Attached files
| file | filename |
|---|---|
| 8-K - FORM 8 K - Stem Cell Therapy International, Inc. | d8k.htm |
 INVESTOR PRESENTATION January, 2010 Exhibit 99.1 |
 Welcome to AmStem “Fulfilling the worldwide promise of stem
cells” Safe Harbor Statement
Certain oral statements made by management of Stem Cell Therapy International, Inc. (“the Company”) from time to time, including certain statements contained herein, that are not historical facts are “forward-looking statements” within the meaning of the Private Securities
Act of 1995. Because such statements involve risks and uncertainties, actual results may
differ materially from those expressed or implied by such forward-looking statements.
Forward- looking statements are statements regarding the intent, belief or current
expectations, estimates or projections of the Company, its directors or its officers about the
Company and the industry in which it operates, and include among other items, statements
regarding the Company’s business and growth strategies and its future financial
performance. Although the Company believes that its expectations are based on reasonable
assumptions, it can give no assurance that the anticipated results will occur. When used in
this report, the words “expects,” “anticipates,” “intends,”
“plans,” “believes,” “seeks,” “estimates,” and similar expressions are generally intended to identify forward-looking statements. Important factors
that could cause the actual results to differ materially from those in forward-looking statements
include, among other items, management’s ability to successfully implement its business and
growth strategies, including its ability to raise additional capital. The Company disclaims
any intention or obligation to update or revise forward-looking statements, whether as a
