Attached files
| file | filename |
|---|---|
| EX-99.1 - EX-99.1 - CEPHALON INC | a10-3156_1ex99d1.htm |
| 8-K - 8-K - CEPHALON INC | a10-3156_18k.htm |
Exhibit 99.2
|
|
Mepha Acquisition Conference Call February 1, 2010 |
|
|
Safe Harbor Statement This presentation contains forward-looking statements that involve risks and uncertainties. These statements may concern, among other things, our business strategy and market opportunities, the development of pharmaceutical products, and future financial and operating results. Additional information that may affect our business and financial prospects, as well as factors that would cause our actual performance to vary from our expectations, may be found in our filings with the Securities and Exchange Commission. |
|
|
Cephalon Perspective How Does Mepha Add To Cephalon? Increased global reach: We can leverage our current and future products through Mepha $400 million of incremental sales: Mepha doubles the size of our international business with a sound, diverse product portfolio Increased balance: We now serve all 3 types of pharmaceutical products (branded, branded generics, and generics) Immediately accretive: Expect immediate accretion to adjusted EPS* and cash from operations Substantial synergies: Expect to achieve revenue, operational, and tax synergies Consistent with our business strategy: We seek to build a growing diversified bio-pharmaceutical company * See our quarterly earnings press releases and Form 8-k filed on the same dates for reconciliation of non-GAAP figures to GAAP. |
