to its bankruptcy, is declared bankrupt, becomes insolvent, makes an assignment for the benefit of creditors, goes into liquidation or receivership, a proceeding is commenced against it which will substantially impair its ability to perform hereunder or such Party otherwise loses legal control of its business;
(b) Either Party may terminate this Agreement upon the occurrence of a material breach by the other Party (including, but not limited to, DISTRIBUTOR’s failure to promptly pay sums owing to UT), which breach has not been cured within thirty (30) days of written notice of such breach from the non-breaching Party;
(c) Either Party may terminate this Agreement upon written notice if an event of Force Majeure continues for more than sixty (60) days as provided in Section 15.4;
(d) The Parties may agree in writing to terminate this Agreement for their mutual convenience at any time and for any reason, subject to such terms and conditions as they may then adopt;
(e) Either Party may terminate this Agreement at any time, with or without cause, by written notice to the other Party, which shall be effective one hundred and eighty days (180) days after its date; and
(f) If at any time in the future, a change in the reimbursement of UT Product or legal requirements of payers would (a) require the Parties to renegotiate or alter significant terms of this Agreement, or (b) result in a substantial adverse change in the respective financial benefits or burdens accruing to any Party under the terms of this Agreement, then upon written request by either Party in the case of (a), or the affected Party in the case of (b), the Parties shall endeavor in good faith to renegotiate and modify the terms of this Agreement to comply with such new requirements or avoid such substantial adverse change. If the Parties are unable to agree to such modifications within one hundred twenty (120) days of receipt of the written request, then either Party (in the case of (a)), or the adversely affected Party (in the case of (b)) may terminate this Agreement immediately upon expiration of the one hundred twenty (120) day period.
15.3 Partial Termination. In the event that either Party shall have the right pursuant to the provisions of Section 15.2 to terminate this Agreement in its entirety, that Party may elect, in its sole discretion, to terminate this Agreement solely as it applies to a portion of the Territory, or, if applicable, any category of Customer.
15.4 Rights and Obligations on Termination. If this Agreement is terminated for any reason, the Parties shall have the following rights and obligations:
(a) Termination of this Agreement shall not release either Party from the obligation to make payments of all amounts then or thereafter due and payable, and shall not release UT from its obligations to provide UT Product to DISTRIBUTOR at DISTRIBUTOR’s request to service its existing patients as of the effective termination date and until such existing patients are transitioned to another
distributor. DISTRIBUTOR and UT shall use their Best Efforts to achieve such transition as expeditiously as possible after the effective termination date;
(b) Each Party’s respective obligations of confidentiality under Article 13 and record retention under Article 18 shall survive as provided in such articles;
(c) Each Party’s respective obligations under Section 7.3, ‘Payment Terms; Invoices,’ Section 9.2, ‘Compliance with Laws,’ the indemnification provisions of Article 12, this Article 16 and Article 17, ‘Dispute Resolution,’ shall survive termination of this Agreement; and
(d) UT shall cause other entities to undertake, or shall otherwise relieve DISTRIBUTOR of its obligations and all costs relating to all PAP Patients, and shall complete such transition or relief with respect to such patients no later than one hundred and eighty (180) days from the termination date. DISTRIBUTOR agrees to use its Best Efforts to cooperate with such transfer.
ARTICLE 16: DISPUTE RESOLUTION
16.1 Negotiation. The Parties agree to consult and negotiate in good faith to try to resolve any dispute, controversy or claim that arises out of or relates to this Agreement. Except as provided in Section 16.2, no formal dispute resolution shall be used by either Party unless and until senior executive officers of each Party shall have attempted to meet in person to achieve such an amicable resolution.
16.2 Reservation for Litigation. Notwithstanding Section 16.3 below, each Party expressly reserves the right to seek judicial relief from a court of competent jurisdiction if the other Party is or appears to be in violation of such other Party’s obligations of non-use and non-disclosure under Article 10 above and each Party expressly reserves the right to seek injunctive or similar equitable relief for any violation or breach hereunder.
16.3 Arbitration. Subject to the reservation of the Parties under Section 16.2 above, any dispute, controversy or claim that arises out of or relates to this Agreement that is not resolved under Section 16.1 shall be settled by final and binding arbitration in accordance with the Commercial Arbitration Rules of the American Arbitration Association (“AAA”) in effect on the Effective Date, as modified by Section 16.4 below. Judgment upon the award rendered by the arbitrators may be entered in any court of competent jurisdiction. The place of arbitration shall New York, New York, U.S.A. The arbitration shall be conducted in the English language by three (3) neutral arbitrators selected by mutual agreement of the Parties or, if that is not possible within thirty (30) days of the initial demand for such arbitration, by the AAA.
16.4 Special Rules. Notwithstanding any provision to the contrary in the AAA’s rules, the Parties hereby stipulate that any arbitration hereunder shall be subject to the following special rules:
(a) Each Party shall have the right to request from the arbitrators, and the arbitrators shall order upon good cause shown, reasonable and limited pre-hearing discovery, including (i) exchange of witness lists, (ii) depositions under oath of named witnesses, (iii) written interrogatories, and (iv) document requests;
(b) Upon conclusion of the pre-hearing discovery, the arbitrators shall promptly hold a hearing upon the evidence to be presented by the Parties and shall promptly render a written opinion and award;
(c) The arbitrators may not award or assess punitive damages against either Party; and
(d) Each Party shall bear its own costs and expenses of the arbitration and one-half (1/2) of the fees and costs of the arbitrators, subject to the power of the arbitrators, in their sole discretion, to award all such reasonable costs, expenses and fees to the prevailing Party.
ARTICLE 17: RECORDS
During the term hereof and for three (3) years thereafter, or such longer period as may be required by Applicable Laws, DISTRIBUTOR shall maintain accurate records as required to meet Applicable Laws. Except as otherwise required by Applicable Laws, DISTRIBUTOR shall provide UT with access to any reasonably requested documentation related solely to this Agreement during reasonable business hours. UT shall give DISTRIBUTOR seven (7) days’ prior written notice of such examinations, which will not occur more than once annually, and such examinations shall be undertaken only to such extent necessary to verify that the DISTRIBUTOR has complied with the terms of this Agreement.
