Attached files
| file | filename |
|---|---|
| EX-21.1 - EX-21.1 - SOMANETICS CORP | k48822exv21w1.htm |
| EX-32.1 - EX-32.1 - SOMANETICS CORP | k48822exv32w1.htm |
| EX-14.1 - EX-14.1 - SOMANETICS CORP | k48822exv14w1.htm |
| EX-31.2 - EX-31.2 - SOMANETICS CORP | k48822exv31w2.htm |
| EX-31.1 - EX-31.1 - SOMANETICS CORP | k48822exv31w1.htm |
| EX-23.1 - EX-23.1 - SOMANETICS CORP | k48822exv23w1.htm |
Table of Contents
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
(Mark One)
| þ | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended November 30, 2009
or
| o | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to
Commission file number 0-19095
SOMANETICS CORPORATION
(Exact name of registrant as specified in its charter)
| MICHIGAN | 38-2394784 | |
| (State or other jurisdiction of | (I.R.S. Employer | |
| incorporation or organization) | Identification No.) |
| 2600 Troy Center Drive, Troy, Michigan | 48084-4771 | |
| (Address of principal executive offices) | (Zip Code) |
Registrant’s telephone number, including area code: (248) 244-1400
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class | Name of each exchange on which registered | |
| Common Shares, par value $.01 per share | The NASDAQ Stock Market |
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in
Rule 405 of the Securities Act. Yes o No þ.
Indicate by check mark if the registrant is not required to file reports pursuant to
Section 13 or Section 15(d) of the Act. Yes o No þ.
Indicate by check mark whether the registrant (1) has filed all reports required to be filed
by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or
for such shorter period that the registrant was required to file such reports), and (2) has been
subject to such filing requirements for the past 90 days. Yes þ No o
Indicate by check mark whether the registrant has submitted electronically and posted on its
corporate Web site, if any , every Interactive Data File required to be submitted and posted
pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period
that the registrant was required to submit and post such files).
Yes o No o
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation
S-K is not contained herein, and will not be contained, to the best of registrant’s knowledge, in
definitive proxy or information statements incorporated by reference in Part III of this Form 10-K
or any amendment to this Form 10-K. þ
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated
filer, a non-accelerated filer or a smaller reporting company. See the definitions of “large
accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the
Exchange Act. (Check one):
| Large accelerated filer o | Accelerated filer þ | Non-accelerated filer o (Do not check if a smaller reporting company) |
Smaller reporting company o |
Indicate by check mark whether the registrant is a shell company (as defined in
Rule 12b-2 of the Exchange Act). Yes o No þ.
The aggregate market value of the common shares held by non-affiliates of the registrant as of
May 29, 2009 (the last business day of the registrant’s most recently completed second fiscal
quarter), computed by reference to the closing sale price as reported by NASDAQ on such date, was
approximately $199,500,000.
The number of the registrant’s common shares outstanding as of February 2, 2010 was 11,946,102.
Documents Incorporated by Reference
Portions of the Proxy Statement for the 2010 Annual Meeting of Shareholders, scheduled to be held
April 21, 2010, are incorporated by reference in Part III, if the Proxy Statement is filed no later
than March 29, 2010.
SOMANETICS CORPORATION
ANNUAL REPORT ON FORM 10-K
FOR THE FISCAL YEAR ENDED NOVEMBER 30, 2009
TABLE OF CONTENTS
| PAGE | ||||||||
| 2 | ||||||||
| 2 | ||||||||
| 21 | ||||||||
| 34 | ||||||||
| 34 | ||||||||
| 34 | ||||||||
| 34 | ||||||||
| 35 | ||||||||
| 37 | ||||||||
| 37 | ||||||||
| 40 | ||||||||
| 41 | ||||||||
| 53 | ||||||||
| 54 | ||||||||
| 73 | ||||||||
| 73 | ||||||||
| 73 | ||||||||
| 74 | ||||||||
| 74 | ||||||||
| 74 | ||||||||
| 74 | ||||||||
| 74 | ||||||||
| 75 | ||||||||
| 76 | ||||||||
| 76 | ||||||||
| EX-14.1 | ||||||||
| EX-21.1 | ||||||||
| EX-23.1 | ||||||||
| EX-31.1 | ||||||||
| EX-31.2 | ||||||||
| EX-32.1 | ||||||||
Table of Contents
PART I
ITEM 1. BUSINESS
Overview
We develop, manufacture and market the INVOS System, a non-invasive patient monitoring system
that provides accurate, real-time blood oxygen measurements in the brain and elsewhere in the body
in tissues beneath the sensor in patients greater than 2.5 kilograms, and continuously measures
changes in blood oxygen levels for individuals of any weight. The INVOS System is the only
commercially-available cerebral/somatic oximeter proven to improve outcomes.
The brain is the organ least tolerant of oxygen deprivation. Without sufficient oxygen, brain
damage may occur within minutes, which can result in paralysis, other disabilities or death. Brain
oxygen information, therefore, is important, especially in surgical procedures requiring general
anesthesia and in other critical care situations with a high risk of the brain getting less oxygen
than it needs. The INVOS System consists of a portable monitoring system, including proprietary
technology, which is used with multiple single-use disposable sensors, called SomaSensors or
OxyAlert sensors. During our fiscal year ended November 30, 2009, net revenues from disposable
sensors comprised approximately 81% of our net revenues. As of November 30, 2009, we had an
installed base of 2,927 INVOS System monitors in the United States in 782 hospitals, and during
fiscal 2009 we sold 502,026 sensors worldwide.
Clinical studies have shown that using the INVOS System to monitor and provide information to
help manage the regional brain blood oxygen saturation of patients is associated with significantly
fewer incidences of major organ dysfunction, which can significantly improve patient outcomes and
reduce hospital costs. During fiscal 2004, the results of the first prospective, randomized,
blinded intervention trial were presented, and the results were published in the January 2007 issue
of a peer-reviewed anesthesia journal. The study showed that when the INVOS System was used to
monitor and provide information to help manage the regional brain blood oxygen saturation of
coronary artery bypass surgery patients, the occurrence of major organ morbidity or mortality was
reduced from 11% to 3% and patients with major organ morbidity or mortality have significantly
longer length of stay in the intensive care unit, or ICU, than those without. Additionally, in
2004, the results of a large retrospective review showed a statistically significant greater than
50% reduction (2.01% versus 0.97%) in the incidence of permanent stroke when information from the
INVOS System was used to help manage brain blood oxygen saturation of cardiac surgery patients.
The results also showed a reduced length of hospital stay and reduced incidence of prolonged
ventilation when the INVOS System was used.
Our INVOS System has U.S. Food and Drug Administration, or FDA, clearance in the United States
for use on adults, children, infants and neonates. We target the sale of the INVOS System for use
in surgical procedures and other critical care situations with a high risk of oxygen imbalances.
We initially focused our marketing efforts primarily on adult and pediatric cardiac surgeries and
carotid artery surgeries. In fiscal 2005, we initiated selling and marketing efforts for the INVOS
System in the pediatric ICU, and in fiscal 2008 we expanded the use of our INVOS System in the
pediatric and neonatal ICUs with the launch of our smaller sensor. Some of our potential future
markets may include other major surgeries involving high risk patients. While our initial focus
has been commercializing the INVOS System to measure blood oxygen saturation changes in the brain,
many clinicians in the pediatric and neonatal ICU use the INVOS System to assess changes in oxygen
saturation in regions of the body other than the brain in addition to cerebral oxygen saturation.
In November 2005, we received 510(k) clearance from the FDA to market our INVOS System to
monitor changes in somatic tissue blood oxygen saturation in regions of the body other than the
brain in patients with or at risk for restricted blood flow. In May 2008, we received 510(k)
clearance from the FDA to market our INVOS System to monitor changes in blood oxygen saturation in
any tissues beneath the sensor, not limited to brain and somatic tissue, in any individual. In
April 2009, we received 510(k) clearance from the FDA to expand the indications for use to reflect
the INVOS System’s ability to provide accurate, immediate blood oxygen saturation measurements in
patients greater than 2.5 kilograms at risk for restricted or no blood flow, in addition to our
previous FDA clearance to measure changes in blood oxygen saturation in any individual. In
addition, this most recent 510(k) clearance expanded the labeling for our INVOS System to include
the following new labeling claims:
2
Table of Contents
| • | The measurement of regional cerebral oxygen saturation (rSO2) is an indication of whether oxygen delivery to the brain is adequate. Prolonged declines in rSO2 are indicative of, or may result in, potential brain injury. |
| • | When used as an indication of compromised cerebral oxygenation, interventions to return the patient’s rSO2 to baseline using the INVOS System have been shown to improve outcomes after surgery. |
| • | In neonates, infants and children, cerebral and somatic rSO2 provide noninvasive indications of oxygen changes in the cerebral and peripheral circulatory systems and may provide an early indication of oxygen deficits associated with impending shock states and anaerobiosis. |
Our four-channel cerebral and somatic INVOS System monitor, which we launched in the second
quarter of 2006, can display information from four disposable sensors. This feature allows for the
simultaneous monitoring of blood oxygen saturation in tissues beneath the sensor in four different
places in the body in patients greater than 2.5 kilograms at risk for restricted or no blood flow,
and also allows for the simultaneous monitoring of changes in blood oxygen saturation in four
different places in the body in all individuals.
We are sponsoring and evaluating sponsorship of clinical trials which may allow us to more
actively target the sale of the INVOS System for use in high risk patient populations and other
patient populations. There are also numerous other independent clinical studies currently
evaluating the use of the INVOS System.
In November 2008, we acquired substantially all of the assets of ICU Data Systems, Inc. ICU
Data Systems developed a patented technology that integrates data from a broad array of hospital
bedside devices, such as physiological monitors, ventilators and infusion devices, into a single
bedside display for comparison, data management and storage. The data integration technology
allows customized presentation of data from various bedside devices for comparison on the same
display and on the same timeline. The device can also calculate and display derived parameters, or
calculated parameters based on the combination of two or more discrete parameters. In addition,
the device can produce user-defined, automated event marks and alerts. All resulting information
can be stored for inclusion in the patient record and clinical research. We launched our
newly-acquired data integration technology, as a stand-alone device that we call Vital Sync™, in
the third quarter of fiscal 2009. The INVOS System is one of many devices whose data can be
integrated into the stand-alone device. To support the addition of the derived parameter features
to the system, we have filed a new FDA 510(k) premarket notification in the fourth quarter of
fiscal 2009. In addition, we expect to invest to combine the ICU Data Systems and INVOS System
technologies in a single product in fiscal 2010. Upon completion of development of a single
product combining the Vital Sync System with our INVOS System technology, we also plan to pursue a
new FDA 510(k) clearance for this integrated device for a launch currently expected in mid-2011.
We sell the INVOS System and Vital Sync System through a direct sales team in the United
States, consisting of salespersons and clinical specialists, the size of which has increased from
53 persons at the end of fiscal 2007 to 63 persons at the end of fiscal 2009, and an independent
sales representative firm. Outside the United States, we have distribution agreements with
independent distributors for the INVOS System, including Covidien in Europe, Canada, the Middle
East and South Africa, and Edwards Lifesciences Ltd. in Japan. During fiscal 2009, we extended our
distribution agreement with Covidien for three years beginning in February 2010 and in December
2009, we extended our distribution agreement with Edwards through fiscal 2014. We expect to
increase the size of our U.S. direct sales team in fiscal 2010 and have hired and may hire
additional direct salespersons and clinical specialists in Europe to support Covidien. Our net
revenues have increased from $38.6 million in the fiscal year ended November 2007 to $50.0 million
in fiscal 2009, representing a compounded annual growth rate of 13.8%. As a percentage of net
revenues, our gross margin decreased from 88% in fiscal 2007 to 87% in fiscal 2009.
Our Corporate Information
We were incorporated under the laws of the State of Michigan in 1982. Our principal executive
offices are located at 2600 Troy Center Drive, Troy, Michigan 48084-4771, and our telephone number
is (248) 244-1400. Our website address is www.somanetics.com. The information on, or that can be
accessed through, our website is not a part of this report. Unless the context indicates
otherwise, as used in this report, the terms “Somanetics,”
3
Table of Contents
“Somanetics Corporation,” the “Company,” “we,” “us” and “our” refer to Somanetics Corporation,
a Michigan corporation.
Somanetics®, INVOS®, SomaSensor®, Window to the
Brain®, Reflecting the Color of Life®, Enlightening Medicine®,
OxyAlert®, OxyAlert NIRSensor®, and iCuro® are our United States
registered trademarks. Each of the other trademarks, trade names or service marks appearing in this
report is either pending registration or belongs to its respective holder.
Industry
Market Opportunity
We believe that in the United States in 2008 there were approximately five million surgeries
involving elderly patients who, due to the type of surgery, age of the patient or other factors,
have a higher risk of developing post-operative complications. Such surgeries include cardiac
surgeries, carotid surgeries, orthopedic surgeries and other major general surgeries involving
elderly patients. In addition, we believe that there are other patient populations, such as
non-elderly adult, pediatric and neonatal patients, undergoing major surgeries and patients
undergoing ICU treatment or in other critical care situations that face a high risk of tissue
oxygen imbalances.
Hospitals in the United States have economic incentives to control health care costs. They
often receive a fixed fee from Medicare, managed care organizations and private insurers based on
the disease diagnosed, rather than on the services actually performed. Therefore, hospitals are
increasingly focused on avoiding unexpected costs, such as those associated with increased hospital
stays of patients with brain or other organ damage or other adverse outcomes following surgery or
ICU treatment. The costs to the health care system associated with adverse surgical and ICU
outcomes and lengthened hospital stays can be significant. In addition, lack of immediate
knowledge about blood oxygen levels in areas such as the brain or other tissues can result in
unnecessary medical treatments and associated costs. With the increasing focus by hospitals on
avoiding unexpected costs, especially in the operating room, ICU and other critical care areas, we
believe that there are significant incentives to evaluate and adopt new monitoring technologies
which could provide information to improve patient care and reduce costs.
Brain Oxygen Imbalances and Its Effects
Oxygen is carried to the brain by hemoglobin in the blood. Hemoglobin passes through the
lungs, bonds with oxygen and is pumped by the heart through arteries and capillaries to the brain.
Brain cells extract oxygen and the blood carries away carbon dioxide through the capillaries and
veins back to the lungs.
The brain is the human organ least tolerant of oxygen deprivation. Without sufficient oxygen,
brain damage may occur within minutes, which can result in paralysis, severe and complex
disabilities, or death. Undetected brain hypoxia, which is a condition in which there is a
decrease of oxygen supply to the brain even though there is adequate blood flow, and ischemia, a
condition in which blood flow, and thus oxygen, is restricted to a part of the body, are common
causes of brain damage and death during and after many surgical procedures and in other critical
care situations.
Brain oxygen imbalances can be caused by several factors, including changes in arterial blood
oxygen saturation, which is the percentage of oxygenated hemoglobin contained in a given amount of
blood which carries oxygen in the arteries to the tissues of the body, blood flow to the brain,
hemoglobin concentration and oxygen consumption by the brain.
Brain oxygen information is important in surgical procedures requiring general anesthesia, in
other critical care situations with a high risk of brain oxygen imbalances, as well as in the
treatment of patients with head injuries or strokes. Once alerted to these imbalances, medical
professionals can use this and other information to take corrective action through the introduction
of medications, anesthetic agents or mechanical intervention, potentially improving patient
outcomes and reducing the costs of care. Immediate and continuous information about changes in
brain oxygen levels also provides immediate feedback regarding the adequacy of the selected
therapy. Equally important, without information about brain oxygen levels, therapy that may not be
necessary might be initiated in an
4
Table of Contents
attempt to ensure adequate brain oxygen levels and may have an adverse impact on patient
safety and increase hospital costs.
Our Solution
Our INVOS System is a non-invasive patient monitoring system that provides accurate, real-time
blood oxygen measurements in the brain and elsewhere in the body in tissues beneath the sensor in
patients greater than 2.5 kilograms, and continuously measures changes in blood oxygen levels for
individuals of any weight. We believe that our INVOS System addresses the market’s need for a
solution that is non-invasive, continuous, immediate, effective and easy to use. The INVOS System
is the only commercially-available cerebral/somatic oximeter proven to improve outcomes. The INVOS
System, which is predominantly used in hospital critical care areas such as operating rooms and
ICUs, consists of a portable monitoring system, including proprietary technology, which is used
with multiple single-use disposable sensors. For multi-channel cerebral monitoring, disposable
sensors are placed on both sides of a patient’s forehead and are connected to the monitor. The
INVOS System uses our proprietary technology to analyze information received from the disposable
sensors and provides a continuous digital and trend display of the blood oxygen saturation in the
area of the body under the sensors. Our four-channel cerebral and somatic INVOS System monitor,
which we launched in the fiscal 2006, can display information from four disposable sensors. This
feature allows for the simultaneous monitoring of blood oxygen saturation in tissues beneath the
sensor in four different places in the body in patients with or at risk for restricted blood flow.
Surgeons, anesthesiologists, pediatric and neonatal ICU physicians, and other medical
professionals can use the information provided by the INVOS System, in conjunction with other
available information, to identify brain and other tissue oxygen imbalances and take necessary
corrective action, potentially improving patient outcomes and reducing the costs of care. Once the
cause of a cerebral or other tissue oxygen imbalance is identified and therapy is initiated, the
INVOS System provides immediate feedback regarding the adequacy of the selected therapy. It can
also provide medical professionals with an additional level of assurance when they make decisions
regarding the need for therapy.
Unlike some existing monitoring methods, the INVOS System functions even when the patient is
unconscious, lacks a strong peripheral pulse or has suppressed neural activity. The measurement
made by the INVOS System is dominated by information from the blood in the veins, where the balance
of oxygen supply and demand can be more effectively assessed. Therefore, it responds to the
changes in factors that affect the balance between oxygen supply and demand, including changes in
arterial oxygen saturation, blood flow, hemoglobin concentration and oxygen consumption. The INVOS
System responds to global changes in brain or other tissue oxygen levels and to events that affect
oxygen levels in the region beneath the sensor.
5
Table of Contents
The following table summarizes some of the principal features and related benefits of the
INVOS System:
| Features | Benefits | |
Non-invasive
|
• Reduced risk to patients and medical professionals |
|
| • Consistent with market trend toward less invasive medical procedures | ||
Continuous Information
|
• Immediate information regarding oxygen imbalances to help guide therapeutic interventions | |
| • Trend information, rather than at a single point in time | ||
4-Channel Monitoring
|
• Simultaneous cerebral and other tissue monitoring | |
| • Provides more data points to help manage patient care | ||
Cost-Effective
|
• Low cost relative to traditional brain monitoring methods | |
| • Small portion of the total cost of the procedures in which it is used | ||
| • Information can potentially improve patient outcomes and reduce the overall cost of care | ||
Easy to Use
|
• Does not require a dedicated technician to operate or interpret | |
| • Automatic sensor calibration | ||
| • Simple user interface and controls | ||
Effective in Difficult Circumstances
|
• Provides information when the patient is unconscious,
lacks a strong peripheral pulse or has suppressed neural
activity, specifically during cardiac arrest,
hypothermia, hypertension, hypotension and hypovolemia |
|
Portable/Compatible
|
• Placed at patient’s bedside | |
| • Lightweight | ||
| • Can be integrated or interfaced with existing multi-modality systems |
ICU Data Systems, Inc.
In November 2008, we acquired substantially all of the assets of ICU Data Systems, Inc. ICU
Data Systems developed a patented technology that integrates data from a broad array of hospital
bedside devices, such as physiological monitors, ventilators and infusion devices, into a single
bedside display for comparison, data management and storage. The data integration technology
allows customized presentation of data from various bedside devices for comparison on the same
display and on the same timeline. The device can also calculate and display derived parameters, or
calculated parameters based on the combination of two or more discrete parameters. In addition,
the device can produce user-defined, automated event marks and alerts. All resulting information
can be stored for inclusion in the patient record and clinical research.
We launched our newly-acquired data integration technology, as a stand-alone device that we
call Vital Sync™, in the third quarter of fiscal 2009. The INVOS System is one of many devices
whose data can be integrated into the stand-alone device. To support the addition of the derived
parameter features to the system, we have filed a new FDA 510(k) premarket notification in the
fourth quarter of fiscal 2009. In addition, we expect to invest to combine the ICU Data Systems
and INVOS System technologies in a single product in fiscal 2010. Upon completion of development
of a single product combining the Vital Sync System with our INVOS System technology, we also plan
to pursue a new FDA 510(k) clearance for this integrated device for a launch currently expected in
mid-2011.
Business Strategy
Our objective is to establish the INVOS System as a standard of care in surgical procedures
requiring general anesthesia and in other critical care situations. Key elements of our strategy
include to:
6
Table of Contents
| • | Target Surgical Procedures and Other Critical Care Situations with a High Risk of Oxygen Imbalances. We target surgical procedures and other critical care situations with a high risk of oxygen imbalances. Some of our current and potential future markets include cardiac surgeries, carotid artery surgeries, pediatric and neonatal ICU applications, orthopedic surgeries and other major surgeries involving high risk patients. We believe that the medical professionals involved in these surgeries and ICU treatments are most aware of the risks of brain and other damage resulting from oxygen imbalances. Therefore, we believe that it will be easier to demonstrate the clinical importance of the information provided by the INVOS System to these professionals and potentially gain market acceptance for our products in connection with these surgeries and ICU treatments. | ||
| • | Sponsor Clinical Studies to Promote Expanded Acceptance of the INVOS System. We believe that our INVOS System has been evaluated in over 700 presentations, study abstracts and published papers. During the second quarter of fiscal 2004, results of both the first prospective, randomized clinical trial and a larger retrospective review evaluating the INVOS System were presented, which we believe have contributed to the INVOS System gaining further market penetration. In addition, in January 2007 the results of the first prospective, randomized clinical trial mentioned above were published in a peer-reviewed anesthesia journal. We plan to sponsor clinical studies using the INVOS System to demonstrate its benefits. We are also sponsoring and evaluating sponsorship of other clinical trials which may allow us to more actively target the sale of the INVOS System for use in other high risk patient populations and other patient populations. We use the results of clinical studies to help convince the medical community of the clinical importance of the information provided by the INVOS System. We also sponsor peer-to-peer educational opportunities and promote use of the INVOS System in regional centers of influence that we believe will influence its adoption by others. In early 2008, The Society of Thoracic Surgeons began collecting cerebral oximetry information as part of its STS Adult Cardiac Surgery Database, which is used to develop practice standards intended to improve qualify and safety. | ||
| • | Invest in Sales and Marketing Activities. We continue to increase our investment in our distribution network consisting of our direct sales employees, independent sales representative firm and distributors. We sell the INVOS System and Vital Sync System through a direct sales team in the United States, the size of which has increased from 53 persons at the end of fiscal 2007 to 63 persons at the end of fiscal 2009, and an independent sales representative firm. We expect to increase the size of our U.S. direct sales team in fiscal 2010 and have hired and may hire additional direct salespersons and clinical specialists in Europe to support Covidien and to sell our Vital Sync™ System. We participate in trade shows and medical conferences, ongoing peer-to-peer educational programs and targeted public relations opportunities. | ||
| • | Interface and Integrate Our Technology into Other Manufacturers’ Multi-Modality Systems. There are many existing monitoring systems in the operating room and the ICU. We would like to interface with these monitors. We have interfaced the INVOS System with the Philips Medical Systems’ VueLink System to provide data, alarm events and status messages from the INVOS System on any monitor that accepts the VueLink module, a multi-parameter monitor. This enables oximetry data from our INVOS System to be displayed on the VueLink screen and integrated with other vital patient information. We plan to support the interface and integration of our INVOS System technology with other medical device manufacturers to expand the installed base of INVOS System monitors and increase the demand for our sensors. We expect that such arrangements will provide another distribution channel for our INVOS System. We launched our newly-acquired data integration technology, as a stand-alone device that we call Vital Sync™, in the third quarter of fiscal 2009. The INVOS System is one of many devices whose data can be integrated into the stand-alone device. To support the addition of the derived parameter features to the system, or calculated parameters based on the combination of two or more discrete parameters, we have filed a new FDA 510(k) premarket notification in the fourth quarter of fiscal 2009. In addition, we expect to invest to combine the ICU Data Systems and INVOS System technologies in a single product in fiscal 2010. Upon completion of development of a single product combining the Vital Sync System with our INVOS System technology, we also plan to pursue a new FDA 510(k) clearance for this integrated device for a launch currently expected in mid-2011. |
7
Table of Contents
| • | Develop Additional Applications and Markets for the INVOS System. We have developed a smaller sensor for use with infants and neonates, and are making other advances to the design and performance features of the INVOS System, including the disposable sensor. We are also evaluating additional potential market segments for our INVOS System, such as use in orthopedic surgeries and other major surgeries, in the adult ICU, in assessing individuals with sleep disorders, and for applications of the technology to monitor other tissues. We are also exploring several novel near-infrared spectroscopy and imaging technologies and products under a Contract Development Agreement with Shirley Research Corporation, an Exclusive Sublicense Agreement with Raba Equity Partners II, LLC (“Raba coreFoundry”), and a Development and Exclusive License Agreement with an inventor and his company. See “NeuroPhysics Corporation and Shirley Research Corporation”, “Raba Equity Partners II, LLC ” and “Compartment Syndrome” below. Pursuit of some of these potential market segments may require additional FDA clearance. We believe that these natural extensions of our technology will increase our market potential without the more significant risks and costs associated with developing entirely new products. |
The INVOS System
Components of the INVOS System
The INVOS System consists of a portable monitoring system, including proprietary technology,
which is used with multiple single-use disposable sensors.
| • | Monitor. Our oximeter is a portable monitor that uses our proprietary technology to analyze information received from the disposable sensors. It provides a continuous digital and trend display of the blood oxygen saturation in the region of the body under the sensors. The monitor includes menus for users to set high and low audible alarms, customize the display and retrieve data. Single-function keys allow users to silence alarms, mark important events, store data for up to 28 surgical procedures, and retrieve data by USB storage device or through a direct link to a computer. Our four-channel cerebral and somatic INVOS System monitor, which we launched in fiscal 2006, measures approximately 11 inches wide, 9 inches high, and 7 inches deep and weighs approximately 11 pounds. We provide a one-year warranty on the monitor. As of November 30, 2009, we had an installed base of 2,927 INVOS System monitors in the United States in 782 hospitals. | ||
| • | Disposable sensors. Each single-use disposable sensor contains a light source and two light detectors. For multi-channel cerebral monitoring, disposable sensors are placed on both sides of a patient’s forehead and are connected to the monitor, which allows for monitoring both sides of the brain. Our four-channel cerebral and somatic INVOS System monitor, which we launched in fiscal 2006, can display information from four sensors. This feature allows for the simultaneous monitoring of blood oxygen saturation in tissues beneath the sensors in four different places in the body in patients with or at risk for restricted blood flow. The number of sensors used depends on the application. The INVOS System is being used to monitor simultaneously the brain and other tissue initially for patients in the pediatric and neonatal ICU and for monitoring other, non-brain tissue alone, and we expect that it will later also be used on adults for other, non-brain tissue. The disposable sensors contain information that is processed by the INVOS System allowing it to automatically calibrate each sensor. During our fiscal year ended November 30, 2009, net revenues from our disposable sensors comprised approximately 81% of our net revenues. During fiscal 2009 we sold 502,026 sensors worldwide. |
Overview of INVOS Technology
Our proprietary In Vivo Optical Spectroscopy, or INVOS, technology is based primarily on the
physics of optical spectroscopy. Optical spectroscopy is the interpretation of the interaction
between matter and light. Spectrometers and spectrophotometers function primarily by shining light
through matter and measuring the extent to which the light is transmitted through, scattered by or
absorbed by the matter. Physicians and scientists can use spectrophotometers to examine human
blood and tissue. Although most human tissue is opaque to ordinary light, some wavelengths
penetrate tissue more easily than others. Therefore, by shining appropriate wavelengths of light
into the body and measuring its transmission, scattering and absorption, or a combination of each,
physicians can
8
Table of Contents
obtain information about the matter under analysis. Optical spectroscopy generates no
ionizing radiation and produces no known hazardous effects.
By identifying the hemoglobin and the oxygenated hemoglobin and measuring the relative amounts
of each, oxygen saturation of hemoglobin can be measured. However, traditional optical
spectroscopy was generally not useful when the substances to be measured were surrounded by, were
behind or were near bone, muscle or other tissue, because they produce extraneous data that
interferes with analysis of the data from the area being examined.
We have developed a method of reducing extraneous spectroscopic data caused by surrounding
bone, muscle and other tissue. This method, which is embedded in our INVOS System, allows us to
gather information about portions of the body that previously could not be analyzed using
traditional optical spectroscopy. The INVOS System measurement is made by our disposable sensors
transmitting low-intensity visible and near-infrared light through a portion of the body and
detecting the manner in which the molecules of the exposed substance interact with light at
specific wavelengths.
Each single-use disposable sensor contains a light source and two light detectors. The dual
detector design of the sensor enables us to measure scattered light intensities from the
intermediate tissues of skin, muscle and bone in a separate process. While both detectors receive
similar information about the tissue between the sensor and the area under examination, the
detector further from the light source detects light that has penetrated deeper into the body, and,
therefore, receives more information specific to the brain or other tissue under examination than
does the detector closer to the light source. By comparing the two measurements, our INVOS
technology is able to suppress the influence of the tissues between the sensor and the brain or
other tissue under examination to provide a measurement of brain or other tissue blood oxygen
saturation.