result of new information, future events, or otherwise. |
 Welcome
to AmStem “Fulfilling the worldwide promise of
stem cells” Agenda • Company Overview • Overview of Strategic Plan and Target Markets • Technology and Science • Future Research and Development • Financial Information |
 Welcome
to AmStem “Fulfilling the worldwide promise of
stem cells” Biotechnology company in the field of regenerative medicine Controls one of the largest, fully accredited Cord Blood banks Umbilical cord blood is a popular source of stem cells for research and treatment Exclusive distributor of SteMixx Clinically proven Stem Cell facial cream Clinical trials underway Focus on utilizing stem cells derived from cord blood to treat Buerger’s disease and other incurable diseases Innovative science with substantial IP ownership 19 Patents and patents pending across the world |
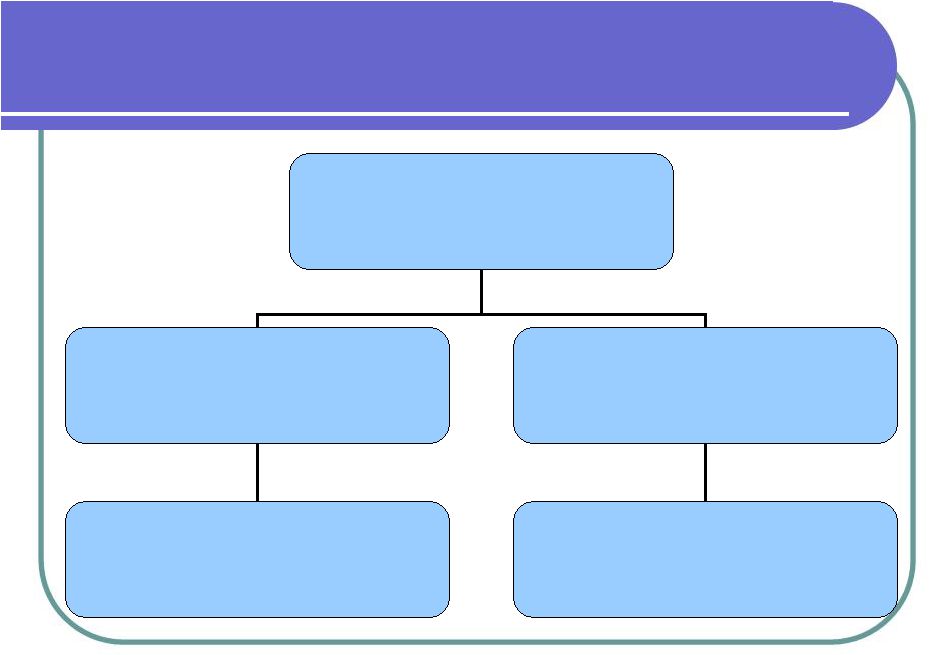 Corporate Structure AmStem Corporation (SCII) AmStem International 100% owned by SCII Histostem Co. Ltd. 90% owned by SCII Distributor of cosmetic products, Treatments Cord Blood Bank Research and clinical trials Manufacture cosmetics |
 Corporate Snapshot • OTCBB SCII • Stock Price Range 12 Months $.03 - $.50 • Shares Outstanding* 193 million • Public Float 33 million • Warrants Outstanding 13 million • Revenues (TTM)** $3.8 million • Net Income (TTM)** $0.6 million • Number of Employees* 50 • Primary US Office San Francisco, CA All based on 1/15/10 *Post Merger ** Histostem financials according to Korean GAAP |
 Current Revenue Streams Korean sales of Stem Cell facial cream Cord Blood banking for future use Medical treatment using Stem Cells Stem Cells provided to researchers & physicians • Predecessor brand to SteMixx for U.S., E.U. markets • Potential antilogous and allergenic transplant use for blood relatives • Full Korean FDA approval for emergent, incurable conditions • Valuable resource for clinical trials, treatments, and study