ARTICLE 18: ARTICLE 18 GENERAL PROVISIONS
18.1 Entire Agreement. This Agreement constitutes the entire agreement of the Parties with respect to the subject matter hereof and supersedes all the Parties’ previous or contemporaneous correspondence, term sheets, understandings, agreements and representations, oral or written between the Parties.
18.2 Assignment. Neither Party shall assign or otherwise transfer its rights or obligations under this Agreement except with the prior written consent of the other Party, which shall not be unreasonably withheld or delayed; provided, however, that no such consent shall be required and either Party may transfer all rights and obligations arising hereunder to an entity if it is: (a) an Affiliate; (b) the successor in interest by reason of sale, merger or operation of law; or (c) has acquired all or substantially all of the assets and business. Any unauthorized attempted assignment or delegation shall be null and void and of no force or effect.
18.3 Subcontracting. DISTRIBUTOR shall not, without the prior written approval of UT, appoint any distributors or agents to act on behalf of DISTRIBUTOR (collectively, “Sub-Distributors”) to distribute UT Product within the Territory, other than any of its Affiliates. DISTRIBUTOR shall at all times remain fully liable for the performance of any approved Sub-Distributors and DISTRIBUTOR shall provide UT with a written acknowledgement executed by each Sub-Distributor that it has read this Agreement and agrees to be bound by its terms and conditions, including those contained in the attachments hereto. Notwithstanding the forgoing, DISTRIBUTOR may subcontract portions of certain limited functions and responsibilities of this Agreement, provided that the subcontractor performs in a manner conforming to this Agreement, subcontractor enters into a confidentiality agreement no less extensive than required by this Agreement; and DISTRIBUTOR retains full responsibility and liability for the performance of the subcontracted service. At no time shall DISTRIBUTOR subcontract
all or substantially all of any given function to a third party without the prior written consent of UT.
18.4 Amendment. This Agreement may not be modified or amended, in whole or in part, except by a written agreement signed by both Parties, and specifically stating that it modifies or amends this Agreement.
18.5 Severability. If one or more of the provisions of this Agreement is subsequently declared invalid or unenforceable, this Agreement shall be treated as though that provision were not in this Agreement, and this shall not affect the validity or enforceability of the remaining provisions of this Agreement (unless those provisions that are invalidated or unenforceable are clearly material and inseparable from the other provisions). The Agreement as modified shall be applied and construed to reflect substantially the good faith intent of the Parties and to achieve the economic effects originally intended by the terms hereof.
18.6 Notices; Language. Except as may be otherwise provided in this Agreement, any notice, demand or request given, made or required to be made shall be in writing and shall be effective, unless otherwise provided herein, either (a) when delivered in person to the other Party, or (b) on the same business day that it is transmitted by facsimile to the facsimile number (s) set forth below, with electronic confirmation of receipt, if transmitted prior to 5:00 p.m. Eastern time on such business day, or on the first business day following such transmission if transmitted after 5:00 p.m. Eastern Time or if transmitted on a day other than a business day; provided a hard copy is deposited within one (1) day after such transmissions in the U.S. mail, postage prepaid, and addressed as set forth below for notices by U.S. mail; or (c) on the third business day following its deposit in the U.S. mail, postage and addressed as follows:
|
If to UT: |
|
United Therapeutics Corporation |
|
|
|
1110 Spring Street |
|
|
|
Silver Spring, Maryland 20910 |
|
|
|
Attention: John Ferrari, Chief Financial Officer |
|
|
|
Telefax: 301-608-9291 |
|
|
|
|
|
|
|
With a copy to: |
|
|
|
United Therapeutics Corporation |
|
|
|
1735 Connecticut Ave. NW |
|
|
|
Washington, DC 20009 |
|
|
|
Attention: Paul Mahon, EVP & General Counsel |
|
|
|
Telefax: 202-483-4005 |
|
|
|
|
|
If to DISTRIBUTOR: |
|
Accredo Health Group |
|
|
|
1640 Century Center Parkway |
|
|
|
Memphis, TN 38134 |
|
|
|
Attention: Michael R. Hess, Chief Counsel Accredo |
|
|
|
Telefax: 901-261-6840 |
18.7 Waiver. Either Party’s failure or delay in exercising any remedy for default shall not be deemed a waiver of that or any subsequent defaults of that provision or of any other provision hereof. No waiver shall be effective unless made in writing with specific reference to the relevant provision(s) of this Agreement and signed by a duly authorized representative of the Party granting the waiver.
18.8 Counterparts. This Agreement shall be executed in two (2) or more counterparts in the English language, each of which shall be deemed an original, which taken together shall constitute one and the same instrument.
18.9 Governing Law. Except as provided by federal law, this Agreement shall be governed by, and interpreted and construed in accordance with, the laws of the State of New York, excluding (a) any conflict-of-laws rule or principle therein contained under which any other law would be made applicable
18.10 Relationship. This Agreement does not make either Party the employee, agent or legal representative of the other Party for any purpose whatsoever. Neither Party is granted any right or authority to assume or to create any obligation or responsibility, express or implied, on behalf of or in the name of the other Party. In fulfilling its obligations pursuant to this Agreement each Party shall be acting as an independent contractor and shall not be deemed to have formed any partnership, joint venture or other relationship
18.11 Headings. The headings contained in this Agreement are for reference purposes only and shall not affect in any way the meaning or interpretation of this Agreement.
18.12 Signature Authority. Each signatory to this Agreement has signature authority and, is empowered on behalf of his or her respective Party to execute this Agreement.
18.13 Cumulative Remedies. Except as expressly provided in this Agreement, and to the extent permitted by Applicable Laws, any remedies described in this Agreement are cumulative and not alternative to any other remedies available at law or equity.