Applications and Market Segments
We target the sale of the INVOS System for use in surgical procedures and other critical care
situations with a high risk of oxygen imbalances. We believe that our INVOS System has
applications for cerebral and other tissue monitoring in the following key market segments:
| • | Cardiac and Carotid Artery Surgery. Until fiscal 2005, we focused our marketing efforts primarily on cardiac and carotid artery surgeries. We believed it would be easier to demonstrate clinical importance of the information provided by the INVOS System and potentially gain market acceptance for our products in connection with these surgeries. Moreover, much of the earliest clinical data regarding the use of the INVOS System involved these surgeries. In September 2000, we received 510(k) clearance from the FDA to market the model 5100 INVOS System in the United States. Unlike earlier models, the model 5100 INVOS System has the added capability of being able to monitor pediatric patients. After receiving this clearance, we expanded our marketing efforts to include pediatric cardiac surgeries. | ||
| • | Pediatric and Neonatal ICU. In fiscal 2005, we initiated selling and marketing efforts for the INVOS System in the pediatric ICU. In the first half of fiscal 2008, we expanded the use of our INVOS System in the pediatric and neonatal ICUs with the launch of our smaller sensor. Our four-channel cerebral and somatic INVOS System monitor, which we launched in fiscal 2006, can display information from four disposable sensors. This feature allows for the simultaneous monitoring of blood oxygen saturation in tissues beneath the sensor in four different places in the body in patients with or at risk for restricted blood flow. The INVOS System is being used to monitor simultaneously the brain and other tissue initially for patients in the pediatric and neonatal ICU. | ||
| • | Other Applications. We are also sponsoring and evaluating sponsorship of other clinical trials which may allow us to more actively target the sale of the INVOS System for use in other high risk patient populations and other patient populations. In addition, we are evaluating other potential market segments for our INVOS System, such as use in orthopedic surgeries and other major surgeries, in the adult ICU, in assessing individuals with sleep disorders, and for applications of the technology in other tissues in the body. |
9
Table of Contents
In November 2008, we acquired substantially all of the assets of ICU Data Systems, Inc., a
technology development company. See “ICU Data Systems, Inc.” above. ICU Data Systems, Inc.
developed a patented technology that integrates data from a broad array of hospital bedside
devices, such as physiological monitors, ventilators and infusion devices, into a single bedside
display for comparison, data management and storage. We launched our newly-acquired data
integration technology, as a stand-alone device that we call Vital Sync™, in the third quarter of
fiscal 2009. The product is expected to be used primarily on patients undergoing surgeries or who
in the ICU and who are being monitored by multiple monitors. The INVOS System is one of many
devices whose data can be integrated into the stand-alone device. To support the addition of the
derived parameter features to the system, or calculated parameters based on the combination of two
or more discrete parameters, we have filed a new FDA 510(k) premarket notification in the fourth
quarter of fiscal 2009. In addition, we expect to invest to combine the ICU Data Systems and INVOS
System technologies in a single product in fiscal 2010. Upon completion of development of a single
product combining the Vital Sync System with our INVOS System technology, we also plan to pursue a
new FDA 510(k) clearance for this integrated device for a launch currently expected in mid-2011.
We are also exploring several novel near-infrared spectroscopy and imaging technologies and
products under a Contract Development Agreement with Shirley Research Corporation, an Exclusive
Sublicense Agreement with Raba Equity Partners II, LLC (“Raba coreFoundry”), and a Development and
Exclusive License Agreement with an inventor and his company. See “NeuroPhysics Corporation and
Shirley Research Corporation”, “Raba Equity Partners II, LLC” and “Compartment Syndrome” below.
Pursuit of some of these potential market segments may require additional FDA clearance. We
believe that these natural extensions of our technology will increase our market potential without
the more significant risks and costs associated with developing entirely new products.
Clinical Development
We believe that favorable peer-reviewed publication is a key element to the INVOS System’s
success. Accordingly, we support clinical research programs with third-party clinicians and
researchers intended to demonstrate the need for the INVOS System and the clinical importance of
the information it provides with the specific objective of publishing the results in peer-reviewed
journals. The research includes studies comparing patients managed using information provided by
the INVOS System with other patients, based on measures of patient outcome and hospital costs,
including patient length of stay, length of time on the ventilator, cognitive dysfunction and
incidence of stroke. In addition to the studies described below, we believe that our INVOS System
has been evaluated in over 700 presentations, study abstracts and published papers. During fiscal
2004, results of the studies described below were presented, which we believe have contributed to
the INVOS System gaining further market penetration. In addition, in January 2007 the results of
the first prospective, randomized clinical trial mentioned below were published in a peer-reviewed
anesthesia journal.
Murkin Study
In the second quarter of 2004, the results of the first prospective, randomized, blinded
intervention study using the INVOS System were presented. The study showed a statistically
significant reduction in incidences of major organ dysfunction when the INVOS System was used to
provide information to help manage regional brain blood oxygen saturation in coronary artery bypass
surgery patients. The 200-patient study was conducted by John Murkin, M.D., professor of
anesthesiology at the University of Western Ontario, and was presented at Outcomes 2004:
Neurobehavioral Assessment, Physiological Monitoring and Cerebral Protective Strategies held in Key
West, Florida. The data and results of the intervention study reported on by Dr. Murkin at
Outcomes 2004 were published as John M. Murkin, M.D., et al., Monitoring Brain Oxygen Saturation
During Coronary Bypass Surgery: A Randomized, Prospective Study, in Anesthesia and Analgesia.
(January 2007).
Patients undergoing coronary artery bypass surgery were randomly assigned to the control or
intervention group. Patients in both groups were monitored with the INVOS System during their
operations, but the monitor display in the control group (100 patients) was covered and patients’
treatments were managed routinely. In the intervention group (100 patients) the patients’
treatments were managed using information from the INVOS System, and the patients received a
pre-determined series of interventions to maintain the INVOS System’s index of regional cerebral
blood oxygen saturation within 75% of baseline values taken at the beginning of the operation.
10
Table of Contents
Independent observers assessed all of the patients for predefined clinical outcomes. The
complication criteria were those reported by cardiac surgeons to the Society of Thoracic Surgeons
National Database. These complications consist of common adverse outcomes following cardiac
surgery, such as stroke, respiratory failure, renal failure and other major morbidities.
Dr. Murkin found that regional brain oxygen desaturations were quite common and are related to
major organ dysfunction. The intervention group experienced statistically significantly fewer
incidents of major organ dysfunction than the control group: three patients in the intervention
group experienced incidents of major organ morbidity or mortality, compared to 11 patients in the
control group. With respect to stroke specifically, one patient in the intervention group
experienced a stroke, compared to four patients in the control group. The difference was not
statistically significant.
A financial analysis of Dr. Murkin’s data was conducted by Leaden Hickman, Ph.D., assistant
professor, health sciences and administration at the University of Michigan, and Dr. Murkin. This
analysis was presented at Outcomes 2005: Neurobehavioral Assessment, Physiological Monitoring and
Cerebral Protective Strategies held in Key West, Florida in May 2005. The analysis showed
measurable cost differences between the intervention and control groups. Total cost per patient
was lower in the intervention group than in the control group ($14,921 vs. $15,619). This
difference was not statistically significant. The potential complication avoidance results in a
total savings of $231,540, or a savings of $1,158 per patient averaged over the entire study group.
The data and results of the financial analysis conducted by Leaden Hickman, Ph.D., and presented
at Outcomes 2005, have not been published in a peer-reviewed publication.
Goldman Study
In the second quarter of 2004, the results of a retrospective, blinded intervention study
using the INVOS System were presented. The study showed a statistically significant reduction in
permanent stroke when information from the INVOS System was used to help manage regional brain
blood oxygen saturation in cardiac surgery patients. The principal investigator in the
2,279-patient study was Scott Goldman, M.D., chairman of the department of surgery at
Pennsylvania-based Main Line Health Center, Lankenau Hospital. Findings from the study were
presented at the Cardiothoracic Techniques and Technologies Annual Meeting in March 2004 and were
published as Scott Goldman, M.D., et al., Optimizing Intraoperative Cerebral Oxygen Delivery Using
Noninvasive Cerebral Oximetry Decreases the Incidence of Stroke for Cardiac Surgical Patients, in
The Heart Surgery Forum #2004-1062 (September 2004).
The study included all patients who underwent cardiac surgery for any reason at the Lankenau
Hospital and Institute for Medical Research from July 1, 2000 to June 30, 2003. The control group
consisted of 1,245 patients who underwent surgery in the 18 months before cerebral oximetry
monitoring with the INVOS System was introduced at the hospital on January 1, 2002. The study
group consisted of 1,034 patients who underwent surgery during the following 18 months and were
monitored with the INVOS System. Operative techniques were modified in the study group to maintain
cerebral oximetry values at or near the pre-operative baseline throughout the surgery. The study
group included a significantly sicker population of patients than the control group, as determined
by pre-operative New York Heart Association, or NYHA, classification and co-morbidities.
The incidence of permanent stroke in the study group (0.97%) was statistically significantly
less than in the control group (2.01%), despite a sicker population according to the higher NYHA
class of the study group. Although the incidence of permanent stroke was lower in the study group,
the incidence of all neurologic dysfunction, including stroke and transient ischemic attack, was
similar in the two groups. The proportion of patients requiring prolonged ventilation also was
statistically significantly smaller in the study group, 6.8%, compared to 10.6% in the control
group. Total ventilator time was statistically significantly shorter in the study group (four
hours) than the control group (five hours). The length of hospital stay was similar overall in the
two groups, but was statistically significantly shorter in the study group when examined by
pre-operative NYHA classifications of patients.
Dr. Goldman’s later analysis of these data concluded that the difference in incidence of
cerebrovascular accidents, or CVA, between the two groups translated into a potential avoidance of
12 CVAs in the study group and approximately $254,214 in direct costs and more than $425,000 in
total costs.
11
Table of Contents
Other Future Studies
We are sponsoring and evaluating sponsoring other clinical trials which may allow us to more
actively target the sale of the INVOS System for use in other high risk patient populations and
other patient populations. In addition, in early 2008, The Society of Thoracic Surgeons began
collecting cerebral oximetry information as part of its STS Adult Cardiac Surgery Database, which
is used to develop practice standards intended to improve qualify and safety.
Marketing, Sales and Distribution
Marketing
We market the INVOS System primarily to cardiac and vascular surgeons, anesthesiologists,
pediatric and neonatal ICU physicians, and other medical professionals. We believe that these
specialists are the medical professionals most aware of the risks of brain and other damage
resulting from oxygen imbalances. We intend to market our Vital Sync System to the same
specialists and medical professionals as our INVOS System.
We believe that favorable peer-reviewed publication is a key element to the INVOS System’s
success. Accordingly, we support clinical research programs with third-party clinicians and
researchers intended to demonstrate the need for the INVOS System and the clinical importance of
the information it provides with the specific objective of publishing the results in peer-reviewed
journals. The research includes studies comparing patients managed based on information provided
by the INVOS System with similarly situated patients not managed based on information provided by
the INVOS System, based on measures of patient outcomes and hospital costs, including patient
length of stay, length of time on the ventilator, cognitive dysfunction and incidence of stroke.
We attend trade shows and medical conferences to promote the INVOS System and Vital Sync
System, and to meet medical professionals with an interest in performing research and reporting
their results in peer-reviewed medical journals and at major international medical conferences. We
also sponsor peer-to-peer educational opportunities, promote use of the INVOS System in regional
centers of influence that we believe will influence its adoption by others, and participate in
targeted public relations opportunities.
Sales and Distribution
We sell the INVOS System and Vital Sync System through a direct sales team in the United
States, consisting of salespersons and clinical specialists, the size of which has increased from
53 persons at the end of fiscal 2007 to 63 persons at the end of fiscal 2009, and an independent
sales representative firm. We expect to increase the size of our U.S. direct sales team in fiscal
2010. We believe the selling cycle for the INVOS System is approximately six to nine months,
although given the current economic downturn in the United States and abroad, we believe that the
sales cycle has been and may continue to be lengthened significantly.
Outside the United States, we have distribution agreements with independent distributors
covering 69 countries for the INVOS System. Our distributors for the INVOS System include
Covidien, in Europe, the Middle East, South Africa and Canada, and Edwards Lifesciences Ltd. in
Japan. During fiscal 2009, we extended our distribution agreement with Covidien for three years
beginning in February 2010 and in December 2009, we extended our distribution agreement with
Edwards through fiscal 2014. We have hired and may hire additional direct salespersons and
clinical specialists in Europe to support Covidien and to sell our Vital Sync™ System.
We also have an international sales consultant. For fiscal 2009, 20% of our net revenues were
represented by international sales.
We offer a no capital cost sales program in the United States whereby we ship the INVOS System
monitor to the customer at no charge. It has been our experience that many hospitals in the United
States prefer to use this method to acquire INVOS System monitors.
We did not have any backlog of firm orders as of January 10, 2010 or as of January 10, 2009.
We generally do not have a backlog of firm orders because we generally ship product upon receipt of
a customer order.
12
Table of Contents
For a description of sales to major customers, see Note 9 of Notes to Financial Statements
included in Item 8 of this report. Covidien was our largest customer in fiscal 2009, 2008 and
2007. We are dependent on our sales to Covidien and Edwards Lifesciences, and the loss of either
of them as a customer would have an adverse effect on our business, financial condition and results
of operations in the near-term, until such time as they could be replaced as our distributor in the
respective market.
Our international sales were $9,854,238 for the fiscal year ended November 30, 2009,
$9,420,472 for the fiscal year ended November 30, 2008 and $7,024,902 for the fiscal year ended
November 30, 2007, including approximately $6,650,000 in fiscal 2009, $6,698,000 in fiscal 2008 and
$5,043,000 in fiscal 2007 to Covidien, our distributor in Europe, the Middle East, South Africa and
Canada, and approximately $2,305,000 in fiscal 2009, $2,118,000 in fiscal 2008 and $1,431,000 in
fiscal 2007 to Edwards Lifesciences Ltd., our distributor in Japan. See Note 9 of Notes to
Financial Statements. For a description of the breakdown of sales between INVOS System monitors
and sensors, see “Management’s Discussion and Analysis of Financial Condition and Results of
Operations — Results of Operations” in Item 7 of this report.
Research and Development
Our research and development activities are conducted internally by a staff consisting of 16
employees. We have developed a smaller sensor for use with infants and neonates, and are making
other advances to the design and performance features of the INVOS System, including the disposable
sensor. We are also working to interface our INVOS System with multi-functional monitors provided
by other manufacturers. We launched our newly-acquired data integration technology, as a
stand-alone device that we call Vital Sync™, in the third quarter of fiscal 2009. The INVOS System
is one of many devices whose data can be integrated into the stand-alone device. To support the
addition of the derived parameter features to the Vital Sync System, or calculated parameters based
on the combination of two or more discrete parameters, we have filed a new FDA 510(k) premarket
notification in the fourth quarter of fiscal 2009. In addition, we expect to invest to combine the
ICU Data Systems and INVOS System technologies in a single product in fiscal 2010. Upon completion
of development of a single product combining the Vital Sync System with our INVOS System
technology, we also plan to pursue a new FDA 510(k) clearance for this integrated device for a
launch currently expected in mid-2011.
Our research, development and engineering expenditures were $3,947,509 during fiscal 2009,
$1,259,227 during fiscal 2008 and $668,815 during fiscal 2007. We expect our research, development
and engineering expenses to increase in fiscal 2010 from the level in 2009, excluding the
$2,000,000 expense under our Exclusive Sublicense Agreement and our Development and Exclusive
License Agreement, described below. We expect this increase primarily as a result of the hiring of
additional research and development personnel, development costs associated with the integration of
the INVOS System and Vital Sync System, development costs associated with our Exclusive Sublicense
Agreement with Raba Equity Partners II, LLC (“Raba coreFoundry”), development costs associated with
our Contract Development Agreement with Shirley Research Corporation, and development costs
associated with advances to the design and performance features of the INVOS System, including the
disposable sensor.
NeuroPhysics Corporation and Shirley Research Corporation
We entered into a Contract Development and Exclusive Licensing Agreement with NeuroPhysics
Corporation as of September 18, 2006. The agreement provided us with feasibility research,
contract development and consulting services and certain ownership and licensing rights, subject to
the rights of the United States Federal government, to intellectual property and technical
knowledge associated with several novel near-infrared spectroscopy, or “NIRS”, and imaging
technologies and products under development at NeuroPhysics. We paid an initial license fee of
$1,000,000. We terminated this agreement in February 2009, except for various provisions regarding
our ownership of the technology related to the potential new products. In February 2009, we
entered into a similar agreement with Shirley Research Corporation and Hugh F. Stoddart and Hugh A.
Stoddart, formerly of NeuroPhysics Corporation, and have agreed to pay monthly development and
consulting fees of $15,000 a month during the term of the agreement and a royalty on future sales
of the new products.
Shirley Research Corporation is in the early stage of feasibility research and development of
several NIRS-based technologies and products, including a novel approach to depth resolved NIRS
measurements. In addition to
13
Table of Contents
this NIRS-based, depth-resolved technology, products that may be developed under the agreement
include (1) a fetal cerebral oximetry system, (2) a monitor for measuring oxygen saturation in deep
tissues for assessing hemorrhagic shock, (3) devices to assess tumors or swelling containing blood,
including in the brain of head trauma victims and neonates with intra-ventricular hemorrhage, (4) a
continuous and non-invasive blood gas monitor, and (5) a new imaging concept intended to improve
resolution and expand the applicability of endoscopes. We may terminate any or all of the projects
under this agreement at any time. We might not be able to develop these technologies or products
into commercially viable products, and competitors might develop and market similar products before
we do.
Raba Equity Partners II, LLC (“Raba coreFoundry”)
We entered into an Exclusive Sublicense Agreement with Raba Equity Partners II, LLC (“Raba
coreFoundry”) in October 2009. Under terms of the agreement, we have obtained exclusive rights,
subject to specified rights of the U.S. Government and rights retained by The Johns Hopkins
University, to make, use and sell products using new cerebral autoregulation technology developed
at The Johns Hopkins University. Integration of this technology into our INVOS®
Cerebral/Somatic Oximeter would yield the first noninvasive monitor providing cerebral
autoregulation data for routine clinical use. The cerebral autoregulation technology is associated
with two pending patents. We paid Raba coreFoundry up-front, non-refundable payments of $1.8
million and will pay a royalty on future revenue associated with products using the licensed
technology. The up-front payments were accounted for as research and development expenses in the
fourth quarter of fiscal 2009.
Cerebral autoregulation refers to the body’s ability to maintain constant blood flow to the
brain despite changes in blood pressure. In many critical care situations, the brain’s
autoregulation can become impaired, making it vulnerable to changes in blood pressure and to
potential brain injury due to loss of this critical protective mechanism. For example, blood
pressure below the patient’s autoregulation threshold can result in cerebral ischemia, while blood
pressure above the patient’s upper limit of autoregulation can result in cerebral bleeding.
Patients at highest risk of impaired cerebral autoregulation include those undergoing certain
surgical procedures, such as cardiovascular, neurological and major orthopedic procedures, and
liver transplants. Others at risk include patients with traumatic brain injury, those who have
suffered a stroke, infants on ventilators and premature babies.
We plan to use this patent-pending method of combining blood pressure measurements and signals
from the INVOS System to continuously monitor and display cerebral autoregulatory function
information. We plan to file a new 510(k) premarket notification with the FDA for the new module
and pursue a product launch in the first half of 2011.
Compartment Syndrome
We entered into a Development and Exclusive Agreement with an inventor and his company in
November 2009 to develop and market a product that uses INVOS technology and methods and means
described in the inventor’s patent to monitor, detect and assess acute compartment syndrome in
parts of the human body. Under terms of the agreement, we have obtained exclusive rights to the
rights of the inventor under a pending patent regarding methods and means for using near infrared
spectroscopy to monitor, detect and assess acute compartment syndrome in parts of the human body,
such as the legs, but only in the field of monitoring, detecting and assessing compartment
syndrome. We paid the inventor’s company an up-front, non-refundable payment of $200,000 and will
pay an additional $300,000 upon obtaining 510(k) clearance for the product and a royalty on future
revenue associated with products using the licensed technology. The up-front payment was accounted
for as research and development expenses in the fourth quarter of fiscal 2009.
The U.S. Department of Defense has provided a grant for the research and development of a
monitor and sensors to monitor, detect and assess compartment syndrome. As part of the agreement,
over a three-year period, we expect to receive approximately $2.1 million for product and prototype
sales and research and development fees, derived from the grant, for its work to develop the
compartment syndrome product.
Compartment syndrome is a limb and life-threatening condition that occurs with the compression
of nerves, blood vessels and muscle inside a closed space (compartment) within the body. This
leads to tissue death due to the lack of oxygenation as the blood vessels are compressed by the
raised pressure within the compartment.
14
Table of Contents
Manufacturing
We assemble the INVOS System and Vital Sync System in our facilities in Troy, Michigan, from
components purchased from outside suppliers. We assemble the INVOS System and Vital Sync System to
control their quality and costs and to permit us to make changes to them faster than we could if
third parties assembled them. Although we believe that most components are generally available
from several potential suppliers, we depend on one supplier for one of our components for the INVOS
System. We are not aware of any validated alternative supplier for this component, although we are
currently in the process of evaluating a second source of supply and are carrying approximately a
six-month supply of this component. Moreover, we typically use one supplier for custom-designed
components, including the unit enclosure, the printed circuit boards, other mechanical components
and the disposable sensor. We are currently dependent on one manufacturer of the sensor and
another component of the INVOS System, and we believe that it would require approximately four to
five months to change sensor suppliers. We do not currently intend to manufacture on a commercial
scale the disposable sensor or the components of the INVOS System.
We received ISO 13485 certification and met the requirements under the European Medical Device
Directive to use the CE Mark, thereby allowing us to continue to market our INVOS System and
disposable sensor in the European Economic Community. Our most recent ISO 13485 compliance
surveillance audit occurred in July 2009.
Competition
There are numerous and varied forms of patient monitoring products that constitute our
competition. Other cerebral and somatic oximeters are just one form of these competing products.
In the United States, we believe there are currently three other companies with FDA clearance to
sell a cerebral oximeter. In December 2005, CAS Medical Systems, Inc. announced that it received
510(k) clearance for a cerebral oximeter, and it began sales of its product during 2007. In
August 2009, we filed a patent infringement action against CAS Medical Systems, Inc. See Item 3
“Legal Proceedings” in this report. During 2009, one other U.S. company announced that it received
510(k) clearance for a cerebral oximeter, and it began sales of its product late in 2009. Outside
the United States, several Japanese manufacturers offer a cerebral oximeter for sale in that
country and primarily for research in other parts of the world, but, to our knowledge, none has
pursued FDA clearance for its product in the United States. We are aware that several companies
and individuals are engaged in the research and development of non-invasive cerebral oximeters, and
we believe that there are several others who may begin development of a cerebral oximeter. Other
companies have FDA clearance for somatic oximeters in the United States. Competition might cause
our sales cycle to lengthen to the extent that customers take longer to make purchasing decisions.
Competition might also reduce our gross margins and market share and prevent us from achieving
further market penetration. Competitors might be more successful than we are in obtaining FDA
clearance with broader claims in their labeling or more successful than we are in manufacturing and
marketing their products and may be able to take advantage of the significant time and effort we
have invested to gain medical acceptance of cerebral oximetry.
We compete with numerous medical equipment companies and medical device integration companies
for the portions of hospital budgets allocated to capital equipment and for the limited amount of
space on a patient’s forehead for sensors. The medical products industry is characterized by
extensive research and development and intense competition in an increasingly cost-conscious
environment. Some of these potential competitors have well-established reputations, customer
relationships and marketing, distribution and service networks. Some of them have substantially
longer histories in the medical products industry, larger product lines and greater financial,
technical, manufacturing, research and development and management resources than we do. Many of
these competitors and potential competitors have long-term product supply relationships with our
potential customers. These competitors and potential competitors might be able to use their
resources, reputations and ability to leverage existing customer relationships to give them a
competitive advantage over us, including in securing forehead sensor space for their products and
dollars from hospital capital equipment budgets to purchase their products. They might also
succeed in developing products that are at least as reliable and effective as our products, that
make additional measurements, that are less costly than our products or that provide alternatives
to our products. Competitors might be more successful than we are in manufacturing and marketing
their products and may be able to take advantage of the significant time and effort we have
invested to gain medical acceptance of cerebral oximetry.
15
Table of Contents
We believe that a manufacturer’s reputation for producing accurate, reliable, effective,
sterile, patented and technically advanced products, clinical literature associated with leaders in
the field, references from users, features (speed, safety, ease of use, patient and surgeon
convenience and range of applicability), product effectiveness and price are the principal
competitive factors in the medical products industry.
Proprietary Rights Information
We have 10 United States patents and two patents in various foreign countries. These patents
expire on various dates from June 2010 to October 2019. We currently have 15 patent applications
pending in the United States, including one reissuance application, and have patent applications in
various foreign countries with respect to aspects of our technology.
In September 2003, we were issued a new patent by the United States Patent and Trademark
Office covering, among other things, the application of non-invasive, near-infrared spectroscopy to
measure continuously and substantially concurrently a blood metabolite (such as oxygen saturation)
in at least two separate internal regions of the brain. This patent is now the subject of a
reissue proceeding in the United States Patent and Trademark Office. We requested the reissuance
of this patent because we believe that we are entitled to broader claims than those that were
originally issued. CAS Medical Systems, Inc. has requested re-examination of this patent, and the
reissuance and re-examination proceedings have been combined. The outcome of the reissue and
re-examination proceeding cannot be predicted, and the claims which ultimately issue may be broader
in scope than the original claims, they may be narrower in scope than the original claims, they may
be the same in scope as the original claims or they may be rejected. The corresponding Australian
patent for “Multi-Channel, Noninvasive, Tissue Oximeter” issued in December 2003, will expire in
October 2019. This patent is pending in other markets outside the United States. We believe the
inventions covered in this patent are important to providing a high quality cerebral oximeter in
the clinical setting.
Our other patents cover methods and apparatuses for introducing light into a body part and
receiving, measuring and analyzing the transmitted light and its interaction with tissue. These
methods also involve receiving, measuring and analyzing the light transmissivity of various body
parts of a single subject, as well as of body parts of different subjects, which provides a
standard against which a single subject can be compared.
Many other patents have previously been issued to third parties involving optical spectroscopy
and the interaction of light with tissue, some of which relate to the use of optical spectroscopy
in the area of brain metabolism monitoring, the primary use of the INVOS System. We are not aware
of any infringement of the claims of any issued patents by our products or by their methods of
use, and no charge of patent infringement has been asserted against us.
In addition to our patent rights, we have obtained United States Trademark registrations for
our trademarks “SOMANETICS,” “INVOS,” “SOMASENSOR,” “WINDOW TO THE BRAIN,” “REFLECTING THE COLOR OF
LIFE,” “ENLIGHTENING MEDICINE,” “OXYALERT,” “OXYALERT NIRSENSOR,” “ICURO,” “SCR,” and “SCR” and
design. We have trademark applications pending for “AUC,” “AREA UNDER THE CURVE,” “SOMANETICS
ALLIANCE,” “SOMANETICS ALLIANCE” and design, the “ALLIANCE” logo, “Vital Sync,” and “PUTTING
PATIENT DATA TO WORK FOR YOU.” We have also obtained registrations for our SOMANETICS mark in more
than 30 foreign jurisdictions, with additional foreign applications for this mark pending, and we
have the marks “INVOS,” “SOMASENSOR,” BRAINSENSOR SOMASENSOR,” “OXYALERT,” “SCR,” “SCR” and design,
and “VITAL SYNC” registered or pending in other foreign countries.
We also rely on trade secret, copyright and other laws and on confidentiality agreements to
protect our technology, but we believe that neither our patents nor our other legal rights will
necessarily prevent third parties from developing or using a similar or a related technology to
compete against our products. Moreover, our technology primarily represents improvements or
adaptations of known optical spectroscopy technology, which might be duplicated or discovered
through our patents, reverse engineering or both.
In addition, ICU Data Systems developed a patented technology that integrates data from a
broad array of hospital bedside devices into a single bedside display for comparison, data
management and storage. The patent
16
Table of Contents
underlying the technology is licensed by us from the University of Florida College of Medicine
and Engineering and expires in 2023.
In addition, Raba Equity Partners II, LLC and The Johns Hopkins University developed a
patent-pending method of combining blood pressure measurements and signals from the INVOS System to
continuously monitor and display cerebral autoregulatory function information. The patent-pending
underlying the technology is licensed by us from Raba Equity Partners II, LLC and, if issued, the
patents would expire in 2027 and 2028.
Government Regulation
Our products are medical devices subject to extensive regulation by the U.S. Food and Drug
Administration, or FDA, under the Federal Food, Drug, and Cosmetic Act, or FDCA. FDA regulations
govern, among other things, the following activities that we perform:
| • | product development; | ||
| • | product testing; | ||
| • | product manufacturing; | ||
| • | product labeling; | ||
| • | product storage; | ||
| • | premarket clearance or approval; | ||
| • | advertising and promotion; | ||
| • | product sales and distribution; and | ||
| • | product event reports and complaint handling. |
Medical devices to be commercially distributed in the U.S. must receive either 510(k)
clearance or a Premarket Approval, or “PMA”, from the FDA pursuant to the FDCA, prior to marketing.
Devices deemed to pose relatively less risk are placed in either class I or II, which generally
requires the manufacturer to submit a premarket notification requesting permission for commercial
distribution; this is known as 510(k) clearance. Most lower risk, or class I, devices are exempted
from this requirement. Devices deemed by the FDA to pose the greatest risk, such as
life-sustaining, life-supporting or implantable devices, or devices deemed not substantially
equivalent to a previously 510(k) cleared device or a preamendment class III device for which PMA
applications have not been called, are placed in class III requiring PMA approval.
510(k) Clearance Pathway. To obtain 510(k) clearance, a manufacturer must submit a premarket
notification demonstrating that the proposed device is substantially equivalent in intended use and
in safety and effectiveness to a previously 510(k) cleared device or a device that was in
commercial distribution before May 28, 1976 for which the FDA has not called for submission of PMA
applications. The FDA’s 510(k) clearance pathway usually takes from three to six months, but it
can last longer.
After a device receives 510(k) clearance, any modification that could significantly affect its
safety or effectiveness, or that would constitute a major change in its intended use, requires a
new 510(k) clearance or could require a PMA approval. The FDA requires each manufacturer to make
this determination in the first instance, but the FDA can review any such decision. If the FDA
disagrees with a manufacturer’s decision not to seek a new 510(k) clearance, the agency may
retroactively require the manufacturer to seek 510(k) clearance or PMA approval. The FDA also can
require the manufacturer to cease marketing and/or recall the modified device until 510(k)
clearance or PMA approval is obtained, to redesign the device or to submit new data or information
to the FDA.
In October 1997, we obtained FDA clearance for an earlier generation INVOS System
incorporating advances in our INVOS technology. In September 2000, we received 510(k) clearance
from the FDA to market the model 5100 INVOS System in the United States. Unlike earlier models,
the model 5100 INVOS System has the added capability of being able to monitor pediatric patients.
