Attached files
| file | filename |
|---|---|
| EX-21 - EXHIBIT 21 - CAMBREX CORP | ex21.htm |
| EX-24 - EXHIBIT 24 - CAMBREX CORP | ex24.htm |
| EX-23 - EXHIBIT 23 - CAMBREX CORP | ex23.htm |
| EX-10.2 - EXHIBIT 10.2 - CAMBREX CORP | ex10_2.htm |
| EX-10.21 - EXHIBIT 10.21 - CAMBREX CORP | ex10_21.htm |
| EX-32 - EXHIBIT 32 - CAMBREX CORP | ex32.htm |
| EX-31.2 - EXHIBIT 31.2 - CAMBREX CORP | ex31_2.htm |
| EX-31.1 - EXHIBIT 31.1 - CAMBREX CORP | ex31_1.htm |
UNITED
STATES
SECURITIES
AND EXCHANGE COMMISSION
WASHINGTON,
D.C. 20549
FORM
10-K
x ANNUAL REPORT PURSUANT
TO SECTION 13 OR 15(d) OF THE
SECURITIES
EXCHANGE ACT OF 1934
For the
fiscal year ended December 31, 2009
OR
o TRANSITION REPORT
PURSUANT TO SECTION 13 OR 15(d) OF
THE
SECURITIES EXCHANGE ACT OF 1934
For the
transition period from to
Commission
file number 1-10638
CAMBREX
CORPORATION
(Exact
name of registrant as specified in its Charter)
|
Delaware
|
22-2476135
|
|
(State
or other jurisdiction of incorporation or organization)
|
(I.R.S.
Employer Identification No.)
|
|
One
Meadowlands Plaza,
|
|
|
East
Rutherford, New Jersey
|
07073
|
|
(Address
of principal executive offices)
|
(Zip
Code)
|
|
Registrant's
telephone number, including area code: (201)
804-3000
|
|
|
Securities
registered pursuant to Section 12(b) of the Act:
|
|
|
Title of each class
|
Name of each
exchange on which
registered
|
|
Common
Stock, $.10 par value
|
New
York Stock Exchange
|
Securities registered pursuant to
Section 12 (g) of the Act: (None)
Indicate
by check mark whether the Registrant is a well-known seasoned issuer, as defined
in Rule 405 of the Securities Act.
Yes o. No
ý.
Indicate
by check mark if the Registrant is not required to file reports pursuant to
Section 13 or Section 15(d) of the Act. Yes o. No
ý.
Indicate
by check mark whether the registrant (1) has filed all reports required to be
filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the
preceding 12 months (or for such shorter period that the registrant was required
to file such reports), and (2) has been subject to such filing requirements for
the past 90 days. Yes ý. No
o.
Indicate
by check mark whether the registrant has submitted electronically and posted on
its corporate Web site, if any, every Interactive Data File required to be
submitted and posted pursuant to Rule 405 of Regulation S-T during the preceding
12 months (or for such shorter period that the registrant was required to submit
and post such files). Yes o No
o The registrant is
not yet subject to this requirement.
Indicate
by check mark if disclosure of delinquent filers pursuant to Item 405 of
Regulation S-K is not contained herein, and will not be contained, to the best
of the registrant's knowledge, in definitive proxy or information statements
incorporated by reference in Part III of this Form 10-K or any amendment to this
Form 10-K. o
Indicate
by check mark whether the registrant is a large accelerated filer, an
accelerated filer, a non-accelerated filer or a smaller reporting
company. See definition of “accelerated filer and large accelerated
filer” in Rule 12b-2 of the Exchange Act. (Check one):
|
Large
accelerated filer o
|
Accelerated
filer ý
|
Non-accelerated
filer o
|
Smaller
reporting company o
|
Indicate
by check mark whether the Registrant is a shell company (as defined in Rule
12b-2 of the Act). Yes o. No
ý.
The
aggregate market value of the voting stock held by non-affiliates of the
registrant was approximately $119,178,870 as of June 30, 2009.
APPLICABLE
ONLY TO CORPORATE REGISTRANTS
As of
January 31, 2010, there were 29,319,872 shares outstanding of the registrant's
Common Stock, $.10 par value.
DOCUMENTS
INCORPORATED BY REFERENCE
Portions
of the Registrant’s definitive Proxy Statement for the 2010 Annual Meeting are
incorporated by reference into Part III of this Report.
CAMBREX CORPORATION AND SUBSIDIARIES
INDEX
TO ANNUAL REPORT ON
FORM
10-K FILED WITH THE
SECURITIES
AND EXCHANGE COMMISSION
For
the Year Ended December 31, 2009
|
Item No.
|
PART
I
|
Page No.
|
|
1
|
2
|
|
|
1A
|
7
|
|
|
1B
|
14
|
|
|
2
|
14
|
|
|
3
|
14
|
|
|
4
|
14
|
|
|
PART
II
|
||
|
5
|
15
|
|
|
6
|
17
|
|
|
7
|
19
|
|
|
7A
|
32
|
|
|
8
|
33
|
|
|
9
|
72
|
|
|
9A
|
72
|
|
|
9B
|
73
|
|
|
PART
III
|
||
|
10
|
74
|
|
|
11
|
75
|
|
|
12
|
75
|
|
|
13
|
75
|
|
|
14
|
75
|
|
|
PART
IV
|
||
|
15
|
76
|
PART I
Item
1 Business
General
Cambrex Corporation (the "Company" or
"Cambrex"), a Delaware corporation, began business in December
1981. Cambrex is a life sciences company that provides products and
services that accelerate and improve the development and commercialization of
new and generic therapeutics. The Company primarily supplies its
products and services worldwide to pharmaceutical and generic drug
companies. Cambrex has three operating segments, which are
manufacturing facilities that have been aggregated as one reportable
segment. The Company's overall strategy is to: grow its portfolio of
custom development projects, especially those in the later stages of the
clinical trial process, secure long-term supply agreements to produce active
pharmaceutical ingredients (“APIs”) and intermediates for newly approved drug
products; expand sales of products and projects based on its proprietary
technologies; and partner with generic drug companies to grow the Company’s
extensive portfolio of generic APIs. The Company also seeks to
demonstrate excellence in regulatory compliance, environmental, health and
safety performance, and customer service.
The Company uses a consistent business
approach:
|
|
·
|
Niche
Market Focus: The Company participates in niche markets where
significant technical expertise provides a competitive advantage and
market differentiation.
|
|
|
·
|
Market
Leadership: The Company secures leading market positions
through excellent customer service, proprietary technologies, specialized
capabilities and an outstanding regulatory record and leverages these
capabilities across the market segments in which it
participates.
|
|
|
·
|
New
Products and Services: The Company continues to invest in
research and product development in order to introduce innovative products
and services to accelerate revenue growth, provide a competitive advantage
and maintain its leading market
positions.
|
|
|
·
|
Operational
Excellence: The Company maintains its commitment to continually
improve productivity and customer service levels and maintains excellent
quality and regulatory compliance
systems.
|
|
|
·
|
Acquisition
and Licensing: The Company may drive growth in strategic
business segments through the prudent acquisition of products, product
lines, technologies and capabilities to enhance the Company's position in
its niche markets.
|
As part
of the process of evaluating strategic alternatives to enhance shareholder
value, the sale of two businesses within the former Human Health segment was
completed in October 2006 and the sale of the businesses that comprised the
Bioproducts and Biopharma segments was completed in February 2007, and
accordingly, these businesses are being reported as discontinued operations in
all periods presented.
Market
Overview and Growth Drivers
The Company participates in markets
that serve the healthcare industry. Customers include companies that
discover and commercialize new small molecule human therapeutics using organic
chemistry and generic drug companies.
The aging population, continued
investment in healthcare research and drug development, and the necessity to
develop life saving therapeutics to address unmet needs drives business growth
in life sciences companies. Aging "baby boomers" in the United
States, Europe and Japan may provide an enormous healthcare
opportunity. This group typically has more education, a higher
socio-economic level and higher demands for healthcare services than previous
generations.
_____________
(dollars
in thousands, except share data)
Demand for Cambrex products and
services is dependent upon some of its customers’ continuing access to financial
resources to advance their research and development (“R&D”) projects for
therapeutic candidates from the laboratory to the clinic, and eventually, to the
patient. Healthcare investment comes from a variety of
sources. Large pharmaceutical and biotechnology companies spend
billions on drug discovery and development. Macro-economic conditions
can have an impact on the availability of funding for the Company’s customers,
especially those customers dependent upon venture capital and other private
sources of funding.
Once a drug is identified, companies
need to develop a robust process for the manufacture of clinical and commercial
quantities. Product testing, analytical methods and quality processes
need to be integrated into the manufacturing process. This is a critical step to
getting a commercially viable drug to market. Cambrex excels in the
manufacture and testing of APIs and drug substances at laboratory, clinical and
commercial scale and specializes in optimizing manufacturing
processes.
Demand for outsourced services from
pharmaceutical companies continues to grow. Large pharmaceutical and
biotechnology companies may outsource the development and manufacturing of a
drug substance to manage multiple internal priorities, access new technologies
or additional capacity, preserve needed capital or ensure multiple sources of
supply. Many emerging pharmaceutical and generic drug companies
outsource all process development and manufacturing. Cambrex is
particularly well positioned to assist drug companies with these much needed
services for traditional APIs.
New drugs are typically
patented. When the patent expires, the drug may be manufactured and
marketed in its generic form. Growth in the generic drug market is
driven by the continuing stream of drug patents that will expire in the future
and favorable market forces that encourage the use of generic pharmaceuticals as
a more cost effective health care alternative to higher-priced branded
drugs. In the United States and many countries in Europe, governments
and prescription benefit management companies provide incentives for generic
substitution to reduce costs. Cambrex manufactures nearly 70 generic
APIs, typically in relatively small quantities for use in niche
therapeutics.
The market for human therapeutics is
regulated by the Food and Drug Administration (“FDA”) in the United States and
other regulatory agencies throughout the world. These agencies
oversee and regulate the development, manufacturing and commercialization
process for APIs and regulated intermediates. Excellent regulatory
and quality systems are essential to serve the industry.
Asian competitors have increased their
capabilities in drug substance manufacturing and finished dosage form drugs in
recent years. There has been a growing impact on the volumes sold of
the Company’s niche products and the presence of these competitors in the market
has resulted in downward pricing pressure on generic APIs and certain
development services for clinical phase products. Regulatory
compliance and product quality may determine the long term impact of these
competitors.
Development
of the Business
The
discussion below provides insight to the general development of our business,
including the material acquisitions and dispositions of assets over the past
five years.
In
October 2006, the Company sold two businesses within the former Human Health
segment for nominal consideration. As a result of this transaction,
these businesses are being reported as discontinued operations in all periods
presented.
In
February 2007, the Company completed the sale of the businesses that comprised
the Bioproducts and Biopharma segments to Lonza for total cash consideration of
$463,914, including working capital adjustments. As a result of this
transaction, these businesses are being reported as discontinued operations in
all periods presented.
_____________
(dollars
in thousands, except share data)
In January 2008, the Company acquired
AS ProSyntest, a privately held API research and development company located in
Tallinn, Estonia. ProSyntest, renamed Cambrex Tallinn, has strengths
in cost effective chemical route selection and sample generation, rapid scale up
of products at kilo lab scale, as well as chiral and organometallic
chemistries.
Products
The Company uses its technical
expertise in a wide range of chemical processes to meet the needs of its
customers for high quality products and services for specialized applications.
The Company’s business is primarily
comprised of the custom development and manufacture of pharmaceutical
ingredients derived from organic chemistry. Products and services are
supplied globally to innovative and generic drug companies. Products
include APIs and advanced pharmaceutical intermediates. Services
include custom development and current Good Manufacturing Practices (“cGMP”)
manufacturing services.
Products and services are sold to a
diverse group of several hundred customers, with one customer, Gyma Laboratories of
America, Inc. (“Gyma”), a distributor representing multiple customers,
accounting for 11.5% of 2009 sales. One product, a gastro-intestinal
API sold to multiple customers, accounted for 12.7% of 2009 sales. No
one customer accounted for more than 10% of 2009 sales of this
product.
This
table summarizes gross sales by product groups:
|
2009
|
2008
|
2007
|
||||||||||
|
APIs
and pharmaceutical intermediates
|
$ | 212,644 | $ | 220,722 | $ | 220,386 | ||||||
|
Other
|
23,633 | 28,896 | 32,188 | |||||||||
|
Total
|
$ | 236,277 | $ | 249,618 | $ | 252,574 | ||||||
The following table shows gross sales
to geographic area for the years ended December 31, 2009, 2008 and
2007:
|
2009
|
2008
|
2007
|
||||||||||
|
North
America
|
$ | 80,830 | $ | 86,631 | $ | 85,644 | ||||||
|
Europe
|
136,534 | 143,542 | 150,692 | |||||||||
|
Asia
|
10,495 | 11,440 | 9,125 | |||||||||
|
Other
|
8,418 | 8,005 | 7,113 | |||||||||
|
Total
|
$ | 236,277 | $ | 249,618 | $ | 252,574 | ||||||
Marketing
and Distribution
The Company's products generally
include higher value, low-to-medium volume niche products requiring significant
technical expertise to develop and manufacture. Marketing generally
requires significant cooperative effort among a highly trained sales and
marketing staff, a scientific staff that can assess the technical fit and
estimate manufacturing economics, manufacturing and engineering staff to scale
up the chemical process and business unit management to determine the strategic
and operational fit. The process to take a client's project from the
clinical trial stage to a commercial, approved therapeutic may take from two to
ten years. The Company uses sales agents and independent distributors
in those areas where they are deemed to be more effective or economical than
direct sales efforts.
_____________
(dollars
in thousands, except share data)
Raw
Materials
The
Company uses a wide array of raw materials in its businesses. For its
products, the Company generally will attempt to have a primary and secondary
supplier for its critical raw materials. Prices for these raw
materials are generally stable except for the petroleum-based solvents where
prices can vary with market conditions.
Research
and Development
The Company's R&D program is
designed to increase the Company's competitiveness by improving its technology
and developing processes for the manufacture of new products to meet customer
requirements. The goals are to introduce innovative and proprietary
products, improve manufacturing processes to reduce costs, improve quality and
increase capacity, to identify market opportunities that warrant significant
technical expertise, and offer the prospects of a long-term, profitable business
relationship. R&D activities are performed at all of the
Company's manufacturing facilities in both the United States and
Europe. Approximately 120 employees are at least partially involved
in R&D activities worldwide.
In December 2007 the Company
consolidated its United States R&D activities and small scale API production
into its facility in Charles City, Iowa. As a result of the
consolidation, the New Jersey R&D facility was closed as of December 31,
2008.
The Company spent $7,929, $7,590 and
$12,157 in 2009, 2008 and 2007, respectively, on R&D efforts.
Patents
and Trademarks
The Company has patent protection
covering certain products, processes and services. In addition, the
Company also relies on know-how and trade secrets (related to many of its
manufacturing processes and techniques not generally known to other companies)
for developing and maintaining its market position. The Company
currently owns 12 issued patents and has 8 patent applications pending in
the United States and owns 26 patents and has 14 patent applications pending in
foreign countries covering various technologies. The Company seeks to
protect its proprietary technology and prepares new patent applications as
decisions are made to patent new inventions.
The patent rights the Company considers
most significant to its business are the following: (i) U.S. Patent Nos.
6,828,336 and 6,586,449 and 26 foreign counterparts are part of its APIs and
pharmaceutical intermediates product group, relate to its nicotine polacrilex
resin products and methods of manufacturing and expire on May 28, 2022; (ii)
U.S. Patent Nos. 7,172,885, 7,247,460, 7,264,952, 7,267,969, 7,276,360, and
7,319,027, are part of its APIs and pharmaceutical intermediates product
group, relate to thermostable omega-transaminases and expire on
December 12, 2024; and (iii) U.S. Patent No. 6,025,516 is part of its APIs and
pharmaceutical intermediates product group, relates to a method of
synthesizing the 13-position sidechain of the drug paclitaxel and its analogs
and expires on October 14, 2018.
The Company's products and services are
sold around the world under trademarks that are owned by the
Company. These include PROFARMACO, which is registered around the
world as a word and design mark, and CAMOUFLAGE, which has been registered in
Europe and is the subject of a United States trademark
application. Rights in these trademarks will exist at least as long
as the Company continues to use each of these trademarks.
The Company has entered into a license
agreement which gives the Company the exclusive rights to certain intellectual
property, including know-how and technology, relating to the development and
manufacture of chirally pure bulk actives. The Company has also
entered into a license agreement for the worldwide exclusive right to
manufacture and sell a product that is part of its APIs and pharmaceutical
intermediates product group.
_____________
(dollars
in thousands, except share data)
Competition
The Company has at least 25 primary API
and advanced intermediate competitors throughout Western Europe and the U.S. and
many more competitors within various segments of the markets the Company serves,
including a growing number of competitors in Asia, Eastern Europe and other
low-cost areas. The Company believes that low cost providers have had
the impact of driving prices down for many products and services for which the
Company competes to provide, and the Company anticipates that it will face
increased competition from these providers in the future. It is
expected that regulatory compliance, product quality and logistics will
determine the extent of the long term impact of these competitors in the primary
markets that the Company serves. If the Company perceives significant
competitive risk and a need for technical or financial commitment, it generally
attempts to negotiate long term contracts or guarantees from its
customers.
Environmental
and Safety Regulations and Proceedings
General: Certain
products manufactured by the Company involve the use, storage and transportation
of toxic and hazardous materials. The Company's operations are
subject to extensive laws and regulations relating to the storage, handling,
emission, transportation and discharge of materials into the environment and the
maintenance of safe working conditions. The Company maintains
environmental and industrial safety, health compliance programs and training at
its plants and believes that its manufacturing operations are in compliance with
all applicable safety, health and environmental laws.
Prevailing legislation tends to hold
companies primarily responsible for the proper disposal of their wastes even
after transfer to third party waste disposal facilities. Moreover,
other future developments, such as increasingly strict environmental, safety and
health laws and regulations, and enforcement policies there under, could result
in substantial costs and liabilities to the Company and could subject the
Company's handling, manufacture, use, reuse, or disposal of substances or
pollutants at its plants to more rigorous scrutiny than at present.
Known environmental matters which may
result in liabilities to the Company and the related estimates and accruals are
summarized in Note 18.
Present and Future Environmental
Expenditures: The Company’s policy is to comply with all legal
requirements of applicable environmental, health and safety laws and
regulations. The Company believes it is in compliance with such
requirements and has adequate professional staff and systems in place to remain
in compliance. In some cases, compliance can only be achieved by
capital expenditures and the Company made capital expenditures of $2,211, $1,760
and $2,060 in 2009, 2008 and 2007, respectively, for environmental
projects. As the environmental proceedings in which the Company is
involved progress from the remedial investigation and feasibility study stage to
implementation of remedial measures, related expenditures may
increase. The Company considers costs for environmental compliance to
be a normal cost of doing business and includes such costs in pricing
decisions.
Employees
At December 31, 2009, the Company had
854 employees worldwide (628 of whom were from international operations)
compared with 856 employees at December 31, 2008 and 844 at December 31,
2007.
Non-U.S. production, administration,
scientific and technical employees are represented by various local and national
unions. The Company believes its labor relations are
satisfactory.
Seasonality
The Company experiences some
seasonality primarily due to planned plant shutdowns by the Company and certain
customers in the third quarter. Operating results for any quarter,
however, are not necessarily indicative of results for any future
period. In particular, as a result of various factors including, but
not limited to, acquisitions, plant shutdowns, and the timing of large contract
revenue streams, the Company believes that period-to-period comparisons of its
operating results should not be relied upon as an indication of future performance.
_____________
(dollars
in thousands, except share data)
Export
and International Sales
The Company exports numerous products
to various areas, principally Western Europe, Asia and Canada. Export
sales from the Company’s domestic operations in 2009, 2008 and 2007 amounted to
$25,768, $24,602 and $28,821, respectively. Sales from international
operations were $151,759, $167,911, and $171,145 in 2009, 2008 and 2007,
respectively. Refer to Note 16.
|
Item 1A.
|
Risk
Factors
|
Factors That May Affect Future
Results
The following risk factors and other
information included in this Annual Report on Form 10-K should be carefully
considered. If any of the following risks occur, the Company’s
business, financial condition, operating results and cash flows could be
materially adversely affected. The risks and uncertainties described
below are not the only ones the Company faces. Additionally, risks
and uncertainties not presently known to the Company or that it currently deems
immaterial also may impair its business, financial condition, operating results
and cash flows in the future.
Risks Relating to Cambrex’s
Business
Companies
may discontinue or decrease their usage of Cambrex’s services.
The Company has observed increasing
pressure on the part of its customers to reduce spending, including the use of
its services and products, as a result of negative macro-economic trends and
various market dynamics specifically affecting the pharmaceutical
industry. These customers could discontinue or decrease their usage
of Cambrex’s services and products, including as a result of the global economic
slowdown.
New technologies, competition or a
reduction in demand for Cambrex’s products could reduce sales.
The
markets for the Company’s products are competitive and price
sensitive. The Company’s competitors may lower prices on products in
the future and the Company may, in certain cases, respond by lowering its
prices. Conversely, failure to anticipate and respond to price
competition may hurt Cambrex’s market share. Some of the Company’s competitors
also have significant financial, operational, sales and marketing resources, and
experience in R&D which may reduce the Company’s level of
business. Companies may develop new technologies that would compete
with the Company’s products or render its products obsolete. Several
of Cambrex’s customers, especially those that buy its generic APIs, have
internal capabilities similar to Cambrex’s. In addition, demand for
the Company’s products may weaken due to a reduction in R&D budgets, loss of
distributors or other factors.
The
Company believes that customers in its markets display loyalty to their initial
supplier of a particular product. Therefore, it may be difficult to
generate sales to potential customers who have purchased products from
competitors. To the extent the Company is unable to be the first to
develop and supply new products, its competitive position may
suffer.
The
Company’s failure to obtain new contracts or renew existing contracts may
adversely affect its business.
Many of
Cambrex’s contracts are short-term in duration. As a result, the Company
must continually replace its contracts with new contracts to sustain its
revenue. In addition, certain of the Company’s long-term contracts may be
cancelled or delayed by clients for any reason upon notice. Multiple
cancellations or non-renewals of significant contracts could materially impact
the Company’s business.
_____________
(dollars
in thousands, except share data)
Failure
to obtain products and raw materials from third-party manufacturers could affect
Cambrex’s ability to manufacture and deliver its products.
The
Company relies on third-party manufacturers to supply many of its raw materials
and intermediates. In addition, the Company has a single source for
supplies of some raw materials to its products. Manufacturing
problems may occur with these and other outside sources. If such
problems occur, the Company cannot ensure that it will be able to manufacture
its products profitably or on time.
Disruptions
to the Company’s manufacturing operations could adversely affect its
results.
Due to
heavy reliance on manufacturing and related operations to produce and distribute
the products the Company sells, the Company could be adversely affected by
disruptions of these operations. Any significant disruption of those
operations for any reason, such as labor unrest, power interruptions, fire, or
other events beyond the Company’s control could adversely affect its sales and
customer relationships and therefore adversely affect its business. While
insurance coverage may reimburse the Company, in part, for profits lost from
such disruptions, any sustained reduction in the Company’s ability to provide
these products would negatively impact its sales growth expectations, cash flows
and profitability.
Failure
to win early stage business opportunities can cause difficulty in winning future
opportunities with that customer.
Certain
products the Company sells are incorporated into its customers’ drug
manufacturing processes. In some cases, once a customer chooses a particular
product for use in a drug manufacturing process, it is unlikely that the
customer will later switch to a competing alternative. In many cases, the
regulatory approvals related to a drug product will specify the products
qualified for use in its making. Obtaining the regulatory approvals needed for a
change in the manufacturing process is time consuming, expensive and uncertain.
Accordingly, if a customer does not select the Company’s products or services
early in its manufacturing design phase for any number of reasons, the Company
may lose the opportunity to participate in the customer’s manufacturing of such
product. Because the Company faces competition in this market from other
companies, it is at risk that its competitors could win significant early
business with customers making it difficult for the Company to recover
late-stage opportunities with higher volumes.
Litigation
may harm the Company or otherwise negatively impact its management and financial
resources.
Complex
or extended litigation could cause the Company to incur large expenditures and
distract its management. For example, lawsuits by employees,
stockholders, counterparties to acquisition and divestiture contracts,
collaborators, distributors, customers, or end-users of the Company’s products
or services could be very costly and substantially disrupt its
business. Disputes from time to time with such companies or
individuals are not uncommon, and the Company cannot be assured that it will
always be able to resolve such disputes out of court or on terms favorable to
the Company.
Refer to
Note 18 for a discussion of the Company’s environmental and legal
matters.
Incidents
related to hazardous materials could adversely affect the Company.
Portions
of the Company’s operations require the controlled use of hazardous
materials. Although the Company is diligent in designing and
implementing safety procedures to comply with the standards prescribed by
federal, state, local and foreign regulations, including the European
Commission’s Registration, Evaluation and Authorization of Chemicals (“REACH”)
regulation, the risk of accidental contamination of property or injury to
individuals from these materials cannot be completely eliminated. In
the event of such an incident, the Company could be liable for any damages which
could adversely affect its business. Additionally, any incident could
shut down the Company’s research and manufacturing facilities and
operations.
_____________
(dollars
in thousands, except share data)
The
Company generates waste that must be transported to approved storage, treatment
and disposal facilities. The transportation and disposal of such
waste are required to meet applicable state and federal statutes and
regulations. The storage, treatment and disposal of such waste
potentially exposes the Company to environmental liability if, in the future,
such transportation and disposal are deemed to have violated such statues or
regulations or if the storage, treatment and disposal facilities are inadequate
and are proved to have damaged the environment.
The
Company is also party to several environmental remediation investigations and
cleanups and, along with other companies, has been named a potentially
responsible party (“PRP”) for certain waste disposal sites.
Refer to
Note 18 for a discussion of the Company’s environmental and legal
matters.
Potential
product liability claims, errors and omissions claims in connection with
services the Company performs and potential liability under indemnification
agreements between the Company and its officers and directors could adversely
affect the Company.
The
Company manufactures products intended for use by the public. These
activities could expose the Company to risk of liability for personal injury or
death to persons using such products, even though the Company does not presently
market or sell the products to end users. The Company seeks to reduce
its potential liability through measures such as contractual indemnification
provisions with customers (the scope of which may vary from
customer-to-customer, and the performances of which are not secured), exclusion
of services requiring diagnostic or other medical services, and insurance
maintained by customers. The Company could be materially and
adversely affected if it were required to pay damages or incur defense costs in
connection with a claim that is outside the scope of the indemnification
agreements, if the indemnity, although applicable, is not performed in
accordance with its terms or if the Company’s liability exceeds the amount of
applicable insurance or indemnity. In addition, the Company could be
held liable for errors and omissions in connection with the services it
performs. The Company currently maintains product liability and
errors and omissions insurance with respect to these risks. There can
be no assurance, however, that the Company’s insurance coverage will be adequate
or that insurance coverage will continue to be available on terms acceptable to
the Company.
The
Company also indemnifies its officers and directors for certain events or
occurrences while the officer or director is, or was, serving at the Company’s
request in such capacity. The maximum potential amount of future
payments the Company could be required to make under these indemnification
agreements is unlimited; however, the Company has a Director and Officer
insurance policy that covers a portion of any potential exposure. The
Company could be materially and adversely affected if it were required to pay
damages or incur legal costs in connection with a claim above its insurance
limits.
While
the Company has what it believes to be adequate insurance coverage, any claims
beyond its insurance coverage may result in substantial costs and a reduction in
its available capital resources.
The
Company maintains property insurance policies covering physical damage to its
equipment, facilities, buildings and inventory; employer’s liability insurance
generally covering death or work injury of employees; product liability
insurance covering product liability claims arising from the use, consumption or
operation of its products; public liability insurance covering certain incidents
to third parties that occur on or in the premises of the company; business
interruption insurance and directors and officers liability insurance, among
others. The Company does not maintain key man life insurance on any
of its senior management or key personnel. The Company’s insurance
coverage, however, may not be sufficient to cover any claim for product
liability, damage to its fixed assets or injury to its employees.
Loss
of key personnel could hurt the Company.
The
Company depends on its ability to attract and retain qualified scientific and
technical employees as well as a number of key executives. There can
be no assurance the Company will be able to retain key personnel, or to attract
and retain additional qualified employees. The Company’s inability to
attract and retain key personnel would have a material adverse effect on the
Company’s business.
_____________
(dollars
in thousands, except share data)
The
Company has made significant capital investments to its facilities to meet its
potential future needs and, as a result, the Company depends on the success of
attracting new and retaining existing customers’ projects and their continued
business.
The
Company has recently made substantial investments in all of its manufacturing
facilities. With the completion of these new facilities, the
Company’s fixed costs have increased. If the Company is not able to
utilize the facilities to capacity, its margins could be adversely
affected.
Global
growth is subject to a number of economic risks.
The
current global economy affects businesses such as Cambrex’s in a number of
ways. The current equity market and tightening of credit in financial
markets adversely affects the ability of the Company’s customers to obtain
financing for significant purchases and operations and could result in a
decrease in or cancellation of orders for its products and services as well as
impact the ability of the Company’s customers to make payments. The Company
believes that cash flows from operations, along with funds available from a
revolving line of credit, will be adequate to meet the operational and debt
servicing needs of the Company, but no assurances can be given that this will
continue to be the case. Given the current state of the worldwide
credit markets, there is a risk that the funds available to be drawn under the
Company’s revolving line of credit may not be available in the event of the
failure of one or more participant banks. Strengthening of the rate
of exchange for the U.S. dollar against certain major currencies such as
the Euro, Swedish krona and other currencies also adversely affects the
Company’s results.
The
Company has a significant amount of debt.
The
Company has a $200,000 revolving credit facility of which $120,800 was
outstanding at December 31, 2009. This facility expires in April of
2012. If the Company is unable to generate sufficient cash flow or
otherwise obtain funds necessary to make required payments on the credit
facility, it will be in default. This current debt arrangement
requires the Company to comply with specified financial ratios. The Company’s
ability to comply with these ratios may be affected by events beyond its
control.
Even if the Company is able to meet its
debt service obligations, the amount of debt it has could adversely affect the
Company by limiting its ability to obtain any necessary financing in the future
for working capital, capital expenditures, debt service requirements, or other
purposes. It also places the Company at a disadvantage relative to
its competitors who have lower levels of debt, while making it more vulnerable
to a downturn in its business or the economy in general. It also
requires the Company to use a substantial portion of its cash to pay principal
and interest on its debt, instead of investing those funds in the
business.
The
Company’s liquidity, business, financial condition, results of operations and
cash flows could be materially and adversely affected if the financial
institutions which hold its funds fail.