18.14 Anti-Kickback Law Compliance. Each party agrees that it shall not violate the federal anti-kickback statute, set forth at 42 U.S.C § 1320a-7b(b) (“Anti-Kickback Statute”), the federal “Stark Law,” set forth at 42 U.S.C § 1395nn, or the Public Contracts Anti-Kickback Law with respect to the performance of its obligations under this Agreement. The Parties intend to treat all discounts (including, but not limited to, prompt payment discounts) payable by UT hereunder as “discounts or other reductions in price” pursuant to the Anti-Kickback Statute, and to comply with the discount safe harbor set forth at 42 C.F.R. § 1001.952(h). Accordingly, the Parties agree that: (i) DISTRIBUTOR shall, as appropriate, disclose all discounts received hereunder to representatives of Medicare, Medicaid, any governmental authority, and Federal health care programs (as defined under 42 U.S.C. 1320a-7b(f)) (collectively, “governmental entities”) upon request in accordance with 42 C.F.R. 1001.952(h); and (ii) UT shall, if required and as appropriate, properly report all discounts paid hereunder to the appropriate governmental entities for purposes of determining “Best Price” under the Medicaid rebate program and for purposes of determining AMP or ASP under Medicare, if applicable. During the Term of this Agreement, each Party shall take all actions necessary and appropriate to ensure that it complies with all Applicable Laws, including, without limitation, the Anti-Kickback Statute, the Stark Law and HIPAA as set forth above; and any laws and regulations relating to the terms of this arrangement as required. In addition, Accredo is subject to Medco Health Solution’s Code of Conduct and its policies and procedures relating to compliance with the above-named laws. These polices and procedures, along with the Code of Conduct, are available on Medco’s internet site (www.medco.com).
18.15 HIPAA Compliance. DISTRIBUTOR shall only provide information to UT in a manner consistent with the Health Insurance Portability and Accountability Act of 1996, as amended, 42 U.S.C. § 1320d, et seq., and the implementing regulations promulgated thereunder (collectively referred to herein as “HIPAA”). Accordingly, the Parties agree
that DISTRIBUTOR shall only provide UT with information that is de-identified in accordance with HIPAA’s de-identification provision, 45 C.F.R. § 164.514(b)(2), unless DISTRIBUTOR: (i) has on file a valid, HIPAA-compliant authorization for each patient whose protected health information (“PHI”) is sought to be disclosed; or (ii) authorization is not required under Applicable Laws in order to disclose the PHI.
18.16 Nothing herein shall be construed to limit DISTRIBUTOR from entering into other agreements with other manufacturers or wholesalers that allow DISTRIBUTOR to dispense products that compete with UT’s Products. Notwithstanding the preceding sentence, DISTRIBUTOR warrants and represents that it will not disparage UT or UT Product
18.17 Each Party shall promptly notify the other Party upon learning of any activity that appears to improperly or inappropriately portray or affect the other Party, its products or Affiliates.
18.18 The Parties do not intend for this Agreement to benefit any Third Party and, therefore, there are no third party beneficiaries to this Agreement.
IN WITNESS WHEREOF, the Parties have caused this Agreement to be executed by their duly authorized representatives as of the Effective Date.
|
UNITED THERAPEUTICS |
|
ACCREDO HEALTH GROUP |
||
|
CORPORATION |
|
|
||
|
|
|
|
||
|
|
|
|
||
|
By |
/s/ Jay A. Watson |
|
By |
/s/ Michael R. Hess |
|
|
Jay A, Watson, Pharm.D. |
|
|
Michael R. Hess |
|
|
Vice President, Strategic Operations |
|
|
Chief Counsel, Accredo |
|
|
and Logistics |
|
|
|
Attachment A
Prices
UT Product
|
UT Product Name |
|
NDC |
|
Strength |
|
Price |
|
|
Tyvaso Starter Kit |
|
66302-206-01 |
|
0.6 mg/ml |
|
TBA |
|
|
Tyvaso Re-Supply Kit |
|
66302-206-02 |
|
0.6 mg/ml |
|
TBA |
|
|
Tyvaso Supplemental Refill (4ct) |
|
66302-206-03 |
|
0.6 mg/ml |
|
TBA |
|
NDC 66302-206-01 Tyvaso Starter Kit includes:
· 28 ampoules of Tyvaso
· 2 Sets of Autoclavable Parts
· 2 Tyvaso Inhalation Devices
· 2 AC Power Adapters
· 1 Rechargeable Battery Pack
· 1 Car Power Cord
· 1 Leather Carrying Case
· 32 Medicine Cups
· 64 Filter Membranes
· 1 Nose Clip
· 1 Measuring Cup
· 1 Safety Box
· 2 Sets of Safety Plugs
NDC 66303-206-02 Tyvaso Re-Supply Kit includes
· 28 ampoules of Tyvaso
· 1 Set of Autoclavable Parts
· 32 Medicine Cups
· 64 Filter Membranes
NDC 66302-206-03 Tyvaso Supplemental Refill includes
· 4 ampoules of Tyvaso
UT shall notify the DISTRIBUTOR in writing of any change (and the amount of the change) in the Price of any respective UT Product during the term of this Agreement in the same time and manner as it notifies other similarly situated distributors.
UT shall provide DISTRIBUTOR with a current list of Tyvaso prices to Discounted Entities, including FSS prices, Federal Ceiling Prices, and prices to section 340B entities, and shall promptly notify Distributor of any and all changes in such prices as well as the effective dates of such changes.
