Attached files
Table of Contents
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, DC 20549
FORM 10-K
| x | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2009
OR
| ¨ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to .
Commission File Number: 000-50855
Auxilium Pharmaceuticals, Inc.
(Exact name of registrant as specified in its charter)
| Delaware | 23-3016883 | |
| (State or other jurisdiction of incorporation or organization) |
(I.R.S. Employer Identification No.) | |
| 40 Valley Stream Parkway Malvern, PA |
19355 | |
| (Address of principal executive offices) | (Zip Code) | |
(484) 321-5900
(Registrant’s telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Securities Exchange Act of 1934:
| Title of Each Class | Name of Each Exchange on Which Registered | |
| Common Stock, Par Value $0.01 Per Share | The NASDAQ Global Market |
Securities registered pursuant to Section 12(g) of the Securities Exchange Act of 1934:
None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes x No ¨
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Exchange Act. Yes ¨ No x
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes ¨ No ¨
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes x No ¨
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See definitions of “large accelerated filer,” “accelerated filer,” and “smaller reporting company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer | x | Accelerated filer | ¨ | |||
| Non-accelerated filer | ¨ | Smaller reporting company | ¨ | |||
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ¨ No x
The aggregate market value of the voting and non-voting common equity held by non-affiliates of the registrant, based on the last sale price for such stock on The NASDAQ Global Market as of June 30, 2009, was approximately $1.3 billion.
As of February 19, 2010, the number of shares outstanding of the issuer’s common stock, $0.01 par value per share, was 47,243,440.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the Registrant’s definitive Proxy Statement for its 2010 Annual Meeting of Stockholders are incorporated by reference into Part III of this Form 10-K to the extent stated herein. Such proxy statement will be filed with the Securities and Exchange Commission within 120 days of the registrant’s fiscal year ended December 31, 2009.
Table of Contents
AUXILIUM PHARMACEUTICALS, INC.
FORM 10-K
December 31, 2009
TABLE OF CONTENTS
Table of Contents
This Report on Form 10-K contains forward-looking statements within the meaning of Section 21E of the Securities Exchange Act of 1934, as amended, that involve risks and uncertainties. All statements other than statements of historical information contained herein are forward-looking statements and may contain projections relating to financial results, economic conditions, trends and known uncertainties. These statements are not guarantees of future performance or events. Our actual results may differ materially from those discussed in this Report. You should review the “Risk Factors” section of this Report for a discussion of the important factors that could cause actual results to differ materially from those described in or implied by the forward-looking statements contained in this Report. Readers are cautioned not to place undue reliance on these forward-looking statements, which reflect management’s analysis, judgment, belief or expectation only as of the date hereof. We undertake no obligation to publicly reissue these forward-looking statements to reflect events or circumstances that arise after the date hereof.
We obtained the market and competitive position data used throughout this Report from our own research, surveys or studies conducted by third parties and industry or general publications. Industry publications and surveys generally state that they have obtained information from sources believed to be reliable, but do not guarantee the accuracy and completeness of such information. While we believe that each of these studies and publications is reliable, we have not independently verified such data. Similarly, we believe our internal research is reliable but it has not been verified by any independent sources.
This Report may include discussion of certain clinical studies relating to our products and/or product candidates. These studies typically are part of a larger body of clinical data relating to such products or product candidates, and the discussion herein should be considered in the context of the larger body of data.
As used herein, the terms “Company”, “Auxilium”, “we”, “us”, or “our”, refer to Auxilium Pharmaceuticals, Inc.
| ITEM 1. | Business |
Company Overview
Company Description
We are a specialty biopharmaceutical company with a focus on developing and marketing products to predominantly specialist audiences, such as urologists, endocrinologists, certain targeted primary care physicians, hand surgeons, subsets of orthopedic, general, and plastic surgeons who focus on the hand, and rheumatologists. We currently have approximately 540 employees, including a sales and marketing organization of approximately 315 people. We reported revenues in 2009 of $164 million, an increase of 30.8% over the $125.4 million reported in 2008.
We currently market two products in the United States (“U.S.”):
Testim® is a proprietary, topical 1% testosterone once-a-day gel indicated for the treatment of hypogonadism. Hypogonadism is defined as reduced or absent secretion of testosterone which can lead to symptoms such as low energy, loss of libido, adverse changes in body composition, irritability and poor concentration. Testim has been approved in the U.S. by the U.S. Food and Drug Administration (“FDA”) and according to National Prescription Audit (“NPA”) data from IMS Health, Inc. (“IMS”), a pharmaceutical market research firm, Testim’s share of total prescriptions for the gel segment of the U.S. testosterone replacement therapy (“TRT”) market was 22.3% for the month of December 2009, and 22.0% for the full year 2009.
XIAFLEX™ (collagenase clostridium histolyticum) is a proprietary, injectable collagenase enzyme for the treatment of Dupuytren’s contracture (“Dupuytren’s”). XIAFLEX received approval from the FDA on
2
Table of Contents
February 2, 2010 for the treatment of adult Dupuytren’s patients with a palpable cord. Dupuytren’s is a condition that affects the connective tissue that lies beneath the skin in the palm. The disease is progressive in nature. Typically, nodules develop in the palm as collagen deposits accumulate. As the disease progresses, the collagen deposits form a cord that stretches from the palm of the hand to the base of the finger. Once this cord develops, the patient’s fingers contract and the function of the hand is impaired. Prior to approval of XIAFLEX, surgery was the only effective treatment. We expect to begin shipping XIAFLEX to our distribution partners in early March 2010 in advance of a launch planned for the end of March 2010.
We also have received marketing approval for Testim in Belgium, Canada, Denmark, Finland, Germany, Greece, Iceland, Ireland, Italy, Luxembourg, the Netherlands, Norway, Portugal, Spain, Sweden and the United Kingdom (“U.K.”). Ferring International Center S.A. (“Ferring”) and Paladin Labs Inc. (“Paladin”) commercialize Testim on our behalf in certain European countries and Canada, respectively.
Our current product pipeline includes:
Phase II:
| • | XIAFLEX for the treatment of Peyronie’s disease (“Peyronie’s”) |
| • | XIAFLEX for the treatment of Adhesive Capsulitis (“Frozen Shoulder syndrome”) |
Phase I:
| • | AA4010, treatment for overactive bladder using our transmucosal film delivery system |
| • | A Fentanyl pain product using our transmucosal film delivery system. |
In addition to the above, we have the rights to develop other compounds for the treatment of pain using our transmucosal film delivery system and other products using our transmucosal film technology for treatment of urologic disease and for hormone replacement. We also have the option to license additional indications for XIAFLEX other than dermal products for topical administration.
Company Strategy
Our goal is to build a leading specialty biopharmaceutical company by focusing on disease states that we believe can be addressed through sales to predominantly specialist audiences, such as urologists, endocrinologists, certain targeted primary care physicians, hand surgeons, subsets of orthopedic, general and plastic surgeons who focus on the hand, and rheumatologists. We have experienced development, regulatory and commercial teams that have a proven track record. We intend to draw upon our track record and experience to successfully develop our product candidates. Our strategy will focus on developing and commercializing XIAFLEX, optimizing Testim’s commercial success and in-licensing additional product candidates and acquiring companies. We believe that we have in place the commercial infrastructure necessary to commercialize Testim for hypogonadism and XIAFLEX for Dupuytren’s in the U.S. We have entered into a development, commercialization and supply agreement with Pfizer, Inc. (“Pfizer”) for XIAFLEX for the treatment of Dupuytren’s and Peyronie’s in Europe and certain Eurasian countries, and we are currently evaluating how to most effectively commercialize XIAFLEX in these two indications in the other territories of the world. Ferring and Paladin commercialize Testim on our behalf in certain European countries and Canada, respectively.
Developing and Commercializing XIAFLEX. We believe that XIAFLEX has the potential to become a blockbuster. For Dupuytren’s and Peyronie’s, we believe there is a large unmet medical need for a non-surgical solution to these debilitating diseases. On February 2, 2010, we received FDA approval of XIAFLEX for Dupuytren’s, and the data from our well-controlled phase II studies in Peyronie’s are encouraging. The product has received orphan drug designation in the U.S. for both indications, and our preliminary market research leads us to believe that urologists, hand surgeons, subsets of orthopedic, general, and plastic surgeons who focus on the
3
Table of Contents
hand, and rheumatologists would be very likely to use the product to delay or avoid surgery. Based on market research that we conducted several years ago, physicians indicate that there are potentially 450,000 patients on an annual basis in the U.S. and Europe who could be candidates for XIAFLEX with approximately 240,000 for the treatment of Dupuytren’s and approximately 210,000 for the treatment of Peyronie’s. These patients represent an annual market potential in excess of $1 billion.
In 2004, we obtained the exclusive worldwide rights to develop, market and sell products containing XIAFLEX, other than dermal formulations labeled for topical administration. Specifically, our license from BioSpecifics Technologies Corp. (“BioSpecifics”) includes the rights to XIAFLEX for the treatment of Dupuytren’s, Peyronie’s and Frozen Shoulder syndrome with the exclusive option to license additional indications. In December 2008, we entered into a collaboration with Pfizer in which we sub-licensed our commercialization rights for Dupuytren’s and Peyronie’s for the 27 countries which were included in the European Union (“EU”) at that time and 19 additional Eastern European and Eurasian countries. We plan to prioritize our development of additional pipeline indications for XIAFLEX. We have previously taken a license for the development and commercialization of Frozen Shoulder syndrome, but other areas of interest could include cellulite and lipoma.
Launching XIAFLEX in Dupuytren’s. Upon FDA approval of XIAFLEX for Dupuytren’s, we offered full time employment to a new group of field sales representatives and believe that these employees will be adequately trained and prepared for a launch planned for the end of March 2010. We expect to begin shipping XIAFLEX to our distributors the week of March 1, 2010 with product being available for shipment to physicians during the week of March 8, 2010. Our plans for commercializing XIAFLEX include the steps outlined below.
| • | We plan on selling XIAFLEX in the U.S. through a team of approximately 100 professionals, including approximately 75 sales representatives supplemented by sales managers, reimbursement specialists and managed market account directors. |
| • | A staff of 11 medical science liaisons will provide medical support for the product. |
| • | To supplement the efforts of our field organization, we intend to implement a number of marketing and medical education programs. We anticipate that these programs will include: a reimbursement assistance hotline; billing assistance guides; claims tracking assistance; participation at national medical meetings; advertisements in medical journals; launch speaker programs; and a unique computerized simulation system that will allow physicians to gain hands-on experience with the XIAFLEX injection technique. |
| • | Pursuant to a Risk Evaluation and Mitigation Strategy (“REMS”) required by the FDA, XIAFLEX can only be used by healthcare providers with experience in injection procedures of the hand and in the treatment of Dupuytren’s. A physician wishing to use XIAFLEX must attest that they have completed the XIAFLEX training program and the site of care must be enrolled before we will ship XIAFLEX to that physician. As a result, we plan to provide training on the proper use and potential risks of XIAFLEX to hand, orthopedic, plastic and general surgeons and rheumatologists who are familiar with the diagnosis and treatment of Dupuytren’s. We have identified approximately 7,000 of these physicians who will be the focus of our initial commercial effort. The XIAFLEX training materials are available via multiple media platforms and review reconstitution, injection technique, manipulation and the safety profile of XIAFLEX. |
| • | We have established a distribution network that will allow health care providers to access XIAFLEX through specialty distributors and specialty pharmacies, or in the institutional setting, after they have undergone training on XIAFLEX and its administration. |
4
Table of Contents
| • | Our reimbursement specialists will continue working with health care payors, both commercial and Medicare, to establish reimbursement processes. In addition, our reimbursement specialists, reimbursement hotline, managed market account directors and field sales representatives will work closely with physician offices to provide assistance as questions and issues arise with reimbursement. |
| • | We will launch XIAFLEX for Dupuytren’s without product and procedure specific reimbursement codes. In late 2009, we applied for product specific reimbursement codes (commonly referred to as “J” codes and “C” codes), and supplemented these applications with the confirmation of approval by the FDA in February 2010. We believe that we will receive a XIAFLEX specific J code effective beginning January 1, 2011. We have begun the process of working with the various hand professional medical societies that will assist us in applying for XIAFLEX specific procedural reimbursement code(s) (“CPT codes”). We believe that we will apply for these CPT codes prior to the end of 2010 and that we may have CPT codes beginning January 1, 2012. |
We will monitor the effectiveness of the various programs outlined above and may modify, eliminate or develop new programs as we believe are warranted.
Optimizing Testim’s Commercial Success. Testim global revenues for 2009 were $160.5 million, a 28.1% increase over the $125.2 million recorded in 2008. In the U.S., Testim revenues were $151.9 million, a 23.2% increase over 2008’s revenues of $123.3 million. Testim has demonstrated an ability to capture market share in the gel segment of the TRT market and the total TRT market, but in 2009 its market share in the gel segment of the TRT market was consistent with 2008. According to IMS, Testim’s gel share was 22.5% in December 2009 compared to 22.6% in December 2008. Fluctuations in share will occur with wins and losses on the managed care front. We had some losses within our managed care accounts in early 2009 that impacted our share growth adversely, but we were able to overcome that through growth in other managed care accounts. We believe that we can continue to increase sales of Testim by capturing market share, expanding the use of TRT through increased physician and patient awareness and benefiting from the on-going demographic changes that could result in additional patient candidates for TRT treatment.
| • | Increase Testim market penetration. Through on-going evaluation of physician targeting and greater sales efficiencies, we intend to improve the effectiveness of our sales force. We will continue to work with accredited education groups, thought leaders and professional organizations to further educate physicians about the diagnosis of hypogonadism and the benefits of TRT, including Testim. We are focusing approximately 150 sales representatives on urologists, endocrinologists and certain targeted primary care physicians. This allows us to reach the physicians who prescribe approximately 50% of TRT prescriptions. We also have entered into collaborations with partners for the international commercialization of Testim. Paladin launched Testim in Canada during 2007, and Ipsen Pharma GmbH (“Ipsen”) promoted Testim in Europe beginning in 2005. In November 2008, we agreed with Ipsen to terminate the distribution agreement between the parties and transfer all marketing authorizations for Testim in Europe to Ferring. Ferring is now responsible for commercializing Testim in Europe. |
| • | Expand market opportunities for Testim and TRT. A 2006 study that was published in The International Journal of Clinical Practice showed 39% of men over 45 years of age have low testosterone (total testosterone levels below 300 ng / dL). According to the U.S. Census Bureau, there were 43.6 million men aged 45 to 84 with hypogonadism in 2000, and these figures were expected to increase to 54.6 million by 2010. As a result, we expect to see an increase in the number of potential patients over the coming years. |
In addition, it has been demonstrated that low testosterone is a comorbid condition with others such as diabetes, obesity, hyperlipidemia, chronic pain and HIV/AIDS. With improved
5
Table of Contents
medical and patient education around the implications of low testosterone and the favorable risk/benefit profile of treating with TRT, we believe physicians will be more proactive in identifying hypogonadism and treating patients. In 2009, the total prescriptions written in the gel market increased 14.9% over 2008.
We will continue to incorporate focused educational efforts on both physicians and consumers to create an increased awareness of both the prevalence and medical need for identifying and treating hypogonadism.
| • | Continue to defend Testim intellectual property. In October 2008, we and our licensor, CPEX Pharmaceuticals, Inc. (“CPEX”) received notice that Upsher-Smith Laboratories, Inc. (“Upsher-Smith”) filed an abbreviated new drug application (“ANDA”) containing a paragraph IV certification seeking approval from the FDA to market a generic version of Testim prior to the January 2025 expiration of CPEX’s U.S. Patent No. 7,320,968 (the “968 Patent”). The ‘968 Patent, which Bentley Pharmaceuticals, Inc. (“Bentley”) assigned to CPEX in June 2008, covers a method for maintaining effective blood serum testosterone levels for treating a hypogonadal male using Testim and is listed in Approved Drug Products with Therapeutic Equivalence Evaluations (commonly known as the “Orange Book”), published by the FDA. The paragraph IV certification sets forth allegations that the ‘968 Patent will not be infringed by Upsher-Smith’s manufacture, use or sale of the product for which the ANDA was submitted. In December 2008, we filed a patent infringement lawsuit against Upsher-Smith which remains pending. Upsher-Smith has alleged that its proposed generic version of Testim would not infringe the ‘968 patent, and that the ‘968 patent is invalid. Under the Hatch-Waxman Act, final FDA approval of Upsher-Smith’s proposed generic product will be stayed until the earlier of 30 months from receipt of the paragraph IV certification (April 2011) or resolution of the patent infringement lawsuit. Should Upsher-Smith receive a tentative approval of its generic version of Testim from the FDA, it cannot lawfully launch its generic version of Testim in the U.S. before the earlier of the expiration of the currently pending 30-month stay or a district court decision in its favor. In October 2009, six additional patents covering Testim were issued and have been listed in the Orange Book. These additional patents may provide us with further market protection. |
| • | Filing of a Citizen’s Petition. In February 2009, we filed a Citizen’s Petition with the FDA requesting that Upsher-Smith be required to perform a variety of studies to demonstrate that the inactive ingredients and formulation of the proposed product do not compromise its safety or efficacy. An FDA response to our Citizen’s Petition was received in August 2009. The FDA agreed with some of the statements made in the Citizen’s Petition regarding the testing required for generic versions of Testim and disagreed with other statements. Although not commenting upon any filing in particular, the FDA did state that “the practical effect of this determination is that any application for a testosterone gel product that has different penetration enhancers than the reference listed drug cannot be submitted as an abbreviated new drug application (ANDA) and, instead, will have to be submitted as a NDA under section 505(b) of the [Federal Food, Drug, and Cosmetic Act].” |
In-licensing Product Candidates and Acquiring Companies. We believe that we can leverage our expertise in clinical development, regulatory strategy and execution and commercialization of specialty pharmaceutical and bio-pharmaceutical products to in-license additional product candidates or acquire companies with product candidates. Although we may seek to find products that have a therapeutic fit with XIAFLEX or Testim (i.e. products that would be utilized by urologists, endocrinologists, certain targeted primary care physicians, hand surgeons, subsets of orthopedic, general, and plastic surgeons who focus on the hand, and rheumatologists), we believe that the core competencies we have with respect to drug development and commercialization can be applied to a number of specialty focused therapeutic areas.
6
Table of Contents
XIAFLEX Opportunity
XIAFLEX is a proprietary, injectable collagenase enzyme we in-licensed from BioSpecifics. Under our agreement with BioSpecifics, we have exclusive worldwide rights to develop, market and sell certain products containing XIAFLEX, other than dermal formulations labeled for topical administration.
XIAFLEX for Dupuytren’s
On February 2, 2010, we received approval from the FDA to begin the sale of XIAFLEX for Dupuytren’s in adult patients with a palpable cord. Currently, we are preparing for a launch of XIAFLEX for Dupuytren’s planned for the end of March 2010. We expect to begin shipping XIAFLEX to our distributors the week of March 1, 2010 with product being available for shipment to physicians during the week of March 8, 2010.
Dupuytren’s disease state and market: Dupuytren’s is a condition that affects the connective tissue that lies beneath the skin in the palm. The disease is progressive in nature. Typically, nodules develop in the palm as collagen deposits accumulate. As the disease progresses, the collagen deposits form a cord that stretches from the palm of the hand to the base of the finger. Once this cord develops, the patient’s fingers contract and the function of the hand is impaired.
XIAFLEX will offer the first and only approved nonsurgical solution to fulfill the unmet need of physicians and patients who desire a nonsurgical alternative. According to Dupuytren’s Disease by Leclercq published in 2000, the global prevalence of Dupuytren’s among the Caucasian population is estimated to range from 3% to 6%. Common co-morbidities include diabetes, epilepsy, alcoholism or HIV. Dupuytren’s is most common in men. It usually starts in adult life, with a peak incidence in people aged 40 to 60. In about 50% of patients, the disease affects both hands, and recurrence after surgical treatment is common (as high as 30% the first two years post-surgery; and 55% within 10 years of surgery).
We believe XIAFLEX as an alternative to surgery will be desired as it:
| • | delivers improvement to 0 to 5 degrees of normal joint contracture without surgery for many patients; |
| • | safeguards patients from the complications commonly associated with surgery; |
| • | simplifies treatment by offering a convenient in-office procedure alternative; |
| • | streamlines recovery for a rapid return to daily activities; and |
| • | significantly reduces costly and time-consuming physical therapy associated with surgery. |
Prior to approval of XIAFLEX for Dupuytren’s, surgery was the only treatment option. Surgical techniques used to treat patients with Dupuytren’s fall into two major groups: fasciotomy, including needle aponeurotomy, which is simply cutting the Dupuytren’s cord and fasciectomy which is removing the cords of the diseased tissue. There are risks associated with surgery which include scarring, infection, hematoma, skin necrosis, finger stiffness and injury to nerves and blood vessels. Several non-operative treatments, including radiotherapy, steroids, interferon and topical vitamin E, have been tried in Dupuytren’s, but none has been adopted in general use nor proven effective. Although preliminary observations suggest that intralesional interferon gamma may reduce the size of Dupuytren’s cords (Plast Reconstr Surg. 1994 May;93(6):1224-35), further data are needed to assess whether it offers any clinical benefits.
Clinical data supporting efficacy of XIAFLEX for Dupuytren’s: The efficacy of 0.58 mg of XIAFLEX was evaluated in two randomized, double-blind, placebo-controlled, multi-centered trials in 374 adult patients with Dupuytren’s (“CORD I” and “CORD II”). At study entry, patients must have had (1) a finger flexion contracture with a palpable cord in at least one finger (other than the thumb) of 20° to 100° in a metacarpophalangeal (“MP”) joint or 20° to 80° in a proximal interphalangeal (“PIP”) joint and (2) a positive “table top test” defined as the inability to simultaneously place the affected finger(s) and palm flat against a table
7
Table of Contents
top. Patients could not have received a surgical treatment (e.g., fasciectomy, fasciotomy) on the selected primary joint within 90 days before the first injection of study medication and patients could not have received anticoagulation medication (except for up to 150 mg of aspirin per day) within seven days before the first injection of study medication.
The cord affecting the selected primary joint received up to three injections of 0.58 mg of XIAFLEX or placebo on Days 0, 30, and 60. About 24 hours after each injection of study medication, if needed, the investigator manipulated (extended) the treated finger in an attempt to facilitate rupture of the cord (finger extension procedure). Following manipulation, patients were fitted with a splint, instructed to wear the splint at bedtime for up to four months, and instructed to perform a series of finger flexion and extension exercises each day.
The primary endpoint was to evaluate the proportion of patients who achieved a reduction in contracture of the selected primary joint (MP or PIP) to within 0° to 5° of normal, 30 days after the last injection of that joint on days 30, 60, or 90 (after up to 3 injections). A greater proportion of XIAFLEX-treated patients compared to placebo-treated patients achieved the primary endpoint (see Table below).
Percentage of Patients Who Achieved Reduction in Contracture of the
Primary Joint to 0° to 5° After Up to 3 Injections in CORD I and II
| Treated Joint | CORD I | CORD II | ||||||
| XIAFLEX | Placebo | XIAFLEX | Placebo | |||||
| All Joints (MP and PIP) Difference (CIe) |
N=203 | N=103 | N=45 | N=21 | ||||
| 64% 57% (47%, 67%) |
7% — |
44% 40% (14%, 62%) |
5% — | |||||
| MP Joints Difference (CIe) |
N=133 | N=69 | N=20 | N=11 | ||||
| 77% 69% (57%, 79%) |
7% — |
65% 56% (19%, 83%) |
9% — | |||||
| PIP Joints Difference (CIe) |
N=70 | N=34 | N=25 | N=10 | ||||
| 40% 34% (14%, 52%) |
6% — |
28% 28% (-10%, 61%) |
0% — | |||||
| e | 95% confidence interval |
Insurance coverage: In our clinical studies, the average age of diagnosis was 62.3 years, and we believe patients are typically diagnosed after the age of 40 years. As a result, we anticipate approximately an equal split of patients having Medicare and commercial insurance. We believe that Medicare is required to provide reimbursement for this procedure via Medicare Part B coverage, which includes physician administered therapies. With commercial (private) insurance carriers, we believe that the product will require prior authorization in many cases with coverage that will vary based on the individual policies of the payors. However, for both Medicare and commercial insurance, we initially anticipate slow and variable reimbursement for XIAFLEX. This will exist as healthcare providers will be required to utilize miscellaneous J codes and C codes and miscellaneous or proxy CPT codes. This miscellaneous coding will result in manual adjudication of these claims causing slow and variable reimbursement of claims for the product and the physician procedures. We believe that we will obtain a product specific J code that can be utilized in January 2011, and specific CPT procedural codes in January 2012.
8
Table of Contents
Patent coverage. In addition to the marketing exclusivity which comes with its orphan drug status in the U.S., the enzyme underlying XIAFLEX for Dupuytren’s is covered by a use patent in the U.S. that expires in 2014. The patent is limited to the use of the enzyme for the treatment of Dupuytren’s within certain dose ranges. In January 2006, we filed a patent application with regard to the composition and manufacturing process for XIAFLEX. If this patent is granted, it would expire in January 2027, subject to patent term adjustments.
Regulatory. FDA has required a REMS program for XIAFLEX, which consists of a communication plan and a medication guide. This REMS is designed (1) to evaluate and mitigate known and potential risks and serious adverse events; (2) to inform healthcare providers about how to properly inject XIAFLEX and perform finger extension procedures; and, (3) to inform patients about the serious risks associated with XIAFLEX. We plan to market XIAFLEX to physicians who are experienced in injection procedures of the hand and treatment of Dupuytren’s and will only provide access to XIAFLEX after physicians have attested to completion of a training program. The training program is available as a video or written manual and demonstrates proper use and administration of XIAFLEX, as well as an overview of both identified and potential risks with XIAFLEX. As a condition of approval for XIAFLEX for Dupuytren’s, FDA required a REMS. Potential risks and serious adverse events associated with XIAFLEX include tendon rupture, serious adverse reactions affecting the injected extremity, and the potential risk of serious hypersensitivity reactions (including the potential for anaphylaxis).
XIAFLEX Potential Future Indications
Peyronie’s
Disease state and market: Peyronie’s is characterized by a plaque or hard lump that occurs on the penis. It begins as a localized inflammation, which progresses to a hardened scar on the shaft of the penis that reduces flexibility and causes the penis to bend during erection. This may cause pain on erection and a deformity that may prevent sexual intercourse altogether. Our market research indicates that, aside from the physical consequences, depression and loss of self-esteem are common in men with Peyronie’s. According to Peyronie’s Disease, A Guide to Clinical Management by Levine, published in 2007, the prevalence of Peyronie’s has been increasing during the last 30 years and now ranges between 3.7% and 7.1%. However, the actual prevalence may be higher because of patients’ reluctance to report this embarrassing condition to their physicians. The pathogenesis of Peyronie’s appears to be multifactorial, with an interplay of genetic predisposition, trauma, and tissue ischemia. Generally, Peyronie’s is treated by urologists. To date, no medical therapy has been proven effective for the treatment of Peyronie’s. Surgical treatment may be an option for some patients, although complications as well as loss of penile length can occur. Administration of XIAFLEX could be an in-office procedure in which the product is injected directly into the plaque causing the penile curvature.
Current treatment options: There are no medical therapies approved for the treatment of Peyronie’s. However, a number of medical therapies have been used to treat Peyronie’s including Vitamin E, Colchicine, Potassium Aminobenzoate (Potaba), Tamoxifen, Interferon a2a, and Corticosteroids. Current surgical therapies include the following procedures: plication, (which involves gathering tissue on the outer side of the bend usually resulting in shortening and straightening of the penis), graft techniques (which involves replacing scar tissue with grafts of tissue from another site), and prosthetic implants (which involves synthetic cylinders that are implanted in the penis to produce a functional erection).
R&D status: We completed a phase IIb randomized, double-blind, placebo-controlled study designed to assess the safety and efficacy of XIAFLEX when administered two times a week every six weeks for up to three treatment cycles (2 x 3) in subjects with Peyronie’s. The study was conducted at 12 sites throughout the U.S., and patients were monitored for 36 weeks following the first injection. The trial was designed primarily to validate a proprietary Peyronie’s Patient Reported Outcome questionnaire (“PRO”) which will measure several domains of patients’ sexual quality of life over a 36 week period. The four domains measured by the PRO are penile pain, Peyronie’s bother, intercourse discomfort and intercourse constraint. We intend to use the PRO in its entirety, or in part as determined after consultation with the FDA, for our phase III Peyronie’s trials, where it is intended
9
Table of Contents
that it would be the primary or co-primary endpoint. Patients had to have a penile contracture between 30 and 90 degrees to be included in the study. Patients were stratified by the degree of penile curvature (i.e. 30 degrees to 60 degrees versus 60 to 90 degrees) and then randomized into four treatment groups to receive either XIAFLEX or placebo with or without modeling of the penile plaque. Modeling refers to massaging of the plaque after the second injection of a treatment series and is intended to maximize the enzymatic effect of the XIAFLEX injection in the plaque. Patients were randomized in a 3:1 ratio of XIAFLEX to placebo and a 1:1 ratio to receive penile plaque modeling or no modeling.
Top-line data demonstrated that XIAFLEX improved penile curvature when compared to placebo with a mean improvement of 29.7% vs. 11%, respectively (P=0.001). In addition, the PRO domain of Peyronie’s symptom bother was significantly improved in XIAFLEX treated subjects compared to placebo. The use of modeling also demonstrated significant improvement in XIAFLEX subjects compared to placebo, 32.4% improvement in curvature compared to a 2.5% worsening of curvature in placebo subjects (p<0.001). In the no-modeling group, XIAFLEX subjects experienced a 27.1% mean improvement in penile curvature, which was not statistically different from the 27.9% mean improvement for subjects receiving the placebo. The remaining three domains did not show statistical significance between XIAFLEX and placebo. The most common adverse events reported in the phase IIb trial were injection site bruising, injection site pain, injection site edema, contusion, penile edema and penile pain. There was a total of five (3.4%) subjects who experienced eight non-fatal serious adverse events, none of which were considered to be related to XIAFLEX. We were looking to accomplish two goals with this study. First, we wanted to validate the Peyronie’s Disease PRO as a tool that we can use in future pivotal studies. Second, we were looking to establish an acceptable primary endpoint related to the PRO that can be used for those pivotal phase III trials. Although we will need to confirm these positions with the FDA and other regulatory agencies, we believe that we will be able to validate the PRO and move XIAFLEX into phase III development in the second half of 2010. The Company expects to have an end of phase II meeting with the FDA to discuss these issues in the second quarter of 2010.
Frozen Shoulder Syndrome
Disease state and market: Frozen Shoulder syndrome is a disorder of diminished shoulder motion, characterized by restriction in both active and passive range of motion of the shoulder joint. Frozen Shoulder syndrome usually affects patients aged 40-70 years. According to Harrison’s Principles of Internal Medicine, it is estimated that 3% of people develop Frozen Shoulder syndrome over their lifetime and that women tend to be affected more frequently than men. The condition may affect both shoulders, either simultaneously or in sequence, in up to 16% of patients. Recurrence of Frozen Shoulder syndrome is common within five years of the onset of the disorder. According to Dahan et. al. in the 2005 eMedicine article “Adhesive Capsulitis,” a higher incidence of Frozen Shoulder syndrome exists among patients with diabetes (10-20%) compared to the general population (2-5%) and incidence among patients with insulin-dependent diabetes is even higher (36%), with an increased frequency of bilateral shoulder involvement.
Current treatment options: The most common treatment for Frozen Shoulder syndrome is extensive physical therapy, corticosteroids and/or arthroscopy. Drugs are used to manage pain, but none have been demonstrated to have an impact on Frozen Shoulder syndrome.
R&D status: BioSpecifics has conducted a phase II clinical trial using XIAFLEX for the treatment of Frozen Shoulder syndrome. Three different doses of the enzyme were compared to placebo in this prospective, randomized, 60-subject trial. The results from this trial suggest that local injection of the enzyme is well-tolerated and may be effective in patients suffering from Frozen Shoulder syndrome. Additional studies are needed to assess the optimal dose and dosing regimen of XIAFLEX for this indication.
Other
Under our agreement with BioSpecifics, we have exclusive worldwide rights to develop, market and sell products containing XIAFLEX, other than dermal formulations labeled for topical administration. We may
10
Table of Contents
expand our agreement with BioSpecifics, at our option, to cover other indications as they are developed by us or BioSpecifics. We plan to prioritize our development of additional pipeline indications for XIAFLEX. In addition to the indications mentioned above, other areas of interest could include cellulite and lipoma.
Testim Opportunity
Testim is a proprietary, topical 1% testosterone once-a-day gel that treats hypogonadism by restoring testosterone blood levels back to normal for a 24-hour period following application. Testim is packaged in convenient, easy-to-open, single-use tubes. Patients apply Testim once per day to the upper arms and shoulders, enabling Testim’s active ingredient, testosterone, to be absorbed through the skin and into the bloodstream.
Hypogonadism market
Hypogonadism, or low testosterone, is a disorder that affects millions of men in the U.S. A 2006 study in The International Journal of Clinical Practice showed that 39% of men over 45 years of age have low testosterone (total testosterone levels below 300 ng / dL). Hypogonadism also affects a high percentage of men with certain co-morbidities regardless of age. This study documented associations with other co-morbidities such as: Obesity (52%), Diabetes (50%) and Rheumatoid Arthritis (47%)1. Other articles have highlighted associations with other conditions: HIV (30%)2, Erectile Dysfunction (18%)3 and up to 74% of men being treated with high doses of opioids for chronic pain4. The effects of low testosterone lead to symptoms such as low energy, diminished libido, adverse changes in body composition, irritability and poor concentration. According to a 2004 study in the Journal of Clinical Endocrinology & Metabolism, the impact of these symptoms on an afflicted person can be significant5.
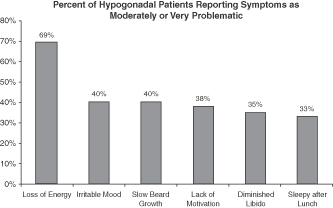
TRT is the standard treatment for hypogonadism. According to IMS, the total number of prescriptions within the TRT market has grown from 1.0 million to 3.9 million prescriptions between 2000 and 2009. Before 2000, the TRT market consisted of oral, injectable and patch therapies. In 2000, the first topical gel was introduced, and this product segment has been the driver of much of the total growth in the TRT market. According to IMS, the gel segment accounted for approximately 71% of the total prescriptions in the TRT market in the fourth quarter of 2009. Despite this growth, we estimate that approximately 10% of men with
| 1 | Mulligan T. et al. Int J. Clin Pract 2006 |
| 2 | Dobs A.S. Clin Endocrinol Metab. 1998 |
| 3 | Bodie J. et al. J. Urol. 2003 |
| 4 | Daniell H.W. J. Pain 2002 |
| 5 | Kelleher S. et al. J. Clin. Endocrinol Metab. 2004 |
11
Table of Contents
hypogonadism currently receive TRT. According to our physician research, this low diagnosis rate stems primarily from low patient and physician awareness of the symptoms, treatment options and monitoring requirements.
Testim has captured a significant portion of the gel prescription growth since its launch in early 2003, and held a 22.3% prescription share of this market in December 2009. In 2009, prescriptions of Testim grew by 14.9% compared to 2008. The gel market continued to be a primary driver of the TRT market in 2009. All other formulations (injectables, patches, orals and buccals) combined increased 24.1% year over year. We believe this growth (primarily in injectables) is attributable to the economic downturn with financially impacted patients opting for lower priced generic injectables.
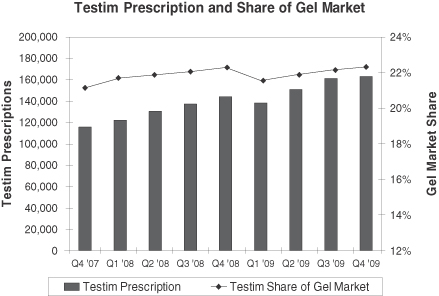
Source: IMS NPA
We believe Testim’s growth since 2006 has been due to Testim’s favorable clinical profile and our increased sales and marketing efforts. According to IMS, Testim’s market share in the TRT gel market was 22.5% in December 2009 compared to 22.6% in December 2008. Fluctuations in share will occur with wins and losses on the managed care front. We had some losses in managed care accounts in early 2009 that impacted our share growth adversely, but we were able to overcome that through growth in other managed care accounts. Our Testim sales organization of approximately 150 representatives promotes Testim to urologists, endocrinologists and certain targeted primary care physicians. We believe this focused, consistent messaging will continue to drive the recognition of hypogonadism and increase Testim prescriptions.
Clinical data supporting the advantages of Testim. We have designed and conducted our clinical trials to provide prescribing physicians with comprehensive information regarding Testim’s benefits. Since 2001, we have completed 16 clinical studies involving approximately 1,800 patients including the largest placebo controlled study (n=400) ever conducted to evaluate the benefits and risks of TRT. We conducted five of these studies using Testim and other TRT products, including a transdermal patch and the other commercial gel. The results from these five studies, taken together, demonstrate that in patients with hypogonadism, testosterone levels directly correlate with patient symptom improvement.
In October 2007, the manuscript “Efficacy of Testosterone Gel Substitution (Androgel® or Testim) among sub-optimally responsive hypogonadal men” by L. Lipshultz, E. Grober, M. Khera, M. Espinoza was posted in the International Journal of Impotence Research web site and later published in International Journal of Impotence Research. The data from this independent study show improvements in symptoms of decreased libido,
12
Table of Contents
energy levels and erectile function in 76 percent of hypogonadal men after switching brands of TRT gel. Total testosterone levels increased significantly in patients who switched to Auxilium’s Testim 1% (testosterone gel) following suboptimal response to Solvay S.A.’s Androgel (testosterone gel) 1% but did not increase significantly in patients who switched from Testim to Androgel.
Cost effectiveness and convenience. We believe that, based on clinical data regarding testosterone levels in patients, market feedback from physicians, and the results of our clinical trials, a higher percentage of patients are treated with only one tube of Testim per day (one dose per day) compared to patients being treated with the other commercial gel who may need more than one dose of that product per day.
Broad prescription coverage. Currently, we have broad third-party payor reimbursement for Testim and Testim is covered by the majority of reimbursement plans.
Patent coverage. In the U.S., the ‘968 patent, which Bentley assigned to CPEX in June 2008, covers a method for maintaining blood serum testosterone levels for treating a hypogonadal male using Testim and is listed in the Orange Book. The ‘968 patent expires in January 2025. Six additional patents issued covering Testim in October 2009 and have been listed in the Orange Book. These patents expire in April 2023. CPEX also has filed continuation applications that are pending. In October 2008, we and our licensor, CPEX, received notice that Upsher-Smith filed an ANDA containing a paragraph IV certification seeking approval from the FDA to market a generic version of Testim prior to the January 2025 expiration of ‘968 Patent. The paragraph IV certification sets forth allegations that the ‘968 Patent will not be infringed by Upsher-Smith’s manufacture, use or sale of the product for which the ANDA was submitted. In December 2008, we filed a patent infringement lawsuit against Upsher-Smith which remains pending. Upsher-Smith has alleged that its proposed generic version of Testim would not infringe the ‘968 patent, and that the ‘968 patent is invalid. Under the Hatch-Waxman Act, final FDA approval of Upsher-Smith’s proposed generic product will be stayed until the earlier of 30 months beginning on the date of receipt of the paragraph IV certification (April 2011) or resolution of the patent infringement lawsuit. Should Upsher-Smith receive a tentative approval of its generic version of Testim from the FDA, it cannot lawfully launch its generic version of Testim in the U.S. before the earlier of the expiration of the currently pending 30-month stay or a district court decision in its favor. Upsher-Smith will also not be able to lawfully launch a generic version of Testim in the U.S. without the necessary final approval from the FDA.
Regulatory. On May 7, 2009, FDA announced that it was requiring the manufacturers of two prescription topical testosterone gels, Solvay S.A. and Auxilium, to make changes to the prescribing information and develop REMS for the products. FDA stated that it was requiring this action after it became aware, through spontaneous post-marketing adverse event reports and peer-reviewed biomedical literature, of cases of secondary exposure of children to testosterone due to drug transfer from adult males using testosterone gel drug products (“transference”). FDA considered this information to be “new safety information.” We believe that all topical testosterone gels have a potential for transference. Testim’s prescribing information has described the risk and procedures for avoidance of transference since the product was launched in 2003. The changes to the prescribing information for Testim include a “boxed warning”, which is used to highlight warning information that is especially important to the prescriber. The goal of the REMS is to inform patients about the serious risks associated with the use of Testim and AndroGel. The REMS includes assessments and a Medication Guide to inform patients. The revised prescribing information and REMS for Testim was approved on September 18, 2009.
In February 2009, we filed a Citizen’s Petition with the FDA requesting that Upsher-Smith be required to perform a variety of studies to demonstrate that the inactive ingredients and formulation of the proposed product do not compromise its safety or efficacy. An FDA response to our Citizen’s Petition was received in August 2009. The FDA agreed with some of the statements made in the Citizen’s Petition regarding the testing required for generic versions of Testim and disagreed with other statements. Although not commenting upon any filing in particular, the FDA did state that “the practical effect of this determination is that any application for a testosterone gel product that has different penetration enhancers than the reference listed drug cannot be
13
Table of Contents
submitted as an abbreviated new drug application (ANDA) and, instead, will have to be submitted as a NDA under section 505(b) of the [Federal Food, Drug, and Cosmetic Act].”
Transmucosal Film Technology
We are developing product candidates using an oral transmucosal drug delivery system based on patented technology licensed from Formulation Technologies, L.L.C., doing business as PharmaForm. (In 2007, PharmaForm was acquired by AKELA Pharma Inc., formerly known as LAB International Inc.) The basis of this technology is a film that adheres to the upper gum. We have the exclusive worldwide right to use this technology platform in therapeutic products that contain hormones or that treat urologic disorders and in certain pain products. We currently have two product candidates using this technology:
| • | AA4010, our therapy to treat overactive bladder, in phase I of development for which we expect to seek a partner to further develop and commercialize the product; and |
| • | A Fentanyl pain product candidate in the phase I of development for the management of pain for which we expect to seek a partner to further develop and commercialize the product. |
Licensing
We have entered into agreements for the licensing of technology and products. We intend to pursue other licensing agreements and collaborations in the future. We have secured collaboration partners for the sale of products in geographic locations where we do not have our own sales force. We may pursue other collaborations in the future.
XIAFLEX
BioSpecifics
In June 2004, we entered into a development and license agreement with BioSpecifics and amended the agreement in May 2005, December 2005 and December 2008 (the “BioSpecifics Agreement”). Under the BioSpecifics Agreement, we were granted exclusive worldwide rights to develop, market and sell certain products containing BioSpecifics’ enzyme, which we refer to as XIAFLEX. Our licensed rights concern the development and commercialization of products, other than dermal formulations labeled for topical administration, and currently, our licensed rights cover the indications of Dupuytren’s, Peyronie’s and Frozen Shoulder syndrome. We may further expand the BioSpecifics Agreement, at our option, to cover other indications as they are developed by us or BioSpecifics.
The BioSpecifics Agreement extends, on a country-by-country and product-by-product basis, for the longer of the patent life, the expiration of any regulatory exclusivity period or 12 years. We may terminate the BioSpecifics Agreement upon 90 days prior written notice. See “Patents and Proprietary Rights” below for a discussion of the patents we license from BioSpecifics.
We are responsible, at our own cost and expense, for developing the formulation and finished dosage form of products and arranging for the clinical supply of products except for costs paid to third parties to develop the lyophilization of the injection formulation which shall be shared equally by Auxilium and BioSpecifics.
BioSpecifics has the option, exercisable no later than six months after FDA approval of the first New Drug Application (“NDA”) or Biologics License Application (“BLA”) with respect to a product, to assume the right and obligation to supply, or arrange for the supply from a third party other than a back-up supplier qualified by us, of a specified portion of our commercial product requirements for territories outside those licensed to
14
Table of Contents
Pfizer. The BioSpecifics Agreement provides that we may withhold a specified amount of a milestone payment until:
| • | BioSpecifics executes an agreement, containing certain milestones, with a third party for the commercial manufacture of the product; |
| • | BioSpecifics commences construction of a facility, compliant with current Good Manufacturing Practice (“cGMP”), for the commercial supply of the product; or |
| • | 30 days after BioSpecifics notifies us in writing that it will not exercise the supply option. |
If BioSpecifics exercises the supply option, commencing on a specified date, BioSpecifics will be responsible for supplying either by itself or through a third party other than a back-up supplier qualified by us, a specified portion of the commercial supply of the product for territories outside those licensed to Pfizer. If BioSpecifics does not exercise the supply option, we will be responsible for arranging for the entire commercial product supply. In the event BioSpecifics exercises the supply option by August 2, 2010, we and BioSpecifics are required to use commercially reasonable efforts to enter into a commercial supply agreement on customary and reasonable terms and conditions which are not worse than those with back-up suppliers qualified by us.
We must pay BioSpecifics on a country-by-country and product-by-product basis a specified percentage of net sales for products covered by the BioSpecifics Agreement. Such percentage may vary depending on whether BioSpecifics exercises the supply option. In addition, the percentage may be reduced if:
| • | BioSpecifics fails to supply commercial product supply in accordance with the terms of the BioSpecifics Agreement; |
| • | market share of a competing product exceeds a specified threshold; or |
| • | we are required to obtain a license from a third party in order to practice BioSpecifics’ patents without infringing such third party’s patent rights. |
As a result of the December 2008 amendment to our license with BioSpecifics, which became effective when we executed the agreement with Pfizer, we owe BioSpecifics 8.5% of the upfront and milestone payments received from Pfizer. With regard to any other sublicensee, we must pay BioSpecifics a specified percentage of sublicense income we receive from any other sublicensee, including upfront payments and milestone payments, and we must pay BioSpecifics an amount equal to a specified mark-up of the cost of goods sold for products sold by us that are not manufactured by or on behalf of BioSpecifics, provided that, in the event BioSpecifics exercises the supply option, no payment will be due for so long as BioSpecifics fails to supply the commercial supply of the product in accordance with the terms of the BioSpecifics Agreement.
Finally, we will be obligated to make contingent milestone payments upon the filing of regulatory applications and receipt of regulatory approval. As a result of the acceptance of the EU Marketing Authorization Application (“MAA”) for XIAFLEX for Dupuytren’s on January 21, 2010, we are obligated to pay BioSpecifics approximately $1.3 million as their share of the $15 million milestone payment we received from Pfizer in February 2010. As a result of the U.S. approval of XIAFLEX for Dupuytren’s on February 2, 2010, we are obligated to pay BioSpecifics $1 million. Additional milestone obligations will be due upon acceptance of filings or approvals, or if we exercise an option to develop and license XIAFLEX for additional medical indications. Pursuant to the BioSpecifics Agreement, the milestone for each additional indication is $500,000, except for cellulite which is $1 million.