The
Company has significant funds held in bank deposits, money market funds and
other accounts at certain financial institutions. A significant
portion of the funds held in these accounts exceed insurable
limits. If any of the financial institutions where the Company has
deposited funds were to fail, the Company may lose some or all of its deposited
funds that exceed the insurance coverage limit. Such a loss would have a
material and adverse effect on the Company’s liquidity, business, financial
condition, results of operations and cash flows.
A
payment failure by any large customer or multiple smaller customers could
adversely affect the Company’s cash flows and profitability.
Historically,
the Company has not experienced any significant bad debt or collection problems,
but such problems may arise in the future. The failure of any of the Company’s
customers to make timely payments could require the Company to write off
accounts receivable or increase provisions made against its accounts receivable,
either of which could adversely affect the Company’s cash flows and
profitability.
_____________
(dollars
in thousands, except share data)
The
Company has significant inventories on hand.
The
Company maintains significant inventories and has an allowance for slow-moving
and obsolete inventory. Any significant unanticipated changes in future product
demand or market conditions, including the current uncertainty in the global
market, could also have an impact on the value of inventory and adversely impact
the Company’s results of operations.
International
unrest or foreign currency fluctuations could adversely affect the Company’s
results.
The
Company’s international revenues, which include revenues from its non-U.S.
subsidiaries and export sales from the U.S., represent the majority of its
product revenues.
There are
a number of risks arising from the Company’s international business,
including:
|
|
·
|
the
possibility that unfriendly nations or groups could boycott its
products;
|
|
|
·
|
general
economic and political conditions in the markets in which it
operates;
|
|
|
·
|
potential
increased costs associated with overlapping tax
structures;
|
|
|
·
|
more
limited protection for intellectual property rights in some
countries;
|
|
|
·
|
unexpected
changes in regulatory requirements;
|
|
|
·
|
the
difficulties of compliance with a wide variety of foreign laws and
regulations;
|
|
|
·
|
longer
accounts receivable cycles in certain foreign countries;
and
|
|
|
·
|
import
and export licensing requirements.
|
In
addition, a significant portion of the Company’s business is conducted in
currencies other than the U.S. dollar, which is its reporting
currency. The Company recognizes foreign currency gains or losses
arising from its operations in the period incurred. As a result,
currency fluctuations between the U.S. dollar and the currencies in which the
Company does business have caused, and will continue to cause, foreign currency
transaction gains and losses. The Company cannot predict the effects
of exchange rate fluctuations upon its future operating results because of the
number of currencies involved, the variability of currency exposures, and the
potential volatility of currency exchange rates. The Company engages
in limited foreign exchange hedging transactions to mitigate the impact of this
volatility on its operations, but its strategies are short-term in nature and
may not adequately protect its operating results from the full effects of
exchange rate fluctuations.
Cambrex’s
operating results may unexpectedly fluctuate in future periods.
The
Company’s revenue and operating results have fluctuated, and could continue to
fluctuate, on a quarterly basis. The operating results for a
particular quarter may be lower than expected as a result of a number of
factors, including, but not limited to, the timing of contracts; the delay or
cancellation of a contract; the mix of services provided; seasonal slowdowns in
different parts of the world; the timing of start-up expenses for new services
and facilities; changes in government regulations; and unfavorable exchange
rates with the U.S. dollar. Because a high percentage of the
Company’s costs are relatively fixed in the short term, such as the cost of
maintaining facilities and compensating employees, any one of these factors
could have a significant impact on the Company’s quarterly
results. In some quarters, the Company’s revenue and operating
results may fall below the expectations of securities analysts and investors due
to any of the factors described above. If such event occurred, sales
of common stock by existing holders would cause the trading price of the
Company’s common stock to decline, even if the decline in revenue did not have
any long-term adverse implications for the Company’s business.
_____________
(dollars
in thousands, except share data)
The
possibility the Company will be unable to protect its technologies could affect
its ability to compete.
The
Company’s success depends to a significant degree upon its ability to develop
proprietary products and technologies. However, the Company cannot be
assured that patents will be granted on any of its patent
applications. The Company also cannot be assured that the scope of
any of its issued patents will be sufficiently broad to offer meaningful
protection. The Company has patents issued in selected countries,
therefore, third parties can make, use, and sell products covered by its patents
in any country in which the Company does not have patent
protection. In addition, issued patents or patents the Company
licenses could be successfully challenged, invalidated or circumvented so that
its patent rights would not create an effective competitive
barrier. The Company provides its customers the right to use its
products under label licenses that are for research purposes
only. These licenses could be contested, and the Company cannot be
assured that it would either be aware of an unauthorized use or be able to
enforce the restrictions in a cost-effective manner.
If a
third party claimed an intellectual property right to technology the Company
uses, it may need to discontinue an important product or product line, alter its
products and processes, defend its right to use such technology in court or pay
license fees. Although the Company may, under these circumstances,
attempt to obtain a license to such intellectual property, it may not be able to
do so on favorable terms, or at all. Additionally, if Cambrex’s
products are found to infringe on a third party’s intellectual property, the
Company may be required to pay damages for past infringement, and lose the
ability to sell certain products or receive licensing revenues.
The
Company could be subject to goodwill impairment charges in the
future.
Under
U.S. GAAP, the Company is required to evaluate goodwill for impairment at least
annually. If the Company determines that the fair value is less than
the carrying value, an impairment loss will be recorded in the Company’s
statement of operations. The determination of fair value is a highly
subjective exercise and can produce significantly different results based on the
assumptions used and methodologies employed. If the Company’s
projected long-term sales growth rate, profit margins or terminal rate are
considerably lower and/or the assumed weighted average cost of capital is
considerably higher, future testing may indicate impairment and the Company
would have to record a non-cash goodwill impairment loss in its statement of
operations.
Assessments
by various tax authorities may be materially different than the Company has
provided for and it may experience significant volatility in its annual and
quarterly effective tax rate.
As a
matter of course, the Company is regularly audited by federal, state, and
foreign tax authorities. From time to time, these audits result in
proposed assessments. In recent years, the Company utilized
significant tax attributes in the form of foreign tax credits and U.S. net
operating loss (“NOL”) carryforwards to reduce or eliminate potential tax
expense related to the repatriation of funds into the U.S. resulting from the
sale of the businesses that comprised the Bioproducts and Biopharma segments in
2007. While the Company believes that it has adequately provided for
any taxes related to these items, and taxes related to all other aspects of its
business, any such assessments or future settlements may be materially different
than it has provided.
The
Company may pursue transactions that may cause it to experience significant
charges to earnings that may adversely affect its stock price and financial
condition.
The Company regularly reviews potential
transactions related to technologies, products, product rights and businesses
complementary to its business. These transactions could include
mergers, acquisitions, divestitures, strategic alliances or licensing
agreements. In the future, the Company may choose to enter into these
transactions at any time. As a result of acquiring businesses or
entering into other significant transactions, the Company may experience
significant charges to earnings for merger and related expenses. If
the Company is not able to successfully integrate the acquired business to
create the advantages the acquisition was intended to create, it may affect the
Company’s results of operations and the market price of its common
stock. Furthermore, if the Company is unable to improve the operating
margins of acquired businesses or operate them profitably, it may be unable to
achieve its growth strategy.
_____________
(dollars
in thousands, except share data)
Risks Related to Cambrex’s
Industry
Any
significant change in government regulation of the drug development process
could have a material adverse effect on the Company.
The
manufacturing of pharmaceutical products is subject to extensive regulation by
governmental authorities, including the FDA and comparable regulatory
authorities in other countries. The Company’s business, as well as
its customer’s business depends in part on strict government regulation of the
drug development process. Legislation may be introduced and enacted
from time to time to modify regulations administered by the FDA and governing
the drug approval process. Any significant reduction in the scope of
regulatory requirements or the introduction of simplified drug approval
procedures could have a material adverse effect on the Company’s
business.
Violations of cGMP and other government
regulations could have a material adverse effect on the Company.
All
facilities and manufacturing techniques used for manufacturing products for
clinical use or for commercial sale in the United States must be operated in
conformity with cGMP regulations as required by the FDA and other comparable
regulatory authorities in other countries and for certain products, the Drug
Enforcement Agency. The Company’s facilities are subject to scheduled
periodic regulatory and customer inspections to ensure compliance with cGMP and
other requirements applicable to such products. A finding that the
Company had materially violated these requirements could result in regulatory
sanctions including, but not limited to, the FDA withholding approval of new
drug applications or supplements and the denial of entry into the U.S. of
products manufactured at non-compliant foreign facilities, the loss of a
customer contract, the disqualification of data for client submissions to
regulatory authorities and a mandated closing of the Company’s
facilities. Any such violations would have a material adverse effect
on the Company’s business. Cambrex’s customers are typically subject
to the same, or similar, regulations and any such violations or other actions by
regulatory agencies, including, but not limited to, plant shutdowns or product
recalls that eliminate or reduce the Company’s sale of its products or services
could negatively impact the Company’s business.
The
outsourcing trend in the preclinical and clinical stages of drug research and
development may decrease, which could slow the Company’s growth.
The
success of the Company’s business depends to a certain extent on the number of
contracts and the size of the contracts that it may obtain from pharmaceutical
companies. Over the past several years, the Company has benefited from increased
levels of outsourcing by pharmaceutical companies of their drug R&D
activities. A slowing of the outsourcing trend could result in a diminished
growth rate in the Company’s sales and adversely affect its business, financial
condition and results of operations.
Available
Information
This annual report on Form 10-K, the
Company’s quarterly reports on Form 10-Q, the Company’s current reports on Form
8-K, and amendments to those reports filed or furnished pursuant to Section
13(a) or 15(d) of the Securities Exchange Act of 1934, are made available free
of charge on the Company’s Internet website www.cambrex.com as
soon as reasonably practicable after such material is electronically filed with
or furnished to the SEC. The most recent certifications by the
Company’s Chief Executive Officer and Chief Financial Officer pursuant to
Section 302 of the Sarbanes-Oxley Act of 2002 are filed as exhibits to this
Annual report on Form 10-K. Last year the Company filed with the New York
Stock Exchange the Annual Chief Executive Officer Certification as required by
Section 303A.12.(a) of the New York Stock Exchange Listed Company
Manual.
Reports filed by the Company with the
SEC may be read and copied at the SEC’s Public Reference Room at 100 F Street,
NE, Washington, DC 20549. Information on the operation of the Public
Reference Room may be obtained by calling the SEC at
1-800-SEC-0330. The SEC also maintains an Internet site at www.sec.gov that
contains reports, proxy and information statements and other information
regarding issuers that file electronically with the SEC.
_____________
(dollars
in thousands, except share data)
The following corporate governance
documents are available free of charge on the Company’s website: the
charters of its Audit, Regulatory Affairs, Compensation and Governance
Committees, its Corporate Governance Guidelines and its Code of Business Conduct
and Ethics. These corporate governance documents are also available
in print to any stockholder requesting a copy from its corporate secretary at
its principal executive offices. Information contained on its website
is not part of this report. The Company will also post on its website
any amendments to or waivers of its Code of Business Conduct and Ethics that
relate to its Chief Executive Officer, Chief Financial Officer and Principal
Accounting Officer.
|
Item
1B
|
Unresolved
Staff Comments
|
None.
|
Item
2
|
Properties.
|
Set forth below is information relating
to manufacturing facilities owned by the Company as of December 31,
2009:
|
Operating
|
||||||
|
Location
|
Acreage
|
Subsidiary
|
Product Lines
Manufactured
|
|||
|
Charles
City, Iowa
|
57
acres
|
Cambrex
|
APIs,
Pharmaceutical Intermediates, Imaging
|
|||
|
Charles
City, Inc.
|
Chemicals,
Animal Health Products and Fine
|
|||||
|
Custom
Chemicals
|
||||||
|
Karlskoga,
Sweden
|
42
acres
|
Cambrex
|
APIs,
Pharmaceutical Intermediates,
|
|||
|
Karlskoga
AB
|
Imaging
Chemicals and Fine Custom Chemicals
|
|||||
|
Paullo
(Milan), Italy
|
13
acres
|
Cambrex
|
APIs
and Pharmaceutical Intermediates
|
|||
|
Profarmaco
Milano S.r.l.
|
The Company leases 10,000 square feet
in Tallinn, Estonia which has a lease term ending May 2014. In
addition, the Company owns a six acre site and buildings in North Haven,
Connecticut, and a three acre site and buildings in Carlstadt, New
Jersey. The Company believes its operating facilities to be in good
condition, well-maintained and adequate for its current needs.
In December 2007 the Company
consolidated its United States R&D activities and small scale API production
into its facility in Charles City, Iowa. As a result of the
consolidation, the Company’s New Jersey R&D facility was closed as of
December 31, 2008. The lease will continue through December
2010.
Most of the Company's products and
services are provided from multi-purpose facilities. Each product has
a unique requirement for equipment, and occupies such equipment for varying
amounts of time. It is generally possible, with proper lead time and
customer and regulatory approval (if required), to transfer the manufacturing of
a particular product to another facility should capacity constraints
dictate.
|
Item
3
|
Legal
Proceedings
|
See "Environmental and Safety
Regulations and Proceedings" under Item 1 and Note 18 with respect to various
proceedings involving the Company in connection with environmental
matters. The Company is party to a number of other proceedings also
discussed in Note 18.
|
Item 4
|
Submission of Matters to a
Vote of Security Holders
|
None
_____________
(dollars
in thousands, except share data)
PART II
|
Item
5
|
Market for the Registrant's
Common Equity, Related Stockholder Matters and Issuer Purchases of Equity
Securities
|
The Company’s common stock, $.10 par
value is listed on the New York Stock Exchange (“NYSE”) under the symbol
CBM. The following table sets forth the closing high and low sales
price of the common stock as reported on the NYSE:
|
2009
|
High
|
Low
|
||||||
|
First
Quarter
|
$ | 5.24 | $ | 1.50 | ||||
|
Second
Quarter
|
4.48 | 2.27 | ||||||
|
Third
Quarter
|
6.51 | 3.89 | ||||||
|
Fourth
Quarter
|
7.17 | 5.17 | ||||||
|
2008
|
High
|
Low
|
||||||
|
First
Quarter
|
$ | 10.96 | $ | 6.93 | ||||
|
Second
Quarter
|
7.28 | 5.51 | ||||||
|
Third
Quarter
|
7.97 | 5.45 | ||||||
|
Fourth
Quarter
|
6.14 | 2.45 | ||||||
As of January 29, 2010, the Company
estimates that there were approximately 1,435 beneficial holders of the
outstanding common stock of the Company.
2009
Equity Compensation Table
The following table provides
information as of December 31, 2009 with respect to shares of common stock that
may be issued under the Company’s existing equity compensation
plans.
|
Column (a)
|
Column (b)
|
Column (c)
|
||||||||||
|
Plan category
|
Number of securities to be issued upon exercise of
outstanding options, warrants and rights
|
Weighted average exercise price of outstanding
options, warrants and rights
|
Number of securities remaining for future issuance
under equity compensation plans (excluding securities reflected in column
(a))
|
|||||||||
|
Equity
compensation plans approved by security holders
|
1,756,699 | $ | 11.16 | 430,880 | ||||||||
|
Equity
compensation plans not approved by security holders
|
263,670 | $ | 12.02 | 17,150 | ||||||||
|
Total
|
2,020,369 | $ | 11.27 | 448,030 | ||||||||
The
material features of the equity compensation plan under which equity securities
are authorized for issuance that was adopted without stockholder approval are
described below:
2000
Employee Performance Stock Option Plan
The 2000
Employee Stock Option Plan (the “2000 Plan”) is used to fund awards for
Non-Executive Employees of the Company. The 2000 Plan is administered by
the Compensation Committee of the Board of Directors, and that Committee may
delegate responsibilities to others to assist in administering the 2000
Plan. The total number of shares of Common Stock which may be issued on
exercise of stock options shall not exceed 500,000 shares, subject to adjustment
in accordance with the Plan. No participant shall be granted options to
purchase more than 100,000 shares of common stock in any twelve month
period. The options shall be priced at fair market value on the date of
grant and shall expire up to 10 years after the date of grant. If the
employment of a participant terminates, other than as a result of death,
disability or retirement, all unexercised awards shall be cancelled
immediately. In the event of death, disability or retirement, the options
will expire one year from the date of the event.
_____________
(dollars
in thousands, except share data)
Comparison
of Five-Year Cumulative Total Returns
The following graph compares the
Company’s cumulative total stockholder return for a five-year period, with a
performance indicator of the overall stock market, the S&P 500 Index and the
S&P 1500 Pharmaceuticals Index which the Company believes more closely
reflects the industry within which the Company operates. Prices are
as of December 31 of the year indicated.
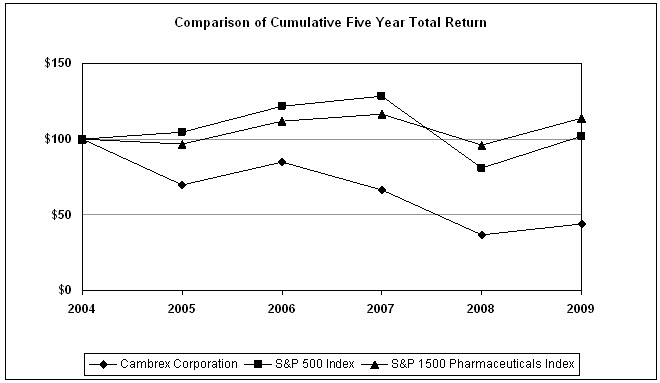
The Company’s commercial activities are
focused on manufacturing and marketing to customers concentrated in the Life
Sciences Industry (including pharmaceutical chemicals and
intermediates). Although the Company’s products are diverse, making
it difficult to select a comparative peer group, the Company believes that the
S&P 1500 Pharmaceuticals Index is a reasonable, publicly available
comparison group for the commercial activities on which it currently
focuses. The S&P 1500 Pharmaceuticals Index is comprised of 18
pharmaceutical companies within the S&P 1500 Composite Index as of December
31, 2009.
_____________
(dollars
in thousands, except share data)
|
Item 6
|
Selected Financial
Data
|
The following selected consolidated
financial data of the Company for each of the years in the five year period
ended December 31, 2009 are derived from the audited financial statements for
2009, 2008, 2007 and 2006 and the books and records of the Company for 2005,
respectively, including all adjustments necessary for discontinued operations
presentation. The consolidated financial statements of the Company as
of December 31, 2009 and 2008 and for each of the years in the three year period
ended December 31, 2009 and the reports of independent registered public
accounting firm thereon are included elsewhere in this annual
report. In October 2006, the Company sold two businesses within the
former Human Health segment and in February 2007 the Company completed the sale
of the businesses that comprised the Bioproducts and Biopharma segments
(excluding certain liabilities). See Note 19. As a result,
these businesses are being reported as discontinued operations for all periods
presented. The data presented below should be read in conjunction
with the financial statements of the Company and the notes thereto and
"Management's Discussion and Analysis of Financial Condition and Results of
Operations" included elsewhere herein.
|
Years Ended December 31,
|
||||||||||||||||||||
|
2009(1)
|
2008(2)
|
2007(3)
|
2006(4)
|
2005(5)
|
||||||||||||||||
|
INCOME
DATA:
|
||||||||||||||||||||
|
Gross
sales
|
$ | 236,277 | $ | 249,618 | $ | 252,574 | $ | 236,659 | $ | 223,565 | ||||||||||
|
Net
revenues
|
234,550 | 249,228 | 252,505 | 235,073 | 224,213 | |||||||||||||||
|
Gross
profit
|
70,278 | 73,743 | 91,232 | 83,858 | 86,911 | |||||||||||||||
|
Selling,
general and administrative expenses
|
35,711 | 40,521 | 48,858 | 58,279 | 56,109 | |||||||||||||||
|
Research
and development expenses
|
7,929 | 7,590 | 12,157 | 10,813 | 11,946 | |||||||||||||||
|
Restructuring
expenses
|
- | 4,695 | 6,073 | - | - | |||||||||||||||
|
Strategic
alternative costs
|
- | 1,515 | 31,127 | 2,958 | - | |||||||||||||||
|
Operating
profit/(loss)
|
26,638 | 19,422 | (6,983 | ) | 11,808 | 18,856 | ||||||||||||||
|
Interest
expense/(income), net
|
4,634 | 3,668 | (485 | ) | 5,478 | 3,089 | ||||||||||||||
|
Other
(income)/expense, net
|
(641 | ) | 754 | 725 | (17 | ) | 201 | |||||||||||||
|
Income/(loss)
before income taxes
|
22,645 | 15,000 | (7,223 | ) | 6,347 | 15,566 | ||||||||||||||
|
Provision
for income taxes
|
12,253 | 7,071 | 6,288 | 14,513 | 25,322 | |||||||||||||||
|
Income/(loss)
from continuing operations
|
10,392 | 7,929 | (13,511 | ) | (8,166 | ) | (9,756 | ) | ||||||||||||
|
Income/(loss)
from discontinued operations, including gains/(losses) from dispositions,
net of tax
|
- | - | 222,759 | (21,706 | ) | (100,702 | ) | |||||||||||||
|
Income/(loss)
before cumulative effect of a change in accounting
principle
|
10,392 | 7,929 | 209,248 | (29,872 | ) | (110,458 | ) | |||||||||||||
|
Cumulative
effect of a change in accounting principle
|
- | - | - | (228 | ) | - | ||||||||||||||
|
Net
income/(loss)
|
10,392 | 7,929 | 209,248 | (30,100 | ) | (110,458 | ) | |||||||||||||
|
EARNINGS
PER SHARE DATA:
|
||||||||||||||||||||
|
Earnings/(loss)
per common share (basic):
|
||||||||||||||||||||
|
Income/(loss)
from continuing operations
|
$ | 0.36 | $ | 0.27 | $ | (0.47 | ) | $ | (0.30 | ) | $ | (0.37 | ) | |||||||
|
Income/(loss)
from discontinued operations, including gains/(losses) from dispositions,
net of tax
|
$ | - | $ | - | $ | 7.77 | $ | (0.81 | ) | $ | (3.81 | ) | ||||||||
|
Cumulative
effect of a change in accounting principle
|
$ | - | $ | - | $ | - | $ | (0.01 | ) | $ | - | |||||||||
|
Net
income/(loss)
|
$ | 0.36 | $ | 0.27 | $ | 7.30 | $ | (1.12 | ) | $ | (4.18 | ) | ||||||||
|
Earnings/(loss)
per common share (diluted):
|
||||||||||||||||||||
|
Income/(loss)
from continuing operations
|
$ | 0.36 | $ | 0.27 | $ | (0.47 | ) | $ | (0.30 | ) | $ | (0.37 | ) | |||||||
|
Income/(loss)
from discontinued operations, including gains/(losses) from dispositions,
net of tax
|
$ | - | $ | - | $ | 7.77 | $ | (0.81 | ) | $ | (3.81 | ) | ||||||||
|
Cumulative
effect of a change in accounting principle
|
$ | - | $ | - | $ | - | $ | (0.01 | ) | $ | - | |||||||||
|
Net
income/(loss)
|
$ | 0.36 | $ | 0.27 | $ | 7.30 | $ | (1.12 | ) | $ | (4.18 | ) | ||||||||
|
Weighted
average shares outstanding:
|
||||||||||||||||||||
|
Basic
|
29,241 | 29,116 | 28,683 | 26,816 | 26,456 | |||||||||||||||
|
Diluted
|
29,267 | 29,161 | 28,683 | 26,816 | 26,456 | |||||||||||||||
|
DIVIDENDS
PER COMMON SHARE
|
$ | - | $ | - | $ | 14.03 | $ | 0.12 | $ | 0.12 | ||||||||||
_____________
(dollars
in thousands, except share data)
|
Years Ended December 31,
|
||||||||||||||||||||
|
2009(1)
|
2008(2)
|
2007(3)
|
2006(4)
|
2005(5)
|
||||||||||||||||
|
BALANCE
SHEET DATA: (at end of period)
|
||||||||||||||||||||
|
Working
capital
|
$ | 94,362 | $ | 74,376 | $ | 69,148 | $ | 117,616 | $ | 139,207 | ||||||||||
|
Total
assets
|
351,515 | 341,072 | 373,462 | 606,376 | 612,472 | |||||||||||||||
|
Long-term
debt
|
120,800 | 123,800 | 101,600 | 158,600 | 182,060 | |||||||||||||||
|
Total
stockholders' equity
|
103,270 | 74,786 | 102,057 | 246,646 | 243,251 | |||||||||||||||
|
(1)
|
Net
income includes tax expense of approximately $5,300 for an estimate of an
international tax liability related to a 2003
transaction.
|
|
(2)
|
Income
from continuing operations include pre-tax charges of $1,515 within
operating expenses for the costs related to strategic alternatives, $4,695
within operating expenses for restructuring costs and $1,040 within
operating expenses related to a former CEO's
retirement.
|
|
(3)
|
Loss
from continuing operations include pre-tax charges of $31,127 within
operating expenses for the costs related to strategic alternatives, $6,073
within operating expenses for restructuring costs and $841 within interest
expense for the write-off of unamortized debt costs. Income
from discontinued operations include the gain on sale of the businesses
that comprised the Bioproducts and Biopharma business segments of
$235,489, expense of $4,636 for the Rutherford litigation settlement and
expense of $1,000 for an adjustment to an environmental reserve at a
Rutherford Business site.
|
|
(4)
|
Loss
from continuing operations include pre-tax charges of $2,958 within
operating expenses for external advisor costs related to divestitures,
$5,272 within interest expense due to the pre-payment of a portion of the
Company’s long-term debt and tax expense of $1,696 related to prior years
returns included in the provision for income taxes. Loss from
discontinued operations include the loss on the sale of two businesses
within the former Human Health segment of $23,244, expense of $200 for an
adjustment to an environmental reserve at a Rutherford Business site,
$2,092 for a goodwill impairment charge, $1,791 due to the acquisition of
Cutanogen and $1,475 for the write-down of an investment in equity
securities.
|
|
(5)
|
Loss
from continuing operations include pre-tax charges for executive severance
of $4,223 and an increase in an environmental reserve of $1,300 recorded
in operating expenses, a tax benefit due to a favorable Swedish court
decision of $3,329 and an increase in valuation allowances against
domestic deferred tax assets totaling $16,926 within the provision for
income taxes. Loss from discontinued operations include pre-tax
charges for goodwill impairment of $76,385, long-lived asset impairment
charge of $30,792 and a tax benefit related to the long-lived asset
impairment of $1,673.
|
_____________
(dollars
in thousands, except share data)
|
Item 7
|
Management's Discussion and
Analysis of Financial Condition and Results of
Operations
|
Executive
Overview
The
Company’s business consists of three manufacturing facilities. These
facilities primarily manufacture APIs, ingredients derived from organic
chemistry and pharmaceutical intermediates.
The
following significant events, which are explained in detail on the following
pages, occurred during 2009:
|
|
·
|
The
Company’s Board of Directors approved the termination of its
postretirement employee benefit plan resulting in a benefit, recorded in
Operating expenses, of approximately
$1,200.
|
|
|
·
|
The
Company recorded tax expense of approximately $5,300 for an estimate of an
international tax liability related to a 2003
transaction.
|
Sales in
2009 decreased 5.3% to $236,277 from $249,618 in 2008. Sales in 2009
were unfavorably impacted 4.1% as a result of foreign currency
exchange.
The
Company experienced lower generic API sales due to competitive
pricing. Sales of controlled substances, which the Company defines as
drugs falling under Schedule II of the U.S. Drug Enforcement Agency's
classification system, showed strong growth in 2009. The Company
also continues to develop several new products utilizing its proprietary
polymeric drug delivery technology. Sales of a feed additive were
lower as a result of exiting the product line in 2008.
The
Company maintained a robust pipeline of custom development projects during
2009 and its portfolio currently includes 12 products for which the Company
expects to manufacture products for its customers’ phase III
clinical trials. With a broad portfolio of products and services in
the API market, the Company remains profitable and has a solid platform for
future growth.
Gross margins in 2009 increased to
29.7% from 29.5% in 2008. Excluding a 1.6% favorable impact from
foreign currency, gross margins decreased 1.4%. The lower margins are
due primarily to lower pricing during 2009.
One customer accounted for 10% or more
of 2009 gross sales. Gyma, a distributor representing multiple
customers, accounted for 11.5% of 2009 sales.
The Company recorded tax expense of
$12,253 in 2009 compared to $7,071 in 2008. The tax provisions in
2009 and 2008 are primarily affected by the non-recognition of tax benefits in
the U.S. where losses are incurred and the Company records valuation allowances
against the benefits. The 2009 tax provision also includes a charge
of approximately $5,300 for an estimate of an international tax liability
related to a 2003 transaction. The 2008 provision also includes
benefits due to the expiration of statutes of limitations on certain tax
positions, benefits for tax loss carrybacks and credits, and incremental
benefits of the project to streamline the Company’s legal
structure.
The Company reported net income of
$10,392, or $0.36 per diluted share in 2009, compared to $7,929, or $0.27 per
diluted share in 2008.
Critical
Accounting Policies
The Company’s critical accounting
policies are those that require the most subjective or complex judgments, often
as a result of the need to make estimates about the effect of matters that are
inherently uncertain. The Company bases its estimates on historical
experience and on other assumptions that are deemed reasonable by management
under each applicable circumstance. Actual results or amounts could
differ from estimates and the differences could have a material impact on the
consolidated financial statements. A discussion of the Company’s
critical accounting policies, the underlying judgments and uncertainties
affecting their application and the likelihood that materially different amounts
would be reported under different conditions or using different assumptions, is
as follows:
_____________
(dollars
in thousands, except share data)
Revenue Recognition
Revenues are generally recognized when
title to products and risk of loss are transferred to
customers. Additional conditions for recognition of revenue are that
collection of sales proceeds is reasonably assured and the Company has no
further performance obligations.
The Company has certain contracts that
contain multiple deliverables. These deliverables often include
process development services and commercial production and are divided into
separate units of accounting if certain criteria are met, including whether the
delivered element has stand-alone value to the customer and whether there is
objective and reliable evidence of the fair value of the undelivered
items. The consideration the Company receives is allocated among the
separate units based on their respective fair values, and the applicable revenue
recognition criteria are applied to each of the separate units.
For contracts that contain
milestone-based payments, the Company recognizes revenue using the proportional
performance method based on the percentage of costs incurred relative to the
total costs estimated to be incurred to complete the
contract. Revenue recognition computed under this methodology is
compared to the amount of non-refundable cash payments received or contractually
receivable at the reporting date and the lesser of the two amounts is recognized
as revenue at each reporting date. The proportional performance
methodology applied by the Company for revenue recognition, utilizes an input
based measure, specifically labor costs, because the Company believes the use of
an input measure is a better surrogate of proportional performance than an
output based measure, such as milestones.