Attachment B
UT Trademarks Logotypes and Trade Names
UNITED THERAPEUTICS
UNITED THERAPEUTICS CORPORATION LOGO
REMODULIN
MEDICINES FOR LIFE
Attachment C
Patient Assistance Program Guidelines
The PAP will be administered in accordance with UT SOPs that will require at a minimum the activities described in this Agreement and this Attachment C. DISTRIBUTOR is responsible for collecting the necessary patient demographics, financial, and clinical information and the oversight of the completion of the PAP enrollment form application. In addition, the DISTRIBUTOR will follow these guidelines and associated SOPs (as developed by UT) and will make the necessary decisions for patient acceptance into the PAP. DISTRIBUTOR will maintain a detailed and retrievable record of all communications, correspondence, and activities related to the PAP including dispensing of UT Product, supplies, and equipment without charge to the PAP Included Patient, and will use reasonable attempts to aid in securing third party payer benefits for each PAP Included Patient where applicable. In addition, DISTRIBUTOR shall take all necessary steps to preserve the confidentiality of Included Patient information gathered under the PAP in accordance with all federal, state and local laws relating to patient privacy, except as such confidentiality may otherwise be expressly waived by Included Patients. If and when Included Patients are presented to DISTRIBUTOR who do not meet the designated UT criteria, then DISTRIBUTOR will consult with UT prior to any decision regarding PAP acceptance.
Indigent Patients
Included Patients who meet the UT PAP criteria for indigent patients will qualify for the PAP for a period up to one (1) year from the date of acceptance into the PAP. DISTRIBUTOR will reevaluate Included Patient household income information, health insurance status, and out-of-pocket medication and supply expenses each quarter to assure continued eligibility. At the end of the one (1) year period, the PAP Included Patient will be required to re-enroll (as they did with the initial application) and provide proof of income or lack of third party payer information eligibility.
Under-Insured/”GAP” Coverage Patients
Included Patients who meet the under-insured/GAP coverage patient criteria will qualify for the PAP for a period up to a maximum of six (6) months or until he/she is able to obtain third party payer or similar reimbursement capabilities (whichever comes first). DISTRIBUTOR will reevaluate Included Patient household income information, health insurance status, and out-of-pocket medication and supply expenses each quarter to assure continued eligibility.
Potential Exhaustion of Insurance Coverage
Included Patients who meet the PAP criteria for potential exhaustion of insurance coverage will qualify for the PAP program for a period up to one (1) year from the date of acceptance into the PAP, except that eligibility will terminate at any earlier time that the Included Patient obtains a new source of insurance coverage (including supplemental coverage) and no longer meets the applicable criteria. DISTRIBUTOR will reevaluate Included Patient household income information, health insurance status, and out-of-pocket medication and supply expenses each quarter to assure continued eligibility.
Further DISTRIBUTOR Responsibilities:
DISTRIBUTOR will use Best Efforts to secure all information to support eligibility of PAP Included Patients PRIOR to any commitments for start of care. However, it may not always be possible to secure all information in the event of a medical need which could cause delays with the start of care. Therefore, DISTRIBUTOR may obtain at the very minimum a completed and
signed enrollment form and use its best judgment as to the Included Patient’s eligibility for acceptance prior to receiving all required supporting documentation. This will constitute a 28-30 day grace period for which the DISTRIBUTOR will not be liable for UT Product, pumps/devices, and supporting pump/device supplies from the initial start of care for a PAP Included Patient. At the end of the grace period, UT will not provide or reimburse for additional UT Product (or related supplies) unless DISTRIBUTOR obtains the required supporting documentation and determines that the PAP Included Patient is eligible for the PAP.
Attachment D
UT Warranty
UT warrants that all of its Product shall as of the date such Product arrives at DISTRIBUTOR’s Designated Shipment Location: (i) be free from defects in design, material and workmanship; (ii) be in compliance with all applicable law and regulation, including without limitation all regulatory requirements of the FDA, including those related to the adulteration or misbranding of Product within the meaning of Section 501 and 502 of the Food Drug and Cosmetics Act; (iii) not be articles which may not be introduced into interstate commerce pursuant to the requirements of Sections 505, 514, 515, 516 or 520 thereof; (iv) be manufactured in accordance with current FDA Good Manufacturing Practice as required by 21 C.F.R. 210 and 820; (v) are fit for the ordinary purposes for which such Products are intended; and (vi) are not infringing upon the patents or trademarks of any third party.
Attachment E
Designated Shipment Locations and Designated Storage Locations
|
Name/Address/Phone/Fax |
|
Name/Address/Phone/Fax |
|
Name/Address/Phone/Fax |
|
|
|
|
|
|
|
Accredo
Health Group, Inc. |
|
Accredo
Health Group, Inc. |
|
Accredo
Health Group, Inc. |
|
|
|
|
|
|
|
Accredo
Health Group, Inc. |
|
Accredo
Health Group, Inc. |
|
Accredo
Health Group, Inc. |
|
|
|
|
|
|
|
Accredo
Health Group, Inc. |
|
Accredo
Health Group, Inc. |
|
Accredo
Health Group, Inc. |
|
|
|
|
|
|
|
Accredo
Health Group, Inc. |
|
Accredo
Health Group, Inc. |
|
AHG
of New York, Inc. |
|
|
|
|
|
|
|
Accredo
Health Group, Inc. |
|
Accredo
Health Group, Inc. |
|
Accredo