Pfizer
In December 2008, we entered into a development, commercialization and supply agreement with Pfizer (the “Pfizer Agreement”). Under the Pfizer Agreement, we granted to Pfizer the right to develop and
15
Table of Contents
commercialize, with the right to sublicense, XIAFLEX for the treatment of Peyronie’s and Dupuytren’s in the 27 member countries of the EU as it existed as of the effective date of the Pfizer Agreement (Austria, Belgium, Bulgaria, Cyprus, Czech Republic, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Ireland, Italy, Latvia, Lithuania, Luxembourg, Malta, the Netherlands, Poland, Portugal, Romania, Slovakia, Slovenia, Spain, Sweden and the U.K.), as well as Albania, Armenia, Azerbaijan, Belarus, Bosnia & Herzegovina, Croatia, Georgia, Iceland, Kazakhstan, Kirghiz Republic, Macedonia, Moldova, Montenegro, Norway, Serbia, Switzerland, Tajikistan, Turkey and Uzbekistan (the “Pfizer Territory”). Subject to each party’s termination rights, the term of the Agreement extends on a country-by-country and product-by-product basis from the date of the Agreement until the latest of (i) the date on which the product is no longer covered by a valid patent or patent application in such country, (ii) the 15th anniversary of the first commercial sale by Pfizer of XIAFLEX in a given country after the receipt of required regulatory approvals and (iii) a generic entry or competitive product containing the same active ingredient with respect to XIAFLEX in such country (the “Term”).
In addition to the $75 million upfront payment we received in December 2008 and the $15 million milestone payment we received in February 2010 for acceptance of the MAA in the EU, Pfizer may make up to an additional $395 million in potential payments upon the achievement of certain specified additional regulatory and commercial milestones for XIAFLEX (on an indication-by-indication basis or for the product as a whole, as the case may be). Subject to the requirement to make certain specified minimum commercialization payments, Pfizer will make commercialization payments to us based on a percentage of the aggregate annual net sales of the Product in the Pfizer Territory on a quarterly basis.
Pfizer will obtain XIAFLEX exclusively from us. We are primarily responsible for development activities prior to granting of product approval, and Pfizer is primarily responsible for development activities in the Pfizer Territory thereafter. We control product development at all times outside of the Pfizer Territory. Development costs will be borne by the party incurring such costs; provided, that the parties will share development costs associated with activities solely associated with obtaining product approval in the Pfizer Territory. However, Pfizer may, on an indication-by-indication basis, recoup its fifty percent (50%) share of such pre-approval development costs by means of an off-set against the milestone payment due upon first commercial sale for such indication. Pfizer is responsible for preparation of regulatory materials necessary for obtaining and maintaining regulatory approvals in the Pfizer Territory, and Pfizer will be responsible for regulatory costs associated with product approval in the Pfizer Territory. Pfizer may also recoup up to $2.5 million with respect to such regulatory costs related to Dupuytren’s. Pfizer is solely responsible for commercializing XIAFLEX in the Pfizer Territory during the term of the Pfizer Agreement and is solely responsible for costs associated with commercializing XIAFLEX in the Pfizer Territory.
Either party may terminate the Pfizer Agreement as a result of the other party’s breach or bankruptcy. Pfizer may terminate the Pfizer Agreement at will; provided that, during a specified period, such termination right is subject to the occurrence of certain specified events relating to the product, product development and regulatory approval.
Testim
CPEX Pharmaceuticals
In May 2000, Bentley granted us an exclusive, worldwide, royalty-bearing license to make and sell products incorporating its patented transdermal gel formulation technology that contains testosterone (the “May 2000 License”). We produce Testim under the May 2000 License. The term of the May 2000 License is determined on a country by country basis and extends until the later of patent right termination in a country or 10 years from the date of first commercial sale. In May 2001, Bentley granted us similar rights for a product containing another hormone, the term of which is perpetual. Under these agreements, we are required to make up-front and milestone payments upon contract signing, the decision to develop the underlying product, and the
16
Table of Contents
receipt of FDA approval. In June 2008, CPEX was spun out of Bentley and is the assignee of certain Bentley assets, including the license agreements and patents we licensed under those agreements. See “Patents and Proprietary Rights” for a discussion of the patents we license from CPEX.
Ipsen
We entered into a license and distribution agreement with Ipsen in March 2004 (the “Ipsen Agreement”). Under the Ipsen Agreement, we granted Ipsen an exclusive license to use and sell Testim on a worldwide basis outside the U.S., Canada, Mexico, and Japan, and in January 2008, we amended that agreement to exclude, in addition, China, Poland, Russia, and South Korea and their territories and possessions (the “Ipsen Territory”). In November 2008, we agreed with Ipsen to terminate the Ipsen Agreement and transfer the marketing authorizations required to promote and sell Testim in the Ipsen Territory to Ferring. In the fourth quarter 2009 the last of the marketing authorizations for the 15 countries included in the Ipsen Territory were transferred to Ferring and the Ipsen Agreement has terminated.
Ferring
In November 2008, we entered into a distribution and license agreement with Ferring (the “Ferring Agreement”). Pursuant to the Ferring Agreement, we appointed Ferring as our exclusive distributor of Testim in Belgium, Denmark, Finland, Germany, Greece, Iceland, Ireland, Italy, Luxembourg, The Netherlands, Norway, Portugal, Spain, Sweden and the U.K. (the “Ferring Territory”). We also granted Ferring an exclusive, royalty-bearing license to import, market, sell and distribute Testim in the Ferring Territory. The exclusive appointment and license commenced on a country-by-country basis upon the transfer of the relevant marketing authorizations from Ipsen. Ferring is required to purchase all Testim supply from us and to make certain sales milestone and royalty payments. In addition, Ferring was required to make certain upfront and milestone payments to the Company related to the transfer of the marketing authorizations in each country within the Ferring Territory.
Paladin
We entered into a license and distribution agreement with Paladin in December 2006. We granted Paladin an exclusive license to use and sell Testim in Canada. The terms of this agreement require Paladin to purchase all Testim supply from us and to make up-front, milestone and royalty payments.
Transmucosal Film Technology
PharmaForm
In June 2003, we entered into a license agreement with PharmaForm. Under this agreement, as amended, we were granted an exclusive, worldwide, royalty-bearing license to develop, make and sell products that contain hormones or that are used to treat urologic disorders incorporating PharmaForm’s oral transmucosal film technology for which there is an issued patent in the U.S. The term of this license agreement is for the life of the licensed patents.
In February 2005, we entered into an additional license agreement with PharmaForm. Under this agreement, we were granted exclusive, worldwide royalty-bearing rights to develop, manufacture and market eight compounds using PharmaForm’s proprietary transmucosal film technology for the management of pain, including acute and chronic pain. As of the fourth anniversary of the effective date, the agreement covers only those compounds for which we have initiated formulation development. The term of this license agreement continues on a product by product and country by country basis until the later of ten years after the first commercial sale or last to expire patent covering the licensed technology. The compounds that may be developed
17
Table of Contents
include opioids as well other types of analgesics. In addition, we will have on-going royalty payment obligations to PharmaForm. The timing of the remaining payments, if any, is uncertain.
Manufacturing
We currently use, and expect to continue to depend on, contract manufacturers to manufacture Testim and handle certain aspects of the manufacture of XIAFLEX. We may depend on contract manufacturers to manufacture any products for which we receive marketing approval and to produce sufficient quantities of our product candidates for use in preclinical and clinical studies. We and our contract manufacturers are subject to an extensive governmental regulation process. Regulatory authorities in our markets require that drugs be manufactured, packaged and labeled in conformity with cGMP. The cGMP requirements govern quality control of the manufacturing process and documentation policies and procedures. We have established an internal quality control and quality assurance program, including a set of standard operating procedures and specifications that we believe is cGMP-compliant.
Testim
DPT. Testim is manufactured for us by DPT Laboratories, Ltd. (“DPT”) pursuant to a manufacturing and supply agreement that expires on December 31, 2010. During the term of the agreement, subject to our right to qualify and order Testim from a back-up supplier, DPT is required to manufacture all of our worldwide commercial requirements of Testim. The manufacturing fees paid to DPT are based upon the specified annual volume of Testim ordered by us. If we order less than a specified amount of Testim in any calendar year, the parties are required to negotiate to establish new manufacturing fees, with the understanding that DPT will continue to produce Testim for a specified minimum period in order to enable us to identify and utilize another supplier. DPT may terminate the agreement only upon our insolvency or bankruptcy or upon our breach of the agreement. We have qualified Contract Pharmaceuticals Limited Canada (“CPL”) as a back-up supplier.
XIAFLEX
Horsham. In September 2006, we leased a 50,000 square foot biological manufacturing facility in Horsham, Pennsylvania that we are using to produce the active ingredient of XIAFLEX. In 2009, we also leased approximately 56,000 square feet of laboratory, warehouse and office space in two other Horsham locations. We believe we have sufficient capacity to manufacture our commercial supply needs for the foreseeable future, and we have the ability to increase capacity at our Horsham facilities if needed.
Subject to BioSpecifics’ option to assume the right and obligation to supply, or arrange for the supply from a third party, of a specified portion of our commercial XIAFLEX requirements (see full discussion of the BioSpecifics Agreement above), we expect to be the supplier of the active pharmaceutical ingredient for commercial supply of XIAFLEX. We use a contract manufacturer, Hollister-Stier Laboratories LLC (“Hollister- Stier”), to fill and lyophilize the XIAFLEX bulk drug substance and produce sterile diluent, and we use a contract packager, Catalent Pharma Solutions, LLC (“Catalent”) to label and package XIAFLEX.
Hollister-Stier. In June 2008, we entered into a supply agreement with Hollister-Stier pursuant to which Hollister-Stier fills and lyophilizes the XIAFLEX bulk drug substance that we manufacture and produces sterile diluent. The initial term of the supply agreement is three years, and it automatically renews for subsequent two year terms, unless terminated earlier.
BioSpecifics. Under the terms of the BioSpecifics Agreement, BioSpecifics has the option, exercisable no later than six months after FDA approval of the first BLA with respect to XIAFLEX, to assume the right and obligation to supply, or arrange for the supply from a third party other than a back-up supplier qualified by us, of
18
Table of Contents
a specified portion of commercial product required by us for territories outside those licensed by Pfizer. The BLA for XIAFLEX for the treatment of Dupuytren’s was approved on February 2, 2010, and BioSpecifics’ supply option is exercisable no later than August 2, 2010. The terms of the BioSpecifics Agreement are summarized above.
Competition
TRT Market Competition
The TRT market is highly competitive. Our success will depend, in part, on our ability to protect our share of the market from the competition. Potential competitors in North America, Europe and elsewhere include major pharmaceutical companies, specialty pharmaceutical companies and biotechnology firms, universities and other research institutions and government agencies.
Other pharmaceutical companies may develop generic versions of Testim or any products that compete with Testim that do not infringe our patents or other proprietary rights, and, as a result, our business may be adversely affected. For example, because the ingredients of Testim are commercially available to third parties, it is possible that competitors may design formulations, propose dosages or develop methods of administration that would be outside the scope of the claims of one or more, or of all, of the patent rights that we in-license. This would enable their products to effectively compete with Testim. Governmental and other pressures to reduce pharmaceutical costs may result in physicians writing prescriptions for these generic products. Increased competition from the sale of competing generic pharmaceutical products could cause a material decrease in revenue from Testim.
AndroGel
The primary competition for Testim in the TRT market is AndroGel (testosterone gel) 1% CIII, marketed by Solvay Pharmaceuticals, Inc. (“Solvay”). In February 2010, Abbot Laboratories, Inc. (“Abbott”) completed an acquisition of Solvay. Solvay launched AndroGel three years prior to Testim giving the brand a significant advantage in the TRT gel market. Watson Pharmaceuticals, Inc. (“Watson”) began co-promoting AndroGel with Solvay in late 2006, and Par Pharmaceutical Companies, Inc. (“Par”) began co-promoting AndroGel in early 2007, each pursuant to patent lawsuit settlement agreements with Solvay. In January 2009, the U.S. Federal Trade Commission (“FTC”) and a number of private parties filed actions against Solvay, Watson and Par alleging that their respective 2006 patent lawsuit settlements related to AndroGel are unlawful. All actions were consolidated in the U.S. District Court for the Northern District of Georgia. In February 2010, the court dismissed the FTC’s claims and some of the private party claims. Certain private party claims remain pending. Solvay and its co-promotion partners collectively had more than 600 sales representatives detailing AndroGel although it remains unclear what resources Abbott will put behind the brand. According to IMS, as of December 31, 2009, AndroGel accounted for 77.7% of the gel prescriptions.
Other Delivery Options
Testim also competes with other TRT products such as short-acting injectables, patches, orals, a buccal tablet and injectable pellets. The injectables have experienced significant growth in the market due to their low cost compared to the transdermal options. This growth coincides with the economic downturn that the U.S. experienced in 2009. There are also a significant number of patients who prefer getting a shot every two to three weeks instead of utilizing a daily application of another product. Physicians perceive the disadvantage of the injectables as being the fact that many patients’ testosterone levels rise past the normal barrier of 1000 ng/dl which leads to a wide fluctuation of testosterone levels in a patient and the potential for side effects such as gynecomastia. Androderm® is a transdermal testosterone patch marketed by Watson. Androderm, the leading patch product, has decreased in share in the last year and accounted for approximately 5.9% of total TRT prescriptions in December 2009, down from 6.5% in December 2008. The buccal therapy accounted for less than 1% of total TRT prescriptions in December 2009. Testopel® is an implantable testosterone pellet that is administered every four to six months.
19
Table of Contents
Generic Competition
In July 2003, Watson and Par filed ANDAs with the FDA to be approved as generics for AndroGel. In response to these ANDAs, Solvay filed patent infringement lawsuits against these two companies to block the approval and marketing of the generic products. In 2006, all the subject companies reached an agreement pursuant to which Watson and Par agreed not to bring a generic to Androgel to the market until August 2015. During this time frame, Solvay, Watson and Par will co-promote AndroGel. In June 2009, Perrigo Israel Pharmaceuticals, Inc. (“Perrigo”) filed an ANDA with a paragraph IV certification seeking the approval of a generic version of Androgel in the United States. The paragraph IV certification procedure challenges a U.S. patent relating to Androgel which runs through the next decade. In its letter to the company, Perrigo asserted that it did not infringe the AndroGel patent because its proposed testosterone gel product contains a different formulation than the formulation protected by the AndroGel patent. In contrast, the proposed testosterone gel products that were the subject of earlier ANDAs filed by other companies, and which were the subject of prior patent litigation brought by the company, purported to be identical to the AndroGel formulation. To date, Solvay has not initiated patent infringement litigation against Perrigo. The introduction of a generic version of Androgel would have an adverse impact on the branded gel market, including taking a significant portion of branded Androgel business. Although Testim has a BX rating, meaning that pharmacists are prohibited from substituting Testim with Androgel, or a generic version of Androgel, a less expensive generic testosterone gel product could still have a material adverse affect on Testim market share and/or Testim’s formulary status, and therefore could have a material negative impact on our financial condition and results of operations.
In October 2008, we and our licensor, CPEX, received notice that Upsher-Smith filed an ANDA containing a paragraph IV certification seeking approval from the FDA to market a generic version of Testim prior to the January 2025 expiration of ‘968 Patent. The ‘968 Patent, which Bentley assigned to CPEX in June 2008, covers a method for maintaining effective blood serum testosterone levels for treating a hypogonadal male using Testim and is listed in the Orange Book, published by the FDA. The paragraph IV certification sets forth allegations that the ‘968 Patent will not be infringed by Upsher-Smith’s manufacture, use or sale of the product for which the ANDA was submitted. In December 2008, we filed a patent infringement lawsuit against Upsher-Smith that remains pending. Upsher-Smith has alleged that its proposed generic version of Testim would not infringe the ‘968 patent, and that the ‘968 patent is invalid. Under the Hatch-Waxman Act, final FDA approval of Upsher-Smith’s proposed generic product will be stayed until the earlier of 30 months from receipt of the paragraph IV certification (April 2011) or resolution of the patent infringement lawsuit. Should Upsher-Smith receive a tentative approval of its generic version of Testim from the FDA, it cannot lawfully launch its generic version of Testim in the U.S. before the earlier of the expiration of the currently pending 30-month stay or a district court decision in its favor. In October 2009, six additional patents covering Testim were issued and have been listed in the Orange Book. These additional patents may provide us with further market protection.
Upsher-Smith will not be able to lawfully launch a generic version of Testim in the U.S. without the necessary final approval from the FDA. In February 2009, the Company filed a Citizen’s Petition with the FDA requesting that Upsher-Smith be required to perform a variety of studies to demonstrate that the inactive ingredients and formulation of the proposed product do not compromise its safety or efficacy. An FDA response to the Company’s Citizen’s Petition was received in August 2009. The FDA agreed with some of the statements made in the Citizen’s Petition regarding the testing required for generic versions of Testim and disagreed with other statements. Although not commenting upon any filing in particular, the FDA did state that “the practical effect of this determination is that any application for a testosterone gel product that has different penetration enhancers than the reference listed drug cannot be submitted as an abbreviated new drug application (ANDA) and, instead, will have to be submitted as a NDA under section 505(b) of the Act.”
Other pharmaceutical companies may develop generic versions of any non-biologic products that we commercialize that do not infringe our patents or other proprietary rights. Governmental and other cost containment pressures may result in physicians writing prescriptions for these generic products or allow physicians to substitute AB-rated generics.
20
Table of Contents
Future Delivery Options
In addition to potential generic competition, several other pharmaceutical companies have TRT products in development that may be approved for marketing in the U.S. and the rest of the world. Based on publicly available information, we believe that these products are in the development phases noted below.
Phase III—In 2009, Endo Pharmaceuticals (“Endo”) acquired Indevus Pharmaceuticals, Inc. (“Indevus”), the makers of Aveed (previously known as “Nebido” in the U.S.) as well as the U.S. marketing rights for Prostrakan’s Fortesta (also known as Fortigel, a 2% gel). Aveed’s Prescription Drug User Fee Act (“PDUFA”) date was September 2, 2009 and the FDA announced that it would require an additional 90 days to review the filing. In December 2009, Endo received a complete response letter from the FDA, asking for more information on rare but serious incidents of anaphylactic reactions and pulmonary oil microembolism among patients taking Aveed. The FDA also said in its complete response letter that Endo’s risk mitigation program for the drug is inadequate. It is not known whether Endo will resubmit the application for Aveed. The FDA also sent a complete response letter to Endo regarding Fortesta in October 2009. Endo has announced that it expects to respond to the FDA’s concerns with respect to Fortesta by mid-2010.
Abbott (formerly Solvay) is awaiting FDA feedback on Androgel 1.62%, a high concentration gel. The PDUFA date was at the end of December 2009. However, the FDA notified Abbott that the agency extended its review of the NDA for Androgel 1.62% by 90 days. Abbott expects the FDA to issue an action by the end of the first quarter 2010.
BioSante Pharmaceuticals/Teva Pharmaceutical Industries Ltd. are developing a testosterone gel (Bio-T-Gel) for male hypogonadism and have announced they expect to submit a NDA in the first half of 2010. Acrux Limited is developing a metered-dose lotion which completed phase III in the third quarter of 2009 and was submitted to the FDA in January 2010.
Phase II—Clarus Therapeutics, Inc. is developing an oral testosterone which is in phase II.
Other—Another potential introduction into the marketplace is an oral therapy called Androxal from Repros Therapeutics, Inc. (“Repros”). This molecule is designed to restore normal testosterone production in males rather than externally replacing testosterone like the current alternatives. However, Repros has been informed by the FDA that testosterone levels or Quality of Life measures will not be acceptable endpoints for phase III trials for hypogonadism. Hence, Repros has announced that it is considering other potential indications. Repros also announced that they participated in a teleconference with the Division of Reproductive and Urologic Products of the FDA on January 25, 2010. The primary purpose of the meeting was to gain a better understanding of the FDA’s position regarding the use of Repros’ oral Androxal® product in the treatment of men with secondary hypogonadism wishing to preserve their fertility. Repros’ data supports the notion that the negative feedback of exogenous testosterone administered by any route suppresses the hypothalamic–pituitary axis and hence spermatogenesis. The discussion with the FDA focused on two issues. First, the FDA requested that Repros propose a label that better defines the population of individuals for whom Repros believes will benefit from the use of Androxal. Second, the FDA requested Repros conduct a literature review of the incidence of infertility associated with the use of exogenous testosterone as supportive of Repros’ data. The FDA requested that Repros provide such information. During the course of the meeting Repros noted that if the FDA concurred with the proposed indication, Repros would like to request a special protocol assessment (“SPA”). The FDA responded that the issues noted above should be resolved first before undertaking a SPA.
MacroChem Corporation has developed a testosterone gel that has completed phase I and is currently looking for a partner to assist with entering phase II development. Other new treatments are being sought for TRT, including a new class of drugs called Selective Androgen Receptor Modulators (“SARMs”), as well as enhanced hydroalcoholic gel formulation of hydrotestosterone. These products are in development and their future impact on the treatment of testosterone deficiency is unknown. Lipocine Inc. (“Lipocine”) is also
21
Table of Contents
developing an oral testosterone product that we believe is in phase I at this time. In May 2009, Solvay, recently acquired by Abbott, announced a license agreement with Lipocine that provides Solvay exclusive rights to develop and commercialize an oral formulation of testosterone.
Competition for XIAFLEX for Dupuytren’s
Halozyme Therapeutics (“Halozyme”) is researching HTI-501, a human lysosomal proteinase that degrades collagen. According to Halozyme, HTI-501 may be useful in the treatment of both medical and aesthetic dermatologic conditions such as cellulite, Dupuytren’s and Peyronie’s and pre-clinical investigations are ongoing.
Biologics or “biotech drugs”, such as XIAFLEX for Dupuytren’s, are not currently considered susceptible to an abbreviated approval procedure, either due to current U.S. law or FDA practice in approving biologic products. However, the U.S. Congress is expected to continue to explore, and ultimately enact, legislation that would establish a procedure for the FDA to accept ANDA-like abbreviated applications for the approval of “follow-on,” “biosimilar” or “comparable” biotech drugs. Such legislation is also expected to contain provisions concerning some period of market exclusivity for biologics or “biotech drugs.” Such legislation has already been adopted in the EU.
Other Competition
We face extensive competition in the acquisition or in-licensing of pharmaceutical products or small companies to enhance our portfolio of products. A number of more established companies, which have strategies to in-license or acquire products, may have competitive advantages, as may other emerging companies taking similar or different approaches to product acquisitions. In addition, a number of established research-based pharmaceutical and biotechnology companies may acquire products in late stages of development to augment their internal product lines. These established companies may have a competitive advantage over us due to their size, resources and experience.
Government Regulation
Government authorities in the U.S., at the federal, state, and local level, and foreign countries extensively regulate, among other things, the following areas relating to our products and product candidates:
| • | research and development; |
| • | testing, manufacture, labeling and distribution; |
| • | advertising, promotion, sampling and marketing; and |
| • | import and export. |
All of our products require regulatory approval by government agencies prior to commercialization. In particular, human therapeutic products are subject to rigorous preclinical and clinical trials to demonstrate safety and efficacy and other approval procedures of the FDA and similar regulatory authorities in foreign countries. Various federal, state, local, and foreign statutes and regulations also govern testing, manufacturing, labeling, distribution, storage and record-keeping related to such products and their promotion and marketing. The process of obtaining these approvals and the compliance with federal, state, local, and foreign statutes and regulations require the expenditure of substantial time and financial resources. In addition, the current regulatory and political environment at FDA could lead to increased testing and data requirements which could impact regulatory timelines and costs.
We have received marketing approval for Testim for the indication of male hypogonadism in the U.S., Belgium, Denmark, Finland, Germany, Greece, Iceland, Ireland, Italy, Luxembourg, the Netherlands, Norway, Portugal, Spain, Sweden and the U.K. Approval in the specified European countries was via the Mutual
22
Table of Contents
Recognition Procedure. In May 2006, we received marketing approval for Testim in Canada. In Europe, Testim has been launched in Belgium, Denmark, Finland, Germany, Greece, Ireland, Italy, Luxembourg, The Netherlands, Portugal, Spain, Sweden, and the U.K. In December 2007, Ipsen notified Auxilium that a decision was made (for non-safety reasons) to withdraw Testim from the market in Denmark, Finland, and Sweden. In November 2008, we agreed with Ipsen to terminate the Ipsen Agreement and transfer the European marketing approvals from Ipsen to Ferring. All marketing approvals were transferred from Ipsen to Ferring in 2009.
We have received marketing approval for XIAFLEX for the treatment of Dupuytren’s in the U.S in adult patients with a palpable cord. Our partner Pfizer has filed for approval of XIAFLEX for the treatment of Dupuytren’s in Europe.
None of our other product candidates has received marketing approval.
U.S. Government Regulation
In the U.S., the FDA regulates drugs under various laws including the Federal Food, Drug, and Cosmetic Act and implementing regulations. If we fail to comply with the applicable requirements at any time during the product development process, approval process, or after approval, we may become subject to administrative or judicial sanctions. These sanctions could include:
| • | refusal to approve pending applications; |
| • | withdrawals of approvals; |
| • | clinical holds; |
| • | warning letters; |
| • | product recalls and product seizures; and |
| • | total or partial suspension of our operations, injunctions, fines, civil penalties or criminal prosecution. |
Any agency enforcement action could have a material adverse effect on us.
Currently there is a substantial amount of congressional and administration review of the FDA and the regulatory approval process for drug candidates in the U.S. As a result, there may be significant changes made to the regulatory approval process in the U.S.
The steps required before a drug may be marketed in the U.S. include:
| • | preclinical laboratory tests, animal studies, formulation, and stability studies; |
| • | submission to the FDA of an investigational new drug exemption (“IND”) which must become effective before human clinical trials may begin; |
| • | execution of adequate and well-controlled clinical trials to establish the safety and efficacy of the product for each indication for which approval is sought; |
| • | submission to the FDA of a NDA or BLA; |
| • | satisfactory completion of an FDA inspection of the manufacturing facility or facilities at which the product is produced to assess compliance with cGMP; and |
| • | FDA review and approval of the NDA or BLA, or any supplements thereto, including, if applicable, a determination of its controlled substance schedule. |
Preclinical studies generally are conducted in laboratory animals to evaluate the potential safety and activity of a product. Violation of the FDA’s good laboratory practices regulations can, in some cases, lead to
23
Table of Contents
invalidation of the studies, requiring these studies to be replicated. In the U.S., drug developers submit the results of preclinical trials, together with manufacturing information and analytical and stability data, to the FDA as part of the IND, which must become effective before clinical trials can begin in the U.S. An IND becomes effective 30 days after receipt by the FDA unless before that time the FDA raises concerns or questions about the proposed clinical trials outlined in the IND. In that case, the IND sponsor and the FDA must resolve any outstanding FDA concerns or questions before clinical trials can proceed. If these concerns or questions are unresolved, the FDA will not allow the clinical trials to commence.
Clinical trials involve the administration of the investigational product candidate or approved products to human subjects under the supervision of qualified investigators. Clinical trials are conducted under protocols detailing, among other things, the objectives of the study, the parameters to be used in assessing the safety and the effectiveness of the drug. Typically, clinical evaluation involves a time-consuming and costly three-phase sequential process, but the phases may overlap. Each trial must be reviewed, approved and conducted under the auspices of an independent institutional review board, and each trial must include the patient’s informed consent. Phase II and III trials must be registered in a government database of trials. The following sets forth a brief description of the typical phases of clinical trials:
| Phase I | Refers typically to closely monitored clinical trials and includes the initial introduction of an investigational new drug into human patients or healthy volunteer subjects. Phase I clinical trials are designed to determine the safety, metabolism and pharmacologic actions of a drug in humans, the potential side effects of the product candidates associated with increasing drug doses and, if possible, to gain early evidence of the product candidate’s effectiveness. Phase I trials also include the study of structure-activity relationships and mechanism of action in humans, as well as studies in which investigational drugs are used as research tools to explore biological phenomena or disease processes. During phase I clinical trials, sufficient information about a drug’s pharmacokinetics and pharmacological effects should be obtained to permit the design of well-controlled, scientifically valid phase II studies. The total number of subjects and patients included in phase I clinical trials varies, but is generally in the range of 20 to 80 people. | |
| Phase II | Refers to controlled clinical trials conducted to evaluate appropriate dosage and the effectiveness of a drug for a particular indication or indications in patients with a disease or condition under study and to determine the common short-term side effects and risks associated with the drug. These clinical trials are typically well-controlled, closely monitored and conducted in a relatively small number of patients, usually involving no more than several hundred subjects. Phase II studies can be sequenced as phase IIa or phase IIb. | |
| Phase III | Refers to expanded controlled and uncontrolled clinical trials. These clinical trials are performed after preliminary evidence suggesting effectiveness of a drug has been obtained. Phase III clinical trials are intended to gather additional information about the effectiveness and safety that is needed to evaluate the overall benefit-risk relationship of the drug and to provide an adequate basis for physician labeling. Phase III trials usually include from several hundred to several thousand subjects. | |
| Phase IV | Refers to trials conducted after approval of a new drug and which explore approved uses and approved doses of the product. These trials must also be approved and conducted under the auspices of an institutional review board. Phase IV studies may be required as a condition of approval. | |
Clinical testing may not be completed successfully within any specified time period, if at all. The FDA closely monitors the progress of each of the first three phases of clinical trials that are conducted in the U.S. and may, at its discretion, reevaluate, alter, suspend or terminate the testing based upon the data accumulated to that point and the FDA’s assessment of the risk/benefit ratio to the patient. The FDA can also provide specific guidance on the acceptability of protocol design for clinical trials. The FDA or we may suspend or terminate
24
Table of Contents
clinical trials at any time for various reasons, including a finding that the subjects or patients are being exposed to an unacceptable health risk. The FDA can also request additional clinical trials be conducted as a condition to product approval. During all clinical trials, physicians monitor the patients to determine effectiveness and to observe and report any reactions or other safety risks that may result from use of the drug candidate.
Assuming successful completion of the required clinical trial, drug developers submit the results of preclinical studies and clinical trials, together with other detailed information including information on the chemistry, manufacture and control of the product, to the FDA, in the form of an NDA or BLA, requesting approval to market the product for one or more indications. In most cases, the NDA/BLA must be accompanied by a substantial user fee. The FDA reviews an NDA/BLA to determine, among other things, whether a product is safe and effective for its intended use. FDA can determine that a REMS is necessary to ensure that the benefits of the drug outweigh its risks. The REMS includes periodic assessments of the strategy and can include potential patient and healthcare provider communications plans, training obligations, monitoring, registries and other elements to ensure the drug can be used safely.
Before approving an application, the FDA will inspect the facility or facilities where the product is manufactured. The FDA will not approve the application unless cGMP compliance is satisfactory. The FDA will issue an approval letter if it determines that the application, manufacturing process and manufacturing facilities are acceptable. If the FDA determines that the application, manufacturing process or manufacturing facilities are not acceptable, it will outline the deficiencies in the submission in the form of a Complete Response Letter and will often request additional testing or information. Notwithstanding the submission of any requested additional information, the FDA ultimately may decide that the application does not satisfy the regulatory criteria for approval and refuse to approve the application.
The testing and approval process requires substantial time, effort and financial resources, which may take several years to complete. The FDA may not grant approval on a timely basis, or at all. We may encounter difficulties or unanticipated costs in our efforts to secure necessary governmental approvals, which could delay or preclude us from marketing our products. Furthermore, the FDA may prevent a drug developer from marketing a product under a label for its desired indications or place other conditions, including restrictive labeling, REMS, or restrictions on distribution as a condition of any approvals, which may impair commercialization of the product. After approval, some types of changes to the approved product, such as adding new indications, manufacturing changes and additional labeling claims, are subject to further FDA review and approval.
If the FDA approves the NDA or BLA, the drug can be marketed to physicians to prescribe in the U.S. After approval, the drug developer must comply with a number of post-approval requirements, including delivering periodic reports to the FDA (i.e., annual reports), submitting descriptions of any adverse reactions reported, field alert reports, biological product deviation reporting, complying with drug sampling and distribution requirements, completion of post-marketing clinical trials or patient registries, completion of additional non-clinical studies or any other agreed upon procedure. The holder of an approved NDA/BLA is required to provide updated safety and efficacy information and to comply with requirements concerning advertising and promotional labeling. Also, quality control and manufacturing procedures must continue to conform to cGMP after approval. Drug manufacturers and their subcontractors are required to register their facilities and are subject to periodic unannounced inspections by the FDA to assess compliance with cGMP which imposes procedural and documentation requirements relating to manufacturing, quality assurance and quality control. Accordingly, manufacturers must continue to expend time, money and effort in the area of production and quality control to maintain compliance with cGMP and other aspects of regulatory compliance. The FDA may require post-market testing and surveillance to monitor the product’s safety or efficacy, including additional studies to evaluate long-term effects.
In addition to studies requested by the FDA after approval, a drug developer may conduct other trials and studies to explore use of the approved drug for treatment of new indications, which require submission of a
25
Table of Contents
supplemental or new NDA and FDA approval of the new labeling claims. The purpose of these trials and studies is to broaden the application and use of the drug and its acceptance in the medical community.
Supplementing our own production capabilities, we use, and will continue to use, third-party manufacturers to produce our products in clinical and commercial quantities. Future FDA inspections may identify compliance issues at our facilities or at the facilities of our contract manufacturers that may disrupt production or distribution, or require substantial resources to correct. In addition, discovery of problems with a product or the failure to comply with requirements may result in restrictions on a product, manufacturer or holder of an approved NDA/BLA, including withdrawal or recall of the product from the market or other voluntary or FDA-initiated action that could delay further marketing. Newly discovered or developed safety or effectiveness data may require changes to a product’s approved labeling, including the addition of new warnings and contraindications. Also, new government requirements may be established that could delay or prevent regulatory approval of our products under development.
The Controlled Substances Act imposes various registration, record-keeping and reporting requirements, procurement and manufacturing quotas, labeling and packaging requirements, security controls, prescription and order form requirements and restrictions on prescription refills on some kinds of pharmaceutical products. A principal factor in determining the particular requirements of this act, if any, applicable to a product is its actual or potential abuse profile. A pharmaceutical product may be listed as a Schedule I, II, III, IV or V substance, with Schedule I substances considered to present the highest risk of substance abuse and Schedule V substances the lowest. Testosterone, the active drug substance in Testim, has been scheduled under the Controlled Substances Act as a Schedule III substance. All regular Schedule III drug prescriptions must be signed by a physician and may not be refilled. Furthermore, the amount of Schedule III substances we can obtain for clinical trials and commercial distribution is limited by the Drug Enforcement Agency (“DEA”), and our quota may not be sufficient to complete clinical trials or meet commercial demand, if any. In addition to federal scheduling, Testim is subject to state-controlled substance regulation and may be placed in more restrictive schedules than those determined by the DEA and the FDA. However, to date, with the exception of the State of New York, which has given testosterone a schedule II classification, testosterone has not been placed in a more restrictive schedule by any state.
Foreign Regulation
Whether or not we obtain FDA approval for a product, we must obtain approval of a clinical trial or product by the comparable regulatory authorities of foreign countries before we can commence clinical trials or marketing of the product in those countries. The approval process varies from country to country, and the time may be longer or shorter than that required for FDA approval. The requirements governing the conduct of clinical trials, product licensing, pricing and reimbursement also vary greatly from country to country. Although governed by the applicable country’s regulations, clinical trials conducted outside of the U.S. typically are administered with the three-phase sequential process that is discussed above under “U.S. Government Regulation”. The foreign equivalent of an IND, a Clinical Trial Application (“CTA”), is a prerequisite to performing pilot studies or phase I clinical trials in certain European countries.
Under EU regulatory systems, we may submit MAAs either under centralized or decentralized procedures. The centralized procedure is mandatory for certain biotechnology derived products and medicines for new active substances for certain diseases. It is also mandatory for orphan drugs but is optional for other new active substances or novel products with innovative characteristics. Approval via the centralized procedure leads to a single marketing authorization that is valid throughout the EU community. Two decentralized procedures exist as alternatives for approval of products that are not obligated to be submitted by the centralized procedure. The first is the Mutual Recognition procedure (“MRP”) which provides for mutual recognition of national approval decisions. Under this procedure, the holder of a national marketing authorization for a product may submit an application to the remaining member states. Within 90 days of receiving the applications and assessment report, each member state must decide whether to recognize approval. The second procedure, the
26
Table of Contents
Decentralized Procedure, was established in 2005. This procedure is available to products that have not been approved anywhere in the EU The MAA is submitted simultaneously in the reference member state chosen by the applicant and in other member states as desired by applicant. The reference member state carries out the main assessment and consults with the other member states during the process. Like the MRP, approval via this process results in national marketing approvals in the territories where scientific approval was received. All products, irrespective of the route of filing, are afforded 10 years of market exclusivity and eight years of data protection on approval. An additional year of market exclusivity, providing a total of 11 years of market exclusivity, may be obtained for products on approval of an additional indication where that indication represents a significant clinical benefit and significant pre-clinical or clinical studies were carried out in support of the additional indication.
We received approval for Testim in the U.K. in June 2003. A mutual recognition application based on the U.K. approval was initiated in March 2004. The procedure was completed in June 2004 and resulted in scientific approval in the following 14 additional countries: Belgium, Denmark, Finland, Germany, Greece, Iceland, Ireland, Italy, Luxembourg, The Netherlands, Norway, Portugal, Spain and Sweden. Marketing approvals have been issued in each of these countries. Testim has been launched in Belgium, Denmark, Finland, Germany, Greece, Ireland, Italy, Luxembourg, The Netherlands, Portugal, Spain, Sweden, and the U.K. In addition, in May 2006, we received marketing approval for Testim in Canada. In December 2007, Ipsen notified Auxilium that a decision was made by Ipsen (for non-safety reasons) to withdraw Testim from the market in Denmark, Finland, and Sweden. In November 2008, we agreed with Ipsen to terminate the Ipsen Agreement and transfer the European marketing approvals from Ipsen to Ferring. All marketing approvals were transferred from Ipsen to Ferring in 2009.
Our partner Pfizer has filed a MAA for approval of XIAFLEX for the treatment of Dupuytren’s in Europe under the centralized procedure. In January 2010 Pfizer was notified that the European scientific/technical review procedure for the MAA for XIAFLEX for the treatment of Dupuytren’s contracture commenced.
Furthermore, regulatory approval of prices is required in most countries other than the U.S. We face the risk that the resulting prices would be insufficient to generate an acceptable return to us or our collaborators.
Fraud and Abuse Laws
We are subject to various federal and state laws pertaining to health care fraud and abuse, including anti-kickback laws, false claims laws and physician self-referral laws. Violations of these laws are punishable by criminal, civil and/or administrative sanctions, including, in some instances, imprisonment and exclusion from participation in federal and state health care programs, including Medicare, Medicaid and veterans’ health programs. Because of the far-reaching nature of these laws, there can be no assurance that the occurrence of one or more violations of these laws would not result in a material adverse effect on our business, financial condition and results of operations.
Anti-Kickback Laws. Our operations are subject to federal and state anti-kickback laws. Certain provisions of the Social Security Act prohibit entities such as us from knowingly and willingly offering, paying, soliciting or receiving any form of remuneration (including any kickbacks, bribe or rebate) in return for the referral of items or services for which payment may be made under a federal health care program, or in return for the recommendation, arrangement, purchase, lease or order of items or services for which payment may be made under a federal health care program. Violation of the federal anti-kickback law is a felony, punishable by criminal fines and imprisonment for up to five years or both. In addition, the Department of Health and Human Services may impose civil penalties and exclude violators from participation in federal health care programs such as Medicare and Medicaid. Many states have adopted similar prohibitions against payments intended to induce referrals of products or services paid by Medicaid or other third party payors.
27
Table of Contents
Physician Self-Referral Laws. We also may be subject to federal and/or state physician self-referral laws. Federal physician self-referral legislation (the “Stark law”) prohibits, subject to certain exceptions, a physician from referring Medicare or Medicaid patients to an entity to provide designated health services, including, among other things, certain radiology and radiation therapy services and clinical laboratory services in which the physician or a member of his immediate family has an ownership or investment interest or has entered into a compensation arrangement. The Stark law also prohibits the entity receiving the improper referral from billing any good or service furnished pursuant to the referral. The penalties for violations include a prohibition on payment by these government programs and civil penalties for participation in a circumvention scheme. Various state laws also contain similar provisions and penalties.
False Claims. The federal False Claims Act imposes civil and criminal liability on individuals or entities who submit (or cause the submission of) false or fraudulent claims for payment to the government. Violations of the federal False Claims Act may result in penalties equal to three times the damages which the government sustained, an assessment, civil monetary penalties and exclusion from participation in the Medicare and Medicaid programs.
The federal False Claims Act also allows a private individual to bring a qui tam suit on behalf of the government against an individual or entity for violations of the False Claims Act. In a qui tam suit, the private plaintiff is responsible for initiating a lawsuit that may eventually lead to the government recovering money of which it was defrauded. After the private plaintiff has initiated the lawsuit, the government must decide whether to intervene in the lawsuit and become the primary prosecutor. In the event the government declines to join the lawsuit, the private plaintiff may choose to pursue the case alone, in which case the private plaintiff’s counsel will have primary control over the prosecution (although the government must be kept apprised of the progress of the lawsuit). In return for bringing the suit on the government’s behalf, the statute provides that the private plaintiff is entitled to receive up to 30% of the recovered amount from the litigation proceeds if the litigation is successful plus reasonable expenses and attorneys fees. Recently, the number of qui tam suits brought against entities in the health care industry has increased dramatically. In addition, a number of states have enacted laws modeled after the False Claims Act that allow those states to recover money which was fraudulently obtained from the state.
Other Fraud and Abuse Laws. The Health Insurance Portability and Accountability Act of 1996 created, in part, two new federal crimes: Health Care Fraud and False Statements Relating to Health Care Matters. The Health Care Fraud statute prohibits the knowing and willful execution of a scheme or artifice to defraud any health care benefit program. A violation of the statute is a felony and may result in fines and/or imprisonment. The False Statements statute prohibits knowingly and willfully falsifying, concealing or covering up a material fact by any trick, scheme or device or making any materially false, fictitious or fraudulent statement in connection with the delivery of or payment for health care benefits, items or services. A violation of this statute is a felony and may result in fines and/or imprisonment.
An increasing number of states are passing legislation requiring the reporting and disclosure of gifts or other value given to health care providers, the disclosure of certain advertising and promotion expenditures, the disclosure of product pricing information, the licensing of sales representatives, the adoption of codes of conduct that meet state requirements and the posting of our compliance plan on our Web site.
Compliance Program
The healthcare laws and fraud and abuse laws applicable to our business are complex and subject to variable interpretations. We maintain certain compliance review, education and training and other programs to further our commitment to high standards of ethical and legal conduct and to minimize the likelihood that we would engage in conduct or enter into arrangements in violation of applicable authorities. For example, we have (i) established a compliance team consisting of representatives from our Legal, Human Resources, Regulatory Affairs/Quality Assurance and Medical Affairs/Safety departments that meets regularly; (ii) established a
28
Table of Contents
compliance hotline that permits our employees to report anonymously any compliance issues that may arise; and (iii) instituted other safeguards intended to help prevent any violations of the applicable fraud and abuse laws and healthcare laws, and to remediate any situations that could give rise to violations. We also review our transactions and agreements, both past and present, to help assure they are compliant.
Through our compliance efforts, we constantly strive to structure our business operations and relationships with our customers to comply with all applicable legal requirements. However, it is possible that governmental entities or other third parties could interpret these laws differently and assert non-compliance with respect to our business operations and relationships including these isolated arrangements. While there have been no claims asserted against us for alleged non-compliance with fraud and abuse laws and other healthcare laws, if a claim were asserted and we were not to prevail, possible penalties and sanctions could have a material effect on our financial statements or our ability to conduct our operations.
Third-party Reimbursement and Pricing Controls
In the U.S. and elsewhere, sales of pharmaceutical products depend in significant part on the availability of reimbursement to the consumer from third-party payors, such as government and private insurance plans. Third-party payors increasingly are challenging the prices charged for medical products and services and implementing other cost containment mechanisms. It is, and will be, time consuming and expensive for us to go through the process of maintaining or seeking reimbursement to the consumer for our products from Medicaid, Medicare and private payors. Our products may not be considered cost effective, and coverage and reimbursement may not be available or sufficient to allow us to sell our products on a competitive and profitable basis, potentially resulting in contract changes with these major payors.
We believe that payors will cover the XIAFLEX treatment regimen for Dupuytren’s. However, without product and procedure specific codes, we will need to set the expectation with physicians that reimbursement, for both the product and the physician’s procedures, may be slow and initially inconsistent.
The use of a miscellaneous J code for XIAFLEX means that payor’s computer systems may flag claims for XIAFLEX reimbursement for manual adjudication. This may result in a 90 to 120 day payment cycle for the drug. We are prepared to offer extended dating to physicians in order to minimize or eliminate the amount of time that they will be out of pocket for XIAFLEX. We have amended our J code application that we filed last year for the FDA approval received, and we expect that a XIAFLEX specific J code will be issued in January 2011, which will result in electronic adjudication of claims thereafter.
The physicians will also have decisions to make regarding how to get reimbursed for their services associated with treating a XIAFLEX patient. We believe that physicians will be reimbursed for these procedures, but as with the product reimbursement, the time for payment may be initially slow and inconsistent. We have been working with the hand societies on possible procedure reimbursement strategies and feel that we are well positioned to have an application filed for CPT codes by the end of 2010, which may result in XIAFLEX specific CPT codes becoming available in 2012.
Our sales representatives have extensive experience in assisting physicians with reimbursement of breakthrough drugs. These representatives will be supplemented with field based reimbursement specialists and a reimbursement hotline. For patients who may have difficulty affording XIAFLEX, we will be establishing a patient assistance program and supporting a co-pay foundation. We believe these efforts around reimbursement issues will position us well for the successful launch of XIAFLEX for Dupuytren’s.
In many foreign markets, including the countries in the EU, pricing of pharmaceutical products is subject to governmental control. In the U.S., there have been, and we expect that there will continue to be, a number of federal and state proposals to implement similar governmental pricing control. While we cannot
29
Table of Contents
predict whether such legislative or regulatory proposals will be adopted, the adoption of such proposals could have a material adverse effect on our business, financial condition and profitability.
Patents and Proprietary Rights
Our success will depend in part on our ability to protect our existing products and the products we acquire or in-license by obtaining and maintaining a strong proprietary position both in the U.S. and in other countries. To develop and maintain such a position, we intend to continue relying upon patent protection, trade secrets, know-how, continuing technological innovations and licensing opportunities. In addition, we intend to seek patent protection whenever appropriate for any products or product candidates and related technology we develop or acquire in the future.