Amounts billed in advance are recorded
as deferred revenue on the balance sheet. Since payments received are typically
non-refundable, the termination of a contract by a customer prior to its
completion could result in an immediate recognition of deferred revenue relating
to payments already received not previously recognized as revenue.
Sales terms to certain customers
include rebates if certain conditions are met. Additionally, sales
are generally made with a limited right of return under certain
conditions. The Company estimates these rebates and returns at the
time of sale based on the terms of agreements with customers and historical
experience and recognizes revenue net of these estimated costs which are
classified as allowances and rebates.
The Company bills a portion of freight
cost incurred on shipments to customers. Freight costs are reflected
in cost of goods sold. Amounts billed to customers are recorded
within net revenues.
Asset Valuations and Review for
Potential Impairments
The review of long-lived assets,
principally fixed assets and other amortizable intangibles, requires the Company
to estimate the undiscounted future cash flows generated from these assets
whenever events or changes in circumstances indicate that the carrying value may
not be fully recoverable. If undiscounted cash flows are less than
carrying value, the long-lived assets are written down to fair
value.
The review of the carrying value of
goodwill and indefinite lived intangibles is done annually or whenever events or
changes in circumstances indicate that the carrying value may not be fully
recoverable utilizing a two-step process. In the first step, the fair
value of the reporting units is determined using a discounted cash flow model
and compared to the carrying value. If such analysis indicates that
impairment may exist, the Company then estimates the fair value of the other
assets and liabilities utilizing appraisals and discounted cash flow analyses to
calculate an impairment charge.
The determination of fair value is
judgmental and involves the use of significant estimates and assumptions,
including projected future cash flows primarily based on operating plans,
discount rates, determination of appropriate market comparables and perpetual
growth rates. These estimates and assumptions could have a
significant impact on whether or not an impairment charge is recognized and the
magnitude of any such charge.
_____________
(dollars
in thousands, except share data)
Environmental and Litigation
Contingencies
The Company periodically assesses the
potential liabilities related to any lawsuits or claims brought against
it. See Note 18 for a discussion of the Company’s current
environmental and litigation matters, reserves recorded and its position
with respect to any related uncertainties. While it is typically very
difficult to determine the timing and ultimate outcome of these actions, the
Company uses its best judgment to determine if it is probable that the Company
will incur an expense related to a settlement for such matters and whether a
reasonable estimation of such probable loss, if any, can be made. If
probable and estimable, the Company accrues for the costs of clean-up,
settlements and legal fees. If the aggregate amount of the liability
and the timing of the payment is fixed or reasonably determinable, the Company
discounts the amount to reflect the time value of money. Given the
inherent uncertainty related to the eventual outcome of litigation and
environmental matters, it is possible that all or some of these matters may be
resolved for amounts materially different from any provisions that the Company
may have made with respect to their resolution.
Income
Taxes
The Company applies an asset and
liability approach to accounting for income taxes. Deferred tax
assets and liabilities are recognized for the expected future tax consequences
of temporary differences between the financial statement and tax basis of assets
and liabilities using enacted tax rates in effect for the year in which the
differences are expected to reverse. The recoverability of deferred
tax assets is dependent upon the Company’s assessment that it is more likely
than not that sufficient future taxable income will be generated in the relevant
tax jurisdictions to utilize the deferred tax assets. In the event
the Company determines that future taxable income will not be sufficient to
utilize the deferred tax assets, a valuation allowance is
recorded. The Company’s valuation allowances primarily relate to
federal NOL carryforwards, foreign tax credits, and alternative minimum tax
credits in the U.S., where profitability is uncertain, and NOL carryforwards in
certain state and foreign jurisdictions with little or no history of generating
taxable income or where future profitability is uncertain.
Employee
Benefit Plans
The Company provides a range of
benefits to certain employees and retired employees, including pensions, post
employment and health care benefits. The Company records annual
amounts relating to these plans based on calculations, which include various
actuarial assumptions, including discount rates, assumed rates of return,
turnover rates, and health care cost trend rates. The Company reviews
its actuarial assumptions on an annual basis and makes modifications to the
assumptions based on current rates and trends when it is deemed appropriate to
do so. The effect of the modifications is generally recorded and
amortized over future periods. The Company believes that the
assumptions utilized for recording obligations under its plans are
reasonable.
The discount rate used to measure
pension liabilities and costs is selected by projecting cash flows associated
with plan obligations which were matched to a yield curve of high quality
bonds. The Company then selected the single rate that produces the
same present value as if each cash flow were discounted by the corresponding
spot rate on the yield curve.
Results
of Operations
2009 Compared to
2008
Gross sales for 2009 decreased 5.3% to
$236,277 from $249,618 in 2008. Gross sales were unfavorably impacted
in 2009 by 4.1% due to strength in the U.S. dollar primarily versus the Euro and
Swedish krona.
_____________
(dollars
in thousands, except share data)
The
following table summarizes gross sales by product groups:
|
2009
|
2008
|
|||||||
|
APIs
and pharmaceutical intermediates
|
$ | 212,644 | $ | 220,722 | ||||
|
Other
|
23,633 | 28,896 | ||||||
|
Total
|
$ | 236,277 | $ | 249,618 | ||||
Sales of APIs and pharmaceutical
intermediates in 2009 of $212,644 were $8,078 or 3.7% below the prior
year. Excluding the unfavorable impact due to foreign exchange rates,
sales were up 0.6%. Higher sales were driven by higher demand for
drug delivery products, controlled substances and custom development
products. These increases were mostly offset by lower revenues
for two products for which long-term contracts are in effect, and
lower volumes and pricing of generic APIs.
Other sales in 2009 of $23,633 were
$5,263 or 18.2% below the prior year. Excluding the unfavorable
impact due to foreign exchange, these sales were down 14.9%. The
decrease in sales is due primarily to lower sales of a feed additive product
line that the Company exited in 2008 and lower sales of specialty
additives.
Gross profit in 2009 was $70,278
compared to $73,743 in 2008. Gross margins in 2009 increased to 29.7%
from 29.5% in 2008. Excluding a 1.6% favorable impact from foreign
currency, gross margins decreased 1.4%. The lower margins are due
primarily to lower pricing during 2009.
Selling, general and administrative
expenses of $35,711 or 15.1% of gross sales in 2009 decreased from $40,521 or
16.2% in 2008. This decrease is due primarily to a favorable impact
from foreign currency (approximately $2,400), a benefit from terminating the
postretirement employee benefit plan (approximately $1,200), higher 2008 expense
related to the former CEO’s retirement (approximately $1,000) and lower
insurance premiums, recruiting expense and professional fees (approximately
$1,600), partially offset by higher legal fees (approximately
$1,200).
Research
and development expenses of $7,929 were 3.4% of gross sales in 2009, compared to
$7,590 or 3.0% of gross sales in 2008. The increase is primarily due
to higher costs related to the development of new products and technology
platforms. The impact of foreign currency reduced R&D expenses by
approximately $550.
Restructuring
expenses for 2008 were $4,695, consisting of rent and related costs at the New
Jersey R&D facility and costs associated with the restructuring of the
corporate office.
Strategic
alternative costs for 2008 were $1,515, consisting of costs associated with a
project to streamline the Company’s legal structure, change-in-control benefits
and costs associated with the modification of employee stock options due to the
payment of the special dividend in connection with the 2007 divestiture of the
businesses that comprised the Bioproducts and Biopharma segments.
Operating profit was $26,638 in 2009
compared to $19,422 in 2008. The increase is due to lower strategic
alternative and restructuring costs and lower spending as discussed above,
partially offset by lower gross profit. The 2008 results include
strategic alternative and restructuring costs of $1,515 and $4,695,
respectively.
Net
interest expense was $4,634 in 2009 compared to $3,668 in 2008. This
increase is due primarily to lower capitalized interest of $1,355 due to the
completion of a large capital project and lower interest income as a result of
lower interest rates. The increase was partially offset by lower
interest expense on the Company’s debt as a result of lower average interest
rates partially offset by higher average debt. The average interest
rate was 3.8% and 4.9% in 2009 and 2008, respectively.
_____________
(dollars
in thousands, except share data)
The
Company recorded tax expense of $12,253 in 2009 compared to $7,071 in
2008. The tax expense for 2009 and 2008 includes a $103 and $5,537
valuation allowance, respectively, to offset benefits generated
from domestic losses and tax credits, and losses in certain foreign
jurisdictions. These valuation allowances result from the Company’s
recent history of domestic and certain foreign losses and its short-term
projections for losses in the relative jurisdictions. Since 2003, the
Company has maintained a full valuation allowance on the tax benefits arising
from domestic pre-tax losses.
The
Company will continue to record a full valuation allowance, primarily on its
domestic net deferred tax assets and indefinite lived intangibles, until an
appropriate level of domestic profitability is sustained or tax strategies can
be developed that would enable the Company to conclude that it is more likely
than not that a portion of the domestic net deferred tax assets would be
realized. If the Company continues to report pre-tax losses in the United
States and certain foreign jurisdictions, income tax benefits associated with
those losses will not be recognized and, therefore, those losses would not be
reduced by such income tax benefits. The carryforward periods for domestic
federal foreign tax credits, NOLs, research and experimentation tax credits and
alternative minimum tax credits are 10 years, 20 years, 20 years and an
indefinite period, respectively. As such, improvements in domestic pre-tax
income in the future may result in these tax benefits ultimately being
realized. However, there is no assurance that such improvements will be
achieved.
In 2009,
the Company’s Italian subsidiary was examined by the Italian tax authorities,
who challenged the business purpose of a 2003 transaction in which a new
subsidiary was created, and the deductibility of certain intercompany
transactions. In the fourth quarter of 2009, the tax authorities
notified the Company that they disagreed with the Company’s responses to their
formal assessments. The Company has analyzed the issues in accordance
with guidance on uncertain tax positions and has recorded an increase to its tax
expense of approximately $5,300. Settlement discussions with the tax
authorities are ongoing.
In 2009,
the Company’s Swedish subsidiary was examined by the Swedish tax authorities,
who questioned certain significant intercompany balances and
transactions. The Company filed responses to the inquiries in the
fourth quarter of 2009. The Company expects it to take several months
before a formal audit report is issued. If the tax authorities were
to disagree with the Company’s position on unresolved issues, the Company
estimates the preliminary assessment would be approximately $200. The
Company has analyzed these issues in accordance with guidance on uncertain tax
positions and believes its reserves are adequate, and intends to defend
itself.
Net income in 2009 was $10,392, or
$0.36 per diluted share, versus $7,929, or $0.27 per diluted share in
2008.
2008 Compared to
2007
Gross sales for 2008 decreased 1.2% to
$249,618 from $252,574 in 2007. Gross sales were favorably impacted
in 2008 by 2.5% due to the weakness in the U.S. dollar primarily versus the Euro
and Swedish krona.
The
following table summarizes gross sales by product groups:
|
2008
|
2007
|
|||||||
|
APIs
and pharmaceutical intermediates
|
$ | 220,722 | $ | 220,386 | ||||
|
Other
|
28,896 | 32,188 | ||||||
|
Total
|
$ | 249,618 | $ | 252,574 | ||||
Sales of APIs and pharmaceutical
intermediates of $220,722 were comparable to the prior
year. Excluding the favorable impact due to foreign exchange rates,
sales were down 2.4%. Lower sales were driven by lower volumes of a
diuretic API, lower demand for custom development and drug delivery products as
well as lower pricing for a gastro-intestinal API due to the renegotiation of a
long-term contract. These decreases were partially offset by higher
sales of controlled substances and higher demand for a central nervous system
API.
_____________
(dollars
in thousands, except share data)
Other sales of $28,896 were $3,292 or
10.2% below the prior year. Excluding the favorable impact due to
foreign exchange, these sales were down 12.1%. The decrease in sales
is due primarily to lower sales of a feed additive product line that the Company
exited in the third quarter of 2008 and lower sales of polymer
products.
Gross profit in 2008 was $73,743
compared to $91,232 in 2007. Gross margins in 2008 decreased to 29.5%
from 36.1% in 2007. The lower margins are due primarily to lower
pricing and higher production costs partially offset by proceeds from an
insurance settlement related to business interruption. The insurance
settlement contributed 0.3% to gross margins. The impact of foreign
currency exchange was negligible.
Selling, general and administrative
expenses of $40,521 or 16.2% of gross sales in 2008 decreased from $48,858 or
19.3% in 2007. Administrative expenses decreased primarily due to
lower personnel costs resulting from reduced staffing at corporate headquarters
(approximately $3,200), lower bonus expense (approximately $2,500) and lower
legal fees (approximately $2,500) partially offset by an unfavorable impact from
foreign currency (approximately $1,100).
Research
and development expenses of $7,590 were 3.0% of gross sales in 2008, compared to
$12,157 or 4.8% of gross sales in 2007. The decrease is primarily due
to the Company’s decision in 2007 to consolidate its New Jersey R&D facility
with its R&D operations in Iowa to create increased operating
efficiencies. The Company also utilized certain R&D personnel on
custom development projects resulting in these costs being classified as cost of
goods sold. The impact of foreign currency was
negligible.
Total restructuring expenses for 2008
and 2007 were $4,695 and $6,073, respectively. Restructuring expenses
include the reduction of employee positions at the corporate office and the
consolidation of the Company’s R&D activities and small scale API production
with its facility in Iowa.
During
2007, the Company announced plans to eliminate certain employee positions at the
corporate office upon completion of the sale of the businesses that comprised
the Bioproducts and Biopharma segments. This plan included certain
one-time benefits for terminated employees. Costs related to these
plans are recorded as restructuring expenses in the income statement. The
Company recognized expense of $805 and $4,014 in 2008 and 2007, respectively,
related to this plan.
In
December of 2007, the Company consolidated its United States R&D activities
and small scale API production with its facility in Charles City, Iowa. The
Company recognized restructuring expenses in 2007 of $2,059 related to this
consolidation. This charge included the present value of the
remaining lease payments under the Company’s current operating lease at the New
Jersey R&D facility (reduced by estimated sublease rentals) of
$998. The operating lease expires in December
2010. In accordance with accounting guidance, the fair value of
the liability recorded at the cease-use date factored in the remaining lease
rentals, reduced by estimated sublease rentals that could be reasonably obtained
for the property. The Company consulted with local real estate
brokers at that time to determine what reasonable sublease rentals could be
obtained. The Company has not been able to sublease the property and
interest dramatically decreased during the fourth quarter of
2008. Due to the lack of interest, the Company consulted with its
real estate broker and determined that the possibility of obtaining a sublease
was extremely low. As a result, during the fourth quarter of 2008,
the Company increased the reserve related to the remaining lease payments by
$2,388. This amount assumes the Company will not obtain a sublease
for the facility. In addition to increasing the reserve, the Company
incurred costs of $1,502 related to lease payments, utilities and severance
during 2008. Costs related to this consolidation are recorded as
restructuring expenses on the income statement.
Total
strategic alternative costs for 2008 and 2007 were $1,515 and $31,127,
respectively. Strategic alternative costs include expenses that the
Company has incurred related to the decision to sell the businesses that
comprised the Bioproducts and Biopharma segments in February 2007, costs
associated with a project to streamline the Company’s legal structure and costs
associated with the exit of a feed additives product line. These costs are not
considered part of the restructuring program or a part of discontinued
operations under current accounting guidance.
_____________
(dollars
in thousands, except share data)
Strategic
alternative costs for 2008 include $1,385 related to the project to streamline
the Company’s legal structure, costs associated with the modification of
employee stock options due to the payment of the special dividend in connection
with the divestiture of $102 and change of control expense of
$28. Costs for 2007 include change of control expenses totaling
$20,025 related to the 2007 divestiture of the businesses that comprised the
Bioproducts and Biopharma segments, retention bonuses of $6,780, costs
associated with the stock option modification of $2,854 and external advisor
costs of $456.
During
the fourth quarter of 2007 the Company committed to a plan to exit a feed
additive product line. The equipment used in producing this product
will be dismantled and disposed subsequent to the completion of
production. Production continued through the third quarter of
2008. The Company recorded $1,012 for the asset retirement obligation
in 2007. This charge is recorded as strategic alternative costs in
the income statement.
Operating profit was $19,422 in 2008
compared to an operating loss of $6,983 in 2007. The increase is due
to lower strategic alternative and restructuring costs and lower corporate
spending partially offset by lower gross margins. The 2008 results
include strategic alternative and restructuring costs of $1,515 and $4,695,
respectively. The 2007 results include strategic alternative and
restructuring costs of $31,127 and $6,073, respectively.
Net
interest expense was $3,668 in 2008 compared to net interest income of $485 in
2007 primarily reflecting interest income in 2007 due to interest earned on the
proceeds from the sale of the businesses that comprised the Bioproducts and
Biopharma segments. Higher average debt partially offset by lower
interest rates contributed to higher interest expense. Additionally,
2007 includes the acceleration of unamortized origination fees related to the
repayment of a prior credit facility of $841. The average interest
rate was 4.9% and 6.9% in 2008 and 2007, respectively.
The
Company recorded tax expense of $7,071 in 2008 compared to $6,288 in 2007.
The tax expense for 2008 includes a $5,537 valuation allowance to offset
benefits generated from domestic tax credits, and losses in
certain foreign jurisdictions. These valuation allowances result
from the Company’s recent history of domestic and certain foreign losses and its
short-term projections for losses in the relative
jurisdictions. Since 2003, the Company has maintained a full
valuation allowance on the tax benefits arising from domestic pre-tax
losses.
In
connection with the sale of the businesses that comprised the Bioproducts and
Biopharma businesses in 2007, the Company utilized domestic federal NOLs and
foreign tax credits for which a full valuation allowance was provided for at
December 31, 2006, to eliminate the U.S. income tax on this transaction.
U.S. income tax related to distributions repatriated from foreign entities in
2008 has been offset by foreign tax credits for which a full valuation allowance
was provided for at December 31, 2007.
Income from continuing operations in
2008 was $7,929, or $0.27 per diluted share, versus a loss of $13,511, or $0.47
per diluted share in 2007.
_____________
(dollars
in thousands, except share data)
Liquidity
and Capital Resources
During 2009 cash and cash equivalents
on hand increased $19,825 to $52,365. The year over year
strength in the Euro and Swedish krona favorably impacted the translated cash
balances by $1,881. During 2009, cash flows from operations provided $34,392,
compared to $4,989 in the same period a year ago. The increase
in cash flows from operations in 2009 versus 2008 is due primarily to the
unusually large cash payments required in 2008 related to change-in-control and
restructuring payments, better inventory management and higher net
income.
Cash flows used in investing activities
in 2009 of $12,520 primarily reflect cash payments related to capital
expenditures of $12,587 compared to $29,378 in 2008. The majority of
funds in 2009 were used for a new mid-scale Pharma manufacturing facility in
Karlskoga, Sweden which was substantially completed as of March 31, 2009 and
capital improvements to existing facilities. For 2010, capital
expenditures are expected to be approximately $12,000 to $15,000.
Cash flows used in financing activities
in 2009 of $3,928 mainly reflect the pay down of debt. In 2008 the
Company had a net increase in bank debt of $22,142.
In April
2007, the Company entered into a $200,000 five-year Syndicated Senior Revolving
Credit Facility (“Credit Facility”) which expires in April 2012. The
Company pays interest on this Credit Facility at LIBOR plus 1.25% - 2.00% based
upon certain financial measurements. The Credit Facility also includes
financial covenants regarding interest coverage and leverage ratios. The
Company was in compliance with all financial covenants at December 31,
2009. The Credit Facility is collateralized by dividend and
distribution rights associated with a pledge of a portion of stock that the
Company owns in a foreign holding company. This foreign holding company owns a
majority of the Company's non-U.S. operating subsidiaries. As of
December 31, 2009 there was $120,800 outstanding.
The Company has employed a plan to
mitigate interest rate risk by entering into interest rate swap agreements to
convert floating rates to fixed interest rates. As of December 31,
2009, the Company had three interest rate swaps in place with an aggregate
notional value of $60,000, at an average fixed rate of 4.48%, and with maturity
dates of October 2010. The Company’s strategy has been to cover a
portion of outstanding bank debt with interest rate protection. At
December 31, 2009, the coverage was approximately 50% of the Company’s variable
interest rate debt.
The 2009 and 2008 weighted average
interest rate for long-term bank debt was 3.8% and 4.9%,
respectively.
Contractual
Obligations
At December 31, 2009, the Company’s
contractual obligations with initial or remaining terms in excess of one year
were as follows:
|
Total
|
2010
|
2011
|
2012
|
2013
|
2014+ | |||||||||||||||||||
|
Long
term debt
|
$ | 120,800 | $ | - | $ | - | $ | 120,800 | $ | - | $ | - | ||||||||||||
|
Interest
on debt
|
6,941 | 3,833 | 2,331 | 777 | - | - | ||||||||||||||||||
|
Operating
leases
|
5,038 | 1,822 | 490 | 405 | 364 | 1,957 | ||||||||||||||||||
|
Purchase
obligations
|
7,415 | 6,452 | 963 | - | - | - | ||||||||||||||||||
|
Contractual
cash obligations
|
$ | 140,194 | $ | 12,107 | $ | 3,784 | $ | 121,982 | $ | 364 | $ | 1,957 | ||||||||||||
In addition to the contractual
obligations listed above, the Company expects to contribute approximately $1,041
in cash to its two U.S. defined-benefit pension plans in 2010. See
Note 15 for detail on the Company’s unfunded balance related to its pension
plans. Also not included in the table above is $6,649 of uncertain
tax positions due to uncertainties surrounding the timing of the
obligation. See Note 8.
See Notes 9, 15, 17 and 18 for
additional information regarding the Company’s pension plans, debt and other
commitments.
_____________
(dollars
in thousands, except share data)
The Company’s forecasted cash flow from
future operations may be adversely affected by various factors including, but
not limited to, declines in customer demand, increased competition, the
deterioration in general economic and business conditions, returns on assets
within the Company’s domestic pension plans that are significantly below
expected performance, as well as other factors. See the Risk Factors
section of this document for further explanation of factors that may negatively
impact the Company’s cash flows. Any change in the current status of
these factors could adversely impact the Company's ability to fund operating
cash flow requirements.
Market
Risks
Currency
Risk Management
The Company's primary market risk
relates to exposure to foreign currency exchange rate fluctuations on
transactions entered into by international operations which are primarily
denominated in the U.S. dollar, Euro and Swedish krona. The Company
currently uses foreign currency exchange forward contracts to mitigate the
effect of short-term foreign exchange rate movements on the Company's local
operating results. As a matter of policy, the Company does not hedge to protect
the translated results of foreign operations. The notional amount of
these contracts as of December 31, 2009 was $15,781. Unrealized
foreign exchange contract losses do not subject the Company's actual results to
risk as gains or losses on these contracts are undertaken to offset gains or
losses on the transactions that are hedged. The foreign exchange
contracts have varying maturities with none exceeding twelve
months.
With respect to the contracts
outstanding at December 31, 2009, a 10% fluctuation of the local currency over a
one-year period would cause $1,562 pre-tax earnings to be at risk. This is based
on the notional amount of the contracts, adjusted for unrealized gains and
losses, of $15,618. These calculations do not include the impact of exchange
gains or losses on the underlying positions that would offset the gains and
losses of the derivative instruments.
Interest Rate Management
The Company has employed a plan to
mitigate interest rate risk by entering into interest rate swap agreements to
convert floating rates to fixed interest rates. As of December 31,
2009, the Company had three interest rate swaps in place with an aggregate
notional value of $60,000, at an average fixed rate of 4.48%, and with maturity
dates in October 2010. The Company’s strategy has been to cover a
portion of outstanding bank debt with interest rate protection. At
December 31, 2009, the coverage was approximately 50% of the Company’s variable
interest rate debt. At December 31, 2009, the Company had variable
debt of $120,800, of which $60,000 is fixed by interest rate
swaps. Holding all other variables constant, if the LIBOR portion of
the weighted average interest rates in the variable rate debt increased by 100
basis points, the effect on our earnings and cash flows would have been higher
interest expense of $608.
Contingencies
The Company is subject to various
investigations, claims and legal proceedings covering a wide range of matters
that arise in the ordinary course of its business activities. The
Company continually assesses all known facts and circumstances as they pertain
to all legal and environmental matters and evaluates the need for reserves and
disclosures as deemed necessary based on these facts and
circumstances. These matters, either individually or in the
aggregate, could have a material adverse effect on the Company's financial
condition, operating results and cash flows in a future reporting
period.
Environmental
In connection with laws and regulations
pertaining to the protection of the environment, the Company and its
subsidiaries are a party to several environmental proceedings and remediation
investigations and cleanups and, along with other companies, have been named a
PRP for certain waste disposal sites ("Superfund
sites"). Additionally, the Company has retained the liability for
certain environmental proceedings associated with the discontinued operations of
the Rutherford Chemicals business.
_____________
(dollars
in thousands, except share data)
Each of these matters is subject to
various uncertainties, and it is possible that some of these matters will be
decided unfavorably against the Company. The resolution of such
matters often spans several years and frequently involves regulatory oversight
or adjudication. Additionally, many remediation requirements are not
fixed and are likely to be affected by future technological, site, and
regulatory developments. Consequently, the ultimate liability with
respect to such matters, as well as the timing of cash disbursements cannot be
determined with certainty.
In
matters where the Company has been able to reasonably estimate its liability,
the Company has accrued for the estimated costs associated with the study and
remediation of Superfund sites not owned by the Company and the Company's
current and former operating sites. These accruals were $6,163 and
$6,226 at December 31, 2009 and 2008, respectively. The decrease in
the accrual includes payments of $310 partially offset by increases to reserves
of $110 and the impact of currency of $137. Based upon available
information and analysis, the Company's current accrual represents management's
best estimate of the probable and estimable costs associated with environmental
proceedings including amounts for investigation fees where full remediation
costs may not be estimable at the reporting date.
CasChem
As a result of the sale of the Bayonne,
New Jersey facility, the Company became obligated to investigate site conditions
and conduct required remediation under the New Jersey Industrial Site Recovery
Act. The Company submitted a sampling plan to the New Jersey
Department of Environmental Protection (“NJDEP”) and is awaiting
approval. The results of the completed and proposed sampling, and any
additional sampling deemed necessary, will be used to develop an estimate of the
Company's future liability for remediation costs, if any.
Cosan
In response to the NJDEP, the Company
completed its initial investigation and submitted the results of the
investigation and a proposed Remedial Action Work Plan (“RAW”) to the NJDEP for
its Cosan Clifton, New Jersey site. The NJDEP subsequently rejected the
RAW and requested additional investigative work at the site and that work is
on-going. The reserve was $1,164 at December 31, 2009 which is based on
the initial remedial action plan. The results of the additional
investigative work may impact the remediation plan and costs.
Additionally,
the Company has recorded a liability of $916 for the Cosan Carlstadt, New Jersey
site based on the investigations completed to date and the proposed RAW
submitted to the NJDEP for their approval. The NJDEP has subsequently
required the Company to perform additional investigative work prior to approval
of the RAW. The results of this additional investigative work may impact
the remediation plan and costs.
Berry’s
Creek
The Company received a notice from the
United States Environmental Protection Agency (“USEPA”) that two former
operating subsidiaries of the Company are considered PRPs at the Berry’s Creek
Superfund Site in New Jersey. The operating companies are among many
other PRPs that were listed in the notice. Pursuant to the notice, the
PRPs have been asked to perform a remedial investigation and feasibility study
of the Berry’s Creek Site. The Company has joined the group of PRPs and
filed a response to the USEPA agreeing to jointly negotiate to conduct or fund
an appropriate remedial investigation and feasibility study of the Berry’s Creek
Site. The PRPs have engaged consultants to evaluate investigation and
remedial alternatives and develop a method to allocate related costs among the
PRPs. As of December 31, 2009, the Company’s reserve was $309 to
cover the initial phase of investigation based on a tentative agreement on the
allocation of the site investigation costs among the PRPs. The
investigation is expected to take several years and at this time it is too early
to predict the extent of any additional liabilities.
_____________
(dollars
in thousands, except share data)
Nepera,
Inc. – Maybrook and Harriman Sites
Nepera,
Inc. (“Nepera”) is named a PRP of the Maybrook Site in Hamptonburgh, New York by
the USEPA in connection with the disposition, under appropriate permits, of
wastewater at that site prior to Cambrex's acquisition of Nepera in
1986. The USEPA also issued the Company a Notice of Potential
Liability and the Company signed a Consent Decree to complete the Record of
Decision (“ROD”) and has provided the USEPA with appropriate financial
assurance, including a letter of credit to guarantee the obligation under the
Consent Decree.
Nepera is
also named a responsible party of the Harriman, New York production facility by
the New York State Department of Environmental Conservation. A final
ROD was issued which describes the remediation plan for the
site. Implementation of the ROD is on-going.
As of
December 31, 2009, the reserve recorded on the books was $1,300 and represents
the Company’s best estimate to complete both RODs.
Solvent Recoveries Superfund
Site
A subsidiary of the Company is one of
approximately 1,300 PRPs at a Superfund site (“the Site”) in Southington,
Connecticut, once operated by Solvent Recoveries, Inc. The PRP group
has completed a Remedial Investigation/Feasibility Study and the USEPA has
proposed remediation of the Site. In 2008, the Company agreed to
enter into a consent decree and settlement with the other PRPs and the USEPA
whereby the Company agreed to pay a settlement amount of $353 with an initial
payment of $106 and the remaining $247 to be paid in installments over time as
the remediation proceeds. The Company has reserved for the unpaid
portion of the settlement and has entered into a letter of credit to guarantee
the payment obligation under the settlement.
Newark Bay Complex
Litigation
CasChem and Cosan have been named as
two of several hundred third-party defendants in a third-party complaint filed
in February 2009, by Maxus Energy Corporation (“Maxus”) and Tierra Solutions,
Inc. (“Tierra”). The original plaintiffs include the NJDEP, the
Commissioner of the NJDEP and the Administrator of the New Jersey Spill
Compensation Fund, which originally filed suit in 2005 against Maxus, Tierra and
other defendants seeking recovery of cleanup and removal costs for alleged
discharges of dioxin and other hazardous substances into the Passaic River,
Newark Bay, Hackensack River, Arthur Kill, Kill Van Kull and adjacent waters
(the “Newark Bay Complex”). Maxus and Tierra are now seeking
contribution from third-party defendants, including subsidiaries of the Company,
for cleanup and removal costs for which each may be held liable in the lawsuit.
Maxus and Tierra also seek recovery for cleanup and removal costs that each has
incurred or will incur relating to the Newark Bay Complex. The
Company expects to vigorously defend against the lawsuit. At this time it is too
early to predict whether the Company will have any liability in this
matter.
The Company is involved in other
environmental matters where the range of liability is not reasonably estimable
at this time and it is not determinable when information will become available
to provide a basis for adjusting or recording an accrual, should an accrual
ultimately be required.