|
 Future
Revenue Streams U.S., E.U. sales of Stem Cell facial cream Medical treatment using Stem Cells Stem Cells provided to researchers & physicians • SteMixx product packaged and ready for distribution • Valuable resource for clinical trials, treatments, and study • Replication of Stem Cell medical facilities internationally • Registration with NBMD, NCBI, and other accepted orgs R&D for clinical trials and medical products • Pay-for-treatment clinical trials internationally • Development of proprietary pipeline and U.S. NDAs |
 Management Team David L. Stark President & CEO • Appointed CEO in April 2008 • Formerly the Director of the National Institute of Clinical Research (NICR) Andrew Norstrud, CPA CFO • Appointed CFO in September 2007 • Former accountant with Grant Thornton LLP, PricewaterhouseCoopers LLP Dr. Hoon Han, MD. PhD President – Histostem Korea • Internationally recognized and distinguished scientist in Stem Cell and regenerative treatment |
 Agenda • Company Overview • Technology and Science • Overview of Strategic Plan and Target Markets • Future Research and Development • Financial Information Welcome to AmStem “Fulfilling the worldwide promise of stem
cells” |
 U.S.
and E.U. Patents NEW! US Patent Approved Jan 2010 – pending paperwork |
 Asia
Patents Six more patents pending – all are planned to be filed internationally |