In November 2005, we received 510(k) clearance from the FDA to market our INVOS System to monitor
changes in somatic tissue blood oxygen saturation in regions of the body other than the brain in
patients with or at risk for restricted blood flow. In May 2008, we received 510(k) clearance from
the FDA to market our INVOS System to monitor changes in blood oxygen saturation in any tissues
beneath the sensor, not limited to brain and somatic tissue, in any individual. In April 2009, we
received 510(k)
17
Table of Contents
clearance from the FDA to expand the indications for use to reflect the INVOS System’s ability
to provide accurate, immediate blood oxygen saturation measurements in patients greater than 2.5
kilograms at risk for restricted or no blood flow, in addition to our previous FDA clearance to
measure changes in blood oxygen saturation in any individual. In addition, this most recent 510(k)
clearance expanded the labeling for our INVOS System to include the following new marketing claims:
| • | The measurement of regional cerebral oxygen saturation (rSO2) is an indication of whether oxygen delivery to the brain is adequate. Prolonged declines in rSO2 are indicative of, or may result in, potential brain injury. | ||
| • | When used as an indication of compromised cerebral oxygenation, interventions to return the patient’s rSO2 to baseline using the INVOS System have been shown to improve outcomes after surgery. | ||
| • | In neonates, infants and children, cerebral and somatic rSO2 provide noninvasive indications of oxygen changes in the cerebral and peripheral circulatory systems and may provide an early indication of oxygen deficits associated with impending shock states and anaerobiosis. |
PMA Approval Pathway. A product not eligible for 510(k) clearance must follow the PMA
approval pathway, which requires proof of the safety and effectiveness of the device to the FDA’s
satisfaction. The PMA approval pathway is much more costly, lengthy and uncertain. It generally
takes from one to three years or even longer. A PMA application must provide extensive preclinical
and clinical trial data and also information about the device and its components regarding, among
other things, device design, manufacturing and labeling. As part of the PMA review, an advisory
panel of experts from outside the FDA may be convened to review and evaluate the application and
provide recommendations to the FDA as to the approvability of the device. The FDA may or may not
accept the panel’s recommendation. In addition, the FDA will typically inspect the manufacturer’s
facilities for compliance with Quality System Regulation, or QSR, requirements, which impose
elaborate testing, control, documentation and other quality assurance procedures.
Upon approval, the PMA can include postapproval conditions that the FDA believes necessary to
ensure the safety and effectiveness of the device including, among other things, restrictions on
labeling, promotion, sale and distribution. Failure to comply with the conditions of approval can
result in material adverse enforcement action, including the loss or withdrawal of the approval.
In addition, modifications made to the device that affect its safety or effectiveness, including,
for example, certain modifications to the indications for use, manufacturing process, labeling or
design, require the submission and approval of a PMA Supplement. Supplements to a PMA often require
the submission of the same type of information required for an original PMA, except that the
supplement is generally limited to that information needed to support the proposed change from the
product covered by the original PMA.
None of our marketed products have required the submission or approval of a PMA.
Clinical Trials. A clinical trial is almost always required to support a PMA application and
is sometimes required for a premarket notification. All clinical studies of investigational
devices must be conducted in compliance with FDA’s requirements. If an investigational device
could pose a significant risk to patients (as defined in the regulations), the FDA must approve an
Investigational Device Exemption, or IDE, application prior to initiation of investigational use.
An IDE application must be supported by appropriate data, such as animal and laboratory test
results, showing that it is safe to test the device in humans and that the testing protocol is
scientifically sound. FDA typically grants IDE approval for a specified number of patients to be
treated at specified study centers. A nonsignificant risk device does not require FDA approval of
an IDE. Both significant risk and nonsignificant risk investigational devices require approval
from institutional review boards, or IRBs, at the study centers where the device will be used or
from private IRBs.
During the study, the sponsor must comply with the FDA’s IDE requirements for investigator
selection, trial monitoring, reporting, and record keeping. The investigators must obtain patient
informed consent, rigorously follow the investigational plan and study protocol, control the
disposition of investigational devices, and comply with all reporting and record keeping
requirements. Most IDE requirements apply to all investigational devices,
18
Table of Contents
whether considered significant or nonsignificant risk. Prior to granting PMA approval and at
times 510(k) clearance, the FDA typically inspects the records relating to the conduct of the study
and the clinical data supporting the application for compliance with IDE requirements.
Postmarket. After a device is placed on the market, numerous regulatory requirements apply.
These include: the QSR, labeling regulations, the FDA’s general prohibition against promoting
products for unapproved or “off-label” uses, the Medical Device Reporting regulation (which
requires that manufacturers report to the FDA if their device may have caused or contributed to a
death or serious injury or malfunctioned in a way that would likely cause or contribute to a death
or serious injury if it were to recur), and the Reports of Corrections and Removals regulation
(which requires manufacturers to report recalls, field and other remedial actions to the FDA if
initiated to reduce a risk to health posed by the device or to remedy a violation of the FDCA
caused by the device that may present a risk to health, and maintain records of other corrections
or removals).
FDA enforces these requirements by inspection and market surveillance. If the FDA finds a
violation, it can institute a wide variety of enforcement actions, ranging from a public warning
letter to more severe sanctions such as:
| • | fines, injunctions, consent decrees and civil penalties; | ||
| • | repair, replacement, refunds, recall or seizure of products; | ||
| • | limitations on exports; | ||
| • | operating restrictions or partial suspension or total shutdown of production; | ||
| • | refusing or delaying our requests for 510(k) clearance or PMA approval of new products or new intended uses; | ||
| • | withdrawing 510(k) clearance or PMA approvals already granted; and | ||
| • | criminal prosecution. |
Our most recent FDA QSR inspection occurred in July 2009.
If any of our current or future FDA clearances or approvals are rescinded or denied, sales of
our applicable products in the United States would be prohibited during the period we do not have
such clearances or approvals. In such cases we would consider shipping the product internationally
and/or assembling it overseas if permissible and if we determine such product to be ready for
commercial shipment. The FDA’s current policy is that a medical device that is not in commercial
distribution in the United States, but which needs 510(k) clearance to be commercially distributed
in the United States, can be exported without submitting an export request and prior FDA clearance
under certain conditions.
We are also subject to numerous federal, state and local laws relating to such matters as safe
working conditions, manufacturing practices, environmental protection, fire hazard control and
disposal of hazardous or potentially hazardous substances.
Seasonality
Our business is seasonal. Our fourth quarter has typically been our strongest quarter due to
a larger number of patients undergoing procedures using the INVOS System, including sensors, and
higher INVOS System monitor revenues associated with hospital budgeting cycles.
Employees
As of February 1, 2010, we had 133 full-time employees, including 73 in sales and marketing,
16 in research and development, 15 in general and administration and 29 in manufacturing, quality
and service. We also employed one part-time individual in general and administration. In
addition, we use one contract employee and we use one consultant. We believe that our future
success is dependent, in large part, on our ability to attract and retain highly qualified
managerial, sales, marketing, technical and manufacturing personnel. We expect to add additional
sales and marketing and research and development employees in fiscal 2010. Our employees are not
represented by a union or subject to a collective bargaining agreement. We believe that our
relations with our employees are good.
19
Table of Contents
Insurance
Because the INVOS System and the Vital Sync System are intended to be used in hospital
critical care units with patients who may be seriously ill or may be undergoing dangerous
procedures, we might be exposed to serious potential product liability claims. We have obtained
product liability insurance with a liability limit of $5,000,000. We also maintain coverage for
property damage or loss, general liability, business interruption, travel-accident, directors’ and
officers’ liability and workers’ compensation. We do not maintain key-man life insurance.
Where You Can Get Information We File With The SEC
We file annual, quarterly and special reports, proxy statements and other information with the
Securities and Exchange Commission. You can read and copy any materials we file with the
Securities and Exchange Commission at the Securities and Exchange Commission’s Public Reference
Room at 100 F Street, N.E., Washington, D.C. 20549. You may obtain information on the operation
of the Public Reference Room by calling the Securities and Exchange Commission at 1-800-SEC-0330.
The Securities and Exchange Commission also maintains an Internet site that contains reports, proxy
and information statements and other information regarding issuers, such as us, that file
electronically with the Securities and Exchange Commission. The address of the Securities and
Exchange Commission’s website is http://www.sec.gov.
We also maintain a website at http://www.somanetics.com. We make available free of charge on
or through our website, our annual report on Form 10-K, quarterly reports on Form 10-Q, current
reports on Form 8-K and amendments to those reports filed or furnished pursuant to Section 13(a) or
15(d) of the Exchange Act as soon as reasonably practicable after we electronically file such
material with, or furnish it to, the Securities and Exchange Commission. We will voluntarily
provide electronic or paper copies of our filings free of charge upon request.
This report includes statistical data that were obtained from industry publications. These
industry publications generally indicate that the authors of these publications have obtained
information from sources believed to be reliable but do not guarantee the accuracy and completeness
of their information. While we believe these industry publications to be reliable, we have not
independently verified their data.
20
Table of Contents
ITEM 1A. RISK FACTORS
An investment in our common shares involves a high degree of risk. You should carefully
consider the specific factors described below, together with the cautionary statement under the
caption “Forward — Looking Statements” below and in Item 7 of this report and the other information
included in this report, before purchasing our common shares. The risks described below are not
the only ones that we face. Additional risks that are not yet known to us or that we currently
think are immaterial could also impair our business, financial condition or results of operations.
If any of the following risks actually occurs, our business, financial condition or results of
operations could be adversely affected. In such case, the trading price of our common shares could
decline, and you may lose all or part of your investment.
Risks Relating to Our Business
Our future growth depends on increased market acceptance of our INVOS System in existing market
segments and market acceptance in new market segments.
Since sales of the INVOS System, including disposable sensors, currently account for
substantially all of our revenues, our future growth will depend on the degree to which our INVOS
System is accepted by hospitals and clinicians in our existing market segments and in new market
segments, such as the neonatal ICU, major surgeries involving high risk patients and other
applications. There are numerous factors that could adversely impact market acceptance of our
INVOS System.
Part of our marketing strategy is to encourage and support clinical research programs. We
depend on favorable peer-reviewed publication and successful clinical use of our products for our
success. The INVOS System has not had extensive clinical use in the new market segments. We
cannot assure you that additional research papers will be published or that any such papers will
conclude that the INVOS System provides information that is clinically important. In addition,
researchers might publish results that do not support the clinical importance of the information
provided by the INVOS System or that conclude that another product provides better or more
important information. Performance problems or adverse research results could prevent acceptance
of the product in existing and new market segments, adversely affect our reputation in the medical
community, result in unexpected expense and adversely affect future sales.
In addition, we compete with numerous medical equipment companies and medical device
integration companies for the portions of hospital budgets allocated to capital equipment and for
the limited amount of space on a patient’s forehead for sensors. Sales of our INVOS System and
Vital Sync System might be limited or delayed because of resistance to major capital equipment
expenditures by hospital purchasing committees, especially during the current economic conditions.
Even if we are successful in convincing physicians, other medical professionals and hospital
purchasing committees that the INVOS System or Vital Sync System provides valuable benefits, they
might be unwilling or unable to commit funds to the purchase of the INVOS System or Vital Sync
System due to budgetary constraints. Moreover, even if one or two units are sold to a hospital, we
believe that it will take additional time and experience with the INVOS System or Vital Sync System
before additional medical professionals in the hospital might be interested in using the INVOS
System or Vital Sync System in other procedures or other areas of the hospital.
Sales of all of our products might be limited because hospitals might fear that the cost of a
new device or product will lower their profits because medical insurers generally fix reimbursement
amounts for the procedures in which our products are generally used. Moreover, medical
professionals may be reluctant to use our INVOS System or Vital Sync System in some new market
segments, particularly those involving diagnostic applications, unless they receive reimbursement
from medical insurers for using the system. Our INVOS System is not currently cleared by the FDA
for use in the diagnosis of disease states. Additionally, the INVOS System or Vital Sync System is
not currently approved for separate reimbursement, and we might not be able to obtain reimbursement
for these uses of our INVOS System or Vital Sync System.
If the INVOS System fails to achieve market acceptance in existing or new market segments or
if these market segments fail to develop as rapidly as expected, our business, financial condition
and results of operations
21
Table of Contents
could be adversely affected and our plan to increase our investments in our direct sales team
and our research and development team might not produce favorable results.
We are dependent on our international distributors for a substantial portion of our sales, and
their failure to sell our products adequately would adversely affect our business.
We are dependent on our distributors to generate all of our international sales. These
independent distributors might fail to commit the necessary resources to market and sell our
products at the level we expect, especially as significant customer education and long lead times
are typically required to market and sell our products successfully. If our distributors fail to
market, promote and sell our products adequately, our business, financial condition and results of
operations would be adversely affected. We might not be able to engage additional distributors on
a timely basis, enter into other third-party marketing arrangements or retain or replace our
existing distributors, when required. If we are unable to engage, replace or retain distributors,
our ability to market and sell our products internationally could be adversely affected. In
addition, if any of our distributor arrangements are terminated or discontinued, we will likely be
faced with increased costs as we attempt to replace these arrangements, and the terminated
distributors might begin to sell a competitor’s product. Even if we are able to engage new
distributors or retain existing ones, they might incur conflicting obligations to sell other
companies’ products or they might distribute other products that provide greater revenues to them
than are provided by our products.
Covidien, our international distributor in Europe, the Middle East, South Africa and Canada
for our INVOS System, accounted for 13%, 14% and 13% of our net revenues for fiscal years 2009,
2008 and 2007, respectively. Edwards Lifesciences Ltd. is our international distributor in Japan
for our INVOS System. The loss of either of these distributors could have an adverse effect on our
business, financial condition and results of operations.
The global economic crisis has had and may continue to have a negative effect on our business
and operations.
The global economic crisis has caused, among other things, lower business spending, which has
had and may continue to have a negative effect on our business and results of operations, including
an increase in the sales cycle for our products. Many of our customers and suppliers have been
affected by the current economic turmoil. Current or potential customers and suppliers may no
longer be in business, may be unable to fund purchases or determine to reduce purchases, all of
which have led and could continue to lead to delays in purchases of our products and customer
payment delays. Further, suppliers may not be able to supply us with needed components on a timely
basis, may increase prices or go out of business, which could result in our inability to meet
customer demand or affect our gross margins. The timing and nature of any recovery in the economy
remains uncertain, and there can be no assurance that market conditions will improve in the near
future or that our results will not be materially and adversely affected. Such conditions make it
very difficult to forecast operating results, make business decisions and identify and address
material business risks.
We currently depend on single-source suppliers for key components of the INVOS System, and the
loss of any of these suppliers could harm our ability to manufacture and sell our products,
increase the cost of our components or delay our clinical trials.
We are dependent on various suppliers for manufacturing the components for our INVOS System
and Vital Sync System. Although we believe that most components are generally available from
several potential suppliers, we depend on one supplier for one of our components. We are not aware
of any validated alternative supplier for this component, although we are currently in the process
of evaluating a second source of supply. Moreover, we typically use one supplier for
custom-designed components, including the unit enclosure, the printed circuit boards, other
mechanical components and the disposable sensor. Sensors represented approximately 81% of our net
revenues in fiscal 2009. Engaging additional or replacing existing suppliers of custom-designed
components is costly and time consuming. We estimate that it would require approximately four to
five months to change sensor suppliers. We do not intend to maintain significant inventories of
components, other than an approximate six-month supply of the one component for which we currently
have no alternative supplier. If we fail to obtain custom-designed components from our sole
suppliers, if we lose any of our present suppliers and cannot replace them on a timely basis when
necessary, if there is an interruption of production at one or more of our suppliers, or if any
supplier is otherwise unable or unwilling to meet our requirements at current prices or at all, our
ability to
22
Table of Contents
manufacture and sell our products would be impaired or we might have to pay higher prices for
our components or our clinical trials could be delayed. In addition, because we do not have
long-term agreements with our suppliers, we might be subject to unexpected price increases which
might adversely affect our profit margins.
In addition, we do not have direct control over the activities of our suppliers and are
dependent on them for quality control, capacity, processing technologies and, in required cases,
compliance with FDA Quality System Regulation requirements. If we are unsuccessful in managing our
suppliers, our business could be adversely affected.
We may become subject to competition which may adversely affect us.
There are numerous and varied forms of patient monitoring products that constitute our
competition. Other cerebral and somatic oximeters are just one form of these competing products.
In the United States, we believe there are currently three other companies with FDA clearance to
sell a cerebral oximeter. In December 2005, CAS Medical Systems, Inc. announced that it received
510(k) clearance for a cerebral oximeter, and it began sales of its product during 2007. In
August 2009, we filed a patent infringement action against CAS Medical Systems, Inc. See Item 3
“Legal Proceedings” in this report. During 2009, one other U.S. company announced that it received
510(k) clearance for a cerebral oximeter, and it began sales of its product late in 2009. Outside
the United States, several Japanese manufacturers offer a cerebral oximeter for sale in that
country and primarily for research in other parts of the world, but, to our knowledge, as of yet,
none has pursued FDA clearance for its product in the United States. We are aware that several
companies and individuals are engaged in the research and development of non-invasive cerebral
oximeters, and we believe that there are several others who may begin development of a cerebral
oximeter. Other companies have FDA clearance for somatic oximeters in the United States.
Competition might cause our sales cycle to lengthen to the extent that customers take longer to
make purchasing decisions. Competition might also reduce our gross margins and market share and
prevent us from achieving further market penetration. Competitors might be more successful than we
are in obtaining FDA clearance with broader claims in their labeling or more successful than we are
in manufacturing and marketing their products and may be able to take advantage of the significant
time and effort we have invested to gain medical acceptance of cerebral oximetry.
We compete with numerous medical equipment companies and medical device integration companies
for the portions of hospital budgets allocated to capital equipment and for the limited amount of
space on a patient’s forehead for sensors. Some of these potential competitors have
well-established reputations, customer relationships and marketing, distribution and service
networks. Some of them have substantially longer histories in the medical products industry,
larger product lines and greater financial, technical, manufacturing, research and development and
management resources than we do. Many of these competitors and potential competitors have
long-term product supply relationships with our potential customers.
We also compete with companies that have longer operating histories, more established products
and greater resources than we do for, among other things, forehead monitoring space, limited
hospital capital budgets and alternative products.
The medical products industry is characterized by extensive research and development and
intense competition in an increasingly cost-conscious environment. Some of these competitors and
potential competitors have well-established reputations, customer relationships and marketing,
distribution and service networks. Some of them have substantially longer histories in the medical
products industry, larger product lines and greater financial, technical, manufacturing, research
and development and management resources than we do. Many of these competitors and potential
competitors have long-term product supply relationships with our potential customers. These
competitors and potential competitors might be able to use their resources, reputations and ability
to leverage existing customer relationships to give them a competitive advantage over us, including
in securing forehead sensor space for their products and dollars from hospital capital equipment
budgets to purchase their products. They might also succeed in developing products that are at
least as reliable and effective as our products, that make additional measurements, that are less
costly than our products or that provide alternatives to our products.
23
Table of Contents
If we fail to manage our growth effectively, our business and operating results could be harmed.
If we experience growth in our business, our growth could place a significant strain on our
management, customer service, operations, sales and administrative personnel and other resources.
To serve the needs of our existing and future customers, we will be required to train, motivate and
manage qualified employees. We have incurred and will continue to incur significant costs to
retain qualified management, sales and marketing, engineering, production, manufacturing and
administrative personnel, as well as expenses for marketing and promotional activities. Our
ability to manage our planned growth depends upon our success in expanding our operating,
management and information and financial systems, which might significantly increase our operating
expenses.
We have invested substantial resources to develop the INVOS System. We expect to continue to
invest resources to develop advances to the design and performance features of the INVOS System,
including the disposable sensor, to interface our INVOS System with multi-functional monitors
provided by other manufacturers, to further develop our Vital Sync System, and to combine the Vital
Sync System and INVOS System technologies in a single product. In addition, we expect to invest
resources in development costs associated with our Exclusive Sublicense Agreement with Raba Equity
Partners II, LLC, our Contract Development Agreement with Shirley Research Corporation and our
Development and Exclusive License Agreement with an inventor and his company. New products require
extensive testing and regulatory clearance before they can be marketed, and substantial customer
education concerning the product’s use, advantages and effectiveness. We might not be able to
develop commercially viable products. We might not be able to manage effectively our future
growth, and if we fail to do so, our business, financial condition and results of operations would
be adversely affected.
Patients might assert product liability claims against us.
Because we test, market and sell patient monitoring devices, patients might assert product
liability claims against us. The INVOS System and the Vital Sync System are used in operating
rooms and other critical care hospital units with patients who might be seriously ill or might be
undergoing dangerous procedures. On occasion, patients on whom the INVOS System or Vital Sync
System is being used may be injured or die as a result of their medical treatment or condition. We
might be sued because of such injury or death, and regardless of whether we are ultimately
determined to be liable or our products are determined to be defective and a contributing factor in
such injury or death, we might incur significant legal expenses not covered by insurance. In
addition, product liability litigation could damage our reputation and impair our ability to market
our products, regardless of the outcome. Litigation could also impair our ability to retain
product liability insurance or make our insurance more expensive. We have product liability
insurance with a liability limit of $5,000,000. This insurance is costly and even though it has
been obtained, we might not be able to retain it. Even if we are able to retain this insurance, it
might not be sufficient to protect us in the event of a major defect in the INVOS System or Vital
Sync System. If we are subject to an uninsured or inadequately insured product liability claim
based on the performance of the INVOS System or Vital Sync System, our business, financial
condition and results of operations could be adversely affected.
If we fail to obtain and maintain necessary U.S. Food and Drug Administration clearances for our
products and indications or if clearances for future products and indications are delayed or not
issued, our business would be harmed.
Our products are classified as medical devices and are subject to extensive regulation in the
United States by the FDA and other federal, state and local authorities. These regulations relate
to manufacturing, labeling, sale, promotion, distribution, importing and exporting and shipping of
our products. In the United States, before we can market a new medical device, or a new use of, or
claim for, an existing product such as the INVOS System or our Vital Sync System, we must first
receive either 510(k) clearance or premarket approval from the FDA, unless an exemption applies.
Both of these processes can be expensive and lengthy. The FDA’s 510(k) clearance process usually
takes from three to six months, but it can last longer. The process of obtaining premarket approval
is much more costly and uncertain than the 510(k) clearance process. It generally takes from one to
three years, or even longer, from the time the premarket approval application is submitted to the
FDA until an approval is obtained.
In order to obtain premarket approval and, in some cases, a 510(k) clearance, a product
sponsor must conduct well-controlled clinical trials designed to test the safety and effectiveness
of the product. Conducting
24
Table of Contents
clinical trials generally entails a long, expensive and uncertain process that is subject to
delays and failure at any stage. The data obtained from clinical trials may be inadequate to
support approval or clearance of a submission. In addition, the occurrence of unexpected findings
in connection with clinical trials may prevent or delay obtaining approval or clearance. If we
conduct clinical trials, they may be delayed or halted, or be inadequate to support approval or
clearance.
Medical devices may be marketed only for the indications for which they are approved or
cleared. The FDA may fail to approve or clear indications that are necessary or desirable for
successful commercialization. Indeed, the FDA may refuse our requests for 510(k) clearance or
premarket approval of new products, or new intended uses or modifications to existing products.
Our clearances can be revoked if safety or effectiveness problems develop.
The FDA might require us to obtain a new clearance to label or promote the INVOS System for
specific patient subgroups; if we fail to obtain such clearances, our sales and revenues may be
adversely affected.
Our INVOS System 510(k) clearance states that the prospective clinical value of the INVOS
System has not been demonstrated in patients with specific disease states. If we wish to label or
promote more actively the INVOS System for specific types of patients, the FDA may require us to
obtain a new 510(k) clearance and would likely carefully scrutinize the data support for any such
claim. We cannot assure you that the FDA would grant additional 510(k) clearances in a timely
fashion, or at all, or that the FDA would not require us to undertake the more burdensome premarket
approval process as a prerequisite for marketing the INVOS System with this type of specific claim.
Any of the above could delay our ability to market and sell new products or to promote the INVOS
System for specific patient subgroups and would thereby have an adverse effect on our business,
financial condition and results of operations.
After clearance or approval of our products, we are subject to continuing regulation by the FDA,
and if we fail to comply with FDA regulations, our business could suffer.
Even after clearance or approval of a product, we are subject to continuing regulation by the
FDA, including the requirements that our facility be registered and our devices listed with the
agency. We are subject to Medical Device Reporting regulations, which require us to report to the
FDA if our products may have caused or contributed to a death or serious injury or malfunction in a
way that would likely cause or contribute to a death or serious injury if the malfunction were to
recur. We must report corrections and removals to the FDA where the correction or removal was
initiated to reduce a risk to health posed by the device or to remedy a violation of the FDCA
caused by the device that may present a risk to health, and maintain records of other corrections
or removals. The FDA closely regulates promotion and advertising, and our promotional and
advertising activities could come under scrutiny. If the FDA objects to our promotional and
advertising activities or finds that we failed to submit reports under the Medical Device Reporting
regulations, for example, the FDA may allege our activities resulted in violations of law.
The FDA and state authorities have broad enforcement powers. Our failure to comply with
applicable regulatory requirements could result in enforcement action by the FDA or state agencies,
which may include any of the following sanctions:
| • | untitled letters, warning letters, fines, injunctions, consent decrees and civil penalties; | ||
| • | repair, replacement, refunds, recall or seizure of our products; | ||
| • | limitations on exports; | ||
| • | operating restrictions or partial suspension or total shutdown of production; | ||
| • | refusing or delaying our requests for 510(k) clearance or premarket approval of new products or new intended uses; | ||
| • | withdrawing 510(k) clearance or premarket approvals that have already been granted; and | ||
| • | criminal prosecution. |
If any of these events were to occur, they could harm our business.
25
Table of Contents
We have modified some of our products without FDA clearance. The FDA could retroactively
determine that the modifications were improper and require us to stop marketing and recall the
modified products.
Any modifications to one of our FDA-cleared devices that could significantly affect its safety
or effectiveness, or that would constitute a major change in its intended use, requires a new
510(k) clearance or a premarket approval. We may be required to submit extensive pre-clinical and
clinical data depending on the nature of the changes. We may not be able to obtain additional
510(k) clearances or premarket approvals for modifications to, or additional indications for, our
existing products in a timely fashion, or at all. Delays in obtaining future clearances or
approvals would adversely affect our ability to introduce new or enhanced products in a timely
manner, which in turn would harm our revenue and operating results. We have made modifications to
our devices in the past, such as changes to the disposable sensor, and may make additional
modifications in the future that we believe do not or will not require additional clearances or
approvals. We believe that these changes do not require the submission of a new 510(k) notice. If
the FDA disagrees, and requires new clearances or approvals for the modifications, we may be
required to recall and to stop marketing the modified devices, to redesign our products or submit
new data or information to the FDA. This could harm our operating results.
If we fail to comply with the FDA’s Quality System Regulation, our manufacturing operations
could be halted, and our business would suffer.
We are currently required to demonstrate and maintain compliance with the FDA’s Quality System
Regulation, or QSR. The QSR governs the methods and documentation of the design, testing, control,
manufacturing, labeling, quality assurance, packaging, storage and shipping of our products. The
FDA enforces the QSR through periodic inspections, which may be unannounced. We have been, and
anticipate in the future being, subject to such inspections. Our failure to comply with the QSR or
to take satisfactory corrective action in response to an adverse QSR inspection could result in
enforcement actions, including a public warning letter, a shutdown of or restrictions on our
manufacturing operations, delays in approving or clearing a product, refusal to permit the import
or export of our products, a recall or seizure of our products, fines, injunctions, civil or
criminal penalties, or other sanctions, any of which could cause our business and operating results
to suffer.
Our products may in the future be subject to product recalls that could harm our reputation,
business and financial results.
The FDA and similar foreign governmental authorities have the authority to require the recall
of commercial products in the event of material deficiencies or defects in design or manufacture.
In the case of the FDA, the authority to require a recall must be based on an FDA finding that
there is a reasonable probability that the device would cause serious injury or death. In addition,
foreign governmental bodies have the authority to require the recall of our products in the event
of material deficiencies or defects in design or manufacture. Manufacturers may, under their own
initiative, recall a product if any material deficiency in a device is found. A government-mandated
or voluntary recall by us or one of our distributors could occur as a result of component failures,
manufacturing errors, design or labeling defects or other deficiencies and issues. Recalls of any
of our products would divert managerial and financial resources and have an adverse effect on our
financial condition and results of operations. The FDA requires that certain classifications of
recalls be reported to FDA within 10 working days after the recall is initiated. Companies are
required to maintain certain records of recalls, even if they are not reportable to the FDA. A
future recall announcement could harm our reputation with customers and negatively affect our
sales. In addition, the FDA could take enforcement action against us if we fail to properly report
field actions to the FDA.
If our products cause or contribute to a death or a serious injury, or malfunction in certain
ways, we will be subject to medical device reporting regulations, which can result in voluntary
corrective actions or agency enforcement actions.
Under the FDA medical device reporting regulations, medical device manufacturers are required
to report to the FDA information that a device has or may have caused or contributed to a death or
serious injury or has malfunctioned in a way that would likely cause or contribute to death or
serious injury if the malfunction of the device or one of our similar devices were to recur. If we
fail to report these events to the FDA within the required timeframes, or at all, FDA could take
enforcement action against us. Any such adverse event involving our products also could result in
future voluntary corrective actions, such as recalls or customer notifications, or agency action,
26
Table of Contents
such as inspection or enforcement action. Any corrective action, whether voluntary or
involuntary, as well as defending ourselves in a lawsuit, will require the dedication of our time
and capital, distract management from operating our business, and may harm our reputation and
financial results.
Failure to obtain or maintain regulatory approval in foreign jurisdictions would prevent us from
marketing our products abroad.
We market our products through distributors in foreign markets. In order to market our
products in the European Community and many other foreign jurisdictions, we must obtain separate
regulatory approvals. We depend on our distributors to obtain and maintain certain of these
regulatory approvals. The approval procedure varies among countries and can involve additional
requirements and testing, and the time required to obtain approval may differ from that required to
obtain FDA clearance. The foreign regulatory approval process may include all of the risks
associated with obtaining FDA clearance in addition to other risks. Our distributors might not be
able to obtain or maintain foreign approvals on a timely basis or at all. Clearance by the FDA
does not ensure approval by regulatory authorities in other countries, and approval by one foreign
regulatory authority does not ensure approval by regulatory authorities in other foreign countries
or approval or clearance by the FDA. Failure to obtain or maintain regulatory approval in foreign
jurisdictions would prevent us from marketing our products abroad.
Federal regulatory reforms may adversely affect our ability to sell our products profitably.
From time to time, legislation is drafted and introduced in Congress that could significantly
change the statutory provisions governing clearance or approval, manufacture and marketing of a
device. In addition, FDA regulations and guidance are often revised or reinterpreted by the agency
in ways that may significantly affect our business and our products. We cannot predict whether
legislative changes will be enacted or FDA regulations, guidance or interpretations changed, and
what the impact of such changes, if any, may be.
The lengthy sales cycle for the INVOS System could cause variability in our operating results.
The decision-making process for our INVOS System customers is often complex and
time-consuming. We believe the period between initial discussions with a potential customer and a
sale of even one unit is approximately six to nine months. The process can be delayed as a result
of hospital capital budgeting procedures. In addition, the process has been and may continue to be
delayed significantly as a result of the current economic downturn in the United States and abroad.