Litigation and Other
Matters
Lorazepam
and Clorazepate
In 1998 the Company and a subsidiary
were named as defendants (along with Mylan Laboratories, Inc. (“Mylan”) and Gyma
in a proceeding instituted by the Federal Trade Commission (“FTC”) in the United
States District Court for the District of Columbia (the “District
Court”). Suits were also commenced by several State Attorneys’
General and class action complaints by private plaintiffs in various state
courts. The suits alleged violations of the Federal Trade Commission
Act arising from exclusive license agreements between the Company and Mylan
covering two APIs (Lorazepam and Clorazepate). The FTC and Attorneys’
General suits were settled in February 2001.
All cases
have been resolved except for one brought by four health care insurers. In 2008
the District Court, in this remaining case, entered judgment after trial against
Mylan, Gyma and Cambrex in the amount of $8,355, payable jointly and severally,
and also a punitive damage award against each defendant in the amount of
$16,709. In addition, the District Court ruled that the defendants were
also subject to a total of approximately $7,000 in prejudgment interest.
The parties will appeal the awards.
Cambrex
paid $12,415 in exchange for a release from Mylan and full indemnity in 2003
against future costs or liabilities in related litigation brought by purchasers,
as well as potential future claims related to this matter. Cambrex expects
any payment of the judgment against it to be made by Mylan under the indemnity
described above.
Other
The
Company has commitments incident to the ordinary course of business including
corporate guarantees of certain subsidiary obligations to the Company’s lenders
related to financial assurance obligations under certain environmental laws for
remediation; closure and third party liability requirements of certain of its
subsidiaries and a former operating location; contract provisions for
indemnification protecting its customers and suppliers against third party
liability for manufacture and sale of Company products that fail to meet product
warranties and contract provisions for indemnification protecting licensees
against intellectual property infringement related to licensed Company
technology or processes.
Additionally, as permitted under
Delaware law, the Company indemnifies its officers, directors and employees for
certain events or occurrences while the officer, director or employee is, or
was, serving at the Company’s request in such capacity. The term of the
indemnification period is for the officer's, director's or employee’s lifetime.
The maximum potential amount of future payments the Company could be required to
make under these indemnification agreements is unlimited; however, the Company
has a Director and Officer insurance policy that covers a portion of any
potential exposure. The Company currently believes the estimated fair
value of its indemnification agreements is not material based on currently
available information, and as such, the Company has no liabilities recorded for
these agreements as of December 31, 2009.
Cambrex's subsidiaries are party to a
number of other proceedings that are not considered material at this
time.
Impact
of Recent Accounting Pronouncements
Fair
Value Measurements
The Company adopted the Financial
Accounting Standards Board’s (“FASB”) Statement “Fair Value Measurements”
related to nonfinancial assets and nonfinancial liabilities effective January 1,
2009. This statement defines fair value, establishes a framework for
measuring fair value in GAAP, and expands disclosures about fair value
measurements. This statement applies whenever another standard
requires (or permits) assets or liabilities to be measured at fair
value. The standard does not expand the use of fair value to
any new circumstances. The effect of adopting this pronouncement did
not have a material impact on the Company’s financial position or results of
operations.
In January 2010, the FASB issued “Fair
Value Measurements and Disclosures - Improving Disclosures about Fair Value
Measurements”. This statement requires some new disclosures and
clarifies some existing disclosure requirements about fair value measurement as
set forth in FASB Statement “Fair Value Measurement”. The amendments
are effective for interim and annual reporting periods beginning after December
15, 2009, except for the disclosures about purchases, sales, issuances, and
settlements in the roll forward of activity in Level 3 fair value
measurements. Those disclosures are effective for fiscal years
beginning after December 15, 2010, and for interim periods within those fiscal
years. The effect of adopting this pronouncement will not have an
impact on the Company’s financial position or results of
operations.
Disclosures
about Derivative Instruments and Hedging Activities
The Company adopted the FASB’s
Statement “Disclosures about Derivative Instruments and Hedging Activities”
effective January 1, 2009. This statement requires enhanced
disclosures about derivative and hedging activities and thereby improves the
transparency of financial reporting. This statement also encourages,
but does not require, comparative disclosures for earlier periods at initial
adoption. The effect of adopting this pronouncement did not have an
impact on the Company’s financial position or results of
operations.
Employers’ Disclosures about
Postretirement Benefit Plan Assets
The Company adopted the FASB’s
statement “Employers’ Disclosures about Postretirement Benefit Plan Assets”
effective December 31, 2009. This statement provides guidance on
additional disclosures about plan assets of a defined benefit pension or other
postretirement plan. Upon initial application, the provisions of this
pronouncement are not required for earlier periods that are presented for
comparative purposes. The effect of adopting this pronouncement did not have an
impact on the Company’s financial position or results of
operations.
Subsequent Events
The Company adopted the FASB’s
Statement “Subsequent Events” effective June 30, 2009. This statement
establishes general standards of accounting for, and disclosure of, events that
occur after the balance sheet date but before financial statements are issued or
are available to be issued. This statement requires the disclosure of
the date through which an entity has evaluated subsequent events and the basis
for that date, that is, whether that date represents the date the financial
statements were issued or were available to be issued. The effect of
adopting this pronouncement did not have a material impact on the Company’s
financial position or results of operations.
FASB Accounting Standards Codification and the
Hierarchy of GAAP
The Company adopted the FASB’s
Statement “The FASB
Accounting Standards Codification and the
Hierarchy of Generally Accepted Accounting Principles” effective September 30,
2009. This statement provides for the FASB Accounting Standards
Codification to
become the single official source of authoritative, nongovernmental U.S.
GAAP. This statement does not change GAAP but reorganizes the
literature.
Measuring Liabilities at Fair
Value
The Company adopted the FASB’s update
“Fair Value Measurements and Disclosures —Measuring Liabilities at Fair
Value.” This update provides amendments to “Fair Value Measurements
and Disclosures – Overall”, for the fair value measurement of
liabilities. This update provides clarification for circumstances in
which a quoted price in an active market for the identical liability is not
available, how to estimate the fair value of a liability and how to determine
the quoted price. The amendments in this update reduce potential
ambiguity in financial reporting when measuring the fair value of
liabilities. The effect of adopting this pronouncement did not have a
material impact on the Company’s financial position or results of
operations.
Revenue Arrangements with Multiple
Deliverables
In September 2009, the Emerging Issues
Task Force (“EITF”) issued “Revenue Arrangements with Multiple
Deliverables.” This issue addresses how to determine whether an
arrangement involving multiple deliverables contains more than one unit of
accounting, and how to allocate the consideration to each unit of
accounting. This issue eliminates the use of the residual value
method for determining allocation of arrangement consideration; and allows the
use of an entity's best estimate to determine the selling price if vendor
specific objective evidence and third-party evidence can not be
determined. This issue also requires additional disclosure to provide
both qualitative and quantitative information regarding the significant
judgments made in applying this issue. In addition, for each
reporting period in the initial year of adoption, this issue requires disclosure
of the amount of revenue recognized subject to the measurement requirements of
this issue and the amount of revenue that would have been recognized if the
related transactions were subject to the measurement requirements of Issue
00-21. This issue is effective for revenue arrangements entered into
or materially modified in fiscal years beginning after June 15,
2010. Early adoption is permitted. The Company is
currently evaluating the potential impact of this issue.
_____________
(dollars
in thousands, except share data)
Forward-Looking
Statements
This document may contain
“forward-looking statements” within the meaning of the Private Securities
Litigation Reform Act of 1995 and Rule 3b-6 under The Securities Exchange Act of
1934, as amended, including, without limitation, statements regarding expected
performance, especially expectations with respect to sales, research and
development expenditures, earnings per share, capital expenditures,
acquisitions, divestitures, collaborations, or other expansion
opportunities. These statements may be identified by the fact that
they use words such as “expects,” “anticipates,” “intends,” “estimates,”
“believes” or similar expressions in connection with any discussion of future
financial and operating performance. Any forward-looking statements
are qualified in their entirety by reference to the factors discussed throughout
this Form 10-K. Any forward-looking statements contained herein are
based on current plans and expectations and involve risks and uncertainties that
could cause actual outcomes and results to differ materially from current
expectations including, but not limited to, global economic trends,
pharmaceutical outsourcing trends, competitive pricing or product developments,
government legislation and regulations (particularly environmental issues), tax
rate, interest rate, technology, manufacturing and legal issues, including the
outcome of outstanding litigation disclosed in the Company’s public filings, the
Company’s ability to satisfy the continued listing standards of the New York
Stock Exchange, changes in foreign exchange rates, uncollectable receivables,
loss on disposition of assets, cancellation or delays in renewal of contracts,
lack of suitable raw materials or packaging materials, the Company’s ability to
receive regulatory approvals for its products and other factors described under
the caption “Risk Factors That May Affect Future Results” in this Form
10-K. Any forward-looking statement speaks only as of the date on
which it is made, and the Company undertakes no obligation to publicly update
any forward-looking statement, whether as a result of new information, future
events or otherwise. New factors emerge from time to time and it is
not possible for the Company to predict which will arise. In
addition, the Company cannot assess the impact of each factor on the Company’s
business or the extent to which any factor, or combination of factors, may cause
actual results to differ materially from those contained in any forward-looking
statements.
|
Item 7a
|
Quantitative and Qualitative
Disclosures about Market
Risk
|
The information required in this
section can be found in the “Market Risks” section of Item 7 on page 27 of this
Form 10-K.
|
Item 8
|
Financial Statements and
Supplementary Data
|
The following consolidated financial
statements and selected quarterly financial data of the Company are filed under
this item:
|
Page
Number
|
|
|
(in this Report)
|
|
|
Reports
of Independent Registered Public Accounting Firm
|
34
|
|
Consolidated
Balance Sheets as of December 31, 2009 and 2008
|
36
|
|
Consolidated
Statements of Operations for the Years Ended December 31, 2009, 2008 and
2007
|
37
|
|
Consolidated
Statements of Stockholders' Equity for the Years Ended December 31, 2009,
2008 and 2007
|
38
|
|
Consolidated
Statements of Cash Flows for the Years Ended December 31, 2009, 2008 and
2007
|
39
|
|
Notes
to Consolidated Financial Statements
|
40
|
|
Selected
Quarterly Financial and Supplementary Data (unaudited)
|
71
|
The consolidated financial statements
and financial statement schedule are filed pursuant to Item 15 of this
report.
_____________
(dollars
in thousands, except share data)
Report
of Independent Registered Public Accounting Firm
To the
Board of Directors and Stockholders of Cambrex Corporation,
We have
audited the accompanying consolidated balance sheets of Cambrex Corporation as
of December 31, 2009 and 2008 and the related consolidated statements of
operations, stockholders’ equity, and cash flows for each of the three years in
the period ended December 31, 2009. In connection with our audits of the
financial statements, we have also audited the financial statement schedules
listed in the accompanying index. These financial statements and schedules are
the responsibility of the Company’s management. Our responsibility is to express
an opinion on these financial statements and schedules based on our
audits.
We
conducted our audits in accordance with the standards of the Public Company
Accounting Oversight Board (United States). Those standards require
that we plan and perform the audit to obtain reasonable assurance about whether
the financial statements are free of material misstatement. An audit also
includes examining, on a test basis, evidence supporting the amounts and
disclosures in the financial statements, assessing the accounting principles
used and significant estimates made by management, as well as evaluating the
overall presentation of the financial statements and schedules. We
believe that our audits provide a reasonable basis for our opinion.
In our
opinion, the consolidated financial statements referred to above present fairly,
in all material respects, the financial position of Cambrex Corporation at
December 31, 2009 and 2008, and the results of its operations and its cash flows
for each of the three years in the period ended December 31, 2009, in conformity with
accounting principles generally accepted in the United States of
America.
Also, in
our opinion, the financial statement schedules, when considered in relation to
the basic consolidated financial statements taken as a whole, present fairly, in
all material respects, the information set forth therein.
We also
have audited, in accordance with the standards of the Public Company Accounting
Oversight Board (United States), Cambrex Corporation’s internal control over
financial reporting as of December 31, 2009, based on criteria established in
Internal Control – Integrated
Framework issued by the Committee of Sponsoring Organizations of the
Treadway Commission (COSO) and our report dated February 11, 2010 expressed an
unqualified opinion thereon.
/s/ BDO
Seidman, LLP
Woodbridge,
NJ
February
11, 2010
Report
of Independent Registered Public Accounting Firm
To the
Board of Directors and Shareholders of Cambrex Corporation,
We have
audited Cambrex Corporation’s internal control over financial reporting as of
December 31, 2009, based on criteria established in Internal Control – Integrated
Framework issued by the Committee of Sponsoring Organizations of the
Treadway Commission (the COSO criteria). Cambrex Corporation’s management is
responsible for maintaining effective internal control over financial reporting
and for its assessment of the effectiveness of internal control over financial
reporting, included in the accompanying “Item 9A, Management’s Report on
Internal Control Over Financial Reporting”. Our responsibility is to express an
opinion on the company’s internal control over financial reporting based on our
audit.
We
conducted our audit in accordance with the standards of the Public Company
Accounting Oversight Board (United States). Those standards require that we plan
and perform the audit to obtain reasonable assurance about whether effective
internal control over financial reporting was maintained in all material
respects. Our audit included obtaining an understanding of internal control over
financial reporting, assessing the risk that a material weakness exists, and
testing and evaluating the design and operating effectiveness of internal
control based on the assessed risk. Our audit also included performing such
other procedures as we considered necessary in the circumstances. We believe
that our audit provides a reasonable basis for our opinion.
A
company’s internal control over financial reporting is a process designed to
provide reasonable assurance regarding the reliability of financial reporting
and the preparation of financial statements for external purposes in accordance
with generally accepted accounting principles. A company’s internal control over
financial reporting includes those policies and procedures that (1) pertain to
the maintenance of records that, in reasonable detail, accurately and fairly
reflect the transactions and dispositions of the assets of the company; (2)
provide reasonable assurance that transactions are recorded as necessary to
permit preparation of financial statements in accordance with generally accepted
accounting principles, and that receipts and expenditures of the company are
being made only in accordance with authorizations of management and directors of
the company; and (3) provide reasonable assurance regarding prevention or timely
detection of unauthorized acquisition, use, or disposition of the company’s
assets that could have a material effect on the financial
statements.
Because
of its inherent limitations, internal control over financial reporting may not
prevent or detect misstatements. Also, projections of any evaluation of
effectiveness to future periods are subject to the risk that controls may become
inadequate because of changes in conditions, or that the degree of compliance
with the policies or procedures may deteriorate.
In our
opinion, Cambrex Corporation maintained, in all material respects, effective
internal control over financial reporting as of December 31, 2009, based on the
COSO criteria.
We also
have audited, in accordance with the standards of the Public Company Accounting
Oversight Board (United States), the consolidated balance sheets of Cambrex
Corporation as of December 31, 2009 and 2008, and the related consolidated
statements of income, stockholders’ equity, and cash flows for each of the three
years in the period ended December 31, 2009 and our report dated February 11,
2010 expressed an unqualified opinion thereon.
/s/ BDO
Seidman, LLP
Woodbridge,
NJ
February
11, 2010
CAMBREX
CORPORATION AND SUBSIDIARIES
|
CONSOLIDATED
BALANCE SHEETS
|
||||||||
|
(dollars
in thousands, except share data)
|
||||||||
|
December 31,
|
||||||||
|
2009
|
2008
|
|||||||
|
ASSETS
|
||||||||
|
Current
assets:
|
||||||||
|
Cash
and cash equivalents
|
$ | 52,365 | $ | 32,540 | ||||
|
Trade
receivables, less allowances of $627 and $1,105 at respective
dates
|
32,025 | 36,685 | ||||||
|
Inventories,
net
|
58,369 | 61,133 | ||||||
|
Prepaid
expenses and other current assets
|
6,654 | 8,798 | ||||||
|
Total
current assets
|
149,413 | 139,156 | ||||||
|
Property,
plant and equipment, net
|
161,149 | 161,500 | ||||||
|
Goodwill
|
36,360 | 35,374 | ||||||
|
Other
non-current assets
|
4,593 | 5,042 | ||||||
|
Total
assets
|
$ | 351,515 | $ | 341,072 | ||||
|
LIABILITIES
AND STOCKHOLDERS' EQUITY
|
||||||||
|
Current
liabilities:
|
||||||||
|
Accounts
payable
|
$ | 17,038 | $ | 19,700 | ||||
|
Accrued
expense and other current liabilities
|
38,013 | 45,080 | ||||||
|
Total
current liabilities
|
55,051 | 64,780 | ||||||
|
Long-term
debt
|
120,800 | 123,800 | ||||||
|
Deferred
income tax
|
17,305 | 16,138 | ||||||
|
Accrued
pension and postretirement benefits
|
40,963 | 44,165 | ||||||
|
Other
non-current liabilities
|
14,126 | 17,403 | ||||||
|
Total
liabilities
|
248,245 | 266,286 | ||||||
|
Commitments
and contingencies (see Notes 17 and 18)
|
||||||||
|
Stockholders'
equity:
|
||||||||
|
Common
Stock, $.10 par value; authorized 100,000,000 issued 31,408,778 and
31,406,778 shares at respective dates
|
3,140 | 3,140 | ||||||
|
Additional
paid-in capital
|
100,497 | 99,881 | ||||||
|
Retained
earnings
|
22,345 | 11,960 | ||||||
|
Treasury
stock, at cost, 2,121,372 and 2,224,613 shares at respective
dates
|
(18,109 | ) | (19,014 | ) | ||||
|
Accumulated
other comprehensive loss
|
(4,603 | ) | (21,181 | ) | ||||
|
Total
stockholders' equity
|
103,270 | 74,786 | ||||||
|
Total
liabilities and stockholders' equity
|
$ | 351,515 | $ | 341,072 | ||||
See
accompanying notes to consolidated financial statements.
CAMBREX
CORPORATION AND SUBSIDIARIES
|
CONSOLIDATED
STATEMENTS OF OPERATIONS
|
||||||||||||
|
(dollars
in thousands, except share data)
|
||||||||||||
|
Years Ended December 31,
|
||||||||||||
|
2009
|
2008
|
2007
|
||||||||||
|
Gross
Sales
|
$ | 236,277 | $ | 249,618 | $ | 252,574 | ||||||
|
Allowances
and rebates
|
1,402 | 2,099 | 1,368 | |||||||||
|
Net
sales
|
234,875 | 247,519 | 251,206 | |||||||||
|
Other
revenues
|
(325 | ) | 1,709 | 1,299 | ||||||||
|
Net
revenues
|
234,550 | 249,228 | 252,505 | |||||||||
|
Cost
of goods sold
|
164,272 | 175,485 | 161,273 | |||||||||
|
Gross
profit
|
70,278 | 73,743 | 91,232 | |||||||||
|
Selling,
general and administrative expenses
|
35,711 | 40,521 | 48,858 | |||||||||
|
Research
and development expenses
|
7,929 | 7,590 | 12,157 | |||||||||
|
Restructuring
expenses
|
- | 4,695 | 6,073 | |||||||||
|
Strategic
alternative costs
|
- | 1,515 | 31,127 | |||||||||
|
Operating
profit/(loss)
|
26,638 | 19,422 | (6,983 | ) | ||||||||
|
Other
(income)/expenses
|
||||||||||||
|
Interest
income
|
(234 | ) | (802 | ) | (5,199 | ) | ||||||
|
Interest
expense
|
4,868 | 4,470 | 4,714 | |||||||||
|
Other
(income)/expenses, net
|
(641 | ) | 754 | 725 | ||||||||
|
Income/(loss)
before income taxes
|
22,645 | 15,000 | (7,223 | ) | ||||||||
|
Provision
for income taxes
|
12,253 | 7,071 | 6,288 | |||||||||
|
Income/(loss)
from continuing operations
|
10,392 | 7,929 | (13,511 | ) | ||||||||
|
Income
from discontinued operations, including gains from dispositions, net of
tax
|
- | - | 222,759 | |||||||||
|
Net
income
|
$ | 10,392 | $ | 7,929 | $ | 209,248 | ||||||
|
Basic
earnings/(loss) per share
|
||||||||||||
|
Income/(loss)
from continuing operations
|
$ | 0.36 | $ | 0.27 | $ | (0.47 | ) | |||||
|
Income
from discontinued operations, including gains from dispositions, net of
tax
|
$ | - | $ | - | $ | 7.77 | ||||||
|
Net
income
|
$ | 0.36 | $ | 0.27 | $ | 7.30 | ||||||
|
Diluted
earnings/(loss) per share
|
||||||||||||
|
Income/(loss)
from continuing operations
|
$ | 0.36 | $ | 0.27 | $ | (0.47 | ) | |||||
|
Income
from discontinued operations, including gains from dispositions, net of
tax
|
$ | - | $ | - | $ | 7.77 | ||||||
|
Net
income
|
$ | 0.36 | $ | 0.27 | $ | 7.30 | ||||||
|
Weighted average shares
outstanding:
|
||||||||||||
|
Basic
weighted average shares outstanding
|
29,241 | 29,116 | 28,683 | |||||||||
|
Effect
of dilutive stock options and restricted stock*
|
26 | 45 | - | |||||||||
|
Diluted
weighted average shares outstanding
|
29,267 | 29,161 | 28,683 | |||||||||
* For
2007, the effect of stock options and restricted stock would be anti-dilutive
and is therefore excluded.
See
accompanying notes to consolidated financial statements.
CAMBREX CORPORATION AND
SUBSIDIARIES
|
CONSOLIDATED
STATEMENTS OF STOCKHOLDERS' EQUITY
|
||||||||||||||||||||||||||||||||
|
(dollars
in thousands, except share data)
|
||||||||||||||||||||||||||||||||
|
|
Common
Stock
|
Additional
|
Accumulated
Other
|
Total
|
||||||||||||||||||||||||||||
|
Shares
Issued
|
Par
Value ($.10)
|
Paid-In
Capital
|
Retained
Earnings
|
Treasury
Stock
|
Comprehensive
(Loss)/Gain
|
Comprehensive
(Loss)/Income
|
Stockholders'
Equity
|
|||||||||||||||||||||||||
|
Balance
at December 31, 2006
|
30,188,017 | $ | 3,015 | $ | 241,360 | $ | 28,860 | $ | (20,832 | ) | $ | (5,757 | ) | $ | 246,646 | |||||||||||||||||
|
Comprehensive
income
|
||||||||||||||||||||||||||||||||
|
Net
income
|
209,248 | 209,248 | 209,248 | |||||||||||||||||||||||||||||
|
Other
comprehensive income/(loss)
|
||||||||||||||||||||||||||||||||
|
Foreign
currency translation adjustment
|
15,684 | |||||||||||||||||||||||||||||||
|
Unrealized
losses on hedging contracts, net of tax of $107
|
(1,385 | ) | ||||||||||||||||||||||||||||||
|
Pensions,
net of tax of $346
|
7,734 | |||||||||||||||||||||||||||||||
|
Reclass
adjustment for gain on marketable securities included in net earnings, net
of tax of $0
|
(1,117 | ) | ||||||||||||||||||||||||||||||
|
Other
comprehensive income
|
20,916 | 20,916 | 20,916 | |||||||||||||||||||||||||||||
|
Total
comprehensive income
|
$ | 230,164 | ||||||||||||||||||||||||||||||
|
Disposition
of business - pension
|
1,320 | 1,320 | ||||||||||||||||||||||||||||||
|
Cash
dividends and return of capital at $14.03 per share
|
(169,782 | ) | (234,077 | ) | (403,859 | ) | ||||||||||||||||||||||||||
|
Purchase
of treasury stock
|
(59 | ) | (59 | ) | ||||||||||||||||||||||||||||
|
Exercise
of stock options
|
1,175,101 | 121 | 21,777 | 21,898 | ||||||||||||||||||||||||||||
|
Deferred
compensation
|
8,771 | 1 | 207 | 62 | 270 | |||||||||||||||||||||||||||
|
Vested
restricted stock
|
27,811 | 3 | (446 | ) | 443 | - | ||||||||||||||||||||||||||
|
Stock
option modification
|
2,535 | 2,535 | ||||||||||||||||||||||||||||||
|
Stock
option expense
|
711 | 711 | ||||||||||||||||||||||||||||||
|
Restricted
stock expense
|
2,431 | 2,431 | ||||||||||||||||||||||||||||||
|
Balance
at December 31, 2007
|
31,399,700 | $ | 3,140 | $ | 98,793 | $ | 4,031 | $ | (20,386 | ) | $ | 16,479 | $ | 102,057 | ||||||||||||||||||
|
Comprehensive
loss
|
||||||||||||||||||||||||||||||||
|
Net
income
|
7,929 | 7,929 | 7,929 | |||||||||||||||||||||||||||||
|
Other
comprehensive loss
|
||||||||||||||||||||||||||||||||
|
Foreign
currency translation adjustment
|
(16,830 | ) | ||||||||||||||||||||||||||||||
|
Unrealized
losses on hedging contracts, net of tax of $322
|
(2,962 | ) | ||||||||||||||||||||||||||||||
|
Pensions,
net of tax of $145
|
(17,868 | ) | ||||||||||||||||||||||||||||||
|
Other
comprehensive loss
|
(37,660 | ) | (37,660 | ) | (37,660 | ) | ||||||||||||||||||||||||||
|
Total
comprehensive loss
|
$ | (29,731 | ) | |||||||||||||||||||||||||||||
|
Purchase
of treasury stock
|
(50 | ) | (50 | ) | ||||||||||||||||||||||||||||
|
Exercise
of stock options
|
2,301 | 18 | 18 | |||||||||||||||||||||||||||||
|
Deferred
compensation
|
4,777 | 59 | 170 | 229 | ||||||||||||||||||||||||||||
|
Vested
restricted stock
|
(1,252 | ) | 1,252 | - | ||||||||||||||||||||||||||||
|
Stock
option modification
|
102 | 102 | ||||||||||||||||||||||||||||||
|
Stock
option expense
|
582 | 582 | ||||||||||||||||||||||||||||||
|
Restricted
stock expense
|
1,545 | 1,545 | ||||||||||||||||||||||||||||||
|
Performance
stock expense
|
34 | 34 | ||||||||||||||||||||||||||||||
|
Balance
at December 31, 2008
|
31,406,778 | $ | 3,140 | $ | 99,881 | $ | 11,960 | $ | (19,014 | ) | $ | (21,181 | ) | $ | 74,786 | |||||||||||||||||
|
Comprehensive
income
|
||||||||||||||||||||||||||||||||
|
Net
income
|
10,392 | 10,392 | 10,392 | |||||||||||||||||||||||||||||
|
Other
comprehensive income
|
||||||||||||||||||||||||||||||||
|
Foreign
currency translation adjustment
|
9,819 | |||||||||||||||||||||||||||||||
|
Unrealized
gains on hedging contracts, net of tax of $304
|
2,450 | |||||||||||||||||||||||||||||||
|
Pensions,
net of tax of $204
|
4,309 | |||||||||||||||||||||||||||||||
|
Other
comprehensive income
|
16,578 | 16,578 | 16,578 | |||||||||||||||||||||||||||||
|
Total
comprehensive income
|
$ | 26,970 | ||||||||||||||||||||||||||||||
|
Adjustment
to cash dividend on restricted stock
|
(7 | ) | (7 | ) | ||||||||||||||||||||||||||||
|
Purchase
of treasury stock
|
(25 | ) | (25 | ) | ||||||||||||||||||||||||||||
|
Exercise
of stock options
|
2,000 | 9 | 9 | |||||||||||||||||||||||||||||
|
Deferred
compensation
|
(102 | ) | 264 | 162 | ||||||||||||||||||||||||||||
|
Vested
restricted stock
|
(666 | ) | 666 | - | ||||||||||||||||||||||||||||
|
Stock
option modification
|
94 | 94 | ||||||||||||||||||||||||||||||
|
Stock
option expense
|
554 | 554 | ||||||||||||||||||||||||||||||
|
Restricted
stock expense
|
658 | 658 | ||||||||||||||||||||||||||||||
|
Performance
stock expense
|
69 | 69 | ||||||||||||||||||||||||||||||
|
Balance
at December 31, 2009
|
31,408,778 | $ | 3,140 | $ | 100,497 | $ | 22,345 | $ | (18,109 | ) | $ | (4,603 | ) | $ | 103,270 | |||||||||||||||||
See
accompanying notes to consolidated financial statements.
