Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Chelsea Therapeutics International, Ltd. | d8k.htm |
 February 2010 Exhibit 99.1 © 2004-2010 Chelsea Therapeutics, Inc.
|
 2 Forward-Looking Statement Forward-Looking Statement This presentation is being provided for informational and discussion purposes. This presentation is not intended to provide and should not be relied upon as investment advice or an opinion regarding the appropriateness or suitability of any investment.
Nothing herein should be construed to be an offer to sell, or a solicitation of an offer to
buy, any securities. This presentation contains forward-looking statements regarding future events. These
statements are just predictions and are subject to risks and uncertainties that could cause
the actual events or results to differ materially. These risks and uncertainties
include failure to get regulatory approval for our product candidates, market acceptance for approved products, management of rapid growth, risks of regulatory review and clinical trials, intellectual property risks, and the need to acquire additional products. The
reader is referred to the documents that Chelsea Therapeutics International, Ltd. files from time to time with the Securities and Exchange Commission. |
 3 Chelsea Overview Chelsea Overview Developing Two Major Assets With Multiple, High-Potential Indications • Droxidopa: • Neurogenic Orthostatic Hypotension*(Northera™) • Intradialytic Hypotension* • Fibromyalgia • Adult Attention Deficit and Hyperactivity Disorder • Freezing of Gait* • Chronic Fatigue • Other norepinephrine related indications • Non-Metabolized Antifolates (CH-1504/CH-4051/CH-4446/Others): • Rheumatoid Arthritis • Psoriasis • Crohn’s Disease • Ankylosing Spondylitis • Uveitus • Oncology • Other Inflammatory Diseases *Approved indication in Japan
|
 Preclinical
Phase I Phase II Phase III Droxidopa: NOH (Northera) Study 302: PD, MSA, PAF Study 301: PD, MSA, PAF Study 306: PD Fibromyalgia Intradialytic Hypotension Metabolically Inert Antifolates: Rheumatoid Arthritis CH-4051 CH-1504 Other Autoimmune: CH-4051 Investigator Studies: AdultADHD: Droxidopa Chronic Fatigue Droxidopa 4 Strong and Balanced Pipeline Strong and Balanced Pipeline Data: Q3 09 Data: Q3 10 Data: Q2 11 Data: Q4 10/Q1 11 Data: Q1 09 Data: Q1 09 Data: Q2 11 Data: Q2 09 Data: 2011 Data: 2011 |
 5 Neurogenic Neurogenic Orthostatic Hypotension Orthostatic Hypotension • Neurogenic Orthostatic Hypotension (NOH) • Sudden fall in BP when standing from a sitting/lying position • Symptoms include lightheadedness, dizziness, falling, syncope • Caused by diminished synthesis and/or release of the NE used by autonomic nerves to regulate blood vessels and heart • 80% of all incidence of orthostatic hypotension is neurogenic in nature • Generates significant health care costs and impact on quality of life • Associated with 3 Primary Patient Groups |