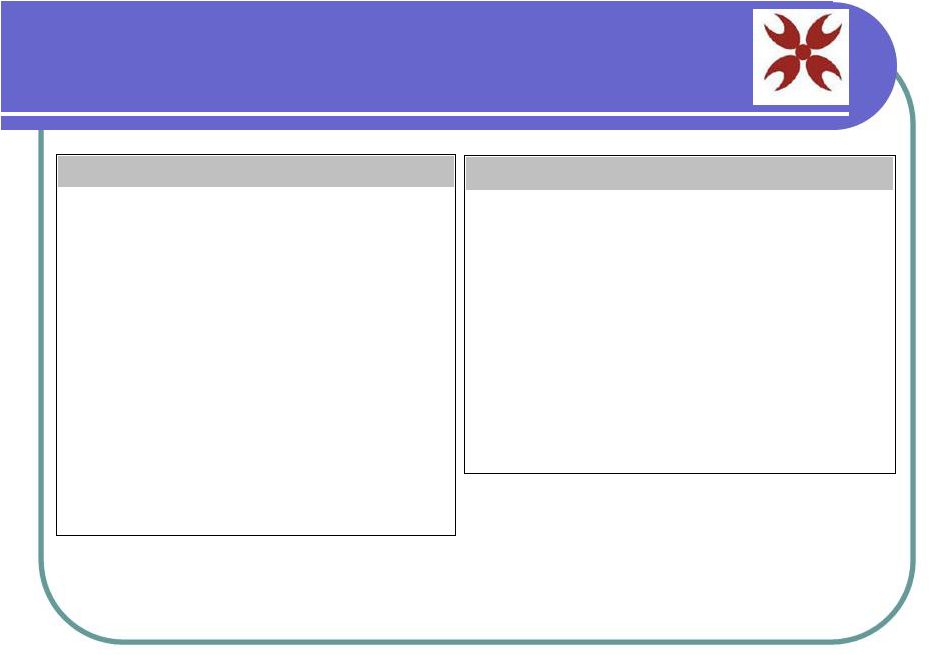 Current Clinical Studies in Korea Condition University/Hospital Phase Patients Treated Anti-Aging New Life Clinic / Dr. Hoon Hahn Phase 1-2 500+ Alopecia (balding) New Life Clinic / Dr. Hoon Hahn Phase 2 56 Diabetes New Life Clinic / Dr. Hoon Hahn Phase 1-2 37 Liver Cirrhosis Han yang University Medical Center / Dr. Hwon Kyum Park Phase 2 31 Cerebral Infarction Center / Dr. Hwon Kyum Park, Kon Kuk Medical Center/ Dr. Sang Keun Chang Phase 2 24 Buerger's Disease Han yang University Medical Center / Dr. Hwon Kyum Park Phase 2 18 ALS Han yang University Medical Center / Dr. Hwon Kyum Park Phase 2 12 Spinal Cord Injury Hany yang University Medical Center / Dr. Hwon Kyum Park, Kon Kuk Medical Center/ Dr. Sang Keun Chang Phase 1-2 10 Condition University/Hospital Phase Patients Treated Heart Disease New Life Clinic / Dr. Hoon Hahn Phase 1 5 Chronic Renal Failure New Life Clinic / Dr. Hoon Hahn Phase 1 5 Alzheimer's Disease New Life Clinic / Dr. Hoon Hahn, Kon Kuk University Medical Center / Dr. Sang Keun Chang Phase 1-2 4 Crohn's Disease New Life Clinic / Dr. Hoon Hahn Phase 1 1 Multiple Sclerosis New Life Clinic / Dr. Hoon Hahn, Kon Kuk University Medical Center / Dr. Sang Keun Chang Phase 1 1 Parkinson's Disease New Life Clinic / Dr. Hoon Hahn, Kon Kuk University Medical Center / Dr. Sang Keun Chang Pending 0 |
 Agenda • Company Overview • Technology and Science • Overview of Strategic Plan and Target Markets • Future Research and Development • Financial Information Welcome to AmStem “Fulfilling the worldwide promise of stem
cells” |
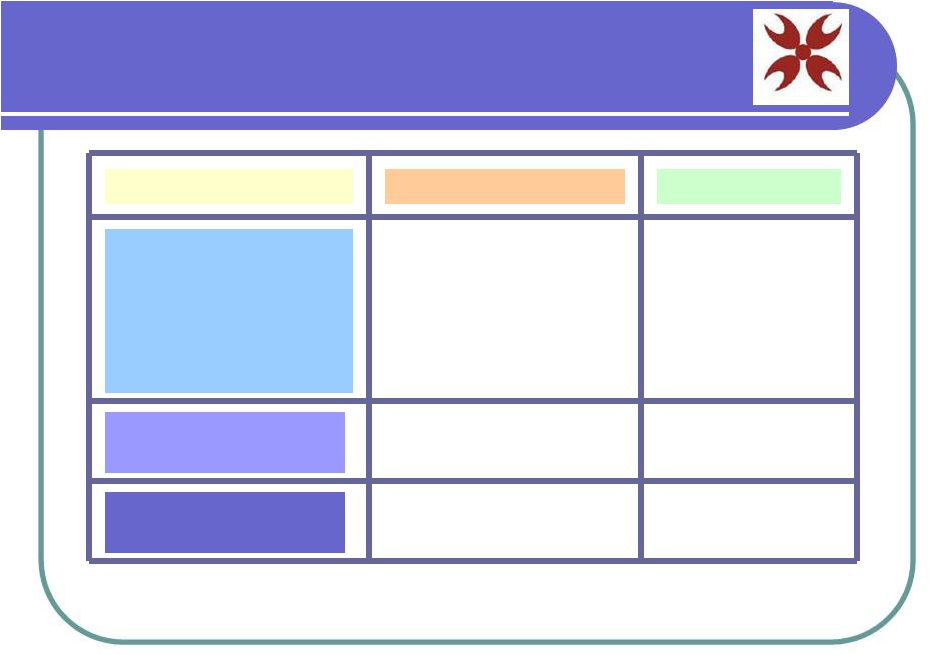 Market Overview AmStem Product Target Market Market Size Stem Cell Facial Cream Stem Cell Injections for Hair Loss Cord Blood Bank and Stem Cells Skin Care Anti-aging facial care U.S. Medical Retail World Medical Retail $65.7 Billion $14.9 Billion $870 Million $1.8 Billion Medical Treatment and research $18 - 25 Million* *Preliminary Estimate $2.5 Billion* Hair regeneration |