CAMBREX
CORPORATION AND SUBSIDIARIES
|
CONSOLIDATED
STATEMENTS OF CASH FLOWS
|
||||||||||||
|
(dollars
in thousands)
|
||||||||||||
|
Years Ended December 31,
|
||||||||||||
|
2009
|
2008
|
2007
|
||||||||||
|
Cash
flows from operating activities:
|
||||||||||||
|
Net
income
|
$ | 10,392 | $ | 7,929 | $ | 209,248 | ||||||
|
Adjustments
to reconcile net income to cash flows:
|
||||||||||||
|
Depreciation
and amortization
|
20,505 | 21,055 | 19,878 | |||||||||
|
Increase
in inventory reserve
|
4,196 | 2,916 | 2,709 | |||||||||
|
Stock
based compensation included in net income
|
1,281 | 1,967 | 3,142 | |||||||||
|
Deferred
income tax provision
|
(287 | ) | (23 | ) | 4,209 | |||||||
|
Strategic
alternative and restructuring charges
|
- | 2,987 | 17,693 | |||||||||
|
Write-off
of debt origination fees
|
- | - | 841 | |||||||||
|
Stock
option modification
|
94 | 102 | 2,535 | |||||||||
|
Foreign
tax reserve
|
5,330 | - | - | |||||||||
|
Other
|
(450 | ) | 1,884 | 447 | ||||||||
|
Changes
in assets and liabilities:
|
||||||||||||
|
Trade
receivables
|
5,930 | 5,547 | (4,542 | ) | ||||||||
|
Inventories
|
712 | (8,612 | ) | (6,329 | ) | |||||||
|
Prepaid
expenses and other current assets
|
2,083 | 7,264 | 1,131 | |||||||||
|
Accounts
payable and other current liabilities
|
(13,038 | ) | (36,509 | ) | (17,919 | ) | ||||||
|
Other
non-current assets and liabilities
|
(2,356 | ) | (1,518 | ) | 550 | |||||||
|
Discontinued
operations:
|
||||||||||||
|
Gain
on sale of businesses
|
- | - | (235,489 | ) | ||||||||
|
Rutherford
settlement, net of tax
|
- | - | 4,172 | |||||||||
|
Changes
in operating assets and liabilities
|
- | - | (5,428 | ) | ||||||||
|
Other
non-cash charges
|
- | - | 2,359 | |||||||||
|
Net
cash provided by/(used in) operating activities
|
34,392 | 4,989 | (793 | ) | ||||||||
|
Cash
flows from investing activities:
|
||||||||||||
|
Capital
expenditures
|
(12,587 | ) | (29,378 | ) | (25,927 | ) | ||||||
|
Acquisition
of business, net of cash
|
- | (1,271 | ) | - | ||||||||
|
Other
investing activities
|
67 | 12 | 887 | |||||||||
|
Discontinued
operations:
|
||||||||||||
|
Capital
expenditures
|
- | - | (530 | ) | ||||||||
|
Proceeds
from sale of business
|
- | - | 466,277 | |||||||||
|
Other
investing activities
|
- | - | 11 | |||||||||
|
Net
cash (used in)/provided by investing activities
|
(12,520 | ) | (30,637 | ) | 440,718 | |||||||
|
Cash
flows from financing activities:
|
||||||||||||
|
Dividends
and return of capital
|
(889 | ) | - | (402,389 | ) | |||||||
|
Long-term
debt activity (including current portion):
|
||||||||||||
|
Borrowings
|
23,600 | 61,600 | 151,500 | |||||||||
|
Repayments
|
(26,600 | ) | (39,458 | ) | (208,755 | ) | ||||||
|
Proceeds
from stock options exercised
|
9 | 18 | 21,898 | |||||||||
|
Other
financing activities
|
(48 | ) | (50 | ) | (59 | ) | ||||||
|
Discontinued
operations:
|
||||||||||||
|
Long-term
debt activity (including current portion):
|
||||||||||||
|
Repayments
|
- | - | (254 | ) | ||||||||
|
Net
cash (used in)/provided by financing activities
|
(3,928 | ) | 22,110 | (438,059 | ) | |||||||
|
Effect
of exchange rate changes on cash and cash equivalents
|
1,881 | (2,410 | ) | 2,876 | ||||||||
|
Net
increase/(decrease) in cash and cash equivalents
|
19,825 | (5,948 | ) | 4,742 | ||||||||
|
Cash
and cash equivalents at beginning of year
|
32,540 | 38,488 | 33,746 | |||||||||
|
Cash
and cash equivalents at end of year
|
$ | 52,365 | $ | 32,540 | $ | 38,488 | ||||||
|
Supplemental
disclosure:
|
||||||||||||
|
Interest
paid, net of capitalized interest
|
$ | 4,906 | $ | 4,126 | $ | 5,003 | ||||||
|
Income
taxes paid
|
$ | 9,617 | $ | 10,342 | $ | 17,869 | ||||||
See
accompanying notes to consolidated financial statements.
CAMBREX
CORPORATION AND SUBSIDIARIES
NOTES
TO CONSOLIDATED FINANCIAL STATEMENTS
(dollars
in thousands, except share data)
|
(1)
|
The
Company
|
Cambrex Corporation and Subsidiaries
(the “Company” or “Cambrex”) primarily provides products and services worldwide
to pharmaceutical companies and generic drug companies. The Company
is dedicated to providing essential products and services to accelerate drug
discovery, development and manufacturing processes for human
therapeutics. The Company’s products consist of active pharmaceutical
ingredients (“APIs”) and pharmaceutical intermediates produced under Food and
Drug Administration current Good Manufacturing Practices for use in the
production of prescription and over-the-counter drug products and other fine
custom chemicals derived from organic chemistry. Cambrex has three
operating segments, which are manufacturing facilities, that have been
aggregated as one reportable segment.
In February 2007, the Company completed
the sale of the businesses that comprised the Bioproducts and Biopharma segments
(excluding certain liabilities) for total cash consideration of $463,914,
including working capital adjustments. As a result of this
transaction, the Company reported a gain of $235,489 in 2007 and all periods
presented reflect the results of these businesses as discontinued
operations. Refer to Note 19 for a complete discussion of
discontinued operations.
Interest
expense was allocated to discontinued operations based upon net assets
consistent with the EITF’s “Allocations of Interest on Discontinued
Operations.”
The Company has evaluated subsequent
events through the issuance date of this Form 10-K.
|
(2)
|
Summary
of Significant Accounting Policies
|
Principles of
Consolidation
The consolidated financial statements
include the accounts of the Company and its wholly-owned
subsidiaries. All significant inter-company balances and transactions
have been eliminated in consolidation.
Cash Equivalents
Temporary cash investments with an
original maturity of less than three months are considered cash
equivalents. The carrying amounts approximate fair
value.
Allowance for Doubtful
Accounts
The Company maintains allowances for
doubtful accounts relating to estimated losses resulting from customers being
unable to make required payments. Allowances for doubtful accounts
are based on historical experience and known factors regarding specific
customers and the industries in which those customers operate. If the
financial condition of the Company's customers were to deteriorate,
resulting in their ability to make payments being impaired, additional
allowances would be required.
Concentrations
of credit risk
Financial instruments that potentially
subject the Company to concentrations of credit risk consist principally of cash
and equivalents and accounts receivable. The Company maintains cash and
equivalents with high quality financial institutions. Concentrations of credit
risk with respect to accounts receivable are limited due to the Company's large
number of customers and their dispersion throughout the world.
CAMBREX
CORPORATION AND SUBSIDIARIES
NOTES
TO CONSOLIDATED FINANCIAL STATEMENTS - (Continued)
(dollars
in thousands, except share data)
|
(2)
|
Summary
of Significant Accounting Policies
(continued)
|
Derivative Instruments
Derivative financial instruments are
used by the Company primarily for hedging purposes to mitigate a variety of
working capital, investment and borrowing risks. The Company
primarily uses foreign currency forward contracts to minimize foreign currency
exchange rate risk associated with foreign currency
transactions. Gains and losses on these hedging transactions are
generally recorded in earnings in the same period as they are realized, which is
usually the same period as the settlement of the underlying
transactions. The Company uses interest rate swap instruments only as
hedges or as an integral part of borrowing. As such, the differential
to be paid or received in connection with these instruments is accrued and
recognized in income as an adjustment to interest expense.
The Company formally documents all
relationships between hedging instruments and hedged items, as well as its risk
management objectives and strategies for undertaking various hedging
relationships. All cash flow hedges are linked to transactions and
the Company assesses effectiveness at inception and on a quarterly
basis. If it is determined that a derivative instrument is not highly
effective or the transaction is no longer deemed probable of occurring, the
Company discontinues hedge accounting and recognizes the ineffective portion in
current period earnings.
Inventories
Inventories are stated at the lower of
cost, determined on a first-in, first-out basis, or market. The
determination of market value involves assessment of numerous factors, including
costs to dispose of inventory and estimated selling prices. Reserves
are recorded to reduce carrying value for inventory determined to be damaged,
obsolete or otherwise unsaleable.
Property, Plant and
Equipment
Property, plant and equipment is stated
at cost, net of accumulated depreciation. Plant and equipment are
depreciated on a straight-line basis over the estimated useful lives for each
applicable asset group as follows:
|
Buildings
and improvements
|
20
to 30 years, or term of lease if applicable
|
|
Machinery
and equipment
|
7
to 15 years
|
|
Furniture
and fixtures
|
5
to 7 years
|
|
Computer
hardware and software
|
3
to 7 years
|
Expenditures for additions, major
renewals or betterments are capitalized and expenditures for maintenance and
repairs are charged to income as incurred.
When assets are retired or otherwise
disposed of, the cost and related accumulated depreciation are removed from the
accounts, and any resulting gain or loss is reflected in operating
expenses. Interest is capitalized in connection with the construction
and acquisition of assets that are capitalized over longer periods of time for
larger amounts. The capitalized interest is recorded as part of the
cost of the asset to which it relates and is amortized over the asset’s
estimated useful life. Total interest capitalized in connection with
ongoing construction activities in 2009, 2008 and 2007 amounted to $677, $2,032
and $1,123, respectively.
Impairment of Goodwill
The Company reviews the carrying value
of goodwill to determine whether impairment may exist on an annual basis or
whenever it has reason to believe goodwill may not be
recoverable. The annual impairment test of goodwill is performed
during the fourth quarter of each fiscal year. The Company did not have a
goodwill impairment for the years ended December 31, 2009, 2008 and
2007.
CAMBREX
CORPORATION AND SUBSIDIARIES
NOTES
TO CONSOLIDATED FINANCIAL STATEMENTS - (Continued)
(dollars
in thousands, except share data)
|
(2)
|
Summary
of Significant Accounting Policies
(continued)
|
Goodwill impairment is determined using
a two-step process. The first step of the goodwill impairment test is
used to identify potential impairment by comparing the fair value of each
reporting unit, determined using various valuation techniques, with the primary
technique being a discounted cash flow analysis, to its carrying
value. If the fair value of a reporting unit exceeds its carrying
amount, goodwill of the reporting unit is considered not impaired and the second
step of the impairment test is unnecessary. If the carrying amount of
a reporting unit exceeds its fair value, the second step of the goodwill
impairment test is performed to measure the amount of impairment loss, if
any. The second step of the goodwill impairment test compares the
implied fair value of the reporting unit’s goodwill with the carrying amount of
that goodwill. If the carrying amount of the reporting unit’s
goodwill exceeds the implied fair value of that goodwill, an impairment loss is
recognized in an amount equal to that excess.
The impairment test for other
intangible assets not subject to amortization consists of a comparison of the
fair value of the intangible asset with its carrying value. If the
carrying value of the intangible asset exceeds its fair value, an impairment
loss is recognized in an amount equal to that excess.
Impairment of Long-Lived
Assets
The Company assesses the impairment of
its long-lived assets, including amortizable intangible assets, and property,
plant and equipment, whenever economic events or changes in circumstances
indicate that the carrying amounts of the assets may not be
recoverable. Long lived assets are considered to be impaired when the
sum of the undiscounted expected future operating cash flows is less than the
carrying amounts of the related assets. If impaired, the assets are
written down to fair market value.
Revenue Recognition
Revenues are generally recognized when
title to products and risk of loss are transferred to customers.
Additional conditions for recognition of revenue are that collection of sales
proceeds is reasonably assured and the Company has no further performance
obligations.
The
Company has certain contracts that contain multiple deliverables. These
deliverables often include process development services and commercial
production and are divided into separate units of accounting if certain criteria
are met, including whether the delivered element has stand-alone value to the
customer and whether there is objective and reliable evidence of the fair value
of the undelivered items. The consideration the Company receives is
allocated among the separate units based on their respective fair values, and
the applicable revenue recognition criteria are applied to each of the separate
units.
For contracts that contain
milestone-based payments, the Company recognizes revenue using the proportional
performance method based on the percentage of costs incurred relative to the
total costs estimated to be incurred to complete the contract. Revenue
recognition computed under this methodology is compared to the amount of
non-refundable cash payments received or contractually receivable at the
reporting date and the lesser of the two amounts is recognized as revenue at
each reporting date. The proportional performance methodology applied
by the Company for revenue recognition, utilizes an input based measure,
specifically labor costs, because the Company believes the use of an input
measure is a better surrogate of proportional performance than an output based
measure, such as milestones.
Amounts billed in advance are recorded
as deferred revenue on the balance sheet. Since payments received are typically
non-refundable, the termination of a contract by a customer prior to its
completion could result in an immediate recognition of deferred revenue relating
to payments already received not previously recognized as
revenue.
CAMBREX
CORPORATION AND SUBSIDIARIES
NOTES
TO CONSOLIDATED FINANCIAL STATEMENTS - (Continued)
(dollars
in thousands, except share data)
|
(2)
|
Summary
of Significant Accounting Policies
(continued)
|
Sales terms to certain customers
include rebates if certain conditions are met. Additionally, sales are
generally made with a limited right of return under certain conditions.
The Company estimates these rebates and returns at the time of sale based on the
terms of agreements with customers and historical experience and recognizes
revenue net of these estimated costs which are classified as allowances and
rebates.
The Company bills a portion of freight
cost incurred on shipments to customers. Freight costs are reflected
in cost of goods sold. Amounts billed to customers are recorded within net
revenues.
Income Taxes
The Company and its eligible
subsidiaries file a consolidated U.S. income tax return. Certain
subsidiaries which are consolidated for financial reporting are not eligible to
be included in the consolidated U.S. income tax return. Cambrex has not
provided U.S. federal income and withholding taxes on its undistributed earnings
from foreign operations as of December 31, 2009 because it intends to reinvest
such earnings indefinitely outside of the United States. If Cambrex
were to distribute these earnings, it is anticipated that foreign tax credits
would be available under current law to significantly reduce or eliminate the
resulting U.S. income tax liability. Determination of the amount of
unrecognized deferred tax related to these earnings is not
practical.
Use of Estimates
The preparation of financial statements
in conformity with U.S. GAAP requires management to make estimates and
assumptions that affect the reported amounts of assets and liabilities and
disclosure of contingent assets and liabilities at the date of the financial
statements and the reported amounts of revenues and expenses during the
reporting period. Actual results could differ from those
estimates.
Environmental Costs
The Company is subject to extensive and
changing federal, state, local and foreign environmental laws and regulations,
and has made provisions for the estimated financial impact of environmental
cleanup related costs. The Company’s policy is to accrue
environmental cleanup related costs of a non-capital nature, including estimated
litigation costs, when those costs are believed to be probable and can be
reasonably estimated. The quantification of environmental exposures
requires an assessment of many factors, including changing laws and regulations,
advancements in environmental technologies, the quality of information available
related to specific sites, the assessment stage of each site investigation,
preliminary findings and the length of time involved in remediation or
settlement. Such accruals are adjusted as further information
develops or circumstances change. For certain matters, the Company
expects to share costs with other parties. Costs of future
expenditures for environmental remediation obligations are not discounted to
their present value unless the aggregate amount of the liability and the timing
of cash payments are fixed or reasonably determinable. Recoveries of
environmental remediation costs from other parties are recorded as assets when
their receipt is deemed certain.
Foreign Currency
The functional currency of the
Company's foreign subsidiaries is the applicable local currency. The
translation of the applicable foreign currencies into U.S. dollars is performed
for balance sheet accounts using current exchange rates in effect at the balance
sheet date and for revenue and expense accounts and cash flows using average
rates of exchange prevailing during the year. Adjustments resulting
from the translation of foreign currency financial statements are accumulated in
a separate component of stockholders' equity until the entity is sold or
substantially liquidated. Gains or losses relating to transactions of
a long-term investment nature are accumulated in stockholders'
equity. Gains or losses resulting from foreign currency transactions
are included in the results of operations as a component of other revenues in
the consolidated income statement. Foreign currency net transaction
(losses)/gains were ($1,006), $1,183 and $260 in 2009, 2008 and 2007,
respectively.
CAMBREX
CORPORATION AND SUBSIDIARIES
NOTES
TO CONSOLIDATED FINANCIAL STATEMENTS - (Continued)
(dollars
in thousands, except share data)
|
(2)
|
Summary
of Significant Accounting Policies
(continued)
|
Earnings per Common Share
All diluted earnings per share are
computed on the basis of the weighted average shares of common stock outstanding
plus common equivalent shares arising from the effect of dilutive stock options
and restricted stock units, using the treasury stock method.
For the years ended December 31, 2009,
2008 and 2007, shares of 2,106,556, 1,648,193, and 1,171,895, respectively, were
not included in the calculation of diluted shares outstanding because the effect
would be anti-dilutive.
Comprehensive Loss
Included within accumulated other
comprehensive loss for the Company are; foreign currency translation
adjustments, changes in the fair value related to derivative instruments
classified as cash flow hedges, net of related tax benefit and changes in the
pensions, net of tax. Total comprehensive income/(loss) for the years
ended December 31, 2009 and 2008 are included in the Statements of Stockholders’
Equity.
The components of accumulated other
comprehensive loss in stockholders’ equity are as follows:
|
2009
|
2008
|
|||||||
|
Foreign
currency translation
|
$ | 16,029 | $ | 6,210 | ||||
|
Unrealized
loss on hedging contracts, net of tax
|
(1,806 | ) | (4,256 | ) | ||||
|
Pensions,
net of tax
|
(18,826 | ) | (23,135 | ) | ||||
|
Total
|
$ | (4,603 | ) | $ | (21,181 | ) | ||
|
(3)
|
Impact
of Recently Issued Accounting
Pronouncements
|
Fair
Value Measurements
The Company adopted the Financial
Accounting Standards Board’s (“FASB”) Statement “Fair Value Measurements”
related to nonfinancial assets and nonfinancial liabilities effective January 1,
2009. This statement defines fair value, establishes a framework for
measuring fair value in GAAP, and expands disclosures about fair value
measurements. This statement applies whenever another standard
requires (or permits) assets or liabilities to be measured at fair
value. The standard does not expand the use of fair value to
any new circumstances. The effect of adopting this pronouncement did
not have a material impact on the Company’s financial position or results of
operations.
In January 2010, the FASB issued “Fair
Value Measurements and Disclosures - Improving Disclosures about Fair Value
Measurements”. This statement requires some new disclosures and
clarifies some existing disclosure requirements about fair value measurement as
set forth in FASB Statement “Fair Value Measurement”. The amendments
are effective for interim and annual reporting periods beginning after December
15, 2009, except for the disclosures about purchases, sales, issuances, and
settlements in the roll forward of activity in Level 3 fair value
measurements. Those disclosures are effective for fiscal years
beginning after December 15, 2010, and for interim periods within those fiscal
years. The effect of adopting this pronouncement will not have an
impact on the Company’s financial position or results of
operations.
CAMBREX
CORPORATION AND SUBSIDIARIES
NOTES
TO CONSOLIDATED FINANCIAL STATEMENTS - (Continued)
(dollars
in thousands, except share data)
|
(3)
|
Impact
of Recently Issued Accounting Pronouncements
(continued)
|
Disclosures
about Derivative Instruments and Hedging Activities
The Company adopted the FASB’s
Statement “Disclosures about Derivative Instruments and Hedging Activities”
effective January 1, 2009. This statement requires enhanced
disclosures about derivative and hedging activities and thereby improves the
transparency of financial reporting. This statement also encourages,
but does not require, comparative disclosures for earlier periods at initial
adoption. The effect of adopting this pronouncement did not have an
impact on the Company’s financial position or results of
operations.
Employers’
Disclosures about Postretirement Benefit Plan Assets
The Company adopted the FASB’s
statement “Employers’ Disclosures about Postretirement Benefit Plan Assets”
effective December 31, 2009. This statement provides guidance on
additional disclosures about plan assets of a defined benefit pension or other
postretirement plan. Upon initial application, the provisions of this
pronouncement are not required for earlier periods that are presented for
comparative purposes. The effect of adopting this pronouncement did not have an
impact on the Company’s financial position or results of
operations.
Subsequent Events
The Company adopted the FASB’s
Statement “Subsequent Events” effective June 30, 2009. This statement
establishes general standards of accounting for, and disclosure of, events that
occur after the balance sheet date but before financial statements are issued or
are available to be issued. This statement requires the disclosure of
the date through which an entity has evaluated subsequent events and the basis
for that date, that is, whether that date represents the date the financial
statements were issued or were available to be issued. The effect of
adopting this pronouncement did not have a material impact on the Company’s
financial position or results of operations.
FASB Accounting Standards Codification and the
Hierarchy of GAAP
The Company adopted the FASB’s
Statement “The FASB
Accounting Standards Codification and the
Hierarchy of Generally Accepted Accounting Principles” effective September 30,
2009. This statement provides for the FASB Accounting Standards
Codification to
become the single official source of authoritative, nongovernmental U.S.
GAAP. This statement does not change GAAP but reorganizes the
literature.
Measuring Liabilities at Fair
Value
The Company adopted the FASB’s update
“Fair Value Measurements and Disclosures —Measuring Liabilities at Fair
Value.” This update provides amendments to “Fair Value Measurements
and Disclosures – Overall”, for the fair value measurement of
liabilities. This update provides clarification for circumstances in
which a quoted price in an active market for the identical liability is not
available, how to estimate the fair value of a liability and how to determine
the quoted price. The amendments in this update reduce potential
ambiguity in financial reporting when measuring the fair value of
liabilities. The effect of adopting this pronouncement did not have a
material impact on the Company’s financial position or results of
operations.
CAMBREX
CORPORATION AND SUBSIDIARIES
NOTES
TO CONSOLIDATED FINANCIAL STATEMENTS - (Continued)
(dollars
in thousands, except share data)
|
(3)
|
Impact
of Recently Issued Accounting Pronouncements
(continued)
|
Revenue Arrangements with Multiple
Deliverables
In September 2009, the Emerging Issues
Task Force (“EITF”) issued “Revenue Arrangements with Multiple
Deliverables.” This issue addresses how to determine whether an
arrangement involving multiple deliverables contains more than one unit of
accounting, and how to allocate the consideration to each unit of
accounting. This issue eliminates the use of the residual value
method for determining allocation of arrangement consideration; and allows the
use of an entity's best estimate to determine the selling price if vendor
specific objective evidence and third-party evidence can not
be determined. This issue also requires additional disclosure to
provide both qualitative and quantitative information regarding the significant
judgments made in applying this issue. In addition, for each
reporting period in the initial year of adoption, this issue requires disclosure
of the amount of revenue recognized subject to the measurement requirements of
this issue and the amount of revenue that would have been recognized if the
related transactions were subject to the measurement requirements of Issue
00-21. This issue is effective for revenue arrangements entered into
or materially modified in fiscal years beginning after June 15,
2010. Early adoption is permitted. The Company is
currently evaluating the potential impact of this issue.
|
(4)
|
Goodwill
|
The changes in the carrying amount of
goodwill for the years ended December 31, 2009 and 2008 are as
follows:
|
Balance
as of January 1, 2008
|
$ | 35,552 | ||
|
Acquisition
of business
|
1,489 | |||
|
Translation
effect
|
(1,667 | ) | ||
|
Balance
as of December 31, 2008
|
35,374 | |||
|
Translation
effect
|
986 | |||
|
Balance
as of December 31, 2009
|
$ | 36,360 |
|
(5)
|
Net
Inventories
|
Inventories
are stated at the lower of cost, determined on a first-in, first-out basis, or
market.
Net
inventories consist of the following:
|
December 31,
|
||||||||
|
2009
|
2008
|
|||||||
|
Finished
goods
|
$ | 26,549 | $ | 24,657 | ||||
|
Work
in process
|
18,361 | 22,372 | ||||||
|
Raw
materials
|
9,887 | 10,688 | ||||||
|
Supplies
|
3,572 | 3,416 | ||||||
|
Total
|
$ | 58,369 | $ | 61,133 | ||||
The
components of inventory stated above are net of reserves of $11,947 and $9,753
as of December 31, 2009 and 2008, respectively.
CAMBREX
CORPORATION AND SUBSIDIARIES
NOTES
TO CONSOLIDATED FINANCIAL STATEMENTS - (Continued)
(dollars
in thousands, except share data)
|
(6)
|
Property,
plant and equipment
|
Property,
plant and equipment consist of the following:
|
December 31,
|
||||||||
|
2009
|
2008
|
|||||||
|
Land
|
$ | 4,219 | $ | 4,127 | ||||
|
Buildings
and improvements
|
90,072 | 83,939 | ||||||
|
Machinery
and equipment
|
325,322 | 282,119 | ||||||
|
Furniture
and fixtures
|
1,867 | 1,954 | ||||||
|
Construction
in progress
|
10,999 | 31,451 | ||||||
|
Total
|
432,479 | 403,590 | ||||||
|
Accumulated
depreciation
|
(271,330 | ) | (242,090 | ) | ||||
|
Net
|
$ | 161,149 | $ | 161,500 | ||||
Depreciation expense was $20,501,
$21,051 and $19,799 for the years ended December 31, 2009, 2008 and 2007,
respectively. Total capital expenditures in 2009 were
$12,587.
|
(7)
|
Accrued
Expense and Other Current
Liabilities
|
The components of accrued expenses and
other current liabilities are as follows:
|
December 31,
|
||||||||
|
2009
|
2008
|
|||||||
|
Salaries
and employee benefits payable
|
$ | 14,817 | $ | 12,369 | ||||
|
Taxes
payable and related reserves
|
6,827 | 1,948 | ||||||
|
Restructuring
and strategic alternatives
|
4,412 | 8,131 | ||||||
|
Deferred
revenue
|
3,224 | 4,426 | ||||||
|
Hedges
payable
|
2,038 | 5,027 | ||||||
|
Commissions
|
1,609 | 3,759 | ||||||
|
Other
|
5,086 | 9,420 | ||||||
|
Total
|
$ | 38,013 | $ | 45,080 | ||||
|
(8)
|
Income
Taxes
|
Income/(loss)
before income taxes consist of the following:
|
December 31,
|
||||||||||||
|
2009
|
2008
|
2007
|
||||||||||
|
Domestic
|
$ | (1,272 | ) | $ | (15,756 | ) | $ | (48,634 | ) | |||
|
International
|
23,917 | 30,756 | 41,411 | |||||||||
|
Total
|
$ | 22,645 | $ | 15,000 | $ | (7,223 | ) | |||||
CAMBREX
CORPORATION AND SUBSIDIARIES
NOTES
TO CONSOLIDATED FINANCIAL STATEMENTS - (Continued)
(dollars
in thousands, except share data)
|
(8)
|
Income
Taxes (continued)
|
The
provision for income taxes consist of the following
provisions/(benefits):
|
December 31,
|
||||||||||||
|
2009
|
2008
|
2007
|
||||||||||
|
Current:
|
||||||||||||
|
Federal
|
$ | (240 | ) | $ | (897 | ) | $ | (8,317 | ) | |||
|
State
|
86 | 120 | 380 | |||||||||
|
International
|
12,694 | 7,871 | 10,016 | |||||||||
| 12,540 | 7,094 | 2,079 | ||||||||||
|
Deferred:
|
||||||||||||
|
Federal
|
$ | 204 | $ | 204 | $ | 172 | ||||||
|
International
|
(491 | ) | (227 | ) | 4,037 | |||||||
| (287 | ) | (23 | ) | 4,209 | ||||||||
|
Total
|
$ | 12,253 | $ | 7,071 | $ | 6,288 | ||||||
The
provision for income taxes differs from the statutory federal income tax rate of
35% for 2009, 2008 and 2007 as follows:
|
December 31,
|
||||||||||||
|
2009
|
2008
|
2007
|
||||||||||
|
Income
tax provision/(benefit) at U.S federal statutory rate
|
$ | 7,926 | $ | 5,250 | $ | (2,528 | ) | |||||
|
State
and local taxes, net of federal income tax benefits
|
30 | 33 | 73 | |||||||||
|
Effect
of foreign income taxed at rates other than the U.S. federal statutory
rate
|
(962 | ) | (2,744 | ) | (27 | ) | ||||||
|
Disallowed
compensation
|
- | - | 6,711 | |||||||||
|
Foreign
income inclusions
|
- | - | 2,361 | |||||||||
|
Tax
credits
|
(135 | ) | (788 | ) | - | |||||||
|
Tax
benefit from income from discontinued operations
|
- | - | (7,915 | ) | ||||||||
|
Indefinite-lived
intangibles
|
204 | 204 | 172 | |||||||||
|
Adjustments
for prior years' taxes
|
5,006 | (562 | ) | (536 | ) | |||||||
|
Net
change in valuation allowance
|
103 | 5,537 | 7,816 | |||||||||
|
Other
|
81 | 141 | 161 | |||||||||
|
Total
|
$ | 12,253 | $ | 7,071 | $ | 6,288 | ||||||
Disallowed
compensation represents the tax effects of change-in-control payments made to
certain executives as a result of the sale of the businesses that comprised the
Bioproducts and Biopharma segments. See Note 19. The tax
benefits for these payments were permanently disallowed for U.S. federal and
state income tax purposes. Tax benefit from income of discontinued operations
represents the tax benefit of domestic losses in continuing operations that were
recognized for accounting purposes due to domestic income reported within
discontinued operations. Adjustments for prior year’s taxes include
tax expense of approximately $5,300, including interest and penalties of
approximately $2,400, for an estimate of an international tax liability related
to a 2003 transaction.
CAMBREX
CORPORATION AND SUBSIDIARIES
NOTES
TO CONSOLIDATED FINANCIAL STATEMENTS - (Continued)
(dollars
in thousands, except share data)
|
(8)
|
Income
Taxes (continued)
|
The
components of deferred tax assets and liabilities as of December 31, 2009 and
2008 relate to temporary differences and carryforwards as follows:
|
December 31,
|
||||||||
|
2009
|
2008
|
|||||||
|
Current
deferred tax assets:
|
||||||||
|
Inventory
|
$ | 2,445 | $ | 1,266 | ||||
|
Receivables
|
18 | 106 | ||||||
|
Legal
and related reserves
|
856 | 1,737 | ||||||
|
Other
|
29 | 955 | ||||||
|
Current
deferred tax assets
|
3,348 | 4,064 | ||||||
|
Valuation
allowances
|
(3,038 | ) | (3,616 | ) | ||||
|
Total
current deferred tax assets
|
$ | 310 | $ | 448 | ||||
|
Current
deferred tax liabilities:
|
||||||||
|
Other
|
$ | 163 | $ | 116 | ||||
|
Total
current deferred tax liabilities
|
$ | 163 | $ | 116 | ||||
|
December 31,
|
||||||||
|
2009
|
2008
|
|||||||
|
Non-current
deferred tax assets:
|
||||||||
|
Foreign
tax credit carryforwards
|
$ | 54,869 | $ | 50,523 | ||||
|
Environmental
|
1,620 | 1,689 | ||||||
|
Net
operating loss carryforwards (domestic)
|
3,135 | 2,661 | ||||||
|
Net
operating loss carryforwards (foreign)
|
201 | 227 | ||||||
|
Employee
benefits
|
12,857 | 14,143 | ||||||
|
Restructuring
|
516 | 1,172 | ||||||
|
Research
& experimentation tax credit carryforwards
|
1,019 | 1,309 | ||||||
|
Alternative
minimum tax credit carryforwards
|
3,266 | 3,266 | ||||||
|
Property,
plant and equipment
|
3,473 | 1,187 | ||||||
|
Other
|
3,515 | 4,714 | ||||||
|
Non-current
deferred tax assets
|
84,471 | 80,891 | ||||||
|
Valuation
allowances *
|
(77,330 | ) | (75,614 | ) | ||||
|
Total
non-current deferred tax assets
|
7,141 | 5,277 | ||||||
|
Non-current
deferred tax liabilities:
|
||||||||
|
Property,
plant and equipment
|
9,094 | 6,750 | ||||||
|
Intangibles
|
8,104 | 7,488 | ||||||
|
Indefinite-lived
intangibles
|
1,940 | 1,736 | ||||||
|
Foreign
tax allocation reserve
|
5,308 | 5,441 | ||||||
|
Total
non-current deferred tax liabilities
|
$ | 24,446 | $ | 21,415 | ||||
|
Total
net non-current deferred tax liabilities
|
$ | 17,305 | $ | 16,138 | ||||
*In
addition to the effect of the domestic and foreign valuation allowances
reflected in the current effective tax rate, the valuation allowance has changed
due to currency translation adjustments.