 6 Northera Northera Overview Overview • “Prodrug” of Norepinephrine: Allows Autonomic Nervous System to Naturally Resume Normal Function • Enviable Safety and Efficacy Profile: Million+ years of Japanese Post-Approval Patient Data • SAEs never statistically significantly exceeded placebo in any clinical trial to date • Therapeutic use of peripheral dopa-decarboxylase inhibitors (DDI) in PD patients does not blunt effect of Northera • Sinemet® – a synthetic metabolic precursor to Dopamine • Effectively treats movement disorders caused by Dopamine deficits • Northera™ – a synthetic metabolic precursor to Norepinephrine • Effectively treats signs and symptoms of dysautonomia caused by norepinephrine deficits Sinemet ® Northera™ |
 7 Original Northera Original Northera Phase III Program: NOH Phase III Program: NOH • Study 302: Pivotal Proof of Efficacy – Withdrawal Design • Study 303: Long-Term Safety Extension to Study 302 – includes long-term efficacy assessment after 3 months • Study 301: Pivotal Proof of Efficacy – Induction Design • Study 304: Long-Term Safety Extension to Study 301 • Study 305: 24 HR Blood Pressure Monitoring Study – subgroup of 301 patients |
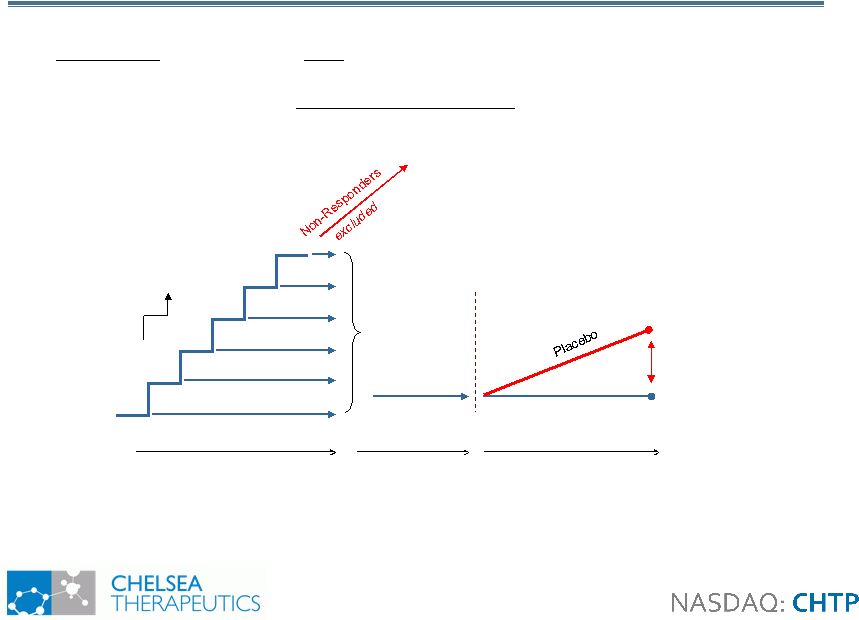 8 Study 302: Design Study 302: Design • Withdrawal design study in 101 NOH patients with primary autonomic failure • Primary Efficacy Measure: OHSA item 1 (dizziness) 100mg 200mg 300mg 400mg 500mg 600mg Up to 2 weeks (open-label) 1 week (open- label) Northera™ 2 weeks (blinded) Randomization Northera™ Visit |
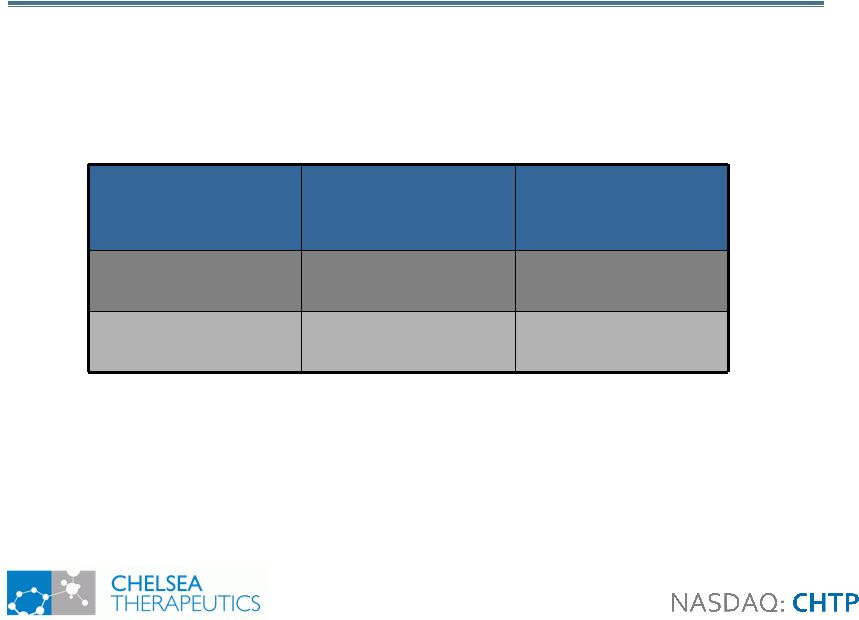
 9 Study 302: Demographics Study 302: Demographics All Patients PD Patients Only Placebo Northera Placebo Northera Number of Patients 51 50 23 21 Age (Years) 66.6 63.1 69.5 72.4 Gender (% Male) 62.8 60.0 73.9 71.4 Mean Northera at Titration (tid) 361 mg 412 mg 387 mg 352 mg DDI Use 29 (57%) 28 (56%) 23 (100%) 21 (100%) |
 10 No Significant Adverse Events No Significant Adverse Events • Midodrine label: supine hypertension (>200mmHg) =13.4% • Most common adverse events reported: • Falls: 2% Northera, 12% placebo • Headache: 2% Northera, 8% placebo • Supine Hypertension: BP (mm HG) Number of Placebo Patients Number of Northera Patients 180-200 3 (6%) 6 (12%) ? 200 0 1 (2%) |
 11 Key Symptomatic Efficacy Outcome Measures Key Symptomatic Efficacy Outcome Measures • Orthostatic Hypotension Questionnaire: OHQ • Orthostatic Hypotension Symptom Assessment: OHSA 1. Dizziness, lightheadedness, feeling faint, or feeling like you might blackout 2. Problems with vision (blurring, seeing spots, tunnel vision, etc.) 3. Weakness 4. Fatigue 5. Trouble concentrating 6. Head/neck discomfort • Orthostatic Hypotension Daily Activities Scale: OHDAS 1. Standing short time 2. Standing long time 3. Walking short time 4. Walking long time |
 Broad