The scope of the intellectual property rights held by pharmaceutical firms involves complex legal, scientific and factual questions and consequently is generally uncertain. In addition, the coverage claimed in a patent application can be significantly reduced before the patent is issued. As a result, we do not know whether any of our current patent applications, or the products or product candidates we develop, acquire or license will result in the issuance of patents or, if any patents are issued, whether they will provide significant proprietary protection or will be challenged, circumvented or invalidated. Because patent applications in the U.S. and some other jurisdictions are sometimes maintained in secrecy until patents issue, and since publication of discoveries in the scientific or patent literature often lags behind actual discoveries, we cannot be certain of the priority of inventions covered by pending patent applications. Moreover, we may have to participate in interference proceedings declared by the USPTO or a foreign patent office to determine priority of invention, or in opposition proceedings in a foreign patent office, either of which could result in substantial cost to us, even if the eventual outcome is favorable to us. There can be no assurance that the patents, if issued and challenged, in a court of competent jurisdiction would be found valid or enforceable. An adverse outcome could subject us to significant liabilities to third parties, require disputed rights to be licensed from third parties or require us to cease using such technology.
Although we believe these patent applications, if they issue as patents, will provide a competitive advantage, the patent positions of pharmaceutical and biotechnology companies are highly uncertain and involve complex legal and factual questions. We may not be able to develop patentable products or processes and may not be able to obtain patents from pending applications. Even if patent claims are allowed, the claims may not issue, or in the event of issuance, may not be sufficient to protect our technology. In addition, any patents or patent rights we obtain may be circumvented, challenged or invalidated by our competitors.
While we attempt to ensure that our product candidates and the methods we employ to manufacture them do not infringe other parties’ patents and proprietary rights, competitors or other parties may assert that we infringe on their proprietary rights. Additionally, because patent prosecution can proceed in secret prior to issuance of a patent, third-parties may obtain other patents without our knowledge prior to the issuance of patents relating to our product candidates which they could attempt to assert against us.
Although we believe that our product candidates, production methods and other activities do not currently infringe the intellectual property rights of third parties, we cannot be certain that a third party will not challenge our position in the future. If a third party alleges that we are infringing its intellectual property rights, we may need to obtain a license from that third party, but there can be no assurance that any such license will be available on acceptable terms or at all. Any infringement claim that results in litigation could result in substantial cost to us and the diversion of management’s attention away from our core business and could also prevent us from marketing our products. To enforce patents issued to us or to determine the scope and validity of other parties’ proprietary rights, we may also become involved in litigation or in interference proceedings declared by the USPTO, which could result in substantial costs to us or an adverse decision as to the priority of our inventions. We may be involved in interference and/or opposition proceedings in the future. We believe there will continue to be litigation in our industry regarding patent and other intellectual property rights.
30
Table of Contents
We also rely on trade secret protection for our confidential and proprietary information. No assurance can be given that others will not independently develop substantially equivalent proprietary information and techniques or otherwise gain access to our trade secrets or disclose such technology or that we can meaningfully protect our trade secrets.
It is our policy to require our employees, consultants, outside scientific collaborators, sponsored researchers and other advisors to execute confidentiality agreements upon the commencement of employment or consulting relationships with us. These agreements provide that all confidential information developed or made known to the individual during the course of the individual’s relationship with us is to be kept confidential and not disclosed to third parties except in specific circumstances. In the case of employees, the agreements provide that all inventions conceived by the individual shall be our exclusive property. There can be no assurance, however, that these agreements will provide meaningful protection or adequate remedies for our trade secrets in the event of unauthorized use or disclosure of such information.
TESTIM. We have licensed the underlying technology for Testim from CPEX. The patents and patent applications that we license from CPEX for Testim cover methods, compositions and formulations for administering drugs, such as testosterone, across the skin and mucus membranes. In the U.S., the ‘968 patent covers a method for maintaining effective blood serum testosterone levels for treating a hypogonadal male using Testim and is listed in Approved Drug Products with therapeutic Equivalence Evaluations (commonly known as the “Orange Book”), published by the FDA. The ‘968 patent expires in January 2025. In October 2009, six additional patents covering Testim were issued and have been listed in the Orange Book. They each expire in April 2023. CPEX also has filed continuation applications that are pending. In Canada, there are two patents covering Testim, one that expired in January 2010 and another that expires in April 2023. The patent issued by the European Patent Office covering Testim expires in April 2023. CPEX also has submitted a patent application in Japan that is awaiting examination. All of these patents and any patents that issue from the pending applications are included in the CPEX License. Testosterone, the active ingredient in Testim, does not have patent protection and is included in competing TRT products.
In October 2008, we and our licensor, CPEX, received notice that Upsher-Smith filed an ANDA containing a paragraph IV certification seeking approval from the FDA to market a generic version of Testim prior to the January 2025 expiration of ‘968 Patent. The ‘968 Patent, which Bentley assigned to CPEX in June 2008, covers a method for maintaining effective blood serum testosterone levels for treating a hypogonadal male using Testim and is listed in the Orange Book. The paragraph IV certification sets forth allegations that the ‘968 Patent will not be infringed by Upsher-Smith’s manufacture, use or sale of the product for which the ANDA was submitted. In December 2008, we filed a patent infringement lawsuit against Upsher-Smith (the “Upsher-Smith Litigation”) which remains pending. Upsher-Smith has alleged that its proposed generic version of Testim would not infringe the ‘968 patent, and that the ‘968 patent is invalid. Under the Hatch-Waxman Act, final FDA approval of Upsher-Smith’s proposed generic product will be stayed until the earlier of 30 months beginning on the date of receipt of the paragraph IV certification (April 2011) or resolution of the patent infringement lawsuit. Should Upsher-Smith receive a tentative approval of its generic version of Testim from the FDA, it cannot lawfully launch its generic version of Testim in the U.S. before the earlier of the expiration of the currently pending 30-month stay or a district court decision in its favor. Upsher-Smith will also not be able to lawfully launch a generic version of Testim in the U.S. without the necessary final approval from the FDA. We have incurred, and will continue to incur, costs related to the Upsher-Smith Litigation and regulatory actions and may incur costs related to any other legal and regulatory actions that we may undertake. The Upsher-Smith Litigation could result in a finding that Upsher-Smith’s proposed testosterone product does not infringe the ‘968 Patent or that the ‘968 Patent is invalid and/or unenforceable. An adverse outcome in the Upsher-Smith Litigation or any such legal action could result in one or more generic versions of Testim being launched in the U.S. before the expiration of the ‘968 Patent in January 2025.
31
Table of Contents
XIAFLEX. We licensed XIAFLEX, our product for the treatment of Dupuytren’s, and product candidate for the treatment of Peyronie’s and Frozen Shoulder syndrome, from BioSpecifics. In addition to the marketing exclusivity which comes with its orphan drug status in the U.S. as a treatment for Dupuytren’s and Peyronie’s, the enzyme underlying this product candidate is covered by two use patents in the U.S., one for the treatment of Dupuytren’s and one for the treatment of Peyronie’s. The Dupuytren’s patent expires in 2014, and the Peyronie’s patent expires in 2019. Both patents are limited to the use of the enzyme for the treatment of Dupuytren’s and Peyronie’s within certain dose ranges. While we believe that trials in Peyronie’s will demonstrate that effective dosing will fall within the patented range, currently, there is not enough data to establish whether the ultimate approved product will be covered by the patents. While XIAFLEX does not have orphan drug status for any indication in Europe, foreign patents cover these products in certain countries, and on approval of XIAFLEX for a first indication in Europe, we expect the product will benefit from 10 years of market exclusivity and 8 years of data exclusivity. We may obtain an additional year of market exclusivity if the regulatory authorities approve an additional indication that they determine to represent a significant clinical benefit.
In January 2005, a patent application was filed with regard to XIAFLEX for the treatment of Frozen Shoulder syndrome. If this patent is granted, it would expire in 2026, subject to patent term adjustments. In January 2006, we filed a patent application with regard to the composition and manufacturing process for XIAFLEX; if this patent is granted, it would expire in January 2027, subject to patent term adjustments.
TRANSMUCOSAL FILM TECHNOLOGY. We have licensed the transmucosal film technology for our overactive bladder and pain product candidates from PharmaForm. Our transmucosal film product candidates are covered by an issued formulation patent in the U.S. and two international patent applications remain pending in several countries worldwide, including the U.S. The issued formulation patent expires in 2020.
Orphan Drug Status
The Orphan Drug provisions of the Federal Food, Drug, and Cosmetic Act provide incentives to drug and biologics suppliers to develop and supply drugs for the treatment of rare diseases, currently defined as diseases that affect fewer than 200,000 individuals in the U.S. or, for a disease that affects more than 200,000 individuals in the U.S. and for which there is no reasonable expectation that the cost of developing and making available in the U.S. a drug for such disease or condition will be recovered from its sales in the U.S. Under these provisions, a supplier of a designated orphan product can seek tax benefits, and the holder of the first FDA approval of a designated orphan product will be granted a seven-year period of marketing exclusivity for that product for the orphan indication. The marketing exclusivity of an orphan drug would prevent other sponsors from obtaining approval of the same drug for the same indication except under limited circumstances. It would not prevent other drugs from being approved for the same indication.
The FDA granted orphan drug status to XIAFLEX in the U.S. for each of the treatments of Dupuytren’s and Peyronie’s. The designations for the treatment of Dupuytren’s and Peyronie’s have been transferred to us. Orphan drug status means that, because XIAFLEX was the first product to receive FDA approval for the orphan indication of Dupuytren’s, another application to market the same drug for the same indication may not be approved, except in limited circumstances, for a period of up to seven years in the U.S. With an approval date of February 2, 2010, the exclusivity period for XIAFLEX for Dupuytren’s would expire February 2, 2017.
The Hatch-Waxman Act
Under the U.S. Drug Price Competition and Patent Term Restoration Act of 1984, known as the Hatch-Waxman Act, newly approved drugs and indications benefit from a statutory period of non-patent marketing exclusivity. The Hatch-Waxman Act provides five-year marketing exclusivity to the first applicant to gain approval of an NDA for a new chemical entity, meaning that the FDA has not previously approved any other new
32
Table of Contents
drug containing the same active ingredient. The Hatch-Waxman Act prohibits an ANDA where the applicant does not own or have a legal right of reference to all the data required for approval, to be submitted by another company for another version of such drug during the five-year exclusive period. Protection under the Hatch-Waxman Act will not prevent the filing or approval of another full NDA, however, the applicant would be required to conduct its own pre-clinical, adequate and well-controlled clinical trials to demonstrate safety and effectiveness. The Hatch-Waxman Act also provides three years of marketing exclusivity for the approval of new NDAs with new clinical trials for previously approved drugs and supplemental NDAs, for example, for new indications, dosages, or strengths of an existing drug, if new clinical investigations are essential to the approval. This three-year exclusivity covers only the new changes associated with the supplemental NDA and does not prohibit the FDA from approving ANDAs for drugs containing the original active ingredient or indications.
If an ANDA applicant were to file a paragraph IV certification under the Hatch-Waxman Act in connection with the submission to the FDA of an ANDA for approval of a generic version of any of our products for which we believed we held a valid patent, then we could initiate a lawsuit against the applicant claiming patent infringement and defending the relevant patent’s validity and enforceability. Depending on the facts and circumstances, the FDA may stay the approval of the ANDA for a generic version of any of our products for 30 months so long as we initiate litigation against the filer of the ANDA within 45 days of receiving the paragraph IV certification. If a court found that one of our patents was invalid or not infringed, then the FDA would be permitted to approve the competitor’s ANDA resulting in a competitive generic product. In the event that the FDA did not grant the 30 month stay, FDA would be permitted to approve the competitor’s ANDA; however, we could engage in legal proceedings, such as an injunction, to attempt to preclude the generic competitor from entering the market during the pendency of the patent litigation, but we may not prevail in which event the competitor could enter the market, despite the ongoing patent litigation.
The Hatch-Waxman Act also permits a patent extension term of up to five years as compensation for patent term lost during product development and the FDA regulatory review process. However, patent extension cannot extend the remaining term of a patent beyond a total of 14 years. The patent term restoration period is generally one-half the time between the effective date of an IND and the submission date of an NDA, plus the time between the submission date of an NDA and the approval of that application. Only one patent applicable to an approved drug is eligible for the extension and it must be applied for prior to expiration of the patent. The USPTO, in consultation with the FDA, reviews and approves the application for patent term extension.
Employees
We currently have approximately 540 employees. We believe that our relations with our employees are good, and we have no history of work stoppages. Generally, our employees are at-will employees. However, we have entered into employment agreements with certain of our executive officers.
Available Information
We file annual, quarterly and current reports, proxy statements and other information with the Securities and Exchange Commission, or SEC. You may read and copy any document we file with the SEC at the SEC’s public reference room at 100 F. Street, N.E., Washington, DC 20549, at prescribed rates. Please call the SEC at 1-800-SEC-0330 for further information on the public reference room. You may also obtain our SEC filings free of charge from the SEC’s Internet Web site at www.sec.gov.
Our Internet Web site address is www.auxilium.com. We make available free of charge through our Web site’s “For Investors” page most of our filings with the SEC, including our annual report on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K and other information. These reports and information are available as soon as reasonably practicable after such material is electronically filed with or furnished to the SEC.
33
Table of Contents
Our Board of Directors has various committees including an audit and compliance committee, compensation committee and nominating and corporate governance committee. Each of these committees has a formal charter. We also have Corporate Governance Guidelines and a Code of Conduct. Copies of these charters, guidelines and codes, and any waivers or amendments to such codes which are applicable to our executive officers, senior financial officers or directors, can be obtained free of charge from our Web site. The references to our Web site and the SEC’s Web site are intended to be inactive textual references only, and the contents of those Web sites are not incorporated by reference herein.
In addition, you may request a copy of the foregoing filings, charters, guidelines and codes, and any waivers or amendments to such codes which are applicable to our executive officers, senior financial officers or directors, at no cost by writing us at the following address or telephoning us at the following telephone number:
Auxilium Pharmaceuticals, Inc.
40 Valley Stream Parkway
Malvern, PA 19355
Attention: Investor Relations
Telephone: (484) 321-5900
34
Table of Contents
| ITEM 1A. | Risk Factors |
In addition to the other information included in this Report, the following factors should be considered in evaluating our business and future prospects. Any of the following risks, either alone or taken together, could materially and adversely affect our business, financial position or results of operations. If one or more of these or other risks or uncertainties materialize, or if our underlying assumptions prove to be incorrect, our actual results may vary materially from what we projected. There may be additional risks that we do not presently know or that we currently believe are immaterial which could also impair our business or financial position.
Risks Related to Commercialization
If medical doctors do not prescribe our products or the medical profession or patients do not accept our products, our ability to grow or maintain our revenues will be limited.
Our business is dependent on market acceptance of our products by physicians, healthcare payors, patients and the medical community. Medical doctors’ willingness to prescribe, and patients’ willingness to accept, our products depend on many factors, including:
| • | perceived safety and efficacy of our products; |
| • | convenience and ease of administration; |
| • | prevalence and severity of adverse side effects in both clinical trials and commercial use; |
| • | availability of alternative treatments; |
| • | cost effectiveness; |
| • | effectiveness of our marketing strategy and the pricing of our products; |
| • | publicity concerning our products or competing products; and |
| • | our ability to obtain third-party coverage or reimbursement for our products and, in the case of XIAFLEX™ (collagenase clostridium histolyticum) for Dupuytren’s contracture (“Dupuytren’s”), the procedures performed by physicians while treating patients with XIAFLEX. |
Even though we have received regulatory approval for Testim® for the treatment of hypogonadism and XIAFLEX for the treatment of adult Dupuytren’s patients with a palpable cord, and even if we receive regulatory approval and satisfy the above criteria for any of our product candidates, physicians may not prescribe, and patients may not accept, our products if we do not promote our products effectively. Factors that could affect our success in marketing our products include:
| • | the adequacy and effectiveness of our sales force and that of any co-promotion partners or international partner’s sales force; |
| • | the adequacy and effectiveness of our production, distribution and marketing capabilities and those of our international partners; |
| • | the success of competing products, including generics, and treatments; and |
| • | the availability and extent of reimbursement from third-party payors for our products and, in the case of XIAFLEX, the procedures performed by physicians while treating patients with XIAFLEX. |
If any of our products or product candidates fails to achieve market acceptance, we may not be able to market and sell the products successfully, which would limit our ability to generate revenue and could harm our business.
35
Table of Contents
If third-party payors do not reimburse customers for our products or any of our product candidates that are approved for marketing, they might not be used or purchased, and our revenues and profits will not grow.
Our revenues and profits depend heavily upon the availability of coverage and reimbursement for the use of our products, and any of our product candidates that are approved for marketing, from third-party healthcare and state and federal government payors, both in the United States (“U.S.”) and in foreign markets. Reimbursement by a third-party payor may depend upon a number of factors, including the third-party payor’s determination the product is:
| • | competitively priced; |
| • | safe, effective and medically necessary; |
| • | appropriate for the specific patient; |
| • | cost-effective; and |
| • | neither experimental nor investigational. |
Since reimbursement approval for a product is required from third-party and government payors, seeking this approval, particularly when seeking approval for a preferred form of reimbursement over other competitive products, is a time-consuming and costly process. Third-party payors may require cost-benefit analysis data from us in order to demonstrate the cost-effectiveness of any product we might bring to market. For any individual third-party payor, we may not be able to provide data sufficient to gain reimbursement on a similar or preferred basis to competitive products or at all. Once reimbursement at an agreed level is approved by a third-party payor, we may lose that reimbursement entirely or we may lose the similar or better reimbursement we receive compared to competitive products. As reimbursement is often approved for a period of time, this risk is greater at the end of the time period, if any, for which the reimbursement was approved.
We may face additional challenges with regard to initial reimbursement which could affect our ability to successfully launch and commercialize XIAFLEX for Dupuytren’s, including:
| • | the variability of reimbursement rates likely to be caused by the use of miscellaneous drug codes and procedure codes may discourage physicians from providing XIAFLEX to certain or all patients depending on their insurance coverage; |
| • | the initial use of “miscellaneous drug codes” for billing XIAFLEX until such time as XIAFLEX-specific drug codes are approved could result in slow and/or inaccurate reimbursement and thereby discourage product use; |
| • | payor and physician confusion surrounding appropriate procedure codes to bill for the administration and follow up procedure related to XIAFLEX until such time as XIAFLEX-specific drug codes are approved could result in slow claims processing times and/or insufficient reimbursement to physicians and discourage physician use; |
| • | physicians may have, or believe they have, an economic incentive to perform Dupuytren’s surgery and therefore discourage use; |
| • | continued downward pressure on reimbursement rates for services and for products administered incident to a physician’s service may discourage uptake or continued usage; |
| • | physicians may decide that managing specialty biologics such as XIAFLEX in their practices, including all of the requirements of the Risk Evaluation and Mitigation Strategy (“REMS”) is too complex and cumbersome for them to undertake or continue; |
| • | an increase in insurance plans that place more of a cost share liability onto patients may limit patients willingness to pay for XIAFLEX and thereby discourage uptake; and |
36
Table of Contents
| • | unforeseen changes in federal health care policy guidelines may negatively impact a physician practice’s willingness to provide novel treatments. |
Our products and any of our product candidates, if approved, may face competition from lower cost generic or follow-on products.
Testim is approved under the provisions of the U.S. Food, Drug and Cosmetic Act that renders it susceptible to potential competition from generic manufacturers via the Abbreviated New Drug Application (“ANDA”) procedure. Generic manufacturers pursuing ANDA approval are not required to conduct costly and time-consuming clinical trials to establish the safety and efficacy of their products; rather, they are permitted to rely on the innovator’s data regarding safety and efficacy. Thus, generic manufacturers can sell their products at prices much lower than those charged by the innovative pharmaceutical companies who have incurred substantial expenses associated with the research and development of the drug product.
The ANDA procedure includes provisions allowing generic manufacturers to challenge the effectiveness of the innovator’s patent protection long prior to the generic manufacturer actually commercializing their products through the paragraph IV certification procedure. In recent years, generic manufacturers have used paragraph IV certifications extensively to challenge patents on a wide array of innovative pharmaceuticals, and we expect this trend to continue and to implicate drug products with even relatively small total revenues.
In October 2008, we and our licensor, CPEX Pharmaceuticals, Inc. (“CPEX”), received notice that Upsher-Smith Laboratories, Inc. (“Upsher-Smith”) filed an ANDA containing a paragraph IV certification seeking approval from the U.S. Food and Drug Administration (“FDA”) to market a generic version of Testim prior to the January 2025 expiration of CPEX’s U.S. Patent No. 7,320,968 (the “968 Patent”). The ‘968 Patent, which Bentley Pharmaceuticals, Inc. (“Bentley”) assigned to CPEX in June 2008, covers a method for maintaining effective blood serum testosterone levels for treating a hypogonadal male using Testim and is listed in Approved Drug Products with Therapeutic Equivalence Evaluations (commonly known as the “Orange Book”), published by the FDA. The paragraph IV certification sets forth allegations that the ‘968 Patent will not be infringed by Upsher-Smith’s manufacture, use or sale of the product for which the ANDA was submitted. In December 2008, we filed a patent infringement lawsuit against Upsher-Smith (the “Upsher-Smith Litigation”) that remains pending. Upsher-Smith has alleged that its proposed generic version of Testim would not infringe the ‘968 patent, and that the ‘968 patent is invalid. Under the Hatch-Waxman Act, final FDA approval of Upsher-Smith’s proposed generic product will be stayed until the earlier of 30 months from receipt of the paragraph IV certification (April 2011) or resolution of the patent infringement lawsuit. Should Upsher-Smith receive an approval of its generic version of Testim from the FDA, it cannot lawfully launch its generic version of Testim in the U.S. before the earlier of the expiration of the currently pending 30-month stay or a district court decision in its favor.
In February 2009, the Company filed a Citizen’s Petition with the FDA requesting that Upsher-Smith be required to perform a variety of studies to demonstrate that the inactive ingredients and formulation of the proposed product do not compromise its safety or efficacy. An FDA response to the Company’s Citizen’s Petition was received in August 2009. The FDA agreed with some of the statements made in the Citizen’s Petition regarding the testing required for generic versions of Testim and disagreed with other statements. Although not commenting upon any filing in particular, the FDA did state that “the practical effect of this determination is that any application for a testosterone gel product that has different penetration enhancers than the reference listed drug cannot be submitted as an abbreviated new drug application (ANDA) and, instead, will have to be submitted as a NDA under section 505(b) of the [Federal Food, Drug, and Cosmetic Act].”
The introduction of a generic version of Testim or Androgel could have an adverse impact on the branded gel market. Although Testim has a BX rating, meaning that pharmacists are prohibited from substituting Testim with Androgel, or a generic version of Androgel, a less expensive testosterone gel product could still have a material adverse affect on Testim market share and/or Testim’s formulary status, and therefore could have a material negative impact on our financial condition and results of operations.
37
Table of Contents
Biologics or “biotech drugs”, such as XIAFLEX for Dupuytren’s, are not currently considered susceptible to an abbreviated approval procedure, either due to current U.S. law or FDA practice in approving biologic products. However, the U.S. Congress is expected to continue to explore, and ultimately enact, legislation that would establish a procedure for the FDA to accept ANDA-like abbreviated applications for the approval of “follow-on,” “biosimilar” or “comparable” biotech drugs. Such legislation is also expected to contain provisions concerning some period of market exclusivity for biologics or “biotech drugs.” Such legislation has already been adopted in the EU.
If product liability lawsuits are brought against us, we may incur substantial liabilities.
The commercialization of our products and the clinical testing, manufacture and commercialization of our product candidates, if approved, involves significant exposure to product liability claims. We have clinical trial and product liability insurance that covers our products and the clinical trials of our product candidates that we believe is adequate in both scope and amount and has been placed with what we believe to be reputable insurers. We are self-insured for the first $1.0 million of liability under these policies. Our product liability policies have been written on a claims-made basis. If any of our product candidates are approved for marketing, we may seek additional coverage. We cannot predict all of the adverse health events that our products or product candidates may cause. As a result, our current and future coverages may not be adequate to protect us from all the liabilities that we may incur. If losses from product liability claims exceed our insurance coverage, we may incur substantial liabilities that exceed our financial resources. Whether or not we are ultimately successful in product liability litigation, such litigation could consume substantial amounts of our financial and managerial resources, and might result in adverse publicity, all of which would impair our business. We may not be able to maintain our clinical trial insurance or product liability insurance at an acceptable cost, if at all, and this insurance may not provide adequate coverage against potential claims or losses. If we are required to pay a product liability claim, we may not have sufficient financial resources and our business and results of operations may be harmed.
Testim competes in a very competitive market, and if we are unable to compete effectively with the other companies that produce products for the treatment of urologic or sexual health disorders, our ability to generate revenues will be limited.
The testosterone replacement therapy (“TRT”) market is highly competitive. Our success will depend, in part, on our ability to protect our share of the market from the competition. Potential competitors in North America, Europe and elsewhere include major pharmaceutical companies, specialty pharmaceutical companies and biotechnology firms, universities and other research institutions and government agencies.
Other pharmaceutical companies may develop generic versions of Testim or any products that compete with Testim that do not infringe our patents or other proprietary rights, and, as a result, our business may be adversely affected. For example, because the ingredients of Testim are commercially available to third parties, it is possible that competitors may design formulations, propose dosages or develop methods of administration that would be outside the scope of the claims of one or more, or of all, of the patent rights that we in-license. This would enable their products to effectively compete with Testim. Governmental and other pressures to reduce pharmaceutical costs may result in physicians writing prescriptions for these generic products. Increased competition from the sale of competing generic pharmaceutical products could cause a material decrease in revenue from Testim.
AndroGel
The primary competition for Testim in the TRT market is Androgel (testosterone gel) 1% CIII, marketed by Solvay Pharmaceuticals, Inc. (“Solvay”). In February 2010, Abbott Laboratories, Inc. (“Abbott”) completed an acquisition of Solvay.. Solvay launched AndroGel three years prior to Testim giving the brand a significant advantage in the TRT gel market. Watson Pharmaceuticals, Inc. (“Watson”) began co-promoting AndroGel with Solvay in late 2006, and Par Pharmaceutical Companies, Inc. (“Par”) began co-promoting AndroGel in early 2007, each pursuant to patent lawsuit settlement agreements with Solvay. In January 2009, the
38
Table of Contents
U.S. Federal Trade Commission (“FTC”) and a number of private parties filed actions against Solvay, Watson and Par alleging that their respective 2006 patent lawsuit settlements related to AndroGel are unlawful. All actions were consolidated in the U.S. District Court for the Northern District of Georgia. In February 2010, the court dismissed the FTC’s claims and some of the private party claims. Certain private party claims remain pending. Solvay and its co-promotion partners collectively had more than 600 sales representatives detailing AndroGel although it remains unclear what resources Abbott will put behind the brand. According to IMS, as of December 31, 2009, AndroGel accounted for 77.7% of the gel prescriptions.
Other Delivery Options
Testim also competes with other TRT products such as short-acting injectables, patches, orals, a buccal tablet and injectable pellets. The injectables have experienced significant growth in the market due to their low cost compared to the transdermal options. This growth coincides with the economic downturn that the U.S. experienced in 2009. There are also a significant number of patients who prefer getting a shot every two to three weeks instead of utilizing a daily application of another product. Physicians perceive the disadvantage of the injectables as being the fact that many patients’ testosterone levels rise past the normal barrier of 1000 ng/dl which leads to a wide fluctuation of testosterone levels in a patient and the potential for side effects such as gynecomastia. Androderm® is a transdermal testosterone patch marketed by Watson. Androderm, the leading patch product, has decreased in share in the last year and accounted for approximately 5.9% of total TRT prescriptions in December 2009, down from 6.5% in December 2008. The buccal therapy accounted for less than 1% of total TRT prescriptions in December 2009. Testopel® is an implantable testosterone pellet that is administered every four to six months.
Generic Competition
In July 2003, Watson and Par filed ANDAs with the FDA to be approved as generics for AndroGel. In response to these ANDAs, Solvay filed patent infringement lawsuits against these two companies to block the approval and marketing of the generic products. In 2006, all the subject companies reached an agreement pursuant to which Watson and Par agreed not to bring a generic to Androgel to the market until August 2015. During this time frame, Solvay, Watson and Par will co-promote AndroGel. In June 2009, Perrigo Israel Pharmaceuticals, Inc. (“Perrigo”) filed an ANDA with a paragraph IV certification seeking the approval of a generic version of Androgel in the United States. The paragraph IV certification procedure challenges a U.S. patent relating to Androgel which runs through the next decade. In its letter to the company, Perrigo asserted that it did not infringe the AndroGel patent because its proposed testosterone gel product contains a different formulation than the formulation protected by the AndroGel patent. In contrast, the proposed testosterone gel products that were the subject of earlier ANDAs filed by other companies, and which were the subject of prior patent litigation brought by the company, purported to be identical to the AndroGel formulation. To date, Solvay has not initiated patent infringement litigation against Perrigo. The introduction of a generic version of Androgel would have an adverse impact on the branded gel market, including taking a significant portion of branded Androgel business. Although Testim has a BX rating, meaning that pharmacists are prohibited from substituting Testim with Androgel, or a generic version of Androgel, a less expensive generic testosterone gel product could still have a material adverse affect on Testim market share and/or Testim’s formulary status, and therefore could have a material negative impact on our financial condition and results of operations.
In October 2008, we and our licensor, CPEX, received notice that Upsher-Smith filed an ANDA containing a paragraph IV certification seeking approval from the FDA to market a generic version of Testim prior to the January 2025 expiration of ‘968 Patent. The ‘968 Patent, which Bentley assigned to CPEX in June 2008, covers a method for maintaining effective blood serum testosterone levels for treating a hypogonadal male using Testim and is listed in the Orange Book, published by the FDA. The paragraph IV certification sets forth allegations that the ‘968 Patent will not be infringed by Upsher-Smith’s manufacture, use or sale of the product for which the ANDA was submitted. In December 2008, we filed a patent infringement lawsuit against Upsher-Smith that remains pending. Upsher-Smith has alleged that its proposed generic version of Testim would not infringe the ‘968 patent, and that the ‘968 patent is invalid. Under the Hatch-Waxman Act, final FDA approval of Upsher-Smith’s proposed generic product will be stayed until the earlier of 30 months from receipt
39
Table of Contents
of the paragraph IV certification (April 2011) or resolution of the patent infringement lawsuit. Should Upsher- Smith receive a tentative approval of its generic version of Testim from the FDA, it cannot lawfully launch its generic version of Testim in the U.S. before the earlier of the expiration of the currently pending 30-month stay or a district court decision in its favor. In October 2009, six additional patents covering Testim were issued and have been listed in the Orange Book. These additional patents may provide us with further market protection.
Upsher-Smith will not be able to lawfully launch a generic version of Testim in the U.S. without the necessary final approval from the FDA. In February 2009, the Company filed a Citizen’s Petition with the FDA requesting that Upsher-Smith be required to perform a variety of studies to demonstrate that the inactive ingredients and formulation of the proposed product do not compromise its safety or efficacy. An FDA response to the Company’s Citizen’s Petition was received in August 2009. The FDA agreed with some of the statements made in the Citizen’s Petition regarding the testing required for generic versions of Testim and disagreed with other statements. Although not commenting upon any filing in particular, the FDA did state that “the practical effect of this determination is that any application for a testosterone gel product that has different penetration enhancers than the reference listed drug cannot be submitted as an abbreviated new drug application (ANDA) and, instead, will have to be submitted as a NDA under section 505(b) of the [Federal Food, Drug, and Cosmetic Act].”
Other pharmaceutical companies may develop generic versions of any non-biologic products that we commercialize that do not infringe our patents or other proprietary rights. Governmental and other cost containment pressures may result in physicians writing prescriptions for these generic products or allow physicians to substitute AB-rated generics.
Future Delivery Options
In addition to potential generic competition, several other pharmaceutical companies have TRT products in development that may be approved for marketing in the U.S. and the rest of the world. Based on publicly available information, we believe that these products are in the development phases noted below.
Phase III—In 2009, Endo Pharmaceuticals (“Endo”) acquired Indevus Pharmaceuticals, Inc. (“Indevus”), the makers of Aveed (previously known as “Nebido” in the U.S.) as well as the U.S. marketing rights for Prostrakan’s Fortesta (also known as Fortigel, a 2% gel). Aveed’s Prescription Drug User Fee Act (“PDUFA”) date was September 2, 2009 and the FDA announced that it would require an additional 90 days to review the filing. In December 2009, Endo received a complete response letter from the FDA, asking for more information on rare but serious incidents of anaphylactic reactions and pulmonary oil microembolism among patients taking Aveed. The FDA also said in its complete response letter that Endo’s risk mitigation program for the drug is inadequate. It is not known whether Endo will resubmit the application for Aveed. The FDA also sent a complete response letter to Endo regarding Fortesta in October 2009. Endo has announced that it expects to respond to the FDA’s concerns with respect to Fortesta by mid-2010.
Abbott (formerly Solvay) is awaiting FDA feedback on Androgel 1.62%, a high concentration gel. The PDUFA date was at the end of December 2009. However, the FDA notified Abbott that the agency extended its review of the NDA for Androgel 1.62% by 90 days. Abbott expects the FDA to issue an action by the end of the first quarter 2010.
BioSante Pharmaceuticals/Teva Pharmaceutical Industries Ltd. are developing a testosterone gel (Bio-T-Gel) for male hypogonadism and have announced they expect to submit a NDA in the first half of 2010. Acrux Limited is developing a metered-dose lotion which completed phase III in the third quarter of 2009 and was submitted to the FDA in January 2010.
Phase II—Clarus Therapeutics, Inc. is developing an oral testosterone which is in phase II.
Other—Another potential introduction into the marketplace is an oral therapy called Androxal from Repros Therapeutics, Inc. (“Repros”). This molecule is designed to restore normal testosterone production in
40
Table of Contents
males rather than externally replacing testosterone like the current alternatives. However, Repros has been informed by the FDA that testosterone levels or Quality of Life measures will not be acceptable endpoints for phase III trials for hypogonadism. Hence, Repros has announced that it is considering other potential indications. Repros also announced that they participated in a teleconference with the Division of Reproductive and Urologic Products of the FDA on January 25, 2010. The primary purpose of the meeting was to gain a better understanding of the FDA’s position regarding the use of Repros’ oral Androxal® product in the treatment of men with secondary hypogonadism wishing to preserve their fertility. Repros’ data supports the notion that the negative feedback of exogenous testosterone administered by any route suppresses the hypothalamic–pituitary axis and hence spermatogenesis. The discussion with the FDA focused on two issues. First, the FDA requested that Repros propose a label that better defines the population of individuals for whom Repros believes will benefit from the use of Androxal. Second, the FDA requested Repros conduct a literature review of the incidence of infertility associated with the use of exogenous testosterone as supportive of Repros’ data. The FDA requested that Repros provide such information. During the course of the meeting Repros noted that if the FDA concurred with the proposed indication, Repros would like to request a special protocol assessment (“SPA”). The FDA responded that the issues noted above should be resolved first before undertaking a SPA.
MacroChem Corporation has developed a testosterone gel that has completed phase I and is currently looking for a partner to assist with entering phase II development. Other new treatments are being sought for TRT, including a new class of drugs called Selective Androgen Receptor Modulators (“SARMs”), as well as enhanced hydroalcoholic gel formulation of hydrotestosterone. These products are in development and their future impact on the treatment of testosterone deficiency is unknown. Lipocine Inc. (“Lipocine”) is also developing an oral testosterone product that we believe is in phase I at this time. In May 2009, Solvay, recently acquired by Abbott, announced a license agreement with Lipocine that provides Solvay exclusive rights to develop and commercialize an oral formulation of testosterone.
If testosterone replacement therapies are perceived, or are found, to create health risks, our sales of Testim may decrease and our operations may be harmed.
Publications have, from time to time, suggested potential health risks associated with TRT. Potential health risks are described in various articles, including a 2002 article published in Endocrine Practice and a 1999 article published in the International Journal of Andrology. The potential health risks detailed are fluid retention, sleep apnea, breast tenderness or enlargement, increased red blood cells, development of clinical prostate disease, including prostate cancer, increased cardiovascular disease risk and the suppression of sperm production. In April 2009, FDA informed the Company that it had become aware, through spontaneous post-marketing adverse event reports and peer-reviewed biomedical literature, of cases of secondary exposure of children to testosterone due to drug transfer from adult males using testosterone gel drug products. FDA considered this information to be “new safety information” and requested changes to the prescribing information for Testim, including a “boxed warning”, which is used to highlight warning information that is especially important to the prescriber. FDA also required a REMS that includes assessments and a Medication Guide to inform patients. It is possible that studies on the effects of TRT could demonstrate these or other health risks. This, as well as negative publicity about the risks of hormone replacement therapy, including TRT, could adversely affect patient or prescriber attitudes and impact Testim sales. In addition, investors may become concerned about these issues and decide to sell our common stock. These factors could adversely affect our business and the price of our common stock.
International commercialization of our products and our product candidates faces significant obstacles.
We may commercialize some of our products, and product candidates, if they are approved, internationally on our own or through collaborative relationships with foreign partners. We have limited foreign regulatory, clinical and commercial resources. We may not be able to enter into collaboration agreements with appropriate partners for important foreign markets on acceptable terms, or at all. Future collaborations with foreign partners may not be effective or profitable for us.
41
Table of Contents
We could be negatively impacted by future interpretation or implementation of federal and state fraud and abuse laws, including anti-kickback laws, false claims laws and federal and state anti-referral laws.
We are subject to various federal and state laws pertaining to health care fraud and abuse, including anti-kickback laws, false claims laws and physician self-referral laws. Violations of these laws are punishable by criminal and/or civil sanctions, including, in some instances, imprisonment and exclusion from participation in federal and state health care programs, including Medicare, Medicaid, Department of Defense and Veterans’ health programs. We have not been challenged by a governmental authority under any of these laws and believe that our operations are in compliance with such laws.
However, because of the far-reaching nature of these laws, we may be required to alter one or more of our practices to be in compliance with these laws. Health care fraud and abuse regulations are complex, and even minor, inadvertent irregularities can potentially give rise to claims that the law has been violated. Any violations of these laws could result in a material adverse effect on our business, financial condition and results of operations. If there is a change in law, regulation or administrative or judicial interpretations, we may have to change our business practices or our existing business practices could be challenged as unlawful, which could have a material adverse effect on our business, financial condition and results of operations.
We could become subject to false claims litigation under federal or state statutes, which can lead to civil money penalties, criminal fines and imprisonment, and/or exclusion from participation in federal health care programs. These false claims statutes include the federal False Claims Act, which allows any person and/or the government to bring suit alleging the false or fraudulent submission of claims for payment under federal programs or other violations of the statute and to share in any amounts paid by the entity to the government in fines or settlement. Such suits, known as qui tam actions, have increased significantly in recent years and have increased the risk that companies like us may have to defend a false claim action. We could also become subject to similar false claims litigation under state statutes. If we are unsuccessful in defending any such action, such action may have a material adverse effect on our business, financial condition and results of operations.
We may be exposed to liability claims associated with the use of hazardous materials and chemicals.
Our research and development activities and our commercial product, Testim, involve the use of testosterone and large amounts of alcohol which are classified as hazardous materials and chemicals. XIAFLEX, approved in the U.S. for the treatment of Dupuytren’s, and in development for the treatment of Peyronie’s and Frozen Shoulder syndrome, is a biologic product. Biologic products may present a manufacturing health hazard due to risk of infection with the bacterial cell line used to produce the product or with potential bacteriophage contamination with the fermentation. Although we believe that our safety procedures for using, storing, handling, manufacturing and disposing of these materials comply with federal, state and local laws and regulations, we cannot completely eliminate the risk of accidental injury or contamination from these materials. In the event of such an accident, we could be held liable for any resulting damages and any liability could materially adversely affect our business, financial condition and results of operations. In addition, the federal, state and local laws and regulations governing the use, manufacture, storage, handling and disposal of hazardous or radioactive materials and waste products may require us to incur substantial compliance costs that could materially adversely affect our business and financial condition. To our knowledge, we have not been the subject of any investigation by any agency or authority for failure to comply with any rules or regulations applicable to hazardous materials or chemicals. We do not maintain specific insurance for the handling of biological, hazardous and radioactive materials. We have contracts with third-party providers for the storage and disposal of hazardous waste and believe that any claims against us in these areas would be the responsibility of these third parties. However, we may be held responsible for these claims despite the fact that we have contracted with third parties for the storage and disposal of hazardous waste. If we are exposed to these types of claims, we could be held responsible for liabilities that exceed our financial resources, which could severely affect our operations.
42
Table of Contents
Risks Related to the Manufacture of XIAFLEX
We have limited experience in manufacturing pharmaceutical and biologic products and may encounter difficulties in the manufacture of the active ingredient of XIAFLEX at our facilities in Horsham, Pennsylvania which could materially adversely affect our results of operations or delay or disrupt manufacture of XIAFLEX and development timelines.
The manufacture of pharmaceutical and biologic products requires significant expertise and capital investment. Although we leased our facilities in Horsham in order to have direct control over the manufacturing of the active ingredient of XIAFLEX, for which we expect to be the sole supplier, we have limited experience in manufacturing XIAFLEX or any other pharmaceutical product. Biologics, such as XIAFLEX, require processing steps that are highly complex and more difficult than those required for most chemical pharmaceuticals. We may encounter difficulties with the manufacture of the active ingredient of XIAFLEX which could delay or disrupt our manufacture of XIAFLEX and development timelines, require write-offs which may affect our financial results, result in product recalls, or otherwise materially affect our results of operations. These problems with manufacturing may include:
| • | our ability to develop, implement and improve our internal manufacturing capability; |
| • | difficulties with production and yields, including the viability of the working cell bank and cell growth at lower than expected levels, scale-up and achieving adequate capacity for such supply; |
| • | availability of raw materials and supplies; |
| • | contamination issues; |
| • | equipment failures; |
| • | quality control and assurance; |
| • | shortages of qualified personnel; |
| • | compliance with strictly enforced federal, state and foreign regulations; and |
| • | lack of capital funding. |
Furthermore, our manufacturing operations expose us to a variety of significant risks, including:
| • | product defects and potential product liability claims; |
| • | contamination of product or product loss; |
| • | environmental liabilities or claims resulting from our production process or contamination at our Horsham facilities; |
| • | sudden loss of inventory; |
| • | reduction of the commercialization payments owed by Pfizer under our agreement and Pfizer gaining the right to engage a third party manufacturer for XIAFLEX; |
| • | Pfizer Inc. (“Pfizer”) termination of the license agreement for breach of contract; and |
| • | inability to manufacture products at a cost that is competitive with third party manufacturing operations. |
If we are unable to maintain regulatory approval for XIAFLEX, we may not have an alternate use for our Horsham facilities but will be required to make payments under our lease.
We have entered into leases for our facilities in Horsham, the first of which expires on January 1, 2017. If we are unable to maintain regulatory approval for XIAFLEX for Dupuytren’s, we may not have an alternate use for the Horsham facilities but will be required to make payments under our leases. As of December 31, 2009, the total future minimum lease payments of these leases during their initial noncancellable terms are approximately $29.5 million.
43
Table of Contents
Our Horsham facilities are subject to regulatory oversight, which may delay or disrupt our development and commercialization efforts for XIAFLEX.
We must ensure that all of the processes, methods, equipment and facilities employed in the manufacture of XIAFLEX at our Horsham facility are compliant with the current Good Manufacturing Practices (“cGMP”). The cGMP requirements govern quality control of the manufacturing process and documentation policies and procedures. Compliance with cGMP requires record keeping and quality control to assure that the clinical and commercial product meets applicable specifications and other requirements. Some of our Horsham facilities were inspected by regulatory agencies after the filing for approval of XIAFLEX with regulatory authorities. If a subsequent inspection by regulatory authorities indicates that there are deficiencies including non-compliance with regulatory requirements, we could be required to take remedial actions, stop production or close ours Horsham facilities, which would disrupt the manufacturing processes, limit the supplies of XIAFLEX and delay clinical trials and subsequent licensure. If we fail to comply with these requirements, we may not be permitted to sell our products or may be limited in the jurisdictions in which we are permitted to sell them.
Future noncompliance with any applicable regulatory requirements may result in refusal by regulatory authorities to allow use of XIAFLEX made at our Horsham facilities in clinical trials, refusal of the government to allow distribution of XIAFLEX for commercialization, criminal prosecution and fines, recall or seizure of products, total or partial suspension of production, prohibitions or limitations on the commercial sale of products or refusal to allow the entering into of federal and state supply contracts.
Risks Related to Our Dependence on Third-Party Manufacturers and Service Providers
Since we currently rely on third-party manufacturers, suppliers and packagers, we may be unable to control the availability or cost of manufacturing and packaging our products, which could adversely affect our results of operations.
We currently do not manufacture Testim or any of our product candidates, except for the active ingredient for XIAFLEX for which we are the sole source of supply. Hollister-Stier Laboratories LLC (“Hollister-Stier”) fills and lyophilizes the XIAFLEX bulk drug substance that we manufacture and produces sterile diluent. Testim is manufactured for us by DPT Laboratories, LTD. (“DPT”), under a contract that expires on December 31, 2010. We have qualified a back-up supplier to manufacture Testim. We also rely on third parties for certain packaging services for our products.
The manufacture of pharmaceutical products requires significant expertise and capital investment. Hollister-Stier, DPT, our back-up supplier for Testim, or any other third-party manufacturer or packager may encounter difficulties in production. These problems may include:
| • | difficulties with production costs and yields; |
| • | availability of raw materials and supplies; |
| • | quality control and assurance; |
| • | damage to, or complete loss of, raw materials, supplies or finished product; |
| • | shortages of qualified personnel; |
| • | compliance with strictly enforced federal, state and foreign regulations; and |
| • | lack of capital funding. |
Hollister-Stier, DPT, or any of our other third-party manufacturers and packagers may not perform as agreed or may terminate its agreement with us, which would adversely impact our ability to produce and sell our products or produce our product candidates for use in clinical trials. The number of third-party manufacturers with the expertise, required regulatory approvals and facilities to manufacture bulk drug substance on a
44
Table of Contents
commercial scale is limited, and it would take a significant amount of time to arrange and receive regulatory approval for alternative arrangements. We may not be able to contract for the manufacturing of our products or any of our product candidates on acceptable terms, if at all, which would materially impair our business.
Any of these factors could increase our costs and result in us being unable to effectively commercialize or develop our products. Furthermore, if any third-party manufacturer fails to deliver the required commercial quantities of finished product on a timely basis and at commercially reasonable prices, we may be unable to meet the demand for our products and we may lose potential revenues.