CAMBREX
CORPORATION AND SUBSIDIARIES
NOTES
TO CONSOLIDATED FINANCIAL STATEMENTS - (Continued)
(dollars
in thousands, except share data)
|
(8)
|
Income
Taxes (continued)
|
The Company establishes a valuation
allowance against deferred tax assets when it is more likely than not that the
Company will be unable to realize those deferred tax assets in the future.
Based on the Company’s current and past performance, cumulative losses in recent
years resulting from domestic operations, the market environment in which the
Company operates, and the utilization of past tax attributes, the Company has
established a valuation allowance of $80,157 against a portion of its domestic
deferred tax assets. However, the Company has not recorded a
valuation allowance against domestic deferred tax assets which are offset by
domestic deferred tax liabilities that are expected to reverse in the
future. With respect to the Company’s foreign deferred tax assets, the
Company has recorded a valuation allowance of $211 as of December 31,
2009.
The Company expects to maintain a full
valuation allowance against its net domestic deferred tax assets (primarily
foreign tax credits), subject to the consideration of all prudent and feasible
tax planning strategies, until such time as the Company attains an appropriate
level of future domestic profitability and the Company is able to conclude that
it is more likely than not that its domestic deferred tax assets are
realizable.
The domestic valuation allowance for
the years ended December 31, 2009, 2008 and 2007 increased by $1,168, increased
by $15,095 and decreased by $26,506, respectively. The 2009 increase in the
domestic valuation allowance was allocated as follows: The valuation allowance
was increased by $130 for domestic losses, and increased by a net amount of
$1,038 for prior year deferred tax amounts and domestic gains and losses
included in other comprehensive income. The 2008 increase in the domestic
valuation allowance was allocated as follows: The valuation allowance
was increased by $4,469 for domestic losses and increased by $10,626 for
domestic gains and losses included in other comprehensive loss. The 2007
decrease in the domestic valuation allowance was allocated as follows: The
valuation allowance was increased by a net amount of $10,354 for domestic losses
in continuing operations and agreed tax audit adjustments for prior year
deferred tax amounts, decreased by $31,584 for discontinued operations, and
decreased by $5,276 for domestic gains and losses included in other
comprehensive income.
The foreign valuation allowance for the
years ended December 31, 2009, 2008 and 2007 decreased by $30, $707 and $55,
respectively. The 2009 decrease in the foreign valuation allowance
was allocated as follows: The valuation allowance was decreased $27 for foreign
income and decreased by $3 for prior year deferred tax amounts and currency
translation adjustments included in other comprehensive income. The
2008 decrease in the foreign valuation allowance was allocated as follows: The
valuation allowance was decreased by $707 for foreign income. The 2007 decrease
in the foreign valuation allowance was allocated as follows: The valuation
allowance was decreased by $10 for foreign income in continuing operations, and
decreased by $45 for currency translation adjustments included in other
comprehensive income.
Under the tax laws of the various
jurisdictions in which the Company operates, NOLs may be carried forward or
back, subject to statutory limitations, to reduce taxable income in future or
prior years. The domestic federal NOLs total approximately $3,841 and will
expire in 2029. The domestic state NOLs total approximately $19,127,
and will expire in 2016. The foreign NOLs were approximately $741.
NOLs in foreign jurisdictions will carry forward indefinitely.
As of December 31, 2009, $54,869 of
domestic federal foreign tax credits, $1,019 of research & experimentation
tax credits and $3,266 of alternative minimum tax credits were available as
credits against future U.S. income taxes. Under the U.S. Internal Revenue Code,
these will expire in 2012 through 2018, and 2020 through 2027, respectively. The
alternative minimum tax credit carryforwards have no expiration date. All
domestic credits are offset by a full valuation allowance.
The
Company has not provided U.S. federal income and withholding taxes on its
undistributed earnings from foreign operations as of December 31, 2009 because
it intends to reinvest such earnings indefinitely outside of the
U.S. Determination of the amount of unrecognized deferred tax related
to these earnings is not practical. However, in 2008, the Company did
repatriate $16,263 of cash resulting from the sale of its foreign businesses
within the Bioproducts segment and
the sale of two foreign businesses within the former Human Health
segment. The Company provided for the tax effect of this in its 2007
tax provision. The Company also settled several intercompany loans in
2008 as part of its project to streamline the Company’s legal structure, and
provided for the tax effects in its 2008 tax provision.
CAMBREX
CORPORATION AND SUBSIDIARIES
NOTES
TO CONSOLIDATED FINANCIAL STATEMENTS - (Continued)
(dollars
in thousands, except share data)
|
(8)
|
Income
Taxes (continued)
|
As of January 1, 2009 the Company had
approximately $1,697 of unrecognized tax benefits, excluding gross interest and
penalties. During 2009, the Company increased its unrecognized
tax benefits by $133 for current year positions and $2,881 for prior years’
positions, which are offset by a decrease in unrecognized tax benefits of $113,
due to the expiration of a statute of limitations period and foreign currency
translation. Of the total balance of unrecognized benefits at
December 31, 2009 $3,868, if recognized, would affect the effective tax
rate.
In the
next twelve months the Company may decrease its reserve for unrecognized tax
benefits for intercompany transactions by approximately $250 mainly due to the
expiration of a statute of limitation period. This item could impact
the income tax provision.
The following table summarizes the
activity related to the Company’s unrecognized tax benefits as of December 31,
2009, 2008 and 2007:
|
2009
|
2008
|
2007
|
||||||||||
|
Balance
at January 1
|
$ | 1,697 | $ | 5,116 | $ | 5,522 | ||||||
|
Gross
increases related to current period tax positions
|
133 | 96 | 128 | |||||||||
|
Gross
increases/(decreases) related to prior period tax
positions
|
2,881 | (2,896 | ) | (109 | ) | |||||||
|
Expiration
for statute of limitations for the assessment of taxes
|
(193 | ) | (401 | ) | (377 | ) | ||||||
|
Foreign
currency translation
|
80 | (218 | ) | (48 | ) | |||||||
|
Balance
at December 31
|
$ | 4,598 | $ | 1,697 | $ | 5,116 | ||||||
Gross
interest and penalties for 2009, 2008 and 2007 of $2,795, $333 and $420,
respectively, related to the above unrecognized tax benefits are not reflected
in the table above. In 2009, 2008 and 2007, the Company accrued
$2,529, $79 and $142, respectively, of interest and penalties in the income
statement. Consistent with prior periods, the Company recognizes
interest and penalties within its income tax provision.
In
September 2008, the Company was selected for a random IRS examination for tax
year 2006. The examination is in process and to date only a small
adjustment to tax credits has been agreed on. Tax years 2007 and
forward remain open to examination within the U.S. The Company is
also subject to exams in its significant foreign jurisdictions for 2005 and 2007
forward.
The
Company is also subject to audits in various states for various years in which
it has filed income tax returns. In June 2009, the Company finalized
a New Jersey examination of its open tax years, with no material
adjustments. Previous state audits have resulted in immaterial
adjustments. Open years for the majority of states where the Company
files are 2006 and forward.
In 2009,
the Company’s Italian subsidiary was examined by the Italian tax authorities,
who challenged the business purpose of a 2003 transaction in which a new
subsidiary was created, and the deductibility of certain intercompany
transactions. In the fourth quarter of 2009, the tax authorities
notified the Company that they disagreed with the Company’s responses to their
formal assessments. Accordingly the Company has recorded an increase
to its tax expense of approximately $5,300. Settlement discussions
with the tax authorities are ongoing.
CAMBREX
CORPORATION AND SUBSIDIARIES
NOTES
TO CONSOLIDATED FINANCIAL STATEMENTS - (Continued)
(dollars
in thousands, except share data)
|
(8)
|
Income
Taxes (continued)
|
In 2009,
the Company’s Swedish subsidiary was examined by the Swedish tax authorities,
who questioned certain significant intercompany balances and
transactions. The Company filed responses to the inquiries in the
fourth quarter of 2009. The Company expects it to take several months
before a formal audit report is issued. If the tax authorities were
to disagree with the Company’s position on unresolved issues, the Company
estimates the preliminary assessment would be approximately $200. The
Company has analyzed these issues in accordance with guidance on uncertain tax
positions and believes its reserves are adequate, and intends to defend
itself.
|
(9)
|
Long-term
Debt
|
In April
2007, the Company entered into a $200,000 five-year Syndicated Senior Revolving
Credit Facility (“Credit Facility”) which expires in April 2012. The
Company pays interest on this Credit Facility at LIBOR plus 1.25% - 2.00% based
upon certain financial measurements. The Credit Facility also includes
financial covenants regarding interest coverage and leverage ratios. The
Company was in compliance with all financial covenants at December 31,
2009. The Credit Facility is collateralized by dividend and
distribution rights associated with a pledge of a portion of stock that the
Company owns in a foreign holding company. This foreign holding company owns a
majority of the Company's non-U.S. operating subsidiaries. As of
December 31, 2009 there was $120,800 outstanding. The 2009 and 2008
weighted average interest rate for long-term bank debt was 3.8% and 4.9%,
respectively.
|
(10)
|
Derivatives
and Hedging Activities
|
The
Company operates internationally and is exposed to fluctuations in foreign
exchange rates and interest rates in the normal course of
business. These fluctuations can increase the costs of financing,
investing and operating the business. The Company uses derivative
financial instruments to reduce these exposures to market risks resulting from
fluctuations in interest rates and foreign exchange rates.
By
nature, all financial instruments involve market and credit
risks. The Company is exposed to credit losses in the event of
nonperformance by the counterparties to the contracts. While there
can be no assurance, the Company does not anticipate non-performance by these
counterparties.
Foreign Currency Forward
Contracts
The Company's policy is to enter into
forward exchange contracts to hedge forecasted cash flows associated with
foreign currency transaction exposures which are accounted for as cash flow
hedges, as deemed appropriate. This hedging strategy mitigates the
impact of short-term foreign exchange rate movements on the Company's operating
results primarily in Sweden and Italy. The Company's primary market
risk relates to exposures to foreign currency exchange rate fluctuations on
transactions entered into by these international operations that are denominated
primarily in U.S. dollars, Swedish krona, and euros. As a matter of
policy, the Company does not hedge to protect the translated results of foreign
operations.
The
Company's forward exchange contracts substantially offset gains and losses on
the transactions being hedged. The forward exchange contracts have
varying maturities with none exceeding twelve months. The Company makes net
settlements for forward exchange contracts at maturity, based upon negotiated
rates at inception of the contracts.
All
forward contracts outstanding at December 31, 2009 have been designated as cash
flow hedges and, accordingly, changes in the fair value of these derivatives are
not included in earnings but are included in accumulated other comprehensive
(loss)/income (“AOCI”). Changes in the fair value of the derivative
instruments reported in AOCI will be recorded into earnings as a component of
product revenue or expense, as applicable, when the forecasted transaction
occurs. The ineffective portion of all hedges is recognized in
current-period earnings and is immaterial to the Company's financial
results.
CAMBREX
CORPORATION AND SUBSIDIARIES
NOTES
TO CONSOLIDATED FINANCIAL STATEMENTS - (Continued)
(dollars
in thousands, except share data)
|
(10)
|
Derivatives
and Hedging Activities (continued)
|
The notional amounts of foreign
exchange forward contracts were $15,781 and $20,568 at December 31, 2009 and
2008, respectively.
Included in AOCI is the fair value of
the Company’s forward exchange contracts which is a gain of $310 and a loss of
$678 as of December 31, 2009 and 2008, respectively. These
gains and losses are located under the captions “Prepaid expenses and other
current assets” and “Accrued expenses and other current liabilities” on the
balance as of December 31, 2009 and 2008, respectively.
The
Company recognized a pre-tax gain in other comprehensive income from foreign
exchange contracts of $988 in 2009. The Company reclassified a
pre-tax loss of $678 from AOCI into other revenue related to foreign exchange
forward contracts in 2009. Assuming current market conditions
continue, the entire amount recorded in AOCI related to foreign exchange forward
contracts is expected to be recorded into other revenue within the next 12
months to reflect the fixed prices obtained from the forward
contracts.
Interest Rate Swap
Agreements
The
Company enters into interest rate swap agreements to reduce the impact of
changes in interest rates on its floating rate debt. The swap
agreements are contracts to exchange floating rate for fixed interest payments
periodically over the life of the agreements without the exchange of the
underlying notional debt amounts.
All swap
contracts outstanding at December 31, 2009 have been designated as cash flow
hedges and, accordingly, changes in the fair value of derivatives are recorded
each period in AOCI. Changes in the fair value of the derivative
instruments reported in AOCI will be recorded into earnings in the period in
which earnings are impacted by the variability of the cash flows of the hedged
item. The ineffective portion of all hedges will be recognized in
current-period earnings and has been immaterial to the Company's financial
results.
As of
December 31, 2009, the Company had three interest rate swaps in place with an
aggregate notional value of $60,000, at an average fixed rate of 4.48%, all with
maturity dates of October 2010. The Company’s strategy has been to
cover a portion of its outstanding bank debt with interest rate
protection. At December 31, 2009, the coverage was approximately 50%
of the Company’s variable interest rate debt. At December 31, 2009
the Company had variable debt of $120,800, of which $60,000 is fixed by interest
rate swaps. Interest expense under these agreements, and the
respective debt instruments that they hedge, are recorded at the net effective
interest rate of the hedged transactions. The fair value of these
agreements were based on quoted market prices and was in a loss position of
$2,038 and $3,541 at December 31, 2009 and 2008 respectively. This
loss is reflected in the balance sheet under the caption “Accrued expense and
other current liabilities.”
The
Company increased other comprehensive income $1,503 related to interest rate
swaps in 2009. The Company reclassified a pre-tax loss of $2,515 from
AOCI into interest expense related to interest rate swaps in
2009. Assuming current market conditions continue, approximately
$2,038 is expected to be reclassed out of AOCI into interest expense within the
next 12 months.
|
(11)
|
Fair
Value Measurements
|
U.S. GAAP
establishes a valuation hierarchy for disclosure of the inputs to the valuations
used to measure fair value. This hierarchy prioritizes the inputs into three
broad levels as follows: Level 1 inputs are quoted prices (unadjusted) in active
markets for identical assets or liabilities; Level 2 inputs are quoted prices
for similar assets and liabilities in active markets, quoted prices for
identical or similar assets in markets that are not active, inputs other than
quoted prices that are observable for the asset or liability, including interest
rates, yield curves and credit risks, or inputs that are derived principally
from, or corroborated by, observable market data through correlation; Level 3
inputs are unobservable inputs based on the Company’s assumptions used to
measure assets and liabilities at fair value. A financial asset or liability’s
classification within the hierarchy is determined based on the lowest level
input that is significant to the fair value measurement. Valuation
techniques used need to maximize the use of observable inputs or minimize the
use of unobservable inputs.
CAMBREX
CORPORATION AND SUBSIDIARIES
NOTES
TO CONSOLIDATED FINANCIAL STATEMENTS - (Continued)
(dollars
in thousands, except share data)
|
(11)
|
Fair
Value Measurements (continued)
|
The following tables provide the assets
and liabilities carried at fair value measured on a recurring basis as of
December 31, 2009 and 2008:
|
Fair Value Measurements at December 31, 2009
using:
|
||||||||||||||||
|
Description
|
Total
|
Quoted Prices in Active Markets for Identical
Assets (Level 1)
|
Signifcant Other
Observable Inputs
(Level 2)
|
Significant Unobservable Inputs (Level
3)
|
||||||||||||
|
Foreign
currency forwards, assets
|
$ | 310 | $ | - | $ | 310 | $ | - | ||||||||
|
Interest
rate swaps
|
(2,038 | ) | - | (2,038 | ) | - | ||||||||||
|
Total
|
$ | (1,728 | ) | $ | - | $ | (1,728 | ) | $ | - | ||||||
|
Fair Value Measurements at December 31, 2008
using:
|
||||||||||||||||
|
Description
|
Total
|
Quoted Prices in Active Markets for Identical
Assets (Level 1)
|
Signifcant Other
Observable Inputs
(Level 2)
|
Significant Unobservable Inputs (Level
3)
|
||||||||||||
|
Foreign
currency forwards, assets
|
$ | 808 | $ | - | $ | 808 | $ | - | ||||||||
|
Foreign
currency forwards, liabilities
|
(1,486 | ) | - | (1,486 | ) | - | ||||||||||
|
Interest
rate swaps
|
(3,541 | ) | - | (3,541 | ) | - | ||||||||||
|
Total
|
$ | (4,219 | ) | $ | - | $ | (4,219 | ) | $ | - | ||||||
The Company’s derivative assets and
liabilities include foreign exchange forward contracts and interest rate swap
contracts that are measured at fair value using observable market inputs such as
forward rates, interest rates, the Company’s credit risk and its counterparties’
credit risks. Based on these inputs, the derivative assets and liabilities are
classified within Level 2 of the valuation hierarchy. Based on the Company’s
continued ability to enter into forward contracts and interest rate swaps, the
Company considers the markets for its fair value instruments to be
active.
As of December 31, 2009 there has not
been any significant impact to the fair value of the Company’s derivative
liabilities due to its own credit risk. Similarly, there has not been
any significant adverse impact to the Company’s derivative assets based on the
Company’s evaluation of its counterparties’ credit risks.
The Company’s financial instruments
also include cash and cash equivalents, accounts receivables, accounts payables
and accrued liabilities. The carrying amount of these instruments
approximates fair value because of their short-term nature.
CAMBREX
CORPORATION AND SUBSIDIARIES
NOTES
TO CONSOLIDATED FINANCIAL STATEMENTS - (Continued)
(dollars
in thousands, except share data)
|
(12)
|
Strategic
Alternative and Restructuring
Charges
|
Strategic Alternative Costs
Strategic
alternative costs include expenses that the Company incurred related to the
decision to sell the businesses that comprised the Bioproducts and Biopharma
segments in February 2007, costs associated with a project to streamline the
Company’s legal structure and costs associated with the exit of a feed additives
product line. These costs are not considered part of the restructuring program
or a part of discontinued operations under current accounting
guidance.
Strategic
alternative costs for 2008 were $1,515 consisting primarily of costs associated
with the project to streamline the Company’s legal structure, change-in-control
benefits and costs associated with the modification of employee stock options
due to the payment of the special dividend in connection with the discussed
above. Strategic alternative costs for 2007 were $31,127 consisting
primarily of change-in-control benefits, retention bonuses, costs associated
with the stock option modification, external advisor costs and the costs to exit
a feed additive product line.
Restructuring
Expenses
Corporate
Office Restructuring
During
2007, the Company announced plans to eliminate certain employee positions at the
corporate office upon completion of the sale of the businesses that comprised
the Bioproducts and Biopharma segments. This plan included certain
one-time benefits for terminated employees. Costs related to these
plans are recorded as restructuring expenses in the income statement. The
Company recognized expense of $805 and $4,014 in 2008 and 2007, respectively,
related to this plan.
Consolidation
of Domestic Research and Development Activities
In
December 2007, the Company consolidated its United States research and
development (“R&D”) activities and small scale API production with its
facility in Charles City, Iowa. The restructuring reserve at December
31, 2008 consisted of the remaining lease payments and related costs under the
Company’s current operating lease at the New Jersey R&D
facility. The operating lease expires in December
2010. Costs related to this consolidation are recorded as
restructuring expenses on the income statement. The Company
recognized expense of $3,890 and $2,059 in 2008 and 2007, respectively, related
to this plan.
The following table reflects the
activity related to the restructuring reserves through December 31,
2009:
|
December
31, 2007
|
2008 Activity
|
December
31, 2008
|
2009 Activity
|
December
31, 2009
|
||||||||||||||||||||||||
|
Reserve Balance
|
Expense
|
Cash Payments
|
Reserve Balance
|
Expense
|
Cash Payments
|
Reserve Balance
|
||||||||||||||||||||||
|
Employee
termination costs
|
$ | 1,168 | $ | 849 | $ | (1,555 | ) | $ | 462 | $ | - | $ | (462 | ) | $ | - | ||||||||||||
|
Lease
payments and related costs
|
998 | 2,396 | (373 | ) | 3,021 | (132 | ) | (1,416 | ) | 1,473 | ||||||||||||||||||
| $ | 2,166 | $ | 3,245 | $ | (1,928 | ) | $ | 3,483 | $ | (132 | ) | $ | (1,878 | ) | $ | 1,473 | ||||||||||||
This reserve will be paid in full by
December 31, 2010. Total restructuring expenses for 2008 and 2007
were $4,695 and $6,073, respectively.
CAMBREX
CORPORATION AND SUBSIDIARIES
NOTES
TO CONSOLIDATED FINANCIAL STATEMENTS - (Continued)
(dollars
in thousands, except share data)
|
(13)
|
Stockholders'
Equity
|
The Company has two classes of common
shares which are Common Stock and Nonvoting Common Stock. Authorized
shares of Common Stock were 100,000,000 at December 31, 2009 and
2008. Authorized shares of Nonvoting Common Stock were 730,746 at
December 31, 2009 and 2008. Nonvoting Common Stock with a par value
of $.10 has equal rights with Common Stock, with the exception of voting
power. Nonvoting Common Stock is convertible, share for share, into
Common Stock, subject to any legal requirements applicable to holders
restricting the extent to which they may own voting stock. As of
December 31, 2009 and 2008, no shares of Nonvoting Common Stock were
outstanding. The Company has authorized 5,000,000 shares of Series
Preferred Stock, par value $.10, issuable in series and with rights, powers and
preferences as may be fixed by the Board of Directors. At December
31, 2009 and 2008, there was no preferred stock outstanding.
In May
2007, the Company paid a special dividend of $14.00 per share to its
shareholders resulting in a reduction in stockholders’ equity of $403,033. The
effect on stockholders’ equity was a reduction to retained earnings of $233,251,
representing total accumulated earnings as of the date of declaration, with the
remainder representing a return of capital of $169,782. As of
December 31, 2009, cash disbursements were $402,858 and $175 was accrued related
to dividends on unvested restricted stock. The Company no longer pays
a quarterly dividend.
The Company held treasury shares of
2,121,372 and 2,224,613 at December 31, 2009 and 2008, respectively, which are
primarily used for issuance to employee compensation plans.
At December 31, 2009 there were 448,030
authorized shares of Common Stock reserved for issuance through stock option
plans.
|
(14)
|
Stock
Based Compensation
|
The
Company recognizes compensation costs for stock option awards to employees based
on their grant-date fair value. The value of each stock option is
estimated on the date of grant using the Black-Scholes option-pricing
model. The weighted-average fair value per share for the stock
options granted to employees for the years ended December 31, 2009, 2008 and
2007 were $3.31, $1.72 and $5.44, respectively.
The
following assumptions were used in determining the fair value of stock options
for grants issued in 2009, 2008 and 2007:
|
2009
|
2008
|
2007
|
|||
|
Expected
volatility
|
38.78%-65.11%
|
33.30%
- 38.78%
|
34.38%
- 36.90%
|
||
|
Expected
term
|
4.75
years
|
4.75
years
|
3.75
- 4.75 years
|
||
|
Risk-free
interst rate
|
2.38%-2.77%
|
2.77%
- 3.08%
|
4.30%
- 4.85%
|
The
Company does not have any publicly traded stock options; therefore, expected
volatilities are based on historical volatility of the Company’s
stock. The risk-free interest rate is based on the yield of a
zero-coupon U.S. Treasury bond whose maturity period approximates the option’s
expected term. The expected term was utilized based on the
“simplified” method for determining the expected term of stock options in Staff
Accounting Bulletin (“SAB”) No. 107, “Share-Based Payment.” The
Company also considered SAB No. 110 when determining the expected term of stock
options.
For 2009,
2008, and 2007, the Company recorded $554, $555 and $379, respectively, in
selling, general and administrative expenses for stock options. In
addition the Company recorded $27 in restructuring expenses in 2008 and $282 and
$50 in strategic alternative costs and restructuring expenses, respectively, in
2007 for stock options related to the change in control agreements and the
reduction in workforce in 2007 and 2008. As of December 31, 2009, the
total compensation cost related to unvested stock option awards granted to
employees but not yet recognized was $2,722. The cost will be
amortized on a straight-line basis over the remaining weighted-average vesting
period of 3.1 years.
CAMBREX
CORPORATION AND SUBSIDIARIES
NOTES
TO CONSOLIDATED FINANCIAL STATEMENTS - (Continued)
(dollars
in thousands, except share data)
|
(14)
|
Stock
Based Compensation (continued)
|
In
addition, for 2009, 2008 and 2007 the Company recorded $94, $102 and $2,535,
respectively, for expenses associated with a stock option modification due to a
special dividend declared in 2007. As of December 31, 2009, the total
compensation cost related to unvested stock option awards that were modified but
not yet recognized was $54. The cost will be amortized on a
straight-line basis over the remaining weighted-average vesting period of 0.6
years.
Cambrex
senior executives participate in an executive incentive plan which rewards
achievement with restricted stock units. Awards are made annually if
certain targets are met and vest in one-third increments on the first, second
and third anniversaries of the grant. On the third anniversary of the
grant, restrictions on sale or transfer are removed and shares are issued to
executives. In the event of termination of employment or retirement,
the participant is entitled to the vested portion of the restricted stock units
and forfeits the remaining amount; the three-year sale and transfer restriction
remains in place. For certain employees with employment contracts,
all shares vest upon certain events, including a change in
control. In the event of death or permanent disability, all shares
vest and the deferred sales restriction lapses. These awards are
classified as equity awards. Certain other employees are eligible to
receive restricted stock as part of the stock-based compensation
plan. These awards cliff vest on the third anniversary of the grant
date.
For 2009,
2008, and 2007, the Company recorded $658, $1,327, and $705, respectively, in
selling, general and administrative expenses for restricted stock. In
addition, the Company recorded $24 in restructuring expenses in 2008 and $1,554
and $172 in strategic alternative costs and restructuring expenses,
respectively, in 2007 for restricted stock. As of December 31, 2009
the total compensation cost related to unvested restricted stock granted but not
yet recognized was $344. The cost will be amortized on a straight-line basis
over the remaining weighted-average vesting period of 0.7 years.
In May 2008 the Company granted a
target award of 43,000 performance shares, with a potential award of up to
86,000 shares to the current CEO. These performance shares are
dependent upon the Company’s performance measured against certain financial
metrics over a three year period beginning July 1, 2008, as compared to an
external peer group. The Company is currently recognizing expense
related to 43,000 shares over the vesting period, which assumes that the CEO
will be compensated at target. The Company will assess performance at
each reporting period and adjust accordingly. For 2009 and 2008 the
Company recorded $69 and $34, respectively, in selling, general and
administrative expense related to these performance shares.
CAMBREX
CORPORATION AND SUBSIDIARIES
NOTES
TO CONSOLIDATED FINANCIAL STATEMENTS - (Continued)
(dollars
in thousands, except share data)
|
(14)
|
Stock
Based Compensation (continued)
|
The
following table is a summary of the Company’s stock option activity issued to
employees and related information:
|
Weighted Average
|
||||||||||||
|
Number of Shares
|
Exercise Price
|
Options Exercisable
|
||||||||||
|
Outstanding
at December 31, 2006
|
2,754,893 | $ | 28.48 | 2,517,941 | ||||||||
|
Granted
|
152,675 | 14.99 | ||||||||||
|
Exercised
|
(1,202,752 | ) | 18.21 | |||||||||
|
Forfeited
or expired
|
(233,059 | ) | 22.52 | |||||||||
|
Outstanding
at December 31, 2007
|
1,471,757 | 20.15 | 1,293,108 | |||||||||
|
Granted
|
744,000 | 4.82 | ||||||||||
|
Exercised
|
(2,301 | ) | 7.47 | |||||||||
|
Forfeited
or expired
|
(622,587 | ) | 19.17 | |||||||||
|
Outstanding
at December 31, 2008
|
1,590,869 | 14.07 | 757,050 | |||||||||
|
Granted
|
533,000 | 6.07 | ||||||||||
|
Exercised
|
(2,000 | ) | 4.40 | |||||||||
|
Forfeited
or expired
|
(101,500 | ) | 17.19 | |||||||||
|
Outstanding
at December 31, 2009
|
2,020,369 | 11.27 | ||||||||||
|
Exercisable
at December 31, 2009
|
$ | 18.35 | 886,579 | |||||||||
In May 2007, the Company paid a special
dividend of $14.00 per share. As a result, the market price of the
stock declined by approximately $14.00 per share from the prior day’s close and
therefore, all outstanding options were modified to reduce the exercise price by
$14.00 per share.
The aggregate intrinsic value for all
stock options exercised for the years ended December 31, 2009, 2008 and 2007
were $4, $4 and $2,866, respectively. The aggregate intrinsic value
for all stock options outstanding as of December 31, 2009 was
$628. The aggregate intrinsic value for all stock options exercisable
as of December 31, 2009 was $170.
CAMBREX
CORPORATION AND SUBSIDIARIES
NOTES
TO CONSOLIDATED FINANCIAL STATEMENTS - (Continued)
(dollars
in thousands, except share data)
|
(14)
|
Stock
Based Compensation (continued)
|
A summary of the Company’s nonvested
stock options and restricted stock as of December 31, 2009 and changes during
the years ended December 31, 2009, 2008 and 2007 are presented
below:
|
Nonvested
Stock Options
|
Nonvested
Restricted Stock
|
|||||||||||||||
|
Number of Shares
|
Weighted-Average Grant-Date Fair
Value
|
Number of Shares
|
Weighted-Average Grant-Date Fair
Value
|
|||||||||||||
|
Nonvested
at January 1, 2007
|
236,952 | $ | 21.39 | 165,868 | $ | 22.02 | ||||||||||
|
Granted
|
152,675 | $ | 14.99 | 125,489 | $ | 17.09 | ||||||||||
|
Vested
during period
|
(137,145 | ) | $ | 16.57 | (123,494 | ) | $ | 21.55 | ||||||||
|
Forfeited
|
(73,833 | ) | $ | 19.79 | (33,962 | ) | $ | 20.91 | ||||||||
|
Nonvested
at December 31, 2007
|
178,649 | $ | 11.34 | 133,901 | $ | 18.11 | ||||||||||
|
Granted
|
744,000 | $ | 4.82 | 122,872 | $ | 8.74 | ||||||||||
|
Vested
during period
|
(69,963 | ) | $ | 10.95 | (102,858 | ) | $ | 12.52 | ||||||||
|
Forfeited
|
(18,867 | ) | $ | 11.50 | (10,588 | ) | $ | 16.52 | ||||||||
|
Nonvested
at December 31, 2008
|
833,819 | $ | 5.55 | 143,327 | $ | 13.38 | ||||||||||
|
Granted
|
533,000 | $ | 6.07 | 36,918 | $ | 3.90 | ||||||||||
|
Vested
during period
|
(218,737 | ) | $ | 5.77 | (86,453 | ) | $ | 11.31 | ||||||||
|
Forfeited
|
(14,292 | ) | $ | 6.56 | (3,106 | ) | $ | 15.27 | ||||||||
|
Nonvested
at December 31, 2009
|
1,133,790 | $ | 5.74 | 90,686 | $ | 11.43 | ||||||||||
|
(15)
|
Retirement
Plans and Other Postretirement
Benefits
|
Domestic Pension Plans
The Company maintains two U.S.
defined-benefit pension plans (“Domestic Pension Plans”). Benefits
for the salaried and certain hourly employees are based on salary and years of
service, while those for employees covered by a collective bargaining agreement
are based on negotiated benefits and years of service. The Company's
policy is to fund pension costs currently to the full extent required by the
Internal Revenue Code.