These delays could have an adverse effect on our business, financial condition and results of
operations and cause variability in our operating results from quarter to quarter, which could
cause fluctuations in the trading price of our common shares.
Sales employee and clinical specialist employee turnover could have an adverse effect on our
business and cause variability in our operating results.
As we expand the number of our sales employees and clinical specialist employees, and alter
our sales territories, we increase the chance of sales employee turnover. We have incurred and
expect to continue to incur significant costs to hire and train new qualified sales employees. In
addition, the process of replacing sales employees can lengthen our sales cycle. These delays
could have an adverse effect on our business, financial condition and results of operations and
could cause variability in our operating results from quarter to quarter, which could adversely
affect the price of our common shares.
If we are unable to obtain or maintain intellectual property rights relating to our technology
and products, the commercial value of our technology and products will likely be adversely
affected and our competitive position could be harmed.
Our success and ability to compete depends in part upon our ability to obtain protection in
the United States and other countries for our products by establishing and maintaining intellectual
property rights relating to or incorporated into our technology and products. We own or license a
variety of patents and patent applications in the United States and corresponding patents and
patent applications in certain foreign jurisdictions. Pending and future
27
Table of Contents
patent applications owned or licensed by us may not issue as patents or, if issued, may not
issue in a form that will be commercially advantageous to us. Even if issued, patents may be
challenged, narrowed, invalidated or circumvented, which could limit our ability to stop
competitors from marketing similar products or limit the length of term of remaining patent
protection we may have for our products. In addition, already issued patents owned or licensed by
us may not be valid or enforceable. Further, even if valid and enforceable, these already issued
patents may not be sufficiently broad to prevent others from marketing competitive products,
despite our patent rights. Changes in either patent laws or in interpretations of patent laws in
the United States and other countries may diminish the value of our intellectual property or narrow
the scope of our patent protection.
For example, one of our significant patents is the subject of a reissue and re-examination
proceeding in the U.S. Patent and Trademark Office. Our reissue application was filed for the sole
purpose of seeking to broaden certain claims. CAS Medical Systems, Inc. has requested
re-examination of this patent, and the reissuance and re-examination proceedings have been
combined. We cannot predict the outcome of this proceeding, which may result in some or all of the
claims being maintained, broadened, narrowed or rejected.
The validity of our patents depends, in part, on whether prior art references disclosed or
rendered obvious our inventions as of the filing date of our patent applications. It is possible
that all relevant prior art may not have been identified, such as U.S. and foreign patents or
published applications or published scientific literature, that could adversely affect the validity
of our issued patents or the patentability of our pending patent applications. For example, patent
applications in the United States are maintained in confidence for up to 18 months after their
filing. In some cases, however, patent applications remain confidential in the U.S. Patent and
Trademark Office for the entire time prior to issuance as a U.S. patent. Patent applications filed
in countries outside the United States are also not typically published until at least 18 months
from their first filing date. Similarly, publication of discoveries in the scientific or patent
literature often lags behind actual discoveries.
We may initiate litigation to enforce some of our patent rights, which may prompt our
adversaries in such litigation to challenge the validity, scope or enforceability of our patents.
If a court decides that one or more of our patents are not valid, not enforceable or of a limited
scope, our rights to stop others from using our inventions may be compromised. For example, during
2009, we filed a patent infringement action against CAS Medical Systems, Inc., one of our
competitors. The defendant in the litigation has answered the complaint denying that it infringes
any asserted patent and asserting defenses and counterclaims, including those for patent invalidity
and/or unenforceability, and antitrust violations. The defendant was also the party that requested
re-examination of one of our patents.
The outcome of this litigation and any other litigation to enforce our patent rights is
subject to substantial uncertainties, especially in medical device-related patent cases that may,
for example, turn on the interpretation of patent claim language by the court which may not be to
our advantage, and also the testimony of experts as to technical facts upon which experts may
reasonably disagree. We expect our involvement in such intellectual property litigation to result
in significant expense.
We also cannot be certain that we were the first to invent the cerebral oximeter technologies
upon which our patents are based or that we were the first to file patent applications based upon
those technologies, in those foreign jurisdictions where patent rights are granted to the first to
file as opposed to the first to invent. In the event that a third party has also filed a U.S.
patent application covering our cerebral oximeter devices, the sensors used with these devices, or
a similar invention, we may have to participate in an adversarial proceeding known as an
interference, which is declared by the U.S. Patent and Trademark Office to determine priority of
invention in the United States. It is possible that we may be unsuccessful in the interference,
resulting in a loss of some or all of our U.S. patent claims. We may also face similar proceedings
outside the United States, including oppositions, to determine priority of invention or
patentability. Even if we are successful in these proceedings, we may incur substantial costs, and
the time and attention of our management and scientific personnel will be diverted in pursuit of
these proceedings. Moreover, the laws or enforcement procedures of some foreign jurisdictions may
not protect intellectual property rights to the same extent as in the United States, and many
companies have encountered significant difficulties in protecting and defending such rights in
foreign jurisdictions. If we encounter such difficulties or if we are otherwise precluded from
effectively protecting our intellectual property rights in foreign jurisdictions, we may incur
substantial costs and our business prospects could be substantially harmed.
28
Table of Contents
We rely on trade secret and copyright protection to protect our interests in proprietary
information and know-how, and for processes for which patents are undesirable to obtain or are
difficult to obtain or enforce. We may not be able to protect our trade secrets or copyrights
adequately. For example, none of our copyrights have been registered with the U.S. Copyright
Office, which limits our ability to sue for and collect damages from third party infringers. In
addition, we rely on non-disclosure and confidentiality agreements with employees, consultants and
other parties to protect, in part, trade secrets and other proprietary technology. These
agreements may be breached, and we may not have adequate remedies for any breach. Moreover, others
may independently develop equivalent proprietary information, and third parties may otherwise gain
access to our trade secrets and proprietary knowledge. Any disclosure of confidential data into
the public domain or to third parties could allow our competitors to learn our trade secrets and
use the information in competition against us.
If we are found to infringe or are alleged to infringe any third party intellectual property
rights, then our business may be adversely affected.
There are numerous U.S. and foreign issued patents and pending patent applications owned by
third parties with patent claims in the fields of cerebral and somatic oximetry, and other areas
that are the focus of our product development efforts. We are aware of patents owned by third
parties, to which we do not have licenses, that relate to, among other things, optical spectroscopy
and the interaction of light with tissue and optical spectroscopy in the area of brain metabolism.
For example, possible competitors own patents that are directed to the non-invasive determination
of blood oxygen saturation levels with a near infra-red spectrophotometric sensor and to an
apparatus for measuring oxygen saturation in blood using two different wavelengths of light. There
may be other patents in addition to those of which we are aware that relate to aspects of our
technology and that may materially and adversely affect our business. Moreover, because patent
applications can take many years to issue as patents, there may be currently pending but
unpublished patent applications, unknown to us, which may later result in issued patents that pose
a material risk to us.
We may pose a threat to companies that own or control patents relating to cerebral oximetry
systems or their components, or to the manufacture and use of such systems, and one or more third
parties may file a lawsuit asserting a patent infringement claim against the manufacture, use or
sale of the INVOS System based on one or more of these patents. We are not aware of any
infringement of the claims of any issued patents by our products, and no charge of patent
infringement has been asserted against us. However, potential competitors would have more
incentive to assert infringement claims or challenge our patents if a more significant market for
the INVOS System develops.
Whether the manufacture, sale or use of the INVOS System, or whether any products under
development would, upon commercialization, infringe any patent claim will not be known with
certainty unless and until a court interprets the patent claim in the context of litigation. If an
infringement allegation is made against us, we may seek to invalidate the asserted patent claim
and/or to allege non-infringement of the asserted patent claim. In order for us to invalidate a
U.S. patent claim, we would need to rebut the presumption of validity afforded to issued patents in
the United States with clear and convincing evidence of invalidity, which is a high burden of
proof.
The outcome of infringement litigation is subject to substantial uncertainties, especially in
medical device-related patent cases that may, for example, turn on the interpretation of patent
claim language by the court which may not be to our advantage, and also the testimony of experts as
to technical facts upon which experts may reasonably disagree. Our defense of an infringement
litigation lawsuit could result in significant expense. Regardless of the outcome, infringement
litigation could significantly disrupt our marketing, development and commercialization efforts,
divert our management’s attention and quickly consume our financial resources.
In the event that we are found to infringe any valid claim in a patent held by a third party,
we may, among other things, be required to:
| • | pay damages, including up to treble damages and the other party’s attorneys’ fees, which may be substantial; |
29
Table of Contents
| • | cease the development, manufacture, importation, use and sale of products that infringe the patent rights of others, including our INVOS System and Vital Sync System, through a court-imposed sanction called an injunction; | ||
| • | expend significant resources to redesign our technology so that it does not infringe others’ patent rights, or to develop or acquire non-infringing intellectual property, which may not be possible; | ||
| • | discontinue manufacturing or other processes incorporating infringing technology; and/or | ||
| • | obtain licenses to the infringed intellectual property, which may not be available to us on acceptable terms, or which may not be available at all. |
Any development or acquisition of non-infringing products or technology or licenses could
require the expenditure of substantial time and other resources and could have a materially adverse
effect on our business and financial results. If we are required to, but cannot, obtain a license
to valid patent rights held by a third party, we would likely be prevented from commercializing the
relevant product, or from further manufacture, sale or use of the relevant product. If we need to
redesign products or need to develop new methods to avoid third-party patents, we may suffer
significant regulatory delays associated with conducting additional studies or submitting
technical, manufacturing or other information related to the redesigned product and, ultimately, in
obtaining approval.
While our products are in clinical trials, and prior to commercialization, we believe our
activities in the United States related to the submission of data to the FDA fall within the scope
of the exemptions that cover activities related to developing information for submission to the FDA
and fall under general investigational use or similar laws in other countries. However, the U.S.
exemptions would not cover the manufacturing, sale or use of products which are no longer in
clinical trials, or other activities in the United States that support overseas clinical trials if
those activities are not also reasonably related to developing information for submission to the
FDA. In any event, the fact that no third party has asserted a patent infringement claim against
us to date should not be taken as an indication, or as a level of comfort, that a patent
infringement claim will not be asserted against us prior to or upon commercialization.
Some of our agreements, including our distribution and sales representative agreements require
us to indemnify the other party in certain circumstances where our products have been found to
infringe a patent or other proprietary rights of others. An indemnification claim against us may
require us to pay substantial sums to the indemnified party, including its attorneys’ fees.
Our success depends on our ability to attract and retain key personnel.
Our future performance depends in significant part on the continued service of our senior
management, including Bruce J. Barrett, our President and Chief Executive Officer, and various
scientific, technical and manufacturing personnel. Our loss of any of these key personnel could
have an adverse effect on us. We do not maintain key-man life insurance on any of our key
personnel, and our employment agreement with Mr. Barrett currently expires June 17, 2011. In
addition, if we are unable to attract, retain and motivate additional, highly-skilled employees
required for the expansion of our operations, our business, financial condition and results of
operations could be adversely affected. We cannot assure you that we will be able to retain our
existing personnel or attract additional, qualified persons when required and on acceptable terms.
Any acquisitions that we make could disrupt our business and harm our financial condition.
From time to time, we evaluate potential strategic acquisitions of complementary businesses,
products or technologies, as well as consider joint ventures and other collaborative projects. For
example, in November 2008, we acquired substantially all of the assets of ICU Data Systems, Inc., a
technology development company, for approximately $2,000,000 in cash plus the assumption of
specified liabilities. In October 2009, we entered into an Exclusive Sublicense Agreement with
Raba Equity Partners II, LLC and paid up-front, non-refundable payments of $1.8 million to Raba
Equity Partners II, LLC. In November 2009, we entered into a Development and Exclusive Agreement
with an inventor and his company and paid an up-front, non-refundable payment of $200,000 and will
pay an additional $300,000 upon obtaining 510(k) clearance for the product. We may not be able to
identify appropriate acquisition candidates or strategic partners, or successfully negotiate,
finance or integrate any businesses, products or technologies that we acquire. Any acquisition we
pursue could diminish our cash otherwise
30
Table of Contents
available to us for other uses or be dilutive to our shareholders, and could divert
management’s time and resources from our core operations.
We have broad discretion to determine how to allocate our cash, cash equivalents, marketable
securities and investments and may not use them effectively.
As of November 30, 2009, we have approximately $79,733,000 of cash, cash equivalents,
marketable securities and long-term investments on hand, which provides us with flexibility in
implementing our business plans and responding to future business conditions and opportunities. We
have broad discretion to determine how to allocate our cash, cash equivalents, marketable
securities and investments. If we fail to apply these funds effectively, the failure could result
in financial losses that could have a material adverse effect on our business and cause the price
of our common shares to decline. We intend to keep sufficient cash, cash equivalents, marketable
securities and investments in cash and bank accounts to avoid becoming an inadvertent investment
company subject to regulation under the Investment Company Act of 1940. The remaining cash, cash
equivalents, marketable securities and investments are expected to be invested in short-term, U.S.
government or other investment grade, interest-bearing investments. These restrictions on our
investments might limit the income otherwise available from investing these funds, lowering our
income and potentially decreasing our earnings and the price of our common shares.
Risks Relating to Our Common Shares
Provisions of our corporate charter documents and Michigan law may delay or prevent attempts by
our shareholders to change our management and hinder efforts to acquire a controlling interest
in us.
Our board of directors has the authority, without further approval of our shareholders, to
issue preferred shares having such rights, preferences and privileges as the board may determine.
Any such issuance of preferred shares could, under some circumstances, have the effect of delaying
or preventing a change in control of us and might adversely affect the rights of holders of common
shares. In addition, we are subject to Michigan statutes regulating business combinations and
takeovers, which might also hinder or delay a change in control of our company. Anti-takeover
provisions that could be included in the preferred shares when issued and the Michigan statutes
regulating business combinations and takeovers can depress the market price of our securities and
can limit the shareholders’ ability to receive a premium on their shares by discouraging takeover
and tender offer bids, even if such events could be viewed as beneficial by our shareholders.
Our directors serve staggered three-year terms, and directors may be removed only for cause by
a vote of the holders of a majority of the shares entitled to vote at an election of directors.
Our Restated Articles of Incorporation also set the minimum number of directors constituting the
entire board at three and the maximum at fifteen, and they require approval of holders of 90% of
our voting shares to amend these provisions. Our bylaws contain procedures, including notice
requirements, for nominating persons for election to our board of directors. These provisions
could have an anti-takeover effect by making it more difficult to acquire our company by means of a
tender offer, a proxy contest or otherwise or by removing incumbent officers and directors. These
provisions could delay, deter or prevent a tender offer or takeover attempt that a shareholder
might consider in his or her best interests, including those attempts that might result in a
premium over the market price for the common shares held by our shareholders.
The market price of our common shares has been volatile and may continue to remain so.
The market price of our common shares has been volatile. The following could cause the market
price of the common shares to continue to fluctuate substantially:
| • | changes in our quarterly financial condition or operating results; | ||
| • | changes in general conditions in the economy; | ||
| • | changes in the financial markets; | ||
| • | changes in the medical equipment industry; |
31
Table of Contents
| • | changes in financial estimates by securities analysts or differences between those estimates and our actual results; | ||
| • | the liquidity of the market for the common shares; | ||
| • | developments with respect to patents and proprietary rights; | ||
| • | publication of clinical research results regarding our products; | ||
| • | changes in health care policies in the United States or foreign countries; | ||
| • | grants or exercises of stock options or warrants; | ||
| • | news announcements; | ||
| • | litigation involving us; | ||
| • | actions by governmental agencies, including the FDA, or changes in regulations; and | ||
| • | other developments affecting us or our competitors. |
In particular, the stock market might experience significant price and volume fluctuations that
might affect the market price of the common shares for reasons that are unrelated to our operating
performance and that are beyond our control.
We have never paid cash dividends on our capital stock, and we currently do not expect to pay
any cash dividends in the foreseeable future.
We have never paid cash dividends on our common shares and currently do not expect to pay such
dividends in the foreseeable future. We currently intend to retain any future earnings for use in
our business or in our share repurchase program. The payment of any future dividends will be
determined by the board in light of the conditions then existing, including our financial condition
and requirements, future prospects, restrictions in financing agreements, business conditions and
other factors deemed relevant by the board. As a result, capital appreciation, if any, of our
common shares will be your sole source of gain for the foreseeable future.
The market price of the common shares might be lower because of shares eligible for future sale
and shares reserved for future issuance upon the exercise of options and warrants we have
granted.
Future sales of substantial amounts of common shares in the public market or the perception
that such sales could occur could adversely affect the market price of the common shares. Any
substantial sale of common shares or even the possibility of such sales occurring may have an
adverse effect on the market price of the common shares. As of February 1, 2010, we have
outstanding options and warrants to purchase an aggregate of 1,814,637 common shares. We have also
reserved up to an additional 305,593 common shares for issuance upon exercises of options or awards
of restricted stock or restricted stock units which have not yet been granted or awarded under our
stock incentive plans. We have effective registration statements for the shares underlying these
options and stock awards. Therefore, except for volume limitations imposed by Securities and
Exchange Commission Rule 144 on affiliates, these shares are freely tradable. The market price of
our common shares could fall if the holders of these shares sell them or are perceived by the
market as intending to sell them.
Forward-Looking Statements
Some of the statements in this report are forward-looking statements. These forward-looking
statements include statements relating to our performance in the sections entitled “Risk Factors,”
“Management’s Discussion and Analysis of Financial Condition and Results of Operations” and
“Business” and elsewhere in this report. Forward-looking statements include statements regarding
the intent, belief or current expectations of us or our management, including statements preceded
by, followed by or including forward-looking terminology such as “may,” “will,” “should,”
“believe,” “expect,” “anticipate,” “plan,” “intend,” “propose,” “estimate,” “continue,” “predict”
or similar expressions, with respect to various matters.
These statements involve known and unknown risks, uncertainties and other factors which may
cause our actual results, performance time frames or achievements to be materially different from
any future results, performance, time frames or achievements expressed or implied by the
forward-looking statements. We discuss many of these risks, uncertainties and other factors in
this report in greater detail in this Item 1A above under the heading “Risk Factors.” Given these
risks, uncertainties and other factors, you should not place undue reliance on
32
Table of Contents
these forward-looking statements. Also, these forward-looking statements represent our
estimates and assumptions only as of the date of this report. You should read this report and the
documents that we have filed as exhibits and incorporated by reference into this report completely
and with the understanding that our actual future results may be materially different from what we
expect. We hereby qualify all of our forward-looking statements by these cautionary statements.
All forward-looking statements in this report are based on information available to us on the
date of this report. We do not undertake to update any forward-looking statements that may be made
by us or on our behalf in this report or otherwise.
33
Table of Contents
ITEM 1B. UNRESOLVED STAFF COMMENTS
None.
ITEM 2. PROPERTIES
Our new headquarters, office, assembly and storage facility is located in an approximately
48,000 square foot, stand-alone building in Troy, Michigan. We lease approximately 48,000 square
feet, approximately 36,000 square feet of which is office space for sales and marketing,
engineering, accounting and other administrative activities. We lease this space for a lease term
that began December 15, 2009 and expires March 31, 2017, with two consecutive options to renew the
lease for a term of five years each. The minimum monthly lease payment through March 31, 2011 is
approximately $36,900 (except that rent is abated for the first three months of the term),
excluding other occupancy costs, and it will increase to approximately $42,800 during the term of
the lease, excluding other occupancy costs. Rent expense will be recorded on a straight-line basis
over the lease term, including consideration of lease incentives. Somanetics also pays other
occupancy costs relating to the premises, including utilities, taxes and insurance.
The new premises replace our existing headquarters, manufacturing facility and warehouse space
in Troy, Michigan, which we currently lease under a lease that expires March 31, 2010. We believe
that this new facility is suitable and adequate for our present needs and for the foreseeable
future and will allow for substantial expansion of our business and number of employees.
ITEM 3. LEGAL PROCEEDINGS
On August 7, 2009, we filed a patent infringement action against CAS Medical Systems, Inc. in
the United States District Court for the Eastern District of Michigan. The complaint asserts that
CAS Medical’s FORE-SIGHT® Cerebral Oximeter willfully infringes upon one or more of
Somanetics’ patents. The complaint also asserts that CAS Medical has engaged in unfair competition
and false advertising, by making false or misleading statements in connection with its advertising
and promotion of FORE-SIGHT, and false or misleading statements related to Somanetics’ products.
The complaint seeks, among other things, compensation for damages and an injunction against CAS
Medical from infringing upon Somanetics’ patents. CAS Medical Systems, Inc. has answered the
complaint denying that it infringes any asserted patent and asserting defenses and counterclaims,
including those for patent invalidity and/or unenforceability, and antitrust violations.
ITEM 4. SUBMISSION OF MATTERS TO A VOTE OF SECURITY HOLDERS
No matter was submitted to a vote of security holders during the fourth quarter of the fiscal
year ended November 30, 2009.
34
Table of Contents
SUPPLEMENTAL ITEM. EXECUTIVE OFFICERS OF THE REGISTRANT
Our current executive officers and the positions held by them are as follows:
| Executive | ||||||||||
| Name | Officer Since | Age | Position | |||||||
Bruce J. Barrett |
6/94 | 50 | President and Chief Executive Officer | |||||||
Arik A. Anderson |
01/09 | 43 | Senior Vice President, R&D and Operations | |||||||
William M. Iacona |
12/00 | 39 | Vice President, Chief Financial Officer, Controller and Treasurer | |||||||
Dominic J. Spadafore |
8/02 | 50 | Senior Vice President, U.S. Sales and Marketing | |||||||
Mary Ann Victor |
1/98 | 52 | Vice President, Chief Administrative Officer, General Counsel and Secretary | |||||||
Our officers serve at the discretion of the board of directors.
Biographical Information
Mr. Bruce J. Barrett has served as our President and Chief Executive Officer and as one of our
directors since June 1994. Earlier in his career, Mr. Barrett served as the Director, Hospital
Products Division, for Abbott Laboratories, Ltd., a health care equipment manufacturer and
distributor, and as the Director, Sales and Marketing, for Abbott Critical Care Systems, a division
of Abbott Laboratories, Inc., a health care equipment manufacturer and distributor. While at
Abbott Critical Care Systems, Mr. Barrett managed Abbott’s invasive oximetry products for
approximately four years. Prior to joining Abbott Laboratories, he served as the group product
manager of hemodynamic monitoring products of Baxter Edwards Critical Care, an affiliate of Baxter
International, Inc., another health care equipment manufacturer and distributor. Mr. Barrett
received a B.S. degree in marketing from Indiana State University and an M.B.A. degree from Arizona
State University. Mr. Barrett is a party to an employment agreement with us that requires us to
elect him to the offices he currently holds.
Mr. Arik A. Anderson has served as our Senior Vice President, R&D and Operations since
January 2009. From October 2007 until January 2009, he served as our Senior Vice President,
Research and Development. From July 2005 until joining Somanetics, Mr. Anderson served as Director
of Product Development for Delphi Medical Systems, a provider of technology and products to the
infusion, respiratory care, vital signs monitoring and power mobility medical device markets. He
was in charge of a team of 45 engineers in the United States, Mexico and India who supported
existing products and developed next generation products. From April 2004 until July 2005, Mr.
Anderson was the President and Chief Executive Officer of Tasso Solutions, a product development
and manufacturing consulting firm specializing in helping companies outsource design and
manufacturing work. From December 2002 until April 2004, Mr. Anderson was the Vice President of
Engineering Services for TriVirix International, a design and manufacturing services company
focused specifically on the medical device industry, serving customers such as Johnson & Johnson
and Medtronic. In this capacity, Mr. Anderson was in charge of 50 engineers for TriVirix in the
U.S. and Europe. Mr. Anderson received a Bachelor of Science degree in Electrical and Computer
Engineering from the University of Wisconsin – Madison.
Mr. William M. Iacona has served as our Vice President and Chief Financial Officer since
January 2006, as our Treasurer since February 2000 and as our Controller since April 1997.
From December 2000 until January 2006, he served as our Vice President, Finance. Before joining
us, he was in the Finance Department of Ameritech Advertising Services, a telephone directory
company and a division of Ameritech Corporation (now AT&T), and was on the audit staff of
Deloitte & Touche LLP, independent auditors. He is a certified public accountant and received a
B.S. degree in accounting from the University of Detroit.
Mr. Dominic J. Spadafore has served as our Senior Vice President, U.S. Sales and Marketing,
since December 2007. From August 2002 until December 2007, he served as our Vice President, U.S.
Sales and Marketing. Mr. Spadafore previously served, from July 2000 until July 2002, as National
Sales and Clinical Director of the Cardiac Assist Division of Datascope Corporation, a medical
device company that manufactures and markets healthcare products including medical devices used in
high-risk cardiac patients. In this position, Mr.
35
Table of Contents
Spadafore supervised approximately 50 sales and
clinical personnel and approximately $80 million in domestic
revenues. From July 1997 until July 2000, he served as Western Area Manager of the Patient
Monitoring Division of Datascope Corporation, and prior to that he held field sales representative
and regional manager positions with progressive responsibilities with Datascope Corporation.
Earlier in his career Mr. Spadafore was a sales representative with the Upjohn Company, a
pharmaceutical manufacturer, and a sales representative with White and White Incorporated, a
medical supply distributor. He received a BA degree in pre-medicine from Oakland University. Mr.
Spadafore is a party to an employment agreement with us that requires us to elect him to the office
he currently holds.
Ms. Mary Ann Victor has served as our General Counsel since September 2009, as our Vice
President, Chief Administrative Officer since January 2006 and as our Secretary since January 1998.
From January 1998 until January 2006, she served as our Vice President, Communications and
Administration. Prior to that she was our Director, Communications and Administration. Her prior
experience includes various investor relations and public relations positions with publicly-held
companies. She also is an attorney and practiced with the law firm Varnum Riddering Schmidt &
Howlett. Ms. Victor received a B.S. in political science from the University of Michigan and a
J.D. from the University of Detroit.
36
Table of Contents
PART II
ITEM 5. MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED SHAREHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
Our common shares trade on The NASDAQ Global Market under the trading symbol “SMTS.” The
following table sets forth, for the periods indicated, the range of high and low sales prices of
our common shares as reported by NASDAQ.
| High | Low | |||||||
Fiscal Year Ended November 30, 2008 |
||||||||
First Quarter |
$ | 29.53 | $ | 19.45 | ||||
Second Quarter |
28.00 | 10.01 | ||||||
Third Quarter |
26.17 | 16.76 | ||||||
Fourth Quarter |
25.21 | 14.11 | ||||||
Fiscal Year Ended November 30, 2009 |
||||||||
First Quarter |
$ | 18.25 | $ | 12.09 | ||||
Second Quarter |
17.25 | 10.18 | ||||||
Third Quarter |
18.48 | 12.94 | ||||||
Fourth Quarter |
17.23 | 12.90 | ||||||
As of February 1, 2010, we had 494 shareholders of record of our common shares.
We have never paid cash dividends on our common shares and currently do not expect to pay such
dividends in the foreseeable future. We currently intend to retain any future earnings for use in
our business or in our share repurchase program. The payment of any future dividends will be
determined by the board in light of the conditions then existing, including our financial condition
and requirements, future prospects, restrictions in any financing agreements, business conditions
and other factors deemed relevant by the board.
Performance Graph
The following line graph compares for the fiscal years ended November 30, 2005, 2006, 2007,
2008 and 2009 (1) the yearly percentage change in our cumulative total shareholder return (i.e.,
the change in share price divided by the initial share price, expressed as the resulting value of a
$100 investment; we have not paid cash dividends) on our common shares, with (2) the cumulative
total return of The Russell 2000 Index and with (3) the cumulative total return on the NASDAQ
Medical Devices Index.
37
Table of Contents
COMPARISON OF CUMULATIVE TOTAL RETURN*
AMONG SOMANETICS CORPORATION,
THE RUSSELL 2000 INDEX, AND
NASDAQ MEDICAL DEVICES INDEX**
AMONG SOMANETICS CORPORATION,
THE RUSSELL 2000 INDEX, AND
NASDAQ MEDICAL DEVICES INDEX**
Comparison of Cumulative Five Year Total Return
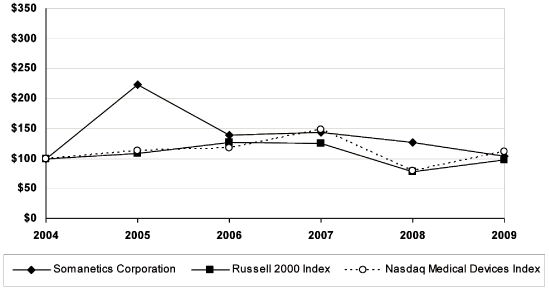
| 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | |||||||||||||||||||
Somanetics Corporation |
100.00 | 222.69 | 139.77 | 144.16 | 126.37 | 103.24 | ||||||||||||||||||
The Russell 2000 Index |
100.00 | 108.14 | 127.00 | 125.50 | 78.49 | 97.75 | ||||||||||||||||||
NASDAQ Medical Devices Index |
100.00 | 113.23 | 117.33 | 148.09 | 78.74 | 111.89 | ||||||||||||||||||
Assumes $100 invested on November 30, 2004 in Somanetics Corporation common shares, The Russell
2000 Index and the NASDAQ Medical Devices Index.
| * | Total return assumes reinvestment of dividends. | |
| ** | Fiscal Year ending November 30. |
38
Table of Contents
Unregistered Sales of Equity Securities and Use of Proceeds
The following table provides information with respect to any purchase made by or on behalf of
us or any affiliated purchaser of our common shares for each month during the fourth quarter ended
November 30, 2009:
| Total | ||||||||||||||||
| Number of | Approximate | |||||||||||||||
| Shares | Dollar Value | |||||||||||||||
| Purchased | of Shares | |||||||||||||||
| Total | as Part of | That May Yet | ||||||||||||||
| Number of | Average | Publicly | Be Purchased | |||||||||||||
| Shares | Price Paid | Announced Plans | Under the Plans | |||||||||||||
| Period | Purchased | Per Share | or Programs | or Programs | ||||||||||||
September 1-30, 2009 |
— | — | — | 13,550,580 | ||||||||||||
October 1-31, 2009 |
— | — | — | 13,550,580 | ||||||||||||
November 1-30, 2009 |
— | — | — | 13,550,580 | ||||||||||||
Total |
0 | N/A | 0 | |||||||||||||
On April 3, 2008, we publicly announced that our Board of Directors authorized the repurchase
of up to $15 million of our common shares. Purchases may be made from time to time in the open
market or in privately negotiated transactions. The prices, timing and amount of, and purposes
for, any purchases will be determined by management. On May 9, 2008, we publicly announced that
our Board of Directors approved an increase in the limit on the share repurchase program and
authorized the repurchase of up to an additional $15 million of our common shares, and on July 1,
2008, we publicly announced that our Board of Directors approved an increase in the limit on the
share repurchase program and authorized the repurchase of up to an additional $15 million of our
common shares, for a total of $45 million of our common shares under the repurchase program.