We currently rely on single source suppliers for certain raw materials and services for manufacturing XIAFLEX and the loss of any of these suppliers could prevent us from selling XIAFLEX, which would materially harm our business.
We rely on third-party suppliers for our supply of raw materials for the manufacture of the XIAFLEX bulk drug substance. Certain raw materials are available to us from only limited sources and are sole sourced. We do not have supply agreements in place with all of our raw material suppliers. We rely on one supplier for lyophilization and sterile diluent and we rely on one other supplier for labeling and packaging of drug product. If any of the suppliers stops manufacturing, or if we are unable to procure raw materials or services on commercially favorable terms, or if we are not able to obtain them in a timely manner, we may be unable to continue to produce or sell XIAFLEX on commercially viable terms, if at all. In addition, the limited number of suppliers of these raw materials and services with whom we do not have supply agreements in place may provide such companies with greater opportunity to raise their prices. Any increase in price for these raw materials or services may reduce our gross margins.
We rely on a single source supplier and a limited number of suppliers for two of the primary ingredients for Testim and the loss of any of these suppliers could prevent us from selling Testim, which would materially harm our business.
We rely on third-party suppliers for our supply of testosterone and CPD, two key ingredients of Testim. Testosterone is available to us from only two sources. We rely exclusively on one outside source for our supply of CPD. We do not have any agreements with these suppliers regarding these key ingredients. If either of the two sources that produce testosterone stops manufacturing it, or if we are unable to procure testosterone on commercially favorable terms, or if we are not able to obtain it in a timely manner, we may be unable to continue to produce or sell Testim on commercially viable terms, if at all. In addition, if our third-party source of CPD stops manufacturing pharmaceutical grade CPD, or does not make CPD available to us on commercially favorable terms, we may be unable to continue to produce or sell Testim on commercially viable terms, if at all. Furthermore, the limited number of suppliers of testosterone and CPD may provide such companies with greater opportunity to raise their prices. Any increase in price for testosterone or CPD may reduce our gross margins.
XIAFLEX is distributed through a small managed network of third-party distributors and we rely on a single third party as a contact center of approved healthcare providers; if any of the distributors fail or refuse to distribute XIAFLEX on commercially favorable terms, or at all, or if the contact center fails or refuses to perform its function, our business could be adversely affected.
We distribute XIAFLEX through a small managed network of third party specialty distributors, specialty pharmacies and a third party logistics supplier that generally sell, distribute or provide XIAFLEX to hospitals or physicians. In addition, we rely on a single third party as a contact center service for approved customers. All of our XIAFLEX shipments are through these distributors. Our business would be harmed if any of these distributors refused to distribute XIAFLEX or refuse to purchase XIAFLEX on commercially favorable terms to us. It is possible that distribution partners could decide to change their policies or fees, or both, in the future. This could result in their refusal to distribute smaller volume products, or cause higher product distribution costs, lower margins or the need to find alternative methods of distributing products. Such alternative methods may not
45
Table of Contents
exist or may not be economically viable. In addition, we are subject to a number of risks associated with our dependence on third parties for distribution of XIAFLEX which could adversely affect sales of XIAFLEX and our business, including:
| • | the single third party contact center we rely on could fail or refuse to provide service, or suffer systems failures; |
| • | one of the small number of third party distributors we rely on to warehouse and distribute XIAFLEX could experience damage to, or complete loss of, XIAFLEX inventory in its possession; and |
| • | one of the small number of third party distributors we rely on to warehouse and distribute XIAFLEX could fail or refuse to comply with regulatory or contractual obligations, which could negatively impact sales of XIAFLEX. |
If we are unable to grow our sales, marketing or distribution capabilities or enter into agreements with third parties to perform some of these functions, we will not be able to grow our business.
As an organization, we have a limited history in sales, marketing and distribution. To directly market and distribute Testim, XIAFLEX for Dupuytren’s in the U.S., or any of our product candidates which receive regulatory approval, we must continue to enhance our sales and marketing efforts and our distribution capabilities. For our direct sales efforts, we will need to hire additional salespeople in order to grow and manage any turnover that occurs in our sales force. For some market opportunities, we may need to enter into co-promotion agreements or other licensing arrangements with pharmaceutical or biotechnology firms in order to increase the commercial success of our products.
We may not be able to grow our direct sales, marketing and distribution capabilities or enter into future co-promotion or similar licensing arrangements with third parties in a timely manner, or on acceptable terms. To the extent that we enter into future co-promotion or other licensing arrangements, our product revenues may be lower than if we directly marketed and sold our products, and some or all of the revenues we receive will depend upon the efforts of third parties, and these efforts may not be successful or our future partners may not devote sufficient resources to our products. Additionally, building marketing and distribution capabilities may be more expensive than we anticipate, requiring us to divert capital from other intended purposes or preventing us from building our marketing and distribution capabilities to the desired levels.
Our third-party manufacturers are subject to regulatory oversight, which may delay or disrupt our development and commercialization efforts.
Third-party manufacturers of our products or product candidates must ensure that all of the processes, methods and equipment are compliant with cGMP, and conduct extensive audits of vendors, contract laboratories and suppliers. The cGMP requirements govern quality control of the manufacturing process and documentation policies and procedures. Compliance by third-party manufacturers with cGMP requires record keeping and quality control to assure that the product meets applicable specifications and other requirements. Manufacturing facilities are subject to inspection by regulatory agencies at any time. If an inspection by regulatory authorities indicates that there are deficiencies, third-party manufacturers could be required to take remedial actions, stop production or close the facility, which would disrupt the manufacturing processes and limit the supplies of Testim or our product candidates. If they fail to comply with these requirements, we also may be required to curtail the clinical trials of our product candidates, which are also supplied by these manufacturers, and may not be permitted to sell our products or may be limited in the jurisdictions in which we are permitted to sell them.
Over 90% of our Testim shipments are to only three customers; if any of these customers refuse to distribute Testim on commercially favorable terms, or at all, our business will be adversely affected.
We sell Testim to wholesale drug distributors and chain drug stores who generally sell products to retail pharmacies, hospitals and other institutional customers. We do not promote Testim to these customers, and they
46
Table of Contents
do not determine Testim prescription demand. However, over 90% of our product shipments during the period covered by this Report were to only three customers: AmerisourceBergen Corporation, Cardinal Health, Inc. and McKesson Corporation. Our business would be harmed if any of these customers refused to distribute Testim or refuse to purchase Testim on commercially favorable terms to us.
It is possible that wholesalers could decide to change their policies or fees, or both, in the future. This could result in their refusal to distribute smaller volume products, or cause higher product distribution costs, lower margins or the need to find alternative methods of distributing products. Such alternative methods may not exist or may not be economically viable.
Due to our reliance on contract research organizations or other third parties to assist us in conducting clinical trials, we are unable to directly control all aspects of our clinical trials.
Currently, we rely in part on third parties to conduct our clinical trials. As a result, we have had and will continue to have less control over the conduct of the clinical trials, the timing and completion of the trials and the management of data developed through the trial than would be the case if we were relying entirely upon our own staff. Communicating with outside parties can also be challenging, potentially leading to mistakes as well as difficulties in coordinating activities. Outside parties may:
| • | have staffing difficulties; |
| • | fail to comply with contractual obligations; |
| • | experience regulatory compliance issues; or |
| • | undergo changes in priorities or may become financially distressed. |
These factors may adversely affect their willingness or ability to conduct our trials. We may experience unexpected cost increases that are beyond our control. Problems with the timeliness or quality of the work of a contract research organization may lead us to seek to terminate the relationship and use an alternative service provider. However, making this change may be costly and may delay our trials, and contractual restrictions may make such a change difficult or impossible. Our contracts with the contract research organizations on which we currently rely are generally terminable upon 30-days prior written notice. If we must replace any of these contract research organizations or any other contract research organization we may use in the future to conduct our clinical trials, our trials may have to be suspended until we find another contract research organization that offers comparable services. The time that it takes us to find alternative organizations may cause a delay in the commercialization of our product candidates or may cause us to incur significant expenses to replicate data that may be lost. Although we do not believe that the contract research organizations on which we rely offer services that are not available elsewhere, it may be difficult to find a replacement organization that can conduct our trials in an acceptable manner and at an acceptable cost. Any delay in or inability to complete our clinical trials could significantly compromise our ability to secure regulatory approval of the relevant product candidate and preclude our ability to commercialize the product, thereby limiting our ability to generate revenue from the sales of product candidates, which may result in a decrease in our stock price.
Risks Related to Intellectual Property
We have only limited patent protection for our products and our product candidates, and we may not be able to obtain, maintain and protect proprietary rights necessary for the development and commercialization of our products or our product candidates.
Our business and competitive positions are dependent upon our ability to obtain and protect our proprietary position for our products and our product candidates in the U.S., Europe and elsewhere. Because of the substantial length of time and expense associated with development of new products, we, along with the rest of the biopharmaceutical industry, place considerable importance on obtaining and maintaining patent protection for
47
Table of Contents
new technologies, products and processes. The patent positions of pharmaceutical, biopharmaceutical and biotechnology companies, including ours, are generally uncertain and involve complex legal and factual questions. Our and our licensors’ patents and patent applications may not protect our technologies and products because, among other things:
| • | there is no guarantee that any of our or our licensors’ pending patent applications will result in issued patents; |
| • | we may develop additional proprietary technologies that are not patentable; |
| • | there is no guarantee that any patents issued to us, our collaborators or our licensors will provide us with any competitive advantage or cover our product candidates; |
| • | there is no guarantee that any patents issued to us or our collaborators or our licensors will not be challenged, circumvented or invalidated by third parties; and |
| • | there is no guarantee that any patents previously issued to others or issued in the future will not have an adverse effect on our ability to do business. |
We attempt to protect our intellectual property position by filing or obtaining licenses to patent applications and, where appropriate, patent applications in other countries related to our proprietary technology, inventions and improvements that are important to the development of our business.
Testosterone, the active ingredient in Testim, is off-patent and is included in competing TRT products. In the U.S., the ‘968 patent covers a method for maintaining blood serum testosterone levels for treating a hypogonadal male using Testim and is listed in the Orange Book. The ‘968 patent expires in January 2025. Six additional patents issued covering Testim in October 2009 and have been listed in the Orange Book. They expire in April 2023. CPEX also has filed continuation applications that are pending.
In October 2008, we and our licensor, CPEX, received notice that Upsher-Smith filed an ANDA containing a paragraph IV certification seeking approval from the FDA to market a generic version of Testim prior to the January 2025 expiration of ‘968 Patent. The ‘968 Patent, which Bentley assigned to CPEX in June 2008, covers a method for maintaining effective blood serum testosterone levels for treating a hypogonadal male using Testim and is listed in the Orange Book. The paragraph IV certification sets forth allegations that the ‘968 Patent will not be infringed by Upsher-Smith’s manufacture, use or sale of the product for which the ANDA was submitted. In December 2008, we filed the Upsher-Smith Litigation which remains pending. Upsher-Smith has alleged that its proposed generic version of Testim would not infringe the ‘968 patent, and that the ‘968 patent is invalid. Under the Hatch-Waxman Act, final FDA approval of Upsher-Smith’s proposed generic product will be stayed until the earlier of 30 months beginning on the date of receipt of the paragraph IV certification (April 2011) or resolution of the patent infringement lawsuit. Should Upsher-Smith receive an approval of its generic version of Testim from the FDA, it cannot lawfully launch its generic version of Testim in the U.S. before the earlier of the expiration of the currently pending 30-month stay or a district court decision in its favor. We have incurred, and will continue to incur, costs related to the Upsher-Smith Litigation and regulatory actions. We may incur costs related to any other legal and regulatory actions that we may undertake. The Upsher-Smith Litigation could result in a finding that Upsher-Smith’s proposed testosterone product does not infringe the ‘968 Patent or that the ‘968 Patent is invalid and/or unenforceable. An adverse outcome in the Upsher-Smith Litigation or any such legal action could result in one or more generic versions of Testim being launched in the U.S. before the expiration of the ‘968 Patent in January 2025. The introduction of a generic version to Testim or AndroGel could have a material adverse affect on our ability to successfully execute our business strategy to maximize the value of Testim as we continue to develop our product pipeline and therefore could have a material negative impact on our financial condition and results of operations.
In Canada, there are two patents covering Testim, one that expired in January 2010 and another that expires in April 2023. The patent issued by the European Patent Office covering Testim expires in April 2023. CPEX
48
Table of Contents
also has submitted a patent application in Japan that is awaiting examination. All of these patents and any patents that issue from the pending applications are included in our license from CPEX.
XIAFLEX, licensed from BioSpecifics Technologies Corp. (“BioSpecifics”) is covered by two method of use patents in the U.S., one for the treatment of Dupuytren’s and one for the treatment of Peyronie’s disease (“Peyronie’s”). The Dupuytren’s patent, which was issued in December 2007 after a reissue proceeding, expires in 2014, and the Peyronie’s patent expires in 2019. The patents are limited to the use of XIAFLEX for the treatment of Dupuytren’s and Peyronie’s, respectively, with certain dose ranges and/or concentration ranges. In January 2005, a patent application was filed with regard to XIAFLEX for the treatment of Frozen Shoulder syndrome. If this patent is granted, it would expire in 2026 or later if patent term adjustments are granted. In January 2006, we filed a patent application with regard to the manufacturing process for XIAFLEX; if this patent is granted, it would expire in January 2027, subject to patent term adjustments. Currently there is not enough data to establish whether any approved product for Peyronie’s or Frozen Shoulder syndrome will be covered by these patents. Foreign patents and pending applications may also cover these products in certain countries depending upon the concentration and dosage ranges of such products.
Our PharmaForm transmucosal film product candidates are covered by an issued formulation patent in the U.S. and two international patent applications remain pending in several countries worldwide, including the U.S. The issued formulation patent expires in 2020.
The standards that the USPTO and its foreign counterparts use to grant patents are not always applied predictably or uniformly and can change. Limitations on patent protection in some countries outside the U.S. and the differences in what constitutes patentable subject matter in these countries, may limit the protection we seek outside of the U.S. For example, methods of treating humans are not patentable subject matter in many countries outside of the U.S. In addition, laws of foreign countries may not protect our intellectual property to the same extent as would laws of the U.S. Also, some countries will not grant patents on patent applications that are filed after the public sale or disclosure of the material claimed in the patent application. The U.S., by contrast, allows a one year grace period after public disclosure in which to file a patent application. In determining whether or not to seek a patent or to license any patent in a particular foreign country, we weigh the relevant costs and benefits, and consider, among other things, the market potential of our product candidates in the jurisdiction, and the scope and enforceability of patent protection afforded by the law of the jurisdiction. Failure to obtain adequate patent protection for our proprietary product candidates and technology would impair our ability to be commercially competitive in these markets. Accordingly, we do not know the degree of future protection for our proprietary rights or the breadth of claims allowed in any patents issued to us or others.
We intend to seek patent protection when appropriate for any products or product candidates we acquire in the future. However, any patent applications for future products may not issue as patents, and any patent issued on such products may be challenged, invalidated, held unenforceable or circumvented. Furthermore, the claims in patents which have been issued on products we may acquire in the future may not be sufficiently broad to prevent third parties from commercializing competing products. If we fail to obtain adequate patent protection for our products, our ability to compete could be impaired.
Technology in-licensed by us is important to our business. We may not control the patent prosecution, maintenance or enforcement of our in-licensed technology. Accordingly, we may be unable to exercise the same degree of control over this intellectual property as we would over our internally developed intellectual property. For example, in-licensed patents for which our rights are limited to a particular field of use could be or become licensed in other fields to other licensees and, therefore, become subject to enforcement by such other licensees without our participation. Consequently, such licensed patents could be held invalid or unenforceable or could have claims construed in a manner adverse to our interests in litigation, which we would not control or to which we would not be a party. If any of the intellectual property rights of our licensors is found to be invalid, this could have a material adverse impact on our operations.
49
Table of Contents
If we are not able to protect and control unpatented trade secrets, know-how and other technological innovation, we may suffer competitive harm. We also rely on trade secrets, know-how and technology, which are not protected by patents, to maintain our competitive position. However, trade secrets are difficult to protect. To maintain the confidentiality of trade secrets and proprietary information, we generally seek to enter into confidentiality agreements with our employees, consultants and collaborators upon the commencement of a relationship with us. However, we may not obtain these agreements in all circumstances. Nor can we guarantee that these agreements will provide meaningful protection, that these agreements will not be breached, or that we will have an adequate remedy for any such breach. In addition, adequate remedies may not exist in the event of unauthorized use or disclosure of this information. Others may have developed, or may develop in the future, substantially similar or superior know-how and technology. The loss or exposure of our trade secrets, know-how and other proprietary information, as well as independent development of similar or superior know-how, could harm our operating results, financial condition and future growth prospects. Many of our employees and consultants were, and many of our consultants may currently be, parties to confidentiality agreements with other companies. Although our confidentiality agreements with these employees and consultants require that they do not bring to us, or use without proper authorization, any third party’s proprietary technology, if they violate their agreements, we could suffer claims or liabilities.
If we breach any of the agreements under which we license commercialization rights to products or technology from others, we could lose license rights that are critical to our business.
We are a party to a number of license agreements by which we have rights to use the intellectual property of third parties that are necessary for us to operate our business. In particular, we have obtained the exclusive right to develop and commercialize Testim pursuant to a license agreement with CPEX. CPEX may unilaterally terminate the agreement if we fail to make payments under this agreement and this failure continues for a period of 30 days following written notice to us by CPEX. If the agreement is properly terminated by CPEX, we may not be able to manufacture or sell Testim.
Additionally, we have obtained exclusive rights to make and sell products that are used for hormone replacement, to treat any type of urologic disorder or as a pain therapy incorporating PharmaForm’s transmucosal film technology. This agreement continues for the life of the licensed patent rights. Either party may terminate this agreement under certain events of bankruptcy or insolvency by the other party. PharmaForm may unilaterally terminate this agreement if:
| • | we fail to make payments under this agreement and this failure continues for a period of 30 days following written notice to us by PharmaForm; or |
| • | we fail to initiate clinical trials within two years of availability of final formulation in quantities adequate for clinical testing and associated documentation for clinical trials. |
If our agreement with PharmaForm is properly terminated by PharmaForm, we may not be able to execute our strategy to develop, manufacture or sell transmucosal film products. To the extent that unpatented PharmaForm trade secrets are necessary for the manufacture of the transmucosal product candidates, our license to such trade secrets would expire with the expiration of the licensed patents in 2020 or later and potentially impair our continued commercialization of our transmucosal product candidates thereafter.
We have also obtained exclusive worldwide rights from BioSpecifics to develop, market and sell products, other than dermal formulations labeled for topical administration, that contain BioSpecifics’ enzyme, which we refer to as XIAFLEX, for the treatment of Dupuytren’s, Peyronie’s and Frozen Shoulder syndrome. We may expand the agreement, at our option, to license products which contain BioSpecifics’ enzyme for other indications, excluding dermal formulations labeled for topical administration, as they are developed by BioSpecifics or by us. This agreement continues on a product by product and country by country basis until the later of:
| • | the last to expire valid claim of a patent covering such product; |
50
Table of Contents
| • | the expiration of the regulatory period conveyed by orphan drug designation with respect to such product; and |
| • | 12 years. |
Upon expiration of the agreement pursuant to the preceding sentence, we will have a fully paid-up license to the products developed under the agreement. Either party may terminate this agreement in the event of bankruptcy or insolvency by the other party. Additionally, either party may terminate this agreement if the other party is in material breach of its obligations under the agreement which continues for a period of 90 days following receipt of written notice of such material breach. We may terminate this agreement in its entirety, or on a country by country basis, on an indication by indication basis, or on a product by product basis, at any time upon 90 days prior written notice to BioSpecifics. If this agreement is properly terminated by BioSpecifics, we may not be able to execute our strategy to commercialize XIAFLEX for Dupuytren’s or to develop and commercialize XIAFLEX for the treatment of Peyronie’s or Frozen Shoulder syndrome or future product candidates utilizing BioSpecifics’ enzyme.
We expect to enter into additional licenses in the future. These licenses may impose various development, commercialization, funding, royalty, diligence or other obligations on us. If we breach any of these obligations, the licensor may have the right to terminate the license or render the license non-exclusive, which could make it impossible for us to develop, manufacture or sell the products covered by the license.
Disputes may arise with respect to our licensing agreements regarding manufacturing, development and commercialization of any products relating to our in-licensed intellectual property. These disputes could lead to delays in or termination of the development, manufacture and commercialization of our products or our product candidates or to litigation.
We may not be able to obtain or maintain orphan drug exclusivity for our products or product candidates, and our competitors may obtain orphan drug exclusivity prior to us, which could significantly harm our business.
Some jurisdictions, including Europe and the U.S., may designate drugs intended to treat relatively small patient populations as orphan drugs. The FDA granted orphan drug status to XIAFLEX in the U.S. for the treatment of Dupuytren’s and Peyronie’s. Orphan drug designation must be requested before submitting an application for marketing authorization. Orphan drug designation may not convey any advantage in, or shorten the duration of, the regulatory review and approval process, but does make the product eligible for orphan drug exclusivity and specific tax credits in the U.S. Generally, if a product with an orphan drug designation subsequently receives the first marketing approval for the indication for which it has such designation, the product is entitled to orphan drug exclusivity. Orphan drug exclusivity means that another application to market the same drug for the same indication may not be approved, except in limited circumstances, for a period of up to 10 years in Europe and for a period of seven years in the U.S. Maintaining orphan drug designations and orphan drug exclusivity for XIAFLEX for the treatment of Dupuytren’s and Peyronie’s may be critical to their success. Our competitors may obtain orphan drug exclusivity for products competitive with our product candidates before we do, in which case we would be excluded from that market. Even if we obtain orphan drug exclusivity for any of our product candidates, we may not be able to maintain it. For example, if a competitive product is shown to be different or clinically superior to our product, any orphan drug exclusivity we have obtained will not block the approval of such competitive product. In addition, even if we obtain orphan drug exclusivity for any of our product candidates, a viable commercial market may never develop and we may never derive any meaningful revenues from the sales of these products.
Our ability to market our products may be impaired by the intellectual property rights of third parties.
Our commercial success will depend in part on not infringing the patents or proprietary rights of third parties. We are aware of competing intellectual property relating to the TRT gel market. While we currently
51
Table of Contents
believe we have freedom to operate in the TRT gel market, others may challenge our position in the future. We are also aware of third-party patents relating to PharmaForm’s transmucosal film delivery system that may impair PharmaForm’s freedom to operate in the manufacture of product candidates using a transmucosal film delivery system. There has been, and we believe that there will continue to be, significant litigation in the pharmaceutical industry regarding patent and other intellectual property rights.
Third parties could bring legal actions against us claiming damages and seeking to enjoin clinical testing, manufacturing and marketing of the affected product or products. A third party might request a court to rule that the patents we in-license are invalid or unenforceable. In such a case, even if the validity or enforceability of those patents were upheld, a court might hold that the third party’s actions do not infringe the patent we in-license thereby, in effect, limiting the scope of our patent rights. If we become involved in any litigation, it could consume a substantial portion of our resources, regardless of the outcome of the litigation. If any of these actions are successful, in addition to any potential liability for damages, we could be required to obtain a license to continue to manufacture or market the affected product, in which case we may be required to pay substantial royalties or grant cross-licenses to our patents. However, there can be no assurance that any such license will be available on acceptable terms or at all. Ultimately, we could be prevented from commercializing a product, or forced to cease some aspect of our business operations, as a result of patent infringement claims, which could harm our business.
If our products or our future products infringe the intellectual property of our competitors or other third parties, we may be required to pay license fees or cease these activities and pay damages, which could significantly harm our business.
Even though our products and our product candidates may be covered by patents, they may nonetheless infringe the patents or violate the proprietary rights of third parties. In these cases, we may be required to obtain licenses to patents or proprietary rights of others in order to continue to sell and use our products and develop and commercialize our product candidates. We may not, however, be able to obtain any licenses required under any patents or proprietary rights of third parties on acceptable terms, or at all. Even if we were able to obtain rights to a third party’s intellectual property, these rights may be non-exclusive, thereby giving our competitors potential access to the same intellectual property.
Third parties may assert patent or other intellectual property infringement claims against us, or our licensors or collaborators, with respect to technologies used in potential product candidates. Any claims that might be brought against us relating to infringement of patents may cause us to incur significant expenses and, if successfully asserted against us, may cause us to pay substantial damages. We may not have sufficient resources to effectively litigate these claims. Even if we were to prevail, any litigation could be costly and time-consuming and could divert the attention of our management and key personnel from business operations. In addition, any patent claims brought against our licensors or collaborators could affect their ability to carry out their obligations to us.
Furthermore, if a patent infringement suit were brought against us, or our licensors or collaborators, the development, manufacture or potential sale of product candidates claimed to infringe a third party’s intellectual property may have to cease or be delayed. Ultimately, we may be unable to commercialize one or more of our product candidates, our patent claims may be substantially limited or may have to cease some portion of our operations as a result of patent infringement claims, which could severely harm our business.
We may become involved in disputes with Pfizer or potential future collaborators over intellectual property ownership, and publications by our research collaborators and scientific advisors could impair our ability to obtain patent protection or protect our proprietary information, which, in either case, could have a significant impact on our business.
Inventions discovered under research, material transfer or other such collaborative agreements, including our collaboration agreement with Pfizer, may become jointly owned by us and the other party to such agreements
52
Table of Contents
in some cases, and the exclusive property of either party in other cases. Under some circumstances, it may be difficult to determine who owns a particular invention, or whether it is jointly owned, and disputes could arise regarding ownership of those inventions. These disputes could be costly and time consuming, and an unfavorable outcome could have a significant adverse effect on our business if we were not able to protect or license rights to these inventions. In addition, our research collaborators and scientific advisors may have contractual rights to publish our data and other proprietary information, subject to our prior review. Publications by our research collaborators and scientific advisors containing such information, either with our permission or in contravention of the terms of their agreements with us, may impair our ability to obtain patent protection or protect our proprietary information, which could significantly harm our business.
Upsher-Smith’s ANDA filed with a Paragraph IV certification under the Hatch-Waxman Act related to Testim could have a material adverse effect on our financial condition and results of operations as it could result in the introduction of a generic product prior to the expiration of the patent covering Testim, as well as in significant legal expenses and diversion of management time.
In October 2008, we and our licensor, CPEX, received notice that Upsher-Smith filed an ANDA containing a paragraph IV certification seeking approval from the FDA to market a generic version of Testim prior to the January 2025 expiration of the ‘968 Patent. The ‘968 Patent, which Bentley assigned to CPEX in June 2008, covers a method for maintaining effective blood serum testosterone levels for treating a hypogonadal male using Testim and is listed in the Orange Book. The paragraph IV certification sets forth allegations that the ‘968 Patent will not be infringed by Upsher-Smith’s manufacture, use or sale of the product for which the ANDA was submitted. In December 2008, we filed the Upsher-Smith Litigation which remains pending. Upsher-Smith has alleged that its proposed generic version of Testim would not infringe the ‘968 patent, and that the ‘968 patent is invalid. Under the Hatch-Waxman Act, final FDA approval of Upsher-Smith’s proposed generic product will be stayed until the earlier of 30 months beginning on the date of receipt of the paragraph IV certification (April 2011) or resolution of the patent infringement lawsuit. Should Upsher-Smith receive an approval of its generic version of Testim from the FDA, it cannot lawfully launch its generic version of Testim in the U.S. before the earlier of the expiration of the currently pending 30-month stay or a district court decision in its favor. We have incurred, and will continue to incur, costs related to the Upsher-Smith Litigation and regulatory actions. We may incur costs related to any other legal and regulatory actions that we may undertake. The Upsher-Smith Litigation could result in a finding that Upsher-Smith’s proposed testosterone product does not infringe the ‘968 Patent or that the ‘968 Patent is invalid and/or unenforceable. An adverse outcome in the Upsher-Smith Litigation or any such legal action could result in one or more generic versions of Testim being launched in the U.S. before the expiration of the ‘968 Patent in January 2025. Since Testim and XIAFLEX for the treatment of Dupuytren’s in the U.S. are currently our only approved products, the introduction of a generic version of Testim could have a material adverse affect on our ability to successfully execute our business strategy to maximize the value of Testim as we commence the commercial launch of XIAFLEX for Dupuytren’s and continue to develop our product pipeline and therefore could have a material negative impact on our financial condition and results of operations.
We may have to engage in costly litigation to enforce or protect our proprietary technology or to defend challenges to our proprietary technology by our competitors, which may harm our business, results of operations, financial condition and cash flow.
The pharmaceutical field is characterized by a large number of patent filings involving complex legal and factual questions, and, therefore, we cannot predict with certainty whether our licensed patents will be enforceable. Competitors may have filed applications for or have been issued patents and may obtain additional patents and proprietary rights related to products or processes that compete with or are similar to ours. We may not be aware of all of the patents potentially adverse to our interests that may have been issued to others. Litigation may be necessary to protect our proprietary rights, and we cannot be certain that we will have the required resources to pursue litigation or otherwise to protect our proprietary rights.
53
Table of Contents
Competitors may infringe our patents or successfully avoid them through design innovation. To prevent infringement or unauthorized use, we may need to file infringement lawsuits, which are expensive and time-consuming. In any such proceeding, a court may decide that a patent of ours or one that we have licensed is not valid or is unenforceable, may narrowly interpret our patent claims or may refuse to stop the other party from using the technology at issue on the grounds that our patents do not cover its technology. In particular, if a competitor were to file a paragraph IV certification under the U.S. Drug Price Competition and Patent Term Restoration Act of 1984, known as the Hatch-Waxman Act, in connection with that competitor’s submission to the FDA of an ANDA for approval of a generic version of any of our products for which we believed we held a valid patent, then we could initiate a lawsuit against such competitor claiming patent infringement and defending the relevant patent’s validity and enforceability. Depending on the facts and circumstances, the FDA may stay the approval of the ANDA for a generic version of any of our products for 30 months so long as we initiate litigation against the filer of the ANDA within 45 days of receiving the paragraph IV certification. If a court found that one of our patents was invalid or not infringed, then the FDA would be permitted to approve the competitor’s ANDA resulting in a competitive generic product. In the event that the FDA did not grant the 30 month stay, FDA would be permitted to approve the competitor’s ANDA; however, we could engage in legal proceedings, such as an injunction, to attempt to preclude the generic competitor from entering the market during the pendency of the patent litigation, but we may not prevail in which event the competitor could enter the market, despite the ongoing patent litigation.
In October 2008, we and our licensor, CPEX, received notice that Upsher-Smith filed an ANDA containing a paragraph IV certification seeking approval from the FDA to market a generic version of Testim prior to the January 2025 expiration of ‘968 Patent. The ‘968 Patent, which Bentley assigned to CPEX in June 2008, covers a method for maintaining effective blood serum testosterone levels for treating a hypogonadal male using Testim and is listed in the Orange Book. The paragraph IV certification sets forth allegations that the ‘968 Patent will not be infringed by Upsher-Smith’s manufacture, use or sale of the product for which the ANDA was submitted. In December 2008, we filed the Upsher-Smith Litigation which remains pending. Upsher-Smith has alleged that its proposed generic version of Testim would not infringe the ‘968 patent, and that the ‘968 patent is invalid. Under the Hatch-Waxman Act, final FDA approval of Upsher-Smith’s proposed generic product will be stayed until the earlier of 30 months beginning on the date of receipt of the paragraph IV certification (April 2011) or resolution of the patent infringement lawsuit. Should Upsher-Smith receive an approval of its generic version of Testim from the FDA, it cannot lawfully launch its generic version of Testim in the U.S. before the earlier of the expiration of the currently pending 30-month stay or a district court decision in its favor. We have incurred, and will continue to incur, costs related to the Upsher-Smith Litigation and regulatory actions. We may incur costs related to any other legal and regulatory actions that we may undertake. The Upsher-Smith Litigation could result in a finding that Upsher-Smith’s proposed testosterone product does not infringe the ‘968 Patent or that the ‘968 Patent is invalid and/or unenforceable. An adverse outcome in the Upsher-Smith Litigation or any such legal action could result in one or more generic versions of Testim being launched in the U.S. before the expiration of the ‘968 Patent in January 2025. Since Testim is currently our only marketed product, the introduction of a generic version could have a material adverse affect on our ability to successfully execute our business strategy to maximize the value of Testim as we continue to develop our product pipeline and therefore could have a material negative impact on our financial condition and results of operations.
Trade secrets may not provide adequate protection for our business and technology.
We also rely on trade secrets to protect our technology, especially where we believe patent protection is not appropriate or obtainable. However, trade secrets are difficult to protect. While we use reasonable efforts to protect our trade secrets, our or our potential collaborators’ employees, consultants, contractors or scientific and other advisors may unintentionally or willfully disclose our information to competitors. If we were to enforce a claim that a third party had illegally obtained and was using our trade secrets, our enforcement efforts would be expensive and time consuming, and the outcome would be unpredictable. In addition, courts outside the U.S. are sometimes less willing to protect trade secrets. Moreover, if our competitors independently develop equivalent
54
Table of Contents
knowledge, methods or know-how, it will be more difficult or impossible for us to enforce our rights and our business could be harmed.
Risks Related to Collaborators
We are dependent upon our collaborative relationship with Pfizer to further develop and commercialize XIAFLEX. There may be circumstances that delay or prevent Pfizer’s ability to develop and commercialize XIAFLEX.
In December 2008, we entered into a development, commercialization and supply agreement with Pfizer (the “Pfizer Agreement”) wherein we granted to Pfizer the right to develop and commercialize, with the right to sublicense, XIAFLEX for the treatment of Peyronie’s and Dupuytren’s in 27 member countries of the European Union, as well as certain other Eurasian countries (the “Pfizer Territory”). Under our agreement Pfizer is solely responsible for regulatory approval, and commercialization, of XIAFLEX in the Pfizer Territory, so we are entirely dependent on Pfizer for the successful completion of those activities.
We are subject to a number of risks associated with our dependence on our collaborative relationship with Pfizer, including:
| • | adverse decisions by Pfizer regarding the amount and timing of resource expenditures for the development and commercialization of XIAFLEX; |
| • | possible disagreements as to the timing, nature and extent of our development plans, including clinical trials or regulatory approval strategy; |
| • | the right of Pfizer to terminate its collaboration agreement with us on limited notice upon the occurrence of certain defined events; |
| • | loss of significant rights if we fail to meet our obligations under the collaboration agreement; |
| • | withdrawal of support by Pfizer following change of Pfizer corporate strategy or due to competing priorities; |
| • | changes in key management personnel at Pfizer that are members of the collaboration’s various operating committees; and |
| • | possible disagreements with Pfizer regarding the collaboration agreement or ownership of proprietary rights. |
Due to these factors and other possible disagreements with Pfizer, we may be delayed or prevented from further developing, manufacturing or commercializing XIAFLEX in the Pfizer Territory, or we may become involved in litigation or arbitration, which would be time consuming and expensive.
The term of the Pfizer Agreement extends, on a country by country basis, until the latest of (i) the date in which XIAFLEX is no longer covered by a valid patent claim in such country, (ii) the 15th anniversary of the first commercial sale by Pfizer of XIAFLEX in a given country and (iii) entry of a generic with respect to XIAFLEX in such country. Pfizer can terminate upon the occurrence of certain defined events. If Pfizer were to unilaterally terminate, we would need to undertake development and marketing activities for XIAFLEX in the Pfizer Territory solely at our own expense and/or seek another partner for some or all of these activities in the Pfizer Territory. If we pursued these activities in the Pfizer Territory on our own, it would significantly increase our capital and infrastructure requirements, and might limit the indications we are able to pursue and could prevent us from effectively developing and commercializing XIAFLEX. If we sought to find another pharmaceutical company partner for some or all of these activities, we may not be successful in such efforts, or they may result in a collaboration that has us expending greater funds and efforts than our current relationship with Pfizer.
55
Table of Contents
In general, we cannot control the amount and timing of resources that Pfizer may devote to our collaboration. If Pfizer fails to assist in the further development or the commercialization of XIAFLEX, or if Pfizer’s efforts are not effective, our business may be negatively affected. We are relying on Pfizer to obtain regulatory approvals for and successfully commercialize XIAFLEX in the Pfizer Territory. Our collaboration with Pfizer may not be successful. If Pfizer ceases developing and commercializing XIAFLEX, we would have to seek additional sources for funding and may have to delay, reduce or eliminate one or more of our commercialization and development programs for XIAFLEX or our other product candidates. If we and Pfizer cannot agree on the development plan for XIAFLEX, development and commercialization progress could be significantly delayed or halted.
We are dependent on the efforts of Pfizer to market and promote XIAFLEX.
Under the Pfizer Agreement, Pfizer will exclusively promote XIAFLEX in the Pfizer Territory, and we have only a limited ability to direct Pfizer in its commercialization of XIAFLEX in the Pfizer Territory. We are thus solely dependent on Pfizer to successfully promote XIAFLEX to physicians and customers in the Pfizer Territory. If Pfizer fails to adequately market and promote XIAFLEX in the Pfizer Territory, we may be unable to obtain any remedy against Pfizer and sales of XIAFLEX may be harmed, which would negatively impact our business, results of operations, cash flows and liquidity due to reduced milestone and royalty payments under the Pfizer Agreement.
If Pfizer’s business strategy changes, it may adversely affect our collaborative relationship.
Any decision by Pfizer to either reduce or eliminate its participation in the Dupuytren’s or Peyronie’s indications, to emphasize other competitive agents currently in its portfolio at the expense of XIAFLEX, or to add additional competitive agents to its portfolio, could reduce its financial incentive to continue to develop, seek regulatory approval for, or commercialize XIAFLEX. Such a change in Pfizer’s business strategy may adversely affect activities under its agreement with us, which could cause significant delays and funding shortfalls impacting the activities under the collaboration and seriously harming our business.
We are dependent on the funding by Pfizer for the development of XIAFLEX.
The financial returns to us, if any, under our collaboration agreement with Pfizer depend in large part on the achievement of development and commercialization milestones, and royalties from sales in the Pfizer Territory. Therefore, our success in the Pfizer Territory, and any associated financial returns to us and our investors, will depend in part on the performance of Pfizer under the agreement. If Pfizer fails to perform or satisfy its obligations to us, the development, regulatory approval or commercialization of XIAFLEX in the Pfizer Territory would be delayed or may not occur and our business and prospects could be materially and adversely affected.
Subject to certain limitations set forth in the Pfizer Agreement, Pfizer may terminate on limited notice to us upon the occurrence of certain defined events. If Pfizer terminates, we may be unable to fund the development costs of XIAFLEX and other product candidates on our own and may be unable to find a new collaborator, which could have a material adverse effect on our business.
Risks Related to Development of Our Product Candidates
We may not be able to develop product candidates into viable commercial products, which would impair our ability to grow and could cause a decline in the price of our stock.
The process of developing product candidates involves a high degree of risk and may take several years. Product candidates may fail to reach the market for several reasons, including:
| • | clinical trials may show our product candidates to be ineffective or not as effective as anticipated or to have harmful side effects or any unforeseen result; |
56
Table of Contents
| • | our inability to enroll patients in clinical trials within the expected timeframes; |
| • | our inability to obtain authorization from FDA or other regulatory authority to initiate clinical trials within the expected timeframes; |
| • | product candidates may fail to receive regulatory approvals required to bring the products to market; |
| • | manufacturing costs and delays and manufacturing problems in general, the inability to scale up to produce supplies for clinical trials or commercial supplies, or other factors may make our product candidates uneconomical; |
| • | the proprietary rights of others and their competing products and technologies may prevent our product candidates from being effectively commercialized or to obtain exclusivity; and |
| • | failure to meet one or more of management’s target product profile criteria. |
Success in the preclinical and early clinical trials does not ensure that large-scale clinical trials will be successful. Clinical results are frequently susceptible to varying interpretations that may delay, limit or prevent regulatory approvals. The length of time necessary to complete clinical trials and to submit an application for marketing approval for a final decision by a regulatory authority varies significantly and may be difficult to predict. Currently, there is substantial congressional and administration review of the regulatory approval process for drug candidates in the U.S. Any changes to the U.S. regulatory approval process could significantly increase the timing or cost of regulatory approval for our product candidates making further development uneconomical or impossible.
In addition, developing product candidates is very expensive and will have a significant impact on our ability to generate profits. Factors affecting our product development expenses include:
| • | changes to the regulatory approval process for product candidates in those jurisdictions, including the U.S., in which we may be seeking approval for our product candidates; |
| • | the cost and timing of manufacturing clinical or commercial supplies of product candidates, including the cost and timing of the implementation of any necessary corrective actions; |
| • | regulatory approval of trade names for our product candidates and the timing thereof; |
| • | our ability to raise any additional funds that we need to complete our trials; |
| • | the number and outcome of clinical trials conducted by us and/or our collaborators; |
| • | the number of products we may have in clinical development; |
| • | in-licensing or other partnership activities, including the timing and amount of related development funding, license fees or milestone payments; and |
| • | future levels of our revenue. |
Our product development efforts also could result in large and immediate write-offs, significant milestone payments, incurrence of debt and contingent liabilities or amortization of expenses related to intangible assets, any of which could negatively impact our financial results. Additionally, if we are unable to develop our product candidates into viable commercial products, we will be reliant solely on sales of our currently approved products for our revenues, potentially limiting our growth opportunities.
57
Table of Contents
If clinical trials for our product candidates are delayed, we would be unable to commercialize our product candidates on a timely basis, which could materially harm our business.
Clinical trials that we may conduct, or that may be conducted by our partners, may not begin on time or may need to be restructured or temporarily suspended after they have begun. Clinical trials can be delayed or may need to be restructured for a variety of reasons, including delays or restructuring related to:
| • | changes to the regulatory approval process for product candidates in those jurisdictions, including the U.S., in which we may be seeking approval for our product candidates; |
| • | obtaining an investigational new drug exemption (“IND”), or other regulatory approval to commence a clinical trial; |
| • | timing of responses required from regulatory authorities; |
| • | negotiating acceptable clinical trial agreement terms with prospective investigators or trial sites; |
| • | obtaining institutional review board, or equivalent, approval to conduct a clinical trial at a prospective site; |
| • | recruiting subjects to participate in a clinical trial; |
| • | competition in recruiting clinical investigators; |
| • | shortage or lack of availability of clinical trial supplies from external and internal sources; |
| • | the need to repeat clinical trials as a result of inconclusive results or poorly executed testing; |
| • | failure to validate a patient-reported outcome questionnaire; |
| • | the placement of a clinical hold on a study; |
| • | the failure of third parties conducting and overseeing the operations of our clinical trials to perform their contractual or regulatory obligations in a timely fashion; |
| • | exposure of clinical trial subjects to unexpected and unacceptable health risks or noncompliance with regulatory requirements, which may result in suspension of the trial; and |
| • | manufacturing issues associated with clinical supplies. |
Prior to commencing clinical trials in the U.S., we must have an effective IND for each of our product candidates. INDs have been filed and are effective for XIAFLEX for the treatment of Peyronie’s, and XIAFLEX for the treatment of Frozen Shoulder syndrome. The foreign equivalent of an IND, a Clinical Trial Application (“CTA”) is a prerequisite to performing pilot studies or phase I clinical trials in many European countries.
We have four projects in clinical development, specifically XIAFLEX for the treatment of Peyronie’s and Frozen Shoulder syndrome, Fentanyl transmucosal film and AA4010. Completion of clinical trials for each product candidate will be required before commercialization. With regard to the development of XIAFLEX for the treatment of Peyronie’s, the FDA required that certain additional pre-clinical studies be completed prior to the initiation of the phase IIb study or any other human study for Peyronie’s which resulted in a delay in commencement of our phase IIb study. We have completed these pre-clinical studies and the U.S. phase IIb trial of XIAFLEX for the treatment of Peyronie’s and announced top line efficacy and safety results in December 2009. If we experience delays in, or termination of, clinical trials, or fail to enroll patients in clinical trials in a timely manner, or if the cost or timing of the regulatory approval process increases, our financial results and the commercial prospects for our product candidates will be adversely impacted. In addition, our product development costs would increase and our ability to generate additional revenue from new products could be impaired.
58
Table of Contents
Adverse events or lack of efficacy in our clinical trials may force us to stop development of our product candidates, prevent regulatory approval of our product candidates, or impact our existing products which could materially harm our business.
Patients participating in the clinical trials of our product candidates may experience serious adverse health events. A serious adverse health event includes death, a life-threatening condition, hospitalization, disability, congenital anomaly, or a condition requiring intervention to prevent permanent impairment or damage. The occurrence of any of these events could interrupt, delay, suspend or halt clinical trials of our product candidates and could result in the FDA, or other regulatory authorities, denying approval of our product candidates for any or all targeted indications. Also, the occurrence of any of these events could impact our existing products. An institutional review board or independent data safety monitoring board, the FDA, other regulatory authorities or we may suspend or terminate clinical trials at any time. Our product candidates may prove not to be safe for human use. Any delay in the regulatory approval of our product candidates could increase our product development costs and allow our competitors additional time to develop or market competing products. If our product candidates do not receive the necessary regulatory approval, we will be reliant on sales of our currently approved products as our sole sources of revenue.
Top line efficacy and safety results for the XIAFLEX Peyronie’s phase IIb clinical trial were announced in December 2009. The data demonstrated that XIAFLEX improved penile curvature when compared to placebo with a mean improvement of 29.7% vs. 11%, respectively (P=0.001). In addition, the PRO domain of Peyronie’s symptom bother was significantly improved in XIAFLEX treated subjects compared to placebo. The use of modeling also demonstrated significant improvement in XIAFLEX subjects compared to placebo, 32.4% improvement in curvature compared to a 2.5% worsening of curvature in placebo subjects (p<0.001). In the no-modeling group, XIAFLEX subjects experienced a 27.1% mean improvement in penile curvature, which was not statistically different from the 27.9% mean improvement for subjects receiving the placebo. The remaining three domains did not show statistical significance between XIAFLEX and placebo. The most common adverse events were injection site bruising, injection site pain, injection site edema, contusion, penile edema, and penile pain. There were also a total of five (3.4%) subjects who experienced eight non fatal serious adverse events, none of which were considered to be related to XIAFLEX.
Our failure to successfully in-license or acquire additional technologies, product candidates or approved products could impair our ability to grow.
We intend to in-license, acquire, develop and market additional products and product candidates so that we are not solely reliant on sales from our currently approved products for our revenues. Because we have limited internal research capabilities, we are dependent upon pharmaceutical and biotechnology companies and other researchers to sell or license products or technologies to us. The success of this strategy depends upon our ability to identify, select and acquire the right pharmaceutical product candidates, products and technologies. To date, we have in-licensed the formulation technology underlying Testim from CPEX, the transmucosal film technology underlying AA4010 and our Fentanyl transmucosal film product candidate from PharmaForm, and the enzyme underlying XIAFLEX from BioSpecifics.