The net periodic pension expense for
2009, 2008 and 2007 is based on a twelve month period and on valuations of the
plans as of January 1. However, the reconciliation of funded status
is determined as of a December 31 measurement date for 2009 and 2008 and a
September 30 measurement date for 2007. The FASB eliminated the
Company’s option to measure the pension and other postretirement benefits plans’
benefit obligations, assets and net periodic cost at a date prior to December
31. Therefore, the pension and postretirement benefits plans, which
were measured as of September 30 in 2007, have been measured as of December 31,
2009 and 2008. The Company elected to use the 15-month alternative to
determine 2008 pension cost. The portion of expense attributed to the
remaining three months of 2007 was charged directly to retained earnings, with
accumulated other comprehensive loss adjusted to reflect the amortization
amounts. The change in measurement date did not have a material
impact on the Company’s financial position or results of
operations.
CAMBREX
CORPORATION AND SUBSIDIARIES
NOTES
TO CONSOLIDATED FINANCIAL STATEMENTS - (Continued)
(dollars
in thousands, except share data)
|
(15)
|
Retirement
Plans and Other Postretirement Benefits
(continued)
|
The
Company also has a Supplemental Executive Retirement Plan (“SERP”) for key
executives. This plan is non-qualified and unfunded.
In July
2008, the Board of Directors of the Company amended the SERP plan to allow for
lump sum payments effective January 1, 2009. If the lump sum value as
of January 1, 2009 was greater than $10, it will be paid in 10 equal actuarial
equivalent installments; all others will be paid as a lump
sum. Retirees as of January 1, 2009 were allowed a one-time election
in 2008 to continue under their current form of payment or switch to the 10 year
installment option. All retirees chose the 10 year installment
option.
International Pension
Plans
A foreign
subsidiary of the Company maintains a pension plan (“International Pension
Plan”) for their employees that conforms to the common practice in their
respective country. Based on local laws and customs, this plan is
unfunded.
Other Postretirement
Benefits
Cambrex provided limited
post-retirement health and life insurance benefits ("postretirement benefits")
to all eligible retired employees. Certain subsidiaries and all
employees hired after December 31, 2002 (excluding those covered by collective
bargaining) were not eligible for these benefits. Effective December
31, 2009, the Company terminated these postretirement benefits for all
participants resulting in a benefit of approximately $1,200.
Savings Plan
Cambrex
makes available to all domestic employees a savings plan as permitted under
Sections 401(k) and 401(a) of the Internal Revenue Code. Employee
contributions are matched in part by Cambrex. The cost of this plan
amounted to $631, $592 and $608 in 2009, 2008 and 2007,
respectively.
Other
The
Company has a non-qualified Compensation Plan for Key Executives (“the Deferred
Plan”). Under the Deferred Plan, officers and key employees may elect
to defer all or any portion of their pre-tax earnings or to elect to defer
receipt of the Company’s stock which would otherwise have been issued upon the
exercise of the Company’s options. Included within other liabilities
at December 31, 2009 and 2008 there is $2,747 and $3,012, respectively,
representing the Company’s obligation under the plan. The Company
invests in certain mutual funds and as such, included within other assets at
December 31, 2009 and 2008 is $2,747 and $3,012, respectively, representing the
fair value of these funds. The fair values of these mutual funds are
based on quoted market prices in active markets (Level 1). Total
shares held in trust as of December 31, 2009 and 2008 were 151,385 and 195,851,
respectively, and are included as a reduction of equity at cost. The
value of the shares held in trust and the corresponding liability of $845 and
$905 at December 31, 2009 and 2008, respectively, have been recorded in
equity. The Deferred Plan is not funded by the Company, but the
Company has established a Deferred Compensation Trust Fund which holds the
shares issued.
CAMBREX
CORPORATION AND SUBSIDIARIES
NOTES
TO CONSOLIDATED FINANCIAL STATEMENTS - (Continued)
(dollars
in thousands, except share data)
|
(15)
|
Retirement
Plans and Other Postretirement Benefits
(continued)
|
The
benefit obligations as of December 31, 2009 and 2008 are as
follows:
|
Pension Plans
|
||||||||||||||||||||||||||||||||
|
Domestic
|
SERP
|
International
|
Postretirement Plans
|
|||||||||||||||||||||||||||||
|
|
2009
|
2008
|
2009
|
2008
|
2009
|
2008
|
2009
|
2008
|
||||||||||||||||||||||||
|
Change
in benefit obligation
|
||||||||||||||||||||||||||||||||
|
Benefit
obligation, beginning of year
|
$ | 58,529 | $ | 57,451 | $ | 5,784 | $ | 5,207 | $ | 16,634 | $ | 18,563 | $ | 1,858 | $ | 1,757 | ||||||||||||||||
|
Service
cost
|
- | - | - | - | 533 | 520 | 26 | 25 | ||||||||||||||||||||||||
|
Interest
cost
|
3,427 | 3,513 | 279 | 303 | 747 | 831 | 110 | 109 | ||||||||||||||||||||||||
|
Plan
participants' contributions
|
- | - | - | - | - | - | 12 | 20 | ||||||||||||||||||||||||
|
Actuarial
loss/(gain)
|
1,869 | 1,234 | 275 | (135 | ) | (594 | ) | 757 | (155 | ) | (4 | ) | ||||||||||||||||||||
|
Benefits
paid
|
(2,739 | ) | (3,431 | ) | (800 | ) | (107 | ) | (468 | ) | (460 | ) | (26 | ) | (65 | ) | ||||||||||||||||
|
Plan
amendments
|
- | - | - | 516 | - | - | - | - | ||||||||||||||||||||||||
|
Effect
of eliminating early measurement date
|
- | (238 | ) | - | - | - | - | - | 16 | |||||||||||||||||||||||
|
Curtailments
|
- | - | - | - | - | - | (1,825 | ) | - | |||||||||||||||||||||||
|
Foreign
exchange
|
- | - | - | - | 1,639 | (3,577 | ) | - | - | |||||||||||||||||||||||
|
Benefit
obligation, end of year
|
$ | 61,086 | $ | 58,529 | $ | 5,538 | $ | 5,784 | $ | 18,491 | $ | 16,634 | $ | - | $ | 1,858 | ||||||||||||||||
The plan
assets and funded status of the Domestic Pension Plans as of December 31, 2009
and 2008 are as follows:
|
2009
|
2008
|
|||||||
|
Change
in plan assets
|
||||||||
|
Fair
value of plan assets, beginning of period
|
$ | 37,311 | $ | 49,985 | ||||
|
Actual
return on plan assets
|
7,489 | (12,342 | ) | |||||
|
Contributions
|
1,161 | 3,194 | ||||||
|
Benefits
paid
|
(2,739 | ) | (3,431 | ) | ||||
|
Effect
of eliminating early measurement date
|
- | (95 | ) | |||||
|
Fair
value of plan assets, end of period
|
$ | 43,222 | $ | 37,311 | ||||
|
Funded
status
|
(17,864 | ) | (21,218 | ) | ||||
|
Accrued
benefit cost, end of period
|
$ | (17,864 | ) | $ | (21,218 | ) | ||
The funded status of the SERP plan was
($5,538) and ($5,784) as of December 31, 2009 and 2008,
respectively. The funded status of the International Pension Plan was
($18,491) and ($16,634) as of December 31, 2009 and 2008,
respectively.
The amounts recognized in accumulated
other comprehensive loss as of December 31, 2009 and 2008 consist of the
following:
|
Pension Plans
|
||||||||||||||||||||||||||||||||
|
Domestic
|
SERP
|
International
|
Postretirement Plans
|
|||||||||||||||||||||||||||||
|
2009
|
2008
|
2009
|
2008
|
2009
|
2008
|
2009
|
2008
|
|||||||||||||||||||||||||
|
Actuarial
loss
|
$ | 17,450 | $ | 20,690 | $ | 815 | $ | 540 | $ | 3,812 | $ | 4,591 | $ | - | $ | 824 | ||||||||||||||||
|
Prior
service cost
|
932 | 1,368 | 459 | 516 | (51 | ) | (58 | ) | - | (541 | ) | |||||||||||||||||||||
| $ | 18,382 | $ | 22,058 | $ | 1,274 | $ | 1,056 | $ | 3,761 | $ | 4,533 | $ | - | $ | 283 | |||||||||||||||||
CAMBREX
CORPORATION AND SUBSIDIARIES
NOTES
TO CONSOLIDATED FINANCIAL STATEMENTS - (Continued)
(dollars
in thousands, except share data)
|
(15)
|
Retirement
Plans and Other Postretirement Benefits
(continued)
|
The
components of net periodic benefit cost are as follows:
|
Pension Plans
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
Domestic
|
SERP
|
International
|
Postretirement Plans
|
|||||||||||||||||||||||||||||||||||||||||||||
|
2009
|
2008
|
2007
|
2009
|
2008
|
2007
|
2009
|
2008
|
2007
|
2009
|
2008
|
2007
|
|||||||||||||||||||||||||||||||||||||
|
Components
of net periodic benefit cost
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
Service
cost
|
$ | - | $ | - | $ | 1,000 | $ | - | $ | - | $ | 53 | $ | 533 | $ | 520 | $ | 462 | $ | 26 | $ | 25 | $ | 21 | ||||||||||||||||||||||||
|
Interest
cost
|
3,427 | 3,513 | 3,597 | 279 | 303 | 300 | 747 | 831 | 665 | 110 | 109 | 107 | ||||||||||||||||||||||||||||||||||||
|
Expected
return on plan assets
|
(2,924 | ) | (4,086 | ) | (3,733 | ) | - | - | - | - | - | - | - | - | - | |||||||||||||||||||||||||||||||||
|
Amortization
of prior service cost
|
625 | 532 | 206 | 57 | - | 1 | (6 | ) | (7 | ) | (7 | ) | (156 | ) | (155 | ) | (156 | ) | ||||||||||||||||||||||||||||||
|
Recognized
actuarial loss
|
355 | - | 209 | - | 5 | 17 | 130 | 125 | 75 | 52 | 56 | 65 | ||||||||||||||||||||||||||||||||||||
|
Curtailments
|
- | - | 414 | - | - | 15 | - | - | - | (1,178 | ) | - | ||||||||||||||||||||||||||||||||||||
|
Net
periodic benefit cost
|
$ | 1,483 | $ | (41 | ) | $ | 1,693 | $ | 336 | $ | 308 | $ | 386 | $ | 1,404 | $ | 1,469 | $ | 1,195 | $ | (1,146 | ) | $ | 35 | $ | 37 | ||||||||||||||||||||||
The sale of the businesses that
comprised the Bioproducts and Biopharma segments in February 2007 required the
Company to recognize a curtailment charge of $337 for the Domestic Pension Plans
and $11 for the SERP plan in 2007 which is recorded in discontinued
operations. In April 2007, the Board of Directors of the Company
approved the suspension of the Domestic Pension Plans and SERP plan effective
August 31, 2007. As a result, the Company was required to recognize a
curtailment charge of $77 for the Domestic Pension Plans and $4 for the SERP
plan in 2007.
The estimated amounts that will be
amortized from accumulated other comprehensive loss into net periodic cost in
2010 are as follows:
|
Pension Plans
|
||||||||||||
|
Domestic
|
SERP
|
International
|
||||||||||
|
Actuarial
loss
|
$ | 429 | 33 | $ | (104 | ) | ||||||
|
Prior
service cost
|
436 | 57 | 6 | |||||||||
|
Total
|
$ | 865 | $ | 90 | $ | (98 | ) | |||||
Major assumptions used in determining
the benefit obligations are presented in the following table:
|
2009
|
2008
|
|||||||
|
Discount
rate:
|
||||||||
|
Domestic
Pension Plans
|
5.90 | % | 6.00 | % | ||||
|
SERP
|
4.15 | % | 5.60 | % | ||||
|
International
Pension Plan
|
4.70 | % | 4.40 | % | ||||
|
Postretirement
Plans
|
5.90 | % | 6.00 | % | ||||
|
Rate
of compensation increase:
|
||||||||
|
International
Pension Plan
|
3.00 | % | 3.00 | % | ||||
CAMBREX
CORPORATION AND SUBSIDIARIES
NOTES
TO CONSOLIDATED FINANCIAL STATEMENTS - (Continued)
(dollars
in thousands, except share data)
|
(15)
|
Retirement
Plans and Other Postretirement Benefits
(continued)
|
Major assumptions used in determining
the net benefit cost are presented in the following table:
|
2009
|
2008
|
2007
|
||||||||||
|
Discount
rate:
|
||||||||||||
|
Domestic
Pension Plans
|
6.00 | % | 6.25 | % | 6.00 | % | ||||||
|
SERP
|
5.60 | % | 6.00 | % | 6.00 | % | ||||||
|
International
Pension Plan
|
4.70 | % | 4.40 | % | 4.25 | % | ||||||
|
Postretirement
Plans
|
6.00 | % | 6.25 | % | 6.00 | % | ||||||
|
Expected
return on plan assets:
|
||||||||||||
|
Domestic
Pension Plans
|
8.00 | % | 8.00 | % | 8.00 | % | ||||||
|
Rate
of compensation increase:
|
||||||||||||
|
Domestic
Pension Plans
|
N/A | N/A | 5.00 | % | ||||||||
|
SERP
|
N/A | N/A | 5.00 | % | ||||||||
|
International
Pension Plan
|
3.00 | % | 3.00 | % | 3.00 | % | ||||||
In making its assumption for the
long-term rate of return on plan assets, the Company has utilized historical
rates earned on securities allocated consistently with its
investments. The discount rate was selected by projecting cash flows
associated with plan obligations, which were matched to a yield curve of high
quality corporate bonds. The Company then selected the single rate
that produced the same present value as if each cash flow were discounted by the
corresponding spot rate on the yield curve.
The
aggregate Accumulated Benefit Obligation (“ABO”) of $61,086 exceeds plan assets
by $17,864 as of December 31, 2009 for the Domestic Pension
Plans. The aggregate ABO is $17,605 for the International Pension
Plan as of December 31, 2009. The International Pension Plan is
unfunded.
The Company expects to contribute
approximately $1,041 in cash to the Domestic Pension Plans in
2010. The Company does not expect to contribute cash to its
International Pension Plan in 2010.
The following
benefit payments, which reflect expected future service, as appropriate, are
expected to be paid:
|
Pension Plans
|
||||||||||||
|
Domestic
|
SERP
|
International
|
||||||||||
|
2010
|
$ | 2,939 | $ | 720 | $ | 551 | ||||||
|
2011
|
$ | 3,113 | $ | 720 | $ | 583 | ||||||
|
2012
|
$ | 3,254 | $ | 720 | $ | 597 | ||||||
|
2013
|
$ | 3,275 | $ | 720 | $ | 682 | ||||||
|
2014
|
$ | 3,465 | $ | 720 | $ | 746 | ||||||
|
2015-2019
|
$ | 18,190 | $ | 3,600 | $ | 4,169 | ||||||
CAMBREX
CORPORATION AND SUBSIDIARIES
NOTES
TO CONSOLIDATED FINANCIAL STATEMENTS - (Continued)
(dollars
in thousands, except share data)
|
(15)
|
Retirement
Plans and Other Postretirement Benefits
(continued)
|
The investment objective for the
Domestic Pension Plan’s assets is to achieve long-term growth with exposure to
risk at an appropriate level. The Company invests in a diversified
asset mix consisting of equities (domestic and international) and taxable fixed
income securities. Assets are managed to obtain the highest total
rate of return in keeping with a moderate level of risk. The target
allocations for plan assets are 30% - 80% equity securities, 25% - 45% U.S.
fixed income and 0% - 10% all other investments. Equity securities
primarily include investments in large-cap and small-cap companies, mostly in
the U.S., Fixed income securities include high quality corporate bonds and U.S.
government securities. Other types of investments include real asset
funds, consisting primarily of investments in commodities, and Treasury
Inflation-Protected Securities (“TIPS”).
The fair values of the Company’s
pension plan assets by asset category are as follows:
|
Fair Value Measurements at December 31, 2009
using:
|
||||||||||||||||
|
Asset Category
|
Total
|
Quoted Prices in Active Markets for Identical
Assets (Level 1)
|
Significant Other Observable
Inputs
(Level 2)
|
Significant Unobservable Inputs (Level
3)
|
||||||||||||
|
Equity
securities:
|
||||||||||||||||
|
U.S.
companies
|
$ | 15,173 | $ | - | $ | 15,173 | $ | - | ||||||||
|
International
companies
|
8,270 | - | 8,270 | - | ||||||||||||
|
U.S.
fixed income
|
14,415 | - | 12,430 | 1,985 | ||||||||||||
|
Commodities
|
3,292 | - | 3,292 | - | ||||||||||||
|
TIPS
|
2,072 | - | 2,072 | - | ||||||||||||
| $ | 43,222 | $ | - | $ | 41,237 | $ | 1,985 | |||||||||
The
following table sets forth a summary of the changes in the fair value of the
Domestic Plan’s Level 3 assets for the year ended December 31,
2009:
|
Group
Annuity Contract
|
||||
|
Balance,
December 31, 2008
|
$ | 1,917 | ||
|
Actual
return on plan assets:
|
||||
|
Relating
to assets still held at the reporting date
|
122 | |||
|
Purchases,
issuances, and settlements
|
(54 | ) | ||
|
Balance,
December 31, 2009
|
$ | 1,985 | ||
CAMBREX
CORPORATION AND SUBSIDIARIES
NOTES
TO CONSOLIDATED FINANCIAL STATEMENTS - (Continued)
(dollars
in thousands, except share data)
|
(16)
|
Foreign
Operations and Sales
|
The following summarized data
represents the gross sales and long lived tangible assets for the Company’s
domestic and foreign entities for 2009, 2008 and 2007:
|
Domestic
|
Foreign
|
Total
|
||||||||||
|
2009
|
||||||||||||
|
Gross
sales
|
$ | 84,518 | $ | 151,759 | $ | 236,277 | ||||||
|
Long-lived
assets
|
39,227 | 158,282 | 197,509 | |||||||||
|
2008
|
||||||||||||
|
Gross
sales
|
$ | 81,707 | $ | 167,911 | $ | 249,618 | ||||||
|
Long-lived
assets
|
42,621 | 154,257 | 196,878 | |||||||||
|
2007
|
||||||||||||
|
Gross
sales
|
$ | 81,429 | $ | 171,145 | $ | 252,574 | ||||||
|
Long-lived
assets
|
42,103 | 159,106 | 201,209 | |||||||||
Export sales, included in domestic
gross sales, in 2009, 2008 and 2007 amounted to $25,768, $24,602, and $28,821,
respectively.
Sales to geographic area consist of the
following:
|
2009
|
2008
|
2007
|
||||||||||
|
North
America
|
$ | 80,830 | $ | 86,631 | $ | 85,644 | ||||||
|
Europe
|
136,534 | 143,542 | 150,692 | |||||||||
|
Asia
|
10,495 | 11,440 | 9,125 | |||||||||
|
Other
|
8,418 | 8,005 | 7,113 | |||||||||
|
Total
|
$ | 236,277 | $ | 249,618 | $ | 252,574 | ||||||
This
table summarizes gross sales by product groups:
|
2009
|
2008
|
2007
|
||||||||||
|
APIs
and pharmaceutical intermediates
|
$ | 212,644 | $ | 220,722 | $ | 220,386 | ||||||
|
Other
|
23,633 | 28,896 | 32,188 | |||||||||
|
Total
|
$ | 236,277 | $ | 249,618 | $ | 252,574 | ||||||
One customer, Gyma, a distributor
representing multiple customers, accounted for 11.5% of consolidated gross sales
for 2009. Two customers each account for 10% of consolidated gross
sales for the years ended December 31, 2008 and 2007. One customer,
Warner Chilcott plc, with which a long-term sales contract is in effect, account
for 10.0% and 11.2% of consolidated sales for 2008 and 2007,
respectively. The second customer, Gyma, accounted for 11.8% and
12.5% for 2008 and 2007, respectively.
CAMBREX
CORPORATION AND SUBSIDIARIES
NOTES
TO CONSOLIDATED FINANCIAL STATEMENTS - (Continued)
(dollars
in thousands, except share data)
|
(17)
|
Commitments
|
The Company has operating leases
expiring on various dates through the year 2019. The leases are
primarily for the rental of office space, office and laboratory equipment and
vehicles. At December 31, 2009, future minimum commitments under
non-cancelable operating lease arrangements were as follows:
|
Year
ended December 31:
|
||||
|
2010
|
$ | 1,822 | ||
|
2011
|
490 | |||
|
2012
|
405 | |||
|
2013
|
364 | |||
|
2014
|
376 | |||
|
2015
and thereafter
|
1,581 | |||
|
Total
commitments
|
$ | 5,038 | ||
Total operating lease expense was
$1,978, $2,270 and $2,270 for the years ended December 31, 2009, 2008 and 2007,
respectively.
The Company is party to several
unconditional purchase obligations resulting from contracts that contain legally
binding provisions with respect to quantities, pricing and timing of
purchases. The Company’s purchase obligations mainly include
commitments to purchase raw materials. At December 31, 2009 future
commitments under these obligations were as follows:
|
Year
ended December 31:
|
||||
|
2010
|
$ | 6,452 | ||
|
2011
|
963 | |||
|
2012
|
- | |||
|
2013
|
- | |||
|
2014
|
- | |||
|
Total
commitments
|
$ | 7,415 | ||
|
(18)
|
Contingencies
|
The Company is subject to various
investigations, claims and legal proceedings covering a wide range of matters
that arise in the ordinary course of its business activities. The
Company continually assesses all known facts and circumstances as they pertain
to all legal and environmental matters and evaluates the need for reserves and
disclosures as deemed necessary based on these facts and
circumstances. These matters, either individually or in the
aggregate, could have a material adverse effect on the Company's financial
condition, operating results and cash flows in a future reporting
period.
Environmental
In connection with laws and regulations
pertaining to the protection of the environment, the Company and its
subsidiaries are a party to several environmental proceedings and remediation
investigations and cleanups and, along with other companies, have been named
potentially responsible parties (“PRP”) for certain waste disposal sites
("Superfund sites"). Additionally, the Company has retained the
liability for certain environmental proceedings associated with the sale of the
Rutherford Chemicals business.
CAMBREX
CORPORATION AND SUBSIDIARIES
NOTES
TO CONSOLIDATED FINANCIAL STATEMENTS - (Continued)
(dollars
in thousands, except share data)
|
(18)
|
Contingencies
(continued)
|
Each of these matters is subject to
various uncertainties, and it is possible that some of these matters will be
decided unfavorably against the Company. The resolution of such
matters often spans several years and frequently involves regulatory oversight
or adjudication. Additionally, many remediation requirements are not
fixed and are likely to be affected by future technological, site, and
regulatory developments. Consequently, the ultimate liability with
respect to such matters, as well as the timing of cash disbursements cannot be
determined with certainty.
In matters where the Company has been
able to reasonably estimate its liability, the Company has accrued for the
estimated costs associated with the study and remediation of Superfund sites not
owned by the Company and the Company's current and former operating
sites. These accruals were $6,163 and $6,226 at December 31, 2009 and
2008, respectively. The decrease in the accrual includes payments of
$310 partially offset by increases to reserves of $110 and the impact of
currency of $137. Based upon available information and analysis, the
Company's current accrual represents management's best estimate of the probable
and estimable costs associated with environmental proceedings including amounts
for investigation fees where full remediation costs may not be estimable at the
reporting date.
CasChem
As a result of the sale of the Bayonne,
New Jersey facility, the Company became obligated to investigate site conditions
and conduct required remediation under the New Jersey Industrial Site Recovery
Act. The Company submitted a sampling plan to the New Jersey
Department of Environmental Protection (“NJDEP”) and is awaiting
approval. The results of the completed and proposed sampling, and any
additional sampling deemed necessary, will be used to develop an estimate of the
Company's future liability for remediation costs, if any.
Cosan
In response to the NJDEP, the Company
completed its initial investigation and submitted the results of the
investigation and a proposed Remedial Action Work Plan (“RAW”) to the NJDEP for
its Cosan Clifton, New Jersey site. The NJDEP subsequently rejected the
RAW and requested additional investigative work at the site and that work is
on-going. The reserve was $1,164 at December 31, 2009 which is based on
the initial remedial action plan. The results of the additional
investigative work may impact the remediation plan and costs.
Additionally,
the Company has recorded a liability of $916 for the Cosan Carlstadt, New Jersey
site based on the investigations completed to date and the proposed RAW
submitted to the NJDEP for their approval. The NJDEP has subsequently
required the Company to perform additional investigative work prior to approval
of the RAW. The results of this additional investigative work may impact
the remediation plan and costs.
Berry’s
Creek
The Company received a notice from the
United States Environmental Protection Agency (“USEPA”) that two former
operating subsidiaries of the Company are considered PRPs at the Berry’s Creek
Superfund Site in New Jersey. The operating companies are among many
other PRPs that were listed in the notice. Pursuant to the notice, the
PRPs have been asked to perform a remedial investigation and feasibility study
of the Berry’s Creek Site. The Company has joined the group of PRPs and
filed a response to the USEPA agreeing to jointly negotiate to conduct or fund
an appropriate remedial investigation and feasibility study of the Berry’s Creek
Site. The PRPs have
engaged consultants to evaluate investigation and remedial alternatives and
develop a method to allocate related costs among the PRPs. As of
December 31, 2009 the Company’s reserve was $309 to cover the initial phase of
investigation based on a tentative agreement on the allocation of the site
investigation costs among the PRPs. The investigation is expected to
take several years and at this time it is too early to predict the extent of any
additional liabilities.
CAMBREX
CORPORATION AND SUBSIDIARIES
NOTES
TO CONSOLIDATED FINANCIAL STATEMENTS - (Continued)
(dollars
in thousands, except share data)
|
(18)
|
Contingencies
(continued)
|
Nepera,
Inc. – Maybrook and Harriman Sites
Nepera,
Inc. (“Nepera”) is named a PRP of the Maybrook Site in Hamptonburgh, New York by
the USEPA in connection with the disposition, under appropriate permits, of
wastewater at that site prior to Cambrex's acquisition of Nepera in
1986. The USEPA also issued the Company a Notice of Potential
Liability and the Company signed a Consent Decree to complete the Record of
Decision (“ROD”) and has provided the USEPA with appropriate financial
assurance, including a letter of credit to guarantee the obligation under the
Consent Decree.
Nepera is
also named a responsible party of the Harriman, New York production facility by
the New York State Department of Environmental Conservation. A final
ROD was issued which describes the remediation plan for the
site. Implementation of the ROD is on-going.
As of
December 31, 2009, the reserve recorded on the books was $1,300 and represents
the Company’s best estimate to complete both RODs.
Solvent
Recoveries Superfund Site
A subsidiary of the Company is one of
approximately 1,300 PRPs at a Superfund site (“the Site”) in Southington,
Connecticut, once operated by Solvent Recoveries, Inc. The PRP group
has completed a Remedial Investigation/Feasibility Study and the USEPA has
proposed remediation of the Site. In 2008, the Company agreed to
enter into a consent decree and settlement with the other PRPs and the USEPA
whereby the Company agreed to pay a settlement amount of $353 with an initial
payment of $106 and the remaining $247 to be paid in installments over time as
the remediation proceeds. The Company has reserved for the unpaid
portion of the settlement and has entered into a letter of credit to guarantee
the payment obligation under the settlement.
Newark
Bay Complex Litigation
CasChem and Cosan have been named as
two of several hundred third-party defendants in a third-party complaint filed
in February 2009, by Maxus Energy Corporation (“Maxus”) and Tierra Solutions,
Inc. (“Tierra”). The original plaintiffs include the NJDEP, the
Commissioner of the NJDEP and the Administrator of the New Jersey Spill
Compensation Fund, which originally filed suit in 2005 against Maxus, Tierra and
other defendants seeking recovery of cleanup and removal costs for alleged
discharges of dioxin and other hazardous substances into the Passaic River,
Newark Bay, Hackensack River, Arthur Kill, Kill Van Kull and adjacent waters
(the “Newark Bay Complex”). Maxus and Tierra are now seeking
contribution from third-party defendants, including subsidiaries of the Company,
for cleanup and removal costs for which each may be held liable in the
lawsuit. Maxus and Tierra also seek recovery for cleanup and removal
costs that each has incurred or will incur relating to the Newark Bay
Complex. The Company expects to vigorously defend against the
lawsuit. At this time it is too early to predict whether the Company will have
any liability in this matter.
The Company is involved in other
environmental matters where the range of liability is not reasonably estimable
at this time and it is not determinable when information will become available
to provide a basis for adjusting or recording an accrual, should an accrual
ultimately be required.
CAMBREX
CORPORATION AND SUBSIDIARIES
NOTES
TO CONSOLIDATED FINANCIAL STATEMENTS - (Continued)
(dollars
in thousands, except share data)
|
(18)
|
Contingencies
(continued)
|
Litigation and Other
Matters
Lorazepam
and Clorazepate
In 1998 the Company and a subsidiary
were named as defendants (along with Mylan Laboratories, Inc. (“Mylan”) and Gyma
in a proceeding instituted by the Federal Trade Commission (“FTC”) in the United
States District Court for the District of Columbia (the “District
Court”). Suits were also commenced by several State Attorneys’
General and class action complaints by private plaintiffs in various state
courts. The suits alleged violations of the Federal Trade Commission
Act arising from exclusive license agreements between the Company and Mylan
covering two APIs (Lorazepam and Clorazepate). The FTC and Attorneys’
General suits were settled in February 2001.
All cases have been resolved except for
one brought by four health care insurers. In 2008 the District Court, in this
remaining case, entered judgment after trial against Mylan, Gyma and Cambrex in
the amount of $8,355, payable jointly and severally, and also a punitive damage
award against each defendant in the amount of $16,709. In addition, the
District Court ruled that the defendants were also subject to a total of
approximately $7,000 in prejudgment interest. The parties will appeal the
awards.