During the fiscal year 2008, we repurchased 1,805,129 common shares at an average price of $17.42
per share and an aggregate cost of $31,449,420. All of the shares were purchased by us in
open-market transactions pursuant to this publicly-announced share repurchase program. The program
does not have an expiration date, except upon purchase of the maximum authorized dollar amount of
our common shares.
39
Table of Contents
ITEM 6. SELECTED FINANCIAL DATA
You should read the following selected financial data together with our financial statements
and related notes included in Item 8 of this report and with “Management’s Discussion and Analysis
of Financial Condition and Results of Operations” included in Item 7 of this report. We have
derived the statement of operations data for the years ended November 30, 2009, 2008 and 2007 and
the balance sheet data as of November 30, 2009 and 2008 from our audited financial statements,
which are included in Item 8 of this report. We have derived the statement of operations data for
the years ended November 30, 2006 and 2005 and the balance sheet data as of November 30, 2007, 2006
and 2005 from our audited financial statements, which are not included in this report. Our
historical results for any prior period are not necessarily indicative of results to be expected
for any future period.
| Year Ended November 30, | ||||||||||||||||||||
| 2009 | 2008 (4) | 2007 | 2006 | 2005 | ||||||||||||||||
| (in thousands, except per share data) | ||||||||||||||||||||
Statement of Operations Data: |
||||||||||||||||||||
Net revenues |
$ | 50,014 | $ | 47,455 | $ | 38,586 | $ | 28,701 | $ | 20,509 | ||||||||||
Cost of sales |
6,581 | 6,249 | 4,726 | 3,566 | 2,601 | |||||||||||||||
Gross margin |
43,433 | 41,206 | 33,860 | 25,135 | 17,908 | |||||||||||||||
Operating expenses: |
||||||||||||||||||||
Research, development and engineering (1) |
3,947 | 1,259 | 669 | 1,582 | 526 | |||||||||||||||
Selling, general and administrative (2) |
29,621 | 26,166 | 22,269 | 16,485 | 13,241 | |||||||||||||||
Total operating expenses |
33,568 | 27,425 | 22,938 | 18,067 | 13,767 | |||||||||||||||
Operating income |
9,865 | 13,781 | 10,922 | 7,068 | 4,141 | |||||||||||||||
Other income: |
||||||||||||||||||||
Interest income |
1,063 | 2,630 | 4,008 | 2,582 | 310 | |||||||||||||||
Total other income |
1,063 | 2,630 | 4,008 | 2,582 | 310 | |||||||||||||||
Income before income taxes |
$ | 10,928 | $ | 16,411 | $ | 14,930 | $ | 9,650 | $ | 4,451 | ||||||||||
Income tax (expense) benefit (3) |
(4,110 | ) | (5,965 | ) | (5,248 | ) | 750 | 3,300 | ||||||||||||
Net income |
$ | 6,818 | $ | 10,446 | $ | 9,682 | $ | 10,400 | $ | 7,751 | ||||||||||
Net income per common share — basic |
$ | .57 | $ | .82 | $ | .73 | $ | .83 | $ | .75 | ||||||||||
Net income per common share — diluted |
$ | .53 | $ | .76 | $ | .67 | $ | .75 | $ | .66 | ||||||||||
Weighted average number of common shares
outstanding — basic |
12,069 | 12,671 | 13,213 | 12,463 | 10,322 | |||||||||||||||
Weighted average number of common shares
outstanding — diluted |
12,936 | 13,672 | 14,384 | 13,824 | 11,798 | |||||||||||||||
| As of November 30, | ||||||||||||||||||||
| 2009 | 2008 (4) | 2007 | 2006 | 2005 | ||||||||||||||||
| (in thousands) | ||||||||||||||||||||
Balance Sheet Data: |
||||||||||||||||||||
Cash, cash equivalents, securities and investments |
$ | 79,733 | $ | 69,996 | $ | 85,804 | $ | 71,571 | $ | 13,148 | ||||||||||
Working capital |
64,251 | 65,640 | 62,998 | 57,968 | 18,044 | |||||||||||||||
Total assets |
101,288 | 87,968 | 104,984 | 92,423 | 29,719 | |||||||||||||||
Total liabilities |
3,255 | 3,120 | 2,819 | 2,205 | 1,878 | |||||||||||||||
Retained Earnings (Accumulated deficit) |
216 | (6,603 | ) | (17,049 | ) | (26,731 | ) | (37,131 | ) | |||||||||||
Total shareholders’ equity |
98,033 | 84,848 | 102,165 | 90,218 | 27,841 | |||||||||||||||
| (1) | Includes a $1,000,000 expense in fiscal 2006 in connection with our Contract Development and Exclusive Licensing Agreement we entered into with NeuroPhysics Corporation, and a $2,000,000 expense in fiscal 2009 in connection with our Exclusive Sublicensing Agreement with Raba Equity Partners II, LLC and our Development and Exclusive License Agreement with an inventor and his company. | |
| (2) | Includes an impairment expense of $929,093 in fiscal 2005 in connection with the write-off of an intangible asset. | |
| (3) | Fiscal 2006 and 2005 amounts are net of a fourth quarter reversal of a portion of our income tax valuation allowance in the amount of $4,068,613 and $4,837,420, respectively. | |
| (4) | Fiscal 2008 amounts reflect 1) our acquisition of substantially all of the assets of ICU Data Systems, Inc. in November 2008, and 2) our common share repurchase program. See Notes 2 and 8 of Notes to Financial Statements in Item 8. |
40
Table of Contents
ITEM 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
Forward-Looking Statements
You should read the following discussion and analysis of our financial condition and results
of operations together with our financial statements and the related notes and other financial data
included elsewhere in this report. Some of the information contained in this discussion and
analysis or set forth elsewhere in this report, including information with respect to our plans and
strategy for our business, includes forward-looking statements that involve risks and
uncertainties. You should review the “Risk Factors” section of this report for a discussion of
important factors that could cause actual results to differ materially from the results described
in or implied by the forward-looking statements contained in the following discussion and analysis.
See also “Forward-Looking Statements” in Item 1A of this report.
Overview
We develop, manufacture and market the INVOS System, a non-invasive patient monitoring system
that provides accurate, real-time blood oxygen measurements in the brain and elsewhere in the body
in tissues beneath the sensor in patients greater than 2.5 kilograms, and continuously measures
changes in blood oxygen levels for individuals of any weight. Our four-channel cerebral and
somatic INVOS System monitor can display information from four disposable sensors simultaneously.
The INVOS System is the only commercially-available cerebral/somatic Oximeter proven to improve
outcomes. In May 2008, we received 510(k) clearance from the FDA to market our INVOS System to
monitor changes in blood oxygen saturation in any tissues beneath the sensor, not limited to brain
and somatic tissue, in any individual. In April 2009, we received 510(k) clearance from the FDA to
expand the indications for use to reflect the INVOS System’s ability to provide accurate, immediate
blood oxygen saturation measurements in patients greater than 2.5 kilograms at risk for restricted
or no blood flow, in addition to our previous FDA clearance to measure changes in blood oxygen
saturation in any individual. In addition, this most recent 510(k) clearance expanded the labeling
for our INVOS System to include the following new marketing claims:
| • | The measurement of regional cerebral oxygen saturation (rSO2) is an indication of whether oxygen delivery to the brain is adequate. Prolonged declines in rSO2 are indicative of, or may result in, potential brain injury. | |
| • | When used as an indication of compromised cerebral oxygenation, interventions to return the patient’s rSO2 to baseline using the INVOS System have been shown to improve outcomes after surgery. | |
| • | In neonates, infants and children, cerebral and somatic rSO2 provide noninvasive indications of oxygen changes in the cerebral and peripheral circulatory systems and may provide an early indication of oxygen deficits associated with impending shock states and anaerobiosis. |
In November 2008, we acquired substantially all of the assets of ICU Data Systems, Inc., a
technology development company, for approximately $2,000,000 in cash plus the assumption of
specified liabilities. ICU Data Systems has developed a patented technology that integrates data
from a broad array of hospital bedside devices, such as physiological monitors, ventilators and
infusion devices, into a single bedside display for comparison, data management and storage. We
launched our newly-acquired data integration technology, as a stand-alone device that we call Vital
Sync™, in the third quarter of fiscal 2009. The INVOS System is one of many devices whose data can
be integrated into the stand-alone device. To support the addition of the derived parameter
features to the system, or calculated parameters based on the combination of two or more discrete
parameters, we have filed a new FDA 510(k) premarket notification in the fourth quarter of fiscal
2009. In addition, we expect to invest to combine the ICU Data Systems and INVOS System
technologies in a single product in fiscal 2010. Upon completion of development of a single
product combining the Vital Sync System with our INVOS System technology, we also plan to pursue a
new FDA 510(k) clearance for this integrated device for a launch currently expected in mid-2011.
41
Table of Contents
Net Revenues and Cost of Sales
We derive our revenues primarily from sales of INVOS Systems, although we derived modest
revenues in fiscal 2009 from sales of our Vital Sync System, which we launched as a stand-alone
device in the third quarter of 2009 through our direct sales team. In the United States, such
sales are made primarily to hospitals through our direct sales team and an independent sales
representative firm. Outside the United States, we have distribution agreements with independent
distributors for the INVOS System, including Covidien in Europe, Canada, the Middle East and South
Africa, and Edwards Lifesciences Ltd. in Japan. Our cost of sales represent the cost of producing
monitors and disposable sensors. Revenues from outside the United States contributed 20% to our
fiscal 2009 net revenues. As a percentage of net revenues, the gross margins from our
international sales are typically lower than gross margins from our U.S. sales, reflecting the
difference between the prices we receive from distributors and from direct customers.
We recognize revenue when there is persuasive evidence of an arrangement with the customer,
the product has been delivered, the sales price is fixed or determinable, and collectability is
reasonably assured. The product is considered delivered to the customer once we have shipped it,
as this is when title and risk of loss have transferred. Payment terms are generally net 30 days
for U.S. sales and net 60 days or longer for international sales.
Our INVOS System revenues are derived from the sale of monitors and our disposable sensors.
We intend that disposable sensors will form the basis of a recurring revenue stream. In addition,
we offer to our customers in the United States a no capital cost sales program whereby we ship the
INVOS System monitor to the customer at no charge. Under this program, we do not recognize any
revenue upon the shipment of the monitor. At the time of shipment of the monitor, we capitalize
the monitor as an asset and depreciate this asset over five years, and this depreciation is
included in cost of goods sold. We recognize sensor revenue when we receive purchase orders and
ship the product to the customer.
Operating Expenses
Selling, general and administrative expenses generally consist of:
| • | salaries, wages and related expenses of our employees and consultants; | ||
| • | sales and marketing expenses, such as employee sales commissions, commissions to independent sales representatives, travel, entertainment, advertising, education and training expenses, depreciation of demonstration monitors and attendance at selected medical conferences; | ||
| • | clinical research expenses, such as costs of supporting clinical trials; and | ||
| • | general and administrative expenses, such as the cost of corporate operations, professional services, stock compensation, insurance, warranty and royalty expenses, investor relations, depreciation and amortization, facilities expenses and other general operating expenses. |
We have increased the size of our U.S. direct sales team from 55 persons at the end of fiscal
2008 to 63 persons at the end of fiscal 2009. We expect to increase the size of our U.S. direct
sales team in fiscal 2010 and have hired and may hire additional direct salespersons and clinical
specialists in Europe to support Covidien. We also expect selling, general and administrative
expenses to increase in fiscal 2010, primarily as a result of the lease agreement that we have
entered into for our new corporate headquarters and assembly and storage facility, and the patent
infringement action that we have filed against CAS Medical Systems, Inc.
Research, development and engineering expenses consist of:
| • | salaries, wages and related expenses of our research and development personnel and consultants; | ||
| • | costs of various development projects; and | ||
| • | costs of preparing and processing applications for FDA clearance of new products. |
We expect our research, development and engineering expenses to increase in fiscal 2010 from
the level in 2009, excluding the $2,000,000 expense under our Exclusive Sublicense Agreement and
Development and Exclusive License Agreement, described below. We expect this increase primarily as
a result of the hiring of
42
Table of Contents
additional research and development personnel, development costs associated with the
integration of the INVOS System and Vital Sync System, development costs associated with our
Exclusive Sublicense Agreement with Raba Equity Partners II, LLC (“Raba coreFoundry”), development
costs associated with our Contract Development Agreement with Shirley Research Corporation, and
development costs associated with advances to the design and performance features of the INVOS
System, including the disposable sensor.
Deferred Tax Assets and Impairment Charges
For the fiscal year ended November 30, 2007, we recognized income tax expense at an estimated
effective tax rate of 35% on our statement of operations. This income tax expense included a
non-cash tax expense on our statement of operations for fiscal 2007 of $5,076,276. In November
2007, we wrote off obsolete inventory of $180,521.
For the fiscal year ended November 30, 2008, we recognized income tax expense at an estimated
effective tax rate of 36% on our statement of operations. This income tax expense included a
non-cash tax expense on our statement of operations for fiscal 2008 of $5,586,906. In addition,
during fiscal 2008 we recognized deferred tax assets related to the previous exercise of stock
options of approximately $1,012,000. These assets were recognized in additional paid in capital on
our balance sheet because they were utilized and reduced current taxes payable in 2008.
For the fiscal year ended November 30, 2009, we recognized income tax expense at an estimated
effective tax rate of 38% on our statement of operations. This income tax expense included a
non-cash tax expense on our statement of operations for fiscal 2009 of $3,715,516. In addition,
during fiscal 2009, we recognized deferred tax assets primarily related to the previous exercise of
stock options of approximately $4,580,000. These assets were recognized in additional paid in
capital on our balance sheet because they were utilized and reduced current taxes payable in 2009.
Results of Operations
Fiscal Year Ended November 30, 2009 Compared to Fiscal Year Ended November 30, 2008
Net Revenues. Our net revenues increased $2,558,117, or 5%, from $47,455,617 in the fiscal
year ended November 30, 2008 to $50,013,734 in the fiscal year ended November 30, 2009. The
increase in net revenues is primarily attributable to:
| • | an increase in U.S. sales of $2,124,351, or 6%, from $38,035,145 in fiscal 2008 to $40,159,496 in fiscal 2009. The increase in U.S. sales was primarily due to an increase in sales of the disposable sensor of $4,737,655, or 16%, primarily as a result of a 12% increase in sensor unit sales. This increase was partially offset by a decrease in sales of the INVOS System monitor in the United States of $2,737,468, or 36%, primarily as a result of the current economic downturn in the United States that is affecting hospital budget spending and lengthening the sales cycle for our INVOS System monitor; and | ||
| • | an increase in international sales of $433,766, or 5%, from $9,420,472 in fiscal 2008 to $9,854,238 in fiscal 2009. The increase in international sales was primarily due to increased sales of our disposable sensor of $1,174,742, or 30%, primarily as a result of increased purchases of approximately $832,000 by Covidien in Europe and $235,000 by Edwards Lifesciences in Japan. This increase was partially offset by a decrease in sales of the INVOS System monitor internationally of $740,976, or 13%, primarily due to reduced purchases by Covidien in Europe as a result of the current economic downturn internationally that is affecting hospital budget spending and lengthening the sales cycle for our INVOS System monitor. In fiscal 2009 and fiscal 2008, international sales represented 20% of our net revenues. Purchases by Covidien accounted for 13% of net revenues in fiscal 2009, compared to 14% in fiscal 2008. |
In the United States, we sold 323,156 disposable sensors in fiscal 2009, and internationally,
we sold 178,870. We placed 404 INVOS System monitors in the United States and 525 internationally
in fiscal 2009, and our installed base of INVOS System monitors in the United States was 2,927, in
782 hospitals, as of November 30, 2009.
43
Table of Contents
Sales of our products as a percentage of net revenues were as follows:
| Fiscal Year Ended November 30, | ||||||||
| Product | 2009 | 2008 | ||||||
Sensors |
81 | % | 72 | % | ||||
INVOS System Monitors |
19 | % | 28 | % | ||||
Total |
100 | % | 100 | % | ||||
We believe that the current economic downturn in the United States and abroad could continue
to significantly lengthen the sales cycle for our products and reduce the growth in our net
revenues in fiscal 2010. We expect international net revenues to increase beginning in February
2010 as a result of new prices negotiated as part of our distribution agreement extension with
Covidien.
Gross Margin. Gross margin as a percentage of net revenues was 87% for the fiscal year ended
November 30, 2009 and November 30, 2008. During fiscal 2009, we realized a 3% increase in the
average selling price of disposable sensors in the United States as a result of increased sales of
our pediatric sensor, which sells for a higher price than the adult sensor. The increase in
average selling prices described above was primarily offset by decreased sales of the INVOS System
monitor to pediatric hospitals in the United States during fiscal 2009. We expect international
gross margins to increase beginning in February 2010 as a result of new prices negotiated as part
of our distribution agreement extension with Covidien.
Research, Development and Engineering Expenses. Our research, development and engineering
expenses increased $2,688,282, or 213%, from $1,259,227 in fiscal 2008 to $3,947,509 in fiscal
2009. The increase is primarily attributable to $1,800,000 in up-front, non-refundable payments in
connection with our Exclusive Sublicense Agreement we entered into with Raba Equity Partners II,
LLC (“Raba coreFoundry”) in the fourth quarter of fiscal 2009 and a $200,000 up-front,
non-refundable payment in connection with our Development and Exclusive License Agreement with an
inventor and his company in the fourth quarter of fiscal 2009. The increase is also attributable
to an increase in salaries of $412,829, primarily due to the addition of research and development
personnel in fiscal 2008 and 2009, and increased development costs and expenses associated with our
Contract Development Agreement with Shirley Research Corporation and the development of our Vital
Sync System of $229,106. We expect our research, development and engineering expenses to increase
in fiscal 2010 from the level in 2009, excluding the $2,000,000 expense under our Exclusive
Sublicense Agreement, and Development and Exclusive License Agreement, described below. We expect
this increase primarily as a result of the hiring of additional research and development personnel,
development costs associated with the integration of the INVOS System and Vital Sync System,
development costs associated with our Exclusive Sublicense Agreement with Raba Equity Partners II,
LLC (“Raba coreFoundry”), development costs associated with our Contract Development Agreement with
Shirley Research Corporation, and development costs associated with advances to the design and
performance features of the INVOS System, including the disposable sensor.
Selling, General and Administrative Expenses. Selling, general and administrative expenses
increased $3,454,498, or 13%, from $26,166,120 for the fiscal year ended November 30, 2008 to
$29,620,618 for the fiscal year ended November 30, 2009, primarily due to:
| • | a $2,261,456 increase in salaries, wages and related expenses, primarily as a result of an increase in the number of employees, principally in sales and marketing (from an average of 105 employees for the fiscal year ended November 30, 2008 to an average of 123 employees for the fiscal year ended November 30, 2009) and an increase in employee insurance premiums and salaries of existing employees; | ||
| • | an $782,640 increase in travel, marketing and selling-related expenses as a result of our increased sales personnel and increased sales and marketing activities, including trade shows, sales training and advertising expenses; | ||
| • | a $359,477 increase in professional service fees, primarily due to increased legal and accounting fees associated with the establishment of Somanetics International BV and the related branches and operations, and legal fees associated with the patent infringement action that we have filed against CAS Medical Systems, Inc., in addition to other corporate and intellectual property matters; |
44
Table of Contents
| • | a $291,558 increase in office and administrative expenses, primarily due to the addition of new employees during fiscal 2009 and the establishment of Somanetics International BV and the hiring of employees in the related branches and operations, and costs associated with terminating our CorRestore license; | ||
| • | a $278,088 increase in stock compensation expense due to stock compensation issued to our officers, employees, directors and one of our consultants in fiscal 2008 and 2009; | ||
| • | a $217,112 increase in accrued incentive compensation expense due to our fiscal year 2009 financial performance, primarily sales and operating income compared to targets in accordance with the 2009 incentive compensation plans; | ||
| • | a $187,451 increase in clinical research expenses, primarily as a result of educational grants and clinical trials evaluating the use of our INVOS System; and | ||
| • | a $182,197 increase in recruiting and training, primarily as a result of an increase in the number of employees, principally in sales and marketing. |
These increases were partially offset by a $635,653 decrease in commissions paid to our independent
sales representative firms as a result of fewer independent sales representative firms in fiscal
2009, and a $523,298 decrease in commissions paid to our sales employees as a result of lower than
expected fiscal 2009 sales.
We expect our selling, general and administrative expenses to increase in fiscal 2010,
primarily as a result of our hiring additional direct sales personnel in fiscal 2009 and 2010, as a
result of the lease agreement that we have entered into for our new corporate headquarters and
assembly and storage facility, and the patent infringement action that we filed against CAS Medical
Systems, Inc.
Other Income. During fiscal 2009, interest income decreased to $1,063,147, from $2,629,967
in fiscal 2008, primarily due to decreased interest rates, in addition to the use of cash for the
repurchase of common shares in 2008, and our decreased cash and cash equivalents balances. These
decreases were partially offset by our increased investment balances and cash provided by operating
activities.
Income Taxes. As of November 30, 2009, we recognized income tax expense at an estimated
effective tax rate of 38% on our statement of operations. In addition, during fiscal 2009 we
recognized deferred tax assets primarily related to the exercise of stock options of approximately
$4,580,000. These assets were recognized as an increase in additional paid in capital on our
balance sheet because they were utilized and reduced current taxes payable. As of November 30,
2008, we recognized income tax expense at an estimated effective tax rate of 36% on our statement
of operations.
Fiscal Year Ended November 30, 2008 Compared to Fiscal Year Ended November 30, 2007
Net Revenues. Our net revenues increased $8,869,785, or 23%, from $38,585,832 in the fiscal
year ended November 30, 2007 to $47,455,617 in the fiscal year ended November 30, 2008. The
increase in net revenues is primarily attributable to:
| • | an increase in U.S. sales of $6,474,215, or 21%, from $31,560,930 in fiscal 2007 to $38,035,145 in fiscal 2008. The increase in U.S. sales was primarily due to an increase in sales of the disposable sensor of $5,619,820, or 23%, primarily as a result of a 16% increase in sensor unit sales. In addition, sales of the INVOS System monitor in the United States increased $932,969, or 14%, primarily as a result of increased purchases by pediatric hospitals; and | ||
| • | an increase in international sales of $2,395,570, or 34%, from $7,024,902 in fiscal 2007 to $9,420,472 in fiscal 2008. The increase in international sales was primarily due to increased purchases of our INVOS System monitor and disposable sensors of $1,655,456 by Covidien in Europe, and $686,471 by Edwards Lifesciences in Japan. In fiscal 2008, international sales represented 20% of our net revenues, compared to 18% of our net revenues in fiscal 2007. Purchases by Covidien accounted for 14% of net revenues in fiscal 2008, compared to 13% in fiscal 2007. |
In the United States, we sold 288,797 disposable sensors in fiscal 2008, and internationally,
we sold 135,850. We placed 517 INVOS System monitors in the United States and 621 internationally
in fiscal 2008, and
45
Table of Contents
our installed base of INVOS System monitors in the United States was 2,523, in 714 hospitals,
as of November 30, 2008.
Sales of our products as a percentage of net revenues were as follows:
| Fiscal Year Ended November 30, | ||||||||
| Product | 2008 | 2007 | ||||||
Sensors |
72 | % | 73 | % | ||||
INVOS System Monitors |
28 | % | 27 | % | ||||
Total |
100 | % | 100 | % | ||||
Gross Margin. Gross margin as a percentage of net revenues was 87% for the fiscal year ended
November 30, 2008 and 88% for the fiscal year ended November 30, 2007. The decrease in our gross
margin percentage is primarily attributable to increased international sales, due to lower margins
we receive on sales to our international distributors. This decrease was partially offset by a 6%
increase in the average selling price of disposable sensors in the United States, which is
attributable to increased sales of our pediatric sensors, which sell for a higher price than the
adult sensor.
Research, Development and Engineering Expenses. Our research, development and engineering
expenses increased $590,412, or 88%, from $668,815 in fiscal 2007 to $1,259,227 in fiscal 2008.
The increase is primarily attributable to an increase in salaries of $279,487 due to the addition
of research and development personnel in fiscal 2007 and 2008, and increased costs associated with
advances to the design and performance features of our INVOS System monitor and disposable sensors
of $214,803.
Selling, General and Administrative Expenses. Selling, general and administrative expenses
increased $3,896,936, or 17%, from $22,269,184 for the fiscal year ended November 30, 2007 to
$26,166,120 for the fiscal year ended November 30, 2008, primarily due to:
| • | a $2,275,304 increase in salaries, wages, commissions and related expenses, primarily as a result of an increase in the number of employees, principally in sales and marketing (from an average of 88 employees for the fiscal year ended November 30, 2007 to an average of 105 employees for the fiscal year ended November 30, 2008) and an increase in salaries of existing employees; | ||
| • | an $673,212 increase in travel, marketing and selling-related expenses as a result of our increased sales personnel and increased sales and marketing activities, including sales training and trade shows; | ||
| • | a $557,282 increase in stock compensation expense due to stock compensation issued to our officers, employees, directors and one of our consultants in fiscal 2006, 2007 and 2008; | ||
| • | a $465,769 increase in professional service fees, primarily due to increased legal, auditing and tax fees; | ||
| • | a $217,099 increase in accrued incentive compensation expense due to our fiscal year 2008 financial performance, primarily increased sales and operating income in accordance with the 2008 incentive compensation plans; and | ||
| • | a $173,672 increase in office and facility expenses primarily as a result of increased employees. |
These increases were partially offset by a $273,905 decrease in commissions paid to our independent
sales representative firms as a result of fewer independent sales representative firms in fiscal
2008, and a $182,919 decrease in clinical research expense primarily as a result of a grant made in
fiscal 2007 for the support of neonatal research.
Other Income. During fiscal 2008, interest income decreased to $2,629,967, from $4,008,537
in fiscal 2007, primarily due to decreased interest rates, decreased investment balances, and the
use of cash for the repurchase of common shares, partially offset by our increased cash and cash
equivalents balances as a result of cash provided by operating activities and maturities and
redemptions of investments.
Income Taxes. As of November 30, 2008, we recognized income tax expense at an estimated
effective tax rate of 36% on our statement of operations. In addition, during fiscal 2008 we
recognized deferred tax assets related
46
Table of Contents
to the exercise of stock options of approximately $1,012,000. These assets were recognized in
additional paid in capital on our balance sheet because they were utilized and reduced current
taxes payable. As of November 30, 2007, we recognized income tax expense at an estimated effective
tax rate of 35% on our statement of operations.
Effects of Inflation
We do not believe that inflation has had a significant impact on our financial position or
results of operations in the past three years.
Liquidity and Capital Resources
General
Our principal sources of operating funds have been the proceeds from sales of our common
shares and cash provided by operating activities. See Statements of Shareholders’ Equity of our
financial statements included elsewhere in this report.
As of November 30, 2009, we did not have any outstanding or available debt financing
arrangements, we had working capital of $64.3 million and our primary sources of liquidity were
$29.0 million of cash and cash equivalents, $24.8 million of marketable securities and $26.0
million of long-term investments. Marketable securities and long-term investments consist of
Aaa-rated United States Government agency bonds, and cash and cash equivalents are currently
invested in bank savings accounts and money market accounts, pending their ultimate use.
In April 2008, our Board of Directors authorized the repurchase of up to $15 million of our
common shares. Purchases may be made from time to time in the open market or in privately
negotiated transactions. The prices, timing and amount of, and purposes for, any purchases will be
determined by management. In May 2008, our Board of Directors authorized the repurchase of up to
an additional $15 million of our common shares, and in July 2008, our Board of Directors authorized
the repurchase of up to an additional $15 million of our common shares, for a total of $45 million
of our common shares under the repurchase program. During fiscal 2008, we repurchased 1,805,129
common shares at an average price of $17.42 per share and an aggregate cost of $31,449,420, leaving
$13,550,580 in dollar value of shares that may yet be purchased under the repurchase program. In
the first quarter of 2010, we have resumed purchases of our common shares under our share
repurchase program.
We launched our newly-acquired data integration technology, as a stand-alone device that we
call Vital Sync™, in the third quarter of fiscal 2009. To support the addition of the derived
parameter features to the system, or calculated parameters based on the combination of two or more
discrete parameters, we have filed a new FDA 510(k) premarket notification in the fourth quarter of
fiscal 2009. In addition, we expect to invest to combine the ICU Data Systems and INVOS System
technologies in a single product in fiscal 2010. Upon completion of development of a single
product combining the Vital Sync System with our INVOS System technology, we also plan to pursue a
new FDA 510(k) clearance for this integrated device for a launch currently expected in mid-2011.
We expect our research, development and engineering expenses to increase in fiscal 2010 as a result
of development costs associated with the integration of the INVOS System and Vital Sync System.
We entered into a Contract Development and Exclusive Licensing Agreement with NeuroPhysics
Corporation as of September 18, 2006. The agreement provided us with feasibility research,
contract development and consulting services and certain ownership and licensing rights, subject to
the rights of the United States Federal government, to intellectual property and technical
knowledge associated with several novel near-infrared spectroscopy, or “NIRS”, and imaging
technologies and products under development at NeuroPhysics. We terminated this agreement in
February 2009, except for various provisions regarding our ownership of the technology related to
the potential new products. In February 2009, we entered into a similar agreement with Shirley
Research Corporation and Hugh F. Stoddart and Hugh A. Stoddart, and have agreed to pay monthly
development and consulting fees of $15,000 a month during the term of the agreement and a royalty
on future sales of the new products.
47
Table of Contents
On August 7, 2009, we filed a patent infringement action against CAS Medical Systems, Inc. in
the United States District Court for the Eastern District of Michigan. The complaint asserts that
CAS Medical’s FORE-SIGHT® Cerebral Oximeter willfully infringes upon one or more of
Somanetics’ patents. The complaint also asserts that CAS Medical has engaged in unfair competition
and false advertising, by making false or misleading statements in connection with its advertising
and promotion of FORE-SIGHT, and false or misleading statements related to Somanetics’ products.
The complaint seeks, among other things, compensation for damages and an injunction against CAS
Medical from infringing upon Somanetics’ patents. CAS Medical Systems, Inc. has answered the
complaint denying that it infringes any asserted patent and asserting defenses and counterclaims,
including those for patent invalidity and/or unenforceability, and antitrust violations. We expect
our selling, general and administrative expenses to increase in fiscal 2010, in part as a result of
this patent infringement action.