We have a limited number of product candidates in our development pipeline. We may not be able to successfully identify any other commercial products or product candidates to in-license, acquire or internally develop. Moreover, negotiating and implementing an economically viable in-licensing arrangement or acquisition is a lengthy and complex process. Other companies, including those with substantially greater resources, may compete with us for the in-licensing or acquisition of product candidates and approved products. We may not be able to acquire or in-license the rights to additional product candidates and approved products on terms that we find acceptable, or at all. If we are unable to in-license or acquire additional commercial products or product candidates, we may be reliant solely on sales of our currently approved products for revenues. As a result, our ability to grow our business or increase our profits could be severely limited.
59
Table of Contents
If we engage in any acquisition, we will incur a variety of costs, and we may never realize the anticipated benefits of the acquisition.
Since our inception, we have acquired the underlying technology for our products and product candidates through in-licensing arrangements. The underlying technology for Testim was licensed from CPEX. The transmucosal film technology underlying AA4010 and the pain product candidates was licensed from PharmaForm. XIAFLEX for Dupuytren’s, Peyronie’s and Frozen Shoulder syndrome, was licensed from BioSpecifics. One of our strategies for business expansion is the acquisition of additional products and product candidates. We may attempt to acquire these product candidates, or other potentially beneficial technologies, through in-licensing or the acquisition of businesses, services or products that we believe are a strategic fit with our business. If we undertake an acquisition, the process of integrating any newly acquired business, technology, service or product into our existing operations could be expensive and time consuming and may result in unforeseen operating difficulties and expenditures and may divert significant management attention from our ongoing business operations. Moreover, we may fail to realize the anticipated benefits of any acquisition for a variety of reasons, such as an acquired product candidate proving to not be safe or effective in later clinical trials or not reaching its forecasted commercial potential. We may fund any future acquisition by issuing equity or debt securities, which could dilute your ownership percentage or limit our financial or operating flexibility as a result of restrictive covenants related to new debt. Acquisition efforts can consume significant management attention and require substantial expenditures, which could detract from our other programs. In addition, we may devote resources to potential acquisitions that are never completed. If we are unable to acquire and successfully integrate product candidates through in-licensing or the acquisition of businesses, services or products, we may remain reliant solely on sales of our currently approved products for revenue. In pursuing our acquisition strategy, we may expend significant management time, consulting costs and legal expenses without consummating a transaction.
Risks Related to Regulatory Approval of Our Product Candidates
We are subject to numerous complex regulatory requirements and failure to comply with these regulations, or the cost of compliance with these regulations, may harm our business.
The testing, development, manufacture and distribution of our products are subject to regulation by numerous governmental authorities in the U.S., Europe and the rest of the world. These regulations govern or affect the testing, manufacture, safety, labeling, storage, record-keeping, approval, distribution, advertising and promotion of our products and our product candidates, as well as safe working conditions and the experimental use of animals. Noncompliance with any applicable regulatory requirements can result in refusal of the government to approve facilities for testing or manufacture of products as well as refusal to approve products for commercialization. Noncompliance with any applicable regulatory requirements also can result in criminal prosecution and fines, recall or seizure of products, total or partial suspension of production, prohibitions or limitations on the commercial sale of products or refusal to allow the entering into of federal and state supply contracts. FDA and comparable governmental authorities have the authority to withdraw product approvals that have been previously granted. Currently, there is a substantial amount of congressional and administrative review of the FDA and the regulatory approval process for drug candidates in the U.S. As a result, there may be significant changes made to the regulatory approval process in the U.S. In addition, the regulatory requirements relating to the manufacturing, testing, labeling, promotion, marketing and distribution of our products may change in the U.S. or the other jurisdictions in which we may have obtained or be seeking regulatory approval for our products or product candidates. Such changes may increase our costs and adversely affect our operations.
Testosterone is listed by the U.S. Drug Enforcement Agency (“DEA”) as a Schedule III substance under the Controlled Substances Act of 1970. The DEA classifies substances as Schedule I, II, III, IV or V substances, with Schedule I substances considered to present the highest risk of substance abuse and Schedule V substances the lowest risk. Our pain transmucosal film product candidates may also involve the use of scheduled substances. Scheduled substances are subject to DEA regulations relating to manufacturing, storage, distribution and
60
Table of Contents
physician prescription procedures. For example, all regular Schedule III drug prescriptions must be signed by a physician and may not be refilled. Furthermore, the amount of Schedule III substances we can obtain for clinical trials and commercial distribution is limited by the DEA and our quota may not be sufficient to complete clinical trials or meet commercial demand, if any.
Entities must be registered annually with the DEA to manufacture, distribute, dispense, import, export and conduct research using controlled substances. State controlled substance laws also require registration for similar activities. In addition, the DEA requires entities handling controlled substances to maintain records and file reports, follow specific labeling and packaging requirements, and provide appropriate security measures to control against diversion of controlled substances. Failure to follow these requirements can lead to significant civil and/or criminal penalties and possibly even lead to a revocation of a DEA registration.
Products containing controlled substances may generate public controversy. As a result, these products may have their marketing rights or regulatory approvals withdrawn. Political pressures and adverse publicity could lead to delays in, and increased expenses for, and limit or restrict the introduction and marketing of our product candidates. For some scheduled substances or any product, the FDA may require us to develop a comprehensive risk management program to reduce the inappropriate use of our products and product candidates, including the manner in which they are marketed and sold, so as to reduce the risk of improper patient selection and diversion or abuse of the product. Developing such a program in consultation with the FDA may be a time-consuming process and could delay approval of any of our product candidates. Such a program or delays of any approval from the FDA could increase our product development costs and may allow our competitors additional time to develop or market competing products.
As a condition for approval of XIAFLEX for Dupuytren’s, we are required to comply with post-marketing requirements. Failure to comply with these requirements or any future post-marketing requirements, or the cost of compliance with such requirements, may harm our business.
FDA can establish requirements for approved products with which we must comply. For example, the law allows FDA to require us as the sponsor of a marketing application to conduct and report the results of certain studies or clinical trials for certain purposes (“post-marketing requirements”) if FDA makes certain findings required by the statute. Failure to report or conduct the studies is considered a violation and can result in enforcement action. Additionally, FDA can request that we voluntarily conduct studies or clinical trials to address questions or concerns (“post-marketing commitments”). These studies or clinical trials could be time-consuming and costly and the results could have negative effects on our ability to market the product.
As a condition of approval for XIAFLEX for Dupuytren’s, FDA required a single post-marketing requirement and several post-marketing commitments. The post-marketing requirement is to conduct a study to evaluate the potential for antibodies to XIAFLEX to interfere with certain other human proteins that are similar to the proteins in XIAFLEX. No new clinical studies are required as part of this evaluation. The post-marketing commitments are generally related to the manufacturing and testing of XIAFLEX. The results of the required and voluntary investigations could be time-consuming and costly and the results could have negative effects on our ability to market the product.
For XIAFLEX for Dupuytren’s and Testim, we are required to implement a REMS. Failure to comply, or the cost of compliance with such REMS or any future REMS, may harm our business.
FDA is authorized to require us as the sponsor of an approved or unapproved marketing application to submit a proposed REMS if the FDA determines that a REMS is necessary to ensure that the benefits of a drug outweigh the risks of the drug. FDA can require submission of a REMS prior to approval of a product or after approval of a product if FDA becomes aware of new safety information. If FDA determines that a REMS is necessary to ensure that the benefits of the drug outweigh the risks, FDA will determine which elements of a REMS are necessary and will approve the REMS once the FDA has determined that the proposed REMS will
61
Table of Contents
ensure that the benefits of the drug outweigh the risks, and the other relevant statutory criteria are met. Each REMS must include a timetable for assessments of the effectiveness of the REMS in mitigating the identified risks and can be required to include other elements, such as a medication guide, a communication plan or other elements to assure safe use. REMS that are required by FDA are subject to inspection and are enforceable. Failure to comply with the requirements of the approved REMS can render the drug misbranded. A violation of a REMS requirement is subject to civil penalties. Complying with the requirements of a REMS can be costly and time-consuming and adversely affect our operations.
As a condition of approval for XIAFLEX for Dupuytren’s, FDA required a REMS. The goal of the REMS is to inform and train healthcare providers about the risks of tendon rupture, serious adverse reactions affecting the injected extremity, and the potential risk of serious hypersensitivity reactions (including the potential for anaphylaxis) associated with XIAFLEX. The REMS consists of a medication guide, a communication plan, and a timetable for submission of assessments of the REMS. The communication plan includes a Dear Healthcare Provider Letter and educational materials (i.e., training guide and procedure training video).
On May 7, 2009, FDA announced that it was requiring the manufacturers of two prescription topical testosterone gels, Solvay S.A. and Auxilium, to make changes to the prescribing information and develop REMS for the products. FDA stated that it was requiring this action after it became aware, through spontaneous post-marketing adverse event reports and peer-reviewed biomedical literature, of cases of secondary exposure of children to testosterone due to drug transfer from adult males using testosterone gel drug products (“transference”). FDA considered this information to be “new safety information.” We believe that all topical testosterone gels have a potential for transference. Testim’s prescribing information has described the risk and procedures for avoidance of transference since the product was launched in 2003. The changes to the prescribing information for Testim include a “boxed warning”, which is used to highlight warning information that is especially important to the prescriber. The goal of the REMS is to inform patients about the serious risks associated with the use of Testim and AndroGel. The REMS includes assessments and a Medication Guide to inform patients. The revised prescribing information and REMS for Testim was approved in September 2009.
When we seek approval for our drug products in other countries, we are subject to numerous complex regulatory requirements and if approval is denied or limited in another country, and/or if another country imposes post-marketing requirements, that decision could affect our ability, or that of our partners, to market the drug in other countries.
A Marketing Authorization Application (“MAA”) has been filed for XIAFLEX for the treatment of Dupuytren’s in the EU by our partner, Pfizer, using the centralized procedure. If major objections are raised during the review procedure, Pfizer may not receive marketing approval and would be unable to commercialize the product in the EU. Alternatively, the marketing authorization may be subject to conditions for approval or post authorization obligations. Such conditions or obligations may be costly and time consuming to fulfill and may affect our operations. For example, additional clinical data may be required to confirm the safety and/or efficacy profile of the product in the target patient population. In addition, marketing authorizations are subject to periodic reviews which if negative could affect the ability of Pfizer to commercialize the product in the EU.
Further regulatory filings are planned by our partner Ferring in 2010 in order to gain additional marketing approvals for Testim in the EU. The filings will be made via the Mutual Recognition procedure. If major objections are raised and upheld during the review procedure, in addition to preventing approval in the new countries, this could also negatively impact the approvals in the existing countries and could thereby impact Ferring’s ability to commercialize the product.
Additionally, failure to comply with or changes to the regulatory requirements that are applicable to our products or our other product candidates may result in a variety of consequences, including the following:
| • | restrictions on our products or manufacturing processes; |
62
Table of Contents
| • | warning letters; |
| • | withdrawal of a product or a product candidate from the market; |
| • | voluntary or mandatory recall of a product or a product candidate; |
| • | fines against us; |
| • | suspension or withdrawal of regulatory approvals for a product or a product candidate; |
| • | suspension or termination of any of our ongoing clinical trials of a product candidate; |
| • | refusal to permit import or export of our products; |
| • | refusal to approve pending applications or supplements to approved applications that we submit; |
| • | denial of permission to file an application or supplement in a jurisdiction; |
| • | product seizure; and |
| • | injunctions, consent decrees, or the imposition of civil or criminal penalties against us. |
If our product candidates are not demonstrated to be sufficiently safe and effective, they will not receive regulatory approval and we will be unable to commercialize them.
The regulatory approval process typically is extremely expensive, takes many years and the timing or likelihood of any approval cannot be accurately predicted. INDs have been filed and are effective for XIAFLEX for the treatment of Peyronie’s and XIAFLEX for the treatment of Frozen Shoulder syndrome. The foreign equivalent of an IND, a CTA, is a prerequisite to performing pilot studies or phase I clinical trials in many European countries. CTAs were filed and approved in select European countries for open label phase III studies for XIAFLEX for the treatment of Dupuytren’s. Additional CTAs have been submitted for a long term follow-up study with approval received in two out of four countries to date.
As part of the regulatory approval process, we must conduct preclinical studies and clinical trials for each product candidate to demonstrate safety and efficacy. The number of preclinical studies and clinical trials that will be required varies depending on the product candidate, the indication being evaluated, the trial results and regulations applicable to any particular product candidate. Top line efficacy and safety results for the XIAFLEX Peyronie’s phase IIb clinical trial were announced in December 2009. The data demonstrated that XIAFLEX improved penile curvature when compared to placebo with a mean improvement of 29.7% vs. 11%, respectively (P=0.001). In addition, the PRO domain of Peyronie’s symptom bother was significantly improved in XIAFLEX treated subjects compared to placebo. The use of modeling also demonstrated significant improvement in XIAFLEX subjects compared to placebo, 32.4% improvement in curvature compared to a 2.5% worsening of curvature in placebo subjects (p<0.001). In the no-modeling group, XIAFLEX subjects experienced a 27.1% mean improvement in penile curvature, which was not statistically different from the 27.9% mean improvement for subjects receiving the placebo. The remaining three domains did not show statistical significance between XIAFLEX and placebo. The most common adverse events were injection site bruising, injection site pain, injection site edema, contusion, penile edema, and penile pain. There were also a total of five (3.4%) subjects who experienced eight non fatal serious adverse events, none of which were considered to be related to XIAFLEX.
The results of preclinical studies and initial clinical trials of our product candidates do not necessarily predict the results of later-stage clinical trials. Product candidates in later stages of clinical trials may fail to show the desired safety and efficacy despite having progressed through initial clinical trials. We cannot assure you that the data collected from the preclinical studies and clinical trials of our product candidates will be sufficient to support FDA or other regulatory approval. In addition, the continuation of a particular study after review by an institutional review board or independent data safety monitoring board does not necessarily indicate that our product candidate will achieve the clinical endpoint.
63
Table of Contents
The FDA and other regulatory agencies can delay, limit or deny approval for many reasons, including:
| • | changes to the regulatory approval process for product candidates in those jurisdictions, including the U.S., in which we may be seeking approval for our product candidates; |
| • | a product candidate may not be deemed to be safe or effective; |
| • | the ability of the regulatory agency to provide timely responses as a result of its resource constraints; |
| • | the manufacturing processes or facilities may not meet the applicable requirements; and |
| • | changes in their approval policies or adoption of new regulations may require additional clinical trials or other data. |
Any delay in, or failure to receive, approval for any of our product candidates or the failure to maintain regulatory approval for our products could prevent us from growing our revenues or achieving profitability
Risks Related to Compliance
Our corporate compliance program cannot guarantee that we are in compliance with all potentially applicable regulations.
We are a relatively small company and we rely heavily on third parties to conduct many important functions. As a biopharmaceutical company, we are subject to a large body of legal and regulatory requirements. In addition, as a publicly traded company we are subject to significant regulations, including the Sarbanes-Oxley Act of 2002 (“SOA”), some of which have either only recently been adopted or are currently proposals subject to change. We cannot assure you that we are or will be in compliance with all potentially applicable laws and regulations. Failure to comply with all potentially applicable federal and state laws and regulations could lead to the imposition of fines, result in our exclusion from participation in state and federal healthcare programs, cause the value of our common stock to decline, impede our ability to raise capital or lead to the de-listing of our stock.
Our controls over external financial reporting may fail or be circumvented.
We regularly review and update our internal controls, disclosure controls and procedures, and corporate governance policies. In addition, we are required under the SOA to report annually on our internal control over financial reporting. If we, or our independent registered public accounting firm, determine that our internal control over financial reporting is not effective, this shortcoming could have an adverse effect on our business and financial results and the price of our common stock could be negatively affected. This reporting requirement could also make it more difficult or more costly for us to obtain certain types of insurance, including director and officer liability insurance, and we may be forced to accept reduced policy limits and coverage or incur substantially higher costs to obtain the same or similar coverage. Any system of internal controls, however well designed and operated, is based in part on certain assumptions and can provide only reasonable, not absolute, assurances that the objectives of the system are met. Any failure or circumvention of the controls and procedures or failure to comply with regulation concerning control and procedures could have a material effect on our business, results of operation and financial condition. The impact of these events could also make it more difficult for us to attract and retain qualified persons to serve on our board of directors, our board committees and as executive officers.
Risks Related to Employees and Growth
If we are not able to retain our current management team or attract and retain qualified scientific, technical and business personnel, our business will suffer.
We are dependent on the members of our management team for our business success. In addition, an important element of our strategy is to leverage the development, regulatory and commercialization expertise of
64
Table of Contents
our current management in our development activities. Our employment agreements with our executive officers are terminable on short notice. The loss of key employees may result in a significant loss in the knowledge and experience that we, as an organization, possess and could cause significant delays, or outright failure, in the further commercialization of our products or the development and commercialization of our product candidates. If we are unable to attract and retain qualified and talented senior management personnel, our business may suffer.
To grow we will need to hire a significant number of qualified personnel on a timely basis. However, there is intense competition for human resources, including management in the technical fields in which we operate, and we may not be able to attract and retain qualified personnel necessary for the successful commercialization of our products and the development and commercialization of our product candidates. As we grow, the inability to attract new employees when needed or to retain existing employees could severely limit our growth and harm our business.
Our operations may be impaired unless we can successfully manage our growth.
We currently have approximately 540 employees. In order to continue to achieve our business goals, we currently anticipate that we will need to add employees to our existing departments. Expansion may place a significant strain on our management, operational and financial resources. Moreover, higher than expected market growth of our products, the acquisition or in-licensing of additional products, as well as the development and commercialization of our other product candidates or marketing arrangements with third parties, could accelerate our hiring needs beyond our current expectations. To manage further growth, we will be required to continue to improve existing, and implement additional, operational and financial systems, procedures and controls, and hire, train and manage additional employees. Our current and planned personnel, systems, procedures and controls may not be adequate to support our anticipated growth and we may not be able to hire, train, retain, motivate and manage required personnel. Our failure to manage growth effectively could limit our ability to achieve our business goals. In addition, turnover in our direct sales force or marketing team could adversely affect product and future product sales growth.
Risks Related to Our Financial Results and Need for Additional Financing
We incurred significant losses since our inception and may not achieve profitability in the foreseeable future.
We have incurred significant losses since our inception, including net losses of $53.5 million for the twelve months ended December 31, 2009. As of December 31, 2009, we had an accumulated deficit of $323.9 million. We expect to continue to incur substantial expenses as we:
| • | market and sell our only approved products, Testim for the treatment of hypogonadism in the U.S., Canada and Europe, and XIAFLEX for Dupuytren’s for adult patients with a palpable cord, in the U.S., through our sales force; |
| • | develop XIAFLEX for the treatment of Peyronie’s and Frozen Shoulder syndrome; AA4010, our overactive bladder transmucosal film product candidate; and our Fentanyl transmucosal film product candidate and any other product candidates that we develop, license or acquire; |
| • | manufacture XIAFLEX; |
| • | seek regulatory approval for our product candidates; |
| • | prepare for the launch of and commercialize any of our product candidates that receive marketing approval or any approved products that we acquire or in-license; and |
| • | acquire or in-license new technologies or development stage or approved products. |
65
Table of Contents
Accordingly, we expect to continue to incur substantial additional losses through the successful launch of XIAFLEX for Dupuytren’s. In order to achieve and maintain profitability, we will need to generate significantly greater revenues from Testim and XIAFLEX for Dupuytren’s. If we fail to achieve profitability within the time frame expected by investors, the value of our common stock may decline substantially.
Our future results are unpredictable, and therefore, our common stock is a highly speculative investment.
Our future results are unpredictable and our success is dependent upon many factors. Accordingly, you must consider our prospects in light of the risks and difficulties we may encounter. The risks and difficulties that we may encounter include:
| • | successfully launching XIAFLEX for Dupuytren’s in the U.S.; |
| • | increasing the sales of Testim; |
| • | manufacturing sufficient quantities of XIAFLEX; |
| • | successfully developing, obtaining marketing approval for and manufacturing or having manufactured our current product candidates, including XIAFLEX for the treatment of Peyronie’s and Frozen Shoulder syndrome; |
| • | successfully identifying and developing new product candidates; |
| • | effectively commercializing any approved product candidates that we develop, including XIAFLEX or any approved products that we acquire; |
| • | responding to competitive pressures from other businesses, including the launch of any competitive products, including generics; |
| • | Identifying and negotiating favorable agreements with third parties for the manufacture, distribution, marketing and sales of our approved products; and |
| • | effectively managing our relationships with vendors and third parties. |
All of our revenues to date have been generated from the sale of Testim or out-licensing of Testim and XIAFLEX, and, if these revenues do not grow, our launch of XIAFLEX for Dupuytren’s in the U.S. is not successful and we cannot commercialize new products, we will not become profitable
Our only products with marketing approval are Testim and XIAFLEX for Dupuytren’s and our product revenues to date have been generated solely from the sale of Testim and out-licensing of Testim and XIAFLEX. Until such time as we develop, acquire or in-license additional products that are approved for marketing, we will be relying on Testim and XIAFLEX for all of our revenues. Accordingly, our success depends significantly on our ability to increase sales of Testim and successfully launch and grow sales of XIAFLEX in the U.S. for Dupuytren’s. Our sales and marketing efforts may not be successful in increasing prescriptions for our products. Sales of our products are subject to the following risks, among others:
| • | growth of the overall androgen market which may be influenced by the sales and marketing efforts of our competitors; |
| • | development and growth of a market for XIAFLEX as an alternative to the current practice of treating Dupuytren’s through surgical procedures and expectant management; |
| • | acceptance by the medical community or the general public of our products as safe or effective therapies; |
| • | increasing awareness and continued acceptance of hypogonadism by the medical community, regulators or the general public as a medical disorder requiring treatment; |
| • | illegal substitution by pharmacists of AndroGel for Testim; |
66
Table of Contents
| • | pressures from existing or new competing products, including generic products, that may provide therapeutic, convenience or pricing advantages over Testim or may garner a greater share of voice; |
| • | failure of third-party payors to provide coverage and sufficient reimbursement; |
| • | failure of third-party payors to provide coverage and sufficient reimbursement for the procedures performed by physicians using XIAFLEX to treat Dupuytren’s; and |
| • | impact of coverage and reimbursement changes. |
For the foreseeable future, if we are unable to grow sales of our products and out-licensing revenues, we will be unable to increase our revenues or achieve profitability and we may be forced to delay or change our current plans to develop other product candidates.
If our financial resources and sources of liquidity are not sufficient, we may be required to limit, scale back or cease our operations.
Based on our current plans and expectations, we believe that our existing financial resources and sources of liquidity will be sufficient to meet our anticipated operating requirements until such time as we are profitable. Our future funding requirements will depend on many factors, including:
| • | our ability to successfully launch and increase sales in the U.S. of XIAFLEX for Dupuytren’s; |
| • | Testim market acceptance, sales growth and the impact of the ANDA filed by Upsher-Smith containing a paragraph IV certification seeking approval from the FDA to market a generic version of Testim, including the cost of pursuing all available legal and regulatory options in defense of Testim; |
| • | our ability to realize sales efficiency and effectiveness of our sales force; |
| • | growth of the overall androgen market which may be influenced by the sales and marketing efforts of our competitors; |
| • | entry into the marketplace of competitive products; |
| • | third-party payor coverage and reimbursement for our products; |
| • | third-party payor coverage and sufficient reimbursement for our procedures performed by physicians using XIAFLEX to treat Dupuytren’s; |
| • | the cost of manufacturing, distributing, marketing and selling our products; |
| • | the scope, rate of progress and cost of our product development activities; |
| • | future clinical trial results; |
| • | the terms and timing of any future collaborative, licensing, co-promotion and other arrangements that we may establish; |
| • | the cost and timing of regulatory approvals; |
| • | the cost of any regulatory enforcement activities; |
| • | the accuracy of market research regarding the commercial potential of any of our products or product candidates; |
| • | the market acceptance and sales growth of any of our products or product candidates upon regulatory approval; |
| • | the costs of supplying and commercializing our products and product candidates; |
| • | increased administrative costs to support a growing infrastructure; |
67
Table of Contents
| • | acquisition of a company or in-licensing costs; |
| • | the effect of competing technological and market developments; |
| • | changes to the regulatory approval process for product candidates in those jurisdictions, including the U.S., in which we may be seeking approval for our product candidates; |
| • | the cost of filing, prosecuting, defending and enforcing any patent claim and other intellectual property rights, including costs associated with the Upsher Smith Litigation and the outcome thereof; |
| • | the extent to which we acquire or invest in businesses, products and technologies; and; |
| • | the timing of payment of milestones from licensing activities. |
These factors could result in variations from our currently projected operating and liquidity requirements. If our existing resources are insufficient to satisfy our liquidity requirements, we may need to borrow money or sell additional equity or debt securities. We may not be able to borrow money on commercially reasonable terms, particularly in light of the recent global economic crisis which has caused significant volatility in the credit and equity market. Moreover, the terms of the sale of any equity or debt securities may not be acceptable to us and could result in substantial dilution of your investment. If we are unable to obtain this additional financing, we may be required to:
| • | reduce the size or scope, or both, of our sales and marketing efforts for Testim, XIAFLEX or any of our future products; |
| • | delay or reduce the scope of, or eliminate one or more of our planned development, commercialization or expansion activities; |
| • | seek collaborators for our product candidates at an earlier stage than otherwise would be desirable and on terms that are less favorable than might otherwise be available, and/or |
| • | relinquish, license or otherwise dispose of rights to technologies, product candidates or products that we currently market or would otherwise seek to develop or commercialize ourselves on terms that are less favorable than might otherwise be available. |
Our revenues, operating results and cash flows may fluctuate in future periods and we may fail to meet investor expectations, which may cause the price of our common stock to decline.
Variations in our quarterly operating results are difficult to predict and may fluctuate significantly from period to period. We plan to launch XIAFLEX for Dupuytren’s in the U.S. in late March 2010. Because XIAFLEX for Dupuytren’s is a new product, its sales prospects are uncertain. We cannot predict with certainty the timing or future level of sales in the U.S. of XIAFLEX for Dupuytren’s. If our quarterly sales or operating results fall below the expectations of investors or securities analysts, including investors and securities analysts’ expectations regarding the success of the launch of XIAFLEX, the price of our common stock could decline substantially. In addition to the other factors discussed under these “Risk Factors,” specific factors that may cause fluctuations in our operating results include:
| • | demand and pricing for our products, including any change in wholesaler purchasing patterns or cost structures for our products or their services; |
| • | growth of the overall androgen market which may be influenced by the sales and marketing efforts of our competitors; |
| • | prescription levels relating to physician and patient acceptance of, and prescription costs for our products or any of our future products; |
| • | prescription levels in the chain of distribution; |
68
Table of Contents
| • | government or private healthcare reimbursement policies, including the timing to establish reimbursement processes for XIAFLEX; |
| • | changes to the regulatory approval process for product candidates in those jurisdictions, including the U.S., in which we may be seeking approval for our product candidates; |
| • | any regulatory actions that could limit the use of our marketed products, such as REMS and boxed warnings; |
| • | introduction of competing products, including generics; |
| • | any interruption in the manufacturing or distribution of our products or any of our future products; |
| • | the timing and results of clinical trials for our product candidates; |
| • | the costs incurred in the manufacture of our products and our product candidates; |
| • | our operating expenses, many of which are relatively fixed; |
| • | timing and costs associated with any new product or technology acquisitions we may complete; |
| • | timing and costs associated with any new out-licensing agreements we may enter; |
| • | variations in our rates of product returns, allowances and rebates and discounts; |
| • | Pfizer’s performance under the license agreement for XIAFLEX; and |
| • | milestone payments. |
As a result of these factors, we believe that period-to-period comparisons of our operating results are not a good indication of our future performance.
The effects of the recent global economic crisis may impact our business, operating results or financial condition.
U.S. and foreign markets have recently experienced historic dislocations and liquidity disruptions which have caused a general tightening in the credit markets, lower levels of liquidity, increases in the rates of default and bankruptcy, and extreme volatility in credit, equity and fixed income markets. While there has been improvement in the U.S. and foreign markets in recent months, access to the credit, equity and fixed income markets remains limited. These macroeconomic developments could negatively affect our business, operating results or financial condition in a number of ways. For example:
| • | current or potential customers may be unable to fund purchases, which could cause them to delay, decrease or cancel purchases of our products or to not pay us or to delay paying us for previously purchased products; |
| • | current or potential patients may be unable or unwilling to pay for pharmaceutical prescriptions; and |
| • | third-party manufacturers and service providers may be unable to provide contracted goods or services. |
In addition, financial institution failures may make it difficult either to obtain financing for potential operating needs or for investing activities, including the financing of any future acquisitions. Also, our investment policy, which includes short-term debt securities, is generally subject to general credit, liquidity, counterparty, market and interest rate risks that may be exacerbated by the recent global financial crisis. If the banking system or the fixed income, credit or equity markets remain volatile, our investment portfolio may be impacted and the values and liquidity of our investments could be adversely affected.
Legislative or regulatory reform of the healthcare system may affect our ability to sell our products profitably.
In both the United States and certain foreign jurisdictions, there have been a number of legislative and regulatory proposals to change the healthcare system in ways that could impact our ability to sell our products
69
Table of Contents
profitably. In recent years, new legislation has been proposed in the United States at the federal and state levels that would effect major changes in the healthcare system, either nationally or at the state level. Recently, President Obama and members of Congress have proposed significant reforms. On November 7, 2009, the House of Representatives passed, and on December 24, 2009, the Senate passed health care reform legislation that would require most individuals to have health insurance, establish new regulations on health plans, create insurance pooling mechanisms and a government health insurance option to compete with private plans and other expanded public health measures. This legislation would also reduce Medicare spending on services provided by hospitals and other providers. It is still too early to determine the impact of the proposed legislation on the pharmaceutical industry and our business. Further federal and state proposals are likely. However, an expansion in government’s role in the U.S. healthcare industry may lower reimbursements for our products, reduce medical procedure volumes and adversely affect our business, possibly materially. In addition, the potential for adoption of these proposals could affect our ability to raise capital, obtain additional collaborators and market our products. Our results of operations could be adversely affected by future health care reforms.
We entered into a two year revolving credit agreement (“Revolving Line of Credit Agreement”), dated as of July 31, 2009, with Silicon Valley Bank (“SVB”). The terms of our Revolving Line of Credit Agreement may restrict our current and future operations, which would adversely affect our ability to respond to changes in our business and to manage our operations.
Our Revolving Line of Credit Agreement requires us to maintain certain financial ratios and contains affirmative and negative covenants (including, but not limited to, limitations on the incurrence of indebtedness, asset dispositions, acquisitions, investments, dividends and other restricted payments, liens and transactions with affiliates) which may restrict our current and future operations. Our Revolving Line of Credit Agreement also requires us to maintain certain financial ratios. To secure the repayment of any amounts borrowed under this Revolving Line of Credit Agreement, we granted to SVB a first priority security interest in all of our assets, including our intellectual property and our rights under license agreements granting us rights to intellectual property. We also agreed not to pledge or otherwise encumber our intellectual property assets without SVB’s approval.
As of the date hereof, we do not have an outstanding balance on the Revolving Line of Credit Agreement. However, were we to draw on the Revolving Line of Credit Agreement, in the event of an event of default, SVB has the right to declare the amounts borrowed under the Revolving Line of Credit Agreement immediately due and payable, and terminate all commitments to extend further credit. An event of default under the Revolving Line of Credit Agreement includes, among other things, the failure to make payments when due, breaches of representations, warranties or covenants, the occurrence of certain insolvency events, or the occurrence of an event which could have a material adverse effect on us. If we were unable to repay those amounts, SVB could proceed against the collateral granted pursuant to the Revolving Line of Credit Agreement. If SVB accelerates the repayment of our borrowings, we cannot assure you that we will have sufficient cash on hand to repay the amounts borrowed under the Revolving Line of Credit Agreement.
Risks Related to Stock Market Price
The market price of our common stock is likely to be volatile.
Prior to our initial public offering in July 2004, there was no public market for our common stock. Since our initial public offering, our stock price has, at times, been volatile. We cannot assure you that an active trading market for our common stock will exist at any time. Holders of our common stock may not be able to sell shares quickly or at the market price if trading in our common stock is not active. Substantially all of our outstanding shares of our common stock are eligible for sale in the public market. For the 30 days prior to the end of the period covered by this Report, our average daily trading volume was approximately 575,000 shares. Sales of a substantial number of shares of our common stock in the public market, or the perception in the market that holders of a large number of shares intend to sell shares, could depress the market price of our common stock and
70
Table of Contents
impair our ability to raise capital through the sale of additional equity securities, even if our business is doing well. In addition, the market price of our common stock could be subject to wide fluctuations in price in response to various factors, many of which are beyond our control, including:
| • | our failure to achieve our operational and research and development goals; |
| • | changes to the regulatory approval process for product candidates in those jurisdictions, including the U.S., in which we may be seeking approval for our product candidates; |
| • | market acceptance and sales growth of our products; |
| • | growth of the overall androgen market, which may be influenced by the sales and marketing efforts of our competitors; |
| • | entry into the marketplace of competitive products including generics; |
| • | developments concerning therapies that compete with Testim in the treatment of hypogonadism in adult males; |
| • | our ability to manufacture any products to commercial standards; |
| • | results of our clinical trials; |
| • | the regulatory status of our product candidates; |
| • | failure of any of our product candidates, if approved, to achieve commercial success; |
| • | regulatory developments in the U.S. and foreign countries; |
| • | developments or disputes concerning our patents or other proprietary rights; |
| • | public concern over our drugs; |
| • | litigation involving us, our general industry or both; |
| • | future sales of our common stock; |
| • | changes in the structure of healthcare payment systems, including developments in price control legislation; |
| • | departure of key personnel; |
| • | the degree to which any co-promotion, licensing or distribution arrangements generate revenue; |
| • | period-to-period fluctuations in our financial results or those of companies that are perceived to be similar to us; |
| • | announcements of material events by those companies that are our third-party manufacturers and services providers, our partners, our competitors or perceived to be similar to us; |
| • | changes in estimates of our financial results or recommendations by securities analysts; |
| • | investors’ general perception of us; and |
| • | general economic, industry and market conditions. |
Furthermore, market prices for securities of pharmaceutical, biotechnology and specialty biopharmaceutical companies have been particularly volatile in recent years. The broad market and industry factors may seriously harm the market price of our common stock, regardless of our operating performance. If any of these risks occurs, it could cause our stock price to fall and may expose us to class action lawsuits that, even if unsuccessful, could be costly to defend and a distraction to management.
71
Table of Contents
Possible dilutive effect of outstanding options and warrants.
At the end of the period covered by this Report, stock options to purchase 5,359,110 shares of common stock and warrants to purchase 102,524 shares of common stock were outstanding. In addition, as of December 31, 2009, a total of 2,444,722 stock options are available for grant under our 2004 Equity Compensation Plan amended and restated as of December 1, 2009. A total of 1,874,113 of the outstanding options and warrants are “in the money” and exercisable as of December 31, 2009. “In the money” means that the current market price of the common stock is above the exercise price of the shares subject to the warrant or option. The issuance of common stock upon the exercise of these options and warrants could adversely affect the market price of the common stock or result in substantial dilution to our existing stockholders.
Provisions in our certificate of incorporation and bylaws and under Delaware law may prevent or frustrate a change in control in management that stockholders believe is desirable.
Provisions of our certificate of incorporation and bylaws may discourage, delay or prevent a merger, acquisition or other change in control that stockholders may consider favorable, including transactions in which you might otherwise receive a premium for your shares. These provisions may also prevent or frustrate attempts by our stockholders to replace or remove our management. These provisions include:
| • | limitations on the removal of directors; |
| • | advance notice requirements for stockholder proposals and nominations; |
| • | the inability of stockholders to act by written consent or to call special meetings; and |
| • | the ability of our board of directors to designate the terms of, and issue, new series of preferred stock without stockholder approval, which could be used to institute a rights plan that would work to dilute the stock ownership of a potential hostile acquirer, effectively preventing acquisitions that have not been approved by our board of directors. |
The affirmative vote of the holders of at least two-thirds of our shares of capital stock entitled to vote is necessary to amend or repeal the above provisions of our certificate of incorporation. In addition, absent approval of our board of directors, our bylaws may only be amended or repealed by the affirmative vote of the holders of at least two-thirds of our shares of capital stock entitled to vote.
In addition, Section 203 of the General Corporation Law of the State of Delaware prohibits a publicly held Delaware corporation from engaging in a business combination with an interested stockholder, generally a person which together with its affiliates owns or within the last three years has owned 15% of our voting stock, for a period of three years after the date of the transaction in which the person became an interested stockholder, unless the business combination is approved in a prescribed manner. Accordingly, Section 203 may discourage, delay or prevent a change in control of our company.
| ITEM 1B. | Unresolved Staff Comments |
None.
| ITEM 2. | Properties |
Malvern. We currently lease approximately 62,092 square feet of space for our headquarters in Malvern, Pennsylvania, of which approximately 57,130 square feet is dedicated to office space and approximately 4,962 square feet is dedicated to warehousing and packaging operations. The initial term of this lease ends on December 31, 2013.
72
Table of Contents
Horsham. In Horsham, Pennsylvania, we lease a 50,000 square foot biological manufacturing facility that we are using to produce the active ingredient of XIAFLEX and approximately 56,000 square feet of laboratory, warehouse and office space in two other Horsham locations. The initial term of these leases ends on January 1, 2017, March 31, 2017 and July 31, 2022, respectively. In general, our properties are well maintained, adequate and suitable for the purposes for which they are used.
| ITEM 3. | Legal Proceedings |
On December 4, 2008, we and CPEX filed a lawsuit against Upsher-Smith Laboratories, Inc. (“Upsher-Smith”) for infringement of CPEX’s U.S. Patent No. 7,320,968 (“the ‘968 Patent”), which covers Testim®, 1% testosterone gel. The lawsuit was filed in the United States District Court for the District of Delaware. We and CPEX are seeking a judgment (1) declaring that Upsher-Smith’s act of filing the Abbreviated New Drug Application seeking approval from the FDA to market a generic version of Testim prior to the January 2025 expiration of the ‘968 Patent is an act of infringement, (2) declaring that the making, using or selling of the product for which Upsher-Smith is seeking approval infringes the ‘968 Patent, (3) ordering that the effective date of FDA’s approval of the product not be until the expiration of the ‘968 Patent, (4) enjoining Upsher-Smith from making, using or selling the product for which it seeks approval until the expiration of the ‘968 Patent, and (5) awarding Auxilium and CPEX their costs and expenses in the action. The companies filed this lawsuit under the Hatch-Waxman Act in response to the notice from Upsher-Smith of its filing of an ANDA with the FDA containing a Paragraph IV certification under 21 U.S.C. Section 355(j) for testosterone gel. Upsher-Smith has alleged that its proposed generic version of Testim would not infringe the ‘968 patent, and that the ‘968 patent is invalid. The Paragraph IV certification notice states that Upsher-Smith does not believe that the testosterone gel product for which it is seeking approval infringes the ‘968 patent and that it seeks to market its generic product before the expiration of the ‘968 patent. The ‘968 Patent is listed in Approved Drug Products with Therapeutic Equivalence Evaluations (commonly known as the “Orange Book”), published by the FDA, and will expire in January 2025. The lawsuit remains pending.
| ITEM 4. | Submission of Matters to a Vote of Security Holders |
No matters were submitted to the vote of stockholders through the solicitation of proxies or otherwise during the quarter ended December 31, 2009.
73
Table of Contents
| ITEM 5. | Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities |
Market Information
Our common stock is traded on The NASDAQ Global Market under the symbol “AUXL.” The following table sets forth the high and low closing sales prices per share for our common stock for the periods indicated, as reported by The NASDAQ Global Market:
| Year Ended December 31, 2009: |
High | Low | ||||
| First Quarter |
$ | 34.67 | $ | 25.56 | ||
| Second Quarter |
$ | 31.38 | $ | 22.07 | ||
| Third Quarter |
$ | 36.63 | $ | 27.95 | ||
| Fourth Quarter |
$ | 35.30 | $ | 29.63 | ||
| Year Ended December 31, 2008: |
High | Low | ||||
| First Quarter |
$ | 35.40 | $ | 23.00 | ||
| Second Quarter |
$ | 36.14 | $ | 28.00 | ||
| Third Quarter |
$ | 42.35 | $ | 31.76 | ||
| Fourth Quarter |
$ | 32.12 | $ | 16.59 | ||
Holders of Record
As of February 19, 2010, there were approximately 80 holders of record of our common stock. Because many of such shares are held by brokers and other institutions on behalf of stockholders, the Company is unable to estimate the total number of stockholders represented by these record holders.
Dividends
We have never paid or declared any cash dividends on our common stock. We currently intend to retain any earnings for future growth and, therefore, do not expect to pay cash dividends in the foreseeable future.
Securities Authorized for Issuance Under Equity Compensation Plans
The information under the heading “Equity Compensation Plan Information” in our definitive Proxy Statement for our Annual Meeting of Stockholders to be held on June 3, 2010, to be filed with the SEC, is incorporated herein by reference.
Recent Sales of Unregistered Securities
During the quarter ended December 31, 2009, we did not issue any unregistered shares of our common stock.
74
Table of Contents
Comparative Stock Performance Graph
The graph below compares the cumulative total stockholder return on our common stock with the cumulative total stockholder return of (i) the NASDAQ Composite Index, and (ii) the NASDAQ Biotechnology Index, assuming an investment of $100 on December 31, 2004, in each of our common stock; the stocks comprising the NASDAQ Composite Index; and the stocks comprising the NASDAQ Biotechnology Index.
Comparison of Cumulative Total Return* Among Auxilium Pharmaceuticals, Inc, the NASDAQ Composite Index, and the NASDAQ Biotechnology Index
| * | Total return assumes $100 invested on December 31, 2004 in our common stock, the NASDAQ Composite Index, and the NASDAQ Biotechnology Index and reinvestment of dividends through fiscal year ended December 31, 2009. |
| 12/31/2004 | 12/31/2005 | 12/31/2006 | 12/31/2007 | 12/31/2008 | 12/31/2009 | |||||||||||||
| Auxilium Pharmaceuticals, Inc. |
$ | 100.00 | $ | 62.15 | $ | 165.99 | $ | 338.87 | $ | 321.36 | $ | 338.76 | ||||||
| NASDAQ Biotechnology Index |
$ | 100.00 | $ | 102.84 | $ | 103.89 | $ | 108.65 | $ | 94.93 | $ | 109.77 | ||||||
| NASDAQ Composite Index |
$ | 100.00 | $ | 101.37 | $ | 111.03 | $ | 121.92 | $ | 72.49 | $ | 104.31 | ||||||
75
Table of Contents
| ITEM 6. | Selected Financial Data |
SELECTED CONSOLIDATED FINANCIAL DATA
(In thousands, except share and per share data)
The following selected consolidated financial data should be read in conjunction with “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our consolidated financial statements and the related notes appearing elsewhere in this Report. The consolidated statements of operations data for the years ended December 31, 2009, 2008 and 2007 and the consolidated balance sheet data as of December 31, 2009 and 2008 have been derived from our audited consolidated financial statements and related notes, which are included elsewhere in this Report. The consolidated statement of operations data for the years ended December 31, 2006 and 2005 and the consolidated balance sheet data as of December 31, 2007, 2006 and 2005 have been derived from audited financial statements which do not appear in this Report. The historical results presented are not necessarily indicative of results to be expected in any future period.
| Years Ended December 31, | ||||||||||||||||||||
| 2009 | 2008 | 2007 | 2006 | 2005 | ||||||||||||||||
| Consolidated Statement of Operations Data: |
||||||||||||||||||||
| Net revenues |
$ | 164,039 | $ | 125,377 | $ | 95,734 | $ | 68,490 | $ | 42,804 | ||||||||||
| Operating expenses: |
||||||||||||||||||||
| Cost of goods sold |
37,077 | 29,486 | 24,609 | 17,692 | 13,119 | |||||||||||||||
| Research and development (1) |
51,398 | 54,497 | 42,802 | 37,900 | 24,301 | |||||||||||||||
| Selling, general, and administrative (1) |
129,181 | 89,482 | 72,768 | 61,277 | 43,935 | |||||||||||||||
| Total operating expenses |
217,656 | 173,465 | 140,179 | 116,869 | 81,355 | |||||||||||||||
| Loss from operations |
(53,617 | ) | (48,088 | ) | (44,445 | ) | (48,379 | ) | (38,551 | ) | ||||||||||
| Interest income, net |
160 | 1,801 | 3,740 | 2,429 | 1,496 | |||||||||||||||
| Other income (expense), net |
— | — | — | 2 | (1,203 | ) | ||||||||||||||
| Net loss applicable to common stockholders |
$ | (53,457 | ) | $ | (46,287 | ) | $ | (40,705 | ) | $ | (45,948 | ) | $ | (38,258 | ) | |||||
| Basic and diluted net loss per common |
$ | (1.22 | ) | $ | (1.12 | ) | $ | (1.07 | ) | $ | (1.48 | ) | $ | (1.54 | ) | |||||
| Weighted average common shares |
43,650,775 | 41,272,557 | 38,187,662 | 30,956,889 | 24,913,087 | |||||||||||||||
|
(1) includes the following amounts of |
||||||||||||||||||||
| Research and development |
$ | 5,048 | $ | 3,140 | $ | 1,180 | $ | 397 | $ | — | ||||||||||
| Selling, general and administrative |
12,852 | 8,602 | 4,857 | 2,685 | 272 | |||||||||||||||
| (1) | The Company adopted the current authoritative guidance for shares-based payments using the modified prospective transition method, which requires the application of this accounting guidance as of January 1, 2006, the first day of the Company’s calendar year. The Company’s consolidated financial statements as of, and for each of the years ended, December 31, 2009, 2008, 2007 and 2006 reflect the current guidance. In accordance with the modified prospective method, the Company’s consolidated financial statements for periods prior to 2006 have not been restated for, and therefore do not include, compensation expense for stock option awards. |
76
Table of Contents
| (2) | The increase in weighted average common shares outstanding and the corresponding decrease in basic and diluted net loss per common share in 2009, 2007, and 2006 is primarily the result of the issuance of common shares in the September 2009, June 2007 and September 2006 public offerings. |
| As of December 31, | |||||||||||||||
| 2009 | 2008 | 2007 | 2006 | 2005 | |||||||||||
| Consolidated Balance Sheet Data: |
|||||||||||||||
| Cash and cash equivalents |
$ | 181,977 | $ | 113,943 | $ | 70,290 | $ | 44,835 | $ | 31,380 | |||||
| Short-term investments |
— | — | 5,800 | 9,909 | 25,350 | ||||||||||
| Total assets |
260,564 | 171,187 | 106,979 | 76,759 | 72,695 | ||||||||||
| Total stockholders’ equity |
120,519 | 35,266 | 64,016 | 42,329 | 39,873 | ||||||||||
| ITEM 7. | Management’s Discussion and Analysis of Financial Condition and Results of Operations |
You should read the following discussion and analysis of our financial condition and results of operations together with our consolidated financial statements and the related notes appearing at the end of this Report. Some of the information contained in this discussion and analysis or set forth elsewhere in this Report, including information with respect to our plans and strategy for our business and related financing, includes forward-looking statements that involve risks and uncertainties. You should review the “Risk Factors” section of this Report for a discussion of important factors that could cause actual results to differ materially from the results described in or implied by the forward-looking statements contained in the following discussion and analysis.