Symptomatic and Functional Benefits Broad Symptomatic and Functional Benefits * p<0.05 ** p<0.01 Favors Northera Favors Placebo |
 Study 302:
Benefit on OHQ Composite Scores Study 302: Benefit on OHQ Composite Scores Composite Score Best Capture Full Therapeutic Benefit of Northera 13 |
 14 Study 302: Results Summary Study 302: Results Summary • Northera™ treatment associated with positive trends in 13 of 14 prospectively identified individual outcome variables • 16 of 17 including composite scores • 5 prospectively defined endpoints achieved statistical significance • Symptomatic/functional benefit Scorecard Droxidopa 5 Midodrine 0 • Greatest improvement in activities of daily living • Most robust treatment effect in PD subgroup • Post Hoc analysis of OHQ composite significant (p<0.05) • Significant improvement for both the clinician-recorded (p<o.o5) and patient- recorded (p<o.o1) CGI-severity scale • Reduction in self-reported falls • 6 placebo patients reported falls • 1 Northera patient reported falls • Safe and very well tolerated |
 Incorporating
Key Findings from 302 Incorporating Key Findings from 302 • Met with FDA to Discuss Results and Key Learnings from Study 302 • Provided all data and sensitivity testing on outcome scales • Indicate benefit of using OHQ composite score • Open Label Response Criteria Not Optimal Enrichment Technique • BP responders didn’t correlate with efficacy outcome parameter • Item #1 (Dizziness) Does Not Fully Capture Treatment Effect • Item 1 scores don’t correlate to Global Impressions of Disease Severity (CGI-S) • Behavior modification occurs • Patients doing poorly don’t challenge themselves therefore don’t get dizzy • Adjusted Clinical Program in Response to New Data 15 |
 History of
Successful FDA Interactions History of Successful FDA Interactions • Granted Orphan Status • Granted SPA for Study 301 • Granted Fast Track Status • Met with FDA in November 2009 • Granted Increase in Study 301 patient enrollment to 150 • Increases power/robustness of outcome and viability of overall NDA submission • Improves likelihood of positive outcome • Granted Request to Change Primary Outcome in Ongoing Study 301 • Confirmed SPA for Study 301 following change in endpoint 16 |
 17 Current Northera Current Northera Phase III Program: NOH Phase III Program: NOH • Study 302: Pivotal Proof of Efficacy – Withdrawal Design • Study 303: Long-Term Safety Extension to Study 302 – includes long-term efficacy assessment after 3 months • Study 301: Pivotal Proof of Efficacy – Induction Design Change in Primary Endpoint to OHQ composite Increasing Enrollment to 150 patients • Study 304: Long-Term Safety Extension to Study 301 • Study 305: 24 HR Blood Pressure Monitoring Study – subgroup of 301 patients • Study 306: Pivotal Proof of Efficacy – Induction Design NOH in Parkinson’s Patients Only – targeting most robust effect Primary Endpoint: OHQ composite |