Health Group, Inc. |
|
|
|
|
|
|
|
Accredo
Health Group, Inc. |
|
Accredo
Health Group, Inc. |
|
Home
HealthCare Resources, Inc. |
|
|
|
|
|
|
|
Accredo
Health Group, Inc. |
|
Accredo
Health Group, Inc. |
|
Accredo
Health Group, Inc. |
|
|
|
|
|
|
|
Accredo
Health Group, Inc. |
|
Accredo
Health Group, Inc. |
|
Accredo
Health Group, Inc. |
|
|
|
|
|
|
|
Accredo
Health Group, Inc. |
|
Accredo
Health Group, Inc. |
|
Accredo
Health Group, Inc. |
|
|
|
|
|
|
|
Accredo
Health Group, Inc. |
|
Hemophilia
Resources of America, Inc. |
|
Accredo
Health Group, Inc. |
|
|
|
|
|
|
|
BioPartners
in Care, Inc. |
|
Accredo
Health Group, Inc. |
|
Accredo
Health Group, Inc. |
|
|
|
|
|
|
|
Accredo
Health Group, Inc. |
|
Accredo
Health Group, Inc. |
|
Accredo
Health Group, Inc. |
|
Name/Address/Phone/Fax |
|
Name/Address/Phone/Fax |
|
Name/Address/Phone/Fax |
|
|
|
|
|
|
|
Accredo
Health Group, Inc. |
|
Critical
Care Systems, Inc. |
|
Critical
Care Systems, Inc. |
|
|
|
|
|
|
|
Accredo
Health Group, Inc. |
|
Critical
Care Systems, Inc. |
|
Critical
Care Systems, Inc. |
|
|
|
|
|
|
|
Critical
Care Systems, Inc. |
|
Critical
Care Systems, Inc. |
|
Critical
Care Systems, Inc. |
|
|
|
|
|
|
|
Critical
Care Systems, Inc. |
|
Critical
Care Systems, Inc. |
|
Critical
Care Systems, Inc. |
|
|
|
|
|
|
|
Critical
Care Systems, Inc. |
|
Critical
Care Systems, Inc. |
|
Critical
Care Systems, Inc. |
|
|
|
|
|
|
|
Critical
Care Systems, Inc. |
|
Critical
Care Systems, Inc. |
|
Critical
Care Systems, Inc. |
|
|
|
|
|
|
|
Critical
Care Systems, Inc. |
|
Critical
Care Systems, Inc. |
|
Critical
Care Systems, Inc. |
|
|
|
|
|
|
|
Critical
Care Systems, Inc. |
|
Critical
Care Systems, Inc. |
|
Critical
Care Systems, Inc. |
|
|
|
|
|
|
|
Critical
Care Systems, Inc. |
|
Critical
Care Systems, Inc. |
|
Critical
Care Systems, Inc. |
|
|
|
|
|
|
|
Critical
Care Systems, Inc. |
|
Critical
Care Systems, Inc. |
|
Critical
Care Systems, Inc. |
|
|
|
|
|
|
|
Critical
Care Systems, Inc. |
|
Critical
Care Systems, Inc. |
|
Critical
Care Systems, Inc. |
|
|
|
|
|
|
|
Critical
Care Systems, Inc. |
|
Critical
Care Systems, Inc. |
|
Critical
Care Systems, Inc. |
|
Name/Address/Phone/Fax |
|
Name/Address/Phone/Fax |
|
Name/Address/Phone/Fax |
|
|
|
|
|
|
|
Critical
Care Systems, Inc. |
|
Critical
Care Systems, Inc. |
|
Critical
Care Systems, Inc. |
|
|
|
|
|
|
|
Critical Care Systems, Inc. |
|
Critical
Care Systems, Inc. |
|
Critical
Care Systems, Inc. |
Attachment F
Inventory Data Reports
The following reports will be completed timely as indicated herein and provided to UT Management via electronic mail in Microsoft Word® or Excel® file formats or comma delimited (“CSV”) files and without cost or fee charges to UT.
DEFINITIONS
Reports
Written reports as described in this Attachment F.
Patient Starts
The initiation of commercial Product on an Included Patient for treatment.
Patient Discontinuations
When UT Product is no longer required by the Included Patient for any variety of medical or physical reasons.
Patient Assistance Program (PAP)
A US based program for Included Patients receiving UT Product who are either indigent, under insured, or in jeopardy of losing insurance due to therapy costs. The program allows for access to Product at no charge and demonstration of financial hardships through an enrollment process is required prior coverage. US Distributors are designated administrators of the program on behalf of UT in accordance with this Agreement.
Product Forecasts
Non-binding detailed reports by product size with reasonable estimates of use over a period of no less than 12 calendar months spanning January through December. Forecasts are updated each quarter during the year with revised 12 month calendar forecasts occurring annually. Forecasts may also include planned purchases of equipment or supplies to support the UT Product, where applicable and requested by UT.
Purchase Orders
An official and binding document, generated by the Distributor, to guarantee a request to purchase and pay at a contractual rate for the UT Products.
PROCEDURES: REPORTS
Reports will be provided to UT on a monthly and quarterly basis based on the type of the Report and data collected. These Reports are outlined below and are referenced as Attachments elsewhere in Attachment F (listed as Exhibits 1-4). Reports will consist of:
|
Report Name |
|
Frequency |
|
Due Following |
|
Exhibits |
|
|
|
|
|
|
|
|
|
Product Utilization Report |
|
Monthly |
|
10th of each month |
|
1 |
|
|
|
|
|
|
|
|
|
Medicaid Utilization Report |
|
Monthly |
|
10th of each month |
|
2 |
|
340B Covered Entity Reconciliation Report |
|
Monthly |
|
10th of each month |
|
3 |
|
Product Forecast |
|
Quarterly |
|
10th of month after each quarter |
|
4 |
Product Utilization Report
The monthly Product Utilization Report provides details of Product dispensing activities to commercial (reimbursable) and PAP Included Patients for the Territory. Exhibit 1 contains a sample Report form.
The Product Utilization Reports will be provided to UT by the DISTRIBUTOR no later than the 10th of each month using only the UT approved electronic form.
The Report contains the following three sections:
Product Utilization Data (by size) & Ordering Patients / Month
· Total Number of Tyvaso Starter Kits, Tyvaso Re-Supply Kits, Tyvaso Supplemental Refill dispensed/sold during the reporting period.
· Total number of orders for the package sizes dispensed/sold accessing the UT Product.
Commercial Inventory On Hand Summary values:
· An Inventory count of UT Product by package size at the end (last day) of the reporting period month.
· A realistic average dispensing/sold quantity of UT Product (a previous 3 month average is recommended).
· Actual Inventory days on hand which is automatically calculated by the Inventory count and average dispensing/sold quantities. The Inventory days on hand are measured based on a 28 day period.