Overview
We are a specialty biopharmaceutical company with a focus on developing and marketing products to predominantly specialist audiences, such as urologists, endocrinologists, certain targeted primary care physicians, hand surgeons, subsets of orthopedic, general, and plastic surgeons who focus on the hand, and rheumatologists. We currently have approximately 540 employees, including a sales and marketing organization of approximately 315 people. We reported revenues in 2009 of $164 million, an increase of 30.8% over the $125.4 million reported in 2008.
We currently market two products in the United States (“U.S.”):
Testim® is a proprietary, topical 1% testosterone once-a-day gel indicated for the treatment of hypogonadism. Hypogonadism is defined as reduced or absent secretion of testosterone which can lead to symptoms such as low energy, loss of libido, adverse changes in body composition, irritability and poor concentration. Testim has been approved in the U.S. by the U.S. Food and Drug Adminsitration (“FDA”). According to National Prescription Audit (“NPA”) data from IMS Health, Inc. (“IMS”), a pharmaceutical market research firm, Testim’s share of total prescriptions for the gel segment of the U.S. testosterone replacement therapy (“TRT”) market was 22.3% for the month of December 2009, and 22.0% for the full year 2009.
XIAFLEX™ (collagenase clostridium histolyticum) is a proprietary, injectable collagenase enzyme for the treatment of Dupuytren’s contracture (“Dupuytren’s”). XIAFLEX received approval from the FDA on February 2, 2010 for the treatment of adult Dupuytren’s patients with a palpable cord. Dupuytren’s is a condition that affects the connective tissue that lies beneath the skin in the palm. The disease is progressive in nature. Typically, nodules develop in the palm as collagen deposits accumulate. As the disease progresses, the collagen deposits form a cord that stretches from the palm of the hand to the base of the finger. Once this cord develops, the patient’s fingers contract and the function of the hand is impaired. Prior to approval of XIAFLEX, surgery was the only effective treatment. We expect to begin shipping XIAFLEX to our distribution partners in early March 2010 in advance of a launch planned for the end of March 2010.
77
Table of Contents
We also have received marketing approval for Testim in Belgium, Canada, Denmark, Finland, Germany, Greece, Iceland, Ireland, Italy, Luxembourg, the Netherlands, Norway, Portugal, Spain, Sweden and the United Kingdom (“U.K.”). Ferring International Center S.A. (“Ferring”) and Paladin Labs Inc. (“Paladin”) commercialize Testim on our behalf in certain European countries and Canada, respectively.
Our current product pipeline includes:
Phase II:
| • | XIAFLEX for the treatment of Peyronie’s disease (“Peyronie’s”) |
| • | XIAFLEX for the treatment of Adhesive Capsulitis (“Frozen Shoulder syndrome”) |
Phase I:
| • | AA4010, treatment for overactive bladder using our transmucosal film delivery system |
| • | A Fentanyl pain product using our transmucosal film delivery system. |
In addition to the above, we have the rights to develop other compounds for the treatment of pain using our transmucosal film delivery system and other products using our transmucosal film technology for treatment of urologic disease and for hormone replacement. We also have the option to license additional indications for XIAFLEX other than dermal products for topical administration.
Operational Highlights
On February 2, 2010, we received marketing approval from the FDA for XIAFLEX for Dupuytren’s for the treatment of adult patients with a palpable cord. Currently, we are preparing for a launch of XIAFLEX for Dupuytren’s planned for the end of March 2010. We expect to begin shipping XIAFLEX to our distributors the week of March 1, 2010 with product being available for shipment to physicians during the week of March 8, 2010. We will market and sell XIAFLEX in the U.S. through a team of approximately 100 field sales managers and representatives, reimbursement specialists and managed market account directors. In addition, a staff of 11 medical science liaisons will provide medical support for XIAFLEX. We have established a distribution network that will allow health care providers to access XIAFLEX through specialty distributors and specialty pharmacies or in the institutional setting after they have undergone training on XIAFLEX and its administration. The FDA has required a risk evaluation and mitigation strategy (“REMS”) program for XIAFLEX, which consists of a communication plan and a medication guide. This REMS is designed (1) to evaluate and mitigate known and potential risks and serious adverse events; (2) to inform healthcare providers about how to properly inject XIAFLEX and perform finger extension procedures; and, (3) to inform patients about the serious risks associated with XIAFLEX. We plan to market XIAFLEX to physicians who are experienced in injection procedures of the hand and treatment of Dupuytren’s and will only provide access to XIAFLEX after physicians have attested to completion of a training program. The training program will be available as a video or written manual and demonstrates proper use and administration of XIAFLEX, as well as an overview of both identified and potential risks with XIAFLEX.
On January 21, 2010 the Company and its European partner, Pfizer Inc. (“Pfizer”), announced the European scientific/technical review procedure for the Marketing Authorization Application (“MAA”) for XIAFLEX for the treatment of Dupuytren’s commenced. As a result of accomplishing this milestone, the Company received $15 million from Pfizer and will make a payment of approximately $1.3 million to its licensor BioSpecifics Technologies Corp. (“BioSpecifics”).
In August 2009, the Company received the FDA’s response to the Company’s Citizen’s Petition regarding Testim. The FDA agreed with some of the statements made in the Citizen’s Petition regarding the testing required for generic versions of Testim and disagreed with other statements. Although not commenting upon any filing in particular, the FDA did state that “the practical effect of this determination is that any
78
Table of Contents
application for a testosterone gel product that has different penetration enhancers than the reference listed drug cannot be submitted as an abbreviated new drug application (ANDA) and, instead, will have to be submitted as a NDA under section 505(b) of the Act.”
On August 5, 2009 the Company secured a two year $30 million revolving credit line from Silicon Valley Bank.
On September 30, 2009 the Company completed the sale of 3.45 million shares of its common stock at a price of $34.50 per share, resulting in proceeds to the Company, net of offering expenses and underwriting discounts and commissions, of approximately $115.8 million.
On May 7, 2009, FDA announced that it was requiring the manufacturers of two prescription topical testosterone gels, Solvay S.A. and Auxilium, to make changes to the prescribing information and develop a REMS for the products. FDA stated that it was requiring this action after it became aware, through spontaneous post-marketing adverse event reports and peer-reviewed biomedical literature, of cases of secondary exposure of children to testosterone due to drug transfer from adult males using testosterone gel drug products (“transference”). FDA considered this information to be “new safety information”. We believe that all topical testosterone gels have a potential for transference. Testim’s prescribing information has described the risk and procedures for avoidance of transference since the product was launched in 2003. The changes to the prescribing information for Testim include a “boxed warning”, which is used to highlight warning information that is especially important to the prescriber. The goal of the REMS is to inform patients about the serious risks associated with the use of Testim and AndroGel. The REMS includes assessments and a Medication Guide to inform patients. The revised prescribing information and REMS was approved on September 18, 2009.
Net revenues. To date, substantially all of our net revenues have been generated by the sales of Testim and out-licensing of Testim and XIAFLEX. We sell Testim in the U.S. to pharmaceutical wholesalers. We do not anticipate that sales of Testim outside of the U.S., pursuant to our current agreements for international distribution rights of Testim, will have a material impact on our revenues or profitability. We will begin selling XIAFLEX through a network of wholesalers, specialty distributors and specialty pharmacies in 2010. We expect our revenues to fluctuate due to:
| • | launch of, market acceptance and pricing of XIAFLEX; |
| • | market acceptance and pricing for Testim, including any change in wholesaler purchasing patterns; |
| • | regulatory approvals and market acceptance of new and competing products, including generics; |
| • | government or private healthcare reimbursement policies for XIAFLEX for and Testim; and |
| • | promotional efforts of our competitors. |
We use a third-party logistics company, ICS, a division of AmerisourceBergen Corporation (“ABC”) for commercial distribution of Testim and XIAFLEX. The majority of Testim sales in the U.S. are to pharmaceutical wholesalers that, in turn, distribute product to chain and other retail pharmacies, hospitals, mail-order providers and other institutional customers. Over 90% of Testim purchases are from three customers: ABC, Cardinal Health, Inc. and McKesson Corporation. All XIAFLEX sales in the U.S. will be to a small network of third party specialty distributors and specialty pharmacies that will generally distribute XIAFLEX to hospitals and to physician practices.
Outside of the U.S., we currently rely on third parties to market, sell and distribute our products. For Canada, we have an agreement with Paladin to market and distribute Testim. We also have an agreement with Ferring to market and distribute Testim on a worldwide basis outside the U.S., Canada, Mexico, Japan, China, Poland, Russia, and South Korea and their territories and possessions. In December 2008, we entered into a
79
Table of Contents
development, commercialization and supply agreement with Pfizer (the “Pfizer Agreement”) under which we sub-licensed our commercialization rights for Dupuytren’s and Peyronie’s for the 27 countries which at that time were included in the European Union (“EU”) and 19 additional Eastern European and Eurasian countries (the “Pfizer Territory”). We are currently evaluating our options for selling XIAFLEX in other indications and territories throughout the rest of the world.
The up-front payment we have received under the Pfizer Agreement is being amortized, net of associated transaction costs, over the estimated term of the agreement. The agreement also includes payment of increasing, tiered, double-digit royalties on all revenues booked by Pfizer in its territory. The up-front and milestone payments we have received from Ferring and Paladin are also being amortized to revenue over the respective contact life of each of these agreements.
Under contractual agreements with our three largest wholesalers of Testim, we pay a fee for service to the wholesalers based on shipment activity. The agreements also provide for targeted levels of required distributor inventory. Otherwise, distributors independently manage their inventories with no intervention by us. Aside from the service fees required under these agreements, we do not offer incentives for wholesalers to take shipment of product.
We record sales of Testim net of allowances for prompt payment discounts, fees to wholesalers based on shipment activity under the terms of wholesaler service agreements, managed care contract rebates and government health plan charge backs, product coupons and product returns. Total product sales allowances have amounted to 23.0%, 20.3% and 18.8% of U.S. Testim revenue for the years ended December 31, 2009, 2008 and 2007, respectively. The year-over-year growth of this percentage results primarily from our entry into additional pricing contracts with managed care providers and from product coupon programs designed to increase revenue growth.
Cost of goods sold. Substantially all of our cost of goods sold currently relate to the sale of Testim. With the commercial launch of XIAFLEX, we expect that cost of sales will increase commensurate with its sales volume with the anticipated market acceptance of the product.
Costs of goods consist of the following types of costs:
| • | raw materials; |
| • | fees paid to Testim and XIAFLEX contract manufacturers and related costs; |
| • | costs of the operation of the Horsham manufacturing facilities, |
| • | Testim royalty payments, which currently consist solely of payments due to CPEX; |
| • | XIAFLEX royalty payments to BioSpecifics discussed below; |
| • | personnel costs associated with quality assurance and manufacturing oversight; and |
| • | distribution costs, including warehousing, freight and product liability insurance. |
We do not anticipate any material changes in our gross margin rate on Testim sales in the U.S. We anticipate our gross margins for Testim sales outside the U.S. will continue to be significantly lower than those seen in the U.S. This is due to a combination of factors which include the terms of our distribution agreements.
As we launch XIAFLEX in the U.S., we may experience variability in our gross margin rate and it is difficult to estimate when we will achieve a more predictable steady state. Factors influencing the gross margin on XIAFLEX will include the selling price per vial and the cost of product manufactured. Although we will utilize a standard costing system for the recognition of cost of goods sold, it is not uncommon in biologics manufacturing to experience unusual charges and write offs as production is ramping up.
80
Table of Contents
Under our license with BioSpecifics, we paid BioSpecifics 8.5% of the upfront payment received from Pfizer and will owe them 8.5% of any milestone payments received from Pfizer under the Pfizer Agreement. The $6,375,000 payment to BioSpecifics as a result of the $75,000,000 up-front Pfizer payment was recorded as a deferred charge and is also being amortized on a straight-line basis to Cost of goods sold over the estimated life of the Pfizer Agreement. We are also obligated to pay BioSpecifics 8.5% of all milestone payments received from Pfizer under the Pfizer Agreement, a specified percentage of XIAFLEX sales by Pfizer, and on a country-by-country and product-by-product basis a specified royalty percentage, which may vary dependent on certain specified circumstances, of all other XIAFLEX sales.
Research and development. Our research and development expenses consist of:
| • | salaries and expenses for our development personnel; |
| • | costs of the operation of the Horsham facilities, except for those costs associated with the production of saleable XIAFLEX inventory which was capitalized during 2009. In future years, we expect the majority of the costs incurred at Horsham to be included in cost of goods sold; however, there are certain research related activities taking place in Horsham and these costs will be included as research and development; |
| • | payments to consultants, investigators, contract research organizations and manufacturers in connection with our preclinical and clinical trials; |
| • | costs of developing and obtaining regulatory approvals; and |
| • | product license and milestone fees paid prior to regulatory approval. |
Due to the significant risks and uncertainties inherent in the clinical development and regulatory approval processes, the cost to complete projects in development cannot be reasonably estimated. XIAFLEX is in phase II of development for the treatment of Peyronie’s disease and Frozen Shoulder syndrome AA4010 and a Fentanyl pain product using transmucosal film technology are in phase I of development. Results from clinical trials may not be favorable. Further, data from clinical trials is subject to varying interpretation, and may be deemed insufficient by the regulatory bodies reviewing applications for marketing approvals. As such, clinical development and regulatory programs are subject to risks and changes that may significantly impact cost projections and timelines. We expect our research and development expenses in 2010 for our current products to be comparable to 2009 spending, as we focus on development of XIAFLEX for Peyronie’s, Frozen Shoulder syndrome and potential other indications in life cycle management projects with respect to XIAFLEX.
However, the current regulatory and political environment at the FDA, could lead to increased data requirements which could impact regulatory timelines and costs. We could also experience further significant increases in our expenditures to develop any other potential new product candidates that we would in-license or acquire.
Selling, general and administrative. Selling, general and administrative expenses consist primarily of salaries and other related costs for personnel, marketing and promotion costs, professional fees and facilities costs. We anticipate increases in selling, general and administrative expenses due to sales and marketing costs associated with Testim and XIAFLEX, hiring of additional personnel, stock compensation expense, information technology expense, investor relations and other activities associated with operating as a publicly traded company.
81
Table of Contents
Results of Operation
Years Ended December 31, 2009 and 2008
Net revenues. Net revenues for the two years ended December 31, 2009 comprise the following:
| Year ended December 31, | % Change | ||||||||||||||
| 2009 | 2008 | Change | |||||||||||||
| (in thousands) | |||||||||||||||
| Gross Testim U.S. revenues |
$ | 197,322 | $ | 154,718 | $ | 42,604 | 27.5 | % | |||||||
| Less- Revenue allowances |
(45,383 | ) | (31,392 | ) | 13,991 | 44.6 | % | ||||||||
| Net Testim U.S. revenues |
151,939 | 123,326 | 28,613 | 23.2 | % | ||||||||||
| International revenues- |
|||||||||||||||
| Testim shipments |
1,962 | 979 | 983 | ||||||||||||
| Testim contract revenue |
6,570 | 935 | 5,635 | ||||||||||||
| XIAFLEX contract revenue |
3,568 | 137 | 3,431 | ||||||||||||
| Total International revenues |
12,100 | 2,051 | 10,049 | 490.0 | % | ||||||||||
| Total revenues |
$ | 164,039 | $ | 125,377 | $ | 38,662 | 30.8 | % | |||||||
| Revenue allowance as a percentage of gross U.S. Testim revenues |
23.0 | % | 20.3 | % | 2.7 | % | |||||||||
The increase in gross Testim U.S. revenues for 2009 compared to 2008 resulted primarily from substantial growth in Testim prescriptions resulting from increased demand and increases in pricing. According to NPA data from IMS, a pharmaceutical market research firm, Testim total prescriptions for 2009 grew 14.9% compared to 2008. This prescription growth was consistent with prescription growth of the overall gel market. We believe that Testim prescription growth in the 2009 period over the 2008 period was driven by physician and patient acceptance that Testim provides better patient outcomes, and the continued focus of our sales force on the promotion of Testim to urologists, endocrinologists and select primary care physicians. Gross Testim U.S. revenues for 2009 also benefited from price increases having a cumulative impact of 10% over the comparable 2008 period. Revenue allowances for 2009 over 2008 increased disproportionately in comparison to the increase in related gross revenues because of increases in the percentages of revenue allowance for product rebates as Testim became more widely prescribed by managed care providers and because of increased coupon usage. Contract revenues shown in the above table represent the amortization of deferred up-front, milestone and royalty payments we have received under our out-licensing agreements. International Testim contract revenue represents the amortization of payments received under the license and distribution agreements with Ipsen Pharma GmbH (“Ipsen”), Paladin and Ferring. During 2009, the remaining $6.4 million balance of milestone payments received from Ipsen was recognized as revenue in connection with the termination of the Ipsen relationship and transfer of the marketing authorizations in Europe to our current Testim partner, Ferring. This one-time revenue benefit accounted for the increase in International Testim contract revenue for 2009 over 2008. International XIAFLEX contract revenue represents the amortization of payments received under the Pfizer Agreement which commenced in December 2008.
Cost of goods sold. Cost of goods sold were $37.7 million and $29.5 million for the years ended December 31, 2009 and 2008, respectively. The increase in cost of goods sold in 2009 over 2008 was directly attributable to the increase in Testim units sold. Gross margin for U.S. Testim revenues was 76.9% in 2009 compared to 76.7% for 2008. The improvement in U.S. gross margin in 2009 over 2008 reflects primarily the impact of year-over-year price, net of increases in rebates and coupon usage. Overall gross margin on net revenues increased to 77.4% in 2009 compared to 76.5% for 2008. This increase primarily results from the increase in International contract revenues, including the one-time revenue benefit related to the Ipsen termination.
82
Table of Contents
Research and development expenses. Research and development expenses were $51.4 million and $54.5 million for the years ended December 31, 2009 and 2008, respectively. The reduction in spending was the result of lower clinical development activity related to XIAFLEX for Dupuytren’s contracture, partially offset by increased manufacturing development costs in Horsham prior to commencement of commercial production activity in 2009 and additional stock-based compensation expense.
Selling, general and administrative expenses. Selling, general and administrative expenses were $129.2 million and $89.5 million for the years ended December 31, 2009 and 2008, respectively. The increase in 2009 over 2008 was due primarily to selling and marketing expenses increasing by $31.0 million resulting from higher investments in headcount related to the XIAFLEX commercial team, pre-launch marketing for XIAFLEX and promotional spending for Testim. General and administrative expenses increased by $8.7 million as a result of additional stock-based compensation expense and higher legal expenses.
Interest income Interest income was $0.2 million and $1.8 million for the years ended 2009 and 2008, respectively, and relates primarily to interest earned on investment of available cash.
Income Taxes. At December 31, 2009, we had Federal tax return net operating loss carryforwards of approximately $209.4 million, which will expire in 2019 through 2028, if not utilized. In addition, we had overall state tax return net operating loss carryforwards of approximately $178.9 million, of which $88.0 million relate to Pennsylvania, which will expire 2010 through 2029 if not utilized. Future utilization of Pennsylvania net operating losses is limited to the greater of 20% of Pennsylvania taxable income or $3.0 million per year. At December 31, 2009, we had federal research and development credits of approximately $6.5 million that will expire in 2020 through 2029, if not utilized.
The Tax Reform Act of 1986 (the “Act”) provides for a limitation on the annual use of net operating loss and research and development tax credit carryforwards following certain ownership changes (as defined in the Act) that could limit our ability to utilize these carryforwards. During 2008 and 2009, we conducted a study to determine whether we have experienced any such ownership changes. Based on this study, we have concluded that we have undergone multiple ownership changes in previous years. Accordingly, our ability to utilize the aforementioned carryforwards will be limited on an annual basis. In addition, approximately $10.7 million and $9.4 million of Federal and state net operating loss carryforwards, respectively, may expire prior to utilization.
Years Ended December 31, 2008 and 2007
Net revenues. Net revenues for the two years ended December 31, 2008 comprise the following:
| Year ended December 31, | % Change | ||||||||||||||
| 2008 | 2007 | Change | |||||||||||||
| (in thousands) | |||||||||||||||
| Gross Testim U.S. revenues |
$ | 154,718 | $ | 114,069 | $ | 40,649 | 35.6 | % | |||||||
| Less—Revenue allowances |
(31,392 | ) | (21,410 | ) | 9,982 | 46.6 | % | ||||||||
| Net Testim U.S. revenues |
123,326 | 92,659 | 30,667 | 33.1 | % | ||||||||||
| International revenues— |
|||||||||||||||
| Testim shipments |
979 | 2,077 | (1,098 | ) | |||||||||||
| Testim contract revenue |
935 | 998 | (63 | ) | |||||||||||
| XIAFLEX contract revenue |
137 | — | 137 | ||||||||||||
| Total International revenues |
2,051 | 3,075 | (1,024 | ) | -33.3 | % | |||||||||
| Total revenues |
$ | 125,377 | $ | 95,734 | $ | 29,643 | 31.0 | % | |||||||
| Revenue allowance as a percentage of gross U.S. Testim revenues |
20.3 | % | 18.8 | % | 1.5 | % | |||||||||
83
Table of Contents
The increase in gross Testim U.S. revenues for 2008 compared to 2007 resulted primarily from substantial growth in Testim prescriptions resulting from increased demand and increases in pricing. According to NPA data from IMS, a pharmaceutical market research firm, Testim total prescriptions for 2008 grew 26.5% compared to 2007. We believe that Testim prescription growth in the 2008 period over the 2007 period was driven by physician and patient acceptance that Testim provides better patient outcomes, the shift in prescriptions away from the testosterone patch product and the other gel product to Testim, and the continued focus of our sales force on the promotion of Testim to urologists, endocrinologists and select primary care physicians. Net revenues for 2008 also benefited from price increases having a cumulative impact of 9% over the comparable 2007 period. Revenue allowances for 2008 over 2007 increased disproportionately in comparison to the increase in the related gross revenues because of increases in the percentages of revenue allowance for product rebates as Testim became more widely prescribed by managed care providers and higher coupon usage.
Cost of goods sold. Cost of goods sold were $29.5 million and $24.6 million for the years ended December 31, 2008 and 2007, respectively. The increase in cost of goods sold in 2008 over 2007 was directly attributable to the increase in Testim units sold. Gross margin for U.S. Testim revenues was 76.7% in 2008 compared to 75.5% for 2007. Overall gross margin on net revenues was 76.5% in 2008 compared to 74.3% for 2007. The improvement in overall gross margin in 2008 over 2007 reflects the impact of year-over-year price increases, the decline in low margin international shipments, and the benefit of volume related manufacturing fee reductions, offset in part by increased coupon usage.
Research and development expenses. Research and development expenses were $54.5 million and $42.8 million for the years ended December 31, 2008 and 2007, respectively. The increase in 2008 over 2007 was primarily due to the increased spending for clinical development of XIAFLEX, preparing for the BLA filing through headcount additions, and additional stock-based compensation expense.
Selling, general and administrative expenses. Selling, general and administrative expenses were $89.5 million and $72.8 million for the years ended December 31, 2008 and 2007, respectively. The increase in 2008 over 2007 was due primarily to selling and marketing expenses increasing by $10.5 million resulting from higher investment in promotional spending for Testim, and pre-launch marketing for XIAFLEX. General and administrative expenses increased by $6.2 million as a result of additional stock-based compensation expense, higher legal expenses, investments in information technology and increases in administrative headcount.
Interest income. Interest income was $1.8 million and $3.7 million for the years ended 2008 and 2007, respectively, and relates primarily to interest earned on investment of available cash.
Sources and Uses of Cash
Cash provided by (used in) operations was $(41.4) million, $46.7 million, and $(30.5) million for 2009, 2008 and 2007, respectively. Cash used in operations for 2009 resulted primarily from operating losses, net of stock compensation expenses and other non-cash charges. Cash used in operations for 2009 included the payment $9.4 million of costs accrued in 2008 in connection with the Pfizer Agreement and an $9.8 million increase in inventory which was primarily due to our build of XIAFLEX for future commercial use in preparation for the its launch. Cash provided by operations for 2008 resulted primarily from the $77.0 million of up-front cash payments received under the Pfizer Agreement and the license and distribution agreement with Ferring which were recorded as deferred revenue, and the collection of $3.7 million in tenant improvement allowances for the Horsham manufacturing facility which was recorded as deferred rent, partially offset by the operating loss, net of stock compensation expense and other non-cash charges, for the year and a $4.3 million increase in inventory which was primarily due to our decision to increase the finished goods inventory level on hand and available for sale. Negative operating cash flow during 2007 was caused primarily by operating losses, net of stock compensation expenses and other non-cash charges.
84
Table of Contents
Cash used in investing activities was $10.6 million, $9.8 million, and $0.4 million for 2009, 2008 and 2007, respectively. Investing activities in each period represent primarily our investments in property and equipment together with, in 2008 and 2007, the net effect of purchases and redemptions of investments for operational cash needs. Our investments in property and equipment relate primarily to improvements made to our Horsham biological manufacturing facility and our information technology infrastructure in preparation for the production of XIAFLEX.
Cash provided by financing activities was $120.0 million, $7.0 million, and $56.3 million in 2009, 2008 and 2007, respectively. Cash provided by financing activity in 2009 results primarily from the $115.8 million in net proceeds we received in the September 2009 common stock offering and $5.1 million in cash receipts from stock option exercises and employee stock purchases. Cash provided by financing activity in 2008 results primarily from the $0.9 million cash received from warrant exercises and $6.6 million in cash receipts from stock option exercises and employee stock purchases. Cash provided by financing activities in 2007 results primarily from the $49.9 million in net proceeds we received in the June 2007 registered direct offering and $6.4 million in cash receipts from stock option exercises and employee stock purchases.
Liquidity and Capital Resources
Since inception through December 31, 2009, we have financed our product development, operations and capital expenditures primarily from private and public sales of equity securities. Since inception through December 31, 2009, we received net proceeds of approximately $403.4 million from our initial public offering, registered direct offering, private and public sales of securities and the exercise of stock options and warrants, including our offering of 3,450,000 shares of common stock completed on September 30, 2009 for which we received proceeds of approximately $115.8 million. We had approximately $182.0 million and $113.9 million in cash and cash equivalents as of December 31, 2009 and December 31, 2008, respectively.
On August 4, 2009, we entered into the Revolving Line of Credit Agreement with SVB which provides us a credit commitment of up to $30.0 million, subject to certain limitations discussed below, and provides SVB with a first priority lien on all our assets as security for borrowings. It also provides for a one-time increase in the amount of the credit commitment of up to $10.0 million upon mutual agreement of the parties. As of December 31, 2009, we had no outstanding borrowing under this agreement.
The financial covenants contained in the Revolving Line of Credit Agreement are a quarterly and cumulative profitability and loss restriction, and a minimum liquidity ratio (“Minimum Quick Ratio”), defined as the ratio of unrestricted cash and cash equivalents, plus net accounts receivable, divided by current liabilities plus, without duplication, all outstanding credit extensions owed to SVB, but excluding deferred revenue and deferred rent. We may not be able to borrow under the Revolving Line of Credit Agreement, or borrowings outstanding may be declared due and payable, if we (x) exceed a quarterly maximum net loss of between $10 million and $30 million, depending on the quarter at issue, during the initial portion of the term of the agreement, (y) fall below a quarterly minimum net income of between $5 million and $10 million, depending on the quarter at issue, during the later portion of the term of the agreement, or (z) fail to maintain a monthly and quarterly Minimum Quick Ratio of between 0.80:1.00 and 1.25:1.00, depending on the month or quarter at issue. Further, when “Net Liquidity”, defined as unrestricted cash and cash equivalents at SVB less borrowings from SVB, is greater than $15.0 million there are no restrictions on the amount which may be borrowed under the credit facility. When Net Liquidity is less than $15.0 million, the amount which may be borrowed under the credit facility is limited to specified percentages of certain customer accounts receivables.
We believe that our current financial resources and sources of liquidity will be adequate for the Company to fund our anticipated operations until such time as we are profitable based on our current plans and expectations. We may, however, elect to raise additional funds prior to this time in order to enhance our sales and marketing efforts for additional products we may acquire, commercialize any product candidates that receive regulatory approval, acquire or in-license approved products or product candidates or technologies for
85
Table of Contents
development and to maintain adequate cash reserves to minimize financial market fundraising risks. Insufficient funds may cause us to delay, reduce the scope of, or eliminate one or more of our development, commercialization or expansion activities. Our future capital needs and the adequacy of our available funds will depend on many factors, including:
| • | our ability to successfully launch and increase sales in the U.S. of XIAFLEX for Dupuytren’s; |
| • | third-party payor coverage and reimbursement for our products; |
| • | the cost of manufacturing, distributing, marketing and selling our products; |
| • | the scope, rate of progress and cost of our product development activities; |
| • | the costs of supplying and commercializing our products and product candidates; |
| • | the effect of competing technological and market developments; |
| • | the cost of filing, prosecuting, defending and enforcing any patent claims and other intellectual property rights, including costs associated with the matter of Auxilium Pharmaceuticals, Inc. and CPEX Pharmaceuticals, Inc. vs. Upsher-Smith Laboratories, Inc. filed on December 4, 2008 in the United States District Court for the District of Delaware and the outcome thereof; |
| • | the extent to which we acquire or invest in businesses and technologies, although we currently have no commitments or agreements relating to any of these types of transactions; and |
| • | entry into the marketplace of competitive products. |
If additional funds are required, we may raise such funds from time to time through public or private sales of equity or debt securities or from bank or other loans. Financing may not be available on acceptable terms, or at all, and our failure to raise capital when needed could materially adversely impact our growth plans and our financial condition or results of operations. Additional equity financing, if available, may be dilutive to the holders of our common stock and may involve significant cash payment obligations and covenants that restrict our ability to operate our business.
We currently have filed with the SEC a registration statement, which became effective on September 19, 2007, that allows for the potential future sale by us, from time to time, in one or more offerings, of up to $31 million of certain debt and equity instruments specified therein.
Contractual Commitments
We are involved with in-licensing of products which are generally associated with payments to the partner from whom we have licensed the product. Such payments frequently take the form of:
| • | an up-front payment, the size of which varies depending on the phase of the product and how many other companies would like to obtain the product, which is paid very soon after signing a license agreement; |
| • | milestone payments which are paid when certain parts of the overall development program are accomplished, or in some cases, when a patent issues; |
| • | payments upon certain regulatory events, such as the filing of an IND, an NDA or BLA, approval of an NDA or BLA, or the equivalents in other countries; |
| • | payments based on a percentage of sales; and |
| • | payments for achievement of certain sales thresholds, such as a payment due the first year a product achieves $100 million in sales. |
86
Table of Contents
We may also out-license products, for which we hold the rights, to other companies for commercialization in other territories, or at times, for other uses. When this happens, the payments to us would also take the same form as described above.
Summary of Contractual Commitments
The following summarizes our contractual commitments as of December 31, 2009 (in thousands):
| Total | Less than 1 year |
1-3 years |
3-5 years |
5+ years | |||||||||||
| Operating leases |
$ | 35,396 | $ | 5,344 | $ | 9,952 | $ | 8,890 | $ | 11,210 | |||||
| Purchase commitment (1) |
190 | 190 | — | — | — | ||||||||||
| $ | 35,586 | $ | 5,534 | $ | 9,952 | $ | 8,890 | $ | 11,210 | ||||||
| (1) | Amount represents a minimum annual manufacturing fee of $0.2 million. |
In early 2010, we received marketing approval from the FDA for XIAFLEX for the treatment of Dupuytren’s and we received a $15 million milestone payment from Pfizer. In connection with these events, we owe BioSpecifies approximately $2.3 million. In addition, we may be obligated to pay $26.2 million of contingent milestone payments in the future, representing our milestone commitments to BioSpecifics for $4.0 million, CPEX for $1.0 million and PharmaForm for $21.2 million. These contingent milestones relate primarily to manufacturing process development, filing of regulatory applications and receipt of regulatory approval. We do not expect to pay any of these contingent milestone payments through December 31, 2010. Further, we owe BioSpecifics a specified percentage of XIAFLEX sales by Pfizer, and on a country-by-country and product-by-product basis a specified royalty percentage, which may vary dependent on certain specified circumstances, of all other XIAFLEX sales, and we owe CPEX a specified percentage royalty of Testim sales.
Off-Balance Sheet Arrangements
We do not have any off-balance sheet arrangements as defined in Item 303(a) (4) of Regulation S-K.
Critical Accounting Policies and Significant Judgments and Estimates
Our management’s discussion and analysis of our financial condition and results of operations discusses our consolidated financial statements, which have been prepared in accordance with U.S. generally accepted accounting principles. The preparation of these financial statements requires us to make estimates and assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the financial statements as well as the reported revenues and expenses during the reporting periods. We base our estimates on historical experience and on various other factors that we believe are reasonable under the circumstances, the results of which form the basis for making judgments about the carrying value of assets and liabilities that are not readily apparent from other sources. We are subject to uncertainties that may cause actual results to differ from these estimates, such as changes in the healthcare environment, competition, legislation and regulation. We believe the following accounting policies, which have been discussed with our audit committee, are the most critical because they involve the most significant judgments and estimates used in the preparation of our consolidated financial statements:
| • | revenue recognition; |
| • | estimating the value of our equity instruments for use in stock-based compensation calculations; and |
| • | inventory. |
87
Table of Contents
Revenue Recognition. In the U.S., we sell Testim to pharmaceutical wholesalers. Prior to the units being dispensed through patient prescriptions, these companies have the right to return Testim for any reason for up to one-year after product expiration. Accordingly, Testim revenue is recognized net of estimated product returns based on our historical experience. In addition, we must make estimates for discounts and rebates provided to third-party payors and coupons provided to patients. Testim is covered by Medicare, Medicaid and numerous independent insurance and pharmacy benefit managers (“PBMs”). Some of these programs have negotiated rebates. We accrue the contractual rebates per unit of product for each individual plan using the most recent historically invoiced plan prescription volumes, adjusted for each individual plan’s prescription growth or contraction, based on data acquired from IMS. In addition, we provide coupons to physicians for use with prescriptions as promotional incentives. We utilize a contract service provider to process and pay claims to patients for actual coupon usage. As Testim becomes more widely used and as we continue to add managed care and PBMs, actual results may differ from our previous estimates. To date, our estimates have not resulted in any material adjustments to our operating results.
The collaboration and out-license agreements we have entered into contain multiple elements. Where we have continuing performance obligations, license and milestone payments are recognized together with up-front payments over the term of the arrangement as we complete our performance obligations, unless the delivered elements have stand alone value to the customer and there is objective, reliable evidence of fair value of the undelivered elements in the arrangement. In the case where the arrangement is considered to be a single unit of accounting, all cash flows under the arrangements are aggregated and recognized as revenue over the estimated term of the arrangement. In addition, unless evidence suggests otherwise, revenue from consideration received is recognized on a straight-line basis over the expected period of the arrangement during which we have continuing performances obligations. In arrangements where we have separate units of accounting, revenue from milestone payments are recognized as revenue upon achievement of the milestone only if the following conditions are met: the milestone payments are non-refundable; achievement of the milestone was not reasonably assured at the inception of the arrangement; substantive effort is involved in achieving the milestone; and the amount of the milestone is reasonable in relation to the effort expended or the risk associated with achievement of the milestone.
For Testim, we currently have license and distribution agreements with Paladin to distribute Testim in Canada and with Ferring to market and distribute Testim worldwide, except for the U.S., Canada, Mexico, Japan, Poland, Russia, South Korea and China, their territories and possessions. Under these agreements, the distributor and the licensee are each required to purchase Testim from us and make up-front, milestone and royalty payments. Product shipments are subject to return and refund only if the product does not comply with technical specifications or if any of the agreements are terminated due to our nonperformance. We are obligated under these agreements to provide multiple deliverables, including the license/product distribution rights, regulatory filing services and commercial product supply. These agreements are treated as single units of accounting as the Company since, at the date of at the agreements, we did not have evidence to support that the consideration for the undelivered item, Testim units to be shipped, is at fair value. We have deferred revenue recognition for non-refundable up-front and milestone payments until at least the first product shipment under each agreement. Upon product shipment, non-refundable up-front and milestone revenue is recognized as revenue on a straight line basis over the remaining contract term. The amortization period is based upon contractual terms of the respective agreement. In July 2009, we began to amortize up-front and milestones received under Ferring agreement non-refundable up-front and milestone revenue as revenue on a straight-line basis over the contract term which is estimated to be 120 months. In May 2006, we began to amortize milestones received under Paladin agreement over its estimated term of 16 years. When earned by us in future periods, additional milestone payments achieved will be amortized over the remaining estimated life of the contracts.
88
Table of Contents
We record sales of Testim net of the following allowances: (a) prompt payment discounts, (b) fees to wholesalers based on shipment activity under the terms of wholesaler service agreements, (c) product returns, (d) managed care contract rebates and government health plan charge backs, and (e) product coupons. The table below provides the balances of accruals relating to each of these allowances as of December 31, 2009 and 2008.
| December 31, | ||||||
| 2009 | 2008 | |||||
| (in thousands) | ||||||
| Accounts receivable reserves: |
||||||
| Prompt pay discounts |
$ | 375 | $ | 295 | ||
| Accrued liabilities: |
||||||
| Wholesaler service agreements |
2,282 | 1,380 | ||||
| Product returns |
3,478 | 2,528 | ||||
| Managed care contract rebates |
9,546 | 7,452 | ||||
| Product coupons |
911 | 630 | ||||
| Subtotal |
16,217 | 11,990 | ||||
| Total |
$ | 16,592 | $ | 12,285 | ||
The nature of each of these allowances and the methodology we use to determine the reserve accrual with respect to each allowance are described as follows:
| (a) | Prompt pay discounts—Prompt payment discounts are offered to all wholesalers in return for payment within 30 days following the invoice date. Based on historical experience, which indicates that virtually all wholesaler payments reflect a deduction for prompt payment discounts, we record sales net of the discount amount. We adjust the reserve at the end of each reporting period to approximate the percentage discount applicable to the outstanding gross accounts receivable balances. |
| (b) | Wholesaler service agreements—Under contractual agreements with our three largest wholesalers (sales to which collectively represented over 90% of 2009 shipments to all of our customers), we provide a fee for service based on shipment activity. The fee rates are set forth in the individual contracts. We track shipments to each wholesaler every period and accrue a liability relating to the unpaid portion of these fees by applying the contractual rates to such shipments. |
| (c) | Product returns—Our return policy permits product returns from wholesalers during a period from six months prior to the product’s expiration date until 12 months subsequent to the expiration date. However, once dispensed by a pharmacy, product may not be returned. In order to estimate product returns, we monitor the remaining shelf life of the product when shipped to customers, actual product returns by individual production batches, NPA data (representing retail prescription information) obtained from IMS, and estimated inventory levels in the distribution channel. By analyzing these factors, we estimate return rate for product sales that remain subject to return and record a return reserve. |
| (d) | Managed care contract rebates and government health plan charge-backs—Managed care contract rebates and government health plan charge backs are rebates and payments provided by us under agreements with private and government health plans. We accrue the contractual rebates per unit of product for each individual plan using the most recent historically invoiced plan prescription volumes, adjusted for each individual plan’s prescription growth or contraction, based on data acquired from IMS. The accrual is continually validated through the payment process, typically within a three month cycle. |
| (e) | Product coupons—Product coupons offer patients the ability to receive free or discounted product through their prescribing physician, to whom we provide an inventory of coupons. We use |
89
Table of Contents
| a third party administrator who invoices us on a monthly basis for the cost of coupons redeemed in the period. Prior to the completion of the financial statements, we generally receive invoices for which the reserve was established. At the end of a reporting period, the accrual for product coupons represents these unpaid invoice amounts and an estimate for unexpired coupons (if any) outstanding. We base our estimates on the historical coupon redemption rate. We maintain the accrual for unexpired coupons based on inventory in the distribution channel and the historical coupon usage, and adjust the accrual whenever changes in coupon usage rate occur. |
The following table provides a roll-forward of the revenue allowances discussed above, in the aggregate, for the years ended December 31, 2009, 2008 and 2007.
| Year ended December 31, | ||||||||||||
| 2009 | 2008 | 2007 | ||||||||||
| (in thousands) | ||||||||||||
| Beginning balance |
$ | 12,285 | $ | 7,670 | $ | 5,882 | ||||||
| Current estimate related to sales in current period |
42,680 | 31,338 | 21,370 | |||||||||
| Current estimate related to sales in prior periods |
(206 | ) | 53 | 40 | ||||||||
| Actual returns / credits in current period related to sales in current period |
(28,564 | ) | (20,221 | ) | (14,155 | ) | ||||||
| Actual returns / credits in current period related to sales in prior period |
(9,603 | ) | (6,555 | ) | (5,467 | ) | ||||||
| Ending balance |
$ | 16,592 | $ | 12,285 | $ | 7,670 | ||||||
In December 2008, we entered into a development, commercialization and supply agreement with Pfizer (the “Pfizer Agreement”). Upon contract signing, Pfizer paid us a $75.0 million non-refundable upfront payment. We are recognizing this $75.0 million up-front payment as revenue, net of associated transaction costs of $3.7 million, over the term of the agreement which is currently estimated to be 20 years. In February 2010, Pfizer paid us a $15.0 million non-refundable milestone payment. In addition, Pfizer may make up to $395 million in potential payments upon the achievement of certain specified additional regulatory and commercial milestones for XIAFLEX. While development costs will be paid by the Party incurring such costs, Pfizer may, on an indication-by-indication basis, recoup its fifty percent (50%) share of such pre-approval territory specific development costs by means of an off-set against the milestone payment due upon first commercial sale for such indication. In addition, Pfizer can recoup up to $2.5 million of territory specific regulatory costs associated with product approval related to Dupuytren’s.
Subject to the requirement to make certain specified minimum commercialization payments, Pfizer will also make commercialization payments to us based on a percentage of the aggregate annual net sales of XIAFLEX in the Pfizer Territory on a quarterly basis. The percentage of Pfizer’s aggregate annual net sales to be paid to us increases in accordance with the achievement of specified thresholds of aggregate annual net sales of XIAFLEX in the Pfizer Territory and decreases if a generic to XIAFLEX or a pharmaceutical product containing the same active ingredient as XIAFLEX is marketed in the Pfizer Territory and the market share of such products exceeds a specified threshold. As defined in the contract, the amount of commercialization payments that Pfizer owes us will be reduced upon the occurrence of a supply shortage.
Valuation of Equity Instruments used in Stock-Based Compensation. We measure stock-based compensation cost for all share-based awards made to employees and directors, including stock options and employee stock purchases, at the grant date based on the fair value of the award. The fair value of stock options is estimated using the Black-Scholes option-pricing model. Pre-vesting forfeitures are estimated in the determination of total stock-based compensation cost based on Company experience. The value of the portion of the award that is ultimately expected to vest is expensed ratably over the requisite service period as compensation expense in the consolidated statement of operations. The determination of fair value of share-based payment awards on the grant date requires significant judgment. Assumptions concerning our stock price volatility and
90
Table of Contents
projected employee exercise behavior over the contractual life of the award can significantly impact the estimated fair value of an award. Given the limited history of our Company, such assumptions are principally based on the observed history of other companies, which may not be reflective of the patterns we will experience. If our actual experience differs significantly from the assumptions used to compute stock-based compensation cost, or if different assumptions had been used, we may have recorded too much or too little expense for our share-based payment awards.
Inventory. We state inventories at the lower of cost or market, as determined using the first-in, first-out method. Inventory costs are based on our judgment of probable future commercial use and net realizable value. If inventory production does not meet approved or anticipated specifications, it is expensed upon determination of such status. With respect to the pre-approval XIAFLEX inventories, we assessed the probability of FDA approval to be highly probable. As discussed in Note 16 of the Notes to the Consolidated Financial Statements, on February 2, 2010 we received marketing approval from the FDA for XIAFLEX.
Recent Accounting Pronouncements
In April 2009, the FASB issued authoritative guidance on the recognition and presentation of other than temporary impairments of investments in debt and equity securities in certain circumstances. This guidance assists both issuers and users of financial statements in determining whether a market is active or inactive and whether a transaction is distressed, and is intended to make the existing accounting more operational. Also in April 2009, the FASB issued authoritative guidance for interim disclosures about fair value of financial instruments. This guidance requires disclosures about fair value of financial instruments for interim reporting periods of publicly traded companies as well as annual financial statements. In May 2009, the FASB issued authoritative guidance that establishes general standards of accounting for and disclosure of events that occur after the balance sheet date but before financial statements are issued or are available to be issued. These pronouncements became effective during 2009 and did not have any significant impact on our consolidated financial statements.
In September 2009, the FASB ratified the consensus reached by the Emerging Issues Task Force (“EITF”) that revises the authoritative guidance for revenue arrangements with multiple deliverables that contain more than one unit of accounting. The guidance addresses how to determine whether an arrangement involving multiple deliverables contains more than one unit of accounting and how the arrangement consideration should be allocated among the separate units of accounting. For the Company, the issue is effective for revenue arrangements entered into or materially modified after December 31, 2010, although early adoption and/or retroactive application are permitted. We are currently assessing the impact that this guidance may have on our consolidated financial statements.
| ITEM 7A. | Quantitative and Qualitative Disclosures About Market Risk |
We do not use derivative financial instruments or derivative commodity instruments for trading purposes. Our financial instruments consist of cash, cash equivalents, short-term investments, trade accounts receivable, long-term investments in auction rate securities (“ARS”), accounts payable and long-term obligations. We consider investments that, when purchased, have a remaining maturity of 90 days or less to be cash equivalents.
We invest in marketable securities in accordance with our investment policy. The primary objectives of our investment policy are to preserve principal, maintain proper liquidity to meet operating needs and maximize yields. Our investment policy specifies credit quality standards for our investments. The maximum allowable duration of a single issue is three months. Our investment policy permits us to invest in ARS with maturities of greater than three months, provided that the interest reset and auction date for the ARS is less than three months. Since February 10, 2008, the auctions for these securities have failed. Since we are unable to predict when the market for these securities will recover, the investments we have in these securities are classified as non-current
91
Table of Contents
and are reported as “Long-term investments” at fair value of $3.1 million, which is $0.7 million below cost, in the Consolidated Balance Sheet at December 31, 2009. The decline in value of these investments is recorded as an unrealized loss in accumulated other comprehensive income. Our ability to access in the near term the funds invested in ARS is dependent on the success of future scheduled auctions, finding a buyer outside the auction process or a decision of the issuer of the securities to call the securities. If the uncertainties in the credit and capital market continue, these markets deteriorate further or there are ratings downgrades on any of the ARS we hold, we may be required to recognize an impairment charge to earnings, if the decline in the value of these securities is assessed to be other than temporary.