 18 Study 301: Revised Design Study 301: Revised Design • Induction design study in150 NOH patients with primary autonomic failure • Primary efficacy measure: OHQ composite • Special Protocol Assessment 100mg 200mg 300mg 400mg 500mg 600mg Washout Randomization Placebo Up to 2 weeks (open-label) 1 week (open- label) 1 weeks (blinded) Visit |
  19 Study 306: NOH in Parkinson’s Disease Study 306: NOH in Parkinson’s Disease Randomization Visit 2 Up to 2 weeks 8 weeks N= 42 N= 42 Visit 1 Placebo, TID 100mg 200mg 300mg 400mg 500mg 600mg 100mg 200mg 300mg 400mg 500mg 600mg Northera™, TID Visit 3a, 3b, 3c…. Visit 7 2 weeks • Enriched Heterogeneous population: 84 PD Patients with NOH • Longer Treatment duration allows active arm to improve and placebo to fail • Primary efficacy measure: OHQ composite • Starting Q2 2010, top-line data Q2 2011 OR Double-Blind Treatment Double-Blind Titration |
 • Differentiated Profile • Treats underlying cause of NOH • Ideal candidate for first line therapy • Benign safety profile a significant benefit for patients taking multiple medications • Significant Upside Potential: 20 Northera Northera NOH Value Proposition NOH Value Proposition US TID Sales* $300-375 million + QD Formulation + Better PD Efficacy + Midodrine Withdrawn + ROW *Assumptions: Price: $30/day,Compliance: 70% |
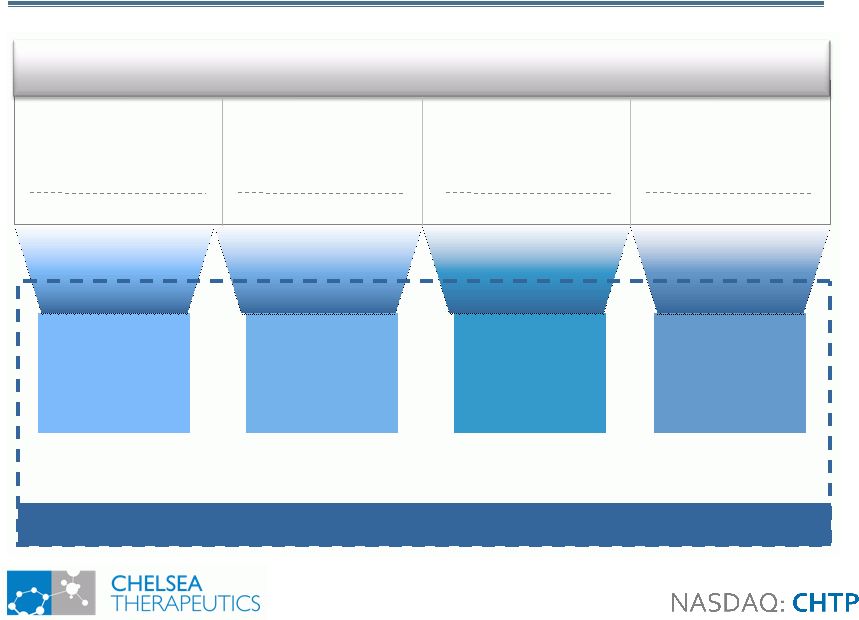 Droxidopa: Label
Expansion Opportunities Droxidopa: Label Expansion Opportunities 21 Fibromyalgia Chronic Fatigue Syndrome Adult Attention Deficit Disorder Intradialytic Hypotension CHTP: Phase II CHTP: Phase II Investigator: Phase II Investigator: Phase II Norepinephrine Related Disorders 15-25% of all hemodialysis patients suffer from IDH Chronic Pain Disorder that Affects 5.8 million Americans Second Most Common Neuropsychiatric Disorder Affecting 4.4% of US adults Debilitating Fatigue Disorder Affecting 1 - 4 million Americans Droxidopa Droxidopa + Carbidopa Droxidopa + Carbidopa Droxidopa + Carbidopa Future Growth Opportunities |
 22 Novel Antifolates Novel Antifolates with Blockbuster Potential with Blockbuster Potential • First non-metabolized oral antifolates • Engineered for improved safety and increased tolerability vs. methotrexate • Potential first line and combination treatment for rheumatoid arthritis • Not competing with Biologics • Methotrexate (MTX) remains most widely prescribed RA treatment: • Limitations are AEs and long-term safety concerns • Undergoes significant metabolism Hydroxylated Polyglutamylated • An estimated 1/3 of patients who continue treatment with MTX only achieve a partial
efficacy response • Additional high potential global markets • Psoriasis, other immunological disorders and Cancer |