Cambrex paid $12,415 in exchange for a
release from Mylan and full indemnity in 2003 against future costs or
liabilities in related litigation brought by purchasers, as well as potential
future claims related to this matter. Cambrex expects any payment of the
judgment against it to be made by Mylan under the indemnity described
above.
Other
The
Company has commitments incident to the ordinary course of business including
corporate guarantees of certain subsidiary obligations to the Company’s lenders
related to financial assurance obligations under certain environmental laws for
remediation; closure and third party liability requirements of certain of its
subsidiaries and a former operating location; contract provisions for
indemnification protecting its customers and suppliers against third party
liability for manufacture and sale of Company products that fail to meet product
warranties and contract provisions for indemnification protecting licensees
against intellectual property infringement related to licensed Company
technology or processes.
Additionally, as permitted under
Delaware law, the Company indemnifies its officers, directors and employees for
certain events or occurrences while the officer, director or employee is, or
was, serving at the Company’s request in such capacity. The term of the
indemnification period is for the officer's, director's or employee’s lifetime.
The maximum potential amount of future payments the Company could be required to
make under these indemnification agreements is unlimited; however, the Company
has a Director and Officer insurance policy that covers a portion of any
potential exposure. The Company currently believes the estimated fair
value of its indemnification agreements is not material based on currently
available information, and as such, the Company has no liabilities recorded for
these agreements as of December 31, 2009.
Cambrex's subsidiaries are party to a
number of other proceedings that are not considered material at this
time.
CAMBREX
CORPORATION AND SUBSIDIARIES
NOTES
TO CONSOLIDATED FINANCIAL STATEMENTS - (Continued)
(dollars
in thousands, except share data)
|
(19)
|
Discontinued
Operations
|
In February 2007, the Company completed
the sale of the businesses that comprised the Bioproducts and Biopharma segments
(excluding certain liabilities) to Lonza for total cash consideration of
$463,914, including working capital adjustments. As a result of this
transaction, the Company reported a gain of $235,489 in 2007 and all periods
presented reflect the results of these businesses as discontinued
operations.
In July 2007 the Company entered into a
settlement agreement settling litigation which had been commenced by the
purchasers of the Rutherford Business in April 2006. As a result of this
settlement, the Company’s 2007 results include a charge of $4,041, net of tax of
$595, recorded in discontinued operations. In addition, during
2007 the Company recorded expense of $1,000 for an adjustment to an
environmental reserve at a Rutherford Business site. Refer to Note 18
for a complete discussion on these matters.
The following table shows revenues and
income from the discontinued operations:
|
2007
|
||||
|
Revenues
|
$ | 20,335 | ||
|
Pre-tax
income of discontinued operations
|
$ | 545 | ||
|
Gain
on sale of Bioproducts and Biopharma segments
|
235,489 | |||
|
Rutherford
litigation settlement
|
(4,636 | ) | ||
|
Rutherford
environmental reserve adjustment
|
(1,000 | ) | ||
|
Income
from discontinued operations before income taxes
|
230,398 | |||
|
Provision
for income taxes
|
7,639 | |||
|
Income
from discontinued operations, including gains from dispositions, net of
tax
|
$ | 222,759 | ||
The 2007 provision for income taxes
includes $7,915 of expense for discontinued operations taxable
income.
CAMBREX
CORPORATION AND SUBSIDIARIES
SELECTED
QUARTERLY FINANCIAL AND SUPPLEMENTARY DATA - UNAUDITED
(in
thousands, except per share data)
|
1st
|
2nd
|
3rd
|
4th
|
|||||||||||||
|
Quarter
|
Quarter
|
Quarter
|
Quarter (1)
|
|||||||||||||
|
2009
|
||||||||||||||||
|
Gross
sales
|
$ | 60,000 | $ | 59,766 | $ | 57,802 | $ | 58,709 | ||||||||
|
Net
revenues
|
61,032 | 59,281 | 56,370 | 57,867 | ||||||||||||
|
Gross
profit
|
19,133 | 19,683 | 16,948 | 14,514 | ||||||||||||
|
Net
income/(loss)
|
4,738 | 5,459 | 2,963 | (2,768 | ) | |||||||||||
|
Earnings/(loss)
per share of common stock:(6)
|
||||||||||||||||
|
Basic
|
0.16 | 0.19 | 0.10 | (0.09 | ) | |||||||||||
|
Diluted
|
0.16 | 0.19 | 0.10 | (0.09 | ) | |||||||||||
|
Average
shares:
|
||||||||||||||||
|
Basic
|
29,200 | 29,222 | 29,253 | 29,286 | ||||||||||||
|
Diluted
|
29,203 | 29,247 | 29,303 | 29,286 | ||||||||||||
|
|
||||||||||||||||
|
|
1st
|
2nd
|
3rd
|
4th
|
||||||||||||
|
Quarter (2)
|
Quarter (3)
|
Quarter (4)
|
Quarter (5)
|
|||||||||||||
|
2008
|
||||||||||||||||
|
Gross
sales
|
$ | 61,706 | $ | 66,226 | $ | 56,508 | $ | 65,178 | ||||||||
|
Net
revenues
|
60,990 | 65,813 | 58,292 | 64,133 | ||||||||||||
|
Gross
profit
|
21,929 | 19,811 | 16,235 | 15,768 | ||||||||||||
|
Net
income/(loss)
|
4,246 | 1,836 | 2,797 | (950 | ) | |||||||||||
|
Earnings/(loss)
per share of common stock:(6)
|
||||||||||||||||
|
Basic
|
0.15 | 0.06 | 0.10 | (0.03 | ) | |||||||||||
|
Diluted
|
0.15 | 0.06 | 0.10 | (0.03 | ) | |||||||||||
|
Average
shares:
|
||||||||||||||||
|
Basic
|
29,035 | 29,090 | 29,163 | 29,175 | ||||||||||||
|
Diluted
|
29,093 | 29,101 | 29,178 | 29,175 | ||||||||||||
|
(1)
|
Net
income includes tax expense of approximately $5,300 for an estimate of an
international tax liability related to a 2003
transaction.
|
|
(2)
|
Net
income includes pre-tax charges of $177 within operating expenses for the
costs related to strategic alternatives and $634 within operating expenses
for restructuring costs.
|
|
(3)
|
Net
income includes pre-tax charges of $398 within operating expenses for the
costs related to strategic alternatives, $514 within operating expenses
for restructuring costs and $597 within operating expenses for the
acceleration of equity awards related to the former CEO's
retirement.
|
|
(4)
|
Net
income includes pre-tax charges of $833 within operating expenses for the
costs related to strategic alternatives, $321 within operating expenses
for restructuring costs and $35 within operating expenses for the
modification of equity awards related to the former CEO's
retirement.
|
|
(5)
|
Net
loss includes pre-tax charges of $107 within operating expenses for the
costs related to strategic alternatives, $3,226 within operating expenses
for restructuring costs and $408 within operating expenses related to the
former CEO's retirement.
|
|
(6)
|
Earnings
per share calculations for each of the quarters are based on the weighted
average number of shares outstanding for each period, as such, the sum of
the quarters may not necessarily equal the earnings per share amount for
the year.
|
|
Item 9
|
Changes in and Disagreements
with Accountants on Accounting and Financial
Disclosure
|
None.
|
Item 9A
|
Controls and
Procedures
|
Conclusion
Regarding the Effectiveness of Disclosure Controls and Procedures
The Company maintains disclosure
controls and procedures as defined in Rules 13a-15(e) and 15d-15(e) under the
Securities Exchange Act of 1934, as amended (the “Exchange Act”) that are
designed to ensure that information required to be disclosed in its reports
filed or submitted under the Exchange Act is processed, recorded, summarized and
reported within the time periods specified in the SEC’s rules and forms, and
that such information is accumulated and communicated to the Company’s
management, including the Company’s Chief Executive Officer and Chief Financial
Officer, as appropriate, to allow for timely decisions regarding required
disclosure. In designing and evaluating the disclosure controls and procedures,
management recognizes that any controls and procedures, no matter how well
designed and operated, can provide only reasonable assurance of achieving the
desired control objectives, and management is required to apply its judgment in
evaluating the cost-benefit relationship of possible controls and
procedures.
As required by SEC Rule 13a-15(b), the
Company carried out an evaluation, under the supervision and with the
participation of management, including the Company’s Chief Executive Officer and
Chief Financial Officer, of the effectiveness of the design and operation of the
Company’s disclosure controls and procedures as of the end of the period covered
by this Annual Report. Based on this evaluation, our Chief Executive Officer and
Chief Financial Officer have concluded that as of December 31, 2009, our
disclosure controls and procedures are effective to ensure that information
required to be disclosed by us in the reports that we file or submit under the
Exchange Act is (i) recorded, processed, summarized and reported, within the
time periods specified in the SEC’s rules and forms and (ii) accumulated and
communicated to management, including our chief executive officer and chief
financial officer, as appropriate to allow timely decisions regarding required
disclosure.
Management's
Report on Internal Control Over Financial Reporting
Our
management is responsible for establishing and maintaining adequate internal
control over financial reporting, as defined in Exchange Act Rule 13a-15(f).
Internal control over financial reporting is a process designed to provide
reasonable assurance regarding the reliability of financial reporting and the
preparation of financial statements for external purposes in accordance with
accounting principles generally accepted in the United States, and include those
policies and procedures that:
|
|
·
|
Pertain
to the maintenance of records, that in reasonable detail, accurately and
fairly represent the transactions and dispositions of the assets of the
Company,
|
|
|
·
|
Provide
reasonable assurance that transactions are recorded as necessary to permit
preparation of financial statements in accordance with generally accepted
accounting principles, and that receipts and expenditures are being made
only in accordance with authorizations of management and the Board of
Directors of the Company, and
|
|
|
·
|
Provide
reasonable assurance regarding prevention or timely detection of
unauthorized acquisition, use or disposition of the Company’s assets that
could have a material effect on the financial
statements.
|
Because
of its inherent limitations, internal control over financial reporting may not
prevent or detect misstatements. Also, projections of any evaluation of
effectiveness to future periods are subject to the risk that controls may become
inadequate because of changes in conditions, or that the degree of compliance
with the policies or procedures may deteriorate.
Under the
supervision and with the participation of our management, including our
principal executive officer and principal financial officer, we carried out an
evaluation of the effectiveness of our internal control over financial reporting
as of December 31, 2009 based on the Internal Control—Integrated
Framework issued by the Committee of Sponsoring Organizations of the
Treadway Commission ("COSO"). Our management concluded that based on
its assessment, our internal control over financial reporting was effective as
of December 31, 2009. Effectiveness of our internal control over
financial reporting as of December 31, 2009 has been audited by BDO Seidman,
LLP, an independent registered public accounting firm, as stated in their report
which appears elsewhere herein.
Changes
in Internal Control over Financial Reporting
There were no changes in our internal
control over financial reporting identified in connection with the evaluation
required by paragraph (d) of Exchange Act Rules 13a-15 or 15d-15 that occurred
during our last fiscal quarter that have materially affected, or are reasonably
likely to materially affect, our internal control over financial
reporting.
|
Item 9B
|
Other
Information
|
None.
PART III
|
Item
10
|
Directors, Executive Officers
and Corporate Governance.
|
Executive
Officers of the Registrant
The following table lists the officers
of the Company:
|
Name
|
Age
|
Office
|
|
Steven
M. Klosk*
|
52
|
President,
Chief Executive Officer
|
|
James
G. Farrell
|
43
|
Vice
President and Corporate Controller
|
|
Paolo
Russolo*
|
65
|
President,
Cambrex Profarmaco Milano
|
|
Gregory
P. Sargen*
|
44
|
Vice
President & Chief Financial Officer
|
|
F.
Michael Zachara*
|
46
|
Vice
President, General Counsel and Corporate Secretary
|
|
*Executive
Officer
|
The Company's executive officers are
elected by the Board of Directors and serve at the Board's
discretion.
Mr. Klosk
joined Cambrex in October 1992 and has served as President & Chief Executive
Officer since May 2008. He also became a member of the Board of
Directors in May 2008. Mr. Klosk joined the Company as Vice
President, Administration. He was appointed Executive Vice President,
Administration in October 1996 and was promoted to the position of Executive
Vice President, Administration and Chief Operating Officer for the Cambrex
Pharma and Biopharmaceutical Business Unit in October 2003. In
January 2005, Mr. Klosk assumed direct responsibility for the leadership of the
Biopharmaceutical Business Unit as Chief Operating Officer. In August
2006, Mr. Klosk assumed the responsibility of the Pharma business as Executive
Vice President and Chief Operating Officer – Biopharma & Pharma and in
February 2007 was appointed to Executive Vice President, Chief Operating Officer
& President, Pharmaceutical Products and Services. From 1988
until he joined Cambrex, Mr. Klosk was Vice President, Administration and
Corporate Secretary for The Genlyte Group, Inc. From 1985 to 1988, he
was Vice President, Administration for Lightolier, Inc., a subsidiary of The
Genlyte Group, Inc.
Mr. Farrell joined Cambrex in September
2005 and has served as Vice President and Corporate Controller since July
2007. Mr. Farrell previously held the position of Corporate
Controller. Mr. Farrell was employed during a part of 2008 by PDI,
Inc. as Vice President and Corporate Controller/Interim Chief Financial
Officer. Mr. Farrell returned to Cambrex in late 2008. From 1994
until 2005, he was with Ingersoll-Rand Company, most recently as Director,
Accounting Policy, Procedures and External Reporting. Mr. Farrell was
with Ernst & Young from 1988 to 1994, most recently as Audit
Manager.
Dr. Russolo is President, Profarmaco
Milano and joined the Company in 1994 with the acquisition of Profarmaco Nobel
S.r.l. in Milan Italy, where he served as Managing Director since
1982. Dr. Russolo joined Profarmaco Nobel S.r.l. in
1971. Upon the acquisition of Profarmaco Nobel S.r.l., Dr. Russolo
continued serving in the role of Managing Director until 2000, when he was
appointed to President, Cambrex Profarmaco Business Unit. Upon the
completion of the sale of the Landen facility Dr. Russolo assumed his current
position.
Mr. Sargen joined Cambrex in February
2003 and has served as Vice President and Chief Financial Officer since February
2007. Mr. Sargen previously held the position of Vice President,
Finance. Previously, he was with Exp@nets, Inc. from 1999 through
2002, serving in the roles of Executive Vice President, Finance/Chief Financial
Officer and Vice President/Corporate Controller. From 1996 to 1998,
he was with Fisher Scientific International’s Chemical Manufacturing Division,
serving in the roles of Vice President, Finance and Controller. Mr.
Sargen has also held various positions in finance, accounting and audit with
Merck & Company, Inc., Heat and Control, Inc., and Deloitte &
Touche.
Mr. Zachara joined Cambrex in June 2008
and has served as Vice President, General Counsel and Corporate Secretary since
February 2009. Mr. Zachara formerly held the position of Assistant
General Counsel and Assistant Corporate Secretary. Previously, he was
with Sun Chemical Corporation from 1997 to 2008 as Senior Corporate Attorney,
Assistant Secretary and Director of Real Estate. From 1994 to 1997,
he was with Brown & Wood LLP, a New York firm as Associate, Real
Estate/Environmental Department. Mr. Zachara has also held positions
with Shanley & Fisher, P.C. and James C. Anderson Associates.
|
Item 11
|
Executive
Compensation.
|
|
Item 12
|
Security Ownership of Certain
Beneficial Owners and Management and Related Stockholder
Matters.
|
|
Item 13
|
Certain Relationships and
Related Transactions and Director
Independence.
|
|
Item 14
|
Principal Accountant Fees and
Services.
|
The remaining information called for by
Part III is hereby incorporated by reference to the information set forth under
the captions “Principal Stockholders,” “Common Stock Ownership by Directors and
Executive Officers,” “Board of Directors,” “Election of Directors,” “Section
16(a) Beneficial Ownership Reporting Compliance,” “Code of Ethics,”
“Compensation Committee Interlocks and Insider Participation,” “Compensation
Committee Report on Executive Compensation,” “Executive and Other Compensation,”
“Executive and Other Compensation,” “Audit Committee Report” and “Principal
Accounting Firm Fees” in the registrant's definitive proxy statement for the
Annual Meeting of Stockholders, to be held April 22, 2010, which meeting
involves the election of directors, which definitive proxy statement is being
filed with the Securities and Exchange Commission pursuant to Regulation
14A.
PART IV
|
Item
15
|
Exhibits and
Financial Statement
Schedules
|
(a) 1. The
following consolidated financial statements of the Company are filed as part of
this report:
|
Page
Number
|
|
|
(in this report)
|
|
|
Financial
Statements:
|
|
|
Reports
of Independent Registered Public Accounting Firm
|
34
|
|
Consolidated
Balance Sheets as of December 31, 2009 and 2008
|
36
|
|
Consolidated
Statements of Operations for the Years Ended December 31, 2009, 2008 and
2007
|
37
|
|
Consolidated
Statements of Stockholders’ Equity for the Years Ended December 31, 2009,
2008 and 2007
|
38
|
|
Consolidated
Statements of Cash Flows for the Years Ended December 31, 2009, 2008 and
2007
|
39
|
|
Notes
to Consolidated Financial Statements
|
40
|
|
Selected
Quarterly Financial and Supplementary Data (unaudited)
|
71
|
(a) 2. (i) The
following schedule to the consolidated financial statements of the Company as
filed herein and the Report of Independent Registered Public Accounting Firms
are filed as part of this report.
|
Page
Number
|
|
|
(in this report)
|
|
|
Schedule
II – Valuation and Qualifying Accounts
|
77
|
All other schedules are omitted because
they are not applicable or not required or because the required information is
included in the consolidated financial statements of the Company or the notes
thereto.
(a) 3. The
exhibits filed in this report are listed in the Exhibit Index on pages 79 -
81.
SCHEDULE
II
CAMBREX
CORPORATION
VALUATION
AND QUALIFYING ACCOUNTS
FOR
THE YEARS ENDED DECEMBER 31, 2009, 2008 and 2007
(dollars
in thousands)
|
Column A
|
Column B
|
Column C
|
Column D
|
Column E
|
||||||||||||||||
|
Additions
|
||||||||||||||||||||
|
Charged/
|
Charged/
|
|||||||||||||||||||
|
Balance
|
(Credited)
to
|
(Credited)
to
|
Balance
|
|||||||||||||||||
|
Beginning
|
Cost
and
|
Other
|
End
of
|
|||||||||||||||||
|
of Year
|
Expenses
|
Accounts
|
Deductions
|
Year
|
||||||||||||||||
|
Description
|
||||||||||||||||||||
|
Year
ended December 31, 2009:
|
||||||||||||||||||||
|
Doubtful
trade receivables and returns and allowances
|
$ | 1,105 | $ | (191 | ) | $ | 31 | $ | 318 | $ | 627 | |||||||||
|
Deferred
tax valuation allowance
|
79,230 | 103 | 1,035 | - | 80,368 | |||||||||||||||
|
Year
ended December 31, 2008:
|
||||||||||||||||||||
|
Doubtful
trade receivables and returns and allowances
|
$ | 560 | $ | 600 | $ | (41 | ) | $ | 14 | $ | 1,105 | |||||||||
|
Deferred
tax valuation allowance
|
64,842 | 3,762 | 10,626 | - | 79,230 | |||||||||||||||
|
Year
ended December 31, 2007:
|
||||||||||||||||||||
|
Doubtful
trade receivables and returns and allowances
|
$ | 571 | $ | 55 | $ | 35 | $ | 101 | $ | 560 | ||||||||||
|
Deferred
tax valuation allowance
|
91,403 | (21,241 | ) * | (5,320 | ) | - | 64,842 | |||||||||||||
*
Includes $(31,584) related to discontinued operations.
SIGNATURES
Pursuant to the requirements of Section
13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly
caused this report to be signed on its behalf by the undersigned, thereunto duly
authorized.
|
CAMBREX
CORPORATION
|
|||
|
By
|
/s/ Gregory P. Sargen
|
||
|
Gregory
P. Sargen
|
|||
|
Vice
President and Chief Financial Officer
|
|||
|
Date:
|
February
11, 2010
|
||
Pursuant to the requirements of the
Securities Exchange Act of 1934, this report has been signed below by the
following persons on behalf of the registrant and in the capacities and on the
dates indicated.
| Signature |
Title
|
Date
|
|||
|
/s/
|
STEVEN M. KLOSK
|
President
and Chief Executive Officer
|
|
)
|
|
|
Steven
M. Klosk
|
|||||
|
/s/
|
GREGORY P. SARGEN
|
Vice
President and Chief Financial
|
)
|
||
|
Gregory
P. Sargen
|
Officer
(Principal Financial Officer and Accounting Officer)
|
||||
|
/s/
|
JOHN R. MILLER*
|
Chairman
of the Board of Directors
|
)
|
||
|
John
R. Miller
|
|||||
|
/s/
|
DAVID R. BETHUNE *
|
Director
|
)
|
||
|
David
R. Bethune
|
|||||
|
/s/
|
ROSINA B.DIXON*
|
Director
|
)
|
||
|
Rosina
B. Dixon, M.D.
|
|||||
|
/s/
|
ROY W. HALEY*
|
Director
|
)
|
||
|
Roy
W. Haley
|
|||||
|
/s/
|
KATHRYN RUDIE HARRIGAN*
|
Director
|
)
|
||
|
Kathryn
Rudie Harrigan, PhD
|
|||||
|
/s/
|
LEON J. HENDRIX, JR.*
|
Director
|
)
February 11, 2010
|
||
|
Leon
J. Hendrix, Jr.
|
|||||
|
/s/
|
ILAN KAUFTHAL*
|
Director
|
)
|
||
|
Ilan
Kaufthal
|
|||||
|
/s/
|
WILLIAM KORB*
|
Director
|
)
|
||
|
William
Korb
|
|||||
|
/s/
|
PETER G. TOMBROS*
|
Director
|
)
|
||
|
Peter
G. Tombros
|
|||||
|
*By
|
/s/ STEVEN M.
KLOSK
|
)
|
|||
|
Steven
M. Klosk
|
|||||
|
Attorney-in-Fact
|
|||||
EXHIBIT
INDEX
|
Exhibit No.
|
Description
|
|
|
3.1
|
--
|
Restated
Certificate of Incorporation of registrant, as
amended.(N).
|
|
3.2
|
--
|
By
Laws of registrant, as amended.(N).
|
|
4.1
|
--
|
Form
of Certificate for shares of Common Stock of registrant.(A - Exhibit
4(a)).
|
|
10.1
|
--
|
2009
Long Term Incentive Plan (X – Exhibit 1).
|
|
--
|
Directors’
Compensation Program.(K).
|
|
| 10.8 | -- | Asset purchase agreement dated as of August 7, 2003 between Rutherford Acquisition Corporation and Cambrex Corporation and The Sellers listed in the asset in the asset Purchase agreement.(Y). |
|
10.9
|
--
|
Credit
Agreement dated as of April 6, 2007 between Cambrex Corporation, the
subsidiary borrowers party hereto, the subsidiary guarantors party hereto,
the lenders party hereto and JP Morgan Chase Bank, N.A., as Administrative
Agent.(U).
|
| 10.10 | -- | Settlement Agreement and Release and Environmental Escrow Agreement dated July 30, 2007 between Rutherford Chemicals LLC, Vertellus Specialties Holdings UK Ltd. (formerly Rutherford Chemicals UK Ltd.), Vertellus Specialties UK Ltd. (formerly Seal Sands Chemicals Ltd.), and Vertellus Specialties Holdings Corp. (formerly Rutherford Chemicals Holdings Corp.), and Cambrex Corporation, Nepera, Inc., CasChem Inc., Zeeland Chemicals, Inc., Nepcam, Inc., and Cambrex Ltd.(Z). |
|
10.12
|
--
|
Supplemental
Executive Retirement Plan Change of Control
Amendment.(W).
|
|
10.16
|
--
|
1994
Stock Option Plan.(C).
|
|
10.17
|
--
|
1996
Performance Stock Option Plan.(G).
|
|
10.18
|
--
|
1998
Performance Stock Option Plan.(H).
|
|
10.19
|
--
|
2000
Employee Performance Stock Option Plan.(H).
|
|
10.20
|
--
|
Form
of Employment Agreement (amended and restated) between the registrant and
its executive officers named in the Revised Schedule of Parties thereto.(O
– Exhibit 10.20) (as amended (P) Exhibit 10.20.1).
|
|
--
|
Revised
Schedule of Parties (Exhibit 10.20 hereto).(E).
|
|
|
10.22
|
--
|
Cambrex
Corporation Savings Plan.(B).
|
|
10.23
|
--
|
Cambrex
Corporation Supplemental Retirement Plan.(D).
|
|
10.25
|
--
|
Employment
Agreement dated February 6, 2007 between the registrant and Gregory P.
Sargen.(V).
|
|
10.29
|
--
|
Deferred
Compensation Plan of Cambrex Corporation (as amended and restated as of
March 1, 2001).(O).
|
|
10.32
|
--
|
Employment
Agreement dated February 6, 2007 between the registrant and Paolo
Russolo.(V).
|
|
10.33
|
--
|
2001
Performance Stock Option Plan.(I).
|
|
10.34
|
--
|
2003
Performance Stock Option Plan.(I).
|
|
10.35
|
--
|
2004
Performance Incentive Plan.(J).
|
|
10.36
|
--
|
Directors’
Common Stock Fee Payment Plan.(J).
|
|
10.38
|
--
|
2004
Incentive Plan.(L).
|
|
10.39
|
--
|
Separation
and General Release Agreement.(M).
|
| 10.41 | -- | Administrative Consent order dated September 16, 1985 of the New Jersey Department of Environmental Protection to Cosan Chemical Corporation.(A-Exhibit 10(q)). |
|
10.42
|
--
|
Registration
Rights Agreement dated as of June 5, 2006 between the registrant and
American Stock Transfer and Trust Company.(F).
|
|
10.46
|
--
|
Stock
Purchase Agreement dated October 23, 2006 between Lonza America Inc.,
Lonza Bioproducts AG, Lonza Sales AG, Lonza Group Limited and Cambrex
Corporation and Subsidiaries.(S – Exhibit 10.1).
|
|
10.47
|
--
|
Agreement
to Lift Sales Restrictions on Certain Vested
Options.(Q).
|
|
10.48
|
--
|
Agreement
to Accelerate Vesting of Certain Options.(R).
|
|
16.1
|
--
|
PricewaterhouseCoopers
LLP Letter.(T).
|
|
--
|
Subsidiaries
of registrant.(E).
|
|
|
--
|
Consent
of BDO Seidman LLP to the incorporation by reference of its report herein
in Registration Statement Nos. 333-57404, 333-22017, 33-37791, 33-81780,
33-81782, 333-113612, 333-113613, 333-129473 and 333-136529 on Form S-8 of
the registrant.(E).
|
|
_______________
See
legend on page 81
EXHIBIT
INDEX
|
Exhibit No.
|
Description
|
|
|
--
|
Powers
of Attorney to sign this report.(E).
|
|
|
--
|
CEO
Certification pursuant to Rule 13a – 14(a) and Rule 15d – 14(a) of the
Securities Exchange Act, as amended.(E).
|
|
|
--
|
CFO
Certification pursuant to Rule 13a – 14(a) and Rule 15d – 14(a) of the
Securities Exchange Act, as amended.(E).
|
|
|
--
|
CEO
and CFO Certification pursuant to 18 U.S.C. Section 1350, as adopted
pursuant to Section 906 of the Sarbanes-Oxley Act of
2002.(K).
|
|
_______________
See
legend on following page
EXHIBIT
INDEX
|
(A)
|
Incorporated
by reference to the indicated Exhibit to registrant's Registration
Statement on Form S-1 (Registration No.
33-16419).
|
|
(B)
|
Incorporated
by reference to registrant's Registration Statement on Form S-8
(Registration No. 33-81780) dated July 20,
1994.
|
|
(C)
|
Incorporated
by reference to registrant's Registration Statement on Form S-8
(Registration No. 33-81782) dated July 20,
1994.
|
|
(D)
|
Incorporated
by reference to the registrant's Annual Report on Form 10-K for
1994.
|
|
(E)
|
Filed
herewith.
|
|
(F)
|
Incorporated
by reference to the registrant’s Registration Statement on Form 8-A dated
May 25, 2006.
|
|
(G)
|
Incorporated
by reference to registrant’s Registration Statement on Form S-8
(Registration No. 333-22017) dated February 19,
1997.
|
|
(H)
|
Incorporated
by reference to registrant’s Registration Statement on Form S-8
(Registration No. 333-57404) dated March 22,
2001.
|
|
(I)
|
Incorporated
by reference to registrant’s Registration Statement on Form S-8
(Registration No. 333-113612) dated March 15,
2004.
|
|
(J)
|
Incorporated
by reference to registrant’s Registration Statement on Form S-8
(Registration No. 333-113613) dated March 15,
2004.
|
|
(K)
|
Furnished
herewith.
|
|
(L)
|
Incorporated
by reference to registrant’s Registration Statement on Form S-8
(Registration No. 333-129473) dated November 4,
2005.
|
|
(M)
|
Incorporated
by reference to the registrant’s Current Report on Form 8-K dated January
4, 2006.
|
|
(N)
|
Incorporated
by reference to registrant’s Quarterly Report on Form 10-Q for the period
ending March 31, 2007.
|
|
(O)
|
Incorporated
by reference to registrant’s Annual Report on Form 10-K for year end 2005
filed May 26, 2006.
|
|
(P)
|
Incorporated
by reference to registrant’s Quarterly Report on Form 10-Q for the period
ending September 30, 2006.
|
|
(Q)
|
Incorporated
by reference to registrant’s Current Report on Form 8-K dated November 7,
2006.
|
|
(R)
|
Incorporated
by reference to registrant’s Current Report on Form 8-K dated June 7,
2005.
|
|
(S)
|
Incorporated
by reference to registrant’s Current Report on Form 8-K filed October 24,
2006.
|
|
(T)
|
Incorporated
by reference to registrant’s Current Report on Form 8-K filed March 21,
2007.
|
|
(U)
|
Incorporated
by reference to registrant’s Current Report on Form 8-K filed April 11,
2007.
|
|
(V)
|
Incorporated
by reference to registrant’s Annual Report on Form 10-K for year end 2006
filed on March 15, 2007.
|
|
(W)
|
Incorporated
by reference to registrant’s Quarterly Report on Form 10-Q for the period
ending June 30, 2008.
|
|
(X)
|
Incorporated
by reference to registrant’s Definitive Proxy Statement for the 2009
Annual Meeting of Stockholders filed on March 20,
2009.
|
|
(Y)
|
Incorporated by reference to the registrant’s Current Report on Form 8-K dated November 10, 2003. |
|
(Z)
|
Incorporated by reference to the registrant’s Quarterly Report on Form 10-Q for the period ending September 30, 2007. |
81