Our investment portfolio is subject to interest rate risk, although limited given the nature of the investments, and will fall in value in the event market interest rates increase. All our cash and cash equivalents at December 31, 2009, amounting to approximately $182.0 million, were maintained in bank demand accounts, money market accounts and U.S. government backed securities. We do not hedge our interest rate risks, as we believe reasonably possible near-term changes in interest rates would not materially affect our results of operations, financial position or cash flows.
Transactions relating to Auxilium UK, Limited are recorded in pounds sterling. Upon consolidation of this subsidiary into our consolidated financial statements, we translate the balance sheet asset and liability accounts to the U.S. dollar based on exchange rates as of the balance sheet date; balance sheet equity accounts are translated into the U.S. dollar at historical exchange rates; and all statements of operations and cash flows amounts are translated into the U.S. dollar at the average exchange rates for the period. Exchange gains or losses resulting from the translation are included as a separate component of stockholders’ equity. In addition, we conduct clinical trials in Australia and certain European countries, exposing us to cost increases if the U.S. dollar declines in value compared to the Australian Dollar and the Euro. We do not hedge our foreign exchange risks, as we believe reasonably possible near-term fluctuations of exchange rates would not materially affect our results of operations, financial position or cash flows.
92
Table of Contents
| ITEM 8. | Financial Statements and Supplementary Data |
AUXILIUM PHARMACEUTICALS, INC. AND SUBSIDIARIES
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
93
Table of Contents
Report of Independent Registered Public Accounting Firm
To Board of Directors and Stockholders of Auxilium Pharmaceuticals, Inc.:
In our opinion, the consolidated financial statements listed in the accompanying index present fairly, in all material respects, the financial position of Auxilium Pharmaceuticals, Inc. and its subsidiaries at December 31, 2009 and 2008, and the results of their operations and their cash flows for each of the three years in the period ended December 31, 2009 in conformity with accounting principles generally accepted in the United States of America. Also in our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of December 31, 2009, based on criteria established in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). The Company’s management is responsible for these financial statements, for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in Management’s Report on Internal Control Over Financial Reporting appearing under Item 9A. Our responsibility is to express opinions on these financial statements, and on the Company’s internal control over financial reporting based on our integrated audits. We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the financial statements are free of material misstatement and whether effective internal control over financial reporting was maintained in all material respects. Our audits of the financial statements included examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. Our audit of internal control over financial reporting included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audits also included performing such other procedures as we considered necessary in the circumstances. We believe that our audits provide a reasonable basis for our opinions.
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
/s/ PricewaterhouseCoopers LLP
Philadelphia, PA
February 26, 2010
94
Table of Contents
AUXILIUM PHARMACEUTICALS, INC. AND SUBSIDIARIES
Consolidated Balance Sheets
(In thousands, except share and per share amounts)
| December 31, | ||||||||
| 2009 | 2008 | |||||||
| Assets | ||||||||
| Current assets: |
||||||||
| Cash and cash equivalents |
$ | 181,977 | $ | 113,943 | ||||
| Accounts receivable, trade |
18,266 | 14,352 | ||||||
| Accounts receivable, other |
1,323 | 2,610 | ||||||
| Inventories |
19,554 | 9,466 | ||||||
| Prepaid expenses and other current assets |
3,670 | 2,466 | ||||||
| Total current assets |
224,790 | 142,837 | ||||||
| Property and equipment, net |
24,227 | 16,552 | ||||||
| Long-term investments |
3,118 | 3,419 | ||||||
| Other assets |
8,429 | 8,379 | ||||||
| Total assets |
$ | 260,564 | $ | 171,187 | ||||
| Liabilities and Stockholders’ Equity | ||||||||
| Current liabilities: |
||||||||
| Accounts payable |
$ | 6,711 | $ | 1,444 | ||||
| Accrued expenses |
51,571 | 46,693 | ||||||
| Deferred revenue, current portion |
4,211 | 6,922 | ||||||
| Deferred rent, current portion |
883 | 439 | ||||||
| Total current liabilities |
63,376 | 55,498 | ||||||
| Deferred revenue, long-term portion |
69,703 | 73,152 | ||||||
| Deferred rent, long-term portion |
6,966 | 7,271 | ||||||
| Commitments and contingencies (Note 11) |
— | — | ||||||
| Stockholders’ equity: |
||||||||
| Preferred stock, $0.01 par value per share, 5,000,000 shares authorized, no shares issued or outstanding |
— | — | ||||||
| Common stock, $0.01 par value per share; authorized 120,000,000 shares; issued 47,317,602 and 42,414,136 shares at December 31, 2009 and 2008, respectively |
473 | 424 | ||||||
| Additional paid-in capital |
446,876 | 307,659 | ||||||
| Accumulated deficit |
(323,912 | ) | (270,455 | ) | ||||
| Treasury stock at cost 84,373 and 47,219 shares at December 31, 2009 and 2008, respectively |
(2,137 | ) | (1,081 | ) | ||||
| Accumulated other comprehensive loss |
(781 | ) | (1,281 | ) | ||||
| Total stockholders’ equity |
120,519 | 35,266 | ||||||
| Total liabilities and stockholders’ equity |
$ | 260,564 | $ | 171,187 | ||||
See accompanying notes to consolidated financial statements.
95
Table of Contents
AUXILIUM PHARMACEUTICALS, INC. AND SUBSIDIARIES
Consolidated Statements of Operations
(In thousands, except share and per share amounts)
| Years Ended December 31, | ||||||||||||
| 2009 | 2008 | 2007 | ||||||||||
| Net revenues |
$ | 164,039 | $ | 125,377 | $ | 95,734 | ||||||
| Operating expenses: |
||||||||||||
| Cost of goods sold |
37,077 | 29,486 | 24,609 | |||||||||
| Research and development* |
51,398 | 54,497 | 42,802 | |||||||||
| Selling, general and administrative* |
129,181 | 89,482 | 72,768 | |||||||||
| Total operating expenses |
217,656 | 173,465 | 140,179 | |||||||||
| Loss from operations |
(53,617 | ) | (48,088 | ) | (44,445 | ) | ||||||
| Interest income |
452 | 1,801 | 3,744 | |||||||||
| Interest expense |
(292 | ) | — | (4 | ) | |||||||
| Net loss |
(53,457 | ) | (46,287 | ) | (40,705 | ) | ||||||
| Basic and diluted net loss per common share |
$ | (1.22 | ) | $ | (1.12 | ) | $ | (1.07 | ) | |||
| Weighted average common shares outstanding |
43,650,775 | 41,272,557 | 38,187,662 | |||||||||
|
* includes the following amounts of stock-based compensation expense: |
||||||||||||
| Research and development |
$ | 5,048 | $ | 3,140 | $ | 1,180 | ||||||
| Selling, general and administrative |
12,852 | 8,602 | 4,857 | |||||||||
See accompanying notes to consolidated financial statements.
96
Table of Contents
AUXILIUM PHARMACEUTICALS, INC. AND SUBSIDIARIES
Consolidated Statements of Stockholders’ Equity and Comprehensive Loss
Year Ended December 31, 2009
(In thousands, except share amounts)
| Common stock | Additional paid-in capital |
Accumulated deficit |
Treasury stock | Accumulated other comprehensive income (loss) |
Total | ||||||||||||||||||||||
| Shares | Amount | Shares | Cost | ||||||||||||||||||||||||
| Balance, December 31, 2008 |
42,414,136 | $ | 424 | $ | 307,659 | $ | (270,455 | ) | 47,219 | $ | (1,081 | ) | $ | (1,281 | ) | 35,266 | |||||||||||
| Net loss |
(53,457 | ) | — | (53,457 | ) | ||||||||||||||||||||||
| Unrealized loss on long-term investments |
499 | 499 | |||||||||||||||||||||||||
| Foreign currency translation adjustment |
1 | 1 | |||||||||||||||||||||||||
| Total comprehensive loss |
(53,457 | ) | 500 | (52,957 | ) | ||||||||||||||||||||||
| Public offering, net of transaction costs |
3,450,000 | 34 | 115,746 | — | — | — | — | 115,780 | |||||||||||||||||||
| Cashless exercise of common stock warrants |
857,947 | 9 | (9 | ) | — | — | — | — | — | ||||||||||||||||||
| Exercise of common stock options |
396,088 | 4 | 3,602 | — | — | — | — | 3,606 | |||||||||||||||||||
| Employee Stock Purchase Plan purchases |
60,655 | 1 | 1,507 | — | — | — | — | |
— 1,508 |
| |||||||||||||||||
| Issuance of restricted stock |
101,500 | 1 | (1 | ) | — | — | — | — | — | ||||||||||||||||||
| Issuance in payment of Board fees |
5,026 | — | 154 | — | — | — | — | 154 | |||||||||||||||||||
| Stock based compensation |
32,250 | — | 18,218 | — | — | — | — | 18,218 | |||||||||||||||||||
| Treasury stock acquisition |
— | — | — | — | 37,154 | (1,056 | ) | — | (1,056 | ) | |||||||||||||||||
| Balance, December 31, 2009 |
47,317,602 | $ | 473 | $ | 446,876 | $ | (323,912 | ) | 84,373 | $ | (2,137 | ) | $ | (781 | ) | $ | 120,519 | ||||||||||
See accompanying notes to consolidated financial statements.
97
Table of Contents
AUXILIUM PHARMACEUTICALS, INC. AND SUBSIDIARIES
Consolidated Statements of Stockholders’ Equity and Comprehensive Loss
Year Ended December 31, 2008
(In thousands, except share amounts)
| Common stock | Additional paid-in capital |
Accumulated deficit |
Treasury stock | Accumulated other comprehensive income (loss) |
Total | ||||||||||||||||||||||
| Shares | Amount | Shares | Cost | ||||||||||||||||||||||||
| Balance, December 31, 2007 |
40,768,809 | $ | 408 | $ | 288,122 | $ | (224,168 | ) | 26,276 | $ | (322 | ) | $ | (24 | ) | 64,016 | |||||||||||
| Net loss |
(46,287 | ) | — | (46,287 | ) | ||||||||||||||||||||||
| Unrealized loss on long-term investments |
(1,181 | ) | (1,181 | ) | |||||||||||||||||||||||
| Foreign currency translation adjustment |
(76 | ) | (76 | ) | |||||||||||||||||||||||
| Total comprehensive loss |
(46,287 | ) | (1,257 | ) | (47,544 | ) | |||||||||||||||||||||
| Cashless exercise of common stock warrants |
789,913 | 8 | (8 | ) | — | — | — | — | — | ||||||||||||||||||
| Exercise of common stock options |
645,562 | 6 | 5,460 | — | — | — | — | 5,466 | |||||||||||||||||||
| Exercise of common stock warrants |
153,022 | 2 | 893 | — | — | — | — | 895 | |||||||||||||||||||
| Employee Stock Purchase Plan purchases |
47,587 | — | 1,184 | — | — | — | — | |
— 1,184 |
| |||||||||||||||||
| Issuance of restricted stock |
2,750 | — | — | — | — | — | — | ||||||||||||||||||||
| Issuance in payment of Board fees |
6,493 | — | 199 | 199 | |||||||||||||||||||||||
| Stock based compensation |
— | — | 11,742 | — | — | — | — | 11,742 | |||||||||||||||||||
| Adjustment of financing transaction costs |
— | — | 67 | — | — | — | — | 67 | |||||||||||||||||||
| Treasury stock acquisition |
— | — | — | — | 20,943 | (759 | ) | — | (759 | ) | |||||||||||||||||
| Balance, December 31, 2008 |
42,414,136 | $ | 424 | $ | 307,659 | $ | (270,455 | ) | 47,219 | $ | (1,081 | ) | $ | (1,281 | ) | $ | 35,266 | ||||||||||
See accompanying notes to consolidated financial statements.
98
Table of Contents
AUXILIUM PHARMACEUTICALS, INC. AND SUBSIDIARIES
Consolidated Statements of Stockholders’ Equity and Comprehensive Loss
Year Ended December 31, 2007
(In thousands, except share amounts)
| Common stock | Additional paid-in capital |
Accumulated deficit |
Treasury stock | Accumulated other comprehensive income (loss) |
Total | |||||||||||||||||||||||
| Shares | Amount | Shares | Cost | |||||||||||||||||||||||||
| Balance, December 31, 2006 |
35,677,008 | $ | 357 | $ | 225,577 | $ | (183,463 | ) | 13,377 | $ | (113 | ) | $ | (29 | ) | 42,329 | ||||||||||||
| Net loss |
(40,705 | ) | — | (40,705 | ) | |||||||||||||||||||||||
| Foreign currency translation Adjustment |
5 | 5 | ||||||||||||||||||||||||||
| Total comprehensive loss |
(40,705 | ) | 5 | (40,700 | ) | |||||||||||||||||||||||
| Public offering, net of transaction costs |
3,672,000 | 37 | 50,061 | — | — | — | — | 50,098 | ||||||||||||||||||||
| Exercise of common stock warrants |
12,751 | — | 74 | — | — | — | — | 74 | ||||||||||||||||||||
| Issuance of restricted stock |
15,000 | — | — | — | — | — | — | — | ||||||||||||||||||||
| Cashless exercise of common stock Warrants |
460,295 | 5 | (5 | ) | — | — | — | — | — | |||||||||||||||||||
| Exercise of common stock options |
826,034 | 8 | 5,228 | — | — | — | — | 5,236 | ||||||||||||||||||||
| Employee Stock Purchase Plan purchases |
106,451 | 1 | 1,150 | — | — | — | — | 1,151 | ||||||||||||||||||||
| Cancellation of restricted stock |
(730 | ) | — | — | — | — | — | — | — | |||||||||||||||||||
| Stock based compensation |
— | — | 6,037 | — | — | — | 6,037 | |||||||||||||||||||||
| Treasury stock acquisition |
— | — | — | — | 12,899 | (209 | ) | (209 | ) | |||||||||||||||||||
| Balance, December 31, 2007 |
40,768,809 | $ | 408 | $ | 288,122 | $ | (224,168 | ) | 26,276 | $ | (322 | ) | $ | (24 | ) | $ | 64,016 | |||||||||||
See accompanying notes to consolidated financial statements.
99
Table of Contents
AUXILIUM PHARMACEUTICALS, INC. AND SUBSIDIARIES
Consolidated Statements of Cash Flow
(In thousands, except share and per share amounts)
(In thousands)
| Years Ended December 31, | ||||||||||||
| 2009 | 2008 | 2007 | ||||||||||
| Cash flows from operating activities: |
||||||||||||
| Net loss |
$ | (53,457 | ) | $ | (46,287 | ) | $ | (40,705 | ) | |||
| Adjustments to reconcile net loss to net cash provided by (used in) operating activities: |
||||||||||||
| Depreciation and amortization |
4,066 | 2,168 | 1,404 | |||||||||
| Stock-based compensation |
17,900 | 11,742 | 6,037 | |||||||||
| Changes in operating assets and liabilities: |
||||||||||||
| Increase in accounts receivable |
(2,627 | ) | (4,666 | ) | (2,522 | ) | ||||||
| Increase in inventories |
(9,770 | ) | (4,324 | ) | (2,335 | ) | ||||||
| Decrease (increase) in prepaid expenses, other current assets and other assets |
(1,570 | ) | 1,018 | (935 | ) | |||||||
| Increase in accounts payable and accrued expenses |
10,117 | 11,549 | 9,105 | |||||||||
| Increase (decrease) in deferred revenue |
(6,160 | ) | 69,826 | (731 | ) | |||||||
| Increase in deferred rent |
139 | 5,686 | 226 | |||||||||
| Net cash provided by (used in) operating activities |
(41,362 | ) | 46,712 | (30,456 | ) | |||||||
| Cash flows from investing activities: |
||||||||||||
| Purchases of property and equipment |
(11,421 | ) | (11,013 | ) | (4,478 | ) | ||||||
| Purchases of short-term investments |
— | (2,400 | ) | (16,902 | ) | |||||||
| Redemptions of short-term investments |
— | 2,400 | 21,011 | |||||||||
| Redemptions of long-term investments |
800 | 1,200 | — | |||||||||
| Net cash used in investing activities |
(10,621 | ) | (9,813 | ) | (369 | ) | ||||||
| Cash flows from financing activities: |
||||||||||||
| Proceeds from common stock offerings, net of transaction costs |
115,780 | 67 | 50,098 | |||||||||
| Borrowings under Revolving Line of Credit |
30,000 | — | — | |||||||||
| Payments under Revolving Line of Credit |
(30,000 | ) | — | — | ||||||||
| Proceeds from warrant exercises |
— | 894 | 74 | |||||||||
| Payments on debt financings |
— | (5 | ) | (61 | ) | |||||||
| Employee Stock Purchase Plan purchases |
1,508 | 1,184 | 1,150 | |||||||||
| Proceeds from exercise of common stock options |
3,606 | 5,466 | 5,236 | |||||||||
| Common stock issued in payment of board fees |
154 | 199 | — | |||||||||
| Purchases of treasury stock |
(1,056 | ) | (759 | ) | (209 | ) | ||||||
| Net cash provided by financing activities |
119,992 | 7,046 | 56,288 | |||||||||
| Effect of exchange rate changes on cash |
25 | (292 | ) | (8 | ) | |||||||
| Increase (decrease) in cash and cash equivalents |
68,034 | 43,653 | 25,455 | |||||||||
| Cash and cash equivalents, beginning of period |
113,943 | 70,290 | 44,835 | |||||||||
| Cash and cash equivalents, end of period |
$ | 181,977 | $ | 113,943 | $ | 70,290 | ||||||
See accompanying notes to consolidated financial statements.
100
Table of Contents
AUXILIUM PHARMACEUTICALS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(1) Organization and Description of Business
Auxilium Pharmaceuticals, Inc. along with its subsidiaries, or the Company, is a specialty biopharmaceutical company with a focus on developing and marketing products to predominantly specialist audiences, such as urologists, endocrinologists, certain targeted primary care physicians, hand surgeons, subsets of orthopedic, general, and plastic surgeons who focus on the hand, and rheumatologists. Through December 31, 2009, the Company had one marketed product, Testim, a proprietary, topical 1% testosterone gel indicated for the treatment of hypogonadism. In the fourth quarter of 2002, the Company received approval from the U.S. Food and Drug Administration (“FDA”) to market Testim. The Company launched Testim in the first quarter of 2003. The Company currently markets Testim in the U.S. through its own sales force. Testim is also approved for marketing in Belgium, Canada, Denmark, Finland, Germany, Greece, Iceland, Ireland, Italy, Luxembourg, The Netherlands, Norway, Portugal, Spain, Sweden and the United Kingdom. The Company currently relies on third parties to market, sell and distribute Testim outside of the U.S.
On February 2, 2010, the FDA approved XIAFLEX™ (collagenase clostridium histolyticum) for Dupuytren’s contracture (“Dupuytren’s”) for the treatment of adult patients with a palpable cord. The Company expects to launch this product at the end of March 2010 through its own sales force which it plans to train prior to launch. The Company has entered into a development, commercialization and supply agreement with Pfizer (the “Pfizer Agreement”) under which it sub-licensed certain development and commercialization rights for Dupuytren’s and Peyronie’s disease (“Peyronie’s”) for the 27 countries which were then included in the European Union (“EU”) and 19 additional Eastern European and Eurasian countries (the “Pfizer Territory”). On January 21, 2010 the Company and Pfizer announced the European scientific/technical review procedure for the Marketing Authorization Application (“MAA”) for XIAFLEX for the treatment of Dupuytren’s commenced.
The Company has four projects in clinical development. XIAFLEX is in phase II of development for the treatment of Peyronie’s and Adhesive Capsulitis (“Frozen Shoulder syndrome”). The Company’s transmucosal film product candidates for the treatment of overactive bladder (“AA4010”) and Fentanyl pain product are in phase I of development. The Company has rights to additional pain products and products for hormone replacement and urologic disease using its transmucosal film delivery system. The Company expects to seek partners to further develop these transmucosal film technology product candidates. The Company also has options to all indications using XIAFLEX for non-topical formulations.
101
Table of Contents
(2) Summary of Significant Accounting Policies
(a) Principles of Consolidation
The accompanying consolidated financial statements include the accounts of Auxilium Pharmaceuticals, Inc. and its wholly owned subsidiaries. All intercompany balances and transactions have been eliminated in consolidation.
(b) Use of Estimates
The preparation of financial statements in conformity with U.S. generally accepted accounting principles requires management to make estimates and assumptions that affect the reported amounts of assets, liabilities, and disclosure of contingencies at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from these estimates.
(c) Translation of Foreign Financial Statements
The Company established a foreign subsidiary in the United Kingdom in 2000, which uses the pound sterling as its functional currency. Assets and liabilities of the Company’s foreign subsidiary are translated at the year-end rate of exchange. The statements of operations for this subsidiary are translated at the average rate of exchange for the year. Gains or losses from translating foreign currency financial statements are accumulated in other comprehensive income (loss) in stockholders’ equity.
(d) Fair Value of Financial Instruments
Management believes that the carrying amounts of the Company’s financial instruments, including cash, cash equivalents, accounts receivable, restricted cash deposits, long-term investments, accounts payable, accrued expenses and notes payable approximate fair value due to the short-term nature of those instruments.
(e) Revenue Recognition
Revenue from sales of Testim is recognized when the following four revenue recognition criteria are met: persuasive evidence of an arrangement exists, delivery has occurred or services have been rendered, the selling price is fixed or determinable, and collectability is reasonably assured. Additionally, revenue arrangements with multiple deliverables are divided into separate units of accounting if the deliverables in the arrangement meet the following criteria: the delivered item has value to the customer on a standalone basis; there is objective and reliable evidence of the fair value of undelivered items; and delivery of any undelivered item is probable. Otherwise, the arrangement is accounted for as a single unit of accounting.
The Company sells Testim to pharmaceutical wholesalers, who have the right to return purchased product prior to the units being dispensed through patient prescriptions, and to its international distributors. Testim is shipped F.O.B. destination point. Transfer of ownership and risk of loss for this product passes to the customer at the point that the product is received by the customer. Since this product is subject to return, revenue is reduced by an estimate of future product returns based on historical experience. Testim revenue is also reduced for estimates of allowances for cash discounts, rebates and patient coupons based on historical experience, and current contract prices and terms with customers. If actual, or expected, customer returns and revenue allowances differ from previous estimates, adjustments of these estimates would be recognized in the period such facts become known.
102
Table of Contents
The following individual customers each accounted for at least 10% of total product shipments for any of the respective periods:
| Years Ended December 31, |
|||||||||
| 2009 | 2008 | 2007 | |||||||
| AmerisourceBergen Corporation |
18.0 | % | 17.3 | % | 15.2 | % | |||
| Cardinal Health, Inc. |
40.8 | % | 34.4 | % | 27.9 | % | |||
| McKesson Corporation |
37.0 | % | 41.1 | % | 50.1 | % | |||
| 95.8 | % | 92.8 | % | 93.2 | % | ||||
The collaboration and out-license agreements the Company has entered into contain multiple elements. Where the Company has continuing performance obligations, license and milestone payments are recognized together with up-front payments over the term of the arrangement as the performance obligations are completed, unless the delivered elements have stand alone value to the customer and there is objective, reliable evidence of fair value of the undelivered elements in the arrangement. In the case where the arrangement is considered to be a single unit of accounting, all cash flows under the arrangements are aggregated and recognized as revenue over the estimated term of the arrangement. In addition, unless evidence suggests otherwise, revenue from consideration received is recognized on a straight-line basis over the expected period of the arrangement during which continuing performances obligations exist. In arrangements where we have separate units of accounting, revenue from milestone payments are recognized as revenue upon achievement of the milestone only if the following conditions are met: the milestone payments are non-refundable; achievement of the milestone was not reasonably assured at the inception of the arrangement; substantive effort is involved in achieving the milestone; and the amount of the milestone is reasonable in relation to the effort expended or the risk associated with achievement of the milestone.
(f) Cash Equivalents and Long-term Investments
We consider our investments classified as Cash equivalents and Long-term investments to be “available for sale”. Cash equivalents include only securities having a maturity of three months or less at the time of purchase. They are classified as short term and stated at market value.
Long-term investments are carried at fair value. Unrealized gains and losses on Cash equivalents and Long-term investments have been recorded as a separate component of Stockholders’ equity in Accumulated other comprehensive loss. All realized gains and losses on these investments are recognized in results of operations.
(g) Accounts Receivable
Accounts receivable, trade consist of amounts due from wholesalers for the purchase of Testim. Ongoing credit evaluations of customers are performed and collateral is generally not required.
Accounts receivable, trade are net of allowances for cash discounts, actual returns and bad debts of $473,000 and $406,000 at December 31, 2009 and 2008, respectively.
103
Table of Contents
The following individual customers each accounted for at least 10% of accounts receivable, trade on either of the respective dates:
| December 31, | ||||||
| 2009 | 2008 | |||||
| AmerisourceBergen Corporation |
20.8 | % | 20.5 | % | ||
| Cardinal Health, Inc. |
40.9 | % | 36.9 | % | ||
| McKesson Corporation |
33.8 | % | 36.3 | % | ||
| 95.5 | % | 93.7 | % | |||
(h) Inventories
Inventories are stated at the lower of cost or market, as determined using the first-in, first-out method.
In 2009, the Company commenced production of XIAFLEX for the treatment of Dupuytren’s for future commercial use in preparation for the launch of this product. Inventory costs associated with Testim and XIAFLEX are based on management’s judgment of probable future commercial use and net realizable value. In the case of XIAFLEX, management assessed the probability of FDA approval to be highly probable. On February 2, 2010, the FDA approved the product. If inventory production does not meet approved or anticipated specifications, it is expensed upon determination of such status.
(i) Concentration of Supply
The Company has limited sources of supply for raw materials for its products. The Company attempts to mitigate the risk of supply interruption by maintaining adequate safety stock of raw materials and by scheduling production runs to create safety stock of finished goods. The Company evaluates secondary sources of supply for all its raw materials and finished goods. The Company does not have any long-term minimum commitments for finished goods production or raw materials (see Note 11).
(j) Property and Equipment
Property and equipment are recorded at cost. Maintenance and repairs are charged to expense as incurred, and costs of improvements are capitalized. Depreciation is recognized using the straight-line method based on the estimated useful life of the related assets. Amortization of leasehold improvements is recognized using the straight-line method based on the shorter of the estimated useful life of the related assets or the lease term.
(k) Long-Lived Assets
The Company reviews long-lived assets, such as property and equipment, for potential impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. Recoverability of assets to be held and used is measured by a comparison of the carrying amount of an asset to estimated undiscounted future cash flows expected to be generated by the asset. If the carrying amount of an asset exceeds its estimated future cash flows, an impairment charge is recognized in the amount by which the carrying amount of the asset exceeds the fair value of the asset. As of December 31, 2009, the Company has not revised the remaining useful lives of any significant assets.
Included in Other assets as of December 31, 2009 and 2008 is the unamortized balance of $6,044,000 and $6,363,000, respectively, of the license agreement payment to BioSpecifics Technologies Corp (“BioSpecifics”), associated with the upfront payment from Pfizer (see Note 8). This payment is being amortized over the estimated 240-month life of this agreement.
104
Table of Contents
(l) Research and Development Costs
Research and development costs include salaries and related expenses for development personnel, as well as costs of operation of the Horsham manufacturing facilities and fees and costs paid to external service providers. Costs of external service providers include both clinical trial costs and the costs associated with non-clinical support activities such as toxicology testing, manufacturing process development and regulatory affairs. External service providers include contract research organizations, contract manufacturers, toxicology laboratories, physician investigators and academic collaborators. Research and development costs, including the cost of product licenses prior to regulatory approval, are charged to expense as incurred.
(m) Income Taxes
Income taxes are accounted for under the asset-and-liability method. Deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases and operating loss and tax credit carryforwards. Valuation allowances are established when necessary to reduce deferred tax assets to the amount expected to be realized. Deferred tax assets and liabilities are measured at the balance sheet date using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in the period such tax rate changes are enacted. Interest and penalties related to uncertain tax positions are classified as income tax expense.
(n) Stock-Based Compensation
The Company measures the compensation costs for all share-based awards made to the Company’s employees and directors, including stock options and employee stock purchases under the Company’s employee stock purchase plan, based on fair values on the date of grant. The fair value of stock options is estimated using the Black-Scholes option-pricing model. Pre-vesting forfeitures are estimated in the determination of total stock-based compensation cost based on Company experience. The value of the portion of the award that is ultimately expected to vest is expensed ratably over the requisite service period as compensation expense in the consolidated statement of operations. For awards that limit performance requirements to continuing service, the Company uses the straight-line method to amortize compensation cost for the full award to expense over their vesting period. For awards with other performance requirements, the graded vesting method of amortization is utilized under which the cost of each vesting tranche of an award is amortized to expense over the period from grant to vesting date.
(o) Comprehensive Income (Loss)
Comprehensive income is defined as the change in equity of a business enterprise during a period from transactions and other events and circumstances from non-owner sources, including foreign currency translation adjustments and unrealized gains and losses on marketable securities. The Company’s comprehensive loss is presented within the accompanying Consolidated Statements of Stockholders’ Equity and Comprehensive Loss.
(p) Net Loss Per Common Share
The basic net loss per common share is computed by dividing the net loss applicable to common stockholders for the period by the weighted average number of common shares outstanding during the period reduced, where applicable, for unvested outstanding restricted shares.
105
Table of Contents
The following table sets forth the computation of basic and diluted net loss per common share (in thousands, except share and per share amounts):
| Years Ended December 31, | ||||||||||||
| 2009 | 2008 | 2007 | ||||||||||
| Numerator: |
||||||||||||
| Net loss |
$ | (53,457 | ) | $ | (46,287 | ) | $ | (40,705 | ) | |||
| Denominator: |
||||||||||||
| Weighted-average common shares outstanding |
43,836,887 | 41,441,916 | 38,408,314 | |||||||||
| Weighted-average unvested restricted common shares subject to forfeiture |
(186,112 | ) | (169,359 | ) | (220,652 | ) | ||||||
| Shares used in calculating net loss applicable to common stockholders per share |
43,650,775 | 41,272,557 | 38,187,662 | |||||||||
| Basic and diluted net loss per common share |
$ | (1.22 | ) | $ | (1.12 | ) | $ | (1.07 | ) | |||
Diluted net loss per common share is computed giving effect to all dilutive potential common stock, including options, and warrants. Diluted net loss per common share for all periods presented is the same as basic net loss per common share because the potential common stock is anti-dilutive. Anti-dilutive common shares not included in diluted net loss per common share are summarized as follows:
| December 31, | ||||||
| 2009 | 2008 | 2007 | ||||
| Common stock options |
5,359,110 | 4,425,624 | 3,776,105 | |||
| Warrants |
102,524 | 1,124,768 | 2,281,939 | |||
| Restricted common stock |
168,500 | 142,000 | 198,072 | |||
| 5,630,134 | 5,692,392 | 6,256,116 | ||||
(q) Segment Information
The Company is managed and operated as one business. The entire business is managed by a single management team that reports to the chief executive officer. The Company does not operate separate lines of business or separate business entities with respect to any of its product candidates. Accordingly, the Company does not prepare discrete financial information with respect to separate product areas and does not have separately reportable segments.
(r) Advertising Costs
Advertising costs, included in selling, general and administrative expenses, are charged to expense as incurred. Advertising expenses for the years ended December 31, 2009, 2008 and 2007 were $229,000, $403,000 and $926,000, respectively.
(s) New Accounting Pronouncements
In April 2009, the FASB issued authoritative guidance on the recognition and presentation of other than temporary impairments of investments in debt and equity securities in certain circumstances. This guidance assists both issuers and users of financial statements in determining whether a market is active or inactive, and whether a transaction is distressed and is intended to make the existing accounting more operational. Also in April 2009, the FASB issued authoritative guidance for interim disclosures about fair value of financial instruments. This guidance requires disclosures about fair value of financial instruments for interim reporting periods of publicly traded companies as well as annual financial statements. In May 2009, the FASB issued
106
Table of Contents
authoritative guidance that establishes general standards of accounting for and disclosure of events that occur after the balance sheet date but before financial statements are issued or are available to be issued. These pronouncements became effective during 2009 and did not have any significant impact on the Company’s financial statements.
In September 2009, the FASB ratified the consensus reached by the Emerging Issues Task Force (EITF) that revises the authoritative guidance for revenue arrangements with multiple deliverables that contain more than one unit of accounting. The guidance addresses how to determine whether an arrangement involving multiple deliverables contains more than one unit of accounting and how the arrangement consideration should be allocated among the separate units of accounting. For the Company, the issue is effective for revenue arrangements entered into or materially modified after December 31, 2010, although early adoption and/or retroactive application are permitted. The Company is currently assessing the impact that this guidance may have on its consolidated financial statements.
(3) Fair Value Measurements
The authoritative literature for fair value measurements established a three-tier fair value hierarchy, which prioritizes the inputs in measuring fair value. These tiers are as follows: Level 1, defined as observable inputs such as quoted market prices in active markets; Level 2, defined as inputs other than the quoted prices in active markets that are either directly or indirectly observable; and Level 3, defined as significant unobservable inputs (entity developed assumptions) in which little or no market data exists.
As of December 31, 2009, the Company held certain investments that are required to be measured at fair value on a recurring basis. The following tables present the Company’s fair value hierarchy for these financial assets as of December 31, 2009 and 2008 (in thousands):
| December 31, 2009 | ||||||||||||
| Fair Value | Level 1 | Level 2 | Level 3 | |||||||||
| Cash and cash equivalents |
$ | 181,977 | $ | 181,977 | $ | — | $ | — | ||||
| Long-term investments: |
||||||||||||
| Auction rate securities |
$ | 3,118 | — | — | 3,118 | |||||||
| Total financial assets |
$ | 185,095 | $ | 181,977 | $ | — | $ | 3,118 | ||||
| December 30, 2008 | ||||||||||||
| Fair Value | Level 1 | Level 2 | Level 3 | |||||||||
| Cash and cash equivalents |
$ | 113,943 | $ | 113,943 | $ | — | $ | — | ||||
| Long-term investments: |
||||||||||||
| Auction rate securities |
$ | 3,419 | — | — | 3,419 | |||||||
| Total financial assets |
$ | 117,362 | $ | 113,943 | $ | — | $ | 3,419 | ||||
The following table summarizes the changes in the financial assets measured at fair value using Level 3 inputs for the years ended December 31, 2009 and 2008 (in thousands):
| Year ended December 31, |
||||||||
| Long-term investments |
2009 | 2008 | ||||||
| Beginning balance |
$ | 3,419 | $ | — | ||||
| Transfers into Level 3 |
— | 5,800 | ||||||
| Settlements |
(800 | ) | (1,200 | ) | ||||
| Unrealized gain (loss)- included in other comprehensive income |
499 | (1,181 | ) | |||||
| Ending balance |
$ | 3,118 | $ | 3,419 | ||||
107
Table of Contents
(4) Cash and Cash Equivalents
Cash and cash equivalents include only securities having a maturity of three months or less at the time of purchase. The following summarizes cash, cash equivalents and short-term investments (in thousands):
| December 31, | ||||||
| 2009 | 2008 | |||||
| Cash and Cash Equivalents |
||||||
| Demand deposits |
$ | 7,192 | $ | 3,660 | ||
| Money market accounts |
172,335 | 75,758 | ||||
| Commercial paper |
— | 1,997 | ||||
| Obligations of U.S. Government |
2,450 | 31,499 | ||||
| Corporate notes |
— | 1,029 | ||||
| $ | 181,977 | $ | 113,943 | |||
(5) Inventories
Inventories consist of the following (in thousands):
| December 31, 2009 |
December 31, 2008 | |||||
| Commercial: |
||||||
| Raw materials |
$ | 4,420 | $ | 3,085 | ||
| Work-in-process |
1,777 | 341 | ||||
| Finished goods |
5,986 | 6,040 | ||||
| Total Commercial |
12,183 | 9,466 | ||||
| Pre-approval: |
||||||
| Raw materials |
903 | — | ||||
| Work-in-process |
6,468 | — | ||||
| Total Pre-approval |
7,371 | — | ||||
| $ | 19,554 | $ | 9,466 | |||
On February 2, 2010, the FDA approved XIAFLEX for the treatment of Dupuytren’s. Pre-approval inventory at December 31, 2009 represents raw materials and work-in-process inventories of XIAFLEX that have been capitalized as inventory based on management’s judgment of the probable future use and net realizable value of these inventories.
108
Table of Contents
(6) Property and Equipment
Property and equipment consists of the following (in thousands):
| Estimated useful life |
December 31, | |||||||||
| 2009 | 2008 | |||||||||
| Office furniture, computer equipment and software |
3 to 5 years | $ | 8,000 | $ | 3,965 | |||||
| Manufacturing equipment |
3 to 10 years | 4,267 | 3,894 | |||||||
| Laboratory equipment |
7 years | 1,288 | 547 | |||||||
| Leasehold improvements |
lease term | 14,300 | 10,071 | |||||||
| 27,855 | 18,477 | |||||||||
| Less accumulated depreciation and amortization |
(9,406 | ) | (5,686 | ) | ||||||
| 18,449 | 12,791 | |||||||||
| Construction-in-progress—laboratory and manufacturing equipment |
5,778 | 3,761 | ||||||||
| $ | 24,227 | $ | 16,552 | |||||||
Depreciation expense was $3,733,000, $2,130,000 and $1,315,000 for the years ended December 31, 2009, 2008 and 2007, respectively.
(7) Long-term Investments
Long-term investments at December 31, 2009 and 2008 consist of auction-rate securities (“ARS”) with original maturities ranging up to 40 years. ARS have interest reset dates of 28 or 35 days. The reset date is the date in which the underlying interest rate is revised based on a Dutch auction and the underlying security may be sold. Since February 2008, the auctions for these securities have failed. Since the Company is unable to predict when the market for these securities will recover, these investments are classified as long-term. These investments are carried at fair value which is below cost. The unrealized loss on these investments at December 31, 2009 and 2008 is included in Other comprehensive income since the Company concluded that such losses are temporary in nature.
(8) Collaboration and License Agreements
(a) BioSpecifics
In June 2004, the Company entered into a development and license agreement with BioSpecifics and amended such agreement in May 2005, December 2005 and December 2008 (the “BioSpecifics Agreement”). Under the BioSpecifics Agreement, the Company was granted exclusive worldwide rights to develop, market and sell certain products containing BioSpecifics’ enzyme XIAFLEX. The Company’s licensed rights concern the development and commercialization of products, other than dermal formulations labeled for topical administration. XIAFLEX for the treatment of Dupuytren’s received FDA approval on February 2, 2010. Currently, the Company is developing XIAFLEX for the treatment of Peyronie’s disease and Frozen Shoulder syndrome. The Company may expand the BioSpecifics Agreement, at its option, to cover other indications as they are developed by the Company or BioSpecifics.
The BioSpecifics Agreement extends, on a country-by-country and product-by-product basis, for the longer of the patent life, the expiration of any regulatory exclusivity period or 12 years. The Company may terminate the BioSpecifics Agreement with 90 days written notice.
The Company must pay BioSpecifics on a country-by-country and product-by-product basis a specified percentage of net sales for products. Such percentage may vary depending on whether BioSpecifics exercises the supply option. If BioSpecifics exercises its supply option, commencing on a specified date, BioSpecifics will be
109
Table of Contents
responsible for supplying either by itself or through a third party other than a back-up supplier qualified by the Company, a specified portion of the commercial supply of the product required outside the territory licensed to Pfizer pursuant to the Pfizer Agreement described in (b) below. In addition, the percentage may be reduced if (i) BioSpecifics fails to supply commercial product supply in accordance with the terms of the BioSpecifics Agreement; (ii) market share of a competing product exceeds a specified threshold; or (iii) the Company is required to obtain a license from a third party in order to practice BioSpecifics’ patents without infringing such third party’s patent rights.
As a result of the December 2008 amendment to the BioSpecifics Agreement, which became effective when we executed the agreement with Pfizer, the Company must pay BioSpecifics 8.5% of the upfront and milestone payments received from Pfizer. With regard to any other sublicensee, the Company must pay BioSpecifics a specified percentage of sublicense income it receives from any other sublicensee, including upfront payments and milestone payments, and an amount equal to a specified mark-up of the cost of goods sold for products sold by the Company that are not manufactured by or on behalf of BioSpecifics, provided that, in the event BioSpecifics exercises the supply option, no payment will be due for so long as BioSpecifics fails to supply the commercial supply of the product in accordance with the terms of the BioSpecifics Agreement.
The amount of $6,375,000 payable to BioSpecifics as a result of the $75,000,000 up-front Pfizer payment was recorded as a deferred charge and is being amortized on a straight-line basis to Cost of goods sold over the estimated 240 month life of the Pfizer Agreement. At December 31, 2009 and 2008, the unamortized balance of $6,044,000 and $6,363,000, respectively, is included in Other assets.
Finally, the Company is obligated to make contingent milestone payments upon the filing of regulatory applications and receipt of regulatory approval. Additional milestone obligations will be due if we exercise an option to develop and license XIAFLEX for additional medical indications. Pursuant to the BioSpecifics Agreement, the milestone for each additional indication is $500,000, except for cellulite which is $1,000,000.
(b) Pfizer
In December 2008, the Company entered into a development, commercialization and supply agreement with Pfizer (the “Pfizer Agreement”). Under the Pfizer Agreement, the Company granted to Pfizer the right to develop and commercialize, with the right to sublicense, XIAFLEX for the treatment of Peyronie’s and Dupuytren’s in the 27 member countries of the EU as it existed as of the effective date of the Pfizer Agreement (Austria, Belgium, Bulgaria, Cyprus, Czech Republic, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Ireland, Italy, Latvia, Lithuania, Luxembourg, Malta, the Netherlands, Poland, Portugal, Romania, Slovakia, Slovenia, Spain, Sweden and the U.K.), as well as Albania, Armenia, Azerbaijan, Belarus, Bosnia & Herzegovina, Croatia, Georgia, Iceland, Kazakhstan, Kirghiz Republic, Macedonia, Moldova, Montenegro, Norway, Serbia, Tajikistan, Switzerland, Turkey and Uzbekistan (the “Pfizer Territory”). Subject to each party’s termination rights, the term of the Pfizer Agreement extends on a country-by-country and product-by-product basis from the date of the Pfizer Agreement until the latest of (i) the date on which the product is no longer covered by a valid patent or patent application in such country, (ii) the 15th anniversary of the first commercial sale by Pfizer of XIAFLEX in a given country after the receipt of required regulatory approvals and (iii) a generic entry or competitive product containing the same active ingredient with respect to the XIAFLEX in such country (the “Term”).
Upon contract signing, Pfizer paid the Company a $75,000,000 non-refundable upfront payment. This up-front payment, net of associated transaction costs of $3,656,000 has been deferred and is being recognized as revenue on a straight-line basis over the term of the agreement which is currently estimated to be 20 years. The resulting amortization included in Net revenues for 2009 and 2008 amounted to $3,568,000 and $137,000, respectively. In February 2010 Pfizer paid the Company an additional $15,000,000 milestone and may make up to $395,000,000 in potential payments upon the achievement of certain specified additional regulatory and commercial milestones for XIAFLEX (on an indication-by-indication basis or for the product as a whole, as the
110
Table of Contents
case may be). Of these potential payments, $135,000,000 in milestones may be paid to us relating to the achievement of certain specified regulatory milestones, and the remaining $260,000,000 that may be made relate to the achievement of certain specified commercial milestones. Subject to the requirement to make certain specified minimum commercialization payments, Pfizer will make commercialization payments to us based on a percentage of the aggregate annual net sales of XIAFLEX in the Pfizer Territory on a quarterly basis. The percentage of Pfizer’s aggregate annual net sales to be paid to us increases in accordance with the achievement of specified thresholds of aggregate annual net sales of XIAFLEX in the Pfizer Territory and decreases if a generic to XIAFLEX or a pharmaceutical product containing the same active ingredient as XIAFLEX is marketed in the Pfizer Territory and the market share of such products exceeds a specified threshold.
Development costs will be borne by the Party incurring such costs; provided, that the Parties will share development costs associated with activities solely associated with obtaining product approval in the Pfizer Territory. However, Pfizer may, on an indication-by-indication basis, recoup its fifty percent (50%) share of such pre-approval development costs by means of an off-set against the milestone payment due upon first commercial sale for such indication. Pfizer will be responsible for regulatory costs associated with product approval in the Pfizer Territory, and Pfizer may also recoup up to $2,500,000 with respect to such regulatory costs related to Dupuytren’s. Pfizer is solely responsible for costs associated with commercializing XIAFLEX in the Pfizer Territory.
The Company is primarily responsible for development activities prior to granting of product approval, and Pfizer is primarily responsible for development activities in the Pfizer Territory thereafter. All development activities will be undertaken pursuant to a defined plan. Pfizer is responsible for preparation of regulatory materials necessary for obtaining and maintaining regulatory approvals. Pfizer is solely responsible for commercializing XIAFLEX in the Pfizer Territory during the term of the Pfizer Agreement subject to a defined plan. The Company will control product development at all times outside of the Pfizer Territory.
Either Party may terminate the Pfizer Agreement as a result of the other party’s breach or bankruptcy. Pfizer may terminate the Pfizer Agreement at will; provided that, during a specified period, such termination right is subject to the occurrence of certain specified events relating to the product, product development and regulatory approval.
(c) CPEX Pharmaceuticals
In May 2000, Bentley granted the Company an exclusive, worldwide, royalty-bearing license to make and sell products incorporating its patented transdermal gel formulation technology that contains testosterone (the “May 2000 License”). The Company produces Testim under the May 2000 License. The term of the May 2000 License is determined on a country by country basis and extends until the later of patent right termination in a country or 10 years from the date of first commercial sale. In May 2001, Bentley granted the Company similar rights for a product containing another hormone, the term of which is perpetual. Under these agreements, the Company is required to make up-front and milestone payments upon contract signing, the decision to develop the underlying product, and the receipt of FDA approval. In June 2008, CPEX was spun out of Bentley and is the assignee of certain Bentley assets, including the license agreements and patents we licensed under those agreements.
In addition to its royalty obligations, the Company is obligated to pay certain upfront payments and milestones under the license agreements with CPEX. Through December 31, 2009, the Company has made aggregate upfront and milestone payments under the two license agreements with CPEX of $625,000. If all events under the CPEX license agreements occur, the Company would be obligated to pay a maximum of an additional $1,025,000 in milestone payments.