 23 CH-1504: Phase II Proof-of-Concept CH-1504: Phase II Proof-of-Concept • 12-week trial comparing CH-1504 to MTX in 200 MTX naive RA patients • 0.25, 0.5 or 1.0 mg daily dose of CH-1504 vs. 20 mg weekly dose of MTX • Achieved Primary Objective by Demonstrating Comparable ACR 20 Response Rates To MTX and Improved Safety Profile • No Drop outs Resulting From GI Tolerability Issues in Any CH-1504 Arm
|
 24 CH-4051: Greater Efficacy/Safety CH-4051: Greater Efficacy/Safety • Key preclinical findings suggests: • Superior preclinical efficacy to both CH-1504 MTX • Enzymatic activity (DHFR inhibition) • Uptake by Reduced Folate Carrier • Increases activity in CIA model over both CH-1504 and MTX • Improved safety/toxicity • Phase I SAD study & MAD study: • Significantly higher doses tolerated than CH-1504 • No serious adverse events • GI and reversible LFT elevations at highest doses • Improved bioavailability vs. CH-1504 • Plasma concentrations suggest superior efficacy over MTX based on animal models
|
 25 CH-4051 Phase II: MTX Partial Responders RA CH-4051 Phase II: MTX Partial Responders RA 2 weeks 12 weeks N= 50 Up to 2 weeks 20 mg/wk MTX + FOLATE 3.0 mg CH-4051/daily + FOLATE 3.0 mg CH-4051/daily 1.0 mg CH-4051/daily 0.3 mg CH-4051/daily N= 50 N= 50 N= 50 N= 50 + 4 weeks • 12-week, 5-arm trial comparing CH-4051 to MTX in 250 RA patients • MTX Partial Responders • Primary efficacy measure: ACR Hybrid Score • Starting Q2 2010, top-line data Q2 2011 Double-Blind Treatment MTX Washout |
 Efficacy
Analysis Using Hybrid ACR Efficacy Analysis Using Hybrid ACR • Hybrid ACR Score (hACR). • Endorsed by ACR, FDA and key opinion leaders • More sensitive to treatment effects than traditional ACR 20/50/70 • Starts with ACR 20/50/70 as a basis--permits greater precision than discrete ACR
scores Example: If a patient meets traditional criteria for ACR20 (minimum 20% improvement), but really has 35% improvement in all signs and symptoms--that patient is hACR35 • If the study shows statistically significant benefits in hACR for CH4051: • Second part of primary analysis tests ACR 20/50/70 • "step-down" procedure used--no statistical penalty 26 |
 27 Financial Summary Financial Summary Capitalization as of January 31, 2009 Common Stock 33,500,406 Warrants 4,060,758 Options 4,606,430 Fully Diluted42,167,594 Cash as of September 30, 2009 $30 million Expected Q4 09 Cash$22 million |
 28 Looking Ahead…. Looking Ahead…. Finalize Protocol Northera PIII: NOH Study 306 Q1 10 Top-line Results Northera: Study 305 Q1 10 Initiate Northera PIII: NOH Study 306 Q2 10 Top-line Results Northera: Study 303 Q2 10 Initiate CH-4051 PII: RA Q2 10 Top-line Results Northera PIII: NOH Study 301 Q3 10 Interim Results Droxidopa PII: Fibromyalgia H2 10 Top-line Results Northera PIII: NOH Study 306 Q2 11 Northera NDA: NOH Q2 11 • Operational Focus • Drive Northera Registration Program/NDA • Advance CH-4051 in RA and Droxidopa in additional indications • Execute strategic, cash generating licensing/partnership opportunities • Upcoming Milestones |