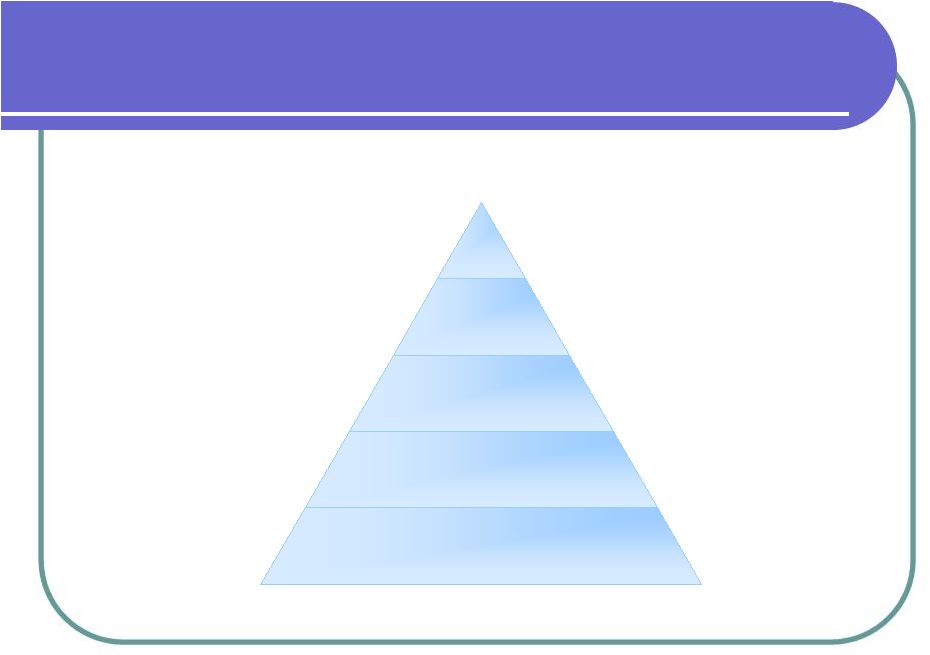 Business Model SCII Level 4: Clinical Trial Development Level 3: Medical Treatment Level 2: Cosmetic Revenue Level 1: Cord Blood Bank Goal: Infiltrate between 0.5 – 1% of market; initial sales of $30-$65 million Goal: Infiltrate between 1–2% of market; initial sales of $2.5 billion market Goal: Infiltrate between 2 – 5% of market share |
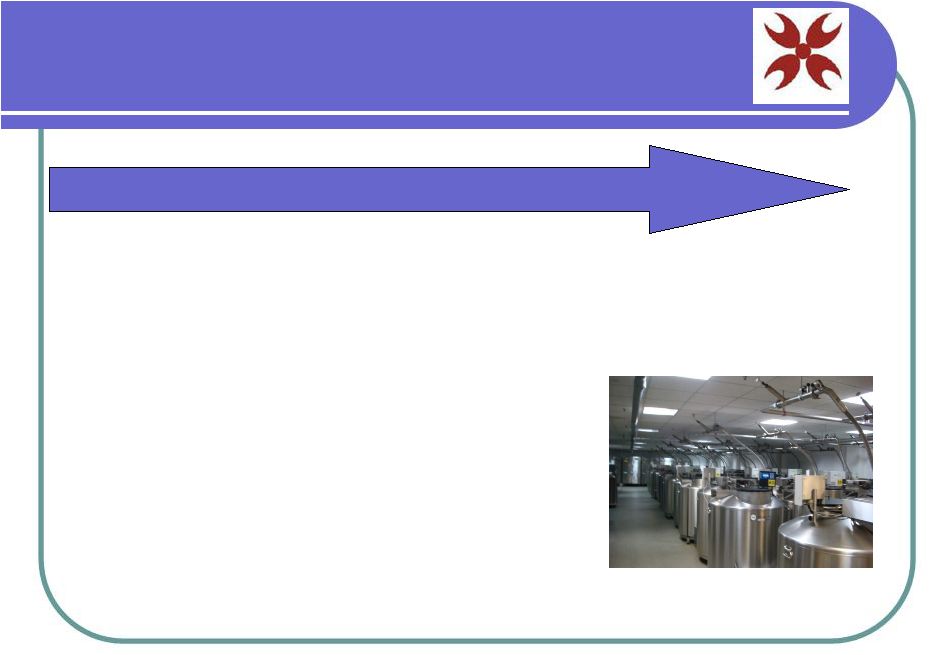 Biological Sales • One of world’s largest Cord Blood bank • Highly desirable genetic profiles • Stem Cells or CBUs • Goal = 2 - 5% of market share LEVEL 1: Cord Blood Bank Sales |
 Cord
Blood Bank Over 80,000 banked CBUs and growing Ethically collected and documented repository State-of-the-art facility manned by MD/Ph.D level technicians |
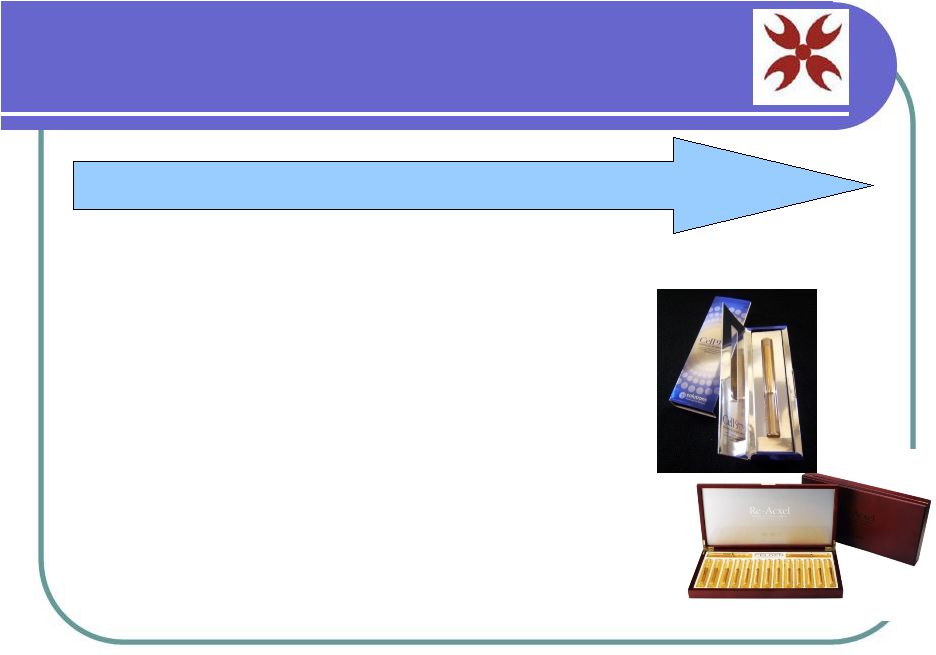 Cosmetic Revenue LEVEL 2: Stem Cell Cosmetic Products • Facial cream ready for immediate distribution in Korean and English • SteMixx, initial product – Researching other uses • Currently being sold in Korea • Expansion through distributors or our supply channel USA/EU • High margins |
 SteMixx – Facial Cream Stem cells = building blocks of the body Ability to renew many major organs and skin SteMixx nourishes body’s cells with essential nutrients Harnesses power of Human Conditioned Stem Cell Media (HCSM) Nutrient rich blend of biocompatible peptides extracted from potent stem cells in umbilical cord blood KFDA proven to rejuvenated skin will break adhesions that cause wrinkles |
 Medical Treatment • Full Korean FDA approval and patents for treatment • Only company currently offering this type of treatment • Impressive early clinical trial results for several new treatments in process of last two years • Goal = break into the market in next 180 days • High-end market LEVEL 3: Stem Cell Injection for Hair Loss |