|
|
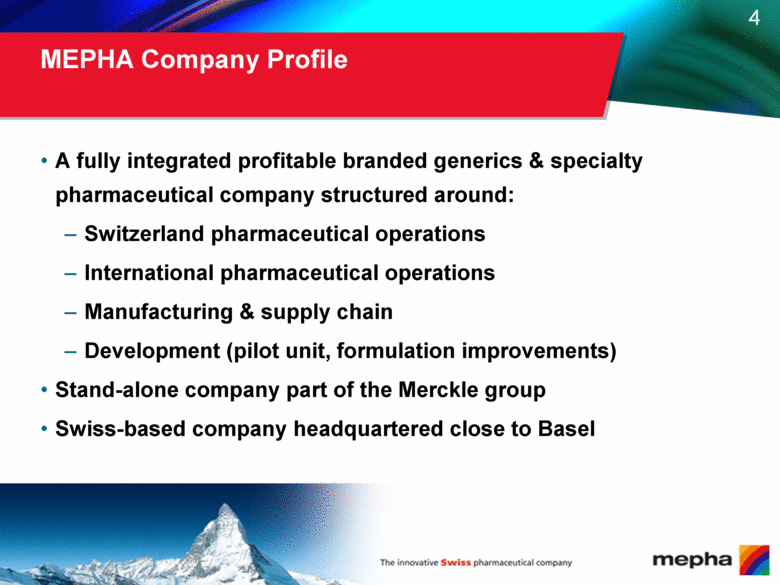
MEPHA Company Profile A fully integrated profitable branded generics & specialty pharmaceutical company structured around: Switzerland pharmaceutical operations International pharmaceutical operations Manufacturing & supply chain Development (pilot unit, formulation improvements) Stand-alone company part of the Merckle group Swiss-based company headquartered close to Basel |
|
|
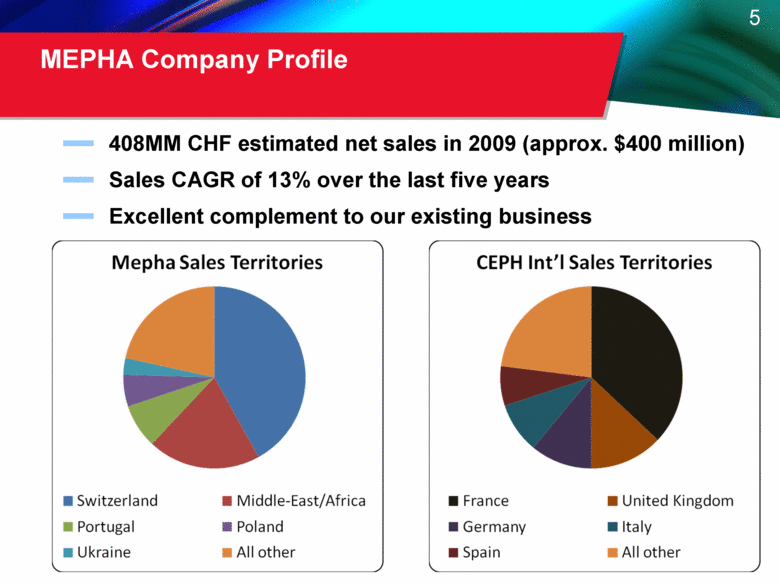
MEPHA Company Profile 408MM CHF estimated net sales in 2009 (approx. $400 million) Sales CAGR of 13% over the last five years Excellent complement to our existing business |
|
|
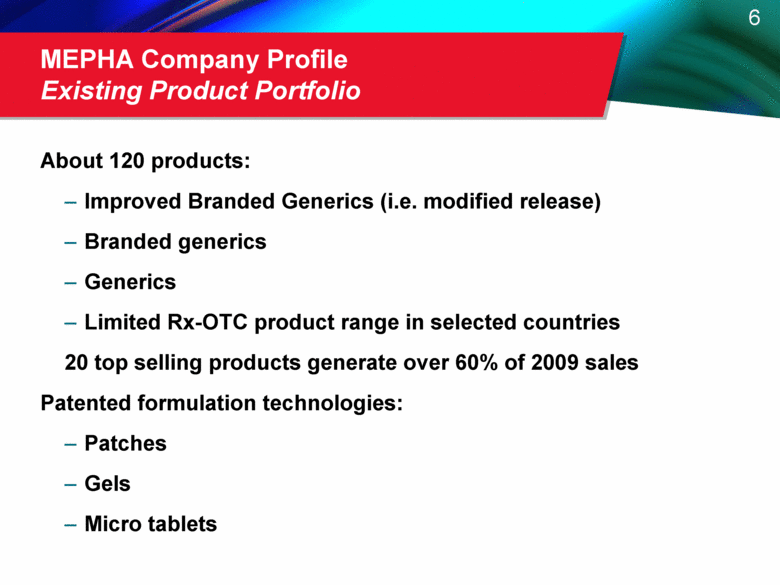
MEPHA Company Profile Existing Product Portfolio About 120 products: Improved Branded Generics (i.e. modified release) Branded generics Generics Limited Rx-OTC product range in selected countries 20 top selling products generate over 60% of 2009 sales Patented formulation technologies: Patches Gels Micro tablets |
|
|
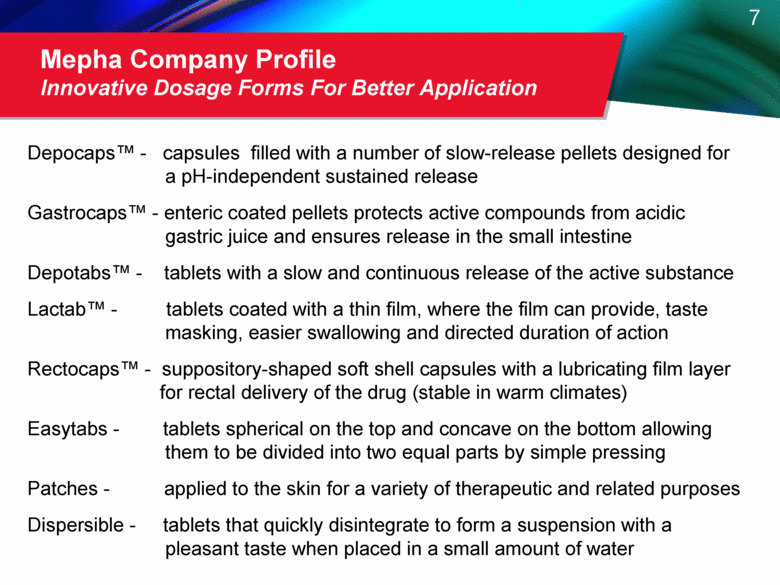
Mepha Company Profile Innovative Dosage Forms For Better Application Depocaps™ - capsules filled with a number of slow-release pellets designed for a pH-independent sustained release Gastrocaps™ - enteric coated pellets protects active compounds from acidic gastric juice and ensures release in the small intestine Depotabs™ - tablets with a slow and continuous release of the active substance Lactab™ - tablets coated with a thin film, where