· Purchase Order (PO) Requests for quantities expected to be purchased in order to meet both regular commercial activity and maintain a contractual on hand Inventory balance. (Note: An actual Purchase Order should accompany the Report)
· An Adjusted Inventory quantity is automatically calculated as the sum of the physical Inventory count plus the expected purchases from the PO.
· The Adjusted Inventory Days on Hand. This automatically calculated field is based on the previous data entries and will confirm if the new purchases plus actual Inventory, divided by the average dispensing/sold product will maintain the contractually required Inventory levels.
Patient Assistance Program for consigned inventory to support PAP Included Patients:
· The total Included Patient census on PAP at the beginning of the reporting period.
· The total Included Patient census on PAP at the end of the reporting period.
· Consignment PAP Inventory count of UT Product by package size at the end (last day) of the reporting period month.
· A realistic average estimate of Consigned PAP Inventory dispensed of UT Product (a previous 3 month average is recommended).
· Actual PAP Consigned Inventory days on hand which is automatically calculated by the PAP Consigned Inventory count and average dispensing/sold quantities. The PAP Consignment Inventory days on hand are measured based on a 28 day period.
· PAP Consignment Purchase Order (PO) Requests (if needed for the next period). It is recommended to request a PO for approximately of a 3 to 4 months worth of Consigned Product based on current use. (Note: An actual PO for Consigned Product should accompany the report).
· An Adjusted PAP Consigned Inventory quantity is automatically calculated as the sum of the PAP Consigned physical inventory count plus any expected Consigned Products from the PO.
· The Adjusted PAP Consignment Inventory Days on Hand. This automatically calculated field is based on the previous data entries and will confirm if the new purchases plus actual Inventory, divided by the average dispensing of PAP Consignment product levels.
Medicaid Utilization Report
The monthly Medicaid Utilization Report provides the information necessary for UT to manage its Medicaid-related programs. UT is a participant in the Federal Fee Schedule (“FFS”) and for the Centers of Medicare and Medicaid Services (“CMS”). Participation requirements are for provisions of rebates to CMS for those patients receiving UT Products who are covered by individual State Medicaid programs. Exhibit 2 contains a sample Report form.
The Medicaid Utilization Report will be provided to UT by the DISTRIBUTOR no later than the 10th of each month using only the UT approved electronic form.
The Report includes summary of activity for the reporting period (month) that includes a unique patient identifier number (HIPAA compliant), the UT Product size), the quantity dispensed during the reporting period, the Distributor’s internal State Medicaid identification number/description, the State of the Program (abbreviated), and any other descriptions or comments to support the data.
340B Covered Entity Reconciliation Report
The monthly 340B Covered Entity Reconciliation Report provides the records that are required in order for the Distributor to recover the loss on Product cost incurred due to UT’s participation in the FFS and CMS. Exhibit 3 contains a sample Report form.
The 340B Covered Entity Reconciliation Report will be provided to UT by the DISTRIBUTOR no later than the 10th of each month IF any transactions to 340B Covered Entities occurred. Any Reports filed for previous months not reported to UT will be denied for refund. If no activity of 340B sales occurs during the month, no Report submission will be required.
Public Health Services (“PHS”) pricing programs as part of FFS participation requires discounted pricing to be offered under the FFS program title known as 340B with whom 340B eligible hospitals or clinics (known as 340B covered entities) are entitled to receive products from FFS/PHS Participating manufactures at reduced price.
In order to facilitate 340B covered entities to obtain the reduced prices, DISTRIBUTOR will offer 340B prices at rates regularly updated and provided by UT when a 340B covered entity identifies itself and requests such prices. If the DISTRIBUTOR purchased UT Product from UT at transfer prices higher than the 340B price, UT will provide payment to the DISTRIBUTOR for the difference between the DISTRIBUTOR’s transfer price and the 340B price sold to a 340B covered entity.
The Report must include the following elements:
· 340B Covered Entity Name
· 340B Identification Number
· Date of UT Product Sale
· Quantity of UT Product Sold
· 340B Ceiling Price (per product NDC /sold) — provided by UT
· Total 340B Sales (unit 340B Ceiling price times quantity sold)
· Distributor Transfer price (per product NDC/ sold)
· Transfer price extension (Transfer price time the unit quantity sold)
· Refund due
PROCEDURES: FORECASTS
Forecasts
The non-binding forecasts will be based on reasonable estimates of expected purchases and be presented in Excel Spreadsheet or similar electronic format listed by month and totaled for the calendar year. The forecasts will list each UT Product size for a particular drug category and may include medical devices (such as Infusion Pumps) or supplies needed from UT contracted equipment distributors to support the Product.
At the end of each Calendar quarter, DISTRIBUTOR will revise and update its twelve (12) month rolling non-binding forecasts for future quarters for that twelve (12) month period based on changes in demand and the market. These revised non-binding Forecasts will be provided to UT no later than the 10th day of first month in the new calendar quarter unless otherwise specified in this Agreement using the UT approved electronic format (refer to Exhibit 4).