In October 2009, we entered into an Exclusive Sublicense Agreement with Raba Equity Partners
II, LLC (“Raba coreFoundry”). We paid Raba coreFoundry up-front, non-refundable payments of $1.8
million and will pay a royalty on future revenue associated with products using the licensed
technology. The up-front payment was accounted for as a research and development expense in the
fourth quarter of fiscal 2009. We plan to file a new 510(k) premarket notification with the FDA to
support marketing the new module in the U.S. and pursue a product launch in the first half of 2011.
In November 2009, we entered into a Development and Exclusive Agreement with an inventor and
his company to develop and market a product that uses INVOS technology and methods and means
described in the inventor’s patent to monitor, detect and assess acute compartment syndrome in
parts of the human body. We paid the inventor’s company an up-front, non-refundable payment of
$200,000 and will pay an additional $300,000 upon obtaining 510(k) clearance for the product and a
royalty on future revenue associated with products using the licensed technology. The up-front
payment was accounted for as research and development expenses in the fourth quarter of fiscal
2009. The U.S. Department of Defense has provided a grant for the research and development of a
monitor and sensors to monitor, detect and assess compartment syndrome. As part of the agreement,
over a three-year period, we expect to receive approximately $2.1 million for product and
prototype sales and research and development fees, derived from the grant, for its work to develop
the compartment syndrome product.
See “Operating Expenses” above for a description of our expected increased research and
development commitments expected in fiscal 2010 as a result of these agreements.
We believe that cash, cash equivalents, marketable securities and long-term investments on
hand at November 30, 2009 will be adequate to satisfy our operating and capital requirements for
more than the next twelve months.
Cash Flows From Operating Activities
Net cash provided by operations during fiscal 2009, 2008 and 2007 was $5,539,819, $15,874,473
and $13,081,989, respectively. In fiscal 2009, cash was provided primarily by:
| • | $8,646,991 of income before income taxes and non-cash depreciation, amortization, stock compensation expense and excess tax benefits from stock option exercises; and | ||
| • | a $195,439 increase in accounts payable, primarily as a result of increased inventory and operating expenses, partially offset by more timely payments made to vendors. |
Cash provided by operations in fiscal 2009 was partially offset by:
| • | a $1,384,230 increase in inventories, primarily due to lower than expected fiscal 2009 sales and the acquisition of components associated with our disposable sensors and our INVOS System monitor due to anticipated sales; inventories on our balance sheet increased less because we capitalized INVOS System monitors to property and equipment that are being used as demonstration units and no capital cost sales equipment, as described below; | ||
| • | a $1,016,839 increase in accounts receivable, primarily as a result of higher fourth quarter sales in fiscal 2009 than in the fourth quarter of fiscal 2008, the timing of more of the sales in the fourth quarter of fiscal |
48
Table of Contents
| 2009 towards the end of the quarter than in the fourth quarter of fiscal 2008 and less timely collections in fiscal 2009 than in fiscal 2008; |
| • | a $489,990 increase in prepaid expenses, primarily due to prepaid expenses related to our planned move into our new corporate headquarters and assembly and storage facility in December 2009; | ||
| • | a $230,000 increase in deferred income tax benefits as a result of payments made for estimated alternative minimum tax that we expect will result in future tax credits when we use our net operating loss carryforwards; | ||
| • | a $121,432 increase in accrued interest income, primarily due to our increased marketable securities and long-term investment balances, partially offset by decreased interest rates; and | ||
| • | a $60,120 decrease in accrued liabilities, primarily as a result of a decrease in accrued state, local and property taxes as a result of payments made in 2009, and a decrease in accrued sales commissions as a result of lower than expected 2009 sales, partially offset by an increase in accrued legal fees associated with the patent infringement action that we have filed against CAS Medical Systems, Inc., and an increase in accrued incentive compensation due to our fiscal year 2009 financial performance, primarily sales and operating income compared to targets in accordance with the 2009 incentive compensation plans. |
We expect our working capital requirements to increase as sales increase.
The increase in inventories described above is greater than shown on our balance sheet because
it includes INVOS System monitors that we capitalized because they are being used as demonstration
units and no capital cost sales equipment. We capitalized $722,121 of costs from inventory for
INVOS System monitors being used as demonstration units and no capital cost sales equipment at
customers during fiscal 2009, compared to $644,814 in fiscal 2008. As of November 30, 2009, we
have capitalized $4,285,163 in costs for INVOS System monitors being used as demonstration and no
capital cost sales equipment, and these assets have a net book value of $1,868,706. We depreciate
these assets over five years.
We believe that the current economic downturn in the United States and abroad could lengthen
our collection cycle for our accounts receivable and, as a result, increase our accounts receivable
balances.
Cash Flows From Investing Activities
Net cash used in investing activities in fiscal 2009 and 2007 was $18,491,637 and $10,127,748,
respectively, and net cash provided by investing activities in fiscal 2008 was $17,220,458. In
fiscal 2009, we invested $58,528,869 in marketable securities and long-term investments, partially
offset by maturities of marketable securities and long-term investments of $40,590,275.
Cash Flows From Financing Activities
Net cash provided by financing activities in fiscal 2009 and 2007 was $4,749,950 and
$1,483,867, respectively, and net cash used in financing activities in fiscal 2008 was $29,101,767.
During fiscal 2009, we received $4,579,947 in excess tax benefits from the previous exercise of
stock options, and $170,003 in proceeds from the issuance of 52,800 common shares as a result of
the exercise of stock options in 2009 by our employees, directors and an officer.
In the first quarter of 2010, we have resumed purchases of our common shares under our share
repurchase program.
49
Table of Contents
Contractual Obligations
The following information is provided as of November 30, 2009 with respect to our known
contractual obligations specified in the following table, aggregated by type of contractual
obligation:
| Payments due by period | ||||||||||||||||||||
| Less | More | |||||||||||||||||||
| than 1 | 1-3 | 3-5 | than 5 | |||||||||||||||||
| Contractual Obligations | Total | year | years | years | years | |||||||||||||||
Long-term debt obligations |
— | — | — | — | — | |||||||||||||||
Capital lease obligations |
— | — | — | — | — | |||||||||||||||
Operating lease obligations |
$ | 3,412,000 | $ | 365,000 | $ | 911,500 | $ | 957,600 | $ | 1,177,800 | ||||||||||
Purchase obligations |
6,225,700 | 6,218,700 | 7,000 | — | — | |||||||||||||||
Other long-term liabilities |
— | — | — | — | — | |||||||||||||||
Purchase obligations consist primarily of purchase orders executed for inventory components.
Off-Balance Sheet Arrangements
We do not have any off-balance sheet arrangements or financing activities.
New Accounting Pronouncements
On July 1, 2009, the Financial Accounting Standards Board (“FASB”) Accounting Standards
Codification (“Codification” or “ASC”) became the single source of authoritative U.S. GAAP (other
than rules and interpretive releases of the U.S. Securities and Exchange Commission). The
Codification is topically based with topics organized by ASC number and updated with Accounting
Standards Updates (“ASUs”). ASUs replace accounting guidance that was historically issued as
Statements of Financial Accounting Standards (“SFAS”), FASB Interpretations (“FIN”), FASB Staff
Positions (“FSP”), Emerging Issues Task Force (“EITF”) Abstracts and other types of accounting
standards. The Codification became effective during our fourth quarter of fiscal 2009, and
disclosures within this Annual Report on Form 10-K have been updated to reflect the change.
Critical Accounting Policies
We believe our most significant accounting policies relate to our accounting treatment of
stock compensation of employees, our accounting treatment for income taxes, our revenue recognition
associated with our no capital cost sales program and our recognition of a technology acquisition
cost intangible asset and goodwill.
Stock Compensation
Generally accepted accounting principles, or GAAP, requires that compensation costs related to
share-based payment transactions, including stock options, stock appreciation rights and restricted
stock be recognized in the financial statements. The issuance of stock compensation under the 2005
Stock Incentive Plan in fiscal 2009, 2008, 2007 and 2006 had an impact on our financial statements.
During fiscal 2009, we granted 68,250 stock options to our employees, directors and an
officer, and we issued 17,588 restricted common shares to our employees and an officer. During
fiscal 2008, we granted 253,000 stock options to our employees, directors and officers, and we
issued 75,273 restricted common shares to our officers and employees. During fiscal 2007, we
granted 96,000 stock options to our employees, directors and one of our officers, and we issued
9,000 restricted common shares to one of our officers. These stock options and restricted shares
were issued at the market price on the date of grant, and they vest and are expensed in the
financial statements over five years. As a result of the stock options and restricted common
shares that we granted during
50
Table of Contents
fiscal 2009, 2008, 2007 and 2006, we have recorded $1,616,678 in fiscal 2009, $1,338,590 in
fiscal 2008, and $781,308 in fiscal 2007 in stock compensation expense.
As of November 30, 2009, there was $4,437,246 of total unrecognized compensation cost related
to nonvested share-based compensation awards granted under the 2005 Plan. That cost is expected to
be recognized over a weighted average period of 3 years. As of November 30, 2008, there was
$5,247,791 of total unrecognized compensation cost related to nonvested share-based compensation
awards granted under the 2005 Plan. That cost is expected to be recognized over a weighted average
period of 4 years. As of November 30, 2007, there was $3,606,518 of total unrecognized
compensation cost related to nonvested share-based compensation awards granted under the 2005 Plan.
That cost was expected to be recognized over a weighted average period of 4 years. No
modifications were made to any share awards that required an accounting charge, and no cash was
paid for share-based liabilities during fiscal 2009, 2008 or 2007.
The fair value of each option grant was estimated on the date of grant using the Black-Scholes
option-pricing model with the following weighted-average assumptions for fiscal 2009, 2008 and
2007: expected volatility (the measure by which the stock price has fluctuated or is expected to
fluctuate during the period) 53.97% (59.30% for 2008 and 47.00% for 2007), risk-free interest rate
(approximate U.S. Treasury yield in effect at the time of grant) of 2.50% (2.35% for 2008 and 5.00%
for 2007), expected lives of approximately 6 years, and a dividend yield of 0%. The fair value of
the restricted common shares was estimated based on the market value of the common shares on the
date of issuance. Different assumptions could significantly change the calculated grant date fair
value and, therefore, the amount of stock compensation expense we recognize over the vesting period
of the awards. We believe, however, that our estimates are appropriate.
Income Taxes
We have performed the required assessment of positive and negative evidence regarding
realization of our deferred tax assets in accordance with GAAP, including our past operating
results, the existence of cumulative losses over our history up to the most recent seven fiscal
years, and our forecast for future net income. Our assessment of our deferred tax assets included
making assumptions about our net revenues and pre-tax income in future years, making allowance for
the uncertainties regarding, among other things, our future net revenues, the rate of adoption of
our products in the marketplace and the competition in the marketplace. As of November 30, 2009,
we have concluded that it is more likely than not that approximately $2,847,000 of such assets will
be realized. The remaining tax benefits of $2,590,000 relating to stock option exercises will not
be recognized on our financial statements until they reduce current taxes payable. The benefit,
when recognized, will increase additional paid in capital. During fiscal 2009, we recognized
deferred tax assets of approximately $4,580,000 in additional paid in capital because they were
utilized and reduced current taxes payable.
For the fiscal year ended November 30, 2009, we recorded income tax expense at an estimated
effective tax rate of 38%, for the year ended November 30, 2008 we recorded income tax expense at
an estimated effective tax rate of 36%, and for the year ended November 30, 2007 we recorded income
tax expense at an estimated effective tax rate of 35%.
Given the assumptions inherent in our financial plans, it is possible to calculate a different
value for our deferred tax asset by changing one or more of the variables in our assessment.
However, we believe that our evaluation of our financial plans was reasonable, and that the
judgments and assumptions that we made at the time of developing the plan were appropriate.
No Capital Cost Sales Revenue Recognition
We offer to our customers in the United States a no capital cost sales program whereby we ship
the INVOS System monitor to the customer at no charge. Under this program, we do not recognize any
revenue upon the shipment of the INVOS System monitor. At the time of shipment of the monitor, we
capitalize the INVOS System monitor as an asset and depreciate this asset over five years. We
recognize sensor revenue when we receive purchase orders and ship the product to the customer. We
believe this is consistent with our stated revenue recognition policy, which is compliant with the
Financial Accounting Standards Board (“FASB”) Accounting
51
Table of Contents
Standards Codification (“ASC”) 605 “Revenue Recognition” and Securities and Exchange
Commission Staff Accounting Bulletin (“SAB”) No. 104.
Technology Acquisition Costs Intangible Asset and Goodwill
Technology acquisition costs and goodwill are related to our November 2008 acquisition of
substantially all of the assets of ICU Data Systems, Inc., a technology development company, for
approximately $2,000,000 in cash plus the assumption of specified liabilities. Goodwill represents
the amount by which the purchase price of the acquired business exceeds the estimated fair value of
the net tangible and separately identifiable intangible assets of the acquired business, in
addition to transaction costs recorded at cost. Goodwill is not amortized, but is tested at least
annually for impairment. The technology acquisition costs intangible asset has an estimated useful
life of 20 years, based on several patents that we have filed related to the technology, and is
being amortized on a straight-line basis over the estimated useful life. Intangible assets and
goodwill are reviewed annually for impairment at the end of our fiscal year, and whenever events or
changes in circumstances indicate that the carrying value of the asset may not be recovered. We
evaluate impairment by comparing the fair value of the intangible asset, determined using a cash
flow method, with its carrying value.
We estimated the value of the technology acquisition costs intangible asset based on a
valuation model that included estimating the future cash flows of the technology and discounting
the net cash flows back to their present value using an appropriate risk-adjusted rate of return
(discount rate). The discount rate used was determined at the time of the acquisition in
accordance with accepted valuation methods. Our assessment of the estimated fair value included
making assumptions about the expected net revenues and operating income related to the acquired
technology in future years, making allowance for the uncertainties regarding, among other things,
the time and cost associated with the further advancement of the design and performance of the
technology, the rate of adoption of the technology, and the potential for competition related to
the technology. As of November 30, 2009, the carrying value of the technology acquisition costs
intangible asset was $234,003, and the carrying value of the goodwill was $1,783,712.
Given the assumptions inherent in our valuation model, it is possible to calculate a different
value for our technology acquisition costs intangible asset by changing one or more of the
variables within our model. However, we believe that our evaluation of our valuation model was
reasonable, and that the judgments and assumptions that we made in our valuation model were
appropriate.
52
Table of Contents
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
The table below provides information about our financial instruments that are sensitive to
changes in interest rates, consisting of investments in United States government agency bonds and
treasury bills. For these financial instruments, the table presents principal cash flows and
related weighted average interest rates by expected maturity dates. Weighted average fixed rates
are based on the contract rates. The actual cash flows of all instruments are denominated in U.S.
dollars. We invest our cash on hand not needed in current operations in United States government
agency bonds and treasury bills with varying maturity dates with the intention of holding them
until maturity.
November 30, 2009
Expected Maturity Dates By Fiscal Year
Expected Maturity Dates By Fiscal Year
| 2010 | 2011 | 2012 | 2013 | 2014 | Thereafter | Total | Fair Value | ||||||||||||||||||||||||||
Investments: |
|||||||||||||||||||||||||||||||||
Marketable Securities and Long-term Investments: |
|||||||||||||||||||||||||||||||||
Fixed Rate ($) |
24,763,854 | 20,000,000 | 1,010,681 | 4,994,314 | — | — | 50,768,849 | 51,155,992 | |||||||||||||||||||||||||
Average interest rate |
.94 | % | 1.84 | % | 2.35 | % | 5.05 | % | N/A | N/A | 1.73 | % | |||||||||||||||||||||
November 30, 2008
Expected Maturity Dates By Fiscal Year
Expected Maturity Dates By Fiscal Year
| 2009 | 2010 | 2011 | 2012 | 2013 | Thereafter | Total | Fair Value | |||||||||||||||||||||||||
Investments: |
||||||||||||||||||||||||||||||||
| Marketable Securities and Long-term Investments: | ||||||||||||||||||||||||||||||||
Fixed Rate ($) |
19,992,545 | 3,845,241 | — | 4,000,000 | 4,992,469 | — | 32,830,255 | 32,823,806 | ||||||||||||||||||||||||
Average interest rate |
.53 | % | 4.72 | % | N/A | 5.00 | % | 5.05 | % | N/A | 2.25 | % | ||||||||||||||||||||
During fiscal 2009, we invested approximately $8,000,000 of our cash in two new bonds with
maturity dates in fiscal 2009, $27,500,000 of our cash in four new bonds with maturity dates in
fiscal 2010, $22,000,000 of our cash in two new bonds with maturity dates in fiscal 2011 and
$1,000,000 of our cash in one new bond with a maturity date in fiscal 2012. Two bonds and two of
our treasury bills matured for approximately $28,000,000, three of our bonds that were due to
mature in 2010, 2011 and 2012 were called for approximately $11,000,000, and one of our bonds due
to mature in 2010 returned approximately $1,500,000 in principal in 2009.
53
Table of Contents
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
MANAGEMENT’S REPORT ON INTERNAL CONTROL OVER FINANCIAL REPORTING
The management of Somanetics Corporation is responsible for establishing and maintaining
adequate internal control over financial reporting. Somanetics Corporation’s internal control
system was designed to provide reasonable assurance to the Company’s management and board of
directors regarding the preparation and fair presentation of published financial statements. All
internal control systems, no matter how well designed, have inherent limitations. Therefore, even
those systems determined to be effective can provide only reasonable assurance with respect to
financial statement preparation and presentation.
Somanetics Corporation management assessed the effectiveness of the Company’s internal control
over financial reporting as of November 30, 2009. In making this assessment, it used the criteria
set forth by the Committee of Sponsoring Organizations of the Treadway Commission (COSO) in
Internal Control-Integrated Framework. Based on our assessment, we have concluded that, as of
November 30, 2009, the Company’s internal control over financial reporting is effective based on
those criteria.
Somanetics Corporation’s independent registered public accounting firm, that audited the
financial statements prepared by the Company included in Item 8 of this report, has issued a report
on the financial statements and on the effectiveness of the Company’s internal control over
financial reporting, which is included on the next page.
January 21, 2010
54
Table of Contents
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Shareholders of
Somanetics Corporation
Troy, Michigan
Somanetics Corporation
Troy, Michigan
We have audited the accompanying balance sheets of Somanetics Corporation (the “Company”) as of
November 30, 2009 and 2008, and the related statements of operations, shareholders’ equity, and
cash flows for each of the three years in the period ended November 30, 2009. We also have audited
the Company’s internal control over financial reporting as of November 30, 2009, based on criteria
established in Internal Control — Integrated Framework issued by the Committee of Sponsoring
Organizations of the Treadway Commission. The Company’s management is responsible for these
financial statements, for maintaining effective internal control over financial reporting, and for
its assessment of the effectiveness of internal control over financial reporting, included in the
accompanying Management’s Report on Internal Control Over Financial Reporting. Our responsibility
is to express an opinion on these financial statements and an opinion on the Company’s internal
control over financial reporting based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight
Board (United States). Those standards require that we plan and perform the audit to obtain
reasonable assurance about whether the financial statements are free of material misstatement and
whether effective internal control over financial reporting was maintained in all material
respects. Our audits of the financial statements included examining, on a test basis, evidence
supporting the amounts and disclosures in the financial statements, assessing the accounting
principles used and significant estimates made by management, and evaluating the overall financial
statement presentation. Our audit of internal control over financial reporting included obtaining
an understanding of internal control over financial reporting, assessing the risk that a material
weakness exists, and testing and evaluating the design and operating effectiveness of internal
control based on the assessed risk. Our audits also included performing such other procedures as
we considered necessary in the circumstances. We believe that our audits provide a reasonable
basis for our opinions.
A company’s internal control over financial reporting is a process designed by, or under the
supervision of, the company’s principal executive and principal financial officers, or persons
performing similar functions, and effected by the company’s board of directors, management, and
other personnel to provide reasonable assurance regarding the reliability of financial reporting
and the preparation of financial statements for external purposes in accordance with generally
accepted accounting principles. A company’s internal control over financial reporting includes
those policies and procedures that (1) pertain to the maintenance of records that, in reasonable
detail, accurately and fairly reflect the transactions and dispositions of the assets of the
company; (2) provide reasonable assurance that transactions are recorded as necessary to permit
preparation of financial statements in accordance with generally accepted accounting principles,
and that receipts and expenditures of the company are being made only in accordance with
authorizations of management and directors of the company; and (3) provide reasonable assurance
regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the
company’s assets that could have a material effect on the financial statements.
Because of the inherent limitations of internal control over financial reporting, including the
possibility of collusion or improper management override of controls, material misstatements due to
error or fraud may not be prevented or detected on a timely basis. Also, projections of any
evaluation of the effectiveness of the internal control over financial reporting to future periods
are subject to the risk that the controls may become inadequate because of changes in conditions,
or that the degree of compliance with the policies or procedures may deteriorate.
55
Table of Contents
In our opinion, the financial statements referred to above present fairly, in all material
respects, the financial position of Somanetics Corporation as of November 30, 2009 and 2008, and
the results of its operations and its cash flows for each of the three years in the period ended
November 30, 2009, in conformity with accounting principles generally accepted in the United States
of America. Also, in our opinion, the Company maintained, in all material respects, effective
internal control over financial reporting as of November 30, 2009, based on the criteria
established in Internal Control — Integrated Framework issued by the Committee of Sponsoring
Organizations of the Treadway Commission.
/s/ DELOITTE & TOUCHE LLP
Detroit, Michigan
February 3, 2010
February 3, 2010
56
Table of Contents
SOMANETICS CORPORATION
BALANCE SHEETS
| November 30, | ||||||||
| 2009 | 2008 | |||||||
ASSETS |
||||||||
CURRENT ASSETS: |
||||||||
Cash and cash equivalents (Note 2) |
$ | 28,964,273 | $ | 37,166,141 | ||||
Marketable securities |
24,763,854 | 19,992,545 | ||||||
Accounts receivable |
8,878,942 | 7,862,103 | ||||||
Inventory (Note 2) |
3,622,531 | 2,960,422 | ||||||
Prepaid expenses |
1,087,450 | 597,460 | ||||||
Accrued interest receivable |
138,099 | 16,667 | ||||||
Deferred tax asset — current (Note 5) |
51,060 | 164,615 | ||||||
Total current assets |
67,506,209 | 68,759,953 | ||||||
PROPERTY AND EQUIPMENT: (Note 2) |
||||||||
Demonstration and no capital cost sales equipment at customers |
4,285,163 | 3,919,296 | ||||||
Machinery and equipment |
1,886,582 | 1,638,597 | ||||||
Furniture and fixtures |
545,796 | 504,485 | ||||||
Leasehold improvements |
197,450 | 197,450 | ||||||
Total |
6,914,991 | 6,259,828 | ||||||
Less accumulated depreciation and amortization |
(3,966,645 | ) | (3,418,697 | ) | ||||
Net property and equipment |
2,948,346 | 2,841,131 | ||||||
OTHER ASSETS: |
||||||||
Long-term investments |
26,004,995 | 12,837,710 | ||||||
Deferred tax asset — non-current (Note 5) |
2,795,963 | 1,587,977 | ||||||
Intangible assets, net (Note 2) |
234,003 | 246,318 | ||||||
Goodwill (Note 2) |
1,783,712 | 1,679,713 | ||||||
Other |
15,000 | 15,000 | ||||||
Total other assets |
30,833,673 | 16,366,718 | ||||||
TOTAL ASSETS |
$ | 101,288,228 | $ | 87,967,802 | ||||
LIABILITIES AND SHAREHOLDERS’ EQUITY |
||||||||
CURRENT LIABILITIES: |
||||||||
Accounts payable |
$ | 1,466,497 | $ | 1,271,058 | ||||
Accrued liabilities (Notes 4 and 6) |
1,788,552 | 1,848,672 | ||||||
Total current liabilities |
3,255,049 | 3,119,730 | ||||||
COMMITMENTS AND CONTINGENCIES (Note 6) |
||||||||
SHAREHOLDERS’ EQUITY: (Note 3) |
||||||||
Preferred shares; authorized, 1,000,000 shares of $.01 par
value; no shares issued or outstanding |
— | — | ||||||
Common shares; authorized, 20,000,000 shares of $.01 par
value; issued and outstanding, 12,104,462 shares at November 30, 2009,
and 12,034,074 shares at November 30, 2008 |
121,045 | 120,341 | ||||||
Additional paid-in capital |
97,696,229 | 91,330,305 | ||||||
Retained Earnings (Accumulated deficit) |
215,905 | (6,602,574 | ) | |||||
Total shareholders’ equity |
98,033,179 | 84,848,072 | ||||||
TOTAL LIABILITIES AND SHAREHOLDERS’ EQUITY |
$ | 101,288,228 | $ | 87,967,802 | ||||
See notes to financial statements
57
Table of Contents
SOMANETICS CORPORATION
STATEMENTS OF OPERATIONS
| For the Years Ended November 30, | ||||||||||||
| 2009 | 2008 | 2007 | ||||||||||
NET REVENUES (Notes 2 and 9) |
$ | 50,013,734 | $ | 47,455,617 | $ | 38,585,832 | ||||||
COST OF SALES |
6,580,766 | 6,249,256 | 4,726,146 | |||||||||
Gross margin |
43,432,968 | 41,206,361 | 33,859,686 | |||||||||
OPERATING EXPENSES: |
||||||||||||
Research, development and engineering
(Note 2) |
3,947,509 | 1,259,227 | 668,815 | |||||||||
Selling, general and administrative |
29,620,618 | 26,166,120 | 22,269,184 | |||||||||
Total operating expenses |
33,568,127 | 27,425,347 | 22,937,999 | |||||||||
OPERATING INCOME |
9,864,841 | 13,781,014 | 10,921,687 | |||||||||
OTHER INCOME: |
||||||||||||
Interest income |
1,063,147 | 2,629,967 | 4,008,537 | |||||||||
Total other income |
1,063,147 | 2,629,967 | 4,008,537 | |||||||||
INCOME BEFORE INCOME TAXES |
$ | 10,927,988 | $ | 16,410,981 | $ | 14,930,224 | ||||||
INCOME TAX EXPENSE (Note 5) |
(4,109,508 | ) | (5,964,734 | ) | (5,247,943 | ) | ||||||
NET INCOME |
$ | 6,818,480 | $ | 10,446,247 | $ | 9,682,281 | ||||||
NET INCOME PER COMMON
SHARE — BASIC (Note 2) |
$ | .57 | $ | .82 | $ | .73 | ||||||
NET INCOME PER COMMON
SHARE — DILUTED (Note 2) |
$ | .53 | $ | .76 | $ | .67 | ||||||
WEIGHTED AVERAGE NUMBER OF
COMMON SHARES OUTSTANDING —
BASIC (Note 2) |
12,069,361 | 12,671,452 | 13,213,428 | |||||||||
WEIGHTED AVERAGE NUMBER OF
COMMON SHARES OUTSTANDING —
DILUTED (Note 2) |
12,935,886 | 13,671,730 | 14,384,445 | |||||||||
See notes to financial statements
58
Table of Contents
SOMANETICS CORPORATION
STATEMENTS OF SHAREHOLDERS’ EQUITY
| Additional | Retained Earnings | Total | ||||||||||||||||||||||
| Common | Share | Paid-In | (Accumulated | Shareholders’ | Comprehensive | |||||||||||||||||||
| Shares | Value | Capital | Deficit) | Equity | Income | |||||||||||||||||||
Balance at December 1, 2006 |
13,163,627 | $ | 131,636 | $ | 116,817,012 | $ | (26,731,103 | ) | $ | 90,217,545 | ||||||||||||||
For cash, exercise of stock options |
271,334 | 2,714 | 1,481,153 | 1,483,867 | ||||||||||||||||||||
Stock compensation expense |
— | 781,308 | 781,308 | |||||||||||||||||||||
Restricted share grant |
9,000 | 90 | (90 | ) | — | |||||||||||||||||||
Net income and comprehensive income |
— | 9,682,281 | 9,682,281 | $ | 9,682,281 | |||||||||||||||||||
Balance at November 30, 2007 |
13,443,961 | $ | 134,440 | $ | 119,079,383 | $ | (17,048,822 | ) | $ | 102,165,001 | ||||||||||||||
For cash, exercise of stock options |
319,969 | 3,199 | 1,332,129 | 1,335,328 | ||||||||||||||||||||
Stock compensation expense |
— | 1,338,590 | 1,338,590 | |||||||||||||||||||||
Restricted share grant |
75,273 | 753 | (753 | ) | — | |||||||||||||||||||
Repurchase of common shares |
(1,805,129 | ) | (18,051 | ) | (31,431,369 | ) | (31,449,420 | ) | ||||||||||||||||
Income tax benefit from stock option exercises |
— | 1,012,325 | 1,012,325 | |||||||||||||||||||||
Net income and comprehensive income |
— | 10,446,247 | 10,446,247 | $ | 10,446,247 | |||||||||||||||||||
Balance at November 30, 2008 |
12,034,074 | $ | 120,341 | $ | 91,330,305 | $ | (6,602,574 | ) | $ | 84,848,072 | ||||||||||||||
For cash, exercise of stock options |
52,800 | 528 | 169,475 | 170,003 | ||||||||||||||||||||
Stock compensation expense |
— | 1,616,678 | 1,616,678 | |||||||||||||||||||||
Restricted share grant |
17,588 | 176 | (176 | ) | — | |||||||||||||||||||
Income tax benefit from stock option exercises |
— | 4,579,947 | 4,579,947 | |||||||||||||||||||||
Net income and comprehensive income |
— | 6,818,480 | 6,818,480 | $ | 6,818,480 | |||||||||||||||||||
Balance at November 30, 2009 |
12,104,462 | $ | 121,045 | $ | 97,696,229 | $ | 215,905 | $ | 98,033,179 | |||||||||||||||
See notes to financial statements
59
Table of Contents
SOMANETICS CORPORATION
STATEMENTS OF CASH FLOWS
| For the Years Ended November 30, | ||||||||||||
| 2009 | 2008 | 2007 | ||||||||||
CASH FLOWS FROM OPERATING ACTIVITIES: |
||||||||||||
Net income |
$ | 6,818,480 | $ | 10,446,247 | $ | 9,682,281 | ||||||
Adjustments to reconcile net income to net
cash provided by operations: |
||||||||||||
Income tax expense (benefit) |
3,715,516 | 5,586,906 | 5,076,276 | |||||||||
Depreciation and amortization |
1,076,264 | 951,906 | 806,034 | |||||||||
Stock compensation expense |
1,616,678 | 1,338,590 | 781,308 | |||||||||
Excess tax benefits from stock option exercises |
(4,579,947 | ) | (1,012,325 | ) | — | |||||||
Changes in assets and liabilities net of effects from
purchase of ICU Data Systems, Inc.: |
||||||||||||
Accounts receivable (increase) |
(1,016,839 | ) | (375,532 | ) | (2,746,528 | ) | ||||||
Accrued interest receivable (increase) decrease |
(121,432 | ) | 534,450 | (199,451 | ) | |||||||
Inventory (increase) |
(1,384,230 | ) | (1,606,952 | ) | (658,895 | ) | ||||||
Deferred income tax benefit (increase) |
(230,000 | ) | (252,488 | ) | (400,960 | ) | ||||||
Prepaid expenses (increase) |
(489,990 | ) | (36,575 | ) | (66,063 | ) | ||||||
Accounts payable increase |
195,439 | 153,055 | 72,276 | |||||||||
Accrued liabilities (decrease) increase |
(60,120 | ) | 147,191 | 541,711 | ||||||||
Accrued income tax expense decrease |
— | — | 194,000 | |||||||||
Net cash provided by operating activities |
5,539,819 | 15,874,473 | 13,081,989 | |||||||||
CASH FLOWS FROM INVESTING ACTIVITIES: |
||||||||||||
Purchases of marketable securities and
long-term investments |
(58,528,869 | ) | (35,021,241 | ) | (63,795,275 | ) | ||||||
Proceeds from maturities of marketable securities and
long-term investments |
40,590,275 | 54,822,160 | 54,000,000 | |||||||||
Payment for purchase of ICU Data Systems, Inc. |
(104,000 | ) | (1,926,031 | ) | — | |||||||
Acquisition of property and equipment |
(449,043 | ) | (654,430 | ) | (332,473 | ) | ||||||
Net cash (used in) provided by investing activities |
(18,491,637 | ) | 17,220,458 | (10,127,748 | ) | |||||||
CASH FLOWS FROM FINANCING ACTIVITIES: |
||||||||||||
Repurchase of common shares |
— | (31,449,420 | ) | — | ||||||||
Excess tax benefits from stock option exercises |
4,579,947 | 1,012,325 | — | |||||||||
Proceeds from issuance of common shares due to
exercise of stock options |
170,003 | 1,335,328 | 1,483,867 | |||||||||
Net cash provided by (used in) financing activities |
4,749,950 | (29,101,767 | ) | 1,483,867 | ||||||||
NET (DECREASE) INCREASE IN CASH AND
CASH EQUIVALENTS |
(8,201,868 | ) | 3,993,164 | 4,438,108 | ||||||||
CASH AND CASH EQUIVALENTS,
BEGINNING OF PERIOD |
37,166,141 | 33,172,977 | 28,734,869 | |||||||||
CASH AND CASH EQUIVALENTS,
END OF PERIOD |
$ | 28,964,273 | $ | 37,166,141 | $ | 33,172,977 | ||||||
Supplemental Disclosure of Non cash investing activities: |
||||||||||||
Demonstration and no capital cost sales equipment capitalized
from inventory (Note 2) |
$ | 722,121 | $ | 644,814 | $ | 833,069 | ||||||
Supplemental Disclosure of Taxes paid: |
||||||||||||
Federal and state income taxes (Note 5) |
$ | 653,992 | $ | 600,317 | $ | 572,627 | ||||||
See notes to financial statements
60
Table of Contents
SOMANETICS CORPORATION
NOTES TO FINANCIAL STATEMENTS
1. Organization and Operations
We are a Michigan corporation that was formed in 1982. We develop, manufacture and market the
INVOS® System, a non-invasive patient monitoring system that provides accurate,
real-time blood oxygen measurements in the brain and elsewhere in the body in tissues beneath the
sensor in patients greater than 2.5 kilograms, and continuously measures changes in blood oxygen
levels for individuals of any weight. The INVOS System measurement is made by transmitting
low-intensity visible and near-infrared light through a portion of the body with disposable
sensors, and detecting the manner in which the exposed substance interacts with light at specific
wavelengths. The INVOS System is the only commercially-available cerebral/somatic Oximeter proven
to improve outcomes. The principal markets for our products are the United States, Europe, and
Japan.