 Clinical Trials • Buerger’s Disease in Saudi Arabia = lucrative clinical trial market – People will pay to be in any clinical trial with a potential cure • Impressive Phase 1-2 clinical trial results in Korea • Years ahead of the competition • Many disease conditions: LEVEL 4: Clinical Trial Development Buerger’s Disease, Alzheimer’s, ALS, Parkinson’s, Aging, Cirrhosis, Spinal Injury, Diabetes Mellitus, & other degenerative diseases |
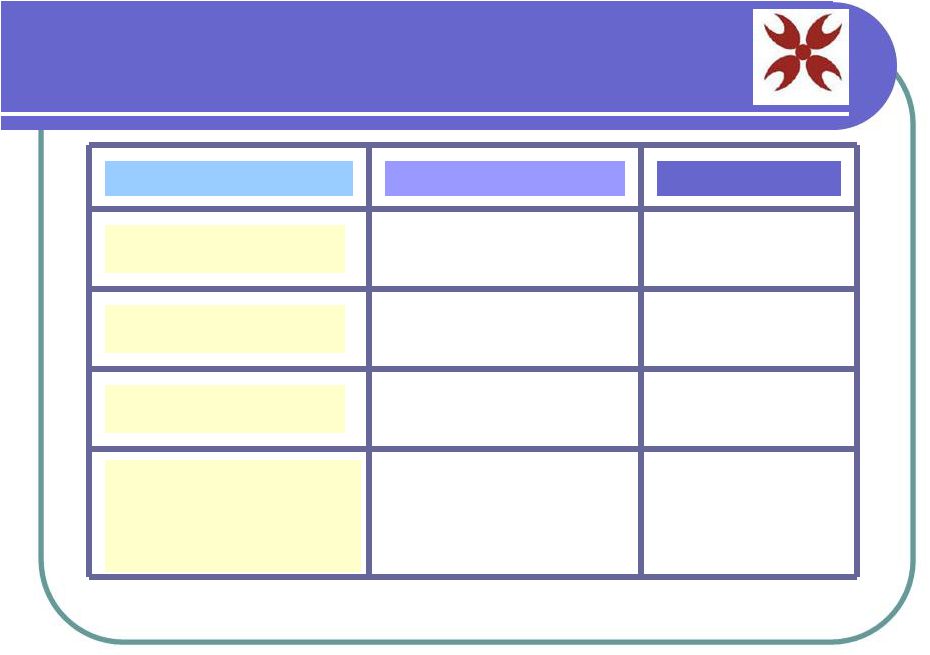 Planned Clinical Trials Condition or Disease Trial Location Phase Korea and/or Mexico Phase 2 Phase 2 Hair Regeneration Buerger’s Disease Alzheimer’s, ALS, Parkinson’s, Cirrhosis, Spinal Injury, Diabetes, Etc. Saudi Arabia Korea and Mexico Phase 2-3 SC Facial Cream Korea & U.S. Marketing |
 Agenda • Company Overview • Technology and Science • Overview of Strategic Plan and Target Markets • Future Research and Development • Financial Information Welcome to AmStem “Fulfilling the worldwide promise of stem
cells” |
 Mid-to-Long Term Development Areas Level 1: Optimize Donor Cord Transplantation • NMDP Certification of Korean Site • Franchise storage facility Level 2: Korean and Mexico Medical Tourism • Cancun Stem Cell clinic (established) • Hair regeneration and anti-aging Level 3: Relicense Current Technologies • Academic and private sector research • U.S., E.U. and Asia (already selling in 5 major hospitals in China) Level 4: Additional Clinical Trials • Diabetes, Degenerative diseases • Maintain good clinical practices at FDA standards for future IND applications
|