EXHIBITS TO ATTACHMENT F
Exhibit 1
Monthly Product Utilization Report

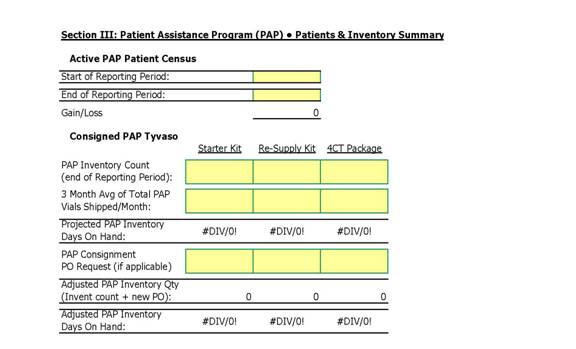
Exhibit 2
Monthly Medicaid Utilization Report
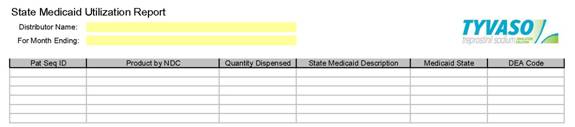
Exhibit 3
340B Covered Entity Reconciliation Report
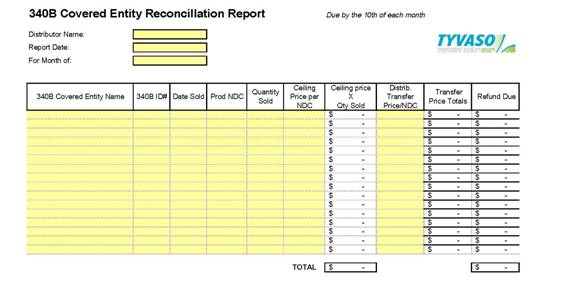
![]()
Exhibit 4
Product Forecast (example):
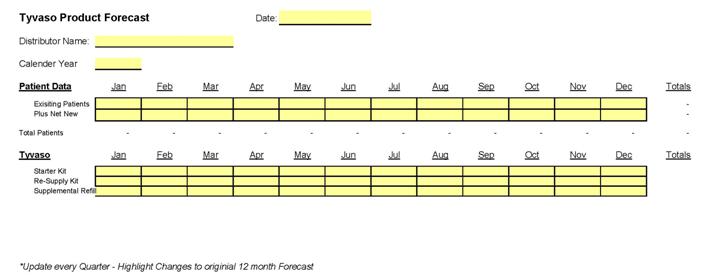
Attachment G
Tyvaso Education & Compliance Program
THIS CHART NEEDS TO BE UPDATED TO MATCH TEXT
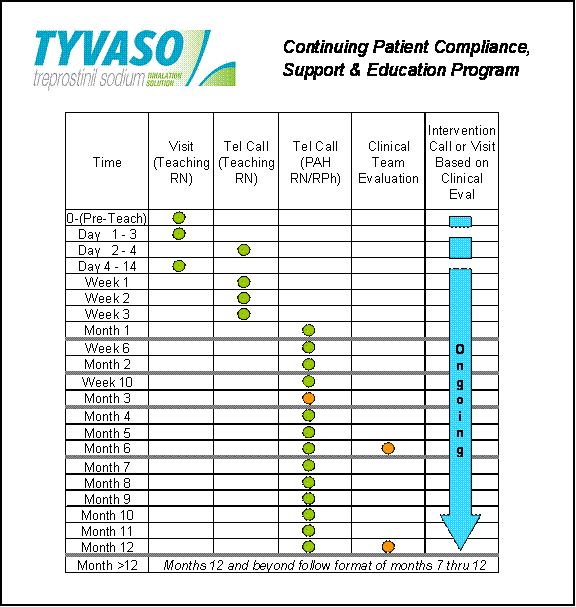
The Tyvaso™ Continuing Patient Compliance,
Support and Education Program
|
TIME |
|
ACTIVITY |
|
|
|
|
|
Day Zero |
|
· Pre — teach (as needed)* |
|
|
|
|
|
Day 1 – 3 |
|
· Teaching RN initiation visit* |
|
|
|
|
|
Day 2 – 3 |
|
· RN post start telephonic status check/assessment* |
|
|
|
|
|
Day 4 – 14 |
|
· RN visit within first 3 to 14 days post-start. Evaluation and review. |
|
|
|
|
|
Week 1,2,3 |
|
· RN telephonic status check/assessment minimum of weekly contact. |
|
|
|
|
|
Week 4/Month 1 |
|
· Telephonic status check/assessment with transfer to PAH RN/RPh** as required. (Reorder Call) |
|
|
|
|
|
Week 6/Month 1.5 |
|
· PAH RN/RPh telephonic status check/assessment |
|
|
|
|
|
Week 8/Month 2 |
|
· Telephonic status check/assessment with transfer to PAH RN/RPh** (Reorder Call) |
|
|
|
|
|
Week 10/Month 2.5 |
|
· PAH RN/RPh telephonic status check/assessment |
|
|
|
|
|
Week 12/Month 3 |
|
· Telephonic status check/assessment with transfer to PAH/RNRPh** (Reorder Call) · 90 day Risk Stratification Review |
|
|
|
|
|
Month 4,5 |
|
Telephonic status check/assessment with transfer to PAH RN/RPh** (Reorder Call) |
|
|
|
|
|
Month 6 |
|
· Telephonic status check/assessment PAH RN/RPh** (Reorder Call) · Clinical Team Evaluation and Treatment Goal Planning |
|
|
|
|
|
Months 7 thru 11 |
|
· Telephonic status check/assessment with transfer to PAH RN/RPh** (Reorder Call) |
|
|
|
|
|
Month 12 |
|
· Telephonic status check/assessment PAH RN/RPh** (Reorder Call) · Clinical Team Evaluation and Treatment Goal Planning |
|
|
|
|
|
Months 13 plus |
|
· Follows format of months 7 through 12 |
*These activities are considered standard activities for a specialty pharmacy and should be included under this Agreement at no additional charge.
**In each case throughout this Exhibit G, the reference to “PAH RN/RPh” means that such activity requires a Registered Nurse or Registered Pharmacist with the required expertise and training in PAH.
The Tyvaso™ Continuing Patient Compliance,
Support and Education Program
Description of Activities
Subject to patient’s consent, the Teaching RN will complete a minimum of two (2 teaching sessions within the first fourteen (14) days of therapy. Additionally, a clinicianwill contact the patient telephonically in weeks one, two, and three of therapy. During these teaching visits and telephonic touch points, the clinician will review with the patient in detail:
· TYVASO
· Education Tools — (Example 1 of Exhibit A to this Attachment G). Includes UT/Lung Rx materials that can be used to supplement DISTRIBUTOR-developed educational materials. Exhibit A will be updated as needed.