In September 2000 we received FDA clearance to market our model 5100 INVOS System in the
United States, which has the added capability of being able to monitor pediatric patients. In
November 2005, we received 510(k) clearance from the FDA to market our INVOS System to monitor
changes in somatic tissue blood oxygen saturation in regions of the body other than the brain in
patients with or at risk for restricted blood flow. In May 2008, we received 510(k) clearance from
the FDA to market our INVOS System to monitor changes in blood oxygen saturation in any tissues
beneath the sensor, not limited to brain and somatic tissue, in any individual. In April 2009, we
received 510(k) clearance from the FDA to expand the indications for use to reflect the INVOS
System’s ability to provide accurate, immediate blood oxygen saturation measurements in patients
greater than 2.5 kilograms at risk for restricted or no blood flow, in addition to our previous FDA
clearance to measure changes in blood oxygen saturation in any individual. In addition, this most
recent 510(k) clearance expanded the labeling for our INVOS System to include new marketing claims.
In November 2008, we acquired substantially all of the assets of ICU Data Systems, Inc., a
company that has developed a patented technology that integrates data from a broad array of
hospital bedside devices, such as physiological monitors, ventilators and infusion devices, into a
single bedside display for comparison, data management and storage. We launched our newly-acquired
data integration technology, as a stand-alone device that we call Vital Sync™, in the third quarter
of fiscal 2009. The INVOS System is one of many devices whose data can be integrated into the
stand-alone device. To support the addition of the derived parameter features to the system, or
calculated parameters based on the combination of two or more discrete parameters, we have filed a
new FDA 510(k) premarket notification in the fourth quarter of fiscal 2009. In addition, we expect
to invest to combine the ICU Data Systems and INVOS System technologies in a single product in
fiscal 2010. Upon completion of development of a single product combining the Vital Sync System
with our INVOS System technology, we also plan to pursue a new FDA 510(k) clearance for this
integrated device for a launch currently expected in mid-2011.
2. Summary of Significant Accounting Policies
Cash Equivalents consist of short-term, interest-bearing investments maturing within three
months of our acquisition of them.
Marketable Securities and Long-Term Investments consist of Aaa-rated United States government
agency bonds and treasury bills, classified as held to maturity, maturing approximately nine months
to five years from the date of acquisition, are stated at an amortized cost of $50,768,849, and
have a market value of $51,155,992 at November 30, 2009.
Inventory is stated at the lower of cost or market on a first-in, first-out (FIFO) basis.
Inventory consists of:
61
Table of Contents
SOMANETICS CORPORATION
NOTES TO FINANCIAL STATEMENTS — (Continued)
| November 30, | ||||||||
| 2009 | 2008 | |||||||
Purchased components |
$ | 2,358,037 | $ | 2,141,050 | ||||
Finished goods |
1,042,205 | 587,808 | ||||||
Work in process |
222,289 | 231,564 | ||||||
Total |
$ | 3,622,531 | $ | 2,960,422 | ||||
Property and Equipment are stated at cost. Depreciation and amortization are computed using
the straight-line method over the estimated useful lives of the assets, which range from two to ten
years. Depreciation expense was $1,063,948, $948,809 and $799,122 for the fiscal years ended
November 30, 2009, November 30, 2008 and November 30, 2007, respectively. We offer to our United
States customers a no capital cost sales program whereby we ship the INVOS System monitor to the
customer at no charge. The INVOS System monitors that are shipped to our customers are classified
as no capital cost sales equipment and are depreciated over five years to cost of goods sold. All
other depreciation expense is recorded as a selling, general and administrative expense. As of
November 30, 2009, we have capitalized $4,285,163 in costs for INVOS System monitors being used as
demonstration and no capital cost sales equipment, and these assets had a net book value of
$1,868,706. As of November 30, 2008, we have capitalized $3,919,296 in costs for INVOS System
monitors being used as demonstration and no capital cost sales equipment, and these assets had a
net book value of $1,820,503. Property and equipment are reviewed for impairment whenever events
or changes in circumstances indicate that the net book value of the asset may not be recovered.
Intangible Assets and Goodwill consist of technology acquisition costs and goodwill. The
carrying amount and accumulated amortization of these technology acquisition costs are as follows:
| November 30, | ||||||||
| 2009 | 2008 | |||||||
Technology acquisition costs |
$ | 246,318 | $ | 246,318 | ||||
Less: accumulated amortization |
(12,315 | ) | — | |||||
Total |
$ | 234,003 | $ | 246,318 | ||||
Amortization expense was $12,315 for fiscal year ended November 30, 2009 for our technology
acquisition costs intangible asset. Amortization expense of $3,097 for the fiscal year ended
November 30, 2008 and $6,912 for the fiscal year ended November 30, 2007 related to an intangible
asset that has a net book value of zero at November 30, 2009.
Technology acquisition costs and goodwill are related to our November 2008 acquisition of
substantially all of the assets of ICU Data Systems, Inc., a technology development company, for
approximately $2,000,000 in cash plus the assumption of specified liabilities. Goodwill represents
the amount by which the purchase price of the acquired business exceeds the estimated fair value of
the net tangible and separately identifiable intangible assets of the acquired business, in
addition to transaction costs recorded at cost. Goodwill is not amortized, but is tested at least
annually for impairment. The technology acquisition costs intangible asset has an estimated useful
life of 20 years, based on several patents that we have filed related to the technology, and will
be amortized on a straight-line basis over the estimated useful life. Intangible assets and
goodwill are reviewed annually for impairment at the end of our fiscal year, and whenever events or
changes in circumstances indicate that the carrying value of the asset may not be recovered. We
evaluate impairment by comparing the fair value of the intangible asset, determined using a cash
flow method, with its carrying value.
No amortization expense was recorded related to the technology acquisition costs intangible
asset for 2008 because the transaction closed in November 2008. Amortization expense for each of
the next 20 fiscal years related to the technology acquisition costs intangible asset is expected
to be approximately $12,300. As of November 30, 2008, the carrying value of the technology
acquisition costs intangible asset was $246,318, and the carrying value of the
62
Table of Contents
SOMANETICS CORPORATION
NOTES TO FINANCIAL STATEMENTS — (Continued)
goodwill was $1,679,713. As of November 30, 2009, the carrying value of the technology
acquisition costs intangible asset was $234,003, and the carrying value of the goodwill was
$1,783,712. The increase in goodwill during fiscal 2009 related to an additional payment in
conjunction with our acquisition of substantially all of the assets of ICU Data Systems, Inc.
We estimated the value of the technology acquisition costs intangible asset based on a
valuation model that included estimating the future cash flows of the technology and discounting
the net cash flows back to their present value using an appropriate risk-adjusted rate of return
(discount rate). The discount rate used was determined at the time of the acquisition in
accordance with accepted valuation methods. Our assessment of the estimated fair value included
making assumptions about the expected net revenues and operating income related to the acquired
technology in future years, making allowance for the uncertainties regarding, among other things,
the time and cost associated with the further advancement of the design and performance of the
technology to ready it for market launch, the rate of adoption of the technology once it is
launched into the marketplace, and the potential for competition related to the launched
technology.
Revenue Recognition, including direct sales, sales through sales representatives and sales to
international distributors, occurs for our products when there is persuasive evidence of an
arrangement with the customer, the product has been delivered, the sales price is fixed or
determinable, and collectability is reasonably assured. The product is considered delivered to the
customer once we have shipped it, as this is when title and risk of loss have transferred. We have
considered the Financial Accounting Standards Board (“FASB”) Accounting Standards Codification
(“ASC”) 605 “Revenue Recognition” and Securities and Exchange Commission Staff Accounting Bulletin
(“SAB”) No. 104 in determining our revenue recognition policy.
Research, Development and Engineering costs are expensed as incurred.
Net Income Per Common Share — basic and diluted is computed using the weighted average number
of common shares outstanding during each period. Weighted average shares outstanding — diluted,
for the years ended November 30, 2009, November 30, 2008 and November 30, 2007, includes the
potential dilution that could occur for common shares issuable under stock options. As of November
30, 2009, 2008 and 2007, the difference between weighted average shares — diluted and weighted
average shares — basic is calculated as follows:
| 2009 | 2008 | 2007 | ||||||||||
Weighted average shares — basic |
12,069,361 | 12,671,452 | 13,213,428 | |||||||||
Add: effect of dilutive common
shares |
866,525 | 1,000,278 | 1,171,017 | |||||||||
Weighted average shares — diluted |
12,935,886 | 13,671,730 | 14,384,445 | |||||||||
At November 30, 2009, there were 458,750 stock options outstanding that were excluded from the
computation of net income per common share — diluted, as the exercise price of these options
exceeded the average market price per share of our common shares. At November 30, 2008 and 2007,
there were no stock options outstanding that were excluded from the computation of net income per
common share — diluted. In addition, at November 30, 2009, we had outstanding 1,814,637 stock
options to purchase common shares, as of November 30, 2008, we had outstanding 1,822,187 stock
options to purchase common shares and as of November 30, 2007, we had outstanding 1,895,656 stock
options to purchase common shares.
Common Share Repurchase Program In April 2008, our Board of Directors authorized the
repurchase of up to $15 million of our common shares. Purchases may be made from time to time in
the open market or in privately negotiated transactions. The prices, timing and amount of, and
purposes for, any purchases will be determined by management. In May 2008, our Board of Directors
authorized the repurchase of up to an additional $15 million of our common shares, and in July
2008, our Board of Directors authorized the repurchase of up to an additional $15 million of our
common shares, for a total of $45 million of our common shares under the repurchase program.
During fiscal 2008, we repurchased 1,805,129 common shares at an average price of $17.42 per share
and an aggregate cost of $31,449,420. All of the shares were purchased by us in open-market
transactions pursuant to this
63
Table of Contents
SOMANETICS CORPORATION
NOTES TO FINANCIAL STATEMENTS — (Continued)
publicly-announced share repurchase program. The program does not have an expiration date,
except upon purchase of the maximum authorized dollar amount of our common shares.
Accounting Pronouncements On July 1, 2009, the Financial Accounting Standards Board (“FASB”)
Accounting Standards Codification (“Codification” or “ASC”) became the single source of
authoritative U.S. GAAP (other than rules and interpretive releases of the U.S. Securities and
Exchange Commission). The Codification is topically based with topics organized by ASC number and
updated with Accounting Standards Updates (“ASUs”). ASUs replace accounting guidance that was
historically issued as Statements of Financial Accounting Standards (“SFAS”), FASB Interpretations
(“FIN”), FASB Staff Positions (“FSP”), Emerging Issues Task Force (“EITF”) Abstracts and other
types of accounting standards. The Codification became effective during our fourth quarter of
fiscal 2009, and disclosures within this Annual Report on Form 10-K have been updated to reflect
the change.
Use Of Estimates The preparation of financial statements in conformity with generally accepted
accounting principles requires us to make estimates and assumptions that affect the reported
amounts of assets and liabilities, the disclosure of contingent assets and liabilities, and the
reported amounts of revenues and expenses for each fiscal period. Actual results could differ from
those estimated.
3. Stock Offerings and Common Shares
During fiscal 2009, we issued 52,800 common shares as a result of stock option exercises by
our employees, directors and an officer for gross proceeds to us of $170,003. During fiscal 2008,
we issued 319,969 common shares as a result of stock option exercises by our employees and
directors for gross proceeds to us of $1,335,328. During fiscal 2007, we issued 271,334 common
shares as a result of stock option exercises by our employees, a former director and a consultant
for gross proceeds to us of $1,483,867.
Common shares reserved for future issuance upon exercise of stock options as discussed above
at November 30, 2009, are as follows:
1991 Incentive Stock Option Plan |
14,663 | |||
1997 Stock Option Plan |
1,023,508 | |||
2005 Stock Incentive Plan |
1,026,139 | |||
Options Granted Independent of Option Plans |
55,000 | |||
Total shares reserved for future issuance |
2,119,310 | |||
4. Accrued Liabilities
Accrued liabilities consist of the following:
| November 30, | ||||||||
| 2009 | 2008 | |||||||
Incentive Compensation |
$ | 1,185,225 | $ | 978,520 | ||||
Sales Commissions |
425,969 | 637,516 | ||||||
Professional Fees |
159,458 | 39,500 | ||||||
Warranty |
17,900 | 19,440 | ||||||
Taxes |
— | 121,683 | ||||||
Clinical Research |
— | 51,671 | ||||||
401(k) Match |
— | 342 | ||||||
Total |
$ | 1,788,552 | $ | 1,848,672 | ||||
5. Income Taxes
Deferred income taxes reflect the estimated future tax effect of (1) temporary differences
between the amount of assets and liabilities for financial reporting purposes and such amounts as
measured by tax laws and regulations and (2) net operating loss and tax credit carryforwards. Our
deferred tax assets primarily represent the
64
Table of Contents
SOMANETICS CORPORATION
NOTES TO FINANCIAL STATEMENTS — (Continued)
tax benefit of net operating loss carryforwards and research and general business tax credit
carryforwards. We had deferred tax assets of approximately $5,437,000 as of November 30, 2009,
which include approximately $2,590,000 related to the exercise of stock options, which will not be
recognized as deferred tax assets on our balance sheet until such time as they are utilized and
reduce current taxes payable. If realized in the future, these tax benefits will be recognized in
additional paid in capital. During fiscal 2009, we recognized deferred tax assets of approximately
$4,580,000 in additional paid in capital because they were utilized and reduced current taxes
payable. The change in the valuation related to future stock option tax benefits is attributable
to current year tax deductions from stock options exercised. We had deferred tax assets of
approximately $8,706,000 as of November 30, 2008, which include approximately $6,953,000 related to
the exercise of stock options, as described above. We have used a statutory federal income tax
rate of 34% when calculating our deferred tax assets. We paid income taxes in fiscal 2009, 2008
and 2007 of approximately $230,000, $252,000 and $401,000 respectively for alternative minimum tax
due. The amounts paid for alternative minimum tax in fiscal 2009, 2008 and 2007 have been recorded
as tax credit carryforward deferred tax assets on our balance sheet, as shown below.
The components of deferred income tax assets as of November 30, 2009 and 2008 were as follows:
| November 30, | ||||||||
| 2009 | 2008 | |||||||
| (in thousands) | ||||||||
Net operating loss carryforwards |
$ | 2,641 | $ | 7,119 | ||||
Stock compensation expense |
1,475 | 885 | ||||||
Other |
66 | 85 | ||||||
Basis difference of fixed assets and intangibles |
196 | (212 | ) | |||||
Alternative minimum tax credit carryforward |
883 | 653 | ||||||
Research and general business tax credit carryforwards |
176 | 176 | ||||||
Subtotal |
5,437 | 8,706 | ||||||
Reduction for future stock option tax benefits valuation |
(2,590 | ) | (6,953 | ) | ||||
Deferred tax asset |
$ | 2,847 | $ | 1,753 | ||||
The items accounting for the difference between income taxes computed at the federal statutory
rate and the provision for income taxes are as follows:
| For the Fiscal Year Ended November 30, | ||||||||||||
| 2009 | 2008 | 2007 | ||||||||||
Taxes at U.S. statutory rate — 34% |
$ | 3,715,516 | $ | 5,579,734 | $ | 5,076,276 | ||||||
State and local income taxes |
338,826 | 325,763 | 122,347 | |||||||||
Nondeductible meals and entertainment |
55,166 | 59,237 | 49,320 | |||||||||
Income tax expense from
continuing operations |
$ | 4,109,508 | $ | 5,964,734 | $ | 5,247,943 | ||||||
Effective tax rate |
37.6 | % | 36.3 | % | 35.1 | % | ||||||
| The components of current and deferred federal and state income tax expense are as follows: | ||||||||||||
| For the Fiscal Year Ended November 30, | ||||||||||||
| 2009 | 2008 | 2007 | ||||||||||
Federal income tax expense — current |
$ | 230,000 | $ | 252,488 | $ | 206,960 | ||||||
Federal income tax expense — deferred |
3,510,516 | 5,360,450 | 4,891,042 | |||||||||
State income tax expense — current |
338,826 | 325,763 | 122,347 | |||||||||
State income tax expense — deferred |
30,166 | 26,033 | 27,594 | |||||||||
Income tax expense from
continuing operations |
$ | 4,109,508 | $ | 5,964,734 | $ | 5,247,943 | ||||||
65
Table of Contents
SOMANETICS CORPORATION
NOTES TO FINANCIAL STATEMENTS — (Continued)
We have performed the required assessment of positive and negative evidence regarding
realization of our deferred tax assets in accordance with generally accepted accounting principles,
including our past operating results, the existence of cumulative losses over our history up to the
most recent seven fiscal years, and our forecast for future net income. Our assessment of our
deferred tax assets included making assumptions about our net revenues and pre-tax income in future
years, making allowance for the uncertainties regarding, among other things, our future net
revenues, the rate of adoption of our products in the marketplace and the competition in the
marketplace. As of November 30, 2009, we have concluded that it is more likely than not that
approximately $2,847,000 of such assets will be realized. The remaining tax benefits of $2,590,000
relating to stock option exercises will not be recognized on our financial statements until they
reduce current taxes payable. The benefit, when recognized, will increase additional paid in
capital.
For the fiscal year ended November 30, 2009, we recognized income tax expense in our statement
of operations of $4,109,508, which consisted of income tax expense recorded at an estimated
effective tax rate of 38%. For the fiscal year ended November 30, 2008, we recognized an income
tax expense of $5,964,734, which consisted of income tax expense recorded at an estimated effective
tax rate of 36%. In addition, for the fiscal year ended November 30, 2007, we recognized an income
tax expense of $5,247,943, which consisted of income tax expense recorded at an estimated effective
tax rate of 35%.
As of November 30, 2009, net operating loss carryforwards of approximately $7.6 million were
available for Federal income tax purposes for future years. Research and business general tax
credits of approximately $176,000 are also available to offset future taxes, and alternative
minimum tax credits of approximately $883,000 are also available to offset future taxes. These
losses and credits expire, if unused, at various dates from 2010 through 2025.
Use of our net operating loss carryforwards, tax credit carryforwards and certain future
deductions could be restricted, in the event of future changes in our equity structure, by
provisions contained in the Tax Reform Act of 1986.
6. Commitments and Contingencies
On July 22, 2009, Somanetics entered into a new lease agreement for an approximately 48,000
square foot, stand-alone office, assembly and storage facility. The lease term began December 15,
2009, and expires March 31, 2017 and grants Somanetics two consecutive options to renew the lease
for a term of five years each. The minimum monthly lease payment during the term through March 31,
2011 will be approximately $36,900 (except that rent is abated for the first three months of the
term), excluding other occupancy costs, and it will increase to approximately $42,800 during the
term of the lease, excluding other occupancy costs. Rent expense will be recorded on a
straight-line basis over the lease term including consideration of lease incentives. Somanetics
also pays other occupancy costs relating to the premises, including utilities, taxes and insurance.
The new premises replace our existing headquarters, office, assembly and warehouse space in
Troy, Michigan, which we currently lease under a lease that expires March 31, 2010. In addition,
on November 14, 2008, we assumed a lease for approximately 900 square feet of office space used
primarily for engineering activities. This lease is on a month-to-month basis until terminated by
lessor or tenant.
Operating lease expense for the years ended November 30, 2009, 2008 and 2007 was approximately
$201,500, $168,200, and $164,300, respectively. Approximate future minimum lease commitments are
as follows:
66
Table of Contents
SOMANETICS CORPORATION
NOTES TO FINANCIAL STATEMENTS — (Continued)
| Year ending November 30, | ||||
2010 |
$ | 365,100 | ||
2011 |
450,100 | |||
2012 |
461,400 | |||
2013 |
472,900 | |||
2014 |
484,700 | |||
2015 |
497,000 | |||
2016 |
509,600 | |||
2017 |
171,200 | |||
Total |
$ | 3,412,000 | ||
We have executed purchase orders for inventory components representing purchase obligations of
approximately $6,225,700 as of November 30, 2009.
In December 1991, we amended and restated our profit sharing plan to include a 401(k) plan
covering substantially all employees. Under provisions of the plan, participants may contribute,
annually, between 1% and 25% of their compensation. In November 2004, our board of directors
approved matching contributions to the 401(k) Plan equal to $2 for every $1 contributed by Company
employees to the 401(k) Plan at each payroll date on or after January 1, 2005, up to a Company
contribution of 4% of the employee’s compensation, and continuing until terminated by further
action of the board of directors. In addition, at the discretion of the board of directors, we may
make other annual discretionary contributions to the plan. Matching contributions made for fiscal
2009, 2008 and 2007 were approximately $463,000, $428,000 and $338,000, respectively.
As of November 30, 2009, we have amended and restated employment agreements or amended and
restated change in control agreements with all of our officers. The employment agreement with our
Senior Vice President, U.S. Sales and Marketing and the change in control agreements with seven of
our officers provide for severance benefits equal to one year’s salary upon termination of
employment without cause or for good reason 90 days before to one year after a change in control of
the Company that occurs by June 17, 2011. In addition, on June 17, 2008, we amended and restated
our employment agreement with our President and Chief Executive Officer that was scheduled to
expire April 30, 2009. The amended and restated agreement provides for severance benefits
consisting of fringe benefits for one year (two years if termination is in connection with a Change
in Control) and a lump sum payment equal to one year’s salary (two years if termination is in
connection with a Change in Control), plus the target bonus for the year of termination (which must
be at least 65% of his salary) (two times the target bonus if termination is in connection with a
Change in Control), plus a pro rata bonus through the date of termination upon termination of his
employment without cause or for good reason. His amended and restated employment agreement expires
June 17, 2011, unless earlier terminated as provided in the agreement, except that the term is
automatically extended for additional one-year periods effective one year before it would otherwise
expire (i.e., so that the remaining term will be two years), unless either party provides the other
with notice that the term will not be extended and such notice provided at least one year before
the term would otherwise expire. All officers have agreed not to compete with us and not to
solicit our employees during specified periods following the termination of employment, and they
have agreed to various confidentiality obligations. The estimated financial exposure of these
employment agreements, upon a change of control of the Company and termination of all of the
executives without cause, is approximately $2,446,000.
We entered into a Contract Development and Exclusive Licensing Agreement with NeuroPhysics
Corporation as of September 18, 2006. The agreement provided us with feasibility research,
contract development and consulting services and certain ownership and licensing rights, subject to
the rights of the United States Federal government, to intellectual property and technical
knowledge associated with several novel near-infrared spectroscopy, or “NIRS”, and imaging
technologies and products under development at NeuroPhysics. We paid an initial license fee of
$1,000,000. We terminated this agreement in February 2009, except for various provisions
regarding our ownership of the technology related to the potential new products. In February 2009,
we entered into a similar agreement with Shirley Research Corporation and Hugh F. Stoddart and Hugh
A. Stoddart, and have agreed to pay monthly development and consulting fees of $15,000 a month
during the term of the agreement and a royalty on future sales of the new products.
67
Table of Contents
SOMANETICS CORPORATION
NOTES TO FINANCIAL STATEMENTS — (Continued)
In November 2009, we entered into a Development and Exclusive Agreement with an inventor and
his company to develop and market a product that uses INVOS technology and methods and means
described in the inventor’s patent to monitor, detect and assess acute compartment syndrome in
parts of the human body. We paid the inventor’s company an up-front, non-refundable payment of
$200,000 and will pay an additional $300,000 upon obtaining 510(k) clearance for the product and a
royalty on future revenue associated with products using the licensed technology.
On August 7, 2009, Somanetics filed a patent infringement action against CAS Medical Systems,
Inc. in the United States District Court for the Eastern District of Michigan. The complaint
asserts that CAS Medical’s FORE-SIGHT® Cerebral Oximeter willfully infringes upon one or
more of Somanetics’ patents. The complaint also asserts that CAS Medical has engaged in unfair
competition and false advertising, by making false or misleading statements in connection with its
advertising and promotion of FORE-SIGHT, and false or misleading statements related to Somanetics’
products. The complaint seeks, among other things, compensation for damages and an injunction
against CAS Medical from infringing upon Somanetics’ patents. CAS Medical Systems, Inc. has
answered the complaint denying that it infringes any asserted patent and asserting defenses and
counterclaims, including those for patent invalidity and/or unenforceability, and antitrust
violations.
We may become subject to product liability claims by patients or physicians, and may become a
defendant in product liability or malpractice litigation. We have obtained product liability
insurance and an umbrella policy. We might not be able to maintain such insurance or such
insurance might not be sufficient to protect us against product liability.
7. Stock Option Plans
In February 1991 and January 1997, we adopted stock option plans, and in February 2005, we
adopted a stock incentive plan, for our key employees, directors, consultants and advisors and,
under the 2005 plan, independent contractors and agents. On April 19, 2007, our shareholders
approved an amendment to the Somanetics Corporation 2005 Stock Incentive Plan to increase the
number of common shares reserved for issuance under the 2005 Plan.
The stock option plans provided for our issuance of options to purchase a maximum of 115,000
common shares under the 1991 plan and 2,560,000 common shares under the 1997 plan. The 2005 plan
permits us to grant stock options, including both nonqualified options and incentive options,
restricted stock and restricted stock units, up to 1,200,000 common shares. In addition, we
granted options to employees independent of the plans. Options granted generally have a 10-year
life, and vest over a three-year period, except the options granted in fiscal 2005 vested on
November 30, 2005 and the options and restricted stock granted in fiscal 2006, fiscal 2007, fiscal
2008 and fiscal 2009 vest over a five-year period. Awards and expirations under the 1991 plan,
1997 plan, 2005 plan and independent of the plans during the years ended November 30, 2009, 2008
and 2007 are listed below.
At November 30, 2009, no additional options may be granted under the 1991 plan or the 1997
plan, and 304,673 common shares were available to be granted or awarded under the 2005 plan.
During fiscal 2009, we granted 68,250 stock options to employees, directors and an officer, at
the market price on the date of grant, during fiscal 2008, we granted 253,000 stock options to our
employees, directors and officers, at the market price on the date of grant, and during fiscal
2007, we granted 96,000 stock options to our employees, directors and one of our officers, at the
market price on the date of grant. During fiscal 2009, we also issued 8,588 restricted common
shares to our employees in January 2009 and 9,000 restricted common shares to an officer in April
2009 valued at the market price on the date of grant of $16.31 and $14.77 respectively. During
fiscal 2008, we also issued 5,273 restricted common shares to our employees in January 2008 and
70,000 restricted common shares to our officers in March 2008 valued at the market price on the
date of grant of $21.81 and $12.61 respectively. During fiscal 2007, we issued 9,000 restricted
common shares to our officers valued at the market price on the date of grant of $18.93. These
stock options and restricted shares vest and are expensed in the financial statements over five
years. During fiscal 2009, 117,400 stock options and 30,455 restricted common shares vested,
during fiscal 2008, 66,800 stock options and 15,400 restricted common shares vested, and during
fiscal 2007, 47,800 stock options and 13,600 restricted common shares vested. As a result of the
stock options and restricted common
68
Table of Contents
SOMANETICS CORPORATION
NOTES TO FINANCIAL STATEMENTS — (Continued)
shares that we granted during fiscal 2006, 2007, 2008 and 2009, we have recorded $1,616,678 in
stock compensation expense in fiscal 2009. As a result of the stock options and restricted common
shares that we granted during fiscal 2006, 2007 and 2008, we have recorded $1,338,590 in stock
compensation expense in fiscal 2008. As a result of the stock options and restricted common shares
that we granted during fiscal 2006 and 2007, we recorded $781,308 in stock compensation expense in
fiscal 2007.