the film can provide, taste masking, easier swallowing and directed duration of action Rectocaps™ - suppository-shaped soft shell capsules with a lubricating film layer for rectal delivery of the drug (stable in warm climates) Easytabs - tablets spherical on the top and concave on the bottom allowing them to be divided into two equal parts by simple pressing Patches - applied to the skin for a variety of therapeutic and related purposes Dispersible - tablets that quickly disintegrate to form a suspension with a pleasant taste when placed in a small amount of water |
|
|
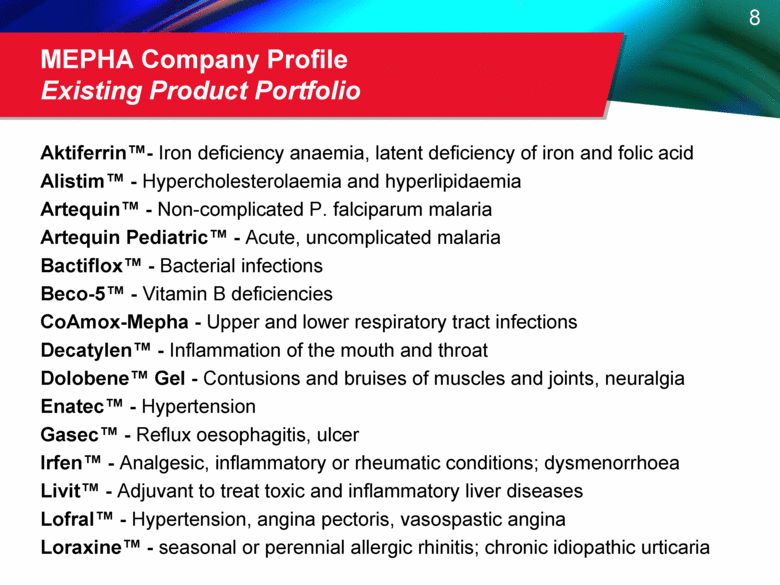
MEPHA Company Profile Existing Product Portfolio Aktiferrin™ - Iron deficiency anaemia, latent deficiency of iron and folic acid Alistim™ - Hypercholesterolaemia and hyperlipidaemia Artequin™ - Non-complicated P. falciparum malaria Artequin Pediatric™ - Acute, uncomplicated malaria Bactiflox™ - Bacterial infections Beco-5™ - Vitamin B deficiencies CoAmox-Mepha - Upper and lower respiratory tract infections Decatylen™ - Inflammation of the mouth and throat Dolobene™ Gel - Contusions and bruises of muscles and joints, neuralgia Enatec™ - Hypertension Gasec™ - Reflux oesophagitis, ulcer Irfen™ - Analgesic, inflammatory or rheumatic conditions; dysmenorrhoea Livit™ - Adjuvant to treat toxic and inflammatory liver diseases Lofral™ - Hypertension, angina pectoris, vasospastic angina Loraxine™ - seasonal or perennial allergic rhinitis; chronic idiopathic urticaria |
|
|
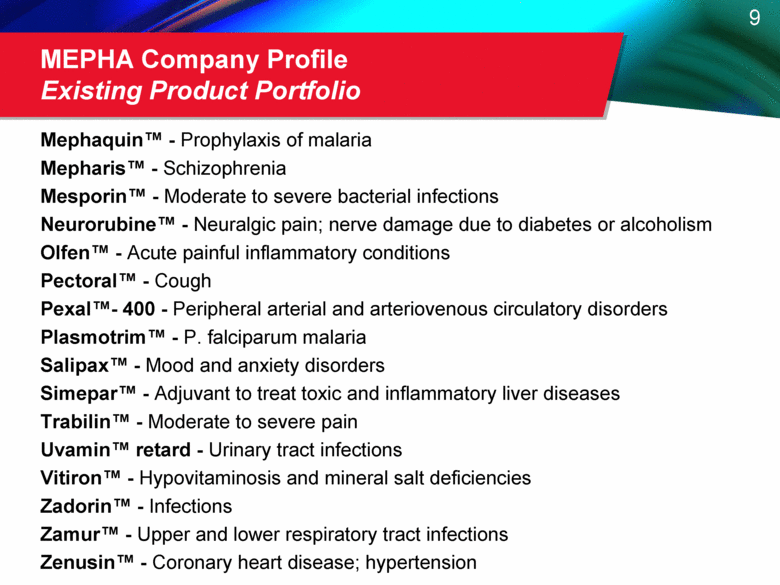