 Mid-to-Long Term Growth Areas Optimize Donor Cord Transplantation • NMDP Certification of Korean Site • Franchise storage facility Korean and Mexico Medical Tourism • Cancun Stem Cell clinic (established) • Hair regeneration and anti-aging Relicense Current Technologies • Academic and private sector research • U.S., E.U. and Asia (already selling in 5 major hospitals in China) Additional Clinical Trials • Diabetes, Degenerative diseases • Maintain good clinical practices at FDA standards for future IND applications
|
 Agenda • Company Overview • Technology and Science • Overview of Strategic Plan and Target Markets • Future Research and Development • Financial Information Welcome to AmStem “Fulfilling the worldwide promise of stem
cells” |
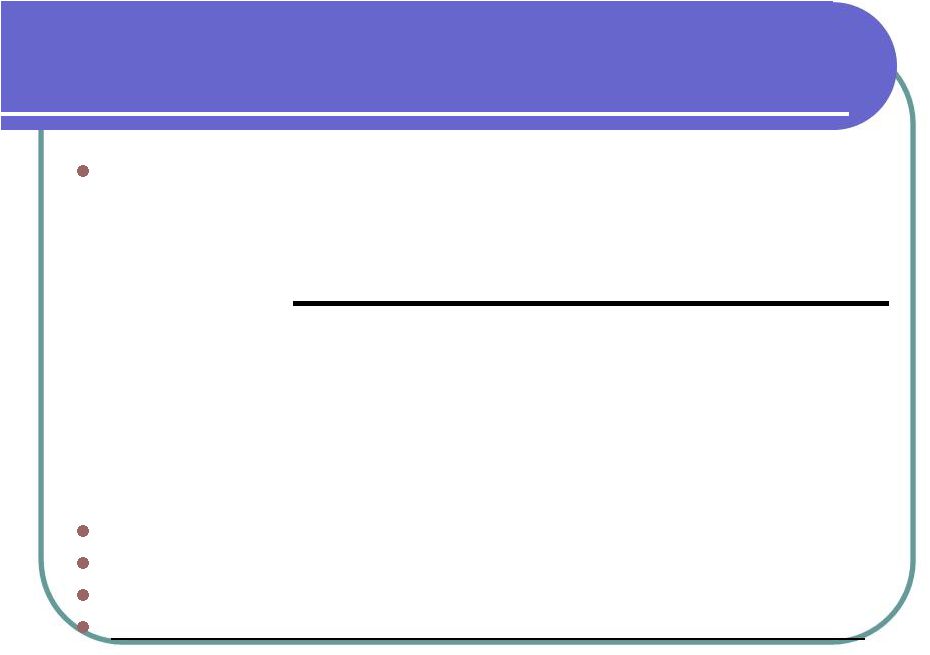 Histostem Financials All Numbers based on Korean GAAP Conversion Rate 1 won = $0.00085 2008 and 2007 audited by KPMG Estimate could be significantly different from final numbers Historical track record of revenues and profitability 2009 2008 2007 Estimate Audited Audited Revenue 5,687,724 $ 3,836,190 $ 3,897,187 $ Gross Profit 4,920,671 $ 2,974,543 $ 3,610,820 $ Gross Profit % 87% 78% 93% Net Income 1,122,978 $ 632,153 $ 110,851 $
Interest Expense Not Estimated 613,410 $ 704,606 $
|
 Investment Considerations Histostem possesses a proven track record of sales and net income – Operating cash flow to fund clinical trial developments Controls one of the largest, fully accredited Cord Blood banks – Umbilical cord blood is a popular source of stem cells for research and treatment Exclusive distributor of SteMixx facial cream Clinical trials underway – Buerger’s Disease Focus on utilizing stem cells derived from cord blood to treat many incurable diseases Innovative science with substantial IP ownership – 19 Patents and patents pending across the world |
 Any
Questions AmStem Corporation Investor Relations (813) 283-2556 IR@amsteminc.com |