111
Table of Contents
(d) Ipsen
The Company entered into a license and distribution agreement with Ipsen in March 2004 which granted Ipsen an exclusive license to use and sell Testim on a worldwide basis outside the U.S., Canada, Mexico, and Japan, and in January 2008, we amended that agreement to exclude, in addition, China, Poland, Russia, and South Korea and their territories and possessions.
In November 2008, the Company agreed with Ipsen to terminate its distribution agreement for Testim and transfer the marketing authorizations required to promote and sell Testim in the territory to Ferring. The transfer of the marketing authorizations for the 15 countries previously held by Ipsen (Belgium, Denmark, Finland, Germany, Greece, Iceland, Ireland, Italy, Luxembourg, Netherlands, Norway, Portugal, Spain, Sweden and the U.K.) was completed in late 2009. Under the termination agreement, the Company paid Ipsen $750,000 in 2008 and has made additional payments in 2009 to Ipsen of $1,750,000 upon transfer of the marketing authorizations. The remaining balance of deferred revenue associated with the Ipsen agreement, amounting to approximately $6,641,000 after deducting these payments, was recognized as revenue over the life of the termination agreement. The amount of Ipsen contract revenue included in Net revenues for 2009, 2008 and 2007 amounted to $6,365,000, $916,000 and $698,000.
(e) Ferring
In November 2008, the Company entered into a distribution and license agreement with Ferring. Pursuant to the agreement, the Company appointed Ferring as its exclusive distributor of Testim in certain European countries. The Company also granted Ferring an exclusive, royalty-bearing license to import, market, sell and distribute Testim in these countries. The exclusive appointment and license commenced on a country-by-country basis upon the transfer of the relevant marketing authorizations from Ipsen. Ferring is required to purchase all Testim supply from us and to make certain sales milestone and royalty payments. In addition, Ferring made to the Company up-front payment and milestone payments upon the transfer to it of the marketing authorizations in each European country within the territory which totaled $6,200,000. These payments were deferred and, commencing in July 2009, are being recognized as revenue on a straight-line basis over the contract term which is estimated to be 120 months. The resulting amortization included in Net revenues for 2009 was $174,000.
(f) Paladin
The Company entered into a license and distribution agreement with Paladin in December 2006. We granted Paladin an exclusive license to use and sell Testim in Canada. The terms of this agreement require Paladin to purchase all Testim supply from the Company. Paladin made up-front payments amounting to $500,000 upon contract signing and is required to make milestone and royalty payments. The up-front payments received from Paladin are being recognized as revenue on a straight-line basis over the contract term which is estimated to be 192 months. When earned by us in future periods, additional milestone payments achieved will be amortized over the remaining estimated of the contract. The resulting amortization included in Net revenues for 2009 and 2008 amounted to $31,000 and $19,000, respectively.
(g) PharmaForm
In June 2003, the Company entered into a license agreement with PharmaForm. Under this agreement, as amended, the Company was granted an exclusive, worldwide, royalty-bearing license to develop, make and sell products that contain hormones or that are used to treat urologic disorders incorporating PharmaForm’s oral transmucosal film technology for which there is an issued patent in the U.S. The term of this license agreement is for the life of the licensed patents.
In February 2005, the Company entered into an additional license agreement with PharmaForm. Under this agreement, the Company was granted exclusive, worldwide royalty-bearing rights to develop, manufacture
112
Table of Contents
and market eight compounds using PharmaForm’s proprietary transmucosal film technology for the management of pain, including acute and chronic pain. As of the fourth anniversary of the effective date, the agreement covers only those compounds for which we have initiated formulation development. The term of this license agreement continues on a product by product and country by country basis until the later of ten years after the first commercial sale or last to expire patent covering the licensed technology. The compounds that may be developed include opioids as well other types of analgesics. In addition, we will have on-going royalty payment obligations to PharmaForm. The timing of the remaining payments, if any, is uncertain.
(9) Revolving Credit Agreement
On August 4, 2009, the Company entered into a two year revolving credit agreement (“Revolving Line of Credit Agreement”) with Silicon Valley Bank (“SVB”). The Revolving Line of Credit Agreement provides a credit commitment of up to $30.0 million, subject to certain limitations discussed below, and provides SVB with a first priority lien on all our assets as security for borrowings. It also provides for a one-time increase in the amount of the credit commitment of up to $10.0 million upon mutual agreement of the parties. As of December 31, 2009, there were no outstanding borrowings under the Line of Credit Agreement.
Interest on borrowings under the Revolving Line of Credit Agreement accrues at SVB’s prime rate plus 0.50 percent, subject to a minimum prime rate of 4.00 percent. The interest rate on borrowings will reduce to SVB’s prime rate upon achievement by the Company of certain financial measurements. The financial covenants contained in the Revolving Line of Credit Agreement are a quarterly and cumulative profitability and loss restriction, and a minimum liquidity ratio (“Minimum Quick Ratio”), defined as the ratio of unrestricted cash and cash equivalents, plus net accounts receivable, divided by current liabilities plus, without duplication, all outstanding credit extensions owed to SVB, but excluding deferred revenue and deferred rent. The Company must not (x) exceed a quarterly maximum net loss of between $10 million and $30 million, depending on the quarter at issue, during the initial portion of the term of the agreement, and (y) fall below a quarterly minimum net income of between $5 million and $10 million, depending on the quarter at issue, during the later portion of the term of the agreement. In addition, the Company must maintain a monthly and quarterly Minimum Quick Ratio of between 0.80:1.00 and 1.25:1.00, depending on the month or quarter at issue. If such financial covenants are not maintained, the Company may not be able to borrow under the agreement or amounts outstanding under the agreement may be declared due and payable.
When “Net Liquidity”, defined as unrestricted cash and cash equivalents at SVB less borrowings from SVB, is greater than $15.0 million there are no restrictions on the amount which may be borrowed under the credit facility. When Net Liquidity is less than $15.0 million, the amount which may be borrowed under the credit facility is limited to specified percentages of certain customer accounts receivables. The Company incurs (1) an annual commitment fee of $150,000, (2) a fee of 0.50 percent per annum on the average unused balance of the commitment and (3), if Net Liquidity is less than $7.5 million, a monthly collateral handling fee of 0.0625 percent of the average monthly amount borrowed. The Revolving Line of Credit Agreement can be terminated by the Company at any time, but is subject to a 2.00 percent fee if such termination is made prior to August 4, 2010 and 1.00 percent fee if such termination is made after August 3, 2010 but prior to the maturity date.
113
Table of Contents
(10) Accrued Expenses
Accrued expenses consist of the following (in thousands):
| December 31, | ||||||
| 2009 | 2008 | |||||
| Payroll and related expenses |
$ | 13,013 | $ | 8,283 | ||
| Royalty expenses |
5,206 | 10,541 | ||||
| Research and development expenses |
2,498 | 4,926 | ||||
| Sales and marketing expenses |
9,827 | 2,491 | ||||
| Testim rebates, discounts and returns accrual |
16,218 | 11,990 | ||||
| Pfizer Agreement expenses |
— | 3,000 | ||||
| Other expenses |
4,809 | 5,462 | ||||
| $ | 51,571 | $ | 46,693 | |||
(11) Commitments and Contingencies
(a) Leases
The Company leases its main office space and its Horsham, Pennsylvania manufacturing facility under two noncancellable operating leases that expire in 2013 and 2017, respectively. Supporting warehouse, laboratory and office space in Horsham are also leased under noncancellable operating leases that will expire in 2017 and 2022, respectively. These leases include periods of free rent and escalating minimum rent payments, and provide allowances to improve the leased facility and other lease incentives. The Company records rent expense for the minimum lease payments on a straight-line basis over the noncancellable lease term. The Company has recorded the cost of the improvements as a fixed asset and the allowance as a deferred rent credit. The Company amortizes the leasehold improvement asset over the shorter of the life of the improvements or the life of the lease. The Company amortizes the deferred rent credit as a reduction of rent expense on a straight-line basis over the life of the lease. The Company also leases office equipment and automobiles. Rent expense was $4,154,000, $4,001,000 and $3,482,000 for the years ended December 31, 2009, 2008, and 2007, respectively.
The lease agreement for the manufacturing facility in Horsham, Pennsylvania has an initial term ending January 1, 2017 and may be extended for two consecutive five-year periods. The Company maintains a bank letter of credit in the amount of $1,900,000 as a security deposit for this lease which is collateralized by a bank deposit of equal amount. This $1,900,000 bank deposit is included in other long-term assets at December 31, 2009 and 2008. Under the lease agreement, the remaining balance of tenant improvements of $3,250,000 is available to the Company and will expire, if not used, by December 31, 2010.
Future minimum lease payments under noncancellable operating leases for manufacturing facilities, office space, equipment and automobiles as of December 31, 2009 are as follows:
| January 1, 2010 to December 31, 2010 |
$ | 5,317,000 | |
| January 1, 2011 to December 31, 2011 |
$ | 5,070,000 | |
| January 1, 2012 to December 31, 2012 |
$ | 4,790,000 | |
| January 1, 2013 to December 31, 2013 |
$ | 4,987,000 | |
| January 1, 2014 to December 31, 2014 |
$ | 3,903,000 | |
| January 1, 2015 and thereafter |
$ | 11,210,000 |
(b) Supply Agreements
The Company has a supply agreement for the production of Testim with DPT Laboratories, Ltd. (“DPT”) which expires on December 31, 2010. In connection with the agreement, the Company purchased approximately $1.1 million of packaging equipment that is used by DPT in Testim production. The Company
114
Table of Contents
owns the equipment. The packaging equipment was placed in service at the end of 2003 and is being amortized over the estimated life of the equipment. Although the Company is not required to purchase any minimum amount of Testim from DPT, it is required to pay a minimum annual manufacturing fee of $190,000.
On June 26, 2008, the Company entered into a supply agreement (the “Supply Agreement”) with Hollister-Stier Laboratories LLC (“Hollister-Stier”), pursuant to which Hollister-Stier manufactures commercial batches of XIAFLEX. The Supply Agreement sets forth specifications, specific services, timelines, pricing, and responsibilities of the parties. The Supply Agreement is effective for an initial term of three years, unless terminated earlier for cause, and is automatically renewed thereafter for subsequent two year terms, unless or until either party provides notification prior to expiration of the then current term of the contract.
The Company is required to purchase a specified percentage of its total forecasted volume of XIAFLEX from Hollister-Stier each year. This purchase obligation is only relieved in the event that Hollister-Stier is not able to supply XIAFLEX within the timeframe established under such forecasts. In the event the Company does not order forecasted batches, it is responsible for the aggregate amounts of components and raw materials purchased by Hollister-Stier to manufacture XIAFLEX for the first twelve (12) months in each forecast, unless Hollister-Stier is unable to supply XIAFLEX in a timely manner. The Supply Agreement provides for cross-indemnification of the parties with Hollister-Stier’s indemnification obligation to the Company for third party claims being limited to $5 million.
(c) Research, Development and License Commitments
As discussed in Note 6, the Company has entered into various licensing agreements. Through December 31, 2009, the Company has made aggregate milestone payments under all agreements of $10,125,000. The Company could make an additional $27,225,000 of contingent milestone payments under all agreements if all underlying events occur over the life of the agreements.
(12) Income Taxes
The components of the net deferred tax assets are as follows (in thousands):
| December 31, | ||||||||
| 2009 | 2008 | |||||||
| Net operating losses |
$ | 64,403 | $ | 74,118 | ||||
| Research and development credit |
6,513 | 5,547 | ||||||
| Depreciation and amortization |
629 | 2,518 | ||||||
| Accruals and reserves |
9,527 | 7,994 | ||||||
| Deferred revenue |
25,388 | 3,286 | ||||||
| Stock compensation |
10,441 | 5,847 | ||||||
| Other temporary differences |
89 | 79 | ||||||
| Gross deferred tax assets |
116,990 | 99,389 | ||||||
| Deferred tax assets valuation allowance |
(116,990 | ) | (99,389 | ) | ||||
| $ | — | $ | — | |||||
In assessing the realizability of deferred tax assets, the Company considers whether it is more likely than not that some portion or all of the deferred tax assets will not be realized. The ultimate realization of deferred tax assets is dependent upon the generation of future taxable income during the periods in which the temporary differences representing net future deductible amounts become deductible. Due to the Company’s history of losses, the deferred tax assets are fully offset by a valuation allowance at December 31, 2009 and 2008. The valuation allowance in 2009 increased by $17,601,000 over 2008 related primarily to additional net operating losses incurred by the Company.
115
Table of Contents
At December 31, 2009, the Company had Federal tax return net operating loss carryforwards of approximately $209,400,000 which will expire in 2019 through 2028, if not utilized, and of which $23,000,000 is a result of windfall stock compensation deductions. The recorded deferred tax asset amount of $64,403,000 for net operating losses shown in the above table is net of these windfall stock compensation deductions which, when realized, will be recorded directly to additional paid-in capital and of the change in ownership limitation discussed below. The Federal research and development credits of $6,513,000 at December 31, 2009 shown in the above table will expire in 2021 through 2029, if not utilized.
In addition, the Company had overall state tax return net operating loss carryforwards of approximately $178,900,000, of which $88,000,000 relate to Pennsylvania, which expires in 2010 through 2029 if not utilized. Future utilization of Pennsylvania net operating loss carryforwards is limited to the greater of 20% of Pennsylvania taxable income or $3.0 million per year.
The Tax Reform Act of 1986 (the “Act”) provides for a limitation on the annual use of net operating loss and research and development tax credit carryforwards following certain ownership changes (as defined by the Act) that could limit the Company’s ability to utilize these carryforwards. Generally, a change in ownership of a company of greater than 50% within a three-year period results in an annual limitation on that company’s ability to utilize its carryforwards from the tax periods prior to the ownership change.
The Company has conducted a study to determine whether it has experienced any ownership changes, as defined by the Act. As a result of the study the Company has concluded that it has undergone multiple ownership changes in previous years. Accordingly, the Company’s ability to utilize the aforementioned carryforwards will be limited on an annual basis. The Company believes that such limitations may result in approximately $10,700,000 and $9,400,000 of Federal and state net operating loss carryforwards, respectively, expiring prior to utilization.
The Company and its subsidiaries file income tax returns in the U.K., U.S. and local tax jurisdictions in the U.S. Presently, the Company has not been contacted by the Internal Revenue Service or any state tax jurisdictions for examination of its income tax returns for open periods. As the Company has generated losses since inception, all periods from 1999 through 2009, are open to examination.
As of December 31, 2009, the Company believes that there are no significant uncertain tax positions, and no amounts have been recorded for interest and penalties. The Company does not anticipate any events that would require it to record a liability related to any uncertain tax position.
(13) Common Stock and Redeemable Convertible Preferred Stock
(a) Common Stock
The Company is authorized to issue 120,000,000 shares of common stock, with a par value of $0.01 per share.
On June 8, 2007, the Company completed a registered direct offering in which the Company issued 3,672,000 shares of common stock at a price of $14.50 per share resulting in proceeds to the Company, net of placement agent fees and other transaction costs, of approximately $49,866,000.
On September 30, 2009, the Company completed the sale of 3,450,000 shares of common stock at a price of $34.50 per share in an underwritten public offering resulting in proceeds, net of offering expenses and underwriting discounts and commissions, of approximately $115,780,000.
(b) Preferred Stock
The Company is authorized to issue 5,000,000 shares of preferred stock, with a par value of $0.01 per share. No preferred stock is issued or outstanding.
116
Table of Contents
(14) Warrants
As of December 31, 2009, the Company had 102,524 common stock warrants outstanding which have an exercise price of $5.84 per share and expire in June of 2010.
(15) Employee Stock Benefit Plans
(a) Employee Stock Purchase Plan
Under the Company’s 2006 Employee Stock Purchase Plan (“ESPP”), as approved by the stockholders of the Company, employees may purchase shares of the Company’s common stock at a 15% discount through payroll deductions. Employees may contribute up to 10% of their compensation to the ESPP. The purchase price is 85% of the fair value per share of common stock on the date the purchase period begins or the date on which the purchase period ends, whichever is lower. The ESPP restricts the maximum number of shares that an employee may purchase to 15,000 shares during each semi-annual purchase period and to $25,000 worth of common stock during each year. There is a maximum of 300,000 shares issuable under the ESPP. In January 2007, July 2007, December 2007, June 2008, December 2008, June 2009 and December 2009 employees purchased 39,721, 37,920, 28,810, 25,602, 21,985, 29,546 and 31,109 shares of common stock at a price of $6.8595, $12.4865, $14.0505, $25.4915, $24.1740, $24.2080 and $25.4830 per share, respectively. At December 31, 2009, there were 60,655 shares available for future grant under the ESPP.
(b) Stock Options and Stock Awards
Under the Company’s 2004 Equity Compensation Plan, amended and restated December 1, 2009, (the “2004 Plan”), as approved by the stockholders of the Company, qualified and nonqualified stock options and stock awards may be granted to employees, non-employee directors and service providers. In June 2009, the stockholders authorized the increase of shares authorized for issuance under the 2004 Plan by 2,650,000 shares from 8,000,000 shares to 10,650,000 shares. The Compensation Committee of the Board of Directors (the “Compensation Committee”) administers the 2004 Plan and has delegated to each of the Company’s Chief Executive Officer and Chief Financial Officer the authority to grant stock options to newly hired employees and promoted employees below the Vice President level within specified parameters. Beginning in 2008, the members of the Board of Directors may annually elect to receive all, or a designated portion, of their fees in the form of common stock instead of cash. The shares issued pursuant to such elections by Board members are issued under the 2004 Plan. During the year ended December 31, 2009 and 2008, such issuances amounted to 5,026 and 6,493 shares having an aggregate fair value of $154,000 and $199,000, respectively, on the dates of issuance. Otherwise, the Company has, to date, granted only nonqualified stock options and restricted stock awards under the 2004 Plan. Stock options are granted with an exercise price equal to 100% of the market value of the common stock on the date of grant, and generally have a ten-year contractual term and vest no later than four years from the date of grant (with some providing for automatic vesting upon a change of control of the Company). The Company issues new shares of common stock upon exercise of stock options. At December 31, 2009, there were 2,444,722 shares available for future grants under the 2004 Plan.
117
Table of Contents
(c) General Stock Option Information
The following tables summarize stock option activity for the three years ended December 31, 2009:
| Years Ended December 31, | ||||||||||||
| 2009 | 2008 | 2007 | ||||||||||
| Options outstanding: |
||||||||||||
| Outstanding at beginning of period |
4,425,624 | 3,776,105 | 3,548,903 | |||||||||
| Granted |
1,592,547 | 1,568,359 | 1,339,240 | |||||||||
| Exercised |
(396,088 | ) | (645,562 | ) | (826,034 | ) | ||||||
| Cancelled |
(262,973 | ) | (273,278 | ) | (286,004 | ) | ||||||
| Outstanding at end of period |
5,359,110 | 4,425,624 | 3,776,105 | |||||||||
| Exercisable at end of period |
2,177,464 | 1,418,731 | 1,153,052 | |||||||||
| Weighted average exercise prices: |
||||||||||||
| Outstanding at beginning of period |
$ | 17.67 | $ | 10.27 | $ | 7.40 | ||||||
| Granted |
28.21 | 32.00 | 15.29 | |||||||||
| Exercised |
9.10 | 8.47 | 6.34 | |||||||||
| Cancelled |
23.30 | 19.48 | 9.53 | |||||||||
| Outstanding at end of period |
21.16 | 17.67 | 10.27 | |||||||||
| Exercisable at end of period |
14.75 | 9.36 | 7.21 | |||||||||
During the year ended December 31, 2009, the Company granted 1,592,547 standard non-qualified stock options to employees and directors to purchase shares of the Company’s common stock pursuant to the 2004 Plan. The options expire ten years from date of grant and their exercise prices represent the closing price of the common stock of the Company on the respective dates that the options were granted. The standard non-qualified stock options vest no later than four years from the grant date, assuming continued employment of the grantee.
Of the 262,973 options cancelled during 2009, 251,503 represented unvested options forfeited with an average exercise price of $23.02 and 11,470 represented vested options cancelled with a weighted average exercise price of $29.41. The aggregate intrinsic value of options outstanding and of exercisable options as of December 31, 2009 was $51,835,119 and $34,735,743, respectively. These aggregate intrinsic values represent the total pretax intrinsic value, based on the Company’s stock closing price of $28.98 as of December 31, 2009, that would have been received by the option holders had all option holders exercised their options as of that date.
The total intrinsic value of options exercised in 2009, 2008 and 2007 was $8,389,000, $15,925,000 and $9,119,000, respectively. As of December 31, 2009, the weighted average remaining contractual life of outstanding options and of exercisable options was 7.72 and 6.77 years, respectively.
The total number of in-the-money options exercisable as of December 31, 2009 was 1,771,589.
During 2008, the Company granted to certain officers 180,000 performance-based options having an exercise price of $32.72. The performance goal for all 180,000 options was met during 2009 and vest at a rate of 33-1/3% on each 10 month anniversary of the BLA filing date of February 27, 2009. These options provided for immediate vesting upon a change in control of the Company while the grantee is employed by, or providing service to the Company.
(d) Restricted Stock Information
During 2009, the Company granted 79,500 performance-based restricted stock awards to certain officers. The amount of shares ultimately earned, if any, is dependent on the timing of the (1) first commercial sale of XIAFLEX after FDA approval, or (2) a change in control of the Company. The amount of restricted stock awards ultimately earned will vest at the rate of 33 1/3 % on the date of achievement of the performance goal and
118
Table of Contents
on the first and second anniversary of this date. During 2008, the Company established a performance plan for employees responsible for the submission of the BLA to the FDA. Under this plan, 22,000 shares of restricted stock were awarded to these employees in early 2009 and the restrictions on these shares lapsed on April 28, 2009 with the FDA acceptance of the BLA.
The compensation cost of restricted stock awards is determined by their intrinsic value on the grant date. The following table summarizes the restricted stock activity for the three years ended December 31, 2008:
| 2009 | 2008 | 2007 | ||||||||||
| Nonvested shares: |
||||||||||||
| At beginning of period |
142,000 | 198,072 | 232,544 | |||||||||
| Granted |
101,500 | 2,750 | 15,000 | |||||||||
| Vested |
(75,000 | ) | (58,822 | ) | (48,742 | ) | ||||||
| Forfeited |
— | — | (730 | ) | ||||||||
| At end of period |
168,500 | 142,000 | 198,072 | |||||||||
| Weighted average grant date fair value: |
||||||||||||
| At beginning of period |
$ | 8.07 | $ | 7.92 | $ | 7.47 | ||||||
| Granted |
29.88 | 32.67 | 13.39 | |||||||||
| Vested |
15.57 | 8.72 | 7.48 | |||||||||
| Forfeited |
— | — | 7.50 | |||||||||
| At end of period |
17.87 | 8.07 | 7.92 | |||||||||
(e) Stock Awards
During 2009, the Company also issued to certain officers and other employees 32,250 shares of common stock having a grant date fair value of $30.00 per share, or $967,500 in the aggregate.
(f) Valuation and Expense Information
Total stock-based compensation costs charged against income for the year ended December 31, 2009, 2008 and 2007 amounted to $17,900,000, $11,742,000 and $6,037,000, respectively. Stock-based compensation costs capitalized as part of inventory amounted to $318,000 at December 31, 2009, and were insignificant in prior periods.
The Company measures the cost of share-based compensation awards at fair value on the date of grant using the Black-Scholes model and applying the assumptions in the following table. The expected volatility is based on the blended historical volatility of the Company and the historical volatility of peer group companies. The Company uses the simplified calculation of expected option life prescribed in the guidance issued by the Securities and Exchange Commission because the Company’s history is inadequate to determine a reasonable estimate of the option life. The dividend yield is determined based on the Company’s history to date and management’s estimate of dividends over the option life. The risk-free interest rate is based on the U.S. treasury yield curve in effect at the time of the grant.
| Year Ended December 31, | |||||||||
| 2009 | 2008 | 2007 | |||||||
| Weighted average assumptions: |
|||||||||
| Expected life of options (in years) |
6.17 | 6.17 | 6.09 | ||||||
| Risk-free interest rate |
2.52 | % | 3.13 | % | 4.59 | % | |||
| Expected volatility |
50.26 | % | 47.90 | % | 50.18 | % | |||
| Expected dividend yield |
0.00 | % | 0.00 | % | 0.00 | % | |||
The weighted-average grant date fair value of the options issued in 2009, 2008 and 2007 was $14.33, $16.11 and $8.30, respectively. As of December 31, 2009, there was approximately $25,875,000 of total unrecognized stock-based compensation cost related to all share-based payments that will be recognized over the
119
Table of Contents
weighted- average period of 2.21 years. Future grants will add to this total, whereas future amortization and the vesting of existing grants will reduce this total.
(16) Subsequent Events
On January 21, 2010, Pfizer announced the European scientific/technical review procedure for the MAA for XIAFLEX for the treatment of Dupuytren’s commenced. As a result of accomplishing this milestone, the Company received a $15 million milestone payment from Pfizer. On February 2, 2010, the Company received marketing approval from the FDA for XIAFLEX for Dupuytren’s. In connection with these events, the Company owes BioSpecifics approximately $2.3 million.
(17) Unaudited Quarterly Financial Information (in thousands, except share and per share amounts):
| 2009 | ||||||||||||||||
| 1st Qtr | 2nd Qtr | 3rd Qtr | 4th Qtr | |||||||||||||
| Net revenues |
$ | 34,674 | $ | 39,194 | $ | 42,143 | $ | 48,027 | ||||||||
| Cost of goods sold |
7,877 | 9,239 | 9,545 | 10,416 | ||||||||||||
| Research and development expenses* |
13,534 | 13,734 | 12,754 | 11,375 | ||||||||||||
| Selling, general and administrative expenses* |
26,403 | 29,383 | 34,685 | 38,711 | ||||||||||||
| Loss from operations |
(13,140 | ) | (13,162 | ) | (14,841 | ) | (12,475 | ) | ||||||||
| Net loss |
(13,234 | ) | (13,374 | ) | (14,949 | ) | (11,899 | ) | ||||||||
| Basic and diluted net loss per common share (1) |
(0.31 | ) | (0.32 | ) | (0.35 | ) | (0.25 | ) | ||||||||
| Weighted average basic and diluted common shares outstanding |
42,320,072 | 42,008,009 | 42,761,821 | 47,001,779 | ||||||||||||
|
|
||||||||||||||||
| * includes the following amounts of stock-based compensation expense: |
||||||||||||||||
| Research and development |
$ | 1,132 | $ | 1,752 | $ | 1,136 | $ | 1,027 | ||||||||
| Selling, general and administrative |
3,398 | 3,117 | 3,001 | 3,336 | ||||||||||||
| 2008 | ||||||||||||||||
| 1st Qtr | 2nd Qtr | 3rd Qtr | 4th Qtr | |||||||||||||
| Net revenues |
$ | 27,117 | $ | 30,901 | $ | 32,585 | $ | 34,775 | ||||||||
| Cost of goods sold |
5,999 | 7,409 | 7,821 | 8,257 | ||||||||||||
| Research and development expenses* |
13,191 | 13,435 | 13,279 | 14,591 | ||||||||||||
| Selling, general and administrative expenses* |
21,020 | 22,173 | 21,931 | 24,358 | ||||||||||||
| Loss from operations |
(13,093 | ) | (12,116 | ) | (10,446 | ) | (12,431 | ) | ||||||||
| Net loss |
(12,314 | ) | (11,726 | ) | (10,107 | ) | (12,319 | ) | ||||||||
| Basic and diluted net loss per common share (1) |
(0.30 | ) | (0.29 | ) | (0.24 | ) | (0.29 | ) | ||||||||
| Weighted average basic and diluted common shares outstanding |
40,670,290 | 41,050,599 | 41,388,176 | 41,972,506 | ||||||||||||
|
|
||||||||||||||||
| * includes the following amounts of stock-based compensation expense: |
||||||||||||||||
| Research and development |
$ | 516 | $ | 648 | $ | 893 | $ | 1,084 | ||||||||
| Selling, general and administrative |
1,597 | 2,128 | 2,295 | 2,581 | ||||||||||||
| (1) | The sum of the quarterly loss per share amounts may differ from the full year amount due to changes in the number of shares outstanding during the year. |
120
Table of Contents
| ITEM 9. | Changes in and Disagreements with Accountants on Accounting and Financial Disclosure |
None.
| ITEM 9A. | Controls and Procedures |
Evaluation of Disclosure Controls and Procedures
Our Chief Executive Officer and Chief Financial Officer have concluded, based on their respective evaluations as of the end of the period covered by this Report, that our disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act) as of the end of the period covered by this Report are effective to provide reasonable assurance that the information required to be disclosed in the reports we file under the Exchange Act is (i) recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms and (ii) is accumulated and communicated to management as appropriate to allow timely decisions regarding required disclosures. A controls system cannot provide absolute assurances, however, that the objectives of the controls system are met, and no evaluation of controls can provide absolute assurance that all control issues and instances of fraud, if any, within a company have been detected.
Management’s Annual Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting. Internal control over financial reporting is defined in Rules 13a-15(f) and 15d-15(f) promulgated under the Exchange Act as a process designed by, or under the supervision of, our Chief Executive Officer and Chief Financial Officer and effected by our Board of Directors, management and other personnel, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles, and includes those policies and procedures that:
(i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of our assets;
(ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that our receipts and expenditures are being made only in accordance with authorization of our management and directors of our company; and
(iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of our assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Our management assessed the effectiveness of our internal control over financial reporting as of December 31, 2009. In making this assessment, management used the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission (“COSO”) in Internal Control-Integrated Framework. Based on this assessment and those criteria, management concluded that our internal control over financial reporting was effective as of December 31, 2009.
The effectiveness of the Company’s internal control over financial reporting as of December 31, 2009 has been audited by PricewaterhouseCoopers, LLP, an independent registered public accounting firm, as stated in their report which appears herein.
121
Table of Contents
Changes in Internal Control over Financial Reporting
No change in our internal control over financial reporting occurred during our fiscal year ended December 31, 2009 that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
| ITEM 9B. | Other Information |
None
122
Table of Contents
| ITEM 10. | Directors, Executive Officers and Corporate Governance |
The information required by this item is incorporated herein by reference to the sections captioned “Election of Directors,” “Executive Officers,” “Corporate Governance,” and “Section 16(a) Beneficial Ownership Reporting Compliance” in our definitive Proxy Statement relating to our 2010 Annual Meeting of Stockholders.
We have adopted a Code of Conduct that applies to all of our directors, officers and employees. Our Code of Conduct contains provisions that satisfy the standards for a “code of ethics” set forth in Item 406 of Regulation S-K of the rules and regulations of the Securities and Exchange Commission. Our Code of Conduct also contains a special Code of Ethics that is applicable to our Chief Executive Officer and our senior financial officers. Our Code of Conduct is available under the heading “For Investors—Corporate Governance” on our Internet Web site, the address of which is www.auxilium.com. The information contained on our Internet Web site is not incorporated by reference into this Report and should not be considered part of this or any other report that we file with or furnish to the SEC.
To the extent that we amend any provision of our Code of Conduct or grant a waiver from any provision of our Code of Conduct that is applicable to any of our directors or our principal executive officer, principal financial officer, principal accounting officer or controller or persons performing similar functions, we intend to satisfy our disclosure obligations under applicable SEC rules by posting such information on our Internet Web site under the heading “For Investors—Corporate Governance.”
| ITEM 11. | Executive Compensation |
The information required by this item is incorporated herein by reference to the section captioned “Executive Compensation” in our definitive Proxy Statement relating to our 2010 Annual Meeting of Stockholders.
| ITEM 12. | Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters |
The information required by this item is incorporated herein by reference to the sections captioned “Security Ownership of Certain Beneficial Owners and Management” and “Equity Compensation Plan Information” in our definitive Proxy Statement relating to our 2010 Annual Meeting of Stockholders.
| ITEM 13. | Certain Relationships and Related Transactions, and Director Independence |
The information required by this item is incorporated herein by reference to the section captioned “Certain Relationships and Related Party Transactions” in our definitive Proxy Statement relating to our 2010 Annual Meeting of Stockholders.
| ITEM 14. | Principal Accountant Fees and Services |
The information required by this item is incorporated herein by reference to the section captioned “Ratification of Selection of Independent Registered Public Accounting Firm” in our definitive Proxy Statement relating to our 2010 Annual Meeting of Stockholders.
123
Table of Contents
| ITEM 15. | Exhibits and Financial Statement Schedules |
| (a) | The following documents are filed as part of this Report: |
| (1) | Consolidated Financial Statements |
| See Index to Consolidated Financial Statements on page 93. |
| (2) | Financial Statement Schedules |
| All schedules to the consolidated financial statements are omitted as the required information is either inapplicable or presented in the consolidated financial statements. |
| (3) | Exhibits |
| The information required by this Item is set forth in the Exhibit Index hereto which is incorporated herein by reference. |
| (b) | Exhibits |
The information required by this Item is set forth in the Exhibit Index hereto which is incorporated herein by reference.
124
Table of Contents
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this Report to be signed on its behalf by the undersigned, thereunto duly authorized.
| AUXILIUM PHARMACEUTICALS, INC. | ||||
| Date: February 26, 2010 |
By:
|
/s/ Armando Anido | ||
| Armando Anido | ||||
| Chief Executive Officer and President | ||||
POWER OF ATTORNEY
KNOW ALL PERSONS BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints Armando Anido and James E. Fickenscher as true and lawful attorneys-in-fact and agents with full power of substitution and resubstitution, for him and in his name, place and stead, in any and all capacities to sign this Report filed herewith and any or all amendments to said Report, and to file the same, with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission granting unto said attorneys-in-fact and agents the full power and authority to do and perform each and every act and thing requisite and necessary to be done in and about the foregoing, as to all intents and purposes as he might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents or any of them, or his substitute, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Exchange Act of 1934, this Report has been signed by the following persons on behalf of the Registrant in the capacities and on the dates indicated.
| Signature |
Title |
Date | ||
| /s/ Armando Anido
Armando Anido |
Chief Executive Officer and President (Principal Executive Officer) and Director | February 26, 2010 | ||
| /s/ James E. Fickenscher
James E. Fickenscher |
Chief Financial Officer (Principal Financial and Accounting Officer) |
February 26, 2010 | ||
| /s/ Rolf A. Classon
Rolf A. Classon |
Chairman | February 26, 2010 | ||
| /s/ Al Altomari
Al Altomari |
Director | February 26, 2010 | ||
| /s/ Edwin A. Bescherer, Jr.
Edwin A. Bescherer, Jr. |
Director | February 26, 2010 | ||
| /s/ Philippe O. Chambon
Philippe O. Chambon |
Director | February 26, 2010 | ||
| /s/ Oliver S. Fetzer
Oliver S. Fetzer |
Director | February 26, 2010 | ||
| /s/ Renato Fuchs
Renato Fuchs |
Director | February 26, 2010 | ||
| /s/ Dennis H. Langer
Dennis H. Langer |
Director | February 26, 2010 | ||
| /s/ William T. McKee
William T. McKee |
Director | February 26, 2010 | ||
Table of Contents
| Exhibit No. |
Description | |
| 3.1 | Fifth Restated Certificate of Incorporation of the Registrant (filed as Exhibit 3.1 to the Registrant’s Quarterly Report on Form 10-Q filed on August 16, 2004, File No. 000-50855, and incorporated by reference herein). | |
| 3.2 | Amended and Restated Bylaws of the Registrant (filed as Exhibit 3.2 to the Registrant’s Quarterly Report on Form 10-Q filed on August 16, 2004, File No. 000-50855, and incorporated by reference herein). | |
| 4 | Third Amended and Restated Investors Rights Agreement, dated October 31, 2003, by and among the Registrant and parties listed therein (filed as Exhibit 4.2 to the Registrant’s Registration Statement on Form S-1, Registration No. 333-114685, and incorporated by reference herein). | |
| 10.1* | License Agreement, dated May 31, 2000, as amended, between Bentley Pharmaceuticals, Inc. and the Registrant (filed as Exhibit 10.2 to the Registrant’s Quarterly Report on Form 10-Q, filed on May 8, 2009, File No. 000-50855, and incorporated by reference herein). | |
| 10.2* | License Agreement, dated May 31, 2001, as amended, between Bentley Pharmaceuticals, Inc. and the Registrant (filed as Exhibit 10.2 to the Registrant’s Registration Statement on Form S-1, Registration No. 333-114685, and incorporated by reference herein). | |
| 10.3* | License Agreement, dated June 20, 2003, between Formulation Technologies L.L.C. d/b/a PharmaForm and the Registrant (filed as Exhibit 10.3 to the Registrant’s Registration Statement on Form S-1, Registration No. 333-114685, and incorporated by reference herein). | |
| 10.4 | Amendment, dated April 19, 2004, to License Agreement between Formulation Technologies, L.L.C. d/b/a PharmaForm and the Registrant, dated June 20, 2003 (filed as Exhibit 10.20 to the Registrant’s Registration Statement on Form S-1, Registration No. 333-114685, and incorporated by reference herein). | |
| 10.5 | Amendment No. 2, dated June 17, 2004, to License Agreement between Formulation Technologies L.L.C. d/b/a PharmaForm and the Registrant, dated June 20, 2003 (filed as Exhibit 10.25 to the Registrant’s Registration Statement on Form S-1, Registration No. 333-114685, and incorporated by reference herein). | |
| 10.6* | License Agreement, dated January 26, 2005, between Formulation Technologies, L.L.C. d/b/a PharmaForm and the Registrant (filed as Exhibit 10.1 to the Registrant’s Quarterly Report on Form 10-Q filed on May 8, 2009, File No. 000-50855, and incorporated by reference herein). | |
| 10.7* | The Amended and Restated Development and License Agreement, dated December 11, 2008, by and between BioSpecifics Technologies Corp. and the Registrant (filed as Exhibit 10.2 to the Registrant’s Current Report on Form 8-K filed on December 18, 2008, File No. 000-50855, and incorporated by reference herein). | |
| 10.8* | License and Distribution Agreement, dated March 26, 2004, between Ipsen Pharma GmbH and the Registrant (filed as Exhibit 10.7 to the Registrant’s Registration Statement on Form S-1, Registration No. 333-114685, and incorporated by reference herein). | |
| 10.9* | Manufacturing Agreement, dated April 23, 2002, between DPT Laboratories, Ltd. and Registrant (filed as Exhibit 10.5 to the Registrant’s Registration Statement on Form S-1, Registration No. 333-114685, and incorporated by reference herein). | |
| 10.10* | First Amendment, dated May 28, 2002 to Manufacturing Agreement between DPT Laboratories, Ltd. and the Registrant, dated April 23, 2002 (filed as Exhibit 10.6 to the Registrant’s Registration Statement on Form S-1, Registration No. 333-114685, and incorporated by reference herein). | |
| 10.11* | Third Amendment, dated September 14, 2005, to Manufacturing Agreement between DPT Laboratories, Ltd. and the Registrant, dated April 23, 2002 (filed as Exhibit 10 to the Registrant’s Current Report on Form 8-K filed on September 20, 2005, File No. 000-50855, and incorporated by reference herein). | |
Table of Contents
| Exhibit No. |
Description | |
| 10.12* | Termination Agreement, dated November 24, 2008, by and between Ipsen Pharma GmbH and the Registrant (filed as Exhibit 10.14 to the Registrant’s Annual Report on Form 10-K for the year ended December 31, 2008, File No. 000-50855, and incorporated by reference herein). | |
| 10.13 | Agreement of Lease, dated December 31, 2004, between Liberty Property Limited Partnership, as landlord, and Auxilium Pharmaceuticals, Inc., as tenant, as amended by the First Amendment to Agreement of Lease, dated as of June 1, 2006, by and between Auxilium Pharmaceuticals, Inc. and Liberty Property Limited Partnership and the Second Amendment to Agreement of Lease, dated February 22, 2008, between Auxilium Pharmaceuticals, Inc. and Liberty Property Limited Partnership (filed as Exhibit 99.1 to the Registrant’s Current Report on Form 8-K filed on February 28, 2008, File No. 000-50855, and incorporated by reference herein). | |
| 10.14 | Form of Securities Purchase Agreement, dated June 28, 2005 (filed as Exhibit 10 to the Registrant’s Current Report on Form 8-K filed on July 1, 2005, File No. 000-50855, and incorporated by reference herein). | |
| 10.15 | Form of Amendment to Securities Purchase Agreement, dated December 30, 2005 (filed as Exhibit 10 to the Registrant’s Current Report on Form 8-K filed on January 6, 2006, File No. 000-50855, and incorporated by reference herein). | |
| 10.16#† | Registrant’s 2004 Equity Compensation Plan, amended and restated as of December 1, 2009. | |
| 10.17# | Form of Nonqualified Stock Option Agreement for awards under the Registrant’s 2004 Equity Compensation Plan, including provision for immediate vesting upon a change of control of the Registrant (filed as Exhibit 10.1 to the Registrant’s Current Report on Form 8-K filed on March 22, 2005, File No. 000-50855, and incorporated by reference herein). | |
| 10.18# | Form of Nonqualified Stock Option Agreement for awards under the Registrant’s 2004 Equity Compensation Plan (filed as Exhibit 10.2 to the Registrant’s Current Report on Form 8-K filed on March 22, 2005, File No. 000-50855, and incorporated by reference herein). | |
| 10.19# | Form of Incentive Stock Option Agreement for awards under the Registrant’s 2004 Equity Compensation Plan, including provision for immediate vesting upon a change of control of the Registrant (filed as Exhibit 10.3 to the Registrant’s Current Report on Form 8-K filed on March 22, 2005, File No. 000-50855, and incorporated by reference herein). | |
| 10.20# | Form of Incentive Stock Option Agreement for awards under the Registrant’s 2004 Equity Compensation Plan (filed as Exhibit 10.4 to the Registrant’s Current Report on Form 8-K filed on March 22, 2005, File No. 000-50855, and incorporated by reference herein). | |
| 10.21# | Registrant’s 2004 Employee Stock Purchase Plan (filed as Exhibit 10.24 to the Registrant’s Registration Statement on Form S-1, Registration No. 333-114685, and incorporated by reference herein). | |
| 10.22#† | Amended and Restated Employment Agreement, dated December 19, 2008, by and between the Registrant and James E. Fickenscher. | |
| 10.23#† | Amended and Restated Employment Agreement, dated December 18, 2008, by and between the Registrant and Jyrki Mattila, M.D., Ph.D. | |
| 10.24#† | Amended and Restated Employment Agreement, dated December 17, 2008 by and between the Registrant and Jennifer Evans Stacey. | |
| 10.25#† | Amended and Restated Employment Agreement, dated December 15, 2008, by and between the Registrant and Armando Anido. | |
| 10.26#† | Amended and Restated Employment Agreement dated, December 17, 2008, by and between the Registrant and Dr. Anthony DelConte. | |
| 10.27#† | Amended and Restated Employment Agreement, dated December 19, 2008, by and between the Registrant and Roger D. Graham, Jr. | |
Table of Contents
| Exhibit No. |
Description | |
| 10.28# | Form of Restricted Stock Grant Agreement for awards under the Registrant’s 2004 Equity Compensation Plan, as amended (filed as Exhibit 10.2 to the Registrant’s Quarterly Report on Form 10-Q filed on May 7, 2007, File No. 000-50855, and incorporated by reference herein). | |
| 10.29# | Form of Nonqualified Stock Option Agreement for awards to Non-Employee Directors under the Registrant’s 2004 Equity Compensation Plan, as amended (filed as Exhibit 10.3 to the Registrant’s Quarterly Report on Form 10-Q filed on May 8, 2009, File No. 000-50855, and incorporated by reference herein). | |
| 10.30# | Form of Nonqualified Stock Option Agreement for awards to Executive Officers under the Registrant’s 2004 Equity Compensation Plan, as amended (filed as Exhibit 10.4 to the Registrant’s Quarterly Report on Form 10-Q filed on May 8, 2009, File No. 000-50855, and incorporated by reference herein). | |
| 10.31* | Supply Agreement, dated June 26, 2008, between the Registrant and Hollister-Stier Laboratories LLC (filed as Exhibit 10.1 to the Registrant’s Quarterly Report on Form 10-Q filed on August 8, 2008, File No. 000-50855, and incorporated by reference herein). | |
| 10.32 | First Amendment to Lease Agreement, dated September 29, 2008, by and between the Registrant and ARE-PA Region No. 6, LLC (filed as Exhibit 99.1 to the Registrant’s Current Report on Form 8-K filed on October 1, 2008, File No. 000-50855, and incorporated by reference herein). | |
| 10.33* | Development, Commercialization and Supply Agreement dated December 17, 2008, by and among, the Registrant, Auxilium International Holdings, Inc. and Pfizer, Inc. (filed as Exhibit 10.1 to the Registrant’s Current Report on Form 8-K filed on December 18, 2008, File No. 000-50855, and incorporated by reference herein). | |
| 10.34* | Loan and Security Agreement, dated August 4, 2009, between Silicon Valley Bank, Auxilium Pharmaceuticals, Inc., Auxilium International Holdings, Inc. and Auxilium U.S. Holdings, LLC (filed as Exhibit 10.2 to the Registrant’s Quarterly Report on Form 10-Q filed on August 10, 2009, File No. 000-50855, and incorporated by reference herein). | |
| 10.35# | Auxilium Pharmaceuticals, Inc. Non-Employee Director Compensation Plan, effective as of July 1, 2009 (filed as Exhibit 10.1 to the Registrant’s Quarterly Report on Form 10-Q filed on August 10, 2009, File No. 000-50855, and incorporated by reference herein). | |
| 21 | Subsidiaries of Registrant (filed as Exhibit 21 to the Registrant’s Annual Report on Form 10-K for the year ended December 31, 2007 and incorporated by reference herein). | |
| 23† | Consent of PricewaterhouseCoopers LLP. | |
| 24† | Power of Attorney (included on signature page). | |
| 31.1† | Certification of Armando Anido, the Registrant’s Principal Executive Officer, required by Rule 13a-14(a) or Rule 15d-14(a). | |
| 31.2† | Certification of James E. Fickenscher, the Registrant’s Principal Financial Officer, required by Rule 13a-14(a) or Rule 15d-14(a). | |
| 32† | Certification of Armando Anido, the Registrant’s Principal Executive Officer, and James E. Fickenscher, the Registrant’s Principal Financial Officer, required by Rule 13a-14(b) or Rule 15d-14(b) and Section 1350 of Chapter 63 of Title 18 of the U.S. Code (18 U.S.C. 1350). | |
| * | Certain information in this exhibit has been omitted pursuant to an Order Granting Confidential Treatment issued by the SEC. |
| † | Filed herewith. |
| # | Indicates management contract or compensatory plan or arrangement. |