MEPHA Company Profile Existing Product Portfolio Mephaquin™ - Prophylaxis of malaria Mepharis™ - Schizophrenia Mesporin™ - Moderate to severe bacterial infections Neurorubine™ - Neuralgic pain; nerve damage due to diabetes or alcoholism Olfen™ - Acute painful inflammatory conditions Pectoral™ - Cough Pexal™ - 400 - Peripheral arterial and arteriovenous circulatory disorders Plasmotrim™ - P. falciparum malaria Salipax™ - Mood and anxiety disorders Simepar™ - Adjuvant to treat toxic and inflammatory liver diseases Trabilin™ - Moderate to severe pain Uvamin™ retard - Urinary tract infections Vitiron™ - Hypovitaminosis and mineral salt deficiencies Zadorin™ - Infections Zamur™ - Upper and lower respiratory tract infections Zenusin™ - Coronary heart disease; hypertension |
|
|
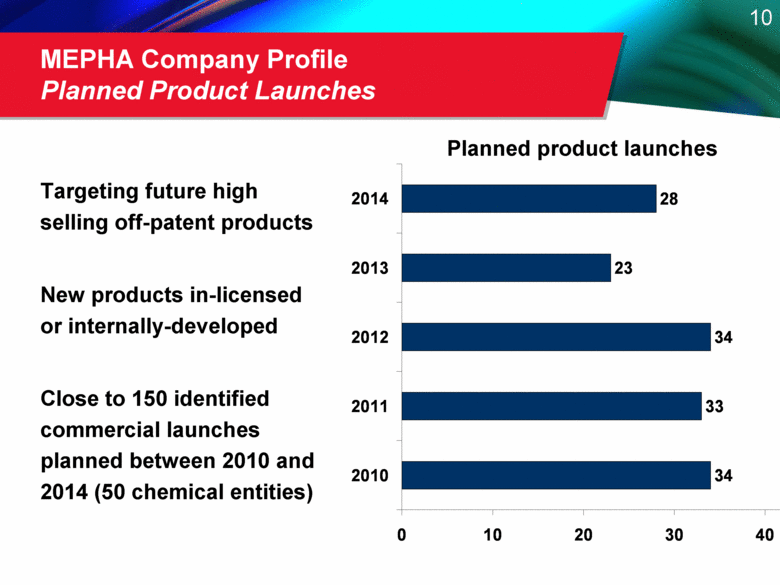
MEPHA Company Profile Planned Product Launches Targeting future high selling off-patent products New products in-licensed or internally-developed Close to 150 identified commercial launches planned between 2010 and 2014 (50 chemical entities) Planned product launches 34 33 34 23 28 0 10 20 30 40 2010 2011 2012 2013 2014 |
|
|
Cephalon With The Addition Of Mepha Global Bio-pharmaceutical company Sales presence in 100 countries 3,800 employees 30 percent of sales outside United States Diverse product portfolio Increasingly stable, and profitable business Deep pipeline: oncology, pain, inflammatory diseases, branded generics Significant cash generator |
|
|
Thank you! February 1, 2010 |