As of November 30, 2009, there was $4,437,246 of total unrecognized compensation cost related
to nonvested share-based compensation awards granted under the 2005 Plan. That cost is expected to
be recognized over a weighted average period of 3 years. As of November 30, 2008, there was
$5,247,791 of total unrecognized compensation cost related to nonvested share-based compensation
awards granted under the 2005 Plan. That cost is expected to be recognized over a weighted average
period of 4 years. As of November 30, 2007, there was $3,606,518 of total unrecognized
compensation cost related to nonvested share-based compensation awards granted under the 2005 Plan.
That cost was expected to be recognized over a weighted average period of 4 years.
The fair value of each option grant is estimated on the date of grant using the Black-Scholes
option-pricing model with the following weighted-average assumptions used for 2009, 2008 and 2007:
expected volatility (the measure by which the stock price has fluctuated or is expected to
fluctuate during the period) of 53.97% for 2009 (59.30% for 2008 and 47.00% for 2007), risk-free
interest rate (approximate U.S. Treasury yield in effect at the time of grant) of 2.50% for 2009
(2.35% for 2008 and 5.0% for 2007), expected lives of 6 years for 2009 (6 years for 2008 and 2007)
and dividend yield of 0%. The fair value of restricted common shares was estimated based on the
market value of the common shares on the date of issuance.
A summary of our stock option activity and related information for the years ended November
30, 2009, 2008 and 2007 is as follows:
| 2009 | 2008 | 2007 | ||||||||||||||||||||||
| Weighted | Weighted | Weighted | ||||||||||||||||||||||
| Average | Average | Average | ||||||||||||||||||||||
| Common | Exercise | Common | Exercise | Common | Exercise | |||||||||||||||||||
| Shares | Price | Shares | Price | Shares | Price | |||||||||||||||||||
Options outstanding |
||||||||||||||||||||||||
December 1, |
1,822,187 | $ | 8.12 | 1,895,656 | $ | 6.75 | 2,071,990 | $ | 6.01 | |||||||||||||||
Options granted |
68,250 | 14.77 | 253,000 | 13.56 | 96,000 | 19.25 | ||||||||||||||||||
Options exercised |
(52,800 | ) | 3.22 | (319,969 | ) | 4.17 | (271,334 | ) | 5.47 | |||||||||||||||
Options canceled |
(23,000 | ) | 18.15 | (6,500 | ) | 14.92 | (1,000 | ) | 18.85 | |||||||||||||||
Options outstanding |
||||||||||||||||||||||||
November 30, |
1,814,637 | 8.38 | 1,822,187 | 8.12 | 1,895,656 | 6.75 | ||||||||||||||||||
Options exercisable |
||||||||||||||||||||||||
November 30, |
1,411,587 | $ | 6.42 | 1,352,587 | $ | 5.51 | 1,609,456 | $ | 4.76 | |||||||||||||||
As of November 30, 2009, the aggregate intrinsic value of stock options outstanding was
$12,067,394, and the aggregate intrinsic value of stock options exercisable was $11,799,187. As of
November 30, 2008, the aggregate intrinsic value of stock options outstanding was $17,165,002, and
the aggregate intrinsic value of stock options exercisable was $16,271,622. As of November 30,
2007, the aggregate intrinsic value of stock options outstanding was $25,136,399, and the aggregate
intrinsic value of stock options exercisable was $24,544,204. The total intrinsic value of options
exercised during fiscal 2009, 2008 and 2007 was $568,889, $5,589,429 and $3,707,681, respectively.
69
Table of Contents
SOMANETICS CORPORATION
NOTES TO FINANCIAL STATEMENTS — (Continued)
A summary of the price ranges of our stock options outstanding and exercisable as of November
30, 2009 is as follows:
| Options outstanding | Options exercisable | |||||||||||||||||||||||
| Weighted | Weighted | Weighted | Weighted | |||||||||||||||||||||
| Average | Average | Average | Average | |||||||||||||||||||||
| Range of Exercise | Options | Exercise | Remaining | Options | Exercise | Remaining | ||||||||||||||||||
| Prices | Outstanding | Price | Life (years) | Exercisable | Price | Life (years) | ||||||||||||||||||
$1.70 - $10.00 |
1,014,638 | $ | 2.90 | 2.34 | 1,014,638 | $ | 2.90 | 2.34 | ||||||||||||||||
$10.01 - $15.00 |
439,503 | 13.36 | 7.38 | 213,253 | 13.47 | 6.03 | ||||||||||||||||||
$15.01 - $19.93 |
360,496 | 17.74 | 7.03 | 183,696 | 17.70 | 6.86 | ||||||||||||||||||
Total |
1,814,637 | $ | 8.38 | 4.49 | 1,411,587 | $ | 6.42 | 3.49 | ||||||||||||||||
The weighted-average grant-date fair value of options granted during fiscal 2009, 2008 and
2007 was $7.81, $7.83 and $9.97, respectively. The total fair value of shares vested during fiscal
2009, 2008 and 2007 was $1,532,494, $936,463 and $713,021, respectively. No modifications were
made to any share awards that required an accounting charge, and no cash was paid for share-based
liabilities during fiscal 2009, 2008 and 2007.
8. Acquisitions
In November 2008, we acquired substantially all of the assets of ICU Data Systems, Inc., a
technology development company, for approximately $2,000,000 in cash plus the assumption of
specified liabilities.
Upon our acquisition of ICU Data Systems, Inc.’s assets in November 2008, the purchase price
was allocated among the net tangible assets, other identifiable intangible assets and goodwill, as
required by the relevant accounting standards. The value assigned to the identified intangible
asset was based on a valuation model that included estimating the future cash flows of the
technology and discounting the net cash flows back to their present value using an appropriate
risk-adjusted rate of return (discount rate). See Note 2. The discount rate used was determined
at the time of the acquisition in accordance with accepted valuation methods. Our assessment of
the estimated fair value included making assumptions about the expected net revenues and operating
income related to the acquired technology in future years, making allowance for the uncertainties
regarding, among other things, the time and cost associated with the further advancement of the
design and performance of the technology to ready it for market launch, the rate of adoption of the
technology once it is launched into the marketplace, and the potential for competition related to
the launched technology.
Goodwill represents the amount by which the purchase price of the acquired business exceeds
the estimated fair value of the net tangible and separately identifiable intangible assets of the
acquired business, in addition to transaction costs recorded at cost. Goodwill is not amortized,
but is tested at least annually for impairment. The technology acquisition costs intangible asset
has an estimated useful life of 20 years, based on several patents related to the technology, and
will be amortized on a straight-line basis over the estimated useful life. Intangible assets and
goodwill are reviewed annually for impairment at the end of our fiscal year, and whenever events or
changes in circumstances indicate that the carrying value of the asset may not be recovered. The
company evaluates impairment by comparing the fair value of the intangible asset, determined using
a cash flow method, with its carrying value. As of November 30, 2009, the carrying value of the
technology acquisition costs intangible asset was $234,003, and the carrying value of the goodwill
was $1,783,712.
9. Major Customers and Foreign Sales
Covidien, our international distributor in Europe, the Middle East, South Africa and Canada
for our INVOS System, accounted for 13% of net revenues for the fiscal years ended November 30,
2009 and November 30, 2007, and 14% of net revenues for the fiscal year ended November 30, 2008.
70
Table of Contents
SOMANETICS CORPORATION
NOTES TO FINANCIAL STATEMENTS — (Continued)
Additionally, foreign net revenues for the fiscal year ended November 30, 2009 were
$9,854,238, for the fiscal year ended November 30, 2008 were $9,420,472, and for the fiscal year
ended November 30, 2007 were $7,024,902.
10. Segment Information
We operate our business in one reportable segment, the development, manufacture and marketing
of medical devices. Our products have similar characteristics, customers, distribution and
marketing strategies, and are subject to similar regulatory requirements. In making operating and
strategic decisions, our management evaluates net revenues based on the worldwide net revenues of
our products, and also profitability on an enterprise-wide basis due to shared costs.
Approximately 100% of our net revenues in fiscal 2009, 2008 and 2007 were derived from our INVOS
System product line.
11. Subsequent Events
On January 20, 2010, our board of directors approved an amendment to the Somanetics
Corporation 2005 Stock Incentive Plan to increase the number of common shares reserved for issuance
under the 2005 plan by 600,000 shares, from 1,200,000 shares to 1,800,000 shares, subject to
shareholder approval at the 2010 Annual Meeting of Shareholders.
During the first quarter of 2010, we have repurchased 157,440 common shares for approximately
$2,532,000 under our share repurchase program.
We evaluated all subsequent events from the date of the balance sheet through February 3, 2010,
which represents the date these financial statements are being filed with the SEC. Except as
described in the preceding paragraph, there were no events or transactions occurring during this
subsequent event reporting period which require recognition or disclosure in the financial
statements.
71
Table of Contents
QUARTERLY INFORMATION (unaudited)
The following is a summary of our quarterly operating results for the fiscal years ended
November 30, 2009 and 2008:
| Quarter | ||||||||||||||||
| First | Second | Third | Fourth* | |||||||||||||
Year Ended November 30, 2009 |
||||||||||||||||
Net revenues |
$ | 11,155,354 | $ | 11,831,560 | $ | 12,513,135 | $ | 14,513,685 | ||||||||
Gross margin |
9,574,873 | 10,227,805 | 10,970,234 | 12,660,056 | ||||||||||||
Net income |
1,302,179 | 1,777,565 | 2,229,046 | 1,509,690 | ||||||||||||
Net income per common
share — basic |
$ | 0.11 | $ | 0.15 | $ | 0.18 | $ | 0.13 | ||||||||
Net income per common
share — diluted |
$ | 0.10 | $ | 0.14 | $ | 0.17 | $ | 0.12 | ||||||||
Year Ended November 30, 2008 |
||||||||||||||||
Net revenues |
$ | 8,693,274 | $ | 12,740,063 | $ | 12,367,988 | $ | 13,654,292 | ||||||||
Gross margin |
7,676,450 | 11,060,807 | 10,650,130 | 11,818,974 | ||||||||||||
Net income |
1,028,429 | 3,052,252 | 3,046,454 | 3,319,112 | ||||||||||||
Net income per common
share — basic |
$ | 0.08 | $ | 0.23 | $ | 0.25 | $ | 0.28 | ||||||||
Net income per common
share — diluted |
$ | 0.07 | $ | 0.21 | $ | 0.23 | $ | 0.25 | ||||||||
| * | Fourth quarter 2009 research, development and engineering expenses included $2,000,000 of up-front, non-refundable payments in connection with two license agreements entered into in that quarter. |
72
Table of Contents
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
None.
ITEM 9A. CONTROLS AND PROCEDURES
Our management has evaluated, with the participation of our principal executive and principal
financial officers, the effectiveness of our disclosure controls and procedures and of our internal
control over financial reporting, both as of November 30, 2009. Based on their evaluation, our
principal executive and principal financial officers have concluded that these controls and
procedures are effective as of November 30, 2009. See Item 8 of this report for Management’s
Report on Internal Control Over Financial Reporting and our Independent Registered Public
Accounting Firm’s Report, which are incorporated in this Item 9A by reference. There was no change
in our internal control over financial reporting identified in connection with such evaluation that
occurred during our fourth fiscal quarter ended November 30, 2009 that has materially affected, or
is reasonably likely to materially affect, our internal control over financial reporting.
Disclosure controls and procedures are our controls and other procedures that are designed to
ensure that information required to be disclosed by us in the reports that we file or submit under
the Exchange Act is recorded, processed, summarized and reported, within the time periods specified
in the Securities and Exchange Commission’s rules and forms. Disclosure controls and procedures
include, without limitation, controls and procedures designed to ensure that information required
to be disclosed by us in the reports that we file or submit under the Exchange Act is accumulated
and communicated to our management, including our principal executive and principal financial
officers, as appropriate to allow timely decisions regarding required disclosure.
Internal control over financial reporting is a process designed by, or under the supervision
of, our principal executive and principal financial officers, and effected by our board of
directors, management and other personnel, to provide reasonable assurance regarding the
reliability of financial reporting and the preparation of financial statements for external
purposes in accordance with generally accepted accounting principles and includes those policies
and procedures that: (1) pertain to the maintenance of records that in reasonable detail accurately
and fairly reflect our transactions and dispositions of assets, (2) provide reasonable assurance
that transactions are recorded as necessary to permit preparation of financial statements in
accordance with generally accepted accounting principles, and that our receipts and expenditures
are being made only in accordance with authorizations of our management and directors, and (3)
provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition,
use or disposition of our assets that could have a material effect on the financial statements.
ITEM 9B. OTHER INFORMATION
None.
73
Table of Contents
PART III
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
The information required by this Item 10 regarding our executive officers is included in the
Supplemental Item in Part I of this report, and is incorporated in this Item 10 by reference. The
information required by this Item 10 regarding our directors will be set forth under the captions
“Election of Directors” and “Biographical Information” in our Proxy Statement in connection with
the 2010 Annual Meeting of Shareholders scheduled to be held April 21, 2010, and is incorporated in
this Item 10 by reference. The information required by this Item 10 concerning compliance with
Section 16(a) of the Securities Exchange Act of 1934 will be set forth under the caption “Section
16(a) Beneficial Ownership Reporting Compliance” in our Proxy Statement in connection with the 2010
Annual Meeting of Shareholders scheduled to be held April 21, 2010, and is incorporated in this
Item 10 by reference.
The information required by this Item 10 concerning our Code of Business Conduct and Ethics
will be set forth under the caption “Code of Business Conduct and Ethics” in our Proxy Statement in
connection with the 2010 Annual Meeting of Shareholders scheduled to be held April 21, 2010, and is
incorporated in this Item 10 by reference. The information required by this Item 10 concerning the
procedures by which security holders may recommend nominees to our board of directors will be set
forth under the caption “Corporate Governance — Nominating Committee” in our Proxy Statement in
connection with the 2010 Annual Meeting of Shareholders scheduled to be held April 21, 2010, and is
incorporated in this Item 10 by reference. The information required by this Item 10 concerning our
Audit Committee and our Audit Committee financial experts will be set forth under the caption
“Corporate Governance — Audit Committee” and “Corporate Governance — Audit Committee Financial
Expert” in our Proxy Statement in connection with the 2010 Annual Meeting of Shareholders scheduled
to be held April 21, 2010, and is incorporated in this Item 10 by reference.
ITEM 11. EXECUTIVE COMPENSATION
The information required by this Item 11 concerning executive compensation will be set forth
under the caption “Executive Compensation” in our Proxy Statement in connection with the 2010
Annual Meeting of Shareholders scheduled to be held April 21, 2010, and is incorporated in this
Item 11 by reference. The information required by this Item 11 concerning Compensation Committee
Interlocks and Insider Participation will be set forth under the caption “Corporate Governance —
Compensation Committee Interlocks and Insider Participation” in our Proxy Statement in connection
with the 2010 Annual Meeting of Shareholders scheduled to be held April 21, 2010, and is
incorporated in this Item 11 by reference. The information required by this Item 11 concerning the
Compensation Committee Report will be set forth under the caption “Corporate Governance —
Compensation Committee Report” in our Proxy Statement in connection with the 2010 Annual Meeting of
Shareholders scheduled to be held April 21, 2010, and is incorporated in this Item 11 by reference.
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER
MATTERS
The information required by this Item 12 concerning security ownership of certain beneficial
owners and management will be set forth under the captions “Voting Securities and Principal Holders
— Principal Holders of Our Voting Securities” and “Election of Directors” in our Proxy Statement
in connection with the 2010 Annual Meeting of Shareholders scheduled to be held April 21, 2010, and
is incorporated in this Item 12 by reference. The equity compensation plan information required by
this Item 12 will be set forth under the caption “Equity Compensation Plan Information” in our
Proxy Statement in connection with the 2010 Annual Meeting of Shareholders scheduled to be held
April 21, 2010, and is incorporated in this Item 12 by reference.
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
The information required by this Item 13 concerning transactions with related persons, if any,
will be set forth under the caption “Certain Transactions” or “Compensation Committee Interlocks
and Insider Participation” and under the caption “Review, Approval or Ratification of Transactions
with Related Persons” in our Proxy Statement in connection with the 2010 Annual Meeting of
Shareholders scheduled to be held April 21, 2010, and is incorporated in this Item 13 by reference.
The information required by this Item 13 concerning director
74
Table of Contents
independence will be set forth under
the caption “Corporate Governance — Independence” in our Proxy Statement in connection with the
2010 Annual Meeting of Shareholders scheduled to be held April 21, 2010, and is incorporated in
this Item 13 by reference.
ITEM 14. PRINCIPAL ACCOUNTANT FEES AND SERVICES
The information required by this Item 14 concerning principal accountant fees and services
will be set forth under the caption “Independent Accountants” in our Proxy Statement in connection
with the 2010 Annual Meeting of Shareholders scheduled to be held April 21, 2010, and is
incorporated in this Item 14 by reference.
75
Table of Contents
PART IV
ITEM 15 EXHIBITS AND FINANCIAL STATEMENT SCHEDULES
| (a) | (1) | Financial Statements | |
| Our financial statements for the following years are included in response to Item 8 of this report: | |||
| Report of Independent Registered Public Accounting Firm | |||
| Balance Sheets — November 30, 2009 and 2008 | |||
| Statements of Operations — For Each of the Three Years in the Period Ended November 30, 2009 | |||
| Statements of Shareholders’ Equity — For Each of the Three Years in the Period Ended November 30, 2009 | |||
| Statements of Cash Flows — For Each of the Three Years in the Period Ended November 30, 2009 | |||
| Notes to Financial Statements | |||
| (2) | Financial Statement Schedules | ||
| None. | |||
| (3) | Exhibits | ||
| The Exhibits to this report are as set forth in the “Exhibit Index” on pages 78 to 80 of this report. Each management contract or compensatory plan or arrangement filed as an exhibit to this report is identified in the “Index to Exhibits” with an asterisk before the exhibit number. | |||
76
Table of Contents
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934,
the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto
duly authorized.
| Somanetics Corporation | ||||
| Date: February 3, 2010 | By: | /s/ Bruce J. Barrett | ||
| Bruce J. Barrett | ||||
| President & Chief Executive Officer | ||||
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been
signed below by the following persons on behalf of the registrant and in the capacities and on the
dates indicated.
| Signature | Title | Date | ||
/s/ BRUCE J. BARRETT
|
President and Chief Executive Officer and a Director | February 3, 2010 | ||
Bruce J. Barrett
|
(Principal Executive Officer) | |||
/s/ WILLIAM M. IACONA
|
Vice President and Chief Financial Officer, | February 3, 2010 | ||
William M. Iacona
|
Controller, and Treasurer | |||
| (Principal Financial Officer and Principal Accounting Officer) | ||||
/s/ JAMES I. AUSMAN
|
Director | February 2, 2010 | ||
James I. Ausman, M.D., Ph.D. |
||||
/s/ DANIEL S. FOLLIS
|
Director | February 2, 2010 | ||
Daniel S. Follis |
||||
/s/ JOHN P. JUMPER
|
Director | February 2, 2010 | ||
John P. Jumper |
||||
/s/ RICHARD R. SORENSEN
|
Director | February 2, 2010 | ||
Richard R. Sorensen |
77
Table of Contents
EXHIBIT INDEX
| Exhibit | Description | |
3(i)
|
Restated Articles of Incorporation of Somanetics Corporation, incorporated by reference to Exhibit 3(i) to the Company’s Quarterly Report on Form 10-Q for the quarter ended February 28, 1998. | |
3(ii)
|
Amended and Restated Bylaws of Somanetics Corporation, incorporated by reference to Exhibit 3(ii) to the Company’s Current Report on Form 8-K dated November 2, 2007 and filed November 5, 2007. | |
10.1
|
Lease Agreement, dated September 10, 1991, between Somanetics Corporation and WS Development Company, incorporated by reference to Exhibit 10.3 to the Company’s Quarterly Report on Form 10-Q for the quarter ended August 31, 1991. | |
10.2
|
Extension of Lease, between Somanetics Corporation and WS Development Company, dated July 22, 1994, incorporated by reference to Exhibit 10.11 to the Company’s Quarterly Report on Form 10-Q for the quarter ended August 31, 1994. | |
10.3
|
Change in ownership of Lease Agreement for 1653 E. Maple Road, Troy, MI 48083, dated September 12, 1994, between Somanetics Corporation and First Industrial, L.P., incorporated by reference to Exhibit 10.12 to the Company’s Quarterly Report on Form 10-Q for the quarter ended August 31, 1994. | |
10.4
|
Second Addendum, between Somanetics Corporation and First Industrial Mortgage Partnership, L.P., dated April 14, 1997, incorporated by reference to Exhibit 10.1 to the Company’s Quarterly Report on Form 10-Q for the quarter ended May 31, 1997. | |
10.5
|
Third Amendment, between Somanetics Corporation and First Industrial Mortgage Partnership, L.P., dated April 23, 1999, incorporated by reference to Exhibit 10.2 to the Company’s Quarterly Report on Form 10-Q for the quarter ended May 31, 1999. | |
10.6
|
Fourth Amendment, between Somanetics Corporation and First Industrial Mortgage Partnership, L.P., dated April 13, 2000, incorporated by reference to Exhibit 10.1 to the Company’s Quarterly Report on Form 10-Q for the quarter ended May 31, 2000. | |
10.7
|
Fifth Amendment, between Somanetics Corporation and First Industrial Mortgage Partnership, L.P., dated January 22, 2003, incorporated by reference to Exhibit 10.7 to the Company’s Annual Report on Form 10-K for the fiscal year ended November 30, 2002. | |
10.8
|
Sixth Amendment, between Somanetics Corporation and First Industrial Mortgage Partnership, L.P., dated April 21, 2004, incorporated by reference to Exhibit 10.1 to the Company’s Quarterly Report on Form 10-Q for the quarter ended May 31, 2004. | |
10.9
|
Seventh Amendment, between Somanetics Corporation and First Industrial Mortgage Partnership, L.P., dated April 16, 2009, incorporated by reference to Exhibit 10.1 to the Company’s Quarterly Report on Form 10-Q for the quarter ended May 31, 2009. | |
10.10
|
Lease, dated as of July 22, 2009, between Kirts Office Center Associates, L.L.C. and Somanetics Corporation, incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K dated July 22, 2009 and filed on July 27, 2009. | |
*10.11
|
Somanetics Corporation Amended and Restated 1991 Incentive Stock Option Plan, incorporated by reference to Exhibit 10.5 to the Company’s Annual Report on Form 10-K for the fiscal year ended November 30, 1991. | |
*10.12
|
Fourth Amendment to Somanetics Corporation 1991 Incentive Stock Option Plan, incorporated by reference to Exhibit 10.7 to the Company’s Annual Report on Form 10-K for the fiscal year ended November 30, 1992. | |
*10.13
|
Amended and Restated Fifth Amendment to Somanetics Corporation 1991 Incentive Stock Option Plan, incorporated by reference to Exhibit 10.10 to the Company’s Annual Report on Form 10-K for the fiscal year ended November 30, 1995. | |
*10.14
|
Somanetics Corporation 1997 Stock Option Plan, incorporated by reference to Exhibit 10.9 to the Company’s Annual Report on Form 10-K for the fiscal year ended November 30, 1996. | |
*10.15
|
First Amendment to Somanetics Corporation 1997 Stock Option Plan, incorporated by reference to Exhibit 10.11 to the Company’s Annual Report on Form 10-K for the fiscal year ended November 30, 1997. | |
*10.16
|
Second Amendment to Somanetics Corporation 1997 Stock Option Plan, incorporated by reference to Exhibit 10.12 to the Company’s Annual Report on Form 10-K for the fiscal year |
78
Table of Contents
EXHIBIT INDEX
| Exhibit | Description | |
| ended November 30, 1998. | ||
*10.17
|
Third Amendment to Somanetics Corporation 1997 Stock Option Plan, incorporated by reference to Exhibit 10.14 to the Company’s Annual Report on Form 10-K for the fiscal year ended November 30, 1999. | |
*10.18
|
Fourth Amendment to Somanetics Corporation 1997 Stock Option Plan, incorporated by reference to Exhibit 10.16 to the Company’s Annual Report on Form 10-K for the fiscal year ended November 30, 2000. | |
*10.19
|
Fifth Amendment to Somanetics Corporation 1997 Stock Option Plan, incorporated by reference to Exhibit 10.1 to the Company’s Quarterly Report on Form 10-Q for the quarter ended February 28, 2002. | |
*10.20
|
Sixth Amendment to Somanetics Corporation 1997 Stock Option Plan, incorporated by reference to Exhibit 10.18 to the Company’s Annual Report on Form 10-K for the fiscal year ended November 30, 2002. | |
*10.21
|
Somanetics Corporation 2005 Stock Incentive Plan, incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K, dated February 24, 2005 | |
*10.22
|
First Amendment to Somanetics Corporation 2005 Stock Incentive Plan, incorporated by reference to Exhibit 10.3 to the Company’s Current Report on Form 8-K, dated January 17, 2007 and filed January 23, 2007. | |
*10.23
|
Second Amendment to Somanetics Corporation 2005 Stock Incentive Plan, incorporated by reference to Exhibit 10.2 to the Company’s Current Report on Form 8-K, dated January 20, 2010 and filed January 26, 2010. | |
*10.24
|
Somanetics Corporation 2009 Executive Officer Incentive Compensation Plan, incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K, dated January 21, 2009 and filed January 23, 2009. | |
*10.25
|
Somanetics Corporation 2010 Executive Officer Incentive Compensation Plan, incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K, dated January 20, 2010 and filed January 26, 2010. | |
*10.26
|
Amended and Restated Employment Agreement between Somanetics Corporation and Bruce J. Barrett, dated as of June 17, 2008, incorporated by reference to Exhibit 99.3 to the Company’s Current Report on Form 8-K, dated June 17, 2008 and filed June 23, 2008. | |
*10.27
|
Amended and Restated Employment Agreement between Somanetics Corporation and Dominic J. Spadafore, dated as of June 17, 2008, incorporated by reference to Exhibit 99.2 to the Company’s Current Report on Form 8-K, dated June 17, 2008 and filed June 23, 2008. | |
*10.28
|
Form of Amended and Restated Change in Control Agreement, between Somanetics Corporation and executive officers, dated as of June 17, 2008, incorporated by reference to Exhibit 99.1 to the Company’s Current Report on Form 8-K, dated June 17, 2008 and filed June 23, 2008. | |
*10.29
|
Form of Director Stock Option Agreement, incorporated by reference to Exhibit 10.30 to the Company’s Annual Report on Form 10-K for the fiscal year ended November 30, 2004. | |
*10.30
|
Form of Officer Non-Qualified Stock Option Agreement, incorporated by reference to Exhibit 10.31 to the Company’s Annual Report on Form 10-K for the fiscal year ended November 30, 2004. | |
*10.31
|
Form of Employee Non-Qualified Stock Option Agreement, incorporated by reference to Exhibit 10.32 to the Company’s Annual Report on Form 10-K for the fiscal year ended November 30, 2004. | |
*10.32
|
Form of Incentive Stock Option Agreement, incorporated by reference to Exhibit 10.33 to the Company’s Annual Report on Form 10-K for the fiscal year ended November 30, 2004. | |
*10.33
|
Form of 2005 Stock Incentive Plan Incentive Stock Option Agreement, incorporated by reference to Exhibit 10.1 to the Company’s Quarterly Report on Form 10-Q for the quarter ended May 31, 2005. | |
*10.34
|
Form of 2005 Stock Incentive Plan Officer Non-Qualified Stock Option Agreement, incorporated by reference to Exhibit 10.2 to the Company’s Quarterly Report on Form 10-Q for the quarter ended May 31, 2005. | |
*10.35
|
Form of 2005 Stock Incentive Plan Non-Officer Non-Qualified Stock Option Agreement, incorporated by reference to Exhibit 10.3 to the Company’s Quarterly Report on Form 10-Q for |
79
Table of Contents
EXHIBIT INDEX
| Exhibit | Description | |
| the quarter ended May 31, 2005. | ||
*10.36
|
Form of 2005 Stock Incentive Plan Director Stock Option Agreement, incorporated by reference to Exhibit 10.4 to the Company’s Quarterly Report on Form 10-Q for the quarter ended May 31, 2005. | |
*10.37
|
Form of Restricted Stock Agreement, incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K, dated June 29, 2006 and filed July 5, 2006. | |
*10.38
|
Stock Option Agreement, dated as of August 1, 2002, between Somanetics Corporation and Dominic J. Spadafore, incorporated by reference to Exhibit 10.3 to the Company’s Quarterly Report on Form 10-Q for the quarter ended August 31, 2002. | |
*10.39
|
Summary of Outside Director Compensation, incorporated by reference to Item 8.01 to the Company’s Current Report on Form 8-K dated May 7, 2009 and filed May 8, 2009. | |
10.40
|
Contract Development Agreement, dated as of February 6, 2009 among Somanetics Corporation, Shirley Research Corporation, Hugh F. Stoddart, and Hugh A. Stoddart, incorporated by reference to Exhibit 99.1 to the Company’s Current Report on Form 8-K, dated February 6, 2009 and filed February 10, 2009. | |
10.41
|
Current Form of Somanetics Corporation Confidentiality Agreement used for testing hospitals and clinics, incorporated by reference to Exhibit 10.22 to the Company’s Annual Report on Form 10-K for the fiscal year ended November 30, 1992. | |
10.42
|
Current Form of Somanetics Corporation Confidentiality Agreement used for the Company’s employees and agents, incorporated by reference to Exhibit 10.3 to the Company’s Quarterly Report on Form 10-Q for the quarter ended August 31, 1992. | |
14.1
|
Somanetics Corporation Code of Business Conduct and Ethics, as re-adopted December 8, 2009. | |
21.1
|
Subsidiaries of the Registrant. | |
23.1
|
Consent of Deloitte & Touche LLP. | |
31.1
|
Certifications of Chief Executive Officer Pursuant to Rule 13a-14(a), as Adopted Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. | |
31.2
|
Certifications of Chief Financial Officer Pursuant to Rule 13a-14(a), as Adopted Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. | |
32.1
|
Certifications of Chief Executive Officer and Chief Financial Officer Pursuant to 18 U.S.C. Section 1350, as Adopted Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. |
80