· Disease information
· Dosing and Administration
· Use of one ampule per day
· Daily documentation in Patient Diary
· Daily disposal of unused drug
· Titration schedule (prescribed per breath)
· Missed Doses
· Managing Side Effects
· Establish treatment goals
· Storage/Handling/Cleaning
· TYVASO Inhalation System
· Education Tools - Information For Use Manual, Patient DVD, Patient Breath Counter, Patient Diary and Quick Start Mat
· Preparing for treatment
· Using the TYVASO Inhalation System
· Assembly
· Use of distilled water only
· Inhaling Technique
· Audiovisual prompts
· Cleaning and maintenance
· Use of distilled water only
· Alarms/Troubleshooting
· Recording number of breaths per treatment sessions
· Use of Breath Counter/Patient Diary
· Fitting 4 treatment sessions into your day
· DISTRIBUTOR Services
· Refills services
· Monthly Touch points (Clinical Risk/Assessments & Medication Resupply)
· Emergency/After Hours Support
· Reimbursement Services
After the first month of therapy, the patient should be competent in the administration of TYVASO and use of the TYVASO Inhalation System. From this point in the Continuing Patient Compliance, Support and Education Program, Accredo will continue to monitor the patient telephonically on a bi-weekly basis in the second and third month of the program, and monthly there after. These ongoing telephonic touch points will include a Risk Stratification Review.
A typical Risk Stratification Review call should cover the following topics:
· Disease: Assess changes in signs and symptoms of disease
· Dose: Confirm the current dose
· Confirm use of one ampule per day and discard of any remaining solution
· Confirm proper breath tracking per treatment session
· Review titration schedule, if applicable
· Side effects: Assess extent and duration of side effects.
Develop solution to minimize side effects.
Consult with prescribing physician if necessary.
· Device: Confirm proper use of the TYVASO Inhalation System.
Confirm use of distilled water only
· Goals: Measure attainment for treatment goals
· Support: Encourage patient to use educational/support tools provided by the manufacturer
(PEER/Integrated Persistency Program). Increase patient awareness of PAH support groups.
Based on information received from the Risk Stratification Review the patient will be identified as one of the following:
1) Compliant
2) Compliant risk — requires clinical intervention
3) Non-compliant - requires clinical intervention
· Compliant: With minimal side effects, the patient is showing benefit from TYVASO and taking treatments 4x a day, between 3 - 9 breaths per session, discarding any remaining solution at the end of each day.
· Compliant Risk: The PAH RN/RPh has documented interventions for device management issues, non-compliant, side effect management, or lack of efficacy.
· Non-compliant: The PAH RN/RPh has multiple documented interventions for non-compliance in addition to lack of efficacy.
If it is determined that a patient is in the compliant risk or non-compliant category, the PAH RN/RPh will intervene appropriately. Intervention may require a home visit and include the following:
· Additional disease education
· Continued education on the use of the TYVASO Inhalation Device
· Review proper Inhalation technique
· Side effect management
· Dose escalation/titration
· Strategies to fit four inhalation sessions into a day
· Reassessment of treatment goals
· Consultation with the prescribing physician
Upon reaching the 180th day of therapy, DISTRIBUTOR’s clinical team will perform a Clinical Team Evaluation and Treatment Goal Plan for the patient. This will include a clinical contact (either PAH RN or RPh) contacting the patient for assessment. After the assessment, the physician will be contacted to review the patients progress to date and therapeutic goals for the next six months.
In addition to the clinical support outlined above, DISTRIBUTOR will contact the patient telephonically on a monthly basis to process refills, ensure the patient is using one ampule per day, confirm the patient is properly tracking number of breaths per treatment session, using distilled water only and resolve reimbursement issues.
Service Fees
|
Description of CPCSEP Service |
|
Service Fee |
|
Teaching RN visit within 4 to 14 days post-start |
|
$250 per visit |
|
Teaching RN telephonic status check/assessment weeks 1, 2 and 3 |
|
$52 per completed call |
|
PAH RN/RPh telephonic status check/assessment |
|
$52 per completed call |
|
Clinical Team Evaluation and Treatment Goal Planning |
|
$150 per session (including completed communication with physician) |
The Parties agree that the activities described in Attachment G occurring from Day Zero through Day 3 (including the initial visit from the Teaching RN and the post start telephonic status check/assessments) are considered standard activities for a specialty pharmacy and should be included under this Agreement at no additional charge.
Note: The CPCSEP Services and corresponding Service Fees will be evaluated by UT on an annual basis and UT may terminate all or a portion of the CPCSEP Services at any time upon thirty (30) days prior written notice to DISTRIBUTOR without any additional fee or expense.
Attachment G “Exhibit A”
|
Education Theme |
|
Education Tool Description |
|
Item # |
|
The welcome letter provides on overview of the TYVASO Patient Tool Kit components. |
|
TYVASO Patient Tool Kit Welcome Letter (Tool kit contains: Patient Video, Patient Brochure, Treatment Tracker, Breath Counter & Quick Start Mat) |
|
TBD |
|
Educational video designed to: |
|
|
|
|
|
|
|
|
|
|
|
1. Teach the patient how to clean, assemble and store the inhalation system. |
|
TYVASO Patient Video |
|
TBD |
|
2. Proper inhalation technique and use of the inhalation system. |
|
|
|
|
|
Disease overview: what is PAH, causes and symptoms of PAH, and treatment options. TYVASO overview: what is TYVASO, how does TYVASO help, and how do I take TYVASO. |
|
TYVASO Patient Brochure |
|
TBD |
|
A daily diary to assist the patient with tracking breathes and cycles. |
|
TYVASO Treatment Tracker |
|
TBD |
|
A table top tool to assist the patient with tracking breaths and cycles. |
|
TYVASO Breath Counter |
|
TBD |
|
Reinforce important information such as: inhalation technique, treatment sessions and how to operate the inhalation system. |
|
TYVASO Inhalation System Quick Start Mat |
|
TBD |
